Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/58/02. The contractual start date was in June 2010. The draft report began editorial review in December 2015 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Irwin Nazareth is a member of the National Institute for Health Research Health Technology Assessment Funding Commissioning Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Gilbert et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The problem
Smoking is the leading cause of ill health and mortality, and remains a major public health problem. Approximately 80,000 deaths in England in 2009 were caused by smoking1 and around 5% of all hospital admissions for those aged ≥ 35 years in 2011/12 were attributable to the habit. 2 Although the prevalence of smoking in the adult population in Great Britain has fallen by more than half since 1974, from 46% to 19% in 2013, the fall in prevalence has slowed and has changed little since 2007. 2 Furthermore, the gap in smoking prevalence between those in professional and managerial occupations and those in routine and manual workers shows no sign of diminishing; those living in the most deprived areas are more than twice as likely to smoke as those living in the least deprived areas. 3
A key objective of every UK government over the last two decades has been to reduce the prevalence of smoking,1,4 and various initiatives have been introduced aimed at reducing tobacco use. One of the key strategies to help current smokers quit was to implement government-funded specialist smoking cessation services in Health Action Zones in 1999, which were then rolled out throughout England in 2000. 5 These specialist services were established by Primary Care Trusts, operating predominantly in primary care settings, and offered intensive advice and support to smokers motivated to quit, in group or one-to-one sessions. Early evaluations suggested that the services were effective in their aim of supporting smokers to quit6,7 and were reaching smokers from more-deprived groups. 8 Since their introduction, the services, now known as the NHS Stop Smoking Services (SSSs), have continued to evolve. The most significant change took place during the course of this research in April 2013, when commissioning of local SSSs was transferred from the NHS to the local authority. The result of this was the tendering out of previously in-house services, leading to some SSSs being run by private and voluntary sector companies.
According to the latest figures available, 61% of smokers indicated more than ‘a little’ inclination to give up, and 26% of all smokers had made an attempt to quit in the previous year. 9 This figure has changed little over the years, but along with this evidence that the majority of smokers want to quit, there is a large literature suggesting that, despite this desire, programmes of support are consistently underused. The majority of smokers do not want to participate in formal cessation programmes but prefer to quit on their own. 10–13 More recent surveys and reviews have confirmed that this has changed little; although the trend for unassisted quit attempts may be decreasing, effective treatments remain widely underused and the majority of quit attempts are still unassisted. 14–16 The proportion of smokers in England using the SSSs is similarly low. Estimates in 2001–2 suggest that 2% of the adult smoking population in England set a quit date using SSSs. 8 In 2009, although 43% of smokers had sought some kind of advice or help to quit, the majority of these used self-help leaflets and books, and only 15% had asked a health professional for help. Just 8% were referred or self-referred to a stop smoking group. 9 West and Brown17 report that, of all quit attempts reported in 2011, 46.5% were unaided and only 4.1% reported using the SSS. Furthermore, figures from the Health and Social Care Information Centre show that since 2012 the number of smokers attending the SSS has a continuing downward trend. 18 Thus, these clinical interventions, provided free of charge by the NHS, reach relatively few self-selected smokers and only a small proportion of the total population of smokers in England.
Recruitment methods to cessation services generally employ a reactive approach, in which smokers are expected to seek out help and approach the service themselves. 11 General practitioners (GPs) and health professionals are encouraged to offer brief advice and to provide referral to services, but in 2008–9 only 55% of smokers reported being given advice, and only 8% were referred to the services. 9 Moreover, these smokers were generally expected to follow up their referral and contact the service themselves to make the appointment. There is a wide range of factors that will deter smokers from seeking help, these include a lack of time, lack of availability and accessibility of times and locations, perceived inappropriateness of the service, a perception that help is not necessary, a sense of a lack of empathy from health professionals and not wanting the social stigma associated with participation in formal programmes, as well as a lack of readiness to quit. 13,19,20
The problem to be addressed then is, given that the majority of smokers say they want to quit, how can more smokers be persuaded and motivated to take the plunge and seek, or accept, help to quit, which would lead to more successful quit attempts.
Rationale for intervention
Mass mailing and proactive strategy
Studies suggest that the direct marketing approach has potential as a population-based strategy for recruiting smokers into support services, and could provide treatment access to individuals who might not otherwise seek cessation care. Paul et al. 20 explored the acceptability of direct marketing and proactive contact offering cessation services to smokers. The authors reported that 92.8% of the sample found it acceptable for the health service to contact people to offer assistance and 55.7% said they were likely to take up the offer of individual counselling. This could be an overestimation of actual take-up of the service, but suggests that proactive contact is acceptable and that smokers are open to the idea of intensive counselling. The importance of proactively encouraging smokers to quit has also been demonstrated in studies exploring recruitment to telephone quit lines, lending support to the ‘cold call’ telephone approach. These studies suggested that demand and interest in using services or receiving information about quitting may be greater than is reflected in current usage rates, and that proactively offering services could result in an increase in uptake. 21–24 A recent systematic review of recruitment methods for smoking cessation programmes suggested that personal tailored messages and proactive and intensive recruitment strategies can enhance recruitment. 25 This review confirmed the conclusion reached by McDonald26 that interpersonal strategies have a positive effect on recruitment into smoking cessation programmes.
Lichtenstein and Hollis27 employed a more proactive recruitment method. They invited smokers attending a medical appointment to an immediate intervention where they were offered information about what attendance at the service would involve, and a strong referral message to the service. Attendance at the first session of the cessation programme increased to 11.3%, compared with 0.006% in a control group who received brief advice only. Fiore et al. 28 also showed that many primary care patients identified as smokers will accept treatment ‘if it is free, appropriately incorporated into the health-care delivery system to ensure convenience, and encouraged through proactive recruitment’.
In line with these findings, a major UK study used a proactive strategy to identify individual smokers and inform them about available cessation services. In a cluster randomised controlled trial, Murray et al. 29 identified all patients in general practices recorded as current smokers or with no status recorded. These patients were proactively informed by letter about the SSSs and given the option of being contacted by an advisor. Smokers in practices allocated to the intervention group indicating that they would like to speak to an advisor were contacted within 8 weeks by a researcher trained as an advisor and offered advice and an appointment. Smokers in control group practices received no further contact. Overall, the proportion of current smokers expressing interest was 13.8%, suggesting that more than the current 5% of the smoking population setting quit dates within the NHS were interested in receiving help. Furthermore, Murray et al. 29 reported a 7.7% absolute increase in smokers using the SSSs in the intervention group over the control group at the 6-month follow-up, and an increase of 1.8% in validated abstinence in those smokers requesting contact over the control group (4% vs. 2.2%).
This study by Murray et al. 29 was the first in the UK to assess a proactive method of recruitment to attract smokers into the SSSs. It demonstrated the potential to increase attendance, and also indicated that novel methods of marketing are needed in order to engage interested smokers to encourage use of the SSSs.
Individual computer-tailoring and risk information
One possible way of enhancing recruitment is to use individual characteristics to personalise and tailor communications. Computer-based systems can generate highly tailored materials, defined as ‘any combination of information or change strategies intended to reach one specific person and based on characteristics unique to that person’. 30 This technology offers a method for personalisation of communications to patients and can include an individualised risk communication element based on an individual’s own risk factors, more personally relevant to the consumer than information about population ‘average’ risks. 31
The use of fear in health promotion has been the subject of debate and is somewhat controversial, with claims that ‘shock tactics’ do not work, are too frightening, or can backfire and prove to be counterproductive by prompting a maladaptive behavioural response. There is also a general notion that healthy lifestyle campaigns and anti-smoking messages should be positive and reflect non-smoking role models rather than dealing with the ‘scary’ health consequences of smoking. 32 However, in a review of studies on fear appeals, Sutton33 concluded that increases in fear in communications are associated with increases in acceptance of the recommended action, in a linear relationship. Providing recipients with a reassuring message that adopting the recommended action would be effective, together with clear advice on how to go about it strengthens intentions to follow the advised course of action. 34
Fear messages about smoking can indeed push people to attempt to quit. The fear induced by such messages can be dealt with adaptively by a behavioural response that removes the reason to be fearful, such as quitting smoking, or maladaptively by, for example, denying the truth or personal relevance of the threat. 32 The likelihood of eliciting the desired response can be maximised by empowering the recipient and giving reassurance that it is possible, and also providing a ‘helping relationship’ that is needed to succeed. 35 This has been demonstrated to good effect in mass media campaigns, particularly that of the Australian national anti-smoking advertising campaign, which used graphic fear-based messages as a dominant part of the communication strategy, but tagged with the national quit helpline number and an additional advertisement encouraging calls to that number. 36 This strategy is also consistent with social cognitive models such as the health belief model, which posits that a greater perceived risk of a disease and perceived efficacy of the action in preventing it is associated with increased participation in a recommended behaviour. The health belief model also highlights the importance of providing a specific cue to action, which can act as a trigger and increase compliance with the recommended behaviour. 37,38
Additional justification for this approach lies in the evidence that smokers do not fully acknowledge their own personal vulnerability. Data show unequivocally that smokers acknowledge that their risks of health problems are higher than those of non-smokers. However, studies indicate that they substantially underestimate their own personal risk and tend to conclude that they are less likely to suffer health effects than other smokers. 39 A large literature demonstrates this ‘optimistic bias’ or ‘unrealistic optimism’. Weinstein et al. 40 provided further clear evidence that smokers engage in risk minimisation by convincing themselves that they are not as much at risk as other smokers. Moreover, smokers do not perceive the relationship between the amount smoked and their perceived risk. Thus, even if people are aware of the well-publicised risks, they resist the idea that the risks apply to them, and a key factor is getting participants to acknowledge that these risks are personally relevant.
Computer-tailoring can be used to customise an individual’s risk factors, enhancing perceived personal relevance and helping to overcome the tendency to deny that the information in the tailored messages applies to the recipient. These personal communications can both inform the smoker of their own personal risk, while at the same time promoting confidence and providing the helping relationship that is essential to encouraging acceptance of the advice and following the recommended course of action. These basic tenets were put together in the 3Ts (Tension, Trigger, Treatment) model proposed by West and Sohal. 41 They proposed that triggers can lead to sudden changes and that creating motivational tension can trigger action in smokers who are predisposed, or motivated, to change. The immediate availability of treatment can then prompt action.
Research has shown that individually tailored self-help materials have a small but useful effect over generic materials on smoking cessation. 42 The addition of personalised risk communication that is more personally relevant to the consumer has also been found to increase uptake of screening. 31 Computer technology can be used to produce a communication that combines the tenets of this model, and can also be combined with proactive recruitment methods with the potential to engage with and recruit a larger proportion of the smoking population in a relatively inexpensive way.
Opportunity to experience a support service without commitment
In addition to the factors noted as barriers to the use of support services, the literature also suggests that many smokers are unaware of, or have insufficient knowledge of or inadequate information about, the services available. 20,43 This lack of knowledge can also lead to the belief that ‘it wouldn’t help me anyway’. 20 The combination of ‘why quit’ messages, hard-hitting messages about the consequences of tobacco use and ‘how to quit’ messages, supportive and positive and emphasising quitting resources, was recommended in the Global Dialogue for Effective Stop Smoking Campaigns,44 an international review of the literature. This report also recommended that promotional efforts need to both build awareness that getting help will increase the chances of success and build awareness of and comfort with the quitting services.
Lichtenstein and Hollis27 demonstrated how a proactive and personal approach can be combined with an opportunity to gain more information about the service and what it involves at a no-commitment introductory session. Their intervention included an assessment, measurement of expired-air carbon monoxide (CO) level with an interpretation, video testimonials and the opportunity to ask questions, a voucher fee waiver and immediate scheduling of the smoker for the group. Thus, including a personal invitation with an appointment to a no-commitment introductory session offers the opportunity to gain an insight into what the service can offer, and also has the potential to increase service use.
This research study brings together evidence on proactive and direct mail recruitment, on personalised computer-tailoring and risk information, and on offering the opportunity to experience a support service without commitment, to evaluate a complex intervention comprising all of these elements, in encouraging and increasing attendance at the English SSSs.
The intervention is further enhanced by the addition of a repeated personal letter, with a further invitation sent 3 months after the original to all participants who fail to attend a taster session. This is consistent with recommendations made by Lichtenstein and Hollis27 who proposed that, with repeated advice over time, a greater proportion will be likely to respond.
Aims and objectives
Principal research question
We hypothesised that smokers, identified from general practice records, sent brief personal tailored letters based on characteristics available in their primary care medical records and on a short screening questionnaire, and invited to a ‘Come and Try it’ taster session designed to inform them about the SSSs, were more likely to attend the services than those who received a standard generic letter advertising the service.
Primary objective
The primary objective of the study was to assess the relative effectiveness and cost-effectiveness of a complex intervention, consisting of proactive recruitment by a brief personal letter tailored to individual characteristics available in medical records, and an invitation to a ‘Come and Try it’ taster session to provide information about the SSSs, over a standard generic letter advertising the service, on attendance at the SSSs of at least one session.
Secondary objectives
The secondary objectives were to (1) assess the relative effectiveness of the intervention on biochemically validated 7-day point prevalent abstinence rates at the 6-month follow-up; (2) compare the cost-effectiveness of the intervention; (3) assess the relative effectiveness on additional periods of abstinence measured by self-report of not smoking for periods of 24 hours to 3 months at the 6-month follow-up; (4) assess the number of smokers attending the taster session and the number of smokers completing the 6-week NHS smoking cessation course; (5) assess the number of quit attempts made and any reduction in daily cigarette consumption; (6) explore the effectiveness of the intervention by socioeconomic status and social deprivation; (7) explore reasons for non-attendance and barriers to attendance at the SSS; and (8) determine predictors of attendance at the services and at the taster sessions (in the intervention group).
Chapter 2 Methods
Study design and setting
The Start2quit study was a pragmatic two-arm randomised controlled trial of a complex intervention, utilising general practices in England to recruit smokers into the trial.
Trial management and conduct
The trial was conducted in two stages. A pilot phase was conducted in seven practices covered by two SSSs between January and December 2011. The aims of the pilot phase were to assess the feasibility of the procedure, to ascertain recruitment rates and assess the uptake of the taster sessions, and to establish that the uptake of smoking cessation services in the intervention group was at least as good as that in the control group (i.e. the difference in proportions of intervention minus control was greater than zero).
The methodology of the pilot phase was essentially the same as the full trial, to enable combination of the data from both phases for analysis. However, lessons learnt on recruitment strategies from the pilot phase were applied to the main trial.
The trial was originally funded for 48 months. Additional funding, approved in July 2012 to carry out additional work, increased the size of the sample and extended the trial by 12 months.
Amendments to the final protocol including the trial extension are presented in Appendix 1. The report of the pilot phase submitted to the Health Technology Assessment (HTA) programme in January 2012 is in Appendix 2.
The study was approved by the London–Surrey Borders Research Ethics Committee and received approval from the local NHS trusts.
Participants
Target population
The target group was smokers motivated to quit who had not attended SSSs in the previous 12 months. We aimed to target smokers in areas of high deprivation and ethnic minorities, where smoking prevalence is high.
Inclusion/exclusion criteria
All current smokers willing to participate and returning the signed consent form, aged ≥ 16 years, able to read English, motivated to quit and who had not attended SSSs in the previous 12 months were eligible for inclusion in the study. For the purposes of this research, motivation to quit was defined as answering ‘yes’ to either or both of the following questions:
-
Are you seriously thinking of quitting in the next 6 months?
-
Would you think of quitting if appropriate help was offered at a convenient time and place?
Exclusion criteria were minimal because the aim was to recruit all smokers into the services. However, smokers aged < 16 years were excluded because of the need for parental consent to participate for this age group, and any patients identified considered by the GP to be unsuitable for the project, for example the severely or terminally ill, were excluded.
Recruitment procedure
Stop Smoking Service and practice recruitment
We worked with the National Institute for Health Research Primary Care Research Network (PCRN) to identify SSSs willing to participate in the trial, and to then identify and recruit practices in the selected SSS areas. We aimed to select more practices in areas of high deprivation and of large ethnic communities to ensure full representation of smokers most in need of help, and to maximise the generalisability of the results. The SSSs and PCRNs assisted in identifying sufficient practices serving these populations.
The trial was originally planned to cover 10 different areas served by a SSS, and we initially recruited Camden (North London) and Oxfordshire SSSs and practices in those areas for the pilot phase of the trial. The extension of the trial and the increase in the size of the sample meant that the number of SSSs was increased to 18 in areas representative of the English SSS.
Participant recruitment
All smokers aged ≥ 16 years were identified from their medical records in participating practices. GPs screened the list to exclude anyone they deemed to be unsuitable for the research, for example severely or terminally ill, and the list was also screened to ensure that only one person from the same address was selected. All remaining persons on the list were sent a letter from their GP inviting them to participate in the trial, together with a participant information sheet describing the research, a consent form and a screening questionnaire. Participants were asked to consent to the use of information from their medical records and information given in the screening questionnaire to send them information about quitting, and for the researchers to access relevant data from their attendance at the SSSs. Data generated from the screening questionnaire were used both to assess the criteria for inclusion in the trial, to provide information for the computer-generated tailored intervention letter and to provide baseline characteristics. Participants returned these questionnaires to the practice using a Freepost envelope. Non-responders were sent a reminder and duplicate questionnaire after 3 weeks. All smokers returning the signed consent form and who were eligible to participate were randomised to the intervention or the control group. Patients had the opportunity to decline to participate but to return the questionnaire with basic information to update their smoking status in their GP practice records.
All recruitment materials can be found in Appendix 3. The baseline questionnaire is included in Appendix 4.
Interventions
Control group
Participants allocated to the control group were sent a standard generic letter from the GP practice advertising the local SSS and the therapies available, and asking the smoker to contact the service to make an appointment to see an advisor.
Intervention group
Participants allocated to the intervention group received the following:
-
a brief personalised and tailored letter sent from the GP that included information specific to the patient, using information obtained from the screening questionnaire and from their medical records
-
a personal invitation and appointment to attend a ‘Come and Try it’ taster session to find out more about the services, run by advisors from the local SSS
-
a repeated personal letter with a further invitation sent 3 months after the original to all participants who failed to attend a taster session following the first letter and invitation.
Each of these components is described in the following sections in detail.
Development of the tailored intervention letter
The overall objectives of the letter were to communicate personal risk level if the person continues to smoke, using individualised information on the risk of serious illness, and to encourage attendance at the SSS.
Letter content
The letter was tailored to the individual using characteristics from practice records (gender and age) and confirmed by the baseline screening questionnaire, information obtained from the screening questionnaire (number of cigarettes per day and previous quit attempts) and information from medical records about diagnosed conditions on the Quality and Outcomes Framework (QOF) register. The final list of diseases consisted of all cancers (excluding lung because of its terminal nature); myocardial infarction; coronary heart disease and heart failure (combined because of terminology and the difficulty of telling someone they have a ‘weak heart’); lone atrial fibrillation; stroke and transient ischaemic attack; diabetes; epilepsy; hypertension; hypothyroidism; asthma; chronic obstructive pulmonary disease (COPD); dementia (if the patient was able to consent); depression and severe mental illnesses (schizophrenia and bipolar disorders); and obesity. Possibilities around co-occurrence of diseases were considered and messages created for the following: COPD and heart failure; COPD and asthma; coronary heart disease and hypertension, stroke, dementia, severe mental illnesses, diabetes, obesity; and for multiple conditions. Because of additional risks associated with smoking for women who are pregnant or taking the contraceptive pill or hormone replacement therapy, personal risk information was also included for these smokers.
Personal risk information can be presented as an absolute or relative risk score, categorised, or as a list of the individual’s risk factors. As it was felt not appropriate to provide specific probability figures without the opportunity to discuss them with a health professional, risk was classified as high, very high or extremely high compared with non- or ex-smokers, as recommended by Edwards et al. 31
The offer of help was tailored to previous quit experience, and the letter was accompanied by a personal invitation to the taster session with details of time and place.
The content of the letter was developed in collaboration with GPs and primary care experts with knowledge of medical information available in records. Two service users also contributed.
Letter structure
The letter was headed ‘Personal Health Risk Report and Taster Session Invitation’. It consisted of four sections (Table 1). The amount of tailoring was maximised within the constraints of the short screening questionnaire and a brief letter, so that the final communication consisted of two pages. The section headings were coloured as a traffic light system, using red for the risks, orange to encourage the person to prepare to stop and green for the invitation to the taster session.
| Section | Objectives | Information included | Source |
|---|---|---|---|
| 1. Introduction | To explain the purpose of letter, so that the individual will know that it is a personalised letter based on their assessment | ||
| 2. Personal risk in terms of dependence and general health | To tell the individual his or her dependence in the context of norms | Dependence | Questionnaire |
| To indicate a category of risk according to dependency in terms of the number of cigarettes smoked per day, the number of QOF-registered conditions and age | Age | Medical records | |
| Number of QOF diseases | |||
| 3. Disease-specific health risks and the benefits of quitting | To make the individual aware of the personal health consequences of continuing to smoke, and their own individual risk of serious illness in relation to dependence and own health status | Dependence | Questionnaire |
| To make the individual anxious because of perception of their own personal risk | Age | ||
| To change the individual’s balance of perceived ‘benefits’ against their understanding of the harm caused by smoking | Gender | Medical records | |
| QOF disease | |||
| 4. Invitation to taster session | To remind the individual that help and support is immediately available | Previous quit attempts | Questionnaire |
| To encourage them to seek out support and use the resources available |
The letter was generated by a computer program, signed by the GP and sent from the practice. The first letter was posted to the participant within 3 weeks of returning the completed questionnaire. A second identical letter and invitation was sent 3 months later to every participant who had not attended one of the earlier taster sessions.
Examples of the personal letter are included in Appendix 5.
Development of the taster session and training
The goal of the taster session was to offer information about the SSS, promote the service, address any concerns or queries smokers may have about the service provided and encourage sign-up to a course. It was not intended to replicate the first session of a course.
A draft of the content of the taster sessions was prepared by one of the co-investigators (SG), and was developed into a standard protocol in consultation with the research team. The final standardised protocol also included items from the NHS Centre for Smoking Cessation and Training’s Standard Treatment Programme46 to ensure conformity with national guidelines, and a detailed manual was produced. The standard protocol for the taster session included:
-
a motivational element, congratulating attendees on coming to the session
-
an introduction to the SSS, emphasising that it is a free service and based on well-researched evidence
-
emphasising the importance of stopping smoking and outlining the benefits of quitting, both health and financial/lifestyle
-
information about the services offered, outlining the structure the treatment programme in one-to-one or group sessions, the length of sessions and of the course
-
information about what to expect when they attend and the content of advice, for example emphasising that no-one will be forced to quit, but will be helped to explore the reasons for and against wanting to give up smoking, and helped to develop strategies to resist smoking after their quit date
-
interaction between attendees, discussing, among other things, reasons for stopping
-
discussion about withdrawal symptoms, with information about the available medications and range of nicotine replacement therapy (NRT) products available
-
the opportunity to have a measurement of CO level taken, with an interpretation
-
a 5-minute digital versatile disk showing group and one-to-one sessions in progress, and testimonials from previous successful attendees, produced by University College London (UCL) Media Services in collaboration with Camden SSS
-
the opportunity to ask questions about the service
-
details of how to contact the service and a clear and persuasive invitation to sign up for a group or individual session.
Between two and seven advisors in each SSS, already trained to give smoking cessation advice in group and one-to-one sessions, attended a 2-hour training session to enable them to facilitate the taster sessions according to the standardised protocol and manual. The training sessions took place within each SSS and were led by two members of the team (either HG or SG), and included an explanation and clarification of the study protocol and procedures, and specified the exact information to be delivered in the session. Only trained advisors led the taster sessions, and each session was run either by one advisor with additional administrative support provided by one other, or the presentation was divided between two advisors. They were encouraged to introduce themselves and describe their background and expertise to reassure attendees of their credibility and expertise.
It was intended that each SSS should run between 4 and 12 taster sessions, depending on the number of participants recruited and the area covered by the SSS, and that up to 50 participants were invited to each session, which lasted approximately 1 hour. Sessions were held in the early evening normally beginning between 18.00 and 19.00. On arrival attendees were asked to sign an attendance sheet provided by the research team. An evaluation form was developed for immediate assessment of attendees views of the session, the form included space to indicate interest in signing up to the SSS. Advisors encouraged attendees to complete these evaluation forms before leaving the session and, when possible, participants were given a date and time of a group or one-to-one session before they left. The taster session protocol in included in Appendix 6.
Fidelity
The training session included a basic introduction to the methodology of randomised controlled trials and of uniformity of an intervention. Thus, training emphasised the importance of standardising taster sessions, and of delivering all protocol-specified content, while allowing for differences in the organisation of the individual SSSs and also allowing for advisors to deliver the information naturally, as they would in their smoking cessation clinics. To assess fidelity to the protocol, the taster sessions were, with the consent of the attendees, audio-recorded. Advisors also completed a personal details form, gathering data on gender, age, highest educational qualification, type of smoking cessation training, time since smoking cessation training, number of patients seen in the previous 6 months and job title to account for differences in ‘therapist effects’.
Procedure and baseline data management
Procedure
Recruitment, collection of baseline data and delivery of the intervention took place over 4 years, between January 2011 to June 2011 for the pilot phase, and between January 2012 and December 2014 for the main trial. The procedure in each practice took 12 weeks to complete, during which time the research assistants visited the practice on four occasions, assisting practice staff with mailing invitation letters and questionnaires to patients, processing returned questionnaires, and generating tailored and generic intervention letters. A series of purpose-written computer programs written in Visual Basic for Applications (1997–2003, Microsoft Corporation, Redmond, WA, USA) that read and write Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and Microsoft Word® (Microsoft Corporation, Redmond, WA, USA) files facilitated these processes, and were installed on laptops for use in the practices.
The practice staff initially ran a search using the practice computer system to identify all patients recorded as smokers. The GPs then screened the list for exclusions. Research assistants visited the practice to first run the ‘Check for Duplicates’ computer program, which randomly selected one smoker from each address on the list, and then using the ‘Invite’ program-generated letters inviting smokers to participate, which were then mailed. Research assistants visited the practice a second time to process returns, using a third computer program ‘Risk’ which allocated identification numbers and randomised participants to the control or intervention group. This computer program also combined the data from the baseline questionnaire and medical records with the correct messages from a message library, written using Microsoft Word, to generate tailored letters and invitations to the taster session for those participants randomised to the intervention, and the generic letters for control participants. These were then mailed to the participants. The first taster session was held approximately 2 weeks after this mailing. Research assistants visited the practice on two further occasions to process responses from patients in the same manner as previously described. Further taster sessions were timed accordingly.
Three months later, all participants in the intervention group who had previously been invited to attend a taster session but had not attended were sent a second intervention letter (identical to the first) and a further invitation to a taster session (Figure 1).
FIGURE 1.
Study schedule showing the duration and timing of the procedure, intervention and follow-up. RA, research assisstant. Reproduced from Gilbert et al. 45 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Patients who returned the questionnaire with written consent but who were not eligible to take part in the study were sent a letter thanking them for responding and informing them that they did not fit the study criteria, and patients who returned questionnaires outside the time frame for processing were sent a similar letter informing them that the recruitment period had ended. Both of these letters were sent from the general practice and contained information about the local SSS, advising the smoker to contact the service for more information or to speak to an advisor.
Security and baseline data management
The patient-level data collected in this trial comprised information downloaded from practice records and information provided by participants on the consent form and baseline questionnaire. The information from the practice record was used to generate letters inviting patients to participate in the trial. It was also used, along with baseline questionnaire information, to generate the tailored and generic letters.
All data files and backup media remained in the practice until all eligible participants had been randomised and the tailored and generic letters generated. At this point data files were transferred to the study centre at UCL using proprietary encryption software (TrueCrypt, V61.1; TrueCrypt Foundation, Henderson, NV, USA).
At the end of recruitment in each practice anonymised average data on patients who were invited to participate in the study but did not respond were collected to establish the external validity of our results. These anonymised data comprised gender, date of birth and postcode. Date of birth was converted to age at the time of the invitation and the postcode converted to an Index of Multiple Deprivation (IMD) score via GeoConvert (2011, UK Data Service Census Support, University of Essex, University of Manchester). IMD is the government’s official measure of multiple deprivation at the small-area level, which provides a relative ranking of areas across England according to their level of deprivation. Names and all other identifying data were removed.
Randomisation and blinding
Randomisation, at the level of the study participant, was embedded into the computer program using permuted blocks. Participants were randomised in the ratio 3 : 2 (intervention to control) within the practice, stratified by gender and using a block size of five. For each practice, a computer program was run to create two randomisation tables, one for men and one for women. Each table consisted of 500 rows. In one column, there was a sequence of 2s and 1s in blocks of five (e.g. 1, 1, 2, 2, 1, where 1 and 2 were intervention and control, respectively). This sequence was created by listing all possible permutations of three 1s and two 2s (10 in all), then repeatedly selecting one permutation at random (with replacement) and adding each selection to the sequence. This procedure used the random number generating function rnd in Microsoft Visual Basic for Applications. For each table, the randomise statement was used to initialise the random number generator with a seed based on the system timer. Having created the tables for a given practice, another computer program was used to allocate participants from that practice to an intervention group by selecting the first unused code (1 or 2) from the table for men or the table for women, depending on the participant’s gender, and then marking that code as used. If the information about gender was missing for a participant, the randomisation table to be used was selected at random. Any imbalances were controlled for in the statistical analysis using covariates that were identified prior to examining the trial data. The use of a computer program that enforced randomisation after consent and baseline data entry ensured that concealment was preserved and differential entry prevented.
It was not possible to blind participants to the receipt of a personally tailored letter and invitation to a taster session. Although the personal letter was generated in the practice by a research assistant, the remainder of the research team in all cases were blind to the allocation of the participant, which was enforced by the data management. GPs and practice staff were not aware of their patients’ allocation. In follow-up interviews, the interviewer was blinded to the allocation of the respondent in order to avoid bias in outcome assessment. The interviewers could become unblinded during the course of the interview when participants were asked about the receipt of the letter and attendance at the taster session; however, the main outcome questions were asked at the start of the interview.
By randomising at the level of participant rather than by practice, there was a slight risk of contamination by communication between patients at the same practice allocated to different intervention groups. To reduce this risk we (1) ensured that only one person from the same household received a screening questionnaire; (2) monitored attendance at the taster sessions, to ensure that anyone attending who had not received an invitation was recorded and checked against participants in the control group; (3) kept a record of attendance at the taster sessions; and (4) measured the amount of contamination at follow-up by asking participants whether or not they had attended a taster session and, if not, whether or not they personally knew or had spoken to anyone else who had been invited to a taster session.
Follow-up data collection and evaluation procedure
At the end of the 6-month follow-up period in each SSS, valid data of attendance were collected from the SSSs using NHS monitoring data collected by smoking cessation advisors. A list of participants recruited from the particular SSS was sent to the local collaborator, who searched their user database for each participant named. For each participant whose name was present in the database, and had attended the service between the study entry date and the 6-month follow-up date, a case report form was completed. Data were collected on dates of attendance, agreed quit date, 4-week follow-up date, total number of sessions attended and treatment outcome. We also collected data on the type of advisor, the type and setting of support received, and pharmacological support used.
Research interviewers, independent from the service providers, conducted a computer-assisted telephone interview 6 months after the date of randomisation to assess self-reported SSS attendance, current smoking status, daily cigarette consumption, reasons for non-attendance and barriers to attendance in all participants.
Procedures were applied to maximise retention of participants at the 6-month follow-up. Interviewers made a maximum of 10 attempts to contact a participant by telephone. If, after 10 attempts, the interviewers had been unable to speak to a participant in person, they sent a text message prompting a response back to the mobile phone from which it was sent. The participant was sent the same message a second time if no response was received after 3 days, and, if no response was received after a further 3 days, the participant was sent a paper version of the follow-up questionnaire to complete and return by post. The paper questionnaire was also sent to participants unable to complete the telephone interview but willing to complete and return a postal questionnaire. A decision was taken late on in the trial to send a reminder for this postal questionnaire. If a participant did not fully complete or did not wish to complete the telephone interview, the interviewer attempted to ask the participant four basic questions most relevant to the primary and main secondary outcome.
Participants claiming 7-day abstinence at the 6-month follow-up were asked to provide a salivary cotinine sample to biochemically validate 7-day point prevalent smoking cessation. 47 Samples were obtained by post using a saliva sample kit. 48 Use of NRT at the time the sample was taken was assessed by questionnaire, as the cotinine content can be affected by continued use, and taken into account when the results of the analysis were received. To maximise return of samples, a £5 Marks and Spencer voucher was included with each kit, and a further £5 voucher was sent on return of the sample. Participants were contacted by a research interviewer to remind them to return their kit if saliva samples were not returned within 7 days, and after 10 unsuccessful attempts to contact the participant, they were sent a reminder text message. If the interviewer was successful in contacting the participant but their sample was still not returned after 7 days, the participant was sent the same reminder text message.
Figure 1 shows detail of the timing of assessments, intervention and follow-up.
Measures
Baseline measures
Inclusion criteria (age, intention and motivation to quit, and previous SSS attendance), demographics (gender, marital status, qualifications, employment and ethnicity), self-reported health, dependence on nicotine (number of cigarettes per day and time from waking to first cigarette), smoking history (age started and previous quit attempts), determination and confidence to quit were assessed in the baseline screening questionnaire.
Outcome measures
Primary outcome
The proportion of people entering the smoking cessation service (i.e. attending the first session of a 6-week course) over a period of 6 months from the receipt of the invitation letter, as measured by the NHS records of attendance at the SSSs.
Secondary outcomes
-
Seven-day point prevalent abstinence at the 6-month follow-up, validated by salivary cotinine for all participants reporting abstinence in both the intervention and control groups.
-
Additional periods of abstinence measured by self-report only: 24-hour and 7-day point prevalent, 1- and 3-month prolonged abstinence.
-
Three-month prolonged abstinence, measured by self-report and validated.
-
Self-reported changes in daily cigarette consumption, quit attempts, and changes in motivation and intention to quit in continuing smokers.
-
The number completing the 6-week NHS course.
Process measures
-
The number of smokers attending the taster session (intervention group only).
-
Self-reported attendance data.
-
Perception of the taster session.
-
Perception of the personal invitation letters.
-
Reasons for non-attendance at the taster session and barriers to attendance at the NHS services.
The number attending the taster sessions was taken from records, all other process measures were included in the follow-up interview 6 months after the date of randomisation. Perception of the taster session was also assessed by an evaluation form immediately after each session.
Reasons for non-attendance at the taster session and barriers to attendance at the NHS services were assessed using open questions. In addition, all participants who reported not attending the SSS were asked to complete the 40-item Treatment Barriers Questionnaire (TBQ), validated on a US population,49 to assess in more depth reasons and barriers to the use of the SSS.
Health economic measures
The economic component estimated the cost of providing the interventions, using primary cost data from a NHS and Personal Social Services perspective, as recommended by National Institute for Health and Care Excellence (NICE) guidance. 50 We also measured patients’ use of health and social care services using a comprehensive service use questionnaires. Quality-adjusted life-years (QALYs) were calculated from the European Quality of Life-5 Dimensions (EQ-5D) questionnaire using the area-under-the-curve method. 51 A cost-effectiveness analysis (CEA) was undertaken to compare the tailored letter plus the taster session and the generic letter. In addition to the within-trial CEA, lifetime health-care cost savings and QALY gains associated with the two interventions were estimated based on a decision-analytic model. 52
Sample size and power calculations
Evidence from the study by Murray et al. 29 suggested that attendance at NHS services could be increased by 7.7% (from 8.9% to 16.6%) using a proactive intervention. To detect an effect of this size at 90% power and an alpha of 0.05 required a sample of 420 participants per group. However, in the absence of other similar trials, we conservatively assumed that the uptake of services in those who received the tailored letter and the taster session could be lower than that reported by Murray et al. 29 Therefore, we assumed an estimated increase of 4.6% [from 8.9% to 13.5%, odds ratio (OR) 1.65] requiring 1029 participants per group, 2058 in all, to detect this difference as statistically significant at the 5% level with 90% power.
We originally planned to recruit practices from 10 different SSSs. The taster sessions in each SSS were to be run by the same four advisors comprising 10 therapist clusters. Thus, before adjusting for clustering we would expect 103 patients per cluster. Although the intervention was manualised and structured training run to reduce the variability between the interventions delivered in each SSS, we decided to account for any persistent therapist effects that might apply to those randomised to receive a taster session. The literature reporting intracluster correlations is scarce; however, Adams et al. 53 found a single study54 of smoking cessation delivered in pharmacies, which reported an intracluster correlation coefficient (ICC) of 0.007: this shrank to 0 after adjustment. We therefore assumed an ICC of 0.005 for our study. Allowing for this ICC, coupled with a therapist cluster size of 103, required our existing sample size to be inflated by a factor of 1.51 only in the intervention group, where the effects would occur. Thus, 1554 would receive the tailored letter and taster session, with 2583 participants in total.
The study by Murray et al. 29 also found validated quit rates at 6 months of 4% in the intervention group, compared with 2.2% in the control group (a difference of 1.8%). With our planned sample size of 2583 participants, we had < 80% power to detect a difference of 1.8%. However, if the quit rate were to double from 2.2% to 4.4% (a difference of 2.2%), we still had 80% power to detect such a difference.
An extension to the trial was funded to permit evaluation with adequate power of the intervention effect on 7-day point prevalent abstinence at the 6-month follow-up. This required an 80% increase in the sample size to 1793 in the control group and 2707 in the intervention group (assuming the same therapist effect as the original protocol), giving a total of 4500. This would give 85.4% power to detect a difference of 1.8% at the 5% significance level, assuming quit rates of 4% compared with 2.2% in the intervention and control groups, respectively. The same sample size would have 95% power to detect the difference between quit rates of 4.4% and 2.2% (doubling of quit rate), respectively.
Practices generally identify 13–22% of their patients as smokers,55 depending on the characteristics of the patient population and the accuracy and completeness of the records. We initially estimated that six practices in each of 10 SSSs, with a list size of > 4000, would give approximately 240,000 patients and, assuming a conservative smoking prevalence of 15% in patients aged ≥ 16 years, 36,000 smokers. Based on previous studies,29,56 we estimated a response rate from two mailings of 7% from smokers motivated to quit, securing 2520 participants and meeting the requirements of the original sample size calculation. The extension to the trial required an additional 2000 participants and, based on recruitment figures at the time the extension was funded, we estimated that an additional eight SSSs (48 practices) would recruit 2060 participants, giving a total of 4580 and meeting the requirement of the new power calculation.
Interim analyses and stopping guidelines
The study was initiated with a pilot phase conducted in seven practices recruited from two SSSs. This was intended to be approximately 20% of the original total sample. The criteria for judging the success of the pilot phase and proceeding to full trial was based on:
-
achieving a 7% response rate (i.e. a mean of 42 participants per practice giving consent and agreeing to randomisation) in the first seven practices
-
a preliminary analysis that suggested that the uptake of smoking cessation services in the intervention group was greater than in the control group (i.e. the difference in proportions, intervention minus control, was greater than zero).
No other stopping rules were applied.
Statistical methods
All main analysis comparing groups for primary and secondary outcomes, and additional subgroup and adjusted analyses was conducted using Stata version 13 (StataCorp LP, College Station, TX, USA).
Baseline characteristics of participants were summarised in terms of the mean, standard deviation (SD), median, minimum, maximum and number of observations and categorical data in terms of frequency counts and percentages. No formal statistical tests were performed.
Comparison of proportions was carried out for binary outcomes between the intervention and the control groups (entry to smoking cessation service, point prevalent and prolonged abstinence, number completing the 6-week SSS course). Univariable logistic regression analysis was carried out to take into account clustering at the SSS level, and multivariable logistic regression was also carried out to take into account any imbalance in important baseline characteristics known to predict smoking cessation outcomes, nominated prior to examination of the trial data, between the groups. Both unadjusted and adjusted estimates are reported. The unadjusted analysis is considered to be the primary analysis. The size of the difference between treatments is expressed as an OR including 95% confidence interval (CI) from logistic regression, with appropriate allowance for clustering.
The therapists were SSS based rather than practice based, and we initially intended that the therapist effect be accounted for by allowing greater variance between SSSs in the intervention group than in the control group, so that the difference in variance would represent the therapist effect. In fact, we discovered that the estimated variance for the primary outcome was slightly lower in the intervention group, rendering it impossible to fit a model including a special clustering effect for participants only assigned to the intervention. Hence, we allowed only for variance between SSSs, assuming it to be the same in the two groups, and thus fitted a random intercepts model.
Self-reported changes in daily cigarette consumption (the difference between cigarette consumption at baseline and at the 6-month follow-up) is a continuous variable and was compared with the two-sample t-test and with multiple linear regression to account for important baseline characteristics. ORs for the difference in means is quoted together with the 95% CI.
Furthermore, we estimated the ICC for our primary outcome and for the validated 7-day abstinent outcome. The ICC for a binary outcome can be estimated as:
The term σu2 can be interpreted as the component of outcome variance because of differences between SSSs, the denominator as the total variance and ρ as the proportion of the total outcome variance that is due to between-cluster variation. 57
Loss to follow-up after randomisation is reported. Analysis is based on intention to treat; that is, we assume that all randomised participants received the treatment that they were randomised to, and all those lost to follow-up are assumed to be still smoking.
Levels of significance
During the course of running the trial but prior to locking the database, based on further expert statistical advice, we devised an analysis plan for interpreting significance levels for analysis on multiple outcomes of interest. Hence, the interpretation of the results of the trial for the primary outcome, (1) engagement with SSS, and the main secondary outcome, (2) 7-day point prevalent abstinence, was governed by an alpha spending plan that preserved the study-wise alpha for (1) and (2). We hypothesised that these outcomes fall naturally into a hierarchy with (1) as a step prior to (2). We employed a hierarchical monitoring plan in which alpha was spent first on (1) and the remaining alpha was available for (2). The simple formula below describes alpha allocation in the hierarchy:
where subscript 2 = two sided; s = study-level critical alpha (0.05); e = engagement with SSS; and c = 7-day point prevalent abstinence. Thus, if the p-value for attendance at SSS was 0.02, there remains a p-value of 0.031 to spend on the second outcome of 7-day point prevalent abstinence and a p-value for that outcome of < 0.031 would be considered significant. If the p-value for the primary outcome (difference in smoking cessation service attendance) was > 0.05, then the overall study would be considered neutral and any finding on the second outcome considered exploratory with a nominal p-value.
Likewise, if there was a significant decrease in attendance within the intervention arm over the control arm, the second outcome would be considered exploratory with a nominal p-value.
Subgroup analyses
In order to assess whether or not the intervention was any more effective for any particular subgroup of smokers, we explored interactions between intervention and deprivation (defined in fifths), intervention and gender, and intervention and age (defined by categories 16–39 years, 40–64 years and ≥ 65 years), for the primary outcome (attendance) and 7-day point prevalent abstinence at the 6-month follow-up. We had planned at an early stage to analyse the interaction with deprivation,58 and with gender and age when drawing up our analysis plan,59 prior to the end of data collection.
Subsidiary analyses
We also explored any delayed effect of sending repeat reminders to smokers on the uptake of service, and any differences in attendance due to seasonal variations.
Patient and public involvement
This trial was embedded in the NHS through the inclusion of a SSS manager as a coapplicant, who was involved in all stages of the research, from design and conduct to analysis. In addition, a past successful user of the Camden SSS was invited onto the Trial Management Group and has been involved in the study from the design stage onwards. The service user contributed to the design of both parts of the intervention and to the conduct of the trial and collection of data.
Another past user of the Camden SSS also contributed to the development of the intervention; thus, two service users were involved in the development of both parts of the intervention. They were consulted on the content of the brief personal letter at all stages of development, and were also consulted on the protocol for the taster sessions. Both service users also narrated their own experiences of quitting and these were used to create the video that formed a part of the taster session.
The Trial Management Group member was fully involved at all management meetings. Considerable effort was put into increasing response rate to the follow-up, and the service user was particularly helpful with suggestions of how to maximise this response, using her perception as a user to propose the use of text messaging and how the texts should be phrased. She also added greatly to the discussion of the results and of the practical implications of this method of recruitment to the SSSs.
Thus, the interests of all parties and the views of the public have been fully represented in the conduct of the study.
Chapter 3 Results
Recruitment and participant flow
Practice recruitment
Eighteen SSSs spread across England, located in both high and low areas of deprivation and representing both large and small organisations, agreed to participate in the trial (Figure 2). Ninety-nine practices within the participating SSS areas, identified and approached by PCRNs, agreed to participate. The number of practices per SSS ranged from 3 to 10, and the practice list size ranged from 2205 to 26,000 (mean, 9723; median, 9725). Cumulative list sizes, shown in Table 2, ranged from 17,617 in Medway to 106,424 in Durham and Darlington. Current cigarette smokers aged 16–99 years were identified from computer records in participating practices (n = 141,488; 14.7% of the total list size). The proportion of smokers identified in each practice ranged between 4.8% and 39.7%, and within each SSS from 9.7% to 20.81%.
FIGURE 2.
Map showing level of socioeconomic deprivation in England and SSSs participating in Start2quit.

| ID | SSS | Number of practices | Cumulative list size | Mean % smokers | Mean % smokers range between practices | Mean practice IMD score |
|---|---|---|---|---|---|---|
| 1 | Camden | 3 | 19,791 | 20.81 | 7.03–39.73 | 22.98 |
| 2 | Oxfordshire | 4 | 42,394 | 17.88 | 16.48–20.21 | 19.24 |
| 3 | Medway | 4 | 17,617 | 18.01 | 15.69–24.04 | 22.29 |
| 4 | Eastern and Coastal Kent | 4 | 43,814 | 14.43 | 13.31–15.23 | 16.14 |
| 5 | Lincolnshire | 6 | 47,000 | 12.40 | 6.83–21.91 | 12.30 |
| 6 | Essex | 3 | 46,916 | 12.11 | 6.65–19.65 | 24.27 |
| 7 | Cornwall | 7 | 65,528 | 17.42 | 13.27–22.20 | 29.06 |
| 8 | Derby | 4 | 47,307 | 14.47 | 8.17–18.62 | 49.67 |
| 9 | Brent | 4 | 21,905 | 11.74 | 8.38–14.78 | 41.86 |
| 10 | Plymouth | 6 | 55,344 | 19.11 | 10.79–24.72 | 32.41 |
| 11 | Swindon | 8 | 68,583 | 13.48 | 8.25–19.15 | 22.73 |
| 12 | Durham and Darlington | 10 | 106,424 | 16.11 | 7.27–25.11 | 28.63 |
| 13 | Hampshire | 9 | 99,829 | 11.15 | 5.98–16.53 | 20.68 |
| 14 | Portsmouth | 6 | 55,367 | 12.94 | 8.75–16.53 | 22.73 |
| 15 | Staffordshire | 7 | 65,239 | 19.29 | 16.27–23.87 | 21.74 |
| 16 | Barnsley | 4 | 46,488 | 19.96 | 15.22–30.54 | 29.75 |
| 17 | Buckinghamshire | 4 | 65,580 | 9.70 | 5.40–15.47 | 6.86 |
| 18 | Coventry | 6 | 47,422 | 13.34 | 4.79–19.80 | 24.98 |
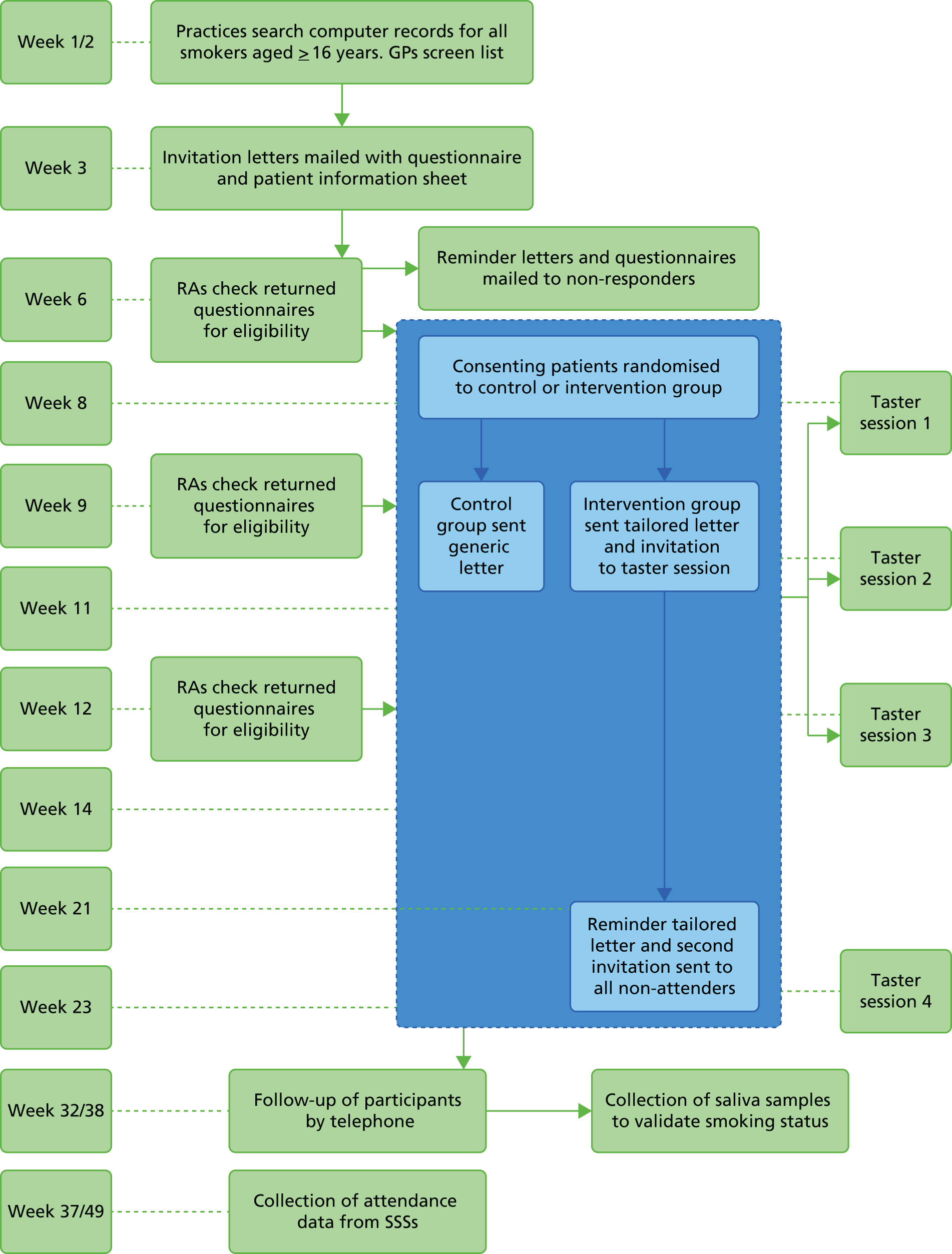
The study targeted higher-risk groups; therefore, practices in areas of high deprivation were preferentially selected as determined by the practice postcode converted to an IMD score (the government’s official measure of multiple deprivation at the small-area level). A majority (54.6%) of practices were located in areas of high deprivation (i.e. within the two highest quintiles of IMD scores, a score > 21.34) (Figure 3). This suggests that the catchment area for these practices were within areas of high deprivation or that a higher number of participants were living in highly deprived areas; however, the postcode of the practice does not always indicate the deprivation status of the catchment area. Nevertheless, a comparison of the mean IMD scores of all smokers identified for each practice with the IMD of the practice indicated a similar trend, and there was good correlation between smokers’ IMD scores and those of the practices (r = 0.80) (Figure 4).
FIGURE 3.
Percentage of practices (%) within each IMD quintile.
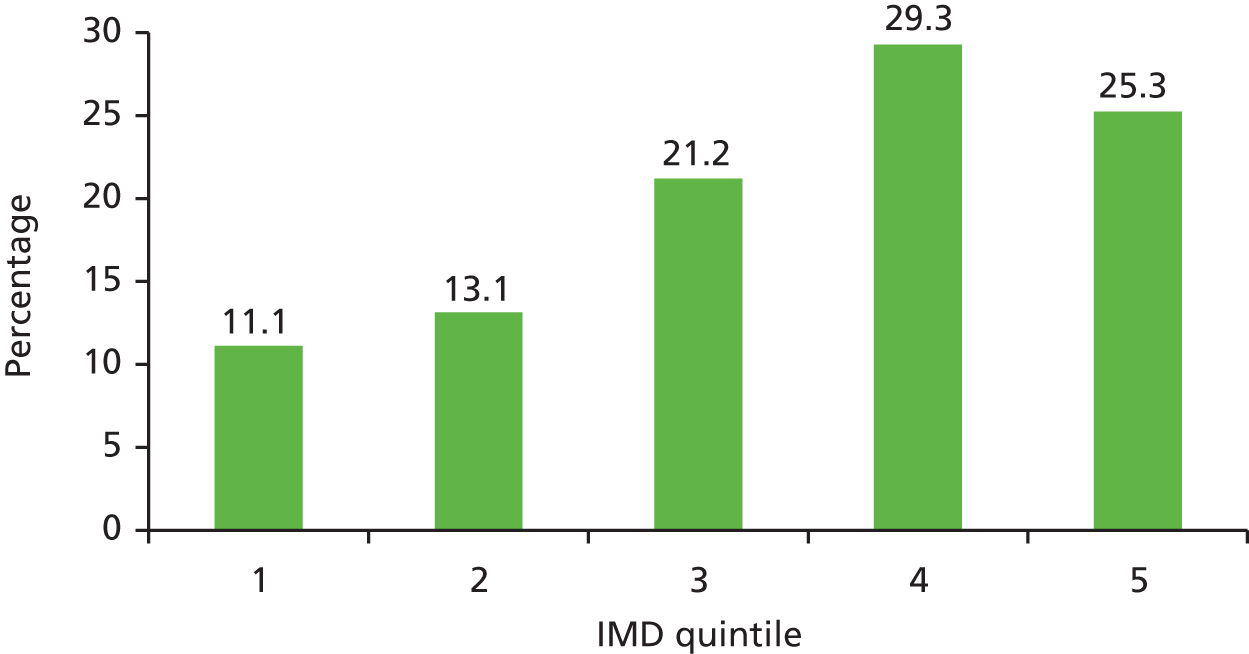
FIGURE 4.
The IMD scores of practices compared with the mean IMD scores of all smokers living in the practice catchment area.
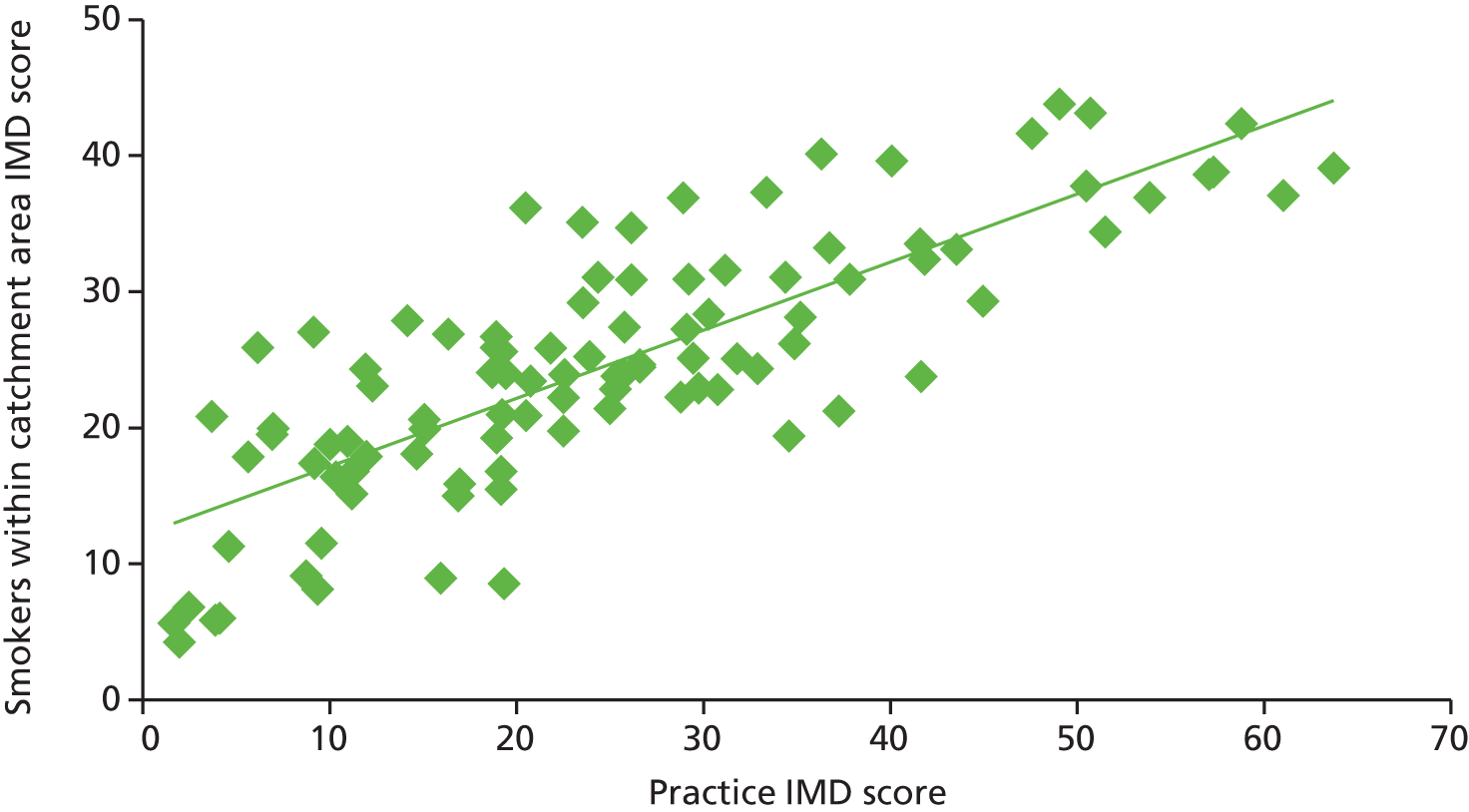
One would also expect to find a high proportion of smokers identified in areas of high deprivation, indicated by a high IMD score, and in many cases there appears to be a close match. However, in some areas the percentage was lower than would be expected, and this may be accounted for by a high ethnic population, in which smoking prevalence may be lower in women, for example in Derby and Brent (Figure 5).
FIGURE 5.
The IMD scores of practices and the proportion of smokers identified in the practice. Derby and Brent are highlighted in blue.
Participant recruitment
The recruitment of participants for the pilot phase of the trial was conducted between January and March 2011. Recruitment for the main phase began in January 2012 and was completed in October 2013.
General practitioners excluded 4186 patients considered to be unsuitable to take part in the study. Reasons for exclusion included patients unable to understand English sufficiently, serious pre-existing condition or terminal illness, and severe cognitive, mental or psychological impairment. However, most practices did not give the reasons for exclusion and we are unable to estimate numbers in each category. A further 25,086 patients were excluded because of duplicate addresses, ensuring that only one person from the same address was selected. All remaining persons on the list (n = 112,216) were invited to participate in the trial. Of these, 21,971 (19.6%) replied. However, 5333 replied to say they were non-smokers and 420 were returned to the practices unopened as a result of incorrect addresses or deceased. Records are not always accurate, and those returned from non-smokers are likely to represent only a portion of those recorded as smokers but are actually non-smokers. Thus, our best estimate of total potentially eligible is 106,463 (see Figure 6).
The total number of questionnaires returned from potentially eligible participants was 16,638 and, of these, 10,380 declined to take part in the study but returned the questionnaire with basic information only to update their smoking status in their practice records. A further 1874 were willing to take part in the study but did not fit the inclusion criteria, leaving 4384 participants enrolled in the trial, representing a response rate of 4.1%. Of these, 2636 were allocated to the intervention group and 1748 to the control group. One participant from the intervention group withdrew from the study before follow-up commenced and 4383 were analysed. See the Consolidated Standards of Reporting Trials (CONSORT) flow chart (Figure 6) for details.
FIGURE 6.
The CONSORT diagram of recruitment and flow of participants through the trial. a, Percentage of total list size; b, total invitations sent minus non-smokers and wrong address/deceased; c, of total potentially eligible. Adapted from Gilbert et al. 45 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license.

Follow-up
Follow-up data collection took place 6 months post randomisation and was conducted between August and November 2011 for the pilot phase and between August 2012 and July 2014 for the main study.
Complete validation data of attendance at the SSS were obtained for each participant from SSSs at the end of the quarter following the end of the 6-month follow-up period in each area. Additional data were obtained by telephone interview or postal questionnaire. In total, 2910 (66.4%) completed the full telephone interview, 302 (6.9%) completed a shorter paper version of the follow-up questionnaire returned by post and an additional 160 (3.7%) completed the four basic questions related to the primary outcome, giving a total response rate of 3372 (76.9%). There was no difference in follow-up response between the treatment groups: 76.7% and 77.3% in the intervention and control groups, respectively. The reasons for loss to follow-up were declined to complete the interview (n = 150), not able to be contacted (n = 857), and died within the 6-month follow-up period (n = 4) (see Figure 6). The timing of completing the follow-up ranged from 20 days prior to the due date (180 days after randomisation) to 194 days after the due date (mean, 27.53 days; median, 22 days). This was not statistically different between the intervention and the control groups.
There were large differences in recruitment between SSSs, ranging from 2.3% in Brent to 6.7% in Oxfordshire, and also in follow-up response. There were also large variations between practices within SSSs. We deliberately included some practices in areas with high ethnic minority populations, for example in Derby and Brent, but in these practices recruitment was especially low (Table 3).
| ID | SSS | Recruitment rate (%) | Recruitment rate range between practices (%) | 6-month follow-up response (%) | 6-month follow-up response range between practices (%) |
|---|---|---|---|---|---|
| 1 | Camden | 3.2 | 2.7–5.8 | 65.6 | 61.0–78.6 |
| 2 | Oxfordshire | 6.7 | 5.5–9.2 | 72.1 | 69.7–74.1 |
| 3 | Medway | 5.1 | 1.8–5.9 | 72.2 | 68.2–80.0 |
| 4 | Eastern and Coastal Kent | 5.5 | 3.5–8.2 | 80.4 | 72.3–85.7 |
| 5 | Lincolnshire | 3.1 | 2.0–3.6 | 82.8 | 82.6–83.3 |
| 6 | Essex | 5.0 | 3.0–6.1 | 75.3 | 57.1–87.0 |
| 7 | Cornwall | 5.3 | 4.3–6.5 | 76.9 | 74.6–80.0 |
| 8 | Derby | 3.4 | 1.7–4.1 | 67.0 | 60.0–72.5 |
| 9 | Brent | 2.3 | 1.4–3.9 | 79.2 | 68.4–100 |
| 10 | Plymouth | 3.3 | 1.6–5.1 | 70.2 | 64.3–75.5 |
| 11 | Swindon | 4.7 | 3.8–6.1 | 74.9 | 68.3–79.3 |
| 12 | Durham and Darlington | 4.0 | 2.3–5.7 | 76.5 | 61.5–82.6 |
| 13 | Hampshire | 4.6 | 2.7–5.6 | 79.6 | 72.3–86.7 |
| 14 | Portsmouth | 3.0 | 1.3–5.1 | 82.9 | 74.3–96.8 |
| 15 | Staffordshire | 3.8 | 2.0–6.1 | 81.1 | 75.0–84.3 |
| 16 | Barnsley | 3.2 | 2.7–4.4 | 76.8 | 72.3–79.7 |
| 17 | Buckinghamshire | 4.5 | 3.7–5.4 | 85.3 | 82.0–93.3 |
| 18 | Coventry | 2.7 | 1.9–4.9 | 87.0 | 77.8–100 |
Biochemical validation of 7-day abstinence
Of the 630 participants who answered ‘not at all’ in response to the question ‘How often do you currently smoke cigarettes or rollups?’ at the follow-up, and who were asked to provide a salivary cotinine sample to validate abstinence, 595 (94.4%) agreed to send a saliva sample for analysis and 443 (70.3%) returned a sample; 399 (63.3%) samples were sent for analysis. Samples from 44 participants who reported that they had resumed smoking between follow-up and returning the sample were not analysed.
Of the samples analysed, 345 (54.8%) were validated (249 had a cotinine content of < 12 ng/ml, 36 were from participants using NRT and 60 from participants were using e-cigarettes). Of the 54 samples not validated, 30 had a cotinine content of > 12 ng/ml and were from participants not using NRT or e-cigarettes, five samples came from participants who had smoked in the previous 6 days and 19 samples were of insufficient volume to be analysed. There was no difference between the intervention and control groups.
Characteristics of participants
Baseline
Table 4 shows the demographic and smoking characteristics by intervention and control group.
| Characteristic | Group | Total (N = 4383) | |
|---|---|---|---|
| Intervention (N = 2635; 60.1%) | Control (N = 1748; 39.9%) | ||
| Demographics | |||
| Gender, n (%) | |||
| Male | 1345 (51.0) | 886 (50.7) | 2231 (50.9) |
| Female | 1290 (49.0) | 862 (49.3) | 2152 (49.1) |
| Age (years) | |||
| Mean (SD) | 49.2 (14.3) | 49.5 (14.3) | 49.3 (14.3) |
| Range | 16–88 | 16–89 | 16–89 |
| Marital status, n (%) | |||
| Single | 664 (25.2) | 444 (25.4) | 1108 (25.3) |
| Living with a spouse | 1429 (54.2) | 961 (55.0) | 2390 (54.5) |
| Separated/divorced | 392 (14.9) | 252 (14.4) | 644 (14.7) |
| Widowed | 134 (5.1) | 83 (4.8) | 217 (5.0) |
| Missing | 16 (0.6) | 8 (0.5) | 24 (0.6) |
| Employment status, n (%) | |||
| Unemployed | 287 (10.9) | 190 (10.9) | 477 (10.9) |
| Paid employment | 1422 (53.9) | 903 (51.7) | 2325 (53.1) |
| Full-time student | 44 (1.7) | 32 (1.8) | 76 (1.7) |
| Home maker | 104 (4.0) | 89 (5.1) | 193 (4.4) |
| Retired | 495 (18.8) | 344 (19.7) | 839 (19.1) |
| Disabled/too ill to work | 254 (9.6) | 171 (9.8) | 425 (9.7) |
| Missing | 29 (1.1) | 19 (1.1) | 48 (1.1) |
| Highest qualification, n (%) | |||
| None | 672 (25.5) | 460 (26.3) | 1132 (25.8) |
| GCSE/CSE/O Level | 1042 (39.5) | 655 (37.5) | 1697 (38.7) |
| A Level | 306 (11.6) | 232 (13.3) | 538 (12.3) |
| Degree/equivalent | 454 (17.2) | 301 (17.2) | 755 (17.2) |
| Postgraduate | 82 (3.1) | 34 (2.0) | 116 (2.7) |
| Missing | 79 (3.0) | 66 (3.8) | 145 (3.3) |
| Ethnic background, n (%) | |||
| White | 2522 (95.7) | 1669 (95.5) | 4191 (95.6) |
| Black | 29 (1.1) | 22 (1.3) | 51 (1.2) |
| Asian | 36 (1.4) | 29 (1.7) | 65 (1.5) |
| Other | 27 (1.0) | 23 (1.3) | 50 (1.1) |
| Missing | 21 (0.8) | 5 (0.3) | 26 (0.6) |
| Deprivation (IMD score), n (%) | |||
| Quintile 1 | 334 (12.7) | 215 (12.3) | 549 (12.5) |
| Quintile 2 | 378 (14.4) | 244 (14.0) | 622 (14.2) |
| Quintile 3 | 574 (21.8) | 392 (22.4) | 966 (22.0) |
| Quintile 4 | 677 (25.7) | 453 (25.9) | 1130 (25.8) |
| Quintile 5 | 653 (24.8) | 436 (24.9) | 1089 (24.9) |
| Missing | 19 (0.7) | 8 (0.5) | 27 (0.6) |
| Live with smokers, n (%) | |||
| No | 1791 (68.0) | 1177 (67.3) | 2968 (67.7) |
| Yes | 835 (31.7) | 567 (32.4) | 1402 (32.0) |
| Missing | 9 (0.3) | 4 (0.2) | 13 (0.3) |
| Smoking characteristics | |||
| Daily smokers, n (%) | 2401 (91.1) | 1616 (92.5) | 4017 (91.6) |
| Non-daily smokers, n (%) | 214 (8.1) | 126 (7.2) | 340 (7.8) |
| Missing, n (%) | 20 (0.8) | 6 (0.3) | 26 (0.6) |
| Cigarettes per day | |||
| Mean (SD) | 16.1 (8.6) | 16.8 (9.9) | 16.4 (9.2) |
| Range | 0.1–80 | 0.3–99 | 0.1–99 |
| Missing, n (%) | 10 (0.4) | 9 (0.5) | 19 (0.4) |
| Time from waking to first cigarette, n (%) | |||
| < 5 minutes | 568 (21.6) | 414 (23.7) | 982 (22.4) |
| 6–30 minutes | 1186 (45.0) | 802 (45.9) | 1988 (45.4) |
| 31–60 minutes | 436 (16.6) | 246 (14.1) | 682 (15.6) |
| 1–2 hours | 222 (8.4) | 152 (8.7) | 374 (8.5) |
| > 2 hours | 215 (8.2) | 132 (7.6) | 347 (7.9) |
| Missing | 8 (0.3) | 2 (0.1) | 10 (0.2) |
| Nicotine dependence score (0–6)a | |||
| Mean (SD) | 2.57 (1.49) | 2.67 (1.52) | 2.61 (1.51) |
| Low (score 0–2), n (%) | 1094 (41.5) | 669 (38.3) | 1763 (40.2) |
| Medium (score 3), n (%) | 850 (32.3) | 581 (33.2) | 1431 (32.7) |
| High (score 4–6), n (%) | 673 (25.5) | 487 (27.9) | 1160 (26.5) |
| Missing, n (%) | 18 (0.7) | 11 (0.6) | 29 (0.7) |
| Age started smoking (years) | |||
| Mean (SD) | 16.5 (4.5) | 16.5 (4.6) | 16.5 (4.5) |
| Range | 6–55 | 1–51 | 1–55 |
| Missing (%) | 12 | 7 | 19 |
| Intention and motivation to quit | |||
| When planning to quit, n (%) | |||
| In next 2 weeks | 481 (18.3) | 315 (18.0) | 796 (18.2) |
| Next 30 days | 606 (23.0) | 380 (21.7) | 986 (22.5) |
| Next 6 months | 1103 (41.9) | 759 (43.4) | 1862 (42.5) |
| Not in the next 6 months | 333 (12.6) | 218 (12.5) | 551 (12.6) |
| Missing | 112 (4.3) | 76. (4.4) | 188 (4.3) |
| Longest previous quit attempt, n (%) | |||
| < 24 hours | 243 (9.2) | 172 (9.8) | 415 (9.5) |
| 1–6 days | 474 (17.9) | 286 (16.4) | 760 (17.3) |
| 1–4 weeks | 436 (16.6) | 282 (16.2) | 718 (16.4) |
| > 1 month | 1454 (55.2) | 986 (56.4) | 2440 (55.7) |
| Missing | 28 (1.1) | 22 (1.3) | 50 (1.1) |
| Previously attended SSS, n (%) | |||
| No | 1763 (66.9) | 1135 (64.9) | 2898 (66.1) |
| Yes | 872 (33.1) | 613 (35.1) | 1485 (33.9) |
| ‘How much do you want to quit?’ (1 = not at all, 5 = extremely) | |||
| Mean score (SD) | 3.74 (0.91) | 3.79 (0.90) | 3.76 (0.91) |
| Not at all, n (%) | 14 (0.5) | 7 (0.4) | 21 (0.5) |
| A little, n (%) | 235 (8.9) | 152 (8.7) | 387 (8.8) |
| Moderately, n (%) | 714 (27.1) | 429 (24.5) | 1143 (26.1) |
| Very much, n (%) | 1117 (42.4) | 759 (43.4) | 1876 (42.8) |
| Extremely, n (%) | 549 (20.8) | 393 (22.5) | 942 (21.5) |
| Missing, n (%) | 6 (0.2) | 8 (0.5) | 14 (0.3) |
| ‘How determined are you to quit?’ (1 = not at all, 5 = extremely) | |||
| Mean score (SD) | 3.74 (0.93) | 3.75 (0.93) | 3.74 (0.93) |
| Not at all, n (%) | 26 (1.0) | 19 (1.1) | 45 (1.0) |
| A little, n (%) | 228 (8.7) | 160 (9.2) | 388 (8.9) |
| Moderately, n (%) | 739 (28.1) | 435 (24.9) | 1174 (26.8) |
| Very much, n (%) | 1047 (39.7) | 736 (42.1) | 1783 (40.7) |
| Extremely, n (%) | 588 (22.3) | 383 (21.9) | 971 (22.2) |
| Missing, n (%) | 7 (0.3) | 15 (0.9) | 22 (0.5) |
| ‘How confident are you that you can quit?’ (1 = not at all, 5 = extremely) | |||
| Mean score (SD) | 2.73 (1.07) | 2.69 (1.06) | 2.71 (1.07) |
| Not at all, n (%) | 362 (13.7) | 248 (14.2) | 610 (13.9) |
| A little, n (%) | 704 (26.7) | 480 (27.5) | 1184 (27.0) |
| Moderately, n (%) | 1024 (38.9) | 680 (38.9) | 1704 (38.9) |
| Very much, n (%) | 372 (14.1) | 229 (13.1) | 601 (13.7) |
| Extremely, n (%) | 169 (6.4) | 105 (6.0) | 274 (6.3) |
| Missing, n (%) | 4 (0.2) | 6 (0.3) | 10 (0.2) |
| Health | |||
| Health problems (self-reported), n (%) | |||
| No | 1969 (74.7) | 1280 (73.2) | 3249 (74.1) |
| Yes | 592 (22.5) | 424 (24.3) | 1016 (23.2) |
| Missing | 74 (2.8) | 44 (2.5) | 118 (2.7) |
| Health problems (number of QOF diseases recorded), n (%) | |||
| 0 | 1422 (54.0) | 957 (54.8) | 2379 (54.3) |
| 1 | 758 (28.8) | 459 (26.3) | 1217 (27.8) |
| 2 | 313 (11.9) | 222 (12.7) | 535 (12.2) |
| 3 | 106 (4.0) | 75 (4.3) | 181 (4.1) |
| 4 | 29 (1.1) | 25 (1.4) | 54 (1.2) |
| 5 | 5 (0.2) | 7 (0.4) | 12 (0.3) |
| 6 | 1 (0.04) | 3 (0.2) | 4 (0.1) |
| 7 | 1 (0.04) | 0 (0.0) | 1 (0.02) |
| Pregnant | 2 (0.1) | 9 (0.5) | 11 (0.3) |
| HRT | 35 (1.3) | 19 (1.1) | 54 (1.2) |
| Contraceptive pill | 124 (4.7) | 76 (4.4) | 200 (4.6) |
The sample was 50.9% male and had a mean age of 49.3 years; 54.5% lived with a spouse or partner and 95.6% were of a white ethnic background. Half of the sample (50.7%) were living in areas of high deprivation, defined as IMD quintiles 4 and 5, and 32% of the sample were living in a household with another smoker. In terms of nicotine dependence, approximately one-quarter were highly dependent; however, a high proportion fell into the low-dependence category, probably accounted for by the non-daily smokers (7.8%), who were not excluded from this study. The mean age at which participants started to smoke was 16.5 years. Regarding intention to quit and motivation, 42.5% were planning to quit some time in the next 6 months, 55.7% had previously quit for > 1 month and 33.9% had previously attended the SSS (but not in the previous 12 months). Motivation and determination to quit were relatively high (mean 3.76 and 3.74, respectively, scored on a 1–5 scale), but confidence in this ability to quit was lower (mean 2.71). Although 74.1% reported having no health problems linked to smoking, 45.7% were recorded as having at least one QOF disease.
Characteristics associated with study attrition
There were differences between those who completed the follow-up interview, either by telephone or by post, and those who did not complete. Participants who did not complete were younger (mean age 45.2 years vs. 50.5 years; p < 0.001), more likely to be living in a highly deprived area [IMD quintile 5 (27.7%) vs. IMD quintile 1 (16.4%); p < 0.001] and were more likely to be single (30.1% vs. 20.6%; p < 0.001). Non-completers had a higher dependence score (mean score 2.73 vs. 2.57; p = 0.004), were more likely to have not previously quit for > 24 hours [longest quit attempt < 24 hours (29.6%) vs. > 1 month (21.7%); p = 0.003] and were more likely to intend to quit in the next 2 weeks than to intend to quit in the next 6 months (28% vs. 19.2%, respectively; p = 0.001). Non-completers also scored higher on wanting and determination to quit (3.86 vs. 3.73 and 3.85 vs. 3.71, respectively; both p < 0.001) and were more confident in their ability to quit (2.79 vs. 2.69; p = 0.005). Participants who completed the follow-up were more likely to have previously attended the SSS (20% vs. 24.6%; p = 0.001). Completion was not related to self-reported or recorded health status (Table 5).
| Characteristic | Follow-up | p-value | |
|---|---|---|---|
| Completed (N = 3354; 76.9%) | Not completed (N = 1005; 23.1%) | ||
| Age (years) | |||
| Mean (SD) | 50.51 (13.75) | 45.28 (15.29) | < 0.001 |
| Deprivation (IMD score), n (%) | |||
| Quintile 1 | 459 (83.6) | 90 (16.4) | < 0.001 |
| Quintile 2 | 493 (79.3) | 129 (20.7) | |
| Quintile 3 | 742 (76.8) | 224 (23.2) | |
| Quintile 4 | 872 (77.2) | 258 (22.8) | |
| Quintile 5 | 787 (72.3) | 302 (27.7) | |
| Marital status, n (%) | |||
| Single | 774 (69.9) | 334 (30.1) | < 0.001 |
| Not single | 2580 (79.4) | 671 (20.6) | |
| Mean nicotine dependence score (0–6) | |||
| Mean (SD) | 2.57(1.49) | 2.73 (1.54) | 0.004 |
| Longest previous quit attempt, n (%) | |||
| < 24 hours | 292 (70.4) | 123 (29.6) | 0.003 |
| 1–6 days | 572 (75.3) | 188 (24.7) | |
| 1–4 weeks | 557 (77.6) | 161 (22.4) | |
| > 1 month | 1911 (78.3) | 529 (21.7) | |
| When planning to quit, n (%) | |||
| In next 2 weeks | 573 (72) | 223 (28) | 0.001 |
| Next 30 days | 755 (76.6) | 231 (23.4) | |
| Next 6 months | 414 (77.8) | 1448 (22.2) | |
| Not in next 6 months | 445 (80.8) | 106 (19.2) | |
| Previously attended SSS, n (%) | |||
| No | 2184 (75.4) | 714 (24.6) | 0.001 |
| Yes | 1188 (80.0) | 297 (20.0) | |
| ‘How much do you want to quit?’ (1–5) | |||
| Mean (SD) | 3.73 (0.9) | 3.86 (0.91) | < 0.001 |
| ‘How determined are you to quit?’ (1–5) | |||
| Mean (SD) | 3.71 (0.94) | 3.85 (0.93) | < 0.001 |
| ‘How confident are you that you can quit?’ (1–5) | |||
| Mean (SD) | 2.69 (1.06) | 2.79 (1.09) | 0.005 |
External validity
Anonymised data of patients who were invited to participate in the study but did not accept were compared with the data of the participants to explore whether or not the sample was representative of the whole population of smokers. Numbers in the anonymised data set are higher than those stated in Figure 6 because of errors in the initial search identifying smokers, leading to a difference in the electronic anonymised data retrieved from practices.
Overall, differences were found between participants and non-participants in gender, age and deprivation (Table 6). Males were under represented in the sample (50.9% vs. 54.3%); however, the difference was small, and may only be significant as a result of the large sample size. The difference was more pronounced in some SSSs than others (Figure 7). Participants were significantly older than non-participants (mean age 49.3 years vs. 43.3 years) and this was reproduced consistently in each SSS. The IMD score was significantly different between participants and non-participants, but the difference was small (1.18); however, there was large variation in IMD scores between individual SSSs. In areas where the population had higher IMD scores, for example Derby SSS and Durham and Darlington SSS, the mean IMD score of participants was lower than that of non-participants, whereas in areas of lower deprivation (Buckinghamshire and Swindon SSSs) the sample recruited tended to be more deprived than the average (Figure 8).
| Characteristic | Participants (n = 4383) | Non-participants (n = 106,451) | Absolute difference (95% CI) | p-value |
|---|---|---|---|---|
| Male, n (%) | 2231 (50.9) | 56,046 (52.65) | 1.7 (–3.3 to –0.2) | < 0.001 |
| Missing | 0 (0) | 3212 (3.0) | ||
| Mean age (years), mean (SD) | 49.31 (14.29) | 43.29 (15.92) | 6.01 (5.53 to 6.49) | < 0.001 |
| Missing | 0 | 983 (0.9) | ||
| IMD score, mean (SD) | 24.28 (14.21) | 25.46 (14.63) | –1.18 (–1.63 to –0.74) | < 0.001 |
| Missing | 27 (0.03) | 3561 (3.4) |
FIGURE 7.
Percentage male participants and non-participants by SSS area.
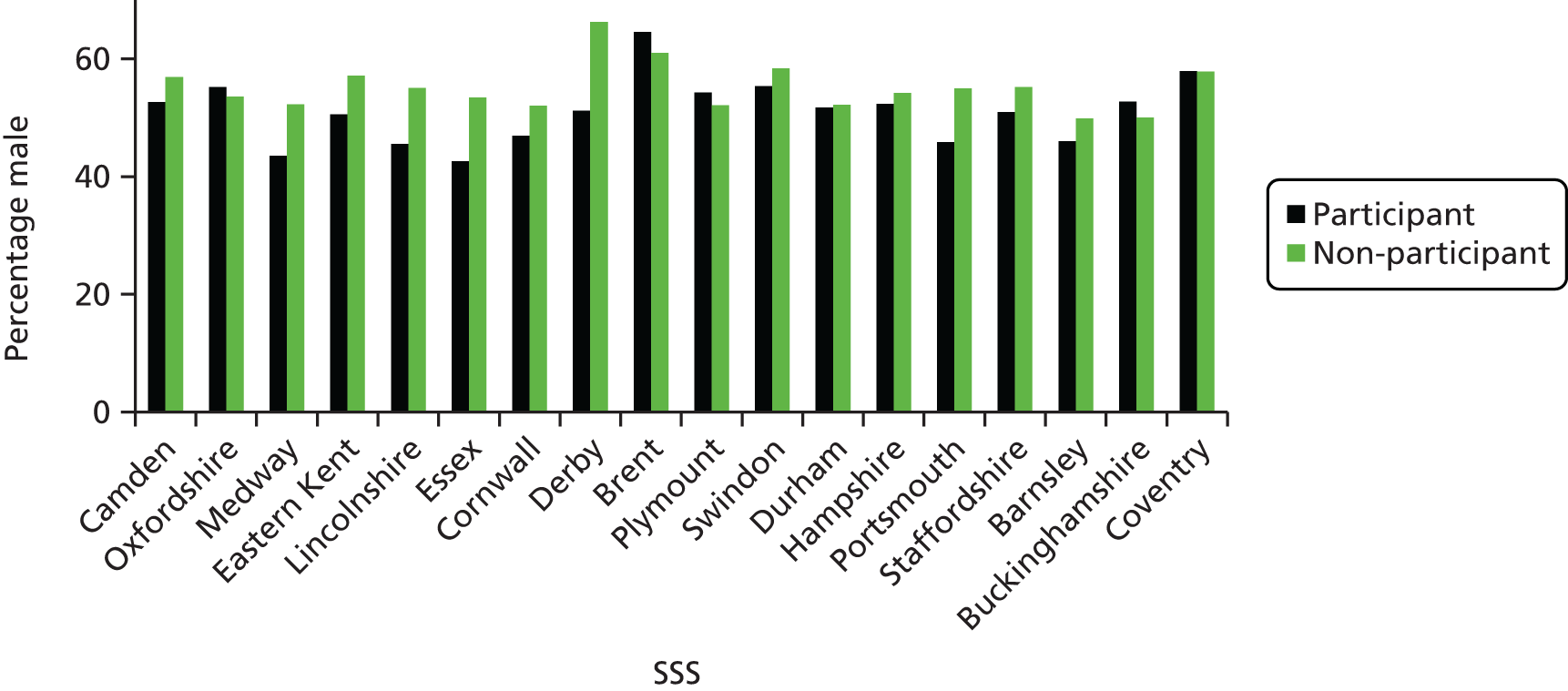
FIGURE 8.
The IMD scores of participants and non-participants by SSS area.
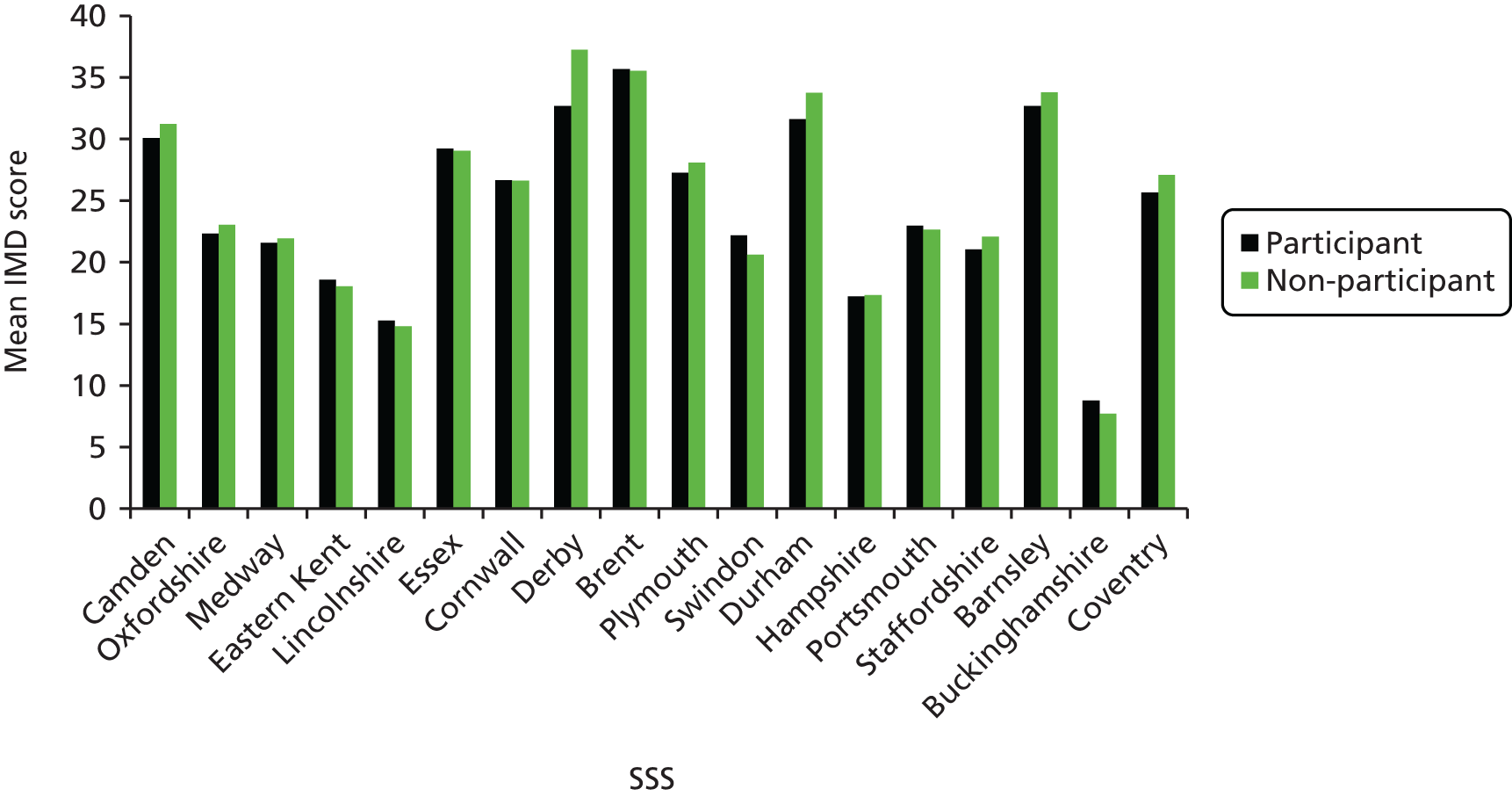
Overall, although the sample is consistently older than the average smoker in all areas, in terms of gender and IMD score the differences are small; therefore, we can probably consider the sample to be representative and have reasonable external validity.
Outcomes
Primary outcome
The proportion of people entering the smoking cessation service (i.e. attending the first session of a 6-week course) over a period of 6 months from the receipt of the invitation letter, as measured by records of attendance at the SSSs, was significantly higher in the intervention group than in the control group (17.4% vs. 9.0%; unadjusted OR 2.12, 95% CI 1.75 to 2.57; p < 0.001) (Table 7).
| Outcome | Group, n (%) | Estimates | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||||
| Intervention (N = 2635) | Control (N = 1748) | OR (95% CI) | p-value | n | OR (95% CI) | p-value | n | |
| Attendance at SSS | 458 (17.4) | 158 (9.0) | 2.12 (1.75 to 2.57) | < 0.001 | 4383 | 2.20 (1.80 to 2.70) | < 0.001 | 4081 |
| 7-day point prevalent abstinence (validated) | 236 (9.0) | 97 (5.6) | 1.68 (1.32 to 2.15) | < 0.001 | 4383 | 1.67 (1.29 to 2.14) | < 0.001 | 4081 |
| 7-day point prevalent abstinence (self-reported) | 424 (16.1) | 187 (10.7) | 1.61 (1.34 to 1.94) | < 0.001 | 4383 | 1.62 (1.34 to 1.97) | < 0.001 | 4081 |
| 24-hour point prevalent abstinence (self-reported) | 445 (16.9) | 201 (11.5) | 1.57 (1.31 to 1.88) | < 0.001 | 4383 | 1.57 (1.31 to 1.89) | < 0.001 | 4081 |
| 1-month prolonged abstinence (self-reported) | 357 (13.6) | 151 (8.6) | 1.67 (1.36 to 2.04) | < 0.001 | 4383 | 1.70 (1.38 to 2.10) | < 0.001 | 4081 |
| 3-month prolonged abstinence (self-reported) | 240 (9.1) | 103 (5.9) | 1.61 (1.26 to 2.04) | < 0.001 | 4383 | 1.64 (1.28 to 2.11) | < 0.001 | 4081 |
| 3-month prolonged abstinence (validated) | 150 (5.7) | 60 (3.4) | 1.70 (1.25 to 2.31) | 0.001 | 4383 | 1.68 (1.23 to 2.30) | 0.001 | 4081 |
| Number completing 6-week NHS course | 382 (14.5) | 123 (7.0) | 2.24 (1.81 to 2.78) | < 0.001 | 4383 | 2.30 (1.84 to 2.87) | < 0.001 | 4081 |
Secondary outcomes
The main secondary outcome of 7-day point prevalent abstinence at the 6-month follow-up, validated by salivary cotinine, was significantly higher in the intervention than in the control group (9.0% vs. 5.6%; unadjusted OR 1.68, 95% CI 1.32 to 2.15; p < 0.001). All other periods of abstinence measured by self-report (24-hour and 7-day point prevalent abstinence, 1- and 3-month prolonged abstinence), 3-month-validated prolonged abstinence and the number completing the 6-week SSS course were significantly higher in the intervention group than in the control group (see Table 7).
There was a slight reduction in daily cigarette consumption in those continuing to smoke in both the intervention and the control groups, and the proportion of these who had made a quit attempt (approximately one-quarter) was similar in both groups. Intention and motivation to quit changed little in these continuing smokers, and there was no difference between the groups (Table 8).
| Self-reported change | Group | Total (N = 3737) | |
|---|---|---|---|
| Intervention (N = 2190) | Control (N = 1547) | ||
| Mean change in daily cigarette consumption (SD) | –2.6 (6.4) | –2.7 (6.7) | –2.6 (6.5) |
| Made a quit attempt, n (%) | 528 (24.1) | 359 (23.2) | 887 (23.7) |
| Changes in motivation and intention, mean (SD) | |||
| Mean change in intention to quit (3 to –3) | –0.28 (1.11) | –0.28 (1.07) | –0.28 (1.1) |
| Mean change in ‘want to quit’ (4 to –4) | 0.05 (1.11) | 0.04 (1.08) | 0.05 (1.08) |
| Mean change in ‘determined to quit’ (4 to –4) | –0.01 (1.24) | 0.04 (1.25) | 0.01 (1.24) |
| Mean change in ‘confident can quit’ (4 to –4) | 0.10 (1.31) | –0.02 (1.22) | 0.05 (1.27) |
Subgroup analyses
Subgroups
Table 9 shows subgroup interactions in outcomes.
| Outcome | Group, n (%) | OR (95% CI) | p-for-interaction | Number of participants in stratum | |
|---|---|---|---|---|---|
| Intervention (N = 2635) | Control (N = 1748) | ||||
| Attendance at SSS | |||||
| Interaction with gender (stratified analysis) | 0.01 | 4383 | |||
| Male | 255 (19.0) | 71 (8.0) | 2.70 (2.04 to 3.57) | 2231 | |
| Female | 203 (15.7) | 87 (10.1) | 1.67 (1.28 to 2.19) | 2152 | |
| Interaction with age (years) (stratified analysis) | 0.65 | 4383 | |||
| 16–40 | 77 (11.6) | 31 (7.2) | 1.69 (1.09 to 2.63) | 1095 | |
| 40–64 | 283 (18.0) | 92 (8.9) | 2.28 (1.78 to 2.94) | 2616 | |
| ≥ 65 | 98 (24.8) | 35 (12.6) | 2.33 (1.52 to 3.57) | 672 | |
| Interaction with deprivation (stratified analysis) | 0.001 | 4383 | |||
| IMD quintile 1 | 58 (17.4) | 22 (10.2) | 1.85 (1.09 to 3.14) | 549 | |
| IMD quintile 2 | 70 (18.5) | 19 (7.8) | 2.65 (1.55 to 4.54) | 622 | |
| IMD quintile 3 | 106 (18.5) | 17 (4.3) | 5.00 (2.94 to 8.49) | 966 | |
| IMD quintile 4 | 113 (16.7) | 47 (10.4) | 1.76 (1.22 to 2.54) | 1130 | |
| IMD quintile 5 | 109 (16.7) | 53 (12.2) | 1.46 (1.02 to 2.07) | 1089 | |
| 7-day point prevalent abstinence (validated) | |||||
| Interaction with gender (stratified analysis) | 0.01 | 4383 | |||
| Male | 131(9.7) | 39 (4.4) | 2.37 (1.63 to 3.42) | 2231 | |
| Female | 105 (8.1) | 58 (6.7) | 1.23 (0.88 to 1.72) | 2152 | |
| Interaction with age (years) (stratified analysis) | 0.72 | 4383 | |||
| 16–40 years | 46 (6.9) | 21 (4.9) | 1.47 (0.86 to 2.50) | 1095 | |
| 40–64 years | 150 (9.5) | 57 (5.5) | 1.83 (1.33 to 2.51) | 2616 | |
| ≥ 65 years | 40 (10.1) | 19 (6.9) | 1.53 (0.87 to 2.70) | 672 | |
| Interaction with deprivation (stratified analysis) | 0.68 | 4383 | |||
| IMD quintile 1 | 34 (10.2) | 15 (7.0) | 1.51 (0.80 to 2.85) | 549 | |
| IMD quintile 2 | 44 (11.6) | 18 (7.4) | 1.63 (0.91 to 2.90) | 622 | |
| IMD quintile 3 | 60 (10.5) | 23 (5.9) | 1.92 (1.16 to 3.18) | 966 | |
| IMD quintile 4 | 58 (8.6) | 19 (4.2) | 2.14 (1.26 to 3.64) | 1130 | |
| IMD quintile 5 | 39 (6.0) | 22 (5.0) | 1.18 (0.69 to 2.03) | 1089 | |
The effect of the intervention on attendance at the SSS was greater for males (19% vs. 8%; OR 2.70, 95% CI 2.04 to 3.57) than for females (15.7 vs. 10.1%; OR 1.67, 95% CI 1.28 to 2.19), and the interaction term was significant (p-for-interaction = 0.01). There was also a differential effect for males and females for validated 7-day point prevalent abstinence (p-for-interaction = 0.01). The intervention was more effective for males (OR 2.37, 95% CI 1.63 to 3.42) than for females (OR 1.23, 95% CI 0.88 to 1.72). Although attendance at the SSS in the intervention group was similar in all IMD quintiles, it was lower in the control group for participants in quintiles 2–4 (medium deprivation) than for those in quintiles 1 or 5, thus the differential was significantly greater (p-for-interaction = 0.001). Abstinence was lower overall in the higher-deprivation groups, but with no significant interaction. There were no significant interactions by age category. Both attendance and abstinence increased with age in both groups.
Effect of repeat reminders
We also explored any effect on the uptake of service of sending repeat reminders to smokers approximately 9–15 weeks (63–105 days) after randomisation. Mean time in days from randomisation to attendance in valid cases (i.e. dates reported by SSSs falling within the 6-month time frame) was 47.35 days (SD 37.91 days) in the intervention group and 55.99 days (SD 49.01 days) in the control group. The most frequent time for attendance in the intervention group was between 19 and 29 days (mode 21 days), with no noticeable increase between 63 and 105 days. In the control group, multiple modes were 15, 40 and 62 days, but there was no clear pattern in the peaks (Figure 9).
FIGURE 9.
Time from randomisation to attendance at the SSS in the (a) intervention group (n = 445) and (b) the control group n = 147. Reproduced from Gilbert et al. 45 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Seasonal variation
To assess any seasonal variation in attendance and any differences between the groups, we examined attendance by quarter and by group. Attendance was significantly higher overall in earlier months (17.4% in January to March vs. 11.4% in October to December; p < 0.001), but the relative effect between groups was not significantly different (Table 10).
| Season of recruitment | Group, n (%) | Total, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| January–March | 159 (21) | 61 (12.1) | 220 (17.4) |
| April–June | 133 (16.9) | 41 (7.7) | 174 (13.2) |
| July–September | 79 (17.9) | 21 (7.3) | 100 (13.7) |
| October–December | 87 (13.4) | 35 (8.3) | 122 (11.4) |
| Total | 458 (17.4) | 158 (9) | 616 (14.1) |
Variation in outcome by Stop Smoking Service
To assess any variation in the outcome by SSS we examined attendance and 7-day-validated abstinence in each SSS area by treatment group. Overall attendance at the SSSs varied from 2.1% to 23.1%, and attendance varied from 3.3% to 28.3% in the intervention group and from 0% to 16.7% in the control group. In all SSSs, the number attending was higher in the intervention group than in the control group (Figure 10). The ICC for the overall attendance was 0.031 (95% CI 0.01 to 0.09), suggesting that around 3% of participants’ tendency to attend the SSS was explained by the SSS in which they were located.
FIGURE 10.
Percentage of participants attending SSSs by treatment group and by SSS.
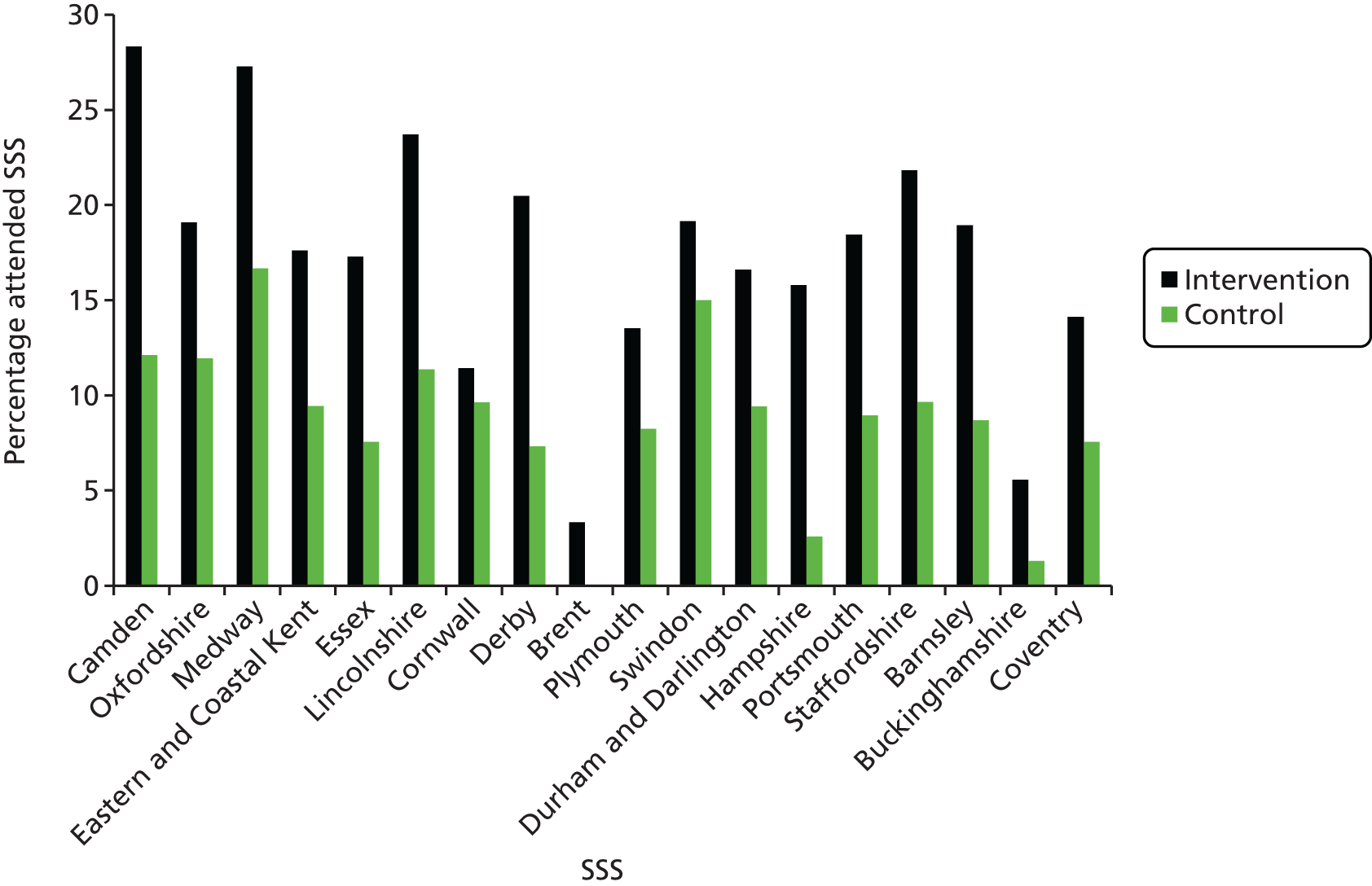
The proportion of participants achieving validated 7-day abstinence varied from 2.1% to 13.4% overall, and from 3.3% to 16% and from 0% to 10.5% in the intervention and control groups, respectively (Figure 11). The ICC for 7-day abstinence was 0.034 (95% CI 0.011 to 0.096), again suggesting that around 3% of participants’ tendency to quit smoking was explained by the SSS in which they were located.
FIGURE 11.
Percentage of all participants with validated 7-day abstinence by treatment group and by SSS.
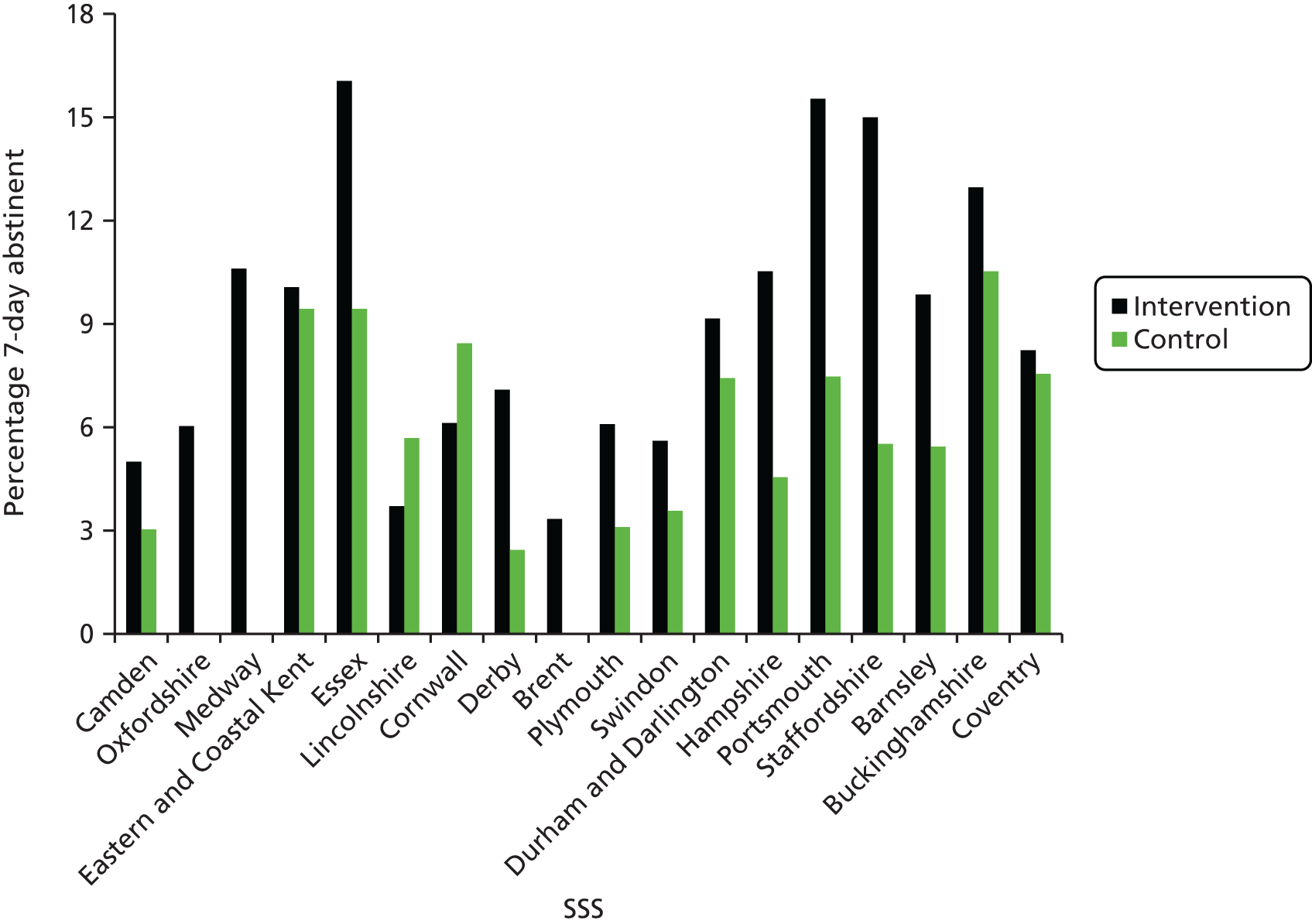
Chapter 4 Process evaluation and subsidiary analysis
The intervention developed and employed in the Start2quit study was a complex one consisting of a personal risk letter and an invitation to a taster session provided by the local SSS. In complex interventions such as this, the elements act both on their own and in conjunction with each other. It is therefore important to try to understand the range of effects and to examine the data to determine the active components, and identify the causal mechanisms through which treatment operates. 60 Process data are the sum of all data collected to document the conduct of the study61 and can be used to explore the way in which the intervention was implemented and the acceptability and feasibility of the intervention, and can also provide insight into the way the ingredients of the intervention facilitate the outcome.
In this chapter we report on process evaluations that were embedded into the trial, including a descriptive analysis of adherence to the intervention and its association with outcomes, data collected to evaluate and assess how acceptable and appropriate participants found the intervention, and an assessment of the fidelity to the protocol of delivery of the taster sessions. We also report on reasons for non-attendance in an additional study exploring barriers to attendance at the SSS.
A more detailed analysis is planned to examine the moderators and mediators of the intervention effect, and the relationships between the various components of the intervention and the outcome in an attempt to explore the active ingredients of the intervention and how they exert their effect. However, it is not possible to report a full assessment of intervention processes within the constraints of the HTA-funded research. These will be examined in more detail in future outputs.
Conduct of and adherence to the intervention, association with outcomes and self-reported attendance
Background
Adherence can be defined as the degree to which behaviour coincides with the recommendations of health-care providers. 62 In trials this can be applied to the intervention as a recommended strategy and accurate measurement of adherence behaviour is necessary to be able to assess whether or not the outcomes can be attributed to the recommended strategy. Any decisions or recommendations based on the outcome depend on valid and reliable measurement of adherence to the treatment,63 and inadequate adherence can adversely impact the effectiveness of an intervention.
One component of this intervention consisted of the opportunity to gain more information about the SSS and what it involves at a no-commitment taster session. This session was part of the strategy to encourage attendance at SSSs and, therefore, attendance at the session was hypothesised to be a major influence on the decision to attend the SSS. Not all participants took up the offer of a taster session. In order to assess the impact of the taster sessions on the primary outcome of the study, details of the provision and attendance at the taster sessions are reported. In addition to the original primary outcome (attendance at the SSS), the trial was powered to assess 7-day point prevalent abstinence at the 6-month follow-up, validated by salivary cotinine analysis. This allowed assessment of whether or not this intervention, and subsequent attendance at the SSS, also translated to increased quit rates. Thus, the first aim of this part of the analysis was to examine the flow of participants from attendance at the taster session, through attendance at the SSS, to achieving validated 7-day abstinence, and the associations between attendance and abstinence.
One approach to measuring adherence behaviour is to ask participants for their subjective ratings of attendance. However, literature on adherence to both medical and behavioural regimes has shown that the subjective reports of patients are problematic. Inaccuracies can result and adherence can be either over- or underestimated. 63 More reliable objective strategies were used in this study to measure attendance both at the taster session and at the SSS. However, self-reported adherence can be revealing, and the second aim of this section was to describe the self-reported attendance, and the amount of agreement and disagreement with the objective measures of attendance.
Methods
Measures
Full details of taster sessions held in each area were documented. Local advisors delivering the session were provided with a list of participants invited to each session, and participants were asked to sign in on arrival. The number attending the taster sessions was taken from these records. Attendance at the SSS was the primary outcome measure and was the valid data of attendance collected from the SSSs. The main secondary outcome of 7-day biochemically validated abstinence was used to define abstinence.
Self-reported attendance at the taster sessions and at the SSS were included in the follow-up interview 6 months after the date of randomisation.
Respondents were asked ‘Do you remember receiving an invitation from your GP to attend a “Come and Try it” taster session to introduce you to the Stop Smoking Service?’. If they did remember, they were asked ‘Did you attend a “Come and Try it” taster session?’. Regarding attendance at the SSS, all respondents were asked ‘In the last 6 months have you tried to make an appointment with the NHS Stop Smoking Service?’. Respondents answering ‘yes’ to this question were then asked ‘Were you successful in making an appointment with the Stop Smoking Service?’, and if they answered ‘yes’ to this they were asked ‘How many times in the last 6 months have you attended any appointments with the Stop Smoking Service?’. Respondents who reported attending were also asked ‘How helpful was attending the Stop Smoking Service to you in quitting or attempting to quit smoking?’. Respondents who completed the basic questions were asked only one question ‘Have you attended any appointments with the NHS Stop Smoking Service in the last 6 months?’. (See Appendix 4 for full follow-up questionnaires.)
Participants
Data from records of attendance include data from all participants. Analysis of the self-reported data includes data from those participants who completed the follow-up in any form (i.e. by telephone, post or by answering the basic questions) (Table 11).
| Method of Completion | Group, n (%) | Total, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| Telephone interview | 1740 (66.0) | 1170 (66.9) | 2910 (66.4) |
| Postal questionnaire | 175 (6.6) | 127 (7.3) | 302 (6.9) |
| Basic questions | 105 (4.0) | 55 (3.1) | 160 (3.7) |
| Total | 2020 (76.7) | 1352 (77.3) | 3372 (76.9) |
Analysis
Frequencies and proportions of participants attending the taster sessions, attending the SSS and achieving validated 7-day point prevalent abstinence were calculated. A rudimentary analysis using a series of chi-squared tests examined the differences in abstinence between the groups according to their attendance at the taster session and at the SSS.
Results
Taster session organisation
A total of 146 taster sessions were organised across the 18 SSS areas, between 4 and 12 in each area depending on the number of participants recruited and the area covered by the SSS. Of these, 106 sessions were first sessions, offered immediately after the first intervention mailing, and 40 were follow-up sessions to which all participants who had not attended one of the first sessions were invited. Of the 146 planned sessions, 131 went ahead, but 15 were cancelled because of low or no attendance.
The mean number of participants invited to an initial session was 24.4 (range 2–78) and to the follow-up session was 49.62 (range 7–168) participants. The numbers varied according to the area covered and the number recruited. Because this varied widely, and some areas were widespread, few people were invited to some sessions. Higher numbers were invited to the follow-up sessions because it was found in the pilot phase that numbers attending the follow-up session were low. Attendance at sessions that went ahead was between 1 and 19 (mean 5.64) participants.
Taster session and Stop Smoking Service attendance and smoking status outcome
Of the 2635 participants in the intervention group invited to attend a taster session, 739 (28%) attended.
In the intervention group more participants who had attended a taster session attended the SSS than those who had not (45.7% vs. 6.3%), and more participants who attended a taster session and the SSS achieved 7-day abstinence (28.7%) than those who only attended the taster session (10%) or only attended the SSS (17.5%). Participants who did not attend a taster session nor the SSS had the lowest rates of validated 7-day abstinence (4.4% and 4.7% in the intervention and control groups, respectively) (Figure 12).
FIGURE 12.
Flow chart showing numbers attending a taster session, the SSS and achieving a validated 7-day point prevalent abstinence in the intervention and the control groups.

Self-reported attendance data
In the intervention group, 1387 (68.6%) participants said that they remembered receiving an invitation to a taster session and 468 (23.2%) reported attending. In addition, 259 (19.2%) participants from the control group also said that they remembered receiving an invitation and 16 (1.2%) said that they attended.
Overall, 1067 (31.6%) respondents reported that they had tried to make an appointment with the SSS: 773 (38.3%) in the intervention group and 294 (21.8%) in the control group. One-quarter of participants (881, 26.1%) reported that they were successful in making an appointment [643 (31.8%) and 238 (17.6%) in the intervention and control groups, respectively] and 820 reported attending the SSS at least once [592 (29.3%) and 228 (16.9%) in the intervention and control groups, respectively] (Table 12). The largest proportion (22.6%) said they attended only once, but one person reported attending up to 48 times.
| Self-reported attendance | Group, n (%) | Total, n (%) (N = 3372) | |
|---|---|---|---|
| Intervention (n = 2020) | Control (n = 1352) | ||
| Remembered invitation to taster session | 1387 (68.6) | 259 (19.2) | |
| Attended a taster session | 468 (23.2) | 16 (1.2) | |
| Tried to make appointment with SSS | 773 (38.3) | 294 (21.8) | 1067 (31.6) |
| Successfully made appointment with SSS | 643 (31.8) | 238 (17.6) | 881 (26.1) |
| Attended SSS at least once | 592 (29.3) | 228 (16.9) | 820 (24.3) |
Self-reported attendance at taster sessions or at the SSS did not correspond to the recorded attendance and validated attendance data from the records of the participating SSSs.
Of the 468 intervention participants who reported attending a taster session, 16 were not recorded as attending, whereas 12 were recorded as attending but did not recall attendance (Table 13). Furthermore, 16 participants in the control group said that they had attended a taster session. Of all participants who completed the follow-up and reported attending the SSS, 429 (intervention, n = 328; control, n = 101) were validated by SSS records, 89 (intervention, n = 66; control, n = 23) did not report attending but according to SSS records did attend and a further 391 (intervention, n = 264; control, n = 127) said that they attended but their attendance was not validated by records (Table 14). Agreement on taster session attendance was much higher at 98% than for attendance at the SSS. Agreement on attendance at the SSS was slightly higher in the control group than in the intervention group (88.9% vs. 83.6%).
| Recorded attendance n (%) | |||
|---|---|---|---|
| Attended | Did not attend | ||
| Self-reported attendance n (%) | Reported attending | 452 (32.6) | 16 (1.2) |
| Did not report attending | 12 (0.8) | 907 (65.4) | |
| Intervention group (N = 2020) | Attendance validated by records n (%) | ||
|---|---|---|---|
| Attended | Did not attend | ||
| Self-reported attendance n (%) | Reported attending at least once | 328 (16.2) | 264 (13.1) |
| Did not report attending | 66 (3.3) | 1362 (67.4) | |
| Control group (N = 1352) | Attendance validated by records n (%) | ||
| Attended | Did not attend | ||
| Self-reported attendance n (%) | Reported attending at least once | 101 (7.5) | 127 (9.4) |
Respondents who reported attending were asked ‘How helpful was attending the Stop Smoking Service to you in quitting or attempting to quit smoking?’ and 309 (73.9% of those whose attendance was validated) rated attending the service as very or extremely helpful on a scale of 1 to 5 (mean 4.09).
Summary
The opportunity to find out more about the SSS by attending a taster session was taken up by less than one-third of participants. The large difference of 40% in SSS attendance between attendees and non-attendees at taster sessions suggests that the session was successful in encouraging uptake of the service, although it is possible that the more motivated smokers were more likely to attend the taster session and also more likely to attend the SSS.
Participants in both the intervention and control groups were more likely to be abstinent if they had attended the SSS. This increase in the proportion abstinent suggests that attending the SSS is more likely to result in abstinence than quitting alone, and confirms the consistent evidence that individual counselling increases the likelihood of cessation. 64,65 Participants who received the intervention and attended the SSS were most likely to be abstinent, whereas participants who received the intervention but did not attend the SSS were not more likely to quit than those in the control group who had not attended the SSS. Attending either the taster session or the SSS (i.e. having some contact with the support services or an advisor) was beneficial, although clearly attending the SSS was most beneficial, and the abstinence figures for the intervention group suggest a possible added benefit of receiving a personal risk letter.
The self-reported SSS attendance figures reflect the effectiveness of the intervention. However, the discrepancy between self-reported attendance at the SSS and the objective measures confirmed the inadequacy of subjective measurement data. Recall or retrospective bias can account for some of this discrepancy, but there is also an issue of interpretation or misunderstanding. Although every effort was made to word the questions clearly, there may have been a lack of differentiation in the minds of the respondents between the taster session and the SSS course, which could account for the slightly better agreement in the control group than in the intervention group. Alternatively, participants may have received advice from elsewhere and not have signed up to a SSS course. In addition, social desirability, the desire to give the impression of compliance, could explain some of the discrepancy. Overall, this demonstrates the importance of collecting objective data, when possible.
Perception of the intervention
Background
Thorough evaluation of an intervention should include an assessment of how acceptable and appropriate participants found the intervention. Participant satisfaction is an important determinant of the potential impact, as it is associated with adherence to the recommended advice and compliance to medical regimes in primary health care. 66,67 This is particularly important when evaluating personal health communication. The superiority of tailored health communications depends on how well received the material was by the target population. 68 Furthermore, assessing the perception of the tailored letter used in this study is crucial because of the use of personal information related to diseases. Issues related to health typically tend to be anxiety-laden and associated with negative rather than positive feelings,69 and a fear-driven appeal message presenting individuals with information about themselves can prompt a maladaptive behavioural response by, for example, denying the truth or personal relevance of the threat. 32
The focus of this section is to evaluate the perception of both the personal risk letters and of the taster session, assessed using measures from previous trials of tailored feedback,70,71 and included in the telephone interview at the 6-month follow–up, using both closed and open questions. Perception of the taster session was also assessed by an evaluation form completed immediately following each session.
Methods
Measures
Respondents to the telephone interview were asked if they remembered receiving a letter from their GP about the NHS SSSs. Those who did remember were asked if they had read the letter and if they had discussed the letter with others. They were then asked a series of questions about their views of the letter and how it made them feel, measured using a five-point Likert scale (1 = not at all, 5 = extremely). A series of similar questions was asked about the taster session of those respondents who reported attending a session.
In a combination of closed and open questions, respondents who self-reported having attended the SSS or having quit were asked to what they attributed their decision to attend the SSS and to what they attributed their decision to quit.
Respondents who said they remembered a letter were asked ‘Did you make the appointment with the Stop Smoking Service as a result of receiving this letter or was it something else?’. Participants who remembered the letter and said they attended a taster session were also asked the question ‘Did you make the appointment with the Stop Smoking Service as a result of attending this “Come and Try it” taster session or was it something else?’. Regarding the decision to quit, all respondents replying ‘not at all’ to the question ‘(How often) do you currently smoke cigarettes or rollups?’ were asked if they had quit as a result of receiving a letter (personal or generic, depending on the group allocation). Respondents who self-reported having quit and also reported attending a taster session were also asked ‘Have you quit smoking as a result of attending the taster session?’. In all of these questions, if respondents reported that making an appointment with the SSS was not as a result of the letter but a result of something else, interviewers probed for their reason for making the appointment.
Respondents who reported attending a taster session were also asked whether attending the taster session or receiving the letter was more important in their decision to attend the SSS and, if they reported having quit, were asked whether attending the taster session or receiving the letter was more important in their decision to quit smoking. (See Appendix 4 for full follow-up questionnaires.)
The questions also incorporated reasons for attendance and non-attendance at the taster session using open questions. Respondents who reported attending the taster session were asked in an open question their reasons for deciding to attend, and respondents who remembered the invitation but did not attend were asked for their reasons for not attending the taster session.
Participants who said they remembered the invitation but did not attend were asked if they would have attended the taster session if they had received a text or telephone reminder. A control measure was included to see whether or not any of the participants had spoken to a taster session attendee.
All taster session attendees were asked to complete an evaluation form immediately following the taster session. Questions matched those of the follow-up interview and also allowed space for free-text comments.
Participants
The data included in this analysis on the perception of the intervention were from those participants who completed the telephone interview [total, n = 2910 (66.4%); intervention, n = 1740 (66.0%); control, n = 1170 (66.9%)].
For the assessment of the taster session and decision to attend the SSS, the analysis includes only those who were validated as attending the SSS or the taster session. However, for the decision to quit, the self-reported smoking status was used. This referred to their perceived state at the time of the follow-up, not to their validated smoking status and, therefore, probably reflects a truer perception of their decision to quit.
Analysis
Proportions and means were calculated to describe the perception of the personal risk letter. Chi-squared and t-tests were used to compare the perception of the risk letter with that of the generic letter. However, these results should be interpreted with caution because of the number of comparisons made. For the perception of the taster session, the proportions endorsing each statement at the 6-month follow-up were calculated and were compared descriptively with the proportions endorsing each statement on the evaluation form immediately following each session. The free-text comments in the evaluation forms were analysed by two of the authors, both thematically and also for content to classify the comments into positive, negative and neutral categories.
The decision to take action as a result of the intervention and the attribution for these decisions are described using frequencies and proportions, and comments from probes were content analysed. Chi-squared tests were used to compare the intervention and control groups on their decisions concerning the letter. However, these are self-selected subgroups and the results should be interpreted with caution.
Results
Perception of the personal risk letter
Of those who completed the telephone follow-up, 2605 (89.5%) remembered receiving a letter. The numbers responding to individual questions on the perception of the letter ranged from 2327 to 2453. The results are shown in Table 15.
| Perception | Group | p-value | |
|---|---|---|---|
| Intervention (N = 1740) | Control group (N = 1170) | ||
| Remembered letter, n (%) | 1570 (90.2) | 1035 (88.5) | 0.138 |
| Read letter, n (%) | 1517 (87.2) | 977 (83.5) | 0.004 |
| Discussed letter, n (%) | 654 (37.6) | 348 (29.7) | < 0.001 |
| Easy to read,a mean (SD) | 4.75 (0.57) | 4.73 (0.65) | 0.255 |
| Easy to understand,a mean (SD) | 4.78 (0.53) | 4.75 (0.60) | 0.196 |
| Written for me,a mean (SD) | 3.43 (1.38) | 3.22 (1.37) | < 0.001 |
| Contained new information,a mean (SD) | 3.12 (1.37) | 3.11 (1.36) | 0.966 |
| Interesting,a mean (SD) | 3.73 (1.17) | 3.67 (1.20) | 0.227 |
| Useful,a mean (SD) | 3.83 (1.28) | 3.72 (1.23) | 0.038 |
| Felt more confident,a mean (SD) | 3.18 (1.42) | 2.95 (1.42) | < 0.001 |
| Felt more determined,a mean (SD) | 3.36 (1.41) | 3.10 (1.40) | < 0.001 |
| Liked the tone,a mean (SD) | 3.68 (1.10) | 3.54 (1.14) | 0.003 |
| Liked the appearance,a mean (SD) | 3.59 (1.12) | 3.51 (1.16) | 0.075 |
| Angry,a mean (SD) | 1.27 (0.81) | 1.22 (0.71) | 0.147 |
| Anxious,a mean (SD) | 1.54 (1.06) | 1.46 (0.96) | 0.054 |
| Depressed,a mean (SD) | 1.31 (0.83) | 1.30 (0.82) | 0.695 |
| Optimistic,a mean (SD) | 2.99 (1.34) | 2.83 (1.31) | 0.003 |
Participants in the intervention group were not more likely than those in the control group to remember receiving a letter (90.2% vs. 88.5%), but were more likely to read the letter (87.2% vs. 83.5%; p = 0.004) and to discuss the letter with others (37.6% vs. 29.7%; p = 0.001) (see Table 15). Participants in the intervention group who received the tailored letter found it to be more personally relevant to them [3.43 vs. 3.22 (on a 1–5 scale); p < 0.001] and reported that it made them feel more confident towards quitting (3.18 vs. 2.95; p < 0.001), more determined towards quitting (3.36 vs. 3.10; p < 0.001) and made them feel more optimistic (2.99 vs. 2.83; p = 0.003) than those in the control group who received the standard letter. They also said that they liked the tone of the letter more than those who had received the standard letter (3.68 vs. 3.54; p = 0.003).
The personal risk letter was considered to be highly acceptable, with the majority of respondents rating it very or extremely easy to read [1421 (95.8%)], and very or extremely easy to understand [1440 (96.8%)], and approximately two-thirds finding it interesting [888 (61.2%)] and useful [960 (66%)]. Very few respondents reported feeling very or extremely angry [60 (4.1%)], anxious [122 (8.2%)] or depressed [67 (4.5%)]. The majority reported low ratings of ‘not at all’ or ‘a little’ on these measures, whereas 565 (67%) rated the letter as making them feel at least moderately optimistic.
Perception of the taster session in the intervention group
Of 1740 participants in the intervention group who completed the telephone interview, 1387 said that they remembered receiving an invitation to a taster session and 468 reported attending a taster session, although 16 of these were not validated by records. Thus, 452 respondents in the intervention group reported attending a taster session, were validated by records of attendance and gave their views on the session in the telephone interview. This analysis is based on these 452 participants. A further 12 participants in the control group also said that they remembered attending a taster session, but were excluded from the analysis. The evaluation form was completed by 637 attendees. Some of the respondents completed only the follow-up or only the evaluation form; approximately two-thirds completed both.
The taster sessions were viewed positively by the majority of attendees. Over 90% of respondents found the session very or extremely easy to understand, and > 70% found it interesting and useful. Although 91.5% expressed the intention to sign up immediately following the session, at the 6-month follow-up 69.9% reported that the session made them more inclined to make an appointment with the SSS. As previously noted, validated records show that 338 (45.7%) attended the SSS following attendance at the taster session. In general, the taster session was rated more highly immediately following the session than at the 6-month follow-up (Table 16).
| Perception | Evaluation, n (%) | |
|---|---|---|
| 6 months after taster session (N = 452) | Immediately after taster session (N = 637) | |
| Taster session was easy to understand | 417 (92.3) | 610 (95.7) |
| Taster session contained new information | 254 (56.2) | 415 (65.2) |
| Taster session was interesting | 334 (73.9) | 534 (83.8) |
| Taster session was useful | 329 (72.8) | 559 (87.8) |
| Taster session made me more inclined to make an appointment (follow-up) | 316 (69.9) | |
| Intend to make an appointment (evaluation form) | 583 (91.5) | |
| Taster session made me feel more confident | 318 (70.4) | 410/556 (73.7) |
| Taster session made me feel more determined | 326 (72.1) | 441/556 (79.3) |
Of the 637 attendees who completed the evaluation forms, 528 left comments. The content analysis overwhelmingly confirmed that the taster sessions were seen as helpful and encouraging, with 91.7% of the comments being positive, 3% negative and 5.3% neutral. The most common words used to describe the session were ‘informative’ (used by 99 attendees), ‘interesting’ (by 70 attendees), ‘friendly’ (by 31 attendees) and ‘encouraging’ (by 21 attendees).
Comments suggested that the advisors did not exert any pressure on the attendees to sign up, and the attendees felt relaxed. For example:
. . . well organised and informative and the first time I have tried ‘group therapy’. I was anxious about group therapy as giving up but it was all cool and helpful.
Male, 54 years
. . . very welcoming and informal and friendly informative and non-judgmental.
Male, 61 years
. . . very good. Without trying to ‘preach’ or exert undue pressure.
Male, 70 years
. . . relaxed atmosphere and ‘no lectures’.
Female, 68 years
Comments also confirmed that some smokers are not aware of the service and what is offered, or how to go about quitting, illustrated by:
. . . informative and knowing I can discuss my problems with someone has made my decision to try to quit easier.
Female, 64 years
. . . nice to know that such a service exists.
Male 50 years
. . . quite enlightening.
Female, 68 years
. . . an interesting concept.
Male, 62 years
Other comments suggest that the taster session gave people new information and encouraged them to look at things in a different way, for example:
. . . puts things into perspective
Male, 52 years
and that the sessions are motivating and encouraging.
. . . time well spent – increasing my determination to quit.
Male, 61 years
Actions as a result of the intervention
Decision to attend the Stop Smoking Service
Three hundred and fifty-three respondents (intervention, n = 273; control, n = 80) who answered the question ‘Did you make the appointment with the Stop Smoking Service as a result of receiving this letter or was it something else?’ said that they attended the SSS and were validated by SSS records. Of these, 277 (78.5%) said that they attended because of the letter [224 (82.1%) vs. 53 (66.3%) in the intervention and control groups, respectively; p = 0.002].
In the intervention group, 258 respondents also said that they attended a taster session and were asked the question ‘Did you make the appointment with the Stop Smoking Service as a result of attending this “Come and Try it” taster session or was it something else?’. Of these, 193 (74.8%) said that they made the appointment because of the letter, 170 (65.9%) because of the taster session and 151 (58.5%) answered yes to both questions. These participants were also asked whether attending the taster session or receiving the letter was more important in their decision to attend the SSS: 141 (54.7%) said both equally, 19 said the taster contributed more and 20 said the letter contributed more (Table 17).
| Decision | Group, n (%) | p-value | |
|---|---|---|---|
| Intervention | Control | ||
| Decision to attend the SSS | |||
| Completed interview and attendance valid (n = 353) | |||
| Made appointment as result of letter | 224/273 (82.1) | 53/80 (66.3) | 0.002 |
| Attended the SSS and taster session (n = 258) | |||
| Made appointment as result of letter | 193 (74.8) | ||
| Made appointment as result of taster | 170 (65.9) | ||
| Made appointment as result of letter and taster | 151 (58.5) | ||
| Decision to attend SSS equal letter and taster | 141 (54.7) | ||
| Decision to quit smoking | |||
| Completed interview, said quit smoking and answered the question ‘Have you quit smoking as a result of receiving this letter?’ (n = 504) | |||
| Quit smoking as result of letter | 220/349 (63.0) | 63/155 (40.7) | < 0.001 |
| Quit smoking and attended taster session (n = 226) | |||
| Quit smoking as result of letter | 158 (69.9) | ||
| Quit smoking as result of taster | 153 (67.7) | ||
| Quit smoking as result of letter and taster | 131 (58.0) | ||
| Decision to quit equal letter and taster | 112 (49.6) | ||
Decision to quit
Six hundred and thirty respondents self-reported having quit and, of the 504 participants who answered the question ‘Have you quit smoking as a result of receiving this letter?’, 283 (56.2%) replied that they had [intervention, n = 220 (63%); control, n = 63 (40.7%); p < 0.001].
Two hundred and twenty-six self-reported having quit, attended a taster session (validated by records) and were asked ‘Have you quit smoking as a result of attending the taster session?’. Of these, 158 (69.9%) said that they quit smoking as a result of the letter, 153 (67.7%) said they quit smoking as a result of the taster session and 131 (58%) answered ‘yes’ to both questions. These participants were also asked whether attending the taster session or receiving the letter was more important in their decision to quit smoking: 112 (49.6%) said both were equally important, 32 said the taster session was more important and 17 said the letter was more important (see Table 17).
Open questions
Interviewers probed the 75 respondents who reported that making an appointment with the SSS was not as a result of the letter but because of something else, for a reason for making the appointment. The most frequently given reason concerned health (n = 23, 30.7%). Other frequently given reasons suggested the respondent wanted to stop anyway and the letter came at the right time to prompt them to make an appointment (n = 21, 28%), for example ‘I’ve been thinking about it so this was a nudge’ or ‘I was going to do it anyway’.
Reasons for attendance and non-attendance at the taster session
Comments analysed were limited to those from validated attendees (n = 451). A majority (239, 53%) said that they attended the taster session because they wanted to quit and saw this as a way of getting help, although a substantial minority (n = 77, 17.1%) said that they attended out of curiosity and ‘wanted to see what it was about’.
Of intervention group participants, 907 gave a reason for not attending the taster session. Many said that they were too busy and had other commitments (n = 367, 40.5%), or that the taster sessions were held at inconvenient times or locations (n = 221, 24.4%). Some reported that they ‘didn’t really want to quit’ or that they were ‘not ready for it’, and a small number wanted to quit on their own or ‘do it my way’.
Some respondents in the control group (n = 243) reported that they remembered getting an invitation and were therefore also asked this question. Most cited other commitments (n = 72, 29.6%), no desire to quit (n = 33, 13.6%) or wanting to quit alone (n = 35, 14.4%). However, as these participants did not receive an invitation to a taster session they would be referring to the service itself not to the taster session.
Supplementary questions
The majority of respondents who said that they remembered the invitation but did not attend (n = 633/902, 70.2%) suggested that a text or telephone reminder would not influence their decision not to attend.
The control measure showed that 14 participants in the intervention group and 30 in the control group had spoken to a taster session attendee.
Summary
This analysis of the perception of the intervention suggested that both parts were well received by the target population. The personal letter was well accepted, and participants who received this personal letter as opposed to the generic letter were more likely to read it and to discuss it with others. They saw it as personally relevant and it appeared to increase their confidence and determination to quit. With regard to whether or not the intervention group found that the letter may have caused upset, few respondents reported feeling very or extremely angry, anxious or depressed.
The taster sessions were also viewed positively by the majority of attendees. However, actual attendance at the SSS resulting from the session was lower than the stated intention to sign up immediately following attendance at the taster session. The comments left by attendees immediately after the session also endorsed the positive view of the sessions, suggested that the taster sessions were reassuring and built the intended awareness and comfort with the services. They also offered some insight into the reasons smokers might not attend, such as the fear of criticism and victimisation.
Questions asking respondents to attribute their decision to attend the SSS or to quit might suggest that, for many smokers, both the letter and the taster session worked in synergy to encourage attendance and attempting to quit. However, in view of the response rate to these questions and possible problems of interpretation and recall, there is insufficient evidence to corroborate this conjecture and further research is necessary.
Additional reasons given for making an appointment with the SSS and for attending the taster session indicated that smokers who attended were thinking of quitting and were prompted by the communication to act on their plans. Similarly, reasons for not attending the taster session suggested that quitting was not a priority and other commitments were more important at this time.
Finally, the additional question included to assess whether or not reminders would encourage attendance suggested that it would not be worthwhile. The control question did not suggest contamination bias as a result of a large number of control participants having access to information from the taster sessions.
Assessment of the fidelity of the delivery of the taster sessions
Background
Treatment fidelity refers to the extent to which an intervention that is delivered matches that described in the intervention manual. Knowing the fidelity compared with the manual is important for interpretation of results, as without this knowledge it is impossible to determine how much the intervention in question is the primary mechanism in any changes observed. 72
Assessment of the fidelity of delivery in complex behavioural interventions requires authors to report the content of the intervention, the characteristics of those delivering the intervention, the setting, the length and adherence of the delivery to the protocol. However, many studies fail to report the content of the intervention or the content and duration of training, and a recent systematic review found that few studies of psychosocial treatments evaluated treatment fidelity. 73 For behavioural interventions, in particular, variation in delivery quality can strongly mediate intervention effectiveness. This failure to account for fidelity may lead to underestimation of the intervention effects and faulty conclusions that interventions are not effective, when in reality they were not implemented as intended, or to the acceptance of statistically effective interventions, which differ greatly from their initial design. 72
The use of present/absent checklists has been identified as the most reliable method to monitor the fidelity of delivery of study interventions. 72,74,75 Key to this method is the prespecified intervention content or ‘treatment manual’, a manual containing explicit guidelines about the content and method of delivery of an intervention to ensure that all providers receive the same training and information. In addition to identifying the extent of fidelity of delivery, it is also important to understand the ‘essential’ components of an intervention. 76,77 A taxonomy of behaviour change techniques (BCTs) has been developed and modified to apply to behavioural support for smoking cessation. 78
Procedures to assess the fidelity of delivery of the taster sessions were embedded into the trial. We aimed to answer the following research questions:
-
To what extent did advisors adhere to protocol-specified content and BCTs?
-
Were advisor or session characteristics related to adherence to protocol-specified content?
-
Was adherence to protocol-specified content related to participants’ attendance at the SSS or to validated 7-day point prevalent abstinence?
Method
Sampling frame
A total of 146 taster sessions were organised across the 18 SSS areas. Of these, 131 went ahead as planned and 15 were cancelled because of lower than expected attendance rates. Only trained advisors led the taster sessions, and each session was either run by one advisor with additional administrative support provided by one other, or the presentation was divided between the two advisors, with one advisor leading and the other supplementing some of the content. To assess fidelity to the protocol, the taster sessions were, with the consent of the attendees, audio-recorded.
Of the 131 sessions delivered, 93 (71%) were recorded. The remainder were not recorded because of forgotten recording equipment, equipment failure or because one or more participants attending the session declined to consent to the recording. Owing to the quantity and length of the recordings one session by each lead advisor (when available) was randomly selected (n = 41, 31.3% of sessions delivered) for transcription and analysis.
To ensure that those selected were representative of the total taster sessions, analysed sessions were compared with those not analysed for session characteristics and outcome variables. There were no significant differences in length of sessions, number of attendees or in outcomes (SSS attendance or 7-day point prevalent abstinence at the 6-month follow-up).
Measures
Adherence measures
The taster session protocol contained 73 specified behaviours (Table 18), all of which were either specific information that the advisors should communicate (e.g. that the first SSS session involves discussion of reasons for and against smoking) or instructions that they should follow (e.g. ask attendees how many of them enjoy smoking). A coding frame was developed by one of the authors based on this protocol, and this was verified by two additional researchers.
| BCTa | Description | Component behaviours in taster session manual (n = 73) |
|---|---|---|
| Give information on stop smoking medication | Explain the benefits of medication, safety, potential side effects, contraindications, how to use them most effectively and how to get them; advise on the most appropriate medication for the smoker and promote effective use | 1. Use of medication as important part of quitting |
| 2. Nicotine deprivation may lead to withdrawal symptoms | ||
| 3. Medication available to reduce cravings while adjusting to not smoking | ||
| 4. How NRT works | ||
| 5. Types of NRT available | ||
| 6. Bupropion (Zyban®, GlaxoSmithKline, London, UK) and varenicline (Champix®, Pfizer, New York, NY, USA) and how they can help reduce desire to smoke | ||
| Boost motivation and self-efficacy | Give encouragement and bolster confidence in ability to stop | 1. Congratulate attendees on coming to the session |
| 2. Attending session suggests motivation to quit | ||
| 3. This an important step in process of quitting | ||
| 4. Positives of this, being something to prepare for | ||
| 5. Good way of proving that attendees are doing something good for their health | ||
| Build general rapport/emphasise empathy of SSS advisors | Establish a positive, friendly and professional relationship with the smoker and foster a sense that the smoker’s experiences are understood | 1. Introduce self and describes personal background |
| 2. Explain understanding of SSS advisors that smoking is something attendees enjoy | ||
| 3. Support in event of ‘slip up’ | ||
| 4. SSS can help work out cause of slip up and work out strategies for avoiding future occurrences | ||
| 5. Recap: thank attendees for attending | ||
| Elicit and answer questions | Prompt questions from the smoker and answer clearly and accurately | 1. Ask for questions |
| Elicit client views | Prompt the client to give views on smoking, smoking cessation and any aspects of the behavioural support programme | 1. Encourage participation |
| 2. Encourage attendee participation | ||
| 3. Encourage participation on withdrawal symptoms | ||
| Emphasise choice | Emphasise client choice within the bounds of evidence-based practice | 1. Making decision in first session after weighing up pros and cons |
| 2. Emphasise that they will not be told to quit | ||
| Explain expectations regarding treatment | Explain to the smoker the treatment programme, what it involves, the active ingredients and what it requires of the smoker | 1. NHS SSS supports smokers to stop smoking completely, not to cut down |
| 2. First session as preparation for stopping smoking | ||
| 3. First session involves discussion of reasons for and against smoking | ||
| 4. Setting of quit date will be encouraged during first few sessions | ||
| 5. Emphasise that weekly contact is extremely important | ||
| 6. Explain that this is why weekly contact is so important | ||
| Explain purpose of CO monitoring | Explain to the smoker the reasons for measuring CO at different time points (e.g. before and after the quit date) | 1. Introduce test for CO present in body |
| 2. Explain its use in NHS courses | ||
| 3. Mention that it will be possible to compare this reading with one they have later at NHS SSS after they quit | ||
| Explain the importance of abrupt cessation | Explain why it is better to stop abruptly rather than cut down gradually if at all possible | 1. Not a single puff rule and its effectiveness |
| Give options for support with the SSS | Give information about options for additional support when these are available (e.g. websites, self-help groups, telephone helpline) | 1. How many sessions in a course |
| 2. Courses can be run by Stop Smoking advisor or practice nurse | ||
| 3. Minimum number of sessions following quit date | ||
| 4. Give detail on length of sessions | ||
| Identify reasons for wanting and not wanting to stop smoking | Help the smoker to arrive at a clear understanding of his or her feelings about stopping smoking, why it is important to stop and any conflicting motivations | 1. Ask attendees how many of them enjoy smoking |
| 2. Identify reasons for wanting and not wanting to stop smoking | ||
| 3. Ask attendees why they are considering quitting smoking | ||
| Measure CO | Measure CO concentration | 1. Offer attendees opportunity to have CO levels read |
| 2. Encourage all attendees to have reading taken | ||
| Provide information on consequences of smoking and smoking cessation | Give, or make more salient, information about the harm caused by smoking and the benefits of stopping; distinguish between the harms from smoking and nicotine; debunk myths about low tar and own-roll cigarettes and cutting down | 1. Short-term benefits of quitting |
| 2. Long-term benefits of quitting | ||
| 3. Explain that CO is a poisonous gas contained in cigarette smoke | ||
| 4. Explain nature of toxicity of CO | ||
| 5. Good news that levels of CO drop very quickly once they stop smoking | ||
| 6. Immediately improves circulation and chance of any related health problems | ||
| Provide information on withdrawal symptoms | Describe to smokers what are, and are not, nicotine withdrawal symptoms, how common they are, how long they typically last, what causes them and what can be done to alleviate them | 1. Ask attendees for any common withdrawal symptoms |
| 2. Mention common symptoms if none is suggested by attendees (e.g. stress/anger/lower concentration/increased appetite) | ||
| 3. Emphasise that not everyone will experience these symptoms | ||
| Summarise information/confirm client decisions | Provide a summary of information exchanged and establish a clear confirmation of decisions made and commitments entered into | 1. Recap: mention that there are benefits to quitting in both long and short term |
| 2. Recap: mention that attending a course will make it four times more likely that they will have a successful quit attempt | ||
| 3. Recap: mention the courses will help develop strategies to avoid smoking | ||
| 4. Recap: mention they will also receive information on available medications | ||
| 5. Recap: remind attendees to complete an evaluation form and return it to an advisor | ||
| 6. Recap: emphasise that immediate sign up to a SSS course is possible | ||
| Importance of behaviour changeb | Detail the role habits play in smoking and emphasise the help the SSS can provide in breaking the associations between smoking and situational triggers | 1. Explain habitual nature of smoking |
| 2. Trigger points | ||
| 3. Importance of developing strategies to break the association between these trigger points and smoking | ||
| 4. NHS SSS support of behaviour change | ||
| 5. Emphasise medication not being miracle cure and behaviour change is also needed | ||
| Promote NHS SSSb | Detail the success rates of the SSS and explain how SSS advisors can help smokers stop smoking and remain quit in the long term | 1. Explain NHS SSS is based on well-researched evidence |
| 2. Attending a NHS course has been proven to be the best way to help people quit | ||
| 3. Services are free | ||
| 4. Those attending course are four times more likely to stop and stay stopped than those who try and quit on their own | ||
| 5. Remaining sessions are for support | ||
| 6. Help in developing strategies to avoid smoking is key aspect of NHS SSS course | ||
| 7. Able to find out more about NRTs at NHS SSS | ||
| 8. Advisors can aid in choosing between different forms of NRT | ||
| 9. Able to find out more about these medications from NHS SSS | ||
| 10. Support available from NHS SSS advisors in this process | ||
| 11. Mention potential sign up | ||
| 12. Show DVD to attendees |
The behaviours specified in the coding frame were independently classified by two researchers into component BCTs using an established taxonomy of 45 smoking cessation BCTs. 78 Following discussion it was decided that most of the protocol-specified behaviours were represented by the 15 BCTs. The remaining BCTs, detailed in the taxonomy by Michie et al. ,78 were not used as they were not applicable to the taster sessions. In addition, two novel BCTs were developed to account for the remaining behaviours that did not fit into the existing 45 BCTs proposed by the taxonomy: ‘promote the SSS’ and ‘importance of behaviour change’. These BCTs accounted for behaviours that were uniquely important to the aims of the taster sessions, encouraging a commitment to changing behaviour by quitting smoking utilising SSS support (see Table 18).
Session characteristics
Taster session characteristics included the structure of the session (one advisor or two providing content), the length of the session and number of attendees.
Advisor characteristics
Advisors completed a short questionnaire at the time of training. Data gathered included gender, age, highest educational qualification, type of smoking cessation training, time since smoking cessation training, employer, job title and number of patients seen in the previous 6 months.
Outcome variables
The outcome variables were the proportion of attendees at each session who subsequently attended the SSS and the proportion of attendees at each session who were found to be validated 7-day point prevalent abstinent at the 6-month follow-up.
Procedure and analysis
All transcripts were anonymised for SSS area and advisor. Every transcript was coded by two researchers, with 25% additionally coded by a third. Average inter-rater reliability for coding was 86% (range 68–99%) across sessions. All disagreements were resolved through discussion between researchers. The data from the coding frames were double-entered into a spreadsheet and discrepancies were corrected.
The fidelity of each taster session was expressed as the percentage of overall protocol-specified behaviours that were delivered; that is, the number of protocol-specified behaviours applied by the advisor divided by the total number of behaviours (e.g. number of behaviours applied by the advisor = 50/total number of behaviours = 73, fidelity = 68.5%). Adherence to each BCT was measured as the number of behaviours applied by the advisor within each BCT divided by the total number classified within each.
Mean and median adherence to protocol-specified behaviours was calculated, and medians for each BCT. t-tests and analysis of variance were used to assess differences in adherence to the protocol by advisor characteristics and advising structure. Correlations were computed to explore associations between adherence to protocol-specified behaviours and the length of session, and between adherence and the main outcome measures of attendance at the SSS and 7-day point prevalent abstinence at the 6-month follow-up.
Results
Session characteristics
Session duration ranged from 14 minutes to 1 hour 18 minutes (mean 43 hours 4 minutes). Twenty-seven sessions (65.85%) were facilitated by one lead advisor with minimal administrative support by an additional advisor and 14 (34.15%) were split between two advisors, with one taking the lead. The number of smokers attending a session ranged from 1 to 17 (mean 5.41), the number of people attending in 11 of the sessions was four.
Advisor characteristics
The majority (73.17%) of advisors were female and 51.22% were aged 45–54 years. The majority (73.17%) were educated to degree level or higher, 41.46% had received smoking cessation training to National Centre for Smoking Cessation and Training stage 1 and 2 certification with various additional training, and 60.98% had received training within the previous 3 years. All were employed by the SSS, and 70.73% were employed as SSS advisors. Most (90.24%) had seen ≥ 21 patients in the last 6 months (Table 19).
| Characteristic | Number of participants (% of total) |
|---|---|
| Gender | |
| Female | 30 (73.17) |
| Male | 11 (26.82) |
| Age (years) | |
| 18–44 | 12 (29.27) |
| 45–54 | 21 (51.22) |
| ≥ 55 | 8 (19.51) |
| Highest qualification | |
| A Level or lower | 11 (26.83) |
| Degree or higher | 30 (73.17) |
| Smoking cessation training | |
| NCSCT stage 1 certification/plus additional training | 8 (19.51) |
| NCSCT stage 1 and 2 certification or SCTRP | 15 (36.59) |
| NCSCT stage 1 and 2 certification plus additional training | 17 (41.46) |
| Missing | 1 |
| Time since stage 2 training (years) | |
| 1–3 | 25 (60.98) |
| ≥ 4 | 11 (26.83) |
| Missing | 5 |
| Employer | |
| NHS SSS | 100 (100) |
| Job title | |
| SSS advisor | 29 (70.73) |
| SSS manager | 4 (9.76) |
| Healthy lifestyle advisor | 2 (4.88) |
| Administrator | 3 (7.32) |
| Other | 1 (2.44) |
| Missing | 2 |
| Number of patients in previous 6 months | |
| < 21 | 3 (7.32) |
| > 20 | 37 (9.24) |
| Missing | 1 |
Adherence
A median of 71.23% (mean 68.53%) of protocol-specified behaviours was delivered across sessions, ranging from 29% to 96%. As can be seen in Figure 13, low-adherence sessions were not concentrated in particular SSS areas.
FIGURE 13.
Percentage adherence to manual-specified content in each analysed taster session.

Median fidelity to specific BCTs across sessions varied from 50% (‘Summarise information’ and ‘Give options for support’) to 100% in five of the BCTs. However, two of the five consisted of only one behaviour, and another two consisted of only two behaviours. The fifth one, ‘provide information on the consequences of smoking and smoking cessation’, consisted of six behaviours and had the highest adherence (Figure 14).
FIGURE 14.
Median fidelity to BCTs across sessions.
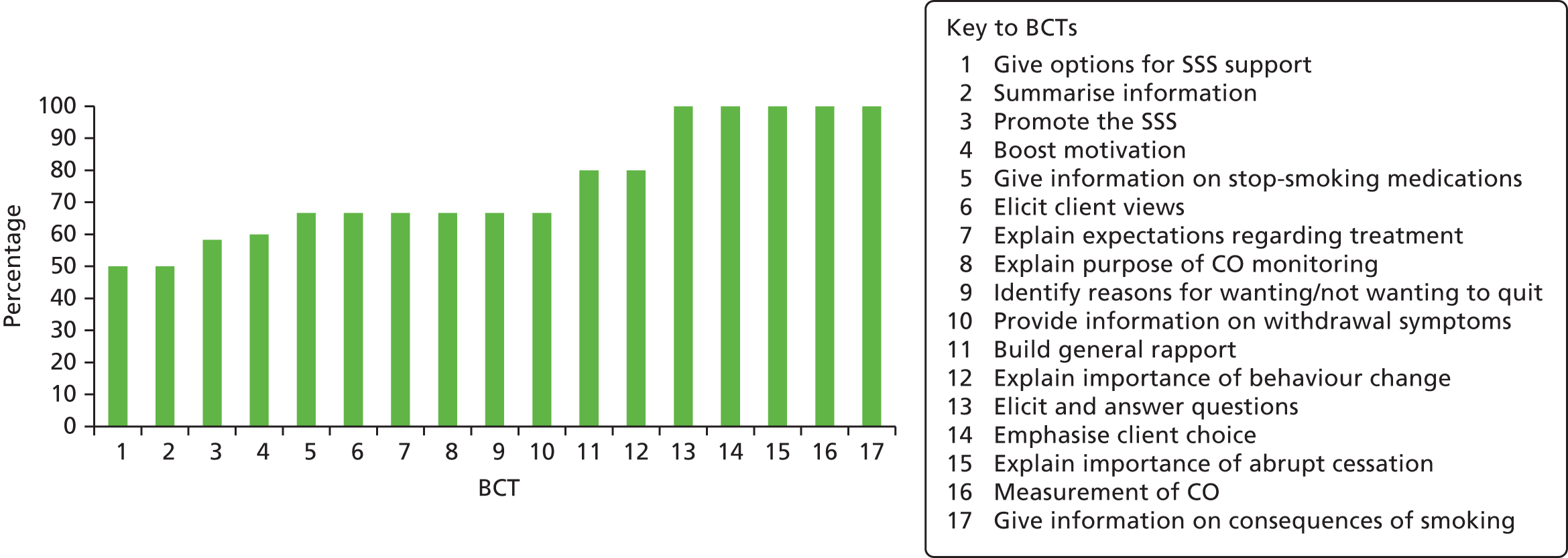
Association between adherence and session and advisor characteristics
Sessions in which the advisor was assisted by a second advisor were found to have significantly higher adherence levels than sessions run by one advisor alone (75.73% vs. 64.79%; p = 0.044). There was a negative correlation between session length and adherence; thus, shorter session length was associated with increased adherence to protocol-specified content (r = –0.351, n = 41; p < 0.025). Female advisors had significantly higher levels of adherence to protocol-specified behaviours than male advisors (72.19% vs. 58.53%; p = 0.018). Level of adherence also varied with advisors’ age: those aged 45–54 years were significantly more adherent (75.41%) than advisors who were younger (61.87%) or older (60.45%) (p = 0.021).
Study outcomes and association with adherence
The proportion of attendees who subsequently attended the SSS ranged from 0% to 100% (mean 44.94%), and the mean proportion of attendees who were found to be validated as 7-day point prevalent abstinent at the 6-month follow-up was 20.21% (range 0–66.67%). No correlation was found between adherence to protocol-specified content and the proportion of attendees attending the SSS, or between adherence and the proportion who were 7-day point prevalent abstinent.
Discussion
The observed median of 71.2% adherence to specified behaviours is higher than that reported by many other similar studies. 79–81 The standardised training in helping smokers to quit that all advisors had received, in addition to having previous experience of running individual or group stop smoking courses, could account for this. However, although the adherence of many was reasonably high, one-quarter fell below 50% and one as low as 29%.
Overall, adherence was greater in sessions that were run jointly by two advisors. The second advisor possibly acted as a safety net, delivering key items that the first advisor failed to present. In addition, presenting in pairs could have mitigated feelings of nervousness for some advisors who had less experience of facilitating groups. Shorter sessions were associated with higher levels of adherence, and being female and in the mid-range age category were significantly associated with adherence to the protocol. Of the BCTs, ‘provide information on consequences of smoking and smoking cessation’ had the highest median percentage fidelity. Information on the consequences of smoking is likely to be a common area of focus for SSS advisors as the health impact of smoking is the most common reason for smokers to attempt to quit.
Adherence to the protocol was not related to the main outcome of attendance at the SSS or to abstinence from smoking. Responding to an invitation to a smoking cessation study and then attending a session to find out more about the SSS indicates a high level of interest in both quitting smoking and getting support to do so, and indicates generally high levels of motivation of attendees. This enthusiasm could indicate that the presentation of protocol-specified content was less important than theorised, as attendees were already feeling inclined to sign up for a SSS course.
The study is limited by focusing solely on adherence to the protocol and neglecting more subtle competency-related variables and non-specific effects, such as communication characteristics (e.g. providing examples to clarify points, and empathetic tone). In addition, a number of advisors provided extra information, both relevant and irrelevant, which was not analysed.
The results of this evaluation indicate that the study intervention is not likely to have been significantly impacted by issues of fidelity, and we can have greater confidence that variability in the main outcome of the study is not a result of variability in SSS advisor adherence to the protocol of the taster sessions.
An exploration of the reasons for non-attendance and barriers to attendance at the Stop Smoking Service
Reasons for non-attendance at the SSS and barriers to attendance at the services were assessed using open questions in the 6-month follow-up. In addition, all participants who completed the telephone follow-up interview and who reported not attending the SSS were asked to complete the TBQ to assess in more depth barriers to the use of the SSS.
Background
Until recently, little research had been devoted to identifying and removing barriers to entering stop smoking programmes, prompting Fiore et al. 65 in the US Department of Health and Human Services guidelines to stress the importance of further investigation in order to identify and remove barriers to treatment. Research both before and since this call has identified that major barriers include a lack of awareness, low expectations of effectiveness, most smokers underestimating the benefits of treatment and a belief that motivation and willpower are sufficient. 10,11,16,43,82–86 The belief that ‘if you really want to quit smoking you will succeed on your own as well as you would with help’ is prevalent. 16 Concern about lack of empathy and notions that treatments are unavailable and hard to access also permeate the literature. 43,82,83,85,86
Copeland et al. 49 adopted a more structured approach to identifying the most common reasons for not seeking treatment and developed a validated 40-item measure of reasons for not entering smoking cessation programmes. Copeland et al. 49 developed and used the TBQ, specifically with low socioeconomic smokers in the USA, to assess barriers to entering smoking cessation programmes and to relate the barriers to demographics, stage of readiness to quit smoking and dependence.
As part of the Start2quit study we adapted the TBQ and distributed it to a sample of English smokers in order to explore the barriers to seeking help and support to quit in the UK, specifically to attending the English SSSs. The aims of the study were:
-
to identify perceived factors that prevent smokers from seeking help in a UK population
-
to examine the factor structure of the adapted TBQ and investigate whether or not it shows the same dimensions across different groups.
In a more detailed analysis, logistic regressions were conducted to determine the most important barriers and whether or not they differ according to demographic and smoking-related characteristics. However, this work was not within the remit of this report and a more extensive analysis and interpretation will be reported in a future output.
Method
Participants
Participants who completed the 6-month follow-up, by telephone or by post, and reported not attending the SSS (n = 2331) were asked for their reasons for non-attendance in an open question. Participants who completed the follow-up by telephone were also asked to complete a further postal questionnaire, the TBQ. Those who agreed (n = 1597) were mailed a TBQ to complete and return in a postage-paid envelope. Participants who did not reply after 2 weeks were sent a duplicate TBQ. A total of 758 completed TBQs were returned.
Measures
Participant characteristics were assessed at baseline: demographics (age, gender, marital status, ethnicity and education), social deprivation (IMD score) and socioenvironmental measures (living with other smokers), smoking history (age starting smoking, previous quit attempts), nicotine dependence (measured by number of cigarettes smoked daily and time from waking to first cigarette), intention to quit and motivation, determination and confidence to quit (all rated on a five-point Likert-type scale; 1 = not all, 5 = extremely). Previous attendance at the SSS and self-reported health problems associated with smoking were also included.
An open question asking for reasons for not trying to make an appointment with the SSS was included in the 6-month follow-up.
The TBQ was adapted from the 40-item TBQ developed by Copeland et al. 49 and previously validated in the USA, and comprised 36 items describing possible reasons why smokers do not enter SSS programmes. Items that did not apply to an English population (e.g. insurance coverage) were removed and British spelling was used throughout. The TBQ was piloted with SSS managers to test for appropriateness to the SSS. Participants responded to each item using a five-point Likert scale (1 = strongly disagree, 5 = strongly agree) (see Appendix 4).
Analysis
Frequencies and means were calculated for baseline characteristics of the sample. The proportion of missing data on the TBQ ranged from 0.3% to 15.6% per item (mean 3.2%). All 758 responses were entered into the analysis. Exploratory factor analysis using principal components analysis (PCA) with varimax rotation was conducted to investigate the underlying structure of the data. To determine the number of factors to retain, the scree criterion87 was applied. Items with factor loadings of at least 0.40 and with no cross-loadings were retained for rotation. Interitem reliability (coefficient alpha) was determined for each of the factors. The effect on alpha of deleting any one item was examined and any item that did not appear to substantially improve measurement was eliminated. The mean score of the remaining items that loaded onto each factor was calculated to construct a scale for each factor, and Pearson’s correlations were used to examine their inter-relationships.
Results
Reasons for not making an appointment with Stop Smoking Service
Of the 2133 responses to the open question asking for reasons for not trying to make an appointment with the SSS, 25.5% implied that they were not ready to quit or were unmotivated to do so, 20.7% indicated that they were too busy and had conflicting commitments, and 16.6% preferred to approach the task of quitting alone rather than use any kind of support. A further 9% were unsure of the support from the SSS or had previously tried to quit using the SSS.
Treatment Barriers Questionnaire response
Of the 1597 participants who reported not attending the SSS and were mailed a TBQ, 758 (47.5%) completed and returned the questionnaire. There were a number of differences overall between responders and non-responders to TBQ. Responders were significantly older (51.6 vs. 48.49 years; p < 0.001) and less likely to have qualifications to Advanced Level (39.2% vs. 29.7%; p < 0.001). They were less likely to be intending to quit in the next 30 days (31% vs. 40.9%; p < 0.001), and less motivated (3.56 vs. 3.7; p = 0.002) and less determined to quit (3.55 vs. 3.72; p < 0.001) than non-responders. They were also more likely to have quit in the past for > 1 month (62.2% vs. 53.5%; p = 0.003).
Participant characteristics
Demographic and smoking characteristics are shown in Table 20. Although participants were randomised 2 : 1 to the intervention and control groups, fewer participants in the control group attended the SSS; thus, respondents to the TBQ are almost equally divided between intervention and control groups (52% vs. 48%). The mean age of respondents was 51.6 years (SD 12.75 years), and 50.4% were female.
| Characteristic | Respondents |
|---|---|
| Intervention (%) | 394 (52) |
| Demographics | |
| Gender, n (%) | |
| Male | 376 (49.6) |
| Age (years) | |
| Mean (SD) | 51.6 (12.75) |
| Marital status, n (%) | |
| Married/living with spouse | 445 (58.7) |
| Highest qualification, n (%) | |
| A Level or higher | 288 (38) |
| Ethnic background, n (%) | |
| White | 735 (97) |
| Deprivation (IMD score), n (%) | |
| Mean IMD score (SD)a | 23.52 (14.19) |
| Quintile 1 | 102 (13.5) |
| Quintile 2 | 113 (14.9) |
| Quintile 3 | 181 (23.9) |
| Quintile 4 | 184 (24.3) |
| Quintile 5 | 174 (23.0) |
| Live with smokers, n (%) | |
| Yes | 236 (31.1) |
| Smoking characteristics | |
| Nicotine dependence score (0–6)b | |
| Mean (SD) | 2.46 (1.53) |
| Low (score = 1–2), n (%) | 333 (43.9) |
| Medium (score = 3), n (%) | 234 (30.9) |
| High (score = 4–6), n (%) | 184 (24.3) |
| Mean age started smoking (years), (SD) | |
| < 14 | 116 (15.3) |
| 14–16 | 377 (49.7) |
| > 16 | 265 (35.0) |
| Intention and motivation to quit | |
| When planning to quit, n (%) | |
| In next 2 weeks | 99 (13.1) |
| Next 30 days | 126 (16.6) |
| Next 6 months | 352 (46.4) |
| Not within 6 months | 149 (19.7) |
| Longest previous quit attempt, n (%) | |
| < 24 hours | 58 (7.7) |
| 1–6 days | 111 (14.6) |
| 1–4 weeks | 115 (15.2) |
| > 1 month | 468 (62.2) |
| Previously attended SSS, n (%) | |
| Yes | 262 (34.6) |
| ‘How much do you want to quit?’ (1 = not at all, 5 = extremely) | |
| Mean score (SD) | 3.56 (0.93) |
| ‘How determined are you to quit?’ (1 = not at all, 5 = extremely) | |
| Mean score (SD) | 3.55 (0.97) |
| ‘How confident are you that you can quit?’ (1 = not at all, 5 = extremely) | |
| Mean score (SD) | 2.58 (1.06) |
| Health | |
| Health problems (self-reported), n (%) | |
| Yes | 178 (23.5) |
Principal components analysis
The PCA with varimax rotation yielded seven factors as the most interpretable and explaining 53.06% of total variance. Thirty items were retained and the scales were named for their item content: (1) work and time constraints (five items, mean 2.65, SD 0.92); (2) smokers should quit on their own (six items, mean 2.64, SD 0.73); (3) nothing can help in quitting smoking (five items, mean 2.91, SD 0.76); (4) lack of interest in quitting (four items, mean 1.97, SD 0.69); (5) lack of social support to attend SSSs (four items, mean 1.70, SD 0.65); (6) lack of privacy at SSS programmes (two items, mean 2.84, SD 1.04); and (7) lack of information on SSS (four items, mean 2.73, SD 0.74). Coefficient alphas ranged from 0.85 to 0.63 for the seven scales. Table 21 displays the factor structure of the TBQ, coefficient alphas and the amount of total variance explained for each factor. Mean scores were calculated for each scale and interscale correlations are shown in Table 22.
| Scale (coefficient alpha reliability, total variance explained) | Loading |
|---|---|
| 1. Work and time constraints (α = 0.85, 14.81% of total variance) | |
| My work schedule would prevent me from attending a regularly scheduled programme | 0.846 |
| My work schedule is too hectic | 0.843 |
| I don’t have the time to commit to a programme | 0.801 |
| I can’t afford to spend my time that way | 0.722 |
| Those programmes are too time-consuming | 0.608 |
| 2. Smokers should quit on their own (α = 0.75, 9.93% of total variance) | |
| Any smoker can quit on his/her own if he/she puts his/her mind to it | 0.724 |
| I should be able to quit on my own | 0.711 |
| I can quit whenever I want to on my own | 0.692 |
| People shouldn’t need help in quitting smoking | 0.565 |
| Most smokers don’t need that kind of help to quit smoking | 0.537 |
| I plan to quit on my own soon | 0.536 |
| 3. Nothing can help in quitting smoking (α = 0.74, 8.19% of total variance) | |
| I’ve tried quitting smoking in the past, and just couldn’t do it | 0.674 |
| I will end up smoking again | 0.619 |
| I’ll just hear things I’ve heard over and over again about smoking | 0.615 |
| I don’t think I can quit smoking, regardless of what I do | 0.595 |
| I won’t learn anything new and helpful | 0.587 |
| 4. Disinterest in quitting (α = 0.70, 6.54% of total variance) | |
| I don’t want to give up smoking | 0.744 |
| I like smoking and don’t want to give up | 0.692 |
| I don’t think smoking is really that bad for me | 0.649 |
| I’m young and healthy and don’t need to quit right now | 0.595 |
| 5. Lack of social support to attend SSSs (α = 0.63, 5.08% of total variance) | |
| I can’t afford childcare | 0.797 |
| There is nobody who could watch my children | 0.733 |
| My health problems prevent me from getting out | 0.599 |
| My spouse/partner smokes and I wouldn’t want to quit without him/her | 0.420 |
| 6. Lack of privacy at SSS programmes (α = 0.70, 4.58% of total variance) | |
| Most programmes are conducted in groups and I am not comfortable meeting in a group | 0.744 |
| I wouldn’t want to talk about my smoking with total strangers | 0.735 |
| 7. Lack of information on SSSs (α = 0.68, 3.92% of total variance) | |
| I am not aware of any programmes in this area | 0.741 |
| There is no service near my home | 0.681 |
| I don’t know much about what programmes do to help smokers quit | 0.679 |
| I would need more information on specific programmes to make a decision whether or not I would attend | 0.612 |
| Scale | Scale | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1: work and time constraints | – | 0.15 | 0.16 | 0.08 | 0.22 | 0.22 | 0.23 |
| 2: smokers should quit on their own | – | –0.09 | 0.06 | 0.07 | 0.17 | 0.03 | |
| 3. nothing can help in quitting smoking | – | 0.43 | 0.17 | 0.25 | 0.16 | ||
| 4: lack of interest in quitting | – | 0.12 | 0.08 | 0.03 | |||
| 5: lack of social support to attend SSSs | – | 0.18 | 0.23 | ||||
| 6: lack of privacy at SSS programmes | – | 0.11 | |||||
| 7: lack of information on SSSs | – | ||||||
Discussion
Exploratory PCA to identify barriers to entering the SSS yielded a 30-item, seven-factor solution, accounting for 53.06% of the variance. Work and time constraints accounted for the highest proportion of variance (14.81%); the notion that smokers should quit on their own and that nothing can help in quitting smoking were the next highest (9.93% and 8.19%, respectively). Other barriers emerging were lack of interest in quitting, lack of social support to attend, lack of privacy at SSS programmes and a lack of information about the SSS.
The results of the PCA were similar to those reported by the developers of the original US questionnaire. 49 Both analyses extracted a seven-factor solution comprising very similar scales. ‘Work and time constraints’ and ‘smokers should quit on their own’ correspond to those found by Copeland et al. 49 ‘Opinions about professional assistance’ and ‘misinformation about professional assistance’ correspond to the combined and renamed ‘nothing can help in quitting smoking’, and this factor is more specific in representing the belief that support offered is ineffective. Although ‘preparedness to quit’ and ‘lack of interest in quitting’ equate and represent smokers not ready to quit, in our study lack of interest in quitting accounted for only 6.54%. This might be because all participants in the present study had already expressed an interest in quitting prior to taking part in the study. The remaining three factors were also similar but were renamed to be more appropriate for each scale. Responses to the open questions also support this interpretation. The most frequently given answers relate to the factors accounting for most of the variance, with the only difference being that ‘lack of interest in quitting’ has the highest frequency of responses.
The results were also consistent with previous research suggesting that time constraints, disbelief in the efficacy of programmes, lack of childcare and transportation, and lack of knowledge about smoking cessation and support programmes are the main perceived barriers to entering smoking cessation programmes. 16,43,82–86 Although other commitments are often put forward as a reason for not attending, the notion that motivation is up to them and support can only help them stay abstinent88 is prevalent. There is a need to challenge the commonly held perception that really wanting to quit is sufficient. 88 A culture change is necessary and health-care professionals need to become more proactive84 if use of support services globally is to be increased.
The size of the sample, more than adequate to meet the requirement of a factor analysis and large enough to allow cross-validation of the questionnaire,89 is a strength of this study. However, the smokers that participated in the present study were recruited for the larger study and were somewhat motivated to quit; therefore, the sample may not be fully representative of the wider population of smokers in England.
This analysis has shown the validity of the TBQ on an English population of smokers motivated to quit smoking following an explicit invitation to attend the SSS (either with risk information and taster session invitation or without). This validation of the TBQ will also enable the comparison of data across studies and populations. Delineating the most common reasons for not seeking treatment could lead to changes to the advertising of services or in information given to smokers by clinicians and could increase treatment seeking. 82
Chapter 5 Health economics
Introduction
A CEA was conducted alongside the Start2quit trial to establish the value for money of personal tailored risk information and taster sessions compared with the standard generic letter advertising the SSS.
The objectives of the CEA were:
-
to identify the resources used and costs associated with delivering the trial interventions
-
to measure the participants’ use of health and social care services
-
to compare the estimated mean cost per participant between the two treatment groups
-
to estimate the health benefits of the trial interventions using QALYs calculated from the EQ-5D questionnaire
-
to compare the cost difference between the two treatment groups with difference in effectiveness and generate incremental cost-effectiveness ratios (ICERs)
-
to test the uncertainty of the calculated ICERs using the bootstrapping method and to generate cost-effectiveness acceptability curves (CEACs) to demonstrate the probability of the intervention being cost-effective over and above the generic letter.
Methods
A full CEA was conducted following the methods of technology appraisal recommend by NICE. 50 The analysis was conducted on an intention-to-treat basis, in which all participants were analysed as randomised. The study was performed from the NHS/Personal Social Services perspective to reflect the English NHS decision-making framework, with costs expressed in UK pounds (£) at a 2012–13 price base. The follow-up for the analysis was 6 months from randomisation. All costs were inflated to 2012–13 price levels, when necessary, using the Hospital and Community Health Services pay and price inflation index. 90
Assessment of costs
A micro-costing approach was applied to estimate the costs of the two trial interventions. The costing methods involved three steps: (1) identifying the relevant cost items (identification), (2) measuring the use of the identified cost items (measurement) and (3) placing a value on these cost items (valuation). 91
Measurement of resource use
Health-care utilisation was systematically collected for each participant alongside the trial. Two main components of health-care resources were used in the trial.
First, the use of resources associated with the delivery of the trial interventions was recorded. This included staff time, consumables (such as postage and printing) and resources required for the training of advisors in the delivery of the taster sessions. Second, patients’ use of health and social care services was recorded using comprehensive service-use questionnaires, as used previously in a number of trials. Table 23 lists the resource items for the two trial interventions. Only resources directly related to the intervention in practice were included in the costing exercise. Resources that were used solely for the purpose of research were excluded.
| Stage | Group | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Cost items | Included | Cost items | Included | |
| Before randomisation | ||||
| Cost of screening for eligible smokers | GP time (2–5.5 hours per PCT) | No | GP time (2–5.5 hours per PCT) | No |
| Other practice staff | Yes | Other practice staff | Yes | |
| Cost of recruiting | Baseline questionnaire (printing) | Yes | Baseline questionnaire (printing) | No |
| Cover letter from GP (printing) | Yes | Cover letter from GP (printing) | No | |
| Postage (envelope, stamps) | Yes | Postage (envelope, stamps) | No | |
| Staff time to prepare and post the letter | Yes | Staff time to prepare and post the letter | No | |
| After randomisation: intervention : control = 3 : 2 | ||||
| Cost of intervention letters | Personalised tailored letter (printing) | Yes | Standard generic letter advertising the SSS (printing) | Yes |
| Postage (envelope, stamps) | Yes | Postage (envelope, stamps) | Yes | |
| Staff time to prepare and post the letter | Yes | Staff time to prepare and post the letter | Yes | |
| Cost of taster session | Training costs taster sessions (staff time, material, equipment, overheads) | Yes | N/A | N/A |
| Cost of providing taster sessions (staff time, material, equipment, overheads) | Yes | N/A | N/A | |
| Cost of SSS service taken up | Cost of SSS attendance (sessions × unit costs) | Yes | Cost of SSS attendance (sessions × unit costs) | Yes |
| Cost of other smoking cessation aids | Non-pharmacological smoking cessation aids | Non-pharmacological smoking cessation aids | ||
| GP visit | Yes | GP visit | Yes | |
| Practice nurse | Yes | Practice nurse | Yes | |
| Pharmacist | Yes | Pharmacist | Yes | |
| NHS Smoking Helpline | Yes | NHS Smoking Helpline | Yes | |
| Other smoking helpline | Yes | Other smoking helpline | Yes | |
| Internet | Yes | Internet | Yes | |
| Pharmacological smoking cessation aids | Pharmacological smoking cessation aids | |||
| NRT | Yes | NRT | Yes | |
| Bupropion | Yes | Bupropion | Yes | |
| Varenicline | Yes | Varenicline | Yes | |
| Cost of wider health-care resource use | GP visit | Yes | GP visit | Yes |
| Prescriptions | Yes | Prescriptions | Yes | |
| Day case | Yes | Day case | Yes | |
| Inpatient (cost per night) | Yes | Inpatient (cost per night) | Yes | |
| Outpatient attendance | Yes | Outpatient attendance | Yes | |
| A&E attendance | Yes | A&E attendance | Yes | |
Smokers aged ≥ 16 years were identified from their medical records by practice staff from participating practices. GPs from these practices then carried out further screening to exclude smokers deemed to be unsuitable for the research, such as those who were severely or terminally ill. GPs spent approximately 2.5 hours screening each practice’s smokers list for eligibility, but GP time was spent for research purposes and was not considered part of the intervention cost.
All eligible smokers were then sent a baseline questionnaire with a cover letter from their GP. A Freepost envelope was included for the return of the questionnaire to the practice. For non-respondents, a reminder and duplicate questionnaire was sent to the smoker 3 weeks after the first mailing. The cost components of the baseline questionnaire consisted of consumables such as printing, envelopes and postage, and staff time used to prepare and post the letters. It is noted that, although baseline questionnaires were sent to both trial groups, the cost was included only for the intervention group because the questionnaire was needed to gather information for generating the tailored letter. For the control group, generic letters would be sent to smokers directly after screening if implemented in the practice. Therefore, the cost of the baseline questionnaire was considered a research cost and excluded from the analysis.
Smokers who returned a completed baseline questionnaire, signed the consent form and were eligible to participate were randomised in a ratio of 3 : 2 to the intervention or the control group. The control group received a standard generic letter advertising the SSS and asking the smoker to contact the service to make an appointment to see an advisor. The relevant resource use includes stationery, mailing and staff time to prepare and post the letter.
In the intervention group, participants were sent a brief personalised and tailored letter based on their medical records and information provided in the returned baseline questionnaire. The resource used for generating the tailored letter includes consumables (printing, envelopes and postage), and staff time used to prepare and post the letters. The intervention letter also included an invitation and an appointment to attend a ‘Come and Try it’ taster session. Resource use associated with the taster session includes training and time of facilitators, equipment and venue overheads.
In addition to the intervention-related resource use, data on health resource use beyond the trial interventions were collected alongside the trial. These resources include the use of the SSSs, other smoking cessation aids and wider health-care resource use.
Numerical information on attendance at SSSs and the intervention type for each participant was provided by the SSS. Participants were asked at follow-up about the smoking cessation aids and other health-care services they had used in the previous 6 months. Smoking cessation aids included pharmacological (NRT, bupropion and varenicline) and non-pharmacological interventions (GP, nurse, pharmacist visits, smoking helplines). Wider health resource use consisted of primary health-care services (visits to primary care professionals) and secondary health-care services (hospital admissions and outpatient attendances).
Unit costs for resource use
The total costs of the identified resources were calculated using national unit costs from a range of published sources. The use of national unit costs can eliminate the difference in local costs among different sites and reflect the actual cost after generating the intervention to the real practice. 50 Table 24 shows unit costs employed to calculate the total cost per participant in the trial. The main sources from which unit costs were obtained include the Personal Social Services Research’s Unit Costs of Health and Social Care 2013,90 the NHS Reference Costs 2012–13,95 Prescription Cost Analysis England 201396 and the 2008 Smoking Cessation Services: Costing Report. 94
| Item | Unit | Cost (£) | Source |
|---|---|---|---|
| Smoking cessation aids | |||
| Non-pharmacological smoking cessation aids | |||
| GP visit | 10-minute brief advice session | 40 | Curtis90 |
| Practice nurse | 10-minute brief advice session | 7 | Curtis90 |
| Pharmacist | 10-minute brief advice session | 11 | Curtis90 |
| NHS Smoking Helpline | £5.90 per call at 2008–9 price | 6.40 | Wu et al.92 |
| Other smoking helpline | £5.90 per call at 2008–9 price | 6.40 | Wu et al.92 |
| Pharmacological smoking cessation aids | |||
| NRT | Per prescription item | 21 | Health and Social Care Information Centre93 |
| Bupropion | Per prescription item | 38 | Health and Social Care Information Centre93 |
| Varenicline | Per prescription item | 34 | Health and Social Care Information Centre93 |
| NHS SSSs | |||
| Group session | Per person per session | 4.60 | NICE94 |
| Individual session | Per person per session | 17 | NICE94 |
| Telephone | £5.90 per call at 2008–9 price | 6.40 | Wu et al.92 |
| Drop-in | Per person per session | 17 | NICE94 |
| Couple/family | Per person per session | 8.50 | NICE94 |
| Wider health-care resource use | |||
| GP visit | Visit (average 11.7 minutes) | 37 | Curtis90 |
| Practice nurse visit | Visit (average 15.5 minutes) | 11 | Curtis90 |
| Day case | FCE | 693 | Department of Health95 |
| Inpatient (cost per night) | Per bed-night | 542 | Department of Health95 |
| Outpatient attendance | FCE | 108 | Department of Health95 |
| A&E attendance | FCE | 114 | Department of Health95 |
Health outcome measures
In addition to the clinical outcome measures used in the statistical analysis (the uptake of SSS and quit rates), health benefits were measured in QALYs in the economic evaluation. Unlike clinical outcomes such as quit rates, which can only be compared between smoking interventions, QALYs are a standard and internationally recognised method enabling comparisons across different health-care programmes. QALYs take into account both quantity and health-related quality of life and are recommended by NICE technology appraisal guidance. 50
In this study, QALYs were calculated as the area under the curve, following the trapezium rule, using utility scores measured by EQ-5D questionnaires at baseline and at the 6-month follow-up. 51 An EQ-5D questionnaire is a self-complete questionnaire that evaluates participants’ quality of life on five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has three levels, indicating no problem, some or a moderate problem and a major problem. Based on their combined answers to the five questions, participants could be classified as being in 1 of 245 possible health states (the 243 states arising from the EQ-5D, plus unconscious and deceased). A preference-based index utility score was derived for each participant’s response to the EQ-5D questionnaire using the UK population tariff calculated by a time trade-off method. 97,98 Perfect health has a utility value of ‘1’ and death was assigned a value of ‘0’; some health states may be considered worse than death and have negative scores.
Cost-effectiveness analysis
To assess the relative cost-effectiveness of the two trial interventions, we employed an incremental CEA to compare the mean difference in costs with the mean difference in health benefits. The ICER was used to combine costs and health benefits in a single measure to which a decision rule for cost-effectiveness can be applied:99
where E represents the change in effects and C represents the costs of intervention, measured in monetary units, and subscripts ‘I’ and ‘C’ refer to intervention and control arm, respectively. In this study, three sets of ICER were conducted using the three outcome measures using the following formulas.
-
Cost per additional attendee to the SSS:
(4)ICER1=ΔCΔE=(Average cost of the tailored letter +average cost of the taster session)−average cost of the generic letterSSS attendance rateI − SSS attendance rateC.
-
Cost per additional quitter at 6 months:
(5)ICER2=ΔCΔE=(Average cost of the tailored letter +average cost of the taster session +average SSS costI + health-care costI)−(Average cost of the generic letter +average SSS costC + health-care costC)Quit rate at 6 monthsI−Quit rate at 6 monthsC.
-
Cost per additional QALY gained at 6 months:
(6)ICER3=ΔCΔE=(Average cost of the tailored letter +average cost of the taster session +average SSS costI + health-care costI)−(Average cost of the generic letter +average SSS costC + health-care costC)QALY gain at 6 monthsI−QALY gain at 6 monthsC.
Uncertainty assessment
A feature of CEA is the uncertainty that exists around the ICER. In this study, uncertainty around the decision to adopt the intervention is assessed through a non-parametric bootstrap resampling technique. Non-parametric bootstrap is a method that resamples observations from the original samples. 93 Bootstrapping has been proposed as an efficient approach for calculating the confidence limits for the ICER, as its validity does not depend on any specific form of underlying distribution. 100–103 We performed the bootstrap replications 5000 times; for each bootstrapped resample, an estimate of the differential costs and QALYs was calculated and 95% CIs for the ICERs were constructed based on the bootstrapping results. 101,102 In addition, cost-effectiveness planes (CEPs) and CEACs were plotted based on the outcomes of the bootstrap iterations. 104
Handling missing data
Missing data are a common and considerable problem in most economic evaluations alongside clinical trials because of the high missing rate of the health-care resource-use questionnaires. In this study, missing data were handled using Rubin’s multiple imputation method. 99,105–107 We assumed any missing data to be missing at random when the probability of the data being missing can depend on the observed values of the individual but not on the missing variable values of the individual. A total of 50 imputed data sets were generated to ensure efficient and reproducible estimates.
Sensitivity analysis
Besides the multiple imputation analysis, a sensitivity analysis was undertaken to repeat the CEA using complete cases, whereby the results are analysed only for those participants who had both completed cost and outcome data at the same time. Because the intervention of interest in this study was a multicomponent intervention, the costs and effects of each intervention element were explored as the second part of the sensitivity analysis.
Long-term costs and outcomes predictions
The within-trial analysis shows the short-term cost-effectiveness of the interventions. However, in common with many preventative interventions, many studies on smoking cessation have demonstrated that the majority of benefits are gained from the reduced risk of developing smoking-related diseases, such as lung cancer, myocardial infarction, COPD and stroke, and the reduced health-care costs and improved health-related quality of life it brings over a longer time period. 108,109 These benefits may not be evident until later in life; hence, the 6-month follow-up period of the trial is not sufficient to capture the real value of the smoking interventions. 99,107,110 Therefore, in this study, besides the within-trial analysis, we included an estimate of lifetime health-care cost savings and QALY gains associated with the two interventions.
The method employed in this study’s long-term CEA was based on an economic model that was developed to estimate smoking-related costs and consequences in adults in England. 52 This is a Markov model that simulated the pathway between smoking and four main smoking-related conditions (lung cancer, myocardial infarction, COPD and stroke) and evaluated the related costs of current smokers, ex-smokers and never-smokers. However, this study only reported the cost savings from quitting smoking. In the absence of estimated lifetime health outcomes, we combined and utilised another English study by Vogl et al. ,111 which reported health-related quality of life by smoking status.
Both future costs and health outcomes were discounted at 3.5% per annum when necessary, in line with the NICE guidelines for the technology appraisal methods. 50 Similar to the short-term CEA, ICERs were calculated and the uncertainty of the calculated ICERs were tested using the bootstrapping method.
Results
A total of 4384 participants were recruited to the trial. One participant withdrew from the study, leaving 4383 participants analysed in the economic evaluation: 2636 in the intervention group and 1748 in the control group (see Figure 6). The base-case CEA was based on a multiple-imputed data set, in which all the missing values were imputed using the multiple imputation method.
Costs
Intervention costs reflect the value of resources required to deliver the trial interventions. Table 25 summarises the average cost of each intervention component for both treatment groups. The cost of screening was £0.30 per person recruited into the trial. For the intervention group, the cost of the baseline questionnaire used to gather information for generating the tailored invitation letter was £32 per person. The average costs of the tailored and generic invitation letter were £13 and £0.60 per participant, respectively. The average cost of the taster session was £27 per person per session; this included £0.10 for the training manual and £26 to provide the session. Four participants in the control group attended the taster sessions; the costs of these sessions were included in the control group according to the intention-to-treat principle.
| Intervention component | Group | |
|---|---|---|
| Intervention | Control | |
| Screening | £0.30/participant | £0.30/participant |
| Baseline questionnaire | £32/participant | N/A |
| Tailored letter | £13/participant | N/A |
| Generic letter | N/A | £0.60/participant |
| Taster sessions | ||
| Training manual | £0.10/session | £0.10/session |
| Providing taster sessions | £26/session | £26/session |
Table 26 and Figure 15 show the mean cost for the interventions and the subsequent use of health services over the 6-month follow-up period. The mean intervention cost in the intervention group was £54 (SD £12) per participant, whereas the corresponding average cost in the control group was only £0.90 (SD £2) per participant. Costs associated with smoking cessation, including use of SSSs, and pharmacological and non-pharmacological cessation aids, were significantly higher in the intervention group than in the control group. The wider health resource-use cost was also higher in the intervention group, but the difference here is insignificant.
| Resource category | Group (£), mean cost (SD) | Differencea (£) (95% CI) | |
|---|---|---|---|
| Intervention (n = 2635) | Control (n = 1748) | ||
| Intervention cost | 54 (12) | 0.90 (1) | 53 (52 to 53) |
| SSS attendance cost | 11 (34) | 5 (23) | 6 (4 to 8) |
| Non-pharmacological cessation aids | 44 (32) | 40 (26) | 4 (2 to 6) |
| Pharmacological cessation aids | 61 (49) | 50 (32) | 10 (8 to 13) |
| Wider health-care resource-use cost | 608 (2175) | 583 (1860) | 25 (–99 to 149) |
| Total cost | 777 (2176) | 679 (1860) | 98 (–26 to 222) |
| Adjusted total costb | 760 (2039) | 669 (2059) | 92 (–32 to 216) |
FIGURE 15.
Cost difference between two intervention groups.
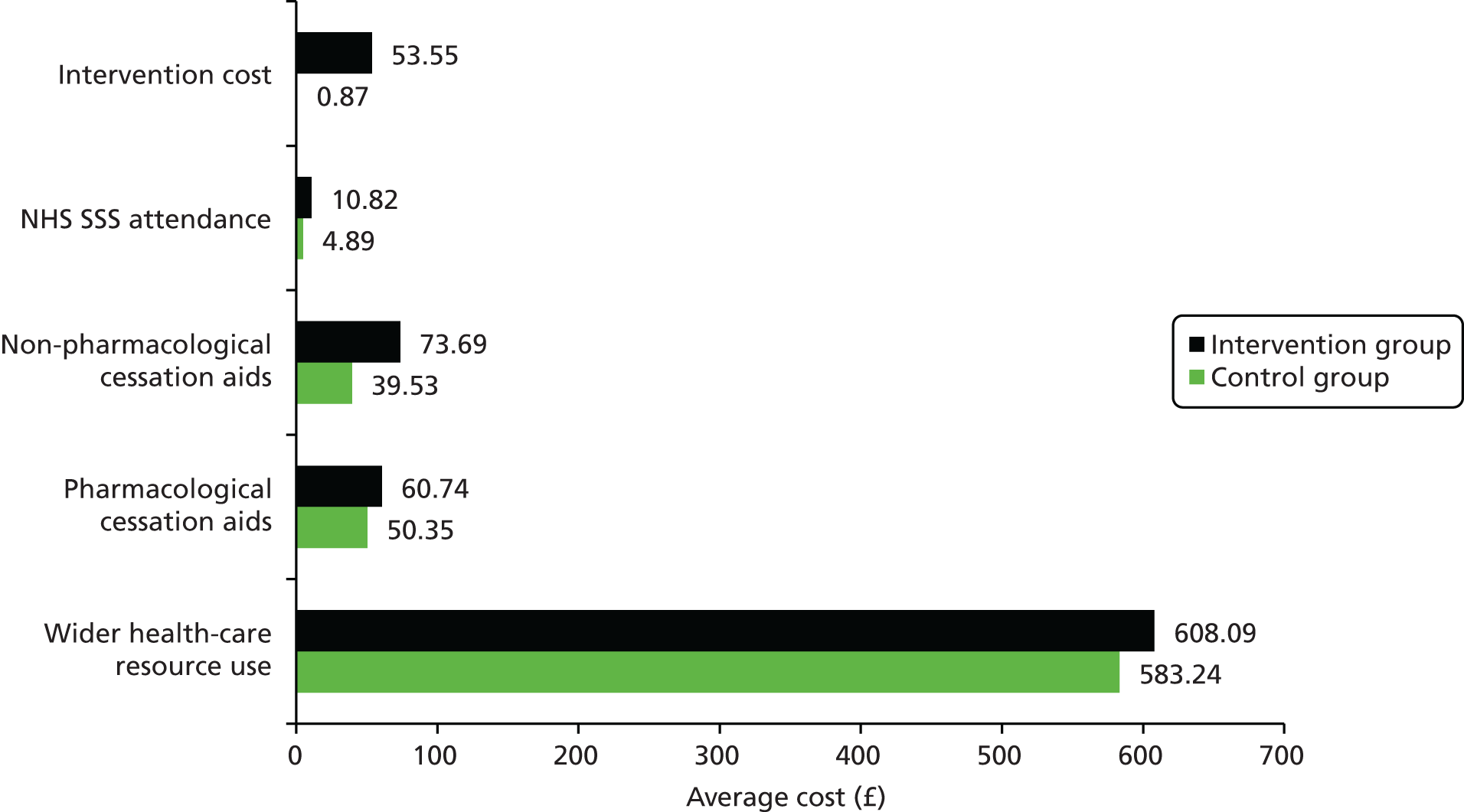
The estimated total mean costs over the 6-month follow-up period were £777 (SD £2176) in the intervention group and £679 (SD £1860) in the control group. Participants’ average cost relies heavily on their wider health resource use over the past 6 months. The cost difference between the two groups was £98 (95% CI –£26 to £222). After adjusting for baseline resource use, the adjusted cost difference was £92 (95% CI –£32 to £216). The 95% CIs indicate that the total cost differences were not statistically significant between the two treatment groups.
Outcomes: utility and quality-adjusted life-years
The primary health economic outcome was QALY gains over 6 months, estimated using the EQ-5D. Mean EQ-5D scores in each intervention group at both baseline and the 6-month follow-up are reported in Table 27. At baseline, the estimated between-group difference (intervention – control) in EQ-5D score was –0.004. The results show that mean EQ-5D scores were improved over the study period for both trial arms. The estimated between-group difference (intervention – control) in EQ-5D score was 0.004 (95% CI –0.015 to 0.030) at 6-month follow-up. This indicates that compared with the control group, the mean EQ-5D score was lower in the intervention group at baseline, whereas after 6 months the mean EQ-5D score was higher in the intervention group.
| Outcome | Group, mean (SD) | Differencea (95% CI) | |
|---|---|---|---|
| Intervention (n = 2635) | Control (n = 1748) | ||
| Baseline EQ-5D scores | 0.751 (0.304) | 0.755 (0.292) | –0.004 (–0.022 to 0.014) |
| 6-month follow-up EQ-5D scores | 0.771 (0.311) | 0.76 (0.312) | 0.004 (–0.015 to 0.030) |
| QALY | 0.381 (0.141) | 0.380 (0.136) | 0.0001 (–0.009 to 0.009) |
| Adjusted QALYb | 0.382 (0.046) | 0.3802 (0.046) | 0.0015 (–0.001 to 0.004) |
Based on the EQ-5D scores, at both the baseline and the 6-month follow-up, QALYs were calculated and reported in Table 27. The number of unadjusted average QALYs per participant over the 6 months was 0.0001 (95% CI –0.008 to 0.009), that is, they were higher in the intervention group than in the control group. After adjustment for baseline utility scores, the results demonstrate that QALY gains were slightly higher in the intervention group compared with the control group (the difference in QALYs was 0.002, 95% CI –0.001 to 0.004). However, the QALY differences between treatment groups were not statistically significant.
Cost-effectiveness analysis and uncertainty
We conducted three sets of cost-effectiveness analyses using three outcome measures in this study. The results are reported as incremental cost per outcome of interest, cost per additional attendee to the SSS, cost per additional quitter and cost per QALY. The results of the analyses are presented in Tables 28–30.
| Outcome | Group | |
|---|---|---|
| Intervention (N = 2635) | Control (N = 1748) | |
| Intervention cost, mean (SD) | £54 (£12) | £0.90 (£1) |
| Cost differencea (95% CI) | £53 (£52 to £53) | |
| Attendance at SSS, n (%) | 458 (17.4) | 158 (9.0) |
| OR (95% CI) | 2.12 (1.75 to 2.57); p < 0.001 | |
| ICER1 (cost per additional person attend SSS) (95% CI) | £627 (£620 to £634) | |
| Smoking outcome | Group, n quitter (%) | Differencea | ||
|---|---|---|---|---|
| Intervention (N = 2635) | Control (N = 1748) | |||
| OR (95% CI) | ICER2 (cost per additional quitter), £ (95% CI) | |||
| 24-hour point prevalent abstinence (self-reported) | 445 (16.9) | 201 (11.5) | 1.57 (1.31 to 1.88) | 1700 (–602 to 4001) |
| 7-day point prevalent abstinence (validated) | 236 (9.0) | 97 (5.6) | 1.68 (1.32 to 2.15) | 2689 (–952 to 6329) |
| 7-day point prevalent abstinence (self-reported) | 424 (16.1) | 187 (10.7) | 1.61 (1.34 to 1.94) | 1699 (–601 to 3998) |
| 1-month prolonged abstinence (self-reported) | 357 (13.6) | 151 (8.6) | 1.67 (1.36 to 2.04) | 1866 (–660 to 4392) |
| 3-month prolonged abstinence (validated) | 150 (5.7) | 60 (3.4) | 1.70 (1.25 to 2.31) | 4053 (–1435 to 9541) |
| 3-month prolonged abstinence (self-reported) | 240 (9.1) | 103 (5.9) | 1.61 (1.26 to 2.04) | 2849 (–1008 to 6706) |
| Outcome | Group | Differencea (95% CI) | |
|---|---|---|---|
| Intervention (n = 2635) | Control (n = 1748) | ||
| Total cost,b mean (SD) | £777 (2176) | £679 (1860) | £98 (–£26 to £222) |
| QALY,b mean (SD) | 0.381 (0.141) | 0.380 (0.136) | 0.0001 (–0.009 to 0.008) |
| ICER (cost per QALY gained) (95% CI)b | £862,629 (–£742,154 to £1,159,241) | ||
| Adjusted total cost,c mean (SD) | £760 (£2039) | £669 (£2059) | £92 (–£32 to £216) |
| Adjusted QALY,c mean (SD) | 0.382 (0.046) | 0.380 (0.046) | 0.002 (–0.001 to 0.004) |
| ICER (cost per QALY gained) (95% CI)c | £59,401 (–£604,833 to £644,486) | ||
First, after participants received the invitation letter and taster session, 458 (17.4%) smokers in the treatment group and 158 (9.0%) smokers in the control group attended the SSS (see Table 28). The average costs incurred in the intervention group and control group were £54 and £0.90, respectively, resulting in an ICER of £627 per additional attendee to the SSS. The ICER indicates that, when comparing the tailored letter and taster sessions with the generic letter, a cost of £627 was incurred to enable one more smoker to take up the SSS.
Second, combining differential costs of the treatment groups with the differential quit rates at the 6-month follow-up, the ICERs are listed in Table 29. The main quit rate used in this trial was biochemical validation of 7-day abstinence; the corresponding ICER was £2689 (95% CI –£952 to £6329) per additional quitter. This means that if the decision-makers were willing to pay more up to £2689 for an additional quitter, the tailored letter and taster session would be the preferred option; otherwise, usual care should be adopted. ICERs using other periods of abstinence measures (from 24-hour self-reported point prevalence to 3-month-validated prolonged abstinence) are also reported in Table 29. The cost per additional quitter ranges from £1699 to £4053 using the multiple imputation data set.
Third, we compared the effectiveness of the two interventions using QALYs as health outcome measures (see Table 30). It was found that the intervention was associated not only with a slight gain in QALYs following adjustment for baseline EQ-5D, but also with higher costs than the control condition. This generates an ICER of £59,401 (95% CI –£604,833 to £644,486) per QALY gained.
However, the differences in both QALYs and costs are not statistically significant, indicating that there may be some uncertainty surrounding the ICERs. We employed the non-parametric bootstrapping method to investigate the uncertainty over mean differences. A scatterplot of the 5000 bootstrapped incremental mean costs and mean QALYs pairs is presented on the CEP (Figure 16). The horizontal axis divides the plane according to incremental cost (positive above, negative below) and the vertical axis divides the plane according to incremental effect (positive to the right, negative to the left). 99 The axes divide the plane into four quadrants through the origin; in the south-east quadrant, the intervention group is less costly and more effective than the standard letter and the intervention is considered more cost-effective. In the north-west quadrant, the opposite is true and the control is more cost-effective, whereas in the north-east and south-west quadrants, the decision depends on the maximum willingness-to-pay threshold. The slope of the two lines in Figure 16 shows the NICE range of decision-making thresholds: £20,000–30,000 per additional QALY gained. The proportion of the scatterpoints that fall to the south and east of the willingness-to-pay threshold line is the probability that the intervention is more cost-effective than the standard letter. Using the results of the bootstrapped replicates, we also generated a CEAC (Figure 17), which provides a plot of probabilities that the intervention is being cost-effective (y-axis) against all potential values of willingness-to-pay thresholds (x-axis).
FIGURE 16.
Cost-effectiveness plane (multiple imputation analysis).
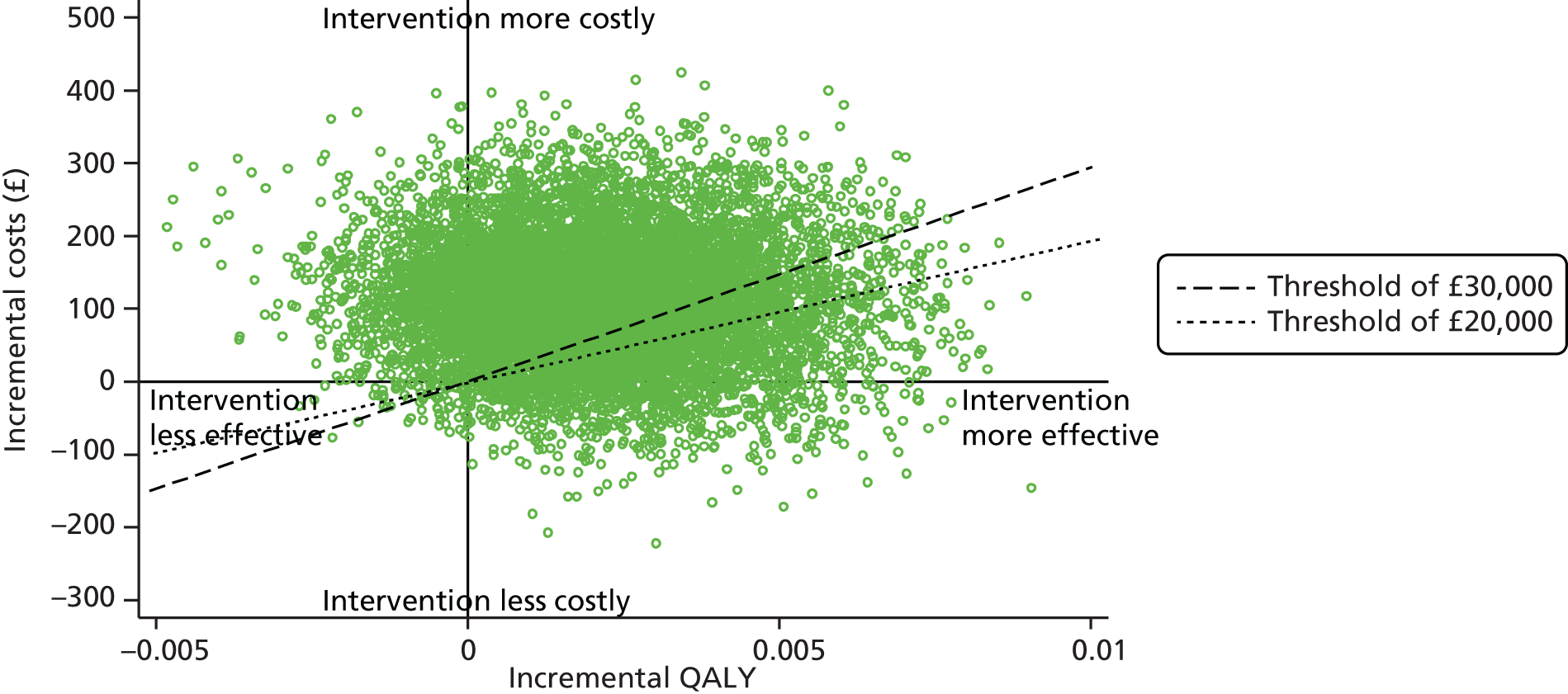
FIGURE 17.
Cost-effectiveness acceptability curve (multiple imputation analysis).

In Figure 16, the majority of the plots in the CEP fall in the north-east quadrant, indicating that the intervention is likely to be more effective, but also more expensive, than the control. The CEAC (see Figure 17) illustrates that, when using the NICE decision-making threshold range of £20,000–30,000 per QALY gained, the tailored letter and taster session has a 20–27% probability of being considered cost-effective at 6 months. Only at higher willingness-to-pay thresholds (>£59,401 per QALY) does the intervention becomes more likely to be cost-effective than the control.
Sensitivity analysis (complete-case analysis)
In order to explore the potential impact of missing data on estimated treatment effects and costs, a sensitivity analysis was conducted based on the complete cases. In this trial, completed costs and outcome data at the same time were available for 2775 participants (63% of all participants), 1667 in the intervention group and 1108 in the control group. The number and percentage of missing data with regard to each cost component are summarised in Table 31. Table 31 also shows the mean costs of the complete cases by treatment groups and resource categories. Tables 32–34 list the results of the CEA using 2775 complete cases.
| Resource category | Group | Difference,b (£) (95% CI) | |||
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Missing, n (%) | Average cost (£),a mean (SD) (n = 1667) | Missing, n (%) | Average cost (£),a mean (SD) (n = 1108) | ||
| Intervention cost | 0 (0) | 55 (13) | 0 (0) | 0.90 (2) | 54 (53 to 55) |
| SSS attendance cost | 0 (0) | 13 (36) | 0 (0) | 5 (24) | 8 (5 to 10) |
| Non-pharmacological cessation aids | 902 (34) | 22 (47) | 585 (33) | 17 (39) | 5 (1 to 8) |
| Pharmacological cessation aids | 752 (29) | 30 (70) | 467 (27) | 16 (47) | 14 (10 to 19) |
| Wider health-care resource-use cost | 903 (34) | 727 (2481) | 586 (34) | 685.42 (2265) | 42 (–140 to 224) |
| Total cost | 968 (37) | 846 (2484) | 640 (37) | 724 (2268) | 122 (–60 to 305) |
| Adjusted total cost | 968 (37) | 851 (2455) | 640 (37) | 724 (2465) | 127 (–60 to 314) |
| Complete casesa | Group | |
|---|---|---|
| Intervention (N = 1667) | Control (N = 1108) | |
| Intervention cost, mean (SD) | £55 (£13) | £0.90 (£2) |
| Differenceb (95% CI) | £54 (£53 to £55) | |
| Attendance at SSS, n (%) | 334 (20.04) | 102 (9.21) |
| OR (95% CI) | 2.47 (1.94 to 3.16); p < 0.001 | |
| ICER1 (cost per additional attendee to the SSS) (95% CI) | £498 (£491 to £504) | |
| Complete casesa | Group | Differenceb (95% CI) | ||
|---|---|---|---|---|
| Intervention (N = 1667) | Control (N = 1108) | |||
| Adjusted total cost, mean (SD) | £851 (£2455) | £724 (£2465) | £127 (£60 to £314) | |
| Smoking outcome | Quitter, n (%) | Quitter, n (%) | OR (95% CI) | ICER2 (cost per additional quitter), £ (95% CI) |
| 24-hour point prevalent abstinence (self-reported) | 383 (22.98) | 168 (15.16) | 1.67 (1.36 to 2.05) | 1629 (–765 to 4022) |
| 7-day point prevalent abstinence (validated) | 214 (12.84) | 87 (7.85) | 1.73 (1.32 to 2.27) | 2552 (–1199 to 6303) |
| 7-day point prevalent abstinence (self-reported) | 365 (21.90) | 157 (14.17) | 1.70 (1.38 to 2.10) | 1647 (–773 to 4067) |
| 1-month prolonged abstinence (self-reported) | 303 (18.18) | 126 (11.37) | 1.73 (1.37 to 2.18) | 1870 (–878 to 4618) |
| 3-month prolonged abstinence (validated) | 135 (8.10) | 51 (4.60) | 1.83 (1.30 to 2.60) | 3640 (–1710 to 8990) |
| 3-month prolonged abstinence (self-reported) | 204 (12.24) | 82 (7.40) | 1.74 (1.33 to 2.31) | 2631 (–1236 to 6497) |
| Complete casesa | Group | Differenceb (95% CI) | |
|---|---|---|---|
| Intervention (n = 1667) | Control (n = 1108) | ||
| Total cost,c mean (SD) | £846 (£2484) | £724 (£2268) | £122 (–£60 to £305) |
| Adjusted total cost,d mean (SD) | £851 (£2455) | £724 (£2465) | £127 (£60 to £314) |
| Baseline EQ-5D scores, mean (SD) | 0.7569 (0.3054) | 0.763 (0.2962) | –0.0061 |
| 6-month follow-up EQ-5D scores, mean (SD) | 0.7751 (0.3108) | 0.7677 (0.3112) | 0.0073 (–0.0163 to 0.0310) |
| QALY,c mean (SD) | 0.383 (0.1437) | 0.3827 (0.1422) | 0.0003 (–0.0106 to 0.0112) |
| ICER,c mean (95% CI) | £407,933 (–£418,734 to £591,198) | ||
| Adjusted QALY,d mean (SD) | 0.3841 (0.0524) | 0.3815 (0.0526) | 0.0026 (0.04365 to 0.0553) |
| ICER,d mean (95% CI) | £49,842 (–£425,064 to £536,813) | ||
To make it easier to compare the primary analysis and sensitivity analysis, Table 35 summarises the main results of the two analyses. When excluding participants with incomplete data, there is little change in the results. The results show that, for both treatment groups, the average costs were slightly higher in the complete cases, and the gains in effectiveness increased slightly too. The ICERs of the complete-case analyses were estimated at £498 (95% CI £491 to £504) per additional attendee to the SSS, £2552 (95% CI –£1199 to £6303) per additional quitter and £49,842 (95% CI –£425,064 to £536,813) per QALY gained when comparing the intervention with the control group.
| Outcome | Analysis | |||
|---|---|---|---|---|
| Multiple imputation | Complete case | |||
| Intervention (N = 2635) | Control (N = 1748) | Intervention (N = 1667) | Control (N = 1108) | |
| Intervention cost, mean (SD) | £54 (£12) | £0.90 (£1) | £55 (£13) | £0.90 (£2) |
| Attendance at SSS, n (%) | 458 (17.4) | 158 (9.0) | 334 (20.04) | 102 (9.21) |
| ICER1 (cost per additional attendee to the SSS) (95% CI) | £627 (£620 to £634) | £498 (£490 to £504) | ||
| Adjusted total cost, mean (SD) | £760 (£2039) | £669 (£2059) | £851.03 (£2455) | £724 (£2465) |
| Cost difference (95% CI) | £92 (–£32 to £216) | £127 (–£60 to £314) | ||
| 7-day point prevalent abstinence, n (%) (validated) | 236 (8.96) | 97 (5.55) | 214 (12.84) | 87 (7.85) |
| ICER2 (cost per additional quitter) (95% CI) | £2689 (–£952 to £6329) | £2552 (–£1199 to £6303) | ||
| Adjusted QALY, mean (SD) | 0.382 (0.046) | 0.38 (0.046) | 0.384 (0.052) | 0.382 (0.05) |
| QALY difference (95% CI) | 0.0015 (–0.001 to 0.004) | 0.003 (–0.044 to 0.055) | ||
| ICER3 (cost per QALY gained) (95% CI) | £59,401 (–£604,833 to £644,486) | £49,842 (–£425,064 to £536,813) | ||
Similar to the multiple imputation analysis, we re-ran the bootstrapping approach to generate the CEP (Figure 18) and CEAC (Figure 19). The probability that the intervention would be considered more cost-effective than the control at different decision thresholds was 24% at £20,000 and 34% at £30,000 per QALY gained. The results of the complete-case analysis have been shown to be consistent with the results of the multiple imputation analysis at the end of the 6-month follow-up.
FIGURE 18.
Cost-effectiveness plane (complete-case analysis).
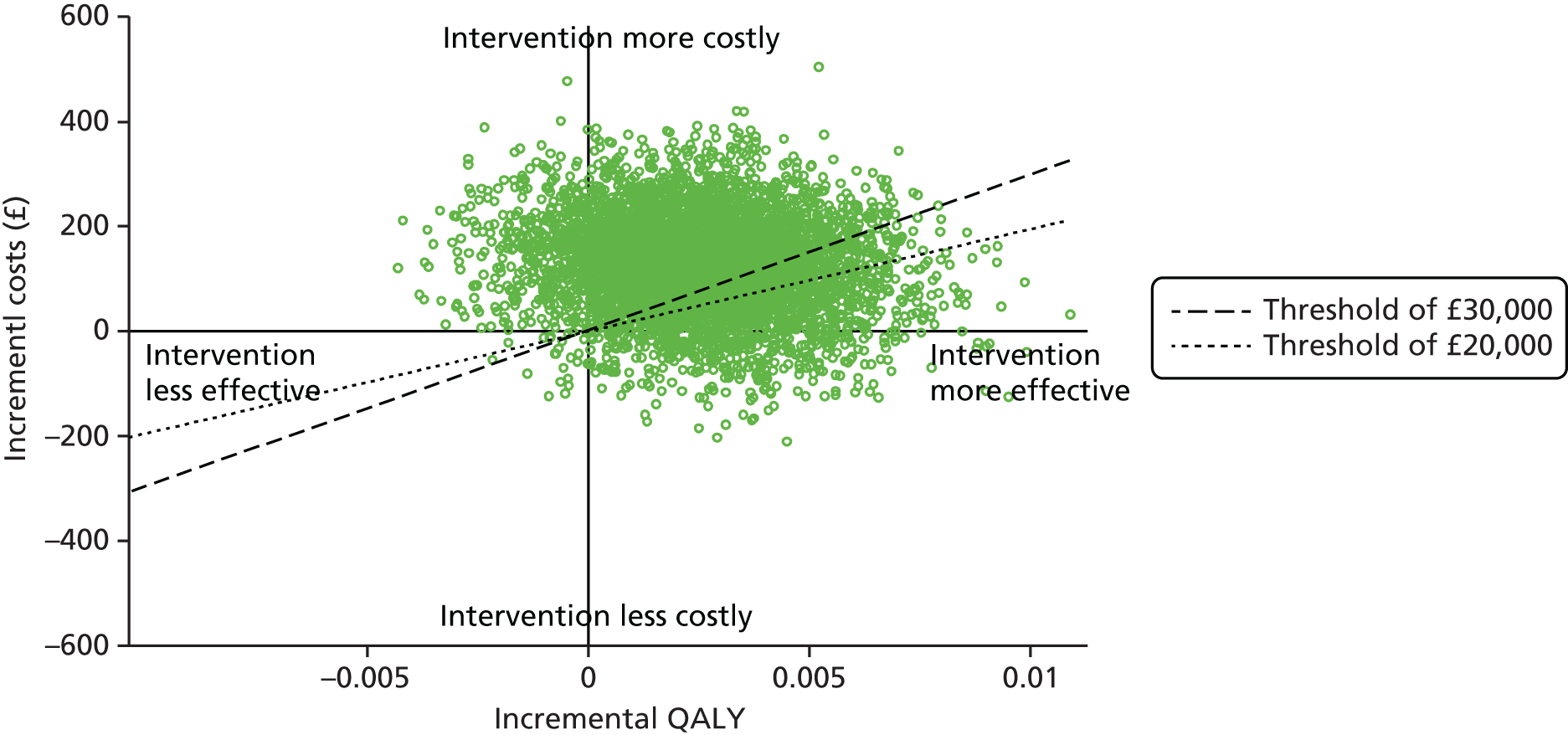
FIGURE 19.
Cost-effectiveness acceptability curve (complete-case analysis).
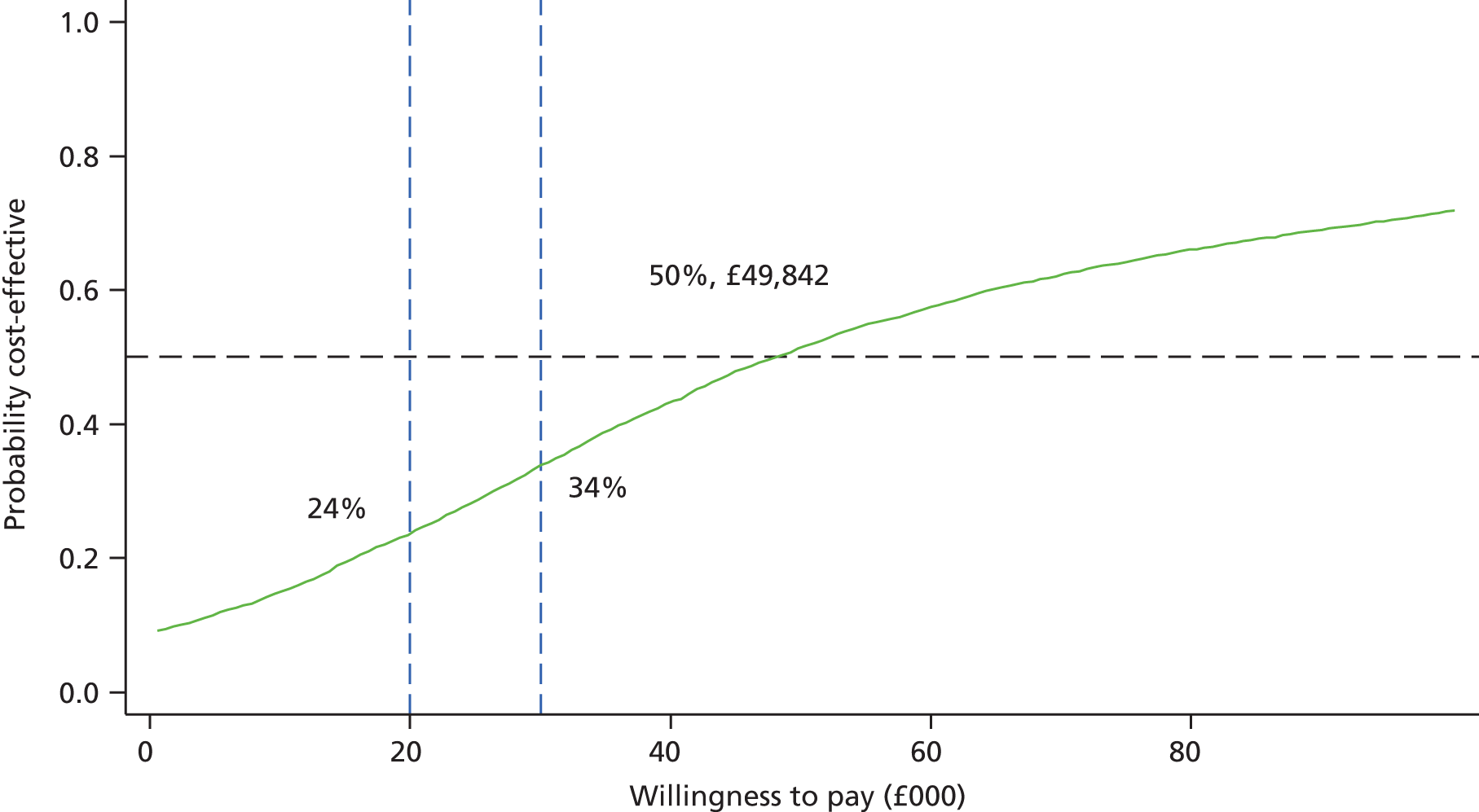
Sensitivity analysis (intervention components)
The intervention designed in this trial consists of more than one component. We broke down the costs and effects of each component to explore the most cost-effective part of the intervention in terms of increasing the SSS attendance. The 4383 participants were divided into three groups according to the treatment they received: 1748 received the standard generic letter, 1896 received a personal tailored letter and 739 participants attended taster sessions after they received the tailored letter.
Table 36 reveals the results of the CEA comparing the three groups for the three outcomes. When there are more than two strategies involved in an incremental CEA, ICERs were calculated following three steps. First, the alternatives were ranked from the cheapest to the most expensive choice. Second, strategies that were more costly, but also less effective than any previous strategy, were excluded from the ICER calculation (known as dominance). Third, and finally, the ICERs were recalculated for the remaining strategies, from the least to most expensive.
| Intervention received | Number of participants | Mean intervention cost (£) (SD) | Attended SSS, n (%) | ICER1 (£) |
|---|---|---|---|---|
| Generic letter only | 1748 | 0.87 (1) | 158 (9.0) | – |
| Tailored letter only | 1896 | 46 (0) | 120 (6.3) | Dominated |
| Tailored letter plus taster session | 739 | 73 (0) | 338 (45.7) | 196 |
| Intervention received | Number of participants | Adjusted mean total cost at 6 months (£) (SD) | Validated 7-day point prevalent abstinence, n (%) | ICER2 (£) |
| Generic letter only | 1748 | 669 (2059) | 97 (5.6) | – |
| Tailored letter plus taster session | 739 | 669 (1430) | 137 (18.5) | 6 |
| Tailored letter only | 1896 | 796 (2105) | 99 (5.2) | Dominated |
| Intervention received | Number of participants | Adjusted mean total cost at 6 months (£) (SD) | Adjusted QALY at 6 months (SD) | ICER3 (£) |
| Generic letter only | 1748 | 669 (2059) | 0.380 (0.046) | – |
| Tailored letter plus taster session | 739 | 669 (1430) | 0.383 (0.031) | 300 |
| Tailored letter only | 1896 | 796 (2105) | 0.381 (0.046) | 63,400 |
The first part of the results shows that smokers who received only the personal tailored letter but did not attend the taster session were least likely (6.3%) to take up the SSS, with an average cost of £46 per participant (see Table 36). Therefore, the tailored letter was dominated by the standard generic letter, which only cost £0.87 per person, with 9.0% of these attending the SSS. The results indicate that about half (45.7%) of smokers who received the taster session followed by a tailored letter attended the SSS. The ICER of comparing the tailored letter plus the taster session to generic letter was estimated as £196 per additional attendee to the SSS.
Similarly, using validated 7-day abstinence as an outcome, the tailored letter was dominated by the tailored letter plus taster session, as it is more costly and less effective (see Table 36). Comparing the tailored letter plus taster session with the generic letter, the ICER was as low as £6 per additional quitter. When the QALY was used as the outcome measure, the ICER was estimated at £300 per QALY gained comparing tailored letter plus taster session with the generic letter. When comparing the tailored letter alone with the tailored letter plus taster session, the ICER was £63,400 per QALY gained. Given the NICE decision-making thresholds range of £20,000–30,000 per QALY gained, interventions below the lower threshold will be acceptable as a cost-effective intervention, and interventions that are above the upper threshold will be rejected. Therefore, the tailored letter plus taster session is considered as the optimal strategy among the three alternatives in the sensitivity analysis.
Long-term costs and outcomes predictions
The CEA extrapolated to the lifetime time horizon used QALYs as the effectiveness/outcome measure. The lifetime accumulative QALY gains by gender and age group are summarised in Table 37. QALYs were reported by participants’ smoking status, which includes ex-occasional smokers (who have only smoked once or twice), ex-regular smokers (who used to smoke sometimes but have now quit), light smokers (who smoke fewer than 10 cigarettes a day), moderate smokers (who smoke between 10–19 cigarettes a day) and heavy smokers (who smoke 20 or more cigarettes a day). The long-term analysis used a lifetime horizon, effectively covering all participants’ remaining lifetime between the age they entered the trial until the time they reach the national average life expectancy at birth. It is reported that the average life expectancy at birth was 81 years (79 years for males and 83 years for females) in 2013 in the UK. 112 Table 38 reports the accumulative lifetime QALY gains after discounting. In this study, QALYs and costs were both discounted at 3.5% per year. Overall lifetime health costs due to smoking-related diseases for both smokers and ex-smokers were derived from the published economic model and are reported in Table 39.
| Smoking status | Lifetime QALY gain (years) | ||||||
|---|---|---|---|---|---|---|---|
| Male | 16–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–74 years | 75–79 years |
| Ex-occasional smoker | 65.603 | 46.986 | 37.555 | 28.379 | 19.675 | 11.574 | 3.706 |
| Ex-regular smoker | 64.914 | 46.461 | 37.155 | 28.097 | 19.501 | 11.481 | 3.679 |
| Light smoker | 64.196 | 46.010 | 36.744 | 27.742 | 19.216 | 11.299 | 3.615 |
| Moderate smoker | 63.341 | 45.433 | 36.267 | 27.368 | 18.946 | 11.131 | 3.556 |
| Heavy smoker | 61.915 | 44.463 | 35.492 | 26.764 | 18.505 | 10.858 | 3.463 |
| Female | 16–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–74 years | 75–83 years |
| Ex-occasional smoker | 49.664 | 45.974 | 36.868 | 27.877 | 19.284 | 11.354 | 3.540 |
| Ex-regular smoker | 49.002 | 45.369 | 36.381 | 27.509 | 19.030 | 11.203 | 3.494 |
| Light smoker | 48.622 | 44.997 | 36.059 | 27.245 | 18.827 | 11.079 | 3.448 |
| Moderate smoker | 48.006 | 44.425 | 35.590 | 26.874 | 18.557 | 10.909 | 3.389 |
| Heavy smoker | 46.874 | 43.377 | 34.747 | 26.225 | 18.095 | 10.629 | 3.293 |
| Smoking status | Discounted lifetime QALY gain (years) | ||||||
|---|---|---|---|---|---|---|---|
| Male | 16–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–74 years | 75–79 years |
| Ex-occasional smoker | 22.421 | 20.530 | 18.032 | 14.924 | 11.165 | 6.370 | 3.347 |
| Ex-regular smoker | 22.137 | 20.287 | 17.832 | 14.776 | 11.067 | 6.320 | 3.322 |
| Light smoker | 21.992 | 20.117 | 17.648 | 14.589 | 10.903 | 6.218 | 3.264 |
| Moderate smoker | 21.732 | 19.874 | 17.424 | 14.394 | 10.749 | 6.124 | 3.211 |
| Heavy smoker | 21.274 | 19.463 | 17.060 | 14.078 | 10.497 | 5.971 | 3.127 |
| Female | 16–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–74 years | 75–83 years |
| Ex-occasional smoker | 22.201 | 20.558 | 18.379 | 15.590 | 12.267 | 8.062 | 5.385 |
| Ex-regular smoker | 21.901 | 20.288 | 18.136 | 15.385 | 12.105 | 7.956 | 5.315 |
| Light smoker | 21.762 | 20.132 | 17.980 | 15.234 | 11.973 | 7.863 | 5.246 |
| Moderate smoker | 21.497 | 19.885 | 17.749 | 15.026 | 11.797 | 7.737 | 5.156 |
| Heavy smoker | 20.997 | 19.423 | 17.332 | 14.661 | 11.499 | 7.531 | 5.010 |
| Smoking status | Gender | |
|---|---|---|
| Men | Women | |
| Current smokers | ||
| Before discounting | £22,386 | £17,559 |
| Discounted | £7029 | £4792 |
| Ex-smokers | ||
| Before discounting | £18,044 | £14,076 |
| Discounted | £5023 | £3360 |
| Difference between smokers and ex-smokers | ||
| Before discounting | £4342 | £3482 |
| Discounted | £2006 | £1432 |
Participants in the trial were simultaneously assigned different lifetime costs and QALYs in accordance with their smoking status and characteristics. The lifetime costs and QALYs were adjusted by the 6-month costs and QALYs reported in the previous within-trial analysis, and the average lifetime costs and QALYs of each intervention before and after discounting are presented in Table 40. Participants who received the tailored intervention were expected to have a health-care cost savings of £210 (before discounting) and £74 (after discounting) over their lifetime compared with those who received usual care. Meanwhile, they also have higher lifetime QALY gains of 0.470 (before discounting) and 0.196 (after discounting) than people in the usual-care group. The results suggest that over the participants’ lifetime, tailored letters and taster sessions generate more QALY gains with less cost; in other words, the intervention is more cost-effective than the control condition.
| Outcome | Before discounting | Discounted | ||
|---|---|---|---|---|
| Intervention group (n = 2635) | Control group (n = 1748) | Intervention group (n = 2635) | Control group (n = 1748) | |
| Lifetime cost, mean (SD) | £19,390 (£2776) | £19,601 (£2787) | £5775 (£1109) | £5848 (£1114) |
| Cost difference (intervention – control) (95% CI) | –£210 (–£432 to £11) | –£74 (–£162 to £15) | ||
| Lifetime QALY gains, mean (SD) | 27.009 (11.894) | 26.539 (11.943) | 13.974 (4.424) | 13.778 (4.442) |
| QALY difference (intervention – control) (95% CI) | 0.470 (–0.478 to 1.419) | 0.196 (–0.157 to 0.549) | ||
| ICER (cost per QALY gained) (95% CI) | –£447 (–£4368 to £3646) | –£376 (–£3881 to £3207) | ||
The long-term CEA results are also summarised in Table 40, indicating a lifetime horizon ICER of –£447 (95% CI –£4368 to £3646) per QALY and discounted ICER of –£376 (95% CI –£3881 to £3207) per QALY. Figures 20 and 21 present CEACs for long-term cost-effectiveness. The first CEAC (see Figure 20), using results before discounting, illustrates that the probability of the tailored letter and taster session being cost-effective was between 83.4% and 83.5% at a willingness-to-pay threshold range of £20,000–30,000 per QALY gained. Figure 21 shows the discounted CEAC, which indicates that the probability of the intervention being cost-effective compared with generic letter was 86.1% at £20,000 per QALY and 86.0% at £30,000 per QALY.
FIGURE 20.
Lifetime CEAC (before discounting).

FIGURE 21.
Lifetime CEAC (discounted).

Summary/conclusion
This economic evaluation assessed the cost-effectiveness of the personal tailored risk information and taster sessions compared with the standard generic letter advertising the SSS.
The mean intervention cost was £54 (SD £12) per participant in the intervention group and £0.90 (SD £2) per participant in the control group. Taking into consideration the wider health resource use, smokers who received the intervention had non-significantly higher total mean costs over the 6-month follow-up period (mean cost difference £98, 95% CI –£26 to £222). After adjusting for baseline resource use, the adjusted cost difference was £92 (95% CI –£32 to £216).
The clinical outcomes used in the economic evaluation were reported in Chapter 3, that is, the uptake of SSS and quit rates. It is recommended by NICE that cost-effectiveness be expressed in terms of cost per QALY. In this study, there was a non-significant trend towards improved QALYs associated with the intervention (unadjusted mean QALY difference of 0.0001, 95% CI –0.008 to 0.009). After adjustment for baseline utility, the difference in adjusted QALYs was 0.002 (95% CI –0.001 to 0.004).
The base-case within-trial CEA was based on a multiple-imputed data set in which all the missing values were imputed using a multiple imputation method. The ICER associated with the intervention was estimated at £627 per additional attendee to the SSS, £2689 for an additional quitter and £59,401 per QALY gained. The CEAC illustrates that, when using the NICE decision-making threshold range of £20,000–30,000 per QALY gained, the tailored letter and taster session has a 20–27% probability of being considered cost-effective at 6 months. Only at higher willingness-to-pay thresholds (> £59,401 per QALY) does the intervention become more likely to be cost-effective compared with the control.
Complete-case analysis was conducted as part of the sensitivity analysis to explore the impact of missing data. The results show that for both trial arms, mean costs and effectiveness were higher in the complete cases. The ICERs of the complete-case analyses were estimated at £498 (95% CI £491 to £504) per additional attendee to the SSSs, £2552 (95% CI –£1199 to £6303) per additional quitter and £49,842 (95% CI –£425,064 to £536,813) per QALY gained when comparing the intervention with the control group. The probability that the intervention would be considered more cost-effective than the control was 24–34% at the £20,000–30,000 per QALY threshold. The complete-case results appeared to be robust compared with analyses including imputed data.
This study uses a multicomponent intervention and, hence, the costs and effects of each intervention element were explored in the sensitivity analysis. Participants who received a tailored letter but did not attend any taster session were found to have the lowest rates of SSS attendance and smoking cessation, and were associated with higher costs than those who received only the generic letter. When comparing the participants who received both tailored letter and taster session with participants who received the generic letter, the ICER was estimated as £196 per additional attendee to the SSS, £6 per additional quitter and £300 per QALY gained. Considering that the majority of the cost (£46 out of £72) incurred in the intervention group was for sending tailored letters, these results may suggest ways in which the intervention in this trial can be improved. For example, cheaper alternatives such as e-mails can be used to replace the traditional postal questionnaire to reduce the cost of the intervention.
It may take several years before the health benefits of smoking cessation interventions start to have an impact. In this study, we extrapolated the lifetime cost-effectiveness of the two trial interventions in addition to the within-trial analyses. The total discounted lifetime health-care cost was lower in the intervention group at £5775 than in the control group at £5848, with a cost saving of £74 (95% CI –£15 to £163). However, the intervention group had higher lifetime QALY gains than the control group (difference in adjusted QALYs of 0.196, 95% CI –0.157 to 0.549). This gives a lifetime horizon discounted ICER of –£376 (95% CI –£3881 to £3207) per QALY. The probability of the intervention being more cost-effective compared with the standard generic letter was 86.1% at £20,000 per QALY and 86.0% at £30,000 per QALY.
In conclusion, the economic evaluation of the Start2quit trial has provided evidence showing that the personal tailored information and taster sessions results in better clinical outcomes. However, the ICERs for the intervention compared with the standard generic letter are high and suggest that it is unlikely to be a cost-effective option in the short term; however, quitting smoking yields far greater health-care cost savings and health benefits over the long term through the reduced risk of smoking-related diseases. The long-term results indicate that over a lifetime horizon, the tailored letter and taster sessions is more effective and less costly than the generic letter, and has a probability of being more cost-effective > 86% using the £20,000–30,000 per QALY gained decision-making threshold.
Chapter 6 Discussion
Main outcome and effectiveness
The aim of the Start2quit study was to assess the effectiveness of a complex intervention designed to increase attendance at the SSSs in England. Attendance at the first session of a 6-week SSS course was significantly higher in the intervention group than in the control group, increasing by 8.4%. This intervention, utilising computer-tailoring to deliver personal risk information together with an invitation to attend a no-commitment ‘taster’ session designed to offer information about the SSS, more than doubled attendance at the SSS compared with the control group, which received a standard generic invitation to contact the service (OR 2.12). Furthermore, participants in the intervention group were more than twice as likely to attend all appointments and complete the 6-week SSS course as control participants (OR 2.24). Results also showed a significantly higher rate of validated 7-day point prevalent abstinence at the 6-month follow-up in the intervention group, an absolute increase of 3.4% over the control group. Abstinence rates were consistently higher for all periods of abstinence at the 6-month follow-up, both point prevalent and prolonged. This finding is important as it demonstrates that the intervention also translated to increased quit rates.
These results compare favourably with previous research of Murray et al. 29 and of Lichtenstein and Hollis,27 on which the Start2quit study had built. Both of these previous studies had attempted to combine a proactive approach with informing smokers about local services. Murray et al. 29 reported a 7.7% absolute increase in smokers using the SSS over a control group, and Lichtenstein and Hollis27 found a striking improvement in attendance at both the first session and the last session of a cessation programme. Murray et al. 29 had also found an increase of 1.8% in 7-day-validated quit rate, although this was only in a post hoc evaluation of a subgroup of smokers requesting help, not in the whole sample between the intervention and the control groups.
The intervention was found to be more effective for some groups of smokers than for others. Although the effect of the intervention on attendance at the SSS was significant for both males and females, it was more pronounced for males, and this differential effect was also observed for 7-day point prevalent abstinence at the 6-month follow-up. There was also a significant difference in attendance by level of social deprivation. However, this appears to be a result of lower attendance in the control group by smokers in mid-levels of social deprivation rather than the intervention having a greater effect on this group. There was no significant difference in abstinence by social deprivation, and neither attendance nor abstinence differed by age category.
The intervention was further enhanced by the addition of a repeated personal letter and invitation sent 3 months after the original, to all participants who failed to attend a taster session after the first invitation. The pattern of attendance over the 6 months suggested that there was little effect on attendance at the SSS of sending these repeat reminders to smokers. There was also little difference in attendance due to seasonal variations.
Recruitment, retention and generalisability of the trial findings
The strategy of proactive recruitment approach using mass mailing aimed to recruit a more representative sample of smokers and to reach smokers who would otherwise, using more traditional reactive recruitment methods, not be aware of the research or of the SSS.
Although 19.6% of those invited replied to the invitation, many of these replied to say that they were non-smokers and others declined to participate but wished to update their GP records. The response rate of 4.1% from smokers willing to take part is lower than in some studies using this recruitment method. For example, Murray et al. 29 reported a response rate of 31% from 24 practices located in one area of Nottingham. Our lower response could be due to a more intensive intervention or longer assessment questionnaire. In addition, differences in the definition of consent and stricter criteria for inclusion reduced the possibility of being eligible for the research. The local nature of the research in Nottingham may also have encouraged participation, whereas the Start2quit study, as a national one, was more remote and could have deterred some smokers from taking part. In addition, records are not always accurate, and inaccuracies in recording and in the practice search could also have led to the lower response rate. The exact number of non-smokers wrongly recorded is not known, as some responded to update their current smoking status on the GP records, but it is possible that the others did not do so. Nevertheless, the requirement to screen and record smoking status does allow for the identification of smokers and patients with chronic diseases. In comparison with the more traditional reactive approach to recruitment, where researchers advertise and wait for response, the size and the representativeness of the sample is high. 26,113,114
An additional advantage of the proactive recruitment method is the ability to target at-risk groups, and a priority of the SSS is to help disadvantaged people and to deliver cessation services to poorer communities. 8 Therefore, practices in areas of high deprivation were preferentially selected, with the result that over half of the practices were located in areas of high deprivation. Unfortunately, although this meant that about half were sampled from areas of higher deprivation, this was to the detriment of achieving a higher response rate, as people living in deprived areas, in general, had lower response rates.
The total response and recruitment varied greatly between areas and between practices within an area. Location of the practice is a major influence on the recruitment rate to trials of smoking cessation. 115 Response rates in some areas of < 2% may be explained by very high deprivation, large ethnic populations, a younger population or practice characteristics, such as a larger list size.
Validation of attendance at the SSS was obtained for each participant from the relevant SSSs. Thus, 100% follow-up was obtained for the primary outcome. Completion of the follow-up, either by interview or by questionnaire, was obtained from 76.9% of the sample, a high proportion in comparison with some smoking studies. For example, Murray et al. 29 obtained a 48% follow-up rate. However, validation of self-reported abstinence was low. Although most participants claiming abstinence agreed to send a saliva sample, samples were received from only two-thirds of those reporting abstinence, and sent for analysis. Reasons for not returning the sample could include a return to smoking; therefore, our assumption that all those who were not validated had not quit was a cautious one, and our results reflect the most conservative estimate. There is no reason to assume any differential in return, or validation, between the groups.
A strength of this study was the collection of anonymised data of those invited but who did not agree to take part in the study, in order to explore the external validity of the sample. This allowed us to establish that, although the sample was significantly older than the average smoker in all areas, the differences in gender and deprivation were small, and we can consider the sample to have reasonable external validity.
Interpretation, acceptance and feasibility
The intervention used in this study employed a two-pronged approach consisting of the use of computer-tailoring technology to send personal risk information in a letter inviting smokers to attend a taster session. This session offered smokers the opportunity to learn more about the services before committing to and signing up to a 6-week course.
The inclusion of individual risk information in the personal invitation to a taster session was justified by evidence, both empirical and theoretical, that fear messages, when accompanied by a cue to action, will increase the likelihood of following the recommended behaviour. 16,36,37 Analysis of the perception of the personal letter suggested that the letter was well accepted. Participants who received the personal letter perceived it as more positive than how control group participants perceived the generic letter. They were more likely than recipients of the generic letter to read and discuss the letter with others, they felt that it was more personally relevant to them, and reported that they felt more confident and determined towards quitting. It is important to establish the correct balance between anxiety and reassurance,16 there is a risk that this direct personal approach could induce too much fear or create hostility. Our concern was that our communication would upset participants and, thus, prompt a maladaptive response. In fact, very few respondents reported perceiving the letter as antagonistic or depressing, and < 10% of recipients reported feeling anxious because of the letter. It could be argued that insufficient concern was induced; however, the immediate offer of support was intended to reassure recipients and reports of anxiety could be offset by this offer. The low anxiety could indicate that the balance of risk information with awareness of the availability of support was appropriate.
Reports from participants who took up the offer of attendance at a taster session were also positive, and the taster sessions were seen as helpful and encouraging. Ratings immediately following the taster session were higher than those at the 6-month follow-up, and reported intention to sign up at both times was higher than the actual attendance. Although this retrospective or recall bias can represent a threat to the internal validity of studies using self-reported data, in this case it illustrates the tendency of participants to report past events from the perspective of the present situation. In the case of smokers, their recollection of the event could be affected by their behaviour since the taster session, that is, their success or otherwise in attempting to stop smoking. The recollection of the smokers who did not quit, or who did not attend the SSS, would account for the lower ratings at the follow-up. Nevertheless, comments from attendees suggested that the taster sessions were reassuring and built the intended awareness and comfort with the services.
A limitation of the study is that it is not possible to disentangle these two parts of the intervention. The Global Dialogue for Effective Stop Smoking Campaigns44 particularly recommended the use of a combination of ‘why quit’ and ‘how to quit’ messages, hard-hitting messages about the consequences of tobacco use paired with a supportive and positive message emphasising quitting resources, and giving smokers hope that they can succeed. Indeed, when participants were asked whether attending the taster session or receiving the letter was more important in their decision to attend the SSS, most said both equally contributed to their decision. Although many cited health concerns as a reason for attending, the letter coming at the right time to prompt them to make an appointment was also cited. However, despite many participants reporting that both the letter and the taster session prompted attendance at the SSS, few attended the SSS without first attending the taster session. Thus, it is likely that once prompted to attend an introductory no-commitment session, the sessions did help smokers to feel empowered and hopeful about quitting, and encouraged them to accept the support offered by the SSS, implying that both parts of the intervention together are needed to prompt uptake of the service. Although we can speculate that both the personal letter and risk information prompted attendance at the taster session, it is not known how much attendance would have occurred if smokers had received a standard generic invitation to a taster session.
That the intervention also translated to increased quit rates is an important outcome of this study. Of the two previous studies on which this study built, Lichtenstein and Hollis27 did not follow up to discover if attendance led to an increased quit rate, and Murray et al. 29 failed to show a difference between point prevalent abstinence rates, either validated or self-reported, between the intervention and control groups, at the 6-month follow-up.
Concern has been expressed that, although proactive recruitment is effective and it is possible to raise awareness of services and encourage use of treatment services, smokers recruited proactively may be less likely than self-referred patients to quit. 116 Although traditionally smokers with an intention to quit smoking in the next 2 weeks are targeted by the SSS for attendance, studies have shown that smokers stating no plans to quit can be engaged in quitting activity117,118 and that some smokers do quit without entering a preparation stage. 41,119 Thus, we deliberately used wider criteria for enrolment in this study, and included those whose intention to quit was more distant, or who were not planning to quit but expressed an interest in receiving help. Despite recruiting a high proportion (42.5%) of participants who intended to quit in the next 6 months (but not in the next 30 days), the proportion of validated 7-day abstinent who had received a personal risk letter and had attended the SSS was comparable with estimates of longer-term abstinence in recipients of treatment from the SSS. 6
Tzelepis et al. ,24 in a study of telephone counselling among cold-called smokers, found that the proportion achieving prolonged abstinence was lower than corresponding rates in treatment seekers, and that proactive recruitment to a quitline was more effective in smokers who are intending to quit in the next 30 days. However, Tzelepis et al. also pointed out that if counselling were offered only to smokers ready to quit, a large proportion of proactively recruited smokers would miss out on getting effective support. 120 Borland et al. 121 recently demonstrated the superiority in longer-term outcome of structured planning over unstructured, more spontaneous quit attempts. Balmford and Borland88 have also suggested that treatments do not only help during a quit attempt and help to prevent relapse, but can encourage and increase motivation to quit. Thus, recruiting smokers who might have more distal plans to quit may be beneficial in increasing readiness to quit and in planning and preparing to quit, and might result in more successful attempts and long-term abstinence than if these smokers are left to make abrupt unplanned attempts on their own.
What was clear in these results was that attendance at the SSS greatly impacted on quit rates. Participants in both the intervention and control groups who attended the SSS were more likely to achieve 7-day abstinence at the follow-up than those who did not. It is difficult to compare these figures with official SSS figures, as different abstinence periods and definitions of abstinence are used. It does, however, support the efficacy of the SSS in increasing the success of quit attempts over unsupported attempts, and endorses the notion that smokers who take advantage of stop smoking programmes have a greater chance of stopping smoking and remaining abstinent than those who try to quit on their own. Nevertheless, the quit rate of around 5% in those who did not attend (in both treatment groups) is higher than one would expect in spontaneous quit rates,122,123 suggesting that a prompt of any kind through mass mailing can have a positive effect.
In addition, it was found that abstinence was substantially higher in participants who received the intervention and attended the SSS than in control group participants who had attended but received only the generic letter. One interpretation of this higher quit rate in SSS attendees in the intervention group is that the additional components of the intervention, both the personal risk letter and the introductory session, played some part in increasing motivation. This motivation was then augmented by the support given by the SSS advisors, resulting in a higher abstinence rate. Again, over half of the respondents reported that both the personal letter and the taster session were equally important in their decision to quit. However, it was not possible to validate these claims in the current analysis and further research is necessary to disentangle the effects of each of the components of this intervention.
Although the efficacy of the SSS in increasing the success of quit attempts was evident, some variation was found in both attendance and in 7-day validated abstinence between SSSs. At the best-performing SSS, the rate of attendance was 28.3% in the intervention group, and overall 7-day abstinence, combining both groups, was 13.4%. There is known to be wide variation in outcomes between SSSs. 123–125 Abstinence rates can vary significantly depending on which practitioner and type of practitioner was seen, and it is known that some types of intervention (group or one-to-one) are more effective than others. 126,127 Abstinence rates can also be affected by other factors, such as the dependence and deprivation level of the population served,128 and it is likely that these factors were at least partly responsible for the variation in abstinence rates seen in this study. However, differences in organisation and service characteristics could be a more central influence on attendance following the taster session. Advisors were trained to standardise taster sessions according to a protocol, and the analysis of adherence to the protocol showed that the delivery of the intervention did not influence subsequent attendance. Thus, local service characteristics and organisational factors are likely candidates that could account for the variation in the number of participants attending the first session of a SSS course. The organisation of the SSSs could have played a part in terms of how soon the clients were seen, how many different locations and times were offered and, importantly, how responsive the services were to smokers wanting to accept the offer of treatment when they indicated their intention to sign up to a course. It is reasonable to assume that those SSSs that were able to offer appointments at the time of the taster session were more successful in recruiting smokers to their service, as motivation can quickly wane if immediate action is not taken. Unfortunately, this information was not available from the SSSs, so it is not possible to confirm this supposition.
During the recruitment period for this study, SSSs were going through a significant change in commissioning. The tendering out of previously in-house services led to some SSSs being run by private and voluntary sector companies. We do not know how this might have positively or negatively influenced our findings. The most likely effect was that those SSSs that were least confident and organised in the changeover would decline to participate and this would reduce the generalisation of our results.
Our analysis showed that the intervention was more effective for some subgroups of smokers. Whereas typically more women than men attend and set a quit date with the services,18 more men than women were encouraged by this intervention to attend. In addition, slightly more men than women achieved abstinence, and this corresponds to the success rate of giving up smoking reported by the SSS, which is higher among men than among women (52% of men compared with 50% of women), although in this study the differential was a result of fewer men in the control group achieving abstinence. This suggests that the trend for greater attendance by women could be reversed by using these measures to encourage men to use the support and, thus, reduce the number of unaided and unsuccessful attempts made by men to quit smoking.
Traditionally, smokers in employment classified as ‘routine and manual occupations’, as classified by a methodology adapted for use in NHS Smoking Cessation Services,96 have the highest number of people setting a quit date and successfully quitting, accounting for about one-quarter. In our study, attendance at the SSS in the intervention group was similar for all levels of deprivation but lower in the control group for participants in areas of medium deprivation. This, again, suggests that a group who would not normally seek help were encouraged to do so by this intervention. However, even though attendance was as high in smokers from areas of higher deprivation, abstinence rates tended to be lower overall in these smokers, with no differential effect. Murray et al. 29 also reported a linear trend where the desire to talk with an advisor increased with the amount of deprivation, and the low abstinence rates in these smokers in areas of high deprivation suggests that this expressed need for help does not translate to an increase in abstinence in those who need it most. Unacceptable smoking-related health inequalities persist, and one of the aims of this study was to target more deprived smokers and encourage them to get help. The problem may be one of keeping these smokers in the programmes, and addressing the particular problems faced by them, rather than encouraging them to attend in the first place.
This leads to a consideration of the factor analysis of barriers to attendance at the SSS. This exploration suggested that one of the main reasons for non-attendance is work and time constraints, also endorsed by answers to open questions in which many said that they were too busy and had other commitments to attend the SSS or the taster session, or that that the sessions were held at inconvenient times or locations. These constraints can apply through all social strata, and to both men and women. The people from areas of lower deprivation might genuinely work longer hours and have less time, whereas the more highly deprived might not have the support for childcare and transportation, and are not able to get to clinics. Our exploratory factor analysis also showed that a disbelief in the efficacy of programmes was evident, confirming an issue that is prevalent in the literature across the globe. This lack of faith in programmes of support, together with pressure from work or lack of support, particularly in lower socioeconomic smokers, can combine to deter attendance, and calls for interventions designed to change beliefs to increase the initiation of evidence-based treatment. 85 Reassurance of the effectiveness of the programmes of support and of the empathy of advisors, in the form of introductory sessions, in addition to the provision of clinics that are easily and immediately accessed at convenient times, could help to overcome these barriers and increase uptake.
Cost-effectiveness
To our knowledge, Start2quit is the first large randomised controlled trial that has assessed the cost-effectiveness of a complex intervention designed to increase attendance at the SSS in the UK. The CEA was conducted to establish the value for money of the personal risk information and taster sessions compared with the standard generic letter. The mean intervention cost was high at £54 per participant, whereas the corresponding average cost in the control group was only £0.90 per participant. The within-trial CEA generated ICERs of £627 per additional attendee to the SSSs and £2689 for an additional quitter. This means, if the decision-makers are willing to pay more than £627 for an additional service user or £2689 for an additional quitter, the tailored letter and taster session would be the preferred option, otherwise usual care should be adopted.
When using QALY as a standard health outcome measurement, the short-term ICER was estimated at £59,401 per additional QALY gained. When using the NICE decision-making threshold range of £20,000–30,000 per QALY gained, the tailored letter and taster session has a 20–27% probability of being considered cost-effective at 6 months. Only at higher willingness-to-pay thresholds (> £59,401 per QALY), does the intervention become more likely to be cost-effective compared with the control.
However, it is important to consider that quitting smoking yields far greater health-care cost savings and health benefits over the long term through the reduced risk of smoking-related diseases. The long-term benefits from the smoking control interventions may take several years before starting to have an impact. After taking into account the lifetime cost savings and health benefits from stopping smoking, the long-term results indicate that the intervention is more effective and less costly than the generic letter. Over a lifetime horizon, the intervention has a probability of being more cost-effective using the £20,000–30,000 per QALY gained decision-making threshold range.
Participants who received the tailored letter but did not attend any taster session were found to have the lowest rates of SSS attendance and smoking cessation, and when costs and effects of each intervention element were explored in a sensitivity analysis, it was found that these participants were associated with higher costs than those who received solely the generic letter. When comparing the participants who received both the tailored letter and taster session with participants who received the generic letter, the ICER was estimated as £195 per additional attendee to the SSS, £66 per additional quitter and £300 per QALY gained. The results indicate that providing participants with both a tailored letter and taster session improved the cost-effectiveness, compared with using the tailored letter alone. Therefore, further research should aim to increase attendance of taster sessions.
Postage accounted for a high proportion of the cost in the intervention group, and adaptations could be introduced that could, while retaining the personal nature of the letter, reduce the amount of assessment necessary. If assessment could be achieved through cheaper alternatives, such as e-mails used to replace the traditional postal questionnaire, it might be possible to attain the same results at lower cost. In addition, the tailored letter did not include information promoting the local availability of the service, and adding a SSS advertising component to the tailored letter might increase uptake of the service without attendance at a taster session.
Strengths and limitations
This trial has demonstrated several methodological strengths. This is the first and largest study to examine the effect of using introductory taster sessions to encourage people to attend a smoking cessation service. We recruited 4383 people from 18 regions across England. The collection of anonymised data of those invited but who did not agree to take part in the study allowed us establish that the representativeness of the sample was acceptable.
Another major strength of the study was that for the main outcome we were able to collect complete data from SSS records. Although we were reliant on the accuracy of reporting from the SSSs, this represents a significant improvement on the self-report outcome reported by Murray et al. ,29 which relied on self-reporting and, in addition, had a low follow-up completion rate of 48%. We demonstrated a discrepancy between recalled attendance and validated attendance. Thus, our study represents a much more accurate outcome, not affected by poor recall or misunderstanding of what constituted attendance at the SSS, and not over-reported because of the social desirability response bias of the intervention group, which would involve wanting to report higher attendance.
A further important strength was that smoking outcome data were collected, allowing us to define the ultimate efficacy in terms of abstinence, rather than only at the attendance point. Although abstinence could be estimated based on quit rates within SSSs, different definitions of abstinence have been used, which do not allow comparison with other smoking cessation studies. Furthermore, it allowed us to show that smokers recruited proactively to the SSS can have outcomes as successful as self-referred clients.
By randomising at the level of participant rather than by practice, there was a slight risk of contamination by communication between patients at the same practice allocated to different intervention groups. We built into the trial safeguards against contamination by ensuring that only one person from the same household received a screening questionnaire and by monitoring attendance at the taster sessions, to ensure that anyone attending who had not received an invitation was recorded and checked against participants in the control group. We also kept a record of attendance at the taster sessions and measured the amount of contamination at follow-up by asking participants who had not attended a taster session whether or not they personally knew or had spoken to anyone else who had been invited or attended to a taster session. The results suggested that few control participants had access to information from the taster sessions and, thus, there was no contamination bias.
Finally, an assessment of the fidelity of the delivery of taster sessions to the protocol was embedded into the trial. In general, adherence to the protocol was high, although variable. But adherence to the protocol was not related to the main outcome of attendance at the SSS or to abstinence from smoking. The first part of the intervention consisting of the personal risk letter was tailored to the individual’s personal characteristics by computer and, therefore, the format was standard for all participants, and all advisors had received standardised training to deliver the taster sessions. Thus, we can be sure that the outcome is a result of the intervention and not a result of other non-specified variables.
There were also some limitations to the study, in addition to the difficulty of dismantling the two components of the intervention, which has been already discussed.
Although the recruited SSSs were spread across England, were located in both high and low areas of deprivation and represented both large and small organisations, we did include only a sample of areas. Eighteen of the 151 SSSs were included, and these participating SSSs may not necessarily be representative of all SSSs in England. It is likely that those agreeing to participate were the more organised and enterprising ones. That is not to say, however, that the less organised SSSs would be less successful in encouraging uptake were they to introduce these procedures and offer introductory sessions to encourage attendance. Recruitment took place at a time when SSSs were undergoing changes in commissioning and we do not know how this affected the decision to take part or not.
In addition, although our proactive recruitment strategy was a strength of the study, the recruitment rate was low, at 4%. As a result, we recruited only a small proportion of smokers in each area. Comparison of some demographics confirmed that the sample was reasonably representative of smokers in terms of gender and deprivation, but we failed to recruit sufficient younger smokers. It is important to attract this population as attendance at the SSS at present tends to be concentrated in older age groups. There was a large difference in response from different SSS areas. Although we aimed to target areas with a high number of ethnic minorities, the response rate in these areas was particularly low and, thus, in areas with a very low response rate the generalisability of the results may be reduced.
We had initially hypothesised that the intervention’s effectiveness might differ by socioeconomic group and analysis of the interaction between the intervention and deprivation was prespecified. Before any analysis began, we extended our planned investigation to investigate interactions of intervention with age group and gender also. These were the only prespecified analyses of interactions.
Observational data were used for the primary outcome rather than subjective self-reported data. However, the collection of these outcome data depended on accurate recording and submission by local SSS collaborators, and on the collaborators being blind to group assignment. There may have been some cases in which loss of blinding occurred, and SSS advisors knew to which group the client had been assigned, leading to bias in treatment.
Finally, assumptions were made for the long-term CEA. To maintain consistency with the Markov model we adopted to generate lifetime cost savings from the interventions, participants’ smoking status was assumed to stay unchanged after the trial period. Specifically, we assumed that those participants who managed to quit at the end of the trial will stay abstinent from smoking for the rest of their life, and smokers who have not given up will carry on smoking until they die. However, in real life, many quitters may relapse to smoking again and some smokers may quit smoking without any aid at some point. Therefore, further research with longer follow-up periods or models that allow for smoking relapse and spontaneous abstinence are recommended.
Chapter 7 Conclusions
Main conclusion
The Start2quit trial built on previous work by Murray et al. 29 and added to the evidence that a proactive approach can be successful in reaching more smokers and informing them of the SSSs and, consequently, increase uptake of the service. We introduced a more intensive intervention to deliver personalised risk information and provide a no-commitment introductory session designed to inform smokers about the service and what it offers. The results showed that reaching out to smokers with more individualised personal invitations can more than double attendance at the SSS. We also demonstrated that the increased attendance translated into increased quit rates. The acceptability of both parts of the intervention was established.
Although the results of the within-trial CEA indicate that the personal risk information and taster sessions is less likely to be a cost-effective option in short term than the generic letter, the long-term predictions suggest that over a lifetime horizon the intervention is more effective and less costly than the generic letter. Some adaptation, such as using e-mail to replace the traditional postal questionnaire, might reduce the postage costs without reducing the impact and, thus, could increase the viability of the strategy as a means to increase uptake of the SSSs.
Recommendations for research
The complexity and interaction between the two components of this intervention make it difficult to identify how the components work and to determine which component is the most essential. The first priority for future research following on from this study would be to dismantle the components of the intervention in a factorial study to assess their separate effects, and to identify the mechanisms of action.
Other recommendations for future research include:
-
Further investigation into the long-term abstinence of smokers proactively recruited compared with those who self-refer, to determine whether or not the quit rates persist into longer-term abstinence of ≥ 12 months.
-
More exploration of the barriers to seeking help and to attendance at support services could suggest changes to the format, content and timing of the introductory session. Although this research has suggested the value of an introduction of some kind to the service, attendance at these sessions could still be higher, and subsequent attendance at the SSSs could also be improved. Different formats of introductory session might be attractive to different smokers, and qualitative research might suggest ways to increase attendance, for example an open invitation to family and friends to allow more widespread promotion of the service, and possibly engage with more members of a community.
-
Qualitative work to break down the components of the personal risk letter and to investigate, for example, which type of smoker is likely to be prompted by the contents to attend (i.e. what works and for whom?).
-
Despite the proactive recruitment strategy, response to the initial questionnaire was relatively low. Experimentation with reactive and opportunistic recruitment could suggest ways in which initial recruitment to the research could be improved.
Implications for health care
Until 2011 the number of smokers accessing the SSSs had been increasing. However, recent data have shown a significant decrease in the past few years. 18 This trend is regrettable, as services offer smokers a significantly higher chance of stopping smoking than trying to quit without support.
The evidence suggests that a programme of proactive recruitment can be effective in raising awareness of the SSS and personal invitations, with or without additional risk information, may also offer the services an opportunity to promote the service in the form of introductory sessions to emphasise its approachability and empathy.
The cost of this intervention in its entirety may be a problem for some providers. The use of computer programs is expensive and increases the cost for each additional quitter. Adaptations to the intervention could be introduced, with the potential to retain the impact on attendance. Health professionals are currently encouraged to routinely refer smokers to local SSSs, but the provision of very brief advice in the form of ‘ask, advise, act’ is insufficient and many referrals are not followed up by the patient, or by the health-care professional. A more proactive approach of ‘ask, advise, contact’, in which smokers are contacted proactively by the service on receipt of their contact information, can reduce patient barriers to receiving treatment, and also has high potential to increase uptake. 128
The results of the adherence to the delivery of the intervention would suggest that the protocol for the taster session is appropriate. Adaptations to suit the local features and attributes could be implemented, with mechanisms to ensure that interested clients are offered early and convenient appointments with the service.
In the short term, the personal risk information and taster session is an expensive intervention compared with the generic letter. However, over a lifetime horizon, the intervention has an 86% probability of being more cost-effective than the generic letter. Successful implementation of the intervention is likely to yield greater health-care cost savings and health benefits over the long term through the reduced risk of smoking-related diseases. These results suggest that use of personal risk information and taster session rather than generic letters has potential long-term costs and health benefits.
Intellectual property and adoption of positive elements of the approach in the NHS
This approach is one that could be adopted by commissioners, who may in the future request cessation services to run introductory taster sessions with no obligation for patients to sign up. If such taster sessions are available, tailored letters to patients who are smokers may be used to prompt their attendance at taster sessions.
There is a possibility that the intellectual property generated around the tailored letter generation could be licensed out. However, the software we have used in the study is cumbersome to use and requires manual input, and is therefore unlikely to suitable to transfer into practice as it is. There may be letter-generating modules in general practice computer patient record systems, although there are multiple providers of such systems and, therefore, adaption or custom plug-in software would need to be developed for each.
Acknowledgements
The successful completion of the Start2quit study would not have been possible without the support and input from numerous colleagues and associates. We would like to thank the following:
-
Additional members of the research team: Sally Skipper (Trial Manager from September 2010 to March 2011) who set up the procedures, and Danielle Stoller, Kirsty Bennet and Andrea Hamill who provided administrative support to the trial and contributed to recruiting participants and collecting data, both baseline and follow-up, and with data organisation and analysis.
-
Members of the Trial Steering Committee for their advice and support: Dr Andy McEwen (UCL) (Chairperson from September 2010 to January 2011), Professor Tim Coleman (University of Nottingham) (Chairperson from February 2011 to November 2015), Dr Caroline Free (London School of Hygiene & Tropical Medicine), Dr Michael Ussher (St George’s, University of London) and Professor Toby Prevost (King’s College London).
-
User representative Sue Dowd sat on both the Trial Management Group and the Trial Steering Committee and provided advice from a service user perspective on the development of the intervention, and contributed to the progress and conduct of the trial.
-
Other contributors to programme development were GPs in the Department of Primary Care at UCL who kindly gave advice on the development of the personal risk letter: Dr Kate Walters, Professor Steve Iliffe, Dr Mary Howman, Dr Anita Berlin and Dr Geoff Wong.
-
Staff from the local UK PCRNs and Comprehensive Local Research Networks, who assisted with recruitment and service support costs, and local Research and Development Leads who helped to facilitate the research permissions for each area.
-
The local collaborators, service managers, advisors and administration staff of the 18 participating SSSs.
-
Practice managers and administrative staff at all of the participating general practices.
-
Research interviewers who carried out the telephone follow-up interviews.
Contributions of authors
Hazel Gilbert (Principal Research Fellow, Health Psychology) is the Principal Investigator, was involved in the conception of the study and the design and development of the intervention, with overall responsibility for the co-ordination and delivery of the study. She contributed to all outputs from the study, drafted and edited the final report, and is leading the dissemination of results at national and international conferences and to local SSSs.
Stephen Sutton (Professor of Behavioural Science) was involved in the conception and design of the study, the development of the intervention and contributed to drafting the final report, revising it and providing detailed feedback.
Richard Morris (Professor of Medical Statistics) is the trial statistician and advised on matters relating to power and sample size calculations and randomisation, on the analysis of the data and contributed to drafting the final report, revising it and providing detailed feedback.
Irene Petersen (Reader in Epidemiology and Statistics) is a statistician responsible for the analysis of the data and contributed to drafting the final report, revising it and providing detailed feedback.
Qi Wu (Research Fellow, Health Economics) conducted the analysis, interpretation and report writing for Chapter 5.
Steve Parrott (Senior Research Fellow, Health Economics) has contributed to matters relating to economic issues, and was involved in the conduct, analysis, interpretation and report writing for Chapter 5.
Simon Galton (SSS Manager) assisted with the design and service delivery aspects of the study, developed the taster sessions and advisor training protocol, and commented on the final report.
Dimitra Kale (Research Associate) was engaged in the recruitment of participants and data collection, has assisted with analysis of data, and conducted the analysis, interpretation and report writing for the barriers to attendance study (part of Chapter 4).
Molly Sweeney Magee (Research Associate) was engaged in the recruitment of participants and data collection, has assisted with analysis of data, and conducted the analysis, interpretation and report writing for the fidelity study (part of Chapter 4).
Leanne Gardner (Trial Manager) assisted with the design, oversaw the co-ordination and delivery of the study, prepared the pilot phase report and commented on the final report.
Irwin Nazareth (Professor of Primary Care) was involved in the conception and design of the study, the development of the intervention and contributed to drafting the final report, revising it and providing detailed feedback.
All authors made substantial contributions to conception and design and/or to acquisition of data and/or to analysis and interpretation of data. All authors read and approved the final manuscript.
Publications
Gilbert H, Sutton S, Morris R, Petersen I, Galton S, Wu Q, et al. Effectiveness of personalised risk information and taster sessions to increase the uptake of smoking cessation services (Start2quit): a randomised controlled trial. Lancet 2017; in press.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Healthy Lives, Healthy People: A Tobacco Control Plan for England. London: The Stationery Office; 2011.
- Office for National Statistics . Statistical Bulletin: Adult Smoking Habits in Great Britain, 2013 2014. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/compendium/opinionsandlifestylesurvey/2015-03-19/adultsmokinghabitsingreatbritain2013 (accessed 23 February 2015).
- Office for National Statistics . Do Smoking Rates Vary Between More and Less Advantaged Areas? 2014. http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/rel/disability-and-health-measurement/do-smoking-rates-vary-between-more-and-less-advantaged-areas-/2012/sty-smoking-rates.html (accessed 23 February 2015).
- Smoking Kills: A White Paper on Tobacco. London: The Stationery Office; 1998.
- McNeill A, Raw M, Whybrow J, Bailey P. A national strategy for smoking cessation treatment in England. Addiction 2005;100:1-11. http://dx.doi.org/10.1111/j.1360-0443.2005.01022.x.
- Ferguson J, Bauld L, Chesterman J, Judge K. The English smoking treatment services: one-year outcomes. Addiction 2005;100:59-6. http://dx.doi.org/10.1111/j.1360-0443.2005.01028.x.
- Ferguson J, Bauld L, Chesterman J, Judge K. The English smoking treatment services: short-term outcomes. Addiction 2005;100:46-58. http://dx.doi.org/10.1111/j.1360-0443.2005.01028.x.
- Chesterman J, Judge K, Bauld L, Ferguson J. How effective are the English smoking treatment services in reaching disadvantaged smokers?. Addiction 2005;100:36-45. http://dx.doi.org/10.1111/j.1360-0443.2005.01026.x.
- Lader D. Smoking-Related Behaviour and Attitudes, 2008/09. Report Number 40. London: Office for National Statistics; 2009.
- Owen N, Davies MJ. Smokers’ preferences for assistance with cessation. Prev Med 1990;19:424-31. http://dx.doi.org/10.1016/0091-7435(90)90040-Q.
- Lichtenstein E, Glasgow RE. Smoking cessation: what have we learned over the past decade?. J Consult Clin Psychol 1992;60:518-27. http://dx.doi.org/10.1037/0022-006X.60.4.518.
- Fiore MC, Novotny TE, Pierce JP, Giovino GA, Hatziandreu EJ, Newcomb PA, et al. Methods used to quit smoking in the United States. Do cessation programs help?. JAMA 1990;263:2760-5. http://dx.doi.org/10.1001/jama.1990.03440200064024.
- Schmid TL, Jeffery RW, Hellerstedt WL. Direct mail recruitment to home-based smoking and weight control programs: a comparison of strategies. Prev Med 1989;18:503-17. http://dx.doi.org/10.1016/0091-7435(89)90009-1.
- Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119-22. http://dx.doi.org/10.1016/j.amepre.2004.09.007.
- Edwards SA, Bondy SJ, Callaghan RC, Mann RE. Prevalence of unassisted quit attempts in population-based studies: a systematic review of the literature. Addict Behav 2014;39:512-19. http://dx.doi.org/10.1016/j.addbeh.2013.10.036.
- Hammond D, McDonald PW, Fong GT, Borland R. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction 2004;99:1042-8. http://dx.doi.org/10.1111/j.1360-0443.2004.00754.x.
- West R, Brown J. Smoking and Smoking Cessation in England in 2011: Findings from the Smoking Toolkit Study n.d. www.smokinginengland.info/latest-statistics/ (accessed 9 March 2015).
- Lifestyles Statistics Team, Statistics on NHS Stop Smoking Services in England, April 2014 to March 2015. Leeds: Health and Social Care Information Centre; 2015.
- Lowry RJ, Hardy S, Jordan C, Wayman G. Using social marketing to increase recruitment of pregnant smokers to smoking cessation service: a success story. Public Health 2004;118:239-43. http://dx.doi.org/10.1016/j.puhe.2003.09.010.
- Paul CL, Wiggers J, Daly JB, Green S, Walsh RA, Knight J, et al. Direct telemarketing of smoking cessation interventions: will smokers take the call?. Addiction 2004;99:907-13. http://dx.doi.org/10.1111/j.1360-0443.2004.00773.x.
- Van Deusen AM, Hyland A, Abrams SM, Celestino P, Mahoney MC, Cummings KM. Smokers’ acceptance of ‘cold calls’ offering quitline services. Tob Control 2007;16:i30-2. http://dx.doi.org/10.1136/tc.2007.020578.
- Cunningham JA, Ferrence R, Cohen J, Adlaf EM. Interest in self-help materials among a general population sample of smokers. Addict Behav 2003;28:811-16. http://dx.doi.org/10.1016/S0306-4603(01)00274-X.
- Tzelepis F, Paul CL, Walsh RA, Wiggers J, Duncan SL, Knight J. Active telephone recruitment to quitline services: are nonvolunteer smokers receptive to cessation support?. Nicotine Tob Res 2009;11:1205-15. http://dx.doi.org/10.1093/ntr/ntp125.
- Tzelepis F, Paul CL, Wiggers J, Walsh RA, Knight J, Duncan SL, et al. A randomised controlled trial of proactive telephone counselling on cold-called smokers’ cessation rates. Tob Control 2011;20:40-6. http://dx.doi.org/10.1136/tc.2010.035956.
- Marcano Belisario JS, Bruggeling MN, Gunn LH, Brusamento S, Car J. Interventions for recruiting smokers into cessation programmes. Cochrane Database Syst Rev 2012;12. http://dx.doi.org/10.1002/14651858.cd009187.pub2.
- McDonald PW. Population-based recruitment for quit-smoking programs: an analytic review of communication variables. Prev Med 1999;28:545-57. http://dx.doi.org/10.1006/pmed.1998.0479.
- Lichtenstein E, Hollis J. Patient referral to a smoking cessation program: who follows through?. J Fam Pract 1992;34:739-44.
- Fiore MC, McCarthy DE, Jackson TC, Zehner ME, Jorenby DE, Mielke M, et al. Integrating smoking cessation treatment into primary care: an effectiveness study. Prev Med 2004;38:412-20. http://dx.doi.org/10.1016/j.ypmed.2003.11.002.
- Murray RL, Coleman T, Antoniak M, Stocks J, Fergus A, Britton J, et al. The effect of proactively identifying smokers and offering smoking cessation support in primary care populations: a cluster-randomized trial. Addiction 2008;103:998-1006. http://dx.doi.org/10.1111/j.1360-0443.2008.02206.x.
- Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: the case for tailoring print materials. Ann Behav Med 1999;21:276-83. http://dx.doi.org/10.1007/BF02895958.
- Edwards AGK, Evans R, Hood K, Elwyn GJ. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.cd001865.pub2.
- Hill D, Chapman S, Donovan R. The return of scare tactics. Tob Control 1998;7:5-8. http://dx.doi.org/10.1136/tc.7.1.5.
- Sutton SR, Eiser JR. Social Psychology and Behavioural Medicine. Chichester: Wiley; 1982.
- Sutton S. Shock tactics and the myth of the inverted U. Br J Addict 1992;87:517-19. http://dx.doi.org/10.1111/j.1360-0443.1992.tb01953.x.
- Hastings G, MacFadyen L. Controversies in tobacco control: the limitations of fear messages. Tob Control 2002;11:73-5. http://dx.doi.org/10.1136/tc.11.1.73.
- Wakefield M, Freeman J, Donovan R. Recall and response of smokers and recent quitters to the Australian National Tobacco Campaign. Tob Control 2003;12:i15-22. http://dx.doi.org/10.1136/tc.12.suppl_2.ii15.
- Sheeran P, Abraham C, Connor M, Norman P. Predicting Health Behaviour: Research and Practice with Social Cognition Models. Buckingham: Open University Press; 1996.
- Curry SJ, Taplin SH, Anderman C, Barlow WE, McBride C. A randomized trial of the impact of risk assessment and feedback on participation in mammography screening. Prev Med 1993;22:350-60. http://dx.doi.org/10.1006/pmed.1993.1029.
- Weinstein ND. Accuracy of smokers’ risk perception. Nicotine Tob Res 1999;1:123-30. http://dx.doi.org/10.1080/14622299050011721.
- Weinstein ND, Marcus SE, Moser RP. Smokers’ unrealistic optimism about their risk. Tob Control 2005;14:55-9. http://dx.doi.org/10.1136/tc.2004.008375.
- West R, Sohal T. ‘Catastrophic’ pathways to smoking cessation: findings from national survey. BMJ 2006;332:458-60. http://dx.doi.org/10.1136/bmj.38723.573866.AE.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3. http://dx.doi.org/10.1002/14651858.cd001118.pub2.
- Roddy E, Antoniak M, Britton J, Molyneux A, Lewis S. Barriers and motivators to gaining access to smoking cessation services amongst deprived smokers – a qualitative study. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-147.
- Global Dialogue for Effective Stop Smoking Campaigns . Lessons Learned from International Literature Review and Unpublished Campaign Results 2006. www.cancer.org/acs/groups/content/@internationalaffairs/documents/document/acspc-034151.pdf (accessed 2 March 2015).
- Gilbert H, Sutton S, Morris R, Petersen I, Galton S, Wu Q, et al. Effectiveness of personalised risk information and taster sessions to increase the uptake of smoking cessation services (Start2quit): a randomised controlled trial. Lancet 2017.
- McEwen A. NHS Centre for Smoking Cessation and Training’s Standard Treatment Programme: One-to-One Smoking Cessation Support. London: NHS Centre for Smoking Cessation and Training; 2011.
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull 1992;111:23-41. http://dx.doi.org/10.1037/0033-2909.111.1.23.
- Etter JF, Perneger TV, Ronchi A. Collecting saliva samples by mail. Am J Epidemiol 1998;147:141-6. http://dx.doi.org/10.1093/oxfordjournals.aje.a009426.
- Copeland AL, Businelle MS, Stewart DW, Patterson SM, Rash CJ, Carney CE. Identifying barriers to entering smoking cessation treatment among socioeconomically disadvantaged smokers. J Smok Cessat 2010;5:164-71. http://dx.doi.org/10.1375/jsc.5.2.164.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; n.d.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Ali S, Godfrey CA, Parrott S. Economic Model of Adult Smoking Related Costs and Consequences for England. London: Public Health Research Consortium; 2011.
- Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol 2004;57:785-94. http://dx.doi.org/10.1016/j.jclinepi.2003.12.013.
- Sinclair HK, Bond CM, Lennox AS, Silcock J, Winfield AJ, Donnan PT. Training pharmacists and pharmacy assistants in the stage-of-change model of smoking cessation: a randomised controlled trial in Scotland. Tob Control 1998;7:253-61. http://dx.doi.org/10.1136/tc.7.3.253.
- Gilbert H, Nazareth I, Sutton S. Assessing the feasibility of proactive recruitment of smokers to an intervention in general practice for smoking cessation using computer-tailored feedback reports. Fam Pract 2007;24:395-400. http://dx.doi.org/10.1093/fampra/cmm028.
- Gilbert HM, Sutton SR, Leurent B, Alexis-Garsee C, Morris RW, Nazareth I. Characteristics of a population-wide sample of smokers recruited proactively for the ESCAPE trial. Public Health 2012;126:308-16. http://dx.doi.org/10.1016/j.puhe.2011.11.010.
- Eldridge S, Kerry S. A Practical Guide to Cluster Randomised Trials in Health Services Research. Chichester: Wiley; 2012.
- Gilbert H, Sutton S, Morris R, Parrot S, Galton S, Nazareth I. Evaluating the effectiveness of using personal tailored risk information and taster sessions to increase the uptake of smoking cessation services: study protocol for a randomised controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-195.
- Petersen I, Morris R. Statistical Analysis Plan For: A Randomised Trial to Increase the Uptake of Smoking Cessation Services Using Personal Targeted Risk Information and Taster Sessions 2014. www.ucl.ac.uk/start2quit/Publications (accessed 20 April 2016).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1258.
- Vitolins MZ, Rand CS, Rapp SR, Ribisl PM, Sevick MA. Measuring adherence to behavioral and medical interventions. Control Clin Trials 2000;21:188-94. http://dx.doi.org/10.1016/S0197-2456(00)00077-5.
- Sabaté E. Adherence to Long-Term Therapies – Evidence for Action. Geneva: World Health Organization; 2003.
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.cd001292.pub2.
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 Update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care 2008;53:1217-22.
- Pascoe GC. Patient satisfaction in primary health care: a literature review and analysis. Eval Program Plann 1983;6:185-210. http://dx.doi.org/10.1016/0149-7189(83)90002-2.
- Vosbergen S, Laan EK, Colkesen EB, Niessen MA, Kraaijenhagen RA, Essink-Bot M-L, et al. Evaluation of end-user satisfaction among employees participating in a web-based health risk assessment with tailored feedback. J Med Internet Res 2012;14. http://dx.doi.org/10.2196/jmir.2067.
- Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring Health Messages: Customising Communication with Computer Technology. Mahwah, NJ: Lawrence Erlbaum Associates; 2000.
- Enwald HPK, Kortelainen T, Leppäluoto J, Keinänen-Kiukaanniemi S, Jämsä T, . Oinas-Kukkonen . Perceptions of fear appeal and preferences for feedback in tailored health communication. An explorative study among prediabetic individuals. Inform Res 2013;18.
- Sutton S, Gilbert H. Effectiveness of individually tailored smoking cessation advice letters as an adjunct to telephone counselling and generic self-help materials: randomized controlled trial. Addiction 2007;102:994-1000. http://dx.doi.org/10.1111/j.1360-0443.2007.01831.x.
- Bennett K, Gilbert H, Sutton S. Computer-tailored smoking cessation advice matched to reading ability: perceptions of participants from the ESCAPE trial. Patient Educ Couns 2015;98:1577-84. http://dx.doi.org/10.1016/j.pec.2015.06.013.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23. http://dx.doi.org/10.1037/0278-6133.23.5.443.
- Perepletchikova F, Treat TA, Kazdin AE. Treatment integrity in psychotherapy research: analysis of the studies and examination of the associated factors. J Consult Clin Psychol 2007;75:829-41. http://dx.doi.org/10.1037/0022-006X.75.6.829.
- Santacroce SJ, Maccarelli LM, Grey M. Intervention fidelity. Nurs Res 2004;53:63-6. http://dx.doi.org/10.1097/00006199-200401000-00010.
- Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: assessment of adherence and competence. J Consult Clin Psychol 1993;61:620-30. http://dx.doi.org/10.1037/0022-006X.61.4.620.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-40.
- Michie S. What works and how? Designing more effective interventions needs answers to both questions. Addiction 2008;103:886-7. http://dx.doi.org/10.1111/j.1360-0443.2007.02112.x.
- Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011;36:315-19. http://dx.doi.org/10.1016/j.addbeh.2010.11.016.
- Dane AV, Schneider BH. Program integrity in primary and early secondary prevention: are implementation effects out of control?. Clin Psychol Rev 1998;18:23-45. http://dx.doi.org/10.1016/S0272-7358(97)00043-3.
- Hardeman W, Michie S, Fanshawe T, Prevost AT, Mcloughlin K, Kinmonth AL. Fidelity of delivery of a physical activity intervention: predictors and consequences. Psychol Health 2008;23:11-24. http://dx.doi.org/10.1080/08870440701615948.
- Lorencatto F, West R, Bruguera C, Michie S. A method for assessing fidelity of delivery of telephone behavioral support for smoking cessation. J Consult Clin Psychol 2014;82:482-91. http://dx.doi.org/10.1037/a0035149.
- Hughes JR, Marcy TW, Naud S. Interest in treatments to stop smoking. J Subst Abuse Treat 2009;36:18-24. http://dx.doi.org/10.1016/j.jsat.2008.04.002.
- Vogt F, Hall S, Marteau TM. Examining why smokers do not want behavioral support with stopping smoking. Patient Educ Couns 2010;79:160-6. http://dx.doi.org/10.1016/j.pec.2009.10.007.
- van Rossem C, Spigt MG, Kleijsen JR, Hendricx M, van Schayck CP, Kotz D. Smoking cessation in primary care: exploration of barriers and solutions in current daily practice from the perspective of smokers and healthcare professionals. Eur J Gen Pract 2015;21:111-17. http://dx.doi.org/10.3109/13814788.2014.990881.
- Christiansen BA, Reeder KM, TerBeek EG, Fiore MC, Baker TB. Motivating low socioeconomic status smokers to accept evidence-based smoking cessation treatment: a brief intervention for the community agency setting. Nicotine Tob Res 2015;17:1002-11. http://dx.doi.org/10.1093/ntr/ntu345.
- Smith AL, Carter SM, Chapman S, Dunlop SM, Freeman B. Why do smokers try to quit without medication or counselling? A qualitative study with ex-smokers. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2014-007301.
- Cattell RB. The scree test for the number of factors. Multivariate Behav Res 1966;1:245-76. http://dx.doi.org/10.1207/s15327906mbr0102_10.
- Balmford J, Borland R. What does it mean to want to quit?. Drug Alcohol Rev 2008;27:21-7. http://dx.doi.org/10.1080/09595230701710829.
- de Vet HC, Adèr HJ, Terwee CB, Pouwer F. Are factor analytical techniques used appropriately in the validation of health status questionnaires? A systematic review on the quality of factor analysis of the SF-36. Qual Life Res 2005;14:1203-18. http://dx.doi.org/10.1007/s11136-004-5742-3.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Drummond M, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford: Oxford University Press; 2001.
- Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost-effectiveness of computer-tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine Tob Res 2014;16:270-8. http://dx.doi.org/10.1093/ntr/ntt136.
- Statistics on NHS Stop Smoking Services in England 1 April 2013 to 31 March 2014. Final Report. Leeds: Health and Social Care Information Centre; 2014.
- Smoking Cessation Services: Costing Report. London: NICE; 2008.
- NHS Reference Costs 2012–13. London: Department of Health; 2013.
- Prescription Cost Analysis, England–2013. Leeds: Health and Social Care Information Centre; 2014.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics; 1995.
- Drummond M, Sculpher M, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Stat Med 1996;15:1447-58. http://dx.doi.org/10.1002/(SICI)1097-0258(19960715)15:13<1447::AID-SIM267>3.0.CO;2-V.
- Willan AR, O’Brien BJ. Confidence intervals for cost-effectiveness ratios: an application of Fieller’s theorem. Health Econ 1996;5:297-305. http://dx.doi.org/10.1002/(SICI)1099-1050(199607)5:4<297::AID-HEC216>3.0.CO;2-T.
- Severens JL, De Boo TM, Konst EM. Uncertainty of incremental cost-effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 1999;15:608-14.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Coyle D, Davies L, Drummond MF. Trials and tribulations. Emerging issues in designing economic evaluations alongside clinical trials. Int J Technol Assess Health Care 1998;14:135-44. http://dx.doi.org/10.1017/S0266462300010588.
- Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011;342.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38142.554479.AE.
- Rasmussen SR, Prescott E, Sørensen TI, Søgaard J. The total lifetime costs of smoking. Eur J Public Health 2004;14:95-100. http://dx.doi.org/10.1093/eurpub/14.1.95.
- Hlatky MA, Owens DK, Sanders GD. Cost-effectiveness as an outcome in randomized clinical trials. Clin Trials 2006;3:543-51. http://dx.doi.org/10.1177/1740774506073105.
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-203.
- Global Health Observatory Data Repository: Life Expectancy, Data by Country. Geneva: World Health Organization; n.d.
- Hughes JR, Giovino GA, Klevens RM, Fiore MC. Assessing the generalizability of smoking studies. Addiction 1997;92:469-72. http://dx.doi.org/10.1111/j.1360-0443.1997.tb03378.x.
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Individual differences in adoption of treatment for smoking cessation: demographic and smoking history characteristics. Drug Alcohol Depend 2008;93:121-31. http://dx.doi.org/10.1016/j.drugalcdep.2007.09.005.
- Gilbert H, Leurent B, Sutton S, Morris R, Alexis-Garsee C, Nazareth I. Factors predicting recruitment to a UK wide primary care smoking cessation study (the ESCAPE trial). Fam Pract 2012;29:110-17. http://dx.doi.org/10.1093/fampra/cmr030.
- Bauld L. Reaching smokers: how can we encourage more people to use effective treatment?. Addiction 2008;103:1007-8. http://dx.doi.org/10.1111/j.1360-0443.2008.02255.x.
- Pisinger C, Vestbo J, Borch-Johnsen K, Jorgensen T. Smoking cessation intervention in a large randomised population-based study. The Inter99 study. Prev Med 2005;40:285-92. http://dx.doi.org/10.1016/j.ypmed.2004.06.001.
- Gilbert HM, Leurent B, Sutton S, Alexis-Garsee C, Morris RW, Nazareth I. ESCAPE: a randomised controlled trial of computer-tailored smoking cessation advice in primary care. Addiction 2013;108:811-19. http://dx.doi.org/10.1111/add.12005.
- Ferguson SG, Shiffman S, Gitchell JG, Sembower MA, West R. Unplanned quit attempts – results from a U.S. sample of smokers and ex-smokers. Nicotine Tob Res 2009;11:827-32. http://dx.doi.org/10.1093/ntr/ntp072.
- Tzelepis F, Paul CL, Walsh RA, Wiggers J, Duncan SL, Knight J. Predictors of abstinence among smokers recruited actively to quitline support. Addiction 2013;108:181-5. http://dx.doi.org/10.1111/j.1360-0443.2012.03998.x.
- Borland R, Balmford J, Swift E. Effects of encouraging rapid implementation and/or structured planning of quit attempts on smoking cessation outcomes: a randomized controlled trial. Ann Behav Med 2015;49:732-42. http://dx.doi.org/10.1007/s12160-015-9706-3.
- West R, May S, West M, Croghan E, McEwen A. Performance of English stop smoking services in first 10 years: analysis of service monitoring data. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4921.
- Brose LS, West R, McDermott MS, Fidler JA, Croghan E, McEwen A. What makes for an effective stop-smoking service?. Thorax 2011;66:924-6. http://dx.doi.org/10.1136/thoraxjnl-2011-200251.
- McDermott MS, Beard E, Brose LS, West R, McEwen A. Factors associated with differences in quit rates between ‘specialist’ and ‘community’ stop-smoking practitioners in the English stop-smoking services. Nicotine Tob Res 2013;15:1239-47. http://dx.doi.org/10.1093/ntr/nts262.
- Bauld L, Chesterman J, Judge K, Pound E, Coleman T. English Evaluation of Smoking Cessation Services (EESCS) . Impact of UK National Health Service smoking cessation services: variations in outcomes in England. Tob Control 2003;12:296-301. http://dx.doi.org/10.1136/tc.12.3.296.
- Brose LS, McEwen A, West R. Does it matter who you see to help you stop smoking? Short-term quit rates across specialist stop smoking practitioners in England. Addiction 2012;107:2029-36. http://dx.doi.org/10.1111/j.1360-0443.2012.03935.x.
- McDermott MS, West R, Brose LS, McEwen A. Self-reported practices, attitudes and levels of training of practitioners in the English NHS Stop Smoking Services. Addict Behav 2012;37:498-506. http://dx.doi.org/10.1016/j.addbeh.2012.01.003.
- Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med 2013;173:458-64. http://dx.doi.org/10.1001/jamainternmed.2013.3751.
- Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health 2010;32:71-82. http://dx.doi.org/10.1093/pubmed/fdp074.
- Murray RL, Coleman T, Antoniak M, Fergus A, Britton J, Lewis SA. The potential to improve ascertainment and intervention to reduce smoking in primary care: a cross sectional survey. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-6.
- West R. Key Performance Indicators: Smoking and Smoking Cessation in England: Findings from the Smoking Toolkit Study. London: Smoking in England; n.d.
- Lowey H, Tocque K, Bellis MA, Fullard B. Smoking cessation services are reducing inequalities. J Epidemiol Community Health 2003;57:579-80. http://dx.doi.org/10.1136/jech.57.8.579.
- Deprivation in Camden: Analysis of 2007 Indices of Deprivation. London: London Borough of Camden; 2008.
- 2001 Census Key Statistics. London: Office for National Statistics; 2003.
- Oxford City Council . Oxford Population by Ethnic Group, 2009 n.d. www.oxford.gov.uk/Direct/Populationbyethnicgroup2009.pdf (accessed 16 January 2012).
- Oxfordshire Data Observatory . SOA Analysis of Oxfordshire’s Most Deprived Areas 2007. http://portal.oxfordshire.gov.uk/content/public/OCP/UO/themes/poverty/IMD_07/ID07_by_SOA.pdf (accessed 16 January 2012).
Appendix 1 Changes to protocol
The following changes to the protocol were made during the course of the research.
12 January 2011
-
To establish external validity of our results, anonymised average data will be collected from practices on patients who are invited to participate in the study but do not respond, in order to compare them with the responders on certain variables. At the end of recruitment in each practice, the names of all participants who have responded will be removed from the original list. For those remaining, the names will be removed, along with any other identifying data (i.e. address and NHS number), leaving gender, date of birth and postcode. The date of birth will be converted to age, and the postcode will be converted to a deprivation score (IMD via GeoConvert), and then removed from the spreadsheet. This spreadsheet, containing three variables – gender, age and IMD score – with no identifier, will then be taken to UCL and used to calculate means for these variables for each practice, and will be compared with these variables in responders. This will enable us to identify differences between those who have responded and those who have not, in order to establish whether or not our sample is generalisable to the rest of the practice population.
25 July 2011
-
If a smoker is not eligible to take part in the study we will send an ‘Ineligible’ letter to these individuals, indicating that they are unable to be included in the study. Furthermore, some smokers may return their questionnaires outside the timeframe for processing, in which case we will send these individuals a ‘Late Responder’ letter. Both letters will be sent from the surgery and will advertise the local NHS SSS and advise the smoker to contact the service for more information or to speak to an advisor.
10 August 2011
-
Participants will be asked to fill out a taster session evaluation form at the end of each session. The purpose of this evaluation form is to provide an ongoing assessment of participants’ perceptions of the taster session.
-
With participant consent, each taster session will be audio-recorded to ensure standardisation of delivery of taster sessions throughout the trial.
7 December 2011
-
All self-reported attendance will be validated by records of attendance. All participants will be asked if they attended a taster session; those answering negatively will be asked if they know or have spoken to anyone who attended a taster session.
-
Participants claiming 7-day abstinence will be asked to provide a saliva sample to biochemically validate 7-day point prevalent smoking cessation at this 6-month follow-up. Those who agree will be sent a saliva sample kit which they will be required to complete themselves and return by post. They will also be asked to complete a consent form and a short questionnaire to confirm their current smoking status and NRT usage. By way of compensation for participants’ time and to maximise kit return, a £5 Marks and Spencer voucher will be included with each postal kit.
9 May 2012
-
Advisors will be required to complete a Stop Smoking Advisor Personal Details Form. The information provided will ensure that ‘therapist effects’ are accounted for in the fidelity analysis.
-
To maximise retention of participants at the 6-month follow-up (70% retention obtained in pilot phase), additional procedures will be utilised to obtain follow-up data.
-
Interviewers will make a maximum of 10 attempts (based on experience in current and previous studies) to contact a participant by telephone at varying times of day and on different days. If a call to a participant is answered, interviewers will aim to complete the full follow-up telephone interview. However, if a participant does not fully complete or does not wish to complete this telephone follow-up interview, the interviewer will attempt to ask the participant four basic questions most relevant to the outcome measures. If a participant is unable to be contacted, a paper version of the follow-up questionnaire will be sent by post. This paper questionnaire will also be sent to participants who are unable to complete the telephone interview but are willing to fill in a paper questionnaire.
-
If, after 10 attempts on different days and times, the interviewers have been unable to speak to a participant in person, they will attempt to leave a message, either with another person or on an answer phone/voicemail. At this time, participants will also be sent a text message, prompting a response back to the mobile phone from which it was sent. This message will state, ‘six months ago you agreed to complete a follow-up phone interview for UCL smoking study Start2quit. Please text/call to let us know when we can contact you’. If a participant replies, they will be given a date and time convenient to them to complete the telephone interview. If no response is received after 3 days, the participant will be sent the same message a second time. If no response is received after a further 3 days, the participant will be sent a postal questionnaire.
-
-
All participants who report not attending the SSS will be asked to complete a further postal questionnaire. The 40-item TBQ has been validated on a US population and will be used to assess in more depth reasons and barriers to the use of the NHS SSS. We will validate this instrument on this UK population.
-
If saliva samples are not returned within 7 days, participants will be contacted by a research interviewer who will remind them to return their kit. Interviewers will make a maximum of 10 attempts to contact the participant at varying times of day on different days, as before, ensuring that the correct postal address is held for the participant. After 10 unsuccessful attempts to contact the participant, they will be sent a text message stating ‘Don’t forget to send your UCL smoking study saliva sample back to receive your additional £5 M&S’. If the interviewer is successful in contacting the participant but their sample is not returned within 7 days, the participant will be sent the same text message reminding them to return their kit. On the return of a saliva sample kit, the participant will be sent an additional £5 Marks and Spencers voucher thanking them for returning their kit.
16 October 2012
-
If the TBQ is not returned within 2 weeks, a reminder will be sent to prompt the participant to return the questionnaire.
12 November 2013
-
Additional funding granted: £631,263
-
Additional time granted: 12 months
-
In response to an invitation from the HTA programme, for projects successfully recruiting participants, to fund additional work which will build on and extend the studies.
Reasons for additional funding
The Start2quit study, assessing the effectiveness on attendance at the NHS services of proactive recruitment by a brief personal tailored letter and invitation to a taster session, is currently recruiting successfully. To maximise the utility of the study, the following additional work will build on and enhance its value.
-
It is important to know whether or not this intervention also translates to increased quit rates, and if the quit rates in people attending as a result of this intervention differ from the usual quit rates in NHS services. As well as powering the trial for the original primary outcome (the proportion of people entering the SSS over a period of 6 months), we will now also power the trial for the secondary outcome of 7-day point prevalent abstinence at the 6-month follow-up, validated by salivary cotinine analysis.
Assuming quit rates of 4% versus 2.2% in the intervention and control groups (mimicking the findings of Murray et al. 29), an 80% increase in the sample size is required, to 1793 in the control group and 2707 in the intervention group (assuming the same therapist effect as the original protocol), giving a total of 4500. A sample of this size would give 85.4% power to detect a difference of 1.8% at the 5% significance level. The same sample size would have 95% power to detect the difference between quit rates of 4.4% and 2.2% (doubling of quit rate).
Based on present recruitment figures, we estimate that with an additional eight SSSs (48 GP practices) we will recruit another 2060 participants, meeting the requirement of the power calculation.
-
Identification of barriers to attendance and the reasons for non-attendance in this sample, following an explicit invitation (either with risk information and taster session invitation or without), will help to develop strategies to overcome the barriers, and to allocate resources to encourage attendance, and thus increase the potential to recruit the optimum number of smokers to the services.
We are currently assessing barriers to attendance at the NHS services using an open question. For the remainder of the study we will use the TBQ, a 40-item measure of reasons for not entering smoking cessation programmes49 that has been recently validated on a low socioeconomic status population in the USA. This questionnaire will allow us to assess different aspects of smoker’s decisions to attend a group or therapy session and highlight any misconceptions or lack of awareness of the service offered. It will also allow us to explore any associations with demographic and dependence factors, as well as validating the questionnaire on a UK population. Participants who report not attending the services will be sent the questionnaire by post.
The TBQ will be mailed to approximately 3500 participants who report not attending the SSS and who agree to complete an additional questionnaire.
-
Taster sessions are being recorded to ensure fidelity to the protocol. Assessing this fidelity can help to address factors that might have impacted on subsequent attendance and quit rates. Extra time for a full analysis of these tapes, using thematic analysis, will allow the exploration of differences in style and delivery of the intervention and their impact on subsequent attendance.
Full analysis of the audio-recording of the taster sessions, using thematic analysis, will be carried out to address factors that might have impacted on subsequent attendance and quit rates.
Appendix 2 Pilot assessment report

Project number: HTA 08/58/02
A randomised trial to increase the uptake of smoking cessation services using personal targeted risk information and taster sessions
Pilot assessment report
Principal investigator: Hazel Gilbert.
Trial team
Trial manager: Leanne Gardner.
Assistant trial manager: Danielle Stoller.
Research assistants: Dimitra Kale and Molly Sweeney Magee.
Co-investigators
Irwin Nazareth, Richard Morris, Stephen Sutton, Irene Peterson and Simon Galton.
Research Department of Primary Care and Population Health
UCL
Royal Free and University College Medical School
London NW3 2PF
Introduction
Background
The NHS SSSs are effective in helping people to quit smoking,129 and, although 74% of current smokers in 2007 reported that they wanted to quit, only a tiny proportion (< 5%)130–132 made use of the free service provided by the NHS. A wide range of factors, such as lack of availability and accessibility, perceived inappropriateness of the service, a perception that help is not necessary, or a sense of a lack of empathy from health professionals, as well as a lack of readiness to quit, will bar smokers from seeking help.
Although recruitment methods to cessation services generally employ a reactive approach, in which smokers are expected to seek out and approach the service,20 evidence suggests that if smokers are proactively and personally invited to use the services, the resultant use will be higher than standard referral by health professionals, or open advertising. 27 The development of computer-tailored self-help materials, intended to meet the needs of one specific person, based on characteristics unique to that person,30 offers a method for further personalising communications to patients and has the potential to engage with and recruit a larger proportion of the smoking population in a relatively inexpensive way. In addition, previous research29 has shown that inviting smokers to attend a no-commitment introductory session designed to offer information about what attendance at the service would involve, together with a strong referral message to the service, can increase attendance.
Thus, the Start2quit intervention is employing a two-pronged approach to encourage people to attend the SSSs, that consists of using computer-tailoring technology to send personal risk information to smokers, and an invitation to a taster session to allow them to find out more about the service before committing and signing up to a 6 week course.
Principal research question
We hypothesise that smokers, identified from general practice records, sent brief personal tailored letters based on characteristics available in their primary care medical records and on a short screening questionnaire, and invited to a ‘Come and Try it’ taster session designed to inform them about the SSSs, are more likely to attend the services than those who receive a standard generic letter advertising the service.
Primary objective
To assess the relative effectiveness on attendance at the SSSs, of proactive recruitment by a brief personal letter, tailored to individual characteristics available in medical records, and invitation to a taster session to provide information about the SSSs, over a standard generic letter advertising the service.
Design and procedure
Start2quit is a randomised controlled trial of a two-component intervention conducted in two stages. First, a pilot trial to be carried out in 12 practices covered by two SSSs was scheduled to run from June 2010 to December 2011. Procedure to the full trial in a further 48 practices covered by eight SSSs is dependent on the success of the pilot in terms of recruitment, the rate of attendance at the taster session, and the uptake of SSSs. The methodology of the pilot phase is essentially the same as the full trial to enable combination of the data from both phases for analysis. However, lessons learnt on recruitment strategies from the pilot phase will be applied to the main trial.
The target group is smokers motivated to quit who have not attended the SSSs in the last 12 months. We will also target smokers in areas of high deprivation and ethnic minorities, where smoking prevalence is high.
Procedure
All smokers aged ≥ 16 years are identified from their medical records in participating practices. After screening by GPs to exclude anyone deemed to be unsuitable, all remaining persons on the list are sent a screening questionnaire with a cover letter from their GP, a participant information leaflet describing the research, and a consent form to participate in the trial. Participants are asked to provide consent for the release of relevant data from their attendance at the SSSs to the researchers, used to validate attendance and quit rates. Participants have the opportunity to decline to participate but to return the questionnaire with Section A only completed to update their smoking status in their records.
All current smokers willing to participate and returning the signed consent form, aged ≥ 16 years, able to read English, motivated to quit and have not attended the SSSs in the previous 12 months are eligible to participate. Exclusion criteria are minimal because the aim is to recruit all smokers into the SSSs.
Interventions
The control group receive a standard generic letter sent from the surgery advertising the SSS and asking the smoker to contact the service to make an appointment to see an advisor.
The intervention group receive a brief motivational letter offering personalised risk information and help to improve their condition by quitting smoking, sent from the GP. This contains information specific to the patient, including an invitation and an appointment to attend a ‘Come and Try it’ taster session run by advisors from the local SSS to find out more about the services. Each SSS runs between 4 and 12 taster sessions, and attendees are encouraged to sign up to a group or one-to-one session at the end of the taster session. Participants who fail to attend will receive a further invitation 3 months later to encourage attendance.
Pilot phase
Objectives
The aims of the pilot phase of the study were:
-
to assess the feasibility of the procedure in terms of:
-
searching medical records and mailing screening questionnaires
-
the randomisation and generation of the tailored letters
-
delivery of the intervention.
-
-
to ascertain recruitment rates
-
to assess the uptake of the taster sessions and subsequent attendance at the SSSs.
The criteria for judging the success of the pilot phase and proceeding to full trial is based on:
-
achieving a 7% response rate (i.e. a mean of 42 participants per practice giving consent and agreeing to randomisation) in the first 12 practices
-
a preliminary analysis that suggests that the difference in uptake of SSSs between the intervention and control groups is greater than zero.
Successful achievement of recruitment, the rate of uptake in attendance at the taster session, and the uptake of SSSs in the intervention group will allow progression to the main trial in a further eight areas that are representative of the SSS.
Planned recruitment
We planned to recruit practices and participants through the PCRN, in two areas selected for the pilot phase. Practices generally identify 13% to 22% of their patients as smokers,55 depending on the characteristics of the patient population, and the accuracy and completeness of the records. Based on the response rate from previous and ongoing studies29,56 we estimated a response rate of 7% from smokers motivated to quit, from two mailings. By selecting six practices in each of the two SSSs with a list size of > 4000, we aimed to secure 504 participants to the pilot phase of the randomised controlled trial.
Progress
The pilot phase for Start2quit was carried out between September 2010 and December 2011.
Research and development approval
The two original SSSs selected for the pilot phase of Start2quit were Camden and Berkshire East. Although provisional approval was gained from Berkshire East Primary Care Trust (PCT), the SSS manager made a late decision to withdraw from the trial. With the help of the CLRN lead in the Thames Valley (Dot Powers), we were able to recruit Oxfordshire SSS as the second area for the pilot phase. Research and development approval was granted by our lead NHS site, Camden PCT, on 16 December 2010, followed by site-specific approval for Oxfordshire PCT on 21 January 2011.
1. Assessing the feasibility of the procedure in terms of:
Searching medical records and mailing screening questionnaires
During the pilot phase, procedures for searching medical records and mailing screening questionnaires for the recruitment of participants were implemented. A search strategy for identifying current smokers aged over 16 years on computerised records at GP practices was created and used by practice staff. Although there were small differences in the codes that practices use to record patients’ smoking status, our search strategy was effective in identifying potential participants for this project. Using computer programs written specifically for Start2quit, invite letters were generated in practices and sent with screening questionnaires to smokers identified in the initial search. One such program was designed to successfully remove duplicate addresses, ensuring that only one smoker per address was invited to the study. Sending invite letters and questionnaires with pre-addressed Freepost envelopes ensured that responses were returned to the practices without charge to the participants. The mailing of the invites was performed by trained practice staff aided by members of the research team and instruction manuals (Practice Manual and Research Manual).
The randomisation and generation of the tailored letters
The subsequent randomisation of fully consenting, eligible participants was performed independently by a computer program designed during the pilot phase. This program also generated the intervention and control letters. Data from questionnaires and medical records of consented participants were collated and entered on to an Excel spreadsheet. Using this spreadsheet, the computer program was able to randomly assign each participant to the control or intervention group and then generate the personal tailored risk letters and taster session invitation cards for participants in the intervention group, and control letters for participants in the control group. This program was run effectively by trained research staff visiting the practices. Some minor amendments to the intervention and control letters were required because of differences in the organisation of SSSs, but these changes did not hamper the progress of the pilot phase. As a result of lessons learnt during the pilot phase of Start2quit, minor amendments have been made to all computer programs used in the study. Many of these changes have made the programs more ‘user-friendly’ and have reduced process time.
Delivery of the intervention
Procedures for the delivery of taster sessions to participants in the intervention group were also created during the pilot phase of Start2quit. In collaboration with advisors from the SSSs, a protocol for running taster sessions and an Advisor Training Manual were prepared to ensure standardisation of the delivery of the sessions. Advisors, trained by the research team to run taster sessions, ran these sessions between March and July 2011 in Camden and Oxfordshire SSSs. In total, four taster sessions were run by Camden SSS and 10 sessions were run by Oxfordshire SSS (five sessions in Oxford and five sessions in Banbury). In each area, participants were invited to one of three initial taster sessions. If they failed to attend, they were then invited to another taster session held 3 months later. Depending on participant numbers in each area, either one or two more sessions at this 3-month time point were required. Throughout the pilot phase, a number of documents required for the effective running of taster sessions were found to be required. These included an audio-recording consent form, evaluation form and an advisor details form.
2. Ascertain recruitment rates
The rate of participant recruitment to the trial throughout the pilot phase was determined and is presented in the Results section of this report.
3. Assessment of the uptake of the taster sessions and subsequent attendance at the Stop Smoking Services
The number of participants who were randomised to the intervention arm and, subsequently, attended a taster session was recorded throughout the pilot phase and is presented in the Results section of this report.
The primary outcome of attendance at the SSSs for Start2quit is ascertained from records at the SSS. SSS records are updated quarterly and, therefore, not all participant data are available at present. Data from Oxfordshire SSS will be available in March 2012. Therefore, we will use participant self-reported attendance as a proxy measure for the primary outcome for the pilot analysis described in this report.
Self-reported attendance was collected by research telephone interviewers, independent from the service providers and research team, via follow-up interviews with participants 6 months after the date of randomisation. A computer program, which guides the telephone interviewers through this process was developed and used during the pilot phase. A Telephone Interviewer Manual was designed to ensure standardisation of the delivery of the interviews. These follow-up interviews were conducted between September 2011 and December 2011 for all participants in Camden and Oxfordshire.
Participant self-reported attendance at the SSSs was obtained from the 6-month follow-up interviews, which contained the following questions relevant to this outcome measure:
-
Have you tried to make an appointment with the SSS (this includes making an appointment with a practice nurse or health-care assistant at your surgery for stop smoking advice)?
-
Were you successful in making an appointment with the SSS?
-
How many times in the last 6 months have you attended any appointments with the SSS (this includes making an appointment with a practice nurse or health-care assistant at your surgery for stop smoking advice)?
If the participants answered ‘yes’ to the first two questions, and reported at least one appointment in responding to question 3, then they were classified as having attended with the SSS. All others were classified as not having attended. Analysis followed the intention-to-treat principle. Those lost to follow-up were assumed to have not attended the SSS.
Descriptive analysis was carried out. For each treatment group the proportion of participants who attended out of all participants in that group was calculated. These proportions are presented for all participants and for each SSS separately.
Results
Recruitment
Practices
In collaboration with the PCRN in each area, we recruited seven GP practices, three in Camden and four in Oxfordshire, between December 2010 and February 2011. Although we originally intended to recruit 12 practices, the total number of smokers approached to take part in the study over the seven practices (7792) was greater than the original target (7200 smokers).
The two SSSs that participated in the pilot phase (Camden and Oxfordshire) both have areas of high deprivation and above-average ethnic diversity. 133–135 IMD 2007 average scores were used to assess the deprivation scores for each area involved in the pilot phase of the trial. These scores provide a population-weighted average of the combined IMD scores for the particular neighbourhoods (called lower-layer super output areas) in a particular district. Data from Camden (IMD 2007) indicate that the borough is within the 20% most deprived areas on five of the six key measures. 133 Oxfordshire also covers some areas where deprived and ethnic minorities form a high proportion of the population. The non-white population is 26.8% in Camden, and the proportion born outside the UK and Eire is 33.6%. 134 In Oxfordshire, it has been reported that 19.6% of the population is non-white. 135
The study aimed to target areas within Camden and Oxfordshire that were relatively deprived, within the limitations of co-ordinating sites for ‘taster’ sessions, GP practices declining to participate and time constraints. We were successful in recruiting a representative selection of practices including some in deprived areas (e.g. practice 5 was located in an area within the 20% most deprived areas in England, and within the 10% most deprived area in England in the areas of education, skills and training and crime). 136
Participants
Figure 22 shows a flow diagram of recruitment progress through the pilot phase of the trial.
FIGURE 22.
Flow diagram of the progress of the pilot phase of the Start2quit trial. a, This figure relates to the proxy measure (self-reported attendance at the SSSs) for the primary outcome of the pilot phase, which was obtained through follow-up telephone interview. We expect to collect primary outcome data of attendance at the SSSs for 100% of participants from records held at each SSS involved in the study. We have assurance from the Oxfordshire SSS that these data will be available to us in March 2012.
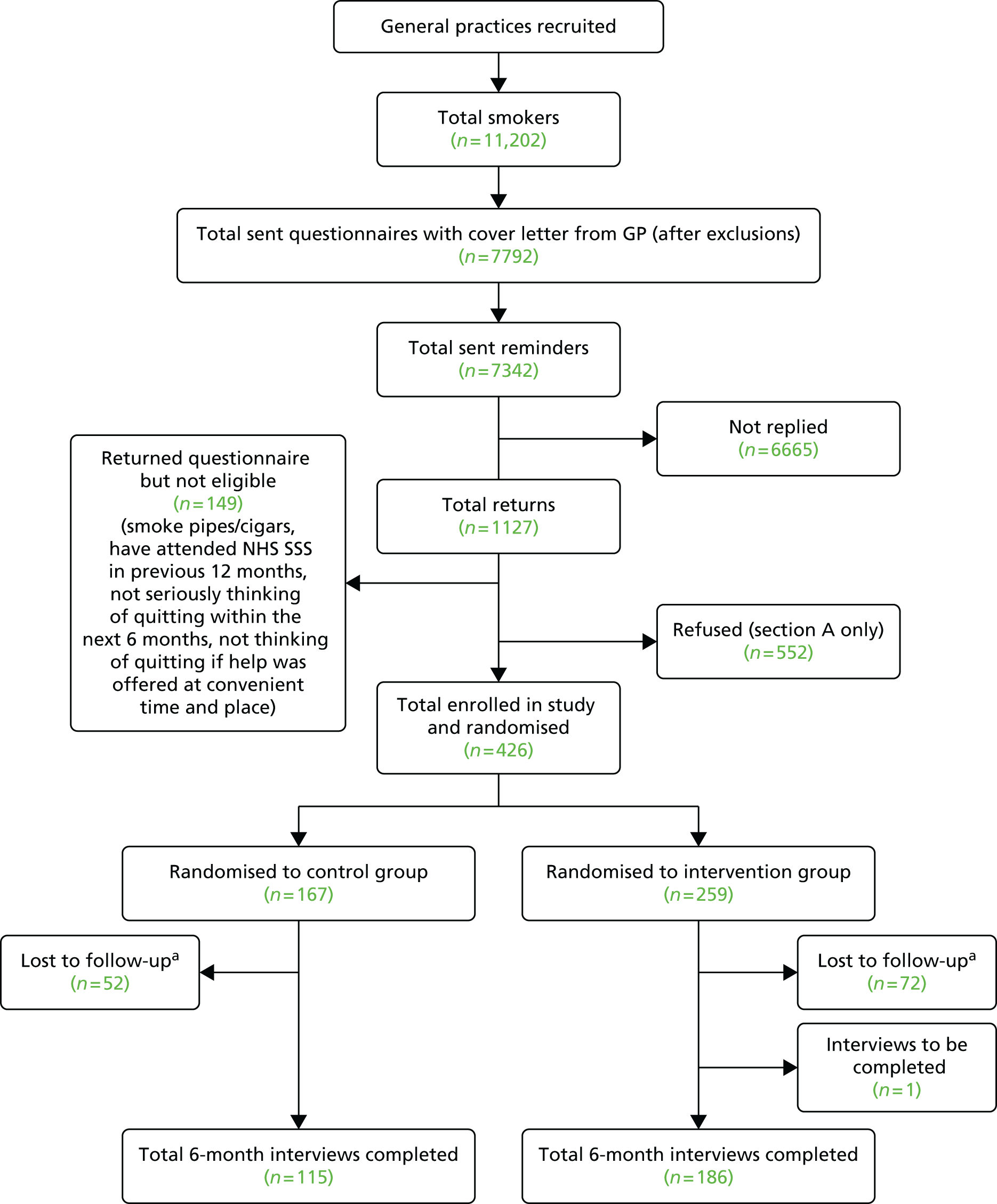
Although 11,202 smokers were initially identified, screening questionnaires with cover letters were mailed to 8548 smokers. This decrease was largely due to the necessity of selecting only one smoker per address. In addition, some patients identified as smokers were found to be ineligible after the mailing had taken place (n = 756; incorrectly coded, wrong address, deceased or ineligible due to answers in the questionnaire; see Table 41 for figures) and were therefore deleted from the denominator, leaving a total of 7792 eligible smokers who were mailed a questionnaire and invited to participate in the study. Reminder invitation letters were sent out to non-responders (n = 7342).
| Practice | Practice size | Smokers | Invitation | Invitations (ineligible + non-smoker + wrong address/deceased) | Ineligible | Non-smoker | Wrong address/deceased | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replies | Willing to participate | Eligible | Ineligible | Section A only | |||||||||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | ||||||||
| Practice 1 (Camden) | 11,800 | 1850 | 1567 | 1506 | 21 | 40 | 0 | 140 | 9.3 | 62 | 4.1 | 41 | 2.7 | 21 | 1.4 | 78 | 5.2 |
| Practice 2 (Camden) | 4251 | 299 | 246 | 238 | 8 | 0 | 0 | 38 | 16.0 | 22 | 9.2 | 14 | 5.9 | 8 | 3.4 | 16 | 6.7 |
| Practice 3 (Camden) | 7340 | 1486 | 1182 | 1160 | 12 | 9 | 1 | 103 | 8.9 | 50 | 4.3 | 38 | 3.3 | 12 | 1.0 | 53 | 4.6 |
| Practice 4 (Oxfordshire) | 8087 | 1333 | 1028 | 986 | 20 | 18 | 4 | 183 | 18.6 | 113 | 11.5 | 93 | 9.4 | 20 | 2.0 | 70 | 7.1 |
| Practice 5 (Oxfordshire) | 10,265 | 2075 | 1579 | 1279 | 19 | 279 | 2 | 159 | 12.4 | 91 | 7.1 | 72 | 5.6 | 19 | 1.5 | 68 | 5.3 |
| Practice 6 (Oxfordshire) | 16,721 | 2852 | 2298 | 2187 | 54 | 53 | 4 | 403 | 18.4 | 189 | 8.2 | 135 | 6.2 | 54 | 2.5 | 214 | 9.8 |
| Practice 7 (Oxfordshire) | 7321 | 1297 | 648 | 436 | 15 | 191 | 6 | 101 | 23.2 | 48 | 11.0 | 33 | 7.6 | 15 | 3.4 | 53 | 12.2 |
| Total | 8548 | 7792 | 149 | 590 | 17 | 1127 | 14.5 | 575 | 7.3 | 426 | 5.5 | 149 | 1.9 | 552 | 7.1 | ||
The total number returning the questionnaire was 1127 (14.5%). Of these, 552 (7.1%) completed section A only to update their practice records and were not willing to participate. A total of 575 (7.3%) gave consent and agreed to randomisation, and 149 of these were not eligible to participate, leaving 426 (5.5%) enrolled in the study (Table 41). To account for therapist effects, the sample size in the intervention group was inflated by 1.51 and eligible participants were randomised to the intervention or control group at a ratio of 3 : 2 within each practice. Thus, 259 participants were randomised to the intervention and 167 to the control group. The overall number in each group conformed closely to the randomisation ratio. The response rate varied greatly between GP practices (ranging from 2.7% to 9.4%) and between the two SSSs (Camden, mean 3.2%; Oxfordshire, mean 6.8%; Figure 23).
FIGURE 23.
Response rates for practices in the pilot phase.
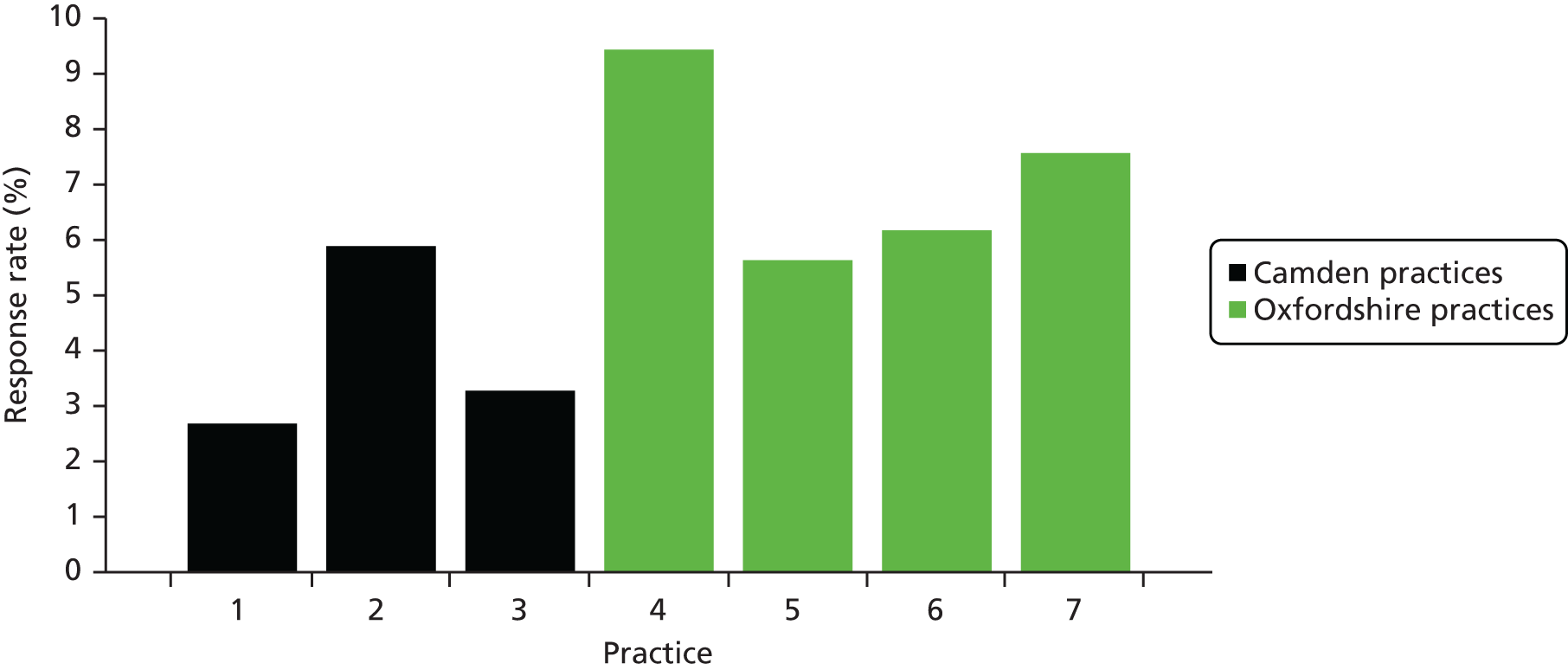
Attendance at taster sessions
In the pilot phase, taster sessions were run by Camden and Oxfordshire SSSs. As mentioned earlier, participants were invited to one of three initial taster sessions. If they failed to attend, they were then invited to another taster session 3 months later. A total of four taster sessions were held in Camden and a total of 10 sessions were held in Oxfordshire, split evenly between the two areas in which GP practices were recruited from in Oxfordshire (Oxford and Banbury).
Attendance rates at the three initial taster sessions were broadly consistent (Figure 24). However, it is important to note that there was relatively low attendance at the taster sessions held 3 months later. In total, 87 out of 259 participants randomised to the intervention group (33.6%) attended a taster session.
FIGURE 24.
Taster session attendance rates.
Follow-up response rate
The completion rate for the follow-up interviews was 70.7%. The response was equivalent in the intervention and control groups (71.8% vs. 68.9%). Self-reported attendance at the SSSs obtained from these interviews will be used as the proxy measure of the primary outcome in the pilot phase.
Attendance at the Stop Smoking Services
Self-report data confirm that the uptake of attendance at the SSSs was greater in the intervention group (70/259; 27%) than in the control group (21/167; 12.6%). Table 42 shows the differences in attendance at the SSSs between the intervention and control groups. These figures assume that those who were lost to follow-up did not attend the SSSs.
| Intervention, f (%) | Control, f (%) | |
|---|---|---|
| All participants (n = 426) | 70/259 (27.0) | 21/167 (12.6) |
| Camden (n = 93) | 14/60 (23.3) | 1/33 (3.0) |
| Oxfordshire (n = 333) | 56/199 (28.1) | 20/134 (14.9) |
Discussion
This report and analysis has set out to demonstrate that the aims of the pilot phase of the study have been met.
The first aim of assessing the feasibility of the procedure in terms of searching medical records and mailing screening questionnaires, generating the tailored letters, and randomising participants and delivering the intervention has been met. These procedures have been carried out successfully. Minor revisions to the programs and procedures will ensure their smooth running in the main trial. Manuals have been produced documenting all procedures to ensure the standardisation of all procedures in the main trial.
Variation in the delivery of services in different areas has meant that we have had to introduce a little more flexibility in the personnel delivering the taster sessions, and in the procedure for signing up smokers to attend the services, and allow more flexibility to fit in with the existing service delivery. Minor revisions to the procedure have been made to account for these variations.
The second aim was to ascertain recruitment rates. The proportion of people giving consent and agreeing to randomisation was 7.3%, meeting the target of 7%. However, not all of those willing to participate satisfied the inclusion criteria and thus the proportion eligible to participate was 5.5%, although this varied greatly between the two SSSs and between GP practices. While in Camden, one practice fell far short of the target at 2.7% enrolment, the highest enrolment from a practice in Oxfordshire was well over, at 9.4%. The low response rate observed in Camden is in accordance with previous studies115 that have shown London-based primary care recruitment rates to be lower than those outside London. Thus, taking the recruitment from Oxfordshire alone as an estimate, an enrolment rate of approximately 7% for the main trial is achievable in areas that are outside the London area. This is highly relevant as all future recruitment centres will take place outside London.
The third aim was to assess the uptake of the taster sessions and subsequent attendance at the SSSs, on which the criteria for judging the success of the pilot phase and proceeding to full trial is based. Attendance at taster sessions of 33.6% was considered acceptable, and service managers were pleased with the result, expressing the opinion that smokers went along who would not have otherwise considered using the SSSs. The uptake of the SSSs in the intervention group was more than twice that of the control group in Camden, and substantially higher than the control group in Oxfordshire. Therefore, the criterion of a greater than zero difference for attendance at the SSS between the intervention and control groups has been satisfied.
Some problems with the NHS Permission Process have been encountered by the project during the pilot phase, leading to delays in obtaining ethics approval letters, and in gaining approval for Service Support Costs from the comprehensive local research network (CLRN). In addition, we suffered a major setback when Berkshire East dropped out of the pilot phase at a late stage, leaving us short of one study site, less than 2 months before the start of recruitment for the pilot phase. Thanks to the CLRN lead in Thames Valley, we were able to promptly engage with Oxfordshire SSS, and have subsequently been able to meet the project milestones.
Conclusion and future timetable
We have met the criteria of a 7% response rate for those giving consent and agreeing to randomisation. The number enrolled in the trial fell slightly below this, but this was mainly because of low recruitment in Camden. We are now focusing on a representative selection of SSSs across England, outside London, and are confident that we can meet the target for main trial.
We have also demonstrated in our preliminary analysis that the difference in uptake of SSSs between the intervention and control groups is greater than zero. Although the numbers taking part in this phase are low, and cannot be generalised, we believe that the results shown here warrant the continuation of this trial to evaluate the intervention across a representative selection of SSSs across England.
The SSSs in Medway, Eastern and Costal Kent, Essex and Lincolnshire have already agreed to participate in the trial. We believe that with a timely start to the main trial in these areas we will be able to meet recruitment targets and proceed to a satisfactory conclusion of the trial. The timetable shows how we plan to meet the project milestones and to reach our target for recruitment (Figures 25 and 26).
FIGURE 25.
Actual recruitment vs. target recruitment for Start2quit.
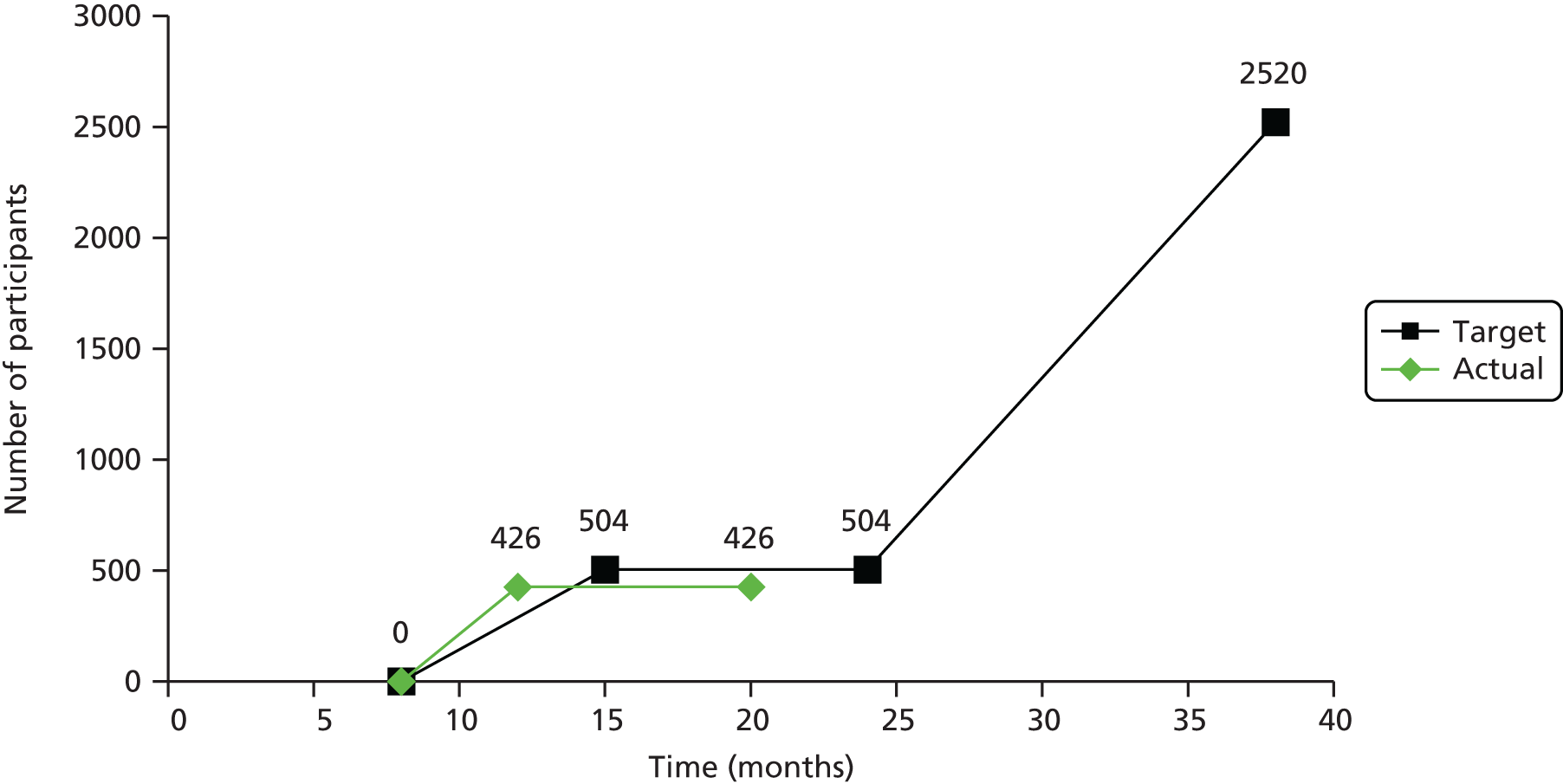
FIGURE 26.
Timetable for Start2quit. R&D, research and development.

Appendix 3 Recruitment materials
Invitation letter
Patient information sheet
Consent form
Late response letter
Standard ineligible letter
Appendix 4 Questionnaires and data collection forms
Baseline smoking questionnaire
Case report form
Stop smoking advisor details questionnaire
Taster session evaluation form
Follow-up questionnaires
Telephone interview
Postal questionnaire
Basic questions
Stop Smoking Service validation form
Treatment Barriers Questionnaire
Appendix 5 Intervention materials
Personal tailored risk intervention letter
Taster session invitation
Generic control letter
Appendix 6 Taster session training manual and protocol
List of abbreviations
- BCT
- behaviour change technique
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- CO
- carbon monoxide
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- NICE
- National Institute for Health and Care Excellence
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- PCA
- principal components analysis
- PCRN
- Primary Care Research Network
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- SD
- standard deviation
- SSS
- Stop Smoking Service
- TBQ
- Treatment Barriers Questionnaire
- UCL
- University College London