Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0608-10163. The contractual start date was in September 2010. The final report began editorial review in October 2017 and was accepted for publication in January 2019. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Cordingley et al. This work was produced by Cordingley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Cordingley et al.
SYNOPSIS
Context of the IMPACT programme
Psoriasis is a common, lifelong inflammatory skin disease, the severity of which can range from limited disease involving a small body surface area to extensive skin involvement. 1 It is associated with high levels of physical and psychosocial disability2–4 and a range of comorbidities, and it is currently incurable.
The majority of patients develop the first signs of psoriasis before the age of 40 years;5 thus, the disease makes significant demands on these patients through their productive adult life. 6 The long-term, visible and fluctuating nature of psoriasis means that its effects can be as disabling as other long-term conditions, with significant experiences of stigmatisation, low self-esteem and even suicidal thinking. 7–9 The relationships between physical or clinical severity of psoriasis and the degree of psychological or social disability relating to the condition are complex. It is not unusual for individuals with psoriasis affecting small body surface area to experience significant and disabling impairment to quality of life. 10 However, most of those with moderate to severe psoriasis experience some level of psychological distress at some point in their lives, depending on the time of onset. 11
In the UK, the majority of patients with psoriasis are managed in primary care by their general practitioner (GP) or, occasionally, by primary care dermatology services. At the time of the original IMPACT programme proposal in 2009, we had indications that dissatisfaction with psoriasis care was widespread and a large survey from the Psoriasis Association of the UK and Ireland reported that one-quarter of respondents had not consulted a health-care professional about their psoriasis in the previous 2 years. 12 There was evidence that, even in secondary care services, clinicians did not detect or attend to high levels of associated distress. 11
Psoriasis and comorbidities
Common comorbidities of psoriasis include psoriatic arthritis (PsA),13,14 Crohn’s disease,15 metabolic syndrome16–19 and non-alcoholic fatty liver disease. 20 In addition, people with psoriasis often have conditions that are associated with an increased risk of cardiovascular disease (CVD) such as obesity,21 hypertension, hyperlipidaemia22,23 and type 2 diabetes. 24
In the few years prior to initiation of the Identification and Management of Psoriasis Associated ComorbidiTy (IMPACT) programme, the findings of a number of studies supported the idea of a direct association between psoriasis and the development of CVD,18,25 with some estimating that the relative risk of CVD in those with severe psoriasis is triple that in the non-psoriasis population. 26 The main hypothesis accounting for the association was that increased systemic inflammation, as may occur in psoriasis, exacerbates other chronic inflammatory processes, including the development of atherosclerosis, which could lead to myocardial infarction (MI) or stroke. 15,27 However, the possible link between psoriasis and CVD is complex for several reasons: people with psoriasis are more likely to have unhealthy lifestyles (increased likelihood of smoking, little physical activity and obesity),28 there is a higher prevalence of co-existing CVD risk factors (such as type 2 diabetes, hypertension and hyperlipidaemia)23 and therapies for psoriasis may increase (e.g. ciclosporin)28 or decrease (e.g. methotrexate)29 the CVD risk. All of these aspects may confound the associations between psoriasis and other disorders. 30,31
There were some preliminary research findings that indicated that reducing behavioural CVD risk factors through, for example, weight loss, improving diet and/or increasing physical activity can reduce psoriasis severity32,33 at the same time as reducing CVD risk. However, our research team had recognised that psychological distress can impede people’s capacity to make behavioural changes that would improve both skin and heart health. This led to a question about whether or not it would be possible to develop interventions that would reduce psoriasis severity and risks of associated comorbidities and that took account of the existing challenges faced by some people with psoriasis.
Health-care professionals managing people with psoriasis could be well placed to support patients’ lifestyle behaviour change (LBC) in terms of timeliness of patient contact and access; however, the capacity of services to deliver behavioural change interventions was unknown. There was no published literature on whether or not LBC skills are included in the training curricula for relevant health-care professionals, or whether health-care professionals were equipped with the knowledge, skills and confidence to manage psoriasis as a complex, long-term condition, including supporting patients with lifestyle change. The views of health-care professionals about managing patients with psoriasis in primary care were also unknown.
Importance and relevance of the IMPACT programme
All evidence at the time of the original IMPACT programme proposal highlighted the enormous unmet need in terms of service access and provision for individuals with psoriasis, particularly in primary care, despite growing awareness in the dermatology community of very significant levels of distress and psychological and social impacts in this group. Unmet needs included extremely limited access to appropriate treatments, poor referral practices and the limited knowledge base of primary care professionals and patients. Knowledge about comorbidities, their association with disease mechanisms, and the impact of psoriasis was also poor in primary care and this corresponded with an apparently low prioritisation of service provision or development. It was clear that a large segment of the population with psoriasis were not benefiting from either recent developments in the understanding of the disease or interventions that could reduce their risk of comorbidities.
The growing interest in the apparent increase in risk for CVD conferred by psoriasis was an additional impetus. Again, the emerging evidence about risk mapped onto clinical observations in the dermatology community that poor health behaviours were more prevalent in patients with psoriasis than in patients with other skin diseases. 34 The third component in this mix, one that was identified quickly because of the strong interest in behavioural medicine in the existing dermatology research group, was the potential role of psychological and behavioural factors as important mediators and/or moderators of these risk factors. Thus, a comprehensive research strategy was developed to investigate whether or not it would be possible to identify opportunities in existing health-care pathways that would help us, first, to identify those at most risk of psoriasis-related comorbidities and, second, to intervene to improve health outcomes in an economically efficient way.
Original aims and objectives
The programme of work set out to identify optimal methods of investigating and managing psoriasis-related comorbidities. It broadly aimed to apply existing knowledge of psoriasis as a complex, multifaceted condition requiring holistic management to find ways to improve the care and self-care of people living with this condition.
The original IMPACT programme application proposed five workstreams (Figure 1) to address gaps in knowledge and thereby provide a platform from which to develop services. Each workstream was set up to address specific aims, with the findings from workstreams 1–4 being used to inform the development of interventions based on primary care to improve outcomes for people with psoriasis addressed in workstream 5.
FIGURE 1.
Relationships between IMPACT programme workstreams.

Modifications to original aims
During a number of the studies, opportunities were identified to supplement the original plans with additional studies; these included workstreams 2, 3 and 4 (Table 1). Thus, as well as meeting our original objectives, we undertook additional work.
| Workstream | Programme objective | Study undertaken | Report section |
|---|---|---|---|
| 1 | Objective 1: to confirm which patients with psoriasis are at highest risk of developing additional long-term conditions and identify service use and costs to patient | Study 1.i: epidemiology of psoriasis and costs of care. Systematic reviews | Epidemiology of psoriasis and its association with risk of cardiovascular disease |
| Study 1.ii: association between psoriasis and risk of CVD (1.i/ii) | Epidemiology of psoriasis and its association with risk of cardiovascular disease | ||
| Study 1.iii: modelling of health-care costs for people with psoriasis | Health economics modelling of costs of interventions for people with psoriasis | ||
| 2 | Objective 2: to apply knowledge about risk of comorbid disease to the development of targeted screening services to reduce risk of further disease and to investigate how this affects patient experience | Study 2.i: screening in primary care for CV risk in psoriasis patients | Cardiovascular risk in patients with psoriasis: screening study and pulse wave velocity measures in people with psoriasis |
| Study 2.ia: assessing the impact of psoriasis severity on artery health (pulse wave velocity) | Cardiovascular risk in patients with psoriasis: screening study and pulse wave velocity measures in people with psoriasis | ||
| Study 2.ii: mixed-methods interview study of patients and practitioners in primary care screening | Cardiovascular disease risk communication and reduction in psoriasis | ||
| Study 2.iii: experimental study of message-framing | |||
| 3 | Objective 3: to learn from patients with psoriasis about helpful and unhelpful coping (self-management) strategies | Study 3.i: main community qualitative study of people with psoriasis | Coping with psoriasis: learning from patients |
| Study 3.ii: adjunct qualitative study with GPs | |||
| 4 | Objective 4: to identify the barriers for professionals providing patients with support in LBC | Study 4.i: content analysis of core training competencies for health-care professionals | Understanding the professional role in supporting lifestyle change in patients with psoriasis |
| Study 4.ii: in-depth qualitative interview study of health-care professionals | |||
| Study 4.iii: observational study of available support information | |||
| Study 4.iv: a survey of DSNs | |||
| 5 | Objective 5: to develop patient self-management resources and staff training packages to improve the lives of people with psoriasis | Study 5.i: design, development and evaluation of materials to support patient self-management | Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management |
| Study 5.ii: design, development and evaluation of a training programme for individuals | Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis | ||
| Study 5 iii: health economics stated preference survey | Valuing the interventions with a stated preference survey |
An economic model was planned as part of workstream 1 to explore whether or not systematic screening of people with psoriasis for CVD risk factors in primary care is cost-effective compared with usual care. This plan was modified because the modelling work fitted better as part of workstream 5. The reasons for this change are given as follows. First, the findings of workstream 1 indicated that psoriasis alone was not independently associated with the risk of major cardiovascular (CV) events over the follow-up period, although people with psoriasis also have a higher rate of acquired CVD risk factors. Second, workstreams 2, 3 and 4 found that many psoriasis patients are not engaged with NHS services and that effective control of CVD risk factors known to the patient and the general practice may be clinically more important than the detection of new risk factors. Third, the economic systematic review and findings from workstreams 3 and 4 suggested that it would be more informative to focus available resources on developing a more complex economic model to account for the interactions between patient and health-care professional characteristics, treatment and organisational factors. Finally, the interventions designed in workstream 5 are designed to provide information about the lifestyles associated with CVD risk and how to manage them. Accordingly, a more complex model was designed to account for the interactions between patients and health-care practitioners and includes CVD risk and management as part of the treatment and care events that a patient may experience. This model can be adapted to include new interventions for explicit management of known CVD risk factors in people with psoriasis as they are developed.
Other changes to our original plans occurred because of major changes to NHS funding and/or service structures. These changes meant that we needed to adjust our approach, particularly in relation to the final workstreams, which occurred towards the second half of the funding period. For example, the end of the administrative role of primary care trusts (PCTs) meant that we no longer had a direct link with the original PCT partner in research. In addition, the roles of health trainers had not been rolled out as we had anticipated for the final workstream; indeed, some of our original NHS partners removed these services altogether. Furthermore, the transition of some public health-care services to local authorities rather than the NHS meant that these services were further separated from the GP links that had been there when the original proposal was submitted. Although these did necessitate adjustments to the original plans, we were still able to meet the objectives by, for example, adapting the staff development intervention to general practice and secondary care dermatology services.
Patient and public involvement throughout the programme
The Psoriasis Association has worked closely with the IMPACT programme team from inception. Helen McAteer, Chief Executive Officer (CEO) of the Psoriasis Association, is a co-investigator on the research programme, contributing to the research strategy and successful delivery of the programme. An IMPACT programme research user group (RUG) (comprising patients and carers) was set up in the first year of the programme grant to advise and work in partnership with the research team across workstreams at a practical and logistical level. In addition, three patient representatives were recruited to sit on the steering committee and contribute to the annual independent expert advisory panel meetings from a patient and public involvement (PPI) perspective. More detail on the PPI approach can be found in Patient, public and practitioner involvement in the IMPACT programme.
Programme management
Management systems were set up from the beginning of the IMPACT programme to ensure effective running and timely delivery of the ambitious programme. There were three main management structures. Day-to-day management was overseen by the programme manager with regular input from the principal investigator. In each workstream, academic leads managed their research teams.
The independent advisory panel comprised individuals with an expertise in psoriasis or inflammatory disease and three patient representatives. All panel members were independent of the programme research team. An annual report was presented to this group followed by a full day’s face-to-face meeting with all researchers and investigators. At the end of this meeting the independent panel presented a written response to the programme team. Full investigator meetings (FIMs) comprised the principal investigator and all co-investigators. These were held monthly to monitor progress. Three patient representatives sat on the independent advisory panel. These individuals also attended RUG meetings. More details about the roles and experiences of patients and research users are provided in Patient, public and practitioner involvement in the IMPACT programme. Communication between co-investigators and researchers was aided by the fact that research staff and academics often spanned more than one workstream. Considerable planning and time were invested in annual whole-day training and development events in which all co-investigators and researchers presented and discussed findings with each other. These served as opportunities to interrogate findings and develop strategies for achieving the next research objectives, but were also important for team building. Extensive advance preparation for these meetings meant that they were very productive and focused. Particular attention was paid to supporting patient representatives to take an active part in these meetings (see Patient, public and practitioner involvement in the IMPACT programme).
Programme achievements
The programme has achieved its intended objectives, including the final aim of fully evaluated interventions for patients and professionals (workstream 5). In addition, we have improved understanding of the complex relationship between psoriasis and CVD (workstreams 1 and 2), undertaken in-depth qualitative research (workstreams 3 and 4) and conducted discrete event simulation (DES) modelling to capture the complexity of psoriasis, lifestyle and patient–health care relationships and estimate the cost-effectiveness of new interventions (workstream 5).
The work has been disseminated via academic, professional and public involvement routes including publication in academic journals, professional resource materials, presentations and a number of high-profile public engagement events. Highlights include:
-
17 peer-reviewed journal papers with at least three more in preparation
-
23 peer-reviewed oral and 43 peer-reviewed poster presentations
-
two successful national public engagement events, one city-wide and one national
-
sets of evaluated patient materials with outcomes assessed
-
an evaluated training intervention for health-care professionals.
Epidemiology of psoriasis and its association with risk of cardiovascular disease
Workstream 1 studies 1.i and 1.ii (Figure 2) address objective 1 of the programme: to confirm which patients with psoriasis are at highest risk of developing additional long-term conditions.
FIGURE 2.
Workstream 1: relationship of studies 1.i and 1.ii to other IMPACT programme workstreams.
Publications relating to this section and workstream are listed in Publications and cited throughout this section.
Prevalence and incidence of psoriasis: a systematic review
The worldwide epidemiology of psoriasis is poorly understood. A systematic review that provided a detailed critique of the existing literature on the worldwide incidence and prevalence of psoriasis was undertaken as part of workstream 1, comparing studies in relation to geography, age and gender. See Parisi et al. 1
The results from the systematic review confirmed that psoriasis is a common disease, and is less common in children and more common in adults. Prevalence rates showed a worldwide geographic variation that probably reflects the fact that psoriasis is a complex disease influenced by both genetic and environmental factors. The incidence of psoriasis in the UK is estimated to be 140 per 100,000 person-years. 35 Studies on the prevalence of psoriasis in the UK highlight that the disease is uncommon before the age of 9 years (0.55%)36 and has an estimated prevalence of between 1.30% [95% confidence interval (CI) 1.21% to 1.39%]37 and 2.60% (95% CI 2.47% to 2.78%)38 in adults and between 1.48% (95% CI 1.20% to 1.80%) and 1.87% (95% CI 1.89% to 1.91%)6,36,39 taking into account all ages.
Furthermore, some studies have found an increasing trend in the prevalence of psoriasis with age;36,39 however, there is no agreement about whether or not the prevalence differed between men and women. 36,39 The systematic review served to inform the recently published World Health Organization’s Global Report On Psoriasis40 and a subsequent call for further epidemiological research on the disease.
Systematic review of published economic evaluations
Although the economic systematic review and economic model of costs were both conceived as part of workstream 1, they were developed in parallel with other workstreams. In particular, the economic model drew on the findings of these to inform its structure and to populate it with data. The methods and results of the systematic review are reported here and those of the economic model are reported in Valuing the interventions with a stated preference survey.
A systematic review of published economic evaluations was conducted to understand what was known about the cost-effectiveness of psoriasis management. 41 The review’s aims were to identify full economic evaluations that compared the costs and health benefits of alternative interventions, summarise what was known about the relative cost-effectiveness of different interventions and assess the quality of the evidence, uncertainties and evidence gaps. The review highlighted inconsistencies between analyses and uncertainties where models have so far struggled to accurately characterise the disease. The review informed the need to develop a new model structure and the direction such a model should take.
The databases EMBASE, MEDLINE and NHS Economic Evaluation Database (NHS EED) were searched for full economic evaluations in January 2012, and updated with a search of NHS EED in April 2014. Predefined inclusion and exclusion criteria were used to screen abstracts and titles and select papers for review. Studies were included in the review if they compared services or treatments for psoriasis in adult patients, measured health outcomes and costs, and used either primary data or synthesised data in a full economic evaluation. The search identified 1355 articles. Three reviewers independently performed the primary and secondary screening of identified papers, cross-checking their results with each other. The full paper was obtained and reviewed for titles and abstracts when one or more reviewer was uncertain. Any discrepancies or uncertainties about the inclusion of papers in the secondary screening were resolved by discussion between the reviewers, with reviewer four acting as arbiter for any remaining uncertainty or disagreement. Two reviewers extracted data from the studies included for full review. Predefined data extraction forms and quality assessment forms were used (see Appendix 1). A total of 37 papers met the inclusion criteria and reported 71 treatment comparisons. The treatments evaluated in the 71 comparisons were systemic (n = 45), topical (n = 22), phototherapies (n = 14) and combinations (n = 4). The 37 economic papers reviewed mainly evaluated individual therapeutic agents rather than packages of care. Typically, these evaluations did not consider the context in which the treatment was delivered. Four articles and seven comparisons directly addressed the organisation and delivery of care. These included a programme to support patients’ self-management at home, online care management and home-based phototherapy. Four of the papers explored differing time and convenience demands on patients.
The review indicated that most of the economic evaluations were modelling studies, synthesising data from several sources. In summary, the systematic review identified a number of key areas of uncertainty in the existing psoriasis economic literature, which future economic analyses should seek to improve on. A limited effectiveness evidence base is over-represented in the available economic evidence and, overall, there was a lack of high-quality head-to-head clinical comparisons of different interventions. Repeated use of previous model structures was commonplace. When different sources of evidence or models have been used, uncertainty persists owing to diversity in setting, perspective and study design.
Many of the studies were limited in terms of reporting the methods used. In addition, the short-term follow-ups used in clinical trials of new interventions meant that 27 out of 37 economic evaluations were restricted to a time horizon of ≤ 1 year, despite the chronic nature of psoriasis. The review found that parameter uncertainty was not typically incorporated into analyses to a suitable degree.
Although the current evidence base is inconclusive about the relative cost-effectiveness of individual treatments, it contributes valuable data and methods to inform complex decisions and develop robust evaluation methods.
Risk of major cardiovascular events in patients with psoriasis: a population cohort study using the Clinical Practice Research Datalink
The severity of psoriasis can range from limited disease involving small body surface area to extensive skin involvement. As described in Synopsis, people affected by the disease often have an impaired quality of life. People with psoriasis may have other comorbid conditions such as obesity, hypertension, hyperlipidaemia and diabetes mellitus, which are associated with an increased risk of CVD.
In the last decade, a number of studies have suggested an association between psoriasis and CVD. It has been argued that increased systemic inflammation in those with psoriasis exacerbates other chronic inflammatory diseases including atherosclerosis, which could lead to MI or stroke (see Synopsis).
However, any purported association between psoriasis and CVD is complex for several reasons: those with psoriasis are more likely to engage in unhealthy lifestyle habits (increased likelihood of smoking, low levels of physical activity and obesity),28 have higher prevalence of CVD risk factors (e.g. diabetes mellitus, hypertension and hyperlipidaemia)23 and therapies that may raise (e.g. ciclosporin)28 or lower (e.g. methotrexate)29 the CVD risk. Each of these are aspects that may confound the association between the two conditions. 30
After controlling for several major CVD risk factors, a number of studies have suggested an increased risk of fatal and non-fatal CVD events in patients with psoriasis. 18,42–51 By contrast, other studies have concluded that psoriasis is not an independent risk factor for CVD. 52–55 In a recent systematic review, Samarasekera et al. 56 concluded that a possible association between severe psoriasis and CVD may exist; however, the authors warn that the existing studies were limited by failing to adequately adjust for important risk factors.
Inflammatory arthritis, a common comorbidity in patients with psoriasis and a recognised risk factor for CVD,57–59 has rarely been considered as a possible confounder. It is also important to note that, in many studies using electronic medical record databases, severe psoriasis is typically defined by exposure to systemic or biologic therapies that may also be used to treat inflammatory arthritis. This raises the possibility of misclassification of severe psoriasis when not taking account of the presence of inflammatory arthritis. Furthermore, little consideration has been given in earlier studies to the time-varying nature of the development of risk factors, or the severity of psoriasis. 30
A large population-based cohort study was undertaken with the aim of investigating whether or not psoriasis is an independent risk factor for major CV events [including MI, acute coronary syndrome (ACS), unstable angina and stroke] when taking into account relevant risk factors for CVD. See Parisi et al. 30
Methods
Study design
Using a primary care database from the UK [specifically the Clinical Practice Research Datalink (CPRD)], a population-based cohort study was conducted that included people with and people without psoriasis. The CPRD comprises the entire medical history (demographics, treatments, clinical events, test results and referrals to hospitals) of patients registered in a general practice in the UK. The database is broadly representative of the UK population in terms of age and gender. The protocol of this study was approved by the CPRD’s Independent Scientific Advisory Committee (protocol reference number 11_134A). The study is reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 60
At the time data were extracted (September 2012), data were available for 652 practices and > 12 million patients.
Study population
An inception cohort of adult (aged ≥ 20 years) patients with psoriasis and a matched comparison group of up to five people without psoriasis were identified between 1994 and 2009. Patients with and patients without psoriasis were included if they had no history of CVD or diabetes mellitus before index date (first diagnosis of psoriasis or corresponding consulting date for comparison patients) and were registered for ≥ 2 years in the general practice before entry into the study cohort.
Comparison patients were individuals who had never received a diagnosis for psoriasis. For each patient with psoriasis up to five comparison patients were matched on age, gender and general practice. Furthermore, to ensure that comparison patients visited the practice at approximately the same time as when patients with psoriasis received their first diagnosis of psoriasis, comparison patients were also matched on index date (psoriasis diagnosis date) in a 6-month window. All patients were followed up from their respective index date (first diagnosis of psoriasis) or consulting date (for the comparison cohort) and ended at the earliest date of the occurrence of a major CV event (MI, ACS, unstable angina or stroke), transfer out of the practice, death date or end of follow-up (31 December 2011).
Definition of exposure
Patients with a first diagnosis of psoriasis between 1994 and 2009 and received a recognised treatment for psoriasis {(emollients, topical treatment, phototherapy, systemic therapy or biologics [National Institute for Health and Care Excellence (NICE) clinical guideline CG153]} were included in the cohort. 61 Patients were classified as having severe psoriasis if they had received a systemic treatment (acitretin, etretinate, ciclosporin, hydroxycarbamide, methotrexate, fumaric acid), phototherapy or a biologic therapy (etanercept, adalimumab, infliximab, ustekinumab, efalizumab); if they had received none of these they were classified as having mild psoriasis. 30
Outcome of interest
The outcome of interest was a combined CV end point, including the first event of fatal or non-fatal MI, ACS, unstable angina or stroke. In the main analysis, the outcome of interest was identified using data from CPRD by using the Read code classification, which is a hierarchical coding system used to record diagnoses in primary care. 62 To assess the robustness of the main findings to potential outcome misclassification, in a sensitivity analysis, the combined CV end point was identified using primary care data (CPRD) and the national mortality records [from the Office for National Statistics (ONS)] of those patients in practices providing linked data between CPRD and ONS. In this situation, both Read codes and the International Classification of Diseases codes were used.
Covariates
To assess whether or not there is a relationship between psoriasis and risk of major CV events, a statistical model was built including multiple risk factors (other than psoriasis). This approach will help us to understand whether or not any observed association between psoriasis and major CV events could be accounted for by other concomitant diseases/risk factors that are known to be linked to CVD. Risk factors included in the model were age, gender, depression and calendar year calculated at baseline and having or developing severe psoriasis or inflammatory arthritis (which included both diagnostic codes for PsA or rheumatoid arthritis). Diabetes mellitus, chronic kidney disease, hypertension, hyperlipidaemia, atrial fibrillation (AF), transient ischaemic attack, congestive heart failure, thromboembolism, valvular heart disease and smoking status were modelled as time-varying risk factors; therefore, an individual’s classified status could change during the study period. Psoriasis was defined as being severe from the date of first exposure to phototherapy, systemic or biologic treatment. It was considered as severe from that point onward. Smoking status was classified as ‘current’, ‘former’, ‘never smoker’ or ‘unknown smoking status’. This enabled switching from one smoking class to another during follow-up. 30 Additional risk factors such as a high body mass index (BMI) score and a high score on the Index of Multiple Deprivation (IMD),63 which is a measure of socioeconomic status, were included only in sensitivity analyses owing to the high proportion of missing data.
All code lists used for the exposure and outcome are available for download [URL: www.clinicalcodes.org (accessed 15 May 2019)]. 64
Statistical analysis
Medians and interquartile ranges were used to summarise continuous variables; proportions were used to summarise discrete covariates. The combined CV end-point events and incidence rates per 1000 person-years with 95% CIs were calculated for patients with and patients without psoriasis, and by disease severity. The estimate of the age- and gender-adjusted hazard ratios (HRs) and 95% CIs for each variable were made using Cox proportional hazard regression. Cox regression with a shared frailty model was used to estimate the fully adjusted HRs and 95% CIs. The assumption of proportionality for each variable and the model overall was tested by using Schoenfeld residuals.
Using the initial cohort identified from CPRD, multiple sensitivity analyses were performed. These included assessing whether or not a more complex or parsimonious multivariate model (by including different sets of risk factors) would change the conclusions. BMI and IMD scores contained a high proportion of missing data; therefore, they were included in the model only in sensitivity analyses. Smoking status had a small proportion of missing values; therefore, it was included in the main analysis and a category was introduced to account for missing values. Accordingly, sensitivity analyses explored the impact of using multiple imputation to estimate missing BMI and IMD scores so that they could be included in the model.
Additional sensitivity analyses were (1) including only those patients with at least one GP visit per year, (2) including only those patients with ≥ 6 months of follow-up, (3) testing for an interaction between the presence of psoriasis or severe psoriasis with age and (4) additional adjustment for patients exposed to methotrexate, ciclosporin or oral retinoids.
Finally, a subgroup analysis was performed by linking data from CPRD to the mortality data contained in the ONS. The nested cohort included patients identified from CPRD practices linked to the ONS and who had an index date (first diagnosis of psoriasis or corresponding consulting date) between 1998 and 2009; they were followed up until 2011. The outcome was the first fatal or non-fatal major CV event (MI, ACS, unstable angina or stroke) recorded in CPRD or ONS.
All statistical analyses were performed using Stata® 12 (StataCorp LP, College Station, TX, USA).
Results
The number of patients meeting all inclusion criteria who formed the final cohort was 48,523 patients with psoriasis and 208,187 comparison patients. Overall, there was a higher proportion of females (56.40%) and the median age at index date was 47 years (see table 1 of Parisi et al. 30).
At baseline, patients with psoriasis had a higher prevalence of the majority of risk factors (inflammatory arthritis, hypertension, hyperlipidaemia, depression, current or ex-smoker, overweight or obese) than the comparison group (see original table 1). At the end of follow-up, patients with psoriasis had a higher prevalence of all time-varying risk factors except for AF, transient ischaemic attack and congestive heart failure (see original table 2). Inflammatory arthritis was present in 2.39% of patients with psoriasis and 0.98% of the comparison patients at baseline. It was also present in 4.69% of the patients with psoriasis and 1.38% of the comparison patients by the end of follow-up. In addition, at baseline, 1.03% of patients with psoriasis had been treated with phototherapy or systemic or biologic therapies. This increased to 4.29% of patients by the end of follow-up. Just over 50% (50.62%) of those treated with systemic or biologic therapies had also been diagnosed with inflammatory arthritis by the end of the follow-up period. 30
The most commonly used systemic treatment used in patients with psoriasis was methotrexate. Original table 3 shows the distribution of phototherapy, systemic therapy or biologic received by patients with psoriasis.
Patients included in the study were followed up for a median time of 5.2 years. Over this period, 1257 (2.59%) patients with psoriasis had a major CV event, compared with 4784 (2.30%) comparison patients (see original table 4 of the published paper). The unadjusted incidence rate of a major CV event per 1000 person-years was higher in the psoriasis group than in the comparison group [4.13 per 1000 person-years, 95% CI 3.91 to 4.36, and 3.87 per 1000 person-years, 95% CI 3.76 to 3.98, respectively] (see original table 4).
There were time-varying effects related to risk of outcome for hypertension, transient ischaemic attack, AF and gender (revealed in Schoenfeld residuals). Allowing each of these variables to have different effects for the first 3 years of follow-up and the later follow-up did, however, remove the non-proportionality (p = 0.12).
In the age- and gender-adjusted analysis, all the variables tested were significantly associated with the risk of major CV events. In particular, the HRs of major CV events due to psoriasis and severe psoriasis were 1.10 (95% CI 1.04. to 1.17) and 1.40 (95% CI 1.07 to 1.84), respectively. However, both HRs were attenuated and became non-significant in the fully adjusted model (HR 1.02, 95% CI 0.95 to 1.08, and HR 1.28, 95% CI 0.96 to 1.69, respectively) (original table 5 of the published paper).
In addition, in the multivariate analysis, each of the following risk factors was significantly related to the risk of major CV events (original table 5): inflammatory arthritis (HR 1.36, 95% CI 1.18 to 1.58), diabetes mellitus (HR 1.18, 95% CI 1.06 to 1.31), chronic kidney disease (HR 1.18, 95% CI 1.07 to 1.31), hypertension (HR 1.37, 95% CI 1.29 to 1.45), transient ischaemic attack (HR 2.74, 95% CI 2.41 to 3.12), AF (HR 1.54, 95% CI 1.36 to 1.73), valvular heart disease (HR 1.23, 95% CI 1.05 to 1.44), thromboembolism (HR 1.32, 95% CI 1.17 to 1.49), chronic heart failure (HR 1.57, 95% CI 1.39 to 1.78), depression (HR 1.16, 95% CI 1.01 to 1.34), current smoker (HR 2.18, 95% CI 2.03 to 2.33), age (HR 1.07, 95% CI 1.07 to 1.07) and male gender (HR 1.83, 95% CI 1.69 to 1.98). Hyperlipidaemia was not significantly related to the risk of major CV events (HR 1.04, 95% CI 0.96 to 1.11).
Interaction terms between psoriasis or severe psoriasis and age were tested; however, they were not included in the multivariate model because they were non-significant (p = 0.40 and p = 0.25, respectively).
The sensitivity analyses did not change the main findings. The HRs of major CV events due to psoriasis or severe psoriasis when taking into account the different sets of risk factors can be found in original table 6 of the published paper. In particular, the HR of major CV events due to severe psoriasis changed from 1.28 (95% CI 0.96 to 1.69) to 1.46 (95% CI 1.11 to 1.92) when inflammatory arthritis was not included in the model.
When using multiple imputation and adding BMI and IMD scores to the fully adjusted model, the HRs of major CV events were > 1 but still non-significant (see original table 6).
Likewise, the results obtained by analysing data from patients with at least one GP visit per year, patients with ≥ 6 months’ follow-up or results that took into account patients exposed to methotrexate, ciclosporin or oral retinoids gave the same results as the main findings (see original table 6).
Finally, the subgroup analysis that analysed data from CPRD linked to the ONS mortality data and patient-level socioeconomic status (IMD) yielded similar results to the main findings. Here, the fully adjusted HRs of major CV event due to psoriasis and severe psoriasis were 1.02 (95% CI 0.93 to 1.11) and 1.10 (95% CI 0.72 to 1.68), respectively (see original table 6).
Discussion
This study investigates whether or not psoriasis is independently associated with a higher risk of CVD. The results confirm that patients with psoriasis have more prevalent comorbid conditions associated with CVD. However, taking into account other established risk factors for CVD, psoriasis itself was not found to be directly associated with short- to medium-term (3–5 years) risk of major CV events. These results highlight that individuals who have psoriasis co-occurring with inflammatory arthritis have a 36% higher risk of a major CV event than those who do not.
Similar to other studies,18,23,46 our research highlights that patients with psoriasis have a higher prevalence of CVD risk factors. Our findings are comparable to those reported by Brauchli et al. 52 and Wakkee et al. ,55 neither of which found an overall higher risk of MI associated with psoriasis. However, our research is more robust because the sample size was larger, 55 we used more stringent inclusion criteria (e.g. psoriasis cases were identified on the bases of diagnosis and treatment received)52 and in our analysis we accounted for important confounders including inflammatory arthritis. 52,55
Our finding that psoriasis is not an independent risk factor for a major CVD event was in contrast to other influential studies that reported that it was an independent risk factor. 42,44,65
In a study that utilised the Health Improvement Network database, Ogdie et al. 65 reported an increased risk of major adverse CV events in patients with either mild or severe psoriasis. It is possible that the cohort of patients with psoriasis used in that study had a longer disease duration than patients identified in our cohort. Different study designs were used (prevalent vs. incident cohort). An important issue is raised by the use of a prevalent cohort, namely that it is associated with the problem of left censoring. For example, those with the most severe psoriasis or CVD could have died prior to cohort entry, meaning that these studies can address only the question of what happens to individuals with psoriasis who have already survived. 30
Another study that reported an increased and significant risk of coronary heart disease in patients with severe psoriasis was conducted by Dregan et al. ,44 using the CPRD; however, it is possible that they misclassified severe psoriasis on the basis of treatments received without taking account of the possibility of comorbid inflammatory arthritis. 30
Using a Danish nationwide database, Ahlehoff et al. 42 reported increased risks of CVD in patients with severe psoriasis with and without PsA. However, in that study, patients with severe psoriasis were classified using hospitalisation for psoriasis or PsA, which could be subject to surveillance bias. 30,42 Furthermore, comorbidities were assigned by linking with prescribed treatments instead of diagnostic codes, which could lead to potential misclassification.
There are a number of additional differences between our study and earlier studies. We examined a wider range of risk factors in the analyses41,43,64 and modelled these to account for development of new risk factors over time. We examined psoriasis severity as a time-varying covariate, so that patients with severe psoriasis become ‘at risk’ only once they started phototherapy, systemic therapy or biologic therapies (rather than simply whether or not they had ‘ever’ been exposed to systemic treatment. That approach would have meant that an individual would have been classified as having severe psoriasis for the whole observation period).
Some strengths and limitations of the study can be listed. Selection bias was minimised, as was information bias and detection bias by identifying patients from the same database. We selected patients with and patients without psoriasis from the same general practice and during the same time-window.
Furthermore, our findings were consistent after multiple sensitivity and subgroup analyses. Several additional strengths can be identified in our study. (1) Important confounders were taken into account to investigate the association between psoriasis and major CV events. These included both traditional and non-traditional CVD risk factors, in particular inflammatory arthritis. (2) We present a large population-based study representative of the UK. (3) Only those with at least a diagnostic code of psoriasis plus a treatment for psoriasis were included in the cohort to minimise the risk of disease misclassification. (4) More advanced methodology was employed, such as the use of the shared frailty model. These methods take better account of the matched nature of the data, and the use of time-varying covariates. 30
Some limitations also need to be taken into account. Given that this was an observational study, the risk of residual confounding was considered. The CPRD is a primary care database and therefore diagnoses of psoriasis were not necessarily confirmed by dermatologists; phototherapy and systemic and biologic therapies were used as a surrogate to classify disease severity rather than standard clinical assessments, such as the Psoriasis Area and Severity Index (PASI) or the body surface area affected by psoriasis. The size of the group of individuals with or developing severe psoriasis may be underpowered to investigate that end point of interest. The duration of follow-up was > 5 years (on average) for those with psoriasis and > 3 years for those who developed severe psoriasis during the observation period. It may be the case that chronic inflammation takes longer to produce adverse CV outcomes and therefore studies involving longer follow-up periods are recommended. 30
Due to the small number of patients exposed to biologics it was not possible to discern whether or not biologic therapy reduces the risk of major CV events. The specific aim of the current study was to investigate whether or not psoriasis was independently associated with the risk of major CV events. Thus, to minimise the risk of bias, patients from our cohort who had a history of either CVD or diabetes mellitus were excluded. 30
Key conclusions
-
Adults with psoriasis are more likely to have prevalent conditions associated with CVD.
-
However, psoriasis alone is not independently associated with the short- to medium-term (i.e. 3–5 years) risk of major CV events, after adjusting for important CVD risk factors.
-
Despite this, the co-occurrence of inflammatory arthritis and psoriasis is an independent risk factor for major CV events.
Implications
-
Screening psoriasis patients for inflammatory arthritis is important, as is screening to prevent development of CVD risk factors in people with psoriasis.
-
Patients with inflammatory arthritis are at an increased risk of CVD; this may be an additional reason to minimise a patient’s cumulative inflammatory burden.
Cardiovascular risk in patients with psoriasis: screening study and pulse wave velocity measures in people with psoriasis
Workstream 2 (Figure 3) addresses objective 2 of the IMPACT programme: to apply knowledge about the risk of comorbid disease to the development of targeted screening services to reduce the risk of further disease.
FIGURE 3.
Workstream 2: relationship of study 2.i to other IMPACT programme workstreams.
This section describes a study66 and a substudy. The first part of this section briefly summarises a study of screening for CVD risk in primary care. 66 The second part of this section reports the small substudy that investigated the utility of pulse wave velocity (PWV) measurement as a potential target method for screening.
Screening for cardiovascular disease risk in primary care
Background
Since 2006, the possibility that people with psoriasis experience an increased rate of fatal and non-fatal CVD in comparison with the general population has been an increasing focus of discussion. 67 At the time of this study it was unclear whether such an enhanced risk was due to an increased prevalence of traditional CV risk factors (e.g. hypertension, obesity, dyslipidaemia); an increased prevalence of some of the more recently recognised CV risk factors, such as physical inactivity and depression; or the severity of the psoriasis. There was very little information on the prevalence of traditional CV risk factors in patients with psoriasis in the UK and this small body of research may have been limited by selection bias, inappropriate choice of control groups or reliance on risk factors measured for other clinical reasons.
Primary care patients aged ≥ 40 years are supposed to be offered CV risk assessment as a matter of routine in the UK. However, this is not the case for patients aged < 40 years. This could be a problem for those diagnosed with psoriasis if, as reported by Gelfand et al. ,18 they have up to three times the expected CVD risk. Without robust data it would be difficult to make a case for systematic screening of patients with psoriasis for CV risk factors over and above what might be offered to the general population. 31,67–69
Previous primary care-based studies have neither assessed how systematic screening for CVD risk factors might influence their estimated prevalence in patients with psoriasis nor estimated the potential benefits of identifying such risk factors and subsequent intervention.
Objectives
Our objectives were to:
-
investigate whether or not screening for CVD risk factors in primary care could influence the estimated prevalence of CVD in patients with psoriasis
-
assess whether or not the prevalence of screen-detected CVD risk factors or estimated CVD risk varies by age, psoriasis severity and the presence of PsA
-
estimate the clinical benefits of normalising modifiable risk factors.
Method
Exposure and outcome measures
A number of exposure and outcome measures were included in the study. These were PsA, severe psoriasis, hypercholesterolaemia, type 2 diabetes, hypertension, alcohol excess, suboptimal levels of risk factors on therapy, rheumatoid arthritis and chronic kidney disease. These are defined in Rutter et al. 66
Selection of general practices, participants and sample size
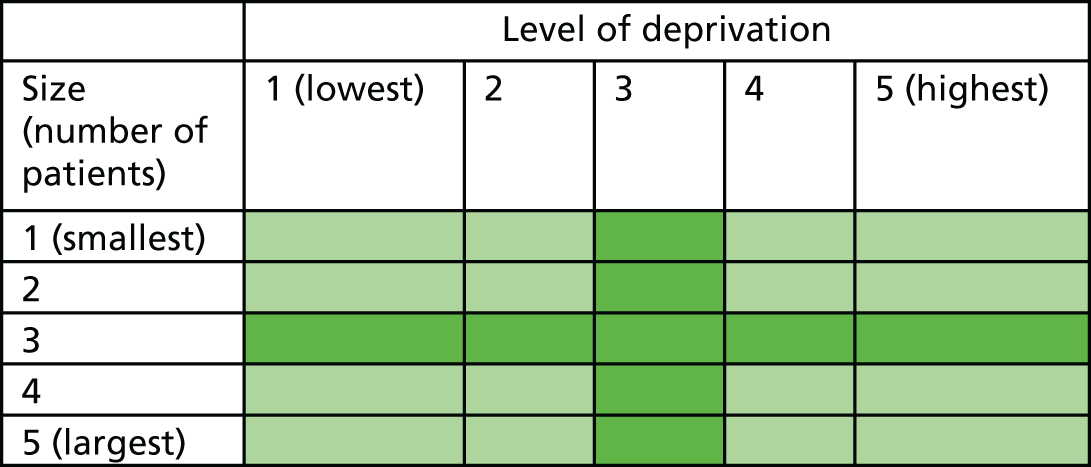
General practices from two PCTs in north-west England were identified. We aimed to recruit large and small general practices from deprived and affluent areas by grouping eligible general practices in quintiles by size, using the numbers of registered patients, and by level of deprivation, using the IMD (based on practice postcode). A five-by-five sampling frame was constructed (Figure 4) and practices in the nine middle quintiles were removed. Practices were then randomly selected from the remaining four quadrants: small and deprived, small and affluent, large and deprived, and large and affluent. Removing the nine middle quintiles increased the likelihood of ensuring maximum differences between the quadrants.
FIGURE 4.
Sampling frame for practices.
Forty-four eligible general practices were sent letters of invitation and information sheets and were followed up by telephone, e-mail or visit. Only five practices were recruited via this route; consequently, the recruitment strategy was widened to include three more PCTs. In these PCTs, practices were identified via the comprehensive local research network. In total, 13 practices were finally recruited and reimbursed for their participation (i.e. block payments were made to participating practices to compensate for time incurred by administrators identifying psoriasis patients in each practice).
We aimed to recruit 320 people to yield estimates of the prevalence of CVD risk factors to within ≈1.9% of the true value for rarer factors and to within ≈5.3% of the true value for common factors.
Accordingly, we aimed to include ≈80 people with psoriasis in each of four age/sex categories: males aged < 40 years; females aged < 40 years; males aged ≥ 40 years and females aged ≥ 40 years. The age of 40 years was the cut-off because routine screening for CVD risk factors is usually offered routinely once every 5 years to all patients aged ≥ 40 years. Participating general practices identified patients with psoriasis aged > 18 years using Read codes known to map to the condition and medications/topical preparations for psoriasis (see Rutter et al. 66). Exclusion criteria were severe mental health problems, no capacity to consent, recent bereavement and terminal illness.
Eligible patients were sent an invitation from their own GP to attend a CVD risk screening assessment at their general practice. The invitation included an information leaflet about the study and a reply slip on which the patient was asked to indicate if they had psoriasis and if they were interested in participating. Smaller practices mailed all eligible patients on their list; larger practices mailed an agreed number (depending on practice capacity), using a randomised numbered list to select invitees. Patients replying positively were telephoned by a researcher, who arranged attendance for CVD risk factor screening at the patient’s own general practice.
Data collection
The study was approved by the local Research Ethics Committee (REC) [North West Research Committee, Greater Manchester East (reference number: 11/NW/0654)]. All participants gave informed written consent before any data collection. Data were collected from the patient (via a self-completed questionnaire in advance of their appointment), from the practice (from medical records) and from a face-to-face assessment, and included items needed to calculate the individual probability of a CV event over the next 10 years using the standard UK CVD risk calculator QRISK®2 (ClinRisk Ltd, Leeds, UK).
When patients consented to having their medical records examined, a designated member of the practice staff recorded (if appropriate):
-
most recent cholesterol reading and pre-treatment value
-
most recent blood pressure reading and pre-treatment value
-
relevant medication
-
information about the following known CVD risk factors – smoking status, hypertension, hyperlipidaemia, type 2 diabetes, AF, chronic kidney disease and rheumatoid arthritis.
Information from the medical records, along with self-reported risk factors, was used to assess whether or not risk factors were newly detected by screening.
Patients were asked about their history of psoriasis; family history of psoriasis; previous treatments for psoriasis; current medication; history of CV conditions including heart attack, stroke, angina and AF; family history of CV conditions; and history of rheumatoid arthritis, chronic kidney disease, depression and type 2 diabetes.
The practice nurse or GP then:
-
Recorded the current smoking status and number of units of alcohol consumed per week reported by the patient.
-
Generated the patient’s BMI. Each practice was provided with a new set of weighing scales for this purpose to ensure consistent/accurate measurements.
-
Measured hip and waist circumferences (cm).
-
Measured sitting blood pressure three times in the same arm after a 5-minute rest and the mean of the last two readings. Each practice was provided with a new blood pressure monitor for this purpose.
-
Took fasting blood samples, to be analysed at a local hospital biochemistry department for the measurement of glycosylated haemoglobin (HbA1c), fasting lipids [total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides], fasting glucose, and liver and renal function.
If patients were found to have high levels of anxiety or depression, they were notified using a standardised form containing their Hospital Anxiety and Depression Scale (HADS) scores, with accompanying advice to speak to their GP.
Patients were recalled for follow-up discussion of the screening if deemed necessary by the practice.
Statistical methods
Data are reported as mean [standard deviation (SD)] or median (range), depending on distribution. Groups were compared using chi-squared tests or Fisher’s exact tests for categorical data and Student’s t-tests or Mann–Whitney U-tests for continuous data.
The prevalence of risk factors was compared with that in a general population sample drawn from the 2011 Health Survey for England (HSE). 70 To facilitate the comparison, sampling weights were used to standardise HSE estimates to the age, ethnicity and gender distribution of the psoriasis sample.
The individualised probability of a CV event over the next 10 years (using QRISK2) was calculated for patients not already deemed to be at high CVD risk (see above) by using Stata 13 plugins and the QRISK®-2013 source code (ClinRisk Ltd). Also using the QRISK2 calculator, the ‘optimised CVD risk’ was calculated for each of these individuals using an ideal set of values for modifiable risk factors (no smoking, cholesterol/HDL ratio 5, systolic blood pressure of 140 mmHg, BMI of 25 kg/m2), but leaving non-modifiable factors such as age, gender, diabetes and family history unchanged. The absolute change in predicted risk through risk factor optimisation was calculated as the optimised minus the predicted risk before risk factor optimisation.
A p-value of < 0.05 was considered statistically significant. All analyses were performed using Stata 13.
Results
Practice and participant recruitment
Practice sizes ranged from 3070 to 16,746 registered patients; IMD scores ranged from 48.79 (least deprived) to 9.77 (most deprived). Details can be found in Appendix 2.
The process of participant recruitment is shown in Figure 5. A total of 1446 people with psoriasis were invited to attend for risk factor review, of whom 287 (19.8%) completed the review.
FIGURE 5.
Flow chart of responses.
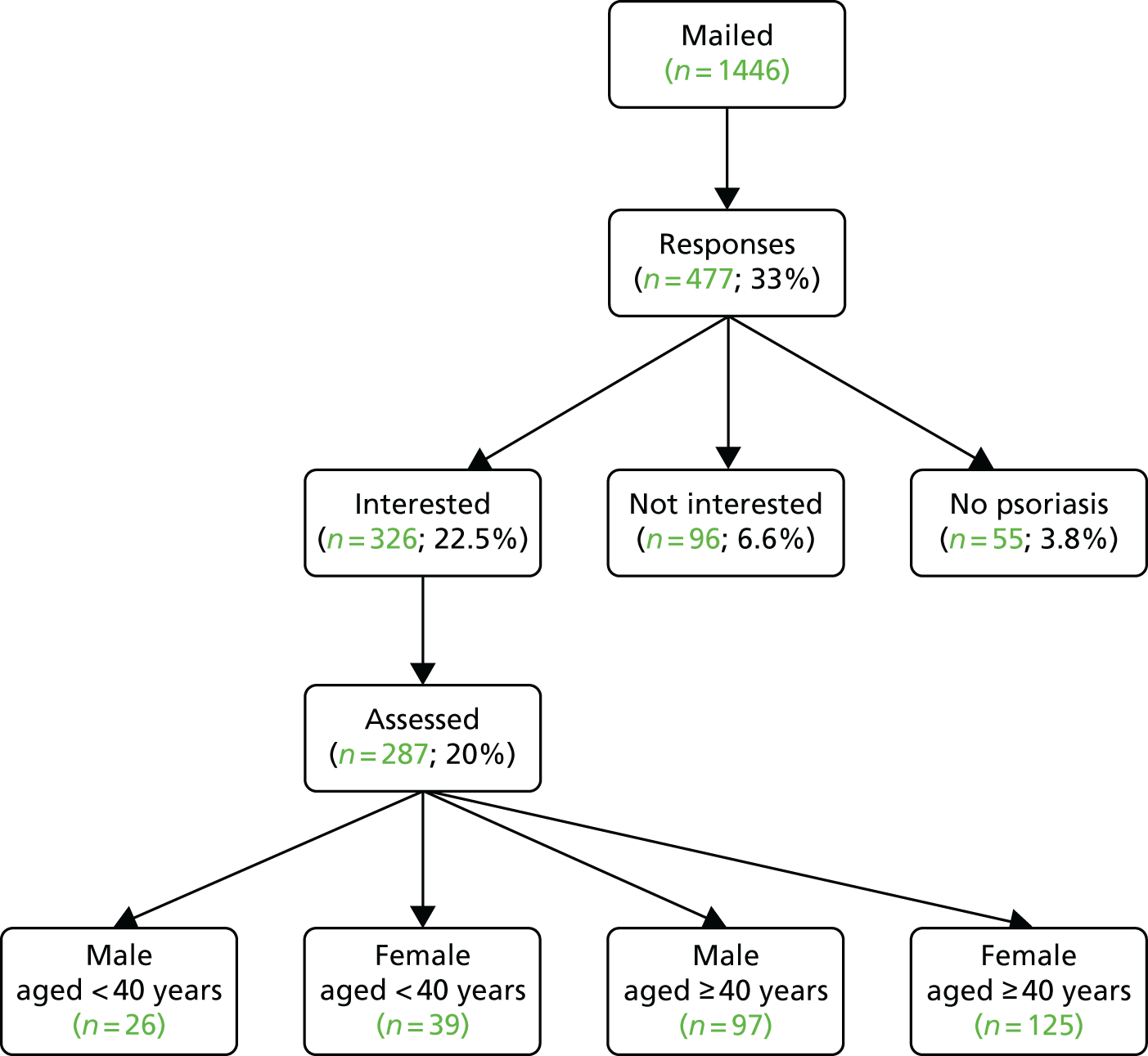
Nine practices (P1, P2, P4, P5, P6, P7, P8, P12 and P13) provided more detailed information about patient response to the screening invitation, permitting a breakdown of response rates by patient age and sex (Table 2). Acceptance rates were lower among younger (< 40 years) than among older (≥ 40 years) patients; there were no appreciable differences by gender.
| Patient sex, age (years) | Invited (n) | Attended (n) | Response rate (%) |
|---|---|---|---|
| Male, < 40 | 231 | 25 | 11 |
| Female, < 40 | 209 | 39 | 19 |
| Male, ≥ 40 | 346 | 82 | 24 |
| Female, ≥ 40 | 444 | 117 | 26 |
| All patients | 1230 | 263 | 21 |
Of those screened, 173 (60%) patients were invited to a follow-up appointment by their practice, 109 patients were not given a follow-up appointment and the outcome for five patients was unknown.
Nearly one-quarter of participants had severe psoriasis and around one-third were clinically obese. One-third of participants self-reported high cholesterol levels and high blood pressure and over two-thirds of these people were receiving medication for these conditions (lipid-lowering or antihypertensive medications). A history of coronary or cerebrovascular disease or AF was self-reported by < 10% of participants.
We found that nearly half the cohort had at least one screen-detected risk factor and one-fifth had two or more risk factors for CVD. There was no evidence that the prevalence of screen-detected risk factors varied by age, psoriasis severity or the presence of self-reported PsA. Comparison with the weighted HSE data suggests differences between our study participants and the general population on one risk factor (where p < 0.001). Hypertension was higher (p < 0.001) in study participants than in the general population. This applied to the full cohort (n = 287) and the subset of people (n = 269) who were not taking ciclosporin, which can cause hypertension.
Among the study participants receiving treatment for CVD risk factors, nearly half (46%) had suboptimal therapy levels for blood pressure and/or total cholesterol and one-quarter (26%) of participants had suboptimal levels for glucose assessed by HbA1c.
In our study, just over one-third of participants not already considered ‘high risk’ according to NICE guidelines were assessed to be at high risk of CVD using the 10-year risk threshold of > 10% (QRISK2). 53 The mean predicted lifetime CVD risk estimated by QRISK2 was 35%. Thirteen per cent of participants had a high lifetime CVD risk of > 50%. After adjusting for age, there were no statistically significant differences in CVD risk between participants who had severe psoriasis and self-reported PsA and those without these conditions.
Discussion
To our knowledge, this is the first UK primary care-based study to report that CVD risk factor screening augments the estimated prevalence of CVD risk factors in psoriasis, and it is the first study to assess lifetime CVD risk and the potential benefits of optimising modifiable CVD risk factors. We have shown that the proportion of patients with screen-detected CVD risk factors was unrelated to age, psoriasis severity or the presence of self-reported PsA. When known and screen-detected risk factors were considered, hypertension was more prevalent in patients with psoriasis, in keeping with data from two other studies. 53,71
Although we had an adequate sample size, we did not show statistically significant differences in the age- and sex-adjusted prevalence of obesity, smoking or diabetes compared with the general population. Although more than one in three participants (37%) were at high 10-year CVD risk, only one in eight (13%) participants overall had a high lifetime risk of > 50%. Therefore, the estimation of lifetime risk does not appear to be particularly beneficial in patients with psoriasis.
We observed many patients with suboptimal management of known risk factors. Previous studies have shown undertreatment of CVD risk factors in patients with psoriasis72 and one study suggests that hypertension is more difficult to manage in these patients,73 perhaps because psoriasis or its treatment somehow increases blood pressure or reduces concordance with therapy.
A few studies have reported the prevalence of screen-detected CVD risk factors in patients with psoriasis in highly selected patient groups. 74–77 Several large studies, some of which were population based,23,78 have reported a higher prevalence of known CVD risk factors including hypertension, dyslipidaemia, diabetes, obesity, smoking, renal disease and metabolic syndrome. 23,78–82 However, control groups in these studies included people with ‘forms of dermatitis’,81 other hospitalised patients77,82 and people taking part in other research projects,77 which makes interpretation, comparison and clinical implications challenging. Other studies have reported the prevalence of known CVD risk factors in patients with psoriasis but without a control group. 83,84 Our study demonstrated that using a standard approach to CVD screening of patients with psoriasis who attend primary care is an effective means of identifying individuals with CVD risk factors who would have otherwise been missed.
Strengths and limitations
Strengths of the study include (1) we selectively sampled practices across a broad range of socioeconomic settings to ensure a meaningful sample size in four age/sex subgroups; (2) the prevalence of important CVD risk factors was established with clinically meaningful precision in these subgroups; (3) we reported how CVD risk factor screening, when added to information on known risk factors, influenced the detected prevalence of CVD risk factors; (4) we used QRISK2 to estimate the 10-year risk of CV events in patients with psoriasis not already judged to be at high CVD risk; (5) psoriasis severity was assessed; (6) we compared prevalence data to population-based controls matched for age, sex and ethnicity; (7) we assessed the benefits of optimising risk factors within 10-year and lifetime time frames; and (8) these data can be used to inform policy on primary care-based risk factor screening.
Limitations of the study include (1) risk factors were measured on one occasion, which may have led to some misclassification; (2) we did not validate diagnoses of psoriasis or PsA; (3) our cohort was largely white, limiting the generalisability of findings; (4) the participation rate was 20%; and (5) men aged < 40 years were under-represented. We suspect that patients who did not participate would be more likely to have an adverse risk factor profile than those who enrolled. Response rates were lower in men than in women, suggesting that men would be less likely to attend for routine screening, even though, being at higher risk, they are more likely to benefit from intervention.
Clinical implications
Cardiovascular risk factor screening is a means of identifying a high proportion of patients (1) at high CVD risk, (2) with screen-detected risk factors and (3) with suboptimally managed known risk factors. ‘Health checks’ have recently come under criticism for failing to improve long-term health outcomes,85,86 perhaps because of overmedication or social class biases in attendees. 87 An alternative explanation has been made by our research team in a parallel study that recorded and analysed CVD consultations (see Cardiovascular disease risk communication and reduction in psoriasis). The study found that risk information was seldom discussed with patients; instead, practitioners prioritised information collection (see Cardiovascular disease risk communication and reduction in psoriasis). 88 Thus, although the current study has shown that screening identifies modifiable CVD risk factors, caution is required before recommending this as a strategy.
Accordingly, our findings in this study need to be considered alongside those showing limited response of clinicians to identified risk before universal CVD screening for people with psoriasis can be recommended. 88
Workstream 2.i substudy: assessing the impact of psoriasis severity on artery health (pulse wave velocity)
Background
As already stated, emerging evidence indicates that there is an association between psoriasis and CVD. 18,89–93 Arterial stiffness is an important surrogate marker of CVD and can be assessed non-invasively at the brachial artery by measuring the velocity of the arterial pulse wave occurring after cardiac contraction. This measurement is known as the PWV94–96 and is a strong predictor of CV events and all-cause mortality. 97
We have previously shown that patients with PsA have a higher risk of CV events than the general population. 30 Measurements of arterial stiffness could identify patients at higher risk of CVD and be incorporated in screening algorithms to identify individuals for more intensive risk factor intervention. Previous studies have indicated that psoriasis is associated with increased arterial stiffness and arterial stiffness has been found to be higher in patients with PsA than in the general population. 98,99 However, no study has either assessed whether or not arterial stiffness is related to the presence of PsA in a cohort of patients with psoriasis or related the age at onset of psoriasis or its severity to arterial stiffness.
Aims
To assess whether PWV is related to (1) the severity of psoriasis, (2) the presence of PsA and/or (3) the age at onset of psoriasis.
Method
Selection of participants
Thirteen general practices across north-west England identified patients with psoriasis and invited them for a CVD risk assessment (study 2.i). This PWV substudy recruited from the 287 people who attended the CVD risk assessments (Figure 6).
FIGURE 6.
Recruitment to PWV substudy.
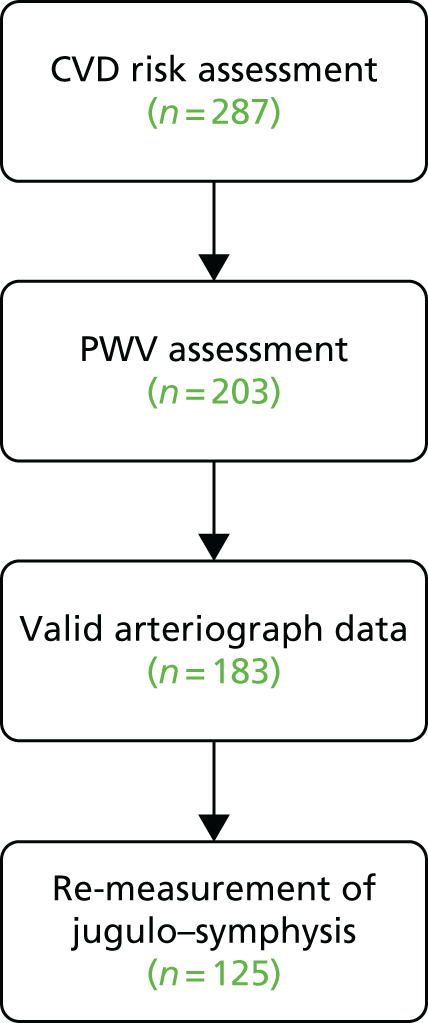
Data collection
Patients attended their own general practice surgery or a local dermatology research department for PWV measurement. Haemodynamic factors, including PWV, were measured using an arteriography device. 100 In addition, a research nurse also conducted a skin assessment using the PASI and patients completed the Simplified Psoriasis Index (SPI)101 assessment. Data on CVD risk factors, and other relevant demographic and medical data, were collected as described in Screening for cardiovascular disease risk in primary care.
The PWV data for the study were collected by four research nurses using three arteriography devices. Early analysis and reliability testing revealed that there was no significant difference in measurements between devices, but there was a significant difference between individual nurses’ measurements (between-observer and within-observer variation); the differences were accounted for by inconsistency in the calliper measurement between the suprasternal notch and the symphysis pubis, known as the jugulo–symphysis measurement used in the calculation of PWV. To reduce observer error and to improve the accuracy of this study, the jugulo–symphysis distance was remeasured in as many participants as possible by a single research assistant.
Data handling
All data were entered into a study-specific database by the research nurse conducting the assessment or by a researcher who entered the data from the hard copy of the information collected by the research nurse. All data were double-checked for correct entry.
Data analysis
As PWV was skewed, it was log-transformed before analysis. Linear regression was used to assess the associations between PWV and CV risk factors, with log PWV as the outcome variable. Severe psoriasis and PsA were also evaluated as potential determinants of PWV: severe psoriasis was defined as Self-Administered Psoriasis Area and Severity Index (SAPASI)102 score of > 10 or use of a disease-modifying therapy [psoralen and ultraviolet A (PUVA), methotrexate, ciclosporin, acitretin, fumaric acid esters, etanercept, adalimumab, infliximab or ustekinumab]. Psoriatic arthropathy was defined as positive responses to any three out of the first five Psoriasis Epidemiology Screening Tool questions or a positive response to the question ‘Have you ever been told that you have arthritis associated with psoriasis?’.
Relationships between arterial stiffness and measures of psoriasis were adjusted for traditional CVD risk factors, psychological variables and the presence of PsA (when appropriate).
Data analysis was performed using the Stata 13 package. Two-tailed p-values of < 0.05 were considered statistically significant.
Sample size
In a small study of 52 patients and 50 controls, Yiu et al. 103 showed that patients with psoriasis had higher PWV than control participants [mean 14.5 m/second (SD 2.5 m/second) vs. mean 13.2 m/second (SD 1.6 m/second), respectively; p < 0.01]. Using this difference in means (1.3 m/second) and the SD data provided, we would have 87% power to detect this difference (two-tailed, p < 0.05) with 50 individuals in each group. Therefore, a sample size of 102 (52 patients and 50 controls) would be adequately powered to demonstrate a 1.3-m/second difference in PWV between study groups. This difference in PWV is likely to be clinically important; a recent meta-analysis has suggested that an increase in aortic PWV of 1.3 m/second would be predicted to be associated with a multivariable-adjusted risk increase of 18% for total CV events and 20% for CV mortality and total mortality. 97
Our assessment included multivariable-adjusted analysis including several continuous and categorical covariates [psoriasis (yes/no), QRISK2, HbA1c, metabolic syndrome (yes/no), physical activity, depression], which increased the sample size by a modest amount.
Results
A total of 125 subjects were recruited: 66 female and 58 male. The median age was 58 years [interquartile range (IQR) 45–66 years]. There were 27 subjects with severe psoriasis and 44 with PsA. The median SAPASI score was 3.6 (IQR 1.4–6.2).
Mean PWV was 8.83 m/second (95% CI 8.08 to 9.58 m/second) in subjects with severe psoriasis and 8.80 m/second (95% CI 8.40 to 9.19 m/second) in those without; the difference was not statistically significant (p = 0.9). Controlling for CV risk factors as psoriasis-associated factors had little effect on the difference between these two groups (see Appendix 3, Table 12). PWV was significantly higher in subjects with PsA (mean 9.34 m/second, 95% CI 8.74 to 9.95 m/second) than in those without (mean 8.52 m/second, 95% CI 8.11 to 8.93 m/second; p-value for difference = 0.03). However, controlling for age and sex reduced the difference between the two groups until it was no longer statistically significant. The difference remained non-significant after controlling for CV risk factors and disease characteristics (see Appendix 3, Table 13).
There was no significant association between PWV and SAPASI score (p = 0.23). However, after controlling for age and gender, PWV increased with increasing SAPASI score (increase of 1.1% per unit increase in SAPASI score, 95% CI 0.3% to 1.8%) because the higher SAPASI scores tended to occur in younger subjects. This association was reduced to a non-significant level by controlling for CV risk factors (see Appendix 3, Tables 12–14).
There was a tendency for PWV to be higher in subjects with a higher age at onset of psoriasis (p = 0.001), but this effect became non-significant after controlling for age. Controlling for CV risk factors and psoriasis-related factors had no further impact on this association.
Conclusions
Main findings
There was no significant relationship between arterial stiffness and either the severity of psoriasis or patient age at onset after adjusting for age. This is in contradiction to other, smaller, studies that appeared to demonstrate a positive association between increased arterial stiffness and psoriasis. 103–105 Patients with PsA had higher levels of arterial stiffness and were older than patients without the condition, but this relationship became non-significant after adjusting for age. It is likely that the current study is a true representation owing to the removal of confounders and the larger sample size.
Clinical implications
The results suggest that patients with either severe psoriasis or early-onset PsA do not have higher levels of arterial stiffness than those with clinically less severe disease. The results do not support screening patients with psoriasis for arterial stiffness as a means of identifying individuals at high CVD risk. However, patients with psoriasis do have an increased prevalence of traditional risk factors and comorbid conditions associated with CVD. Thus, this still suggests that lifestyle modification and behaviour change to reduce acquired risk factors for CVD are an important part of managing psoriasis patients.
Strengths and limitations
The study involved a large, well-characterised patient cohort; however, the absence of a control population without psoriasis means that there was potential for limited statistical power to identify significant relationships.
Key conclusions
-
Screening for CVD risk factors in patients with psoriasis identified that approximately 40% of individuals were at high (> 20%) CVD risk over the next 10 years and had potentially modifiable CVD risk factors.
-
Management was suboptimal in a high proportion of patients with known risk factors.
-
In a group of patients with psoriasis, disease severity, age at onset of psoriasis and the presence of PsA were not independently related to higher levels of arterial stiffness.
-
There was no increased prevalence of raised arterial stiffness in patients with psoriasis or PsA.
Implications
-
These data provide valuable augmenting information about the prevalence of CVD risk factors and the potential value of primary care-based screening of patients with psoriasis.
-
It is important to intervene early to identify or prevent the development of poor lifestyle behaviours in patients with psoriasis with appropriate lifestyle management and behaviour change interventions.
-
Such interventions will, in turn, not only militate against development of CVD, but optimise psoriasis outcomes and improve the management of psoriasis per se (e.g. as has been shown for weight reduction).
-
There is no support for screening for arterial stiffness in clinical practice.
Cardiovascular disease risk communication and reduction in psoriasis
Workstream 2 (Figure 7) addresses objective 2 of the IMPACT programme: to apply knowledge about the risk of comorbid disease to the development of targeted screening services to reduce the risk of further disease and to investigate how this affects patient experience.
FIGURE 7.
Workstream 2: relationship of studies 2.ii and 2.iii to other IMPACT programme workstreams.
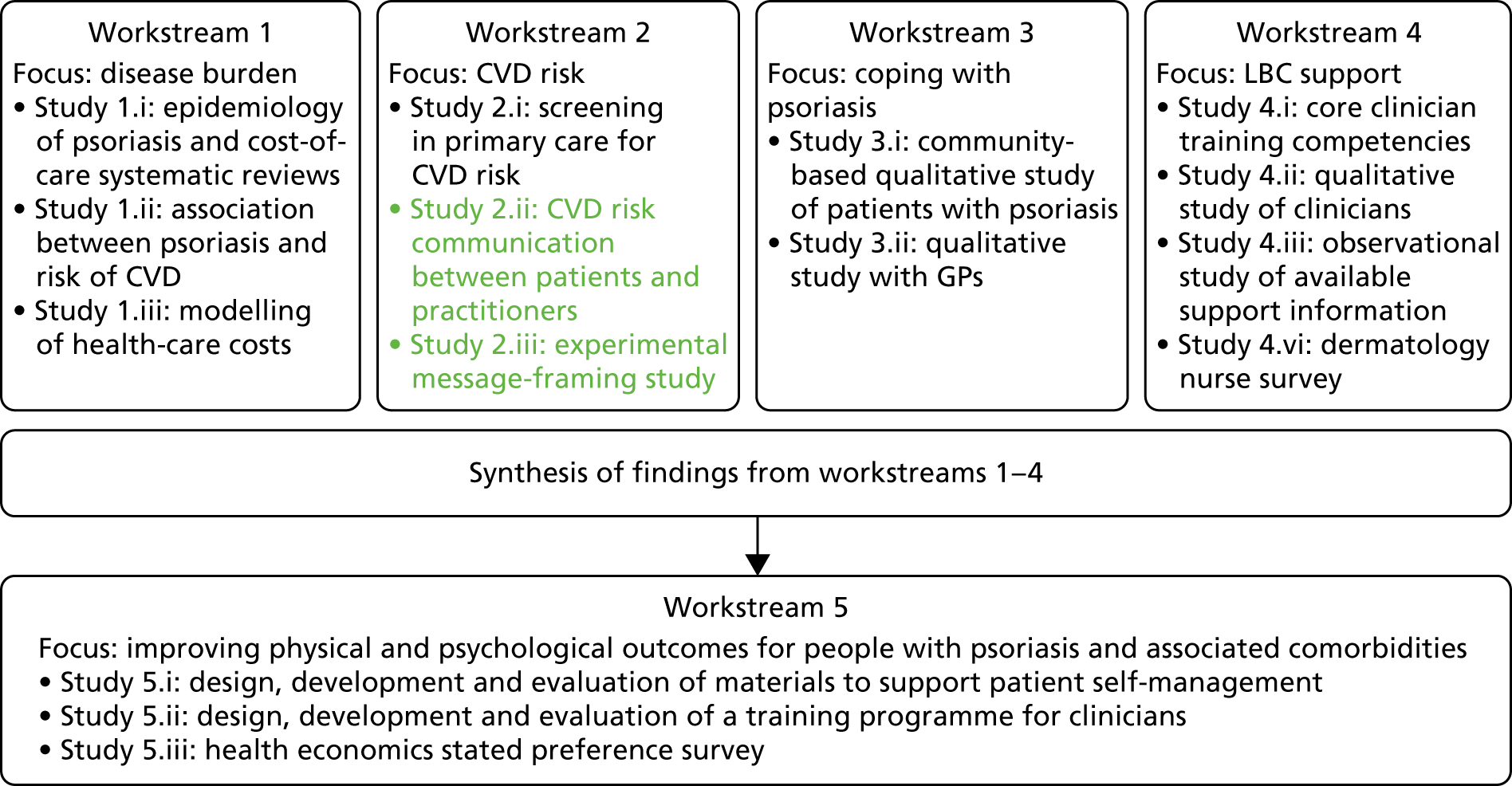
Publications relating to this section and workstream are listed in Publications and cited throughout this section.
Background
It has been highlighted that the precise nature of the relationship between CVD and psoriasis is not yet fully understood. It is likely to be multifactorial, involving inflammatory, genetic and behavioural processes. People with psoriasis appear, in general, to have higher levels of unhealthy lifestyle behaviours than the general population. As well as reducing the risk of developing CVD, there is evidence that behavioural strategies such as weight loss, healthy diet and increasing activity can also be beneficial in reducing psoriasis severity. 32,33,106–109 In addition, the high levels of psychological distress and/or low mood associated with psoriasis4,7 can compound an unhealthy lifestyle and inhibit a patient’s capacity to manage the challenge of making LBCs. 110
Although psoriasis is demanding for patients to live with and there is evidence for the benefits of lifestyle change as part of management, this is often overlooked by health-care professionals providing care. 11,111–113 Informing patients who are living with an already challenging condition that their disease may be an additional risk factor for CVD or other comorbidities is not neutral information. There is evidence that informing individuals of increased risk of disease without also providing strategies for reducing that risk has little effect on health protective behaviour or can make the situation worse. 114 This may act as a motivator for people to engage with health protective behaviours; however, disempowered patients may feel that they have even less control over their health than before. Furthermore, attempts to standardise written risk information sometimes result in patients significantly overestimating risk115 because perception of personal risk is complex and open to bias. 116,117
Message-framing
Message-framing – emphasising the benefits (‘gain framed’) or costs (‘loss framed’) of a behaviour or course of action – is important in risk communication116 and has been shown to affect behavioural outcomes. 118 The effects of message-framing may be different for different health-related scenarios, for example in the context of ‘prevention’ or low-risk behaviours, such as reducing alcohol, versus ‘detection’ or high-risk behaviours, such as cancer screening. 116 The effects of message-framing may also differ in relation to immediate versus future health consequences. 119 Emotional response to health messages is another factor that can also affect decision-making and behavioural outcomes. 120 These factors may be important in the context of communicating risk to people with psoriasis who might be motivated to make LBCs through a desire to either improve their immediate psoriasis symptoms or reduce their future risk of CVD.
In summary, it was unknown if, in a population of patients who are already identified as potentially psychologically vulnerable, (1) communicating CVD risk enables patients with psoriasis to understand their own personal risk and motivates or empowers them to reduce their risk rather than add to their distress or (2) message-framing affects patients’ behavioural intentions.
Aim
Workstream 2 aimed to explore risk communication to identify which communication techniques are most useful and/or effective in supporting psoriasis patients to understand risks associated with comorbidities and ways to minimise risk through health-related behavioural strategies. This was achieved through two related studies. These studies are summarised here but are fully reported in two published papers (Nelson et al. 88 and Keyworth et al. 121) and in a doctoral thesis that formed part of the IMPACT programme122 (see Appendix 4).
Study 2.ii: qualitative examination of cardiovascular disease risk communication in primary care
This study investigated how patients and primary care clinicians (GPs and practice nurses) perceived/experienced the process of assessing for and communicating about CVD risk in CVD screening consultations. The focus was on which risk communication techniques are used by GPs and practice nurses and how these affect patients’ understanding of risk in the context of psoriasis.
Study 2.iii: experimental study of message-framing
This study aimed to investigate the impact of health risk information on intentions to make healthy lifestyle changes in people with psoriasis and to determine whether gain-framed health messages (e.g. ‘if you change your lifestyle, your psoriasis will be better’) or loss-framed health messages (e.g. ‘if you don’t change your lifestyle, you will get CVD) are more effective in prompting intentions to change behaviour. It also aimed to investigate whether people’s behavioural intentions are driven by a desire to improve immediate psoriasis symptoms or reduce future risk of CVD. This work has been published. 123
Study 2.ii: qualitative examination of cardiovascular disease risk communication in primary care
Methods
Data collection and analysis
This was a qualitative study that was ‘nested’ in the main screening study (study 2.i) of workstream 2 (Cardiovascular risk in patients with psoriasis). Ethics approval was obtained from the North West – Greater Manchester East REC (reference number: 11/NW/0654). Patients (aged < 40 years and ≥ 40 years) and practitioners (GPs and practice nurses) from primary care practices participating in the screening study consented to have their CVD risk assessment/follow-up consultations audio-recorded. The aim was to capture as many audio-recordings as possible and use them to inform subsequent sampling and also data collection by playing extracts of the recorded consultations to stimulate discussion in interviews, a procedure termed ‘tape-assisted recall’. Consent to approach participants for interview was also obtained, with the aim of purposively sampling patients and practitioners based on (1) salient issues arising in consultations and (2) diversity of personal characteristics.
Audio-recordings
Audio-recordings were analysed with a qualitative content analysis124 using a framework of topics relevant to CVD risk assessment/management (e.g. biomedical, behavioural and psoriasis-specific factors) to guide critical listening/coding of audio-recordings. Coding enabled categorisation of practitioners’ communication techniques in terms of how they attempted to support patients to understand their own personal risk and ways to reduce risk. This categorisation informed subsequent in-depth interviews with both patients and practitioners about their experiences of risk communication consultations. See Nelson et al. 88
In addition, a subset of audio-recordings (selected for a spread of practices and practitioners taking part in the screening study) were subject to detailed quantitative content analysis125 using a pre-determined coding frame to identify the type of information provided by practitioners in consultations (i.e. generic or individualised, verbal or numerical descriptors of risk or both) and how the information was conveyed (i.e. if interpretation of risk information and/or specific advice on how to modify risk was provided to patients). See Keyworth et al. 121
Interviews
Interviews with both patients and practitioners were guided by interview schedules developed from the literature, from the qualitative content analysis of the consultations and through discussion among the team (Table 3).
| Patient interviews | Practitioner interviews |
|---|---|
| General questions | |
|
Reasons for taking part in the study Understanding of the study/consultation purpose Understanding of CVD risk and link with psoriasis Perception of personal risk Any changes in views about health since talking part |
Reasons for taking part in the study Understanding of the study Type and amount of information given to patients Strategies used to communicate risk to patients Techniques used to address patients’ LBC Barriers to/facilitators of doing lifestyle change work Training needs |
| Specific questions linked to recording excerpts | |
|
Understanding of risk information conveyed by practitioner and ways to reduce risk Perceptions of sources of information, support for LBC |
Reflections on what was happening in the consultation, including aims, intentions, intended messages, techniques used, impressions of patients’ understanding, impressions of patients’ emotional reactions, and use of personalised/general strategies |
The tape-assisted recall approach enabled interview questions to be grounded in specific instances arising in consultations when practitioners and patients discussed these issues. Interviews were audio-recorded, transcribed verbatim and transferred to NVivo 10 (QSR International, Warrington, UK) for data management. 126 Patients were asked how they understood their own level of risk, knowledge of methods to reduce risk, intentions to act to reduce risk, ability to undertake potential lifestyle changes and anxiety in discussing risk. These components linked to the theory of planned behaviour,127 which highlights key factors that predict health-protective behaviours. Clinicians were asked about their perceived ability to pick up on client distress, whether or not they addressed all of a patient’s concerns and key discussion points in the CVD risk consultations. Principles of framework analysis128 guided the analysis of the interview data and enabled a framework of key concepts and themes to be generated.
Results
Practitioners in 10 of the 13 general practices participating in the overall workstream 2 screening study (study 2.i) agreed to have their consultations audio-recorded (four GPs, seven practice nurses and two research nurses). In total, 130 separate audio-recordings of CVD risk assessment consultations and 15 audio-recordings of follow-up consultations were captured. These involved 13 different practitioners and 131 patients (because one patient had the follow-up but not the initial consultation recorded). Capturing follow-up audio-recordings in particular proved to be very problematic because appointments were not conducted in dedicated clinics in the same way that assessment appointments were. Twenty-nine in-depth interviews with patients with psoriasis were then conducted (12 patients aged < 40 years; 17 patients aged ≥ 40 years). In-depth interviews with 12 primary care practitioners (six practice nurses, two research nurses and four GPs) were carried out. (Sampling for qualitative interviews was brought to a close when the data set was judged to offer sufficiently rich and detailed accounts of patient and practitioner experiences.)
Analysis focused on how risks were discussed in consultations, what methods of communicating risk information were used and if practitioners investigated patients’ knowledge of risk reduction or offered support about risk reduction/referral.
Findings
Audio-recordings
Findings highlighted the limited discussion of risk and LBC between practitioners and patients, with practitioners prioritising recording of information over responding to patient cues for discussion or as opportunities for intervention. The data contained a few examples of skilled, patient-centred discussion in a minority (2/13) of practitioners (both involved a practice nurse with training in behaviour change techniques). However, more commonly, practitioners appeared to miss opportunities to address patient cues for discussion of CVD risk and/or lifestyle management (Table 4 contains consultation extracts). There was also limited evidence of practitioners using skills in exploring and addressing low mood and its link with either CVD risk factors, such as excessive alcohol use/smoking, or psoriasis.
| Extract | Interpretation (linked to core issues from consultation analysis) |
|---|---|
| Missed opportunities | |
| Extract 1 (consultation 80) | |
| GP3:So it works out at 42 units a week . . . | |
| Patient:I’m just a normal bloke who goes to the pub on a Friday night, that’s all! | Patient reports drinking above recommended weekly units of alcohol, uses humour to deflect discussion |
| GP3:Yeah, yeah, so it’s all right, it’s just that you know the rec– . . . we just need to advise you what the current guidance is, OK? | GP side-steps discussion, falls back on standard recommendations rather than addressing individual need |
| Patient:[laughs] [pause] | No verbal response from patient |
| GP3:Right, now, let’s have a look at your medications | GP changes topic to focus on recording medication use |
| Extract 2 (consultation 16) | |
| Patient:I’m desperately trying to lose weight . . . | While being weighed, patient gives clear cue about needing weight management support |
| PN1:That’s 78.2 kg, OK . . . [typing] which comes to 12 st 4 Ib. | Nurse focused on recording patient’s weight |
| Patient:[gasps] | Patient gives clear non-verbal cue indicating emotional impact |
| PN1:See what you were before . . . [typing] more or less 5 kg – a stone . . . | Nurse does not respond to emotional cue and points out patient’s weight gain |
| Patient:Yeah . . . I put on a lot when I was first pregnant . . . | Patient gives reason for weight gain |
| PN1:Yeah, 68 kg there, weren’t you? Have you ever smoked? | Nurse misses/blocks patient cue for discussion of weight and moves on to record smoking status, shuts down discussion |
| Extract 3 (consultation 102) | |
| PN8:We’ve got you down as 10 units [of alcohol] per week last time | |
| Patient:Yeah, I’ve cut it down last – er – I’ve been cutting down | Patient offers cue for possible discussion of own successful behaviour change |
| PN8:Right | Nurse does not respond to patient cue, closes down discussion |
| Patient:I don’t really drink . . . I’m trying to lose weight, you see. Now if you’d have said psoriasis impacting weight, I’d have said that’s what’s caused all my weight problems since I was 7 [laughs] – I would, honestly! | Patient cues for discussion a second time, links own alcohol behaviour change with goal of weight loss and raises further link between psoriasis and weight |
| PN8:Right, um, are you on any statins? | Nurse fails to respond to specific concerns of patient, misses opportunity to discuss alcohol, weight and lifestyle–psoriasis links, closes down discussion, focuses on recording medication use |
| Used opportunities | |
| Extract 1 (consultation 30) | |
| PN2:You are 13 st 6 lb and it’s saying at the most you should be 9 st 11 lbs you see, on the computer, so . . . | Nurse uses ‘guiding’ style and graphical representation of BMI categories to support patient’s understanding of appropriate weight |
| Patient:So I’m in the obese scale? | Patient verbalises concern about weight information provided |
| PN2:Yeah. So what we gonna do? What do you think you can do exercise-wise? They say if you pick something that you like you will maintain it and keep doing it, you know what I mean? Rather than me saying to you,‘Right, you need to go for a walk five times a week for half an hour’. You need to find something that you like doing, really, to maintain it and keep it going |
Nurse responds directly to patient’s concern, signals that she will support patient, explores patient’s confidence to make a change Gives rational for increasing/maintaining physical activity, encourages individualised choice Moves towards planning for change |
| Extract 2 (consultation 61) | |
| PN9:But first of all, just as a first thought . . . is doing a food diary | Nurse suggests a food diary as part of individual action planning with patient |
| Patient:Right, yeah! | Patient expresses interest in food diary activity |
| PN9:And actually writing down for a week what you have during the day and when you have it and absolutely writing everything down, even if it’s just a few crisps | Nurse focuses on detail of food diary with patient |
| Patient:Even if it’s just a few crisps or whatever, yeah, yeah, course, yeah | Patient expresses interest in plan |
| PN9:Yeah, and then I can see you again with that and we have the option of referring you to the health trainer . . . We do have other referral procedures where we can actually send you to the Active Living Team . . . It’s – which fits into your lifestyle and which you would prefer to do? . . . If we could initially ask you to do a food diary, then see me in a week to 10 days with that? |
Nurse offers review/follow-up to discuss food diary, offers possibility of further options for more individualised support Encourages individualised choice Makes a plan with timeline for follow-up |
| Patient:Food diary, of course, yep, no problem | Patient expresses interest in plan |
In addition, 44 audio-recordings in total were sampled for the detailed quantitative content analysis using a coding frame to record specific techniques used by practitioners to communicate risk information. There was variation in the type of risk information communicated to patients. Generic risk information given alone was rare (two out of 44 consultations), with individualised information given more frequently on its own (23 out of 44 consultations) or in combination with generic information (19 out of 44 consultations). The most frequently used method of communicating magnitude of risk was a verbal descriptor accompanied by a numerical descriptor (28 out of 44 consultations), rather than a verbal descriptor alone (16 out of 48 consultations). No practitioner used numerical descriptors of risk alone. When risk factors were discussed with patients (156 instances across all consultations), interpretation was provided on 131 occasions. However, specific advice about behavioural strategies to modify risk was given in fewer than half (60 out of 156; 38%) of consultations.
Practitioner interviews
Practitioners described their role to be that of standard advice-giver, yet, paradoxically, report this strategy to be ineffective in helping patients with risk reduction. More commonly, nurses (who were expected to have more of a role in conducting routine risk assessment consultations than GPs) were not confident about supporting patients with risk reduction as part of their professional role, believing themselves to be ineffective and untrained to deliver effective behaviour change support.
In addition, practitioners often minimised or downplayed identified CVD risk factors during consultations with the result that risk was often understated or normalised (i.e. framed as common and expected). For example, they tended to emphasise risk factors that were absent for patients (e.g. no family history of CVD) alongside discussing those that were present (e.g. high cholesterol). Practitioners also downplayed the extent to which identified risk factors were present. They would say something like ‘it’s high but only a bit high’ or ‘it’s only just out of range’. They tended to normalise the presence of risk factors as being common in today’s society. Practitioners also reported in interviews that their own perceptions of risk and risk factors influenced their attitudes and perceptions about the seriousness of both. In some cases, practitioners’ beliefs contradicted health-care recommendations (e.g. little risk was associated with drinking excess alcohol or being overweight). In general, these factors seemed to account for practitioners’ reported approach to practice as being generally reactive rather than preventative in nature.
Patient interviews
Patients sometimes interpreted the lack of personalised engagement from clinicians in consultations as a signal that the risk/lifestyle modification information imparted was not relevant to them. Many reported taking away from CVD risk consultations little new understanding of their own personal risk and how to reduce it. Some reported strong emotional reactions to risk discussions such as experiencing health behaviour conversations as emotionally laden and feeling burdened to make lifestyle changes. Nonetheless, patients viewed primary care clinicians as being in an influential position to help them understand CVD risk and make lifestyle changes and wanted to discuss these issues more in consultations with their doctor or nurse.
Conclusions
Screening for CVD risk provides opportunities for practitioners and patients to discuss current and future health status and enables practitioners to offer brief behaviour change interventions. Such opportunities may be missed because of a practitioner focus on data-recording rather than intervention in consultations, minimising risk and avoiding discussion with patients of the emotional impact of risk information, as well as providing standardised rather than specific/personalised advice about how to modify CVD risk.
Study 2.iii: experimental study of message-framing
Data collection and analysis
A community sample of participants with psoriasis was recruited to an online questionnaire to investigate the impact of health risk information on reported intentions to make healthy lifestyle changes. Ethics approval was obtained from the university REC (reference number: 13118). A two-by-two between-participant design was used to randomly allocate individuals to one of four evidence-based health message types (message frame: loss or gain; message focus: immediate psoriasis symptom reduction or future CVD risk reduction). The primary outcome measure was behavioural intentions to change. A series of two-way between-group analyses of variance tests were conducted to explore the impact of message frame and message focus on behavioural intentions.
Results
A total of 217 participants were randomised to one of the four conditions. There was a significant message frame × message focus interaction for reported behavioural intention to reduce alcohol intake (p = 0.023). Loss-framed messages (e.g. ‘if you don’t change your lifestyle you will get CVD’) were more effective for intentions to reduce future risk of CVD whereas gain-framed messages (e.g. ‘if you change your lifestyle your psoriasis will be better’) were more effective for intentions to reduce immediate psoriasis symptoms. Behavioural intention scores for increasing exercise and improving diet were not statistically significant. See Keyworth et al. 123
Conclusions
Message effectiveness about the benefits of reducing alcohol in the management of psoriasis is dependent on how the message is constructed. When presented with messages about short-term psoriasis symptom reduction, gain-framed messages were more effective than loss-framed messages in increasing behavioural intentions. Conversely, when presented with messages about long-term CVD risk reduction, loss-framed messages were more effective than gain-framed messages.
All findings from study 2.ii informed the planning and development of the IMPACT workstream 5 patient materials and practitioner training intervention, which both include risk reduction strategies.
Key conclusions
-
In consultations for CVD risk communication in people with psoriasis, opportunities to support patients’ understanding of risk and strategies to reduce risk were missed.
-
Most practitioners did not feel equipped to provide tailored, person-centred risk communication and LBC support.
-
In addition, the way practitioners frame health risk messages may affect behavioural intentions in people with psoriasis depending on the health benefit being emphasised.
Coping with psoriasis: learning from patients
Workstream 3 (Figure 8) addresses objective 3 of the IMPACT programme: to learn from people with psoriasis about coping (self-management) strategies.
FIGURE 8.
Workstream 3: relationship of studies 3.i and 3.ii to other IMPACT programme workstreams.
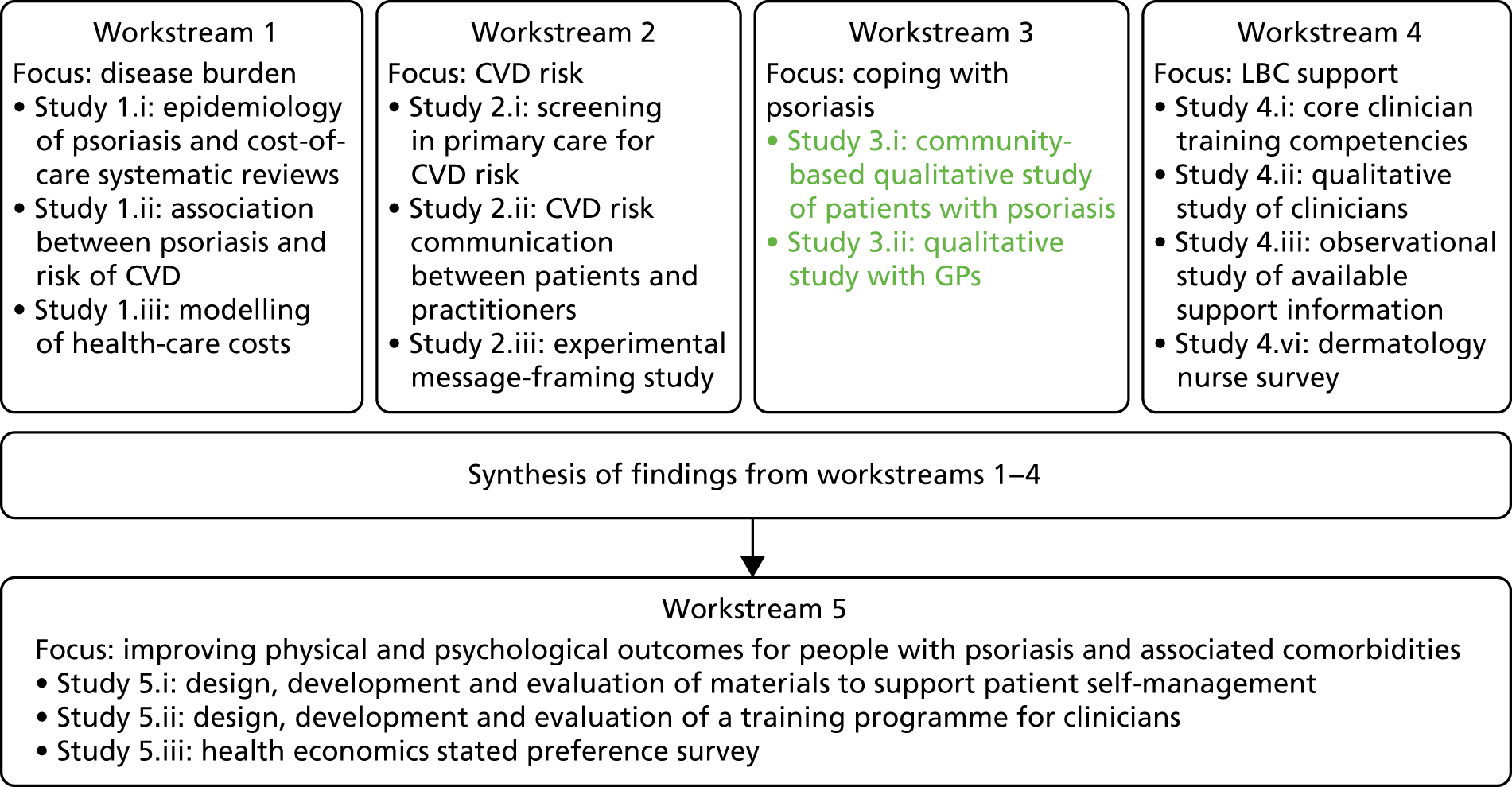
Publications relating to this section and workstream are listed in Publications and cited throughout this section.
Background
As described in Synopsis, psoriasis is a common, lifelong inflammatory skin disease. It is associated with high levels of physical and psychosocial disability2–4 and is incurable. Given that the majority of patients develop the first signs of psoriasis before 40 years of age, the disease can make significant demands throughout productive adult life. 6 The long-term, visible and fluctuating nature of psoriasis means that its effects can be as disabling as, and possibly even more disabling than, other long-term conditions, with significant experiences of stigmatisation, low self-esteem and even suicidal thinking. 7–9 The relationship between physical or clinical severity of psoriasis and the degree of psychosocial disability relating to the condition is complex. It is not unusual for individuals with psoriasis of very limited extent to have significant and disabling impairment to quality of life10 and health-care professionals often fail to detect psychological distress in patients. 11
As outlined in earlier sections, there are a range of comorbidities associated with psoriasis. Of particular relevance to this chapter is the finding that people with psoriasis are more likely to have increased acquired risk of some comorbidities owing to higher rates of unhealthy lifestyle behaviours, including smoking, excess alcohol consumption, obesity and/or being sedentary (see Cardiovascular risk in patients with psoriasis). Although there is some evidence that weight loss, healthy diet and/or increasing physical activity can reduce psoriasis severity,32,33,106,108,109 the relationship between psychological distress and unhealthy lifestyle can be bidirectional, impeding people’s capacity to manage the challenges of making behavioural changes.
The majority of patients with psoriasis are managed in primary care; however, there is evidence that these patients may be managed inadequately or inappropriately in this setting. 129,130 Surveys from patient organisations in both the UK and USA indicate that dissatisfaction with psoriasis care is widespread. 12,131,132 A large survey from the Psoriasis Association in the UK reported that a quarter of respondents had not consulted a health-care professional about their psoriasis in the previous 2 years;12 in a more recent update, one-third of respondents reported having never consulted a doctor about psoriasis and another 19% had consulted a doctor about psoriasis no more than once per year. 132 In addition, treatment adherence is low, with reported rates of adherence to recommended regimens being between 22% and 67%. 133 Although some people have unanswered concerns about adverse treatment effects,134 others express their belief that they are not receiving effective treatments. 133,134
This literature suggests that many people with psoriasis may be missing out on (and perhaps unaware of) recent developments in treatment options or may be self-managing their condition suboptimally. The NICE guideline for the assessment and management of psoriasis135 recommends that psoriasis is recognised and managed as a complex, long-term condition linked with physical and psychological comorbidities; however, evidence suggests that it may not currently be managed in this way. Against this backdrop, it was unknown how people living with psoriasis cope with and self-manage the condition, meaning in-depth perspectives from affected people were missing from the literature. The views of GPs about managing patients with psoriasis in primary care were also unknown.
Aim
The aim of workstream 3 was to explore the perspectives of people with psoriasis about effective coping responses and self-care strategies through:
-
In-depth qualitative interviews with people with psoriasis (study 3.i: main patient study).
An explicit aim of this work was to learn about the coping strategies used by individuals who were currently functioning well despite severe disease and how people seek or choose not to seek help from health-care professionals and services. See Nelson et al. 112 As an adjunct to this work, a brief subsidiary study (study 3.ii) aimed to gather the views of health-care professionals managing people with psoriasis.
-
Qualitative interviews with GPs about experiences of managing psoriasis in primary care (study 3.ii: adjunct GP study).
This study aimed to explore GP views and experiences of their own practice of psoriasis management. See Nelson et al. 111
Approval was obtained from the University of Manchester’s REC for the patient and GP studies (reference numbers 10325 and 11353, respectively) and from the Greater Manchester Primary Care Research Governance Partnership (ReGroup) for the GP study (reference number 2011/280).
Methods
Data collection and analysis (main patient study)
Given the evidence that people with psoriasis may disengage from health-care services for psoriasis management, the study sample was recruited from community sources (i.e. not through primary or secondary care health-care services) across Greater Manchester by distributing information through websites and in community locations such as libraries, places of religious worship, shops and community centres. Sampling was purposive for maximum variation on age, gender, ethnicity, socioeconomic background and self-identified severity of psoriasis including duration and treatment.
Data were gathered from participants in a location convenient to them. This qualitative study used face-to-face interviews to gather views about coping with psoriasis including experiences of consultations with practitioners. Ethics approval was provided by the university REC (reference number: 10325). In-depth, face-to-face, semistructured qualitative interviews were conducted using a topic guide developed from the existing psoriasis literature as well as the theoretical frameworks of illness beliefs136 and coping/appraisal. 137 These theoretical approaches conceptualise coping as an evaluative process by which individuals appraise both the source of stress and the coping resources available to them. The appraisal process accounts for individual differences in response to similar levels of adversity. Participants were asked about physical, emotional and social effects of living with psoriasis and their coping strategies (both problem- and emotion-focused, including use of social support, self-care strategies, medication use and choices as well as use of health-care services). Interviews were audio-recorded with consent, transcribed verbatim and transferred to the NVivo computer package for qualitative data management. 126 Data were analysed concurrently with data collection according to principles of framework analysis128 to code for salient themes and produce a thematic framework of key concepts using constant comparison techniques. 138
Data collection and analysis (adjunct general practitioner study)
General practitioners in Greater Manchester primary care practices were invited by e-mail to take part in an interview and purposively sampled for diversity on gender, age, ethnicity and size of practice. A topic guide was developed from the relevant literature and used to guide interviews in which GPs were asked about how they managed patients with psoriasis in the primary care setting. Interviews were audio-recorded, transcribed verbatim and, as above, also analysed using principles of framework analysis and constant comparison to generate key concepts. The NICE guideline135 (which focuses on the importance of two main elements: the assessment and the management of psoriasis) was used to identify main themes about GPs’ attitudes to managing psoriasis.
Results
Response to advertising was rapid, with approximately 90 people with psoriasis expressing interest in being interviewed. Purposive sampling was used to select 29 people to take part in interviews, enabling achievement of a community sample of patients who were diverse in terms of age, gender, socioeconomic background, ethnicity, self-identified severity of psoriasis and duration and treatment of psoriasis.
In total, 14 GPs (a mixture of salaried, partner and trainee GPs) who were diverse in age and gender as well as in geographical location and practice size were interviewed for the practitioner study.
Findings (main patient study)
Participants described marked experiences of stress and distress in relation to living with psoriasis (including itching, flaking and painful skin, and lowered mood, self-confidence and self-image due to concerns about their appearance and feelings of low control over psoriasis symptoms). These demands were perceived to go unacknowledged in consultations with health-care professionals. Participants gave accounts of poor experiences in the management of psoriasis by health-care practitioners (such as perceived lack of expertise and a failure to recognise and manage psoriasis as a complex, long-term condition with appropriate monitoring, review and referral to specialist services). There were reports of coping with the demands of the condition against the perceived lack of support from services by disengaging from consulting about psoriasis or seeking alternative opinions and treatments outside formal health-care services.
Analysis also enabled identification of the function, range and use of coping/self-care strategies among people with psoriasis. Participants reported a variety of goals in relation to coping with their condition, for example reducing symptoms and psychological distress, managing uncertainty and gaining control over symptoms. Although at the outset the study had a particular focus on identifying positive coping strategies, during data collection and analysis it became clear that there were higher than expected levels of distress among the study sample and evidence of common use of less helpful coping strategies in this group (such as minimising psoriasis as unimportant, disengaging from services, stopping treatment use, avoiding social contact, bottling up emotions and acting on angry feelings as well as hypervigilance in hiding affected skin). Some effective coping responses/self-care strategies were found (such as learning to be open about psoriasis with inquisitive people, taking care of general health, seeking emotional support from trusted people, focusing on positive aspects of life, pacing activity to conserve energy and suggesting alternative treatment strategies when consulting with their health-care professionals). We acknowledge that the high levels of distress found could be due to self-selection bias, with ‘most distressed’ people responding to advertising; however, participants interviewed were often working, studying and functioning in roles as parents or carers. Moreover, there were no strong indicators that this sample was atypical.
The analysis also identified a number of culturally specific cross-cutting themes that were particularly salient for participants with a South Asian background. These were linked specifically with high levels of experienced stigmatisation due to the visible nature of psoriasis, which could affect an individual’s standing in their family and wider community. Table 5 presents illustrative data extracts from the patient study.
| Themes | Data extracts |
|---|---|
| Physical/emotional stress and distress | You’re stressful because you feel crap, your body’s itchy and when you’re irritable you’re unhappyP12: white male, aged 44 years |
| Need for emotional stress and distress to be acknowledged in consultations | Nobody’s ever asked me, even my GP, ‘how bad is it? Does it trouble you?’P16: white male, aged 39 yearsYou don’t get a chance to express yourself . . . If [doctors] are not inviting or understanding enough, what’s the point of talking to them about something that deep?P20: British Pakistani male, aged 37 years |
| Need for social stress and distress to be acknowledged in consultations | I showed [the GP] and he said, ‘well, it’s not that bad’. That really upset me because it probably isn’t bad compared to other people, but you don’t want that from your doctor, you want some empathyP3: white female, aged 28 years |
| Control issues | There’s nothing you can do. There’s not a hope in hell that [creams] control it at all. There’s no pointP8: white male, aged 28 years[Psoriasis] has [a] mind of its own, completelyP1: white male, aged 43 years |
| Perceived lack of expertise/ability to help | [The GP] can’t do anything. What can he do when he says there’s no cure – just try this ointment?P13: Pakistani/European female, aged 70 years[The dermatologist] prescribed medicine which didn’t completely work and said, ‘we don’t know nothing much about it’, because the medicines weren’t that goodP26: Bangladeshi male, aged 20 years |
| Perceived lack of management/monitoring of psoriasis as a long-term condition | I’ve got angina issues – same client with two different problems – given loads of time for one [angina] but none for the other [psoriasis]P20: British Pakistani male, aged 38 years |
| Lack of ongoing discussion with GPs for psoriasis treatment review | [Restricted prescribing of steroid cream] annoys me and I know there’s probably a reason for it, but I’ve never understood [it]. They say it thins your skin – well, I prefer thin skin than psoriasis!P3: white female, aged 28 years |
| Perceived restrictions on referrals to specialist care for psoriasis | [The GP] was reluctant to refer me to a dermatologist – did eventually, but it took a long timeP9: white male, aged 23 yearsTo be told you’re not in a worse enough condition to get any further help is annoyingP16: white male, aged 39 years |
| Ceasing to consult owing to belief that nothing can/will be done to help | When I go to my doctors, it’s not really about psoriasis, because they’re not going to be able to help meP23: Indian male, aged 32 years |
| Ceasing to consult owing to prioritising other conditions before psoriasis | There’s a tendency to think GPs have better things to do . . . I am not going to die from [psoriasis]P10: white male, aged 20 years |
| Consulting outside the UK health-care system | Whenever I go [to Pakistan], I pay for my treatment, because if I hadn’t paid for [dermatology consultation] I would never have had [the diagnosis]. My repeat prescription would have kept on coming and [psoriasis] would have got worseP18: Pakistani female, aged 47 years |
Findings (adjunct general practitioner study)
General practitioners reported assessment and management of psoriasis that were not in line with NICE recommendations. First, some GPs recognised psoriasis as a complex condition but most viewed it primarily as a skin complaint. No GP reported undertaking a structured assessment of the impact of psoriasis on patients to incorporate discussion of possible physical and psychological comorbidity. In particular, they reported minimising the potential emotional and social effects of the condition on people’s lives in consultations. Second, GPs did not view or manage psoriasis as a long-term condition with regular monitoring, review and appropriate referral as they would for other long-term conditions seen in primary care. Addressing lifestyle issues in relation to psoriasis was also not a priority. However, most GPs indicated low levels of expertise and confidence to manage psoriasis, citing lack of undergraduate and postgraduate training in dermatology. Illustrative data extracts are presented in Table 6.
| Themes | Data extracts (GPs and patients) |
|---|---|
| Assessing psoriasis and being assessed | I think of it primarily as a skin complaint . . . I am aware that there is . . . systemic problems . . . but, I think in my own mind, I sometimes find it difficult to put the two togetherGP5It’s a skin condition but it goes beyond that. It doesn’t stay there. It sort of runs deeply . . . there was discomfort on me. It got to the stage where I was thinking about it so much I actually cancelled meeting my friendsP17 |
| Physical assessment | I probably don’t examine them enough because I look at what they show me . . . so I’ve never even thought about the discussion . . . and, well, obviously I feel uncomfortable undressing . . .GP3[The GP] has never looked . . . never examined me for my skin . . . in 6 years or something like thatP8 |
| Psychological assessment | I haven’t got any patients who are distressed to the point of not wanting to go out, not wanting to do anything, where it impacts on their lives . . . mostly because I think we manage their psoriasis sufficiently wellGP10You don’t really get a chance to express yourself. I’ve not been to hospital yet with [psoriasis], so I don’t know if somebody is going to be able to listen to what is actually going on for me. I kind of feel embarrassed going out. There’s no one listening to thatP20 |
| Managing psoriasis as a long-term condition | So much of the success of psoriasis treatments is based on patients’ ability to manage it themselves and apply stuff appropriately . . . We can prescribe anything we like, but if the patient isn’t using it correctly, it’s probably not going to workGP11I get fed up . . . You can use [creams] for a period of time but eventually they stop working . . . My psoriasis will keep growing and the cream will lose its effect. So I generally don’t put anything on my skinP8 |
| Management/monitoring of psoriasis | Patients’ willingness and desire to be regularly reviewed is very variable. A lot of them, even if you say come back in a month . . . they don’t come back, they don’t really want to see you againGP8There was never any kind of follow-up to it and I never went to the GP for any other reason, over the 10-year periodP11 |
| Expertise in psoriasis | There wasn’t a huge amount of dermatology [training] . . . I would probably do more if I knew moreGP9GPs just basically didn’t know what to do with it because they don’t come across it, or if they do come across it they have limited knowledge of itP6 |
All findings from these studies informed the planning and development of the IMPACT programme workstream 5 new patient materials and practitioner training intervention.
Key conclusions
-
The daily demands on people living with psoriasis are strong in terms of physical, psychological and social challenges.
-
Patients feel unsupported by health-care services based on negative experiences of limited support.
-
Despite NICE recommendations for the management of psoriasis, psoriasis is not yet recognised and managed as a long-term, complex condition. This seems to translate as minimal acknowledgement of the emotional and social needs that need to be addressed alongside accurate diagnosis, regular review and appropriate referral.
-
Individuals may disengage with consulting about psoriasis, leading to uncontrolled symptoms and suboptimal self-management.
-
The self-reported levels of expertise and confidence of GPs managing patients with psoriasis in primary care are low compared with other long-term conditions.
Implications
-
Services need to be designed that address the needs of psoriasis patients, recognise it as a lifelong, potentially life-ruining, complex condition that requires holistic support to address physical, psychological and social effects and comorbidities.
-
This would involve timely diagnosis, monitoring and regular review as well as referral to specialist services.
-
Current levels of demand may be an underestimation because of low levels of engagement by patients due to low expectations of service provision.
Findings from this workstream informed the planning and development of the IMPACT programme workstream 5 practitioner training intervention and the new patient materials intervention.
Published outputs from the IMPACT programme112,113 were and cited in the British Psychological Society (BPS) consultation response to the revised NICE guidance (CG153)61 and the IMPACT programme team’s recommendations for NICE quality standards as part of the consultation process.
Understanding the professional role in supporting lifestyle change in patients with psoriasis
Workstream 4 (Figure 9) addresses objective 4 of the IMPACT programme: to identify the barriers to providing patients with useful LBC advice.
FIGURE 9.
Workstream 4: relationship to other IMPACT programme workstreams.
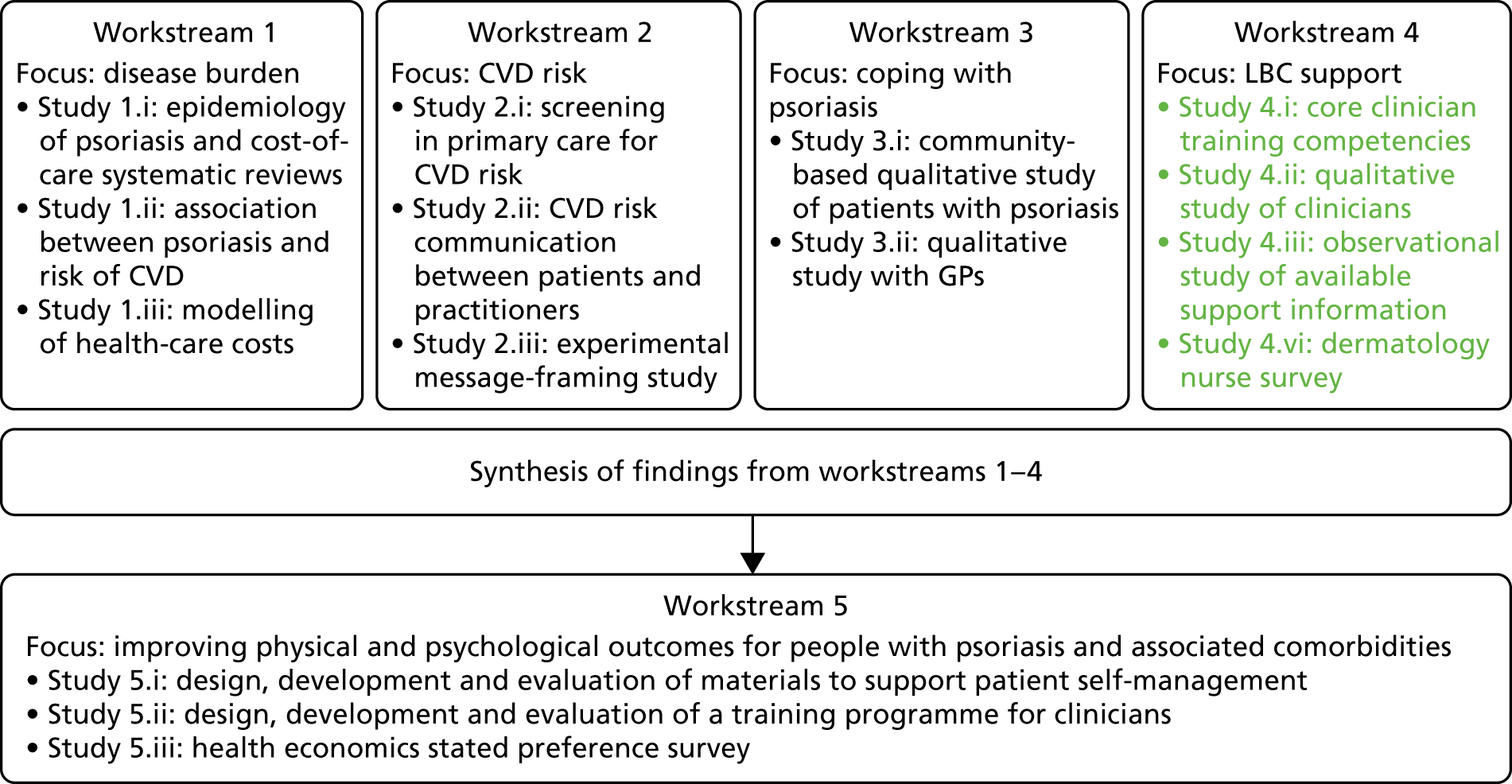
Publications relating to this section and workstream are listed in Publications and cited throughout this section.
Background
As all earlier sections highlight, the lifelong, inflammatory nature of psoriasis can generate significant physical, psychological and social demands for affected people that are not always recognised by health-care professionals (e.g. GPs, dermatologists, specialist dermatology nurses) and can lead to disengagement from health-care services and/or suboptimal self-management. In addition, comorbidities of psoriasis are not well recognised by many patients or primary care practitioners. The relationship between psoriasis and increased risk of comorbidities including CVD is complex and likely to be multifactorial, involving inflammatory, genetic and behavioural processes. Patients with psoriasis are also likely to have increased acquired risk due to a higher likelihood of smoking, excess alcohol consumption, obesity and a sedentary lifestyle. These behaviours can be compounded by the low mood and psychological distress common in psoriasis, which can hinder a person’s capacity to manage their lifestyle, making psoriasis particularly challenging to live with.
Addressing patients’ lifestyle as part of patient care is known to be effective in the management of other long-term conditions139–142 and there is some evidence that weight loss, healthy diet and increased physical activity may reduce psoriasis severity. 32,33,106–109 Health-care professionals managing people with psoriasis could, with appropriate training, support patients’ LBCs. However, to the best of our knowledge, there was no published literature on whether or not LBC skills are included in the training curricula for such health-care professionals, or whether or not professionals are equipped with the knowledge, skills and confidence to manage psoriasis as a complex, long-term condition, including supporting patients with lifestyle changes.
In addition, recent research indicates that brief, subtle changes to the environment can also influence health-related behaviours, such as healthier food choices and increasing physical activity in positive ways,143–145 and that exposure to written and/or visual LBC information in health-care settings may facilitate health promotion to improve patients’ knowledge and attitudes to behaviour change. 146 However, little was known about the nature and quality of lifestyle-related information that patients with psoriasis may be exposed to in the clinic setting.
In summary, it was unknown if and how patients with psoriasis were being supported to develop and maintain healthy lifestyle behaviours as part of self-management.
Aim
Workstream 4 aimed to identify the barriers to effectively supporting patients with psoriasis to develop and/or maintain a healthy lifestyle through four inter-related studies.
Study 4.i: content analysis of core training competencies relating to lifestyle behaviour change skills for health-care professionals
This study aimed to assess the extent to which LBC skills are included in the postgraduate training curricula of dermatologists, dermatology specialist nurses (DSNs), GPs and general practitioners with a special interest (GPSIs) in dermatology. 147
Study 4.ii: in-depth qualitative interview study of health-care professionals to examine how they conceptualise and manage psoriasis, including gauging attitudes to providing lifestyle behaviour change support as part of patient care
This qualitative study aimed to assess the experiences of dermatologists, specialist nurses and GPs in managing psoriasis and, in particular, providing strategies and referral routes to support lifestyle change (e.g. weight reduction, increased physical activity, reduction in smoking or alcohol use) for patients and the barriers to conducting this work in practice.
Study 4.iii: observational study of the prevalence, nature and quality of lifestyle behaviour change information available to patients with psoriasis in clinic settings
This study aimed to investigate whether or not the setting of the patient waiting room currently promotes appropriate LBC information for psoriasis patients by providing up-to-date information linking lifestyle with disease (general and specific) and high-quality links to support LBC.
Study 4.iv: survey of dermatology specialist nurses to assess views on behaviour change skills training needs
This study aimed to assess the views of DSNs on training needs in relation to supporting behaviour change in people with psoriasis. The aim was to enable planning of the level and type of training required for dermatology specialist staff who are likely to have fewer opportunities to undertake skills support in the area of LBC.
It was intended that findings from all four studies would inform the development of effective new approaches (IMPACT programme workstream 5 interventions) to help front-line staff assess and manage psoriasis more holistically and overcome any barriers to supporting patients’ LBCs.
Study 4.i: content analysis of health-care professionals’ core training competencies
Methods
Data collection and analysis
A content analysis of post-qualification professional core competency documents across general practice and dermatology was carried out to assess whether or not LBC support is included as either a general or a dermatology-specific aspect of patient management. Eleven core competency documents for health-care professionals (GP: five documents; dermatologist: one document; specialist dermatology nurse: one document; GPSI in dermatology: four documents) were collated and searched for terms associated with health promotion or LBC as part of the professional role. A coding scheme linked to the Prevention and Lifestyle Behaviour Change competence framework148 was developed to examine the context of these instances and whether or not the domains of knowledge, skills, attitudes and behaviours were included as explicit training competencies or requirements for qualification.
Results
In the 11 curriculum documents analysed, 67 instances of terms related to LBC and health promotion were found. Most were found in the GP curriculum (62.7%), followed by the specialist nurse curriculum (20.9%) and dermatologist curriculum (16.4%). There were no instances in the curriculum of GPSIs in dermatology. The majority of terms were related to awareness-raising alone, with no instances linked to the skills required for long-term behaviour change facilitation (Figure 10).
Of the 67 occurrences found in the curricula, around one-third related to being aware of opportunities to introduce LBC; approximately one-fifth related to being able to identify and signpost to LBC support (however, behaviour change techniques known to enable these competencies were not included); half the instances were unable to be mapped to the Prevention and Lifestyle Behaviour Change competence framework148 and none of the core curricula related to provision of long-term support and LBC facilitation. In summary, the core practitioner training documents showed few clearly specified learning outcomes or recommendations relating to LBC knowledge, skills and attitudes. There were few references to recognised LBC techniques (see Keyworth et al. 147 for supporting data).
Conclusion
Study 4.i highlighted the lack of systematic training for practitioners who may be managing patients with psoriasis to develop appropriate skills and knowledge. Post-qualification health-care professional curricula could be improved by including more explicit LBC skills training.
Study 4.ii: in-depth qualitative interview study of health-care professionals about supporting patients with lifestyle behaviour change
Data collection and analysis
Health-care professionals were first contacted in writing through their professional organisations or via public general practice lists with snowball sampling as a second step when recruitment proved challenging (i.e. health-care professionals did not readily respond to invitations via their organisations and responded better when known peers facilitated invitations personally). In this way, individual participants were able to identify other potential interviewees to approach. This study was approved by the University of Manchester’s REC (reference number: 12017).
A topic guide developed from the literature guided semistructured interviews with health-care professionals managing people with psoriasis. Participants were asked about their attitudes and knowledge in relation to supporting lifestyle changes for people with psoriasis as part of psoriasis management, as well as their experiences of practice and strategies used, with a focus on their:
-
levels of knowledge (including on alcohol consumption, smoking/smoking cessation, obesity, low activity levels, CVD risk, low mood associated with psoriasis)
-
attitudes to lifestyle change support or advice-giving (clinical and managerial priorities, actual and perceived staff roles)
-
understanding of barriers to engaging in behavioural change support for patients (environmental, social/cultural norms, low mood)
-
perceptions of barriers for practitioners (knowledge, ‘embeddedness’ of LBC, training/skills, preservation of therapeutic relationship, patient burden)
-
attitudes to the use of patient information (leaflets, booklets, or known referral routes to services such as smoking cessation)
-
actual behaviours used to address LBC.
In recognition of the potentially sensitive nature of the interview questions, all interviews were undertaken by experienced interviewers trained in asking questions to probe adequately and sensitively to get beyond socially desirable answers. Interviews were audio-recorded, transcribed and analysed using principles of framework analysis,128 with a model of evidence-based factors known to influence behaviour change149 and the Self-Regulatory/Common Sense Model (SRM/CSM) of illness representations150 as frames to consider the data.
Results
In total, 23 in-depth interviews were conducted with clinicians (seven consultant dermatologists, six DSNs, five GPSIs in dermatology and five regular GPs in primary care).
Findings highlighted that, although most clinicians recognised the importance of LBC in psoriasis management, they did not see it as part of their professional role to support patients with behaviour change. Lack of time and prioritising other aspects of care such as diagnosis and medications management were cited as underlying reasons, in addition to a belief that addressing alcohol use, smoking or weight loss was a potential threat to harmonious relationships with patients. Clinicians were pessimistic about patients’ motivation to change as well as their own influence in helping patients make behavioural changes. Table 7 presents illustrative data extracts to support the analysis (see Nelson et al. 113).
| Themes | Data extracts |
|---|---|
| Lifestyle change support not part of role | I don’t see that my clinic should be dealing with someone’s obesity, no, I don’t. Somebody’s clinic should – but not mine!CD1My specialty is to improve skin. I have a generalised knowledge for alcoholism, smoking . . . but my specialty is the skin, so that’s where I would put the most input inDSN2 |
| Prioritising ‘other’ aspects of care over lifestyle support | The medication really is what we’re prioritising – establish the diagnosis, quantify the severity of it and decide on an appropriate treatment planCD3 |
| Preserving relationships with patients over lifestyle support | You don’t want to lose your credibility with [patients]. Some lifestyle issues you can’t over-push because they just wouldn’t come back and see youGP4 |
| Fatalistic thinking | I don’t think we’re that effective at influencing [patient behaviour], I don’t think I am that effective at influencing it. They’re the exception, [patients] who do something about lifestyle. A lot do exactly the opposite of what you want them to do!CD4Some patients really don’t want [lifestyle support] or don’t want to know. I actually had a patient say, ‘I’ve come here for my skin, not for that’!DSN5 |
| Admonishing patients/using scare tactics | If you say, ‘have you thought about stopping smoking?’, one or two will be very angry and another 3% might stop. If you really try your scare tactics, you might get another one or twoGPSI4 |
| Using established behaviour change techniques | I‘ll talk first of all about what’s achievable for that patient and how they can aim to try and make small steps lead to big changesCD6 |
| Lack of formal training in lifestyle management | I had communication training but not changing people’s lifestylesCD5 |
| Need for clinicians to be trained in LBC | I think to do [lifestyle change support] properly, patients need the proper level of support, and to do that you need a proper level of training, and just dabbling in things isn’t going to provide the necessary tools for the patientsGPSI3Many times I didn’t know how to tell [a patient] they should lose weight without making them feel embarrassed. Or alcohol . . . it’s a delicate subject. [We need] to be trained on how to approach a patient better, and to be more convincingCD7 |
| Formalising service structures for lifestyle support | If we formally built it into our nursing role, that would be good. To actually – ‘OK, as part of our assessment we’re going to weigh you . . . going to ask you about . . .’ It could be something we could look at incorporating in a more formal way so that patients can start to link their general health with [lifestyle behaviours]DSN1 |
In addition, practitioners held a range of different ‘personal models’ of psoriasis. Most reported working with incoherent models in mind, for example holding a ‘sophisticated’ understanding of psoriasis as a complex condition while paradoxically managing the condition in a ‘linear’ skin-focused way. Practitioners who reported working to an incoherent personal model also reported frustration in relation to managing psoriasis and satisfaction only when patients’ skin improved. Table 8 presents illustrative data extracts to support the analysis (see Chisolm et al. 151).
| Health profession | Personal model constructs (beliefs, affect, behaviour related to psoriasis) | Illustrative quote | Description of personal model |
|---|---|---|---|
| Dermatology specialist nurse | Identity beliefs: psoriasis is a complex, multifaceted condition | We’re aware of general health and its impact on psoriasis as well as psoriasis’ impact on general health; I think it’s a mutual process |
Sophisticated model (i.e. psoriasis as a complex, long-term condition) Coherent model depicting psoriasis as a complex, long-term condition and reports clinical behaviours that mirror beliefs addressing these complexities |
| Cause beliefs: lifestyle factors can cause exacerbations of psoriasis | I always like to mention it and say ‘did you know, smoking and drinking can worsen your skin?’ | ||
| Consequence beliefs: psoriasis can affect engagement in lifestyle behaviours | Having psoriasis is stressful, so obviously people use drinking and smoking as a way to cope | ||
| Timeline beliefs: long-term condition that is improved but not cured by treatment | They generally start to feel better and come out of themselves a bit more, you know, albeit temporary because it doesn’t cure, it just improves it for a while | ||
| Control beliefs: confident that light treatment and lifestyle change can improve psoriasis | For 90% [of] people with psoriasis, the light therapy will improve their skin . . . getting enough sleep, avoiding stress, eating healthily is all going to benefit both their health and their skin | ||
| Affective experiences: high job satisfaction related to improvements in patients’ well-being | You see them, sort of, blossoming . . . it’s a lovely job for that . . . hugely satisfying because you see a difference in them and just how their demeanour, they’re taller, they’re happier, they feel much much better. And then they feel more able to cope | ||
| Clinical management behaviour: reports actively raising and addressing links between general health and well-being and psoriasis with patients during consultations | You wouldn’t ignore it if there were things that you felt were relevant to their health . . . I think we try to proactively bring things up, you know, for instance, very overweight or a smoker or a heavy drinker . . . our role more is to help them understand that their general health is relevant to the skin | ||
| GP with dermatology special interest | Identity beliefs: psoriasis is a skin problem | Let’s see if we can get the skin problem . . . let’s get this rash sorted out |
Linear (i.e. psoriasis as a simple skin condition) Despite acknowledgement of psoriasis-related complexities, skin-focused thinking is prevalent along with primarily skin-focused management strategies. Lack of understanding of psoriasis as a long-term condition also evident |
| Cause beliefs: psoriasis is exacerbated by environmental factors, smoking and obesity | We live in dry homes with dry carpets and air conditioning in offices and things, and air-conditioned cars and then we take the grease off our skin and we are surprised that dry skin conditions like psoriasis are aggravated | ||
| Consequence beliefs: psoriasis can have social and psychological consequences | I have little doubt that smoking makes psoriasis much worse | ||
| Timeline beliefs: psoriasis is episodic in nature; it resolves with treatment for long periods of time but can return | [Treatments] can keep psoriasis completely at bay for years . . . They start seeing their psoriasis coming back up and start stepping up their treatments | ||
| Control beliefs: psoriasis can be completely cleared and returned to looking ‘normal’ through treatment regimens | You can get psoriasis completely under control with aggressive therapy . . . avoiding soaps and detergents, keeping skin really well oiled, a little bit of natural sunlight therapy | ||
| Affective experiences: frustrated with long-term nature of psoriasis; optimistic about treatments | I’m optimistic, we’ve got some fantastic treatments | ||
| Clinical management behaviour: manages psoriasis as a standalone skin disease, unassociated with any comorbidities; does not address relevant lifestyle factors with patients | I have the privilege of only having to deal with a skin disease not having to think about unrelated problems . . . things like irritable bowel syndrome, heart disease or hypertension |
For the most part, limited knowledge and skills in ‘whole-person’ management, including LBC skills, underpinned these beliefs and attitudes. Nonetheless, some clinicians identified a need for training to enable them to manage psoriasis as a complex, long-term condition involving comorbidities and to support patients with LBCs.
Conclusions
There are low levels of both knowledge and skills among professionals about managing psoriasis as a complex, long-term condition, including addressing LBCs. There is also a lack of structured support in both primary and secondary care for this work. Training to broaden professionals’ conceptualisations of psoriasis and incorporate evidence-based LBC skills in consultations could enable better patient assessment and management.
Study 4.iii: observational study of lifestyle behaviour support information for patients in clinic settings
Data collection and analysis
Health centres were randomly selected from a full, publicly available list. In a non-participant observation study, exploratory observational methods were used to record the prevalence and quality of leaflets and posters signposting LBC (whether general or dermatology specific) in health centre waiting areas for patients with psoriasis in both primary and secondary care. A structured observation schedule was developed to guide data collection (see Chisholm et al. 151). Ethics approval was obtained from the University of Manchester REC (reference number: 12017). Content analysis was used to identify frequency, characteristics and standard of materials. A series of quality indicators guided rating of the materials’ quality in terms of visual condition and visibility/accessibility to patients.
Results
From the 24 health centres observed, 262 sources of lifestyle information were identified. These were mainly categorised into generic posters/displays not specific to psoriasis (n = 113) and generic leaflets/flyers not specific to psoriasis (n = 98). Information was of poor quality, as well as being poorly displayed, and there was no evidence of high-quality psoriasis-specific information being made available to patients.
Conclusions
Study 4.iii found that little emphasis is given to the role of lifestyle as a health risk in patients with psoriasis. Evidence about the use of environmental cues to prompt behaviour change could inform the design and display of lifestyle information.
Study 4.iv: online survey of dermatology specialist nursing staff
Methods
Data collection and analysis
Dermatology specialist nurses were approached through the British Dermatological Nursing Group (BDNG) and invited to take part in an online anonymous survey about training needs in relation to supporting patients with LBCs (see Appendix 5). This included whether or not DSNs perceived that they had the knowledge, skills and confidence to address behaviour change with patients in consultations and views about which practitioners were responsible for engaging in this type of activity. Responses were analysed with descriptive statistics to generate frequencies and percentages.
Results
Analysis of 77 participant responses indicated that DSNs generally expressed confidence in being able to address lifestyle change with patients with psoriasis. However, only 19% reported having knowledge of evidence-based techniques that could be used in consultations. On a scale from 1 to 7, with 7 being the highest level of confidence, the mean scores given by DSNs were between 4.3 and 4.0 for addressing smoking cessation, alcohol reduction, physical activity, diet and weight loss. There were differences in the degree to which respondents believed that different health-care professionals had a role to play in addressing LBC with patients with psoriasis: primary care-based practice nurses (100%); GPs (95%); DSNs (90%); dermatologists (73%) and GPSIs (70%).
Conclusions
Findings from this survey suggest that LBC skills training needs careful planning to tailor interventions in the most appropriate ways and deliver them in the most appropriate settings. Uptake of training may be low if health-care professionals fail to identify the relevance of acquiring such skills.
Key conclusions
-
The role of lifestyle behaviours in the management of psoriasis is under-recognised.
-
LBC skills/competencies are poorly specified in education and training curricula with little or no reference to evidence-based approaches.
-
Health-care professionals managing people with psoriasis are not currently equipped with the knowledge, skills and confidence to manage it as a complex, long-term condition. This includes the provision of LBC support to patients as a part of their professional role.
-
Current practice does not utilise evidence-based approaches to design and present LBC patient information in clinic environments.
Implications
-
Health-care professionals and services could better utilise evidence-based skills training to support patients with LBC and use best-practice design principles to improve and better target materials for patients with psoriasis.
Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management
Workstream 5 study 5.i (Figure 11) addresses objective 5 of the IMPACT programme: to devise an evidence based, acceptable, feasible and accessible primary care-based intervention to improve self-care and coping for people with psoriasis.
FIGURE 11.
Workstream 5: relationship of study 5.i to other IMPACT programme workstreams.
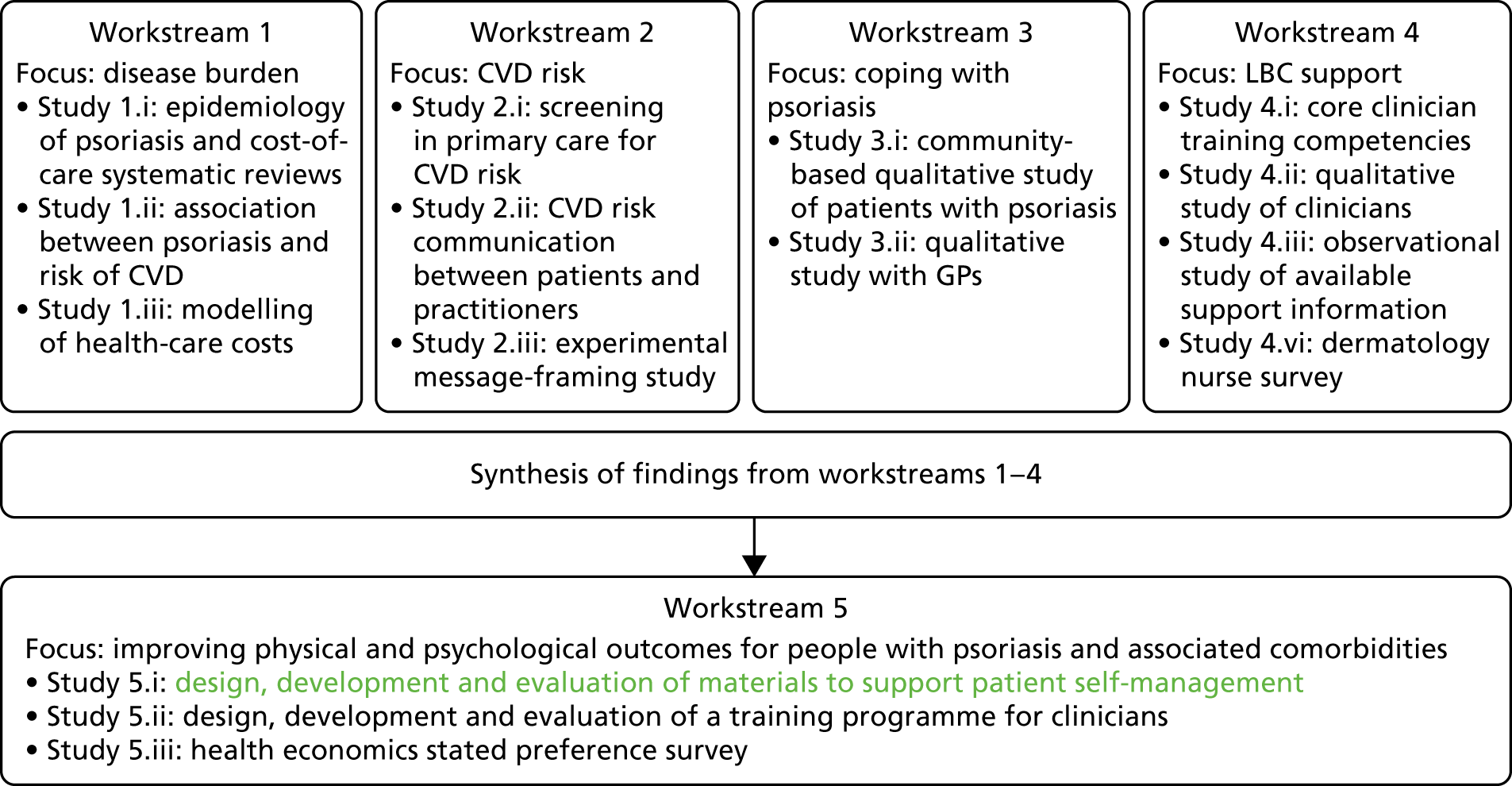
Publications relating to this section and workstream are listed in Publications and cited throughout this section.
Background
Evidence from workstreams 1–4 suggested that patients’ understanding of psoriasis as an immune-mediated inflammatory disease involving comorbidities and the need for self-management was limited. 152 In addition, current patient information about psoriasis does not explain comorbidity risk in ways that are tailored to the individual, or provide a rationale for lifestyle modification. Clinicians are also currently not trained to support ‘whole-person’ psoriasis care. Consequently, patients’ understanding of disease mechanisms and resultant self-care activities are often mismatched, their sense of personal control of psoriasis hampered and coping repsonses adversely affected. 112 Furthermore, informing patients with psoriasis about additional health risks has the potential to increase anxiety further in an already anxious population.
The Common Sense Model of Self-Regulation of Health and Illness (CS-SRM)136 highlights the influence of an individual’s illness and treatment beliefs (i.e. how they perceive their illness or condition and its treatment) on subsequent coping and self-management. 153 The beliefs identified in the model (i.e. symptoms, cause, chronicity, control/cure and consequences)154 can predict outcomes in a range of health-related conditions including psoriasis. 155 Thus, going beyond educational approaches that aim to increase knowledge alone to change patients’ understanding of psoriasis may help to reduce anxiety and increase both ‘illness coherence’ and motivation for improved self-management.
The broad aim of this study was to develop and test the feasibility/acceptability of Psoriasis and Wellbeing (PSO WELL®) patient materials as the patient-focused aspect of workstream 5, using evidence from IMPACT programme workstreams 1–4, theory from the field of illness beliefs as outlined above and guidance from the Medical Research Council (MRC) framework for complex interventions. 156,157 Full details of the aims, methods and results of the study are available in Nelson et al. 158 The study set out to develop and test new materials to:
-
improve patients’ illness coherence (i.e. broaden understanding of psoriasis as a systemic long-term condition without increasing anxiety)
-
improve patients’ understanding of psoriasis treatments and their relevance by helping them appreciate the connection between disease mechanisms and treatment/self-care choices, including changing lifestyle behaviours
-
identify any motivational aspects of the intervention that patients perceived as supporting them to change thoughts, emotions or behaviours (i.e. the mechanisms of action).
Methods
The PSO WELL materials comprised 15 paper-based leaflets that were developed iteratively in consultation with key stakeholders and informed by principles of health literacy and communication (Tables 9 and 10 and Figure 12).
| Principles | Techniques | Aim |
|---|---|---|
| Broadening understanding (beliefs) about psoriasis and treatment options |
Clearly written, accurate information directly addressing key beliefs in CS-SRM Use of analogies/metaphors to communicate complex disease/treatment mechanisms Directly addressing common emotional responses to psoriasis |
Improve illness coherence Improve understanding of relapsing–remitting nature of psoriasis Improve understanding of links between treatment options and underlying disease processes |
| Recommendations for writing effective health communication |
Textual/graphical coherence Attention to readability/usability Chunking information into ‘bite-size’ sections Pilot-testing with target audience |
Promote optimum information processing |
| Content of personal relevance to people with psoriasis |
Inclusion of issues/experiences grounded in in-depth qualitative research with patients/clinicians managing psoriasis Advice/suggestions from current best evidence-based self-management approaches |
Promote understanding of comorbidities Provide rationale for personally relevant LBC/medication adherence |
| Positive messages |
Use of self-affirmation to build confidence and motivation Hopeful tone Modelling functional coping |
Counteract possible increase in anxiety resulting from new understanding of psoriasis as lifelong, incurable inflammatory condition |
| Leaflet set | Leaflet titles | Content |
|---|---|---|
| Introductory (two leaflets) | Psoriasis: New for You; Knowing Your Psoriasis | Signposting for patients with a new diagnosis of psoriasis; signposting for patients with a long-standing diagnosis of psoriasis |
| Understanding psoriasis as a complex long-term condition (three leaflets) | Changing Phases; Linking Mind and Body; Your Journey with Psoriasis | What is psoriasis?; associated comorbidities; CVD risk; mood factors |
| Medications management (six leaflets) | Treatment Overview; Topical Treatments; Emollients; Steroid-Based Topicals; Light Therapy; Systemics and Biologics | Explanation of first-, second- and third-line treatments for psoriasis and relevance for stages of disease; practical tips for treatment use; reflective activities to enhance adherence |
| Lifestyle management (four leaflets) | Why Does My Weight Matter?; Keeping Active Feeling Good; Smokeless; Is Your Glass Half Full? | Weight/nutrition; physical activity; smoking; alcohol |
FIGURE 12.
The PSO WELL patient materials: design.

The study took the form of a within-group, before-and-after feasibility/acceptability study involving primary care patients with psoriasis who had previously given permission to be contacted about psoriasis research as part of study 2.i (University of Manchester REC; reference number: 14269).
The following data were collected pre intervention by post:
-
the Revised Illness Perceptions Questionnaire (IPQ-R)159 modified for psoriasis;158 one domain, ‘illness coherence’, was the primary outcome
-
two additional items written in the style of IPQ-R items asking participants to score whether or not (1) they had a ‘clear picture of understanding of other conditions that are associated with psoriasis (sometimes called comorbidities)’ and (2) they believed that ‘a healthy lifestyle can improve my psoriasis or psoriasis flares’158
-
HADS to assess anxiety and depression160
-
SPI to assess current psoriasis severity, historical course of the condition and perceived psychological/social impact. 101
Following completion and return of baseline assessments, PSO WELL materials were posted to participants. Participants were given 2 weeks to reflect on the leaflets and identify information therein that was (1) new or (2) made them think differently about psoriasis and its management. Participants were also asked to rate the ‘relevance’ of each of the PSO WELL leaflets as follows: ‘not relevant now’, ‘highly relevant now’ or ‘would have been relevant in the past’.
After returning the completed ‘relevance’ chart and leaflets, the IPQ-R items and HADS assessment were repeated post intervention, with the addition of two simple numerical rating scales developed in-house that asked participants to score any changes in understanding and anxiety (positive, negative or none) that they perceived to have taken place following interaction with the materials (see figure 2 in Nelson et al. 158 for the study process flow chart).
A purposively sampled subset of participants was invited to participate in an in-depth, individual interview based on a flexible topic guide to evaluate the usability and acceptability of the materials.
Data analyses
Descriptive statistics were collated to describe the demographic profile of the research participants, the perceived relevance of the PSO WELL materials and numerical rating scale data for self-reported changes in understanding and anxiety. Paired sample t-tests were used to assess change in illness perceptions, anxiety and depression scores before and after exposure to the materials.
Qualitative data collection and analysis were carried out using constant comparison138 and explored for similarities and differences within and across interviews using principles of framework analysis161 to generate key concepts.
Results
Fifty-eight participants out of a total of 127 invited participants completed the relevance chart and 55 completed the pre- to post-intervention questionnaires. Nineteen out of 24 invited participants took part in an in-depth interview. Demographic data for the study samples (both quantitative and qualitative) can be found in Nelson et al. 158
Changes to illness coherence and beliefs about control
Post exposure to the PSO WELL materials, a statistically significant difference between the means was found in the primary outcome of illness coherence [16.2 to 17.51; t(55) = –3.48; p = 0.001 (two-tailed)], indicating a large effect size (η2 = 0.19). This indicates an improvement in participants’ understanding of their psoriasis, on average. Notably, both personal control and treatment control domains also improved post intervention, with a large [t(55) = –2.98; p = 0.004 (two-tailed); η2 = 0.14] and moderate [t(55) = –2.08; p = 0.042 (two-tailed); η2 = 0.08] effect size, respectively (see table 4 in Nelson et al. 158 for significant IPQ-R domain changes).
The materials resulted in changes in understanding of specific aspects of psoriasis: 31 out of 54 (57%) participants reported not having a clear understanding of comorbidities pre intervention, which reduced to 19 (35%) participants post intervention; 31 out of 53 (58%) participants did not know about the relevance of lifestyle behaviours pre intervention, which reduced to 19 (36%) participants post intervention (similarity in reported frequencies is coincidental).
Changes to anxiety and depression scores
There were no clinically or statistically significant increases in HADS scores after exposure to the materials, implying that improvements in understanding of the nature of psoriasis occurred without a corresponding increase in either anxiety or depression. Cronbach’s alpha scores for the IPQ-R and the HADS domains range from 0.91 to 0.72, indicating high levels of internal reliability (see Nelson et al. 158).
Numerical rating scale scores
Eighty per cent of participants reported that their understanding of psoriasis increased post intervention; none reported that their anxiety increased after reading the leaflets and 16% reported reduced anxiety (see Nelson et al. 158).
Leaflet relevance
The majority of participants indicated that the content of the leaflets was highly relevant. Notably, many indicated that it would have had relevance eariler in the course of their disease (see Nelson et al. 158).
Process evaluation
Three key themes explained participants’ views of the PSO WELL materials, including the influences that they perceived the materials had on changes in understanding in relation to psoriasis (Table 11 provides illustrative data extracts). Additional data are provided in the full paper. 158
| Subtheme | Data extract |
|---|---|
| Theme 1: seeing the bigger picture – psoriasis as more than a skin condition | |
|
Newly discovered aspects of psoriasis Learning about comorbidities |
I didn’t realise that psoriasis and arthritis could be combinedP1: female, aged 65 years |
| Learning about lifestyle behaviour links | I didn’t realise smoking and alcohol could affect [psoriasis]P3: female, aged 42 years |
| Learning about treatments | I wasn’t aware there were other types of treatment . . . light therapy and thatP5: male, aged 69 years |
| Learning about disease/treatment mechanisms | Nobody had ever explained why you get . . . plaques . . .P17: female, aged 32 years |
| Enablers of learning | |
| Plain language style | The [materials] were at the right level without patronising peopleP4: female, aged 57 years |
| Ease of navigation | [Leaflets] were uniform, so they’d all look nice and you’d choose your colour . . . and could go straight to itP9: female, aged 59 years |
| Chunking of information | It’s little bits at a time, little bits of information . . . bite-sized, that’s itP7: female, aged 45 years |
|
Positive/hopeful tone Unintended consequences Disappointment in health-care services |
The way it’s written isn’t threatening at all, it just makes it plain that there could be a linkP2: male, aged 62 yearsIt was a bit disappointing really that my doctor had never said there are new thingsP5: male, aged 69 years |
| Self-blaming | Having the leaflets, it made me angry with myself for not looking things upP8: female, aged 31 years |
| Regret that information not made available earlier | If we had this back then it would’ve been brilliant . . . I wouldn’t have felt so alone with [psoriasis]P6: male, aged 44 years |
| Theme 2: personalising psoriasis – developing illness coherence | |
|
Making personal sense of psoriasis Finding a ‘reference point’ |
By having the leaflets – they said – ‘if this doesn’t work, you can try this’P8: female, aged 31 years |
| Linking disease aspects to self | I recognised the sort of things that were me . . . from reading all the different things and relating certain aspects to myself . . .P4: female, aged 57 years |
| New awareness of bi-directional links | I realised being withdrawn had made my psoriasis worse but hadn’t . . . linked that one relates to the otherP8: female, aged 31 years |
| Enablers of engagement with materials | |
| Personally relevant/resonant content (acknowledging emotional impact) | I thought . . . yes, that’s how it is . . . there it is on the page . . . it’s not just a skin condition, it’s the whole range of emotions that come with it as wellP6: male, aged 44 years |
| Perceived trustworthiness of the materials | The source of the information is the thing . . . if you can go to a trusted source . . . the NHS is very much in that categoryP18: male, aged 61 years |
|
Perceived high quality of materials’ content and design Changing emotions and cognitions (feelings and thoughts) |
[Leaflets] were quite professional . . . and . . . attractive . . . you want to pick them upP17: female, aged 32 years |
| Reduced feelings of self-blame/increased confidence | It’s not my fault is one of the biggest things. By knowing all these things affect [psoriasis], it’s not something I’ve done . . . that’s made this happen . . .P8: female, aged 31 years |
| Increased feelings of self-esteem (being valued) | The way it was presented . . . actually made me feel for once somebody was trying to make me feel valued about itP19: female, aged 67 years |
| Bringing previously ‘unconscious’ thoughts to the fore | I kind of made a personal link and I thought – hey – when I get stressed there are times when my psoriasis starts getting worse. Maybe I can do something about itP17: female, aged 32 years |
| Self-questioning about managing psoriasis differently | Those leaflets made me think, well actually, I should be more proactiveP3: female, aged 42 years |
| Theme 3: managing psoriasis differently – making changes? | |
|
Materials as ‘stand-alone’ tool Consulting in a different way Using medications differently |
That leaflet was good in that it made me go back and think about [medication] and I got on a different topical lotionP3: female, aged 42 yearsI make sure I look after [my skin] properly and put the creams on right way . . . I take more care . . .P1: female, aged 65 years |
|
Taking action to address lifestyle behaviour Perceived change mechanisms |
[Re-joining Weight Watchers] was after that [reading leaflets] . . . because I felt better. Somebody understood me, understood my condition, understood how I feelP7: female, aged 45 years |
| Tracking psoriasis with body diagram | I’m shocked looking now . . . when I completed the leaflets I felt I was in a good place, but my psoriasis is much better months along the line than it wasP3: female, aged 42 years |
| Identifying concerns and priorities with activities/charts | Seeing it written down made me realise that there were phases. It . . . made me realise what was bothering meP4: female, aged 57 years |
| Identifying goals and strategies with activities/charts | You need a strategy . . . you need a plan . . . and I started to . . . write down what I need to doP1: female, aged 65 years |
| Increasing intention/motivation to make changes through engagement with activities | Being able to put that tick . . . gave you a boost – like – yeah, you’re doing what you need to do, you’re going to get thereP8: female, aged 31 years |
|
Materials as a shared management tool Materials as a useful tool to share with doctor |
It would open up a conversation, if [the GP] was to say – we’ve got these leaflets – where are you?P5: male, aged 69 years |
| Shared tool as an incentive to change | The doctor could actually use [leaflet] and say – look – what we’re going to do is I need you to keep a diary, to keep noting and . . . I want you to fill this inP17: female, aged 32 years |
| Materials as a support for clinicians’ practice | I imagine [materials] would help GPs massively because I wonder how much GPs actually update their information about [skin] conditionsP3: female, aged 42 years |
| Resistance to using the materials | |
| Psoriasis experienced as mild/not bothersome | I don’t think I was so keen on the [activities] . . . because it’s saying how much is [psoriasis] affecting you at the moment and it wasn’t affecting me . . . my psoriasis isn’t normally that badP9: female, aged 59 years |
| Feeling ‘bombarded’ with LBC messages in general | I’m not sure anything in the [weight] leaflet would help . . . it’s on the telly all the time, there’s stop smoking things all overP9: female, aged 59 years |
| Perceiving messages as rigid/rule based | For me personally it smacks a bit of work . . . you went on various courses and it would be ‘let’s have a target here’ and . . . ‘we need to write an action plan!’ . . . oh crikey here we go againP13: female, aged 64 years |
Theme 1: seeing the bigger picture – psoriasis as more than a skin condition
From exposure to the PSO WELL materials, participants reported gaining insight into aspects of psoriasis that had been unknown or unexplained before, including psoriasis as a complex long-term condition involving associated comorbidities and lifestyle links with a full range of treatments available. Elements that were perceived to encourage learning and reassurance were a plain language style, colour coding and easy navigation, ‘chunking’ of information and the leaflet’s positive/hopeful tone. Some unintended consequences of exposure to the new materials were identified, including participants’ disappointment in health-care services (and in some cases themselves) for not providing/seeking relevant information before.
Theme 2: personalising psoriasis – developing illness coherence
Participants perceived that the materials had helped with ‘making sense’ of psoriasis by linking disease aspects to their personal experience more directly (i.e. developing illness coherence). A particularly new aspect was recognising links between psoriasis, mood and lifestyle. The relevant and meaningful content of the materials was reported as an enabler of sense-making and the perceived trustworthiness/high quality of the materials also enabled participants to engage with the messages therein. Some changes in cognitive and emotional responses to psoriasis were reported from interaction with the leaflets, which appeared to encourage a sense of personal control and prompt thinking about ways to self-manage differently.
Theme 3: managing psoriasis differently – making changes?
Some participants reported having used the materials as a tool to make immediate changes to self-management, finding the activity sections useful. Others perceived the materials as useful for shared management of psoriasis in consultation with health-care professionals. A few participants were resistant to using the materials at all, especially where they had pre-existing negative attitudes to LBC in particular and could experience these messages as threatening.
Discussion
This study was the first to develop, test and evaluate materials to change patient understanding of psoriasis as a complex long-term condition involving comorbidities and lifestyle behavioural factors. Targeting patients’ understanding of disease and treatment as an unaddressed need identified from previous IMPACT work111–113,158 represents a new way of thinking about health communication in the psoriasis field.
The new PSO WELL resources are acceptable to patients and can improve understanding of psoriasis, illness coherence and sense of control without increases in anxiety. In addition, the materials may be able to change emotional and cognitive responses and increase motivation and intention to improve self-management behaviours. Several mechanisms of action were identified: (1) accurate content that directly targeted illness beliefs; (2) design values such as attention to readability (plain language, navigation, chunking of information) that appeared to optimise information-processing162 and high-quality materials conveying that patients were valued (this contrasted markedly with the lack of design values in health communication identified in our previous work); (3) personally meaningful information that acknowledged the potential emotional impact of psoriasis directly and offered a rationale for self-management rather than merely aiming to increase knowledge alone, as many educational interventions do; and (4) a positive tone that appeared to reassure participants and encourage them to engage with the materials. This may have counteracted potentially threatening health messages about psoriasis as a complex long-term condition.
Although this intervention took the form of relatively simple paper leaflets, participants’ accounts suggest that the written word – the right language in context – is important for clarifying or reinforcing messages that may have only been ‘tentatively’ known before. In addition, the study suggests that changing understanding may be a precursor to behaviour change. A trial of an intervention to modify patients’ illness and treatment beliefs in relation to another long-term condition, which was based on the same theoretical model of illness beliefs as this study, resulted in behavioural changes that were maintained for at least 2 years. 163 Our lower-cost intervention may have similar outcomes.
Strengths and limitations
This was a within-group, pre–post test study to test the feasibility and acceptability of new patient resources for psoriasis. The intervention was not tested against a no-intervention control group; therefore, caution is needed when interpreting the results. However, exposure to the intervention was restricted to a 2-week period and this minimises the likelihood of other factors causing a change in participants’ understanding within that time frame. Participants were self-selecting and may have been more likely to be engaged with health messages. However, we measured the effects of the PSO WELL patient intervention and evaluated its process in depth to identify perceived mechanisms of action and these can usefully inform next phase of the research. Some patients perceived that the PSO WELL materials could be a useful for managing their psoriasis in partnership with their clinician. The parallel PSO WELL practitioner intervention (see Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis) showed positive effects on clinicians’ knowledge and skills in managing psoriasis164 and the materials could be used by patients in collaboration with clinicians who have undertaken the training.
Creating an e-health PSO WELL platform to deliver patient materials
Background
The materials developed and described above aimed to address a number of patient needs. A remaining challenge was to ensure patient access to the support materials. Without the direct link that would have been afforded by face-to-face contact between patients and health trainers (see Synopsis), our team felt that there would be limited opportunities for follow-up or consolidation of changes, which may have been initiated by the leading clinician (GP or dermatologist). One way to overcome this would be the availability of an electronic health (e-health) platform that enables the patients to access and use the new online IMPACT programme materials developed in workstream 5. There have been major changes in the field of online health delivery systems since the programme started.
At the time that the original proposal was written (2010) we had anticipated production of digital versatile discs (DVDs) as the electronic resource. However, online systems for delivering interventions have changed enormously and findings from our recent research (and that of others) highlighted the need and expectation for integrated systems that would enable NHS staff to manage the links to their patients. Although there are increasing numbers of health applications (apps) available,165 usage by patients with long-term conditions drops dramatically in a very short space of time unless the process is co-managed by health-care providers. In addition, before costly electronic interventions are developed, it is important to establish if the target audience has the capacity or willingness to use the technology. Health practitioners and patients express concerns about health-related online interventions such as equity of access caused by ‘digital divides’ that may include age, education and technology ownership. Other concerns are changes to the doctor–patient relationship, the ability to process and understand complex information, and privacy and confidentiality.
The most effective systems allow clinical staff to track usage and patients to share data. We identified a commercial partner (company Z) that had already developed an online shared management support service for patients with type 2 diabetes mellitus that is currently in full (commercial) use in NHS England and was considered ‘first in class’ and ‘best in class’ by NHS England, with content certified against current NICE guidance.
The aims of this part of workstream 5 were to:
-
identify whether or not patients would like to access support via online systems
-
define the scope of the e-health platform for psoriasis based on company Z’s approach and on materials developed earlier in workstream 5
-
specify the functional requirements of this e-health platform as a functioning service that can be integrated into current NHS systems
-
build a low-fidelity prototype to demonstrate the potential for integrating this work with an existing chronic-disease software platform that has been proven to provide the highest levels of data security and privacy
-
develop a full costing plan for each of the main features to assess economic feasibility
-
assess acceptability to patients with psoriasis and health-care practitioners.
Methods
Patient use of digital platforms
We surveyed a community sample of 120 psoriasis patients and found that the majority regularly accessed digital platforms such as websites and tablet/smartphone apps, including those individuals aged ≥ 65 years. Primary care patients with psoriasis previously identified as part of workstream 2 using Read codes and history of treatment for psoriasis and who agreed to be contacted by the research team were sent the Patient Readiness to Engage in Health Information Technology (PRE-HIT) questionnaire. 166 The PRE-HIT questionnaire assesses the technical skills, motivation and concerns of prospective patient populations being targeted to use online or mobile tools.
The PSO WELL service builds on the previous, extensive experience of company Z in designing and developing patient self-management systems and utilises the research findings and patient materials developed by the IMPACT programme team. The specification process focused on developing and adapting proven self-management approaches for psoriasis.
A process utilising pathway mapping, stakeholder analysis and problem definition was used to understand current psoriasis self-management and UK clinical and care pathways for the treatment of psoriasis and PsA. This process was used to define a broad scope and technical specification for PSO WELL. Interviews with patients and health-care professionals were used to refine the specification and define a core set of functionalities for development of a low-fidelity prototype for user acceptance testing. User acceptance testing was performed at two stages to refine the proposal and to gain feedback and evaluation of the proposed core functionality of the approach.
The data generated in the project were used to validate the scope and technical specification of the PSO WELL e-health platform concept, informing minimally viable product development, development roadmap and stakeholder engagement plans.
Findings and outputs
Survey results
A total of 118 people aged 25–91 years (mean 56 years; 58% female) completed the questionnaire. Education levels ranged from no formal qualification to doctorate level. Details of responses can be found in Appendix 6; however, the main conclusions from the survey were, first, that there were no gender differences in technology usage and, second, that although older people were less likely to engage with digital platforms than younger people, the majority of those aged > 65 years were regularly using smartphones or electronic tablets.
Pathway mapping and stakeholder identification
To inform the design of the e-health platform for people with psoriasis, detailed process mapping and stakeholder analysis was undertaken to outline the current care pathways and experiences of people with psoriasis in the UK. This process was directly informed by the data gathered in all workstreams (Figure 13).
FIGURE 13.
Pathway map illustrating the (preliminary) data-gathering approach used to identify pathways and key stakeholders.
Problem identification and solution generation
From this analysis, the key requirements of the system were identified, linked on the process map and used to generate solutions. Potential solutions were generated and cross-checked against the identified problems. From the solutions generated, the specifications for an e-health platform for psoriasis should achieve the following:
-
provide personalised health care tailored to individual medical and personal history and preferences
-
change a person’s understanding of their condition
-
change people’s (patient and health-care professional) attitudes
-
give permission to challenge
-
re-engage patients with their treatment and management of their condition (positive messaging to encourage re-engagement)
-
provide tools for effective self-management and reinforce messages and inter-relatedness of issues
-
support behaviour change (goals, reinforcement, support)
-
integration into the health-care system to support collaborative care models
-
improved/increased monitoring
-
provide alternative channels for referral.
Finally, typical ‘user stories’ were generated to illustrate how the identified solutions could be realised in software. A ‘user story’ refers to a patient’s experiences of accessing health care for their psoriasis. Information was drawn from clinical data on average length of time to receive treatment or time from symptom onset to consultation with a GP or a dermatologist. This was supplemented with data from workstream 3 that provided summaries of people’s experiences of coping with psoriasis. The existing PSO WELL branding was used as a starting point for new designs and development of a low-fidelity prototype (paper model) to illustrate the key functions and data flows of PSO WELL. These were demonstrated to patients and health-care professionals in focus groups and their responses collected.
Key functions of PSO WELL
Through the approach outlined above, a list of core functions was defined that addressed the identified needs of patients and clinicians in receiving and delivering high-quality psoriasis health care.
The plan following the end of this programme of research is that these core functions will be integrated into a dynamic system based on company Z’s digital therapeutic self-management approach, allowing a high degree of individual personalisation. This approach, in conjunction with the intended integration of PSO WELL into clinical pathways, could support patients by considering individual lifestyles and preferences to optimise self-management and potentially improve clinical engagement and response.
Data flows in dynamic and interactive systems are difficult to represent in a flow chart; however, Figure 14 illustrates the functional domains and features of the proposed system and indicates which aspects of patient care are affected.
FIGURE 14.
Functional domains, matrix and development roadmap for PSO WELL.

Costs of development of the PSO WELL e-health platform
Company Z provided the researchers with a fully costed project plan for the development of several versions of the e-health platform. Costs depend on the range and selection of functions chosen; however, all costs cover key components such as data security, data management and ongoing servicing and support costs.
Key conclusions
-
New theory-based, high-quality PSO WELL materials are acceptable to patients, feasible to use and can improve understanding of psoriasis and illness coherence.
-
Materials appear to broaden understanding of psoriasis as a systemic condition and increased participants’ sense of control without raising anxiety.
-
Patients with a wide range of ages and years with psoriasis would value using patient materials to learn about self-management and to track their symptoms and flares using data shared with their health-care professionals.
-
Patients would like both paper-based materials and access to an interactive e-health platform that links directly with NHS service delivery.
Implications
-
Carefully formulated patient materials have the potential to change people’s beliefs about a condition and make self-management activities more salient.
-
There are opportunities for improving psoriasis patients’ optimism about the likely benefits of effective self-management.
-
There are materials available for distribution and use by health-care professionals to increase patients’ likelihood of engaging in effective self-care.
Key outputs
-
A set of high-quality patient materials for use in clinical practice (both primary and secondary care settings).
-
A low-fidelity prototype of an e-health platform to deliver patient materials and self-management tools that are acceptable to patients with psoriasis.
-
Full costings for a range of functions that can be delivered via an e-health platform that is fully integrated with current NHS systems to enable both patient and practitioner access to support materials and individualised patient-specific data.
Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis
Workstream 5 study 5.ii (Figure 15) addresses objective 5 of the IMPACT programme: to develop and evaluate training for health-care staff to improve targeted services for people with psoriasis. This objective was modified from the original, which read ‘to devise evidence-based training for primary care staff to improve access to targeted services for people with psoriasis’.
FIGURE 15.
Workstream 5: relationship of study 5.ii to other IMPACT programme workstreams.
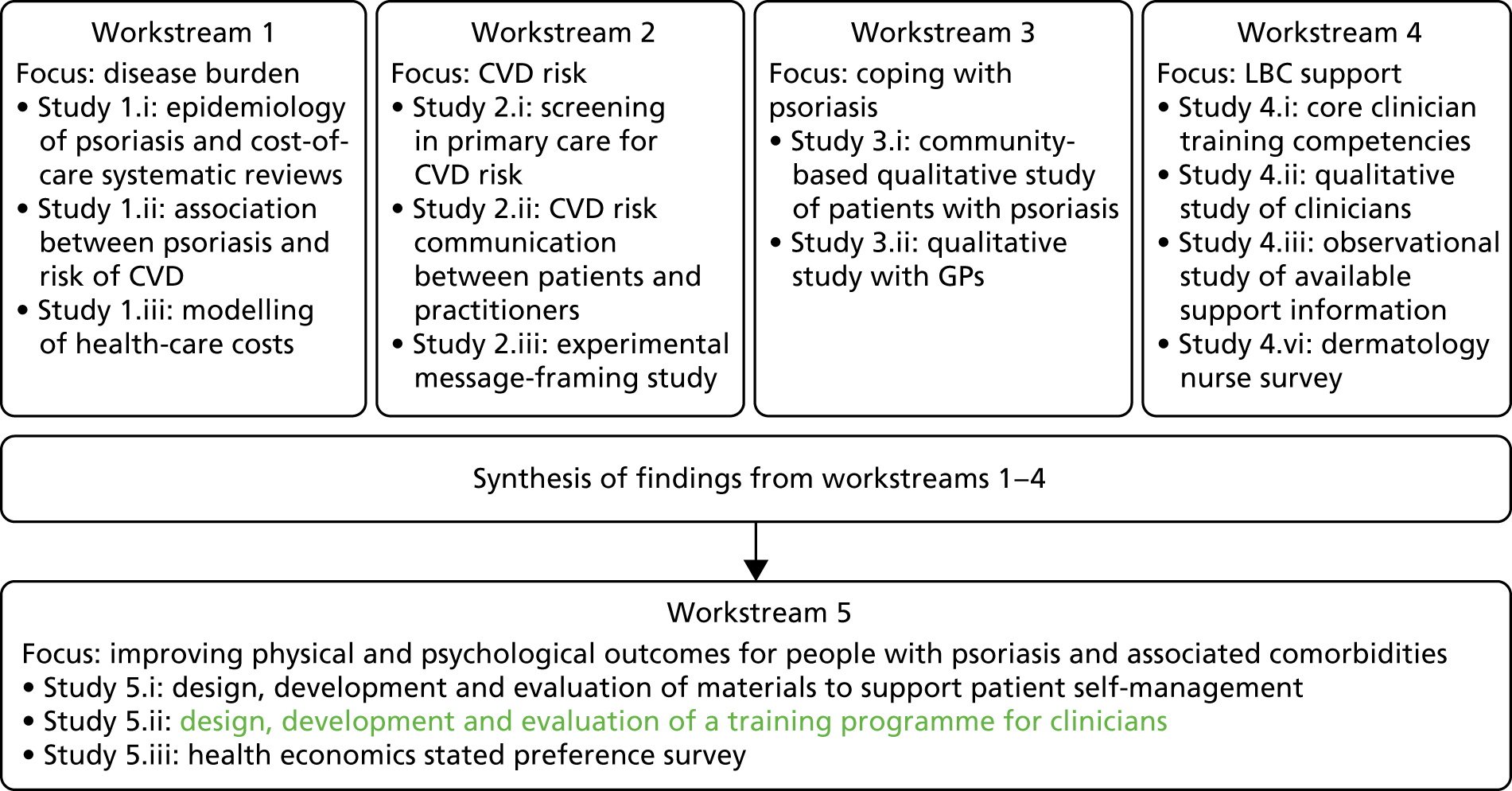
The publication relating to this section and workstream is listed in Publications and cited throughout this section.
Background
A number of key findings, discussed in earlier chapters, led the team to identify the need to develop a patient-centred approach to support healthy living in people with psoriasis. Although psoriasis was found not to confer an independent risk of CVD, risk factors for CVD were particularly common in patients with psoriasis. Screening for comorbidities including inflammatory arthritis rarely occurred (workstreams 1 and 2). Low mood and low engagement in formal psoriasis management by psoriasis patients indicated that health-care practitioners may need a new approach to support whole-person care in this group (workstreams 2, 3 and 4). Findings also indicated that most health-care professionals had little exposure to the principles of LBC and none had received specific skills training in this area as part of standard professional development pathways (workstreams 3 and 4).
Patients may experience suboptimal care because practitioners do not recognise psoriasis as a complex immune-mediated long-term condition. Practitioners miss opportunities to reduce risks of both psychological and physical comorbidities, having received limited training to support them with LBC.
Lifestyle behaviour change intervention approaches are more effective if they have a strong theoretical basis and utilise established techniques consistent with underlying theory. 167 Motivational interviewing is a consultation approach that aims to enhance patients’ motivation to change health-threatening behaviours by eliciting their personal resources, goals and action plans. 168 It offers a framework that health practitioners can use to address patients’ beliefs about an illness and its treatment, with the aim of increasing self-efficacy, a construct that predicts intention to change behaviour (and actual behaviour change). Motivational interviewing has a good evidence base in both the addictions and physical health/long-term conditions fields169,170 but has yet to be applied and tested in psoriasis. Developing a consultation approach that is consistent with motivational interviewing may help to address clinicians’ lack of confidence and skills to support LBC in people with psoriasis, as highlighted in our previous research. 113
Informed by the findings of workstreams 1–4, we developed the PSO WELL training programme for practitioners. This intervention was structured around a motivational interviewing approach to the management of long-term conditions, focusing on the importance of LBCs for optimal medical and self-management in psoriasis and how clinicians can integrate this fully in dermatology consultations.
Aim
We aimed to develop, deliver and evaluate an effective, feasible and acceptable training programme for experienced clinicians who manage individuals with psoriasis, which would extend their skill set to encompass a patient-centred, tailored and integrated LBC consultation incorporating motivational interviewing techniques. As part of a 1-day training programme, we addressed two components (increasing knowledge about psoriasis and developing LBC support skills) by:
-
disseminating the latest CVD risk research findings to clinicians and offering them strategies for communicating these to patients in a systematic way (including exploring a conceptual model of how behavioural factors can have an impact on psoriasis and how these can be addressed in consultations in a way that is consistent with the NICE recommendations for psoriasis)135
-
presenting the case for patient-centred consultations that convey hope in order to encourage optimal self-management and reduce distress
-
building the necessary skills to support confidence among participants to conduct an integrated psoriasis management consultation that addresses mood and supports patient self-management skills.
Methods
Intervention content and delivery
The 1-day intensive skills-based PSO WELL training programme taught an integrated patient-centred assessment consultation, conducted in the spirit of motivational interviewing (i.e. with an ethos of collaboration, evoking patients’ existing knowledge and supporting their autonomy), aimed at developing key skills in motivational interviewing processes (i.e. engaging, focusing, evoking and planning) and techniques to support patient choice and LBC. The training took place away from participants’ places of work in convenient and central locations and was delivered by IMPACT programme team members trained in motivational interviewing. A mix of presentations and skill-building activities was delivered along with opportunities for individual coaching during the session. The following areas were covered in the training package: (1) CVD risk communication, (2) assessing psoriasis, (3) motivational interviewing skills development and (4) long-term condition management strategies [see the IMPACT programme website171 for a video overview of the training: www.impactpsoriasis.org.uk/practitioners/ (accessed 5 August 2019)]. A workbook provided more detailed background information to the training components and reinforced key messages (Figure 16).
FIGURE 16.
Workbook and training materials used for PSO WELL training.

Evaluation design
A pilot before-and-after study was conducted to explore the efficacy of the PSO WELL small-group motivational interviewing-based training. We hypothesised that practitioners attending the training intervention would, following the training, display improvements in knowledge about psoriasis (including related comorbidities and associated risk factors) as well as demonstrating motivational interviewing skills to optimise behaviour change with patients. Training measures (described below) were taken immediately before and after the training session on the same day.
Participant recruitment
We invited a range of practitioners who manage patients with psoriasis to attend the training, including generalists and specialists from primary and secondary health-care settings. Recruitment sources included the British Association of Dermatologists (BAD); the Scottish and Welsh Dermatological Societies; the Primary Care Dermatology Society; primary care Clinical Commissioning Groups (CCGs) (e.g. through the North Manchester CCG bulletin); nurse specialist groups and individuals (e.g. the BDNG, the Dermatology Nurses Arena meeting and the IMPACT programme expert dermatology nurse group lead); AbbVie Inc. (North Chicago, IL, USA); NHS dermatology departments across Wales, England and Scotland; and IMPACT programme research events. All participants provided written informed consent prior to beginning the study. The recruitment advert is provided in Appendix 7. Ethics approval was obtained from the University of Manchester REC (reference number: 14223).
Before-and-after measures
Participants completed pre- and post-training measures immediately prior to and following the PSO WELL training programme as follows.
Behaviour change skills
Participants provided two brief (10-minute) audio-recorded extracts of a consultation with a standardised patient actor (patient actors are individuals trained to act as a real patient to simulate a set of symptoms or problems) before and after the training programme, which were objectively scored by an independent coding team using the Behaviour Change Counselling Index (BECCI),172 a valid and reliable 11-item behaviour change skills checklist that assesses core motivational interviewing competencies.
Audio-recordings were reviewed by a panel of seven trained BECCI raters and tested for inter-rater reliability. Intraclass correlation coefficients (ICCs) were calculated using a team of the seven raters comparing two audio-recordings each against a ‘gold standard’ scorer (motivational interviewing trainer); thus, inter-rater reliability was obtained for 15% of the total data set (total n = 110 audio-recordings). ICCs indicated ‘almost perfect’ agreement173 on mean BECCI score (ICC 0.93, 95% CI 0.75 to 0.98; p < 0.001) and total BECCI score (ICC 0.93, 95% CI 0.77 to 0.98; p < 0.001). When considering inter-rater reliability more closely on each BECCI domain score, raters obtained ‘substantial’ to ‘almost perfect’ agreement as follows: domain 1 (ICC 0.81, 95% CI 0.33 to 0.95; p < 0.05), domain 2 (ICC 0.94, 95% CI 0.78 to 0.82; p < 0.001), domain 3 (ICC 0.78, 95% CI 0.11 to 0.94; p < 0.005) and domain 4 (ICC 0.90, 95% CI 0.68 to 0.97; p < 0.001). For domain titles and descriptions, see Appendix 8, Methods. The primary outcome was change in clinician consultation activity consistent with the motivational interviewing skills in training (BECCI). A change of 0.8 in the before-and-after score was considered adequate to assess responsiveness on this measure,23 although a power calculation indicated that the number of participants needed to achieve 80% power to detect a significant difference of 0.4 in scores on the BECCI scale with a p-value of < 0.05 was 41 practitioners in total. We adopted this as our minimum number to include in the study.
In addition, BECCI raters estimated the percentage of time that clinicians talked during consultations compared with patients. This additional measure, included as an optional independent item in the BECCI manual,172 is recommended as a useful extra indication of a consultation approach consistent with motivational interviewing. Practitioners are expected to talk less than patients (i.e. < 50% of the total consultation time) if adopting a motivational interviewing approach during interactions with patients.
In addition to assessment of practitioner skills using the BECCI measure, data were also collected from standardised patient actors to capture their impressions of consultations before and after training. This comprised a five-point Likert scale with five items assessing patient actor perceptions about the extent to which they felt that (1) they were listened to, (2) the clinician understood how they were feeling, (3) they had confidence in being able to change their behaviour, (4) the clinician provided constructive information and (5) in the consultation they talked more than the clinician about ways to improve their health. Open-ended written feedback was also provided by patient actors to obtain unanticipated impressions about practitioners’ communication skills and these data were interrogated for common themes using content analysis.
Knowledge of psoriasis
A set of eight questions (22 items in total) assessing current knowledge about psoriasis and associated comorbidities was devised for this study by senior members of the IMPACT programme team and examined by the wider dermatology team for accuracy as well as ease/difficulty of use.
This knowledge questionnaire was used to determine if the educational component of the training was effective in changing practitioners’ understanding of psoriasis as a complex, long-term condition. Topics covered in the measure included awareness of psoriasis-related comorbidities as well as the prevalence of and associations between psoriasis and lifestyle/mood factors including smoking, alcohol use, obesity, physical inactivity, anxiety, depression and stress. Participants could score a maximum of 35 points on the measure comprising three sections of questions on comorbidities (maximum of 9 points), prevalence of psoriasis-associated lifestyle/mood factors (maximum of 7 points) and associations between psoriasis and lifestyle/mood (maximum of 19 points).
Feasibility and acceptability interviews
In addition, qualitative acceptability and feasibility interviews were conducted (either face to face or by telephone) with a subset of trainees at least 2 weeks post training to elicit views and experiences of the training and impressions of how the intervention might work in the clinical environment. Semistructured interviews were guided by questions in two key areas: (1) acceptability of the training content and delivery and (2) feasibility of practitioners’ ability to both attend and engage with the training.
Data analysis
Quantitative outcome measures (motivational interviewing skills and knowledge about psoriasis) were analysed using within-group comparisons to assess potential change over time. Depending on the nature of the data, both parametric and non-parametric tests were used. Low levels of missing data (fewer than three items on any measure) were managed using imputation of the mean value for that scale or subscale. Larger levels of missing data were managed by multiple imputation.
Qualitative interviews were transcribed verbatim and imported into NVivo 10. Data were categorised using content analysis and principles of thematic analysis. 174 Familiarisation with the data was followed by initial open coding in which any data relevant to the research question were highlighted and noted. Patterns in the data were then grouped into categories and further organised into superordinate themes. Disconfirming (i.e. atypical) cases were identified and highlighted to examine the extent of differing views identified in the sample.
Results
It was not possible to identify exact numbers of clinicians invited because recruitment was conducted largely via third parties that forwarded invitations to whole organisation/membership lists. However, in total, 61 clinicians (mainly from secondary care) attended the PSO WELL training. (Details of sample characteristics are provided in Chisholm et al. 164) Pre- and/or post-training audio-recorded consultations were unavailable for six of the 61 participants (three participants were not sufficiently confident to complete the consultations with patient actors, one was absent post training and two technical faults occurred; these six audio-recordings were omitted from the analysis and used for training of the BECCI rater team). In total, 110 audio-recordings were included in the BECCI analysis.
Behaviour change skills
A paired-samples t-test was conducted to evaluate the impact of the PSO WELL training on clinicians’ (n = 55) motivational interviewing skills (BECCI measure). There was a statistically significant increase in BECCI scores from time 1 (mean 0.50, SD 0.47) to time 2 (mean 1.26, SD 0.71) [t(54) = 8.37; p < 0.0005 (two-tailed)]. The mean increase in BECCI scores was 0.76 with a 95% CI ranging from 0.57 to 0.94. The eta-squared statistic (η2 = 0.56) indicated a large effect size. 27
Figures 17 and 18 illustrate the overall change in mean BECCI score following training, as well as the change in practitioner motivational interviewing skills across each of the four BECCI domains (domain 1, agenda setting; domain 2, the why and how of behaviour change; domain 3, the whole consultation; and domain 4, talking about targets).
FIGURE 17.
Mean BECCI score before and after attending PSO WELL training (n = 55). Max, maximum. Reproduced from Chisholm et al. 164 © 2016 British Association of Dermatologists. Reproduced with permission from John Wiley & Sons.
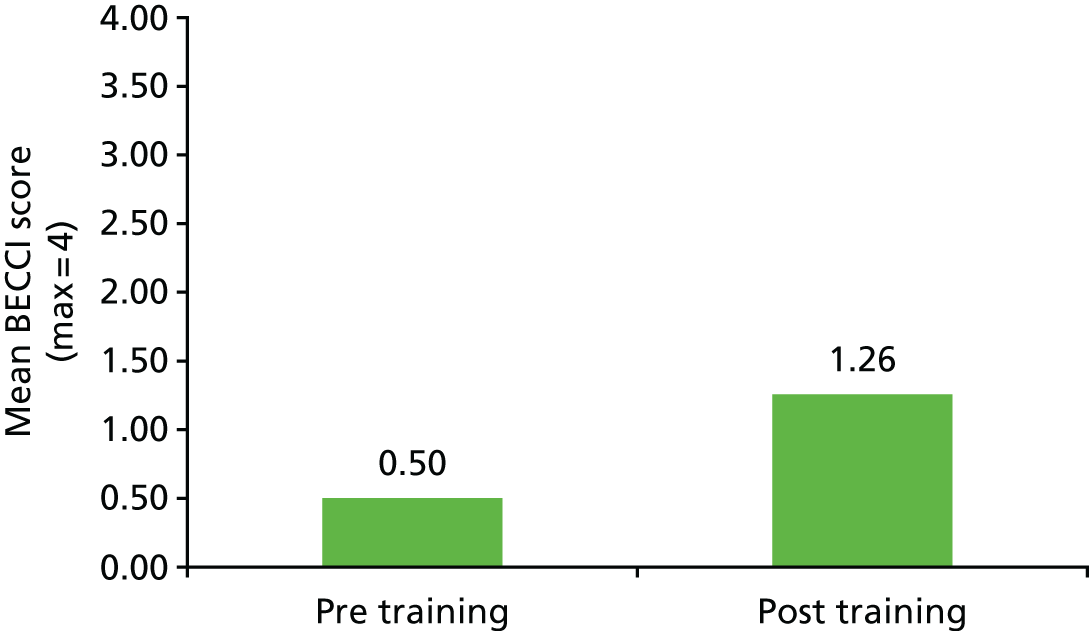
FIGURE 18.
Mean BECCI domain scores before and after attending PSO WELL training (n = 55). Reproduced from Chisholm et al. 164 © 2016 British Association of Dermatologists. Reproduced with permission from John Wiley & Sons.
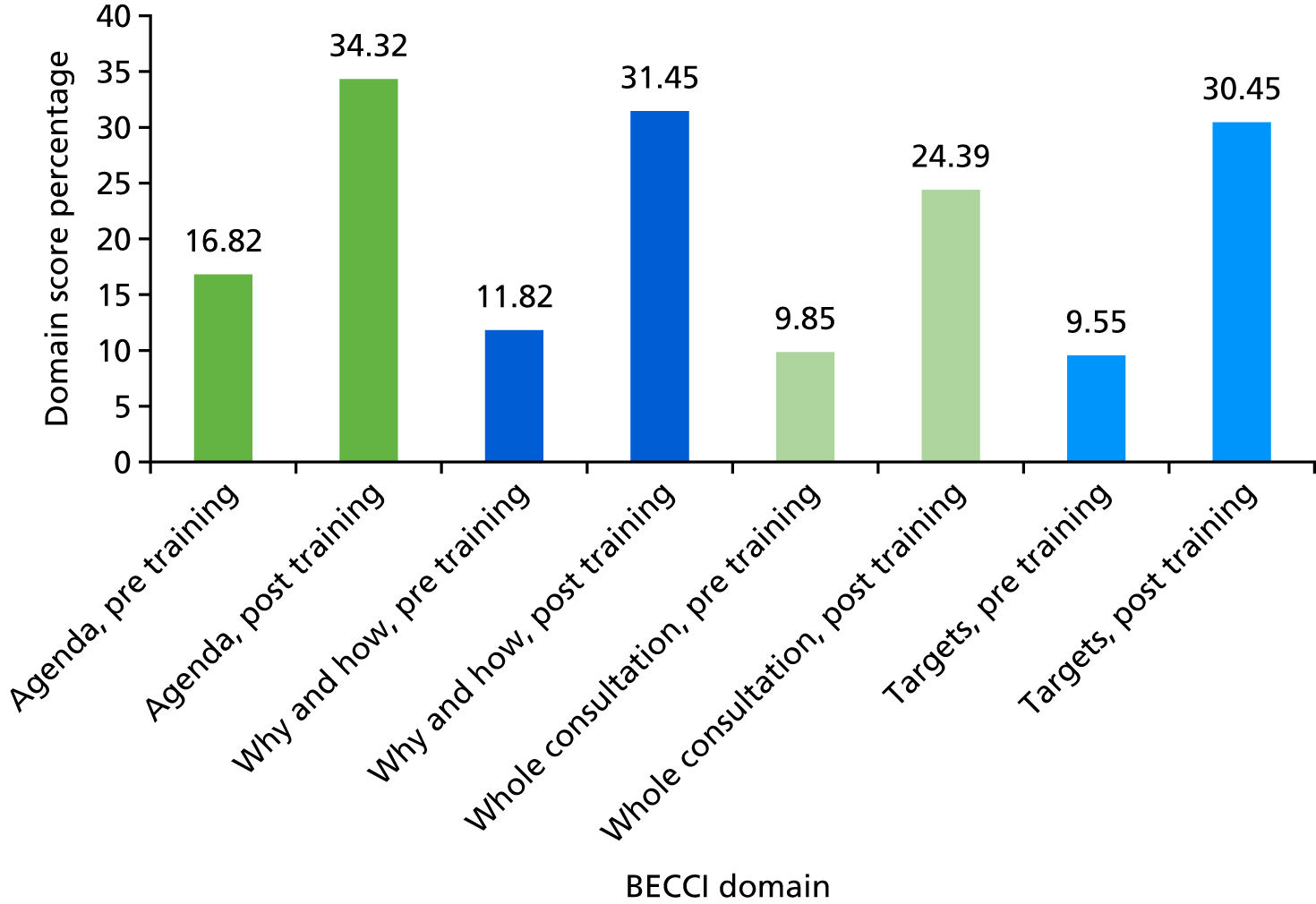
As a treatment fidelity check, the extent to which participants are adopting a consistent motivational interviewing approach is measured by the ratio of clinician-to-patient talk time. Practitioners are likely to be using this approach if they speak less than the patient in the consultation overall (i.e. < 50% total consultation time). Trained, blinded BECCI raters estimated the percentage of time clinicians spoke during consultations in comparison to patients. Figure 19 illustrates the mean estimated clinician talk time, indicating an 11.18% reduction following the training.
FIGURE 19.
Mean estimated clinician talk time (% of total consultation) before and after attending PSO WELL training (n = 55). Reproduced from Chisholm et al. 164 © 2016 British Association of Dermatologists. Reproduced with permission from John Wiley & Sons.
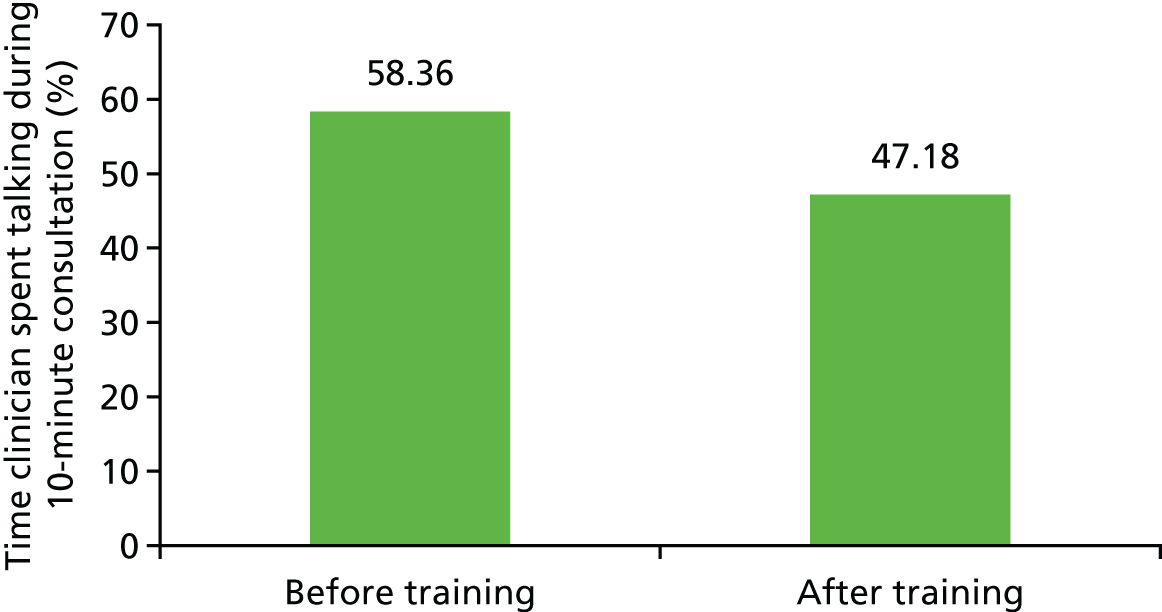
Patient actor feedback
Descriptive analyses of patient actor feedback before and after training are illustrated in Figure 20. It should be emphasised that these are impressions based on the experience of these individuals in training undergraduate medical students and postgraduate medical practitioners. These data suggest that patient actors felt that practitioners’ consultation approaches improved following training. Open-ended written feedback also indicates that patient actors were more satisfied following training. Content analysis of the patient actor feedback highlighted that actors perceived shifts in the way in which practitioners (1) approached consultations in general, (2) guided the focus of the consultation topics and (3) approached management planning. Actors reported that, following the training, they were more satisfied with consultations because practitioners adopted a more collaborative style, attended more to patients’ agendas, acknowledged their thoughts and feelings more and actively linked psoriasis to associated lifestyle behaviours. They also perceived that practitioners developed concrete action plans during consultations (which had been absent prior to training), judged to be led more by patients’ rather than practitioners’ ideas.
FIGURE 20.
Mean Likert scale scores of patient actors by item and total score before and after training (n = 46). Higher scores indicate improved outcomes. Max, maximum; Q, question. Reproduced from Chisholm et al. 164 © 2016 British Association of Dermatologists. Reproduced with permission from John Wiley & Sons.
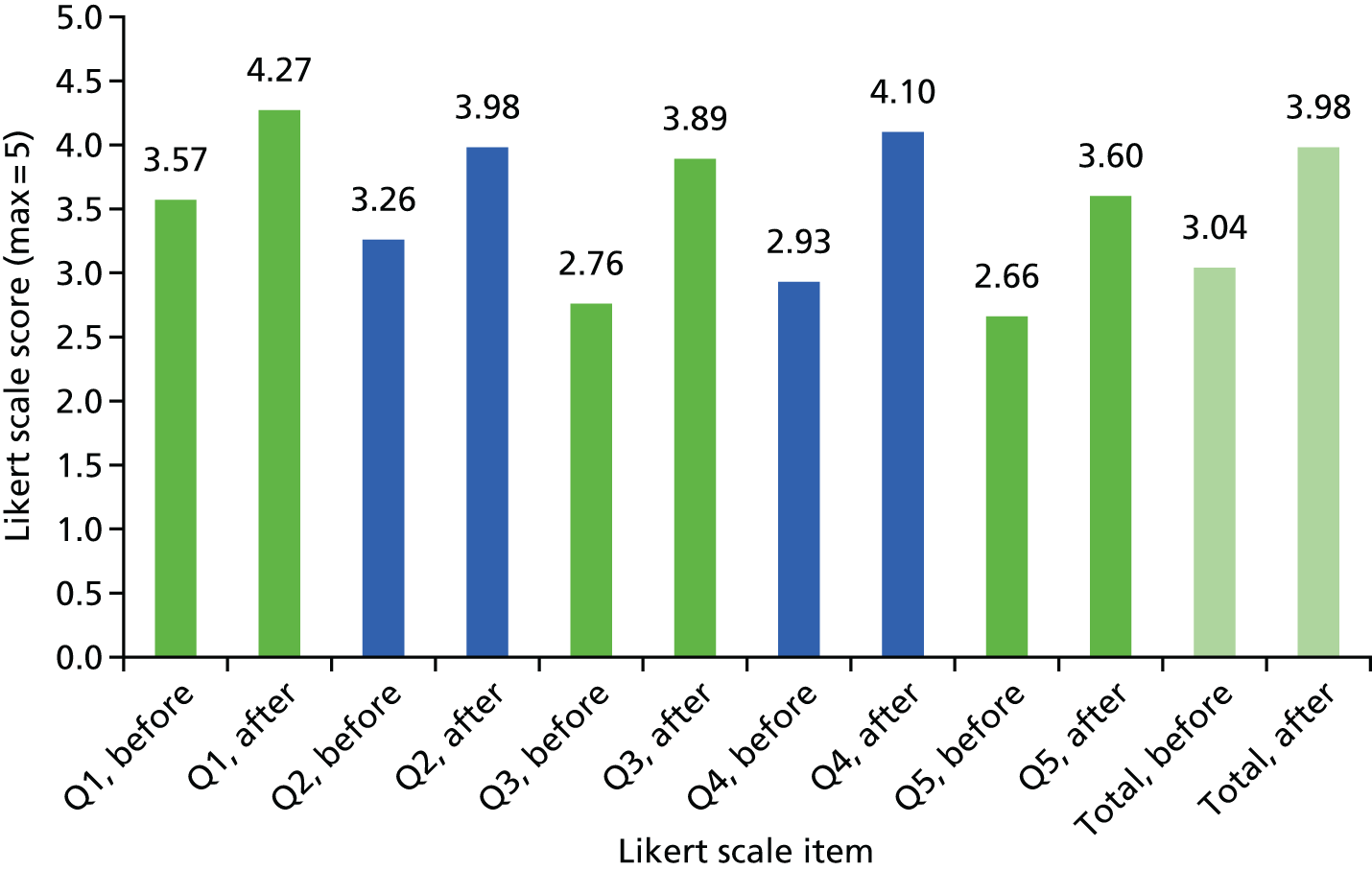
Knowledge of psoriasis
Participants’ knowledge about behavioural and mood factors related to psoriasis (e.g. obesity, smoking, alcohol use, physical inactivity, stress, low mood and anxiety) significantly increased following participation in the training. Specifically, paired-sample t-tests highlighted increases in participants’ knowledge of risk factor prevalence [t(60) = 4.30; p < 0.001] as well as the probable mechanisms linking psoriasis and risk factors [t(60) = 7.12; p < 0.001]. However, participant knowledge of psoriasis-related comorbidities (e.g. CVD, PsA and inflammatory bowel disease) did not increase significantly post training (p = 0.096).
Acceptability and feasibility interviews
A total of 18 practitioners was purposively sampled for qualitative interviews (four male, 14 female; eight specialist dermatology nurses, seven consultant dermatologists, two registrar dermatologists and one GP). Analysis of interview data highlighted five core themes illustrating practitioners’ views and experiences of attending the training and completing the associated research measures: (1) delivery of the training, (2) utility of training content, (3) skills acquisition and scope for implementation, (4) interest and ability to attend and (5) trade-offs when undergoing assessment.
In summary, practitioners valued the opportunity to share and learn from others’ experiences, practise skills and obtain coaching/feedback. Clinicians judged the training to have been engaging and the content to be transferable across clinical settings, reporting that it encouraged consideration of their own consultation style, the complexities of psoriasis and, in particular, how to adopt a whole-patient approach to care. The training provided new understanding about effective and ineffective strategies to help patients change health-related behaviours and increased clinicians’ confidence to attempt new approaches in practice. Practitioners found the 1-day course to be intensive and felt that they learned more than expected through training that was deemed relevant and appealing to a range of other practitioners working with people with psoriasis. Patient actors used as part of the skills assessment were felt to be realistic, although some clinicians found the assessments daunting. See Chisholm et al. 164 for more details.
Key conclusions
-
Motivational interviewing-based, 1-day PSO WELL training increased clinicians’ knowledge and skills in relation to behaviour change in the context of managing psoriasis and promoted a more patient-centred consultation style in follow-up evaluation consultations.
-
Following training, clinicians demonstrated increased understanding of the links between psoriasis and lifestyle/mood factors and were able to integrate discussion of behaviour change into consultations.
-
Patient actors perceived shifts in the consultation style used by clinicians following training that were consistent with core components of motivational interviewing (e.g. collaborative approach to care, empathy and ability to elicit patient-led solutions and management plans).
-
Participants judged the training programme to be feasible to conduct and evaluate in existing health-care settings, and clinicians were satisfied with the training content, delivery and assessment methods.
-
It is possible to shift thinking about psoriasis from a simple skin condition to a complex inflammatory condition that confers increased CVD risk in some patients.
-
The challenge of recruiting participants from primary care (particularly GPs) to attend a 1-day training programme remains a barrier to improving health outcomes for people with psoriasis.
Implications
-
Members of staff whose role it is to promote behaviour change in patients with psoriasis to whom such change is relevant could benefit from this brief, effective intervention.
-
This programme could be viewed as an important part of training for medical and nursing staff managing psoriasis patients with elevated CVD risk.
-
A patient outcome trial of these techniques is required to establish efficacy in health gain.
Key outputs
-
Evaluated training materials and full-day training for clinicians managing individuals with psoriasis in primary and secondary care.
Valuing the interventions with a stated preference survey
Workstream 5 (Figure 21) addresses objective 5 of the IMPACT programme: to improve physical and psychological outcomes for people with psoriasis and associated comorbidities.
FIGURE 21.
Workstream 5: relationship of study 5.iii to other IMPACT programme workstreams.
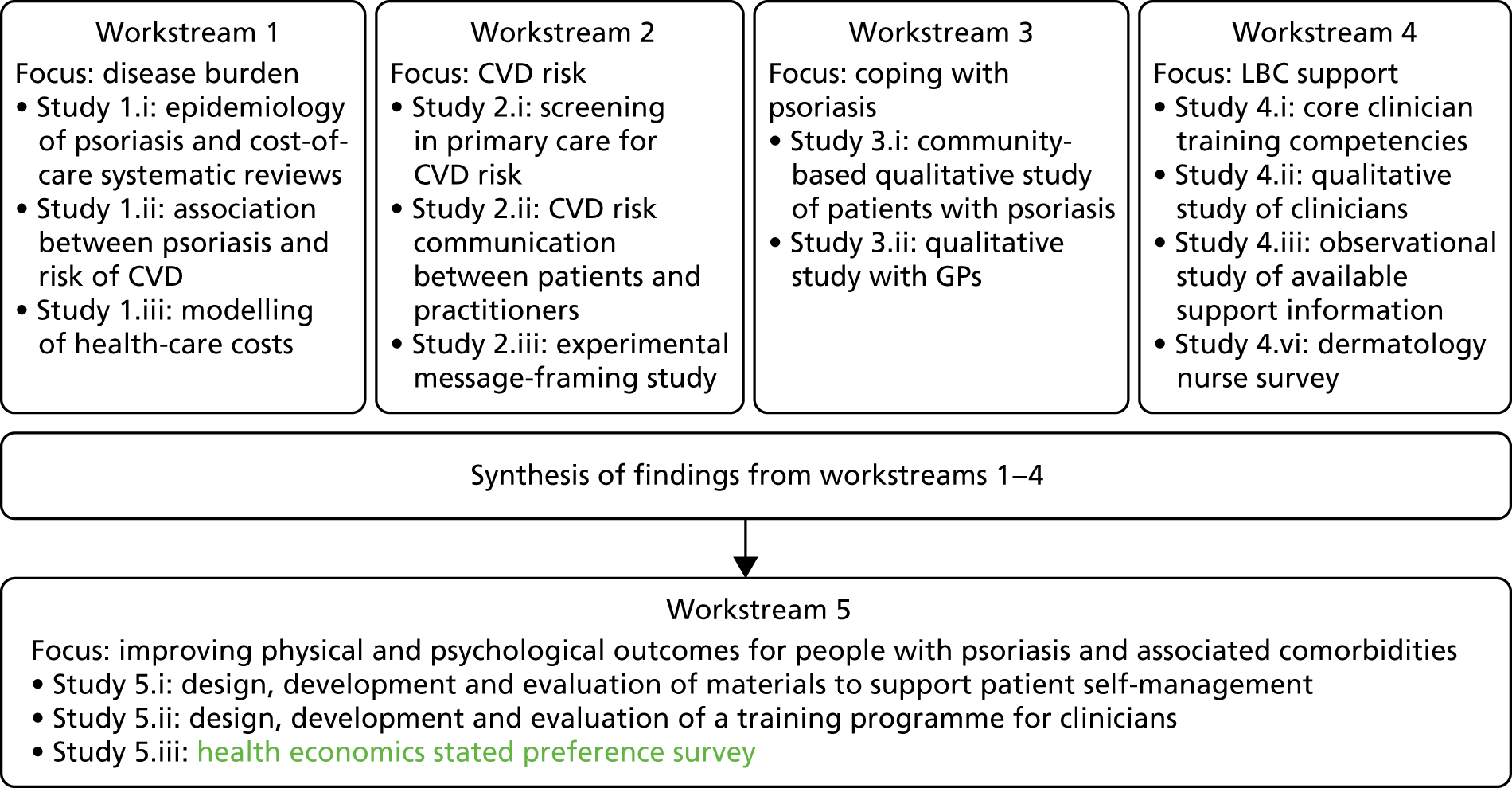
Background
The IMPACT research programme has shown that psoriasis is a complex condition with long-term consequences. People with psoriasis often have risk factors for other conditions, such as CVD. The lifestyle of a person can affect their psoriasis symptoms and their risk of CVD. The PSO WELL intervention includes patient information materials [described in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management] and training for clinicians [described in Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis]. Together the interventions aim to help a person with psoriasis understand (1) what psoriasis is and how it is treated and (2) how their lifestyle choices (for example weight, physical activity levels and smoking) may affect both their psoriasis symptoms and their risk of other illnesses such as CVD.
The intervention also aims to provide a more personalised, tailored approach to psoriasis care and to support a person with psoriasis to identify and make any changes to their lifestyle that they think are needed (e.g. stopping smoking, managing their weight or using prescribed medications).
The analysis of the acceptability and feasibility of the two components of the PSO WELL intervention indicates that each of the components is acceptable and feasible [reported in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis]. However, it was beyond the scope of the evaluation to assess the relative importance of different features of the overall PSO WELL intervention.
A stated preference study was designed to find out about the preferences of people with psoriasis for key aspects of the patient materials and clinician training interventions (referred to in this section as the PSO WELL intervention). Surveys of this kind present a series of choices and ask participants to select which options from that particular combination of options they prefer. The stated preference survey adds to the information generated in the feasibility and acceptability evaluations of the patient materials and clinician training. The survey was designed once the assessments of the PSO WELL interventions were completed. This section summarises the methods and results of the survey. Appendix 8 describes these in more detail and Appendix 9 presents the participant information and survey materials.
Aims and objectives
The overall aim was to provide additional information about the acceptability of and preferences for the different objectives and components of the PSO WELL intervention [described in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis]. Key objectives were to quantify the preferences of people with psoriasis for the different components of the intervention and to assess the relative importance of each component.
Methods
Survey design
In line with the aim and objectives, the stated preference survey was designed to compare the intervention components only, so an opt-out or status quo option was not included in the design. A discrete choice experiment design was used to estimate the main effects for each component or attribute of the intervention. This assumes that there are no interactions between the attributes included. The survey asked participants to consider a series of choice questions (also referred to as choice sets). Each choice question gave participants two descriptions, or scenarios, of different aspects of the PSO WELL intervention and asked them to choose which they preferred. The decision to limit each choice question to two scenarios was based on discussion with the multidisciplinary team leading the design of the PSO WELL intervention and evaluation. This was to help minimise the perceived burden on participants.
Through discussion with the IMPACT programme research team, four different aspects or attributes to describe the PSO WELL intervention were identified. These comprised two attributes that described components of the intervention and two attributes that described desired outcomes of the intervention. The choice of attributes and levels, as well as how they were described, was informed by the qualitative research reported in Cardiovascular disease risk communication and reduction in psoriasis, Coping with psoriasis: learning from patients and Understanding the professional role in supporting lifestyle change in patients with psoriasis to develop the PSO WELL intervention, as well as the results of the development and feasibility studies [described in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis]. Draft versions of the survey were reviewed by members of the IMPACT programme team and RUG and simplified in response to comments.
The attributes and levels included in the survey were as follows:
-
Information, defined for participants as ‘general information and exercise leaflets to take away and read and work through in your own time. The information describes psoriasis and its treatment, lifestyle factors that may affect psoriasis and health, how to manage psoriasis and how to make lifestyle changes’. These can be given as printed leaflets or online. The levels for this attribute were (1) no information and exercises to take away, (2) printed information and exercises to take away and (3) printed and online information and exercises to take away.
-
Clinic visit, defined for participants as ‘whether your clinician gives you personalised information (about your psoriasis and its treatment, lifestyle factors that may affect your psoriasis and your health, how to manage your psoriasis) and helps you to identify and make lifestyle changes’. The levels for this attribute were (1) no personalised information or help to make lifestyle changes, (2) clinician gives me personalised information about my psoriasis and health and (3) clinician gives me personalised information about my psoriasis and health and support to make lifestyle changes.
-
Understanding, defined for participants as ‘your understanding about what psoriasis is, what the treatments are and how lifestyle choices may affect both your psoriasis symptoms and risk of other illnesses such as CVD’. The levels for this attribute were (1) worse than before, (2) same as before and (3) better than before.
-
Ability, defined for participants as ‘your ability to manage your psoriasis and/or make changes to your lifestyle to improve your psoriasis and health’. The levels for this attribute were (1) worse than before, (2) same as before and (3) better than before.
A fifth attribute was added about the length of time it would take to read and use the information, manage psoriasis and make any lifestyle changes. The levels were 30 minutes, 1 hour, 2 hours, 3 hours, 4 hours and 8 hours. Time is used as a measuring rod to estimate how much participants are willing to spend or trade to get their preferred level of each attribute (see Appendix 8, Methods, Survey design: time attribute).
The number of attributes and levels gives 486 possible combinations of components of the PSO WELL intervention. It was not feasible to include all these in a single survey in this study. A fractional factorial design175,176 was used to select a sample of 18 choice sets that was efficient and orthogonal (see Appendix 8, Methods, Fractional factorial design). The 18 choice questions were randomly divided into two questionnaires of nine choice sets each. Figure 22 gives an example choice question.
FIGURE 22.
Example choice question.
The survey also included demographic questions (age, gender, age at diagnosis, types of clinician seen, treatments received) and a measure of overall health [the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)].
Sample size
There is no agreed formula for working out sample sizes. One approach is to use the number of choice sets in the fractional factorial design, assuming that the true probability of choosing option 1 or option 2 is 50% and that an acceptable error for this probability is ± 2.5%. This gives a minimum sample of 85 complete responses for each choice set. We anticipated that approximately 30–40% of participants would complete all the choice sets in a questionnaire, meaning that 213 to 283 participants would be needed to get 85 complete responses per choice question. Stated preference survey guidelines suggest that a sample of 500 participants is sufficient for a statistical analysis that also accounts for heterogeneity. 177 Accordingly, we aimed to recruit 500 participants. Post hoc sample size estimates for each attribute and level were also conducted (see Appendix 8, Methods, Sample size estimate).
Participant sample, recruitment and survey administration
The target population for the survey was people with psoriasis because they represent the perspective of the intended users. The inclusion criteria were that participants had a diagnosis of psoriasis (defined as ‘told by a doctor that they have psoriasis’), were aged ≥ 18 years and lived in the UK. The criterion regarding location reflects the fact that the interventions were developed in the UK health-care context.
The survey was developed for participants to complete online. Ethics approval was provided by the University of Manchester REC (reference number: 16325). The stated preference survey was advertised via social media (see Appendix 8, Methods, Sample size estimate), specifically targeting people with psoriasis. Participants who responded to the adverts were given a link to the survey materials (see Appendix 8, Methods, Sample size estimate).
Analysis
The survey data were analysed using descriptive statistics to summarise participant characteristics and regression analysis of responses to the choice sets (see Appendix 8, Methods, Analysis). The results (coefficients) from the regression analysis give an indication of the strength of preference for levels 2 and 3 compared with the base or reference level (level 1) within each attribute. Time was used as a common measure to compare the strength of preferences between attributes. One approach is to estimate the amount of time that a person would be willing to spend (or trade) to get their preferred level of an attribute. This is known as the marginal rate of substitution (MRS) and is estimated by dividing the coefficient for each attribute level by the coefficient for the time attribute. The results of the regression analyses were used to estimate the relative amount of time participants were willing to trade for the different attributes. The primary analysis used a conditional logit model to estimate the main effects for participants who completed all the choice questions. Sensitivity analyses explored whether or not the MRS differed if a random parameters logit model was used and the impact on the results of including participants who completed one or more and seven or more choice questions. Subgroup analysis explored the impact of participant characteristics on the MRS and the relative importance of each attribute level.
Results
Participants
Overall, 526 eligible participants were recruited and 250 (48%) of these participants completed all choice questions (see Appendix 8, Figure 71). This met our initial recruitment and sample size targets, with a slightly higher rate of people completing all choice sets. Nevertheless, post hoc sample size estimates suggest that for one attribute level (printed information) the sample size was not sufficient to identify preferences (compared with no information) at a statistically significant level.
Participants’ characteristics are reported in full in Appendix 8, Table 15 and summarised here. Participants who did not answer any choice questions (160 out of 526; 30%) also did not complete the questions about their sociodemographic characteristics or treatment, which were at the end of the survey. In total, 260 out of 366 (71%) participants who answered one or more choice questions also answered one or more demographic or treatment questions (see Appendix 8, Results, Participant characteristics and time to complete survey). Of those who answered one or more demographic or treatment questions, the majority were women (164/260; 63%). The average age of participants was 48 years [standard error (SE) 1, 95% CI 46 to 50, n = 257] and the average age at diagnosis was 22 years (SE 1, 95% CI 20 to 24, n = 252). The EQ-5D-5L health status measure was used to estimate the utility of participants. The utility score is anchored by 0 (dead) and 1 (full health). The average utility value of participants with complete EQ-5D-5L data depended on the value set used. Using the crosswalk value set, which maps the utility weights from the three-level version of the EuroQol-5 Dimensions (EQ-5D) to the five-level version, the average utility was 0.71 (SE 0.01, 95% CI 0.68 to 0.74, n = 255). Using the utility weights developed specifically for the five-level version of the EQ-5D, the average utility value was 0.80 (SE 0.01, 95% CI 0.77 to 0.82, n = 255). Of the participants who reported the types of health-care practitioner they had ever seen (n = 258), most had been cared for by both a GP and a hospital doctor and nurse (212/258; 82%) at some point. Nearly all participants had used psoriasis creams, ointments, lotions or shampoo at some time since being diagnosed with psoriasis (255/260; 98%); over half had used PUVA treatment (161/260; 62%) and just under half had been prescribed tablets (114/260; 44%). Just under one-fifth of participants had been prescribed injections (48/260; 19%).
Including all eligible respondents, the average time to complete the survey was 8 minutes (SD 22 minutes), although this ranged from < 1 minute to 416 minutes (see Appendix 8, Results, Participant characteristics and time to complete survey). Five participants had > 1 hour between the start and finish times (range 96–416 minutes). For 240 out of 250 (96%) of those who completed all choice questions, the mean time taken was lower than or within the time range given in the participant instructions (10–20 minutes).
Regression analysis indicated that there were no differences in all but one participant characteristic between those who took survey 1 or survey 2 and answered one or more choice questions. The exception to this was that participants who took survey 2 were more likely to see their GP and hospital doctor or nurse (compared with GP only) than those who took survey 1 (logistic regression; p = 0.004). The analysis also indicated that, for participants completing one or more choice questions, whether they chose option 1 or option 2 in each choice set was not dependent on their characteristics (see Appendix 8, Tables 16 and 17).
Choice data
The analyses of the choice data are described in detail in Appendix 8, Tables 18 and 19. The results are briefly summarised here. For the attributes about information, clinician visits, understanding of psoriasis and comorbidity, and ability to make lifestyle changes, participants preferred higher levels (levels 2 or 3) of each attribute rather than the reference or base level (level 1), which is in line with what was expected (Figure 23). In addition, the strength of preference measured by the coefficients was statistically significant for both levels compared with the lowest or reference level for these attributes. The exception to this was the information attribute, for which only the coefficient for printed and online information materials (level 3) was statistically significantly different from the base level of no information. The post hoc sample size estimates indicate that substantially more participants are needed to identify a statistically significant coefficient for the level of printed information materials only (see Appendix 8, Table 20).
FIGURE 23.
Coefficients for categorical attribute levels, effects coded, participants completing all choice questions, n = 250.
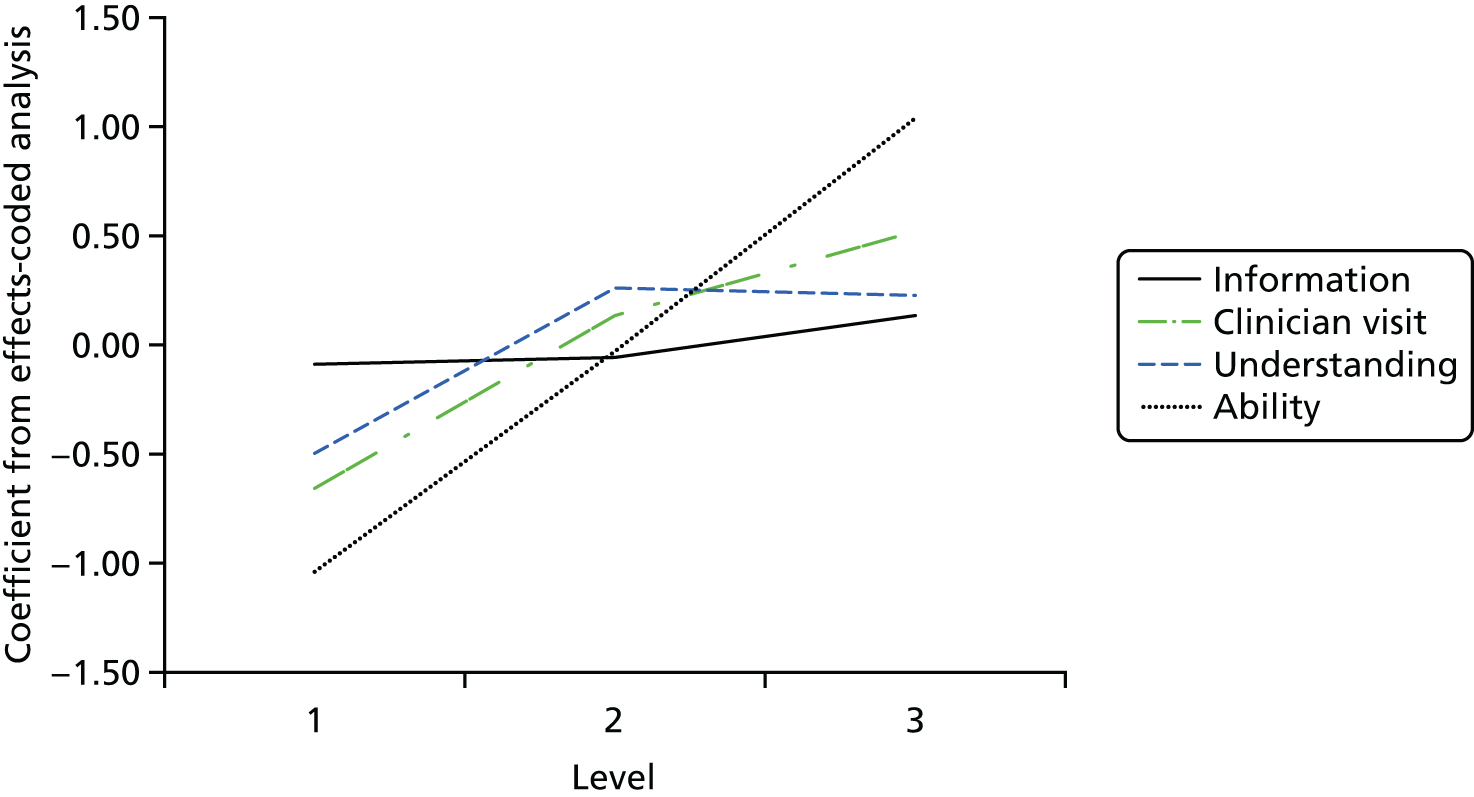
The coefficient for time was negative and statistically significant. This is in line with expectations that, all other things being equal, participants would prefer to spend less rather than more time managing their psoriasis and making lifestyle changes. There is some variation in this. Participants preferred to spend more time than 30 minutes (the reference level) and up to 2 hours per week. If they needed to spend more time than this, then they preferred to trade fewer rather than more hours per week (Figure 24). Although this appears to be counterintuitive, it may be plausible if the participants receive little support to help them self-manage their psoriasis and make lifestyle choices. The qualitative studies in this research programme suggest that this may be the experience of patients.
FIGURE 24.
Coefficients for time attribute, effects coded, participants completing all choice questions, n = 250.
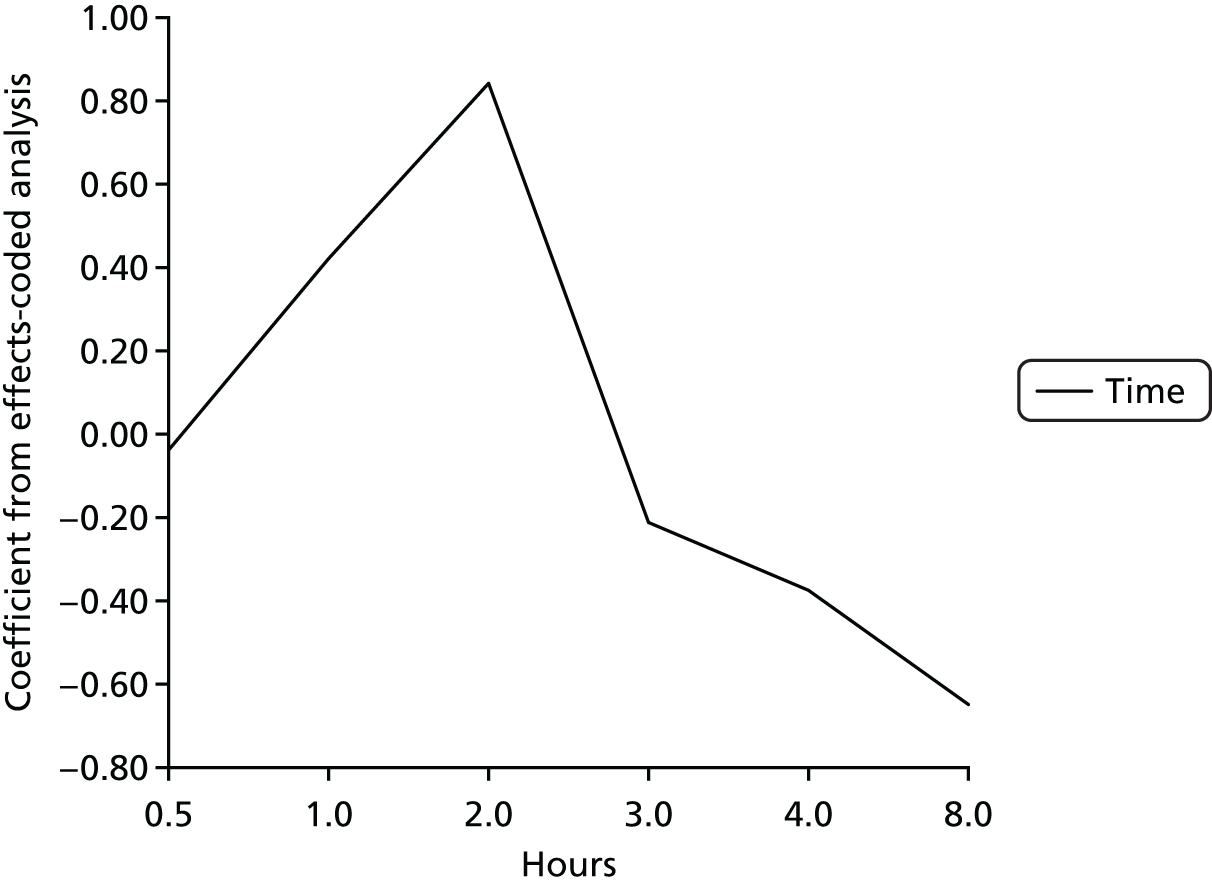
Willingness to trade time for preferred attributes
The MRS analysis is described in Appendix 8, Tables 21–24. An overview of the results is given here. The data suggest that participants were willing to trade 3 hours (95% CI 1 to 5 hours) per week for general information and exercise leaflets to take away and read and work through in their own time if they could have the information in print and online. They were not willing to spend more than half an hour (95% CI –2 to 2 hours) to have printed information only.
Participants were willing to spend around 10 hours (95% CI 6 to 13 hours) per week to understand and manage their psoriasis and make lifestyle changes if it meant that their understanding (about what psoriasis is, what the treatments are and how lifestyle choices may affect their psoriasis symptoms or risk of other illnesses) was no worse or better than before the intervention. Participants were willing to spend a similar amount of time (9 hours, 95% CI 6 to 13 hours) if it meant that they received personalised information at clinic visits (about their psoriasis and its treatment, lifestyle factors that may affect their psoriasis and health, and how to manage their psoriasis). To have a clinic visit that also helped them to identify and make lifestyle changes, participants were willing to trade 14 hours per week (95% CI 10 to 19 hours).
Participants were willing to spend 25 hours a week (95% CI 17 to 32 hours) if it meant that their ability to manage their psoriasis and/or make changes improved.
Discussion
Overall, the stated preference survey results suggest that participants valued improvements in their ability to manage psoriasis and make lifestyle changes resulting from interventions. The MRS analysis suggested that they were willing to trade 25 hours (95% CI 17 to 32 hours) of their time per week to achieve this level of improvement. The next most important attribute was that clinic visits include personalised information and support for lifestyle changes. Relative to the other attributes, the generic, theory-based information materials were less important. Nevertheless, participants were willing to trade 3 hours per week to have these information materials in printed and online versions. It was not possible to assess the relative importance of printed information materials only.
Limitations
The stated preference survey was advertised using social media and e-mail lists and administered online. This limits the population of potential participants to those who use social media and/or are engaged with the Psoriasis Association and research. Participants who responded to the survey were self-selected. These factors may limit how representative the study sample of responders is of people with diagnosed psoriasis.
In addition, it is important to remember that the participants did not see the actual PSO WELL patient or training materials and thus they were responding to hypothetical options rather than making choices about the actual materials developed by this team. Thus, these findings need to be read in the context provided in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis.
The stated preference survey asked participants to complete nine choice tasks. Each choice task described the hypothetical options in a standardised form using four descriptors of a component of the option (attribute), which could take one of four levels. This increases the complexity of the survey and the chance that respondents may not complete the survey as expected, increasing uncertainty about the interpretation of results. In line with recommended practice, the descriptions of the attributes and levels were derived from the qualitative research reported in Cardiovascular disease risk communication and reduction in psoriasis, Coping with psoriasis: learning from patients and Understanding the professional role in supporting lifestyle change in patients with psoriasis to develop the PSO WELL intervention as well as the results of the development and feasibility studies [described in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis]. 175,176,178,179 Draft versions of the survey were reviewed by the multidisciplinary IMPACT programme team and RUG and simplified in response to comments. This included reducing the number of options considered by each participant from 18 to nine and simplifying the descriptions used.
Participants who did not answer any choice questions (160/526 participants; 30%) also did not complete the questions about their sociodemographic characteristics or treatment, which were at the end of the survey. This introduces uncertainty about the representativeness and robustness of the results. In total, 260 out of 366 (71%) participants who answered one or more choice questions also answered one or more demographic or treatment questions. This lack of data means that it is not possible to assess whether or not there were any differences between those who did and those who did not answer any, or all, choice questions. As is typical with anonymous online surveys, it was not possible to collect comprehensive data about the number of people who saw the adverts and e-mail invitations or who then visited the website to look at the details about the survey.
The participants who completed one or more demographic questions were similar in age (median 47 years, IQR 26 years) to those in the epidemiological cohort study (study 1.ii) reported in Epidemiology of psoriasis and its association with risk of cardiovascular disease (median 47 years, IQR 25 years). 30 The mean age of the survey participants is also within the range of those reported in the systematic review conducted as part of study 1.i (mean 42 years, range 50 years; 12 studies)1 and a systematic review of studies including psoriasis patients. 180
There were more women in the stated preference survey sample (63%) than in the epidemiological cohort study (53%). Other large studies included in the systematic review by Parisi et al. 1 suggest a similar prevalence of psoriasis in men and women. However, the subgroup analyses suggest that the results of the choice analyses are similar between women and men. Of those survey participants who reported treatments they had ever had, 63% had received phototherapy or systemic or biologic treatments (assumed to be tablets or injections for psoriasis). The epidemiological cohort study reported that 1% of participants had severe psoriasis at baseline (defined as having phototherapy or systemic or biologic treatment) and 4% had severe psoriasis at follow-up. 30 In terms of overall health, the survey participants had mean EQ-5D utility values (estimated from the crosswalk mapping system to facilitate comparison with the older three-level version) of 0.71 (95% CI 0.68 to 0.74). This was within the range of values reported in a systematic review of studies using the three-level version of the EQ-5D (0.52–0.9). 180
The stated preference survey used a binary design so that each choice set included two options of different attribute levels but did not include a third opt-out or status quo option. The binary, forced-choice approach assumes that respondents would choose to use unappealing options in practice. 175 Research exists to show that some people with psoriasis in the UK do not engage with health-care services for a variety of reasons. In addition, the design means that the survey did not address preferences for the characteristics of the PSO WELL intervention compared with current usual care. Choice sets that include three or more options may also be more statistically efficient than binary designs. 181 Nevertheless, the design is consistent with the aims and objectives of the survey. These were to provide additional information about preferences for the different components and desired outcomes of the PSO WELL intervention, quantify the preferences of people with psoriasis for the different components of the intervention and assess the relative importance of each component. The IMPACT programme team felt that the binary design also reduced the burden on participants. Overall, the design was 94% efficient compared with an optimal binary choice design and 65% efficient compared with a six-choice design for a main effects analysis.
The stated preference survey used a main effects design, which assumes that each of the attributes is independent. If preferences for one or more attribute levels are dependent on the value of a second attribute level, then the analysis could produce biased estimates of preferences. However, a design that allows statistical analysis of main effects and interactions typically requires a higher number of choice sets. The attributes were selected through a process of discussion with the IMPACT programme research team, taking into account the need for independence between attribute levels, the research on which the PSO WELL intervention was based and the early stage of development and evaluation of the intervention. There was also a need to balance the wish to understand how preferences changed with different levels of the attributes within the resources available (including participant numbers) against the demands of a design that was sufficient to estimate main effects and at least two-way interactions.
The survey recruited 526 eligible participants and 250 of these completed all choice questions, which was above the initial sample size estimates. Post hoc sample size estimates indicated that the number of complete responses was sufficient for the statistical analysis for all but one of the intervention attribute levels (see Appendix 8, Table 20); this was the level of printed information materials (versus no information materials). The coefficient for this level was not statistically significantly different from the reference level of no information materials; Figure 23 illustrates the similarity in preferences for the levels of no information materials and printed information materials. However, the post hoc sample size estimate indicates that over 9000 to 9600 completed responses would be needed for the analysis of this level. This means that there is insufficient power to identify whether preferences for the printed information materials level did or did not differ from the no information materials level. However, the results and sample size estimates for printed information may also reflect the preferences of the sample of people who completed the survey. As discussed above, these may be people with more severe psoriasis and who may be more likely to be engaged with social/online media than the broader population of people with psoriasis. It is also possible that participants implicitly compared the theory-based, printed information materials to the printed information currently available and from the descriptions provided did not see any differences between the two.
The results indicate that participants would prefer to spend > 30 minutes per week to understand and manage psoriasis and make lifestyle changes but only up to 2 hours per week. After that, participants would prefer to spend less rather than more time. This appears to be counterintuitive. In addition, the results imply that participants may be willing to trade up to 25 hours of their time per week to achieve improvements in their ability to manage psoriasis and make lifestyle changes. The results for the time attribute and MRS may reflect the situation if the participants in the survey received little support to help them in managing psoriasis and lifestyle choices. It may also be a limitation of the design of the survey, which used 30 minutes as the lowest level of time spent per week for the time attribute and 8 hours as the highest level. These times were informed by the need to manage psoriasis on a daily basis and the amount of time participants may spend on additional self-management and lifestyle changes.
Overall, limitations to the design of the stated preference survey mean that further qualitative and experimental research is needed to assess the potential impact and preferences of people with psoriasis for the different components of the PSO WELL interventions.
Conclusions
Overall, the stated preference survey suggests that participants valued improvements in their ability to manage psoriasis and make lifestyle changes. Findings indicated that individuals were willing to trade 25 hours of their time per week to achieve improvement. The next most valued attribute was clinic visits that include personalised information and support for lifestyle changes. The theory-based patient materials were valued less; however, participants were still willing to trade 3 hours per week to have these.
The survey participants appeared to have severe psoriasis. This means that it is not possible to draw conclusions about the relevance and applicability of the results to the broader population of people with less severe psoriasis. There were also limitations in the design of the survey that may affect the validity of the results.
Implications for policy and further research
The survey results suggest that, for people with severe psoriasis, the ability to manage psoriasis and make lifestyle changes is important, along with personalised information and support to make lifestyle changes provided as part of clinic visits. These are key components of the PSO WELL training intervention for clinicians and support the potential for it to be of value to patients. These aspects were valued the most by the respondents. However, understanding psoriasis and access to printed and online information were positively valued by the survey participants who were willing to spend 3–10 hours per week in exchange for these aspects of the PSO WELL intervention.
Implications for research
There were some differences between the findings of this study and those reported in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management. The survey recruitment methods used in the stated preference study meant that the sample of people who visited the survey website were more likely to be those who were active on social media, were comfortable using online survey methods and possibly had more severe psoriasis. Invitations via the Psoriasis Association and the IMPACT programme mailing lists may also indicate that participants were more aware of treatment and engaged with psoriasis-related research. Further research to explore the preferences of people with less severe psoriasis and whether or not preferences differ by stage and severity of disease is warranted. This may be particularly important to understand the results for the information attribute.
Health economics modelling of costs of interventions for people with psoriasis
Although the economic model was conceived as part of workstream 1 (Figure 25), it was developed in parallel with other workstreams and drew on data and findings from workstreams 2 and 5 to inform the model structure and to populate the model with data. Accordingly, the methods and results are reported here rather than in Epidemiology of psoriasis and its association with risk of cardiovascular disease.
FIGURE 25.
Workstream 1: relationship of study 1.iii to other IMPACT programme workstreams.
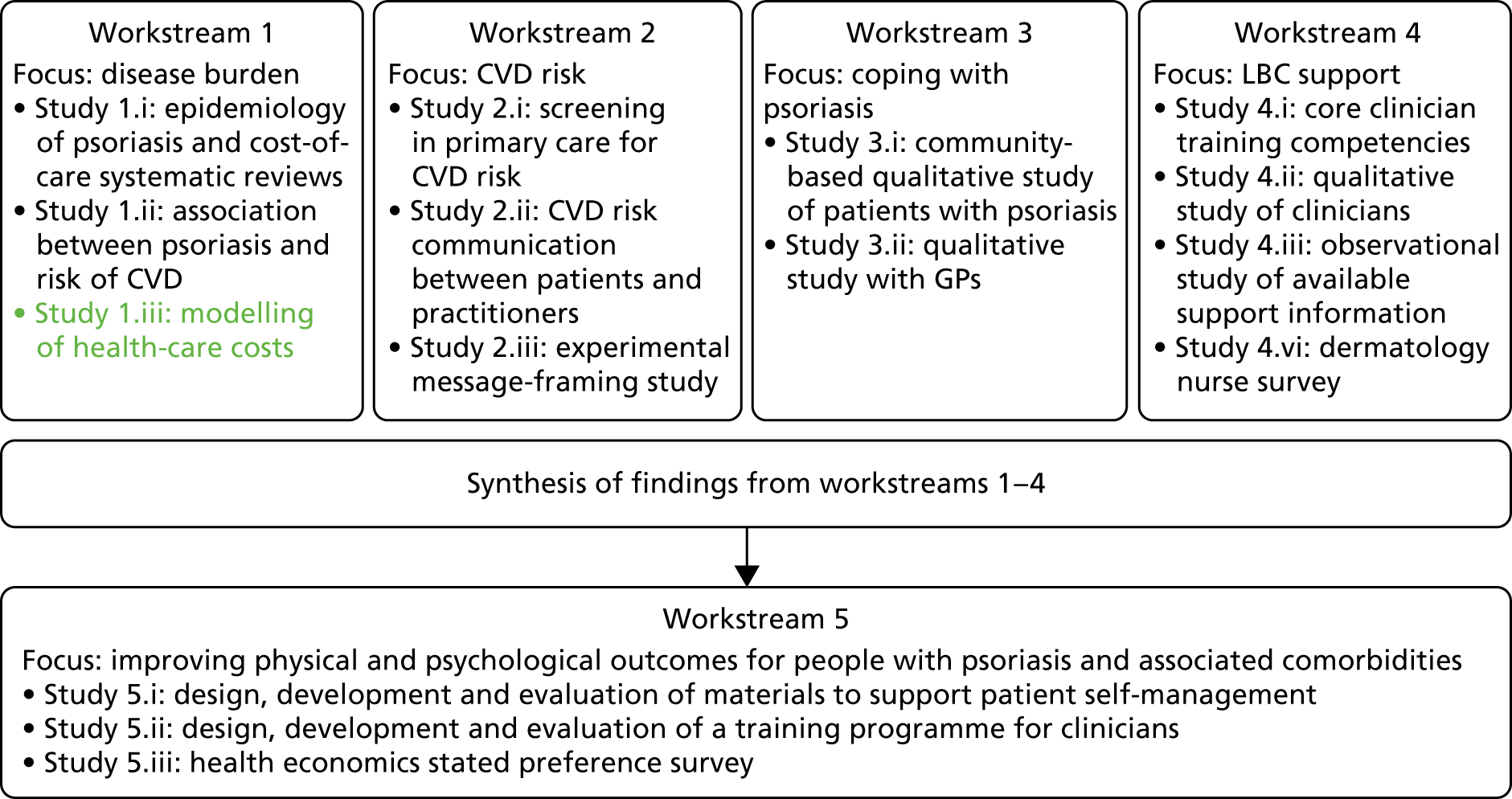
The data used in the model come primarily from workstreams 2 and 5 plus data from the economic review and other published sources.
Economic model
Population, intervention, comparator, outcome, time (PICOT) statement:
-
The target population for the economic model was patients with mild, moderate or severe psoriasis. The model used the sample of patients in the cohort study in IMPACT workstream 2, utilising the rich data set containing 287 patients. This assumes that patients recruited to the study were representative of the target population.
-
The intervention comprised the PSO WELL clinician training and patient information materials as a package of care.
-
The comparator was treatment as usual (TAU), defined as the usual management of psoriasis patients in primary and secondary care without any PSO WELL patient information materials or clinician training taking place.
-
The primary outcome of the economic analysis was the incremental cost-effectiveness ratio (ICER). This was estimated as the net cost of the intervention divided by the net health benefit. The measure of health benefit for the primary analysis was the quality-adjusted life-year (QALY).
-
The time horizon for the evaluation was 10 years from entry to the model.
Approach
The economic model aims to provide an assessment of the costs and outcomes of usual care and an exploratory evaluation of the cost-effectiveness of the PSO WELL intervention compared with TAU. The perspective is the UK health and social care system. This was used to define the range of costs included in the analyses. The time horizon for the primary analysis was 10 years, the maximum of previous analyses identified in the literature review. A longer horizon would stretch the limited evidence available too far, whereas shorter time horizons are assessed in scenario analyses. The key outcomes were incremental health benefit, measured in QALYs, and incremental costs. These were used to calculate the ICER, which is the primary outcome.
Model development
The model was intended to be a simplification of psoriasis care in real life, rather than to incorporate all the possible characteristics and interactions of patients, health-care professionals and treatment. Accordingly, the model was designed to incorporate key features identified in the different components of the IMPACT research programme.
Published economic evidence and guidelines61,182–188 combined with the early evidence from the qualitative research components of the IMPACT research programme were used to characterise current management of psoriasis and develop a preliminary version of the IMPACT programme cost-effectiveness model. Key decisions about model goals, structure and assumptions were made by the multidisciplinary IMPACT programme research team through a structured programme of consultation. The process involved discussion papers, short surveys, one-to-one and small group meetings and workshops at FIMs over the course of the IMPACT programme [see Appendix 10 for further details and for the completed International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines checklist189]. The model was also discussed with the Independent Expert Advisory Committee at key points in development. This evidence-led fine-tuning helped ensure that the economic model structure provided a meaningful and accurate characterisation of the key components of the psoriasis care pathways from the perspectives of patients and clinicians.
Psoriasis is a complex condition with great diversity of potential patient pathways. Although the individual treatments used for psoriasis appear simple, they are prescribed in a complex package of care and interactions between patient and clinician. A simple characterisation of the pathway through different treatments indicates patients starting with topical therapies and stepping up to phototherapies and/or systemic non-biological and biological treatment if symptoms are not adequately managed. These steps in treatment are also referred to as the treatment ladder, reflecting both the move from first- to second- and third-line therapies and the increasing toxicity associated with second- and third-line treatment.
In reality, progression from one treatment to the next is determined by a number of other factors. For example, the point at which a person with psoriasis seeks treatment varies – this may be when symptoms first appear, when topical therapy is an appropriate first line of treatment or when symptoms have progressed to be more severe, requiring early systemic treatment. Patients starting with topical therapy may disengage with treatment and not seek further care until the symptoms require treatment with the more advanced systemic therapies.
In addition, evidence provided in earlier chapters suggests that physical, social and emotional challenges are important aspects of psoriasis, but clinicians do not always broach these topics during consultations. 111,112 This means that the interaction between patients and clinicians can also play an important role in prescribing and medication usage as well as the success of psoriasis management. Finally, treatment depends on the interface between primary and secondary care and interactions between generalist and specialist health-care professionals.
The modelling studies included in the systematic review typically used either decision tree models or Markov models. Decision tree models compare ‘branches’ of treatment strategies, made up of all possible treatment decisions. Characterising the complexity of treatment and interactions noted above would make a decision tree unwieldy, with each possible long-term event or treatment decision exponentially increasing the overall number of branches and treatment strategies being compared.
A Markov model is made up of a number of unique health states between which simulated patients move over time. Each health state is associated with certain health or cost outcomes, permitting a simulated patient cohort to move between these. Typically, any individual patient’s route to a particular health state is not considered and takes little or no account of their characteristics or how long they have lived with the disease. It is clear from the outcomes of workstreams 3 and 4 that this would be a limiting assumption in a model for psoriasis care, as patient history is highly important; for example, did their clinician provide adequate information? Did the patient see the same clinician or a different one at follow-up? These historical events and characteristics have a bearing on a patient’s probability of experiencing another event; this restricts the usefulness of decision tree or Markov models.
These factors point to the need for an approach that can incorporate the complexities of psoriasis and psoriasis care. Agent-based modelling imitates key actions and interactions of individuals or agents (e.g. patients and health-care professionals) and organisations. The aim is to assess what the effects of these interactions might be on the system as a whole. DES models allow an individual patient to take a random walk through a range of possible events, explicitly taking account of the diversity of potential patient pathways in psoriasis. A hybrid model that incorporates both agent-based and DES approaches was chosen. This allows a large number of potential courses of disease and treatment and allows repeated interactions between the different individuals (agents) in the model. 190
Overview of economic model structure
The structure of the economic model is illustrated in Figure 26 and briefly described in this section. Further technical details about the structure are given in Appendix 11.
FIGURE 26.
Economic model structure.
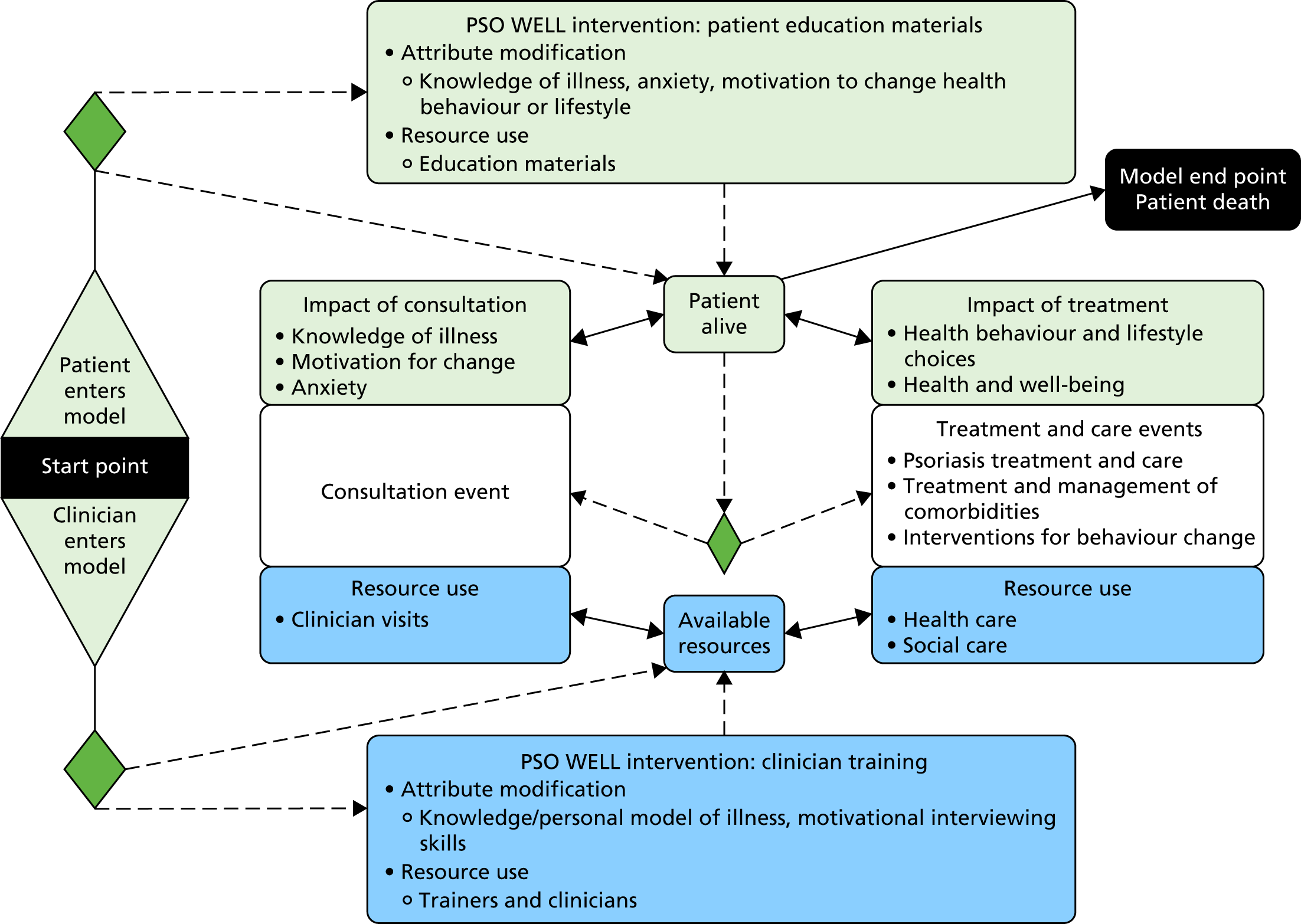
The model compares TAU with the PSO WELL intervention plus TAU. The patient information materials and clinician training developed and evaluated in workstream 5 (see Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis) are considered together as a single PSO WELL package. It is assumed that the distribution of patient materials and training of clinicians occur right at the start of the simulation. The key differences between the PSO WELL arm and the TAU arm is the assumption that (1) the patient materials could change the likelihood that patients make beneficial changes to their lifestyle and health behaviours and (2) training makes clinicians more likely to provide appropriate treatment and use motivational interviewing techniques. These, in turn, influence patient health, need for health care and health-care costs.
At the start of each simulated patient journey, the patient enters the model with a profile of sociodemographic and health characteristics. The sociodemographic characteristics include age, gender, ethnicity and Townsend deprivation score for the local area where the patient lives. The health characteristics include the severity of psoriasis, PsA, anxiety and depression, BMI, type 2 diabetes, MI and risk of future MI or stroke. Treatment history attributes include prior and current psoriasis treatments. Lifestyle behaviours included are alcohol consumption, extent of physical activity and smoking status. 147
Psoriasis severity determines what type of clinician a person sees routinely and what treatments are prescribed. The interactions between patients and practitioners determine many events that happen during a patient’s journey through the model. The model focuses on interactions between the patient and their GP and between the patient and the dermatology service and also includes the wider costs and health impacts of the comorbidities associated with psoriasis. The patient uses health-care resources (e.g. interacting with clinicians at clinical consultations, receiving treatment) until either the model time horizon is reached or the patient dies. The number of health-care services used and the costs depend on each person’s characteristics and how they interact with the health-care system. At the same time, the patient may or may not make changes to their lifestyle. These changes will then influence their health and need for health care.
Outcomes are recorded at the end of the simulation to calculate mean and total costs and QALYs across the whole simulated population, for usual care alone and for usual care plus the PSO WELL interventions.
The model includes a number of assumptions to simplify the components and the patient’s journey through the model and to estimate parameters the data used. The model assumptions are described in detail in Appendix 11, Key components and assumptions, and the data assumptions are included in Appendix 11, Tables 26–30. The key assumptions about the patient’s journey include:
-
A proportion of clinicians will act in accordance with clinical guidelines. This will be higher for clinicians who receive the training intervention than for those who do not receive the training intervention.
-
If a clinician acts in accordance with clinical guidelines, then the model assumes that this clinician’s treatment decisions are made in line with evidence-based guidelines. 135
-
If a clinician does not act in accordance with clinical guidelines, then the clinician will, in the model, prescribe phototherapy if required; the specific type of phototherapy regimen is selected at random by the model. If a patient with severe psoriasis has previously received phototherapy, then a systemic therapy is prescribed; again, the type of systemic therapy is selected at random by the model.
-
A clinician’s propensity to use motivational interviewing skills during consultations depends on their knowledge of and ability to use motivational interviewing skills and their assessment of the relevance of these for the patient and for treatment decision.
-
A patient’s time until next consultation depends on their current psoriasis treatment –
-
routine GP appointments occur every 6 months to review progress with topical therapy
-
an appointment is scheduled to take place 2 weeks after referral from a dermatologist, a psoriasis flare or patient help-seeking following the PSO WELL intervention
-
routine dermatologist consultations occur every 10–16 weeks depending on the specific complex treatment regimen.
-
-
The treating clinician may identify the need for a formal lifestyle or comorbidity intervention. To manage an identified comorbidity, it is assumed that patients will receive the next available intervention in the relevant treatment sequence derived from clinical guidelines182–187
-
The patient will agree to a lifestyle intervention only if it is a behaviour that they are intent on changing.
Key variables and data
The variables, data sources and parameter estimates are detailed in Appendix 11, Key variables; Appendix 11, Parameter estimates and data sources for the economic model; and Appendix 11, Tables 26–32. The model includes psoriasis treatment effects that were identified from the economic systematic review [Dermatology Life Quality Index (DLQI) and PASI]. These were converted to a common measure for the model using published algorithms (see Appendix 11, Key variables, Treatment effects). The model includes the outcomes of behaviour change and comorbidity interventions, which were identified from clinical guidelines61,182,183,185–187,191 and the sources used to develop the guidelines.
Psoriasis flares occur in the model, potentially causing people with mild disease to progress to severe psoriasis. A flare will cause an increase in psoriasis symptoms and/or decrease in treatment outcomes. It is assumed that even if patients with severe psoriasis have their symptoms effectively managed, they cannot return to having mild or moderate psoriasis (see Appendix 11, Key variables, Psoriasis flares).
Quality-adjusted life-years were used as the measure of total health benefit experienced by simulated patients over the course of their journey through the model. A QALY measures the length of survival and adjusts this to take into account any ill health during that time. For example, a person in full health for a year will have a QALY of 1, whereas a person who is in less than full health may have a QALY of 0.25. Death is assigned a value of zero. The model allows a simulated patient’s health and QALYs to vary as events occur and their attributes change (see Appendix 11, Key variables, Quality-adjusted life-years). Time to mortality was predicted from all-cause mortality. 192 The only cause of early mortality is fatality due to MI193 or stroke (see Appendix 11, Key variables, Mortality). 194
Various health-care resources and costs are required throughout the simulation (see Appendix 11, Key variables, Costs and resource use). These included the costs of the PSO WELL interventions and other health-care costs. The costs of the clinician training element depended on the number of clinicians trained as well as the number of psoriasis patients managed by each clinician. For the primary analysis, we conservatively assumed that each clinician managed only one patient, which increases the unit cost per patient of the training. A sensitivity analysis was used to explore the impact of increasing the number of patients treated by each clinician.
Other health-care costs included the costs of GP and dermatologist consultations, topical treatment, phototherapy, non-biologic and biologic systemic therapies and the costs of interventions to support lifestyle changes and to manage comorbidities.
All costs and QALYs were discounted at an annual rate of 3.5% to account for social time preferences as recommended for the UK. 195
Analysis
The model produced simulated pairs of net costs and net outcomes. These were used to generate cost-effectiveness acceptability curves, as recommended by NICE for health technology appraisals,195 and to estimate the probability that the PSO WELL intervention is cost-effective compared with TAU. The cost-effectiveness acceptability approach revalues effects or benefits in monetary terms. However, in the UK there is no universally agreed monetary value for the types of health benefit measures (such as QALYs or relapse-free years) typically used in cost-effectiveness analyses. An approach used in health care is to ask the question: what is the maximum amount that decision-makers are willing to pay to gain one unit of health benefit? The simulated net QALYs were revalued using a range of maximum willingness-to-pay (WTP) values that a decision-maker may be willing to pay to gain 1 QALY. The WTP thresholds used ranged from £1 to £50,000 to gain 1 QALY. This range of values was based on the range of WTP values historically implied by NICE decisions. 196
The model consists of an inner loop that simulates one patient journey for each simulated patient. There is an outer loop that repeats the inner loop 250 times. Each of these iterations uses a new set of values for the model variables. Therefore, if one patient journey is modelled, a total of 500 unique patient experiences are simulated (250 for the TAU arm and 250 for the PSO WELL arm). This addresses the uncertainty that comes from the differences between patients in terms of their sociodemographic and health characteristics. The patients included in the model were the 287 people included in the screening study of CVD risk in patients with psoriasis (study 2.i). This gives 71,750 unique patient experiences (250 journeys × 287 patients) for the TAU arm and for the PSO WELL arm of the model. This adds in the uncertainty in the data used to estimate the variables or parameters included in the model. All results presented are therefore probabilistic.
The model structure and simplifying assumptions introduce another source of uncertainty owing to structural or design decisions. Threshold and scenario analyses were used to assess the impact of these on the results. The threshold analysis explored the impact of increasing the number of patients who could benefit from the PSO WELL intervention. The scenario analyses looked at the impact of changing key assumptions used in the model. The technical development and data analysis were undertaken in R version 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Primary analysis
The detailed results of the primary and sensitivity analyses are given in Appendix 11, Tables 32 and 34, and Appendix 11, Figure 72. In the primary analysis, the model predicts that TAU is associated with 4.74 QALYs (95% CI 4.66 to 4.83 QALYs) per patient over 10 years (see Appendix 11, Table 34). In terms of costs, TAU is expected to incur a total cost of £26,242 (95% CI £25,852 to £28,452) per patient. The PSO WELL arm of the model generates 4.75 QALYs (95% CI 4.64 to 4.82 QALYs) per patient, with a mean cost of £27,801 (95% CI £24,902 to £27,466) per patient.
Overall, the model predicts a small gain in health benefit of 0.01 QALYs for PSO WELL (95% CI –0.10 to 0.13 QALYs) and with a mean net cost of £838 compared with TAU (95% CI –£1106 to £2593). This difference is smaller than the clinician intervention cost per patient (£1133) and may indicate that minor cost savings are made elsewhere to offset it. However, the 95% CI for the difference in costs crosses zero, indicating that the difference in cost could have occurred by chance.
The ICER is £72,802 per QALY. If decision-makers are willing to pay £20,000 to gain 1 QALY, then the probability that the PSO WELL intervention is cost-effective is 34%. This means that if only one patient per clinician trained benefits from the PSO WELL intervention, it is unlikely to be a cost-effective use of NHS resources (see Appendix 11, Table 34, and Appendix 11, Figures 72 and 73).
Threshold analysis
Threshold analysis was used to explore whether or not the relative cost-effectiveness of the PSO WELL intervention package improved if more patients could benefit from clinician training. This indicates that if decision-makers are willing to pay £20,000 to gain one QALY, the PSO WELL intervention may be cost-effective if a trained typical clinician group (average of 10 clinicians per group) manages ≥ 22 psoriasis patients (see Appendix 11, Figure 74). This equates to each clinician applying their new knowledge and skills to two patients in the year following training, thereby generating more health gains and costing less per patient. PSO WELL dominates TAU, simultaneously improving health outcomes and causing a net saving if 40 patients per clinician group (or four patients per clinician) could benefit from the clinician training.
Scenario analysis
Scenario analyses were used to assess whether or not the probability that the clinician training was cost-effective changed if alternative assumptions about key model inputs were changed (see Appendix 11, Table 35). For comparability of results, we assumed that 10 patients stand to benefit from training a typical clinician group of 10 clinicians.
The PSO WELL intervention is more likely to be cost-effective if the effect of training is maintained for > 1 year. If the impact of the clinician training is maintained for the 10-year time span of the model, then incremental costs fall and the probability that PSO WELL is cost-effective increases to 44%. If the number of patients benefiting per clinician group trained is increased to 16, the probability that PSO WELL is cost-effective increases to 52%.
The cost-effectiveness of PSO WELL improves if all dermatologists always act in a guideline-concordant manner and 20 patients benefit per clinician group (or two patients per clinician trained), at which point PSO WELL has a 50% probability of being cost-effective. This result suggests that a large benefit may be achieved if GPs correctly refer severe psoriasis patients to secondary care when it is guaranteed to be guideline appropriate. PSO WELL is less likely to be cost-effective over shorter model durations (suggesting that cost offsets are realised over longer durations) or if the PSO WELL development costs are included on top of delivery costs.
Discussion
This exploratory cost-effectiveness analysis estimates that the PSO WELL interventions have the potential to be cost-effective for the NHS. The likelihood of this depends on the number of patients who stand to benefit from a typical group of GPs and dermatologists being exposed to the intervention. If a trained group manages more patients, then the clinician intervention cost is spread over a larger potential pool of people who can benefit. If around 22 patients are managed per group of 10 clinicians, our analysis suggests that PSO WELL may be cost-effective. This is less likely to be true if costs incurred in developing the interventions are included in the analysis, rather than considered to be sunk costs.
Despite its exploratory nature, this evaluation possesses a number of strengths. First, as a DES, the model allows for repeated interactions between clinicians and patients, and contains a large number of psoriasis treatment options, health-related behaviours and comorbidities. This would be impractical with alternative models. Second, model development involved regular collaboration between multidisciplinary experts in the IMPACT programme team. Furthermore, a good-practice checklist was used to validate the model throughout its development (provided in Appendix 10). 189
Nevertheless, there were a number of challenges to address in building the model. As a consequence, the model required a number of simplifying assumptions to make the evaluation feasible. In addition, limitations with the evidence available meant that some assumptions about the model inputs were required. These mean that the evaluation does have some limitations, which also highlight where further research may be useful.
Our analysis used the sample of participants recruited into the CVD risk factor screening study (in workstream 2) as the source of data about patient baseline characteristics. 66 A key reason for this was the availability of detailed information about the study participants’ health and lifestyle characteristics. However, for the reasons noted in Cardiovascular risk in patients with psoriasis, the sample may not be representative of the target population of people with psoriasis for the economic evaluation in terms of CVD risk. If this sample of patients has a higher CVD risk than the wider population of people with psoriasis, then this may overestimate the impact of the intervention on patients’ overall health and QALYs and underestimate the occurrence of events that incur health-care costs. Even so, the analyses of the CPRD data (see Epidemiology of psoriasis and its association with risk of cardiovascular disease) do indicate that people with psoriasis have higher rates of CVD than the general population.
Our model omits a number of features that would ideally be included for the simulation to accurately reflect reality. Simplifications have been made to the psoriasis treatment logic. These include assuming random treatment selection among clinicians who do not follow published national guidelines, excluding contraindications to treatment and excluding the role of dermatology clinic nurses. Further work is needed to understand whether this would under- or overestimate the relative cost-effectiveness of the PSO WELL intervention.
Non-adherence to treatment by patients is also missing from the model, which may have a significant impact on the effectiveness of psoriasis therapies. 133 If the patient materials and/or clinician training improve patient adherence with psoriasis treatments or increase engagement with health care, then our evaluation may underestimate the health benefits of the PSO WELL intervention and underestimate its relative cost-effectiveness. However, improved adherence or engagement may also mean that people with psoriasis use services more intensively and/or use a wider range of health-care services. This would increase the costs and reduce the potential cost-effectiveness of the intervention.
The potential role and effectiveness of the patient education intervention is limited in the model. Its only positive effect is that a patient may choose to seek a GP consultation. In reality, psoriasis education might benefit patients more broadly. This could be by helping patients to manage their psoriasis and adhere to treatment. Patients may also be motivated to change their lifestyle. The benefits of these changes are not captured in the model, which may underestimate the cost-effectiveness of the PSO WELL intervention.
The clinician intervention is more influential in the model; however, we assume that its benefit expires after 1 year, a potentially conservative assumption that underestimates the cost-effectiveness of the PSO WELL intervention. The scenario analyses indicated that increasing the time for which the training is effective decreases costs and increases patient health benefit.
The probability that a clinician acts in accordance with the guidelines affects participants’ health in terms of the effectiveness of the treatments they receive. There was limited information to inform the estimate of whether or not a clinician acted in accordance with the guidelines. For the primary analysis, it was assumed that 78% of PSO WELL-trained clinicians would use treatment guidelines appropriately, compared with 59% of clinicians who did not receive the PSO WELL training. 130,164 If the difference in appropriate use of treatment guidelines is lower than assumed, the results will overestimate the cost-effectiveness of the PSO WELL intervention. If the difference is higher than assumed, our results will underestimate the cost-effectiveness of the intervention. The scenario analyses indicate that this is an important variable in the model, suggesting an area for further evaluation of the intervention.
An important consideration is how to appropriately distribute the cost of the clinician intervention to accurately estimate the cost-effectiveness of PSO WELL The true cost-effectiveness depends on the number of psoriasis patients able to benefit. The present primary analysis assumes that each clinician will be able to apply their new skills and knowledge to only one patient, following training, which is a conservative, minimum figure. The threshold and scenario analyses indicate that the cost-effectiveness of the PSO WELL intervention is sensitive to the number of patients who could benefit from clinician training. Further research is needed to clarify the number of patients with whom each clinician could realistically use their knowledge and skills.
Finally, only one intervention is included in the analysis: the package of patient materials and clinician training combined. This is owing to the low cost per patient of the patient information materials component of PSO WELL and limited information about the relative effectiveness of the patient materials compared with clinician training. As further evidence becomes available it will be possible to evaluate each of the components individually.
Despite these limitations, our model provides initial information to inform future research and evaluate the PSO WELL intervention as new evidence becomes available. The model also provides a structure that incorporates the complexities of psoriasis treatment and interactions between patients and clinicians that affect the effectiveness and cost-effectiveness of any treatment. The model can be adapted to evaluate new approaches to psoriasis management that address the range of issues found to affect patient care that were identified by the IMPACT programme. Given this, the analysis presented provides a valuable preliminary estimate of the health economic outcomes associated with the primary care management of psoriasis as a complex condition, and of the PSO WELL interventions.
Summary of the health economic analysis
We undertook a systematic review of cost-effectiveness analyses, which found that there was little consensus about the cost-effectiveness of treatments and care strategies for individuals with psoriasis. The analyses were limited by a lack of quality evidence and frequently provided only a limited reporting of methods. We developed a new microsimulation model to evaluate the potential economic acceptability of the PSO WELL interventions developed. The simulation model utilised inputs from parallel IMPACT programme workstreams, the systematic literature review, clinical guidelines and expert opinion within the project team. The model was used to provide preliminary estimates of the economic outcomes, including cost-effectiveness, associated with the PSO WELL interventions. The exploratory analysis indicated that the interventions developed in this programme of work have the potential to be cost-effective and identified areas where more work is needed.
Key conclusion
The PSO WELL intervention was found to be associated with a small QALY gain per patient and a net cost comparable with TAU; however, the cost-effectiveness of the PSO WELL intervention depends on how many patients are expected to benefit from improvements in clinician knowledge and skills and how long those improvements are expected to be maintained.
Implications
-
If each of the clinicians trained as part of the PSO WELL intervention routinely managed more than one psoriasis patient and more than one psoriasis patient benefited from that training, the intervention cost per group trained could generate health benefits more efficiently. Our analysis suggests that the combined patient and clinician interventions may be a cost-effective use of NHS resources if a typical group of 10 clinicians trained manages around 22 psoriasis patients.
-
We recommend research into the following areas to improve the data available for future economic analyses and to reduce uncertainty about the results:
-
We recommend quantitative and qualitative research, including integrated economic and clinical randomised controlled trials, in the following areas – assessment of the number of patients likely to benefit from clinician training and the time the effectiveness of training is likely to last.
-
Quantification and modelling of the benefits of the patient information materials on patients’ ability to manage their psoriasis and broader health.
-
Evaluation of how clinicians who do not follow published national guidelines select treatment and what treatments are typically used in this situation.
-
Evaluation of the impact of the PSO WELL interventions on patient adherence and engagement with psoriasis treatment and health-care services and clinician adherence/concordance with treatment guidelines.
-
Extending the model to include new evidence and incorporate contraindications to treatment as well as the role of dermatology clinic nurses.
-
Patient, public and practitioner involvement in the IMPACT programme
Introduction
Patient and public involvement in research is a core principle of the National Institute for Health Research (NIHR) and a PPI strategy is a necessary component of NIHR grant applications. PPI in research has been evident since the 1990s and is now accepted widely in the health research arena. However, during the development and submission of the IMPACT programme grant application, advice and support for investigators in relation to PPI in research was relatively sparse. Over the course of the IMPACT programme, there has been a rapid growth in PPI advisory groups and guidance. 197 Consequently, choosing the right PPI strategy for the IMPACT programme and enabling it to evolve over time has not been without challenges. Lessons have been learned along the way. Nonetheless, the IMPACT programme can report several important PPI successes, including a stable RUG of 20 members and growing, a website with a worldwide following and a highly commended public engagement series of events focused on psoriasis.
The IMPACT programme benefited from establishing early links with the Psoriasis Association – the largest national organisation representing patients with psoriasis. In addition, some members of the research team had already developed or contributed to patient research/community health action groups in other settings. This was a good foundation and ensured an enduring commitment to understanding and incorporating the patient viewpoint into the IMPACT programme. This commitment was visible in both the design of the research programme and its PPI structure. This chapter describes (1) the PPI structure of the IMPACT programme, (2) the involvement, participation and engagement with patients and the public that occurred throughout the programme and (3) the outcomes and the reflections of those involved in the PPI work.
The INVOLVE definitions of involvement, participation and engagement198 are used throughout this report, which are as follows:
-
involvement – members of the public are actively involved in research projects and in research organisations
-
participation – people take part in a research study
-
engagement – information and knowledge about research is provided and disseminated.
The term ‘public’ in PPI is generally used to describe patients, potential patients, carers and people who use health and social care services, as well as people from organisations that represent people who use services. PPI specifically does not include the perspectives of people who have a professional role in health-care services. However, for the purposes of this report we also include non-academic health-care professionals within our remit for a number of reasons. Part of our research focused on implementing changes to health-care practice, thus making health-care professionals’ activities the target, as well as the focus, of the research. Professionals have also taken part in IMPACT programme activity in various guises: as advisers for our research strategy, as research participants and as recipients of research results. Health-care professionals were therefore directly affected by the IMPACT programme work, and raising awareness of optimal psoriasis management among all clinicians involved in the care of this patient group continues to be a goal of this and future research for our team.
Involvement
Pre-grant award PPI: patient representative co-investigators
Between 2007 and 2010, prior to the grant award, a former CEO of the Psoriasis Association, Gladys Edwards, and Toni Doyle, local NHS manager for patient experience, were co-applicants on the grant application and were involved in the development of the proposal. In fact, Gladys Edwards provided continuous encouragement and expertise during the grant writing and development process. Figure 27 shows the timeline between the initial proposal development and receipt of funding.
FIGURE 27.
The IMPACT programme timeline from proposal development to funded programme of research. PGfAR, Programme Grants for Applied Research.
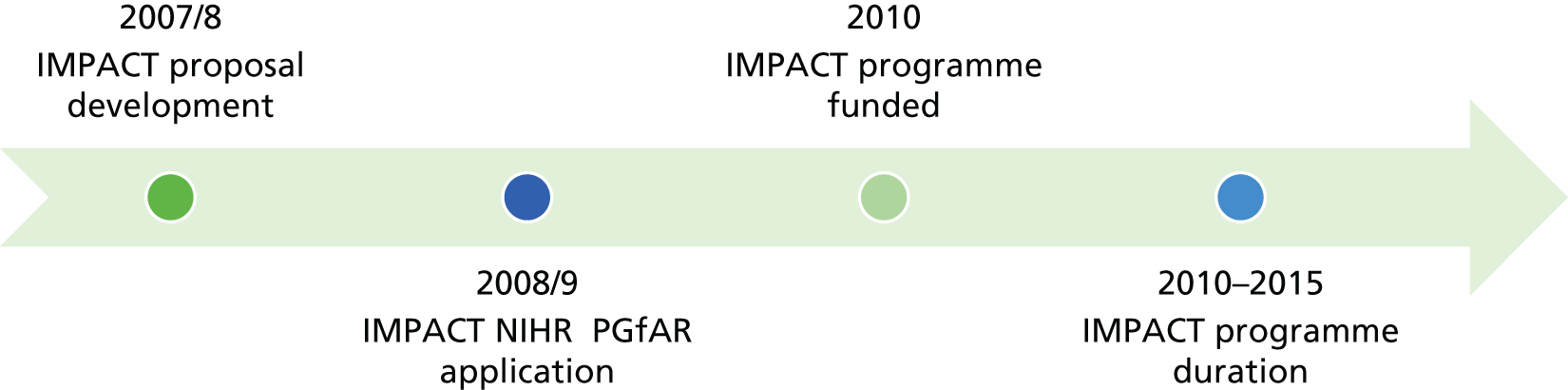
Helen McAteer replaced Gladys Edwards as CEO of the Psoriasis Association in 2010 and has been closely involved in all aspects of the IMPACT programme to date. The team has therefore been committed to PPI from inception, and the contribution of patient representatives as co-investigators from this early juncture has enabled valuable lessons to be learned, which will undoubtedly improve involvement and engagement with PPI partnerships in future research projects.
Post-grant award PPI: recruiting for involvement
Programme launch event
Patients and their relatives attended a launch event at the start of the programme, which had been advertised in the three local dermatology centres. The event consisted of a series of presentations, refreshments and an opportunity for patient and professional attendees to ask questions.
Research user group
During the first 12 months of the IMPACT programme, Toni Doyle and Alison Littlewood, IMPACT Programme Manager, developed the IMPACT and psoriasis RUG. Posters and flyers were designed to advertise this new PPI group. All advertising material was checked by the Salford patient information group before use. Posters were displayed in general practice surgeries and dermatology clinics across the three partner PCTs (Salford; East Lancashire; and Ashton, Leigh and Wigan) asking for individuals with an interest in psoriasis research to join the new group. The Psoriasis Association also advertised the group on its website and in its quarterly magazine Pso. Uptake was initially slow, so, to improve reach, pharmacies across the patch were asked and agreed to include in all prescription bags (including in those containing non-psoriasis-related medication) mini flyers advertising the RUG.
The IMPACT programme steering committee patient representatives
In addition to the RUG, Alison Littlewood and Toni Doyle advertised the role of steering committee patient representative. A patient representative advertisement was used to find three representatives who could contribute objective and balanced advice to the IMPACT research programme, drawing on personal experience of psoriasis and the wider experience of people with psoriasis. Figure 28 shows the components of PPI both pre and post grant across the IMPACT programme.
FIGURE 28.
The PPI roles that have contributed to the IMPACT programme of research.
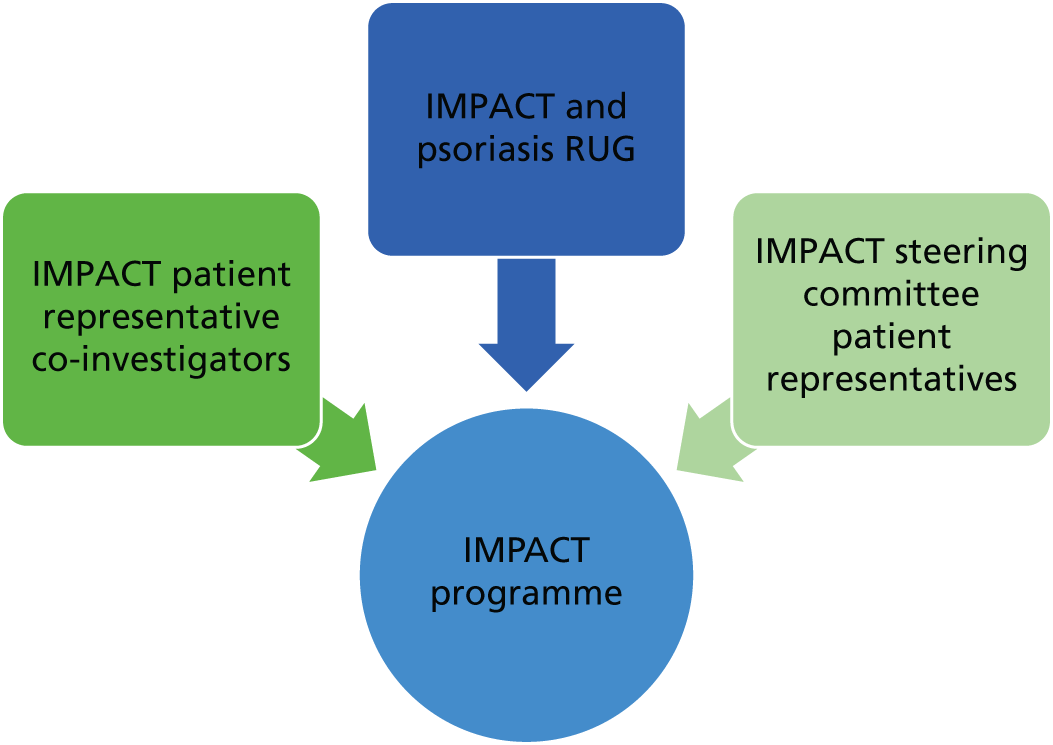
Finding the right people: research user group and patient representatives
Primary criteria for membership of the IMPACT RUG were as follows: (1) having an active interest in psoriasis – either having psoriasis or caring for someone with psoriasis, (2) being located in the north-west of England and (3) having an interest in advising psoriasis research investigators with the aim of improving care for people with psoriasis. Three steering committee patient representatives were recruited from the existing RUG and from a local Psoriasis Association support group. These representatives were invited to join the IMPACT steering committee for biannual meetings, one of which included an annual meeting overseen by the independent expert advisory panel.
Methods of involvement: patients, the public and practitioners – maximising participation
Research user group
Finding the best time to meet
In the early stages of establishing the RUG, recruitment was challenging and membership (as well as meeting attendance) fluctuated. To address this, an ‘expression of interest’ form was devised for interested individuals to download from the IMPACT programme website or request by phone from Alison Littlewood. This form collected individuals’ contact details, current age and age at psoriasis onset and, to facilitate more appropriate scheduling of events, also gathered people’s preferred attendance times for meetings. The information gathered indicated that members’ preferences varied, so meetings were held at different times, including early evening to make it more accessible for those working during the day. It proved difficult to attract younger people to the RUG and, although varying the time of meetings encouraged more young people to come forward, it has remained challenging to engage patients in this age group.
Reimbursement
The RUG members’ travel expenses were reimbursed and they received a fee in line with the payment structure suggested by INVOLVE199 for specific activities. The university reimbursement procedure proved slow, with some payments taking an unacceptability long time to be made (in some instances, months after the meeting). In addition, the forms that participants were required to complete were complicated and off-putting for some participants and our team were concerned that this could be a potential barrier to future involvement. To alleviate delays, the programme manager organised a cash advance process to ensure that all members could be paid their fee or reimbursed travel expenses in cash on the day on which they attended a meeting.
Research user group meeting format
To ensure that meetings were viewed as accessible and engaging, the format/style was informal, with questions from the RUG encouraged throughout. At each meeting, research team members were in attendance to present an aspect of their research and interact with participants so that each could get to know the group better and vice versa. Interactive workshops were held to address specific areas of the research programme in more depth (this was particularly helpful during the development of the patient and practitioner interventions in workstream 5) and guest speakers such as Professor Chris Griffiths and Helen McAteer were invited to the meetings when appropriate.
Training
The initial RUG meetings included a ‘training’ element to incorporate an overview of the aims and objectives of the IMPACT research programme and explore INVOLVE’s ‘opportunities for involvement’ cycle. 199 Training on meeting participation and the research process was included on both a formal and informal basis, often addressed as a topic within a broader meeting. In addition, some members attended PPI events organised by the Citizen Scientist initiative at Salford Royal NHS Foundation Trust.
Research user group characteristics 2011–16
The first RUG meeting was held in October 2011 and, over the course of the programme, meetings were held three or four times per year, with various members of the IMPACT team in attendance, depending on the focus of the discussion.
As of 2019, the RUG comprises approximately 20 members (and this has been a fairly consistent size for the group) who contribute to the research both at meetings and via e-mail. There is a good gender balance in the group, which reflects the fact that the prevalence of psoriasis is similar in both sexes, and membership includes black and minority ethnic individuals. A major challenge has been to extend the age range to include more young people (aged < 25 years) and to ensure social and ethnic diversity in the RUG, particularly in relation to educational attainment. We undertook a separate project to examine the difficulties for younger people: it appears that the self-consciousness associated with visible psoriasis is particularly impactful at a younger age, with individuals expressing major concerns about the idea of mixing with strangers.
The current RUG membership has stabilised and there is a well-established partnership between the RUG and the IMPACT programme researchers; however, there is also a steady flow of interest from new members through our website contact form.
The IMPACT programme patient representatives
Recruitment and development of the role
The three steering committee patient representatives attended initial meetings with research team representatives and the programme manager. During the meeting, we outlined the IMPACT programme aims and methods, committee meeting procedures and the expectations of the patient representative role, including the terms of reference (see Appendix 12). These define the purpose of the steering committee and representatives’ responsibilities. To facilitate the involvement and participation of each patient representative, a ‘buddy’ system was implemented. Karen Kane took on the role of managing this system and worked closely with the group before, during and after meetings. Figure 29 gives an outline of the IMPACT programme management and reporting structure.
FIGURE 29.
The IMPACT programme management structure.
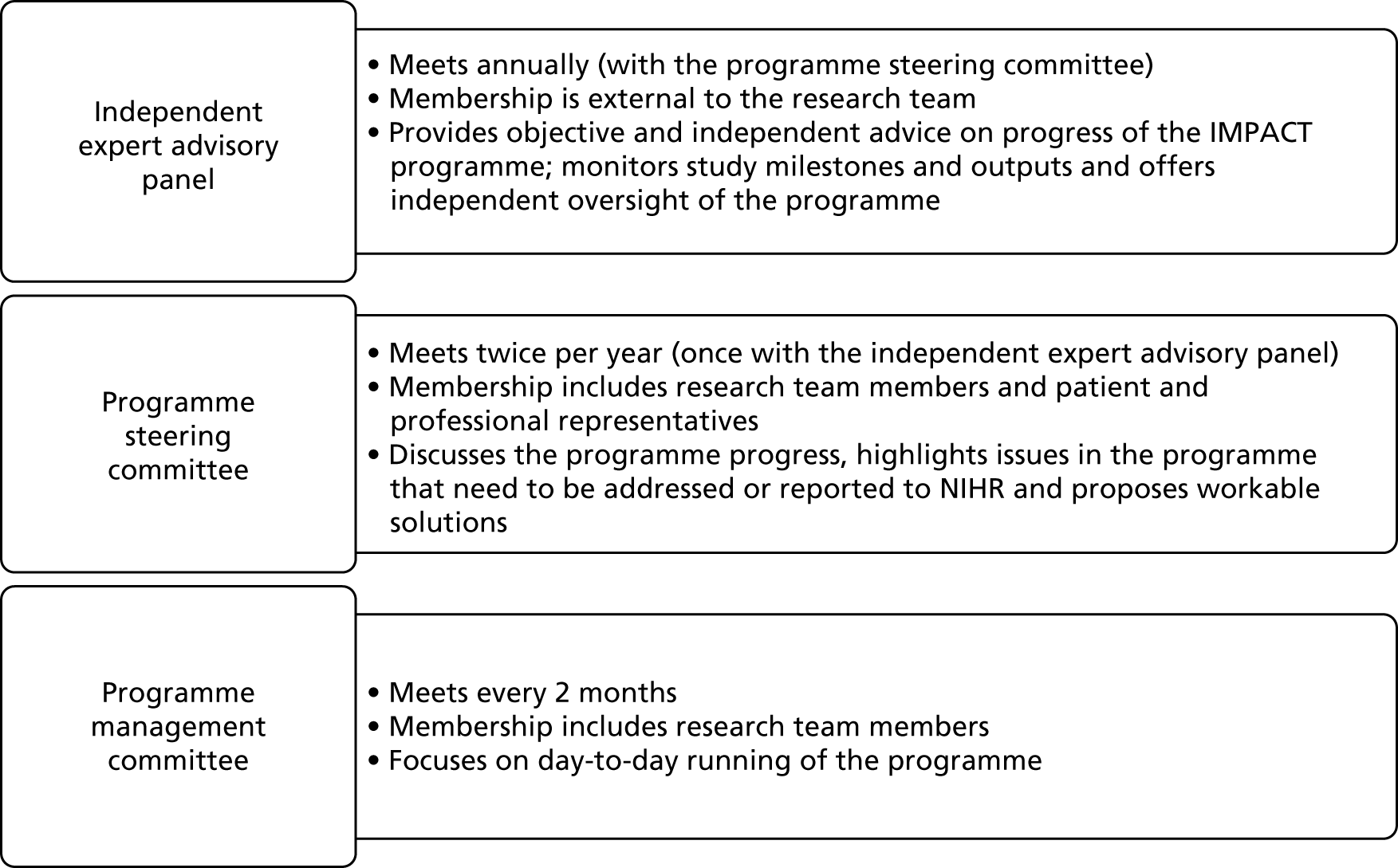
Professional representatives: expert group consultation
To gain an insight into health-care professionals’ perceptions about the work being conducted in the IMPACT programme, three professional representative groups were invited to provide their views and opinions via face-to-face group meetings or telephone interviews. Members of the IMPACT programme team engaged with specialist dermatology nurses, consultant dermatologists and GPSIs in dermatology. The core aims of these ‘expert group consultations’ were to (1) identify professionals’ views about the ongoing work, (2) highlight important issues in psoriasis care that remain unaddressed by the IMPACT programme and (3) discover what professionals believe services for people with psoriasis should ideally look like. Together, findings from these consultations were used to guide the IMPACT programme team in gauging how specific research workstreams were identifying gaps and challenges in current care and how future research could be shaped to address remaining challenges.
Expert dermatology nurse group
In February 2013, several members of the IMPACT programme team attended a psoriasis expert group workshop facilitated by IMPACT programme co-investigators Christine Bundy and Lis Cordingley. The group comprised six specialist dermatology nurses with extensive experience in the field. Some members of the group were responsible for developing the specialist dermatology nurse’s curriculum as part of the BDNG. This expertise helped the IMPACT programme team gain valuable insights into the current training curriculum.
The workshop provided an opportunity to share knowledge and ideas on how IMPACT programme workstream 5 could progress with regard to developing training for health-care professionals managing people with psoriasis. The expert group provided vital insights around the training needs of clinicians, particularly for practice nurses and DSNs, to develop the workstream 5 training package. Discussions about curriculum development were also useful in informing the work of workstream 4 in relation to health-care professional training needs in the context of supporting lifestyle change in patients with psoriasis.
Consultant dermatologists
During 2013 and 2014 there were four opportunities to speak to consultant dermatologists about their views on how training for dermatologists could be developed. Two of these were preceptor meetings (in which expert dermatologists provide in-depth training and supervision) run from a designated International Centre of Excellence at Manchester (Salford Royal NHS Foundation Trust) to train specialist dermatologists in specific aspects of psoriasis management. They are focused largely on management of severe psoriasis. The programmes are attended by around 40 delegates, on average. Other opportunities arose at two UK dermatology national conferences at which members of the IMPACT programme research team were presenting early findings. Dermatologists were asked what key elements needed to be included in an effective training programme to broaden the assessment and management of psoriasis and comorbidities. They were also asked about the training formats that they favoured and which locations were most likely to facilitate attendance. Dermatologists indicated that they were familiar with skills-based training and that this, rather than lecture-based training, was preferred in most cases. Many identified the barriers to communication between primary care and secondary care services, including the limited information that is passed to secondary care at the point of patient referral.
General practitioners with a special interest in dermatology
During July and August 2013, 20 GPSIs practising across the UK were invited to participate in a telephone interview with one of two members of the research team: Anna Chisholm or Christina Pearce. Twelve GPSIs took part and provided insights that usefully informed the work being conducted in workstream 4 (lifestyle behaviours in psoriasis) as well as the development of workstream 5 (health-care practitioner training). In relation to supporting patients with LBCs, GPSIs reported that knowledge of the relevance of lifestyle factors in psoriasis management was increasing, but that people with psoriasis are not routinely provided with this kind of support. As this insight corresponded with findings from workstreams 2 and 4, the GPSIs were prompted during the interviews to consider how services in this area could be improved.
The GPSIs identified a range of ways in which current psoriasis services could be improved to ‘integrate’ LBC support, including (1) what services should involve (e.g. patient management plan cards, longer consultations, psoriasis update training, communication skills training), (2) how improvement could be achieved (e.g. in-house training, 3-day courses, website or e-learning, establishing champions for more ‘integrated’ services), (3) where psoriasis services should be delivered (e.g. primary care settings, social or community centres, secondary care) and (4) who should deliver services (e.g. multidisciplinary teams, specialist nurses, whole practices, psychologists or lifestyle specialists). The information gathered from this consultation exercise enabled the IMPACT programme investigators to gauge the applicability of IMPACT programme findings to current real-world contexts, and to shape the design and implementation of the workstream 5 health-care practitioner training.
Patient representatives: expert group consultation
In addition to consultations with professional representatives, the team ran a series of workshops to consult patients on the development of the workstream 5 intervention. Three interactive workshops (two in 2013 and one in 2014) helped the team primarily develop and refine what would become the PSO WELL patient materials intervention [details in Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management]. Two further workshops were run with our health technology consultants in 2017 to assess the viability of the proposals for creating the PSO WELL online platforms. During the 2014 workshops, patients were further consulted about the development of the PSO WELL practitioner intervention [details in Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis].
The aim of the expert group consultations was to enable patients affected by psoriasis to identify what was missing from current services to facilitate a more ‘holistic’ or ‘integrated’ approach to managing the condition. At the 2013 meetings, patients identified key elements for management as follows:
-
regular examination of the impact of psoriasis on skin but also on life (i.e. appropriate assessment)
-
regular review of treatments and efficacy
-
help for patients to learn how to self-review, including knowing how mood affects psoriasis and vice versa
-
support for patients to understand how to use medications in the right way
-
shared management (between clinicians and patients), with equal weight being given to each party’s expertise as well as filling each other’s gaps in knowledge
-
help to identify and practise coping strategies to address the impact of psoriasis in different areas of life
-
seeing psoriasis care as long term with strategic lifelong planning but emphasising hope for the future
-
accurate information and support on general health, nutritional aspects and fitness (at different levels and in different formats, e.g. written/web based)
-
support for practitioners to develop knowledge and skills in psoriasis management (e.g. to better understand psoriasis, the potentially distressing nature of the condition and the role of mood; to be able to talk with patients without embarrassment about appearance/intimate concerns and to support them sensitively with risk/lifestyle factors and address these issues in ways that are tailored to the individual; to be able to discuss treatment options knowledgeably).
At the 2014 meeting, early versions of the newly developing PSO WELL patient materials (based on findings from workstreams 1–4 and the contributions gathered at the 2013 patient workshops) were shared with participants so that they could contribute to the next iteration of the materials. Figure 30 shows patient representatives actively contributing to this work as part of the 2014 workshop.
FIGURE 30.
A RUG meeting: focus on IMPACT patient materials. Permissions obtained from all attendees.

Key points highlighted by the expert group participants were as follows:
-
tone was kind; messages were accurate, hopeful and not ‘scary’
-
easy to read; ‘empowering’
-
the term ‘relapsing and remitting’ condition was novel and interesting
-
links between the immune system and mood needed to be drawn out more
-
mood topics needed some rewording and reordering to sound more positive and balanced
-
visibility of the condition should be mentioned in the section referring to mood and psoriasis
-
information on emollients was new
-
addition of emoticons/icons would aid understanding
-
the idea that ‘if a treatment is ineffective, that’s OK, you can go back’ was new and could encourage confidence to ask for different treatment
-
lifestyle information was empowering – actions you can take for yourself
-
tips on habit-breaking in the alcohol section should be included
-
action planning sections should come full circle, for example include follow-up or revisiting goals
-
information on ‘who could help you’ is needed.
Views from attendees about the early version materials offered invaluable learning for the IMPACT programme researchers to take forward in the next phase of intervention development.
Participation
In the IMPACT programme of work, research involvement and research participation have influenced each other. As the research has evolved, the team has expressly taken a proactive, flexible approach to the design and conduct of later studies on the basis of what was learned from participants in earlier stages. Specifically, in workstreams 2, 3 and 5, patients were research participants through in-depth qualitative interviews to gather perspectives on living with psoriasis and experiences of health-care services. In addition, clinicians took part in workstream 4, which gathered their perspectives on providing psoriasis care. Lessons learned from each of these studies influenced the way other parts of the programme were conducted.
For example, participants in workstream 3 sometimes indicated that they had a limited understanding of psoriasis and its treatments and were experiencing poor levels of support from health-care services, which in some cases resulted in disengagement with health-care services. These reflections influenced the way that the researchers (who were now alert to the possibility that participants may be reluctant to take part in research through health-care services) approached recruitment/engagement in workstream 2. Our approach was to take nothing for granted, recognising that patients may feel disempowered or neglected, and make extra efforts to engage and recruit them by going back to first principles and carefully presenting the rationale for any research activity. We also ensured that each researcher coming in contact with people with psoriasis was aware of findings from previous IMPACT programme studies and the need for particular empathy and sensitivity in interactions with patients. In the same way, the experience of interacting with patients and working alongside practitioners in workstream 2 had some influence on the design of workstream 4. The experiential learning as well as the research outcomes from all three workstreams fed into the design and content of workstream 5. The directions of influence between workstreams are depicted in Figure 31.
FIGURE 31.
Directions of participants’ influence on the research.

Findings from early IMPACT programme workstreams encouraged the research team to develop a ‘customer-based’ approach to recruitment and retention on the basis that participants are choosing to ‘spend’ time on a study. Examples of this approach include the inclusion of:
-
reply slips on which a potential participant can indicate how they wish to be contacted (mail, e-mail or telephone) and preferred contact times (morning, afternoon, evening, weekdays or weekends)
-
good-quality study documentation (professional graphic design and print)
-
timely responses to telephone and e-mail queries and feedback
-
free-text boxes at the end of questionnaires for feedback and general comments.
Giving participants the opportunity to feed back to the IMPACT programme team throughout the research process meant that timely and often useful small adjustments to the research plan could be made without affecting the fidelity of the study. For example, we instigated an appointment telephone and text reminder service for the CVD risk screening study of workstream 2 after a number of participants told us that they now relied on reminders for medical appointments. We also started to include a summary sheet of the most frequently asked questions after many people told us that the four-page patient information sheet was too long to read. We were particularly pleased to receive this patient feedback on the final workstream 5 participant questionnaire:
I have really enjoyed taking part in this research which I hope will have a positive outcome in terms of future benefit to psoriasis patients. I am surprised to say that in a strange way this research, especially the leaflets, has made me feel more valued.
Workstream 5 patient participant
Engagement
The internet and social media
The IMPACT programme website [www.impactpsoriasis.org.uk (accessed 12 October 2019)] is a platform for communication, information and dissemination. Study news, updates and recently published work are regular features and it is a place where members of the public can find out more about the IMPACT programme mission. Since October 2014, when the website was relaunched, there have been > 6000 new visitors to the site from across all continents and > 1000 hits on the information specifically aimed at patients, members of the public and practitioners.
To engage with the wider social media community both in the UK and internationally, the IMPACT programme Twitter (Twitter, Inc., San Francisco, CA, USA) account, @impactpsoriasis, has been an important tool for news and networking. A range of information is publicised via this account, including study updates and psoriasis-specific information of interest to our followers, who include members of the public, health-care practitioners and individuals and organisations interested in dermatology research. We have > 1400 followers worldwide.
Manchester Psoriasis Shout Out!® 2014: a festival bringing academics, researchers, clinicians, patients and members of the public together
The Manchester Psoriasis Shout Out!® (a collaboration between University of Manchester, Salford Royal NHS Foundation Trust, and Manchester Academic Health Science Centre) was a week-long series of events in 2014 aimed at raising awareness about psoriasis, engaging members of the public in psoriasis research taking place in Greater Manchester and discussing opportunities for involvement. This event was also a great chance to listen to thoughts and views from members of the public and health-care practitioners and to disseminate IMPACT programme findings.
As part of the Manchester Psoriasis Shout Out!, the IMPACT programme team held a practitioner-focused networking event. This was a chance to showcase research findings directly to health-care practitioners managing patients with psoriasis and also introduce the workstream 5 PSO WELL clinician training package developed from IMPACT programme phase I (workstreams 1–4). The opportunity for practitioners to ask questions about the training package and register interest in taking part in the workstream 5 study formed part of our recruitment strategy.
Members of the RUG, the patient representatives and the local Salford Psoriasis Association group attended events and assisted in staffing (together with researchers and clinicians) the Manchester Psoriasis Shout Out! trailer during the week. Photographs from the event are included in Figure 32 and some of the RUG members took part in the films created for the event, which can be found online [www.psoriasisshoutout.co.uk/#!films/c1ql4 (accessed 12 October 2019)].
FIGURE 32.
Photographs of events undertaken as part of Manchester Psoriasis Shout Out! 2014. (a) The IMPACT workstream 5 PSO WELL launch at the Midland Hotel, Manchester. Pictured left to right: Alison Littlewood, Christine Bundy, Christopher EM Griffiths and Lis Cordingley; (b) the Manchester Psoriasis Shout Out! trailer in St Ann’s Square, Manchester. Pictured: Karen Kane; (c) the Manchester Psoriasis Shout Out! flash mob in full flow, St Ann’s Square, Manchester; (d) the Manchester Psoriasis Shout Out! poetry event, Cornerhouse, Manchester; (e) patient and public question and answer session: ask the experts, Salford Royal NHS Foundation Trust. Pictured: Christopher EM Griffiths; (f) the Manchester Psoriasis Shout Out! fashion show finale, Trafford Centre, Manchester. Permissions obtained for photographs.






Outcomes and reflection
The outcomes of PPI are often experiential rather than quantifiable for all involved (patients, patient representatives, the public, researchers and managers) and therefore are difficult to measure. 200 With this in mind, we include some reflections of those involved along with more concrete outcomes.
The flow chart in Patient and Public Involvement in Health and Social Care Research: A Handbook for Researchers199 shows different stages of incorporating PPI into the research process; we have used this as our basis for describing outcome and reflection.
Identifying and prioritising, design, and development of the grant proposal
Identifying and prioritising, design, and development of the grant proposal are grouped together as these three stages are not linear activities but an iterative, interconnected process in which earlier stages continue to be revisited until the grant proposal takes its final form. In the early stages of the IMPACT programme proposal, Gladys Edwards and Helen McAteer, representing the Psoriasis Association, were involved in identifying and prioritising research questions to be addressed in the grant application along with the design of research questions and methods. Helen McAteer reviewed participant recruitment strategies and, once the IMPACT programme was funded, supported recruitment by providing information to the Psoriasis Association membership on how to participate in workstream 3, ‘coping with psoriasis’, which yielded a good response. From the outset, there has been patient representation, with Gladys Edwards, Helen McAteer and Toni Doyle all key co-investigators throughout the development of the grant proposal. At the very start of IMPACT programme development, a Psoriasis Association survey about poor experiences of care was a major influence on the IMPACT grant application. The NHS patient experience representative ran a series of patient experience meetings (including dermatological conditions) across Salford PCT. The challenge to engaging in PPI work in the design and development stages is establishing a wider group of patient advisors in the area of research and not only those who are already members of active patient associations when few funds are available and fixed-term research staff are not yet in post because the grant is not yet awarded. 201 The RUG and the patient representatives on the newly formed steering committee in effect had less influence over the core design of the first four workstreams of the IMPACT research programme because these had already been planned during the grant application process. However, the design and content of workstream 5 was developed using both the evidence gathered from research participants in earlier workstreams as well as the input of the RUG members (including the patient representatives on the steering committee). The perception that it is not possible to influence a programme of activity that is already in motion can be frustrating. This has been reported by patient advisors recruited to projects that have already been established202 and has also been expressed by IMPACT programme patient representatives during the course of the programme. As this current programme of research ends, we have come full circle through all the research stages and can consider what should be done next. We continued to develop our ties with the Psoriasis Association and, in addition, developed a local, ‘grassroots’-level RUG group, which has continued to engage and develop.
Undertaking and managing
As depicted in Figure 29, the IMPACT programme management structure included patient representation at both programme management committee (HM and TD) and programme steering committee (HM, TD and three patient representatives) level. This was crucial to the running of the research programme.
Members of the RUG have commented and advised on several aspects of the research, including recruitment strategies, questionnaire content and design, and participant information. These are in addition to the development of the workstream 5 PSO WELL patient resources designed to support patients with psoriasis to self-manage in better ways. Prior to the formation of the group, local Psoriasis Association members commented on workstream 3 recruitment materials and an established primary care user group was contacted to comment on workstream 2 recruitment materials.
Although commenting on patient information sheets and other study documents can be seen as tokenistic (which, if there are no other opportunities for involvement, may be more likely), having patient/public input into these is a valuable adjunct to the work of the researchers. Regardless of the research team’s writing skills, testing study documents with people who have experience of the particular condition can often increase the relevance of documentation to that specific patient group, which in turn makes a successful study more likely. Such reviewing activity often plays to the strengths of some RUG members whose day-to-day life, training and employment enable them to use well-developed editorial skills to enhance research materials that are aimed at patients.
It is difficult to measure the impact of this type of input in a quantifiable way, though it is suggested that those studies that use PPI in some form are more likely to reach 90% of target recruitment. 203 Workstream 2 reached 90% of its target recruitment and all the qualitative parts of the programme recruited and sampled widely to reach data saturation on main themes. Although this cannot be attributed entirely to RUG involvement (other strategies to increase participation such as widening our geographic area of recruitment were introduced),204 the expertise of RUG members in reviewing procedures and materials for the study undoubtedly contributed to successful recruitment.
An often forgotten outcome of this type of exercise may be the enduring effect on researchers. As researchers, we learn through secondary experience (gained from the RUG group and participants in the study) what it feels like to receive, make sense of and respond to the documents we send out and the procedures we ask to be completed. This continuous exposure influences the way we construct our participant materials, collect data and think about recruitment in all future research activity.
Analysing and interpreting
Results across the programme have been presented to RUG members at regular junctures to compare the findings with their experiences. It has been an interesting process and we uncovered some knowledge gaps among RUG members, in particular with respect to the existing links between psoriasis and unhealthy lifestyle behaviours. This challenged the assumptions of the researchers that newly emerging evidence about the influences of lifestyle on psoriasis had already been cascaded to and assimilated by patients and health practitioners. This led the team to think more about dissemination and future implementation challenges for the intervention developed in the final phase of the programme.
Dissemination
Timely dissemination during the IMPACT programme has ensured that research findings are published and publicised quickly, with delivery methods aimed appropriately for our wide range of stakeholders. A quarterly IMPACT programme newsletter is distributed to all investigators, researchers, patient representatives, RUG members, professional representatives, independent expert advisory panel members, steering committee members and the research sponsor covering research progress and highlighting findings.
Patient and practitioner dissemination has taken different forms over the course of the research programme.
Patients and the public
The Psoriasis Association has been integral to publicising IMPACT programme work, with regular articles featuring in their newsletter Pso – an opportunity to share information with their membership. Team members have also presented IMPACT programme and associated work at the annual conference of the Psoriasis Association, which is primarily attended by members. In addition, one of our patient representatives, Pat Bowker, also runs a local Psoriasis Association group through which she is able to inform the group of the IMPACT programme’s research and findings.
Following the publication of study results, participant summaries have been written and circulated to those who took part in the research, giving an outline of key findings. Our RUG has been involved in reviewing patient summaries for our website, which cover each workstream.
One of our patient representatives and member of the RUG, Sabera Kazi, presented her experience of being involved in research at a ‘Medicine & Me’ meeting focusing on psoriasis. This event, hosted by the Royal Society of Medicine in association with the Psoriasis Association, was for health-care professionals, people with psoriasis and their families and carers to hear from medical professionals, researchers and fellow patients about living with psoriasis and the latest research.
Practitioners
Health-care practitioners involved in psoriasis management contributed to various aspects of the research programme. This could include working in one of the medical practices involved in workstream 2 and potentially taking part in the CVD risk screening study, participating in workstream 4 qualitative interviews to explore barriers to and facilitators of giving lifestyle behaviour advice or being recruited to take part in the workstream 5 PSO WELL practitioner training intervention. IMPACT programme findings have been published in journals targeting practitioners and also presented at events such as Primary Care Dermatology Society meetings, BDNG meetings, BPS psychodermatology meetings and the BAD annual conference. These outputs have been crucial in getting IMPACT programme findings to those working at the coalface in clinical practice.
Implementation
The input of the RUG and the study participants has had considerable influence on the development of the patient materials, making them relevant and increasing validity. Our ongoing RUG meetings are helping the research team think about future developments and how the final interventions may be tested in a real-world situation.
Monitoring and evaluation
The management structure as outlined in Figure 29 was instigated to ensure that the programme had independent oversight. The independent expert advisory panel met with the steering committee once per year to monitor programme progress. Three patient representatives sat on the steering committee (Pat Bowker, Paul Fitzpatrick and Stephen Kownacki), contributing to meetings and providing oversight from a patient perspective.
We find that, although it may be possible to measure input into PPI in terms of financial and time costs, it is difficult to track all decisions made through the influence of PPI and the specific outcome of those decisions. 200 In addition, it is also difficult to report PPI activity within scientific papers to allow scrutiny by our peers. It has been reported that PPI activity is often under-reported because of limited word count and is not included in journal guidelines. 200,205
The patient representatives and two of the researchers most closely involved with them (KK and AL) used the normalisation process theory (NPT) toolkit to evaluate the role of the patient representative and their place in the programme. 206 NPT encourages exploration of and reflection on the collective and individual work needed to embed a practice in the normal way of working, allowing potential barriers and enablers to be recognised. This exercise has allowed us to develop lessons to be learned and recommendations for future development of both the RUG and the patient representative role along with more involvement from the research team. To supplement this evaluation, the RUG completed a survey about their thoughts on the structure and remit of the RUG and how both could be improved.
Lessons learned and recommendations
The team has been committed to PPI and engagement from the outset, but has taken time to reflect on and evaluate the various aspects of involvement to identify what could be improved next time. The whole research programme has benefited greatly from PPI, including excellent links with the Psoriasis Association and its invaluable input along the way, the wide range of engagement and involvement activities feeding into the programme, the buddy system implemented to support the three patient representatives on the steering committee and the authentic and honest working relationship developed between the research team and the patient representatives, which has helped with evaluating engagement.
Lessons learned
Several lessons have been learned along the way. Key insights include the importance of:
-
Involving the RUG and patient representatives from the outset. Although IMPACT programme phase 2 afforded opportunity for wider PPI, phase 1 was under way by the time the group was formed, making it challenging for members to fully contribute in the early stages. Involving the group from the grant-writing stage would ensure full participation in the identification and prioritising of research questions. With the psoriasis RUG now established, future programmes will benefit from having close links with this expert group from the beginning.
-
Ensuring an explicit PPI strategy is set from the start of the programme, with key milestones throughout each workstream, to which the PPI members and research team are fully committed.
-
Implementing a more defined reporting structure between the patient representatives and the RUG members to incorporate wider viewpoints and for the patient representatives to use the RUG as a sounding board for some of their ideas.
Implications for PPI in further research
The IMPACT programme PPI experience has highlighted a number of considerations for PPI (including the involvement of health-care practitioners) in research projects and programmes:
-
establishing a clear PPI strategy (when, where, how, who, etc.) as early as possible to encourage ownership and commitment from stakeholders
-
setting clear terms of reference for PPI to clarify obligations and rights within the research project (e.g. expectations for meeting attendance and contribution, rights to information/acknowledgement)
-
setting goals for PPI alongside an evaluation framework to facilitate understanding of the tangible as well as hidden benefits (or, indeed, unintended consequences) associated with PPI in research
-
implementing a robust reporting structure between PPI groups and the research team
-
keeping PPI visible on the research programme agenda by ensuring activity is highlighted at each FIM, and all members of the team link with the programme’s PPI activity in the course of the research
-
making sure appropriate training is available for PPI members and researchers to maximise participation (e.g. what PPI is, getting involved in PPI, research meeting skills, research methods skills)
-
ensuring quick methods of payment/remuneration are in place to minimise the exclusion of disadvantaged groups from participation.
Conclusion: where do we go from here?
The IMPACT programme has incorporated PPI in various forms across all components of its research and the benefits of this involvement are evident in the relevance of research findings to both patients and practitioners. There is no doubt that the PPI partnerships built up over the past 5 years have put us in a strong position to ensure authentic engagement at the start of future research projects and programmes. The RUG has continued to build capacity and links with the rapidly developing north-west PPI infrastructure. Over the past 5 years, the RUG members have developed their competencies and capabilities in parallel with the active researchers and are important partners in the development and delivery of new research proposals. Lessons learned along the way have informed our ongoing research plans and several projects have incorporated and benefited from active PPI partnerships from the outset.
Tales from the heart and the head: lessons from the IMPACT programme
Context and aims of the IMPACT programme application to the National Institute for Health Research
At the time the original application was submitted, interest in and understanding of psoriasis and its comorbidities was undergoing a period of rapid development. In both the academic and clinical dermatology communities, psoriasis had started to be recognised as an incurable complex immune-mediated disease with high numbers of associated comorbidities. Evidence for an association between psoriasis and CVD was growing and a number of influential publications raised the possibility that psoriasis may be a direct contributor to the development of CVD. These publications generated a number of hypotheses such as the ‘psoriatic march’ and counter-hypotheses such as ‘observational bias’ to account for the observed associations.
Others had approached the issue of CV-related comorbidities from a different perspective. There was increasing acknowledgement of the poor lifestyle of many individuals with psoriasis, and over this period a number of researchers recognised the contribution of behavioural factors. These were identified as risk factors for psoriasis onset, psoriasis symptom exacerbation and comorbid CV risk or disease.
High levels of psychological comorbidity and poor quality of life were well recognised by dermatologists; however, these were not well managed even in specialist settings, with seminal work in this field having been undertaken by individuals who initiated the IMPACT programme application. In addition, the links between psychological well-being and behaviours that potentially increased CV risk were starting to be considered. This perspective recognised that poor mood may drive the unhealthy behaviours that, in turn, may increase both psoriasis severity and CVD risk. This standpoint was added to the list of potential hypotheses considered by our research team to account for the increased observed risk of CVD in psoriasis patients. It is important to note that, at this stage, IMPACT programme investigators recognised that these were not necessarily competing hypotheses and that, indeed, multiple pathways to those disease end points were probable.
Although these developments were high on the agenda of psoriasis investigators, they had not reached the primary care setting, nor did they appear to influence the clinical management of psoriasis across settings. Psoriasis was generally treated episodically as primarily a ‘skin complaint’, in sharp contrast to other inflammatory conditions such as inflammatory arthritis or inflammatory bowel disease. There was both anecdotal and some observed evidence that assessment of psoriasis severity was rare in primary care. A report commissioned by the Psoriasis Association revealed a high degree of patient dissatisfaction, and indications that poor assessment of both disease and impact underpinned this response.
Taken together, these elements provided the backdrop to the proposed IMPACT NIHR research programme, the overarching aims of which were to:
-
investigate further the nature of the associations between psoriasis and CV and psychological comorbidities
-
investigate possible clinical prevention and response strategies
-
learn more about patients’ experiences of psoriasis, particularly their experiences of primary care management
-
devise effective responses to this common, incurable condition that affects > 2% of the population.
Clinical and research contexts of the work
The clinical and research contexts in which the IMPACT programme was undertaken have shifted significantly over the course of the research. For example, the emphasis placed on primary care as the focal point for the research needed to be modified, not least because policy changes had consequences for the engagement of general practices. Although this has been recognised for many years, academic and clinical staff highlighted as factors additional issues such as downwards pressure on referrals, worsening morale and GP shortages, all of which served to reduce the motivation of clinicians to engage. In addition, the introduction of CCGs and the end of PCTs changed the nature of relationships between service providers.
In the future, it is possible that other policy developments may provide opportunities for innovations in care. The current NICE guidelines for psoriasis (assessment and management) and the new NICE guidelines for PsA each reinforce some of the messages from the IMPACT programme, namely the importance of screening for and responding to illness-specific patient distress and its negative effects on patients’ abilities to self-manage effectively. At the NHS organisational level, changes such as the establishment of the Greater Manchester Health and Social Care Partnership may allow innovations in service configuration (in terms of both delivery of rapid, personalised patient care and professional training).
Key conclusions from the IMPACT programme
Psoriasis and cardiovascular disease
The IMPACT programme found that there is an increased risk of CVD for patients with severe psoriasis (for definitions of severity see Epidemiology of psoriasis and its association with risk of cardiovascular disease) and that psoriasis patients in general have higher than expected levels of CV risk factors when compared with non-psoriasis populations.
However, importantly and in contrast to the highly cited and influential work of Gelfand et al. , and using the same data source, we concluded that psoriasis alone is not an independent risk factor for CVD. Indeed, our work found that co-occurrence of psoriasis and inflammatory arthritis is a risk factor for CVD. It seems highly likely that the Gelfand study neither identified nor controlled for inflammatory arthritis.
Screening for cardiovascular disease risk
Our team carried out CVD risk screening for patients with psoriasis (aged both < 40 years and ≥ 40 years) attending primary care practices in the north of England. Practitioners were asked to follow their usual CVD screening procedures, which typically involved blood tests and either one or two consultations. Follow-up consultations were usually undertaken by telephone or face-to-face appointments with nursing staff. Primary care screening of psoriasis patients identified a high proportion as being at ‘high risk’ of CVD, with potentially modifiable CVD risk factors ascertained. For example, over half of screened psoriasis patients had a very high waist circumference measurement and 29% had a raised blood pressure. A high proportion of those with previously known risk factors did not appear to be in receipt of effective interventions and those with PsA were at highest risk.
Although these data seem to indicate the potential value of screening, the parallel mixed-methodology arm of the screening study produced new insights into why CVD screening may be ineffective. There is an assumption that if a problem is identified via screening then it will lead to intervention. Our team found very little evidence of clinicians intervening, with many using screening as primarily a data-recording process rather than a ‘teachable moment’ for their patients. In the majority of interactions, health-care professionals did not expand on or tailor the risk information provided for the patient. In some cases, risk reduction was advised; however, specific advice or support was very rarely given. There were very few examples of the use of established behaviour change techniques even for those patients who actively viewed the consultation as an opportunity to discuss changes to their health risk status. Practitioners and patients had different perspectives on the consultation as providing a potential opportunity to intervene. Practitioners often viewed the encounter as unlikely to lead to changes, whereas patients generally saw practitioners as influential in supporting lifestyle management.
Strategies for communicating CV risk information varied widely between clinicians. In general, clinicians were more likely to discuss biomedical risk factors, that is, factors such as hypertension or hyperlipidaemia, most of which are managed pharmacologically. Disappointingly, the percentage of consultations in which clinicians engaged in discussions about behavioural risk factors (physical activity levels, diet, alcohol consumption or smoking) ranged between 27% and 40%. Even fewer of these consultations included specific discussions with patients or interventions to support patients to reduce risk factors.
Capacity of services to reduce cardiovascular risk factors for patients with psoriasis
Even if health-care professionals seeing psoriasis patients had a broad understanding of the CVD risk factors associated with the disease, IMPACT programme research has identified two crucial factors that mitigate against any likely improvement in psoriasis-associated CV risk. The first is a clear deficit in the knowledge and skill sets of both primary and secondary care clinicians in behaviour change techniques. These include a range of evidence-based techniques that are most likely to engage individuals in improved health behaviours. Across all IMPACT workstreams, but most noticeably in workstreams 2 and 4, practitioners indicated that they had received little exposure to behaviour change interventions, which were also absent from the curricula used for both undergraduate and postgraduate training of GPs, nurses and dermatologists. Additional in-depth interviews with practitioners indicated that they held narrow biomedical ‘personal models’ of psoriasis in which management strategies were restrictive and ‘skin focused’. They rarely identified a role for contributory lifestyle behaviours or saw the need to address these.
The second factor is the low level of understanding of the links between lifestyle behaviour, CVD risk and psoriasis outcomes in patients with psoriasis. At one of our earliest patient engagement meetings, participants let it be known that they did not understand why our research focus was on lifestyle health behaviours. Few had been aware of psoriasis-associated comorbidities. A small number had been told to avoid alcohol because of potential interactions with medication (usually methotrexate), but none had been aware of the links between psoriasis severity and weight, for example. The observation study undertaken as part of workstream 4 demonstrated that there were no psoriasis-specific materials for patients on lifestyle behaviours and how they might contribute both to psoriasis severity and to CV risk. Furthermore, even generic LBC materials were either badly displayed or not available in health settings most likely to be used by psoriasis patients.
Thus, IMPACT programme researchers identified correspondingly limited ‘models’ or understanding of psoriasis held by both practitioners and patients. The challenge where patients were concerned would be how to broaden patients’ understanding of psoriasis without raising levels of poor mood or adding to feelings of helplessness and hopelessness, particularly in patients with high (albeit largely unrecognised) levels of illness burden.
Psychological well-being
As anticipated, both the CPRD study and the screening study identified higher levels of low mood than those in matched populations. In the CPRD, just over 4% of those with psoriasis had a concomitant Read code for depression. In the CV screening study, a much larger number of patients reported high HADS scores, indicating likelihood of anxiety and/or depression (data available from programme team). These were also the psoriasis patients most likely to engage in unhealthy behaviours. These findings reinforced reported experiences of our patient RUG members and the in-depth qualitative findings of the workstream 3 study into coping with psoriasis.
Workstream 3 provided new insights into the experiences of people with psoriasis. Although there is extensive research evidence demonstrating that psoriasis is a stigmatising condition that has a major impact on quality of life and well-being, participants in workstream 3 confirmed that this is rarely, if ever, discussed in primary care consultations. The absence of discussions that acknowledged the demands of living with psoriasis or the significant self-management challenges associated with treatment adherence contributed markedly to patients’ feelings of low self-worth. Patients described withdrawing from primary care services and their low expectations of good-quality long-term care. Findings from this workstream also raised questions about how many people have been missed who would fulfil criteria for referral to secondary care because they had disengaged with services or how many remained unaware of the significant developments in psoriasis treatment that have occurred over the last decade.
New paradigm for psoriasis management
Consideration and discussion of IMPACT programme findings, including those undertaken as part of the synthesis phase, consolidated growing awareness of a need for a different approach to psoriasis management in both primary and secondary care. The paradigm shift would be about moving people’s ‘working model’ of psoriasis management from an approach that was ‘skin focused’ to one that recognised psoriasis as a complex long-term condition. This would mean a focus on the relapsing–remitting nature of psoriasis, the management (or even prevention of) physical and psychological comorbidities and, finally, a new recognition of the role of health behaviours in determining the severity and course of psoriasis and comorbidity risks. The team recognised that this is what occurred in the field of diabetes management > 20 years ago, when significant energy was subsequently directed to the transformation of diabetes services.
Following the planned evidence synthesis process undertaken by the team, two broad areas for intervention were identified. The first target was to improve the capacity of the health-care workforce to address the needs of people with psoriasis and the second was to help empower people with psoriasis by giving them a broader understanding of psoriasis including potential comorbidities and how to minimise them. These included an ‘understanding’ or knowledge component, an attitudinal component and a skills component:
-
Knowledge component – the target was to broaden both patients’ and health-care practitioners’ understanding of psoriasis as a long-term condition, with associated comorbidities, and as one in which health behaviours contribute to both onset and severity.
-
Attitudinal component – for health-care practitioners, the target was to raise the profile of psoriasis as more than a ‘simple skin condition’ and as an area worthy of their skilled attention; the target for patients was to transform attitudes of passive acceptance (or even hopelessness) to ones of assertive partnership with their health-care practitioners.
-
Skills component – for practitioners, the skills improvement target was an increased capacity to perform integrated clinical management combining physical, psychological and behavioural components to improve outcomes while recognising the limited time constraints in which they are working; for patients, the skills component was focused on self-management, getting more out of health-care consultations, improved medication adherence and the ability to engage with relevant healthy lifestyle behaviours.
The outcomes of both the patient- and practitioner-focused components of workstream 5 have been very promising. The team identified means of improving understanding, attitudes and skills in both groups as well as providing some solid preliminary evidence for likely mechanisms of action and stakeholder acceptability. Importantly, the lessons learned from this work are likely to be relevant to other long-term conditions and their management. Finally, a practical contribution has been made in terms of the production and evaluation of very high-quality practitioner training materials and patient support materials that are now available for dissemination and further evaluation.
Strengths and limitations
Each of the studies that make up the IMPACT programme had strengths and limitations, which are reported in detail in the previous chapters and published outputs of the work.
A key strength of the programme as a whole included the multiple opportunities for extensive discussion of methods and findings for each study with the whole IMPACT programme team. This was facilitated by the management structure, the FIMs, the independent advisory panel meetings and engagement of patient representatives and the RUG throughout. The process actively supported input to the design and interpretation of each study from all the different research and clinical disciplines and from the perspectives of different stakeholders. The design of the programme meant that, although each study could stand alone, in terms of contribution to the evidence base there were clear synergies between the studies. Both these factors contributed to methodological developments (described in Methodological developments and lessons learned) as well as new evidence and the PSO WELL intervention.
Nevertheless, there are limitations. These include the quality and robustness of the available data for the studies in workstream 1 that used secondary data sources and systematic review methods; identification, selection and recruitment issues that may affect the representativeness and generalisability of the quantitative and qualitative research in workstreams 2, 3, 4 and 5; and time, resource and organisation constraints that limited the design and scope of the quantitative evaluations in studies 2.i and 2.ii and workstream 5.
Methodological developments and lessons learned
Qualitative research in dermatology
The IMPACT programme has resulted in a number of methodological innovations, the most notable being the application of qualitative and mixed-methods approaches to dermatology research for the first time. Qualitative research methods had rarely been used in dermatology research and the IMPACT programme is viewed as pioneering in this respect. Qualitative and mixed-methodology studies of the experiences of both patients and practitioners led to a number of new findings and insights that were subsequently utilised in designing the (workstream 5) interventions.
As well as standard semistructured interview and focus group approaches, qualitative data elicitation techniques included tape-assisted recall in workstream 2 and structured discussions with the multidisciplinary team of IMPACT programme experts to develop the models for the DES in workstream 1. Mixed methodological techniques included a broad range of techniques including completion of mixed open and closed question response sheets by professional ‘simulated’ patients, actors trained to respond as if they were a patient and stimulus response sheets containing a mixture of open and closed items completed by real patients in response to receiving the patient materials as part of workstream 5. The team also recognised that it may be challenging to ask patients to articulate why they found particular aspects of the interventions useful and so developed a system that allowed patients to simply identify sections of materials that they believed had changed their understanding of their condition using symbols. A structured observational study was undertaken to identify the quality and relevance of existing patient materials for individuals with psoriasis. As part of the evidence synthesis phase, stimulus materials were used to trigger structured discussions, which were recorded on specially designed tables; data from these were then collated and used as a focus for the final IMPACT programme workstream.
The visibility, impact and high quality of qualitative and mixed-methodology research outputs led to the IMPACT programme lead qualitative researcher, Pauline A Nelson, being appointed as the first qualitative research section editor of the British Journal of Dermatology. This appointment was heralded by the editor in the most recent British Journal of Dermatology position statement207 and the need for qualitative research in dermatology highlighted in one of Nelson’s early editorials:208
These findings often go beyond ‘static’ description to a dynamic explanation of the data . . . We owe it to patients . . . and . . . professionals to push methodological boundaries in the field, not only making qualitative methods an established component of dermatology research but also moving beyond the more conventionally employed interview and focus group.
Nelson208
Methods summary
The range of methods used enabled the team to apply key steps and processes and issues identified in the MRC framework,156 which was designed to facilitate the development of complex health interventions. These also included testing the use of existing or adapted quantitative measures and the development of new measures in the context of psoriasis. This pioneering work has value to both this team and others undertaking dermatology-related research.
Implications of IMPACT findings
There are a wide range of implications for practice from the findings of the IMPACT Programme Grants for Applied Research programme. These are outlined below in relation to clinicians, patients and services.
Key messages and implications for practice
Clinicians
Several key implications were identified in relation to clinicians:
-
Primary care clinicians were particularly challenging to engage; however, it is crucial that GPs become familiar with and subsequently apply the NICE guidance for psoriasis in their daily practice.
-
There is a need to offset the common perception of some clinicians (in both primary and secondary care) that delivering ‘whole-person’ psoriasis care is additional to their core work and potentially damaging to their relationships with patients. The message that these new ways of working add value but not volume to their existing workload needs to be communicated to clinicians.
-
Together with using motivational interviewing techniques, dermatology staff, with additional training, could identify risks, limit disease impact on physical and psychological well-being and incorporate use of the new patient materials in their psoriasis consultations.
-
The PSO WELL training needs to be embedded in professional curricula (where training in behaviour change techniques is currently missing).
Patients
Several key implications were identified in relation to patients:
-
The need to broaden understanding of psoriasis in a way that provides a logical rationale for effective methods of self-management.
-
The patient materials may act as a prompt to re-engage previously demoralised patients with services.
-
How we provide health messages is important. The design quality of materials, credibility and message-framing are all linked to engagement and effectiveness.
Services
Several key implications for services were identified:
-
The importance of rapid access to personalised health-care provision for people newly diagnosed with psoriasis to prevent accumulation of risk factors for psoriasis-associated comorbidities.
-
Effective recording and sharing of disease activity and comorbidity risk data between services and with patients.
-
Annual screening for CVD risk factors and inflammatory arthritis.
-
The need for rapid and effective dissemination of these research findings to those responsible for the clinical commissioning of services.
-
Existing whole-person care models, such as those currently in place for diabetes management, are applicable to people with psoriasis.
Implications for future research: remaining questions
Although the IMPACT programme has identified and addressed a number of key research issues pertaining to the identification and management of psoriasis associated comorbidities, a number of important questions emerged from the research undertaken that were beyond the scope of the programme, but which remain to be answered.
Epidemiology and mechanisms
From the epidemiological findings, mechanisms by which inflammatory arthritis drives CVD risk are still unknown. Similarly, although we identified links between low mood and risk, the specific direction of the relationship remains unclear. One limitation to the conclusions that could be drawn from the epidemiological work was the need to restrict inclusion criteria to those individuals for whom psoriasis developed in early adulthood (at minimum age of 20 years). There may be a different risk profile (and/or different mechanisms) for those who developed psoriasis in childhood, in whom onset following infection is more common.
The PSO WELL interventions
From the intervention stage (workstream 5), outstanding questions include whether or not (1) practitioners can implement and sustain the learning from the PSO WELL training in real-world clinical situations (and which types of practitioners are best placed to deliver this new way of working to psoriasis patients), (2) they can also incorporate effective use of the PSO WELL patient materials alongside their newly acquired knowledge and skills (and indeed whether or not and how this should be tested), (3) combining the PSO WELL clinician training and patient materials intervention would have a greater effect than using each separately and (4) the interventions are actually cost-effective when applied in practice.
Acknowledgements
We are grateful to all patients, research participants, NHS staff and other stakeholders who contributed to the success of this research.
The IMPACT programme manager was Alison Littlewood. Administrative support was provided by Sue Bailey and Vaila Mallace, with thanks to Gemma Boswell and Shakil Chowdhury.
Members of the IMPACT programme steering committee: Pat Bowker, Robert Chalmers, Paul Fitzpatrick, Karina Jackson, Stephen Kownacki (chairperson), Sabera Kazi and Christopher Main.
Former IMPACT programme team members: Professor Bonnie Sibbald and Mrs Toni Doyle.
Associated researchers: Rachael Thorneloe, Laura Howells, Helen Kitchen, Susie Moschogianis, Alasdair Henry and Gemma Shields.
Graphic designers: Spoken-Image (Manchester, UK) (www.spoken-image.com) and David Webb (http://david-webb.co.uk).
Mapmyhealth Ltd (Nottingham, UK) (www.mapmyhealth.co.uk).
The team would like to thank Salford Royal NHS Foundation Trust and the five former primary care trusts in the north-west of England involved in workstream 2.
Contributions of authors
The corresponding author is Lis Cordingley (https://orcid.org/0000-0001-7675-240X), who is the guarantor of this report and led the writing team of Pauline A Nelson (https://orcid.org/0000-0003-4162-4736), Linda Davies (https://orcid.org/0000-0001-8801-3559) and Christopher EM Griffiths (https://orcid.org/0000-0001-5371-4427). They were responsible for drafting chapters or significant sections of the report, responding to reviewers’ comments and updating subsequent versions.
Additional contributors to individual chapters are given below. Where chapters are drawn from earlier publications arising from this work, readers are advised to consult the original work for details of specific contributions to those works.
Darren Ashcroft (https://orcid.org/0000-0002-2958-915X) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease (lead) and Cardiovascular risk in patients with psoriasis.
Christine Bundy (https://orcid.org/0000-0002-5981-3984) contributed to Understanding the professional role in supporting lifestyle change in patients with psoriasis; Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management; and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis (lead).
Carolyn Chew-Graham (https://orcid.org/0000-0002-9722-9981) contributed to Cardiovascular risk in patients with psoriasis, Cardiovascular disease risk communication and reduction in psoriasis and Coping with psoriasis: learning from patients.
Anna Chisholm (https://orcid.org/0000-0002-2054-7340) contributed to Cardiovascular disease risk communication and reduction in psoriasis; Understanding the professional role in supporting lifestyle change in patients with psoriasis; Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis associated co-morbidities and the role of self-management; and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in psoriasis.
Jamie Elvidge contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease; Valuing the interventions with a stated preference survey; and Health economics modelling of costs of interventions for people with psoriasis.
Matthew Hamilton (https://orcid.org/0000-0001-7407-9194) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease, Valuing the interventions with a stated preference survey and Health economics modelling of costs of interventions for people with psoriasis.
Rachel Hilton contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease; Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis associated co-morbidities and the role of self-management; and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in psoriasis.
Karen Kane (https://orcid.org/0000-0001-8117-8318) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease; Cardiovascular risk in patients with psoriasis; Cardiovascular disease risk communication and reduction in psoriasis; Understanding the professional role in supporting lifestyle change in patients with psoriasis; Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis associated co-morbidities and the role of self-management; Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in psoriasis; and Patient, public and practitioner involvement in the IMPACT programme (joint lead).
Christopher Keyworth (https://orcid.org/0000-0002-7815-6174) contributed to Cardiovascular disease risk communication and reduction in psoriasis and Understanding the professional role in supporting lifestyle change in patients with psoriasis.
Alison Littlewood (https://orcid.org/0000-0002-8766-9190) contributed to SYNOPSIS, Cardiovascular risk in patients with psoriasis and Patient, public and practitioner involvement in the IMPACT programme (joint lead).
Karina Lovell (https://orcid.org/0000-0001-8821-895X) contributed to SYNOPSIS and Valuing the interventions with a stated preference survey.
Mark Lunt (https://orcid.org/0000-0002-2391-5575) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease, Cardiovascular risk in patients with psoriasis and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in psoriasis.
Helen McAteer contributed to SYNOPSIS; Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis associated co-morbidities and the role of self-management; and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in psoriasis.
Dionysios Ntais (https://orcid.org/0000-0002-5853-5548) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease, Valuing the interventions with a stated preference survey and Health economics modelling of costs of interventions for people with psoriasis.
Rosa Parisi (https://orcid.org/0000-0002-0968-9153) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease.
Christina Pearce (https://orcid.org/0000-0002-7393-191X) contributed to Cardiovascular disease risk communication and reduction in psoriasis; Understanding the professional role in supporting lifestyle change in patients with psoriasis; Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management; and Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis.
Martin Rutter (https://orcid.org/0000-0001-6380-539X) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease and Cardiovascular risk in patients with psoriasis (lead).
Deborah Symmons (https://orcid.org/0000-0002-8625-1200) contributed to Epidemiology of psoriasis and its association with risk of cardiovascular disease and Cardiovascular risk in patients with psoriasis.
Helen Young (https://orcid.org/0000-0003-1538-445X) contributed to Cardiovascular risk in patients with psoriasis.
Publications
Workstream 1
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–85. https://doi.org/10.1038/jid.2012.339
Hamilton MP, Ntais D, Griffiths CE, Davies LM. Psoriasis treatment and management – a systematic review of full economic evaluations. Br J Dermatol 2015;172:574–83. https://doi.org/10.1111/bjd.13486
Parisi R, Rutter MK, Lunt M, Young HS, Symmons DPM, Griffiths CEM, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the Clinical Practice Research Datalink. J Invest Dermatol 2015;135:2189–97. https://doi.org/10.1038/jid.2015.87
Workstream 2
Keyworth C, Nelson PA, Chew-Graham CA, Kane K, Pearce CJ, Griffiths CEM, et al. Communicating cardiovascular disease risk to people with psoriasis: what techniques do practitioners use? Int J Behav Med 2016;23:168–78. https://doi.org/10.1007/s12529-015-9517-8
Nelson PA, Kane K, Chisholm A, Pearce CJ, Keyworth C, Rutter MK, et al. ‘I should have taken that further’: missed opportunities during cardiovascular risk assessment in patients with psoriasis in UK primary care settings – a mixed-methods study. Health Expect 2016;19:1121–37. https://doi.org/10.1111/hex.12404
Rutter MK, Kane K, Lunt M, Cordingley L, Littlewood A, Young HS, et al. Primary care-based screening for cardiovascular risk factors in patients with psoriasis. Br J Dermatol 2016;175:348–56. https://doi.org/10.1111/bjd.14557
Keyworth C, Nelson PA, Bundy C, Pye SR, Griffiths CEM, Cordingley L. Does message framing affect changes in behavioural intentions in people with psoriasis? A randomized exploratory study examining health risk communication. Psychol Health Med 2018;23:763–78. https://doi.org/10.1080/13548506.2018.1427876
Workstream 3
Cordingley L, Nelson PA, Griffiths CE, Chew-Graham CA. Beyond skin: the need for a new approach to the management of psoriasis in primary care. Br J Gen Pract 2012;62:568–9. https://doi.org/10.3399/bjgp12X658133
Nelson PA, Barker Z, Griffiths CE, Cordingley L, Chew-Graham CA, IMPACT Team. ‘On the surface’: a qualitative study of GPs’ and patients’ perspectives on psoriasis. BMC Fam Pract 2013;14:158. https://doi.org/10.1186/1471-2296-14-158
Nelson PA, Chew-Graham CA, Griffiths CE, Cordingley L, IMPACT Team. Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol 2013;168:354–61. https://doi.org/10.1111/j.1365-2133.2012.11217.x
Workstream 4
Keyworth C, Nelson PA, Chisholm A, Griffiths CE, Cordingley L, Bundy C, Identification and Management of Psoriasis-Associated Co-morbidiTy (IMPACT) team. Providing lifestyle behaviour change support for patients with psoriasis: an assessment of the existing training competencies across medical and nursing health professionals. Br J Dermatol 2014;171:602–8. https://doi.org/10.1111/bjd.13067
Nelson PA, Keyworth C, Chisholm A, Pearce CJ, Griffiths CE, Cordingley L, et al. ‘In someone’s clinic but not in mine’: clinicians’ views of supporting lifestyle behaviour change in patients with psoriasis – a qualitative interview study. Br J Dermatol 2014;171:1116–22. https://doi.org/10.1111/bjd.13231
Keyworth C, Nelson PA, Griffiths CEM, Cordingley L, Bundy C. Do English healthcare settings use ‘Choice Architecture’ principles in promoting healthy lifestyles for people with psoriasis? BMC Health Serv Res 2015;15:215.
Chisholm A, Nelson PA, Pearce CJ, Keyworth C, Griffiths CE, Cordingley L, Bundy C, IMPACT Team. The role of personal models in clinical management: exploring health care providers’ beliefs about psoriasis. Br J Health Psychol 2016;21:114–34. https://doi.org/10.1111/bjhp.12148
Workstream 5
Chisholm A, Nelson PA, Pearce CJ, Littlewood AJ, Kane K, Henry AL, et al. Motivational interviewing-based training enhances clinicians’ skills and knowledge in psoriasis: findings from the PSO WELL® study. Brit J Dermatology 2017;176:677–86. https://doi.org/10.1111/bjd.14837
Nelson PA, Kane K, Pearce CJ, Bundy C, Chisholm A, Hilton R, et al. ‘New to me’: changing patient understanding of psoriasis and identifying mechanisms of change. The Pso Well® patient materials mixed-methods feasibility study. Br J Dermatol 2017;177:758–70. https://doi.org/10.1111/bjd.15574
PhD thesis (associated with workstreams 2 and 4)
Keyworth C. Risk Communication and Lifestyle Behaviour Change in People with Psoriasis. PhD thesis. Manchester: University of Manchester; 2015. URL: www.escholar.manchester.ac.uk/api/datastream?publicationPid=uk-ac-man-scw:266982&datastreamId (accessed 28 January 2019).
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85. https://doi.org/10.1038/jid.2012.339.
- Anstey A, McAteer H, Kamath N, Percival F. Extending psychosocial assessment of patients with psoriasis in the UK, using a self-rated, web-based survey. Clin Exp Dermatol 2012;37:735-40. https://doi.org/10.1111/j.1365-2230.2012.04457.x.
- de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc 2004;9:140-7. https://doi.org/10.1046/j.1087-0024.2003.09110.x.
- Kimball AB, Gieler U, Linder D, Sampogna F, Warren RB, Augustin M. Psoriasis: is the impairment to a patient’s life cumulative?. J Eur Acad Dermatol Venereol 2010;24:989-1004. https://doi.org/10.1111/j.1468-3083.2010.03705.x.
- Lebwohl M. Psoriasis. Lancet 2003;361:1197-204. https://doi.org/10.1016/S0140-6736(03)12954-6.
- Nevitt GJ, Hutchinson PE. Psoriasis in the community: prevalence, severity and patients’ beliefs and attitudes towards the disease. Br J Dermatol 1996;135:533-7. https://doi.org/10.1111/j.1365-2133.1996.tb03826.x.
- Baker CS, Foley PA, Braue A. Psoriasis uncovered: measuring burden of disease impact in a survey of Australians with psoriasis. Australas J Dermatol 2013;54:1-6. https://doi.org/10.1111/ajd.12010.
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Academy Dermatol 1999;41:401-7. https://doi.org/10.1016/S0190-9622(99)70112-X.
- Singhal A, Ross J, Seminog O, Hawton K, Goldacre MJ. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med 2014;107:194-20. https://doi.org/10.1177/0141076814522033.
- Leary M, Rapp SR, Berbst K, Exum ML, Feldman SR. Interpersonal concerns and psychological difficulties of psoriasis patients: effect of disease severity and fear of negative evaluation. Health Psychology 1998;17:1-7. https://doi.org/10.1037/0278-6133.17.6.530.
- Richards HL, Fortune DG, Weidmann A, Sweeney SK, Griffiths CE. Detection of psychological distress in patients with psoriasis: low consensus between dermatologist and patient. Br J Dermatol 2004;151:1227-33. https://doi.org/10.1111/j.1365-2133.2004.06221.x.
- Beresford A. Psoriasis Association Members’ Questionnaire: Report Prepared by Independent Market Researcher 2002.
- Christophers E, Barker JN, Griffiths CE, Daudén E, Milligan G, Molta C, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J Eur Acad Dermatol Venereol 2010;24:548-54. https://doi.org/10.1111/j.1468-3083.2009.03463.x.
- Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 2003;4:441-7. https://doi.org/10.2165/00128071-200304070-00001.
- Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263-71. https://doi.org/10.1016/S0140-6736(07)61128-3.
- Gisondi P, Tessari G, Conti A, Piaserico S, Schianchi S, Peserico A, et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol 2007;157:68-73. https://doi.org/10.1111/j.1365-2133.2007.07986.x.
- Cohen AD, Dreiher J, Shapiro Y, Vidavsky L, Vardy DA, Davidovici B, et al. Psoriasis and diabetes: a population-based cross-sectional study. J Eur Acad Dermatol Venereol 2008;22:585-9. https://doi.org/10.1111/j.1468-3083.2008.02636.x.
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735-41. https://doi.org/10.1001/jama.296.14.1735.
- Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatol Treat 2008;19:5-21. https://doi.org/10.1080/09546630701364768.
- Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol 2009;51:758-64. https://doi.org/10.1016/j.jhep.2009.04.020.
- Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med 2007;167:1670-5. https://doi.org/10.1001/archinte.167.15.1670.
- Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol 2005;125:61-7. https://doi.org/10.1111/j.0022-202X.2005.23681.x.
- Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829-35. https://doi.org/10.1016/j.jaad.2006.08.040.
- Lee MS, Lin RY, Lai MS. Increased risk of diabetes mellitus in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population-based cohort study. J Am Acad Dermatol 2014;70:691-8. https://doi.org/10.1016/j.jaad.2013.11.023.
- Gelfand JM, Kurd SK, Neimann AL, Shin DB, Margolis DJ, Troxel AB. Letter: psoriasis and risk of myocardial infarction–reply. JAMA 2007;297:361-3. https://doi.org/10.1001/jama.297.4.362-b.
- Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol 2007;143:1493-9. https://doi.org/10.1001/archderm.143.12.1493.
- Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 2011;20:303-7. https://doi.org/10.1111/j.1600-0625.2011.01261.x.
- Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. J Invest Dermatol 2009;129:1601-3. https://doi.org/10.1038/jid.2009.55.
- Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology 2010;49:295-307. https://doi.org/10.1093/rheumatology/kep366.
- Parisi R, Rutter MK, Lunt M, Young HS, Symmons DPM, Griffiths CEM, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the Clinical Practice Research Datalink. J Invest Dermatol 2015;135:2189-97. https://doi.org/10.1038/jid.2015.87.
- Prey S, Paul C, Bronsard V, Puzenat E, Gourraud PA, Aractingi S, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol 2010;24:23-30. https://doi.org/10.1111/j.1468-3083.2009.03564.x.
- Gisondi P, Del Giglio M, Di Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr 2008;88:1242-7.
- Rucević I, Perl A, Barisić-Drusko V, Adam-Perl M. The role of the low energy diet in psoriasis vulgaris treatment. Coll Antropol 2003;27:41-8.
- McAleer MA, Mason DL, Cunningham S, O’Shea SJ, McCormick PA, Stone C, et al. Alcohol misuse in patients with psoriasis: identification and relationship to disease severity and psychological distress. Br J Dermatol 2011;164:1256-61. https://doi.org/10.1111/j.1365-2133.2011.10345.x.
- Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007;143:1559-65. https://doi.org/10.1001/archderm.143.12.1559.
- Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol 2005;141:1537-41. https://doi.org/10.1001/archderm.141.12.1537.
- O’Neill P, Kelly P. Postal questionnaire study of disability in the community associated with psoriasis. BMJ 1996;313:919-21. https://doi.org/10.1136/bmj.313.7062.919.
- Kay L, Parry-James J, Walker D. The prevalence and impact of psoriasis and psoriatic arthritis in the primary care population in North East England. Arthritis Rheum 1999;42.
- Seminara NM, Abuabara K, Shin DB, Langan SM, Kimmel SE, Margolis D, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011;164:602-9. https://doi.org/10.1111/j.1365-2133.2010.10134.x.
- World Health Organization (WHO) . Global Report on Psoriasis 2016. http://apps.who.int/iris/handle/10665/204417 (accessed 16 June 2017).
- Hamilton MP, Ntais D, Griffiths CE, Davies LM. Psoriasis treatment and management – a systematic review of full economic evaluations. Brit J Dermatol 2015;172:574-83. https://doi.org/10.1111/bjd.13486.
- Ahlehoff O, Gislason GH, Charlot M, Jørgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011;270:147-57. https://doi.org/10.1111/j.1365-2796.2010.02310.x.
- Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J 2012;33:2054-64. https://doi.org/10.1093/eurheartj/ehr285.
- Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837-44. https://doi.org/10.1161/CIRCULATIONAHA.114.009990.
- Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol 2009;129:2411-18. https://doi.org/10.1038/jid.2009.112.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol 2008;159:895-902. https://doi.org/10.1111/j.1365-2133.2008.08707.x.
- Li WQ, Han JL, Manson JE, Rimm EB, Rexrode KM, Curhan GC, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol 2012;166:811-18. https://doi.org/10.1111/j.1365-2133.2011.10774.x.
- Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population-based study in Taiwan. J Am Acad Dermatol 2011;64:495-501. https://doi.org/10.1016/j.jaad.2010.01.050.
- Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol 2007;156:271-6. https://doi.org/10.1111/j.1365-2133.2006.07562.x.
- Mallbris L, Akre O, Granath F, Yin L, Lindelöf B, Ekbom A, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol 2004;19:225-30. https://doi.org/10.1023/B:EJEP.0000020447.59150.f9.
- Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 2010;31:1000-6. https://doi.org/10.1093/eurheartj/ehp567.
- Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol 2009;160:1048-56. https://doi.org/10.1111/j.1365-2133.2008.09020.x.
- Dowlatshahi EA, Kavousi M, Nijsten T, Ikram MA, Hofman A, Franco OH, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the Rotterdam Study. J Invest Dermatol 2013;133:2347-54. https://doi.org/10.1038/jid.2013.131.
- Stern RS, Huibregtse A. Very severe psoriasis is associated with increased noncardiovascular mortality but not with increased cardiovascular risk. J Invest Dermatol 2011;131:1159-66. https://doi.org/10.1038/jid.2010.399.
- Wakkee M, Herings RM, Nijsten T. Psoriasis may not be an independent risk factor for acute ischemic heart disease hospitalizations: results of a large population-based Dutch cohort. J Invest Dermatol 2010;130:962-7. https://doi.org/10.1038/jid.2009.321.
- Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol 2013;133:2340-6. https://doi.org/10.1038/jid.2013.149.
- Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167-72.
- John H, Kitas G. Inflammatory arthritis as a novel risk factor for cardiovascular disease. Eur J Intern Med 2012;23:575-9. https://doi.org/10.1016/j.ejim.2012.06.016.
- Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol 2011;7:399-408. https://doi.org/10.1038/nrrheum.2011.75.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. https://doi.org/10.1136/bmj.39335.541782.AD.
- National Institute for Health and Care Excellence . Psoriasis: The Assessment and Management of Psoriasis [NICE Guidelines CG153] 2012. www.nice.org.uk/guidance/cg153 (accessed 28 January 2019).
- Robinson D, Schulz E, Brown P, Price C. Updating the Read Codes: user-interactive maintenance of a dynamic clinical vocabulary. J Am Med Inform Assoc 1997;4:465-72. https://doi.org/10.1136/jamia.1997.0040465.
- Ministry of Housing, Communities and Local Government . English Indices of Deprivation 2010: Guidance 2011. www.gov.uk/government/publications/english-indices-of-deprivation-2010-guidance (accessed 21 May 2014).
- Springate DA, Kontopantelis E, Ashcroft DM, Olier I, Parisi R, Chamapiwa E, et al. ClinicalCodes: an online clinical codes repository to improve the validity and reproducibility of research using electronic medical records. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0099825.
- Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2015;74:326-32. https://doi.org/10.1136/annrheumdis-2014-205675.
- Rutter MK, Kane K, Lunt M, Cordingley L, Littlewood A, Young HS, et al. Primary care-based screening for cardiovascular risk factors in patients with psoriasis. Br J Dermatol 2016;175:348-56. https://doi.org/10.1111/bjd.14557.
- Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol 2013;69:1014-24. https://doi.org/10.1016/j.jaad.2013.06.053.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes 2012;2. https://doi.org/10.1038/nutd.2012.26.
- Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: a systematic review of the literature. J Gen Intern Med 2011;26:1036-49. https://doi.org/10.1007/s11606-011-1698-5.
- Craig R, Mindell J. Health Survey for England 2011: Health, Social Care and Lifestyles. Leeds: NHS Digital; 2012.
- Ma C, Schupp CW, Armstrong EJ, Armstrong AW. Psoriasis and dyslipidemia: a population-based study analyzing the National Health and Nutrition Examination Survey (NHANES). J Eur Acad Dermatol Venereol 2014;28:1109-12. https://doi.org/10.1111/jdv.12232.
- Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0036342.
- Armstrong AW, Lin SW, Chambers CJ, Sockolov ME, Chin DL. Psoriasis and hypertension severity: results from a case-control study. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0018227.
- Kimball AB, Leonardi C, Stahle M, Gulliver W, Chevrier M, Fakharzadeh S, et al. Demography, baseline disease characteristics and treatment history of patients with psoriasis enrolled in a multicentre, prospective, disease-based registry (PSOLAR). Br J Dermatol 2014;171:137-47. https://doi.org/10.1111/bjd.13013.
- Ma L, Li M, Wang H, Li Y, Bai B. High prevalence of cardiovascular risk factors in patients with moderate or severe psoriasis in northern China. Arch Dermatol Res 2014;306:247-51. https://doi.org/10.1007/s00403-013-1437-3.
- Kimball AB, Szapary P, Mrowietz U, Reich K, Langley RG, You Y, et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol 2012;67:76-85. https://doi.org/10.1016/j.jaad.2011.06.035.
- Warnecke C, Manousaridis I, Herr R, Terris DD, Goebeler M, Goerdt S, et al. Cardiovascular and metabolic risk profile in German patients with moderate and severe psoriasis: a case control study. Eur J Dermatol 2011;21:761-70. https://doi.org/10.1684/ejd.2011.1467.
- Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Br J Dermatol 2011;165:1037-43. https://doi.org/10.1111/j.1365-2133.2011.10494.x.
- Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol 2012;132:556-62. https://doi.org/10.1038/jid.2011.365.
- Shapiro J, Cohen AD, David M, Hodak E, Chodik G, Viner A, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol 2007;56:629-34. https://doi.org/10.1016/j.jaad.2006.09.017.
- Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and cardiovascular risk factors: a case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol 2012;66:252-8. https://doi.org/10.1016/j.jaad.2010.11.046.
- Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res 2006;298:321-8. https://doi.org/10.1007/s00403-006-0703-z.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology 2012;225:121-6. https://doi.org/10.1159/000342180.
- Mazlin MB, Chang CC, Baba R. Comorbidities associated with psoriasis: data from the Malaysian psoriasis registry. Med J Malaysia 2012;67:518-21.
- Krogsbøll LT, Jørgensen KJ, Gøtzsche PC. Universal health checks should be abandoned. BMJ 2013;347. https://doi.org/10.1136/bmj.f5227.
- Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C, Gøtzsche PC. General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e7191.
- Gøtzsche PC, Jørgensen KJ, Krogsbøll LT. General health checks don’t work. BMJ 2014;348. https://doi.org/10.1136/bmj.g3680.
- Nelson PA, Kane K, Chisholm A, Pearce CJ, Keyworth C, Rutter MK, et al. ‘I should have taken that further’: missed opportunities during cardiovascular risk assessment in patients with psoriasis in UK primary care settings – a mixed-methods study. Health Expect 2016;19:1121-37. https://doi.org/10.1111/hex.12404.
- Friedewald VE, Cather JC, Gordon KB, Kavanaugh A, Ridker PM, Roberts WC. The editor’s roundtable: psoriasis, inflammation, and coronary artery disease. Am J Cardiol 2008;101:1119-26. https://doi.org/10.1016/j.amjcard.2007.11.032.
- Friedewald VE, Cather JC, Gelfand JM, Gordon KB, Gibbons GH, Grundy SM, et al. AJC editor’s consensus: psoriasis and coronary artery disease. Am J Cardiol 2008;102:1631-43. https://doi.org/10.1016/j.amjcard.2008.10.004.
- Kimball AB, Guerin A, Latremouille-Viau D, Yu AP, Gupta S, Bao Y, et al. Coronary heart disease and stroke risk in patients with psoriasis: retrospective analysis. Am J Med 2010;123:350-7. https://doi.org/10.1016/j.amjmed.2009.08.022.
- Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 2009;145:700-3. https://doi.org/10.1001/archdermatol.2009.94.
- Shelling ML, Federman DG, Prodanovich S, Kirsner RS. Psoriasis and vascular disease: an unsolved mystery. Am J Med 2008;121:360-5. https://doi.org/10.1016/j.amjmed.2008.01.025.
- Baulmann J, Schillings U, Rickert S, Uen S, Düsing R, Illyes M, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens 2008;26:523-8. https://doi.org/10.1097/HJH.0b013e3282f314f7.
- Horváth IG, Németh A, Lenkey Z, Alessandri N, Tufano F, Kis P, et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens 2010;28:2068-75. https://doi.org/10.1097/HJH.0b013e32833c8a1a.
- Rajzer MW, Wojciechowska W, Klocek M, Palka I, Brzozowska-Kiszka M, Kawecka-Jaszcz K. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens 2008;26:2001-7. https://doi.org/10.1097/HJH.0b013e32830a4a25.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318-27. https://doi.org/10.1016/j.jacc.2009.10.061.
- Costa L, Caso F, D’Elia L, Atteno M, Peluso R, Del Puente A, et al. Psoriatic arthritis is associated with increased arterial stiffness in the absence of known cardiovascular risk factors: a case control study. Clin Rheumatol 2012;31:711-15. https://doi.org/10.1007/s10067-011-1892-1.
- Soy M, Yildiz M, Sevki Uyanik M, Karaca N, Güfer G, Piskin S. Susceptibility to atherosclerosis in patients with psoriasis and psoriatic arthritis as determined by carotid-femoral (aortic) pulse-wave velocity measurement. Rev Esp Cardiol 2009;62:96-9. https://doi.org/10.1016/S0300-8932(09)70027-2.
- Jatoi NA, Mahmud A, Bennett K, Feely J. Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens 2009;27:2186-91. https://doi.org/10.1097/HJH.0b013e32833057e8.
- Chularojanamontri L, Griffiths CE, Chalmers RJ. The Simplified Psoriasis Index (SPI): a practical tool for assessing psoriasis. J Invest Dermatol 2013;133:1956-62. https://doi.org/10.1038/jid.2013.138.
- Feldman SR, Fleischer AB, Reboussin DM, Rapp SR, Exum ML, Clark AR, et al. The self-administered psoriasis area and severity index is valid and reliable. J Invest Dermatol 1996;106:183-6. https://doi.org/10.1111/1523-1747.ep12329912.
- Yiu KH, Yeung CK, Chan HT, Wong RM, Tam S, Lam KF, et al. Increased arterial stiffness in patients with psoriasis is associated with active systemic inflammation. Br J Dermatol 2011;164:514-20. https://doi.org/10.1111/j.1365-2133.2010.10107.x.
- Bicer A, Acikel S, Kilic H, Ulukaradag Z, Karasu BB, Cemil BC, et al. Impaired aortic elasticity in patients with psoriasis. Acta Cardiol 2009;64:597-602. https://doi.org/10.2143/AC.64.5.2042688.
- Gisondi P, Fantin F, Del Giglio M, Valbusa F, Marino F, Zamboni M, et al. Chronic plaque psoriasis is associated with increased arterial stiffness. Dermatology 2009;218:110-13. https://doi.org/10.1159/000182256.
- Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk of incident psoriasis. Arch Dermatol 2012;148:918-24. https://doi.org/10.1001/archdermatol.2012.943.
- Hamminga EA, van der Lely AJ, Neumann HA, Thio HB. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses 2006;67:768-73. https://doi.org/10.1016/j.mehy.2005.11.050.
- Jensen P, Zachariae C, Christensen R, Geiker NR, Schaadt BK, Stender S, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol 2013;149:795-801. https://doi.org/10.1001/jamadermatol.2013.722.
- Naldi L, Conti A, Cazzaniga S, Patrizi A, Pazzaglia M, Lanzoni A, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol 2014;170:634-42. https://doi.org/10.1111/bjd.12735.
- Poikolainen K, Reunala T, Karvonen J, Lauharanta J, Kärkkäinen P. Alcohol intake: a risk factor for psoriasis in young and middle aged men?. BMJ 1990;300:780-3. https://doi.org/10.1136/bmj.300.6727.780.
- Nelson PA, Barker Z, Griffiths CE, Cordingley L, Chew-Graham CA. IMPACT Team . ‘On the surface’: a qualitative study of GPs’ and patients’ perspectives on psoriasis. BMC Fam Pract 2013;14. https://doi.org/10.1186/1471-2296-14-158.
- Nelson PA, Chew-Graham CA, Griffiths CE, Cordingley L. IMPACT Team . Recognition of need in health care consultations: a qualitative study of people with psoriasis. Br J Dermatol 2013;168:354-61. https://doi.org/10.1111/j.1365-2133.2012.11217.x.
- Nelson PA, Keyworth C, Chisholm A, Pearce CJ, Griffiths CE, Cordingley L, et al. ‘In someone’s clinic but not in mine’: clinicians’ views of supporting lifestyle behaviour change in patients with psoriasis – a qualitative interview study. Br J Dermatol 2014;171:1116-22. https://doi.org/10.1111/bjd.13231.
- Senior V, Smith JA, Michie S, Marteau TM. Making sense of risk: an interpretative phenomenological analysis of vulnerability to heart disease. J Health Psychol 2002;7:157-68. https://doi.org/10.1177/1359105302007002455.
- Knapp P, Raynor DK, Berry DC. Comparison of two methods of presenting risk information to patients about the side effects of medicines. Qual Saf Health Care 2004;13:176-80. https://doi.org/10.1136/qhc.13.3.176.
- Kahneman D, Tversky A. Prospect Theory: an analysis of decision under risk. Econometrica 1979;47:263-91. https://doi.org/10.2307/1914185.
- Weinstein ND. Why it won’t happen to me: perceptions of risk factors and susceptibility. Health Psychol 1984;3:431-57. https://doi.org/10.1037/0278-6133.3.5.431.
- Rothman AJ, Martino SC, Bedell BT, Detweiler JB, Salovey P. The systematic influence of gain- and loss-framed messages on interest in and use of different types of health behavior. Pers Soc Psychol Bull 1999;25:1355-69. https://doi.org/10.1177/0146167299259003.
- O’Connor DB, Warttig S, Conner M, Lawton R. Raising awareness of hypertension risk through a web-based framing intervention: does consideration of future consequences make a difference?. Psych Health Med 2009;14:213-9. https://doi.org/10.1080/13548500802291618.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making 2007;27:696-713. https://doi.org/10.1177/0272989X07307271.
- Keyworth C, Nelson PA, Chew-Graham CA, Kane K, Pearce CJ, Griffiths CEM, et al. Communicating cardiovascular disease risk to people with psoriasis: what techniques do practitioners use?. Int J Behav Med 2016;23:168-78. https://doi.org/10.1007/s12529-015-9517-8.
- Keyworth C. Risk Communication and Lifestyle Behaviour Change in People With Psoriasis 2015.
- Keyworth C, Nelson PA, Bundy C, Pye SR, Griffiths CEM, Cordingley L. Does message framing affect changes in behavioural intentions in people with psoriasis? A randomized exploratory study examining health risk communication. Psychol Health Med 2018;23:763-78. https://doi.org/10.1080/13548506.2018.1427876.
- Schreier M. Qualitative Content Analysis in Practice. Thousand Oaks, CA: Sage Publications; 2012.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88. https://doi.org/10.1177/1049732305276687.
- Richards L. Using NVivo in Qualitative Research. Thousand Oaks, CA: Sage Publications; 1999.
- Ajzen I. Perceived behavioral control, self-efficacy, locus of control, and the theory of planned behavior. J Appl Soc Psychol 2006;32:665-83. https://doi.org/10.1111/j.1559-1816.2002.tb00236.x.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Thousand Oaks, CA: Sage Publications; 2003.
- Gillard SE, Finlay AY. Current management of psoriasis in the United Kingdom: patterns of prescribing and resource use in primary care. Int J Clin Pract 2005;59:1260-7. https://doi.org/10.1111/j.1368-5031.2005.00680.x.
- Griffiths CEM, Taylor H, Collins SI, Hobson JE, Collier PA, Chalmers RJG, et al. The impact of psoriasis guidelines on appropriateness of referral from primary to secondary care: a randomized controlled trial. Br J Dermatol 2006;155:393-400. https://doi.org/10.1111/j.1365-2133.2006.07354.x.
- Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol 2001;137:280-4.
- Psoriasis Association . The Patient Experience. A Survey of Members of The Psoriasis Association 2010.
- Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol 2013;168:20-31. https://doi.org/10.1111/bjd.12039.
- Thorneloe RJ, Bundy C, Griffiths CE, Ashcroft DM, Cordingley L. Nonadherence to psoriasis medication as an outcome of limited coping resources and conflicting goals: findings from a qualitative interview study with people with psoriasis. Br J Dermatol 2017;176:667-76. https://doi.org/10.1111/bjd.15086.
- National Institute for Health and Care Excellence (NICE) . Psoriasis: Assessment and Management (CG153) 2012. http://guidance.nice.org.uk/CG153/Guidance (accessed 28 January 2019).
- Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: using common sense to understand treatment adherence and affect cognition interactions. Cognit Ther Res 1992;16:143-63. https://doi.org/10.1007/BF01173486.
- Lazarus RS, Folkman S. Stress, Appraisal and Coping. New York, NY: Springer; 1984.
- Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: Sage Publications; 2008.
- Estabrooks PA, Nelson CC, Xu S, King D, Bayliss EA, Gaglio B, et al. The frequency and behavioral outcomes of goal choices in the self-management of diabetes. Diabetes Educ 2005;31:391-400. https://doi.org/10.1177/0145721705276578.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. IMAGE Study Group . Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-119.
- Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, et al. American Diabetes Association . Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies – a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004;27:2067-73. https://doi.org/10.2337/diacare.27.8.2067.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343:16-22. https://doi.org/10.1056/NEJM200007063430103.
- Eves FF, Olander EK, Nicoll G, Puig-Ribera A, Griffin C. Increasing stair climbing in a train station: the effects of contextual variables and visibility. J Environ Psychol 2009;29:300-3. https://doi.org/10.1016/j.jenvp.2008.10.002.
- Marteau TM, Ogilvie D, Roland M, Suhrcke M, Kelly MP. Judging nudging: can nudging improve population health?. Br Med J 2011;342. https://doi.org/10.1136/bmj.d228.
- Rozin P, Scott S, Dingley M, Urbanek JK, Jiang H, Kaltenbach M. Nudge to nobesity I: minor changes in accessibility decrease food intake. Judgm Decis Mak 2011;6:323-32.
- Hollands GJ, Shemilt I, Marteau TM, Jebb SA, Kelly MP, Nakamura R, et al. Altering micro-environments to change population health behaviour: towards an evidence base for choice architecture interventions. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-1218.
- Keyworth C, Nelson PA, Chisholm A, Griffiths CE, Cordingley L, Bundy C. Identification and Management of Psoriasis-Associated Co-morbidiTy (IMPACT) team . Providing lifestyle behaviour change support for patients with psoriasis: an assessment of the existing training competencies across medical and nursing health professionals. Br J Dermatol 2014;171:602-8. https://doi.org/10.1111/bjd.13067.
- NHS Yorkshire and the Humber . Prevention and Lifestyle Behaviour Change: A Competence Framework 2010. www.champspublichealth.com/sites/default/files/prevention%20and%20lifestyle%20behaviour%20change.pdf (accessed 1 August 2014).
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. ‘Psychological Theory’ Group . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Leventhal H, Nerenz D, Steele D, Baum A, Singer J. A Handbook of Psychology and Health. Hillsdale, NJ: Erlbaum; 1984.
- Chisholm A, Nelson PA, Pearce CJ, Keyworth C, Griffiths CE, Cordingley L, et al. IMPACT Team . The role of personal models in clinical management: exploring health care providers’ beliefs about psoriasis. Br J Health Psychol 2016;21:114-34. https://doi.org/10.1111/bjhp.12148.
- Cordingley L, Nelson PA, Griffiths CE, Chew-Graham CA. Beyond skin: the need for a new approach to the management of psoriasis in primary care. Br J Gen Pract 2012;62:568-9. https://doi.org/10.3399/bjgp12X658133.
- Petrie KJ, Jago LA, Devcich DA. The role of illness perceptions in patients with medical conditions. Curr Opin Psychiatry 2007;20:163-7. https://doi.org/10.1097/YCO.0b013e328014a871.
- Peyrot M, Rubin RR. Behavioral and psychosocial interventions in diabetes: a conceptual review. Diabetes Care 2007;30:2433-40. https://doi.org/10.2337/dc07-1222.
- Fortune DG, Richards HL, Griffiths CE, Main CJ. Targeting cognitive-behaviour therapy to patients’ implicit model of psoriasis: results from a patient preference controlled trial. Br J Clin Psychol 2004;43:65-82. https://doi.org/10.1348/014466504772812977.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Nelson PA, Kane K, Pearce CJ, Bundy C, Chisholm A, Hilton R, et al. ‘New to me’: changing patient understanding of psoriasis and identifying mechanisms of change. The Pso Well® patient materials mixed-methods feasibility study. Br J Dermatol 2017;177:758-70. https://doi.org/10.1111/bjd.15574.
- Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The revised Illness Perception Questionnaire (IPQ-R). Psychol Health 2002;17:1-16. https://doi.org/10.1080/08870440290001494.
- Zigmond AS, Snaith RP. The Hospital Anxiety And Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Ritchie J, Spencer E, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Rowlands G, Protheroe J, Winkley J, Richardson M, Seed PT, Rudd R. A mismatch between population health literacy and the complexity of health information: an observational study. Br J Gen Pract 2015;65:379-86. https://doi.org/10.3399/bjgp15X685285.
- Cunningham MA, Swanson V, Holdsworth RJ, O’Carroll RE. Late effects of a brief psychological intervention in patients with intermittent claudication in a randomized clinical trial. Br J Surg 2013;100:756-60. https://doi.org/10.1002/bjs.9100.
- Chisholm A, Nelson PA, Pearce CJ, Littlewood AJ, Kane K, Henry AL, et al. Motivational interviewing-based training enhances clinicians’ skills and knowledge in psoriasis: findings from the Pso Well® study. Br J Dermatol 2017;176:677-86. https://doi.org/10.1111/bjd.14837.
- Vaghefi I, Tulu B. The continued use of mobile health apps: insights from a longitudinal study. JMIR Mhealth Uhealth 2019;7. https://doi.org/10.2196/12983.
- Koopman RJ, Petroski GF, Canfield SM, Stuppy JA, Mehr DR. Development of the PRE-HIT instrument: Patient Readiness to Engage in Health Information Technology. BMC Fam Pract 2014;15. https://doi.org/10.1186/1471-2296-15-18.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. https://doi.org/10.1080/08870446.2010.540664.
- Rollnick S, Kinnersley P, Stott N. Methods of helping patients with behaviour change. BMJ 1993;307:188-90. https://doi.org/10.1136/bmj.307.6897.188.
- Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001;96:1725-42. https://doi.org/10.1080/09652140120089481.
- Knight KM, McGowan L, Dickens C, Bundy C. A systematic review of motivational interviewing in physical health care settings. Br J Health Psychol 2006;11:319-32. https://doi.org/10.1348/135910705X52516.
- The University of Manchester . IMPACT Psoriasis 2019. www.impactpsoriasis.org.uk (accessed 5 August 2019).
- Lane C, Huws-Thomas M, Hood K, Rollnick S, Edwards K, Robling M. Measuring adaptations of motivational interviewing: the development and validation of the behavior change counseling index (BECCI). Patient Educ Couns 2005;56:166-73. https://doi.org/10.1016/j.pec.2004.01.003.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. PharmacoEconomics 2008;26:661-77. https://doi.org/10.2165/00019053-200826080-00004.
- Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 2013;16:3-13. https://doi.org/10.1016/j.jval.2012.08.2223.
- de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient 2015;8:373-84. https://doi.org/10.1007/s40271-015-0118-z.
- Coast J, Horrocks S. Developing attributes and levels for discrete choice experiments using qualitative methods. J Health Serv Res Policy 2007;12:25-30. https://doi.org/10.1258/135581907779497602.
- Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health: a checklist – a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011;14:403-13. https://doi.org/10.1016/j.jval.2010.11.013.
- Møller AH, Erntoft S, Vinding GR, Jemec GB. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas 2015;6:167-77. https://doi.org/10.2147/PROM.S81428.
- Burgess L, Street DJ. Optimal designs for choice experiments with asymmetric attributes. J Stat Plan Inference 2005;134:288-301. https://doi.org/10.1016/j.jspi.2004.03.021.
- NationaI Institute for Health and Care Excellence (NICE) . Hypertension: The Clinical Management of Primary Hypertension in Adults [NICE Guidelines CG127] 2011. www.nice.org.uk/guidance/cg127 (accessed 28 January 2019).
- NationaI Institute for Health and Care Excellence (NICE) . Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children [NICE Guidelines CG43] 2006. www.nice.org.uk/Guidance/CG43 (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Alcohol-Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence [NICE Guidelines CG115] 2011. www.nice.org.uk/guidance/cg115 (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification [NICE Guidelines CG181] 2014. www.nice.org.uk/guidance/cg181 (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Depression in Adults: Recognition and Management [NICE Guidelines CG90] 2009. www.nice.org.uk/guidance/cg90 (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Generalised Anxiety Disorder and Panic Disorder in Adults: Management [NICE Guidelines CG113] 2011. www.nice.org.uk/guidance/cg113 (accessed 28 January 2019).
- NHS . Overview: Psoriasis 2015. www.nhs.uk/conditions/psoriasis/ (accessed 2015).
- Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Möller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Med Decis Making 2012;32:701-11. https://doi.org/10.1177/0272989X12455462.
- Heeg BMS, Damen J, Buskens E, Caleo S, de Charro F, van Hout BA. Modelling approaches: the case of schizophrenia. PharmacoEconomics 2008;26:633-48. https://doi.org/10.2165/00019053-200826080-00002.
- National Institute for Health and Care Excellence (NICE) . Alcohol-Use Disorders: Prevention [NICE Guidelines PH24] 2010. www.nice.org.uk/guidance/ph24 (accessed 28 January 2019).
- Office for National Statistics (ONS) . National Life Tables, UK: 2011 to 13 2014. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014-09-25 (accessed 2014).
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Luengo-Fernandez R, et al. Coronary Heart Disease Statistics. London: British Heart Foundation; 2012.
- Stroke Association . State of the Nation Stroke Statistics 2015.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 [NICE Article PMG9] 2013.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. https://doi.org/10.1136/bmj.329.7459.224.
- National Institute for Health Research. Going the Extra Mile: Improving the Nation’s Health and Wellbeing through Public Involvement in Research 2014.
- National Institute for Health Research . What Is Public Involvement in Research? 2015. www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/ (accessed 2015).
- National Institute for Health Research . Patient and Public Involvement in Health and Social Care Research: A Handbook for Researchers 2014.
- Barber R, Boote JD, Parry GD, Cooper CL, Yeeles P, Cook S. Can the impact of public involvement on research be evaluated? A mixed methods study. Health Expect 2012;15:229-41. https://doi.org/10.1111/j.1369-7625.2010.00660.x.
- Thompson J, Barber R, Ward PR, Boote JD, Cooper CL, Armitage CJ, et al. Health researchers’ attitudes towards public involvement in health research. Health Expect 2009;12:209-20. https://doi.org/10.1111/j.1369-7625.2009.00532.x.
- Supple D, Roberts A, Hudson V, Masefield S, Fitch N, Rahmen M, et al. From tokenism to meaningful engagement: best practices in patient involvement in an EU project. Res Involv Engagem 2015;1. https://doi.org/10.1186/s40900-015-0004-9.
- Ennis L, Wykes T. Impact of patient involvement in mental health research: longitudinal study. Br J Psychiatry 2013;203:381-6. https://doi.org/10.1192/bjp.bp.112.119818.
- Staley K. ‘Is it worth doing?’ Measuring the impact of patient and public involvement in research. Res Involv Engagem 2015;1. https://doi.org/10.1186/s40900-015-0008-5.
- Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-89.
- NoMAD . Normalization Process Theory 2015. www.normalizationprocess.org/npt-toolkit/ (accessed 2014).
- Anstey A, Reynolds NJ. What does the BJD now stand for? A position statement. Br J Dermatol 2015;172:1463-5. https://doi.org/10.1111/bjd.13855.
- Nelson PA. Getting under the skin: qualitative methods in dermatology research. Br J Dermatol 2015;172:841-3. https://doi.org/10.1111/bjd.13720.
- University of York . NHS Economic Evaluation Database Handbook 2007. www.york.ac.uk/inst//crd/pdf/nhseed-handbook2007.pdf (accessed 29 January 2015).
- Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol 2009;27:469-74.
- Hensher D, Rose J, Greene W. Applied Choice Analysis: A Primer. Cambridge: Cambridge University Press; 2005.
- Daly A, Dekker T, Hess S. Dummy coding vs effects coding for categorical variables: clarifications and extensions. J Choice Model 2016;21:36-41. https://doi.org/10.1016/j.jocm.2016.09.005.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health 2016;19:300-15. https://doi.org/10.1016/j.jval.2016.04.004.
- Ryan M, Skatun D, Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht: Springer; 2008.
- Devlin NJ, Krabbe PFM. The development of new research methods for the valuation of EQ-5D-5L. Eur J Health Econ 2013;14:1-3. https://doi.org/10.1007/s10198-013-0502-3.
- Staidle JP, Dabade TS, Feldman SR. A pharmacoeconomic analysis of severe psoriasis therapy: a review of treatment choices and cost efficiency. Expert Opin Pharmacother 2011;12:2041-54. https://doi.org/10.1517/14656566.2011.590475.
- Blank PR, Blank AA, Szucs TD. Cost-effectiveness of oral alitretinoin in patients with severe chronic hand eczema: a long-term analysis from a Swiss perspective. BMC Dermatol 2010;10. https://doi.org/10.1186/1471-5945-10-4.
- Feldman SR, Garton R, Averett W, Balkrishnan R, Vallee J. Strategy to manage the treatment of severe psoriasis: considerations of efficacy, safety and cost. Expert Opin Pharmacother 2003;4:1525-33. https://doi.org/10.1517/14656566.4.9.1525.
- Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol 2014;28:333-7. https://doi.org/10.1111/jdv.12106.
- Ahn CS, Gustafson CJ, Sandoval LF, Davis SA, Feldman SR. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol 2013;14:315-26. https://doi.org/10.1007/s40257-013-0030-z.
- Sawyer L, Samarasekera EJ, Wonderling D, Smith CH. Topical therapies for the treatment of localized plaque psoriasis in primary care: a cost-effectiveness analysis. Br J Dermatol 2013;168:1095-105. https://doi.org/10.1111/bjd.12261.
- Jonas DE, Garbutt JC, Amick HR, Brown JM, Brownley KA, Council CL, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2012;157:645-54. https://doi.org/10.7326/0003-4819-157-9-201211060-00544.
- NHS Choices . Alcohol Units 2015. www.nhs.uk/Livewell/alcohol/Pages/alcohol-units.aspx (accessed May 2016).
- National Institute for Health and Care Excellence (NICE) . Physical Activity: Brief Advice for Adults in Primary Care [NICE Guidelines PH44] 2013. www.nice.org.uk/guidance/ph44 (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Physical Activity: Exercise Referral Schemes [NICE Guidelines PH54] 2014. www.nice.org.uk/guidance/ph54/ (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Smoking Cessation Services [NICE Guidelines PH10]. 2008. www.nice.org.uk/guidance/ph10 (accessed 28 January 2019).
- Department of Health and Social Care (DHSC) . Statistics on Smoking Cessation Services in the Health Action Zones in England, April 1999 to March 2000 2000. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/publicationsandstatistics/statistics/statisticalworkareas/statisticalpublichealth/dh_4083852 (accessed 28 January 2019).
- Department of Health and Social Care (DHSC) . Statistics on Smoking Cessation Services in Health Authorities: England April 2000 to March 2001 2001. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Pressreleases/DH_4010832 (accessed 28 January 2019).
- Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther 2010;35:139-51. https://doi.org/10.1111/j.1365-2710.2009.01085.x.
- Roberts J, Lenton P, Keetharuth AD, Brazier J. Quality of life impact of mental health conditions in England: results from the adult psychiatric morbidity surveys. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/1477-7525-12-6.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL, Drummond MF, et al. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Curtis L. Unit Costs of Health and Social Care 2014. Kent: Personal Social Services Research Unit, University of Kent; 2014.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2013 to 2014. 2015. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 28 January 2019).
- Murphy SM, Edwards RT, Williams N, Raisanen L, Moore G, Linck P, et al. An evaluation of the effectiveness and cost effectiveness of the National Exercise Referral Scheme in Wales, UK: a randomised controlled trial of a public health policy initiative. J Epidemiol Community Health 2012;66:745-53. https://doi.org/10.1136/jech-2011-200689.
- NHS Digital . Statistics on NHS Stop Smoking Services in England: April 2014 to September 2014 2015. www.hscic.gov.uk/catalogue/PUB16345 (accessed 28 January 2019).
- Vodermaier A, Millman RD. Accuracy of the Hospital Anxiety and Depression Scale as a screening tool in cancer patients: a systematic review and meta-analysis. Support Care Cancer 2011;19:1899-908. https://doi.org/10.1007/s00520-011-1251-4.
- Joint Formulary Committee . British National Formulary 2015. www.medicinescomplete.com/mc/bnf/current/.
- NHS Digital . Prescription Cost Analysis: England, 2014 2015. www.hscic.gov.uk/catalogue/PUB17274 (accessed 28 January 2019).
- Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ 2009;339. https://doi.org/10.1136/bmj.b4229.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Brindle P. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart 2008;94:34-9. https://doi.org/10.1136/hrt.2007.134890.
- Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum 2009;61:233-9. https://doi.org/10.1002/art.24172.
- NHS Choices . Psoriasis: Treatment 2015. www.nhs.uk/conditions/psoriasis/pages/treatment.aspx (accessed 28 January 2019).
- National Institute for Health and Care Excellence (NICE) . Psoriasis – Infliximab: Costing Template [TA134] 2008. www.nice.org.uk/resource/ta134/html/p/ta134-psoriasis-infliximab-costing-template?id=xxn7x7ytnvugwuf2wqvbhkkfae (accessed 28 January 2019).
- Carter CT, Martin S, DiBonaventura M, Annunziata K, Freedman D. Patient-Reported Psoriasis Disease Flaring and Impact of Flare Frequency on Humanistic Outcomes n.d.
Appendix 1 Selection criteria and data extraction forms used for systematic review of economic evaluations
This appendix links with Epidemiology of psoriasis and its association with risk of cardiovascular disease.
Selection criteria used in the systematic review
| Inclusion criteria | Exclusion criteria |
|---|---|
Studies were included in the review if they satisfied any of the following conditions:
|
Studies were excluded from the review if they satisfied any of the following conditions:
|
Quality assessment was performed, based on the NHS EED quality assessment guidelines. 36 The quality of all the studies was assessed by the reviewer and 10% of the studies were cross-assessed by the second reviewer to ensure consistency and certainty. 209 The quality assessment included the following key elements:
-
methods for deriving the effectiveness data
-
measurement of resource data
-
valuation of resource data
-
measurement and valuation of health benefits (utilities)
-
method of synthesising the costs and effects
-
analysis of uncertainty
-
generalisability of the results.
Data extraction form 1
| Author and year | Disease type and severity | Study design | Evaluation type | Setting | Perspective | Country |
|---|---|---|---|---|---|---|
| E.g. moderately severe – plaque | Model or trial analysis | CBA, CEA, CMA or CUA | E.g. primary care, outpatient | E.g. health-care system, societal |
Data extraction form 2
| Author and year | Biological therapies | Non-biological systemic therapies | Phototherapies | Topical (mono)-therapies | Topical combination therapies | Service organisation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 . . . | N1 | N2 . . . | P1 | P2 . . . | Tm1 | Tm2 . . . | Tc1 | Tc2 . . . | S1 | S2 . . . | |
| Study 1 | T | C | ||||||||||
| Study 2 | ||||||||||||
| Study n | ||||||||||||
Data extraction form 3
| Author and year | Population characteristics | Costs included | Adverse events included | Currency and price-year | Measure of health benefit | Model structure and sensitivity analysis | Time horizon | Discounting |
|---|---|---|---|---|---|---|---|---|
| E.g. yes (provided), limited detail | E.g. direct medical | Yes/no | E.g. £, 2007 | E.g. QALY, PASI75 | E.g. Markov, no sensitivity analysis | E.g. 1 year | E.g. 3.5% per year |
Data extraction form 4
| Author and year | Study funder and employers of authors | Potential financial interest | Are study results consistent with potential financial interest? |
|---|---|---|---|
Appendix 2 Characteristics of practices participating in workstream 2 screening study
This appendix links with Cardiovascular risk in patients with psoriasis.
| Practice ID | Number of registered patients | IMD | Practice type |
|---|---|---|---|
| P1 | 16,746 | 19.40 | Large deprived |
| P2 | 1086 | 14.81 | Small deprived |
| P3 | 3058 | 26.31 | Small affluent |
| P4 | 8762 | 19.93 | Small deprived |
| P5 | 12,875 | 35.07 | Large affluent |
| P6 | 5581 | 48.79 | Small affluent |
| P7 | 13,295 | 14.79 | Large deprived |
| P8 | 8049 | 44.63 | Small affluent |
| P9 | 3070 | 10.33 | Small deprived |
| P10 | 3647 | 10.76 | Small deprived |
| P11 | 12,606 | 13.20 | Large deprived |
| P12 | 8687 | 19.58 | Small deprived |
| P13 | 10,244 | 9.77 | Large deprived |
Appendix 3 Results from pulse wave velocity study
This appendix links with Cardiovascular risk in patients with psoriasis.
| Covariates | Severe psoriasis,a mean (SD) | p-value | Psoriatic arthritis,b mean (SD) | p-value | ||
|---|---|---|---|---|---|---|
| Yes (n = 27) | No (n = 97) | Yes (n = 44) | No (n = 80) | |||
| Unadjusted | 8.83 (8.08 to 9.58) | 8.80 (8.40 to 9.19) | 0.94 | 9.34 (8.74 to 9.95) | 8.52 (8.11 to 8.93) | 0.03 |
| Model 1: age and sex | 9.04 (8.35 to 9.73) | 8.84 (8.42 to 9.27) | 0.59 | 9.10 (8.55 to 9.65) | 8.74 (8.28 to 9.20) | 0.25 |
| Model 2: model 1 + ethnicity and deprivation | 9.07 (8.38 to 9.76) | 8.82 (8.40 to 9.24) | 0.49 | 9.12 (8.57 to 9.66) | 8.71 (8.25 to 9.16) | 0.19 |
| Model 3: model 2 + presence of known CVDc | 9.06 (8.37 to 9.76) | 8.81 (8.38 to 9.24) | 0.48 | 9.11 (8.56 to 9.66) | 8.69 (8.22 to 9.16) | 0.19 |
| Model 4: model 3 + smoking, diabetes, sBP and LDL cholesterol | 8.69 (7.95 to 9.43) | 8.54 (8.06 to 9.01) | 0.65 | 8.76 (8.16 to 9.37) | 8.45 (7.95 to 8.95) | 0.29 |
| Model 5: model 4 + CRP | 9.12 (8.35 to 9.88) | 8.88 (8.36 to 9.39) | 0.52 | 9.18 (8.52 to 9.83) | 8.78 (8.25 to 9.32) | 0.23 |
| Model 6: model 5 + eGFR | 8.63 (6.91 to 10.36) | 8.41 (6.92 to 9.91) | 0.53 | 8.62 (7.06 to 10.18) | 8.22 (6.75 to 9.69) | 0.19 |
| Model 7: model 6 + depression score (HADS-D) + anxiety score (HADS-A) | 8.57 (7.82 to 9.32) | 8.52 (8.05 to 8.99) | 0.89 | 8.64 (8.00 to 9.28) | 8.48 (7.98 to 8.98) | 0.61 |
| Model 8: model 7 + severe psoriasis | 8.69 (7.95 to 9.43) | 8.54 (8.06 to 9.01) | 0.65 | 8.74 (8.11 to 9.36) | 8.44 (7.93 to 8.94) | 0.32 |
| Model 9: model 7 + PsA | 8.54 (7.75 to 9.32) | 8.44 (7.93 to 8.94) | 0.76 | 8.76 (8.16 to 9.37) | 8.45 (7.95 to 8.95) | 0.29 |
| Covariates | Psoriasis severity (SAPASI quartiles), mean (SD) | p-value | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Model 1: age and sex | 8.17 (7.57 to 8.78) | 9.03 (8.36 to 9.70) | 9.03 (8.35 to 9.71) | 9.40 (8.57 to 10.23) | 0.0524 |
| Model 2: model 1 + ethnicity and deprivation | 8.20 (7.59 to 8.80) | 8.97 (8.31 to 9.64) | 9.02 (8.34 to 9.71) | 9.31 (8.48 to 10.13) | 0.0885 |
| Model 3: model 2 + presence of known CVDa | 8.18 (7.57 to 8.79) | 9.03 (8.34 to 9.71) | 9.12 (8.39 to 9.86) | 9.32 (8.49 to 10.15) | 0.0687 |
| Model 4: model 3 + smoking, diabetes, sBP and LDL cholesterol | 8.10 (7.45 to 8.74) | 8.72 (8.00 to 9.44) | 8.88 (8.08 to 9.67) | 8.80 (7.91 to 9.68) | 0.2762 |
| Model 5: model 4 + CRP | 8.34 (7.61 to 9.06) | 8.88 (8.09 to 9.67) | 9.01 (8.13 to 9.88) | 8.81 (7.87 to 9.75) | 0.5187 |
| Model 6: model 5 + eGFR | 8.11 (6.35 to 9.86) | 8.66 (6.91 to 10.40) | 8.77 (6.88 to 10.66) | 8.56 (6.58 to 10.55) | 0.5125 |
| Model 7: model 6 + depression score (HADS-D) + anxiety scores (HADS-A) | 8.12 (6.35 to 9.89) | 8.64 (6.86 to 10.41) | 8.80 (6.88 to 10.72) | 8.58 (6.59 to 10.58) | 0.5437 |
| Model 8: model 7 + PsA | 8.05 (6.23 to 9.88) | 8.59 (6.78 to 10.39) | 8.74 (6.78 to 10.70) | 8.53 (6.50 to 10.56) | 0.5342 |
| Covariates | Age at diagnosis of psoriasis, mean (SD) | p-value | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Null | 8.16 (7.54 to 8.77) | 8.43 (7.79 to 9.07) | 9.17 (8.48 to 9.86) | 9.66 (8.92 to 10.41) | 0.0096 |
| Model 1: age and sex | 8.59 (8.01 to 9.17) | 8.82 (8.14 to 9.50) | 9.33 (8.63 to 10.04) | 9.08 (8.37 to 9.80) | 0.3622 |
| Model 2: model 1 + ethnicity and deprivation | 8.64 (8.04 to 9.23) | 8.82 (8.14 to 9.51) | 9.31 (8.60 to 10.01) | 8.98 (8.26 to 9.70) | 0.4863 |
| Model 3: model 2 + presence of known CVDa | 8.61 (8.01 to 9.22) | 8.80 (8.11 to 9.50) | 9.30 (8.59 to 10.01) | 8.97 (8.25 to 9.69) | 0.4755 |
| Model 4: model 3 + smoking, diabetes, sBP and LDL cholesterol | 8.34 (7.67 to 9.00) | 8.48 (7.78 to 9.18) | 8.82 (8.12 to 9.53) | 8.81 (8.07 to 9.54) | 0.6294 |
Appendix 4 PhD thesis abstract: risk communication and lifestyle behaviour change in people with psoriasis
This appendix links with Cardiovascular disease risk communication and reduction in psoriasis. Reproduced with permission from Keyworth. 122
PhD thesis title: risk communication and lifestyle behaviour change in people with psoriasis
PhD thesis abstract
People with psoriasis are known to engage in high levels of unhealthy lifestyle behaviours that may lead to poorer psoriasis outcomes and increase the risk of CVD. Thus, helping individuals with psoriasis understand the link between behaviours and health risks, that is health risk communication and direct support for LBC are important aspects in optimal management of psoriasis, a long-term inflammatory skin condition.
There are two aspects of the literature that remain unclear: first, whether or not adequate support is given to patients to enable them to understand that the links between lifestyle behaviours and health outcomes is part of psoriasis patient management strategies; and, second, whether or not there is agreement around effective health risk communication techniques. This programme of research aimed to examine these gaps in the literature using four related studies.
The first study used content analysis to examine general and dermatology-specific health-care professionals’ core training competencies for evidence of skills relating to LBC. An important finding was the lack of explicit skills relating to LBC and changing understanding of health risks. There was little or no reference to recognised LBC techniques that could be used to support and facilitate LBC with patients.
The second study used observational techniques to examine messages about the links between behaviour and health outcomes and LBC signposting (such as leaflets or posters about healthy living) for patients with psoriasis in primary and secondary care patient waiting areas. There was little evidence of psoriasis-specific information about healthy living. Generic information (not specifically about psoriasis) was often of poor quality and poorly displayed and did not conform to evidence-based recommendations for effective LBC signposting.
The third study combined observational and qualitative techniques to examine how health-care professionals communicate information about CVD risk to patients and the role of LBC in reducing risk in the context of primary care risk assessments with people with psoriasis. A key finding was that interpretation of risk information was not always linked to specific advice about how to modify each risk factor. Discussion was mostly instructional rather than a shared collaborative discussion about behaviour change and risk reduction.
The fourth study used experimental methods to examine the effects of message-framing theory as a health risk communication strategy on reported behavioural intentions in people with psoriasis. An important finding was that, for messages about psoriasis symptom reduction, gain-framed (positively framed) messages were more effective in increasing behavioural intentions for reducing alcohol consumption. Conversely, for messages about CVD risk reduction, loss-framed (negatively framed) messages were more effective for increasing behavioural intentions to reduce alcohol consumption.
The body of work presented in this thesis demonstrates that much needs to be done to increase the skill sets of health-care professionals to help people with psoriasis recognise the specific links between their own health behaviours and health outcomes. In addition, specific recommendations have been suggested as a way of improving risk communication strategies, such as using theory-based personally relevant health information for people with psoriasis.
Lay abstract
Psoriasis is a long-term skin condition that often appears as skin redness and ‘plaques’ on the surface of the skin. People with psoriasis are known to engage in an unhealthy lifestyle (increased alcohol intake, smoking, insufficient physical activity and poor diet). This worsens psoriasis and leads to the additional risk of CVD (e.g. heart attack). Telling people about these risks should be a key part of health-care management in patients with psoriasis. Yet there is limited evidence of the best way of doing this, in a way that leads people to live healthier lives and reduce long-term health risks.
The programme of research aimed to examine this using four research studies.
The first study examined whether or not the core training goals for primary and secondary health-care professionals (e.g. doctors, nurses, dermatologists) included information about the importance of healthy lifestyle. An important finding was the lack of reference to specific ways that health-care professionals could encourage and support patients to live healthier lifestyles (e.g. setting small achievable goals, setting an action plan).
The second study examined information available to patients about healthy living (such as leaflets or posters about healthy living) in primary and secondary care patient waiting areas (e.g. doctor’s surgeries). There was little evidence of psoriasis-specific information about healthy living. Generic information (not specifically about psoriasis) was sometimes available but the quality was often poor and not displayed in a way that patients could read and understand the information.
The third study examined how doctors and nurses talk about CVD to patients with psoriasis. A key finding was the lack of specific advice about how patients could have healthier lifestyles to reduce long-term health risks.
The fourth study examined the effect of differently worded health messages (positively worded or negatively worded) on whether or not people would decide to make healthy changes to their lifestyle (e.g. increasing physical activity). When patients were told that healthy living improves the visible appearance of psoriasis, positively worded messages were more effective than negative ones in encouraging people to consider making lifestyle changes. When patients were told that healthy living would reduce the likelihood of CVD (e.g. heart attack), negatively worded messages were more effective.
The research presented in this thesis suggests that more attention could focus on encouraging patients with psoriasis to live healthier lives. Specific ways of improving how patients with psoriasis are told about this involves appropriately worded, personally relevant health information.
Appendix 5 The dermatology specialist nurse survey of lifestyle management
This appendix links with Understanding the professional role in supporting lifestyle change in patients with psoriasis.
Appendix 6 Patient Readiness to Engage in Health Information Technology survey: responses from 127 individuals
This appendix links with Psoriasis and Wellbeing (PSO WELL): developing patient materials to broaden understanding of psoriasis as a long-term condition, psoriasis-associated comorbidities and the role of self-management.
The PRE-HIT questionnaire was adapted with permission from Koopman et al. 166 for use in the IMPACT programme. Adaptations were minimal and comprised only changes from US to UK terminology/spelling.
A total of 127 people returned the survey:
-
age 25–90 years (mean 57 years, median 59 years)
-
41% male and 59% female.
The technology-based questions of the survey may have influenced who responded but both groups had previously given us e-mail addresses or mobile phone numbers with which to contact them (data from workstream 2).
The responding group included more individuals who used e-mail than the non-responding group: 60% e-mail compared with 46%. However, mobile phone use was higher in the non-responding group: 50% mobile phone for responding group compared with 60% in the non-responding group.
The PRE-HIT questionnaire results (scores ≤ 100)
Note that the higher the score, the more likely a person is to engage:
-
mean 69.03, median 69.00 (minimum 33, maximum 95)
-
wide distribution of scores
-
no gender differences
-
the greater the age, the less likely to engage.
FIGURE 33.
The PRE-HIT scores by gender. (a) Female; and (b) male.
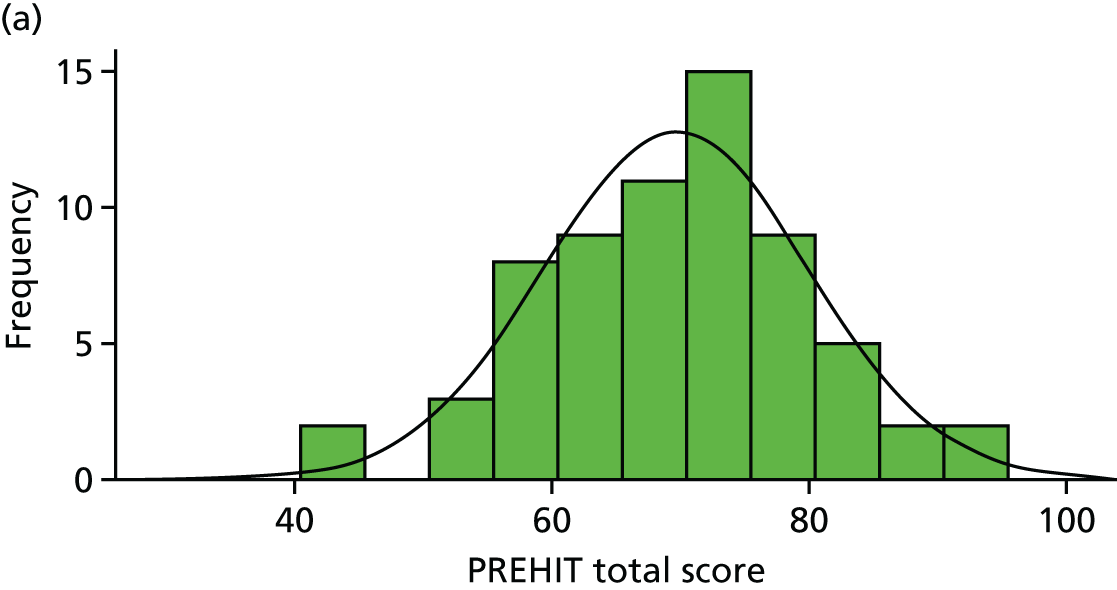
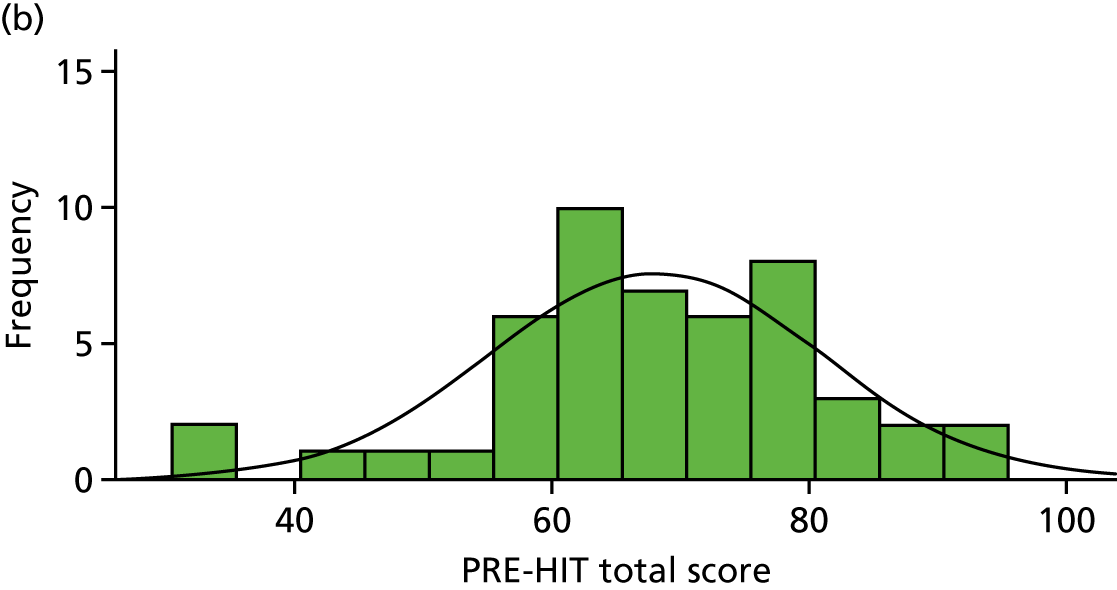
FIGURE 34.
Scatterplot of the PRE-HIT scores and age showing negative correlation between age and score. R2 linear = 0.424.
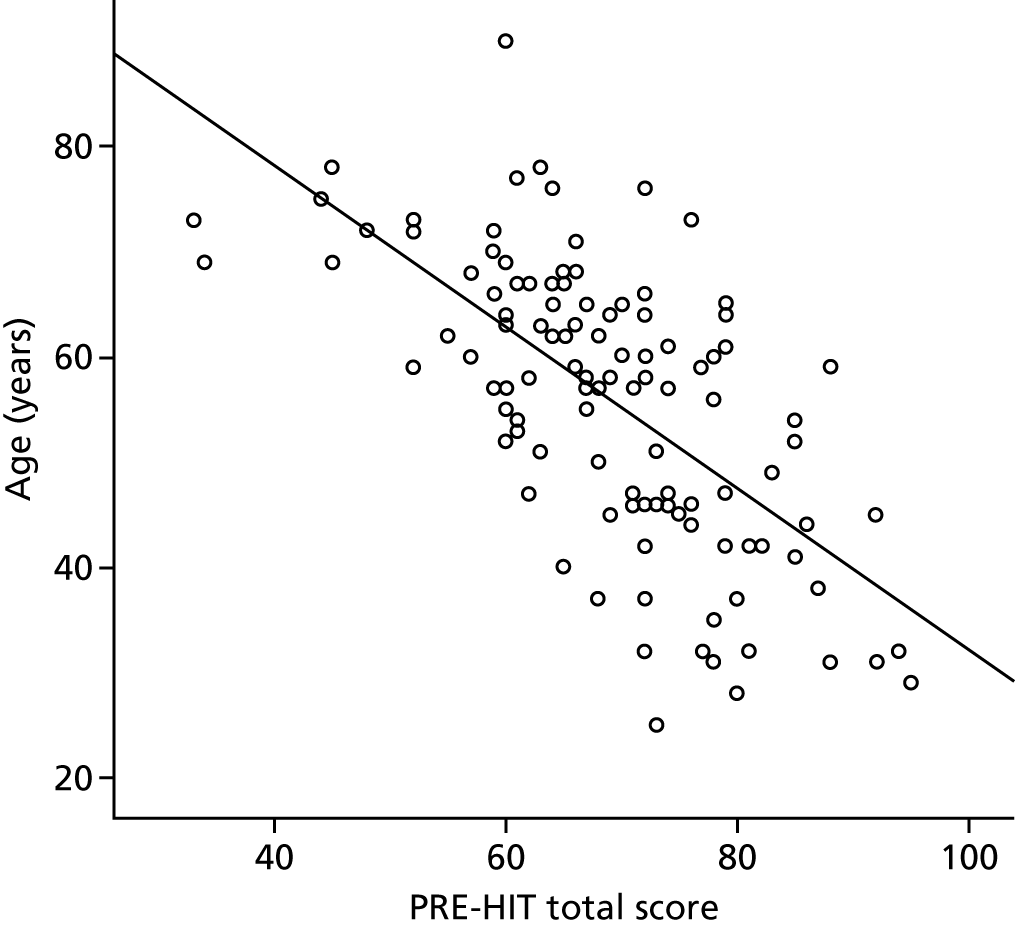
Mobile phone expertise scores (from two questions)
FIGURE 35.
The PRE-HIT mobile phone expertise total scores showing no significant difference between men and women.
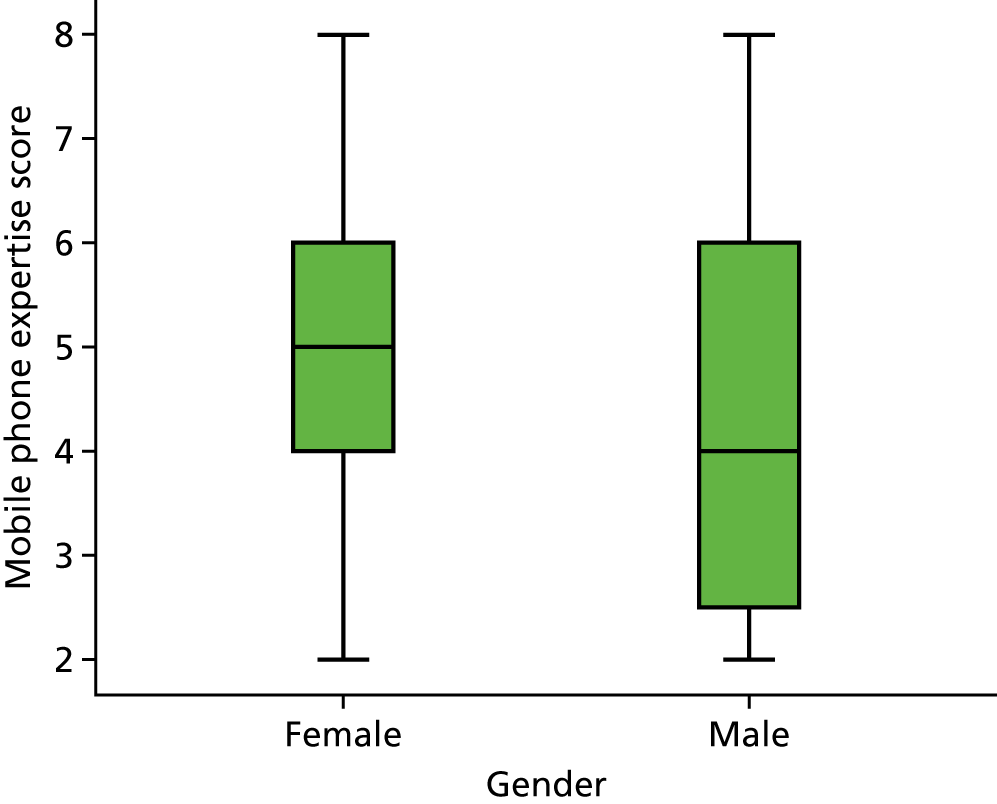
FIGURE 36.
Responses to PRE-HIT question 12 (‘I go online using my mobile phone’) responses by gender.
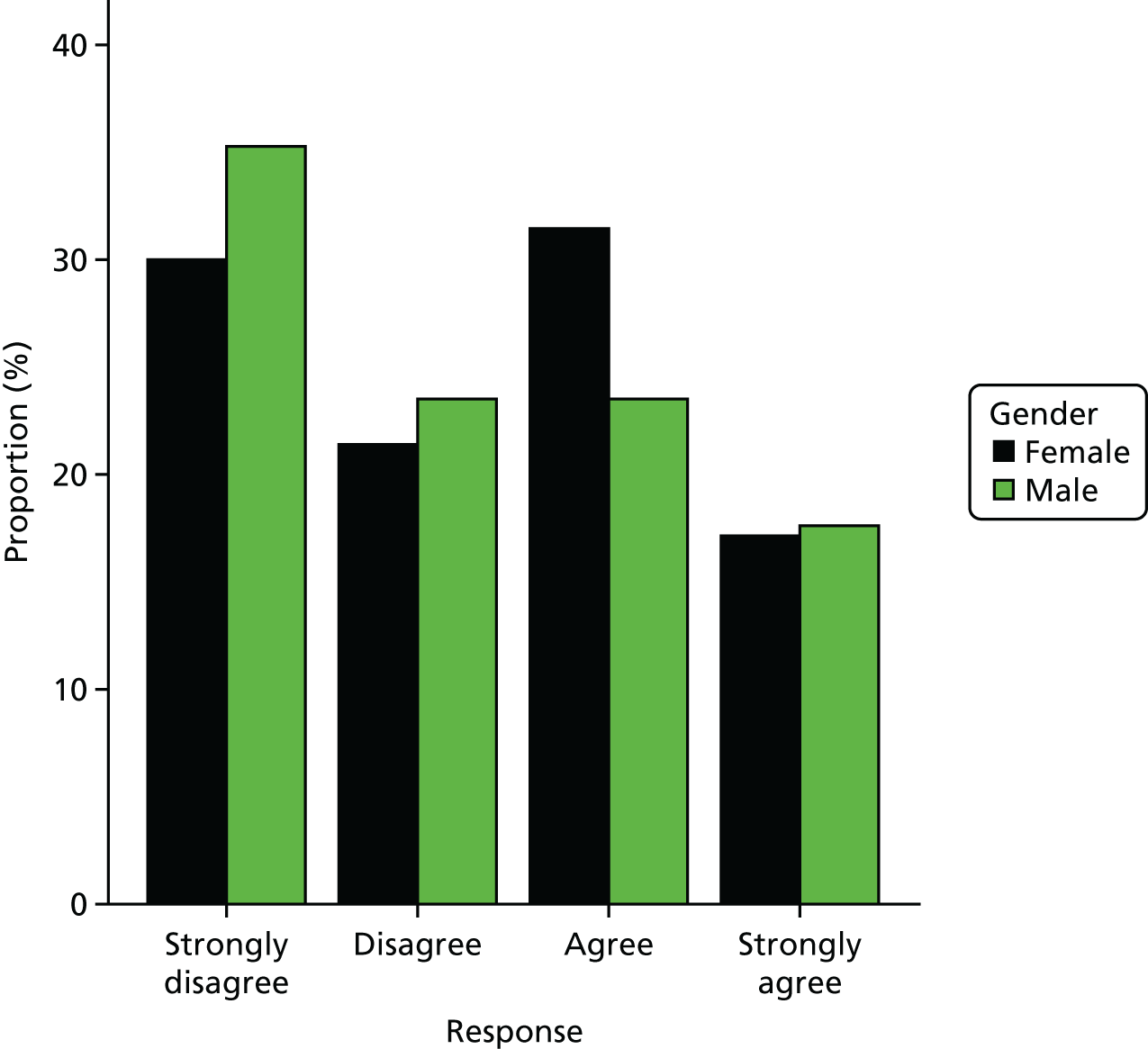
FIGURE 37.
Responses to PRE-HIT question 13 (‘I use my mobile phone to text people almost every day’) responses by gender.
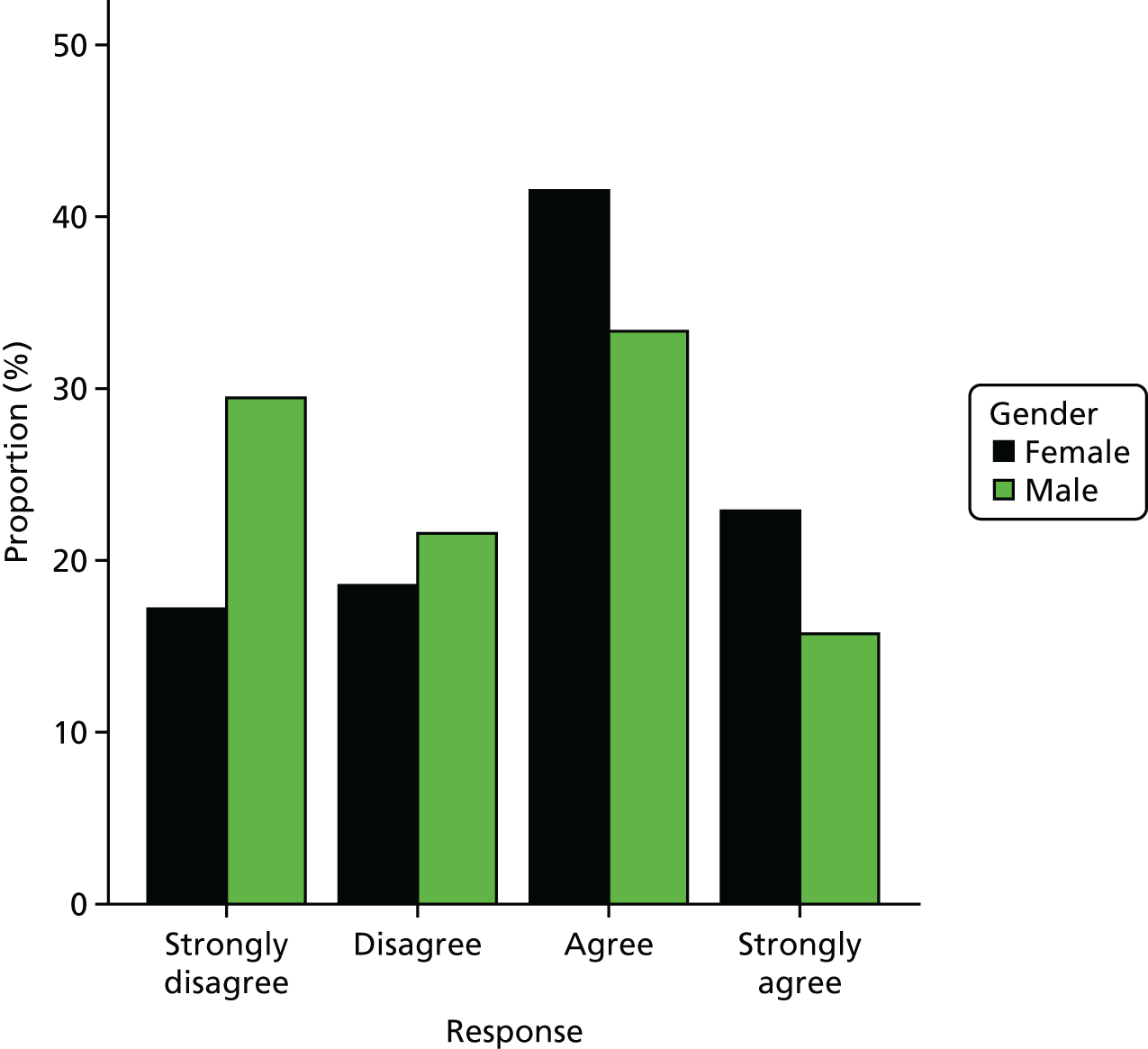
Computer internet experience expertise scores (from four questions)
Note that the lower the score, the less able a person is to use the computer/internet.
FIGURE 38.
The PRE-HIT computer internet experience expertise total scores by gender.
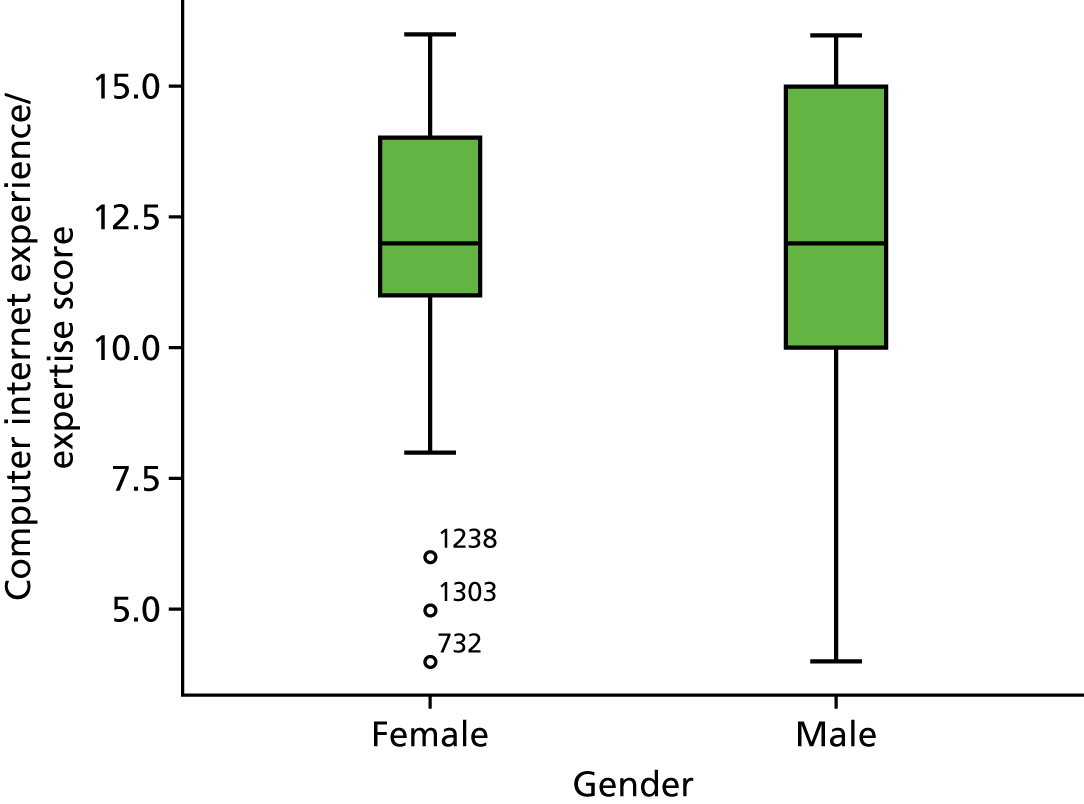
FIGURE 39.
Responses to PRE-HIT question 14 (‘If I went on the computer, I would be able to figure out most problems that I might run into’) by gender.
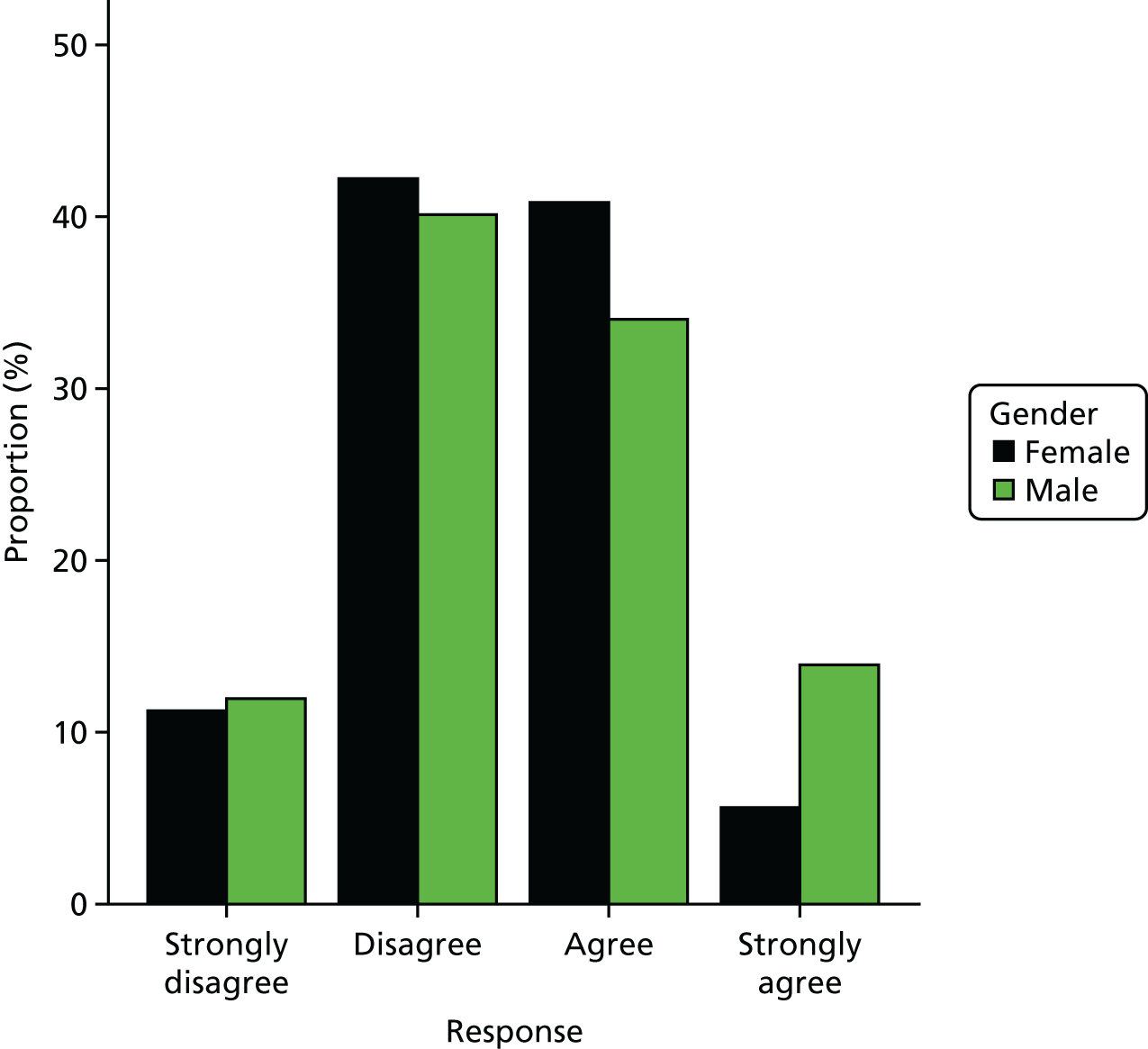
FIGURE 40.
Responses to PRE-HIT question 16 (‘If I went on the computer, I would have access to the internet’) by gender.
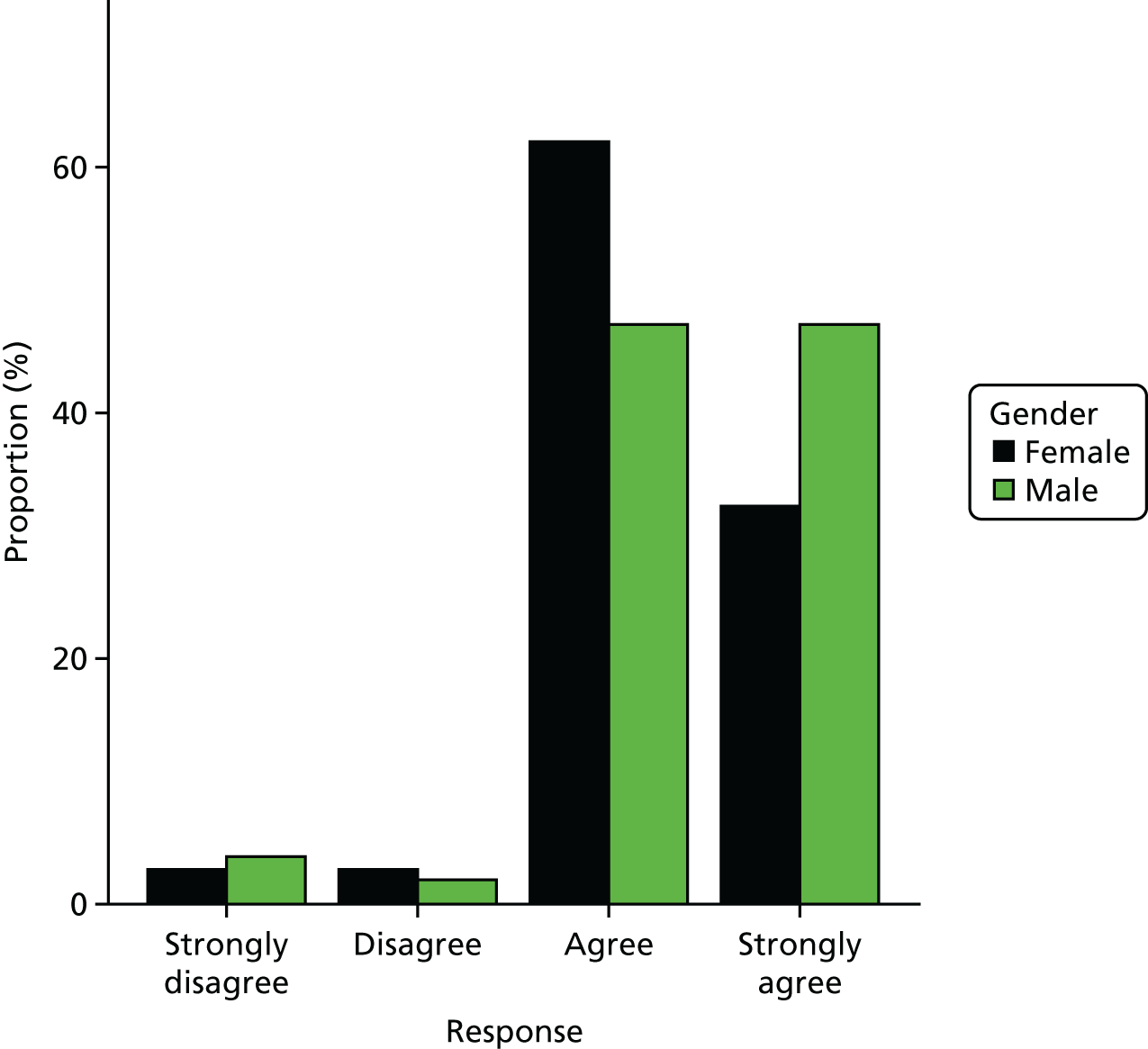
FIGURE 41.
Responses to PRE-HIT question 17 (‘If I went on the internet, I would find using it to be easy’) by gender.
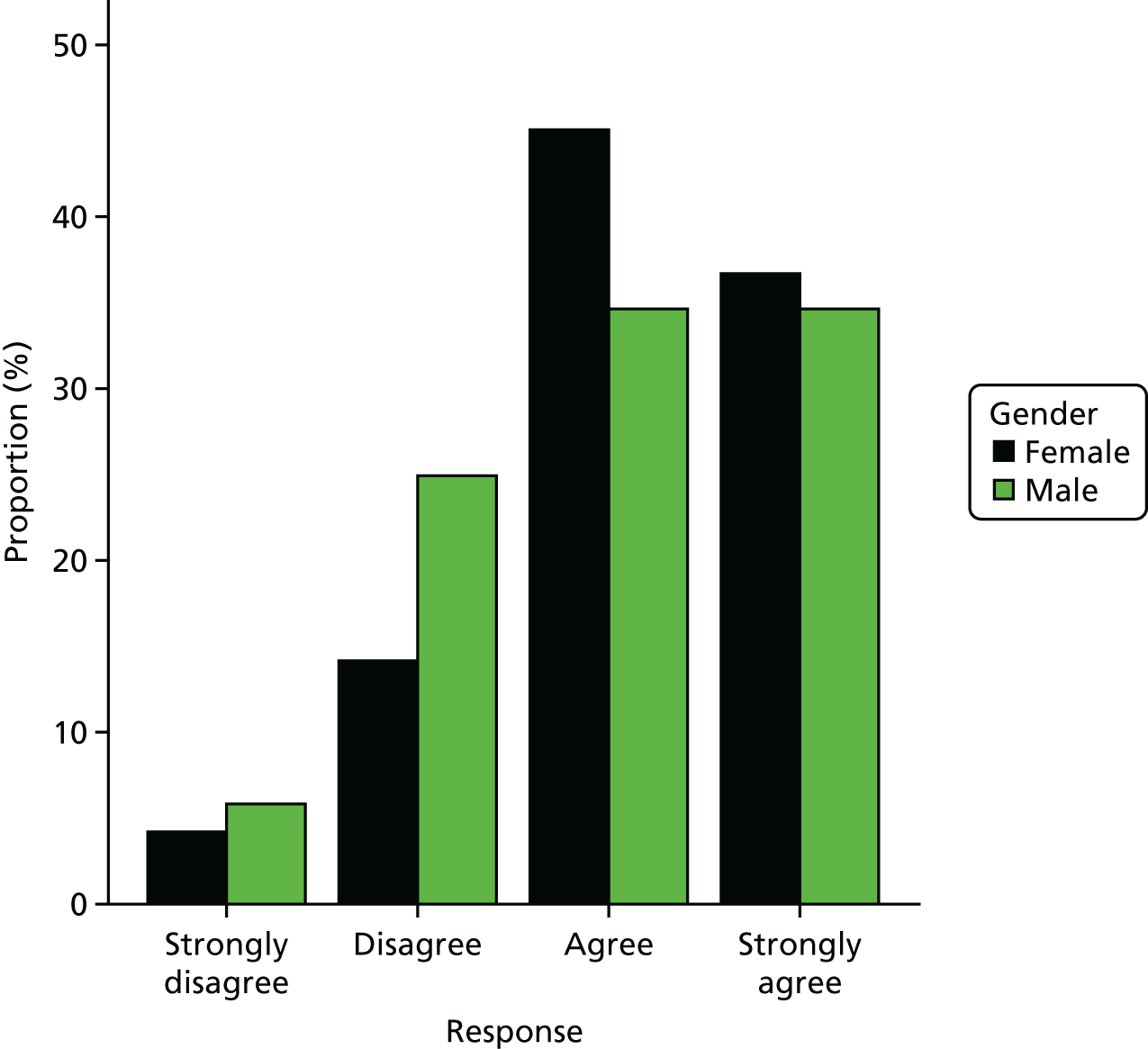
FIGURE 42.
Responses to PRE-HIT question 18 (‘If I went on the internet, I would find using e-mail to be easy’) by gender.

Computer anxiety scores (from four questions)
Note that the lower the score, the greater the computer anxiety experienced by a person.
FIGURE 43.
The PRE-HIT computer anxiety scores by gender.
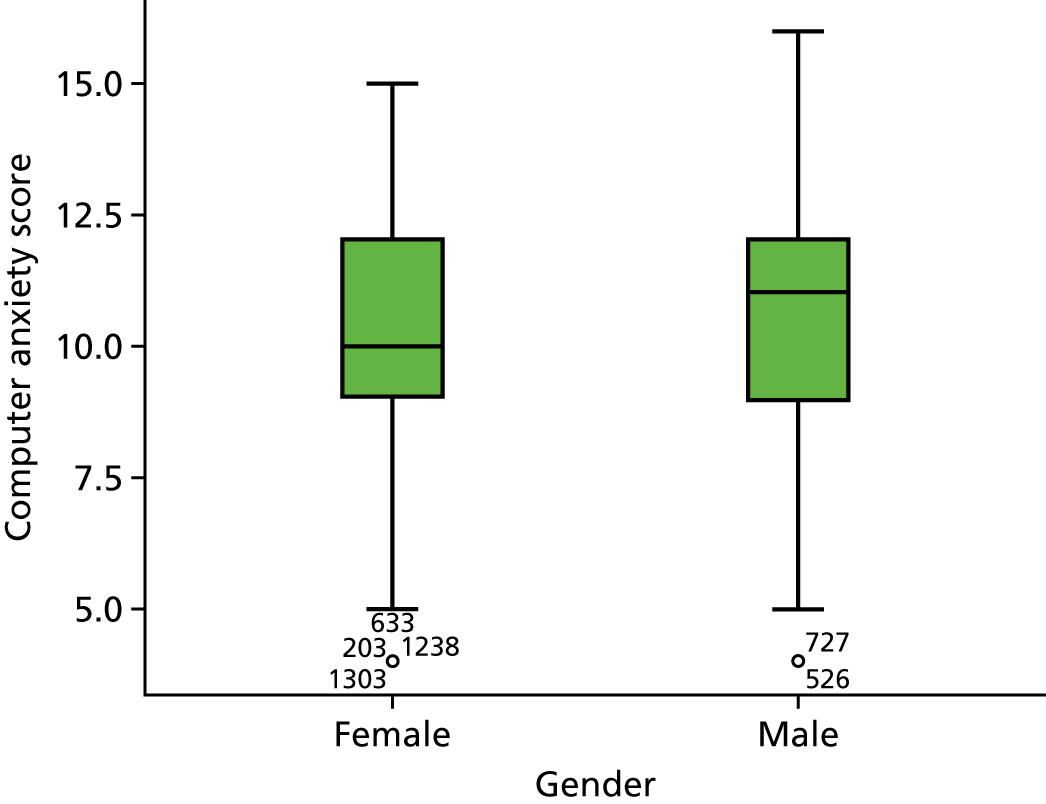
FIGURE 44.
Responses to PRE-HIT question 15 (‘If I went on the computer, I would find using it to be frustrating’) by gender.

FIGURE 45.
Responses to PRE-HIT question 21 (‘If I went on the internet, I would get frustrated with the amount of information I found about health’) by gender.
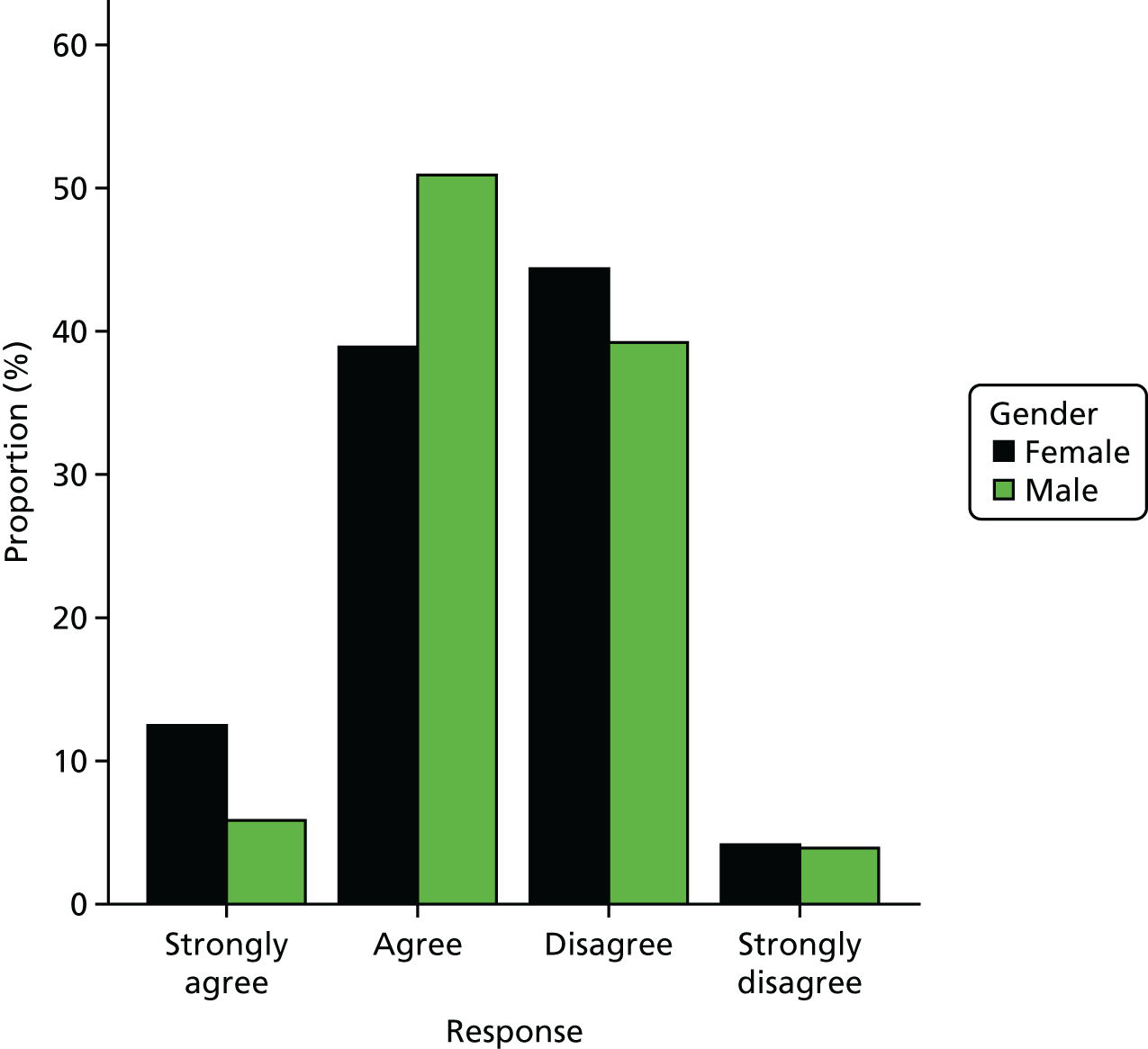
FIGURE 46.
Responses to PRE-HIT question 22 (‘If I went on the internet, I would find searching for information would be stressful’) by gender.
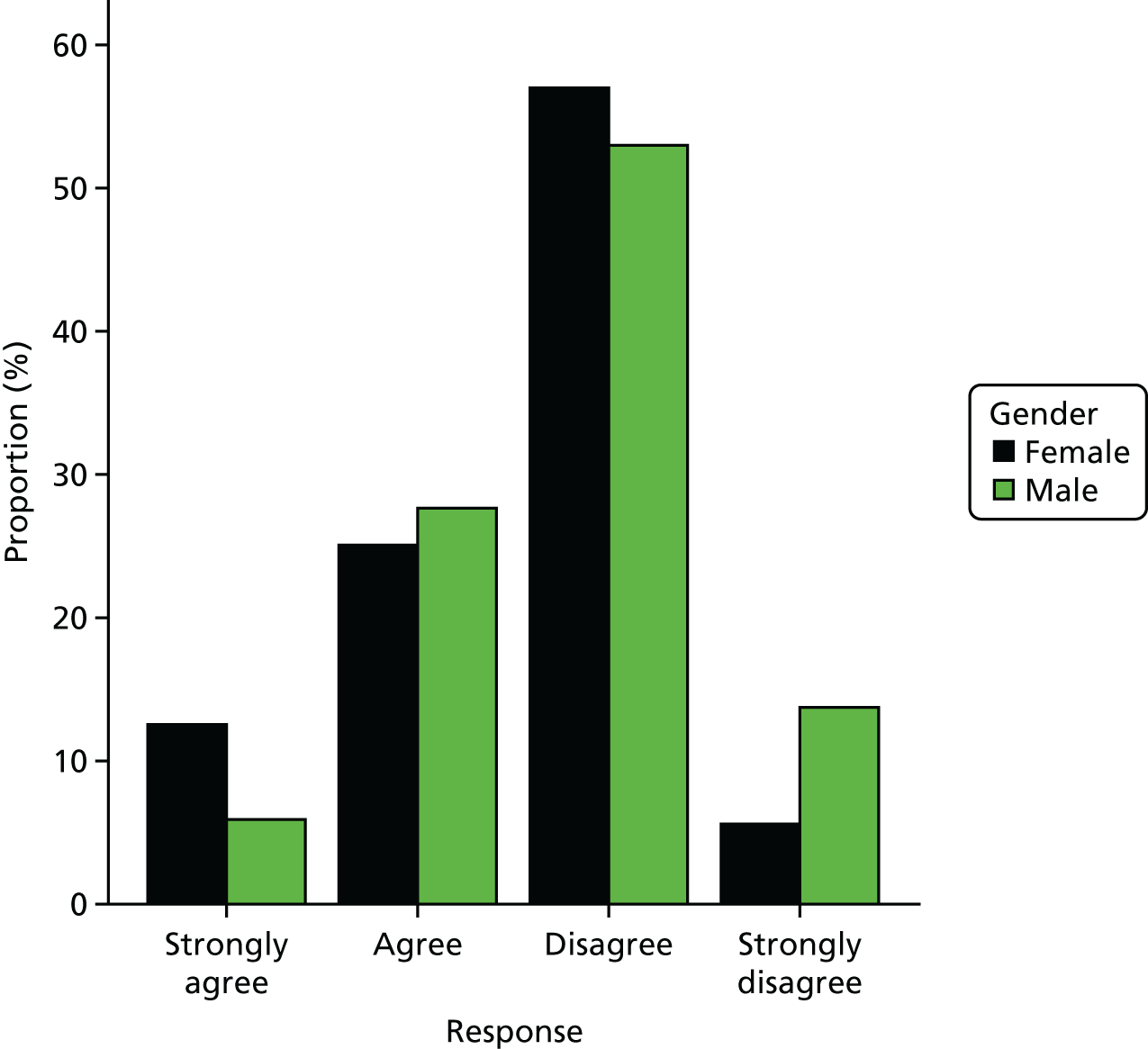
FIGURE 47.
Responses to PRE-HIT question 23 (‘If I went on the internet, I would find sorting through information to be too time-consuming’) by gender.
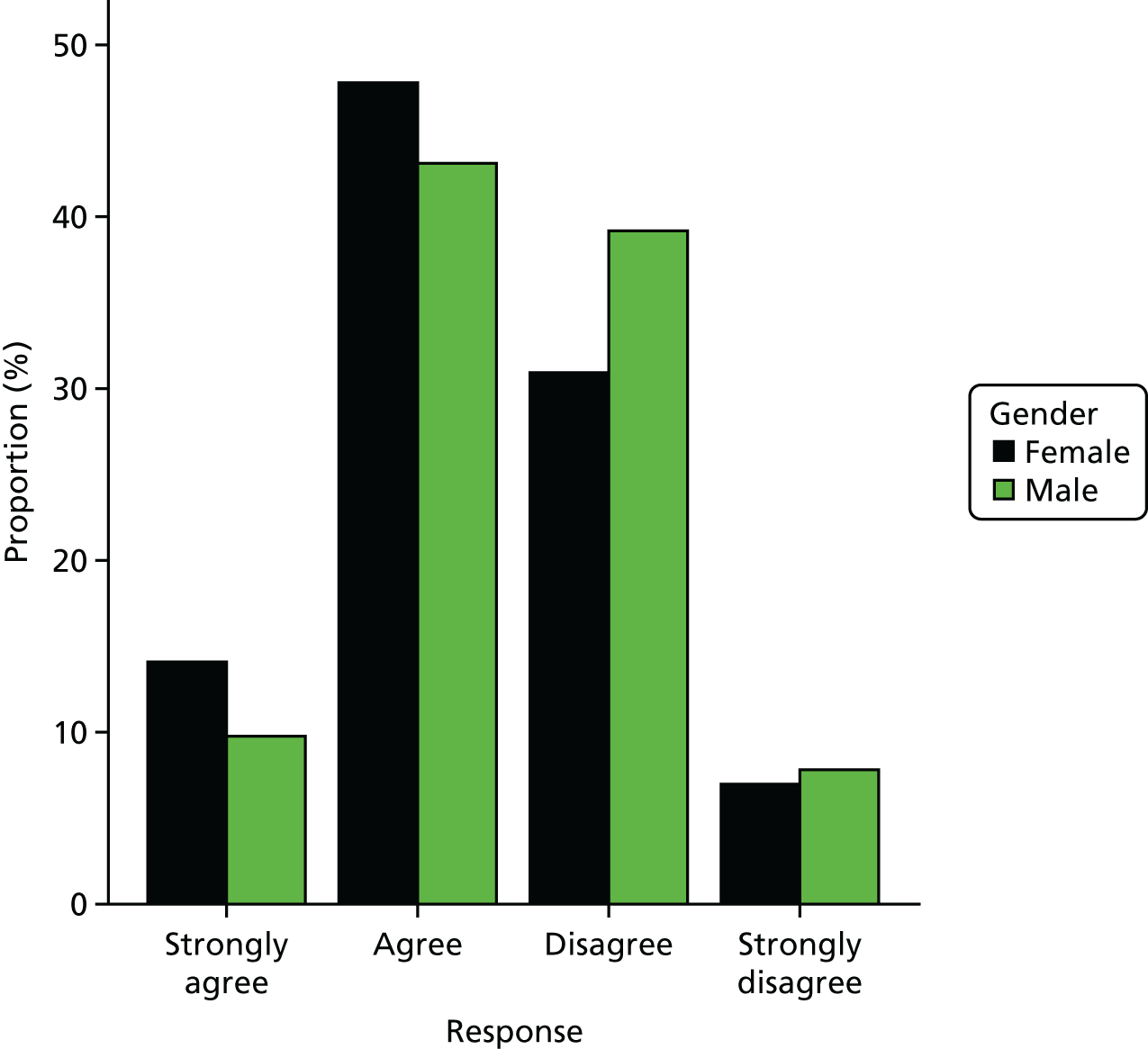
Internet privacy concern scores (from two questions)
Note that the lower the score, the greater the concern.
FIGURE 48.
The PRE-HIT internet privacy concern scores by gender.
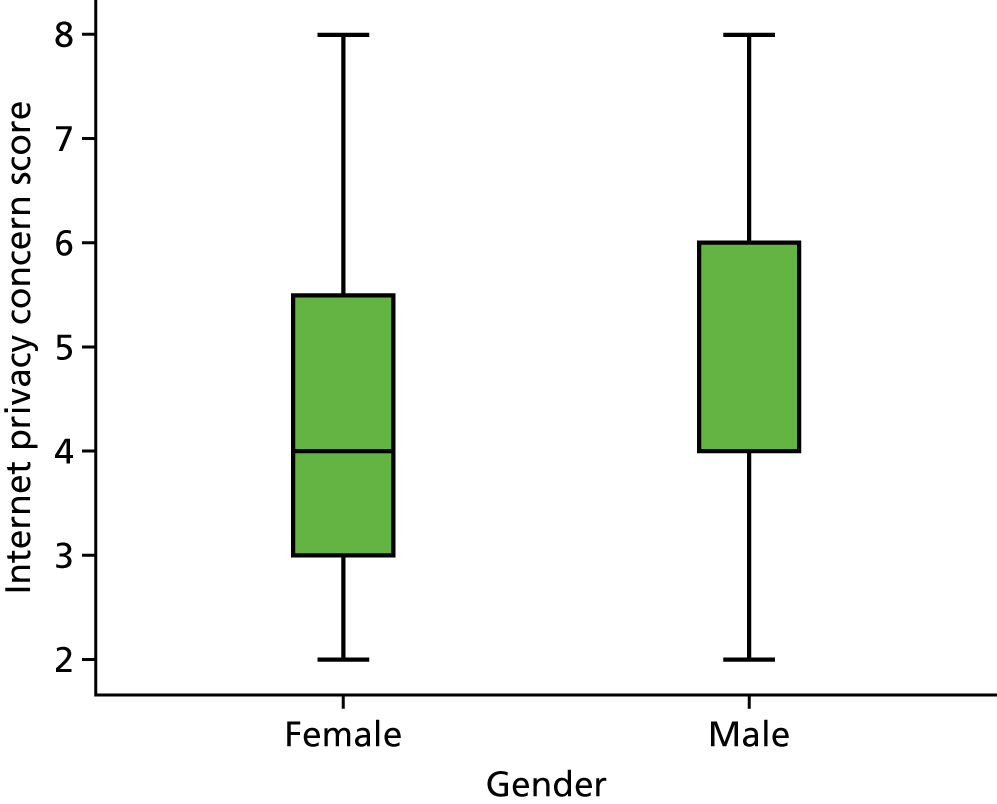
FIGURE 49.
Responses to PRE-HIT question 19 (‘If I went on the internet, I would be very concerned about giving any personal information’) by gender.
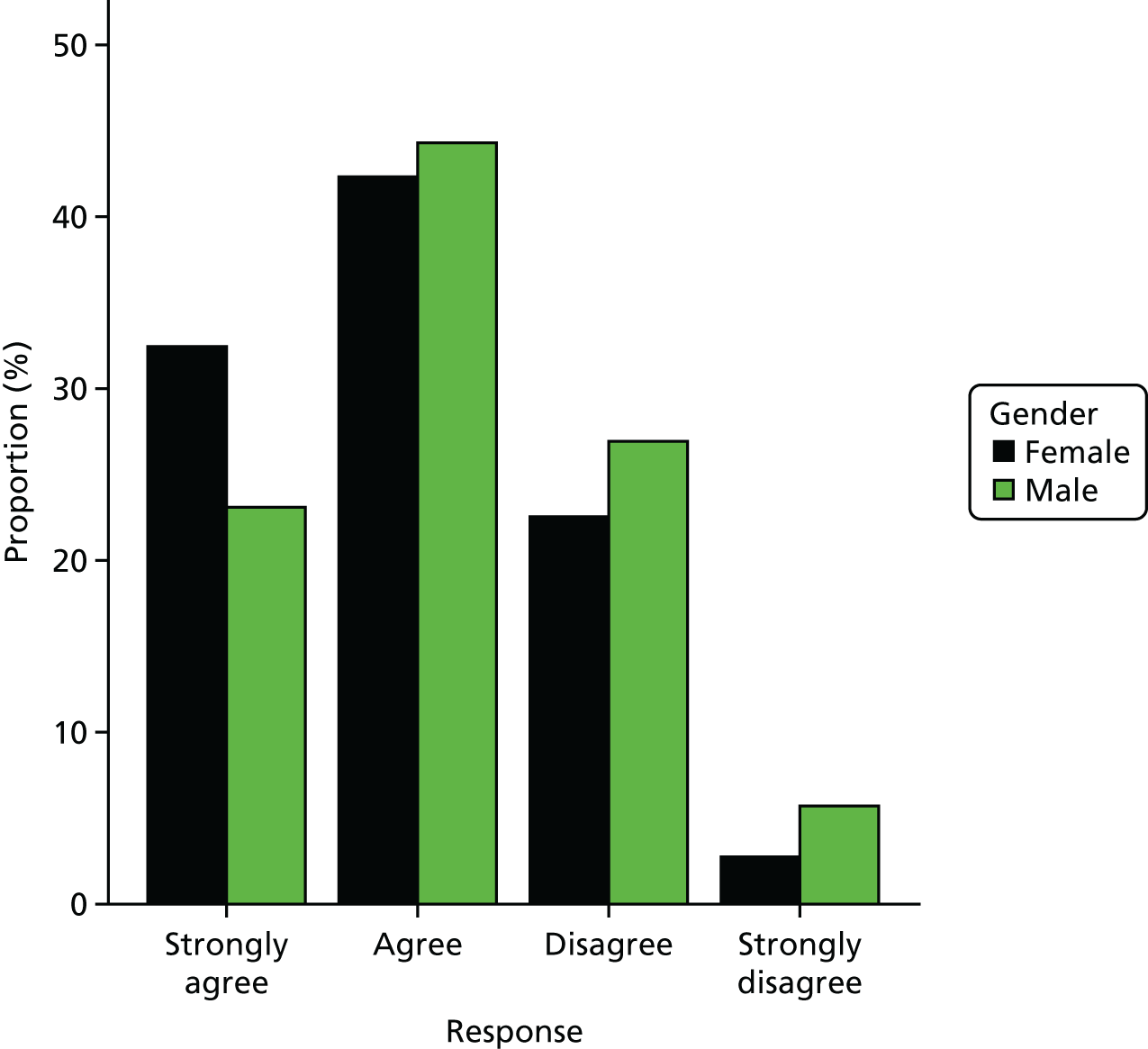
FIGURE 50.
Responses to PRE-HIT question 20 (‘If I went on the internet, I would be concerned it would lead to invasions of my privacy’) by gender.
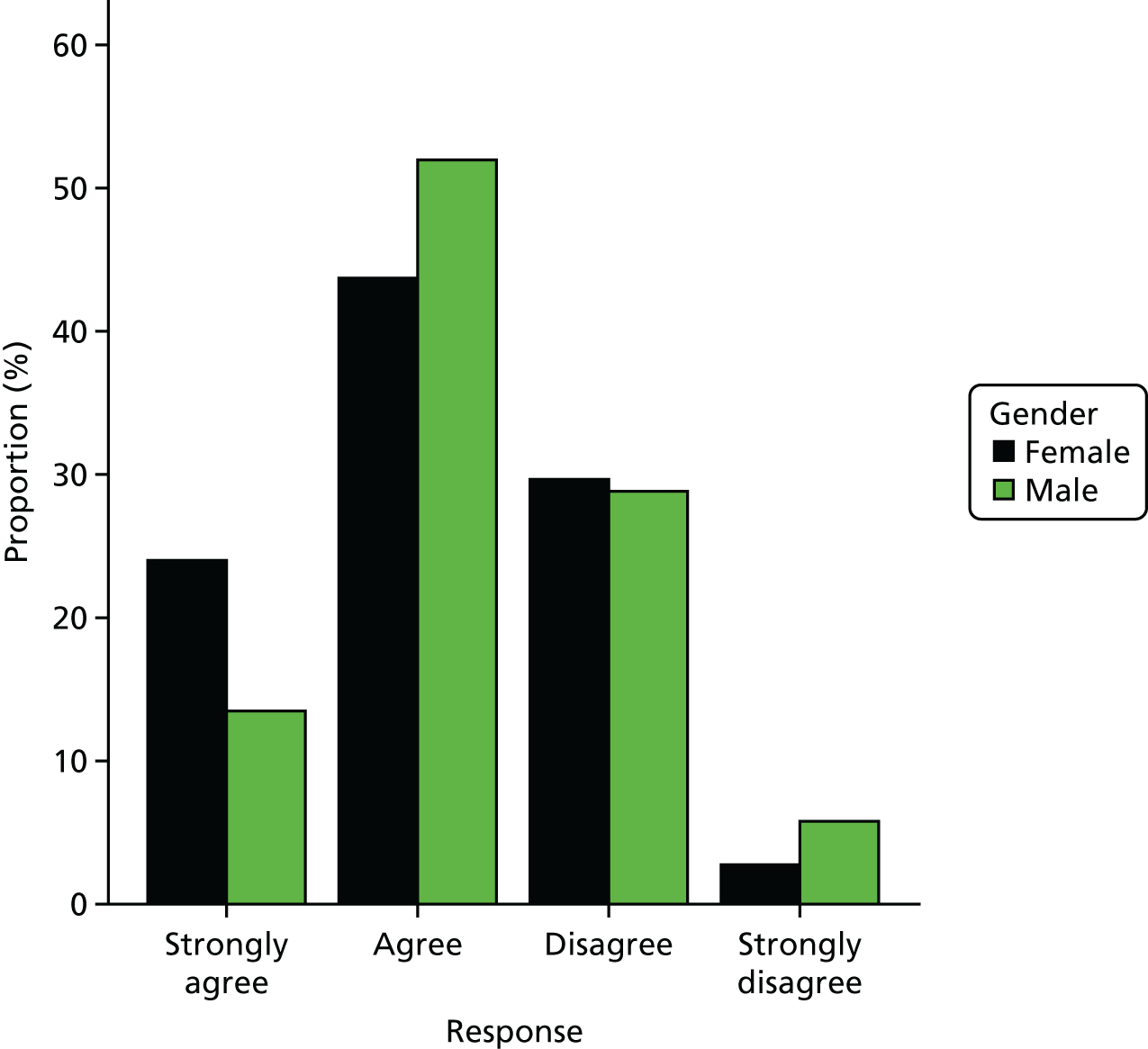
Health information need scores (from five questions)
Note that the lower the score, the less information is needed.
FIGURE 51.
The PRE-HIT health information need total scores by gender.
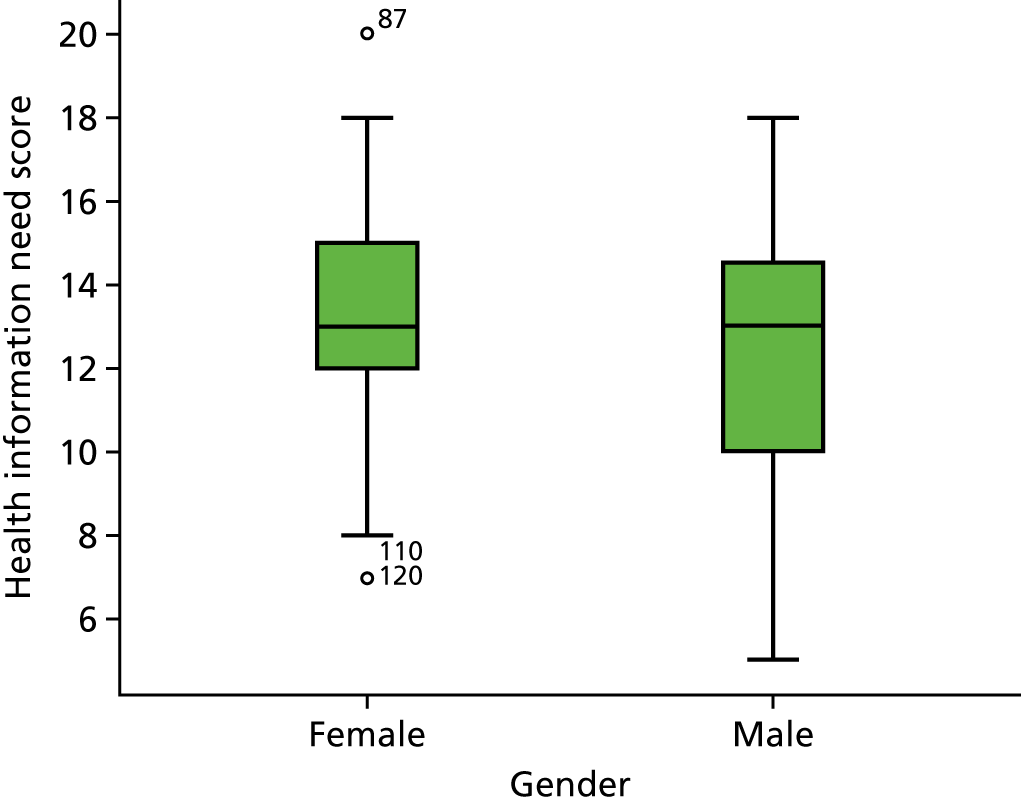
FIGURE 52.
Responses to PRE-HIT question 24 (‘If I went on the internet, I would use it to look up things so that I would not worry about them any more’) by gender.
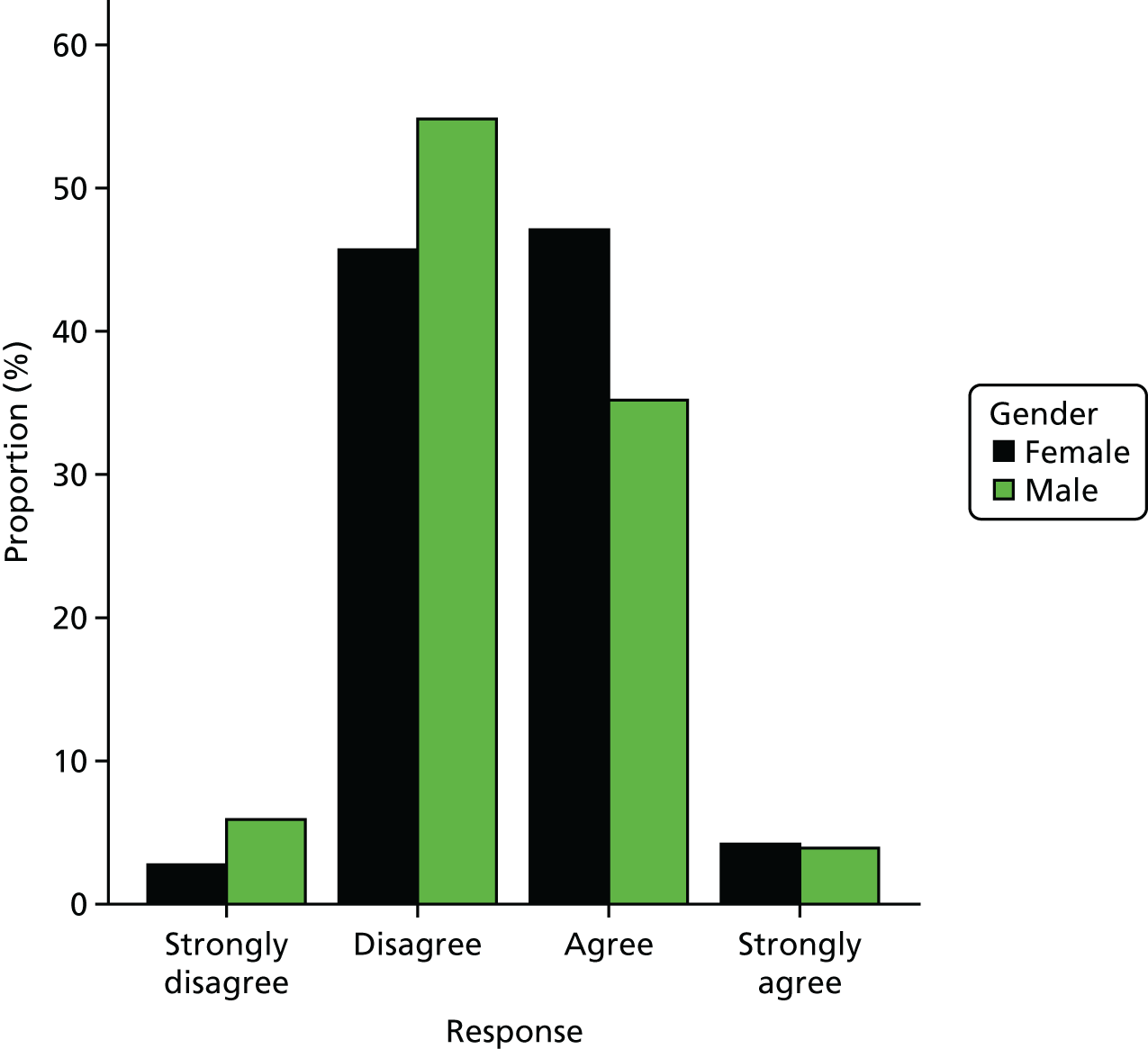
FIGURE 53.
Responses to PRE-HIT question 25 (‘If I went on the internet, I would use it to look up information about herbals and/or supplements’) by gender.

FIGURE 54.
Responses to PRE-HIT question 26 (‘If I went on the internet I would use it to look up symptoms’) by gender.

FIGURE 55.
Responses to PRE-HIT question 27 (‘If I went on the internet I would use it to search for information about my health’) by gender.
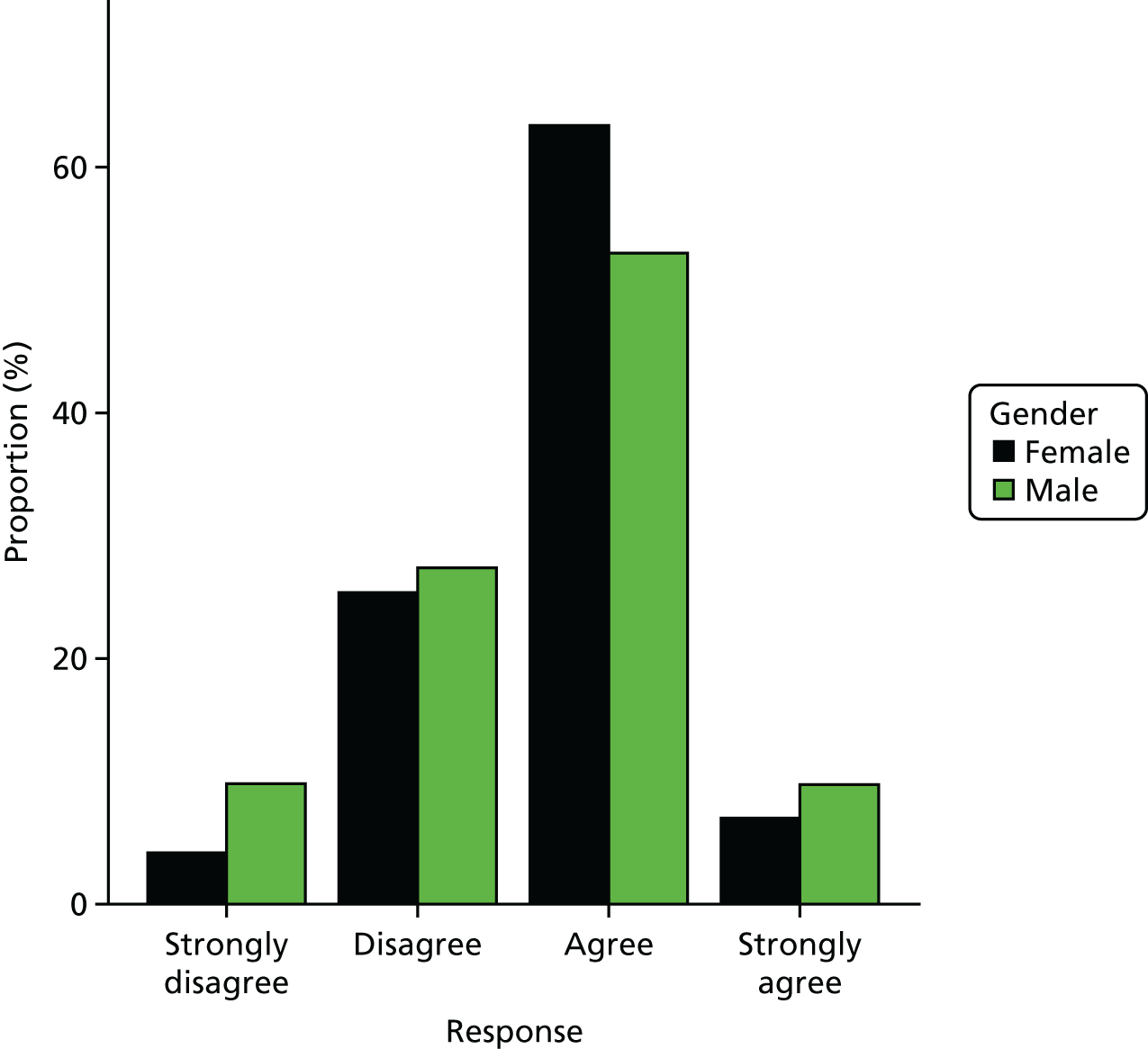
FIGURE 56.
Responses to PRE-HIT question 28 (‘If I went on the internet I would use the internet to find information about medications’) by gender.
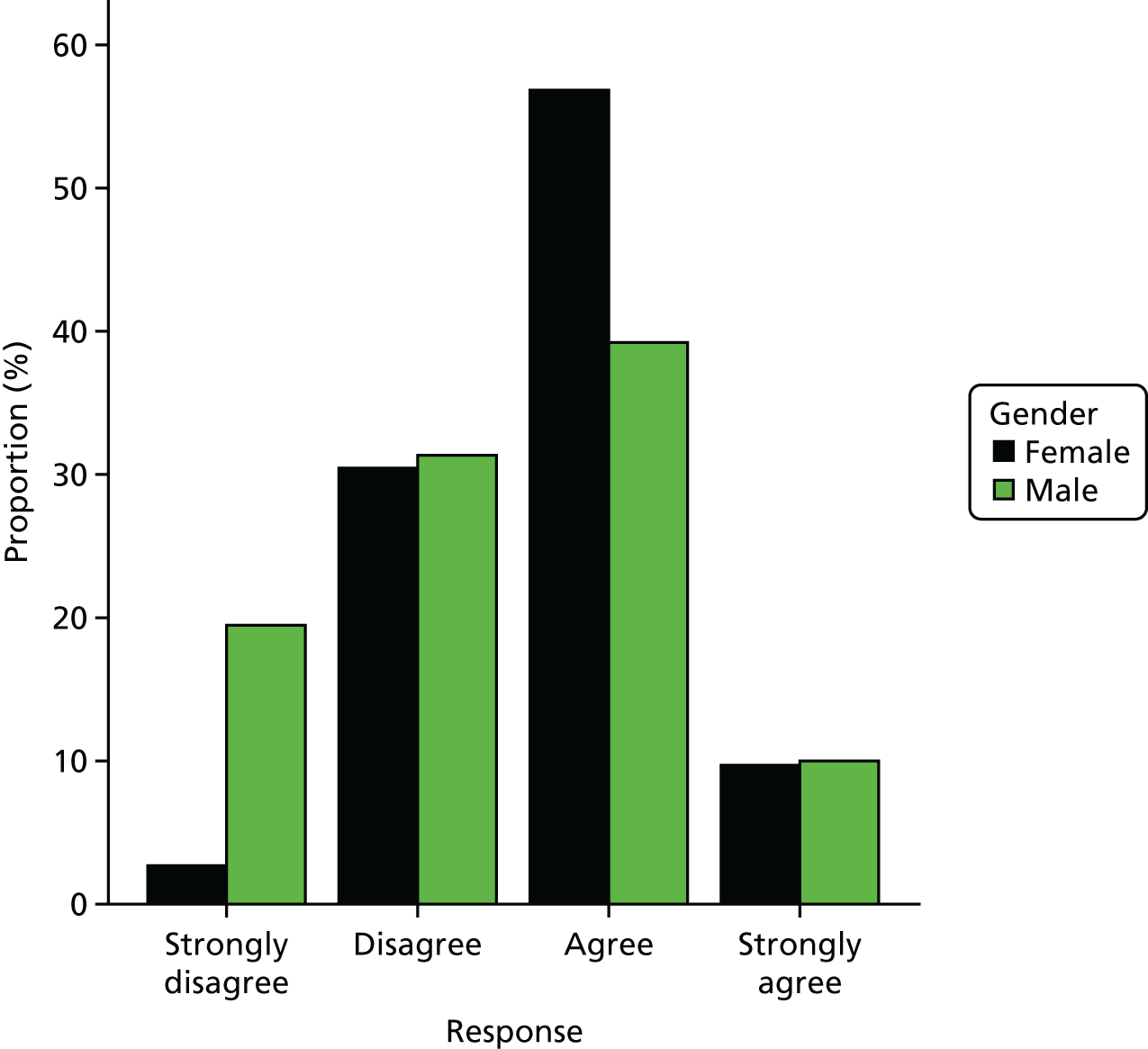
‘No news is good news’ scores (from three questions)
Note that the lower the score, the greater the belief in ‘no news is bad news’ (i.e. the greater the information anxiety).
FIGURE 57.
The PRE-HIT ‘no news is good news’ total scores by gender.
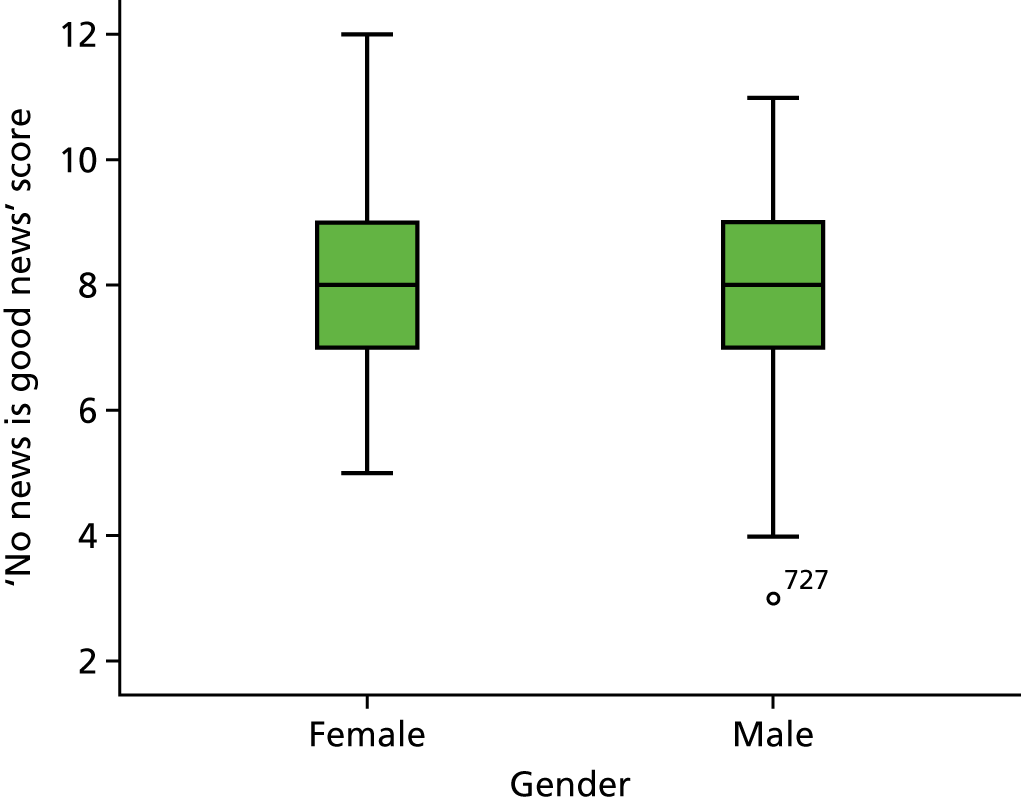
FIGURE 58.
Responses to PRE-HIT question 2 (‘People today want to know too much about their health’) by gender.
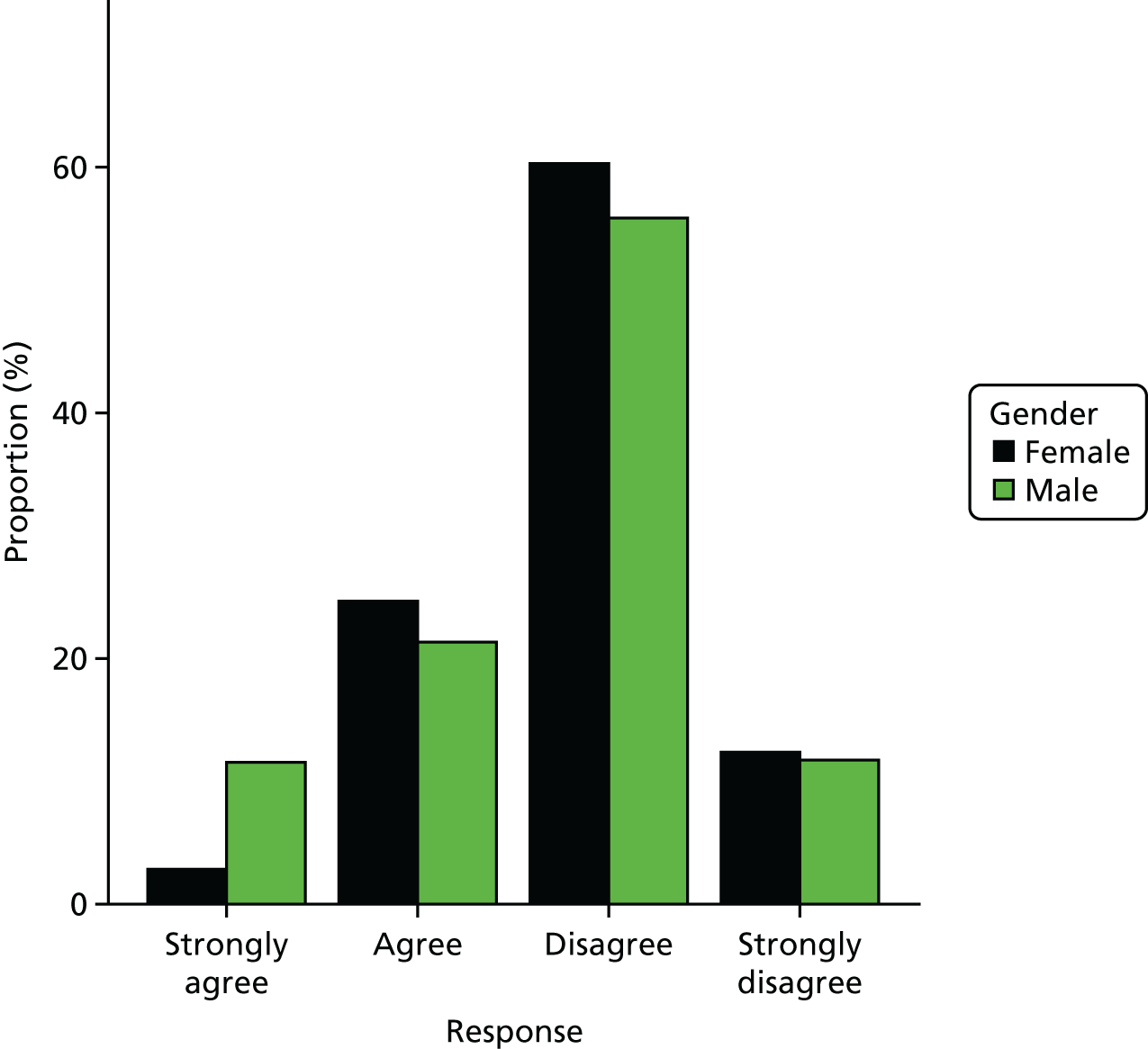
FIGURE 59.
Responses to PRE-HIT question 3 (‘Regarding my health, I agree with the statement “No news is good news” ’) by gender.
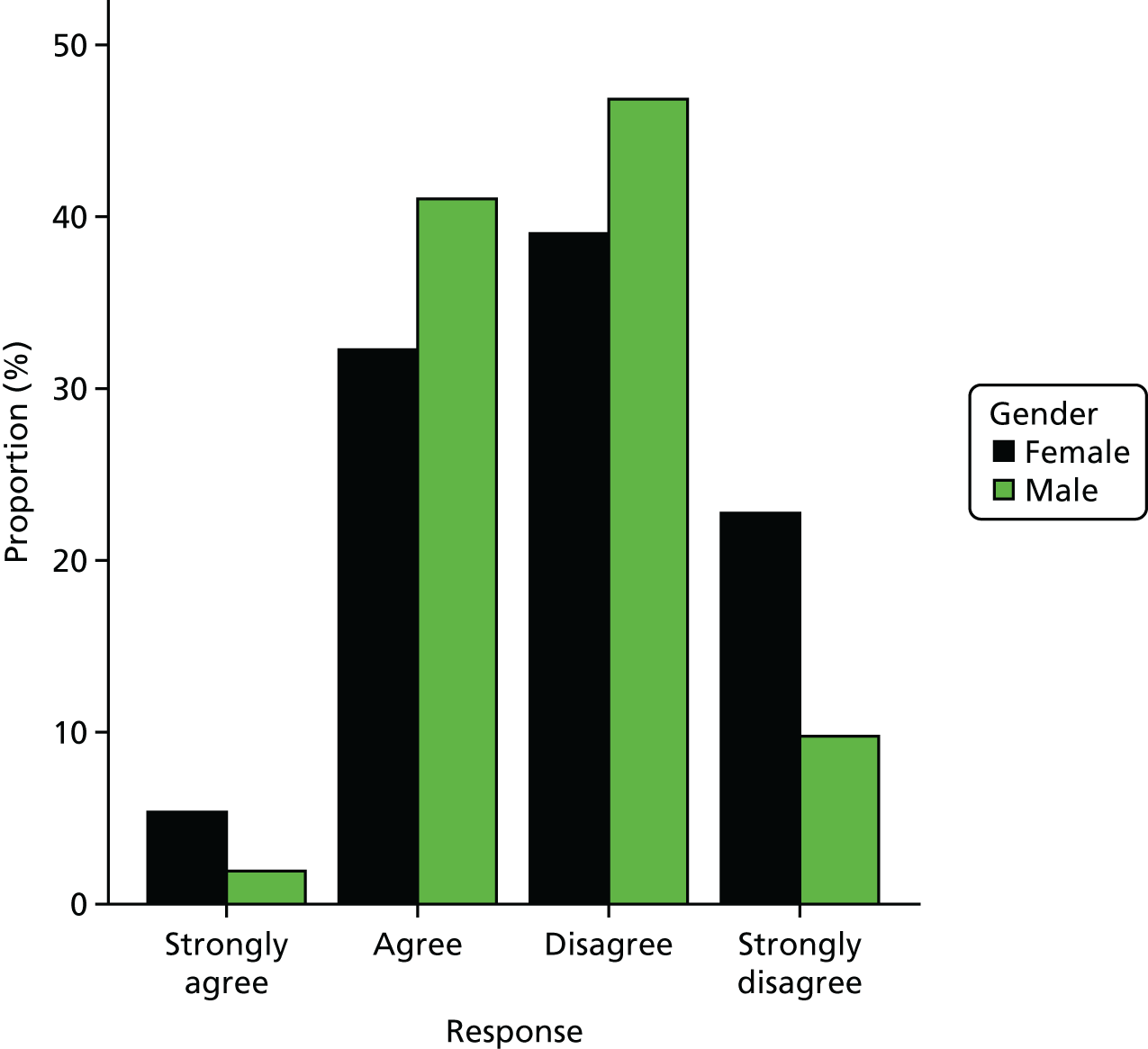
FIGURE 60.
Responses to PRE-HIT question 6 (‘I am concerned about what I might find if I look up health issues on the internet’) by gender.
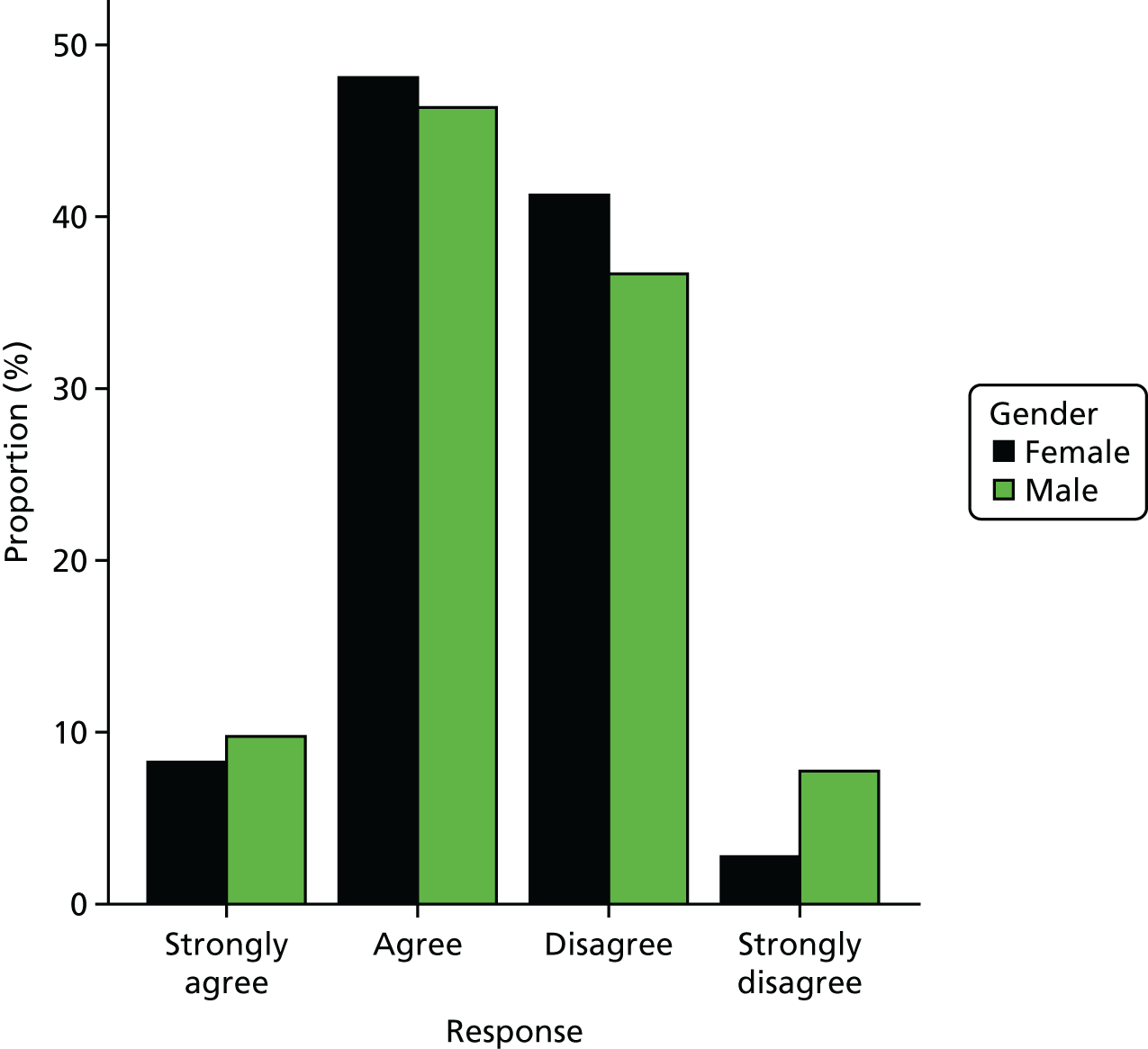
Relationship with doctor (from three questions)
Note that the lower the score, the stronger the relationship.
FIGURE 61.
The PRE-HIT relationship with doctor scores by gender.

FIGURE 62.
Responses to PRE-HIT question 1 (‘I let my doctor handle the details of my health’) by gender.
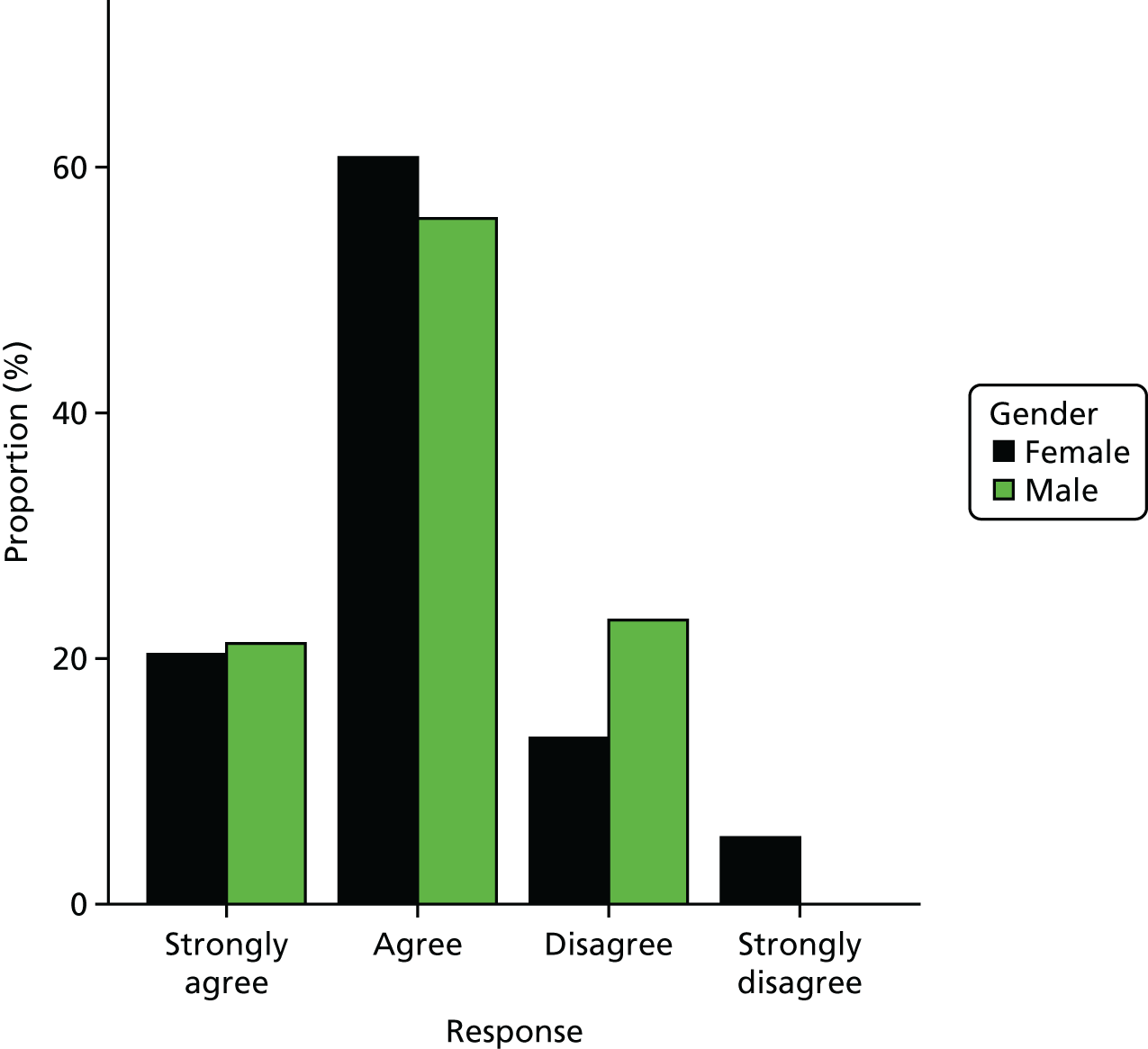
FIGURE 63.
Responses to PRE-HIT question 4 (‘Doctors are my most trusted source of health information’) by gender.
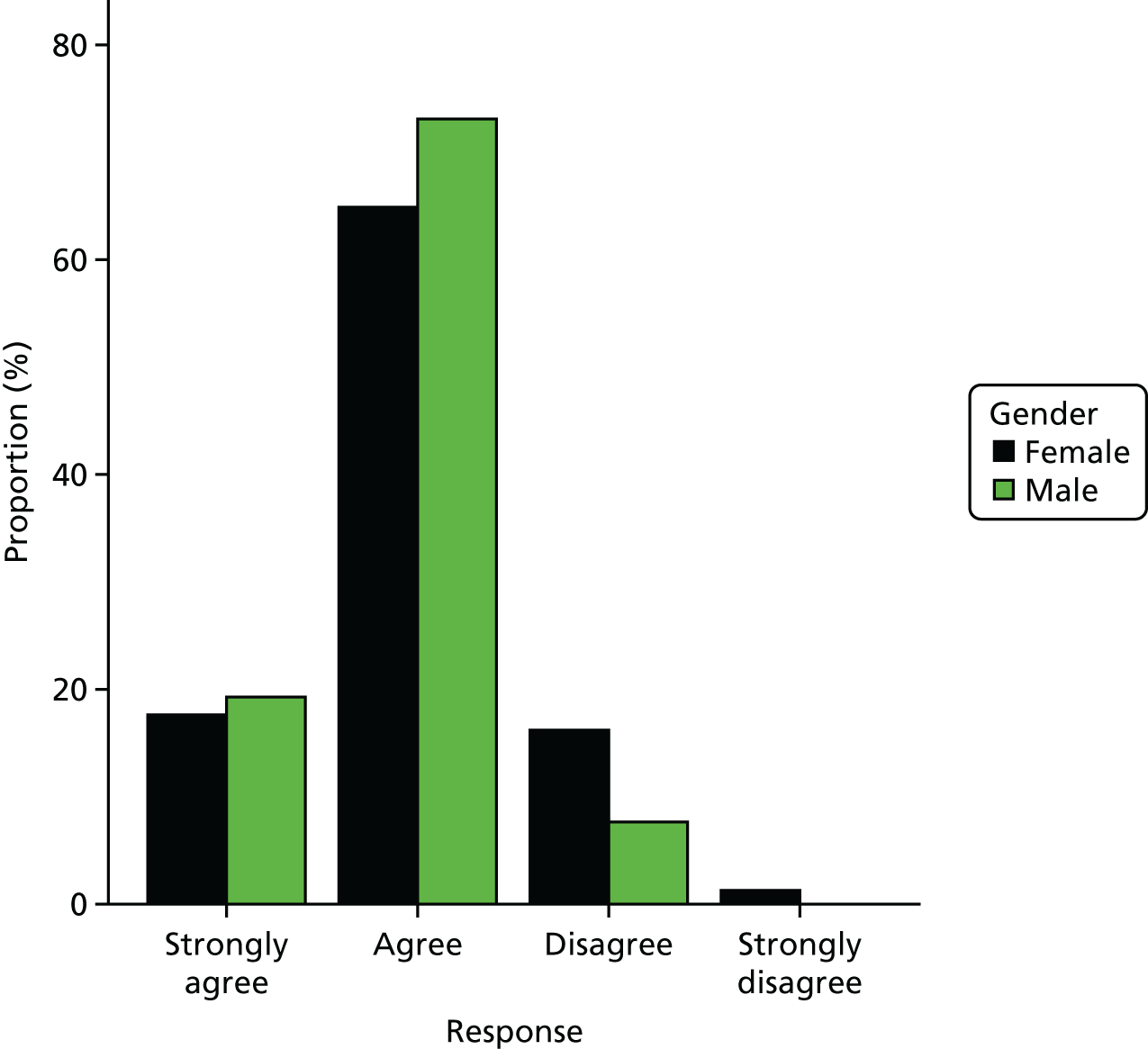
FIGURE 64.
Responses to PRE-HIT question 7 (‘When I have a health concern, my first step is to contact my doctor’s office’) by gender.
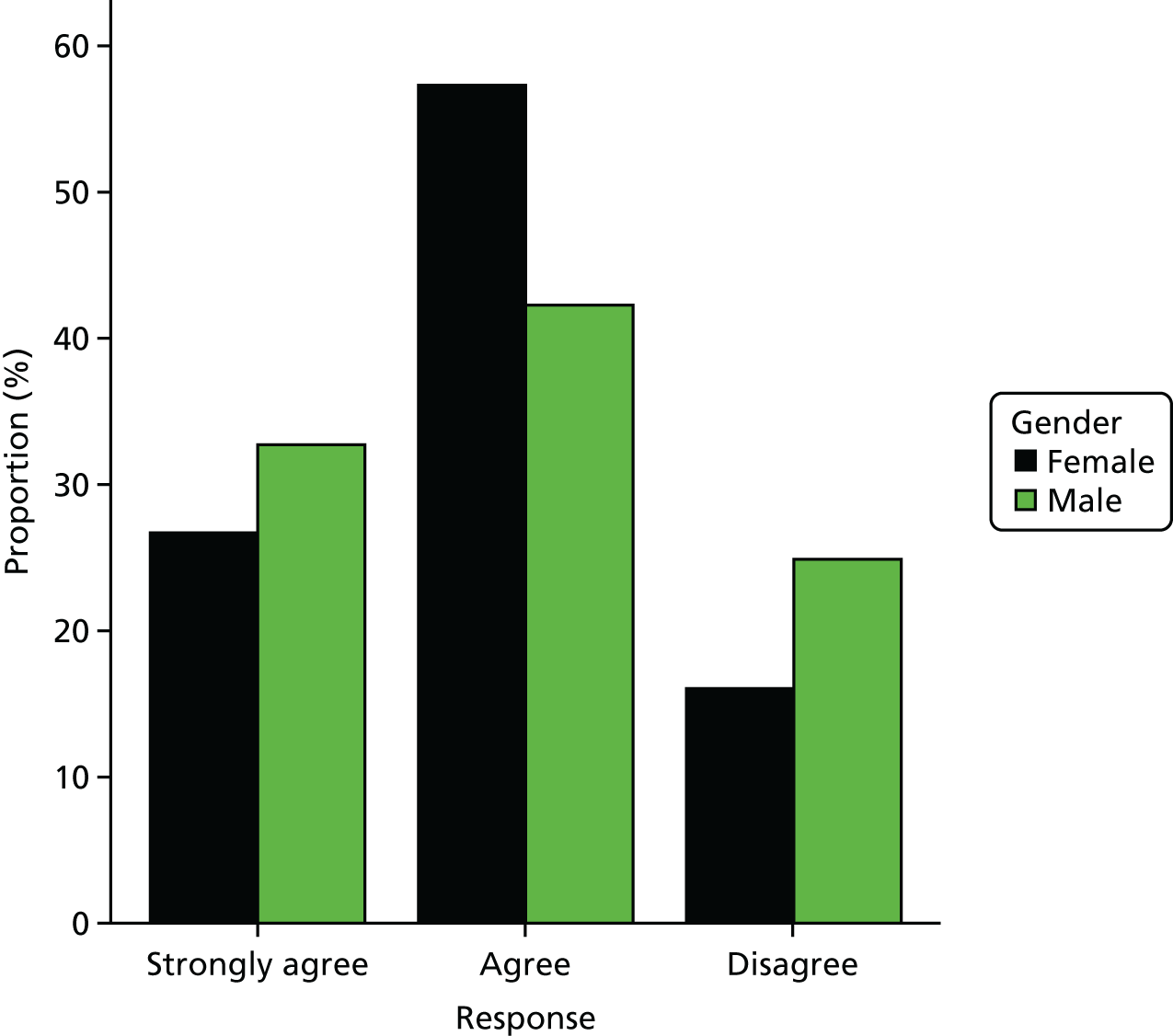
Preferred mode of interaction scores (from five questions)
Note that the lower the score, the greater the preference for GP consultation.
FIGURE 65.
The PRE-HIT preferred mode of interaction scores by gender.
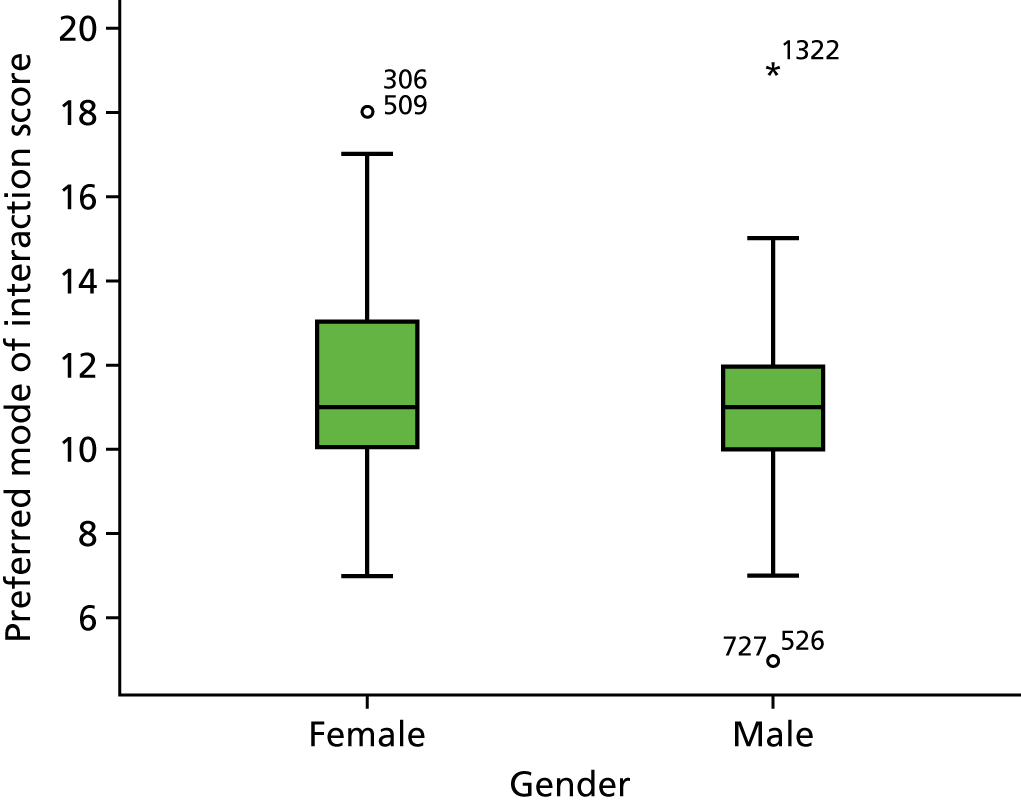
FIGURE 66.
Responses to PRE-HIT question 5 (‘I trust the internet as a source for health information’) by gender.
FIGURE 67.
Responses to PRE-HIT question 8 (‘Looking up health concerns on the internet is more convenient for me than contacting the doctor’s surgery’) by gender.
FIGURE 68.
Responses to PRE-HIT question 9 (‘If possible, I would prefer calling my doctor’s surgery to e-mailing them’) by gender.

FIGURE 69.
Responses to PRE-HIT question 10 (‘If possible, I would e-mail my doctor because it is easier than making a visit to the surgery’) by gender.
FIGURE 70.
Responses to PRE-HIT question 11 (‘Looking up information online about medications is easier than asking my doctor’) by gender.
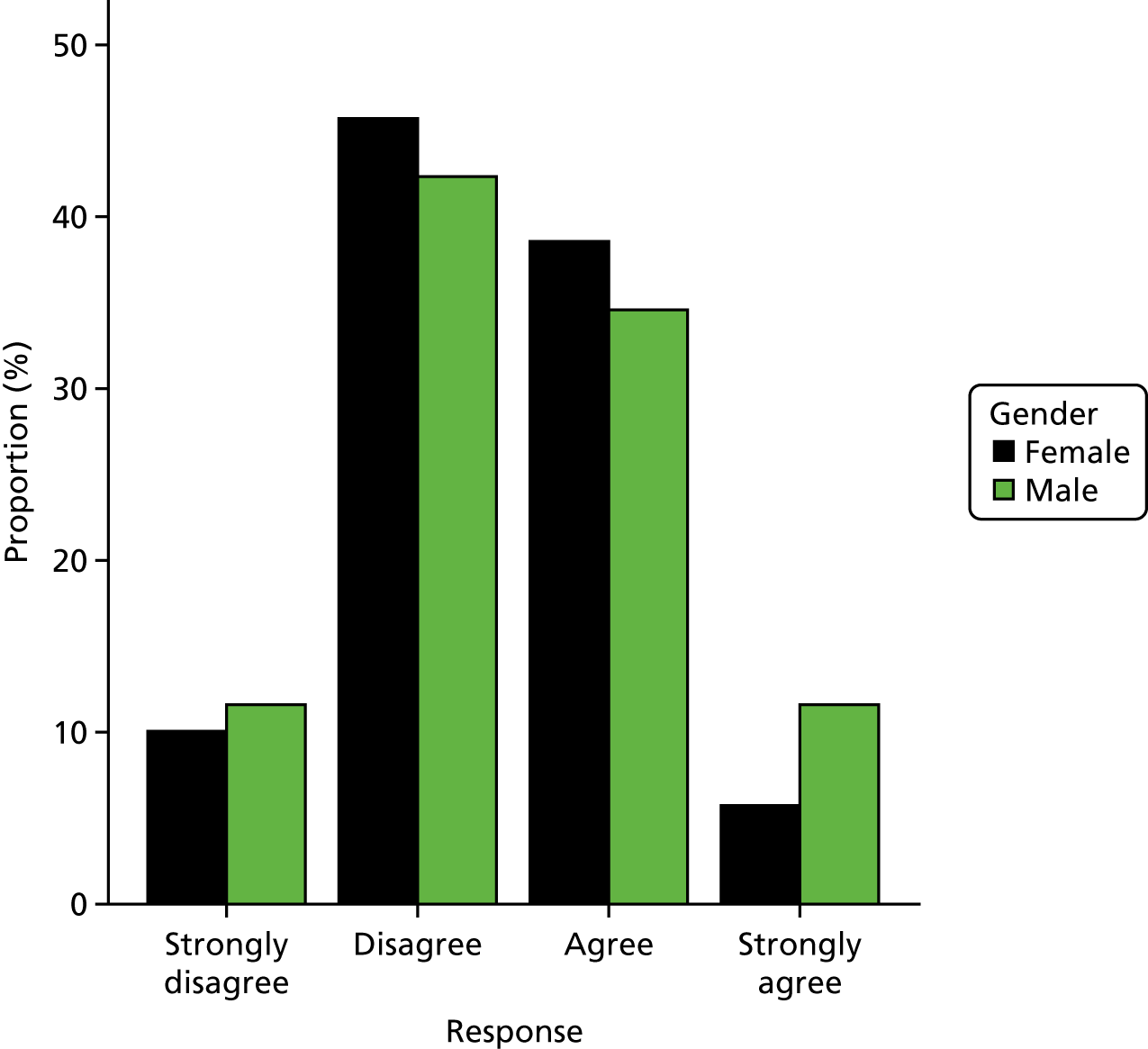
Appendix 7 Recruitment advert for PSO WELL Training
This appendix links with Psoriasis and Wellbeing (PSO WELL): developing a patient-centred approach using motivational interviewing skills with dermatology clinicians to support healthy living in people with psoriasis.
Appendix 8 Detailed methods and results of the stated preference survey
This appendix links with Valuing the interventions with a stated preference survey.
Methods
Survey design: time attribute
A fifth attribute was added to the stated preference survey about the length of time it would take for patients to read and use the information, manage their psoriasis and make any lifestyle changes. Many stated preference surveys use cost as a quantitative attribute to assess the amount of money that respondents are willing to pay in exchange for their preferred type of intervention. In this survey, time is used rather than cost. On the basis of the work to develop the intervention and discussions with the IMPACT research team, it was felt that time better reflected the trade-offs relevant to users of psoriasis care, which is not directly charged for in the NHS. It was anticipated that, overall, participants would prefer to spend less rather than more time to understand and manage their psoriasis. To explore this in the analysis, the time attribute had six levels (30 minutes, 1 hour, 2 hours, 3 hours, 4 hours and 8 hours). The decision to use 30 minutes as the base level was based on the nature of psoriasis and the need to manage the condition on a daily basis.
Fractional factorial design
The five attributes, combined with the number of levels (four attributes with three levels and one attribute with six levels), gives a total number of 486 scenarios describing different combinations of the features of the PSO WELL patient material and clinician training intervention. It is not feasible to design a survey to assess preferences for all of these scenarios. To address this, a fractional factorial design was used to reduce the number of scenarios by selecting a sample of possible combinations that covers the combinations and effects of interest. 175 A published design catalogue (http://neilsloane.com/oadir/MA.18.3.6.6.1.txt; accessed 28 January 2019) and modulo arithmetic were used to generate an efficient, orthogonal fractional factorial design176 to estimate the main effects of each attribute. This gave a design with 18 choice sets. The efficiency of the design was assessed using online software (Discrete Choice Experiments). The design was estimated to be 94% efficient compared with an optimal two-choice design and 65% efficient compared with a six-choice design for a main-effects analysis.
Sample size estimate
There is no clear formula for working out sample sizes, but an initial estimate was derived based on the number of choice sets in the fractional factorial design211 for a main-effects design. Based on this approach, for each choice set it was assumed that the true probability of choosing option 1 is 50% and the probability of choosing option 2 is also 50%. It was also assumed that an acceptable error for this probability is ± 2.5% in the sampled population. With a survey of 18 choice sets, this equates to a minimum of 170 completed questionnaires in total (85 × 2 questionnaires of nine choice sets). Based on previous experience, we anticipated that approximately 30–40% of participants would complete all the choice sets in a questionnaire. This indicates that 213–283 participants are needed to get 85 complete responses per choice question. A higher number of responses will be required if there is significant heterogeneity in the sample of participants. Stated preference survey guidelines suggest that 500 participants is sufficient for a statistical analysis that accounts for heterogeneity. Accordingly, we initially aimed to recruit 500 participants.
Post hoc sample size estimates used the approach recommended by de Bekker-Grob et al. 177 to estimate the numbers needed for a main-effects analysis that can identify statistically significant preferences for each level of each attribute. The hypotheses for the sample size estimates were that participants would prefer:
-
printed information and printed plus online information to no information
-
personalised information and personalised information plus support for lifestyle changes to no information or support
-
understanding of psoriasis, treatments and health to be the same as or better than before rather than worse than before
-
ability to manage psoriasis and make lifestyle choices to be the same as or better than before rather than worse than before
-
to spend less rather than more time to manage their psoriasis.
Sample sizes were estimated for time as a continuous variable. Inputs to the sample size calculations included the coefficients from the main analysis (used as effect size estimates), the study design, 5% statistical significance level and 80% statistical power. The calculations were done in R version 3.2.0, based on the code published by de Bekker-Grob et al. 177
Participant recruitment and survey administration
The stated preference survey was advertised via social media websites [e.g. Twitter and Facebook (Facebook, Inc., Menlo Park, CA, USA)], specifically targeting psoriasis pages or groups to recruit participants. The Psoriasis Association also advertised the survey on their website, newsletter and through social media channels. Psoriasis Association members and IMPACT programme participants (who had agreed to be contacted about further research) were e-mailed. The survey was developed for participants to complete online.
Participants who responded to the adverts followed a web link to see the survey instructions, a cover letter, a participant information sheet, survey instructions and the survey itself. The survey was then randomly selected for the participant to complete. Participants were not offered any incentives to complete the survey.
The survey software used was LimeSurvey (LimeSurvey GmbH, Hamburg, Germany), managed and hosted on a University of Manchester website.
Analysis
Descriptive statistics were used to summarise participant characteristics and the survey responses. Logistic regression was used to explore whether or not there were differences in respondent characteristics between the survey versions and the option chosen for each choice set.
Effects and dummy coding of the attributes give different absolute estimates of the coefficients or strength of preferences. However, the relative difference for each categorical attribute level compared with the lowest (reference) level will be the same for the two coding methods. 212 Accordingly, the primary, sensitivity and subgroup analyses used dummy coding of the attributes to explore how the coefficients (or preferences) changed for each level. Effects coding was used to retrieve the preference weight (coefficient) for the reference level to assess the functional form of the attributes. 175,213
The primary analysis of the choice questions included only participants with complete responses to these questions. The responses to the choice questions were analysed with a conditional logistic regression model using maximum likelihood estimation to estimate the preference weights (coefficients) for each of the attributes. This model is a simple fixed-effects model that takes into account the panel nature of the data and is widely applied to analyse discrete choice data in health care. 175,213,214
The coefficients for each attribute tell us how strongly users feel that it is an important part of the PSO WELL intervention. Random utility theory assumes that a responder chooses between two options by interpreting the information described as a set of characteristics and selects the one that provides the highest utility overall. Based on this assumption, the results of the regression analyses were used to calculate MRS between the different attributes. This allowed us to estimate the relative time participants were willing to trade for the different features and assess how important they are (e.g. how much does the provision of information materials matter to users compared with other aspects, such as their ability to make lifestyle changes?). The MRS was calculated in Stata 13 as the coefficient for each attribute level divided by the time coefficient using the non-linear combination of estimates command.
The conditional logit model assumes that, across all participants, all the choice questions/tasks measure utility equally well. If this is not the case then the coefficients from the regression cannot be used to assess the relative importance of different levels across attributes (scale heterogeneity). However, the overall aim of the analysis was to estimate the MRS or willingness to spend time to gain the preferred level of each attribute. This deals with the issue of scale heterogeneity by essentially converting each coefficient to the same scale.
The assumption of fixed effects means that the choice of options in a choice set is determined by the attributes and levels assigned to the option and assumes that there are no systematic, unobserved differences in preferences between respondents. The sensitivity analysis included a random parameters logistic regression model to explore whether or not relaxing this assumption affected the marginal rates of substitution.
The data were downloaded and prepared for analysis in IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA) and analysed in Stata 13.
Results
A total of 526 participants accessed the survey and were eligible to take part (Figure 71). Of these, 366 (69%) participants answered one or more choice questions and 250 (48%) participants answered all choice questions.
FIGURE 71.
Flow diagram of participants’ responses, excluding duplicates.
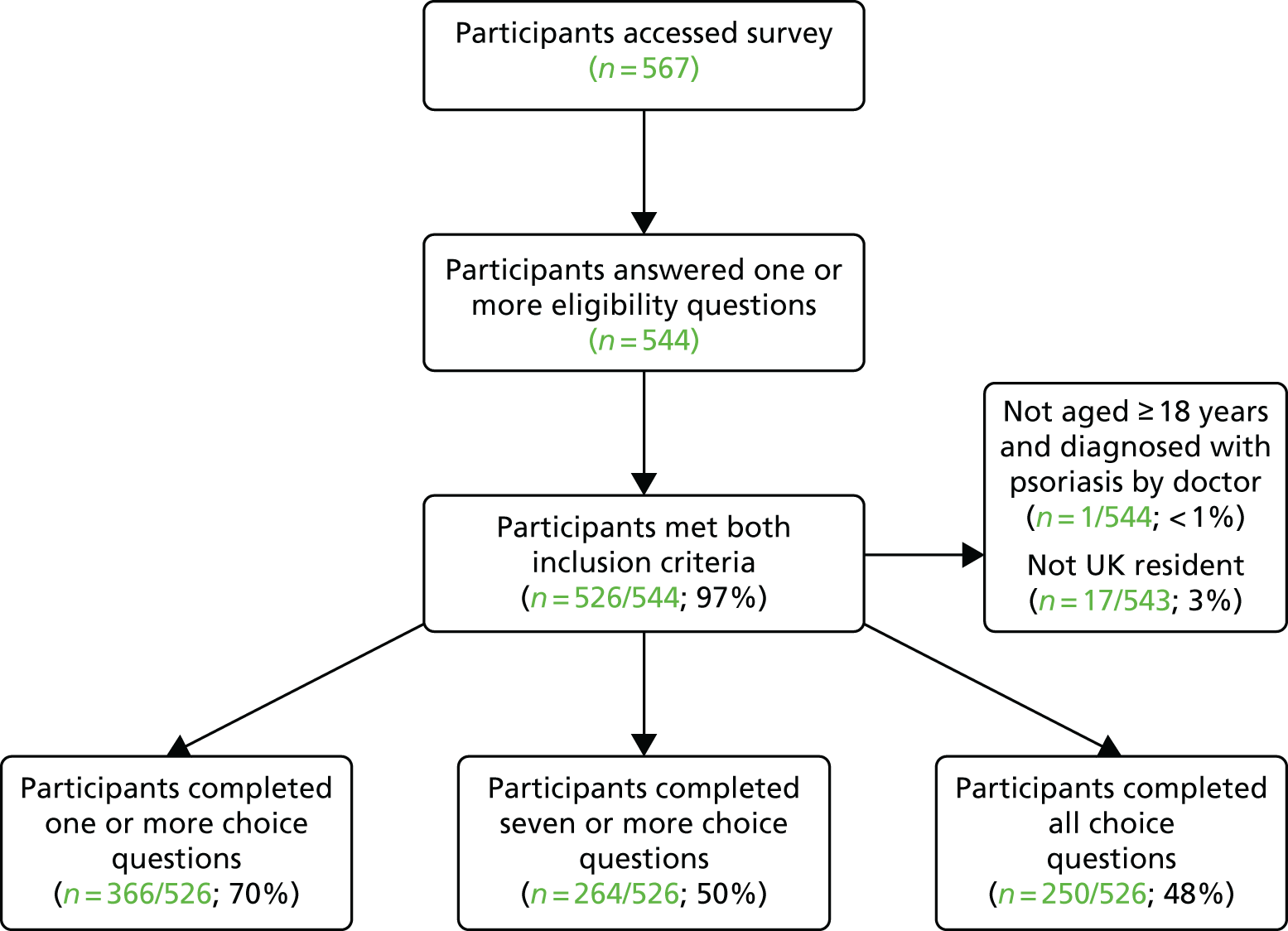
Participant characteristics and time to complete survey
The characteristics of participants are summarised in Table 15. The characteristics of participants completing surveys 1 and 2 are presented in Table 16 and characteristics of participants choosing option 1 or 2 are presented in Table 17. Participants who did not answer any choice questions (160/526; 30%) also did not complete the questions about their sociodemographic characteristics or treatment, which were at the end of the survey. Out of the 116 people who completed one or more but not all nine of the choice questions, 11 (5%) answered one or more of the sociodemographic questions. This lack of data means that it is not possible to assess whether or not there were any differences between those who did and those who did not answer any, or all, choice questions. As is typical with anonymous online surveys, it was not possible to collect comprehensive data about the number of people who saw the adverts or e-mail invitations or who then visited the website to look at the details about the survey.
| Demographic and clinical characteristics | n/N (%) | Bootstrapped 95% CI (%) |
|---|---|---|
| Answered one or more demographic or treatment questions | 260/366 (71) | 67 to 76 |
| Female | 164/260 (63) | 57 to 69 |
| Type of health-care practitioner ever seen | ||
| GP only | 40/258 (16) | 11 to 21 |
| GP and hospital doctor or nurse | 212/258 (82) | 77 to 87 |
| Other | 4/258 (2) | < 1 to 3 |
| Ever used one or more prescribed treatments | 255/260 (98) | 96 to 100 |
| Types of prescribed treatment ever used | ||
| Creams/ointments/lotions/shampoo | 255/260 (98) | 96 to 100 |
| PUVA | 161/260 (62) | 56 to 68 |
| Tablets | 114/260 (44) | 38 to 50 |
| Injections | 48/260 (19) | 14 to 24 |
| Demographic and clinical characteristics | Mean (SE); n | 95% CI (%) |
| Number of types of treatment ever used | 2 (< 1); 260 | 2.1 to 2.4 |
| Age (years) | 48 (1); 257 | 46 to 50 |
| Age when first diagnosed with psoriasis (years) | 22 (1); 252 | 20 to 24 |
| EQ-5D utility value (Devlin et al. value set215) | 0.80 (0.01); 255 | 0.77 to 0.82 |
| EQ-5D utility value (crosswalk value set180) | 0.71 (0.01); 255 | 0.68 to 0.74 |
| Survey 2 (compared with survey 1) | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Female | –0.33 | 0.30 | –1.09 | 0.275 | –0.93 to 0.26 |
| Age (years) | < 0.01 | 0.01 | 0.32 | 0.751 | –0.02 to 0.02 |
| Age when first diagnosed with psoriasis (years) | < 0.01 | 0.01 | 0.18 | 0.860 | –0.02 to 0.02 |
| GP and hospital doctor or nurse (compared with GP only) | 1.33 | 0.47 | 2.85 | 0.004 | 0.41 to 2.24 |
| Types of treatments | |||||
| Creams/ointments/lotions/shampoo | –0.47 | 1.46 | –0.32 | 0.749 | –3.33 to 2.40 |
| PUVA | –0.65 | 0.34 | –1.91 | 0.056 | –1.32 to 0.02 |
| Tablets | –0.04 | 0.31 | –0.15 | 0.884 | –0.64 to 0.56 |
| Injections | –0.18 | 0.38 | –0.46 | 0.647 | –0.93 to 0.58 |
| EQ-5D utility value (Devlin et al. value set215) | 0.23 | 0.74 | 0.31 | 0.757 | –1.21 to 1.67 |
| Completed all choice questions | –0.24 | 0.78 | –0.31 | 0.754 | –1.76 to 1.28 |
| Constant | 0.06 | 1.82 | 0.03 | 0.973 | –3.50 to 3.62 |
| Chose option 2 (vs. chose option 1) | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Female | –0.05 | 0.10 | –0.49 | 0.626 | –0.24 to 0.14 |
| Age | < –0.01 | < 0.00 | –0.40 | 0.689 | –0.01 to 0.01 |
| Age when first diagnosed with psoriasis (years) | < 0.00 | < 0.00 | 0.44 | 0.661 | –0.01 to 0.01 |
| GP and hospital doctor | 0.05 | 0.15 | 0.36 | 0.721 | –0.24 to 0.35 |
| Types of treatments | |||||
| Creams/ointments/lotions/shampoo | 0.71 | 0.54 | 1.32 | 0.188 | –0.35 to 1.77 |
| PUVA | 0.02 | 0.11 | 0.21 | 0.834 | –0.19 to 0.24 |
| Tablets | –0.01 | 0.10 | –0.13 | 0.894 | –0.21 to 0.18 |
| Injections | 0.13 | 0.13 | 0.99 | 0.321 | –0.12 to 0.37 |
| EQ-5D utility value (Devlin et al. value set215) | 0.09 | 0.24 | 0.38 | 0.703 | –0.38 to 0.57 |
| Constant | –1.01 | 0.58 | –1.73 | 0.083 | –2.14 to 0.13 |
Including all eligible respondents, the average time to complete the survey was 8 minutes (SD 22 minutes), although this ranged from < 1 minute to 416 minutes. Five participants had > 1 hour between the start and finish times (range 96–416 minutes). There were no differences in average time between those who started survey 1 (mean 7 minutes, SD 12 minutes; n = 251) and those who started survey 2 (mean 9 minutes, SD 28 minutes; n = 275) (p = 0.362). As expected, there were differences in the time taken between those who completed one or more choice questions (mean 10 minutes, SD 24 minutes; n = 366) and those who did not (mean 3 minutes, SD 14 minutes; n = 160). This difference was statistically significant (p < 0.001). Similarly, there were differences in the time taken between those who completed all choice questions (mean 12 minutes, SD 29 minutes; n = 250) and those who did not (mean 5 minutes, SD 9 minutes; n = 116; p = 0.005). For 240 (96%) of the 250 of those who completed all choice questions, the mean time taken was lower than or within the time range given in the participant instructions (10–20 minutes).
Analysis of choice data
Effects coding was used in a conditional logistic regression model to allow estimation of the coefficient for the base level and to plot the coefficients as shown in Figures 23 and 24. The full results of the primary analysis of preferences, using dummy-coded attributes, are shown in Table 18. For the information attribute, Figure 23 illustrates the similarity in preferences for levels 1 (no information) and 2 (printed information materials).
| Coefficient | SE | z | p-value | 95% CI | |
|---|---|---|---|---|---|
| Information materials (reference level is no information) | |||||
| Printed information | 0.02 | 0.08 | 0.29 | 0.774 | –0.13 to 0.18 |
| Printed and online information | 0.21 | 0.07 | 2.79 | 0.005 | 0.06 to 0.35 |
| Clinician gives personalised information about psoriasis/support for lifestyle changes (reference level is no information or support) | |||||
| Personalised information | 0.73 | 0.08 | 9.47 | 0.000 | 0.58 to 0.89 |
| Personalised information and support for lifestyle changes | 1.13 | 0.08 | 14.02 | 0.000 | 0.97 to 1.29 |
| Understanding of psoriasis, treatments and health (reference level is worse than before) | |||||
| Same as before | 0.75 | 0.08 | 9.95 | 0.000 | 0.61 to 0.90 |
| Better than before | 0.76 | 0.08 | 9.65 | 0.000 | 0.60 to 0.91 |
| Ability to manage psoriasis and make lifestyle changes (reference level is worse than before) | |||||
| Same as before | 0.98 | 0.08 | 12.77 | 0.000 | 0.83 to 1.13 |
| Better than before | 1.92 | 0.10 | 19.83 | 0.000 | 1.73 to 2.11 |
| Time (hours) | –0.08 | 0.01 | –6.16 | 0.000 | –0.10 to –0.05 |
The results of the primary analysis of preferences using dummy-coded attributes are shown in Table 18. For the attributes about information, clinician visits, understanding of psoriasis and comorbidity and ability to make lifestyle changes, these analyses indicate that participants preferred higher levels (levels 2 or 3) of each attribute rather than the reference or base level, which is in line with what was expected. In addition, the strength of preference measured by the coefficients was statistically significant for both levels compared with the lowest or reference level for these attributes. The exception to this was the information attribute, for which only the coefficient for printed and online information materials (level 3) was statistically significantly different from the base level of no information.
When treated as a continuous linear variable, the coefficient for time was negative and statistically significant, as shown in Table 18. This is in line with expectations that, all other things being equal, participants would prefer to spend less rather than more time to manage their psoriasis and make lifestyle changes. However, when time was analysed as a categorical variable, the results indicate that participants preferred to spend up to 2 hours rather than the base level (30 minutes) to manage their psoriasis (Table 19 and see Figure 24). At and beyond 2 hours, participants preferred to spend less time (compared with the base level) to manage their psoriasis. In addition, the level of 3 hours was not statistically significantly different to the base level of 30 minutes (see Table 19).
| Coefficient | SE | z | p-value | 95% CI | |
|---|---|---|---|---|---|
| Information materials (reference level is no information) | |||||
| Printed information | 0.02 | 0.08 | 0.24 | 0.809 | –0.14 to 0.18 |
| Printed and online information | 0.21 | 0.08 | 2.79 | 0.005 | 0.06 to 0.36 |
| Clinician gives personalised information about psoriasis/support for lifestyle changes (reference level is no information or support) | |||||
| Personalised information | 0.79 | 0.08 | 9.53 | 0.000 | 0.63 to 0.96 |
| Personalised information and support for lifestyle changes | 1.18 | 0.09 | 13.51 | 0.000 | 1.01 to 1.35 |
| Understanding of psoriasis, treatments and health (reference level is worse than before) | |||||
| Same as before | 0.74 | 0.08 | 9.66 | 0.000 | 0.59 to 0.89 |
| Better than before | 0.72 | 0.08 | 8.88 | 0.000 | 0.56 to 0.87 |
| Ability to manage psoriasis and make lifestyle changes (reference level is worse than before) | |||||
| Same as before | 0.99 | 0.08 | 12.48 | 0.000 | 0.84 to 1.15 |
| Better than before | 2.07 | 0.10 | 20.19 | 0.000 | 1.87 to 2.27 |
| Time (hours) (reference level is 0.5 hours) | |||||
| 1 | 0.46 | 0.18 | 2.57 | 0.010 | 0.11 to 0.81 |
| 2 | 0.88 | 0.18 | 5.01 | 0.000 | 0.54 to 1.23 |
| 3 | –0.17 | 0.17 | –1.02 | 0.308 | –0.50 to 0.16 |
| 4 | –0.34 | 0.14 | –2.34 | 0.019 | –0.62 to –0.06 |
| 8 | –0.61 | 0.11 | –5.64 | 0.000 | –0.82 to –0.40 |
The post hoc estimates of the sample size needed to identify a statistically significant coefficient for each attribute level are shown in Table 20. If time is treated as a continuous variable, the results show that the number of participants who completed all choice questions is sufficient to identify statistically significant main effects for all but one of the attribute levels. The exception is level 1 (printed information) of the information materials attribute, which would require a sample size of 9373. If time is treated as a categorical variable, the number of complete responses required is 9598 for level 1 of the information materials attribute and 744 for the 3-hour level of the time attribute.
| Attribute and level | Sample size when time is a continuous variable (n) | Sample size when time is categorical variable (n) |
|---|---|---|
| Information materials (reference level is no information) | ||
| Printed information | 9373 | 9598 |
| Printed and online information | 101 | 100 |
| Clinician gives personalised information about psoriasis/support for lifestyle changes (reference level is no information or help) | ||
| Personalised information | 9 | 9 |
| Personalised information and support for lifestyle changes | 4 | 4 |
| Understanding of psoriasis, treatments and health (reference level is worse than before) | ||
| Same as before | 8 | 8 |
| Better than before | 9 | 8 |
| Ability to manage psoriasis and make lifestyle changes (reference level is worse than before) | ||
| Same as before | 5 | 5 |
| Better than before | 2 | 2 |
| Time is a continuous variable | 22 | – |
| Time (hours), categorical variable (reference level is 0.5 hours) | ||
| 1 | – | 106 |
| 2 | – | 29 |
| 3 | – | 744 |
| 4 | – | 137 |
| 8 | – | 25 |
Time willing to trade
The time that participants are willing to spend (or trade) to gain the level of each attribute level that they prefer is shown in Table 21. The results suggest that improvements in ability to manage psoriasis and make lifestyle changes, along with clinic visits that include personalised information and support to make lifestyle changes, were the most important components of the PSO WELL intervention. The information materials are the least important components. The sensitivity and subgroup analyses indicated that there were no differences in the relative importance of the different attribute levels according to the analysis methods or participants’ demographic and treatment characteristics (Tables 22–24). The 95% CIs overlapped, indicating no statistically significant differences compared with the primary analysis.
| Attribute level | Primary analysis, MRS (95% CI) | Relative importance (from highest to lowest time willing to trade) |
|---|---|---|
| Ability to manage psoriasis and make lifestyle changes is better than before | 25 (17 to 32) | 8 |
| Clinic visit includes personalised information and support for lifestyle changes | 14 (10 to 19) | 7 |
| Ability to manage psoriasis and make lifestyle changes is same as before | 13 (8 to 17) | 6 |
| Understanding of psoriasis, treatments and health is same as before | 10 (6 to 13) | 4 |
| Understanding of psoriasis, treatments and health is better than before | 10 (6 to 13) | 4 |
| Clinic visit includes personalised information | 9 (6 to 13) | 3 |
| Printed and online information | 3 (1 to 5) | 2 |
| Printed information | < 1 (–2 to 2) | 1 |
| Time levels 3 and 4 combined | Random parameter logistic analysis | Participants completed one or more choice questions | Participants completed seven or more choice questions | |
|---|---|---|---|---|
| Information materials (reference level is no information) | ||||
| Printed information | < 1 (–2 to 3) | 1 (–1 to 4) | 1 (–1 to 2) | 1 (–1 to 3) |
| Printed and online information | 3 (1 to 5) | 5 (1 to 8) | 2 (0 to 5) | 3 (1 to 5) |
| Clinician gives personalised information about psoriasis/support for lifestyle changes (reference level is no information or help) | ||||
| Personalised information | 10 (6 to 14) | 14 (8 to 20) | 9 (6 to 13) | 9 (6 to 13) |
| Personalised information and support for lifestyle changes | 16 (10 to 21) | 21 (13 to 29) | 15 (10 to 21) | 15 (10 to 20) |
| Understanding of psoriasis, treatments and health (reference level is worse than before) | ||||
| Same as before | 11 (7 to 14) | 12 (7 to 17) | 10 (6 to 13) | 9 (6 to 13) |
| Better than before | 11 (6 to 15) | 14 (8 to 20) | 10 (6 to 13) | 9 (6 to 13) |
| Ability to manage psoriasis and make lifestyle changes (reference level is worse than before) | ||||
| Same as before | 14 (9 to 19) | 17 (10 to 25) | 13 (8 to 17) | 12 (8 to 17) |
| Better than before | 27 (18 to 35) | 35 (21 to 48) | 25 (16 to 33) | 24 (17 to 32) |
| Age (years) | Gender | EQ-5D utility | ||||
|---|---|---|---|---|---|---|
| < 40 | ≥ 40 | Male | Female | < 0.70 | ≥ 0.70 | |
| Information materials (reference level is no information) | ||||||
| Printed information | 1 (–2 to 4) | –1 (–3 to 2) | < 1 (–3 to 3) | 1 (–2 to 3) | –2 (–3 to 7) | 1 (–1 to 3) |
| Printed and online information | 3 (0 to 6) | 3 (0 to 5) | 1 (–2 to 4) | 3 (1 to 6) | 4 (–1 to 10) | 2 (< 1 to 4) |
| Clinician gives personalised information about psoriasis/support for lifestyle changes (reference level is no information or help) | ||||||
| Personalised information | 8 (3 to 13) | 10 (6 to 15) | 8 (3 to 14) | 10 (6 to 14) | 10 (1 to 18) | 9 (6 to 13) |
| Personalised information and support for lifestyle changes | 14 (6 to 21) | 15 (8 to 21) | 13 (5 to 20) | 15 (9 to 21) | 16 (3 to 28) | 14 (9 to 19) |
| Understanding of psoriasis, treatments and health (reference level is worse than before) | ||||||
| Same as before | 8 (3 to 13) | 11 (6 to 16) | 9 (3 to 15) | 10 (5 to 14) | 13 (2 to 23) | 9 (5 to 12) |
| Better than before | 8 (3 to 14) | 11 (5 to 16) | 8 (3 to 14) | 10 (6 to 15) | 14 (3 to 26) | 8 (5 to 12) |
| Ability to manage psoriasis and make lifestyle changes (reference level is worse than before) | ||||||
| Same as before | 12 (5 to 19) | 13 (7 to 19) | 12 (5 to 19) | 12 (7 to 17) | 12 (2 to 23) | 12 (8 to 17) |
| Better than before | 21 (10 to 21) | 26 (16 to 37) | 23 (11 to 36) | 24 (15 to 33) | 21 (5 to 38) | 24 (16 to 33) |
| GP only | GP and hospital doctor or nurse | Used two or fewer treatments | Used more than two treatments | |
|---|---|---|---|---|
| Information materials (reference level is no information) | ||||
| Printed information | 1 (–4 to 5) | < 1 (–2 to 3) | 1 (–2 to 3) | < 1 (–4 to 3) |
| Printed and online information | 6 (< 0 to 13) | 2 (< 1 to 4) | 3 (0 to 5) | 2 (–1 to 6) |
| Clinician gives personalised information about psoriasis/support for lifestyle changes (reference level is no information or help) | ||||
| Personalised information | 7 (< 1 to 14) | 10 (6 to 14) | 8 (4 to 12) | 11 (4 to 19) |
| Personalised information and support for lifestyle changes | 17 (3 to 31) | 14 (9 to 19) | 14 (8 to 20) | 15 (6 to 24) |
| Understanding of psoriasis, treatments and health (reference level is worse than before) | ||||
| Same as before | 8 (4 to 15) | 10 (6 to 13) | 9 (5 to 13) | 10 (4 to 17) |
| Better than before | 8 (4 to 16) | 10 (6 to 14) | 9 (5 to 14) | 11 (4 to 18) |
| Ability to manage psoriasis and make lifestyle changes (reference level is worse than before) | ||||
| Same as before | 15 (2 to 28) | 12 (7 to 16) | 11 (7 to 16) | 14 (6 to 23) |
| Better than before | 27 (5 to 50) | 23 (15 to 31) | 23 (14 to 32) | 27 (12 to 42) |
For the information attribute, the printed information level was included in the primary analysis to illustrate its relative importance, even though it was not statistically different from the base level of no information. The sensitivity analysis indicated that there were no differences in the overall results from combining levels 1 and 2 into one level. Similarly, the time level for 3 hours (which was not statistically significant) was included in the primary analysis. In the sensitivity analysis, this was combined with the level of 4 hours. Table 22 indicates that this did not affect the relative time participants were willing to trade to gain improvements in the different components of the intervention.
Appendix 9 Stated preference survey materials
This appendix links with Valuing the interventions with a stated preference survey.
Appendix 10 Economic model development and International Society for Pharmacoeconomics and Outcomes Research checklist
This appendix links with Health economics modelling of costs of interventions for people with psoriasis.
| Meeting 1 | Meeting 2 | Meeting 3 | Meeting 4 |
|---|---|---|---|
| FIM workshop preceded by survey | FIM workshop preceded by survey | FIM only | FIM only |
|
|
|
Finalisation of outstanding model assumptions to ensure plausible primary outputs |
Model validation using the International Society for Pharmacoeconomics and Outcomes Research checklist189
| Evidence of good practice | Met by model? | Discussion |
|---|---|---|
| 1. DES models should be used when the problem under study involves constrained or limited resources. DES is also an attractive option in non-constrained models when there are interactions between individuals, populations, and/or their environment; when time-to-event is best described stochastically rather than with fixed time intervals and time dependencies are important; when individual pathways through the model are influenced by multiple characteristics of the entity; and when recording individual entity experience is desirable | Yes |
Although the model does not contain limited resources, outcomes are driven by interactions between patients and clinicians Time to event in psoriasis is stochastic in nature; ‘flare-ups’ and longer-term events are not characterised by a pattern The pathway of an individual patient is influenced by his or her own set of attributes, affecting service use and health outcomes Recording the experience of individual patients best captures the complex nature of psoriasis care, rather than making general assumptions or relying on population means |
| 2. Constrained-resource models should consider the effect of alternative strategies on health-related outcomes and not focus solely on measures of resource utilisation and system capacity. The omission of health-related outcomes from a model should be justified | N/A |
The model does not include constrained resources Both health outcomes and cost outcomes are important outputs from the model |
| 3. The need to model constrained resources should be carefully considered | N/A | The model does not include constrained resources |
| 4. If downstream decisions can have significant effects on the differences in costs or outcomes, the model should be structured to facilitate analyses of alternative downstream decisions | Yes | The model accounts for downstream decisions of clinicians and patients, which are in turn influenced by initial experience/non-experience of the intervention. These downstream decisions can be examined in sensitivity analysis through amended parameter values |
| 5. If parameter values are elicited from experts, uncertainty around the elicited values should be represented, and the elicited values should be validated | Yes | Expert opinion was not used to inform parameter values directly |
| 6. If confidence in the elicited values is low, resulting analysis should be viewed only as a starting point for what-if analyses, and for estimating the value of collecting additional data | No | Confidence in some parameters is low, so the model is viewed as a starting point for what-if analyses, with extensive threshold and scenario analyses. These have been used to identify the range of further information required rather than directly estimating the value of collecting additional information |
| 7. If the decision is made to modify the original structure owing to data constraints, the new structure must be carefully analysed to understand the effects of modifications so as to inform decision-makers of the additional uncertainty introduced. Explicit considerations of the size and likely direction of the effects of the modification should be presented | Yes | Model structure was developed iteratively but was always intended as a DES model, of which the over-riding structure has not changed |
| 8. When modelling clinical practice, it should not be assumed that relevant guidelines are actually applied | Yes | We estimated that only a proportion of clinicians acted in a guideline-concordant manner and assumed this differed between clinicians who received the training intervention and those who did not. In any model run, if a clinician was designated to act in a guideline-concordant manner, then it was assumed that all treatment decisions followed the guideline. If the clinician was designated as not acting in a guideline-concordant manner, then treatment types were selected by the model |
| 9. Ideally, clinical and administrative decision algorithms should be based on analyses of observed decisions. If that is infeasible, algorithms should be developed with relevant personnel, and validated using routinely collected data (e.g. extracting data from patient records) | Yes | Opinion elicited from team-informed decision algorithms, in particular with regard to psoriasis treatment decisions |
| 10. Where feasible, when estimating times to competing events, methods of analysis that estimate the timing of competing events jointly are preferred to approaches that estimate separate time to event curves for each event | Yes | The time to event associated with a variable is re-estimated once a related event or attribute has occurred or changed |
| 11. Where possible, progression of continuous disease parameters and the likelihood of related events should be defined jointly to maintain the discrete event nature of DES (e.g. sample the continuous measure at which an event occurs, and then sample the time at which that level is reached). | Yes | Time to event and magnitude of event are defined simultaneously |
| 12. To simplify debugging and updating, submodels should be used to structure the model. When comparing strategies within the same system, submodels common to all strategies should be defined once and called from each strategy | Yes | Functions are defined at the start of the model and are used throughout the simulation |
| 13. For structural sensitivity analyses, alternative structures should be implemented within a single DES | Yes | Sensitivity analysis can be conducted by modifying the input CSV files and then re-running the original code |
| 14. Analysts should ensure that ongoing risks remain active over the relevant time horizon | Yes | The model does not shut down risks prematurely |
| 15. Implementation should account only for the outputs required for validation and final analyses. If individual-level data are required, outputs should be stored as attributes; otherwise, aggregated values should be collected | Yes | The model allows both for individual data to be calculated and collected and for individual data to be switched off for more efficient model runs |
| 16. The choice between using general programming or dedicated DES software should be informed by the relative importance of flexibility and execution speed (the former) versus modelling efficiency, automated structure and transparency (the latter) | Yes | The required complexity of the decision problem led to the decision to develop the model in R |
| 17. Analysts should test the stability of outputs generated by similarly specified model runs using alternative random number seeds to perform several independent runs and identify the number of entities, replication duration or number of replications (using the same inputs) required to ensure that the distribution of outputs is stable (e.g. less than a 5% or 1% difference between output values across model runs) | Yes | Multiple random number seeds are used in probabilistic sensitivity analysis, which allows for the comparison of results by seed. It is possible to vary time horizon and number of replications per patient to limit variation between model runs |
| 18. Use of variance reduction techniques is recommended. Balance should be sought between using simple techniques such as extending model runs or matching baseline characteristics, and more sophisticated methods available in dedicated DES software or requiring coding in more generic software. The balance trades off coding time versus improvements in run times and results’ accuracy | Yes | It is possible to reduce variation in baseline characteristics and modify the number of model runs |
| 19. If the number of strategies to compare is large or there are many structural assumptions to test, then factorial design and optimum seeking approaches should be used | N/A | The number of strategies compared for this analysis is not large. Structural assumptions were selected as those to which the model results might be most sensitive |
| 20. When run times for probabilistic sensitivity analysis are constrained, the optimal combination of run size (per input parameter set) and numbers of alternative inputs tested should be estimated empirically to optimise the precision of the outputs of interest | N/A | The run times for probabilistic analysis were not constrained (typically around 1 day) |
| 21. When computing time precludes adequate representation of all potential strategies and parameter uncertainty, meta-modelling should be used | N/A | The present analysis has focused on one comparison between two strategies only; therefore, meta-modelling was not considered to be necessary |
22. If the system to be modelled is not empty at the start of the time horizon to be evaluated, a warm-up period should be used to build the system up to the starting point provided:
|
N/A | The model system is empty at the start |
| 23. Animated representation that displays the experience of events by individuals is recommended as a means of engaging with users and helping to debug through identification of illogical movements | No | No animation of the model is currently planned |
| 24. Both general and detailed representations of a DES structure and logic should be reported to cover the needs of different users. Detailed event documentation figures are also of benefit to the modeller when returning to the model after a period of absence | Yes | Detailed documentation, explanatory commenting of model code and diagrammatic representation have been developed concurrently with the model |
Appendix 11 Economic model structure, components, assumptions and results
This appendix links with Health economics modelling of costs of interventions for people with psoriasis.
Model structure
Treatment as usual
At the start of each simulated patient journey, the patient enters the model with a profile of sociodemographic and health characteristics. The sociodemographic characteristics include age, gender, ethnicity and Townsend deprivation score for the local area the patient lives in. The health characteristics include the severity of psoriasis, PsA, anxiety and depression, BMI, type 2 diabetes, MI and risk of future MI or stroke. Treatment history attributes include prior and current psoriasis treatments. Lifestyle behaviours included are alcohol consumption, extent of physical activity and smoking status. 147 It is assumed that the likelihood that patients are willing to change their behaviours is the same for each behaviour (reduce alcohol consumption, stop smoking, improve diet and physical activity or make no change to any of these behaviours).
Until either the model time horizon is reached or the patient dies, the patient uses health-care resources (e.g. interacting with clinicians at clinical consultations, receiving treatment). The number of health-care services used and the costs depend on each person’s characteristics and how they interact with the health-care system. At the same time, the patient may or may not make changes to their lifestyle that influence their health and need for health care. These events directly affect the costs and QALYs accumulated by the patient. Outcomes are recorded at the end of the simulation so that mean and total costs and QALYs across the whole simulated population can be calculated for each arm of the model.
The model focuses on interactions between the patient and their GP and between the patient and the dermatology service. This is based on the assumption that these are the key relationships that will influence psoriasis treatment. However, the model does also include the wider costs and health impacts of the comorbidities associated with psoriasis. Whenever a patient attends a clinical consultation at either a general practice or dermatology clinic, the model records an interaction between the patient and the clinician (clinical agent). For simplicity, the model assumes that clinical agents possess two key characteristics (attributes) that affect their interaction with the patient and influence patients’ health-care costs and health outcomes. The first is the clinician’s propensity to make treatment decisions that are in line with evidence-based guidelines. This depends on their knowledge of relevant guidelines and assessment of their relevance to the patient and treatment decision required. The second characteristic is the clinician’s propensity to use motivational interviewing skills during consultations. Again, this depends on the clinician’s knowledge of and ability to use motivational interviewing skills and their assessment of the relevance of these for the patient and treatment decision.
Treatment as usual plus PSO WELL
The PSO WELL treatment arm is structured in the same way as the usual care arm but with the addition of the PSO WELL interventions. The intervention arm of the model is, therefore, TAU plus the patient materials and clinician training, considered together as a single PSO WELL package. It is assumed that the distribution of patient materials and training of clinicians occur right at the start of the simulation. The key differences between the PSO WELL arm and the TAU arm is the assumption that (1) the patient materials could change the likelihood that patients make beneficial changes to their lifestyle and health behaviours and (2) training makes clinicians more likely to provide appropriate treatment and use motivational interviewing techniques. These in turn influence patient health, need for health care and health-care costs.
The PSO WELL patient education intervention potentially motivates a patient to seek a GP appointment. In the IMPACT feasibility study, 27.3% (15/55) of participants who received the patient education materials intended to seek more information about psoriasis within 4 weeks. In the model, this is the likelihood that a patient will seek a consultation following the intervention. In the usual care arm, no new help-seeking behaviour is assumed.
The clinician training intervention is more influential in the model, increasing the likelihood that clinicians make guideline-concordant decisions and use motivational interviewing skills. 164 GPs respond well to direct information about the management of psoriasis; 78% of referrals to secondary care were considered appropriate from GPs who received guidelines developed by dermatologists, compared with 59% from other GPs. 130 The model utilises these results as the probability that a clinician will act in a guideline-concordant manner in treatment and referral decisions or deploy motivational interviewing skills during a consultation. For clinicians on the PSO WELL model arm, this probability is 78% compared with 59% of TAU clinicians. This differential is assumed to last for 1 year, after which the likelihood is 59% for all clinicians.
Key components and assumptions
This section describes the key components and interactions of the model. The variables for the model are reported in Key variables; the parameter estimates and data sources are reported in Parameter estimates and data sources for the economic model.
Agent interactions at consultations
Patient–practitioner interactions at consultations, where diagnoses and treatment decisions are made, determine many events that happen during a patient’s journey through the model. Psoriasis severity determines what type of clinician a person sees routinely and what treatments are prescribed. All patients are assumed to have a GP interaction at the start of care. Non-severe psoriasis patients have their care managed by a GP, whereas severe psoriasis patients then have their psoriasis managed by a dermatologist. A GP can refer a patient to a dermatologist for management (who may accept or reject the referral). A dermatologist can refer a patient to a GP for non-psoriasis issues while maintaining management of the patient’s psoriasis care.
At a consultation, the clinician first judges the patient’s psoriasis severity. If the GP diagnoses mild psoriasis, a topical therapy is prescribed216 and a future check-up is scheduled. If the GP diagnoses severe psoriasis, the patient is referred to a dermatologist to potentially receive a more complex therapy: phototherapy, non-biologic systemic therapy or biologic systemic therapy. 216
A GP might misdiagnose a person with mild psoriasis as having a more severe condition. The probability of this happening may differ between TAU and PSO WELL clinician training. Similarly, a GP could classify severe psoriasis patient as having mild disease and prescribe a topical therapy. We assume that dermatologists will always correctly refer a person with mild psoriasis back to primary care.
Psoriasis treatment
People with mild psoriasis can progress through the sequence of topical therapies derived from current clinical guidelines:
-
topical corticosteroid once daily plus vitamin D once daily (NICE guidelines CG153)61
-
if there is insufficient control from (1), change treatment to vitamin D twice daily (NICE guidelines CG153)61
-
if there is insufficient control from (2), change treatment to corticosteroid twice daily (NICE guidelines CG153)61
-
if a patient exhausts all available topical therapies and they still have mild psoriasis, re-prescribe corticosteroid twice daily.
People with severe psoriasis who enter the model will start treatment with phototherapy if they have previously received only topical treatment. If the patient has previously received a complex therapy, the clinician will initiate the appropriate treatment in the sequence according to current guidelines. 61
A clinician who follows the clinical guidelines will stop prescribing ultraviolet B (UVB) phototherapy if the patient has received 10 courses in total or a maximum of 175 sessions (assumes a typical course consists of 2.5 sessions per week for 7 weeks). 188 Any complex treatment for severe psoriasis will be stopped if it does not lead to a treatment response (defined as a reduction in PASI score of ≥ 75% or a 50–75% reduction in PASI plus a reduction of at least 5 points on the DLQI measure). This is the guideline decision rule for biologic therapies61 and is assumed to apply for all systemic treatments for severe psoriasis.
A clinician who does not act in accordance with the guidelines will, in the model, prescribe phototherapy if required. However, in this case there is no clear information about which type of phototherapy will be prescribed. Accordingly, when clinicians do not act in accordance with the guidelines, the specific type of phototherapy is selected at random by the model. If a patient with severe psoriasis has previously received phototherapy, but the clinician does not adhere to the relevant clinical guidelines, it is assumed that a systemic therapy is prescribed. In this case, the specific type of systemic therapy will be selected at random by the model.
The model does not incorporate potential contraindications or side effects associated with severe psoriasis treatments.
Scheduling consultations
A patient’s time until next consultation depends on their current psoriasis treatment. Routine GP appointments occur every 6 months to review progress with topical therapy. An appointment is scheduled for 2 weeks after referral from a dermatologist, a psoriasis flare or patient help-seeking following the PSO WELL intervention. Routine dermatologist consultations occur every 10–16 weeks depending on the specific complex treatment regimen.
Lifestyle and comorbidity interventions
A clinician with a good knowledge of psoriasis and awareness of related health issues may identify the need for a formal lifestyle or comorbidity intervention. The patient will agree to a lifestyle intervention only if it is a behaviour they are intent on changing. To manage an identified comorbidity, patients will receive the next available intervention in the relevant treatment sequence derived from clinical guidelines. 182–187 If the end of a particular comorbidity treatment sequence is reached and further treatment is required, patients will continue to receive the last intervention given. Patients who have behavioural interventions will receive each behavioural treatment sequence only once.
Key variables
Key sources of model inputs include data from the IMPACT programme, published evidence identified in the literature review, clinical guidelines130,182–187 and expert opinion (Tables 26–32).
| Input parameter | Description and distribution |
|---|---|
| Age (years) | Mean 53.0 (SD 15.5) |
| Deprivation score (Townsend) for the local area of the patient | Mean –0.22 (SD 3.25) |
| Ethnicity | White 97.2%, Indian 1.8%, remainder split equally between Pakistani, Bangladeshi, other Asian, Black Caribbean, Black African, Chinese and other |
| Gender | Female 57.1%, male 42.9% |
| Height (cm) | Female: mean 162.8 (SD 6.0); male: mean 174.8 (SD 7.8) |
| Weight (kg) | Female: mean 76.5 (SD 17.2); male: mean 87.4 (SD 16.2) |
| DLQI score | Mean 4.60 (SD 4.79) |
| PASI score | Mean 4.17 (SD 3.83) |
| Presence of severe psoriasis | 17.3% |
| History of biologic systemic therapy | 18.4% |
| History of non-biologic systemic therapy | 29.2% |
| History of phototherapy | 59.2% |
| History of topical therapy | Assumed to be 100% |
| Alcohol consumption (units per week) | Female aged < 40 years, mean 4.6 (SD 6.7); female aged ≥ 40 years, mean 7.6 (SD 9.2); male aged < 40 years, mean 9.3 (SD 12.4); male aged ≥ 40 years, mean 19.5 (SD 31.0) |
| Exercise level risk (physical activity) | 29.1% likely to take a value of 1 (binary variable indicating high risk of inactivity) |
| Intent to change behaviour | Distribution: attribute is equally likely (20%) to take any value from 1 to 5 |
| Anxiety (HADS) | Mean 7.1 (SD 4.7) |
| Anxiety, history of treatment for | 27.5% |
| AF, presence of | 7.2% likely in patients aged > 40 years |
| Cardiac disease, presence of | 7.0% |
| Cardiac disease, family history of | 23.3% |
| Cholesterol ratio (total high density) | Female aged < 40 years, mean 3.26 (SD 1.1); female aged ≥ 40 years, mean 3.5 (SD 1.3); male aged < 40 years, mean 3.7 (SD 1.2); male aged ≥ 40 years, mean 4.0 (SD 1.2) |
| Depression (HADS) | Mean 4.5 (SD 3.7) |
| Depression, history of treatment of | 27.5% |
| Diabetes (type 2), presence of | 5.6% |
| PsA, presence of | Aged < 40 years: 6.3%; aged ≥ 40 years: 19.9% |
| Smoking status: current smoker | 18.2% |
| Stroke, previous event | 0.7% |
| SBP (mmHg) | Female aged < 40 years, mean 112.2 (SD 10.4); female aged ≥ 40 years, mean 131.3 (SD 17.8); male aged < 40 years, mean 129.4 (SD 15.0); male aged ≥ 40 years, mean 136.6 (SD 17.4) |
| SBP (mmHg), current treatment | 32.9% likely to be receiving antihypertensive medication if indicated |
| Treatment | PASI effect | DLQI effect | QoL effect |
|---|---|---|---|
| Topical therapiesa | |||
| 1. Corticosteroid and Vitamin D, OD | Assumption: PASI score is less than clearance threshold in responders | Assumption: change in DLQI estimated based on change in QOL using published algorithm217 | 71% responders |
| 2. Vitamin D, BD | 54% responders | ||
| 3. Corticosteroid, BD | 62% responders | ||
| Phototherapyb | |||
| 1. UVB | PASI75 responders: 70%218 | Change in DLQI –8.5216 | Utility calculated as normal |
| 2. PUVA | PASI75 responders: 83%216 | Change in DLQI –8.5 (assumed equal to UVB)216 | |
| 3. UVB plus topical | PASI75 responders: 62%218 | Change in DLQI –8.5216 | |
| Non-biologic systemic therapyc | |||
| 1. Methotrexate | PASI75 responders: 48%216 | Mapping: PASI reduction > 75%, DLQI change –9.4;219 PASI reduction 50–75%, DLQI change –6.1; PASI reduction < 50%, DLQI change –5.2 | Utility calculated as normal |
| 2. Ciclosporin | PASI75 responders: 70%216 | ||
| 3. Acitretin | PASI75 responders: 30%216 | ||
| Biologic systemic therapyb | |||
| 1. Adalimumab | PASI75 responders: 72%220 | Change in DLQI –11.5220 | Utility calculated as normal |
| 2. Etanercept | PASI75 responders: 33%216 | Change in DLQI –7.0216 | |
| 3. Ustekinumab | PASI75 responders: 67%216 | Change in DLQI –9220 | |
| 4. Infliximab | PASI75 responders: 80%216 | Change in DLQI –9.7216 | |
| Indication | Description | Treatment effect size | Time to relapse |
|---|---|---|---|
| Behavioural interventions | |||
| Alcohol consumption | Mean reduction in drinks per week,191,222 converted to units per week by assuming 2 units per drink223 |
Brief GP-led intervention: single brief intervention, –7.3 units per week Extended GP-led intervention: multiple brief interventions, –8.8 units per week Specialised alcohol service referral: multiple extended interventions, –5.1 units per week Distributions: normal |
1 year; distribution: exponential |
| Diet | Weighted mean difference in weight, intervention vs. usual care183 |
Brief GP-led intervention: behaviour therapy, –4.3 kg Dietitian referral: motivated to maintain 600 kcal deficit, –5.3 kg Distributions: normal |
18 months (evidence reflects high uncertainty beyond 18 months); distribution: exponential |
| Exercise | Proportion of patients achieving dichotomous improvement in exercise vs. control;224 weight loss as a result of improved exercise vs. control183 |
Brief GP-led intervention: brief advice, 27.6%; weight change due to physical activity, –3.1 kg Exercise referral scheme: same effectiveness as brief intervention; little to no evidence of increased effectiveness225 Distributions: normal |
1 year; distribution: exponential |
| Smoking status | Probability of success of intervention in causing smoking cessation226,227 |
Brief GP-led intervention: intermediate services, 34% Smoking cessation service referral: specialist services, 49% Distributions: beta |
13% quit for 1 year (then relapse assumed). Of remainder: 39% relapse at 3 months, 29% relapse at 6 months, 17% relapse at 9 months and 15% relapse at 1 year.228 Distributions: exponential |
| Comorbidity interventions | |||
| Anxiety | Standardised mean difference in anxiety score187 |
Low intensity psychological: non-facilitated self-help, combined GAD and anxiety, –0.74 Medication: sertraline effect vs. placebo, –0.28 High-intensity psychological CBT vs. control, –0.63 Distributions: normal |
6 months; distribution: exponential |
| Cardiac disease (MI) | Event risk based on QRISK®2 (ClinRisk Ltd, Leeds, UK) algorithm | Treatment effect not applicable | Relapse not applicable |
| Cholesterol ratio high | Evidence in clinical guidelines only reports a mean change in LDL cholesterol level.185 Cannot easily be converted to a change in cholesterol-to-HDL ratio. Assumption that a decrease in LDL with no change in HDL approximates the change in cholesterol ratio |
Statins: atorvastin reduction in LDL (no change in HDL), 40–50%229 Assumption: reduction in cholesterol–to-HDL ratio of midpoint (45%) Distribution: beta |
Ongoing treatment, effect maintained |
| Depression | Standardised mean difference in depression score186 |
Medication: amitriptyline, –0.61 Psychological intervention: CBT, –0.89 Combination therapy: same effectiveness as CBT. No evidence of differential efficacy to CBT alone Distributions: normal |
1 year (assumption); distribution: exponential |
| Diabetes, type 2 | Event risk based on QDiabetes® (ClinRisk Ltd, Leeds, UK) algorithm | Treatment effect not applicable | Relapse not applicable |
| PsA | Event risk estimated from literature (41) | Treatment effect not applicable | Relapse not applicable |
| Stroke | Event risk based on QRISK®2 algorithm | Treatment effect not applicable | Relapse not applicable |
| Elevated SBP | Evidence in clinical guidelines reports a treatment effect associated with diuretics only.182 Pooled change in trough SBP vs. placebo obtained from Cochrane review229 |
Antihypertensive medication: pooled treatments, –8.5 Distribution: normal |
Ongoing treatment, effect maintained |
| Patient attribute | Utility decrement (SE) |
|---|---|
| Age (years), decrement per year | –0.002 (0.001)230 |
| Anxiety (HADS anxiety score) | |
| ≥ 4 HADS anxiety < 8 | –0.032 (0.006)230 |
| ≤ 8 HADS anxiety | –0.068 (0.012)230 |
| Depression (HADS depression score) | |
| ≥ 4 HADS depression < 7 | –0.052 (0.007)230 |
| ≥ 7 HADS depression < 11 | –0.069 (0.009)230 |
| ≥ 11 HADS depression | –0.120 (0.011)230 |
| ≥ 16 | –0.177 (0.021)230 |
| Diabetes, type 2 | –0.071 (0.005)231 |
| Gender, male | 0.015 (0.004)230 |
| Presence of severe psoriasis | –0.050 (0.010)a |
| PsA | –0.093 (0.005)230 |
| Recovery period immediately following CV event | –0.100 (0.020)b |
| Skin complaint | –0.019 (0.008)c |
| Long-term decrement following CV event | –0.025 (0.006)230 |
| Patient education intervention | Clinician training intervention | ||||
|---|---|---|---|---|---|
| Delivery resource | Total cost (£) | Unit cost per patient (£) | Delivery resource | Total cost (£) | Unit cost per group (£) |
| Production of materials | 1540.00 | 18.55 | Production of training materials | 3763.40 | 627.23 |
| Delivery, including packaging and postage | 457.87 | 5.52 | Delivery, including venue hire, travel costs, staff time and practitioner time forgonea | 65,328.00a | 10,888.00a |
| Primary analysis unit cost | – | 24.07 | Primary analysis unit cost | – | 11,515.23b |
| Design, including meetings, content development and testing | 1989.70 | 239.65 | Design, including content development and pilot training | 5979.89 | 996.65 |
| Marketing materials | 307.50 | 3.70 | Marketing materials | 368.50 | 61.42 |
| Unit cost (in scenario analysis) | – | 243.35c | Unit cost (in scenario analysis) | – | 1058.07c |
| Reason for intervention | Position of intervention in treatment sequence | ||
|---|---|---|---|
| First | Second | Third | |
| Alcohol consumption: 15 units per week for females, 22 units per week for males191 |
GP-led brief advice Unit cost: £38233 |
Extended brief intervention (assumed 3 × standard consultation), £114233 | Referral to community alcohol services, £121234 |
| Dietary behaviour: BMI > 25 kg/m2 | Referral to community dietitian, £80234 | None | |
| Physical activity: suitable if patient’s current exercise status is considered to be ‘at risk’ (inactive) | Exercise referral scheme,a £385235 | None | |
| Smoking status: suitable for improvement if patient is currently a light, moderate or heavy smoker | Referral to stop smoking services, £224236 | None | |
| Anxiety: HADS score of ≥ 8237 | Low-intensity psychological therapy, £17233 | 8-week course of sertraline with two GP visits, £79238 | 15 psychologist-led CBT sessions, £2070233 |
| Cholesterol ratio elevated: QRISK®2 score of ≥ 10185 | Ongoing management with statins, £23–35 per year by prior CVD status238,239 | ||
| Depression:186 HADS score of ≥ 7237 | 3-month course of citalopram with four GP visits, £156238 | 16 CBT sessions, £1488233 | Combination therapy, £1644 |
| Diabetes, type 2: risk of event calculated using QDiabetes® algorithm240 | Ongoing cost of diabetes management, £537 per year241 | ||
| MI: risk of event calculated using QRISK®2 algorithm242 | Event cost, £3425 (limited evidence regarding ongoing costs associated with the event)234 | ||
| PsA: rate of diagnosis estimated from literature243 | Ongoing maintenance, £10,846 per year | ||
| Elevated SBP: SBP ≥ 140 mmHg182 | Ongoing management with ACE inhibitor, £17–18 per year (by age and ethnicity) | ||
|
Stroke Risk of event calculated using QRISK®2 algorithm242 |
Event cost, £5763 (limited evidence regarding ongoing costs associated with the event)234 | ||
| Cost item | Description | Unit cost, £ (SD) |
|---|---|---|
| Topical therapies | ||
| Corticosteroid, OD | 4-week course, betamethasone valerate 0.1% or mometasone furoate 0.1% | 2.93 (0.57)238,239 |
| Vitamin D, OD | 4-week course, calcipotriol | 24.04 (–)238 |
| Vitamin D, BD | 4-week course, 2 × vitamin D OD | 48.08 (–)238 |
| Corticosteroid, BD | 4-week course, 2 × corticosteroid OD | 5.86 (1.14)238 |
| Phototherapy | ||
| Phototherapy session (assumed equal for UVB and PUVA) | Equal likelihood of two or three sessions per week and 6, 7 or 8 weeks per course244 | 92.33 per session;234 expected course cost, 1615.69 |
| Non-biologic systemic | ||
| Methotrexate | 14-week course | 8.40 (–)238 |
| Ciclosporin | Neoral® (no generic), 12-week course | Pack cost, £17–62;238 unit cost per dose calculated by patient weight |
| Acitretin | Neotigason® (no generic), 12 week course | 75.09 (11.34)238,239 |
| Biologic systemic | ||
| Adalimumab | Humira® (no generic), 16-week course | Drug, 3521.40 (–);238 administration, 0 (self-administered) |
| Etanercept | Enbrel® (no generic), 12-week course | Drug, 2145.12 (–);238 administration, 0 (self-administered) |
| Ustekinumab | Stelara®, 16-week course | Drug, 6441.00 (–);238 administration, 0 (self-administered) |
| Infliximab | Remicade® (no generic), 10-week course | Vial cost, £420238 (whole vials required calculated by patient weight); administration by infusion, 1738.92 (622.53)234,245 |
Treatment effects
Psoriasis treatment effects (see Table 27) were identified from the literature review. Only studies that included all regimens within a particular type of therapy were considered. If multiple studies reported different effect sizes for equivalent treatments, the largest was selected. For simplicity, treatment effects occur immediately in the model.
The studies in the systematic review used a range of measures to quantify treatment effects. The two main measures used were, first, the DLQI, which assesses the impact of psoriasis on aspects of a patient’s life (symptoms and feelings, daily activities, leisure, personal relationships) and whether or not the psoriasis treatment is a problem. Whether or not the psoriasis treatment is a problem is particularly relevant for topical therapies. The second measure is the PASI, which measures severity of psoriasis symptoms. The model required a single measure of treatment response. The DLQI was considered to better reflect patients’ concerns about quality of life. Accordingly, published algorithms were used to generate a common measure from the data collected in the systematic review. The following algorithm217 was used to estimate the impact of topical therapies on DLQI:
For systemic therapies, the identified treatment effect was the proportion of patients who experienced a ≥ PASI75 (75% improvement in PASI score) response. Based on this, the following relationship was used to estimate the impact of systemic treatments on DLQI score (Table 33). 219
| PASI response | Associated DLQI change |
|---|---|
| > PASI75 | –9.36 |
| PASI50–75 | –6.12 |
| < PASI50 | –5.17 |
Treatment effects of behaviour change and comorbidity interventions were obtained from the sources used in developing clinical guidelines. 130,182–187 These effects are realised for a finite period of time before relapsing to prior values, with time to relapse based on the available evidence. Details of the effects and time to relapse are given in Table 28.
Psoriasis flares
Psoriasis flares occur in the model, potentially causing mild psoriasis to progress to severe psoriasis. It is estimated that 35.5% of psoriasis patients experience a flare by 4 weeks;221 from this, the model predicts a time to flare. A flare will cause an increase in DLQI score (estimated as the reported difference in DLQI scores of patients with frequent flares and infrequent flares). 246 If the DLQI score exceeds 10, the patient is classified as having severe psoriasis. It is assumed that even if patients with severe psoriasis have their symptoms effectively managed they cannot return to having mild or moderate psoriasis.
Quality-adjusted life-years
Quality-adjusted life-years were used as the measure of total health benefit experienced by simulated patients over the course of their journey through the model. A QALY measures the length of survival and adjusts the life-year to take into account any ill health during that time. This is done by estimating utility weights that reflect a person’s preferences for different health states. Utility weights are typically measured on a scale from 0 to 1. A person in full health for 1 year will have a utility value of 1 and a QALY of 1. A person who is in less than full health for 1 year may have a utility value of 0.25 and a QALY of 0.25. Death is assigned a value of 0. Some of the measures used to generate utility values also allow a person with very poor health to have utility values of < 0. The model allows a simulated patient’s health status to vary as events occur and their attributes change. If the simulated patient has clinical characteristics (e.g. severe psoriasis symptoms or diabetes) or experiences events (e.g. side effects or stroke) that indicate that they are in less than full health, they are assigned a reduction in utility (also termed a utility decrement). The patient’s overall utility for 1 year is estimated by subtracting any utility decrements from 1 (see Table 29). For example, if a patient has PsA, the associated decrement (–0.093)230 is incurred, giving a utility score of 0.907. To ensure that the cumulative impact of several utility decrements does not overestimate the total negative impact of characteristics and events occurring at once, only the largest decrement is applied in full at each utility update.
Mortality
Time to mortality is predicted based on all-cause mortality. 192 The only cause of early mortality included in the model is fatality due to MI (15.1% in females, 10.6% in males)193 and stroke (12.5%). 194 These were thought to be the most relevant to the higher CVD risk seen in people with psoriasis1,30,66 and the focus of the PSO WELL intervention.
Costs and resource use
Various health-care resources are required throughout the simulation. The PSO WELL interventions require two types: the resources needed to deliver the intervention (direct provision costs) and the resources used to develop the interventions. These are treated as sunk costs for conceptualisation and planning. Our primary analysis included only the delivery costs of the PSO WELL interventions, implying that all necessary development had taken place. Total delivery costs, obtained directly from the IMPACT programme records, were £24 per patient for the information materials and £11,515 per group of 10 clinicians trained (see Table 30). For the primary analysis, we conservatively assumed that each clinician trained would manage only one patient who benefited from the clinician training intervention. This was varied in threshold analysis. The average group size for clinician training was 10 participants.
Other health-care costs were sourced from UK sources (see Tables 31 and 32). The basic cost per consultation is £38 for a GP visit and £93–109234 for a dermatologist appointment. Topical and non-biologic systemic therapies were costed using the average of available generic treatments in the British National Formulary238 weighted by the total number of each pack prescribed in England in 2014. 239 The cost of phototherapy (£92) is incurred per session. 234 Biologics included in the model are all self-administered, with the exception of infliximab, which incurs an additional administration cost as it is delivered by intravenous infusion. The costs of providing interventions to support lifestyle changes and to manage comorbidities were also included and are detailed in Table 31. Clinical guidelines were used to determine typical treatment regimens. 182–187
Costs and QALYs were discounted at an annual rate of 3.5% to account for social time preferences as recommended in the UK. 195
Parameter estimates and data sources for the economic model
Most of the patient attribute values in the model are based on a cohort study of psoriasis and CV events of 287 individuals with psoriasis66 (see Table 26). The model estimates any missing data using the mean and dispersion of the observed data.
Severe psoriasis is present in 17.3% of patients entering the simulation. 66 Severe psoriasis is defined as a DLQI score of > 10, a PASI score of ≥ 10 or previous treatment with a biologic systemic therapy.
Other health-related patient characteristics include anxiety and depression scores, PsA, BMI, type 2 diabetes and CV markers. Risks of future MI, stroke and of diabetes are estimated using published risk algorithms. 240,242 Lifestyle behaviours included are alcohol consumption, extent of physical activity and smoking status. 147 Treatment history attributes include prior and current psoriasis treatments. Patients are assumed equally likely to be willing to improve their alcohol consumption, diet, physical activity, smoking or none of these behaviours.
| Costs | PSO WELL (£) | TAU (£) | Incremental difference (£) |
|---|---|---|---|
| 1. Mean | 1. 26,242 | 1. 27,081 | 1. 838 |
| 2. SD | 2. 794 | 2. 793 | 2. 1103 |
| 3. 95% CI | 3. 24,902 to 27,466 | 3. 25,852 to 28,452 | 3. –1106 to 2593 |
| QALYs | PSO WELL | MAU | Incremental difference |
| 1. Mean | 1. 4.737 | 1. 4.748 | 1. 0.012 |
| 2. SD | 2. 0.052 | 2. 0.053 | 2. 0.070 |
| 3. 95% CI | 3. 4.643 to 4.815 | 3. 4.659 to 4.833 | 3. –0.097 to 0.127 |
| ICER (£) | 72,802 | ||
| Net monetary benefit per patient (if willing to pay £20,000 per QALY) (£) | –607 | ||
| Probability PSO WELL is cost-effective (ICER ≤ £20,000) (%) | 34 | ||
| Number of patients required to benefit per clinician group to achieve ICER ≤ £20,000 | 22 | ||
| Alternative model assumption | PSO WELL net cost (£) (SD; 95% CI) | PSO WELL net QALYs (£) (SD; 95% CI) | ICER (£) | Probability PSO WELL is cost-effective at WTP threshold of £20,000 per QALY (%) | Number of patients benefiting per 10 clinicians trained to achieve ICER ≤ £20,000 |
|---|---|---|---|---|---|
| Development costs associated with PSO WELL included in its model costs | 1630 (1155; –326 to 3412) | 0.014 (0.075; –0.115 to 0.140) | 117,023 | 22 | 514 |
| PSO WELL impact on clinicians is maintained for 10 years (i.e. does not deteriorate) | 675 (1162; –1226 to 2539) | 0.015 (0.071; –0.103 to 0.121) | 45,665 | 44 | 16 |
| All dermatologists make guideline-concordant treatment decisions always | 1034 (1043; –717 to 2777) | 0.024 (0.079; –0.116 to 0.148) | 43,208 | 36 | 20 |
| Model duration reduced from 10 years to 1 year | 1102 (186; 819 to 1388) | 0.010 (0.006; 0.001 to 0.020) | 108,418 | 0 | 50 |
| Model duration reduced from 10 years to 3 years | 1078 (383; 466 to 1733) | 0.019 (0.019; –0.013 to 0.049) | 55,382 | 10 | 26 |
FIGURE 72.
Plot of primary analysis PSA results (10 patients benefiting from PSO WELL per clinician group). Each incremental output point represents the mean result of 287 patients simulated through the model in both treatment arms; hence, only 250 incremental output points are shown.
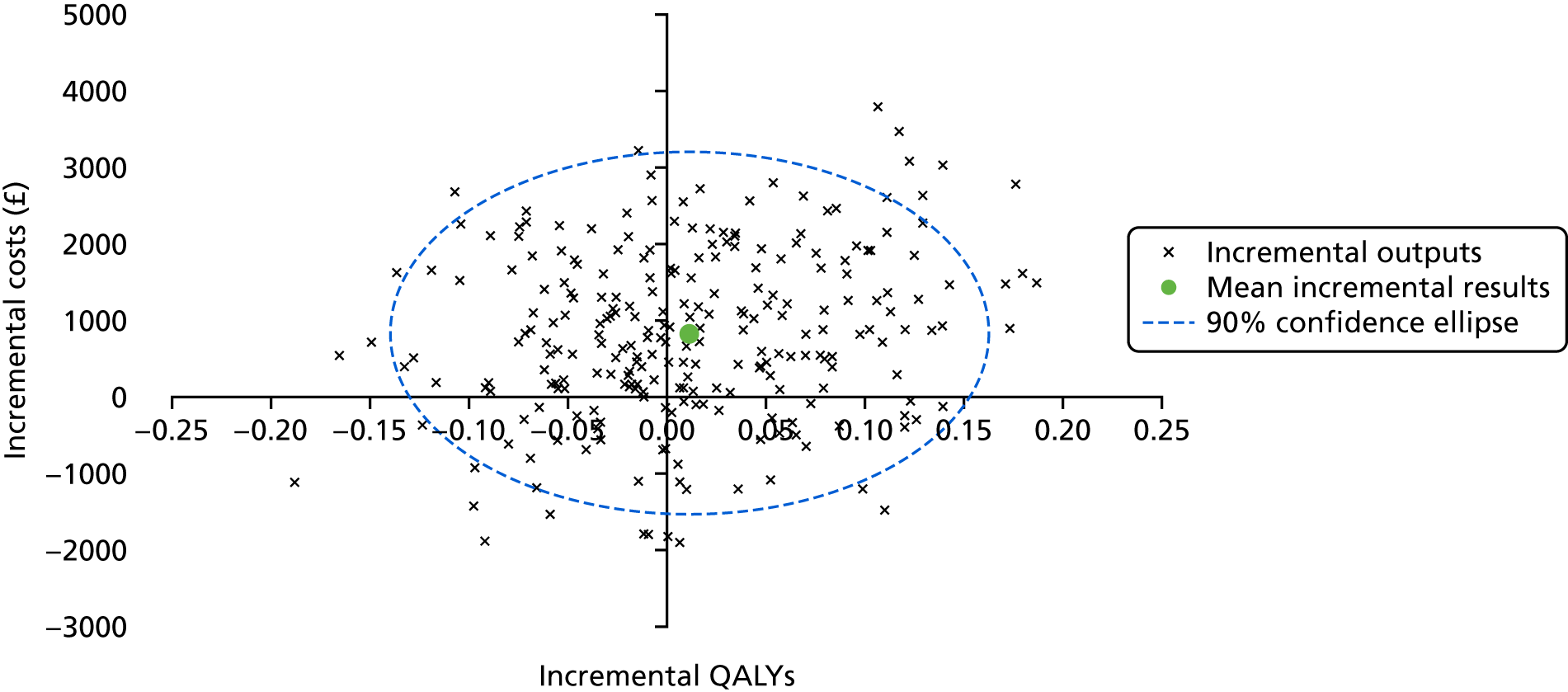
FIGURE 73.
Primary analysis cost-effectiveness acceptability curve.
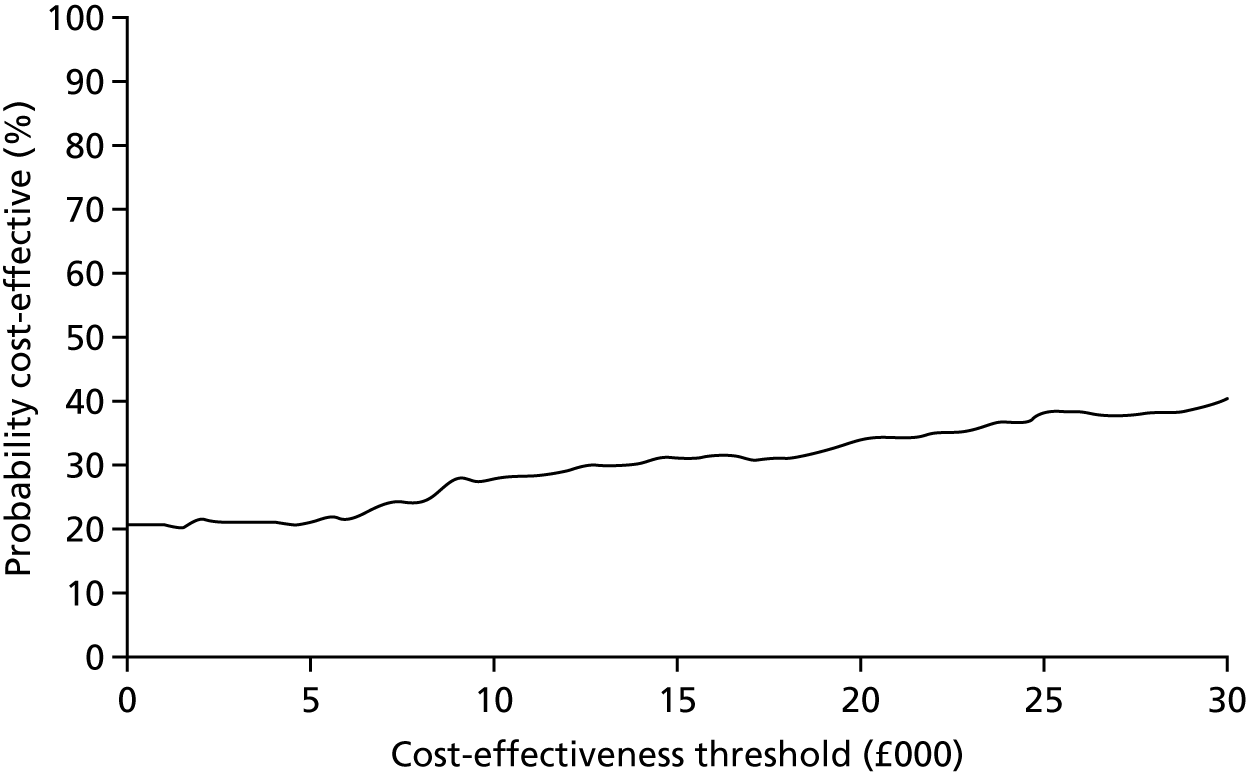
FIGURE 74.
Threshold analysis varying the number of patients benefiting from PSO WELL.
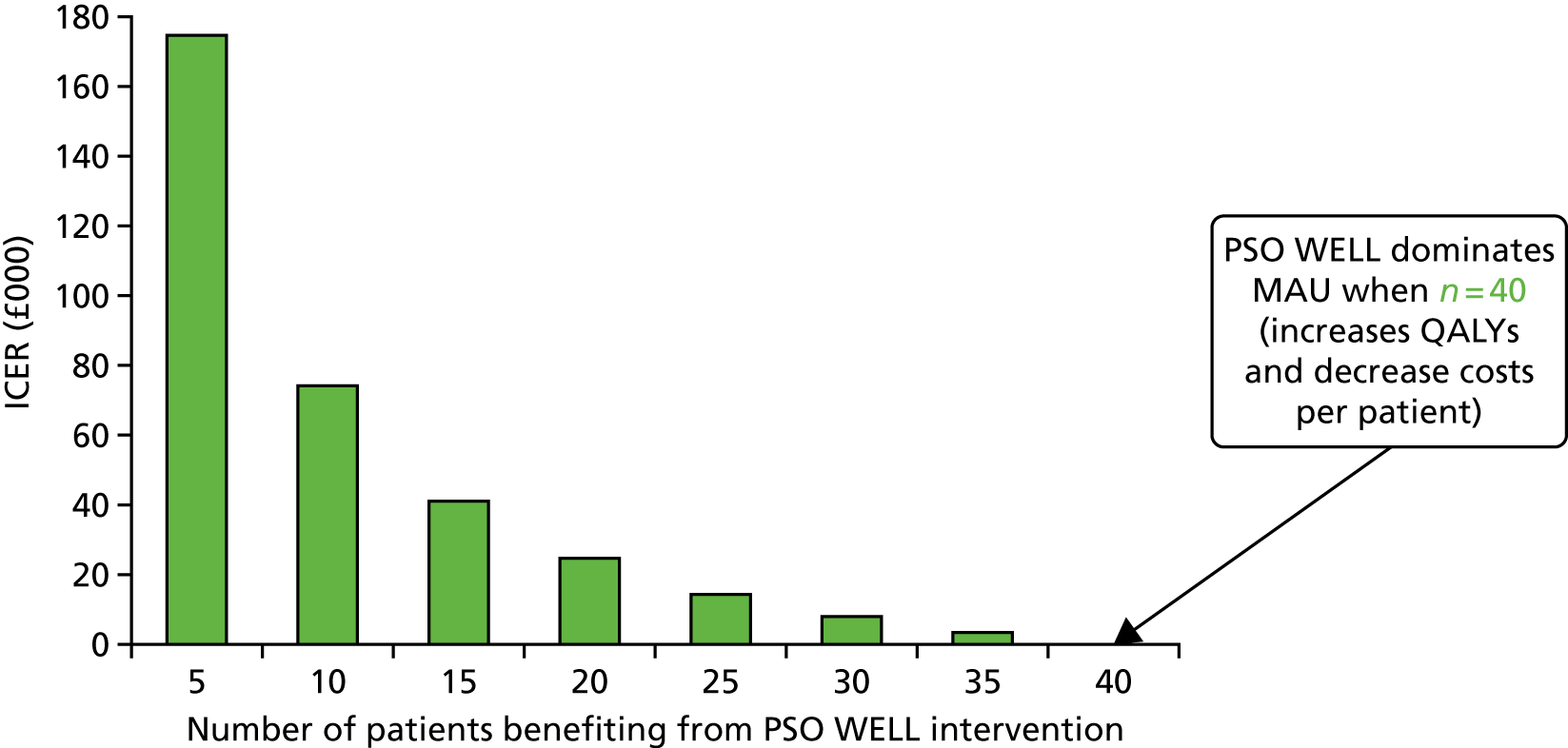
Appendix 12 The IMPACT programme steering committee: patient representative terms of reference
This appendix links with Patient, public and practitioner involvement in the IMPACT programme.
The IMPACT programme of research
The major objective of the IMPACT programme is to apply the best available knowledge to improve services for the care of people with psoriasis. This research is funded by NIHR.
Purpose of the IMPACT programme steering committee
The overall purpose of the IMPACT programme steering committee is to bring together researchers, health-care deliverers (dermatologists, clinicians, GPs and NHS managers), psoriasis experts and patient representatives to advise on the work of the IMPACT programme team. This work includes the development of patient self-care support materials and training for professionals to improve patient care and access to targeted high-quality services.
The programme steering group will:
-
discuss the progress of the IMPACT programme
-
highlight any issues and difficulties that need to be reported to NIHR (such as problems with recruitment or study progress, deviation from the original research plan and any concerns over the management and conduct of the programme) and discuss workable solutions
-
discuss findings that are of direct benefit to patients and decide how these findings should be presented to both national and local patient groups.
Patient representative responsibilities
Patient representatives will be expected to:
-
attend two programme steering committee meetings per year
-
work constructively as part of the programme steering committee to support the aims of the IMPACT programme
-
take into account the responsibilities, viewpoints and expertise of other members
-
discuss and comment on items during meetings and raise issues for consideration
-
read information circulated prior to the meeting and, when appropriate, provide comments from a patient/public perspective including –
-
contributing views and experience about patient self-care strategies to cope with psoriasis
-
helping to identify barriers to practitioners providing psoriasis patients with useful advice about LBCs
-
provide unbiased, balanced views including, when appropriate, personal views as well as the views of others not represented in the group on experiences of psoriasis
-
advise on methods to share IMPACT findings with psoriasis patient groups both locally and nationally
-
respect any requests for confidentiality, and declare any conflicts of interest if they arise.
-
Membership
-
Chairperson and IMPACT principal investigator (Professor Christopher EM Griffiths).
-
Programme co-applicants.
-
Programme manager (Alison Littlewood).
-
Administrative lead at Salford Royal NHS Foundation Trust.
-
Psoriasis Association representative.
-
Patient representatives from three PCTs (Salford; Ashton, Leigh and Wigan; and East Lancashire).
-
Professional representatives from three PCTs (Salford; Ashton, Leigh and Wigan; and East Lancashire).
-
Research representative.
Frequency of meetings
Meetings will be held every 6 months. The travel expenses of patient representatives will be reimbursed at each meeting. Please note that ad hoc meetings will be convened if urgent matters requiring discussion arise.
List of abbreviations
- ACS
- acute coronary syndrome
- AF
- atrial fibrillation
- BAD
- British Association of Dermatologists
- BDNG
- British Dermatological Nursing Group
- BECCI
- Behaviour Change Counselling Index
- BMI
- body mass index
- BPS
- British Psychological Society
- CCG
- Clinical Commissioning Group
- CEO
- chief executive officer
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- CV
- cardiovascular
- CVD
- cardiovascular disease
- DES
- discrete event simulation
- DLQI
- Dermatology Life Quality Index
- DSN
- dermatology specialist nurse
- e-health
- electronic health
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FIM
- full investigator meeting
- GP
- general practitioner
- GPSI
- general practitioner with a special interest
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycosylated haemoglobin
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- HSE
- Health Survey for England
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IMPACT
- Identification and Management of Psoriasis Associated ComorbidiTy
- IPQ-R
- Revised Illness Perceptions Questionnaire
- IQR
- interquartile range
- LBC
- lifestyle behaviour change
- LDL
- low-density lipoprotein
- MI
- myocardial infarction
- MRC
- Medical Research Council
- MRS
- marginal rate of substitution
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- ONS
- Office for National Statistics
- PASI
- Psoriasis Area and Severity Index
- PASI75
- 75% improvement in Psoriasis Area and Severity Index score
- PCT
- primary care trust
- PPI
- patient and public involvement
- PsA
- psoriatic arthritis
- PSO WELL®
- Psoriasis and Wellbeing
- PRE-HIT
- Patient Readiness to Engage in Health Information Technology
- PUVA
- psoralen and ultraviolet A
- PWV
- pulse wave velocity
- QALY
- quality-adjusted life-year
- REC
- Research Ethics Committee
- RUG
- research user group
- SAPASI
- self-administered Psoriasis Area and Severity Index
- SD
- standard deviation
- SE
- standard error
- SPI
- Simplified Psoriasis Index (formerly Salford Psoriasis Index)
- TAU
- treatment as usual
- UVB
- ultraviolet B
- WTP
- willingness to pay

