Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1005. The contractual start date was in August 2007. The final report began editorial review in February 2013 and was accepted for publication in July 2014. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Iliffe et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 EVIDEM-ED: a cluster-randomised controlled trial to improve early diagnosis and clinical management of dementia in primary care
Abstract
Aim To test a customised educational intervention developed for general practice, promoting both earlier diagnosis of dementia and concordance with management guidelines.
Design/method The intervention, based on practice-based workshops, was tested in an unblinded cluster randomised controlled trial (RCT) with a pre- and post-intervention design, with two arms: usual care compared with the educational intervention. Twenty-three general practices participated and the records of 1072 patients with dementia were audited. Our primary outcome was an increase in the proportion of patients with dementia who received at least two documented dementia-specific management reviews per year. Secondary outcomes were practitioner concordance with management guidelines in a subset of 167 patients with dementia; satisfaction and met need among 84 carers; and attitudes, knowledge and confidence with dementia diagnosis and management in general practitioners (GPs) and practice nurses.
Findings The estimated odds ratio (OR) of having two or more reviews in the intervention group compared with the usual care group was 0.83 [95% confidence interval (CI) 0.52 to 1.33; p = 0.44]. Case detection rates were unaffected by the intervention. The estimated incidence rate ratio (IRR) for the intervention group compared with the usual care group from multilevel Poisson regression modelling was 1.03 (95% CI 0.57 to 1.86, p = 0.93). Carers’ recall of advice given suggested that a large minority had not received the information recommended by the National Institute for Health and Clinical Excellence (NICE) dementia guidelines. Carers of patients taking cholinesterase inhibitors reported more advice on some aspects of care.
Discussion An educational intervention customised to the needs of each practice did not appear to alter the documentation of clinical management of patients with dementia, nor did it increase case identification.
Background: why this study was necessary
Demography and impact
There is increasing interest in earlier diagnosis, because of:
-
the ageing of the population and the rising prevalence of dementia
-
the costs of care for people with dementia, and
-
the perceived benefits for the person with dementia (PWD) and their carers of early intervention, with concerns about delays in diagnosis, especially in primary care.
Dementia is one of the main causes of disability in later life; in terms of the Global Burden of Disease, it contributes 11.2% of all years lived with disability, higher than stroke (9.5%), musculoskeletal disorders (8.9%), heart disease (5%) and cancer (2.4%). 1 The total costs of caring for people with dementia in the UK have been estimated at between £17B and £18B per year2 – more than heart disease (£4B), stroke (£3B) and cancer (£2B).
Delayed recognition
Dementia syndromes are underdiagnosed and undertreated in primary care in all countries3,4 with an estimated 50% of primary care patients of more than 65 years not diagnosed by their primary care physicians. 5,6 The complex reasons for this include patient, family, practitioner and service causes, and are discussed in detail elsewhere. 7
The evidence suggests that there are tangible benefits to earlier recognition of dementia. Early disclosure of the diagnosis seems to be what people with dementia want to have8 and younger professionals want to give. 9 The benefits of making a diagnosis include ending uncertainty about the cause of symptoms and behaviour change, with greater understanding of problems; giving access to appropriate support; promoting positive coping strategies; and facilitating the planning and fulfilment of short-term goals. 10,11 There is also the potential for using cholinesterase inhibitor medication to modify symptoms and delay the need to seek care home moves among people with dementia.
Enhancing skills in primary care
The insidious nature of dementia means that it is most likely to present as a problem within primary care, but there are obstacles to earlier recognition in this setting. Therefore, considerable efforts have been made to provide educational programmes to enhance the diagnostic skills of primary care practitioners. Because of the apparent time constraints in primary care consultations, much research focus has been on the development of brief screening tests for assessing cognitive function. However, despite the availability of user-friendly cognitive function tests, there has been little evidence of improvement in primary care recognition of, and response to, dementia syndromes over the decade since the introduction of cholinesterase inhibitors. The UK evidence is particularly compelling on this point, especially from early educational interventions,12 and recent analyses by members of the EVIDEM (Evidence-based Interventions in Dementia) programme of incidence and prevalence in a large GP data set. 13
We find persuasive the argument that the problem of underdiagnosis is probably due to the interaction of case complexity, pressure on time and the negative effects of reimbursement systems that do not reward time commitment and systematic follow-up. 14,15 However, in our view there is also considerable evidence that the main problem is not that primary care practitioners simply lack diagnostic skills, but that they lack the resources and management skills in both clinical management and in prioritisation of the needs of their patients with dementia. We have presented the evidence for this elsewhere. 16
The response of primary care to the needs of people with dementia may be due, in large part, to the limited availability of support services but this does not mean that an educational intervention is unlikely to have an effect. Improved confidence in clinical knowledge and skills is still needed to allow practitioners to improve their performance with this patient group. It does mean that the effects of an educational intervention would probably be weak, unless other obstacles to earlier diagnosis and better management of dementia were reduced. Education alone does not usually change practice.
Fortuitously, three changes in UK NHS funding, in the law and in NHS priorities, created an environment conducive to changes in practice and thereby opening an opportunity to test an educational intervention in optimal circumstances. In 2006, the introduction of the Quality and Outcomes Framework (QOF), led to financial incentives for primary care to establish a dementia register and carry out annual reviews of patients. This was followed by the implementation of the Mental Capacity Act (MCA, 2005)17 in 2007, which clarified the law on the assessment of capacity to make decisions. The third was the launch of the National Dementia Strategy (NDS) in 2009,18 which prioritised improvements in the care of people with dementia, along the entire disease trajectory, and introduced new resources (such as ‘dementia advisors’ in some areas). GPs came under increasing pressure from primary care trusts (PCTs) to make diagnoses earlier, and to limit the prescription of antipsychotic drugs for behavioural and psychological symptoms of dementia (BPSD). They also began to experience rising demand for advice about memory loss symptoms, and to more frequently encounter situations in which they had to make difficult decisions about the best interests of people with dementia.
The timing for an educational intervention around dementia diagnosis and management in primary care could hardly have been better. Practitioners were under pressure to improve the quality of the care they gave, and were reimbursed for doing so, but knew that they lacked knowledge and skills and so were motivated to learn. 19
Components of the trial
The EVIDEM-ED trial began with literature reviews that scoped the area, followed by co-design of an educational intervention. This was field tested for feasibility and acceptability before being tested in a definitive cluster randomised trial.
Literature reviews
In order to inform the planned trial of an educational intervention designed to change clinical practice, we carried out two literature reviews. The first was a rapid appraisal of the published, English language literature on barriers to earlier diagnosis of dementia. The second was a review of interventions in primary care designed to alter clinical practice with patients with dementia.
Review 1: Investigating Barriers to Good Practice
A full account of this review appears elsewhere. 20
Method
Publications in English, up to August 2009, relating to barriers to the recognition of dementia were identified by a broad search strategy, using electronic databases MEDLINE, EMBASE and PsycINFO. Exclusion criteria included non-English language, studies about pharmacological interventions or screening instruments, and settings without primary care. Figure 1 shows the selection process for papers.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for EVIDEM-ED: Review 1, Investigating Barriers to Good Practice.
Results
Eleven empirical studies21–31 were found (see Appendix 1). The main themes from the qualitative studies were lack of support, time constraints, financial constraints, stigma, diagnostic uncertainty and disclosing the diagnosis. Quantitative studies yielded diverse results about knowledge, service support, time constraints and confidence. The factors identified in qualitative and quantitative studies were grouped into three categories: patient factors, GP factors and system characteristics. These are discussed in detail in the published review. 20
Conclusion
Much still needs to be done in service development and provision, GP training and education, and the eradication of stigma attached to dementia to improve the early detection and management of dementia. Implementation of dementia strategies should include attention to all three barriers, and further research should focus on their interaction.
Review 2: Changing Service Provision and Clinical Practice
We conducted a second review of potential solutions to the problem of underperformance in primary care. This second review aimed to identify and appraise empirical studies of interventions designed to improve the performance of primary care practitioners. A full account appears elsewhere. 32
Methods
A rapid appraisal approach was adopted to inform the implementation of the NDS in England,18 introduced in 2009. To avoid delays in the analysis and completion of the review, papers that were not easily accessible were not included. Publications about the detection and management of dementia in the community were searched for using MEDLINE, EMBASE and PsycINFO, without restricting the date or language of publication. Searches were carried out up to February 2010. A broad search strategy was adopted and search terms are listed in Box 1. This was executed as a two-step process with educat* added as the second step, after all of the other search terms. Bibliographies of articles discovered were also examined for additional relevant literature.
Dementia OR Cognitiv* Impair* OR Alzheimer’s Disease
AND
Primary Care OR General Practi* OR Family Pract*
AND
Diagnos* OR Manag*
AND
Educat*
This search resulted in a total of 4579 articles. To prevent narrowing of the search scope and therefore potentially reducing the search sensitivity, the terms were not refined, but instead each title was reviewed with its abstract if available to ascertain its relevance. A number of exclusion criteria were then applied. All studies of pharmacological interventions were excluded, as were studies of the performance of cognitive function tests. Publications were excluded if they reported on the diagnosis or treatment of dementia in anywhere but a primary care setting (for example, the benefits of respite in long-term care facilities); if they related to a population outside the scope of this review (for example, interventions for caregiver health); or if they were clinical discussions about dementia diagnoses or care. Letters were also excluded, as were publications in languages other than English. Two other relevant articles were found, one directly from bibliographic searching, and a second was sourced following a recommendation from an expert in the field. Figure 2 shows the search process, presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 33
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for EVIDEM-ED: Review 2, Changing Service Provision and General Practice.
After applying these criteria, 162 articles remained, but 70 of these were not readily obtainable and these were also excluded for pragmatic reasons; 77 of the remaining 92 papers were excluded after analysis because they were clinical reviews, guidelines, studies of barriers or studies without interventions that were designed to change practice. References in the 92 papers were reviewed to identify other intervention studies.
Data were extracted from each study report to allow comparison of interventions and to assess the quality of study designs. The characteristics chosen for comparison (including location and type of study, size, recruitment process, methodology, outcome measures, results and conclusions) and a detailed description of each intervention can be found in the published review. 32 Randomised trials were assessed for quality using the Physiotherapy Evidence Database (PEDro) scale. 34 The PEDro scale was chosen because its assessment of blinding is very relevant to empirical studies in dementia. In quality-rating scales that consider double-blinding as the central methodological issue, studies would lose points for failing to be blinded. The PEDro rating scale divides blinding into participants, therapists and assessors, recognising that although not all of the components of trials can be blinded, it is preferable that some groups are blinded rather than none at all. 35
Results
Fifteen studies were identified of which one used qualitative methods only and three were unpublished. Of the 11 studies29–31 included in the review, 10 were RCTs (see Appendix 1). Six21–24,27,30 reported educational interventions and five25,26,28,29,31 trialled service redesigns, either by changing the service pathway or by introducing case management. Educationally, only facilitated sessions and decision-support software improved GPs’ diagnosis of dementia, as did trials of service pathway modification. Some of the case management trials showed improved stakeholder satisfaction, decreased symptoms, and care that was more concordant with clinical guidelines.
Conclusion
The quality of the studies varied considerably. Education interventions are effective when learners are able to set their own educational agenda. Although modifying the service pathway and using case management can assist in several aspects of dementia care, these would require the provision of extra resources, and their value is yet to be tested in different health systems.
Aims and objectives of the EVIDEM-ED study
Using the insights from the two reviews, we designed an educational intervention for general practice, combining timely diagnosis and psychosocial support around the period of diagnosis in concordance with management guidelines. 36
The objectives of the EVIDEM-ED project were to:
-
develop an educational intervention suitable for the workplace use that has the potential to change management practice in dementia care among GPs and practice nurses
-
include shared care guidelines for medication use by patients with dementia, as part of management in general practice
-
develop and test electronic resources that promote the above objectives.
Developing the educational intervention
The theoretical basis of the intervention is described in full elsewhere. 16 In summary, the educational intervention was constructed using the following principles:
-
Diagnosis in primary care depends upon pattern recognition and the use of ‘illness scripts’ (more or less complex representations of diseases) in non-linear pathways to find the most probable explanations for presenting symptoms.
-
The first stage of diagnosing is not testing, but starting to suspect the possibility that a dementia syndrome may be emerging (the trigger phase). The doctor needs an index of suspicion to construct a diagnosis, which is often ‘triggered’ by a symptom within the patient’s story.
-
A specific problem with dementia is that all involved – patients, families and GPs – are reluctant to diagnose dementia, a serious and largely unmodifiable disease, which carries a huge burden of stigma.
-
The diagnostic task is complex, therefore, because the accumulating cognitive impairments are occurring within complex personalities, themselves embedded in social relationships.
-
Any educational intervention needs to be flexible enough to correct context-specific deficits. In other words, there is no ‘cook book’ or ‘one size fits all’ grand intervention that will alter clinical behaviour in primary care.
A workplace approach
In primary care settings, the most effective educational methods hinge on adult learning approaches – problem-solving, small group work, solution focus, ‘academic detailing’ (on site rather than off site) – that permit flexibility in learning and allow adaptation of guidelines to local circumstances.
A co-design approach to the production of an Educational Needs Assessment (ENA) tool was adopted in order to gain the insights and experiences of a range of practitioners. 37 This involved an expert group of designers and an expert panel of ‘critical friends’ working in an iterative technology development process38 to develop a prototype ENA tool for dementia diagnosis and management. The prototype was refined and subsequently ‘field tested’ with volunteer practices. A full description of this process can be found elsewhere. 39
The expert group
The multidisciplinary expert group was made up of three GPs, an old age psychiatrist, a carer and a psychologist, and was attended by three members of the research team. Members of the expert group were chosen on the basis of their expertise or experience in dementia care, or in professional education, or both. The aim of the expert group was to decide which skills and attributes were essential for a primary care team to possess in order to deliver effective care for patients with dementia.
The expert group was given three objectives: to (1) design a care pathway that would assist practitioners in earlier diagnosis and enhance subsequent clinical management (the care pathway); (2) identify the attributes a practice would need to implement the care pathway (the task matrix); and (3) use the task matrix to derive a set of questions that would identify the practice’s learning needs (the ENA).
The expert group was also asked to frame its work in terms of adult learning approaches, in other words, that learning would be problem-solving, case based and usable by practitioners at different points on the spectrum from novice to expert. 40,41
A modified nominal group technique was used with the expert group to develop the prototype ENA tool. Nominal groups are potentially powerful learning and development tools. 42 They have a particularly useful role in analysing health-care problems43 and can help bridge the gap between researchers and practitioners. 44
The expert group met initially to decide what the elements of a training programme designed to change practice in dementia should be. This meant identifying what tasks were needed to be performed in order to identify patients with dementia, and to care for them appropriately in the primary care setting. The expert group designed a flow chart and identified elements of the diagnostic process outlining the pathway that a clinician might follow once they suspect that a patient has dementia (see Appendix 3, Figure 29). In addition, the elements of a training programme for primary care teams were identified (see Appendix 4). From this, the expert group developed questions that would identify the practice’s strengths and weaknesses in the care of people with dementia.
This cyclical process of adapting and refining ideas took place over one calendar year, and necessitated four meetings of the expert group. The prototype ENA was sent to the expert panel after the fourth meeting of the expert group.
The expert panel
The expert panel comprised a group of external, independent people who had registered their interest in the EVIDEM project by subscribing to a mailing list on the website. The expert panel had 13 members (one of whom dropped out in the course of the development process), with a mix of carers, patients, and professionals, including two GPs, a social worker, a practice nurse and an Admiral Nurse (a community nurse specialising in support of people with dementia and carers). Seven panel members were carers of people with dementia.
Expert panel members were blinded to each other as well as to the expert group members. They received and sent comments on all the documents by e-mail or post. The purpose of the panel was to review the proposals made by the expert group to ensure that no themes were omitted and to decide how comprehensive, valid and feasible the ENA was as a tool. They were also charged with assessing if the development of the prototype concurred with known factors favouring the adoption of an innovation, using literature that had been circulated to them in advance. When this had been completed the expert panel returned their comments and suggestions to the expert group for review.
Field-testing the Educational Needs Assessment tool
After incorporation of the feedback from the expert panel, a prototype version of the needs assessment tool suitable for field testing was prepared. This is shown in Table 1.
| Question | What the answers tell us | What we do |
|---|---|---|
| 1. How would you rate your current care for people with dementia and their carers (using a simple scale of good enough/satisfactory/needs substantial improvement)? | Answers will indicate whether focused educational input is needed or broader input (this is a very subjective assessment – the practice may be better or worse than it thinks) | Gives the research team some sense of scale of need and time commitment, and may permit preliminary selection of learning materials and resources |
| 2. What grounds or criteria is your rating based on? | Identifies more clearly the areas of strength and weakness, from practice perspective. e.g. Is the major problem with diagnosis, or disclosure of the diagnosis, or judging impairment, or knowing what the appropriate responses and resources are? | Sense of priorities for learning will begin to emerge here |
| 3. Does the number of people in your practice diagnosed with dementia correlate with the local prevalence figures? | Reflects (1) local demography and (2) under-recognition | GPs tend to overestimate prevalence and likely future workload, so some reframing possible (we need epidemiological data) |
| 4. How do you arrive at your decision for diagnosis of dementia? | Tells us about the diagnostic procedure followed in the practice. It will also inform us on who makes the diagnosis | Helps identify roles within the practice team. Skill mix and experiences within the group can then be shared between colleagues with the opportunity for peer-to-peer learning |
| 5. How many older people with suspected dementia did you refer last year? | Reveals the practice culture (transfer of responsibility to specialist services vs. GP care) | We will know if we need to increase their capacity to provide GP care or simply reinforce existing good practice |
| 6. After diagnosis, what follow-up do you provide to people with dementia and their carers? | Opens up discussion about (1) systematisation of care within the practice and (2) resources available to the practice | (1) Case management methods (2) Local (and national) directory of resources |
| 7. Are you using a shared care protocol for cholinesterase inhibitors? If ‘yes’ then (1) who was involved in producing the protocol, and (2) who is involved in its implementation (e.g. hospital consultants, community psychiatric nurses, Care of Older People team)? | Awareness of protocol (if it exists), and its appropriateness for general practice | Rehearse use of (GP developed) shared care protocol |
| 8. How effective do you think cholinesterase inhibitors are and how effective have you found them in your practice? | Awareness of realistic likely impact of cholinesterase inhibitors | Discussion of trial data on cholinergic drug effects |
| 9. What non-pharmacological alternatives do you have available to help your patients (and their carers)? | Will indicate extent of networking with local services and identify practice resources usable by people with dementia | Provision of information about cognitive reframing, other psychosocial support methods |
| 10. Based on your experience, what do you think are the important quality markers in caring for people with dementia? (What would you want for yourself?) | Elicits both clinical and personal experience; may provide very useful case vignettes | Fit the practice’s conception of quality markers to the NICE/SCIE guideline indicators36 |
| 11. Is there anything that you would like improve? If yes, what is it and why would you like it to change? | Prioritisation of learning needs | Highly focused educational input |
This ENA template was then field tested in volunteer general practices. The practices were based in north west and north east London, and were recruited directly by the EVIDEM programme as part of a RCT of an educational intervention. Practices were informed about the process and asked to choose either field-test status or RCT participation. The first five respondents wanting to join the field test were enrolled.
To carry out an ENA, two members of the research team held a group meeting at each practice. A team member experienced in group-based learning in general practice (the expert tutor), facilitated discussion of the needs assessment tool. The other acted as a participant observer, ensuring that all questions were asked and clarifying points where necessary, as well as taking notes about the assessment process. The practices were asked to invite whichever members of staff they thought should participate, including attached staff from community services. The times of meetings were left to the practice to choose. The research project paid for lunch when lunchtime meetings were preferred.
An educational prescription was agreed at the end of each needs assessment process, and up to three follow-up visits were arranged to work through the themes identified for the educational prescription. The educational prescriptions were used to collect and collate learning materials for each practice, and the subsequent workshops were also used to revisit and revise the questions in the ENA. The research team assembled material in written and electronic form for each of the items on the educational prescription workshops led by the expert tutor who had facilitated the ENA process, again with a participant observer from the research team.
Table 2 shows the characteristics of the practices in the field test, the participation of different disciplines, the number of workshops held with each practice, and the themes identified in their ‘educational prescription’.
| Practice | List size | Non-clinical staff present? | Community staff present? | Diagnostic methods | Shared care protocol | BPSD management | Carers needs and quality markers | MCA 200517 and legal issues | Complex case discussion | Improved service awareness and collaboration | Care planning | No. of workshops |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Inner city, GP led | 6300 | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | – | 3 |
| 2. Outer city, GP led | 9100 | – | – | ✓ | – | – | ✓ | ✓ | ✓ | ✓ | – | 2 |
| 3. Outer city, nurse led | 6000 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | 3 |
| 4. Outer city, GP-led training practice | 5200 | – | – | ✓ | – | ✓ | ✓ | ✓ | – | – | ✓ | 2 |
| 5. Inner city, GP led | 10,500 | – | – | ✓ | ✓ | – | ✓ | – | – | ✓ | ✓ | 3 |
As a result of the field testing, the variety of learning materials used was broadened to include more reference material for use during sessions. The timing planned for topics was also amended and the expert tutors became more knowledgeable and aware of areas of need that were consistent across individuals and groups. The practices involved in the field test made it clear that they did not want decision-support software embedded in their electronic medical record systems but instead preferred simple, easily accessible guidance in an electronic format that functioned as ‘look-up’ documents.
The educational prescriptions developed through the ENA appeared acceptable and useful in volunteer practices. The time commitment (no more than 4 hours, spread out at the practice’s discretion) appeared manageable. The pilot group of practices prioritised diagnosis, assessment of carers’ needs, quality markers for dementia care in general practice, and the implications of the MCA 200517 for clinical practice. The content of the ENA seemed to be comprehensive, in that no new topics were identified by practices in the field trial. On the basis of this pilot, the ENA tool was used in the full trial.
Developing a learner’s manual
The themes identified by practitioners in the field test were researched by EVIDEM staff and a learner’s manual was constructed (available via www.EVIDEM.org.uk). This manual was designed in a modular form with Microsoft Word version 97–2004 (Microsoft Corporation, Redmond, WA, USA) documents that could be accessed during consultations if necessary. The content of the learner’s manual was developed using the expertise of all members of the EVIDEM team. The manual contains modules on the following:
-
dementia diagnosis and cognitive assessment tools
-
differential diagnosis of dementia by illness and types
-
disclosure of dementia diagnosis and cultural diversity
-
use of cholinesterase inhibitors, NICE/Social Care Institute for Excellence (SCIE) guidelines and shared care of medication
-
management of BPSD and Department of Health (DH) guidelines for using antipsychotic drugs
-
non-pharmacological interventions for dementia
-
medication review and dementia
-
needs of people with dementia and primary care
-
case studies for management of people with dementia
-
carer needs and support in general practice
-
end-of-life care
-
holistic care in dementia – personhood approach
-
MCA, advance directives and Lasting Power of Attorney (LPA)
-
Independent Mental Capacity Advocates (IMCAs) and Court of Protection
-
financial and legal guidance: where to go for help
-
dementia and driving.
Implementing the EVIDEM-ED trial
Trial design
The effectiveness of an educational intervention combining practice-based workshops and computer-based support was tested in a pragmatic, unblinded, cluster RCT, with a pre and post design, and with two arms: usual care compared with educational intervention [see CONSORT (Consolidated Standards of Reporting Trials) diagram in Figure 3]. The researchers were aware of group allocation but carers and people with dementia were not informed. A full description of the trial design – including management structure, participant involvement and patient and public involvement (PPI) – is available in the published protocol. 45
FIGURE 3.
EVIDEM-ED: CONSORT flow diagram.
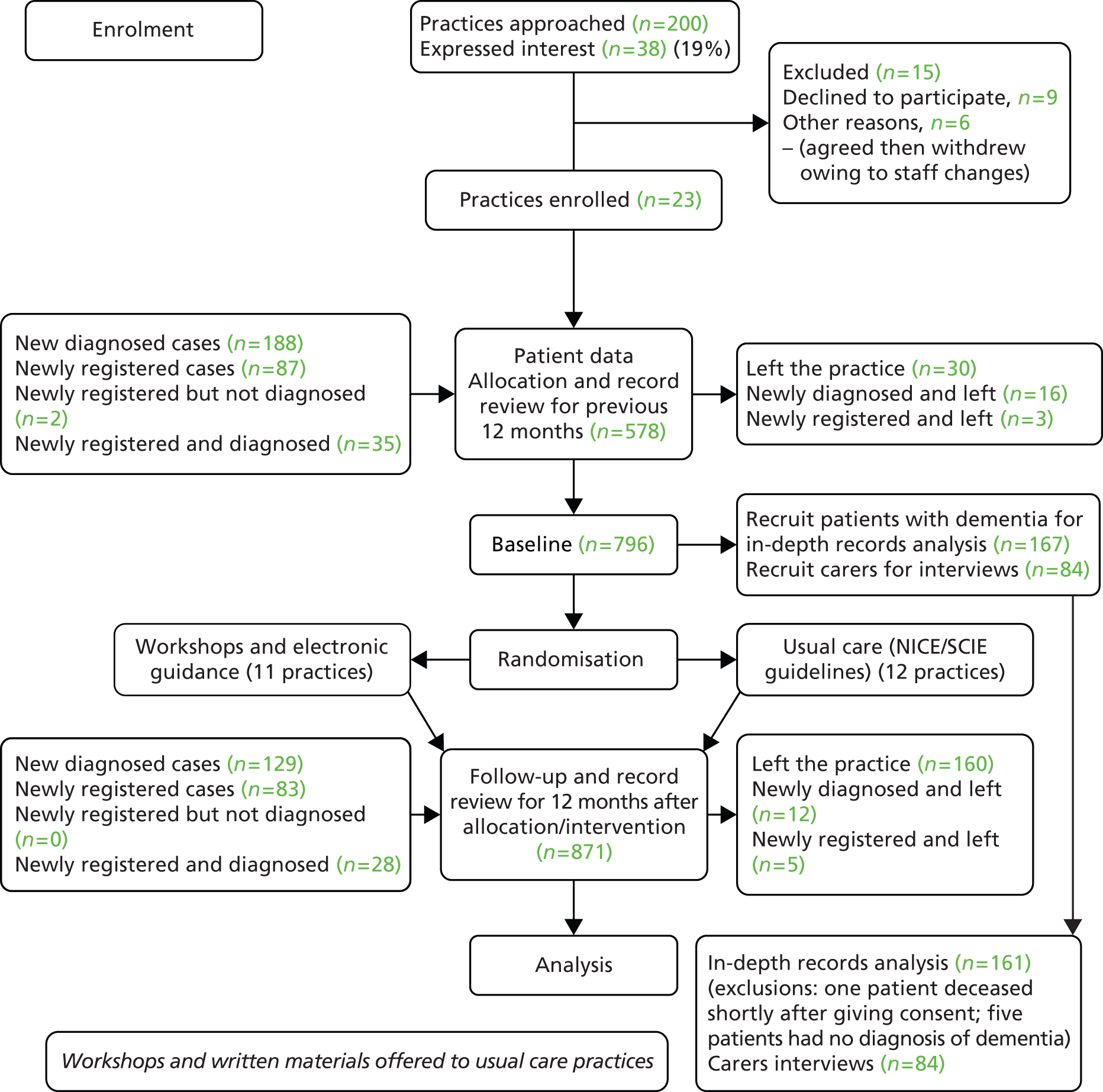
Standard significance tests assume that random sampling has taken place, and that the behaviour or knowledge of any one individual is not affected by others in the sample. However, this study, like many in the health service field, is based on clusters of individuals who may influence each other – in this case colleagues in a practice who share information or similar views, or patients who receive similar treatment. 46 This ‘clustering effect’ can mean that false conclusions are drawn about relationships in the data. This effect was controlled for by using cluster membership (i.e. respondents in each practice).
Study setting
The study took place within primary care practices in the geographical area covered by the North Thames (NT) Dementias and Neurodegenerative Diseases Research Network (DeNDRoN) – Metropolitan North London, Essex, Hertfordshire and Bedfordshire. Approval for the trial was received from Southampton & South West Hampshire Research Ethics Committee (REC) (A): reference 09/H0502/77.
The educational intervention
The educational intervention consisted of practice-based workshops with a tailored curriculum designed by a multidisciplinary expert group and supplemented by electronic resources. The educational interventions reflected different approaches to adult learning, namely workshops directly relevant to clinical practice, allowing learning to occur through peer reflection about real cases, and electronic resource materials suitable for ‘real-time’ use in consultations.
Recruitment
Primary care practices
Interested general practices in the North Thames DeNDRoN were identified in collaboration with the local Primary Care Research Networks (PCRNs): the Primary Care Research Network-Greater London (PCRN-GL) and the Primary Care Research Network-East of England (PCRN-EoE). Practices were contacted by the trial research team, by letter and awareness raising through general practice educational meetings, and regular newsletters.
Inclusion criteria
The inclusion criteria for practices in this study were (1) routine data collection from clinical encounters on electronic medical records and (2) team commitment to participate in educational workshops held in the practice. (All staff working in the practice were eligible to participate in the study.)
Exclusion criteria
Practices that did not routinely capture clinical data in electronic records were excluded.
Patients with dementia and their carers
An in-depth study of medical care for patients with dementia was carried out in a subset of the practice populations. Patients identified by the practice were invited by their usual GP to permit analysis of their medical records, and their carers (where known) were invited to participate in face-to-face interviews, with telephone follow-up 12 months later.
Inclusion criteria
Being on the QOF register with a Read Code for dementia of any type documented in their medical records, with no lower age limit.
Exclusion criteria
Exclusion criteria were (1) participants who were unable to speak English, for whom there was no available interpreter; (2) either the patient or carer was involved in concurrent research; (3) the key clinical professional working with the patient felt that an approach to the person with dementia or their carer would be inappropriate or may increase distress; and (4) any other important reason that the lead clinical professional might have about why the person with dementia or their carer should not be contacted.
Every effort was made to include those who met the inclusion criteria and who might adequately understand verbal explanations or written information given in English.
Outcome measurements
Primary outcome, measured in the audit sample
We derived our primary outcome measure and the effect size from discussions with practitioners in the feasibility phase of the trial. The consensus was that the clinical tasks involved in providing good-quality care required at least two encounters per year, and that the educational intervention would promote this effectively in the majority of those in the intervention arm. Our hypothesis was, therefore, that the proportion of patients receiving two dementia-specific management reviews per year would increase between the intervention and control groups of patients by 50%, that is, 20% (usual care) compared with 70% (intervention), after the introduction of the educational intervention. Data relating to dementia-specific patient reviews and consultations relevant to dementia management were extracted from the practice records, first by electronic searches and then by hand-searching by research clinicians. Read-Coded dementia reviews were counted for each patient with dementia in the 12 months prior to intervention/randomisation and the 12 months following intervention/randomisation. Using the same time periods, all face-to-face consultations in which any aspect of dementia was documented as Read Code or free text were counted as ‘opportunistic reviews’, regardless of the problem triggering the consultation.
Sample size
Based on the study having 90% power to detect as significant, using a 5% two-tailed significance level, a 50% difference in the proportion of individuals with two or more dementia-related GP visits (usual care 20% vs. intervention 70%), the required sample size, based on individual randomisation, would be 23 per group – a total of 46 individuals. However, owing to the use of cluster randomisation, the total required sample size needed to be inflated in order to take account of this clustering. The number of patients recruited per practice was also inflated in order to maintain the sample sizes in the presence of attrition. 47,48 With 20 practices (10 per arm), the power to detect the differences postulated would be maintained if the intraclass correlation coefficient were of the order ≤ 0.37. Thus the effective sample size with 10 patients per cluster and 20 practices needed to be 200. If the expected attrition rate as 1/3 (33.3%) then 15 patients would need to be recruited per practice in order to maintain the sample size of 10 per practice.
Secondary outcomes, measured in the subset of patients with dementia who permitted review of their medical records
Concordance with guidelines
Documented concordance with the best practice recommendations from the intervention were assessed by the researchers examining the medical records of the subset of patients who had agreed to participate in the in-depth study. A medical records data extraction pro forma based on the intervention and best practice guidelines was developed (see Appendix 7).
Medical records at each practice were reviewed and analysed by independent clinical researchers, and diagnostic and management actions were coded using the pro forma, noting whether or not the action was taken in primary care.
Concordance with guidelines for diagnosis was operationalised by first identifying the index consultation. This was taken as the first consultation, communication or other entry in the patient record which indicated that dementia was being considered as a possible diagnosis – that is, by the use of the Read Code and/or the recording of symptoms of dementia. Examples of symptoms included were short-term memory loss, confusion, wandering, personality change and depression. The content of all consultations in the 6 months prior to the index consultation were reviewed. A count of specific actions recorded as part of the index consultation and/or in the period leading to formal diagnosis was then made. The actions were informed by the NICE/SCIE dementia guidelines36 and agreed by the Project Management Group (see Appendix 2) as reflecting the working definition of good practice in the diagnosis of dementia. They were as follows:
Diagnosis in primary care
-
Request for blood tests.
-
Cognitive testing completed.
-
Informant history taken.
-
Referral to consultant, nursing or secondary care.
-
Depression and/or psychosis considered.
-
Carer concerns recorded.
-
Behavioural and psychological symptoms related to dementia recorded (apart from depression, elsewhere classified).
Information was given by the practice to either the carer or patient or both, on:
-
signs and symptoms
-
course and prognosis
-
treatments
-
local care and support services
-
support groups
-
sources of financial and legal advice and advocacy
-
medicolegal issues
-
local information sources, including libraries and voluntary organisations.
The following actions were coded at time of index or up to formal diagnosis:
-
anti-dementia medication prescribed
-
medication review undertaken
-
computed tomography (CT)/magnetic resonance imaging (MRI) scan requested.
Management in primary care
-
Had antipsychotic medication been prescribed?
-
Evidence of:
-
consent and capacity; evidence that the provisions of the MCA 2005 have been followed and evidence of recall, reasoning, decision-making, and, if relevant, agreement from next of kin, carers or other consultees?
-
care plan
-
BPSD addressed and managed
-
advance statements
-
living will (advance decision to refuse treatment)
-
LPA
-
preferred priorities
-
direct payments/personal budgets, functional abilities, activities of daily living (ADL) or the use of global assessments
-
discussion about carers’ needs.
-
Information was given by the practice to either the carer or patient or both, on:
-
local care and support services
-
sources of financial and legal advice and advocacy
-
medicolegal issues.
No attempt was made to measure the number of times a particular action – for example, record of contact with carers – was recorded, but only whether or not such contact was recorded in the pre-intervention and/or post-intervention periods by a member of the practice.
Measurement of unmet needs in patients and carers
Unmet needs were captured using a structured interview schedule with carers, developed by the research team in a previous trial. 21
Consent
People with dementia were identified by practice managers and their lead clinician checked whether or not they fulfilled the inclusion criteria. Before seeking consent from patients to participate in the study, practitioners were asked for their opinion about the capacity of the person with dementia to give informed consent to taking part in the study, using the MCA 200517 as the framework for their judgement.
For patients judged as having decision-making capacity, the following information was posted to the person with dementia: (1) a covering letter explaining the involvement of the practice and signed by the lead clinician; (2) a participant information sheet; and (3) a response letter and prepaid envelope to be returned to the research team. A researcher arranged to see those patients with dementia and their carers who expressed an interest in participating in the study. This encounter took place at the preference of the carer, at the patient’s home, in the GP practice or at the research team’s offices at the Royal Free Hospital. Its purpose was to seek consent from the carer and consent/assent from the person with dementia, where possible, for their participation in the study and for allowing access to patient records.
For those judged by the lead clinician to lack capacity to give informed consent, a consultee (as defined in the MCA 200517) was identified and consulted about possible involvement of the person with dementia in the trial. The relevant clinician wrote to the consultee, providing full information about the trial.
Figure 4 shows the process of identifying participants and how they were approached, recruited and consented/consulted about EVIDEM-ED.
FIGURE 4.
Flow chart of how participants were identified, approached, recruited and consented/consulted about EVIDEM-ED (2009–10).

Randomisation and masking
Participating practices were randomised to intervention or usual care arms by an independent researcher using a computer randomisation programme. 49 Independent clinicians undertaking record reviews could have deduced the allocation of the practices and could have become unblinded.
The process for obtaining participant informed consent was in accordance with the REC guidance, the MCA 200517 and good clinical practice (GCP).
With the informed consent of the person with dementia or following discussion with their consultee, members of the research team examined medical records in the GP surgery, using the pro forma to guide data extraction. No personalised information was recorded or retained by the researcher, and each case was allocated a unique study number for the purposes of recording data. All other information was stored in accordance with the Data Protection Act (1998). 50
Statistical analyses
Primary outcome: number of reviews
Multilevel logistic regression modelling was used to compare the proportion of patients who had two or more reviews during the 12 months post intervention/randomisation (post period) between the usual care and intervention arms. Two-level random-effects models were fitted in order to take account of the cluster randomisation. The models adjusted for the proportion of patients within each practice with two or more reviews during the 12 months pre intervention/randomisation (pre period). Separate analyses of the dementia related reviews, opportunistic reviews and the total reviews were undertaken.
As sensitivity analyses, the above were repeated only for those patients with information available for the full 12-month pre and post periods.
Diagnoses
Multilevel Poisson regression modelling was used to compare the diagnoses rates, in those aged ≥ 65 years, between the intervention and usual care arms in the post period. The number of newly diagnosed patients was used as the outcome with the practice list size of those aged ≥ 65 years used as the exposure. Two-level random-effects models were fitted in order to take account of the cluster randomisation. The number of diagnoses during the pre period was adjusted for in the model.
Multilevel logistic regression modelling was undertaken to compare concordance with management guidelines in the post period between the intervention and usual care arms using two-level random-effects models. Models were adjusted for the concordance levels in the pre period.
Findings
Of 200 practices we approached, 23 agreed to participate and provided the required level of access and data. The practices came from 12 different primary care organisations in urban, semirural and rural areas. Practice enrolment was from 2008 to 2010. Practices received a mean of three workshops, including the needs assessment workshop at the beginning of the trial (range 2–4). These were staggered across the practices and took place from 2009 to 2011.
Practice information
Eleven practices were randomised to the intervention arm and 12 to the usual care arm. The number of patients with dementia per practice ranged from 5 to 123 in the intervention arm and from 6 to 108 in the usual care arm. The characteristics of the practices by arm of study are shown in Table 3. There was a slightly higher mean list size, a slightly higher mean deprivation score, and a slightly higher mean number of GPs in the intervention practices.
| Variable | Summary measure | Usual care practices (n = 12) | Intervention practices (n = 11) |
|---|---|---|---|
| No. of GPs | Mean (SD) | 4.6 (2.7) | 5.1 (2.1) |
| Median | 5.5 | 5 | |
| Min. | 1 | 2 | |
| Max. | 9 | 9 | |
| List size | Mean (SD) | 7892 (4684) | 8382 (4711) |
| Median | 9239 | 6849 | |
| Min. | 1133 | 2682 | |
| Max. | 14,358 | 19,323 | |
| Deprivation scorea | Mean (SD) | 19.9 (10.0) | 20.4 (7.6) |
| Median | 17.5 | 22.0 | |
| Min. | 7 | 8 | |
| Max. | 40 | 29 | |
| Care homes | No. of practices with patients residing in care homes | 9 | 9 |
| Min. per practice | 0 | 0 | |
| Max. per practice | 6 | 15 | |
| Total no. homes per group | 17 | 30 |
Patient information
A total of 1072 (intervention 512, usual care 560) patients had information available in their medical records at audit showing the number of reviews (dementia/opportunistic/total) in the 12 months (or a proportion of) before intervention or randomisation and/or the 12 months (or a proportion of) after. Of those, 61% (n = 313) were female in the intervention group and 70% (n = 382) were female in the usual care group. The mean age for those people with dementia in the intervention group was 83 standard deviation (SD) 8.7; minimum (min.) 33; maximum (max.) 104 years and 83 (SD 8.8; min. 55; max. 109) years for those in the usual care group.
Dementia management reviews
The mean total number of dementia management reviews (planned and opportunistic) for people with dementia in the intervention group in the period before randomisation was 0.89 (SD 0.92; min. 0; median 1; max. 4). For the period after randomisation it was 0.89 (SD 1.09; min. 0; median 1; max. 8).
For those people with dementia in the usual care group prior to the intervention, the mean total number of dementia management reviews (planned and opportunistic) was 1.66 (SD 1.87; min. 0; median 1; max. 12). For the period after intervention it was 1.56 (SD 1.79; min. 0; median 1; max. 11). This difference at baseline appears to have been due to one practice in the usual care group having medical responsibility for several care homes.
Primary outcomes
The numbers of each type of review for each patient in the pre and post periods were dichotomised for the primary analyses. These were classified according to whether the individual had < 2 or ≥ 2 dementia management reviews. A summary can be seen in Table 4.
| Variable | Usual care practice patients (%) | Intervention practice patients (%) |
|---|---|---|
| Dementia (pre) | 15.1 | 4.9 |
| Dementia (post) | 9.6 | 6.1 |
| Opportunistic (pre) | 21.3 | 6.6 |
| Opportunistic (post) | 21.4 | 8.3 |
| Total (pre) | 39.0 | 18.2 |
| Total (post) | 35.9 | 19.8 |
Estimated ORs (odds of having two or more reviews in the intervention group vs. the usual care group), along with the p-values and 95% CIs for those with a full and partial data period are presented in Table 5.
| Reviews (≥ 2 vs. < 2) | OR | 95% CI | p-value |
|---|---|---|---|
| For all cases including proportion of data collection period pre and/or post | |||
| Dementia | 0.94 | 0.33 to 2.62 | 0.899 |
| Opportunistic | 0.96 | 0.53 to 1.74 | 0.890 |
| Total | 1.05 | 0.72 to 1.53 | 0.811 |
| For full pre and post data period | |||
| Dementia | 0.83 | 0.32 to 2.10 | 0.688 |
| Opportunistic | 0.62 | 0.25 to 1.56 | 0.310 |
| Total | 0.83 | 0.52 to 1.33 | 0.444 |
There were no significant differences in recording of dementia management reviews for patients diagnosed by the current practice between the usual care and intervention arms.
Case detection
In order to get a realistic estimate of case identification that was comparable with studies published elsewhere we analysed rates of diagnoses by age group.
Pre period: in the pre-intervention/randomisation 12-month period, there were 239 newly diagnosed cases of dementia. Eleven of these (4.6%) were in people aged < 65 years. Of the 228 newly diagnosed cases in those aged ≥ 65 years, 99 were in the usual care practices and 129 in the intervention practices.
Post period: in the post-intervention/randomisation 12-month period there was a total of 169 newly diagnosed cases of dementia. Six of these (3.7%) were in people aged < 65 years. Of the 163 newly diagnosed cases in those aged ≥ 65 years, 78 were in the usual care practices and 85 in the intervention practices.
The primary analysis considered diagnosis rates in those registered with the practice list and this was used as the denominator for the calculation of rates. For the usual care practices combined the total patient population was 15,699 compared with 11,541 for the intervention practices.
Table 6 shows the case detection rates in the pre-intervention/randomisation and the post-intervention/randomisation periods. These are displayed separately for the intervention practices (combined) and the usual care practices (combined). The rates by practice were also calculated and the min. and max. rates, across the practices, are also displayed in Table 6.
| Rates | Usual care practices (%) | Intervention practices (%) | |
|---|---|---|---|
| Pre period | Combined | 0.63 | 1.12 |
| Min. | 0.00 | 0.17 | |
| Max. | 4.40 | 3.45 | |
| Post period | Combined | 0.50 | 0.74 |
| Min. | 0.00 | 0.00 | |
| Max. | 4.10 | 1.06 | |
Case detection rates were unaffected by the intervention. The estimated IRR for the intervention compared with the usual care group from the multilevel Poisson regression modelling was 1.03; the p-value was 0.927 (95% CI 0.57 to 1.86).
Secondary outcome analysis
An in-depth study of the medical records of 167 people with dementia was carried out to capture the detail of clinical management before and after diagnosis. Nineteen of the 23 practices in the trial invited patients with dementia and/or their carers to permit analysis of medical records; four practices were unable to send invitations at baseline, for organisational reasons. The total number of people invited was 763, of whom 190 expressed an interest (25%) and, after further discussion with researchers, 167 (22%) allowed review of medical records. The medical records of some of the 167 participants were incomplete because they had joined their practice after the index consultation, or after diagnosis, and the previous records had not reached their new practice. One hundred and sixty-one had documentary evidence of a formal diagnosis, and 136 had documentary evidence of the index consultation and a formal diagnosis. Figure 5 shows the characteristics of the secondary outcome sample.
FIGURE 5.
EVIDEM-ED: patient medical record data characteristics.

Symptoms were recorded at index consultation for 101 patients, and, of those, 39% had one or two symptoms, 45% had three or four symptoms, and 17% had five or more symptoms recorded.
Comorbidity and repeat medication use
Forty-two members of the subset (25.3%) had 0–3 comorbidities recorded, 64 (38.6%) had 4–6 comorbidities and 60 (36.1%) had 7 or more comorbidities. Data on comorbidities were missing for one member of the subset. Six members of the subset (3.6%) had no repeat medications documented in the medical record, 59 (35.5%) had 1–5 medications, 82 (49.4%) had 6–10 medications and 19 (11.4%) had more than 10 medications. Data on repeat prescription of medication were missing for one member of the subset.
Table 7 shows the characteristics of dementia type by randomisation group.
| Characteristics | Randomisation group | ||||
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Age, years | Mean | 82 | 81 | ||
| SD | 8 | 8 | |||
| Min. | 57 | 60 | |||
| Max. | 102 | 97 | |||
| n | 97 | 64 | |||
| n | (%) | n | (%) | ||
| Gender | Male | 29 | 29.9 | 29 | 45.3 |
| Female | 68 | 70.1 | 35 | 54.7 | |
| Total | 97 | 100.0 | 64 | 100.0 | |
| Location | Community | 55 | 59.8 | 40 | 62.5 |
| Care home | 37 | 40.2 | 24 | 37.5 | |
| Total | 92 | 100.0 | 64 | 100.0 | |
| Diagnosis | Senile dementia/dementia | 28 | 28.9 | 12 | 18.8 |
| Alzheimer’s disease | 41 | 42.3 | 21 | 32.8 | |
| Vascular dementia | 13 | 13.4 | 17 | 26.6 | |
| Dementia with Lewy bodies | 0 | 0 | 3 | 4.7 | |
| Mixed dementia | 8 | 8.2 | 4 | 6.3 | |
| Mild cognitive impairment | 3 | 3.1 | 3 | 4.7 | |
| Other | 4 | 4.1 | 4 | 6.3 | |
| Total | 97 | 100.0 | 64 | 100.0 | |
Concordance with diagnostic and management guidelines
Table 8 shows the concordance of medical records with guidelines about the diagnostic process in the 136 people with dementia for whom we had an index consultation and a formal diagnosis. Table 9 shows the concordance of medical records with guidelines about management after diagnosis in the same number of cases.
| Diagnostic actions within primary care concordant with guidelines (N = 136) | n (%) |
|---|---|
| Request for blood tests documented (full or partial screen according to NICE guidelines) | 76 (56) |
| Results of cognitive testing | 53 (39) |
| Informant history documented | 78 (57) |
| Referral to specialists at index consultation | 87 (64) |
| Depression or psychosis considered | 52 (38) |
| Carer concerns recorded | 74 (54) |
| BPSD considered, apart from depression | 42 (31) |
| Documentation of information given to carer or patient or both on: | |
| Signs and symptoms | 5 (4) |
| Course and prognosis | 0 (0) |
| Treatment option | 18 (13) |
| Care and support available | 20 (15) |
| Support groups available | 7 (5) |
| Financial, legal and advocacy advice | 11 (8) |
| Medicolegal matters | 12 (9) |
| Local services (e.g. libraries, voluntary organisations) | 4 (3) |
| Management actions (taken after formal diagnosis) | n (%) |
|---|---|
| Anti-dementia drugs prescribed | 71 (52) |
| Medication review | 56 (41) |
| Referred for CT/MRI scan | 7 (5) |
| Prescribed antipsychotic drugs | 26 (19) |
| Consent and capacity; evidence that the provisions of the MCA have been followed and evidence of recall, reasoning, decision-making and, if relevant: | 33 (24) |
| Agreement from next of kin? | 15 (11) |
| Care plan made | 70 (52) |
| BPSD addressed and managed | 101 (74) |
| Discussion of advance statements recorded | 4 (3) |
| Discussion of living will (advance decision to refuse treatment) recorded | 1 (1) |
| Discussion of LPA recorded | 12 (9) |
| Preferred priorities for care (e.g. DNAR order) | 12 (9) |
| Discussion of direct payments/personal budgets recorded | 4 (3) |
| Functional abilities, ADL or global assessments documented | 74 (54) |
| Discussion about carers needs | 45 (33) |
| Evidence that information was given by the practice to either the carer or patient or both, on: | |
| Local care and support services | 5 (4) |
| Sources of financial and legal advice and advocacy | 6 (4) |
| Medicolegal issues | 16 (12) |
| Local information sources, including libraries and voluntary organisations | 3 (2) |
Two-thirds of patients were referred for specialist assessment at the index consultation. When referral to a specialist was documented at the index consultation, 58 (69%) were made to old age psychiatrists, 14 (17%) to memory clinics, four to neurologists (5%) and five (6%) to geriatricians. NICE guidelines for diagnostic work-up were adhered to in the majority of patients, with documentation of the blood screen, informant history and carer concerns, but not for cognitive function testing. Among those with cognitive test results documented, 30 (58%) were for the Mini Mental State Examination (MMSE),52 two (4%) were for the Six Item Cognitive Impairment Test (6CIT) and 19 (37%) were for the Abbreviated Mental Test Score (AMTS).
A locum reading the medical records of patients in the subset for the time period between index consultation and formal diagnosis would only occasionally learn what information had been given about signs and symptoms of dementia, the disease course and prognosis, the resources available locally that could provide support, and the relevant medicolegal concerns.
The number receiving cholinesterase inhibitors or memantine (Ebixa®, Lundbeck) (n = 71) was similar to the number with a formal diagnosis of Alzheimer’s disease or mixed dementia (n = 74). Among those receiving anti-dementia medication, 56 (82%) received donepezil (Aricept®, Eisai & Pfizer), 7 (10%) received galantamine (Reminyl®, Janssen), 2 (3%) received rivastigmine (Excelon®, Novartis) and one received memantine. These medications were offered but declined by two patients.
One in five were prescribed antipsychotic medication, of which the most commonly used was quetiapine (Seroquel®, AstraZeneca) (n = 13, 52%), followed by amisulperide (patent expired 2008, multiple manufacturers/trade names) (n = 4, 16%), haloperidol (Haldol®, Johnson & Johnson) (n = 2, 8%) and risperidone (Risperdol®, Johnson & Johnson) (n = 2, 8%); six individuals received aripipprazole (Aripiprex®, Otsuka and Bristol-Myers Squibb) or olanzapine (Zyprexa®, Eli Lilly and Company).
One in four records showed that mental capacity had been assessed. The majority of records showed evidence of care planning, assessment of, and response to, BPSD and functional assessment.
A locum encountering these patients and reading their medical records would not know whether they had considered or made any decisions about advanced statements, ‘living wills’, Power of Attorney (POA), preferred priorities of care, use of direct payments or personal budgets, sources of support and medicolegal concerns, such as driving.
Concordance with diagnostic guidelines at baseline differed between those patients from practices subsequently allocated to intervention or usual care groups, as shown in Table 10.
| Diagnostic action | Randomisation group | |||||
|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||
| n | % | Total | n | % | Total | |
| Dementia blood screen | 47 | 81.0 | 58 | 29 | 69.0 | 42 |
| Cognitive function test | 34 | 58.6 | 58 | 17 | 39.5 | 43 |
| Informant history considered | 45 | 78.9 | 57 | 32 | 71.1 | 45 |
| Referral made | 50 | 87.7 | 57 | 37 | 84.1 | 44 |
| Depression/psychosis considered | 30 | 52.6 | 57 | 22 | 51.2 | 43 |
| Carer concerns | 47 | 81.0 | 58 | 26 | 60.5 | 43 |
| BPSD recorded | 27 | 47.4 | 57 | 15 | 34.9 | 43 |
| Signs and symptoms | 1 | 2.1 | 48 | 4 | 8.9 | 45 |
| Course and prognosis | 0 | 0.0 | 47 | 0 | 0.0 | 45 |
| Treatment | 13 | 27.7 | 47 | 5 | 11.1 | 45 |
| Care and support | 11 | 22.4 | 49 | 9 | 20.0 | 45 |
| Support groups | 3 | 6.1 | 49 | 4 | 9.1 | 44 |
| Financial, legal and advocacy | 7 | 13.7 | 51 | 4 | 8.9 | 45 |
| Medicolegal matters | 4 | 8.0 | 50 | 8 | 17.8 | 45 |
| Local services | 2 | 4.0 | 50 | 2 | 4.4 | 45 |
| Anti-dementia drugs prescribed | 40 | 66.7 | 60 | 31 | 58.5 | 53 |
| Medication review | 34 | 65.4 | 52 | 22 | 51.2 | 43 |
Concordance with management guidelines at baseline differed between those patients from practices subsequently allocated to intervention or usual care groups, as shown in Table 11. Concordance with management guidelines at follow-up is shown in Table 12.
| Management action | Randomisation group | |||||
|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||
| n | % | Total | n | % | Total | |
| Prescribed antipsychotic drugs | 18 | 25.4 | 71 | 8 | 12.9 | 62 |
| Valid patient consent | 20 | 28.2 | 71 | 12 | 20.3 | 59 |
| Recall and reasoning | 8 | 11.6 | 69 | 6 | 10.2 | 59 |
| Care plan | 40 | 59.7 | 67 | 30 | 50.0 | 60 |
| BPSD mentioned/managed | 58 | 81.7 | 71 | 43 | 71.7 | 60 |
| Advanced statements | 2 | 2.9 | 70 | 2 | 3.3 | 60 |
| Living will | 1 | 1.4 | 69 | 0 | 0.0 | 60 |
| LPA | 10 | 14.5 | 69 | 2 | 3.3 | 60 |
| Preferred place of care | 11 | 15.7 | 70 | 1 | 1.7 | 60 |
| Direct payments | 3 | 4.3 | 70 | 1 | 1.7 | 60 |
| Functional abilities/ADL | 52 | 74.3 | 70 | 22 | 36.7 | 60 |
| Carer’s needs | 23 | 37.7 | 61 | 22 | 37.9 | 58 |
| Care and support services | 22 | 34.9 | 63 | 12 | 20.0 | 60 |
| Support groups | 0 | 0.0 | 64 | 5 | 8.3 | 60 |
| Financial and legal advocacy | 4 | 6.2 | 65 | 2 | 3.3 | 60 |
| Medicolegal issues | 10 | 15.4 | 65 | 6 | 10.0 | 60 |
| Local services | 3 | 4.8 | 63 | 0 | 0.0 | 60 |
| Management action | Randomisation group | |||||
|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||
| n | % | Total | n | % | Total | |
| Prescribed antipsychotic drugs | 22 | 22.7 | 97 | 7 | 11.3 | 62 |
| Valid patient consent | 25 | 26.3 | 95 | 5 | 8.1 | 62 |
| Recall and reasoning | 18 | 20.0 | 90 | 3 | 4.8 | 62 |
| Care plan | 52 | 54.2 | 96 | 27 | 43.5 | 62 |
| BPSD mentioned/managed | 59 | 61.5 | 96 | 45 | 73.8 | 61 |
| Advanced statements | 5 | 5.2 | 96 | 3 | 4.8 | 62 |
| Living will | 2 | 2.1 | 95 | 2 | 3.2 | 62 |
| LPA | 8 | 8.3 | 96 | 4 | 6.6 | 61 |
| Preferred place of care | 13 | 13.5 | 96 | 5 | 8.1 | 62 |
| Direct payments | 3 | 3.2 | 94 | 2 | 4.0 | 50 |
| Functional abilities/ADL | 63 | 65.6 | 96 | 30 | 48.4 | 62 |
| Carer’s needs | 25 | 27.5 | 91 | 18 | 30.5 | 59 |
| Care and support services | 15 | 16.9 | 89 | 7 | 11.7 | 60 |
| Support groups | 0 | 0.0 | 93 | 2 | 3.3 | 60 |
| Financial and legal advocacy | 2 | 2.1 | 95 | 1 | 1.7 | 60 |
| Medicolegal issues | 11 | 11.5 | 96 | 4 | 6.7 | 60 |
| Local services | 0 | 0.0 | 93 | 0 | 0.0 | 48 |
Table 13 shows ORs (with 95% CIs, p-values and numbers included in each model) from models which take into account the practice clustering and are adjusted for baseline levels of concordance. ORs represent the odds of concordance for the intervention compared with usual care practices. There are only two statistically significant differences, both favouring the usual care group. Participants from practices in the intervention group were less likely to have details of valid patient consent, and of care plans, documented in their medical records.
| Management action | OR (95% CI); p-value (n) |
|---|---|
| Prescribed antipsychotic drugs | 0.75 (0.20 to 2.76); 0.664 (131) |
| Valid patient consent | 0.18 (0.03 to 0.94); 0.042 (127) |
| Recall and reasoning | 0.08 (0.01 to 1.24); 0.071 (121) |
| Care plan | 0.44 (0.20 to 0.98); 0.045 (125) |
| BPSD mentioned/managed | 1.65 (0.59 to 4.59); 0.339 (128) |
| Advanced statements | 0.49 (0.07 to 3.35); 0.470 (128) |
| Living will (advance decision to refuse treatment) | 0.58 (0.05 to 6.55); 0.659 (127) |
| LPA | 0.52 (0.13 to 2.14); 0.363 (126) |
| Preferred place of care | 1.17 (0.04 to 35.94); 0.926 (128) |
| Direct payments/personal budgets | 1.39 (0.18 to 10.77); 0.756 (116) |
| Functional abilities/ADL | 0.65 (0.24 to 1.79); 0.407 (128) |
| Carer’s needs | 1.10 (0.11 to 11.04); 0.938 (116) |
| Care and support services | 0.74 (0.20 to 2.72); 0.646 (118) |
| Support groups | 8857496 (0 to ∞); 0.995 (121) |
| Financial and legal advocacy | 2310740 (0 to ∞); 0.995 (123) |
| Medicolegal issues | 0.51 (0.15 to 1.81); 0.300 (123) |
| Local services | Unable to estimate as a result of all values being zero |
Professionals’ knowledge and skills analysis
Practitioners’ knowledge about dementia
Knowledge about dementia was measured by a self-completed 14-item multiple-choice quiz, for questions which were drawn from two US instruments: the University of Alabama at Birmingham Alzheimer’s Disease Knowledge Test53 and the Alzheimer’s Disease Knowledge Test. 54 The questions reflected the content of the educational intervention, and covered current and future prevalence, risk factors, diagnosis (including differential diagnosis), medication and management (see Appendix 5). Where necessary, wording was adapted to reflect development in knowledge about dementia or cultural differences. A scoring system of ‘correct’ = + 1, ‘do not know’ = 0, ‘wrong’ = –1 was adopted to maximise variance and to allow investigation of the mix of the three types of answer. These scores were transformed to give a theoretical range of –100 to 10055 (see Appendix 5). The scores of GPs on knowledge skills and attitudes were compared with those obtained using the same instrument in the previous trial of educational interventions, in 2001–2.
Professional knowledge, skills and attitudes at baseline: general practitioners
Ninety-two GPs completed questionnaires at baseline, with very few missing data except for age. There were no statistically significant differences in knowledge, skills and attitudes between GPs in the two arms of the trial. Forty-six (50%) were women, and their age range was 26–65 years, mean = 37 years, median = 41 years.
Sixty-six (72%) were principals, 12 were salaried (13%), six were registrars (6.5%) and four were locums or categorised themselves as ‘other’ (8.5%). Sixty-one (66%) had worked in old age psychiatry, elderly medicine or general psychiatry.
Eleven (12%) had discussed the implications of the NDS18 in the practice, 16 (17%) had discussed the NDS in a professional development setting and seven (7.5%) had discussed it with specialist colleagues. Fifteen (7.5%) had been offered training in dementia diagnosis and/or management by local specialist services. Twenty-five (27%) had discussed the implications of the MCA 200517 with their practice, 31 (34%) had discussed it in a professional development setting, and 15 (16%) had discussed it with a specialist colleague.
Confidence in reaching a diagnosis was similar to the earlier findings, but respondents were significantly more confident about advising on management of BPSD in 2009–10 than in 2001–2, as shown by Table 14.
| Confidence in: | 2001–2 | 2009–10 | p-value |
|---|---|---|---|
| Reaching a diagnosis | 81 (64%) | 63 (68.5%) | n.s. |
| Advising on BPSD management | 40 (32%) | 41 (45%) | 0.03 |
At both time periods, about one in five GPs disagreed with the statement that much can be done to improve the quality of life of people with dementia or their carers. A similar proportion disagreed that families would rather be told the diagnosis as soon as possible (Table 15).
| Attitude | Agreementb | p-value | |
|---|---|---|---|
| 2001–2 | 2009–10 | ||
| Much can be done to improve the quality of life of carers of people with dementia | 105/124 (84%) | 74/92 (81%) | n.s. |
| Families would rather be told about their relative’s dementia as soon as possible | 99/120 (83%) | 76/92 (83%) | n.s. |
| Much can be done to improve the quality of life of people with dementia | 98/124 (77%) | 77/91 (84%) | n.s. |
| Providing diagnosis is usually more helpful than harmful | 79/122 (65%) | 66/92 (72%) | n.s. |
| Managing dementia is more often frustrating than rewarding | 46/124 (38%) | 31/92 (34%) | n.s. |
| Dementia is best diagnosed by specialist services | 41/125 (33%) | 41/91 (44%) | 0.03 |
| Patients with dementia can be a drain on resources with little positive outcome | 17/118 (14%) | 11/92 (12%) | n.s. |
| It is better to talk to the patient in euphemistic terms | 11/117 (9%) | 15/92 (16%) | n.s. |
| There is little point in referring families to services as they do not want to use them | 4/121 (3%) | 4/92 (4%) | n.s. |
| The primary care team has a very limited role to play in the care of people with dementia | 6/125 (5%) | 9/92 (10%) | n.s. |
Respondents were significantly more likely to agree that dementia is best diagnosed by specialist services in 2009–10 than in 2001–2, although at both time periods only a minority agreed with this proposition.
The 2009–10 respondents were significantly less familiar with management of dementia-related symptoms, availability of services or how to access them, than respondents in 2001–2 (Table 16).
| Perceived barrier | 2001–2 | 2009–10 | p-value |
|---|---|---|---|
| Too busy/not enough time during surgery visits | 98/118 (83%) | 69/92 (75%) | n.s. |
| Lack of social service support available to practice | 69/118 (58%) | 54/92 (59%) | n.s. |
| Lack of funding within the practice | 54/118 (46%) | 35/92 (38%) | n.s. |
| Lack of team staff in the practice | 60/118 (50%) | 40/91 (44%) | n.s. |
| Unfamiliar with advances in the management of dementia related symptoms | 53/119 (45%) | 61/92 (66%) | < 0.001 |
| Unfamiliar with available services to help keep patients at home | 49/119 (41%) | 57/92 (62%) | < 0.01 |
| Unsure how to refer patients to available services to help keep them at home | 29/119 (24%) | 33/92 (36%) | 0.04 |
Differences between the two time points in perceived difficulties in diagnosis and management of patients with dementia are shown in Table 17. Not all questions were the same at the two time points.
| Perceived difficulty | Mean score | |
|---|---|---|
| 2001–2 (scale 1–7) | 2009–10 (scale 1–6) | |
| Responding to co-existing behaviour problems | 4.6 | 3.7 |
| Discussing the probable diagnosis: | 4.5 | 3.6 |
| With the co-ordinating support services for carers and people with dementia | 4.3 | – |
| With the co-ordinating support services for people with dementia | – | 3.8 |
| With the co-ordinating support services for carers | – | 3.8 |
| Responding to the family’s concerns: | 3.9 | – |
| Getting information about support services for carers and people with dementia | 3.8 | – |
| Getting information about support services for people with dementia | – | 4.1 |
| Getting information about support services for carers | – | 4.2 |
| Responding to co-existing psychiatric problems | 3.8 | 3.9 |
| Discussing the probable diagnosis with the family | 3.5 | 3.3 |
| Reaching a probable diagnosis yourself | 3.4 | 3.3 |
| Getting information about anti-dementia medication | 3.4 | 3.5 |
| Getting specialist assessment services advice by telephone | 2.8 | 3.5 |
A consistent one in five respondents in 2009–10 cannot answer the questions, suggesting that they are not engaged with patients with dementia (Table 18).
| Resource | Available and satisfactory | Available but not satisfactory | Needed, but not available | Not needed | Cannot say | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2001–2, n = 126 | 2009–10, n = 92 | 2001–2 | 2009–10 | 2001–2 | 2009–10 | 2001–2 | 2009–10 | 2001–2 | 2009–10 | |
| Information about what old age psychiatry services offer | 67 (59%) | 33 (36%) | 34 (30%) | 31 (34%) | 10 (9%) | 15 (16%) | 0 (0%) | 0 (0%) | 14 (11%) | 13 (14%) |
| Protocol for assessment and investigation of a patient with possible dementia | 27 (32%) | 28 (30%) | 10 (12%) | 24 (26%) | 43 (51%) | 22 (24%) | 2 (2%) | 1 (1%) | 43 (34%) | 17 (18.5%) |
| Brief screening instrument for early identification | 46 (48%) | 37 (40%) | 12 (13%) | 19 (21%) | 31 (33%) | 15 (16%) | 3 (3%) | 1 (1%) | 32 (25%) | 19 (21%) |
| Nurse with mental health training working in association with the practice | 37 (34%) | 2 (2%) | 8 (7%) | 4 (4%) | 54 (50%) | 64 (70%) | 7 (7%) | 4 (4%) | 19 (15%) | 18 (20%) |
| Shared care protocol for cholinesterase inhibitors | – | 10 (11%) | – | 13 (14%) | – | 37 (40%) | – | 7 (8%) | – | 25 (27%) |
| Information about benefits (attendance allowance, council tax, etc.) | 48 (46%) | 13 (14%) | 26 (25%) | 17 (18.5%) | 25 (24%) | 37 (40%) | 3 (3%) | 6 (6.5%) | 23 (18%) | 18 (20%) |
Nearly four in five GPs responding in 2009–10 thought that dementia prevalence would double by 2021, and 41% estimated the average caseload as ≥ 21. The overestimation on both questions is consistent with findings from 2001–2 (Table 19).
| Question | Correct n (%) | p-value | |
|---|---|---|---|
| 2001–2, N = 126 | 2009–10, N = 92 | ||
1. A GP with a list of 1500–2000 people can expect to have the following number of people with dementia on their list:
|
48 (38) | 32 (35) | n.s. |
2. By 2021, the prevalence of dementia in the general population in the UK is expected to:
|
14 (11) | 16 (17) | n.s. |
3. One of the risk factors for the development of Alzheimer’s disease is:
|
71 (58) | 55 (60) | n.s. |
4. All of the following are potentially treatable aetiologies of dementia except:
|
107 (86) | 71 (77) | n.s. |
5. A patient suspected of having dementia should be evaluated as soon as possible as:
|
108 (86) | 73 (79) | n.s. |
6. Which of the following procedures is required to definitely confirm that symptoms are due to dementia?
|
25 (21) | 14 (15) | n.s. |
7. Which of the following is not a necessary part of the initial evaluation of a patient with possible dementia?
|
110 (87) | 84 (91) | n.s. |
8. A sudden onset of confusion, disorientation and inability to sustain attention – this presentation is most consistent with the diagnosis of:
|
121 (96) | 85 (92) | n.s. |
9. Which of the following is nearly always present in dementia?
|
115 (92) | 85 (92) | n.s. |
10. Which of the following clinical findings best differentiates vascular dementia from Alzheimer’s disease?
|
89 (71) | 68 (74) | n.s. |
11. The effect of anti-dementia drugs is to:
|
114 (91) | 72 (78) | < 0.01 |
12. Which statement is true concerning the treatment of dementia patients who are depressed?
|
91 (72) | 53 (58) | 0.01 |
Respondents in 2009–10 were significantly less likely to answer correctly the questions about the effect on anti-dementia drugs (cholinesterase inhibitors) and the treatment of depression in patients with dementia.
Carers’ perceptions
Participants
Ninety carers of patients with dementia expressed interest in being interviewed about the quality of dementia care. Of these, 84 gave consent for two interviews, one at baseline and then again 12 months later. Six carers who had expressed interest did not consent to an interview, either because of the death of the person with dementia or because they changed address and could not be contacted.
Depending on the preference of the carers, the interviews took place in the following places: the carer’s home (n = 23, 27.4%); the patient’s home (n = 4, 4.8%); carer and patient’s home (n = 46, 54.8%); the GP practice (n = 6, 7.1%); carer’s place of work (n = 3, 3.6%); and the research department at University College London - Royal Free Hospital (n = 2, 2.4%). All participants received a copy of the signed and dated consent forms, and the original was retained in the Trial Master File. A third copy was filed in the PWD’s medical notes at their GP practice. The interviews took, on average, 98 (SD 33, range 30–205) minutes to complete.
A structured interview schedule was used, derived from the instrument used in the previous trial,56 and is shown in Appendix 6. Interviews were tape recorded with permission, and transcribed.
Results
Carer and patient characteristics
Of the 84 carers who completed interviews, 63 (75%) were women and 21 (25%) men. Their age ranged from 36 to 88 years, with a mean age of 66 years. More than one-third were spouse carers (wives 36%, husbands 13%) and 44% were adult children (daughters 33%, sons 11%), with the remaining 7% of carers being sisters or daughters-in-law.
Their relatives with dementia had been diagnosed, on average, 43 months prior to interview (SD 42.9 months, range 1–168 months) and 79 of the people with dementia (94%) lived in their own home. See Table 20 for further sociodemographic information of dementia patients and their carers. Table 21 shows that the care recipients had substantial functional losses and a high prevalence of BPSD.
| Demographic information | Patient: mean (SD, range) | Carer: mean (SD, range) |
|---|---|---|
| Age (years) | 80.5 (9, 57–97) | 65.7 (12.6, 36–88) |
| Time since diagnosis (months) | 43 (42.9, 1–168) | – |
| Age of leaving school (years) | 15.3 (1.6, 11–18) | 16.65 (2.5, 13–29) |
| Demographic information | Patient: n (%) | Carer: n (%) |
| Further education (e.g. diploma, university) | 25 (29.8) | 44 (52.4) |
| Gender | ||
| Female | 44 (52.4) | 63 (75) |
| Male | 40 (47.6) | 21 (25) |
| Marital status | ||
| Widowed | 40 (47.6) | – |
| Married/co-habiting | 41 (48.8) | 72 (85.7) |
| Single | 1 (1.2) | 8 (9.5) |
| Divorced | 2 (2.4) | 4 (4.8) |
| Ethnic status | ||
| White – UK | 68 (81) | 73 (86.9) |
| White – Irish | 2 (2.4) | 3 (3.6) |
| White – other | 7 (8.3) | 3 (3.6) |
| Black – Caribbean | 1 (1.2) | 1 (1.2) |
| Black – African | 2 (2.4) | – |
| Black – other | – | 1 (1.2) |
| Indian | 3 (3.6) | 3 (3.6) |
| Bangladeshi | 1 (1.2) | – |
| Employment status | ||
| Working full time | 9 (10.7) | |
| Working part time | 12 (14.3) | |
| Unable to work for health reasons | 1 (1.2) | |
| Homemaker | 4 (4.8) | |
| Retired | 51 (60.7) | |
| Other (e.g. freelance) | 7 (8.3) | |
| Relationship with patient | ||
| Wife | 30 (35.7) | |
| Husband | 11 (13.1) | |
| Daughter | 28 (33.3) | |
| Son | 9 (10.7) | |
| Other (e.g. sister, daughter-in-law) | 6 (7.2) | |
| Type of dementia | ||
| Alzheimer’s disease | 29 (34.5) | |
| Vascular | 10 (11.9) | |
| Lewy body | 4 (4.8) | |
| Pick’s disease | 2 (2.4) | |
| Mixed (e.g. Alzheimer’s disease and vascular) | 9 (10.7) | |
| Dementia | 14 (16.7) | |
| Other (e.g. unclear) | 16 (19) | |
| Place of residence | ||
| Own home (with or without spouse) | 79 (94) | |
| With relative | 5 (6) | |
| Type of disability | Yes, na (%) | No, na (%) |
|---|---|---|
| Able to manage personal care | 42 (50.6) | 41 (49.4) |
| Able to manage medication | 13 (16.7) | 65 (83.3) |
| Able to prepare food | 21 (26.6) | 58 (73.4) |
| Mobility within the house | 64 (79) | 17 (21) |
| Mobility outside the house | 43 (55.1) | 35 (44.9) |
| Able to cope with toileting | 56 (67.5) | 27 (32.5) |
| Able to do shopping | 12 (14.8) | 69 (85.2) |
| Able to do housework | 20 (25) | 60 (75) |
| Able to manage money and bills | 8 (9.8) | 74 (90.2) |
| Verbally aggressive | 37 (45.1) | 45 (54.9) |
| Physically aggressive | 15 (18.3) | 67 (81.7) |
According to carer reports, almost half of the sample taking anti-dementia medication had Alzheimer’s disease (n = 25, 46.3%), compared with 10% (n = 3) of those not on medication.
Table 22 shows that the majority of carers recalled receiving advice or support in only 6 of 15 domains: home adaptations, the care recipient’s driving, benefits and grants, legal concerns, respite (day care) and in how they were coping. Despite the requirements of an annual dementia review, which should include the assessment of carer needs, only half of the carers included in the study had been asked how they were coping and 60% had not been asked about their fears or anxieties.
| Type of advice – been asked/given advice about: | Yes, na (%) | No, na (%) |
|---|---|---|
| How to manage verbal aggression | 6 (16.2) | 31 (83.8) |
| How to manage physical aggression | 4 (26.7) | 11 (73.3) |
| What to tell a relative re. diagnosis | 6 (7.4) | 75 (92.6) |
| Keeping a relative independent | 13 (15.5) | 71 (84.5) |
| Home adaptations and equipment | 46 (58.2) | 33 (41.8) |
| The care recipient’s driving | 22 (55) | 18 (45) |
| Benefits/grants | 45 (54.2) | 38 (45.8) |
| Costs of services | 27 (32.1) | 57 (67.9) |
| Legal aspects | 44 (52.4) | 40 (47.6) |
| Day care | 44 (53) | 39 (47) |
| Respite options | 29 (37.2) | 49 (62.8) |
| How the carer is coping | 44 (52.4) | 40 (47.6) |
| Fears and anxieties | 33 (39.8) | 50 (60.2) |
| Future care plans and expectations | 13 (15.7) | 70 (84.3) |
| Reference material | 40 (48.2) | 43 (51.8) |
Fifty-four of the 84 carers reported that the person they care for was taking cholinesterase inhibitors. Comparison of advice and support received between the groups taking/not taking cholinesterase inhibitors is shown in Table 23. The carers of those patients taking cholinesterase inhibitors reported significantly more advice about the care recipient’s driving, significantly more enquiries about their own coping and significantly more discussion about their fears and anxieties.
| Been asked/given advice about: | On cholinesterase inhibitors (N = 54),a n (%) | Off cholinesterase inhibitors (N = 30),a n (%) | χ2; p-value | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| How to manage verbal aggression | 5 (20.8) | 19 (79.2) | 1 (7) | 12 (92) | n.s. |
| How to manage physical aggression | 3 (30) | 7 (70) | 1 (20) | 4 (80) | n.s. |
| What to tell a relative about diagnosis | 6 (11.8) | 45 (88.2) | 0 (0) | 30 (100) | n.s. |
| Keeping a relative independent | 9 (16.7) | 45 (83.3) | 4 (13.3) | 26 (86.7) | n.s. |
| Home adaptations and equipment | 28 (57.1) | 21 (42.9) | 18 (60) | 12 (40) | n.s. |
| Relative driving | 20 (66.7) | 10 (33.3) | 2 (20) | 8 (80) | 6.599; < 0.025 |
| Benefits/grants | 32 (60.4) | 21 (36.6) | 13 (43.3) | 17 (56.7) | n.s. |
| Costs of services | 18 (33.3) | 36 (66.7) | 9 (30) | 21 (70) | n.s. |
| Legal aspects | 32 (59.3) | 22 (40.7) | 12 (40) | 18 (60) | n.s. |
| Day care | 29 (54.7) | 24 (45.3) | 15 (50) | 15 (50) | n.s. |
| Respite options | 17 (34) | 33 (66) | 12 (42.9) | 16 (57.1) | n.s. |
| How the carer is doing | 35 (64.8) | 19 (35.2) | 9 (30) | 21 (70) | 9.372; < 0.002 |
| Fears and anxieties | 26 (49.1) | 27 (50.9) | 7 (23.3) | 23 (76.7) | 5.292; < 0.025 |
| Future care | 8 (15.1) | 45 (84.9) | 5 (16.7) | 25 (83.3) | n.s. |
| Reference material | 29 (54.7) | 24 (45.3) | 11 (36.7) | 19 (63.3) | n.s. |
Qualitative results
Questions with open-ended responses were included in the interview. Box 2 displays a summary of three themes arising; perceptions of support from primary care, expectations of primary care, and the need for proactive care. Full analysis of the qualitative data will be reported in a separate publication.
Overall, few carers had contact with primary care and had relatively low expectations of their GP. However, those who did have contact with the practice were generally satisfied with the care they received.
They kind of, I mean you get the proactivity in terms of coming in and have the blood tests, because she’s got diabetes etc. But we didn’t really get any dementia help from him [the GP]. And, you know, to be honest, when she had these water infections and we’d ring and say, ‘Oh look she’s really bad,’ they really didn’t come and see her. They said, ‘Well there’s not a lot that we can do for her really.’ And even when she went to hospital, they said, ‘Well why don’t you just ring 999?’
Daughter, 55, of care home resident, not on cholinesterase inhibitors
I did go and see the GP; I mean they were always very nice. And if they came to see mum here, and they’d always, you know, sometimes they’d say to me, ‘How are you?’ you know. You know, while they, you know, as we were showing them out etc., you know, ‘Are you okay? I know it’s hard.’ But, and then once when I was really feeling not well myself, I did go and see the GP just to have some blood tests and things, you know, because I was really at a bit of a low ebb. And, you know . . . we chatted about the caring.
Daughter, 55, of care home resident, not on cholinesterase inhibitors
No, nothing at all. The bottom line is, I don’t think, then again I don’t think anybody can do anything for us, for me. We’ll just have to help ourselves.
And in terms of support then, how is that for you?
There is no support.
Wife, 66, husband living at home, on cholinesterase inhibitors
Yes we’ve been in and out of there [the practice]. And he’s brilliant, I have to say . . . Yes, he’s been a brilliant support, you know . . . It is, I must admit, you know, a couple of times he’s said, ‘And how are you?’ you know, and I think that’s just so nice really.
Daughter, 47, mother living in her own home, on cholinesterase inhibitors
Well it was mum’s GP that said really you do need to get this Power of Attorney done, and a couple of times I’ve been, he’s said, ‘Have you done it yet, have you done it yet?’ And I said, ‘I will do,’ and he said, ‘Honestly,’ he said, ‘If your mum goes into hospital and I’m not around, you know, they won’t listen to you.’ And he is lovely. He really is. But then you talk to nearly anybody in the town and he’s caring right the way across the board, you know, right through the young . . . He’s lovely and he sits there and, you know, he says to mum, he held her hand the other day and he said, ‘You know what’s the matter with you,’ he said, ‘You worry too much.’ And she said, ‘I know, I’ve always been the same.’ And he said, ‘I know.’ And she just said, you know, ‘Thank you so much.’ And I thought that goes such a long way.
Daughter, 47, mother living in her own home, on cholinesterase inhibitors
No, no. I don’t find them very helpful at all actually.
And what about for you as carers, do they ever ask you if you’re okay or if you need anything?
No never.
Daughter-in-law, 52, mother-in-law living in her own home
Theme 2: expectations of primary care
Well I think the GP could have put me in touch with social services a bit quicker because I was fighting to get mum some care, because I was doing it all myself. And I think the GP could have been a bit more sympathetic or as he’s seen her probably over the years, he could have shared the experience. But he didn’t. He, it was just, all I ever got from him was, ‘Take her home, make her comfortable. What do you want me to do? Do you want me to put her in a home? Do you want me to do this?’ All the things I didn’t want him to do.
Daughter, 50, mother living in her own home, not on anti-dementia medication
Is there anything else you would say to her GP that they could improve?
Well, to take it a bit more seriously, I think. I think there is a matter of fact isn’t it? Yes, and just to, you know, be able to help people that don’t understand dementia at all. You know, if you don’t, I mean the first time I’d heard of it was when mum got it. And then I still didn’t understand what it was all about. You don’t know what to ask, do you? I remember sitting in the surgery thinking, ‘Well what do I ask next? Is she going to get better?’ They never said to me that she wasn’t going to get better.
Daughter, 50, mother living in her own home, not on anti-dementia medication
So I guess they can only do so much. And they were always very nice and we talked to them on the phone, etc.
Daughter, 55, of care home resident, not on cholinesterase inhibitors
The bottom line is, I don’t think, there again I don’t think anybody can do anything for us, for me. We’ll just have to help ourselves.
Wife, 66, husband living at home, on cholinesterase inhibitors
Theme 3: proactive care
Yes you’re very loathe because you’re always conscious that the doctor’s time is valuable, you know you’ve already waited an hour to actually see them past the appointment time and you’re very grateful you’re not being given the bum’s rush. But you don’t think to say, ‘You know, is there anything locally that mum could go to?’ because you think it’s ‘the doctor’.
Daughter of care home resident, (carer, age unknown), no longer on cholinesterase inhibitors
I can only, I don’t know really. I mean they, they always seem quite independent of everything else. And I know they’ve got a lot of demands on them. Again I suppose if they worked with a key worker so they, they did their visits or they – and the idea of them looking at reviewing such a vulnerable person’s health proactively does seem quite a good idea. I don’t know how that fits in with the opportunity cost of resourcing and expenses.
Son, 58, mother living in own home, on cholinesterase inhibitors
So ideally that’s what I would like. I would like some sort of, I don’t know how they could do this, but they should be, I would like them to have some sort of recognition that all people who are carers are really under a huge strain that cannot be seen. And I mean, you know, I mean I know – I don’t want preferential treatment for me, but I want preferential treatment for carers.
Wife, 72, husband living at home, not on cholinesterase inhibitors
Discussion
The EVIDEM-ED trial builds on an earlier study,21 and developed and tested an intervention customised to the educational needs of individual practices. The deliverables from this programme include an educational intervention for general practice and practice nursing, combining timely dementia diagnosis and psychosocial support around the period of diagnosis, with components appropriate to later stages of the disease trajectory. It also includes electronic resources on the same themes, together with shared care guidelines for medication use.
The English policy imperatives and financial incentives for dementia diagnosis and management have created an ideal environment for a trial of an educational intervention designed to improve clinical practice in primary care – the educational intervention as developed following the Medical Research Council (MRC)’s recommendations for complex interventions,56 with strong elements of co-design to gain the insights and experiences of a range of practitioners. 37 The ENA deployed in this trial is an example of a strategy aimed to improve quality of care by overcoming the translation block that obstructs the diffusion of clinical guidelines and knowledge into practice. 57
In this study we found no significant improvement in case identification or documentation of dementia management reviews after an educational intervention tailored to practice educational needs. This is despite the financial gain to practices for increasing the number of cases identified and reviewed annually. There are several possible reasons for this.
The intervention may have been too weak to change practice. More workshops may have been needed, with reinforcement or mentoring of practitioners over longer periods of time. This level of educational input was not practicable in this trial, and we doubt that it would be feasible in real-world primary care organisations.
Physicians have a limited ability to accurately self-assess their competence. 58 Although the ENA was designed as a group process to offset this tendency, more external assessment may have been needed to truly tailor the intervention to needs.
Practitioners’ knowledge, skills and attitudes in 2009–10 were similar to those recorded using the same instrument in 2001–2. Differences in responses between the two time periods suggested that GPs were less confident about diagnosis and overall clinical management of dementia in 2009–10 (with BPSD as an exception).
Carers’ recall of advice given to help them manage the person with dementia suggested that a large minority had not received the information recommended by the NICE dementia guidelines. 36,59 However, carers of patients taking cholinesterase inhibitors reported more advice on some aspects of care than those whose care recipients did not take this medication.
Carers’ generally positive attitudes towards general practice, despite their limited contacts with the care recipients’ GPs, is similar to findings from the earlier trial. 60
Limitations of the study
It is possible that the medical records did not capture important changes in the quality of management after the educational intervention, which were not captured in our evaluation of secondary outcomes, but our creation of a category of ‘opportunistic dementia review’ fitted with clinical practice and allowed a generous interpretation of clinical activity. Many patients with dementia joined or left during the pre and post periods, truncating the data collection time so that the length of follow-up may have been too short to capture a difference. The number of dementia management reviews was higher in the non-intervention group at baseline; this could be the consequence of one practice in the usual care group having medical responsibility for several care homes. Distribution of newsletters and guidelines to usual care arm practices may have had an effect on their patients’ behaviour.
The study took place in south east England with practices that were innovative early adopters, not typical general practices, and local educational programmes that had been developed to implement the NDS may have influenced practice activities, although we found no evidence of this. The volunteer practices were probably different from others, in that they wanted to take part in a pilot educational programme about dementia. However, the results of this study have wider implications, particularly about the value of tailoring educational interventions.
The intervention was developed in ways consistent with current understanding of how effective interventions are made. Nevertheless, there may have been deficiencies in the development process. For example, the views of people with dementia and their family carers may not have had sufficient weight. Some professional perspectives may have been too powerful; the absence of response from expert panel members to the ENA questions themselves could be a sign of this. The expert group may not have used the expert panel’s critical comments sufficiently, resulting in an oversimplification of the ENA. Finally, the expert tutor may have had an effect on the use of the ENA and the ‘filling’ of the educational prescription, even although we used a participant observer to avoid idiosyncratic interpretations of the group discussions.
Conclusions
This study suggests that a tailored educational intervention aimed at GPs does not improve dementia case identification or documentation of clinical management for people with dementia, even when policy pressure encourage changes in clinical practice and the reimbursement system rewards it.
Implementation studies
There was considerable demand for the EVIDEM-ED intervention even before the results of the RCT were available. Its use has been tested as follows:
-
Thames Valley Health Innovation and Education Cluster ran sessions with 13 practices in 2011. The sessions aimed to improve GP knowledge around dementia, and to improve care for patients and their carers. The sessions were facilitated by a group of five postgraduate psychology students from the University of Reading, who received 1 day of training on small group facilitation and the use of the training tool.
-
Cambridge Community Mental Health Team ran sessions, for which a consultant in old age psychiatry acted as a group facilitator.
-
Central and North West London (CNWL) NHS Foundation Trust demonstrated the approach to specialist registrars in old age psychiatry, so that they could test it in general practices.
-
NHS Wales has run two training sessions for GPs in the use of ENA.
-
Two research projects using the EVIDEM-ED approach have been submitted for funding, one from Johns Hopkins Medical School in the USA and the other from the University of Auckland in New Zealand.
Research capacity building
Three academic GP registrars have learned research skills by working within the EVIDEM-ED project: Dr Louise Pealing, Dr Tamar Koch and Dr Alexandra Davidson.
Dr Sarah Voss in Swindon obtained funding for a parallel project on promoting person-centred care in general practice for people with dementia and their families. Details of this initiative are reported elsewhere. 61 This nested project was funded by the Alzheimer’s Society and its outputs have been incorporated into EVIDEM-ED’s learners’ manual (see: www.EVIDEM.org.uk).
Changes to protocol
No changes to the original protocol were made.
List of appendices
Appendix 1 Chapter 1: Findings from literature review of interventions in primary care designed to alter clinical practice with patients with dementia.
Appendix 2 Chapter 1: Advisory and steering group membership.
Appendix 3 Chapter 1: Diagnostic and management processes identified by the expert team.
Appendix 4 Chapter 1: Changing clinical practice in dementia: elements of a training programme – for primary care team.
Appendix 5 Chapter 1: General practitioner questionnaire.
Appendix 6 Chapter 1: Carer semi-structured interview schedule.
Appendix 7 Chapter 1: Medical records data extraction tool – baseline (time 1).
Chapter 2 EVIDEM-E: exercise as a therapy for behavioural and psychological symptoms of dementia – a randomised controlled trial of clinical effectiveness and cost-effectiveness
Abstract
Background Exercise could be beneficial for some BPSD.
Objectives To review the evidence and cost-effectiveness about exercise and BPSD, and to design and test a simple dyadic exercise regimen.
Study overview (1) Literature review: a rapid appraisal of the literature showed that exercise programmes for people with dementia have been poorly conceptualised. It is unclear which aspects of physical activity produce best outcomes. (2) RCT: a pragmatic, randomised, controlled, single-blind, parallel-group trial of 12 weeks of tailored walking for community-dwelling individuals with dementia who had BPSD, and for their carers. A total of 906 people with dementia were invited; 131 were randomised [64 treatment as usual (TAU), 67 intervention], and 57 TAU and 59 in the intervention arm were analysed. The primary outcome was change in the Neuropsychiatric Inventory62 (NPI) score.
Results There was no significant between-group difference in NPI score at week 12 [adjusted difference in means (intervention minus control) = –0.41, 95% CI –7.37 to 4.32; p = 0.6]. Caregiver’s burden, as measured by the Zarit Burden Interview (ZBI), doubled by week 12 for the TAU group but decreased in the intervention group (OR = 0.18, 95% CI 0.05 to 0.69; p = 0.01). Average cost of the exercise intervention was £284 per dyad. Within a subsample, the exercise intervention had a significantly higher cost from a societal perspective (mean difference £2728.60, p = 0.05) but a non-significantly lower cost from a health and social care (HSC) perspective.
Conclusion Regular simple exercise does not improve the BPSD but does attenuate rising caregiver burden.
Background: why this study was necessary
Dementia is associated with a cluster of non-cognitive symptoms and behaviours that are an integral part of the syndrome. Commonly described by some clinicians as BPSD, they include disturbed perception, thought content, mood or behaviour. 63 Although recognised as core to the phenomenology of dementia in Alzheimer’s disease seminal case studies,64 these symptoms have received relatively little attention from the research community. This is surprising given that > 80% of people with dementia will experience BPSD at some point during the course of their illness. 65 BPSD have long been commonly associated with reduction in the quality of life for the person as well as their carers;66 increase of caregiver stress,67 higher costs of care;68 and premature moves to long-term care facilities. 69
Although there is some evidence supporting the treatment of BPSD with antipsychotic drugs, there have been increasing concerns over the safety of these drugs for people with dementia. 70 Several studies have indicated that there is a long-term risk of mortality in patients with dementia who take antipsychotic medication. 71,72 There are few other safe pharmacological interventions with a proven evidence base.
Non-pharmacological alternatives that have been reported to have a positive effect include music therapy, bright light therapy, behavioural interventions and exercise. 73 As well as the obvious physical impact, exercise has been demonstrated to have a positive effect on cognitive symptoms and mood. 74,75 Interventions such as exercise may offer a safer effective alternative to pharmacological treatment, could empower individuals with dementia and may reduce carers’ burden.
Heyn et al. 76 carried out meta-analysis of exercise interventions for people with dementia and reported data on 30 trials of exercise. The authors analysed trials that included exercise regimens promoting strength, cardiovascular fitness or flexibility and analysed them for functional, cognitive or behavioural outcomes. They found a positive effect of exercise on behavioural outcomes (effect size = 0.54; 95% CI 36 to 72).
However, the trials included in the meta-analysis do not provide a full picture of the effectiveness of exercise for BPSD for a number of reasons. First, there was considerable heterogeneity among the interventions, and exercise was often combined with other behavioural interventions; consequently, it is difficult to isolate the impact that exercise had on behavioural outcomes. Second, some exercise regimens were complex and required a degree of physical fitness that would preclude many older adults with complex physical problems and moderate or significant dementia from performing them. These complex exercise regimens were potentially unsustainable without the support of trained therapists. The relatively high cost of delivering specialist input for such regimens may prevent such interventions being used more widely. Finally, most trials included in the analysis were relatively small, with only two reporting samples including > 100 participants.
Because of this limited evidence base, we designed a pragmatic, randomised, controlled, single-blind, parallel-group trial to evaluate the effectiveness of a simple exercise regimen as a therapy for BPSD.
Research questions:
-
What is the current evidence around the effects of exercise on BPSD, and how can this evidence shape the design of this trial?
-
Does a simple dyadic exercise regimen improve outcomes for people with BPSD and their carers?
-
What are participants’ views about, and experiences of, the dyadic exercise regimen?
Study methods and findings
Components of the study
The first question was addressed through a review of the literature; this informed the focus and design of the clinical trial to answer the second and third research questions (ISRCTN01423159). As a consequence of unforeseen difficulties with recruitment, a fourth question arose: Why is it difficult to recruit participants from a seemingly large population and supportive clinical service? To answer this question we added a small substudy that incorporated a focus group with recruiting clinicians.
Literature review
Methods
A rapid appraisal approach was adopted to inform the design and implementation of a RCT of exercise as therapy for BPSD. This review, rather than systematically aggregating data, has adopted a critical interpretive approach,77 the purpose of which is to construct theories grounded in research, develop an understanding about the potential effect of exercise on BPSD and to generate practical methods to evaluate these effects. This broad aim led to the generation of three research questions: (1) Does exercise ameliorate BPSD?; (2) How has exercise been conceptualised and how does this relate to the findings? and (3) What are the main limitations and methodological shortcomings of recent studies? Full details of the methods used in this literature review were published. 78
Inclusion/exclusion criteria
Although the aim of this review was to be as inclusive as possible with the current relevant literature, we recognised the importance of setting boundaries concerning papers to include. 77 Studies and review articles were selected that met the following criteria:
-
Individual studies must measure the efficacy of exercise as a therapy for BPSD. Exercise is defined as physical activity that is a planned, structured and repetitive programme.
-
Review articles must examine intervention studies assessing the efficacy of exercise as a therapy for BPSD.
-
Papers must have been published in peer-reviewed journals using relevant keywords appearing as an important factor in the title or abstract. Studies examining combined interventions (i.e. combining exercise with another psychosocial intervention) were excluded, as these are unable to establish the absolute effect of exercise.
The database search was constructed using the following strategies and searched up to January 2011.
-
MEDLINE, EMBASE, PsycINFO and PubMed were searched using a combination of keywords: ‘physical activity and dementia/Alzheimer’s disease’, ‘exercise and dementia/Alzheimer’s disease’, ‘non-pharmacological interventions and dementia’, ‘exercise, sleep disturbance and dementia/Alzheimer’s disease’, ‘exercise, apathy and dementia/Alzheimer’s disease’, ‘exercise, aggression and dementia/Alzheimer’s disease’, ‘exercise, wandering/repetitive behaviours and dementia/Alzheimer’s disease’, ‘exercise, depression/mood and dementia/Alzheimer’s disease’.
-
Other sources were also searched including reference lists and book chapters.
Results
Seven hundred and twenty-three articles were screened, of which 16 met the inclusion criteria. Of these, 10 were review articles and six were additional papers not included in these reviews (see the PRISMA chart in Figure 6 for the derivation of the selected papers). We examined each paper included in the reviews to assess their inclusiveness and their contribution to answering our three questions.
FIGURE 6.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart describing the search process of finding articles examining the efficacy of exercise on BPSD.
-
Does exercise reduce BPSD? Using very stringent inclusion criteria, the Cochrane review by Forbes et al. 79 concluded that there is insufficient evidence of the effectiveness of physical activity programmes on affective symptoms in older persons with dementia to warrant commissioning new services or decommissioning existing ones. They argue that until further methodologically robust studies are conducted and published, making firm recommendations for clinical practice about the importance, type and duration of exercise as a therapy for BPSD will not be possible. The existing research literature, although thin, does offer insights into potential effects of physical activity on BPSD and hints at the impact (or not) of exercise on behavioural and psychological symptoms not included in the Cochrane review (e.g. sleep disruptions, wandering, repetitive behaviours, anxiety and apathy).
-
How has exercise been conceptualised and how does this relate to the findings? Exercise programmes for people with dementia have been poorly conceptualised and it is unclear which aspects of physical activity (e.g. type and duration) provide better results than others. We need to understand further what behavioural and psychological symptoms respond and to what type of physical activity intervention, and how exercise may work, for whom and under what circumstances. Indeed, exercise may affect various BPSD in different ways. The current evidence cautiously indicates that its effect on some symptoms (e.g. agitation, anxiety) may be more rapid than on others (e.g. depression).
-
What are the main limitations and methodological shortcomings of recent studies? The methodological shortcomings of current work in this area are substantial – very few studies have robust methodologies. Few use randomised controlled designs and few have a sample size with enough power. In addition, many studies have short follow-up periods, which could potentially produce a type 2 error, that is, an effect of exercise on some symptoms may not be evident after only a short period. Many also rely on findings from mixed dementia groups while ignoring the potential differences between different types of dementias. Moreover, the intervention itself was often a heterogeneous one using different modalities of exercise (i.e. strength building, cardiovascular fitness or flexibility) and/or combined with other interventions (e.g. carer education). In such instances it remains unclear which particular components of an intervention are effective.
Trial objectives
Objectives
Primary objective
To determine the effectiveness of a dyadic exercise regimen delivered through a programme of incremental walking for treating BPSD (as defined by NPI scores) compared with TAU.
Secondary objectives
To determine the effect of this dyadic exercise regimen on (1) the quality of life of people with dementia; (2) psychotropic medication usage; (3) moving to a residential care facility (care home); (4) mortality levels; and (5) caregivers’ perceived level of burden. We also carried out an economic evaluation of the intervention.
Method
Trial design
We conducted a pragmatic, randomised, controlled, single-blind, parallel-group trial of a dyadic exercise regimen (tailored walking) for community-dwelling individuals with dementia who had BPSD, and for their carers. Individuals with a diagnosis of dementia, or suspected dementia, who had a carer who was willing and able to be a co-participant in the exercise regimen, were eligible for recruitment.
After screening and consent, participants were randomly allocated to one of two arms: the intervention arm, which received an individually tailored regimen of walking (see below for details) in addition to TAU or the control arm which received TAU only. TAU included home care, attendance at day-care facilities, visits by health professionals, hospital clinic visits, receipt of medication, respite care and so on. The trial protocol is shown in Appendix 8.
Both the intervention and TAU arms were assessed for all outcomes at weeks 6 and 12. Further telephone contact took place at 26 weeks to assess adverse events (AEs), mortality, change in domiciliary status and adherence to exercise regimen. The consent procedure, standard operating procedure for monitoring AEs, risk assessment tools and risk management pathway are shown in Appendices 9–13.
An independent randomisation officer (RO) assigned participants using a computerised algorithm and informed the participants, the participant’s GP and exercise therapist of allocation status. Other study personnel were kept blind to randomisation status.
Recruitment and baseline assessment of participants
Participants with a clinical diagnosis of dementia or ‘suspected dementia’ with at least one significant BPSD defined by the NPI62 (excluding the domains of delusions and hallucinations) were eligible for the trial (Figure 7). Diagnosis of dementia was confirmed using International Classification of Diseases, Tenth Edition (ICD-10) Diagnostic Criteria for Research (DCR-10). 80 A risk assessment was performed to assess the suitability of participants for the intervention at baseline. This assessment included measurement for risk of falls using the Falls Risk Assessment Tool (FRAT)81 and Timed Unsupported Steady Standing (TUSS) assessment. 82
FIGURE 7.
EVIDEM-E: CONSORT diagram.
Interventions
Physical exercise was delivered as an individually tailored regimen of walking, designed to become progressively intensive and last between 20 and 30 minutes. This was facilitated by a registered exercise professional qualified in Instructing Physical Activity and Exercise [National Vocational Qualification (NVQ) Level 3] and delivered to participants in the intervention arm of the trial in and around their own home. The exercise therapist facilitated physical exercise in the participant–carer dyad with the expectation that the dyad would perform the exercise regimen regularly and independently of the therapist at least five times per week. In order to attempt to control for the social effects of the therapist contact, the exercise therapist provided input for only the first 6 weeks of the trial, but participants were requested to continue exercising for 12 weeks. The intervention protocol is shown in Appendix 16.
The intervention group dyad was asked to complete a visual analogue scale, the Rate of Perceived Exertion (RPE). 83 Perceived exertion is based on the physical sensations that a person experiences during physical activity, including increased heart rate, increased respiration or breathing rate, increased sweating and muscle fatigue. Although this is a subjective measure, a person’s exertion rating may provide a fairly good estimate of the actual heart rate during physical activity.
All participants were asked to record their daily activities throughout the 12 weeks of participation using a diary that was designed to meet the specification of this study (see Appendices 14 and 15).
Setting
This study was conducted in inner city, urban and semirural locations in and around London. Recruitment began in EVIDEM’s host trust, with approaches to memory clinic teams and community teams working with older adults to identify and approach people with dementia who would fit the trial’s inclusion and exclusion criteria (see below), and progressively expanded the range of potential recruitment sources, which included:
-
North Thames DeNDRoN, which has a registry of people with dementia interested in participating in research (DemReg).
-
GP practices working with other projects in the EVIDEM programme.
-
Memory assessment services and community mental health services in six NHS Trusts: CNWL NHS Foundation Trust, West London Mental Health NHS Trust, East London NHS Foundation Trust, Surrey and Borders Partnership NHS Foundation Trust, Barnet, Enfield and Haringey Mental Health NHS Trust and South Essex Partnership University NHS Foundation Trust.
-
Self-referral in response to publicity about the trial through various media channels, including television and radio [British Broadcasting Corporation (BBC)], websites (EVIDEM and Alzheimer’s Society), local support groups (Housing 21 and Alzheimer’s Society’s Memory Cafés), carer information events (‘Service user network information day’ and ‘Admiral Nurses Carer Information Day’) and newsletters, for example the Alzheimer’s Society’s ‘Living with Dementia’.
The EVIDEM-E team held a focus group to identify barriers to recruitment, and the outcomes of these are reported below (see Findings) and also in a research letter in the International Journal of Geriatric Psychiatry. 84
Outcome measures
All outcomes were measured at baseline and after 6 and 12 weeks of participation.
The primary outcome measures were the behavioural and psychological symptom scores measured by the NPI62 at 12 weeks’ follow-up (Table 24 provides a full description of outcome assessment).
| Day | Administrator | Intervention/assessment |
|---|---|---|
| 0 | RW | ICD-10, MMSE, NPI, demographics, CSRI, DEMQOL-Proxy, GHQ, ZBI, medication, vital signs |
| 0 | Randomisation | Allocation and diaries |
| 1–2 | Exercise therapist | RPE timed walk, diarya |
| 3–4 | Exercise therapist | RPE timed walka |
| 5–8 | Exercise therapist | Telephone contact – advice and guidancea |
| 6–8 | Exercise therapist | RPE timed walka |
| 13–17 | Exercise therapist | Telephone contact – advice and guidancea |
| 17–28 | Exercise therapist | Telephone contact as neededa |
| 40–46 | Independent researcher | NPI, diary reminder |
| 40–46 | RW | DEMQOL-Proxy, GHQ, ZBI, medication, vital signs |
| 40–46 | Exercise therapist | RPE timed walka |
| 80–88 | Independent researcher | NPI, diary reminder |
| 80–88 | RW | DEMQOL-Proxy, GHQ, ZBI, medication, vital signs |
| 80–88 | Exercise therapist | RPE timed walka |
| 90–99 | RW | Collection of diaries |
| 182–196 | RW | Telephone contact, check for change of status (residence and mortality) |
Secondary outcome measures were:
-
participants’ mental health measured using the General Health Questionnaire (GHQ)85
-
participants’ quality of life measured using Dementia Quality of Life (DEMQOL)-Proxy86
-
carers’ burden measured using the short ZBI87
-
service use and costs, measured using the Client Service Receipt Inventory (CSRI). 88
Participants’ level of physical activity and compliance with the intervention were measured by carers using daily diaries and the RPE scale,83 and blood pressure and heart rate monitoring were carried out by the researcher. Domiciliary change and mortality at 26 weeks was ascertained by the researcher over the telephone.
Sample size
Assuming 80% of participants in the TAU group will have BPSD measured by the NPI at the 12-week follow-up, and based on an anticipated between-group (TAU minus intervention) absolute risk difference of 30% in the proportion of people with improved BPSD (defined as at least three-point improvement), we calculated that a sample size of 146 participants would provide 90% power to detect this difference with a 5% (two-sided) significance level, allowing for an anticipated 20% attrition rate.
Randomisation
With consent to participation, individuals were randomly allocated to receive TAU or exercise in addition to TAU (intervention). The randomisation ratio for the two groups was 1 : 1 (intervention : TAU). Randomisation was performed by the central co-ordinating centre using a computer algorithm of a simple randomisation protocol. A RO performed the randomisation and communicated notifications to participants, carers and the therapist, but not the research worker (RW).
Concealment of individual’s group allocation was preserved until individuals had committed to the study. The nature of the intervention rendered it impossible for participants to be blind to allocation. Although it was difficult for the RW to be blind to the allocation of participants because of their involvement in patient recruitment and assessments, several strategies were implemented to minimise unblinding of the RW. The efficacy of blinding was assessed at each time point using a three-point Likert-type scale polarised by the two arms and including a ‘not sure’ level as the centre rating. Telephone assessments were used when possible to reduce the likelihood of the RW observing treatment. For the statistical analysis of participants’ data, the statistician, the RW and principal investigator remained blind to the study group allocation of the participants.
Analytic methods
An analysis plan was agreed and locked before analysis began. The randomisation code was not revealed until analysis had been completed. Descriptive statistics for the baseline demographic and the outcome data are presented for each group. Categorical data are presented as numbers and percentages, and continuous data as means, SDs and ranges.
Continuous outcomes were compared using analysis of covariance (ANCOVA), which included the relevant baseline scores.
Participants were categorised into two groups: those who had a clinically significant reduction in BPSD (NPI score) of three points or more, and those who did not. We used binary logistic regression to analyse the difference in the proportions of those who had a clinically significant reduction in NPI score between the TAU and intervention groups at 12 weeks.
All analyses were undertaken using the statistics package SPSS™ version 20 [Statistical Package for the Social Sciences (SPSS), IBM Ltd., Armonk, NY, USA: www-01.ibm.com/software/uk/analytics/spss/] before the breaking of the randomisation key and the analyses were carried out blindly by the RW.
Economic analysis
Resource use and cost measures
Data on utilisation of care and support services were captured through the CSRI,88 completed with the assistance of the RW. The CSRI was completed twice (baseline and 12 weeks), on each occasion asking about service use retrospectively over the previous 3 months. Data were collected on all HSC services, equipment and adaptations, medication and unpaid carer inputs.
Unit costs used were drawn, where possible, from the Personal Social Services Research Unit (PSSRU) compendium for 201189 and reflect long-run marginal opportunity costs. The British National Formulary database was used to obtain medication costs. 90 Market sources were used to estimate costs for equipment and adaptations to homes when these were not available in the PSSRU compendium. Although most unit costs were found at 2011 prices, in other cases available figures were adjusted to the 2011 price level. When services would continue to provide a benefit for > 1 year, costs were annuitised using the HM Treasury recommended annual discount rate of 3.5%. 91 Unpaid care costs were estimated using an hourly rate equal to the National Minimum Wage, under the assumption that this was a potential opportunity cost for the unpaid carers.
Cost-effectiveness analyses
Cost-effectiveness analyses were conducted from two perspectives: HSC and societal. A HSC perspective considers those costs incurred by organisations providing HSC. A societal perspective considers costs incurred by all members of society collectively. In this instance, the difference between the two perspectives is the inclusion of informal (unpaid) carer costs in the societal perspective only. The main cost-effectiveness analyses from each perspective compared the exercise regimen and control on cost and mean difference in composite NPI score. Secondary cost-effectiveness analyses compared the exercise regimen and control on cost and each of the following outcomes in turn: the ZARIT caregiver burden inventory (ZBI), DEMQOL-Proxy, GHQ and a measure of quality-adjusted life-years (QALYs) generated from DEMQOL-Proxy scores. Scores on the outcome variables for which lower scores show better outcomes have been reversed in order to have a more intuitive interpretation for the economic analysis. For each cost-effectiveness analysis, there were four potential outcomes.
The exercise regimen is:
-
more effective (has superior outcomes) and less expensive than usual care
-
less effective and more expensive than usual care
-
both less expensive and less effective than usual care, or
-
both more expensive and more effective than usual care.
Strong dominance of one intervention over another is described in cases (i) and (ii), and the decision of whether or not to implement the new intervention is typically straightforward, although the potential presence of measurement error must be considered. For cases (iii) and (iv), the decision-maker must weigh up the differences in outcomes and costs before choosing whether or not to implement the exercise regimen. The value or weight attached to differences in outcomes will play a part in deciding whether or not to proceed with implementing the new intervention. In such cases, the typical approach would first involve the calculation of the incremental cost-effectiveness ratio (ICER), ICER = ΔC/ΔE, where ΔC is the mean cost difference between (in this case) the exercise regimen and control, and ΔE is the difference in mean outcome.
Each ICER was estimated with the Seemingly Unrelated Regression (SUR)92 model using Stata version 13 (StataCorp LP, College Station, TX, USA, http://stata.com/). Each cost and outcome measure, in turn, was regressed on treatment allocation, controlling, respectively, for cost and outcome at baseline. In sensitivity analysis, we controlled additionally for participant age at baseline, gender, ethnicity, marital status, education level, whether or not living in a care home, MMSE52 score at baseline, carer’s age and gender. Regression models were bootstrapped with 1000 replications in order to address possible data skewness. Multiple imputation (using 10 imputed data sets) was used to deal with missing values in some outcomes and covariates. 93
The formula net benefit (NB) = λ × ΔE – ΔC was used to calculate NBs, which, in addition to using mean cost and outcome differences as with ICER calculations, used a range of hypothetical values of willingness to pay (λ) for an additional unit on a given outcome measure. Cost-effectiveness acceptability curves (CEACs) were then plotted for the primary outcome (NPI) using the NB values calculated for each value of willingness to pay within the range of £0–10,000. This showed the probability of the exercise regimen being cost-effective over the values of willingness to pay considered.
The economic analysis was conducted using Stata version 13.
Protocol changes
No significant protocol changes have been made to this trial (see Appendix 8).
Ethical arrangements
This study was approved by Outer North East London REC (ref. 09/H0701/67), and nine minor amendments were requested and approved.
Findings
Description of the sample
Recruitment and follow-up began in 2010 and ended in 2012. One hundred and thirty-one participant dyads were randomised to receive either the exercise regimen in addition to TAU (intervention) or TAU only (control) (see Figure 7). Eighty-nine per cent of dyads completed the trial, with seven and eight dyads lost from the TAU group and the intervention groups, respectively. The sample (people with dementia and carers) was predominantly female (women 163: men 99). Participants with dementia were typically older than the carer participants (78.85 ± 7.1 years vs. 63 ± 16.2 years). Most of the participants with dementia were living at home and were being cared for by partners or adult children. Alzheimer’s disease was the most prevalent type of dementia (n = 82, 62.6%), and most participants had been diagnosed within the previous 2 years (n = 73, 56%). Report of a fall in the previous year was more common for people with dementia (80%) than for carers (34%). Quality of life for people with dementia at baseline as measured by the DEMQOL-Proxy was relatively good (101.3 ± 13.9). Behavioural and psychological symptoms as measured by the NPI [30.6 (SD 17.7), range 4–80] were similar to that reported in the original validation paper. 81 Participants were dichotomised according to validated thresholds for ‘caseness’ relating to psychological well-being (GHQ) (range 0–84, threshold ≥ 23)84 and for caregiver burden (ZBI) (range 0–28, threshold ≥ 24). 94 Thirty-six carers (27%) reached the validated threshold for ‘caseness’ relating to psychological well-being (GHQ)84 and 25 (19%) for caregiver burden (ZBI). 94 Heart rate and blood pressure readings were generally within the normal range in our sample. Table 25 provides the descriptive analysis.
| Participant | Control (N = 64) | Intervention (N = 67) | ||
|---|---|---|---|---|
| n | Mean ± SD (range)/frequency (percentage) | n | Mean ± SD (range)/frequency (percentage) | |
| Person with dementia | ||||
| Age, years | 64 | 78 ± 7.4 (58–99) | 67 | 79 ± 6.8 (64–97) |
| Gender: female | 64 | 39 (60.9) | 67 | 35 (52.2) |
| Ethnicity: white | 64 | 50 (78.1) | 67 | 56 (83.6) |
| Marital status: married | 63 | 45 (71.4) | 67 | 46 (68.6) |
| FRAT score: ≥ 2 | 64 | 36 (56.3) | 67 | 27 (40.3) |
| Living: private residence | 64 | 57 (89.1) | 67 | 59 (88.1) |
| Years’ education | 60 | 11.92 ± 5.9 (0–36) | 63 | 12.1 ± 4.1 (6–23) |
| MMSE | 64 | 14.9 ± 8.7 (0–29) | 67 | 16.3 ± 7.4 (0–30) |
| Alzheimer’s disease | 59 | 38 (64.4) | 65 | 44 (67.7) |
| Years since diagnosis: ≤ 2 years | 61 | 38 (64.4) | 59 | 35 (54.7) |
| Carer | ||||
| Age, years | 59 | 60.9 ± 17 (22–88) | 53 | 65.4 ± 14.9 (27–89) |
| Gender: female | 64 | 39 (60.9) | 67 | 50 (74.6) |
| FRAT score: = 0 | 64 | 46 (71.9) | 67 | 40 (59.7) |
| Relationship: ‘partner or spouse’ | 64 | 35 (54.7) | 67 | 42 (62.7) |
| Carer distress (NPI) | 64 | 11.9 ± 8.1 (0–39) | 67 | 11.8 ± 8.9 (0–38) |
Exercise uptake
Self-reported walking time (diary) appeared to differ between the groups. The TAU group increased walking by just over 2 minutes at week 6 in comparison with week 1, but by week 12 their reported walking time had decreased by almost eight minutes in comparison with week 6. Participants in receipt of the intervention reported increasing their walking time by 6 minutes at week 6 and retained this change at week 12.
Analysis of primary outcomes
Adjusting for baseline NPI scores, there was no significant difference in NPI score at 12 weeks (β = –0.41; p = 0.6, 95% CI –7.37 to 4.32), where ‘β’ represents the difference in mean NPI scores (intervention minus TAU groups) at week 12. In addition, there was no significant difference in the proportions of participants reaching a clinically significant reduction of three or more points of NPI score at week 12 compared with baseline between the TAU and intervention groups (OR = 1.41; p = 0.36, 95% CI 0.67 to 3.01) (Table 26).
| Control frequency (%) | Intervention frequency (%) | ORa | 95% CI | p-value |
|---|---|---|---|---|
| 33/57 (57.9%) | 39/59 (66.1%) | 1.41 | 0.67 to 3.01 | 0.36 |
| Mean ± SD | Mean ± SD | βb | ||
| 25.6 ± 16.6 | 23.9 ± 20.6 | –1.53 | –7.37 to 4.32 | 0.60 |
Analysis of secondary outcomes
Caregiver’s burden as measured by the ZBI doubled by week 12 for the TAU group participants but decreased from 23% to 17% for those in the intervention group (OR = 0.18; p = 0.01; 95% CI 0.05 to 0.69). There were no statistically significant differences between the groups at week 12 regarding carers’ mental health (GHQ), carers’ distress (NPI) and quality of life of participants with dementia (DEMQOL-Proxy) (Table 27).
| Outcome | Week | Control frequency (%) | Intervention frequency (%) | ORa (95% CI) | p-value |
|---|---|---|---|---|---|
| NPI | Baseline | 64 | 67 | ||
| 6 | 32/56 (57.1) | 39/62 (62.9) | 1.27a (0.61 to 2.66) | 0.52 | |
| GHQ | Baseline | 46/63 (73) | 48/67(71.6) | ||
| 6 | 24/57 (42.1) | 16/62 (25.8) | 0.42b (0.18 to 1.00) | 0.05 | |
| 12 | 24/56 (43) | 17/55 (31) | 0.59b (0.24 to 1.43) | 0.19 | |
| ZBI | Baseline | 52/62 (83.9) | 50/65 (76.9) | ||
| 6 | 14/55 (25.5) | 14/59 (23.7) | 0.48b (0.14 to 1.67 | 0.25 | |
| 12 | 18/56 (32) | 10/57 (17.5) | 0.18b (0.05 to 0.69) | 0.01 | |
| Mean ± SD | Mean ± SD | β c | |||
| NPI | 6 | 26.6 ± 17.5 | 25.7 ± 20.5 | –0.81 (–6.08 to 4.45) | 0.76 |
| DEMQOL-Proxy | 6 | 101.1 ± 14.9 | 103.6 ± 11.9 | 1.27 (–2.33 to 4.86) | 0.49 |
| 12 | 101 ± 13.5 | 104 ± 10 | 2.62 (–0.78 to 6.02) | 0.09 | |
| NPI carer distress | 6 | 11.07 ± 7.2 | 11.5 ± 8.5 | –0.06 (–2.25 to 2.14) | 0.96 |
| 12 | 9.98 ± 5.9 | 10.9 ± 9.3 | 1.14 (–1.31 to 3.58) | 0.76 | |
Follow-up at week 26
No major changes were reported at the 26-week follow-up. Four individuals had moved from their family homes to care homes. Three of those individuals were in the control group and one in the intervention group (OR = 0.38; p = 0.42; 95% CI 0.04 to 3.85).
Three deaths were reported at week 26: two from the control group and one from the intervention group. This difference was not significant (OR = 1.76; p = 0.55; 95% CI 0.28 to 11.07).
No significant difference was reported in receipt of antipsychotic medication (OR = 1.48; p = 0.68; 95% CI 0.23 to 9.52).
Qualitative data
Diaries were returned by 90 (69%) participant dyads: 52 (78%) from the intervention group and 38 (60%) from the TAU group. Participants in both groups reported positive and negative aspects of walking outside, and barriers that affect this activity. Weather was reported as the main factor that affected walks. Participants reported that during the walks they enjoyed the nature, socialisation, gaining a sense of achievement, feeling independent and feeling less agitated (for the person with dementia). Some of the disadvantages reported were overexertion, physical pain, feeling alone, difficult terrain and noise. Planned walks were cancelled because of unfavourable weather conditions, poor health, carer unavailability, PWD feeling agitated, and being busy indoors.
It was not possible to perform further detailed analyses nor draw conclusions from the diaries contents because of variation in completion.
Recruitment
With early recruitment low and an obvious incongruity between verbal support and limited promotion of the study, we invited clinicians (team managers, nurses, occupational therapists, physiotherapists and psychologists) to a focus group to discuss their perceptions of research, impediments to their role as recruiters, and potential solutions to the study’s recruitment difficulties.
The group described how clinicians are increasingly challenged by patients ‘informed about the latest research’. The group indicated they often had to provide counter evidence in response to ‘headline’, and sometimes ‘sensationalised’ research conclusions. Together with patients’ limited understanding of varying quality in research and the importance of clinical judgement for each individual, this could have (in the group’s view) an adverse impact on the patient–clinician relationship. When recruiting to research, the group felt responsible – as the source of the research invitation – for assuming a degree of accountability for the research project’s value and conduct (Box 3, quotation a). This responsibility was experienced as unreasonable, as they often had no part in the study’s design and administration, and so there was a risk that recruiting might be perceived as potentially unrewarding, or worse, detrimental to patients. Although the group perceived the value of research, they also identified several concerns about recruiting their patients to EVIDEM-E (see Box 3, quotes b–d). Understandably, recruitment was regarded as a low priority in consultations and all too easily ‘slipped off the radar’ (see Box 3, quote e1). However, the group did suggest ways to enhance recruitment to EVIDEM-E (see Box 3, quotes f–i). All three suggestions aimed to enhance the participants’ experience opposed to theirs as recruiters, and the first suggestion involved increasing clinician workload.
Recruitment to the trial improved when alternative mechanisms were tried, including recruitment from a registry of patients and carers held by North Thames DeNDRoN Dementia Register study (DemReg). Eight participants were recruited to the trial in the first 6 weeks of working with the DemReg registry.
What if they agree due to your relationship with them, when you’ve said this thing is going to be great . . . ?
b. Overburdening ‘vulnerable’ individuals with voluminous Participant Information Sheets/invitation
I was just wondering if you could take it (information pack) out and talk them (participants) through, rather than this big pack arriving. I’d be thinking ‘wow’ I thought I was just going out on a walk . . .
c. Anxiety over the patients’ feelings after researcher withdrawal
From our perspective they have participated in research . . . but they got nothing to say: thank you very much. I think that kind of puts people off.
d. Concern that participants would be dissatisfied when allocated to a control group
I’m thinking about when putting someone forward, they’ve agreed to go forward with it, only for them not to be put in the category where they actually get the intervention. I think this maybe holds people [professionals] back from encouraging people [clients].
We have to tell the client what is it they get out of this . . . what if they go on the control group and get nothing?
e. Low priority in individual clinical context
A lot of people just want practical advice and support . . . if someone is, let’s say, incontinent. What can I do, what can I put in place? It’s the practical solution to a problem that they have. And even if we say that we bring experience, knowledge and information that doesn’t really matter because there is something that is going on at that time that needs to be sorted.
I don’t see it as likely that patients that will be offered some sort of treatment will ask: ‘why are you saying this is the best treatment?’ and ask for a justification. Many of our patients would be grateful because what they are looking for is an outcome, they are not interested how we get there. They just want positive outcome.
f. Continuation of treatment: themselves to be trained to deliver the EVIDEM-E intervention
I think it would be really useful if we could do it (exercise intervention) ourselves and have a go. With proper training and under you observing it, I don’t see a reason why we couldn’t.
Would it be possible, not as part of the trial, simply a form of walking with somebody who has got some sort of behavioural problems, and we can try it out after being trained by your exercise therapist.
g. Feedback provided face to face to participants
You need to talk to people. The feedback is very important, whether it (the study) has success or not. At the end they (participants) should be given some feedback.
h. Provision of carer respite
There is a lot going on for carers at that particular time. And I think that whilst in their heart of hearts they might want to be involved and participate, but if the services are not going in to enable them to have a good night sleep, or maybe incontinence worry is a primary concern . . . A lot of times carers will actually say: I want a break. I want someone to physically do it (walking) for me. I’ve been up all night, they’ve been going to the toilet, they are restless, agitated, they ask repetitive questions. And it will be really nice that while the therapist takes them out the carer can put their feet up for 10, 15, 20 minutes. That’s the expectation of the carer, that someone will do that. That’ll be the motivation.
i. Tangible rewards
What’s in it for us?
Description of the economic analysis subsample
Complete CSRIs were received from 74 dyads at baseline and 67 dyads at the 12-week follow-up. However, depending on the outcome variable, the matching sample for the economic analysis varied from 49 to 52 dyads. Using multiple imputation techniques we were able to achieve a sample size of 52 dyads (22 control; 30 intervention). Hereafter, our findings on service use, costs and cost-effectiveness are based on the 52 dyads in this subsample, which is just less than half of the sample available for the main outcome analysis (116 dyads).
Table 28 presents descriptive statistics for the economic analysis subsample. We tested if the economic analysis subsample was significantly different from the original sample of 131 dyads by using the Wilcoxon rank-sum test for continuous variables and the Fisher’s exact test (or Pearson’s chi-squared test when appropriate) for binary variables. We found no significant differences between the ‘economic subsample’ and the full sample at 95% level of confidence, although a p-value of 0.07 was found for the difference in MMSE score at baseline. Using the same method we compared the intervention and control groups, and, again, did not find any significant baseline difference at 95%, with the smallest p-value (again equal to 0.07) being associated with the ‘primary education or less’ variable, implying that people included in the final economic analyses sample were perhaps less likely to have a limited education.
| Variable | Treatment group | p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Total | Control vs. intervention | Estimation sample vs. original sample | ||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||||
| Age, years | 22 | 78.4 | 9.07 | 30 | 78.6 | 7.6 | 52 | 78.5 | 8.2 | 0.66 | 0.91 | |
| Carer’s age, years | 19 | 62.2 | 17.11 | 25 | 63.6 | 16.4 | 44 | 63.0 | 16.5 | 0.88 | 0.96 | |
| MMSE | 22 | 17.5 | 8.17 | 30 | 13.6 | 7.4 | 52 | 15.3 | 7.9 | 0.07 | 0.58 | |
| NPI | 22 | 32.9 | 19.06 | 30 | 31.6 | 19.2 | 52 | 32.1 | 19.0 | 0.89 | 0.62 | |
| ZBI | 21 | 17.0 | 7.74 | 29 | 19.0 | 9.0 | 50 | 18.1 | 8.5 | 0.34 | 0.19 | |
| DEMQOL-Proxy | 22 | 100.7 | 16.28 | 30 | 103.6 | 12.5 | 52 | 102.4 | 14.2 | 0.82 | 0.29 | |
| Utility score (based on DEMQOL-Proxy) | 22 | 0.71 | 0.15 | 30 | 0.72 | 0.15 | 52 | 0.72 | 0.14 | 0.62 | 0.71 | |
| GHQ | 22 | 19.7 | 10.88 | 30 | 17.9 | 9.1 | 52 | 18.7 | 9.8 | 0.53 | 0.87 | |
| n | % | Cases | n | % | Cases | n | % | Cases | ||||
| Gender: female | 22 | 59.1 | 13 | 30 | 53.3 | 16 | 52 | 55.8 | 29 | 0.78 | 0.89 | |
| Carer’s gender: female | 22 | 68.2 | 15 | 30 | 66.7 | 20 | 52 | 67.3 | 35 | 1.00 | 0.90 | |
| Ethnicity: white | 22 | 81.8 | 18 | 30 | 90.0 | 27 | 52 | 86.5 | 45 | 0.44 | 0.18 | |
| Married or in a civil partnership | 22 | 68.2 | 15 | 30 | 73.3 | 22 | 52 | 71.2 | 37 | 0.76 | 0.62 | |
| Living alone | 22 | 31.8 | 7 | 30 | 26.7 | 8 | 52 | 28.8 | 15 | 0.76 | 0.66 | |
| Paid carer | 22 | 4.5 | 1 | 30 | 6.7 | 2 | 52 | 5.8 | 3 | 1.00 | 1.00 | |
| Living in a care home | 22 | 9.1 | 2 | 30 | 13.3 | 4 | 52 | 11.5 | 6 | 1.00 | 0.98 | |
| Primary education or less | 21 | 4.8 | 1 | 28 | 3.6 | 1 | 52 | 3.8 | 2 | 1.00 | 0.07 | |
| Further education | 21 | 9.5 | 2 | 28 | 17.9 | 5 | 52 | 13.5 | 7 | 0.68 | 0.18 | |
| Dementia severity | Mild | 22 | 50.0 | 11 | 28 | 28.6 | 8 | 50 | 38.0 | 19 | 0.15 | 0.67 |
| Moderate | 22 | 31.8 | 7 | 28 | 35.7 | 10 | 50 | 34.0 | 17 | 1.00 | 0.78 | |
| Marked | 22 | 18.2 | 4 | 28 | 35.7 | 10 | 50 | 28.0 | 14 | 0.22 | 0.86 | |
Average intervention cost per dyad
Total intervention cost was calculated by multiplying unit cost per visit (£60) or unit cost per telephone call (£10) by the number of contacts between the exercise professional and each dyad. Average intervention cost per dyad was £284 (range £190–320).
Cost analyses
In Table 29 we present service utilisation rates by people within the study by treatment group and overall. At baseline, there were no significant differences in use of services by treatment group. Overall, at baseline, 12% of people with dementia made use of accommodation services, 58% hospital services and 71% community services. Most sample members (85%) reported use of unpaid care.
| Variable | Treatment group: no. and percentage using services | Fisher’s exact test | |||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | Total | p-value | ||||
| Pre baseline (3 months) | |||||||
| Residential care/accommodation | 2 | 9 | 4 | 13 | 6 | 12 | 1.00 |
| Hospital services | 14 | 64 | 16 | 53 | 30 | 58 | 0.57 |
| Community services | 14 | 64 | 23 | 77 | 37 | 71 | 0.36 |
| Equipment and adaptations | 12 | 55 | 20 | 67 | 32 | 62 | 0.40 |
| Day services | 8 | 36 | 13 | 43 | 21 | 40 | 0.78 |
| Medications | 22 | 100 | 29 | 97 | 51 | 98 | 1.00 |
| Unpaid care | 19 | 86 | 24 | 80 | 43 | 83 | 0.72 |
| n | 22 | 100 | 30 | 100 | 52 | 100 | |
| Follow-up (1–3 months) | |||||||
| Residential care/accommodation | 1 | 5 | 3 | 10 | 4 | 8 | 0.63 |
| Hospital services | 16 | 73 | 14 | 47 | 30 | 58 | 0.09 |
| Community services | 12 | 55 | 21 | 70 | 33 | 63 | 0.38 |
| Equipment and adaptations | 12 | 55 | 14 | 47 | 26 | 50 | 0.78 |
| Day services | 10 | 45 | 12 | 40 | 22 | 42 | 0.78 |
| Medications | 22 | 100 | 29 | 97 | 51 | 98 | 1.00 |
| Unpaid care | 18 | 82 | 26 | 87 | 44 | 85 | 0.71 |
| n | 22 | 100 | 30 | 100 | 52 | 100 | |
At 12 weeks’ follow-up, overall proportions remained fairly similar. However, the two treatment groups seem to have separated in terms of use of hospital services: more than 70% of the control group reported using hospital or similar services compared with only 47% of the intervention group, although this difference is not significant (p = 0.09). For other service categories, as at baseline, the Fisher’s exact test did not show any significant differences.
Table 30 displays baseline and 12-month follow-up service use costs.
| Variable | Control (n = 22) | Intervention (n = 30) | Total (n = 52) | Bootstrapped test: adjusted p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | ||
| Pre-baseline costs (£) | |||||||||||||
| Accommodation | 951.7 | 3080.3 | 0 | 10,468 | 1300.9 | 3478.1 | 0 | 13,182 | 1153.2 | 3288.8 | 0 | 13,182 | 072 |
| Hospital services | 513.6 | 747.8 | 0 | 2566.0 | 577.5 | 1248.9 | 0 | 5217.0 | 550.4 | 1057.4 | 0 | 5217.0 | 0.83 |
| Community services | 575.5 | 1108.9 | 0 | 4355.3 | 682.4 | 1010.9 | 0 | 3061.6 | 637.2 | 1044.2 | 0 | 4355.3 | 0.72 |
| Equipment and adaptations | 68.2 | 135.7 | 0 | 502.5 | 112.0 | 158.6 | 0 | 459.1 | 93.4 | 149.5 | 0 | 502.5 | 0.30 |
| Day services | 270.2 | 550.0 | 0 | 2,055.6 | 259.6 | 492.5 | 0 | 1778.9 | 264.1 | 512.3 | 0 | 2055.6 | 0.94 |
| Medications | 275.7 | 194.3 | 3 | 882.8 | 272.8 | 177.0 | 0 | 691.3 | 274.0 | 182.7 | 0 | 882.8 | 0.96 |
| Total HSC | 2654.8 | 3756.8 | 315.7 | 16,121 | 3205.1 | 3595.1 | 129.4 | 13,747 | 2,972.3 | 3638.2 | 129.4 | 16,121 | 0.61 |
| Unpaid care | 6563.3 | 4953.9 | 0 | 15,366 | 7812.1 | 6273.3 | 0 | 24,870 | 7283.8 | 5733.3 | 0 | 24,870 | 0.42 |
| Total societal | 9218.2 | 5647.8 | 465.9 | 21,113 | 11,017 | 5719.5 | 691.3 | 25,145 | 10,256 | 5704.5 | 465.9 | 25,145 | 0.24 |
| 12-month follow-up costs (£) | |||||||||||||
| Accommodation | 632.7 | 2967.7 | 0 | 13,919 | 697.0 | 2361.4 | 0 | 10,468 | 669.8 | 2607.4 | 0 | 13,919 | 0.80 |
| Hospital services | 461.0 | 937.2 | 0 | 4425.7 | 146.7 | 255.9 | 0 | 898.0 | 279.7 | 650.7 | 0 | 4425.7 | 0.08 |
| Community services | 270.4 | 707.0 | 0 | 3229.0 | 390.5 | 782.2 | 0 | 3919.9 | 339.7 | 746.5 | 0 | 3919.9 | 0.65 |
| Equipment and adaptations | 103.0 | 189.7 | 0 | 710.4 | 89.0 | 160.3 | 0 | 641.5 | 94.9 | 171.7 | 0 | 710.4 | 0.25 |
| Day services | 270.5 | 519.5 | 0 | 1,937.0 | 229.1 | 476.8 | 0 | 1541.7 | 246.6 | 490.7 | 0 | 1937.0 | 0.78 |
| Medications | 246.3 | 169.4 | 6 | 672.8 | 285.2 | 172.9 | 0 | 783.7 | 268.7 | 170.9 | 0 | 783.7 | 0.04 |
| Total HSC | 1983.8 | 3080.5 | 85.7 | 14,528 | 1837.5 | 2511.8 | 118.4 | 11,367 | 1899.4 | 2738.7 | 85.7 | 14,528 | 0.41 |
| Intervention | 0 | 0 | 0 | 0 | 284.0 | 43.2 | 190.0 | 320.0 | 163.8 | 145.4 | 0 | 320.0 | – |
| Total HSC plus intervention | 1983.8 | 3080.5 | 85.7 | 14,528 | 2121.5 | 2509.7 | 417.5 | 11,627 | 2063.2 | 2737.5 | 85.7 | 14,528 | 0.76 |
| Unpaid care | 5820.7 | 6750.9 | 0 | 28,626 | 8411.6 | 5727.0 | 0 | 24,570 | 7315.5 | 6251.9 | 0 | 28,626 | 0.24 |
| Total societal plus intervention | 7804.5 | 6859.0 | 85.7 | 29,735 | 10,533 | 5890.7 | 532.5 | 29,271 | 9378.7 | 6399.7 | 85.7 | 29,735 | 0.31 |
The summary statistics suggest that a sizeable proportion of total HSC service costs is related to use of accommodation services, which, at baseline, resulted in a higher cost on average for the intervention group, although the difference was not significant (tested using an adjusted regression model with 1000 bootstrap replications). 93 Apart from accommodation, hospital and community services displayed the highest aggregate costs. Total HSC costs appeared to be higher for the intervention group (£3205) than the control group (£2655), although this difference was not significant. At baseline, the intervention group was found to have higher costs, on average, than the control group, even when considering the provision of unpaid care (£6500 vs. £7800), leading to an average societal cost of £9200 for the control group and £11,000 for the intervention group.
At follow-up, accommodation costs were more similar between the two groups than at baseline, whereas the average cost of hospital care appeared to be higher in the control group than the intervention group, although the difference was not significant. Interestingly, for both groups there was reduction in HSC service costs (£1984 vs. £1838), largely due to reduction in use of paid accommodation. The only adjusted difference found to be significant (p = 0.04) relates to the use of medications, which was higher for the intervention group.
Provision of unpaid care was greater, and hence more costly, in the intervention than the control group (£8400 vs. £5800). This difference may not be surprising given the nature of the intervention. Total societal cost, including intervention and provision of unpaid care, was £10,500 for the intervention group compared with £7800 for the control group, although after adjustment for baseline covariates this difference was not significant.
Cost-effectiveness analysis
In Table 31 we report the incremental costs and incremental effects for the primary and secondary outcomes.
| Perspective | Incremental cost (£, 2010–11): mean, 95% bootstrap CI | Incremental effect: mean, 95% bootstrap CI | ICER | ||||
|---|---|---|---|---|---|---|---|
| Mean | Upper CI | Lower CI | Mean | Upper CI | Lower CI | ||
| HSC: 0–12 weeks | |||||||
| NPI | –168.6 | –1232.8 | 895.6 | 4.07 | –4.65 | 12.79 | Intervention dominant |
| ZBI | –170.8 | –1234.6 | 893.1 | 1.54 | –1.78 | 4.86 | |
| DEMQOL-Proxy | –165.6 | –1251.7 | 920.6 | 2.87 | –1.94 | 7.68 | |
| QALY (DEMQOL-Proxy) | –169.7 | –1240.0 | 900.5 | 0.0055 | –0.0031 | 0.0140 | |
| GHQ | –173.6 | –1235.8 | 888.6 | 4.19 | –0.55 | 8.93 | |
| Societal: 0–12 weeks | |||||||
| NPI | 1686.4 | –1407.1 | 4780.0 | 4.01 | –4.72 | 12.73 | 421 |
| ZBI | 1641.1 | –1497.8 | 4780.0 | 1.56 | –1.75 | 4.86 | 1055 |
| DEMQOL-Proxy | 1635.9 | –1520.9 | 4792.6 | 2.82 | –1.97 | 7.61 | 580 |
| QALY (DEMQOL-Proxy) | 1565.8 | –1592.6 | 4724.2 | 0.0055 | –0.0031 | 0.0140 | 286,440 |
| GHQ | 1657.3 | –1471.8 | 4786.4 | 4.23 | –0.50 | 8.97 | 392 |
Incremental cost-effectiveness ratios would typically be calculated by dividing incremental costs by incremental benefits, but these have not been presented in Table 31 because neither incremental costs nor incremental effects were significantly different between trial arms. From a HSC perspective, we found that incremental costs were negative, that is, the intervention group had lower costs than the control group (about £170 less) and incremental effects were positive, that is, the intervention group achieved better outcomes. Although none of these differences were significant at the 95% level, for illustrative purposes we note that, from a societal perspective, the ICER for the NPI outcome measure was £421. Figures 8 and 9 show CEACs for the primary outcome measure (NPI) from a HSC and societal perspective, respectively.
FIGURE 8.
Cost-effectiveness acceptability curve: exercise regimen vs. usual care; HSC perspective, with effectiveness measured on the NPI scale.
FIGURE 9.
Cost-effectiveness acceptability curve: exercise regimen vs. usual care; societal perspective, with effectiveness measured on the NPI scale.
From a HSC perspective (see Figure 8) the CEAC suggests that, at a willingness to pay of £500 per incremental improvement in outcome (i.e. per 1-point difference in NPI score), the exercise regimen is cost-effective with probability of > 80%. From a societal perspective (see Figure 9), the ICER was £421 per incremental difference in NPI score. If a reduction of at least eight points in the NPI score can be considered clinically meaningful95 then this result suggests that the cost of achieving a meaningful improvement is £3368.
Table 32 presents results for a sensitivity analysis that, in addition to baseline costs and baseline outcomes, also controlled for participant age at baseline, gender, ethnicity, marital status, education level, whether living in a care home, MMSE score at baseline, carer age and gender. This analysis gave results that are consistent with the main analysis.
| Perspective | Incremental cost (£, 2010–11): mean, 95% bootstrap CI | Incremental effect: mean, 95% bootstrap CI | ICER | ||||
|---|---|---|---|---|---|---|---|
| Mean | Upper CI | Lower CI | Mean | Upper CI | Lower CI | ||
| HSC: 0–12 weeks | |||||||
| NPI | –159.6 | –1267.8 | 948.5 | 2.46 | –7.59 | 12.50 | Intervention dominant |
| ZBI | –155.9 | –1254.7 | 942.9 | 0.56 | –3.90 | 5.02 | |
| DEMQOL-Proxy | –156.5 | –1256.3 | 943.3 | 2.55 | –3.32 | 8.41 | |
| QALY (DEMQOL-Proxy) | –156.7 | –1257.4 | 944.1 | 0.0066 | –0.0026 | 0.0157 | |
| GHQ | –155.5 | –1250.6 | 939.5 | 4.00 | –1.92 | 9.91 | |
| Societal: 0–12 weeks | |||||||
| NPI | 1,018.8 | –2331.0 | 4368.7 | 2.42 | –7.54 | 12.39 | 421 |
| ZBI | 992.5 | –2384.6 | 4369.6 | 0.58 | –3.86 | 5.02 | 1,711 |
| DEMQOL-Proxy | 978.1 | –2403.7 | 4359.9 | 2.46 | –3.40 | 8.33 | 397 |
| QALY (DEMQOL-Proxy) | 954.0 | –2444.5 | 4352.4 | 0.0065 | –0.0025 | 0.0155 | 146,437 |
| GHQ | 1,004.8 | –2349.2 | 4358.8 | 4.07 | –1.79 | 9.93 | 247 |
Discussion
Summary of the main findings
The study comprised three inter-related components: a literature review; a RCT; and some qualitative exploration of experiences of the conduct and participation in the trial. In the literature review we described the limited, but promising, evidence to support the use of exercise as a therapy for BPSD. The main shortcoming in the reported literature was the limitations of the research methods in the existing evidence base, something we sought to address with our RCT. The findings from the literature review were used to design a methodologically robust trial of an exercise programme for the purpose of ameliorating BPSD. Our main finding was that the exercise programme did not produce a significant clinical benefit for BPSD in this population. Importantly, however, we did find that caregiver burden was significantly better at the end of the trial for those in receipt of the exercise programme. Finally, significant – and unexpected – challenges of recruiting to the trial via clinicians were explored in a focus group. Clinicians reported significant anxieties recruiting to our relatively ‘low-risk’ trial that were most readily overcome through the use of a well-defined systematic recruitment and feasibility tool.
Towards a research agenda: from the literature to a randomised controlled trial
Behaviours that challenge caregivers – particularly agitation, psychosis and sleep disturbance – are often central to predicting institutionalisation, probably because of the reported negative impact for caregivers. 96–98 Research that identifies optimal physical activity modalities, frequency, intensity and duration for persons with different types and severity of dementia has been lacking and will be important to inform commissioners of the value of exercise programmes in this population.
Appropriateness, safety and motivation to participate are critical to the success of exercise programmes, and may be particularly complex for older adults with dementia. For example, adherence may be influenced by the nature of dementia itself. Particular symptoms, such as apathy, may have a proportionally greater impact (relative to other BPSD) on adherence, which could be further impacted upon by context76 (e.g. paid carers often view apathy differently to unpaid carers).
Exercise appears to be beneficial in reducing some BPSD, especially depressed mood and agitation, and may also improve sleep and reduce ‘wandering’. However, research into the efficacy of exercise with the therapeutic aim of improving other important symptoms, such as anxiety, apathy and repetitive behaviours, was weak. Although some studies suggest that walking for at least 30 minutes, several times per week, may enhance outcomes, the beneficial effect of exercise type, its duration and frequency are unclear. Indeed, studies examining the effect of daily exercise had more favourable outcomes.
Results from the trial
Although overall BPSD were lower at week 12 in the group that received the exercise in comparison with the TAU group, this difference was not statistically significant. This is not consistent with the meta-analysis reported by Heyn et al. 76 There are three possible explanations for this: first, exercise is not a clinically effective therapy for BPSD; second, our exercise regimen has not had sufficient intensity to effect an impact on the participants; or, third, the effect size is smaller than we anticipated.
Importantly, our secondary analyses revealed a significant benefit for carers by attenuating burden across the trial duration. This is important as caregiver burden is strongly linked to use of health, social and medical services by both the person with dementia and their caregiver. 65–68 Given the absence of impact on behavioural and psychological symptoms, it is unclear why caregivers from the TAU group reported having a significant worsening of burden in comparison with those in receipt of the intervention. The increase may have been due to worsening burden because of BPSD, differential physical burden between the two groups (there was a slightly higher number of physical conditions in the TAU group at baseline) or unhappiness among carers about not being randomised to the intervention. Conversely, participation in exercise in the intervention group may have attenuated the perception of burden.
The economic evaluation looked at costs and cost-effectiveness. At 12 weeks, average costs from a societal perspective for the group following the exercise regimen were non-significantly higher than for the control group getting TAU. From a HSC system perspective, costs for the exercise group were non-significantly lower.
When considering cost-effectiveness for each outcome measure there were no significantly different incremental costs or incremental effects. The sensitivity analysis conducted (adjusting for baseline sociodemographic variables) generated findings that were consistent with the main analyses.
Recruitment to clinical trials
Researchers aim to work to high professional standards of GCP and require all study participants to be consenting volunteers, who are aware of their right of refusal without fear of detriment. When people lack capacity, ethics committees require rigorous protocols to safeguard participants’ wishes and welfare. We believe that all people, whether they have dementia or not, should have the right to participate in research. We recognise that people take part for many reasons, including altruism and a sense of purpose. Within a society that often patronises and demeans older people, many clinicians and researchers are passionate advocates of enlarging patient choice. In our experience people who are not interested in participating in research have a range of direct and indirect ways of showing this. Despite all of these safeguards, clinicians appear apprehensive about recruiting even for relatively low-risk studies like EVIDEM-E. Therefore, we support recent calls to quantify clinicians’ impact on recruitment and test ways of improving accruals. 99 We also agree more is needed to understand the experience of participating as a ‘control’. The response from the teams was positive, but actual recruitment was much slower than anticipated. Six participants were recruited to the trial through memory clinics and community mental health teams over a 6-month period from a patient population exceeding 2000 people with dementia.
There are several ways in which researchers can incentivise clinicians’ involvement in research. The focus group indicated their involvement should bring tangible reward for individual clinicians and participants. It was not enough, in the group’s view, that NHS trusts financially gain through involvement with research – how these resources enhance frontline clinical services should be made explicit. Encouraging a sense of ownership is a well-established tool for engendering investment, and we attempted this by including clinicians on our advisory groups. However, it is likely that a more widespread and involved approach is needed to improve practitioner engagement. Consulting clinicians on dissemination strategies might help them see the pathway from project to practice more clearly. Finally, developing flexible quick guides to recruitment may instil confidence when discussing individual projects with patients.
The approaches suggested by the focus group would all maintain clinicians at the centre of the recruitment process. An alternative could be direct contact between researchers and patients, which could potentially resolve many recruitment challenges for clinician and researcher while improving patient choice. In collaboration with the North Thames DeNDRoN, we helped to develop a registry of people with dementia who were interested in research (‘DemReg’),100 which appears to be an efficient recruitment mechanism. This registry is discussed in detail in Chapter 6 of this report.
Strengths of the trial design
The process of designing the study was challenging to the research team. It saw the project team – supported by an independent steering group – attempt to strike a balance between achieving a feasible and sustainable intervention that could be widely adopted in the target population while maintaining scientific rigour in its evaluation.
Strengths of this study include the relatively large sample size, with low attrition rates; use of well-validated outcome measures; a pragmatic exercise programme that does not require specialist training or equipment; and efforts to control for the impact of therapist contact.
The trial design addressed other important factors: a more homogeneous sample in terms of diagnosis, severity of disease and mobility; a sample size with sufficient power to detect an effect (positive or negative) of clinically significant magnitude; and blinded and objective outcome ratings.
Limitations of the study
Careful consideration was given to blinding the researcher to the allocation status of the participants. The single-blind design left study personnel vulnerable to unmasking group allocation during data collection with participants. To mitigate this impact we decided to include a second, independent, researcher who collected our primary outcome data (NPI) at 6 and 12 weeks. However, a risk remained that the dyad would divulge information about the group to which they have been allocated. The efficacy of maintenance of blinding was assessed at each time point, by personnel collecting data who recorded the arm to which they believed each dyad was randomised.
Separating the effect of psychosocial contact (from the exercise therapist) from the exercise intervention has proved to be the biggest challenge in our study. We addressed this problem by introducing two periods during the intervention: 1–6 weeks, whereby the participants in the intervention group were trained and supported by an exercise therapist, and 6–12 weeks, when participants were encouraged to continue exercising but had no contact with the therapist. Although we tried to control for contacts between participants and the exercise therapist, we could not control for the social contact between the person with dementia and their carer. Dyadic walking may have encouraged not only physical activity, but also psychosocial support from the carer as well.
We used outcome measures that are well validated and widely used in research with individuals with dementia. We also gave considerable thought to the issue of measuring physical activity. Particular consideration was given to the use of equipment by the participants to record their individual activities, for example GPS (Global Positioning System) receivers and pedometers. However, we decided not to use such instruments in order to avoid excessive intrusion, and to prevent the instruments affecting exercise behaviour in their own right. Similarly, participants’ levels of physical activity was evaluated through measurements of the participants’ self-reported RPE. The intervention delivered by the therapist centred on monitoring and increasing activity intensity utilising the RPE, and so could be provided only to the exercise group.
Assessing physical activity with minimum intrusion to study participants is challenging and we relied on participants’ self-reports through daily dairies. Self-report is prone to information bias, especially when participants are not blinded to their group allocation.
The economic evaluation adopted a HSC perspective for some analyses and a societal perspective for others to ensure that unpaid carer inputs were not overlooked. A range of outcome measures was examined, including QALYs generated from a dementia-specific measure. A limitation of the economic analyses was the sample size, which was 50% lower than the sample for the main outcomes analysis because service-use data were not collected. This loss of statistical power limits the conclusions that can be drawn. Another possible limitation is that only 85% of study participants provided information about support received from unpaid carers, yet we know that all sample members had a carer. It is difficult to estimate accurately the amount of time an unpaid carer spends with someone with dementia.
Conclusions
There are two key implications that we can draw from this study. First, recruitment of people with dementia via clinical sources is resource intensive and research registers can significantly increase the efficiency of this process. This is critical to ensure that large clinical trials are feasible. Second, regular walking is not effective at reducing BPSD but does seem to attenuate rising caregiver burden. We cannot be sure whether this was because of the exercise per se, increased psychosocial effect between carer and person with dementia or a placebo effect. Neither incremental costs nor incremental effects (for any outcome measure considered) were significantly different between the intervention and control groups, which suggests that the exercise regimen is unlikely to be cost-effective. Further research should seek to confirm our findings and clarify the active component.
Building research capacity
The exercise therapist (James Lee) used data from this study towards his undergraduate degree; he received the Bob Finney Memorial prize for this work and graduated with first-class honours. The RW (Arlinda Cerga-Pashoja) has registered for a Doctor of Philosophy (PhD) and passed her MPhil–PhD viva in 2011. In addition, David Lowery worked in a postdoctoral capacity and contributed to an expert therapy development group of another National Institute for Health Research (NIHR)-funded research programme (WHELD) on behalf of the EVIDEM group. The study team members have also contributed in delivering educational programmes to the CNWL staff through summer schools.
Voluntary RWs have given an invaluable input in the EVIDEM-E study. Nine volunteers helped with data entering, designing databases and collecting outcomes. They gained research experience and had the opportunity to be trained in GCP.
Changes to protocol
No changes from the original protocol were made.
List of appendices
Appendix 8 Chapter 2: Research protocol EVIDEM-E study.
Appendix 9 Chapter 2: Consenting protocol – standard operating procedures.
Appendix 10 Chapter 2: Standard operating procedure for monitoring of adverse events.
Appendix 11 Chapter 2: Risk assessment and management tool.
Appendix 12 Chapter 2: Serious adverse event reporting form.
Appendix 13 Chapter 2: Risk management pathway.
Appendix 14 Chapter 2: Diary – intervention group, example page.
Appendix 15 Chapter 2: Diary – control group, example page.
Appendix 16 Chapter 2: Intervention protocol.
Chapter 3 EVIDEM-C: promoting continence and managing incontinence with people with dementia living at home
Abstract
People with dementia experience persistent problems with toileting and incontinence, which are difficult to manage, problematic for carers and one factor in the decision to move to a care home. There is little clinical guidance for primary care professionals tailored to this population. This series of studies, shaped by MRC framework101 for the development of complex interventions, investigated (1) the incidence of the problems for people with dementia living in their own homes; (2) the published evidence for management; (3) the experience; and (4) the strategies and issues faced by people with dementia, their carers and the professionals trying to support them, as well as the feasibility of testing different designs of continence pads and tools to aid primary care nurses in tailoring their advice, management and support.
The study has added new evidence about the incidence, experience and management of toileting difficulties (TDs) and incontinence for this population. The reasons for the toileting and incontinence problems are multifactorial, and a mosaic of strategies is required to both reveal the problems and manage them in ways that are acceptable to the person with dementia and family members. This study suggests that there are strategies and responses that primary care professionals and others can utilise to encourage greater openness, thereby lessening the taboo of incontinence within the stigma of dementia. It remains to be seen if these approaches, combined with more emphasis of effective containment of excreta, will influence decisions about relocation of people with dementia to care homes.
Introduction
Dementia is one of the most disabling, distressing and burdensome diseases. Supporting people with dementia and their family carers to live in their own homes is a major HSC policy objective in the UK102,103 and in other countries. 104–106 There are many dimensions to the problems faced by people with dementia and their family carers as the dementia progresses. 107,108 Many are well understood and the strategies required from HSC services are recognised. 102,103 However, toileting problems and incontinence in people with dementia are problems which are poorly understood and for which there is little guidance for HSC professionals working in community settings.
Background: the overall need for the EVIDEM-C study
Family carers have reported incontinence as the most problematic symptom to manage. 109 Incontinence contributes significantly to both family and carer burden associated with supporting a person with dementia,110 and also to the decision to seek residence in a group or care home. 111
Dementia is a clinical syndrome with a trajectory of progressive loss in cognition and abilities in undertaking ADL, including personal toileting and physical functioning. 112 BPSD113 can manifest as inappropriate voiding and toileting behaviours. 114 Extrinsic factors, such as attitudes of ‘therapeutic nihilism’ (i.e. attitudes of nothing can be done to help) by professionals and unadapted environments compound these impairments. 115
The high prevalence of incontinence in people with dementia who are resident in care homes is well documented,116 as are the research and guidance for managing the problems for people living in these settings. 117 However, international estimates suggest over two-thirds of all people with dementia live in their own homes. 118,119 In reviewing clinical and social care guidance for best practice in managing toileting and incontinence problems in people with dementia living at home, and supporting their family carers, it became evident that there was a significant knowledge deficit.
There are internationally agreed definitions of types of urinary incontinence (UI) and faecal incontinence (FI). 120 ‘Functional incontinence’ is a term sometimes used when factors outside the lower urinary tract (such as environmental features or cognitive impairment) result in incontinence. Although internationally agreed algorithms for assessment and treatment of UI and FI inform national clinical guidance, the guidance on incontinence management either pointed to the evidence gaps for frail older people121 or excluded people with cognitive impairment. 122 Available guidance on primary care management of dementia did not refer to incontinence management. 123 The NICE and SCIE guidelines on supporting people with dementia and their carers did not review evidence on incontinence management, offering the view that combined interventions are more likely to support and maintain independence in the person with dementia. 124
Against this background EVIDEM-C (2007–12) investigated the promotion of continence and management of UI and FI in people with dementia living in their own homes. The specific research questions it addressed were:
-
What is the prevalence of different types of incontinence problems experienced by people with dementia, living at home?
-
What is the evidence for different strategies and interventions in managing incontinence in people with dementia living at home?
-
What are the perceived factors that support or detract from the use of different strategies and interventions from the perspective of informal carers, people with dementia, generalist and specialist health-care staff, social workers and social care?
-
What are the experiences, strategies, impact and cost consequences of managing incontinence problems in people with dementia living at home, and how do they change over time?
-
What are the feasibility and acceptability of developing specific interventions to promote continence and manage incontinence with people with dementia and their carers at home?
In addition, the study aimed to develop educational materials for those working in community and primary care settings to assist in the promotion of continence and management of incontinence in people with dementia living at home.
The research approach and design
The research approach was one of critical realism125 which allows an integration of both subjective and objective research approaches. In taking this approach, the multifaceted nature of the issues and problems in promoting continence and managing incontinence in the home, as well as the perspectives of multiple and diverse stakeholders, was examined in the light of the evidence of effectiveness. This research approach allowed the study to acknowledge the complexity of the issues. The EVIDEM-C study drew on the MRC Framework101 for developing complex interventions in stepwise phases. The study had four interlinked phases:
-
Reviewing the evidence about prevalence and effective interventions (see Phase 1: reviewing the evidence for incidence, prevalence and effective strategies, below)
-
Exploring the experiences and strategies used by people with dementia and their carers to manage incontinence, and the impact and consequences of that incontinence (see Phase 2: exploring the experience, strategies, impact and consequences, below)
-
Testing the feasibility of an identified intervention (see Phase 3: investigating the feasibility, effectiveness and acceptability of an identified strategy, below)
-
Developing educational resources (see Phase 4: developing and testing resources for practice, below).
The overall protocol can be found in Appendices 17–23 and research instruments and tools are provided in Appendices 26–34. The study was supported by an advisory group, which brought different types of expertise (carers, professionals – primary care, dementia specialists and continence specialists) to inform and help shape the study. It was chaired by a former carer of a person with dementia. Patient and public involvement representatives were present at every meeting, and performed co-production roles in shaping the research activities, such as agreeing the intervention to be tested in Phase 3, reviewing research materials, such as interview topic guides, and assisting in recruitment of participants.
Phase 1: reviewing the evidence for incidence, prevalence and effective strategies
In this phase, three reviews were undertaken and a nested study of a secondary data analysis of a primary care database was carried out to establish the prevalence and incidence of incontinence in people with dementia. Each is presented below.
A systematic review of the evidence about prevalence
Although the presence of incontinence symptoms is well documented in people resident in care homes,116 there is currently little evidence available for clinicians, commissioners and service planners concerning the scale of the problems in the larger population of people with dementia living at home.
Methods
Aims and objectives
To identify the prevalence of UI and FI experienced by people with dementia, living at home.
Study design: a systematic literature review
Search procedure
We searched the following electronic databases: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, British Nursing Index (BNI) and The Cochrane Library [including Database of Abstracts of Reviews of Effects (DARE) and National Technical Information Service (NTIS)], from 1 January 1990 to 1 September 2008, and then searches were updated to 2012 week 13 (4 April). In addition, ‘lateral searching’ techniques124 were used for key authors and cited references. Search terms are given in Table 33.
| Area | Search terms (Medical Subject Headings and keywords) |
|---|---|
| Population characteristics | exp Fecal Incontinence/or exp Urinary Incontinence/ exp Delirium, Dementia, Amnestic, Cognitive Disorders/or exp Dementia/ dementia.mp. exp Aged |
| Setting | Community dwelling.mp Community.mp |
| Research field of enquiry | Exp prevalence Prevalence.mp. Exp Needs assessment |
Screening as per the inclusion and exclusion criteria (Table 34) and data extraction were undertaken by two researchers. Judgements of risk of bias in the studies127 used criteria recommended for prevalence studies. 128 A narrative analysis was undertaken because of the heterogeneity in the included studies.
| Criteria | |
|---|---|
| Inclusion | Community-based observational studies Data on UI and/or FI in people with cognitive impairment or dementia Resident in their own homes English language |
| Exclusion | Studies not reporting empirical data of UI and/or FI Settings of hospital, nursing homes, cares homes, group homes Excluded people with dementia or cognitive impairment Data from the population of interest not identifiable in the results Published in languages other than English |
Results
The PRISMA33 flow diagram in Figure 10 reports the results of the search. From 427 references, eight studies129–136 met the inclusion criteria and are summarised in Table 35. No studies reported incidence. Seven studies provided prevalence rates as findings that were incidental to their primary question. Study populations, size of sample and assessment tools were varied, including the Care Needs Assessment Pack for Dementia (CareNap-D),138 Cognitive Performance Score (CPS),139 the ICD-10,80 the UK Office of Population Censuses and Surveys (OPCS) Disability Survey 1985140 and the MMSE. 52 Assessment of risk of bias found that all studies had some aspect that was judged to be a risk; this has been reported in full elsewhere. 137 The reported prevalence rates are presented in Table 36. The prevalence of UI ranged from 1.1% in a general community population to 38% in those receiving home care services. Prevalence of FI ranged from 0.9% in a community population to 27% in a sample attending an old age psychiatry outpatient clinic.
FIGURE 10.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of search results for prevalence studies.
| Study author, year, location | Population | No. with cognitive impairment or dementia and method of assessment | Method of assessment of UI and FI |
|---|---|---|---|
| Chung,129 2006, Hong Kong | Convenience sample recruited from the Alzheimer’s Disease Association, memory clinics and outpatient clinics n = 197 Mean age 77 years; 64% female |
197 with a confirmed diagnosis of dementia or Alzheimer’s disease | UI/FI determined by self and carer report to researcher using an amended CareNap-D138 |
| Landi et al.,130 2003, Italy | Patients enrolled in home health-care programmes and Silver Network Home Care Project n = 5372 Mean age 78.6 (SD 9.5) years; 59% female |
In text, number not given but states 30% with moderate to severe cognitive impairment, determined by CPS139 (CPS > 2) | UI determined by a single self-report question with five-point scale as part of the enrolment assessment to the home care service completed by a health professional (GP, geriatrician, nurses) |
| Meaney et al.,131 2005, Ireland | Patients, consecutively referred, attending an old age psychiatry outpatient clinic, meeting ICD-10 criteria and living in the community n = 82 Mean age 76 (SD 7.8) years; 55 = female, 27 = male |
82 with ICD-1080 diagnosis of dementia | UI/FI determined by self and carer report to project nurse using (CareNap-D)138 |
| Mohide et al.,132 1988, Canada | Patients receiving home care services aged > 16 years n = 2801 Mean age not given; number by sex not given |
Number not given Method of determining cognitive disabilities not given |
UI determined by study developed continence assessment form completed by health professional (unspecified) |
| Nakanishi et al.,133 1997, Japan | A randomly selected (unspecified) sample of community residing people aged > 65 years from a computerised sex–age register in one city n = 1405 Mean age not given; number by sex not given |
Number not given Dementia determined by intellectual functioning subscale of OPCS Disability Survey 1985140 |
UI and FI determined by self-report using OPCS Disability Survey 1985140 to welfare commissioners |
| Ouslander et al.,134 1990, USA | Community-residing patients with a dementia diagnosis attending a community facility whose family carers volunteered for ‘help with stress and burden’ n = 184 Mean age: incontinent 76.28 (SD 8.10) years; continent 75.84 (SD 7.73) years Number by sex not given |
A total of 184 with a clinical diagnosis of dementia | UI determined by report of carer to unspecified researcher Part of study developed memory and behaviour checklist |
| Rait et al.,135 2005, UK | General practice registered patients, aged > 75 years, approached as part of a RCT of the methods of assessment of older people; computer randomised to universal or targeted arm n = 15,051 (subjects in universal arm) of whom 14,621 completed MMSE52 47% aged 75–79 years, 61.5% female |
n = 2682 with cognitive impairment determined by MMSE52 < 23/24 | UI once a week or more often single question by self-report to research nurse |
| Teri et al.,136 1989, USA | Patients with diagnosis of an Alzheimer’s disease-type dementia attending a geriatric clinic selected (from case notes) as meeting criteria of diagnosis (aged between 55 and 85 years and community residing) for the study n = 56 Mean age 71 (range 55–85) years 43% female, 57% male |
56 with a clinical diagnosis of Alzheimer’s disease-type dementia | UI and FI determined by report by caregivers to trained interviewer; no tool specified |
| Study | Reported UI and FI rates | ||||
|---|---|---|---|---|---|
| UI | FI | ||||
| Unspecified frequency | ≥ 1 per day | ≥ 1 per week | Night only | ||
| Chung 2006129 | – | – | – | 21% of 197 | 18% of 197 |
| Landi et al. 2003130 | 38% of 5372 Relative risk of UI in those with a CPS 2–4 compared with CPS 0–1: 2.03 (95% CI 1.88 to 2.18), and in those with a CPS > 4 compared with CPS 0–1: 2.97 (95% CI 2.78 to 3.18) |
– – |
– – |
– – |
– – |
| Meaney et al. 2005131 | 34% of 82 | – | – | 34% of 82 | 27% of 82 |
| Mohide et al. 1988132 | 10% of 2801 | – | – | – | – |
| Nakanishi et al. 1997133 | 1.1% of 1405 | – | – | – | 0.9% of 1405 |
| Ouslander et al. 1990134 | – | 24% of 184 | 11% of 184 | – | – |
| Rait et al. 2005135 | – – |
– – |
8.8% of 2465 Relative risk of UI in people with MMSE score < 24 compared with MMSE score ≥ 24: 2.03 (95% CI 1.73 to 2.36) |
– – |
– – |
| Teri et al. 1989136 | – | – | 11% of 56 | – | 7% of 56 |
Discussion
This review found no studies reporting incidence and a wide variation in reported prevalence rates of UI and/or FI in people with dementia living in their own homes. This is explained, in part, by the variety of populations and assessment tools, and the lack of uniformity in criteria for defining incontinence and dementia. These methodological challenges in assessing prevalence rates have been well documented for both incontinence116 and dementia,141 including the issues of underreporting through embarrassment and stigma. Consequently, nearly all of the studies may have underestimated prevalence. The rates were incidental findings in nearly all studies and the one study designed to address the prevalence question of interest had a small population, identified through carers who identified themselves as experiencing high levels of stress. 134
To our knowledge, this is the first review addressing this question of prevalence. The greatest weakness of the study is the search strategy, which may have overlooked other studies with incidental prevalence findings in studies, and the exclusion of studies not reported in the English language. The study is reported in more detail elsewhere. 137
Conclusion
There is currently no definitive evidence of incidence or prevalence of UI or FI in people with dementia living at home, and further research is required to establish population-level data.
A cohort study of the incidence and management of incontinence in primary care
The absence of robust data on prevalence or incidence of incontinence in people with dementia living in the community led the research team to undertake a nested study investigating these questions and management strategies in primary care.
Methods
Aims and objectives
To investigate the incidence of UI and FI in community-dwelling people aged 60–89 years with dementia, and management strategies in primary care.
Study design
A cohort study of patient data from The Health Improvement Network (THIN), a UK general practice patient database. 142
Procedures
We compared two cohorts: all adults with dementia aged 60–89 years with at least 6 months’ data from 1 January 2001 to 31 December 2010, and a stratified (by age and gender) random sample of four times as many adults without dementia. IRRs, adjusted for potential confounders (age, gender and comorbidity), were calculated using multilevel Poisson regression.
Ethical arrangements
The NHS South-East Multicentre REC approved the scheme for THIN to obtain and provide anonymous patient data to researchers, and scientific approval for this study was obtained from the THIN Scientific Review Committee142 in October 2011.
Results
There were 1,246,963 eligible participants from 487 general practices, of whom 54,816 entered the dementia cohort and 205,795 were selected for the non-dementia cohort. During the 10-year follow-up, incontinence (urinary, faecal and double) was newly recorded for 8987 (16%) people with dementia and 23,083 (11%) without dementia. Table 37 reports the incidence of UI and FI per 1000 person-years at risk (PYAR) in men and women with and without dementia. The IRRs, adjusted for age, sex and comorbidity, for UI were 3.2 (95% CI 2.7 to 3.7) in men and 2.7 (2.3 to 3.2) in women. The IRRs for FI were 6.0 (95% CI 5.1 to 7.0) in men and 4.5 (3.8 to 5.2) in women.
| Type of incontinence | Men | Women | ||
|---|---|---|---|---|
| Without dementia: rate per 1000 PYAR (95% CI) | With dementia: rate per 1000 PYAR (95% CI) | Without dementia: rate per 1000 PYAR (95% CI) | With dementia: rate per 1000 PYAR (95% CI) | |
| Urinary | 19.8 (19.4 to 20.3) | 42.3 (40.9 to 43.8) | 18.6 (18.2 to 18.9) | 33.5 (32.6 to 34.5) |
| Faecal | 3.1 (2.9 to 3.3) | 11.1 (10.4 to 11.9) | 3.6 (3.5 to 3.8) | 10.1(9.96 to 10.6) |
Fifteen and 18% of people with and without dementia respectively were prescribed medication for UI. Crude and adjusted IRRs are shown in Table 38.
| Incidence of action | Crude IRR (95% CI) | IRR adjusted for sex, age and comorbidity (95% CI) |
|---|---|---|
| Incidence of drug treatment for UI | ||
| Both sexes | 1.3 (1.2 to 1.4) | 2.2 (1.4 to 3.7) |
| Incidence of long-term catheterisation | ||
| Men | 1.3 (1.2 to 1.4) | 1.6 (1.3 to 1.9) |
| Women | 1.9 (1.8 to 2.1) | 2.3 (1.9 to 2.8) |
The incidence of catheterisation could be analysed in 47,066 people with UI over 163,735 PYAR. The IRRs comparing people with dementia to those without are given in Table 38. The results in full are presented elsewhere. 143
Discussion
Dementia is associated with at least a doubling in the incidence rate of incontinence (any type) in community-dwelling primary care patients. To our knowledge this is the first report of incidence of incontinence from the clinical records of community-dwelling people with dementia.
Patients aged 60–89 years with UI and dementia received drug treatments at more than twice the rate of patients without dementia but of the same sex, age and comorbidity. This difference was attenuated with age but remained evident. There is some evidence to suggest that, in general, older adults may not receive optimum treatment for UI. 144,145 Indwelling urinary catheters were recorded at more than double the rate in women with dementia, compared with those without dementia of the same age and comorbidity. The difference was attenuated with comorbidities but not age. Indwelling catheters are associated with discomfort and risk of infection among other problems. 146,147 The clinical reasoning for the greater use of drug treatment and indwelling catheters in this population needs investigation.
The study has a number of limitations. The data are from primary care clinical records, kept for that purpose rather than the questions of this study. The different general practices do not record uniformly all types of information regarding the patient, for example, treatment or non-pharmaceutical supplies from other health-care services. Despite this the data provide insights not offered elsewhere.
Conclusions
This large cohort study establishes the incidence of incontinence among people with dementia living in the community. The incidence of incontinence is more than double – and for some age groups treble – for people with dementia compared with people without dementia of the same age, gender and comorbidity. The clinical reasoning in the primary care management of UI in people with dementia requires further investigation.
A systematic review of interventions and strategies
Urinary and faecal symptoms from underlying pathology in the genitourinary system have established pharmacological and/or surgical treatments. 120 Conservative interventions (i.e. non-pharmacological and non-surgical86 are also important. Incontinence problems in people with dementia are multifactorial116 and many aspects require conservative management. This review addressed the question: what is the evidence for effectiveness of conservative interventions for the prevention or management of incontinence in community-dwelling people with dementia or cognitive impairment?
Methods
Aims and objectives
To identify and assess the empirical evidence for the effectiveness of conservative interventions for the prevention or management of incontinence in community-dwelling people with dementia or cognitive impairment.
Study design
A systematic literature review.
Search procedure
We searched electronic databases: MEDLINE, EMBASE, CINAHL, PsycINFO, BNI, Care.data and The Cochrane Library [including DARE, NTIS and System for Information on Grey Literature in Europe (SIGLE)], Social Science Citation Index (SSCI), AgeInfo, National Research Register (NRR), PapersFirst (conference presentations), and the specialised register of the Cochrane Effective Practice and Organisation of Care (EPOC) Group, Dissertation Abstracts, DH and similar websites. The databases were searched from their start date to 2012 week 13 (4 April). In addition ‘lateral searching’ techniques126 were used for key authors and cited references. Search terms were used in combination (Box 4). Abstracts were screened by two researchers for inclusion. For ambiguous abstracts the full text was retrieved and read. Data were extracted against predefined categories by one researcher and confirmed by a second.
FI or UI.
Delirium, dementia, amnestic, cognitive disorders or dementia.
Aged.
Elderly.
Caregivers.
Caregiver burden.
Spouses.
Family.
Significant other.
Home care services.
Housebound patients.
Community living.
Community dwelling.
Home nursing.
Ambulatory care.
Ambulatory care nursing.
Self care.
Home nursing, professional.
Home health care.
Home health agencies.
Homemaker services.
Community health services.
Social support.
Volunteer workers.
Toilet facilities.
Occupational therapy.
ADL.
ADL or adl.
Toilet.
Toilet training.
Attitude to health.
Adaptation, psychological.
Behaviour therapy.
Inclusion and exclusion criteria
Inclusion Interventional studies addressing problems of incontinence (UI and/or FI) experienced in people with dementia or cognitive problems in the setting of the person’s home, and reporting in the English language.
Exclusion Papers not reporting empirical, observational data of interventions or strategies addressed at problems of incontinence; settings of hospital, nursing homes, care homes or group residential homes or populations; papers excluding people with cognitive impairment or dementia or where they were included but not identifiable in the results; papers not published in the English language.
Results
The search results are presented in the PRISMA flow diagram (Figure 11) and three papers148–150 met the inclusion criteria. Each study was assessed for risk of bias. 127 Because of the heterogeneity of the interventions and outcomes it was not appropriate to undertake a meta-analysis, and a narrative summary of findings is presented. The characteristics of the three studies are presented in Table 39.
FIGURE 11.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the results of search for conservative intervention studies.

| Authors, year, location | Research question or aim | Study design | Participants | Intervention | Control |
|---|---|---|---|---|---|
| Gitlin and Corcoran,148 1993, USA, community | To test the effectiveness of an intervention to expand caregiver problem-solving and use of environmental solutions for problems with bathing and incontinence | Randomised two-group experimental pilot study | Recruited spouse carers residing with a spouse diagnosed with moderate Alzheimer’s disease via network of social service agencies Thirty-seven spouse carers recruited and 17 randomly assigned (unspecified) to the treatment group |
Five visits by OT over 3 months using a framework from the competence–environmental literature. Visit 1, problem identification and review of current strategies; visit 2, identification of environmental influences, education and development of plan; visit 3 implementation of plan; visit 4 expansion of plan; visit 5 review and closure | An attention control group receiving home-making services |
| Jirovec and Templin,149 2001, USA, community | To determine if functional incontinence, i.e. urine loss caused by factors outside the lower urinary tract, could be reduced in memory-impaired incontinent elders who had an individualised toileting programme (IST) | Two-by-two mixed design analysis by variance (group by time) | Recruited by advertisement for carers of ‘memory-impaired’ elders Total n = 118 dyads recruited Seventy-seven randomly assigned to intervention (38 to bimonthly follow-up and 38 to 6-month follow-up) and 41 to control At 6 months 74 remained in study (44 intervention group and 30 control), i.e. 37% attrition rate; 14 carers found that participating ‘was too much for them’, two carers became ill or could not be reached; 19 elders moved to a nursing home; nine people died |
1. IST carers recorded usual voiding patterns (most around every 2 hours) and agreed an IST schedule. Not specified if IST day only or night too 2. Carers taught about age-related bladder changes to understand urgency in toileting behaviours 3. Carers taught about the importance of insuring adequate fluid intake 4. Home environments assessed for obstacles to urine control and advice given 5. Pamphlet of teaching protocol, written at sixth-grade level, left with carer 6. Monthly telephone calls to review toileting schedule and difficulties, keep carers alert to intervention strategies, ensure carers offered elders six to eight glasses of fluid a day and retain in study |
Control group paid US$25 per visit Monthly telephone calls to maintain commitment to study and provide ‘friendly visits’ |
| Enberg et al.,150 2002, USA, community | To examine the short-term effectiveness of PV in cognitively impaired housebound older adults | Exploratory study Prospective, controlled crossover design, for which the usual care controls crossed over to the intervention following the 8-week observation |
Participants recruited via Home Health Nursing Services had to have a MMSE52 score of < 24 and a resident carer Recruited patients = 19 and carers = 16 Nine randomised to the intervention and 10 to control Six of nine in the intervention group completed the intervention (two died and one carer became ill) |
Prompted voiding instruction to carers in 8-weekly sessions in patients’ homes by NPs. PV described as a behavioural therapy by which carers approached patients every 2 hours to ask if wet or dry, to check and to praise if dry. Patients were also asked if they would like to use the toilet. Those asking for the toilet were helped to get there and those not asking were encouraged to do so Carers also encouraged to eliminate caffeine from care recipient’s diet. If they had enuresis caregivers were encouraged to limit fluids in the evening |
The NPs visited every 1–2 weeks to provide ‘socialisation’ (attention control) of an average of 35 minutes without discussing incontinence |
Two studies were described as exploratory and pilot studies, recruiting fewer than 20 dyads. 148,150 All had a control group, although one reported no data on these people. 148 All had risk of bias to the internal validity, presented previously in detail. 150 The interventions were advice and education aimed at the family carer, for them to act upon. Two included education for toileting: prompted voiding (PV)148 and an individualised toileting schedule,150 together with other aspects of continence education. The interventions involved between weekly149 and monthly150 contact with the health professional for the duration of the study. Toileting schedule advice was the least acceptable to carers in the occupational therapist (OT) intervention study. 148 The study of individualised scheduled toileting presented statistics indicating a significant reduction in UI in the intervention arm149 but on our re-analysis of the data provided this was not confirmed. 151 Using the intention-to-treat method the PV study found no statistical differences between groups on any UI outcome but noted there were clinically significant reductions in episodes of UI for some patients. 150 The results in full detail have been published previously. 148
Discussion
Only three studies146–148 of conservative interventions were found, dating from over a decade ago. Two were described as exploratory and pilot studies and all had some risk of bias to internal validity. None provided evidence of effectiveness of the interventions. All three provided evidence as to the difficulties in recruiting to such studies. All three provided evidence as to issues in acceptability and feasibility for some carers in agreeing with and implementing the professional advice or instruction.
The review has limitations in that while the search was designed to be comprehensive, criteria such as English language only may have resulted in the omission of some studies. However, to our knowledge this is the first systematic review addressing the question of evidence of effective interventions for this population group. Previous reviews have not differentiated between evidence from community-dwelling or within-an-institution populations although much of the evidence relates to those within care homes,152,153 or has not drawn on a systematic approach. 154 This lack of attention to the very different environments (both physically and in availability of people to help) of an individual’s home from a care home is apparent in many clinical guidelines. 155,156
Conclusions
There is little evidence of effective interventions for managing incontinence in people with dementia living at home. There is an urgent need for both research and clinical guidance for health professionals tailored to the setting in which the majority of people with dementia live.
A review of English community health services continence policies and clinical guidance
Local clinical guidelines for incontinence, produced by specialist continence services, are an important source of evidence to guide clinical practice for primary care professionals, particularly community nurses. A major part of district nurses’ and community nurses’ work is in supporting older people with incontinence problems who live at home. 157 Specialist continence services include nurses who work closely with a lead medical consultant, and across primary care, acute care and in care homes, although there is wide variation in provision. 158 In the absence of nationally available clinical guidelines, as described in the introduction to this chapter, this review sought to understand the extent to which local guidelines had been tailored to address the needs of people with dementia or cognitive impairment. The advisory group considered this an important review in light of the findings of the early parts of Phase 2 (see Exploring the experience, strategies, impact and consequences, below).
Methods
Aims
To investigate if the clinical guidance for incontinence management provided by specialist continence services in England addresses the problems and issues experienced by people with dementia living at home and their carers.
Study design
A review by documentary analysis159 of clinical policies and guidance from a sample of at least 25% of specialist continence services covering 152 PCT areas and distributed across the 10 Strategic Health Authority regions of England. Documents were obtained through internet searches on public domain NHS websites and requests to specialist continence nurses identified through a national directory. 160 The framework for analysis was designed in conjunction with the advisory group.
Ethical arrangements
The NHS Research Ethics Service (NRES) advised that this was a service evaluation not requiring NHS ethics committee review. The study followed university research ethics requirements.
Results
Ninety-eight documents from 38 local community health service specialist continence services, located across all 10 strategic health authority areas, were obtained and analysed.
Of the 38 local services, 19 (50%) made no mention of dementia or cognitive impairment in their documents. Only in the documents of three services were nurses offered detailed guidance about the management of incontinence for people with dementia at home. The remaining 16 organisations provided guidance that mentioned dementia or cognitive impairment in assessment processes, but there was very little evidence of any subsequent advice or mechanism for producing specifically tailored care plans, such as a dementia care pathway. Only one organisation specified that the service user should be given any information about the consultation, and that was a copy of the referral form for incontinence pads with delivery information on it.
Ten (26%) of the 38 local organisations referred to a leaflet entitled Continence in the Confused Elderly (anonymous) to be given to family carers. The carers in the advisory group reviewed this leaflet and concluded that it had deficiencies in content, information, tone and style. People with dementia were identified as a special case in the documentation of only one service, which warranted the provision of additional continence products. Only one service made reference to addressing the problems of delivery of NHS continence pads to people with dementia living alone at home.
Discussion
Fifty per cent of sampled organisations had clinical guidance on incontinence which made no mention of dementia. A small minority provided examples of good practice. These good practice examples focused on person-centred assessment and responsiveness to the described problems, an approach advocated for some time in the care of people with dementia generally,115 and by gerontological nurses and dementia specialists. 161 A more detailed discussion of the results has been published previously. 162
To our knowledge, this is the first study exploring the local guidance to primary care professionals in meeting the continence needs of people with dementia living at home. The study has limitations in that the representativeness of the documents is unknown, although the geographical spread, method of requesting from specialist nurses and internet searches would suggest that the variety has been captured.
Conclusion
Local clinical guidance on incontinence management rarely addresses the specific needs of people with dementia living at home or their carers. There is much room for improvement and providing evidence-based guidance to frontline staff on the specific problems of people with dementia and their carers. This review led the advisory group to prioritise for Phase 4: the development and testing of dementia specific continence assessment tools and guidance for the use of primary care nurses working with people with dementia living in their own home. The review also pointed to the importance of the involvement of carers of people with dementia in continence service developments.
Phase 2: exploring the experience, strategies, impact and consequences
In Phase 2, interlinked primarily qualitative studies were undertaken. All four studies were informed by the background and rationale described in the introductory section of this chapter, and these will not be repeated in each of the following sections. For each study therefore the details of the methods, results, discussion and conclusion only are given here.
The views of people with dementia
We could find no accounts from the perspective of the person with dementia on issues of incontinence. The purpose of this study was to investigate the PWD’s perceptions of managing problems of incontinence.
Methods
The study approach was in the interpretative tradition163 and the design was of qualitative guided conversations, a recommended research technique for use with people with dementia. 164 A purposive sample of people with dementia was recruited through primary care, specialist community mental health services and voluntary organisations. Conversations were taperecorded with permission, transcribed and thematically analysed,165 using NVivo™ version 8, 2008 software package (QSR International, Warrington, UK: www.qsrinternational.com/default.aspx).
Ethics
The ethical issues we considered were capacity to consent, within the guidance of the MCA;17 assessment of continuing consent during the conversation and non-verbal signs of wishing to finish; and preparing researchers to manage distress and act on information that might suggest that a vulnerable adult was being neglected or harmed. A favourable opinion was given by Camden and Islington NHS local REC (08/H0722/60 2008) (see Appendix 24).
Results
It took a period of 1 year to recruit seven people with mild to moderate dementia and UI problems. They described a range of problems from urgency (which they could not respond to in time) to nocturnal enuresis. All described feelings of shame and humiliation as in this example:
There’s always the fear and sometimes I don’t get there in time and I find myself with wet pyjamas and it’s personally very embarrassing.
Man with dementia 04
Strategies included planning activities outside the house only when they knew they could access toilets, accepting help from their spouse (although this sometimes caused tension) and using a variety of absorbent materials for containment, for example towels. Descriptions of the use of incontinence pads included problems of fit and acceptability, as in this example.
I’m so small you know, and I’ve been cutting them [incontinence pads] and the whole flat is covered in bits of, what do you call it, small pieces, fluff, every day fluff . . . they’re so big, I’ve got to cut them.
Woman with dementia 01
Strategies also included using euphemisms in describing the problems, using humour to overcome embarrassment and trying to protect family members from spillage by clearing up or dealing with urine or wet clothes themselves. The detailed results are reported elsewhere. 158
Discussion
To our knowledge, this is the first study reporting people with dementia’s perceptions of these problems of incontinence. The descriptions of embarrassment and shame are no different from those given by other adults. 166 The desire to protect spouses or other family members from the ‘pollution of bodily waste’167 has not been reported before and may have two unintended consequences. The first is that it may increase family carer stress if the person cannot appropriately deal with leakage and excreta. The second is that the family carer may not understand the motivation for such acts and interpret these as the need to protect their dignity by concealment from everyone, including health professionals who could potentially help. Although the study has limitations through size and difficulty in recruitment, it does provide insights that have not been reported previously.
Conclusion
People with dementia are able to describe in detail their experiences and management of incontinence. Their desire to protect their own dignity, but also protect others, may lead to unintended consequences and delays in seeking wider help for such problems.
Views of family carers on the problems of incontinence and effective strategies to manage them
We could find no study investigating family carers’ strategies for managing incontinence. The purpose of this study was to investigate family carers’ perceptions of the range of incontinence problems and their strategies for managing them.
Methods
The research approach was in the interpretative tradition. 164 A purposive sample of family carers of people with dementia, living in their own homes, was recruited through primary care, specialist community mental health services and voluntary organisations. Qualitative semi-structured interviews (SSIs), based on an open-ended topic guide,168 were conducted either face to face or by telephone, recorded by tape, transcribed and thematically analysed,164 using NVivo™ version 8, 2008 software package.
Ethics
Researchers were prepared to manage participant distress appropriately, and also situations that might suggest a vulnerable person was being neglected or harmed. A favourable review was given by Camden and Islington NHS local REC (08/H0722/60 2008).
Results
Thirty-two carers were interviewed. They described a range of problems from supporting the person to remain independent in toileting, through to dealing with inappropriate behaviours, to containing and managing incontinence. Key themes were identified: active problem-solving, protecting the dignity of the person with dementia, problematic responses from health services and unintended consequences. All carers actively used problem-solving strategies but sometimes these were not acceptable or understood by the person with dementia, particularly as the dementia progressed. Most carers reported protecting the person’s dignity by not seeking health professionals’ help, often until the point of crisis.
I didn’t want people to know about her not managing and incontinence . . . I felt her dignity, you know . . . She was so in herself a very dignified lady . . . If she’d have known she’d have been horrified.
Husband c6
Once the carer had decided to seek help, the responses from health professionals were reported to be often less than helpful, and carers reported local health service policies on access to continence products to be inconsistent and often inappropriate to their circumstances. A few carers reported strategies for managing toileting and incontinence that had the potential for distress and harm to the person with dementia. These findings are reported in full elsewhere169 in a paper that was awarded the Royal College of General Practitioners’ Research Paper of the Year 2011, in the category of dementias and neurodegenerative diseases.
Discussion
The reluctance to seek help for toileting and incontinence problems by carers of people with dementia as they seek to preserve their dignity and personhood115 has not been reported before, although the low rate of seeking help for incontinence symptoms is well documented in older adults. 170 The variability and sometimes inappropriate responses of primary care professionals has not been reported previously, to our knowledge. Management strategies were mediated by their acceptability to both the PWD, the carer and other family members. A key influence in carers’ management strategies was to ensure that excreta were well contained, an important goal identified by expert panels for incontinence management in frail older people. 117 Although the study has limitations because of its sample size, the diversity of the population and the sampling until saturation in data ensured depth and breadth in the findings.
Conclusions
There is a wide range of types of incontinence problems that change over time, and carers managing them are often poorly supported by primary care professionals. Primary care professionals could be more proactive in enquiry, repeated over time, about toileting and incontinence problems; alert to strategies that have the potential for negative effects; and develop their repertoire of referrals, advice and information to reduce crisis and problems.
Views of primary care, social care and specialist mental health staff in community settings on effective strategies
We could find no studies reporting HSC professionals’ perceptions of effective strategies for the management of incontinence in this population. The aim of this study was to investigate the perceived range of problems and effective strategies.
Method
The study design was in the interpretative tradition using qualitative individual and group interviews,171 with HSC professionals purposively sampled to ensure a range of generalist and specialist community services, disciplines, grades of staff and those with qualifications regulated through legislation and those without. Participants were approached through organisations that had agreed to participate in the EVIDEM programme and at EVIDEM educational events; these were mainly in north and central London and Hertfordshire. Data were recorded in field notes and in flip charts kept as part of the group discussions, transcribed to electronic documents and thematically analysed.
Ethics
The NRES advised us that the study did not require review as it was a service evaluation. The study followed university research ethics requirements.
Results
We recruited 140 participants from a wide range of staff groups (Table 40), and they took part in 30 individual interviews and group interviews. Collectively, they described a wide range of toileting and incontinence problems, with a wide range in the number of problems reported. Some informants mentioned only one or two types of problems, whereas dementia specialist staff, such as the Admiral Nurses, described greater numbers of both physical problems and dementia-related problems.
| Staff group | No. |
|---|---|
| Dementia specialist social care staff | 40 |
| LA social workers and care managers | 14 |
| Continence nurse specialists | 9 |
| GPs | 3 |
| Admiral Nurses (specialist dementia care nurses) | 10 |
| Consultant old age psychiatrists and medical team | 6 |
| Community mental health services for older people and memory service team members (nurses, assistants, psychologists managers) | 33 |
| Occupational therapists | 6 |
| District and community nurses | 3 |
| Voluntary organisations advice and carers support staff | 15 |
| Total | 139 |
Collectively, they reported a wide repertoire of strategies but many offered only one or two strategies that reflected their type of service. A commonly reported strategy was to refer the patient to another service, in many cases the district nursing service. The small number of district nurse informants focused mainly on using continence pads as a containment strategy. Many informants considered that the local NHS provision of incontinence advice and absorbent products did not address the needs of people with dementia and their carers; in some instances these deficiencies were seen as contributing directly to decisions to move the person to a care home. Other system-level problems were identified, such as the lack of acknowledgement of toileting support in social care assessments.
Discussion and conclusion
To our knowledge there are no other published studies exploring multidisciplinary and multiagency perspectives on managing incontinence for people with dementia living at home, although there are some that have considered the issue from the perspective of care home staff. 172 The study has limitations in that views offered by a small number of informants may not be representative of others throughout England. However, the intention was not to undertake a representative survey but to understand the breadth of issues and strategies. The findings suggest that many staff groups have relatively limited knowledge of the range of problems that can be experienced with a concomitant limited repertoire of responsive strategies. However, the cumulative responses also point to a mosaic of practical, psychological, behavioural, biomedical, environmental and supportive strategies that can be utilised. One question is whether knowledge alone of this mosaic of strategies can influence primary care professional practice in addressing these types of problems. The respondents also pointed to failures of publicly funded systems in addressing these problems, which raises the question of whether or not new commissioning processes and initiatives, such as personal budgets, will be better able to address them.
Experience, including impact and consequences, of people with dementia and their family carers over time
Dementia follows a course of progressive disablement over a number of years. 173 The experience, impact, cost and consequences over time are poorly described in relation to incontinence and TDs (defined as a range of problems associated with loss of independence in toileting or behavioural symptoms, sometimes resulting in soiled clothing or excreta uncontained in pads or voiding inappropriately). This study investigated the experiences, impacts, costs and consequences of incontinence in people with dementia and their family carers over a period of up to 3 years.
Method
This was a longitudinal mixed-methods174 study investigating the experience of a purposive sample of people with dementia with continence problems living at home, and of their carers. Personal and general invitations to participate were distributed via community organisations, social care organisations, specialist community mental health services for older adults in five London boroughs, and GPs in eight PCT areas. Data collection was by serial qualitative interviews. 175 In addition, validated instruments were used to characterise the person with dementia who met the criteria of the ICD-10. 80 Their mental state and abilities were described by the MMSE,52 the NPI62 and the Disability Assessment for Dementia (DAD),176 their TDs and incontinence by an adapted International Incontinence Modular Questionnaire [International Consultation on Incontinence Questionnaire (ICIQ)]177 and their health-related quality of life using the DEMQOL. 86 Also recorded were the service use and cost consequences using the CSRI Parts 1 and 2,88 as well as the carers’ health-related quality of life [EuroQol: European Quality of Life-5 Dimensions (EQ-5D)],178 and burden (ZBI87 and NPI Caregiver Distress Scale179). Analysis of the qualitative data was thematic,165 through comparisons over time and between cases using the NVivo 8 software package and longitudinal charting. Statistical analyses of quantitative data were undertaken using SPSS version 20 and Stata version 13 software. Costs were derived from the PSSRU Unit Costs of Health and Social Care 201189 and estimates using the Office for National Statistics 2011 Annual Earnings Survey. 180
Ethics
Ethical considerations included those described above (see p. 84). The study was given a favourable review by the Camden and Islington NHS local REC 08/HO722/77 in 2008.
Results
Thirty-four people with dementia and 29 family carers were recruited over 2 years (1 December 2008 to 31 December 2010). During the period of the study, 10 people with dementia died, six moved to care homes and one withdrew. Because of the long recruitment period the remaining participants were in the study for different lengths of time (see Figure 12) but many family carers provided retrospective data on entering the study to describe their experiences over a longer period of time (Table 41). The characteristics of participants at the first interview are provided in Table 42.
FIGURE 12.
Flow diagram of participants over time.
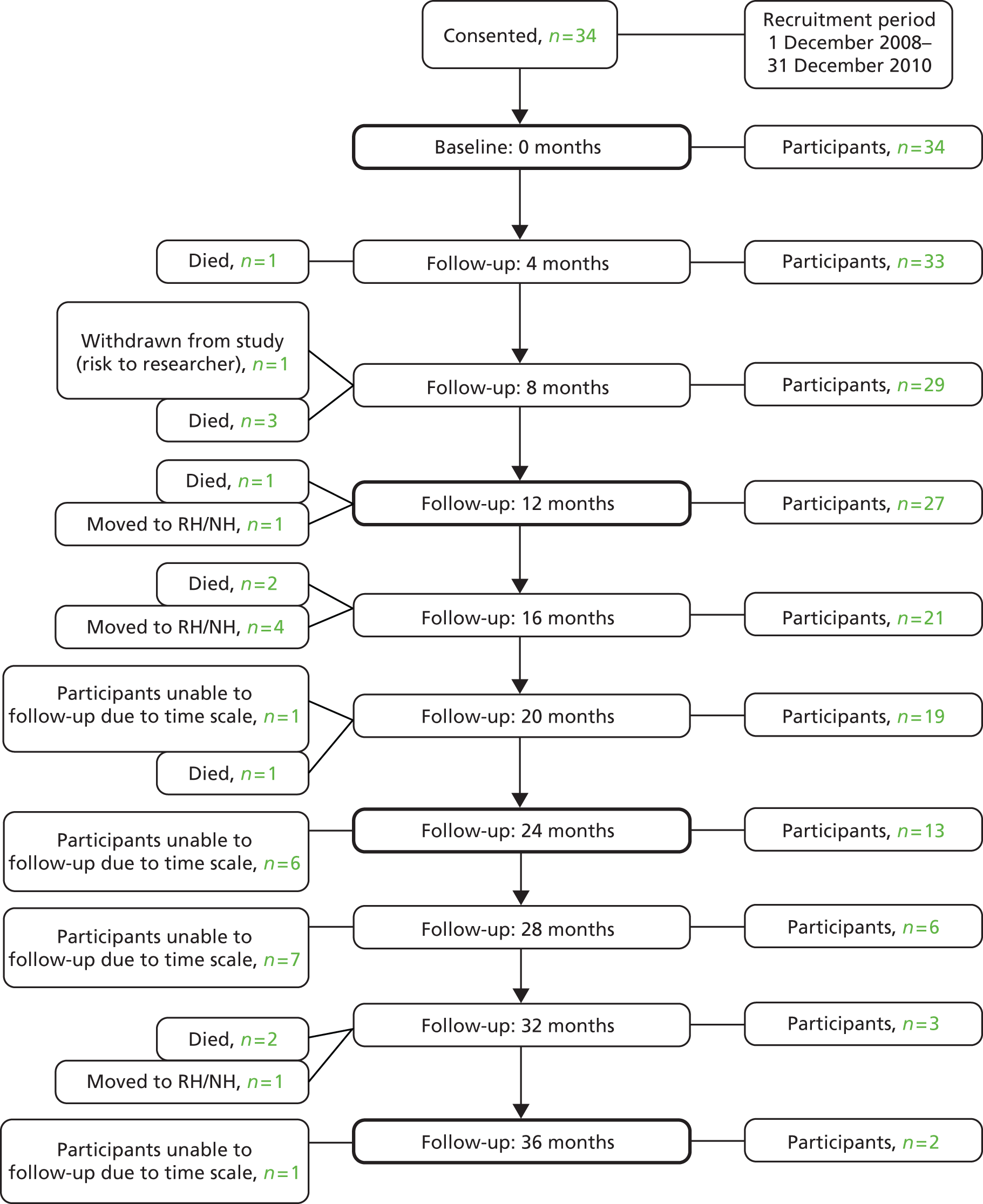
| Months of reported data | No. of participants |
|---|---|
| 0–12 | 2 |
| 13–24 | 11 |
| 25–36 | 15 |
| 37+ | 6 |
| Characteristics | Person with dementia (N = 34) | Carer (N = 29) |
|---|---|---|
| Male, n (%) | 10 (29.4) | 13 (44.8) |
| Female, n (%) | 24 (70.6) | 16 (55.2) |
| Age, years (mean/range) | 81.9 (62–98) | 68.1 (44–88) |
| Relationship to carer (N = 29), n (%) | ||
| Spouse | 17 (58.6) | – |
| Adult–child | 11 (37.9) | – |
| Sibling | 1 (3.5) | – |
| Ethnicity, n (%) | ||
| White British | 24 (70.6) | 21 (72.4) |
| White other | 4 (11.8) | 1 (3.4) |
| White Irish | 3 (8.8) | 3 (10.3) |
| Black British | 1 (2.9) | 1 (3.4) |
| Black Caribbean | 1 (2.9) | 1 (3.4) |
| Black African | 1 (2.9) | 1 (3.4) |
| Chinese | 0 (0) | 1 (3.4) |
| English not first language | 6 (17.6) | 2 (6.9) |
| Person with dementia living alone | 8 (23.5) | – |
| Person with dementia median measure scores (range) | ||
| MMSE52 (possible score 0–30) (n = 14) | 18 (2–26) | – |
| DAD176 (possible score 0–100%) (n = 33) | 15 (0–95) | – |
| DEMQOL86 (possible score 28–116) (n = 8) | 87 (52–101) | – |
| NPI62 (possible score 0–120) (n = 24) | 18 (0–69) | – |
| Carer measure scores (mean/range) | ||
| EQ-5D178 (possible score 0–100) (n = 24) | – | 75 (40–95) |
| NPI carer distress179 (possible score 0–50) (n = 24) | – | 10 (0–32) |
| ZBI87 (possible score 0–88) (n = 27) | – | 30 (11–60) |
The types of toileting and incontinence problems are described in Table 43.
| Problem | Baseline | 12 months | 24 months | 36 months |
|---|---|---|---|---|
| TDs | 44.1% (15/34) | 21% (4/19) | 11% (1/9) | 0% (0/2) |
| Urinary (UI) | 29% (10/34) | 31% (6/19) | 11% (1/9) | 0% (0/2) |
| Faecal (FI) | 9% (3/34) | 5% (1/19) | 0% (0/9) | 0% (0/2) |
| Double (UI plus FI) | 56% (19/34) | 53% (10/19) | 89% (8/9) | 100% (2/2) |
The progression of the dementia was evident in participants and was reflected in the DAD scores, which showed a significant reduction over time in ability from a median score of 15 to 10 (p = 0.003 by Wilcoxon signed-rank test). A series of basic regression models were fitted to the data. Inter-participant variability was allowed for by random-effect parameter, and bootstrap CIs were used to account for floor and ceiling effects in the outcomes. The effects on each of the carers’ measures were adjusted for the person with dementia’s NPI measure (Table 44). The carers’ health-related quality of life scores were significantly negatively affected by the presence of FI (–5.3, CI –10.4 to –0.1, significant at the 5% level) but not by TDs or UI. The carers’ distress scores (in response to BPSD) were significantly negatively affected in the presence of TDs (6.5, CI 0.6 to 12.3, significant at the 5% level) and UI (5.2, CI 2.7 to 7.6, significant at the 5% level) but not in the presence of FI.
| Carer measure | Presence of TDs (95% CI) | TDs adjusted for NPI (95% CI) | Presence of UI (95% CI) | UI adjusted for NPI (95% CI) | Presence of FI (95% CI) | FI adjusted for NPI (95% CI) |
|---|---|---|---|---|---|---|
| EQ-5D178 | 0.1 (–5.6 to 5.7) | 3.6 (–4.0 to 11.2) | –0.8 (–12.0 to 10.5) | –2.8 (–18.0 to 12.4) | –5.3a (–10.4 to –0.1) | –5.1 (–12.4 to 2.3) |
| Caregiver Distress Scale179 | 6.5a (0.6 to 12.3) | N/A | 5.2a (2.7 to 7.6) | N/A | 1.8 (–2.2 to 5.8) | N/A |
| ZBI87 | 4.9 (–0.7 to 10.4) | 2.4 (–6.0 to 10.8) | 0.1 (–11.3 to 11.5) | –4.1 (–19.6 to 11.4) | –0.1 (–4.8 to 4.6) | –2.9 (–9.0 to 3.3) |
A number of themes were identified: changes in problems over time; the processes of normalisation, both in managing toileting changes over time and also in managing excreta out of place; the iatrogenic effects of hospital inpatient stays and respite stays in care homes on both the PWD’s toileting abilities and physical health (e.g. the development of pressure ulcers); the interplay of decreased mobility and incontinence; and the perceived positive impact of both paid help in personal care, which the carer controlled, and good excreta containment on reducing carer stress. Figure 13 illustrates some of these themes through the changes and experiences over time of one person with dementia and their family carer. The presence of double incontinence (UI and FI) compared with one type of problem only (TD, UI or FI) was found to significantly increase the costs by 89% from a HSC perspective and by 69% from a societal perspective (Table 45). The HSC perspective includes costs both provided by the NHS or government and also privately purchased. Societal costs include costs of the time of the informal or family carers.
FIGURE 13.
A longitudinal chart of one participant’s experience. PPA, primary progressive aphasia.
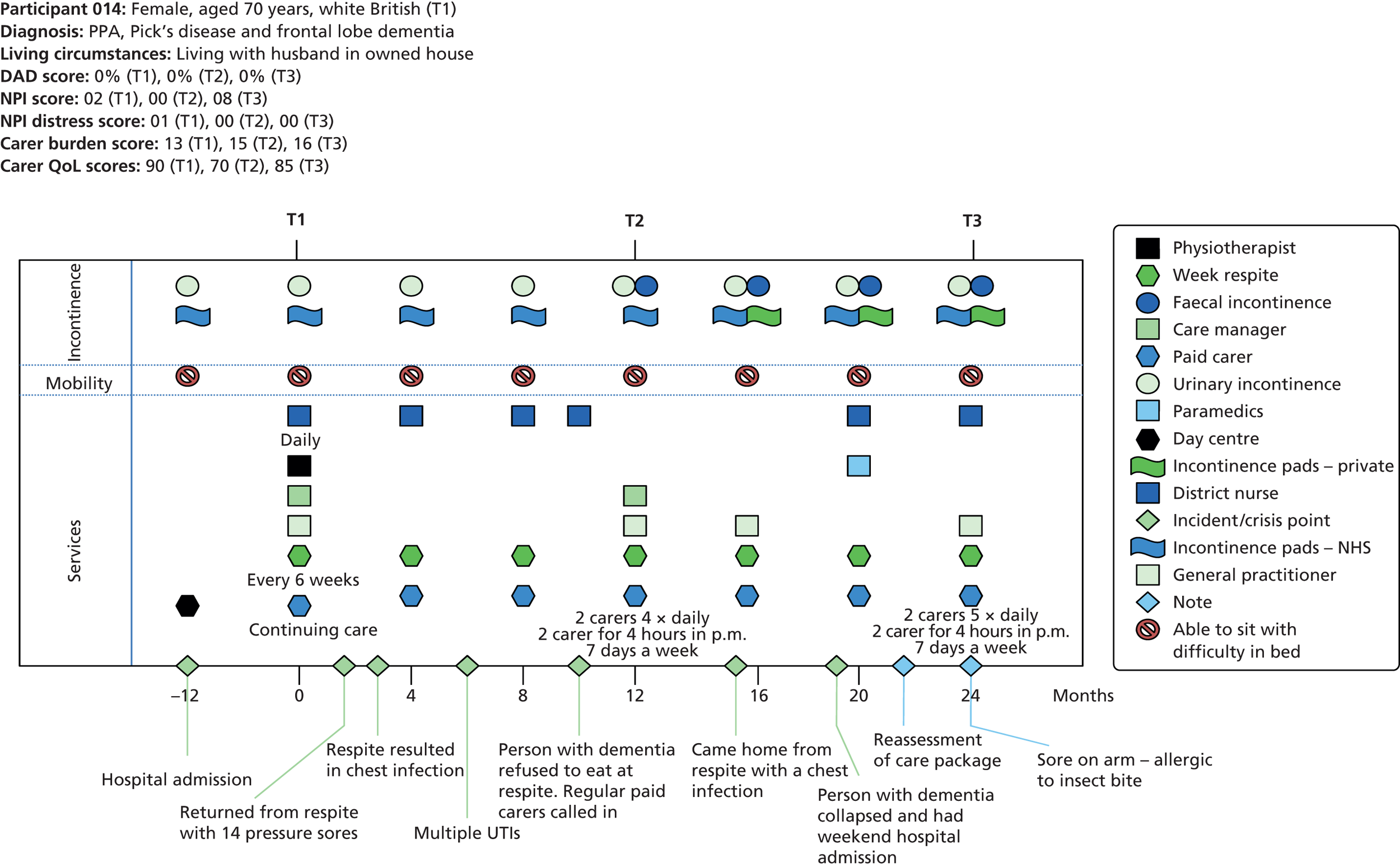
| Issue present, and significance | 3-month mean costs in pounds sterling (SD): n = 34 | ||
|---|---|---|---|
| HSC (SD) | Societal (SD) | n | |
| One type only (TDs or UI or FI) | 4200.9 (4110.8) | 7464.6 (4885.1) | 15 |
| Double incontinence (UI and FI) | 8207.5 (6722.2) | 12431.3 (7163.7) | 19 |
| p-value (2 > 0 or 1) | 0.03 | 0.01 | |
Discussion and conclusion
To our knowledge this is the first study reporting experiences of incontinence over time, and impacts upon this patient population and their carers. The small sample is a limitation but provides insights into the experience, types, costs and impact of incontinence that are not provided elsewhere. The results in full are discussed elsewhere. 169 The negative impact of the presence of FI on carer quality of life warrants further investigation. The perceived positive impact of assistance in personal care tasks, which are within the control of the carer, and effective containment of excreta require further investigation, in particular whether or not it influences or defers decisions about moving to a care home.
Phase 3: investigating the feasibility, effectiveness and acceptability of an identified strategy
Effective containment of excreta was identified as very important to family carers, both in discussing their experiences and also in its impact on factors such as reported burden (Phases 2 and 3). Carers within and outwith the advisory group prioritised the investigation of designs of incontinence pads to meet the needs of people with dementia and their carers. The one previous study of acceptability and effectiveness of different pad design did not include people with dementia living at home. 181 The study aimed to investigate the feasibility of ascertaining acceptability, effectiveness and associated costs of different types of incontinence pad designs as used by people with dementia living at home and also to provide data to inform both future research design and also service providers.
Method
This was an observational study based on the methods of a previous clinical trial with cognitively intact adults181 and informed by the family carers in the advisory group. The study aimed to recruit a sample of 40 family carers, diverse in their, and the PWD’s, characteristics, for example age, gender, stage of dementia. Family carers were recruited through voluntary organisations across Greater London, specialist mental health services for older people in North West London, and general practices and a community continence service in South West London. At time point 1, the family carers were instructed in observational and diary data collection. They were asked to complete the diary sheets for 1 week. Data characterising the person with dementia (DAD,176 NPI62) and the family carer burden176 and the type of incontinence (ICIQ)177 were also collected. At time point 2, the family carers were interviewed about the experience of data collection and their diaries retrieved. Analysis of quantitative data was undertaken using the SPSS version 20 software package. Analysis of the qualitative data was undertaken thematically. 165
Ethics
Ethical issues were as outlined previously (see p. 84). The study was given a favourable review by the NHS South East REC (10/H1102/34) in 2010.
Results
Twenty-five family carers of people with dementia living at home were recruited to the study over 18 months. There was diversity in the sample of family carers, the person they cared for and the toileting and incontinence problems (Table 46).
| Characteristics | Family carer (N = 25) | Person with dementia (N = 25) |
|---|---|---|
| Female, n (%) | 19 (76) | 17 (68) |
| Male, n (%) | 6 (24) | 8 (32) |
| Age, years (median/range) | 63 (32–89) | 84 (64–90) |
| Relationship, n (%): | ||
| Spouse | 11 (44) | |
| Adult–child | 11 (44) | |
| Sibling and other | 3 (12) | |
| Ethnicity, n (%): | ||
| White British | 19 (76) | 22 (88) |
| White other | 4 (16) | 2 (8) |
| White Irish | 1 (4) | 0 (0) |
| Black Caribbean | 3 (12) | 1 (4) |
| English not first language, n (%) | 3 (12) | |
| ZBI87 possible score 0–88: median (range) | 45 (8–60) | |
| DAD176 possible score 0–100%: median (range) | 23 (0–83) | |
| NPI62 possible score 0–120: median (range) | 21 (0–72) | |
| TDs only | 1 (4%) | |
| UI only | 4 (16%) | |
| FI only | 1(4%) | |
| TDs + UI | 3 (12%) | |
| UI + FI | 8 (32%) | |
| TDs + UI + FI | 8 (32%) |
The range and funding source of aids and adaptations used are described in Table 47.
| Items used | Provided by or funding source (n = 24) | ||||
|---|---|---|---|---|---|
| Own | NHS | LA | NHS + own | LA + NHS + own | |
| Incontinence pads | 8 (33%) | 10 (42%) | 0 (0%) | 5 (21%) | 1 (4%) |
| Equipment (e.g. commodes) | 5 (21%) | 6 (25%) | 0 (0%) | 13 (54%) | |
| Adaptation of home (17 had no adaptations) | 6 (25%) | 1 (4%) | |||
There was also diversity in the designs of incontinence pads used, which changed for some individuals when outside the home and between day and night-time (Table 48).
| Time period | Design of incontinence pad used (n = 27a/n = 25 nightb)a | |||||
|---|---|---|---|---|---|---|
| Large shaped inserts | Pull-ups | Small inserts | Nappy | T-shaped nappy | Combination of two types at once | |
| Daytimec | 10 | 10 | 4 | 1 | 0 | 2 |
| Night-time | 8 | 8 | 1 | 1 | 1 | 5 |
Those who were recruited demonstrated high levels of commitment to the study’s aims. Twenty (80%) of the carers were able to complete some days of the diary sheets (mean number of days 8, range 2–16 days). Carers entered data about each change of pads; the number of times they entered data ranged from 4 to 56. One aspect was leakage of excreta outside the pad, for example onto clothes. The rate of leakage by pad design is reported in Table 49.
| Design | No. of pad changes | No. of leakage events (%) |
|---|---|---|
| Large shaped insert pads | 201 | 51 (24) |
| Pull-ups | 218 | 41 (19) |
| Nappy | 19 | 4 (21) |
| T-shaped nappy | 8 | 2 (25) |
| Small insert pad | 19 | 1 (0.05) |
Analysis of data on the acceptability and effectiveness of different designs of incontinence pads revealed several important factors. Designs of pads were acceptable to the carer when they could safely and efficiently be changed by the carer (and others such as day centre staff). Safety factors related to designs that allowed the carer to change the pad and hold the person steady if necessary (i.e. to minimise risks of a fall). Efficiency factors related to speed in changing, to cause least disturbance to the person and with minimal effort on the part of the carer. Effectiveness related to the ability of the design to ensure containment of excreta.
It was evident that the required specifications of pads changed according to location (e.g. the use of public toilets or at home), the availability of the carer or others to change the pad and whether a pad was to be used in the day or at night. At night, 40% of the sample (n = 10) used either a more absorbent pad than in the day, a different design or two designs in combination. The results are described in full elsewhere. 169
Discussion and conclusion
To our knowledge this is the first study exploring preferences and effectiveness of incontinence pad design when used by people with dementia living at home and their family carers. Considerable endeavour and resources went into the recruitment process, which was much slower than anticipated, and we were unable to recruit the planned numbers: future studies will need to take great care with recruitment strategies. There are limitations due to the sample size but the study provides data that will be used to inform the development of a clinical trial protocol. It also provides insights into the specifications in effectiveness and acceptability of different pad designs for people with dementia living at home not provided elsewhere.
Phase 4: developing and testing resources for practice
Drawing on the evidence of the earlier studies, a priority for the family carers in the advisory group was practical resources to assist primary care professionals to both recognise the range of problems faced by people with dementia and their carers and promote a variety of strategies that would be helpful.
This phase of the study therefore undertook the development and testing of a continence assessment tool for people with dementia, to be used by primary care professionals, primarily community nurses. It also undertook the development and testing of supporting educational materials.
Developing and testing a continence assessment tool and resources
The aim of this study was to develop and test a continence assessment tool and supporting resources for people with dementia, to be used by primary care professionals, primarily community nurses.
Methods
An adapted three-stage Delphi consultation study182 was used. Stage 1 brought together an expert group of carers, dementia specialist workers from the voluntary sector and health professionals. This group examined commonly used continence assessment tools, and discussions were facilitated to describe a broad range of principles and issues that would underpin an assessment tool designed to address the needs of people with dementia. At stage 2 a prototype dementia-focused continence assessment tool was developed using the data generated in stage 1, asking for agreement or disagreement to items plus suggestions for further items. This was used to consult, in writing, both the expert group in stage 1 and also a further group of carers and specialist continence professionals. The prototype was further adapted. At stage 3 a different, wider group of experts (carers and professionals) was consulted in writing. They were sent the draft dementia-focused assessment tool (Figure 14) together with a questionnaire to test its face and content validity. Recipients were asked (1) whether or not the tool would improve recognition of the problems (face validity) and (b) to rate each item for importance and identify missing or unnecessary items (content validity). It was emphasised that the tool’s remit was a supplement to the continence assessment tools and guidance currently used, hence it was called an add-on tool. Participants were also sent supporting resource materials on which to comment (Table 50).
FIGURE 14.
The EVIDEM-C add-on continence assessment tool.
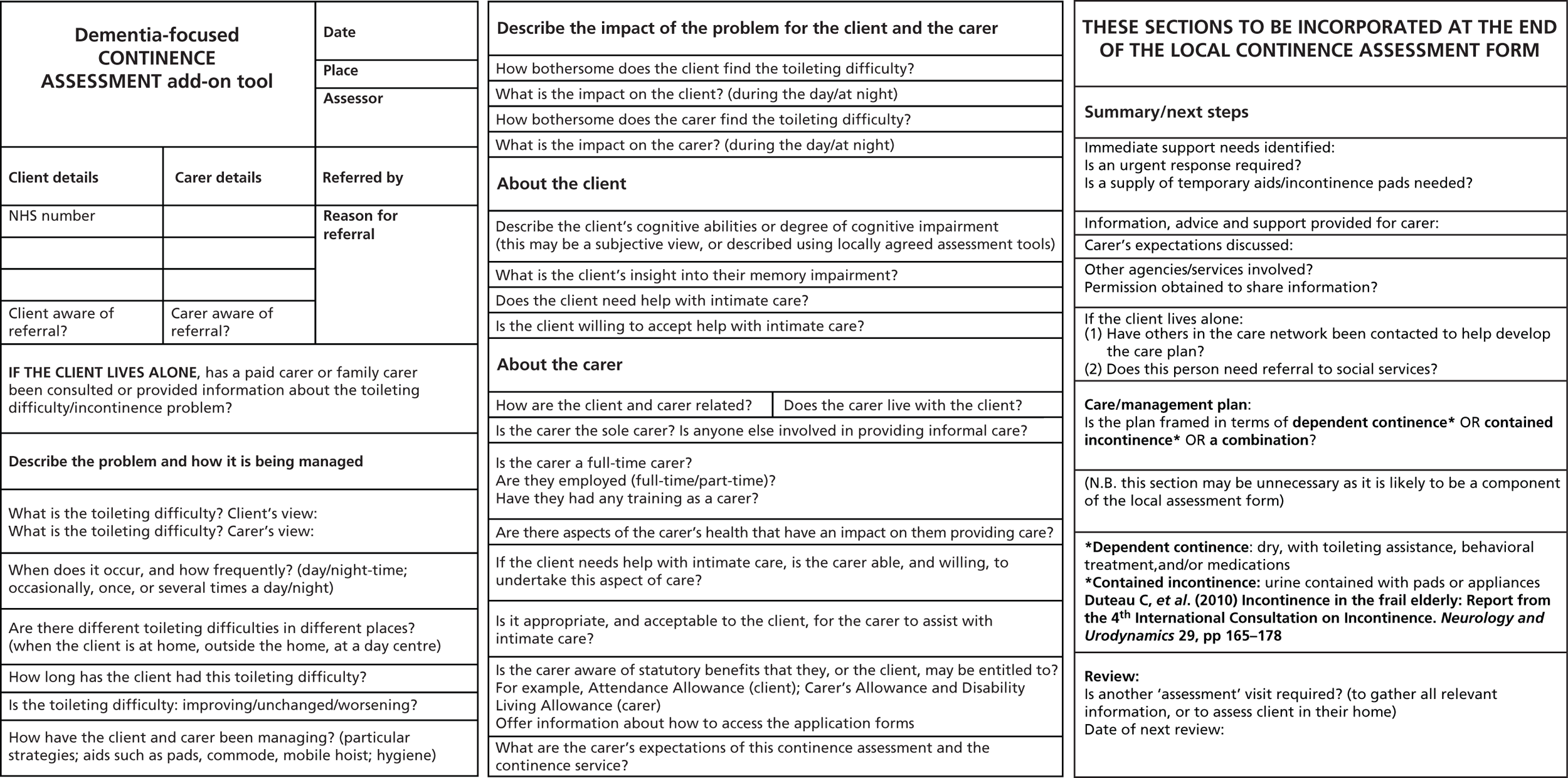
| Toileting/continence issue | Examples of suggested actions/strategies | |||
|---|---|---|---|---|
| Techniques/knowledge/activities | Environmental/aids | Further professional assessment or source of advice | ||
| 1 | Recognising the need to void but unable to act on it in a timely way | Awareness of aspects of the person’s behaviour that may signal the need to void, for example unexplained restlessness, tugging at clothes Map usual voiding patterns and plan support accordingly PV if acceptable |
||
| 2 | Apathetic or not acting on the need to void | Map usual voiding patterns and plan support accordingly PV if acceptable |
Consider assessment by another professional for other problems, such as depression, e.g. GP, CPN | |
| 3 | Difficulty in locating the toilet in the home | Map usual voiding patterns and plan support accordingly PV if acceptable |
Toilet signage | Occupational therapist PromoCon |
| 4 | Difficulty in recognising the toilet | Map usual voiding patterns and plan support accordingly PV if acceptable |
Contrast and lighting, e.g. different coloured toilet set to toilet bowl Declutter the bathroom |
Occupational therapist PromoCon |
| 5 | Unable to perform all difference of actions to void in the right place | Map usual voiding patterns and plan support accordingly Assist men to sit to urinate Family carer of opposite sex may need advice/explanation on how to assist in this |
Consider ways in which the task could be simplified, e.g. pull-up trousers/jogging bottoms rather than zips | Occupational therapist PromoCon |
| 6 | Unable to perform all personal hygiene afterwards | Map usual voiding patterns and plan support accordingly Family carer of opposite sex may need advice/explanation on how to assist in this Consider wipes rather than toilet paper |
Advice from: Continence specialist nurse Occupational therapist PromoCon |
|
| 7 | Physically unable to get to/use the toilet in a timely way and/or has dexterity problems which make it difficult to manage clothes and personal hygiene | Grab rails Raised toilet seats Commodes Urine bottles Extended wipers Easier clothing (Velcro® closures/elasticated waists) |
Consider potential for assessment by other professional, e.g. GP and/or physiotherapist and/or occupational therapist PromoCon Disabled Living Foundation |
|
| 8 | Locks toilet door and is then unable to comprehend how to unlock it | Remove or disable the toilet door lock | Occupational therapist PromoCon |
|
| 9 | Uses inappropriate places or receptacles | Use behavioural management techniques, e.g. the ABC approach (Antecedents, Behaviour and Consequences) to then decide appropriate strategies: What happened, when? In what circumstances? With what consequences? Plan strategies based on this information |
Consider strategies to make it easier to locate a toilet Place appropriate receptacle in the place used (if appropriate) |
Advice from: CPN Occupational therapist PromoCona |
| 10 | Aids not acceptable or not helpful | Consider different designs | Advice from: Occupational therapist PromoCona Disabled Living Foundation |
|
| 11 | Resists assistance in toileting | Check awareness of good communication techniques for people with dementia to reduce anxiety/fear/embarrassment/reluctance Use behavioural management techniques to problem solve |
Consider potential for assessment and advice by other professional, e.g. GP, CPN, clinical psychologist | |
| 12 | Restlessness/does not remain on/at the toilet long enough to void | Check awareness of good communication techniques for people with dementia to reduce anxiety/fear/embarrassment/reluctance (Information Sheet 5) Use behavioural management techniques to problem solve |
Consider other reasons for restlessness, e.g. pain Consider potential for assessment and advice by other professional, e.g. GP, CPN |
|
| 13 | Resists changing of body-worn pads | Check awareness of good communication techniques for people with dementia to reduce anxiety/fear/embarrassment/reluctance (Info. Sheet 5) Use behavioural management techniques to problem solve |
Consider a different design of body-worn pads | Advice from: Continence specialist nurse PromoCona Disabled Living Foundation Consider potential for assessment and advice by other professional, e.g. GP, CPN, clinical psychologist |
| 14 | Removes all body-worn pads (dry and/or wet) | Use behavioural management techniques to problem solve (Information Sheet 4) | Consider a different design of body-worn pads, e.g. pull-up pant designs | Advice from: Continence specialist nurse PromoCona |
| 15 | Leakage from body-worn pads | Ensure the product is being used correctly Ensure other issues are not interfering with the product’s effectiveness, e.g. over-liberal use of barrier creams |
Consider a different design or absorbency of body-worn pads (Information Sheet 6) Information about, and opportunities to test different designs of pads |
Advice from: Continence specialist nurse PromoCona |
| 16 | Problems voiding at night | Consider pattern of fluid intake, aiming for 1.5 l per day but reducing intake and type in the evening (cross reference to fluids and diet information in local assessment tools) | Night-time lights with motion sensors Consider commode/urine bottles for night-time use |
Consider whether medications contributing to nocturia and refer for review as appropriate Consider potential for other professional to address any causes of nocturia, e.g. GP Advice from: OT |
| 17 | Problems in managing outside the home | Consider carrying a change of lower garments in case of ‘accidents’ | Provide information on RADARb keys | |
| 18 | Concerns about ‘smells’ | Awareness of smell-neutralising products Consider removing items where accidental spills may not be noticed, e.g. toilet mats |
Advice from: Continence specialist nurse PromoCona |
|
| 19 | Problems in faecal matter in the wrong place (smearing, clearing up inappropriately and concealment of faeces) | Consider whether there are problems of constipation which can be addressed (local guidance applies) NB. The innate response of curiosity can mean people investigate (feel for) the ‘problem’ which often results in faeces on hands Use behavioural management techniques to problem solve |
Toilet signage | Consider potential for assessment and advice by other professional, e.g. GP, CPN, clinical psychologist, continence specialist nurse PromoCona |
| 20 | Problems in that the person takes multiple doses of laxatives, having forgotten taking them before, resulting in diarrhoea | Consider the use of medication aids such as dosette boxes Advise on medication storage |
Consider potential for assessment and advice by other professional, e.g. GP | |
| 21 | Problems of disposal of used body-worn pads | Awareness of principles of hygiene and waste disposal | ||
| 22 | Problems of concealment of soiled clothing or pads | Use behavioural management techniques to problem solve | Consider potential for assessment and advice by other professional, e.g. GP, CPN, clinical psychologist | |
Ethics
The NHS research ethics service advised the researchers that the study did not require NHS research ethics review. The study adhered to university ethical conduct of research requirements.
Results
The three stages of the Delphi consultation involved 46 carers and professionals (Figure 15).
FIGURE 15.
The three-stage Delphi consultation and participants.
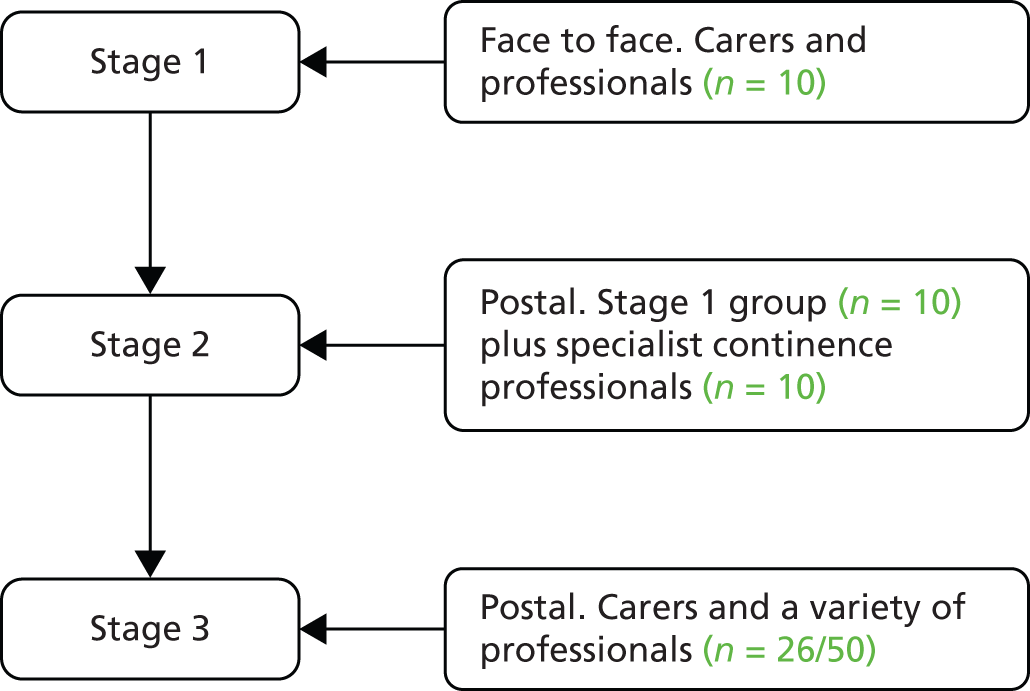
At stage 1, the key principle agreed by the group was that the typical NHS continence assessment tools should be turned ‘upside down’, and that the first question in the tool should be an enquiry about the problem and its impact as defined by the person (if able) and their carer. At stage 3, 26 experts, of the 50 invited, returned the questionnaire (Table 51).
| Respondents’ area of expertise | No. of responses |
|---|---|
| Carer | 8 |
| GP | 2 |
| Geriatrician/psychogeriatrician | 1 |
| Continence nurse specialist | 3 |
| District nurse/community nurse | 7 |
| OT | 2 |
| Other | 3 |
| Total | 26 |
The majority of respondents (87%) perceived the tool as being likely to improve primary care professionals’ recognition of the toileting and incontinence problems experienced by people with dementia and their family carers. The GPs could see the value of the tool as an educational rather than a practice aid. All agreed that the first two questions, on the problem and the impact, were important. Most agreed on the importance of the items within the tool, although not all items were supported equally. For example, the carers did not rank the question regarding awareness of benefits as important. Caveats to the value of the tool on its own were added, such as the need for training. The results in full are described elsewhere. 183
Discussion and conclusion
The face and content validity of the dementia-focused continence assessment tool were established. The study had limitations in the response rate from some groups but provides a breadth of opinion not provided elsewhere on such an assessment tool. The involvement of family carers (i.e. experts by experience) in the first two stages ensured that the perspective of service users was present. Continence services in the UK have been repeatedly criticised for the absence of service user involvement. 158 To our knowledge there have been no other studies developing continence assessment tools tailored to people with dementia. Using a three-stage adapted Delphi consultation technique we created a tool (see Figure 14) along with supporting resources (Table 50) that is person centred120 rather than constructed from a biomedical perspective. The challenge, as pointed out by a number of participants, is how to ensure that those using the tool also adopt a person-centred approach and have a repertoire of strategies to address the problems identified.
Summary of the main findings
This study investigated ways in which toileting and incontinence problems could be addressed for people with dementia living in their own homes. The study design drew on the MRC framework for the development of complex interventions. 101
The first phase was of one of review. Little evidence was found on prevalence, effective conservative interventions or local clinical guidance or provision of NHS-funded incontinence products tailored to the needs of this population. Our nested cohort study provides data on incidence of incontinence in this population for the first time.
The second phase explored the experiences and strategies of people with dementia, their family carers – including longitudinally – and of HSC professionals. People with dementia and their family carers experienced multiple impacts, including humiliation, distress, quality of life, and financial and iatrogenic consequences from some care services. The carer strategies emphasised preservation of dignity and effective containment of excreta. Decision-making balanced both the needs of the person with dementia and the family members. Some solutions had the potential to have unintended, negative consequences for the person with dementia. HSC professionals varied in the extent to which they recognised the range of problems and, consequently, in their repertoire of possible strategies.
The third phase explored the feasibility of investigating the effectiveness and acceptability of different designs of incontinence pads with people with dementia. Although there were significant problems in recruitment, those who volunteered were committed to the study. The study also revealed the types of criteria family carers used in assessing designs of incontinence pads. This included safety and efficiency in changing pads as well as effective containment of excreta. These specifications changed in different settings, for example outside the home and at night-time.
In the fourth phase, a continence assessment tool (Figure 14) and supporting resource (see Table 50) were designed with an expert group and bench tested through a Delphi process. This used a person-centred approach and was tailored to the needs of people with dementia living at home. It was found to have good face and content validity, although it raised questions regarding whether or not it and the supporting materials alone could change professional practice. In addition, material from this and other phases were used to provide continuing professional development education materials for GPs,184 community nurses185 and social care staff.
Strengths and limitations
These have been described at each phase. In overview, the study strengths were the presentation of new knowledge not previously reported in each phase. The iterative processes between the phases allowed for greater reflexivity and responsiveness to both findings and the input from carers of people with dementia as well as professionals. The main weakness lies in the failure to always achieve the planned sample size, and the consequences of this for external validity. However, dementia and incontinence are both stigmatised conditions and the discussion of excreta, particularly faeces, is often a taboo subject in all societies. 167 This study was successful in engaging people with dementia and family carers in research on incontinence, and is a strength in both the research approaches and findings. The failure to always engage practitioners in studies indicates that this population and these types of problems are not necessarily perceived as a priority.
Interpretation of the findings
The study has addressed all of the research questions presented at the beginning of the chapter.
First, each phase of the study was able to add to the body of knowledge that primary care practitioners and commissioners can draw upon, because there is very little other evidence or guidance available tailored to this population. 117,118 Toileting and incontinence problems are neglected among those who have been, until relatively recently, an overlooked population: people with dementia living at home.
Second, the depth and breadth of the description of the problem of incontinence and its impact, provided in this study, expand and illuminate accounts of people with dementia in care homes186 and studies of older adults with incontinence but without cognitive impairment. 166,170
Third, a key message from this study for primary care professionals was to proactively enquire at each contact about toileting/bladder and bowel problems. The range of problems (from those amenable to medical treatment such as urinary tract infections through to voiding in inappropriate places) suggested a stepwise approach to identifying suitable and acceptable management strategies. The findings indicated that primary care professionals need a repertoire of strategies that draws on knowledge of cultural, psychological, behavioural, biomedical, technical and environmental aspects, as well as local systems, in order to help solve practical problems. This study has been able to develop resources for primary care practitioners that take cognisance of all factors (see Figure 14 and Table 50). There is little other research detailing this mosaic of interlocking incontinence problems, although it is evident in the report of eight case studies in Australia. 187 Expert opinion tends to focus on physical practicalities117 or behavioural techniques188 as solutions. It has highlighted for the first time that some strategies adopted by carers (family and paid care workers) may be unintentionally detrimental to the person and that health-care professionals need to be alert to this possibility.
Fourth, the study has developed and tested the first person-centred tool for continence assessment by primary care nurses, tailored to people with dementia living at home. This requires further field testing and evaluation.
Fifth, the study has demonstrated the high priority that family carers place on adequate containment of excreta, and its link with carer quality of life. The evidence of less-than-helpful responses from some NHS continence services supports other accounts from service users about variation in service quality and lack of professional help. 145 This study provides further evidence to support a continence paradigm shift from cure and prevention to very good containment as proposed by the International Continence Society expert group on UI in frail elders. 117 A pilot study has been conducted that investigated the acceptability and effectiveness of different continence pad designs with people with dementia living at home and their carers. The results indicate that a clinical trial of designs of pads is feasible, although with caveats on recruitment, as discussed below.
Sixth, the study highlighted the difficulties in recruiting people with dementia and their family carers to observational studies that address issues of incontinence. This has also been reported in studies attempting investigations of interventions in the USA149,150 and Australia. 187 The influence of embarrassment and stigma on clinical practice and research for both dementia and incontinence is well documented. 4,116
Conclusions
Incontinence has been described as a ‘geriatric giant’,189 a significant problem among older adults, and the evidence from this study suggests that it is yet to be ‘tamed’ for those with dementia living at home. The reasons are multifactorial and a mosaic of strategies is required to both reveal the problems and manage them in ways that are acceptable to the person with dementia and their family members. This study suggests that there are strategies and responses that primary care professionals and others can use to encourage greater openness that may reduce the taboo of incontinence within the stigma of dementia. It remains to be seen if these approaches, combined with more emphasis on effective containment of excreta, influence the point at which decisions are made for a person with dementia to move to a care home.
Impact
In addition to the impact through the presentations at events attended by HSC professionals, people with dementia and their family carers (see Tables 70 and 71), the study can point to two other types of impact. The first was the award to the study by the Royal College of General Practitioners for Research Paper of the Year 2011 in the category of dementias and neurodegenerative diseases. 169 The second relates to the invitations to provide materials on incontinence and dementia for the continuing professional development of:
-
GPs by the British Medical Journal Quality group184
-
nurses by the Royal College of Nursing-published Nursing Standard185
-
adult care staff by the South East England DH and the Social Care & Local Partnerships Programme.
Building research capacity
Laura Cole, research associate, has undertaken part-time doctoral study registered at St George’s University of London.
Changes to protocol
Owing to recruitment problems, the continence element of the programme did not undertake a longitudinal cohort study. Instead, a longitudinal qualitative study was agreed and undertaken. (Agreed by the Programme Director, December 2010.)
List of appendices
Appendix 17 Chapter 3: Overall EVIDEM-C protocol 2007.
Appendix 18 Chapter 3: Protocol – Exploring issues and solutions in promoting continence and managing incontinence with people with memory problems living at home and their carers, 2008.
Appendix 19 Chapter 3: Protocol – Professional views of current issues and solutions in promoting continence and managing incontinence with people with memory and cognition problems living at home.
Appendix 20 Chapter 3: Protocol – Investigating the experience of managing continence problems over time, version 2, 2010.
Appendix 21 Chapter 3: Protocol – Incidence and management of incontinence in general practice patients with dementia; an analysis of THIN data, 2011.
Appendix 22 Chapter 3: Protocol – Investigating the acceptability, effectiveness and associated costs of different types of absorbent products used for incontinence by people with memory problems living at home, 2010.
Appendix 23 Chapter 3: Protocol – a modified Delphi consultation to develop a dementia-focused continence assessment tool for use with people with dementia living at home, 2010.
Appendix 24 Chapter 3: Adoption by research networks and research permissions.
Appendix 25 Chapter 3: EVIDEM-CL1 March 2011 – briefing for Local Authority Adult Services managers and social workers.
Research instruments and toolsAppendix 26 Chapter 3: Aide-memoire for guided conversations with the person with dementia.
Appendix 27 Chapter 3: Aide-memoire in the qualitative interview study with family carers.
Appendix 28 Chapter 3: Interview tools to characterise the person with dementia and the carer in the longitudinal study and absorbent pads study.
Appendix 29 Chapter 3: Validated tools used in the longitudinal study and the absorbent pads study.
Appendix 30 Chapter 3: Toileting difficulties and incontinence questions used in addition to the ICIQ-UI and ICIQ-N, for the longitudinal study and the absorbent pads study.
Appendix 31 Chapter 3: Further incontinence questions about pad usage for the longitudinal study.
Appendix 32 Chapter 3: Additional table in the Client Service Receipt Inventory (part 1) longitudinal and absorbent pads study.
Appendix 33 Chapter 3: Data collection tools for examining feasibility and acceptability in the absorbent pads study.
Appendix 34 Chapter 3: Additional Table in the Client Service Receipt Inventory (part 1) for the absorbent pads study.
This includes the overall EVIDEM-C protocol as written in 2007, plus the subsequent protocols written for individual elements. Study tools and instruments are in Appendices 26–34. Appendix 24 lists the applications made for study adoption, research governance permissions and NHS service support costs for research. Details of research ethics review are given with each study in the main report.
Chapter 4 EVIDEM-EoL: quality of care at the end of life
Abstract
Timely and appropriate agreements between visiting health-care professionals and care home staff regarding end-of-life care for older people with dementia (OPWD) are difficult to reach. This study tracked care received by 133 OPWD over 18 months in six residential care homes, including medication review and economic evaluation. Interviews with residents, care home and visiting NHS staff focused on their views of end-of-life care for OPWD and ways of working. Just over 20% of the resident cohort died. There were no significant differences between those who died and those still alive in terms of sex, age, care home, duration of prior residence or a formal diagnosis of dementia. Sedative load was not significant but inappropriate prescribing was. Providing end-of-life care to OPWD was characterised by three types of uncertainty: treatment uncertainty related to difficulties in prognostication and how to interpret and manage key events and symptoms; relational uncertainty related to roles, responsibilities and relationships among those involved; and service uncertainty related to service capability.
Phase 2 used a co-design approach [Appreciative Inquiry (AI)] with three of the six care homes that built on existing relationships and expertise and, when appropriate, end-of-life care tools. The intervention was evaluated in terms of its ability to address the different types of uncertainty. AI did not increase resource use and there was a reduction in hospital costs. The intervention supported a shift in care home culture that could mitigate uncertainties inherent to end-of-life care of OPWD and different ways of working between NHS and care home staff.
Introduction
Background: the need for this study
People with dementia have a limited life expectancy and the type of end-of-life care that they need has been widely discussed. 190–193 These discussions focus particularly on the place of care and on the adaptation of existing end-of-life care pathways, but people with dementia experience different trajectories of functional decline and have different needs. 194,195
Communication problems, confusion, functional loss, nutritional deficiencies and complications, such as infections and incontinence, can compound the distress of a person who is dying, challenge clinicians’ knowledge and expertise,196 and result in inappropriate medical interventions and referral to hospital. 197–199
There are significant methodological challenges in researching end-of-life care for people with dementia. 200,201 Most research in this area tends to rely on proxy or retrospective accounts of particular symptoms and events. Fewer studies have addressed the environment and organisation of care, or decisions by different practitioners on individual patient outcomes.
In the UK, the quality of care of people with dementia in care homes is variable. 202–204 The cognitive ability and dependency of residents vary between care homes, and there is a considerable overlap in residents’ needs between homes with on-site nursing and those without. 205 Many factors influence how care homes work with primary health-care services. These include ownership, staff turnover, the qualifications of the care home manager, access to specialist palliative care, financial incentives offered to GPs, and the history of innovation within the home. 206–210 There is little evidence that care home residents, relatives and staff are involved in identifying priorities for end-of-life care for people with dementia, or in developing models of care that go beyond the individual patient-focused encounter. There is a need to develop and test dementia-specific approaches to end-of-life care for this often marginalised population. 211,212
The NHS End of Life Care Programme for care homes209 seeks to ensure that when residents are dying they can avoid emergency hospital admissions and be supported by care home and NHS staff who are skilled in providing end-of-life care. Care homes are being encouraged to use end-of-life care support tools, such as the Gold Standards Framework (GSF),213 Preferred Priorities for Care,214 and the Liverpool Care Pathway (LCP). 215 These interventions increase staff confidence and knowledge, reduce unplanned hospital admissions, and allow more people to die in their preferred place of care. 216,217
Research questions
This study had two phases. In the first phase the research question was, what are the pathways to death of OPWD living in a care home? In the second phase the research question was, can an intervention be developed to promote integrated working between primary care and care home providers, to improve end-of-life care for OPWD?
Methods
The methodology used in Phases 1 and 2 of the study is detailed in the study protocols shown in Appendices 35 and 36.
Phase 1 objectives
Phase 1 objectives were to:
-
characterise OPWD living in care homes and describe their respective pathways to death, including a survival analysis to identify indicators of the end of life
-
describe the care and support needs of this population, and of their carers
-
establish how care home staff and NHS primary care practitioners define, assess and provide end-of-life care for this population
-
describe how different contexts and models of care in care home environments influence experiences of end-of-life care
-
identify the treatments and interventions received (including medication and potentially inappropriate prescribing), and services and resources used, leading up to death.
Study design
Phase 1 used a prospective, mixed-methods design including case notes analysis, interviews, mapping of service use and economic evaluation. Data from residents’ care notes were extracted at three time points (Table 52). Care notes of residents who had either died or left the home before the end of the data collection period were reviewed to the date of death or leaving the home.
| Time point | Dates |
|---|---|
| Baseline | March 2009 to July 2009 |
| Time point 2 | July 2009 to November 2009 |
| Time point 3 | December 2009 to April 2010 |
Unstructured conversational interviews were undertaken with a purposively selected sample of older care home residents with dementia. The manager and, where possible, deputy manager, as well as two NHS professionals (e.g. GP and district nurse) with ongoing involvement with each home were interviewed. Emergency care practitioners and paramedics were also interviewed to establish how they interpreted their responsibilities when called to an older person with dementia in a care home. Care home notes of older people who died during Phase 1 were reviewed to document services received prior to death, the place of death and the care provided. These reviews were complemented by interviews with care home staff. Interview schedules and data extraction forms are shown in Appendices 37–46. Interview information sheets are shown in Appendices 49–51.
Inclusion and exclusion criteria
Inclusion and exclusion criteria for care homes and OPWD are detailed in Appendix 35. Inclusion criteria for care home residents were age ≥ 65 years, and a documented diagnosis of dementia or an assessment by the senior care worker that their level of cognitive impairment and behaviour were consistent with a diagnosis of dementia. Exclusion criteria were people with dementia who did not speak English, those whom the care home manager thought it inappropriate to approach (e.g. people in the terminal stage of the disease) and individuals who lacked capacity to consent and for whom a consultee could not be identified.
Information sources
Care home characteristics were collected using Annual Quality Assurance Assessment (AQAA) forms completed for the study by the care home managers (Table 53). Missing data were obtained through Care Quality Commission (CQC) listings and care home managers’ interviews.
| Care home | |||||
|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 |
| April 2007 | July 2008 | March 2009 | June 2008 | March 2009 | March 2009 |
Sample
Details of the recruitment process used for both the care homes and the OPWD are reported elsewhere210 (see also Appendix 35). A total of 214 residents were eligible for participation in the study across the six care homes. Care homes were identified using the CQC (previously known as the Commission for Social Care Inspection) directory of care homes. Inclusion criteria were that the care home was for older people, provided personal care and specialist support in dementia care, had a minimum of 20 beds and there was no onsite nursing provision. To optimise the opportunities for participation, a further inclusion criterion was that the last CQC inspection report was favourable and that care home staff considered that they have a good working relationship with their local primary care services. Of the 10 care homes approached, six agreed to participate. The six care homes had a mix of ownership and geographical location.
A staged approach to the recruitment of OPWD was adopted, involving separate meetings with staff, residents and relatives. Individual consent was secured with residents with dementia who could consent in the moment and for those without capacity, through letters and follow-up contact with consultees.
Minimum data set
Areas of data collection are detailed in Appendix 35. Participants’ health and care needs over time were documented, as well as service utilisation (using an adapted version of the CSRI). 88 Additional data on residents were obtained using the DAD,176 the Barthel ADL Index,218 the Cohen–Mansfield Agitation Inventory (CMAI),219 and the Cornell Scale for Depression in Dementia,220 completed by home managers and senior carers.
Analytic methods
Case notes review and interviews
Data extracted from residents’ care notes were analysed using SPSS version 20. Interviews were digitally recorded, anonymised and analysed thematically using NVivo version 8. Two researchers undertook the analysis and discussion of key themes and issues.
Review of resident deaths
Health events and care received in the month preceding death were extracted from care notes and through interviews with care home managers and/or senior care workers. Data were summarised in timelines depicting deceased residents’ end-of-life trajectories, using Microsoft Visio™ software version 2010 (Microsoft Corporation, Redmond, WA, USA). Two researchers were involved in defining dying trajectories, using an inductive approach to classification of Visio timelines.
Medication review
Residents’ medical administration records were reviewed to (1) determine the sedative load and use of sedative and psychotropic medications and (2) estimate the prevalence of potentially inappropriate prescribing (PIP) among OPWD in care homes. To determine the sedative load and use of sedative and psychotropic medications, regular medications were classified using the Anatomical Therapeutic Chemical classification system and individual sedative loads were calculated using a model to quantify the cumulative effect of taking multiple drugs with sedative properties (see Parsons et al. 221 for further detail). The prevalence of PIP was determined using 31 of the 65 Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria (see Parsons et al. 221 for further detail). 222
Characteristics of older people who died compared with those alive
Examination of characteristics of older people who died compared with those alive using Kaplan–Meier methods (significance values where quoted are for the log-rank test) was undertaken to examine if there were indicators that residents were reaching end of life. The analysis was restricted to residents who met the following criteria: (1) residents who were alive and resident in the care home at the date of the baseline data collection and (2) residents who had a valid date at which their survival time was censored (at date of either death or transfer, or recorded alive at date of last data collection).
Costing analysis
Objectives
-
To compare the resource utilisation patterns between residents who were alive and dead by the end of Phase 1.
-
To establish the estimated costs for supporting end-of-life care for OPWD in care homes.
Descriptive statistics derived using SPSS version 20 were used to report estimated service use and costs. The Wilcoxon rank-sum test (two independent samples test) was used to detect differences in service utilisation and costs between residents who were alive and who had died by the end of Phase 1. HSC unit costs estimates were taken from the Unit Costs of Health and Social Care 2010. 223 Medication costs were estimated using information from the British National Formulary. 224 The monthly accommodation cost per resident was based on the average of the accommodation costs between the two time points. For those residents with missing records, the overall average accommodation cost was used.
Ethical considerations
The protocol of Phase 1 of the EVIDEM-EoL study entitled ‘Changing practice in dementia care in care homes: developing and testing evidence-based interventions at the end of life’ (REC reference: 08/H0502/74) received a favourable ethical opinion from the Southampton & South West Hampshire REC (A) on 14 July 2008.
Public involvement
Two members of the Public Involvement in Research Group (PIRG) based at the University of Hertfordshire provided support to the study. Both were recruited because of their particular expertise and experience of dementia and mental health services. One member of PIRG was a member of the project advisory group, attended coffee mornings at the care homes to explain the study to residents, was involved in some of the qualitative analysis, including the interviews with residents, and commented on the final report. Both members commented on various study materials throughout the project, including care home newsletters. The steering group was chaired by a member of the PIRG who had been a carer of someone with dementia and three other members of the group were PPI representatives, all of whom had been carers of people with dementia who had died in a care home.
Phase 2: Intervention objectives
The intervention phase objectives were to:
-
identify interventions/ways of working to support integrated working between care home staff and primary health-care services
-
test ways that primary health-care services and care home staff can work together to reflect the priorities, experiences and concerns of OPWD living in care homes at the end of life
-
consider how available end-of-life care frameworks help primary care and care home staff to manage uncertainty at the end of life
-
identify facilitators and barriers to sustaining effective end-of-life care for people with dementia living in care homes.
Study design
Phase 2 used a co-design approach (AI)225,226 to bring together care home and visiting NHS staff to identify what worked well in end-of-life care for people with dementia, and to use that as a basis to plan and implement change. The exploration and enhancement of what is working well in an organisation is often framed as a ‘4-D cycle’ (Box 5). The cycle is often conceptualised as discrete steps, although the process is non-linear, iterative and ongoing.
Appreciating the best of what is Conversations between participants (within the chosen topic area) reveal stories of people working at their best. In the EVIDEM-EoL study the topic was the best experience of collaborative working in caring for a resident dying with dementia in a care home.
DreamEnvisaging what might be The stories are collectively discussed in order to generate new ideas, understanding and agreeing a shared future image of the future. This shared future is often summed up in a statement of intent. For example, in the EVIDEM-EoL study, one care home created the future ideal: ‘All residents who wish to can live and die here, their home’.
DesignTogether, create innovative ways to make it happen Participants choose and prioritise what needs to change to enable participants to move towards the agreed future and identify resources to enable these changes to occur. Within the EVIDEM-EoL study the design phase focused on one context-specific innovation per site.
DestinySustaining the change Together, participants think about how to involve the wider organisation and systems necessary to ensure changes are sustained and spread.
The 4-D cycle was implemented over three facilitated hour-long AI sessions over 6 months. The AI method and session design are detailed in Appendix 47 (for further detail regarding the design of the intervention, see Nicholson et al. 227). Phase 2 tracked the events and care experienced by OPWD living in the care homes who participated in the intervention phase. Data were extracted from residents’ care notes at two time points, staggered over 7 months of data collection that ran from January to July 2011.
Baseline data extraction coincided with the start of the intervention (Table 54). Researchers reviewed participating residents’ care notes over the 3 months prior to the start of the intervention or closely thereafter and again in July 2011 after the third and final round of intervention sessions in the care homes. Baseline data were collected in this way for 90% of the study sample. Baseline data for the remaining residents (n = 8) were collected between mid-March and mid-April 2011 owing to delays in obtaining consent.
| Session | Care home | ||
|---|---|---|---|
| CH1 | CH5 | CH6 | |
| 1 | 25 January 2011 | 3 February 2011 | 19 January 2011 |
| 2 | 15 March 2011 | 24 March 2011 | 14 March 2011 |
| 3 | 28 June 2011 | 29 June 2011 | 20 June 2011 |
Impact of the Appreciative Inquiry intervention
The impact of the AI intervention on integrated working in end-of-life care was evaluated through analysis of resident deaths following the intervention and resource use before and after the intervention.
Resident deaths were examined through care note reviews, complemented by interviews with care home staff, for changes in services received prior to death, place of death and care provided following the intervention.
Resource use was examined among residents who participated in both phases of the study (see Costing Analysis, below, for further details).
Finally, postintervention interviews with all participants following the third and final AI session (see Appendix 47, interview schedule) were undertaken to examine perceived changes in working practices.
Minimum data set
A minimum data set of information was generated, as in Phase 1. Additional data on residents were obtained using the Clinical Dementia Rating Scale,228 completed by care home managers and senior carers.
Sample
Care home and resident samples
The six care homes that participated in Phase 1 received feedback and summaries of the Phase 1 findings, and all care homes were then invited to participate in Phase 2. Three of the six homes agreed to join the intervention phase, with a total of 154 residents eligible for participation.
Intervention sample
The GP practices and district nurses approached in Phase 2 were the same as those recommended to researchers in Phase 1. In two of the practices [attached to care homes 5 and 6 (CH5, CH6)] the GPs who had participated in Phase 1 both suggested other GPs at the practice to interview. Only the district nurse associated to CH6 participated in both phases of the study.
Care homes involved staff as they were able, with interviews being completed with all of the managers. The manager of CH5 attended two sessions before leaving her post. This manager had not yet been replaced by the third AI session, which was held with the GP and deputy manager only.
Relatives sample
A significant amendment was made to the Phase 2 protocol in 2011 following a recommendation from the study’s advisory group to include relatives of care home residents both living and deceased. Relatives were recruited via study information leaflets left in the care homes, and through care home managers. Researchers also approached potential participants at relative group meetings normally held in the care home. Five participants were recruited in this way, including one relative living at the care home with his/her partner.
Analytic methods
Interviews and care note data were analysed as in Phase 1. The facilitated meetings were digitally recorded, anonymised and analysed thematically using NVivo version 8 to organise and manage the analysis. Two researchers were involved in the analysis and discussion of key themes and issues.
Costing analysis
Objectives
-
To establish the estimated costs for supporting end-of-life care for OPWD in care homes for Phase 2.
-
To compare the resource utilisation patterns between the same residents who were in both phases.
Patterns of service utilisation and estimation of costs were carried out as in Phase 1.
Ethical considerations
The intervention phase of the EVIDEM-EoL study entitled ‘EVIDEM-End of Life: Changing practice in dementia care in care homes: developing and testing evidence-based interventions at the end of life – Phase 2’ (REC reference: 10/H0502/55) received a favourable ethical opinion from the Southampton & South West Hampshire REC (A) on 10 August 2010.
Results from Phase 1
Care home characteristics
Care homes were a mix of provider type, size, location, building structure and religious affiliation (Table 55).
| Characteristic | Care home | |||||
|---|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | |
| Provider type | Private not for profit | Private not for profit | Private not for profit | Voluntary | Private | Private |
| No. of places | 46 | 62 | 60b | 66 | 67 | 57 |
| No. of dementia places | 46 | 61 | 60 | 66 | 67 | 57 |
| Location | Suburban | Urban | Suburban | Urban | Rural | Rural |
| Building | Former LA | Purpose built | Purpose built | Purpose built | Conversion | Purpose built |
| Religious affiliation | No | No | No | Yes | No | No |
Resident funding status and care home fees, as well as staff numbers, working patterns and qualifications are shown in Tables 56–58.
| Characteristic | Care home | ||||||
|---|---|---|---|---|---|---|---|
| CH1 (n = 44) | CH2 (n = 58) | CH3 (n = 57) | CH4 (n = 62) | CH5 (n = 66) | CH6 (n = 54) | Total N (%) | |
| Provider type | Private not for profit | Private not for profit | Private not for profit | Voluntary | Private | Private | |
| Privately funded | 7 (15.9%) | 0 (0%) | 32 (56.1%) | 47 (75.8%) | 17 (25.8%) | 27 (50.0%) | 130 (38.1%) |
| Publicly funded | 37 (84.1%) | 58 (100%) | 25 (43.9%) | 15 (24.2%) | 49 (74.2%) | 27 (50.0%) | 211 (61.9%) |
| Characteristic | Care home | ||||||
|---|---|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Average | |
| Min. cost per week (£) | Unavailable | 495 | 595 | 525 | 500 | 420 | 507 |
| Max. cost per week (£) | Unavailable | 580 | 695 | 640 | 660 | 600 | 635 |
| Cost range (£) | Unavailable | 85 | 100 | 115 | 160 | 180 | 128 |
| Characteristic | Care home | |||||
|---|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | |
| Total no. of caring staff | 41 | 52 | 43 | 42a | 40 | 41 |
| Full time, n (%) | 5 (12.2) | 14 (26.9) | 20 (46.5) | 23 (54.8)a | (59)c | 26 (63.4) |
| Part time, n (%) | 36 (87.8) | 38 (73.1) | 23 (53.5) | 19 (45.2)a | (41)c | 15 (36.6) |
| Agency (temporary) care staff | 0 | –b | –b | 1a | –b | –b |
| Full-time direct care workers to residents | 0.89 | 0.9 | 0.75 | 0.68 | 0.61 | 0.76 |
| Non-caring staff members | 21 | 11 | 32 | –b | 11 | 22 |
| Direct care workers holding NVQ at Level 2 or above, n (%) | 14 (34.1) | 31 (59.6) | 19 (44.2) | 19 (47.5) | 26% (exact number unavailable) | 29 (70.7) |
| Direct care workers turnover in past 12 months | 13 (1FT;12 PT) | 25 (FT/PT not specified) | 5 (FT/PT not specified) | 4 in past 6 monthsa | 6 (FT/PT not specified) | 8 (FT/PT not specified) |
| Manager’s time in post, years | 4.5 | 2 | 3 | 3 | 3 | 6 |
| Manager dementia traininga | Yes | Yes | Yes | Yes | Yes | Yes |
| Manager end-of-life traininga | Yes | No | No | Yes | Yes | Yes |
| Volunteersa | Yes | No | Yes | Yes | No | No |
Organisation of care
All of the care homes operated a key worker system with a named care worker. Most operated teams headed by a senior care worker/team leader and tried to keep the same teams for continuity of care. Only one care home had permanent night staff. Training in most care homes was ongoing and in-house (see Appendix 55, Table 106). Frameworks for structured end-of-life care, such as the LCP215 or GSF213 for Care Homes, appeared rarely, if ever used (see Appendix 55, Table 107).
Resident needs
Resident needs are shown in Appendix 56, Table 108. Overall, care home 1 is characterised by the highest concentration of dependent older people with advanced dementia. Residents at care homes 3 and 4 had similar levels of dependency but less of dementia-related dysfunction. Residents in care homes 5 and 6 were more heterogeneous, evidenced by wider ranges of cognitive and functional ability.
Reported number and location of resident deaths in the year prior to the study are shown in Table 59. Between 30% and 50% of residents who had died in the year prior to the study died in hospital or in a hospice.
| No. of deaths in year prior to study | Care home | |||||
|---|---|---|---|---|---|---|
| CH1: 12 | CH2: 12 | CH3: 16 | CH4: 14 | CH5: 10 | CH6: 3 | |
| At home, n (%) | 7 (58.3) | 7 (58.3) | 11 (68.8) | 7 (50.0) | 7 (70.0) | 2 (66.6) |
| At hospital or hospice, n (%) | 5 (41.6) | 5 (41.6) | 5 (31.3) | 7 (50.0) | 3 (30.0) | 1 (33.3) |
| Short-term/temporary residents in last 12 months before completion of AQAA | 51 | 63 | 73 | 1 | 12 | 3 |
Access to primary and specialist palliative care
General practitioners
Care homes arrangements with GPs differed considerably (see Appendix 57, Table 109). For example, some GPs had set days to visit (but would also visit at other times if necessary), whereas others visited on request only.
District nurses
All the care homes had frequent visits from district nurses. These nurses also provided diabetes care, wound and continence care, as well as ordering equipment from medical loans or equipment services, for example pressure-relieving mattresses. Their involvement in end-of-life care was variable and in one care home the district nursing team had no history of such involvement.
Out-of-hours general practitioners and emergency ambulance services
Care homes frequently called on the services of out-of-hours (OOH) GPs. The decision to call the emergency services for distressing symptoms was often linked to staff confidence in managing end-of-life care.
Specialist care
None of the care homes had Admiral Nurse support or used night-sitter services. Counsellor services for staff were available on request via GP referral for only two of the six care homes. Only three of the care homes received any kind of regular support from a Community Mental Health Team. Three out of the six care homes reported never receiving support from palliative care specialists. The care homes that had access to specialist services reported variable patterns of contact, and in one home the service was restricted to residents with cancer.
Phase 1 resident baseline characteristics (n = 133)
Recruitment and retention
One hundred and thirty-three OPWD were recruited, representing 62% of the population (see Appendix 58, Table 110).
Resident age, length of stay, admission route into the home
Appendix 58 (Tables 111 and 112) shows resident characteristics, including age, length of stay and admission route into the home. Median age at admission ranged from 82.7 years in CH5 to 87.5 years in CH3. Length of residency ranged from 0.2 years in CH6 to 3.4 years in CH2. A total of 43% of residents were admitted to the care home from their own home; 6% were admitted from a relative’s home; 29% from hospital; 11% from another care home; and 11% from sheltered/warden-controlled housing.
Cognitive function, activities of daily living, behavioural status, distressing behaviour
Over one-quarter 26.3% (n = 35) of the sample did not have a formal diagnosis of dementia recorded (see Appendix 58, Table 113) but were identified by care home staff as having memory problems and/or behaviours consistent with a diagnosis of dementia (e.g. memory lapses, disorientation to time and place, lack of ability to problem solve, needing help/prompts for personal care). Of those who had a formal diagnosis of dementia, 70% did not have the source of their dementia diagnosis recorded.
Resident needs in relation to ADL were documented in the areas of continence, personal care, maintaining a safe environment, and eating and drinking, and are shown (see Appendix 58, Tables 114 and 115).
Assessment of all residents using validated tools including the DAD scale,176 CMAI219 and Cornell Scale for Depression in Dementia220 confirmed the presence of symptoms that were consistent with a diagnosis of dementia (Tables 60 and 61). Care home 1 was characterised by a high concentration of dementia-related disability, as evidenced as by DAD scores ranging from 0% to 20% (see Table 61). Care homes 5 and 6 are characterised by wide ranges of disability levels as evidenced by DAD scores ranging from 2.5% (i.e. more dysfunction) to 100% (no disability).
| DAD | Care home | ||||||
|---|---|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Total | |
| n | 18 | –b | 15 | 22 | 32 | 14 | 101 |
| Median | 11.25 | –b | 12.5 | 10 | 29.14 | 40 | 17.5 |
| Mean (SD) | 10.42 (6.26) | –b | 17.50 (15.50) | 16.88 (16.90) | 32.88 (25.54) | 35.50 (24.77) | 23.47 (21.83) |
| Range | 0–20.00 | –b | 0–42.50 | 0–48.72 | 2.50–100.00 | 2.50–100.00 | 0–100.00 |
| Characteristic | Care home | ||||||
|---|---|---|---|---|---|---|---|
| CH1 (n = 19) | CH2 | CH3 (n = 15) | CH4 (n = 22) | CH5 (n = 32) | CH6 (n = 14) | % of total N = 102 | |
| CMAI219 (n = 102) | |||||||
| Aggressive behaviour, n (%) | 3 (15.8) | –b | 2 (13.3) | 4 (18.2) | 6 (18.8) | 1 (7.1) | 16 (15.7) |
| Physically non-aggressive behaviour, n (%) | 8 (42.1) | –b | 6 (40.0) | 12 (54.5) | 15 (46.9) | 11 (78.6) | 52 (51.0) |
| Verbally agitated behaviour, n (%) | 11 (57.9) | –b | 9 (60.0) | 11 (50.0) | 14 (43.8) | 7 (50.0) | 52 (51.0) |
| Cornell Scale for Depression in Dementia220 (n = 101) | |||||||
| Indicating probable depression (i.e. score of ≥ 12), n (%) | 5 (27.8) | –b | 0 (0) | 0 (0) | 5 (15.6) | 2 (14.3) | 12 (11.9) |
Around 15% of the overall sample displayed aggressive behaviour and around 50% of the sample displayed both physically non-aggressive and verbally agitated behaviour. Only 12% of the overall sample was assessed on the Cornell Scale for Depression in Dementia scale as exhibiting symptoms of depression (see Table 61).
Average number of comorbidities and prescribed medications
Residents had a wide range of documented conditions and symptoms, with just under half experiencing three or more comorbidities (Table 62). Residents were prescribed a median of seven medications, with a range of 0–17 (Table 63).
| Comorbidities | Care home | ||||||
|---|---|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Total | |
| ≥ 3, n (%) | 9 (45) | 13 (52) | 3 (18.8) | 7 (30.4) | 22 (64.7) | 4 (26.7) | 58 (43.6) |
| Characteristic | Care home | ||||||
|---|---|---|---|---|---|---|---|
| CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Total | |
| Mean no. medications (SD) | 7.60 (3.35) | 7.70 (4.19) | 7.07 (4.73) | 6.87 (2.88) | 7.91 (2.87) | 6.67 (4.13) | 7.40 (3.56) |
| Median no. medications | 6.5 | 7 | 6 | 7 | 8 | 6 | 7 |
| Range | 3.0–15.0 | 2.0–17.0 | 0.0–17.0 | 1.0–13.0 | 2.0–14.0 | 2.0–15.0 | 0.0–17.0 |
Sedative load of medications
Analysis of sedative load and use of sedative and psychotropic medications among participating residents showed that approximately one-third of residents were not taking any medications with sedative properties at each time point, whereas a significant proportion of residents had a low sedative load score of 1 or 2 (54.8%, 59.0% and 57.1% at baseline and time points 2 and 3, respectively). Sedative load scores were similar throughout the study period for residents with dementia in each of the care homes, and lower than previously reported in studies conducted in long-term care (for further details, see Parsons et al. 222).
Evaluation of potentially inappropriate prescribing
Analysis of the prevalence of PIP using STOPP criteria shows that 46.2% and 40.9% of the sample were prescribed at least one potentially inappropriate medication (PIM) at time points 2 and 3, respectively. Long-term (i.e. > 1 month) use of neuroleptic drugs, non-steroidal anti-inflammatory drug use for > 3 months and proton pump inhibitors use at maximum therapeutic dosage for > 8 weeks were the most prevalent PIMs in this study population (for further details, see Parsons et al. 222).
Health events
Range of health events
Health-related events recorded in care notes were reviewed in order to capture possible signs of physical decline (see Appendix 58, Table 116). The proportion of residents for whom an event was recorded at each time point over the course of Phase 1 is shown in Appendix 58, Table 117. Problems relating to eating, drinking and falls were among the most frequent events, whereas pain, distress and decreased mobility were documented relatively less often. The number of falls across care homes per time point is shown in Appendix 58, Table 118. Across homes, 22%, 31% and 30% of residents had sustained a fall in the 3 months preceding data extraction at baseline, time point 2 and time point 3, respectively.
Advance Care Planning
Documented involvement in end-of-life discussions is shown in Appendix 58 (see Table 119). Forty per cent of residents had no evidence of end-of-life discussions recorded in their care notes. It is worth noting that end-of-life discussions as recorded in the care notes pertained for the most part to decisions regarding faith-based and post-death arrangements (e.g. religion and funeral arrangements) rather than care-related preferences (i.e. resuscitation status; significant carers, family members whom the resident would want to be present at the end of life) (Table 64).
| No evidence of resuscitation discussions, n (%) | 56 (42.11) |
| Evidence of resuscitation discussions, n (%) | 22 (16.54) |
| No evidence of discussions on end-of-life wishes, n (%) | 53 (39.85) |
| Unavailable, n (%) | 2 (1.50) |
Deaths over the course of Phase 1
Just over 20% (n = 27) of the sample died over the course of Phase 1, including 15 residents in the care homes and 12 in, or on their way to, hospital (see Appendix 58, Table 120). A further six residents (n = 5) were discharged from the care home into a care home with nursing over the course of the study.
Health events and service utilisation in the month leading up to death
The most widely reported symptoms among residents in the month preceding death were problems with eating and drinking (18 of the 22 residents for whom data were available), perceived general deterioration (14 out of 22), breathing problems (13 out of 22), infection (9 out of 22) and increased sleepiness (9 out of 22). These mirror events and symptoms as they were recorded in the care notes across the sample (i.e. residents both alive and dead at the end of Phase 1) and over the 12-month period (see Appendix 58, Tables 114 and 115), and suggest that care home staff do not perceive any significant differences in symptoms between residents who are close to death and those who are not, nor any sudden changes in symptoms in the month preceding death. Only 3 out of 22 deaths reportedly involved any pain management. Examination of service utilisation in the month preceding death reveals that only 2 out of the 22 resident deaths, for which data was available, reportedly had received specialist services support (i.e. hospice nurse and Twilight Nurse). Both cases involved a resident with a cancer diagnosis.
Characteristics of older people who died compared with those still alive
The typical illness trajectory of OPWD is one of progressive functional decline over a prolonged period. Among the 127 residents with available data there were 17 deaths. Six residents had moved from the care home and 104 residents were alive at end of the study. There were no evident differences in survival comparing 80- to 89-year-olds with those aged ≥ 90 years. Survival rates appeared slightly lower for males but this difference was not significant (p = 0.096). The absence or presence of a formal diagnosis of dementia (p = 0.67) was not a relevant indicator for impending death. The mortality rate was highest in care home 6, but the 98% CIs indicate the relative imprecision of these estimates, given the small number of deaths per care home. Our results suggest that for care home staff and visiting health-care practitioners, it is difficult to predict how long this period will last or to differentiate even in hindsight between residents who are dying and those who are not.
Dying trajectories
Residents’ trajectories to death were examined in depth, and summarised from care home notes and staff accounts in the form of Visio timelines. Classification of Visio summary timelines resulted in three distinct end-of-life trajectories: anticipated, unexpected and uncertain dying.
Trajectory A, anticipated dying Anticipated death with planned end-of-life care in the care home. These residents were recognised to be approaching death and decisions were made about providing for end-of-life care in the care home (Figure 16). In all cases, care continued in the home up to death; none were transferred to hospital or hospice. Twelve cases fitted into this model – the majority of the deaths.
FIGURE 16.
Anticipated dying: an illustration.
Trajectory B, unexpected dying Acute illness or sudden event leading to death in the care home or emergency admission to hospital where the resident subsequently died. Only two deaths were classified as ‘unexpected’. In both cases, residents had been stable in the care home and then suffered an unexpected acute event (i.e. pulmonary embolus and breathing difficulties), which led to unexpected death in the care home or emergency admission to hospital (Figure 17).
FIGURE 17.
Unexpected dying: an illustration.
Trajectory C, uncertain dying Diagnostic uncertainty or disagreement as to the best course of action that led to hospital admission and death in hospital. For these residents the period before death appeared to be a time of diagnostic uncertainty. They were unwell but it was not evident to the care home staff that they were close to the end of life. Resident deaths that fit into this category were further classified into treatment uncertainty (n = 5) and service uncertainty (n = 3).
Treatment uncertainty These residents died in hospital following admittance for reasons such as treatment of infection, not responding to oral antibiotics or for further investigation of an illness for which the cause was not clear (Figure 18). Equally, there were residents who were still alive at the end of data collection, whose experience of episodes of ill health did not signal their impending death within the next 6 months (Figure 19).
FIGURE 18.
Treatment uncertainty of a deceased resident: an illustration. A&E, accident and emergency; ADA, after death analysis; T/c, telephone call.
FIGURE 19.
Treatment uncertainty of a living resident: an illustration. A&E, accident and emergency; OT, occupational therapist; T/c, telephone call.
Service uncertainty Three deaths occurred in the midst of disagreement as to where the resident should be cared for and by whom, leading to prolonged stays in hospital awaiting placement in a care home with nursing, and in one case to discharge from the hospital back to the care home just 1 day before death (Figure 20). Finally, in four of the cases it was not possible to categorise the death because of limited information.
FIGURE 20.
Service uncertainty: an illustration. CHM, care home manager; TLC, tender loving care.
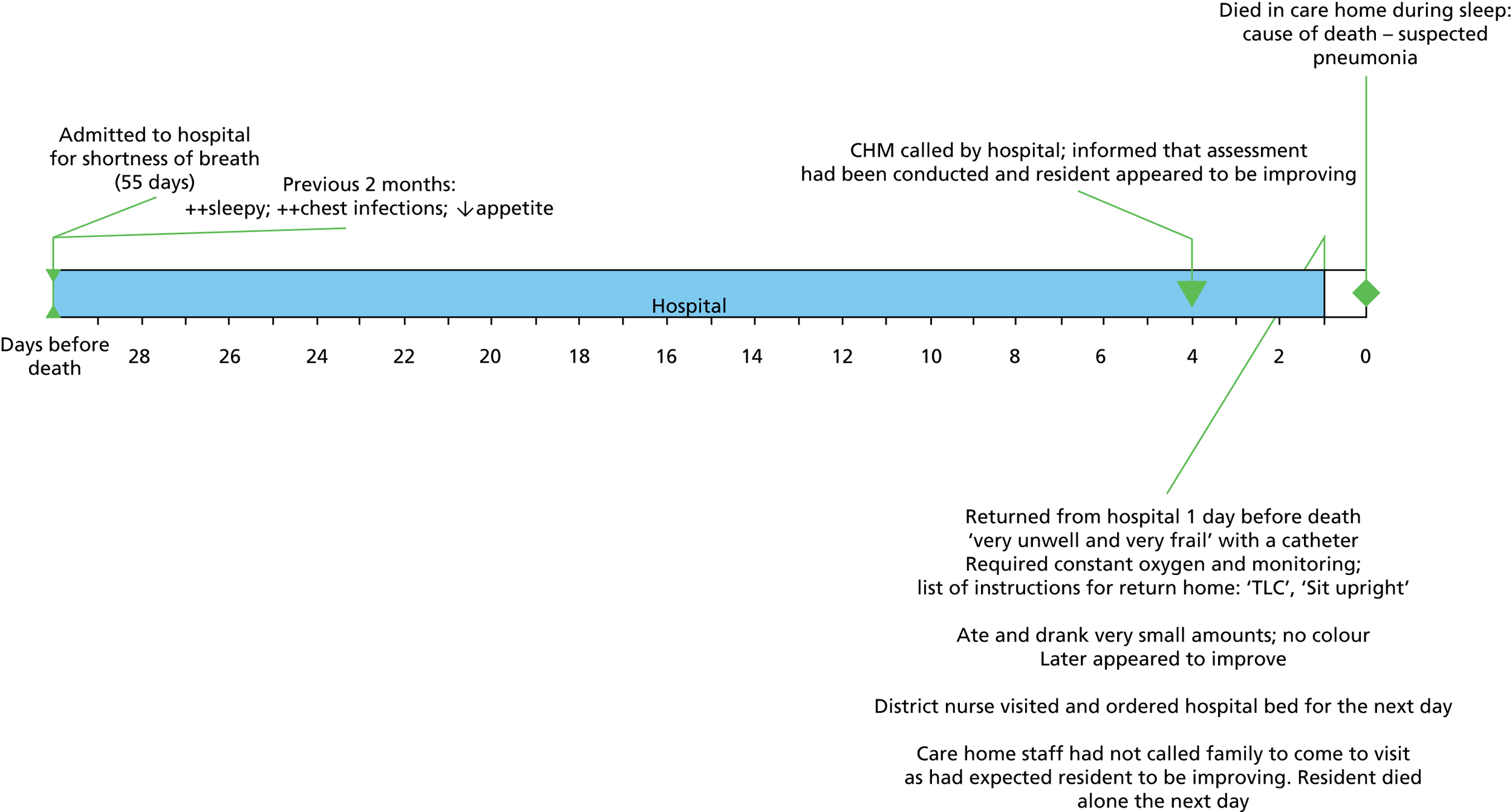
Living and working with uncertainty
This section synthesises the qualitative data from Phase 1 interviews with OPWD (n = 18), care home staff (n = 33), GPs (n = 5), district nurses (n = 5) and emergency ambulance services staff (n = 3), which focused on the experience of providing and receiving care. Interviews illustrated the issues and decisions that led up to the events observed in the resident dying trajectories outlined above, and made explicit how the experience of dying with dementia is shaped by multiple factors and not just symptom-related issues.
Interview data indicate that care home staff members were more likely than NHS staff to have undertaken dementia training (see Appendix 60, Tables 124 and 125). Only two GPs (out of five) and two district nurses (also out of five) reported having received some form of training in dementia care. All but one member of care home managerial staff had received dementia training. None of the GPs had undergone any further specialist training in geriatric or end-of-life care.
Residents’ interviews
When discussing end-of-life priorities and needs, the recurrent and overarching theme was one of uncertainty about their future and how they would be involved in decision-making. Among older people, important themes were uncertainty about the progression of their disease, how they understood living in a care home and who they identified as the person(s) who understood them. Place of death was not necessarily a meaningful measure of effective end-of-life care. Preferences and expectations about end-of-life care were blurred with the everyday experiences of care. Findings demonstrated the importance of ongoing review and discussion with people whose dementia might wrongly be seen as precluding meaningful discussion. Residents’ uncertainty about the future and being in the care homes is discussed in detail by Goodman et al. 229
Care home and NHS staff interviews
Uncertainty was also a dominant theme in the staff interviews. It was expressed in terms of diagnostic uncertainty and clinical decision-making at different time points, and staff uncertainty about roles and responsibilities within care homes and NHS services, including questions about workforce capacity and uncertainty when accessing primary and community care services.
Uncertainty in the resident trajectory
Knowing when someone with dementia was actively dying was very difficult. Although care home and NHS staff knew signs of deterioration such as weight loss, reduced function, chest infections and increased apathy were significant indicators, these were often not documented, were diffuse, and could either be present for long periods of time, or, with treatment, might resolve. This variable path to death was recognisable after death but not always in the moment. Treatment uncertainty affected decision-making about withdrawal of treatments and referral to hospital, and how information about the person with dementia was communicated to family members and within/across organisations.
Clinical decision-making in care homes was not seen as the work of one person and had to take multiple perspectives into account. For care home staff, decision-making about quality of life related to the responsiveness of the resident, their ability to still interact and take an interest in their surroundings. For clinical staff it was whether the treatment was unduly prolonging a life that was naturally coming to an end (and one that they themselves would not want to live). When the dying period was protracted it was difficult to maintain both communication – between clinicians, care home staff and family members – and responsiveness in times of crisis.
Examples of when staff had worked together well suggested that there had been time to discuss symptoms and involve relatives, and that the pathway to death had been clear. NHS staff seem to have had very little input to the care of some residents who died, and it was unclear if this was because it was not required or because their needs were not obvious and they had been overlooked.
Uncertainty in roles, relationships and responsibilities
At the outset of the study, NHS and care home staff believed that they had good working relationships but communication and responsibility for decision-making were often unclear. To provide end-of-life care NHS staff relied on care home staff to describe what was normal for the older person, and to inform decisions to visit, treat, liaise with family and involve other services. Interviews highlighted persistent themes of uncertainty in how these roles and relationships were negotiated. When it was clear that someone was terminally ill, and in the last days of life, some (but not all) care home staff wanted something, such as the LCP,215 to give them permission to talk about dying, liaise with NHS staff and help structure their activities. However, two managers commented that the LCP meant that primary care staff ‘took over’ end-of-life care, which ‘medicalised’ dying, excluded the involvement of staff and complicated communication between them.
Paramedics interviewed in this phase reported that they were sometimes used as a ‘go-between’, and the ‘easy option’ when a GP was unavailable to attend or when an inexperienced care worker was unsure what to do. This was an area that was also raised during post-death analysis interviews by some care home managers. It suggested a sense that these two participant groups lacked the authority and opportunity to discuss and debate the choices and decisions that staff make in emergency situations. 230
Uncertainty about access to, and capacity of, services
There was uncertainty within and across groups about the value of focusing on end of life, and whether or not care homes were the best place to die. Only GPs were convinced that end of life for people with dementia should be in the care homes. District nurses and care home managers, for different reasons, were unsure.
For care home managers, it was because their focus was on the living; consequently, talking about dying was often felt to be inappropriate. District nurses, regular visitors to care homes and some paramedics were concerned that neither they nor the care home staff had the capacity to care for people at the end of life. This view was supported by examples of care home staff working unpaid overtime when a resident was dying because there was limited cover at night. GPs also hinted at the possibility that some OPWD were overlooked because reviewing their care would generate more work for their already full caseloads.
Uncertainty about access to, and capacity of, services to provide end-of-life care was related to the limited responsiveness of primary care services and the eligibility of residents for specialist palliative care services and equipment (even if they could be provided in time). Similarly, there was evidence of people dying in hospital while waiting for a placement to a care home with nursing because the hospital staff were uncertain if the care home could provide end-of-life care.
Service utilisation and associated costs
Distribution of service use per month
All care homes residents are entitled to the full range of NHS primary, community and hospital-based care support but were found to access only a limited number of services on a regular basis. Three main categories of service were defined:
-
Hospital services were limited to emergency (ambulance), accident and emergency (A&E), inpatient and outpatient services.
-
Community health services included district nursing as well as OOH GP services.
-
GP services.
Use of these services was examined among all residents for whom data were available. There were no significant differences between residents who were alive at the end of the study period and those who had died, in terms of age on admission, gender, reason for admission, admission route and number of long-term conditions (Table 65).
| Characteristic | Overall (n = 133) | Alive (n = 116) | Dead (n = 17) | p-valuea |
|---|---|---|---|---|
| Age on admission (years): mean (SD) | 84.0 (6.7) | 84.0 (6.9) | 84.4 (5.5) | 0.984 |
| Gender: % female | 77.4 | 79.3 | 64.7 | 0.178 |
| Reason for admission (%) | 0.513 | |||
| Isolation | 5.7 | 6.8 | 0.0 | |
| Not coping | 44.3 | 43.2 | 50.0 | |
| Bad health | 18.2 | 16.2 | 28.6 | |
| Needs not being met | 25.0 | 25.7 | 21.4 | |
| Other (note: missing for 45 individuals) | 6.8 | 8.1 | 0.0 | |
| Admitted from (%) | 0.0.728 | |||
| Own home | 43.3 | 42.9 | 46.7 | |
| Relative’s home | 5.8 | 6.7 | 0.0 | |
| Hospital | 29.2 | 29.5 | 26.7 | |
| Other care home | 10.8 | 10.5 | 13.3 | |
| Sheltered housing/warden controlled | 10.8 | 10.5 | 13.3 | |
| No. of long-term conditions: mean (SD) | 2.4 (1.5) | 2.4 (1.5) | 2.4 (1.5) | 0.844 |
Service use for the whole sample is shown in Appendix 59, Table 121, and for those alive at the end of 12 months plus those who had died. In accordance with other research that has found that costs are usually highest for people 1 year before death,231 service use was significantly greater among those individuals who had died in all three categories of service.
Distribution of costs
The distribution of health-care costs for the overall sample is shown in Appendix 59 (see Table 122), and for those alive at 12 months compared with those individuals who died. Generally, each care home resident in Phase 1 incurred about £2549 per month in terms of accommodation, health service use and medication consumption. Given the significant differences in service use, it was not surprising to observe that primary care, hospital and community health-care costs were significantly greater for those individuals who died than those alive at the end of 12 months. Including the costs of accommodation and medicines did not alter this finding, despite accommodation costs being much greater than all of the other health-care cost components.
Multivariate analysis of total costs
Table 66 presents the results of a multivariate analysis of total costs (including accommodation and medication costs). A generalised linear model was used to estimate the relative effects of individual characteristics on total costs. Death significantly increased total costs. The marginal effect of death on total costs was a £379 increase (other characteristics at their average values). Results also suggest that individuals admitted from a relative’s home or from another care home incurred significantly lower total costs than those admitted from their own home. Further investigation regarding route of admission of older people into care homes may be warranted.
| Characteristic | Marginal effect | 95% CI | p-value |
|---|---|---|---|
| Age at admission, years | –56.2 | –111.1 to –1.3 | 0.05 |
| Gender: male | –478.6 | –1336.4 to 379.3 | 0.27 |
| Admitted from: | |||
| Relative’s home | –1527.3 | –2333.5 to –721.3 | 0.00 |
| Hospital | –330.4 | –1037.4 to 376.7 | 0.36 |
| Other care home | –377.7 | –1154.0 to 398.5 | 0.34 |
| Other/sheltered housing/warden controlled . . . | –346.4 | –1145.9 to 453.0 | 0.40 |
| No. of long-term conditions | 90.5 | –138.2 to 319.3 | 0.44 |
| Died | 2592.7 | 432.1 to 4753.2 | 0.02 |
Results from Phase 2
Phase 1 identified uncertainty at all levels as a potential barrier to the delivery of effective end-of-life care for OPWD in residential care homes. This analysis led us to design an intervention that could address these different but overlapping levels of uncertainty by building on existing relationships, strengths and achievements and, when appropriate, end-of-life care resources. This section considers how AI (and the tools that emerged in the process of implementing it) was effective in addressing the different types of uncertainty experienced by care home and NHS staff. The findings from Phase 2 are organised and discussed under three headings.
How AI addressed uncertainty:
-
in interpersonal and interprofessional relationships (Relational uncertainty)
-
in identification and management of end-of-life care for people with dementia and resource use (Treatment uncertainty)
-
around the knowledge and skills required to provide end-of-life care for people with dementia and the capacity of NHS and care home staff and services to provide this (Service uncertainty).
Relational uncertainty
Creating communicative space
Appreciative Inquiry sessions were protected, non-hierarchical meetings and were held in the care home. The starting point was discussion of the participants’ strengths in end-of-life care; it was a space in which care home staff heard first hand (and in most cases for the first time), from visiting NHS staff, that their experience in dementia care and knowledge of the resident was valued and ‘fundamental’ to end-of-life care.
A persistent theme in Phase 1 was concerns about the accountability of care home staff to the regulator (CQC), and, for NHS staff, their personal liability. The AI processes meant that practitioners were able to admit their own fears and concerns, and acknowledge their co-dependency. At the end of the intervention, one GP commented to the care home manager that he used to dread coming to the care home but this was no longer the case.
Participants perceived the most important outcome as appreciating colleagues’ roles in end-of-life care. The GP linked to care home 1 recognised that post AI there was a greater shared understanding of what they were trying to achieve.
It’s (. . .) important to share that we actually have the same goals, that we think the same things are important, it enables easier conversations on a daily basis, because we know where we are all coming from
GP-CH1
Out-of-hours checklist
These discussions demonstrated how important it was for NHS staff to value the knowledge that care home staff had about their residents and dementia. It highlighted why this valuing was less evident when contacting OOH services. Care home staff did not know what information the OOH GP needed. OOH GPs would tell care home staff to call an ambulance when the information received seemed insufficient to make an assessment of the urgency of the problem. It was through ‘appreciating’ the challenges of their respective roles that the development of the OOH checklist arose. The checklist provided sufficient information for the GP and it could be left by the phone ready for OOH call-back. It was a tool developed together that addressed a shared frustration.
Do Not Attempt Cardiopulmonary Resuscitation audit
The AI intervention gave care home staff the confidence to share information and initiate conversations with the primary health-care staff. In care home 5, the GP carried out an audit of Do Not Attempt Cardiopulmonary Resuscitation (DNACPR) forms in April 2011, which revealed only one out of 53 residents (i.e. the total number of residents in the care home, including those not consented to the study) or others on their behalf had completed such a form, but by July 2011 there were seven, and, by March 2012, 25 out of 55 residents (45.5%) had a DNACPR form. Residents identified as nearing the end of life were more likely to be included on the practice palliative care register and have their situation discussed with family members. The process of the DNACPR audit, and the involvement of the GP, triggered conversations across the care home about anticipating end of life, and normalised talking about dying. All three senior care workers interviewed in this care home following the intervention described how they initiated discussions with the GP regarding possible signs of deterioration and transitions to end-of-life care. Indeed, the proportion of care home staff involved in discussions around of life care of residents was observed to have doubled after the intervention (see Appendix 61, Table 126).The GP interviewed after the intervention recognised this change:
The communication (. . .) is no longer doctor–carer, ‘you do this, I’ll do that’ (. . .) there’s an improved confidence with the staff to be able to say, ‘doctor, we’re concerned that this patient is deteriorating, what do you think we should do?’ (. . .) or ‘his family are here’, and then I say, ‘OK, fine, I think we should all come together’.
GP-CH5
Prompt sheet
The explicit focus on end-of-life care started a discussion about care homes as places for living and dying. In care home 1 participants worked together to develop a prompt sheet that could help staff initiate a conversation about preferences and priorities for end-of-life care with residents, using a range of resources including information from the Dying Matters coalition. 232 The AI sessions had sharpened the organisation’s focus on providing end-of-life care:
We do talk about [end of life] (. . .) [but] I think the meetings really helped us to focus a bit more on that (. . .) helps to focus on what we are doing. People are coming here [and] we know that they will eventually pass away (. . .) that is what we should be planning for.
Deputy Care Home Manager-CH1
There was evidence this had made her more receptive to the subject of end-of-life wishes when carrying out care reviews with residents, thus allowing residents to raise and discuss end-of-life topics.
Treatment uncertainty
Symptom management
Uncertainty about symptom management, such as breathlessness, repeated falls and difficulty eating and drinking had been documented in Phase 1, as triggers for admission to hospital or calls to emergency services. During the final AI session, one care home manager expressed concern over ‘eating and drinking’ issues at end of life and personal liability, suggesting that AI was an intervention that could accommodate and address underlying concerns about responsibility alongside clinical decisions. Post intervention there was evidence of consultation with specialist palliative care services and improved understanding about situations when supplementary and enteral nutrition would not be appropriate.
Post-death analysis and use of Visios
Phase 1 made it explicit that NHS staff lacked confidence in their knowledge about dementia, and care home staff their lack of clinical knowledge. The use of Visios in Phase 2 enabled an exploration of what ‘normal’ looks like for the resident with dementia and what symptoms were significant (see Appendix 51). AI balanced a focus on an unplanned hospital admission and death with a discussion of what had been achieved leading up to the resident’s death. It encouraged reflexivity and review, and enabled participants to ‘hold’ situations when it was unclear what the best decision would be, for example when a resident was well in between increasing episodes of ill health. It enabled them to identify and reinforce what had worked well, how to improve care but also accept that in some of the deaths it was only with hindsight that the situations were clear, which led to discussion on how to negotiate uncertainty across organisations and between practitioners.
Documentation change
Finally, the AI intervention addressed treatment uncertainty by making the challenges participants encountered explicit, and by the co-development of documentation that helped bridge the gap between social and medical views of care. This included care plans that used language and instructions that made clear to care staff the medical decisions that had been reached with the GP. End-of-life care tools were being appropriated by care home staff to make explicit the impact that medical decisions had on end-of-life care practices, especially in times of crisis and exacerbation:
Yeah basically he (family member) doesn’t want his mum to go to hospital so it’s not so much a ‘Do Not Resuscitate’ [as a] ‘Don’t Call Paramedics – call GP’
Deputy Care Home Manager-CH6
Service uncertainty
Appreciative Inquiry focused on the capacity and assets of the workforce and the evidence for what was achievable and appreciated. Among care home staff there was evidence of increased confidence that primary health-care services would respond when needed:
That is something that we are really promoting here [with the doctors]. Instead of going to hospital . . . lately quite a few deaths that we had, they passed away in the home. [T]hrough this research, I think we have [discovered] what we can [provide] here in the home with the help of the doctors, rather than have to send someone to hospital for treatment (. . .) now, having the GP involved and everything we know that they can stay here, they can be looked after and cared for here.
Deputy Care Home Manager-CH5
Increased district nurse involvement
Finally, a change brought about through the AI intervention in care home 6 included ‘coffee mornings’ between care home staff and the wider district nursing team to introduce incoming members of staff and discuss any issues (an innovation that was still in place 18 months after the end of data collection). Family members interviewed after a relative’s deaths in this care home also noticed how district nurse involvement had been instrumental in enabling their mother to die in the care home:
They knew my mother didn’t want to go into hospital, they knew what the situation was, um and they . . . the home felt . . . When she spoke to me about, when [Deputy Manager] spoke to me about mother going to hospital, I said can you care for her in the home? Have you got enough you know . . . and she said yes, we have, with the District Nurses coming in
Family member-CH6
Overall, the numbers of primary health-care staff involved in the resident deaths increased. For example the district nurses working with care home 1 who had not been involved in end-of-life care in Phase 1 were consulted for advice, nursing support and reassurance in Phase 2 (Figure 21).
FIGURE 21.
District nurse involvement: an illustration. DN, district nurse; EoL, end of life.
Costing analysis
Comparison of resource utilisation and associated costs was undertaken between the overall sample in Phases 1 and 2. Table 67 shows the costs incurred per resident per month for each category of costs.
| Costs (£) per resident per month | Median | Percentage change in terms of median costs | |
|---|---|---|---|
| Phase 1 | Phase 2 | ||
| Accommodation | 2243.35 | 2380.49 | 6 |
| Medication | 56.54 | 81.17 | 44 |
| Primary care | 76.31 | 83.90 | 10 |
| Hospital | 38.98 | 18.52 | –52 |
| Community health | 45.46 | 7.82 | –83 |
| Total service | 193.98 | 164.52 | –15 |
| Total service and accommodation | 2488.17 | 2539.47 | 2 |
| Total (service, accommodation, medication) | 2548.53 | 2632.61 | 3 |
As the sample size of this study is relatively small, only median values are presented. Each care home resident in Phase 2 incurred about £2633 per month in terms of accommodation, health service use and medication consumption compared with £2549 in Phase 1. Accommodation costs (including care home staff and food and so on) accounted for around 90% of the total costs per month. There was a decrease in community health costs (–83%) and hospital costs (–52%). Medication costs however nearly doubled. Overall, total service costs incurred dropped by 15% from Phase 1 to Phase 2.
One of the most commonly used quantitative means for measuring end-of-life care has been place of death. 233 This study saw similar proportions of residents dying in hospital (55%) and in the care home (45%) in Phase 1 (15 out of 27 deaths in care homes) and Phase 2 (five out of nine deaths in care homes). However, we observed a decrease in the length of inpatient stays and emergency ambulance use specifically, which was found to significantly reduce hospital costs overall.
Costs before and after the intervention were further examined among residents who had participated in both phases of the study. Results are similar to those derived from comparisons between the wider samples and were subject to significance testing. Among residents who participated in both phases of the study (n = 28), hospital and community care costs were significantly lower in Phase 2 than in Phase 1 (p = 0.0456 and p = 0.0001, respectively). However, there was no significant difference between primary care costs between the two phases (p = 0.8376). 234
Discussion
Summary and discussion of Phase 1 findings
Prognostication is difficult for people with dementia. 200,201 Key symptoms and events among OPWD can sometimes be indicative of a transition from living to dying. 235
There were no significant differences between residents who were alive at the end of the study period and those who had died, in terms of age on admission, formal dementia diagnosis, gender, reason for admission, admission route and number of long-term conditions.
There was considerable heterogeneity, however, in the resident population across care homes in terms of length of stay, function, residents with a dementia diagnosis, and individual resident’s use of resources. Future research on end-of-life care for people with dementia needs to consider how the diversity of the care home population affects the experience and knowledge of care home staff about end-of-life care.
Phase 1 showed that in care homes with no on-site nursing there were different pathways to death. Not all residents had advanced dementia and dementia was not always the cause of death. However, dementia did complicate decision-making. There was evidence of attempts to use Advance Care Planning but there was considerable variation in how this was documented, who was involved in the decision-making and whether or not this information was used. Interviews with residents demonstrated that residents’ accounts of care preferences and identification of key care staff had the potential to inform future decision-making and augment the completion of an Advance Care Plan.
In the care home environment, dying with a dementia could be a ‘contested’ process,236 complicated by uncertainty at the individual, service and organisational levels of care. Different participants had considerable discretion about how they engaged in providing end-of-life care for people with dementia, both in the care home and between services. Patterns of working could either foster discussion of residents’ needs or limit opportunities for it.
Access to services was inextricably linked to uncertainty about roles, responsibilities, capacity and knowledge of the workforce, and whether the person with dementia was approaching the end of life. From an organisational perspective, however, there was an additional lack of clarity. This was uncertainty about the appropriateness of service requests, the capacity of the primary care workforce to provide ongoing support, entitlement to specialist services and whether hospital and emergency services believed that care homes were able to provide end-of-life care.
The co-dependency between NHS and care home staff and the examples of when collaborative working mitigated some of the challenges described suggested an intervention that would require the engagement of all those involved, incorporate the residents’ views and preferences and build on relationships that were already in place. It had the potential to address and ‘hold’ the uncertainty experienced when staff are reliant on input from (multiple) external services, as well as answerable for decision-making to family members and the regulator.
Summary and discussion of Phase 2 findings
Although ‘clear care pathways’ for people with dementia at the end of life were desirable, they were often unattainable. AI sought solutions from among those already involved in providing care. As a context-sensitive intervention it addressed the participants’ interests and concerns, and created a process of working that, using Fisher and Ridley’s terms,237 could foster ‘practical certainty’ in situations when uncertainty was an intrinsic and enduring feature. AI’s effectiveness is judged by its ability to create an environment in which participants were able to safely acknowledge their co-dependency and appreciate each other’s role in end-of-life care (i.e. Discovery Phase), imagine the best end-of-life care for people with dementia within the home (i.e. the Dream Phase) and work together to design end-of-life care tools that would help them to attain their ideal (i.e. Design Phase).
The relocation of a person with dementia to a care home can signal a reduction in, or even the end of, the involvement and responsibility of NHS professionals. This study demonstrated the value of an intervention that sustained ongoing negotiated involvement of NHS staff.
Appreciative Inquiry focused on the practical delivery and expectation of care by taking into account the assets of the organisations involved and the capacity of the participants to build on previous achievements. It complemented approaches to dementia care that emphasise what a person retains and has capacity to do. A deficit model of care that starts with failure (e.g. breakdown in communication, knowledge deficits, inappropriate admission and uncontrolled symptoms) can identify service shortcomings, but is less likely to engage with the complex uncertainties that NHS and care home staff encounter, which Phase 1 findings demonstrated. AI did not preclude the use and refinement of existing Advance Care Plans, end-of-life care frameworks and extra training in end-of-life care, all of which aim to address the same issues. 238–240 AI led to their increased use (DNACPR, palliative care register, LCP215). However, its context-specific, non-hierarchical approach to co-design was arguably more flexible and better suited to working across organisations and between public and independent providers. Implementation of AI in Phase 2 helped reduce the use of health services and corresponding costs, and these results were corroborated by the qualitative findings. The total costs incurred by residents were lower in Phase 2 than in Phase 1.
Appreciative Inquiry was an intervention that could accommodate and address underlying concerns about responsibility and liability alongside clinical decisions. The difference between using AI and introducing an established end-of-life tool such as the GSF213 was that AI led to the use and development of end-of-life resources that arose from a change in working relationships. A decision to use a particular tool did not change how care was organised, but rather was itself a result of a change in care practices and working relationships initiated by the AI intervention.
Relative interviews in conjunction with care home notes demonstrated the importance of involving relatives in end-of-life discussions and medical decisions concerning residents and keeping relatives informed of any changes and/or deterioration. The reasons why some relatives wanted more rather than less medical intervention and encouraged hospital admission needs further investigation. One relative interview highlighted the importance of viewing a resident’s death within the context of their life before admission to the care home.
Finally, findings related to medication use in Phase 1 were not explored or challenged in Phase 2, but suggest that sedative load was not as significant as anticipated. Inappropriate prescribing does appear, however, to be an issue among older people both living and dying with dementia, which may warrant further research.
Limitations
The EVIDEM-EoL study is an exploratory study. The results are tentative. AI appears to improve and change working relationships with promising outcomes. It is an approach that fits with others that emphasise relational approaches to care. 241,242 Nevertheless, more research is needed to test these findings further and with more robust controls.
The small sample of care homes and care home residents is a limitation affecting generalisability, although the resident characteristics and size of care homes were consistent with other studies in care homes without on-site nursing. 243–245 Care home notes from which resident baseline characteristics were extracted could be inconsistent across time points. Residents’ long-term conditions are likely to be under-reported as a result. Phase 2 was shorter, which might lead to an underestimate of the impact of the interventions introduced in Phase 2 on resource utilisation. There could also be potential underestimation of the costs of resources to support the care home residents. Outcome measures for end-of-life care were captured in the study but were limited to place of death and evidence of documentation. This made it more difficult to assess the association between resource utilisation (i.e. costs) and the quality of life of the residents in this study.
Conclusion
Dying with, or from, dementia is invariably ‘protracted, unglamorous and ordinary’. 211 The everyday realities of living in a care home, the impact of dementia and other health problems, and the physical and practical isolation from NHS services further complicated that process. Appreciative Inquiry addressed these context-specific challenges, emphasised the capacity and assets of the organisations and services involved, provided a basis from which to co-design new tools and use existing end-of-life care resources.
An important output of this study is the development of a framework that defines the different manifestations or levels of uncertainty, and that can be used to test the comprehensiveness and strengths of future interventions designed to improve end-of-life care for people with dementia in care homes.
As Seymour et al. 246 have observed, for the vast majority of those who die, the promise of hospice and palliative care has never been fulfilled. The rapidly changing demography of ageing and dying requires solutions that are grounded in a reality that can carry the vision of hospice and palliative care into settings with limited access to specialist palliative care. Kellehear and Young247 have argued for a ‘public health of dying’ in as much as care homes are small communities of care, reliant on unqualified staff, family members and a varying range of support services; this study provides a model of relational working that arguably reflects some of those goals and realities. From an economic perspective, the findings have highlighted that there is greater scope for more efficient use of resources. However, further and continuing research on the costing and economic analysis of different end-of-life care models for OPWD in care homes is needed.
Changes to protocol
Phase 1
Phase 1 of the EVIDEM-EoL study entitled ‘Changing practice in dementia care in care homes: developing and testing evidence-based interventions at the end of life’ (REC reference: 08/H0502/74) received a favourable ethical opinion from the Southampton & South West Hampshire REC (A) on 14 July 2008. The Phase 1 protocol was subject to three significant amendments.
-
Amendment 1 sought to offer anonymous resident data to the EVIDEM registry study (see Chapter 6) (approved 30 June 2008). Amendment 1 (REC reference: 08/H0502/74) was approved by the Southampton & South West Hampshire REC (A) on 5 March 2009.
-
Amendment 2 sought to refine the process of consultee engagement. Amendment 2 (REC reference: 08/H0502/74) was approved by the Southampton & South West Hampshire REC (A) on 5 March 2009.
-
Amendment 3 sought to include ambulance staff from the London Ambulance and Paramedic Service and the Bedfordshire and Hertfordshire Paramedic Service in the research study. Amendment 3 (REC reference: 08/H0502/74) was approved by the Southampton & South West Hampshire REC (A) on 11 August 2009.
Phase 2: Intervention phase
Phase 2 of the EVIDEM-EoL study entitled ‘EVIDEM-End of Life: Changing practice in dementia care in care homes: developing and testing evidence-based interventions at the end of life – Phase 2’ (REC reference: 10/H0502/55) received a favourable ethical opinion from the Southampton & South West Hampshire REC (A) on 10 August 2010. Phase 2 of the study was subject to one amendment that sought to include relatives of residents living in participating care homes in the study. Amendment 1 (REC reference: 10/H0502/55) was approved by NRES Committee South Central – Southampton (A) on 27 May 2011.
List of appendices
Appendix 35 Chapter 4: Phase 1 protocol.
Appendix 36 Chapter 4: Phase 2 protocol.
Appendix 37 Chapter 4: Care home manager topic guide.
Appendix 38 Chapter 4: Care worker topic guide.
Appendix 39 Chapter 4: NHS and social services staff topic guide.
Appendix 40 Chapter 4: Emergency services carer topic guide.
Appendix 41 Chapter 4: Care note data extraction form.
Appendix 42 Chapter 4: Care home manager/senior carer topic guide.
Appendix 43 Chapter 4: Care home staff topic guide.
Appendix 44 Chapter 4: NHS staff topic guide.
Appendix 45 Chapter 4: Relatives prompt guide.
Appendix 46 Chapter 4: Emergency services prompt guide.
Appendix 47 Chapter 4: Intervention design, Phase 2.
Appendix 48 Chapter 4: Intervention information – general.
Appendix 49 Chapter 4: Intervention information – care home staff.
Appendix 50 Chapter 4: Intervention information – district nurses/general practitioners.
Appendix 51 Chapter 4: Intervention material – post-death analysis.
Appendix 52 Chapter 4: Participant developed tools – prompt sheet.
Appendix 53 Chapter 4: Participant developed tools – out-of-hours information sheet.
Appendix 54 Chapter 4: EVIDEM-EoL Advisory Group members.
Appendix 55 Chapter 4: Care home characteristics.
Appendix 56 Chapter 4: Care home resident needs and deaths in year prior to study.
Appendix 57 Chapter 4: Care homes’ access to primary and specialist services.
Appendix 58 Chapter 4: Resident baseline characteristics.
Appendix 59 Chapter 4: Service utilisation and associated costs.
Appendix 60 Chapter 4: Sample information – interviews.
Appendix 61 Chapter 4: Phase 2 – documentation changes.
Chapter 5 EVIDEM-MCA: implementing the Mental Capacity Act 2005
Abstract
The MCA 200517 safeguards the rights of people with dementia and carers, enshrining principles of capacity, decision-making and best interests. Our study, designed in four phases (pre diagnosis, immediately after diagnosis, living with dementia, end of life), explored the MCA’s implementation and impact.
We interviewed practitioners from agencies working with people with dementia (n = 272), ‘well’ older people about approaches to long-term planning (n = 37), OPWD (n = 16) and carers (n = 15) about views and experiences of using the MCA to make plans and everyday decisions, and many of these were followed up 9–12 months later. Framework methods were used to analyse and categorise themes.
Baseline interviews indicated limited awareness, knowledge and understanding of the MCA but by follow-up these had grown. The need for training to be a continuous process informed by supervision, rather than one-off events was identified. An ‘information merry-go-round’ for people seeking advice and information was found. Some ‘well’ older people had made financial plans but appeared reluctant to think about HSC preferences and choices. Principles of the MCA, such as ‘best interests’ decision-making, were useful for carers to apply when deciding for their relatives. Few professionals are aware of offences under the MCA and lack confidence in distinguishing criminal acts (ill treatment and wilful neglect) from poor care.
Practitioners working with people with dementia may be uniquely placed to address decision-making and longer-term planning. Specific information and advice can empower people with dementia and carers and minimise risks of abuse and neglect. Implementation of major legal changes, such as the MCA, needs to be managed in all settings and needs long-term commitment.
Introduction
This study investigated one of the major changes affecting day-to-day practice in dementia care in England, a change that also fundamentally enhanced the rights of people with dementia and their carers. The MCA 200517 in England and Wales, implemented in 2007, enshrines much of the practice already established under case law. 248,249 It safeguards people who lack ability to make specific decisions, enhances personal autonomy and enables people to make advance decisions about their care and finances and to refuse treatment. It introduced new proxy decision-making roles to address health, welfare and financial matters, and specialist advocacy when major health and welfare decisions are being considered for people who do not have family or friends. The implications of the MCA for HSC practitioners were initially unclear. 250,251
It had been estimated that over two million people in England and Wales were likely to be personally affected by the provisions of the new MCA. 252 These were expected to include many people with dementia and their carers, their representatives or service providers. 253 The MCA now provides a statutory framework to empower and protect people, notably those who fear cognitive impairment, those who have newly received a diagnosis of dementia, or those who have advancing symptoms who may not be able to make specific decisions at specific times (www.dca.gov.uk). 254 It clarifies who can make decisions, in which situations, and the steps that should be taken when considering these decisions (see Mental Health Foundation, 2012 for a review on the topic). 255 It also enables people to plan ahead in the event of loss of their capacity to make particular decisions. 256
The ability to make choices about future care and treatment decisions led to predictions that the MCA would have great potential to enhance practice and thus contribute to better outcomes for people with dementia and their carers. 257 People with dementia may require specialist advice sensitive to their particular values and circumstances to formulate and communicate their wishes and preferences about care and treatment. However, there are likely to be key transitions or times when advice and assistance may be most pertinent. 258 These include professional encounters, such as the communication of a diagnosis of dementia, transitional periods when planning and setting up care packages, or when facing the end of life. It was predicted that professionals would need to devote time to assisting people with dementia and their carers to benefit from the MCA, and to make sure that they were ready to explain and debate its implications within teams and across agencies. 259 Although savings of professional time were envisaged, there were concerns that practitioners might feel compromised by work pressures or be unable to provide the necessary expertise and support. 260 Furthermore, proactive promotion of the MCA by service managers and professionals may be required if people with dementia are able to maximise their opportunities for planning their care and making their wishes known.
Essentially, the MCA offered people with dementia a crucial opportunity to influence professional decision-making. It confirmed that professionals should presume capacity to make decisions unless proven otherwise and that best interests should be the basis for action if a person is not able to make a specific decision. It has the potential to enhance the confidence of people with dementia and their carers that they are able to shape their current and future care and treatment. Lastly, the new criminal offences of ill treatment and wilful neglect defined by the MCA were designed to strengthen adult protection systems, to the benefit of people with dementia and others who may have particular difficulty in reporting abuse and providing evidence. The full implementation of the MCA in late 2007 provided a unique opportunity to explore the early, then ongoing implementation and impact of the MCA with a focus on its incorporation into practice and service cultures.
Methods
Aims and objectives
Two overarching aims of the study informed the initial study design (see Appendix 62 for the study protocol). These aims were to:
-
identify the implementation issues arising from the introduction of the MCA in the services working with people with dementia and their carers over a 5-year period, in community settings (including care homes)
-
explore and make recommendations about continued professional development programmes about the MCA, and their links to adult safeguarding training and practice.
Study design
The study was designed as four phases to reflect the trajectory of the dementia syndrome: (1) pre diagnosis; (2) post diagnosis; (3) living with dementia after diagnosis; and (4) towards the end of life. Distinct research questions were developed for each phase, incorporating the overarching aims of the study. Participants for each phase were recruited accordingly. The phases, research questions and participants are summarised in Table 68 and details can be found in Appendix 63.
| Phase | Pre diagnosis | Immediately post diagnosis | Living with dementia after diagnosis | End of life |
|---|---|---|---|---|
| Characteristics of phase | Before diagnosis Possibly before contact with specialist HSC services |
Early planning Capacity not likely to be greatly impaired |
Capacity may be impaired Safeguarding against abuse is priority |
Attitudes towards Advance Care Plans Proxy decision-making may be needed |
| Research questions | RQ1: How do ‘well’ older people conceptualise and consider future care needs? RQ2: What support is there for older people and how is it conceptualised by staff? RQ3: What are the knowledge, views, experiences and expectations of the MCA among staff supporting ‘well’ older people living in the community, who may wish to consider planning for their possible futures? |
RQ4: What are the knowledge, views, experiences and expectations of the MCA among staff supporting people in the early stages of dementia? How does this change over time? RQ5: What advice, information and support are available to those keen to plan soon after receiving a diagnosis? RQ6: What framework does the MCA provide for people with dementia and carers living in their own homes when making everyday decisions? |
RQ7: What are the knowledge, views, experiences and expectations of the MCA among staff supporting people in the later stages of dementia, when capacity may be impaired? How does this change over time? RQ8: What advice, information and support are available to those whose relatives with dementia lack capacity? RQ9: What frameworks can be put in place so that people with dementia – with and without capacity – are safeguarded against abuse? Does the MCA help? |
RQ10: What are the knowledge, views, experiences and expectations of the MCA among staff who work with people with dementia at the end of their lives and their carers regarding Advance Care Plans? RQ11: How does the MCA help them make proxy decisions? |
| Research participants | Older people interviews Age Concern (now Age UK) Safeguarding Adult Coordinators |
Alzheimer’s Society Carers’ Centres Safeguarding Adult Coordinators People with dementia plus carers |
Social workers Admiral Nurses People with dementia plus carers Safeguarding Adult Coordinators Alzheimer’s Society staff survey |
Care home staff and managers Care home staff and managers |
To capture the element of change over time in the implementation of the MCA, we incorporated a longitudinal element in our study by interviewing most practitioners at two time points: time 1 and time 2 (and some groups at time 3). As our intention was to investigate how the MCA was becoming embedded in practice settings, the study was designed in order to elicit practitioners’ own experiences over time as a way of capturing changes in their understanding and experiences.
Settings
Participants were interviewed at their convenience. For the majority of practitioners, interviews took place in work hours in a private office or staff room at their place of work, although a small minority opted for telephone interviews. ‘Well’ older people, people with dementia and their carers all preferred interviews to be conducted in their own homes. Three participants offered to be interviewed in the researchers’ office, as they were in the area and their offices were unsuitable (e.g. open plan).
Inclusion and exclusion criteria
No exclusion criteria applied to staff interviews as we sought responses from staff in each service, at all grades and undertaking a range of work with people with dementia. Because of time constraints and work pressures, some staff declined the opportunity to participate. As participation was voluntary, no further invitations were extended.
Inclusion criteria for interviews with ‘well’ older people were that participants had to be aged > 50 years (to explore how people possibly plan ahead as they approach later life) and the ability to participate in an interview in English. No other exclusion criteria were applied.
For interviews with dyads of people with dementia and their carers, the inclusion criteria were the presence of a formal diagnosis of dementia, and the presence of a carer whom they either lived with or saw for more than half the week. An ability to speak English was also necessary. No other exclusion criteria were applied.
Sources
In order to access the wide range of participants needed, different community-based groups were asked to publicise this study as a recruitment strategy. These included advocacy groups, social clubs and social groups, lunch clubs, support groups, Carers’ Centres, Alzheimer’s Society groups, Age Concern (now Age UK), Greater London Forum for Older People, University of the Third Age, local authority (LA) social services/adult services departments, and a selection of care homes in the private and not-for-profit sectors. We were keen to access as diverse a range of participants as possible, and hence recruited from different areas in and around London along with agencies that worked with diverse client groups. As Robson125 suggests, we adopted flexibility in our sampling framework, for instance, although we started with a quota sampling frame, that is, aiming to speak to people with varying job roles and experiences, we also adopted a ‘snowballing’ approach – the practice of asking interview participants for suggestions of other potential participants.
Recruitment and sampling strategy
For all phases of the study, a quota sampling strategy125 was designed to recruit participants who would be willing to share their views with the research team. An invitation letter and details of the study were provided for those who expressed interest. In some cases, contact was initiated by a presentation by a member of the research team on a related topic, at the end of which recruitment was discussed and participants were invited to participate. This proved an effective way to make contact, as it engaged the group or agency from the start and fostered a relationship based on mutual trust. Once permission from senior management had been obtained, invitation letters were cascaded to all staff via e-mails or in hard copy. Voluntary participation, confidentiality and anonymity of responses were emphasised. As noted above, ‘snowballing’ was a further means to recruitment.
Data collection
All who expressed interest were approached, the study was described to them, implications of taking part were explained, assurances of confidentiality were made and consent was obtained. For the analytical method chosen (see Analytic methods, below), SSIs within a structured format are considered most appropriate. For this reason, structured questioning was seen as too restrictive for participants to talk in detail about individual experiences, and open-ended questioning was seen as too broad and reflexive, which could have diluted the focus of this study. SSIs were conducted using topic guides developed in light of the study aims and emerging knowledge about the MCA in practice. Most interviews lasted 30–45 minutes.
To achieve maximum consistency, a similar methodology was applied to interviews with ‘well’ older people, people with dementia and carers. However, the researcher (KS) conducting the interviews adopted a more exploratory approach to the interview when talking with people with dementia and probed where relevant. Her experience in other phenomenological research studies was helpful in engagement and conducting interviews on sensitive subjects.
For the subsample of Alzheimer’s Society staff, the Society had chosen an electronic survey as being the most efficient way to collect information from busy staff, and questions were developed accordingly. This contained as many questions from other interview topic guides as relevant and feasible.
In view of the potential sensitivity and personal nature of the interviews, participants were reassured that their anonymity would be maintained and, therefore, all identifying personal details have been changed or replaced with ellipses and ‘their’ has been used to replace his/her. Participants who requested information about the MCA following the interview were provided with sources of information and specific material that was relevant to their role or interest. Following some interviews, the research team provided training on the MCA to care home staff or information to older people’s groups.
Interview topic guides
Tailored, semi-structured topic guides were developed to explore the research questions at each phase. Topic guides were piloted and discussed with members of the study advisory group. For practitioners this was tailored to activities of the specific agency or professional group being interviewed. The topics covered included:
-
Training The model and effectiveness of training received (if any), the nature of the training, the effectiveness of training in developing expertise and confidence.
-
Roles and activities Any impact of the MCA on specific roles and activities, matters raised by people with dementia and/or carers.
-
Looking ahead Predictions about use and uptake of the MCA and its impact.
-
Personal perspectives Any documents that participants had drawn up for themselves or others, exploring the reasons why/why not.
A longitudinal element was captured in two ways. First, participants were asked to reflect on changes in their practice, and how these may have affected their knowledge and experiences when using and abiding by the MCA. Second, questions were specifically framed to ask participants to describe ‘new’ or ‘recent’ procedures, frameworks, structures and processes encountered following the first interview. Although time 1 and time 2 interviews were not always directly compared, an understanding of findings from time 1 interviews informed the development of the time 2 topic guide, thus further capturing the longitudinal element of this study.
The topic guides for ‘well’ older people in Phase 1, and people with dementia and carers in Phases 2 and 3, incorporated questions around planning (financial, HSC – referred to as ‘welfare’ in the MCA), everyday decision-making, proxy decision-making and sources of support they would consider or had used, or were unknown or unavailable. Appendix 64 includes all topic guides used in the study.
Sample size
Because we used a qualitative methodology, no sample size was determined in advance. Instead, members of staff in participating organisations were approached and the importance of the study explained to them. For qualitative interviews with ‘well’ older people, recruiting and data collection ceased when thematic saturation was achieved, that is, when no new themes appeared to be emerging from the data. The same principles of thematic saturation were followed for interviews with dyads of people with dementia and carers. For this group we anticipated recruiting 12 dyads, allowing for around two dyads to drop out over the course of the longitudinal element of the study.
Analytic methods
All interviews were digitally recorded and transcribed verbatim by an experienced transcriber. All transcripts were checked for clarity, verified by the interviewer for consistencies and subjected to framework analysis. 261,262 Framework analysis is appropriate for this type of evidence-based practice study, as it enables the delineation of themes in relation to prespecified research questions. This enables a priori questions to be answered through the use of qualitative research and is particularly useful in HSC services research. 262 More details of the five main stages in framework analysis can be found in Appendix 65.
Ethical permissions
Ethical requirements were maintained across all phases. Appropriate permissions from senior managers were obtained for interviews with their staff. Local government bodies’ requirements for research governance approvals were met. All participants were approached through staff e-mails or written invitations but voluntary participation was emphasised and confidentiality was assured. We were keen to assure participants that the interview was not a test of them or their service, but simply an exploration of their views.
All data collected were stored in password-protected computers, accessible to only the research team. When reporting findings, any identifying data were removed and only the interviewer who collected the data was able to identify a participant. For interviews with ‘well’ older people, ethical approval was obtained from King’s College London REC (REP(GGS)/08/09–29). For interviews with dyads of people with dementia and carers, ethical approval was obtained from the Social Care REC (09/IEC08/17). For the survey of Alzheimer’s Society staff, relevant data sharing agreements were exchanged and signed.
Results
In order to obtain a multidimensional perspective on the implementation of the MCA, the study data were analysed as a complete data set. Principles of framework analysis enabled the identification of themes in relation to questions from the interview topic guides. Significant cross-cutting themes were delineated from transcripts rather than from each phase. Although the phases helped with designing the study and data collection, we judged this would be a more holistic means of presenting the findings, understanding the data and addressing the overall aims of the study.
Sample characteristics
Overall, semi-structured interviews conducted included: 272 interviews with practitioners over all time points; 37 interviews with ‘well’ older people; 16 interviews with people with dementia; and 15 interviews with carers of people with dementia. We further conducted analyses of 84 responses to a survey of carers undertaken by the EVIDEM-ED team and of 86 survey responses by staff working for the Alzheimer’s Society. See Table 69 for description of the interviews.
| Phase | Pre diagnosis | Post diagnosis (just received) | Post diagnosis (later in trajectory) | Towards end-of-life care |
|---|---|---|---|---|
| Characteristics of phase | Before diagnosis Possibly before contact with HSC services |
Early planning Capacity still likely to not be impaired too much |
Capacity may be impaired Safeguarding against abuse is priority |
Attitudes towards Advance Care Planning Proxy decision-making may need to be made |
| Research participants | Older adult interviews = 37 Age Concern (now Age UK) = 10 Safeguarding Adult Coordinators (time 1) = 13 |
Alzheimer’s Society = 10 Staff from voluntary associations and Carers’ Centres = 15 Safeguarding Adult Coordinators (time 2) = 12 People with dementia and carers = 12 EVIDEM-ED questionnaire survey of carers = 84 |
Social services staff (time 1) = 10 Admiral Nurses (time 1) = 15 Admiral Nurses (time 2) = 15 Safeguarding Adult Coordinators = 15 Alzheimer’s Society staff survey = 86 |
Care home staff (time 1) = 43 Care home staff (time 2) = 28 |
| Total | 272 interviews with practitioners over all time points 37 interviews with ‘well’ older people 16 interviews with people with dementia 15 interviews with carers of people with dementia plus 84 responses to questionnaire 86 survey responses (Alzheimer’s Society) |
|||
Detailed breakdowns of sample characteristics are presented in each of the published papers. In summary, these comprised 10 staff from Age Concern (now Age UK) groups;258 15 staff from Carers’ Centres and other voluntary associations and nine staff from Alzheimer’s Society branches;263 13 Safeguarding Adult Coordinators (SACs) at time 1,264 12 at time 2;265 and 15 at time 3;266,267 28 care home managers and senior care staff and 15 care workers in care homes at time 1;268 and 28 care home staff at time 2 (in preparation); 15 specialist dementia community nurses (Admiral Nurses) at time 1;269 and 15 again at time 2;270 10 social services’ staff at time 1 (in preparation). We also analysed the views of 86 Alzheimer’s Society staff who contributed to a survey about responding to financial abuse of people with dementia and carers. 271
Additionally, 37 ‘well’ older people were interviewed for Phase 1;272 a survey captured responses from 84 carers for Phase 2; and 15 dyads were interviewed comprising a person with dementia and their carer for Phases 2 and 3.
Thematic findings: overall
A number of themes were derived from transcripts. For the purposes of this report, overarching themes are presented (for an overview see Table 70) and between-group differences are highlighted. Detailed findings are reported in the study’s publications. Please refer to Appendix 66 for a narrative description of each theme.
| 3.2.1. Universal appeal of MCA | 3.2.2. Training | 3.2.3. Implementation | 3.2.4. Everyday decision-making | 3.2.5. Long-term planning | 3.2.6. Safeguarding |
|---|---|---|---|---|---|
| 3.2.1.1. Beneficial | 3.2.2.1. Frequency and format of training offered | 3.2.3.1. Quickly and easily adapted into adult safeguarding practices and procedures | 3.2.4.1. Negotiated on regular basis between person with dementia and carer | 3.2.5.1. Planning influenced by personal factors | 3.2.6.1. Inherent safeguarding value of MCA |
| 3.2.1.2. Dignity and rights protected | 3.2.2.2. Challenge of translating training into practice | 3.2.3.2. Confidence and expertise | 3.2.4.2. Past preferences and best interests principal key to making proxy decisions | 3.2.5.2. Financial plans and funeral plans common, less so HSC plans | 3.2.6.2. Financial management and safeguarding against financial abuse is a significant area of concern as capacity deteriorates |
| 3.2.1.3. Familiarity with details not widespread but growing | 3.2.3.3. Risk of information ‘merry-go-round’ | 3.2.4.3. Challenges remain in understanding how best interests can be weighed up – i.e. whose best interest if the well-being of one depends on well-being of the other? | 3.2.5.3. Most patients and carers did not know where to turn – conceptualising debilitating conditions is a good way to start these discussions | 3.2.6.3. Warning signs of financial abuse need to be publicised more to raise awareness among practitioners, community support networks, financial bodies and society in general | |
| 3.2.3.4. MCA expertise at all levels unlikely, but central expertise required | 3.2.4.4. Inequalities in information access remained, in terms of those who had access to family solicitors and knowledgeable family members compared with those who did not | 3.2.6.4. Role of banks and other community support networks |
Universal appeal of the Mental Capacity Act
One of the most important themes to emerge from the study was the wide appeal of the values and principles of the MCA to practitioners working in HSC services, to ‘well’ older people, and to people with dementia and carers. Most of those who knew about it or had experience of using it were strongly positive about the MCA. This wide appeal contained the following three subthemes.
1. Beneficial to range of health, social care and voluntary sector practitioners.
2. Dignity and rights protected of people with dementia and carers.
The second of these two subthemes was illustrated in one of our early interviews with a participant working for a local Age UK group (see below). Although Age UK is not a specialist dementia-focused organisation, its branches and national organisation play a substantial part in advice-giving to older people. This illustrates the value of taking a broad system perspective to dementia care and support. It also highlighted the dimension to the MCA of enhancing the rights of people with dementia rather than seeing it as a professional burden.
I think it would be that it maybe just allowed people to have more rights, be supported in terms of making their decisions, and so I think that is the key thing, and that people are able to retain rights more than they have been able to, and I think the Act is around the best interest of the individual more and something about it being less restrictive in terms of the intervention on behalf of people, so I think it is really about broadening people’s rights.
Age Concern (now Age UK) staff member, CO 02
3. Awareness not widespread but growing.
This third subtheme is illustrated by a short extract from a specialist community nurse working with people with dementia (an Admiral Nurse). This practitioner had yet to call on specialist support but, in practice, her decision-making appeared to rest comfortably on principles of thinking about decisions on the basis of what was in the person’s best interests.
I think the only way really that it has been useful in terms of practising is that it’s at the back of my mind now. When I come into contact with people and decisions have to be made, I am thinking ‘is that something that is in that person’s best interests or is this case where somebody else should be called in?’ So I am more aware but in actual fact there hasn’t been any actual incident where I felt that I have actually put it into practice.
Admiral Nurse 03
Training
We systematically asked all practitioner groups about the training they had received in order to explore the sources of their understanding and knowledge. The frequency and format of training and the challenge of translating training into practice were two subthemes that emerged. The first exposed professionals’ variable introductions to the MCA and ways in which didactic training was not always seen as useful. Those who had missed initial training were not often offered opportunities to remedy this. In services with very high turnover this is a particular problem, as there is little opportunity for peer education. Some participants spoke of the problem of translating training into practice and we found few reports of the MCA being used in supervision. Staff within local authorities with responsibilities for safeguarding, and for the MCA Deprivation of Liberty Safeguards, emerged as valued sources of local knowledge and support.
Implementation
At the level of implementation, most practitioner groups found the MCA easy to apply in everyday practice. Principles of the MCA were universally accepted, and seen as reflecting the values of the Human Rights Act. 273 Most practitioners felt that the MCA generally clarified their thinking around matters such as capacity and making best-interests decisions. Recording decision-making processes was proving relatively straightforward. The following four subthemes emerged.
Quick and easy incorporation into safeguarding practices and procedures
There was strong evidence from interviews that the MCA had made a difference to the safeguarding of people with dementia from abuse and neglect. Prior to the MCA this had been seen as difficult as intervention powers were unclear and individuals’ refusal of support seemed hard to override. Those working in safeguarding services were unanimous that people with dementia were better safeguarded, as the extract below from one interview shows.
I think it gives a framework for evidence decision-making on behalf of someone. I think it also gives us a framework to discuss with families their responsibilities under the MCA framework. It gives people who don’t have capacity a level of protection – capacity has to be made in a particular systematic way and it gives us protection as to why that decision has been made in their best interests.
Safeguarding Adult Coordinator 04
Confidence and expertise
We asked all practitioners to report on their own levels of confidence and expertise in the MCA. Our analysis is reported by professional group. Generally, those working in adult safeguarding scored highest, whereas care home staff were less confident but recognised their own limited expertise, as the quotation below illustrates. This finding has major implications for the wider system of support for people with dementia as lack of familiarity with legal powers and safeguards in one setting may mean that communication between different practitioners is not based on similar understandings. For example, we found that advance statements in a care home might be known to a manager but not other staff and the decision-making authority of a person who had been granted LPA might not be understood by care home staff. These findings chime with some of the findings of EVIDEM-EoL.
My knowledge on a standard basis would be around 2 (out of a possible 5), but if I were to do research for my clients then I would delve into it and on that specific issue, then I would be confident in getting the information. We just offer general advice and up to a level of 2 I would know general advice, and we could research if we don’t know, that is more than adequate [for] my post.
Care home worker 01
Risk of information ‘merry-go-round’
Taking a system-wide approach, this study also found that people with dementia and their carers remain poorly served by some agencies and professionals. The advice and information they seek may not be easily accessible and they run the risk of being passed from agency to agency. Advice to find information online seems particularly inappropriate for many people with dementia: ‘Frustration that people are referred to numerous sources of information which are not able to give them advice or address their specific concerns’.
Mental Capacity Act expertise at all levels is unlikely, but central expertise is required
We noted above that local sources of expertise were emerging but did not always have explicit roles in being the main source of local knowledge. Our linked study, which audited MCA knowledge and practice in an acute hospital,274 also observed the limits of MCA expertise within the hospital and in its own policies and procedures. We do not suspect that this hospital is unique.
Everyday decision-making
The MCA contains principles that support everyday decision-making. However, there was limited evidence of their formal application and operation with respect to people with dementia living in their own homes and their carers. Our interviews with these individuals revealed a number of subthemes that throw light on how decisions are made within such close relationships: negotiated decisions, past preferences, weighing best interests and inequities in access to knowledge. This area had not been investigated previously. Our interviews were with people with early dementia and these themes may not necessarily apply to people whose symptoms are more severe. Four subthemes were identified, the first three of which are illustrated by a quotation from a participant.
Everyday negotiation
I like retirement. I like being at home. We [wife and myself] have breakfast together and decide what we’re going to do all day. Mostly we’re together but if she has one of her Ramblers’ outings, I don’t stop her. I’m not that kind of husband! But mostly we do things together.
Man with dementia
Past preferences being key to making proxy decisions
Oh I don’t ask her what she wants any more. I know what she’ll say anyway – ‘anything you like, you decide’. So I just do what’s best for us both. She has never had sugar in her tea. Never! And lately, she seems to like it! So I let her be, let her have it if that’s what makes her happy. When the sugar runs out, I’ll get some more but I’m not going to break my back getting the sugar for her because she’s never had it before, you know?
Spouse carer of woman with dementia
Weighing best interest decisions
I need to make sure that my health does not suffer. I know I should be doing anything that’s good for him, and I would. But I get so tired these days and I worry . . . if I go what will happen to him? So sometimes I need to force myself to rest . . . for his benefit, if you see what I mean?
Spouse carer of man with dementia
Inequalities in access to information
This subtheme arose in these interviews as well as in those with professional participants. In particular, there appear to be inequalities of access to information among those who do not have close family members or friends, and those who are not familiar with making financial plans and arrangements. Such people may never have consulted a solicitor and may regard themselves as being able to rely on continuity of care – for example, presuming that they will be able to retain their ‘family’ GP if they move to a care home.
Long-term planning
We asked a diverse group of people with dementia, their carers and ‘well’ older people to discuss their thoughts about long-term care needs and planning. They described personal perspectives and circumstances, talked of different types of plans, and some acknowledged not knowing where to turn. Three main subthemes were identified which are illustrated in the quotations below:
Planning influenced by personal perspectives and circumstances
I live for today, tomorrow you die. It has been successful for me so far, I am 79 . . . I enjoy good health and I go away every year and everything.
OA 021, male, age 79 years, black Caribbean
I don’t care if I am dying tomorrow, I said to people ‘look, if it is my turn that God said I should come and join Him’, I said ‘I am prepared, what is the use of worrying?’
OA 035, female, age 65 years, black Caribbean
What the Government have to realise is as I get older my friends get older, so at the moment I am waiting to find out if one friend is going to be buried, another friend has Alzheimer’s and so it goes on and on and on and on, no way can I ask a friend my age. Younger friends lead very busy lives and they have got family problems of their own.
OA 012, female, age 84 years, white British
Financial and funeral plans common, less so health and social care plans
I am hoping that I won’t be looked after [in a care home], but yes I will wait until I cross that bridge, I think.
Is there a reason you have avoided thinking about it now?
No, I have not thought about it, perhaps I need to think about it, I was just interested in getting the money sorted, but you are right, I do need to think about it, haven’t heard about it before. (OA 016, female, age 59, British Indian)
Hopefully I will probably die [in own home] because I don’t want to go into a home, unless I have to, or into an old people’s bed-sit with a warden.
Have you made any written formal plans to ensure this?
No, I think that automatically would happen with the council.
Have you made any provisions to prevent being moved into a home?
No, I don’t know how I can.
OA 023, female, age 68 years, white British
Limited support with knowing where to turn
No (not sure where to seek help around care), I have a financial adviser so he keeps up to date with everything, and, you know, he is very good – like certain policies that I used to have that he felt that I should cash them in, you know, because I have become ill, he said it is no use carrying on with them, so he has advised me to keep a certain life[style], car, and things like that – but certain policies I was paying in monthly and that and he said it is not worth it at the moment so that is very good.
OA 037, female, age 53 years, British Indian
If I made plans in the future, well I could think of an event immediately that would save me the trouble, if my doctor tells me I have only six months to live, something like that. But I think if a doctor told me that I had dementia, then yes, I would be making plans in that case, but I cannot think of anything else which would precipitate that. Well it is all I can think of at the moment, there may well be others you know, or if I have a sudden debilitating illness I suppose, like muscular dystrophy.
OA 010, male, age 70 years, white British
Safeguarding
As noted above, a significant element of the MCA is the safeguards it provides for vulnerable people such as those with dementia. Almost all practitioners noted this in their accounts of everyday practice. Alongside this central tenet, three subthemes emerged, which are illustrated in the quotations below:
Inherent safeguarding ethos
I think it has shifted the relationship between the people who receive services and those who provide services if it’s being implemented properly. And it can be challenged in law. It keeps us in our place. We provide support and expertise but in the end it’s their decision. And in our care management procedures it’s about positive risk taking. Life is about risk and adventure. When I first came into safeguarding it was about protection. If they are being empowered and feeling in control the chances of them being victimised are less.
Safeguarding Adult Coordinator 08
Need for help with financial management and safeguarding
Financial abuse is one of the second biggest abuse (types) that occurs in (area), it’s the second biggest of our alerts. There are a substantial amount of people with memory impairment which leads people to believe that they can get away with it.
Safeguarding Adult Coordinator 07
Warning signs of financial abuse should be publicised
We’ve done one (referral to the Police concerning a person who had gained) Power of Attorney. He befriended a couple when they had capacity. The husband died and the wife gave him Power of Attorney. He moved into the property and he moved her into a small room. The (person granted) Power of Attorney was not willing to spend money on her – for nightdresses and things when these were asked for. We’ve gone to the Court – the Office of Public Guardian has asked for bank statements. We’ve moved the lady to a place of safety; he doesn’t know where she is, while investigations are going on.
Safeguarding Adult Coordinator 019
Role of banks and community support networks in risk reduction
Many of those who had encountered people with dementia (whom they thought were at risk of financial abuse) thought that more could be done to reduce these risks if banks and other financial bodies were more engaged with safeguarding services so that that they could draw attention to emerging concerns about a customer’s vulnerability. Our investigation of this subject was extended by a partnership with the national Alzheimer’s Society in which we offered support in the devolvement of their national staff survey and shared data from our interviews with Adult Safeguarding Coordinators. This partnership established the extent of carers’ concerns about financial exploitation of their relative with dementia and, for the first time, highlighted the growing risks of internet and telephone scams.
Discussion
Strengths of the study
There are two main strengths of this study: (1) the range of practitioner groups that shared their views of understanding and using the MCA in their work and permitted us to see this from a systems perspective and (2) the longitudinal nature of the study that enabled us to explore change in perceptions among the same groups of practitioners, their successors, and among people with dementia and their carers. Together, these contributed to a form of triangulation of data that provided us with a holistic perspective on how the MCA is being implemented and incorporated into practice and everyday decision-making. We gained insight into a breadth of experience, as well as depth over time. Our familiarity with the emerging literature on the MCA led to an invitation to produce an integrative review of the MCA evidence for dementia practitioners. 275,276
An additional strength of this study is its predominant use of qualitative methods, which gave participants the opportunity to discuss their experiences in greater depth than might have been possible from any other method, such as a survey. Qualitative interviews further enabled us to probe appropriately according to the responses given. We also drew on survey methodology when we wanted to obtain a greater number of responses and a broader (rather than deeper) perspective, as in the survey of Alzheimer’s Society practitioners’ views of managing money, and by conducting secondary analysis of the EVIDEM-ED survey of carers’ satisfaction with legal and financial advice, planning and referrals. We drew on national and local media for examples of criminal prosecutions under the MCA (as these are not collected nationally). We also mined our research unit’s unique collection of Serious Case Reviews to investigate their coverage of MCA practice.
Methodologically, the rigour or trustworthiness of the study can be established by three criteria: credibility or validity of study findings, confirmability or dependability of study findings, and transferability or generalisability of the interpretations:
-
Credibility or validity was sought through obtaining a wide range of perspective, over time, on the issue, ensuring that a broad holistic view of the research question was obtained in our data. A commitment to iterative collection and analysis also supported this.
-
Confirmability or dependability of interpretations was established through clear description of analytical methods, peer analysis of data, rigorous paper trails and reflexive thinking on the parts of the researchers.
-
Generalisability or transferability to other settings was sought through detailed discussion with research peers, through the multidisciplinary advisory groups of the MCA study and the research programme as a whole, who offered insights in the transferable elements of our study in various settings.
Limitations of the study
Our study is limited in that participants were not always the same between time 1 and time 2 due to staff changes and differences in availability. We were unable to observe practice and everyday decision-making and relied on participants’ accounts. Our interviews with people with dementia and their carers were undertaken with a group in which dementia symptoms were not severe. Planning and decision-making may change as severity and disability increase.
Interpretation of the study findings in light of previous research
Interviews conducted during time 1 revealed interesting trends related to early implementation and expectations of using the MCA. The value of the MCA in supporting carers was a development first identified in this study during early interviews with Alzheimer’s Society staff. 263 These staff reported that the framework of the MCA was very useful to carers, particularly around long-term care planning and the ‘best interests’ principle. Challenges in proxy decision-making have been recorded277 and, based on this understanding, we investigated how proxy decision-making by carers was developing and changing over time. Although a person with dementia and their carer may require separate advice,278 we found that many of our dyads presented themselves as a unit and talked of the importance of getting advice together so they could discuss the implications with each other ‘there and then’. We found that, if approached sensitively, some dyads welcomed the opportunity to discuss long-term care planning. However, some did not and in many cases, it falls to a skilful practitioner to gauge individuals’ needs and wishes. We also have drawn attention to the potential to think about advance planning and arrangements among people who do not have family or friends who are willing and able to take on such roles.
Safeguarding Adult Coordinators appeared to find the MCA easy to incorporate into their systems of work. 264 Most were well informed about it and acted as sources of expertise locally. Prior to the MCA, many SACs considered there had been a legal vacuum around decision-making. 279 We cautioned at time 1 if this concentration on MCA work could mean that safeguarding work was in danger of being sidelined, further contributing to demands on SACs’ time and resources. By time 2, most SACs continued to be well informed and had greater experience in using the MCA. 265
There is a need, however, for continuous training to be sustained over time, given the high rate of staff turnover in social care settings,280 and even among SACs as our participant groups showed. The extensive investment by the DH in developing training and publicity at the time the MCA was implemented in 2007281 will not be repeated. Having the confidence to use and refer to the MCA also grew, alongside knowledge and experience with using the MCA, but it is unclear how case law and other developments are being fed into practice. At the time of writing (April 2014) Parliament has been debating the new Care Act 2014, as proposed by the White Paper Caring for our Future: Reforming Care and Support,282 and the experiences of the MCA implementation have lessons for how new law can be incorporated into professional transfer training. We have discussed these lessons with stakeholders in Ireland where a new Mental Capacity Bill, similar to the MCA, is shortly to be published and with dementia researchers and gerontologists in Australia, including policy-makers in the state of South Australia.
Our study also highlighted how voluntary sector organisations often served as valuable sources of support and advice to people living in the community but their knowledge varied. Staff from Age Concern (now Age UK) and carers’ organisations were not always specifically trained about the MCA, and tended to rely on signposting to other organisations that they felt would be more knowledgeable about legal matters. 258 Signposting is an important part of any local information strategy and the need for it may be greatest among people lacking confidence, having mixed experiences with ‘officialdom’ and those who are on their own in later life. Age UK at the local level was a major resource for some older people who were keen to discuss and consider long-term care planning. 272 However, our interviews with ‘well’ older people revealed that attitudes to planning could also be based on personal inclinations and long-term habits, as well as preferences. Approaching GPs or primary care services was not generally considered appropriate unless there were health concerns. Professionals working with people with dementia and their carers may need to offer time and a listening ear in order to support people who are feeling that matters are slipping out of their control at many levels.
By time 2, community nurses’ and adult safeguarding staff’s understanding of the MCA was generally more sophisticated. However, specifics of the MCA remained blurred among some and memories of training were dim. Some community nurses, initially themselves often lacking confidence, expressed concern about a lack of understanding of the MCA among other professionals. 270 One exception to this was that many participants at time 2 were aware of the importance of financial planning and that they could promote understanding of this among their clients or service users. 265–267 This confidence was obvious about end-of-life care planning, except among care home managers who described undertaking such discussion with residents on moving into the home, but largely without reference to the MCA.
This study has highlighted that many professionals have experience of family members with dementia, an observation that has not previously been evidenced. At a personal level, some reported finding family decision-making and planning potentially easier under the MCA, but loss of decision-making capacity or family disputes were sources of tension. We note the value of building upon the personal experience of practitioners to promote empathy with family carers in the provision of timely information and advice. 263,268 There have been surprisingly few investigations of the overlap of professional and personal experiences of dementia caring and so this study has added to the literature.
The offences of ill treatment and neglect created by the MCA were poorly understood and their implications have not been reported widely in professional or research-based literature. 39 Understanding among practitioner groups was minimal, as reported in our papers. 269,270 Accounts given appeared to be based on ‘common sense’ rather than legal definitions and thus potentially erroneously downgrading the offence of ill treatment and wilful neglect as poor practice. We therefore investigated this subject more thoroughly through the use of a consensus group. This group recognised the importance of highlighting that poor practice could be addressed by legal sanctions but were unsure how to access emerging case law and to promote learning from it. In conjunction with our understanding that some MCA training has been reported to be didactic and piecemeal, or needs to better reflect practice dilemmas and uncertainties, we have developed resources on this facet of the MCA reflecting the findings of our interviews. These include overviews for dementia practitioners283 and for the care home sector. 284
Conclusions and implications
There are three main lessons to be drawn from this study. For HSC practitioners it is evident that this legislation met a need in practice and that the legislation has largely been easy to work with, that professional values are in accordance with the values and principles of the MCA, and that it has promoted interdisciplinary and interagency working. This is possibly a result of the consensus that shaped the legislation: the involvement of practitioners in the implementation of the MCA and the devising of its Code of Practice. Engagement with practitioners and with stakeholders from patient/user and carer groups may be a valuable consideration for other change processes.
Second, this study has gleaned information about training transfer, the process by which professionals and non-qualified staff (the majority of practitioners working in dementia care) are provided with training while in post and change their practice. One advantage of a longitudinal study is that we were able to discuss training at various intervals, to talk to practitioners about what seemed to work well and whether or not they were able to refresh their knowledge and skills. We found that training was not always welcome or seen as relevant if it was didactic in nature, that there seem to be gaps among hospital-based staff in particular, and that few employers are explicitly checking that their new staff are familiar with the MCA. There was surprisingly little use of the MCA in supervision among professionals. Many practitioners were reliant on local expertise but this ran the risks of overloading certain sources. In light of the high levels of concern about financial abuse of people with dementia expressed by many participants, there may be scope to highlight this among all practitioners – especially to alert them to the targeting of people with dementia by strangers and by electronic or telephone contacts.
The third lesson lies in the potential inequalities of information. Providing information online may be acceptable to professionals but it is not welcomed by many older people or carers. Those with knowledgeable family members or those who have legal advisers are better off in this regard. Information was also not always shared among services, with one particular gap in social care – where some care home staff are not familiar with the implications of the MCA and the legal documents that their residents may have drawn up but which the residents are no longer able to bring to staff’s attention. The key professional here is the person’s GP but the sharing of MCA details with the GP and consequent passing to new care providers was not commonly encountered. We further noted the potential of an ‘information merry-go-round’ locally, as not all voluntary sector staff offering information and advice were confident in their knowledge of the MCA and potentially re-referred people making enquiries. A similar lack of expertise and confidence was also found among some professionals, such as some community nurses, who were otherwise likely to be seen as highly informed about dementia.
We do not suggest that everyone needs to have legal expertise in dementia services. The implication of this study is that it should be available and that information, advice and assistance are not the same. Commissioners may wish to ensure that these three areas are covered. Although memory services, for example, may give early information, some people with dementia and carers do not wish to take this up at the time of diagnosis but when these issues do arise they may not have contact with staff from a memory service. Advice to look ‘online’ may not be adequate or person centred.
Conclusion
This study broke new ground in investigating legislation that has fundamentally altered dementia practice and the experiences of many people with dementia and their carers. Although many people with dementia and carers will not encounter the MCA directly, it has great salience for them and its principles are widely supported by practitioners. Our research has outlined how new law is transferred to services and professionals. It has learned about changes in experience and confidence over time. It has focused on day-to-day decisions, as these are at the heart of human relationships. We are most grateful to all those who have allowed us to hear directly of their practice, plans, decisions and experiences.
Impacts
-
The research team has engaged with members of staff working with CNWL NHS Foundation Trust at events such as the annual summer schools across the study period, providing updates and workshops on aspects of the MCA (see Acknowledgements, EVIDEM-MCA publications and presentations). Discussions have been held with different professional groups and feedback provided to the Trust on MCA staff training.
-
A Dementia E-Learning Package (2012) was commissioned by King’s College School of Nursing and Midwifery and has subsequently been delivered to staff across the NHS South West. This educational package comprises the following modules: Introduction to Legal, Ethical and Cultural Issues in Dementia Care; Developing Competence in Social, Legal and Cultural Context; and Advanced Competence in Social, Legal and Ethical Context of Dementia. These are continuing professional development courses that a range of staff can undertake.
-
The research team was invited to undertake a literature review for the Joseph Rowntree Foundation on improving practice in communication with older people living in housing with care schemes, which made use of the EVIDEM-MCA study’s findings. This was published by the Joseph Rowntree Foundation285 and revised for a peer-reviewed publication. 286
-
The research team worked with Dr Mareeni Raymond on the development of a toolbox for GPs on dementia for BMJ Quality during 2011 to 2012.
-
This study team provided assistance to staff of the Mental Health Foundation as members of its advisory groups on the Mental Capacity Advocates study and Proxy Decision-Making (INDIPS study). The study is represented on the Advisory Group of the NIHR School for Social Care Research (SSCR)-funded study of the MCA Deprivation of Liberty Safeguards.
-
Membership of the Dementia Action Alliance (DAA) was awarded to the Social Care Workforce Research Unit. The DAA is a national consortium of over 100 organisations committed to transforming the quality of life of people living with dementia in the UK and people who care for them. Our application drew attention to work on the EVIDEM programme and other studies. In our application we drew up an action plan to outline how we remain actively committed to involving people with dementia and carers in current and future research (www.dementiaaction.org.uk/info/2/action_plans/117/social_care_workforce_research_unit_kings_college_london).
-
The study contributed to the Alzheimer’s Society’s policy and communications work on safeguarding people with dementia from financial abuse287 (http://alzheimers.org.uk/site/scripts/documents_info.php?documentID = 1770). The research team provided expert review of three of the Society’s fact sheets on Financial Abuse and Making Decisions, which are available to members of the public.
-
The research team has informed student and professional development studies in several UK universities by discussing study proposals and details (e.g. at University of Bedfordshire).
-
The MCA lead at the DH has requested all outputs from this study and pre-publication copies have been sent to her.
Building on research capacity
Nested within the EVIDEM-MCA study was a further NIHR Service Delivery and Organisation-funded research study, ‘The transition from cognitive impairment to dementia: older people’s experiences’. 288 This study complemented the EVIDEM-MCA study by providing the opportunity to investigate the dementia diagnosis transition from the perspective of people receiving this diagnosis and their carers. It highlighted the nuances of providing ‘information’ and the need to think about what information is wanted, understood and retained. The literature review confirmed the professional focus on care and treatment at the end of life (Advance Care Planning), and less so on day-to-day decision-making in dementia. 289 Implications for commissioners and primary care teams were published in the British Journal of General Practice in 2013. 290
A second study was also nested in the EVIDEM-MCA study. Although the EVIDEM-MCA study protocol explained that the focus on the research would be community based, the opportunity arose to work on the findings of an audit covering policy and practice about the MCA in one teaching hospital. This audit provided valuable insight into the implementation of the MCA in this setting, where reasonability appeared to have been diluted and procedural documents were incomplete and overlapping, and where training had been partial and not reflective of the needs of adult learners. 274
Research capacity was further built by the involvement of new and junior staff in the study. This included assistance with interviewing, when senior staff mentored researchers who were new to dementia research, and help with study outputs (from Nigel Charles, now of University of Plymouth, and Jess Harris of King’s College London). The EVIDEM-MCA study has also provided illustrative case examples of approaches to research with care homes as published in the ENRICH Research in Care Homes resources (www.DeNDRoN.nihr.ac.uk/enrich/#.UEcmZcEiaHc).
Changes to protocol
The original protocol aimed to recruit a range of staff from HSC services. This included IMCAs and medical staff using the MCA. We subsequently learned that the DH and others had commissioned studies with IMCAs291,292 and in the field of health care, with patients293 and with health-care practitioners. 254,294,295 There was a clear evidence gap in the views, experiences and expectations of social care staff when using the MCA. Social care practitioners were also those who were most likely to come into contact with people with dementia and carers living in the community. Following guidance from our advisory group, we focused on this particular and diverse group of practitioners, as this was, and remains, the under-researched population and sector.
List of appendices
Appendix 62 Chapter 5: EVIDEM-MCA protocol.
Appendix 63 Chapter 5: Details of the four phases of study design.
Appendix 64 Chapter 5: Semi-structured interview schedules.
Appendix 65 Chapter 5: Five main stages in framework analysis.
Appendix 66 Chapter 5: Narrative account of findings.
Chapter 6 EVIDEM: from cohort to research register
Abstract
The EVIDEM programme aimed to develop a cohort of up to 2000 people with dementia and their carers in order to recruit along the dementia disease trajectory, from diagnosis to end of life. The EVIDEM programme’s position in an NHS Trust allowed the research team to work closely with the North Thames DeNDRoN to create a register for people with dementia and their carers who wanted to become involved in research. The register contributed participants to EVIDEM studies, facilitated other dementia studies in the North Thames DeNDRoN area and provided a model for register development more widely.
A technology development methodology was used to develop the register. The construction and population of a dementia research register was feasible but its initial phase of development was complex because of ethical and organisational difficulties. Recruitment from primary care was particularly costly in staff time and identified only a small number of people with dementia who were not already known to specialist services. Recruiting people with dementia through secondary care was more effective but was also a resource intensive process.
In 21 months of operation across four NHS trusts, clinical researchers invited 1400 people with dementia or their carers to enrol in the register, gained consent from just over 800 and recruited around 230 to studies. To achieve the upper target of 2000, cohort members would take, we estimate, about 4–5 years of consistent recruitment through the existing register.
Background
UK government policy is to maintain people with dementia syndromes in their own homes for as long as possible. 18 However, the needs of people with dementia and their carers are inadequately addressed at all key points in the illness trajectory, from diagnosis through to end-of-life care. 296 Further research is required to understand the barriers to the timely recognition of dementia syndromes in primary care,7 the support needed by people with dementia and their families after diagnosis, the factors predicting relocation of people with dementia syndromes to care homes, the best methods of managing incontinence and challenging symptoms297 and the therapeutic options available to clinicians, which are currently sparse and insufficiently evaluated. 298
Some treatments for people with Alzheimer’s disease have been shown in trials to be modestly effective in modifying symptoms299 and emerging therapies – both pharmacological and psychosocial – will need rigorous evaluation in large-scale trials. The design of these trials will raise many questions, including which populations should be studied, for how long and with which principal and secondary end points?300
These questions may be difficult to answer. Difficulties in ensuring that samples are representative have meant that people with dementia who are recruited to clinical research have been younger than the general population of people with dementia, whereas women, the oldest old and ethnic minorities have been under-represented in study populations. Such under-representation may not always affect the external validity of relative effect estimates, but measures of absolute effectiveness, absolute harm and cost-effectiveness are associated with underlying risk levels in different sociodemographic groups and under-representation of subgroups will bias absolute effect estimates. 301 Research on dementia could gain much from the study of patient populations that more appropriately reflect the population at risk. 302 Age is particularly important given the potential for delays in dementia diagnosis, the inexorable progression of dementia and the diminishing capacity of people with dementia to give informed consent to participate in research.
In theory, primary care-based studies could address these problems of representativeness because of the heterogeneity of the community-dwelling population but, in practice, we know from recent trials that recruitment to studies on dementia through general practice is problematic. 303,304
Promoting dementia research
One of the barriers to recruiting people with dementia to clinical trials is the lack of a suitable tool that would facilitate identification of potential participants with the desired baseline characteristics. Registers for patients with motor neurone disease and Huntington’s disease have been long established but to date there is no equivalent register in the UK for those with dementia syndrome. This situation prompted North Thames DeNDRoN to test the concept of a research register of people with dementia who would express an interest in participating in dementia and cognitive impairment research in general, rather than specific studies. North Thames DeNDRoN was one of the seven regional DeNDRoN networks and was a collaboration between three universities [Imperial College London (ICL), Queen Mary’s University of London (QMUL) and University College London (UCL)] and 36 NHS trusts covering all North London boroughs plus areas of Essex, Hertfordshire and Bedfordshire (26 acute trusts, 10 mental health trusts). It is hosted by one NHS trust.
The EVIDEM programme needed to recruit to five specific studies but also had an interest in creating a cohort of people with dementia to allow studies to take place along the entire trajectory of dementia, from diagnosis to end of life. A joint approach to creating a register and deriving a cohort was a logical way to combine researcher expertise with the research facilitation objectives of DeNDRoN. A full description of the early stages of this project is reported elsewhere,100 and this chapter is based substantially on that account.
Research registers
There is now considerable experience of developing research registers, particularly in North America. Many registers have been developed to facilitate epidemiological studies305 but can also offer an organised and systematic way to maintain contact with participants from previous research and recruit an even more diverse pool of subjects interested in participating in future studies. 306
Research registers have been used in dementia research to study the clinical expression of Alzheimer’s disease307 and to improve the flow of information in order to increase research participation. 308 The US Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)309 has functioned as a vehicle for a wide range of studies and as a mechanism for developing and testing dementia-specific instruments. In 2008, the Leon Thal Symposium proposed the development of a US National Registry and Database to meet the multiple needs of the research field, including the development of a research programme on prevention. 310 Similarly, the European Alzheimer’s Disease Consortium, in 2010, proposed the construction of international research registries for studies of familial Alzheimer’s disease and for therapeutic trials. 311
However, there are difficulties in developing research registries. Although registries based on routinely collected data can offer opportunities for research, they pose problems of data organisation and accuracy for researchers. 312 Prospective collection of additional data requires organised outreach from the registry to patients, providers and staff, integration of the register into pre-existing clinical routines and addition of reminder systems to clinical workstations. 313 Unique challenges in recruiting and retaining participants with neurological disorders for research studies include the cognitive deficits of the participants and the complex ways in which many neurological conditions present. 314
The perceived advantages of a research register were that it could allow prescreening of research-ready populations for different types of study, allow more accurate assessments of study feasibility (because the potential research population would be known), and create the basis for longitudinal studies.
Construction and contents
The research and development questions we asked in the joint EVIDEM and North Thames DeNDRoN project were:
-
Is it feasible to develop and sustain a research register for people with dementia?
-
What are the actions and resources required to develop and implement a dementia research register?
-
What are the clinical requirements for a dementia register for the purpose of clinical trials recruitment?
-
What are the likely recruitment rates to a dementia research register?
Register design
The development of the register was based on a standard technology development methodology, originally derived from the construction of decision support systems. 38 This involves phases of modelling and prototype creation, ‘bench testing’ and refining of the prototype with experts, and then ‘field testing’ of the refined prototype register in exemplar sites. We have used this methodology elsewhere in the EVIDEM programme (see Chapter 1).
A co-design approach was taken,37 bringing together researchers (in the EVIDEM programme), research network developers (in DeNDRoN) and people with dementia and carers, through the PPI arm of DeNDRoN. Expert advisors from the Centre for Health Informatics at University College London were recruited to the design team to develop the electronic database for the register.
The design team met on six occasions during 2008–9 and held monthly teleconferences to review progress. The design team consisted of five members from the EVIDEM programme (Iliffe, Lowery, Rait, Walters, Kharicha) and two from North Thames DeNDRoN, bringing together academic, clinical and research network expertise. The design team developed a prototype register, ‘bench tested’ it with other experts in the field, and then initiated recruitment to it, initially in one specialist pilot site but also in selected general practices.
The proposals for the register were discussed with DeNDRoN’s PPI working group (made up of DeNDRoN regional workers and members of third-sector organisations) and its PPI forum (made up of people with neurodegenerative diseases and their carers).
Modelling, ‘bench testing’ and prototype development
The objectives agreed by the joint design team were to:
-
identify people with dementia and their carers through primary and secondary health care, social care, community care and voluntary sector organisations in the North Thames DeNDRoN region
-
invite patients to join a research register
-
gain consent for a minimum data set of information about patients to be held on the research register
-
enable clinical research staff and registered research staff to search for patients relevant to a set of user-defined parameters, and then use that retrieval set as the basis for making contact (through the patients’ clinicians)
-
enable the register staff to maintain a list of studies to which the patient has been invited, is deciding about, has consented to or is participating in
-
enable appropriate matching of register members to research projects and further anonymised analyses
-
manage all such data securely, using role-based access and maintaining an audit log.
Recruitment to the registry would occur in the geographical area covered by the North Thames DeNDRoN (North London, and parts of Essex, Buckinghamshire and Hertfordshire). Recruitment would be tested in primary care, secondary care, and if possible in social care (e.g. care homes), community health care (e.g. community nursing services, Admiral Nurses) and third-sector (voluntary) organisations (e.g. Alzheimer’s Society).
The target population was defined as people of any age, with any form of dementia, residing in the community or residential care within the defined geographical area.
The inclusion criteria chosen were people with either a formal specialist (imaging/neuropsychological) or informal generalist diagnosis of dementia, as well as participants with cognitive impairment presumed secondary to an underlying neurodegenerative disease. The case definition includes different types of dementia syndrome and people with dementia of differing severity (from early to late dementia as well as mild cognitive impairment). We agreed to include non-ICD-10 Diagnostic and statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) diagnoses but the source of, and basis for, the diagnosis would be a field within the register to allow prospective filtering to match the quality needs of future research projects.
The exclusion criteria we selected were (1) people who do not speak English, for whom an interpreter could not be located and (2) those whose clinician believed would be inappropriate to approach, for specific reasons like receiving end-of-life care, treatment for severe comorbidity or major behaviour disturbance.
Ethical approval
Although the primary aim of the register was to support research, the design team felt that it was essential to seek ethical approval, in part because diminishing capacity to consent to participation in research is a feature of dementia syndrome. In addition, the Data Protection Act50 requires that all patients who are identified for research projects have given their consent to be identified in this way. Although other disease research registers had not sought approval from ethics committees, the design team believed that an ethics committee would provide another layer of expert opinion about how best to explain the purpose of the register. In addition, the rigorous and well-documented consenting process that has to be applied following an ethics committee’s approval provides a clear, auditable and defensible pathway documenting dissemination of information about the register, the assessment of capacity and the storage of clinical documents, should challenges arise. Approval for the trial was received from Southampton & South West Hampshire REC (A): reference 08/H0502/34.
It was also clear that recruitment of large numbers of people with dementia and their carers would require Research Management and Governance approvals across multiple sites and sectors, and information management approvals for data storage. In addition, the joint team had to develop a minimum data set and database, and devise mechanisms for capturing data in primary and secondary care, and through other routes, such as care homes and third-sector organisations.
Constructing the minimum data set
A minimum data set was developed in three stages. In the first stage, comments on the secondary care requirements of the data set were gathered from the North Thames DeNDRoN’s executive board, supplemented by individual discussions with researchers within the local network. In the second stage, face-to-face interviews with 39 GPs were conducted to discuss the potential for using data from the general practice reimbursement mechanism (the ‘QOF’) for dementia. In the third stage, members of North Thames DeNDRoN gave feedback on the minimum data set fields generated in the previous two stages, and the data set was refined based on this feedback. Box 6 shows the contents and data fields of the minimum data set. The design group intended that the minimum data set would evolve over time to be consistent among collaborating centres, as far as pragmatically possible.
-
For all primary care practices we recorded location (PCT) and deprivation index score.
-
For all clinics we recorded specialist and clinic location.
-
For other services we recorded location, e.g. care home, supported accommodation, elderly mentally infirm home, and key worker details.
-
For all participants in the register the following information (extracted from practice or clinic notes) is recorded where possible:
-
demographic details (name, date of birth, gender, marital status, first language, ethnicity, address, postcode, housing status, NHS number)
-
carer information (name, date of birth, gender, address, postcode, relationship to person with dementia)
-
practice details (name, address)
-
specialist details (name, clinic details)
-
cognitive status (date of most recent test and score)
-
functional status (date of most recent test and score)
-
behavioural/neuropsychiatric status
-
investigations (imaging and dates)
-
specific dementia medication
-
comorbidity (e.g. depression, cardiovascular disease, diabetes)
-
history of participation in trials/studies.
-
We were aware that information recorded in notes would be variable across services and sites. This minimum data set was based on data known to be routinely collected in secondary care clinics assessing patients with cognitive disorders, but its applicability to primary care was unclear.
We therefore invited 25 GPs from 10 practices known to be both interested in dementia and engaged in research, to assist us with a study of the data content of the records of patients with dementia. Five accepted the invitation and four (all from different practices) were able to commit time to it. In each practice the electronic medical records were searched by the GPs for patients with dementia, and individual records were scrutinised (‘hand-searched’) for information in the data fields shown in Box 6.
Figure 22 shows how information that could be used to prescreen patients with dementia is distributed across codes, free-text and scanned documents, and which categories (e.g. functional abilities) are commonly missing. This demonstrates the amount of additional work that would be required to use existing GP electronic records as the source of data for a registry. This finding, together with a pilot recruitment study in general practice (see below and Appendix 67) convinced the joint team that the research register should be built primarily from patient populations that were already engaged with specialist services.
FIGURE 22.
Prescreening from GP record. a, Medications may not be accurate in all cases.
Three aspects of the minimum data set were seen as important by both researchers and PPI experts, and confirmed as such by an ethics committee: confidentiality, duplication of information and access.
Confidentiality
A unique identifier is assigned to all participants on the register so that data are held anonymously. A file linking name and unique identifier is stored separately and securely, and in accordance with the Data Protection Act. 50 This will be held until the participant indicates that s/he no longer wishes her/his data to be included on the register, and 6-monthly reviews will allow reaffirmation of register status. Six-monthly reviews were chosen because of the relatively rapid health status changes that can occur in dementia syndrome. It is intended for the register to be comprehensive and to be able to include all patients seen in the North Thames DeNDRoN region in order to be representative of the patient population.
Duplication
Information on whether or not the patient has been/is currently participating in research studies will be included on the register to avoid patients being approached for participation in multiple projects, and register managers will cross check key identifiers (name, date of birth, NHS number) of potential new participants to ensure that people with dementia and their carers are not approached repeatedly.
Access
National and regional researchers wanting to access the registry would need to approach North Thames DeNDRoN in the first instance. Prioritisation of studies within the North Thames DeNDRoN portfolio by local researchers was anticipated and access to the register would reflect this prioritisation. Governance of the registry would be managed through DeNDRoN’s national co-ordinating centre, in the first instance.
Constructing the register
Physical construction of the research register and its use at the first sites provided experience of the practical problems involved in recruiting people with dementia to a research register. These included identification and invitation of potential participants, judgement of capacity, and obtaining both official permission and actual support from practitioners and administrators to recruit through NHS services.
Identification of people with dementia
This was undertaken using medical records complied with the then-current recommendations from the Patient Information Advisory Group. These recommendations allowed only members of the patient’s usual clinical care team to identify patients suitable for the register. The lead clinician (or other member of the normal clinical team responsible for the patient’s care) would then make the first contact with the patients identified, either in a face-to-face meeting or by letter/telephone. This contact would be only to inform the individual or their family about the register; enrolment would usually take place separately from the clinical encounter in which the information about the register was given. There were, in practice, exceptions to this, as some people were keen to enrol immediately rather than wait until their next appointment. In cases when people feel they have had sufficient time to consider their decision, their consent can be taken on the same day as they receive the information. Figure 23 shows the recruitment path that we developed for an individual enrolled through a memory clinic. This process is likely to vary slightly to reflect differing care pathways in different memory clinic services.
FIGURE 23.
The consent process in DemReg.
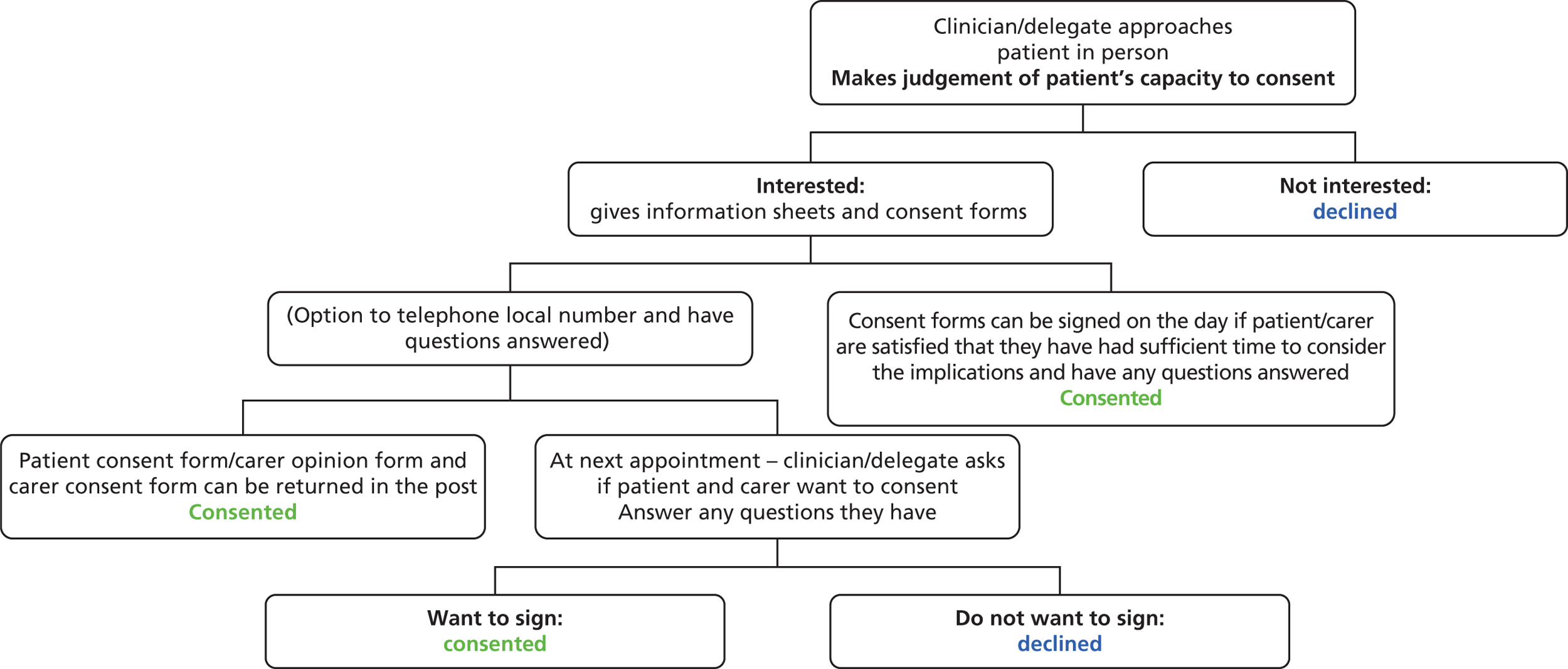
Judgement of capacity
To ensure that people at all stages of the disease process were able to join the register requires judgements about decision-making capacity. (This proved particularly difficult in primary care – see below.) A recent analysis of ability to consent to research in a therapeutic trial of Ginkgo biloba found approximately 70% of individuals with mild-moderate dementia could not give valid consent to research participation. 315 In the case of individuals who are not able to give informed consent, UK MRC and European Union-GCP guidelines and the principles of the MCA 200517 (England and Wales) were followed, and opinion sought from a relevant consultee. This is also in accord with internationally accepted guidelines on research involving human subjects with dementia. 316
Seeking permissions
Research Management and Governance offices at each NHS trust where researchers were interested in setting up the research register were approached for permission to involve trust staff. Seeking multiple permissions across provider organisations in primary and secondary care proved to be a lengthy process, taking up to 5 months per organisation. This process was not made easier by high staff turnover rates in the DeNDRoN research network itself and the time needed for training, Criminal Research Bureau checks and research passport applications for new study officers. The steps required to engage a new clinical site as a recruitment site for the register are summed up in Box 7.
-
A Principal Investigator to act as champion for that site.
-
Local Research Management and Governance approval.
-
Resources for a local co-ordinator, who initially carries out and then co-ordinates data entry, acts as contact person for data queries, and liaises with site staff about recruiting patients. To date, the financial resources have come from different streams of research network funding. New staff may need to be recruited, or honorary contracts established, for those already in other posts.
-
Agreement from local information technology departments who need to give new staff access to electronic databases, and to set up shared drives where none existed previously. The local information governance manager needs to be satisfied about data security.
-
Service manager agreement to provide office space, promote the use of the register to front line clinical staff, allow computer use and staff involvement in seeking consent, as well as facilitating best working practices for each site.
-
Site team involvement in supporting the lead clinician in identification of patients to inform about the study.
The initial phase of recruitment of patients and carers to the research register yielded important lessons about where best to recruit, and about data governance.
Recruitment in secondary care
Recruitment began in the first mental health trust in early March 2009, and three other trusts began patient recruitment in the following 12 months, with four more initiating involvement. Figure 24 shows the gender distribution of those joining the research register, and Figure 25 shows the age distribution. The self-reported ethnicity of those enrolled is shown in Figure 26; about 20% were non-white.
FIGURE 24.
Gender distribution of enrollees in the dementia register.
FIGURE 25.
Age distribution of enrollees in the dementia register.
FIGURE 26.
Ethnicity of enrollees in the dementia register.
Figure 27 shows the rates of invitation and recruitment to the register, and the numbers engaged through the register in trials, from the end of March 2009 to the end of December 2011. Acceptance of the invitation was high, at over 90%, but the rate of recruitment was determined by the pattern of clinic attendances, with a gap of 3–6 months between the invitation to join the register and acceptance for the majority of participants. Figure 28 shows the recruitment to DemReg and to studies across four NHS trusts at the end of February 2012 (when EVIDEM’s involvement in the register’s development process ended).
FIGURE 27.
Recruitment of patients and carers to DemReg, and accrual to studies, March 2009 to December 2011. PIS, patient information sheet.
FIGURE 28.
Recruitment to DemReg and accrual to studies across four trusts, February 2012.
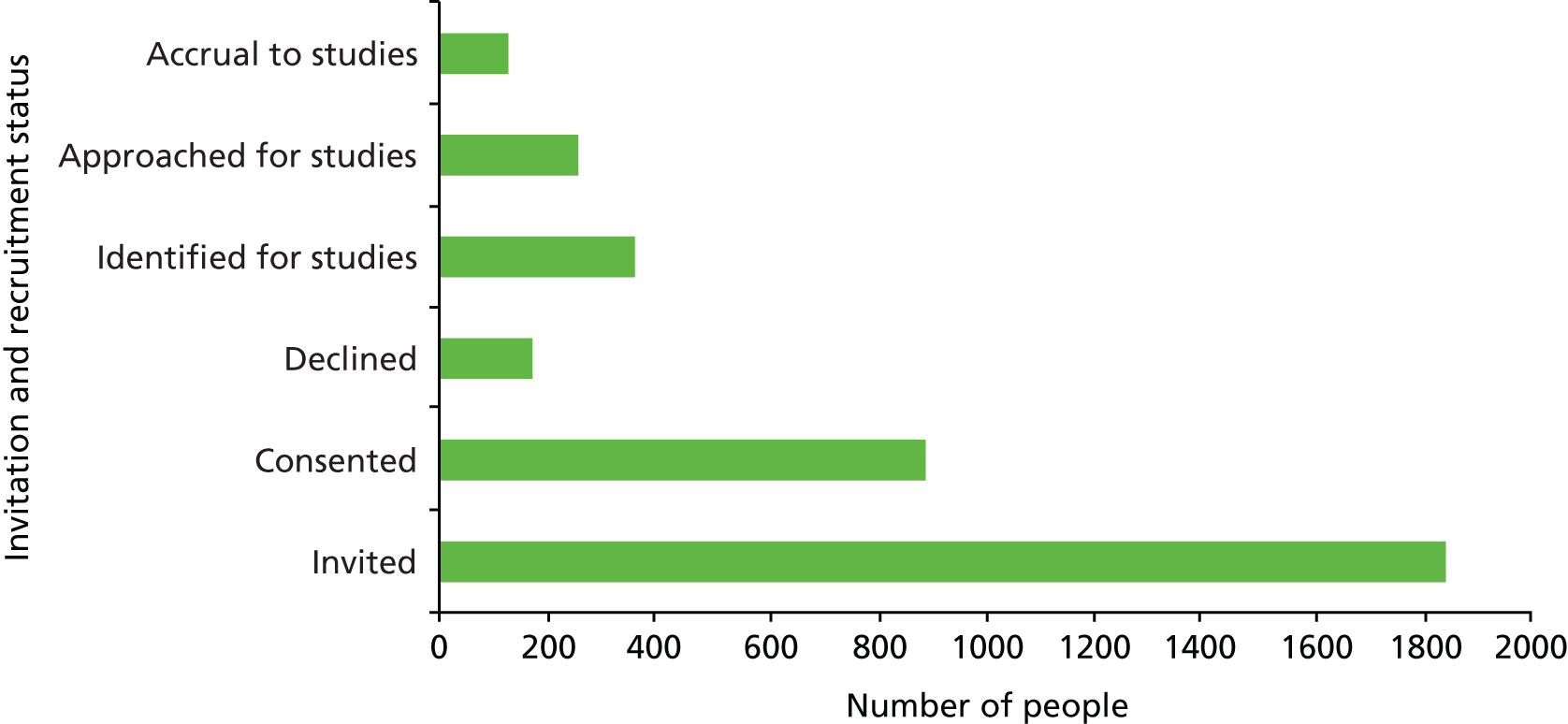
Recruitment in primary care
Tests of recruitment began with five practices that were already part of the EVIDEM programme in autumn 2009. Of 72 people with dementia identified from these five general practices, who were sent information by post about the research register, three people responded that they were not interested in research or finding out more, and 18 people expressed interest and asked for more information. Fifty-one people did not respond to the invitation.
Among those expressing interest in the research register, 10 people were already attending specialist clinics operating the research register, and their details were passed on to the appropriate clinic. Three lived in care homes, and an assistant psychologist obtained the carer’s agreement to gather data for one of them. She did not meet the resident to assess decision-making capacity, but both the GP and the care home manager judged that the resident lacked capacity for this decision but said that the person would have wanted to have participated in the study. The data gathered did not contain information about medication or MMSE score, and for one potential recruit the psychologist extracting the data was unsure which of two documented diagnoses was correct. For the other two individuals the assistant psychologist had to meet relatives (in their role as consultees), as the care home staff did not feel able to give an opinion about participation in research (which they are not able to do under the MCA).
The assistant psychologist also arranged a meeting, at a hospital site, with two of the three people with dementia who were not seen in any other NHS service. The remaining person not seen by any other service lived in an area distant from any facility that the psychologist could invite them to, and the psychologist was reluctant to make home visits, so this individual’s expression of interest was not pursued.
Testing the process of recruitment in general practice was undertaken by the EVIDEM team and in specialist clinics by North Thames DeNDRoN but responsibility for subsequent 6-monthly follow-up and review did not clearly belong to either party and had to be decided through discussion. The situation was complicated by the organisation of research infrastructure in England, where three research networks may be involved in dementia research: DeNDRoN; the PCRN, which recruits GPs for research projects; and the Comprehensive Local Research Network, which funds the involvement of practitioners in research work. These three types of network do not have the same boundaries, so trilateral negotiations were necessary in some locations to allow the EVIDEM team to test recruitment to the register through general practice.
For these reasons, the prototype register was developed first in specialist services (particularly memory clinics), leaving recruitment in primary care (and other settings – see above) to be explored at a later stage.
Data governance
A decision had to be made about who would ultimately hold and be responsible for the data collected in this way. The project information sheets stated that documents and register data would be stored by the register team at North Thames DeNDRoN but, as North Thames DeNDRoN is not a legal entity, documents and register data are stored by West London Mental Health Trust and were accessible by only the clinical and research staff within the involved NHS trust’s boundaries.
In a later development which was not part of the joint EVIDEM/North Thames DeNDRoN project, the register’s software was adapted to fit within clinical systems, to facilitate recruitment and avoid duplicating data entry.
Discussion
Feasibility
The North Thames DeNDRoN dementia register was a pioneering project in the UK. This case study suggests that construction and population of a dementia research register is feasible, but that the initial development is complex because of the ethical safeguards in dementia research and the organisational difficulties in embedding research projects in NHS clinical services. Recruitment from primary care has proved problematic; enrolment of patients is particularly costly in terms of staff time, especially given the very small number of people with dementia identified who were not already known to specialist services. The logistics of recruitment in memory clinics were better because of the concentration of patients and staff as well as the use of care pathways into which the register’s recruitment process could be embedded. Even then, given the time scale of clinic attendance and the restrictions on obtaining informed consent, the recruitment process may take up to 6 months.
Resource issues
Recruiting people with dementia to the register through secondary care is still a resource intensive process. Potential register members need to be identified as suitable, informed about the register, met again to obtain consent and to capture information for the minimum data set, and reviewed every 6 months to confirm their continued interest and update their data.
The preliminary steps in gaining the necessary permissions and resources to establish the register at a new site require effort (a manager able to devote sufficient time) and up to 5 months of preparatory work (decreasing as time and experience informs the process). However, early investment of effort will ensure that not only will local clinical teams be invested in the process, but also that it ensures that data collected thereafter will be both accurate and complete. The costs of developing and running the register were seen as core service support costs and were therefore borne by the NIHR Clinical Research Network. DeNDRoN funded the register’s development in the short term but it is also part of DeNDRoN’s 5-year strategy (2010–15) to develop a broad coalition to secure appropriate funding arrangements for research registers in the longer term.
Clinical data requirements
Identifying the components of a minimum data set was an early achievement of the design team, which is being tested as researchers begin to use the register to recruit to studies. The effectiveness of the register’s minimum data set (as currently designed) as a device for prescreening potential research populations is under investigation.
Recruitment
Acceptance rates are very high in the first clinic to recruit to the register but this may reflect the efforts of register ‘champions’. We have monitored recruitment in more recently recruited clinics, for which there may be less ‘ownership’ and hence less commitment to register development; this gives us an estimate of the likely growth of a dementia research register within usual NHS clinical practice. Easier recruitment may perpetuate potential selection biases and we are not yet able to assess the representativeness of the research-ready population recruited to the register; this is an issue that needs to be revisited as register use becomes a normal process for researchers. We know from analysis of participants in the first site to start recruitment to the register that the register included more women than men, and a substantial number of people from minority ethnic groups and from the ≥ 75 years age group. Ways of increasing recruitment through primary care, and through care homes and social care services, to offset biases inherent in clinic recruitment, need further investigation.
Conclusions
Between them the EVIDEM projects recruited 704 people with dementia to studies, along with 292 carers; EVIDEM-ED’s audit of general practice included 1072 people with dementia at baseline, and at the end of the EVIDEM programme the dementia register held an additional 500 people who were not involved with EVIDEM but who have expressed an interest in being involved in dementia research.
We believe that the register will assist in connecting people with dementia and their carers with high-quality research studies that will help us answer important questions regarding the pathology, clinical pathways, aetiology, experiences of, and best treatments for, neurodegenerative disease.
The primary obstacle to the development of the register has been the complexity of permission processes within the NHS, an experience noted by others. 317 This may change, as the register is adopted by research sites that are not part of the designer group; in new sites, negative staff attitudes and competing clinical priorities may become more salient and more limiting, as found in other registries. 313 A number of important characteristics of the register are awaiting evaluation, including the utility to researchers of the minimum data set and the cost per person recruited to studies through the register.
The success of this prototype will be measured by the proportion of people from the register who participate in research studies and the impact over time that the register has on overall accrual to portfolio studies.
Appendix
Appendix 67 Chapter 6: Proposed data capture fields with definitions for primary care.
Chapter 7 Implications for research, policy and practice
What are the implications of the EVIDEM programme for research, policy and practice? We will attempt to answer these questions in terms of new knowledge and knowledge application, products arising from EVIDEM projects, methodology development in EVIDEM and the implications of the programme for research. We will also illustrate how the programme organisation added value to the individual projects, describing the synergies in the research process, EVIDEM’s ‘offspring’, and research capacity building within the programme.
Diagnosis and management in primary care
New knowledge and knowledge application The EVIDEM-ED trial has shown that a high-intensity, tailored educational intervention in a context of increasing policy pressure (NDS 2009,18 the review of antipsychotic use in 2011), growing resources in some areas (e.g. pilot schemes of dementia advisors, dementia champions in general practice) and financial incentives for general practices (within the QOF reimbursement system) does not increase dementia case recognition or improve the amount or content of clinical management of people with dementia in general practice. Our view is that the less-intense, less-tailored educational activities currently being promoted as part of the NDS are unlikely to change clinical practice in primary care.
EVIDEM-ED products Although the educational intervention proved ineffective in changing documented practice, it was popular with practitioners and researchers. By the end of the EVIDEM programme the methods for carrying out learning needs assessment and the writing of educational prescriptions used in the EVIDEM-ED project had been taken up by practitioners in the English and Welsh NHS, and Ireland, and by researchers in Germany (University of Düsseldorf), the USA (at Johns Hopkins Medical School) and New Zealand (University of Auckland). The clinical themes identified by GPs as priorities for learning in EVIDEM-ED were used in the evaluation of e-learning packages on dementia, funded by the Alzheimer’s Society (see www.alzheimers.org.uk/site/scripts/documents_info.php?document ID = 367).
Methodology development The implementation of ENA and educational prescriptions in general practice (EVIDEM-ED) has tested this approach to learning on a scale that is unusual in educational research, and takes these methods beyond theoretical discussion into practical application.
Implications for research Educational interventions in settings in which a co-ordinated system of dementia case management operated have shown positive effects,318 so the effective change may need to include the additional resource of case management as well as focused education. Future research may need to refocus on the additional resources needed in primary care, as in the CAREDEM study,319 rather than in the acquisition of new skills by the existing workforce.
Exercise as therapy for behavioural and psychological symptoms of dementia
New knowledge and knowledge application The EVIDEM-E trial has demonstrated that exercise as a form of therapy for BPSD is acceptable to people with dementia and their families, and feasible for use by the NHS, but its effectiveness in reducing behavioural and psychological symptoms in people with dementia is not yet established. The secondary, but important, finding of EVIDEM-E is that the exercise intervention seemed to have a strong positive effect on carer’s burden. This is important, as carer’s burden is strongly associated with increased likelihood of relocation to a care home. It does not appear likely to be cost-effective, however, showing no significant cost or effect differences for any of the outcome measures considered.
EVIDEM-E products The EVIDEM-E project has made a video about exercise as therapy in dementia, accessible via www.EVIDEM.org.uk.
Methodology development EVIDEM-E is one of the largest RCTs of exercise for BPSD. This trial has contributed to the debate about designing trials for non-pharmacological studies. Predictably, recruitment has been one of the main challenges encountered. The EVIDEM-E team largely solved the recruitment problems, and, as a consequence, learned lessons that have been reported to the wider research and clinical communities.
Implications for research The walking intervention developed and tested in EVIDEM-E is safe, simple to apply and does not need special training or equipment. Further research should focus on the mechanisms by which exercise affects carers’ burden, the feasibility of deploying this tailored intervention on a wide scale without loss of fidelity, and its long-term impact on BPSD, carer burden and relocation.
Managing incontinence in community-dwelling people with dementia
New knowledge and knowledge application The EVIDEM-C project has found that the incidence of incontinence (urinary and/or faecal) in community-dwelling people with dementia is at least double that in populations matched by age, gender and comorbidity. The use of indwelling urinary catheters, a management strategy discouraged by international and national clinical guidelines, was found to be double the rate in people with dementia compared with a matched population without dementia. It has shown that incontinence in people with dementia is a deeply hidden problem, a taboo concealed within a stigma. Family carers often sought to preserve the person’s dignity by not seeking help, often to the point of crisis. Professional and continence service responses to incontinence often omitted any specific consideration of the effects of dementia. Family carer management strategies emphasised the effective containment of excreta and some solutions had unintended, negative consequences for the person with dementia. FI was shown to be less common than UI among people with dementia, but found to have a greater negative effect on the carer’s quality of life. The presence of FI was found to significantly increase the costs, by almost two-thirds, from both a HSC perspective and a societal perspective, compared with the costs of managing UI. This project made recommendations for practice (routine enquiry by professionals about continence problems at each encounter with carers) and developed an assessment tool for community nurses.
EVIDEM-C products The EVIDEM-C project provides tools and supporting materials for practitioners working with people with dementia and their families, and insights that require further testing and investigation by researchers.
Methodology development The analysis of a GP records database (THIN142) by the EVIDEM-C project has clarified the methods needed to study the epidemiology of incontinence, especially when using routinely collected clinical data. The EVIDEM-C project also developed innovative analyses of costs incurred in the management of incontinence in people with dementia.
Implications for research Strategies and responses that primary care professionals and others can use to encourage greater openness, and which may reduce the taboo of incontinence within the stigma of dementia, need testing in further studies. Similarly, dementia-specific continence assessment tools and guidance for the use of primary care nurses produced by this project need evaluation for their utility and impact.
End-of-life care in dementia
New knowledge and knowledge application The EVIDEM-EoL project showed that the trajectories of the end-of-life in people with dementia are often unclear to care home staff, family members and visiting health-care practitioners. The transition from living to dying with or from dementia is often characterised by uncertainty in three key areas:
-
how different groups should work together and across the health and social (independent) care
-
whether or not a person with dementia is actively dying and how to manage the process and its related symptoms
-
whether or not the resources and services needed to support the person in the care home will always be accessible.
In settings in which there are no on-site clinical practitioners, decision-making in these three areas is a complex process reliant on how roles and relationships are negotiated between family, residents and NHS and care home staff. An intervention such as AI was able to help address ‘relational uncertainties’ about who does what and for whom and to develop productive working relationships between care home practitioners, family members and NHS staff. It fostered rapid and sustained engagement between care homes staff and GPs, did not increase resource use, reduced the use of emergency services and appeared to improve the management of unexpected events, crisis management and unplanned hospital admissions. This has direct implications for practice.
EVIDEM-EoL products The EVIDEM-EoL project has field-tested AI in care homes and developed a framework for the evaluation of end-of-life care interventions for people with dementia in long-term care settings, which is currently being used in a Train the Trainer evaluation funded by East of England Multi-Professional Deanery.
Methodology development The application of AI methods in care homes and primary care (in the EVIDEM-EoL project) is one of the few examples of the practical use of this quality improvement technique. The methodological testing of consent processes for people with dementia in long-term care in the EVIDEM-EoL project adds to our understanding of how to conduct research with people with impaired cognition, and contributed to the NIHR SSCR-commissioned review of research methods for end-of-life care research in social care settings. The evaluation framework for end-of-life interventions in long-term care settings is a novel development. Collaboration with Belfast University in analysis of medication use in care homes (EVIDEM-EoL) is also innovative – see Chapter 4 for details and outputs from this collaboration.
Implications for research AI appears to improve working relationships between NHS professionals and care home staff, with promising outcomes. It is an approach that fits with others which emphasise relational approaches to care. Nevertheless, more research is needed to test these findings further and with more robust controls.
Evaluating the Mental Capacity Act 2005
New knowledge and knowledge application The EVIDEM-MCA project found that dementia care services and practitioners have traditionally not conceptualised their practice as being framed by legal rules. The MCA 200517 was a major challenge to this and the dementia care sector has had to adapt to this fundamental shift. There are lessons here for future changes being proposed for social care law, such as those outlined in the Care and Support White Paper of July 2012. 282
EVIDEM-MCA products The EVIDEM-ED learners’ manual included brief advice for practitioners on capacity assessment and the requirements of the MCA 2005,17 derived from the MCA project. Forty-four tailored learning resources have been prepared by the EVIDEM-MCA project.
Methodology development The longitudinal element of the programme enabled repeat interviews with practitioners and people with dementia and carers; this is a novel approach among some practitioner groups. EVIDEM-MCA for example, interviewed SACs four times, many of whom participated in all rounds. In contrast, on returning to care homes for repeat interviews, few staff interviewed initially were still in post at time 2. Learning from this study has contributed to the NIHR SSCR-commissioned overview of qualitative research with people with dementia.
Implications for research We need to study MCA offences and their role in providing assurance that people with dementia have access to justice and are safeguarded. It is difficult to know if the media coverage of prosecutions under the MCA is having the effect of reducing ill treatment and wilful neglect, but research might establish if emerging case law was facilitating cultural change within care services, by demonstrating that ill treatment is not simply a matter of poor practice, but potentially subject to criminal sanctions. Much dementia care has focused on efforts to improve services, support carers, promote good practice and encourage staff. We do not know if an approach that is more punitive leads to greater intolerance of abuse. We do not know the effects on services such as hospitals and care homes if cases are brought against staff or family carers, or on staff and team dynamics.
A final area for research is that of bridging the gap between professionals and carers. Our finding that many professionals have experience of dementia among family members is probably of no surprise to readers of this report. However, it has not been commented upon or its implications explored. Research might investigate the interface between care work and family care. In relation to the MCA, such personal and professional experiences appear mutually informative.
A dementia cohort
New knowledge and knowledge application The COHORT/REGISTER project, developed jointly with North Thames DeNDRoN, demonstrated that it is feasible to develop, populate and use an ethically acceptable dementia register, housed within a NHS clinical record system. However, we also showed that recruitment to such a register through primary care is costly and yields small numbers of participants, making recruitment through specialist services more cost-effective. The further development of research registers was taken up by DeNDRoN.
COHORT/REGISTER products The North Thames Dementia register (NT DemReg) was adopted and used by five NHS trusts.
Methodology development The development of the North Thames DeNDRoN research register has influenced debates within DeNDRoN about the configuration of recruitment and feasibility assessment tools for neurodegenerative diseases research.
Implications for research The pioneering work of the dementia registry has led to the development of a specification of a national registry by DeNDRoN; this work is under way. There is potential for using the registry not only to identify and recruit research interested people with dementia and their carers, but also as a research database in its own right.
The benefits of programme funding
The EVIDEM programme has had a rich research output because of its multidisciplinary nature and its connectedness to the host NHS trust, CNWL NHS Foundation Trust. Combining the expertise of different organisations and disciplines created synergies in the research programme, allowed EVIDEM to produce ‘offspring’ studies, and contributed to the expansion of research capacity in dementia studies.
Synergies in the research process
The interaction between projects was critical to the success of the EVIDEM programme. Some of the cross-fertilisation was about specific topics, problems and techniques. For example, the EVIDEM-ED project benefited from the knowledge of BPSD within the trial of exercise as therapy, and drew its training materials on the MCA 200517 from EVIDEM-MCA. In turn, EVIDEM-ED provided data from interviews with carers about the experience of managing incontinence for EVIDEM-C, and about sources of legal advice provided to family carers from EVIDEM-MCA. Similarly, EVIDEM-EoL, EVIDEM-C and EVIDEM-MCA shared ethics protocols and supporting consent documents.
All projects benefited from iterative face-to-face debate within the EVIDEM programme about recruitment methods. All projects experienced challenges in recruiting people with dementia and their carers to the EVIDEM studies. The process of recruitment from where there was no on-site research presence or input was both time-consuming and resource intensive. The projects noted how recruitment could be experienced as an additional burden by practitioners, how the stigma of the diagnosis impeded recruitment, how professionals acted as gatekeepers to research in some services, and how among some NHS staff there were concerns about the ownership of research.
We also noted the variable appetite of some professionals to improve care for people with dementia. Although there were some enthusiasts among the generalists (especially GPs and community nurses), these individuals were not necessarily typical of their entire professional groups, which, although not averse to improving care, were not necessarily willing to put in any additional effort into a research activity designed to achieve this.
Nevertheless, the EVIDEM projects recruited 704 people with dementia to studies, along with 292 carers; EVIDEM-ED’s audit of general practice included > 1000 people with dementia, and, at the end of the EVIDEM programme, the dementia register held an additional 500 people who were not involved with EVIDEM but who have expressed an interest in being involved in dementia research. Over 500 professionals – ranging from part-time care home staff and volunteer carer support workers to highly trained psychiatrists and dementia care nurses – were interviewed, took part in group discussions or participated in the research in other ways (e.g. in a Delphi process).
Sharing ideas within the EVIDEM programme also prompted new research initiatives within the programme, such as the use of the THIN database,142 a primary care data set, to gather epidemiological evidence about continence in EVIDEM-C.
The EVIDEM programme has run annual ‘summer schools’ with CNWL NHS Foundation Trust staff using the project themes to update practitioners and managers and using different styles of dissemination events and opportunities, some in the workplace and others organised centrally. Large numbers of practitioners have attended other EVIDEM presentations and new media, such as free online journals and professional outlets, webinars, tweets and blogs, have been used as communication tools.
Offspring from the EVIDEM programme
The EVIDEM programme generated further studies, as shown below.
-
The Transitions study on how people with dementia experienced the process of diagnosis (NIHR Service Delivery and Organisation programme funded; project number 08/1809/229). See www.netscc.ac.uk/hsdr/files/project/SDO_FR_08–1809–229_V01.pdf.
-
A person-centred primary care training package, developed and field-tested in Avon and Wiltshire NHS Trust by Sarah Voss and Rachel Edwards (and funded by the Alzheimer’s Society). See Chapter 1 for details of this project or: www.alzheimers.org.uk/site/scripts/documents_info.php?documentID = 1475&pageNumber = 3.
-
The Optimal study (funded by the NIHR Health Services and Delivery Research programme; project number 11/1021/02) on ways of optimising NHS working with care homes.
-
The EVIDEM-MCA team worked with the Alzheimer’s Society in devising data collection instruments, augmentation of interview data and secondary analysis of findings in its study of Financial Abuse in dementia (The ‘Short Changed’ report:287 see www.alzheimers.org.uk/site/scripts/news_article.php?newsID = 1127).
Research capacity building
The EVIDEM programme has created a fruitful postdoctoral research environment allowing the further development of dementia researchers (Samsi, Thune-Boylé, Lowery). Two new researchers have undertaken PhDs within EVIDEM: (Cerga-Pashoja, Cole), and four early-career GPs have acquired research and writing skills within EVIDEM (Koch, Pealing, Davidson, Venavadem) with another experienced social scientist (Charles) participating in EVIDEM-MCA prior to moving to health services research. One exercise therapist (Lee) used his experience of working on EVIDEM-E for his Bachelor of Science dissertation. One postgraduate (Ng) secured her Master of Science in International Health Policy under the direction of Professor Martin Knapp at the London School of Economics based on her economic evaluation of the end-of-life intervention. Embedding the EVIDEM programme in the CNWL NHS Trust has assisted the trust to increase its research activity and focus it more on older adults. The NT DemReg is a powerful tool for recruiting people with dementia and their carers to studies. The EVIDEM-MCA research team has acted as research advisors on projects covering Mental Capacity Advocates, Indirect or Proxy Holding Personal Budget Arrangements, and the Deprivation of Liberty Safeguards, at the Mental Health Foundation and University of Bristol.
Conclusions
The EVIDEM programme is an addition to the small number of longitudinal studies of the dementia trajectory, and leaves behind a mechanism (the register) for recruiting further cohorts. It has shown the benefits of interdisciplinarity in dementia research, and demonstrated the value of mixed methodologies in addressing complex problems. Grounding the EVIDEM programme in an NHS trust allowed the research teams to work with the diversity of the population and the range of providers operating in dementia services.
The longitudinal element of the programme enabled repeat interviews with practitioners and people with dementia and carers, as described above in the review of EVIDEM-MCA. Both EVIDEM-C and EVIDEM-EoL had longitudinal elements in which they interviewed and followed up people with dementia and family carers for up to 3 (EVIDEM-C) and 4 (EVIDEM-EoL) years.
As discussed above, the testing of recruitment from primary and secondary care has been a feature of all projects, with different methods being used in different settings. The EVIDEM-E project, which experienced slow recruitment initially, benefited most from using the research register. In contrast, and to meet the aims of part of its study, EVIDEM-MCA gained the trust of community and voluntary groups of older people to access ‘well’ older people. EVIDEM-EoL developed and tested recruitment within the care home settings, providing learning which contributed to the DeNDRoN ENRICH research initiative (www.DeNDRoN.org.uk/enrich/).
Finally, the extensive involvement of lay experts at the levels of individual projects as well as in the steering group for the whole programme has shaped the development of methods and had a decisive role in the evolution of projects such as EVIDEM-C and EVIDEM-EoL.
Acknowledgements
We would like to thank Tricia Labro who provided support throughout the programme and helped with correspondence, minutes, matters of finance, organising meetings and conferences.
Chapter 1: EVIDEM-ED
A number of people have contributed to this project. We are extremely grateful to all the people with dementia who gave permission for us to include their clinical experience in the study, as well as the carers who gave so freely of their time and shared their views with us.
We are also grateful to the practice staff and primary care professionals, who were involved with us throughout the study, for the feedback, participation, access and continued interest and support.
We thank the North Thames DeNDRoN, particularly Tania Page, who assisted us with recruitment and raising the profile of the study via newsletters. The study received support from North Thames DeNDRoN and the North Central/North East London Comprehensive Local Research Networks with NIHR Flexibility and Sustainability funding (FSF) support for participating primary care practitioners. We are also grateful to the staff at the Clinical Research Networks and PCRNs, and, particularly, Eleanor Harrison, Gina Johnson and Helen Macdonald, who assisted us with recruitment in the east of England.
We are grateful to members of the study advisory group for sharing their views on various aspects, and for sharing their experience, views and guidance.
We would like to thank Kalpa Kharicha who provided maternity cover, and helped with the recruitment and ethical stages of the study.
In addition, others were involved as identified in Table 71.
| Name and role | Study element to which they contributed | |
|---|---|---|
| Steve Iliffe | Professor of Primary Care for Older People Research Department of Primary Care & Population Health University College London |
Chief Investigator |
| Jane Wilcock | Senior Research Fellow Research Department of Primary Care & Population Health University College London |
Trial Manager for EVIDEM-ED, Academic Programme Manager for EVIDEM |
| Priya Jain | Assistant Research Manager Research Department of Primary Care & Population Health University College London |
Assistant Research Manager for EVIDEM-ED, administrator for EVIDEM |
| Mark Griffin | Statistician Research Department of Primary Care & Population Health University College London |
Statistician for EVIDEM-ED and EVIDEM programme, member of EVIDEM-ED advisory group, member of EVIDEM management group |
| Ingela Thuné-Boyle | Senior Research Fellow and Chartered Health Psychologist Research Department of Primary Care & Population Health University College London |
Responsible for recruitment and consent of people with dementia and their carers, undertook carer interviews |
| Tamar Koch | GP Research Department of Primary Care & Population Health University College London |
Undertook two literature reviews, member of the advisory group |
| Frances Lefford | GP Research Department of Primary Care & Population Health University College London |
Facilitated training in practices, member of expert and steering groups |
| Dave Rapp | GP Research Department of Primary Care & Population Health University College London |
Assisted with data collection for primary and secondary outcome data |
EVIDEM-ED publications and presentations
Peer-reviewed (Table 72) and professional (Table 73) papers have been published throughout the study. In addition, some papers are awaiting publication, and others are under review.
| Authors | Title | Journal |
|---|---|---|
| Iliffe S, Manthorpe J, Drennan V, Goodman C, Warner J | The EVIDEM programme: a test for primary care research in London? | London J Prim Care 2008;1:69–73 |
| Iliffe S, Manthorpe J, Warner J, Drennan V, Goodman C, Rait G, et al. | Making progress in psychosocial research in dementia | Dementia 2008;7:167–74 |
| Iliffe S, Jain P, Wong G, Lefford F, Gupta S, Warner A, et al. | Dementia diagnosis in primary care: looking outside the educational box | Aging Health 2009;5:51–9 |
| Manthorpe J, Iliffe S Samsi K, Cole L, Goodman C, Drennan V, et al. | Dementia and dignity: nursing practice and its dilemmas | Int J Older People Nurs 2010;5:235–44 |
| Iliffe S, Wilcock J, Griffin M, Jain P, Thuné-Boyle I, Koch T, et al. | Evidence-based interventions in dementia: a pragmatic cluster-randomised trial of an educational intervention to promote earlier recognition and response to dementia in primary care (EVIDEM-ED) | Trials 2010;11;13 |
| Koch T, Iliffe S, for the EVIDEM-ED research team | Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: a narrative review | BMC Fam Pract 2010;11:52 |
| Koch T, Iliffe S | The role of primary care in the recognition of and response to dementia | J Nutr Health Aging 2010;14:107–9 (editorial) |
| Iliffe S, Pealing L | Subjective memory complaints: a clinical review | BMJ 2010:340:c1425 (commissioned) |
| Koch T, Iliffe S | Dementia diagnosis and management: a narrative review of changing practice | Br J Gen Pract 2011;61:514–15 |
| Iliffe S, Koch T, Jain P Lefford F, Wilcock J, Wong G, et al. | Developing an educational intervention on dementia diagnosis and management in primary care for the EVIDEM-ED trial | Trials 2012;13:142 |
| Thuné-Boyle I, Wilcock J, Iliffe S | Communicating with carers about dementia | Int J Geriatr Psychiatry 2013;28:438–40 |
| Wilcock J, Iliffe S, Griffin M, Jain P, Thuné-Boyle I, Lefford F, et al. | Tailored educational intervention for primary care to improve the management of dementia: the EVIDEM–ED cluster randomised controlled trial | Trials 2013;14:397 |
| Wilcock J, Jain P, Griffin M, Thuné-Boyle I, Lefford F, Rapp D | Diagnosis and management of dementia in family practice | Aging Ment Health; in press 2015 |
| Feldman L, Wilcock J, Thuné-Boyle I, Iliffe S | Carers’ accounts of help-seeking behaviour for people with dementia symptoms: qualitative study | Dementia; in press 2015 (published online: 23 February 2015 doi:10.1080/13607863.2015.1011082) |
| Gilbert C, Wilcock J, Iliffe S | A comparison of service use by people with dementia a decade apart | Dementia; in press 2015 |
| Authors | Title | Journal |
|---|---|---|
| Iliffe S, Jain P, Wilcock, for the EVIDEM-ED team | Recognition of and response to dementia syndrome in primary care 1 | Innovait 2009;2:230–6 |
| Iliffe S, Jain P, Wilcock J, for the EVIDEM-ED team | Recognition of and response to dementia syndrome in primary care 2 | Innovait 2009;2:237–44 |
| Iliffe S | Detecting dementia | Public Serv Rev Health 2009;20:15–16 |
| Iliffe S | Recognising symptom patterns will aid early diagnosis of dementia | Guidelines in Practice 2010;13:29–35 |
| Pealing L, Iliffe S | A woman with forgetfulness and falls: a case study | BMJ 2011;343:d7412 |
The methods and findings of the EVIDEM-ED trial have been presented at 15 national and international conferences, including the UK National Dementia Congress, the Alzheimer’s Disease International Conference, the International Association of Gerontology and Geriatrics, and the Gerontological Society of America.
Chapter 2: EVIDEM-E
We gratefully acknowledge the contribution of all study participants and their carers for their involvement in the trial. The study was supported by a proactive and valuable steering group that played a pivotal role during challenging times. Particular thanks are extended to Dr Ritchie (Chair).
The study drew upon a wide number of recruitment hubs and we would like to acknowledge the importance these hubs played in ensuring that we achieved our target of the largest sample in the world for such a trial. We give particular acknowledgement to North Thames DeNDRoN and the use of DemReg: Dr Nilforooshan (Surrey and Borders Partnership NHS Foundation Trust) and Dr Regan (West London Mental Health NHS Trust).
We gratefully acknowledge the support of several volunteers who kindly provided their time in exchange for experience of working on a research trial. John Aeron-Smith and Stoyanka Dimitrova collected some data and supported data entry. Data entry was also supported by Anne Theobald, Polly Pulford, Dorrie Mystris and Mirela Sula.
Randomisation was conducted by several specialist registrars (Dr Bailey, Dr Jordanova, Dr Rajenthran and Dr Holzer) over the course of the trial.
All authors, along with the steering group contributed to the design of the study. All authors contributed to the drafting and/or critical revision of this chapter for important intellectual content, approval of the final version, and taking public responsibility for its content.
In addition, others were involved as identified in Table 74.
| Name and role | Study element to which they contributed: | |
|---|---|---|
| James Warner | CNWL NHS Foundation Trust and Imperial College London, Consultant Old Age Psychiatrist | Chief Investigator Conceived the idea of the study, provided clinical oversight, and wrote the first draft of this chapter |
| David Lowery | CNWL NHS Foundation Trust, Senior Research Manager | Oversaw the day-to-day management of the trial, led the qualitative component exploring clinician experiences of recruitment to research trials, led the development of the pilot video toolkit, conducted some of the analysis, and contributed to the interpretation of the results |
| Arlinda Cerga-Pashoja | CNWL NHS Foundation Trust, RW | Collected the majority of the data; entered the majority of the data; wrote the first draft of the analysis plan; conducted the majority of the analysis and contributed to the interpretation of the results |
| Ingela Thuné-Boyle | Research Department of Primary Care & Population Health College London, Senior Research Fellow and Chartered Health Psychologist | Led the literature review; collected a substantial proportion of the data; conducted some of the analysis; and contributed to the interpretation of the results |
| James Lee | Independent contractor, Exercise Therapist | Provided the exercise therapy, contributed to the development of the video toolkit, contributed to some of the analysis of data and interpretation of results |
| Alex Bailey | CNWL NHS Foundation Trust, Consultant Old Age Psychiatrist | Was the principal randomisation officer |
| Raul Bhattacharya | East London NHS Foundation Trust. Consultant Psychiatrist | Was involved in the first stages of the study design including protocol writing and at the later stages as Principal Investigator at East London NHS Foundation Trust |
| Mark Griffin | Research Department of Primary Care & Population Health University College London, Statistician | Provided consultancy for setting up trial databases, oversaw the statistical entry and analysis of the main trial, and contributed to the interpretation of the results |
| Francesco D’Amico | LSE, Research Officer | Conducted the analysis of the economic data, contributed to the interpretation of the results |
| Amritpal Patel | Health Economics Researcher London School of Economics | Conducted the analysis of the economic data and contributed to the interpretation of the results |
| Martin Knapp | London School of Economics, Professor of Social Policy | Supervised the analysis of the economic data and contributed to the interpretation of the results |
| Steve Iliffe | Research Department of Primary Care & Population Health University College London, Professor for Primary Care for Older People | Contributed to the interpretation of the results |
EVIDEM-E publications and presentations
Papers have been published (Table 75) and presentations (Table 76) made throughout the study. In addition, there are papers accepted for publication but not yet published, and others under review.
| Authors | Title | Journal |
|---|---|---|
| Lowery D, Warner J | Behavioural and psychological symptoms of dementia (BPSD): the personal and practical costs of dementia | J Integ Care 2009;17:13–19 |
| Lowery D | Exercise and dementia | Alzheimer’s Society Newsletter, Living with Dementia, July 2010 |
| Cerga-Pashoja A, Lowery D, Bhattacharya R, Griffin M, Iliffe S, Lee J, et al. | Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial | Trials 2010;11:53 |
| Lowery D, Warner J, Cerga-Pashoja A, Thuné-Boyle I, Iliffe S | Clinicians as recruiters to dementia trials: lessons from the EVIDEM-E project | Int J Geriatr Psychiatry 2011;26:765–9 |
| Iliffe S, Curry L, Kharicha K, Rait G, Wilcock J, Lowery D, et al. | Developing a Dementia Registry: a descriptive case study from North Thames DeNDRoN and the EVIDEM programme | BMC Med Res Methodol 2011;11:9 |
| Thuné-Boyle, I, Iliffe, S, Cerga-Pashoja A, Lowery D, Warner J | The effect of exercise on behavioral and psychological symptoms in dementia: towards a research agenda | Int Psychogeriatr 2012. URL: http://dx.doi.org/10.1017/S1041610211002365 |
| Lowery D, Cerga-Pashoja A, Iliffe S, Thuné-Boyle I, Griffin M, et al. | The effect of exercise on behavioural and psychological symptoms of dementia and caregiver’s perceived burden: the EVIDEM-E randomised controlled clinical trial | Int J Geriatr Psychiatry 2014;29:819–27 |
| Presenter | Title | Location and date |
|---|---|---|
| Warner J | The use of Anti-Psychotic Medications in People with Dementia | EVIDEM Summer School, London, UK, 2008 |
| Warner J | Exercising your Mind | Enfield over-50s Group, London, UK, 2009 |
| Warner J | Behavioural and psychological symptoms of dementia and their management | EVIDEM Summer School, CNWL NHS Trust, UK, 2009 |
| Lowery D | EVIDEM-E: A randomised controlled evaluation of a tailored exercise package for individuals with dementia and their carers: the impact on sleep, behaviour and quality of life | Dementia Services Development Centre’s 3rd International Conference, York, UK, 2009 |
| Warner J | The motion: people in the terminal stages of dementia are wasting their families’ lives and the resources of the NHS, as opposer | Dementia Care Congress, Harrogate, UK, 2009 |
| Cerga-Pashoja A | Evaluation of exercise on individuals and their carers: a randomised controlled trial | PRIMENT Clinical Trials Unit, University College London, UK, 2010 |
| Lowery D | Challenging behaviour: meaning and (care) management. The National Dementia Strategy Practice Perspectives: one year on | King’s College London, London, 2010 |
| Lowery D | Evidenced based interventions in dementia: opportunities to take part and change practice in dementia care in the community | Hillingdon Carers Information Day, Hillingdon Civic Centre, 2010, UK |
| Cerga-Pashoja A | The importance of behavioural and psychological symptoms of dementia and a possible therapy: an invitation to participate in EVIDEM-E | Hillingdon Admiral Nurse Service Carers Information Day, Hillingdon Civic Centre, UK, 2010 |
| Warner J | Managing Behavioural and Psychological Symptoms of Dementia: training our colleagues to identify causes and consequences | EVIDEM Summer School, CNWL NHS Trust, UK, 2011 |
| Lowery D | Taking a stroll beyond memory lane: Can walking help with symptoms of dementia? | Alzheimer’s Society Memory Lane Café, London, UK, 2011 |
| Lowery D and Lee J | Providing people with dementia and their carers with tools to improve their levels of physical activity | Dementia Care Congress, Liverpool, UK, 2011 |
| Lowery D | Scientists assess whether exercise helps combat dementia | BBC One/Radio 4 Breakfast News, 2011 |
| Lowery D | Physical Activity: A tool for improving outcomes for people with dementia | Chartered Society of Physiotherapy Annual Congress, Liverpool, UK, 2011 |
| Cerga-Pashoja A | Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial | Presentation for conversion to PhD. Research Department of Primary Care & Population Health, University College London, UK, 2011 |
| Cerga-Pashoja A | Methodological challenges of conducting research in the NHS | Society for Academic Primary Care Regional Meeting, Cambridge, UK, 2011 |
| Lowery D | Factors affecting clinician engagement in recruitment for dementia trials | Gerontological Society of America Annual Scientific Meeting, Boston, USA, 2011 |
| Lee J | The role of applied health research: experiences of implementing an intervention within a trial of exercise therapy for symptoms of dementia | University of Brighton, UK, 2011 |
| Thuné-Boyle I | The effect of exercise on behavioural and psychological symptoms in dementia | Alzheimer’s Disease International, London, UK, 2012 |
| Lowery D | Reducing caregiver burden: a curious corollary of asking a caregiver to do more! In ‘The real dementia challenge: using research to change practice for older people with dementia’, symposium | The British Society of Gerontologists, Oxford, UK, Autumn Meeting, 2013 |
| Lowery D | Reducing caregiver burden: a curious corollary of asking a caregiver to do more! | Dementia Care Congress, Nottingham, UK, 2013 |
| Lowery D | EVIDEM Exercise In ‘Evidence-Based Interventions in Dementia (EVIDEM): Research Across the Dementia Trajectory’, symposium | Gerontological Society of America Annual Scientific Meeting, New Orleans, USA, 2013 |
Chapter 3: EVIDEM-C
The authors all contributed and agreed on the final version of this chapter as shown in Table 77.
| Name and role | Study element to which they contributed | |
|---|---|---|
| Vari Drennan | Professor of Health Policy and Service Delivery | Chief Investigator Conceived, designed, organised the overall and individual elements of the study, including data collection, analysis and interpretation, and drafted this report |
| Laura Cole | Research Associate | Made a contribution to the design of individual elements, the organisation of the study, data collection, analysis and interpretation of the study. In addition, helped to draft and critique the output for important intellectual content |
| Sheila Donovan | Research Fellow | Made a contribution to the design of individual elements, the organisation of the study, data collection, analysis and interpretation of the study. In addition, helped to draft and critique the output for important intellectual content |
| Robert Grant | Senior Research Fellow in quantitative methods | Provided statistical analysis and interpretation, and, in addition, helped to draft this output for important intellectual content |
In addition, we would like to thank others who were involved in individual studies as identified in Table 78.
| Name | Role | Study element contributed to |
|---|---|---|
| Francesco D’Amico | Research Officer, PSSRU London School of Economics | Health economics |
| Anna El-Jouzi | Librarian, St George’s University of London | Systematic review |
| Mandy Fader | Professor of Continence Technology, Faculty of Health Sciences, Southampton University | Systematic review and advisory group member |
| Nan Greenwood | Senior Research Fellow, Faculty of Health and Social Care Sciences, St George’s University of London and Kingston University | Systematic review |
| Steve Iliffe | Professor of Primary Care for Older People, Research Department of Primary Care & Population Health, University College London, London | Chief investigator for the programme |
| Martin Knapp | Director and Professor of Social Policy PSSRU, London School of Economics | Health economics |
| Mary Murrell | Former family carer | Member of advisory group |
| Caroline Norrie | Research Associate, Faculty of Health and Social Care Sciences, St George’s University of London and Kingston University | Survey |
| Irene Peterson | Principal Research Associate, Research Department of Primary Care & Population Health, University College London, London | Cohort study |
| Greta Rait | Senior Lecturer in Primary Care, PRIMENT Clinical Trials Unit, Research Department of Primary Care & Population Health, University College London, London | Systematic review, cohort study and advisory group member |
| Amritpal Redhill | Researcher, PSSRU London School of Economics | Health economics |
| Jacqui Woods | Former family carer | Chair of advisory group |
EVIDEM-C publications and presentations
Papers have been published (Table 79) and presentations [oral (Table 80) and poster (Table 81)] made throughout the study. In addition, there are papers accepted for publication but not yet published, and others under review.
| Authors | Title | Journal |
|---|---|---|
| Drennan V, Cole L | Promoting continence and managing incontinence with people with dementia living at home: one more challenge for integration | J Integ Care 2009;17:15–25 |
| Drennan V, Cole L | Dealing with incontinence | Alzheimer’s Society Magazine 2009:14 |
| Drennan V, Cole L | Exploring Issues and solutions in promoting continence with people with dementia living at home | J Clin Nurs 2010;19(Suppl. 1):100 |
| Drennan VM, Cole L, Iliffe S | A taboo within a stigma? A qualitative study of managing continence in people with dementia living at home | BMC Geriatr 2011;11:75 |
| Drennan VM, Norrie C, Cole L, Donovan S | Addressing incontinence for people with dementia living at home: a documentary analysis of local English community nursing service continence policies and clinical guidance | J Clin Nurs 2012;22:339–46 |
| Drennan V, Rait G, Cole L, Grant R, Iliffe S | The prevalence of incontinence in people with cognitive impairment or dementia living at home: a systematic review | Neurol Urodyn 2013;32:314–24 |
| Title | Event | Date | Location |
|---|---|---|---|
| Management of Incontinence in the Community: Workshop | London Centre for Dementia Care: State of Art in Dementia Care Summer School and Stakeholders event | 1 July 2008 | London |
| Workshop on incontinence as part of Dignity and dementia: where do the problems and solutions lie? | Dementia Congress | 28–30 October 2008 | Bournemouth |
| An overview of the EVIDEM programme and the EVIDEM-C study | Academic Primary Care Seminar St George’s University of London | 4 November 2008 | London |
| Exploring issues and solutions in promoting continence and managing incontinence with people with memory problems and dementia living at home | Faculty of Health and Social Care Sciences Seminar, St George’s University of London | 14 May 2009 | London |
| Comparing the views of people with dementia and their carers with health and social care staff on managing toilet difficulties and incontinence | British Society of Gerontology Annual Conference | 2–4 September 2009 | Bristol |
| Exploring issues and solutions in promoting continence and managing incontinence with people with memory loss and cognitive problems living at home and their carers | Dementia Services Development Centre’s International Conference | 14–16 September 2009 | York |
| Exploring issues of toileting problems and continence promotion for people living at home | EVIDEM Summer School, CNWL NHS Foundation Trust | 11 September 2009 | Hillingdon |
| EVIDEM-C: Exploring issues and solutions in promoting continence and managing incontinence with people with memory problems and dementia living at home | Annual Meeting of the North West of England Annual Association of Continence Advisors | 9 December 2009 | Preston |
| Research Support: EVIDEM-C | Association for Continence Advice (ACA) North East Thames Continence Advisors Group | 22 January 2010 | London |
| The Last Taboo? Managing toileting problems and incontinence with people with dementia living in their own homes | Making Research Count in Social Work and Social Care Practice: The National Dementia Strategy. King’s College London | 1 March 2010 | London |
| Exploring issues and solutions in promoting continence and managing incontinence with people with memory loss and cognitive problems living at home and their carers | Royal College of Nursing International Nursing Research Conference | 11–13 May 2010 | Newcastle |
| The last taboo? Managing toileting problems and incontinence with people with dementia living in their own homes | Dementia Care Conference, Hertfordshire Adult Care Services | 22 June 2010 | Hertfordshire |
| Promoting continence and managing incontinence with people with memory problems living at home | Hillingdon Admiral Nurse Service Carers’ Information Day | 8 July 2010 | Hillingdon |
| Exploring issues and solutions in promoting continence with people with dementia living at home | Fourth European Nursing Congress: Older Persons: The Future Of Care | 4–7 July 2010 | Rotterdam |
| Issues in managing continence problems with people with dementia living at home: the EVIDEM-C study | Faculty of Health and Social Care Sciences Seminar, St George’s University of London | 28 October 2010 | London |
| Managing incontinence and dementia at home: a feasibility study of preferences and effectiveness of different types of absorbent products | Royal College of Nursing Continence Conference | 2–3 November 2010 | York |
| Managing incontinence and dementia at home: a feasibility study of preferences and effectiveness of different types of absorbent products | Royal College of Nursing International Nursing Research Conference | 16–18 May 2011 | Harrogate |
| Issues in managing continence problems with people with dementia living at home: the EVIDEM-C study | CNWL NHS Foundation Trust Occupational Therapists Study Day | 1 September 2011 | London |
| Issues in managing continence problems with people with dementia living at home: the EVIDEM-C study | EVIDEM Summer School and Central and North West London NHS Foundation Trust | 7 October 2011 | London |
| Developing a continence assessment tool for use by district nursing services sensitive to the needs of people with dementia | Royal College of Nursing International Research Conference | 22–24 April 2012 | London |
| Title | Event | Date | Location |
|---|---|---|---|
| EVIDEM-C Promoting continence and managing incontinence with people with dementia and their carers living at home: A review of Evidence | DeNDRoN Conference | 14 October 2008 | Newcastle |
| Promoting continence and managing incontinence with people with dementia and their carers living at home: EVIDEM-C | St George’s University of London Research Conference | 3 December 2008 | London |
| Exploring issues and solutions in promoting continence and managing incontinence with people with dementia living at home and their family carers | St George’s University of London Research Conference | 2 December 2009 | London |
| Managing incontinence at home: preferences, effectiveness and ease of use of different types of absorbent products | The Dementia Services Development Centre’s 4th International Conference: Coming of Age | 19–21 October 2010 | London |
| Managing incontinence at home: preferences, effectiveness and ease of use of different types of absorbent products | St George’s University of London Research Day | 30 November 2011 | London |
| Developing a continence assessment tool sensitive to the needs of people with dementia living at home and their family carers | Alzheimer’s Disease International Conference | 13 April 2012 | London |
Chapter 4: EVIDEM-EoL
A large number of people have contributed to this project. We are very grateful to all of the older people in the care homes, and their relatives, who participated in the study. Hosting a research study in a care home is time-consuming and often inconvenient. We are particularly grateful to the care home managers and staff who welcomed us as a research team, facilitated our work and engaged enthusiastically with all the demands of the project.
We are also grateful to the primary care professionals who were involved with us throughout the study. Their participation, and continued interest made Phase 2 possible.
The study received support from North Thames DeNDRoN and Hertfordshire NHS Community Trust with FSF support.
We are grateful to members of the study advisory group (for membership, see Appendix 54) for their commitment over the 4 years of the study. Their experience, robust views, input and guidance were invaluable.
In addition, others were involved as identified in Tables 82 and 83.
| Name and role | Study element they contributed to | |
|---|---|---|
| Claire Goodman | Professor of Health Care Research, CRIPACC, University of Hertfordshire | Chief Investigator. Conceived the idea of the study, provided clinical oversight and wrote the first draft of this chapter |
| Sarah Amador | Research Fellow, CRIPACC, University of Hertfordshire | Made a significant contribution to the organisation, data collection, analysis and interpretation of Phase 2. In addition, helped draft and critique the output for important intellectual content |
| Elspeth Mathie | Research Fellow, CRIPACC, University of Hertfordshire | Made a significant contribution to the development of the AI intervention, the organisation of the study, data collection, analysis and interpretation of Phase 2 |
| Natasha Baron | Research Assistant, Cambridge Centre for Health Services Research, University of Cambridge | Made a significant contribution to the organisation, data collection, analysis and interpretation of Phase 1 |
| Ina Machen | Research Fellow, CRIPACC, University of Hertfordshire | Made a significant contribution to the organisation, data collection, analysis and interpretation of Phase 1 |
| Name and role | Study element to which they contributed | |
|---|---|---|
| Catherine Evans | NIHR Clinical Lecturer in Palliative Care, Cicely Saunders Institute, King’s College London | Review study set-up and care home recruitment, and member of advisory group |
| Carole Parsons | Lecturer in Pharmacy Practice, School of Pharmacy, Queen’s University Belfast | Analysis of sedative load and potentially inappropriate prescribing |
| Yi Ting Ng | Postgraduate student, London School of Economics | Economic analysis |
| Derek King | Research Fellow, PSSRU, London School of Economics | Economic analysis |
| Caroline Nicholson | Postdoctoral Research Fellow, Florence Nightingale School of Nursing and Midwifery, King’s College London | Development and facilitation of AI intervention |
| Liz Stevenson | Research assistant, CRIPACC, University of Hertfordshire | Data collection in care homes |
| David Stott | CLiCIR, University of Hertfordshire | Survival analysis |
EVIDEM-EoL publications and presentations
Papers have been published (Tables 84–87). A Master’s thesis has been submitted (Table 88). In addition, study newsletters (Table 89), presentations (Table 90) and posters (Table 91) have been made throughout the study.
| Authors | Title | Journal |
|---|---|---|
| Evans C, Goodman C | End-of-life care for people with dementia living in a care home | J Integ Care 2008;16:15–25 |
| Iliffe S, Manthorpe J, Drennan V, Goodman C, Warner J | The EVIDEM programme a test for primary care research in London | London J Prim Care 2008;1:69–73 |
| Robinson L, Iliffe S, Brayne C, Goodman C, Rait G, Manthorpe J, et al. | Primary care and dementia: 1. diagnosis, screening and disclosure | Int J Geriatr Psychiatry 2009;24:9895–901 |
| Goodman C, Evans C | Innovation section: changing practice in dementia care for people in care homes towards the end of life | Dementia 2009;8:424–31 |
| Goodman C, Evans C, Wilcock J, Froggatt K, Sampson E, Drennan V, et al. | End-of-life care for community-dwelling older people with dementia: an integrated review | Int J Geriatr Psychiatry 2010;25:329–37 |
| Robinson L, Iliffe S, Brayne C, Goodman C, Rait G, Manthorpe J, et al., for the DeNDRoN Primary Care Clinical Studies Group | Long-term care at home: psychosocial interventions, information provision, carer support and case management | Int J Geriatr Psychiatry 2010;25:657–64 |
| Manthorpe J, Iliffe S, Samsi K, Cole L, Goodman C, Drennan V, et al. | Dementia, dignity and quality of life: nursing practice and its dilemmas | Int J Older People Nurs 2010;5:235–44 |
| Goodman C, Baron NL, Machen I, Stevenson E, Evans C, Davies SL, et al. | Culture, consent, costs and care homes: enabling older people with dementia to participate in research | Aging Ment Health 2011;15:475–81 |
| Parsons C, Haydock J, Mathie E, Baron N, Machen I, Stevenson E, et al. | Sedative load of medications prescribed for older people with dementia in care homes | BMC Geriatr 2011;11:56 |
| Goodman C | The organisational culture of nursing staff providing long-term dementia care is related to quality of care | Evid Based Nurs 2011;14:88–9 |
| Davies SL, Goodman C, Bunn F, Victor C, Dickinson A, Illiffe S, et al. | A systematic review of integrated working between care homes and health-care services | BMC Health Serv Res 2011;11:320 (highly accessed) |
| McMurdo MET, Roberts H, Parker S, Wyatt N, May H, Goodman C, et al. on behalf of the Age and Ageing Specialty Group, NIHR, Comprehensive Clinical Research Network | Improving recruitment of older people to research through good practice | Age Aging 2011;40:659–65 |
| Goodman C, Davies S | ENRICH: an initiative to facilitate dementia research in care homes | Br J Community Nurs 2012;17:277 |
| Parsons C, Johnston S, Mathie E, Baron N, Machen I, Stevenson I, et al. | Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis | Drugs Aging 2012;29:143–55 |
| Siegel EO, Anderson R, Calkin J, Chu C, Corazzini K, Dellefield ME, et al. | Supporting and promoting personhood in long term care settings: contextual factors | Int J Older People Nurs 2012;7:295–302 |
| Goodman C, Amador S, Elmore N, Machen I, Mathie E | Preferences and priorities for end-of-life care: A qualitative study of older people with dementia resident in care homes. | Int J Nurs Studies 2013;50:1639–74 |
| Amador S, Goodman C, King D, Ng YT, Elmore N, Machen I, et al. | Exploring resource use and associated costs in end-of-life care for older people with dementia in residential care homes | Int J Geriatr Psychiatry 2014;29:758–66 |
| Authors | Chapter title | Book title/editors |
|---|---|---|
| Amador S, Goodman C, King D, Machen I, Elmore N, Mathie E, et al. | Emergency ambulance service involvement with residential care homes in the support of older people with dementia: an observational study | BMC Geriatr (under review) |
| Goodman C, Amador S, Elmore N, Machen I, Mathie E | A framework for the evaluation of interventions and future research in end-of-life care of older people with dementia | In preparation |
| Amador S, Goodman C, Elmore N, Mathie E, Machen I | A modified Appreciative Inquiry in care homes: promoting cooperative working for end-of-life care of older people with dementia | In preparation |
| Authors | Chapter title | Book title/editors |
|---|---|---|
| Wilcock J, Frogatt K, Goodman C | End-of-life care for people with dementia | In Downs M, Bowers B, editors. Excellence in dementia care: Research in to practice. Maidenhead: Open University Press; 2008 |
| Goodman C, Davies S, Leyshon S, Fader M, Norton C, Gage H, et al. | Collaborating with Primary Care: promoting shared working between district nurses and care home staff | In Froggatt K, et al., editors. Understanding Care Homes: A Research and Development Perspective. London: Jessica Kingsley Publishers; 2009 |
| Goodman C, Davies SL | Good practice outside the care homes | In Dening T, Milne A, editors. Mental Health in Care Homes. Oxford: Oxford University Press; 2011 |
| Authors | Chapter title | Book title/editors |
|---|---|---|
| Goodman C, Froggatt K, Mathie E | End of life research methods in social care. Research Methods in Social Care series | NIHR School for Social Care Research. 2012. URL: www2.lse.ac.uk/LSEHealthAndSocialCare/pdf/SSCR-Methods-Review_12_web.pdf |
| Authors | Title | |
|---|---|---|
| Ng YT | Resource implications of supporting end-of-life care for older persons with dementia in the care homes in England | Unpublished Master’s Thesis. London: Department of Social Policy, London School of Economics |
| Authors | Title | Newsletter |
|---|---|---|
| Evans C, Goodman C | Spotlight on the End of Life Project | Alzheimer’s Society magazine, 2008. URL: http://alzheimers.org.uk/site/scripts/documents_info.php?documentID = 809&pageNumber = 3 |
| Evans C, Goodman C | Spotlight on the End of Life Project | EVIDEM Newsletter 2:5 |
| Baron N, Goodman C | An update on the End of Life study | EVIDEM Newsletter 5:3–4 |
| Authors | Title | Event |
|---|---|---|
| Goodman C, Wilcock J, Bisset M, Froggatt K, Drennan V, Blanchard M, et al. | Integrative review of end-of-life care for community-dwelling older people with dementia | Royal Society of Medicine, London, 2008 |
| Goodman C, Evans C | Setting the scene: introduction to providing care in NHS care homes | Royal Society of Medicine, London, 2008 |
| Goodman C, Evans C | EVIDEM-EoL: changing practice in dementia care in care homes: developing and testing evidence based interventions at the end of life | Symposium Presentation King’s College London, London, 2008 |
| Evans C, Goodman C, Davies SL | Research in care homes. Bulletin issue 11 | PCRN_GL Masterclass, 2008 |
| Goodman C | Understanding the experience of living and dying in a care home | Presentation to Quantum Homes, Herts, 2008 |
| Goodman C | Research in end-of-life care in care homes | Dementia and end-of-life care study day St Nicholas Hospice, Ipswich, 2008 |
| Iliffe S, Manthorpe J, Goodman C, Drennan V, Warner J | Dignity and dementia: where do the problems and solutions lie? | Dementia Care Congress, Bournemouth, 2008 |
| Goodman C | Supporting people with dementia and their carers at the end of life in care homes and primary care | EVIDEM Summer School, CNWL NHS Foundation Trust, London 2009 |
| Goodman C, Baron N | Living and dying with dementia in care homes: the EVIDEM-End of Life study | The Dementia Service Development Centre 3rd International Conference, York, 2009 |
| Goodman C, Baron N, Stevenson E, Machen I | Culture consent and care home: enabling older people with dementia to participate in research | British Society of Gerontology Annual Conference, Bristol, 2009 |
| Goodman C | Addressing uncertainty: end-of-life care in residential settings | The National Dementia Strategy Practice Perspectives: One Year On, King’s College London, London, 2010 |
| Goodman C | End-of-life care for people with dementia | DeNDRoN, 2010 |
| Goodman C | EVIDEM-End of Life: recognising and supporting end-of-life care for people with dementia living in care homes | Delivering Better Health Services: The Health Services Research Network, Manchester, 2010 |
| Mathie E, Goodman C | Living and dying in residential care homes: views of residents: ‘a good death’ | NHS Luton and Bedfordshire Quality Summit – Care Homes, Flitwick, 2011 |
| Goodman C | The role of care homes in HSC integration | Navigating the Future: Partnership and Integration in a Changing World. Seminar of ADASS and BGS, London, 2011 |
| Goodman C | Dementia research in care homes | Presentation at Dementia Research: Knowledge into Care. NIHR CLAHRC, Cambridge, 2011 |
| Nicholson C | Good gossip: using appreciative inquiry to further end-of-life care in care homes | Presentation at the Margaret Butterworth Care Home Forum, London, 2011 |
| Goodman C | The role of care homes in HSC integration | Workshop session at Navigating the future: Partnership and Integration in a Changing World. Seminar of ADASS and BGS, London, 2011 |
| Goodman C | New research into partnership working with dementia | Presentation at the Hertfordshire Care Providers Association Members Meeting, Welwyn Garden City, 2011 |
| Mathie E, Goodman C, Nicholson C, Amador S | End of life care in residential care homes – an appreciative inquiry | Presentation at the Assets for Health and Wellbeing Across the Life Course International Conference, British Library Conference Centre, London, 2011 |
| Goodman C | Dementia research in care homes | Presentation at Dementia Research: Knowledge into Care, NIHR CLAHRC, Cambridge, 2011 |
| Goodman C, Mathie E, Nicholson C, Amador S | Dealing with uncertainty at the end of life for people with dementia: appreciative inquiry as an intervention to improve working between primary healthcare and care homes | Presentation at the UK Dementia Congress Liverpool, 2011 |
| Goodman C, Mathie E, Nicholson C, Amador S | Using what we do well to improve end of life care for older people with dementia | Presentation at the Hertfordshire County Council Good Practice in Dementia Care Conference, Hatfield, 2011 |
| Mathie E | EVIDEM-End of Life: working with primary healthcare supporting people with dementia living and dying in care homes | Presentation at Working Together: Valuing Each Other. New Approaches to Working Between Care Homes and Primary Care. Hertfordshire AGENET meeting, Hatfield, 2012 |
| Nicholson C | An appreciative inquiry: end of life care in residential care | Presentation at Working Together: Valuing Each Other. New Approaches to Working Between Care Homes and Primary Care Hertfordshire AGENET meeting, Hatfield, 2012 |
| Mathie E | Supporting people with dementia: living and dying in residential care homes | Community Care Conference, London, 2012 |
| Goodman C, Nicholson C, Mathie E, Amador S | End-of-life care for people with dementia: an intervention to promote integrated working between care home staff and healthcare practitioners | Presentation at the Alzheimer’s Disease International Conference London, 2012 |
| Nicholson C, Mathie E, Machen I, Amador S, Goodman C | Working appreciatively in end-of-life care: an intervention to promote collaborative working between care home staff and healthcare practitioners | Presentation at the RCN UK’s International Nursing Research Conference London, 2012 |
| Goodman C | Care homes: working with and working for the NHS | Presentation at the BGS Community Geriatrics Meeting, The Met Hotel, Leeds, 2012 |
| Goodman C | An intervention to promote integrated working between care homes and healthcare practitioners for end-of-life care of older people with dementia | Presentation at Delivering Better Health Services HSRN symposium, Manchester Central, 2012 |
| Goodman C | Maximising quality in residential care | Presentation at the New Dynamics of Ageing/My Home Life workshop, RIBA, London, 2012 |
| Amador S, Mathie E, Nicholson C, Goodman C | End of life care for people with dementia: researching uncertainty | Presentation at Developing a Research Culture, CLCH NHS Trust Annual Conference, Mary Ward House, London, 2012 |
| Amador S, Mathie E, Nicholson C, Goodman C | Participatory research in care homes: promoting collaborative working between care homes and healthcare professionals for end-of-life care of older people with dementia | Presentation in Conducting Healthcare Research with Care Homes: Residents, Researchers and Healthcare Professional’s Perspectives Symposium, BSG Annual Conference, Keele, 2012 |
| Authors | Title | Event |
|---|---|---|
| Goodman C, Evans C | EVIDEM-End of Life (EoL): changing practice in dementia care in care homes – developing and testing interventions towards the end of life | Poster session at the DeNDRoN Annual Conference, Newcastle upon Tyne, 2008 |
| Goodman C, Baron N, Machen I, Stevenson L | EVIDEM-EoL: living and dying with dementia in care homes | Poster session at the DeNDRoN Annual Conference, 2009 |
| Goodman C, Baron N | Ensuring equity of participation for people with dementia: strategies to support inclusionary research | Poster session at the Biennial symposium of ICCHNR, Edmonton, Canada, 2011 |
| Goodman C, Nicholson C | End-of-life care in residential care homes: an Appreciative Inquiry | Poster session at the fourth National Care Homes Congress, Birmingham, 2011 |
| Nicholson C, Goodman C, Mathie E, Amador S, Baron N, Machen I | End of life care for people with dementia: an intervention to promote collaborative working between care home staff and healthcare practitioners | Poster session at the 9th Palliative Care Congress, The Sage Gateshead, Gateshead, UK, 2012. URL: http://spcare.bmj.com/content/2/Suppl_1/A23.1 |
| Nicholson C, Mathie E, Machen I, Amador S, Goodman C | Working appreciatively in end-of-life care: an intervention to promote collaborative working between care home staff and healthcare practitioners | Poster session at the RCN UK, 2012. The 2012 International Nursing Research Conference |
| Name and role | Study element to which they contributed | |
|---|---|---|
| Jill Manthorpe | Professor of Social Work | Chief Investigator. Conceived the idea of the study, conducted interviews, report writer, member of EVIDEM project team, data analysis and dissemination lead; drafted final report |
| Jessica Harris | Research Associate | Assistance with interviewing at initial stage of the study |
| Kritika Samsi | Lecturer | Project researcher and co-investigator. Interviews, analysis and project manager, assisted with drafting of final report and all study outputs |
Chapter 5: EVIDEM-MCA
A number of people have contributed to this project. We are extremely grateful to all of the participants for their time and for sharing their views. We are most grateful to Joan Rapaport, Hazel Heath, Jess Harris, Philip Rapaport, Tayavnie Nagendran, Nigel Charles and Lynn Phair who conducted a number of interviews and assisted with data analysis. We thank Karishma Chandaria for the opportunity to work with the Alzheimer’s Society and for contributing to our linked study of financial abuse among people with dementia. We are grateful to members of the study advisory group and of the Social Care Workforce Research Unit advisory group for sharing their views on various aspects. Thanks are also due to Steve Chamberlain, Maria Grey, Priya Jain and Frances Lefford for their contributions to a consensus group meeting.
The authors all contributed and agreed on the final version of this chapter as shown in Table 92.
EVIDEM-MCA publications and presentations
Papers have been published (Table 93) throughout the study. In addition, there are papers accepted for publication not yet published and under review (Table 94). Please see Table 95 for a list of all of the presentations at seminars, events and conferences.
| Authors | Title | Journal |
|---|---|---|
| Manthorpe J, Rapaport J, Harris J, Samsi K | Realising the safeguarding potential of the Mental Capacity Act 2005: early reports from adult safeguarding staff | J Adult Protect 2009;11:13–24 |
| Manthorpe J, Samsi K | Implementing the Mental Capacity Act 2005: challenges for commissioners | J Integ Care 2009;17:39–47 |
| Manthorpe J, Iliffe S, Samsi K, Cole L, Goodman C, Drennan V, et al. | Dementia, dignity and quality of life: nursing practice and its dilemmas | Int J Older People Nurs 2010;5:235–44 |
| Manthorpe J, Samsi K, Heath H, Charles N | ‘Early days’: Knowledge and use of the Mental Capacity Act 2005 by care home managers and staff | Dementia 2011;10:283–98 |
| Manthorpe J, Samsi K | Improving practice in communication with older people and support networks living in housing with care schemes: aspirations and ambitions | Br J Soc Work 2012;42:1495–512 |
| Samsi K, Manthorpe J, Rapaport P | ‘As people get to know it more’: Experiences and expectations of the Mental Capacity Act 2005 amongst local information, advice and advocacy services | Soc Pol Soc 2011;10:41–54 |
| Samsi K, Manthorpe J | ‘I live for today’: A qualitative study investigating older people’s attitudes to advance planning | Health Soc Care Community 2011;19:52–9 |
| Samsi K, Manthorpe J, Nagendaran T, Heath H | Challenges and expectations of the Mental Capacity Act 2005: The perspectives of community-based specialist nurses working in dementia care | J Clin Nurs 2012;21:1697–705 |
| Phair L, Manthorpe J | The use of the Mental Capacity Act among hospital patients: findings from a case study of one Acute Hospital Trust in England | J Adult Protect 2012;14:259–70 |
| Manthorpe J, Samsi K, Rapaport J | When the profession becomes personal: dementia care practitioners as family caregivers | Int Psychogeriatr 2012;24:902–10 |
| Manthorpe J, Samsi K, Rapaport J | ‘More of a leg to stand on’: Views and usage of the Mental Capacity Act 2005 among local Alzheimer’s Society and Caregiver groups: findings from the EVIDEM MCA project | Aging Ment Health 2012;16:102–9 |
| Manthorpe J, Samsi K | Mental capacity: the force of law | J Dementia Care 2012;20:12–13 |
| Manthorpe J, Samsi K, Rapaport J | Dementia nurses’ experience of the Mental Capacity Act 2005; a follow-up-study | Dementia 2014;13:131–43 |
| Manthorpe J, Samsi K | Inherently risky? Personal budgets for people with dementia and the risks of financial abuse: findings from an interview-based study with Adult Safeguarding Coordinators | Br J Soc Work 2013;43:889–903 |
| Manthorpe J, Samsi K, Rapaport J | Responding to the financial abuse of people with dementia: a qualitative study of safeguarding experiences in England | Int Psychogeriatr 2012;1–11. doi:10.1017/S1041610212000348 |
| Manthorpe J, Samsi K | Mental Capacity and dementia: A review, Part 1 | J Dementia Care 2012;20:35–8 |
| Manthorpe J, Samsi K | Making Decisions in Dementia Care: Has the Mental Capacity Act Helped Social Work Practice in England and Wales? | Community Care, 28 November 2012. URL: www.communitycare.co.uk/articles/28/11/2012/118730/making-decisions-in-dementia-care-has-the-mental-capacity-act-helped-social-work-practice-in-england-and-wales.htm) |
| Manthorpe J, Samsi K | Mental Capacity and dementia: A review, Part 2 | J Dementia Care 2013;21:35–8 |
| Manthorpe J, Samsi K, Rapaport J | ‘Capacity is the key’: Investigating new legal provisions in England and Wales for adult safeguarding | J Elder Abuse Negl 2013;25:355–73 |
| Samsi K, Manthorpe J | Everyday decision-making amongst people with dementia and carers: findings from a longitudinal interview study of people with dementia and family carers | Int Psychogeriatr 2013;25:949–61 |
| Manthorpe J, Samsi K | Changing practice: adapting to the Mental Capacity Act 2005 | Soc Care Neurodisabil 2013;4:124–33 |
| Manthorpe J, Iliffe S, Goodman C, Drennan V, Warner J | Working together in dementia research: reflections on the EVIDEM programme | Working with Older People 2013;17:138–45 |
| Samsi K, Manthorpe J, Chandaria K | Risks of financial abuse of older people with dementia: findings from a survey of UK voluntary sector dementia community services staff | Journal Adult Protect 2014;16:3 |
| Manthorpe J, Samsi K | Care professionals’ understanding of the new criminal offences created by the Mental Capacity Act 2005 | Int J Geriatr Psychiatry 2014; doi:10.1002/gps.4147 |
| Authors | Title | Journal |
|---|---|---|
| Manthorpe J, Samsi K | Care homes and the Mental Capacity Act 2005: changes in understanding and practice over time | Dementia [being revised] |
| Samsi K | Negotiating capacity and consent in substance misuse | In Crome I, Wu L-T, Rao R, Crome P, editors. Substance Use and Older People. Chichester, West Sussex: John Wiley & Sons Inc.; 2014 |
| Manthorpe J, Samsi K | Briefing note on MCA offences for care homes | National Care Home Forum (in press 2015) |
| Author | Title | Event |
|---|---|---|
| Manthorpe J, Samsi K | Early views of local Alzheimer’s Society staff regarding the Mental Capacity Act 2005 | Dementia Congress, Harrogate, November, 2009 |
| Manthorpe J, Samsi K | ‘Can you help me think about my future?’: Exploring the role of information and advice organisations | Dementia Services Development Centre Annual Conference, York, July, 2009 |
| Samsi K, Manthorpe J, Heath H | Early findings from interviews with care home staff | Care Home Congress, Birmingham, July, 2009 |
| Manthorpe J, Samsi K. | Putting the Mental Capacity Act into practice in care homes: emerging findings and research challenges | National Care Home Research and Development Forum, London, April, 2009 |
| Samsi K, Manthorpe J | Making plans for the unknown: transitions in cognitive impairment Alzheimer’s disease and other dementias | Annual Gerontological Society of America Conference, Atlanta, GA, November, 2009 |
| Manthorpe J, Seminar Series | Money management and the Mental Capacity Act | Centre for Policy on Ageing, London, 2010 |
| Samsi K, Manthorpe J | Planning for retirement and later life: ‘Hope nothing is going to happen and we carry on as long as we can’ | Annual Conference of BSG, London, 5 July, 2010 |
| Samsi K, Manthorpe J | ‘It helps us to give people something concrete that they can work with’: early views of local Alzheimer’s Society staff or the Mental Capacity Act 2005 | North East London NHS Foundation Trust Older People’s Conference, July 2010 |
| Samsi K, Manthorpe J | Futurescopes: older people’s attitudes and knowledge of making plans | Annual Conference of Dementia Services and Development Centre, London, May 2010 |
| Manthorpe J, Samsi K | Developmental steps in building a toolkit to challenge wilful neglect and ill-treatment under the Mental Capacity Act | Dementia Congress, Liverpool, 10 November 2011 (poster) |
| Samsi K, Manthorpe J | Wilful neglect and ill-treatment under the Mental Capacity Act 2005: identifying thresholds and safeguarding pathways | Annual Conference of International Psychogeriatrics Association, Den Haag, Netherlands, 5 September 2011 |
| Samsi K, Manthorpe J | Use and views of the Mental Capacity Act 2005 | Annual Conference of British Society of Gerontology, Plymouth, 10 July 2011 |
| Manthorpe J, Samsi K | Developing a toolkit to understand the offences of wilful neglect and ill-treatment under the Mental Capacity Act | Annual Conference of Gerontological Society of America, Boston, November 2011 |
| Manthorpe J, Samsi K, Chandaria K | Safeguarding people with dementia from financial abuse | Alzheimer’s Disease International Conference, London, 7–9 March 2012 (poster) |
| Manthorpe J, Samsi K | When psychosocial interventions go wrong | INTERDEM meeting, Alzheimer’s Disease International Conference, London, 7 March 2012 |
| Samsi K | Championing dementia | Addressing equalities in older people’s social care, A Joint Conference of Making Research Count, Age UK London and the Social Care Workforce Research Unit, 26 January 2012 |
| Manthorpe J | Lessons from England on mental capacity working | International conference on Squalor and Neglect, Sydney, Australia, 22 February 2012 |
| Manthorpe J | Making decisions in dementia care: how new law helped practice in England and messages for social work practice in other jurisdictions | European Social Work Research Conference, Basle, 23 March 2012 |
| Manthorpe J | Elder abuse and mental capacity (pod broadcast and audience) | Age UK staff group seminar, London, 11 April 2012 |
| Manthorpe J | Care and Control: Mental Health, Mental Capacity and Social Care Futures | Manchester Metropolitan University Social Work Conference, 26 April 2012 |
| Manthorpe J | Interventions in elder abuse | International Elder Abuse Day, National Centre for the study of Elder Abuse, Dublin, 24 June 2012 |
| Manthorpe J, Samsi K | Crime against residents with dementia | Margaret Butterworth Care Home Forum, London, 10 July 2012 |
| Manthorpe J, Samsi K | Everyday independent and joint decision-making by people with dementia and carers | British Society of Gerontology Annual Conference, University of Keele, Stoke, 12 July 2012 |
| Manthorpe J, Samsi K | Preventing financial abuse among people with dementia | British Society of Gerontology Annual Conference, University of Keele, Stoke, 11 July 2012 |
| Manthorpe J, Samsi K | Everyday decision-making among people with dementia and carers | Gerontological Society of America’s 65th Annual Scientific Meeting, San Diego, 14 November 2012 |
| Manthorpe J, Samsi K | Planning in advance for long-term care needs | 2nd International Conference on Evidence-based Policy in Long-term Care, London, September 2012 |
| Manthorpe J, Samsi K, Chandaria K | Who should protect people with dementia from financial abuse? | Alzheimer Europe, Vienna, 6 October 2012 (poster) |
| Samsi K, Manthorpe J | ‘How do carers decide for their relatives with dementia?’ | Alzheimer Europe, Vienna, 6 October 2012 |
| Manthorpe J, Samsi K, Chandaria K | ‘Protecting people with dementia from financial abuse’ (poster) | 7th UK Dementia Congress, Brighton, 30 October to 1 November, 2012 |
| Samsi K, Manthorpe J | Everyday decision-making among people with dementia and carers | Gerontological Society of America’s 65th Annual Scientific Meeting, San Diego, CA, 14 November 2012 |
| Manthorpe J, Samsi K | Use and views of the Mental Capacity Act 2005 | Evidence Based Interventions in Dementia: Findings, London, 16 April 2013 |
| Manthorpe J | Leaning about Lasting Powers of Attorney | Making Research Count, London, 22 September 2013 |
| Manthorpe J, Samsi K | Understanding the new criminal offences created by the Mental Capacity Act 2005 | Annual Conference of the British Society of Gerontology, University of Oxford, 11 September 2013 |
| Manthorpe J, Samsi K | The mistreatment and neglect of people with dementia | Alzheimer Europe, Malta, 12 October 2013 |
| Manthorpe J, Samsi K | From professional to the personal dementia care practitioners as family carers | INTERDEM/Alzheimer Europe, Malta, 10 October 2013 |
| Manthorpe J, Samsi K | Making decisions in dementia care | Gerontological Society of America, New Orleans, 26 November 2013 |
| Manthorpe J | The relevance of the MCA for audiologists in practice | University College London Audiology Masterclass, London, 5 December 2013 |
Chapter 6: EVIDEM: from cohort to research register
The authors all contributed and agreed on the final version of this chapter as shown in Table 96.
| Name and role | Study element to which they contributed | |
|---|---|---|
| Steve Iliffe | Research Department of Primary Care & Population Health, University College London | Project development, management committee member |
| Lisa Curry | West London Mental Health Trust | Cohort manager |
| Jane Wilcock | Research Department of Primary Care & Population Health, University College London | Project development, field research and management committee member |
| Greta Rait | Research Department of Primary Care & Population Health, University College London | Project development, management committee member |
| David Lowery | CNWL NHS Foundation Trust | Management committee member |
| Kalpa Kharicha | Research Department of Primary Care & Population Health, University College London | Management committee member |
| Archana Tapuria | Centre for Health Informatics and Multiprofessional Education, University College London, London | Information technology development |
| Dipak Kalra | Centre for Health Informatics and Multiprofessional Education, University College London, London Information Technology Development | |
| Craig Ritchie | West London Mental Health Trust | Project leader |
EVIDEM: from cohort to research register – publications and presentations
The following peer-reviewed papers have been published (Table 97).
| Authors | Title | Journal |
|---|---|---|
| Iliffe S, Curry L, Kharicha K, Rait G, Wilcock J, Lowery D, et al. | Developing a Dementia Research Registry: a descriptive case study from North Thames DeNDRoN and the EVIDEM programme | BMC Med Res Methodol 2011;11:9 |
Additional publications on behalf of the EVIDEM group: publications and presentations
The following peer-reviewed papers have been published (Table 98).
| Authors | Title | Journal |
|---|---|---|
| Iliffe S, Manthorpe J, Warner J, Drennan V, Goodman C, Rait G, et al. | Making progress in psychosocial research in dementia | Dementia 2008;7:167–74 |
| Iliffe S, Manthorpe J, Drennan V, Goodman C, Warner J | The EVIDEM programme: a test for primary care research in London? | London J Prim Care 2008;1:69–73 |
| Manthorpe J, Iliffe S | The Mental Health of Older People: Taking a Long View | J Integr Care 2008;16:4–13 |
| Manthorpe J | Mental Health in Later Life: Better Outcomes through Wise Commissioning | J Integr Care 2009;17:15–22 |
| Manthorpe J, Iliffe S, Rait G, Goodman C, Drennan V, Warner J | Keeping on Track | Sign post 2009;14:26–30 |
| Manthorpe J, Iliffe S, Samsi K, Cole L, Goodman C, Drennan V, et al. | Dementia, dignity and quality of life: nursing practice and its dilemmas | Int J Older People Nurs 2010;5:235–44 |
| Manthorpe J, Iliffe S, Goodman C, Drennan V, Warner J | Working together in dementia research: reflections on the EVIDEM programme | Working With Older People 2013;17:1–8 |
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Global Burden of Dementia in the Year 2000. Geneva: WHO; 2003.
- Dementia UK: The Full Report. London: Alzheimer’s Society; 2007.
- Pucci E, Angelerie F, Borsetti G, Brizoli E, Cartechini E, Giuliani G, et al. General practitioners facing dementia: are they fully prepared?. Neurol Sci 2004;24:384-9. http://dx.doi.org/10.1007/s10072-003-0193-0.
- Vernooij-Dassen M, Moniz-Cook E, Woods R, De Lepeleire J, Leuschner A, Zanetti O, et al. Factors affecting the timely recognition and diagnosis of dementia in primary care across eight European states: a modified focus group study. Int J Geriatr Psych 2005;20:1-10. http://dx.doi.org/10.1002/gps.1302.
- Tesco, Alzheimer’s Society and Alzheimer (Scotland) . Mapping the Dementia Gap 2013. www.alzheimers.org.uk/dementiamap (accessed December 2013).
- Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. US Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2003;138:927-37. http://dx.doi.org/10.7326/0003-4819-138-11-200306030-00015.
- Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, et al. Primary care & dementia: 1 Diagnosis, screening and disclosure. Int J Geriatr Psych 2009;24:895-901. http://dx.doi.org/10.1002/gps.2204.
- Jha A, Tabet N, Orrell M. ‘To tell or not to tell – comparison of older patients’ reaction to their diagnosis of dementia and depression’. Int J Geriatr Psych 2001;16:879-85. http://dx.doi.org/10.1002/gps.412.
- Sullivan K, O’Conor F. Should a diagnosis of dementia be disclosed?. Ageing Ment Health 2001;5:340-8. http://dx.doi.org/10.1080/13607860120080297.
- Bamford C, Lamont S, Eccles M, Robinson L, May C, Bond J. Disclosing a diagnosis of dementia: a systematic review. Int J Geriatr Psych 2004;19:151-69. http://dx.doi.org/10.1002/gps.1050.
- Pratt R, Wilkinson H. A psychosocial model of understanding the experience of receiving a diagnosis of dementia. Dementia 2003;2:181-99. http://dx.doi.org/10.1177/1471301203002002004.
- Iliffe S, Manthorpe J, Eden A. Sooner or later? Issues in the early diagnosis of dementia in general practice: a qualitative study. Fam Pract 2003;20:376-81. http://dx.doi.org/10.1093/fampra/cmg407.
- Rait G, Walters K, Bottomley C, Petersen I, Iliffe S, Nazareth I. Survival of people with a clinical diagnosis of dementia in primary care. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3584.
- Stoppe G, Knoblauch A, Haak S, Maeck L. Physicians competence regarding the early diagnosis of dementia: differences between family physicians and primary care neuropsychiatrists in Germany. Psychiatr Prax 2007;34:134-8. http://dx.doi.org/10.1055/s-2006-951855.
- Hinton L, Franz C, Reddy G, Flores Y, Kravitz R, Barker J. Practice constraints, behavioural problems and dementia care: primary care physicians perspectives. J Gen Intern Med 2007;22:1625-7. http://dx.doi.org/10.1007/s11606-007-0317-y.
- Iliffe S, Jain P, Wong G, Lefford F, Gupta S, Warner A, et al. Dementia diagnosis in primary care: looking outside the educational box. Ageing Health 2009;5:51-9. http://dx.doi.org/10.2217/1745509X.5.1.51.
- Mental Capacity Act. London: The Stationery Office; 2005.
- National Dementia Strategy. London: The Stationery Office; 2009.
- Turner S, Iliffe S, Downs M, Wilcock J, Bryans M, Levin E, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing 2004;33:461-7. http://dx.doi.org/10.1093/ageing/afh140.
- Koch T, Iliffe S. EVIDEM-ED research team . Rapid appraisal of barriers to the diagnosis and management of patients with dementia in primary care: a narrative review. BMC Fam Pract 2010;11:52-9. http://dx.doi.org/10.1186/1471-2296-11-52.
- Downs M, Turner S, Bryans M, Wilcock J, Keady J, Levin E, et al. Effectiveness of educational interventions in improving detection and management of dementia in primary care: a cluster randomised controlled study. BMJ 2006;332:692-5. http://dx.doi.org/10.1136/bmj.332.7543.692.
- Waldorff F, Almind G, Makela M, Moller S, Waldemar G. Implementation of a clinical dementia guideline: a controlled study on the effect of a multifaceted strategy. Scand J Prim Health Care 2003;21:142-7. http://dx.doi.org/10.1080/02813430310005136.
- Rondeau V, Allain H, Bakchine S, Bonet P, Brudon F, Chauplannaz G, et al. General practice-based intervention for suspecting and detecting dementia in France. Dementia 2008;7:433-50. http://dx.doi.org/10.1177/1471301208096628.
- Chodosh J, Berry E, Lee M, Connor K, DeMonte R, Ganiats T, et al. Effect of a dementia care management intervention on primary care provider knowledge, attitudes, and perceptions of quality of care. J Am Geriatr Soc 2006;54:311-17. http://dx.doi.org/10.1111/j.1532-5415.2005.00564.x.
- Vickrey B, Mittman B, Connor K, Pearson ML, Della Penna RD, Ganiats TG, et al. The effect of a disease management intervention on quality and outcomes of dementia care. Ann Intern Med 2006;145:713-26. http://dx.doi.org/10.7326/0003-4819-145-10-200611210-00004.
- Wenger N, Roth C, Shekelle P, Young RT, Solomon DH, Kamberg CJ, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc 2009;57:547-55. http://dx.doi.org/10.1111/j.1532-5415.2008.02128.x.
- Vollmar H, Mayer H, Ostermann T, Butzlaff ME, Sandars JE, Wilm S, et al. Knowledge transfer for the management of dementia: a cluster-randomised trial of blended learning in general practice. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-1.
- Perry M, Draskovic I, van Achterberg T, Borm GF, van Eijken MI, Lucassen P, et al. Can an EASYcare based dementia training programme improve diagnostic assessment and management of dementia by general practitioners and primary care nurses? The design of a randomised controlled trial. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-71.
- Callahan C, Boustani M, Unverzagt F, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer Disease in primary care: a randomised controlled trial. JAMA 2006;295:2148-57. http://dx.doi.org/10.1001/jama.295.18.2148.
- Fortinsky R, Kulldorff M, Kleppinger A, Kenyon-Pesce L. Dementia care consultation for family caregivers: collaborative model linking an Alzheimer’s Association chapter with primary care physicians. Ageing Ment Health 2009;13:162-70. http://dx.doi.org/10.1080/13607860902746160.
- Clark P, Bass D, Looman W, McCarthy CA, Eckert S. Outcomes for patients with dementia from the Cleveland Alzheimer’s managed care demonstration. Ageing Ment Health 2004;8:40-51. http://dx.doi.org/10.1080/13607860310001613329.
- Koch T, Iliffe S. Dementia diagnosis and management: a narrative review of changing practice. Brit J Gen Pract 2011;61:514-15. http://dx.doi.org/10.3399/bjgp11X588493.
- Moher D, Liberati A, Tetzlaff J, Altman G. PRISMA group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:332-6. http://dx.doi.org/10.1136/bmj.b2535.
- Moseley A, Herbert R, Sherrington C, Maher C. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother 2002;48:43-9. http://dx.doi.org/10.1016/S0004-9514(14)60281-6.
- Bhogal S, Teasell R, Foley N, Speechley M. The PEDro scale provides a more comprehensive measure of methodological quality that the Jadad Scale in stroke rehabilitation literature. J Clin Epidemiol 2005;58:668-73. http://dx.doi.org/10.1016/j.jclinepi.2005.01.002.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: NICE/SCIE; 2006.
- Kaulio M. Customer, consumer and user involvement in product development: a framework and a review of selected methods. Total Qual Manag Bus 1998;9:141-9. http://dx.doi.org/10.1080/0954412989333.
- Wyatt J, Spiegelhalter D. Evaluating medical expert systems: what to test and how?. Med Inform 1990;15:205-17. http://dx.doi.org/10.3109/14639239009025268.
- Iliffe S, Koch T, Jain P, Lefford F, Wong G, Warner A, et al. Developing an educational intervention on dementia diagnosis and management in primary care for the EVIDEM-ED trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-142.
- Iliffe S, Wilcock J, Austin T, Walters K, Rait G, Turner S, et al. Dementia diagnosis and management in primary care: developing and testing educational models. Dementia 2002;1:11-23. http://dx.doi.org/10.1177/147130120200100111.
- Wilcock J, Iliffe S, Walters K, Rait G, Austin T, Turner S, et al. The development of an evidence-based curriculum for dementia care training in general practice. Educat Ageing 2002;17:217-36.
- Dockery G, De Koning K, Martin M. Participatory Research in Health: Issues and Experiences. London: Zed books; 1996.
- Van der Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972;3:337-42. http://dx.doi.org/10.2105/AJPH.62.3.337.
- Carney O, McIntosh J, Worth A. The use of the nominal group technique in research with community nurses. J Adv Nurs 1996;23:1024-9. http://dx.doi.org/10.1046/j.1365-2648.1996.09623.x.
- Iliffe S, Wilcock J, Griffin M, Jain P, Thuné-Boyle I, Koch T, et al. Evidence-based interventions in dementia: a pragmatic cluster-randomised trial of an educational intervention to promote earlier recognition and response to dementia in primary care (EVIDEM-ED). Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-13.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PCJ. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- Roberts C. The implications of variation in outcome between health professionals for the design and analysis of randomised controlled trials. Statist Med 1999;18:2605-15. http://dx.doi.org/10.1002/(SICI)1097-0258(19991015)18:19<2605::AID-SIM237>3.0.CO;2-N.
- Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol 2004;57:785-94. http://dx.doi.org/10.1016/j.jclinepi.2003.12.013.
- Research Randomizer, A Free Online Tool Available for Researchers n.d. www.randomizer.org (accessed June 2013).
- Data Protection Act. London: HMSO; 1998.
- British Department for Communities and Local Government . The Indices of Deprivation 2010 (ID 2010) 2011. www.communities.gov.uk/publications/corporate/statistics/indices2010?view = Standard (accessed December 2013).
- Folstein M, Folstein SE, McHugh P. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Barrett JJ, Haley WE, Harrell LE, Powers RE. Knowledge about Alzheimer’s disease among primary care physicians, psychologists, nurses, and social workers. Adv Biosci 1997;11:99-106.
- Dieckmann L, Zarit S, Zarit J, Gatz M. The Alzheimer’s Disease Knowledge test. Gerontologist 1988;28:402-7. http://dx.doi.org/10.1093/geront/28.3.402.
- Taylor RS, Mears R, Reeves B, Ewings E, Binns S, Keast J, et al. Developing and validating a questionnaire to evaluate the effectiveness of evidence-based practice teaching. Med Educ 2001;35:544-7. http://dx.doi.org/10.1046/j.1365-2923.2001.00916.x.
- A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: MRC; 2000.
- Rubinstein L, Pugh J. Strategies for promoting organisational practice change by advancing implementation research. J Gen Intern Med 2006;21:S58-64. http://dx.doi.org/10.1007/s11606-006-0276-8.
- Davis D, Mazmanian P, Fordis M, van Harrison R, Thorpe K, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence. JAMA 2006;296:1094-102. http://dx.doi.org/10.1001/jama.296.9.1094.
- Dementia: Supporting People with Dementia and their Carers. London: NICE; 2006.
- Downs M, Turner S, Bryans M, Wilcock J, Keady J, Levin E, et al. Family carers’ accounts of primary care services for their relatives with early signs of dementia. Dementia 2006;5:353-73. http://dx.doi.org/10.1177/1471301206067111.
- Edwards R, Voss S, Iliffe S. Education about dementia in primary care: is patient centredness the key?. Dementia 2014;13:111-19. http://dx.doi.org/10.1177/1471301212451381.
- Cummings JL, Mega M, Grey K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. http://dx.doi.org/10.1212/WNL.44.12.2308.
- Finkel S, Burns A. Behavioural and Psychological Symptoms of Dementia (BPSD): a clinical and research update. IPA Bull 1999;16:3-6.
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift für Psychiatrie Und Psychisch-Gerichtliche Medizin 1907;64:146-8.
- Aalten P, DeVugt ME, Lousberg R, Korten E, Jaspers N, Senden B, et al. Behavioural symptoms in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn 2003;15:99-105. http://dx.doi.org/10.1159/000067972.
- Colerick EJ, George LK. Predictors of institutionalisation among caregivers of patients with Alzheimer’s disease. J Am Geriatr Soc 1986;34:492-8.
- Finkel SI, Costa e Silva J, Cohen G, Miller S, Sartorius N. Behavioural and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr 1997;8:497-500. http://dx.doi.org/10.1017/S1041610297003943.
- Rabins PV, Mace NL, Lucas MJ. The impact of dementia on the family. JAMA 1982;248:333-5. http://dx.doi.org/10.1001/jama.1982.03330030039022.
- Cohen-Mansfield J. Assessment of disruptive behaviour/agitation in the elderly: function, methods and difficulties. J Geriatr Psych Neurol 1995;8:52-60.
- Ballard C, Cream J. Drugs used to relieve behavioural symptoms in people with dementia or an unacceptable chemical cosh?. Int Psychogeriatr 2005;17:4-12. http://dx.doi.org/10.1017/S1041610205221026.
- Kales H, Velenstein M, Kim HM, McCarthy JF, Ganoczy D, Cunningham F, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatr 2007;164:1568-76. http://dx.doi.org/10.1176/appi.ajp.2007.06101710.
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, et al. The dementia antipsychotic withdrawal trial (DART-AD): long term follow-up of a randomised placebo-controlled trial. Lancet 2009;8:151-7. http://dx.doi.org/10.1016/S1474-4422(08)70295-3.
- Devanand DP, Lawlor BA. Treatment of Behavioural and Psychological Symptoms of Dementia. London: Martin Dunitz; 2000.
- Mather AS. Effect of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: a randomised control trial. Br J Psychiatry 2002;180:411-15. http://dx.doi.org/10.1192/bjp.180.5.411.
- Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer’s disease. Ageing Ment Health 2008;12:72-80. http://dx.doi.org/10.1080/13607860701529932.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004;85:1694-704. http://dx.doi.org/10.1016/j.apmr.2004.03.019.
- Dixon-Woods M, Cavers D, Agarwal S, Annandale E, Arthur A, Harvey J, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-35.
- Thuné-Boyle ICV, Iliffe S, Cerga-Pashoja A, Lowery D, Warner J. The effect of exercise on behavioural and psychological symptoms of dementia: towards a research agenda. Int Psychogeriatr 2012;24:1046-57. http://dx.doi.org/10.1017/S1041610211002365.
- Forbes D, Thiessen EJ, Blake CM, Forbes SC, Forbes S. Exercise Programs for People with Dementia. Cochrane Database Syst Rev 2013;12. http://summaries.cochrane.org/CD006489/DEMENTIA_exercise-programs-for-people-with-dementia#sthash.McOH30MA.dpuf.
- The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva, Switzerland: World Health Organization; 1993.
- Nandy S, Parsons S, Cryer C, Underwood M, Rashbrook E, Carter Y, et al. Development and preliminary examination of the predictive validity of the Falls Risk Assessment Tool (FRAT) for use in primary care. J Public Health 2004;26:138-43. http://dx.doi.org/10.1093/pubmed/fdh132.
- Studenski S, Duncan PW, Chandler J, Samsa G, Prescott B, Hogue C, et al. Predicting falls: the role of mobility and nonphysical factors. J Am Geriatr Soc 1994;43:297-302.
- Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
- Lowery DP, Warner J, Cerga-Pashoja A, Thuné-Boyle I, Iliffe S. Clinicians as recruiters to dementia trials: lessons from the EVIDEM-E project. Int J Geriatr Psychiatry 2011;26:767-9. http://dx.doi.org/10.1002/gps.2671.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. London: Oxford University Press; 1972.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9100.
- Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist 2001;41:652-7. http://dx.doi.org/10.1093/geront/41.5.652.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Curtis L. Unit Costs of Health and Social Care 2011 n.d. www.pssru.ac.uk/project-pages/unit-costs/2011/index.php (accessed June 2012).
- British National Formulary. London: BMJ Group Pharmaceutical Press; 2009.
- HM Treasury . The Green Book: Appraisal and Evaluation in Central Government 2003. www.gov.uk/government/uploads/system/uploads/attachment_data/file/220541/green_book_complete.pdf (accessed 29 November 2013).
- Zellner A. An efficient method of estimating seemingly unrelated regression equations and tests for aggregation bias. J Am Stat Assoc 1962;57:348-68. http://dx.doi.org/10.1080/01621459.1962.10480664.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Schreiner AS, Morimoto T, Arai Y, Zarit S. Assessing family caregiver’s mental health using a statistically derived cut-off score for the Zarit Burden Interview. Ageing Ment Health 2006;10:107-11. http://dx.doi.org/10.1080/13607860500312142.
- Howard R, Phillips P, Johnson T, O’Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int J Geriatr Psychiatry 2011;26:812-17. http://dx.doi.org/10.1002/gps.2607.
- Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry 1998;13:248-56. http://dx.doi.org/10.1002/(SICI)1099-1166(199804)13:4<248::AID-GPS770>3.0.CO;2-0.
- Schur D, Whitlatch CJ. Circumstances leading to placement: a difficult caregiving decision. Lippincotts Case Manag 2003;8:187-95. http://dx.doi.org/10.1097/00129234-200309000-00002.
- Lindsay J, Anderson L. Dementia/Alzheimer’s disease. BMC Women’s Health 2004;4. http://dx.doi.org/10.1186/1472-6874-4-S1-S20.
- Rendell JM, Merritt RD, Geddes JR. Incentives and disincentives to participation by clinicians in randomised controlled trials. Cochrane Database Syst Rev 2007;2.
- Iliffe S, Curry L, Kharicha K, Rait G, Wilcock J, Lowery D, et al. Developing a Dementia Research Registry: a descriptive case study from North Thames DeNDRoN and the EVIDEM programme. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-9.
- Craig P, Dieppe P, MacIntyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Living Well with Dementia. London: DH; 2009.
- National Dementia Strategy 2010–2013. Edinburgh: Scottish Executive; 2010.
- National Framework for Action on Dementia 2006–2010. Sydney, New South Wales: Department of Health on behalf of AHMC; 2006.
- European Parliament . European Initiative on Alzheimer’s Disease and Other Dementias 2011. www.europarl.europa.eu/oeil/FindByProcnum.do?lang = en&procnum = INI/2010/2084 (accessed July 2012).
- Congress of United States of America . National Alzheimer’s Project Act S. 3036 2011. www.kintera.org/site/pp.asp?c = mmKXLbP8E&b = 5829219 (accessed July 2012).
- Nurock S, Ames D, Burns A, O’Brien J. Dementia. London: Hodder Arnold; 2010.
- Black BS, Rabin PV, Ames D, Burns A, O’Brien J. Dementia. London: Hodder Arnold; 2010.
- Georges J, Jansen S, Jackson J, Meyrieux A, Sadowska A, Selmes M. Alzheimer’s disease in real life: the dementia carer’s survey. Int J Geriatr Psychiatry 2008;23:546-51. http://dx.doi.org/10.1002/gps.1984.
- Hope T, Keene J, Gedling K. Predictors of institutionalisation for people with dementia living at home with a carer. Int J Geriatr Psychiatry 1998;13:682-90. http://dx.doi.org/10.1002/(SICI)1099-1166(1998100)13:10<682::AID-GPS847>3.0.CO;2-Y.
- Luppa M, Luck T, Braehler E, Koenig HH, Riedel-Heller SG. Prediction of institutionalisation in dementia: a systematic review. Dement Geriatr Cogn Disord 2008;26:65-78. http://dx.doi.org/10.1159/000144027.
- Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, . APA Work Group on Alzheimer’s Disease and other Dementias . American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. 2nd edn. Am J Psychiatry 2007;164:5-56.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems n.d. www.who.int/classifications/apps/icd/icd10online/ (accessed August 2012).
- Stokes G. Incontinent or not? Do not label: describe and assess. J Dement Care 1995;3:20-1.
- Kitwood TM. Dementia Reconsidered: The Person Comes First. Buckingham: Open University Press; 1997.
- Milsom I, Altman D, Lapitan MC, Nelson R, Sillén U, Thom D, et al. Incontinence. 2009.
- DuBeau CE, Kuchel GA, Johnson T, Palmer MH, Wagg A. Fourth International Consultation on Incontinence: incontinence in the frail elderly: report from the 4th International Consultation on Incontinence. Neurourol Urodyn 2010;29:165-78. http://dx.doi.org/10.1002/nau.20842.
- Wimo A, Winblad B, Jönsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer’s Dement 2007;3:81-9. http://dx.doi.org/10.1016/j.jalz.2007.02.001.
- Knapp M, Prince M. Dementia UK: Report to the Alzheimer’s Society. London: King’s College London and London School of Economics and Political Science; 2007.
- Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, et al. Incontinence. 4th International Consultation on Incontinence; 2009.
- Scottish Intercollegiate Guideline Network . Management of Urinary Incontinence in Primary Care 2004. www.sign.ac.uk/pdf/sign79.pdf (accessed October 2007).
- National Institute of Clinical Excellence . Urinary Incontinence: The Management of Urinary Incontinence in Women 2006. www.nice.org.uk/nicemedia/pdf/CG40NICEguideline.pdf (accessed December 2007).
- Evidence-Based Guideline for the Primary Care Management of Dementia. Newcastle upon Tyne: Centre for Health Services Research; 1998.
- Dementia. A NICE-SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care. London: The British Psychological Society and the Royal College of Psychiatrists; 2007.
- Robson C. Real Word Research: A Resource for Social Scientists and Practitioner-Researcher. Oxford: Blackwell Publishers; 2002.
- Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 2005;331:1064-5. http://dx.doi.org/10.1136/bmj.38636.593461.68.
- Higgins JT, Green S. Cochrane Handbook for Systematic Reviews of Interventions n.d. www.cochrane-handbook.org/ (accessed August 2012).
- Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can 1998;19:170-6.
- Chung JC. Care needs assessment of older Chinese individuals with dementia of Hong Kong. Ageing Ment Health 2006;10:631-7. http://dx.doi.org/10.1080/13607860600650532.
- Landi F, Cesari M, Russo A, Onder G, Lattanzio F, Bernabei R, et al. Potentially reversible risk factors and urinary incontinence in frail older people living in the community. Age Ageing 2003;32:194-9. http://dx.doi.org/10.1093/ageing/32.2.194.
- Meaney AM, Croke M, Kirby M. Needs assessment in dementia. Int J Geriatr Psychiatry 2005;20:322-9. http://dx.doi.org/10.1002/gps.1284.
- Mohide EA, Pringle DM, Robertson D, Chambers LW. Prevalence of urinary incontinence in patients receiving home care services. CMAJ 1988;139:953-6.
- Nakanishi N, Tatara K, Naramura H, Fujiwara H, Takashima Y, Fukuda H. Urinary and faecal incontinence in a community-residing older population in Japan. J Am Geriatr Soc 1997;45:215-19.
- Ouslander JG, Zarit SH, Orr NK, Muira SA. Incontinence among elderly community-dwelling dementia patients. Characteristics, management, and impact on caregivers. J Am Geriatr Soc 1990;38:440-5.
- Rait G, Fletcher A, Smeeth L, Brayne C, Stirling S, Nunes M. Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age Ageing 2005;34:242-8. http://dx.doi.org/10.1093/ageing/afi039.
- Teri L, Borson S, Kiyak HA, Yamagishi M. Behavioural disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in Alzheimer’s disease. J Am Geriatr Soc 1989;37:109-16.
- Drennan VM, Rait G, Cole L, Grant R, Iliffe S. The prevalence of incontinence in people with cognitive impairment or dementia living at home: a systematic review. Neurourol Urodynam 2013;32:314-24. http://dx.doi.org/10.1002/nau.22333.
- McWalter G, Toner H, McWalter A, Eastwood J, Marshall M, Turvey T. A community needs assessment: the care needs assessment pack for dementia (CareNap-D) its development, reliability and validity. Int J Geriatr Psychiatry 1998;13:16-22. http://dx.doi.org/10.1002/(SICI)1099-1166(199801)13:1<16::AID-GPS721>3.0.CO;2-N.
- Landi F, Tua E, Onder G, Carrara B, Sgadari A, Rinaldi C, et al. The minimum dataset for home care: a valid instrument to assess frail older people living in community. Med Care 2000;38:1184-90. http://dx.doi.org/10.1097/00005650-200012000-00005.
- Martin J, Metzer H, Ellliot D. The Prevalence of Disability Among Adults: OPCS Surveys of Disability in Great Britain, Report 1. London: HMSO; 1988.
- The 10/66 Dementia Research Group . Methodological issues in population-based research into dementia in developing countries. A position paper from the 10/66 Dementia Research Group. Int J Geriatr Psychiatry 2000;15:21-30. http://dx.doi.org/10.1002/(SICI)1099-1166(200001)15:1<21::AID-GPS71>3.0.CO;2-5.
- The Health Improvement Network n.d. www.thin-uk.com/ (accessed August 2012).
- Grant RL, Drennan VM, Rait G, Pettersen L, Iliffe S. Incidence and management of incontinence in older people with and without dementia: a cohort study using a primary care database. PLOS Med 2013;10. http://dx.doi.org/10.1371/journal.pmed.1001505.
- Teunissen D, van den Bosch W, van Weel C, Lagro-Janssen T. Urinary incontinence in the elderly: attitudes and experiences of general practitioners. A focus group study. Scand J Prim Health 2006;24:56-61. http://dx.doi.org/10.1080/02813430500417920.
- Wagg A, Potter J, Peel P, Irwin P, Lowe D, Pearson M. National audit of continence care for older people: management of urinary incontinence. Age Ageing 2008;37:39-44. http://dx.doi.org/10.1093/ageing/afm163.
- Landi F, Cesari M, Onder G, Zamboni V, Barillaro C, Lattanzio F, et al. Indwelling urethral catheter and mortality in frail elderly women living in community. Neurourol Urodynam 2004;23:697-701. http://dx.doi.org/10.1002/nau.20059.
- Pronovost PJ, Bo-Linn GW. Preventing patient harms through systems of care. JAMA 2012;308:769-70. http://dx.doi.org/10.1001/jama.2012.9537.
- Gitlin LN, Corcoran MA. Expanding caregiver ability to use environmental solutions for problems of bathing and incontinence in the elderly with dementia. Technol Disabil 1993;2:12-21.
- Jirovec MM, Templin T. Predicting success using individualised scheduled toileting for memory-impaired elders at home. Res Nurs Health 2001;24:1-8. http://dx.doi.org/10.1002/1098-240X(200102)24:1<1::AID-NUR1001>3.0.CO;2-R.
- Engberg S, Sereika SM, McDowell BJ, Weber E, Brodak I. Effectiveness of prompted voiding in treating urinary incontinence in cognitively impaired homebound older adults. J Wound Ostomy Continence Nurs 2002;29:252-65.
- Drennan VM, Greenwood N, Cole L, Rait G, Grant R, Iliffe S. A systemic review of conservative interventions for addressing incontinence in people with dementia living at home. BMC Geriatr 2012;12. http://dx.doi.org/10.1186/1471-2318-12-77.
- Hagglund D. A systematic literature review of incontinence care for persons with dementia: the research evidence. J Clin Nurs 2010;19:303-12. http://dx.doi.org/10.1111/j.1365-2702.2009.02958.x.
- Bravo C. Urinary and faecal incontinence in dementia. Rev Clin Gerontol 2004;14:129-36. http://dx.doi.org/10.1017/S0959259804001388.
- Yap P, Tan D. Urinary incontinence in dementia: a practical approach. Aust Fam Physician 2006;4:237-41.
- Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1154-66. http://dx.doi.org/10.1212/WNL.56.9.1154.
- Urinary Incontinence in Neurological Disease. Manchester: NICE; 2012.
- First Assessment: A Review of District Nursing Services in England and Wales. London: National Audit Office; 1999.
- The Healthcare Quality Improvement Partnership. London: Royal College of Physicians; 2010.
- Silverman D. Interpreting Qualitative Data: Methods of Analysing Talk, Text, and Interaction. London: Sage; 2006.
- Fermor R. Directory of Community Health Services. Teddington: Keyways Publishing; 2009.
- Adams T, Clarke CL. Dementia Care: Developing Partnerships in Practice. Toronto: Baillière Tindall; 1999.
- Drennan V, Norrie C, Cole L, Donovan S. Addressing incontinence for people with dementia living at home: a documentary analysis of local English community nursing service continence policies and clinical guidance. J Clin Nurs 2013;22:339-46. http://dx.doi.org/10.1111/j.1365-2702.2012.04125.x.
- Crotty M. The Foundations of Social Research: Meaning and Perspective in the Research Process. London: Sage; 1998.
- Wilkinson H. The Perspectives of People with Dementia: Research Methods and Motivations. London: Jessica Kingsley; 2002.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. London: Sage Publications; 1998.
- Horrocks S, Somerset M, Stoddart H, Peters TJ. What prevents older people from seeking treatment for urinary incontinence? A qualitative exploration of barriers to the use of community continence services. Fam Pract 2004;21:689-96. http://dx.doi.org/10.1093/fampra/cmh622.
- Douglas M. Purity and Danger an Analysis of Concepts of Pollution and Taboo. London: Routledge and Keegan; 1966.
- Seale C. The Quality of Qualitative Research. London: Sage; 1999.
- Drennan VM, Cole L, Iliffe S. A taboo within a stigma? A qualitative study of managing incontinence with people with dementia living at home. BMC Geriatr 2011;11. http://dx.doi.org/10.1186/1471–2318–11–75.
- Burgio KL, Ives DG, Locher JL, Arena VC, Kuller LH. Treatment seeking for urinary incontinence in older adults. J Am Geriatr Soc 1994;42:208-12.
- Morgan DL. Focus Groups as Qualitative Research. Thousand Oaks, CA: Sage Publications; 1997.
- Resnick B, Keilman LJ, Calabrese B, Parmelee P, Lawhorne L, Pailet J, et al. Nursing staff beliefs and expectations about continence care in nursing homes. J Wound Ostomy Continence Nurs 2006;33:610-18. http://dx.doi.org/10.1097/00152192-200611000-00004.
- Xie J, Brayne C, Matthews F. Medical Research Council Cognitive Function and Ageing Study collaborators. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 2008;336:258-62. http://dx.doi.org/10.1136/bmj.39433.616678.25.
- Tashakkori A, Teddlie C. Handbook of Mixed Methods in Social and Behavioural Research. Thousand Oaks, CA: Sage Publications; 2003.
- Murray SA, Shiekh A. Serial interviews for patients with progressive diseases. Lancet 2006;368:9001-2. http://dx.doi.org/10.1016/S0140-6736(06)69350-1.
- Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther 1999;53:471-81. http://dx.doi.org/10.5014/ajot.53.5.471.
- Avery K, Donovan J, Peters T, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodynam 2004;23:322-30. http://dx.doi.org/10.1002/nau.20041.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998;46:210-15.
- Office for National Statistics . Annual Hours and Earnings Survey 2011 n.d. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2011 (accessed June 2012).
- Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al. Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product designs. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12290.
- Mullen PM. Delphi: myths and reality. J Health Organ Manag 2003;17:37-52. http://dx.doi.org/10.1108/14777260310469319.
- Drennan VM, Donovan S. Developing and Testing an Additional Continence Assessment Tool for People With Dementia Living at Home: A Modified Delphi Study 2013.
- Drennan VM, Raymond M. Medical care quality improvement in dementia workbook. 2015.
- Drennan VM, Greenwood N, Cole L. Continence care for people with dementia at home. Nurs Times 2014;110.
- Stokes G. Incontinence and Inappropriate Urinating. Bicester: Winslow Press; 1986.
- Bird M, McNess G. Ameliorating Incontinence in Older People with Cognitive Impairment: Eight Case Studies. Australia: Southern Area Health Authority; 2006.
- Stokes G. Challenging Behaviour in Dementias. Milton Keynes: Speechmark Editions; 2003.
- Isaacs B. The Challenge of Geriatric Medicine. London: Oxford University Press; 1992.
- Cox S, Cook A, Hockley J, Clark D. Palliative Care for Older People in Care Homes. Buckingham: Open University Press; 2002.
- Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA 2003;289:2387-92. http://dx.doi.org/10.1001/jama.289.18.2387.
- NHS End of Life Care Programme in Collaboration with the National Council of Palliative Care. London: Department of Health; 2007.
- Darzi A. Our NHS, Our Future. London: The Stationery Office; 2007.
- Mularski RA, Dy SM, Shugarman LR, Wilkinson AM, Lynn J, Shekelle PG, et al. A systematic review of measures of end-of-life care and its outcomes. Health Serv Res 2007;42:1848-70. http://dx.doi.org/10.1111/j.1475-6773.2007.00721.x.
- Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, Mularski RA, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med 2008;148:147-59. http://dx.doi.org/10.7326/0003-4819-148-2-200801150-00010.
- Hughes JC, Jolley D, Jordan A, Sampson EL. Palliative care in dementia: issues and evidence. Adv Psychiatr Treat 2007;13:251-60. http://dx.doi.org/10.1192/apt.bp.106.003442.
- Lloyd-Williams M, Payne S. Can multidisciplinary guidelines improve the palliation of symptoms in the terminal phase of dementia?. Int J Palliat Nurs 2002;8. http://dx.doi.org/10.12968/ijpn.2002.8.8.10680.
- Volicer L, Hurley AC. Management of behavioural symptoms in progressive degenerative dementias. J Gerontol A Biol Sci Med Sci 2003;58:M837-45. http://dx.doi.org/10.1093/gerona/58.9.M837.
- Bayer A. Death with dementia: the need for better care. Age Ageing 2006;35:101-2. http://dx.doi.org/10.1093/ageing/afj033.
- Goodman C, Evans C, Wilcock J, Froggatt K, Drennan V, Sampson E, et al. End of life care for community dwelling older people with dementia: an integrated review. Int J Geriatr Psychiatry 2010;25:329-37. http://dx.doi.org/10.1002/gps.2343.
- van der Steen JT, Albers G, Licht-Strunk E, Muller MT, Ribbe MW. A validated risk score to estimate mortality risk in patients with dementia and pneumonia: barriers to clinical impact. Int Psychogeriatr 2011;23:31-43. http://dx.doi.org/10.1017/S1041610210001079.
- Reilly S, Venables D, Hughes J, Challis D, Abendstern M. Standards of care in day hospitals and day centres: a comparison of services for older people with dementia. Int J Geriatr Psychiatry 2006;21:460-8. http://dx.doi.org/10.1002/gps.1495.
- Venables D, Reilly S, Challis D, Hughes J, Abendstern M. Standards of care in home care services: a comparison of generic and specialist services for older people with dementia. Ageing Ment Health 2006;10:187-94. http://dx.doi.org/10.1080/13607860500310518.
- Goodman C, Froggatt K, Mathie E. End of Life Care. London: NIHR SSCR; 2011.
- Challis D, Mozley CG, Sutcliffe C, Bagley H, Price L, Burns A, et al. Dependency in older people recently admitted to care homes. Age Ageing 2000;29:255-60. http://dx.doi.org/10.1093/ageing/29.3.255.
- Goodman C, Woolley R, Knight D. District nurse involvement in providing palliative care to older people in residential care homes. Int J Palliat Nurs 2003;9:521-7. http://dx.doi.org/10.12968/ijpn.2003.9.12.11987.
- Goodman C, Woolley R, Knight D. District nurses’ experiences of providing care in residential care home settings. J Clin Nurs 2003;12:67-76. http://dx.doi.org/10.1046/j.1365-2702.2003.00678.x.
- Ashton S, McClelland B, Roe B, Mazhindu D, Gandy R. An end-of-life care initiative for people with dementia. Eur J Palliat Care 2009;16:240-3.
- End of Life Care Strategy – Promoting High Quality Care for All Adults at the End of Life. London: DH; 2008.
- Goodman C, Baron NL, Machen I, Stevenson E, Evans C, Davies SL, et al. Culture, consent, costs and care homes: enabling older people with dementia to participate in research. Ageing Ment Health 2011;15:475-81. http://dx.doi.org/10.1080/13607863.2010.543659.
- Small N, Froggatt K, Downs M. Living and Dying with Dementia: Dialogues About Palliative Care. Oxford: Oxford University Press; 2007.
- Inquiry into the Quality of Healthcare Support for Older People in Care Homes: A Call for Leadership, Partnership and Quality Improvement. London: British Geriatrics Society; 2011.
- The National Gold Standards Framework 2006. www.goldstandardsframework.org.uk/ (accessed December 2014).
- National End of Life Care Programme 2007. http://webarchive.nationalarchives.gov.uk/20130718121128/http:/endoflifecare.nhs.uk (accessed December 2014).
- Marie Curie Palliative Care Institute Liverpool 1997. www.mcpcil.org.uk/liverpool-care-pathway/ (accessed December 2004).
- Badger F, Thomas K, Clifford C. Raising standards for elderly people dying in care homes. Eur J Palliat Care 2007;14:238-41.
- Roe B, McClelland RS, Ashton SE, Mazhindu D, Gandy R. An Evaluation to Determine the Impacts of Introducing the Gold Standards Framework (GSF) and Liverpool Care Pathway (LCP) to Staff, Patients/residents, Family Caregivers and Practitioners Involved in the Care of Older People with Dementia in Greater Manchester Care Settings. Liverpool: John Moores University; 2008.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Disabil Rehabil 1988;10:61-3. http://dx.doi.org/10.3109/09638288809164103.
- Cohen-Mansfield J. Agitated behaviors in the elderly: II. Preliminary results in the cognitively deteriorated. J Am Geriatr Soc 1986;34:722-7.
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry 1988;23:271-84. http://dx.doi.org/10.1016/0006-3223(88)90038-8.
- Parsons C, Haydock J, Mathie E, Baron N, Machen I, Stevenson E, et al. Sedative load of medications prescribed for older people with dementia in care homes. BMC Geriatr 2011;11. http://dx.doi.org/10.1186/1471-2318-11-56.
- Parsons C, Johnston S, Mathie E, Baron N, Machen I, Stevenson E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging 2012;29:143-55. http://dx.doi.org/10.2165/11598560-000000000-00000.
- Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU, The University of Kent; 2010.
- British National Formulary. London: Joint formulary. Committee; 2010.
- Cooperrider DL, Srivastva S. Appreciative inquiry in organizational life. Res Organ Change Dev 1987;1:129-69.
- Cooperrider DL, Whitney DK. Collaborating for Change: Appreciative Inquiry. San Francisco, CA: Berrett-Koehler Communications; 1999.
- Nicholson C, Goodman C, Mathie E, Amador S, Baron N, Machen I. End of life care for people with dementia: an intervention to promote collaborative working between care home staff and healthcare practitioners. BMJ Support Palliat Care 2012;2. http://dx.doi.org/10.1136/bmjspcare-2012-000196.66.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72. http://dx.doi.org/10.1192/bjp.140.6.566.
- Goodman C, Amador S, Elmore N, Machen I, Mathie E. Preferences and priorities for ongoing and end-of-life care: a qualitative study of older people with dementia resident in care homes. Int J Nurs Stud 2013;50:1639-47. http://dx.doi.org/10.1016/j.ijnurstu.2013.06.008.
- Amador S, Goodman C, King D, Machen I, Elmore N, Mathie E, et al. Emergency ambulance service involvement with residential care homes in the support of older people with dementia: an observational study. BMC Geriatr 2014;14. http://dx.doi.org/10.1186/1471-2318-14-95.
- Bardsley M, Georghiou T, Dixon J. Social Care and Hospital Use at the End of Life. London: The Nuffield Trust; 2010.
- Seymour JE, French J, Richardson E. Dying Matters Coalition . Dying matters: lets talk about it. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4860.
- Higginson IJ, Astin P, Imperial SD. Where do cancer patients die? Ten-year trends in the place of death of cancer patients in England. Palliat Med 1998;12:353-63. http://dx.doi.org/10.1191/026921698672530176.
- Amador S, Goodman C, King D, Ng YT, Elmore N, Mathie E, et al. Exploring resource use and associated costs in end-of-life care for older people with dementia in residential care homes. Int J Geriatr Psychiatry 2014;29:758-66. http://dx.doi.org/10.1002/gps.4061.
- Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med 2009;361:1529-38. http://dx.doi.org/10.1056/NEJMoa0902234.
- Froggatt K, Hockley J, Parker D, Brazil K. A system lifeworld perspective on dying in long term care settings for older people: contested states in contested places. Health Place 2011;17:263-8. http://dx.doi.org/10.1016/j.healthplace.2010.11.001.
- Fisher M, Ridley S. Uncertainty in end-of-life care and shared decision making. Crit Care Resusc 2012;14:81-7.
- Hockley J, Watson J, Oxenham D, Murray SA. The integrated implementation of two end-of-life care tools in nursing care homes in the UK: an in-depth evaluation. Palliat Med 2010;24:828-38. http://dx.doi.org/10.1177/0269216310373162.
- Hewison A, Badger F, Clifford C, Thomas K. Delivering ‘Gold Standards’ in end-of-life care in care homes: a question of teamwork?. J Clin Nurs 2009;18:1756-65. http://dx.doi.org/10.1111/j.1365-2702.2008.02613.x.
- Badger F, Plumridge G, Hewison A, Shaw KL, Thomas K, Clifford C. An evaluation of the impact of the Gold Standards Framework on collaboration in end-of-life care in nursing homes. A qualitative and quantitative evaluation. Int J Nurs Stud 2012;49:586-95. http://dx.doi.org/10.1016/j.ijnurstu.2011.10.021.
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008;17:1144-63. http://dx.doi.org/10.1111/j.1365-2702.2007.02220.x.
- Small N. Living well until you die. Ann N Y Acad Sci 2007;1114:194-203. http://dx.doi.org/10.1196/annals.1396.019.
- Mathie E, Goodman C, Crang C, Froggatt K, Iliffe S, Manthorpe J, et al. An uncertain future: the unchanging views of care home residents about living and dying. Palliat Med 2012;26:734-43. http://dx.doi.org/10.1177/0269216311412233.
- Davies S, Goodman C, Bunn F, Victor C, Dickinson A, Iliffe S, et al. A systematic review of integrated working between care homes and health care services. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-320.
- Shah SM, Carey IM, Harris T, DeWilde S, Hubbard R, Lewis S, et al. Identifying the clinical characteristics of older people living in care homes using a novel approach in a primary care database. Age Ageing 2010;39:617-23. http://dx.doi.org/10.1093/ageing/afq086.
- Seymour J, Payne S, Chapman A, Holloway M. Hospice or home? Expectations of end-of-life care among white and Chinese older people in the UK. Sociol Health Illn 2007;29:872-90. http://dx.doi.org/10.1111/j.1467-9566.2007.01045.x.
- Kellehear A, Young B, Monroe B, Olivière D. Resilience in Palliative Care: Achievements in Adversity. Oxford: Oxford University Press; 2007.
- Office of Public Sector Information . The Mental Capacity Act 2005 n.d. www.legislation.gov.uk/ukpga/2005/9/contents (accessed December 2014).
- Ministry of Justice . MCA Code of Practice 2007. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/socialcare/deliveringadultsocialcare/mentalcapacity/mentalcapacityactdeprivationoflibertysafeguards/index.htm (accessed December 2014).
- Dawson C, McDonald A. Assessing mental capacity: a checklist for social workers. Practice 2000;12:5-20. http://dx.doi.org/10.1080/09503150008415180.
- Bradley A. The new independent mental capacity advocate service. Working Older People 2007;11:13-6. http://dx.doi.org/10.1108/13663666200700004.
- Bellhouse J, Holland A, Clare I, Gunn M, Watson PC. Capacity-based mental health legislation and its impact on clinical practice: 1. Admission to hospital. J Ment Health Law 2003;9:9-23.
- Alzheimer’s Society . The Right to Decide 2007.
- Schiff R, Sacares P, Snook J, Rajkumar C, Bulpitt CJ. Living wills and the Mental Capacity Act: a postal questionnaire survey of UK geriatricians. Age Ageing 2006;35:116-21. http://dx.doi.org/10.1093/ageing/afj035.
- Mental Capacity and the Mental Capacity Act 2005: A Literature Review. London: Mental Health Foundation; 2012.
- Johns R. Who decides now? Protecting and empowering vulnerable adults who lose the capacity to make decisions for themselves. Brit J Soc Work 2007;37:557-64. http://dx.doi.org/10.1093/bjsw/bcm015.
- White SM, Baldwin TJ. The Mental Capacity Act 2005: implications for anaesthesia and critical care. Anaesthesia 2006;61:381-9. http://dx.doi.org/10.1111/j.1365-2044.2006.04533.x.
- Samsi K, Manthorpe J, Rapaport P. ‘As people get to know it more’: experiences and expectations of the Mental Capacity Act 2005 amongst local information, advice and advocacy services. Soc Policy Soc 2011;10:41-54. http://dx.doi.org/10.1017/S1474746410000370.
- Hotopf M. The assessment of mental capacity. Clin Med 2005;5:580-4. http://dx.doi.org/10.7861/clinmedicine.5-6-580.
- Myron R, Gillespie S, Swit P, Williamson T. Whose Decision? Preparation for and Implementation of the Mental Capacity Act in Statutory and Non-Statutory Services in England and Wales. London: Mental Health Foundation; 2008.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. London: Routledge; 1993.
- Pope C, Ziebland S, Mays N. Qualitative research in healthcare: analysing qualitative data. BMJ 2000;320:114-16. http://dx.doi.org/10.1136/bmj.320.7227.114.
- Manthorpe J, Samsi K, Rapaport J. ‘More of a leg to stand on’: views and usage of the Mental Capacity Act 2005 among local Alzheimer’s Society & Caregiver groups: findings from the EviDEM MCA project. Ageing Ment Health 2012;16:102-9. http://dx.doi.org/10.1080/13607863.2011.628971.
- Manthorpe J, Rapaport J, Harris J, Samsi K. Realising the safeguarding potential of the Mental Capacity Act 2005: Early reports from adult safeguarding staff. J Adult Protect 2009;11:13-24. http://dx.doi.org/10.1108/14668203200900010.
- Manthorpe J, Samsi K, Rapaport J. ‘Capacity is the key’: investigating new legal provisions in England and Wales for adult safeguarding. J Elder Abuse Negl 2013;25:355-73. http://dx.doi.org/10.1080/08946566.2013.770313.
- Manthorpe J, Samsi K, Rapaport J. Responding to the financial abuse of people with dementia: a qualitative study of safeguarding experiences in England. Int Psychogeriatr 2012;24:1454-64. http://dx.doi.org/10.1017/S1041610212000348.
- Manthorpe J, Samsi K, Rapaport J. ‘Inherently risky?’ Personal budgets for people with dementia and the risks of financial abuse: findings from an interview-based study with Adult Safeguarding Coordinators. Brit J Soc Work 2012:1-15.
- Manthorpe J, Samsi K, Heath H, Charles N. ‘Early days’: knowledge and use of the Mental Capacity Act 2005 by care home managers and staff. Dementia 2011;10:283-98. http://dx.doi.org/10.1177/1471301211403970.
- Samsi K, Manthorpe J, Nagendran T, Heath H. Challenges and expectations of the Mental Capacity Act 2005: an interview-based study of community-based specialist nurses working in dementia care. J Clin Nurs n.d.;21:1697-705. http://dx.doi.org/10.1111/j.1365-2702.2011.03912.x.
- Manthorpe J, Samsi K, Rapaport J. Dementia nurses’ experience of the Mental Capacity Act 2005; a follow-up-study. Dementia 2014;13:131-43. http://dx.doi.org/10.1177/1471301212454354.
- Samsi K, Manthorpe J, Chandaria K. Risks of financial abuse of older people with dementia: findings from a survey of UK voluntary sector dementia community services staff. J Adult Protect 2014;16:180-92. http://dx.doi.org/10.1108/JAP-04-2013-0018.
- Samsi K, Manthorpe J. ‘I live for today’: a qualitative study investigating older people’s attitudes to advance planning. Health Soc Care Community 2011;19:52-9. http://dx.doi.org/10.1111/j.1365-2524.2010.00948.x.
- Human Rights Act 1998. London: The Stationery Office; 1998.
- Phair L, Manthorpe J. The use of the Mental Capacity Act among hospital patients: findings from a case study of one Acute Hospital Trust in England. J Adult Protect 2012;14:259-70. http://dx.doi.org/10.1108/14668201211286020.
- Samsi K, Manthorpe J. ‘Mental capacity and dementia: a review. Part 2’. J Dement Care 2013;21:35-8.
- Manthorpe J, Samsi K. ‘Mental capacity and dementia: a review. Part 1’. J Dement Care 2012;20:35-8.
- Baxter K, Glendinning C, Clarke S. Making informed choices in social care: the importance of accessible information. Health Soc Care Community 2008;16:197-20. http://dx.doi.org/10.1111/j.1365-2524.2007.00742.x.
- Purves B, Perry J, O’Connor D, Purves B. Decision-Making, Personhood and Dementia. London: Jessica Kingsley; 2009.
- Penhale B, Perkins N, Pinkney L, Reid D, Hussein S, Manthorpe J. Partnership and Regulation in Adult Protection: the Effectiveness of Multi-Agency Working and the Regulatory Framework in Adult Protection. Sheffield: University of Sheffield, School of Nursing and Midwifery; 2007.
- Hussein S, Stevens M, Manthorpe J. International Social Care Workers in England: Profile, Motivations, Experiences and Future Expectations. London: Social Care Workforce Research Unit, King’s College London; 2010.
- Stanley N, Lyons C, Manthorpe J, Rapaport J, Rapaport P, Carrahar M, et al. Mental Capacity Act 2005: Mental Health Training Set. London: Department of Health; 2007.
- Caring For our Future: Reforming Care and Support. London: The Stationery Office; 2012.
- Manthorpe J, Samsi K. Mental capacity: the force of law. J Dement Care 2012;20:12-3.
- Manthorpe J, Samsi K. Care professionals’ understanding of the new criminal offences created by the Mental Capacity Act 2005 [published online ahead of print 30 May 2014]. Int J Geriatr Psychiatry 2014. http://dx.doi.org/10.1002/gps.4147.
- Samsi K, Manthorpe J. Decision-Making and Communication in Housing with Care. York: Joseph Rowntree Foundation; 2010.
- Manthorpe J, Samsi K. Improving practice in communication with older people and support networks living in housing with care schemes: aspirations and ambitions. Brit J Soc Work 2012;42:1495-512.
- Short Changed – Protecting People with Dementia from Financial Abuse. London: Alzheimer’s Society; 2011.
- Manthorpe J, Samsi K, Campbell S, Abley C, Keady J, Bond J, et al. The Transition from Cognitive Impairment to Dementia: Older People’s Experiences. Southampton: NIHR Service Development and Organisation programme; 2011.
- Robinson L, Gemski A, Abley C, Bond J, Keady J, Campbell S, et al. The transition to dementia: individual and family experiences of receiving a diagnosis: a review. Int Psychogeriatr 2011;23:1026-43. http://dx.doi.org/10.1017/S1041610210002437.
- Manthorpe J, Samsi K, Campbell S, Abley C, Keady J, Bond J, et al. A qualitative study of user and carer experiences of the diagnostic process in dementia: implications for clinicians and commissioners. Br J Gen Pract 2013;63:30-1.
- Redley M, Luke L, Keeley H, Clare I, Holland A. The Evaluation of the Pilot Independent Mental Capacity Advocate (IMCA) Service. 2006.
- Townsley R, Laing A. Effective Relationships, Better Outcomes: Mapping the Impact of the Independent Mental Capacity Advocate Service (1st April 2009 to 31st March 2010). London: Social Care Institute of Excellence; 2011.
- Shah A, Banner N, Heginbotham C, Fulford B. The application of the Mental Capacity Act 2005 among geriatric psychiatry patients: a pilot study. Int Psychogeriatr 2009;21:922-30. http://dx.doi.org/10.1017/S1041610209990391.
- Williams V, Boyle G, Jepson M, Swift P, Williamson T, Heslop P. Making Best Interests Decisions: People and Processes. London: Mental Health Foundation; 2012.
- Wilson E, Seymour JE, Perkins P. Working with the Mental Capacity Act: findings from specialist palliative neurological care settings. Palliat Med 2010;24:396-402. http://dx.doi.org/10.1177/0269216309360739.
- Improving Services and Support for People with Dementia. Report by the Comptroller and Auditor General; HC 604 Session 2006–2007. London: National Audit Office; 2007.
- Forget Me Not 2002: Developing Mental Health Services for Older People in England. London: Audit Commission; 2002.
- Warner JP, Butler RE, Wuntakal B, Tovey D. Clinical Evidence. London: BMJ Books; 2006.
- Burns A, O’Brien J. BAP Dementia Consensus group, clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J Psychopharmacol 2006;20:732-55. http://dx.doi.org/10.1177/0269881106068299.
- Vellas B, Andrieu S, Sampaio C, Wilcock G. European Task Force group disease-modifying trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol 2007;6:56-62. http://dx.doi.org/10.1016/S1474-4422(06)70677-9.
- Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9380.
- Schoenmaker N, Van Gool WA. The age gap between patients in clinical studies and in the general population: a pitfall for dementia research. Lancet Neurol 2004;3:627-30. http://dx.doi.org/10.1016/S1474-4422(04)00884-1.
- Wilcock J, Bryans M, Turner S, Downs M, Iliffe S. Methodological problems in dementia research in primary care: a case study of a randomised controlled trial. Prim Health Res Dev 2007;8:12-21. http://dx.doi.org/10.1017/S1463423607000035.
- McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, van der Meulen J, et al. The Hawthorne effect: a randomised controlled trial. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-30.
- Roos L, Nicol J. A Research register: uses, development, and accuracy. J Clin Epidemiol 1999;52:39-47. http://dx.doi.org/10.1016/S0895-4356(98)00126-7.
- Fitzgerald SG, Kelleher A, Teodorski E, Collins DM, Boninger M, Cooper RA. The development of a nationwide register of wheelchair users. Disabil Rehabil 2007;2:358-65. http://dx.doi.org/10.1080/17483100701745752.
- Fritsch T, McClendon MJ, Smyth KA, Lerner AJ, Chen CH, Petot GJ, et al. Effects of educational attainment on the clinical expression of Alzheimer’s disease: results from a research register. Am J Alzheimers Dis Other Demen 2001;16:369-76. http://dx.doi.org/10.1177/153331750101600606.
- Austrom MG, Bachman J, Altmeyer L, Gao S, Farlow M. A collaborative alzheimer disease research exchange: using a community-based helpline as a recruitment tool. Alzheimer Dis Assoc Disord 2010;24:S49-53.
- CERAD . Consortium to Establish a Registry for Alzheimer’s Disease n.d. http://cerad.mc.duke.edu/ (accessed 20 June 2010).
- Katchachurian ZS. Work Groups A & B of the Leon Thal Symposium 2008 . A roadmap for the prevention of dementia II. Alzheimers Dement 2009;5:85-92. http://dx.doi.org/10.1016/j.jalz.2009.01.021.
- Gauthier S, Garcia A, Sano M, Robert P, Senanarong V, Woodward M, et al. Priorities for research criteria in Alzheimer’s disease. Alzheimers Dement 2010;6:359-62. http://dx.doi.org/10.1016/j.jalz.2010.05.2017.
- Roos LL, Mustard CA, Patrick NJ, Comm B, Mclerran DF, Malenka DJ, et al. Registries and administrative data: organisation and accuracy. Med Care 1993;31:201-12. http://dx.doi.org/10.1097/00005650-199303000-00002.
- Bentley S, Melville J, Berry B, Katon W. Implementing a clinical and research register in obstetrics: overcoming the barriers. Gen Hosp Psychiatry 2007;29:192-8. http://dx.doi.org/10.1016/j.genhosppsych.2007.01.011.
- Newberry A, Sherwood P, Hricik A, Bradley S, Kuo J, Crago E, et al. Understanding Recruitment and Retention in Neurological Research. J Neurosci Nurs 2010;42:47-5. http://dx.doi.org/10.1097/JNN.0b013e3181c1fdd9.
- McCarney R, Fisher P, Iliffe S, van Haselen R, Griffin M, van der Meulen J, et al. Ginkgo biloba for mild to moderate dementia in a community setting: a pragmatic, randomised, parallel-group, double-blind, placebo-controlled trial. Int J Geriatr Psychiatry 2008;23:1222-30. http://dx.doi.org/10.1002/gps.2055.
- Brodaty H, Dresser R, Eisner M, Erkunjuntti T, Gauthier S, Graham N, et al. Alzheimer’s Disease International and International Working Group for Harmonisation of Dementia Drug Guidelines for research involving human subjects with dementia. Alzheimers Dis Assoc Disord 1999;13:71-9. http://dx.doi.org/10.1097/00002093-199904000-00003.
- Rees M, Wells F. Falling research in the NHS. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c2375.
- Cherry D, Hahn C, Vickery B. Educating primary care physicians in the management of Alzheimer’s disease: using practice guidelines to set quality benchmarks. Int Psychogeriatr 2009;21:S44-52.
- Iliffe S, Waugh A, Poole M, Bamford C, Brittain K, Chew-Graham C, et al. The effectiveness of collaborative care for people with memory problems in primary care: results of the CAREDEM case management modelling and feasibility study. Health Technol Assess 2014;18.
- Shekelle PG, MacLean CH, Morton SC, Wenger NS. ACOVE quality indicators. Ann Intern Med 2001;135:653-67. http://dx.doi.org/10.7326/0003-4819-135-8_Part_2-200110161-00004.
- Robert P, Schuck S, Dubois B, Olie JP, Lepine JP, Gallarda T, et al. Screening for Alzheimer’s disease with a short cognitive evaluation battery. Dement Geriatr Cogn Disord 2003;15:92-8. http://dx.doi.org/10.1159/000067971.
- Richardson J. The Easy-Care assessment system and its appropriateness for older people. Nurs Older People 2001;13:17-9.
- Culverwell A, Milne A, Guss R, Tuppen J. Screening for dementia in primary care: how is it measuring up?. Qual Ageing 2008;9:39-44. http://dx.doi.org/10.1108/14717794200800019.
- Buckwalter KC, Maas M, Reed D. Assessing family and staff caregiver outcomes in Alzheimer’s disease research. Alzheimer Dis Assoc Disord 1997;11:105-16.
- Culter NE. Someone I know has Alzheimer’s: a statistical portrait from the First National Opinion Survey of Alzheimer’s Disease. Am J Alzheimer’s Dis Other Demen 1987;2:21-5.
- Gilleard C, Groom F. A study of two dementia quizzes. Br J Clin Psychol 1994;33:529-34.
- Ramsay J, Campbell JL, Schroter S, Green J, Roland M. The General Practice Assessment Survey (GPAS): tests of data quality and measurement properties. Fam Prac 2000;17:372-9.
- Sloper P, Turner S. Adaptation and Help Seeking Strategies in Families of Children with Physical Disabilities. Manchester: Hester Adrian Research Centre, University of Manchester; 1991.
- Bowman KF, Mukherjee S, Fortinksy RH. Exploring strain in community and nursing home family caregivers. J Appl Gerontol 1994;17:371-92.
- Zarit SH. Brief measures of depression and cognitive function. Generations 1997;21:41-3.
- Rowley N. Basic Clinical Science: Describing a Rose with a Ruler. London: Hodder and Stoughton; 1991.
- Livingston G, Burns A, Levy R. Dementia. London: Chapman and Hall; 1994.
- Banerjee S, Murray J, Foley B, Atkins L, Schneider J, Mann A. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry 2003;74:1315-16. http://dx.doi.org/10.1136/jnnp.74.9.1315.
- Mayeux R, Sano M. Treatment of Alzheimer’s disease. N Engl J Med 1999;341:1670-9. http://dx.doi.org/10.1056/NEJM199911253412207.
- Birks JS, Melzer D, Beppu H. Donepezil for Mild and Moderate Alzheimer’s Disease. Cochrane Database Syst Rev 2003;3.
- American Diabetes Association . Exercise and Type 1 Diabetes 2014. www.diabetes.org/food-and-fitness/fitness/exercise-and-type-1-diabetes.html (accessed December 2014).
- Teri L, Logsdon RG, McCurry SM. Predictors non-pharmacological treatment of behavioural disturbance in dementia. Med Clin N Am 2002;86:684-9. http://dx.doi.org/10.1016/S0025-7125(02)00006-8.
- Stevens J, Killeen M. A randomised control trial testing the impact of exercise on cognitive symptoms and disability of residents with dementia. Contemp Nurs 2006;21:32-40. http://dx.doi.org/10.5172/conu.2006.21.1.32.
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer’s Disease: a 1-year randomised, controlled trial. J Am Geriatr Soc 2007;55:158-65. http://dx.doi.org/10.1111/j.1532-5415.2007.01035.x.
- Holmberg SK. Evaluation of a clinical intervention for wanderers on a geriatric nursing unit. Arch Psychiatr Nurs 1997;11:21-8. http://dx.doi.org/10.1016/S0883-9417(97)80046-5.
- Inouye SK, Van Dyck C, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. http://dx.doi.org/10.7326/0003-4819-113-12-941.
- World Medical Association (WMA) . Declaration of Helsinki: Ethical Principles for Medical Research Involving Humans n.d.
- Ouslander JG, Griffiths PC, McConnell E, Riolo L, Kutner M, Schnelle J. Functional incidental training: a randomised, controlled, crossover trial in Veterans Affairs nursing homes. J Am Geriatr Soc 2005;7:1091-100. http://dx.doi.org/10.1111/j.1532-5415.2005.53359.x.
- Eccles M, Clarke J, Livingstone M, Freemantle N, Mason J. North of England Evidence Based Dementia Guideline Development Group . North of England evidence based guidelines development project: guideline for the primary care management of dementia. BMJ 1998;317:802-8. http://dx.doi.org/10.1136/bmj.317.7161.802.
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Alzheimer’s Disease International . Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112-17. http://dx.doi.org/10.1016/S0140-6736(05)67889-0.
- Bosanquet N. The socioeconomic impact of Alzheimer’s disease. Int J Geriatr Psychiatry 2001;3:249-53. http://dx.doi.org/10.1002/gps.332.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37-49. http://dx.doi.org/10.1016/S0090-4295(02)02243-4.
- Norton C, Christiansen J, Butler U, Harari D, Nelson RL, Pemberton J, et al. Incontinence. Plymouth: Health Publication; 2002.
- Hunskaar S, Burgio K, Clarke A, Lapitan MC, Nelson R, Sillen U, et al. Incontinence: Basics and Evaluation. Plymouth: Health Publications; 2005.
- Perry S, Shaw C, Mcgrother C, Matthews RJ, Assassa RP, Dallosso H, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002;4:480-4. http://dx.doi.org/10.1136/gut.50.4.480.
- Fultz NH, Herzog AR. Self-reported social and emotional impact of urinary incontinence. J Am Geriatr Soc 2001;49:892-9. http://dx.doi.org/10.1046/j.1532-5415.2001.49179.x.
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327-36.
- Brown JS, Vittinghoff E, Wyan JF, Stone MC, Nevitt MC, Grady D. Urinary incontinence: does it increase risk for falls and fractures. J Am Geriatr Soc 2000;48:721-5.
- Iliffe S, Lenihan P, Orrell M, Walters K, Drennan V, See Tai S. The development of a short instrument to identify common unmet needs in older people in general practice. Br J Gen Pract 2004;509:914-18.
- Stoddart H, Donovan J, Whitley E, Sharp D, Harvey I. Urinary incontinence in older people in the community: a neglected problem?. Br J Gen Pract 2001;468:548-52.
- Thomas P, Ingrand P, Lalloue F, Hazif-Thomas C, Billon R, Vieban F, et al. Reasons of informal caregivers for institutionalizing dementia patients previously living at home: the Pixel study. Int J Geriatr Psychiatry 2004;2:127-35. http://dx.doi.org/10.1002/gps.1039.
- Wagg A, Peel P, Lowe D, Potter J. Report of the National Audit of Continence Care for Older People (65 Years and Above) in England, Wales & Northern Ireland. London: The Royal College of Physicians; 2006.
- Schneider J, Kavanagh S, Knapp M, Beecahm J, Netten A. Elderly people with advanced cognitive impairment in England: resource use and costs. Ageing Soc 1993;13:27-50. http://dx.doi.org/10.1017/S0144686X00000635.
- Cassells C, Watt E. The impact of incontinence on older spousal caregivers. J Adv Nurs 2003;42:607-16. http://dx.doi.org/10.1046/j.1365-2648.2003.02664.x.
- Forbat L. Listening to carers talking about the subjects of continence and toileting. Nurs Times 2004;2:46-9.
- Potter J, Norton C, Cottenden A. Bowel Care in Older People: Research and Practice. London: Royal College of Physicians; 2002.
- Goodman C, Davies S, Norton C, Leyshon S, Gage H, Fader M, et al. Can Clinical Benchmarking Improve Bowel Care in Care Homes for Older People?. London: Department of Health; 2006.
- Caring for the Carers. London: DH; 1998.
- National Service Framework for Older People. London: DH; 2001.
- Good Practice in Continence Services. London: DH; 2000.
- Incontinence: Causes, Management and Provision of Services. London: RCP; 1995.
- Dixon-Woods M, Agarwal S, Young B, Jones D. Integrative Approaches to Qualitative and Quantitative Evidence. London: Health Development Agency; 2004.
- Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev 2004;1. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD002802/pdf_fs.html (accessed December 2007).
- Robson C. Real World Research. Oxford: Blackwell; 2004.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage; 1997.
- Dixon-Woods M, Shaw RL, Agarwal S, Smith JA. The problem of appraising qualitative research. Qual Saf Health Care 2004;13:223-5. http://dx.doi.org/10.1136/qshc.2003.008714.
- Byford S, Sefton TAJ. Economic evaluation of complex health and social care interventions. Natl Inst Econ Rev 2003;1:98-108. http://dx.doi.org/10.1177/002795010300100114.
- Shaw C, Matthews RJ, Perry SI, Assassa RP, Williams K, Mcgrother C, et al. Leicestershire MRC Incontinence Study Team . Validity and reliability of an interviewer-administered questionnaire to measure the severity of lower urinary tract symptoms of storage abnormality: the Leicester Urinary Symptom Questionnaire. BJU Int 2002;90:205-15. http://dx.doi.org/10.1046/j.1464–410X.2002.02893.x.
- Grimshaw J, Campbell M, Ramsay C. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Safe Health Care 2003;12:47-52. http://dx.doi.org/10.1136/qhc.12.1.47.
- Coren E, Fisher M. The Conduct of Systematic Research Reviews for SCIE Knowledge Reviews. London: Social Care Institute for Excellence; 2006.
- Brown JB, Crabtree BF, Miller WL. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999.
- Crabtree BF, Miller WL. Primary Care Research: A Multi-Method Typology and Qualitative Road Map. Thousand Oaks, CA: Sage; 1992.
- Clarke CL, Keady J, Wilkinson H. The Perspectives of People with Dementia: Research Methods and Motivations. London: Jessica Kingsley; 2002.
- Pratt R, Wilkinson H. The Perspectives of People with Dementia: Research Methods and Motivations. London: Jessica Kingsley Publications; 2002.
- National Institute of Clinical Excellence . Faecal Incontinence: The Management of Faecal Incontinence in Adults 2007. www.nice.org.uk/nicemedia/pdf/CG49NICEGuidance.pdf (accessed December 2007).
- Report of the National Audit of Continence Care for Older People (65 Years And Above) in England, Wales & Northern Ireland. London: Clinical Effectiveness and Evaluation Unit. RCP; 2005.
- Integrated Mental Health Services for Older Adults: A Service Development Guide. London: DH; 2005.
- Thüroff J, Abrams P, Andersson KE, Artibani W, Chartier-Kastler E, Hampel C, et al. Guidelines on Urinary Incontinence 2006. www.uroweb.org/fileadmin/user_upload/Guidelines/16%20Urinary%20Incontinence.pdf (accessed January 2008).
- Payne C, Blaivas J, Brown J, Hirsch M, Peters J, Kusek T, et al. Continence. Paris: International Continence Society & Les Editions; 2005.
- Drennan V, Cole L. Comparing the Views of People With Dementia and Their Carers With Health and Social Care Staff on Managing Toilet Difficulties and Incontinence n.d.
- Glendinning C, Tjadens F, Arksey H, Moree M, Moran N, Nies H. Care Provision within Families and its Socio-Economic Impact on Care Providers. York: Social Policy Research Unit, University of York; 2009.
- Dahlberg L, Bambra C, Demack S. Age and gender of informal carers: a population-based study in the UK. Health Soc Care Community 2007;5:439-45. http://dx.doi.org/10.1111/j.1365-2524.2007.00702.x.
- Glaser K, Evandrou M, Tomassini C. The health consequences of multiple roles at older ages in the UK. Health Soc Care Community 2005;13:470-7. http://dx.doi.org/10.1111/j.1365-2524.2005.00574.x.
- Chappell NL, Reid CR. Burden and well-being among caregivers: examining the distinction. Gerontologist 2002;42:772-80. http://dx.doi.org/10.1093/geront/42.6.772.
- Arksey H. Rationed care: assessing the support needs of informal carers in English Social Services Authorities. J Soc Policy 2002;1:81-101. http://dx.doi.org/10.1017/S0047279402006529.
- Drennan V, Cole L. Promoting Continence and Managing Incontinence with People with Dementia Living at Home: One More Challenge for Integration. J Integr Care 2009;17:15-2. http://dx.doi.org/10.1108/14769018200900004.
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;7591:455-9. http://dx.doi.org/10.1136/bmj.39108.379965.BE.
- Murray SA, Boyd K, Kendall M, Worth A, Benton TF. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ 2002;325:929-32. http://dx.doi.org/10.1136/bmj.325.7370.929.
- Pettigrew A. Longitudinal Field Research on Change: Theory and Practice. Organ Sci 1990;3:267-92. http://dx.doi.org/10.1287/orsc.1.3.267.
- Molloy D, Woodfield K, Bacon J. Longitudinal Qualitative Research Approaches in Evaluation Studies. London: Department of Work and Pensions; 2002.
- Saldana J. Longitudinal Qualitative Research: Analysing Change Through Time. Walnut Creek, CA: Altamira Press; 2003.
- Blythway B, Borat J, Shirani F, Weller S. Conducting Qualitative Longitudinal Research: Fieldwork Experiences. Leeds: University of Leeds; 2010.
- Hartney E, Orford J, Dalton S, Ferrins-Brown M, Kerr C, Maslin J. Untreated heavy drinkers: a qualitative and quantitative study of dependence and readiness to change. Addict Res Theory 2003;5:317-37. http://dx.doi.org/10.1080/1606635031000141094.
- Wenger GC. Advantages gained by combining qualitative and quantitative data in longitudinal study. J Ageing Stud 1999;4:369-76. http://dx.doi.org/10.1016/S0890-4065(99)00015-8.
- Gitlin LN, Schinfeld S, Winter L, Corcoran M, Boyce AA, Hauck W. Evaluating home environments of persons with dementia: interrater reliability and validity of the Home Environmental Assessment Protocol (HEAP). Disabil Rehabil 2002;24:59-71. http://dx.doi.org/10.1080/09638280110066325.
- Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R. CALM-AD Trial Group. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007;14:1382-92. http://dx.doi.org/10.1056/NEJMoa066583.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. http://dx.doi.org/10.1093/geront/20.6.649.
- Corden A, Miller J. Qualitative longitudinal research for social policy: introduction to themed section. Soc Policy Soc 2007:529-32.
- Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2005;14:443-51. http://dx.doi.org/10.1002/pds.1115.
- Goodman C, Drennan V, Davies S, Masey H, Gage H, Scott C, et al. Nurses as Case Managers in Primary Care: the Contribution to Chronic Disease Management. London: HMSO; 2010.
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park, CA: Sage Publications; 1990.
- British Geriatric Society . Continence Best Practice Guide 6.2 2007. www.bgs.org.uk/index.php/topresources/publicationfind/goodpractice/377-continence (accessed December 2014).
- Perry S, Shaw C, Assassa P, Dallosso H, Williams K, Brittain KR, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. J Public Health Med 2000;3:427-34. http://dx.doi.org/10.1093/pubmed/22.3.427.
- Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 2009;1:76-83. http://dx.doi.org/10.1002/pds.1688.
- Davé S, Petersen I, Kalaitzaki E. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf 2009;8:704-7. http://dx.doi.org/10.1002/pds.1770.
- Peritz E. Berkson’s bias revisited. J Chron Dis 1984;12:909-16. http://dx.doi.org/10.1016/0021-9681(84)90067-5.
- Norton PA, MacDonald LD, Sedgwick PM, Stanton SL. Distress and delay associated with urinary incontinence, frequency, and urgency in women. BMJ 1988;297:1187-9. http://dx.doi.org/10.1136/bmj.297.6657.1187.
- NHS Information Centre . Prescription Cost Analysis England 2007 n.d. www.ic.nhs.uk/pubs/prescostanalysis2007 (accessed 10 August 2008).
- Reported in Section 2.12 of Department of Health (2000) Good Practice in Continence Services. London: Department of Health; 1997.
- Listen to Us: Involving People with Dementia in Planning and Developing Services. London: HMSO; 2005.
- Mostwyn J, Bouricer A, Haab F, Koeble H, Rao S, Resnick N, et al. Incontinence. Plymouth: Health Publications; 2005.
- Continence Foundation n.d. www.continence-foundation.org.uk (accessed December 2014).
- Turner DA, Shaw C, McGrowther CW, Dallosso HM, Cooper NJ. MRC Incontinence Team . The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU Int 2004;9:1246-52. http://dx.doi.org/10.1111/j.1464-410x.2004.04806.x.
- Hughes JC, Robinson L, Volicer L. Specialist palliative care in dementia. BMJ 2005;330:57-8. http://dx.doi.org/10.1136/bmj.330.7482.57.
- Sepulveda C, Marlin A, Yoshida T, Ullrich A. Palliative care: the World Health Organisation’s global perspective. J Pain Symptom Manag 2002;24:91-6. http://dx.doi.org/10.1016/S0885-3924(02)00440-2.
- Reilly S, Abendstern M, Hughes J, Challis D, Venables D, Pedersen I. Quality in long-term care homes for people with dementia: an assessment of specialist provision. Ageing Soc 2006;26:649-68. http://dx.doi.org/10.1017/S0144686X06004909.
- Care Homes for Older People: National Minimum Standards, Care Homes Regulations. London: The Sationery Office; 2003.
- Froggatt K, Payne S. A survey of end of life care in care homes: issues of definition and practice. Health Soc Care Community 2006;14:341-8. http://dx.doi.org/10.1111/j.1365-2524.2006.00628.x.
- Guidelines for a Palliative Approach in Residential Aged Care. Canberra: Rural Health and Palliative Care Branch, Australian Government Department of Health and Ageing; 2004.
- Improving Supportive and Palliative Care for Adults with Cancer. London: NICE; 2004.
- Ross M, Fisher R, MacLean MJ. A Guide to End-of-Life Care for Seniors. Ottawa: Health Canada; 2000.
- Glendinning C, Jacobs S, Alborz A, Hann M. A survey of access to medical services in nursing and residential homes in England. Brit J Gen Pract 2002;52.
- Laing W, Buisson I. Care of Elderly People: Market Survey. London: Laing & Buisson; 2007.
- Goodman C, Davies S, Norton C, Fader M, Morris J, Wells M, et al. Can district nurses and care home staff improve bowel care for older people using a clinical benchmarking tool?. Br J Community Nurs 2013;18:580-7. http://dx.doi.org/10.12968/bjcn.2013.18.12.580.
- Netten A. Care Homes for Older People. Volume 1: Facilities, Residents and Costs. Canterbury: PSSRU, University of Kent; 2001.
- Evans C. The Analysis of Experiences and Representations of Older People’s Health in Care Homes to Develop Primary Care Nursing Practice. London: King’s College London; 2008.
- Reed J, Payton VR. Privileging the voices of the older service users. A methodological challenge. Soc Sci Health 1998;4:230-42.
- Conducting Research with People Not Having the Capacity to Consent to their Participation. A Practical Guide for Researchers. Leicester: BPS; 2008.
- Hubbard G, Downs MG, Tester S. Including older people with dementia in research: challenges and strategies. Ageing Ment Health 2003;7:351-62. http://dx.doi.org/10.1080/1360786031000150685.
- Prior L. Using Documents in Social Research. London: Sage Publications; 2003.
- Corner J, Halliday D, Haviland J, Douglas HR, Bath P, Clark D, et al. Exploring nursing outcomes for patients with advanced cancer following intervention by Macmillan specialist palliative care nurses. J Adv Nurs 2003;41:561-74. http://dx.doi.org/10.1046/j.1365-2648.2003.02568.x.
- Coffey AJ, Atkinson PA. Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks, CA; 1996.
- Coast J. Is economic evaluation in touch with society’s health values?. BMJ 2004;329:1233-6. http://dx.doi.org/10.1136/bmj.329.7476.1233.
- Dewing J. From ritual to relationship. Dementia 2002;1:157-71. http://dx.doi.org/10.1177/147130120200100204.
- Dewing J. Understanding Wandering in Persons Living with Dementia. Newcastle upon Tyne: University of Northumbria; 2008.
- Davies SM. Relatives’ Experiences of Nursing Home Entry: A Constructivist Inquiry 2001.
- Robinson L, Hughes J, Daley S, Keady J, Ballard C, Ladislav V. End-of-life care and dementia. Rev Clin Gerontol 2005;15:135-48. http://dx.doi.org/10.1017/S0959259806001833.
- The Preferred Priorities for Care (PPC). London: NHS National End of Life Care Programme; 2007.
- Kellehear A. Dementia and dying: the need for a systematic policy approach. Crit Soc Policy 2009;29:146-57. http://dx.doi.org/10.1177/0261018308098398.
- Reason P, Bradbury H. Handbook of Action Research. London: Sage; 2006.
- Mental Capacity Act 2005 Draft Code of Practice. London: The Stationery Office; 2007.
- The Law and Vulnerable Elderly People. London: Age Concern; 1986.
- Manthorpe J, Samsi K. Mental capacity and dementia: a review, part 1. J Dement Care 2012;20:35-8.
- Glasby J, Kilbride L. Who knows? The provision of information to the carers of people with dementia. Practice 2003;15:51-67. http://dx.doi.org/10.1080/09503150308416934.
- Mental Capacity Assessments: Working in Practice? Initial Findings from the Mental Health Foundation’s Assessment of Mental Capacity Audit Tool (AMCAT). London: Mental Health Foundation; 2010.
- Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage Publications; 2006.
Appendix 1 Chapter 1: Findings from literature reviews of interventions in primary care designed to alter clinical practice with patients with dementia
| Authors, date and country | Size | Recruitment | Intervention | Method | Outcomes | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Waldorff et al. 2003,22 Denmark | 413 GPs in intervention, 122 in control group | GPs identified from medical association and organisational database | Assessment of a multifaceted strategy to implement guidelines | Controlled before-and-after study | Included dementia screening, thyroid function and vitamin B12 tests, cognitive testing and referrals to specialists; also, questionnaire examining acceptability of guidelines | No significant difference between control and intervention group was found in the number of diagnostic evaluations undertaken, the number of ordered, or the amount of cognitive testing performed Most GPs had read the guidelines and found them applicable |
After a multifaceted strategy for implementation, there was no improvement in the adherence to a guideline for diagnosing dementia. However, the outcomes were measured only 4–7 months after implementation, and although the intervention was delivered at GP level, the outcomes were measured at practice level |
| Downs et al. 2006,21 central Scotland and London | 35 practices | Local group meetings, postal and phone invitations, practice visits | Three arms: (1) tutorial on CD-ROM; (2) decision-support software; and (3) practice-based workshops | Unblinded, cluster randomised before-and-after controlled trial | Detection rates. Concordance with guidelines | Significant increase in the detection of dementia in arms 2 and 3 No improvement in adherence to guidelines |
Might be useful to examine potential cumulative effects of combining educational interventions |
| Rondeau et al. 2008,23 France | 684 GPs, 214 specialists, 3021 patients | Random selection from a list of GPs throughout France | Group educational meeting and training in use of neuropsychological tests | Cluster RCT | Primary outcome: Suspicion of dementia by GP Secondary outcome: Diagnosis of dementia by GP |
No difference in diagnosis of dementia in intervention group | Education ineffective |
| Chodosh et al. 2006,24 USA | 166 primary care clinics | Questionnaires sent to all primary care health providers at 16 clinics | Survey of knowledge, attitudes and perceptions following participation in the ACCESS study (Vickrey et al.25) | Survey following a cluster RCT of a care management intervention | Practitioners’ knowledge about capacity determination, evaluation, treatment and patient safety Measures of attitude, and perceptions of quality of care |
Intervention group knew more about determining capacity and more felt that management of dementia patients in primary care was difficult; no other difference in knowledge, attitudes or perceptions of quality of care | Care management programmes may have a positive effect on care despite lack of educational gain |
| Wenger et al. 2009,26 USA | 357 intervention and 287 control community-dwelling patients | Patients aged > 75 years, with difficulty in falls, continence or cognition; two medical groups participated, each having two sites, with one acting as a control and one as an intervention site | Patients’ problems were identified by a telephone call then the physician triggered a condition-specific process of management and resulted with formulating a plan for the patient. Physicians in the intervention group had a 3-hour educational session on ACOVE quality indicators.320 Patients also completed questionnaires. Data were collected from medical records | Controlled trial | Percentage of quality indicators met after 13 months of medical record extraction | For cognitive impairment, care as triggered by quality indicators did not differ; however, more attention was given to caregiver education, decision-making capacity, and discussion about driving occurred in the intervention group | Care did not improve for patients with cognitive impairment. All physicians provided more recommended care for patients who presented with their symptoms (compared with those identified by screening) |
| Vollmar et al. 2010,27 Germany | 389 GPs from 26 QCs | All QCs within a 50-km radius of Witten University were contacted by telephone or letter to the QC moderator GPs were recruited at QC meetings, and required to participate in an extra session and have access to the internet A control group was used for comparison |
QCs were randomly allocated to either arm of the trial: online learning plus structured case discussion (‘blended learning’) or lecture plus structured case discussion. Both groups received pocket books of the guidelines. Control group received only the pocket book. Knowledge tests were carried out pre and post intervention, and at 6 months | Cluster RCT | Primary outcome: Knowledge gain from before to after the intervention Secondary outcome: Comparison of knowledge gain of the two groups |
Both groups showed a significant gain in knowledge following the interventions However, there was no difference in knowledge gains between the two groups Subgroup analysis showed that GPs who self-reported using the online learning had significant knowledge gain compared with the other group The control group showed a knowledge gain that was significantly less than the intervention groups |
Blended learning was not more effective than traditional learning in improving dementia knowledge in GPs. However, when GPs self-report use of online modules, results suggest that blended learning techniques could be more effective |
| Perry et al. 2008,28 Holland | 151 patients | 54 GPs recruited patients > 70 years into DGIP | Secondary analysis of data examining whether the DGIP (an in-home assessment) improves rates of detection of dementia in primary care In-home assessment performed by specialist nurse, assessed function, mood, cognition and caregiver burden A management plan was formulated |
Blinded RCT | Primary outcome: The number of new dementia diagnoses Secondary outcome: The level of under-registration of dementia cases |
The number of dementia diagnoses in the control group (9%) was significantly less than those in intervention group (29%); p = 0.02 | An in-home assessment and management programme is effective in detecting dementia in the primary care setting compared with controls of usual care |
| Callahan 2006,29 USA | 153 patients with Alzheimer’s disease | Primary care centres | Collaborative care management | RCT | NPI cognitive function, carer stress, service use | Intervention group had lower NPI scores but no difference in depression, cognitive status, or functional scores. Carers showed less stress. Intervention group had higher number of contact visits with physician/nurse, but no difference in hospital or nursing home admissions | Collaborative care can reduce behavioural/psychological symptoms in patients, and stress in carers |
| Vickrey 2006,25 USA | 408 patient–caregiver dyads from 18 clinics | Patients identified from organisational database | Dementia care manager with web-based support software for care planning and co-ordination Interactive educational seminars for practitioners in intervention group |
Cluster RCT | Primary outcome: Adherence to guidelines Secondary outcomes: Use of cholinesterase inhibitors, patient QoL, caregivers’ knowledge, QoL, social support and confidence |
Intervention group care more adherent to guidelines, received more community services and were prescribed more cholinesterase inhibitors Caregivers were more confident in intervention group |
Systems change by introducing care managers can improve quality of care, but is costly |
| Fortinsky et al. 2009,30 USA | 84 caregivers | Family caregivers of patients with dementia were recruited from the Alzheimer’s Association and primary care | Intervention group received educational materials which were discussed with a dementia care consultant | Cluster RCT | Primary outcome: Admission to a nursing home in the study period Secondary outcomes: Caregiver self-efficacy, caregiver burden, depression, health, satisfaction with the service |
Those in the intervention group were 40% less likely to end up in a nursing home than those in the control group but this did not achieve statistical significance | No difference was found between groups for secondary outcomes Inadequately powered study |
| Clark 2004,31 USA | 89 participants with a symptom of memory loss or diagnosis of dementia, but no mention of how many in control and how many in intervention groups | Patients registered with Kaiser with diagnosis of dementia on medical records or symptoms indicative of cognitive impairment | Intervention group received care consultation: telephone interaction between Alzheimer’s Association staff and patient/caregiver | RCT | Hospital admissions A&E visits, number of physician contacts, satisfaction with service, depression, strain on patient | Intervention group reported more memory symptoms but were less likely to have hospital admissions or A&E visits, and had fewer physician contacts. Also experienced less embarrassment, isolation and relationship strain | Overall, patients who had a care consultant had lower levels of negative consequences from memory problems and reduced usage of certain services |
| Trial | Description of intervention |
|---|---|
| Waldorff et al. 200322 | Local GPs and specialists collaborated in the design of the multifaceted strategy Interventions included:
|
| Downs et al. 200621 | Three interventions trialled:
|
| Rondeau et al. 200823 | Training on a battery of cognitive screening tests (the Short Cognitive Evaluation Battery321), which tests four cognitive areas that are often impaired in Alzheimer’s disease (with a sensitivity of 93.8%); 2-hour group educational meetings on Alzheimer’s disease and other forms of dementia |
| Chodosh et al. 200624 | Intervention participants were offered five educational modules comprising 100 minutes of presentation and discussion in small-group format (and available on the web). These were:
|
| Wenger et al. 200926 | Educational sessions for practitioners and structured prompts on management and care for patients. For positively screened patients, a prompt initiated collection of specific data, triggered recommendations of specific investigations and suggested specific care processes. The prompt supported the facilitation of an impression and plan for the patient, and included decision-support materials and patient education resources and information about local services. Three-hour educational session learning an ‘efficient approach’ to the condition, using ACOVE quality indicators (ACOVE investigators 2001320). After piloting, practices met to share experiences of clinicians and to modify the structure of the intervention as necessary. Clinicians/practices were able to review and adapt the prompts to suit their local services and personnel |
| Vollmar et al. 201027 | Two interventions:
|
| Perry et al. 200828 | GPs referred patients to the study, who were then randomised to control or intervention. A geriatric specialist nurse visited patients at home for assessment using the EASYcare instrument.322 This tool assesses ADL, mood, cognition and has goal-setting elements. This was followed by up to six visits in 3 months for management planning. The nurse, primary care physician and geriatrician met regularly to discuss cases |
| Callahan et al. 200629 | Collaborative care management, by the primary care physician and a geriatric nurse practitioner, for 12 months. All patient contacts were with the nurse. All intervention patients recommended for anticholinesterase inhibitors. Assessments on patient’s behaviour and memory were made regularly and management planned accordingly. Primary care physicians were consulted to prescribe medications to help if non-pharmacological interventions had not been successful. Caregiver and patient education given, as well as regular psychological support for both caregiver and patient. Weekly meetings with multidisciplinary teams, who reviewed care and adherence to guidelines. Support given by web-based system, which helped with monitoring and multidisciplinary communication |
| Vickrey et al. 200625 | Twenty-three guideline recommendations identified (by a multidisciplinary group) as care goals. The same group designed an assessment and protocols and organised care co-ordination. Care managers (mainly social workers) were trained and carried out the assessments. Follow-up was arranged, based on need but regular 6-monthly format assessments were carried out. Assessments were made, software assisted in providing a care plan, and recommendations to primary care physician were made. The software also facilitated multidisciplinary communication and referrals to other agencies. Up to five interactive seminars of 90 minutes were offered to primary care physicians, educating on issues such as behaviour changes, determining capacity and depression |
| Fortinsky et al. 200930 | Care consultant initiated monthly contacts with patient and caregiver over 12 months. Educational material for caregivers was given to intervention and control groups, including information about the course of disease, legal/financial issues and community services. The intervention group was able to discuss these with the care consultants. Contacts were used to assess symptoms and concerns and compose action plans for caregivers. The agenda was set by the caregiver. Primary care physicians were faxed care plans with the hope that they would discuss/review with caregiver and patient during consultations |
| Clark et al. 200431 | Care consultation offered by Alzheimer’s Association specially trained staff. The care consultants conducted structured assessments and developed care strategies using family and community resources. These might include education/training programmes or support groups. Regular follow-ups arranged initially biweekly then monthly or 3-monthly |
| Study design | Waldorff et al. 200322 | Downs et al. 200621 | Rondeau et al. 200823 | Chodosh et al. 200624 | Wenger et al. 200926 | Vollmar et al. 201027 | Perry et al. 200828 | Callahan et al. 200629 | Vickrey et al. 200625 | Fortinsky et al. 200930 | Clarke et al. 200431 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controlled before-and-after study | Cluster randomised triala | Cluster RCT | Cluster RCT | Controlled trial | Cluster RCT | RCT | RCT | Cluster RCT | Cluster RCT | RCT | |
| Eligibility criteria were specified | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Random allocation to intervention | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Allocation concealed | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes |
| Intervention groups similar at baseline | Nob | Yes | Yes | Yes | Yes | Noc | Yes | Yes | Yes | Nod | Yes |
| Blinding of all participants | No | No | No | No | No | No | No | Yes | No | No | No |
| Blinding of all therapists | No | No | No | No | No | No | No | No | No | No | Yes |
| Blinding of all assessors | No | No | No | No | No | No | No | Yes | No | Partial | No |
| Measures of at least one outcome obtained from > 85% of participants | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Intention-to-treat analysis | Yes | Yes | No | Yes | No | No | No | No | Yes | No | No |
| Results of between interventions group statistical comparisons are reported for at least one outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Study provides both point measures and measures of variability for at least one key outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total (max. points = 11) | 4 | 6 | 5 | 8 | 5 | 5 | 6 | 9 | 8 | 7 | 8 |
Appendix 2 Chapter 1: Advisory and steering group membership
EVIDEM-ED: expert group members
| First name | Role |
|---|---|
| Professor Steve Iliffe | Research team, University College London |
| Ms Jane Wilcock | Research team, University College London |
| Ms Priya Jain | Research team, University College London |
| Dr Geoff Wong | GP with educational role, Queen Mary, University of London, Centre for Health Sciences |
| Dr Frances Lefford | GP with educational role, University College London |
| Dr Alex Warner | GP without educational role, University College London |
| Dr Susham Gupta | Specialist registrar in adult and old age psychiatry, Chelsea and Westminster Hospital |
| Mr Mark Griffin | Statistician, University College London |
| Mr Andrew Kingston | Social worker |
| Mr Horton Kennedy | Carer |
| Ms Kate Wolfe | Dementia and mental health advocate |
| Dr Alexandra Davidson | Clinical research associate |
| Ms Anna Dowrick | Campaigns officer, Alzheimer’s Society |
EVIDEM-ED: expert panel members
| First name | Category |
|---|---|
| Dr James Hickling | GP, Alzheimer’s Society |
| Dr Peter O’Brien | GP without educational role |
| Dr Lucy Farley | GP without educational role |
| Ms Sally Murnaghan | Practice nurse |
| Ms Clare Morris | Admiral Nurse |
| Ms Jane Langton | Social worker |
| Professor John Keady | Educationalist |
| Mr Peter Ashley | Person with dementia |
| Mrs Deborah Stone | Carer |
| Mrs Karen Weech | Carer |
| Mrs Stephanie Palmer | Carer |
| Ms Karen Thompson | Carer |
Appendix 3 Chapter 1: Diagnostic and management processes identified by the expert team
FIGURE 29.
Diagnosis of dementia; care pathway for community-dwelling patients. Hb, haemoglobin; TSH, thyroid-stimulating hormone.
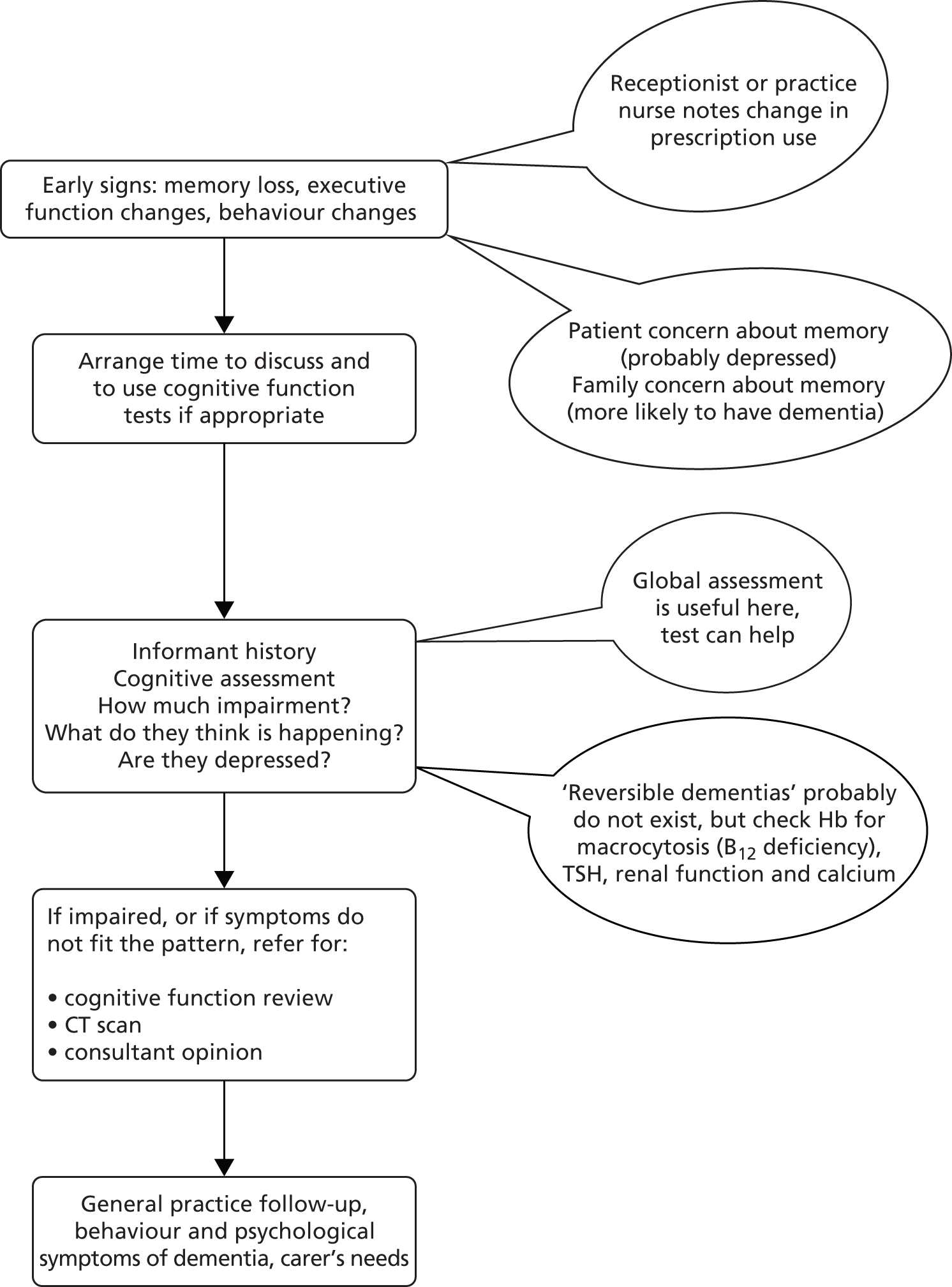
Appendix 4 Chapter 1: Changing clinical practice in dementia: elements of a training programme – for primary care team
| The task | Objectives | How to achieve |
|---|---|---|
| Pattern recognition [interpreting the meaning of accumulating symptom(s)] | Growing personal awareness/knowledge of members of the public, as well as professional experience among practitioners |
|
| Understanding the difficulties of the diagnostic process |
|
|
| A raised profile for dementia in the GP’s work environment (increasing ‘receptiveness’) |
|
|
| Practice team awareness of the issues |
|
|
| Practice systems for intelligence gathering, collation of information and knowledge of individual’s family circumstances and social networks, and responsibility for acting on that gathered knowledge |
|
|
| Continuity of care for individuals, which deepens knowledge, allows observation over time and permits trust to develop |
|
|
| Managing expectations of patients and carers by Primary Care Team |
|
|
| Assessing the degree of impairment | Getting all sides of the story: patient, carer, others (including own team and local social services in case known to them) |
|
| Assess the risks and challenges |
|
|
| Consider other long-term conditions and their relationship to the symptoms of dementia and other functional abilities (hearing or visual impairments, mobility problems) |
|
|
| Using locally preferred (standardised) assessment tools, knowing their limits | ||
| Using tacit knowledge (instincts, hunches, acquired experience) |
|
|
| Self-awareness of changes in thinking abilities (planning, calculating) and recognition of compensatory adaptations by other people (someone else takes over the bill paying) |
|
|
| Discussing possible diagnoses | Disclosure – who, when and to whom? (This is no different from breaking bad news for any other condition.) |
|
| Negotiate disclosure of the diagnosis with patient/carer |
|
|
| Responding | Maintain a positive attitude about dementia: ‘something can be done’ – based on awareness of local resources |
|
| Getting support and involvement of secondary care |
|
|
| Phase in responses involving resources/services |
|
|
| Locate services and assess if they make a difference |
|
|
| Medication for dementia and ‘shared care’ systems |
|
|
| Support for carers (practical, information, psychological support) means understanding how and why family members respond differently to dementia |
|
Appendix 5 Chapter 1: General practitioner questionnaire
General practitioner questionnaire
Thank you for agreeing to complete this questionnaire.
Most responses involve you only circling a number, and we have avoided questions which might require you to refer to your records.
We would ask that when the questionnaires are completed that colleagues do not confer, or cross-check textbooks for responses, because it is very important that we get an accurate picture of any change in knowledge as a consequence of the interventions. The responses will not be divulged to anyone.
Please return to your practice manager or a member of the research team
Jane Wilcock and Steve Iliffe
Research Department of Primary Care & Population Health
Royal Free Campus, Rowland Hill Street, London NW3 2PF
NIHR: Programme Grant for Applied Research for the EVIDEM Research & Development programme Hosted by Central and North West London NHS Foundation Trust in Collaboration with UCL, King’s College London, St George’s University of London, Kingston University London and the University of Hertfordshire.
Appendix 6 Chapter 1: Carer semi-structured interview schedule
EVIDEM-ED: carer schedule
Acknowledgements
Question 10 (Dementia Quiz) includes items from CSRD Nursing Home study (Buckwalter)324 (items 1, 3, 5, 10, 16, 17, 18, 19); Cutler 1987 (items 6, 7, 8);325 Gilleard and Groom 1984 (item 12);326 other quiz items formulated by Steve Turner and Michelle Bryans (items 2, 4, 9, 13, 14, 15, 20) and Steve Turner and Ailsa Cook (item 11).
Questions 18, 28–32, 34–36, 66 and 67 adapted with permission from The General Practice Assessment Survey (GPAS). 327 GPAS is copyright of Safran/The Health Institute and National Primary Care Research and Development Centre.
Questions 21 and 22 from Sloper and Turner 1991. 328
Questions 59–61 adapted from Bowman et al. 1994. 329
Question 63 (Carer Stress Measure) Zarit et al. 1997. 330
Appendix 7 Chapter 1: Medical records data extraction tool – baseline (time 1)
Appendix 8 Chapter 2: Research protocol EVIDEM-E study
Version 8.1 May 2009
Randomised Control Evaluation of Exercise on Individuals with Dementia and their Carers as a Therapy for BPSD
Authors
David Lowery
Research Associate
CNWL FT
Rahul Bhattacharya
Specialist Registrar, Charing Cross Psychiatric Training Scheme
Mark Griffin
Medical Statistician
Arlinda Cerga Pashoja
Research Worker
CNWL NHS FT
James Warner
Senior Lecturer Imperial College London
Consultant in Old Age Psychiatry
Address for correspondence
Dr David Lowery CNWL HQ, Greater London House, Hampstead Road, London NW1 7QY
E-mail: d.lowery@nhs.net
Protocol development history
June–September 2008 – initial development
September 2008 EVIDEM advisory group
October 2009 EVIDEM-E steering group
February 2009 EVIDEM E protocol development group
February 2009 EVIDEM-E steering group
Abbreviations
| AE | Adverse event |
| CNWL NHS | Central & North West London NHS Foundation Trust |
| CRF | Case Report Form |
| DAP | Data Analysis Plan |
| DeNDRoN | Dementia & Neuro-Degenerative Diseases Research Network |
| DMG | Data Monitoring Group |
| EOT | End of Trial |
| ET | Exercise Therapist |
| GCP | Good Clinical Practice |
| ICF | Informed Consent Form |
| TAU | Treatment as Usual |
| NHS | National Health Service |
| PCT | Primary Care Trust |
| PI | Principal Investigator |
| PIS | Participant Information Sheet |
| IR | Independent Researcher |
| RW | Research Worker |
| REC | Research Ethics Committee |
| R&D | Research and Development Department |
| SAE | Serious Adverse Event |
| TSC | Trial Steering Committee |
Summary
Background
Over 700,000 people in the UK have dementia. The deterioration associated with dementia is distressing for patient and carer alike and often leads to institutionalisation. Behavioural and Psychological Symptoms of Dementia (BPSD) are common and are core symptoms of the disease. However, research on interventions has traditionally focused on treating the cognitive components of dementia. Over 80% of people suffering from dementia experience BPSD including symptoms such as agitation and aggression. These symptoms cause considerable distress to the person with dementia and their carers and predict early institutionalisation and death. Historically, BPSD has been managed with medication, typically anti-psychotic drugs. Though effective in symptom control, recent data show that anti-psychotic medications increase mortality and the risk of stroke in people with dementia. Consequently, there is a need to evaluate the impact that non-pharmacological interventions such as physical exercise have on BPSD.
Design
A pragmatic, randomised, single-blind controlled trial. The two arms of the trail are: a graded programme of dyadic exercise (walking) in addition to treatment as usual (TAU) in the intervention arm and treatment as usual alone as the control arm.
Setting
The study will be conducted in a community cohort in the UK nested within the EVIDEM research programme.
Sample
Participants with a clinical diagnosis of dementia or ‘suspected dementia’ (age related difficulty with thinking or memory loss not related to an acute confusional episode) with at least one significant BPSD symptom defined by the Neuropsychiatric Inventory (NPI) (excluding the domains of delusions and hallucinations) will be eligible for the trial. Diagnosis of dementia will be confirmed in accordance with ICD-10 Diagnostic Criteria for Research (DCR-10).
Participants will be recruited directly through the North Thames DeNDRoN’s registry of participants with Dementia, as well as primary and secondary care, carer homes and self-referrals.
Interventions
A 6-week supervised incremental course of walking outside, at a pace and distance to suit the participant and carer for at least 5 days per week. Walking is a useful exercise which is considered acceptable by participants, and is easily delivered and measured (in terms of time and distance) but rarely incorporated in a daily routine. A physiotherapist or exercise therapist will oversee the delivery of the exercise programme. The sustainability of this intervention will be assessed by evaluating adherence and impact during an unsupervised interval (weeks 6–12) and by further telephone contact at 6 months.
Treatment as usual includes attendance at day care facilities, visits by health professionals, receipt of medication.
Outcome assessment
The primary outcome measure will be behavioural and psychological symptoms measured by the Neuropsychiatric Inventory (NPI).
Secondary outcome measures will be:
-
participants’ mental health (GHQ)85
-
participants’ quality of life (DEMQOL-Proxy)86
-
caregivers’ burden of caring (short ZBI)87
-
participants’ level of physical activity and compliance with the intervention (diaries, blood pressure and heart rate monitors)
-
economic evaluation (Client Service Receipt Inventory)88
-
participants’ and carers’ views about the intervention (Diaries and semi-structured questionnaires).
Aims and objectives of the project
Primary objective
To determine the effectiveness of physical exercise delivered through a programme of incremental walking for treating behavioural and psychological symptoms of dementia compared with treatment as usual (TAU).
Secondary objectives
-
To determine the impact of physical exercise (involving the carer) on the carers’ assessment of participant’s functioning and their quality of life.
-
To determine the effect of a dyadic walking programme on caregivers’ perceived level of burden.
-
To explore participants’ views about the intervention and barriers to treatment.
Background
Dementia and BPSD
Dementia is defined as ‘progressive brain atrophy due to nerve cell loss leading to a characteristic worsening of memory and global intellectual deterioration without impairment of consciousness’. 331 The increase in degenerative diseases through the inversion of the age pyramid in industrialised societies has brought about an increase in the prevalence of dementia. Approximately 5% of people aged over 65 years have some form of dementia, with the population prevalence rising to 20% in those aged over 80 years. 332 Over 80% of people with dementia will experience related changes of personality and behaviour, including apathy, aberrant motor behaviour, disinhibition, verbal and physical aggression. 65 Behavioural symptoms sometimes cause distress for people with dementia and their carers, and can often contribute to the breakdown in care at home leading to institutional care. 333
Conventional treatments for BPSD
Conventional treatment for dementia involves the use of cholinesterase inhibiting drugs (such as donepezil, rivastigmine or galantamine). However these drugs are expensive,90 have limited efficacy,334 minimal impact for participant related quality of life335 and the evidence base for impact on BPSD is weak. Though there is some evidence base in treating BPSD with antipsychotics (e.g. haloperidol, risperidone and olanzapine), there has been increasing concerns over the safety of these drugs for people with dementia. In the UK the Medicines and Healthcare products Regulatory Agency (MHRA) has issued a warning about the increased risk of cerebro-vascular events for people taking a course of risperidone and olanzapine. In the US, the Food and Drug Administration (FDA) has issued a warning against the use of all anti-psychotics in dementia. Therefore, in the absence of effective and safe pharmacological options, non-pharmacological alternatives should be explored and appraised more thoroughly.
Health and social care professionals are faced with a significant challenge in managing BPSD, which is critical in enabling participants to continue to live in their own homes. This is made all the more important by the demographical changes of our future. There is insufficient evidence for non-pharmacological interventions in managing BPSD sustainably, and supporting the participant and carer with a degree of safety in such a situation. The use of pharmacological interventions often poses a dilemma in balancing risk and benefit. Interventions such as the exercise dyad may offer a safer alternative, which if effective may not only have all the benefits of an effective intervention, it could also be empowering to the participant and carer due to its dyad modality of delivery.
Exercise as treatment for BPSD
Physical Exercise is defined by the American Diabetes Association as ‘physical activity or anything that gets you moving’. 336 Exercise has an impact on the cardio-vascular and musculo-skeletal systems and has been shown to have a modest positive impact on mood,74 including for those with dementia. 75
There has been some research on non-pharmacological interventions in the treatment of BPSD, mainly in the US. 337 Impact of exercise on cognitive symptoms has also been studied particularly in residential home settings in Australia338 and France. 339 Heyn et al. carried out meta-analysis of exercise in dementia and reported data on 30 trials of exercise. 76 The authors reported on trials that included strength, cardiovascular or flexibility regimes; and analysed for functional, cognitive or behavioural outcomes. The authors reported a significant positive effect size of exercise on behavioural outcomes. However, most trials included in the analysis on behavioural outcomes were relatively small (n < 65), and only two of eight reported on samples that included in excess of 100 participants. Further, exercise interventions were often combined with behavioural interventions and so it is difficult to isolate the impact that exercise has had on behavioural outcomes.
Perhaps, of greater importance, is the feasibility and sustainability of any exercise regime. Many of the trials reported in Heyn et al. ’s meta-analysis were relatively complex, required equipment or sustained interaction with a trained therapist and so are potentially not suitable for the community setting. We are aware of one trial of tailored walking for community-dwelling people with dementia–carer dyads340 that has demonstrated a significant reduction of the frequency of ‘aggressive incidents’ within the dyad. However, this trial was restricted to a relatively small sample and the impact on other important BPSD was not captured. Therefore, we propose a simple walking regime, provided it is of sufficient pace and distance, meets the criteria for exercise yet is more likely to be acceptable and sustainable in a community setting. We believe there is a need to assess the impact of a regular regime of walking on a wide range of behavioural domains by means of a randomised controlled trial.
Experimental design and methods
Study design
We will undertake a pragmatic, randomised, controlled, single-blind, parallel-group trial. Individuals with a diagnosis of dementia or suspected dementia (age related difficulty with thinking or memory loss not related to an acute confusional episode), which will be confirmed with ICD-10 criteria using the DCR-10, will be recruited to the study. In order to be eligible, participants will present with at least one behavioural and psychological symptom in one of the sub-scales of the Neuropsychiatric Inventory (NPI) (except hallucinations or delusions occurring in isolation); and will score at least 2 for severity (‘causes distress’) and 2 for frequency (‘often- about once a week’) in the NPI rating sub-scale. Suitability for inclusion into the trial is assessed, based on availability of an identified carer and ability to perform exercise regime safely.
Community-dwelling individuals including those living in residential and care facilities will be recruited through the EVIDEM network, CNWL NHS Foundation Trust and North Thames DeNDRoN.
Risk assessment will be performed to assess the suitability of participants for the intervention at baseline. Assessment will include measurement for risk of falls through Falls Risk Assessment Tool (FRAT)81 and Timed Unsupported Steady Standing (TUSS)82 If the person has neurological, mobility related or sensory impairment, which might limit their ability to involve in walking regime, attempts will be made to modify their exercise regime with advice from the Association of Chartered Physiotherapists in Exercise Therapy (UK). Acute confusional state will be assessed by The Confusion Assessment Method (CAM). 341
After confirming eligibility and obtaining patient and carer consent to treatment, initial baseline assessments will be carried out. Participants will then be randomised into one of two arms: the treatment arm which includes receipt of the intervention in addition to treatment as usual (TAU) or the control arm which receives TAU only. Both the treatment and control arms will be re-assessed for all outcomes at week 6 and week 12. At 18 weeks, qualitative data will be gathered from a smaller sample of participants using semi-structured questionnaires. Further telephone contact will occur at 26 weeks to assess adverse events and mortality, change in domiciliary status and adherence to exercise regime.
FIGURE 30.
Study design: Recruitment protocol of subjects for structured exercise as an intervention for dementia with BPSD.
Safety variables and end points
Safety variables will include falls’ risk assessments and functional abilities. Safety endpoints will be falls and significant adverse events (AEs) spontaneously reported during the study and discontinuations due to AEs.
Stopping rules and discontinuation
If there is a significant statistical difference (p < 0.05) between the number of reported AE/SAE by the intervention and control groups the TSC and PI will consider the discontinuation of the trial. See risk management procedures (section below).
Interventions
Physical Exercise will be delivered as an individually tailored regime of walking designed to become progressively intensive. This will be facilitated by a qualified exercise therapist or physiotherapist and delivered to participants in the treatment arm of the trial. The exercise therapist would facilitate physical exercise in the participant–carer dyad with the expectation that the participant–carer dyad would perform the exercise regime regularly and independently of the therapist.
Participant involvement
People with a clinician’s diagnosis of dementia or ‘suspected dementia’ (age related difficulty with thinking or memory loss not related to an acute confusional episode) with at least one significant BSPD symptom defined by the Neuropsychiatric Inventory (NPI) (excluding the domains of delusions and hallucinations) would be eligible for the trial. Diagnosis of dementia will be confirmed in accordance with the ICD-10 Diagnostic Criteria for Research (DCR-10).
We will recruit participants with dementia with an identified carer. The carer can be a family member or a professional. We shall ensure about their level of physical health and their ability to support the participant with dementia in the trial.
Recruitment base
Participant recruitment will be both retrospective and prospective: individuals with dementia known to the GP or secondary care will be identified and suspected new cases would be further investigated to confirm diagnosis. Regular reminders about the study will be sent to participating practices and secondary care teams with the network catchments area. A central register of all referrals will be maintained.
There are four potential sources of recruitment:
-
North Thames Dementia & Neuro-Degenerative Diseases Research Network (DeNDRoN) – Registry of people with dementia interested in participating in research (DemReg)
-
GP practices that are affiliated to the EVIDEM Research Group – Practices will be asked to interrogate their lists for people with a diagnosis of dementia or memory impairment
-
Memory assessment services and community mental health services, (e.g. Admiral Nurses) will be recruited via CNWL NHS Foundation Trust
-
Self-referral.
Recruitment process
An assigned clinical support officer (CSO) from DeNDRoN will inspect their registry for people that are eligible to participate in our study. This worker will identify lists of people with dementia who are eligible to participate in our study and post the following information to the person with dementia and the carer:
-
a covering letter signed by the clinical lead for North Thames DeNDRoN
-
a participant information sheet
-
a response letter and stamped addressed envelope.
The clinical support officer from DeNDRoN will contact participants by phone 1 week after the date of the mail-out to confirm interest, presence of BPSD and carer availability. The clinical support officer will ask for permission to pass on their contact details to the research worker. The CSO will provide the trial manager and research worker with details of the interested individuals within 24 hours.
GP practices recruited through the EVIDEM Research Group will be asked to inspect their patient lists using QoF (quality outcomes framework) data and READ codes to identify patients with dementia. Lists of people with dementia identified by practices, Memory Clinics and Community Mental Health Teams will be checked by their lead clinician. Practitioners will be asked for their opinion about the capacity of the person with dementia to give informed consent, using the Mental Capacity Act 2005 as the framework for their judgement.
The practices will post the following information to the person with dementia and/or the carer:
-
a covering letter signed by the lead clinician
-
a participant information sheet
-
a response letter and stamped addressed envelope.
Participants will be directed to return letters to the research team or contact the research team by phone or e-mail. A researcher will arrange to see those people with dementia and their carers who express an interest in participating in the study. During the meeting the researcher will explain the study, respond to any queries, screen for eligibility and seek consent/assent for participation in the study.
For those judged as lacking capacity to consent, a consultee will be identified and consulted about involvement in the trial. In the event that a consultee cannot be identified, the person with dementia will be excluded from participation in the trial.
The researcher will undertake an initial telephone screen to confirm interest, and likelihood of at least one BPSD symptom and then arrange an initial interview at a time and venue convenient to the participants (ideally their home). The potential participants will receive a written and verbal explanation of the trial (appendix 1). If the participant meets inclusion and exclusion criteria we will seek consent from both participant and carer, using the consent protocol. Once obtained (or in the case of an individual who is not capable of giving informed consent, the assent of the participant with agreement of carer in accordance with accepted guidelines),316 participants will proceed to the baseline interview/assessment and then be randomised. As the NPI score is an outcome this will be administered at the baseline interview once consent has been obtained along with the DEMQOL-Proxy, CSRI, GHQ, ZBI and vital signs (i.e. blood pressure and heart rate).
Inclusion criteria
-
Diagnosis of:
-
dementia in primary or secondary care or
-
suspected dementia confirmed by the researcher to ensure the DCR-10 criteria are met.
-
-
Presence of a carer (professional, friend or family member, who does not necessarily have to live with the participant).
-
NPI score in any one sub-set except only hallucination or delusion more than or equal to 2 in severity and more than or equal to 2 in frequency.
-
Consent of participant, or in the case of an individual who is not capable of giving informed consent, the assent of the participant with agreement of carer.
-
Consent of carer.
Exclusion criteria
-
Cardio-respiratory condition, neurological or musculo-skeletal condition of a degree that prevents participation to even the modified exercise regime unsafe or not possible.
-
Three or more falls in the previous year (‘frequent fallers’) assessed by FRAT or high falls risk defined by TUSS.
-
Uncontrolled medical problems, which the GP considers would exclude participants from undertaking the exercise programme; for example, acute systemic illness’ such as pneumonia, poorly controlled angina, acute rheumatoid arthritis, unstable or acute heart failure.
-
Sensory impairment to an extent that prevents dyad facilitated exercise.
-
Participant or carer dissent to engage in the exercise programme.
-
Acute confusional state assessed by The Confusion Assessment Method (CAM).
Informed consent
All participants and their carers will provide written informed consent. The Informed Consent Form (appendix 2 & 3) will be signed and dated by both the patient and carer before they enter the trial. The research worker (RW) will explain the details of the trial and provide a Participant Information Sheet, then allow the patient and carer a minimum of 72 hours to consider whether they would like to be involved in the trial. The research worker will encourage the patient/carer to ask any questions that might help them make a decision on their potential involvement in the trial.
Informed consent will be collected from each participant before they undergo any interventions (including history taking) related to the study. One copy of the Informed Consent Form will be kept by the participant, one will be kept by the research worker, and a third will be retained in the participant’s general practice records.
Where a participant who appeared to be eligible and signed a consent form is subsequently found not to be eligible (e.g. the GP considers they fulfil one of the exclusion criteria) they will not be considered to have entered the study.
Should there be any subsequent amendment to the final protocol, which might affect a participant’s participation in the trial, continuing consent will be obtained using an amended consent form which will be signed by the participant.
Compliance
Compliance will be defined as continuation with the exercise programme. This will be recorded though diarised entries, blood pressure and heart rate monitoring from carers and participants (see Section 4.7.2).
Randomisation
Patient/carer dyads will be randomly allocated to receive treatment as usual (TAU) or exercise therapy in addition to treatment as usual (ET). The randomisation ratio for the two groups will be 1 : 1 (ET : TAU). A computer algorithm will be used to perform the randomisation centrally by an Independent Researcher after the initial interview, who will communicate the results to the participant and carer, and to the exercise therapist; but not the research worker or IR.
Concealment and blinding
In order to maintain blinding, randomisation will be performed independently of the research worker (RW) following baseline evaluation. The exercise programme will be initiated and supervised by an ‘Exercise Therapist’ (ETh). Baseline and subsequent evaluations will be undertaken by RW. An independent researcher (IR) will collect the primary outcome data (NPI) at weeks 6 and 12, which will be completed by telephone with the aim of minimising the risk of un-blinding participants.
Outcome assessment
Assessments will be conducted according to Table 103. Participants will be visited in either their own homes, or a mutually acceptable convenient location (e.g. GP’s surgery), by a trained research worker (RW) masked to arm allocation. All possible measures will be taken to ensure that blinding will be uncompromised and the efficacy of this will be assessed at each time point using a 5 point Likert Scale. Both the IR and RW will complete the scale indicating to which arm they believe each individual dyad is randomised to. The scale will be polarised by the two arms and include a ‘not sure’ level as the centre rating. However, there remains a risk that the dyad will divulge information about the group they have been allocated to. The IR will collect the primary outcome measure (NPI) by telephone. This strategy has been adopted so as to minimise the risk of conversation with the dyad that might un-blind the randomisation.
| Exercise therapist (ET) | Research worker (RW) | Independent researcher 1 (IR1) | Independent researcher 2 (IR2) | |
|---|---|---|---|---|
| Exercise group only | All participants | All participants | All participants | |
| Baseline | Screens for eligibility; Obtains consent; Outcomes assessed; BP & HR at rest assessed |
|||
| T –1 | Randomisation & mails diaries and phone contact to provide instruction about diary completion | |||
| Week 1 | RPE, walk, support to complete diary | |||
| RPE, walk, support to complete diary | ||||
| RPE, walk, support to complete diary | ||||
| Week 2 | Telephone contact | |||
| Week 4 | Telephone contact | |||
| Week 6 | RPE, walk, support to complete diary | Visits: outcomes; Adverse events; BP & HR at rest assessed |
Telephone to complete NPI, remind diary completion | |
| Week 12 | RPE, walk, support to complete diary | Visits: outcomes; Adverse events; BP & HR at rest assessed |
Telephone to complete NPI, remind diary completion | |
| Week 13 | Collects sealed diaries | |||
| Week 16 | Qualitative interviews | |||
| Week 26 | Telephone contact: CSRI Change in domicile? Still exercising? Mortality assessment |
Baseline assessment
In addition to the protocol, socio-demographic data and illness characteristics, including medication, will be sought at recruitment (see Table 103). Confirmation of clinical diagnosis of dementia will be ascertained at the beginning of recruitment into the trial using the ICD-10 criteria, using the DCR manual.
All participant–carer dyads will be provided with diaries to record the level of their physical activity i.e. walking and any difficulties they encountered when walking (see appendix 2.4.1 & 2.4.2). Diaries for the intervention group will differ slightly from the control group. Thus, the dyad on intervention group will also be asked to complete a visual analogue scale (Rating of Perceived Exertion)83 to which they will be trained by the exercise therapist.
Follow-up assessments
The primary outcome assessments include change of behavioural and psychological functioning measured using the NPI. 62 This tool can also be used to derive a score which pertains to the distress caused by each behavioural domain). We can not think of any plausible mechanism by which our intervention might effect psychotic behavioural domains and so we will exclude the domains of delusions and hallucinations from our analysis.
An assessment of caregiver-rated quality of life (DEMQOL-Proxy);86 assessment of general health (GHQ)84 and a caregiver assessment of the burden of caring (short version of the ZBI)94 will also be administered. Participants general level of fitness will also be assessed as heart rate at rest and blood pressure readings.
At week 6 and 12 the NPI, GHQ, DEMQOL-Proxy, ZBI and measurement of vital signs will be repeated in both groups.
In carrying out the NPI assessment we will focus on eight of the ten domains. We will measure the difference between the treatment and control groups in agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/ability and aberrant motor behaviour. We do not expect physical exercise to impact on hallucinations and delusions. The summative mean of the 8 sub-scales is 8.08 (if hallucinations and delusions are included the figure is 8.25). In studies where NPI was measured in the placebo group a mean reduction of 2.2 was observed. 339
The phenomenology of participants’ and cares’ perspectives of exercise and barriers to treatment will be examined with open-ended, in-depth interviews. The interviews will be carried out by the research worker after the quantitative data has been analysed and the randomisation code has been breached. Theoretical sampling will be used to ensure that an initial sample is drawn to include as many as possible of the factors that might affect variability of behaviour. It is anticipated that saturation will be reached after 15–20 interviews.
| Day | Administrator | Assessment schedule | |||||
|---|---|---|---|---|---|---|---|
| Exercise dyad group | Treatment as usual group | ||||||
| Measure | Subject | Measure | Subject | ||||
| 0 | Researcher | ICD-10 | Participant+Carer Participant Participant Carer Carer Participant+Carer Carer Participant Participant+Carer |
ICD-10 | Participant+Carer Participant Participant Carer Carer Participant+Carer Carer Participant Participant+Carer |
||
| MMSE | MMSE | ||||||
| NPI | NPI | ||||||
| Demographics | Demographics | ||||||
| CSRI | CSRI | ||||||
| DEMQOL-Proxy | DEMQOL-Proxy | ||||||
| GHQ | GHQ | ||||||
| ZBI | ZBI | ||||||
| Medication | Medication | ||||||
| Vital signs (BP&HR) | Vital signs (BP&HR) | ||||||
| 0 | Independent Researcher 2 | RANDOMISATION and SEND DIARIES TO ALL | |||||
| 1–2 | Exercise therapist | RPE | Participant+Carer | ||||
| Timed walk | |||||||
| Diary | |||||||
| 3–4 | Exercise therapist | RPE | Participant+Carer Participant+Carer |
||||
| Timed walk | |||||||
| Diary | |||||||
| 6–8 | Exercise therapist | RPE | Participant+Carer | ||||
| Timed walk | |||||||
| Diary | |||||||
| 40–46 |
Independent Researcher 1
(telephone contact) |
NPI | Carer Participant+Carer |
NPI | Carer Participant+Carer |
||
| Remind completion of diaries | Remind completion of diaries | ||||||
| 40–46 | Researcher | DEMQOL-Proxy | Carer Participant+Carer Carer Participant Participant+Carer Participant+Carer |
DEMQOL-Proxy | Carer Participant+Carer Carer Participant Participant+Carer Participant+Carer |
||
| GHQ | GHQ | ||||||
| ZBI | ZBI | ||||||
| Medication | Medication | ||||||
| Adverse events | Adverse events | ||||||
| Vital signs (BP&HR) | Vital signs (BP&HR) | ||||||
| 40–46 | Exercise therapist | RPE | Participant+Carer | ||||
| Timed walk | |||||||
| Diary | |||||||
| 80–88 |
Independent Researcher 1
(telephone contact) |
NPI Remind completion of diaries |
Participant Participant+Carer |
NPI Remind completion of diaries |
Carer Participant+Carer |
||
| 80–88 | Researcher | DEMQOL-Proxy | Carer Participant+Carer Carer Participant Participant+Carer Participant+Carer |
DEMQOL-Proxy | Carer Participant+Carer Carer Participant Participant+Carer Participant+Carer |
||
| GHQ | GHQ | ||||||
| ZBI | ZBI | ||||||
| Medication | Medication | ||||||
| Adverse events | Adverse events | ||||||
| Vital signs (BP&HR) | Vital signs (BP&HR) | ||||||
| 80–88 | Exercise therapist | RPE Timed walk Diary |
Participant+Carer | ||||
| 90–98 | Researcher | Collection of diaries | Participant+Carer | Collection of diaries | Participant+Carer | ||
| 112–120 | Researcher | Qualitative interviews | Participant+Carer | ||||
| 182–196 | Researcher | Telephone contact: | Participant+Carer | Telephone contact: | Participant+Carer | ||
| CSRI | CSRI | ||||||
| Change in domicile? (hospitalisation) | Change in domicile? (hospitalisation) | ||||||
| Still exercising? | Still exercising? | ||||||
| Mortality assessment | Mortality assessment | ||||||
Statistical methods
Sample size
Based on observing a meaningful clinical difference between the groups (0.3) on the primary outcome measure (NPI), using a statistical cut off of p < 0.05 with a two tailed test, a sample size of 146 participants will give 90% power to detect this difference. (calculated by URL: http://www.stat.ubc.ca/∼rollin/stats/ssize/b2.html)
| Intervention | Difference | N (including compensation for 20% attrition) |
|---|---|---|
| 0.4 | 0.4 | 70 (88) |
| 0.5 | 0.3 | 116 (145) |
| 0.6 | 0.2 | 238 (298) |
Therefore, we aim to recruit 146 persons with dementia and their carers in this trial, 73 dyads on each arm.
Data entry
Double entry of data provided in paper form will be undertaken using automated consistency and logical checks. Data will be stored encrypted and password-protected on local drive with weekly backup. The central database will be maintained at CNWL NHS Foundation Trust head quarters. A log will be kept of data amendments. A research diary will be maintained by the researchers, which will be counter-signed monthly by the Principal Investigator.
Data protection
We will be fully compliant with the provisions of the Data Protection Act. 50 All records will be kept in a locked filing cabinet at the study centre. Confidentiality of electronic records will be ensured by password protection. The databases will be designed so that participant names are not shown on screen. Participant names and contact addresses will be deleted from the database at the earliest opportunity (for example, if a participant withdraws from the trial).
Data analysis
Baseline demographic and outcome data will be compared between the two randomisation groups. Categorical data will be analysed using the chi-square test. Continuous data will be analysed using the t-test or, if the data is found to be non-normally distributed, the Wilcoxon Rank-Sum test.
Outcomes at 6 and 12 weeks will be compared between randomisation groups using ANCOVA for continuous data. The relevant baseline scores will be included as the co-variate in order to adjust for any potential baseline differences in the respective outcome. Categorical outcomes will be compared using the chi-square test.
All data will be analysed on an intention-to-treat basis. Where appropriate imputation of missing data will be implemented. Under the assumption that data are missing at random (MAR) multiple imputation will be used.
All analyses will be completed using the statistics package STATA before the breaking of the randomisation key and the blinded analyses will be sent to a third party.
Qualitative data will be simultaneously collected and analysed. In order to enhance reliability, the analysis and coding will be carried out independently and simultaneously by two independent researchers. The analysis will be aided by the use of the NVivo™ computer software package. Line-by-line initial descriptive open coding will be carried out. Low-level categories will emerge from the data, which will be integrated into meaningful units to form higher-level categories, which will be grounded in the data. A coding paradigm will be applied to link categories with one another and create themes. The process of data collection and analysis will continue until theoretical saturation has been achieved.
Adverse events and risk management
Attrition will be carefully monitored to determine its effects on the power of the study. If a dyad withdraw from the exercise component of the trial permission would be sought to continue monitoring outcomes.
Definitions
An adverse event is any unfavourable and unintended sign, symptom, syndrome or illness that develops or worsens during the period of observation in the trial.
An AE does include a/an:
-
exacerbation of a pre-existing illness
-
increase in frequency or intensity of a pre-existing episodic event or condition
-
condition detected or diagnosed after intervention even though it may have been present prior to the start of the trial
-
continuous persistent disease or symptoms present at baseline that worsen following the start of the trial.
An AE does not include a/an:
-
medical or surgical procedure (e.g. surgery, endoscopy, tooth extraction, transfusion); but the condition that leads to the procedure is an AE
-
pre-existing disease or conditions present or detected at the start of the trial that did not worsen
-
situations where an untoward medical occurrence has not occurred (e.g. hospitalisations for cosmetic elective surgery, social and/or convenience admissions).
A Serious Adverse Event (SAE) is any adverse event occurring following study mandated procedures, having received exercise intervention or usual treatment that results in any of the following outcomes:
-
death
-
a life-threatening adverse event
-
inpatient hospitalisation for non elective procedures
-
sudden or rapidly progressive major disablement
-
an event that caused the participant to seek non-routine medical treatment.
Important medical events that may not result in death, be life-threatening, or require hospitalisation may be considered a serious adverse event when, based upon appropriate medical judgement, they may jeopardise the patient or participant and may require medical or surgical intervention to prevent one of the outcomes listed in this definition.
All adverse events will be assessed for seriousness, expectedness and causality.
A distinction is drawn between serious and severe AEs. Severity is a measure of intensity whereas seriousness is defined using the criteria above. Hence, a severe AE need not necessarily be serious.
Reporting of adverse events
All treatment related serious adverse events will be recorded and reported to the TSC and REC as part of the annual reports. Unexpected serious adverse events will be reported within the timeframes to the REC as stated below. The Principal Investigator shall be responsible for all adverse event reporting.
During the trial we will conduct monitoring of adverse events. Participants will be asked to contact the trial site immediately in the event of any serious adverse event. Adverse Events (AEs) will be brought to the attention of the study team by either:
-
Return of AE card Participants will be instructed to complete a coded, Freepost postcard each time they experience an AE, which they have not previously reported to the study centre.
-
Telephone call to the study team Participants will be encouraged to call the study team if they experience any adverse effects during the study.
-
Notification by GP Participant’s doctors will be encouraged to contact the study team of any AEs they are made aware of.
-
Notification by carer The participant’s carer will be encouraged to contact the study team, either by returning the AE postcard or by telephoning the study team, if they observe any AEs in the participant or themselves.
On notification of an AE at the study centre, the Principal Investigator will call the subject and carer for further information. The Principal Investigator shall determine seriousness and causality in conjunction with any treating medical practitioners.
All adverse events will be recorded and closely monitored until resolution, stabilisation, or it has been demonstrated that the study treatment is not the cause.
Risk management
To ensure the safety of the researchers the GP will be asked if there is a history of violence with any participants to be interviewed. In case of participants recruited from residential or nursing homes, staff will be approached for history of violence. Where participants are recruited from secondary care, similar information will be obtained from the secondary care team or CMHT. Falls risk will be minimised by assessment prior to randomisation
Risk issues:
-
inadvertent disclosure of dementia [disclosure of diagnosis rests with lead clinician (GP)]
-
carer suffering from undiagnosed dementia
-
falls (exclude high falls risk; exercise therapist to monitor; adverse event reporting)
-
cardiovascular events (exclude high risk, exercise therapist to monitor; adverse event reporting)
-
worsening of BPSD (exercise therapist to monitor; adverse event reporting)
-
accidents – exercise therapist to assess road sense and undertake location risk assessment (for exercise location) (e.g. traffic density, kerb height, risk of mugging/assault), ability of carer to manage the person with dementia safely in the streets.
Trial intervention related SAEs
A serious adverse event that is deemed directly related to or suspected to be related to the trial intervention shall be reported to the TSC and ethics committee. The reporting form for SAEs is shown in the Appendix 4.
The event shall be reported immediately of knowledge of its occurrence to the Principal Investigator.
The Principal Investigator will:
-
Assess the event for seriousness, expectedness and relatedness to the trial treatment.
-
Take appropriate medical action, which may include halting the trial and inform the sponsor of such action.
-
If the event is deemed related to the trial treatment, shall inform the REC using the reporting form found on the NRES web page within 7 days of knowledge of the event.
-
Within a further 8 days send any follow-up information and reports to the REC.
-
Make any amendments as required to the trial protocol and inform the REC as required.
Removal of participants from interventions, assessments or the trial
Participants may be withdrawn from the trial either at their own request or at the discretion of the Investigator. The participants will be made aware that this will not affect their future care.
The research team will advise discontinuation of exercise intervention or withdrawal from the trial if the exercise intervention poses a hazard to the safety of a participant, or if the participant poses a hazard to the safety of his carer or someone else.
Those who withdraw from the trial or follow-up will not be replaced. Participants should not be accepted as lost to follow-up unless 2 phone calls, letters or visits to the participant and carer have been fruitless.
Feasibility
The design allows for a total recruitment period of 24 months. We have obtained funds to recruit and employ one researcher who is based at CNWL FT HQ. Taking into account leave, sickness we expect 44 working weeks per year from the researcher. On this basis, there are 440 days for recruitment and follow-up. We believe it is possible to undertake an average of two participant contacts per day depending on location and complexity (in addition to other duties such as data entry). We estimate a maximum of 435 face-to-face participant contacts by the researcher in this trial. There will be a maximum of 725 visits by the exercise therapist.
Stopping rule
The stopping rule will be as follows: once all participants have been randomised any recruitment interviews that have been arranged will be honoured. Participants yet to be contacted, as well as those subsequently expressing an interest in the trial, will be sent a letter thanking for their interest but explaining that recruitment for the trial is now closed.
End of trial notifications
Once the trial is completed a summary of the results will be sent to the participants and to their GPs.
Ethical and regulatory aspects
Full ethical approval for the study will be sought from IRAS.
This research will be conducted in accordance with the Declaration of Helsinki. 342 Informed consent will be sought from all participants. However, due to the nature of this study, we anticipate some participants will be unable to give real consent. In the case of subjects who are not able to give informed consent, guidance from the Mental Capacity Act will be followed, and consent sought from the responsible carer. Specifically, as in all biomedical research involving human subjects, the investigators will need to obtain the informed consent of the prospective subject or, in the case of an individual who is not capable of giving informed consent, the proxy agreement of a representative, having given due consideration to advance decisions. This is also in accord with internationally accepted guidelines on research involving human subjects with dementia.
The participants’ GPs will have clinical responsibility for the participant throughout the trial. Study personnel will inform the GP of any adverse events and any significant clinical problems that are brought to the investigators’ attention.
All study records will be securely stored for 10 years after the completion of the study.
We will make every effort to maintain confidentiality of data supplied by participants. Participants will be free to withdraw from the study at any time and will be reassured that doing so will not affect their medical care.
Informed consent and participant information
The process for obtaining participant informed consent will be in accordance with the REC guidance, and GCP and any other regulatory requirements that might be introduced. The researcher and the participant or other legally authorised representative shall both sign and date the Informed Consent Form before the participant can participate in the trial.
The participant will receive a copy of the signed and dated forms and the original will be retained in the Trial Master File. A second copy will be filed in the participant’s medical notes and a signed and dated note made in the notes that informed consent was obtained for the trial.
The decision regarding participation in the study is entirely voluntary. The research worker shall emphasise to them that consent regarding study participation may be withdrawn at any time without penalty or affecting the quality or quantity of their future medical care, or loss of benefits to which the participant is otherwise entitled. No trial-specific interventions will be done before informed consent has been obtained.
The research worker will inform the participant of any relevant information that becomes available during the course of the trial, and will discuss with them, whether they wish to continue with the trial. If applicable they will be asked to sign revised consent forms.
If the Informed Consent Form is amended during the trial, the investigator shall follow all applicable regulatory requirements pertaining to approval of the amended Informed Consent Form by the REC and use of the amended form (including for ongoing participants).
Records
Case report forms
Each participant will be assigned a unique Participant Identification Number for use on CRFs, other trial documents and the electronic database. CRFs will be treated as confidential documents and held securely in accordance with regulations. The investigator will make a separate Trial Recruitment Log (TRL) to record confidential participant information including, name, date of birth and Participant Identification Number. This permits identification of all participants enrolled in the trial, in case additional follow-up is required.
CRFs shall be restricted to those personnel approved by the Principal Investigator and recorded on the ‘Trial Delegation Log.’
All paper forms shall be filled in using black ballpoint pen. Errors shall be lined out but not obliterated by using correction fluid and the correction inserted, initialled and dated.
The Principal Investigator shall sign a declaration ensuring accuracy of data recorded in the CRF.
Source documents
Source documents shall be filed at the investigator’s site and may include but are not limited to consent forms and questionnaires. A CRF may also completely serve as its own source data. Only trial staff as listed on the Delegation Log shall have access to trial documentation other than the regulatory requirements listed below.
Direct access to source data/documents
The CRF and all source documents shall be made available at all times for review by the Principal Investigator, and inspection by the sponsor [Central and North West London NHS Foundation Trust] and relevant regulatory authorities, including the R&D departments.
Ethical issues
It is possible some participants have not been formally diagnosed with dementia or have been diagnosed but have not been told, or have forgotten the diagnosis.
Quality assurance and audit
Research staff training
The researcher conducting the recruitment and outcome interviews will undergo training in administering the relevant questionnaires. In addition researcher will be trained in participant risk assessment.
Monitoring and audit
The Principal Investigator will conduct an internal audit at the study centre every 3 months to ensure: confidentiality and integrity of databases; effectiveness of database backup systems; confidentiality and integrity of paper records; reconciliation of enquiries with enquiry outcome; data entry procedures; numbers allocated to each treatment group; comparison of paper records and electronic records. Each month, the Principal Investigator, together with the study team will review the progress of recruitment by recording number of letters sent, number of enquiries received, number of calls made, number of participants entered, number of participants randomised.
The study team will comply fully with any request for external audit by the sponsors or funding body.
Indemnity arrangements
This project will be indemnified through Central North West London NHS Foundation Trust. This covers participants in the event of negligent or non-negligent harm. Standard NHS indemnities apply.
User and public involvement
User representatives will be involved in the development, implementation and interpretation of the study. This involvement will include: advice on recruiting patients, invitation letters, the design of information leaflets, and research instruments, piloting assessments, helping to assess progress, and contributing to the evaluation of the project, the interpretation of findings and the dissemination of results. User representatives will be invited to trial steering committee meetings and also provide assistance to the study.
Trial Steering Committee
The Trial Steering Committee (TSC) will provide a critical overview of the trial, and will meet at the beginning of the trial and then 6-monthly, unless the Principal Investigator needs to seek its advice on risk management, in which case a special meeting will be convened. The TSC will include the Principal Investigator (JW), the project lead (DL), researchers (RB and AP), independent representatives of relevant voluntary organisations, individuals with expertise in exercise promotion and falls prevention, a statistician, and nominees of the funding body. It will be chaired by an independent investigator with expertise in exercise promotion. Because no medicinal products are being tested and the risks of the kind of exercise that are being promoted are low, a separate data management and ethics committee will not be convened, but responsibility for overview of the risks of the trial will rest with the TSC.
Independent members
Chair – Dr Craig Ritchie, Senior Lecturer, Imperial College
Vice Chair – Clare Leonard, Physiotherapist
-
James Lee, Exercise Therapist
-
Sue Ricketts, Carer
-
Timothy Shore, Assistant Clinical Psychologist
-
Lyn Strother, Carer and EVIDEM advisory group member
-
Fiona Walters, OT
-
Lay representative
EVIDEM members
-
Dr Rahul Bhattacharya. SpR Charing Cross Psychiatric Training Scheme
-
Sandra Brookes, Lead of the Old Age Directorate in CNWL FT
-
Prof Steve Iliffe, Professor of Primary Care for Older People at UCL, and co-director of the Centre for Ageing Population Studies in the Research Department of Primary Care
-
Mark Griffin, Lecturer Medical Statistical Department, UCL
-
Dr David Lowery, EVIDEM Research Programme Manager, CNWL NHS FT
-
Dr James Warner, Consultant Psychiatrist CNWL NHS FT and Senior Lecturer Imperial College School of Medicine
-
Arlinda Cerga-Pashoja, Research Worker
Non-participant observers
-
Jane Wilcock, Senior Research Fellow and EVIDEM Research Programme Manager UCL
The steering group will meet 6-monthly (or more often if necessary).
Methods for disseminating and implementing research results
The systematic review will be forwarded to The Cochrane Library, as will the write-up of the trial for inclusion in their clinical trials register.
The trial will be published in a peer-reviewed medical journal.
Abstracts will be submitted to identified relevant conferences to inform other researchers of the work.
The support of the National Institute for Health Research (NIHR) will be credited in all publications that arise from this project in accordance with the acknowledgment and disclaimer agreed between NIHR and the EVIDEM Consortium.
Project timetable
| Year | Month | General administrative tasks | Milestones | Participant management tasks | Target recruitment (cumulative) |
|---|---|---|---|---|---|
| 2009 | March | Steering Group | Researcher starts, ethics submissions | ||
| April | |||||
| May | |||||
| June | |||||
| July | Ethics and R&D approvals | ||||
| August | Recruitment begins | 0 | |||
| September | Steering Group | 8 | |||
| October | 16 | ||||
| November | Final follow-up begins | 24 | |||
| December | 30 | ||||
| 2010 | January | 38 | |||
| February | 46 | ||||
| March | Steering Group | 54 | |||
| April | 62 | ||||
| May | 70 | ||||
| June | 78 | ||||
| July | 86 | ||||
| August | 92 | ||||
| September | Steering Group | 100 | |||
| October | 108 | ||||
| November | 116 | ||||
| December | 124 | ||||
| 2011 | January | 132 | |||
| February | Recruitment ends | 146 | |||
| March | |||||
| April | Steering Group | ||||
| May | Final follow-up ends | ||||
| June | Analysis and writing up | ||||
| July | |||||
| August | |||||
| September | |||||
| October | |||||
| November | |||||
| December | |||||
| 2012 | January | Steering Group | |||
| February | Final report/publications |
Appendix 9 Chapter 2: Consenting protocol – standard operating procedures
It is important to establish if the potential participant and their carer have the capacity to provide informed consent at each interview. It is vital that the carer can provide informed consent. If the potential participant does not have the capacity to provide informed consent, they must still provide their assent (agree to participate) alongside the informed consent of their carer.
Assessment of capacity is based on current case law applicable in England. To determine if the individual is capable of providing informed consent, it is important to test whether pertinent information about the trial and the alternative (i.e. no trial) can be understood; if it can be retained for a sufficient amount of time to weigh in the balance and reach a decision about participation; and that the person can communicate their decision to participate free from coercion. The process for doing this is as follows:
-
Understanding the trial As well as providing written information sheets, the main points of the trial and the alternative (i.e. no trial) should be provided (and discussed) verbally in a way that the potential participant can understand. These points are:
-
Dementia causes problems with memory and thinking as well as changes in mood and behaviour.
-
Problems with mood and behaviour are usually treated with drugs, which can sometimes have a negative impact on the person taking them.
-
There is some evidence that exercising may be a safe, alternative treatment to BPSD.
-
Walking is a safe way of exercising.
-
Participants will be randomly allocated into two groups: to receive either exercise therapy or care as usual for 12 weeks. There is an equal chance (50 : 50) of receiving either of these two allocations. This is because we do not know for sure if exercise works.
-
A few participants and their carers will be allocated to one further follow-up, at which they will be asked questions about their experiences of the trial. This interview will be audio-recorded.
-
A final telephone contact will occur at week 26, where all participants from the exercise therapy group will be asked a few questions about their current activity levels.
-
Participation is for 12–26 weeks but participants can withdraw at any time without having to give a reason.
-
Participants at risk of falling or who have other physical health problems that may unable them to carry out a walking programme will not be included in the study.
-
An exercise therapist will visit participants at home and establish a programme of walking outside for the participant–carer dyad.
-
The walking programme will be at a pace and distance to suit both the participant and his/her carer.
-
The exercise therapist will visit participants on five occasions. Each visit will last approximately 1–2 hours and will take place in the home of either the participant or their carer.
-
A RW will visit participants on three or four occasions. At each visit, we will complete various questionnaires.
-
All information held about them will be strictly confidential and it will not be possible to identify them from published project data.
-
They do not have to take part and if they do not, the medical care they receive will in no way be altered. They can withdraw at any time.
-
-
Retain and weigh in the balance of the information The length of time they need to retain this information for depends on the individual. If they are happy to give a decision immediately then they need to retain only the information for that amount of time. Similarly, if they ask to think about it and say that they will decide the next day then they need to retain the information until the next day. The best way to test belief and retention of information is to ask the participant to repeat back the relevant information.
-
Communicate a decision free from coercion The individual needs to communicate their decision on participation without any pressure from the carer (if applicable) or the researcher attempting to obtain informed consent.
Once this procedure is followed, the researcher can then assess whether or not she/he feels that the individual has the capacity to give informed consent. If it is decided that they are not so capable then the potential participant may be able to participate but still needs to give their assent to the study (confirmation that they are happy to participate in the absence of full capacity). Therefore, an Assent Form will be completed. Three copies of the Assent Form are then made: one to go to the participant’s GP; one to go to the carer; and one to be kept at the study centre.
Participants with established capacity to consent will complete the Participant’s Consent Form. One copy of the form will go to the participant’s GP; one to the participant; and one to be kept at the study centre.
The carer will complete a Participant–Carer Consent Form, at all occasions. One copy will be kept by the carer and one will be kept at the study centre.
Appendix 10 Chapter 2: Standard operating procedure for monitoring of adverse events
Classification of event
All adverse events will be classified by the Principal Investigator as either:
-
Adverse event (AE) Any unfavourable and unintended sign, symptom or disease temporarily associated with the use of an investigational product, whether or not considered related to the investigational product.
-
Serious adverse event (SAE) An AE that results in death, or is life threatening, or required in-participant hospitalisation, or results in persistent or significant disability or incapacity.
Classification of intensity
Regardless of the classification of AEs as serious/non-serious, the maximal intensity of the event is to be evaluated by the responsible clinician, after consultation with the participant, carer and participant’s GP:
-
Mild no impairment of normal daily activities
-
Moderate impairment of normal daily activities
-
Severe unable to perform normal daily activities.
Classification of causal relationship
Each AE must be classified on the basis of the available data with regard to the presumed causal relationship to one of the following categories:
1 = probable
-
Rational relationship to the time of intake of the investigational medication.
-
AE is already known to be a side effect of the investigational medication or may be expected.
-
Regression or disappearance of the AE after discontinuation of investigational medication or dose reduction.
-
Reappearance of the AE after repeated exposure.
-
AE cannot be explained in a reasonable manner by the clinical state of the participant.
2 = possible
-
Rational relationship to the time of intake of the investigational medication.
-
AE is already known as a side effect of the investigational medication or may be expected.
-
AE could be explained by numerous other factors.
3 = improbable
-
Rational relationship to the time of intake of the investigational medication.
-
AE has not been reported so far as a side effect of the investigational medication or cannot be expected.
-
AE persists after discontinuation of the investigational medication or dose reduction.
-
Repeated exposure does not lead to reappearance of the AE.
-
AE could be explained by numerous other factors.
4 = no relationship
-
No rational relationship to the time of intake of the investigational medication.
-
AE is evidently caused by other factors, for example symptom of a concomitant disease.
5 = unable to evaluate
-
Amount and content of data do not permit a judgement of the relationship to the investigational medication.
Appendix 11 Chapter 2: Risk assessment and management tool
Appendix 12 Chapter 2: Serious adverse event reporting form
Appendix 13 Chapter 2: Risk management pathway
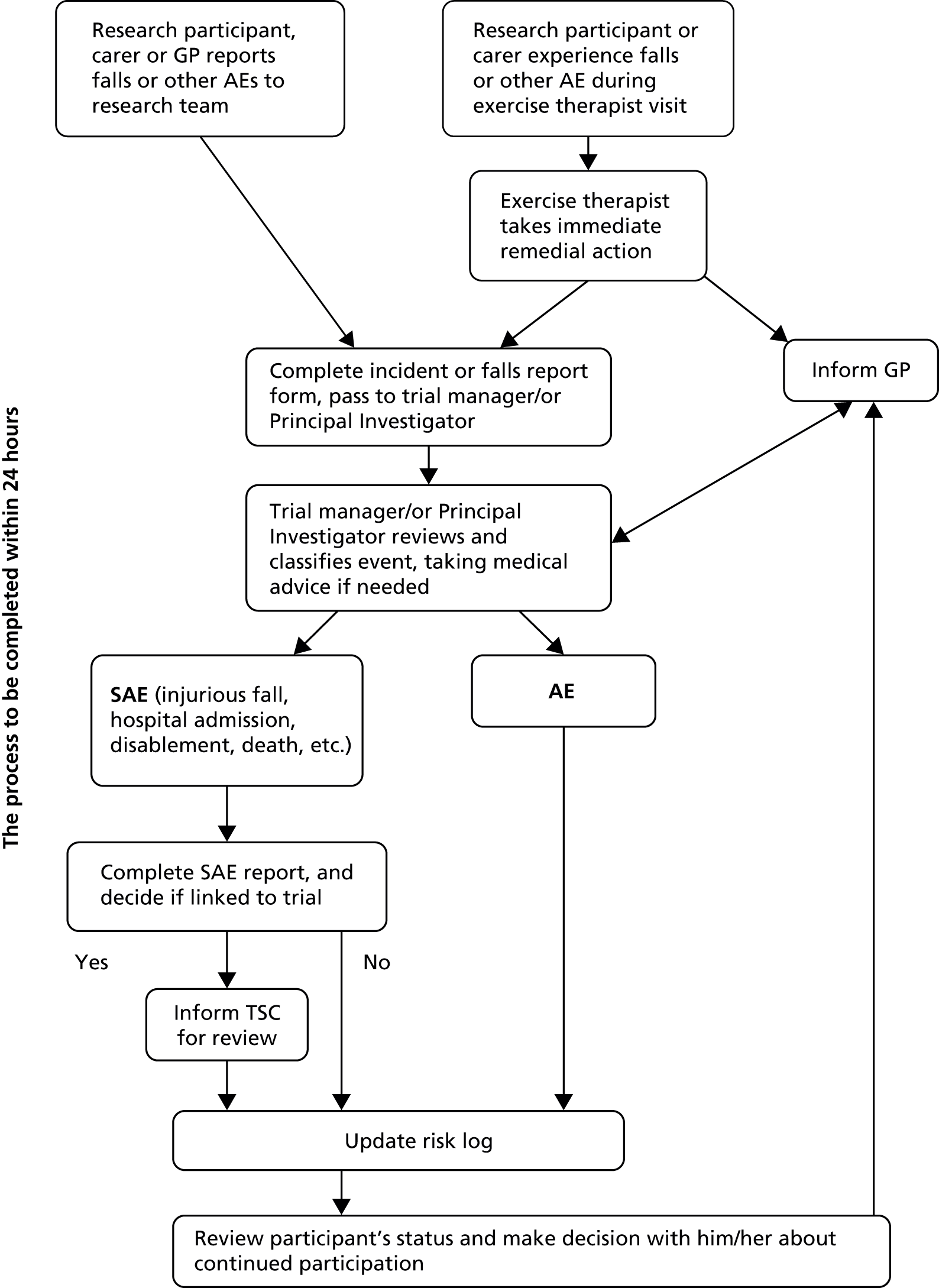
Appendix 14 Chapter 2: Diary – intervention group, example page
Appendix 15 Chapter 2: Diary – control group, example page
Appendix 16 Chapter 2: Intervention protocol
Within 5 working days of randomisation, the consultant is to visit the participant in the participants own home at a time that is convenient to the participant and carer (Visit 1):
-
explain the exercise regime to carer and patient
-
explain diary including the use of a visual analogue scale for Rating of Perceived Exertion (RPE) (Borg)83
-
carry out local risk assessment for Carer–Patient dyad performing walking independently of study personnel
-
where risk is identified, the consultant agrees to record and report such risks to the Trial Manager within 24 hours
-
-
join Carer–Patient dyad on walk of approximately 20–30 minutes (depending on ability) and record time and distance
-
assess RPE and extend exercise to 60–70%, or 12–14 on 20-point scale
-
support completion of diary and facilitate participant completion of RPE
-
inform the Randomisation Officer when the first visit has been completed.
At Visits 2 (must be between 3 and 5 days of first visit) and 3 (must be within 8–10 days of first visit) – the consultant will:
-
inquire and document the dyads’ compliance with the exercise regime, and re-explain the regime/diary/RPE where required
-
identify any barriers to adherence and suggest solutions where possible
-
give general encouragement to maintain the exercise regime.
Up to 28 days from the first visit the consultant will respond to the participant–carer dyad by telephone on an ‘as needs basis’ for information about the exercise programme only.
-
Where participants’ requests extend beyond the exercise regime or the 28-day time period, the consultant will provide them with the contact details of the Trial Manager and log the contact with the Trial Manager within 48 hours.
-
The Consultant will respond to any carer/patient telephone call within 48 hours.
At Visits 4 (must be within 41–45 days of first visit) and 5 (must be within 82–86 days of first visit) – the consultant will:
-
join carer–participant dyad on walk of approximately 20–30 minutes (depending on ability) and record time and distance
-
support completion of diary and facilitate participant’s completion of RPE.
Appendix 17 Chapter 3: Overall EVIDEM-C protocol 2007
Introduction
Urinary and faecal incontinence in people with dementia is a major challenge for carers and professionals in supporting a person with dementia in their own home and a common trigger for admission to long-term care. Continence research and evidence-based guidance either explicitly excludes people with dementia (see for example NICE 2006)122 or addresses those in care home settings (see for example Ouslander et al. 2005). 343 Current evidence-based guidelines on primary care management of dementia does not address incontinence management or its contribution to carer burden. 344 This study is part of a wider programme of research and has the ultimate goal of developing and field testing evidence-based tool kit for the management of urinary and faecal incontinence with carers, community nurses, social care workers and home care staff. The elements within this study include: an investigation into the prevalence of incontinence problems in people with dementia living at home, the identification and testing of feasible and acceptable interventions for continence management in the home, and the development of educational packages tailored to informal carers and different groups of specialist and generalist health and social care professionals.
Background and rationale
Dementia affects over 4% of the people over 60, increasing to 13% for those over the age of 80 in Western Europe with significant projected increases in the next 20 years. 345 Dementia has enormous impact not only for the individual and their family but also for the health and social care system. 346 The median length of survival from diagnosis to death is 8 years, with a trajectory of progressive deterioration in cognition, abilities and physical functioning. In the later stages of the condition, people with dementia can present with complex and challenging problems including aggressive behaviour, restlessness and ‘wandering’, eating problems, incontinence, delusions and hallucinations, and mobility difficulties. Urinary and faecal incontinence are frequent symptoms as the disease progresses. Incontinence is the involuntary leakage of urine or stool or both. 347,348
The prevalence of any type of urinary incontinence in all older adults is between 6% and 10%, with increasing rates associated with old age. 349 It is estimated that 2–5% of adults experience faecal incontinence. 350 Incontinence lowers quality of life and impacts negatively on mental health351,352 as well as creates significant practical and financial problems. There is evidence that incontinence contributes significantly to other major health issues for older people such as falls. 353 Incontinence has been identified by professional and older people as an issue of unmet need in primary care. 354–356
A United Kingdom (UK) population-based study of 15,000 home-dwelling people aged over 75 identified that 18.3% had cognitive impairment and of these 31% had urinary incontinence problems. 135 A national general practice audit of 999 older patients with faecal incontinence identified that 27% had a diagnosis of dementia. 357 Estimates suggest that about 60% of people with cognitive impairment live in their own homes. 358 For this group, there are no data on the prevalence over time of faecal incontinence or urinary incontinence problems to aid in service planning for the support of continence management or estimating cost consequences for households or primary care organisations.
Incontinence problems have been identified as a significant factor in increasing informal carer burden and triggering the admission of people with dementias to care homes. 110,134,356 There is little literature that explores the nature and impact of managing bladder and bowel problems in people with dementia at home by informal carers. Two small scale qualitative investigations into informal carers views (n = 12) on managing frail spouses’ incontinence suggested individual problem solving strategies359,360 that contributed further to carer stress such as decreasing their outside social contact. There is an absence of information that identifies both those aspects of incontinence problems and also the carer and service contexts that contribute to the admission to care homes. Anecdotal evidence suggests that faecal incontinence is the tipping point as far as carers are concerned whether they are able to continue supporting the person with dementia. 361 Evidence from care home settings suggest that carers had difficulty interpreting what the person with dementia wanted and therefore how best to manage their continence e.g. repeated requests to be taken to the toilet and refusal of medication to ease constipation. 362
Assisting older people remain in their own homes, improving continence care and addressing carer burden are priorities for the health and social care improvement and service frameworks. 363–365 People with dementia receive services from both generalist primary care services and specialist mental health care of older people. They may also receive support from social workers (generalist or specialist) and publicly funded social care assistance. Incontinence is a clinical issue that often has low priority in generalist primary care services and specialist mental health care services. Specialist continence services are not currently available in all areas of the UK. 366,367 However, there is no current evidence-based guidance for addressing problems of incontinence in people with dementia living at home. Evidence-based guidance in continence management has been developed by a number of agencies111,122,156,365,368 however, these either point to the evidence gaps in treatment and management for frail older people (e.g. SIGN 2004)121 or exclude people with cognitive impairment (e.g. NICE 2006). 122 Current evidence-based guidelines on primary care management of dementia does not address incontinence management or its contribution to carer burden. 344
Aims and research questions
The aims of this study are to:
-
identify the prevalence of different types of incontinence problems experienced by people with dementia and the associated cost consequences over a 3-year period
-
identify and test feasible and acceptable interventions for continence management in the home.
The specific research questions are:
-
What is the prevalence of different types of incontinence problems experienced by people with dementia, living at home, and their carers, over a 3-year period?
-
What is the evidence for different strategies and interventions in managing incontinence in people with dementia living at home?
-
What are the perceived factors that support or detract from the use of different interventions (identified in question 2) from the perspective of informal carers, generalist and specialist health-care staff, social workers and social care?
-
Are the interventions (identified in questions 2 and 3) feasible, acceptable, effective and appropriate in the home setting from the perspective of the person, their carer(s), professionals and public service delivery?
Method
The overall research approach is one of critical realism369 which allows an integration of both subjective and objective research approaches and makes explicit the interrelationships between context, process, interventions (mechanisms) and outcomes. 370 In taking this approach, the multifaceted nature of the issues and problems in managing incontinence in the home, as well as the perspectives of multiple and diverse stakeholders can be examined in the light of the evidence of effectiveness. This research approach allows the study to acknowledge the complexity of the issues. The study therefore draws on more than one methodology for generating evidence and knowledge. There are three elements leading to the fourth element of producing and field testing educational packages tailored to informal and formal carers and different groups of specialist and generalist health and social care professionals. The three elements are:
Element 1 The establishment of period prevalence of different types of incontinence problems in a community-dwelling cohort through interview survey at three points during the 5-year programme span. This data will be obtained as part of the broader set of enquiries on ‘transitions’ undertaken with a sample of people and their carers enrolled in the parent cohort study.
Element 2 The development of feasible and acceptable interventions for incontinence management through:
-
A systematic review of the evidence, which integrates evidence from both quantitative and qualitative, approaches (Dixon–Woods et al. 2004). 371
-
Qualitative exploration of people with dementia, carers and professionals views of problems and potential interventions through:
-
Up to 25 (or until saturation is reached) semi-structured individual interviews and four group interviews with informal care givers using adapted structured focus group techniques134 to explore perceptions of the problems and acceptability or consequences of different types of interventions.
-
Up to 10 exploratory discussions (rather than semi-structured interviews) with people with dementia regarding their perceptions of problems with going to the toilet or lack of control.
-
Up to eight group interviews with health and social care professionals employing adapted structured focus group techniques125 on their perceptions of the problems and acceptability or consequences of different types of interventions.
-
Element 3 The testing of the identified interventions for feasibility, acceptability and cost consequences372 with up to 40 people with dementia (the exact number to be determined following the evidence revealed by one and their carers over 12 months). The detailed methods for element 3 are dependent on results from element 2. A subsequent protocol will be developed for this.
Element 1 method
This element addresses research question 1. The research design for this element is a repeated descriptive survey. It is a nested study within the broader cohort ‘longitudinal study of transitions’, which addresses a broader range of health and social topics. The survey questions are repeated annually over 3 years. The rationale for embedding this element in a broader study is that most adults find discussion of bowel and bladder problems deeply embarrassing and often shameful. Adults are unlikely to volunteer to participate in a study that focuses on this issue in isolation; however, they are likely to be more willing to discuss it in the wider contexts of their daily life.
The sample
The sample will be people with cognitive impairment, living at home, and their carers within the cohort who have agreed to participate in the ‘longitudinal study of transitions’. The recruitment for this longitudinal study will be recruited via health, social work and social care professionals. Recruitment and consent processes are in line with the overall EVIDEM programme principles.
The data collection and tools
There are no data collection tools on incontinence specifically validated for a population of cognitively impaired people. The study advisory group and user group will provide expert opinion on drawing together the most appropriate and acceptable validated tools (e.g. Leicester Urinary Symptom Questionnaire)373 plus additional questions to describe symptoms, severity, impact, causes and contributing factors e.g. motor/mobility deficits, behavioural problems, management strategies and associated costs. While population-based surveys of incontinence problems have found that postal methods are acceptable (Perry et al. 2002350) it is unlikely to be feasible with this group of people and their carers. It is anticipated that this will be an interviewer delivered survey and that the interviewer will proceed with the interview in conversational ways rather than as a checklist set of questions which are more likely to distress and agitate people with dementia. The survey questions on this topic will be piloted as part of the wider longitudinal survey and amended as necessary. The survey interviews will be undertaken at three points in time over 3 years. The feasibility of carer completed diaries of continence related problems will also be explored as these have been used successfully in some other studies. It is possible that after the first survey, some carer informants may prefer and be willing to complete a postal survey.
Data analysis
The data will be entered on to a SPSS™ software programme and analysed descriptively in the first instance and subsequently for correlates between types of incontinence, management strategies and demographic and mental and physical condition factors.
Timetable
| Year 1 | Year 2 | Year 3 | Year 4 | |
|---|---|---|---|---|
| Advisory group and user group | ✗ | ✗ | ✗ | ✗ |
| Developing data collection tools | ✗ | |||
| Ethics and research governance (linked to broader study) | ✗ | |||
| Recruitment and piloting (linked to broader study) | ✗ | |||
| Recruitment and data collection and analysis time period 1 | ✗ | |||
| Data collection and analysis time period 2 | ✗ | |||
| Data collection and analysis time period 3 | ✗ | |||
| Overall data analysis and report writing | ✗ |
Element 2 method
This element addresses research questions 2 and 3.
The systematic literature search
A systematic review of the published and unpublished literature on continence management community-dwelling older people with dementia and their carers will be undertaken using methods developed by the Cochrane Collaboration374 and the Social Care Institute for Excellence,375 incorporating both qualitative and quantitative evidence. The scope of the review will include:
-
studies that offer continence care, management and educational-based interventions with people with dementia and their carers in primary care settings including patients’ own home
-
studies that consider services operated within developed health economies and focus on research undertaken in the UK, Europe, Australasia and North America where there is a similarity in the age distribution of the populations and comparable methods of health-care delivery.
Excluded from the review will be:
-
Studies that are hospital based and nursing home based. It is not within the scope of this review to include foreign language papers.
The review will then proceed in stages:
-
Search the literature across a wide range of health and social care electronic databases including MEDLINE, EMBASE, CINAHL, PsycINFO BNI, CAREDATA, Cochrane Library (including DARE, NTIS, SIGLE), Social Science Citation index, Age Info, National Research register, Papers First (conference presentations) and the specialised register of the Cochrane Effective Practice and Organisation of Care Group (EPOC), Dissertation Abstracts, Department of Health and similar websites. Preliminary searching will begin with a strategy based on keyword/index (MeSH) terms. The two main themes will be dementia and incontinence. In addition ‘lateral searching’ techniques will be used such as checking reference lists of relevant papers, and using the ‘Cited by’ option on WoS, Google Scholar and Scopus, and the ‘Related articles’ option on PubMed and WoS, as recommended in searching for studies of complex interventions. 126 In addition, leading researchers and expert practitioners in the field will be contacted to help identify unpublished research. Key publications linked to dementia care and incontinence management that are not indexed on databases will be hand searched.
-
Abstracts and brief records from databases will be screened by two researchers for relevance to the research questions 2 and 3 and filed in a bibliographic management package (RefMan 10).
-
A common data extraction sheet will be developed for study quality appraisal and evidence relevant to research questions 2 and 3 (above). Each retrieved study will be assessed by two researchers independently. The initial aim will be to identify and classify interventions to incontinence care for community-dwelling older people with dementia.
-
The evidence will be synthesised and a report produced.
The findings from the review will inform the second element of this phase.
Method: the exploration of perceptions of feasible and acceptable interventions for continence management (the qualitative interview studies)
This stage uses qualitative methods to explore perceptions of feasibility and acceptability of different types of strategies for incontinence management from the perspectives of the three groups of key stakeholders: the people with dementia, the informal carers of people with dementia and professionals (generalist and specialists) providing services to people with dementia in their own home. Three data collection methodologies will be used by:
-
up to 25 (or until saturation is reached) semi-structured individual interviews and four group interviews with informal care givers using adapted structured focus group techniques125 to explore perceptions of the problems and acceptability or consequences of different types of interventions
-
up to 10 exploratory discussions (rather than semi-structured interviews) with people with dementia regarding their perceptions of problems with going to the toilet or lack of control
-
up to eight group interviews with health and social care professionals’ employing adapted structured focus group techniques125 on their perceptions of the problems and acceptability or consequences of different types of interventions.
Sample recruitment
The study user advisory group will be consulted on suitable wording for recruitment materials and data collection tools. Recruitment for carers and people with dementia will take place via local Alzheimer’s Society branches. Recruitment of professionals will take place through local service generalist and specialist services. Each structured focus group will have between 8 and 12 participants.
Data collection and analysis
The structured focus group interviews will be led by an experienced researcher, with another researcher present to take research field notes. 376 The lead researcher will record the key issues and views on flip chart, using some of the techniques of nominal group technique (NGT)43 to focus the discussions on the key research questions. With permission the group discussion will be taped as an aide to the record of the group which will be typed up from the flip charts and the filed notes. The record of the groups interviews will be analysed in NVivo™ software using a template methodology,377 derived from a priori theories derived from the literature review but also allowing new themes and issues to be identified. The semi-structured interviews will be guided by an aide-memoire125 developed from the literature in the first phase. Interviews will be recorded with permission, transcribed (tapes destroyed) and analysed with the same template framework as used for the NGT groups using NVivo™ software. The exploratory discussions with people with dementia are forms of semi-structured interviews that are much more flexible in their approach, using conversational techniques rather than lists of questions,378 to assist participation by people with dementia. The exploratory discussions will focus around views and feelings related to bladder, bowel and toilet problems rather than questions that require detailed recall. 379 With permission the discussions will be recorded, transcribed (recording destroyed) into the NVivo™ software package and analysed as described above. The findings between the different stakeholders will be compared and contrasted and the evidence used with that of the findings from the systematic review to design element 3.
Ethical considerations
Participants’ information sheets will be developed in consultation with the research advisory and study user reference group. Participants will be recruited having been provided with full information about the study, its processes and data uses. The consenting process for people with dementia will conform to that agreed for the EVIDEM research programme and for all participants researchers will make it clear that individuals will be free to withdraw at any point in the research process. The Individuals in interview process will be assured confidentiality and anonymity in data processing (through assignment of a study identification number) and subsequent reporting. Participants in group interviews will be known to each other and each interview will start with group agreements on the level of confidentiality those participants feel comfortable with. Quotations from participants may be used but in a way that will not reveal identities. All data will be kept on password protected computers and locked filing cabinets. Each participant will be made aware that the researchers have a duty of care should any information be revealed that suggests a vulnerable adult is being neglected or abused in any way.
Timescale
| Year 1 | Year 2 | Year 3 | Year 4 | |
|---|---|---|---|---|
| Advisory group and user group | ✗ | ✗ | ✗ | ✗ |
| Systematic literature review | ✗ | |||
| Ethics and research governance for element 2b | ✗ | |||
| Data collection 2b interviews and focus groups | ✗ | |||
| Data analysis and report writing | ✗ | ✗ | ||
| Development of protocols for element 3 | ✗ | ✗ |
Establishment of research and user advisory group
It is anticipated that a group of professionals with interests in the care of people with dementia and incontinence and service users/carers will be formed to advise on the conduct of this work stream. It is anticipated that there will be an initial and annual meeting but most work will be undertaken by e-communication.
Dissemination
The disseminations strategy is part of the overall EVIDEM strategy to submit material for lay and professional audiences, participate in Central and North West London NHS Foundation Trust research events and submit to present orally in relevant conferences.
Appendix 18 Chapter 3: Protocol – exploring issues and solutions in promoting continence and managing incontinence with people with memory problems living at home and their carers, 2008
Introduction
Urinary or faecal incontinence are distressing symptoms for any adult that impact on many facets of an individual’s health and quality of life. Professional knowledge and public awareness is increasing the availability of an evidence base in treatment and best practice. 121,362,380 However, people with memory problems and dementia living at home are one group for whom there is little guidance on promoting continence or managing incontinence. Incontinence problems are a common element in the decisions to move into a care home. 134,356 Continence research and evidence-based guidance either explicitly excludes people with dementia (see for example National Institute of Clinical Excellence 2006)122 or more commonly includes only those people living in care home settings (see for example Ostaszkiewicz et al. 2004). 368 Evidence-based guidelines produced in 1998 for primary care staff on supporting people with dementia and their carers did not address incontinence management. 123 The recent NICE–SCIE guideline on supporting people with dementia and their carers124 does not review evidence in relation to continence promotion but offers the view that combined interventions are more likely to support and maintain independence in the person with dementia.
EVIDEM C is a part of the NIHR funded programme of research (Principal Investigator Professor Steve Iliffe, University College London). It has the ultimate goal of developing and field testing an evidence-based resource for the promotion of continence and management of urinary and faecal incontinence with carers, community nurses, and social care staff.
Background and rationale
Dementia affects over 4% of the people over 60, increasing to 13% for those over the age of 80 in Western Europe with significant projected increases in the next 20 years. 345 Dementia has enormous impact not only for the individual and their family but also for the health and social care system. 119,296,346 The median length of time from diagnosis to death is 10 years for those under 65 at diagnosis and 4 years for those over 80 years. 173 The clinical syndrome of dementia has a trajectory of progressive deterioration in cognition, abilities, and physical functioning. The impairment experienced by the individual is often compounded by extrinsic factors such as attitudes of ‘therapeutic nihilism’115 in professionals, unadapted environments, and social exclusion. As the condition progresses problems may arise in maintaining independence in going to the toilet, in avoiding constipation, and in managing incontinence problems that cannot be resolved. Estimates suggest that there are 500,000 people with dementia in England and two-thirds live at home. 119
Incontinence is the involuntary leakage of urine or stool or both. 347,349 The prevalence of any type of urinary incontinence in all older adults is between 6% and 10%, with increasing rates associated with old age. 349 It is estimated that 2–5% of adults experience faecal incontinence. 350 Incontinence lowers quality of life and impacts negatively on mental health351,352 as well as creates significant practical and financial problems. There is evidence that incontinence contributes significantly to other major health issues for older people such as falls. 353 Incontinence has been identified by professionals and older people as an issue of unmet need in primary care. 354,355,366
A UK population-based study of 15,000 home-dwelling people aged over 75 identified that 18.3% had cognitive impairment and of these 31% had urinary incontinence problems. 135 A national general practice audit of 999 older patients with faecal incontinence identified that 27% had a diagnosis of dementia. 381 Estimates suggest that about 60% of people with cognitive impairment live in their own homes. 358
For this group, there are no data on the changes over time in continence status to aid in service planning for the support of continence management or estimating cost consequences for households or primary care organisations.
Incontinence problems have been identified as a significant factor in increasing informal carer burden and triggering the admission of people with dementias to care homes. 110,134,356 There is little literature that explores the nature and impact of managing bladder and bowel problems in people with dementia at home by informal carers. Two small scale qualitative investigations into informal carers views (total n = 12) on managing frail spouses’ incontinence359,360 suggested that individual problem solving strategies might contribute further to carer stress through decreasing their outside social contact. There is an absence of information that identifies both those aspects of incontinence problems and also the carer and service contexts that contribute to the admission to care homes. Anecdotal evidence suggests that faecal incontinence is the tipping point as far as carers are concerned whether they are able to continue supporting the person with dementia. 361 Evidence from care home settings suggest that formal carers had difficulty interpreting what the person with dementia wanted and therefore how best to manage their continence, e.g. repeated requests to be taken to the toilet and refusal of medication to ease constipation. 362
Assisting older people to remain in their own homes, improving continence care and addressing carer burden are priorities for the health and social care improvement and service frameworks (DOH 1998, 2000, 2001). 363–365 People with dementia receive services from both generalist primary care services and specialist mental health care of older people. They may also receive support from social workers (generalist or specialist) and publicly funded social care assistance. Incontinence is a clinical issue that often has low priority in generalist primary care services and specialist mental health care services. Specialist continence services are not currently available in all areas of the UK. 356 Evidence-based practice guidance is not currently available to assist professionals and staff in generalist services in their support of people with dementia living at home and their carers.
Study aim and research approach
This study draws on the Medical Research Council (MRC 2000)56 Framework for developing complex interventions in using stepwise phases. The study has a number of linked phases commencing with an integrative review of the literature,367 which is currently in progress. The research approach is one of critical realism369 which allows an integration of both subjective and objective research approaches and makes explicit the interrelationships between context, process, interventions (mechanisms) and outcomes. 370 In taking this approach, the multifaceted nature of the issues and problems in promoting continence and managing incontinence in the home, as well as the perspectives of multiple and diverse stakeholders can be examined in the light of the evidence of effectiveness. This research approach allows the study to acknowledge the complexity of the issues.
The research questions for this phase of the study are:
-
What is the evidence for different strategies and interventions in promoting continence and managing incontinence in people with dementia living at home? (Addressed by the integrative review of the literature.)
-
What is the experience and perceptions of people with dementia and their close carers regarding the problems and successful strategies for promoting continence and managing incontinence at home?
The results from this phase will then be used to develop feasibility studies of potential interventions, which will be submitted for peer and ethical review.
The development of this protocol has been informed by the EVIDEM and EVIDEM-C advisory group, which involves service users and carers.
Method
The methodology is qualitative, in the interpretive tradition, using individual and group interviews.
The sample and recruitment
Informal carers and people with dementia (up to 55 people) will be recruited through (a) invitation letters in local North London Alzheimer’s Society branch newsletters and (b) invitation letters being distributed to service users with memory problems and their carers, who might be interested in participating, by professional staff in the day and community services for older people with mental health problems (primarily those provided by Central and North West London NHS Trust). It is anticipated that people with dementia likely to be willing to participate would be at the mild to moderate stage of the disease progression.
Those individuals willing to participate will make contact with the researcher who will send them the participant information leaflet and arrange a time to meet at their home or another community service venue convenient for them. At the meeting the research will be explained and consent sought to participate (see section below on detailed ethical considerations and mental capacity).
Data collection and analysis
Data will be gathered through:
-
Up to 25 (or until saturation is reached) semi-structured individual interviews with close care givers to explore perceptions of the problems, their criteria for success in promoting continence and managing incontinence, and their experience of successful strategies in promoting continence and managing incontinence. These will be face to face or by telephone as the person chooses.
-
Up to four group interviews (approximately five per group) with close care givers using adapted structured focus group techniques369 to gain their views on the feasibility and acceptability of different types of strategies/interventions identified from the literature. From the data gathered, individual interviews will be undertaken with carers and professionals. If group interviews are not feasible logistically or in terms of numbers then individual interviews (face to face or by telephone as the person chooses) will be conducted.
-
Up to 10 exploratory discussions164 with people with mild to moderate dementia regarding their feelings and experience of problems with going to the toilet and different types of help.
Semi-structured interviews with individuals will use an aide memoire to ensure key themes are explored. Interviews will be taped, with permission, transcribed and the tapes deleted. Each transcription will only be known by an identifying number not the person’s name and all items that might identify the informant, such as names, will be made anonymous. The transcriptions will be entered into a software programme, NVivo™, to aid data management. Content analysis370 will be undertaken by two researchers, independently and compared, to aid validation of the analysis.
Small group interviews will be conducted by two researchers. One will lead the discussion using the same topics as in the aide memoire and the other will take notes. The group’s discussion points will be summarised on flip chart to aid verification from the group of the issues raised and views expressed. The notes and flip chart will be transcribed into word documents and entered into NVivo™ for cross comparison and analysis between groups and themes from individual interviews.
Exploratory discussions with individuals with dementia will be much more conversational and centre on two questions:
-
What sort of problems do you have with going to the toilet? and
-
What helps you when you have these problems?
It is anticipated that the conversation is likely to be in the presence of the person’s spouse/close carer if they have one. The emphasis in the exploratory discussion will be on experiences and feelings not ‘facts’. Exploration on views of different types of help will only be explored if the person is comfortable with the discussion and willing to continue. The conversation will be taped, with permission. If permission is not given, permission for taking notes will be sought. The tapes or notes will then be treated in the same way as the transcriptions of the semi-structured interview as above.
Ethical considerations
The research questions under consideration are directly relevant to people who have dementia and it is important to incorporate views of those people experiencing the problems not just the professional views or their family members. 382 People who have dementia may lack capacity to consent. Only those people with dementia with the capacity to make the decision to participate in the research will be included. The procedure for establishing the ability to make the decision will proceed in accordance with the guidance for the Mental Capacity Act 2005. People with dementia will only be approached after their close family carer or a professional who knows them well enough to judge their ability to consent to participation in the study, has discussed the research first and asked whether they would be willing to meet the researcher. This person will explain about the research and the exploratory discussion process using the information leaflet which has been adapted to provide key points in straight forward language. After consent to approach the person has been gained, the researcher will arrange to meet them with their carer or supporting person of their choice. She will repeat the explanations, using the leaflet and check the person is able to consent at that point in time. Key elements in checking will be: whether the person has general understanding of what they are consenting to and why they are being asked to make it, whether the person understands what will happen if they agree to participate in the study, whether they are they able to understand the verbal and/or written information relevant to making a decision whether to participate in the study. A consent form for signing will be offered, although if the informant is unwilling to sign (writing may be difficult) this will not be pursued and verbal consent will be noted. Any sign (verbally or in body language) that the individual is not happy about taking part or continuing to take part in the research will be interpreted as withholding consent, or a desire to withdraw. The researcher will end the conversation, thanking the person for their time and involvement. The researcher will check throughout the discussion that the informant is willing to continue and cease if the person indicates they wish the discussion to end.
Urinary or faecal incontinence are distressing symptoms that are embarrassing to discuss for any adult. Undertaking intimate care associated with incontinence for an adult spouse, relative or loved one is a sensitive issue. All interviews will be conducted by researchers aware of the potential socially problematic nature of the issues that are being discussed. Interviewers will use language understandable and acceptable to the interviewee and make all efforts to lessen any discomfort in discussing the issues. Should an individual become distressed then the interviewer will pause, be sympathetic, and check whether the person wishes to discontinue the interview. The researcher will encourage informants to share their problems and distress with their key health professional, or family doctor as appropriate. All researchers will have contact information on key support agencies to leave with informants.
All informants will be assured of anonymity and confidentiality in the transcription, analysis and reporting of their interview. Any direct quotations used in the report will be non-attributable. At the start of the group interviews, informants will be asked to respect that views and opinions expressed in the interview are held confidential to the group.
Informants will be made aware that if any information is shared that suggests that a vulnerable older adult is suffering neglect or abuse then the researcher has a responsibility to share that information with the service manager (in case of abuse or neglect from a service provider) or in the case of individuals gain their consent to share that information with the named Local Authority Officer for vulnerable adults.
Data protection considerations
All participants will be assigned a code number and this with their contact details will be kept separately and securely from any data collected in the process of the research. This file will be registered with the University data manager. All research data will be identifiable by code number alone. Audiotapes will be transcribed and deleted. The transcribed interviews will be entered stored on a password protected computer, accessible only to the researchers in locked offices the University. Hard copies of data will be stored in locked filing cabinets in the same offices. Research data will be archived for 5 years and then destroyed.
Timescale
Interviews – July and August 2008
Analysis – August and September 2008
Report writing – September 2008
Feedback to participants – end of September 2008
Reports and dissemination
The findings will be written up as a brief report for circulation to participants. A full report will be used as the basis for papers and articles to be submitted to a professional journal and local and national voluntary organisation’s newsletters. An abstract will be submitted for presentation at a service users and professional conference, such as Dementia Congress.
Appendix 19 Chapter 3: Protocol – professional views of current issues and solutions in promoting continence and managing incontinence with people with memory and cognition problems living at home
This protocol concerns the investigation of the views of health and social care professionals and staff of current issues and solutions in promoting continence and managing incontinence with people with memory and cognition problems, and their carers, living at home. (NB. The views of people with these problems and their carers are also being investigated under a separate study protocol.)
Introduction
Urinary or faecal incontinence are distressing symptoms for any adult that impact on many facets of an individual’s health and quality of life. Professional knowledge and public awareness is increasing the availability of an evidence base in treatment and best practice. 119,124,362 However, people with memory problems and dementia living at home are one group for whom there is little guidance on promoting continence or managing incontinence. Incontinence problems are a common element in the decisions to move into a care home. 343,356 Continence research and evidence-based guidance either explicitly excludes people with dementia (see for example National Institute of Clinical Excellence 2006)122 or more commonly includes only those people living in care home settings (see for example Ostaszkiewicz et al. 2004). 368 Evidence-based guidelines produced in 1998 for primary care staff on supporting people with dementia and their carers did not address incontinence management. 123 The recent NICE–SCIE guideline on supporting people with dementia and their carers380 does not review evidence in relation to continence promotion but offers the view that combined interventions are more likely to support and maintain independence in the person with dementia.
This investigation of professional views is part of a larger study (EVIDEM-C) which has the ultimate goal of developing and field testing an evidence-based resource for the promotion of continence and management of urinary and faecal incontinence with carers, community nurses and social care staff. EVIDEM-C is a part of the NIHR funded programme of research (Principal Investigator Professor Steve Iliffe, University College London website: www.evidem.org.uk).
Background and rationale
Dementia affects over 4% of the people over 60, increasing to 13% for those over the age of 80 in Western Europe with significant projected increases in the next 20 years. 345 Dementia has enormous impact not only for the individual and their family but also for the health and social care system. 119,296,346 The median length of time from diagnosis to death is 10 years for those under 65 at diagnosis and 4 years for those over 80 years. 173 The clinical syndrome of dementia has a trajectory of progressive deterioration in cognition, abilities, and physical functioning. The impairment experienced by the individual is often compounded by extrinsic factors such as attitudes of ‘therapeutic nihilism’115 in professionals, un-adapted environments, and social exclusion. As the condition progresses problems may arise in maintaining independence in going to the toilet, in avoiding constipation, and in managing incontinence problems that cannot be resolved. Estimates suggest that there are 500,000 people with dementia in England and two-thirds live at home. 119
Incontinence is the involuntary leakage of urine or stool or both. 347,349 The prevalence of any type of urinary incontinence in all older adults is between 6% and 10%, with increasing rates associated with old age. 349 It is estimated that 2–5% of adults experience faecal incontinence. Incontinence lowers quality of life and impacts negatively on mental health351,352 as well as creates significant practical and financial problems. There is evidence that incontinence contributes significantly to other major health issues for older people such as falls. 353 Incontinence has been identified by professionals and older people as an issue of unmet need in primary care. 354,355,381
A UK population-based study of 15,000 home-dwelling people aged over 75 identified that 18.3% had cognitive impairment and of these 31% had urinary incontinence problems. 135 A national general practice audit of 999 older patients with faecal incontinence identified that 27% had a diagnosis of dementia. 367 Estimates suggest that about 60% of people with cognitive impairment live in their own homes. 356 For this group, there are no data on the changes over time in continence status to aid in service planning for the support of continence management or estimating cost consequences for households or primary care organisations.
Incontinence problems have been identified as a significant factor in increasing informal carer burden and triggering the admission of people with dementias to care homes. 110,134 There is little literature that explores the nature and impact of managing bladder and bowel problems in people with dementia at home by informal carers. Two small-scale qualitative investigations into informal carers views (total n = 12) on managing frail spouses’ incontinence359,360 suggested that individual problem solving strategies might contribute further to carer stress through decreasing their outside social contact. There is an absence of information that identifies both those aspects of incontinence problems and also the carer and service contexts that contribute to the admission to care homes. Anecdotal evidence suggests that faecal incontinence is the tipping point as far as carers are concerned whether they are able to continue supporting the person with dementia. 361 Evidence from care home settings suggest that formal carers had difficulty interpreting what the person with dementia wanted and therefore how best to manage their continence, e.g. repeated requests to be taken to the toilet and refusal of medication to ease constipation. 362
Assisting older people to remain in their own homes, improving continence care and addressing carer burden are priorities for the health and social care improvement and service frameworks (DOH 1998,363 2000,365 2001364). People with dementia receive services from both generalist primary care services and specialist mental health care of older people. They may also receive support from social workers (generalist or specialist) and publicly funded social care assistance. Incontinence is a clinical issue that often has low priority in generalist primary care services and specialist mental health care services. Specialist continence services are not currently available in all areas of the UK. 367 Evidence-based practice guidance is not currently available to assist professionals and staff in generalist services in their support of people with dementia living at home and their carers.
Aims
This investigation is part of a larger study (EVIDEM-C), which has the ultimate goal of developing and field testing evidence-based resources for the promotion of continence and management of urinary and faecal incontinence with people with dementia, close carers, primary care professionals, social care and home care staff. EVIDEM-C draws on the Medical Research Council (MRC 2000)56 Framework for developing complex interventions in using stepwise phases. The research approach is one of critical realism369 which allows an integration of both subjective and objective research approaches and makes explicit the interrelationships between context, interventions (mechanisms) and outcomes. 370 In taking this approach, the multifaceted nature of the issues and problems in promoting continence and managing incontinence in the home, as well as the perspectives of diverse stakeholders can be explored.
This investigation asks the questions:
-
What are the views of health and social care professionals and staff of (a) current issues and (b) currently employed solutions in promoting continence and managing incontinence with people with memory and cognition problems, and their carers, living at home?
-
From professional and clinical experience, are there strategies/advice/interventions/aids/support/technology that appear to be more acceptable and effective in supporting people living at home?
-
From professional and clinical experience, are there gaps in the knowledge and/or provision to appropriately support people and their carers in dealing with these issues?
The results from this investigation will be both fed to local participating services and used to develop feasibility studies of potential interventions, which will be submitted for peer and ethical review.
The development of this protocol has been informed by the EVIDEM-C advisory group, which involves service users and carers.
Methods
The methodology is qualitative, in the interpretative tradition, using small group and individual interviews. The sample is purposive to capture views from a diverse range of professional and staff groups across health and social care services in different inner urban and urban areas within the North Thames DeNDRoN research network area (URL: http://www.dendron.org.uk/rn/north_thames.html).
Professional groups include:
-
staff in older people mental health services providing day, outreach, and domiciliary services (likely staff groups include community mental health nurses, social workers, occupational therapists, admiral nurses)
-
staff in Primary Care Trust Provider Services providing domiciliary services to older people (likely staff groups include district nursing, community nursing, physiotherapists) and continence/bowel and bladder services
-
staff in independent service providers of home care and day centres for older people (likely staff groups include home care organisers, day centre managers)
-
staff in Local Authority Adult Service Departments (likely staff groups include social workers, in house home care managers and carers)
-
general practitioners and practice nurses (approached via the Greater London Primary Care Research Network).
The initial approach will be to a senior manager of the organisation to explain the investigation and seek permission to approach staff to volunteer. Requests for volunteers and participation information sheets will be circulated to staff. If it is not possible to organise small groups in convenient work locations then face-to-face or telephone interviews will be conducted as preferred.
Data will be gathered through:
-
Group interviews (approximately 5–8 per group) using adapted structured focus group techniques (Robson 2004)369 based on the research questions outlined above or
-
Individual semi-structured interviews based on the research questions outlined above. These will be face to face or by telephone as the person chooses.
Small group interviews will be conducted by two researchers. One will lead the discussion using the same topics as in the aide memoire and the other will take notes. The group’s discussion points will be summarised on flip chart to aid verification from the group of the issues raised and views expressed. The notes and flip chart will be transcribed into word documents and entered into NVivo™ for cross comparison and analysis between groups and themes from individual interviews. Semi-structured interviews with individuals will be recorded in field notes to be comparable with the data gathered in the group interviews. These will be entered into MS Word documents and treated as above. Content analysis370 will be undertaken by two researchers, independently, and compared to aid validation of the analysis.
Ethical and data protection considerations
All informants will be assured of anonymity and confidentiality in the transcription, analysis and reporting of their interview. Any direct quotations used in the report will be non-attributable. At the start of the group interviews, informants will be asked to respect that views and opinions expressed in the interview are held confidential to the group.
Informants will be made aware that if any information is shared that suggests that a vulnerable older adult is suffering abuse from a service provider then the researcher has a responsibility to share that information with the service manager.
All participants will be assigned a code number and this with their contact details will be kept separately and securely from any data collected in the process of the research. All data will be identifiable by code number alone. Data collected in group interviews will not have any identifying information on it. Data will be stored on a password protected computer, accessible only to the researchers in locked offices of the University. Hard copies of data will be stored in locked filing cabinets in the same offices and destroyed after the investigation is completed.
This protocol is deemed as service evaluation by the NHS Research Ethics Service and does not require an NHS ethics committee review. The protocol complies with the ethical review procedures of the host University.
Reports and dissemination
The findings will be written up as a brief report for circulation to participants. A full report will be used as the basis for papers and articles to be submitted to professional and service orientated journals. The findings will be used to develop feasibility studies of possible interventions/services and develop an evidence-based resource for the promotion of continence and management of urinary and faecal incontinence with people with dementia, close carers, primary care professionals, social care and home care staff.
Appendix 20 Chapter 3: Protocol – investigating the experience of managing continence problems over time, version 2, 2010
This study protocol originally had greater emphasis on quantitative elements of a cohort study. Unfortunately the difficulties in recruiting people into the study led to an amendment with greater emphasis on the qualitative elements.
Introduction
Urinary or faecal incontinence are distressing symptoms for any adult that impact on many facets of an individual’s health and quality of life. It is estimated that up to a third of people with memory problems living at home have incontinence problems. Incontinence is known to be a significant factor in the decision for people with memory problems and dementias to take up residence in a care home. This is an aspect of the health and well-being of people with memory loss that is poorly described both in its trajectory and the factors that promote the maintenance of continence and the management of incontinence at home. This lack of detailed knowledge of the issues and effective management strategies is reflected in the lack of guidance for people with dementia, their carers and health and social care professionals to address these problems.
This study aims to prospectively follow a group of people with dementia with continence problems and their carers for up to 3 years to describe the type of problems, the management strategies, the lived experience of the family carers and the person with dementia and the impact and cost of managing these problems.
This is one study in a group of studies known as EVIDEM-C which has the ultimate goal of developing and field testing an evidence-based resource for the promotion of continence and management of urinary and faecal incontinence with people with dementia, close carers, community nurses, social care workers and home care staff. In addition data from this study will assist in the planning of future studies which test interventions to help manage incontinence. EVIDEM-C is a part of the NIHR funded programme of research ‘Changing practice in dementia care in the community: developing and testing evidence-based interventions, from timely diagnosis to end of life’. The chief investigator is Professor Steve Iliffe, University College London.
Background and rationale
Dementia affects over 4% of the people over 60, increasing to 13% for those over the age of 80 in Western Europe with significant projected increases in the next 20 years. 345 Dementia has enormous impact not only for the individual and their family but also for the health and social care system. 119,296,346 The median length of time from diagnosis to death is 10 years for those under 65 at diagnosis and 4 years for those over 80 years. 173 The clinical syndrome of dementia has a trajectory of progressive deterioration in cognition, abilities, and physical functioning. The impairment experienced by the individual is often compounded by extrinsic factors such as attitudes of ‘therapeutic nihilism’115 in professionals, unadapted environments, and social exclusion. As the condition progresses problems may arise in maintaining independence in going to the toilet, in avoiding constipation, and in managing incontinence problems that cannot be resolved. In addition, there can be behavioural and psychological problems such as disinhibition, apathy, and faecal smearing. Estimates suggest that there are 500,000 people with dementia in England and two-thirds live at home. 119
Incontinence is the involuntary leakage of urine or stool or both. 347,349 The prevalence of any type of urinary incontinence in all older adults is between 6% and 10%, with increasing rates associated with old age. 349 It is estimated that 2–5% of adults experience faecal incontinence. Incontinence lowers quality of life and impacts negatively on mental health351,352 as well as creates significant practical and financial problems. There is evidence that incontinence contributes significantly to other major health issues for older people such as falls. 353 Incontinence has been identified by professionals and older people as an issue of unmet need in primary care. 354,355,381
A UK population-based study of 15,000 home-dwelling people aged over 75 identified that 18.3% had cognitive impairment and of these 31% had urinary incontinence problems. 135 A national general practice audit of 999 older patients with faecal incontinence identified that 27% had a diagnosis of dementia. 367 Evidence on prevalence of problems is invariably provided at a single time point. However, for a progressive degenerative disorder such as the dementia syndromes the experience from the affected person and their family is one of ongoing changes and adaptations. There are no studies that describe changes over time. As a consequence there are no data on the changes over time in continence status and associated issues to aid in service planning for the support of incontinence management or estimating cost consequences for households or primary care organisations.
Incontinence problems have been identified as a significant factor in increasing informal carer burden and triggering the admission of people with dementias to care homes. 110,134 There is little literature that explores the nature and impact of managing bladder and bowel problems in people with dementia at home by informal carers. Two small scale qualitative investigations into informal carers views (total n = 12) on managing frail spouses’ incontinence359,360 suggested that individual problem solving strategies might contribute further to carer stress through decreasing their outside social contact. There is an absence of information that identifies both those aspects of incontinence problems and also the carer and service contexts that contribute to the admission to care homes. Anecdotal evidence suggests that faecal incontinence is the tipping point as far as carers are concerned whether they are able to continue supporting the person with dementia. 361 Evidence from care home settings suggest that formal carers had difficulty interpreting what the person with dementia wanted and therefore how best to manage their continence e.g. repeated requests to be taken to the toilet and refusal of medication to ease constipation. 362
Assisting older people to remain in their own homes, improving continence care and addressing carer burden are priorities for the health and social care improvement and service frameworks (DOH 1998,363 2000,365 2001364). People with dementia receive services from both generalist primary care services and specialist mental health care of older people. They may also receive support from social workers (generalist or specialist) and publicly funded social care assistance. Incontinence is a clinical issue that often has low priority in generalist primary care services and specialist mental health care services. Specialist continence services are not currently available in all areas of the UK. 367 Evidence-based practice guidance is not currently available to assist professionals and staff in generalist services in their support of people with dementia living at home and their carers.
Professional knowledge and public awareness is increasing the availability of an evidence base in treatment and best practice. 121,380,383 However, people with memory problems and dementia living at home are one group for whom there is little guidance on promoting continence or managing incontinence. Continence research and evidence-based guidance either explicitly excludes people with dementia (see for example National Institute of Clinical Excellence 2006)122 or more commonly includes only those people living in care home settings (see for example Ostaszkiewicz et al. 2004). 369 A recent review of a sample of English Primary Care Trust continence assessment and management policies for primary care nurses162 demonstrated that little guidance was available for this group of patients beyond offering a general leaflet (Continence Foundation undated). Evidence-based guidelines produced in 1998 for primary care staff on supporting people with dementia and their carers did not address incontinence management. 123 The recent NICE–SCIE guideline on supporting people with dementia and their carers124 does not review evidence in relation to continence promotion but offers the view that combined interventions are more likely to support and maintain independence in the person with dementia. International experts in incontinence have reviewed the research evidence concerned with frail elderly people and concluded that there is a paucity of research that specifically consider frail elderly people, including those with cognitive impairment. They recommended that further research is required that considers the prevalence and natural history of urinary incontinence and faecal incontinence, the management and cost consequences. 384
Our earlier study interviewing people with dementia, family carers and health and social care professionals as to effective continence management strategies385 suggested a number of social and contextual factors that may be significant in the experience over time. These factors include access to health and social care services, access to finance to pay for additional continence aids, the gender of the family carer in relation to the gender of the person with dementia as well as the type of relationships e.g. spousal or child. Some of these factors reflect the broader literature on the nature and impact of informal care giving. 56,386–390
Our review of evidence391 has found no studies that explore the experience or impact of managing toileting and incontinence problems in people with dementia living at home and their family carers over time. This study aims to investigate aspects of these questions specifically in a community-dwelling sample of people with dementia and their family carers.
Study aim and research approach
This study aims to prospectively investigate and describe the experience of people with memory loss and continence problems living at home and their family carers over a time period of up to 3 years. The purpose is to inform the development of an education resource, inform the planning of future intervention studies and inform the commissioning and provision of services for people with dementia and their carers at home. The research questions it addresses are:
-
What types of continence promotion problems and incontinence problems are experienced by people with memory loss and dementia living at home over time?
-
What is the range of continence promotion and incontinence management strategies?
-
Which strategies are viewed as most effective?
-
What is the impact and cost consequences of incontinence management for the individuals, their carers and services supporting them? In particular what is the lived experience of managing intimate care involving excreta and what factors influences the ability of the family carer(s) to offer care in these circumstances or decide that alternative caring options must be found?
-
What is the sequence of continence related events that contribute to decisions to seek residential placement for the person with dementia?
The overall EVIDEM-C study draws on the Medical Research Council56,392 Framework for developing complex interventions in using stepwise phases. The study has a number of linked phases commencing with an integrative review of the literature,367 which is currently in progress. The research approach is one of critical realism369 which allows an integration of both subjective and objective research approaches and makes explicit the interrelationships between context, process, interventions (mechanisms) and outcomes. 370 In taking this approach, the multifaceted nature of the issues and problems in promoting continence and managing incontinence in the home, as well as the perspectives of multiple and diverse stakeholders can be examined in the light of the evidence of effectiveness. This research approach allows the study to acknowledge the complexity of the issues.
The EVIDEM research group have assisted in the development of this protocol. This includes:
Professor Steve Iliffe (UCL, primary care)
Dr James Warner (Imperial College, old age psychiatry)
Professor Claire Goodman (University of Hertfordshire, health services research)
Professor Jill Manthorpe (King’s College London, social work and social care)
Dr Greta Rait (UCL, primary care)
Professor Martin Knapp (LSE, economics)
Dr Mark Griffin (UCL, statistics)
Kalpa Kharicha (UCL, health service research and primary care)
David Lowery (CNWL NHS Trust, research management)
Members of the EVIDEM-C advisory group, which involves service users and carers, have also assisted in the development of the protocol.
Methods
This is a prospective longitudinal, descriptive study investigating the experience of up to 30 people with dementia with continence problems, living at home and their carers through qualitative serial interviews. 175,393 The use of qualitative, serial interviews allows the in-depth exploration of the patient with dementia and the family carer experience as it changes over time rather than as a snapshot at one point in time. The relationship and trust between the participant(s) and researcher builds over time, allowing the time for sensitive or complex information to be disclosed in depth. Serial qualitative interviewing yields a detailed and contextualised account of the experience of change and decline in chronic illness, relationships between the cared for and the family carers as they develop strategies to manage distressing and stigmatising symptoms and the response and impact of health and social care services and professionals as the disease progresses and the symptoms change. Longitudinal qualitative studies allow for the exploration of transitions, adaptations and trajectories. 394
The study will be through face-to-face and telephone interviews, repeated at 4-monthly intervals. The initial interview and those at yearly intervals will gather data that characterises the participants using validated tools. At the first interviews carers will be asked to provide retrospective information for the preceding year.
The sample and recruitment
Sample size in qualitative longitudinal research is influenced by the research questions, the population under consideration, the theoretical framing and available resources. 395,396 Examples can be found of studies with samples of 12 or less397 to those with cohorts of 500. 398 The Department of Health funded Bangor Longitudinal study of ageing included a qualitative study of a sub set of 30 people visited 2–4 times a year over 3 years. 399 The sample size in this study has been based on a number of factors. These are (a) the theoretical framing and findings revealed in the earlier study (referred to above), (b) the resources and timescales of the broader EVIDEM-C study and c) experience of the difficulties of trying to recruit participants, who are often already burdened by coping with their day-to-day lives, to a study investigating sensitive, embarrassing and often felt to be stigmatising aspects of their condition.
The aim is to have a sample of up to 30 men and women with dementia and continence problems living at home and their family carers to try and capture diversity in dementia conditions, socio-demographic characteristics, and health and social care service contexts. The sample is intended to be purposive, with a sampling framework informed by theory and earlier research findings, to ensure the greatest diversity in experience. The sample is intended to be selected to include both men and women, people in a range of age bands (age 65–74, 75–84, 85 and over), people from majority and minority ethnic groups, people from a range of socio-economic circumstances (i.e. those on income support and those not) and from a range of service and contextual factors (from deprived inner city areas to wealthier suburban areas). The experience to date is that the sample is one of convenience in that it includes those that volunteer rather than those selected for specific characteristics. In the sample recruited to date (August 2010) there are male and female participants with dementia, in age bands 75–84, and 85 and over, from majority and minority ethnic groups, people from a range of socio-economic circumstances (i.e. those on income support and those not) and from a range of service and contextual factors (from deprived inner city areas to wealthier suburban areas). Participants are living alone, living with spouses and living with daughters as their main family carers.
Inclusion criteria
The person has mild to moderate memory problems and cognition problems (not all people have formal dementia diagnosis) and problems with managing the toilet, remaining continent or incontinence problems.
The person and/or their carer are able to communicate in English.
Exclusion criteria
Neither the person nor their carer can communicate in English.
Recruitment
People with dementia (up to 30 people) and their carers living at home, will be recruited through the following multiple routes:
-
through initial approaches from their general practice (in the London PCTs of Westminster, Kensington & Chelsea, Brent and Harrow, Hounslow, Hillingdon, Hammersmith and Fulham)
-
through initial approaches from their specialist secondary services in Central and North West London NHS Trust older people services e.g. memory clinics, and community mental health teams and services such as Admiral Nurses
-
through the individuals volunteering, having seen the wider EVIDEM publicity or joined the wider EVIDEM cohort of people willing to consider involvement in community-based research studies and the North Thames DemReg
-
through specialist dementia social care services such as Housing 21 in North West London
-
through advertisements and contacts in local Alzheimer’s Associations, Age Concern and Carer groups in North London.
The clinical support officers from the Greater London Primary Care Research Network and the North Thames Dementias & Neurodegenerative Diseases Research Network will assist in general practice and in secondary care services in identifying patients with dementia and continence problems to be approached. Individuals will either be asked in the first instance by their clinician at the end of a consultation whether they would be willing to be approached to learn more about the study or their clinician will write to them asking whether they are willing to be approached to learn more about the study.
It is anticipated that people with dementia likely to be willing to participate would be at the mild to moderate stage of the disease progression. Those individuals willing to participate will make contact with the researcher who will send them the participant information leaflet and arrange a time to meet. At the meeting the research will be explained and consent sought to participate (see section on detailed ethical considerations and mental capacity).
After the first interview, the family carer will be asked if they would be willing to be interviewed again in 4 months time to discuss their experience in the intervening period. If they decline then they will be asked if they are willing to continue in the study by brief telephone contact at 4 and 8 months, followed by being asked to undertake a full interview at 12 months, repeated the following year. Participants are able to decline and drop out of the study at any point without needing to give a reason. Those happy to continue in the qualitative element will be interviewed at 4 and 8 and 12 months of each year they are in the study. After the first interview, the interview can be with the carer alone if the person with dementia decides not to continue or their condition is such that their communication abilities preclude an interview. If an individual moves to a care home then a final interview will be undertaken with the carer and if appropriate, consent sought for a final interview with the affected person.
The data collection and tools
The interviewer will proceed with the interview in conversational ways rather than as a checklist set of questions which are more likely to distress people with dementia. If the interview is proving too long for the individual, the interviewer will arrange, with permission, to return at another time to compete the interview.
Data collection for the person with dementia and as appropriate their carer will include:
-
demographic data
-
medical information, including current other medical conditions, number of births for women, weight and medications
-
characterisation of their mental condition and levels of ability (Mini-Mental State Examination,52 Disability Assessment for Dementia,176 Neuropsychiatric Inventory Questionnaire,62 Cornell Scale for depression in dementia220)
-
characterisation of their continence promotion activities and incontinence problems (ICIQ short form ICIQ-Noctururia,177 additional questions regarding bowel symptoms and management)
-
characterisation of their home and its adaptation to any impairments (adapted Home Environment Assessment Protocol400)
-
characterisation of their quality of Life (DEMQOL, Smith et al. 2005)86
-
characterisation of service use and cost consequences (Client Service Receipt Inventory part 1). 401
Data collection for the qualitative element with the person with dementia descriptive will be by guided conversation as to any views on what helps in dealing with continence problems.
For the carer alone:
-
demographic data
-
characterisation of their level of support to their family member, service use and cost consequences (Client Service Receipt Inventory part 2, Howard et al. 2007)401
-
characterisation of the impact of caring and their quality of life, (caregivers burden scale,402 health-related quality of life EQ-5D, EuroQol 2008)
-
perceptions of the effectiveness of strategies that are most helpful with current problems.
The qualitative element included at the annual and 4-monthly interviews will be undertaken using an aide memoire. This will cover the following topics, explored through open ended, semi-structured questions.
-
The experience of living with someone with toileting problems and incontinence due to dementia.
-
The experiencing of developing strategies in managing the current toileting problems and incontinence problems, including sources of information, advice and practical help.
-
The experience of developing a caring role in providing intimate care that involves managing excreta.
-
Any critical or significant events related to incontinence that have occurred since the last interview and the impact of those events e.g. hospital admission.
-
The extent to which providing intimate care involving excreta influences their view of their ability to continue in a caring role or whether alternatives are being sought.
Data analysis
Descriptive techniques will be used to characterise the participants, summarising information collected through the validated tools.
The analysis of longitudinal qualitative data requires specific techniques to ensure that the temporal nature at the individual and group level is captured. 403 This analysis will be based on methods suggested by Pettigrew (1990)394 Saldana (2003)396 and Lewis (2005)404 and previous experience as part of a NIHR Service Delivery and Organisation study of older adults experience of nurse case management overtime. 405 Data will be entered onto the NVivo™ software package and the organisation of the cases and multiple interviews undertaken as recommended by Saldana (2003). 396 In addition a schematic representation of the individual case narrative overtime will be prepared as recommended by Pettigrew (1990)394 and Lewis (2005)404 and undertaken in a previous study. 405 The analysis will be undertaken at five different levels as described by Lewis (2005)404 and framed by the research questions above:
-
Individual case narratives to capture the trajectory over time, i.e. the course of events, the adaptations, transitions as well any re-interpretations of events and decisions over time.
-
Cross-sectional analysis between cases at specific time points.
-
Identification of themes using the constant comparative method406 and analysis of linkages between themes and participant characteristics over time.
-
Cross-case comparisons of narratives and themes.
-
Between-group comparisons for example by sub groups defined by gender.
Final interpretations will address the research questions as well as generalise the findings at a theoretical level.
Ethical considerations
The research questions under consideration are directly relevant to people who have dementia and it is important to incorporate views of those people experiencing the problems not just the professional views or their family members. 382 People who have dementia may lack capacity to consent. Only those people with dementia with the capacity to make the decision to participate in the research will be included at the first point. The procedure for establishing the ability to make the decision will proceed in accordance with the guidance for the Mental Capacity Act 2005. People with dementia will only be approached after their close family carer or a professional who knows them well enough to judge their ability to consent to participation in the study, has discussed the research first and asked whether they would be willing to meet the researcher. This person will explain about the research and the exploratory discussion process using the information leaflet which has been adapted to provide key points in straight forward language. After consent to approach the person has been gained, the researcher will arrange to meet them with their carer or supporting person of their choice. She will repeat the explanations, using the leaflet and check the person is able to consent at that point in time. Key elements in checking will be: whether the person has general understanding of what they are consenting to and why they are being asked to make it, whether the person understands what will happen if they agree to participate in the study, whether they are they able to understand the verbal and/or written information relevant to making a decision whether to participate in the study. A consent form for signing will be offered, although if the informant is unwilling to sign (writing may be difficult) this will not be pursued and verbal consent will be noted. Any sign (verbally or in body language) that the individual is not happy about taking part or continuing to take part in the research will be interpreted as withholding consent, or a desire to withdraw. The research will end the conversation, thanking the person for their time and involvement. The researcher will check throughout the discussion that the informant is willing to continue and cease if the person indicates they wish the discussion to end.
As this is a study over time the consent process will be repeated before each subsequent contact. Consent will be at subsequent contacts over the 3 years, the person with dementia may have lost the capacity to make a decision to consent, i.e. understand information about the decision to be made, retain that information in their mind, use or weigh that information as part of the decision-making process, or communicate their decision (by talking, using sign language or any other means). In these instances the researcher will consult the family carer (the consultee) in accordance with the code of practice of the Mental Capacity Act 2005 whether the person would want to take part in this further stage of the research. With their agreement in writing, the interviewer would continue. However, the interviewer would stop at any sign that the person wishes to withdraw, as described as above. The interviews in this study offer minimal risk to the person and minimal intrusion (see next paragraph) or interference to their rights.
Urinary or faecal incontinence are distressing symptoms that are embarrassing to discuss for any adult. Undertaking intimate care associated with incontinence for an adult spouse, relative or loved one is a sensitive issue. All interviews will be conducted by researchers aware of the potential socially problematic nature of the issues that are being discussed. Interviewers will use language understandable and acceptable to the interviewee and make all efforts to lessen any discomfort in discussing the issues. Should an individual become distressed then the interviewer will pause, be sympathetic, and check whether the person wishes to discontinue the interview. The researcher will encourage informants to share their problems and distress with their key health professional, or family doctor as appropriate. All researchers will have contact information on key support agencies to leave with informants.
All informants will be assured of anonymity and confidentiality in the transcription, analysis and reporting of their interview. Any direct quotations used in the report will be non-attributable.
Informants will be made aware that if any information is shared that suggests that a vulnerable older adult is suffering neglect or abuse then the researcher has a responsibility to share that information with the service manager (in case of abuse or neglect from a service provider) or in the case of individuals gain their consent to share that information with the named Local Authority Officer for vulnerable adults.
Data protection considerations
All participants will be assigned a code number and this with their contact details will be kept separately and securely from any data collected in the process of the research. All research data will be identifiable by code number alone. The interview data will be entered and stored on a password protected computer, accessible only to the researchers in locked offices of the University. Hard copies of data will be stored in locked filing cabinets in the same offices. Research data will be archived for 5 years and then destroyed.
Timescale
Recruitment will commence in October 2008 and end in December 2010.
Data analysis will be ongoing and completed by January 2012.
A final report will be made in 2012.
Reports and dissemination
The findings will be written up as a brief report for circulation to participants. The full report will be used as the basis for papers and articles to be submitted to a professional journal and local and national voluntary organisation’s newsletters. An abstract will be submitted for presentation at a service users and professional conference, such as Dementia Congress.
Appendix 21 Chapter 3: Protocol – incidence and management of incontinence in general practice patients with dementia: an analysis of THIN data, 2011
Incontinence problems have been identified as a significant factor in increasing carer ‘burden’ and triggering the entry of people with dementias to care homes. 110,134,356 Supporting people with dementia to live at home is a major policy objective. There are currently no primary care-based data available in the UK or elsewhere to provide clinicians or commissioners services with data on the incidence and current management of people with dementia living at home who also have problems with urinary and/or faecal incontinence. This protocol addresses this absence of data through an analysis of general practice records held in the THIN database.
Background
Dementia affects over 4% of the people over 60, increasing to 13% for those over the age of 80 in Western Europe with significant projected increases in the next 20 years. 345 Dementia has enormous impact not only for the individual and their family but also for the health and social care system. 119,296,346 The median length of time from diagnosis to death is 10 years for those under 65 at diagnosis and 4 years for those over 80 years. 173 The clinical syndrome of dementia has a trajectory of progressive deterioration in cognition, abilities, and physical functioning. The impairment experienced by the individual is often compounded by extrinsic factors such as attitudes of ‘therapeutic nihilism’115 in professionals, un-adapted environments, and social exclusion. As the condition progresses problems may arise in maintaining independence in going to the toilet, in avoiding constipation, and in managing incontinence problems that cannot be resolved. Estimates suggest that there are 500,000 people with dementia in England and two-thirds live at home. 119
Incontinence is the involuntary leakage of urine or stool or both. 347,349 The prevalence of any type of urinary incontinence in all older adults is between 6% and 10%, with increasing rates associated with old age. 349 It is estimated that 2–5% of adults experience faecal incontinence. Incontinence lowers quality of life and impacts negatively on mental health351,352 as well as creates significant practical and financial problems. There is evidence that incontinence contributes significantly to other major health issues for older people such as falls. 353 Incontinence has been identified by professionals and older people as an issue of unmet need in primary care. 354,355,381
There are no population surveys that identify the incidence of people with dementia and continence problems living at home. However, there are some indications that they may be a substantial minority. A UK population-based study of 15,000 home-dwelling people aged over 75 identified that 18.3% had cognitive impairment and of these 31% had urinary incontinence problems. 135 A national general practice audit of 999 older patients with faecal incontinence identified that 27% had a diagnosis of dementia. 367 There are some indications that people with dementia and incontinence symptoms are not investigated and managed proactively. 407
There are currently no primary care-based data available in the UK or elsewhere to provide clinicians or commissioners services with data on incidence and the characteristics of people with dementia living at home who also have problems with urinary and/or faecal incontinence.
The experience of people with dementia and their family carers interviewed in a related ongoing study by the authors suggests that:
-
Like the general population408 most delay consulting general practice or any health professional about incontinence problems as they feel embarrassed and humiliated.
-
Family members report avoiding talking to their general practitioner about these issues as they seek to protect the person with dementia’s dignity and public persona. 354 However, as the dementia progresses this becomes unsustainable and the family carers insist on seeking help in order to cope with the wider burden of supporting their family member. This is sometimes reported as part of a crisis situation in which hospitalisation or temporary residence in a care home occurs.
-
Family carers report a variety of responses from general practice when they do seek help: some indicate they get little assessment or help other than the referral for continence pads, others indicate a more detailed investigative approach to identify or discount possible treatable causes of these symptoms, such as urinary tract infections or prostate hypertrophy problems, and then more active management of the problems.
Purpose
This cohort study will describe current practice in continence care for people with dementia, and quantify the burden of disease. It will also examine whether this differs with demographics or health status, or between general practices in ways not explained by patient characteristics.
Hypotheses
The background described above suggests a number of hypotheses:
-
The general practice reported incidence of incontinence problems is higher in the dementia population than in the general population of similar age registered with a GP. This relationship may be confounded by other factors such as age or mobility.
-
The general practice response to people with dementia consulting for incontinence problems, compared to people of similar demographics without dementia, is more likely to be prescription of continence pads, and less likely to be investigation and referral for tractable problems, including surgery, or it may take longer to get referred.
Research questions
-
What is the incidence rate of urinary and/or faecal incontinence, stratified by age and sex, recorded in general practice patients aged over 60:
-
a. overall
-
b. with a diagnosis of dementia and
-
c. without a diagnosis of dementia?
Which covariates affect this relationship (co-morbidity, restricted mobility, Townsend deprivation score)?
-
-
What is the incidence of recorded treatments, management and referrals made for incontinence in patients with and without dementia, stratified by sex and age?
Which covariates affect this relationship (polypharmacy, restricted mobility, Townsend deprivation score)?
Methods
Study design
A cohort study
Data source
Data will be taken from THIN (URL: http://www.epic-uk.org/thin.htm) covering general practices in the United Kingdom (UK) providing data during the period 1 January 2000 – 31 December 2009. This electronic recording scheme is one of the largest UK sources of continuous primary care data on patients’ consultations and prescribing data. It has been widely used for epidemiological studies, including a study of dementia and survival. 13 Anonymised patient data are pre-collected from participating practices. Practices are broadly representative of UK general practices in terms of patients’ age and sex, practice size and geographical distribution. GPs enter medical diagnoses and symptoms using Read codes, a hierarchical recording system used to record clinical summary information. The age, sex, medical diagnosis and symptom records, health promotion activity, referrals to secondary care, prescriptions and quintiles of Townsend deprivation score are recorded for each registered individual.
Inclusion/exclusion criteria
In order to examine the incidence of incontinence (research question 1) we will first identify an ‘exposed cohort’ of individuals aged 60 or above with data available for at least 6 months between 1 January 2000 and 31 December 2009. Any patients with Read codes indicating learning disabilities and specifically Down’s syndrome will be excluded as this is a known risk factor for both dementia and incontinence. Based on the overall age and gender distribution in this cohort we will identify a comparison ‘unexposed cohort’ stratified by age, sex and practice, by randomly selecting from patients over the age of 60 without a record of dementia.
For all research questions, data will be taken from each practice after they have achieved an acceptable level of data quality as defined by the Acceptable Mortality Rate (AMR) date. 409 We will use established methods to identify and exclude likely prevalent cases of incontinence or dementia recorded within 120 days of registration. This cut-off has been established by analysing THIN using the method of Lewis et al., specifically for incontinence and dementia. 403 Pre-existing incontinence and/or dementia will be identified from any time point in the patient’s data, except for ‘incontinence’ codes prior to age 16.
Study variables
Dementia and incontinence will be identified from Read codes and drug codes, excluding single isolated drug codes and any codes flagged on THIN as having errors. Learning disabilities, Down’s syndrome and restricted mobility will similarly be identified from Read codes. Lists of Read codes have been developed in order to identify patients with these conditions. Codes were identified by searching the code dictionary for relevant terms (e.g. continen*, faecal, urin*, dement*, alzheimer*, memor*), then adjacent codes were examined and searches run on other keywords which are suggested by the dictionary definitions. This process has been used on a number of studies using THIN data. 410 Drug/device codes were identified in the same way. All these lists were checked by two clinicians for relevance and completeness.
The first date against a code for dementia, incontinence and restricted mobility will be stored, allowing dementia and mobility to be modelled as time-varying predictors. The first date of each of various types of treatment/referral for incontinence will also be stored for use in research question 2.
We will use drugs prescribed as a proxy index for co-morbidity, calculated from the therapy records by counting the number of distinct BNF sections prescribed within the 6 months preceding the first incontinence code. For those without incontinence, a random date will be selected within their time at risk (uniformly distributed) and 6 months of prescriptions taken prior to that point. These calculations will apply to both exposed and unexposed cohorts.
Year of birth and sex will be extracted from the patient data files. Townsend deprivation scores (in quintiles) will be extracted from the postcode variable information (PVI) file for each practice.
Other variables to be extracted include:
-
practice and patient ID
-
indicator variables for the presence of dementia, incontinence (including pre-existing codes), and different types of treatments or referrals
-
the AMR date for the practice
-
year of birth, registration date, transfer date and death date.
Analysis
For research question 1 (incidence of incontinence in dementia compared to no dementia), incidence rates will be derived for incontinence in people with and without pre-existing dementia, and stratified by age, gender and deprivation. Epidemiological calculations of rate ratios will be used for the stratified analysis. Continuous covariates and inter-practice variation will be accounted for in a multilevel Poisson regression model. Incontinence will be the outcome, dementia the exposure, potential covariates are age, sex, deprivation, polypharmacy, and calendar period, and in a Poisson model time at risk will be an offset variable. This will allow us to find the adjusted relationships between dementia and incontinence and to consider whether the covariates interact (are effect modifiers) with dementia. We will account for variation between practices by modelling this as a random effect in the Poisson regression. Results will be compared with published statistics that are representative of current practice. 116
For research question 2 (access to, and timeliness of, treatment for incontinence in dementia compared to no dementia), another multilevel regression model will be constructed with treatment options for incontinence as the outcomes, dementia as the exposure, and covariates defined as for question 1. A multilevel Poisson regression will be used and the offset and random effect will be defined as above. This will allow us to quantify any differences in access to treatment between those with and without dementia.
Limitations
The data are limited to consultations recorded in general practice and there may be differences in coding by different GPs, which we will investigate where possible. Because the data are drawn from GP consultations, there could be a Berkson’s bias inflating associations between morbidities. This will be minimised by looking over long time periods and measuring the time to first recording (incidence) of a diagnosis or treatment. 411 We also know that people with continence problems can delay seeking help, sometimes by years, so there may be a bias toward earlier recording of incident incontinence in patients with dementia. We will cross-check diagnostic codes against prescriptions and free-text comments for under-recording or delayed recording of diagnoses.
Appendix 22 Chapter 3: Protocol – investigating the acceptability, effectiveness and associated costs of different types of absorbent products used for incontinence by people with memory problems living at home, 2010
Aim
The aim of this study is:
-
to investigate the feasibility of the research methods to ascertain the acceptability, effectiveness and associated costs of different types of absorbent products used for incontinence by people with memory problems living at home
-
to provide preliminary data on the acceptability, effectiveness and associated costs of different types of absorbent products used for incontinence by people with memory problems living at home to inform both future research design and also service providers.
Background and rationale
Urinary or faecal incontinence are distressing symptoms for any adult. Incontinence lowers quality of life and impacts negatively on mental health351,352 as well as creates significant practical and financial problems. Incontinence can lead to social embarrassment, restriction of leisure activity, creation of extra laundry and replacement costs for clothing and bedding, and it can be a source of conflict between individuals and their family (Continence Foundation, undated).
There is no firm evidence of the numbers of people with memory problems living at home who have continence problems. Evidence suggests that up to a third of people with memory problems and dementia living at home experience incontinence problems. 135,381 Dementia affects over 4% of the people over 60, increasing to 13% for those over the age of 80 in Western Europe with significant projected increases in the next 20 years. 345 Dementia has enormous impact not only for the individual and their family but also for the health and social care system. 119,296,346 The clinical syndrome of dementia has a trajectory of progressive deterioration in cognition, abilities, and physical functioning. As the condition progresses problems may arise in maintaining independence in going to the toilet, in avoiding constipation, and in managing incontinence problems that cannot be resolved. In addition, there can be behavioural and psychological problems such as disinhibition, apathy and faecal smearing. 114 Estimates suggest that there are 500,000 people with dementia in England, two-thirds of whom live at home. 119 Incontinence is known to be a significant factor in the decision for people with memory problems and dementias to take up residence in a care home. 110,134,359 There is an absence of information exploring incontinence problems combined with the carer and service contexts that contribute to the admission to care homes.
Incontinence is the involuntary leakage of urine or stool or both. 347,349 There are a number of internationally agreed algorithms for the assessing and treating incontinence symptoms, with the term ‘functional’ incontinence used to describe that which has no physiological basis. 348 UK surveys suggest that even though individuals find their urinary symptoms bothersome and socially disabling, some older people consider them as an inevitable part of ageing and too humiliating to seek help,166 while others view the treatments as cumbersome and too invasive. 412 There is also evidence that health professionals have a nihilistic view of incontinence in older adults, thinking that nothing can be done, and rather than thorough assessment and treatment of the underlying causes they resort to provision of containment devices e.g. pads, as the first option. 407 The International Continence Society suggests that for frail, elderly people (which include people with moderate to severe dementia) the aim of professional input may be to have well contained incontinence. 117
There are no UK estimates of the total public expenditure associated with treating or managing incontinence. There are some indications of the scale of the direct expenditure. It was estimated that the NHS spent £536 million at 1999/2000 prices in treating clinically significant urinary incontinence symptoms in community-dwelling adults. 354 In 2007 nearly 1.5 million NHS prescriptions were filled for incontinence appliances in England at a cost of £33 million. 413 Absorbent incontinence products account for the majority of this spending. 181 They come in a wide range of designs and absorbencies (see table below). Reviews of NHS provision of continence services357,365,382 have suggested that:
-
There are significant geographical variations in eligibility criteria to receive NHS continence services, in access to specialist continence services, and in the range and quantities of treatment provided in primary and secondary care.
-
In addition, a national survey found variations, inflexibility, and arbitrary ceilings on the NHS provision of continence aids e.g. disposable and washable pads, and bed pads. 414
Our recent research involving interviewing people with dementia living at home, their family carers and health and social care staff closely supporting them162 suggested that:
-
Carers and people with dementia often stated preferences for certain types of continence pad designs; this was often but not always for ‘pull-up’ pants.
-
Preferences might be affected by: different degrees of incapacity, different types of behavioural problems and ease of use at night or during the day in the home or ease of use outside the home.
-
Ineffective containment pads increased the financial costs and stress of supporting someone with these problems at home.
There are only currently studies looking at absorbent incontinence products and preferences, ease of use and effectiveness with people living at home who do not have dementias. 169 The following chart indicates the range of products available.
Types of absorbent pad designs
| Designs of body-worn containment pads | Used for which type of incontinence | Design details |
|---|---|---|
| Inserts | Light/moderate/heavy | Disposable inserts for light incontinence often held in place in underwear by an adhesive strip Other types held in place by close fitting underwear or stretch mesh briefs Sometimes have wetness indicator to signal the need for a pad change Sometimes have elasticised gathers of hydrophobic material which are intended to impede lateral leakage |
| Nappies | Moderate/heavy | Adult-sized versions of babies’ nappies (nappies) Disposable nappies usually have elasticated waist and legs and self-adhesive tabs (usually resealable), and often a wetness indicator and standing gathers Washable nappies are fixed with Velcro or press-studs and usually elasticated at the waist and legs |
| T-shaped nappies | Moderate/heavy | To enable users to apply the nappy while the person is standing Fastens round the waist before the pad is pulled through between the legs into position and secured |
| Pull-up disposable pants | Moderate/heavy | Similar in construction to trainer pants for toddlers Those for light incontinence are often know as pants with integral pad |
| Menstrual pads (W) (disposable only) | Light | Have an adhesive strip to adhere to underpants |
| Leafs (M) and pouches (M) | Light | Leafs designed to fit over the penis, pouches designed to fit over the scrotum also Worn with close-fitting underwear or stretch mesh briefs Disposables have an adhesive strip to adhere to underpants |
Research questions
-
Do the research questionnaires capture all aspects of the acceptability, effectiveness, and associated costs of different types of absorbent products used for incontinence by people with memory problems living at home?
-
Is there evidence from this pilot study of the extent to which different types of absorbent products used for incontinence by people with memory problems living at home are acceptable, and effective in dealing with different types of continence in different social situations?
-
Are people with memory problems, their family carers and/or their paid carers willing to participate in this type of research and try different types of absorbent incontinence products?
-
Is it feasible to ask people with memory problems, their family carers and/or paid carers to collect this amount of data?
Method
This pilot study design is informed by the Medical Research Council recommendations for assessing feasibility in developing and testing complex interventions. 56 The design aims to examine key uncertainties as outlined in the questions above and may be refined for future studies. This is a pilot study using primarily quantitative methods but also capturing participants’ views with qualitative methods.
We aim to recruit up to 40 family carers of people with memory problems living at home and using absorbent incontinence products. We also aim to recruit the person with memory problems if they have capacity to consent. We aim for the sample to have a wide variation of demographic characteristics, co-morbidities and health status as a result of recruiting through a NHS continence services and voluntary organisations across London. Interested NHS continence services will be identified through presentations at the London Association of Continence Advisors and then permissions sought with the employing NHS organisation as required under NHS research governance requirements.
The sample
The sample will be a purposive sample of up to 40 family carers, who are supporting their family member in managing continence problems with absorbent pads. In addition those people with memory problems who have capacity to consent will be recruited. The purposive sample will include family carers of people with memory problems who have been using pads for different amounts of time and people who had incontinence problems that pre-dated symptoms of dementia.
Inclusion criteria:
-
The person is a family carer who is supporting their family member with memory problems in managing incontinence with absorbent pads.
-
They are able to communicate in English.
-
The person using the absorbent pads has capacity to give consent.
Exclusion criteria:
-
The family carer cannot communicate in English.
-
The person with memory problems does not have capacity to consent.
Recruitment
Participants will be recruited using several approaches as it is anticipated people will be slow to come forward due to the sensitive nature of the topic. We will ask local (in up to four different areas of London Strategic Health Authority) community service and primary care staff such as district nurses, continence nurses and GPs to identify and initially approach family carers of people with dementia living at home with incontinence and in receipt of absorbent pads from local NHS services. In addition we will place information in local Alzheimer’s Society branch newsletters and in the Dementia Research Network Registry (DemReg) asking people to contact us directly who might be interested in participating. Those individuals willing to participate will make contact with the researchers who will send them the participant information leaflet and arrange a time to meet them in their home. At the meeting the research will be explained and consent sought to participate (see below on detailed ethical considerations and mental capacity).
Data collection
Participants (family carers and people with memory problems) will be seen in their own home and information collected at two points:
-
Time point 1: initial meeting in the person’s home.
-
Time point 2: 8–10 days after time point 1 as suits the participant.
Data collection at time point 1
The following data will be collected using these tools. If the person with dementia has consented then the data will be collected in an interview supported by the family carer. If the person with dementia is unable to consent then the information will be collected from the family carer alone.
-
demographic data about the person with dementia
-
medical information about the person with dementia, including current other medical conditions, weight and medications
-
Disability Assessment for Dementia176
-
characterisation of continence promotion activities and incontinence problems (ICIQ short form ICIQ-Nocturia,177 additional questions regarding bowel symptoms and management)
-
characterisation of service receipt and cost consequences (Client Service Receipt Inventory part 1 section C)401
-
characterisation of mental condition and levels of ability (Mini-Mental State Examination)52 only used if the person with dementia is able to consent.
For the family carer alone:
-
demographic data about the family carer
-
characterisation of the impact of caring and their quality of life (caregivers burden scale)94
-
NPI (Neuropsychiatric Inventory). 62
The researcher will also:
-
introduce the pilot study diaries and recording tools (Pad and leakage diary sheet, and opinion sheet) to be kept for the next seven days (including for night-time); these have been adapted with permission from the research tools used by Dr M Fader et al. 181 for the NIHR Health Technology funded project.
-
enquire whether the family carer would be willing to try pad weighing scales and record the weight (indicates the volume of contents); for those willing, scales will be left alongside the study diaries.
Data collection at time point 2
The researcher will collect the study diaries and recording sheets. The researcher will also ask about the acceptability, ease of completion, level of completion and any other relevant views regarding the study data collection tools (topic guide for evaluation of study research methods).
Flow chart of the study
Data analysis
Quantitative data will be managed through SPSS™ and analysed descriptively to address the research questions. Qualitative data from the second interview will be managed and thematically analysed through a software system such as NVivo™.
Ethical considerations
There are ethical issues related to capacity to consent, distress, confidentiality and safeguarding vulnerable adults.
The research questions under consideration are directly relevant to people who have memory problems and it is important to incorporate views of those people experiencing the problems if possible (Department of Health 2005). 415 People who have memory problems may lack capacity to consent. Only those people with memory problems with the capacity to make the decision to participate in the research will be included.
The procedure for establishing the ability to make the decision will proceed in accordance with the guidance for the Mental Capacity Act 2005. People with memory problems will only be approached after their close family carer or a professional who knows them well enough to judge their ability to consent to participation in the study, has discussed the research first and asked whether they would be willing to meet the researcher. This person will explain about the research and the exploratory discussion process using the information leaflet which has been adapted to provide key points in straight forward language. After consent to approach the person has been gained, the researcher will arrange to meet them with their carer or supporting person of their choice. She will repeat the explanations, using the leaflet and check the person is able to consent at that point in time. Key elements in checking will be: whether the person has general understanding of what they are consenting to and why they are being asked to give it, whether the person understands what will happen if they agree to participate in the study, whether they are they able to understand the verbal and/or written information relevant to making a decision whether to participate in the study. A consent form for signing will be offered, although if the informant is unwilling to sign (writing may be difficult) this will not be pursued and verbal consent will be noted. Any sign (verbally or in body language) that the individual is not happy about taking part or continuing to take part in the research will be interpreted as withholding consent, or a desire to withdraw. The research will end the conversation, thanking the person for their time and involvement. The researcher will check throughout the discussion that the informant is willing to continue and cease if the person indicates they wish the discussion to end.
The interviews in this study offer minimal risk to the person and minimal intrusion (see next paragraph) or interference to their rights. Urinary or faecal incontinence are distressing symptoms that are embarrassing to discuss for any adult. Undertaking intimate care associated with incontinence for an adult spouse, relative or loved one is a sensitive issue. All interviews will be conducted by researchers aware of the potential socially problematic nature of the issues that are being discussed. Interviewers will use language understandable and acceptable to the interviewee and make all efforts to lessen any discomfort in discussing the issues. Should an individual become distressed then the interviewer will pause, be sympathetic, and check whether the person wishes to discontinue the interview. The researcher will encourage informants to share their problems and distress with their key health professional, or family doctor as appropriate. All researchers will have information on key support agencies to leave with informants.
All informants will be assured of anonymity and confidentiality in the transcription, analysis and reporting of their interview. Any direct quotations used in the report will be non-attributable.
Informants will be made aware that if any information is shared that suggests that a vulnerable older adult is suffering neglect or abuse then the researcher has a responsibility to share that information with the service manager (in case of abuse or neglect from a service provider) or in the case of individuals gain their consent to share that information with the named Local Authority Officer for adult safeguarding.
Data protection considerations
All participants will be assigned a code number and this with their contact details will be kept separately and securely from any data collected in the process of the research. All research data will be identifiable by code number alone. The data will be entered and stored on a password protected computer, accessible only to the researchers in locked offices of the University. Hard copies of data will be stored in locked filing cabinets in the same offices. Research data will be archived for 5 years and then destroyed.
Timescale
The timescale for the project is 1 year. Recruitment will commence in June/July 2010 (determined by ethical review and governance procedures). Our experience to date suggests that recruitment will be slow for such a sensitive subject and we anticipate that it may take up to 6 months to recruit. A final report will be written in 2012.
Reports and dissemination
The findings will be written up as a brief report for circulation to participants and in the EVIDEM final report. Papers and articles to be submitted to a professional journal and local and national voluntary organisation’s newsletters. An abstract will be submitted for presentation at a service users and professional conference, such as Dementia Congress.
Appendix 23 Chapter 3: Protocol – a modified Delphi consultation to develop a dementia-focused continence assessment tool for use with people with dementia living at home, 2010
This protocol describes the process for developing and establishing the face and content validity of the EVIDEM-C continence assessment tool to be used with people with dementia living at home and their family carers. (NB. It draws on the background from previous protocols and does not repeat it here.)
Background
EVIDEM-C is a research study investigating issues of continence promotion and incontinence management for people with dementia living at home and their family carers with the ultimate aim of developing resources to assist those working in primary care. Our earlier studies revealed that:
-
people with dementia and their family carers often only sought professional help in toileting and incontinence problems at points for crisis169
-
people with dementia and their family carers often reported difficulties with health professionals recognising and advising on their problems170
-
local NHS continence policies and assessment tools, primarily used by primary care nurses, rarely directly explored the problems of people with dementia in toileting and incontinence or outlined appropriate care pathways. 162
Aim
The aim is to develop and test the face and content validity of a prototype dementia specific assessment tool to be used in tandem with locally agreed continence assessment tools.
Method
To undertake a three-stage adapted Delphi consultation. 182
Stage one will bring together a group of experts in a facilitated series of group activities to review current examples of local continence assessment tools and agree the range of principles to be incorporated in a dementia specific continence assessment tool.
Stage two will consult those in stage one and a wider group of experts by post (or e-mail) on a prototype dementia specific tool for views on whether it addresses the principles and issues identified in stage one plus views on any omitted items.
Stage three will undertake a postal consultation of family carers and professional experts to address the following questions:
-
Is this additional assessment tool likely to improve primary care professionals’ recognition of the toileting and incontinence problems experienced by people with dementia and their family carers? (face validity)
-
Have all the important items associated with toileting problems and incontinence experienced by people with dementia and their family carers, been included? And are there any items which are not important and should not be included? (content validity)
Stage three
The diversity of the sample is important rather than its size to capture the widest variation in views. 183 The aim is for at least five participants in each of eight categories as listed described below:
Stage three proposed participants
| Expert group | No. | Route to approach |
|---|---|---|
| Family carers | 5–10 | EVIDEM-C family carer e-network |
| Voluntary organisations with knowledge and expertise in bladder and bowel problems and dementia carers supporting people with dementia | 5–10 | Members of EVIDEM steering group from these organisations plus direct approaches to Bladder and Bowel Foundation |
| General practitioners | 5–10 | Identified as either published about an aspect of dementia or incontinence |
| Psychogeriatricians | 5–10 | Identified as having published about an aspect incontinence |
| Nurses in the community | 5–10 | Identified as either published about aspects of dementia or incontinence. Also through the e-network of the Queens Nursing Institute |
| Continence nurse specialists | 5–10 | Identified through the Association of Continence Advisors local branches in London and the North West of England |
| Community mental health nurses and Admiral Nurses | 5–10 | Identified through Community Psychiatric Nursing Association, For Dementia and also publishing on aspects of care for people with dementia living at home |
| Others with specific e.g. occupational therapists, Disable Living Advice Centres, PromoCon social workers, social care providers | 5–10 | EVIDEM–C network of advisers and contacts |
Individual experts will be identified through publications in this field, from the advice of the wider EVIDEM team, the EVIDEM-C advisory group and events held in the host NHS Trust. Each identified person will be sent the draft assessment tool and a data collection form. Analysis will be by descriptive statistics for the quantitative elements. Thematic analysis165 of any text received in the comments section.
Ethics
NHS research ethics query service advised that this is not within the domain of the NHS ethics committee. University requirements will be followed. Anonymity and confidentiality of participants will be kept by the use of participant identifying numbers. Data will be kept in locked filing cabinets and on password protected University computers.
Timescale
Stage one will commence in the Autumn 2010.
Stage two, early Winter 2010.
Stage three, Spring 2011.
Reports and dissemination
The findings will be written up as a brief report for circulation to participants and in the EVIDEM final report. Papers and articles to be submitted to a professional journal and local and national voluntary organisation’s newsletters. An abstract will be submitted for presentation at professional conferences.
Appendix 24 Chapter 3: Adoption by research networks and research permissions
The EVIDEM-C study was adopted onto the portfolio of the UK Clinical Research Network (UKCRN) and by the associated research networks for primary care and dementia and neurodegenerative diseases. Individual study elements required permissions from each organisation through which participants were approached. In addition, application and agreement for payment of NHS support to research costs to GPs was required for some elements. This is summarised below in Table 105. Most of these processes required completion of forms specific to that organisation and then correspondence, monitoring returns (from monthly to completions of data collection to annual) and often reports on study completion. Throughout the period of the EVIDEM-C study the research governance processes and the organisations responsible for them repeatedly altered.
| Study element | Research network adoption | UKCRN Portfolio ID | Body/bodies agreeing research access permissions | Body/bodies agreeing NHS service support costs |
|---|---|---|---|---|
| Qualitative interviews study | UKCRN | 4938 (EVIDEM-C-1) | West London Mental Health Research & Development Consortium | |
| DeNDRoN | Brent PCT for 6 North West London PCTs | Brent PCT for North West London PCTs | ||
| Westminster PCT | ||||
| NIHR PCRN | London Borough of Westminster Social Care Services | |||
| Housing 21 | ||||
| For Dementia | ||||
| Age Concern (Westminster) | ||||
| Four local branches of Alzheimer’s Society | ||||
| Longitudinal study | UKCRN | 8928 (EVIDEM-C-2) | West London Mental Health Research & Development Consortium | |
| PCRN– Greater London as above | Brent PCT for 6 North West London PCTs | Brent PCT for North West London PCTs | ||
| Hounslow PCT | ||||
| Westminster PCT | ||||
| London Borough of Westminster Social Care Services | ||||
| Housing 21 | ||||
| For Dementia | ||||
| Age Concern (Westminster) | ||||
| Four local branches of Alzheimer’s Society | ||||
| Absorbent pads study | UKCRN | 10058 (EVIDEM-C-3) | ||
| DeNDRoN as above | ||||
| PCRN-Greater London as above | South East London NHS Research & Development Centre |
South East London NHS R&D Centre | ||
| South West London Primary & Community Care | South West London Primary & Community Care | |||
| West London Mental Health Trust | ||||
| CNWL NHS Foundation Trust | ||||
| Age Concern (Westminster) | ||||
| Eight local branches of the Alzheimer’s Society | ||||
Appendix 25 Chapter 3: EVIDEM-CL1 March 2011 – briefing for Local Authority Adult Services managers and social workers
Meeting the needs of frail and cognitively impaired older people with incontinence in order to maintain independence and minimise the consequences for the person, their family and health and social care services.
Prepared by Vari Drennan, Jill Manthorpe and Steve Iliffe.
Background
Incontinence is leakage of urine or faeces or both. It is a symptom of a bladder or bowel problem. The prevalence of incontinence rises with age, with estimates of up to 15% of older women and 2–11% for older men experiencing daily urinary incontinence (UI). 116 Among people living in care settings the rates are even higher. The risk of women having UI increases with factors such as having three or more children (although this diminishes with increasing age), obesity, diabetes and moderate to severe dementia. 116 We know less about factors that predispose men to UI but these include urinary tract infections, disability and cognitive impairment, brain disorders and prostate problems. Among people aged over 60, rates of faecal incontinence are 5.1% in men and 6.2% in women116 and these increase with age. It can be caused by problems in the gastrointestinal or nervous systems as well as food intolerances, infections and the interplay between loss of mobility and cognition. 416 For some people with physical and/or cognitive impairments incontinence is the result of a lack of aids, unadapted environments, or a lack of timely assistance rather than a bladder or bowel problem.
Incontinence lowers a person’s quality of life and adversely affects mental health. 351 It can be socially embarrassing, restricts social activity, creates extra work, such as laundry, and can mean much money has to be spent on replacement clothing and bedding. It can cause conflict between individuals and their family417 and can make caring harder. Other health problems may get even worse, and it can lead to events such as falls. 353
There are no UK estimates of the total public expenditure associated with treating or managing incontinence. There are some indications of the scale of the direct health service expenditure. It was estimated that the NHS spent £536 million at 1999/2000 prices in treating clinically significant urinary incontinence symptoms in community-dwelling adults. 418 In 2007 nearly 1.5 million NHS prescriptions were filled for incontinence appliances in England at a cost of £33 million. 413 Absorbent products for incontinence account for the majority of this spending. 181
This briefing highlights the key actions in six areas that Local Authority staff and funded services can take in helping to promote continence and manage incontinence to minimise the consequences for the service user, the family carers and the health and social care services. Each action point is elaborated by a brief explanation and followed by a range of questions raising key points for adult service managers and social workers to consider.
Key action points
Assessment before assumptions
In many cases incontinence can be treated and the symptoms stop or lessen but treatment can only start if the person seeks help, usually from their GP or community nurse, who should conduct a thorough assessment. Assumptions that these symptoms should just be managed by absorbent products i.e. incontinence pads, are demoralising for the person and costly to them, their families and any health and social care services.
Key points for social care: many bowel and bladder problems can be treated or the consequences reduced
Are care planners and social care staff actively encouraging and helping service users to consult their GP or community nurse for an assessment if they have these bladder or bowel symptoms or the symptoms change? Do they encourage family carers to seek help? Is this information available to personal assistants? Do the local authority and NHS publish information about this? Is this information given to people in frontline, information, signposting and other community-facing roles? Do social care workers, older people and families have confidence that these problems are addressed by GPs and not seen as ones where nothing can be done because of a person’s age? How are the experiences of those making referrals audited or collated? What is done with any concerns?
Are positive stories about the point of consulting NHS professionals available to social care staff? Are these promoted among staff who may not encounter many professionals or who are worried of being seen as interfering? Are such messages as ‘requests for assessment are welcome’ promoted among families, volunteers and housing support staff?
Promoting continence
The likelihood of bladder and bowel problems increases with age and having other medical problems. Simple steps can help avoid some bladder and bowel problems and associated incontinence. These include:
-
a balanced diet, with sufficient fibre, e.g. five portions of fruit and vegetables a day, 6–8 glasses of fluids a day (approximately 1.2 litres or 2–3 pints) – more in hot weather.
-
general exercise of up to 30 minutes five times a week (adapted exercise for those with disabilities)
-
a toilet routine that allows privacy, dignity and adequate time for their bowels to open; and
-
in the case of those with impaired mobility or dexterity (skill in using hands), adaptations, aids or assistance (such as raised toilet seats, grab rails, elastic waist trousers rather than zips, etc.).
Key points for social care: there are basic steps to promote continence and prevent incontinence
Are basic prevention factors understood, in place in support plans, and promoted in social care contacts with users of services, particularly older people?
Are these subjects covered in reviews? What evidence is available that they are covered in self- or facilitated assessments, support planning and reviews? Would an audit of support plans show that these subjects are being addressed? What outcomes are recorded in this area?
Needing the help of other people with managing toileting
Many older people with health problems and disabilities have limited mobility and reduced dexterity. They may need support in acquiring the best adaptations, aids or assistance to maintain independence in toileting. For some people this will mean that they can only remain continent if they are supported by other people. 117 This has implications for family and other carers and social care supporters. Extensive help will very likely be needed if a person becomes seriously cognitively impaired, for example with Alzheimer’s disease.
Key points for social care: some people will only remain continent with aids, adaptations and assistance
Is publicly funded social care focused on maintaining continence rather than just relying on the premature use of continence pads which lower self esteem and are costly? Can re-ablement and other prevention services be called upon and what are the referral routes? What importance does the Resource Allocation System (RAS) give to these outcomes and what sums are allocated? What information is given about sources of equipment to people paying for all their social care as well as those receiving some support through personal budgets or help with paying care home fees?
Do carers and social care providers have access to training and support to enable them to help people remain continent? What is the access point for carers and social care workers if they have questions? What are the training opportunities for carers and care workers around subjects such as ‘prompted voiding’ (the reminding or helping someone to use the toilet regularly of a person with dementia)? Are these training opportunities available to personal assistants and to carers?
What is the local source of advice for care managers and care staff on aids and adaptations and what is provided through publicly funded joint (NHS and local government) equipment stores? How are such services audited in terms of access and acceptability? Are local older people engaged in activities such as ‘mystery shopping’ to see if such services are accessible and informative? Are charges and payments systems clear and fair?
Are frontline staff well equipped to inform people about sources of information and advice, particularly those not meeting publicly funded social care eligibility criteria? Are information, brokerage and navigation services audited to ensure that they are giving the best support?
Do care managers, brokers, dementia advisers and others supporting people with dementia and their carers consider specifically what assistance with continence might be needed and who is responsible for this? How and where is this recorded?
Do planners and providers consider the needs of people with dementia in the design of day centres and care homes, e.g. toilet signage, adequate lighting, contrasting colours for toilet seats? Are aspects of good design part of neighbourhood and community planning and regeneration?
Managing incontinence
Some people’s incontinence cannot be treated or the underlying condition means they are unable to regain continence e.g. teaching pelvic floor exercises may not work for a person with dementia. The aim then is to manage and contain incontinence and minimise distress to all concerned. It may be possible to manage urinary incontinence and maintain bowel continence by assisting the person to sit on the toilet or commode after a meal (often breakfast), or at the time they usually open their bowels.
Key points for social care: many people who have urinary incontinence contained by pads are able to sit on a toilet or commode to open their bowels
Has the extent of support or aids required to assist someone to open their bowels on a toilet or commode been considered by those supporting people with assessments and in support planning? Is this part of desired outcomes for people affected, including carers? Are the costs of meeting these outcomes included in the Resource Allocation System (RAS)? Is information about help with equipment such as dryers and washing machines from the Social Fund or the voluntary sector clearly signposted to older people, carers and advice sources?
Absorbent products
The aim for the person, their family carers and for health and social care staff is to ensure that the use of incontinence pads leads to the best possible outcomes. Points to consider include ensuring that they:
-
adequately contain the urine and/or faeces without leakage (both during the day and at night)
-
are changed in ways to preserve dignity and as required to avoid skin damage
-
are a design that is acceptable to, and manageable by, the person who is changing the pads
-
are used according to the manufacturers’ instructions
-
are disposed of safely.
Chair and mattress protection may help meet outcomes such as promoting hygiene, in the context other issues such difficulty in pad design or in the case of a person with cognitive impairment who may be unwilling to use pads (dry or wet) or who may disturb or take them off.
The Department of Health has stated that the NHS supplies absorbent continence products according to clinical need364 and has developed standards and criteria for continence services. 365 Social care services managers may wish to consult these if there are disagreements about responsibilities. The Royal College of Physicians’ national audits show great variation in provision and policies. 158
Key points for social care: incontinence products
Are care managers and social care managers aware of local community health services/NHS policies on the provision of NHS-funded continence pads including any eligibility criteria? Has there been discussion of how these are operationalised at a local level, e.g. at Health and Wellbeing Boards or Overview and Scrutiny Committees? Do social care services staff in all sectors know how to refer people for an assessment for these products? Are care managers and care providers aware of other sources of information about these products such as the web-based PromoCon (part of the Disabled Living Foundation), the Disabled Living Foundation and the Bladder and Bowel Foundation Charity? Is information about these readily accessible on the internet and other public information services?
Are care managers and social care providers aware of the local contacts for altering or stopping deliveries of NHS funded pads when the need changes, or the person moves or dies?
How are people given choice and control over the products that are supplied by the NHS?
Do social care staff receive education and training in the correct use of different designs of pads and how to maintain modesty and dignity while changing pads? Do they receive training in hygienic practices and correct disposal? How is this audited? How do complaints about this lead to improvement? How are NHS services informed by local feedback on the quality of the service as well as the quality of the product?
Challenging behaviour and ‘matter’ out of place
Assistance in toileting and intimate, personal care can seem particularly insulting or threatening for someone with dementia and may be a flash point for the person to resist help or become aggressive. Other challenges for carers and staff may include faecal smearing, and lack of hygiene. Visitors may misinterpret care practices and the issue may lead to conflict between carers and care staff. Both older people and care workers may have cultural preferences that need to be considered. The Mental Capacity Act 2005 provides a framework for acting legally and in a person’s best interests in this area of making decisions, as it does in others when a person lacks capacity to make decisions. Some situations may give rise to concerns about safeguarding and advice should be sought on these from safeguarding staff in accordance with local policy and procedures.
Key points for social care: challenging behaviour in toileting and intimate personal care
Have social care providers received training in working with people with dementia and are they knowledgeable about communicating with people with dementia to reduce fear, anxiety and resistance? What support is available to staff and carers in written but also face-to-face learning? Are there opportunities for staff to discuss intimate care in group or personal supervision? How well are interventions, information and support meeting the needs of diverse groups of older people, carers and care workers? Is care informed by the principles and guidance of the Mental Capacity Act when carers and care workers are making decisions about supporting people lacking capacity when they are acting in their best interests, e.g. carrying out care when a person is refusing this?
Are social care staff aware of behavioural management techniques that may help to identify potential causes of challenging behaviours? Are social care staff aware of who to either refer the person to, or encourage the person and their family to approach for assessment and strategies to manage or lessen behavioural and psychological disturbances? What is the referral pathway in the locality and is there evidence that it leads to positive outcomes? Are there any examples that can be used to assure care staff and carers that this is worth the trouble?
Incontinence is humiliating, distressing and embarrassing for any adult. Denial and concealing this problem from others is a common response. In people with dementia this can sometimes mean inappropriate activities, e.g. hiding soiled clothing and forgetting it, wrapping up faeces and hiding them. As the dementia progresses, identifying the correct place to urinate or defecate can also become a problem, as can apathy, and both can result in urine or faeces spillage. Attempts to clear up leakage and ‘matter’ out of place can also be ineffective or make matters worse from a carer’s or care worker’s point of view. If a person has faeces on their hands and tries to remove them (also known as faecal smearing) this can cause great distress. This can occur when the person can no longer manage personal hygiene after defecating or the person has tried to manipulate the pelvic floor or rectum to help defecation, or in severe dementia as an innate response of curiosity to the sensation of faeces exiting the body. These types of behaviours can place additional stress and distress on family carers and care workers, as well as visitors and others living in close proximity.
Key points for social care: when urine and faeces is out of place
Are care managers and care providers aware of contact points for obtaining assessment and advice in preventing and managing these situations? Is access to advice timely and useful? Are people who are not eligible for publicly funded social care services fully included in information and advice resources?
Are carers and social care workers provided with accessible and realistic information on managing spillage and leakage hygienically? Who is responsible for ensuring this information is useful and how is it audited? What is the involvement of carers and frontline staff in the construction of referral pathways and in the production of information? What records are kept to ensure that care practices are lawful and that staff are protected from unfair allegations that they are acting unlawfully? Are risk assessments used to support good practice and decision-making? Are safeguarding staff able to access expertise when responding to referrals or enquiries in this area?
Questions to address in the overall commissioning and provision
What do older people say about local information and services? What information is available to carers and to direct care workers, and is this accurate, sufficient and accessible?
How are local responsibilities for information, equipment, advice and professional interventions divided or managed jointly?
What should be the responses to an actual or future audit of assessments, support plans and reviews covering continence and incontinence?
If managing continence and incontinence is to be part of the outcomes framework in local commissioning, what needs to happen locally for this to be successful?
Acknowledgements
‘This briefing has been developed from independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (Rp-PG-0606–1005 known as EVIDEM evidem.org.uk). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.’
Vari Drennan is Professor of Health Policy and Service Delivery, St George’s University of London and Kingston University; Jill Manthorpe is Professor of Social Work, King’s College London; Steve Iliffe is Professor of Primary Care for Older People, University College London. The EVIDEM programme is funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (Rp-PG-0606–1005). evidem.org.uk
Research instruments and tools
These appendices provide examples of the research instruments and tools used in the different EVIDEM-C studies.
Appendix 26 Chapter 3: Aide-memoire for guided conversations with the person with dementia
Instructions to researcher
Repeat rationale for the study and the process of this data collection. Confirm confidentiality and anonymity in reporting. Confirm that they are willing to carry on with the discussion. Confirm permission to record or not. Confirm that the exploratory discussion can be stopped at any time.
The discussion will start with general enquires of health and well-being to start establishing a non-threatening rapport and set the tone for the conversation about the more sensitive issues.
Discussion should centre around these themes:
-
Some people like you sometimes experience problems with not being able to get to the toilet in time, do you ever have those sort of problems?
-
Conversational probes for types of problems – urge/stress urinary incontinence, nocturia, finding the toilet, managing clothes, etc.
-
-
What helps you when you have that . . . INSERT . . . problems? How do you manage?
-
Conversational probes for types of strategies for addressing those problems and preferences, e.g. containment pads/pants, commode/urinal by bed, manageable clothes.
-
Conversational probes on preferences and feelings about different strategies. How do you feel about managing in that way?
-
-
Some people like you sometimes experience problems with their bowels, getting constipated and then sometimes very loose motions, do you ever have those sorts of problems?
-
Conversational probes for types of problems with bowels.
-
-
What helps you when you have those sorts of problems?
-
Conversational probes for types of strategies and preferences.
-
-
Some people discuss these sorts of problems with their family doctor or with a district or continence nurse? Have you discussed these problems with your doctor or any other professional?
Probes for any views on experience of discussing or receiving help for these problems.
Confirm demographic information:
-
age band, e.g. sixties, seventies, etc.
-
living arrangements, e.g. living with family or alone.
Check whether uses any other health and social care services not mentioned above.
Closure by thanking for their contribution and confirm arrangements for summary of all research findings to be shared.
Appendix 27 Chapter 3: Aide-memoire in the qualitative interview study with family carers
Instructions for researcher
Repeat rationale for the study and the process of this data collection. Confirm confidentiality and anonymity in reporting. Confirm permission to record or not. Repeat that the interview can stop at any time.
The interview will start with general enquires of health and well-being of the close carer and the types of support and help they give to the person with dementia they care for. The interview then moves to the more sensitive issues.
-
Can you describe any problems your (insert – husband/wife, partner, parent, etc.) has with going to the toilet or being incontinent? Probes into:
-
urinary and faecal
-
origin of problems
-
impact of different types of incontinence
-
-
What types of interventions/strategies/support help you in helping support your loved one (a) stay continent and (b) manage their incontinence? Probe:
-
explore strategies for different problems, for day and night-time or for changing circumstances
-
explore whether strategies ideas worked out alone or from suggestions/help from professionals/other carers
-
-
Are there problems/situations that are more difficult to cope with than others? Probe:
-
emotional issues in relation to dealing with adult urine/faeces, intimate tasks
-
successful strategies but with other consequences like restricting outside activities
-
-
Have your discussed these sorts of problems with your family doctor or with continence nurse or any other professional?
-
Are you currently using services/aids/equipment to help manage these problems? If yes what sort, how acquired and what has that experience been like?
-
Confirm demographic information
-
age band, e.g. sixties, seventies
-
record gender
-
ask whether has had a carer’s assessment of need by social services
-
ask whether in receipt of additional financial benefits, e.g. attendance allowance.
-
Closure by thanking for their contribution and confirm arrangements for summary for findings to be shared.
Appendix 28 Chapter 3: Interview tools to characterise the person with dementia and the carer in the longitudinal study and absorbent pads study
Appendix 29 Chapter 3: Validated tools used in the longitudinal study and the absorbent pads study
| Measures | References | Permissions |
|---|---|---|
| Neuropsychiatric Inventory (NPI) | Cummings JL, Mega M, Grey K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–14 | Permission obtained |
| Health-related quality of life for people with dementia (DEMQOL) | Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005:9(10). URL: www.iop.kcl.ac.uk/departments/?locator = 330&context = 773 | Publicly available |
| Mini Mental State Examination (MMSE) | Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 | Publicly available |
| International Consultation on Incontinence Questionnaire: Urinary Incontinence (ICIQ-UI) and Nocturia (ICIQ-N) | Avery K, Donovan J, Peters T, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322–30. | Permissions obtained |
| Disability Assessment for Dementia (DAD) | Gelinas I, Gauthier L, McIntyre M. Development of a functional measure for persons with Alzheimer’s disease: the Disability Assessment for Dementia. Am J Occupat Ther 1999;53:471–81 | Permissions obtained |
| International Statistical Classification of Diseases and Related Health Problems: dementia (ICD-10) | URL: www.who.int/classifications/icd/en/ | Publicly available |
| Health outcomes/quality of life (EQ-5D) | URL: www.euroqol.org/ | Permission obtained |
| Zarit Burden Inventory (ZBI) | Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649–55 | Permission obtained |
| Census ethnicity categories | Office for National Statistics (ONS) | Publicly available |
| Client Service Receipt Inventory (CSRI) | Reference for CALM study: Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donezepil for the Treatment of Agitation in Alzheimer’s Disease. N Engl J Med 2007;357:1382–92 Schneider J, Murray J, Banerjee S, Mann A. EUROCARE: A cross-national study of co-resident spouse carers for people with Alzheimer’s Disease: I factors associated with carer burden. Int J Geriatr Psychiatry 1999;14:651–61 |
Permission obtained |
Appendix 30 Chapter 3: Toileting difficulties and incontinence questions used in addition to the ICIQ-UI and ICIQ-N, for the longitudinal study and the absorbent pads study
Appendix 31 Chapter 3: Further incontinence questions about pad usage for the longitudinal study
Appendix 32 Chapter 3: Additional table in the Client Service Receipt Inventory (part 1) longitudinal and absorbent pads study
| Adaptations, equipment and products | Tick if yes | Type of adaptation or equipment (list all) | Who supplied this? | Who/what organisation paid for this? |
|---|---|---|---|---|
| Changes to service user’s home (e.g. putting in shower cubicle, stair lift) | ||||
| Special equipment (e.g. for mobility, bathing, etc.) | ||||
| Continence products (e.g. pads, pull-up pants) | ||||
| Aids to getting to and using the toilet or protecting bedding/furniture (e.g. grab rails, raised toilet, urinal bottles) | ||||
| Furniture, clothing, bedding or household goods replaced because of incontinence problems |
Appendix 33 Chapter 3: Data collection tools for examining feasibility and acceptability in the absorbent pads study
Appendix 34 Chapter 3: Additional table in the Client Service Receipt Inventory (part 1) for the absorbent pads study
Absorbent products
| Brand | Size | Teardrops | Amount/frequency | Source, e.g. NHS/internet | Cost (if applicable) | Notes |
|---|---|---|---|---|---|---|
Other continence productsa
| Name | Source, e.g. NHS/internet | Cost (if applicable) | Notes |
|---|---|---|---|
EVIDEM C Investigating absorbent products CSRI Version 1 29032010
Appendix 35 Chapter 4: Phase 1 protocol
REC REF 08/H0502/74
Changing practice in dementia care in care homes: developing and testing evidence-based interventions at the end of life
PI: Professor Claire Goodman, University of Hertfordshire, c.goodman@herts.ac.uk
Background
How people with dementia die, and the type of end-of-life interventions they should receive, has been widely discussed and partially introduced across the NHS and care home sector. 191–193 These discussions focus particularly on place of care and the adaptation of existing approaches to provide palliative care for people with dementia. There remains, however, a limited understanding of how this population experience the end of life and if the recognition of an end stage of life in dementia can improve service provision and influence place of care.
Older people with dementia have a limited life expectancy from the time they receive a diagnosis, and particularly on entry to a care home. Cox and Cook190 identify three ways in which people with dementia can die:
-
People who have a diagnosis of dementia at the end of life, but whose death is caused by another medical condition, for example, cancer, or heart disease.
-
People who die due to a complex interplay of mental and physical problems, where the dementia has not impacted greatly on their functioning.
-
People who are described as having end-stage dementia, where the associated consequences of the dementia impact upon all domains of their life and they ultimately die of the complications that go with progressive neurodegeneration.
Each of these different ways will directly influence the place and experience of death for an individual and his/her family members. Death for people with dementia is not a single trajectory, rather patients and carers live with dementia for years often with different trajectories of functional decline and needs. 194,195
Practitioners experience many difficulties in recognising and responding to older people’s needs with dementia at the end of life. Practitioners of all disciplines lack confidence in discussing advance care planning with patients and their families, and find the recognition of when someone is dying, and identification of the kind of support needed, problematic. 197,199 The range of health needs experienced at the end of life by people with dementia is many. Communication problems, confusion, functional loss, nutrition and complications, such as, infections and incontinence, can compound the distress of a person who is dying and challenge clinicians’ knowledge and expertise. 419 Attempting to manage these challenges can result in inappropriate medical interventions and referral to acute care. 198
Few diagnostic and support tools have been developed for primary care, or take into account the range of community-based professionals and support workers that can be involved in caring for people with dementia in a care home. In the United Kingdom, over 13,000 care homes offer personal care and approximately 405,000 older people receive care in these settings. The average care home providing personal care has between 26 and 36 places depending on ownership. 420 Twenty-three per cent of older people over 65 will die in a care home, the majority of whom will die with dementia. It is often assumed that most people with dementia die in a care home with nursing care (a nursing home), but homes with personal care only (a residential home) care for a significant number of older people who have some degree of dementia, which may be unrecognised. 193 Care homes without on-site nursing provision are wholly reliant on primary care services for first contact, referral and access to health-care and end-of-life services. This makes end-of-life care an important issue for a wide, primary care-based workforce.
An integrative review of qualitative and quantitative research on palliative care interventions for community-dwelling older people with dementia (including older people in care homes), demonstrates that research on end-of-life care for people with dementia poses significant methodological challenges. 200 The North American context of care heavily influences this literature where a focus on prognostication is linked to decisions about reimbursement for medical costs and use of more ‘aggressive’ interventions for people with advanced symptoms of dementia, for example, management of pneumonia with intravenous antibiotics. Moreover, the majority of research on end-of-life care for people with dementia tends to rely on proxy or retrospective accounts of particular symptoms and events in the illness. Few studies have addressed the environment and organisation of care, or the affect of different care decisions on patient outcomes.
It is important to recognise that the organisation and context of care home provision for people with dementia is highly variable and heterogeneous. 202,203 This affects individuals’ access to care and experience of support at the end of their lives. The care home sector is a mixed economy of care operating with different patterns of ownership (local authority, private, charitable and voluntary) and size of organisation (e.g. a single independent care home/a commercial chain). The cognitive ability and dependency of residents in care homes also varies by care home, and there is a considerable overlap in residents’ needs between settings with on-site nursing and those without. 205 Despite a requirement by the National Minimum Care Standards for Care Homes that 50% of care staff within a home are trained to NVQ level 2 by 2005,421,422 there is a wide variation in the levels of staff training that care homes offer and expect from their care staff. Many factors influence how care homes work with primary health-care services, and the degree a care home is likely to engage with end-of-life care for their residents. These include factors both within a care home, such as, ownership, care staff turnover, and qualifications held by a care home manager (e.g. a nursing qualification), and service provision to a care home, for example, GP attachment, the payment of a GP retainer, a locality’s (LA/PCT) organisational history of innovation with care homes. 198,199
This study, the EVIDEM-end of life project, has two phases. This protocol addresses Phase 1 of the study. In Phase 1 we aim to establish the different possible pathways to death for older people with dementia living in a care home. The second phase will use these findings to develop and test dementia specific interventions that support health-care professionals, community workers and care home staff in palliative care for older people with dementia in care homes. The Phase 1 findings will directly inform Phase 2 that intends to develop a toolkit for providing end-of-life care for people with dementia resident in a care home.
Aim
To explore and document the need for support and end-of-life care of older people with dementia living in a care home environment.
Objectives
-
To describe the different characteristics of older people with dementia residing in a care home and their respective pathways to death.
-
To describe the care and support needs of older people with dementia living in a care home and their carers.
-
To establish how care staff, and NHS primary care practitioners define, assess and provide end-of-life care for older people with dementia resident in a care home.
-
To describe how different contexts and models of care in care home environments influence an older person with dementia’s experience of end-of-life care.
-
To identify the treatments and interventions received, and services and resources used, leading up to an older person’s death.
-
To establish if dementia specific educational support and assessment tools can improve experiences and outcomes for older people with dementia dying in care homes (this applies to Phase 2 of the study).
Definitions of end-of-life care
End-of-life care is a contested term423 and consequent inconsistencies in understanding can lead to different emphasis in care provision. End-of-life care as a type of service provision is closely related to the palliative care speciality. Palliative care focuses upon the support of people as they live and die with a life limiting illness. The World Health Organization (WHO) defines palliative care as:
An approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual. 420
This WHO stance on palliative care adopts a public health perspective with emphasis on early identification to prevent symptoms and ensure optimal disease management. 420 There is a distinction between generalist and specialist palliative care services:424,425
-
Generalist palliative care services are provided by health and social care practitioners to individuals in whatever care setting they reside.
-
Specialist palliative care services support these generalist services and provide care for people with unresolved symptoms, or complex psychosocial, end-of-life or bereavement issues. Specialist palliative care may be provided in dedicated settings such as hospices, but also in hospitals, people’s homes, and long-term care facilities.
End-of-life care is a broader term than ‘terminal care’. Terminal care is usually associated with the last few days and hours of life, and is based upon the knowledge that an individual is dying, while end-of-life care encompasses more than the terminal care phase. The term ‘end of life’ originates from North America and is particularly used in the context of the care of older people.
End of life care for seniors requires an active, compassionate approach that treats, comforts and supports older individuals who are living with, or dying from, progressive or chronic life-threatening conditions. Such care is sensitive to personal, cultural and spiritual values, beliefs and practices and encompasses support for families and friends up to and including the period of bereavement. 426
Phase I study design
The project uses a two-phase prospective study design to explore and document needs for support and end-of-life care for people with dementia in care homes to develop care practices at the end of life. This proposal refers to Phase 1 only. Phase 1 intends to use documentary review and interviews to prospectively track care needs and service use for people with dementia in care homes over a 2-year period at 4-monthly intervals. The study will involve older people with dementia resident in care homes, care staff, NHS primary care practitioners and social service personnel. The Phase 1 findings will inform Phase 2, developing and evaluating care practices at the end of life for people with dementia in care homes. A separate NRES submission will be made for Phase 2.
Study settings
Six care homes in a range of possible sites within the North Thames DeNDRoN area (i.e. Camden, Barnet, Enfield, Haringey, Islington, Barking and Dagenham, City and Hackney, Havering, Newham, Redbridge, Tower Hamlets, Waltham Forrest, Brent, Hammersmith and Fulham, Ealing Kensington and Chelsea, Harrow, Hillingdon, Hounslow, Westminster, Hertfordshire, Bedfordshire and Essex) and registered with the Commission for Social Care and Inspection (CSCI) as offering personal care and specialist support in dementia care, with up to 50 beds (the median size of a care home in England offering personal care is 35 beds)427 and which, in recent inspections by CSCI, have been assessed as meeting National Minimum Standards. 422 To reflect the diversity of care home provision within England, and London in particular, requires the selection and identification of care homes that reflect a range of providers (e.g. private, voluntary/charitable and national chain), staffing expertise and history of working with NHS services (e.g. use of integrated care pathways, adoption of palliative care frameworks, etc.). The participating care home settings will therefore reflect a mix of ownership to include care homes that are part of a large commercial chain, private, voluntary, charitable and faith-based providers.
Recruitment
In the UK there are over 13,000 care homes that offer personal care and approximately 405,000 older people receiving care in residential settings. 428 Older people in care homes with personal care represent the majority of residents in long-term care. For this population, access to health and palliative care services is mediated wholly through primary care services. This group are the focus of the study.
The care homes
The sensitivity of the focus of the study on end-of-life care for people with dementia, and the need to maintain a relationship with the care home staff over a 2-year period, requires that the recruitment of care homes and participants is undertaken in three stages, namely:
Stage 1: identification of possible care homes
The intrinsic heterogeneity (culture of care, population, staff turnover, income, ownership) of care homes makes identifying representative care homes problematic. The median size of a care home with personal care is 35 places. 427 Using the CSCI Directory of Care Homes and Care services, we will identify eligible care homes from the range of possible sites in the North DeNDRoN area and Sussex on the basis that:
-
The care home is for older people, offering personal care and specialist support in dementia care.
-
The care home does not have on-site nursing care (care homes with mixed provision can be included in the study, we will recruit participants who have been assessed as not requiring nursing care).
-
Has between 20 and 50 places.
-
The most recent CSCI inspection report is favourable, with no ongoing problems or issues identified requiring action.
-
The care homes’ ‘typicality’ is comparable with findings from other national studies and similar to one another.
-
Care home staff consider they have a good working relationship with their local primary care services.
-
The final sample has a mix of ownership (e.g. charity, voluntary, private, corporate).
Stage 2: invitation to express an interest in participation
We will write to the managers of the identified care homes, introducing the study and asking if we can telephone to arrange a meeting at a mutually convenient time with the manager/senior management team to discuss the study’s aim, and what participation will involve. Where several care homes are represented by one provider, we will first approach the owner organisation to gauge their interest and willingness for their care homes to participate, and then write to the relevant care home managers. Managers and liaison staff within a Primary Care Trust (and Local Authority) where a care home has signalled an interest to participate will inform NHS professionals and social service personnel about the study taking place within their respective organisations.
Stage 3: discussion with older people, care staff, NHS and social service personnel
Having met with the care home manager, we will arrange meetings with the different participant groups to introduce ourselves and the study. This will include a care home’s residents’ forum, care home staff (preferably without the care home manager being present) and the relevant primary health care teams, and social service personnel. These will be informal meetings to gauge the different groups’ level of interest and engagement with the study, and identify possible reasons for a care home or NHS staff not taking part (e.g. staff sickness, reorganisation within the care home). We will host coffee mornings/afternoon tea events in interested care homes to encourage discussion and questions about the study.
Once it has been established which six care homes will participate in the study, we will provide all the potential participants (older people, care staff within a care home, and NHS and social service staff providing services to a care home) with information booklets about the study. We will ask to display a poster about the study in each care home to inform visitors that a research study is taking place. All participants will have a minimum of 48 hours to consider information provided about the study before further contact by the researcher to gauge interest or not in the study. To enable the older people (and/or their consultee) to discuss the older person’s participation with family and trusted friends, we will allow a week for them to consider if they would like to take part. It will be made clear from the start that a decision whether to take part in the study or not is entirely voluntary and that their decision will not affect care or working in the care home or PCT in any way. At no stage will care home or NHS staff be involved in providing detailed information about the study to the older people, or in the consenting process.
Older people with dementia
A recent study found that the numbers of older people with a recorded diagnosis of dementia account for 46% of older people resident in care homes with no on-site nursing care. 429 This is consistent with larger surveys of residents of care homes with personal care. The PSSRU report that 22% of residents’ experience severe cognitive impairment430 and 48% mild/moderate impairment. Similarly, a secondary analysis of the Health Survey for England 2000 indicates 24% of residents experience severe impairment,431 but 30% of residents were considered unable to complete a cognitive function test, indicating likely under reporting. In the PSSRU study, most individuals with severe impairment on admission had no documented diagnosis of dementia. 206
To address objectives one, two and five, and capture the range of characteristics, episodes of ill health, care needs and service use experienced by older people with dementia, we will purposively recruit a minimum of 120, and up to 250, older people resident in 10 care homes with personal care and no on-site nursing. The older people approached to participate will either have a recorded diagnosis of dementia in their care notes, or following discussion with their care staff, be identified as having symptoms and behaviour that is consistent with a diagnosis of dementia. Older people will be recruited by project staff using a separate information and consent process, and in discussion with their consultee when a person with dementia lacks capacity to give (informed) consent. Respective inclusion and exclusion criteria comprise:
-
Inclusion criteria Older people aged 65 years and over with a formal diagnosis of dementia (documented medical assessment) or informal diagnosis (considered by care home staff and/or in care notes as having dementia) and a validated measure of cognitive function impairment (undertaken by the researcher), resident in a care home registered to provide personal care only and specialist support in dementia care.
-
Exclusion criteria People who do not speak English for whom an interpreter cannot be located, those whose care home manager considers it inappropriate to approach, and individuals who lack capacity to give an (informed) consent where a consultee cannot be identified to support (or not) the individual’s participation.
Recruitment of the older people will involve a three stage process previously used in care home research431,432 that intends to minimise pressure on individuals to take part and provide opportunities for discussion with others:
-
Stage 1 The respective care home managers will give each older person (and/or their consultee) within the sampling frame a letter of invitation to participate in the study and an information booklet.
-
Stage 2 A member of the care home staff (e.g. the care home manager) will introduce a member of the project staff (e.g. the research fellow) to each older person (and/or their consultee) who has indicated they may wish to participate in the study. A time is agreed with each older person (and/or consultee) and when available, an intermediary, to discuss the study.
-
Stage 3 Meeting with an older person, and an intermediary (if available), such as a care worker or family carer, with whom the older person particularly relates to explain the research study and if in agreement, begin initial consenting. The researcher will ensure that the intermediary is present and able to explain and, if necessary to interpret any areas of concern of lack of understanding. The researcher will ensure that the participant is given full opportunity to have the study explained to them in a way that best meets their individual needs.
In situations where an older person has been formally assessed, and it is documented that they do not have the capacity to consent or take part in an interview, we will ask the older person’s consultee (e.g. a relative) if, based on their knowledge of the older person, allowing a researcher to review his/her care notes would upset or distress them. The care home manager will be asked to write to the consultee about the study and they will be asked to assent to enrolment of the person with dementia. The researcher will arrange to see the person with dementia and his/her consultee to seek their written support for the older person’s participation in the study. In the event that a consultee cannot be identified, then the person with dementia will be excluded from the study.
Amendment – submitted Southampton REC November 2008
The process of engaging consultees in the research study required amendment following a low consultee response rate in the EPOCH-ExPeriences of Older People in Care Homes research study (PI, Claire Goodman). In the event that a nominated consultee does not respond to a care home manager’s letter, additional processes have been put in place to ensure that we have consulted appropriately, comprising:
-
The letter of invitation, sent by the care home manager, requests that the consultee response form is returned (in the SAE) within 2 weeks of receipt.
-
The letter details that if we do not hear from the consultee after 2 weeks we will follow up their response with a telephone call, asking them, if, based on their knowledge of the older person they think they would be interested or not in participating in the study, and allow the researchers to review their medical and nursing notes, and to share anonymous patient data with the EVIDEM Registry. In the event of still not being able to obtain a response from the nominated consultee, we propose to approach the GP, District Nurse or Social Worker, who knows the older person best.
-
If the consultee at any point expresses the opinion that the older person would not wish to take part, that person must be excluded from the study, unless another consultee can be identified.
-
We will then write to consultees who felt the older person would wish to take part, to inform them that the researchers have begun to extract data from the person’s medical and nursing notes.
These additional processes are in keeping with the Mental Capacity Act (2005)17 expectation that ‘reasonable steps’ have been take to identify others who could be consulted about what a prospective participant’s wishes and feelings about participation in the project would be if they had capacity. These changes reflect the British Psychological Society’s recommendations for undertaking research with people who do not have the capacity to consent. 433
The above amendment was approved by the Southampton and South West Hampshire Research Ethics Committee on 6 March 2009, reference no. 08/H0502/74.
Care workers, NHS and social care staff
To address objectives three, four and five (above) we will recruit up to five care home staff from each care home including the care home manager, and up to three NHS professionals (e.g. general practitioner, district nurse and Macmillan nurse) who have ongoing involvement with a care home participating in the study. This will establish how care home, NHS and MHS care staff define end-of-life care for older people with dementia, the assessment and care tools they use, approaches to care, current service provision, access to specialist services and level of training and expertise.
Amendment – submitted Southampton REC July 2009
In addition, early results are indicating that ambulance staff called to the care home will automatically start resuscitation procedures even if a do not resuscitate agreement is in place for the person concerned. We would therefore like to include data from the ambulance service about how they interpret their responsibilities in these situations.
Ethical review and approval for the above amendment has been obtained. The Southampton and South West Hampshire Research Ethics Committee A approved the amendment on 11 August 2009, reference no. 08/H0502/74.
Data collection
In keeping with the study’s aim and objectives, data collection focuses on five main areas, summarised as:
-
Baseline characteristics of the older people with dementia participating in the study, including: age, gender, ethnicity, funding support, level of cognitive and physical function, medical conditions, function and length of stay in the care home.
-
How older people with dementia’s health and care needs change over time, the services they receive, and key events in their lives over the 2 years that affect their health and use of services.
-
Training and experience of care home and NHS staff to provide care to older people with dementia and understanding of older people’s care needs at the end of life.
-
Current assessment and management strategies for people with dementia within the care homes (e.g. assessment tools, care protocols and end-of-life care frameworks).
-
The physical environment, resources, and current access to health-care services of each participating care home.
Older people with dementia: characteristics and change over time
A pilot of the tool to extract data from residents’ care home notes and care plans will be undertaken within two care homes. The development of the tool is informed in particular by previous research projects undertaken in care homes by members of the research team,429,430 the broader EVIDEM research programme, and a further study lead by Professor Claire Goodman; EPOCH-ExPeriences of Older People in Care Homes, examining experiences of living and dying in a care home.
To address objectives one, two and five (above) we will record a baseline for all participants, where possible, the following minimum data set of information (extracted from care home notes and care plans):
-
Demographic details (name, date of birth, gender, marital status, first language, ethnicity).
-
When appropriate: carer information (name, date of birth, gender, address, postcode, relationship to person with dementia).
-
GP and practice details (name, address).
-
Cognitive status: if available, date of most recent test and score. In instances when unavailable, and an individual is considered by care staff to exhibit symptoms likely indicative of dementia, a validated measure of cognitive function will be undertaken.
-
Functional status (date of most recent test and score) and Barthel score in discussion with the older person’s key care worker.
-
Behavioural/neuropsychiatric status.
-
Medication.
-
Co-morbidity (e.g. depression, CVD, diabetes).
We will review existing care plans (including details of advanced advance care plans, and other support tools for end-of-life care and current medication, specifically the use of pain relief and sedatives, and (if held in the care home) residents’ medical notes. These reviews will be repeated every four months over 2 years. Data extraction will focus particularly upon those areas of care that are most significant for end-of-life care. These will include, for example, episodes of ill health, physical care needs, cognition, discussion of advanced care plans, access and use of primary care and specialist services, out of hours services, and bereavement/deaths in each care home. Particular attention will be given to those care needs of older people with dementia that are modifiable and likely to respond to palliative care interventions (e.g. evidence of experiencing pain, agitation, constipation, low mood, confusion, loss of appetite, fatigue).
Where possible, to complement the notes review, brief unstructured interviews are planned with a small purposively selected sample of the older people participating in the study to explore their health, and experiences of living in a care home. Events, reported by care staff, or documented in the notes, that occur during the 4-monthly data collection intervals will inform the purposive selection. Events are those likely indicative of a change in health status and care needs, for example, episodes of ill health, advance care planning. We will also seek interviews in instances of incomplete documentation or an older person’s expression of interest in the study. The interviews with the older participants will help to validate the note review process and identify any changes over time in the older person’s function and cognition that may not have been documented. This will also ensure that as far as is possible the person with dementia’s experience of living and dying in a care home is acknowledged and documented. To facilitate older people’s participation in an interview, we will draw upon different media, for example, ‘talking mats’ used in previous research involving older people with dementia. 434 The media used will inform how interviews are recorded, for example, notes, audio tape.
For those older people who die during the study, care home and GP notes will be retrospectively reviewed, with permission from the respective GP, to establish what services the older person received prior to death, place of death and the care provided. A case study analysis of this group will combine the different data sources for each person (interviews with older people and staff, case notes, field notes, services received) collected over time and allow thematic and outcomes related analysis of the care these older people received.
Amendment – submitted Southampton REC November 2008
Anonymous data from the reviews of residents’ care home notes and care plans (part of the study’s dataset) will be shared with the EVIDEM Registry research study, entitled; ‘A registry of people with dementia using services in the North Thames Dementia and Neurodegenerative Research Network (North Thames DeNDRoN) Region’. The EVIDEM Registry study intends to develop a unique and comprehensive minimum dataset (MDS) of individuals with dementia (or symptoms likely indicative of dementia) at differing stages in the disease trajectory (from diagnosis to nearing the end of life). Recruitment to the Registry is intended through inclusion of anonymous patient data from the four EVIDEM research studies and direct recruitment to the registry (e.g. via general practices).
Ethical review and approval for the EVIDEM Registry has been obtained. The Southampton and South West Hampshire Research Ethics Committee A approved the EVIDEM Registry research study on 30 June 2008, reference no. 08/H0502/34. Ethical approval has also been given for the EVIDEM research study ‘Promoting Continence and Managing Incontinence at Home’ that includes sharing of anonymous patient data with the EVIDEM Registry study. Ethical approval was obtained from Camden and Islington Community Local Ethics Committee on 29 September 2008.
The above amendment was approved by the Southampton and South West Hampshire Research Ethics Committee on 6 March 2009, reference no. 08/H0502/74.
Care home and NHS staff training and experience
To address objectives three, four and five (above), and understand how care home and NHS primary care staff conceptualise, recognise and provide end-of-life care for older people with dementia, we will undertake audio recordings of semi-structured interviews with the care home managers from the participating care homes, and up to five care workers (sampled to reflect the range of experiences and seniority within a care home), up to three NHS staff (i.e. GP, district nurse and palliative care specialist). Semi-structured interviews will cover participants’ length of employment, training, knowledge on end-of-life care and use of relevant tools for the assessment and care of people with dementia, experience and attitudes towards providing end-of-life care for people with dementia, and working with generalist and specialist palliative care services.
Practitioners will be asked to provide up to three examples of where they have provided end-of-life care to an older person with dementia in the study care homes. These examples will help to illustrate what supports and inhibits good end-of-life care for people with dementia.
Emergency care staff
The focus of the interviews was the impact dementia had on the treatment, and care that was provided and the decision-making processes, as well as the policies and protocols used at the end of life. Particular attention was given to the use of Do Not Resuscitate orders and the challenges of adhering to patient wishes, and other challenges that may arise when treating someone who is at the end of life. Finally, two scenarios were described and participants were asked how they would respond to these fictional scenarios and why, if they received the call out. This helped the researchers understand the circumstances and decision-making process when placed in difficult situations.
The care home environment
To inform objectives three and four, in each of the participating care homes we will review care home documentation and policies for evidence of end-of-life care training, use of frameworks and support tools, and guidance on end-of-life care and evidence of the involvement of older people and relatives in end-of-life decision-making. 435 This will include the review of support materials used for training programmes on working with people with dementia and end-of-life care implementing the Mental Capacity Act 2005.
We will also review shared documentation, such as, integrated care pathways, e.g. continence management, and notes that show evidence of older people with dementia’s use of related services, for example, specialist palliative care, hospice support and out of hours services, as well as documenting informal support available to the home (e.g. volunteers, older people advocates and faith organisations).
Analysis of data
Quantitative data
This is a prospective study that aims to describe the symptoms, health needs, care received and experiences of older people with dementia resident in a care home, and their pathway to death. Analysis will focus on physical and mental care needs, social relationships, care interventions, access to support, documented hopes and expectations about death, and access and use of primary care, specialist palliative and psychogeriatric services. Quantitative analysis will include descriptive statistics to summarise the attributes and key events in the lives of the older people over time. This data will provide information about the frequency and pattern of death in the care homes, services that were available to the older people, and the place of death. To ensure the volume of data is managed appropriately, the data will be specifically analysed to consider what it reveals about living in a care home with a diagnosis of dementia and the pathway to death, including:
-
mapping key events
-
episodes of ill health and related prescribing
-
care provision within the care home
-
access to generalist and specialist NHS services
-
place of death, evidence of use and implementation of advance care planning, and care home staff’s expectation (or not) of death and impact on the care provided.
Where older people have received palliative care, analysis will draw on Corner et al.’s436 matrix of evidence for making judgements about the type of palliative care interventions received.
Qualitative data
Summaries of documentary reviews and interview transcripts will be thematically analysed using computer software for qualitative analysis (N7) to facilitate storage, retrieval, and analysis of the data. Data analysis will follow a process of data reduction and data complication to identify prominent themes and their interrelationships. 437 The analysis will involve three stages:
-
a process of familiarisation and categorisation by reviewing and segmenting data into separate and defined categories that are close to the participants’ and documents’ own categories
-
categorical and thematic comparisons within and between groups (e.g. care staff, NHS staff, older people) and the identification of preoccupations, differences and themes
-
data interpretation and recontextualisation to identify thematic relationships and working hypotheses on, for example, pathways to death for people with dementia in care homes.
Credibility of analysis will be achieved through comprehensive data analysis, such as, searching for rival explanations for emergent themes, and peer debriefing with the research team and the steering group.
Economic analysis
The resource implications of providing end-of-life care for people with dementia resident in a care home will be documented and cost calculated. This will include the health and social care services provided by care home staff, and NHS generalist and specialist practitioners. The skill mix and relative contribution of different services will be compared across the different care homes in a cost–consequence framework. 438 This will incorporate the perspectives of health and social care services, older people with dementia, care home staff and other practitioners providing services to the care home.
Ethical issues arising from the study
There are ethical issues about researching sensitive issues and ensuring voluntary and informed consent in a closed community such as a care home. We propose a framework for participatory and inclusionary consent compatible with the Mental Capacity Act 2005. Consent will be seen as an ongoing and context specific process. We will aim whenever possible to include people with dementia or confusion in every stage of the study. It will be stressed throughout the negotiation for access, and within the consent process, that this study is about living in a care home and approaching the end of life. We will also ensure that in each care home processes are in place that support the older person and can address any issues that may arise from participation.
The researchers are very experienced with professional, personal and research experience of working with older people that are vulnerable. Dr Evans as the research fellow is an experienced academic and primary care nurse, who has specialised in the area of gerontology completing a doctoral study that involved working closely with care home staff and interviewing older people in care homes. The researchers will work closely with the senior members of the research team to provide continual supervision, support and guidance. Specific ethical issues arising from this study are:
-
Anonymity and confidentiality All participants will have a unique identification code. No names, or identifying details, will appear on any data collection forms, analysis or draft and final reports. During data collection, access to the names and contact details of participants will be restricted and these will be kept in a password protected computer at the University of Hertfordshire. Those with access will be the research fellow, and members of the research team. All participants will be guaranteed anonymity in written reports, summaries of data analysis and a summary of the findings will be sent out to participants for their comments prior to publication and dissemination. The care homes will not be identifiable and if necessary distinguishing details that are not central to the study will be changed to ensure they are not recognised
-
Consent Care home staff, NHS staff and the older people, may feel they are obliged to take part in the study because either their manager or care worker has identified them as possible participants. They may feel that refusal to participate could adversely affect either their relationships with their employer, or their care worker. In introducing the study to the managers and care home staff, care will be taken to ensure no one feels coerced or obliged to take part. The information sheets stress that participation (or not) in the study will not affect the care they receive or their relations with other people in the care home or primary care services. At each stage of the recruitment process, the researchers will re-iterate that participation is voluntary.
-
People with cognitive impairment/limited understanding The research team in this study have over 10 years experience of working with people who find consent and participation in research difficult, arising from problems of dementia, confusion or illness and fatigue. For people with cognitive impairment, the approach will be informed by the principles of the Mental Capacity Act 2005 that assumes that all adults are capable of giving or refusing consent unless proven otherwise, and that the best interests of the person who lacks capacity are paramount. It is therefore an assumption of the study that older people who may experience short term memory and cognition problems can nevertheless consent in the moment, and that it is the responsibility of the researcher on each occasion to review the study aims, confirm with the participant that they are willing to participate in the study and ensure that they are not alarmed or distressed by the experience. 439,440 At the stage of initial consenting, the researcher will ensure that the participant is given full opportunity to have the study explained to them in a way that best meets their individual needs. If there is an intermediary, such as a care worker or family carer, with whom the older person particularly relates, the researcher will ensure that this intermediary is present and able to explain and, if necessary to interpret any areas of concern of lack of understanding.
In situations where an older person has been formally assessed, and it is documented that they do not have the capacity to consent or take part in an interview, we will ask the older person’s consultee (e.g. a relative) if, based on their knowledge of the older person allowing a researcher to review his/her care notes would upset or distress them (see below).
-
People judged by the practitioner/professional who works most closely with them as lacking capacity to give (informed) consent For those judged by the care worker/care home manager who works most closely with them as lacking the capacity to give consent, or when an older person during the course of the study loses the capacity to consent, a consultee (as outlined in the Mental Capacity Act 2005) will be consulted about the older person’s care notes being reviewed for the purpose of the study. The care home manager will be asked to write to the consultee about the study and they will be asked to assent to enrolment of the person with dementia. The researcher will arrange to see the person with dementia and his/her consultee to seek their written support for the older person’s participation in the study. In the event that a consultee cannot be identified, then the person with dementia will be excluded from the study.
-
Risk There is some risk that involvement in this study may affect an older person’s care by an individual providing or expressing information of which care home and NHS staff were unaware, or through the disclosure of risk. At all stages the researcher will make clear that they cannot be involved in an older person’s care in any way. However, failure to disclose information about observed or reported poor practice may harm an individual particularly if a researcher chooses not to intervene in a potentially damaging situation. 441 If any risk (e.g. evidence of elder abuse, inadequate care, an acute health need) is disclosed or observed, procedures are in place to address this as part of the study protocol and reflect CSCI, PCT and university guidance and procedures. This will be discussed with the care home staff and NHS staff prior to the commencement of data collection.
-
A vulnerable group This research will be conducted with a group of people who are ill, easily tired and who may be vulnerable for a number of reasons. The older people will be treated with dignity and respect at all times, and the researchers who will have contact with the participants are experienced in working with people of very advanced age. Data collection procedures, and participants’ responses, will be carefully monitored and reviewed by the research team throughout the study. The wellbeing and support of the participants in the study are study priorities.
-
Emotional support and supervision for researchers and members of the Public Involvement Group This kind of research is challenging. The contact with the older people and their care workers over time means that the researchers will know some of the older people in the study quite well and be affected if they should die during the study. The research team will meet weekly to debrief and review issues together and meet with their respective research manager (CG) every 2 weeks to ensure adequate support.
Project management and governance
This research will operate under the Research Governance Framework, and the University of Hertfordshire will be the sponsor. As the study involves older people in care homes, and care home staff, we shall also apply for ethical permissions from the relevant social services’ research governance group within the Local Authority departments of social care who work with the participating PCTs.
User and service involvement
An expert advisory panel of representatives from service and the Public Involvement in Research group has been commenting on and influencing the research process throughout, and will continue to do so. There is a heavy reliance on collaboration and co-operation between the research team and service partners.
Dissemination and development of Phase 2
Throughout this study, the identity of all participants and the care homes will be protected and will remain confidential to the study team. All findings will be reported in a way that ensures that no individual, or the study settings, can be identified. A number of dissemination strategies, in partnership with key stakeholders and representative organisations, will be used to ensure the findings inform policy and practice development in end-of-life care for people with dementia. In addition to the traditional methods of dissemination through peer reviewed and professional publication and conference presentations, we propose to disseminate findings through:
-
Presentations to staff, residents and their relatives in the participating care homes and key professional service and user representative groups.
-
Workshops for community and palliative care nurses providing end-of-life care to people with dementia in care homes.
-
Sending a summary report of findings to all participants who wish to receive this.
-
Organisations that the research team have strong links with will be included in a planned process of dissemination, including e-alerts, seminars and publications e.g. Royal College of Nursing, Alzheimer’s Society, National Care Homes Research and Development Forum, DeNDRoN, Dementia Care, Royal Society of Medicine (geriatrics and specialist gerontology section), Queens Nursing Institute, Better Government for Older People, Social services’ research group ‘Making Research Count’, Help the Aged and Age Concern. The planned process will include, for example:
-
Production of summary findings and briefing papers, in collaboration with ‘My Home Life Programme’ for dissemination and discussion with their care home partners (English Community Care Association; National Care Association; National Care Forum), and relative and patient representative organisations.
-
Academic and professional conference presentations (including Dementia and Care Home Congress, and Palliative Care Congress).
-
Links with initiatives by the National Palliative Care Council, Marie Curie, and Alzheimer’s Society for end-of-life care for people with dementia in care homes.
-
The findings from Phase 1 will be fed back to the participants for comment and review. Four care homes from Phase 1 will be involved in Phase 2 of the study. These will be homes interested in developing and testing a toolkit for supporting people with dementia at the end of life. Findings from Phase 1 will inform the development of a protocol to develop and test the toolkit. A separate NRES submission will be made for Phase 2.
Appendix 36 Chapter 4: Phase 2 protocol
REC Ref: 10/H0502/55
EVIDEM-End of Life: Changing practice in dementia care in care homes: developing and testing evidence-based interventions at the end of life – Phase 2.
Introduction
Approximately two-thirds of people living in care homes have limited mental capacity119 and it is estimated that 54% of people with dementia are living in care homes. 2 Research consistently shows that it is very difficult to identify when someone is no longer living with dementia but dying from or with it. 406 Consequently, decisions about when to withhold treatment and how to provide palliative care are often influenced by the context of care, professional, and family beliefs and values. To date, research on end-of-life care for people with dementia has been dominated by US-based research and on prognosis and studies on decision-making on withdrawal of particular treatments (e.g. use of antibiotics and artificial feeding), and interventions to reduce unplanned hospital admissions. 241,442 The majority of research has been conducted in nursing homes rather than residential care homes and the research questions reflect the priorities and concerns of health-care professionals and services.
There is minimal evidence of residents, relatives and care home staff being involved in discussing and identifying research priorities for end-of-life care for people with dementia, or models of care that go beyond the individual patient-focused encounter There is a need to develop and test dementia-specific approaches to providing comfort and avoidance of distress for this vulnerable and often marginalised population that are grounded in the everyday experience of residents and care home life. 211
An emphasis on improving the quality of end-of-life care for older people living in care homes is the focus of the NHS End of Life Care programme for care homes. 209 Within this programme there is a commitment to providing patients with more choice in how they are cared for at the end of their life and where they die. It aims to extend the boundaries of palliative care provision to ensure that when residents in care homes are dying they can avoid emergency hospital admissions and be supported by care home and NHS staff skilled in providing end-of-life care.
Care homes at the time of writing were being encouraged, in collaboration with NHS staff, to use palliative care support tools such as the Gold Standards Framework,213 the Preferred Priorities for Care,443 and the Liverpool Care Pathway. 215 Studies that have evaluated these interventions report an increase in staff confidence and knowledge, a reduction in unplanned hospital admissions, and an increase in people being able to die in their care home as the preferred place of care. 216,217 However, other commentators and researchers have asked whether equivalent improvements can be achieved with the use of alternative approaches to joint working between health-care and care home staff, and if, with these tools, there is a danger of medicalising the dying process. 444 Care homes are a sector that are already under-resourced and the assumption that they can accept end-of-life care responsibilities without appropriate support and resources could have unintended consequences for the overall focus and quality of care that care home staff can provide. 246
This protocol is informed by the NHS End of Life Care Programme and builds upon longitudinal research on how people with dementia live and die in care homes. It will be informed by a participatory research approach that will support collaboration between researchers, care home staff and NHS staff that visit the care homes.
It aims to develop and refine an intervention that encourages integrated working between primary care health services and care home providers, to provide end-of-life care for older people with dementia and develop a resource that can inform future service development and research for people with dementia living and dying in care homes. It builds on initiatives and practice development work that have focused on improving/changing the organisation and quality of end-of-life care within care home settings (for example Hewison et al. 239).
Study aims and objectives:
Aims and objectives:
-
To identify with care home and primary care staff, and relatives, strategies to support integrated working between care home staff and primary health care services for end-of-life care for people with dementia.
-
To test ways that primary health-care services and care home staff can work together to identify resident and organisational aims/outcomes to support end-of-life care that reflect the priorities, experiences and concerns of older people with dementia living in care homes and support required at end of life.
-
To consider how available palliative care support tools and frameworks act as a resource for primary health care services and care home staff, to manage uncertainty at the end of life.
-
To identify facilitators and barriers to collaborative working to support and sustain effective end-of-life care for people with dementia living in care homes.
Background
Phase 1 of EVIDEM-end of life tracked the care received by 133 people with dementia living in six different care homes (residential) during the 18 months of data collection. Twenty-seven (20%) people died. In the event of a person dying, the research team, where possible, conducted post-death analyses, consisting of a final care note review and a brief interview with an appropriate carer or care home manager about the circumstances of the death. Of the 26 post-death analyses complete, 11 (42%) died in hospital. A further six (4.5%) people were lost to follow-up after being transferred to other care homes or nursing homes. How people died was very variable, shaped by which NHS services were involved, and whether it had been formally recognised that they were dying. The most commonly recorded symptoms at the point of death were decreased appetite, increased sleepiness, decreased mobility, and shortness of breath or breathing difficulties. The majority of the people that died saw the GP and/or the district nurse at least once when approaching end of life. Interestingly, 10 of the 27 deaths were recorded as dying out of hours (when out of hours was defined as from 6.30 p.m. to 8.00 a.m. on weekdays and all day at weekends and on bank holidays), and for a further 10, the time of death was unknown. Furthermore, 16 were recorded as being admitted to hospital at least once during the previous year before death, with seven of these returning to the care home, where they later died. It was also found that there were often discrepancies between what was recorded in the care home notes and what the researchers were told at the point of interview, particularly in relation to wishes surrounding preferred place of care.
Interviews with care home staff, district nurses, GPs and paramedics revealed a commitment to providing high quality, end-of-life care that ensured the person with dementia’s symptoms were palliated and they were able to die in their place of choice. However, findings also suggest a lack of confidence and knowledge when supporting people with dementia at the end of life. This was in part because of the heterogeneity of the dying experience for people with dementia and the disconnect between the espoused principles of good end-of-life care and staff’s ability to apply them to particular events and situations within the care homes.
Tools to support assessment and recognition of the need for palliative care were known and valued (mainly by NHS staff), but participants expressed uncertainty about how to use them to support people with dementia at the end of life. Staff struggled to provide examples of how the tools had supported end-of-life care for a person with dementia except in one instance where a palliative care register was cited as having improved communication between GPs and out of hours services.
Health service staff definitions of when someone was approaching the end of life varied, and did not necessarily reflect how older people and care home staff identified their needs for support and their priorities for end-of-life care. For NHS staff there was a proscribed amount of time when they were likely to become involved in providing end-of-life care. Despite recognising dying could be a prolonged process, and their belief in the importance of building relationships, their involvement invariably was during the last week and days of life. Furthermore, whilst there was recognition from all participants that providing end-of-life care for people with dementia was difficult and required extra training and knowledge, there was no consensus as to who had (or should have) this knowledge, or more importantly, should lead and co-ordinate the care. However, GPs were most frequently the person suggested. For care home staff it could be a difficult transition to move from providing every day care to someone with dementia to providing end-of-life care.
NHS staff were regular visitors to the participating care homes and there were frequently occurring opportunities to meet with care home staff to talk about residents. However, these encounters were largely unstructured and specific to particular residents in the care home. Findings suggested that participants would welcome strategies that helped primary health care services and care home staff develop ways of collaborating that could be mutually supportive, address their uncertainties about how to support people with dementia, promote shared learning and incorporate regular review of the care needs of the residents.
The majority of NHS primary care staff, (in particular the district nurses and GPs but not the paramedics) were familiar with palliative care support tools such as the Gold Standards Framework,213 Preferred Priorities for Care443 and the Liverpool Care Pathway,215 but only a minority had used/were using these resources for people with dementia living in care homes. Many were unsure how to take this forward, or if it was their role to do so. An assumption of many of the palliative care tools that care notes can support and guide how practitioners review care together, and document decisions and future plans was not supported by the review of residents’ notes. Phase 1 highlighted that care homes would mainly use notes to record care and past events, and not as a tool to plan and review care with the different services and practitioners involved in the individual’s end-of-life care.
Interviews with older people with dementia demonstrated that they were able to articulate their opinions, preferences and concerns about living in a care home, and for some, anxieties and unmet spiritual needs about approaching the end of life. What they appeared to value was having the time to reflect and talk with someone about their end of life as one element and expression of having meaningful relationships with staff and family members. This was linked to the need to find meaning and purpose to their life in the care home and some resolution of past losses and events that had led to them being admitted. For many, what happened in the future was not as important as how they felt now about living in the care home, or the events that had led to their admission to a care home. Older people were not sure with whom, in the care home or from the primary health care staff that visited the care home, they would or could talk to about their preferences and priorities for care. Longitudinal data did, however, demonstrate that for the majority of residents, there was enough time (at least a year) to initiate and sustain these conversations and, where appropriate to involve relatives in discussions before a crisis or event occurred.
The findings indicate that there is a need to review and develop existing resources and palliative care support tools in two ways. Firstly, to contextualise any intervention to support end-of-life care for people with dementia, as one aspect of care that assumes older people with dementia have the opportunity to discuss and express what is important to them about living in a care home. Secondly, to develop or modify interventions and tools that equips NHS and care home staff, residents and their relatives, to live and work with the uncertainties of providing end-of-life care to people with dementia, and that can be incorporated into, or developed from existing patterns of working and collaboration.
Phase 2 aims to begin to address these issues and develop a model of collaborative working for end-of-life care for people with dementia through working in partnership with care home and primary care staff in three care homes.
Method
Research approach
Phase 2 will use a participatory research approach that is informed by the principles of action research. This is an approach that emphasises shared objective setting, review, and development as part of the research process, and refinement of the intervention. 445 It offers an opportunity to use the findings from Phase 1 to inform problem identification and problem solving, as an activity shared and negotiated between the care home and NHS staff, the research team, and the residents and their families; a process that will aim to develop an intervention that reflects the preoccupations and context of care which is relevant and grounded in everyday practice.
In three care homes that have already participated in Phase 1 of the study we will facilitate regular meetings over 1 year to:
-
review and discuss the findings from Phase 1 of the study together, and their implications for the residents with dementia living in the care home
-
identify from a suggested list of changes (drawn from the findings of Phase 1) an aspect of end-of-life care they would want to focus on
-
agree study objectives and a strategy for change that can be jointly implemented during the data collection period
-
implement, review and refine the intervention
-
evaluate the change against the objectives and desired outcomes.
In the three participating residential care homes, care home staff (including the manager or deputy manager), and the NHS staff that work most closely with them to provide end-of-life care for people with dementia (GPs and district nurses) will meet with the research team.
In the first 3 months of the study, (following ethical and governance approvals and recruitment of residents and staff) at a series of meetings (up to four per care home) facilitated by the researchers, we will discuss the proposed approach, the level of commitment required participants’ preferences for structuring shared working and discussions into existing patterns of contact between primary care and care home staff.
Months 3–6 The participants will review with the research team the findings from Phase 1 and discuss what the findings reveal about how care is provided within the care home and in collaboration with NHS services, key assumptions about what end-of-life care involves and what the priorities are for the different participants. The purpose of the discussions will be to discover how care home and NHS staff interpret the findings, discuss priorities and agree together an aspect of working together that can be improved. Based on the findings we anticipate that the areas they could choose to focus on will be on strategies/interventions that:
-
enable NHS staff and care home staff to better work to create opportunities over time for discussion with the older person, and where appropriate their family, their priorities for end of life and review together the implications this has for how they work together, what tools they use and how they can maintain communication
-
support NHS and care home staff to review and plan together the care of people with dementia who are not well/showing signs of deterioration, but it is unclear if they are dying
-
develop shared approaches when key decisions are needed that involve NHS staff for example need for extra care, management of symptoms experienced by people with dementia as they approach the end of life
-
develop strategies that enable care home and NHS staff to incorporate end-of-life care into every day practice, and to manage uncertainty.
As part of this process, and facilitated by the research team, participants will work together to discuss and refine research questions/objectives, plan achievable change, and evaluate the outcomes and effectiveness of the intervention(s).
Months 5–10 The participants will develop with the research team (and where appropriate, experts in end-of-life care and care of people with dementia), the chosen area of end-of-life care they want to work on together. It is anticipated this will be an iterative process of discussion and review facilitated by the researchers.
Months 10–12 The participants will review progress against the stated objectives and desired outcomes.
In order to understand the impact of the intervention on resident experience over time, and ensure their experience is central to the study, we will track the care received by residents with dementia from each of the participating care homes. This will involve approximately 75 residents in total. We will document their access to and use of health-care services continuity of care and involvement in decision-making. Where possible and appropriate we will conduct discussions with up to five older people with dementia in each care home for up to two times over the year, and their key care workers (including health-care staff), review their case notes to identify any key events, assessment and support tools used, frequency of contact with health care and service uptake.
This data will be compared with equivalent data collected in Phase 1 and show if integrated working to plan for and support end-of-life care makes a difference to the process of care, residents’ health needs, their quality of life, and use of services including unplanned hospital admissions, length of hospital stay and transfers to nursing home care.
We will carefully monitor the resources involved, and estimate the costs of the chosen interventions. We will use interviews, focus groups and documentary review to complement the older person’s experience, and obtain a detailed understanding of the context of the care homes and services that work with them. In addition to the development of the interventions and the related documenting of the process, participants’ views and contributions at each of the three care homes, we will undertake the following:
-
interviews with family carers/relatives (n = 5–8)
-
documentary review of care home and PCT related documentation (e.g. shared protocols/assessment/care planning, joint funding agreements, integrated pathways and service level agreements)
-
interviews with key stakeholders (e.g. PCT manager, palliative care specialist services, paramedic/ambulance services).
Anonymous data from the reviews of residents’ care home notes and care plans (part of the study’s dataset) will be shared with the EVIDEM Registry research study, entitled ‘A registry of people with dementia using services in the North Thames Dementia and Neurodegenerative Research Network (North Thames DeNDRoN) Region’. The EVIDEM Registry study intends to develop a unique and comprehensive minimum dataset (MDS) of individuals with dementia (or symptoms likely indicative of dementia) at differing stages in the disease trajectory (from diagnosis to nearing the end of life). Recruitment to the Registry is intended through inclusion of anonymous patient data from the four EVIDEM research studies and direct recruitment to the registry (e.g. via general practices).
Ethical review and approval for the EVIDEM Registry has been obtained. The Southampton and South West Hampshire Research Ethics Committee A approved the EVIDEM Registry research study on 30 June 2008, reference no. 08/H0502/34. Ethical approval has also been given for the EVIDEM research study ‘Promoting Continence and Managing Incontinence at Home’ that includes sharing of anonymous patient data with the EVIDEM Registry study. Ethical approval was obtained from Camden and Islington Community Local Ethics Committee on 29 September 2008.
Data analysis will establish how the findings from Phase 1 impact on how participants set priorities for end-of-life care and work together and whether the chosen interventions and their implementation influence the process and networks of care available to the older person with dementia.
We anticipate that resident and organisational outcome measures will focus on how older peoples’ views, priorities needs and choices are supported through the chosen interventions, their effect on quality of life and wellbeing, continuity of care, staff satisfaction, use of resources, services, and the costs of implementation. The cross case comparison of how the three care homes implement shared working to improve end-of-life care for people with dementia will enable us to establish how priorities and outcomes are defined, key achievements, and distinguish between those processes of negotiation and development that are common to all settings, and those that are context specific. It will also enable a comparison of the costs and effectiveness of different approaches to end-of-life care.
Recruitment
We will recruit three care homes from the six care homes that participated in Phase 1 of the EVIDEM-End of Life study. All six care homes will receive a summary of the findings from Phase 1 and the proposal for Phase 2. Recruitment will be primarily based on interest and willingness of NHS and care home staff to participate in Phase 2 of the study and commit to a further 18 months of data collection.
If more than three care homes and their partner NHS primary care teams express an interest in participating, we will purposively select three care homes that represent diversity of approaches to end-of-life care and expertise in palliative and dementia care.
The findings from Phase 1 highlighted that the majority of care homes could provide some examples of providing end-of-life care at the patient level. However, even at the individual patient level of care there was a wide range of how dying was defined, how decisions were made about keeping the older person in the care home and the actual intensity and involvement of NHS staff. For example, in terms of the level of care home contact with primary care services, use of support tools such as the Liverpool Care Pathway, involvement of relatives in decision-making, etc.
Inclusion criteria would be:
-
perceived good working relationship between NHS and care home staff and willingness to commit to work together on EVIDEM-EoL
-
ability to commit to a minimum of four face-to-face meetings over the year’s data collection
-
methods of sharing information and some documentation about patients in particular aspects of care for end-of-life care between the care home and primary care staff (GPs, district nurses, specialist palliative care support) linked to the care home.
Exclusion criteria would be:
-
only one of the participant groups (NHS or care home staff) are interested in the study.
Recruitment of health-care professionals
The research team will meet with possible participants, either as a group or as individuals, to discuss the study and answer any questions. The research team will then wait a minimum of a week before seeking practitioners’ individual written consent to participate. All health-care professionals will fit the following criteria:
-
act as the main contact for the care home they work with (where more than one General Practice and attached community nursing team visits the care home we will recruit the team that has the most frequently occurring contact)
-
have been going into the care home in question for at least a year
-
be able to commit to a minimum number (i.e. four) of meetings at the study care home.
Recruitment of care homes
Care homes will be recruited on the basis that:
-
The care home is for older people offering personal care including specialist support for dementia.
-
Can identify NHS primary care staff that they would be able to work with.
-
Be able to commit to a minimum number of meetings at the care home and support staff in their participation (e.g. agree to release them from other duties for the period of the intervention).
-
Regardless of how they are identified initially, recruitment will follow the same process. After an initial meeting between the care home manager and members of the study team, for interested care homes, separate meetings will be set up with care home staff and residents to outline the study, what it involves, give them information sheets and answer any queries. Following these meetings the care home manager will be asked to confirm whether or not the care home has decided to participate. Permission may also need to be sought from the care home organisation it belongs to.
Recruitment of care home staff
Following the initial meeting to explain the study to the staff as a group, give out information sheets and answer queries, those staff who have expressed an interest will be approached individually so they can be given further information and voice any questions. Those agreeing to participate will be given at least 48 hours to decide whether or not they want to participate. They will be asked to sign a consent form endorsing their agreement to take part. It will be emphasised that participation is purely voluntary and that they can change their mind at any time without giving a reason. Where some members of the group do not agree to the interview being recorded, only notes will be taken. Researchers will reiterate to all staff that their participation is voluntary and that they can leave the study at any time.
Recruitment of older people
The older people who will be recruited will fit the following criteria.
Inclusion criteria
-
Resident in care home for a minimum of 4 weeks.
-
Have a diagnosis of dementia and/or be perceived by staff as having dementia.
-
Have the capacity to understand and consent to participate in the study, or have a nominated consultee who could provide an opinion whether the older person, if they had the capacity, would have wanted to take part in the study.
-
Anticipated to be resident in the care home for the coming year.
Exclusion criteria
-
Has a mental health problem (not caused by the ageing process).
-
Does not have capacity to consent in the moment or an identifiable consultee (see above) who can be approached for their opinion.
-
Cannot speak English and an interpreter not available in the participant’s language.
Following the initial meeting, the older person will be provided with an information sheet and consent form and given at least 48 hours to consider the information before further contact by the researcher. For those who are agreeable, a meeting will be set up with a member of the study team to explain what involvement in the study means for them, and answer any questions they may have. All residents will be given at least 48 hours to consider their participation before a member of the research team revisits them to answer any further queries and confirm whether or not they would like to take part. It will be made clear from the start that a decision to take part in the study is entirely voluntary and a decision not to take part will not affect their care in any way.
All of the older people in this study have complex needs, and will include people who are in difficult circumstances, who may be vulnerable, have communication or recall difficulties and may tire easily. At every stage verbal consent to continue will be obtained and the opportunity offered to defer or shorten the time of involvement in the study. Those who have special communication needs will be interviewed using communication aids if appropriate.
Recruitment of family/relatives
Family members and relatives of older people will be invited to attend the initial meeting with residents to give them information about the study, and will also be given an information sheet outlining the study.
At the end of the study we will invite by letter family members of residents who have died during the study to ask if they would be willing to take part in an interview to gain insight into their perspective on their thoughts and views about end-of-life care. Those who express an interest will be asked to contact the researchers for further information. A member of the research team will meet with relatives individually to discuss the study and answer any queries. They will be given at least 48 hours to consider whether or not they would like to participate, after which they will be consented individually. However, it will be impressed upon them that taking part is voluntary and that they may withdraw from the study at any time without giving a reason. The interview will be held in a private room within the care home, the participant’s home, or the host university, depending on which is more convenient for the participants. It is possible that taking part in an interview could trigger distress. The possibility of this will be discussed with participants prior to the interview and what might help to reduce the impact of talking about a recent bereavement. Participants will be offered the option of writing down their thoughts or being interviewed with a friend or relative present if that is preferable to a one-on-one interview. Contact with relatives will not be made before a minimum of 8 weeks has elapsed since the resident has died.
Amendment submitted NRES Committee South Central – Southampton A (May 2011)
Preliminary findings from Phase 2 have demonstrated how influential the relative can be in decision-making around end-of-life care. Following discussion with the EVIDEM-EoL steering group it was agreed that it was important to include the views of relatives of residents living in the care home.
It is proposed to extend the study to include views of all relatives, and not restrict data collection to capture only the views of relatives who have had a relative die in the care home (as stated in the original proposal). The same protocol as before (consenting, information, etc.) will be followed. We will recruit to up to 15 relatives, who will be approached in the first instance by the care staff to see if they are willing to talk. Some of the relatives will have a family member who is taking part in the study and others may not. They will be given at least 48 hours to consider whether or not they would like to participate, after which they will be consented individually. However, it will be impressed upon them that taking part is voluntary and that they may withdraw from the study at any time without giving a reason. The interview will be held in a private room within the care home, the participant’s home, or the host university, depending on which is more convenient for the participants.
The above amendment was approved by the South Central – Southampton A Committee on 27 May 2011 (REC reference 10/H05002/55 – amendment number 1)
Interviews with stakeholders
Semi-structured interviews with NHS health-care professionals and up to three stake holders (e.g. Care Quality Commission, PCT Commissioners, Directors of Primary Care Services) at the end of the study to establish how they understand end-of-life care is provided, how the older person is identified as needing the service, how the older person’s needs are assessed and care is planned, and to what extent the care home staff are involved with this, and the time different team members spend in discussing and planning care/end-of-life intervention, the skills and knowledge needed and range of activities this involves.
Data collection
General
Multiple sources of evidence are needed to provide a full picture of how the different end-of-life care related interventions are implemented and experienced across the three study sites by care home staff, health-care professionals and older people resident in the care homes. By triangulating a range of data sources, it will be possible to demonstrate which characteristics of integrated working are specific to certain circumstances, and which are transferable and can be shown to achieve different types of outcomes for older people. This phase of the study will use mainly qualitative data collection methods including face-to-face interviews; care notes reviews, documentary review and field notes.
Data collection will be undertaken as follows:
-
Where feasible discussions with a number of older people with dementia per care home, over the period of 1 year to establish their views and perspectives of living and dying in the care home and if this changes over the period of the study, if appropriate to the chosen intervention.
-
Two reviews of each older person’s care home case notes including, demographic information, care plans and ongoing updates, at 6-monthly intervals, over the period of 1 year to establish their care, planned and ongoing, health-care services received including any hospitalisations and any changes in their health status and needs.
-
Field notes and observation documenting the detail and outcomes of meetings between the care home and NHS staff and the researchers and the implementation process in the care homes of the chosen interventions.
-
Review of key documents and tools that are shared by the care home staff and health-care professionals such as care pathways, shared notes and assessment tools, to establish the structural and organisational context of end-of-life care.
-
Semi-structured interviews with care home staff at the beginning and end of the study to establish, the support they consider the older person needs when they are dying, how they define end-of-life care, how often they see the health-care professional and where, how the health-care professional and the care home staff work together, and any support and training received from health-care staff, or they would like to receive, and what facilitates and or hinders integrated working with the health-care professional.
-
One focus group will be conducted with up to 10 care home staff of differing levels of experience and seniority. This will be facilitated by two members of the research team and will be recorded if the group consents to this unanimously, otherwise only notes will be taken. The interview will be conducted in a private room in the care home at the convenience of the staff, using a semi-structured format that will focus on their experiences of the implementation of the chosen intervention and working with health-care professionals to provide end-of-life care. It will explore the level of contact they have, how they communicate and feed back information about the older people receiving care, and perceived facilitators and barriers to integrated working with health-care professionals.
-
Semi-structured interviews with NHS health-care professionals and up to three stakeholders (e.g. Care Quality Commission, PCT Commissioners, Directors of Primary Care Services) at the beginning and end of the study to establish how the older person is identified as needing the service, how the older people’s needs are assessed and care is planned, and to what extent the care home staff are involved with this, and the time different team members spend in discussing and planning care/end-of-life intervention, the skills and knowledge needed and range of activities this involves.
-
Interviews (either face to face, or over the telephone) with relatives of older people who have died will be conducted where the relative is able. Contact will not be made before a minimum of 8 weeks has elapsed since the resident has died. Interviews will last up to 30 minutes, and will explore the relatives’ experience of the end-of-life care the older person received, including services and communication with the care home and NHS. Alternatively, the relatives will be given the option to write their thoughts down in the event that they feel an interview may be too distressing.
Data collection: older people
For those older people with dementia, who have the capacity to consent in the moment, they will be interviewed up to two times over the study in a private room within the care home. As the research is being conducted with frail older people who may tire easily, the interviews will last for a maximum of 30 minutes or less if it is apparent that that the older person is tiring. These semi-structured interviews will focus on:
-
their experience of living in a care home and thoughts about end of life
-
the amount of support they need from the care home staff
-
the health-care services they have received
-
how they define their priorities for end of life
-
what indicators/measures they use to assess if care received is effective
-
how they think that care home staff and health-care professionals work together.
For those residents who consent, their care home notes will be reviewed twice, over the period of a year, to coincide with their interviews. The following data will be collected:
-
demographic information
-
health conditions
-
medication
-
use of equipment
-
hospital admissions
-
health-care professionals involved in their care, for which condition, frequency of contact, planned care, location of consultation (i.e. care home or hospital outpatients)
-
care plans
-
any shared assessments/care planning between care home staff and health-care professionals in use
-
activities of daily living – level of support residents need with these
-
evidence of planning for end-of-life care, records of decisions made and where appropriate after death analysis, consisting of a brief interview with a care worker or the care home manager who was involved in the person’s death and a final review of the care plan for that person.
These data will reveal the range and intensity of activities undertaken to support residents’ care, and where relevant, how effective the integrated working for end-of-life care is over time, as measured by outcomes and by the older people themselves, the care home staff and health-care professionals (outcomes are likely to include quality of life, continuity of care, access to, and satisfaction with care, involvement in decision-making on treatments, opportunities to discuss their uncertainties, priorities and preferences and place of care). The data will also identify preferred practitioners and key drivers for the different types of chosen interventions.
Data collection: care home staff
Care home staff who participate in the research and the shared discussions with NHS staff will be interviewed at the beginning and end of the study. The aim of these semi-structured interviews is to establish:
-
the support they consider the older person needs when they are dying
-
how they define end-of-life care
-
how often they see the health-care professional concerned and where
-
how the health-care professional and the care home staff work together, and any support and training received from health-care staff, or they would like to receive
-
what facilitates and or hinders integrated working with the health-care professional.
At the end of the study, one focus group will be conducted with up to 10 care home staff of differing levels of experience and seniority. This will be facilitated by two members of the research team and will be recorded if the group consents to this unanimously otherwise only notes will be taken. The interview will be conducted in a private room in the care home at the convenience of the staff, using a semi-structured format that will focus on their experiences of the implementation of the chosen intervention and working with health-care professionals to provide end-of-life care. It will explore the level of contact that they have, how they communicate and feed back information about the older people receiving care, and perceived facilitators and barriers to integrated working with health-care professionals.
Data collection: health-care professionals and stakeholders
Individual health-care professionals who participate in the group discussions and the development and implementation of the chosen intervention to support end-of-life care will be interviewed at the beginning, and end of the study.
The interview will establish their professional backgrounds, training, workload, funding, and how they work together with the care home. We will investigate their perception of the focus of their activities with the older person and the extent and mechanisms of their integrated working with the care home staff to achieve their care, support or treatment objectives for the older people. In addition we will investigate how they evaluate and reflect on their experience of integrated working to promote end-of-life care for people with dementia through interviews, care plans and notes review, we will aim to establish:
-
how the older person is identified as needing the service
-
how the older person’s needs are assessed and care is planned, and to what extent the care home staff are involved with this
-
the time different team members spend in discussing and planning care/end-of-life intervention, the skills and knowledge needed and range of activities this involves.
Data collection: relatives of residents that have died during Phase 2 and family members of residents who are living in the care home
Based on the findings from Phase 1 we anticipate 20% of residents recruited to the study will die over the data collection period. A minimum of 8 weeks after the death of the older person with dementia (and following discussion with the care home manager to ensure that there are no reasons not to contact the relative) we will invite by letter a family member or significant other, to take part in an interview to gain insight into their perspective on their thoughts and views about end-of-life care. Those that indicate they are willing to take part in an interview will be contacted by phone and their possible participation will be discussed further.
The interview will be semi-structured and seek to explore their experience as a relative visiting the care home what they thought was significant about the last months of the person’s life. As already noted it is possible that the interview will cause distress because of the memories and feelings talking about the deceased person may generate. Every attempt to ameliorate the impact of the interview will be made and participants will be offered the option of writing down their thoughts or being interviewed with a friend or relative present if that is preferable to a one-on-one interview.
Amendment submitted NRES Committee South Central: Southampton A (May 2011)
Interviews with family members of residents who are living in the care home will be open and discursive and ask about their views about living and dying in a care home. We know from securing consultees’ opinions about their relatives’ possible participation in the study that some relatives will want to talk about their regret and guilt about placing a relative in a care home. We will be careful to ensure that participation does not create any further distress and will ensure that sufficient time is allowed in the interview for relatives to express their views and feelings about a range of issues. It is likely that past experiences will influence how they think about end-of-life care for people with dementia. Once we have explained the purpose of the study very few prompts will be used to ensure participant priorities and views are shared. If any issues are raised that cannot be addressed or resolved in the interview that relate to the care home or the care of their relative the researcher will ensure an appropriate person is identified for the relative to talk further with (e.g. care home manager, Age UK advocate).
The above amendment was approved by the South Central: Southampton A Committee on 27 May 2011 (REC reference 10/H05002/55 – amendment number 1).
Analytical synthesis
All interviews will be recorded, transcribed and analysed using NVivo™ software. Organisational, operational and quality review documents will be analysed through the same framework and using the same software. Statistical data from validated assessment tools, and information on the older persons’ use of services, and the professional diaries on service activities will be entered onto an SPSS™ database. The findings generated from the integrated working in the three study sites will be brought together in two units of analysis:
-
the individual care homes
-
cross-case comparisons looking at how the different contexts and mechanisms affect the outcomes for the older person.
To enable comparison and the development of an explanatory model, analysis will then be undertaken within and across sites. Qualitative data analysis will be undertaken using NVivo™ and thematic content analysis to identify key themes and common experiences and priorities of care. Data from the case studies will be analysed to describe the features and impact of end-of-life care on the outcomes. The analysis of outcomes will be guided by the findings of Phase 1 but is likely to be categorised as outcomes for the older person, the care home staff, and the health-care practitioners’ roles, and include the older person’s understanding of their care, access to services, clinical outcomes. Outcomes for the health-care system will include: transparency of care, service utilisation, and staff resources used as a result of working together to develop end-of-life care interventions that are dementia specific.
Economic evaluation
The resource implications of different approaches supporting end-of-life care with care homes will be documented and costed, including the resource implications of developing an intervention this way, the health and social care services delivered, hospitalisations. The contribution of care home staff and health-care professionals will be compared across models and sites in a cost consequences framework. 438 This will incorporate the perspectives of the health-care services, older people, and care home staff.
Ethical issues
Intrusion
Participatory approaches to research are by definition intrusive. There is a risk that individuals participating in the study may feel that their right to self determination is compromised if during the course of the study they do want to participate in the planned change agreed by their peers. For the residents too, the impact of the chosen intervention on the care they receive may be intrusive in an environment that is their home. Integral to the research questions asked, the design of the study, the role of the facilitator and the stages of data collection methods is a consciousness of the need to be encouraging reflection at each stage of the study and be responsive to the participants and the context of care. To this end, ongoing opportunities will be created for individuals to reflect on the study and withdraw from participation and/or highlight if participation in the research is disruptive and intrusive for residents, care home staff and primary care staff. It is a potential finding of the study that certain interventions are judged too intrusive on staff time and responsibilities and the everyday life of residents.
Anonymity and confidentiality
All participants and study sites will have a code number and no names or identifying details will appear on any data collection forms, analysis or draft and final reports. During data collection only the research team will have access to the names and contact details of participants and these will be kept in a password protected computer and double locked filing cabinet, to which only the research team will have access to. All participants will be guaranteed anonymity in written reports and summaries of data analysis. A summary of the findings will be sent out to participants for their comments prior to publication and dissemination.
Consent
It is possible that health-care professionals, older people and care home staff may feel obliged to take part. They may feel that refusal to participate may adversely affect their relationships with their employer or the services involved in providing their care. In introducing the study to the possible participants, care will be taken to ensure no one feels coerced or obliged to take part. The information sheets stress that participation (or not) in the study will not affect the care they receive or their relations with other people in their organisations. At each stage of the recruitment process, the researchers will re-iterate that participation is voluntary.
People with cognitive impairment/limited capacity
The research team has extensive experience of working with people who find consent and participation in research difficult, perhaps because of problems of cognition, confusion, illness or fatigue. For people with dementia whose level of cognitive impairment means they will not be able to consent to participation, the approach taken will be informed by the key principles set out in the Mental Capacity Act17 that assumes that all adults are capable of giving or refusing consent unless proven otherwise, and that the best interests of the person who lacks capacity are paramount. It is, therefore, an assumption of the study that patients who experience short term memory and cognition problems can consent in the moment. It is the responsibility of the researcher on each occasion to review the study aims with the participant, and confirm that they are still willing to participate in the study, ensuring that they are not alarmed or distressed by the experience. 439,440 The study is informed by the principles of inclusionary research whereby every effort will be made to enable people that wish to, to participate, even if that means alternative methods of communication and data collection need to be found.
At the stage of initial consenting the researcher will ensure that the participant is given full and appropriate opportunity to have the study explained to them in a way that best meets their individual needs. If there is an intermediary, such as a family member or key worker, with whom the older person particularly relates, the researcher will ensure that this intermediary is present and able to explain and, if necessary, to interpret any areas of concern or lack of understanding. Where older people are unable to give written consent because of poor vision or if they are unable to write, verbal consent will be recorded by the researcher with a care home staff member as a witness. If the older person is unable to consent in the moment, or loses capacity over the period of the data collection, their consultee will be approached for their opinion on whether this study is something that the older person would have participated in or would wish to continue participating in, if they had the capacity.
Risk
The focus of the study is end-of-life care for older people with dementia. Discussing such sensitive issues may cause distress for the people involved. There is a risk that by expressing their needs and experiences some older people may become distressed, confused or concerned. If this should happen any serious issues will be communicated to the professional whom they identify as knowing them best, and being most involved in their care. There is some risk that involvement in this study may affect residents’ care and outcomes, by older people providing or expressing information of which care home staff were not previously aware. At all stages, the researcher will make clear that they cannot be involved in providing care in any way. However if any risk (e.g. evidence of elder abuse, inadequate care, acute health need) is disclosed, procedures are in place to address this that reflect the care home, PCT and university guidance and procedures The study relies on close collaboration with the participating sites. Part of the recruitment process involves how the study will address incidents where the participants may become distressed, or there is evidence of need for support or review from health and social care services. The researchers will have a protocol describing who to consult and how, which they will follow in all such cases (see EVIDEM-EoL Bad Practice Protocol V2 260608.)
All interviews will be structured so that they minimise the distress caused by the discussion of sensitive issues and focus on collecting data which is essential to each participant. However, the main participants are older people with complex health and social care needs, so it is possible that the older people may become upset. The researchers will treat all participants with respect and dignity. Moreover, the research fellows who are responsible for data collection have extensive experience in interviewing vulnerable older people about sensitive topics.
If the participants express any sensitivity, embarrassment or upset at any point during the interview, the researcher will remind them that they are free to stop the interview at any time. In this situation, the researcher will switch off the recorder immediately. The research team work closely with each of the care homes involved and the key workers. If a resident becomes distressed in the course of an interview the researcher will ask their permission to ensure that their key worker and other appropriate staff members are aware of this.
There is a small risk of disclosure in the study, as the core participant group includes older people with complex health and social care needs. At the beginning of each interview participants will be reminded that anything they say is confidential but that it will not be possible to keep information confidential if there is any indication that they or any other participant is at risk, or if their care services are deficient or there is evidence of abuse or an acute health need.
The procedures for situations where there is evidence of abuse, neglect or criminal disclosure are:
-
if the person is at immediate risk the care home manager will be contacted
-
if there is suspicion of abuse, what the participant reported or was observed will be documented by the researcher, the care home manager or appropriate line manager will be informed and the agreed procedure for reporting suspicion of abuse will be adhered to. The Principal Investigators for the study will also be informed
-
if there is evidence of negligence in the provision of care or significant shortcomings in the care provided to the older person, the research fellow will document the concerns/issues and notify the care home manager or other appropriate line manager and the procedures for registering and addressing a complaint about the standard of care will be followed
-
if during the data collection it becomes apparent that the participant needs medical attention, the researcher will contact a member of the care home staff urgently.
With regards to the NHS professionals and care staff, there is likely to be an increased workload as part of participating in Phase 2. Researchers will make clear what commitments could be involved when explaining the study to potential participants and throughout the recruitment process. Researchers will reiterate that all participants are free to withdraw from the study at any point, without giving a reason.
In addition, there is a risk that the study may highlight areas of tension and disagreement between care home staff and NHS professionals. The researcher designated as the facilitator for the group discussions and meetings has extensive experience in facilitating and managing focus groups and potentially difficult discussions in a range of settings. A semi-structured prompt guide will also be used in aiding the discussions and ensuring they maintain focus.
Interviewing vulnerable older people may be stressful and emotionally demanding.
All researchers will meet with the Principal Investigators regularly to debrief and identify any issues or needs they have for ongoing support.
Conducting interviews with relatives in their own homes incurs a degree of risk to the researcher conducting the interviews. The research team will adhere to the Centre for Research in Primary and Community Care (CRIPACC) lone worker policy.
Vulnerable patient group
This research will be conducted with a diverse group of people who are ill, easily tired and who may be vulnerable for a number of reasons. All participants will be treated with dignity and respect at all times. The researchers are experienced in working with older people who have problems of this nature. Data collection procedures and participant responses will be monitored carefully and reviewed by the research team throughout the study. The wellbeing and support of participants are study priorities.
The research team
The multidisciplinary research team brings together academics and practitioners from nursing, social work, general practice and health economics. The team has worked on a series of research studies, both together and independently, that focus on older people with complex needs living in care homes and community settings. These have included funded work on inter-professional working in: care homes, activity promotion, team working across health and social care, the use of new technologies, case management and shared assessment processes across health and social care to improve care to older people, multi professional networks for the delivery of care to people with long term and disease specific conditions. The Public Involvement in Research Group (PIR Group) has strong links with the voluntary sector and is working closely with INVOLVE. The PIR Group will be contributors to the research design and execution. Members from this group will be involved in the Steering Group for this project and bring to the proposal experience of having been recipients of health and social care services, working with older people’s groups, and involvement in other studies that focused on older people including care homes and project management. The PIR Group comprises users, carers, patients, patient and public involvement links, and representatives from the voluntary sector.
Dissemination
The findings from this study will inform commissioners and providers of services in their decision-making about commissioning and providing end-of-life care services to care homes. It will make explicit the managerial processes and tools that enable better integration of care delivery between health-care professionals and care homes, and demonstrate the ongoing support and training required to achieve meaningful outcomes for the older person and the service.
Dissemination of preliminary findings for consultation and the final report will initially be through the participating study sites, following a report to the NIHR and the organisations of the different practitioners, teams and agencies that are involved in the study. This will be done through workshop events, e-alerts and network meetings.
The research team and Steering Group will disseminate findings nationally through their involvement with bodies such as Age UK, Alzheimer’s Society, National Care Home Research and Development Forum, DeNDroN, PCRN, Better Government for Older People and relevant research networks. Findings will be presented in professional and peer reviewed journals and at conference events across the relevant disciplines.
Appendix 37 Chapter 4: Care home manager topic guide
Appendix 38 Chapter 4: Care worker topic guide
Appendix 39 Chapter 4: NHS and social services staff topic guide
Appendix 40 Chapter 4: Emergency services topic guide
Appendix 41 Chapter 4: Care notes data extraction form
Appendix 42 Chapter 4: Care home manager/senior carer topic guide
Appendix 43 Chapter 4: Care home staff topic guide
Appendix 44 Chapter 4: NHS staff topic guide
Appendix 45 Chapter 4: Relatives prompt guide
Appendix 46 Chapter 4: Emergency services prompt guide
Appendix 47 Chapter 4: Intervention design, Phase 2
Appreciative Inquiry
Devised in the USA as a complement to conventional forms of action research, AI is a research approach, method and philosophy for promoting positive personal or organisational change and development. AI seeks to discover those things that ‘give life’ to people, organisations and human systems when they are most effective and healthy. It is founded on the assumption that inquiry into, and conversation about, strengths, successes, values and hopes triggers change. It assumes that in every situation or organisation something works well and change can be leveraged through discovering, sustaining and spreading these moments of excellence within the wider system. Widely used in public and corporate institutions to develop organisations, the central tenet within AI is that in order to understand a person or situation (necessary for change and development) one needs to appreciate and openly inquire. Thus at the centre of AI participatory research is a subtle but fundamental shift from ‘diagnosis’ (determining why something is like it is) to ‘inquiry’ (questioning to understand another person’s view and thus seeing new perspectives and possibilities within a situation). AI argues that the way people talk about an organisation or situation is important and affects the way people view their work and their role. Furthermore, AI assumes that an organisation is dynamic – with a past, present and future. People within an organisation will have more confidence to move towards the future (the unknown) if they carry forward within their working practices the best parts of their past (the known). The exploration and enhancement of what is going well in a system is often framed as a four-dimensional (4-D) cycle (Figure 31).
FIGURE 31.
The AI 4-D model of positive change.
Modified Appreciative Inquiry for care homes
Appreciative Inquiry Light
Typically, AI interventions work intensely over a period of 2–5 days and assume a stable unitary organisation. In the EVIDEM-EoL study, care home and health-care staff were spread over different locations and with limited time resources. The AI method was modified (Figure 32). Hour-long sessions, were held three times over a period of 6 months in the three care homes. There was a focus on creating a ‘team in the moment’, whose members carried ideas generated back into their own professional place of work.
FIGURE 32.
Modified AI for care homes.
Appreciative Inquiry sessions
All sessions were facilitated by a nurse researcher (‘facilitator’) and attended by at least two other researchers (‘researchers’) from the EVIDEM-EoL team. Sessions brought together care home staff and visiting health-care practitioners with the expressed intent of discussing end-of-life practice. There was considerable preparation before the sessions and follow-up by the research team in between the meetings. The researchers’ role was to ground sessions in actual practice by providing participants with evidence of end-of-life care gathered in Phase 1, as well as evidence from Phase 2, as it became available. Researchers gave absent stakeholders (i.e. residents and relatives, emergency services) a voice over the course of the sessions using interview data collected over the course of the study. Researchers also summarised key themes and points for participants in between sessions, and provided updates in newsletters. Initiatives arising from the intervention were linked to practice development within the wider system by providing care homes with current and up-to-date resources in end-of-life care (Dying Matters leaflets, East of England DNACPR forms) and links to the care homes’ local support networks (local hospices, end-of-life facilitators, Hertfordshire Partnerships).
Session one: appreciation/stories
Prior to session one, participants were sent a letter introducing AI and encouraging reflection and sharing of stories of good practice (see intervention information). This introduction to ‘good gossip’ enabled some familiarity with AI and the emphasis on positive experiences before the structured sessions. Session one used the principles of the Discovery and Destiny phases of AI to reveal current excellent practice, value the shared capacity among participants and together create a shared intent about the future of end of life within that care home. Following a brief introduction participants were paired and invited to tell each other a story of ‘a positive memorable moment of working with others to provide end-of-life care for a resident dying with dementia’. Each pair recounted and listened to experiences of working well and then spent time comparing their stories for > 15 minutes. Each pair reported back to the wider group, and participants were invited to respond to what they had heard. This enabled participants to notice and value the role of each group member. Participants were encouraged to comment on any similarities they had noticed in the stories and together the group constructed a number of key attributes, values, skills and abilities they identified around shared working which were written down, for example ‘families are really important and we value keeping them involved’. The whole group were then asked to imagine the care home 5 years on, and together to think about their best ideal for end-of-life care for people with dementia. Several questions were used to help direct this thinking: i.e. What is different? What is going on in the home? Who is here? How have residents benefited? These future-directed statements were recorded, and the session ended by each participant reflecting on their own practice and shared working.
Session two: positive development of practice
The second round of AI session across care homes built on session one and used the principles of the AI design phase to focus on prioritising and choosing a particular innovation for participants to co-design together. Session two used reflections on real-life practice and end-of-life care within the care home to reflect on collaborative working and tease out the specifics of each innovation.
The meeting began with a check in about session one and anything participants wanted to share. An after death analysis of a care home resident was presented to the group (see Appendix 51) by the research team, using data from care notes, interview with key staff members and the time line of death. The emphasis on using these after-death analyses was on the process of care giving, and the alignment of strengths and adjustment of practice, where necessary, to move towards the future-directed statements. This emphasis on moving care forward to the intended ideal allowed for the telling of real-life/nuanced stories within a framework of generous listening, in which problem talk is framed in appreciation and possibilities, and forward thinking reflection. The tension between reflection on, and judging, specific practices was held by asking each participant to see the case from a variety of different perspectives. Thus, the discussions of the end-of-life pathway within the cases were looked at first from the point of the primary care doctor/district nurse, second from the care home staff perspective and then from the resident and family viewpoint. This proved a powerful tool in keeping participants open to the point of view of others and valuing difference, but with enough detail to enable realistic plans to develop the future-directed statements about the organisation and quality of end of life within the care home. For example, one GP was able to imagine himself as a member of care staff confronted with a collapsed resident, calling OOH for urgent medical advice. He acknowledged the inevitability of calling paramedics when the OOH response was slow, which often ends with a resident being transferred to hospital and possibly dying there. The remainder of session two was given over to the participants prioritising an innovation, and working within the groups to allocate responsibilities as to what needed to happen next, with whom and how to work with wider systems, for example OOH doctors.
Session three: sustaining change
The final session built on the previous two phases and the AI Destiny phase to concentrate on sustainability and embedding the practice innovation into the wider care home system. Although the emphasis throughout the Phase 2 study has been on a specific innovation in each care home, participants has taken the principles of AI into wider everyday practices. Thus, although session three reflected on the particular innovation of each care home and spreading, there was also an emphasis on reflecting on collaborative working and end-of-life care and how this can continue to be developed within each case study site. Also throughout the 6 months the participants had become a stronger group and more self-directing and in this last session the facilitator’s role was more supporter than coach.
Thus the facilitator asked questions of the group to enhance their own learning and capacity to sustain and spread the processes, for example ‘Where are we now compared with where we hoped we would be?’ ‘What more needs to happen?’. Essentially, this allowed each group to negotiate with each other about what realistic sustainability might look like rather than taking on imposed targets from outside. Using this context-based approach, three different structures emerged based on the strengths and interests of participants. One care home is using an existing GP visit as a strategic overview meeting to attend to the development of end-of-life care in the home. Another is creating structures to involve the other GP practice and spread their learning. The third is using AI principles to collaborate more with the district nurses who were unable to continue to attend the facilitated meetings. Participants reflected on the positives but also the challenges of the AI intervention, and set their own plans for furthering the work.
Appendix 48 Chapter 4: Intervention information – general
Appreciative conversations
Part of the EVIDEM project is about acknowledging and sharing what experiences and skills we already have in caring for people with dementia dying in care homes. We know from research that people work best when they work from a place of strength acknowledging what they do well and having conversations with others. These conversations create a shared understanding of what is good practice and what needs to be in place so it can happen more of the time. Usually we ask about things that are broken – the problems – so that we can fix them. In this case, we are trying to look at things at their best – the successes – so that we can find out what works and do more of it.
In January we will be doing this through ‘appreciative conversations’, asking you to tell your stories to other care home staff and the primary care team staff about times when you saw things working at their best in relation to people dying of dementia in your care home.
So, in preparation for this, we would like you to think about a situation where you felt you or the team you were working with, delivered care that allowed a resident to die well?
This may be in relation to symptom control, how you worked with the NHS professionals, or perhaps were able to carry out the wishes of the resident or their family.
Think about
What made the situation really special?
What was your contribution?
How did it feel?
Has it changed you in any way, if so how?
How did the care home or primary care team help you in this?
What were other people doing that helped?
What do you think was really making it work?
Talk about
Find a colleague and talk about it together – are your experiences similar?
We call this Good Gossip and can happen over a tea break, coming on and off a shift, as well as structured meeting times in the course of work.
Appendix 49 Chapter 4: Intervention information – care home staff
Information for care home staff
We are really pleased that you are joining with us in Phase 2 of the EVIDEM project and excited about working with yourselves and the Primary Health Care Team.
What we want to do in January
Our aim is to help you use your experiences and knowledge of what works well to create shared interventions to support and enhance practice. We all want the care of people dying with dementia to be as good as it can possibly be.
What we have asked you to do
We have asked you to tell stories of times when you felt you and the team have delivered care that allowed a resident with dementia to die really well. Although you may have remembered a difficult experience was there a moment when you remembered something good that came out of it? Perhaps you felt proud about how you or your care home handled the difficulty? We are not denying that there are lots of challenges in your work but we are interested in your capacity with your colleagues to overcome these.
The idea of talking about the good things with other colleagues we call Good Gossip because:
-
Good Gossip not only makes you feel better, but also your listener and the organisation in which you are working.
-
You can only gossip by talking to others, no one gossips on their own.
-
Good Gossip is ongoing conversation when you talk and listen to colleagues in your organisation, about what you are proud of, what you do well, what you have seen others do that makes you feel good.
What we want you to do now . . .
Experience and Practice Good Gossip . . . For many of us we need others to help us practice and talk about what we do well. Here are some ways to do that:
-
Think about your wishes for your care home and residents dying of dementia, what you would love to see happening or in place . . .
-
Go tell someone else and ask them to tell you their wishes. If that feels hard then what would things look like if it were a little better in your care home?
-
What would be the smallest thing you could do to bring your wishes into being . . . it may start with saying good morning to someone or learning someone’s name.
Appendix 50 Chapter 4: Intervention information – district nurses/general practitioners
Information for district nurses and general practitioners
The EVIDEM-EoL Phase 1 tracked over 1 year the care received by people with dementia in six care homes. The findings suggested that fundamental to good care is the way of working together of NHS care home staff, family members and, where possible, people with dementia.
Phase 2 is an intervention-based study with three care homes, underpinned by collaborative working. Care home and primary care staff identify an area for improvement around the care of a person dying with dementia, and develop strategies to implement this. To facilitate this we are using an AI approach. The originator David Cooperrider first worked with physicians in USA and noted that when he asked doctors about success rather than failure their energy, enthusiasm and motivation shifted.
Appreciative Intervention is both a philosophy and process that has been widely applied to change in health care. Fundamentally, it is the search for the best in people and their organisations and the assumption that within a system there is always something that is working. This strength-based change uses enquiry, positive questions, and good experiences to explore, discover and open up new possibilities with others who are important in the system.
In January we will begin this through ‘appreciative conversations’, asking you when you saw things working at their best in relation to people dying of dementia in care homes. So in preparation we would like you to think about a situation where you felt you or the team you were working with, delivered care that allowed a resident to die well. This may be in relation to symptom control, how you worked with the care home or were able to carry out the wishes of the resident or their family.
Think about
What made the situation really special?
What was your contribution?
How did it feel?
Has it changed you in any way, if so how?
How did the care home or other primary care team members help you in this?
What do you think was really making it work?
Talk about
Find a colleague and talk about it together, are your experiences similar? We call this Good Gossip and can happen over a break, as well as structured meeting times in the course of work.
Cooperrider D. Appreciative Inquiry: towards a methodology for understanding and enhancing organizational innovation. Doctoral Dissertation. Cleveland, OH: Western Reserve University; 1986.
Reed J. Older people maintaining wellbeing: implications for future developments. Int J Older People Nurs 2008;3:76–8.
Alfred R, Shohet R. ‘What’s the best day you’ve ever had at work?’ Appreciative Inquiry at the Manchester Heart Centre. In Edmonstone J, editor. Building on the Best: An Introduction to Appreciative Inquiry in Health Care. Chichester: Kingsham Press; 2006.
Appendix 51 Chapter 4: Intervention material – post-death analysis
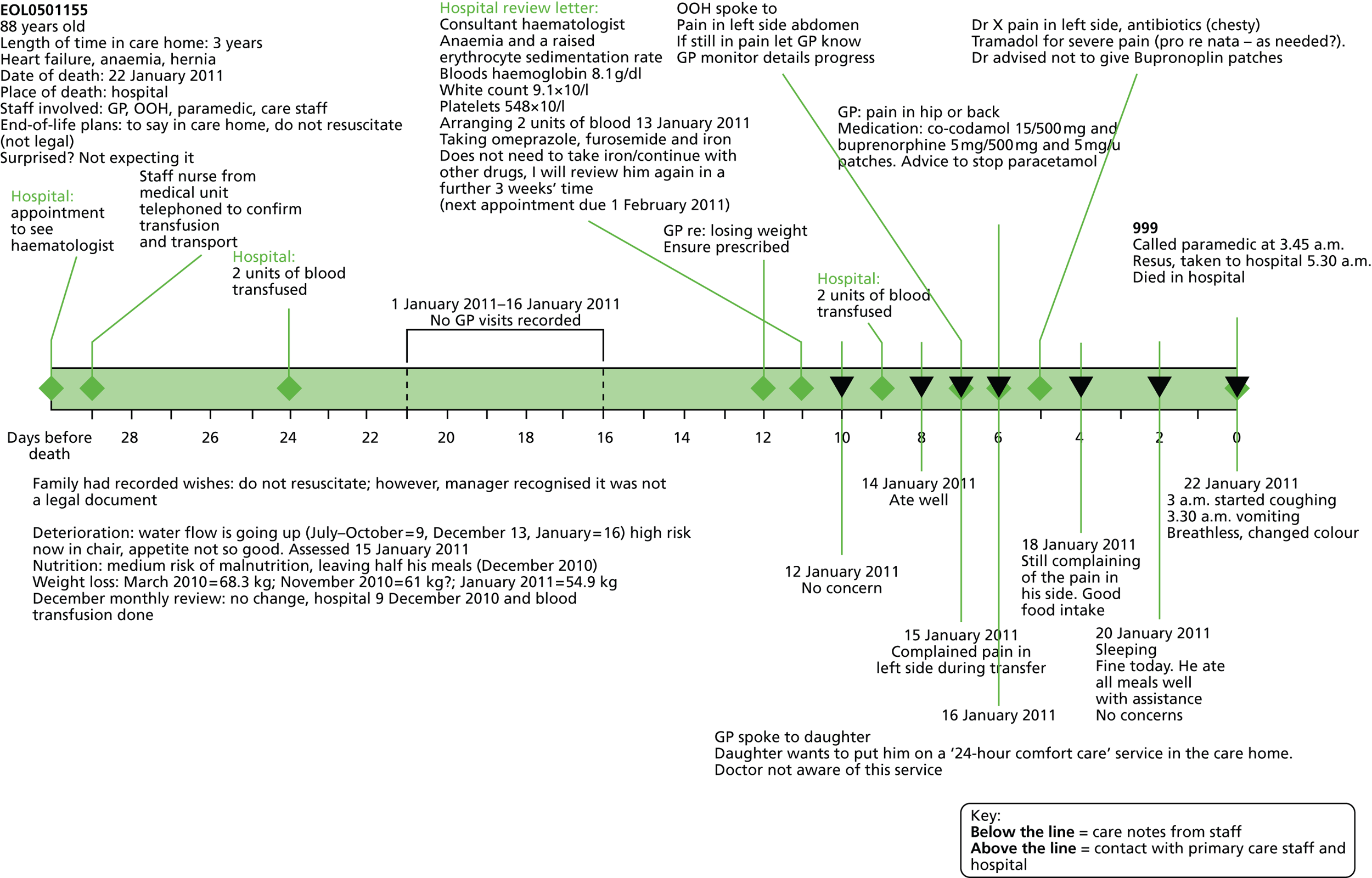
Appendix 52 Chapter 4: Participant developed tools – prompt sheet
Starting to have conversations about dying with residents and relatives
There is something about looking for clues from residents or relatives as to when they might be ready to talk about their wishes and being able to respond outside of a need to fill in a form, for example:
-
when they have received news of a death, may be a friend, family, pet . . .
-
when someone else in the home has died . . .
-
when they have just been through a time of change or something out of the ordinary routine, e.g. a hospital admission/not feeling so well/family member has visited . . .
Sometimes an open question, e.g. ‘How are you today?’ can open up a lot and often it is allowing yourself to not close this down. In one care home, passing by a resident, I said ‘How are you?’ and she said ‘I’m past my sell by date just waiting now to go . . .’ It could be a start of a conversation. My neighbour said to me yesterday, ‘I shouldn’t be here any longer.’ These are openings for us to listen.
Some ways of asking
-
I wondered if you minded thinking about the care you want for the future, for instance when you become unwell or your health starts to deteriorate.
-
I am not bringing this up because you/your relative is ill at the moment but just so that we can plan for the future and provide the best care for you/your relative in the coming months.
-
Some people have very definite views about how they want to be cared for at end of life and others do not want to think about it. We understand everyone is an individual.
-
It has been shown that it is sometimes easier to think about these things while you/residents are well, rather than have to make decisions at short notice.
-
You might not want to talk about this with me at the moment, or ever and that is absolutely fine. We just wanted to ask and hope you don’t mind. Or I can give you a form to take away and we can talk again. You might want to talk to other relatives, chat together or talk to your GP. There are some leaflets.
You (the resident or relative) are welcome to discuss the issues at any time they feel prompted to do so and for whatever reason.
-
We sometimes ask our residents if they have any anxieties about dying when the time comes. Is this something you would like to talk about?’
-
None of us know what the future holds but sometimes it helps to talk about our hopes and fears. Is there is anything you would like to talk about with me or your relative, a GP a spiritual or religious person? Please let me know and we will make some time for a conversation.
From research findings
Residents/relatives may talk informally with carers about their wishes (or what they don’t want, or what happened to a close relative that was good or bad), ask them if this can be recorded in the care notes. Follow up those conversations. It is not just the manager who has the conversations – in one care home the woman who worked in the laundry had the most open discussions with residents and relatives.
Possible times to talk with resident/relative – after a hospital admission, after a death of a fellow resident, when a resident’s health declines.
Advice from another care home manager: start the subject at the initial assessment (6 weeks), give them the form to take away and then review in another few months’ time. Keep offering the opportunity to follow up, especially as health deteriorates. It is easier to have the difficult conversations, if you have already had some earlier.
Some specific questions
Any wishes for your last days?
Where would you like to be cared for in the future? (care home or hospital?) ‘Care home as long as possible?’
Would you want any more hospital admissions? Resuscitation? Transfer to a nursing home?
Examples from other documents/care homes
A Preferred Priorities of Care (final wishes) document can help you/us prepare for the future. It gives you an opportunity to think about, talk about and write down your preferences and priorities for care at the end of life. You do not need to do this unless you want to . . .
-
Remember your views may change over time and you can change what you have written whenever you wish to.
-
What are your preferences and priorities for your future care?
-
Where would you like to be cared for in the future?
A final wishes plan is designed to inform people around me of my ‘wishes’ regarding the atmosphere and environment I would like to be in as my life draws to a close – and any specific requests I have. Please note this is for guidance and not legally binding.
-
Resident Have you spoken to anyone about your wishes? Would you like help to do so?
-
Relative Has your relative ever spoken to you about what they might like?
-
When you (or your relative) comes towards the end of your/their life, what would you/they like around you/them?
-
favourite flowers, music, pictures, favourite perfume, prayers, physical contact, massage, personal care
-
relatives/someone with you
-
any spiritual/religious needs
-
if the condition is prolonged I would/would not like to venture out of my room.
-
-
Subjects you might need to talk about:
-
the type of care someone would like towards the end of their life
-
where they’d like to die
-
whether they have any particular worries they’d like to discuss about being ill and dying
-
how long you would like doctors to keep treating you
-
resuscitation.
-
Some questions about funeral arrangements
(Not the key for advanced planning but sometimes a way to start a conversation; some people may feel more comfortable talking about funeral plans.)
-
I would like my death and funeral to be announced in the following ways
-
My preferred funeral director is
-
The person I would like to deal with my funeral arrangements is
-
The type of funeral I would like is (traditional, less traditional, hymns, music, readings, poems)
-
Buried or cremated
-
Flowers and or donations at the funeral
-
I would like to take with me (leave from); to wear (clothes, jewellery) to take with me (photo, book)
Appendix 53 Chapter 4: Participant developed tools – out-of-hours information sheet
Information out-of-hours services need to know about a resident
Prompt guide for care staff – out-of-hours services information sheet
These are questions for care home staff to think about before ringing OOH services. Please try and have answers ready for as many of these questions as you can.
_________________________Date
| Resident’s name | Answers |
|---|---|
| Medical history/background | |
| Symptoms (what is wrong?) | |
| How long has this been going on? | |
Is this:
|
|
| What were they like before this problem arose? What are they usually like? What is normal for them/what has changed? How has the problem altered/what are they normally like? |
|
| Wishes of the residents | |
| Wishes of the family/relative | |
| Preferred place of care? | |
| (anything else known – resus.?) | |
| Any more hospital admissions? Would resident want to go to hospital? |
|
| What do you want OOH to do? | |
| Why is OOH being called? | |
| Are they acutely unwell now? | |
| Do they need an ambulance (999)? Is it an emergency situation? | |
| Can their symptoms be managed? | |
| Further treatment for infection? | |
| Other |
Appendix 54 Chapter 4: EVIDEM-EoL advisory group members
| Marion Cowe | Member of the Public Involvement Group for CRIPACC (University of Hertfordshire). Lay perspective, former carer of brother with dementia |
| Jaleh von Wagner | Lay perspective. United Carers for Dementia. Carer for 8 years (24/7) of mother, who died from vascular dementia/Alzheimer’s disease then cared for elderly, disabled blind father for further 2.5 years (24/7); with both at the death |
| Rachel Dutton | Member of Housing 21 Association. Specialist area is social care provision for people with dementia towards end of life |
| Catherine Evans | Former EVIDEM-EoL lead researcher; research specialises in older people; background in nursing |
| Hillary Speller (attended one meeting) | Lay perspective. Father died with dementia in distressing circumstances |
| Jane Wilcock | Senior Research Fellow, University College London and EVIDEM Programme Manager |
| Allan Kellehear | Professor of Sociology, University of Bath. Specialist areas include dying, public health and end of life |
| Derek Baker | Lay perspective, wife has dementia and is living in a care home. Chairman of the Mid Essex Branch Committee, Alzheimer’s Society |
| Nicole Jackman | Senior doctor at St Francis Hospice – specialist interest in palliative care for the elderly |
| Louise Robinson | GP academic, University of Newcastle. Specialist areas include dementia (diagnosis, quality of life, end of life), promoting older people’s health |
| Sheila Peace | Professor of Gerontology, The Open University. Specialises in sociology, care homes, end of life and older people |
| Clive Evers | Director of Education and Information, Alzheimer’s Society |
| Ram Awatar | Director of Clinical Services, Nightingale House. Specialist areas include care homes, dementia and older people |
| Karen Harrison Dening | Specialises in research for OPWD and is a consultant Admiral Nurse |
| Priya Jain | Assistant Research Manager, EVIDEM Early Diagnosis |
| Kalpa Kharicha | Senior Research Fellow and EVIDEM Programme Manager (covering Jane Wilcock’s maternity leave) |
| Jan Dewing | Professor at University of Kent at Canterbury and Visiting Professor at the University of Wollongong, Australia. Holds extensive national and international experience, particularly in the field of dementia and care for the elderly, and has made a great contribution to consenting with people who lack capacity |
| Heather Maggs | Member of the Public Involvement Group for CRIPACC (University of Hertfordshire). Lay perspective. Father had dementia. Member of Alzheimer’s Society Quality Research in Dementia |
Appendix 55 Chapter 4: Care home characteristics
| Care home | Organisation of care | Dementia training | Palliative training or other | |
|---|---|---|---|---|
| CH1 | Dedicated Dementia Unit; staff allocation by duty manager night before shift on basis of experience – less experienced placed with more experienced; high level of continuity; key worker | Need to have completed basic dementia training to work on Dementia Unit. Can do further training BTEC diploma over 2.5 days or 1-year course – Champion in Dementia. Yearly update given to staff regarding use of antipsychotics | Informal in-house training provided by district nurses dependent on needs (e.g. stoma care, catheter care). Informal in-house training on mouth care, pressure area care, basics of pain relief and constipation management | |
| CH2 | Team of workers; key worker system; key worker for two to three residents, may change if poor rapport with resident. At beginning of shift, three staff members decide how to allocate work. Duty manager gives handover to all staff for morning shift and again for afternoon shift, which starts at 3.30 pm | On dementia unit all staff will have undertaken basic dementia training | District nurses advise CH staff on mouth care, request turning charts, fluid/food intake charts, monitoring passing of urine | |
| CH3 | Three staff per shift on dementia unit; allocated on daily basis at beginning of shift by senior staff. Communication – verbal and written, notes in diary, district nurse book and GP book | On dementia unit all staff will have undertaken basic dementia training | ||
| CH4 | Dedicated dementia unit; 6- and 12-hour shifts; separate night staff (12-hour shifts). Verbal handover through notes. Verbal to senior who transfers information to team | Training compulsory for all care staff run by organisation – 3 days over 3 weeks, including some homework | ||
| CH5 | Key worker system; hierarchical system; not clear how work organised; night staff entirely separate – not same support system available to them, fewer staff, no senior, theoretically home manager contactable | Training (information on office wall) but not clear what is involved | Informal in-house training provided by district nurses (nursing sisters) in the past, e.g. diabetic training | |
| CH6 | Senior carer on each unit, on each shift. Seniors handover to everybody but anything more specific to do with staff it would be senior to senior | Unclear | ||
| Care home | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 |
|---|---|---|---|---|---|---|
| Use end-of-life care framework | District nurses use LCP | None | LCP used once in conjunction with district nurses | Made enquiries about GSF | Services Director looking at GSF; looked at LCP but not progressed | Looked at LCP but not progressed |
| Other end-of-life arrangements | Organisation-specific end-of-life care plan, staff do not attempt resuscitation (although trained, they wait for paramedics) | Organisation-specific end-of-life care plan | Organisation-specific end-of-life care plan; staff do not resuscitate (although trained, wait for paramedics) | Resuscitation unless chosen otherwise, asked as soon as possible after admission | Organisation-specific end-of-life care; everyone for resuscitation unless otherwise stated | Organisation-specific end-of-life care; everyone for resuscitation unless otherwise stated |
Appendix 56 Chapter 4: Care home resident needs and deaths in year prior to study
| Areas of need | CH1 (N = 44): n (%) | CH2 (N = 58): n (%) | CH3 (N = 57): n (%) | CH4 (N = 62): n (%) | CH5 (N = 66): n (%) | CH6 (N = 54): n (%) |
|---|---|---|---|---|---|---|
| Bedfast | 0 | 0 | 3 (5.3) | 0 | 0 | 0 |
| Help with dressing/undressing | 41 (93.2) | 46 (79.3) | 50 (87.7) | 42 (67.7) | 52 (78.8) | 38 (70.4) |
| Help with washing/bathing | 44 (100.0) | 56 (96.6) | 57 (100.0) | 59 (95.2) | 63 (95.5) | 51 (94.4) |
| Help going to the toilet | 33 (75.0) | 32 (55.2) | 42 (73.7) | 32 (51.6) | 30 (45.5) | 21 (38.9) |
| Singly incontinent | 34 (77.3) | 15 (25.9) | 7 (12.3) | 39 (62.9) | 16 (24.2) | 13 (24.1) |
| Doubly incontinent | 18 (40.9) | 22 (37.9) | 18 (31.6) | 9 (14.5) | 21 (31.8) | 3 (5.6) |
| Dementia | 31 (70.5) | 34 (58.6) | 29 (50.9) | 30 (48.4) | 55 (83.3) | 23 (42.6) |
| Other mental health needs | 1 (2.3) | 6 (10.3) | 1 (1.8) | 0 | 4 (6.1) | 0 |
| Learning disabilities | 1 (2.3) | 1 (1.7) | 2 (3.5) | 0 | 0 | 0 |
| Physical disabilities | 19 (43.2) | 17 (29.3) | 0 | 0 | 0 | 1 (1.9) |
| Two or more staff to help with their care | 14 (31.8) | 11 (19.0) | 8 (14.0) | 14 (22.6) | 13 (19.7) | 4 (7.4) |
| Help/supervision/prompts to eat meals | 23 (52.3) | 17 (29.3) | 11 (19.3) | 23 (37.1) | 29 (43.9) | 4 (7.4) |
| Impaired vision | 24 (54.5) | 13 (22.4) | 25 (43.9) | 37 (59.7) | 42 (63.6) | 2 (3.7) |
| Impaired hearing | 2 (4.5) | 8 (13.8) | 16 (28.1) | 20 (32.3) | 19 (28.8) | 2 (3.7) |
| English not first language | 1 (2.3) | 2 (3.4) | 0 | 0 | 0 | 0 |
| Specialist communication needs | 4 (9.1) | 0 | 0 | 0 | 3 (4.5) | 0 |
| Alcohol dependence | 0 | 1 (1.7) | 0 | 0 | 0 | 1 (1.9) |
| Drug dependence | 0 | 0 | 0 | 0 | 0 | 0 |
Appendix 57 Chapter 4: Care homes’ access to primary and specialist services
| Care service | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 |
|---|---|---|---|---|---|---|
| GP (no. of associated practices; visitation rate) | Two practices; once a week and on request | Two practices; once a week and on request | Four practices; once a week and on request | Four practices; once a week and on request | Three practices (new residents registered with only one); once a month and on request | Two practices; on request and annual medication review |
| GP (no. of visiting GPs) | One regular from each practice but up to two or three GPs from each practice | One regular from each practice but one or more from each practice | One regular from each practice but up to two GPs from each practice | No designated GP. Up to 6 different GPs | One regular from one practice, but up to eight different GPs | Full contact with each practice; eight GPs in one practice; unclear how many GPs in other |
| GP OOH | On request | Every 2 weeks on average | On request | On request | Once a week on average and on request | On request |
| District nurse | On request | Every day (insulin dependent diabetic) and on request | Twice a day (insulin dependent diabetic) and on request | On request | On request | Twice a day (insulin dependent diabetic) and on request |
| Night nurse | On request | Never | On request | Never | Never | Never |
| Community Mental Health Nurse/MHT | Once a week | Once a month (patient review) | On request | Every few months and on request | Once a month | Never; MHT occasionally |
| Admiral Nurse | No | Never | Never | Never | Never | Never |
| Counsellor | On request (GP referral) | In-house trained | Never | On request | Never | Never |
| Palliative care nurse (e.g. Macmillan, Marie Curie) | On request (only for people with cancer) | Never | Never | Once a month | Never | Occasionally (Macmillan by referral from GP) |
| Other | Psychogeriatrician (on request, 3-month wait, GP referral); hospice support – on request (only for people with cancer); night sitter service – on request (only for people with cancer from hospice) | Psychogeriatrician has been involved | None mentioned | Parkinson’s Nurse – once a month and on request; psychiatrist – from time to time; psychogeriatrician – infrequent support | Chaplain – twice a week/once a month (different churches) | None mentioned |
Appendix 58 Chapter 4: Resident baseline characteristics
| Care home | Recruitment (n) | Recruitment rate (%) | Retention (n) | Retention (%) at end of Phase 1 |
|---|---|---|---|---|
| CH1 | 20 | 71.4 | 15 | 75.0 |
| CH2 | 25 | 62.5 | 19 | 76.0 |
| CH3 | 16 | 50.0 | 12 | 75.0 |
| CH4 | 23 | 76.7 | 19 | 82.6 |
| CH5 | 34 | 54.0 | 25 | 73.5 |
| CH6 | 15 | 71.4 | 10 | 66.7 |
| Total | 133 | 62.2 | 100 | 75.2 |
| Care home | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 |
|---|---|---|---|---|---|---|
| Median age at admission, years (mean, SD) | 84.4 (84.4, 6.15) | 87.3 (86.3, 8.55) | 87.5 (86.0, 4.63) | 86.5 (83.7, 5.61) | 82.7 (82.0, 6.56) | 83.7 (82.8, 7.06) |
| Median age at baseline, years (mean, SD) | 88.7 (87.4, 6.16) | 90.4 (89.51, 8.33) | 89.7 (88.7, 5.77) | 88.1 (85.7, 5.85) | 84.9 (83.5, 6.22) | 84.3 (83.7, 7.41) |
| Median length of residency, years (mean, SD) | 2.5 (3.0, 2.16) | 3.4 (3.2, 1.84) | 2.0 (2.8, 2.13) | 1.6 (2.0, 1.77) | 1.3 (1.5, 1.00) | 0.2 (1.0, 2.20) |
| Place of previous residence (N = 120) | n | % |
|---|---|---|
| Own home | 52 | 43.3 |
| Relative’s home | 7 | 5.8 |
| Hospital | 35 | 29.2 |
| Care home | 13 | 10.8 |
| Sheltered housing – warden controlled | 13 | 10.8 |
| Total | 120 | 100.0 |
| Reason for admission (N = 91) | ||
| Following death of spouse | 3 | 3.30 |
| Resident isolated | 5 | 5.50 |
| Concerns about safety | 8 | 8.80 |
| Deterioration of health | 16 | 17.60 |
| Resident unable to cope living independently | 31 | 34.10 |
| Carers no longer able to cope | 11 | 12.10 |
| Short-term stay into long-term placement | 7 | 7.70 |
| Needs unmet in previous accommodation | 4 | 4.40 |
| Other | 6 | 6.60 |
| Total | 91 | 100.0 |
| Recorded diagnosis of dementia (N = 98) | ||
|---|---|---|
| Type | n | % |
| Alzheimer’s disease | 38 | 38.80 |
| Vascular dementia | 16 | 16.30 |
| Mixed | 5 | 5.10 |
| Lewy body | 2 | 2.00 |
| Korsakoff syndrome | 1 | 1.00 |
| Other | 1 | 1.00 |
| Not recorded | 35 | 35.70 |
| No recorded diagnosis | 35 | 26.30 |
| Source | ||
| Neurologist | 1 | 1.00 |
| Social worker | 2 | 2.00 |
| Consultant psychiatrist | 12 | 12.30 |
| Assessment | 4 | 4.10 |
| Brain scan | 1 | 1.00 |
| SMHT | 7 | 7.20 |
| CPN report | 1 | 1.00 |
| Memory clinic | 2 | 2.00 |
| Not recorded | 68 | 69.40 |
| No recorded diagnosis | 35 | 26.30 |
| ADL | Recorded (N, %) | No record (N, %) | Total (N, %) |
|---|---|---|---|
| (In)continence | 131 (100) | 0 (0.0) | 131 (100) |
| Personal care | 131 (99.2) | 1 (0.8) | 132 (100) |
| Maintaining a safe environment | 128 (97.7) | 3 (2.3) | 131 (100) |
| Eating and drinking | 128 (97.0) | 4 (3.0) | 132 (100) |
| Toilet use | 92 (70.2) | 39 (29.8) | 131 (100) |
| Sleeping | 61 (47.7) | 67 (52.3) | 128 (100) |
| Expressing sexuality | 19 (14.5) | 112 (85.5) | 131 (100) |
| Breathing | 13 (9.8) | 119 (90.2) | 132 (100) |
| Function | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Total |
|---|---|---|---|---|---|---|---|
| ADLa | |||||||
| (In)continence, n (%) | |||||||
| Incontinent | 13 (68.4) | 5 (20.8) | 7 (43.8) | 11 (47.8) | 13 (38.2) | 4 (26.7) | 53 (40.5) |
| Occasional accident | 0 (0) | 2 (8.3) | 1 (6.3) | 0 (0) | 2 (5.9) | 1 (6.7) | 6 (4.6) |
| Continent | 6 (31.6) | 17 (70.8) | 8 (50.0) | 12 (52.2) | 19 (55.9) | 10 (66.7) | 72 (55.0) |
| Personal care, n (%) | |||||||
| Needs assistance of up to two people | 4 (21.1) | 8 (33.3) | 7 (43.8) | 18 (78.3) | 7 (20.6) | 6 (40) | 50 (38.2) |
| Needs assistance of one person | 15 (78.9) | 16 (66.7) | 7 (43.8) | 4 (17.4) | 26 (76.5) | 5 (33.3) | 73 (55.7) |
| Independent | 0 (0) | 0 (0) | 2 (12.5) | 1 (4.3) | 1 (2.9) | 4 (26.7) | 8 (6.1) |
| Maintaining a safe environment, n (%) | |||||||
| Immobile | 3 (15.8) | 2 (8.7) | 1 (6.7) | 2 (8.7) | 3 (9.1) | 0 (0.0) | 11 (8.6) |
| Needs supervision | 5 (26.3) | 7 (30.4) | 4 (26.7) | 2 (8.7) | 11 (33.3) | 5 (33.3) | 34 (26.6) |
| Independent (with or without mobility aid) | 11 (57.9) | 14 (60.9) | 10 (66.7) | 19 (82.6) | 19 (57.6) | 10 (66.7) | 83 (64.8) |
| Eating and drinking, n (%) | |||||||
| Full assistance | 7 (35) | 6 (28.6) | 4 (25) | 4 (17.4) | 8 (24.2) | 2 (13.3) | 31 (24.2) |
| Minimal assistance | 3 (15) | 2 (9.5) | 1 (6.3) | 2 (8.7) | 3 (9.1) | 1 (6.7) | 12 (9.4) |
| Independent | 10 (50) | 13 (61.9) | 11 (68.8) | 17 (73.9) | 22 (66.7) | 12 (80) | 85 (66.4) |
| Toilet use, n (%) | |||||||
| Needs assistance of up to two people | 2 (15.4) | 3 (15) | 3 (25) | 0 (0) | 4 (21.1) | 2 (15.4) | 14 (15.2) |
| Needs assistance of one person | 7 (53.8) | 7 (35) | 2 (16.7) | 2 (13.3) | 2 (10.5) | 3 (23.1) | 23 (25) |
| Independent | 4 (30.8) | 10 (50) | 7 (58.3) | 13 (86.7) | 13 (68.4) | 8 (61.5) | 55 (59.8) |
| Event category | Detail as recorded in care notes |
|---|---|
| Infection | Upper respiratory tract, chest, urinary tract, eye, thrush, catheter-induced penile tear, discharge (anal, penile), raised temperature |
| Gastrointestinal | Vomiting, diarrhoea, constipation |
| Pain | Increased pain, headache |
| Stroke/transient ischaemic attack | Suspected stroke or transient ischaemic attack |
| Carcinoma | Suspected or spreading carcinoma |
| Leg/ankle swelling | Leg and/or ankle swelling |
| Tissue viability | Pressure ulcer, blister, tear, soreness |
| Confusion | Increased confusion, absence |
| General deterioration | General ill health, general deterioration, generally unwell, increased frailty, increased weakness |
| Fallsa | Falls |
| Eating and drinking | Loss of appetite, difficulty swallowing, supplementary feeds, unable to eat, unable to drink, weight loss |
| Sleepiness | Increased sleepiness |
| Withdrawal | Increasingly withdrawn, no longer enjoys activities as s/he used to |
| Skin colour | Pale, skin colour change |
| Decreased mobility | Reduced mobility, increased stiffness |
| Incontinence | Urine, faeces |
| Refusing personal care | Repeatedly refusing personal care |
| Breathing | Laboured breathing, breathlessness |
| Agitation | Increased agitation, aggressive behaviour towards other residents |
| Distress | Low mood, tearfulness, ‘wants to die’ |
| Other | Muscle spasms, ‘floppiness’, not his or herself, lethargy, altered level of consciousness, increased difficulty in communicating, disturbed sleep, dehydration, mouth sores, seizure, unsteadiness, nausea, drowsiness, weight gain, shaking, rectal prolapse, renal and heart failure, dizziness, fainting, bleeding (penile, vaginal, nose, blood in stools), lip swelling, face swelling, unresponsive |
| Type of eventb | Baseline (N = 133) | Time point 2 (N = 127) | Time point 3 (N = 112) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Infection | 31 | 23.30 | 20 | 15.70 | 29 | 25.90 |
| Gastrointestinal | 9 | 6.80 | 16 | 12.60 | 27 | 24.10 |
| Pain | 3 | 2.30 | 5 | 3.90 | 3 | 2.70 |
| Stroke/transient ischaemic attack | 2 | 1.50 | 2 | 1.60 | 2 | 1.80 |
| Carcinoma | 1 | 0.80 | 2 | 1.60 | 4 | 3.60 |
| Leg/ankle swelling | 4 | 3.00 | 3 | 2.40 | 4 | 3.60 |
| Tissue viability | 10 | 7.50 | 15 | 11.80 | 11 | 9.80 |
| Confusion | 11 | 8.30 | 13 | 10.20 | 15 | 13.40 |
| General deterioration | 1 | 0.80 | 8 | 6.30 | 4 | 3.60 |
| Fallsc | 29 | 21.80 | 39 | 30.70 | 33 | 29.50 |
| Eating and drinking | 36 | 27.10 | 45 | 35.40 | 37 | 33.00 |
| Sleepiness | 11 | 8.30 | 22 | 17.30 | 36 | 32.10 |
| Withdrawal | 0 | 0.00 | 4 | 3.10 | 8 | 7.10 |
| Skin colour | 0 | 0.00 | 2 | 1.60 | 2 | 1.80 |
| Decreased mobility | 0 | 0.00 | 2 | 1.60 | 4 | 3.60 |
| Incontinence | 15 | 11.30 | 36 | 28.30 | 38 | 33.60 |
| Refusing personal care | 0 | 0.00 | 1 | 0.80 | 3 | 2.70 |
| Other | 8 | 6.00 | 10 | 7.90 | 19 | 17.00 |
| Breathing | 1 | 0.80 | 1 | 0.80 | 3 | 2.70 |
| Agitation | 2 | 1.50 | 9 | 7.10 | 12 | 10.70 |
| Distress | 0 | 0.00 | 2 | 1.60 | 5 | 4.50 |
| Time period | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Total |
|---|---|---|---|---|---|---|---|
| At least one fall up to 3 months preceding baseline or up to the previous date of extraction | |||||||
| Baseline (N = 133), n (%) | 5 (25.0) | 6 (24.0) | 2 (12.5) | 4 (17.4) | 9 (26.5) | 3 (20.0) | 29 (21.8) |
| Time point 2 (N = 127), n (%) | 8 (40.0) | 9 (37.5) | 4 (26.7) | 5 (22.7) | 10 (32.3) | 3 (20.0) | 39 (30.7) |
| Time point 3 (N = 112), n (%) | 9 (47.4) | 2 (10.0) | 2 (14.3) | 9 (42.9) | 6 (23.1) | 5 (41.7) | 33 (29.5) |
| Resident, consultee and family | 8 (6.0) |
| Resident and consultee | 3 (2.3) |
| Resident and family | 7 (5.3) |
| Consultee and family | 10 (7.5) |
| Family | 18 (13.5) |
| Consultee | 14 (10.5) |
| Resident | 10 (7.5) |
| No evidence of discussions on end-of-life wishes | 53 (39.8) |
| Unclear | 10 (7.5) |
| Attrition | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | Total |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| Deceased: care home | EoL0201064 | EoL0301074 | 2 | ||||
| Deceased: hospital | EoL0501171 | 1 | |||||
| Transferred | EoL0501165 | 1 | |||||
| Time point 2 | |||||||
| Deceased: care home | EoL0101015 | EoL0401148 a | 2 | ||||
| Deceased: hospital | EoL0501154 | 4 | |||||
| EoL0501156 | EoL0601223 | ||||||
| EoL0501209 | |||||||
| Transferred | EoL0301083 | EoL0501181 | EoL0601215 | 3 | |||
| Time point 3 | |||||||
| Deceased: care home | EoL0101013 | EoL0301090 | EoL0401127 | EoL0601217 | 6 | ||
| EoL0101021 | EoL0401133a | ||||||
| Deceased: hospital | EoL0101023 | EoL0201044a | EoL0501192 | EoL0601233 | 7 | ||
| EoL0201062a | EoL0501206 | ||||||
| EoL0201067a | |||||||
| Transferred | EoL0501199a | EoL0601221 | 2 | ||||
| End of Phase 1 | |||||||
| Deceased: care home | EoL0101022 | EoL0201056 | EoL0301091 | EoL0401130 | 5 | ||
| EoL0201068 | |||||||
| Total deaths (%) | 5 | 6 | 3 | 4 | 6 | 3 | 27 (20.3) |
| Total transfers (%) | 0 | 0 | 1 | 0 | 3 | 2 | 6 (4.5) |
| Total (%) | 5 (25) | 6 (24) | 4 (25) | 4 (17.4) | 9 (26.5) | 5 (33.3) | 33 (24.8) |
Appendix 59 Chapter 4: Service utilisation and associated costs
| Type of visit | Overall (n = 122) | Alive (n = 105) | Dead (n = 17) | Wilcoxon test p-value |
|---|---|---|---|---|
| Primary care | 0.9 (0.5–1.3) | 0.8 (0.5–1.2) | 2.0 (1.0–3.0) | 0.0006 |
| Hospital | 0.3 (0–0.8) | 0.2 (0–0.7) | 0.8 (0.4–13.8) | 0.002 |
| Community health | 0.4 (0.2–1.1) | 0.4 (0.2–0.7) | 1.1 (0.6–1.6) | 0.008 |
| Costs | Overall (n = 133) | Alive (n = 116) | Dead (n = 17) | Wilcoxon test p-value |
|---|---|---|---|---|
| Primary care | 76 (45–123) | 67 (39–107) | 127 (106–279) | 0.0003 |
| Hospital | 39 (0–199) | 34 (0–111) | 273 (96–6984) | 0.0018 |
| Community health care | 45 (21–92) | 44 (20–83) | 89 (29–167) | 0.0401 |
| Accommodation | 2243 (0–2473) | 2243 (0–2447) | 2243 (1754–2473) | 0.4958 |
| Total care and accommodation | 2488 (413–2890) | 2438 (194–2834) | 2917 (2226–9867) | 0.0152 |
| Medication | 57 (31–92) | 57 (30–95) | 43 (38–66) | 0.4015 |
| Total (including accommodation and medication) | 2549 (463–2974) | 2490 (251–2927) | 2964 (2253–9925) | 0.0168 |
Appendix 60 Chapter 4: Sample information – interviews
| Staff | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 |
|---|---|---|---|---|---|---|
| Care home manager | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Care home deputy manager | ✗ | ✗ | ✗ | ✗ |
| Participant information | CH1 (N = 5) | CH2 (N = 5) | CH3 (N = 3) | CH4 (N = 3) | CH5 (N = 4) | CH6 (N = 4) |
|---|---|---|---|---|---|---|
| Age, years: age range, median (n) | 41–56, 48.7 (3) | 27–58, 41.0 (4) | 46–68, 57.0 (2) | 20–47, 30.0 (3) | 31–46, 40.25 (4) | 18–39, 27.0 (4) |
| FT spread; PT (n) | 1 FT; 4 PT (5) | 3 FT; 2 PT (5) | 1 FT; 2 PT (3) | 3 FT; 0 PT (3) | 3 FT; 0 PT (3) | 2 FT; 1 PT (3) |
| Length of time working in care home, months: range (n) | 1–240 (4) | 11–36 (3) | 50–178 (2) | 30–36 (3) | 24–48 (4) | 3–27 (3) |
| Qualifications: received detail (n) | 4 NVQ2 (4) 2 BTEC (4) | 2 NVQ2 (4) | 1 NVQ2 (2) | 3 NVQ2 (3) | 2 NVQ2 (3) | 2 NVQ3 (3) |
| Dementia training: received (n) | 5 in-house (5) | 5 (5) | 3 (3) | 3 (3) | 4 in-house (4) | 3 (4) |
| End-of-life training: received (n) | 2 (5) | 5 (5) included in general training | 2 (3) | 0 (3) | 0 (4) | 1 (4) |
| Staff | GPs (n = 5) | District nurses (n = 5) | Emergency services (n = 3) |
|---|---|---|---|
| Length of time in post: range, years | 7–18 | 0.5–7 | 6–7 |
| Length of time working with care home: range, years | 4–11 | 0.5–7 | N/A |
| Dementia training | 2/5 | 2/5 | No (MCA only) |
| End-of-life training | No | 4/5 | No |
Appendix 61 Chapter 4: Phase 2 – documentation changes
| Time point | Baseline (n = 74) | Time point 2 (n = 73) |
|---|---|---|
| Evidence of end-of-life discussions: n (%) | 54 (73) | 58 (79.5) |
| Involvement in end-of-life discussions: n (%) | ||
| Resident involvement | 7 (13.0) | 7 (12.1) |
| Family involvement | 34 (63.0) | 44 (75.9) |
| POA | 4 (7.4) | 6 (10.3) |
| Care home staff involvement | 9 (16.7) | 19 (32.8) |
| GP involvement | 4 (7.4) | 10 (17.2) |
| Other (i.e. witness to living will, minister) | 4 (7.4) | 4 (6.9) |
| District nurse involvement | – | 2 (3.4) |
| Hospital Medical Assessment Unit | – | 2 (3.4) |
Appendix 62 Chapter 5: EVIDEM-MCA protocol
Introduction
EVIDEM-MCA is a part of the NIHR-funded EVIDEM programme of research (Principal Investigator Professor Steve Iliffe) and has the ultimate goal of developing an evidence-based resource to enhance practitioners’ awareness of the MCA to the benefit of people with dementia and to develop new knowledge about the interaction between the MCA and adult safeguarding systems and practice. This study will evaluate the impact of the Mental Capacity Act (MCA) 2005 over time, how it affects people with dementia and carers, and develop educational outputs in support of the Act. Based on an investigation into the implementation of the Act at practice level, the study will produce practice guidance on the use of the MCA, with a special focus on its use in adult safeguarding work. The study will take place in the North West London area as part of the EVIDEM programme and will connect to other parts of the programme. Specifically the study will evaluate the impact of the MCA on people with dementia, carers, professionals and the culture of the health and social care organisations. It will produce case series to inform adult protection approaches in the context of the MCA at Trust, local authority and national level.
Background
The Mental Capacity Act 2005 (MCA) in England and Wales, implemented 2007, enshrines much of the practice established under case law to safeguard people who lack ability to make specific decisions, to enhance personal autonomy and to enable people to make advance decisions to refuse treatment. It introduced new proxy decision-making roles to address health, welfare and financial matters and specialist advocacy for people who do not have family or friends where major health and welfare decisions are to be made. The implications of the MCA for health and social care practitioners around the areas of planning and working with others in new roles remain unclear. 250,251
It has been estimated that over two million people in England and Wales may be personally affected by the provisions of the Act. 252 These include many people with dementia and those who are carers, their representatives or service provider. 253 The MCA provides a statutory framework to empower and protect people, notably those who fear cognitive impairment or those who have received a diagnosis of a dementia, or those who have early and advancing symptoms who may not be able to make specific decisions at specific times (www.dca.gov.uk) currently or in the future. 254 It clarifies who can make decisions, in which situations, and the steps that should be taken. It also enables people to plan ahead in the event of loss of capacity to make particular decisions. 256
The procedures and the ability to make choices about future care and treatment decisions mean that the MCA has potential to enhance practice and user empowerment, and thus contribute to better outcomes for service users and carers. 257 People with dementia may require specialist advice sensitive to their particular values and networks to make valid and applicable advance decisions to refuse treatment and to formulate and communicate their wishes and preferences about care and treatment. However, there are likely to be key transitions or times when this type of advice and assistance may be most pertinent. These include encounters, such as the communication of a diagnosis of dementia, transitional periods when planning and setting up care packages or facing end of life. Professionals may need to devote time to assist people with dementia and their carers to benefit from the Act, and to make sure that they are ready to explain and debate its implications within teams and across agencies. 259 Whilst savings of professional time may emerge if people with dementia and carers are better informed and more confident, practitioners may feel compromised by work pressures and unable to provide the necessary support. Furthermore, proactive promotion of the Act on the part of managers and professionals in a range of local settings may be required if people with dementia are to maximise their opportunities for planning their care and making their wishes known. In these respects, the Act offers people with dementia a crucial opportunity to influence professional-decision-making so that capacity is presumed unless proven otherwise, that best interests should be the basis for decisions if a person is not able to make a specific decision, and provides people with a dementia with greater confidence that they are able to shape their future social care and treatment. 209 Lastly, the new criminal offences of ill-treatment and wilful neglect under the Act offer new means of adult protection that may be of benefit to people with dementia. The full implementation of this Act in late 2007 has provided a unique opportunity to explore the early operation of the Act and its incorporation into practice and service cultures.
Rationale for the study
Implemented in 2007, the Mental Capacity Act (2005) has a number of implications for the practice and governance of research, most of which reflect existing good research practice. The specific implications for the responsibilities of local authority social care research governance procedures are given here. More general issues for research involving people who lack capacity are given in the Mental Capacity Act Code of Practice. 446
Section 1 of the MCA establishes some basic principles: that a person must be assumed to have capacity unless it is established that he lacks capacity; that a person is not to be treated as unable to make a decision unless all practicable steps to help him to do so have been taken without success; that a person is not to be treated as unable to make a decision merely because he makes an unwise decision; that an act done, or decision made, under this Act for or on behalf of a person who lacks capacity must be done, or made, in his best interests; that before the act is done, or the decision is made, regard must be had to whether the purpose for which it is needed can be as effectively achieved in a way that is less restrictive of the person’s rights and freedom of action. 249 (Please note that ‘he’ also refers to ‘she’.)
Someone is thought to lack capacity because of ‘impairment or particular problems with the functioning of the mind or brain’ (Section 2 Sub section 1). A person is thought to be unable to make specific decisions if he or she is unable:
-
to understand the information relevant to the decision
-
to retain that information
-
to use or weigh that information as part of the process of making the decision, or
-
to communicate his decision (whether by talking, using sign language or any other means) (Section 3 sub section 1).
However, if a person can understand information with appropriate explanations they are seen as capable of making that decision. Further, a person’s inability to recall information for long periods does not make them incapable under the MCA.
The MCA requires that decisions taken on behalf of people who are deemed to lack capacity are taken explicitly in their best interests. Such decisions should be made after considering all relevant features and not simply on the person’s age, appearance, condition(s) or known behaviour patterns. In particular, those making such decisions should take into account views expressed when the person did have capacity and the possibility he or she might regain capacity in the future.
A person judged to be unable to make decisions or consent under the MCA should be involved as much as possible in making choices and their known or suspected feelings and beliefs need to be taken into account in making any decisions on their behalf. Those acting for someone deemed not to be capable under the MCA should also consult any individual named by the person to consult on such matters and the person’s carers (unpaid or paid). Such individuals include someone granted lasting powers of attorney by the person or a deputy appointed by the Court of Protection to manage his or her affairs (i.e. welfare). In addition to these requirements, the individual making the decision must reasonably believe the course of action is in the best interests of the incapable person.
Study aim and research approach
This study aims to:
-
identify the implementation issues arising from the introduction of the MCA in the services and practices of staff working with people with dementia and their carers over a 5-year period
-
explore and make recommendations about continued professional development programmes around the MCA for staff and their links to adult safeguarding training and practice.
There are five main research questions:
-
In 2007 what challenges face staff when they come across issues of mental capacity in their practice with people with dementia?
-
What are the expectations of professionals working with people with dementia about the MCA?
-
What are the expectations of older people and carers about the MCA?
-
What is revealed by a study of professional records about practitioners’ work in passing information about the MCA to people with dementia and their carers and how does this change over the period of 5 years?
-
What links between the MCA and adult protection arise in practice and is the MCA assisting the safeguarding of people with dementia?
This study has two main phases. Phase 1 involves an audit of staff perceptions of issues arising in decision-making and assessment of capacity among people with dementia, collection of data about training received on the MCA and information provided. Phase 2 will build on this audit to continue to engage with staff about their work in assessing capacity and implementing the provisions of the MCA, will undertake a review of case records, and will seek information from staff and people with dementia and carers about their contact with professionals over issues of mental capacity and decisions about care and treatment.
The results from Phase 1 will thus be used to develop tools for data collection about advice and recommendations (Phase 2). Details of Phase 2 will be submitted for peer and ethical review.
The development of this protocol has been informed by the main EVIDEM advisory group and the EVIDEM-MCA study advisory group, which involve people with experience of using services, carers, practitioners, advocates and managers. The latter has commented in particular on the draft interview schedule and the proposed list of participants.
Phase 1
Methodology
Qualitative research methodology, using individual in-depth interviews will be used over time to obtain a range of perspectives from practitioners working with people with dementia and carers. Interviews will be conducted at baseline and follow-up will be conducted 12–18 months after baseline interviews.
Practitioner sample and recruitment
Participants will be recruited through contact with employers. Interviews will be semi-structured and will start through an audit of training and experiences in the management of issues surrounding decision-making and mental capacity. Those interviewed will also be asked about adult protection connections with the MCA. Interviews with the new IMCA will explore the nature of these roles in the Trust area and their expectations about the size and type of work with people with dementia as the service becomes established.
These first interviews will provide a baseline for the main interviews in 2009, 2010 and 2011 as participants will be asked if we can return to them in future years as part of the study. The interviews will be semi-structured.
The interviews will target specific individuals in specific settings in North London as being likely to have particularly pivotal experiences:
-
community-based mental health nurses whose practice involves the day to day support of carers of people with dementia (Admiral Nurses, n = 15)
-
adult protection staff (n = 10)
-
medical staff with experience of clinical work with people with dementia at times of transition (n = 6)
-
carers, former and current, of people with dementia who have volunteered to assist dementia related studies through the voluntary sector (n = 15)
-
senior social workers/approved social workers/care managers working with people with dementia at times of transition or change in care packages (n = 20)
-
voluntary sector staff supporting people with dementia and carers (n = 30) (Age Concern, Alzheimer Society and Carers Groups)
-
independent mental capacity advocates (IMCAs) (n = 5)
-
care home staff, ranging from managers and deputies (n = 10) to care assistants (n = 15)
-
home care staff (n = 15)
-
older people attending a science interest group; a multicultural social group and an advocacy group (a set of groups chosen for their diversity of memberships) (n = 30).
Staff from a range of agencies will be invited to participate as well as a small number of carers and older people (Phase 2 will be the opportunity to focus on the research questions with people using services for support with dementia or caregiving). Key agencies will include the local authorities, independent sector providers of health and social care services, and support groups for older people and people with dementia and their carers. The community and voluntary sector have been interested in this area for a long time447 and Phase 1 will include interviews with groups that advise older people as well as older people.
Data collection and analysis
Data will be gathered through individual interviews (face to face or by telephone) using a semi-structured interview schedule to ensure key themes are explored. Interviews will focus on the training practitioners had, their experience of the Act and their expectations of it as it was implemented. An attempt to obtain case examples will be made. Interviews will be taped, with permission, transcribed and the tapes deleted and telephone interviews will be recorded in note form. Each transcription will only be known by an identifying number not the person’s name and all items that might identify the informant, such as names or care homes, will be made anonymous. Data will be coded using the Framework qualitative method of data analysis261,262 to draw out issues, concepts and themes. Data analysis will be an iterative process of identifying, organising, refining and re-organising the data into themes and sub-themes to describe the key elements of practice, including describing similarities and differences in training, knowledge and confidence. Background literature will be used to establish consistencies and inconsistencies between the reports of knowledge and practice and to identify the general issues and research questions raised by the data.
Ethical considerations
People will be approached following contact with their employer. This person would explain about the research and pass on an information leaflet which has been adapted to provide key points in straight forward language. After consent to approach the person has been gained the researcher will repeat the explanations and check consent at that point in time. A consent form for signing will be offered, although if the informant is unwilling to sign this will not be pursued and verbal consent will be noted. The researcher will thank the person for their time and involvement and offer to send them a summary of the study’s findings and to leave a short leaflet of information sources about the MCA.
All participants will be assured of anonymity and confidentiality in the transcription, analysis and reporting of their interview. Any direct quotations used in the report will be non-attributable.
Approximate timescale
Development of topic guide – Winter 2007
Phase 1 baseline interviews – Spring to Winter 2008
Analysis and writing up of baseline interviews – Spring 2009
Concurrent Phase 1 follow-up interviews – Spring to Winter 2009
Analysis and writing up of Phase 1 follow-up interviews – Spring 2010 and ongoing
Feedback to participants – ongoing according to their convenience
Reports and dissemination
The findings will be written up as a brief report for circulation to participants. A full report will be used as the basis for papers and articles to be submitted to professional journals and local and national voluntary organisations’ newsletters. An abstract will be submitted for presentation at a service users’ and professional conference, such as the Dementia Congress.
Phase 2
Methodology
This study adopts a longitudinal qualitative, exploratory approach and people with dementia and carers will be interviewed at three or four time points over the course of 2 years. Issues explored will include day-to-day decision-making issues, whether plans and decisions have changed, whether certain individuals or groups have been particularly helpful and to identify whether the MCA 2005 has been helpful or useful in the formalising of these plans and decisions. We are aware that individuals and their carers may choose not to continue for the full duration of the study, and there may be people who die through the course of the study. However, following people with dementia and their carers prospectively will give a more accurate picture of their experiences, as well as facilitate identification of when assistance might have been most beneficial.
Sample and recruitment of people with dementia and carers
This study mainly includes interviews with people with dementia and their family carers. In a small number of cases, professionals involved in care will be approached with permission of the person with dementia and carer. The research team is working closely with local specialist agencies, such as the Alzheimer’s Society and other carers groups in order to recruit people with dementia and carers who will be willing to speak to us. The moderator or the organiser at each of these groups will approach suitable participants and inform them of the research study. Only after both the person with dementia and the carer consent to being approached by the research team will contact details be passed to the research team. A researcher will arrange to meet both of them at a mutually convenient time and location.
In accordance with the principles of the MCA, people with dementia will not be thought to lack capacity and everyone referred for the study will be approached to participate. The procedure for establishing a person’s ability to decide whether or not they want to participate will proceed in accordance with the guidance for the Mental Capacity Act 2005; this will include the following four steps:
-
The Information Sheet will be explained to the person with dementia by the researcher, with any necessary assistance from the carer.
-
The ability to retain the information will be determined by asking the person with dementia to repeat to the researcher the salient points of the Information Sheet, namely what participation will involve, that it is entirely voluntary and that follow-up interviews may be conducted.
-
The researcher will ask the person with dementia whether they are happy with taking part, followed by querying the reason for this; this will again determine that they recognise that participation is voluntary.
-
Finally, all participants will be asked to sign the Consent Form as the means to communicate their decision. If they are unable to sign the form due to any other physical or cognitive problems, their relative or carer will be approached to sign for them, after obtaining verbal assent from the person with dementia. Any sign (verbally or in body language) that the individual is not happy about taking part or continuing to take part in the research will be interpreted as withholding consent, or a desire to withdraw. The researcher will end the conversation, thanking the person for their time and involvement. The researcher will check throughout the discussion that the participant is willing to continue and cease if the person indicates they wish the discussion to end.
Carers will also be asked to participate in the interview. They will be provided with an Information Sheet and the study explained to them by the researcher. Carers willing to participate will be interviewed either before or after the interview with their relative with dementia, depending on their preference. Consent will be obtained from carers prior to interviews and the same procedure for obtaining consent will be followed. However, we are not expecting carers to lack capacity. In total, we are hoping to interview 10 people with dementia and 10 carers and we estimate that moderators of groups that are involved in working with this client group may need to approach around 15 to 20 people for this.
We will also ask people with dementia and carers if it possible for us to speak to their wider social and professional network, such as friends, lawyers, bank managers, in order to obtain a more holistic picture of the role of external sources of support in the making of plans and decisions by people with dementia and carers. If both parties are willing for this contact to be initiated, we will ask for relevant contact details and they will be approached and invited to take part in the study. An Invitation Letter, an Information Sheet and a Consent Form will be sent to all such parties. Similar procedures for obtaining consent will be followed. Interview with this group will be conducted depending on the professional or social role they have played in the life of the person with dementia and carer, based on the anecdotal information supplied by them.
At each subsequent contact, consent to participate will be again obtained, following the same procedure. At subsequent contacts over the 2 years, the person with dementia may have lost the capacity to make a decision to consent i.e. understand information about the decision to be made, retain that information in their mind, use or weigh that information as part of the decision-making process, or communicate their decision (by talking, using sign language or any other means). In these instances, these will be excluded from the study and the researcher will conduct the interview with carer. Consent will be obtained from them prior to conducting the interview. Once again, we are not expecting carers to lack capacity.
Data collection
Making plans and decisions for the future is a sensitive, personal topic for people with dementia as well as carers and supporters. All interviews will be conducted by empathic researchers aware of the potential problematic nature of the issues being discussed and with experience of working with both groups of participants. Interviewers will use language understandable and acceptable to the interviewee. Should an individual become distressed during the interview, the interviewer will pause, offer sympathy and check whether the person wishes to discontinue the interview. All interviewers carry relevant contact information on key support agencies to leave with participants. All participants will be reassured of confidentiality and anonymity in the transcription, analysis and reporting of interviews. Any direct quotations used in the report will be non-attributable.
Participants will be made aware that if any information is shared that suggests that a vulnerable older adult is suffering neglect or abuse then the researcher has a responsibility to share that information with the service manager (in case of abuse or neglect from a service provider) or in the case of individuals, gain their consent to share that information with the named Local Authority Officer for safeguarding adults. In extreme cases, where a crime appears to be suspected, then the researcher will discuss with the Principal Investigator or call the police.
Data analysis
Data will be coded using the Framework Analysis method (Pope et al. , 2000;262 Ritchie and Spencer, 1993261) to draw out issues, concepts and themes. Data analysis will be an iterative process of identifying, organising, refining and re-organising the data into themes and sub-themes to describe the key elements of practice, including day to day support, decision-making and formal and informal sources of support and information with formalising plans. We will use NVivo™, a qualitative data analysis software package that can manage pooled data. The trustworthiness of the data and analysis will be framed in terms of its credibility to others with experience of the topic, transferability to other settings, dependability (depth of description of methods, peer analysis of data, third party evaluation of data gathering) and if possible, confirmability (by independent review of the data). The advisory group (consisting of professionals, carers and people with dementia) will also be consulted during this process in order to refine developing codes. Data will be anonymised before presenting or discussing with any member outside the research team.
Approximate timescale
This project is contracted to end in September 2012. The following are the timescales that have been generated for this phase (Phase 2) of the study:
Development of Phase 2 topic guide – Spring 2010.
Phase 2 baseline interviews – Spring to Winter 2010.
Concurrent Phase 2 follow-up interviews – Winter 2010 to Summer 2011.
Analysis and writing up of Phase 2 interviews – Winter 2011 and ongoing.
Feedback to participants – from Winter 2011 onwards.
Report writing – Spring 2012.
Completion of project: September 2012.
Reports and dissemination
The study will be disseminated in five main ways:
-
A brief report presenting the salient findings in an easy-to-read style will be generated and circulated to participants.
-
A full report to the study funders.
-
This report will serve as the basis for papers and articles which will be submitted to professional journals and local and national voluntary organisations’ newsletters.
-
Abstracts will be regularly submitted for presentation at service users’ and professionals conferences, such as the Dementia Congress, British Society of Gerontology Conference and Alzheimer’s Disease International Conference.
-
The groups and moderators who have helped us with recruitment will be offered presentation days when the researcher will go down and present the study findings either to volunteers and staff who work there or to clients at these centres.
Appendix 63 Chapter 5: Details of the four phases of study design
Phase 1 This phase explored the needs, wishes, experiences and challenges facing ‘‘well’ older people’ (with no reported suspicions of declining mental capacity or dementia) many of whom have little or no contact with health or social care services. The MCA potentially affects this group, as it does us all, by enabling a person to set out their choices, and for these to be respected even if decision-making capacity eventually deteriorates. By setting up a LPA, for example, people may nominate trusted family member(s) (or others) to make decisions on their behalf should they lose the capacity to do so themselves. Our research questions were:
-
RQ1: How do ‘well’ older people conceptualise and consider future care needs? How do they plan for their future within the remit of the MCA?
-
RQ2: What are the knowledge, views, experiences and expectations of the MCA among staff working with ‘well’ older people living in the community who may be interested in making plans for their futures?
We conducted semi-structured qualitative interviews with a diverse population of ‘well’ older people and with information and advice staff working in local Age Concern (now Age UK) offices to answer these research questions.
Phase 2 This phase investigated the needs and experiences of people who had recently been diagnosed with dementia (and their carers) in order to explore their views of short- and longer-term planning. Although decision-making capacity is likely to not be severely impaired in this group, the MCA offers options for planning in such circumstances that may seem more personally relevant. We also wanted to explore the experiences and attitudes of people newly diagnosed with dementia and the practitioners who support them about decision-making. We sought to understand the possible impact of the MCA on everyday decision-making for people living in the community (an under-researched area compared with decision-making at end of life or when major medical options are under consideration). Research questions for Phase 2 were:
-
RQ3: What are the knowledge, views, experiences and expectations of the MCA among staff supporting people in the early stages of dementia? Has this changed over time and how?
-
RQ4: What advice, information and support are available about decision-making after receiving a diagnosis?
-
RQ5: What framework, if any, does the MCA provide people with dementia and carers living in their own homes who make decisions on an everyday basis?
We conducted semi-structured qualitative interviews with four groups: (1) people with dementia and carers; (2) staff at Alzheimer’s Society branches; (3) staff at Carers’ Centres; and (4) SACs, often at the forefront of implementing the MCA.
Phase 3 This phase investigated the needs and experiences of those whose capacity may be impaired, and who as a result of this, need help with decision-making and possibly safeguarding against abuse. As the MCA incorporates provisions for this group, we wanted to explore the training, experiences, attitudes and expectations of practitioners who work with people with dementia and their carers. Research questions for Phase 3 were:
-
RQ6: What are the knowledge, views, experiences and expectations of the MCA among staff supporting people in the later stages of dementia, when capacity may be impaired? Has this changed over time and how?
-
RQ7: What advice, information and support are available to those who are caring for relatives with dementia whose capacity is impaired?
-
RQ8: What arrangements can be put in place so that people with dementia with and without capacity are safeguarded against abuse? Does the MCA help?
Semi-structured qualitative interviews were carried out with four groups: (1) social workers, (2) Admiral Nurses (specialist dementia nurses), (3) people with dementia and carers living in the community, and (4) SACs. An online survey of Alzheimer’s Society staff was also conducted through the Society.
We further undertook specific consultations about an issue that emerged across many of the interviews from this and earlier phases; practitioners’ knowledge of and practice experience with the two new offences of wilful neglect and ill-treatment created by the MCA. Our first consultation event included practitioners from NHS, social care and criminal justice backgrounds on 10 March 2011. Our second sought the views of older people about what type of information they would find helpful, without being alarmist. We met with this group on 15 November 2011 and recorded this discussion. Following these consultations and analysis of the interview data we produced a preliminary paper for the Journal of Dementia Care (Manthorpe and Samsi, 2012)448 and will be writing another paper for an academic journal. We will also be liaising with the SCIE to encourage them to host a version of these on their website.
Phase 4 This final phase of the research study explored the potential relevance of the MCA for people with severe dementia who may be at the end of life. Capacity at this stage is likely to be consistently impaired, proxy decision-making is likely to be needed on a regular basis, and advance care plans may be in place. We sought to elicit the attitudes of staff working in care homes about the practice of decision-making on behalf of or by their residents, and the possible impacts of the MCA on their work more generally. The research questions for this phase were:
-
RQ9: What are the knowledge, views, experiences and expectations of the MCA among staff who work with people with dementia at the end of their lives and their carers regarding advance care plans or other similar plans?
-
RQ10: How does MCA proxy decision-making work in practice?
Semi-structured confidential interviews were conducted with staff in a variety of care homes (small single owner to part of a large chain of care homes) at two time points to ascertain their training, experiences and attitudes towards the MCA.
Nested within Phase 4 was a substudy of an NHS acute hospital trust where several untoward incidents involving patients lacking capacity had indicated that use and knowledge of the MCA were partial among the hospital staff. This substudy engaged with the author of an audit commissioned by the hospital trust to produce a report with lessons for the wider NHS and health-care providers that was made available to the wider public. 274
Appendix 64 Chapter 5: Semi-structured interview schedules
-
Interviews for Practitioners’ Audit Time 1.
-
Interviews for Practitioners’ Audit Time 2.
-
Interviews with ‘well’ older people.
-
Interviews with people with dementia.
-
Interviews with carers.
EVIDEM – Mental Capacity Act 2005
Interviews for Practitioners’ Audit Time 1
Revised according to practitioner group being interviewed
Introductions
-
Explain purpose of this audit, no right or wrong answers but interest in hearing about practitioners’ experiences. Explain part of EVIDEM (info provided).
-
Assurances of confidentiality (draw attention to Consent Form).
-
Seek permission to tape.
Brief details of interviewee
-
Job title.
-
Role (key activities).
-
Qualifications (including registrations/NVQ).
-
Number of years since qualified.
-
Place of work (employer/department/team).
-
Time in present post.
Section 1: training
-
Have you heard of the MCA 2005?
-
If yes, please tell me what you have heard/if you have been involved in implementation.
-
Have you had any training on the Act? If yes, please outline when, where and who supplied it, content.
-
Was this helpful in terms of information? If yes, what in particular? – If no, why not?
-
Has the training proved useful in practice? If yes, please give some examples of how, if no, why do you think this is? e.g. prompts, not my line of work, no one has heard of it . . .
-
Have you explained the MCA to anyone (colleagues or patients/carers)? If yes please outline, with some examples.
-
Have you delivered training yourself? If yes please outline when, where, to whom, how did you do this?
-
Specifically, how confident are you in your own knowledge of the Act, say, if 0 is not at all confident and 5 is very confident, where would you place yourself?
-
And where do you think you should be (0–5)?
Section 2: roles and activity
-
Is the MCA affecting your work? Please outline if yes, and explain which areas or no and why?
-
Where would you seek advice from about mental capacity/making decisions matters if you had any problems?
-
Is there any new paperwork/guidance in place? If yes, where and are you using it?
-
In your opinion, do you think that people with suspected or early dementia will use the MCA to make plans for their future?
-
Is discussion about this something that you might get involved in, in your work?
-
If yes, please outline the possible areas of MCA that are raised in your work e.g. advance decisions about treatment, lasting powers of attorney, assessment of capacity.
-
If no, where do you advise people with early/suspected dementia and their families to go if they have queries or want advice? Would this be within your team/organisation/or elsewhere?
-
-
What do you think might be the advantages of the MCA? (prompt to whom, people with dementia, carers, professionals)
-
And what about any disadvantages (and to whom, why)?
Section 3: looking ahead
-
Have you any predictions about the MCA? (e.g. lots of interest, no real help)
-
Do you think it will be useful in adult protection/safeguarding people with dementia? If yes, please explain. If no, why not?
-
Just briefly, and this is not an exam, but we are trying to find out how familiar these new terms are – have you heard of the following:
-
Independent Mental Capacity Advocates (IMCAs) – if yes, what do they do?
-
Lasting Powers of Attorney – if yes, what can the LPAs do?
-
Deprivation of Liberty or Bournewood safeguards?
-
The new offences of mistreatment and wilful neglect under the MCA?
-
And how would you define ‘capacity’?
-
Section 4: personal perspectives
-
Would you say you have had experiences of looking after someone with dementia in your own family or network, e.g. partner/friend? Could you briefly outline if you think this has affected any of the matters we have been talking about?
-
Finally, have you been thinking of making any plans yourself or for a person you know (e.g. discussion with parents or partner) about this area? If yes, please outline.
End of interview
Demographic details
-
Male/female
-
Age
-
Are you a carer? Or recent experience?
-
Ethnicity (Census categories):
Ethnic group
Choose ONE from A to E, then indicate cultural background.
-
A. White Options of British; Irish or any other White background (please describe).
-
B. Mixed Options of White and Black Caribbean; White and Black African; White and Asian or any other Mixed background (please describe).
-
C. Asian or Asian British Options of Indian; Pakistani; Bangladeshi; any other Asian background (please describe).
-
D. Black or Black British Options of Caribbean; African; any other Black background (please describe).
-
E. Chinese or other ethnic group Options of Chinese; any other (please describe).
-
Conclusion
Thanks and assurances of anonymity
Ask permission to get back in touch over the course of the project to ask for updates/Best method of making contact again? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . [phone number]
Offer to leave information sheet about MCA training materials and contacts
Interviews for Practitioners’ Audit Time 2
Revised according to practitioner group being interviewed
Thank participant for allowing us to talk to them last year about the MCA. We interviewed over a hundred practitioners and have found it really useful to learn what their initial experiences and expectations were. We’d like to ask you a few (five) follow-up questions – is that convenient?
-
When we talked last time, you said that X was happening – is this still the case? (e.g. revised policy/procedures)
-
What are your main experiences of the MCA – now it’s bedded in?
-
Last time you had experience of/did not have experience of . . .
-
Has anything changed? If yes, what?
-
-
Last time you predicted . . .
-
Were you right?
-
What’s happened since?
-
-
In what ways has the Act changed your practice or that of staff team colleagues – if at all? Is there anything you would like to add?
-
Any developments/experiences around the new offences under the Act – wilful neglect or ill-treatment?
-
Any other observations around the links between adult safeguarding and the MCA?
-
Have you made any plans yourself or for your family?
Many thanks for your help – we would be happy to send you a copy of our findings. Would you like this?
Interviews with ‘well’ older people
-
Please can you tell me if you have made any plans for managing your money and so on for now and in the future and what these are?
-
What about a will/any advanced decisions, a LPA.
-
Have you told people any wishes you might have?
-
Why is it that you have this (all aspects) (Why not?)
-
-
What about any plans for your future care if you needed to be looked after?
-
What about where you want to live?
-
Why is it that you have this (all aspects) (Why not?)
-
-
What about any medical plans or decisions?
-
Do you think you might think about making plans in the future (what circumstances/events?) For example:
-
if you got a diagnosis of something serious
-
if you felt you were having memory problems
-
if your family/friends change/move
-
if suggested by family and friends and so on
-
(Why/why not?)
-
-
Have any professionals talked to you about making plans? Explore if HSC or if lawyer/accountant, etc.
-
We would just like to ask if you have heard of any of the following and what they mean to you:
-
The Mental Capacity Act?
-
LPA or Enduring Power of Attorney (EPOA)?
-
Advance decisions? (sometimes termed Living Wills)
-
-
If you have made/will make any plans, do you think that professionals (e.g. doctors, nurses, social workers, care workers) will take any notice of them?
-
Why do you think this? – Do you have any experiences?
-
-
Have you made any decisions on behalf of other people (adults), like relatives when they are poorly, confused?
-
What happened here?
-
Who encouraged you?
-
(Why/why not?)
-
How has this affected how you feel about making plans for your own future?
-
-
Have you ever discussed any of these issues with your friends/family?
-
If you decided to make plans or had a friend who wanted to make plans, whom do you think you would you seek advice from?
-
Is there anything we have not covered that you would like to add?
Demographic details
-
Male/female
-
Age
-
Are you a carer? Or recent experience?
-
Ethnicity (Census categories):
Ethnic group
Choose ONE from A to E, then indicate cultural background.
-
A. White Options of British; Irish or any other White background (please describe).
-
B. Mixed Options of White and Black Caribbean; White and Black African; White and Asian or any other Mixed background (please describe).
-
C. Asian or Asian British Options of Indian; Pakistani; Bangladeshi; any other Asian background (please describe).
-
D. Black or Black British Options of Caribbean; African; any other Black background (please describe).
-
E. Chinese or other ethnic group Options of Chinese; any other (please describe).
-
End of interview
-
Permission to get back in touch over the course of the project to ask for updates.
-
Best method of making contact again? . . . . . . . . . . . . . . . . . . . . . . . [phone number]
Interviews with people with dementia
Introduction and assurance of confidentiality: explain pilot process, informed consent, seek permission to record.
1. Could you tell me what an ordinary day for you is like?
-
Does anyone support or help you in any way? (Who is the main person?)
-
Establish relationship, co-residency, frequency of contact.
-
-
What, if anything, does X (main person) help you with?
-
On an average day, do you have to make a lot of decisions?
-
Explore what these are.
-
Prompts:
-
what time to get up
-
what to wear
-
paying bills
-
going shopping
-
cleaning the house.
-
-
2. Do you make all of your own decisions?
-
Does anyone help you with some of them?
-
For example, banking, bills.
-
Has this always been the case?
-
-
Do you choose who makes decisions for you?
-
Are you happy with decisions you have made recently?
-
Can you tell me about any decisions that you have been happy with?
-
Have you give away responsibility to someone else to make a decision for you that you were unhappy with?
-
-
Can you tell me any specific instances when you might need the help of someone to make a decision for or with you?
-
What about things like managing finances?
-
Paying bills, investments, savings.
-
-
What about things like your health, such as taking medicines, going to the doctor, etc.?
-
Having dental treatment, getting new glasses, hearing aids, etc.
-
-
What about just everyday decisions, like shopping for clothes or deciding what groceries to buy?
-
Lifestyle issues:
-
Driving and car-related items, such as car repairs.
-
Repairs and major household items/decorating.
-
Valued activities and hobbies.
-
-
Looking after yourself (dressing, hairdressing, toileting, washing and so on)
-
Choice about type of care.
-
What about having paid people to help around the house or to help with other things?
-
3. Did you have any plans about what to do in retirement?
-
Did you have any wishes for retired life?
-
Like staying in this house/flat.
-
-
What were these?
-
Did you talk to anyone about this?
-
Have you had to change any plans recently?
4. Did you have any formal or written plans for this time or period of your life?
-
Such as a will.
-
Joint bank accounts/utilities.
-
Insurance policies.
-
An enduring power of attorney or LPA.
-
Statements of wishes.
-
Advance decisions about care and treatment (sometimes called Living Wills).
-
Any other written wishes or plans?
-
When did you set these out and why?
-
5. Has anyone been involved in helping you make any decisions or plans?
-
Family/relatives?
-
Professionals?
-
Medics/nurses/etc.
-
Bank managers/solicitors/accountants/etc.
-
Alzheimer’s Society/Age Concern (now Age UK)/Carers’ centres, etc.
-
-
Friends?
-
How did you feel about this?
-
Do you wish they had?
6. We would like to learn more about how people make everyday decisions. How do you think we might be able to do this best?
-
Prompts:
-
Could you see yourself writing a diary?
-
Telephone calls at regular intervals?
-
Visiting for a talk?
-
Other?
-
How often could we contact you?
-
How can we find the times when people are ‘wondering what to do’, ‘thinking’, ‘pondering’?
-
Using vignettes (think aloud around imaginary case studies).
-
7. Do you think there is anything we have not covered?
8. Would it be possible for us to speak to the main people involved in helping you? Such as your GP/social services person/Community Psychiatric Nurse (CPN)/Alzheimer’s Society? Is there anyone you would particularly recommend?
Thank you for your time today. Offer reassurances of confidentiality and anonymity.
Interviews with carers
Introduction and assurance of confidentiality: explain pilot process, informed consent, seek permission to record.
1. Could you tell me about your role as a person supporting/looking after x.
-
How did it begin?
-
How long have you been involved in caregiving?
-
What do you do for X?
-
Has it changed from when you first started helping him/her?
-
-
(Flesh out relationships and carer role/responsibility).
2. Could you tell me about any decisions you make or think about together with or on behalf of X?
-
Could you tell me who was involved, why they were involved, in what capacity they were involved and how they contributed.
-
Expand around finance (managing money, accounts, bills, etc.).
-
Law.
-
Health (medical, physical, medication, dentist and so on).
-
Consumer decisions, like shopping and deciding what groceries to buy.
-
What about issues of everyday life, such as driving.
-
What about decisions to do with care, such as toileting, washing and so on.
-
What about whether or not they need help?
-
-
Family contact and relationships with others (family, services, etc.)
-
What works well and not so well?
-
What is comfortable for you?
-
What is troubling or tricky?
-
-
Do you tend to give X a chance to make the decision first, even if it’s not something you agree on?
-
Why/why not?
-
3. Could you describe a ‘tricky’ day or ‘tricky event’?
-
How do you deal with making decisions for X?
4. What guides how you make decisions for X?
-
Previous discussions?
-
Can you tell me about the kinds of discussions you have.
-
Context and content of discussions.
-
5. Does what you do come from any plans made by X?
-
Enduring power of attorney.
-
LPA.
-
Statements of wishes.
-
Advance decisions about care and treatment (sometimes called Living Will).
-
Your knowledge of X as a person.
6. Is anyone else involved (professionals, relatives, etc) in helping you making such decisions?
-
Family/relatives?
-
Professionals?
-
Friends?
7. We would like to learn more about how people make decisions and are thinking about decisions on an every day basis, as well as major ones, over a period of time. How do you think we might be able to do this best?
-
Prompts:
-
Diary format?
-
Telephone calls at regular intervals?
-
Visiting for a talk or interview?
-
Other?
-
What intervals would be best to balance the detail of everyday life and yet not being a bother?
-
How can we capture times when people are ‘wondering what to do’, ‘thinking’, ‘pondering’?
-
Using vignettes (think aloud around imaginary case studies).
-
8. Do you think there is anything we have not covered?
Thank you for your time today. Offer reassurances of confidentiality and anonymity.
Appendix 65 Chapter 5: Five main stages in framework analysis
Familiarisation
This early stage is for the researchers to get familiarised with the data and sensitised to early themes. It encourages the research to see the individual differences inherent in transcripts that can sometimes get lost when coding begins. The process of sensitisation to these individual differences also enables the researcher to better identify within- and between-participant differences. In a few cases, the researcher felt the need to revert to the original recorded interview to get a better feel for the data. Any emergent early impressions were noted, relating to reactions to transcript/participant, how they felt as they listened to the participant, and any specifics that they wanted to remember for later. These were jotted on one side of the paper transcript.
Identifying a thematic framework
This stage of framework analysis is commonly referred to as ‘coding’ in other qualitative methodologies. This principally involves identifying key themes, issues or discussion points embedded in the transcript. These are delineated and assigned a ‘code’ or a name that best captures the essence of the theme or issue identified. In framework analysis, ‘a priori issues’ questions can form the basis of the themes – we therefore used the interview topic guide as a starting point for creating overarching categories and any emergent themes from transcripts were coded as responses to each question. As Pope and colleagues describe,262 the outcome of this stage is a ‘detailed index’ of the data into ‘manageable chunks for subsequent retrieval and exploration’, which is what we achieved.
Example
-
We were keen to ascertain the training on the MCA practitioners had been offered. We therefore labelled ‘Training’ as one of our a priori categories and coded (1) ‘no training’, (2) ‘1-day training course’, (3) ‘1+ day training programme’, and (4) ‘MCA training as part of other activities’. We were able to identify and make inferences regarding the amount of training one participant group had had compared with another.
Indexing
Indexing refers to the process of numerically annotating transcripts in order to identify consistencies, which then go on to develop the coding framework. However, we followed this process slightly differently. All of the word codes (as opposed to numerical) that had been generated during stage 2 were listed on a separate sheet of paper. We then grouped together all codes that shared commonalities or consistencies. These clusters were given an appropriate name.
Example
-
One of our interview questions referred to ‘Practice experiences’ – we named this as an overarching category, incorporating (1) ‘intrinsic impact’, (2) ‘community role of X’, (3) ‘role of safeguarding’, and (4) ’change and extension to job role’ as separate codes under it. 269
-
This also helped us to identify and be mindful of individual participant differences. For example, while community nurses saw safeguarding as less of a priority in their jobs during time 1 interviews258 SACs talked about the significant impact of the MCA on their practice experiences. 264
Charting
Framework analysis describes this stage as a process of rearranging the data and thematic framework to create order, not dissimilar to the iterative principle of grounded theory. 449 We applied this stage as a principle for synthesising and developing our final coding framework through a process of abstraction, in order to derive all the detail from the data and ensure that we were coding elements that may have been missed with simply an a priori approach.
Example
-
The question asking all participants about their personal experiences of caregiving (family caring) was asked as a final question to round off the interview. Family caring experiences were commonly reported and participants offered examples of how these had contributed to their professional understanding of dementia and caring. In relation to this, five main codes were developed: (1) ‘informing the professional role’, (2) ‘insight into services’, (3) ‘professional influences on caring’, (4) ‘planning’, and (5) ‘no apparent effect of MCA’.
Mapping and interpretation
Mapping and interpreting essentially are ways of representing pictorially or graphically all of the themes and investigating how each of the themes relates to each other. This detailed exploration of the iteratively developed and revised thematic framework enabled us to gain a clearer understanding and explanation of the ‘bigger picture’. For instance, we examined the association between three categories: (1) ‘training’, (2) ‘(self-reported) knowledge of the MCA’, and (3) ‘self-reported confidence’; and we were able to identify and offer explanations for individual discrepancies between these three categories.
Example
-
In Manthorpe et al. 268 we describe how some participants, with no training and limited self-reported knowledge, reported high levels of confidence in abiding by the Act. We questioned the reliability of some self-reported knowledge about the MCA, and have considered the adequacy of training now the Act is no longer ‘new’ and the potential for mandatory training for all staff.
Appendix 66 Chapter 5: Narrative account of findings
Universal appeal of the Mental Capacity Act
| 3.2.1.1. Beneficial | Practitioners across the range of professional groups felt that the MCA was beneficial as it clarified and consolidated a previously confusing legal system that had baffled older people, and sometimes practitioners. Most thought it enhanced the autonomy of people with impaired cognition, by assuming that people are able to make decisions unless proved otherwise; encouraging professionals to better consider ability to make decisions, and ‘giving a voice to older people at risk’ (Manager 02) |
| 3.2.1.2. Dignity and rights protected | While recognising that individual decision-making was supported (even unwise or ‘eccentric’ decisions), many participants believed that the Act provided better safeguards for people who might be particularly vulnerable to neglect and abuse. Overall, the Act was seen as supporting the rights and dignity and decisions of people with dementia |
| 3.2.1.3. Details not widespread but growing | Alongside the universal appeal of the MCA, however, it was evident that awareness of details and experience of using the Act were still not widespread, but these were steadily growing.294 Those who had direct contact with people with dementia and carers said that the Act made them more aware of questions related to capacity, and one intrinsic impact was the more common practice of now routinely questioning whether a capacity assessment had been done. Understanding of the MCA was seen as increasing overall, and there appeared to be steady advances of understanding, confidence and experience when time 1 and time 2 interviews were compared |
Training
| 3.2.2.1. Frequency and format of training offered | Training appeared to have been available to nearly all practitioners who participated in our study. However, not all had undertaken it for various reasons. There was also variability in the level and type of training each participant had received. Although some participants had undertaken in-house training, others had attended more detailed training provided by external consultants, geared towards professional HSC workers. Some training had lasted for less than an hour, or half a day, whereas others attended day-long sessions. Formats varied too, ranging from conferences, ‘awareness-raising’ sessions, specific to the MCA (e.g. through an employer) or as general updating (part of Continuing Professional Development) Most who had received training deemed it useful, providing a good overview, being informative and helpful in outlining a job-defined formalised code of working and ‘crystallising what [I] do’ (AN13). However, in terms of day-to-day work, some perceived minimal change and reported little benefit from training. Other accounts varied according to participants’ experiences. Although some felt that senior staff were responsible for cascading the information to more junior levels, this did not seem to happen systematically, but occurred instead during staff meetings or by putting items on a notice board to convey the ‘basics’. A number of junior care home workers tended to rely on paperwork and care plans to come from ‘head office’ and demonstrated little knowledge of the MCA. Few professionals had received training that incorporated safeguarding practice and the MCA |
| 3.2.2.2. Challenge of translating training into practice | Training sometimes appeared to be over-didactic, with limited use of case studies and examples. High-level information was imparted at these events, which often resulted in most participants knowing about the general principles of the MCA but not its specifics. Many appeared to use guesswork when asked about specific points, suggesting that they had gleaned overviews of the MCA rather than acquired detailed understanding Ongoing training was considered important, as these provided opportunities to share emerging challenges. A minority of practitioners across all professional groups said they had missed the training offered or had not been able to attend owing to work commitments. Two participants talked of having recently started at a particular organisation and, therefore, had missed training at their old place of work as well as at their new one Given the high turnover of staff and frequent changes to staff and organisational structures, the need for training to be a continuous process was identified. Renewing and refreshing training at local levels could also incorporate new information and developments. Following the Act’s initial implementation, there have been many changes to procedures and structures, as well as results of early evaluations and research findings, all of which could be incorporated into regular training. Use of the Mental Health Foundation’s Assessment of Mental Capacity Audit Tool (AMCAT) (easily accessible and free: URL: www.amcat.org.uk/audit_your_assessment/) material could be enhanced (Mental Health Foundation 2010)450 |
Implementation
| 3.2.3.1. Quick and easy incorporation into safeguarding practices and procedures | Practitioners working in adult safeguarding were especially positive about the Act and considered that it had enhanced their everyday practice. They felt that much of their work was already guided by the principles of the MCA, but considered that the MCA had reinforced these principles. Many of these participants particularly appreciated that the MCA provided a framework for mental capacity assessments. They welcomed the focus on specific rather than global capacity (is the person able to make a specific decision and does it need to be made now?) and the provisions for planning, either by appointment of a LPA or by greater encouragement for people to make their wishes known about future care and treatment.451 Over the different time points, these participants were enthusiastic in extolling the Act’s potential to support defensible decision-making for professionals and carers, protecting people’s rights from abuse, and achieving good outcomes for those who lacked capacity However, all practitioners considered that it would be some time before the MCA was completely embedded in practice. Given that HSC policy and commissioning are increasingly driven by the policy of personalisation, greater clarity will be needed around challenges arising from concerns about safeguarding risks (e.g. of exploitation, abuse and neglect). We were able to witness this major change to adult social care services in the course of the study and have produced some of the first findings about the MCA and its link to personalisation and safeguarding267 |
| 3.2.3.2. Confidence and expertise | We explored practitioners’ level of professional confidence in implementing the MCA. In order to encourage discussion and to avoid the uncertainties of language (e.g. differences between ‘much’, ‘a little’, ‘somewhat’), we used a five-point scale as a means to get practitioners to state how confident they felt, where ‘1’ was ‘not confident’ and ‘5’ was ‘very confidentWe noted some lack of association between confidence and knowledge. Participants who said they were very knowledgeable about the MCA did not always feel confident about implementing it. Perhaps more worryingly, those who indicated that they did not feel knowledgeable about the MCA sometimes indicated higher than expected levels of confidence. A majority of participants, however, talked of feeling confident about other sources if they needed more information Confidence seems likely to accumulate with familiarity and experience in using the Act. Confidence was also related to whether practitioners felt able to access advice and expertise locally There was limited confidence about conducting assessments of capacity, with some practitioners stating their belief that only medical professionals were able to ‘verify’ whether or not a client with dementia had capacity. Although most practitioners were aware in principle that capacity had to be understood and assessed by the person closest to the specific decision, many suggested that, for a formal report of capacity, a medical practitioner was needed. Others held the erroneous view that next-of-kin status granted family members the rights to make decisions for their relatives |
| 3.2.3.3. Risk of information ‘merry-go-round’ | An information ‘merry-go-round’ was evident, by which practitioners appeared to be signposting queries on to other practitioners or agencies who then passed these queries back to them in a potential loop of confusion. There was not always a central point of expertise locally that could deal with queries, signpost appropriately and answer questions that practitioners encountered. Where this was available, it was highly valued An additional challenge was a lack of clarity about the specific content of information and advice and consequently the roles of advice and information agencies. This also led in part to the information ‘merry go-round’ when staff in agencies that provided information were approached for advice that they did not feel equipped to supply. For example, making a LPA involves completing a lengthy form with which some professionals said they did not feel able to assist269 Although some third-sector groups were keen to describe themselves as safeguarding agents and champions of older people, they often perceived their roles as gatekeepers to more detailed sources of information and routes to intervention, rather than providing these themselves. For instance, groups that traditionally provided information and advice, such as Age Concern (now Age UK), felt rightly not able to respond to specialist legal queries falling outside their competence258 Signposting to other services was becoming an expanding task. Although many information-providing agencies were responding to basic enquiries from older people, as well as those closely involved with decision-making, and encouraging drawing up of LPAs, they also suggested other sources of help. When responding to enquiries related to a dementia, many of those providing basic information and advice turned to health practitioners or solicitors as specialists, sometimes to supplement their own in-house national resources, such as telephone helplines. This suggests that sources of information and advice were not clearly mapped at local levels. There is the potential for people to be passed on from agency to agency. Commissioners may wish to satisfy themselves that the local map of information and advice has linear pathways and is not circular |
| 3.2.3.4. MCA expertise at all levels unlikely, but central expertise required | A striking conclusion from interviews held with staff at all levels in care homes was the need for a single authoritative lead on the MCA. Most junior care workers (generally termed ‘care assistants’) and some senior care workers had rudimentary, if any, understanding of the Act and indicated that they would rely on care home managers or senior staff to identify or answer any complex questions.268 Although some care home managers were well informed, others indicated that they too had fairly rudimentary understanding of the Act themselves and would turn to other specialist sources if the need arose. However, there was little consistency in nominations made. They included the home’s regional manager or other headquarter’s staff (in homes that were part of large chains), local social workers, community mental health nurses, the local mental health team, an IMCA helpline, Age Concern (now Age UK) (local voluntary sector) advocates, a GP and solicitors. Each manager’s choices were different and a minority were uncertain of any source of authority, advice or expertise. For example, some managers emphasised the role of the family in making decisions, whereas others emphasised the role of social workers or mental health professionals There appears to be scope for the principles of the Act to be more explicit in everyday practice and record keeping in care homes. Although in most homes participating in this study, there was anecdotal evidence of this (i.e. making best-interests decisions based on a resident’s past preferences), there was little formal record of these procedures which could stand up to legal challenge or regulatory scrutiny. Moreover, some care home staff themselves confessed to a lack of confidence in dealing with more complex cases when quick decisions may need to be made, e.g. about whether a resident lacking capacity could lawfully be moved from a care home to another setting for treatment or assessment or who could consent to treatment. By time 2 some homes had clarified these matters, often through the making of advance care plans or similar decisions being recorded on a person’s move to the care home. Nonetheless, at time 2 there were still care home staff who were unfamiliar with the MCA, largely because they were new in post and new to the work. Some were not familiar with English law and their level of English was basic |
Everyday decision-making
| 3.2.4.1. Everyday negotiation | Analysis revealed a significant theme around the number of everyday decisions people with dementia and carers who lived together made jointly, including what to do, what to wear, what to eat and what holidays to plan. Both people with dementia and carers talked of the potential of making decisions as important to individual autonomy. Most, however, acknowledged that, since the diagnosis of dementia, things had changed and almost half the participants with dementia said they now found it difficult to make decisions Many carers reported trying to actively involve their relative with dementia in decision-making; some provided them with cues or reduced possible stress, for example, by offering fewer options. Some carers revealed that, over time, shared decision-making had given way to them making decisions unilaterally. While some tried to involve their relative with dementia in general or domestic decisions, four carers described deliberately not doing so in order to protect or ‘save’ them from the stress of minor decision-making, in order to better participate in more important decisions. Spousal carers were more likely to make joint decisions through the interchange of everyday conversation Such changes can be seen as part of a life course of changes to family decision-making and so need to be contextualised within biographies and gendered relationships. For example, retirement appeared to have been the point at which many participants’ lifestyles and routines had changed and when joint domestic decision-making often seemed to have started. One man with dementia talked of how, when he was working, he left decision-making about meals to his wife, but since retirement, they had made such decisions together. Similarly, deciding how to spend the day was a joint enterprise for some, another change since retirement |
| 3.2.4.2. Past preferences and best-interest decisions key to making proxy decisions | Many carers were making decisions on behalf of their relative with dementia without reference to the MCA but in line with its values of acting in a person’s best interests and in the least restrictive way. Although most recognised the reasons for having to make such decisions, there were some expressions of frustration when carers felt responsible, strained and confused. Mostly they used their knowledge of their relative’s past habits, preferences, likes and dislikes to help them make decisions. In some cases, however, they confessed to resorting to doing what suited them best. For instance, one carer talked of his wife with dementia never liked having sugar in her tea, although lately this had changed. However, when he ran out of sugar, he reasoned that his wife would not mind as it was in keeping with her past preferences |
| 3.2.4.3. Weighing best-interest decisions | The principle of making decisions in the PWD’s best interests appeared to underlie many carers’ interactions with their relatives with dementia, albeit in different ways. There appeared to be a divide between two groups of carers. One group described their relative’s well-being or best interests being at the heart of decisions that they made on their behalf. A second group described making decisions in terms of what might be beneficial to them both. In many cases, this constituted what was least stressful to carers. Carers in this group felt that maintaining their health was important in being able to support their relative with dementia and, therefore, they adopted the most pragmatic solution they judged necessary for the long term |
| 3.2.4.4. Inequalities in access to information | We asked participants whom they had approached, if anyone, for information and advice when facing major or difficult decisions. Their responses indicated that such decisions rarely occurred in isolation. A range of support was accessed, from friends and family to solicitors and members of support groups. Many of the participants had family members upon whom they felt they could rely, relatives who were sufficiently knowledgeable and whom they felt had their best interests at heart. In most cases, this ‘family’ was adult children; in one case, it was a sister and her family. Adult children were more likely to use the internet and seek other sources of information, and many of our participants felt happy to leave any complex decisions to their children whom they felt were able to access advice and expertise. Although there were no apparent gender differences, adult daughters were more often involved in supporting day-to-day practicalities, whereas adult sons were involved in more long-term care planning. However, from such a small number of participants, it would be premature to make any generalisations. What emerges is a lack of evidence about effective support for older people who have no social support networks that they trust or who are not able to access local support services. This is an area in which research may have much to offer practitioners about how to meet specific needs and how to sustain resourcefulness |
Long-term planning
| 3.2.5.1. Planning influenced by personal factors | A significant theme that emerged was that participants had individual and personal approaches to planning.272 A range of predisposing factors was identified, including disposition, belief systems and spirituality, participants’ living situations, the current (2010–11) uncertain state of the economy, and their confidence and trust in medical practitioners. Some participants declared their personal tendencies and preferences were to avoid planning for the future. They described beliefs such as ‘living for today’, enjoy life to the maximum, not thinking much about the future, and not letting worries and problems affect their life. Making detailed plans for the future had, therefore, not featured in their lives prior to dementia or old age Some participants discussed this disinclination in the context of spiritual beliefs. Illustrating this, apart from funeral arrangements and despite being concerned about finances, one older person had not drawn up a will nor any made health or social care plans. She did not feel that it was appropriate for her to interfere in ‘God’s work’ A significant subtheme was identified from discussions with people living alone and with no relatives. This group spoke about being unable to appoint anyone to hold a LPA or to ensure their wishes and preferences were carried out because they had no direct or even distant next of kin. They highlighted that their friends, being elderly themselves, were compromised in their ability to act as effective attorneys. This particular group indicated the strongest intentions to plan but it was difficult for some to find members of their social networks who could take on the responsibility A small group of participants said that they did not have any finances or valuable personal effects to leave their children and hence viewed planning as irrelevant to their situation. They conceptualised plans in terms of financial transactions or for functions of inheritance and appeared to find it difficult to think about planning for health and personal welfare decisions as matters that might affect them |
| 3.2.5.2. Financial and funeral plans commons, less so HSC plans | Of the arrangements that had been made, the most common were financial. Making a will was frequently mentioned, followed by granting EPOA (the earlier Power that applied to financial decisions alone and not health and welfare decisions), setting up joint bank accounts, taking out health insurance, and consolidating investments and bank accounts. A tendency to plan for financial security did not necessarily mean that individuals also planned for other aspects of their future. For example, one participant talked of her household insurance, health insurance, income protection plans and financial arrangements. Despite having made difficult decisions for her late mother through final stages of cancer, this participant had not considered making plans of this nature for herself although she expressed interest in these when the subject was raised during the interview Funeral arrangements were also discussed, ranging from having a separate bank account for funeral costs, to more elaborately planned procedures, including hymns participants would like to have sung at the ceremony Housing and residential care appeared to be significant discussion points (rather than explicit planning measures) for participants. Most had spoken to partners, family members and close friends about these subjects. Some described how much their home meant to them emotionally and that they hoped to stay in it until the end; others spoke of their willingness to move into residential care in order to relieve their children of caring responsibilities. It appeared, however, that few were aware of the complexities of the social care system in England and did not understand how they could state their preferences or how the MCA could support these There was an absence of HSC plans in the formats permitted under the MCA (Advance Decisions and LPAs) and most participants admitted not having thought about these. They seemed interested in the provisions of the MCA when they were introduced during the interviews and asked for information afterwards. This was in contrast with the many financial arrangements that they had already put in place around joint accounts, direct debit for bills, Powers of Attorney, and wills |
| 3.2.5.3. Limited support with knowing where to turn | Most participants did not know where to turn if they were interested in taking forward plans for the future. The interviews conceptualised possible serious debilitating conditions as a way to start these exploratory discussions about planning. Participants were asked about their familiarity with and understanding of key terms and phrases, such as the MCA, LPAs and Living Wills (the legal term being Advance Decisions but this term was not widely understood). None of the participants had heard of the MCA specifically, despite some understanding that principles of the Act were respected in health and care settings. Some appeared to have an understanding of LPAs, knowing that this involved appointment of a trusted person to manage one’s affairs when one is unable to do so. However, few appeared aware of the change in terms since the introduction of the Act and assumed that the EPOA meant the same as the LPA. Living Wills/Advance Decisions were rarely understood, and, in some cases, associated with what participants called ‘clinics in Switzerland’ and euthanasia. Many participants assumed that Living Wills could not be honoured by medical professionals because assisted suicide is illegal in the UK Participants on the whole appeared unsure where to turn for support with planning. Financial advice appeared easier to come by and participants knew where this could be accessed. Having reliable financial advisers to give sound advice was described positively and participants appeared reassured by them The role of the GP appeared to be crucial. Some participants were unsure if they could rely on their GP, reporting not always seeing the same GP and not feeling particularly supported by the primary health-care team. Others felt they could rely on their GP for advice about making plans and that these would be honoured. These were confident in the GP’s information and advice regarding Living Wills/Advance Decisions. When asked whether any local information and advice agencies would be helpful, white British participants said that they were likely to visit local branches of Age Concern (now Age UK), whereas black Caribbean participants appeared reluctant to share personal information with these voluntary agencies. The latter group felt that if they wanted to make plans they were more likely to consult family members and friends who would direct them to appropriate agencies and people rather than talking to strangers. These findings are indicative but may suggest that professionals should explore people’s confidence in voluntary sector groups as sources of advice when advising people to contact them One reason for making plans was the onset of a debilitating illness. The current high profile of dementia in the media may explain why this condition was generally known about by almost all participants. Dementia was discussed as one of these conditions, when participants speculated that there might be little chance of recovery and the prospect of losing capacity to make decisions was heightened |
Safeguarding
| 3.2.6.1. Inherent safeguarding value | Most participants, including practitioners and some people with dementia and carers, felt that the principles of the MCA provided safeguards to protect the autonomy and well-being of the person with dementia, through respecting capacity, enabling the setting up of LPAs and best-interests decision-making. Most were optimistic about its use and value, and some highlighted that its greatest benefit was in providing safeguards against false allegations of abuse for practitioners and carers as well.451 Many practitioners also considered that carers would benefit from knowing details of the Act, as it would provide them with knowledge of when and how they would be able to make decisions for their relatives if the need arose or to act on their behalves The principles and procedures of the MCA were perceived as placing the individual at the heart of the safeguarding process by those with local responsibilities for adult safeguarding (formerly adult protection, also known as elder abuse) in local authorities (SACs). Rights to draw up a LPA were, in several instances, described as part of ‘protection plans’ (SAC04) and could be one outcome of a safeguarding investigation (SAC07). Access to the IMCA (paid advocacy) service in cases of suspected abuse was universally valued. The Deprivation of Liberty Safeguards (introduced later than the MCA) were providing another layer of protection. In one example, a person’s wish to remain in a care home against family pressure had been supported (SAC05). In another, a case of neglect had emerged (SAC11). (This study did not focus on Deprivation of Liberty Safeguards as there are other major research studies on this subject in progress.) The Act’s potential to shift the boundaries of traditional relationships and to empower vulnerable adults was observed, but a new emphasis on its role in reducing victimisation and positive risk-taking also emerged However, some raised criticisms that there was insufficient publicity about the Act and that written material was not always readily available (most of it is online). One SAC was particularly concerned that there were limited opportunities for people to find out about the Act and considered that at the time of its introduction: ‘a bigger national drive would have been better’ |
| 3.2.6.2. Financial management and safeguarding | Safeguarding Adult Coordinators felt that the MCA might potentially address one significant area of concern for them – money management and financial abuse. They discussed the safeguarding potential (but limited current use) of the MCA in these areas and the hazards that might be faced by carers and people with dementia who had not yet set out prespecified plans, but when the latter had deteriorating capacity. Most SACs interviewed described this area as a major element of their work Current data collection systems make it hard to be specific about the prevalence of actual abuse or the even the composition of formal referrals to SACs. One participant noted that although financial abuse featured in about 12% of local investigations, they lacked information about whether alleged victims had dementia or cognitive impairment. Some described situations as common place in which family care was insufficient, yet services were declined because their cost would reduce the family inheritance. Within families, unusual interest in a person’s financial affairs could be suspicious, with some relatives described as being financially motivated. One SAC reflected ‘having money does not protect you from abuse in old age; it can make you more vulnerable’. Other examples of the abuse of people with dementia encountered following referrals included valuables missing without explanation; undue influence and theft; misuse of direct payments (cash for care); taking the ‘free’ item under a ‘buy one get one free’ supermarket offer; or ‘borrowing’ money from an older vulnerable person Although having dementia in itself was acknowledged as problematic when raising safeguarding alerts (because people might not realise the risk or danger), most practitioners reported using strategies to engage a person with dementia to find out what was happening or of concern. These included face-to-face interviews, involving advocates, liaison with partner agencies (e.g. the police and the Department of Work and Pensions) and exploring legal options. However, they deemed the highest priority was remaining vigilant about the risks of financial abuse In a modified form of data triangulation, interviews with SACs were compared with responses from a survey of staff of the Alzheimer’s Society to see if they considered that money management was a problem for people with dementia. These staff similarly thought that risks of financial abuse were widespread. The MCA and financial systems were reported to be not always sufficiently sensitive to the needs of people with declining, rather than greatly impaired, capacity to make fine judgements and to take account of their changing circumstances |
| 3.2.6.3. Warning signs of financial abuse should be publicised | Participants from the Alzheimer’s Society suggested that there should be greater publicity about the warning signs of financial abuse to raise awareness among practitioners, community support networks such as shops, banks, and post offices, and society in general. In their experience, discussions about managing money were not easy and this suggests that skills may need to be developed in this area of practice. We worked with the Alzheimer’s Society to develop a series of evidence-based policy recommendations to promote the safeguarding and rights of people with dementia, pointing to the provisions of the MCA as helpful for some people lacking decision-making capacity and as part of a prevention or protection plan.287 This study attracted substantial media publicity In addition to individual planning to minimise abuse or to minimise common confusions over money management, SACs referred to other preventative measures including:
Almost all SACs highlighted the potential for people with decision-making capacity to draw up a LPA under the MCA. This was widely regarded as the most significant means of prevention of abuse once mental capacity was severely affected. Allied to these positive views of LPAs, and acknowledging that they had to be drawn up while a person was still able to make this decision, SACs generally expressed confidence in the system of deputies appointed for people without LPAs. One also reported that the Office of the Public Guardian was proving extremely helpful in managing an errant attorney in complex and difficult circumstances. There seems greater scope for professionals who inform people with dementia and their carers about the MCA to highlight the Office of the Public Guardian’s potential role in prevention of exploitation For people with minimal income and savings, the Department of Work and Pensions system of appointeeship (appointment of a suitable person to collect pension or benefits on behalf of another person, typically a relative) was viewed positively but not to the same extent. SACs made criticisms of the lack of checking of those who took on the role of an appointee although even drawing up a LPA, where greater checks and monitoring are required, could not ensure protection, as this example indicated |
| 3.2.6.4. Role of banks and community support networks | The procedures and rules of banks reportedly presented significant barriers to safeguarding people with dementia from financial abuse. Most participants raised this problem and alluded to the impersonal nature of high street banks, bank staff’s reluctance to question suspicious activity and defensiveness about information sharing, even when MCA arrangements had been made. Memory clinic staff and others may wish to suggest that people with a new diagnosis should consider current and future financial arrangements when they are ready to do so. At policy level, as noted above, the Alzheimer’s Society report Short Changed: Protecting People with Dementia from Financial Abuse,287 to which this research contributed, has been a springboard for the Alzheimer’s Society to take forward discussions with the financial services industry |
Appendix 67 Chapter 6: Proposed data capture fields with definitions for primary care
| Date of data extraction | ||
| GP name and contact details | ||
| Search register for: | ||
|---|---|---|
| Dementia syndrome | Suspected | (see below) Allows mild cognitve impairment capture |
| Diagnosed | Diagnosis of dementia | |
| Subtype diagnosed | Subtype diagnosed under ‘organic brain disorders’, i.e. Alzheimer’s disease, vascular, mixed, Lewy body, frontotemporal dementia, Parkinson’s disease dementia, other | |
| or Dementia symptoms | Presence of possible dementia symptoms | Presence of possible dementia indicators such as: st memory loss, memory loss, forgetfulness, behavioural change, disorientation, confusion, personality change, speech problems, deterioration, global deterioration, wandering |
| or Cognitive impairment indicator | MMSE score | Where MMSE score is not available from within the electronic medical record other psychometric tests indicative of cognitive impairment will be captured, e.g. 6CIT, AMTS, ADAS-cog, etc. |
| Then exclude: | ||
| Alcohol dependency | Alcohol dependency | Record of alcohol dependency is required as ‘alcohol problem’ may be insufficient as an exclusion criterion |
| and Opioid dependency | Opioid dependency | |
| and Diagnosis of psychiatric disorder | Diagnosis of major psychiatric disorder | Any diagnosis of major psychiatric disorder would be an exclusion criterion |
| From this modified subpopulation audit and report on: | ||
| Patient gender | Automatic | |
| Patient age | Automatic | |
| Patient contact details | Residential status | Ideally we could like to capture residential status, i.e. own home, sheltered accommodation, care home. However, we will be able to capture only the patient’s address |
| Carer details | Ideally it would be preferable to capture presence of a carer, marital status and carer strain score as a dependency indicator. However, we are unlikely to be able to routinely capture this data and a follow-up call for these details after initial selection criteria have been applied may be required | |
| Comorbidities | Capture all active comorbidities | Automatically identified from active problem list |
| Medication | Capture all medications | The whole medication record can be extracted, this will allow checking for prescription of cholinesterase inhibitors and concomitant medications, such as antidepressants, psychotropics, etc. |
| Contact with secondary care | Professional contacts | List other professional contacts, such as memory clinics. This information will allow a quality assessment of secondary care databases and also give contact details if further information is required; reports may need to be followed up manually |
| Medical imaging | Indication of whether CT scans/MRI scans have been performed, date of scan and results where possible; reports may need to be followed up manually | |
Glossary
- Read Code
- This is the standard clinical terminology system used in general practice in the UK. Read Codes are codes representing clinical terms, which are used by clinicians to record patient findings and procedures across primary and secondary care.
List of abbreviations
- 6CIT
- Six Item Cognitive Impairment Test
- A&E
- accident and emergency
- ADL
- activities of daily living
- AE
- adverse event
- AI
- Appreciative Inquiry
- AMTS
- Abbreviated Mental Test Score
- ANCOVA
- analysis of covariance
- AQAA
- Annual Quality Assurance Assessment
- BNI
- British Nursing Index
- BPSD
- behavioural and psychological symptoms of dementia
- CareNap-D
- Care Needs Assessment Pack for Dementia
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMAI
- Cohen–Mansfield Agitation Inventory
- CNWL
- Central & North West London
- CONSORT
- Consolidated Standards of Reporting Trials
- CPS
- Cognitive Performance Score
- CQC
- Care Quality Commission
- CRF
- Case Report Form
- CRIPACC
- Centre for Research in Primary and Community Care
- CSCI
- Commission for Social Care and Inspection
- CSRI
- Client Service Receipt Inventory
- CT
- computed tomography
- DAA
- Dementia Action Alliance
- DAD
- Disability Assessment for Dementia
- DARE
- Database of Abstracts of Reviews of Effects
- DCR-10
- Diagnostic Criteria for Research
- DEMQOL
- Dementia Quality of Life tool
- DemReg
- Dementia Register study
- DeNDRoN
- Dementia and Neurodegenerative Diseases Research Network
- DH
- Department of Health
- DNACPR
- Do Not Attempt Cardiopulmonary Resuscitation
- ENA
- Educational Needs Assessment
- EPOA
- Enduring Power of Attorney
- EQ-5D
- European Quality of Life-5 Dimensions
- EVIDEM
- Evidence-based Interventions in Dementia
- FI
- faecal incontinence
- FRAT
- Falls Risk Assessment Tool
- GCP
- good clinical practice
- GHQ
- General Health Questionnaire
- GP
- general practitioner
- GSF
- Gold Standards Framework
- HSC
- health and social care
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- ICIQ
- International Consultation on Incontinence Questionnaire
- IMCA
- Independent Mental Capacity Advocate
- IMD
- Index of Multiple Deprivation
- IRR
- incident rate ratio
- LA
- local authority
- LCP
- Liverpool Care Pathway
- LPA
- Lasting Power of Attorney
- max.
- maximum
- MCA
- Mental Capacity Act
- min.
- minimum
- MMSE
- Mini Mental State Examination
- MRC
- Medical Research Council
- MRI
- magnetic resonance imaging
- NB
- net benefit
- NDS
- National Dementia Strategy
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- NPI
- Neuropsychiatric Inventory
- NRES
- NHS Research Ethics Service
- NTIS
- National Technical Information Service
- NVQ
- National Vocational Qualification
- OOH
- out of hours
- OPCS
- Office of Population Censuses and Surveys
- OPWD
- older people with dementia
- OR
- odds ratio
- OT
- occupational therapist
- PCRN
- Primary Care Research Network
- PCRN-GL
- Primary Care Research Network-Greater London
- PCT
- primary care trust
- PEDro
- Physiotherapy Evidence Database
- PhD
- Doctor of Philosophy
- PIM
- potentially inappropriate medication
- PIP
- potentially inappropriate prescribing
- PIRG
- Public Involvement in Research Group
- POA
- Power of Attorney
- PPI
- Patient and Public Involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSSRU
- Personal Social Services Research Unit
- PV
- prompted voiding
- PYAR
- person-years at risk
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RO
- randomisation officer
- RPE
- Rate of Perceived Exertion
- RW
- research worker
- SAC
- Safeguarding Adult Coordinator
- SAE
- serious adverse event
- SCIE
- Social Care Institute for Excellence
- SD
- standard deviation
- SPSS
- Statistical Package for the Social Sciences
- SSCR
- School for Social Care Research
- SSI
- semi-structured interview
- STOPP
- Screening Tool of Older Persons’ potentially inappropriate Prescriptions
- SUR
- Seemingly Unrelated Regression
- TAU
- treatment as usual
- TD
- toileting difficulty
- THIN
- The Health Improvement Network
- TSC
- Trial Steering Committee
- TUSS
- Timed Unsupported Steady Standing
- UI
- urinary incontinence
- WHO
- World Health Organization
- ZBI
- Zarit Burden Interview