Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10147. The contractual start date was in August 2008. The final report began editorial review in March 2014 and was accepted for publication in October 2014. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Adam Gordon declares receiving grants from the British Geriatrics Society during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Gladman et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Life expectancy has risen in the developed world to the extent that most people expect not only to reach retirement age but also to live many more years in good health. But there is a downside. The very last few years of life, whether in the seventh or the tenth decade, are often spent in a vulnerable state with multiple, chronic, disabling physical and mental health conditions and age-related loss of function, as there has been little reduction in the number of years of life people can expect to live with disability. This vulnerable state, frailty, imparts an increased propensity to acute illness and consequent loss of function, which results in the medical crises that drive acute hospital admission. The numbers of frail older people admitted to hospital rises year on year, despite the development of increasingly sophisticated community health and social support services, and this rise in acute admissions causes problems with both the capacity of the system and the quality of care, safety and patient experience. This is one of the major challenges facing health and social care throughout the world, especially as demographic changes have reduced the number of people available to help and care for those who lose their independence. There is a significant cost associated with the final months of life, which appears to be independent of age. 1 This period is most predictable in people with cognitive impairment and associated progressive disability. 2
Comprehensive geriatric assessment
Mindful of these demographic realities, health services have been developed to take account of the problems faced by vulnerable older people. Instead of a health-care service designed to deal with single acute conditions by a single practitioner [such as the typical general practitioner (GP) or emergency department consultation], models of care described as comprehensive geriatric assessment (CGA) have been developed. These are characterised by an assessment of a range of health conditions, functions and activities and the physical and social environment, usually undertaken by a team of different health and social care professionals. Each case is carefully managed so that the team shares information and, using a care plan, provides a sufficient number of interventions to improve the patient’s overall outcome in an iterative manner over time (typically days, weeks or months). The value of such an approach, in principle, has long been firmly established; Stuck et al. ’s3 meta-analysis of 28 randomised controlled trials (RCTs) involving > 10,000 patients, published in 1993, was the first of many reviews that have demonstrated the benefits of CGA over routine care with respect to mortality, institutionalisation, readmission and mental well-being. The most up-to-date review, from Ellis et al. ,4 included 22 RCTs involving 10,315 participants in acute hospital settings and found similar findings, favouring acute units (wards) delivering CGA.
Although most UK hospitals provide geriatric medical wards that aim to deliver the benefits of CGA, it cannot be said that all vulnerable older people, whenever and wherever they face complex health problems, receive these benefits. This is partly because of the lack of models to deliver CGA in non-hospital settings and the relative lack of evidence of benefit of the few alternative models that do exist (e.g. liaison services5).
The Medical Crises in Older People research programme, funded by a National Institute for Health Research (NIHR) Programme Grants for Applied Research (PGfAR) award, began when a small group of clinical academics in geriatric health care in Nottingham sat down and thought about the main research issues affecting their day-to-day practice and where CGA might be helpful. Three broad areas emerged: patients discharged from acute medical units (AMUs); patients with delirium and dementia in general hospitals; and health care for residents of care homes.
Frail older people discharged from acute medical units: the acute medical unit workstream
The first broad area was the care of vulnerable older people presenting to AMUs. The last few decades have witnessed a rising number of patients admitted as an emergency to hospitals and there has been a recognition that it is inefficient to admit them first and then identify their problems. Instead, acute medical assessment units have been developed through which all patients presenting as an emergency are assessed and triaged. Acute medical assessment units (also called medical admissions units) allow for immediate urgent care to be given, enable those who need admission to be correctly identified and allow those who could be managed in an ambulatory setting to be discharged. However, the number of vulnerable older people presenting in crisis to AMUs is rising and there is worrying evidence6 that those who are discharged are prone to re-present or go on to have poor outcomes. This appeared to be a setting where CGA was required but absent.
After the review of the literature, the research involved the undertaking of a cohort study of older people discharged from AMUs to identify and describe the older people coming through the units (Acute Medical Unit Outcome Study; AMOS). A key purpose of this was to test a screening tool (the Identification of Seniors at Risk or ISAR tool7) to enable a high-risk population to be identified, enabling the interventions to focus on this group and hopefully optimise cost-effectiveness. Older patients discharged from AMUs were followed up for 3 months and a range of adverse outcomes was recorded, including death, readmission and decline in physical or mental function and well-being. The health and social care costs incurred were recorded. The degree to which the ISAR tool could distinguish between those with good outcomes and those with poor outcomes and between low and high users of health and social care resources was calculated.
The next stage was the development of an intervention in which geriatricians assessed high-risk patients on the AMU and then case managed them in the community using a wide range of community services until the presenting medical crisis was resolved. The phrase ‘interface geriatrician’ was coined to refer to a geriatrician working in this way, partly in hospital and partly in the community. The justification for this development was on the basis that the absence of specific geriatric medical expertise in this setting was a missing link in the delivery of CGA for these patients. Developing the service required close links between the university research team and the health services to enable the necessary service investment to accompany the research.
The final stage was to evaluate the effect of this intervention in a RCT (Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study; AMIGOS), in which the clinical and economic effects of the interface geriatrician and usual care without interface geriatrician input were compared.
Frail older people with cognitive impairment in general hospitals: the medical and mental health unit workstream
The second broad area of concern was the care of confused older people in hospital with dementia, delirium or both. Psychiatrists have increasingly developed and marshalled the evidence that there is a high prevalence of mental health conditions complicating the care of older people admitted to general hospitals. 8 The care of such people is widely understood to be suboptimal and many of the accusations of poor-quality or undignified hospital care in the NHS relate to people with dementia. For some commentators it is taken for granted that hospitals are bad places for old and frail people and that the policy directive should be towards alternative forms of provision. However, hospital care is often inevitable and desirable; half of all people with hip fractures have dementia and these people need a prompt and skilled operation that cannot be carried out elsewhere. Our group was also worried that this prejudice against hospitals might become a self-fulfilling prophesy: the belief that hospitals are inevitably bad places for older people could be used as justification for not attempting to improve them, thus allowing them to become less suitable. The hospital care of older people with delirium and dementia appeared to be a context in which a particular variant of CGA was required.
An analogy was made between the care of confused older people in hospital at the start of the 21st century and that of patients with stroke some decades earlier. In the past, stroke patients were commonly found in hospital but received no specialist treatment and there was a presumption that little could be done to improve their outcomes. However, repaying investment in innovation and health services research, stroke units were developed during the second half of the 20th century and were proven to have powerful beneficial effects on the outcomes of people with stroke. This evidence has had a transformational effect on the care of people with stroke. We wondered if a specialist ward for confused older people, a medical and mental health unit (MMHU), could have a similar effect.
The first stage of this workstream required a cohort study to identify the numbers of people in hospital with cognitive impairment, their characteristics and their outcomes over the subsequent months. This would allow the needs of these patients to be known in sufficient detail to design an intervention to improve their experiences and outcomes.
The second stage was to develop a specialist MMHU, drawing on not only the cohort study but also on existing literature about practice in dementia care and on a linked observational research study undertaken by the study team about the care of older people with mental health problems in hospital (the Better Mental Health study9).
The third and final stage was to test the clinical effectiveness and cost-effectiveness of this MMHU compared with standard hospital care in a RCT, given the acronym TEAM (Trial of an Elderly Acute care Medical and mental health unit). During the implementation of the workstream, on noting that the plans to evaluate the MMHU had omitted to compare the hospital experiences of patients, a further research grant to do this was sought (Research for Patient Benefit programme, reference number PB-PG-0110–21229 – ‘In a general hospital are older people with cognitive impairment managed better in a specialist unit?’); the results of this aspect of the research are reported as integral to the TEAM study.
Health care for residents of care homes: the care home workstream
The third area that our group identified was the health care of the residents of care homes. The 1980s and 1990s witnessed a huge expansion in the provision of care homes in the UK, from lower levels of provision than in other northern European countries. Around 3% of people aged > 65 years live in a care home in England and Wales: about 300,000 individuals in the UK. 10 By international standards UK care homes are small, with around 20 residents compared with ≥ 100 residents in institutions in countries such as the Netherlands and the USA. In the UK, the National Assistance Act 194811 enabled local authorities rather than the newly formed NHS to provide residential care, with the presumption that the health-care needs of residents would be met by the NHS, just as for people living in their own homes – the primary care system led by GPs contracted to the NHS. These factors meant that UK care homes did not have resident specialist medical staff, unlike those in the Netherlands and the USA. Residents of care homes are typical examples of vulnerable older people, whose ongoing care would be expected to be best if based on the principles of CGA. However, in the UK, primary health care provided by GPs and their teams has been characterised by Black and Bowman12 in an editorial in the BMJ as ‘haphazard’ and ‘idiosyncratic’, which, if correct, would make CGA difficult to deliver. We wanted to explore whether or not the benefits of CGA could be extended to this group.
The original notion of the grant holders was that CGA could be enabled if care home staff providing day-to-day care could use their routine observations to prompt timely health care. Thus, the original plan was to survey care home residents, implement an improved monitoring framework and then evaluate the framework. In fact, it became clear during the early stages of the programme that this original notion was flawed. The provision of health care in care homes appeared more complex than had been anticipated and the barriers to delivering CGA are similarly complex. It did not seem likely that improving the recognition of ill health among care staff alone would be sufficient to mount an effective CGA response. The workstream leads decided that it was necessary to take a step back, ask what was already known and define the problems more closely, rather than assuming that these were adequately specified and hence that proposed interventions could be justified.
The workstream was, therefore, modified to include a review of the literature evaluating interventions in care homes, a cohort study of care home residents and a study to illuminate the delivery of health care in care homes using a qualitative approach. It was therefore decided that the workstream should aim to understand the issues affecting the health care of residents of care homes and would prepare for further research to develop and evaluate rational service models. This was a significant modification to the original plan. On reflection, this was a great advantage of a research programme as opposed to a project: with most forms of project funding the team would not have had the flexibility to do this.
A literature review was required because the research team realised that there was a perception that there was no evidence base for health care in care homes but that this perception probably represented ignorance of the evidence base rather than absence of an evidence base. Without an explicit evidence base, it is difficult to engage policy-makers, commissioners or practitioners and hence compete for a fair position among other health priorities. The most powerful form of evidence base for the effectiveness of interventions is a systematic review of RCT evidence. This was, therefore, what was planned.
The rationale for the cohort study was similar to that used for the other two workstreams; to understand, and hence plan to meet, the health-care needs of residents of care homes, the residents’ problems needed to be described in clinical detail and the changes in their health and the resources that they already use needed to be quantified.
The case studies of existing innovations in the health care of care home residents and the interview study of health care in care homes both aimed to understand, describe and critically appraise the provision of health care in care homes. This knowledge, alongside the measured needs of the residents, seemed essential to the rational development and evaluation of interventions. For the care home workstream, unlike the other two workstreams, there was not a sufficient understanding of health and social care processes to propose feasible and potentially effective interventions. Therefore, the experimentation that health and social care practitioners were already making was explored.
Synthesis
The workstreams had many things in common: the participants in all workstreams were older people with varying degrees of frailty; the research approach intended to use a cohort study followed by development and evaluation of interventions; and all workstreams faced issues around recruitment in the presence of cognitive impairment and around health status measurement in frail older people. We decided to attempt to bring together findings from all three workstreams in a synthesis, with the particular objective of identifying factors that were likely to bring about health-care improvement.
We chose to do this by describing the results of the research programme with reference to an established framework for understanding health care, adapted from Brown and Lilford,13 which applied the input–process–outcome chain (described first by Donabedian14) highlighting three essential measurement points:
-
‘proximal end points’ to describe content
-
‘at the level’ measures to assess fidelity and
-
‘distal end points’ to assess effect.
Structure of this report
The findings of the AMU, MMHU and care homes workstream studies are provided in Chapters 2–4 respectively. Because of the huge amount of work covered by this programme and to avoid duplicate publication, only summaries of the published research are provided. All publications arising from this programme are recorded on the Medical Crises in Older People Discussion Paper Series website [see www.nottingham.ac.uk/mcop/index.aspx (accessed 4 February 2015)]. The findings of the synthesis are reported in Chapter 5. In the final chapter we report briefly on general issues arising from the programme. There were considerable challenges to patient and public engagement in research involving people as vulnerable as those in this programme and over the course of the programme we learnt a lot. Similarly, the research was in many ways innovatory; after all, by studying frail older people we were focusing on those patients who are often excluded from research by virtue of their age or aspects that make them difficult to recruit, retain or measure in research studies. We present a subsection examining the impact of this work to date and discussing the optimisation of its future impact. The very final subsection draws all of the chapters and subsections together to highlight the most significant contributions that the Medical Crises in Older People programme has made to the care of frail older people and briefly outlines the most pressing research and development priorities that arise from this work.
Chapter 2 The acute medical unit workstream
Aim
The overall aim of the AMU workstream was to develop and evaluate services in which geriatricans provided specialist input to the care of frail older people presenting to an AMU but not requiring hospital admission.
Phases
In the first phase a preparatory literature review was carried out. This was followed by a descriptive phase using a cohort study (AMOS) to examine the value of a tool to risk stratify the population. The third phase was a developmental phase during which the services were developed, optimised and described. The fourth and final phase was a RCT to examine the benefits and costs of the novel service compared with those of usual practice (AMIGOS).
The interface between acute hospitals and community care for older people presenting to acute medical units: a mapping review
A preparatory stage before undertaking a systematic review is to undertake a mapping review. These are broad reviews of reviews and are helpful to establish whether or not previous systematic reviews have already been carried out, to appraise the likely extent of the literature and to help clarify the context of the systematic review. This mapping review, which has been published,15 was undertaken as a preliminary step to examine the evidence for interventions for older people at the interface between the community and acute hospital.
A wide range of searchable databases was examined for relevant systematic reviews (see Appendix 2 for the databases searched and the search strategy). Reviews were included if they addressed older people (aged 65+ years) being discharged rapidly (< 72 hours) from hospital and assessed health, function, institutionalisation or cost-related outcomes, including length of stay and readmissions.
In total, 300 individual reviews were identified, seven3,16–21 of which were relevant and of adequate quality [see Appendix 3 for the data extraction (results) table]. Three meta-analyses3,16,17 reported evidence in favour of CGA for frail older patients in acute hospital and, to a lesser extent, community settings. None of them directly assessed the interface for the group of patients discharged from AMUs. Two meta-analyses18,19 addressed alternative locations of care, including hospital-at-home schemes. Both found evidence in favour of CGA, although none was specific to the interface of interest. Two further reviews20,21 addressed the community–hospital interface, although not solely the group of patients attending AMUs. These reviews found evidence in favour of schemes working across the acute hospital–community care interface (e.g. in reducing falls, support for hospital at home and some evidence for community geriatrics). However, there was uncertainty about the role of services based in emergency care settings.
The mapping review showed that there was evidence to support the benefits of CGA in general, with strong evidence for inpatient CGA and weaker evidence for community-based CGA. No review specifically focused on patients discharged from AMUs or emergency departments, but sufficient material was identified to justify a systematic review of primary studies directly related to ‘interface geriatrics’.
A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital
Given that the mapping review demonstrated that there was sufficient material to justify a systematic review of CGA for patients discharged rapidly from hospital, and no previous relevant review on the topic, we went on to perform a systematic review. This work has been published. 22
Standard bibliographic databases were searched for high-quality RCTs of CGA for patients discharged rapidly from hospital (see Appendix 4 for the databases searched and the search strategy). Of the 3399 full citations screened, five trials23–27 were of sufficient quality to be included [see Appendix 5 for the data extraction (results) table]. There was no clear evidence of benefit for CGA interventions in this population in terms of mortality [relative risk (RR) 0.92, 95% confidence interval (CI) 0.55 to 1.52] or readmissions (RR 0.95, 95% CI 0.83 to 1.08) or for subsequent institutionalisation, functional ability, quality of life or cognition.
This review justified the development and evaluation of our intervention.
Umbrella review of tools to assess the risk of poor outcome in older people attending acute medical units
A key step in the work of this workstream was to establish how frail or high-risk older people could be identified in emergency care settings such as AMUs. To do this an umbrella review of reviews was conducted to identify relevant systematic reviews of appropriate tools to assess the risk of functional decline in older people attending AMUs (see Appendix 6 for the databases searched and the search strategy). This work has been published. 28 Umbrella reviews are like mapping reviews in that they are reviews of reviews; however, they focus on a single question rather than also covering contextual issues.
Of the 323 citations identified in the search, four systematic reviews were included,29–32 reviewing nine different tools to assess adverse health outcomes [see Appendix 7 for the data extraction (results) table]. Three assessment tools were considered to be potentially suitable for use: the ISAR tool,7 the Hospital Admission Risk Profile33 and the Triage Risk Screening Tool,34 but only the ISAR tool had evidence to predict all aspects of adverse health outcomes, that is, death, institutionalisation, readmission, resource use and decline in physical or cognitive function.
From these reviews, the ISAR tool was found to be ‘fair’ in terms of sensitivity, specificity and area under a receiver operating characteristic curve. We concluded that the ISAR tool was the most appropriate screening tool to assess the risk of adverse health outcomes in older patients being discharged from an AMU and so it was chosen for the planned intervention and the AMIGOS trial. However, this tool needed to be validated in a UK population.
The Identification of Seniors at Risk score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units: the Acute Medical Unit Outcome Study
Having identified the ISAR tool as the most promising risk assessment tool for our purpose, our next objective was to evaluate whether or not the ISAR tool predicted the clinical outcomes and health and social services costs of older people discharged from AMUs in the UK. This work has been published. 35
A cohort study was performed using receiver operating characteristic curve analysis (area under the curve; AUC) to compare the baseline ISAR score with adverse clinical outcome at 90 days {where adverse outcome was any of death, institutionalisation, hospital readmission, increased dependency in activities of daily living [ADL] [decrease of ≥ 2 points on the Barthel ADL index36], reduced mental well-being [increase of ≥ 2 points on the 12-item General Health Questionnaire (GHQ-12)37] or reduced quality of life [reduction in European Quality of Life-5 Dimensions (EQ-5D) score38]} and health and social services costs over 90 days estimated from routine electronic service records. The setting was two AMUs in the East Midlands, UK (Nottingham and Leicester). Appendix 8 shows the ISAR tool questions, Appendix 9 the baseline patient-identifiable data form, Appendix 10 the baseline patient interview form, Appendix 11 the baseline patient data collection form and Appendix 12 the follow-up patient data collection form.
In total, 667 patients aged ≥ 70 years who had been discharged from an AMU were included. Adverse outcome at 90 days was observed in 76% of participants. The ISAR tool was poor at predicting adverse outcomes (AUC 0.60, 95% CI 0.54 to 0.65) and fair at predicting health and social care costs (AUC 0.70, 95% CI 0.59 to 0.81).
We therefore confirmed that adverse outcomes were common in older people discharged from AMUs. The poor predictive ability of the ISAR tool in older people discharged from AMUs made it unsuitable as a sole tool for use in clinical decision-making, but it was sufficient to identify a higher-risk group suitable for a clinical trial.
The predictive properties of frailty-rating scales in the acute medical unit
Although we went on to use the ISAR tool to select our higher-risk group of patients, we wondered whether or not frailty-rating scales might have more predictive value. We had collected a large number of frailty-related variables as part of the AMOS study, reported in the previous section. We therefore compared the predictive properties of five frailty-rating scales using data collected for the AMOS study. This work has been published. 39
Participants were classified at baseline as frail or non-frail using the five different frailty-rating scales. 40–44 The ability of each scale to predict outcomes at 90 days (mortality, readmissions, institutionalisation, functional decline and a composite outcome comprising any of these) was assessed using the AUC.
In total, 667 participants were studied. According to all scales, frail participants were associated with a significant increased risk of mortality (RR range 1.6–3.1), readmission (RR range 1.1–1.6), functional decline (RR range 1.2–2.1) and the composite adverse outcome (RR range 1.2–1.6). However, the predictive properties of the frailty-rating scales were poor, at best, for all outcomes assessed (AUC ranging from 0.44 to 0.69).
We concluded that frailty-rating scales, like the ISAR tool, were of limited use in risk-stratifying older people being discharged from AMUs and offered no advantage over the ISAR tool in this setting.
Patient-based health and social care costs of older adults discharged from acute medical units
Introduction
The AMOS study also allowed us to produce patient-based UK NHS and social care costs for this group of older patients (aged 70+ years) who attended an AMU and were then discharged home. We estimated these costs partly to ensure that we had robust methods for our later RCT and partly because this group of patients has not previously been studied greatly yet we were aware of great interest elsewhere about the use of resources and hence service costs in this group of patients using emergency and non-elective care. This study has been published. 45
Methods
Data were collected retrospectively for 90 days from recruitment using Electronic Administration Record information extracted from various health-care services. Hospitalisation data were collected for 644 patients in Leicester and Nottingham (23/667 withdrew consent for their resource use data to be obtained). Hospital care data included inpatient stays, day cases and outpatient and critical care. Social care data were obtained for all participants. In a subset of 456 participants (in Nottingham), further approvals and access were gained to obtain data from general practices, ambulance services and intermediate and mental health care services. Resource use was combined with national unit costs46,47 to derive total patient costs. The costing perspective was NHS and local authority (social services) expenditure.
Results
Data were obtained from 48 out of 118 general practices (250/456 Nottingham participants) despite exhaustive attempts to acquire data from all practices. Thus, costs from all sectors were available for 250 participants. The mean (95% CI, median, range) total cost for this subgroup was £1926 (£1579 to £2383, £659, £0–23,612). Secondary care made up 76% of costs. Other costs were for primary care (10.9%), ambulance service use (0.7%), intermediate care (0.2%), mental health care (2.1%) and social care (10.0%). The 10% of the most costly participants accounted for 50% of the overall costs.
Discussion
Secondary care costs were the main cost driver in this patient group. Despite the expectation that this group would mainly incur ambulatory and community costs, many of these costs contributed little in this patient group. Consideration should be given to focusing primarily on secondary care costs in some research instances, such as when scoping work reveals that secondary care costs are likely to be dominant. Nevertheless, in view of the fact that we aimed to influence community care, we elected to use the same methods in our RCT and to ascertain ambulatory and community costs as well as hospital costs.
The role of the interface geriatrician across the acute medical unit–community interface
We proposed that the outcomes of frail older people discharged from AMUs might be improved by ‘interface geriatricians’, geriatricians working across the hospital–community interface. 48 In Nottingham and Leicester the community geriatricians (at the time, five in Nottingham and seven in Leicester) developed this style of working for the subsequent AMIGOS study. Community geriatricians went to the AMU to see higher-risk older patients (identified using the ISAR tool) who had been randomised to the intervention and who were to be imminently discharged. They assessed the patients and then arranged whatever further care they felt was necessary, with the expectation being that this would take place mainly in the community.
A team of interface geriatricians met regularly throughout the AMIGOS study to discuss cases as part of their clinical and professional development. The interface geriatrics style of working grew out of existing practice as the community geriatricians were already experienced in both hospital and community practice. The difference was the focus on this new group of patients who were at higher risk and who were being discharged from an AMU.
Unsurprisingly, perhaps, the interventions undertaken were typical of geriatric medical practice in any other setting. They comprised a comprehensive specialist geriatric medical assessment that included enquiry into mental health issues and cognition, geriatric syndromes and issues of polypharmacy, often employing the use of collateral history taking. A particular feature was that the initial assessment on the AMU was almost always followed by assessment at the patient’s home, which often revealed important diagnostic facts undetected on the AMU. These assessments led to a range of actions such as changes to medication and also communication of the geriatrician’s assessment findings to the patient and primary care staff. Although interface geriatricians often identified clear potential benefits arising from their actions, they were aware that in some cases they were unable to prevent poor outcomes, and for some patients they had little to offer.
This experience demonstrated that interface geriatrics was a feasible option and had the potential to benefit patients. However, warnings were sounded by the clinicians that the benefits of this approach might be limited, with concerns being that community services might not act on the advice given by the interface geriatricians, that the benefits might be diluted in the AMIGOS trial through the inclusion of some low-risk patients (because of the relatively poor discriminatory power of the ISAR tool) and that some of the marginal clinical benefits (such as satisfaction with having an adequate explanation of health conditions) might be difficult to detect using conventional outcome measures.
The Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study
The main objective of this study was to evaluate the addition of specialist geriatric medical input to frail older people attending an AMU and identified as being at high risk of readmission, functional decline or death. This study has been published. 49,50 Appendix 13 shows the patient screening data form, Appendix 14 the baseline patient-identifiable data form, Appendix 15 the patient baseline initial interview form, Appendix 16 the patient baseline initial data collection form, Appendix 17 the patient follow-up data collection form, Appendix 18 the carer baseline data collection form and Appendix 19 the carer follow-up data collection form.
Methods
A multicentre, individual-patient RCT comparing the intervention with usual care was undertaken. The intervention was interface geriatrics,48 as described in the previous section. Patients aged ≥ 70 years discharged from two UK AMUs (Nottingham and Leicester) and scoring ≥ 2 on the ISAR risk screening tool were recruited prior to discharge and randomised to receive the intervention or usual care. Carers of participants were also recruited. Follow-up was by postal questionnaire 90 days after randomisation. The primary outcome was the number of days spent at home (for those admitted from home) or the number of days spent in the same care home (if admitted from a care home). Secondary outcomes included mortality, institutionalisation, hospital resource use and scaled outcome measures (including quality of life, disability and mental well-being).
A postal questionnaire was sent at 90 days to carers or family members for whom there was baseline information. Baseline and follow-up carer measures were:
-
carer strain: Caregiver Strain Index51
-
carer-specific quality of life52
-
generic quality of life: EQ-5D. 38
In view of the small numbers, carer outcomes were not compared between groups.
A purposive sample of patient participants and carer participants was selected to have a semistructured qualitative interview at home 30 days after discharge. Analysis was performed in parallel with recruitment and interview and so the content of the interviews developed as data emerged. The emerging findings were not shared with the community geriatricians during the trial. Selection was determined by the researcher on the basis of emerging themes and recruitment continued until data saturation. The interviews covered the problems that led to admission, what participants perceived happened in hospital, what they wanted and expected, what helped and what did not help, discharge arrangements, resettlement at home, impact on everyday activities, transfer of care to community services and ongoing problems.
In the economic study, 417 participants (205 allocated to the intervention arm) were analysed at 90 days’ follow-up. Data were collected retrospectively for 180 days from recruitment using Electronic Administration Record information extracted from various health-care services, as for the AMOS trial, but in addition the cost of the interface geriatricians was also included. Quality-adjusted life-years (QALYs), based on EQ-5D valuations at baseline and follow-up, were obtained for 254 (60.9%) participants (127 per arm). Multiple imputation by chained equations was applied to deal with missing QALY values. Costs and QALYs were adjusted by baseline characteristics using regression methods. The difference in mean total costs and QALYs between arms and incremental cost-effectiveness ratios (ICERs) were estimated, handling uncertainty by non-parametric bootstrapping.
Results
Of 1001 eligible patients, 433 were recruited: 217 in the control group and 216 in the intervention group. The two groups were well matched for baseline characteristics, and withdrawal rates were similar in both groups (5%).
In total, 201 (98%) received the intervention as intended with 133 (66%) having a response beyond the initial assessment; 122 of these were seen at home. The range of actions taken by the geriatricians was largely as intended and as might be delivered in routine practice, most commonly liaison with other practitioners, medication changes, giving health advice and referral for rehabilitation, further diagnostic tests and additional medical follow-up.
The mean number of days spent at home over 90 days’ follow-up was 80.2 in the control group and 79.7 in the intervention group (95% CI for the difference in means –4.6 days to 3.6 days; p = 0.31). There were no significant differences in any of the secondary outcomes.
In total, 65 carer participants (15%) were recruited from the 433 patient participants but only 46 (11%) were true carers (the others being informants). The 46 true carers gave a median of 2 hours per day physical care and 3 hours per day of supervision. Of these, 17 (37%) carers were spouses and 26 (57%) were children, with three (7%) ‘other’ carers. The mean age of the carers was 61.5 years and 31 (67%) were female, 22 (48%) were co-resident with the patient participant and 15 (33%) were in paid employment. At baseline, carer participants had high carer strain [median Caregiver Strain Index 7, interquartile range (IQR) 3–9] and poor carer quality of life (median carer-specific quality of life 8, IQR 5–9) and the median EQ-5D score was 0.81 (IQR 0.69–0.85). There was no significant change in these variables at follow-up.
In total, 18 older patients and six of their informal carers were interviewed. The thematic analysis revealed six themes, some with subthemes:
-
Staff recognition (subtheme: dispersal of blame). The majority of the patients wished to express the positive attributes of the staff on the AMU, saying that they felt well looked after on the ward. When problems were identified the patients were keen to point out that they did not blame the staff but rather apportioned blame on external factors.
-
Incomplete satisfaction (subthemes: perceived lack of treatment, constant disturbance, waiting, poor communication, discharge uncertainty, carer frustration). Although the patients wanted to portray a positive image of the staff on the AMU, all but two spoke about areas of dissatisfaction and these were clustered around the six subthemes.
-
Stoicism (subthemes: ageing assumptions, modest expectations, minimisation of needs, passive acceptance). There was an underlying attitude of stoicism. Patients did not have high expectations around improving the state of their health in light of the ageing process. Similarly, they were tolerant and understanding of any weaknesses experienced on the AMU. The patients had low expectations of hospital care, resulting in passive acceptance of any weaknesses experienced.
-
Eager to go home. Although the patients recognised that they needed hospital-based assessment, they did not want to remain on the AMU for any longer than was absolutely necessary.
-
Nebulous grasp of the geriatrician role. The patients spoke about the geriatricians possessing a pleasant bedside manner but the majority of patients were unsure what the geriatrician had done for them.
-
Outstanding needs (subthemes: unresolved health issues, unresolved daily living needs, impact on informal carer, value of independence). Patients had both outstanding health needs and daily living needs, which were not addressed as part of their stay on the AMU. These impacted on their informal carers. Despite the help received with daily living activities a lot of the patients voiced a desire to complete these activities themselves rather than have others complete them for them.
In the complete-case economic analysis involving the subgroup of 254 patients with EQ-5D valuations at baseline and follow-up completed, the differences in mean total costs and QALYs (intervention vs. control) were +£138.9 (95% CI –£1139.8 to £1434.5) and 0.004 (95% CI –0.012 to 0.020), respectively, resulting in an ICER of £38,583 per QALY, with a 47% probability of the ICER being < £30,000 per QALY. In the adjusted cost-effectiveness analysis, the differences were +£146.4 (95% CI –£60.6 to £340.7) and 0.002 (95% CI –0.006 to 0.011), respectively, resulting in an ICER of £73,200 per QALY, with a 36% probability of the ICER being < £30,000 per QALY).
In the full-sample economic analysis (imputation of missing QALY values), the mean cost of inpatient care was lower in the intervention arm (–£211.7, 95% CI –£1097.9 to £471.6) whereas all other care costs were higher (social care +£220.1, 95% CI –£299.5 to £691.5; day cases +£155.6, 95% CI £31.1 to £280.3; outpatient care +£46.3, 95% CI –£69.5 to £166.2). The intervention cost was +£115.6 per case (95% CI £106.0 to £125.8). In an adjusted cost-effectiveness analysis, the total cost for the intervention group was higher (+£213.4, 95% CI £94.8 to £331.2) with no QALY gain (–0.001, 95% CI –0.009 to 0.007) and so the intervention was dominated by standard care (3% probability of the ICER being < £30,000 per QALY).
Discussion
This specialist geriatric medical intervention applied to a high-risk population of older people attending and being discharged from AMUs had no impact on patient-level outcomes or subsequent use of secondary care or long-term care. It was not cost-effective.
The interview findings indicated some areas in which AMUs could improve patients’ experiences. They also demonstrated that most patients had background conditions and that the trip to the AMU contributed little to their management from their perspectives and was confined simply to the assessment of an acute medical condition (which for these patients was not sufficient to warrant admission to hospital from the AMU). They illustrate that the interface geriatricians seemed to have little impact on the main issues affecting the health and well-being of these patients, which were not the medical crises that had precipitated their presentation but the underlying health conditions in which these crises arose.
Together, the findings support the deduction from the AMIGOS findings that a more integrated follow-up response after an AMU attendance is warranted, involving chronic disease management if health outcomes are to be improved and preventing hospital admission if costs are to be minimised.
Chapter 3 The medical and mental health unit workstream
Aim
The overall aim of this workstream was to develop and evaluate a specialist unit for people with mental health problems in a general hospital.
Phases
The workstream had three phases. The first was a preparatory phase to describe and understand the nature of people with mental health problems in general hospitals and their carers. This involved a scoping review of mental health problems in older people in hospital and the Better Mental Health cohort study. The second phase was to develop a MMHU. The third phase examined the benefits and costs of the novel service compared with those of usual practice (the TEAM study).
A scoping review of mental health problems in older people in hospital
This review53 helped prepare the research team both for the linked Better Mental Health study and for the preparatory work for this workstream. The review found that mental health problems were common in patients in general hospitals and were associated with worse outcomes than for patients without them. The quality of care for such people, especially those with dementia, was felt to be poor.
The dominant theory on which dementia care was based was a psychosocial one, ‘person-centred care’, based on Tom Kitwood’s concepts of personhood and avoiding ‘malignant social psychology’. 54 This model has been refined to ‘relationship-centred care’, which focuses on relationships. In contrast to person-centred care, the most commonly used theories underpinning the training of general hospital nurses tended to be task focused.
The behaviour disturbances seen in people with dementia were likely to be sensitive to the social and physical environment, offering opportunities to improve care through environmental change. The literature was found to abound with possible interventions in terms of therapies or practices, many of which could be applied to hospitals in the UK, although there was very little written about the use of person-centred care approaches applied to general hospitals.
Dementia care in general hospitals has become an important topic for the NHS in the UK, as evidenced by the National Dementia Strategy published in 2009,55 a year after our programme began. The preferred service model to help meet the needs of patients with mental health problems in hospital was to use old-age liaison psychiatry services, although it was unclear what such services should comprise, there was no firm evidence of cost-effectiveness and it was not clear how they would facilitate person-centred care. 56
Tools such as Dementia Care Mapping,57,58 which examine how care is delivered and how to improve it, were noted. NHS quality improvement tools might also be employed to improve care and there were also other approaches to improve quality of care at the organisational level, although it was not clear whether or not they increased person-centred care. Possible mechanisms to affect change might be through commissioning, legal or regulatory means.
The Better Mental Health cohort study
The purposes of this study were to establish the number of older patients with mental health problems in general hospitals and to measure their health status and outcomes in order to design the RCT of the MMHU. This information could also be of use for the development of other services for this patient group. Papers have been published from this study. 59–62 Appendix 20 shows the screening form, Appendix 21 the patient baseline data form, Appendix 22 the carer baseline form, Appendix 23 the patient outcome form and Appendix 24 the carer outcome form.
Methods
Participants were from two sites at the Nottingham University Hospitals NHS Trust, an 1800-bed teaching hospital providing sole general medical and trauma services for a population of approximately 660,000 people. Individuals aged ≥ 70 years with an unplanned admission to 1 of 12 wards (two trauma orthopaedic wards, three acute geriatric medical wards and seven general medical wards) were eligible for inclusion. Exclusion criteria were unwillingness to be screened, being unconscious or too ill to be interviewed up to the fifth day of admission and an inability to speak English with no available interpreter. Consecutive admissions were identified from the hospital administration computer system and patients were approached between day 2 and day 5 of admission.
A two-stage assessment procedure was used. The first stage identified people unlikely to have a mental health problem. The second stage used more detailed assessments to characterise problems. The first-stage assessment used the Abbreviated Mental Test Score,63 the four-item Geriatric Depression Score,64 the two-item Primary Care Evaluation of Mental Disorders anxiety screen,65 the four CAGE questions for alcohol misuse (Cut down, Anger, Guilt, Eye-opener)66 and a question asking ward staff if there was any other reason to believe that a mental health diagnosis might be present. Participants screening negative for cognitive impairment (Abbreviated Mental Test Score of > 7), depression (four-item Geriatric Depression Score of < 1) and alcohol abuse (CAGE score of < 2) and negative on the mental health diagnosis question, or who scored only on the anxiety questions, were excluded from further study. Patient–carer pairs were recruited from those screening positive for cognitive impairment if a carer could be identified and was willing to participate.
Participants were followed up 180 days after recruitment. Information was collected from the participants, family members and other informal or professional carers. Information on readmissions and total number of days spent in hospital was collected from hospital administration systems. Mortality, and dates and types of care home placements (residential or nursing, permanent or respite) were ascertained from the hospital administration systems, the patients’ GPs, the carer informants or care home. Surviving participants were interviewed at home with a carer or, if this was not possible, by telephone with an informant. Participants were tested for cognitive function and carers provided information on behavioural and psychological symptoms and ADL. Economic data were collected retrospectively for 180 days from recruitment using Electronic Administration Record information, as for the AMOS study (see Chapter 2).
Patient outcomes were survival to 180 days; days spent at home, defined as 180 minus the total number of days spent in hospital, in a care home or dead for patients living in the community at admission and as 180 minus the total number of days spent in hospital, in a new care home or dead for patients living in a care home at admission;67 change in ADL, defined as an increase or decrease of ≥ 2 points on the Barthel index36 at follow-up compared with admission and before the acute illness.
Carer participants were asked at baseline and at 6 months to complete a questionnaire, with help as required, giving demographic and care-giving details. It included the Caregiver Strain Index,51 with a score of ≥ 7 indicating high strain.
Electronic administrative records were sought for 6 months post admission from health services (general practices, hospitals, ambulance transport services, intermediate and mental health-care services) and social care services. Standardised costs were applied to all resource use types.
Of 1004 patients screened, 36% had no mental health problems or had anxiety alone. Of those screening positive, 250 took part in the full study. Adjusting for the two-stage sampling design, 50% of admitted patients aged > 70 years were cognitively impaired, 27% had delirium and 8–32% were depressed. In total, 6% had hallucinations, 8% delusions, 21% apathy and 9% agitation/aggression (of at least moderate severity). Of those with mental health problems, 47% were incontinent, 49% needed help with feeding and 44% needed major help to transfer.
Of the 250 patients recruited to the study, 180 were cognitively impaired and had carers willing to take part. After 6 months, 78 patients (31%) had died and 100 carers were followed up. Carers’ own health, in terms of mobility, usual activities and anxiety, was poor in one-third of cases. At the time of admission, high carer strain was common (42% had a Caregiver Strain Index ≥ 7), particularly among co-resident carers (55%). High levels of behavioural and psychological symptoms at baseline were associated with more carer strain and distress. At follow-up, carer strain and distress had reduced only slightly, with no difference in outcomes for carers of patients who moved from the community to a care home.
The median number of days spent at home for participants was 107.5 days (IQR 0–163 days); 38 (15%) spent > 170 days at home. The mortality at 180 days was 78 (31%), 104 (42%) were readmitted and 46/192 (46%) community-dwelling patients moved to a care home. In surviving participants, half improved in ADL ability at 180 days from admission, but only 24% recovered to their pre-acute illness baseline and 36% showed further decline in function during follow-up.
Health and social care costs were derived for the 247 participants for whom resource use data were available. Primary care data were available for 122 (49%) participants because of the reluctance of some general practices to allow access to data. In this subset with full data, the mean (95% CI, median, range) total cost of care was £9842 (£8573 to £11,256, £7717, £715–48,795). Secondary care contributed > 80% of the costs, with the remaining costs incurred in social care (10.7%), primary care (6.7%) and other sectors (2.1%).
In summary, the Better Mental Health cohort study showed that a large number of older people admitted to a general hospital had mental health problems, particularly cognitive impairment, that their outcomes were poor and that their use of health and social care resources was high.
The development of the medical and mental health unit
We describe elsewhere how the MMHU was developed. 68
The process was guided by discussions held with the acute hospital trust nursing, therapy and medical management; discussions held with the local mental health trust; negotiations with the trust research and development department and the two local commissioning primary care trusts about the funding of the unit (additional funding for staff of £280,000 per year for 3.5 years was granted); and advice from two existing units and from other experts. Other sources of relevant information included the emerging findings of the Better Mental Health cohort study; a book on dementia (and delirium) co-authored by Professor Harwood;69 and a multidisciplinary development group that met monthly, with representation of senior nursing, medical and general management, mental health NHS trust management, allied health professionals and ward staff.
Initially it had been anticipated that the MMHU would care for patients with any significant mental health problem. During the early pilot period it became clear that patients with depression alone did not benefit from being cared for on the MMHU and the criterion for entry was changed to cognitively impaired older people. Most of the patients cared for on the MMHU had dementia or delirium.
The MMHU formally commenced development work on 1 February 2009 and opened for business on 1 June 2009. The ward was formerly a 28-bed acute geriatric medical ward and staff were therefore familiar with the problems of those with combined medical and mental health needs, who made up about 75% of its previous case load.
Admission criteria were kept broad (‘confused and over 65’), allowing for easy case identification and transfer from the AMU and the exercise of discretion in particular cases. Exclusion criteria included those requiring detention under the Mental Health Act 2007;70 acute intoxication and the immediate management of patients with overdose; and an over-riding clinical need for alternative ward facilities.
The predominant philosophy was that of CGA. To enhance the care of those with delirium and dementia, additional aspects beyond the provision of a typical geriatric medical ward were developed. These were enhancing the staffing level and skill mix, introducing the person-centred care approach, a programme of organised activity, improving the environment to make it more suitable for confused patients and introducing a proactive and inclusive approach to family carers.
Comparison of a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial
Papers from this study, the TEAM study, have been published previously. 71–74 Appendix 25 shows the patient baseline data form, Appendix 26 the carer baseline data form, Appendix 27 the patient outcome form, Appendix 28 the carer outcome form, Appendix 29 the medical data form and Appendix 30 the methods for the analysis of the staffing interview.
Methods
Patients were recruited who had been admitted for acute medical care to the Nottingham University Hospitals NHS Trust. Suitable patients were identified on the hospital AMU and were randomly allocated between the MMHU and standard care. Randomised patients were subsequently approached for recruitment to the study. This approach was necessary so that patients could be moved from the admission unit to the wards at any time of the day or day of the week at the pace required for the efficient operation of the hospital, yet allowing sufficient time for patients to be recruited ethically. Participants were aged > 65 years and had been identified by the admissions unit physicians as being ‘confused’. A family member or carer was recruited if available and willing to act as an informant.
Potentially suitable patients were entered into a computerised screening log and, if a bed was available on the MMHU, randomised 1 : 1 between the unit and standard care in a permuted block design, stratified for previous care home residence. Readmitted patients were assigned their original allocation. Regardless of allocation, patients had access to standard medical and mental health services, rehabilitation and intermediate and social care.
Standard care wards included five acute geriatric medical wards and six general (internal) medical wards. Practice on geriatric medical wards was based on CGA and staff had general experience in the management of delirium and dementia. Mental health support was provided, on request, from visiting psychiatrists, on a consultation basis. The 28-bed MMHU was an acute geriatric medical ward with five enhanced components, as described earlier.
The primary outcome was number of days spent at home (or in the same care home) in the 90 days following randomisation. In addition, a range of health status outcomes was measured: quality of life (DEMQoL,75 EQ-5D,76 London Handicap Scale77), behavioural and psychological symptoms [Neuropsychiatric Inventory (NPI)78], dependency in personal ADL,36 cognitive impairment [Mini-Mental State Examination (MMSE)79], carer strain (Carergiver Strain Index51) and carer psychological well-being (GHQ-1237). Carer satisfaction was measured on 10 dimensions of care (overall, admission, car parking, feeding, medical management, being kept informed, dignity and respect, the needs of a confused patient, discharge arrangements, timing of discharge) using Likert scales (very/mostly satisfied, mostly/very unsatisfied; items taken from Counting the Cost80).
Structured non-participant observations of the experience of care on study wards were undertaken using Dementia Care Mapping. 81 Two trained researchers observed the care of 90 randomly subsampled participants. Observations were made every 5 minutes for 6 hours per patient. Clinical staff were not aware which patients were being observed. Quantified mood and engagement scores, activity, noise and staff interactions that significantly addressed or disregarded patients’ emotional and psychological needs (‘personal enhancers’ and ‘personal detractors’) were recorded, according to strict definitions. Inter-rater reliability was assessed throughout the study and was satisfactory (Cohen’s kappa between 0.50 and 0.85).
Outcome assessments were carried out by research staff who were not involved in recruitment or baseline data collection and who were blind to allocation. Carer satisfaction with hospital care was ascertained through a telephone call 1–3 weeks after discharge. Health outcomes were ascertained during interviews with patients and carers at home 90 days (±7 days) after randomisation. Routine health service records were examined for service use, mortality and readmissions.
In total, 40 family carers were purposively recruited from participants in the RCT, 20 from each setting, and took part in face-to-face semistructured interviews. An interview schedule was constructed to ensure that critical topics were covered, such as patient admission and settling in to the ward; carer relationship with staff; the ward environment; patients’ daily routines such as sleeping, meals, hygiene and activities; privacy and dignity; care and medical treatment; and discharge planning. Participants were encouraged to discuss both what they considered worked well and what they considered worked not so well on wards relating to quality of care. Interviews were conducted in the carers’ homes and consent was obtained to audio record interviews. Participants were reassured that privacy, confidentiality and identity would be protected. Interviews were transcribed verbatim and were coded for themes, which were compared and contrasted between settings to provide a detailed understanding of participants’ experiences and if and how the intervention added to carers’ perspectives of the quality of care.
A total of 22 ward staff from the MMHU were purposively recruited to take part in face-to-face semistructured interviews. The breakdown of the staff interviewed was as follows: two deputy ward managers, six general nurses, three mental health nurses, one student nurse, two occupational therapists, three health-care assistants, two activity co-ordinators, one junior doctor, one receptionist and one cleaner. The mean age of the sample was 37 (range 20–64) years and 15 (68%) were female. Length of experience in the profession ranged from 4 months to 29 years. Interviews lasted between 30 and 90 minutes. An interview schedule was constructed to ensure that the following topics were explored: education and training, job satisfaction, care of patients with dementia, team working, communication with carers and organisational barriers to change in practice and culture.
Twenty-six patients were approached and recruited from a sample of cognitively impaired patients aged > 65 years who had been recruited to the study. A trained dementia researcher assessed whether a traditional semistructured interview was appropriate or not by using MMSE scores combined with a general assessment of patients’ current cognitive function and conversation skills. All interviews were conducted on hospital wards and most were carried out at the bedside because of patients’ levels of illness and mobility or lack of an alternative location. Participants lacking capacity or appropriate communication skills were offered an interview using a Talking Mat. This is a low-tech, alternative and augmentative communication tool that uses images to explore specific topics and provides a visual scale that enables people to express their general feelings about individual options. This tool has been successfully used to assist communication for people with cerebral palsy,82 aphasia,83 learning disability84 and Huntington’s disease. 85 Research suggests that this communication tool can be used effectively with people at all stages of dementia. 86 However, using Talking Mats as a research tool in an acute hospital setting had not been done before. We explored its use as an adjunct to the TEAM interview work.
In the economic analysis, 599 (MMHU n = 309) participants were analysed at the 90-day follow-up, at which point 139 (MMHU n = 68) had died. Health (inpatient stays, day cases, outpatient care, critical care, ambulance service use, mental health trust and primary care) and social care resource use data were collected and combined with unit costs (from the NHS and personal social services perspective, cost year 2012/13) to estimate total costs. Primary care and inpatient resource use data were obtained for 468 out of 599 (78.1%) and 595 out of 599 (99.3%) patients respectively. For the remaining services, resource use data were complete. The per-patient additional cost of the MMHU was calculated as the excess health-care costs incurred at this ward over a period of 90 days (trial follow-up) compared with usual care in a general or geriatric ward, averaged across each patient allocated to the MMHU. QALYs, based on EQ-5D valuations at baseline and follow-up, were obtained for 272 out of 599 (45.4%) patients (MMHU n = 139), including 62 (MMHU n = 30) who had died by the follow-up [assumed baseline utility (EQ-5D valuation) until date of death]. In a complete-case cost-effectiveness analysis, including 209 out of 599 (34.9%) patients with complete QALY and cost data, the differences in mean total costs and QALYs between arms and the ICERs were estimated, handling uncertainty by non-parametric bootstrapping. Costs and QALYs were adjusted by baseline characteristics using regression methods. Additionally, a cost analysis was conducted for the complete-case resource use data set [complete inpatient and primary care data, 466 out of 599 (77.8%) patients].
Results
Between July 2010 and December 2011, 310 patients were recruited from the specialist unit and 290 from standard care. The recruitment rate was slightly higher on the specialist unit (71% vs. 66% of those randomised). Those not recruited were of similar age and sex and were from a similar area of residence (postcode), but care home residents assigned to the specialist unit were more likely to be recruited than those assigned to standard care (73% vs. 56%). In total, 462 participants lacked mental capacity, 227 (73%) assigned to the specialist unit and 235 (81%) assigned to standard care.
There was no statistically significant difference in the number of days spent at home between settings [median 51 days MMHU vs. 45 days standard care; 95% CI for difference –12 days to 24 days; p = 0.3). The median index hospital stay was 11 days in both settings and the mortality rates were 22% and 25% (95% CI for difference –9% to 4%), the readmission rates were 32% and 35% (95% CI for difference –10% to 5%) and the new care home admission rates were 20% and 28% (95% CI for difference –16% to 0%) for the MMHU and standard care respectively. Participants on the MMHU spent significantly more time with positive mood or engagement (79% vs. 68%, 95% CI for difference 2% to 20%; p = 0.03) and experienced more staff interactions that addressed their emotional and psychological needs (median four vs. one per observation; p < 0.001). More family carers in the MMHU group than in the standard care group were satisfied with care (overall 91% vs. 83%, 95% CI for difference 2% to 15%) and severe dissatisfaction was reduced in the MMHU group compared with the standard care group (5% vs. 10%, 95% CI for difference –10% to 0%; p = 0.004). There were no significant differences in any of the other outcomes.
In total, 20 carers each from the MMHU and standard care groups were interviewed. In the MMHU group this included two spouses, 13 daughters, two sons, one brother and two granddaughters and in the standard care group this included six spouses, six daughters, one granddaughter, five sons, one sister and one nephew. Seven of the patients from the MMHU were male and 13 were female, with a mean age of 87 (range 83–97) years, and 11 of the patients from standard care were male and nine were female, with a mean age of 85 (range 69–95) years. The main themes identified in exploring carer satisfaction related closely to met or unmet expectations and included activities and boredom, staff knowledge, dignity and personal care, the ward environment and communication between staff and carers. Neither setting was perceived as wholly good or wholly bad; however, greater satisfaction (and less dissatisfaction) with care was experienced by carers from the MMHU group. Carers were aware of improvements relating to activities, the ward environment and staff knowledge and awareness of the appropriate management of dementia and delirium. However, in some cases communication and engagement of family carers was still perceived as insufficient.
Health professionals suggested that working on the MMHU allowed them to provide better care than they had previously done to cognitively impaired patients. The six main improvements experienced by staff were across the following themes: confidence in competence; working with mental health professionals; increased knowledge of dementia; moving towards a person-centred acute model of care; improving coping strategies; and a positive change in attitudes towards patients with cognitive impairment. Staff commented positively about the skills mix of nursing care available to patients on the MMHU, specifically the introduction of three mental health nurses. Participants highlighted that this helped increase staff confidence and morale when staff were faced with unfamiliar or perceived challenging behaviour. Staff further commented that working on a specialised unit for patients with cognitive impairment had greatly increased their knowledge and awareness of dementia and delirium. Staff generally considered that they had a good understanding of the principles of person-centred care. A few nursing staff felt that the acute hospital setting was too task focused and an inappropriate place to deliver person-centred care. The specialist MMHU was considered a busy and sometimes challenging environment for the majority of staff interviewed. However, staff described a strong ward team spirit and supportive culture, which individuals highlighted helped improve stress-related coping strategies when dealing with unfamiliar situations. Participants acknowledged that their confidence in dealing with this patient group had increased. Staff expressed that this was closely related to the different types of training that they had received (educational and practical) for patients with cognitive impairment. Having a greater understanding of both dementia and person-centred care had helped staff display a more positive attitude towards this group of patients. Themes identified by participants with regard to improving patients’ and relatives’ experiences of care were staff–carer communication; staffing levels and resources; balancing an increased risk of falls against allowing patients to walk around freely; and organisational barriers to change in practice.
The use of Talking Mats increased the total number of patients able to be interviewed from eight to 15, but a substantial minority could not be meaningfully interviewed using either method:
-
eight out of 26 (31%) were interviewed conventionally (mean MMSE 19, range 14–24)
-
seven out of 26 (27%) were interviewed using Talking Mats (mean MMSE 9, range 1–18)
-
11 out of 26 (42%) were not interviewed conventionally or using Talking Mats (mean MMSE 12, range 0–24).
All of the eight patients interviewed using traditional semistructured methods were admitted to the MMHU. Five were female and three were male. Six had a prior diagnosis of dementia and all had cognitive impairment. Five themes emerged:
-
Feelings. Most of the participants reported positive emotions such as feeling content and enjoying the ward environment, but there were also less positive feelings such as boredom and isolation.
-
Memory and confusion. Of the eight patients interviewed, four appeared to be aware that they were, or had been, confused while in hospital.
-
Activity. Patients noted a lack of activity on the ward, with many references to them sitting by their bed. Only one patient reported that this was because of ill health. Although some patients felt frustrated by this, others did not seem to mind. Of the patients who talked about the activities room on the MMHU, two noted enjoying their time there and the organised activities conducted within. However, some patients spoke of not wanting to go to the activities room or take part in activities, preferring instead to remain inactive or wait for their family to come to see them instead.
-
Communication. This included lack of communication, communication regarding health and availability of staff to talk to.
-
Staff. Feelings towards staff were almost all positive. Care and kindness shown by staff was repeatedly mentioned. All of the patients reported being able to talk to staff when seeking assistance; however, all of the patients noted a lack of communication as well, with staff not communicating on issues such as health, discharge and patients’ likes and dislikes. Three patients talked to family members while they were in hospital, of whom two reported that their family member acted as a liaison between the patient and staff.
All Talking Mat interviews were conducted on the hospital wards (three on the MMHU and three on the standard care ward) and most took place at the bedside because of patients’ levels of illness, their mobility or the lack of an alternative location. Six of the seven participants who were interviewed using a Talking Mat had a previous diagnosis of dementia and most (n = 5) experienced delirium on admission. Functional abilities were poor and all participants had been acutely unwell, with comorbidities. The mean interview duration was 21 minutes (range 13–35 minutes). Five interviews were cut short because of increased confusion, cognitive decline or the effects of physical illness. Participants’ ability to express feelings about different aspects of the ward varied; with between four and 21 questions being answered. However, all participants were able to provide some information about their experiences. Participants on the MMHU placed 13 cards in the positive response (Thumbs Up) category whereas those receiving standard care placed 11 cards in the positive response category. In total, 22 cards were placed in the middle, neutral, section. Participants on the standard care ward placed more cards in the negative response (Thumbs Down) category (n = 10) than those on the MMHU (n = 5).
Five themes emerged from the data about the Talking Mat method of communication:
-
Communication. Participants understood how to use Talking Mats, which enabled them to express their feelings about aspects of care on the ward. The ability to carry out the interview varied between participants.
-
The person as an individual. Information from the Talking Mats coupled with dialogue expressed during the process provided information about individual differences and built a picture of who the participants were and their experiences of hospital care.
-
Cognitive impairment. Attention fluctuated during the interviews with all participants becoming distracted by something, for example the pictures on the Talking Mat or changing topics. ‘Confusion’ increased for most during the interview and was displayed in different ways, such as expressing delusions or becoming agitated.
-
Physical illness. Talking Mats served as a short-term, helpful distracter for those experiencing pain, although symptoms quickly returned and two interviews were terminated as a result.
-
Environment. Noise and lack of privacy was as much an issue as with standard interview techniques, but because of the frailty of the participants it was even harder to take them off the ward to be interviewed.
In the unadjusted complete-case cost-effectiveness analysis undertaken in the subgroup of 209 (MMHU n = 109) participants with complete QALY and resource use data, the mean total cost was non-significantly lower (–£584.8, 95% CI –£2375.9 to £1085.8) and the QALY gain was non-significantly higher (0.007, 95% CI –0.013 to 0.027) in the MMHU group than in the standard care group, giving a 60% probability of MMHU care being dominant and a 85% probability of the ICER being ≤ £30,000 per QALY. In an adjusted analysis, the mean total cost for the MMHU group was significantly lower than that for the standard care group (–£486.7, 95% CI –£854.6 to –£126.5) with no significant QALY gain (0.0003, 95% CI –0.0108 to 0.0117), giving a 53% probability of MMHU care being dominant and a 95% probability of the ICER being ≤ £30,000 per QALY.
In the cost analysis undertaken in the subgroup of 466 (MMHU n = 241) patients with complete inpatient and primary care data, the mean cost for MMHU patients was non-significantly lower than that for standard care patients for inpatient care (–£151.6, 95% CI –£1066.6 to £750.9), social care (–£304.0, 95% CI –£805.1 to £238.3), and all remaining services except for three services for which the costs were non-significantly higher for MMHU patients: primary care (£23.2, 95% CI –£17.5 to £66.0), ambulance service use (£3.5, 95% CI –£18.3 to £25.7) and mental health care (£9.8, 95% CI –£37.8 to £61.2). The cost of care was non-significantly lower in the MMHU arm (–£521.9, 95% CI –£1523.0 to £547.7) and the incremental total cost (taking into account the extra costs of the MMHU) was also non-significantly lower (–£334.2, 95% CI –£1389.7 to £719.2).
Discussion
Specialist care for people with delirium and dementia provided by the MMHU improved the patient experience and carer satisfaction although there were no convincing benefits in terms of health status or service use outcomes. The qualitative findings confirmed and elaborated on these findings and indicated causal links between the interventions that included the MMHU and the observed outcomes. The economic study showed a high probability that the MMHU was cost-effective, in part because of lower inpatient (despite the cost of the intervention itself) and social care costs.
These findings are valuable because patient experience and carer satisfaction may be more appropriate measures of success for frail older people approaching the end of their life, as these patients were. Not only do these findings support the notion of further development and testing of MMHUs, they also illustrate the broad principle that investment in, and delivery of, best practice in dementia care leads to demonstrable changes in patient experience. These results provide the justification for further investment in and evaluation of such units. They also show that investment in and delivery of best dementia care can be both cost-saving and cost-effective.
The qualitative findings from carers enrich the understanding of what carers identify as important domains of good general hospital care in this patient group. An important finding is that the amount of communication required by family carers cannot be underestimated. We found the extent of this surprising and beyond what we had planned for. New approaches to engagement with family carers are required, including the assessment of expectations and the giving and receiving of information. Meeting this need will require major changes to the way that acute wards operate and the re-prioritisation of staff time to enable this activity. Facilitating more hands-on care by family members may provide the quid pro quo to enable it within resource-constrained health-care systems. Organisational development methodologies should be explored in future attempts to implement such changes, alongside more staff-directed education and training interventions and incorporation in pre-registration education. Nurse leaders will play an important role in creating the conditions for delivering, and fostering a culture that rewards and raises the demand for, relationship-centred care for this population.
The findings of the staff interviews contrasted markedly with those observed only a few years earlier, as reported in the Better Mental Health study, with staff ill-prepared to look after patients with cognitive impairment. 9 Better-educated staff and the development of dementia-friendly environments will help nurture staff confidence and morale in working with this client group as well as improve patients’/family members’ experiences of and satisfaction with care. Developing a unique skill mix in this setting (the introduction of mental health specialist staff) and encouraging multidisciplinary working alongside increased education and training has allowed MMHU staff to share and deliver best practice. Staff who lack the knowledge, skills and confidence to care for dementia patients are unlikely to be able to undertake and support patients’ ongoing personal care needs, especially when faced with challenging or unfamiliar patient behaviour such as aggression, wandering and disrobing or refusing/not wanting to eat, particularly if communication is difficult. The major outstanding need, despite the efforts of the intervention to encourage staff to be proactive towards carers on the MMHU, is communication. Although it can be argued that staff on the MMHU had made positive changes towards the delivery of person-centred care, less success was achieved in the delivery of relationship-centred care. Relationships between staff (especially nurses) and relatives still need reforming, with more partnership and collaboration. Collaboration, in terms of shared decision-making and exchange of knowledge and information, has been shown to be particularly important for relatives’ satisfaction with hospital care of the elderly. 87 Organisational factors identified previously as impeding the development of effective nurse–family collaboration include a task-focused culture and workload; shift patterns and length; lack of training; education given and poor supervision; resistance to change; and bureaucratic issues. 9,88
The five themes arising from the patient interviews (feelings, memory and confusion, activity, communication and staff) illustrate the major domains of hospital experience as contemporaneously elicited. Given that these are the main themes of patient experience, they are the main domains that staff and providers should focus on to improve experience and hence satisfaction. The first two point to the need for staff to acknowledge and understand the feelings experienced by patients, including their own awareness of their cognitive problems, which contrasts with a more task-focused style of care that focuses on personal ADL. The third theme validates the importance of the focus on promoting activity as part of the development of the MMHU. Although the importance of communication and staff kindness is hardly a new observation, this study reminds us (if such reminding should be needed) that people with cognitive impairment remain sensitive to these issues – there is no sense that once one becomes confused one becomes so unaware of surroundings that communication and other behaviours are not appreciated.
The use of Talking Mats increased from only one-third to just over half the proportion of participants who the researchers felt would be able to undergo a meaningful interview. This tool appeared to enable people with more severe cognitive impairment to be interviewed. Thus, although this tool does not provide a solution for eliciting information from all patients in hospital, it appears to offer an improvement over conventional interview approaches.
Chapter 4 The care home workstream
Aim
The overall aim of this workstream was to describe health care for people in care homes in sufficient detail that logical, evidence-based interventions could be proposed and current interventions could be properly evaluated.
Phases
The workstream had three elements. The first was a literature review. The second element was a study to describe the nature of current care home residents and hence estimate their health needs. The third element was an interview study to explain the delivery of health care in care homes.
Literature review of care home randomised controlled trials
Introduction
The first element of the care home workstream was to undertake a systematic review of the RCT evidence for interventions specifically for the residents of care homes. This had not been done previously and it was important to scope the extent of the evidence base to guide the eventual implementation of evidence-based interventions. This work has been published. 89,90
Methods
Appendix 31 shows the databases searched and the search strategy.
Results
In total, 3226 abstracts were identified and 291 articles were reviewed in full. Most were recent (median age 6 years) and from the USA. A wide range of targets and interventions was identified. Targets included behaviour (n = 44 studies), prescribing (n = 20), malnutrition (n = 20), influenza (n = 19), quality of life (n = 18), depression (n = 17), mobility (n = 13), oral health (n = 13), falls (n = 12), quality of care (n = 12) and urinary incontinence (n = 12). Interventions were often mixed and included pharmacological, educational, physical therapeutic and managerial interventions. Appendix 32 shows selected data extraction (results) tables.
Discussion
This study was the first to collate data from all RCTs conducted in care homes and represents an important resource for those providing and commissioning health care for this sector. The evidence base is developing rapidly. Several areas – influenza, falls and mobility – are appropriate for systematic review. For other topics researchers need to focus on outcome measures that can be compared and collated.
A cohort study of the health status and outcomes of care home residents
Introduction
The second element of the care home workstream was to survey residents of care homes. UK care home residents are understood to be poorly served by existing health-care arrangements. The aim of this study was to describe the health, functional status and health-care resource use of a representative cohort of UK care home residents to help estimate their needs and hence the health-care services required to meet these needs. This study has been published. 90,91 Appendix 33 shows the baseline data collection form, Appendix 34 the baseline interview form, Appendix 35 the follow-up data collection form and Appendix 36 the follow-up interview form.
Methods
An 180-day longitudinal cohort study was undertaken of 227 residents across 11 UK care homes (five nursing homes and six residential care homes), selected to be representative of nursing/residential status and dementia registration. 91 The Barthel index measuring dependency in personal ADL,36 MMSE,79 NPI,78 Mini Nutritional Assessment,92 EQ-5D76, GHQ-1237, diagnoses and medications were recorded at baseline and the Barthel index, NPI, GHQ-12 and EQ-5D were recorded at follow-up after 180 days.
A costing study was also performed. Data were collected retrospectively for 180 days from recruitment using information extracted from various health-care services including general practice (including direct and indirect patient-centred events, medication and wound management), hospital care (including inpatient stays, day cases, outpatient care and critical care) and wider secondary care (including the ambulance service, intermediate care and mental health care). The perspective for applying costs was that of the NHS. The differences in mean costs between care home types (nursing/residential) were assessed using Student’s t-tests with non-parametric bootstrapping employed to manage the non-normality of cost data.
Results
In total, 227 out of 323 (70%) residents were recruited. The median (IQR) Barthel index score was 9/20 (2.5–15.5), the median (IQR) MMSE score was 13/30 (4–22) and the median (IQR) number of medications was eight (5.5–10.5). The mean (standard deviation) number of diagnoses per resident was 6.2 (4). In total, 30% of the residents were malnourished and 66% had evidence of behavioural disturbance. Thirty-seven (16%) participants died and one left the area without forwarding details before the follow-up at 180 days. Sixteen of the participants who died were from residential care homes and 21 were from from nursing homes. The death rate varied significantly between individual homes (range 0–32% of respondents; χ2 test p < 0.05).
The median (IQR) Barthel index score fell from 5 (1.5–8.5) at baseline to 3 (0–7) at follow-up for nursing home residents (Wilcoxon signed-rank test p < 0.01) but did not change significantly for residential care home residents or the cohort as a whole over time. There was greater behavioural disturbance in all groups at follow-up, with the median (IQR) NPI score increasing from 3 (0–10.5) to 5 (0–13), from 2 (0–6.5) to 4 (0–10) and from 6 (0–19.5) to 8 (1.5–14.5) for the whole cohort, residential care home residents and nursing home residents respectively (Wilcoxon signed-rank test p < 0.01 for all).
Secondary care resource use data were collected for all 227 participants (103 residential care home and 124 nursing home residents) over the 180 days. Primary care resource use and medication use were collected for a subset of 209 participants (90 residential care home and 119 nursing home residents) whose GPs allowed access. Out of a total of 227 participants 110 (48.5%) used secondary care services and out of a total of 209 participants 181 (86.6%) used either primary or secondary care services. Over the 180-day follow-up period there were 41 hospital admissions that resulted in an overnight stay, comprising 503 inpatient-days, and a further 11 day-case admissions. There were 763 general practice contacts, comprising 264 in-practice consultations and 499 consultations at home.
In the full cohort the mean (95% CI, median, range) total hospital and secondary care costs were significantly higher for patients residing in nursing homes than for those residing in residential care homes (£1254, £855 to £1858, £113, £0–17132 vs. £535, £331 to £922, £0, £0–9781; p < 0.02) and when accounting for all health-care services in the subset the mean (95% CI, median, range) cost was significantly higher for nursing home residents than for residential care home residents (£1669, £1277 to £2316, £556, £0–17566 vs. £945, £691 to £1410, £383, range £0–10399; p < 0.03).
In the full cohort, during the trial period there were 52 inpatient or day-case events across 43 participants; 96% (50/52) of these events were classified as an emergency and 82% (41/50) of the emergency events resulted in an inpatient stay. In the subset, hospital care contributed 59.4% of costs. Other sectors contributing costs were primary care (30.9%), the ambulance service (0.6%) and mental health care (9.0%); no participants used intermediate or critical care. The 10% most costly participants accounted for 51.5% of the overall costs.
Discussion
This cohort study demonstrated highly prevalent dependency, cognitive impairment, mild frequent behavioural symptoms, multimorbidity, polypharmacy and frequent use of NHS resources across both residential care home and nursing care settings. Effective care for such a cohort requires broad expertise from multiple disciplines.
The key cost-generating events external to the care home were hospital inpatient stays, and the results of this study suggest that care home-residing older people mainly require hospital care in emergency situations and that nursing home residents consume external health-care services at a significantly higher cost than residential care home residents. In general, however, the study participants were associated with lower levels of health-care costs from health-care services external to the care home than in the other two studies within the Medical Crises in Older People programme (see Chapter 2, Patient-based health and social care costs of older adults discharged from acute medical units, and Chapter 3, The Better Mental Health cohort study). These findings suggest that the most cost-effective model to provide health care for care home residents would most likely need to focus on preventing admissions to hospital and that it might be more cost-effective if targeted at nursing home residents.
An interview study of the actors involved in the health care of care home residents
There is concern about the quality of health care for care home residents, but little evidence to explain or guide the rational development of improvements to services. We therefore aimed to explain the delivery of health care to residents living in care homes in the UK and hence enable rational service development. This study has been published elsewhere. 93
Methods
This study was a qualitative interview study using a grounded theory approach. It was set in six UK care homes and included primary care professionals serving the homes. In total, there were 32 participants: seven care home managers, two care home nurses, nine care home assistants, six GPs, three dementia outreach nurses, two district nurses, two advanced nurse practitioners and one occupational therapist. Appendix 37 provides further details of the methods.
Results
Five themes were identified:
-
Complex health needs and the unstable and unpredictable nature of residents’ illness trajectories (illustrative quote from GP informant: ‘Because one day, they can be fine, the next day, they stop eating, and then they could linger for months, or the next day, they could die’).
-
A mismatch between health-care requirements and GP time.
-
Reactive or anticipatory health care (illustrative quote from GP informant: ‘In the past, we used to try and do anticipatory things like a little ward round once a week. And I think we just found that it wasn’t making a lot of difference to just letting the staff call us when they needed help. So we were putting more hours in without seeing very much for it’).
-
A dissonance in health-care knowledge and ethos (illustrative quote from GP informant: ‘The average general practitioner isn’t experienced enough . . . and you need a, basically another specialism going in and I think that would deliver better care to the patient’).
-
Tensions in the responsibility for the health care of residents (illustrative quote from a district nurse informant: ‘As a district nurse is a bit of an issue, because there are times when we have to go into a residential home to administer insulin when there are nurses there, trained nurses, and they will not administer the insulin because they’re saying we’re not insured, so that piles even more pressure, even more visits onto the district nurses’).
Care home managers and staff were pivotal to health-care delivery for residents despite their perceived role in social care provision. Formal health care for residents was primarily provided by one or more GPs, often organised to provide a reactive service that did not meet residents’ complex needs. Deficiencies were identified in training required to meet residents’ needs for both care home staff and GPs. Misunderstandings, ambiguities and boundaries around roles and responsibilities of health and social care staff limited the development of constructive relationships.
Discussion
The health care of care home residents was found to be difficult because the residents’ needs were complex and unpredictable. Neither GPs nor care home staff had enough time to meet these needs and many lacked the prerequisite skills and training, irrespective of the model of organisation employed. Anticipatory care was generally held to be preferable to reactive care, but attempts to structure care to make it more anticipatory were dependent on effective relationships between GPs and care home staff and their ability to establish common goals. Roles and responsibilities for many aspects of health care were not made explicit and this risked poor outcomes for residents. These findings help explain the concerns noted about the quality of health care for care home residents in the UK. Missed opportunities for partnership working were described, which should give rise to more rational approaches to service development.
Chapter 5 Synthesis
Introduction
As described in Chapter 1, this synthesis aims to bring together the findings from all three workstreams with the objective of identifying key factors that are likely to influence health-care improvement. To do this we describe the results of the research programme with reference to an established framework for understanding health care, adapted from Brown and Lilford13 (Figure 1), which applied the input–process–outcome chain (described first by Donabedian14) highlighting three essential measurement points: ‘proximal end points’ to describe content, ‘at the level’ measures to assess fidelity and ‘distal end points’ to assess effect. Each of these points may use qualitative or quantitative data. 13
FIGURE 1.
Causal chain for health care. Reproduced from ‘Evaluating service delivery interventions to enhance patient safety’. Celia Brown, Richard Lilford. BMJ 337, p. 162, 2008, with permission from BMJ Publishing Group Ltd.

Figure 2 shows how the model shown in Figure 1 was applied to this programme.
FIGURE 2.
How the model in Figure 1 was applied to this programme. Adapted from ‘Evaluating service delivery interventions to enhance patient safety’. Celia Brown, Richard Lilford. BMJ 337, p. 162, 2008, with permission from BMJ Publishing Group Ltd. DRS-98-R, Delerium Rating Scale–Revised 98; MCOP, Medical Crises in Older People.
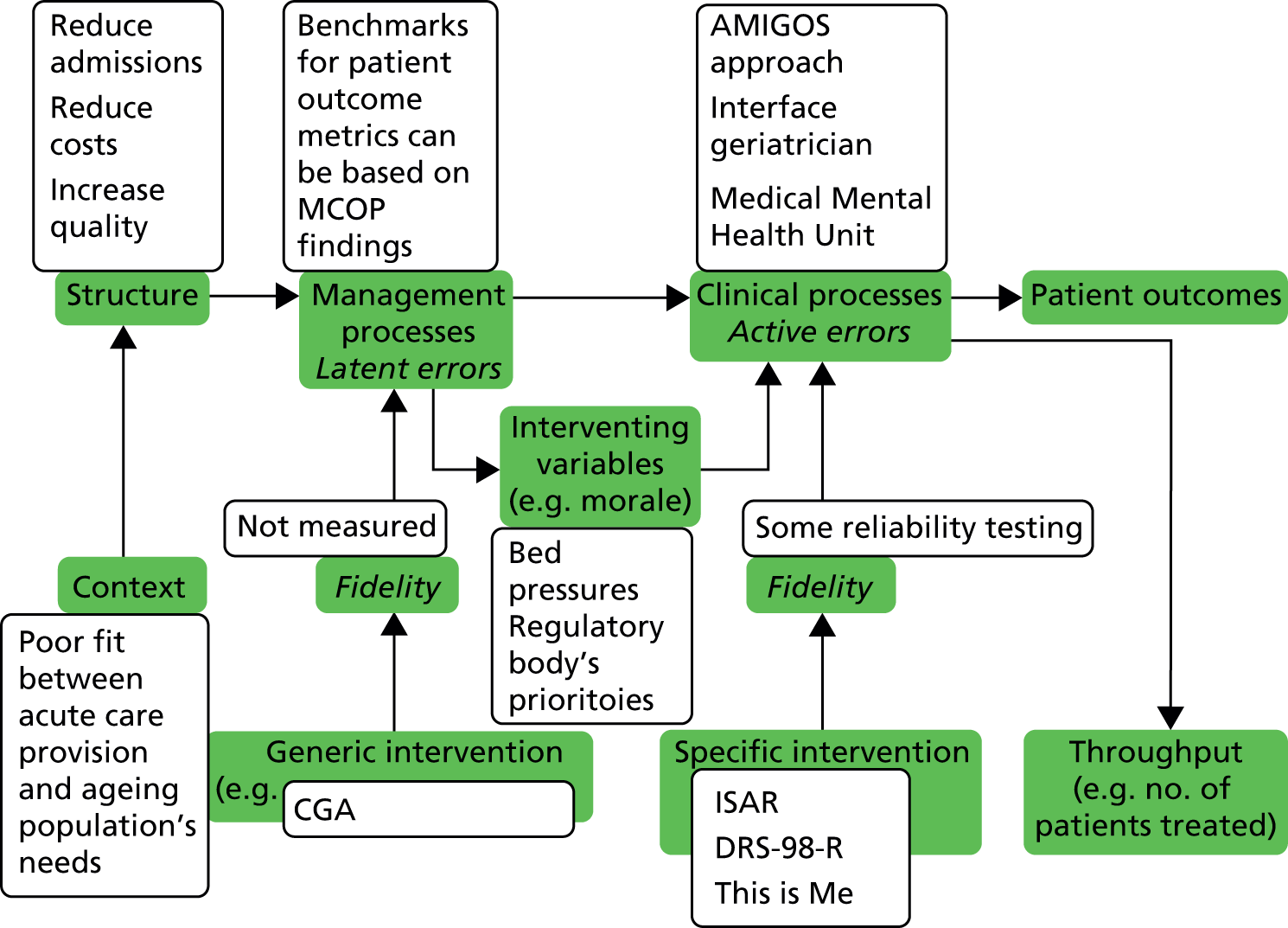
Subsequent sections of this synthesis discuss the programme’s findings under headings taken from Figure 1: context, structure, generic interventions, specific management processes, clinical processes, intervening variables and fidelity.
Context
Although the programme of work summarised here was developed within a health service perspective, demographic, societal and politicoeconomic factors were important contextual considerations. Growing numbers of people surviving into a longer old age is a cause for celebration, but these people also have increasing expectations of health services as health care and technology afford a growing range of interventions to treat disease and ameliorate age-related disability. The investigators’ prior expectations were of a mismatch between the needs of older people and the services provided for them: increasing numbers of older people were presenting to AMUs but these services did not appear to be tailored to their needs; people with cognitive impairment seemed to be very common in hospital but, again, there seemed to be little evidence that hospitals had taken this on board; care home residents seemed to have complex health problems without a service in place designed to deal with such complexity. This mismatch was confirmed by findings from the programme across all workstreams:
-
In the AMU workstream the testimonies of people discharged from AMUs in the AMIGOS study (see Chapter 2) described considerable ongoing and unaddressed health needs and the findings of the AMOS study (see Chapter 2) showed that such people had a measurable decline in their health over time.
-
The MMHU workstream (see Chapter 3) was justified and informed by the findings of our parallel Service Delivery and Organisation (SDO) programme-funded Better Mental Health study,9 which identified two root causes of care failures: (1) inadequate staff training and support in the management of older patients with cognitive impairment and (2) organisational inflexibility, which imposed unrealistic targets on those caring for such patients and detracted from their time and ability to provide appropriate care.
-
The interview study of GPs and care home staff (see Chapter 4) again confirmed a mismatch between need and provision.
Structure
In this analysis, ‘structure’ refers to strategic policy objectives and systems influencing the delivery of health care. In 2010 health expenditure consumed nearly 10% of UK gross domestic product. Despite a consistent trend in England towards reducing state involvement in the provision of health care since 1979, the state continues to fund 80% of health care and the NHS continues to provide most of it, with central commissioning, policy-making and research funding. Preventing the high costs of hospital admissions, through the development of alternatives to inpatient treatment (such as hospital-at-home schemes, day surgery), was a major objective. When admission is inevitable, efforts have focused on minimising the length of stay and increasing bed occupancy rates, to maximise throughput and gain efficiencies of scale. However, this has led to a pressured system:
-
In the AMU workstream the rising number of people attending emergency units was a matter of concern because it was associated with a rising number of admissions. Another central policy factor affecting this workstream was the requirement for patients to spend no longer than 4 hours in an emergency department, with financial penalties for hospital trusts if this target was not met. To ensure that those patients whose assessment and initial treatment would take > 4 hours did not spend > 4 hours in the emergency department, many patients were moved into AMUs and when these became full (as they usually were) there was intense pressure to discharge patients from AMUs. This led, potentially, to under-assessment and this was one of the deficits that the intervention in the AMIGOS trial aimed to overcome. It also made it difficult to carry out the AMIGOS study because patients were often sent home before they could be recruited.
-
In the MMHU workstream the pressure was most obvious in terms of the constant ‘bed crisis’ – when bed usage is close to 100% the system is unable to accommodate natural fluctuations in demand and so there is inevitably a shortage of beds. Such a shortage of beds can threaten the ability to run a trial of a bed-based unit. It can also threaten the running of such units: if bed crises are severe, patients who should not or who do not need to go to a ward may be sent there anyway if there is nowhere else. Confused patients might therefore be sent to units with little or no expertise in their management and patients without cognitive impairment might find themselves on wards mainly for such people and may find this unacceptable. Patients may also be discharged prematurely; carers thought that 22% of standard care patients were discharged too soon and 30% said that they were unprepared for discharge. The extent of this pressure was illustrated by the considerable accommodation required to the TEAM trial design (see Chapter 3), including Zelen-type randomisation (in which random allocation is carried out before recruitment rather than the more usual recruitment before allocation), the complex algorithm to deal with varying numbers of beds and the requirement for 24/7 senior investigator cover to ensure compliance with the algorithm.
Another key strategic and policy objective was care quality. Respect for the individual, dignity in care and person-centred care are stated to be of paramount importance in the planning, delivery and evaluation of health-care interventions. Nevertheless, the evidence suggests that the reality falls short of aspirations. In 2012 the Care Quality Commission review of services found that hospitals were ‘struggling in areas such as dignity and respect, nutrition, care and welfare’94 and the Patients Association published 13 cases of care failures. 95 The unsatisfactory situation was acknowledged by the Prime Minister’s prioritisation of ‘improving care standards’ in 2013. 94 While this programme was being undertaken, in 2011 a collaboration of health-care groups led by the British Geriatrics Society went so far as to describe existing arrangements for health care in care homes as ‘a betrayal of older people, an infringement of their human rights and unacceptable in a civilised society’. 96 We were able to offer some of the findings of this programme for the British Geriatrics Society report.
Generic interventions
Achieving improvements in health care calls for effective implementation, and management is a fundamental part of this process. Figure 1 distinguishes generic interventions from specific, local managerial and professional interventions, which will be discussed separately later. In practical terms, generic interventions are taken to be those that fall into the remit of a chief executive officer overseeing multiple services within one trust. It should be noted that recent reforms, which underpin the formation of NHS foundation trusts, free these health-care providers to determine exactly how desired outcomes are to be achieved, rather than specifying the structures and staffing to be used to do this. In addition, the report of the Francis Inquiry into care failures in mid-Staffordshire,97 which came to light during the period of this programme, criticised attention to targets to the neglect of care quality. Nonetheless, the application of measurable targets and standards, often summarised in ‘dashboards’, is a fundamental part of NHS management and accountability.
The centrally devised NHS Outcomes Framework for 2012–1398 (see Appendix 38) has 12 overarching indicators and a number of these are relevant to this programme. For example, providers were to be monitored in relation to ‘emergency admissions for acute conditions that should not usually require hospital admission’ (3a); ‘emergency readmissions within 30 days of discharge from hospital’ (3b); and ‘patient experience of hospital care’ (4b). Many of the areas highlighted for improvement in the Outcomes Framework pertained to the population of older people, including, for instance, helping older people to recover their independence after illness or injury (improvement 3.6) measured in terms of the proportion of older people (aged ≥ 65 years) who were (1) still at home 90 days after discharge into rehabilitation and (2) offered rehabilitation following discharge from an acute hospital or a community hospital. It is interesting that metrics for none of these quality indicators were known or available to the research staff or the clinicians who aided them in the research. This may partly reflect the lower priority of quality targets compared with other targets, given that such data are difficult to collect.
Findings from the Medical Crises in Older People programme are directly applicable at this level and could be used to support generic interventions through targets for commissioners and providers, for instance in relation to readmission and rehabilitation of specific groups of older people:
-
The AMOS study (see Chapter 2) showed that, among patients aged > 70 years attending AMUs and discharged within 72 hours, 33% were readmitted within 90 days of discharge from hospital and 5% died within this period. These figures provide benchmarks against which to strive for improved outcomes. The AMOS study also identified the costs incurred by these patients and such information can be used to examine where cost efficiencies might be achieved and estimate the limits with regard to how much might be ‘invested to save’.
-
The Better Mental Health cohort study (see Chapter 3) looked at patients aged > 70 years screening positive for mental health problems with admission for > 48 hours and followed this group for 180 days. During this time, 42% of this more dependent population were readmitted to hospital, 31% died and 24% were admitted to a new permanent care placement. In this group, older age, higher comorbidity and poorer nutrition were associated with higher mortality. These results indicate areas to be considered for targeted investment to improve outcomes. The cost results (see Chapter 2) showed this group to be the most resource intensive of those studied in this programme: the mean cost for Better Mental Health cohort patients was £9842 over 6 months compared with a mean cost of £1926 over 3 months in the AMOS cohort and mean costs of £1669 and £945 for nursing and residential care home patients over 6 months, respectively, in the care home cohort study study. Such information can be used to consider where the greatest scope for cost efficiencies lies.
-
The care home cohort study (see Chapter 4) showed how severely disabled, and hence potentially how needy, care home residents were: the median Barthel index score was 9, indicating severe dependency, the median MMSE score was 13, indicating severe cognitive impairment, the median number of medications was eight, indicating that polypharmacy was ubiquitous, the mean number of diagnoses per resident was 6.2, explaining the polypharmacy and indicating extensive multimorbidity, 30% were malnourished and 66% had evidence of behavioural disturbance. These data can be used to determine the services likely to meet the needs of this group of people. The cohort study costing data showed that the health service costs incurred by these residents were relatively low compared with those incurred by other groups. Although they were relatively high users of primary care, the oft-held assumption that these residents are responsible for a vast number of unnecessary hospital admissions was not supported. Thus, strategies focusing on avoiding such admissions may have less impact on overall health-care costs and hospital resource use than might be anticipated.
-
The TEAM study preliminary economic results (see Chapter 3) showed that a MMHU was likely to be cost-effective and that the costs of setting one up could be offset by savings across the health and social care system. Further evaluation of MMHUs is required before more precise and widely generalisable statements can be made about them but there is sufficient justification here for general hospitals to consider investment in MMHUs as a means of improving the quality of care for people with cognitive impairment at a cost that is not prohibitively expensive. The findings are supportive of efforts to invest in dementia care on the basis that this will produce better patient care at an acceptable cost or with cost-savings.
Family carers figure in the NHS Outcomes Framework and health-related quality of life for carers is to be monitored. For acute providers, attention to carers’ needs is seldom a priority; more commonly the focus has been on the staff–patient relationship, with families as rather unwelcome visitors, rather than on the specific and health-related needs associated with the caring role. The findings reported in Chapter 3 of this report and in Bradshaw et al. 62 throw light on the nature of carers of people with cognitive impairment and show how carer stress is closely associated with the severity of patients’ cognitive disability, indicating how to target resources at carers, with the implicit objective of increasing their resilience to care. Our SDO programme-funded Better Mental Health report9 also articulated the perspectives of carers, whose experiences are not appreciated by hospital systems.
Specific management processes
Specific management processes are taken here to be within the control of a ward manager or clinical champion, for example. This level of management is likely to have a direct impact on the experiences of individual staff, patients and their relatives. For instance, in the AMIGOS post-discharge interview study, participants described their experiences of being on an AMU, revealing aspects of dissatisfaction that would be under the control of senior clinical staff. Examples included problems with communication and a lack of focus on patients’ symptoms, which were often left unresolved once a major medical emergency had been ruled out on the basis that they could be dealt with elsewhere. Many of the inferences drawn above about the relevance of the Medical Crises in Older People programme findings to generic interventions can also be applied to managers of a clinical setting: awareness of carers’ needs and fostering positive interactions between cognitively impaired patients and staff are examples of standards that could be adopted by clinical leaders. However, unless there is organisational commitment – generic support – for such improvements, it is unlikely that they will be promoted or resourced and so they may prove unsustainable.
Clinical processes
The AMIGOS intervention, the use of interface geriatricians and the MMHU are examples of innovation in complex clinical processes.
The AMIGOS study showed no benefit of the interface geriatrician intervention and so does not provide a model of care that requires direct implementation. Nevertheless, it is interesting to note that the notion behind this intervention gained considerable traction in the local hospital during the course of the study. At the outset the intervention was almost viewed as a speculative research notion but during the conduct of the study the potential benefit of it became better appreciated. This led to the development (outside of this programme or any evaluative framework) of an AMU geriatrician service in the Nottingham University Hospitals NHS Trust and a frail older persons unit in the University Hospitals of Leicester NHS Trust. These developments coincided with increased national appreciation of the issue of the poor fit between patient need and service provision, which resulted in the publication of the Royal College of Physicians’ Silver Book for acute care,99 which emphasises the need for CGA for frail older people. Members of the programme made significant contributions to the Royal College of Physicians publication.
The MMHU innovation included joint medical and mental health professional staffing; enhanced staff training in delirium, dementia and person-centred dementia care; the provision of organised purposeful activity; environmental modifications to meet the needs of those with cognitive impairment; delirium prevention; and a proactive and inclusive approach to family carers. The patient experience of hospital care for older people was observed directly in the MMHU and on comparator wards, with more positive staff–patient interactions seen in the enhanced ward environment of the MMHU. This may be interpreted as an endorsement of the MMHU approach, in which case the findings offer a model for improving the experience in hospital of frail patients with complex needs that could be adopted if funding and support were available. Indirectly, it indicates that there is a link between the components of the innovation and improved patient and carer experience, which could be used to justify such efforts even if directed across the hospital rather than solely at the development of a single ward.
Clinical processes can be taken to include the use of technology as well as clinical skills, and so the application of structured questionnaires and assessments or care protocols falls into this category of interventions. Although the category embraces a huge number of processes that could conceivably be used to care for older people, we report on the usefulness of two specific assessment tools:
-
The ISAR tool. 7 Although this tool emerged from a literature review28 as the most promising tool to identify people at high risk of poor outcome, when tested in two UK hospitals it did not accurately predict adverse health outcomes such as death, institutionalisation, hospital readmission, increased dependency in ADL, reduced mental well-being or quality of life, or high health and social service costs over 90 days. This therefore meant that the ISAR tool was not a suitable tool to be used alone for individual patient management, such as in access to specific frail elderly care pathways. The five frailty-rating scales we tested were also of limited use in risk-stratifying older people being discharged from AMUs and offered no advantage over the ISAR tool in this setting.
-
The Delirium Rating Scale – Revised 98 (DRS-R-98). 100 This tool was tested as a diagnostic and measurement tool for delirium. Although its properties showed it to be valuable in epidemiological research, like the ISAR tool, it was not accurate enough to be used in clinical management, particularly in settings (such as hospitals) where there is comorbid dementia.
These examples point to a broader observation: measurement tools used in (group) research are often not suitable for individual patients.
Intervening variables
In this framework, ‘intervening’ variables mediate or moderate the associations between management processes and clinical processes or act in an unknown way to influence them. Staff morale is one example and the effect of ‘bed crises’ might be another. In fact, pressures of time and resources are ubiquitous intervening variables in health care. We described earlier how the rigorously monitored 4-hour wait targets in emergency departments meant that busy emergency departments needed to move patients who might breach that target to another setting such as an AMU and that when AMUs became full this created a pressure on staff to discharge or admit patients, possibly at the expense of good communication or comprehensive care.
Although it may not be possible to control for intervening variables, identifying them and bringing them to managers’ attention may go some way towards enabling services to adapt to them or take avoiding action; here, the Medical Crises in Older People programme contributes a number of insights. In our SDO programme-funded Better Mental Health study,9 bed pressures were judged by staff to have conflicted with good care. In the care home interview study (see Chapter 4), apprehension about the regulatory framework, perceived to be threatening and critical, loomed large: evidence emerged that care home staff sometimes avoided health-care tasks that they might have thought appropriate because of fear of criticism if they carried them out.
Fidelity
An often-overlooked aspect of applied research is the assurance of the intervention’s constancy over time and across different study sites or practitioners, called ‘fidelity’. 101 Figure 1 reminds us that, in both management processes and in clinical processes, the reality may fall short of the intention. Therefore, in appraising the outcomes from such processes described we should seek independent verification that they were implemented correctly and competently. In evaluating clinical interventions in particular, trial results might be negative because the intervention was not delivered optimally to the right group and not because the intervention is inherently ineffective. We debate later whether or not the negative findings of both the AMIGOS study and the TEAM study reflect this issue. Measuring the fidelity of the implementation of the MMHU was difficult because no previous model existed against which to compare it. However, patient activity and the quality of person-centred care and staff–patient interactions were judged in the non-participant observer (Dementia Care Mapping) study; in the audit of assessments; in intervention, communication and planning recorded in case notes; and indirectly through the staff, patient and carer interviews.
Summary
This synthesis draws attention to the contextual factors that affect the conduct of research and the potential implementation of the findings. It illustrates that many of the findings, across the workstreams, have potential value at several levels and hence could be of use to policy-makers, commissioners, providers and clinicians. This calls for effective knowledge transfer to these potential users of the research, many of whom are unlikely to read the peer-reviewed publications arising directly from the programme. We describe our approach to this in the impact section of the following chapter.
Chapter 6 Concluding observations
This final chapter reflects on a number of research elements that were part of the overall programme. In this programme applied research was conducted in the NHS but also in care homes, and we reflect on the issues involved. Measurement of health status is central to any quantitative health research and we reflect on the difficulties of doing so in frail older people. Health economics findings are particularly valuable for the implementation of the findings of applied health research and we report on the issues involved from the experience of this programme. Patient and public involvement (PPI) in research has come to be expected in applied health research and we reflect on how this can be achieved when performing research on frail older people. All health research requires close scrutiny with regard to ethical considerations and we report on the particular challenges of the conduct of research in frail older people. It is clearer now than ever that there must be specific knowledge mobilisation processes to ensure that research findings are transferred to those who might make use of them and so we discuss the nature of, and our early experience of, maximising impact. Finally, we conclude by summarising the key achievements of the programme and reflect on the authors’ current understanding of the nature of CGA and how complex interventions for frail older people can be evaluated.
The conduct of applied research in the NHS and care homes
This section illustrates some of the research context issues experienced in the conduct of this programme and how problems were overcome.
Research preparation and conduct
Hospital settings
The Medical Crises in Older People programme was hosted by a NHS trust (Nottingham University Hospitals NHS Trust). The MMHU studies were conducted in this trust and the AMU studies were conducted in this and another NHS trust hospital, the University Hospitals of Leicester NHS Trust. Although there was an expectation that NHS-funded and -hosted research would have simple, implicit and explicit access to the appropriate settings, in practice, research is virtually impossible without the approval of the staff who are required to co-operate and facilitate it. To enhance the prospects of conducting the hospital-based research smoothly, it was important to ensure that the ward staff understood the remit of, justification for, nature of and procedures for each research study and how they would impact on the running of their clinical areas. Introductory discussions and presentations were held with the executives, clinical directors, doctors, matrons, ward managers and sisters, explaining the studies and giving staff the opportunity to discuss the research procedures. Posters and handouts were also made for the ward staff, giving specific details about the procedure for each study. All of these actions were taken before requesting ethics committee and NHS permission, as they helped rehearse in minute detail the precise procedures and requirements of all clinical and research staff. This was part of the ‘set-up’ work. It illustrates the resources required to prepare for ethical and governance permissions and the difficulty of doing so before a study is funded.
Staff on the AMU were extremely busy, potentially to the detriment of patient experience as the AMIGOS post-discharge interviews showed. Thus, it was important for the AMOS and AMIGOS studies, both of which involved the AMU for recruitment, to adjust to this. Research staff had to work with little assistance from ward staff. Acute medical settings can also be quite dramatic places, with ill people, deaths, open grief and suffering, and research staff have to be able to deal with this. Senior clinical researchers were used (from nursing and occupational therapy professional backgrounds), embedded in the wards as far as possible, who could assist the ward staff in minor ways and who would therefore be less likely to be perceived as nuisances and who would be able to deal with the emotional pressure of working in an acute unit. The use of (cheaper) non-clinical researchers may have made it more difficult to conduct these studies in these settings. A possible problem of embedding research staff in this way is that it can cause ambiguity for potential participants in terms of distinguishing between usual care and research. This was dealt with by careful training of the staff regarding this very issue and the explicit requirements of the consenting processes.
The design of the TEAM study was necessarily more highly dependent on the NHS ward staff than that of the AMOS and AMIGOS studies. The design of the study required ward staff on the AMU to identify ‘confused’ older patients being admitted and to randomise them to the MMHU or to standard care. At this point, the patients were not recruited to the TEAM study and this form of allocation was accepted as a clinically justifiable method under clinical governance. However, this gave an opportunity for AMU staff to ignore this process and to attempt to allocate patients directly to wards, based on non-random factors such as perceived ability to benefit or bed availability. The pressure to do so was strong given that the NHS trust had recently embarked on a process of bed closures that had been associated with a constant ‘bed crisis’. If the recruitment process had been regularly bypassed in this way, the pool of potential participants would have been reduced, affecting the potential sample size, and the sample could have been unrepresentative. The way that this was dealt with involved:
-
Mechanisms to bypass randomisation if there were too many empty beds on the MMHU or if there was an ‘extreme’ bed crisis. This was controlled by an algorithm over which staff had no control.
-
Repeat briefings to bed managers to adhere to the algorithm.
-
Repeat calls to senior management.
-
24/7 consultant/senior investigator availability to resolve difficulties (which was required three to four times per week, often in the night).
In addition to the constant availability of senior staff, junior and non-clinical staff required support to ensure that they could withstand the pressures arising from these tensions. This illustrates that there are competing priorities in NHS settings that can hinder the conduct of research and shows the importance of having (expensive) senior clinical researchers as far as possible and clinical academics leading the research who are capable of exerting sufficient influence to protect the research.
Being funded by the NIHR, the research was eligible for ‘NHS research support’. At the time this support referred to research support networks (such as for stroke, mental health and other NHS research priorities), research support specialty topic groups (such as for age and ageing research) and the hospital research and development department. The networks, specialty groups and research and development employed research officers (usually nurses) working in NHS settings to assist in the recruitment of participants to NIHR-funded studies. Thus, this programme of work used research staff who were employed from the research grant itself as well as research support staff employed by the research networks and specialty groups. In this programme, assistance was provided by the Primary Care Research Network, the Mental Health Research Network, the Dementia and Neurological Diseases Research Network (through the Trent Dementia Research Network) and the Age and Ageing Specialty Group. These staff proved to be well skilled (particularly in dealing with mental health issues and older people) and flexible (working evenings or weekends as required) and were embedded with the research grant-funded staff as a single team. They were critical to the successful recruiting of participants to all quantitative studies. None of the studies in this programme struggled to recruit and, although our research team had never had problems recruiting in the past, failure to recruit is a common reason for research failure. The team believe that these experiences underline the value of NHS research support networks.
Care homes
Care homes are the permanent homes for residents and are also businesses that provide income for the owners and staff and they are not part of the NHS; thus, access to these settings for people undertaking NHS research cannot be assumed and participation from staff should not be expected as part of their roles. Care home managers have a duty of care towards their residents and this could result in them declining to facilitate research. There are other issues that may affect this decision, such as concerns that information obtained during the research process could influence care home regulatory processes. To establish a suitable research relationship, the project team began by providing an educational event for all care home staff locally. Only those who responded positively to this were contacted further. A process of getting to know each other then took place. The drawback of this approach is that, although the cohort study of care home residents sampled to ensure a representative sample of home characteristics, this selection was drawn from a subset of homes that may itself not have been typical in that it included only those homes that were keen to engage in research.
During the preparation for the cohort study of care home residents, the research ethics committee required the research team conduct coffee mornings in the homes for residents and families to meet the team. This proved almost completely unproductive as the research team had anticipated, as the residents were hard of hearing and sleepy and many were cognitively impaired. The experience revealed that the widespread misunderstanding of the nature of care home residents may extend to the members of research ethics committees. What was found more useful was to revert to our original plan and use the care home manager to help inform residents and their families of the research and to introduce the research team to individual residents.
The conduct of the cohort study and staff interview study in care homes was relatively simple once access to the residents and staff had been negotiated and agreed with care home managers. Whereas NHS staff can be seen as providing support for research as part of their roles (NHS trusts are paid to support NHS-funded research), this is not the same for care home staff, who work outside the NHS. Care home staff were often paid on an hourly basis (often poorly, with some holding other jobs) and so their involvement in research interviews either had to be at the expense of their employed work or had to be in their own time. These issues are likely to have influenced the selection of staff who were interviewed in favour of more motivated and altruistic staff. Mechanisms to provide incentives, or reduce disincentives, for care homes to participate in research are required, along the lines of specific support networks (e.g. the Enabling Research in Care Homes programme, developed by the Dementia and Neurological Diseases Research Network102).
Measurement in Medical Crises in Older People studies
During the conduct of the Medical Crises in Older People studies, the research team gained considerable expertise in the use of a variety of measures in frail older people.
There is a risk of missing baseline data in studies conducted in NHS settings because of the intense pace of the clinical work, difficulty in locating clinical information and inaccuracy of routinely recorded information and because research activity must not interfere with clinical management, mealtimes and visiting the toilet. Potential participants may have difficulty in concentrating on the researcher in the face of ward noise and distractions (there are few quiet areas in hospital wards or AMUs) and patients obviously wish to interact with their visitors. These concerns were most marked for the research conducted in the AMU. These points emphasise how important it is to keep data collection to a minimum and to have flexible and resourceful research staff.
Losses to follow-up
There is also a risk of missing follow-up data. There are several common reasons for this in studies of frail older people. Obviously, scaled health outcome data at follow-up will be missing in participants who have died over follow-up. Some participants will formally withdraw from the research but many will not formally withdraw but will simply decline to participate in outcome assessment, even if offered a postal questionnaire, telephone support or a home visit. Some will explain that they are too ill to complete questionnaires. This can be seen as implicit withdrawal. Great care has to be taken to strike an acceptable balance between the requirement to gather as much data as possible and placing an undue burden on vulnerable participants. From the experience of the Medical Crises in Older People studies (and previous studies conducted by the research team) the following processes produce the best results:
-
Participants should be informed, when consenting, that outcome assessments will be requested in due course and those who are not willing to undertake these assessments at the outset should not be recruited.
-
With cognitively impaired participants, recruiting a carer is recommended. Such a person can act as an advocate for the participant, can also be an informant and may often be a research subject in their own right. Ethically, including cognitively impaired people in research without a carer remains a challenge.
-
Just before follow-up, hospital records and GPs should be consulted to establish whether or not a participant has died and if any change of address has occurred, such as to a care home.
-
The use of postal questionnaires is adequate for many self-report health outcomes. It is an inexpensive method and avoids researcher bias. Questionnaires can be sent with a reply-paid envelope and covering letter. Participants who do not respond within 2 weeks can be reminded by telephone and those who have not completed all parts of the questionnaire or who give ambiguous answers can be asked to clarify these by telephone. The telephonist can be kept blind to allocation in controlled studies. Non-responding participants can be offered a repeat mailing or a home visit from a researcher (who can also be blinded to allocation in controlled studies).
-
For very frail or cognitively impaired participants, with whom it was not possible to carry out face-to-face interviews, flexibility and initiative was required, such as the collection of as many data as possible from a family member by telephone.
-
A small number of participants will not return a further mailed questionnaire. Some may indefinitely put off visits from researchers or be absent or decline to answer when visited. This should be interpreted as meaning that some do not wish to continue with the study but do not wish to be impolite by openly withdrawing. Some, despite verbal and written assurances, may be unsure about their rights to withdraw or if doing so will have adverse consequences for them. Thus, any further attempts to gather data from them goes beyond what is ethically acceptable and could be seen as harassment. It is important for researchers to discuss individual cases with senior researchers and to not be put under pressure to obtain data ‘at all costs’ or go beyond the process for follow-up that has been approved by the research ethics committee. Some degree of missing data has to be expected in ethically conducted studies of frail older people. The exact process of data collection in those who do not respond but who have not formally withdrawn needs to be clear in the research ethics committee application.
Table 1 shows the number of participants for whom data were missing and the reasons for this in all of the studies referred to in this report.
| Item | Better Mental Health study | AMOS | Care home outcome study | AMIGOS | TEAM |
|---|---|---|---|---|---|
| Participants recruited | 250 | 669 | 227 | 433 | 600 |
| Withdrew consent before baseline data collected | 1 | 2 | 0 | 16 | 0 |
| Baseline data for analysis | 249 | 667 | 227 | 417 | 600 |
| Died before follow-up due | 78 | 34 | 37 | 26 | 139 |
| Died after follow-up but before questionnaire returned | 0 | 7 | 0 | 4 | 0 |
| All subsequent withdrawals | 28 | 132 | 1 | 73 | 78 |
| Did not complete questionnaire | 2 | 23 | 0 | 14 | 0 |
| Did not respond | 26 | 89 | 1 | 55 | 78 |
| Too ill | 0 | 20 | 0 | 4 | 0 |
| Outcome data for analysis | 143 | 494 | 189 | 314 | 383 |
Specific health items
The most overwhelming measurement issues related to the effects of cognitive impairment, and to a lesser degree physical health, on outcome ascertainment:
-
In the care home outcome study GHQ-12 data were often missing. Only the minority with a high MMSE score completed this outcome. It is not a suitable measure of mood or well-being for use in care home residents.
-
In the TEAM study a decision was made that participants with a MMSE score of ≤ 10 were not required to complete the DEMQoL as answers were deemed to be not meaningful; this is specified in the instructions for use, although DEMQol proxy can be used.
-
Care homes often did not record weight and patients who were immobile were hard to weigh, making body mass index and other physical measures difficult to complete. Many participants did not know if they had lost weight.
-
Many participants did not know their height, could not stand to be measured and found it hard to comply with demi-span measures.
-
Simple physical measures such as arm or leg circumference or grip strength were hard to measure because of injuries, dressings, drips, oedema and amputations.
-
Walking tests even in mobile participants could be limited by the environment.
-
Participants were often unclear about the paid carers and other services that they received.
Even using informants could be difficult. Many informants did not have accurate knowledge of personal details such as items in the Barthel index scale (measuring independence in personal ADL) or accurate knowledge of whether or not the participant experienced the symptoms recorded in the NPI (measuring behavioural disturbance).
Days at home: an outcome measure in studies of specialist services providing care for older people67
A common problem in designing research studies into services for older people, particularly those who are likely to be in the last months or years of their life, is to find an outcome measure that adequately reflects their problems and the objectives of care and is sensitive to interventions. Surveys of older people suggest that remaining at home is highly valued and government policy promotes this (improvement area 3.6 of the NHS Outcomes Framework,98 ‘Helping older people to recover their independence after illness of injury’, is assessed by measuring the proportion of older people still at home 91 days after discharge into rehabilitation). ‘Days at home’ was investigated as a potential outcome measure for studies of older people using hospital services. A similar concept of ‘home time’ has recently been explored as an outcome measure for stroke trials. 103,104
Days at home was created as a continuous variable by counting the number of days not dead, in an institution (long term or respite), in hospital or in other health-care facilities. This could also be summarised as the proportion of days spent at home in the follow-up period. It was hypothesised that such a variable would be more sensitive to change than a categorical outcome and would enable more powerful statistical analyses to be performed.
The limitations of using days at home as an outcome measure are that hospital or long-term care is not necessarily a bad outcome and, if widely adopted as an outcome measure, health services could be encouraged to target the measure at the expense of patients’ quality of life. A strength of this measure is that data collection does not require direct patient contact, therefore reducing the burden on participants and their carers.
For the AMOS study, the Better Mental Health cohort studies, the AMIGOS study and the TEAM study, data on date of death and overnight stays in hospital were easily extracted from routine hospital databases. However, information on care home admissions is not collected on one central database and required intensive follow-up by researchers to ascertain and verify dates provided by participants, carers, care homes and general practices. In the AMIGOS study this meant that stays in respite care could not be included in the days at home calculation. In future studies careful planning will be required to ensure that data can be collected for all days not spent at home in the study follow-up period, especially for those participants who do not wish to complete assessments at the end of the study.
The number of days at home was calculated for 598 out of 600 participants in the TEAM study and for 417 out of 433 participants in the AMIGOS study (using the revised definition). This included 77 and 78 participants in the two studies, respectively, who did not complete outcome assessments at follow-up. The mean and median numbers of days at home were greater for participants in the AMIGOS study (mean 80 days, median 90 days) than for participants in the TEAM study (mean 43 days, median 48 days), suggesting that days at home is a valuable measure to discriminate between sicker older patients who are admitted to hospital and a ‘healthier’ group who undergo a short assessment on an AMU and then return home. In both studies associations in the expected directions were observed with baseline variables, which would be considered a priori to be associated with adverse outcomes. This included ADL and cognitive impairment scores (measured in both studies), delirium and behavioural and psychological symptom scores (measured in the TEAM study), and comorbidity and ISAR scores (measured in the AMIGOS study). There was evidence in both studies that the number of days at home was associated with change in ADL score (calculated for the participants not lost to follow-up). Days at home values were smaller and more variable for groups experiencing a decline in ADL score at 90 days’ follow-up than in those with no decline. There remained a large amount of overlap in days at home values between participants with no decline in ADL score and participants with decline in ADL score. The observed associations between number of days at home and change in cognitive impairment and behavioural and psychological symptom scores (in the TEAM study) and EQ-5D health status and GHQ-12 scores (in the AMIGOS study) were much weaker.
Within each study, however, there was a large proportion of participants with the same value for number of days at home: 0 days in the TEAM study for 28% of participants who were not able to return to their usual place of residence after the initial admission and 90 days in the AMIGOS study for 55% of participants who were not readmitted during the follow-up period. This, combined with the left-skewed distribution in both studies, meant that two-part modelling and non-parametric techniques had to be used to evaluate the effects of the interventions, including using bootstrapping to calculate 95% CIs for the intervention effect. No significant differences were found in either study between the intervention group and the standard care group. This does not rule out the possibility that number of days at home can detect a difference as this finding was consistent with there being no significant differences between groups when component outcomes, such as mortality and care home placement, were analysed individually. In the TEAM study there were two distinct parts observed for the days at home distribution and so an overall summary measure (based on the mean or median) may not be that meaningful. For this study, summarising the effect of the intervention in terms of the proportion of patients able to go home and the amount of time spent at home for those returning home may be more relevant to patients. There may still be difficulties in interpreting an intervention effect that is summarised in two parts, especially if there is evidence of a difference between the two groups in the proportion of patients able to go home.
The experience of using number of days at home in the Medical Crises in Older People studies of older people using acute hospital services suggests that it may be a useful overall summary measure to compare different populations but may have little added value over single outcomes when used as an outcome measure in RCTs.
Using electronic sources of data for resource use and economic evaluation
Introduction
Economic evaluations require patient-level resource use information to estimate patient costs. Several methods are available, including questionnaires, diaries and electronic record searches. The National Programme for IT105 prompted UK health and social care services to record patient-level resource use using Electronic Administration Record systems. Because of lack of interoperability between health and social care sectors, and the requirement for multiple service approvals, retrieving Electronic Administration Record information in the UK is labour intensive. However, this method may provide better information than self-report methods, such as the Client Service Receipt Inventory (CSRI), particularly in cognitively impaired people. For this reason, extraction of data from Electronic Administration Records was the primary method for obtaining resource use data across this programme. The CSRI was used as a back-up method and so comparison of the two methods would be possible. Lessons learnt can be divided into obtaining and coding data from multiple sources.
Obtaining data
One of the biggest challenges was gaining access to the service data sets. Consent to access health and social care records was obtained from participants as part of the overall consent process. Gaining access to services subsequent to patient consent, however, was a significant task and highly time-consuming. Anonymity and the protection of personal data are important; the process for anonymising data is not difficult and can be carried out by the service before the data are sent to a researcher if an encrypted patient list and patient identification codes are sent securely to the service for consenting patients. However, it proved particularly difficult to get general practices to agree to share their data, despite researchers being experienced in this area.
The delays between patient consent being gained and obtaining data for analysis varied between 3 and 6 months. This is a significant consideration for project planning. Once access was gained, the data extraction process itself was generally not time-consuming for most services.
Once access was obtained, data were extracted from a wide range of Electronic Administration Record systems. With the exception of primary care, the researchers did not need to have any particular prior knowledge about the electronic system itself, just the parameters available within the system that described resource use at each service. Parameter lists that described resource use at each service and which were important for the research objectives of the Medical Crises in Older People programme were created through discussions with the research team and an analyst at each service. Most, but not all, services employed analysts who knew how to extract the data required. At least one researcher required extensive knowledge of primary care systems to train a larger research team. After training and distribution to the research team of an extraction protocol for primary care systems, most researchers became familiar with the extraction process. By the end of the extraction period all researchers could visit a practice and extract the data by themselves.
Data coding
The data sets obtained from the services were of different designs and used a range of different coding systems. To develop a unified data set it was necessary to code and process the data fairly extensively.
Secondary care data were processed through the Healthcare Resource Group (HRG) toolkit. 106 The data that we obtained are similar to the parameters required to assign the most appropriate HRG4 code to patient-level resource use information. As suggested by Geue107 in ‘Spoilt for choice’, we assigned unit costs to secondary care inpatient and day-case episodes using the HRG4 casemix costing method. HRG4 codes are available in the NHS reference costs46,108 and are suggested by the NHS Health & Social Care Information Centre [www.hscic.gov.uk/ (last accessed 7 April 2015)], Geue107 and ourselves as the gold standard method for attaching unit costs to inpatient and day-case care. We did not agree with the costing algorithm suggested by Geue,107 which used a ‘dominant’ HRG4 code to assign a unit cost (believing that this underestimated the cost of care of frail older people with multiple episodes per spell), and so designed our own costing algorithm.
Primary care data processing was carried out using our specially designed Visual Basic for Applications scripts [the Visual Basic for Applications scripts were originally designed within Microsoft Excel 2003 but were updated to work in Microsoft Excel 2007 and Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA)]. Although not perfect, the scripts provide a simple, efficient method for converting the data from Microsoft Word (Microsoft Corporation, Redmond, WA, USA) format into parameter categories within Microsoft Excel and then into Stata version 11 (StataCorp LP, College Station, TX, USA) for analysis. When possible, we replicated the costing methods described in Curtis47,109 [Personal Social Services Research Unit (PSSRU) costs]. Various extrapolations for time assumptions were used from the PSSRU costs because the variety of different tasks and professionals associated with patient care that were elicited from the primary care electronic system was greater than that listed by Curtis. 47,109 Many of these extra professionals and tasks were not included in the final analysis because this extra information was available in only one of the primary care systems (SystmOne) from which resource use data were extracted. For those professionals and tasks that were kept in the final analysis and for which a time assumption could not be extrapolated from the PSSRU costs, we consulted the wider literature or used expert opinion. In some cases the costs may still be an underestimate of the time taken to perform some tasks but are probably a closer approximation of the true cost of primary care than if only accounting for consultations and ignoring all other patient-centred tasks.
Social care data were plentiful but not always in a standardised format. We processed data on contacts, assessments and care plans only as these are the categories for which we could make assumptions to add unit costs. A number of assumptions were made for the time taken and for who performed a contact or assessment. For social care, costs for care plans were sourced from Curtis. 47,109 For contacts and assessments, the time spent with the professional who performed the contact and assessment was used as the main aspect of the cost for this task. If a unit cost for contact and assessment could not be sourced from Curtis,47,109 the same method as that used to extrapolate a time assumption from the PSSRU costs was used, as has been described for extrapolating a time assumption for primary care resource use.
All other data from the other services were relatively easy to process as the data sets were already in Microsoft Excel format and categorised and assigning a unit cost was relatively simple.
Discussion
In summary, Electronic Administration Records provided more complete patient costs. Although the CSRI can be modified and is simple to administer, poor recall and inadequate detail about the nature of contacts prevented accurate patient-level cost estimation. Using Electronic Administration Records reduces the burden on research participants, which is especially important for frail and cognitively impaired people. Gaining access to Electronic Administration Record systems is labour intensive; however, once access has been gained, these systems provide useful information that can be used for health-care research. If interoperability between services’ electronic systems improves, this will also improve the ability to access this data for research and clinical purposes. 107
Patient and public involvement
One of the intentions of this programme was to conduct research that had been improved by PPI. This is a challenge when carrying out research with frail and cognitively impaired older people.
At the start of the programme, experience of and organisational support for PPI in research were patchy. One initial approach taken was to attempt to work with groups representing older people – the relevant charities. However, it was found that many of these groups were not yet prepared to do this or were overwhelmed by similar requests. Offers were taken up from individuals who came to our notice who wished to assist in research, such as the spouses of patients cared for by the clinical academics. However, some did not have the relevant skills and others could not provide a significant or sustained contribution. Such input was limited to reading patient documentation at the start of some studies and giving opinions on specific potentially contentious ethical points. There were offers of PPI representatives from local emerging groups. These people also had significant other responsibilities and so their contributions were limited. Listening events were undertaken with the Alzheimer’s Society and local dementia carers, with care home providers and providers of services to care homes. Although all of the actions described above helped to explain our research to the public, helped our research team to explain it better to lay audiences and elicited general approval from these representatives of the public, they did not lead to significant ongoing PPI in the research.
Patient and public involvement with research can occur at any part of the research cycle: identifying problems and priorities, research design, research conduct, analysis, reporting, dissemination and wider knowledge transfer leading to implementation. In the case of the Medical Crises in Older People programme, the research team identified the problems and the potential innovations to deal with them and the funding bodies decided on the priorities. The research designs were largely planned by the research team and the work was carried out with little dependence on PPI for its conduct. Towards the later stages of the programme a newly formed PPI group (see below) contributed to some of the analysis and interpretation of some of the qualitative findings of the Medical Crises in Older People programme and charitable bodies became involved in the dissemination of some of the research findings.
Given the modest contribution of PPI to the programme in the early years, and that it was a responsibility of this programme to develop different, sustainable, active processes to improve PPI – if not in this research then in future work – a PPI group was developed in Nottingham to support research on confused older people. There was also an existing generic PPI group in Leicester to support research on frail older people.
Certain principles learned from other research teams informed the important steps when developing these two PPI groups:
-
identifying people who have skills that are useful to research
-
identifying people who can properly represent a constituency or resource
-
making plans that recognise the limitations that PPI personnel have in contributing to research
-
examining potential PPI roles at every stage of the research cycle from the initiation of a research project
-
planning how the group will be sustained
-
attempting to empower PPI personnel to direct their enthusiasm effectively.
The medical and mental health unit patient and public involvement group
The development process was led by a researcher paid by the Medical Crises in Older People grant. An initial information search of the internet and the INVOLVE (a NHS organisation set up to promote the involvement of patients and public in research) and NIHR websites provided information on what PPI was but not how to go about forming such a group. A number of meetings were held with staff with general and research PPI responsibility at the local NHS trust. The trust already had a database of experienced PPI members who had been involved with other PPI groups within the hospital and potential members of the new PPI group were sought from this database. People in this database had already gone through a process of becoming aware of the roles and responsibilities of PPI in research.
Five people attended the first meeting: two of these were sisters and previous carers for their mother who had dementia; the other three had previously worked in mental health services. This meeting involved spending time getting to know one another, learning what experiences or knowledge they had of dementia and of PPI and explaining what they wanted from the group. This programme and further studies were discussed. The PPI lead was overwhelmed by the sense of enthusiasm, friendliness and genuine interest in the research. Members discussed and agreed the times of future meetings.
Great effort was made to remain in regular contact with the members over the following month, as this would be an important time in terms of retaining or losing these members. During this month, other people showed an interest in joining the group, either people known to the original five PPI members or people volunteering from the MMHU under study in the TEAM trial.
In the third month posters and leaflets were designed to hand out around hospital and community buildings promoting the group. These were reviewed by the PPI members before distribution. We also started to talk to local community groups that were supported by the local mental health NHS trust [such as the Black and Minority Ethnic Dementia Community of Interest] in an attempt to gain new members and to raise awareness. Contacts and suggestions were followed up to find suitable groups who might be interested in becoming involved.
Within a few months the group had 14 interested members; not everyone had attended a meeting but interested members had stayed in touch by e-mail and wanted to be part of the group, helping where they could. Members discussed how the they wanted the group to develop and be run – we wanted them to be in a position to offer value to research rather than demanding tasks from them. The group agreed on most things but a couple of questions threw up discussion, namely how wide the scope of the research should be (dementia-only studies or open the research up to all older people) and whether the focus should be on hospital research or should be expanded to the community. These discussions were constructive even though they could have potentially led to a loss of focus.
An example of the approaches needed to encourage involvement is illustrated here. One lady who had looked after her mother who had Alzheimer’s disease until her death was interested in joining the group. However, she lived in a neighbouring county, too far away for her to attend the monthly meetings. The PPI lead e-mailed her regularly on an individual basis as well as sending her the group e-mails and also telephoned her every so often. Including this lady in the group has been of benefit. She always responded to tasks e-mailed out to the group and her input has been just as valuable as the input of those who attended meetings. E-mailing and telephoning her has not really been much of an effort and sending material by post is required for many members who do not use e-mail. However, this is resource intensive.
The initial members of the group were predominantly white, British, middle class and retired. One Pakistani health-care worker joined the group later. He believed that finding black and minority ethnic (BME) members for such a group would be difficult and that BME carers would probably feel anxious about being in the group. He felt that there may be barriers between white and BME races and at the time of writing this issue was not resolved.
The PPI lead attended specific PPI in research conferences hosted by INVOLVE, finding a large and growing community committed to this research contribution. An early priority was to provide general PPI training to group members to enable them to better understand research processes and how things work. Currently, training for PPI personnel tends to be locally arranged and the content of such training is not clearly defined. INVOLVE did not provide training for the group and it has been difficult to find training. This remains a challenge.
After 6 months the group had 20 members. Meetings were usually attended by 10–12 people and tended to focus on the first part of the research cycle: defining problems and priorities from the carer perspective.
Regarding outputs so far, the group has discussed, supported and submitted two NIHR Research for Patient Benefit programme research applications. It has also given advice on two other applications and on PhD research. Group members attended a focus group to plan a research study into continence issues in people with dementia, the outcome of which changed the direction of the research. Interestingly, the findings were so useful that they have been used by the University of Nottingham School of Nursing to rewrite the continence modules for their Bachelor of Science and Master of Science degrees. This illustrates that PPI for research purposes can have unexpected effects beyond the original remit. Group members have also been involved in the analysis phase of current Medical Crises in Older People programme work, assisting with the analysis of observations of staff–patient interactions in hospital. The group has helped organise two dissemination conferences, one for professionals and the other for the public, and has contributed by speaking at them. One member has also had an article relating her experience of her father’s admission to the MMHU published in the BMJ. 110
These outputs illustrate what a viable PPI group can achieve. It should be noted that this required considerable resource input. A lot of time was needed to set up this group and the month-to-month running of the group was also time-consuming. Members of this group, and other PPI groups, stressed the importance of remaining in touch with the research team between meetings rather than just being picked up as and when needed. If ongoing interest is not shown through regular contact, members might not get the acknowledgement that they want and might leave the group. This observation also indicates the sorts of skills required to run a PPI group. Organising the monthly meetings also took time because of having to liaise with the researchers who wanted to present at the meetings and then feeding back to them afterwards. Administrative tasks were also important such as typing up agendas and the minutes of meeting and organising refreshments and lunches.
Finally, this group may still be in the ‘honeymoon’ period and issues about replacing and sustaining the group have not yet been addressed. This is largely related to securing funding but also finding a suitable person to lead the group.
The Leicester patient and public involvement group
In Leicester, the PPI group was formed in 2011, 3 years into the Medical Crises in Older People programme, and was supported by the local NHS research support system (the Comprehensive Local Research Network). Possible members were targeted to represent a range of professional, clinical and lay members from as broad a spectrum as possible: the NHS, academia, patients, social care, the local authority and charities. Local community groups were also approached, including the Leicestershire and Rutland Minority Ethnic Forum. A more formal approach was taken with defined terms of reference, including aims and purpose, roles and responsibilities of the members and methods of communication.
The group consisted initially of 19 members with typically 7–10 members attending each meeting. The lay members were always well represented. Pre-meeting reading was forwarded to all group members with active feedback from those who could not attend. These comments were fed into the meeting and reflected in the minutes, which were then circulated for approval and any study questions. The group usually discussed two proposals at each meeting. Members of the group are co-opted onto project management boards for individual studies that are brought to the group. Professional group members often allow the use of their resources to advertise studies and arrange events such as focus groups for individual researchers who come to the group looking for support. In conjunction with the NIHR Age and Ageing Specialty Group a video was produced featuring the AMIGOS study to encourage older people to participate in research. This can be viewed at www.crncc.nihr.ac.uk/about_us/ccrn/specialty/age_and_ageing (accessed 27 November 2014).
Research ethics
Research into health care for older people who live in care homes or who are in hospital in an AMU or MMHU poses a distinct set of challenges. Many of these people are mentally and physically frail and many lack mental capacity, posing problems related to the ethical recruitment to, and conduct of, studies.
Legal framework for the inclusion of people lacking mental capacity in research
The ethical principles of informed consent date back to the Nuremberg Code (1948) and state that the voluntary consent of subjects is essential in medical research and this requirement has been reiterated through the Helsinki Declarations (1964, 2008). However, it has also been argued that using research findings derived from an unrepresentative sample, for example in the case of the exclusion of large numbers of participants who are unable to provide consent, is itself unethical: some findings may not be properly extrapolated from relatively well, non-cognitively impaired people to those with cognitive impairment and multiple morbidities; there are well-recognised broader benefits of being involved in research that should not be denied to people with cognitive impairment; and people with cognitive impairment who may have wished to have undertaken an altruistic act such as participate in research should not have that wish denied. 111 There has been little research conducted on the implications of excluding patients who lack capacity from research studies but a clinical example of the consequence of not including people with dementia in hypertension trials is that there is genuine uncertainty about the risks and benefits in this group.
The Mental Capacity Act 2005112 came into force in England and Wales in 2007 and provided a legal framework for the inclusion in research of participants who lack capacity. The act stipulates that, whenever possible, consent should be obtained from the participant but that when this is not possible at the time a relative or friend should be consulted. This consultation is aimed at ascertaining the wishes and feelings of the person lacking capacity about taking part in the research. There is no requirement under the Act for this consultation to take place face-to-face and a telephone consultation, as often necessitated in an acute situation, is deemed appropriate. 113 In situations in which a friend or relative is not available, the Act makes provision for the researcher to seek permission from a registered practitioner who is not involved in the research project. These consultees can only guess as to a patient’s wishes in respect of research. Most consultees would reason that an individual would wish to improve medical treatment and provide consent, but this may not actually reflect the prior wishes of the patient. 113
The Mental Capacity Act112 stipulates that studies including patients lacking capacity must be approved by a research ethics committee. There are five basic issues for research ethics committees to consider: ensuring that there is an adequate research design, an acceptable participant selection method, a favourable risk–benefit ratio, documented free and informed consent and compensation for research-related injuries. Committees will approve research projects involving participants who lack mental capacity only if there are reasonable grounds for believing that ‘research of comparable effectiveness’ cannot be carried out if confined to participants who have the capacity to provide consent:114 there has to be a good reason for including people in studies who lack the capacity to give informed consent. Examples would be specific treatments for severe dementia, which obviously should not be tested on people without dementia or on those with only mild dementia.
Practical issues
The Medical Crises in Older People studies were undertaken within the legal framework described above. Several practical ethical issues made them challenging.
There are issues regarding the separation of research from clinical practice: research participants must be aware of, and not be misled about, the difference between research processes and clinical processes. The issue of who first makes contact with potential participants can be difficult. The guidance states that the clinical staff responsible for the patients and not the researchers should be the ones who make first contact with the patients about the research. This means that clinicians are aware of and complicit in the research (assuring safety) and avoids information governance problems (researchers having access to information to see whether or not a patient is suitable before having proper permission to do so). However, busy staff may not have the time to identify suitable patients, leading to under-recruitment. The more that clinical staff are involved, the more there is a risk that patients will be inclined to see participation in research as part of their care, or to feel under an obligation to participate. This may similarly occur in care homes when care home staff make the first approach to residents.
Information governance brings other issues. Research staff have no right to see or use data without the appropriate permission to do so and this includes routine data from those who decline to enter a study that might help characterise those who did not enter and hence estimate any recruitment bias. In the TEAM study, patients were randomly allocated to the MMHU or usual wards under clinical governance procedures and were recruited to the study afterwards. This meant that some patients were randomised who did not become participants, which could have introduced bias. This bias could be examined by looking at routinely collected data (e.g. length of stay, death rates) from all patients randomised, not only those recruited to the study, as might be carried out as part of an audit study. In fact, the officer within the hospital responsible for adherence to legislation pertaining to information governance permitted this under appropriate clinical governance procedures.
In the AMU studies there was a short time during which to recruit participants and assess their capacity. Many potential participants did not have their spectacles or hearing aids and they had little time and a reduced ability to assimilate study information. Research ethics committees tend to require that many points are included in patient information sheets but this can make them difficult to comprehend quickly. However, we were pleased to note that the research ethics committee reviewing the TEAM study gave permission to use single-sided patient information sheets for exactly this reason, with supplementary information available if required. Many patients were anxious and in a state of mental shock such that they felt unable to make decisions without family advice. Despite explicit reassurance, some feared that research involvement might affect their state benefits, that the research was commercially driven or that their details would be made public, and it is therefore important to recognise quite how scared and vulnerable these patients are and feel. Together with the embedding of research staff in the AMU, these issues could have affected recruitment and may have predisposed to a degree of coercion of potential participants; a proportion of potential participants with mental capacity did not give consent and a large proportion of included participants subsequently implicitly withdrew. Recruitment after discharge from the AMU was not appropriate as the AMIGOS study required intervention to begin on the AMU.
Potential participants among care home residents were vulnerable in other ways that could have affected participation: they suffered from fatigue, pain, cognitive impairment, limited functional status, sensory and speech deficiencies, depression and dependency. However, these could be overcome with time and patience.
The use of family or carer consultees has challenges in practice. In the Better Mental Health cohort study 10% of potential participants without capacity had no family member or friend who could act as a consultee and a further 12% of potential participants without capacity had family members or friends who could not be contacted, partly because 6% of potential participants were discharged from hospital quickly, before an appointment with the researcher could be arranged, partly because some families visit in the evening or at weekends only and partly because some family members do not visit at all because of commitments of children or other relatives. Some carers were so distressed by their family member’s admission that they did not want to spend time being interviewed by a researcher. Family consultees may hesitate to agree consent involving their elderly relative in the belief that it is not in their relative’s best interests; on assessing the benefits and risks they may not believe that the benefits of participating will be sufficient because of the imminent end of the elder’s life or because they may not want their relative to be bothered or undergo undue stress.
For the AMOS study, the research ethics committee would not give approval to allow the use of registered medical practitioners as consultees on the basis that it assumed that few people without mental capacity would be discharged without the involvement of a potential consultee. Such an assumption was not unreasonable given that the clinical management of patients is also subject to the same legal framework: decisions on patients lacking mental capacity should be undertaken in their best interests, which involves consultation with members of the family or others who know the patient. In practice, however, many people who the research team assessed as lacking mental capacity to participate in research were discharged from the AMU without the opportunity to identify a consultee. This resulted in recruitment of a lower-risk group than intended.
Findings from the AMOS study were used to advise the research ethics committee for the AMIGOS study that patients lacking mental capacity and without a consultee should be included after consultation with the responsible registered medical practitioner on the AMU, and the ethics committee approved this. In the TEAM study the research ethics committee agreed that professional consultee agreement from the nurse in charge of the ward was acceptable if potential participants had lacked mental capacity to give consent and had no available carers.
A common issue in discussion with research staff is about the disclosure of information given by participants and a duty of care. A non-clinical researcher might respond to a participant describing a physical or mental problem with ‘Oh dear’ or might respond to a request for help with ‘You had better see your GP’, but may wonder if he or she should actively pass the information on to the GP, for example if the patient is housebound or otherwise unable to do so themselves. The sense of a duty of care may be even higher for clinical researchers in such circumstances. Researchers undertaking observations may observe aspects of care that are harmful or substandard, such as observing untended patients at risk of falling. In the Medical Crises in Older People studies, specific arrangements were in place to enable discussion of such concerns and for specific cases to be dealt with by the workstream lead or overall principal investigator. A general rule is that researchers should feel empowered to do what they feel is right under such circumstances, even if it means a non-participant observer becomes a participant observer.
A specific related requirement, stipulated by research ethics committees, was that participants in interview studies should be advised prior to giving consent that, although their replies would be confidential, if they disclosed evidence of harm or wrong-doing the researcher was bound to disclose this information to other relevant people. It is impossible to know if this led to significant self-censorship, but research staff tended to find this clarification helpful.
Impact
General considerations
Research of any sort should, hopefully, eventually lead to ‘impact’ or some sort of consequence that makes the research worthwhile. Impacts can be in health, economic, social, cultural, political or other dimensions. They will usually be indirect: research findings are seen in the light of existing and other emerging findings and it is often the sum of all of these findings that brings about change. Health research, especially when publicly funded, should be expected to lead to patient benefits, but there are other possible impacts of health research such as developing skills in the workforce, stimulating further innovation or supporting industry or improving nations and their citizens in more subtle but still valued ways.
Researchers and those who fund research are concerned that the ‘investment’ in research should ideally reap returns that are obtained as soon as possible and which are as large as possible. There is increasing recognition that much research does not get rapidly taken up and used. This is often referred to as the ‘know–do gap’115 and in biomedical circles is also part of what is known as the ‘second translation gap’. 116 There is a focus on closing the know–do gap by explicit and active means rather than leaving it to chance. The assessment process used to evaluate British universities in 2014, and hence determine their future funding [the Research Excellence Framework – see www.ref.ac.uk/ (accessed 9 February 2015)], included an assessment of research impact, which is intended to be an incentive in this direction.
This applied research programme has generated much new knowledge as summarised in earlier chapters. The purpose of this section is to outline the research team’s understanding of the research impact and to outline the steps taken to date and future plans that will optimise the impact of the Medical Crises in Older People programme.
The nature of impact
A theoretical analysis of impact is beyond the scope of this chapter but one commonly cited classification, which shows the complexity of the nature of impact, is as follows:117,118
-
conceptual impact – opinions, attitudes and knowledge (hearts and minds) are changed as a result of the research
-
instrumental impact – tangible changes occur as a result of the research, for example health outcomes, economic, social, cultural, policy, workforce
-
process impact – conducting the research leads to distinct changes in services or practice
-
symbolic impact – the research evidence is used as a political mechanism to change services or practice.
Demonstrating the impact of research is difficult. It may be hard to show attribution: a single study may provide only part of the information base that leads to a change or impact. There may be a considerable delay between the generation of a piece of new knowledge and its ultimate impact. Seventeen years has been suggested as the average time lag between beginning research to reaching clinical practice, but measuring and defining these gaps is problematic and varies across scientific disciplines. The impact arising from negative intervention studies or simple descriptive studies is harder to characterise than the impact of positive intervention studies that have been implemented. Negative studies can prevent ineffective or unsafe interventions from being implemented, but the effects of this are impossible to measure. Similarly, descriptive studies may improve understanding, but this too is hard to measure. For these reasons, the concept of the ‘pathway to impact’ is used: ultimate and total impacts of research may be impossible to ascertain, but it is possible to enumerate the tangible steps that are expected to lead to impact.
Pathways to impact
Methods and frameworks exist for operationalising the pathway to impact and the ultimate health research impact119–121 and these enable researchers to track the steps towards change and provide evidence of attribution. Understanding these processes allows researchers and those involved in the translation and implementation processes to attempt to speed up and streamline the process of closing the know–do gap. The NIHR, when it funded this research in 2008, defined impact as ‘demonstrable change in NHS practice, service delivery or policy. Effective translation of research findings into improved outcomes for patient and carer benefit’. This was a narrow definition, but the NIHR’s tool to assess steps along the pathway to impact (described later in this paragraph) implied that a more sophisticated definition is understood in practice. The NIHR in 2015 stated that ‘By “impact”, we mean the contribution to benefits to society resulting from the research we fund, including patients, populations, the NHS, health services, the economy and academia’ [see www.nets.nihr.ac.uk/impact (accessed 11 March 2015)]. The Research Excellence Framework 2014 (see www.ref.ac.uk/) used to assess the impact of the work of British universities describes ‘an effect on or changes to the activity, attitude, awareness, behaviour, capacity, opportunity, performance, policy, practice, process or understanding of an audience, beneficiary, community constituency, organisation or individual’. The criteria used to assess impact are ‘reach’ and ‘significance’. Ultimately, the health impacts of research are expected to stretch beyond the NIHR’s 2008 notion of demonstrable ‘patient and carer benefit’ to include increased research capacity; assistance to implementation; contributions to policy or guidance; changes to service delivery; influences on commissioning; stimulation of innovation; benefit to the economy, society, culture or public policy, health, the environment or quality of life; reduced risk or harm; and improved public understanding or behaviour. Capturing evidence of impact is complex and requires systematic identification and subsequent gathering and recording of the elements leading towards it. As part of the NIHR Award Assessment Tool the NIHR developed a checklist for monitoring auditable outputs and events that are assumed to represent stages along the pathway to impact (note that since 2014 the NIHR has used an external system to replace the NIHR Award Assessment Tool). The domains of this tool were:
-
further research funding
-
research collaborations both in the non-profit and the industry sectors
-
research training (undergraduate, postgraduate and professional)
-
academic promotion or establishment
-
generation of research resources (databases, tools, measures, methods, models, etc.)
-
academic publications and presentations
-
non-academic publications, presentations and other outputs (e.g. media)
-
citation in guidelines or reviews
-
research team members contributing to guidelines and reviews
-
use in teaching or training
-
interventions or products (including intellectual property)
-
other.
Knowledge translation and implementation are important elements of the processes by which applied research findings lead to impact. Research organisations with a responsibility for implementation research have examined this area and would include the development of implementation networks and the extent of their reach as an important domain beyond these NIHR domains.
Using this approach, the following paragraphs summarise and reflect on progress along the pathway to impact of the Medical Crises in Older People programme, recognising that impact will continue beyond the lifetime of the research programme.
Further research funding
The Research Excellence Framework explicitly excludes academic impact from its assessment of impact but clearly it was an intention of the Medical Crises in Older People programme that a viable critical research mass was developed, and the award of further research awards is evidence of this. Six further research awards were obtained during the life of the programme, three for a further programme looking at the prevention of falls in people with dementia, one for the evaluation of care home services, one to augment the Medical Crises in Older People programme itself and one to examine the merits of antihypertensive medication in patients with dementia.
Research collaborations
Research collaborations improve research opportunity, are associated with better, less parochial and hence more generalisable research and potentially have greater reach. Significant collaborations arising from the Medical Crises in Older People programme included links to colleagues studying care home medicine in the Netherlands and dementia care in Canada. The strength of the research and the critical mass of researchers was a major factor in the local research implementation organisation [the Collaboration for Leadership in Applied Health Research and Care – Nottinghamshire, Derbyshire and Leicestershire (CLAHRC-NDL); see www.clahrc-ndl.nihr.ac.uk/clahrc-ndl-nihr/research/older-peoples-health-and-wellbeing/index.aspx (accessed 9 February 2015)] adopting an Older People’s Health and Wellbeing theme, with the Medical Crises in Older People programme principal investigator as its lead. This has led to important opportunities to enhance impact.
Research training
Eleven postgraduate students were supported by work associated with the Medical Crises in Older People programme, with many co-supervised by academics outside the host academic division. This has the potential effect of developing a broad research workforce that is skilled and informed about ageing and older people. Numerous undergraduate medical and nursing students also took roles in different aspects of the work, such as literature reviewing, data checking and analysis. This has the potential to affect their future career choices while providing real-life research experience. Nursing staff were involved in data collection and analysis, bridging the gap between academia and clinical practice and promoting theory into practice and evidence-based care. Members of the Nottingham dementia PPI group were trained to support and review new grant proposals, critique research documents, analyse qualitative data, publish documents and present at conferences. The impact of these training and educational opportunities will extend far beyond the lifetime of the Medical Crises in Older People programme.
Academic promotion and establishment
Several formal appointments and promotions of staff occurred during the lifetime of this award, using evidence from participation in this programme. An important step was significant recognition by the associated NHS trust that research into older people was a core part of its research and innovation strategy, with the commitment of significant core funding. This funding was matched by a local wealthy philanthropist to support a clinical/academic post for a Medical Crises in Older People programme researcher to continue knowledge translation and implementation of learning developed during, and beyond, the programme. This will include developing ‘advanced nurse practitioners’ for frail older people. The potential impact of this is considerable; the evidence and good practice from the Medical Crises in Older People programme, developed through this additional funding, could be applied in other national and international health-care settings.
Generation of research resources
Perhaps the main research resource produced by the programme was the MMHU. It was not only an intervention to be tested but can also continue to act as a research and innovation hub, in the same way that coronary care units in the last four decades not only provided acute cardiac care but also facilitated many of the large cardiac studies that transformed cardiac care over this period.
Academic publications and presentations
Publications arising from the Medical Crises in Older People programme are listed on the Medical Crises in Older People Discussion Paper Series website (see www.nottingham.ac.uk/mcop). All publications are open access to increase their potential impact. Numerous presentations have been made across the UK and internationally, both invited and following submission to research conferences. Such presentations are usually limited to impact within the academic sphere. Those with a published abstract are also shown on the Medical Crises in Older People Discussion Paper Series website. An important research output and publication worth noting is one authored by a member of the Nottingham PPI group published in the BMJ. 110 This is a moving personal narrative that describes the carer’s experience of the positive impact of the MMHU: it has both reach and significance.
Non-academic publications, presentations and other outputs
One of the purposes of the Medical Crises in Older People Discussion Paper Series was to disseminate findings that might not be suitable for peer-reviewed research publications but which might be useful for many potential research users (which includes clinicians). An important example is the paper describing the development of the MMHU. Web publishing allows people around the world to freely read about this work.
Other key activities that enable knowledge transfer to audiences who might not read academic journals include presentations and workshops delivered to NHS development organisations and relevant charities, including the Alzheimer’s Society and Age UK.
Another method of knowledge transfer specifically designed to help busy practitioners become aware of research findings is the use of BITEs (Brokering Innovation Through Evidence) [see www.clahrc-ndl.nihr.ac.uk/publications/bites.aspx (accessed 9 February 2015)], which are short, accessible summaries of research developed by CLAHRC-NDL, put into context and circulated directly to relevant practitioners. Several of these arising from the Medical Crises in Older People programme can be seen on the CLAHRC-NDL website.
The use of the media, newspapers and television becomes increasingly possible and important for research that deals with topics of high public relevance. Engagement of the media can enhance public understanding of the science of the research or the general areas that it concerns. Garnering public approval and stimulating interest can enhance the degree to which research findings can influence policy. It can also generate important research consequences such as people wishing to collaborate or participate in the research or study it in more depth. The hospital care of people with dementia became an important priority in the UK mass media during the period of this programme, which afforded many opportunities for radio, newspaper and television engagements. For example, a national newspaper described the MMHU as ‘leading the kindness revolution’ [see www.dailymail.co.uk/health/article-2277169/Inside-hospital-thats-leading-kindness-revolution-Concluding-series-crisis-compassion-nursing.html (accessed 9 February 2015)], reporting examples of good practice and evidence from the Medical Crises in Older People programme, promoting the research to a wide audience and generating much public and political interest. To a lesser extent, the acute care of older people has afforded similar opportunities. Health care in care homes did not have the same opportunities, reflecting lower levels of media interest. The extent to which research findings can drive media priorities is unclear, but it is likely that areas of research with little media interest will find it hard to make use of the media for knowledge transfer.
A final area of knowledge translation and exchange explored in the Medical Crises in Older People programme was the development of a DVD documentary about the MMHU, called ‘Today is Monday’, to illustrate the aspects of care that were tested. A professional film-maker was commissioned to make the documentary and the result was a short high-quality film showing a moving reconstruction of a day in the life of the MMHU. Early feedback from viewers is that its honesty is incredibly powerful, working as a piece of drama as well as being suitable as an educational tool; in fact, the two functions are seen as intertwined. At the time of writing over 500 people had seen this video (and plans are made for many more to do so), the majority of whom would be unlikely to have read our peer-reviewed reports. This is an example of the use of the arts to aid the dissemination, and particularly public understanding, of science. The potential impact of this unique research output is unknown. Thus far, requests have been received to use it as an educational tool for psychiatrists, medics and nurses and to inform policy-makers and commissioners. International interest in this research product extends to Canada, the USA and Australia, providing opportunities for global comparisons and learning. Its potential reach and significance are considerable.
It is not yet clear how important formal academic outputs, non-academic outputs and other activities are to research impact. It seems reasonable to argue that direct impact is unlikely or may be delayed without employing as many different knowledge transfer approaches as possible, but at the same time the ultimate authority of the messages will depend on their scientific rigour, which is tested by being published in peer-reviewed journals. It is not one or the other but both that are necessary.
Guidelines or reviews
The research itself has produced several review articles, as listed on the Medical Crises in Older People website (www.nottingham.ac.uk/mcop), but at the time of writing this report it was too early for research papers from the programme to be highly cited elsewhere.
However, two potentially influential documents were developed during the period of the programme, with significant involvement from Medical Crises in Older People programme personnel who drew on this research. These were the Royal College of Physician’s Silver Book99 on the acute care of older people and the British Geriatrics Society’s Quest for Quality122 on health care for the residents of care homes. This work with the British Geriatrics Society also influenced its Commissioning Guidance for High Quality Health Care for Older Care Home Residents [see www.bgs.org.uk/campaigns/2013commissioning/Commissioning_2013.pdf (accessed 27 November 2014)] and the response from the Royal College of Physicians and the British Geriatrics Society to the Older People’s Commissioner for Wales formal review into the quality of life and care of older people living in residential care in Wales.
Use in teaching or training
At the time of writing the findings of the programme have been used by local educators but there has not been time for this to result in any significant changes to the specific content of undergraduate or postgraduate training. However, emerging collaborations with the East Midlands Local Education and Training Board, whose priority in 2013/14 was frail older people, have provided ongoing knowledge translation opportunities.
Interventions or products (including innovations)
At the time of writing this report, whether the MMHU is seen as a complex intervention that could be replicated or a unit demonstrating a set of principles remains to be seen. Similarly, it is not yet clear whether or not the documentary made on the ward is a product that could be used to guide the development of similar units or the application of the set of principles. However, both have been identified as examples of innovation and dignified compassionate care by clinicians, academics, politicians, journalists and commissioners. Political windows of opportunity exist, allowing initiatives such as these to be shared through considered knowledge translation strategies.
Several examples of innovation seem to have sprung from this work: new ideas for incontinence in people with dementia, enhanced care planning to reduce persistent vocalisation in elements of dementia care,123 interventions to reduce falls in people with dementia, procedures to optimise the use of antihypertensives safely in people with dementia and better models of care for frail older people in emergency settings. 99
Other (including network and reach)
In 2013, England witnessed a major transformation of organisation of the NHS. One development was the creation of Academic Health Sciences Networks. These are regional organisations with several higher-level transformational functions (promoting research, promoting life science industries and developing health informatics, as well as assisting implementation, improving services and developing the workforce) involving health, social care, industry and third-sector providers in the region. As the Medical Crises in Older People programme dealt with high-priority issues through scholarly and evidence-based interventions and evaluations in clinical settings, the principal investigator was invited to lead the frail older people’s clinical theme in the East Midlands Academic Health Sciences Network. 124 Knowledge from the Medical Crises in Older People programme will form the basis of the initial strategic direction of this clinical theme. Developing a network of clinical expertise and evidence-based research through stakeholder engagement and effective knowledge translation and implementation provides ample opportunities to close the know–do gap, building sustainable health services for frail older people across the East Midlands and beyond.
Cost and economic issues for the UK NHS
Multiple health, social, private and voluntary agencies are involved in the care of older people experiencing medical crises. The true economic impact of the health and social care of older people is rarely described appropriately or at all. 125 Resource use data can be collected alongside clinical studies to inform estimates of costs of care. Despite clear recommendations to assess the full opportunity costs of resources consumed and which methods to use,126 only half of published studies measure costs other than secondary care, with even fewer including long-term or social care costs. Criticisms of costing studies in older people include the varying perspectives used, with some focusing on hospitalisation only,127 the use of small biased samples,128,129 the lack of reporting of methods,128 poor or no reporting of distributions of data or variance around point estimates130 and the use of inappropriate analytical techniques. 131 With regard to the costs of providing CGA, reports in the literature range widely and come from a variety of health systems and countries. 130,132,133 The eight studies reporting costs in CGA trials reviewed by Ellis et al. 4 report costs from a hospital perspective only and so are not able to tell us whether costs are shifted to other areas of health care, to social care or to informal carers. Although CGA is unequivocally clinically effective, its cost-effectiveness is less well established.
In our studies we determined that frail older people living in the community consume health and social care from a range of sectors, predominantly through hospitalisations, primary care and social care. Our two economic evaluations were conducted alongside the two RCTs. Both interventions were partly motivated by the desire to reduce hospital costs or at least shift them to community costs if this resulted in better clinical outcomes.
We examined the cost-effectiveness of the MMHU for older people with cognitive impairment admitted to a general hospital. Specialist care for people with delirium and dementia did not demonstrate convincing benefits in terms of health status but appears to be cost-effective compared with standard care through a reduction in social care costs, with secondary care costs marginally lower despite the investment in the unit because of a small reduction in length of stay. We also examined the cost-effectiveness of interface geriatrics. No cost-savings were noted and the intervention was not cost-effective.
Both of these interventions had the potential for resource use, and hence costs, to shift from the secondary care sector to the primary care sector. In the study of the MMHU the main savings that were noted from this investment in secondary care arose in social care. Observations such as these lend weight to the case for the integration of care and budgets between secondary and primary care (vertical integration) and between health and social care (horizontal integration). 45,134
In one of the few studies examining the crossover between health, social and informal care costs,135 a considerable amount of variation in access to social care exists locally compared with access to health care. This then affects the uptake of that social care and the use of informal care to ‘fill the gaps’. Furthermore, a connection was found between the use of social care services and perceived health: those who reported improvement in their health status during the preceding year were more frequent users of social care services. Despite the potential advantage of providing social care rather than health care in certain circumstances, fragmentation between services remains136 and older people themselves are often unable to take control of their own care arrangements. 137 We argue that, until there is closer working between health and social care and between primary and secondary care, it is difficult for the findings of evaluations such as ours to be put into practice. For example, in the current economic climate in the UK, in which there are severe cost constraints on health and social care organisations, the lack of integration means that there is little incentive or possibility for secondary care to make investments (such as in a MMHU) that will reap dividends overall but particularly in social care.
Implications for practice
Although these have been covered in Chapter 5 a summary is given here:
-
Clinicians, commissioners and providers should be aware that older people discharged from AMUs (medical admission units) have a measurable decline in their health over a short period of time. Clinicians’ practice should take due account of this, for example in terms of potential anticipatory actions, providers’ practice should support clinicians in doing so and commissioners’ practice should facilitate the integration of services to enable such actions.
-
Policy-makers should consider, in their practice, the implications of the 4-hour target used in the NHS at the time of this research, given that it may have played a part in the difficulty in delivering CGA to older people with frailty discharged from AMUs.
-
NHS hospital administrators, and those who advise them, should be aware that the practice of aiming for very high levels of bed occupancy may limit the ability of services to function efficiently and for the NHS to fulfil its research functions.
-
Policy-makers and commissioners may wish to consider their practice in the light of the assertion in the Quest for Quality document96 (a document that this research has partly contributed to) that existing arrangements for health care in care homes are ‘a betrayal of older people, an infringement of their human rights and unacceptable in a civilised society’.
-
Commissioners’ and providers’ practice could make use of the findings regarding the outcomes of and costs incurred by older people discharged from AMUs when planning, commissioning and delivering services that affect this group of patients.
-
Commissioners’ and providers’ practice could make use of the findings regarding the outcomes of and costs incurred by older people with mental health problems in hospital when planning, commissioning and delivering services that affect this group of patients.
-
Commissioners’ and providers’ practice could make use of the findings regarding the outcomes of and costs incurred by the residents of care homes when planning, commissioning and delivering services that affect this group of patients.
-
Commissioners and providers wishing to implement MMHUs in their hospitals can be informed by our findings.
-
In the light of our findings about carers, commissioners and providers may wish to challenge their practice in terms of the provision given to the carers of people with mental health problems in hospital. An example that might be considered is whether or not temporary hospital accommodation for the carers of confused older patients in hospital would be desirable and affordable.
-
Providers and commissioners should reconsider their practice if they commission or provide an acute interface geriatrics model as tested in the AMIGOS study in view of its unfavourable cost-effectiveness.
-
Providers’ and clinicians’ practice could be altered in many specific ways by the range of outputs produced in the MMHU workstream, for example the steps taken to develop staff training or the ward environment in this study could inspire others.
-
Clinicians’ and providers’ practice may alter if they have previously worked on the basis that the ISAR tool or frailty rating scales or the DRS-R-98 are useful clinical tools for service delivery and they might reflect more widely on the drawbacks of using measurement tools in clinical practice that have been validated only in their ability to enable statistical testing in quantitative research studies. Although we used Dementia Care Mapping to ascertain and compare outcomes in a RCT, its sensitivity to change in a hospital setting adds further to its potential value in the practice of quality assurance of the care of people with dementia.
Final conclusions
The acute medical unit
The impact section of this report shows that this workstream has demonstrated leadership in the area of transforming the care of older people on AMUs in the UK. The very notion that the AMU is a focal point for the initiation of CGA has been widely accepted. The research work showed that even the best-evidenced simple clinical risk stratification tool, the ISAR tool, is insufficient alone to guide clinical management. The concept of the interface geriatrician has been developed, implemented and tested so that others can develop it further. Such development is required because the addition of an interface geriatrician alone is not sufficient to make a major change to the health and well-being of higher-risk patients discharged from AMUs.
The medical and mental health unit
This workstream showed convincingly the plight and challenges faced by cognitively impaired people in hospital, providing evidence to guide innovation and development elsewhere. It showed that it is possible to develop, in a publicly funded health-care system, a specialist unit for such patients that is demonstrably different from usual care. This will help others to innovate. The unit was tested in a rigorous trial, the first ever in the world. The quality of patients’ experiences was improved and their carers were more satisfied. In other words, their experience of care was more dignified. The effects on ultimate health outcomes were modest but a preliminary cost analysis showed that the costs of developing the ward were offset by subsequent resource use savings across the health and social care system. The economic analysis concluded that the MMHU was likely to be cost-effective. Not only does this provide powerful evidence to counter any lingering sense of hopelessness about the inevitability of loss of dignity for such patients, it also justifies further examination of the use of MMHUs as a means of improving the care of people with cognitive impairment in general hospitals. It also illustrates the general principle that investing in best practice can be affordable and beneficial.
Health care in care homes
This work has been valuable despite the major changes to the research plan. A huge and valued repository of care home literature has been developed, demonstrating convincingly that care home medicine is far from ‘an evidence-free zone’. The complexity of health care in care homes has been illustrated, showing the vital health-care roles that the care home staff play (despite being seen as social care practitioners), and a light has been shone on current primary care arrangements, which a range of national organisations find unacceptable. The findings can help policy-makers, commissioners and practitioners to appreciate the policy, managerial and clinical steps that need to be taken to improve matters, recognising the importance of partnership working.
Programmatic considerations
The programme developed a critical mass of researchers and has increased research capacity in the field of the care of older people. This developed alongside a growing research capability within the NHS, including local trusts and research networks. The crucial mass of researchers held within it a large body of expertise in measurement in frail older people and in the ethical conduct of research in such people. Success in the involvement of patients and the public in research was demonstrated. Early success in the process of closing the know–do gap was demonstrated so that early patient benefits from this work can be achieved.
What is comprehensive geriatric assessment?
A large team of researchers has considered the nature of CGA, from the time when the grant proposal was written through the development phases of the funded work to the evaluation, analysis and reporting of the studies. The team’s understanding of the CGA process deepened over this period. A summary of this follows and in the following section we use this understanding to comment on why neither of our flagship trials, the AMIGOS and TEAM trials, yielded the hoped-for benefits in terms of improved health outcomes at follow-up.
The team came across frequent misunderstandings. CGA was remarkably unknown to people who were not specialists in geriatric medicine (and sometimes to specialists), even those who were unknowingly contributing to it and even though the evidence base had been robustly shown 20 years before the date of this report and it offers a contribution to one of the most pressing health-care problems of the age. The principles of CGA need to be known by all who deal with frail older people and not just specialists in one professional discipline. CGA is a misnomer, which can make it difficult for non-specialists to understand; it is more than assessment, implying a complex process beyond the assessment. CGA can be misunderstood by many as simply another phrase for geriatric medicine or specialist geriatric medical input. Such misunderstandings are understandable in a health service with many unhelpful acronyms and we noted that many people found the acronym an off-putting piece of jargon. Synonyms such as geriatric evaluation and management are likely to offer no advantage.
The assessment part of CGA, to be comprehensive, needs to assess physical and psychiatric conditions (diagnoses such as delirium or dementia and health problems such as falls or immobility), functioning (impairments, activity limitations and participation restrictions), the social environment (such as the social network) and the physical environment (such as the home). Implicitly, the staff carrying out the assessment need to be knowledgeable about these complex areas, which requires good levels of training. Experience in the AMIGOS trial showed that assessments can be comprehensive and brief even if they are not deep. Assessments need to be tailored to the patient group and setting. Patients presenting with falls need assessments targeted towards their likely constellation of problems (such as strength and balance) whereas those with confusion will need assessment focusing more on their cognition, mood and behaviour. Patients in emergency settings require brief high-level assessments whereas those in hospital settings require more detailed assessments. It is inevitable that most instances in which CGA is employed will require a team of different professionals to ensure that all domains have been assessed by a suitably trained person.
Comprehensive geriatric assessment needs to be targeted at those for whom such a complex process is required and justifiable. Frailty describes a state in which patients are vulnerable to deterioration, in which such deterioration can be extreme in the face of challenges that would not trouble a more robust person and in which the recovery after such deterioration can be prolonged or never materialise. CGA should be focused on those who are frail, but it remains difficult to identify these people.
Comprehensive geriatric assessment is more than assessment. Not only is a team usually required to assess the patient, a team is also required to undertake the interventions that arise from that assessment. This means that a care plan should be derived from that assessment, team members should know their responsibilities in terms of delivering the care plan and the effects of doing so need to be monitored by repeated assessments. These steps require some form of case management and team working. The model for CGA working in hospitals is well rehearsed. Medical consultants usually lead defined teams in defined settings with a defined patient case load. Multidisciplinary team meetings enable information flow for the production of coherent care plans, adherence to them and monitoring the response to intervention. The AMIGOS trial experience illustrated the problems of conducting CGA across the secondary–primary care interface and the difficulty of doing so in primary care. In the AMIGOS trial the assessment was carried out by a geriatrician, without direct access to or authority over a multidisciplinary team. As the clinical report about interface geriatrics showed, a far more comprehensive assessment was made after a home visit. However, the care plan advised by the geriatrician was likely to be not as good as one devised with the help of a full multidisciplinary team. Most importantly, there was no clear multidisciplinary team in the community: there were multiple GPs and multiple community teams, all with differing patient caseloads. Few patients used the small intermediate care services and there were no overt mechanisms for multidisciplinary communication. There remains a need for models of community CGA to be demonstrated. The British Geriatrics Society and the Royal College of General Practitioners have proposed such a model,138 and ‘virtual wards’139 represent an attempt to implement such a model, although evidence is still required to determine whether or not these are effective.
The research team offers the following questions to identify whether or not a system is delivering CGA:
-
Is the patient frail?
-
Does the patient undergo an assessment that includes all domains of a CGA?
-
Are the assessments suitable for the type of geriatric condition?
-
Are the assessments suitable for the setting?
-
Is a care plan made based on the CGA?
-
Are there mechanisms to share the production of the care plan and to allocate responsibilities to deliver interventions?
-
Is there evidence that these mechanisms are used?
-
Are the interventions as planned delivered?
-
Is there evidence or later reassessment?
-
Is there evidence of repeated review by the team?
-
Is there evidence of further cycles of planning and action?
Such a framework may help guide the development and evaluation of CGA interventions in novel settings and quality assure those in more familiar settings.
Understanding the findings of the randomised controlled trials
The positive aspects of the AMIGOS trial were that we were able to develop and test a novel intervention targeted at a high-priority group of patients and, along with the AMOS trial, shine a light on this group of patients. This was appreciated by the AMU staff. The positive aspects of the TEAM trial were that we were able to operationalise and test best practice in dementia care in a general hospital setting, conduct the first ever trial of a unit delivering such care in a general hospital and demonstrate that the quality of experience for patients and carers could be improved by doing so, in a cost-effective manner. However, neither RCT significantly improved health outcomes.
There are several possible explanations. It is possible that the TEAM trial was underpowered for some outcomes. There was an 8% absolute reduction in the percentage of patients who moved into long-term care. This could have occurred by chance but might have been statistically significant if the sample size had been larger and would be clinically valuable. It is possible that poor targeting of the intervention in the AMIGOS trial, because of the difficulty of identifying the frailest patients, could have diluted any benefits of the intervention in that study.
It may be that, in the AMIGOS trial, the right variant of CGA was not delivered. In this report we described the intervention as a specialist geriatric medical intervention and not CGA per se. To have made it CGA there would have to have been far greater integration with community services and possibly greater understanding of specialist geriatric care in the community than at present. Similarly, maintaining any advantages brought about by the MMHU after discharge from hospital would have required community services to continue to support patients and carers using similar expertise. We cannot say whether or not this occurred. System-wide change is a challenge for any trials of CGA-like interventions.
We have increasingly wondered whether or not the patients in the Better Mental Health cohort study and the TEAM study were too close to the end of their lives to benefit from CGA. The benefits of CGA in previous studies are typically restorative ones: increased survival and better health. However, many patients in the Better Mental Health cohort study and the TEAM study were clearly close to death and perhaps beyond the scope for recovery. In such patients a palliative care framework would be more suitable. In such a framework, the quality of care prior to death would be the primary outcomes; restorative outcomes such as survival and disability would be secondary outcomes. Days at home would probably not be chosen as the primary outcome measure in, say, a trial of palliative and terminal care. If a palliative and supportive care approach had been taken, the primary outcomes of the TEAM trial might have been patient experience and carer satisfaction, in which case the result would have been seen as a positive one, not a negative one.
Research-commissioning priorities
Further innovation is required in the use of CGA in AMUs and such innovations need to be evaluated. Examples include the use of frail elderly care units on AMUs and integrated services delivering a whole package rather than the more limited one tested in the AMIGOS study. The health problems experienced by those who attend AMUs may not be solved by interventions in the AMU but by interventions in other parts of the wider system either before or after episodes of medical crisis. System changes might require changes to the training and education of the workforce to ensure that staff in all professions and at all levels of seniority are trained to deal with the frail older people that they meet; the identification of at-risk people in the community and developing community-based services that meet their needs before they present to hospital with a medical crisis; developing effective and efficient models of vertical integration so that patients discharged following a medical crisis receive effective and efficient services to meet their underlying problems, and this will also require the development of models of horizontal integration, taking into account the need for the statutory (health and social care) sectors and the voluntary and private sectors to work together.
Further evaluation of other MMHUs is warranted, to examine the generalisability of the TEAM study, and this might enable meta-analyses to see if any modest but not statistically significant findings of the TEAM study are real and if cost-effectiveness can be seen in other settings and contexts. The development and evaluation of different models for enhancing hospital staffing and training and the hospital environment are warranted. However, a MMHU is far from a panacea. Given the high proportion of cognitively impaired people in general hospitals, MMHUs can only ever be part of a complete service, and so there will always be a need for organisational research into the best configurations of service components in a range of different contexts. Furthermore, clinical problems that remained despite the MMHU, such as patients who repetitively shout out on a ward, require the development of new interventions and their evaluation.
Existing innovations in models of health care for the residents of care homes need immediate evaluation. An example in the UK context would be the use of specialist nurses instead of GPs to provide first-line primary care. Such nurses and other models such as comprehensive teams provide the ability to deliver a form of CGA and are likely to prove preferable over ordinary, usual primary care. Complex research designs are required to evaluate these developments and to guide future innovation. There is scope for a subspecialty of care home health care to develop, and complex research studies will be required to evaluate the effect of such a development.
Acknowledgements
Many people contributed to the work presented here but did not meet the criteria for authorship. We would like to thank these people for their invaluable help. They include researchers, the trusts (Nottingham University Hospitals NHS Trust, University Hospitals Leicestershire NHS Trust and Nottinghamshire Healthcare NHS Trust), the British Geriatrics Society (start-up grant for Adam Gordon), Nottingham and Nottinghamshire Social Services, the research networks (Mental Health Research Network and Comprehensive Local Research Network for Trent and Leicestershire), members of the public, the AMIGOS trial geriatricians, the external advisors and the patients and their families. Particular research staff to thank are Casey Quinn, Rachael Taylor, Tony Stevens, Fiona Kearney, Claire Litherland, Caroline May, Catherine Russell, Hilda Parker, Diane Gali, Diane Havard, Geraldine Dowling, Fiona Marshall, Jil Mamza, Anthony Avery, Gertrude Chikura, Finbarr Martin and, last but not least, Gail Arnold for the administrative support that held this whole programme together from start to finish.
Contribution of authors
All authors contributed to the preparation of this report. Further individual contributions are listed below.
Professor John Gladman was the principal investigator, the care home workstream lead, author of the final report and guarantor.
Professor Rowan Harwood was a co-investigator and the MMHU workstream lead.
Dr Simon Conroy was a co-investigator and the AMU workstream lead.
Professor Pip Logan was a co-investigator and programme manager and contributed to the design, conduct and reporting of the care home workstream.
Professor Rachel Elliott was a co-investigator and lead for all costing and health economic studies.
Associate Professor Rob Jones was a co-investigator and contributed to the design and reporting of the MMHU workstream.
Professor Sarah Lewis was a co-investigator and lead of the statistical analyses.
Dr Jane Dyas was a co-investigator and contributed to the design and reporting of the care home workstream.
Professor Justine Schneider was a co-investigator and supported the dissemination of the findings and the overall synthesis.
Professor Davina Porock was a co-investigator and contributed to the design and reporting of the MMHU workstream.
Associate Professor Kristian Pollock was a co-investigator and contributed to the design and reporting of the MMHU workstream.
Dr Sarah Goldberg undertook project management as well as contributing to the design, conduct and reporting of the MMHU workstream.
Dr Judi Edmans undertook project management as well as contributing to the design, conduct and reporting of the AMU workstream.
Associate Professor Adam Gordon undertook project management as well as contributing to the design, conduct and reporting of the care home workstream.
Ms Lucy Bradshaw undertook statistical analyses in all of the workstreams.
Mr Matthew Franklin undertook resource use ascertainment and costing and contributed to the analysis of the economic studies.
Ms Katherine Whittamore contributed to the design, conduct and reporting of the MMHU workstream.
Dr Isabella Robbins contributed to the design, conduct and reporting of the care home workstream.
Mr Aidan Dunphy contributed to the design, conduct and reporting of the AMU workstream.
Dr Karen Spencer contributed to the design, conduct and reporting of the MMHU workstream.
Ms Janet Darby contributed to the design, conduct and reporting of the AMU workstream.
Dr Lukasz Tanajewski contributed to the economic analyses.
Dr Vladislav Berdunov contributed to the economic analyses.
Dr Georgios Gkountouras contributed to the economic analyses.
Ms Pippa Foster contributed to the design, conduct and reporting of the MMHU studies and PPI.
Ms Nadia Frowd contributed to the conduct and reporting of the MMHU studies and PPI.
Publications
Publications, other outputs and indicators of impact arising from this report can be seen in the Impact section of the Medical Crises in Older People Discussion Paper Series website [see www.nottingham.ac.uk/mcop (accessed 12 March 2015)] and many have been cited in this report.
In total, 14 discussion papers have been published to date:
Gladman J. Provision of Medical Care in UK Care Homes. Medical Crises in Older People Discussion Paper Series. Issue 1, June 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue1-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Logan P, Robbins I, Gordon A, Goldberg S. Health Care Services for UK Care Homes. Medical Crises in Older People Discussion Paper Series. Issue 2, July 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue2-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Jurgens F, Harwood R, Goldberg S, Logan P. Better Mental Health in General Hospitals. Medical Crises in Older People Discussion Paper Series. Issue 3, September 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue3-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Harwood RH, Conroy SP, on behalf of the Medical Crises In Older People Study Group. Days at Home: an Outcome Measure in Studies of Specialist Services Providing Care for Older People. Medical Crises in Older People Discussion Paper Series. Issue 4, October 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue4-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Harwood RH, Porock D, King N, Edwards G, Hammond S, Howe L, et al. Development of a Specialist Medical and Mental Health Unit for Older People in an Acute General Hospital. Medical Crises in Older People Discussion Paper Series. Issue 5, November 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue5-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Conroy S, Stevens A, Gladman JRF. The Interface between Acute Hospitals and Community Care for Older People Presenting to Acute Medical Units: a Mapping Review. Medical Crises in Older People Discussion Paper Series. Issue 6, December 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue6-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Goldberg S, Harwood RH, Gladman JRF. Mental Health Problems on Hospital Medical Wards: a Listening Event. Medical Crises in Older People Discussion Paper Series. Issue 7, January 2011. URL: www.nottingham.ac.uk/mcop/documents/papers/issue7-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Chikura G. Nurse Practitioners in UK Care Homes. Medical Crises in Older People Discussion Paper Series. Issue 8, February 2011. URL: www.nottingham.ac.uk/mcop/documents/papers/issue8-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Kearney F, Ali A, Blundell A, Wong R, Laithwaite E, et al. The Role of the Interface Geriatrician across the Acute Medical Unit/Community Interface. Medical Crises in Older People Discussion Paper Series. Issue 9, February 2012. URL: www.nottingham.ac.uk/mcop/documents/papers/issue9-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Harwood RH, Jones RJ, Porock D, Griffiths A, Schneider J, et al. Medical and Mental Health/Better Mental Health Development Study Protocol. Medical Crises in Older People Discussion Paper Series. Issue 10, March 2012. URL: www.nottingham.ac.uk/mcop/documents/papers/issue10-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Edmans JE, Gladman JRF, Havard D. Umbrella Review of Tools to Assess Risk of Poor Outcome in Older People Attending Acute Medical Units. Medical Crises in Older People Discussion Paper Series. Issue 11, June 2012. URL: www.nottingham.ac.uk/mcop/documents/papers/issue11-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Sartain K, Kerr M. Carers’ Perspectives on Hospital Dementia Care. Medical Crises in Older People Discussion Paper Series. Issue 12, January 2014. URL: www.nottingham.ac.uk/mcop/documents/papers/issue-12-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Gladman JRF, Harwood RH, Foster PER, Goldberg S, Davies O, Schneider J, et al. Today is Monday – the Evidence Base behind a Film about the Care of People with Dementia and Delirium in Hospital. Issue 13, March 2014. URL: www.nottingham.ac.uk/mcop/documents/papers/issue13-mcop-issn2044-4230.pdf (accessed 27 November 2014).
Bradshaw LE, Lewis SA, Goldberg SE, Gladman JRF, Harwood RH. Statistical Report for the TEAM trial: Care in Specialist Medical and Mental Health Unit Compared with Standard Care for Older People with Cognitive Impairment Admitted to General Hospital: Randomised Controlled Trial. Issue 14, May 2014. http://www.nottingham.ac.uk/mcop/documents/papers/issue14-mcop-issn2044-4230.pdf (accessed 27 November 2014).
The following are the peer-reviewed journal articles published to date that solely and specifically refer to the work in this programme:
Conroy SP, Stevens T, Parker SG, Gladman JRF. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing 2011;40:436–43. URL: http://dx.doi.org/10.1093/ageing/afr060 (accessed 27 November 2014).
Edmans J, Conroy S, Harwood R, Lewis S, Elliott RA, Logan P, et al. Acute medical unit comprehensive geriatric assessment intervention study (AMIGOS): study protocol for a randomised controlled trial. Trials 2011;12:200. URL: www.trialsjournal.com/content/pdf/1745-6215-12-200.pdf (accessed 7 April 2015).
Goldberg S, Whittamore K, Harwood R, Bradshaw L, Gladman J, Jones R. The prevalence of mental health problems amongst older adults admitted as an emergency to a general hospital. Age Ageing 2011;41:80–6. URL: http://dx.doi.org/10.1093/ageing/afr106 (accessed 27 November 2014).
Harwood RH, Goldberg SE, Whittamore KH, Russell C, Gladman JRF, Jones RG, et al. Evaluation of a medical and mental health unit compared with standard care for older people whose emergency admission to an acute general hospital is complicated by concurrent ‘confusion’: a controlled clinical trial. Trials 2011:12;123. URL: www.trialsjournal.com/content/pdf/1745–6215–12–123.pdf (accessed 27 November 2014).
Bradshaw LE, Goldberg SE, Schneider JM, Harwood R. Carers for older people with co-morbid cognitive impairment in general hospital: characteristics and psychological well-being. Int J Geriatr Psychiatry 2012;28:681–90. URL: http://dx.doi.org/10.1002/gps.3871 (accessed 27 November 2014).
Gordon AL, Logan PA, Jones RG, Forrester-Paton C, Mamo JP, Gladman JRF. A systematic mapping review of randomized controlled trials (RCTs) in care homes. BMC Geriatrics 2012;12:31. URL: http://dx.doi.org/10.1186/1471–2318–12–31 (accessed 27 November 2014).
Bradshaw LE, Goldberg SE, Lewis SA, Whittamore K, Gladman JRF, Jones RG, et al. Six-month outcomes following an emergency hospital admission for older adults with co-morbid mental health problems indicate complexity of care needs. Age Ageing 2013:42;582–8. URL: http://dx.doi.org/10.1093/ageing/aft074 (accessed 27 November 2014).
Edmans J, Bradshaw L, Franklin M, Gladman J, Conroy S. Randomised controlled trial of specialist geriatric medical assessment for patients discharged from hospital acute assessment units. BMJ 2013;347:f5874. URL: www.bmj.com/content/347/bmj.f5874 (accessed 27 November 2014).
Edmans J, Bradshaw L, Gladman JRF, Franklin M, Berdunov V, Elliott R, et al. The Identification of Seniors at Risk (ISAR) score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units. Age Ageing 2013;42:747–53. URL: http://dx.doi.org/10.1093/ageing/aft054 (accessed 27 November 2014).
Goldberg SE, Bradshaw SE, Kearney F, Russell C, Whittamore K, Foster PER, et al. Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347:f4132. URL: http://dx.doi.org/10.1136/bmj.f4132 (accessed 27 November 2014).
Goldberg SE, Harwood RH. Experience of general hospital care in older patients with cognitive impairment: are we measuring the most vulnerable patients’ experience? BMJ Qual Saf 2013;22:1–4. URL: http://qualitysafety.bmj.com/content/22/12/977.full.pdf+html (accessed 7 April 2015).
Robbins IJ, Gordon AL, Dyas JV, Logan PA, Gladman JRF. Explaining the barriers to and tensions in delivering effective health care in UK care homes: a qualitative study. BMJ Open 2013;3:e003178. URL: http://dx.doi.org/10.1136/bmjopen-2013–003178 (accessed 27 November 2014).
Spencer K, Foster P, Whittamore KH, Goldberg SE, Harwood RH. Delivering dementia care differently – evaluating the differences and similarities between a specialist medical and mental health unit and standard acute care wards: a qualitative study of family carers’ perceptions of quality of care. BMJ Open 2013;3:e004198. URL: http://dx.doi.org/10.1136/bmjopen-2013–004198 (accessed 27 November 2014).
Whittamore K, Goldberg S, Gladman JRF, Jones RG, Harwood RH. The diagnosis, prevalence and outcome of delirium in a cohort of older people with mental health problems on general hospital wards. Int J Geriatr Psychiatry 2013;29:32–40. URL: http://dx.doi.org/10.1002/gps.3961 (accessed 27 November 2014).
Wou F, Gladman JRF, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing 2013;42:776–81. URL: http://dx.doi.org/10.1093/ageing/aft055 (accessed 27 November 2014).
Franklin M, Berdunov V, Edmans J, Conroy S, Gladman JRF, Tanajewski L, et al. Identifying patient-level health and social care costs for older adults discharged from acute medical units in England. Age Ageing 2014;43:703–7.
Glover A, Bradshaw LE, Watson N, Laithwaite E, Goldberg SE, Whittamore KH, et al. Diagnoses, problems and healthcare interventions amongst older people with an unscheduled hospital admission who have concurrent mental health problems: a prevalence study. BMC Geriatr 2014;14:43. URL: www.biomedcentral.com/1471-2318/14/43 (accessed 7 April 2015).
Goldberg S, Harwood R, Whittamore K, Pollock K, Gladman JRF. Caring for cognitively impaired older patients in the general hospital: a qualitative analysis of similarities and differences between a specialist medical and mental health unit and standard care wards. Int J Nurs Stud 2014;51:1332–43. URL: http://dx.doi.org/10.1016/j.ijnurstu.2014.02.002 (accessed 27 November 2014).
Gordon AL, Franklin M, Bradshaw L, Logan PA, Elliott RA, Gladman JRF. Health status of UK care home residents – a cohort study. Age Ageing 2014;43:97–103. URL: http://dx.doi.org/10.1093/ageing/aft077 (accessed 27 November 2014).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Seshamani M, Gray A, Economics H. Ageing and health-care expenditure: the red herring argument revisited. Health Econ 2004;13:303-14. http://dx.doi.org/10.1002/hec.826.
- Thomas GM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med 2010;362:1173-80. http://dx.doi.org/10.1056/NEJMoa0909087.
- Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032-6. http://dx.doi.org/10.1016/0140-6736(93)92884-V.
- Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d6553.
- Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med 2013;11. http://dx.doi.org/10.1186/1741-7015-11-48.
- Woodard J, Gladman J, Conroy S. Frail older people at the interface. Age Ageing 2010;39.
- McCusker J, Bellavance F, Cardin S, Trépanier S, Verdon J, Ardman O. Detection of older people at increased risk of adverse health outcomes after an emergency visit: the ISAR screening tool. J Am Geriatr Soc 1999;47:1229-37.
- Who Cares Wins. Improving the Outcome for Older People Admitted to the General Hospital: Guidelines for the Development of Liaison Mental Health Services for Older People. London: Royal College of Psychiatrists; 2005.
- Gladman J, Porock D, Griffiths A, Clissett P, Harwood RH, Knight A, et al. Care of Older People With Cognitive Impairment in General Hospitals 2012. www.nets.nihr.ac.uk/__data/assets/pdf_file/0003/85071/ES-08-1809-227.pdf (accessed 12 March 2015).
- Office for National Statistics . Changes in the Older Resident Care Home Population Between 2001 and 2011 n.d. www.ons.gov.uk/ons/dcp171776_373040.pdf (accessed 11 February 2015).
- National Assistance Act 1948. London: The Stationery Office; 1948.
- Black D, Bowman C. Community institutional care for frail elderly people. Time to structure professional responsibility. BMJ 1997;315:414-15. http://dx.doi.org/10.1136/bmj.315.7106.441.
- Brown C, Lilford R. Evaluating service delivery interventions to enhance patient safety. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2764.
- Donabedian A. Evaluating the quality of medical care. Milbank Q 1966;83:691-729. http://dx.doi.org/10.1111/j.1468-0009.2005.00397.x.
- Conroy S, Stevens A, Gladman JRF. The Interface Between Acute Hospitals and Community Care for Older People Presenting to Acute Medical Units: A Mapping Review 2010. www.nottingham.ac.uk/mcop/documents/papers/issue6-mcop-issn2044-4230.pdf (accessed 27 November 2014).
- Baztan JJ, Suarez-Garcia FM, Lopez-Arrieta J, Rodriguez-Manas L, Rodriguez-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b50.
- Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull 2005;71:45-59. http://dx.doi.org/10.1093/bmb/ldh033.
- Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, et al. A systematic review of discharge arrangements for older people. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6040.
- Shepperd S, Doll H, Angus R, Clarke M, Iliffe S, Kalra L, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD007491.
- Ali W, Rasmussen P. What is the evidence for the effectiveness of managing the hospital/community interface for older people?. N Z Health Technol Assess Rep 2004;7.
- Day P, Rasmussen P. What is the evidence for the effectiveness of specialist geriatric services in acute, post-acute and sub-acute settings? A critical appraisal of the literature. N Z Health Technol Assess Rep 2004;7.
- Conroy SP, Stevens T, Parker SG, Gladman JRF. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing 2011;40:436-43. http://dx.doi.org/10.1093/ageing/afr060.
- Davison J, Bond J, Dawson P, Steen IN, Kenny RA. Patients with recurrent falls attending accident and emergency benefit from multifactorial intervention – a randomised controlled trial. Age Ageing 2005;34:162-8. http://dx.doi.org/10.1093/ageing/afi053.
- Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet 1999;353:93-7. http://dx.doi.org/10.1016/S0140-6736(98)06119-4.
- McCusker J, Dendukuri N, Tousignant P, Verdon J, Poulin de Courval L, Belzile E. Rapid two-stage emergency department intervention for seniors: impact on continuity of care. Acad Emerg Med 2003;10:233-43. http://dx.doi.org/10.1111/j.1553-2712.2003.tb01997.x.
- Mion LC, Palmer RM, Meldon SW, Bass DM, Singer ME, Payne SM, et al. Case finding and referral model for emergency department elders: a randomized clinical trial. Ann Emerg Med 2003;41:57-68. http://dx.doi.org/10.1067/mem.2003.3.
- Caplan GA, Williams AJ, Daly B, Abraham K. A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department – the DEED II study. J Am Geriatrics Soc 2004;52:1417-23. http://dx.doi.org/10.1111/j.1532-5415.2004.52401.x.
- Edmans JE, Gladman JRF, Havard D. Umbrella Review of Tools to Assess Risk of Poor Outcome in Older People Attending Acute Medical Units 2012. www.nottingham.ac.uk/mcop/documents/papers/issue11-mcop-issn2044-4230.pdf (accessed 27 November 2014).
- Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, de Rooij SE, Grypdonck MF. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs 2007;16:46-57. http://dx.doi.org/10.1111/j.1365-2702.2006.01579.x.
- Sutton M, Grimmer-Somers K, Jeffries L. Screening tools to identify hospitalised elderly patients at risk of functional decline: a systematic review. Int J Clin Pract 2008;62:1900-9. http://dx.doi.org/10.1111/j.1742-1241.2008.01930.x.
- McCusker J, Kakuma R, Ibrahamowicz M. Predictors of functional decline in hospitalized elderly patients: a systematic review. J Gerontol Ser A Biol Sci Med Sci 2002;57:M569-77.
- de Saint-Hubert M, Schoevaerdts D, Cornette P, D’Hoore W, Boland B, Swine C. Predicting functional adverse outcomes in hospitalized older patients: a systematic review of screening tools. J Nutr Health Aging 2010;4:394-9. http://dx.doi.org/10.1007/s12603-010-0086-x.
- Sager MA, Rudberg MA, Jalaluddin M, Franke T, Inouye SK, Landefeld CS, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc 1996;44:251-7.
- Meldon SW, Mion LC, Palmer RM, Drew BL, Connor JT, Lewicki LJ, et al. A brief risk stratification tool to predict repeat emergency department visits and hospitalizations in older patients discharged from the emergency department. Acad Emerg Med 2003;10:224-32. http://dx.doi.org/10.1111/j.1553-2712.2003.tb01996.x.
- Edmans J, Bradshaw L, Gladman JRF, Franklin M, Berdunov V, Elliott R, et al. The Identification of Seniors at Risk (ISAR) score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units. Age Ageing 2013;42:747-53. http://dx.doi.org/10.1093/ageing/aft054.
- Wade DT, Collin CL. The Barthel ADL index: a standard measure of physical disability?. Int Dis Studies 1988;10:64-7. http://dx.doi.org/10.3109/09638288809164105.
- Goldberg D. General Health Questionnaire (GHQ-12). Windsor: NFER-NELSON; 1992.
- Brazier J, Walters SJ, Nicholl JP, Kohler B. Using the SF-36 and EuroQol on an elderly population. Qual Life Res 1996;5:195-204. http://dx.doi.org/10.1007/BF00434741.
- Wou F, Gladman JRF, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing 2013;42:776-81. http://dx.doi.org/10.1093/ageing/aft055.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. http://dx.doi.org/10.1093/gerona/56.3.M146.
- Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008;168:382-9. http://dx.doi.org/10.1001/archinternmed.2007.113.
- Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc 2008;56:2211-16. http://dx.doi.org/10.1111/j.1532-5415.2008.02008.x.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009;57:453-61. http://dx.doi.org/10.1111/j.1532-5415.2008.02136.x.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatrics 2008;8. http://dx.doi.org/10.1186/1471-2318-8-24.
- Franklin M, Berdunov V, Edmans J, Conroy S, Gladman JRF, Tanajewski L, et al. Identifying patient-level health and social care costs for older adults discharged from acute medical units in England. Age Ageing 2014;43:703-7. http://dx.doi.org/10.1093/ageing/afu073.
- Department of Health . NHS Reference Costs 2009–10 n.d. www.gov.uk/government/publications/nhs-reference-costs-2009-2010 (accessed 22 March 2015).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Gladman JRF, Kearney F, Ali A, Blundell A, Wong R, Laithwaite E, et al. The Role of the Interface Geriatrician Across the Acute Medical Unit Community Interface 2012. www.nottingham.ac.uk/mcop/documents/papers/issue9-mcop-issn2044-4230.pdf (accessed 27 November 2014).
- Edmans J, Conroy S, Harwood R, Lewis S, Elliott RA, Logan P, et al. Acute medical unit comprehensive geriatric assessment intervention study (AMIGOS): study protocol for a randomised controlled trial. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-200.
- Edmans J, Bradshaw L, Franklin M, Gladman J, Conroy S. Randomised controlled trial of specialist geriatric medical assessment for patients discharged from hospital acute assessment units. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5874.
- Robinson B. C. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344-8. http://dx.doi.org/10.1093/geronj/38.3.344.
- McMillan SC, Mahon M. The impact of hospice services on the quality of life of primary caregivers. Oncol Nurs Forum 1994;21:1189-95.
- Gladman JRF, Jurgens F, Harwood R, Goldberg S, Logan P. Better Mental Health in General Hospitals 2010. www.nottingham.ac.uk/mcop/documents/papers/mcop-issn2044-4230-issue3.pdf (accessed 27 November 2014).
- Adams T. Kitwood’s approach to dementia and dementia care: a critical but appreciative review. J Adv Nurs 1996;23:948-53. http://dx.doi.org/10.1046/j.1365-2648.1996.10613.x.
- Department of Health . Living Well With Dementia: A National Dementia Strategy 2009. www.gov.uk/government/uploads/system/uploads/attachment_data/file/168221/dh_094052.pdf (accessed 11 March 2015).
- Holmes J, Montaña C, Powell G, Hewison J, House A, Mason J, et al. Liaison Mental Health Services for Older People: A Literature Review, Service Mapping and In-Depth Evaluation of Service Models 2010. www.nets.nihr.ac.uk/__data/assets/pdf_file/0017/64502/FR-08-1504-100.pdf (accessed 11 March 2015).
- Brooker D. Dementia care mapping: a review of the research literature. Gerontologist 2005;45:11-8. http://dx.doi.org/10.1093/geront/45.suppl_1.11.
- University of Bradford, School of Health Studies . Welcome to Dementia Care Mapping n.d. www.brad.ac.uk/health/dementia/DementiaCareMapping (accessed 14 January 2010).
- Gladman JRF, Harwood RH, Jones RJ, Porock D, Griffiths A, Schneider J, et al. Medical and Mental Health Better Mental Health Development Study Protocol 2012. www.nottingham.ac.uk/mcop/documents/papers/issue10-mcop-issn2044-4230.pdf.
- Goldberg S, Whittamore K, Harwood R, Bradshaw L, Gladman J, Jones R. The prevalence of mental health problems amongst older adults admitted as an emergency to a general hospital. Age Ageing 2012;41:80-6. http://dx.doi.org/10.1093/ageing/afr106.
- Bradshaw LE, Goldberg SE, Lewis SA, Whittamore K, Gladman JRF, Jones RG, et al. Six-month outcomes following an emergency hospital admission for older adults with co-morbid mental health problems indicate complexity of care needs. Age Ageing n.d.;42:582-8. http://dx.doi.org/10.1093/ageing/aft074.
- Bradshaw LE, Goldberg SE, Schneider JM, Harwood R. Carers for older people with co-morbid cognitive impairment in general hospital: characteristics and psychological well-being. Int J Geriatr Psychiatry 2012;28:681-90. http://dx.doi.org/10.1002/gps.3871.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. http://dx.doi.org/10.1093/ageing/1.4.233.
- Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999;14:858-65. http://dx.doi.org/10.1002/(SICI)1099-1166(199910)14:10<858::AID-GPS35>3.0.CO;2-8.
- Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. JAMA 1994;272:1749-56. http://dx.doi.org/10.1001/jama.1994.03520220043029.
- Ewing JA. Detecting alcholism: the CAGE questionnaire. JAMA 1984;252:1905-7. http://dx.doi.org/10.1001/jama.1984.03350140051025.
- Gladman JRF, Harwood RH, Conroy SP. Medical Crises in Older People Study Group . Days at Home: An Outcome Measure in Studies of Specialist Services Providing Care for Older People 2010. www.nottingham.ac.uk/mcop/documents/papers/issue4-mcop-issn2044-4230.pdf (accessed 27 November 2014).
- Harwood RH, Porock D, King N, Edwards G, Hammond S, Howe L, et al. Development of a Specialist Medical and Mental Health Unit for Older People in an Acute General Hospital 2010. www.nottingham.ac.uk/mcop/documents/papers/issue5-mcop-issn2044-4230.pdf (accessed 27 November 2014).
- Waite J, Harwood R, Morton I, Connelly D. Dementia Care: A Practical Manual. Oxford: Oxford University Press; 2008.
- Mental Health Act 2007. London: The Stationery Office; 2007.
- Goldberg SE, Bradshaw SE, Kearney F, Russell C, Whittamore K, Foster PER, et al. Medical Crises in Older People study group . Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4132.
- Spencer K, Foster P, Whittamore KH, Goldberg SE, Harwood RH. Delivering dementia care differently – evaluating the differences and similarities between a specialist medical and mental health unit and standard acute care wards: a qualitative study of family carers’ perceptions of quality of care. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-004198.
- Goldberg S, Harwood R, Whittamore K, Pollock K, Gladman JRF. Caring for cognitively impaired older patients in the general hospital: a qualitative analysis of similarities and differences between a specialist medical and mental health unit and standard care wards. Int J Nurs Stud 2014;51:1332-43. http://dx.doi.org/10.1016/j.ijnurstu.2014.02.002.
- Whittamore KH, Goldberg SE, Bradshaw LE, Harwood RH. Medical Crises in Older People study group . Factors associated with family caregiver dissatisfaction with acute hospital care of older cognitively impaired relatives. J Am Geriatr Soc 2014;62:2252-60. http://dx.doi.org/10.1111/jgs.13147.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9100.
- EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Harwood RH, Ebrahim S. Manual of the London Handicap Scale. Nottingham: University of Nottingham; 1995.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. http://dx.doi.org/10.1212/WNL.44.12.2308.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Counting the Cost. Caring for People with Dementia on Hospital Wards. London: Alzheimer’s Society; 2009.
- Brooker D. Person-Centred Dementia Care: Making Services Better (Bradford Dementia Group Good Practice Guides). London: Jessica Kingsley; 2006.
- Murphy J. Talking Mats: Speech and Language Research in Practice 1998:11-4. www.scribd.com/doc/218143093/Talking-Mats-Speech-and-language-research-in-practice (accessed 11 March 2015).
- Murphy J. Enabling people with aphasia to discuss quality of life. Br J Ther Rehabil 2000;7:454-7. http://dx.doi.org/10.12968/bjtr.2000.7.11.13835.
- Murphy J, Cameron L. The effectiveness of Talking Mats® with people with intellectual disability. Br J Learn Disabil 2008;36:232-41. http://dx.doi.org/10.1111/j.1468-3156.2008.00490.x.
- Ferm U, Sahlin A, Sundin L, Hartelius L. Using Talking Mats to support communication in persons with Huntington’s disease. Int J Lang Commun Disord 2010;45:523-36. http://dx.doi.org/10.3109/13682820903222809.
- Murphy J, Gray CM, Cox S. Communication and Dementia: Talking Mats – Helping People With Dementia to Express Their Views 2007. www.jrf.org.uk/sites/files/jrf/2128-talking-mats-dementia.pdf (accessed 11 March 2015).
- Lindhardt T, Nyberg P, Hallberg IR. Collaboration between relatives of elderly patients and nurses and its relation to satisfaction with the hospital care trajectory. Scand J Caring Sci 2008;22:507-19. http://dx.doi.org/10.1111/j.1471-6712.2007.00558.x.
- Tadd W, Hillman A, Calnan S, Calnan M, Bayer A, Read S. Dignity in Practice: An Exploration of the Care of Older Adults in Acute NHS Trusts 2011. www.nets.nihr.ac.uk/__data/assets/pdf_file/0007/64546/FR-08-1819-218.pdf (accessed 11 March 2015).
- Gordon AL, Logan PA, Jones RG, Forrester-Paton C, Mamo JP, Gladman JRF, et al. A systematic mapping review of randomized controlled trials (RCTs) in care homes. BMC Geriatr 2012;12. http://dx.doi.org/10.1186/1471-2318-12-31.
- Gordon AL. Does Comprehensive Geriatric Assessment (CGA) Have a Role in UK Care Homes? 2012.
- Gordon AL, Franklin M, Bradshaw L, Logan PA, Elliott RA, Gladman JRF. Health status of UK care home residents – a cohort study. Age Ageing 2013;43:97-103. http://dx.doi.org/10.1093/ageing/aft077.
- Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001;56:M366-72. http://dx.doi.org/10.1093/gerona/56.6.M366.
- Robbins IJ, Gordon A, Dyas J, Logan P, Gladman J. Explaining the barriers to and tensions in delivering effective health care in UK care homes: a qualitative study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003178.
- BBC News . Cameron: Long Way to Go on NHS Care 2013. www.bbc.co.uk/news/health-20898401 (accessed 5 February 2015).
- BBC News . Action Urged over ‘Appalling’ NHS Care 2012. www.bbc.co.uk/news/health-20427439 (accessed 5 February 2015).
- A Quest for Quality in Care Homes. Inquiry into the Quality of Healthcare Support for Older People in Care Homes: A Call for Leadership, Partnership and Quality Improvement. London: British Geriatrics Society; 2011.
- Francis R. Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry. London: The Stationery Office; 2013.
- Department of Health . The NHS Outcomes Framework for 2012–13 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/213711/dh_131723.pdf (accessed 9 February 2015).
- Conroy S. British Geriatrics Society . Quality Care for Older People With Urgent and Emergency Care Needs (‘The Silver Book’) 2012. www.bgs.org.uk/campaigns/silverb/silver_book_complete.pdf (accessed 11 March 2015).
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-Revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001;13:229-42. http://dx.doi.org/10.1176/jnp.13.2.229.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-40.
- National Institute for Health Research . Enabling Research in Care Homes. A Toolkit for Care Home Research n.d. www.dendron.nihr.ac.uk/enrich/ (accessed 6 February 2015).
- Quin TJ, Dawson J, Lees JS, Chang TP, Walters MR, Lees KR. GAIN and VISTA investigators . Time spent at home poststroke: ‘home-time’ a meaningful and robust outcome measure for stroke trials. Stroke 2008;39:231-3. http://dx.doi.org/10.1161/STROKEAHA.107.493320.
- Mishra NK, Shuaib A, Lyden P, Diener HC, Grotta J, Davis S, et al. Home time is extended in patients with ischemic stroke who receive thrombolytic therapy. Stroke 2011;42:1046-50. http://dx.doi.org/10.1161/STROKEAHA.110.601302.
- Department of Health . NHS Informatics. Final Benefits Statement for Programmes Previously Managed under the National Programme for IT n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/205687/Benefits_statement.pdf (accessed 12 March 2015).
- Health & Social Care Information Centre . National Health Service Information Centre HRG-4 Reference Cost Grouper 2009 10 2011. www.ic.nhs.uk/services/the-casemix-service/using-this-service/reference/downloads/costing/hrg4-2010-11-reference-costs-grouper-documentation (accessed 12 March 2015).
- Geue C, Lewsey J, Lorgelly P, Govan L, Hart C, Briggs A. Spoilt for choice: implications of using alternative methods of costing hospital episode statistics. Health Econ 2012;21:1201-16. http://dx.doi.org/10.1002/hec.1785.
- Department of Health . NHS Reference Costs 2011–12 n.d. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed 22 March 2015).
- Curtis L. Unit Costs of Health and Social Care 2012. University of Kent, Personal Social Services Research Unit; 2012.
- Sartain K. An outstanding example of hospital care for patients with dementia. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2513.
- Adamis D. Ethical research in delirium: arguments for including decisionally incapacitated subjects. Sci Eng Ethics 2010;16:169-74. http://dx.doi.org/10.1007/s11948-009-9120-y.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Coats TJ. Consent for emergency care research: the Mental Capacity Act 2005. Emerg Med J 2006;23:893-4. http://dx.doi.org/10.1136/emj.2006.041640.
- Griffith R. A nurse prescriber’s guide to the Mental Capacity Act 2005. Nurse Prescr 2006;4:245-9. http://dx.doi.org/10.12968/npre.2006.4.6.21506.
- Bridging the ‘Know–Do’ Gap. Geneva: World Health Organization; 2006.
- Cooksey D. A Review of Health Research Funding. London: The Stationery Office; 2006.
- Weiss CH. The many meanings of research utilization. Public Adm Rev 1979;39:426-31. http://dx.doi.org/10.2307/3109916.
- Rycroft-Malone J, Wilkinson JE, Burton CR, Andrews G, Ariss S, Baker R, et al. Implementing health research through academic and clinical partnerships: a realistic evaluation of the Collaborations for Leadership in Applied Health Research and Care (CLAHRC). Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-74.
- Banzi R, Moja L, Pistotti V, Facchini A, Liberati A. Conceptual frameworks and empirical approaches used to assess the impact of health research: an overview of reviews. Health Res Policy Syst 2011;9. http://dx.doi.org/10.1186/1478-4505-9-26.
- Kuruvilla S, Mays N, Pleasant A, Walt G. Describing the impact of health research: a research impact framework. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-134.
- Hanney S, Grant J, Wooding S, Buxton MJ. Proposed methods for reviewing the outcomes of health research: the impact of funding by the UK’s ‘Arthritis Research Campaign’. Health Res Policy Syst 2004;2. http://dx.doi.org/10.1186/1478-4505-2-4.
- Thorpe M. Quest for Quality. London: British Geriatrics Society; 2011.
- Howe L. Occupational engagement for patients with dementia and delirium. Occup Ther News 2011;19:30-1.
- East Midlands Academic Health Science Network . Igniting Innovation Prospectus 2012. www.clahrc-ndl.nihr.ac.uk/clahrc-ndl-nihr/documents/central-documents/emahsn-prospectus.pdf (accessed 11 March 2015).
- Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al. A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11150.
- Guide to the Methods of Technology Appraisal. London: NICE; 2004.
- Sartini M, Cristina ML, Spagnolo AM, Cremonesi P, Costaguta C, Monacelli F, et al. The epidemiology of domestic injurious falls in a community dwelling elderly population: an outgrowing economic burden. Eur J Public Health 2010;20:604-6. http://dx.doi.org/10.1093/eurpub/ckp165.
- Timonen L, Rantanen T, Mäkinen E, Timonen TE, Törmäkangas T, Sulkava R. Cost analysis of an exercise program for older women with respect to social welfare and healthcare costs: a pilot study. Scand J Med Sci Sports 2008;18:783-9. http://dx.doi.org/10.1111/j.1600-0838.2007.00752.x.
- Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg 2011;202:511-14. http://dx.doi.org/10.1016/j.amjsurg.2011.06.017.
- Bouman A, van Rossum E, Evers S, Ambergen T, Kempen G, Knipschild P. Multidimensional geriatric assessment: effects on health care use and associated cost of a home visiting program for older people with poor health status: a randomized clinical trial in the Netherlands. J Gerontol A Biol Sci Med Sci 2008;63:291-7. http://dx.doi.org/10.1093/gerona/63.3.291.
- Kehusmaa S, Autti-Rämö I, Valaste M, Hinkka K, Rissanen P. Economic evaluation of a geriatric rehabilitation programme: a randomized controlled trial. J Rehabil Med 2010;42:949-55. http://dx.doi.org/10.2340/16501977-0623.
- Melis RJF, Adang E, Teerenstra S, van Eijken MIJ, Wimo A, van Achterberg T, et al. Multidimensional geriatric assessment: back to the future cost-effectiveness of a multidisciplinary intervention model for community-dwelling frail older people. J Gerontol A Biol Sci Med Sci 2008;63:275-82. http://dx.doi.org/10.1093/gerona/63.3.275.
- Philp I, Mills KA, Thanvi B, Ghosh K, Long JF. Reducing hospital bed use by frail older people: results from a systematic review of the literature. Int J Integr Care 2013;13.
- Johri M, Beland F, Bergman H. International experiments in integrated care for the elderly: a synthesis of the evidence. Int J Geriatric Psychiatry 2003;18:222-35. http://dx.doi.org/10.1002/gps.819.
- Kehusmaa S, Autti-Rämö I, Helenius H, Hinkka K, Valaste M, Rissanen P. Factors associated with the utilization and costs of health and social services in frail elderly patients. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-204.
- Goodwin N, Smith J, Davies A, Perry C, Rosen R, Dixon A, et al. A Report to the Department of Health and NHS Future Forum: Integrated Care for Patients and Populations: Improving Outcomes by Working Together n.d. www.nuffieldtrust.org.uk/sites/files/nuffield/publication/integrated_care_for_patients_and_populations_-_improving_outcomes_by_working_together_jan12.pdf (accessed 11 March 2015).
- Lewis J, West A. Re-shaping social care services for older people in England: policy development and the problem of achieving ‘good care’. J Soc Policy 2014;43:1-18. http://dx.doi.org/10.1017/S0047279413000561.
- British Geriatrics Society and Royal College of General Practitioners . Interface Between Primary and Secondary Medical Care n.d. www.bgs.org.uk/index.php/topresources/publicationfind/goodpractice/360-primarysecondarycareinterface?showall = &start = 2 (accessed 9 February 2015).
- Lewis GH, Georghiou T, Steventon A, Vaithianathan R, Chitnis X, Billings J, et al. Impact of ‘Virtual Wards’ on Hospital Use: A Research Study Using Propensity Matched Controls and a Cost Analysis n.d. www.nets.nihr.ac.uk/__data/assets/pdf_file/0011/87923/FR-09-1816-1021.pdf (accessed 11 March 2015).
Appendix 1 Grant submission documents
Planning for this study began in 2006, followed by a first submission in 2007, an award in 2008 and completion in 2013. The first-stage proposal consisted of < 4500 words. Five external reviews were undertaken and a response to these queries was returned (> 10,000 words) with two further full research trial protocols (7000 and 8000 words). No single final full research proposal was created. In this appendix the first-stage proposal and our response to reviewers are provided, to give an indication of the review process involved. Note that our first-stage proposal included a fourth workstream that was advised against by the reviewers and so was not part of the final agreed research project.
First-stage proposal text
This programme concerns the development and evaluation of specialist services for frail older people with, or at risk of, medical crises. There are four workstreams for different frail patient groups: older people admitted to hospital as a medical emergency with significant co-morbid mental health conditions, older people presenting to the acute medical unit but who are not admitted to hospital, patients with hip fracture, and residents of care homes. The plan of the research is presented by first covering those aspects of the research that are common to all four workstreams, followed by descriptions of the unique aspects of each workstream. The timing of Milestones and Outputs is shown on the Gantt chart (Annex 3), and are listed for each workstream as M1, M2. . . or O1, O2. . ., etc.
1. Common methodological issues
Certain aspects of this research will be common to all four strands. It is through these common processes that the secondary objectives (1–5, Full Form Section 8) will be achieved. These include:
-
a single research programme board, comprising all grantholders
-
a larger reference group, including user groups, Trust personnel, clinical teams & other researchers
-
the development of a common user involvement mechanism
-
a common core baseline and outcome dataset
-
a common intervention ideology (Comprehensive Geriatric Assessment)
-
a shared methodology requiring a preparatory stage, a stage in which a cohort or register study is performed from which risk stratification factors can be identified to target interventions upon those most likely to benefit and from which data to design evaluative studies can be drawn, a stage of service optimisation and characterisation of the complex intervention to be delivered, and a final phase in which an exploratory controlled trial is performed
-
a co-ordinated dissemination and implementation strategy for rapid patient benefit, including a programme web-site
These common aspects will enable the programme to meet its 5 secondary objectives, and justify the need for long term and large scale programme support.
1.1 The common core data set will include:
-
key baseline patient variables and scores related to frailty: socio-demographics, frailty rating scale (Rockwood Frailty Scale) [10.1] proximity to death (Palliative Care Index) [10.2], pain questions, nutrition (Mini Nutrition Assessment) [10.3], co-morbidity (Charlston Comorbidity Index) [10.4], personal ADL (Barthel ADL) [10.5], cognition (MMSE) [10.6], psychiatric health (General Health Questionnaire, GHQ-12) [10.7], behaviour (Neuropsychiatric Inventory, NPI) [10.8] and quality of life (EQ5D) [10.9]. Baseline carer well-being (Caregiver Quality of Life Index (CQLI) [10.10])
-
key patient outcomes associated with frailty: clinical outcomes at 90 days (survival, place of residence, days spent at home, Barthel, NPI, GHQ-12, EQ5D) and resource use over 6 months. Carer well-being at 90 days (CQLI).
1.2 The common approach taken to optimise and characterise the services in each strand will be closely steered by the research programme board to ensure consistency
The objectives in this phase for each strand are:
-
to develop a service that delivers comprehensive geriatric assessment (CGA)
-
to demonstrate that a generalisable service is in place that is fit for purpose and evaluation.
In this programme, comprehensive geriatric assessment is a set of principles rather than a recipe. These principles are that:
-
there are groups of people in the four areas of interest of this programme who can be identified as frail, which means that they have multiple medical, psychiatric and social disadvantages, are usually disabled, are often close to death and are heavy users of services
-
assessment of their physical and mental health and the contextual factors that influence them is required, leading to the delivery of multi-faceted interventions to improve outcomes
It is also a premise that specialist medical expertise, and also expertise in the psychiatric aspects of management of these patients in medical settings, is likely to be lacking in existing, generalist, services.
An iterative, complex human systems approach will be employed to develop services [10.11]. In this approach a service is seen as a complex human system, and defined (in a ‘root definition’) by the interactions between six factors:
-
the patients
-
the staff
-
the interventions made by staff and the changes made to the patients
-
the managerial systems that control the services
-
the national context that influences services
-
the local context that influences these services.
The lead clinician for each strand will develop a root definition for each service, discussing it with the research programme board, and the relevant parts of the programme reference group. Doing so will lead to the identification of obstacles to service implementation including staffing issues, involvement of key personnel, training needs, development of operational protocols, etc. Close working between the research programme board and the NHS Trust is crucial, and has been assured (see Annex 4). The developmental stage will be highly user-sensitive and will make use of public involvement mechanisms in the acute Trust (Nottingham University Hospitals NHS Trust) and a community Trust (Nottinghamshire Country Teaching PCT).
Although the root definition of a service is necessarily largely descriptive, the description will be generalisable by virtue of specific research outputs including:
-
clearly defined and justified criteria to select patients
-
description of staffing numbers, their expertise, and working patterns
-
an observational cohort study and case studies describing evidence of the delivery of comprehensive assessment, appropriate interventions, and outcomes compatible with clinical effectiveness
-
user and staff views.
1.3 Evaluative trial with concurrent qualitative and economic study
For each strand the following specific points describe the general stance this programme will take to the evaluation of these complex interventions:
-
A single centre, single blinded, controlled trial will be performed.
-
Trial protocols will be published.
-
Randomised studies will use web based allocation.
-
Special effort will be made regarding consent and assent procedures, and carers will also be consented since they will also be trial participants (their outcomes will also be measured).
-
Outcomes will be measured and analysed by researchers independent of the service and masked to allocation.
-
Each trial will adhere to standard trial operation procedures.
-
Trials will be powered for meaningful clinical outcomes (for example, days spent at home by 3 months), with the anticipation that larger and multi-centre studies may be required to replicate the findings and improve the precision of estimates of treatment effects on secondary outcomes.
-
The definition of the intervention and control treatments (and the difference between them) will be determined quantitatively using indicators of service delivery but also through qualitative (interview) studies of participants and staff. Such work is important in trials of complex interventions to improve the generalisability of the findings, and to explain the associations between service delivery and outcomes.
-
The perspective for costs taken will be that of the third party payer: NHS, personal social services and private care home sector. Resource use data will be collected for the index admission or care episode, along with subsequent secondary care (inpatient and outpatient episodes), primary and community (geriatric day hospital, GP and social services home care services) contact and specialist accommodation requirements. GP records are a good source for primary care data, but patient or carer reports are more reliable for other service contacts [10.12]. A modified version of the Client Service Receipt Inventory [10.13] will be developed to capture these data across all workstreams. Unit costs will be taken from routine local or national sources, as appropriate. Depending on the level of skewness in the data, differences in costs will be analysed using t-tests or non-parametric bootstrapping. In line with standard practice, the EQ-5D will be used in the generation of QALYs in economic studies to generate conventional incremental cost effectiveness ratios (ICERs). Where appropriate, patients will provide EQ-5D data, and where this is not possible, carers will provide proxy EQ-5D ratings. In view of the limitations of this instrument in this population group, we will also use disease specific quality of life measures in each strand, and examine their relationship to the EQ-5D. QALYs will also be extrapolated to lifetime, and discounted at 3.5% to estimate the cost effectiveness of the interventions over the lifetime of the target population. Uncertainty around ICERs will be expressed using appropriate probabilistic methods including bias-corrected bootstrapping methods. Cost effectiveness acceptability curves and estimates of expected value of information will be generated to inform decision-making and to quantify the costs and benefits of further research.
1.4 The dissemination, implementation and rapid patient benefit strategy
Formal outputs from this programme will appear soon after the second programme year (see Gantt chart) and will continue throughout and beyond the 5 years of the programme. This programme will produce a range of scholarly and practical material relating to frail older people: their outcomes, how they can be assessed, how they can be treated, and ultimately the effect of the use of Comprehensive Geriatric Assessment in their care. The work will be included within the Trust R&D strategy, which itself is liked to the Trust development and implementation strategies. Such close working will help to ensure that the material we produce is in a form that is usable to NHS providers. Close working with user representative groups will ensure that material is also suitable for these users, and hence can be used in public involvement exercises: we will also use the extensive and effective dissemination networks of these groups. Our website will describe our programme and findings for the national and international audience.
1.5 Programme management and planning
The principal investigator is Professor John Gladman who will direct the study researchers (two post-doctoral researchers, three research assistants, and a trial secretary) and oversee the four workstrand leaders.
The main decision making and planning will be undertaken through the regular programme board meetings, which will be monthly for the first year, bimonthly for the next 18 months and 3 monthly thereafter (see Gantt chart).
There will be a larger reference group, which will not meet but be consulted as required throughout the study. The reference group will include NHS managers, ward staff and patient involvement staff, user group representatives
2. Primary objective 1: Evaluation of Medical and Mental Health Unit (MMHU)
2.1 Preparatory phase
We will liaise with the team undertaking the SDO programme literature review of this area that is in progress. Research staff will become familiar with the key papers in this area, and in the use of measurement scales for this patient group. Regulatory approval will be obtained. Months 1–3.
2.2 Selected cohort (register) study
Objectives: to obtain descriptive statistics of the health and service outcomes of people admitted to hospital as a medical emergency who have co-morbid mental health conditions, and to identify predictors of adverse outcomes and use of services.
Design: cohort (register) study of 240 patients.
Inclusion criteria: age > 65, admitted as emergency to medical, surgical or geriatric ward, referred to hospital geriatric liaison team (the Interdisciplinary Discharge Team IDT) and with a co-morbid mental health problem (cognitive impairment/disorder of affect/behaviour disorder).
Exclusion criteria: not resident locally, already deemed terminally ill, other specific medical need (e.g. HDU, surgery).
Baseline measurements: common core baseline dataset (see 1.1) plus CAM [10.14].
Follow up measurements: common core outcome dataset (see 1.1) plus Demqol [10.15].
Analysis: The influence of baseline factors, particularly the co-morbid mental health problems, upon resource use and health outcomes will be examined. A sample of 240 participants (3 recruited per working day for 4 months) is feasible and provides the power to examine 12 predictive variables and estimate the mean length of stay to within 4 days.
Timescale: preparation months 1–3, recruitment months 4–7, follow up to month 13, analysis and reporting to month 19.
Milestones: ethics/R&D approval (M1), commencement/completion of recruiting (M2, M3) and follow up at 3 & 6 months (M4, M5 & M6, M7).
Outputs: internal – mean and SD of key outcomes for evaluative study; external – dissemination of risk stratification findings (O1).
2.3 Service development and characterisation phase
Objectives: to demonstrate the delivery of a feasible, acceptable, evidence-based MMHU; to secure ethical committee and R&D approval for the evaluative study (2.4); to pilot the RCT.
Methods: common approach to service development and characterisation (see 1.2). Entry to the MMHU will be by random allocation during the development phase. This will ensure that acceptability issues (uninformed patients or family may initially have objections to such a unit) are dealt with, that the nature of the patients in the development cohort resembles those in the subsequent evaluative trial (2.4), and provide other pilot information for it.
Setting: current plans are for a secure self contained 20 bedded ward, developed from existing geriatric medical beds, with additional mental health nursing input, and specific arrangements for liaison with specialist, voluntary and community psychiatric services. Professor Harwood (workstrand leader) will have clinical responsibility for patients, and this will be part of his usual clinical NHS duties.
Outcome: the principal outcome is agreement between the research team and the Trust that the service provided by the MMHU is fit for purpose.
Timescale: months 1–24.
Milestones: recruitment of first patient to ward (M8), ethics committee and R&D approval for 2.4 (M9).
Output: internal – detailed descriptive information to support service delivery, pilot trial data (recruitment rates, means and SDs of principal outcomes); external – descriptive report for publication (O2), publication of protocol (O3).
2.4 Evaluative trial
Objective: to compare the effect of a MMHU and usual acute hospital care for older people admitted as a medical emergency with significant co-morbid mental health conditions upon their and their carers’ health outcomes, and upon resource use.
Design: individually randomised controlled trial.
Participants: will be defined during stages 2.2 and 2.3.
Identification and recruitment: referrals will be made using existing systems of nurse-led assessment for older people with potential rehabilitation needs and complex discharge planning (the ‘integrated discharge team’), which includes a specialist mental health nurse. Patient and carer (both) consent or assent will be obtained prior to randomisation. Patients without consent/assent will not be transferred to the MMHU.
Intervention: as per 2.3.
Control: standard care without transfer to the MMHU or involvement of that team (access to usual services – geriatric, psychiatric consultation and intermediate care – will not be affected).
Randomisation: Individual patient, web-based, randomisation. We will devise a mechanism to ensure that delays in transfer (for example due to bed availability) are minimised by not randomising when there is no bed.
Baseline measurements: common core baseline dataset (see 1.1) plus CAM.
Follow up measurements: common core outcome dataset (see 1.1) plus Demqol.
Principal outcome measure: proportion discharged home.
Secondary outcome measures: number of days spent at home over 6 months post randomisation, proportion living at home at 3 months, mortality, total days of hospital stay in 6 months, and scaled functional and quality of life outcomes for patient and carer at 3 months. These outcome measures will necessarily be interviewer-administered.
Economic study: see 1.3.
Concurrent qualitative study: see 1.3.
Sample size: allowing for drop outs, using 1 : 1 randomisation, 400 participants (200 in each arm) will allow us to detect at 90% power a 17% increase in home discharge rate (22% to 39%, or 33% to 50%). This number should be achievable: during 18 months, with a 4 week anticipated length of stay, there should be 360 patients going through the unit.
Timescale: recruit months 25–42, follow up months 28–48, analysis and dissemination months 49–60.
Milestones: first and last recruit (M10, M13); first and last follow up at 3 and 6 months (M11, M12, M14, M15).
Outputs: main publication (O4).
3. Primary objective 2: Evaluation of Acute Medical Unit Comprehensive Geriatric Assessment
3.1 Preparatory stage
An important first step in the delivery of CGA in this setting is to identify those most likely to benefit from it so that this process can be targeted. Our searching has already indicated that a screening tool for use in acute medical settings to predict outcomes already exists, hence our decision in 3.2 to validate or amend this. During this phase, research staff will become familiar with the key papers in this area, and in the use of measurement scales for this patient group. Regulatory approval will be obtained. Months 1–3.
3.2 Cohort study
Objectives: to validate a modified version of the Identification of Seniors At Risk (ISAR) tool – a North American screening tool used to identify older people attending emergency medical settings at risk of severe functional decline, institutionalisation or death [10.16].
Design: cohort study.
Participants: 500 consecutive patients presenting to the Nottingham Acute Medical Unit.
Inclusion criteria: age > 70, not admitted to hospital.
Exclusion criteria: not resident in the Nottingham area.
Baseline measurements: common core baseline dataset (see 1.1).
Follow up measurements: common core outcome dataset (see 1.1).
Analysis: sensitivity, specificity and likelihood ratios will be calculated for severe functional decline (≥ 2 point decrease in Barthel score), institutionalisation and death. Comparisons with previously reported studies will be carried out using Area Under the Curve (AUC) analysis.
Sample size estimation: a sample size of 500 participants, with 10% having adverse outcomes, will allow us to estimate the sensitivity of the ISAR tool to within 14%.
Timescale: preparation months 1–3, recruitment months 4–10, follow up to month 16, analysis and reporting to month 19.
Milestones: ethics and R&D approval (M1), commencement and completion of recruitment (M2, M4) and follow up at 3 and 6 months (M3, M5, M6, M7).
Outputs: internal – mean and SD of key outcomes for evaluative study; external – description of the outcomes of this hitherto little studied group and the validity of the ISAR tool to stratify for poor outcomes (O1).
3.3 Service development and characterisation phase
Objectives: to demonstrate the delivery of feasible, acceptable, evidence-based CGA for frail older patients attending, but not admitted to hospital from, an acute medical unit; to secure ethical committee and R&D approval for the evaluative study (3.4).
Methods: common approach to service development and characterisation (see 1.2). Pilot work along these lines is already in progress (see Full Application Form section 9). It is not necessary to randomise patients during this phase.
Setting and intervention: intervention will be the addition (to all existing care) of specialist geriatric medical assessment and management with active community-based follow-up, using and liaising with rapid access clinics, intermediate care services, community matrons, care home staff and the primary care team. Current service development is being undertaken by Dr Conroy (co-applicant, an experienced Lecturer and SpR in geriatric medicine), but it is planned that this CGA service will be consultant-led. The new clinical work will be supported by NHS support costs which, alongside existing Trust resources for development and programme-funded R&D sessions, will be used to create a new academic post.
Outcome: the principal outcome is agreement between the research team and the Trust that the service is fit for purpose.
Timescale: months 1–22.
Milestones: agreement statement that service is fit for purpose (M8), ethics committee and R&D approval for 3.4 (M9).
Output: internal – detailed descriptive information to support service delivery, pilot trial data (recruitment rates, means and SDs of principal outcomes); external – descriptive report (O2) publication of protocol (O3).
3.4 Evaluative trial
Objective: to compare the effects of CGA and usual care for frail older people presenting to but not admitted to hospital from an acute medical unit upon their and their carers’ health outcomes, and upon resource use.
Design: individually randomised controlled trial.
Participants: will be defined during stages 3.2 and 3.3.
Identification and recruitment: referrals will be made using existing systems of nurse-led assessment for older people on the acute medical unit. Patient and carer consent or assent will be obtained prior to randomisation.
Intervention and control: as per 3.3.
Randomisation: Individual patient, web-based, randomisation.
Pilot trial: a short phase to implement and confirm trial consent/assent, randomisation and follow-up processes.
Baseline measurements: common core baseline dataset (see 1.1).
Follow up measurements: common core outcome dataset (see 1.1).
Principal outcome measure: number of days spent at home over 6 months post randomisation.
Secondary outcome measures: proportion admitted, proportion living at home at 3 months, mortality, total days of hospital stay in 6 months, and scaled functional and quality of life outcomes for patient and carer at 3 months.
Economic study: see 1.3.
Concurrent qualitative study: see 1.3.
Sample size: using pilot data and presuming that a high risk group can be identified after the earlier phases, a sample size of 500 will be required to detect a 20% increase in the number of days spent at home, allowing for drop-outs.
Timescale: recruit months 23–40, follow up months 29–46, analysis and dissemination months 47–60.
Milestones: first and last recruit (M10, M13), first and last follow up at 3 and 6 months (M11, M12, M14, M15).
Outputs: main publication (O4).
4. Primary objective 3: Evaluation of hip fracture Comprehensive Geriatric Assessment
4.1 Introductory comments
The planning of this work strand differs slightly from the previous two, because of its maturity of development: changes to the hip fracture service at NUH NHS Trust are already planned during the early years of the research programme, based upon 8 years of local audit [10.17] and research work, and completion of a major literature review [10.18]. For this reason we expect only a short preparatory phase. The other main difference between this and the other research strands is that a system-wide change to the service is already planned on the basis of service improvement as opposed to the sole introduction of a more discreet research intervention. For this reason we need to use a before and after research design and not a RCT.
At present at NUH NHS Trust there are 3 otherwise undifferentiated trauma wards which receive all trauma patients including hip fractures. There is ad hoc medical liaison and ad hoc nurse-led assessment of patients for their suitability for discharge or transfer of care to other settings. Thus assessment is not standardised, or comprehensive, and in particular pre-operative specialist geriatric medical input is lacking.
It is planned to develop an acute zone (pre-operative and immediate post-operative) within each ward, and also a recovery zone. The reason for developing these zones is to support pathways that focus medical care in the acute zones and rehabilitation inputs in the recovery zones. Routine arrangements for specialist geriatric medical liaison and nurse-led assessments will be implemented in both the acute and recovery zones, thus enabling the routine delivery of Comprehensive Geriatric Assessment (CGA).
The aim of this strand of research will be to examine the effect of the application of this new model of service, compared to the current model. Given this service development, we will not be able to allocate participants on a randomised basis to receive the current and new service. We will, however, be able to describe the current and new services and compare the outcomes achieved by them, comparing a cohort before and after the change of service. Whilst this design is not as powerful as a RCT, we should note that case ascertainment is virtually 100%, all patients with hip fracture will be eligible for study, and time trends in incidence (a rise from 715 cases in 1999 to 780 in 2007) and case mix (e.g. a fall in the proportion coming from nursing homes from 28% to 21% between 2000 and 2006) are known from audit data. These factors increase the likelihood that the before and after cohorts will be comparable and that time-related factors can be adjusted for, and hence that such a study will have reliability similar to that of a RCT.
A consequence of using this research design is that instead of having a cohort study, a developmental phase and an evaluative phase, we will require a pre-intervention phase, a development phase and a post intervention phase.
A final comment about this research strand is that we shall work closely with the highly efficient existing hip fracture audit team, which already collects much of the data required for this research and this enables us to deal economically with a relatively large sample size.
4.2 Before and after (pre- and post-intervention) evaluative study
Objective: to compare the effects of orthogeriatric CGA and usual care for frail older people with hip fracture upon their and their carers’ health outcomes, and upon resource use.
Design: non-randomised, pre and post, controlled trial.
Participants: all patients with hip fracture, with no exclusions.
Identification and recruitment: all patients with hip fracture admitted to the unit for 12 months in the pre-intervention phase and for 12 months in the post intervention phase will be invited to participate. Patient and carer (both) consent or assent will be obtained prior to the collection of research data beyond that already collected as part of the on-going audit process.
Intervention and control: as per 4.1 and 4.3.
Baseline measurements: existing hip fracture audit variables, additional variables from common core baseline dataset (see 1.1).
Follow up measurements: existing hip fracture audit variables, additional common core outcome dataset (see 1.1).
Principal outcome measure: mortality and length of stay.
Secondary outcome measures: proportion discharged home, proportion living at home at 3 months, number of hospital admissions/number of days spent at home over 6 months, total days of hospital stay in 6 months, and scaled functional and quality of life outcomes for patient and carer at 3 months. Economic study: see 1.3.
Concurrent qualitative study: see 1.3.
Sample size: 750 participants in each cohort. This will allow us to detect a drop in mortality from 10% (current) to 6% (reported by other units) or a reduction in length of stay of 4 days.
Timescale: preparation months 1–2, recruitment of pre-intervention cohort 3–14, development phase months 15–20 (see 4.3 below), recruitment of post intervention cohort months 21–32, follow up until month 38, analysis and dissemination months 39–48.
Milestones: ethics and R&D approval (M1), first and last recruit for pre-intervention phase (M2, M5), first and last follow up at 3 and 6 months for intervention phase (M3, M4, M7, M8), recruitment of post intervention cohort (M9, M12), first and last follow up at 3 and 6 months (M10, M11, M13, M14).
Outputs: main publication (O3).
4.3 Service development and characterisation phase
Objectives: to demonstrate the delivery of a feasible, acceptable, evidence-based CGA process for patients with hip fracture
Methods: common approach to service development and characterisation (see 1.2).
Setting: current plans in the Trust are for a new consultant medical post which will enable the improved medical support to the proposed service development described in 4.1.
Outcome: the principal outcome is agreement between the research team and the Trust that the new service delivering orthogeriatric CGA to patients with hip fracture is fit for purpose.
Timescale: months 15–20.
Milestones: creation of acute and recovery zones (M6).
Output: internal – detailed descriptive information to support service delivery; external – description of service (O1).
Response to first stage reviews
Thank you for the letter indicating the intention to award us a Programme grant.
You have raised a number of issues that need to be satisfactorily addressed before an award can be made. Please find below our responses to these issues. Overall, we agree with the issues raised and welcome the opportunity to amend the programme along the lines suggested. We shall also take note of the comments made by the individual reviewers. Please also find:
-
CVs of the qualitative researchers who have agreed to joint the team and letters from each to this effect
-
fuller protocols of workstreams 1 & 2 than were possible in the word limit of the original proposal
-
a revised Gantt chart (on disk only)
-
a revised budget statement (on disk only).
We trust that we have met all the conditions necessary to permit this Programme Grant to be awarded and we are ready and eager to get started as soon as the contracts are signed.
One of the concern areas of the reviewers has been the costs: on the one hand the programme is complimented as ambitious and good value for money (which is what was required and what we aimed for) but on the other hand it is criticised as vulnerable partly due to underfunding. We appreciate these two perspectives. The opportunity to drop workstream 3 is accepted, and we welcome the invitation to recycle the released funds. Furthermore, I would like to point out that we will shortly be appointing a new clinical lecturer, who will be expected to work half time on this programme. Dr Logan (a co-applicant) and I have also been part of a Designated Research Team award, which is a small group of local therapists whose interest is in falls reduction and similar matters in care homes. It is a condition of this award (mainly a research capacity award) that the team works with more senior researchers, and we fully expect this team to work in this programme and indeed be co-located with it. I raise these points to indicate that we will be able to bring other resources into this programme outside the direct NIHR funding itself. Furthermore, we fully expect this programme to act as a platform for further, related research. For example we are already shortlisted for a SDO award about mental health care in institutions which, should we be successful, would only add to our resources and help to secure success.
We are now confident that we will have the resources to complete this programme successfully. The final section of this response letter (section 10) summarises the main changes to the finance sheet as a result of these responses: our proposed research budget remains within the £2M overall limit, does not exceed £400,000 for each study year.
1. Sub-package three is not to be supported
We accept that workstream 3 should be dropped.
In dropping this workstream, this allows us to spread the three RA posts we have requested across the remaining three workstreams. There is clearly a practical advantage of having one RA per project instead of them being split across projects. It also increases the amount of RA time per workstream from 0.75 WTE to 1.0 WTE, and therefore deals with the general issue about having inadequate resources for these ambitious projects.
In dropping this workstream it also releases the consultant research PA sessions that had been intended for the workstream lead. These have been recycled to respond to the further issues raised by the Research Selection Panel, the main costs going to:
-
the research costs of care homes in Workstream 4 (see point 9 below)
-
the research costs of General Practices involved in Workstream 4 (see point 9 below)
-
costs for University staff and facilities involved in this programme
The issue of costs to the University, although not mentioned in the specific list by the Research Selection Panel, was raised by one reviewer, and indeed has been a concern of the research team. Whilst each individual and the University are absolutely enthusiastic about this prestigious award, they are mindful of the need to cover a proportion of the real staff costs the University will incur.
2. Named qualitative researcher
Although the existing study group has considerable expertise in qualitative work of the nature required in this programme (for example the qualitative studies of the dementia services we have studied), we recognise that our most well known work has been our complex RCTs. Nevertheless, the Research Selection Panel’s request gives us the opportunity to invite two of our qualitative research colleagues and collaborators to join. These are Professor Justine Schneider (Professor of Mental Health and Social Care, Institute of Psychiatry, University of Nottingham/Nottinghamshire Mental Healthcare Trust) and Dr Jane Dyas (Primary Care Lead, Trent RDSU). Their CV’s are appended, together with letters confirming their support and anticipated involvement.
Justine Schneider has broad experience of applied research on health and social care for older people. Before taking up a research career she was a social worker with adults for ten years and an ‘informal’ carer for a similar period. She brings a social care perspective to the programme of research. Her research on older and their carers people employs mixed methods, including qualitative and observational approaches: an experimental study of occupational therapy in residential homes for older people; a longitudinal study of informal care for people with dementia; a cross-national study of carer ‘burden’ in dementia; and a large study of quality of life in residential care. She is about to commence an ethnographic study of inpatient care for people with dementia (subject to contract with the SDO). In the present study, she will participate in the reference group, with particular expertise in qualitative methods and social perspectives.
Jane Dyas has broad experience in qualitative and other research particularly in primary care. She is based in the Trent Research Development Unit. She has worked with the applicants in the past and does so now through a study of the use rehabilitation for older people with non-injurious falls who call an ambulance, and she also supports the Designated Research Team which will work with this programme.
3. Qualitative work to be carried out not only during the trials but during the development work
We quite agree that phase I and phase II work is required prior to the formal RCTs. This is planned for the first two years of the programme. We have described this developmental work, and although we have not used the word ‘qualitative’ for this work (whereas we did use this word to describe work running alongside the RCTs), you will see from the description taken from the proposal that it includes both quantitative and qualitative approaches:
1.2 The common approach taken to optimise and characterise the services in each strand will be closely steered by the research programme board to ensure consistency.
The objectives in this phase for each strand are:
-
to develop a service that delivers comprehensive geriatric assessment (CGA)
-
to demonstrate that a generalisable service is in place that is fit for purpose & evaluation
An iterative, complex human systems approach will be employed to develop services [10.11]. In this approach a service is seen as a complex human system, and defined (in a ‘root definition’) by the interactions between six factors:
-
the patients
-
the staff
-
the interventions made by staff and the changes made to the patients
-
the managerial systems that control the services
-
the national context that influences services
-
the local context that influences these services.
The lead clinician for each strand will develop a root definition for each service, discussing it with the research programme board, and the relevant parts of the programme reference group. Doing so will lead to the identification of obstacles to service implementation including staffing issues, involvement of key personnel, training needs, development of operational protocols, etc. Close working between the research programme board and the NHS Trust is crucial, and has been assured (see Annex 4). The developmental stage will be highly user-sensitive and will make use of public involvement mechanisms in the acute Trust (Nottingham University Hospitals NHS Trust) and a community Trust (Nottinghamshire Country Teaching PCT).
Although the root definition of a service is necessarily largely descriptive, the description will be generalisable by virtue of specific research outputs including:
-
clearly defined and justified criteria to select patients
-
description of staffing numbers, their expertise, and working patterns
-
an observational cohort study and case studies describing evidence of the delivery of comprehensive assessment, appropriate interventions, and outcomes compatible with clinical effectiveness
-
user and staff views.
We agree that we should also have included in this brief description that this same process will be used to test the trial processes (recruitment, for example) and choice of outcome measures. We would argue that what we plan is precisely ‘how to pilot the intervention’, although we did not use these exact words. We therefore fully agree that qualitative work should be done prior to the trial stage, and argue that this is what we already intend to do, albeit with some quantitative work which we also think is necessary.
The key object of the developmental work in all three workstreams is, to repeat what we have already stated, to ensure that we have shown that we have developed a service that is fit for evaluation. To re-frame our original protocol, there are three sub-phases that make up the developmental phase.
3.1 The first part of the work towards that will be the register study (MMHU workstream), ISAR evaluation study (AMU CGA workstream) and cohort study (Care Home CGA workstream). These are all required to examine the nature and number of people with the worst outcomes or the greatest use of resources. For the MMHU workstream, the study will show our colleagues in the Trust where the patients are, how they can be identified, how many we will have at any one time, and what the likely throughput will be on the new MMHU. This will allow us together to design our recruitment processes to the number of beds we have available (we anticipate a 20-bedded general medical ward adjacent to a psychiatry ward will be chosen). Similarly the ISAR study will help us to establish which of the very many elderly patients attending and being discharged from the AMU are most at risk and hence most likely to need some sort of intervention focussed upon them (or, conversely, to establish which are stable and low users of resources). The Care Home cohort study will describe patterns of care, identify just how much out of hours care is taking place and the characteristics of which sort of residents this care is focussed upon. The register, ISAR evaluation and cohort studies therefore provide the quantitative information upon which services can be developed.
3.2 The development of the services themselves is when the actual staff, locations and working practices are chosen and assembled. This sub-phase will require a particularly close partnership between local service personnel and the research team, with emphasis upon the service personnel.
For the MMHU it requires deciding precisely on a venue, assembling a team of people to work on it, in-service training, and agreement of procedures and protocols. This is why we have asked, via NHS Support Costs, for mental health nursing staff to help at this phase. Our research team will obtain important views to obtain from staff, users (patients or their carers), co-patients (those without mental health problems), advocates and outside bodies such as the Alzheimer’s Society. Interviews, case studies and focus groups will illuminate problems and potential solutions from these differing perspectives. This is where we will undertake qualitative work and require expertise. Our earlier and on-going literature review will provide the evidence base for proposed treatments and interventions, and also identify and review relevant good practice guidelines and protocols. By these means we expect to develop a realistic, logical intervention, and to have described it and justified its working practices.
For the AMU CGA study, we will need to clarify the job timetable of a geriatrician to ensure the right amount of time is available for clinics, domiciliary visits, etc. Working relationships will need clarifying between secondary, intermediate and primary health care, and social services. The working practices of the existing multidisciplinary geriatric team will need to be adjusted, and again we will draw upon the mental health expertise of the mental health nurse (from NHS support costs). Again, our research team will obtain important views to obtain from hospital staff but also community staff, patients and their carers. Interviews, case studies and focus group will illuminate problems and potential solutions from these differing perspectives.
The Care Home CGA study is more complex than the other two workstreams. This is because we will have two sets of service professionals to deal with: GPs and their primary care teams, and care home staff. Furthermore, we will have more descriptive data to collect. For example, we will run a survey of how care homes record their assessments, and a survey of the nature of GP cover to care homes.
In response to point 9 we have described the financial arrangements we have proposed to incentivise the developmental work. To develop the intervention we intend in this study for a small group to be formed of local GPs and the care homes to which they provide cover. We will examine individual cases as a group of clinicians to arrive at the best way to undertake assessments, record them, and what to do when a trigger observation is made. This process will have a large educational element to it. Our earlier and on-going literature review will provide the evidence base for proposed treatments and interventions, and also identify and review relevant good practice guidelines and protocols.
To aid the later implementation of the intervention we aim to develop a larger care home research group, comprising GPs and primary care staff, care home staff, and representatives of the residents. This group will meet partly for educational purposes, but we will also use them to gather their views and suggestions: some simple surveys, focus groups and interviews will be undertaken.
3.3 A third sub-phase is required to check that the new intervention has been implemented and is working as intended. Clinical effectiveness techniques will be used (for example, audit) to determine whether guidelines for referral are followed, whether protocols and procedures are followed, whether outcomes are compatible with those intended, and to gauge user and staff views.
We shall request approval from the research ethics committee and R&D departments to undertake this developmental work as an entire package of study for each workstream. We will seek approval in a form which allows the work to be done flexibly, according to the local conditions at any moment.
4. Consent and the Mental Capacity Act
This has been an area to which we have devoted considerable thought. We welcome the Research Selection Panel’s request for us to elaborate.
We accept that many of the potential participants of this programme will not have the mental capacity to give real consent to participate in the research – just as they as patients will often not have the capacity to consent to the medical and social interventions that are proposed for them.
We will, of course, submit the research for approval by the appropriate body (the Ethics and R&D Committees) in relation to our Strategic Health Authority under the DoH, comprising the appropriate authority (relating to MCA, 2005, s30(1–6)). We will endeavour to ensure that all our procedures, as outlined then to them, and as implemented, will be in conformity with MCA, 2005, s31, s32 & s33. All the interventions we propose could be argued to be ‘best practice’ and none involve new drugs, surgery or technical procedures. The ‘risks’ would more appropriately be described as the burdens of the data collection processes. We will strive during pilot phases to ensure that the assessment burden is as brief as necessary to answer the questions, and hence minimise this burden, Thereby we will strive to make it more acceptable to potential recruits.
The Panel wisely draws our attention to how gaining consent from older people (who may be cognitively impaired) would be challenging and place pressure on the delivery of the programme. All the research will be overseen by senior clinicians dealing with older people, for whom these ethical and practical dilemmas, related to the consent seeking process and problems with lack of capacity, are literally an everyday occurrence in clinical practice. We are often consulted on these matters by other clinicians and asked to teach on these topics. We would also like to point to our record in research with frail older people to illustrate that we have considerable expertise in doing this at a practical level and delivering results. For example, in our recent RCT of a variant of nasogastric feeding in acute stroke (where most patients did not have capacity, and where the ethical issues and the emotional context were complex) we achieved recruitment rates of 74% of all nasogastric tube-fed patients (abstract to be presented to the British Geriatrics Society April 2008). This expertise will be strongly used to support the researchers with such problem areas.
We re-affirm our plans to recruit researchers with an appropriate previous health professional background such that they have facility with working with these problems, and to ensure that researchers are carefully trained and closely supervised for these areas.
There is no doubt that there will be a sufficient supply of appropriate patients in the clinical setting but it is reasonable to be concerned that delays in going through consent/capacity processes could slow the research plans. As a result of dropping workstream 3, this will give us more researcher time than we had previously allowed, and this additional factor we believe will considerably strengthen our ability to overcome any such threatened delay problems.
We believe our research will be fully consistent with the provisions of the Mental Capacity Act. In relation to Mental Capacity Act issues it may be helpful for you to see what we have drafted to accompany the appropriate section of our Ethics Committee application. Thus, regarding Section 30, supplementary information:
-
What impairing condition(s) will the participants have?
Whilst some participants will be capable some participants will be incapable as a result of suffering from dementia or delirium, or from other disorders such as severe depression or serious learning disability.
-
Justifying the inclusion of participants unable to consent for themselves. It should be clear why the research could not be carried out as effectively if confined to adults capable of giving consent.
Quite clearly, since such people are the main group within our research, to exclude them would invalidate the work almost entirely. The study focuses on how older people, both with impaired capacity as well as those who are capable, are best dealt with in medical crises, to achieve the best outcomes for themselves and their carers, and it is therefore essential that all such patients are included. In many cases mental health or mental capacity problems, perhaps varying, will considerably complicate the medical crisis. This again makes it essential to include all such cases to help to establish the best ways for services to achieve the best results for all involved.
-
How will the capacity of potential participants to consent to the research be assessed? Who in the research team will make the assessment and what knowledge of the participant or relevant training/experience will they have to enable them to undertake it?
The researchers conducting the interviews will have primary responsibility for assessing the capacity of the individual to participate in research, under the direct supervision of the workstream lead. They are expected to be recruited from health professional backgrounds and to already possess considerable experience in these areas. They will receive training from members of the research team, including Professor Gladman and Assoc. Professor Jones, who are experienced in this field, and have given specialist clinical advice on and taught on such matters. Additionally, the researchers will be informed by the clinical teams of all necessary and appropriate issues and information regarding the participant.
-
Does the research have the potential to benefit participants who are unable to consent for themselves?
1 Yes 0 No
-
Will the research contribute to knowledge of the causes or the treatment or care of persons with the same impairing condition (or a similar condition)?
1 Yes 0 No
If Yes, please explain how the research will achieve this:
The study will give us considerable knowledge on how clinical services can be improved to cater better for and to achieve better outcomes with vulnerable older people in medical crises, and especially with those with mental incapacity. The research will have great potential importance for informing improved practice in this area to the benefit of incapable people.
-
Will the research involve any foreseeable risk or burden for these participants, or interfere in any way with their freedom of action or privacy?
1 Yes 0 No
If Yes, please give an assessment below. Highlight any risk, burden, restriction or invasion of privacy specific to these participants and say what will be done to minimise it:
This will be minimal in that all patients will anyway receive normal clinical management, either the standard treatment approach or an intervention package approach expected to be revealed as achieving better results, but all patients will be subject to structured clinical assessments which may be somewhat more extensive than would always be practice. There would be no different impact on their freedom of action or their privacy than would be involved in ordinary clinical practices but the information collection might in some cases be somewhat longer and potentially tiring. The researchers will be trained to be sensitive to such issues and will adapt their approaches to avoid a burdensome approach in interviewing.
The project information would be passed to family carers (or consultees) assenting to participation on behalf of incapable research subjects, under s32 of the Mental Capacity Act, in advance of the involvement of the person lacking capacity, creating another mechanism for the views of the person lacking capacity to be taken into account.
-
What arrangements will be made to identify and consult persons (‘consultees’) able to advise on the inclusion of each individual participant and on their presumed wishes and feelings?
Consultees will be identified by the referring clinician at the time participants are considered for participation. For persons without an obvious family member or similar non-professional carer to serve in this role, an independent advocate, planned to be either from the Alzheimer’s Society or Age Concern, will be approached to act as consultee. We would, of course, also be pleased to work with any such consultees already identified and made available by the Ethics Committee.
-
Is it possible that a participant might need to be treated urgently as part of the research before it is possible to identify and consult a consultee?
0 Yes 1 No
If Yes, say whether arrangements will be made instead to seek agreement from a registered medical practitioner and outline these arrangements. Or, if this is also not feasible, outline how decisions will be made on the inclusion of participants:
-
What arrangements will be made to consult consultees during the course of the research where necessary? What burden could this place on consultees?
The consultee would be approached for involvement once, prior to contact being made with the participant. The consultee could, of course, approach the research team with new information at any time, but it is expected that the interviews with participants would take place promptly following agreement of the consultees. It is therefore not expected that it would be necessary routinely to involve consultees after the initial agreement.
-
What steps will you take, if appropriate, to provide potential participants who are unable to consent for themselves with information about the research, and to consider their wishes and feelings?
This will be approached sensitively, as appropriate, at the pace and to the depth the individual wishes, at the beginning of the interview with the participant, including the presentation of written information.
-
Is it possible that the capacity of participants could fluctuate during the research? How would this be handled?
Individuals’ capacity may vary, for example, according to the time of the day interviews occur. We will seek information on this from the clinical team and we will endeavour to conduct the interview at a time when the participant is at his or her most competent. However, it is essential that the relevant clinical data is collected at the time of the medical crisis rather than sometime later. For all those with incapacity we are involving a consultee from the initial stages of the research for all such participants and this will be on-going, and we will involve such a consultee at any later stage with any individual who subsequently loses capacity during the duration of his/her involvement with the research.
Thereby, we intend that for participants who lose capacity at any stage the research will thus continue to meet the terms of the Mental Capacity Act 2005.
-
What will be the criteria for withdrawal of participants?
Capable individuals will withdraw by indicating their refusal at any stage. For those lacking capacity withdrawal will occur: either: via a communication from the consultee that the individual would wish to be withdrawn from the study, consistent with s 32(5) of the Mental Capacity Act; or, if the participant indicated in any way that he or she wished to be withdrawn from the study, consistent with s 33(5) of the Mental Capacity Act.
-
Describe what steps will be taken to ensure that nothing is done to which participants appear to object (unless it is to protect them from harm or minimise pain or discomfort)?
The views of the participant will be sought at the time the interview is to occur, and if the individual wishes it, additional time can be made available for the individual to consider matters. In addition, the consultees are asked in the information sheet to notify us if there is reason to believe that the participant does object or would object if he or she had capacity to anything we propose.
-
Describe what steps will be taken to ensure that nothing is done which is contrary to any advance decision or statement by the participant?
The information sheet to the consultee directs his or her attention specifically to advance views and decisions of the participant.
5. Co-location and sharing of expertise
5.1 Service co-location
Obviously, it is not possible for the staff delivering all workstreams to be completely co-located, given that they will be in a Medical and Mental Health Unit, on the Acute Medical Unit and in care homes and primary care. However the workstream leads, the posts supported by research support and treatments costs, and the majority of the staff involved in workstreams 1 & 2 will all be co-located in the Health Care of Older People directorate in NUH. This means that they will meet regularly in day to day clinical, research and management settings. We expect communication and interaction between staff to be an everyday occurrence, thereby facilitating the sharing of expertise between workstreams 1 & 2, and with clinical services. The programme of meetings and educational events that we intend to hold will be one means of drawing staff from primary care into this project. Furthermore the Designated Research Team that will work alongside us are primary care clinicians doing part time research. This will also facilitate the sharing of expertise into primary care.
5.2 Research co-location
The research staff employed by the programme grant will have an office in the Queen’s Medical campus of Nottingham University Hospitals (which comprises, in one building, the University Hospital and Medical School) and will therefore be co-located. It will be important for the research office to be separate from the clinical services to avoid unblinding of the researchers and any other form of contamination (see point 6).
6. Detailed study design for the Medical Mental Health Unit and issues of contamination
The detailed study design is appended. The Research Selection Panel in particular was concerned that if the workstream lead was also the clinician involved, that this might introduce ‘contamination’ or some form of bias. Again, we had thought long and hard about this, and found it difficult to provide a brief justification and description of the issues in the final proposal.
We accept that any open (unblinded) service trial is open to biases – such as our trials of a stroke unit or rehabilitation services. A concern is that undue involvement of a researcher to one group of participants only, such as the trial participants, might evoke some sort of response bias either by biasing the proportion of respondents, or by encouraging a more favourable response. The approach we have taken towards this in the past and which we shall use here are:
-
Recruitment should be by a researcher who is not part of the clinical service.
-
This research should recruit participants in a way that establishes the clinical equipoise of the trial: we do not know which is better. This reduces expectation bias, or at least does not encourage it.
-
Follow up should be independent and blinded to allocation status, and independent of the service. This can be achieved by several means including the use of telephone follow up where possible and the use of a researcher who is unaware of allocation. The exact words used when approaching patients to obtain follow up information must be carefully designed to avoid invoking bias. Researchers will be carefully trained and supervised.
-
Outcome measures should be chosen that do not inherently or unfairly favour the intervention, or refer to it.
-
Data should be recorded by someone blinded to the allocation status and independent of the intervention.
-
Data should be analysed blind to the allocation status.
Another concern is that the expertise being developed for the treatment might ‘leak’ into routine, control, practice, and this ‘contamination’ might reduce the observed treatment effect. It is noteworthy that despite this possibility, the benefits of stroke units were still observed in trials. We argue that the correct trial to perform, the correct research question to answer, is ‘what is the benefit of a Medical and Mental Health Unit when compared to optimised ordinary care’. Therefore, if the control treatment is enhanced simply by the presence of the trial, then this is the correct control condition to test. Thus ‘contamination’, to some extent, is not a bad thing. In fact, we suspect that however much ‘contamination’ occurs, it will still not enable non-specialised services to develop the clinical skills and expertise, or the management and organisational practices that would be possible in a specialist unit. By analogy with the stroke unit studies, where the benefit is attributed to ‘co-ordinated care’, we suspect that it will be these elements, the ones that cannot leak and cause contamination, that will be important.
With these safeguards in place, we expect biases to be minimised. We see no reason for the fact that the workstream lead will also be the lead clinician from introducing bias.
7. Workstreams 1 & 2: further details including justification of sample sizes
Full protocols for workstreams 1 and 2 are attached.
Owing to lack of space in the original proposal document, we did not fully explain our sample size calculations. The Research Selection Panel requested more information about the sample size calculations for workstreams 1 and 2. Each of these workstreams has two quantitative elements: a cohort study used largely to prepare for each trial, and the trials themselves. Fuller descriptions of the sample size calculations and reasoning for each part are given below.
7.1 Workstream 1. Evaluation of Medical and Mental Health Unit (MMHU) – Cohort study
The cohort study will recruit 240 participants. This will enable us to estimate the distribution of the proposed key outcomes for the evaluative trial. Based on using a 95% confidence interval for precision of each estimate, the proportion discharged home will be estimated to within 6%, and the length of stay will be estimated to within 4 days (assuming a SD of length of stay of 32 days, based on data from a similar unit in York). This sample size will also enable us to conduct multivariate analysis of outcome predictors with up to 15 explanatory variables, based on the rule that the number of explanatory variables should not exceed the square root of the sample size.
7.2 Workstream 1. Evaluation of Medical and Mental Health Unit (MMHU) – RCT
In the evaluative study, the primary outcome is the proportion discharged home. Assuming that the proportion discharged home in the usual care control group is between 22% and 33% of patients, a sample size of 374 participants (187 randomised to each treatment group) will allow us to detect at 90% power a 17% increase in home discharge rate (22% to 39%, or 33% to 50%). We will recruit 400 participants to allow for drop out. Further details are provided in the full protocol, which explores some the uncertainty around the assumptions for this calaculation.
7.3 Workstream 2. Evaluation of Acute Medical Unit Comprehensive Geriatric Assessment – cohort study (ISAR validation)
In the cohort study, our primary aim is to establish the diagnostic validity of the ISAR tool for adverse outcomes. Assuming an adverse outcome in 10% of the population (n = 50), a sample size of 500 will allow us to estimate the sensitivity of the ISAR tool to within 14% and the specificity to within 5% (using a 95% confidence interval for these proportions).
7.4 Workstream 2. Evaluation of Acute Medical Unit Comprehensive Geriatric Assessment – RCT
In the evaluative trial, we propose that the primary outcome is the number of days spent at home over 6 months post randomisation. This is a novel outcome which aims to combine information on time to death and time in hospital. We will establish the distribution of this outcome within the cohort study, but using pilot data, in a high risk group who were readmitted within 6 months, the mean and SD of days at home in 6 months were 113 days and 55 days respectively. Presuming that a high risk group can be identified after the earlier phases, a sample size of 438 (219 per treatment group) will be required to detect a 20% increase (to 136 days) in the number of days spent at home at 99% power, and a 10% increase (to 124 days) at 85% power. We will recruit 500 participants to allow for drop out. We have aimed for 99% power to allow for the possibility that the distribution of days at home in 6 months will not be normal and we may have to use non-parametric analysis. This will be reassessed following the cohort study and the power calculation revisited as appropriate.
8. Detailed health economic study plan for each workstream
For brevity, we described in the final proposal the overall approach across all workstreams:
The perspective for costs taken will be that of the third party payer: NHS, personal social services and private care home sector. Resource use data will be collected for the index admission or care episode, along with subsequent secondary care (inpatient and outpatient episodes), primary and community (geriatric day hospital, GP and social services home care services) contact and specialist accommodation requirements. GP records are a good source for primary care data, but patient or carer reports are more reliable for other service contacts [10.12]. A modified version of the Client Service Receipt Inventory [10.13] will be developed to capture these data across all workstreams. Unit costs will be taken from routine local or national sources, as appropriate. Depending on the level of skewness in the data, differences in costs will be analysed using t-tests or non-parametric bootstrapping. In line with standard practice, the EQ-5D will be used in the generation of QALYs in economic studies to generate conventional incremental cost effectiveness ratios (ICERs). Where appropriate, patients will provide EQ-5D data, and where this is not possible, carers will provide proxy EQ-5D ratings. In view of the limitations of this instrument in this population group, we will also use disease specific quality of life measures in each strand, and examine their relationship to the EQ-5D. QALYs will also be extrapolated to lifetime, and discounted at 3.5% to estimate the cost effectiveness of the interventions over the lifetime of the target population. Uncertainty around ICERs will be expressed using appropriate probabilistic methods including bias-corrected bootstrapping methods. Cost effectiveness acceptability curves and estimates of expected value of information will be generated to inform decision-making and to quantify the costs and benefits of further research.
Fuller descriptions of the economic approaches we shall use for each workstream, taking into account specific comments by individual reviewers are given below.
The overarching aim of the economic analysis is to assess the relative value for money of each of the applications of CGA. To maximise the transferability of the methods and results obtained, the economic analysis protocol will have a standard structure across the three workstreams, with common data collection and analysis strategies used whenever appropriate. In addition to this, the individual workstreams will have specific issues regarding perspective, resource use and outcome measurement that will need to be managed. The parameters and protocol for collection of those parameters will be piloted, finalised and applied during the cohort study phases, and will then be applied to the evaluative phases. The MMHU and AMU workstreams will some overlap in perspective and resource use categories. The care home workstream will take a similar approach, but has more complex data handling needs due to the multi-level nature of data collected, and the increased number of care models.
Approaches to characterisation and valuation of resource use will be workstream specific due to the complexity of each intervention under evaluation. Details of the approaches are provided in the next section. A consistent methodological approach is presented for the three workstreams, with adaptations for each intervention where necessary. Common approaches will be used to assess utility of outcome for the economic evaluation, and to carry out the economic analysis, and are provided in this section.
8.1 Assessment of utility of outcome for economic analysis
There are criticisms that utility measures such as EQ-5D, used to generate quality adjusted life years (QALYs) are of limited use in older people(1) as they measure factors that may affect health-related quality of life (HRQoL). Thus they are preference-based and capability-based, which may not reflect the actual quality of life of a person with disability.(2) In a study of stroke, a difference in death rates did not translate into a significant different in EQ-5D.(3) This suggests either that the EQ-5D is insensitive, or that increasing the survival of patients does not result in survival with a high quality of life. Barthel ADL explained 37% of the variation in EQ-5D and other outcome measures did not improve explanatory power in that study. Despite these concerns, the EQ-5D has been used to assess interventions in older people.(4–6) Furthermore, in a group of elderly with dementia, QALY weights have been elicited from caregivers and clinicians for use in cost utility analysis.(7;8) Adjusting EQ-5D for cognitive status does not appear to improve validity.(9) The EQ-5D has been used by this research team before to assess the economic impact of an early discharge rehabilitation service for frail older people in the Nottingham area.(6) We consider the EQ-5D the best instrument at present to use in cost utility analyses. In our proposed studies, carers will provide proxy measures of patient EQ-5D at study entry and 90 days later. In line with current practice, utility of patients only will be incorporated into the primary economic analysis.
8.2 Economic analysis
During the evaluative trial phases, the economic data collection protocols will be integrated into the overall data collection process for the trial. Data will be collected as outlined above for each patient in the control and intervention arms. Quality assurance of data entry and preparation for analysis will be completed prior to analysis.
Data analysis will consist of the following stages:
-
descriptive cost and utility reporting; incremental cost effectiveness analysis (probabilistic ICER generation, with cost effectiveness acceptability curve generation).
-
Sensitivity analyses (impact of unit costs, setting, variations in intervention, perspective and other key model parameters).
-
Impact of missing data.
-
Mapping of utility measures onto disease-specific outcomes.
-
Extrapolation of costs and outcomes beyond 6 months to lifetime.
8.3 Evaluation of Medical and Mental Health Unit (MMHU): resource use and costs
The aim of the economic evaluation is to assess the cost effectiveness of an MMHU compared with current alternative practice. This workstream requires resource use and outcome parameters associated with frail elderly admitted to the MMHU or the equivalent service, and their carers, from a full range of perspectives.
8.3.1 Cohort study
During the cohort study, resource use data will be collected to reflect the multiple use of NHS, personal social services (PSS) and informal care resources by this client group in the six month period following a medical emergency admission in the presence of mental health comorbidity. This phase will allow detailed characterisation and development of measurement of current practice. This information is essential to inform the evaluative trial.
Information will be collected on the duration and nature of medical, nursing, social and therapy input, the nature and intensity of personal care, and the overall utilisation of social services, community and informal resources. Measures include length of hospital stay, nursing and medical input. The use of psychiatric liaison services, specialist investigations and outpatient rehabilitation facilities will be recorded. Data on social services utilisation (number and duration of personal care visits, tasks undertaken, aids and appliances supplied) will be obtained from patients and the social services. The client service receipt inventory (CSRI) was developed for use in mental health, (10) has been tested in primary care to assess accuracy of retrospective client reporting of GP visits, (11) and used to assess costs of follow-up care in stroke patients.(3) Costs in this client/patient group are primarily caused by the index admission and follow up secondary, community or specialised accommodation care.(3) Informal care costs are also often significant.(12) An adapted version of the CSRI will be used to assess extra input from family members, friends or voluntary organisations. The categories of data collected for each patient included in the study will be:
NHS perspective:
-
Length of index acute admission.
-
Time spent in care home, or long-stay unit.
-
General practice and other primary care and mental health visits.
-
Home visits from primary care services and primary care/community mental health services.
-
Subsequent planned and unplanned inpatient and outpatient secondary and tertiary care contact.
This list will be finalised during piloting of the cohort study data collection protocol. For each of these categories, the professionals involved, interventions and investigations, length of visit, transport, medicines and living aids will be recorded and costs attached. The sources of data will be finalised as part of this phase. Medical notes and nursing notes will be used, combined with primary care electronic records, and carer report.
Personal social services perspective:
-
Social worker.
-
Community care assistant (home help).
-
Meals on wheels.
-
Day centre.
-
Lunch club.
The range of services provided will be finalised during piloting of the cohort study data collection protocol. The protocol will be piloted and applied during this phase of the workstream. Social services reports will be used, combined with carer reports. The methods used will follow methods recommended by PSSRU for measuring and valuing health and social care in this population.
Informal carer costs will be assessed by use of CSRI, recording number of hours spent in informal care, and using days spent at home by the patient as a proxy measure of carer burden.
Analysis of cohort cost data set will consist of:
-
Generation of means and ranges for individual patient costs, for a range of diagnoses.
-
Derivation of relationship of baseline patient characteristics with resource use.
8.3.2 Service development and characterisation phase
During this phase, characterisation of the intervention will be carried out. This intervention requires the setting up of a 20 bedded ward, developed from existing geriatric medical beds, with additional mental health nursing input, and specific arrangements for liaison with specialist, voluntary and community psychiatric services. Therefore, in discussion with local finance managers and service delivery managers, we will generate information on set-up costs, fixed running (overhead) costs, as well as changes in staffing costs to run the unit. We will carry out observations of staff involved in direct patient care to assess changes in work practice.
In addition, variable costs, i.e. those directly related to patient numbers, will be characterised and valued, using a range of data sources: direct observation, medical and nursing notes, community psychiatric services and social services reports, patient and carer reports. The most reliable data sources will be identified as part of this process. The economic data collection protocol will be finalised prior to completion of this phase. The economic analysis will be carried out excluding set-up costs, but including running costs that are incurred as a result of the new service, which will be presented separately to inform a future business case.
8.4 Evaluation of Acute Medical Unit Comprehensive Geriatric Assessment: resource use and costs
To assess the cost effectiveness of an acute medical unit compared with current alternative practice, this workstream requires resource use and outcome parameters associated with frail older people who attend, but are not admitted to the acute medical unit, and their carers, from a full range of perspectives. This phase will allow detailed characterisation and development of measurement of current practice. This information is essential to inform the evaluative trial.
8.4.1 Development of data collection protocol (cohort study)
Elderly people who present to, but are not admitted from, acute medical units require a wide range of inputs from different parts of the health, personal social services, private and informal care sectors. There is likely to be very wide variation in the types and magnitude of resources consumed, and this phase is essential to develop a data collection method to capture that variation. The development of the data collection protocol will require characterisation of the normal pathways of care, and resources consumed as part of that process. This development and validation will be carried out as part of the cohort study to evaluate the ISAR (Identification of Seniors At Risk) tool.
Information will be collected on the duration and nature of medical, nursing, social and therapy input, the nature and intensity of personal care, care home services and the overall utilisation of social services, community and informal resources. Data on social services utilisation (number and duration of personal care visits, tasks undertaken, aids and appliances supplied) will be obtained from patients and the social services. An adapted version of the CSRI will be used to assess extra input from family members, friends or voluntary organisations.
Analysis of this current practice dataset will consist of:
-
Generation of means and ranges for individual patient costs, for a range of diagnoses (6 months worth of data).
-
Examination of relationship of baseline patient characteristics with resource use.
8.4.2 Service development and characterisation phase
Characterisation of the intervention will be carried out during this phase. This intervention is relatively complex, with the addition to existing care of specialist geriatric medical assessment and management with active community-based follow-up, setting up, using and liaising with rapid access clinics, new use of intermediate care services, community matrons, care home staff and primary care team. There is likely to be a set of changes in work practice from ‘usual care’ to ‘intervention’ that are much more complex than simply adding on the costs of the new services, so detailed assessment of resource use for both arms will be essential.
In discussion with local finance managers and service delivery managers, we will generate information on set-up costs, fixed running (overhead) costs, as well as changes in staffing costs to run each aspect of the service. We will carry out observations of staff involved in direct patient care to assess changes in work practice.
In addition, variable costs, i.e. those directly related to patient numbers, will be characterised and valued, using a range of data sources: direct observation, medical and nursing notes, community matron services, care home and social services reports, patient and carer reports. The most reliable data sources will be identified as part of this process. The economic data collection protocol will be finalised prior to completion of this phase. The economic analysis will be carried out excluding set-up costs, but including running costs that are incurred as a result of the new service, which will be presented separately to inform a future business case.
8.5 Evaluation of Care Home Comprehensive Geriatric Assessment
To assess the cost effectiveness of care home CGA compared with current alternative practice, this workstream requires resource use and outcome parameters associated with frail elderly people residing in care homes, and their carers, from a full range of perspectives.
8.5.1 Development of data collection protocol (cohort study)
Elderly people who reside in care homes require a wide range of NHS services, which are consumed at highly variable rates. This study requires identification of range of settings and key players in secondary, intermediate and primary care, the social services and the third (private and voluntary) sector, and development of methods for quantification of resource use. There is likely to be very wide variation in the types and magnitude of resources consumed, due to the heterogeneity of the client group, the range of care home settings in which they are based, and this phase is essential to develop a data collection method to capture that variation. The development of the data collection protocol will require characterisation of the normal use of NHS and PSS services, pathways of care, and resources consumed as part of that process. As care homes are run both privately as well as publicly, the perspective of this study will be wider, to take this into account.
Information will be collected on the duration and nature of medical, nursing, social and therapy input, the nature and intensity of personal care, care home services and the overall utilisation of social services, community and informal resources. An adapted version of the CSRI will be used to assess extra input from family members, friends or voluntary organisations.
Analysis of this current practice dataset will consist of:
-
Generation of means and ranges for individual resident costs, for a range of diagnoses (6 months worth of data).
-
Examination of relationship of baseline resident characteristics with resource use.
8.5.2 Service development and characterisation phase
Characterisation of the intervention will be carried out during this phase. To reduce variation in care home models of care, for the baseline, the study will be selecting care homes that already provide a certain minimum level of medical care. As this study has a stepped wedge cross over design, once the care home group has been established, it will be necessary to collect ‘normal care’ data prior to introduction of the intervention.
This intervention is again relatively complex, and varied. There is likely to be a set of changes in work practice from ‘usual care’ to ‘intervention’ that are much more complex than simply adding on the costs of the new services, so detailed assessment of resource use for both stages will be essential. Also, costs incurred by private care homes need to be collected. Costs incurred by private care homes (there are no NHS care homes in Nottingham and few social services-run homes) are likely to be highly variable,(13;14), and will require development of specific costing methods, allied to those used by PSSRU, and will need to be controlled for in the cluster-based analysis of costs.(15) We will carry out observations of staff involved in direct resident care to assess changes in work practice. In addition, variable costs, i.e. those directly related to resident numbers, will be characterised and valued, using a range of data sources: direct observation, medical and nursing notes, care home and social services reports, patient and carer reports. The most reliable data sources will be identified as part of this process. The economic data collection protocol will be finalised prior to completion of this phase.
8.6 References
- Katona C, Livingston G, Cooper C, Ames D, Brodaty H, Chiu E. International Psychogeriatric Association Consensus Statement on Defining and Measuring Treatment Benefits in Dementia. Int Psychogeriatr 2007;19:345-54.
- Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people: preferences or capabilities?. Soc Sci Med 2006;62:1891-901.
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost effectiveness and cost utility analyses from a prospective randomised controlled trials. Stroke 2004;35:196-203.
- Fleming SA, Blake H, Gladman JRF, Hart E, Lymbery M, Dewey ME, et al. A randomised controlled trial of a care home rehabilitation service to reduce long-term institutionalisation for elderly people. Age Ageing 2004;33:384-90.
- Flood C, Mugford M, Stewart S, Harvey I, Poland F, Lloyd-Smith W. Occupational therapy compared with social work assessment for older people. An economic evaluation alongside the CAMELOT randomised controlled trial. Age Ageing 2005;34:47-52.
- Miller P, Gladman JRF, Cunliffe AL, Husbands SL, Dewey ME, Harwood RH. Economic analysis of an early discharge rehabilitation service for older people. Age Ageing 2005;34:274-80.
- Honkanen LA, Mushlin AI, Lachs M, Schackman BR. Can hip protector use cost-effectively prevent fractures in community-dwelling geriatric populations?. J Am Geriatr Soc 2006;54:1658-65.
- Bryan S, Hardyman W, Bentham P, Buckley A, Laight A. Proxy completion of EQ-5D in patients with dementia. Qual Life Res 2005;14:107-18.
- Wolfs CAG, Dirksen CD, Kessels A, Willems DCM, Verhey FRJ, Severens JL. Performance of the EQ-5D and the EQ-5D+C in elderly patients with cognitive impairments. Health Qual Life Outcomes 2007;5.
- Chisholm D, Knapp MRJ, Knudsen HC, Amaddeo F, Gaite L, Van Wijngaarden B. Client Socio-Demographic and Service Receipt Inventory – European Version: development of an instrument for international research: EPSILON Study 5. Br J Psychiatry 2000;177:s28-s33.
- Patel A, Rendu A, Moran P, Leese M, Mann A, Knapp M. A comparison of two methods of collecting economic data in primary care. Fam Pract 2005;22:323-7.
- Graff MJL, Adang EMM, Vernooij-Dassen MJM, Dekker J, Jonsson L, Thijssen M, et al. Community occupational therapy for older patients with dementia and their care givers: cost effectiveness study. Br Med J 2008;336:134-8.
- Kavanagh S, Schneider J, Knapp M, BA JB, Netten A. Elderly people with cognitive impairment: costing possible changes in the balance of care. Health Soc Care Community 1993;1:69-80.
- Donaldson C, Bond J. Cost of continuing-care facilities in the evaluation of experimental national health service nursing homes. Age Ageing 1991;20:160-8.
- Johnston K, Gray A, Moher M, Yudkin P, Wright L, Mant D. Reporting the cost effectiveness of interventions with nonsignificant effect differences: example from a trial of secondary prevention of coronary heart disease. Int J Technol Assess Health Care 2003;19:476-89.
9. Re-evaluate costs for workstream 4
We welcome the opportunity to improve the funding of this workstream, and agree that the lack of costs here would have rendered it vulnerable. We still believe that this project is possible, and will depend greatly upon clinical leadership – from the study team and local colleagues.
9.1 Research costs
We now propose to remunerate all care homes who agree to participate in the survey part of the workstream 4, and those involved in the implementation and evaluation stage. This will reflect the extra effort required by them to learn about the project, to amend their way of working (recording and monitoring) and in particular to assist with data collection. We intend to offer £500 to each home in the survey stage, and £1500 for each in the implementation and evaluation stage.
We also intend to remunerate GP practices. We have planned for sessional fees to pilot practices during the developmental stage, and fees to practices for data collection during the trial stage.
9.2 Support costs
We will also require GPs to spend their time or their practice nurses’/community matrons’ time in supporting this research: learning about it, spending more time in care homes until the process beds down and becomes routine. These are support costs and have been calculated on a sessional basis and are shown in the revised budget sheet.
These figures are given in the amended finance sheet. We do not feel that any costs are treatment costs: there are already additional payments made to GPs for looking after people in care homes, and we expect that future contracts will adapt to the elements of practice involved in workstream 4, and we have no reason to assume that they will be any greater than at present.
10. Summary of changes to the finance form
10.1 Costs removed
Staff costs
-
25% WTE consultant research PA salary costs for workstream 3.
-
Reason: workstream 3 now not required.
Direct costs non pay
-
Higher degree fees.
-
Reason: perhaps not true research costs, and we preferred to spend on better staff to safeguard the success of the project.
Indirect costs
-
Overheads for 25% consultant research PA salary.
-
Reason: workstream 3 lead now not on grant.
10.2 Costs inserted or amended
Staff costs
-
Grade of 1.0 WTE post doc researcher increased.
-
Reason: we have an excellent individual in mind who will be available, and have adjusted the salary so that we can employ her immediately. This assures us that we will be able to start this project on time and with a competent pair of hands.
-
Grade of one WTE R&T 4 researcher increased.
-
Reason: we have another excellent individual in mind who will also be available and have adjusted the salary so that we can employ her immediately. This assures us that we will be able to start this project on time and with a competent team.
Direct costs non pay
-
Care home research fees (£4000 year 1, £12,500 in years 3–5).
-
Reason: See point 9. Eight care homes will be involved in the pilot phase, and we will require them to provide us data from their records and to assist us in informing residents and relative to aid recruitment, budgeted at £250 per home. In years 3, 4 & 5 we will be involving 25 homes in the evaluative stage, at £500 per home.
-
GP pilot research fees (£12,000 years 1 & 2).
-
Reason: See point 9. The pilot, developmental stage is crucial to develop a clinically realistic intervention and thus this part of the research process must involve GPs. We estimate that this will need us to work with 6 GPs for 10 sessions over 2 years @ £200 per session.
-
GP data gathering fees (£400 years 1–2, £3000 years 3–5).
-
Reason: See point 9. We will require Practices to give us data for the study from their records. We have budgeted for 8 practices @ £50 per practice during the pilot stage (years 1&2) and for 60 practices @ £50 per practice for the evaluative stage (years 3–5).
-
Co-applicant fees (£34,000 over 5 years).
-
Reason: We are assured of considerable and on-going support from all applicants throughout the project. Had the project been based in the University, a very much larger sum would have been requested to cover academic staff time. This time will be most necessary during the first two years as the workstreams are set up. The size of these fees in this budget has been constrained by the overall budget. Whilst the total amount budgeted is equivalent only to the staff costs (without indirect costs) of around 2 hours per week for one University employed academic over the 5 years (including one of our new qualitative researcher, we have 8 University employed academics), it will give the Lead Applicant the ability to recompense the Divisions of Primary Care, Rehabilitation and Ageing, Epidemiology and Public Health, Psychiatry, the Schools of Pharmacy and of Nursing, and the University of Leicester (where Dr Conroy now works) for staff time and resources.
Indirect costs
-
Slight increase of these in line with changes to the two research staff, at 25%.
11. Conclusion
We trust that in this response letter, and in the further attached information, we have satisfactorily answered each of the queries raised by the Research Selection Panel. We are now confident that we will have the resources to complete this programme successfully.
The main service development ideas in our proposal are already part of the longer term service development strategy for the Nottingham University Hospitals Trust. We have already started discussions to prepare for the staff and service changes. We have already identified some of the key research staff and will shortly begin the public and user engagement process. We will be ready to start on proposed start date of 1 August 2008, subject to the contractual process.
Yours sincerely
Professor John Gladman, on behalf of the co-applicants.
Appendix 2 The interface between acute hospitals and community care for older people presenting to acute medical units: a mapping review – databases and searches
Taken from Conroy S, Stevens A, Gladman JRF. The Interface Between Acute Hospitals and Community Care for Older People Presenting to Acute Medical Units: A Mapping Review. Medical Crises in Older People Discussion Paper Series. Issue 6, December 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue6-mcop-issn2044-4230.pdf (accessed 20 March 2015), reproduced under the terms of the Creative Commons Attribution Non-commercial No derivatives 3.0 Licence (CC BY-NC-ND 3.0) http://creativecommons.org/licenses/by-nc-nd/3.0/.
A systematic approach was taken to the mapping review, in part to pilot a subsequent systematic review. The following databases were searched by a researcher with librarianship skills from inception until September 2009:
-
Ovid MEDLINE(R) (1966+)
-
EMBASE (1980+)
-
British Nursing Index (BNI) (1985+)
-
Health Management Information Consortium (HMIC)
-
The Cochrane Library
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
AgeInfo (www.cpa.org.uk/ageinfo/ageinfo2.html)
-
Applied Social Sciences Index and Abstracts (ASSIA)
-
National Research Register (NRR) Archive (https://portal.nihr.ac.uk/Pages/NRRArchive.aspx)
-
National Information Centre on Health Services Research and Health Care Technology (NICHSR) (www.nlm.nih.gov/nichsr/db.html)
-
Database of Abstracts of Reviews of Effects (DARE)/Health Technology Assessment (HTA) database/NHS Economic Evaluation Database (NHS EED) (www.crd.york.ac.uk/crdweb/).
The following search terms were used (adapted from previous relevant reviews):
-
acute care/sub-acute care/post-acute care/intermediate care/care continuum/integrated care/progressive care/transitional care (identifies the setting)
-
frail/geriatric assessment/health services for the aged/(geriatric unit or specialist geriatric or acute geriatric).mp./((elder$or older or geriatric$or aged) adj3 (unit or specialist)).tw./acute care for elder$.ti./(acute care adj3 elderly).mp./elder$unit$.ab./geriatric$acute care.ab. (identifies the population/process)
-
activities of daily living/cost/cost benefit/cost effectiveness/mortality/health status/length of stay/discharge/readmission/quality of life/satisfaction/carer strain/carer burden (identifies the outcomes).
The search terms were refined for each database, to conform to the appropriate syntax and searching strategy required. Searches were limited to review or review article using the individual database filters.
Appendix 3 Interface between acute hospitals and community care for older people presenting to acute medical units: a mapping review – data extraction (results) table
Taken from Conroy S, Stevens A, Gladman JRF. The Interface Between Acute Hospitals and Community Care for Older People Presenting to Acute Medical Units: A Mapping Review. Medical Crises in Older People Discussion Paper Series. Issue 6, December 2010. URL: www.nottingham.ac.uk/mcop/documents/papers/issue6-mcop-issn2044-4230.pdf (accessed 20 March 2015), reproduced under the terms of the Creative Commons Attribution Non-commercial No Derivatives 3.0 Licence (CC BY-NC-ND 3.0) http://creativecommons.org/licenses/by-nc-nd/3.0/.
| Study, search period of review, type of review, population | Intervention examined | Results/conclusions | CASP quality rating |
|---|---|---|---|
| CGA reviews | |||
| Stuck 19933 Inception–1993 Meta-analysis Population not specified |
CGA, categorised into five different types depending on setting and organisation of delivery compared with usual hospital or community care Categories: (1) GEMU – in hospital, (2) IGCS – in hospital, (3) HAS – community, (4) HHAS – patients recently discharged from hospital, (5) OAS |
Three HHAS studies (the category most similar to the concept of interface geriatrics) were identified, two of which had ambulatory follow-up. The results for these studies are as follows: 12-month mortality RRR 0.89 (95% CI 0.65 to 1.23), living at home at 12 months RRR 1.49 (95% CI 1.12 to 1.98), readmissions RRR 1.03 (95% CI 0.56 to 1.90), physical function at 6 months RRR 0.98 (95% CI 0.59 to 1.63), cognition at ≥ 6 months RRR 0.97 (95% CI 0.63 to 1.48) When all forms of CGA were considered overall, the results are as follows: 6-month mortality RRR 0.86 (95% CI 0.75 to 0.98), living at home RRR 1.26 (95% CI 1.1 to 1.44), readmissions RRR 0.88 (95% CI 0.79 to 0.98), cognition at 6 months RRR 1.41 (95% CI 1.12 to 1.77), physical function at 6 months RRR 1.10 (95% CI 0.89 to 1.36) |
84% |
| Baztan 200916 Inception–31 August 2008 Meta-analysis Older people with acute medical disorders |
CGA in AMUs compared with conventional care units | This review excluded five papers describing non-hospital interventions. Acute geriatric units reduced activity limitation (OR 0.82, 95% CI 0.68 to 0.99) compared with conventional hospital care and increased the likelihood of living at home after discharge (OR 1.3, 95% CI 1.11 to 1.52) but the survival advantage could have occurred by chance (OR 0.83, 95% CI 0.60 to 1.14) | 88% |
| Ellis 200517 Approx. 1987–2004 Meta-analysis Older people in acute hospital care |
GEMUs and IGCS | Inpatient CGA increased the likelihood of returning home from hospital but did not significantly reduce mortality (living at home: OR 1.16, 95% CI 1.04 to 1.30; mortality: OR 0.95, 95% CI 0.87 to 1.05). Most of the benefit was seen in GEMUs with little seen in IGCS: interface with the community was not considered | 81% |
| Comparison of alternative settings | |||
| Parker 200018 1988–April 1999 Meta-analysis Patients aged ≥ 65 years receiving acute, post-acute and subacute rehabilitation care |
To assess the evaluative literature on the costs, quality and effectiveness of different locations of care for older people: (1) admission avoidance, nurse-led beds and early discharge schemes, (2) increased condition-specific expertise in hospital settings such as stroke units, hip units, GAUs and ACE units, (3) rehabilitation (inpatient, community-based and day hospitals) | The focus was on place of care, in particular a comparison of alternatives for similar patients. Inpatient rehabilitation (usually compared with non-specialist inpatient settings) reduced mortality and increased the likelihood of living at home. Nurse-led beds and early supported discharge schemes increased the likelihood of living at home without any adverse effect on mortality Mortality: admissions avoidance OR 0.88 (95% CI 0.53 to 1.47), nurse-led beds OR 1.00 (95% CI 0.62 to 1.60), early discharge OR 0.97 (95% CI 0.71 to 1.32), GAUs and ACE units OR 0.98 (95% CI 0.78 to 1.23), inpatient rehabilitation OR 0.71 (95% CI 0.56 to 0.90), community-based rehabilitation OR 1.07 (95% CI 0.73 to 1.58), day hospital OR 1.30 (95% CI 0.96 to 1.76) Living at home: admission avoidance OR 1.10 (95% CI 0.63 to 1.93), nurse-led beds OR 2.01 (95% CI 1.37 to 2.94), early discharge OR 1.58 (95% CI 1.16 to 2.14), GAUs and ACE units OR 1.26 (95% CI 1.04 to 1.53), inpatient rehabilitation OR 1.61 (95% CI 1.20 to 2.15), community-based rehabilitation OR 1.01 (95% CI 0.42 to 2.46), day hospital OR 0.60 (95% CI 0.27 to 1.37) |
88% |
| Sheppard 200919 Inception–January 2008 Meta-analysis Older people contacting emergency care at home or in the emergency department |
Hospital-at-home schemes (including a multidisciplinary team, the provision of 24-hour cover if required, with access to a doctor, and a safe home environment) compared with inpatient hospital care | Admission-avoidance hospital at home can provide an effective alternative to inpatient care for a selected group of elderly patients otherwise requiring hospital admission, with a trend towards increased readmission: 3-month mortality-adjusted HR 0.77 (95% CI 0.54 to 1.09), 6-month mortality-adjusted HR 0.62 (95% CI 0.45 to 0.87), 3-month readmission or hospitalisation HR 1.49 (95% CI 0.96 to 2.33). Other health outcomes were similar | 94% |
| General reviews | |||
| Ali 200420 1980–2003 Patients aged ≥ 65 years with complex comorbidities who need services between general hospital and home support |
To provide evidence for the effectiveness of any service managing patients across the hospital–community interface | The evidence reviewed concluded that intervention programmes that provide services to reduce and prevent falls are effective in doing so; discharge planning arrangements have some beneficial effects on subsequent readmission to hospital; hospital-at-home schemes as an alternative to acute hospital care have good outcomes for selected patients; emergency department-based studies were insufficient in number and quality to comment on; there is uncertainty over the effectiveness of nurse-led inpatient care for post-acute patients and little is known about community-based nurse-led units; integrated post-discharge home-care programmes guided by a case manager show benefits | 93% |
| Day 200421 1980–2003 Patients aged ≥ 65 years with complex morbidities or at risk of deteriorating function who require rehabilitation following hospitalisation for an acute episode or who have multiple chronic health conditions or changing disabilities or who are frail or who have an unclear diagnosis, atypical presentation of illness or sudden unexplained decline in functional abilities |
To identify and appraise international evidence for the effectiveness of specialist geriatric services | The evidence was generally supportive of specialist geriatric services in community settings – for prevention and supportive discharge. However, benefits were not consistent across all outcomes and were not always clinically significant. There was good evidence for integrated CGA services for orthogeriatric patients, which cover acute care and supported discharge; good evidence for inpatient CGA with ‘medical control’ and long-term follow-up of patients; inconclusive evidence relating to inpatient CGA units (GEMUs/GEUs); good evidence for a CGA approach in the management of stroke and delirium; and a lack of evidence for day hospitals or outpatient CGA. For patients as well as caregivers, targeted comprehensive services (including training and education in addition to assessment and treatment) provided by a multidisciplinary team, tailored to individuals’ needs, appear to be the most effective specialist team service models | 93% |
Appendix 4 A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: databases and searches
Taken from Conroy S, Stevens T, Parker S, Gladman J. A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing 2011;40:436–43 by permission of Oxford University Press.
The following databases were searched from inception until September 2009:
-
Ovid MEDLINE(R) (1966+).
-
EMBASE (1980+).
-
BNI (1985+).
-
HMIC.
-
The Cochrane Library.
-
CINAHL.
-
AgeInfo (www.cpa.org.uk/ageinfo/ageinfo2.html).
-
ASSIA.
-
NRR Archive (http://portal.nihr.ac.uk/Pages/NRRArchive.aspx).
-
NICHSR (www.nlm.nih.gov/nichsr/db.html).
-
DARE/HTA database/NHS EED (www.crd.york.ac.uk/crdweb/).
The following search terms were used (adapted from previous relevant reviews):
-
acute care/sub-acute care/post-acute care/intermediate care/care continuum/integrated care/progressive care/transitional care (identifies the setting)
-
frail/geriatric assessment/health services for the aged/(geriatric unit or specialist geriatric or acute geriatric).mp./((elder$or older or geriatric$or aged) adj3 (unit or specialist)).tw./acute care for elder$.ti./(acute care adj3 elderly).mp./elder$unit$.ab./geriatric$acute care.ab. (identifies the population/process)
-
activities of daily living/cost/cost benefit/cost effectiveness/mortality/health status/length of stay/discharge/readmission/quality of life/satisfaction/carer strain/carer burden (identifies the outcomes).
Appendix 5 A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: data extraction (results) table
| Study | Design | Mean van Tulder score | Setting | Intervention | Model | Population | Primary outcome | Main results (intervention : control) |
|---|---|---|---|---|---|---|---|---|
| Davison 200523 | RCT | 13.5 | Two urban EDs | Hospital-based geriatric assessment and home-based physiotherapy and occupational therapy assessment focusing on falls | Geriatrician led (OPD) | 313 cognitively intact men and women aged > 65 years with a fall or fall-related injury and at least one fall in the preceding year; 159 randomised to the intervention and 154 to usual care | Falls over 1 year | At 12 months: falls 435 : 1251 (387 : 617 excluding outliers); death 3/159 : 5/154; fall-related admission 14/159 : 17/154 |
| Caplan 200427 | RCT | 11.5 | Urban ED | Hospital- or home-based, nurse-led CGA with weekly MDT supported by geriatricians | Nurse led | 739 patients aged > 75 years discharged from the ED; 370 randomised to the intervention and 369 to the control | Hospital admissions in 30 days | 30 days: readmission 42/370 : 51/399 18 months: death 55/370 : 53/399; institutionalisation 32/370 : 28/399; admission 164/370 : 201/399 |
| McCusker 200325 | Pseudo- RCT | 11 | Four urban EDs | Brief standardised geriatric nursing assessment in the ED with geriatrician or emergency physician input as required followed by referral to community services/GPs | Nurse led | 10,826 patients attended the EDs; 7921 were assessed for eligibility and 5766 were excluded; 426 were eligible (high ISAR score) and 388 consented, of whom 178 were randomised to the intervention and 210 were randomised to the control | Primary care physician and ED use over 30 days | 30 days: death 1/166 : 1/179; return ED visit 58/166 : 48/179 |
| Mion 200326 | RCT | 12.5 | Two urban EDs | CGA led by an advanced practice nurse specialising in geriatrics, liaison with emergency staff, referral to community services as appropriate and short-term case management | Nurse led | 2815 patients were screened of whom 987 were eligible, 650 were enrolled and 450 were randomised (226 intervention and 224 control) | Death, repeat ED use, hospitalisation and nursing home transfer at 120 days | 30 days: death 4/326 : 2/324; return ED visit 66/326 : 49/324; institutionalisation 2/326 : 9/324; hospitalisation 46/326 : 46/324 120 days: death 9/326 : 10/324; return ED visit 121/326 : 128/324; institutionalisation 91/326 : 87/324 |
| Close 199924 | RCT | 10.5 | Urban ED | Geriatrician-led day hospital-delivered CGA and single OT home visit. Day hospital referral for MDT if required | Geriatrician led (OPD) | Patients aged > 65 years presenting with a fall to A&E; 1031 were screened of whom 397 were randomised (184 intervention and 213 control) | Falls over 1 year | 12 months: cumulative number of falls 183 : 510; death 19/184 : 27/213; institutionalisation 18/184 : 18/213; hospital admission 69/184 : 97/213 |
Appendix 6 Umbrella review of tools to assess the risk of poor outcome in older people attending acute medical units: databases and searches
Taken from Edmans JE, Gladman JRF, Havard D. Umbrella Review of Tools to Assess Risk of Poor Outcome in Older People Attending Acute Medical Units. Medical Crises in Older People Discussion Paper Series. Issue 11, June 2012. URL: www.nottingham.ac.uk/mcop/documents/papers/issue11-mcop-issn2044-4230.pdf (accessed 20 March 2015), reproduced under the terms of the Creative Commons Attribution Non-commercial No derivatives 3.0 Licence (CC BY-NC-ND 3.0) http://creativecommons.org/licenses/by-nc-nd/3.0/.
A literature review was conducted to identify relevant systematic reviews of appropriate tools to assess the risk of functional decline in older people attending AMUs.
The following databases were searched from inception until 31 December 2011:
-
MEDLINE (1946 to February Week 1 2012)
-
PsycINFO (1806 to February Week 2 2012)
-
CINAHL
-
EMBASE (1980 to 2012 Week 7)
-
Web of Science
-
The Cochrane Library
-
Cochrane Database of Systematic Reviews (CDSR)
-
DARE
-
Cochrane Controlled Trial Register (CCTR).
The following search strategy was used, based on previous relevant reviews:
-
exp Aged/
-
(aged, 80 and over).mp.
-
aged.mp.
-
age*.mp.
-
elder*. 6 aging*.mp.
-
exp Aging/
-
geriatric*.mp.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
-
exp Hospitalization/
-
hospitalised patient.mp.
-
hospital admission.mp.
-
older patient.mp.
-
10 or 11 or 12 or 13
-
9 and 14
-
screening.mp.
-
screening instrument.mp.
-
exp Risk Assessment/
-
geriatric screening.mp.
-
risk assessment.mp.
-
predictors.mp.
-
predict*.mp.
-
predicting,mp.
-
16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
-
functional decline.mp.
-
functional status decline.mp.
-
ADL decline.mp.
-
decreased physical function.mp.
-
decreased physical outcome.mp.
-
impaired physical outcome.mp.
-
ADL status decline.mp.
-
25 or 26 or 27 or 28 or 29 or 30 or 31
-
15 and 24 and 32
-
limit 33 to (English language and humans)
-
limit 34 to ‘review’
Appendix 7 Umbrella review of tools to assess the risk of poor outcome in older people attending acute medical units: data extraction (results) table
Taken from Taken from Edmans JE, Gladman JRF, Havard D. Umbrella Review of Tools to Assess Risk of Poor Outcome in Older People Attending Acute Medical Units. Medical Crises in Older People Discussion Paper Series. Issue 11, June 2012. URL: www.nottingham.ac.uk/mcop/documents/papers/issue11-mcop-issn2044-4230.pdf (accessed 20 March 2015), reproduced under the terms of the Creative Commons Attribution Non-commercial No derivatives 3.0 Licence (CC BY-NC-ND 3.0) http://creativecommons.org/licenses/by-nc-nd/3.0/.
| Characteristics of study | McCusker 200231 | Hoogerduijn 200729 | Sutton 200830 | de Saint-Hubert 201032 |
|---|---|---|---|---|
| Objective | Predict functional decline in older hospitalised patients aged > 60 years – physical decline or nursing home admission | Identify valid, reliable and clinical user-friendly tools to screen for functional decline in older people | Identify screening tools to screen elderly patients aged > 65 years at risk of functional decline presenting to emergency departments, any condition | Identify tools to detect the risk of functional decline at and after discharge |
| Aspects of functional decline considered | ADL ability, nursing home admission, death | ADL ability, nursing home placement, mortality, hospital resource costs | ADL ability, physical function, cognitive function, nursing home admission, quality of life | ADL ability, nursing home admission, death |
| Inclusion criteria | Elderly patients, longitudinal design, one or more predictors of functional decline | Predictors of functional decline, tested in hospital setting, tools to identify risk of functional decline | Age > 65 years, admitted to emergency department, any condition, tools with predictive validity, generalisability, clinical utility and reliability | Age > 65 years, admitted to hospital, cohort study, risk assessment, early evaluation, functional decline, follow-up at and/or after discharge |
| Date of search and databases searched | 1976–98 MEDLINE and hand searching |
1990–February 2005 MEDLINE, PsycINFO, CINAHL, The Cochrane Library, DARE, CDSR, CCTR, reference lists of selected articles |
1990–November 2007 MEDLINE, CINAHL, Ageline, PsycINFO, EMBASE, The Cochrane Library, DARE, CDSR, CCTR |
1970–2007 MEDLINE and 1981–2007 Web of Science plus reference lists of relevant papers |
| Exclusion criteria | Not original study, restricted to specific condition or procedure, intervention as predictor, not hospital setting, not in English | Case reports, commentaries, guidelines | Includes risk factors only, not a screening tool | Studies restricted to a particular setting (e.g. heart failure, hip fracture), community or rehabilitation setting, risk factors only |
Appendix 8 Identification of Seniors at Risk tool questions (answered yes or no)
-
Before the illness or injury that brought you to the emergency department, did you need someone to help you on a regular basis?
-
Since the illness or injury that brought you to the emergency department, have you needed more help than usual to take care of yourself?
-
Have you been hospitalised for one or more nights during the past 6 months (excluding a stay in the emergency department)?
-
In general, do you see well?
-
In general, do you have serious problems with your memory?
-
Do you take more than three different medications every day?
Appendix 9 The Identification of Seniors at Risk score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units: the Acute Medical Unit Outcome Study – baseline patient-identifiable data form
| Hospital number | ||
| Date | ||
| Name | ||
| Date of birth/age | Date of birth: | Age: |
| Gender | Male □ Female □ | |
| Address | ||
| Telephone number | ||
| Immediate discharge destination | ||
| GP | ||
| GP telephone number | ||
| Ethnicity | White | □ |
| Mixed | □ | |
| Asian | □ | |
| Black | □ | |
| Chinese | □ | |
| Other | □ | |
| Mental capacity present | Yes □ No □ | Date: |
| Person who gave assent | ||
| Relationship to participant | ||
| Carer/contact name | ||
| Carer address | ||
Appendix 10 The Identification of Seniors at Risk score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units: the Acute Medical Unit Outcome Study – baseline patient interview form
T his section is to be completed from the medical notes of the participant and by direct interview.
| Date | ||||||
|---|---|---|---|---|---|---|
| Gender | Male □ Female □ | |||||
| Consent/assent | Consent □ Carer assent □ Consultant assent □ | |||||
| Has the participant ever had any of the following medical conditions? | ||||||
| Myocardial infarct | Yes □ No □ | |||||
| Congestive heart failure | Yes □ No □ | |||||
| Peripheral vascular disease | Yes □ No □ | |||||
| Cerebrovascular disease | Yes □ No □ | |||||
| Dementia | Yes □ No □ | |||||
| Chronic pulmonary disease | Yes □ No □ | |||||
| Short of breath | Yes □ No □ | |||||
| Connective tissue disease | Yes □ No □ | |||||
| Ulcer disease | Yes □ No □ | |||||
| Mild liver disease | Yes □ No □ | |||||
| Moderate or severe liver disease | Yes □ No □ | |||||
| Diabetes | Yes □ No □ | |||||
| Hemiplegia | Yes □ No □ | |||||
| Moderate or severe renal disease | Yes □ No □ | |||||
| Renal failure | Yes □ No □ | |||||
| Diabetes with end-organ damage | Yes □ No □ | |||||
| Any tumour | Yes □ No □ | |||||
| Leukaemia | Yes □ No □ | |||||
| Lymphoma | Yes □ No □ | |||||
| Metastatic solid tumour | Yes □ No □ | |||||
| AIDS | Yes □ No □ | |||||
| Did the patient present with any of the following? | ||||||
| Fall | Yes □ No □ | |||||
| Reduced mobility | Yes □ No □ | |||||
| New or increased continence disorder | Yes □ No □ | |||||
| Current pressure sores | Yes □ No □ | |||||
| Dehydration | Yes □ No □ | |||||
| Deteriorated cognitive skills or status in the past 3 months | Yes □ No □ | |||||
| Psychological stress or acute disease in the past 3 months (e.g. bereavement, moved home, been sick) | Yes □ No □ | |||||
| Result of the AMU assessment (as listed in the records). This may be a diagnosis (i.e. a disease), a problem (e.g. a fall) or a symptom (e.g. chest pain). Record diagnoses if made; only record problems or symptoms if not | ||||||
| Medications | ||||||
| Drug | Dose | Frequency | Route | |||
| Total number of different prescription medications taken each day | ||||||
| Neuropsychological problems | ||||||
| Severe dementia or depression □ | ||||||
| Mild dementia or depression □ | ||||||
| No psychological problems □ | ||||||
| Measurements | ||||||
| Height | ||||||
| Weight (use scales) | ||||||
| Demispan | ||||||
| Mid-arm circumference (cm) | Right arm | Left arm | ||||
| Calf circumference (cm) | Right calf | Left calf | ||||
| Grip strength | Right | Left | ||||
| Ability to rise from a chair five times without using his/her arms | Yes □ No □ | Time | ||||
| Ability to walk 2.4 meters (8 feet) | Yes □ No □ | Time | ||||
| Note equipment used | ||||||
| Cognition | ||||||
| MMSE – not reproduced for copyright reasons | ||||||
Appendix 11 The Identification of Seniors at Risk score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units: the Acute Medical Unit Outcome Study – baseline patient data collection form
Appendix 12 The Identification of Seniors at Risk score to predict clinical outcomes and health service costs in older people discharged from UK acute medical units: the Acute Medical Unit Outcome Study – follow-up patient data collection form
Appendix 13 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: patient screening data form
| Ward | |
| Bed number | |
| Date | |
| Completed by | Patient □ Other □ |
| If other, specify relationship to patient: | |
| Identification of Senior at Risk | |
|---|---|
| Before the illness that brought you to the acute medical unit, did you need someone to help you on a regular basis? | Yes □1 No □0 |
| Since the illness or injury that brought you to the acute medical unit, have you needed more help than usual to take care of yourself? | Yes □1 No □0 |
| Have you been hospitalised for one or more nights during the past 6 months (excluding this stay in the acute medical unit)? | Yes □1 No □0 |
| In general, do you see well? | Yes □0 No □1 |
| In general, do you have serious problems with your memory? | Yes □1 No □0 |
| Do you take more than three different medications every day? | Yes □ 1 No □0 |
| Total ISAR score | |
Inclusion criterion for the study is an ISAR score of ≥ 2.
Appendix 14 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: baseline patient-identifiable data form
| Date recruited | ||
| NHS number | ||
| Hospital number | ||
| Name | ||
| Date of birth/age | Date of birth: | Age: |
| Gender | Male □ Female □ | |
| Address | ||
| Telephone number | ||
| In care home at baseline | Yes □ No □ | |
| In care home at outcome | Yes □ No □ | |
| Immediate discharge destination | ||
| GP | ||
| GP telephone number | ||
| Ethnicity | White | □ |
| Mixed | □ | |
| Asian | □ | |
| Black | □ | |
| Chinese | □ | |
| Other | □ | |
| Mental capacity present | Yes □ No □ | Date: |
| Person who gave assent | ||
| Relationship to participant | ||
| Contacted family if recruited with medical practitioner as consultee | Yes □ No □ | Date: |
| Comments | ||
| Randomised | Yes □ No □ | Date: |
| Randomisation code: | ||
| If not randomised, give reason | ||
| Assessed by Front door Assessment and Co-ordination Team (FACT) | Yes □ No □ | |
| Consent to interview study | Yes □ No □ | |
| Carer/contact name | ||
| Carer phone number | ||
| Carer address | ||
Appendix 15 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: patient baseline initial interview form
T his section is to be completed from the medical notes of the participant and by direct interview.
| Date | ||||
|---|---|---|---|---|
| Gender | Male □ Female □ | |||
| Consent type | Consent □ Carer consultee □ Medical practitioner consultee □ | |||
| Cognition | ||||
| MMSE – not reproduced for copyright reasons | ||||
| Result of the AMU assessment (as listed in the records). This may be a diagnosis (i.e. a disease), a problem (e.g. a fall) or a symptom (e.g. chest pain). Record diagnoses if made; only record problems or symptoms if not | ||||
| Assessed by Assessed by Front door Assessment and Co-ordination Team (FACT)/crisis response | Yes □ No □ | |||
| Medications | ||||
| Drug | Dose | Frequency | Route | |
| Total number of different prescription medications taken each day | ||||
| Did the patient present with any of the following? | ||||
| Weight loss | Yes □ No □ | |||
| Fall | Yes □ No □ | |||
| Reduced mobility | Yes □ No □ | |||
| New or increased continence disorder | Yes □ No □ | |||
| Current pressure sores | Yes □ No □ | |||
| Dehydration | Yes □ No □ | |||
| Cognitive impairment/confusion | Yes □ No □ | |||
| Has the participant ever had any of the following medical conditions? | ||||
| Myocardial infarct | Yes □ No □ | |||
| Congestive heart failure | Yes □ No □ | |||
| Peripheral vascular disease | Yes □ No □ | |||
| Cerebrovascular disease | Yes □ No □ | |||
| Dementia | Yes □ No □ | |||
| Chronic pulmonary disease | Yes □ No □ | |||
| Short of breath | Yes □ No □ | |||
| Connective tissue disease | Yes □ No □ | |||
| Ulcer disease | Yes □ No □ | |||
| Mild liver disease | Yes □ No □ | |||
| Moderate or severe liver disease | Yes □ No □ | |||
| Diabetes | Yes □ No □ | |||
| Hemiplegia | Yes □ No □ | |||
| Moderate or severe renal disease | Yes □ No □ | |||
| Diabetes with end-organ damage | Yes □ No □ | |||
| Any tumour | Yes □ No □ | |||
| Leukaemia | Yes □ No □ | |||
| Lymphoma | Yes □ No □ | |||
| Metastatic solid tumour | Yes □ No □ | |||
| AIDS | Yes □ No □ | |||
Appendix 16 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: patient baseline initial data collection form
Appendix 17 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: patient follow-up data collection form
Appendix 18 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: carer baseline data collection form
Appendix 19 Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study: carer follow-up data collection form
Appendix 20 The Better Mental Health cohort study: screening form
Study number ________________________ Ward _______________________
Researcher ___________________________ Date ________________________
-
Can the patient speak enough to communicate? If no, is this due to severe aphasia or tracheostomy? If so, exclude. If not, screen is positive.
-
Is the patient unconscious, drowsy or too unwell to answer? If yes, go straight to question 4 or review to day 5 and exclude if still unable.
| 1. Ask: will you do a short memory test for me? | Correct = 1, wrong or unable = 0 | Positive |
| What is your age? (exact) | Yes No | |
| What is the time? (nearest hour) | ||
| Please repeat the address ‘42 West Street’ and try to remember it | ||
| What is the year? (exact) | ||
| What is the name of this hospital? (any generally accepted) | ||
| Can you tell me what these two people do? (show photographs) | ||
| What is your date of birth? (month and year correct) | ||
| What was the year of the First World War? (accept 1914 or 1914–18) | ||
| What is the name of the current monarch? | ||
| Please count backwards from 20 to 1 (all correct) | ||
| Recall the address | ||
| Total (≤ 7 is positive): | ||
| 2. May I now ask some questions about your mood? | Positive | |
| Are you basically satisfied with life? | No = 1, yes = 0 | Yes No |
| Do you feel that your life is empty | No = 0, yes = 1 | |
| Are you afraid that something bad is going to happen to you? | No = 0, yes = 1 | |
| Do you feel happy most of the time? | No = 1, yes = 0 | |
| Total (≥ 1 is positive): | ||
| In the past month: | Yes = 1, no = 0 | |
| Have you been bothered by nerves, feeling anxious or being on edge? | Yes No | |
| Have you been bothered by worrying about a lot of different things? | ||
| Have you had an anxiety or a panic attack? (sudden feeling of panic) | ||
| Total (≥ 2 is positive): | ||
| 3. Now I’d like to ask some questions about drinking alcohol | Yes = 1, no = 0 | Positive |
| Do you ever drink alcohol? If no, screen negative. If yes, ask: | ||
| Have you ever felt you needed to cut down on your drinking? | Yes No | |
| Have people annoyed you by criticising your drinking? | ||
| Have you ever felt guilty about drinking? | ||
| Have you ever felt you needed a drink first thing in the morning to steady your nerves or to get rid of a hangover (an eye-opener)? | ||
| Total (≥ 2 is positive): | ||
| 4. Is there any other reason to suspect that this person might have a mental health problem (e.g. agitated, confused, appearing to hallucinate, nurses report ‘something odd’) | Yes No | Positive: Yes No |
Study number _________________________
Date _________________________________
Screen positive?
Ask: Would you consider taking part in a research study about mood or memory problems in hospital? Give information sheet and explain as necessary.
Assess capacity
Can the person (free from undue pressure):
-
Understand information about the study?
-
Retain the information (for long enough to make a decision)?
-
Use it to make a decision?
-
Communicate the decision?
If yes to all, patient has capacity. If no to any, patient lacks capacity
Patient has capacity: Yes No
If yes, ask patient if they are willing to take part and take formal consent? Ask patient if you could talk to a family member or carer. Then contact carer for carer participant consent.
If no, contact carer, seek assent and carer participant consent.
Appendix 21 The Better Mental Health cohort study: patient baseline data form
Appendix 22 The Better Mental Health cohort study: carer baseline form
Appendix 23 The Better Mental Health cohort study: patient outcome form
Appendix 24 The Better Mental Health cohort study: carer outcome form
Appendix 25 Comparison of a specialist Medical and Mental Health Unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial: patient baseline data form
Study ID ________________________
This section is to be completed from the medical and nursing notes of the participant by the researcher.
| Has the participant ever had any of the following medical conditions? | |||||||
|---|---|---|---|---|---|---|---|
| Myocardial infarction, heart attack, angina | Yes □ No □ | ||||||
| Stroke or cerebrovascular disease | Yes □ No □ | ||||||
| Heart failure, left ventricular failure, congestive cardiac failure | Yes □ No □ | ||||||
| Other heart disease (atrial fibrillation, valve disease) | Yes □ No □ | ||||||
| Dementia | Yes □ No □ | ||||||
| Chronic obstructive pulmonary disease (COPD) or asthma | Yes □ No □ | ||||||
| Osteoarthritis | Yes □ No □ | ||||||
| Hip fracture | Yes □ No □ | ||||||
| Diabetes | Yes □ No □ | ||||||
| Kidney disease | Yes □ No □ | ||||||
| Cancer | Yes □ No □ | ||||||
| Depression | Yes □ No □ | ||||||
| Arthritis or rheumatism | Yes □ No □ | ||||||
| Eyesight problems (not corrected with glasses) | Yes □ No □ | ||||||
| Hearing difficulties or deafness | Yes □ No □ | ||||||
| Breathlessness or shortness of breath | Yes □ No □ | ||||||
| Paralysis, weakness, loss of arm or leg, hemiplegia, paraplegia | Yes □ No □ | ||||||
| Peripheral vascular disease | Yes □ No □ | ||||||
| Medical history at admission or other relevant information | |||||||
| Did the participant present with any of the following? | |||||||
| Fall | Yes □ No □ | ||||||
| Reduced mobility (off legs) | Yes □ No □ | ||||||
| New or increased continence disorder | Yes □ No □ | ||||||
| Deteriorated cognitive skills or status in the past 3 months | Yes □ No □ | ||||||
| Admission medications (British National Formulary is available on the ward or in the office; please look up spellings of drugs and British National Formulary chapter number – if unsure ask Sarah) | |||||||
| Drug | Dose | Frequency | BNF chapter number | ||||
| Total number of different prescription medications taken each day | |||||||
| Admission/initial modified early warning score. From observations chart. Please circle. Observation chart may put score that doesn’t exist, in which case look at observation and record as coded below | |||||||
| Score | 3 | 2 | 1 | 0 | 1 | 2 | 3 |
| Temperature (°C) | < 35 | – | 35.1–36 | 36.1–38 | – | 38.1–39 | > 39 |
| Systolic blood pressure (mmHg) | < 80 | 81–90 | 91–100 | 101–179 | – | 180–199 | > 200 |
| Heart rate (bpm) | < 40 | 41–50 | – | 51–100 | 101–110 | 111–129 | > 130 |
| Respiratory rate per minute | < 8 | – | – | 9–14 | 15–20 | 21–29 | > 30 |
| Oxygen % | > 40%/10 l | 15 l/minute | |||||
| Conscious level (alert, voice, pain, unresponsive) | New weakness, pupil deficit | Newly confused/agitated | – | A | V | P | U |
| Urine output (ml/kg/h) | < 10 | < 20 | < 30 | Not passed urine in 6 hours | Not passed urine in 12 hours | Not passed urine in 18 hours | |
This section is to be completed from the nursing notes – activities of daily living at current admission.
| Barthel index | ||
|---|---|---|
| How do they manage with grooming? | Needs help with personal care | 0 |
| Independent face/hair/teeth/shaving (implements provided) | 1 | |
| How do they manage with eating? | Unable | 0 |
| Needs help cutting, spreading butter, etc. | 1 | |
| Independent (food provided in reach) | 2 | |
| How do they manage with dressing? | Dependent | 0 |
| Needs help but can do about half unaided | 1 | |
| Independent (including buttons, zips, laces, etc.) | 2 | |
| How do they manage with bathing? | Dependent | 0 |
| Independent (or in shower) | 1 | |
| How do they manage using the toilet? | Dependent | 0 |
| Needs some help but can do something alone | 1 | |
| Independent (on and off, dressing, wiping) | 2 | |
| How do they manage with their bladder? | Incontinent or catheterised and unable to manage | 0 |
| Occasional accident (max. once per 24 hours) | 1 | |
| Continent (for > 7 days) | 2 | |
| How do they manage with their bowels? | Incontinent (or needs to be given enema) | 0 |
| Occasional accident (once per week) | 1 | |
| Continent | 2 | |
| How do they manage with transferring? | Unable – no sitting balance | 0 |
| Major help (one or two people, physical) can sit | 1 | |
| Minor help (verbal or physical) | 2 | |
| Independent | 3 | |
| How do they manage with mobility? | Immobile | 0 |
| Wheelchair independent including corners, etc. | 1 | |
| Walks with help of one person (verbal or physical) | 2 | |
| Independent (but may use any aid, e.g. stick) | 3 | |
| How do they manage with stairs? | Unable | 0 |
| Needs help (verbal, physical, carrying aid, stair lift) | 1 | |
| Independent up and down | 2 | |
This section to be completed from NOTIS – previous hospital stays.
| Has the patient been in hospital in the past year? | Yes □ No □ | |
| If yes, please list the dates and hospital: | ||
| Dates | Hospital | |
|---|---|---|
| From | To | |
Appendix 26 Comparison of a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial – carer baseline data form
Appendix 27 Comparison of a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial – patient outcome form
Appendix 28 Comparison of a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial – carer outcome form
Appendix 29 Comparison of a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial – medical data form
Study ID ________________________
This section is to be completed from the medical notes of the participant by the researcher.
| Has the participant ever had any of the following medical conditions? | |||||||
|---|---|---|---|---|---|---|---|
| Myocardial infarct | Yes □ No □ | ||||||
| Congestive heart failure | Yes □ No □ | ||||||
| Peripheral vascular disease | Yes □ No □ | ||||||
| Cerebrovascular disease | Yes □ No □ | ||||||
| Dementia | Yes □ No □ | ||||||
| Chronic pulmonary disease | Yes □ No □ | ||||||
| Short of breath | Yes □ No □ | ||||||
| Connective tissue disease | Yes □ No □ | ||||||
| Ulcer disease | Yes □ No □ | ||||||
| Mild liver disease | Yes □ No □ | ||||||
| Moderate or severe liver disease | Yes □ No □ | ||||||
| Diabetes | Yes □ No □ | ||||||
| Hemiplegia | Yes □ No □ | ||||||
| Moderate or severe renal disease | Yes □ No □ | ||||||
| Renal failure | Yes □ No □ | ||||||
| Diabetes with end-organ damage | Yes □ No □ | ||||||
| Any tumour | Yes □ No □ | ||||||
| Leukaemia | Yes □ No □ | ||||||
| Lymphoma | Yes □ No □ | ||||||
| Metastatic solid tumour | Yes □ No □ | ||||||
| AIDS | Yes □ No □ | ||||||
| Neuropsychological problems | |||||||
| Severe dementia or depression □ | |||||||
| Mild dementia or depression □ | |||||||
| No psychological problems □ | |||||||
| Did the participant present with any of the following? | |||||||
| Fall | Yes □ No □ | ||||||
| Reduced mobility | Yes □ No □ | ||||||
| New or increased continence disorder | Yes □ No □ | ||||||
| Current pressure sores | Yes □ No □ | ||||||
| Dehydration | Yes □ No □ | ||||||
| Deteriorated cognitive skills or status in the past 3 months | Yes □ No □ | ||||||
| Psychological stress or acute disease in the past 3 months (e.g. bereavement, moved home, been sick) | Yes □ No □ | ||||||
| Admission medications | |||||||
| Drug | Dose | Frequency | BNF chapter number | ||||
| Total number of different prescription medications taken each day | |||||||
| Admission/initial modified early warning score. Please circle | |||||||
| Score | 3 | 2 | 2 | 0 | 1 | 2 | 3 |
| Systolic blood pressure (mmHg) | < 70 | 71–80 | 81–100 | 101–199 | – | > 200 | – |
| Heart rate (bpm) | – | < 40 | 41–50 | 51–100 | 101–110 | 111–129 | > 130 |
| Respiratory rate | – | < 9 | – | 9–14 | 15–20 | 21–29 | > 30 |
| Temperature (°C) | – | < 35 | – | 35–38.4 | – | > 38.5 | – |
| Conscious level (alert, voice, pain, unresponsive) | – | – | – | A | V | P | U |
Appendix 30 Comparison of a specialist medical and mental health unit with standard care for older people with cognitive impairment admitted to a general hospital: a randomised controlled trial – methods for analysis of the staffing interviews
Taken from Spencer K, Foster P, Whittamore KH, Goldberg SE, Harwood RH. Delivering dementia care differently – evaluating the differences and similarities between a specialist medical and mental health unit and standard acute care wards: a qualitative study of family carers’ perceptions of quality of care. BMJ Open 2013;3:e004198. URL: http://bmjopen.bmj.com/content/3/12/e004198.full.pdf+html (accessed 20 March 2015), reproduced under the terms of the Creative Commons Attribution Non-commercial Licence (CC BY-NC 3.0): http://creativecommons.org/licenses/by-nc/3.0/
Interviews were transcribed verbatim and NVivo software (version 10; QSR International, Warrington, UK) was used to facilitate analysis. Data were analysed thematically using a framework analysis that allowed a systematic process to be followed in the development of knowledge and theory. Framework analysis is a flexible approach utilised in health service research that allows all data to be collected and then analysed. The organisation of data within this approach involved a five-stage process: (1) familiarisation, (2) identifying a thematic framework, (3) indexing, (4) charting and (5) mapping and interpretation. Familiarisation with data involved constant comparison across the data to identify categories and themes. Coding transcripts to identify recurrent statements and expressed feelings formed the basis of the thematic framework. Themes were compared and contrasted between settings by indexing, charting and mapping to provide a detailed understanding and interpretation of the participants’ experiences and whether and how the intervention added to the carers’ perspectives of quality of care. All authors met on a regular basis to discuss the development of codes, themes, categories and theories about the phenomenon being studied.
Appendix 31 Literature review of care home randomised controlled trials: databases and searches
Taken from Gordon AL, Logan PA, Jones RG, Forrester-Paton C, Mamo JP, Gladman JRF, et al. A systematic mapping review of randomized controlled trials (RCTs) in care homes. BMC Geriatr 2012;12:31, reproduced under the terms of the Creative Commons Attribution Non-commercial Licence (CC BY 2.0): http://creativecommons.org/licenses/by/2.0/.
MEDLINE (1950 to June 2009) was searched for ‘Nursing Home’, ‘Residential Facilities’ and ‘Homes for the Aged’, combined using the ‘OR’ command. Results were limited to the English language and RCTs. CINAHL with full text (1978 to June 2009) was searched for ‘nursing homes’, ‘residential facilities’ and ‘skilled nursing facilities’, with results limited to RCTs. The Allied and Complementary Medicine Database (AMED) (1985 to June 2009) was searched for ‘Nursing homes’, ‘Long term care’ and ‘Residential facilities’ combined using the ‘OR’ command and ‘Randomized controlled trial’ using the ‘AND’ command. The British Nursing Index (BNI) (1985 to June 2009) was searched for ‘Nursing Homes’, ‘Residential Care’ and ‘Long-term care’. Abstracts were reviewed by a single researcher and articles were included if they described interventions evaluated by a RCT in residential, nursing or care homes.
A keywording strategy was developed by three researchers using an iterative approach and a random sample of 20 articles, which were reviewed repetitively with key descriptors recorded. The researchers met after each iteration and the process concluded when two subsequent reviews identified no new descriptors. The resulting framework described year of publication, country of publication, individual or cluster randomisation, stratified or non-stratified randomisation, method of stratification, blinding strategy (patient/investigators/both/neither), target of intervention, intervention treatment, control treatment, number of subjects (total/intervention/control), number of clusters (total/intervention/control), outcome measures and results. The remaining articles were then divided amongst six reviewers who classified them according to the keywording strategy. As a final measure, all articles were reviewed by the lead researcher with disagreements resolved by consensus.
Appendix 32 Literature review of care home randomised controlled trials: data extraction (results) tables (selected)
Taken from Gordon AL, Logan PA, Jones RG, Forrester-Paton C, Mamo JP, Gladman JRF, et al. A systematic mapping review of randomized controlled trials (RCTs) in care homes. BMC Geriatr 2012;12:31. This work has been reproduced under the terms of the Creative Commons Attribution License (CC BY 2.0): http://creativecommons.org/licenses/by/2.0/. and Gordon AL. Does Comprehensive Geriatric Assessment (CGA) Have a Role in UK Care Homes? PhD thesis. Nottingham: University of Nottingham; 2012.
| Country | Number of articles |
|---|---|
| USA | 145 |
| UK | 24 |
| Netherlands | 23 |
| Canada | 16 |
| Australia | 12 |
| Japan | 8 |
| Type of intervention | No. of studies |
|---|---|
| Pharmacological | 87 |
| Physical therapy | 56 |
| Occupational therapy, aids and appliances | 45 |
| Education of staff | 32 |
| Nutritional | 21 |
| Psychological or behavioural therapy | 15 |
| Home administration | 15 |
| Dental and oral health | 14 |
| Vaccine | 14 |
| Case management/CGA | 10 |
| Nursing interventions not covered elsewhere | 6 |
| Aromatherapy | 1 |
Appendix 33 A cohort study of the health status and outcomes of care home residents: baseline data collection form
This information should be gathered, where possible, without the help of the subject. Care home records and care home staff should be used as the primary informants. Any remaining gaps should be filled by reference to GP or hospital notes should be consulted.
| Study ID | |||
| Marital status | |||
| Type of accommodation (residential home/nursing home) | |||
| Date of admission to care home | |||
| Health | |||
|---|---|---|---|
| Current medical problems/diagnoses | |||
| Medications (drug, dose, frequency, route) | |||
| Has the subject ever had any of the following medical conditions? | |||
| Myocardial infarct | Yes □ No □ | ||
| Congestive heart failure | Yes □ No □ | ||
| Peripheral vascular disease | Yes □ No □ | ||
| Cerebrovascular disease | Yes □ No □ | ||
| Dementia | Yes □ No □ | ||
| Chronic pulmonary disease | Yes □ No □ | ||
| Connective tissue disease | Yes □ No □ | ||
| Ulcer disease | Yes □ No □ | ||
| Mild liver disease | Yes □ No □ | ||
| Diabetes | Yes □ No □ | ||
| Hemiplegia | Yes □ No □ | ||
| Moderate or severe renal disease | Yes □ No □ | ||
| Diabetes with end-organ damage | Yes □ No □ | ||
| Any tumour | Yes □ No □ | ||
| Leukaemia | Yes □ No □ | ||
| Lymphoma | Yes □ No □ | ||
| Moderate or severe liver disease | Yes □ No □ | ||
| Metastatic solid tumour | Yes □ No □ | ||
| AIDS | Yes □ No □ | ||
| Renal failure | Yes □ No □ | ||
| Chronic heart failure | Yes □ No □ | ||
| Short of breath | Yes □ No □ | ||
| Cancer | Yes □ No □ | ||
| Is there evidence that the subject has suffered psychological stress or acute disease in the past 3 months? | Yes □ No □ | ||
| NHS resource use – visiting | |||
| Does the subject have regular visits from any of the following? | |||
| GP | Yes □ No □ Times per week □ | ||
| Physiotherapist | Yes □ No □ Times per week □ | ||
| Podiatrist/chiropodist | Yes □ No □ Times per week □ | ||
| Dentist | Yes □ No □ Times per week □ | ||
| Community matron | Yes □ No □ Times per week □ | ||
| District nurse | Yes □ No □ Times per week □ | ||
| Specialist nurse (if yes, list all relevant specialties below) | Yes □ No □ Times per week □ | ||
| Other (please list) | Yes □ No □ Times per week □ | ||
| Day centres/regular use of resources away from the care home | |||
| Do you attend any centres, etc.? | |||
| Day centre/hospital | Yes □ No □ Times per week □ | ||
| Other (please list) | Yes □ No □ Times per week □ | ||
| Nutrition | |||
| How is the subject fed? | Unable to eat without assistance □ | ||
| Self-fed with some difficulty □ | |||
| Self-fed without any problem □ | |||
| With regard to eating over the last 7 days in particular, how has the subject managed? | Independent □ | ||
| Supervised □ | |||
| Limited assistance □ | |||
| Extensive assistance □ | |||
| Total dependence □ | |||
| Has the subject unintentionally lost weight in the last 12 months | Yes □ No □ | ||
| If ‘yes’, how much weight have they lost over the last 12 months | > 3 kg (6.6 lb) □ 1–3 kg (2.2–6.6 lb) □ No weight loss □ Does not know □ |
||
| Does the subject have a poor appetite (eats less than one-quarter of their meal) | Yes □ No □ | ||
| Has the subject’s food intake declined over the past 3 months because of loss of appetite, digestive problems or chewing or swallowing difficulties? | Severe loss of appetite □ | ||
| Moderate loss of appetite □ | |||
| No loss of appetite □ | |||
| How many full meals does the subject eat daily? | 1 meal □ | ||
| 2 meals □ | |||
| 3 meals □ | |||
| In the subject’s diet, do they manage: | At least one serving of dairy products (milk, cheese, yogurt) per day? Yes □ No □ | If 0 or 1 ‘yes’ □ If 2 ‘yes’ □ If 3 ‘yes’ □ |
|
| Two or more servings of legumes or eggs per week? Yes □ No □ | |||
| Meat, fish or poultry every day? Yes □ No □ | |||
| Does the subject consume two or more servings of fruit or vegetables per day? | Yes □ No □ | ||
| How much fluid (water, juice, coffee, tea, milk, etc.) does the subject consume per day? | < 3 cups □ | ||
| 3–5 cups □ | |||
| > 5 cups □ | |||
| Personal ADL | |||
| How has the subject managed with their personal hygiene over the last 7 days? | Independent □ | ||
| Supervised □ | |||
| Limited assistance □ | |||
| Extensive assistance □ | |||
| Total dependence □ | |||
| How do they manage with grooming? | Needs help with personal care | 0 | |
| Independent face/hair/teeth/shaving (implements provided) | 1 | ||
| How do they manage with eating? | Unable | 0 | |
| Needs help cutting, spreading butter, etc. | 1 | ||
| Independent (food provided in reach) | 2 | ||
| How do they manage with dressing? | Dependent | 0 | |
| Needs help but can do about half unaided | 1 | ||
| Independent (including buttons, zips, laces, etc.) | 2 | ||
| How do they manage with bathing? | Dependent | 0 | |
| Independent (or in shower) | 1 | ||
| How has the subject managed with using the toilet over the last 7 days? | Independent □ | ||
| Supervised □ | |||
| Limited assistance □ | |||
| Extensive assistance □ | |||
| Total dependence □ | |||
| How do they manage with their bowels? | Incontinent (or needs to be given enema) | 0 | |
| Occasional accident (once per week) | 1 | ||
| Continent | 2 | ||
| How do they manage with their bladder? | Incontinent or catheterised and unable to manage | 0 | |
| Occasional accident (max. once per 24 hours) | 1 | ||
| Continent (for > 7 days) | 2 | ||
| How do they manage with regard to using the toilet? | Dependent | 0 | |
| Needs some help but can do something alone | 1 | ||
| Independent (on and off, dressing, wiping) | 2 | ||
| Mobility | |||
| With regard to mobility, is the subject? | Bed or chair bound □ | ||
| Able to get out of bed/chair but does not go out □ | |||
| Goes out □ | |||
| Over the last 7 days in particular, how has the subject been with regard to mobility? | Independent □ | ||
| Supervised □ | |||
| Limited assistance □ | |||
| Extensive assistance □ | |||
| Total dependence □ | |||
| How do they manage with transferring? | Unable – no sitting balance | 0 | |
| Major help (one or two people, physical) can sit | 1 | ||
| Minor help (verbal or physical) | 2 | ||
| Independent | 3 | ||
| How do they manage with mobility? | Immobile | 0 | |
| Wheelchair independent including corners, etc. | 1 | ||
| Walks with help of one person (verbal or physical) | 2 | ||
| Independent (but may use any aid, e.g. stick) | 3 | ||
| How do they manage with stairs? | Unable | 0 | |
| Needs help (verbal, physical, carrying aid) | 1 | ||
| Independent up and down | 2 | ||
| Cognition | |||
| Has the subject suffered deterioration in their cognitive skills or status in the past 3 months? | Yes □ No □ | ||
| Does the subject suffer from? | Severe dementia or depression □ | ||
| Mild dementia □ | |||
| No psychological problems □ | |||
| Behaviour | |||
| Delusions: does the subject have beliefs that you know are not true? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (beliefs present but seem harmless and produce little distress) □ | ||
| Moderate (beliefs are distressing and disruptive) □ | |||
| Marked (beliefs are very disruptive and are a major source of disturbed behaviour) □ | |||
| Hallucinations: does the subject have hallucinations such as false visions or voices? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (hallucinations present but seem harmless and produce little distress) □ | ||
| Moderate (hallucinations are distressing and disruptive) □ | |||
| Marked (hallucinations are very disruptive and are a major source of disturbed behaviour) □ | |||
| Agitation and aggression: does the subject have periods when he/she is agitated or aggressive? Or refuses to co-operate? Or won’t let people help him/her with washing or dressing? Or shouts or swears? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (behaviour is disruptive but can be managed with distraction or reassurance) □ | ||
| Moderate (behaviour is disruptive and difficult to distract or control) □ | |||
| Marked (agitation is very disruptive and a major source of difficulty; there may be a threat of personal harm) □ | |||
| Depression: does the subject seem sad or depressed? Does he or she say that he or she feels sad or depressed? Or a burden, a failure or a bad person? Or say that he/she wishes to die or harm him/herself? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (depression is distressing but usually responds to distraction or reassurance) □ | ||
| Moderate (depression is distressing and depressive thoughts are spontaneously spoken by the subject and are difficult to alleviate) □ | |||
| Marked (depression is very distressing and a major source of suffering for the subject) □ | |||
| Is the subject nervous, anxious, worried or frightened? Is he/she shaky, tense or fidgety? Is he/she afraid to be in particular places or apart from familiar people? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (anxiety is distressing but usually responds to distraction or reassurance) □ | ||
| Moderate (anxiety is distressing and anxiety symptoms are spontaneously voiced by the subject and are difficult to alleviate) □ | |||
| Marked (anxiety is very distressing and a major source of suffering for the subject) □ | |||
| Elation: does the subject seem abnormally cheerful or happy for no reason? Does he/she find things funny that others don’t? Or tell silly jokes or play tricks or pranks? Or boast about abilities or wealth? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (elation is noticeable by friends and family but is not disruptive) □ | ||
| Moderate (elation is noticeably abnormal) □ | |||
| Marked (elation is very pronounced; subject is euphoric and finds everything to be funny) □ | |||
| Apathy and indifference: has the subject lost interest in the world around him/her? Does he or she seem less interested in his/her usual activities and in other people? Or has he or she become less likely to start a conversation? Does he or she seem not to have any motivation or not to care about things any more? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (apathy is noticeable but produces little interference with daily life; only slightly different from usual behaviour; subject responds to suggestions to do things) □ | ||
| Moderate (apathy is very evident; may be overcome with coaxing and encouragement; responds spontaneously only to powerful events such as family visits) □ | |||
| Marked (apathy is very evident and usually fails to respond to any encouragement or external events) □ | |||
| Disinhibition: does the subject seem to act impulsively without thinking about the consequences? Does he/she talk to strangers as if he or she knows them? Or say or do things that are rude or embarrassing? Or hurt people’s feelings? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (behaviour is noticeable but usually responds to distraction or reassurance) □ | ||
| Moderate (behaviour is very evident and difficult to overcome by carer) □ | |||
| Marked (behaviour usually fails to respond to any intervention by carer and is a source of embarrassment or social distress) □ | |||
| Irritability and temper: does the subject get irritated easily? Or impatient? Do his/her moods change quickly? Does he/she get bad tempered? Or angry or argumentative? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (irritability or moodiness is noticeable but usually responds to distraction or reassurance) □ | ||
| Moderate (irritability or moodiness is very evident and difficult to overcome by carer) □ | |||
| Marked (irritability or moodiness is very evident, usually fails to respond to any intervention by carer and is a major source of distress) □ | |||
| Motor behaviour: does the subject pace around or wander? Or engage in repetitive activities, such as opening cupboards or drawers or picking at things or winding threads? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (behaviour is noticeable but produces little interference with daily life) □ | ||
| Moderate (behaviour is very evident but can be overcome by carer) □ | |||
| Marked (behaviour is very evident and usually fails to respond to any intervention by carer and is a major source of distress) □ | |||
| Does the subject have difficulty sleeping? Is he or she up at night (not including getting up once or twice to the toilet)? Does he/she get up at night thinking it is day? Is he/she sleepy during the day? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (every night) □ | |||
| And how severe are the problems? | Mild (night-time behaviours occur but are not particularly disruptive) □ | ||
| Moderate (night-time behaviours occur and disturb the subject and the sleep of the carer; more than one type of night-time behaviour may be present) □ | |||
| Marked (night-time behaviour occurs; several types of night-time behaviour may be present; the subject is very distressed during the night and the sleep of the carer is very disturbed) □ | |||
| Has the subject’s appetite or eating habits changed? Has he/she lost or gained weight or changed the foods that he/she likes? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (change in appetite or eating habits is present but has not led to change in weight and is not disturbing) □ | ||
| Moderate (change in appetite or eating habits is present and has caused a minor change in weight) □ | |||
| Marked (obvious changes in appetite or eating habits are present and have caused weight change; this is embarrassing or otherwise disturbs the subject) □ | |||
Appendix 34 A cohort study of the health status and outcomes of care home residents: baseline interview form
To be conducted by a researcher with the participant. A next-of-kin or care home staff member may be present.
Study ID ___________________________
| Measurements | ||
|---|---|---|
| Height | ||
| Weight | ||
| Calculate body mass index (BMI) (weight in kg)/(height in m)2 | BMI < 19 □ | |
| BMI 19 to < 21 □ | ||
| BMI 21 to < 23 □ | ||
| BMI ≥ 23 □ | ||
| Mid-arm circumference | Right arm | Left arm |
| Mid-calf circumference | Right calf | Left calf |
| Grip strength | Right | Left |
| Can the subject rise from a chair five times without using their arms? | Yes □ No □ | |
| Observations | ||
| Is the subject clinically dehydrated? | Yes □ No □ | |
| Questions | ||
| Pain | ||
| 1. Which statement best describes how you feel at the moment about pain or discomfort? | I have no pain or discomfort □ | |
| I have moderate pain or discomfort □ | ||
| I have extreme pain or discomfort □ | ||
| Health | ||
| 2. In comparison with other people of the same age, how do you consider your health status? | Not as good □ | |
| Does not know □ | ||
| As good □ | ||
| Better □ | ||
| Nutrition | ||
| 3. Do you view yourself as being . . . | Malnourished □ | |
| Having no nutritional problem □ | ||
| Not sure □ | ||
| Personal ADL | ||
| 4. Which statement best describes how you feel at the moment about looking after yourself? | I have no problems with looking after myself □ | |
| I have some problems in washing or dressing □ | ||
| I am unable to wash or dress myself □ | ||
| 5. Which statement best describes how you feel at the moment about daily activities? | I have no problems performing my usual activities □ | |
| I have some problems performing my usual activities □ | ||
| I am unable to perform my usual activities □ | ||
| Mobility | ||
| 6. Which statement best describes how you feel at the moment about your mobility? | I have no problems walking about □ | |
| I have some problems walking about □ | ||
| I am confined to bed □ | ||
| Social networking | ||
| 7. How often do you talk to friends/relatives outside of your care home? | Very often □ | |
| Often □ | ||
| Not very often □ | ||
| Never □ | ||
| Mood | ||
| 8. Over the last few weeks have you recently been able to concentrate on whatever you’re doing? | ||
| Better than usual | □ 0 | |
| Same as usual | □ 1 | |
| Less than usual | □ 2 | |
| Much less than usual | □ 3 | |
| 9. Over the last few weeks have you recently lost much sleep over worry? | ||
| Not at all | □ 0 | |
| No more than usual | □ 1 | |
| Rather more than usual | □ 2 | |
| Much more than usual | □ 3 | |
| 10. Over the last few weeks have you recently felt that you were playing a useful part in things? | ||
| More so than usual | □ 0 | |
| Same as usual | □ 1 | |
| Less useful than usual | □ 2 | |
| Much less useful | □ 3 | |
| 11. Over the last few weeks have you recently felt capable of making decisions about things? | ||
| More so than usual | □ 0 | |
| Same as usual | □ 1 | |
| Less so than usual | □ 2 | |
| Much less than usual | □ 3 | |
| 12. Over the last few weeks have you recently felt constantly under strain? | ||
| Not at all | □ 0 | |
| No more than usual | □ 1 | |
| Rather more than usual | □ 2 | |
| Much more than usual | □ 3 | |
| 13. Over the last few weeks have you recently felt that you couldn’t overcome your difficulties? | ||
| Not at all | □ 0 | |
| No more than usual | □ 1 | |
| Rather more than usual | □ 2 | |
| Much more than usual | □ 3 | |
| 14. Over the last few weeks have you recently been able to enjoy your normal day-to-day activities? | ||
| More so than usual | □ 0 | |
| Same as usual | □ 1 | |
| Less so than usual | □ 2 | |
| Much less than usual | □ 3 | |
| 15. Over the last few weeks have you recently been able to face up to your problems? | ||
| More so than usual | □ 0 | |
| Same as usual | □ 1 | |
| Less so than usual | □ 2 | |
| Much less able | □ 3 | |
| 16. Over the last few weeks have you recently been feeling unhappy and depressed? | ||
| Not at all | □ 0 | |
| No more than usual | □ 1 | |
| Rather more than usual | □ 2 | |
| Much more than usual | □ 3 | |
| 17. Over the last few weeks have you recently been losing confidence in yourself? | ||
| Not at all | □ 0 | |
| No more than usual | □ 1 | |
| Rather more than usual | □ 2 | |
| Much more than usual | □ 3 | |
| 18. Over the last few weeks have you recently been thinking of yourself as a worthless person? | ||
| Not at all | □ 0 | |
| No more than usual | □ 1 | |
| Rather more than usual | □ 2 | |
| Much more than usual | □ 3 | |
| 19. Over the last few weeks have you recently been feeling reasonably happy all things considered? | ||
| More so than usual | □ 0 | |
| About same as usual | □ 1 | |
| Less so than usual | □ 2 | |
| Much less than usual | □ 3 | |
| 20. Do you feel full of energy? | Yes □ No □ | |
| 21. Which statement best describes how you feel at the moment about your mood? | I am not anxious or depressed □ | |
| I am moderately anxious or depressed □ | ||
| I am extremely anxious or depressed □ | ||
| Health economic data – to patient: ‘I’d like to finish off by asking some additional questions about you’ | ||
| 22. How financially well off do you feel in general? | Very well off □ | |
| Well off □ | ||
| Not well off □ | ||
| 23. Do you receive pension credit? | Yes □ | |
| No □ | ||
| 24. What was your highest level of education? | Primary school □ | |
| Secondary school □ | ||
| Vocational training □ | ||
| University/college □ | ||
| MMSE | ||
| Not reproduced for copyright reasons. | ||
Appendix 35 A cohort study of the health status and outcomes of care home residents: follow-up data collection form
This information should be gathered, where possible, without the help of the participant. Care home records and care home staff should be used as the primary informants. Any remaining gaps should be filled by reference to the GP or hospital notes should be consulted.
Study ID _________________________
| Personal ADLs | |||
|---|---|---|---|
| How do they manage with grooming? | Needs help with personal care | 0 | |
| Independent face/hair/teeth/shaving (implements provided) | 1 | ||
| How do they manage with eating? | Unable | 0 | |
| Needs help cutting, spreading butter, etc. | 1 | ||
| Independent (food provided in reach) | 2 | ||
| How do they manage with dressing? | Dependent | 0 | |
| Needs help but can do about half unaided | 1 | ||
| Independent (including buttons, zips, laces, etc.) | 2 | ||
| How do they manage with bathing? | Dependent | 0 | |
| Independent (or in shower) | 1 | ||
| How do they manage with their bowels? | Incontinent (or needs to be given enema) | 0 | |
| Occasional accident (once per week) | 1 | ||
| Continent | 2 | ||
| How do they manage with their bladder? | Incontinent or catheterised and unable to manage | 0 | |
| Occasional accident (max. once per 24 hours) | 1 | ||
| Continent (for > 7 days) | 2 | ||
| How do they manage with regard to using the toilet? | Dependent | 0 | |
| Needs some help but can do something alone | 1 | ||
| Independent (on and off, dressing, wiping) | 2 | ||
| Mobility | |||
| How do they manage with transferring? | Unable – no sitting balance | 0 | |
| Major help (one or two people, physical) can sit | 1 | ||
| Minor help (verbal or physical) | 2 | ||
| Independent | 3 | ||
| How do they manage with mobility? | Immobile | 0 | |
| Wheelchair independent including corners, etc. | 1 | ||
| Walks with help of one person (verbal or physical) | 2 | ||
| Independent (but may use any aid, e.g. stick) | 3 | ||
| How do they manage with stairs? | Unable | 0 | |
| Needs help (verbal, physical, carrying aid) | 1 | ||
| Independent up and down | 2 | ||
| Behaviour | |||
| Delusions: does the subject have beliefs that you know are not true? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (beliefs present but seem harmless and produce little distress) □ | ||
| Moderate (beliefs are distressing and disruptive) □ | |||
| Marked (beliefs are very disruptive and are a major source of disturbed behaviour) □ | |||
| Hallucinations: does the subject have hallucinations, such as false visions or voices? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (hallucinations present but seem harmless and produce little distress) □ | ||
| Moderate (hallucinations are distressing and disruptive) □ | |||
| Marked (hallucinations are very disruptive and are a major source of disturbed behaviour) □ | |||
| Agitation and aggression: does the subject have periods when he/she is agitated or aggressive? Or refuses to co-operate? Or won’t let people help him/her with washing or dressing? Or shouts or swears? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (behaviour is disruptive but can be managed with distraction or reassurance) □ | ||
| Moderate (behaviour is disruptive and difficult to distract or control) □ | |||
| Marked (agitation is very disruptive and a major source of difficulty; there may be a threat of personal harm) □ | |||
| Depression: does the subject seem sad or depressed? Does he or she say that he or she feels sad or depressed? Or a burden, a failure or a bad person? Or he/she wishes to die or harm him/herself? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (depression is distressing but usually responds to distraction or reassurance) □ | ||
| Moderate (depression is distressing and depressive thoughts are spontaneously spoken by the subject and are difficult to alleviate) □ | |||
| Marked (depression is very distressing and a major source of suffering for the subject) □ | |||
| Is the subject nervous, anxious, worried or frightened? Is he/she shaky, tense or fidgety? Is he/she afraid to be in particular places or apart from familiar people? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (anxiety is distressing but usually responds to distraction or reassurance) □ | ||
| Moderate (anxiety is distressing and anxiety symptoms are spontaneously voiced by the subject and are difficult to alleviate) □ | |||
| Marked (anxiety is very distressing and a major source of suffering for the subject) □ | |||
| Elation: does the subject seem abnormally cheerful or happy for no reason? Does he/she find things funny that others don’t? Or tell silly jokes or play tricks or pranks? Or boast about abilities or wealth? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (elation is noticeable by friends and family but is not disruptive) □ | ||
| Moderate (elation is noticeably abnormal) □ | |||
| Marked (elation is very pronounced; subject is euphoric and finds everything to be funny) □ | |||
| Apathy and indifference: has the subject lost interest in the world around him/her? Does he or she seem less interested in his/her usual activities and in other people? Or has he or she become less likely to start a conversation? Or seems not to have any motivation or not to care about things any more? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (apathy is noticeable but produces little interference with daily life; only slightly different from usual behaviour; subject responds to suggestions to do things) □ | ||
| Moderate (apathy is very evident; may be overcome with coaxing and encouragement; responds spontaneously only to powerful events such as family visits) □ | |||
| Marked (apathy is very evident and usually fails to respond to any encouragement or external events) □ | |||
| Disinhibition: does the subject seem to act impulsively without thinking about the consequences? Does he/she talk to strangers as if he or she knows them? Or say or do things that are rude or embarrassing? Or hurt people’s feelings? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (behaviour is noticeable but usually responds to distraction or reassurance) □ | ||
| Moderate (behaviour is very evident and difficult to overcome by carer) □ | |||
| Marked (behaviour usually fails to respond to any intervention by carer and is a source of embarrassment or social distress) □ | |||
| Irritability and temper: does the subject get irritated easily? Or impatient? Do his/her moods change quickly? Does he/she get bad tempered? Or angry or argumentative? | Yes □ No □ | ||
| If yes, how often do these problems occur? | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (irritability or moodiness is noticeable but usually responds to distraction or reassurance) □ | ||
| Moderate (irritability or moodiness is very evident and difficult to overcome by carer) □ | |||
| Marked (irritability or moodiness is very evident, usually fails to respond to any intervention by carer and is a major source of distress) □ | |||
| Motor behaviour: does the subject pace around or wander? Or engage in repetitive activities such as opening cupboards or drawers or picking at things or winding threads? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (behaviour is noticeable but produces little interference with daily life) □ | ||
| Moderate (behaviour is very evident but can be overcome by carer) □ | |||
| Marked (behaviour is very evident and usually fails to respond to any intervention by carer and is a major source of distress) □ | |||
| Does the subject have difficulty sleeping? Is he or she up at night (not including getting up once or twice to the toilet)? Does he/she get up at night thinking it is day? Is he/she sleepy during the day? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (every night) □ | |||
| And how severe are the problems? | Mild (night-time behaviours occur but are not particularly disruptive) □ | ||
| Moderate (night-time behaviours occur and disturb the subject and the sleep of the carer; more than one type of night-time behaviour may be present) □ | |||
| Marked (night-time behaviour occurs; several types of night-time behaviour may be present; the subject is very distressed during the night and the sleep of the carer is very disturbed) □ | |||
| Has the subject’s appetite or eating habits changed? Has he/she lost or gained weight or changed the foods that he/she likes? | Yes □ No □ | ||
| If yes, how often do these problems occur | Occasionally (less than once a week) □ | ||
| Often (about once a week) □ | |||
| Frequently (several times a week but less than every day) □ | |||
| Very frequently (once a day or more) □ | |||
| And how severe are the problems? | Mild (change in appetite or eating habits is present but has not led to change in weight and is not disturbing) □ | ||
| Moderate (change in appetite or eating habits is present and has caused a minor change in weight) □ | |||
| Marked (obvious changes in appetite or eating habits are present and have caused weight change; this is embarrassing or otherwise disturbs the subject) □ | |||
Appendix 36 A cohort study of the health status and outcomes of care home residents: follow-up interview form
To be conducted by a researcher with the participant 6 months after initial interview. A next-of-kin or care home staff member may be present.
Study ID _____________________________
| Questions | |
|---|---|
| Pain | |
| 1. Which statement best describes how you feel at the moment about pain or discomfort? | I have no pain or discomfort □ |
| I have moderate pain or discomfort □ | |
| I have extreme pain or discomfort □ | |
| Personal ADL | |
| 2. Which statement best describes how you feel at the moment about looking after yourself? | I have no problems with looking after myself □ |
| I have some problems in washing or dressing □ | |
| I am unable to wash or dress myself □ | |
| 3. Which statement best describes how you feel at the moment about daily activities? | I have no problems performing my usual activities □ |
| I have some problems performing my usual activities □ | |
| I am unable to perform my usual activities □ | |
| Mobility | |
| 4. Which statement best describes how you feel at the moment about your mobility? | I have no problems walking about □ |
| I have some problems walking about □ | |
| I am confined to bed □ | |
| Mood | |
| 5. Which statement best describes how you feel at the moment about your mood? | I am not anxious or depressed □ |
| I am moderately anxious or depressed □ | |
| I am extremely anxious or depressed □ | |
| 6. Over the last few weeks have you recently been able to concentrate on whatever you’re doing? | |
| Better than usual | □ 0 |
| Same as usual | □ 1 |
| Less than usual | □ 2 |
| Much less than usual | □ 3 |
| 7. Over the last few weeks have you recently lost much sleep over worry? | |
| Not at all | □ 0 |
| No more than usual | □ 1 |
| Rather more than usual | □ 2 |
| Much more than usual | □ 3 |
| 8. Over the last few weeks have you recently felt that you were playing a useful part in things? | |
| More so than usual | □ 0 |
| Same as usual | □ 1 |
| Less useful than usual | □ 2 |
| Much less useful | □ 3 |
| 9. Over the last few weeks have you recently felt capable of making decisions about things? | |
| More so than usual | □ 0 |
| Same as usual | □ 1 |
| Less so than usual | □ 2 |
| Much less than usual | □ 3 |
| 10. Over the last few weeks have you recently felt constantly under strain? | |
| Not at all | □ 0 |
| No more than usual | □ 1 |
| Rather more than usual | □ 2 |
| Much more than usual | □ 3 |
| 11. Over the last few weeks have you recently felt that you couldn’t overcome your difficulties? | |
| Not at all | □ 0 |
| No more than usual | □ 1 |
| Rather more than usual | □ 2 |
| Much more than usual | □ 3 |
| 12. Over the last few weeks have you recently been able to enjoy your normal day-to-day activities? | |
| More so than usual | □ 0 |
| Same as usual | □ 1 |
| Less so than usual | □ 2 |
| Much less than usual | □ 3 |
| 13. Over the last few weeks have you recently been able to face up to your problems? | |
| More so than usual | □ 0 |
| Same as usual | □ 1 |
| Less so than usual | □ 2 |
| Much less able | □ 3 |
| 14. Over the last few weeks have you recently been feeling unhappy and depressed? | |
| Not at all | □ 0 |
| No more than usual | □ 1 |
| Rather more than usual | □ 2 |
| Much more than usual | □ 3 |
| 15. Over the last few weeks have you recently been losing confidence in yourself? | |
| Not at all | □ 0 |
| No more than usual | □ 1 |
| Rather more than usual | □ 2 |
| Much more than usual | □ 3 |
| 16. Over the last few weeks have you recently been thinking of yourself as a worthless person? | |
| Not at all | □ 0 |
| No more than usual | □ 1 |
| Rather more than usual | □ 2 |
| Much more than usual | □ 3 |
| 17. Over the last few weeks have you recently been feeling reasonably happy all things considered? | |
| More so than usual | □ 0 |
| About same as usual | □ 1 |
| Less so than usual | □ 2 |
| Much less than usual | □ 3 |
Appendix 37 An interview study of the actors involved in the health care of care home residents: methods
Taken from Robbins IJ, Gordon AL, Dyas JV, Logan PA, Gladman JRF. Explaining the barriers to and tensions in delivering effective health care in UK care homes: a qualitative study. BMJ Open 2013;3:e003178. URL: http://bmjopen.bmj.com/content/3/7/e003178 (accessed 20 March 2015), reproduced under the term of the Creative Commons Attribution Non-commercial Licence 3.0 (CC BY-NC 3.0) https://creativecommons.org/licenses/by-nc/3.0/.
With the existing paucity of knowledge concerning how health care is delivered in care homes, a grounded theory approach was adopted. A phenomenological interview study was used to understand how formal health care is delivered in care homes. The perspectives of care home staff and primary care services were sought using qualitative interviews that aimed to provide a description of context, different cultures of work, concepts and behaviours and to give parity to accounts from different professional and organisational perspectives.
Semistructured interviews were used, expecting respondents’ time to be limited. In light of the media and regulatory scrutiny, it was anticipated that care home staff might feel defensive and that their care was being judged. Therefore, a hypothetical case vignette was used to help elicit talk and to generate valid data.
The initial intention was for both residential and nursing homes, with and without dementia registration, to be sampled. The aim was to sample participants from the typical range of care staff who work in care homes and from primary care. Initial interviews were therefore planned with managers, nurses and care assistants employed in care homes and GPs, district nurses and allied health professionals providing services from primary care. The data-driven grounded theory approach required theoretical sampling whereby sampling decisions could change as the study progressed to test evolving theoretical constructs.
The managers of all care homes both within Nottinghamshire and within a circle with a 10-mile radius centred on the University of Nottingham Medical School (n = 131) were invited to a care home educational event. Of these, 18 care homes accepted an invitation to take part in a cohort study. Eleven care homes were selected from these for the cohort study using a purposive sampling matrix that reproduced the proportion of residents housed in residential/nursing and dementia-registered homes nationally. All 11 homes from the cohort study were invited to take part in the interview study. Once a home was recruited, individual care home staff were invited to participate through a circular letter and posters placed in staffrooms and on notice boards. Data saturation was reached after six homes were recruited.
General practitioners were approached after recruitment of the care homes. One practice attached to each home was identified and the GP who most frequently provided care was approached. Allied health professionals and district nurses were recruited from contacts made during the conduct of research in GP practices and care homes or were sought out by telephone and letter when their participation was considered to be important to the emerging theoretical framework.
The interviews were completed at a time and place to suit the participants and lasted between 20 and 90 minutes. An interview guide and case vignette guided the interview. Recordings were made using a digital recorder and were transferred to compact discs, transcribed and anonymised. The recordings were erased as soon as the anonymised transcription was verified as a true record by the interviewer.
The interviews were undertaken by IJR and ALG. Neither had direct clinical responsibility for the residents in the care homes but ALG worked as a NHS community geriatrician in the same region.
To understand the complexity of health-care delivery, an iterative process ran in parallel with data collection. After each interview IJR and ALG discussed the interview content, which they checked against interview schedules. The schedules were adapted, with emerging themes to be used in later interviews. Memos were written after interviews, recording ideas and initial analysis. Contradicting evidence was sought in the emerging theories. Recruitment was stopped when data saturation was felt to have been reached. Further analysis was performed using NVivo version 8 to organise the interview data and memos. Coding of all of the data was carried out by IJR and ALG, independently initially, to develop subthemes. The final analysis was triangulated by all authors through team discussions, literature review and the writing phase of this process.
Appendix 38 NHS Outcomes Framework 2012–13
Taken from www.gov.uk/government/publications/nhs-outcomes-framework-2012-to-2013 (accessed 11 February 2015).
-
Domain 1: preventing people from dying prematurely.
-
Domain 2: enhancing quality of life for people with long-term conditions.
-
Domain 3: helping people to recover from episodes of ill health or following injury.
-
Domain 4: ensuring that people have a positive experience of care.
-
Domain 5: treating and caring for people in a safe environment and protecting them from avoidable harm.
Glossary
- Abbreviated Mental Test Score
- A 10-item questionnaire used to screen for cognitive impairment (confusion).
- Activities of daily living
- Refers to activities that are part of normal life. Person activities of daily living refers to activities related to self-care whereas instrumental activities of daily living refers to activities such as household or social activities.
- Acute medical unit
- A short-stay hospital unit used to triage and stabilise patients presenting to hospital as an emergency. Another name for this is a medical assessment unit. In the UK these units differ from emergency departments because they are not limited to a 4-hour stay and they operate some degree of selection of medical (as opposed to surgical or trauma) patients.
- Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study
- One of the studies conducted as part of this research.
- Acute Medical Unit Outcome Study
- One of the studies conducted as part of this research.
- Area under the curve
- A form of mathematical analysis used to measure the discriminating value of a diagnostic test.
- Barthel index
- A 20-point scale measuring the amount of assistance required to undertake 10 different daily activities and to maintain continence.
- Black and minority ethnic
- Terminology used in the UK to describe people of non-white descent.
- Brokering Innovation Through Evidence
- Short, accessible summaries of research, put in context and circulated directly to relevant practitioners, developed by the Collaboration for Leadership in Applied Health Research and Care – Nottinghamshire, Derbyshire and Leicestershire.
- CAGE
- A four-item questionnaire to screen for alcoholism, the letters in the acronym referring to the areas covered in the four questions (Cut down, Anger, Guilt, Eye-opener).
- Client Service Receipt Inventory
- A questionnaire used to record a person’s use of health and social care services.
- Collaboration for Leadership in Applied Health Research and Care – Nottinghamshire, Derbyshire and Leicestershire
- A National Institute for Health Research-supported organisation aiming to conduct implementation research and to facilitate the implementation of research findings.
- Comprehensive geriatric assessment
- A process used to provide health care for older people with frailty.
- Comprehensive Local Research Networks
- Regional networks in England designed to support research in the NHS.
- Confidence interval
- When a mean (average) or some other statistic is calculated, confidence intervals can be calculated to provide an indication of the precision of that statistic. Small samples produce less precise estimates of means or other statistics and larger samples produce more precise estimates. The 95% confidence interval of a point estimate represents the upper and lower boundaries of the point estimate between which one can be 95% confident of the true value of the statistic and hence represents the degree of precision of the point estimate.
- Delirium Rating Scale – Revised 98
- A scale used to diagnose and measure the severity of delirium (acute confusion).
- DEMQoL
- A quality-of-life score for people with dementia.
- Doctor of Philosophy
- A postgraduate academic qualification awarded after ≥ 3 years of study through research. The term is used for a large range of disciplines and not only philosophical studies.
- Electronic Administration Record
- An electronic record kept by providers of health or social care services for administration purposes.
- Emergency department
- Areas of hospitals in which unselected patients presenting as emergencies are seen. In the current UK health-care system it is intended that patients will stay in such units for < 4 hours.
- European Quality of Life-5 Dimensions
- A measure of overall health status.
- General Health Questionnaire – 12 items
- A brief questionnaire measuring mental well-being, focusing largely on depressive and anxiety symptoms.
- General practitioner
- In the UK state-provided health system all citizens can register with a primary care physician, called a general practitioner, who is responsible for the provision and gatekeeping of most health care.
- Geriatric Depression Score – four items
- A brief questionnaire used to screen for depression. There are several other versions of this scale with more items.
- Identification of Seniors at Risk
- A short scale used in emergency care settings intended to distinguish between older people with higher and lower risks of a range of subsequent adverse outcomes.
- Incremental cost-effectiveness ratio
- The ratio between the costs of an intervention and its benefits. It is typically expressed in terms of the cost required to achieve 1 extra year of good-quality life.
- Information technology
- The use of computers and related technology.
- Interquartile range
- A statistical term that gives an indication of the degree of spread in the distribution of a variable and represents the range of values of the variable for the middle 50% of a sample. The sample is arranged in order and split into quarters (quartiles) and the interquartile range gives the lowest value of the second quartile and the highest value of the third quartile.
- Master of Science
- A postgraduate academic qualification awarded after 1 year of study through research.
- Medical and mental health unit
- Name used in this programme of research to describe a specialist hospital ward dedicated to the care of people with delirium and dementia.
- Medical Crises in Older People
- The name of this programme of research.
- Mini-Mental State Examination
- A 30-point score of global cognitive function.
- Mini Nutritional Assessment
- A short scale to assess the nutritional status of a patient.
- National Health Service
- The UK state-funded health-care system.
- National Institute for Health Research
- An arm of the NHS dedicated to the conduct of clinical research.
- Patient and public involvement
- The involvement of patients or other members of the public in the conduct of research, rather than them simply being participants or informants.
- Primary Care Evaluation of Mental Disorders
- A screening questionnaire for depressive symptoms.
- Programme Grant for Applied Research
- A funding stream of the National Institute for Health Research. The research in this report was largely funded by this finding stream.
- Quality-adjusted life-year
- A concept used by health economists to account for both the quantity (survival) and quality of life. For example, 1 quality-adjusted life-year could represent a person living for a year with 100% quality of life or two people living for a year with 50% quality of life.
- Randomised controlled trial
- A form of experimental design used in research in which the outcomes of a group given a new treatment are compared with the outcomes of a group given another treatment (called a control treatment) and in which the research participants are allocated to the treatment group or the control group at random.
- Receiver operating characteristic
- A graphical plot used here to examine the discriminating ability of a diagnostic test.
- Relative risk
- A statistic giving the probability of an event occurring in a treatment group compared with a control group.
- Research for Patient Benefit
- A funding stream of the National Institute for Health Research. Some of the research in this report was funded by this scheme.
- Service Delivery and Organisation
- A funding stream of the National Institute for Health Research. Some of the research presented here draws on research supported by this funding stream.
- Trial of an Elderly Acute care Medical and mental health unit
- One of the studies conducted as part of this research.
List of abbreviations
- ADL
- activities of daily living
- AMIGOS
- Acute Medical Unit Comprehensive Geriatric Assessment Intervention Study
- AMOS
- Acute Medical Unit Outcome Study
- AMU
- acute medical unit
- ASSIA
- Applied Social Sciences Index and Abstracts
- AUC
- area under the curve
- BME
- black and minority ethnic
- BNI
- British Nursing Index
- CAGE
- Cut down, Anger, Guilt, Eye-opener
- CCTR
- Cochrane Controlled Trial Register
- CDSR
- Cochrane Database of Systematic Reviews
- CGA
- comprehensive geriatric assessment
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLAHRC-NDL
- Collaboration for Leadership in Applied Health Research and Care – Nottinghamshire, Derbyshire and Leicestershire
- CSRI
- Client Service Receipt Inventory
- DARE
- Database of Abstracts of Reviews of Effects
- DRS-R-98
- Delirium Rating Scale – Revised 98
- EQ-5D
- European Quality of Life-5 Dimensions
- GHQ-12
- General Health Questionnaire – 12 items
- GP
- general practitioner
- HMIC
- Health Management Information Consortium
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISAR
- Identification of Seniors at Risk
- MMHU
- medical and mental health unit
- MMSE
- Mini-Mental State Examination
- NHS EED
- NHS Economic Evaluation Database
- NICHSR
- National Information Centre on Health Services Research and Health Care Technology
- NIHR
- National Institute for Health Research
- NPI
- Neuropsychiatric Inventory
- NRR
- National Research Register
- PPI
- patient and public involvement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SDO
- Service Delivery and Organisation
- TEAM
- Trial of an Elderly Acute care Medical and mental health unit