Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10428. The contractual start date was in July 2008. The final report began editorial review in July 2014 and was accepted for publication in September 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Cullum et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
A wound is an interruption to skin integrity caused by physical trauma or disease. Everybody experiences wounds and most wounds heal uneventfully; however, a significant minority of people have wounds that are very slow to heal or which do not heal at all. These more complex wounds, which are mainly managed by community nurses, were the focus of this programme of research.
The most common types of chronic wound are leg ulcers (mainly caused by venous and/or arterial disease), pressure ulcers (caused by unrelieved pressure as a result of immobility) and diabetic foot ulcers (caused by vascular and neurological complications of diabetes). 1 Less common chronic wounds include surgical wounds that have broken down. The term ‘chronic wound’ implies a wound of long duration; however, from a clinical perspective, it is often clear that a wound is complex and likely to be longstanding at a fairly early stage. For this reason we have used the term ‘complex wound’ (rather than ‘chronic wound’) throughout this programme of research. We define ‘complex wounds’ as wounds that have superficial, partial or full-thickness skin loss and that are healing by secondary intention.
Wounds healing by secondary intention are those that are left open to granulate and heal from the bottom up (as opposed to those whose edges are brought together and closed by sutures, glue or clips). Reliable estimates of the prevalence and incidence of complex wounds are rare. When we began this research programme it had been reported that up to 32% of hospital inpatients and 7% of community-based patients in the UK have a pressure ulcer at any point in time;2 that approximately 0.05% of people at any time were thought to have open leg ulcers;3 and that foot ulcers were thought to affect 2.0–3.0% of people with diabetes per year. 4,5 One of the aims of the work was to derive better local estimates of prevalence.
A motivating factor for our research was the relative lack of contemporary, high-quality data about how many people are affected by complex wounds, the amount of NHS resource consumed in wound management, the nature of the care that people receive and the outcomes achieved and how these outcomes are experienced. Clearly such intelligence is crucial from a research perspective to both determine the need for future research and prioritise research questions. Just as importantly, better-quality intelligence is needed by the NHS so that access to the right services can be ensured, staff training needs identified and addressed and the delivery of effective (and cost-effective) management implemented. This basic information is also needed to populate cost-effectiveness modelling that is currently being undertaken to establish the cost-effectiveness of treatments currently being used or for value of information analyses to inform future research. For example, we had already encountered a requirement for formal elicitation methods in the absence of published data on the costs, healing and complication rates of severe pressure ulcers and the frequency with which specific treatments are used in the UK. 6,7
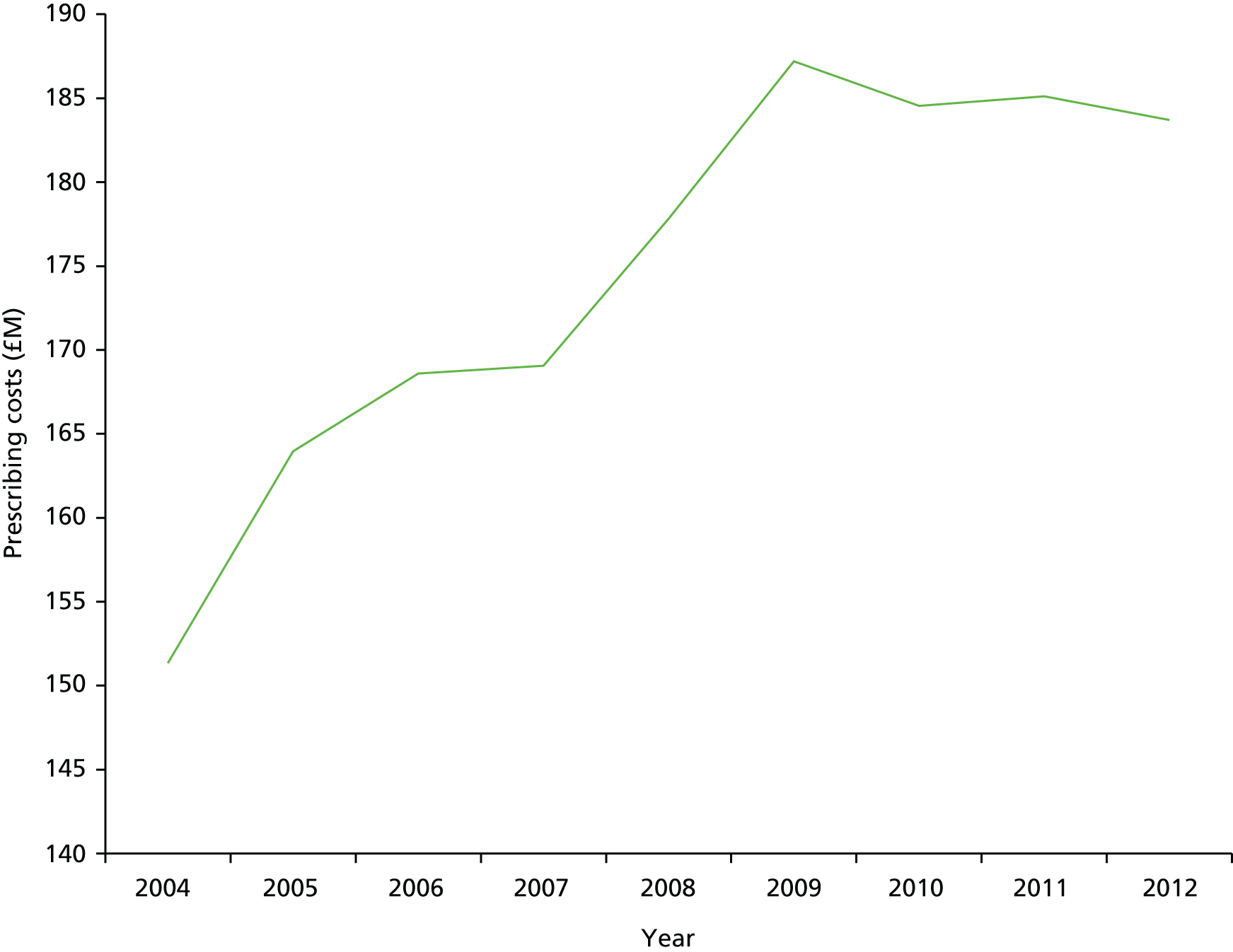
Most people with complex wounds are managed in the community by nurses along with referral to specialist services such as tissue viability, various surgical specialties (e.g. vascular, plastics), dermatology and podiatry as required. Because the care of people with complex wounds is community based and delivered by nurses, there is little or no collection and analysis of routine clinical data, hence our collective lack of basic intelligence. We do know that wound management is costly, although accurate cost data are also hard to find. The NHS (England) expenditure on wound dressing prescribing increased by 21% between 2004 and 2012 (Figure 1), with > 9 million wound dressing items prescribed in the community (England) during 2012 at a cost of £184M. 8 Importantly, however, the main cost drivers of complex wounds are not the dressings themselves but staff time and hospitalisation costs. Added to these there are clearly personal financial costs for patients and their families (because of an inability to work) as well as non-financial impacts on quality of life. Data on the impact of complex wounds on patients were beginning to accumulate in 2008 when we began this work. We had already shown that pressure ulcers had a measurable (negative) impact on quality of life when people with pressure ulcers were compared with others matched for age and comorbidities. 9 Research had also given some insights into the negative impact on patients of leg10,11 and foot12 ulceration.
FIGURE 1.
Prescription cost analysis for wound dressings, 2004–12.
The evidence base for complex wound care
It is surprising that, given the evidence for complex wounds being common and costly and having important negative impacts on quality of life, the evidence base for underpinning prevention and treatment decisions is very poor. In 2005, the National Institute for Health and Care Excellence (NICE)13 published guidelines on the management of pressure ulcers in primary and secondary care. This guideline contained 39 recommendations of which 38 were graded D (evidence extrapolated from observational studies and formal consensus) and one was graded C (directly relevant evidence from observational studies or evidence extrapolated from systematic reviews of observational studies). 14 The Scottish Intercollegiate Guidelines Network (SIGN)15 guideline of 2010 on chronic venous leg ulcer management contained 19 recommendations of which five were graded A, three were graded B, four were graded C and seven were graded D. 14 The NICE16 guideline on foot care in type 2 diabetes included a section devoted to foot ulcer management consisting of 11 recommendations of which three were graded B, one was graded C and seven were graded D. 14 The evidence to support high-quality clinical decision-making is therefore weak.
Our vision in designing a programme of research in complex wounds was to initiate a step change in research, but a calculated one underpinned by good information. Motivated by a belief that most of the existing research had been driven by the needs of the pharmaceutical industry and not patient or service priorities and that the NHS intelligence on the nature, treatment and costs of complex wounds and outcomes for people with complex wounds was poor, we set out to improve the collection and analysis of routine data on complex wounds in community nursing practice. We wanted to accurately determine the number of people with complex wounds in Leeds and the nature of their wounds, care and treatments. A further aim was to gain a patient perspective on future research priorities including the nature of the treatment outcomes that matter most to patients.
Local context
The sine qua non of research funded by the National Institute for Health Research (NIHR) is that it must be relevant for (and when possible embedded in) the NHS. 17 This programme of research grew out of a longstanding research collaboration with the Leeds Tissue Viability Service (now the Leeds Community Wound Prevention and Management Service). This collaboration has resulted in NIHR- and Medical Research Council-funded wounds research and particularly randomised controlled trials (RCTs), with Leeds always being one of the most successful recruiting centres. 18–21 An implicit aim of this research programme was therefore to capitalise on this track record and explore how it could be used to promote much wider collection of data and research involvement of staff beyond those working in the specialist service. An overarching principle was also to ensure that the research that we carry out is of value nationally as well as locally. In several ways, Leeds provides an ideal research laboratory for wounds research: it has a large and diverse population of approximately 751,000, 20% of its population live in some of the most deprived areas of the country22 and there are urban, suburban and rural communities. The population of Leeds is ethnically diverse with approximately 17% of residents from black and minority ethnic groups. 23
Aims and objectives of the programme
This programme of research commenced in 2008 and had the overarching aim of optimising the quality of care and outcomes for people with, or at risk of, complex wounds.
Our objectives were to:
-
assess how high-quality data about complex wounds can be captured effectively for use in both service planning and research while ensuring integration with current clinical data collection systems and minimal impact on staff time
-
investigate whether or not a clinical register of people with complex wounds could give valid estimates of treatment effects, thus reducing dependence on large-scale RCTs
-
identify the most important research questions and outcomes for people with complex wounds from the perspectives of patients, carers and health-care professionals
-
evaluate the potential contributions to decision-making of individual patient data (IPD) meta-analysis and mixed-treatment comparison meta-analysis
-
complete and update Cochrane systematic reviews in topic areas of high priority.
These objectives were refined slightly over the 5-year programme of research in response to research findings and local priorities.
Structure of this report
Chapters 2–4 present the findings of the component workstreams and their substudies. These workstreams are constructed around focused, coherent themes rather than particular methodologies and they vary in the volume of work in each:
-
Chapter 2 outlines the work that relates to objectives 1 and 2 and explores the extent to which it is possible for the NHS to routinely collect high-quality data about people with complex wounds and use these data for research and service delivery
-
Chapter 3 explores service user and service provider perspectives on research in wound care and examines them against the current ‘research evidence terrain’ (objective 3)
-
Chapter 4 outlines the theme of work that ran throughout the research programme, aiming to provide up-to-date summaries of research evidence in areas of uncertainty prioritised by the NHS (objectives 4 and 5).
Each of these chapters includes a brief introduction to the context of the workstream, relevant previous literature, the methods used, the main findings and the key implications.
-
Chapter 5 draws together the overall conclusions and outlines the particular contributions to knowledge that this research has made.
Chapter 2 Data capture and epidemiology (workstream 1)
Abstract
Background
The specific objectives for this workstream were to undertake a high-quality point prevalence survey and care audit of complex wounds and pilot a prospective complex wounds register suitable for both health care and research.
Methods
We undertook a systematic review of complex wounds prevalence studies and a point prevalence survey and audit of people receiving care for a complex wound in Leeds and designed and piloted a new complex wounds register.
Results
There were no previous high-quality prevalence surveys focused on people with all types of complex wound and most previous studies were weak in terms of design and reporting. Our own point prevalence survey estimated a point prevalence of any complex wound of 1.47 per 1000 people [95% confidence interval (CI) 1.38 to 1.56 per 1000 people]. The point prevalence of complex wounds in injecting drug users was 5.64 per 1000 people (95% CI 3.97 to 7.99 per 1000 people). Pressure ulcers and venous leg ulcers were the most common types of complex wound. A total of 195 people with a complex wound were recruited to a complex wounds register pilot by the Leeds Tissue Viability Service and district nurses (26% consent rate). We established the feasibility of correctly identifying and extracting routine NHS data and transferring it into the register; however, comprehensive participant recruitment (which required individual patient consent) and the tracking of individual wounds in people with multiple wounds were challenging and analyses were limited by the lack of available data. Staff feedback highlighted the need for further developments to facilitate routine data collection along with the need to consider a more efficient recruitment process and new information technology (IT) and devices. Smart pens had severe limitations as data collection tools in the context of community wound management.
Conclusions
A complex wounds register that serves both clinical care and research would be valuable. Such a register proved impossible to implement comprehensively. Challenges included an absence of existing electronic routine clinical data collection in the community nursing service, limited IT infrastructure, a requirement for individual participant consent and the difficulty of accurately tracking multiple wounds in the same patient.
Background
Surprisingly little is known about the number, nature and care of people with complex wounds in the UK, nor about outcomes. High-quality epidemiological data are vital in helping health-care providers understand the extent of the condition, the characteristics of patients and how to best plan health-care services. When we began this research programme no comprehensive survey of people with complex wounds in Leeds had been undertaken and there was no reliable information regarding the number of people affected or the nature of the care that they were receiving (including setting). Although our original objective was to establish prospective data capture about people with complex wounds and their care and outcomes, it was anticipated that a preliminary cross-sectional survey in advance of this would enable us to better plan for prospective data collection and inform priorities across other workstreams.
There are real advantages to prospective data collection. A live register of complex wounds would allow monitoring of trends in treatments and outcomes, enable rapid identification of likely patient numbers (and potential participants) for studies, aid research prioritisation and facilitate research implementation. However, we regarded it as premature to commit large resources to the establishment of a complex wounds register without first exploring if it would be feasible to establish and maintain one. A successful register based in community nursing would need careful design to ensure that data collection was feasible within typical work patterns and met both clinical and research needs. It was also important to assess the resources required to ensure the sustainability of a wounds register.
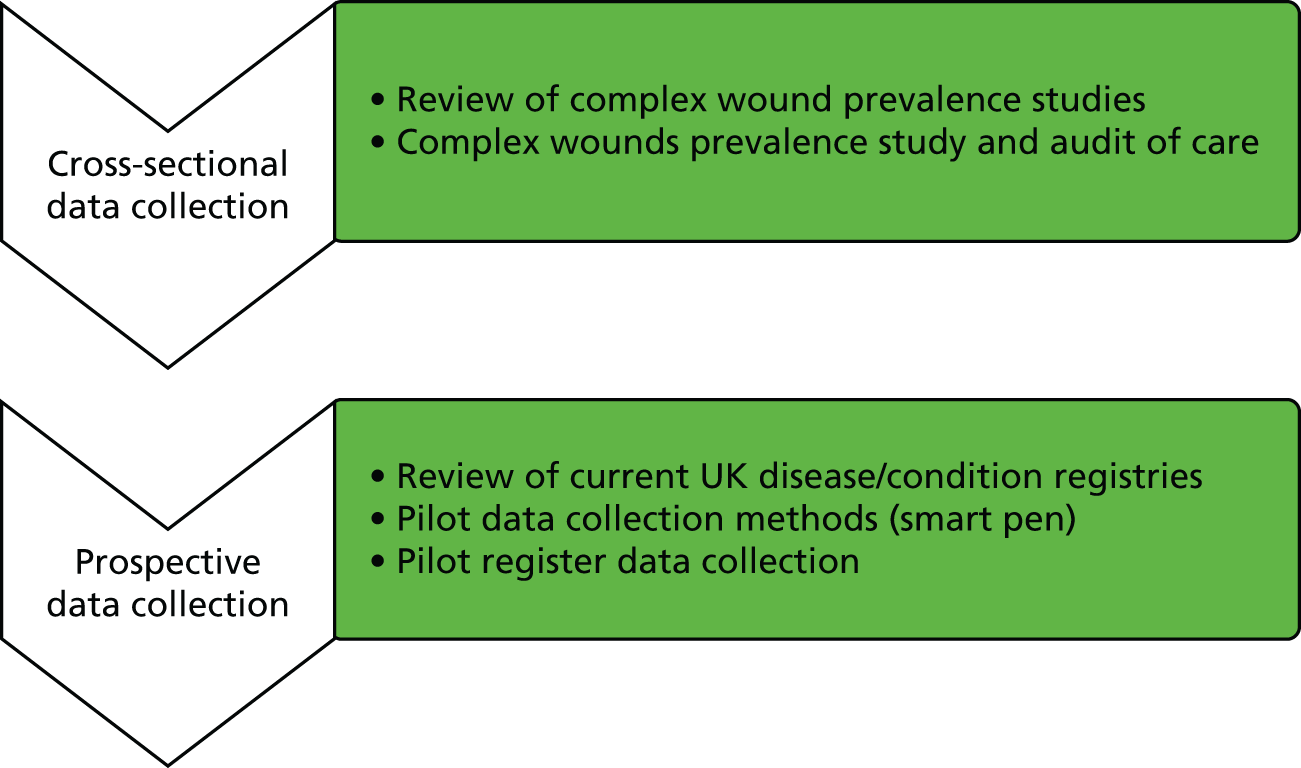
Workstream 1 consisted of two parts (Figure 2). The first part focused on cross-sectional data collection and the second part focused on prospective data collection.
FIGURE 2.
Overview of workstream 1.
Review of complex wound prevalence studies
Background
As part of developing our plans for a cross-sectional survey to estimate the prevalence of complex wounds in a large UK city, we reviewed all cross-sectional studies that had estimated the prevalence of people with complex wounds in community settings.
We were particularly interested in the measures taken in the prevalence studies to ensure the accuracy of the numerator (the number of complex wounds) and the quality of the denominator (the population identified as being at risk) as they are both crucial factors in estimating prevalence. Measures to help ensure the accuracy of the numerator include the diagnostic criterion (or wound definition) used for the inclusion of cases plus the validation process for identified cases and establishing underlying pathology (to identify case subtypes). The quality of the denominator data is also extremely important in calculating estimates of prevalence. Ideally, it should be a geographically defined population, preferably using population statistics that are contemporaneous with the study itself.
Objectives
This review was undertaken to answer the following questions:
-
What types of complex wound have been the focus of prevalence surveys?
-
What diagnostic criteria have been used in complex wound surveys?
-
What data items have been collected in prevalence surveys of complex wounds in community settings?
-
What methods have been used to collect and validate prevalence data (i.e. choice of denominator, sampling strategies, case validation and validation of underlying pathology)?
-
What was the quality of the denominator data used in prevalence calculations?
-
Has the ‘capture–recapture’ technique been used in any community prevalence studies in complex wound care to estimate prevalence in hard-to-reach groups, for example injecting drug users?
-
Have quality-of-life data been assessed in community prevalence studies in complex wound care?
-
What are the current estimates of prevalence of complex wounds in community-dwelling persons?
Methods
A systematic review of the literature was undertaken. Cross-sectional studies were included if they estimated the prevalence of people with complex wounds in community-dwelling populations or whole geographical populations.
A cross-sectional study was defined as ‘a study that examines the relationship between diseases (or other health-related characteristics) and other variables of interest as they exist in a defined population at one particular time’. 24
We included studies of people with complex wounds such as leg ulcers, pressure ulcers and diabetic and non-diabetic foot ulcers plus any other types of complex wounds not included in the above (e.g. surgical wounds healing by secondary intention). Studies conducted solely in non-community settings and single institutions such as hospitals and nursing homes were excluded.
Search strategy
Using OvidSP, an electronic search in MEDLINE (1950 to March Week 2 2009 and updated to March Week 3 2012) was undertaken as follows:
-
exp Skin Ulcer/
-
exp Leg Ulcer/
-
exp Pressure Ulcer/
-
exp Foot Ulcer/
-
exp Diabetic Foot/
-
(skin ulcer$or foot ulcer$or diabetic foot or diabetic feet or leg ulcer$or varicose ulcer$or venous ulcer$or stasis ulcer$or arterial ulcer$or neuropathic ulcer$).tw.
-
((ischaemic or ischaemic) adj (wound$or ulcer$)).tw.
-
(bed sore$or pressure sore$or pressure ulcer$or decubitus ulcer$).tw.
-
(chronic adj (wound$or ulcer$)).tw.
-
or/1–9
-
exp Epidemiology/
-
exp Prevalence/
-
(prevalence or audit or survey).tw.
-
11 or 13 or 12
-
10 and 14
Relevant data items were identified a priori by one author (JH) and extracted from the included studies by the same author. Extracted data items included:
-
country and setting
-
types of wounds and definitions
-
prevalence estimates
-
denominator information
-
sampling strategies
-
method of data collection
-
method of case validation and establishing aetiology
-
range of variables for which data were collected
-
quality-of-life data
-
use of the capture–recapture technique.
Quality assessment of included studies was conducted independently by two reviewers using a recently published 22-item checklist. 25 Agreement between reviewers was also assessed and a third reviewer arbitrated final decisions when required.
A narrative report was written in answer to each of the review questions outlined.
Results
Results of the search and description of the included studies
The electronic search produced 1834 references of which 76 were deemed potentially relevant; the full papers were retrieved for these references. Based on assessment of the full papers, 48 individual studies (in 56 published papers) were included in the review (Table 1). 3,26–80
| Author and year | Population, country, size | Wound type(s) included | Study design/methods | Method of case validation | Method to establish aetiology | Number of wound cases (people); point prevalence estimate (%) (when data provided)a |
|---|---|---|---|---|---|---|
| All wounds or all ‘chronic’ wounds | ||||||
| Gupta 200426 | Sunderpur and Kandwa village, India, n = 6917 | All wounds (acute and chronic) | Door-to-door survey | Physical examination of all wounds | Not stated | All chronic wounds 0.45% (estimated 95% CI 0.40% to 0.50%) |
| Lindholm 199927 | Uppsala county, Sweden, n = 288,433 | Chronic wounds: leg, foot, pressure and others not healed during previous 6 weeks | Survey of all caregivers in the county health-care system | Not undertaken | Not undertaken | All chronic wounds 694, leg and foot ulcers 406, pressure sores 117, other chronic wounds 171; all chronic wounds 0.24% (estimated 95% CI 0.22% to 0.26%), leg ulcers 0.14% (estimated 95% CI 0.13% to 0.16%), pressure sores 0.04% (estimated 95% CI 0.03% to 0.05%), other wounds 0.06% (estimated 95% CI 0.05% to 0.07%) |
| McDermott-Scales 200928 | Republic of Ireland, n = 133,562 | All wounds including leg ulcers, pressure ulcers and other types | Survey of nurses in community care setting | Not undertaken | Not undertaken | All wounds 290, pressure ulcers 76, leg ulcers 55; all wounds 0.20% (estimated 95% CI 0.19% to 0.24%), pressure ulcers 0.056% (estimated 95% CI 0.045% to 0.071%), leg ulcers 0.041% (estimated 95% CI 0.032% to 0.054%) |
| Pieper 199929 | Community patients receiving home care from 13 home-care agencies throughout lower Michigan, USA, n = 2847 | All wounds | Survey of sample of nurses at 13 home care agencies | Not stated | Not stated | All wounds 36.3%, leg ulcers 4.3%, diabetic foot ulcer 2.9%, pressure ulcer 8.1% |
| Rodrigues 200630 | Individuals receiving home care, Québec, Canada | Chronic wounds | Nursing personnel head at each home-care programme asked to complete questionnaire for each patient with chronic wound and a general service centre questionnaire | Not undertaken | Not undertaken | 488; 1.4% |
| Srinivasaiah 200731 | Hull and East Yorkshire, UK, n = 590,000 | Wounds | Survey of all trusts in area | Not undertaken | Not undertaken | Leg and foot ulcers 629, pressure ulcers 294, surgical wounds 699; leg and foot ulcers 0.11% (estimated 95% CI 0.10% to 0.12%), pressure ulcers 0.05% (estimated 95% CI 0.04% to 0.06%) |
| Vowden 200932–35 | Bradford, UK, n = 487,975 | All wounds | Survey of acute trusts, primary care trusts and nursing homes in the Bradford area | Not undertaken | Not undertaken | All wounds 1735, pressure ulcer 363, leg ulcers 482; all wounds 0.36% (95% CI 0.33% to 0.37%), pressure ulcers 0.07% (95% CI 0.06% to 0.08%), leg ulcers 0.10% (95% CI 0.09% to 0.11%) |
| Leg ulcers | ||||||
| Baker 1991,36 199437 | Perth, WA, Australia, n = 238,000 | Chronic leg ulcers | Health professionals and health institutions provided notification of patients and self-referral also available | Clinical assessment in patients’ home or hospital | Venous refilling assessed by photoplethysmography, arterial Doppler ankle–brachial index, absence of pedal pulses | 259; 0.11% (estimated 95% CI 0.10% to 0.12%) |
| Callam 198538 | Lothian and Forth Valley Health Board areas, Scotland, UK, n = 1,000,000 | Chronic leg ulcers | Health-care professionals surveyed to identify all patients with current chronic leg ulcers or who received treatment within 3 past months | Not undertaken | Not undertaken | 1477; 0.15% (estimated 95% CI 0.14% to 0.16%) |
| Clarke-Moloney 200639 | Health Service Executive Mid-Western region, Ireland, n = 339,591 | Leg ulcers | All public health nurses and community nurses asked to complete audit form for each patient with a leg ulcer within the defined period | Not undertaken | As reported by health-care professional | 429; 0.13% (estimated 95% CI 0.11% to 0.14%) |
| Clarke-Moloney 200840 | Health Service Executive Mid-Western region, Ireland, n = 361,028 | Leg ulcers | As for 2006 | Not undertaken | As reported by health-care professional | 395; 0.11% (estimated 95% CI 0.10% to 0.12%) |
| Cornwall 198641 | Regional health district, UK, n = 198,900 | Leg ulcers | Survey of all GPs and long-term care institutions | Clinical assessment of random sample | Doppler ankle pressure measurement, ultrasound for status of veins, photoplethysmography for venous refilling time | 357; 0.18% (estimated 95% CI 0.16% to 0.20%) |
| Forssgren 200842 | Skaraborg city, Sweden, n = 254,111 | Leg ulcers | Survey of all health-care providers within the area | Clinical assessment of a random sample of patients | ABPI, Doppler | 621; 0.24% (estimated 95% CI 0.23% to 0.26%) |
| Franks 199743 | Populations served by health care trust, UK, n = 275,000 | Leg ulcers | Survey of health professionals within one trust | Not undertaken | Not undertaken | 264; 0.10% overall (estimated 95% CI 0.09% to 0.11%) (lower for South Asians) |
| Freak 199544 | Stockport and Trafford Health Authorities, UK, n = 540,000 | Leg ulcers | Survey of key personnel who treat leg ulcers | Not undertaken | Not undertaken | 587; 0.11% (estimated 95% CI 0.10% to 0.12%) |
| Johnson 199545 | Sample of non-institutionalised elderly aged ≥ 60 years, Sydney, Australia, n = 1050 | Leg ulcers | Sample interviewed by trained interviewers using structured questionnaire | Cross-referencing reported cases with those receiving home nursing services for ulcer dressings | Not undertaken | 5; 0.48% (estimated 95% CI 0.20% to 1.11%) |
| Lees 199246 | Community dwellers aged > 45 years, Newcastle Community Health District population, UK, n = 107,400 | Leg ulcers | Survey of all district nurses in the community health district | Not undertaken | Not undertaken | 206; 0.19% (estimated 95% CI 0.17% to 0.22%) |
| Marklund 200047 | All those aged ≥ 70 years, Brålanda, rural Sweden, n = 551 | Leg ulcers, healed and non-healed | Survey (telephone interview or postal questionnaire) of people identified from the population registry | Clinical examination of positive reports and a random sample of negative reports | People with a previous history of leg ulcers were inspected for signs of venous disease, otherwise not undertaken | 46; 8.4% (estimated 95% CI 6.3% to 11.0%) |
| Moffatt 20043 | Wandsworth Community NHS Trust, UK, n = 252,000 | Leg ulcers (excluded patients with isolated foot ulceration) | Case ascertainment by health professionals to identify patients, who were then invited for interview | All identified patients were interviewed/examined | Clinical finding and results from non-invasive vascular investigations including Doppler ABPI and photoplethysmography, Rheo Dopplex II (Huntleigh Ltd., Luton, UK) to assess popliteal reflux and duplex scanning (in a number of patients to confirm findings) | 113; 0.05% (estimated 95% CI 0.04% to 0.05%) |
| O’Brien 200048 | Mid-Western Health Board Region of Ireland, n = 317,069 | Leg ulcers | Survey of health professionals within the region | Not undertaken | Considered established if patients had been investigated before the study with ABPI. Those without ABPI measurement were invited for clinical assessment to establish aetiology | 389; 0.12% (estimated 95% CI 0.11% to 0.14%) |
| Pina 200549 | Unit B2 of sub Região de saúde, Lisbon, Portugal, n = 186,000 | Leg ulcers | Case identification through health professionals | Not undertaken | Not undertaken | 263; 0.14% (estimated 95% CI 0.13% to 0.16%) |
| Walker 2002,50,51 Jull 200452 | North Auckland and Central Auckland Health Districts, New Zealand, n = 540,435 | Leg ulcers | Survey of health professionals and self-notification by patients | Not undertaken | Not undertaken | Point prevalence: 211; 0.04% (estimated 95% CI 0.03% to 0.05%) Period prevalence: 426; 0.08% (estimated 95% CI 0.07% to 0.09%) Point prevalence 39 per 100,000, period prevalence 79 per 100,000 per year |
| Leg and foot ulcers | ||||||
| Andersson 199353 | Gothenburg (n = 430,763), Sweden, n = 5140 | Leg and foot ulcers | Postal survey of random sample of Gothenburg | Sample invited for clinical examination | Ankle/arm index, VariTest apparatus | Reported 97, validated 35; total 2.15%, validated 1.02% |
| Andersson 198454 | Gothenburg, Sweden, n = 434,699 | All ulcers on the feet and legs | Cases identified from patient registers and when this was not available a sample of medical records was scrutinised to obtain an estimate | Not undertaken | Not undertaken | 1377; 0.32% (estimated 95% CI 0.30% to 0.33%) |
| Dale 198355 | Patients aged ≥ 65 years at single group practice, UK | Leg and foot ulcers | Postal survey of random sample of those born between 1900 and 1916 | Clinical examination | Not undertaken | 0.80% |
| Ebbeskog 199656 | South Stockholm Medical Area, Sweden, n = 241,804 | Leg and foot ulcers | Survey of all care providers in an area | Not undertaken | As reported by health-care professional | 294; 0.12% (estimated 95% CI 0.11% to 0.14%) |
| Harrison 2001,57 Lorimer 200358 | One Ontario region, Canada | Leg and foot ulcers | Case finding via administrative and provider databases | Clinical examination (all home-care patients plus convenience sample of long-term care patients). No validation of those identified through family doctor or podiatrists | Doppler | 0.18% |
| Lindholm 199259 | Malmö city, Sweden, n = 232,908 | Leg and foot ulcers | Survey of all health-care providers within the area | Not undertaken | As reported by health-care professional | 275; 0.12% (estimated 95% CI 0.10% to 0.13%) |
| Nelzén 1991,60 199461 | Skaraborg county, Sweden, n = 270,800 | Leg and foot ulcers | Postal survey of all district nurses and practice nurses and outpatient and hospital wards where it was likely that leg ulcer patients were treated | Clinical assessment of a random sample of patients | Doppler, ABPI | 827; 0.31% (estimated 95% CI 0.29% to 0.33%) (3.05 per 1000 population) |
| Nelzén 199662 | Skaraborg county and Malmö city, Sweden, n = 12,000 | Leg and foot ulcers | Postal questionnaire sent to 12,000 randomly selected people aged 50–89 years | Clinical examination of all those reporting an ulcer | Doppler, ABPI | 82; open leg ulcers 0.63%, open plus healed ulcers 2% |
| Öien 200063 | Blekinge county, Sweden, n = 151,610 | Leg and foot ulcers | Survey of all health-care providers within the area | Not undertaken | As reported by health-care professionals or arterial circulation assessment and skin biopsies when health-care professional states ‘unknown’ | 287; 0.19% (estimated 95% CI 0.17% to 0.21%) |
| Wong 200564 | Older people aged ≥ 65 years seen by community nursing, Hong Kong, total number of health records reviewed (those seen by community nursing) n = 671 | Lower limb ulceration | Retrospective review of nursing records of older people receiving community nursing services | Not undertaken | Not undertaken | 86; 12.8% (estimated 95% CI 10.5% to 15.6%) |
| Diabetic foot ulcers | ||||||
| Abbott 200565 | Diabetes, UK, n = 15,111 | Diabetic foot ulcers | Podiatrists examined a randomly selected cohort of diabetic patients attending primary and secondary health-care clinics in six districts of northwest England over 4 years | Not stated | Not applicable | 201; 1.3% (estimated 95% CI 1.2% to 1.5%) |
| Al-Mahroos 200766 | Diabetes, Bahrain, n = 1477 | Diabetic foot ulcers | Cross-sectional study of diabetic patients on routine visits to the six diabetes clinics in Bahrain | Not stated | Not applicable | 87; 5.9% (estimated 95% CI 4.8% to 7.2%) |
| De Sonnaville 199769 | Diabetes managed in primary care, the Netherlands, n = 609 | Foot ulcers | Podiatrist examined all patients managed in a shared care project in primary care | Not stated | Not applicable | 11; 1.8% (estimated 95% CI 1.0% to 3.2%) |
| Kumar 199467 | Sample of type 2 diabetic patients identified from general practices in three cities in northern England, UK, n = 811 | Diabetic foot ulcers | Potential participants identified from records of 37 general practices and invited to take part | Not stated | Not applicable | 43; 5.3% (estimated 95% CI 4.0% to 7.1%) |
| Reid 200668 | Individuals with diabetes living in Norway House, a remote Aboriginal community in northern Manitoba, Canada, n = 169 | Diabetic foot ulcers | Individuals were contacted at clinics etc. and through the media | Not stated | Not applicable | Past or present 25, present 8; past or present 14.8% (estimated 95% CI 10.2% to 20.9%), present 4.7% (estimated 95% CI 2.4% to 9.1%) |
| Tseng 200370 | Diabetes, Taiwan, n = 12,531 | Foot ulcers | Diabetic patients selected for telephone interview | Not undertaken | Not applicable | 369; 2.9% (estimated 95% CI 2.7% to 3.3%) |
| Non-diabetic foot ulcers | ||||||
| Bristow 200871 | Non-diabetic, single health service district, UK, n = 610,805 | Non-diabetic foot ulcers | Sample of health-care professionals asked to prospectively complete a questionnaire when they encountered an ulcer | Not undertaken | As reported by health-care professional | 132; 0.02% (estimated 95% CI 0.02% to 0.03%) |
| Firth 200872 | Patients with rheumatoid arthritis, population served by the hospital, UK, n = 477,800 | Foot ulcers | All patients under the care of consultant rheumatologists at a teaching hospital were surveyed | Clinical examination, case note review and contact with health professionals | Not applicable | Based on self-report 35, after validation 30; based on self-report 4.0% (estimated 95% CI 2.9% to 5.5%), after validation 3.4% (estimated 95% CI 2.4% to 4.8%) (based on 883 respondents) |
| Pressure ulcers | ||||||
| Barbenel 197773 | Greater Glasgow Health Board, Scotland, UK, n = 1,105,000 | Pressure ulcers | Survey of hospital and district nurses | 5% random sample of hospital-based patients but no validation of community-based patients | Not undertaken | 946; 0.09% (estimated from whole population provided in paper) (estimated 95% CI 0.08% to 0.09%) |
| Barbenel 198074 | Borders Health Board, Scotland, UK, n = 99,000 | Pressure ulcers | Survey of hospital and district nurses | Four hospital wards validated but no validation of community-based patients | Not undertaken | 0.09% |
| Bergquist 199975 | Patients receiving home health care, USA, n = 1820 | Pressure ulcers | Retrospective review of admission records | Not undertaken | Not undertaken | 109 (when admitted to home health-care agency); 6.0% admission prevalence (estimated 95% CI 5.0% to 7.2%) |
| Hallett 199676 | Portsmouth Healthcare NHS Trust, UK | Pressure ulcers | Survey of nurses in community services | Not undertaken | Not undertaken | 206; 4.4% (based on community services caseload?) |
| Inman 199877 | District nurse caseload on survey day, UK, n = 1129 (day caseload of district nurses) | Pressure ulcers | Audit of all district nurses in the trust | Not stated | Not stated | 55; 4.9% (estimated 95% CI 3.8% to 6.3%) |
| Meehan 199978 | Patients on home health agencies’ caseload, USA, n = 21,529 | Pressure ulcers | Survey of patients on home health agencies’ caseload by specially trained nurses | Not stated | Not stated | 1455; 6.8% (estimated 95% CI 6.4% to 7.1%) |
| Raghavan 200379 | Spinal injury patients living in the community served by a regional spinal injuries unit and under regular follow-up, UK, questionnaire respondents n = 427 (valid questionnaire respondents) | Pressure ulcers | Postal survey to patients | Not undertaken | Not stated | 99; 23.2% (estimated 95% CI 19.4% to 27.4%) |
| Torrance 199980 | Combined number of hospital beds in the trust and number of patients on district nurses active caseload, UK, approximately n = 1500 | Pressure ulcers | Survey of health-care facilities in an integrated acute and community care trust | Not stated | Not stated | 3% community (1996), 8.5% hospital (1996) |
The studies were predominantly conducted in Europe and industrialised countries (23 in the UK and Ireland and 10 in Sweden) and were published between 1977 and 2009. Most were studies of the general population and 13 were conducted in subgroups of the population, most frequently the elderly and specific groups with conditions such as diabetes, rheumatoid arthritis or spinal injury.
Quality assessment
All included studies had a number of flaws (either minor or major) and instances of poor reporting. Overall, the mean number of flaws and poorly reported items per study was 13.9, ranging from 7 to 20. Instances of poor reporting accounted for the majority of these (mean 9.4, range 5–13). Studies had an average of 2.9 minor flaws (range 1–5) and 1.5 major flaws (range 0–3). The most frequently poorly reported and/or flawed items included:
-
the role of funding organisations
-
conflict-of-interest declarations
-
sampling issues
-
assessment of sampling bias
-
whether or not sampling bias was addressed in the analysis
-
exclusion rates from analysis
-
reliability of the estimates
-
reporting of type of prevalence
-
precision of estimates and prevalence in the total sample (crude or adjusted).
Items tending to have fewer instances of poor reporting and flaws included information about the funding of the study, aims of the studies, study design, sampling method, response rate, the source for measuring prevalence and issues around definitions of outcomes.
Agreement between reviewers for each study was variable. The average agreement for studies (based on the 22 items in the checklist) was 14.9 (67.7%) but agreement ranged between 9 and 22. Items that tended to have higher levels of agreement between reviewers related to funding, conflicts of interest, ethics, sampling frame, assessment of sampling bias and whether or not it was addressed in analysis, reliability of estimates and precision of estimates. Items that tended to have lower levels of agreement between reviewers included the aims of the studies, study design, sampling methods, source to measure prevalence, aspects of definitions of prevalence, measurement of prevalence and reporting of type of prevalence.
Types of complex wounds
Few studies (n = 7)26–35 attempted to measure the prevalence of all wounds or all complex wounds; most focused on people with leg ulcers (n = 15)3,36–52 or leg and foot ulcers (n = 10). 53–64 However, it was not always possible to determine from reports of leg ulcer studies if people with ulcers confined to the foot were excluded. A total of 16 studies investigated either (people with) foot ulcers (diabetic foot ulcers n = 6,65–70 non-diabetic foot ulcers n = 271,72) or pressure ulcers (n = 8). 73–80
Diagnostic criteria
Twenty-one out of 48 studies (46%) reported a diagnostic criterion or definition for the type of wound eligible for inclusion (Table 2). Definitions varied but tended to include a general description of the wound, its location, its duration and the underlying pathology. General descriptions that were used included ulcer, wound, chronic ulcer or wound, open wound or sore, defect in the dermis, break in the skin, full-thickness skin break or defect, localised area of necrosis, destruction of skin and deeper soft tissues, and reference to ulcer staging (e.g. Wagner stages for foot ulcers81).
| Wound type | Definitions (when provided) |
|---|---|
| All complex wounds/all wounds | Chronic wounds: leg, foot and pressure ulcers and other wounds that had not healed during the previous 6 weeks.27 Leg ulcer: ulcers between the knee and malleoli.27 Foot ulcer: ulcers below the malleoli27 |
| Leg ulcer | Chronic ulceration of the leg defined as a defect in the dermis at a site below the knee, persistent for ≥ 1 month41 |
| Chronic leg ulcer: an ulcer below the knee that had been open for ≥ 6 weeks42 | |
| Chronic leg ulcer: an ulcer distal to the knee that does not heal within 6 weeks47 | |
| Leg ulcer: an open wound on the leg that had not healed within the last 4 weeks3 | |
| Leg ulcer: an open sore anywhere below the knee48 | |
| Leg ulcer: an open wound on the leg49 | |
| Leg and foot ulcer | Leg ulcers are situated on the lower legs or feet and are usually caused by altered blood flow53 |
| Open sore below the knee anywhere on the leg or foot that takes > 6 weeks to heal55 | |
| An open sore on the skin of the lower leg, ankle region or the dorsum of the foot, excluding only those clearly caused by pressure necrosis of the heel or bony high points, to neoplasia or severe arterial disease with digital ischaemia44 | |
| Leg ulcer: chronic ulcers below the knee. Foot ulcer: ulcers below the ankle that do not involve higher structures57 | |
| Chronic ulceration: an open wound below the knee (including foot ulcers) that did not heal or was supposed to heal within a 6-week period after onset of ulceration60 | |
| Leg ulcer: any wound below the knee (including the foot) that did not heal within a 6-week period after onset of ulceration, regardless of the cause of the ulcer61 | |
| Leg ulcer: any break in the skin on the lower leg (below the knee) or on the foot, that had been present for > 6 weeks. Healed leg ulcer: a wound that had been resurfaced with epithelium and looked pink, dry and smooth51 | |
| Lower limb ulceration: an open wound below the knee including both foot ulcers and leg ulcers. Pressure ulcers were excluded64 | |
| Diabetic foot ulcer | Foot ulcer: a full-thickness skin break to at least Wagner stage 1, occurring distal to the malleoli65 |
| Foot ulcer: according to Wagner classification and associated pathogens66 | |
| Foot ulcer: according to Wagner scale, stages 1 and 269 | |
| Non-diabetic foot ulcer | Ulcer: an open wound on the foot below the ankle71 |
| Foot ulcer: a full-thickness skin defect occurring in isolation on or below the midline of the malleoli and requiring > 14 days to heal72 | |
| Pressure ulcer | Pressure ulcer: any lesion caused by unrelieved pressure resulting in damage to underlying tissue, usually found over bony prominences78 |
The anatomical location of leg ulcers was most commonly referred to as ‘below the knee’ or ‘lower leg’. Foot ulcers were referred to as ‘below ankle’ or ‘below malleoli’ and pressure ulcers as in an ‘area of bony prominence’. The presence of the wound for ‘≥ 6 weeks’ was the most commonly used diagnostic criterion relating to duration, although presence for ‘≥ 4 weeks’ and ‘> 14 days’ were also used. The underlying pathology was rarely defined but included altered blood flow, immobile individuals and the result of pressure, shear or friction.
Data items
The level of detail provided about the data items collected in the included studies varied but in the main was brief (this may be explained in part by journal specifications on word limits). For example, it was widely reported that ‘demographic’ data were collected but no further details were provided in many cases.
The data items collected are shown in Table 3. The ‘core’ or most frequently collected data items were patient demographics and medical history (including comorbidities), current wound assessment (including vascular assessment) and details of prevention strategies/equipment. There was more variation in the collection of other data items depending on the wound type being studied. For example, studies examining pressure ulcers were more likely to collect information on continence, whereas studies assessing ulcers in people with diabetes and rheumatoid arthritis were more likely to report laboratory tests such as creatinine, glucose and rheumatoid arthritis serology.
| Data category | Category content (when provided) |
|---|---|
| Demographics | Age, sex, ethnicity, geographical location, height, weight, body mass index |
| Medical history and comorbidities | Especially vascular history and surgery, smoking status, neurological disorders (stroke, multiple sclerosis, spinal cord injury), rheumatoid arthritis, kidney failure, diabetes, hepatic cirrhosis, nutrition disorders (cachexia, obesity) and cancer |
| Vascular assessment | Pulses, Doppler, ABPI, presence of oedema, varicose veins and skin condition |
| Neurological assessment | Vibration and temperature perception, reflexes |
| Previous ulcers | If yes, date of previous ulcer, number of episodes, site, type, treatment method, time to heal |
| Current ulcer(s) | Assessment: recurrent or primary ulcer, number, location (drawing or list of anatomical locations), duration, size/measurement, aetiology, wound bed characteristics, exudate, odour, pain, surrounding skin, cellulitis, haemorrhage, staging/grading, self-treatment, suspicion or presence of infection, wound swabbing, MRSA status, systemic infection, lymphangitis, osteomyelitis |
| Measurement by photographs or drawings | |
| Staging/grading: Wagner scale81 (foot ulcers), Torrance82 and NPUAP83 (pressure ulcer), Shea classification84 | |
| Risk score: Waterlow85 and Norton et al.86 (pressure ulcer), Braden scale,87 Maelor score88 | |
| Treatment: cleansing agent used, dressing used, desired dressings but unavailable, use of compression, use of skincare preparation | |
| Setting and frequency of treatment, time taken to travel and treat | |
| Use of miscellaneous items/services, additional procedures | |
| Inpatient days attributable to the wound/whether wound was main reason for admission or delayed discharge (acute care settings) | |
| Referrals: specialist nurse, general practitioner, vascular surgeon, dermatologist, diabetologist, other | |
| Use of prevention strategies/equipment | Compression stockings, seat cushion, mattress, repositioning, inspection |
| Laboratory tests | Urea, creatinine, glucose, rheumatoid arthritis serology |
| Mobility/disability | Paralysis, use of artificial limbs, callipers and wheelchairs |
| Continence | Faecal and urinary incontinence |
| Workforce | Source of referral, care provider details (profession and grade), level of education and training (Doppler, compression bandages, wound care, prevention of recurrence, wound care course) |
| Quality of life | Nottingham Health Profile, SF-36 |
| Social situation/support | Employment status, living alone, marital status |
| Footwear | Shoe fitting, orthotic use |
| Patient concordance | Reasons for non-concordance |
Methods used to collect and validate prevalence data
Sampling strategies
Most studies were surveys of health-care professionals who were asked to complete a questionnaire for each patient they encountered with an eligible wound. Of those employing a sampling strategy, a random sample was identified from the population of interest, whether that was patients with a particular condition (e.g. diabetes65), health-care professionals71 or the wider public/population (e.g. from census data45).
Method of data collection
The method of data collection was not reported in 21 of the studies. When information was provided, the most widely used method was paper-based questionnaires (including postal questionnaires; n = 16). Other methods of data collection included interviews (including telephone interviews with patients), electronic or web-based online questionnaires and questionnaires for which the method was unspecified.
Case validation
Fifteen studies3,26,36,37,41,42,45,53,55,57,58,60–62,72–74 undertook some method of case validation for positive reports of ulcers. The majority of these undertook clinical examination of either all people identified as having a wound or a random sample or convenience sample (e.g. those in hospital but not those in the community). One study cross-referenced self-reported wounds with community nursing services. 45 One study attempted to identify false negatives, conducting a clinical examination of a random sample of patients not reported as having a wound. 47
Validation of underlying pathology in cases
The majority of studies did not describe or report validation of the underlying wound pathology. Six studies39,40,56,59,63,71 accepted the differential diagnosis reported by the care provider.
Eight studies3,37,41,42,48,54,60,62 reported that the ankle–brachial pressure index (ABPI) had been measured to determine whether or not there was any ischaemia. Venous competence was assessed in fewer studies: vein status was assessed using ultrasound in one study;41 venous refilling was assessed by photoplethysmography in two studies;37,41 and popliteal reflux was assessed in a single study using Rheo Dopplex II and duplex scanning. 3
Denominator data
Over half of the studies (n = 27) used a defined geographical population (or representative sample of that population) for the denominator. 3,26–28,31,32,36–44,48–51,53,54,56–63,73,74 The choice of denominator for the remainder of the studies included the total current caseload29,30,64,69,75–78,82 of health-care professionals or the provider organisation, the sample surveyed (not clear if these were representative samples of geographical populations)45–47,55,62,65–68,70 and respondents to the survey. 72,79 Of the studies that used a defined geographical population, just over half (n = 15) reported that the source of the information was census data or some type of official national statistics. The source of information was not stated in the other 12 studies that used a geographical population. Fifteen of the 27 studies that used a geographical population also gave an indication of how contemporary the denominator was in relation to the date that the study was undertaken (they ranged from the same year to up to 6 years preceding the study). Twelve studies did not provide any information on the contemporaneous nature of the denominator.
Capture–recapture technique
The technique of capture–recapture was employed in one of the included studies, although not for a ‘hard-to-reach’ group. 50 Walker et al. 50 conducted a population-based, cross-sectional study in New Zealand in 1998 in which people with leg ulcers were identified by both health-care professionals and self-notification. The team used both traditional and capture–recapture methods to estimate the cumulative incidence and prevalence of leg ulcers. Their results indicated that actual leg ulcer prevalence was six to eight times higher than that observed in the traditional method, although in this study the prevalence estimate derived from traditional reporting methods was much lower than expected (even taking account of differences in methods, etc.).
Quality-of-life data
Two studies52,89 compared quality of life data from individuals with leg ulcers with data from matched control subjects.
Jull et al. 52 (a publication describing the Walker et al. 51 2002 study population) compared Short Form questionnaire-36 items (SF-36) scores of people with leg ulcers with those of a control group randomly selected from the electoral roll using a stratified sampling process and population norms. Cases reported significantly lower mean SF-36 scores than the control group (and the population norms), with an impact similar to that of conditions such as diabetes and arthritis (as reported in the Medical Outcomes Study90).
Using cases from their 2004 study of the prevalence of leg ulceration in a south-west London population,3 Moffatt et al. 89 matched these cases with control subjects from six general practice age/sex registers within the same catchment area. The authors reported that cases had a significantly worse quality of life status than control subjects for all domains on the Nottingham Health Profile.
Current estimates of the prevalence of complex wounds
Estimates of the prevalence of all chronic/complex wounds for individual studies are provided in Table 4 (in which cases are people not wounds). Prevalence estimates varied by wound type. Table 5 reports the range of prevalence estimates for people affected by each wound type.
| Study | Prevalence of chronic (complex) wounds (%) |
|---|---|
| Rodrigues 200630 | 1.40 |
| Gupta 200426 | 0.45 |
| Lindholm 199927 | 0.24 |
| Wound category | Prevalence estimate (%) | |
|---|---|---|
| Minimum | Maximum | |
| All complex wounds | 0.24 | 1.4 |
| Leg ulcers | 0.039 | 0.48 |
| Leg and foot ulcers | 0.1 | 12.8 |
| Diabetic foot ulcers | 1.3 | 5.9 |
| Non-diabetic foot ulcers | 0.02 | 3.39 |
| Pressure ulcers | 0.056 | 23 |
Tables 6–9 summarise the point prevalence estimates from studies in the following wound categories: leg ulcers, leg and foot ulcers, diabetic foot ulcers and pressure ulcers. Data were taken directly from published studies with no pooling or weighting. Cases are people rather than wounds.
| Study | Prevalence estimate (%) |
|---|---|
| Marklund 200047 | 8.40a |
| Johnson 199545 | 0.48 |
| Forssgren 200842 | 0.24 |
| Lees 199246 | 0.19 |
| Cornwall 198641 | 0.18 |
| Callam 198538 | 0.15 |
| Pina 200549 | 0.14 |
| Clarke-Moloney 200639 | 0.13 |
| O’Brien 200048 | 0.12 |
| Baker 1991,36 199437 | 0.11 |
| Clarke-Moloney 200840 | 0.11 |
| Franks 199743 | 0.10 |
| Freak 199544 | 0.11 |
| Moffatt 20043 | 0.05 |
| Walker 2002,50,51 Jull 200452 | 0.04 |
| Study | Prevalence estimate (%) |
|---|---|
| Wong 200564 | 12.8 |
| Nelzén 199662 | 2.00a |
| Andersson 199353 | 1.02 |
| Dale 198355 | 0.80 |
| Nelzén 199662 | 0.63 |
| Andersson 198454 | 0.32 |
| Nelzén 1991,60 199461 | 0.31 |
| Öien 200063 | 0.19 |
| Harrison 2001,57 Lorimer 200358 | 0.18 |
| Ebbeskog 199656 | 0.12 |
| Lindholm 199259 | 0.12 |
Discussion
The existing literature on complex wound prevalence had three main shortcomings. First, there were very few estimates of the prevalence of complex wounds per se, with studies tending to focus on the prevalence of specific types of wounds. More than half of the studies identified (25/48) surveyed people for leg or leg and foot ulcers in isolation; three studies surveyed people with any ‘chronic’ wound (definitions varied); and a further four surveyed people with any kind of wound, including acute wounds. There was no estimate of the impact or demand on services (such as community nursing) of caring for people with any kind of complex wound and surveys focusing on particular wound subgroups are usually reliant on accurate differential diagnosis (few studies had evidence of this). A survey that includes people with any chronic or complex wound is more forgiving in terms of diagnostic accuracy (people may be misclassified as to type of wound but are less likely to be misclassified as to whether or not they have a complex wound).
Second, no study had estimated the prevalence of complex wounds in hard-to-reach groups, for example injecting drug users (although one study28 had included them there was no specific denominator for this subgroup). By contrast, our research programme was, in part, borne out of a strong local perception that chronic skin ulceration in injecting drug users was a growing and difficult issue. This review of previous studies underlined the fact that no data on the prevalence or incidence of skin ulcers in injecting drug users existed.
Third, the pre-existing literature was characterised by huge variability in study design and wound definitions plus common deficiencies in the design and reporting of studies. These issues were likely to affect the validity of the prevalence estimates and make interpretation of the findings extremely difficult.
Complex wounds prevalence survey
Background
High-quality data on complex wounds are important in helping health-care providers plan sufficient and adequate services for the people affected by this condition. This prevalence survey looking at people with complex wounds in Leeds was motivated by a need to (1) estimate the numbers of people with a complex wound in Leeds and the characteristics of the people affected, their wounds and their care and (2) inform plans for a prospective register of people with complex wounds. It was essential that the survey and the register overcame common deficiencies of design covered in the previous section of this report, including focusing on poorly specified subgroups of people with complex wounds74,91 and wide variations and/or lack of clarity regarding wound definitions. 31,32,72 Our aim was to ensure that this survey overcame some of the weaknesses in the existing literature.
Objectives
The overall aim of this research was to investigate the number, nature and care of complex wounds in the geographical population of the city of Leeds, UK.
The specific objectives were to:
-
estimate the overall point prevalence of complex wounds in Leeds
-
estimate the point prevalence of different wound types, namely diabetic foot ulcer, non-diabetic foot ulcer, venous leg ulcer, arterial leg ulcer, arterial/venous leg ulcer, pressure ulcer, dehisced surgical wound, pilonidal sinus, pilonidal abscess, traumatic wound, other surgical wound, fungating carcinoma, burn and any other types of complex wound identified
-
estimate the point prevalence of complex wounds in intravenous drug users
-
determine the characteristics of people with complex wounds
-
describe who manages the care of people with complex wounds
-
describe current wound management received by people for different types of complex wound.
Methods
Study design
A multiservice, cross-sectional survey was undertaken to identify the number, nature and care of complex wounds across the city. The survey was conducted in the following areas: community and primary care services, mental health services, acute services and independent care providers such as nursing homes and hospices.
Study population
The study population consisted of residents of the city of Leeds (population 751,48722).
Inclusion criterion
People were included in the survey if they had at least one complex wound identified during the data collection period (28 February 2011 to 13 March 2011). A complex wound was defined as one that involves superficial, partial or full-thickness skin loss and is healing by secondary intention.
Identification of health-care providers
All those managing the care of complex wounds within the city of Leeds were identified. The services included:
-
community and primary care services (Leeds Community Healthcare NHS Trust and Leeds Primary Care Trust), which covered the tissue viability service, district nursing, practice nurses, children’s nursing services, podiatry, intermediate care team, intermediate care ward, community rehabilitation ward, no fixed abode team and nursing services provided in prisons
-
mental health and learning disability services (Leeds Partnerships NHS Foundation Trust); this included a range of community-based and inpatient services)
-
acute services (Leeds Teaching Hospitals NHS Trust), which included inpatient and outpatient wards and departments
-
independent organisations including nursing homes, hospices and private hospitals.
A data co-ordinator post was established and appointed to at the beginning of the research programme, hosted within the Leeds Community Healthcare NHS Trust. Preparations with health-care providers in Leeds began up to 12 months in advance to optimise participation by establishing an esprit de corps and collaborative relationships. The data co-ordinator identified and engaged with health-care providers and professionals in the months leading up to the survey to introduce, explain and encourage their participation. Educational sessions were provided for health-care professionals prior to the survey to familiarise them with the data capture form and survey procedures. The sessions linked into existing events such as professional or team meetings and scheduled NHS training events within the city. The survey was also publicised and promoted in articles included in city-wide NHS newsletters and bulletins. In the days immediately prior to data collection all care providers were contacted by either e-mail or telephone to remind them about the survey. Finally, project team members were available during the 2-week survey to answer queries and provide further information on how to complete the data capture form.
Data capture form
After deliberation and consultation it became obvious that, because of the working arrangements of community nurses, we required a paper-based survey (see Appendix 1) accompanied by guidance notes regarding completion. It was also apparent that individual patient consent would be a huge barrier to the collection of a comprehensive data set. Having taken advice from the National Information Governance Board, we designed a data capture form that collected anonymised information about patients and their wounds from their care providers. The questionnaire was designed to be completed by the health professional away from the bedside and included the kinds of data that are routinely collected for the purposes of clinical care.
Patient data items collected by the survey included demographics, relevant comorbidities, the number and type of current wounds and current wound treatments; we also recorded wound duration at the time of the survey. Information about individual wound dressings was summarised into different categories. We also collected data about the care provided including the profession of the person completing the form (e.g. nurse, podiatrist), his or her grade and the type of service provider for whom he or she worked.
Definitions
A complex wound was defined as one with superficial, partial or full-thickness skin loss and that was healing by secondary intention.
Types of complex wound were categorised according to the following definitions (these were included in the guidance notes for completion):
-
Foot ulcer in person with diabetes – any open wound present on the foot below the level of the ankle in a person with diabetes.
-
Foot ulcer in person without diabetes – any open wound present on the foot below the level of the ankle in a person without diabetes. 71
-
Leg ulcer – an area of discontinuity of epidermis and dermis on the lower leg, persisting for ≥ 4 weeks. 54 May be caused by venous disease or arterial disease alone or a combination of both.
-
Venous leg ulcer – occurs when deep and/or superficial leg veins become incompetent due to damaged valves or blockages, such as deep-vein thrombosis, leading to increased pressure in the leg veins. 54
-
Arterial leg ulcer – a failure of, or reduction in, the nutritional blood supply to an area of skin, most commonly because of atherosclerosis. 54
-
Arterial/venous leg ulcer – an ulcer caused by a combination of venous and arterial disease.
-
Pressure ulcer – localised injury to the skin and/or underlying tissue usually over a bony prominence as a result of pressure or pressure in combination with shear. A number of contributing or confounding factors are also associated with pressure ulcers; the significance of these factors is yet to be elucidated. 83
-
Dehisced surgical wound – a surgically closed wound (e.g. sutured) that has broken open because of, for example, infection or poor healing.
-
Pilonidal sinus – a sinus tract that commonly contains hairs. It occurs under the skin between the buttocks (the natal cleft), a short distance from the anus.
-
Abscess – collection of pus formed just under the skin. Symptoms include swelling, redness, pain and warmth over the affected area.
-
Traumatic wound – a traumatic wound is a contused wound characterised by torn and irregular edges, the presence of devitalised tissue fragments and the presence of foreign matter (gravel, etc.).
-
Other surgical wound – any other wound following surgical intervention that is healing by secondary intention.
-
Fungating carcinoma – a malodorous, exuding, necrotic skin lesion, the term ‘fungating’ referring to a malignant process. Lesions that have a predominantly proliferative growth pattern may develop into a nodular ‘fungus’ or ‘cauliflower’-shaped lesion.
-
Burn – damage to the skin, and sometimes to underlying tissues, caused by contact of the skin with a hot substance.
-
Other – a complex wound that does not fit into any of the above categories.
Data collection
A pilot study of the data capture form was undertaken with care providers from a range of care settings and professions and amendments were made in the light of feedback received (a substantial amendment was submitted to and approved by Northern and Yorkshire Research Ethics Committee).
Data collection for the survey took place over 2 weeks from 28 February to 13 March 2011. Care providers were asked to complete a data capture form for every patient with a complex wound on their caseload using information from routinely collected data sources, for example case notes or electronic data sources. They were requested to complete the form away from the ‘patient bedside’ and no wounds were inspected for the purposes of the survey. Multiple forms for the same person were avoided by care providers completing only one form per patient during the survey period; if care was shared between services then one care provider was nominated to complete the form.
Reports of previous studies of this kind have discussed low response rates for completion of survey data capture forms in some care settings, for example nursing homes. 27,59 To minimise this we adopted a number of strategies. The data co-ordinator (based in the NHS) identified and engaged with health-care professionals in the months leading up to the survey to explain the survey and encourage their participation. Educational sessions were provided for health-care professionals prior to the survey to familiarise them with the data capture form. Finally, project team members were available during the 2-week survey to answer queries and provide further information on how to complete the data capture form. The project team did not access case notes or have any form of contact with or undertake observation of patients.
Data handling and storage
At the end of the data collection period completed data capture forms were returned to the University of York. Completed forms were scanned by York Trials Unit and the information was stored in a database. Once scanned, the paper forms were locked in a filing cabinet within a locked room with only the immediate research team having access. The database was stored on a password-protected computer, again with access for the immediate research team only.
Analysis
As no hypotheses were formulated, the data were analysed descriptively. The overall point prevalence of complex wounds was estimated using the Leeds population from the 2011 census as the denominator,22 along with prevalence estimates for each type of complex wound. The point prevalence of complex wounds in intravenous drug users was estimated using the total population of intravenous drug users in Leeds as the denominator (n = 5500) (Leeds Drug Service Team, personal communication, 2010). All point prevalence estimates were produced using a binomial proportion and are presented alongside 95% Wilson score CIs.
The following questions to ask of the data were generated through consultation with patients, clinicians and NHS managers in Leeds:
-
What are the characteristics of people with complex wounds?
-
What are the characteristics of those managing the treatment of individuals with complex wounds?
-
What is the nature of the wound management currently provided for people with different types of complex wound?
Ethics
The study was reviewed and approved by the Department of Health Sciences Research Governance Committee, the University of York and the Northern and Yorkshire Research Ethics Committee (reference 10/H0903/41). Research and development departments of the following organisations approved the research (with permissions co-ordinated by the West Yorkshire Comprehensive Local Research Network):
-
NHS Leeds (reference P/0063)
-
Leeds Teaching Hospitals NHS Trust [reference NU10/9532 (43641/WY)]
-
Leeds Partnerships Foundation Trust
-
45 nursing homes in Leeds
-
two hospices: St Gemma’s and Wheatfield’s
-
non-NHS hospitals: the Nuffield Hospital and two Spire hospitals
-
National Offender Management Service, Yorkshire region
-
Her Majesty’s Prison (HMP) Leeds and HMP Wealstun.
Copies of all approvals (and amendments) are available on request.
Results
Leeds population estimates
Table 10 presents population estimates for Leeds based on 2011 census data,22 overall, by sex and by 10-year age category. Over half of the population was aged < 40 years.
| Category | Population, n |
|---|---|
| Total population of Leeds | 751,485 |
| Male | 367,935 |
| Female | 383,550 |
| Age (years) | |
| 0–9 | 88,425 |
| 10–19 | 93,002 |
| 20–29 | 131,734 |
| 30–39 | 103,477 |
| 40–49 | 102,727 |
| 50–59 | 82,344 |
| 60–69 | 70,398 |
| 70–79 | 48,464 |
| 80–89 | 25,932 |
| ≥ 90 | 4982 |
Sample
In total, 1103 forms were returned, each reporting information on an individual with at least one complex wound. Surveys were returned (including records of nil returns) from all services anticipated, reassuring us that adequate data capture had been achieved.
Point prevalence
Information was returned for 1103 individuals and the total population of Leeds using the 2011 census estimate22 was 751,485. Hence, the point prevalence of complex wounds in Leeds was estimated as 1.47 per 1000 population (95% CI 1.38 to 1.56 per 1000 population).
Point prevalence by patient characteristics
The prevalence of complex wounds in females was 1.63 per 1000 (95% CI 1.51 to 1.77 per 1000), whereas that in males was 1.28 per 1000 (95% CI 1.17 to 1.40 per 1000). The prevalence of complex wounds increased with age, being most prevalent in the ≥ 90 years category, with an estimated 22.88 individuals per 1000 having a complex wound (95% CI 19.08 to 27.42 per 1000). The largest number of individuals with a complex wound who were captured by the survey fell into the 80–89 years age group (312/1103, 28.3%).
Point prevalence by wound type
Point prevalence estimates by wound type are presented in Table 11 based on the number of individuals with at least one wound of the relevant type. Pressure ulcers were the most frequent wound type, with a point prevalence of 0.31 per 1000 (95% CI 0.28 to 0.36 per 1000). Venous leg ulcers had a point prevalence of 0.29 per 1000 (95% CI 0.25 to 0.33 per 1000). Fungating carcinomas and burns were the least common wound types captured within the survey.
| Wound type | Frequency | Point prevalence per 1000 | 95% CI per 1000 |
|---|---|---|---|
| Abscess | 33 | 0.04 | 0.03 to 0.06 |
| Burn | 15 | 0.02 | 0.01 to 0.03 |
| Fungating carcinoma | 14 | 0.02 | 0.01 to 0.03 |
| Other | 82 | 0.11 | 0.09 to 0.14 |
| Pilonidal sinus | 25 | 0.03 | 0.02 to 0.05 |
| Pressure ulcer | 236 | 0.31 | 0.28 to 0.36 |
| Traumatic wound | 81 | 0.11 | 0.09 to 0.13 |
| Foot ulcers | 166 | 0.22 | 0.19 to 0.26 |
| Diabetic foot ulcer | 95 | 0.13 | 0.10 to 0.15 |
| Non-diabetic foot ulcer | 71 | 0.09 | 0.07 to 0.12 |
| Leg ulcers | 335a | 0.44 | 0.40 to 0.49 |
| Arterial/venous leg ulcer | 79 | 0.11 | 0.08 to 0.13 |
| Arterial leg ulcer | 38 | 0.05 | 0.04 to 0.07 |
| Venous leg ulcer | 218 | 0.29 | 0.25 to 0.33 |
| Surgical wounds | 156 | 0.21 | 0.18 to 0.24 |
| Dehisced surgical wound | 51 | 0.07 | 0.05 to 0.09 |
| Other surgical wound | 105 | 0.14 | 0.12 to 0.17 |
| Wound type unknown | 25 | ||
| Total | 1168b |
Pressure ulcers were the most prevalent complex wound in women, with a point prevalence of 0.39 per 1000 (95% CI 0.33 to 0.46 per 1000); in men they were the second most prevalent wound type, with a point prevalence of 0.23 per 1000 (95% CI 0.19 to 0.29 per 1000). Venous ulcers were the most frequent wound type in men, with a point prevalence of 0.25 per 1000 (95% CI 0.20 to 0.30 per 1000). Burns and pilonidal sinuses were the least common wounds in females (both with an estimated point prevalence of 0.02 per 1000, 95% CI 0.01 to 0.04 per 1000 and 0.01 to 0.03 per 1000, respectively) and fungating carcinomas were the least common wound type for males (point prevalence 0.01 per 1000, 95% CI 0.01 to 0.02 per 1000). Further details can be found in Appendix 2.
Diabetic foot ulcers were around twice as common in men (0.17 per 1000, 95% CI 0.13 to 0.22 per 1000) than women (0.08 per 1000, 95% CI 0.05 to 0.11 per 1000). Traumatic wounds were around twice as common in women (0.15 per 1000, 95% CI 0.11 to 0.19 per 1000) than men (0.07 per 1000, 95% CI 0.05 to 0.10 per 1000). Pilonidal sinuses were more than twice as frequent in men (0.05 per 1000, 95% CI 0.03 to 0.08 per 1000) as in women (0.02 per 1000, 95% CI 0.01 to 0.03 per 1000).
Point prevalence in intravenous drug users
Of the 1103 individuals with a complex wound, 31 were current (or previous) intravenous drug users. The point prevalence of complex wounds among past or current intravenous drug users was estimated at 5.64 per 1000 (95% CI 3.97 to 7.99 per 1000). Drug use status was unknown for 116 individuals and missing for 30 individuals, accounting for 13.2% of the sample population.
The most prevalent wound type in people with a history of intravenous drug use was venous leg ulcer, with a point prevalence of 4.73 per 1000 (95% CI 3.23 to 6.92 per 1000). We used current local estimates of the number of intravenous drug users in Leeds for the denominator (n = 5500; Leeds Drug Service, personal communication, 2010).
Characteristics of individuals with complex wounds
The characteristics of individuals within the sample are presented in Table 12. There was a slightly higher proportion of females than males (56.8% vs. 42.7%) and most people were white British (91.8%). The youngest person with a complex wound was aged < 1 year and the oldest was aged 108 years. The mean age was 70.06 years [standard deviation (SD) 19.41 years] and the median age was 76 years. Over 60% of people with complex wounds were aged > 70 years.
| Characteristic | Frequency, n | % |
|---|---|---|
| Sex | ||
| Female | 627 | 56.8 |
| Male | 471 | 42.7 |
| Missing | 5 | 0.5 |
| Ethnicity | ||
| White British | 1013 | 91.8 |
| White Irish | 23 | 2.1 |
| White other | 14 | 1.3 |
| Black Caribbean | 11 | 1.0 |
| Asian Indian | 10 | 0.9 |
| Asian Pakistani | 6 | 0.5 |
| Chinese | 5 | 0.5 |
| Asian other | 4 | 0.4 |
| Other | 4 | 0.4 |
| Black African | 2 | 0.2 |
| Asian Bangladeshi | 2 | 0.2 |
| White and black Caribbean | 2 | 0.2 |
| White and Asian | 2 | 0.2 |
| Other mixed background | 1 | 0.1 |
| Black other | 1 | 0.1 |
| Missing | 3 | 0.3 |
| Age (years) | ||
| 0–9 | 3 | 0.3 |
| 10–19 | 20 | 1.8 |
| 20–29 | 30 | 2.7 |
| 30–39 | 50 | 4.5 |
| 40–49 | 61 | 5.5 |
| 50–59 | 99 | 9.0 |
| 60–69 | 137 | 12.4 |
| 70–79 | 256 | 23.2 |
| 80–89 | 312 | 28.3 |
| 90–99 | 110 | 10.0 |
| ≥ 100 | 4 | 0.4 |
| Missing | 21 | 1.9 |
When each wound type was considered separately, those with arterial/venous leg ulcers had the highest mean age at just below 80 years, whereas those with pilonidal sinuses were the youngest (mean age just over 37 years) (see Appendix 2).
Wounds
Specific information was recorded on the questionnaire concerning 1416 wounds. The total number of complex wounds reported in the sample population was 1479 (based on 1085 individuals), with data missing for 18 individuals. Assuming (a conservative) one wound for each of these individuals, the sample population was estimated to have a total of 1497 wounds.
Each person had a mean number of 1.36 (SD 0.99) complex wounds per person. The data were positively skewed and the median number of complex wounds per person was one, despite the range being between one and 20 (75.1% of the sample population had only one complex wound). Males had, on average, more complex wounds per person [mean 1.40 (SD 0.94)] than females [mean 1.30 (SD 0.69)].
Duration of wound was recorded for 1374 of the 1416 wounds (97.0%) in the sample. The mean duration in the sample was 67.5 weeks (SD 187.1 weeks). The median was much shorter (4 months), indicating a positive skew (most wounds within the sample had a smaller than average duration).
The most common underlying cause of the complex wound (based on professional opinion) was pressure/friction/sheer, which was recorded as a reason for 406 wounds (28.7%).
Comorbidities
Table 13 shows the frequencies of prespecified comorbidities. Cardiovascular disease (CVD) was the most common comorbidity (44.8%) and stroke was the least common (8.3%). The numbers of comorbidities per person are presented in Table 14. Most people captured by the survey had one comorbidity (330/1103, 29.9%); 313 people (28.4%) had two comorbidities.
| Comorbidity | Frequency, n | % |
|---|---|---|
| CVD | 494 | 44.8 |
| Arthritis | 298 | 27.0 |
| Diabetes | 231 | 20.9 |
| Peripheral vascular disease | 216 | 19.6 |
| Cancer | 130 | 11.8 |
| Airways | 123 | 11.2 |
| Orthopaedic | 106 | 9.6 |
| Neurological | 100 | 9.1 |
| Stroke | 92 | 8.3 |
| Total | 1790a |
| Total number of prespecified comorbidities per patient | Frequency, n | % |
|---|---|---|
| 0a | 214 | 19.4 |
| 1 | 330 | 29.9 |
| 2 | 313 | 28.4 |
| 3 | 167 | 15.1 |
| 4 | 63 | 5.7 |
| 5 | 15 | 1.4 |
| 6 | 1 | 0.1 |
| Total | 1103 | 100 |
One of the limitations of the survey was that it was not possible to distinguish between a person with no comorbidities and a person with missing comorbidity data as both are classed as having no comorbidities. This could have skewed the mean number of comorbidities towards zero, causing underestimation. Because of this, the mean number of comorbidities is presented twice, once for all individuals and once for individuals with at least one comorbidity. The mean number of comorbidities per person taking into account all individuals in the survey was 1.62 (SD 1.21) and the median number was two comorbidities. The mean number of comorbidities for individuals with at least one comorbidity was 2.01 (SD 1.00).
Diabetic foot ulcers were found to be associated with the highest average number of reported comorbidities at 2.49 (SD 1.03), whereas individuals with pilonidal sinuses had the lowest average number of comorbidities (0.32, SD 0.75).
Other health issues
Table 15 shows summary statistics relating to ‘other’ health issues such as incontinence and mobility.
| Health issue | Frequency, n | % |
|---|---|---|
| Continencea | ||
| No incontinence | 760 | 68.9 |
| Urinary incontinence | 254 | 23.0 |
| Faecal incontinence | 133 | 12.1 |
| Missing | 60 | 5.4 |
| Nutritional status | ||
| No recent weight change | 810 | 73.4 |
| Recent weight loss | 198 | 18.0 |
| Recent weight gain | 55 | 5.0 |
| Missing | 40 | 3.6 |
| Mobility | ||
| Patient walks with difficulty | 452 | 41.0 |
| Patient walks freely | 435 | 39.4 |
| Patient is immobile | 200 | 18.1 |
| Missing | 16 | 1.5 |
| Currently on antibiotics | ||
| No | 849 | 77.0 |
| Yes | 168 | 15.2 |
| Don’t know | 61 | 5.5 |
| Missing | 25 | 2.3 |
Urinary incontinence was reported for nearly one-quarter of the sample (23%) and faecal incontinence for approximately 12%, with 9.3% (103/1103) of the overall sample having both urinary and faecal incontinence. Urinary incontinence was most frequently found in people with at least one pressure ulcer (48.3%, 114/236), as was faecal incontinence (33.9%, 80/236).
The majority of people had not experienced recent weight change (73.4%) and were not currently taking antibiotics (77%). In total, 41.0% walked with difficulty and 39.4% walked freely. Diabetic foot ulcers were most commonly associated with difficulty with walking, with 58.9% (56/95) of individuals with at least one wound of this type reported to have a problem. Non-diabetic foot ulcers, arterial leg ulcers and traumatic wounds were also frequently associated with walking difficulties (53.5%, 52.6% and 51.9%, respectively). Immobility was most frequently associated with pressure ulcers, with 48.3% (114/236) of individuals with at least one pressure ulcer reported as being immobile. This is more than twice the frequency of immobility as in those with non-diabetic foot ulcers (23.9%, 17/71).
Wound management
Health professionals providing care
Table 16 indicates both the professional background and the grade (Agenda for Change band) of those completing the questionnaires. Health professionals caring for people with complex wounds were mainly qualified nurses (accounting for around 66% of the individuals captured by the survey), with 5.9% being podiatrists. The nurses providing care and completing the survey questionnaires were mainly district nurses (reporting on 36.3% of people with complex wounds) followed by practice nurses (25.1%) and specialist nurses (4.7%). Just over 85% (247/289) of the ‘other’ category were also nurses. Thus, 88% of people with complex wounds were receiving nursing care from (and were reported into the survey by) a nurse. The majority (74.4%) were seen by band 5 and band 6 staff (46.1% and 28.4%, respectively).
| Care provider | Frequency, n | % |
|---|---|---|
| Job title | ||
| District nurse | 400 | 36.3 |
| Other | 289 | 26.2 |
| Practice nurse | 277 | 25.1 |
| Podiatrist | 65 | 5.9 |
| Specialist nurse | 52 | 4.7 |
| Missing | 20 | 1.8 |
| Band | ||
| 3 | 27 | 5.4 |
| 5 | 508 | 46.1 |
| 6 | 313 | 28.4 |
| 7 | 77 | 7.0 |
| 8a | 9 | 0.8 |
| Other | 110 | 10.0 |
| Missing | 59 | 5.3 |
Wound consultations
Services providing wound care are presented in Table 17. Individuals were mainly cared for by community NHS services (801/1103, 72.6%). Acute NHS services cared for 9.5% of individuals with complex wounds, and nursing homes and hospices provided services for 7.3% and 0.8% of individuals, respectively. Approximately one-quarter of patients were seen within more than one health-care setting.
| Service | Frequency, n | % |
|---|---|---|
| Community NHS | 801 | 72.6 |
| Acute NHS | 105 | 9.5 |
| Nursing home | 80 | 7.3 |
| Missing | 25 | 2.3 |
| Hospice | 9 | 0.8 |
| Other | 83 | 7.5 |
| General practice | 67 | 6.1 |
| Prison | 8 | 0.7 |
| Residential home | 2 | 0.2 |
| CIC bed | 1 | 0.1 |
| ICT bed | 1 | 0.1 |
| Inpatient | 1 | 0.1 |
| Out-of-hospital care | 1 | 0.1 |
| Private hospital (outpatient) | 1 | 0.1 |
| Not specified | 1 | 0.1 |
| Total | 1103 | 100.0 |
The frequency of wound consultations per week was recorded for 1066 individuals and ranged between 1 and 14. The mean number of consultations per patient per week was 3.15 (SD 2.15) (median of two per week). The duration of the most recent consultation was recorded for 1083 individuals (98.2%) and the mean duration was 28 minutes (SD 43.6 minutes). If it is assumed that the maximum recorded consultation of 20 hours was a recording error, the mean duration was just below 27 minutes (SD 25.2 minutes).
Treatment objectives
The most frequent wound treatment objective was protection (66.5% of 1416 wounds). Encouraging granulation was the second most frequent objective (49.3%), whereas reducing ‘overgranulation’ was the least common treatment objective.
Wound dressings
A primary dressing was reported in 1326 (93.6%) of the 1416 wounds. The five most frequently reported primary dressings were non- and low-adherence wound contact dressings (26.3%), foam (15.9%), iodine-containing low-adherence wound contact dressings (12.4%), spun hydrocolloid/hydrofibre (8.4%) and soft polymers (7.7%). Collagen dressings, hyaluronic acid, silicone sheets or gels, silver-containing silicone sheets or gels and acrylic dressings were not reported as being used as the primary dressing for any wound. A secondary dressing was reported for 678 (47.9%) of the 1416 wounds. The five most frequently reported secondary dressings were non- and low-adherence wound contact (37.9%), foam (30.5%), soft polymer (12.4%), vapour permeable/film (4.3%) and odour absorbent/charcoal (3.1%).
The three most frequently reported dressings for each wound type are presented in Tables 18 and 19 for primary and secondary dressings, respectively.
| Wound type | Wounds with a primary dressing, n (%) | NLA wound contact, n (%) | Foam, n (%) | Iodine NLA wound contact, n (%) | Spun H/H, n (%) | Soft polymer, n (%) | Iodine-containing hydrogel, n (%) | Silver spun H/H, n (%) | Alginate, n (%) | Cavity, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pressure ulcer | 263 (91.6) | 74 (28.1) | 25 (9.5) | 32 (12.2) | ||||||
| Venous leg ulcer | 276 (92.6) | 157 (56.9) | 24 (8.7) | 16 (5.8) | ||||||
| Other surgical wounds | 109 (91.6) | 29 (26.6) | 19 (17.4) | 15 (13.8) | ||||||
| Diabetic foot ulcer | 106 (94.6) | 22 (20.8) | 34 (32.1) | 13 (12.3) | ||||||
| Other wounds | 88 (87.1) | 24 (27.3) | 16 (18.2) | 19 (21.6) | ||||||
| Traumatic wound | 85 (97.7) | 28 (32.9) | 16 (18.8) | 11 (12.9) | ||||||
| Arterial/venous leg ulcer | 45 (42.1) | 10 (9.3) | 10 (9.3) | |||||||
| Non-diabetic foot ulcer | 77 (93.9) | 19 (24.7) | 23 (29.9) | 13 (16.9) | ||||||
| Dehisced surgical wounds | 48 (92.3) | 7 (14.6) | 14 (29.2) | 5 (10.4) | ||||||
| Arterial leg ulcer | 42 (93.3) | 14 (33.3) | 7 (16.7) | 4 (9.5) | ||||||
| Abscessa | 35 (97.2) | 14 (40.0) | 5 (14.3) | |||||||
| Pilonidal sinus | 24 (92.3) | 5 (20.8) | 3 (12.5) | 9 (37.5) | ||||||
| Burna | 15 (93.8) | 3 (20.0) | 6 (40.0) | |||||||
| Fungating carcinomaa | 9 (64.3) | 2 (22.2) | 2 (22.2) |
| Wound type | Wounds with a secondary dressing, n (%) | NLA wound contact, n (%) | Foam, n (%) | Soft polymer, n (%) | Vapour permeable/film, n (%) | Odour absorbent/charcoal, n (%) | Iodine NLA wound contact, n (%) |
|---|---|---|---|---|---|---|---|
| Pressure ulcer | 118 (41.1) | 15 (12.7) | 60 (50.8) | 17 (14.4) | |||
| Venous leg ulcer | 106 (35.6) | 67 (62.3) | 12 (11.3) | 11 (10.4) | |||
| Other surgical wounds | 58 (48.7) | 24 (41.4) | 15 (25.9) | 9 (15.5) | |||
| Diabetic foot ulcer | 73 (65.2) | 23 (31.5) | 37 (50.7) | 4 (5.5) | |||
| Other wounds | 39 (38.6) | 15 (38.5) | 11 (28.2) | 7 (17.9) | |||
| Traumatic wound | 36 (41.4) | 13 (36.1) | 7 (19.4) | 12 (33.3) | |||
| Arterial/venous leg ulcer | 72 (64.3) | 42 (58.3) | 12 (16.7) | 6 (8.3) | |||
| Non-diabetic foot ulcera | 33 (40.2) | 14 (42.4) | 10 (30.3) | ||||
| Dehisced surgical wounds | 35 (67.3) | 8 (22.9) | 14 (40.0) | 6 (17.1) | |||
| Arterial leg ulcer | 25 (55.6) | 13 (52.0) | 8 (32.0) | 2 (8.0) | |||
| Abscess | 26 (72.2) | 6 (23.1) | 6 (23.1) | 6 (23.1) | |||
| Pilonidal sinus | 16 (61.5) | 4 (25.0) | 4 (25.0) | 6 (37.5) | |||
| Burn | 10 (62.5) | 6 (60.0) | 3 (30.0) | 1 (10.0) | |||
| Fungating carcinomab | 5 (35.7) | 2 (40.0) |
A cavity wound dressing (either silver or non-silver containing) was used as the primary dressing for 35 wounds and was used most frequently in relation to ‘other’ surgical wounds and pilonidal sinuses.
Bandaging and hosiery
For all wound types, a non-compression bandage plus wadding was the most frequently reported bandaging (16.5%). Approximately one-quarter of the 455 leg ulcers in the sample was treated using a non-compression bandage (Table 20) and multilayer compression was used for just over one-fifth of these wounds (22.6%).
| Type of bandaging | Frequency, n | % |
|---|---|---|
| Non-compression bandage plus wadding | 116 | 25.5 |
| Multilayer compression | 103 | 22.6 |
| Three-layer reduced compression | 67 | 14.7 |
| Short stretch compression | 50 | 11.0 |
| Two-layer compression | 36 | 7.9 |
| Dressing retention bandage | 8 | 1.8 |
Hosiery was rarely used in wound treatment (53/1416 wounds, 3.7%). When used as a treatment, hosiery was most frequently used for venous leg ulcers (33/298, 11.1%) but was also used in the treatment of some traumatic wounds, diabetic and non-diabetic foot ulcers, ‘other’ wounds, burns and arterial/venous leg ulcers.
There were 218 individuals in the sample population with at least one venous leg ulcer. Of these, 176 (80.7%) were receiving at least one form of compression therapy, most frequently (34.4% of those with a venous leg ulcer) multilayer compression (Table 21). Compression bandaging was used more frequently than compression hosiery.
| Type of compression therapy | Frequency, n | % |
|---|---|---|
| Multilayer compression | 75 | 34.4 |
| Three-layer reduced compression | 38 | 17.4 |
| Short stretch | 33 | 15.1 |
| Two-layer compression | 16 | 7.3 |
| Class 2 hosiery over the counter | 7 | 3.2 |
| 40 mmHg made to measure | 6 | 2.8 |
| 40 mmHg over the counter | 5 | 2.3 |
| Class 1 hosiery over the counter | 5 | 2.3 |
| Class 2 hosiery made to measure | 4 | 1.8 |
| Class 3 hosiery made to measure | 2 | 0.9 |
| Class 3 hosiery over the counter | 0 | 0 |
| Total | 191a | 87.6 |
Equipment and footwear
Table 22 summarises all forms of equipment used. Equipment was mainly pressure-relieving equipment for people with, or at risk of, pressure ulceration. The static, air-filled mattress overlay (Repose®, Frontier Medical Group, Blackwood, South Wales, UK) was the most frequently reported pressure-relieving mattress, used by 15.7% of the sample population. In addition, 8% of patients had a wheelchair and a few used crutches (0.8%). Of the 236 individuals with at least one pressure ulcer, 173 (73.3%) were using a pressure-relieving mattress, most commonly an alternating-pressure mattress replacement (41.6%). A minority of people were using more than two types of mattress (5.2% of 173 patients).
| Equipment | Frequency, n | % |
|---|---|---|
| Pressure-relieving mattresses | ||
| Static, air-filled mattress overlay (Repose®, Frontier Medical Group) | 173 | 15.7 |
| Alternating-pressure mattress replacement | 99 | 9.0 |
| High-specification foam mattress replacement | 85 | 7.7 |
| Foam mattress overlay | 13 | 1.2 |
| Alternating-pressure mattress overlay | 10 | 0.9 |
| Wheelchair | 91 | 8.3 |
| Other equipment | 72 | 6.5 |
| Crutches | 9 | 0.8 |
Use of orthotics is detailed in Table 23. Prescribed footwear was reported for 12.6% of people. Heel pressure relief, footwear adaptations and insoles were reported much less frequently (2.7%, 2.5% and 0.9%, respectively). Of the 95 individuals with at least one diabetic foot ulcer, 62 (65.3%) were using an orthotic device, whereas only 23 out of 71 (32.4%) people with a non-diabetic foot ulcer were using one.
| Footwear | Frequency, n | % |
|---|---|---|
| Prescribed footwear | 179 | 12.6 |
| Other types of footwear (not specified) | 46 | 3.2 |
| Heel pressure relief, e.g. Repose® Heel Trough (Frontier Medical Group), PRAFO® boot (Anatomical Concepts, Inc, Clydebank, UK) | 38 | 2.7 |
| Footwear adaptation | 36 | 2.5 |
| Insoles | 13 | 0.9 |
Medication and other treatments
Topical steroids were being used in the care of 7.2% of people with complex wounds. In total, 19% of patients were taking analgesics and 13.8% were taking antibiotics related to wound infection. Only one patient (who had a venous leg ulcer) was taking pentoxifylline.
In terms of other treatments, a small number of people were reported to be having physiotherapy (4.3%, 47/1103), occupational therapy (2.8%, 31/1103) or hyperbaric oxygen therapy (0.3%, 3/1103).
Discussion
We designed and implemented a survey protocol that included an explicit definition of a complex wound and different types of complex wound. Our review of previous prevalence studies highlighted that most estimates were usually based on either self-report by sampling the general population (or subpopulation, e.g. limited by age) or the case loads of health-care providers. Although case load-based surveys will always underestimate the prevalence because they do not capture people who self-treat, we chose this approach as it was most likely to be practical, less expensive and satisfy the information needs of the NHS. We aimed to be as comprehensive as possible in surveying all kinds of health-care provider and our survey included hospitals, hospices, care homes and prisons.
The prevalence survey gave us up-to-date epidemiological data regarding the number and nature of people with complex wounds in a large UK city (Leeds). These findings are likely to be applicable to other places with a similar demographic profile. Although pressure ulcers, leg ulcers, foot ulcers and surgical wounds were the most common complex wounds, a wide variety of less common complex wounds accounted for one in five of the people identified. This suggests that a more complete understanding of the extent of complex wounds and their impact on society is best achieved by approaching complex wounds as a whole, particularly as people with complex wounds draw on the same services, irrespective of the underlying cause.
To our knowledge, this survey is the first to estimate the prevalence of complex wounds among injecting drug users. The prevalence of complex wounds was higher among injecting drug users than among the general population and these wounds were most commonly venous leg ulcers. Injecting drug users can be a difficult population to access and thus our prevalence estimate might be an underestimate, although linking with services such as the No Fixed Abode team in Leeds likely improved our access to patients who may otherwise have been missed. Use of techniques such as capture–recapture may have further enhanced our estimates. Although most people with complex wounds were aged > 65 years, those with pilonidal sinuses, complex surgical wounds, burns and abscesses were younger. Wound care services may need to be geared towards a primarily elderly population but need the flexibility to deal effectively with the potentially differing needs of a smaller but younger population. Most wound care in the city is undertaken by nurses (in a variety of roles) within community NHS services; the remainder of patients are managed by a wide variety of professions. Fewer than 10% of patients are cared for in acute NHS services and 8% in nursing homes.
Comparisons with other epidemiological studies
Overall, our estimate of the point prevalence of complex wounds is lower than relevant recent estimates (range 2–3.55 per 1000 population). Our lower estimate probably reflects our case definition (several previous studies have included uncomplicated surgical and other acute wounds or differed in other ways28,31,32). Our definition of a complex wound was intended to capture those wounds for whom healing is frequently protracted, for which there are usually comorbidities and for which NHS intervention is often frequent and prolonged.
Our point prevalence estimates for specific wound types were similar to those in previous studies. For example, a leg ulcer point prevalence of 0.44 per 1000 is within the range 0.39–4.8 per 1000 reported by others. 45,50 However, our pressure ulcer point prevalence estimate (0.31 per 1000) was lower than previous estimates from studies using a geographically defined population. Previous estimates of pressure ulcer prevalence have ranged from 0.4 to 230 per 1000 depending on the baseline risk in the survey population; for example, rates in spinal injury patients are particularly high. 27,73,74,79 Our low estimate is probably at least partly due to the high priority placed on pressure ulcer prevention at local and national levels and early implementation of local improvement tools in Leeds, leading to improvements in care. 92 There were also differences in study design, methods and reporting behaviour.
Quality of care
Venous leg ulcers
We were able to compare the management of venous leg ulcers in Leeds with elements of the Royal College of Nursing93 and SIGN94 guidelines, specifically screening for arterial disease using Doppler-aided ABPI; use of compression therapy; use of low-adherence/low-cost dressings; and prescription of pentoxifylline. Overall, adherence to guideline recommendations was generally good. Nearly 70% of people recorded as having at least one venous leg ulcer had an ABPI assessment recorded in their notes (60% for people with at least one leg ulcer of any type). In total, 80% of people with a venous ulcer were receiving some form of compression; 56% of primary dressings and 67% of secondary dressings were non-/low-adherence wound contact dressings. The percentage of people recorded as having an ABPI assessment may be an underestimate if, for instance, patients’ notes were not available when the survey form was completed or the ABPI assessment was carried out by a specialist team (such as a vascular secondary care team) and the recommended treatment was carried out by another care provider who completed the survey form (with the ABPI assessment not necessarily recorded in the notes if carried out by another provider).
Figures for the use of compression therapy and ABPI assessment in patients with venous leg ulcers are very similar to those reported in previous studies in the UK and Ireland. In Bradford, Vowden and Vowden34 reported that a Doppler assessment had been performed in 66% of people with leg ulcers and 75% of people with venous leg ulcers received some sort of compression therapy. A survey of leg ulcer management in Ireland estimated that 75% of limbs with a venous leg ulcer had an ABPI recorded, 50% were receiving high levels of compression therapy and a further 15% were receiving lower levels of compression therapy. 40
Royal College of Nursing guidelines93 recommend that simple low-adherent, low-cost dressings that are acceptable to the patient are used in the management of venous leg ulcers. Similarly, SIGN guidelines94 also recommend the use of simple non-adherent dressings and, in addition, do not recommend the routine use of either honey or silver dressings in the management of venous leg ulcers. The high use of non-/low-adherence wound contact dressings in the management of venous leg ulcers in our survey is particularly encouraging. In contrast, the studies by Vowden and Vowden34 and Clarke-Moloney et al. 40 both reported that the most frequently used dressings for venous leg ulcers were antimicrobial products (Vowden and Vowden34 reported that 41% of primary dressings were antimicrobials and in the Irish survey40 silver-impregnated dressings were the most commonly used dressings). In our survey, antimicrobial dressings were used relatively rarely for venous ulcer management (52/276 cases, 19%) when a primary dressing was reported. Reducing the use of antimicrobial dressings has been a priority in Leeds Community Healthcare NHS Trust, with intensive efforts made to bring about change, and this strategy appears to have been successful.
Scottish Intercollegiate Guidelines Network guidelines94 recommend the use of pentoxifylline in the management of venous leg ulcers; however, we identified only one person who was reported to be receiving pentoxifylline. However, there has been no particular effort to promote the prescribing of pentoxifylline for people with venous leg ulcers in Leeds (or elsewhere as far as we are aware). In December 2012 NHS Leeds recategorised pentoxifylline as suitable for prescribing for people with hard-to-heal leg ulcers in primary care. The findings from our survey will provide a useful benchmark from which to monitor the use of pentoxifylline in the management of venous leg ulcers in Leeds.
Pressure ulcers
We were able to assess adherence to NICE guidelines13 in the management of pressure ulcers in a number of areas: ulcer categorisation, risk assessment, availability and suitability of pressure-relieving equipment and use of suitable wound dressings. Overall, adherence to guidelines was good but some areas for improvement were identified.
According to NICE guidelines, people with existing pressure ulcers should receive ongoing assessment, although there is little evidence to support any particular method of assessment. The Braden risk assessment tool95 is recommended for all Leeds NHS providers. Other care providers such as nursing homes do not necessarily use the Braden risk assessment tool, for example some use the Waterlow score. 96 The survey captured information about those people who had a Braden risk assessment reported in their notes in the last month. In people with at least one pressure ulcer, 57.2% (135/236) were reported as having had a Braden risk assessment completed in the last month, 30.5% (72/236) had not and 12.3% (29/236) of the data were missing. This percentage of those reported as having had a Braden risk assessment may potentially be an underestimate if, for example, a risk assessment had been completed but not monthly or patients’ notes were not available when the survey form was completed.
National Institute for Health and Care Excellence guidelines recommend that all pressure ulcers are categorised according to EPUAP recommendations. We identified that 94.8% (272/287) of pressure ulcers had been either categorised or classed as unstageable. It is encouraging that most pressure ulcers are assigned a category from which clinicians can assess improvement or deterioration of the ulcer.
National Institute for Health and Care Excellence guidelines also recommend that patients with pressure ulcers should have access to appropriate pressure-relieving support surfaces and strategies. In total, 77% of people were reported as being in receipt of one of the listed pieces of pressure-relieving equipment (high-specification foam replacement, static air-filled mattress overlay, foam overlay, alternating pressure overlay, alternating pressure mattress replacement). It was not possible to estimate the proportion of people who had declined pressure-relieving equipment and so the proportion of people having access to pressure relief could be > 77%.
Based on professional consensus (as opposed to research evidence), NICE clinical guidelines recommend the creation of an optimum wound healing environment using modern dressings, for example hydrocolloids, hydrogels, hydrofibres, foams, films, alginates and soft silicones. The dressings identified in this survey in the management of pressure ulcers were largely concordant with NICE guidelines (foam and soft polymer were the most frequently reported dressings for both primary and secondary dressings).
Review of registers
Background
Before developing and piloting our own complex wounds register in Leeds we aimed to learn as much as we could from current and previous chronic disease registers, including how these were defined, how they were operationalised and how data were collected and used. To obtain relevant literature we conducted a systematic review of chronic disease registers (see Appendix 3 for the review protocol). Prior to the review we were not aware of any UK registries of chronic wound care but wanted to confirm that this was the case. We also felt that information from registries of other chronic diseases might provide valuable insights.
Aims and objectives
The aim was to identify, appraise and summarise reports of chronic disease registries.
Specific aims were to:
-
identify chronic diseases (and countries) for which a register had been utilised
-
identify the methods of data collection used
-
summarise the uses of register data
-
describe how the register (and data collection related to it) integrates with other data collection systems in the organisation
-
examine the configuration of registries across multiple specialty teams or across health-care interfaces, for example primary and secondary care.
Methods
Systematic search
Inclusion criteria
Any paper reporting a chronic disease register was included. A chronic disease was defined as a long-lasting or recurrent condition. Registries relating to surgical procedures were excluded. A medical register was defined as a database that met the six characteristics that define a medical database register:97
-
mergeable data: data from many users and patients can be aggregated
-
standardised data set: same variables collected for all patients
-
rules for data collection: protocol-driven data collection
-
observations associated over time: follow-up data for individual patients linked
-
knowledge about patient outcomes: complete ascertainment of end points of patients in the register
-
inclusion principle: the clinical characteristic or diagnosis on which the register is focused and which all registrants share.
Search methods
A MEDLINE search was undertaken using OvidSP and the following search strategy:
-
exp Chronic Disease/ (182,090)
-
2(chronic adj (illness$or disease$)).tw. (25,675)
-
1 or 2 (199,178)
-
exp Registries/ (35,210)
-
(registr$or register$1).tw. (77,603)
-
4 or 5 (92,049)
-
6 and 3 (1409)
This searching was supplemented by less formal internet searching via the search engine Google (Google Inc., Mountain View, CA, USA). Similar terms to those used in the MEDLINE search were used.
Screening process
Screening stage 1
All study titles and abstract (when available) were reviewed. Full papers were obtained when the title and/or abstract suggested that the paper reported:
-
a review of medical registers
-
a study in which a medical data register relating to a chronic condition was discussed or explicitly used as a source of data.
The selection process was carried out by two reviewers. Any disagreement was taken to a third, senior reviewer.
Screening stage 2
Full articles were then screened against the criteria of Drolet and Johnson97 and classified as medical data registers or not. When a paper provided very limited information, that is, a register was mentioned only briefly as a source of data without sufficient information to make an inclusion decision, we followed the methods of Drolet and Johnson97 and searched for further information on MEDLINE and the internet. We had also planned to contact authors if necessary. When no further useful information could be obtained the study was excluded with regard to further data extraction. Again, all phases were conducted by two reviewers.
Results
The MEDLINE search produced > 1400 references and after initial screening 30 potential papers of interest were identified. Based on the available abstracts it was evident that the publications were likely to be of limited value in addressing the aims of the review. Two-thirds of the papers were reports of analyses across a wide variety of conditions using data from a register and were not about the register itself.
The internet search also produced a substantial number of ‘hits’, most of which were of limited relevance because they did not relate to registries about chronic diseases.
However, two significant publications were identified (in addition to that by Drolet and Johnson97) and full reports were obtained. 98,99
Both the Newton and Garner98 and Raftery et al. 99 papers were reviews of registers, clinical databases and routine databases with similar aims and objectives to those of our planned review. In effect, these reviews had already comprehensively addressed what we were setting out to do; therefore, to meet our objective of informing our own register design, this project became a ‘review of reviews’, although this was not the original intent. Drolet and Johnson97 developed a framework for classifying registries. We also identified, through internet searching and reference checking, papers specific to reports of registers/databases of people with complex wounds. 100–108 The reviews identified sufficiently addressed our core questions and provided an adequate basis for the design of our own register. The findings of the reviews are summarised in Table 24.
| Study | Review aims and objectives | Review methods |
|---|---|---|
| Newton 200298 | Commissioned by the Department of Health Policy Research Programme in 2000 in support of the White Paper Saving Lives: Our Healthier Nation.109 The objectives were to:
|
The authors reviewed the literature, wrote to regional directors of research and development, regional directors of public health and district directors of public health. They also contacted < 100 specific individuals in relation to individual registers |
| Raftery 200599 | This was a NIHR Health Technology Assessment programme-commissioned study with the following aims:
|
The authors detail the methods used for each separate objective (see full report). In summary, they used a combination of electronic databases, key literature sources and information from experienced users of routine databases to answer the questions |
| Drolet 200897 | Aimed to develop a more useful categorisation scheme for registries | The project was conducted in three phases (identification of references, preliminary review and framework construction and evaluation), largely following the immersion/crystallisation process used in qualitative research |
Classification and definitions of registers
Newton and Garner98
In their report, Newton and Garner98 draw a distinction between ‘disease register’ (or ‘case register’) and ‘clinical database’. They reserve the term ‘disease register’ for those databases that have a clearly identified denominator population. Clinical databases do not necessarily relate to a defined denominator population and usually include cases from a particular institution or group of institutions. According to this definition, registers can be used for epidemiological research and needs assessment as well as to improve clinical care and service quality and for health technology assessment (HTA). Clinical databases generally have the objectives of quality improvement and HTA and, although cases should be as representative as possible of those in the target population, these databases are not suitable for epidemiological research because of the lack of a clear denominator and they are potentially misleading as indicators of the need for care in the community.
Raftery et al.99
Raftery et al. 99 adopted the following key terms (or definitions) in their review:
-
routine data – there is no unequivocal definition but important characteristics include regular/continuous/periodic collection, use of standard definitions, degree of obligation to collect data completely/regularly and collection at a national or regional level
-
health technologies – all methods used by health professionals to promote health, prevent and treat disease, and improve rehabilitation and long-term care
-
HTA – includes the assessment of:
-
efficacy (patient benefit/harm in experimental and closely monitored studies) and effectiveness (benefit/harm when applied in everyday practice)
-
equity (extent of use by different groups or particular health technologies in relation to some measure of clinical need or perceived fairness)
-
diffusion (factors influencing uptake of health technologies by place and time)
-
costing (cost efficacy, cost-effectiveness, cost of illness, cost–consequences and cost impact).
-
Raftery et al. 99 then developed a classification system for routine databases according to their potential use in HTA (to assess efficacy or effectiveness and, to a large extent, equity and diffusion):
-
Group I databases. These capture information on both the HTA being used and outcomes. They offer the greatest potential for HTA and can be subdivided into three groups:
-
Ia – clinical registries are clinically rich databases that contain data on both the interventions (health technology) and the outcomes (health states) at the patient level. These have frequently been designed for research purposes
-
Ib – clinical administrative databases contain data on health technologies but only limited health state data at the patient level. They are typically designed for administration purposes, for example NHS Hospital Episode Statistics.
-
Ic – population-oriented databases identify the health technology and health state at the population level, for example vaccination programmes.
-
-
Group II databases. These contain information about health technologies in use but not the outcomes associated with these technologies, for example prescribing data that summarise drugs prescribed but not patient outcomes.
-
Group III databases. These contain only health state information but no details of health technologies or interventions received. Group III databases are further subdivided into:
-
IIIa – adverse event reporting and confidential enquiries
-
IIIb – disease/disability-only registers
-
IIIc – health surveys.
-
When considering the use of databases to assess the costs of health technologies, Raftery et al. 99 suggest a classification system that distinguishes between the different ways that health technologies can be costed.
Drolet and Johnson97
Drolet and Johnson97 created a detailed definition for registries in health care and outlined a set of characteristics common to them all. Their framework [MDR-OK – mergeable data (M), data set standardized (D), rules for data collection (R), observations associated over time (O) and knowledge of outcomes (K)] includes six distinguishing features of registries, which were summarised in the methods section.
Number of registers and databases in the UK
Newton and Garner98
Newton and Garner98 reported that the number of disease registers already in existence in England is large, possibly larger than is generally appreciated. Their review was not exhaustive but identified approximately 250 registers. They noted the heterogeneity in every aspect of these registers, reflecting the fragmentation of policy and lack of strategy in this area. Cancer registries were recognised as an exception because there is now a developing national framework and regional structures to support it.
Raftery et al.99
This report identified around 270 databases at the level of the UK, England and Wales or England (> 1000 including databases specific to Scotland, Wales and Northern Ireland). The lack of detail in the review by Newton and Garner98 meant that it was not possible to cross-reference the English registers identified in both reviews, but we assumed that a large number of the registers would have been identified by both reviews. Newton and Garner98 did list six national treatment and device registers by name and these were all also included in the review by Raftery et al. ,99 with Raftery et al. 99 also presenting a more extensive list of named registers from across the UK. Around 60 of the registers identified by Raftery et al. 99 had the potential for HTA and approximately half of these were group I registries. Eighteen clinical registries were identified as having the greatest potential although only two could be directly used in costing. Review of the potential capture of health technologies prioritised by the UK NIHR Health Technology Assessment programme showed that only 10% would be captured in these databases, mainly drugs prescribed in primary care (wound care, specifically for venous leg ulcers, would not be captured by any of the databases).
Main uses of registers
Newton and Garner98
Newton and Garner98 identified the following current uses of registers:
-
Patient care (regular review and recall, structured care programmes, monitoring high-risk groups, managing demand and regulating access, communication and risk stratification).
-
Public health (surveillance, planning the provision of health care, monitoring the impact of preventative measures).
-
Health technology assessment. Databases and registries can make a useful contribution to evaluation if they conform to certain criteria (large numbers of patients defined in the same way, data collected in a standardised fashion, followed over long periods of time using precise definitions applied uniformly). As the authors indicate, although not as rigorous as RCTs for deriving comparative effect estimates, registries have the advantage of being based on more representative populations and often include far larger numbers of cases.
-
Research (descriptive studies, improving the performance of other research designs, studies of processes, hypothesis testing).
Raftery et al.99
Raftery et al. 99 examined the most promising databases that they had identified for the extent to which they had actually been used for HTA. They concluded that:
-
the use of databases for HTA has been limited
-
clinical registers were mainly used for national comparative audits
-
effectiveness assessment is more feasible when relevant outcomes can be readily included (most databases contain insufficient data on health states and lack the detail required for risk adjustment)
-
accessibility is a major barrier to using clinical registers, with their use largely limited to those who ‘own’ them.
Characteristics of successful registers
Newton and Garner98
Newton and Garner98 identified the following factors that seem to consistently predict success:
-
Appropriate multidisciplinary team, including a computer scientist, a biostatistician and someone trained in the clinical domain of the register.
-
Stability of funding – challenging as it may be years before the benefits of a database become apparent.
-
Focused aims – a register should have initial aims that are clear and focused. Although there is often a temptation to collect a large number of data, this may be of marginal value and questionable quality and can interfere with the primary objectives.
-
Data collection systems and a design that relate well to function.
-
Leadership – there should be a clearly identified, senior professional in charge of a register plus a senior administrative officer who is responsible for the quality of all aspects of the register.
-
Ideal physical location (an academic environment, expertise available, ‘neutral ground’, all potential users feel that they have equal access, all disciplines feel comfortable).
Setting up a disease register
Newton and Garner98
Newton and Garner98 identified a number of steps that might be considered when setting up a population-based register:
-
Establish an expert group who can ensure that a register has a sound financial and scientific basis and decide on initial data collection processes and data content.
-
Establish a steering group to ensure that a register is run according to its stated aims and objectives and that the rights of patients are respected. This steering group should include patient/family representation.
-
Notify the information commissioner.
-
Obtain approval from a research ethics committee if the register is to be used for research.
-
Establish arrangements for access to the data.
-
Consider arrangements for data security, including physical security (secure room in a secure building), local security (encryption), technical security (passwords) and procedural security (staff training and written records of procedures).
-
Establish appropriate arrangements for accountability.
-
Publicise the register and its findings. It is essential to promote the register among those who contribute data. Some form of ‘corporate identity’ may be useful to reinforce the presence of the register. The maintenance of good will requires frequent communication with participants and appropriate reporting. It is very important to avoid the perception that data are collected for the sake of it.
Methodological considerations
Newton and Garner98
Newton and Garner98 proposed the following:
-
A clear case definition is fundamental to the success of any register.
-
Methods used to ascertain cases need to be considered carefully. Diagnostic processes must be based on a formal protocol. If clinical assessment is the basis for a diagnosis, it is good practice to assess the reliability of the diagnostic protocol from the outset.
-
Reliable identification of duplicates requires patient identifiers although this is not necessarily straightforward even when these are available.
-
Future development of disease registers should relate to the evolving NHS information strategy,110 for example electronic patient records.
Evaluating the quality and value of registers
Newton and Garner98
Newton and Garner98 suggest the following as characteristics that can be used to assess the quality and value of registers:
-
the public health importance of the topic
-
the system used and its objectives
-
the uses and other outputs of the register
-
an evaluation of the system against a set of technical attributes (sensitivity, timeliness, representativeness, predictive value, accuracy and completeness of descriptive information, simplicity, flexibility and acceptability)
-
the resources used to operate the system and a summary of whether or not the system is meeting its objectives and any modifications required.
Newton and Garner98 also describe methods for assessing the completeness and validity of register data:
-
Completeness – the proportion of all cases in the population that appear in the register.
-
Death certificate method – completeness is the proportion of cases not first identified from a death certificate.
-
Independent case ascertainment – completeness assessed by means of an independent survey, for example a survey of part of the population covered by the register or capture–recapture techniques to compare two incomplete registers (for this method to be valid, the two registers must be assumed to have identified independent random samples of the population of true cases).
-
Historic data methods – data from previous years or some other ‘known’ prevalence/incidence rate are used to predict an expected rate of identification.
-
Validity – the extent to which the information on the register agrees with some external source that objectively measures the same variable.
-
Diagnostic criteria method – register staff examine clinical records to assess the proportion of cases that satisfy strict diagnostic criteria.
-
Re-abstracted record method – a sample of case data is re-abstracted from the original source records and compared with register records.
-
Internal consistency method – a computer programme is used to check the register for illegitimate codes and logical inconsistencies.
Raftery et al.99
Raftery et al. 99 proposed criteria for assessing the validity of databases and then applied these criteria to those routine databases identified as having the most potential for HTA. They distinguished two broad dimensions for assessing validity: coverage and accuracy.
Coverage addresses the following concepts:
-
extent of the data set, that is, the completeness of variables or content validity
-
completeness of data collection by variable, that is, the frequency of missing data
-
completeness of recruitment of units and their representativeness, that is, the characteristics of patients or delivery of the health technology may differ in selected units, giving different results, which could reduce the external validity of the findings on effectiveness, equity and diffusion
-
completeness of recruitment of patients or technologies in each setting (there is a risk of selection bias).
Accuracy relates to:
-
the use of explicit definitions of variables, including in the coding system
-
checks on the reproducibility of coding
-
the extent to which data are audited, both internally (internal consistency) and externally (by an external agency or by comparison with another database).
Raftery et al. 99 reported that, although internal consistency checks of databases were common, relatively few had any form of external audit. Issues around coverage and coding have, in general, received little attention.
Confidentiality and data protection
Newton and Garner98
Newton and Garner98 addressed several issues relating to confidentiality and data protection. Patient consent should be obtained unless there is a good reason not to do so; however, there is empirical evidence that the obligation to express consent results in levels of bias that could invalidate epidemiological research. Registers can rarely be operated using fully anonymised data for four main reasons:
-
it is difficult to avoid or check for duplication of cases with anonymised data
-
construction of longitudinal records for linking exposure and outcome is harder with anonymised data
-
validation of a register against external data sets is more difficult
-
data linkage with other unrelated data sets is more difficult.
However, in the UK, the NHS number is beneficial because, being a unique identifier, it facilitates data linkage even with anonymised data.
Registers must comply with current legislation on data protection (e.g. Data Protection Act 1998111 and Human Rights Act 1998112) and take into account NHS policy on confidentiality. Newton and Garner98 noted that, at the time of writing, the legal position regarding the use of register data was contradictory and uncertain. However, since that time, legislation around confidentiality and data protection has been clarified and amended. Current legislation means that there are only two classes of disease register using identifiable data: those for which explicit consent of the patient is required and those for which it is impracticable to obtain consent and which are covered by regulations under Section 251 of the NHS Act 2006. 113
The important question for health researchers is whether or not registers should be used to support both clinical care and research. It is likely that few registers are capable of both addressing service needs and providing the basis for high-quality science. We were particularly interested in whether or not we could use a wounds register for HTA purposes, including to derive treatment effect estimates in the absence of RCT data. Registers have the advantage of greater external validity as there is likely to be less selection in operation than for recruitment of participants into RCTs. Registers are also likely to have greater sample sizes than many RCTs, particularly in wound care where our own work has shown a median sample size of 63. 114 Of course, the major disadvantage of using such observational data sets for estimating comparative effectiveness is the presence of selection bias and confounding. Furthermore, registers based on routine data may lack the detailed information on comorbidities necessary to adjust for important confounders.
Another question is whether or not the benefits of disease registers are worth the costs. There has been little published on the cost of establishing and running a register. Newton and Garner98 have detailed the costs that need to be considered including set-up costs (hardware, software, IT support and training), ongoing costs (cost of full-time administrator, cost of routine procedures associated with data collection and preparation), development costs (upgrading hardware and enhancements to data collection systems) and private costs (incurred by health professionals, patients and carers, mostly in terms of time but also travel, etc.). They suggest that a high-quality register generally requires a dedicated administrator and therefore has a minimum cost of around £30,000 per annum. 98 Most modest regional or district registers cost between £50,000 and £80,000 per year to perform the central functions of the register. Size, in terms of numbers of cases registered, partly determines cost, as does the desired quality of the data. Raftery et al. 99 also discussed the costs of those databases with the greatest potential for HTA. They reported that, in 2005, the total central cost of health databases was > £50M or around 0.1% of the NHS annual spend. Large variations exist in the annual estimated cost of each database, with the top four, which are clinical administrative databases (Prescription Pricing Authority database, Hospital Episode Statistics, Cancer Registers, General Practice Research Database), accounting for > 80% of the total cost. Most clinical registers claim very low costs per record (possibly because of uncosted, local-level inputs). Many clinical registers receive no formal Department of Health or NHS funding. It is not clear whose responsibility it is to fund registers and stability of funding is crucial. Lead times are often long and substantial costs accrue before useful outputs can be demonstrated. Newton and Garner98 recommend that registers used for research purposes should be developed within integrated research programmes to make the most use of them. They also suggest funding such registers as research infrastructure so that they are not competing for funding with research project proposals.
Databases and registers specific to complex wounds
Our searching identified five registers or clinical databases specifically in the area of patients with complex wounds. Reports of these registers are summarised in Table 25. Scrutiny of the relevant publications suggested that this approach may have promise both for research purposes100 and for the quality of clinical care,105 although it was also clear that there was no tried and tested UK-relevant complex wounds register template that we could replicate locally.
| Register reference | Brief summary |
|---|---|
| Margolis 2003,100 Kantor 2000101 | A US-based project using an existing administrative database (primarily used for ‘billing’ purposes) to identify and track diabetic neuropathic foot ulcers. The papers report findings on the development of a prognostic model to identify ulcers that are not likely to heal and the accuracy of the database data compared with clinical records. Although the database has been used for epidemiological research, there is no discussion of the representativeness and generalisability of the findings or how they dealt with any bias in the sample. An internet search did not find any information about ongoing work |
| Golinko 2009102 | A US-based diabetic foot ulcer database. A wound electronic medical record was created [in Microsoft Access® (Microsoft Corporation, Redmond, WA, USA)] to collect information to populate the database. Variables used in the database were determined by treatment guidelines and protocols. Reports from the database were exported into Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA). The database was used for clinical purposes and as an aid in decision-making to identify non-healing ulcers. An internet search did not find any information about ongoing work |
| Coerper 2004103 | Project based in Germany where 10 wound care centres formed a partnership called German Wound Net that collected centralised and prospective computerised documentation on 4175 patients with chronic wounds in a 2-year pilot study. The published report used the data to produce some descriptive statistics and to conduct a multivariate analysis to identify predictors of healing. The authors also identified a number of other potential uses, including comparative audit between centres and to identify potential participants for RCTs. There is no discussion of the ‘completeness’ (therefore representativeness) of the database or of any quality assessment. A search for the German Wound Net website address detailed in the report was unable to find the site or to identify any ongoing work by German Wound Net |
| Taylor 1999104 | A UK (Salford)-based project in which leg ulcer service records were computerised to improve clinical care and audit processes. Records were previously all paper based. The database was a stand alone piece of work and was not integrated with any broader organisational IT strategies. Unable to find any current details about the database |
| RUT (Register Ulcer Treatment)106–108 | A Swedish initiative developed by a GP, Dr Rut Öien, at the Blekinge Wound Centre. Currently contains details of the majority of ulcer patients in Blekinge county (total population 150,000; most patients with ulcers seen at the wound centre) but is in the process of being rolled out nationally. One of a number of national quality registers that are centrally funded. Infrastructure in the form of specialist centres are also funded by government to start, develop, run and support national quality registers and to improve analyses and use of outcome data. The aim of the quality register is quality improvement of clinical care. RUT participants are assessed at initial presentation/assessment and then at follow-up (healing, death) but not at every treatment. Under Swedish law these national quality registers do not require individual patient consent because of public interest, although recent legislation is expected to include an obligation to let individuals know that their data will be processed (not necessarily in writing) and that an individual’s data should be withdrawn if the individual requests |
Discussion
The review of registers highlighted a number of planning, design and methodological considerations that were crucial to informing the development of our register pilot. We attempted to address and incorporate these as far as possible (and appropriate) within the constraints of a pilot study using routine NHS data. However, while conducting the review and planning the pilot study it became apparent that in the UK there does not appear to be much expert guidance available for those establishing registers, unlike for those planning clinical trials. Perhaps provision of this kind of guidance is worth considering as it could help to ensure that all registers adhere to high-quality standards while also preventing unnecessary duplication and developing a body of expertise. A national strategy for the development of registers along with infrastructure was also recommended by Newton and Garner98 in 2002.
When designing our own register for piloting we endeavoured, when possible, to address those characteristics that the review identified as being associated with successful registers, for example having an appropriate multidisciplinary team and ensuring that there were clearly focused aims for the register. We placed great emphasis on avoiding the collection of a large number of data that we would be unlikely to use, however tempting that was. Instead, we used the findings of our review to stimulate the development of a protocol for a register pilot that had very clear aims and objectives, together with consideration of the more long-term objective of being able to use the register for HTA and quality improvement. These decisions determined the type of data that we planned to collect, namely patient health data, information about health technologies and patient outcome data (including health-related quality-of-life data).
Specific methodological considerations raised by the review were also addressed. First, we had to develop and agree a clear case definition that was relevant for both clinical service and research purposes. The Leeds Community Healthcare NHS Trust Tissue Viability Service already had a working definition of complex wounds and this was adopted for the purposes of the register.
Second, we wanted to be able to differentiate unique cases and avoid duplicates and this would be much easier if data were not anonymised. In addition, we wanted to be able to collect health-related quality-of-life data longitudinally (as the lack of these data is a major barrier to HTA and particularly cost-effectiveness modelling). To do this we would require individual patient consent to participate in the pilot study. In making these decisions we were troubled by the inevitable loss of potential participants that a consent requirement would bring, together with possible selection bias. This selection bias (biased selection of people into the register so that people with particular characteristics were more or less likely to be included) will be a challenge for any future epidemiological research. For example, identification of factors prognostic for specific wound outcomes could be biased and misleading if people with particular prognostic profiles were systematically excluded from the register. However, we could not see a way round this problem and felt that selection bias of this nature was less important in terms of quality improvement and HTA. Recruitment of sufficient numbers of participants for clinical effectiveness and cost-effectiveness analyses was important and therefore maximising recruitment (and understanding factors that promoted it) became one of the specific aims of the pilot study.
Finally, the review by Newton and Garner98 encouraged that those developing registers should explicitly consider the evolving NHS information strategy110 and specifically electronic patient records. It was fortuitous that electronic patient records were evolving in the trust around the same time as the register pilot and we were able to link into these developments.
Smart pen pilot study
Background
One of the perennial challenges of conducting research in real-life clinical settings is the need to balance the requirements of research against the need to minimise the research burden on clinicians and patients. Data collection in community nursing tends to be paper based as the nurses are working away from the office, typically in patients’ own homes, and mobile computing for community nurses was almost non-existent at the time of our prevalence survey and the planning for the register pilot. Furthermore, and crucially, clinical notes tend to stay with patients in the home as an essential clinical reference for the next care provider who visits, thus any method of data collection for both clinical and research purposes needs to reflect this. Although we were developing a protocol for the complex wounds register, an opportunity arose to trial smart pens for data collection purposes within the tissue viability service and we grasped this opportunity as one of the original aims of the research programme was to investigate alternative data collection methods.
The smart pen explored in this pilot was a digitalised pen developed by a company called Destiny Wireless plc [see www.destinywireless.com (accessed July 2011)]. The pen uses paper technology along with ‘Anoto functionality’. It enables users to enter data into digitised forms, transmit the data electronically to the company’s secure servers and then have the data relayed back to their own computer system or destination of choice.
The smart pen looks and operates like an ordinary pen. It has an infrared camera in the nib that captures pen strokes as text is being written onto the bespoke form. Data capture forms must be pre-digitised with a unique dot matrix pattern; each pen stroke on the digitised paper is stored as digital data. Once the ‘send’ box on the form is ticked and the pen is ‘docked’, an automatic router sends the data to their destination. We saw a potential advantage of this combined paper and electronic data capture method in that it would meet the needs of the clinicians for a written clinical record and the needs of research for rapid relay of accurate electronic data without an associated burden on health staff. The data are presented as PDF files and as a database that is populated by the information contained on the completed assessment forms. Free text is ‘recognised’ and inserted into the data extracted.
Aims and objectives
Our overall aim was to establish if the smart pen offered a potential solution to the dual needs of clinical record keeping and research data collection within the context of a complex wounds register. We identified the following objectives:
-
to trial the usability of smart pens within the context of clinical wound assessment
-
to trial the usability of an adapted, revised wound assessment form suitable for use with the smart pens
-
to test the feasibility and reliability of the translation of paper records to electronic data and the production of the form in PDF format
-
to test the population of a database with wound assessment data
-
to test the feasibility of extracting data from a smart pen-derived database to populate a wounds register
-
to test whether or not a PDF of the wound assessment form can be attached to a patient record on SystmOne (the electronic patient record system used within Leeds Community Healthcare Trust; TPP, Leeds, UK).
Methods
Preparatory work for the smart pen pilot
In preparation for the trial of the smart pen by the Leeds Tissue Viability Service, it was necessary to revise the existing wound assessment chart into a format that was suitable for use with the smart pen. The form had to be put onto paper that incorporated a unique dot matrix pattern.
The revised and reformatted wound assessment form was developed by Destiny Wireless over a few weeks, with members of the clinical and research teams consulted with regard to its content and layout. The form was finally signed off as fit for purpose and the necessary volume ordered prior to the beginning of the pilot period.
The required software for the smart pen was downloaded onto the desktop computers used by participating clinicians. Training in the principles of the smart pen and the process of information transfer was delivered by Destiny Wireless on 23 November 2010 to members of the tissue viability service and a member of the patient and public involvement team. Using fictional data, staff filled out wound assessment forms, logged onto the smart pen programme and practised docking their smart pen so that they were conversant with the process. Any technical problems were ascertained and dealt with prior to commencement of the trial.
During the training session it was noted that the ink provided in the smart pens was blue and would therefore not meet the documentation standard of Leeds Community Healthcare NHS Trust. Supplies of black refills were forwarded by post from Destiny Wireless in the days following the training session. The smart pen was trialled between 29 November 2010 and 13 May 2011, when smart pen use was discontinued.
During the trial period, PDF files of the submitted wound assessment forms together with spreadsheets populated with the data were sent by Destiny Wireless to the senior data analyst in the Leeds community informatics team via a secure SystmOne NHS e-mail account. The senior data analyst managed the returns of data and held them on a secure area of the organisation’s network. Each Monday during the pilot period he received the previous week’s data from Destiny Wireless. The final electronic versions of the forms were received on 23 May 2011.
The senior data analyst checked the data for any anomalies; any found were fed back to the clinical team who attempted to improve the quality and consistency of data collection.
Results
Between 29 November 2010 and 23 May 2011 the senior data analyst received 20 data extracts in spreadsheet format and 437 forms in PDF format.
An initial problem that was encountered very early was that patients’ dates of birth were not represented correctly if they were born before the year 2000. All dates of birth were interpreted and represented as 20**, even if patients were born in 19**. This was reported to Destiny Wireless and was rectified within a few weeks of the start of the trial.
Further issues
Another fundamental problem was the ability of the system to correctly interpret clinicians’ handwriting. Each clinician has a different handwriting style; however, the smart pen system was frequently unable to accurately interpret the free text. In an attempt to improve handwriting recognition, clinicians were advised to use lower case characters when completing the wound assessment form. This impacted on the time taken to complete the forms as practitioners were required to pay more attention to the presentation and legibility of the written characters and to spend more time addressing the presentation of their handwriting.
There was frequently illogical output into the spreadsheet because the smart pen engine looks for words in a dictionary and words could not be recognised if they were not in the dictionary; moreover, the system could not interpret punctuation.
Text could also not be interpreted properly if it was not placed wholly within a box, but, unfortunately, busy staff do not always write neatly in boxes.
The design of the smart pen wound assessment form required that it became a four-sided booklet-type form that recorded data from only one wound episode, whereas the assessment form had previously been a double-sided form that allowed the recording of multiple wounds and/or successive wound reassessments. The adapted form did not really serve the clinical purpose: there was no space to record multiple wounds and a consequent requirement for multiple forms. Clinicians felt that the layout of the form was ‘unwieldy.’ As a result of the size of the form, and the increased number of forms required for each patient, paper notes became much bulkier, which began to impact on storage facilities and would have required further consideration in the future.
Direct population of patients’ records on SystmOne by the smart pen system would have been the ideal but, unfortunately, this was not achieved during the trial phase.
Conclusions
Smart pen technology was trialled by clinicians within the Leeds Community Healthcare NHS Trust Tissue Viability Service to test the feasibility of its future use for electronic patient records as well as to enter data into a future complex wounds register. During a 4-month trial period, 437 forms were completed and the data entered into a database. Although, ostensibly, smart pen data entry would meet the needs for a clinical patient record that would remain with the patient and the timely return of clinical data for research purposes, in reality the smart pen system did not meet either clinical or research needs and we decided against its further use and it was therefore not used for data collection in this programme.
Register pilot study
Background
Our review did not identify any recent UK-based registers or clinical databases relating to complex wounds. Review work undertaken in relation to UK-based registers and clinical databases also failed to identify any registers of complex wounds. 98,99
On embarking on this work we saw a number of potential advantages to establishing a disease register for complex wounds. Such a register would be able to provide basic information, currently lacking, concerning the numbers of different types of wound, typical treatments used in the NHS, times to healing, costs of disease and ultimately prognostic information. This type of information is essential for cost-effectiveness modelling (to estimate the cost-effectiveness of treatments in use now) as well as for prioritising future research. We also planned to cautiously investigate the potential for a wounds register to (ultimately) estimate the clinical effectiveness and cost-effectiveness of wound treatments. However, we had no idea whether or not a wounds register would be feasible (e.g. whether or not patients and staff would participate) and what the barriers to establishing one might be. We therefore embarked on a pilot study to investigate the feasibility of a register of people with complex wounds in a large community trust.
Aims and objectives
The overall aim of the pilot study was to assess the feasibility of establishing and populating a complex wounds register. We saw the register as collecting data suitable for research and quality improvement purposes. Aspects of a register that are fundamental to its future potential and which therefore need to be addressed in a feasibility study include recruitment, consent, technical issues and the accuracy and completeness of the data. 98
This pilot study aimed to address the following research questions:
-
Will patients consent to participate in a register of complex wounds and, if not, why not?
-
Can data from consenting patients be accurately identified within routinely collected data?
-
Are participants willing to provide additional self-reported health-state data [European Quality of Life-5 Dimensions (EQ-5D)] and can these data be correctly linked with their routinely collected data?
-
Do health-care professionals collect the data that we need to populate a register? If they do not, what are the reasons for this?
-
How well do health-care professionals collect the data?
-
Can data for the register be pulled out of the NHS databank and transferred safely to the University of York for analysis?
-
How much additional activity does the register generate?
-
What can we do with the data that we have collected?
-
Do the data have value that could pay for the register in the future?
Methods
A pilot study for a ‘register’ of patients receiving care for a complex wound was undertaken, populated by prospective, routinely collected NHS data. Supplementary to the NHS data, self-reported health-state data (EQ-5D) were collected directly from register participants. People were eligible for inclusion in the register if they had a complex wound, that is, one that involved superficial, partial or full-thickness skin loss and was healing by secondary intention (typically pressure ulcers, leg ulcers and dehisced surgical wounds).
The register pilot was to involve all relevant parts of Leeds Community Healthcare NHS Trust, that is, tissue viability, district nursing, general practice-based practice nursing and podiatry.
Routine data collection in the NHS
Data collection was embedded in the routine data systems used by Leeds Community Healthcare NHS Trust. Historically, administration, record keeping and patient case notes in Leeds Community Healthcare NHS Trust were largely paper based with limited use of IT (currently SystmOne software) to store and retrieve detailed clinical information. Paper-based patient case notes were held in patients’ homes, clinics or relevant administrative bases.
SystmOne
Leeds Community Healthcare NHS Trust uses SystmOne software to electronically record details of all patients and basic information about the care that they are receiving, primarily to provide information for commissioners and fulfil contractual obligations. SystmOne had not hitherto been used for the capture of detailed clinical information or as a replacement for paper case notes. However, at the time of study, the method of capture and storage of routine data within Leeds Community Healthcare NHS Trust was in a process of change. SystmOne was being increasingly utilised to manage and store clinical information. Trust services that are predominantly clinic based, such as podiatry, had already begun to use SystmOne to store patient clinical information.
Mobile working practices
Mobile working practices for trust services in which care is provided largely in patients’ homes were also being enhanced and phased in gradually across the trust, allowing increased utilisation of SystmOne. The tissue viability service began to use mobile technology to capture all patient information at this time. Since the introduction of new mobile working practices, the tissue viability service was operating a largely paper-free clinical and administrative record process within SystmOne. Community matrons and the intermediate care team were to be the next wave of services to implement the changes in mobile working.
The change in mobile working practices was made feasible through the use of semirugged computers (ToughBook®, Panasonic). These laptops operate as a desktop, with the ability to withstand the ‘harsh’ environment of mobile working (according to the manufacturer they are capable of withstanding a 30-inch drop). Health-care staff log on via a secure internet connection and input clinical information directly into SystmOne, instead of using paper-based case notes.
Wound assessment form
Data variables required to populate the register consisted of a subset of all of the information routinely collected by health-care professionals. Clinical information about the wound was collected on the wound assessment form. Until recently, the wound assessment form was a paper form completed by the health-care professional, which remained with the patient case notes. However, the advent of SystmOne led to digitisation of the wound assessment form, although the paper form was still available for those trust staff not currently using SystmOne. Tissue viability service staff worked closely with trust IT personnel to develop and launch the wound assessment form in SystmOne for clinical use, at the same time ensuring that the data were suitable to populate a register. The paper version of the wound assessment form was also updated to reflect these changes. To minimise disruption to established practice, the definitions used by the service before the pilot were upheld. Options for completion on most variables are standardised (in drop-down boxes on SystmOne or tick boxes on the paper copy). A copy of the wound assessment form can be found in Appendix 4.
Self-reported health state
Leeds Community Healthcare NHS Trust does not currently routinely collect patient self-reported health-state data; however, collection of these data in the register was required for future cost-effectiveness analysis. We planned to collect health-state data directly from consenting register participants using the EQ-5D questionnaire.
Population
The study population consisted of Leeds residents who were receiving care for a complex wound from Leeds Community Healthcare NHS Trust and/or general practices.
Ethics approval
The study was reviewed and approved by the National Research Ethics Service Committee Yorkshire & The Humber – Bradford (reference 12/YH/0353), the Department of Health Sciences Research Governance Committee, the University of York and Leeds Community Healthcare NHS Trust (reference P/0138). Copies of approvals and amendments are available on request.
Recruitment and consent
The decision for patients to consent to share their data in this study was entirely voluntary. This was made clear in the information provided to them. It was also made clear that their decision would not affect the treatment that they received and that they could withdraw their consent at any time without having to state a reason for doing so. We also recognised that consent is not a ‘one-off’ action but is an ongoing process; in recognition of this, consent would be affirmed every 12 months or each time a patient entered care services when a complex wound developed (whichever came first).
Informed consent to participate in the feasibility study was sought from patients who presented to participating services with a complex wound (incident cases). In addition to incident cases, when recruitment from services commenced, all existing patients with a complex wound were also approached to participate. The recruitment and consent process for existing patients was the same as for incident cases and the process is detailed in the subsequent paragraphs.
An invitation to participate in the study was sent by mail to patients’ homes. This was generated automatically by service administration teams when patients were registered with the service to receive treatment. The mail-out consisted of an introductory letter from the tissue viability service clinical team lead, an information sheet, a consent form and a prepaid return envelope. The information sheet contained details about the study including why it was being undertaken, what the data would be used for, implications for patients and contact details should they require further information or have any questions. Further information was available from the wounds data co-ordinator who was based with the tissue viability service in Leeds Community Healthcare NHS Trust. For patients who were themselves unable to consent, we sought the advice of a consultee (usually a partner or a carer). We asked the consultee whether they thought that the individual would be likely to have objected to taking part.
Patients who were willing to participate were requested to complete, sign and return the consent form in the prepaid envelope to the data co-ordinator (or give the form to the health-care professional caring for the wound, who returned it to the data co-ordinator). A copy of the signed consent form was forwarded to the appropriate service administration team for storage with the patient records (paper or electronic). Administrators were asked to indicate in patients’ individual health-care record within SystmOne that they had consented to participate in the register pilot study. Individual patient consent was indicated using a tick box (‘yes’ or ‘no’). Individual patient NHS numbers were used as unique identifiers and were recorded (by a member of the administration team) on each signed consent form returned to the data controller. A reminder letter was sent by administrative teams to potential participants 1 month after the initial invitation if there had been no response. No further reminders were sent.
For those patients who did not wish to participate, we sought their reasons for refusal by adding to the consent form a free-text box that they could complete, with the form returned to the data co-ordinator in a prepaid envelope. They also had the opportunity to telephone or e-mail the data co-ordinator to discuss their reasons for declining to participate.
The recruitment and consent process varied slightly depending on the clinical service configuration.
Data collection
Routinely collected NHS data
This pilot study aimed to capture routine data from a variety of services. Because of differences in the method of routine data capture, as outlined in the previous section, the data collection process for services differed. For tissue viability and podiatry (whether clinic based or ‘mobile’), clinical information about the wound was input directly into SystmOne on the wound assessment form at each wound consultation and stored in the trust ‘databank’ (FRANK). District nurses and practice nurses continued to complete a paper version of the wound assessment form. Forms were printed specifically for the pilot study to include a ‘carbon copy’ (or multiple copies). The original form remained with the patient notes and at each wound consultation a carbon copy was returned to the data co-ordinator. Paper-based data from district nurses were then entered into SystmOne. Data from practice nurses were entered into a separate spreadsheet. Data were collected from participants for the duration of the study pilot or until wound healing or loss to follow-up.
The SystmOne and paper-based data collection processes are detailed in Figures 3 and 4, respectively.
FIGURE 3.
Electronic data collection process using SystmOne.
FIGURE 4.
Paper-based data collection process. DN, district nurse; PN, practice nurse; WAF, wound assessment form.
Self-reported health-state data
The EQ-5D form along with a covering letter were posted to the home address of register participants four times per year. A prepaid envelope was included for return of the completed form to the University of York.
Data variables
Routinely collected NHS data
Table 26 details the NHS data variables that were used to populate the register (some variables are still under development as the wound assessment form continues to be developed for clinical use in SystmOne).
| Variable | Available response choices (where appropriate and currently available) |
|---|---|
| Name | |
| Address | |
| Postcode | |
| Date of birth | dd/mm/yyyy |
| Sex | Male/female |
| NHS number | |
| Ethnicity | |
| Risk factors | |
| Chemotherapy/radiotherapy | Yes |
| Continence/moisture issues | Yes |
| Diabetes | Yes |
| Elderly | Yes |
| Immunosuppression | Yes |
| Infection | Yes |
| Mobility | Yes |
| Nutrition | Yes |
| Peripheral neuropathy | Yes |
| Poor blood supply | Yes |
| Poor oxygen supply to wound | Yes |
| Recent acute illness/surgery | Yes |
| Steroids | Yes |
| Smoker | Yes |
| BMI | Under development |
| Wound(s) detail | |
| Wound type | Leg ulcer (arterial, venous, mixed), pressure ulcer, diabetic foot ulcer, non-diabetic foot ulcer, dehisced surgical wound, pilonidal sinus, abscess, fungating wound, traumatic wound, burn, other |
| Wound size | Maximum length, maximum width (mm) |
| ABPI | < 0.4, 0.61–0.8, 0.81–1.0, 1.01–1.2, ≥ 1.21, not applicable |
| Start of episode (patient-reported estimate) | Under development |
| Start of treatment | dd/mm/yyyy |
| Treatment | |
| Referral source | |
| Date of wound-related consultation | dd/mm/yyyy |
| Location of care | |
| Consultation by whom | Name of health-care professional |
| Primary wound dressing | Applied (yes/no), name of dressing (free text for trade name) |
| Secondary wound dressing | Applied (yes/no), name of dressing (free text for trade name) |
| Bandaging | Applied (yes/no), name of bandaging (free text for trade name) |
| Hosiery | Applied (yes/no), name of hosiery (free text for trade name) |
| Other current treatments/equipment | Under development |
| Key events | |
| Wound healed | Yes/no, dd/mm/yyyy |
| Death | dd/mm/yyyy |
| Wound-related admission to hospital – admission and discharge dates plus reason | Under development |
| Wound-related GP visit | Under development |
| Wound-related specialist appointment | Under development |
| Amputation | Under development |
Self-reported health-state data
The EQ-5D is a standardised instrument that includes the following five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. 115–117 Each dimension has three levels: no problems, some problems and extreme problems. The respondent is asked to indicate his/her health state by ticking (or placing a cross) in the box against the most appropriate statement in each of the five dimensions.
Data management
All register data referred to hereafter were considered ‘sensitive’ data; these data included personal data that could be used to identify individuals.
Data required for the wounds register were taken from individual health-care records. The retrieval of such data could occur only when patients had consented to the sharing of their data. Individual patient consent was indicated using a tick box (‘yes’ or ‘no’). This tick box was located on a general consenting questionnaire used by health-care practitioners and formed a part of the individual health-care record. The health-care worker or a member of the administrative staff within the trust completed this tick box on receipt of a signed consent form.
Data were drawn from the NHS databank by a senior data analyst (in the Community Informatics Department of Leeds Community Healthcare NHS Trust) and provided by the Community Informatics Department to the University of York every 3 months. The data analyst checked for and removed any duplicate entries.
All data drawn from the NHS databank were transferred to the University of York via a secure NHS e-mail account hosted at both institutions. EQ-5D data returned by participants to the University of York were manually entered into a Microsoft Excel spreadsheet.
All electronic and manual data were securely stored with restricted access.
Third-party data sharing policy
Once patient data were provided to the University of York by Leeds Community Healthcare NHS Trust, the trust was the data controller and the university became the data processor. A third-party data sharing agreement was drafted to ensure safe and effective transfer of shared information.
As the owner of the wounds register, the university became a data controller. A third-party data sharing policy ensured that data were shared with only (1) those organisations that had a legitimate right to view and process that data, having signed up to a third-party data sharing agreement (i.e. Leeds Community Healthcare NHS Trust) or (2) the individuals to whom the data related.
Data analysis
The analysis sought to answer the following research questions.
Will patients consent to participate in a register of complex wounds and, if not, why not?
Patients with a complex wound were identified systematically by administrative staff and mailed information about the register. When patients declined to participate we attempted to establish reasons for non-consent; an opportunity to provide feedback on their reasons for non-consent was included on the consent form and the form could be returned to the data co-ordinator in a prepaid envelope. Alternatively, patients could telephone or e-mail the data co-ordinator with their feedback.
Can data from consenting patients be accurately identified within routinely collected data?
It was important that patients who consented to participate were correctly identified on the routine data system (SystmOne) to ensure that (1) their data could be ‘extracted’ from the NHS databank and (2) no patient who had ‘not consented’ was incorrectly identified as ‘consented’. The signed paper consent forms containing patient information were cross-referenced with information held in SystmOne and any discrepancies reported.
Are participants willing to provide additional self-reported health-state data and can these data be correctly linked with their routinely collected data?
We report the proportion (%) of register participants who returned the EQ-5D questionnaire at each time point. We also assessed the number of participants who could be correctly matched with the information from the databank.
Do health-care professionals collect the data that we need to populate a register? If they do not, what are the reasons for this?
To assess whether health-care professionals collect the data that we need to populate a register we estimated the number (%) of missing data for each register variable. We also investigated differences in the completion rate between the two methods of data collection (electronic data input into SystmOne and paper-based data collection). Feedback sessions were held with the different staff groups to ascertain the barriers and facilitators involved in collecting the data. The feedback sessions were facilitated by a member of the research team (KL, based in the NHS) using a series of semistructured questions. Responses were noted by the facilitator during the sessions. Topic headings were constructed (which were informed by the questions) and populated with themes that were identified from the responses.
How well do health-care professionals collect the data?
To assess the ‘accuracy’ of data, variables were checked for logical inconsistencies and inaccurate codes. For paper-based data, a random sample was selected and cross-referenced with case notes and discrepancies noted. Accuracy of data input from paper copy to register was also cross-referenced in a random sample and discrepancies noted.
Can data for the register be pulled out of the NHS databank and transferred safely to the University of York?
We assessed the feasibility of the trust IT department generating data files for register participants from their databank (FRANK). We also assessed the efficiency and safety of data transfer.
How much additional activity does the register generate?
We estimated the additional activities associated with running a prospective register, including both staff time and resources and materials (disposable) required, for example for printing paper copies of the wound assessment form.
What can we do with the data that we have collected?
We summarise the characteristics of the register population, their wounds and their care and investigate variation based on sex, postcode, etc. The potential for analyses that utilise the longitudinal nature of the data is considered. The rate of healing is explored and the potential to investigate the relative effectiveness of different treatments for healing through propensity scoring and instrumental variable methods is considered.
Do the data have value that could pay for the register in the future?
We originally intended to explore the potential for the register to become self-financing through revenue generated by data access, for example by the pharmaceutical industry. Unfortunately, we did not have sufficient time to complete this work.
Results
Anonymised data from the wound assessment form (pre-pilot data from the tissue viability service only)
The major finding from the analysis of the pre-pilot study data (anonymised) was the difficulty in tracking longitudinal wound data for individuals. This was particularly a problem when an individual had more than one wound of the same type as it was not always possible to differentiate between them and hence follow the trajectory of each wound. For individuals with only one wound similar problems were found in some cases as it was not always clear whether the wound being assessed was the same one as at the previous consultation or was a new wound. The large amount of uncertainty surrounding wound tracking meant that longitudinal methods could not be applied without assumptions relating to wound identity being made.
Over half of the individuals (58.5%) had only one consultation recorded because of the specialist (assessment-focused) service provided by the tissue viability service.
Recruitment
Recruitment took place between September 2012 and September 2013. Recruitment of participants began initially in the Leeds Community Healthcare NHS Trust Tissue Viability Service (from 24 September 2012), followed by recruitment by district nurses (from 21 December 2012), the podiatry service (from 26 February 2013) and practice nurses (from 15 June 2013). Figures for recruitment overall and by service are provided in Table 27. No information packs were sent out to practice nurse patients.
| Recruitment activity | Tissue viability service | District nursing | Podiatry | Total |
|---|---|---|---|---|
| Information packs sent out | 586 | 140 | 7 | 733 |
| Consent forms received | 170 | 25 | 0 | 195 |
| Recruitment rate (%) | 29.0 | 17.9 | 0 | 26.6 |
Reasons for declining to participate
Thirty-nine people (5% of all of the information packs sent out) who did not wish to or who were unable to participate detailed the reasons for their decision. The most commonly cited reasons were:
-
Other physical health problems and illness (e.g. ‘I have read the literature and appreciate your objectives. During the last 2/3 years of cellulitis I have had angina problems, heart damage whilst coping with diabetes, warfarin, insulin and been inundated by research studies, questionnaires etc. I am not interested in further answers on the subject of my “complex wounds” which continue to need bandaging twice weekly and I have appointments to keep with the surgical teams which oversee the treatment’).
-
Advanced age (e.g. ‘I am 93 years old, completely deaf and at the end of my life, everything is worn out, sorry’).
-
Person had dementia (e.g. ‘My mother is in her 98th year with dementia and would not understand anything of this project’).
-
Other reasons given were:
-
just did not want to
-
in hospital
-
person had recently died
-
the wound had healed
-
taking part in other research studies
-
felt unable to make a decision on behalf of the person (who had dementia).
-
Identifying participants on SystmOne
A total of 196 register participants were identified from a combination of the paper consent forms and the extracted register data; 183 (93.4% of 196) had a signed consent form and their data were extracted from the register, and 12 (6.1% of 196) had a signed consent form but their data were not included in the register. Of the 12 people with paper consent forms but no register data, four had not been marked as consented on SystmOne and eight had been marked as consented. One person was included in the register data but did not have a signed consent form (0.5%) and was excluded from further analysis.
The data set
A total of 195 individuals had given consent to being included in the register. Clinical data were collected for 183 of 195 individuals and in total there were 652 clinical entries. There were 12 individuals for whom no clinical data were available; five of these individuals did not have a consultation at which a wound assessment form was completed within the study period and data on the remaining seven were not downloaded (reason unclear, potentially an error during the data extraction process).
The number of entries per patient for those with clinical data ranged from one to 22, with a mean of 3.6 (SD 3.56) and a median of two entries. Approximately one-quarter of patients (47/183, 25.7%) had only one entry in the register and a further quarter (25.7%) had two entries (Figure 5).
FIGURE 5.
Number of register entries per patient.

Potential duplicates with respect to clinical entries were explored based on wound type, date of consultation, wound size and wound identifier. It was estimated that 552 of the 652 clinical entries were unique, 97 entries were repeated once and one entry was potentially duplicated three times. As a wound identifier was not available for all entries it was difficult to be certain which of the potential duplicates were true duplicates. When we were confident that there was a true duplicate entry (because a wound identifier and date of consultation matched), the duplicate was deleted. There were 17 such entries, each of which was duplicated once, leaving 635 clinical entries.
Completeness
It was difficult to calculate a duration of follow-up for the participants in the pilot because when some participants were referred for further wound care to a service not involved in the pilot then data collection ceased or was interrupted. Given that wound care can be delivered by a range of services such as district nursing, podiatry and tissue viability, this issue underlines the need for a system that can be used across services, that is, full data collection coverage. Without this there is a risk, as may have occurred here, of gaps in the data or complete cessation of data collection.
Table 28 shows the completion rate (in relation to the 635 clinical entries) for each variable when data were submitted.
| Variable | Completion rate (%) |
|---|---|
| Wound identifiera | 37.5 |
| ABPIb | 50.8 |
| Name of hosierya | 68.3 |
| Ethnicity | 81.3 |
| Wound maximum width | 87.1 |
| Wound maximum length | 87.7 |
| Wound healed | 89.1 |
| Type of wound | 97.5 |
| Date of wound-related consultation | 98.0 |
| Name of bandaginga | 98.4 |
| Name of secondary dressinga | 99.5 |
| Postcode | 99.7 |
| Name of primary dressinga | 99.8 |
| Consent date | 100.0 |
| NHS number | 100.0 |
| Date of birth | 100.0 |
| Sex | 100.0 |
| Location of care | 100.0 |
| Referral source | 100.0 |
| Event completed by | 100.0 |
| Chemotherapy/radiotherapyc | 100.0 |
| Incontinencec | 100.0 |
| Diabetesc | 100.0 |
| Elderlyc | 100.0 |
| Immunosuppressionc | 100.0 |
| Infectionc | 100.0 |
| Mobilityc | 100.0 |
| Nutritionc | 100.0 |
| Peripheral neuropathyc | 100.0 |
| Poor blood supplyc | 100.0 |
| Poor oxygen supply to woundc | 100.0 |
| Recent acute illness/surgeryc | 100.0 |
| Smoking statusc | 100.0 |
| Steroids or NSAIDsc | 100.0 |
| Primary dressing usedc | 100.0 |
| Secondary dressing usedc | 100.0 |
| Bandage usedc | 100.0 |
| Hosiery usedc | 100.0 |
| Start of treatment date | 100.0 |
| Questionnaire start date | 100.0 |
| Wound healed datea | 100.0 |
Section B of the wound assessment form (see Appendix 4) asks the health professional completing the form to select all appropriate risk factors from a list, meaning that a negative response was reported as ‘null’. Therefore, it was not possible to distinguish between missing data and a negative response, and the assumption that a null response implies a negative response has been made, meaning that the completion rate is classed as 100%. This was also the case in relation to the use of a primary or secondary dressing, bandaging or hosiery and for wound healed status. Variables that this applies to are indicated using a footnote in Table 28.
The completion rate was lowest in relation to wound identifier, which was requested for the 499 entries completed after the first download of data. An entry was provided in 187 of these cases (37.5%).
There was also a low completion rate for the ABPI; a measure was available for 171 of the 335 entries relating to a foot or leg ulcer (51.0%). The ABPI was reported for a larger proportion of SystmOne entries relating to foot or leg ulcers (53.4%, 156/292) than for paper wound assessment form entries (34.9%, 15/43) and was recorded for 31 of the 300 wounds that were neither a foot nor a leg ulcer (10.3%).
Ethnicity data were missing for 43 individuals (22.1%) and a total of 119 entries (18.7%). Completion was slightly higher for paper forms (87.5%) than for SystmOne entry (80.0%). Postcode data were missing for two entries in relation to one individual.
An entry for the type of wound variable was missing for 28 of the 635 clinical entries; completion was slightly higher on SystmOne than on the paper wound assessment form (96.6% vs. 90.3%). When wound type was listed as ‘other’, details were required; there were 38 such wounds and further details were provided for 30 (78.9%).
A numerical value (regardless of correct units) was recorded for 87.7% and 87.1% of entries in relation to wound length and wound width, respectively; completion rates were similar between the different methods of collection.
The name of the primary or secondary dressing was provided in a large proportion of relevant cases. Completion was higher for name of bandaging (when relevant) for SystmOne entries than for paper entries (99.5% vs. 94.3%). Name of hosiery was provided in fewer cases (68.3%), with completion higher on paper wound assessment forms than when data were inputted directly into SystmOne (80.0% vs. 66.7%).
Date of wound-related consultation was missing for 13 entries (2.0%) in relation to three individuals (1.5%), with data missing from a similar proportion of paper and electronic entries.
Accuracy
There were minor inconsistencies in how the NHS systems that we drew data from reported ethnicity. For example, white British was reported as both ‘white British’ and ‘white British – ethnic category 2001’. For 10 entries, ‘ethnic category – 2001 census’ was reported, without specification of ethnic category.
In relation to risk factors, most ‘other’ risk factors were additional to the prespecified list; however, one entry listed chemotherapy under this heading and another detailed recent surgery despite there being specific fields for this information. Another entry reported a wound type in this field.
There was a lot of inconsistency in the unit of measurement used for wound length and width despite dimensions in millimetres being requested. Some entries were provided in centimetres and this was stated, whereas for other entries the units were not specified and could have been either millimetres or centimetres. A handful of entries reported wound dimensions using free text rather than as a numeric value. In addition, some wounds that were reportedly healed had wound dimension recorded within the same entry.
Table 29 shows the numbers of entries in which a specific name was provided for primary and secondary dressings, bandaging and hosiery despite a negative response being recorded for these items. This highlights the difficulty in differentiating between missing and negative data when a response is either ‘yes’ or ‘null’. Just under one-third of entries had actually named a specific dressing (17 entries, 30.4%) when a primary dressing was not reported. Of these 17 entries, 13 were judged to be primary dressings. For secondary dressings, a name of dressing was reported for 18 entries; of these 10 were secondary dressings and five were dressings that are usually classed as primary dressings. A specific type of bandage was recorded for 23 entries when a negative response was given with respect to presence and all of these were classed as bandaging. Both entries specifying a hosiery type when a null response was provided were deemed to be hosiery.
| Treatment | Entries in which treatment indicated to be ‘not present’, n (%) (n = 635) | Name of item recorded when treatment ‘not present’, n (%) |
|---|---|---|
| Primary dressing | 56 (8.8) | 17 (30.4) |
| Secondary dressing | 426 (67.1) | 18 (4.2) |
| Bandage | 382 (60.2) | 23 (6.0) |
| Hosiery | 553 (87.1) | 2 (0.4) |
All start of treatment dates seem reasonable; however, for 79 entries the questionnaire start date was specified as being before the start of treatment date. Wound consultation was also reported as taking place before the start of treatment for 70 entries.
Date of death was recorded for six individuals; two of these dates were after the study end date (and after the date of analysis) and were assumed to be errors. A further two individuals consented and returned an EQ-5D form after their supposed date of death.
Paper wound assessment forms for a randomly selected one-third (n = 9) of the individuals seen by district nurses were checked and discrepancies between the paper form and the data entered onto SystmOne were noted. There was a total of 15 forms for those nine individuals; notes were unavailable for one patient who was in hospital at the time of the check and no paper questionnaires were available for another participant. No discrepancies relating to study variables were found for 14 of 15 wound assessment forms (93.3%). The only discrepancy was that the name of the primary dressing from one form had not been transferred to SystmOne.
The register population
Around half of register patients were male (51.4.8%) and just over 70% were white British or British/mixed British. The minimum age within the sample was 21 years and the maximum age was 99 years. The mean age was 72.4 years (SD 14.51 years) and the median age was 75 years. Over 70% of individuals within the sample were aged > 70 years. No demographic data were available for patients with EQ-5D data only.
Table 30 presents the proportions of register patients with certain risk factors at baseline. Just over one-quarter of the register population had diabetes reported at baseline and one-fifth of the first entries in the register for each individual reported an infection.
| Risk factor | Frequency (%) |
|---|---|
| Continence issues | 14 (7.7) |
| Diabetes | 49 (26.8) |
| Infection | 37 (20.2) |
| Nutrition | 31 (16.9) |
| Peripheral neuropathy | 14 (7.7) |
| Poor blood supply | 25 (13.7) |
| Smoker | 11 (6.0) |
Table 31 shows the frequency of different types of wound as reported at the first visit. In total, 271 wounds were reported at the first visit in 183 patients. Tracking of wounds through the study was difficult because of issues related to the wound identifier when a patient had multiple wounds.
| Type of wound | Frequency |
|---|---|
| Venous leg ulcer | 81 |
| Pressure ulcer | 52 |
| Dehisced surgical wound | 34 |
| Non-diabetic foot ulcer | 26 |
| Arterial/venous leg ulcer | 25 |
| Other wound | 24 |
| Arterial leg ulcer | 8 |
| Diabetic foot ulcer | 8 |
| Traumatic wound | 7 |
| Abscess | 2 |
| Missing | 2 |
| Burn | 1 |
| Pilonidal sinus | 1 |
| Other surgical | 0 |
| Fungating carcinoma | 0 |
Wound length and width are not summarised because of lack of clarity regarding units of measurement in many cases. Because of this and the difficulties in tracking wounds through time, longitudinal methods were also not applied.
Referral source is summarised in Table 32 for each entry with clinical data in the register. Most individuals had one referral source but eight individuals had entries after referrals from two services and one patient was referred by three services. One-third of entries were the result of a referral from district nursing services and a further one-quarter were the result of a referral from general practice.
| Referral source | North-west, n (%) | North-east, n (%) | East, n (%) | South, n (%) | West, n (%) | Out of area/missing, n (%) | Total, n (%) |
|---|---|---|---|---|---|---|---|
| District nursing service | 61 (53.0) | 28 (30.4) | 41 (31.3) | 63 (29.3) | 12 (15.4) | 4 (100.0) | 209 (32.9) |
| General practice | 26 (22.6) | 31 (33.7) | 33 (25.2) | 61 (28.4) | 7 (9.0) | 0 (0.0) | 158 (24.9) |
| Acute trust | 23 (20.0) | 15 (16.3) | 18 (13.7) | 42 (19.5) | 28 (35.9) | 0 (0.0) | 126 (19.8) |
| Private sector | 5 (4.3) | 1 (1.1) | 4 (3.1) | 27 (12.6) | 8 (10.3) | 0 (0.0) | 45 (7.1) |
| Tissue viability service | 0 (0.0) | 2 (2.2) | 18 (13.7) | 4 (1.9) | 2 (2.6) | 0 (0.0) | 26 (4.1) |
| Podiatry service | 0 (0.0) | 10 (10.9) | 7 (5.3) | 0 (0.0) | 8 (10.3) | 0 (0.0) | 25 (3.9) |
| Intermediate care service | 0 (0.0) | 1 (1.1) | 1 (0.8) | 7 (3.3) | 6 (7.7) | 0 (0.0) | 15 (2.4) |
| Residential care service | 0 (0.0) | 0 (0.0) | 7 (5.3) | 1 (0.5) | 4 (5.1) | 0 (0.0) | 12 (1.9) |
| Self-referral | 0 (0.0) | 4 (4.3) | 0 (0.0) | 2 (0.9) | 3 (3.8) | 0 (0.0) | 9 (1.4) |
| Mental health trust | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.9) | 0 (0.0) | 0 (0.0) | 4 (0.6) |
| Community matron | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.4) | 0 (0.0) | 0 (0.0) | 3 (0.5) |
| Hospice | 0 (0.0) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Neurology service | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Total | 115 (100.0) | 92 (100.0) | 131 (100.0) | 215 (100.0) | 78 (100.0) | 4 (100.0) | 635 (100.0) |
Table 33 shows the location of care for patients by area; around two-thirds of consultations took place within the home and the next most common locations were a nursing/residential home or a health centre, accounting for approximately 10% of entries each.
| Location of care | North-west, n (%) | North-east, n (%) | East, n (%) | South, n (%) | West, n (%) | Out of area/missing, n (%) | Total, n (%) |
|---|---|---|---|---|---|---|---|
| Home | 80 (69.6) | 56 (60.9) | 80 (61.1) | 168 (78.1) | 40 (51.3) | 2 (50.0) | 426 (67.1) |
| Nursing/residential home | 7 (6.1) | 2 (2.2) | 12 (9.2) | 28 (13.0) | 12 (15.4) | 0 (0.0) | 61 (9.6) |
| Health centre | 11 (9.6) | 18 (19.6) | 10 (7.6) | 11 (5.1) | 9 (11.5) | 2 (50.0) | 61 (9.6) |
| General practice | 17 (14.8) | 6 (6.5) | 19 (14.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 43 (6.8) |
| Clinic | 0 (0.0) | 10 (10.9) | 7 (5.3) | 0 (0.0) | 17 (21.8) | 0 (0.0) | 34 (5.4) |
| Mental health inpatient | 0 (0.0) | 0 (0.0) | 1 (0.8) | 4 (1.9) | 0 (0.0) | 0 (0.0) | 5 (0.8) |
| South Leeds Independence Centre | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.4) | 0 (0.0) | 0 (0.0) | 3 (0.5) |
| Hospice | 0 (0.0) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| Total | 115 (100.0) | 92 (100.0) | 131 (100.0) | 215 (100.0) | 78 (100.0) | 4 (100.0) | 635 (100.0) |
Face-to-face contact data were available relating to 100 individuals from the date of their consent until July 2013. The number of face-to-face consultations per month ranged from 0 to 30, with a mean of 1.8 (SD 4.93).
Data analyses (including feasibility of propensity scoring and instrumental variables analyses)
There were 31 entries that reported a healed wound relating to 24 individuals. It is difficult to say how many of these entries related to unique wounds because of the deficiencies in the wound identifier, the fact that the type of wound was missing for many of these entries and the issue of possible missed healing events if data collection ceased when a service not participating in the pilot took over wound care. An issue with data transfer meant that on receipt all wounds had a healing date regardless of healing status. These dates were replaced with missing values when wound healed status was not confirmed but confidence in the accuracy of these dates is low. On top of this, five of the 31 entries reporting a healed wound had a start of treatment date after the date of healing date, implying a negative healing time, which is obviously not possible. After excluding these five entries, the minimum reported healing time was 4 days and the maximum was just over 1 year (420 days); the median healing time was approximately 100 days. Summary statistics were recreated once seven potential duplicate entries had been removed to provide a conservative estimate of time to healing. Time to healing ranged between 4 and 420 days although the median time to healing was 88 days. Because of the lack of available data it was not possible to present Kaplan–Meier curves or estimate the median healing time. It was also not possible to examine time to healing for wounds with different prognostic profiles, although this data set holds the potential for the application of survival analysis techniques.
Approximately 30% (31) of the 104 entries with infection indicated as a risk factor were reported as being treated with a silver-containing primary dressing and < 10% of primary dressings for these individuals (nine entries) contained iodine. As only 40 entries (23 individuals) would be available for inclusion in a propensity scoring approach to examine the relative effectiveness of silver- and iodine-containing dressings, the propensity scoring method was not applied.
We estimated the proportion of people with venous leg ulcers who were receiving appropriate compression therapy by analysing the compression used and the ABPI measurement. Of the 166 entries relating to people with a venous leg ulcer, 59 limbs (37 individuals) were recorded as having an ABPI between 0.8 and 1.2 (safe limits for the application of compression). Approximately 85% of these 59 limbs were recorded as receiving compression therapy.
Because of the limitations in the data and the number of data available, instrumental variables and propensity scoring could not be explored. With a longer follow-up providing more data and a reliable wound identifier, it is thought that this exploration might be possible.
European Quality of Life-5 Dimensions questionnaire data
Of the 198 consenting patients, 12 had EQ-5D data only. In total, 157 individuals returned an EQ-5D form at some time point, with 21 individuals returning a form at all three time points. Six individuals returned EQ-5D forms but were not listed as consented; it is believed that there was an error with the patient ID for these individuals but data were deleted as this could not be proven. The response rate for the baseline EQ-5D questionnaire was reasonably high at around 80% but the proportion of those responding then fell rapidly to 40.0% at 3 months and 12.3% at 6 months (Table 34). For an EQ-5D form to be used in analysis, one level had to be chosen for each dimension; if the form was only partially completed a utility value could not be assigned. It was prespecified that if a form was returned > 1 month outside of the expected return date then the data would be discounted to allow for comparability of response.
| Questionnaire status | Baseline, n (%) | 3 months, n (%) | 6 months, n (%) |
|---|---|---|---|
| Complete | 146 (74.9) | 71 (36.4) | 20 (10.3) |
| Partially complete | 11 (5.6) | 7 (3.6) | 4 (2.1) |
| Total returned | 157 (80.5) | 78 (40.0) | 24 (12.3) |
| Total returned within 1 month of expected date | 116 (59.5) | 43 (22.1) | 4 (2.1) |
| Returned on time and complete | 111 (56.9) | 38 (19.5) | 3 (1.5) |
The baseline EQ-5D questionnaire was fully completed by 146 individuals and partially completed by a further 11. The baseline questionnaire was completed before the consent date for four individuals (this was either an error in the date of the questionnaire or on the consent form). It took, on average, just over 1 month to return the baseline questionnaire (mean 1.2 months, SD 1.83 months). In total, 116 questionnaires were returned within 1 month of the expected date (based on date of consent), of which 111 (56.9% of the sample) were complete and hence could be used in the analyses.
The 3-month questionnaire was completed by around 35% of individuals. No questionnaires were returned early but the longest time until return was 9 months after the date of consent. The average return time was approximately 4.5 months (SD 1.05 months). Of the 43 who returned the questionnaire 1 month either side of the expected date, 38 (19.5% of those in the study) completed all dimensions and hence could be included in the analyses.
The 6-month questionnaire was returned by just 24 individuals (12.3%). No questionnaires were returned early but the longest time until return was 10 months after the date of consent. The average return time was approximately 8.5 months (SD 1.06 months). Of the four who returned the questionnaire 1 month either side of the expected date, three (1.5% of those in the study) completed all dimensions and hence could be included in future analyses.
Table 35 shows the frequency of each response level for individuals who returned completed EQ-5D questionnaires within 1 month of the expected date of return. No frequencies are presented at 6 months to protect the anonymity of the three individuals who returned completed forms at this time point. At baseline most of the population scored 2 for mobility; although this was still the most frequent score at 3 months, the proportion of individuals scoring 1 had increased, although at 3 months there was a much smaller sample size. This increase in the proportion scoring 1 at 3 months compared with baseline was seen across all dimensions.
| Dimension | Baseline (n = 111), n (%) | 3 months (n = 38), n (%) |
|---|---|---|
| Mobility | ||
| 1 | 24 (21.6) | 12 (31.6) |
| 2 | 80 (72.1) | 24 (63.2) |
| 3 | 7 (6.3) | 2 (5.3) |
| Self-care | ||
| 1 | 59 (53.2) | 29 (76.3) |
| 2 | 41 (36.9) | 6 (15.8) |
| 3 | 11 (9.9) | 3 (7.9) |
| Usual activities | ||
| 1 | 24 (21.6) | 13 (34.2) |
| 2 | 66 (59.5) | 19 (50.0) |
| 3 | 21 (18.9) | 6 (15.8) |
| Pain/discomfort | ||
| 1 | 21 (18.9) | 11 (28.9) |
| 2 | 76 (68.5) | 23 (60.5) |
| 3 | 14 (12.6) | 4 (10.5) |
| Anxiety/depression | ||
| 1 | 61 (55.0) | 24 (63.2) |
| 2 | 47 (42.3) | 12 (31.6) |
| 3 | 3 (2.7) | 2 (5.3) |
It is possible that the changes seen here could be an artefact of attrition as opposed to reflecting a true change over time. To provide insight into this, the characteristics of EQ-5D respondents at baseline and 3 months were summarised and compared with those of the overall sample population.
Of those providing EQ-5D data at baseline, 51.0% were male, a similar proportion to that in the overall register population; however, at 3 months, 65.7% of respondents were male. The mean ages of respondents at baseline and 3 months (71.3 years and 73.3 years, respectively) were similar to the mean age of 72.4 years in the register population. The median values were also similar. In relation to risk factors, the proportions of smokers and those with a poor blood supply were similar at each time point to those in the overall sample. There were, however, differences in the proportion with diabetes between those providing EQ-5D data (22.5% at baseline and 13.2% at 3 months) and the overall register population (26.8%). The higher proportion of males and lower proportion of people with diabetes at 3 months may have led to the changes in responses, although it is not possible to say this definitively nor for the effect of attrition to be disentangled from ‘true’ changes in the descriptive summary shown in Table 35.
Staff feedback
Four feedback sessions were held, one with tissue viability nurses (n = 6), one each with the two district nursing pilot sites (pilot site 1, n = 2; pilot site 2, n = 12) and one with the podiatry service (n = 1 podiatrist). The sessions took place face to face at ‘team meetings’ except for the podiatry session, which took place on a one-to-one basis. Note that the views expressed relate to the electronic version of the wound assessment form for the tissue viability and podiatry services, and the paper version for district nurses.
In general, staff felt that the wound assessment form was too long, laborious and time-consuming to complete. A particular issue was that only one wound could be recorded per wound assessment form and this was a disadvantage when a patient had multiple wounds and hence multiple wound assessment forms to complete. Generic information also had to be included each time a wound assessment form was completed. For district nurses using the paper version, the writing did not always go through all the carbon layers and some things did not fit into the categories provided and they needed a free-text option. The ‘questionnaire viewer’ facility (introduced part-way through the pilot study) enabled clinicians to view a summary of the electronic wound assessment form by ‘hovering’ the cursor over it and this was regarded positively as an aid to identify the relevant wound assessment form without having to open them all.
Tissue viability nurses reported that they did not complete the wound assessment form if they were busy and tended to record the information elsewhere in the patients’ records on SystmOne (in the patient journal, which is a facility to record free-text notes). If they did complete the wound assessment form, sometimes it would never be opened again and it was not easy to compare past assessments.
District nurses used the paper version and, although complicated at first, they got used to it (this applied more to pilot site 2). They found that it prompted them to carry out wound assessments (and reassessments), undertake Doppler-aided assessment of ABPI and think more about which dressings they used; they felt confident that they were not missing anything. However, they did note that the forms were heavy to carry around and bulky in the patient notes.
Podiatrists tended to use their own wound assessment form that was specific to feet.
The mobile technology (ToughBook) did not always work well for the tissue viability nurses; the signal was sometimes poor and they were unable to complete the form while out in practice. The recent introduction of electronic care plans for district nurses was complementary to the wound assessment form and also prompted reassessment.
Barriers and facilitators
The drop-down boxes on the electronic version of the wound assessment form made it easier to complete. District nurses found the paper version more spaced out than the previous form but were also pleased that the number of reassessment columns had been reduced, as this reduced the number of carbon pages. Having multiple pages/one wound per form made it more difficult to use and it was sometimes difficult to know which wound was which.
District nurses in pilot site 2 addressed the need for extra time to complete the form by scheduling their work to accommodate it.
Tissue viability nurses felt that they had become more comfortable with mobile working technology and this had made it easier to complete the wound assessment form. However, they also felt that a new form was needed that combined with systems already in place in SystmOne and linked with planned new IT devices in the trust. The podiatrist felt that more work with SystmOne was needed to overcome the barriers to using the wound assessment form.
District nurses would have liked the form to be a single page (paper version) or to be able to use the electronic version (using mobile technology). They also wanted the facility to draw a sketch of the wound, inclusion of a body diagram and to be able to include more than one wound on a single wound assessment form. They felt that the description of wound location could be removed.
Tissue viability nurses generally felt that a new form was needed that had drop-down boxes but also ‘fewer clicks’ and the ability to reassess a wound within a single wound assessment form. The podiatrist wanted the form to be simplified and integrated into the current system.
Tissue viability nurses were very supportive of using future technology to develop the wound assessment form, such as integrating more with SystmOne and using devices such as a tablet with a camera that could be used to upload data to SystmOne. District nurses also wanted to be able to use mobile technology to complete the wound assessment form.
Activities associated with the register
We have chosen to represent the resources associated with the establishment and running of the register as ‘activities’ rather than monetary costs at this point as we think that this will be more meaningful and relevant over time. Activities and tasks generated by the register are detailed in Table 36 and relate to those directly associated with setting up and running the register. Other activities at the NHS site for routine data collection and developments are not included here but are discussed in the following section.
| Register activities | Location (NHS or university) | Function performed by |
|---|---|---|
| Set-up activities | ||
| Planning the aims, scope and methods in the form of a protocol and related documents | NHS and university | Collaboration between a number of stakeholders |
| Preparing and submitting ethics application and all other approvals | University | Project support officer and research fellow |
| Liaison with NHS site and university IT department to discuss/agree data transfer issues | NHS and university | Wounds data co-ordinator, research fellow and project support officer |
| Running activities | ||
| Preparing and submitting substantial amendments to ethics committee and all other approvals | University | Project support officer and research fellow |
| Identifying potential participants and preparing and mailing out study information | NHS | Administrator |
| Dealing with incoming paperwork from potential participants and filing it safety and securely | NHS | Administrator and wounds data co-ordinator |
| Recording recruitment details of consenting individuals for upload to public research body (UKCRN) and also NHS electronic patient records | NHS | Administrator and wounds data co-ordinator |
| Data entry into SystmOne for forms completed on paper version of wound assessment form | NHS | Wounds data co-ordinator |
| Preparing and mailing out EQ-5D materials to register participants | NHS | Administrator |
| Data entry of EQ-5D data into spreadsheet for register participants and filing the paper documents safely and securely | University | Project support officer |
| Preparing for and extracting participant data from NHS data warehouse and transfer (via secure e-mail) to register administrator | NHS | Senior analyst in IT |
| Receiving register data and saving and storing electronic files safely and securely | University | Project support officer |
| Uploading recruitment data to public research body (UKCRN) | University | Project support officer |
Other activities
Some activities occurred within the NHS site itself and were not necessarily regarded as register activity. Within the pilot study, the development and implementation of the routine collection of electronic patient data necessitated a great deal of NHS staff engagement including clinical staff (tissue viability service staff), the wounds data co-ordinator and NHS IT service staff. This joint working took place over an extended period of time. There was also clinical staff time required to record patient data in the wound assessment form.
Furthermore, there was an activity implication attached to the patient and/or carer time to read and respond to the study invitation and complete the EQ-5D questionnaire every 3 months.
Discussion
Any register that relies on routine clinical data to populate it is dependent on the clinical and IT interface within the source organisation. The use of IT in patient record keeping and the recording of clinical data within nursing services were undergoing rapid change during the pilot study and continue to do so. Our attempts to integrate the collection of complex wounds data into the clinical information system were largely supported by enthusiastic clinical staff (both tissue viability specialists and general nurses), who demonstrated a willingness to undertake wound assessments and treatment reviews as part of wound management. Their accuracy and completion rates were also generally good. These attributes present an important platform on which to build in the future, particularly if a wounds register is extended across the trust and potentially to other areas/trusts. However, a number of challenges remain regarding the use of routinely collected wounds data for a register. Although we were able to identify and track individual participants using their NHS number, it was much more challenging to track individual wounds in people who had more than one wound. Software limitations meant that each wound assessment form could not be given an individual file name (with identifier) and we had to try to identify individual wounds using information in the form (including wound type, size and location). When the problem was identified (through the pre-pilot data), staff were requested to include Wound1, Wound2 etc. in the wound description section. When this was completed it was possible to track longitudinal data; however, it was not implemented until 3 months into the pilot study and the nurses were not always consistent in recording the information. One possible solution might be further software development so that wounds can be reassessed within the same wound assessment form rather than relying on nurses to manually enter the information. An alternative would be to collect only information about a ‘reference’ wound, perhaps the largest wound.
A key issue is that of coverage of the register and thus data collection. In some cases it was challenging to track participants as they moved between different services for wound care. This made it difficult to report an accurate duration of follow-up data. Full coverage of service providers would be required to ensure full data collection.
Data analysis of the wounds register
A lack of data, because of both poor recruitment and the difficulty of tracking longitudinal data, limited the analyses that we were able to undertake. We had planned to implement propensity scoring and investigate the use of instrumental variables but this was not possible because of the limitations of the data that were collected. Only one-quarter of those approached gave consent and future work should consider alternative approaches to recruitment. It appears that some of the reasons why people do not wish to take part may be because of old age and poor health, which are not easily modifiable. A possible solution would be a two-tier model of participation, in which people could opt out of completion of the EQ-5D (which would remove the need to identify participants). A two-tier model would permit patients to choose their level of involvement while potentially enhancing the overall recruitment rate and subsequent availability of core register data. Ethics approval for this pilot study was based on our following a recruitment process of mailing study information to potential participants. Permission to adopt a recruitment strategy similar to those that we use in wounds trials,18,19,118 which involve recruitment via the clinical care team, would be likely to enhance overall recruitment. Another alternative would be to consider using pseudo-anonymised data, providing the risk of re-identification of participants could be minimised. Although some ‘richness’ of data would be lost (including EQ-5D data), using pseudo-anonymised data would enable larger numbers of participants and a more representative sample.
This pilot study has identified areas for improvement in the characteristics of routinely collected NHS data that are suitable for use in a register. For example, the standardisation of data variables is a key feature of register data. Many of the data variables on the wound assessment form were standardised, such as the ‘wound type’ variable, which had 13 available options for staff to choose from and a limited opportunity for free text. Other variables were less standardised. For instance, the ‘wound length and width’ variable required clinicians to insert numerical details in millimetres; however, this was not always carried out accurately. Extending the standardisation of this variable to provide measurement options could potentially increase its suitability for populating a wounds register. Similarly, because of the sheer number of potential options, the ‘wound dressing’ variable was provided as free text, with clinicians entering the name of the dressing. Further work could usefully investigate how wound dressing data could be further standardised while remaining flexible, to incorporate new dressings as they emerge onto the market and into clinical practice. The need for further data standardisation to populate a register creates an opposing tension with clinical staff, who noted in feedback that they would like more free-text opportunities. Therefore, a careful balance would need to be achieved to meet the needs of clinical record keeping by staff and the need for high-quality data to populate a register.
Another characteristic of the NHS data used in this pilot study that would need to be enhanced if the register was to be continued and/or expanded concerns the implementation of data definitions. Our review work identified that explicit data definitions impact on the value and quality of register data. To date, nurses have relied on clinical judgement when completing the assessment forms and few, if any, operational definitions were available for variables. For the purposes of the complex wounds register pilot we implemented a clear case definition of a complex wound (which was already in use within the trust and wider region) and other wound definitions that had been used in the prevalence survey. Any future wounds register using routinely collected NHS data would need to implement clear operational definitions for all data variables.
Implications for future wounds registers
Wound care in the UK is something of a ‘Cinderella service’, subsumed in community nursing work and captured only as crude activity data. Consequently, the planning of services and new research is hampered by collective ignorance of the numbers of patients affected, treatments used and the likely value of new research. We have previously shown that, historically, primary care databases (General Practice Research Database and The Health Improvement Network) were suboptimal for exploring wound treatments and outcomes because most care is delivered by community nurses and not accurately captured at the practice level. 119 The pilot register work was required because of concerns about the extensive challenges faced in harmonising even routine data collection across the many health-care providers involved in complex wound care. These concerns were borne out by our experience and the collection of data in our pilot relied on the development of a complex infrastructure involving collaboration between multiple stakeholders from the organisations involved. Full coverage of all providers was not possible within the pilot study. Indeed, this infrastructure is a key programme output and, although we suggest that future large-scale data collection is feasible based on the successful collection of valid pilot data, such data collection would require similar close collaboration to support it.
Overall conclusions from workstream 1
A systematic review of previous prevalence surveys of complex wounds indicated a considerable accumulation of published literature. All of the identified studies had methodological flaws that would affect the validity of their prevalence estimates and/or were badly reported. Only a small number of studies have investigated all complex wounds (or all wounds), with most focusing on wounds of a specific aetiology (usually leg ulcers). Estimates of the prevalence of complex wounds ranged between 0.24% and 1.4%. Most previous surveys appear to have used paper-based questionnaires and fewer than one-third implemented any case validation. A defined geographical population was used for the denominator population in half of the studies, with the other half examining a variety of populations. No studies used the capture–recapture technique in hard-to-reach groups. Our scrutiny of the existing literature enabled us to identify the core variables (a minimum data set) to be used in future studies. The findings of this review also enabled us to design a prevalence survey that avoided most of the common pitfalls.
We had originally planned to use the capture–recapture technique to estimate complex wound prevalence in hard-to-reach groups. In the event this proved unnecessary because the targeted provision of health care for drug users and people of no fixed abode in Leeds meant that we had few doubts that we could identify people with complex wounds from these populations. Our own prevalence survey estimated that 1.47 people per 1000 (95% CI 1.38 to 1.56 per 1000) in Leeds were receiving health care for some kind of complex wound. Pressure ulcers were the most common complex wound (point prevalence of 0.31 per 1000) followed by venous leg ulcers (0.29 per 1000). We have produced the first estimate of which we are aware of the prevalence of complex wounds in current or previous intravenous drug users (5.64 per 1000, 95% CI 3.97 to 7.99 per 1000). A clear picture emerges from these data of complex wounds mainly affecting older people with multiple comorbidities. The mean age of those with a complex wound was 70.1 years (SD 19.41 years) and people with a complex wound had an average of two comorbidities, with CVD and arthritis being the most common. Rates of urinary and faecal incontinence were also high at 23% and 12%, respectively. Most people received their wound management from community nurses; most patients received two to three wound care consultations per week, with a mean duration of 28 minutes.
Our review of registers and clinical databases identified five concerning patients with complex wounds, although only one of these was UK based and this was > 10 years old. We designed a complex wounds register with a view to future use in both clinical care and research (e.g. HTA). We worked closely with clinical colleagues and NHS IT professionals to ensure that our register protocol had clear operational definitions for most variables, clear recruitment and consent procedures and a data capture process that would impact minimally on clinical work while providing clinically useful data.
We did not find any guidance and advice for people in the UK who are considering establishing a chronic disease register and we think that such advice would be of enormous value. Such guidance should usefully address important design features to be considered as well as issues around consent and data protection.
Our complex wounds register pilot demonstrated that recruitment to such a register is possible, although the recruitment strategy that we used resulted in only approximately 26% of eligible patients being recruited. Such a low recruitment rate would be unsatisfactory for a full register and would introduce selection bias. A relatively small number of potential participants explained their reasons for non-participation, indicating that recruitment would be likely to be negatively influenced by the age and general health of people with complex wounds (i.e. primarily elderly people with other health conditions/poor general health and dementia).
We found that it was possible to correctly identify the NHS records of consenting register participants, extract data from the NHS databank and securely transfer and store register data.
The feasibility of data analysis could be assessed only to a limited extent within the pilot. We learned that the tracking of individual wounds in patients with multiple wounds over time very difficult. The type of complex wound reported for register participants was similar to what was expected from our prevalence survey, with venous leg ulcers and pressure ulcers being the most frequently reported. Patients who consented to participate in the register pilot were slightly older and more likely to be male when compared with all people with complex wounds in the city.
The median wound healing time for people in the wounds register was just under 3 months; however, this was based on a small number of individuals. There were insufficient data to examine how this may differ for people with different prognostic profiles.
The small number of participants whose wounds healed during follow-up may be because the tissue viability service, by its nature, serves the more complex, hard-to-heal population and this was mainly where we piloted the register. This was also evident in the pre-pilot data (which involved only the tissue viability service), with over half of the individuals having only one consultation. However, expansion to other services to improve coverage in the future would help overcome this issue.
Wound management of register participants was primarily delivered in patients’ own homes; this has implications for the nature of the data collection for a register (currently likely to be on paper using some method of duplication so that a copy stays with the patient and another is sent for data entry).
The day-to-day running of a register generates a considerable number of tasks and processes that require at least one full-time individual with skills in project management, data management, data protection and conducting quality procedures.
Chapter 3 Service user and provider perspectives (workstream 2)
Abstract
Background
Health care and research should be guided by the priorities of patients, carers, the wider public and the NHS. Our objective was to explore the experiences of complex wound care, identify which outcomes matter most to people with complex wounds, compare these with those reported in wounds research and derive a prioritised list of research questions in the area of pressure ulcer prevention and treatment.
Methods
The methods used included semistructured interviews with people affected by complex wounds, carers and health professionals regarding their experiences of complex wound care and desirable treatment outcomes; a systematic review of the design and conduct of RCTs of complex wound treatments; and consultative and deliberative research agenda setting in the area of pressure ulcer prevention and treatment.
Results
Most patients and health professionals viewed healing of the complex wound as the primary treatment goal. Patients were greatly troubled by the socially inhibiting consequences of their complex wound, but wound care services did not focus on the psychological or social impacts. The treatment model was geared to healing, not ‘living with’ a long-term condition. In total, 167 RCTs of complex wound treatments were analysed, of which 69 (41%) did not specify a primary outcome; 40 (24%) had complete healing as the primary outcome, 47 (28%) used a surrogate measure of wound healing and 11 (7%) reported an outcome unrelated to healing. A total of 960 treatment uncertainties were elicited and a top 12 prioritised by patients, carers and health professionals.
Conclusions
There is a mismatch between the nature and quality of RCTs in complex wounds and the kind of research evidence desired by patients, carers and clinicians. It was possible to work with patients, carers and health professionals to identify and prioritise for research the uncertainties in pressure ulcer prevention and treatment. Community nursing management of people with complex wounds may be improved by adopting an approach aimed at helping patients live with a long-term condition.
Background
The primary stated purpose of the UK NHS is the improvement of health-care outcomes, with an imperative to move towards systems of accountability that focus on outcomes achieved for patients rather than on the processes by which they are achieved. 120 Enhancing quality of life for people with long-term conditions has been identified as a key ‘domain’ in the NHS Outcomes Framework. 121 Workstream 2 focused on service user engagement in identifying, measuring and reporting outcomes in chronic wound care and their involvement in the prioritisation of wound-related research questions.
Chronic complex wounds impact on patient morbidity, mortality, daily functioning and quality of life. 9–12 There is evidence from research into other chronic conditions that the outcomes that matter most to patients may not be the primary outcomes measured in RCTs. For example, findings from the OMERACT (Outcome Measures in Rheumatology) collaboration suggest that clinicians and researchers may not realise that certain outcomes are very important for patients. 122 As a result, the OMERACT collaboration advocates the use of core outcome sets designed using consensus techniques. 123 There has been no such initiative in wound care and there has been no systematic exploration of the characteristics of outcomes reported for wound trials and the factors that influence them. If the principal clinical objective in wound care is complete wound healing, this in itself can be measured and presented in a number of ways in RCTs. 124 Conducting trials with lengthy follow-up is costly and time to complete healing and the proportion of wounds completely healed are difficult to measure in trials of short duration. Therefore, studies may opt to report surrogate outcomes including surrogate measures of healing such as change in ulcer size or area. 125–127 A surrogate end point is described by Temple128 as ‘a laboratory measurement or a physical sign used as a substitute for a clinically meaningful endpoint that measures directly how a patient feels, functions or survives’. Surrogates are predictors for the clinically meaningful end point of complete healing. There is evidence that some surrogate markers are robust predictors of healing126 but there may also be the potential for false extrapolation. It is not clear how valuable such healing measures are judged to be by patients. For example, Yudkin et al. 129 warn that surrogates used in diabetes research commonly show much larger responses to treatment than the ‘hard’ outcomes that matter to patients, such as renal and visual impairment or quality of life. They argue that, as a result, widely accepted treatment strategies are based on artificially inflated expectations. Researching surrogate outcomes in shorter trials can cause other problems, for example it is difficult to accurately assess adherence to treatment over time and this may impact on treatment effectiveness. Within this workstream we therefore sought to find out what the important outcomes are from patient, carer and staff perspectives and then systematically summarise the outcomes that are reported in wound trials and compare the two. We also sought to explore whether or not factors such as industry funding of trials may influence outcome selection or some other aspect of trial quality.
As well as getting the outcomes and trial design right, another important aspect related to the conduct of high-quality, relevant research is identifying the right question in the first place. Several authors have written persuasively of the importance of gathering and prioritising patients’ research questions so that future research assesses the things that matter to patients. 130,131
The James Lind Alliance (JLA) was established in 2004 to encourage patients, carers and clinicians to work together to identify and prioritise shared health-care uncertainties, arguing that medical consensus can be flawed and that research into clinical practice and NHS services should identify and address the uncertainties and investigate the outcomes that are of most practical importance to patients, their carers and clinicians. Research priority themes emerging from the deliberations of JLA partnerships to 2012 (across asthma, incontinence, vitiligo, eczema, stroke, prostate cancer, schizophrenia, aspects of balance and type 1 diabetes) emphasise the assessment of treatments in terms of long-term effects (wanted and unwanted) and safety and adverse effects. 132 All aspects of the management of complex wounds are served by a poor evidence base; however, we selected pressure ulcer prevention and treatment as a complex wounds topic on which to embark on a JLA Priority Setting Partnership (PSP). We selected pressure ulcers because they are the most frequent type of complex wound (see Chapter 2) and because their reduction is such a high priority for the NHS. 133
In summary, workstream 2 employed mixed methods to explore the outcomes that matter most to patients undergoing treatment for complex, chronic wounds and how these compare with the outcomes that matter most to health-care professionals and those measured and reported in RCTs in wound care. We also report on the James Lind Alliance Pressure Ulcer Partnership (JLAPUP). Methods and results are reported separately for three studies:
-
The relative importance of wound treatment outcomes to patients, carers and health-care staff (which outcomes would stakeholders like to see measured in wounds research?).
-
A systematic review of complex wounds research (which outcomes are currently measured in wounds research?).
-
Involving patients and clinicians in developing research priorities: the JLAPUP (what are the pressure ulcer research priorities of patients, carers and clinicians?).
This workstream therefore analysed the research that is undertaken in complex wounds and compared it with the research that patients, carers and clinicians want to see.
The relative importance of wound treatment outcomes to patients, carers, health-care staff and policy-makers
Introduction
Although wound healing (a physiological end point involving close monitoring of the wound surface area, wound depth and extent of tissue involvement) is often regarded as the main aim of treatment for health professionals and patients, there has been little published work that identifies and compares the treatment outcomes that matter most to different stakeholders. In addition to healing, other possible outcomes of interest in wound care include the number of dressing changes required by patients and associated resource use; exudate and odour management; product durability; levels of wound infection; and whether or not the wound recurs. Outcomes perceived as being important from the patient perspective include wound debridement,134 pain, dressing comfort, effects on mobility and in/dependence and health-related quality of life. 135 It is noteworthy, however, how absent the patient voice is from wounds research and particularly from conversations about outcomes. For example, the European Wound Management Association Patient Outcome Group is made up of clinicians, academics and industry representatives136 and did not involve patients in drawing up its guidance on wounds research. 134
Objective
Our objective in undertaking this study was to identify the most important outcomes for complex wounds from the perspectives of patients (including intravenous drug users), carers, health-care professionals and policy-makers.
Methods
Interviews
Semistructured interviews using a topic guide were conducted with 33 people with complex wounds receiving care from Leeds Community Healthcare NHS Trust. People were eligible for interview if they were receiving care for pressure ulcers, leg ulcers, foot ulcers associated with diabetes or surgical wounds healing by secondary intention. We particularly sought to include some people with complex wounds associated with current or previous intravenous drug use. The interviews aimed to assess the wound outcomes that mattered most to the interviewees. In contrast to previous research (e.g. Spilsbury et al. 137) the interviews did not focus on any one outcome (i.e. quality of life), but rather allowed participants to generate naturalistic data on what they considered successful in terms of treatment outcomes. Interviews lasting 30–60 minutes took place in participants’ own homes or, when an interviewee was of no fixed abode, in a private room at a referring specialist general practice. One interview was conducted by telephone. Interviews were digitally audio-recorded, transcribed verbatim and subjected to thematic and comparative analyses. 138,139 One interviewee preferred not to be recorded but consented for contemporaneous notes to be taken instead. A written post-interview checklist was completed immediately after each interview recording who was present, the main themes, a note on rapport and any particularly sensitive issues.
Purposive sampling was used to ensure that as broad a range of patient experiences as possible was recorded in the interviews and to meet the wider programme aim of including the views of intravenous drug users, which are seldom recorded in the existing literature. The sample size aimed to maximise diversity across the patient group. 140 This meant adopting an iterative sampling approach, moving back and forth (iterating) between sampling and analysing data so that preliminary analytical findings shaped subsequent sampling choices. A sampling frame was drawn up by the researcher with NHS clinician partners based on their patient profiles. The researcher had no access to patients’ medical records. The clinician partners were asked to review patients and carers according to the sampling frame, make a list and from this list ask patients if they might be interested in taking part in the research. If a patient on the list expressed an interest, a clinician partner approached him or her. Those who agreed were referred to the researcher who then telephoned them to give more details and to ask if she might send out a written information sheet. In addition, one patient participant self-referred to the study after hearing about it at a patient and public involvement event. One carer self-referred and a patient and carer couple asked to be referred by their district nurse in response to articles that they had read about the study in Multiple Sclerosis Society and carer publications.
Eight carers were involved in interviews. Six joined in the interviews above or were asked for additional thoughts after the interview. Two carers, one recruited through the local carers group newsletter, took part in separate interviews in which they were the primary interviewee. Finally, 12 health-care professionals of varying grades and length of service involved in the direct delivery of wound care services were interviewed at their place of work. Three of these had managerial responsibility for teams. One member of the executive management team was also interviewed.
Ethics and informed consent
The study was subject to full ethical review. It received approval from the University of York Department of Health Sciences Research Governance Committee and York Research Ethics Committee (reference 09/H1311/88). The researcher telephoned every patient and carer initially referred to the study by the on-site clinicians. Care was taken to ensure that potential participants did not feel pressured to become involved in the study. They were given time to read through the available information, ask questions and make an informed choice. The nature and design of the study allowed for a ‘cooling off’ period between the patient/carer being approached (or, in some cases, approaching us) and the interview taking place. Each patient participant had at least three opportunities to talk about the study before an interview was arranged. Participants were informed that they could withdraw at any time without giving a reason. They were assured that the decision to withdraw or to not take part would not affect the standard of care received. A consent form was completed before each interview, which provided a fourth opportunity for people to discuss the study and consider their participation. Participants could access further information from the programme website and all additional relevant study information was made available to participants on request. A £10 shopping voucher was given to each lay participant to thank them for taking the time to participate.
To preserve anonymity, the responses of health professionals with very distinctive roles in identifiable places are not linked to particular individuals and quotations refer to a randomly allocated numerical pseudonym. The key for this is available only to the interviewer.
Interview analysis
The analysis of interviews had five stages: reading the text and coding for descriptive labels; sorting for patterns within the data; identifying outliers or negative cases and revising theory accordingly; generalising and refining constructs and theories; and more detailed reflection and revision. Additional fieldwork served to further contextualise the interview data. This including shadowing a tissue viability nurse at another site, attending wounds conferences and training events, recording patient experiences at public engagement events and the development of the JLAPUP. Engagement with the wider sociological and health literature enabled a consideration of the intersections between individual experience and collective practices and the integration of micro-empirical study with macro-theoretical perspectives. 141
Results
Patients
Interviews with 33 people (18 men and 15 women) elicited narrative experiences of developing, having treatment for and living with a chronic, complex wound, The biographical details of the patient interviewees are summarised in Table 37. Some interviewees had multiple wounds and/or more than one type of wound. Fifteen interviewees were receiving treatment for leg ulcers; five of these interviewees had previously received treatment for leg ulcers, as had two further interviewees. Six interviewees had post-operative surgical wounds healing by secondary intention. These are either surgical wounds deliberately left open following surgery to heal from the ‘bottom up’ or the result of closed wounds splitting open because of infection, tissue loss or other factors that prevent them from being stitched or stapled closed again. Five people had foot ulcers associated with diabetes and eight people had pressure ulcers, including one who had acquired a pressure ulcer while in hospital receiving treatment for a diabetic foot ulcer. Two of the eight had heel pressure ulcers, which could also be classified as foot ulcers. One interviewee had a groin wound as a result of intravenous drug use. Six leg ulcers were associated with the interviewees’ use of intravenous drugs.
| Patient ID | Sex | Age (years) | Ethnic origin (self-description) | Age leaving education (years) | Number of wounds | Type of wound | Previous chronic wound | Duration of wound (months) |
|---|---|---|---|---|---|---|---|---|
| LU1 | F | 75 | Scottish | 15 | 2 healed | LU | – | 6 |
| LU2 | F | 80+ | German | 14 | 2 | LU | – | 240 |
| LU3 | M | 53 | English | 16 | 2 | LU | – | 120 |
| LU4 | M | 66 | English | 15 | 1 | LU | – | 48 |
| LU5 | F | 85 | English | 14 | 1 | LU | – | 72 |
| LU6 | F | 97 | English | 15 | 4 | LU | LU | 456 |
| LU7 | F | 88 | English | 14 | 3 | LU | LU | 12 |
| LU8 | F | 81 | English | – | 9 | LU | – | 8 |
| A1 | M | 63 | English | > 18 | 1 | SWHSI | – | > 12 |
| A2 | F | 42 | English | 16 | 1 | SWHSI | – | 8 |
| A3 | F | 59 | White British | > 18 | 1 | SWHSI | – | 8 |
| A4 | F | 57 | White British | 15 | 1 | SWHSI | – | 9 |
| A5 | M | 35 | White British | > 18 | 1 | SWHSI | – | 4 |
| A6 | M | 41 | Sudanese | Student | 1 healed | SWHSI | – | 8 |
| DFU1 | M | 55 | English | 15 | 2 | DFU + PU | – | 3 |
| DFU2 | F | 80 | English | 14 | 1 | DFU | LU | 5 |
| DFU3 | M | 70 | English | 16 | 1 | DFU | DFU | 48 |
| DFU4 | F | 83 | English | 14 | 1 | DFU | – | 8 |
| LDFU5 | M | 72 | English | 14 | 4+ | DFU + LU | LU + DFU | 72 |
| PU1 | M | 40 | Indian born UK | 16 | 2 | PU | PU | 12 |
| PU2 | F | 77 | White British | 15 | 1 | PU | – | 12 |
| PU3 | F | 90 | English | 14 | 1 | PU | LU | ‘Years’ |
| PU4 | F | 72 | English | 15 | 2 | PU | PU | 24 |
| PU5 | M | 57 | English | 15 | 1 | PU | PU | 5 |
| PU6 | M | 53 | White British | > 18 | 1 | PU | PU | 10 |
| PU7 | F | 65 | English | > 18 | 1 | PU | PU + LU | 39 |
| IVG1 | M | 40 | White British | 15 | 2 | GW | – | 4 |
| IVLU1 | M | 35 | White British | 14 | 3 | LU | LU + GW | 45 |
| IVLU2 | M | 36 | White British | 16 | 2 | LU | LU | 24 |
| IVLU3 | M | 45 | White British | 16 | 2 | LU | – | 24 |
| IVLU4 | M | 21 | English | 15 | 1 | LU | – | 36 |
| IVLU5 | M | 33 | White British | 16 | 1 | LU | – | > 84 |
| IVLU6 | M | 39 | English | 16 | 1 | LU | GW | 5 |
Eighteen people reported one wound, eight reported two wounds and five reported three wounds or more (maximum nine); two people were interviewed about wounds that had ‘healed’ but which were still problematic or required ongoing maintenance. Wound duration ranged from a few months up to 38 years. Some people were unclear exactly how long they had had their wound(s) and provided an estimate. Thirteen people previously reported having treatment for a different chronic wound and one person had been experiencing constant quick recurrences of the same leg ulcer for 38 years. The majority of interviewees were managing multiple long-term conditions and disabilities. Those reported included vascular disease, diabetes, arthritis, cancer, opioid addiction, spinal cord injury, multiple sclerosis (MS) and spina bifida. One woman described herself as ‘a walking medical dictionary’ because she had so many conditions (DFU2, female, aged 80 years).
The mean age of interviewees was 60 years; the median age was 59 years, with a range of 21–97 years. The majority of participants were white British (30/33), one was Asian British (Indian), one was Sudanese (seeking asylum) and one was German. English was a second language for two of the interviewees. Five of the interviewees had attended formal education beyond the age of 16 years, two of whom were educated to degree level, and one was currently in higher education. The majority of participants were retired or unemployed. Five were in employment when they developed their wound. Nine reported that they were widowed, four were divorced or estranged from a partner, seven were single and 13 had partners. The partner of one interviewee was in residential care. One interviewee had dependent children living with them. One had been a cocarer for a parent but was recently bereaved. Six of the interviewees were homeless and sleeping rough or in temporary accommodation.
Treatment outcomes most wanted by people with leg ulcers
Fifteen interviewees had leg ulcers. Six of these were men who regarded their leg ulcers as a consequence of intravenous drug use; these men were among the youngest people in the sample. Of those without intravenous drug use involvement, six were women in their 70s, 80s and 90s and three were men in their 50s, 60s and 70s with a range of comorbidities including arterial and venous disease, arthritis, Bowen’s disease, thyroid problems, gout, leukaemia and lymphoedema. Eight out of these nine interviewees said that what they most wanted from treatment was healing:
. . . get it healed up and dried up as quickly as possible.
LU1, female, 75 years
. . . to be cured . . . to be rid of the ulcer.
LU4, male, 66 years
. . . for them to get better.
LU6, female, 97 years
I just want them to heal.
LU7, female, 88 years
. . . not to have the sores.
LU8, female, 81 years
. . . not to have a hole in my leg. For it to heal up.
LU3, male, 53 years
. . . what do I want most? Give me a miracle cure! That would be very welcome. Find a cure, that’s what I want.
LU2, female, 80+ years
One of these interviewees with a leg ulcer for > 10 years was faced with the prospect of amputation but was still hoping that his wound might heal:
Not to have a hole in my leg. For it to heal up. Or, like they did, they said, it’s never going to heal, I think we’ll go down the amputation route . . . I just want it to heal up and that. And if it’s not going to heal up a decision has to be made whether I’m going to have to carry on dressing it forever, or what. But nobody seems to want to make that decision. Because it’s got smaller now . . . And the bloke who were going to chop me leg off went, ahhh you see, good job we didn’t cut your leg off now, isn’t it? And I thought, you were all for it at one bit. You were sat there with your meat cleaver and your sharpener.
LU3, male, 53 years
Another of these interviewees, a man in his early 70s with bilateral leg ulcers and diabetic foot ulcers for > 5 years, had refused amputation. What he most wanted was healing but he also acknowledged that once one wound healed another might break out and that he could have recurrent ulcers until he died:
[What I most want from treatment is] to get them cured, right. You see that one . . . that was about that long. That was painful, right. But it healed 100% up, right. But as that closes up, the other one opens out. So what the hell, you know . . . You talk about throwing your hankie in the sky and clapping hands, right. No . . . the same day as it healed, I got another one. This one I’ve got, nearly 6 years now . . . that can heal and another will break out . . . when’s it going to end? I don’t know. It will end when I die, probably. I mean, I’m 72 now, which I’ve probably lived a bit over time. I think to myself that I’ll take it with me.
LDFU5, male, 72 years
The exception was a woman aged 85 years who had had a leg ulcer for 6 months and had faced barriers in getting access to treatment:
I felt as though I’d hit a brick wall. That nobody were listening to me. Now, I’m 85, but I’m not senile and I’m not stupid.
LU5, female, 85 years
She said what she most wanted was to be listened to and to get it properly treated. Once it was being treated, she most wanted to get the bandages off and ‘not look like Nora Batty’:
Well what I wanted – I didn’t want to be pushed off to one side; people saying, oh come back on . . . And then, no you can’t come Wednesday because there won’t be a nurse here. And you had to fit in round them . . . them receptionists, well [laughter] . . . I wanted access. And I wanted somebody to do something about it. That’s all. I wasn’t asking for a miracle . . . But I just wanted somebody to get something started. [Now] I just want to get these bandages off . . . And not look like Norah Batty.
LU5, female, 85 years
All six men with leg ulcers who were or had been intravenous drug users said that what they most wanted from their treatment was healing:
I just want it to be better, you know, I just want it to get better.
IVLU1, male, 35 years
. . . to heal up.
IVLU3, male, 45 years
. . . just to get them healed up . . . just generally make it better.
IVLU4, male, 21 years
. . . to get it right . . . Get my health back.
IVLU2, male, 36 years
. . . just to get it healed up, and that’s it . . . I just want to get rid of it.
IVLU5, male, 33 years
I basically just want to get better.
IVLU6, male, 39 years
One of these interviewees with a leg ulcer for > 3 years wanted his ulcer to heal but talked about how attending appointments and waiting for it to heal was putting his life on hold:
I just want it to be better, you know, I just want it to get better . . . If that’s [healing] not going to happen, I’d rather have that knowledge now so I know where to take my life in what direction, you know, even if I have to just have it done once a week at least I could go back to some part-time work . . . All I want to do is go back to work. I don’t want to be on the dole . . . make sure it’s clean and doesn’t smell.
IVLU1, male, 35 years
He was coming to terms with possibly irreversible damage caused by intravenous drug use and wanted resolution of his chronic ulcer even if that meant amputation:
I thought I’d done everything right . . . with 5 years of being totally clean after I had the bust artery. Everything felt all right and then all of a sudden I got this just because I scratch my leg basically . . . I’ve even thought about having my leg chopped off and just saying, sod it, just get the leg chopped off and have it healed up as a stump rather than be like I am. I mean the worst I’ve been I’ve wanted that to happen.
IVLU1, male, 35 years
The interviewee with a groin wound had experienced barriers to accessing treatment. What he most wanted was to be listened to and get the treatment he needed:
They doubt your pain . . . Their attitude was that I were a smack head – a heroin addict – nobody says that, ‘a smack head’ . . . You’ve brought it on yourself, tough. Eventually I got what I wanted. I wanted it cut out [groin infection] and I got it cut out.
IVG1, male, 40 years (from contemporaneous notes)
Treatment outcomes most wanted by people with pressure ulcers
Seven interviewees had pressure ulcers. Three were men, all of whom used wheelchairs, two because of spinal cord injury and one because of spina bifida and hydrocephalous. Of the four female interviewees, one had MS and three had multiple comorbidities including cancer, cardiovascular conditions, chronic obstructive pulmonary disease, arthritis and asthma. All required a wheelchair or other mobility aids. Three of the interviews took place at the interviewee’s bedside. Six out of seven interviewees said that what they most wanted from treatment was for their pressure ulcer to heal:
For it [the treatment] to work! . . . [G]et it over and done with . . . I just want rid.
PU1, male, 40 years
I want it clearing up. I want the ulcer to go.
PU2, female, 77 years
I just wish it would hurry up and get better . . . get it right.
PU4, female, 72 years
Ideally get rid of it.
PU5, male, 57 years
To have it operated on . . . I just want it [surgery] to be done as quickly as possible and I want it to be successful and to be mobilised again as fast as I possibly can.
PU6, male, 53 years
To heal and be finished with.
PU7, female, 65 years
Healing and/or getting access to treatment was seen as the key to getting mobile and getting on with life, especially for those forced to live much of their lives in bed. For example:
I just wish it would hurry up and get better and then I can sit up all day . . . I want to go back to going to the day centre as well but I can’t see that happening before Christmas.
PU4, female, 72 years
One interviewee with previous experience of pressure ulcers was frustrated with the time that he had waited to receive surgical treatment:
It was quite obvious by Christmas time that it wasn’t going to heal. It was so deep . . . I would like to know, and I’m going to ask them when I go in, have they ever seen a sore this big, this deep that’s healed itself? I’ve a sneaky feeling the answer is no. So why don’t they operate in the first place because it’s wasting time, wasting money, wasting National Health effort, you know, nurses coming here . . . I just want it [surgery] to be done as quickly as possible and I want it to be successful and to be mobilised again as fast as I possibly can.
PU6, male, 53 years
Most interviewees talked about the difficulty of achieving healing and the fact that even if this wound did heal, they were at risk of developing new ulcers. For example:
I just want it over and done with and hopefully not get another pressure sore again. I know that’s not going to happen, I probably will get another pressure sore.
PU1, male, 40 years
I want the ulcer to go, you know, to not be there anymore. I suppose that’s wishful thinking.
PU2, female, 77 years
One interviewee with a current ulcer for 5 months, experience of previous ulcers and an ongoing risk of pressure ulcers because of spinal cord injury said:
Ideally get rid of it If that can’t happen, to manage it so it doesn’t interfere with your life . . . [What I most want is] the treatment that allows you to get on with living.
PU5, male, 57 years
The exception to the expressed desire for healing was one woman who spent most of her time in bed. She most wanted to keep the wound clean and dressed, fearing neglect, that is, that the nurses would no longer come and attend to it:
I’ll have it for the rest of my life, well what life I’ve got left. It won’t go away love . . . I wouldn’t like to go about without nothing on, a pad on, you know . . . I sometimes think to myself, oh they’ll stop coming soon, you know, they’ll stop coming. It’s got down to twice a week.
PU3, female, 90 years
Five of the seven interviewees with pressure ulcers linked their acquisition to medical interventions including hospitalisation, radiotherapy and an accident with a shoe horn while being fitted for special shoes by orthotics. Two interviewees expressed frustration at not being turned in hospital after being placed on surfaces on which they could not turn themselves. When asked whether he had any coping strategies or advice for others, one of these interviewees (PU1, male, 40 years), who had spina bifida and hydrocephalous, and therefore a lifetime of managing pressure ulcers, said, ‘stay out of hospital’. Although some interviewees were concerned that their ulcers may have been avoidable, one elder interviewee was resigned to acquiring pressure ulcers during hospitalisation: ‘it’s just one of them things that happened you know’ (PU3, female, 90). An interviewee who had heel pressure ulcers from tight leg bandaging while in hospital with pneumonia, acquired during respite care, focused the interview on another ulcer, which she blamed on her own tendency to scratch. Interviewees who were wheelchair users all talked about risk and the complexities of avoiding pressure ulcers while still being able to live a full life.
Treatment outcomes most wanted by people with diabetic foot ulcers
Four out of five interviewees with diabetic foot ulcers said that what they most wanted from treatment was healing:
I want it to get better . . . Just get it healed.
DFU2, female, 80 years
. . . what I most wanted is for them to heal up quickly, which they haven’t done . . . Get them healed fast and get home.
DFU3, male, 70 years
I’m hoping it’ll heal.
DFU4, female, 83 years
To get them cured.
LDU5, male, 72 years
One of these interviewees also said, ‘I want them to take the pain away’ and ‘to make me walk’ (DFU4, female, 83 years). One persisted with treatment rather than amputation despite the gradual loss of toes and a long experience of leg ulcer recurrence:
. . . and then again it’s knowing it’s not going to be a success . . . because it’s like cancer. Although many, many years and moons have gone by, they’re still struggling to find that cure, right, and they haven’t found it yet.
LDFU5, male, 72 years
The exception here was one man facing the prospect of amputation who said what he most wanted was to get mobile again. At the time of the interview he had his wound for 3 months. A severe foot infection resulting in surgery for the removal of toes had been the trigger to finding out that he also had diabetes and needed a coronary artery bypass operation:
. . . so within a week of finding an infection, half my foot’s gone . . . I want to get mobile again . . . I’ve been sat here for 3 month now and it’s doing my head in . . . If you said to me right now, I’m going to perform an operation this afternoon, I’d say take that foot off because I can’t walk on it far, so it might as well not be there, do you know what I mean? I don’t [won’t] class myself as a cripple, alright I’ve had an operation. I might be disabled, I might not be able to drive a wagon again but I’m not housebound and I need mobility, you know, that’s what I need.
DFU1, male, 55 years
He was left with doubts about whether or not the surgeon should have tried to save his foot in the first place:
He was the only doctor available [an accident and emergency rather than diabetes specialist] and they have said, he was thorough and did them a favour . . . [he] is what they call a conservationist, he likes to conserve stuff instead of chop off, get rid. But the amount of money that it must cost the health service to do this, all that time because the [negative pressure wound therapy] you had to pay for daily. The medication that I’m on, the attendance by the nurses, it’s all expenses.
DFU1, male, 55 years
Treatment outcomes that matter most to people with surgical wounds healing by secondary intention
All six of these interviewees wanted healing:
The flesh has merged and we just want that final closure of an epidermis to finish it off.
A1, male, 63 years
I need it to close. I just want it to close. I need to be comfortable. I need it to be quick.
A2, female, 42 years
I just want it over and done with . . . just get rid of it all . . . to heal where it’s going to heal for life and I don’t have to worry about it any more.
A3, female, 59 years
. . . you just want it to heal and, you know, get better.
A4, female, 57 years
. . . to see light at the end of the tunnel . . . a resolution to it . . . I can start doing all the things I used to do.
A5, male, 35 years
One of these interviewees, who was left with a small wound 12 months after treatment, weighed his concern about his wound healing against his cancer survival: ‘these other things [the wound and stoma] are really more secondary. The fact that you’re alive still is the number one consideration’ (A1, male, 63 years). However, he was also concerned about being left with bodily disfigurement:
[T]here wasn’t really a good successful outcome to the healing of my wound anyway because I was still going to be left sort of body dysmorphic at the end of it . . . So other than the healing aspect, I had to deal with the other issues of the muscles not being joined together and just having to accept that situation as being . . . [it’s] the least worst case scenario. I’ve not had a recurrence of cancer, so you’re thankful for that . . . there were six of us [in hospital at the same time] and there are only two of us that are still alive, so you know, it puts it all into perspective.
A1, male, 63 years
This interviewee and two others were also coming to terms with having a colostomy:
[There was] very little preparation beforehand regarding how mentally it would affect me having a colostomy. And the consequences you know of the cancer treatment as well . . . very little counselling other than the night before. Even then I was given some news, he said ‘you realise you could be impotent and lose all erectile function’. I mean these things were just thrown at one.
A1, male, 63 years
All of these interviewees had undergone abdominal surgery. Two interviewees had undergone surgery for a perforated bowel and were being investigated for possible Crohn’s disease or colitis. A woman with an open wound for 8 months was trying to reconcile herself to accepting that there was little that could be done to speed up healing:
It’s like, you know it’s there [the open wound] and you’re putting up with it but it’s very frustrating that it is there and that nobody is doing anything about it but in some ways they can’t. So you’d like somebody to magically do something. Stitch it up and get rid of it but they can’t do it. It’s impossible to do.
A3, female, 59 years
She and others reflected on how the wound had happened and whether or not it might have been prevented:
. . . maybe if they’d stapled it, it wouldn’t have popped. I don’t know. Maybe it would, maybe it wouldn’t, I don’t know. I’d perhaps like to think that it wouldn’t have done because when it’s been cut three times in the same place, to stick it with glue seems a bit – I don’t know. It just literally ‘pfff’ all the way down. Whereas when it was stapled before at least it had a bit of a chance.
A3, female, 59 years
Now I’ve read from doing research from other parties that do colorectal cancers that what is supposed to be a good thing is for you to have a series of antibiotics before you go down to theatre. Now that wasn’t offered to me and neither was it suggested . . . So I do think that if I’d had . . . It would have helped.
A1, male, 63 years
The nurses were a bit concerned because there was a small internal stitch that had been left inside the wound and they thought maybe that was why it wasn’t healing, that maybe this stitch needed taking out.
A4, female, 57 years
I just think to myself if I’d been operated on at the time when they’d seen me and they probably would have saved themselves so much money, so it’s not a complaint about how the treatment went or anything like that or the surgery that I’ve had, it’s just the waiting time that I had to wait to have the initial operation. If that waiting time had been decreased, I don’t think we would have had all the subsequent problems that we’ve had afterwards.
A5, male, 35 years
One woman suspected that a short-term determination to cut costs meant rationing of more-expensive dressings, which she assumed would help her to heal more quickly:
Like I say, a lot of it is down to money, you know, like wasting time with all different dressings and I think they started with the cheapest first and worked their way up to the most expensive. Whereas if somebody has got a reaction to a dressing, try the more expensive one and if that works, well that’s good. It means it’s going to heal quicker. I mean maybe if they’d have done that, put the more expensive one on me first, they might have been able to leave it, you know, a couple of days and it might have healed quicker, so they would have saved money in the long run because, I mean they’re buying dressings all the time.
A4, female, 57 years
Three interviewees developed their wounds after what they had anticipated to be relatively routine or minor operations: a hernia operation, removal of the appendix and a keyhole sterilisation procedure, which ‘failed, so they had to cut me open’ (A2, female, 42 years). There were lots of comments from these interviewees about how waiting for the wound to heal was putting their lives on hold – they had a feeling that they were in limbo and wanted to get back to being able to carry out their usual activities:
[I want] to see light at the end of the tunnel . . . my life has stood still for about 12 months and I’d like to think that there is a resolution to it . . . I can start looking for work again. I can start exercising again. I can start doing all the things I used to do. That’s what I’m looking for more than anything else.
A5, male, 35 years
I need it to be quick . . . and not painful . . . when it’s closed I can get my life back on track and go back to work properly and go back to my job, my friends and I can’t [becomes tearful].
A2, female, 42 years
[I want] speed [laugh]! Because you think, god, how long is it going to take?
A4, W, 57 years
One interviewee who self-referred to the study said that, although his wound was healed, it still caused him pain. He did not know why he had developed the wound in the first place and suspected it was implicated in other health issues currently affecting him:
They have to tell me what is going on . . . why still now I’ve got that [wound] and why my tissue is not healing. Everyone tells me something different . . . Only I want them to, you know, provide for me [answers] like why my wound has been – that pain because sometimes pain is killing me and, you know, I can’t eat and I can’t – so that most I want from them is the treatment . . . to treat my wound [2 years after closure of a wound that was open for 5 months].
A6, male, 41 years
Summary of key findings
When asked what they most want from treatment:
-
Most people (29 out of 33) said that what they most wanted from treatment is healing.
-
Two people who had experienced barriers to getting treatment said that what they most wanted was access to treatment, ‘to be listened to’ (LU5, female, 85 years; IVG1, male, 40 years).
-
One man, who was deciding whether or not to have his foot amputated, said that what he most wanted from treatment was to become mobile again (DFU1, male, 55 years).
-
One woman, who thought she would die with her pressure ulcer, wanted it to be managed and not neglected (PU3, female, 93 years).
When asked what bothered them most about the wound itself, six people said pain, five people said the social embarrassment of the smell, three people said the social embarrassment of leakage, two people said the boredom caused by confinement to the bed/house, two said itching, two said the threat of infection, one said the strangeness of not having pain (neuropathy), some gave more than one answer and some did not answer or said ‘everything’:
. . . how would you like to stand in front of a friend when you smell, it’s like you’ve got body odour or something like that. It’s not nice and then you’ve got the pain as well, so you’re always rubbing it or trying to do something to make yourself comfortable, it’s not right when you’re being out with people to be in that situation. So it’s left me at home, you know . . . For the last 5 years I haven’t had a girlfriend. I’ve just got one recently . . . She said to me that she can smell it but she’s not bothered about it because she knows it’s such and such. She said to me, got to get something sorted out about it. I said I want to do, I don’t want to be like this forever.
IVLU1, male, 35 years
When asked if they could change one thing about the wound what that would be, most people did not opt for incremental change. Instead, they said that they wanted:
To get rid of it.
LU2, female, 80+ years
Take it away. Completely.
LU4, male, 66 years
Just to heal.
LU5, female, 85 years
To heal properly, but to heal properly and to have your legs back.
LU7, female, 88 years
Not to have it full-stop. I don’t want it full-stop. I wouldn’t wish it on my worst enemy.
IVLU1, male, 35 years
Get rid of them.
IVLU2, male, 36 years
I’d make my legs better.
IVLU3, male, 45 years
A new leg. With that not there.
IVLU5, male, 33 years
I’d take it away.
IVLU6, male, 39 years
If I could change it? Just not have it.
DFU2, female, 80 years
Others said that they would speed up healing, pay for skin grafts or go back in time and prevent the triggering incident/injury that started the wound. One interviewee said ‘make me walk’ (DFU4, female, 83) and one said ‘change the pain’ (A6, male, 41 years).
In summary, interviewees wanted to have access to treatment, to be healed and to ‘get their life back’ as soon as possible. The one exception was an elderly woman with pressure ulcers who thought that she would have her ulcer until she died and she most wanted to ensure that the wound would continue to be managed and not neglected. The social consequences of the wound were as troubling to many participants as the physical pain and discomfort.
A shared desire for fast healing and a return to mobility was common among people with different wound types, along with a shared frustration that life had to be put on hold pending healing. Those facing amputation of a limb wanted to know when to give up their hope of healing. Some of those with wounds associated with intravenous drug use were coming to terms with the fact that the underlying damage to their venous system might not be reversible once they stopped injecting drugs. Those with pressure ulcers and a leg ulcer ‘ambassador’ spoke most about their desire for healing in the context of managing the ongoing risk of acquiring another wound once their current ulcer was healed. Many of those with pressure ulcers and surgical wounds were left with patient safety concerns about the cause of wounds that were acquired while in health and care services and whether or not these could have been prevented.
Carers
Eight carers were interviewed; most contributed to an interview with the person they cared for and consented for these comments to be used. Two people were interviewed in their own right, with the person who they cared for being present on both occasions. The biographical details of the interviewed carers are summarised in Table 38.
| Carer ID | Carer age (years) | Relationship to patient | Patient ID | Patient sex | Patient age (years) | Type of wound |
|---|---|---|---|---|---|---|
| C1 | – | Wife | A1 | M | 63 | SWHSI |
| C2 | 55 | Daughter | DFU2 | F | 80 | DFU |
| C3 | 70 | Wife | LU4 | M | 66 | LU |
| C4 | 46 | Sister | PU5 | M | 57 | PU |
| C5 | – | Son | LU6 | F | 97 | LU |
| C6 | – | Husband | PU7 | F | 65 | PU |
| C7 | 86 | Wife | – | M | 89 | LU, PU |
| C8 | – | Wife | DFU3 | M | 70 | DFU |
Ideal treatment outcomes
Most carer interviewees said that ideally what they most wanted from treatment was for the wounds of the person who they were caring for to heal and for things to go back to how they were, but they knew that this was unlikely to happen:
Well, the impossible – as was [things as they were]. But that will never be.
C8
Take them off [her] and put them on me!
C5
Well best of all I would like him to be walking again, but I don’t think that will ever happen now . . . sometimes it seems to bother him a bit and I’d just like it to heal for his sake.
C7
This is rather dwarfed into insignificance compared to the other problems . . . You’re still stuck with it . . . Like old friends who won’t be parted. But we would quite happily be parted . . . I should imagine we’re always going to have to be very careful of it. It’s going to need protecting with a lot of care. But let’s get there first . . . The target’s moving back a bit. It’s moved back hundreds of times, but nevertheless it still would be nice if something could be done [to heal it] . . . [It’s been] three and a quarter years!
C6
The following interviewee was the carer of someone with a pressure ulcer and said that he wanted these wounds to be prevented in the first place:
Yes, we think prevention. It’s a bit annoying that these things are happening so much in hospitals. They do need to be reducing them, because it’s costing the NHS so much . . . orthopaedic people and plastic surgeons. The cost is just ridiculous to be quite honest. I know money’s short but you could save on that if you could stop this sort of thing. But maybe that’s the Holy Grail, that’s easier said than done.
C6
Disagreements about treatment
There were points of conflict between carers and the people they cared for about some interventions. Two were concerned about the reluctance of the person cared for to seek help. One, who feared losing his independence, was reluctant to call the doctor in ‘in case they cart him off’ (C4). Another refused to leave the house for appointments to treat multiple underlying conditions:
I think he’s just given up . . . This is the trouble. This is the worst thing for me. Because I can’t get him to go out . . . every year he’s supposed to go to the eye clinic [because of diabetes] and they would provide an ambulance but he won’t go. And he won’t have his ears tested . . . that’s the thing that I find frustrating. Well you can’t get any further, can you?
C7
Carers also sometimes feared the consequences of the surgical interventions that the person being cared for hoped would help resolve problems with the wound:
And that is the fear. He [the surgeon] can’t control your healing process. You’re diabetic. You’re classed as obese in the sense that you are not the skinniest thing . . . [as a consequence of inactivity because of the wound] so even that is another additional risk problem. The fact that you’ve had all of this . . . if you are worrying about appearances . . . from my point it does not worry me at all. I am probably the only one that sees that and I’m not saying it to make you feel better. I’d rather have you here than take the risk of a cosmetic situation and that’s how I feel and I still feel like that.
C1
You just go from week to week waiting for another ulcer to appear and it dries up and then it comes back and to me, that will be it. That’ll be what his feet will be doing now.
Not when I’ve had my feet straightened.
I don’t know. I don’t know.
Well, hopefully, anyway.
That’s all you can say, but you don’t know, do you? But you see, when he’s [had the operation] – what is it, you’re about 6 weeks aren’t you with them pegs in? So it’s [going to be] even harder.
Some carers had gone through periods when they felt that they were drifting along and there was treatment activity but no real treatment plan. This carer wished he had pushed more to see a tissue viability nurse but he did not know about them at the time:
It just seemed to be a bit of a – not a losing battle, but we weren’t really getting anywhere. It would have been nicer if we could have a tissue nurse in, if there was such a thing . . . We just seemed to be plodding on and not getting anywhere. ‘Til that setback came [heel damage caused by orthotics] and the really prompt reaction – I’m not criticising anybody, but it’s just the way that it . . . We should have pushed more . . . The district nurses, one of them came one day and said . . . I think we’ll be coming in years to come unless we do something. I should have thought of that and pushed it myself earlier on. I didn’t know what to expect. I didn’t know anything about pressure sores at all. I didn’t know much about them other than they were a nuisance.
C6
Most interviewees praised the treatment that the person they cared for had received but some were ambivalent about the effectiveness of the interventions they had seen:
I think she’s always had fairly good treatment from the surgery and the doctors and nurses who have been to her. They’ve always given her – everything that’s new that’s come out, they’ve tried. So I mean, there’s not much else they can do really . . . They actually just heal themselves, I think. They use something that will heal up, and then the next time she gets one they’ll try it and it doesn’t work. So then they try other different things.
C5
Nevertheless, carers appreciated someone coming to ‘keep an eye on it’:
Well all she does, she looks at it, measures it sometimes and changes the plaster and puts cream on his legs. Because his skin’s very dry. But apart from that, nothing. I think more or less to keep an eye on it . . . I don’t know enough about it, really . . . I would have thought that if they cleaned whatever the debris is there it would perhaps heal better, but I don’t know. She did put a little pad on today, because it wasn’t healing, as well as the plaster. So, you know, they do try different things now and again . . . the advantage to them coming, you see, is they can keep an eye on it. Because I mean, I don’t know enough about them.
C7
Over the longer term some of the carers felt that there was a lack of interest in the outcome and that they were being left to get on with it:
But sometimes though because it’s so long and it’s so chronic and these wounds are chronic whether they’re ulcers or . . . you sometimes have to re-address them a little bit further on . . . and you can only re-address them if somebody bothers to ask or if somebody asks, ‘oh has he healed yet?’ or something like that. Nobody knows whether he’s healed or not. I’m sorry but this sounds terribly like moaning and it’s not moaning but how can you possibly know whether a patient has recovered from that unless you enquire to what the outcome is.
C1
The following carer cared for someone with a surgical wound healing by secondary intention. She said that at first there had been lots of people around, to the point when it sometimes felt that ‘your life is not your own’ (C1); however, over time she felt that she lacked the reassurance of nursing back-up or follow-up:
[At the start] you have a lot of support – I don’t know whether you have support but you have a lot of people around you, so you feel like you’ve got that support. Then it became obvious that once . . . in that respect, I think I felt let down . . . in the sense that you needed that kind of nursing support. We didn’t need the physicality, the fact of a nurse being able to do it. I’m perfectly able to do that [change dressings]. If somebody says to me, look let’s try this, do this, I’m perfectly able to do it . . . I wasn’t to start off with . . . I was looking through my fingers when I had to look at him, that’s how scared I was but it’s not a problem anymore. It’s become second nature but I just think maybe a phone call might have been to see how he’s getting along or whatever. I suppose they could feel that if you need them you’ll ring but sometimes that’s not what you want to do . . . Just somebody to pick the phone up and say, do you need anything and then I can say, ‘no I don’t’ or I can ask them, ‘what do you think?’ . . . I know everybody is stretched every which way with whatever and I know there is an older lady on her round that hasn’t got anybody to come in and has to take priority. No harm is going to befall [him].
C1
Some carers performed no physical wound care. This carer had been specifically told not to:
I’ve only changed the plaster once . . . occasionally the catheter leaks – it [the dressing] was wet and came off. So I stuck another one on. They told me off about it. They said, ‘oh you mustn’t do that. You must ring us’. Well, I mean, it doesn’t take a science degree to do that, does it? I thought I’d done quite well . . . I’m not supposed to touch them at all.
C7
Other carers had become used to applying dressings:
When it [pressure ulcer] first appeared it’s not normal for somebody to have a big hole, is it? It was a bit scary at first but obviously over time you get used to it . . . I wouldn’t say that I know too much about them . . . I know how to change his dressing and basically [he] relays it to me and I get all my information from [him].
C4
The following carer said that nurses would answer her questions if asked but, especially as she was involved in dressing the wound, she would like to be more actively included in discussions: ‘the key thing is [to feel] trust’ (C4). Some carers felt cautious about annoying health professionals and the person who they were caring for with their questions:
I think me mam’s one of these, she doesn’t tend to ask. It’s me who asks. Me mam’s more laid back than me. I want to know everything . . . I get worried over her and I’m asking all the time.
C2
We always get a list of questions, don’t we? They think, ‘oh, he’s one of them’.
C6
One couple spoke about the difficulty of having to watch their ‘p’s and q’s’ to not offend health professionals with questions and comments while also being worried about the consequences of disagreements and conflicting advice:
. . . one [surgeon] wanted to take it out and drain it and another one said, no we won’t and . . . and then he thought maybe it would heal on its own. Well it didn’t heal on its own because he had to come home and I had to empty the bag . . . with an abscess . . . oh the stench of that was bad.
C1
One carer felt that she was not very good at asking questions and that health professionals were mostly too busy to talk:
. . . an odd one will say something. Mostly they’re so busy they just come in and bang bang . . . There are some that are very good and talk, but mostly they . . . I mean, they are quite friendly, but they are so busy . . . I think most of them take the attitude that they don’t expect you to understand really. They just sort of get on with their job. An odd one will say something, but not really . . . I’m always interested. I mean, you learn things that way don’t you? . . . I’m not very good at asking questions.
C7
One carer was applying support stockings and compression bandages and felt that she needed more support to make sure she was doing the right thing:
I’m shown something, how to do it, then I can do it . . . I never know, with not being a professional, I’m doing it the right way; the correct way. But obviously, I have been doing it the correct way . . . he can’t bend . . . I mean [he] couldn’t do it himself. But I don’t mind doing it, you know, as long as, shall I say, we both get support . . . And quite frankly, I feel that . . . we’ve been let down by the district nurses. I feel as though, yes, there’s probably other people more important than we are, but for something as serious as [leg ulcers and] lymphoedema, you have to have proper bandaging done, and because, yes, I can do it, but am I doing it right? Am I doing it correctly? Do I know what I’m looking for once the bandages come off and [he] gets a shower and you know he showers his legs. Do I know what I’m looking for? Because I don’t. I don’t know if it’s good or bad. We’ve said this before, haven’t we? . . . And as regards feedback, the only feedback we did get is from [nurse] at the lymphoedema clinic. And she said, yes, they’re going on all right. And she automatically says, but your ulcer is not healing. Have you been to your GP [general practitioner]? . . . And what does the practice nurse do there? ‘Oh yes it’s okay, so don’t bother coming again’. So I mean, honestly, it seemed as though we were between a rock and a hard place.
C3
One carer said that what she would really have liked was a care plan:
. . . it would be wonderful if they could have . . . A care plan and we don’t have that because you do need it. You are scared. You think, what’s going to happen if . . . I mean basically you’re just waiting to see if something rears it head again and then you deal with it. There is none of that continuity there . . . There’s nobody to ask . . . your GP doesn’t know.
C1
There was a lot of sympathy for how busy nurses were and a sense that they were fulfilling physical care tasks but concerns that there was no clear treatment pathway for patients engaged with many different services and that no one was overseeing or managing cases:
I suppose you’re thinking that the district nurse should cover all of those things but sadly a district nurse really goes out to do wound care, this or that. They really can’t go beyond that. They don’t know anything about stomas . . . They certainly can’t deal with psychological issues or anything like that or feelings, so it is a tough one . . . because of the volume of patients that they have and they have to spread themselves thinly, [they] are literally going in . . . to deal with just wound care . . . They’re there to do that specific one task and we’ve had to find out different things even from all your sexual issues and urinating and all that and we had to branch out in all these different directions. Nobody could deal with it and it would have been lovely if somebody could have given us some pointers . . . There’s no one point where you can say, I’ll point you in that direction and I know it’s possible to do it . . . You have to find your own support network basically.
C1
Carers in the study were more likely than patients to express doubt that the interventions being applied to patients would lead to healing. Nevertheless, they wanted health professionals to ‘keep an eye’ on the wound. Patients and carers both felt that waiting for the wound to heal left them in limbo. Carers expressed frustration at a lack of overall case management of the wound and feelings of being abandoned by health professionals and being left to cope with the wound.
Staff
Twelve health-care professionals with responsibility for wound care were interviewed, one man and 11 women. The biographical details of the professional interviewees are summarised in Table 39. Eleven participants described themselves as white British and one as white Irish. The mean age of staff participants was 44 years and the median age was 47 years (range 23–53 years). The mean length of service with the NHS was 19 years and the median was 22 years (range 1–32 years).
| Staff ID | Sex | Age (years) | Ethnic origin (self-description) | Occupational title (band) | Years in NHS |
|---|---|---|---|---|---|
| HPTVN1 | F | 47 | White British | Team leader for tissue viability (8a) | 29 |
| HPNFA2 | F | 48 | White British | Specialist practice nurse (6) | 25 |
| HPPOD3 | F | 52 | White British | Podiatry specialist team leader (8a) | 22 |
| HPPOD4 | F | 23 | White British | Community podiatrist (5) | 1 |
| HPPOD5 | F | 53 | White British | Community podiatrist (6) | 25 |
| HPENDQ | F | 50 | White British | Executive nurse, director of quality | 32 |
| HPTVN7 | F | 47 | White British | Clinical nurse specialist, tissue viability (7) | 22 |
| HPTVN8 | F | 37 | White British | Clinical nurse specialist, tissue viability (7) | 15 |
| HPCN09 | F | 43 | White Irish | Community staff nurse (5) | 22 |
| HPTVN10 | F | 50 | White British | Clinical nurse specialist, tissue viability (7) | 28 |
| HPCN11 | M | 26 | White British | Community nurse specialist (5) | 1 |
| HPDN12 | F | 48 | White British | District nurse (7 protected) | 10 |
Treatment outcomes aimed for and considered most important by staff
Health professionals aimed to make a positive difference for patients although many of them rarely saw the final outcomes of their interventions. When asked what treatment outcomes they aim for, most health professionals said healing, but this was often qualified: ‘you find out that that’s not realistic’ (H3) and ‘there’s clearly going to be some chronic wounds where that’s not a realistic outcome’ (H5). Outcome aims then became ‘improvement’ (H3, H2, H5, H4), for the ‘patient to feel positive’ (H2) and (for tissue viability nurses) determining the cause of the wound and why it is not healing. There were two mentions of finding a treatment with which the patient is able to comply and one mention of the prevention of recurrence.
Methods described to determine the cause of wounds included assessment of circulation, nutrition, sources of pressure damage and levels of activity. Investigation was also said to involve checking that the treatment given is appropriate, advising on lifestyle changes to promote wound healing and referring patients on to appropriate sources of treatment and support including vascular surgeons, smoking cessation services and dieticians.
All health professionals aimed for symptom management outcomes that focused on the removal of slough and the reduction of pain, odour and exudate. If a wound was the result of pressure damage, health professionals ensured the provision of appropriate offloading and pressure-relieving devices and services. Infected wounds were treated with antibiotics.
Two podiatrists said that that the outcome that they aimed for was to find a treatment with which the patient was able to comply. One health professional in a predominantly preventative role (H1) described most of the healing that could be achieved as ‘fragile healing’ and identified the prevention of recurrence as an important treatment outcome.
One tissue viability nurse said that sometimes she thought goals for treatment outcomes were different for patients and health professionals:
I think sometimes our goals are different to their goals . . . Well our goal is we want to heal it but the patients might not be. They might just want . . . I’ve had a patient before in a wheelchair . . . They can’t feel half their body, so they’re really not that fussed. It doesn’t give them any pain but what they don’t want the wound to be, they don’t want to be wet through with exudate and they don’t want it smelling and as long as that’s contained, they can carry on doing what they’re doing and they’re happy.
H10
Most challenging aspects of wound care for staff
Many of the health professionals interviewed said that one of the most challenging things about working with patients with chronic wounds was not seeing or knowing if patients heal. For example, two tissue viability nurses and a podiatrist said that it was challenging not knowing whether or not their work made a difference because they did not find out the outcomes for patients who they had treated:
We don’t get to know what’s happened with them . . . what’s the outcome.
H2
Just finding out when they heal would be helpful . . . do patients that come under our umbrella have different outcomes to the patients that don’t? . . . We don’t know what our outcomes are really . . . we don’t have a service model that takes all patients with wounds [and] follows them through to healing and it’s not really right that we do in lots of ways because otherwise you just end up with a very de-skilled generic nursing population.
H12
. . . you want to see the end of the tale kind of thing.
H10
Most health professionals discussed how repetitive and time-consuming wound care could be and the difficulty of keeping themselves and their patients motivated over long periods of uncertainty about the outcome:
It can be a really slow moving thing . . . it’s having the patience.
H2
The longevity of the wound.
H11
It’s time-consuming [and] trying to keep motivated yourself that this is actually going to heal and hence motivate them.
H1
Most challenging thing is probably . . . Sometimes you feel like you’re going in day in and day out . . . even though you’ve got all the various people on board . . . you’re just going in there and it looks exactly the same and it’s not changing and it’s not getting any better and . . . it’s kind of out of my hands because there is vascular people involved, tissue viability nurses involved and I’m just going in there and just doing what the care plan says basically and you kind of think, it’s up to them isn’t it what they do at the top. I’m just going in bandaging and not seeing any improvement. There is nothing else that you can particularly do really.
H8
Most health professionals described the challenge of getting patients to comply with their advice:
Very challenging is when you can see that you are not going to heal this wound and that is challenging, especially if . . . it’s because they make unhealthy choices . . . this is his choice . . . But we cannot be effective, we cannot help this person, that’s challenging.
H1
. . . sometimes it’s hard to get patients on your side, you know, make them understand that you’re trying to help them . . . You have patients who don’t want to listen, don’t want to help themselves . . . you’ll find those are the ones who have chronic ulcers a lot longer than others.
H2
If they’re non-compliant with their diabetes, if they’re smoking and not looking after themselves or if they’re walking about barefoot and neuropathic, you’re sometimes up against it with people.
H6
In particular, they described the challenges they faced getting people to attend appointments and to comply with advice about managing blood glucose levels and weight, moisturising skin and wearing the right footwear. Nurses raised the challenge of getting leg ulcer patients to go to bed rather than ‘insisting on sitting in a chair’ (H10). Some interviewees acknowledged that it could be difficult for patients to comply with some of the advice they gave:
they’re human beings after all.
H1
One of my most frustrating things is weight management . . . because the [chronic wounds on the] legs are the main concern we tend to be the main person managing . . . care when [actually] . . . The legs are a knock-on effect. The diet and other support and psychological support need to be the main thing . . . it’s really frustrating . . . because they need not just dietary input, they need psychological input as well, support. I think they get a raw deal . . . They don’t get much here . . . just get a dietician visiting every month to 6 weeks when they need somebody going in every week going you’re doing really, really well. Like Weight Watchers at Home service, you know, somebody coming around and giving you loads of encouragement.
H10
Some of the interviewees discussed the challenging issue of ‘ownership’ of the wound and whether or not their services were creating a culture of dependency rather than encouraging behaviour change:
I think as nurses we own patients’ problems, we own their wounds, you know, it’s like if things aren’t improving, you hear district nurses saying all the time, ‘oh well I’ve tried everything, I don’t know what else we can do, I’m really sorry this hasn’t worked, oh let’s try this new dressing’ and yet . . . standing back from it, kind of observing this situation that the patient sat at the side of an ashtray spurring forth tab ends or . . . they’re still grossly obese . . . yet nothing is said about the bit that the patient can do. But you also haven’t done your bit and I think patients do need to do their bit because there is only so much we can influence and control.
H12
Sometimes they don’t think it’s about them, it’s what are people doing for them.
H6
It’s difficult because some patients will come in . . . and you can tell they’re . . . really aggravated with this ulcer and they have, I’d probably say a poor attitude, they just don’t care. Just let you get on with it and they don’t say anything to you and it’s really hard because you want to know what’s going on . . . and especially how they’re dealing with it at home because . . . we find that a lot of patients self-neglect . . . I think it’s denial a lot of it and then some patients will come in and just be like, I really need your help.
H2
. . . we sometimes say the leg belongs to them and not to us because I think over the years and months nurses go in and take over this bandaging and the elderly frail lady at home that doesn’t get any visitors . . . thinks very much the nurses keep coming . . . I think we take over where . . . the leg belongs to the patient and not us . . . we forget to step back . . . you get into this routine.
H4
I think all they think of is, ‘oh well the dressing they’re going to put on there is going to cure it’. Well it’s not, it’s actually . . . them . . . kind of taking control of their treatment in a way, helping themselves and a lot of patients you find really don’t do that. They don’t really help themselves that way.
H8
The challenge of dealing with patient expectations for healing came up in many of the interviews:
Just dealing with their expectations, you know, with them thinking, ‘oh I should be healed by now and it isn’t healing’. I think it’s important that you’ve got to remember that, that ulcer is their ulcer . . . It’s not just what everybody else can do for them.
H6
. . . you go in and you know they’ll go, ‘is it getting any better?’ and it clearly isn’t. Whereas the district nurses might go, ‘oh yeah, yeah’, you know, just to keep them [happy] . . . [the patient] chose to listen to the ones who said it was getting better.
H11
Their expectation I think is quite challenging . . . you can’t dash people’s hopes . . . sometimes they get really frustrated if you keep changing the treatment but if you don’t change it . . . it might end up being a chronic wound that you’re managing forever but when new treatments come out and you try it, you know, that can be quite challenging. You’ve got to really draw on change management techniques haven’t you to change people’s attitudes. If they’ve been having four-layer bandaging forever and we actually turn round and say, oh that’s no good now, I think we’re going to change the dressing – I think that’s probably frustrating.
H9
Many of the responses reflected the difficulties of what one interviewee described as ‘more pressure for delivering complex care in the community at both policy and demographic level’ (H5). The professional motivation of being able ‘to make a difference’ or ‘see people heal’ was particularly challenging when working with some groups of patients:
. . . pressure ulcer patients definitely are, your immobile pressure patients with lots of different comorbidities are sort of heart sink and your grossly obese patients as well like with their swollen wet legs are heart sink because even if you get them to recognise they need to lose weight that’s not something that happens overnight and therefore you’re going to manage the problem in the interim period.
H12
Nurses discussed the challenges of working with people exhibiting signs of self-neglect, for example ‘bad hygiene and [a] house . . . you can’t go into’ (H7), and also issues of safeguarding and the challenges of dealing with particularly unpleasant surgical wounds in a patient’s own home:
So we actually had to go in and open it up like a sandwich and fill it . . . with a filler, fasten it back up, pull it over and tape it on with Hypafix to keep it together, it was disgusting. It wasn’t nice. That was quite difficult for some members of staff because as soon as you take the Hypafix off it just went ‘phew’ . . . there are lots of wounds like that . . . [and] the possibility of safeguarding or, you know, it’s an unstageable wound that we’ve found. Could we have done anything about that? There’s a lot of reflection around wound cause analysis, you know.
H9
Some health professionals described the challenge of delivering a good service within current resources and sometimes the difficulty of understanding the reasoning behind the treatment decisions colleagues were making:
. . . in terms of nurses having enough time and . . . [their] knowledge base . . . are they assessing and picking up what they really need to pick up and then if it starts to deteriorate do they act on it? Or why do people take the decisions that they have taken?
H4
A key consideration in choosing treatments was to minimise the number of visits to patients or appointments at a clinic. Two reasons were given for this: one was to reduce patient disruption and the other was to minimise clinical costs. Health professionals therefore sought ‘products that are going to have the biggest impact on improvement as quickly as possible’ (H5). Some interviewees found it particularly challenging to select the correct treatment choice and to apply their knowledge of the evidence base to patients eager to see healing:
. . . making sure you get the right dressing and the most appropriate for that patient.
H7
. . . for me the challenging one is knowing sometimes what the evidence might say and therefore how do we follow that because . . . you can’t just immediately put the compression on because you need to do pain first and you need to manage the exudate but I need compression but I can’t do that because the patient is in too much pain. What’s causing that pain and how do we unpick that or maybe they’ve got arterial problems, a mixed leg ulcer arterial and venous and how do I balance both or am I waiting for them to go to vascular? But in the meantime a good patient that’s desperate that they’re wanting to move things on.
H4
Sometimes it’s very obvious what needs to be done, you know . . . you’ve got very sort of tangible outcomes . . . Other times it’s not at all easy because there isn’t the armament. You don’t have the armaments available to manage the problem or the things that you’ve got available to you have been rejected by the patient and they’re not interested in pursuing that kind of treatment because they’ve had a bad experience with it previously or they’re quite nervous. So then, you know, you’ve got to do quite a lot of investing time to try and explain the rationale and the research evidence behind things and how you know [that] it’s worked before with other patients and how it’s probably worth a try even though they’ve had an unsuccessful attempt previously.
H12
Some tissue viability nurses expressed frustration at expectations from colleagues who expected them to have a ‘magic dressing’ to cure a chronic wound:
Because we’re the experts, we’ve got the magic dressing haven’t we in our bags! We’ll get tissue viability in, they’ll sort it, there’s the magic dressing. There isn’t one, you know, and they still say that now even when you’re doing the study days. ‘We want to know what dressings to put on’? ‘What dressing for what type of wound’?
H11
. . . when you do training, they often come with this idea that you’re going to tell them what dressing to put on everything and they come with that expectation and when they leave without that information, they’re always desperately disappointed. So we’ve started almost saying, you will not leave today knowing what dressing to put on what wound for every patient because it isn’t about the dressing, it’s about the assessment of the patient and identifying what needs to be sorted out in order to enable healing and the dressing is like your last consideration in a way. It’s just the best plug for the hole.
H12
One interviewee felt that access to dressings was restricted and found this frustrating. She had previous experience at a trust that allowed direct access to a wider range of dressings. These dressings were thought to be effective based on her previous experience of using them:
. . . sometimes it can get quite frustrating when you’re putting on the same dressing all the time and you know that this is really not doing anything and something else would really benefit this patient, but we’re not allowed to access that because those specific dressings and resources are only for specific people in specific teams. I find that really frustrating because . . . I suppose you’ve got to look at cost and all the rest of it . . . [but] I’ve seen something work elsewhere . . . [they] had a cupboard full of all these fantastic dressings, not necessarily expensive but just really good and I saw that first hand that those worked. So I think coming here, it was hard . . . we only have three types of dressings . . . that’s really difficult when you know that these [other] dressings really work on patients . . . when I refer people on, I do make suggestions and say, look I know this works, perhaps try that.
H2
There was a sense from tissue viability nurses that patients shared the idea that there was a ‘magic dressing’ and that this was reinforced by dressing manufacturers and some health-care professionals:
. . . as my colleague . . . says, ‘you wear your glasses to create vision and you’re wearing your bandages to create your circulation’. If you take them off, your circulation will just go back to where it was because [patients] they’ll come to us and say, ‘oh is there not a cream I can put on it or a dressing’ and you’re like, nothing is going to fix it from the outside and it’s the same with pressure if you’re going to sit on your bottom all day, it’s going to be there. No plaster can fix that. There is not a wonder dressing out there that’s going to make that better.
H10
I mean I suppose they [patients] expect a solution and if they’ve got a wound, I suppose they expect some part of that solution is going to be whatever covers the hole. But I think the myth the patients [have] is just reinforced by staff . . . all the adverts that you see, you know, these beautiful bodies that have got dressings on, you know, that just got better magically . . . Nobody ever has the kind of bottom that I have to put dressings on [laugh]. Not on any advert that I’ve ever seen. So it’s just marketing isn’t it and it’s pedalled by industry that the dressing is the solution. Wound care solutions, you know, that’s often what the mantras are of the various companies. They provide wound care solutions.
H12
What most bothers patients (from the staff perspective)
When asked which aspects of having a chronic wound they thought most bothered their patients, the health professionals all identified pain, smell and exudate. Much of the pain and discomfort discussed was associated with compression bandaging and dressing changes. Most also identified restrictions in mobility caused by the wound and by their treatment interventions. Restricted mobility was associated with social isolation, a lack of independence and disengagement from society as a consequence of, for example, being unable to work, being advised to stay in bed or fear that the wound (signified by having to wear potentially leaky and smelly, bulky, pink or brown bandaging, not being able to wear ‘normal’ shoes or carrying a portable negative pressure wound therapy machine) might be negatively perceived by others.
Some health professionals identified patients as being most bothered by feeling fed up and frustrated with waiting for the wound to heal. One tissue viability nurse described some patients enduring ‘an endless rollercoaster of optimism and despair’ (H12). She attributed this to:
. . . new products . . . being prescribed and applied and then that not working and then another product being prescribed, recommended and applied and the optimism raises and then drops again . . . I think because we’re not able to say who won’t heal.
H12
In addition, some nurses identified restrictions on showering and bathing because of the need to keep dressings and bandages dry, the chore of having to ensure the correct dressing supplies were available in the house and occasional discomfort caused by wrongly applied dressings. A podiatrist identified the fear of losing a limb.
Some interviewees identified patients as being most bothered by feelings of intrusion from lots of appointments with different health professionals: ‘I would be very frustrated if I had a lot of different clinicians coming in with perhaps different levels of competency’ (H5). Some said that intrusion into the private space of the home or ‘living at the clinic’ (H1) could leave patients feeling that ‘their life is not their own’ (H1), although others said that they thought some patients particularly welcomed home visits. One community nurse thought that constant appointments could lead to patients ‘feeling old’ and as if they are ‘sponging off the system’ (H8).
Good outcomes from interventions
Health professionals described the personal satisfaction that they felt when patients were ‘pleased with the outcome’, ‘valued your intervention’ and have ‘had a positive outcome as a direct result of your intervention’ (H12). A speedy outcome was particularly welcome:
. . . anything within a reasonable time, if you can get some success and improve their comfort, then that’s what it’s all about really . . . improving people’s lives.
H6
. . . any patients whose wounds have healed are a success story. I mean the quicker the better!
H7
There was a particular sense of satisfaction if the professional’s own intervention was perceived as the one that made a direct difference, resulting in a positive outcome for a patient previously treated unsuccessfully for some time. Examples included:
-
Getting leg ulcer patients into compression who had previously been treated only with dressings: ‘eventually [they] get referred and . . . you get them in[to] compression and then you heal them really quickly’ (H12).
-
Choosing a key dressing or topical treatment: ‘They may have had lots of antibiotics but actually it wasn’t cellulitis it was wet eczema . . . prescribe some topical steroid and come back a week or 2 weeks later and you can clearly see that the patient is a completely different person and their leg is completely different and you go away and say, job well done’ (H4); ‘I packed it with iodine . . . I packed it from the inside and it just healed in no time’ (H2). The subsequent reoccurrence of this wound did not diminish a sense of achievement.
-
Dexterity with dressings: ‘It was such a complicated dressing to get a seal on [negative pressure wound therapy to anus and perineum] . . . It was only me who could ever do it or that’s what they’ve told me!’ (H9).
-
Getting patients with foot ulcers into the right footwear and offloading damaging pressure.
A sense of satisfaction at a good outcome also came from effectively liaising with other health-care services, ‘put[ting] all the things in place’ (H8) and getting agreement to progress a treatment plan. Some health professionals reported satisfaction from identifying the root cause of the problem and from increasing the mobility of patients who are not going to heal: ‘it was never going to heal but she did manage for quite a while and she managed to get out again’ (H9). A health professional who said that the people she saw heal were ‘few and far between’ regarded patients changing some aspects of their damaging lifestyle as a good outcome, for example:
We’ve got one guy . . . he’s been coming to us for about, 6, 7 years . . . and he’s had this chronic leg ulcer and it has actually got smaller and smaller and it’s almost healed and that’s largely because he’s changed his lifestyle. He’s stopped injecting and he’s eating healthier and actually he’s put on an immense amount of weight. He’s trying to reduce his smoking at the moment and he’s actually taken it a bit further, he’s getting quite involved and quite vocal in some of the groups that are going on . . . I consider him a success really if we can just get this last bit of wound healed then I would consider him a success.
H3
Concerns about negative outcomes from interventions
Table 40 summarises the range of concerns that staff raised about the potential negative outcomes for patients associated with particular wound care interventions used.
| Number of staff reporting concerns | Intervention | Negative outcomes |
|---|---|---|
| 7 | Compression bandaging | Pain, discomfort, reduced mobility, social embarrassment leading to reduced social engagement, fixed ankle |
| 7 | Prohibitions and limiting recommendations on footwear | Social embarrassment leading to reduced mobility and reduced social engagement, loss of the freedom to walk barefoot |
| 5 | Dressings | Allergic reactions |
| 4 | Support stockings | No benefit or skin trauma because of an inability to get them on or difficulty with getting them on, social embarrassment for men, wrinkles forming dangerous tourniquet |
| 3 | Negative pressure wound therapy | Reduced mobility/social engagement |
| 3 | Prohibition on getting dressings and bandaging wet | Discomfort of being unable to bathe or shower, social embarrassment, reduced social engagement |
| 3 | Bed rest | Reduced mobility/social engagement, laundry burden for those with leaky legs |
| 3 | Rearranging domestic sphere, e.g. special seating, rearranging furniture, sleeping downstairs | Family disruption, potential expense |
| 2 | Analgesia | Side effects, no benefit because of poor prescribing for neuropathic pain, potential abuse of pregabalin and gabapentin |
| 2 | Contradictory advice on the importance of undertaking exercise for vascular health, keeping limbs raised and keeping off ulcers for wound healing | Confusion and uncertainty, further physiological damage, reduced mobility/social engagement |
| 1 | Antibiotics | Side effects |
| 1 | Topical antiseptics | Side effects |
| 1 | Topical barrier creams | Allergic reactions |
| 1 | Prescription costs | Expense for paying patients |
| 1 | Contradictory advice from the clinical team on limb conservation or amputation | Confusion and uncertainty, reduced mobility/social engagement |
All of the health professionals dealing with people with leg ulcers recognised four-layer compression bandaging as the evidence-based gold standard treatment for achieving healing as an outcome in venous leg ulceration. However, compression bandaging was contraindicated in some cases because of arterial disease or the risk of deep-vein thrombosis, and many interviewees reported examples of patients not being tolerant of it because of discomfort or because they were not able to wear their shoes. This impacted on their mobility and desire to go out:
. . . unless you . . . prescribe special footwear then you’re actually perhaps consigning the person to be stuck in their own home . . . you wouldn’t necessarily need to be house bound if you didn’t have four-layer bandaging. I think some of the things that we do prescribe can really challenge somebody’s lifestyle and sense of independence.
H5
As noted previously, there was also concern about the quest for the ‘magic dressing’ and the potentially disruptive impact of ongoing interventions on patients in terms of being able to get on and live their lives. There was also a concern that the ‘emotional roller coaster . . . cycle of optimism and despair’ may have a negative impact on healing outcomes:
. . . and we don’t have any concept of what the impact of despair has on healing and kind of feeling that everything that everyone has tried is useless and that they’re in a hopeless situation. We haven’t really got an understanding of how that affects us metabolically, physiologically but I think that’s ultimately what we put our patients through a lot of the time.
H12
Discussion
Most patients and health professionals in the study identified healing as a primary treatment outcome and were focused on achieving that healing as quickly as possible while managing pain, infection and discomfort. Most also spoke about the difficulties that they were experiencing achieving that healing and their concerns about preventing recurrence. Health professionals reported a lack of sense of achievement because they rarely saw the outcomes of their interventions, whereas many patients and carers reported feeling left ‘in limbo’ and as if their lives were ‘on hold’. They wanted to ‘get their lives back’.
The treatment model described in the interviews was in keeping with the ‘specialist healing’ rather than ‘chronic care’ route identified by Briggs and Flemming142 in their synthesis of qualitative research on living with leg ulceration. Wound care interventions were largely targeted at the acute phase of what was, for most, a long-term condition, often symptomatic of other underlying conditions. Health professionals reported frustration with unrealistic expectations from patients of short-term healing. However, the specialist healing treatment model may in itself raise or reinforce expectations for fast and complete healing. Some tissue viability nurses identified a cycle of optimism and despair that left patients feeling hopeless and some said that this was fuelled by a futile quest for a ‘magic dressing’ to ‘fix wounds from the outside’. A short-term technical focus on finding the right product to heal the wound also gave rise to concerns about health professionals taking ‘ownership of the wound’ themselves and becoming frustrated with patients not doing more to enable their own healing.
Some carers in the study questioned whether or not the treatment activity that they witnessed was actually leading to any particular outcomes. However, patient and carer interviewees appreciated that health professionals were very busy and were trying different things and welcomed the reassurance of someone ‘keeping an eye on it’. Carers expressed concerns about the difficulty of navigating health services for patients with multiple conditions and the possible negative outcomes for those they cared for of refusing or seeking further interventions. There were also fears from carers about health professionals losing interest over the long term and being left to get on with it, a sense of being abandoned with the wound.
The patients in our study were as troubled by the socially inhibiting consequences of their wound as by the wound itself. This is in keeping with the finding of Corbin and Strauss143 that ‘the main issue for people who are chronically ill is not sickness but their body failure and what it does to their activities and their lives’ (p. 278). Health professionals and patients associated many treatment interventions with negative outcomes including reduced mobility and social embarrassment leading to reduced social engagement. In this broader context, some of what may be viewed as ‘non-compliance’ by a health professional may feel like a strategy of self-care from a patient’s perspective to minimise the impact of a condition and/or a treatment on daily life. Surgical wounds healing by secondary intention and pressure ulcers raised particular safety concerns for patients and their carers when these were thought to be an outcome of health intervention.
Most of the patients in the study were living with multiple conditions that presented a complex mix of physical, social and psychological challenges for them. 144 Wound care services were focused on the physical but not the psychological or social impacts of having a wound. This is part of a wider debate about whether or not the current design of NHS care delivery is suited to the needs of people with chronic conditions and multimorbidity and whether or not these patient groups face barriers to effective self-management/self-care. 145
Health professionals in the study discussed the changes to services that they would like to see to improve outcomes but expressed uncertainty about the future design and direction of wound care services. The study took place during the passing and implementation of the Health and Social Care Act 2012,146 which brought in the most wide-ranging reforms of the NHS since it was founded in 1948 at a time when the UK economy was experiencing one of the most prolonged economic downturns since the economic depression of the 1930s. Concerns about the costs to the NHS of preventing and treating their wounds were raised in many of the patient and carer interviews. One health professional with a managerial overview discussed the problems of a short-term, technical focus on outcomes and expressed a need to make more moves towards a social rather than medical model of care:
. . . [there are] too many people [health professionals] down the garden path all doing a little bit of something . . . if you’re thinking it’s going to be a relatively short-term curative outcome then you perhaps wouldn’t invest quite as much in the self-care approach . . . So I don’t know, perhaps . . . you should always be invested in the self-care approach because actually by people being well informed, they’re more likely to be able to manage prevention in the future . . . we’ve got very much a medical model . . . I think still and although we work towards more social models of care, I think for most people, for myself, as members of the public, you know, I would want something that was healed and so it would take me a little while to accept perhaps that I was in a position where it was chronic and wasn’t going to heal . . . it’s really essential for the future that we start to do that and to change our horizons because there’s no point in setting unrealistic goals.
H5
The study demonstrates the frustrations felt by health professionals, patients and carers in seeking to achieve the desired outcome of healing. It raises questions about determining the balance between care and cure in services for people with chronic, complex wounds who want healing but also want to be able to live as full a life as possible while managing wounds. A short-term focus on achieving a quick technical fix through dressings and other wound care devices relies on the assumption that these devices are effective at achieving healing. A more socially reflexive approach to wound care would consider interventions in the context of the wider impacts of the condition on patients’ lives and seek to mitigate any negative outcomes from interventions for patients and their own capacity for self-care.
Finally, our in-depth qualitative study of patients, carers and health professionals makes no claims to be representative of the views of all people delivering and receiving care for chronic, complex wounds at home. The breadth gained by covering a range of chronic, complex wounds in the study may be at the expense of the particularity and diversity of experience within particular wound types. Intravenous drug users with complex wounds constitute 21% of the sample of people interviewed compared with a point prevalence of 5.64 per 1000 in the prevalence survey. This inclusion brought down the mean age of the overall sample, which is 10 years younger than that captured in our prevalence survey. Referrals of intravenous drug users to the study were exclusively male and increased the proportion of men in the sample (54.54% vs. 42.7% in the prevalence survey).
Funding sources and the quality of reports of complex wounds trials: 2004–11
(Adapted from the previously published paper by Hodgson et al. 114)
Introduction
Randomised controlled trials within the field of complex wounds, as within all areas of medicine, represent an essential part of the evidence base because of their ability to provide unbiased estimates of relative treatment effects. The generation of such unbiased estimates depends on adequate steps being taken to minimise bias. For example, it has been demonstrated that failure to conceal allocation and failure to blind outcome assessors and participants results in biased, larger estimated effect sizes. 147,148
The failure to specify a primary outcome a priori can also introduce bias as authors are free to cherry-pick outcomes for reporting that show a statistically significant difference. 149 As well as methodological features of RCTs that can minimise bias, other elements of study design also impact on the overall quality of evidence generated. These design features include sample size, choice of primary outcome and the duration of post-randomisation follow-up. Trial sample size is important because, although estimates from small trials are not necessarily biased, they will be underpowered to detect anything but the largest treatment effects, also leading small trials to have been associated with publication bias. 150 The choice of primary outcome is similarly important and needs to be meaningful to both clinicians and patients. Duration of follow-up is linked to issues of study power and outcome selection. Important outcomes such as wound healing can take a long time to achieve (often months for chronic wounds). Studies with a short duration of follow-up will potentially miss outcome events and be underpowered. One approach is the selection of other surrogate outcomes that can be measured over a shorter period; however, these may not be valid proxies for, or may be harder to interpret than, clinically meaningful outcomes.
The importance of methodological characteristics such as those outlined above has led to a number of studies being conducted that seek to quantify the methodological quality of samples of RCTs from across medicine151 and within specific areas. 152–154 However, despite the methodological concerns described above, no such overview exists within the area of chronic wounds, an area of medicine in which most treatments are medical devices for which RCT data are not necessary for licensing and marketing.
Previous research in other areas of medicine suggests that funding can have an important impact on a number of trial characteristics. For example, studies have observed that industry-funded drug trials are more likely to report in favour of (the sponsored) treatment than research that is not funded by a commercial organisation. 155,156 Research has shown that commercially funded studies are associated with a shorter duration of follow-up,157 more frequent use of non-active comparators158,159 and lower risk of bias. 156,160,161 In the area of chronic wounds there are few data on the prevalence of industry-funded trials or the influence of industry funding on trial design.
Objectives
-
To critique and summarise the methodological characteristics of RCTs in complex wound care.
-
To determine the prevalence of industry-funded trials in chronic wound care.
-
To investigate whether or not funding source is associated with features of methodological quality in complex wound RCTs.
Methods
Study selection
A library of eligible RCTs in chronic wound care was assembled and used. Eligible studies were:
-
randomised comparisons of treatments for foot, leg or pressure ulcers in any setting and for any category of patient (studies that included other wound types as well were excluded)
-
published between 2004 and 2011 (inclusive)
-
reported in English (because of a lack of translation resources).
Studies were excluded if they were:
-
secondary reports in which the primary report of the main study was referenced
-
conference abstracts (as these typically do not contain sufficient methodological information to assess risk of bias)
-
a trial protocol with no results
-
a cost-effectiveness study (unless they also reported the effectiveness of an intervention)
-
a phase I trial (as these are not aimed at determining effectiveness).
Trials were considered to be a RCT and included if they were described as ‘randomised’ either in the title or in the text of the paper. If no randomisation process was referred to, they were excluded.
This library was constructed by searching the Cochrane Wounds Group Specialised Register of Trials. The register is maintained by the Cochrane Wounds Group and aims to identify all randomised and quasi-RCTs in the area of wounds research. Reports are identified for inclusion in the register by regular searches of a number of databases including MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Cochrane Central Register of Controlled Trials (CENTRAL) along with periodic searches of other databases. Studies included in the register have been coded on several criteria including wound type. A search was therefore carried out using the search terms Pressure* or Venous or Leg* or Ulcer* or Diabet* in the condition field and Treat* in the intervention field.
The titles and abstracts (when available) of the identified studies were screened by a single author to exclude studies that obviously did not meet the inclusion criteria. The full text was obtained for all studies that were potentially relevant. Two authors independently checked the full papers for eligibility. At this stage duplicate reports of the same trial were identified and grouped prior to extraction. These reports were thus extracted as one study with maximal data extraction across all reports available. Any disagreements regarding inclusion/exclusion were resolved through discussion and when agreement could not be reached a third author acted as arbitrator.
Data extraction
For each eligible study relevant data were initially extracted by one of seven authors. A second independent extraction was completed by a single author (blinded to the initial extraction). Any disagreements were resolved through discussion between reviewers. When agreement could not be reached, a single third reviewer arbitrated. The process of data extraction was extensively piloted with data recorded on a Microsoft Access database, using drop-down menus when possible.
Data were extracted on the following characteristics (Table 41):
-
the publishing journal and its impact factor (when multiple reports of the same study were available we used the publication with the highest impact factor here)
-
journal specialty
-
trial design (parallel or other)
-
number of randomised groups
-
wound type
-
treatments evaluated
-
sample size
-
duration of follow-up
-
primary outcome if defined with type of outcome also extracted
-
funding source
-
information on study methodology.
| Extracted item | Brief description |
|---|---|
| Journal specialty | Journals were categorised as general medicine, wounds journal or other specialty journal using MeSH data from the National Library of Medicine |
| Funding | We defined funding source as commercial, non-commercial, mixed commercial and non-commercial, not reported or unclear. The decision regarding funding source was based on published disclosures of full or partial funding. In the absence of a funding disclosure, employment of an author by a commercial enterprise was considered commercial funding even in the presence of any other funding statements. When a conflict of interest statement was recorded as none, ‘non-commercial’ was recorded even if the funding source was not given |
| Primary outcome | Primary or main outcomes defined explicitly or an outcome used in a power calculation or a main outcome described explicitly in primary study objectives |
| Type of outcome | When defined primary outcomes were classified as complete healing if primary outcome was proportion healed or time to complete healing; surrogate healing if the primary outcome was any other healing-related outcome; and non-healing if the primary outcome was a non-healing outcome such as the presence of infection or pain |
| Sequence generation | Method described for generating the randomisation sequence used to allocate participants to study groups, including computer-generated sequences, random number tables and coin toss |
| Allocation concealment | Method described to prevent the individual responsible for enrolling trial participants from knowing or predicting the allocation sequence in advance, including central randomisation or sealed, opaque, sequentially numbered envelopes |
| Blinding of assessors | Outcome assessors had no knowledge of group allocation or it was judged that the outcome and the outcome measurement were unlikely to be influenced by lack of blinding (e.g. mortality) |
| Overall risk | Low risk of bias: study was at low risk of bias in all three domains (sequence generation, allocation concealment and blinding of outcome assessors); unclear risk of bias: study was at unclear risk of bias in one or more domain and at high risk of bias in no domains; high risk of bias: study was at high risk of bias in one or more domains |
When classifying funding source in the absence of a funding disclosure in study reports, we did not consider any of the following as constituting funding by a commercial organisation (i.e. we took a conservative approach):
-
declarations of consulting
-
speaking fees
-
honoraria
-
stock ownership
-
commercial funding of a study product in the absence of other commercial funding for the study.
When a paper explicitly stated that the study was independent, non-commercial funding was recorded, even if the funding source was not provided (as long as there was no evidence in the paper that authors were employed by a commercial organisation). In the absence of a funding disclosure, employment of an author by a commercial enterprise was considered commercial funding even in the presence of any other funding statements. When a conflict of interest statement was recorded as ‘none’, non-commercial was recorded even if the funding source was not reported.
When no funding information was found in a paper, the reviewers checked the International Standard Randomised Controlled Trial Number (ISRCTN) database for a record of the study. When the source of funding field contained relevant information this was extracted. If funding details were not provided in the paper and were not found on this database then ‘not reported’ was recorded.
In situations in which the reviewer was unsure how to class a funding body that had been provided in a report, a Google search was conducted to locate further information to help establish whether the body was commercial or non-commercial. If there was no further information then funding was reported as unclear. If information was found the two reviewers discussed the nature of the funder and classification. When they were unable to make a decision regarding a funder’s status, details were discussed with a third reviewer. A central log of funders was kept for reference so that all reviewers could ensure consistency.
The risk of bias for each study was also assessed using the Cochrane risk-of-bias tool162 for the domains of sequence generation, allocation concealment and blinding of outcome assessors. An overall assessment risk of bias was also made following Cochrane guidelines163 (see Table 41 for definitions used in assessments).
Data analysis
Descriptive summary statistics were calculated for each of the general and methodological items specified and outcomes were stratified by wound type and by funding source. The descriptive summary statistics were then used to compare the methodological quality of commercially funded and non-commercially funded RCTs for which mean differences or odds ratios (ORs) (as appropriate) with 95% CIs were calculated. To assess the difference in study duration between studies with different funding sources mean differences were logged. The results were initially recorded in Microsoft Access and Stata 12 (StataCorp LP, College Station, TX, USA) was used for data analysis.
Results
The results of the study selection process and reasons for exclusion are presented in Figure 6. We identified 647 potentially eligible studies. After an initial assessment of titles and abstracts, 385 studies were excluded and full-text copies of the remaining 262 potentially eligible studies were obtained. Ninety-five studies were excluded for the following reasons: 36 concerned an ineligible wound type; 26 concerned an ineligible study type/design; three were duplicate publications of existing studies; 23 were secondary reports of included studies; one paper was not in English; two were trial protocols; and four studies were excluded because of exceptionally poor reporting that made judgement of whether or not the study met the inclusion criteria impossible. The remaining 167 studies met the inclusion criteria.
FIGURE 6.
Study selection process.
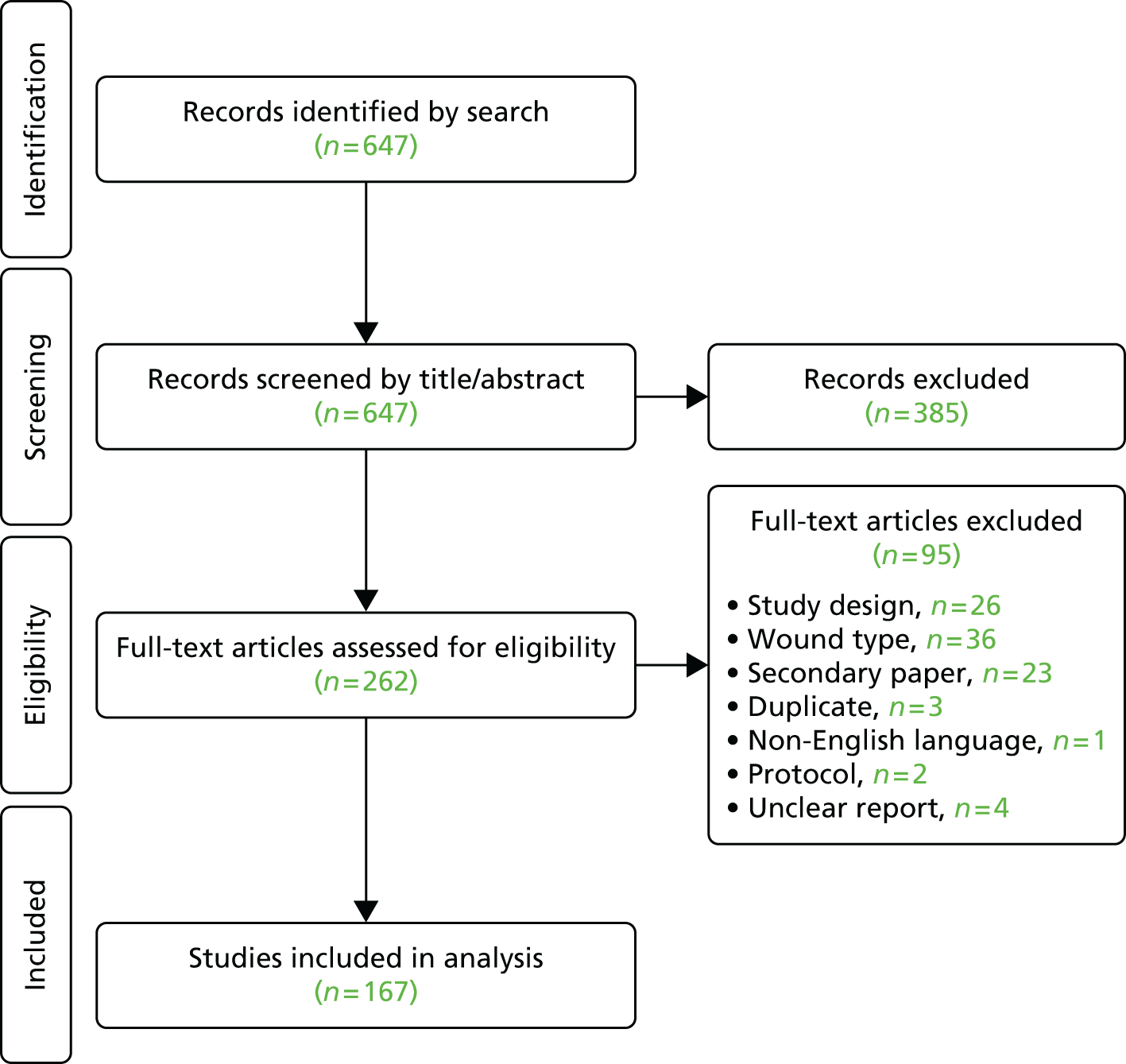
Table 42 presents the general characteristics of the 167 included studies stratified by wound type. Leg ulcers were the most frequently investigated of the three wound types (38%; 63/167), closely followed by foot ulcers (34%; 57/167). Only 19% (31/167) of the studies focused on pressure ulcers over the 8-year period investigated. A further 10% (16/167) of trials investigated more than one wound type.
| Characteristic | All (n = 167), n (%) | Leg ulcers (n = 63), n (%) | Pressure ulcers (n = 31), n (%) | Foot ulcers (n = 57), n (%) |
|---|---|---|---|---|
| Journal type | ||||
| General medical | 11 (7) | 3 (5) | 4 (13) | 2 (4) |
| Wounds specialty | 57 (34) | 26 (41) | 10 (32) | 13 (23) |
| Non-wounds specialty | 99 (59) | 34 (54) | 17 (55) | 42 (74) |
| Impact factor, median (IQR) | 1.93 (1.21–3.01) | 2.38 (1.26–3.21) | 1.63 (1.36–2.48) | 1.48 (0.80–2.90) |
| Funding | ||||
| Commercial | 58 (35) | 24 (38) | 7 (23) | 16 (28) |
| Mixed | 10 (6) | 3 (4.8) | 2 (7) | 5 (8.8) |
| Non-commercial | 55 (33) | 20 (32) | 14 (45) | 16 (28) |
| Unclear/not reported | 44 (26) | 16 (25) | 8 (26) | 20 (35) |
| Study design | ||||
| Parallel | 160 (96) | 58 (92) | 30 (97) | 56 (98) |
| Other | 7 (4) | 5 (8) | 1 (3) | 1 (2) |
| Study groupsa | ||||
| 2 | 144 (90) | 52 (90) | 27 (90) | 51 (91) |
| 3 | 14 (9) | 6 (10) | 2 (7) | 4 (7) |
| 4+ | 2 (1) | 0 (0) | 1 (3) | 1 (2) |
| Intervention | ||||
| Bandages/stockings | 14 (8) | 14 (22) | 0 (0) | 0 (0) |
| Dressings/topical agents | 42 (25) | 20 (32) | 10 (32) | 10 (18) |
| Drugs | 33 (20) | 8 (13) | 4 (13) | 16 (28) |
| Growth factors | 16 (10) | 0 (0) | 0 (0) | 13 (23) |
| Tissue grafts | 11 (7) | 4 (6) | 1 (3) | 5 (9) |
| Other | 51 (31) | 17 (27) | 16 (52) | 13 (23) |
| Comparators | ||||
| Placebo | 50 (30) | 14 (22) | 12 (39) | 18 (32) |
| Usual/standard care | 43 (26) | 20 (32) | 8 (26) | 12 (21) |
| Named comparison | 74 (44) | 29 (46) | 11 (35) | 27 (47) |
The majority of trials were parallel in design (96%, 160/167), with only 4.2% (7/167) using other trial designs (either crossover or factorial). Most (parallel) trials had just two intervention arms (90%, 144/160), 9% (14/160) had three arms and 1% (2/160) had four arms. The most frequent intervention types investigated were dressings and topical agents (25%, 42/167), drugs (20%, 33/167), growth factors (10%, 16/167), bandages and stockings (8%, 14/167) and tissue grafts (7%, 11/167). There were a further 23 different technologies investigated.
The majority of trials were reported in specialty journals (93%, 156/167), with 34% (57/167) published in wounds journals. The remaining studies (7%, 11/167) were published in general medical journals. Of the included studies, 77% (128/167) were published in a journal with an Institute for Scientific Information® impact factor. The median impact factor for the 128 studies was 1.93 [interquartile range (IQR) 1.21–3.01].
In total, 35% (58/167) of the studies were reported as having been commercially funded, 33% (55/167) were not commercially funded and 6% (10/167) had funding from both commercial and non-commercial sources. For the remaining 26% (44/167) of studies either the source of funding was not reported or the status of the funding source was unclear.
Table 43 presents information on a number of methodological characteristics of the included trials. The median number of participants was 60 (IQR 35–99) and there was a median of 28 (IQR 16–48) participants per treatment arm.
| Characteristic | All (n = 167), n (%) | Leg ulcers (n = 63), n (%) | Pressure ulcers (n = 31), n (%) | Foot ulcers (n = 57), n (%) |
|---|---|---|---|---|
| Number of participants, median (IQR) | ||||
| Overall | 60 (35–99) | 81 (43–126) | 44 (26–60) | 50 (30–86) |
| Per treatment arm | 28 (16–47) | 37 (21–62) | 21 (7–30) | 27 (15–43) |
| Duration of follow-up (months), median (IQR) | ||||
| All | 2.8 (1.6–5.6) | 3.0 (2.8–6.0) | 1.9 (0.9–3.3) | 3.0 (1.4–4.7) |
| Primary outcome: complete healing | 5.6 (3.0–6.1) | 6.0 (3.0–12.0) | 3.9 (1.3–6.0) | 4.7 (4.7–5.6) |
| Primary outcome: surrogate healing | 2.8 (1.7–3.7) | 2.8 (1.9–3.0) | 1.6 (0.93–2.8) | 1.9 (1.1–3.4) |
| Primary outcome: non-healing | 1.7 (1.4–2.1) | 1.9 (1.6–36.0) | 1.4 (1.4–1.4) | 1.9 (1.4–3.9) |
| Primary outcome: not defined | 2.8 (1.3–4.3) | 3.0 (1.6–5.6) | 1.8 (0.85–3.0) | 2.8 (0.93–3.7) |
| Primary outcome | ||||
| Complete healing | 40 (24) | 18 (29) | 4 (13) | 16 (28) |
| Surrogate healing | 47 (28) | 15 (24) | 14 (45) | 12 (21) |
| Non-healing | 11 (7) | 3 (5) | 1 (3) | 6 (11) |
| None | 69 (41) | 27 (43) | 12 (39) | 23 (40) |
| Sequence generation | ||||
| Low RoB | 67 (40) | 27 (43) | 15 (48) | 19 (33) |
| Unclear RoB | 98 (59) | 36 (57) | 15 (48) | 37 (65) |
| High RoB | 2 (1) | 0 (0) | 1 (3) | 1 (2) |
| Allocation concealment | ||||
| Low RoB | 41 (25) | 22 (35) | 5 (16) | 11 (19) |
| Unclear RoB | 123 (74) | 41 (65) | 24 (77) | 45 (79) |
| High RoB | 3 (2) | 0 (0) | 2 (7) | 1 (2) |
| Blinding of assessors | ||||
| Low RoB | 56 (34) | 13 (21) | 15 (48) | 23 (40) |
| Unclear RoB | 64 (38) | 24 (38) | 13 (42) | 22 (39) |
| High RoB | 47 (28) | 26 (41) | 3 (10) | 12 (21) |
| Overall RoB | ||||
| Low RoB | 10 (6) | 4 (6) | 2 (7) | 3 (5) |
| Unclear RoB | 107 (64) | 33 (52) | 24 (77) | 41 (72) |
| High RoB | 50 (30) | 26 (41) | 5 (16) | 13 (23) |
The median duration of trial follow-up was 2.8 months (IQR 1.6–5.6 months) and varied significantly depending on the nature of the primary outcome. Studies reporting surrogate measures of healing (e.g. change in ulcer size) had a median follow-up of 2.8 months (IQR 1.7–3.7 months), whereas studies with a primary outcome of either time to complete healing or proportion of wounds completely healed had a median follow-up of 5.6 months (IQR 3.0–6.1 months).
The proportion of trials that defined a primary outcome measure was 59% (98/167). Of those that defined a primary outcome, 41% (40/98) reported a measure of complete healing as the primary outcome (either time to complete healing or proportion of wounds completely healed), 48% (47/98) reported an intermediate (surrogate) measure of healing (e.g. change in ulcer size/area) and 11% (11/98) reported a primary outcome that was unrelated to healing (e.g. pain).
The Cochrane risk-of-bias assessment162 revealed that, of the 167 included trials, only 40% (67/167) described an appropriate method of random sequence generation; in 59% (98/167) of studies the method of random sequence generation was unclear or was not reported and 1% (2/167) of studies reported using inappropriate methods of sequence generation such as alternation or date of birth. Only 25% (41/167) of studies reported adequate methods of allocation concealment such as remote telephone randomisation or sealed, opaque, sequentially numbered envelopes; in 74% (123/167) of studies the method of allocation concealment was unclear or not reported and 2% (3/167) of studies did not adequately conceal allocation. With regard to blinding of outcome assessors, 34% (56/167) of studies were classified as being at low risk of bias, 38% (64/167) had an unclear risk of bias and 28% (47/167) had a high risk of bias. Following Cochrane review guidelines to construct an overall risk-of-bias assessment,163 30% (50/167) of studies were judged to be at high risk of bias, 64% (107/167) had an unclear risk of bias and only 6% (10/167) had a low risk of bias.
Table 44 presents the methodological quality of the included studies stratified by funding source. The sample sizes of commercially funded trials were not statistically significantly different from those of non-commercially funded trials (difference 8 participants, 95% CI –25 to 41 participants). Differences in duration were modelled using a log model because of the data being highly skewed. There was no statistically significant difference in the duration of follow-up between commercially funded and non-commercially funded trials (with commercially funded studies having, on average, 1.3 months less follow-up, 95% CI –1.8 to 1.06 months). Commercially funded trials were no more or less likely to specify a primary outcome than non-commercially funded trials (OR 1.48, 95% CI 0.74 to 2.94) and were no more likely to identify a surrogate healing measure as their primary outcome (OR 0.68, 95% CI 0.29 to 1.57).
| Characteristic | Fully or partly commercially funded (n = 68), n (%) | Non-commercially funded (n = 55), n (%) | Funding unclear/not reported (n = 44), n (%) |
|---|---|---|---|
| Number of participants, median (IQR) | |||
| Overall | 63 (40–117) | 52 (28–91) | 60 (29–98) |
| Per treatment arm | 30 (18–51) | 26 (14–44) | 23 (14–41) |
| Duration of follow-up overall (months), median (IQR) | 2.8 (1.9–5.6) | 3.0 (1.9–6.0) | 2.0 (1.2–3.5) |
| Primary outcome | |||
| Complete healing | 17 (25) | 15 (27) | 8 (18) |
| Surrogate healing | 26 (38) | 11 (20) | 10 (23) |
| Non-healing | 5 (8) | 4 (7) | 2 (5) |
| None | 20 (29) | 25 (45) | 24 (55) |
| Sequence generation | |||
| Low RoB | 23 (34) | 31 (56) | 14 (32) |
| Unclear RoB | 43 (63) | 24 (44) | 30 (68) |
| High RoB | 2 (3) | 0 (0) | 0 (0) |
| Allocation concealment | |||
| Low RoB | 21 (31) | 13 (24) | 7 (16) |
| Unclear RoB | 45 (66) | 41 (75) | 37 (84) |
| High RoB | 2 (3) | 1 (2) | 0 (0) |
| Blinding of assessors | |||
| Low RoB | 23 (34) | 23 (42) | 10 (23) |
| Unclear RoB | 19 (28) | 20 (36) | 25 (57) |
| High RoB | 26 (38) | 12 (22) | 9 (20) |
| Overall RoB | |||
| Low RoB | 3 (4) | 5 (9) | 2 (5) |
| Unclear RoB | 37 (54) | 37 (67) | 33 (75) |
| High RoB | 28 (41) | 13 (24) | 9 (20) |
There was no statistically significant difference between commercially funded and non-commercially funded trials with regard to the likelihood of being classified as having either a high or an unclear risk of bias in the domains of sequence generation (OR 0.63, 95% CI 0.34 to 1.16), allocation concealment (OR 1.08, 95% CI 0.62 to 1.88) or blinding of outcome assessors (OR 0.84, 95% CI 0.47 to 1.50). There was no statistically significant difference between industry-funded and non-industry-funded trials in the odds of being classified as having a high or an unclear risk of bias overall (OR 0.91, 95% CI 0.54 to 1.52).
Discussion
There is clearly a ‘disconnect’ between the number of people with complex wounds and the huge impact that these wounds have on patients and services, and the volume and quality of the evidence base. Our review of the quality and relevance of complex wounds trials revealed a number of problems. Although our prevalence study found that pressure ulcers were the most prevalent type of wound, our methodological review found that only 19% of recent chronic wounds trials investigated pressure ulcers and many of those trials were of poor quality.
A significant proportion of complex wounds trials did not specify a primary outcome, raising the risk of authors cherry-picking results (i.e. presenting outcomes for which there were statistically significant differences). In a related study, Lockyer et al. 164 found that 86% of wound trials that did not define a primary outcome claimed a significant treatment effect. This raises deep suspicion of bias given the inadequate statistical power of trials in this field.
The use of surrogate healing measures (e.g. change in ulcer size) is widespread and the majority of studies that defined a primary outcome used these intermediate measures of healing. The cost of longer-term follow-up is likely to be the primary reason for this. Clearly cost savings are an advantage of using surrogate outcomes and there is some evidence that a number of surrogate end points, such as initial change in ulcer size, are good predictors for the clinically meaningful end point of complete healing for some wounds. 126,127,165 There are, however, a number of reasons to remain cautious about using surrogate end points. First, surrogate measures of healing, such as change in ulcer size or area, introduce the complex issue of how to measure ulcer size and have the potential for inaccuracies and lack of reliability in systems of measurement. A recent systematic review into the performance of instruments for the measurement of pressure ulcers revealed little evidence to support the reliability of different methods of measurement, particularly with regard to ulcer depth and volume. 165 Second, although some studies have shown surrogate healing measures to be good predictors of complete healing, what is less clear is their ability to differentiate between treatment effects. It is not enough that a surrogate predicts the clinical end point, it must also be able to predict changes in the clinical end point because of different treatment effects. 125,166
The average sample size observed in our review was very small. The statistical power of a hypothetical trial involving 60 participants (the median sample size) in which approximately 50% of ulcers heal in 12 weeks18 (the median duration of follow-up) and in which it is assumed that there is a modest effect size of 15%18 would be just 21%. In other words, only one in five of the trials in our sample is sufficiently large to detect a statistically significant treatment effect should one exist. The vast majority of chronic wounds trials are therefore underpowered to detect all but very large effects. Although there is some debate regarding the importance of adequately powering a trial to detect a difference, there is evidence that published small trials yield larger effect sizes than large trials,150 most likely as a result of publication bias. The fact that many chronic wounds trials are small means that it is very important that systematic reviewers check for publication bias whenever possible. Equally, it is important that authors register trials so that reviewers can identify ongoing and potentially unpublished material.
The risk of bias in the reviewed studies was a concern, with only a minority of studies being at low risk of bias. Assessment of bias was, however, hindered by poor reporting, with the vast majority of studies at unclear risk of bias for at least one domain. Although it is encouraging that only a small minority of studies were at high risk of bias for either sequence generation or allocation concealment, it is impossible to make any judgement about the prevalence of high risk-of-bias trials in these domains when such a large proportion of trials are poorly reported. Previous research has suggested that reporting is worse than conduct167,168 and perhaps the majority of studies are at low risk of bias; however, the previous studies167,168 were conducted in highly regulated fields and it would be inappropriate to generalise to the area of chronic wounds trials. Even under the most optimistic scenario (that the unclear risk trials reflect poor reporting rather poor trial methodology) there remains a substantial risk of bias in chronic wounds trials because of almost one-third of trials not blinding outcome assessors.
The methodological quality of commercially funded trials and non-commercially funded trials was not significantly different across all of the included measures of methodological quality. Based on current evidence it is not possible to draw conclusions regarding the influence of funding source on the methodological quality of chronic wounds trials, although we acknowledge the limited power in these analyses, which in turn also limits the conclusions that can be drawn. Furthermore, the overall standard of trial quality was very poor and wound triallists share a culture that international trial design, conduct and reporting standards do not seem to have penetrated. Almost one-quarter of the sample had an unclear funding source – more transparency in declaring support for research will allow more studies to be included in future analyses of this type.
Our results are very similar to those obtained in other methodological reviews that used samples of trials from across medicine, for example that by Hopewell et al. 168 Chronic wounds trials are, therefore, not considerably methodologically weaker than trials conducted in some other areas of medicine. The cohort assessed in the study by Hopewell et al. ,168 however, was much older and dated back to 2000, whereas this study includes trials up to 2011, thus limiting comparability, particularly as the impact of the Consolidated Standards of Reporting Trials169 may mean that trials are now of higher methodological quality than observed by Hopewell et al. 168 It should also be noted that the methodological quality of reports assessed by Hopewell et al. 168 was poor and the fact that chronic wounds trials may be of comparable quality is no reason for complacency or celebration.
James Lind Alliance Pressure Ulcer Partnership
Background
The role of people with chronic, complex wounds in wounds research is usually limited to ‘being objects of study and beneficiaries of research results’. 170 Complementary to our research into which outcomes matter to patients, carers and clinicians, and our analysis of the nature and quality of completed wounds research, we embarked on a study to determine the patient, carer and clinician research priorities in pressure ulcer prevention and treatment. We situated this work firmly within the JLA and chose pressure ulceration as the focus of this work (as opposed to leg ulcers or any other kind of complex wound) because they are the most common complex wound type (see Chapter 2) and because they are a high priority for the NHS. 133 We adopted a mixed-methods, participatory research framework for this priority-setting work. There is no gold standard method for health research topic identification and priority setting and reporting in this area is predominantly descriptive rather than evaluative. 171,172 The JLA approach follows an open and consultative model in which patients, carers and health professionals work together in PSPs to encourage those directly affected by a specific condition to submit their key ‘uncertainties’ (normally in relation to treatment effects). 173 These are then ranked and widely disseminated.
Objectives
Our objectives in this study were to:
-
bring patients, carers and health professionals together to identify and prioritise the top 10 uncertainties, or ‘unanswered questions’, about the effects of pressure ulcer interventions that they agree are most important
-
investigate methodology for patient and public involvement in research priority setting in wound care.
Methods
We began this work by developing a protocol in close consultation with advisers from the JLA (a JLA guidebook has subsequently been published173). Although this work was conducted in close collaboration with the JLA and our methods were informed by the emergent JLA approach, we were also influenced by other approaches and in particular the dialogue model, another framework for research agenda setting, which includes both consultative and deliberative methods and allows for emergent and flexible design. 170
Emergent and flexible design was considered important because a significant proportion of people with pressure ulcers find it difficult to participate in activities outside the home because of their age and comorbidities. Furthermore, and distinct from most other PSPs, there were no existing patient groups specifically representing the interests of people with (or at risk of) pressure ulcers. These factors indicated that it would be necessary to try more than one method and to vary methods between groups to develop an accurate understanding of the clinical uncertainties of pressure ulcer patients, carers and health professionals.
The process, outlined in the protocol and which we followed, is summarised in Figure 7.
FIGURE 7.
Overview of the JLAPUP process. UK DUETs, UK Database of Uncertainties about the Effects of Treatments.
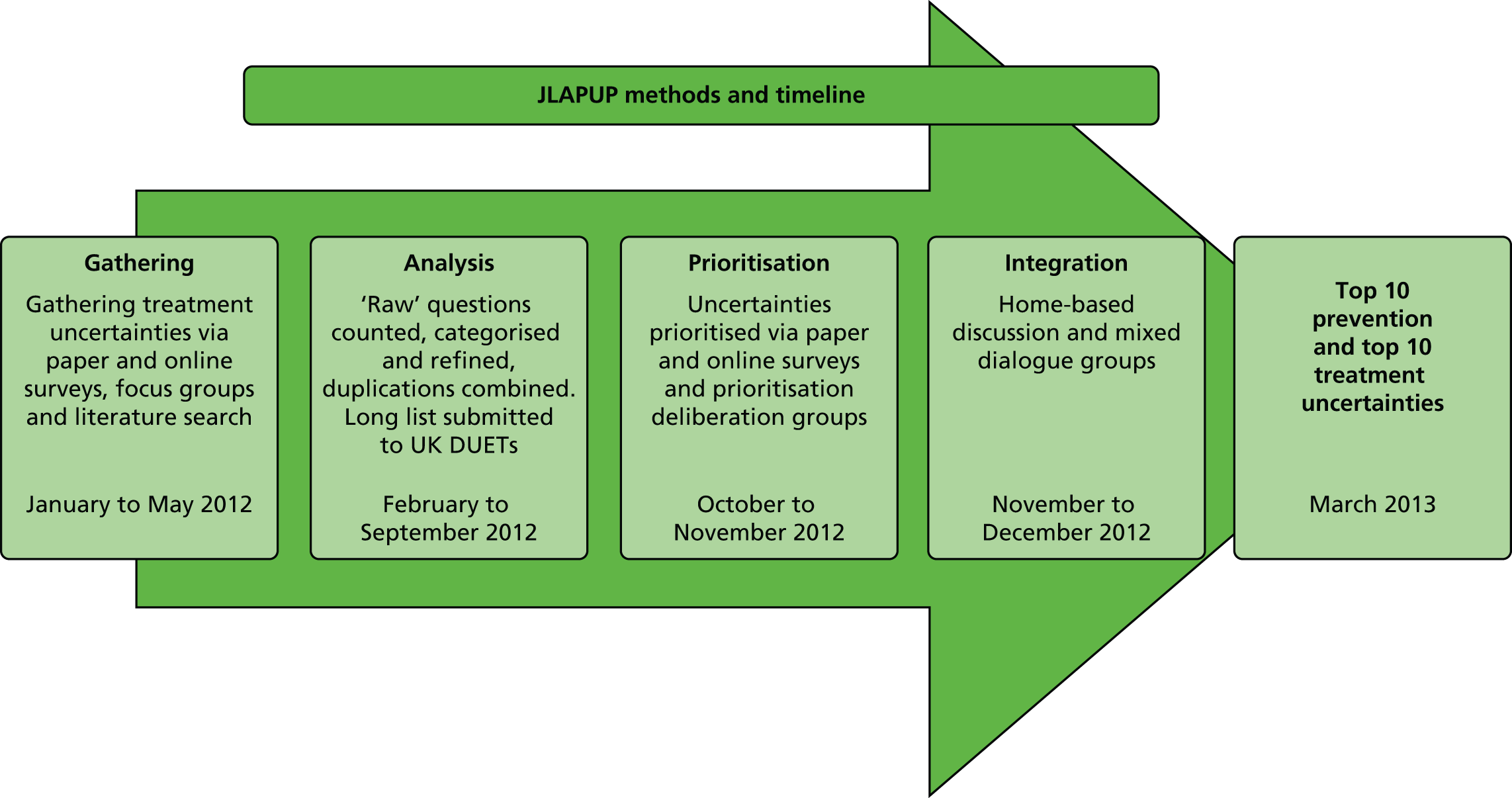
Exploration
During the exploration phase, potential partner organisations and individuals with an interest in pressure ulcers were identified through peer consultation, an internet search and the JLA register of affiliates. An initial search of research recommendations from systematic reviews and informal conversations with health professionals and researchers in the field provided exploratory information about the clinical uncertainties that might concern different stakeholders. A lack of good-quality research evidence and the absence of a patient stakeholder voice in the field were quickly apparent.
Initiation
During the initiation phase, interested organisations were invited to appoint a representative to attend an exploratory meeting. A press release was issued and paper and e-mail invitations were widely disseminated to advertise the event. People with experience of pressure ulcers (either as recipients or as providers of care) came together with representatives of groups/charities with an interest in the management and treatment of pressure ulcers, the JLA and researchers and clinicians from this research programme at a meeting to (1) identify treatment and prevention uncertainties associated with pressure ulcers, (2) identify opportunities for and challenges to the establishment of the JLAPUP, (3) identify ways in which organisations and individuals could be involved in the JLAPUP and (4) establish a steering group to guide the development of the JLAPUP.
It was agreed at the launch meeting that collaboration between parties would be fostered by an independent facilitator from the JLA with no stake in the content of the outcome. We felt that this facilitation would ensure that the priority-setting process was not dominated by any particular interest group and would help to create the conditions for successful participation and dialogue. It was agreed at the first steering group meeting to adopt the draft protocol and to define ‘active partners’ in the partnership as:
-
organisations that were prepared to ask their members to submit uncertainties about pressure ulcer prevention and treatments to help decide priorities for future research
-
supporters who were interested individuals who could inform and support the partnership.
The JLAPUP established a website to promote the partnership’s work, house surveys and encourage wider involvement. Social media were used extensively. A Facebook page (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) [see www.facebook.com/pages/James-Lind-Alliance-Pressure-Ulcer-Partnership/307879132584509 (accessed 11 May 2016)] and a Twitter account (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) (@JLAPUP) were established and used with press releases to encourage participation and dissemination.
Consultation: uncertainty gathering
A range of different methods was used to gather patient, carer and health professional uncertainties. At the beginning of the process, uncertainties were gathered at the launch meeting and some were submitted by e-mail and through a contact form on the JLAPUP website. Paper and online surveys were then developed as the principal means of gathering uncertainties. The paper survey was distributed via active members and at the following NHS sites in the north of England, for which we obtained necessary ethical approval (see Ethics and governance): Leeds Teaching Hospitals NHS Trust, Leeds Community Healthcare NHS Trust and the Golden Jubilee Spinal Injuries Unit, James Cook University Hospital, Middlesbrough. York Teaching Hospital NHS Foundation Trust granted research approval but not until after the survey closing date and so surveys were not distributed there as intended.
The online survey, accessible nationally, was created using SurveyMonkey® (Palo Alto, CA, USA). The online version differed from the paper version because it included a ‘question builder’ option that sought to inform and encourage participants to use a PICO (population, intervention, comparison, outcome174) format when possible. The link to the online survey was widely distributed through a press release, e-mails, Twitter, Facebook and partner organisations. It was live for 4 months. Limited population data were collected from respondents alongside their treatment uncertainties. Participants were asked to identify themselves as someone having or at risk of pressure ulcers, a carer or a health professional. Those who belonged to more than one category were invited to complete the survey from both perspectives. Health professionals were asked for their professional role. Patients were asked for information on age, sex, ethnicity and home/care setting. Carers were asked to complete this information about the person who they cared for.
Uncertainties were gathered face-to-face at a number of relevant patient, carer and health professional events. An interactive stall was set up at large events. Gathering sites included a link nurse training event at Leeds Teaching Hospitals NHS Trust; a nurse training event for staff at the James Cook University Hospital; the Multiple Sclerosis Society Conference in Manchester (13–15 April 2012); the Tissue Viability Society Conference in Kettering (18–19 April 2012); and the York Carers’ Forum (May 2012).
Articles about the JLAPUP and calls for uncertainties were placed in a range of media targeted at stakeholders including the Spinal Injuries Association online newsletter, the magazine of the Multiple Sclerosis Society and Carers News.
Records were kept of the source of all submitted uncertainties (whether patients, carers or health professionals) to enable the uncertainties submitted by different stakeholders to be compared and contrasted and as a means of generating dialogue between stakeholders.
Collation
Paper survey results were manually entered into SurveyMonkey for subsequent analysis. Submissions were categorised as follows:
-
intervention/non intervention
-
originator
-
submission group
-
broad intervention taxonomy derived from the Cochrane Wounds Group
-
detailed intervention taxonomy derived from the Cochrane Wounds Group
-
outcome.
Once all submissions were received, uncertainties/questions were combined when there were duplicates, counted and organised into four data sets, by originator and taxonomy of interventions derived from the Cochrane Wounds Group. The taxonomy was adapted to accommodate the range of submissions received. When the questions related to interventions, these were analysed to form a PICO-style question (noting the journey from initial submission to question) and duplicates combined to form a list of ‘indicative uncertainties’.
Checks were performed on the interpretation and categorisation of submissions by comparing treatments of the same submission by different coders (members of the programme research team and steering group members). These checked for categorisation errors and whether or not the question produced remained faithful to the submission without overinterpreting what was expressed. An ‘uncertainty analysis’ exercise was conducted at a steering group meeting to discuss the interpretation of submissions and their translation into PICO-style uncertainties. A sequential sample showing the journey from submission to question was sent to steering group members including the Programme Grants for Applied Research programme chief investigator, the chair and the JLA representative, to check for ‘justifiability’ of interpretation.
To ensure that identified intervention questions were genuine uncertainties and not answered by existing research, ‘indicative uncertainties’ were then checked against existing systematic reviews by performing an individualised search for each uncertainty in The Cochrane Library. The following databases were accessed: the Cochrane Database of Systematic Reviews; the Database of Abstracts of Reviews of Effects; the Health Technology Assessment database; and, if the uncertainty related to cost-effectiveness, the NHS Economic Evaluation Database. The search was carried out by an experienced systematic reviewer and checked by another. Genuine uncertainties were prepared for publication in a pressure ulcer module within the NICE UK Database of Uncertainties about the Effects of Treatments (UK DUETs).
Research recommendations from systematic reviews were not included in the gathering, analysis or prioritisation process. The steering group decided that the focus of the PSP was collecting and prioritising uncertainties from patients, carers and health professionals, rather than research recommendations arising from systematic reviews (which would be researcher-derived uncertainties).
Research questions that were not about treatment interventions were categorised using the health research classification system of the UK Clinical Research Collaboration [see www.ukcrc.org/research-coordination/health-research-classification-system/ (accessed 11 May 2016)].
Prioritisation
The long list of genuine (rather than ‘indicative’) uncertainties that remained after the evidence check was then reduced to produce a workable shortlist for prioritisation. Questions were ranked and grouped in order of number of times submitted. When there were strong similarities some uncertainties were combined. The criteria for inclusion in the shortlist agreed by the steering group were:
-
all questions that had been submitted more frequently than once by any category (patient, carer or health professional)
-
all questions appearing on more than one list (submitted by one or more categories of patient, carer or health professional)
-
all questions originating from mixed meetings (where patients, carers and health professionals had discussed the uncertainty together)
-
all questions submitted by carers (because there were far fewer of these).
When they felt strongly, steering group members gave each other the opportunity to advance a case for the reinclusion of an excluded question. Questions could only be reincluded if the case was found convincing enough to secure agreement from a majority of the group.
A prioritisation survey was designed in paper and electronic formats in consultation with survey methodologists within the programme team. The aim was to enable stakeholder groups to value the different uncertainties identified during the previous phases and rank these in order of importance, resulting in group-specific research agendas. Discussions extended to consideration of discrete choice experiments and best–worst scaling techniques;175 however, resource constraints meant that the survey had to be designed within the constraints of SurveyMonkey software. The survey design asked people to rate uncertainties according to their importance on a scale of 1–10, to encourage a greater differentiation in responses, with 0 being not at all important, 5 being of average importance and 10 being extremely important, with there also being an option of ‘no view’ or ‘don’t know’. The question order was randomised. Some limited demographic data were collected, in line with the gathering survey but with the addition of a question about wheelchair use. The survey instruments were piloted with a panel of patients to gain feedback on ease of use and clarity of understanding. The survey was amended in the light of the pilot and measures were taken to ensure security and the prevention of multiple entries in the online survey.
The survey was launched with a widely distributed press release. The website was amended with links to the online questionnaire and a Twitter and Facebook campaign was launched. A brief explanatory YouTube video (YouTube, LLC, San Bruno, CA, USA) was created and linked to the online survey and JLAPUP website. Explanatory text for the online survey was amended a number of times in light of comments received from respondents. Online and paper surveys were open for 5 weeks.
Paper survey responses were input into SurveyMonkey and three data sets were produced using Stata 12 for patient, carer and health professional respondents. Data sets included aggregate rankings, average score rating, total number responding, Cochrane broad taxonomy and category of intervention and the frequency with which a question was answered.
Integration
The first stage of the integration phase involved examining the similarities and differences in responses from separate stakeholder group prioritisations. Questions covering similar areas but asked by different groups were thematically combined. As broader questions emerged, efforts were made to retain some of the granularity, the subtle differences and nuances of specific questions being lost through combination.
The steering group and other stakeholders were asked to choose three prevention and three treatment uncertainties from a list of treatment uncertainties that had been raised and prioritised by only one stakeholder group. This gave stakeholders the opportunity to consider the importance of, and a chance to include, uncertainties that may not have occurred to them from their own patient, carer or health professional perspective. These selections were included in a final shortlist of uncertainties agreed as important by all stakeholder groups for deliberation at a final priority-setting meeting. Selections were made using two methods. A facilitated questionnaire was administered at stakeholder events and meetings including events on Worldwide STOP Pressure Ulcer Day. Far fewer patients than health professionals attended these events so an integration workshop was run with the Pressure Ulcer Research Service User Network for the UK (PURSUN UK), which had recently been set up to improve the quality of patient and public involvement in pressure ulcer research.
The second stage of the integration phase involved the conduct of home and bedside interviews with care home residents who are key stakeholders but who are often not included in studies about older people’s health176 and who were under-represented in our consultation phase. Visits to care homes and a rehabilitation centre were arranged to ask which of the treatment uncertainties gathered so far mattered most to residents and whether or not they had others they would like to include. The necessary permissions were secured for these visits (see Ethics and governance).
The final stage of the integration phase was a priority-setting meeting to choose the top 10 treatment uncertainties for future research. The format of the meeting was adapted from the format for final JLA meetings. 173 New methods for use in the meeting were piloted in mini mock workshops and facilitators were given the opportunity to help develop the workshop process through comment and discussion on the workshop outline.
All active members and supporters were invited to take part in the final priority-setting meeting, as were people who had taken part in the process to date who had submitted their details and expressed an interest in future involvement. Before the meeting participants were asked to complete a pre-workshop exercise to choose and rank their top 10 preferred uncertainties from the 30 most frequently submitted and highly ranked questions. Facilitators were given written and oral briefings.
The meeting was staffed by facilitators, a statistician to rank uncertainties, a qualitative researcher who hosted a ‘listening/experience corner’ (which afforded an opportunity for participants to talk outside the confines of the demands of the workshop) and an independent observer. Researcher members of the meeting were non-voting and were asked to act as jargon de-buggers and to offer research clarification when this was requested from members of the meeting (a written glossary of key terms in wounds research was also made available at the meeting). In addition, two people took on the role of Twitter correspondent and photographer. With the meeting’s permission, facilitators made a digital audio-recording of the sessions. Facilitators each had a set of cards that detailed the rankings by stakeholder group to date of each prioritised uncertainty. Facilitators also had a ‘back story’ book that detailed the history of each of the 30 shortlisted uncertainties.
On arrival, participants were greeted, given a workshop pack and directed via a colour-coded badge to a place at one of four tables of mixed groups, which were balanced as far as possible between patients, carers, health professionals and researchers. If participants had not completed the pre-meeting exercise, they were encouraged to do so during arrival refreshments.
The meeting began with a welcome, an explanation of the structure and ground rules for the day and the roles that people would play and a presentation summarising the reasons for forming the PSP, the prioritisation process to date and the task in hand. The four groups then went into breakout groups for round 1. The objectives of round 1 were:
-
introductions
-
identifying similarities and differences between participants’ individual rankings so that they could appreciate the different (or similar) points of view in the group
-
discarding questions not selected by group members as part of their top 10.
After introductions and a recap of the ground rules, group members took turns to place a numbered sticker representing their top-ranked uncertainty on a flip chart display and explain why they had chosen it. The group continued explaining and debating individually ranked uncertainties in rounds until 15 minutes before the end of the session, when everyone was asked to use their numbered stickers to record their full top 10 on the poster. If an uncertainty did not appear on the display, it was eliminated. The rest went forward into round 2. The display gave a picture of shared and divergent priorities. During the refreshment break facilitators divided the 30 A4 uncertainty cards into a pile of uncertainties identified in round 1 and a pile of uncertainties to be eliminated. The uncertainties still in play were represented for the next round by laying out the uncertainty cards in ranking order.
The same groups reconvened for round 2. This focused on creating a group rank order of 15 uncertainties from the shortlisted cards using a ‘diamond nine’ approach. The results from the four workshops were collated over lunch to produce an aggregated top 15. The aggregation process was conducted using a simple points system in which the first-placed uncertainty received 15 points, the second 14 and so on, with uncertainties outside the top 15 receiving a score of zero. The points for each uncertainty were then added together to produce an overall ranking. This was calculated using a Microsoft Excel spreadsheet into which the rankings from each group were entered. The spreadsheet was then linked using a pivot table to a Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation, which could be updated automatically and allowed for the quick presentation of the results of the ranking process to those attending.
A plenary session was then held to report back the results of the first sessions, to reveal the aggregated top 15 and deliberate the results. Participants were allowed to make a case for the reintroduction of excluded uncertainties, with decisions made by airing objections and a show of hands. After lunch participants formed new mixed groups (indicated by a second colour on their badge). Using the aggregated rankings from the morning sessions (with the addition of any uncertainties reintroduced after deliberation) members were asked to rank their top 15 questions. Results were collated and an aggregated ranked top 15 was produced. Groups were asked to particularly aim for agreement about the top 10. An aggregated ranked top 15 was produced during the refreshment break.
The meeting concluded with a final plenary session that presented and deliberated the results. It considered cases for ranking uncertainties more highly and for combining uncertainties. The aim of the session was to choose a final top 10; however, the meeting agreed to adopt a top 12. The meeting was reminded that all of the uncertainties are a resource for researchers, including the original data set, the shortlist and the top 12.
Reporting
All intervention questions without a reliable or complete answer and for which further research is called for were published on NHS Evidence in the UK DUETs at www.library.nhs.uk/duets/SearchResults.aspx?tabID=294&catID=15594 (accessed 20 June 2016).
A press release, newsletter and Twitter campaign were used to publicise the top 12 uncertainties. The results were made available to the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC). Representatives from NETSCC attended a steering group meeting with a view to developing a commissioning brief.
The results have been presented at academic and clinician conferences and published for clinician and academic audiences. 177–179 Non-intervention uncertainties have been collated and are available on request.
Evaluation
Feedback from participants was invited at all meetings and in surveys. Data were used to refine and amend future plans. Steering group members took part in a final evaluation that sought their views on the effectiveness of the process and their levels of involvement. A JLA PSP reporting proforma has been completed. An academic paper critically reflecting on the methodology has been published. 177
Ethics and governance
A submission to the National Research Ethics Service for ethical approval was made to distribute the survey and recruit patients to discussion groups through NHS sites. The Proportionate Review Sub Committee of the NRES Committee East of England – Hertfordshire Research Ethics Committee reviewed the application and gave approval (reference 11/EE/0551). All required research and development approvals were obtained. Ethical approval was also received from the University of York Health Sciences Research Governance Committee.
Results
Consultation: uncertainty gathering
We received 359 submissions from the uncertainty-gathering process, which together generated a total of 960 treatment uncertainties. There were 83 paper survey submissions and 180 online survey submissions (the site was visited 344 times). The remaining submissions were made at meetings, at stalls at events, by telephone, e-mail, on Twitter and at webinars. The number of submissions is not the same as the number of people participating because each meeting, event or webinar counted as one submission source.
Respondents to the consultation were health professionals (43%), patients (37%) and carers (11%), with the remainder from mixed groups (Table 45). The majority of health professionals (45%) were tissue viability nurses. No GPs responded (Table 46).
| Originator | n | % |
|---|---|---|
| Health professional | 409 | 42.6 |
| Patient | 354 | 36.9 |
| Carer | 101 | 10.5 |
| Mixed group | 96 | 10.0 |
| Total | 960 | 100.0 |
| Health professionals | n | % |
|---|---|---|
| Tissue viability nurse/wounds specialist nurse | 143 | 45.0 |
| Hospital nurse | 47 | 14.8 |
| Community-based nurse | 16 | 5.0 |
| GP | 0 | 0 |
| Hospital doctor | 14 | 4.4 |
| Care assistant | 7 | 2.2 |
| Dietitian | 4 | 1.3 |
| Physiotherapist | 9 | 2.8 |
| Other/not specified | 78 | 24.5 |
| Total | 318 | 100.0 |
The number of paper survey returns (n = 83) was disappointing given the investment of time to obtain ethics and governance approval for distribution to patients and carers through the NHS. In total, 49% of completed paper surveys were from patients, 16% were from carers and 35% were from health professionals.
In total, 62% of patient respondents to the survey were female and 38% were male, with 94.3% describing their ethnicity as ‘white British’. The second largest group identified as ‘white – any other background’ (1.9%). Patient respondents to the survey were generally younger and more likely to be living at home than the pressure ulcer population as a whole (Figure 8). People in more formal care settings (including people in nursing homes and hospital inpatients) were under-represented relative to the prevalence survey outlined in Chapter 2 (Figures 9 and 10). Most patient respondents did not describe any comorbidities; 30% said that they had MS and 10% spinal injury (Figure 11).
FIGURE 8.
Age distribution of patient respondents to the uncertainty-gathering survey compared with that of people with pressure ulcers in the prevalence survey (see Chapter 2).
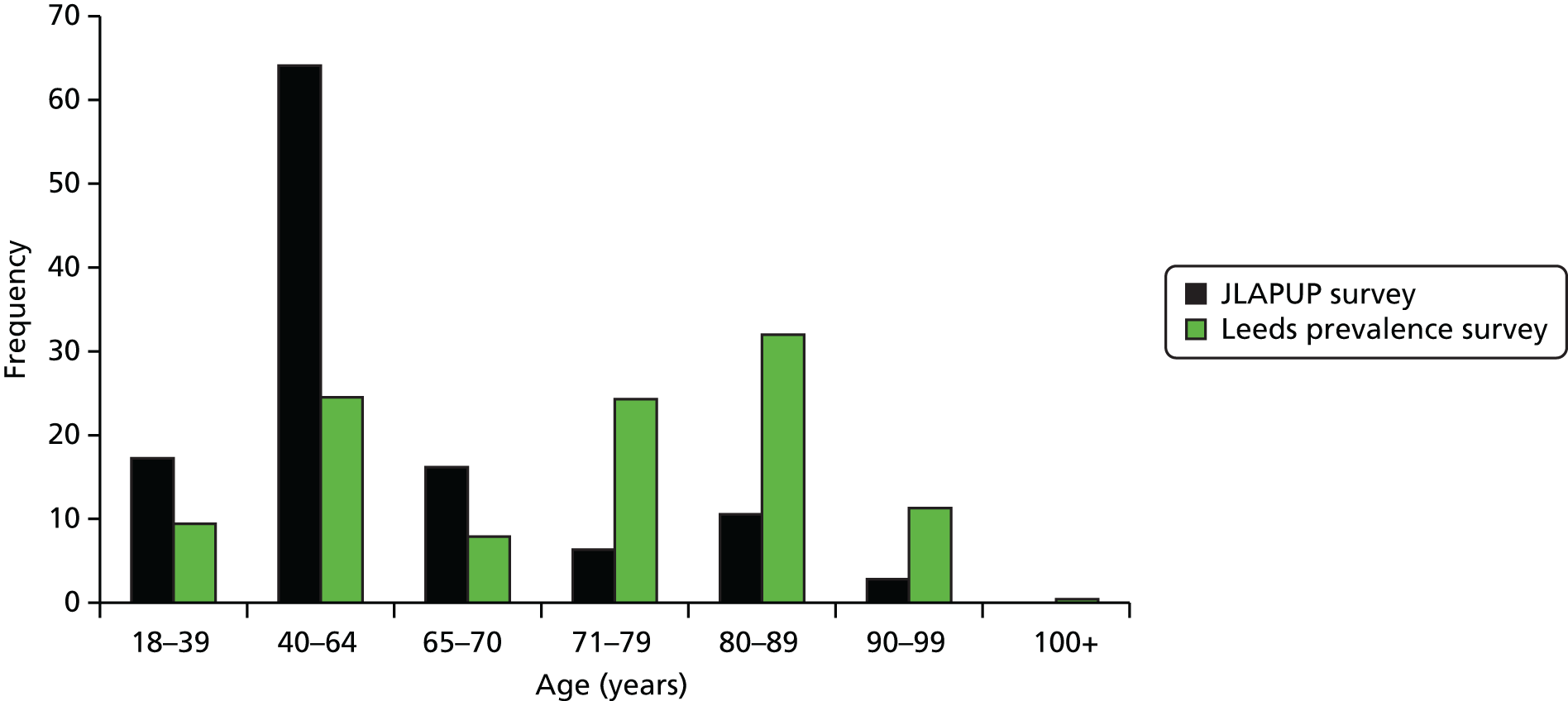
FIGURE 9.
Patient home/care setting of respondents to the JLAPUP uncertainty-gathering survey.
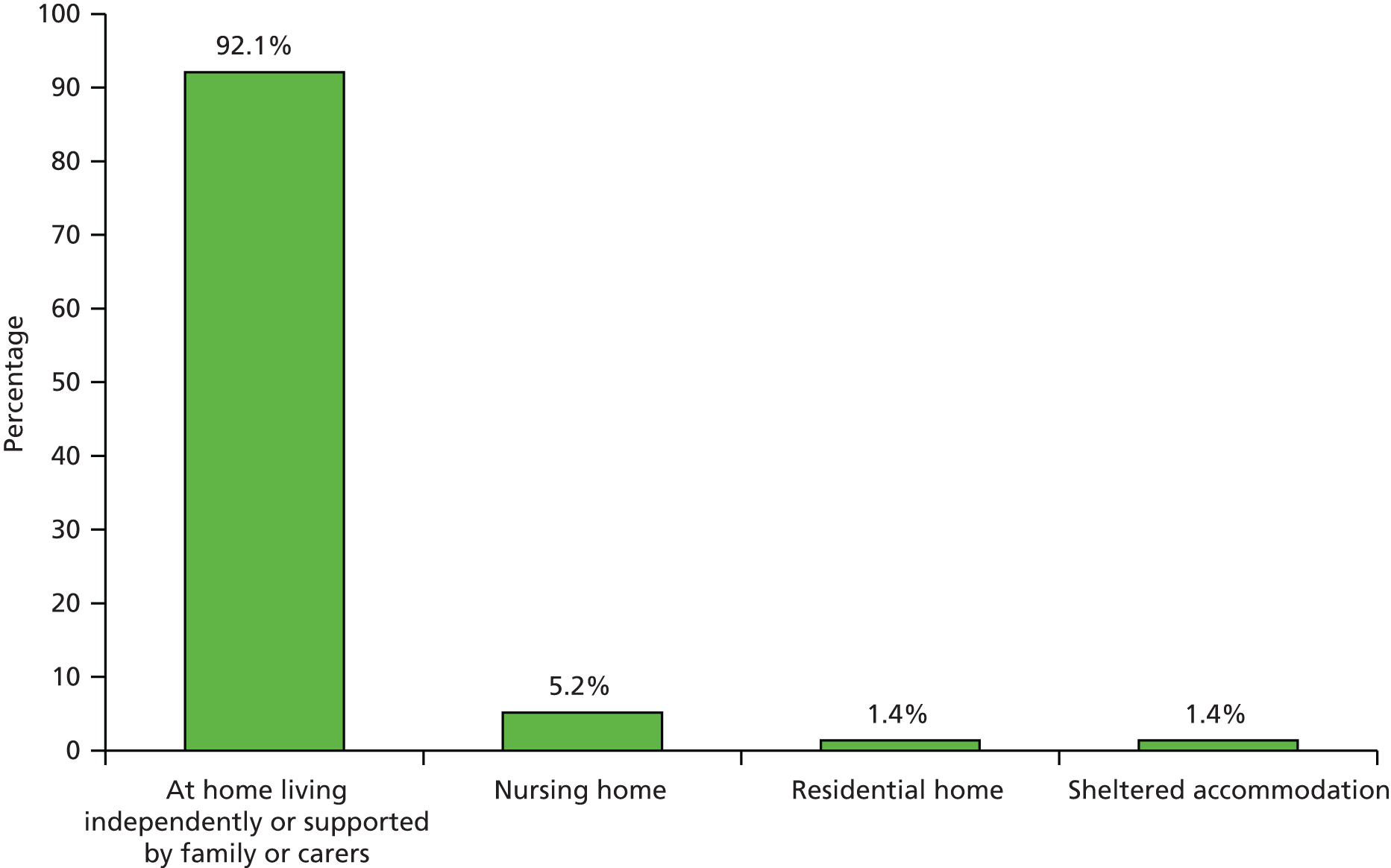
FIGURE 10.
Leeds prevalence survey: location where care delivered.
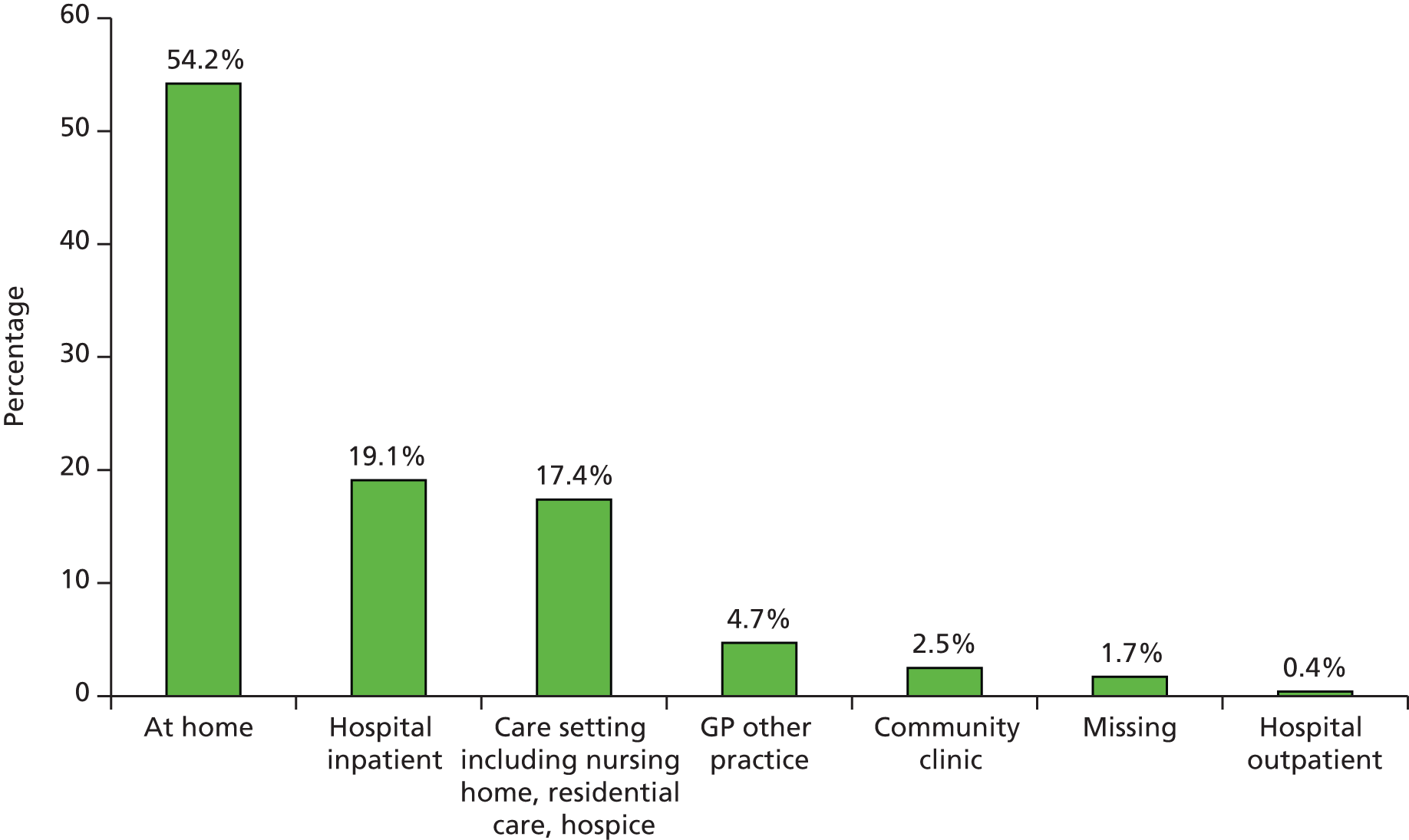
FIGURE 11.
Comorbidities specified in the uncertainty-gathering survey.
The survey comments sections were well used. The range of responses included requests for information, comments on the importance of the uncertainties submitted, views on the priority-setting process and the sharing of experiences. Examples include:
More consultation exercise with no realistic changes to current practices?
Patient comment JLAPUP survey, 3 March 2012
We are confused as to the contradictory responses we have received to our questions about pressure sores and do not know how best to get a real, current one to heal. This is despite at least weekly visits from a district nurse and doctors appointments.
Patient comment JLAPUP survey, 21 February 2012
Feel questions are important. There is currently to my knowledge no accurate and objective way of measuring the changes in pressure ulcer volume or size over time which would give a good indicator of healing and how this relates to the many products all proposing to heal these wounds.
Hospital doctor comment JLAPUP survey, 24 February 2012
My mum was in bed at home for years, and I helped look after her. She was unable to communicate, move etc. due to having Primary Progressive Multiple Sclerosis. She could not say that she was in pain, or communicate with us in any way. We were not told about the possibility or likelihood of her developing pressure ulcers or how to prevent them – and she consequently developed one unfortunately. As many people who develop pressure ulcers will have another condition and may be unable to care for themselves or tell anyone how they are feeling, I think that it is vital that research looks at this aspect, how to care for people, preventing pressure ulcers, helping people to know what to look for and what to do, thinking more about the elderly or people with underlying conditions who aren’t able to say what they need or are feeling, and also making sure that carers understand the risks and how to prevent them.
Carer comment JLAPUP survey, 2 March 2012
Further analysis of the comments may cast some contextualising light on the uncertainties gathered and how people participating understood and engaged with the survey.
Collation
Submissions from all sources were categorised as intervention or non-intervention questions, reworked into a PICO format when possible and categorised according to the adapted Cochrane taxonomy: 960 uncertainties were produced. The majority of uncertainties related to the effectiveness of interventions (71.9%). Of these, the most frequently submitted uncertainties related to methods of pressure and shear reduction and relief (30%) and the organisation of care (30%) (Table 47). Prevention was identified as an outcome in 61% of the intervention uncertainties and healing in 32% (Table 48).
| Interventions | n | % |
|---|---|---|
| Organisation of care | 204 | 29.6 |
| Pressure and shear reduction and relief | 207 | 30.0 |
| Local wound treatment | 70 | 10.1 |
| Managing patients with limited mobility | 51 | 7.4 |
| Risk assessment | 51 | 7.4 |
| Nutrition | 41 | 5.9 |
| Local skin care | 34 | 4.9 |
| Surgery | 10 | 1.4 |
| Other | 15 | 2.2 |
| Physical therapies | 4 | 0.6 |
| Massage | 2 | 0.3 |
| Smoking cessation | 1 | 0.1 |
| Total | 690 | 100.0 |
| Outcomes | n | % |
|---|---|---|
| Prevention | 422 | 61.2 |
| Ulcer healing | 218 | 31.6 |
| Value for money to the NHS | 4 | 0.6 |
| Ulcer-related pain | 6 | 0.9 |
| Patient safety | 4 | 0.6 |
| Reduction in number of dressing changes that an ulcer requires | 5 | 0.7 |
| Secondary prevention | 4 | 0.6 |
| Ability to undertake normal activities | 4 | 0.6 |
| Quality of life | 4 | 0.6 |
| Speed of ulcer healing | 3 | 0.4 |
| Ulcer-related infection | 3 | 0.4 |
| Other | 10 | 1.4 |
| Ulcer-related odour | 2 | 0.3 |
| Ulcer-related leakage | 1 | 0.1 |
| Total | 690 | 100.0 |
Intervention uncertainties were checked against the evidence base to ensure that they were ‘genuine uncertainties’, that is, that there was not an existing, reliable answer in the research literature. All were found to be genuinely uncertain with the exception of one patient submission. A systematic review found reliable evidence for the use of medical grade sheepskins for the prevention of pressure ulcers180 and so this was removed from the list. Partial evidence was found to answer two uncertainties submitted by patients and clinicians: the effectiveness of support surfaces for prevention and the use of nutritional interventions for prevention. The limited nature of the evidence meant that these remained on the list of uncertainties published on UK DUETs.
In total, 270 non-intervention questions were categorised by research activity category according to the UK Clinical Research Collaboration health research analysis taxonomy. Duplicates were combined and those too poorly specified to categorise were removed. This resulted in a final list of 220 unique questions, the majority of which were about the aetiology/prognosis of pressure ulcers (Table 49).
| Non-intervention questions | n | % |
|---|---|---|
| Aetiology/prognosis | 120 | 54.5 |
| Health and social care services research | 43 | 19.5 |
| Management of diseases and conditions | 40 | 18.2 |
| Underpinning research | 16 | 7.3 |
| Evaluation of treatments and therapeutic interventions | 1 | 0.5 |
| Total | 220 | 100.0 |
Prioritisation
In combining questions for the longlisting and shortlisting processes, there was a tension between producing broader issues for prioritisation and maintaining the specifics and authenticity of uncombined questions. The approach taken aimed to maintain a balance between identifying themes and maintaining finer details (specific populations and interventions) to help retain the richness of the submissions because this fine detail would be useful in the design of any subsequent trials. In total, 141 participants completed the online and paper prioritisation surveys in which patients, carers and health professionals prioritised uncertainties that had been raised by their peers (56 from patients, 14 from carers and 71 from health professionals).
In total, 50% of people who completed the survey were health professionals, 40% were patients and 10% were carers. Of the patient respondents, 80% said that they were wheelchair users, 93% were living at home independently or supported by family or carers and 59% were aged 40–64 years. Of the health professionals taking part, 39% were tissue viability nurses, 22% were community nurses and 17% were hospital nurses. Of the carers, 75% were caring for someone who uses a wheelchair, 75% were caring for someone living at home and 44% were caring for someone aged 40–64 years.
In total, 33% of respondents to the online survey used the question builder, with 10% using it for more than one question, and 81% of the people who started the online survey completed it (Table 50).
| Originator | Number starting survey | Number completing survey | % completing survey |
|---|---|---|---|
| Patient | 76 | 56 | 73.7 |
| Carer | 16 | 14 | 87.5 |
| Health professional | 83 | 71 | 85.5 |
| Total | 175 | 141 | 80.6 |
Respondents rated the majority of uncertainties as being of above average importance. On the 10-point scale, patients’ average responses were in the range 6.05–9.14; carers’ average responses were in the range 4.00–9.64 (with 4.00 being an outlier and the remaining uncertainties ranging from 6.62 to 9.64); and health professionals average responses were in the range 6.18–8.80.
The online and paper surveys contained comments and feedback boxes. There were some comments on the difficulty of judging the importance of uncertainties:
You say not to rate all the questions as ‘very important’ but that is difficult when they are!
Patient comment from prioritisation survey, November 2012
This questionnaire for me was pointless as all areas of research are important and how can you value one over the other.
Health professional from prioritisation survey, November 2012
The comments boxes also identified three key issues for the priority-setting process:
-
Some respondents wanted to answer the questions rather than choose which should be researched.
-
Some were not reading the explanation in the website text so the text was enhanced and a brief explanatory video created, linked to the survey and JLAPUP website. This was viewed 93 times and had an impact in reducing the number of telephone and e-mail queries.
-
Throughout the process, the concept of ‘treatment uncertainty’ was a difficult one to explain and people sometimes responded by sharing a range of concerns and experiences or asking for advice.
For example:
I have answered a large amount of questions as don’t know, I’m not a professional carer and therefore don’t feel equipped to answer these questions fully. I do care for my mum 55 hours per week and she has a pressure sore on her heel which began during a 15 week stay in hospital and 20 month on it still hasn’t healed. We are therefore now obsessed with any red mark and ensuring she is repositioned at least every 3–4 hours. In recent weeks my aunt following an operation has developed pressure sores within a month of the operation and I’m alarmed that the nurses visiting are suggesting she put E45 cream onto the sores which is now broken skin, and 2 weeks after these were identified she still doesn’t have any proper antiseptic cream or dressing to help heal. I’m really alarmed that more urgency isn’t taken to try to heal pressure sores immediately they become apparent and that appropriate pressure relieving items are not made available to people at high risk of developing them as a preventative action, there seems to be a view of waiting until the problem has arisen rather than trying to prevent it. That said I’m not a NHS professional and can only provide a view based on my own experiences.
Carer comment from online prioritisation survey, November 2012
Integration
Identifying agreements and disagreements in survey results
The rankings from the prioritisation survey were integrated to provide a picture of the most highly rated kinds of questions that had been identified by all three groups. The shortlist for final deliberation was based on the most highly ranked questions raised and ranked by all three groups. There were 10 topics that were identified and agreed as being very important by all three groups.
-
Repositioning for prevention and healing.
-
How effective is repositioning in the prevention of pressure ulcers?
-
for those in bed – turning self, being turned and changing position (sleep disruption)
-
for wheelchair users repositioning self or being repositioned in chair
-
for people with contracted limbs.
-
-
Ranking health professionals 1; patients/service users 3, 4, 6 and 9; carers 2 and 16.
-
Does regular turning of patients in bed promote healing of pressure ulcers? How often is it best to turn people? For people who require a carer to help them move what is the most effective means of repositioning? For people with limited memory as well as limited mobility would a ‘prompt of routine’ tool that reminds people to reposition themselves help to heal pressure ulcers?
Ranking health professionals 2; patients/service users 6; carers 10.5 and 18.
-
Support surfaces for prevention and healing.
-
What is the relative effectiveness of the different types of pressure-relieving beds, mattresses, overlays and cushions (including cushions for electric and self-propelling wheelchairs) in preventing pressure ulcers? Do they meet the needs of the full range of patients at risk?
-
compared with conventional and other specialist surfaces
-
for those who share a mattress with a partner, people with MS
-
for those with contracted limbs.
-
-
Ranking health professionals 6, 8 and 9; patients/service users 2, 8, 10 and 15; carers 1, 3 and 14.
-
Do pressure-reliving support surfaces, particularly mattresses, help ulcers heal? Are they appropriate and effective for treating the frail and elderly and those with MS?
Ranking health professionals 5 and 8; patients/service users 7; carers 1 and 13.
-
Involvement for prevention.
-
How effective at preventing pressure ulcers is involving (informing/educating/motivating) patients/family/lay carers in patient care?
-
Ranking health professionals 2, 16 and 18; patients/service users 1, 5, 7 and 18; carers 6.5 and 8.
-
Eating and drinking (nutrition/hydration) for prevention and healing.
-
Does improving diet (nutrition) and hydration promote pressure ulcer healing?
-
Ranking health professionals 1; patients/service users 2; carers 5.
-
Does improved diet (nutrition) and hydration promote the prevention of pressure ulcers?
-
for people with MS, spina bifida and spinal injury
-
including effectiveness of high-protein diet, micronutrients and effects of weight loss and gain.
-
Ranking health professionals 7; patients/service users 13 and 21; carers 4.
-
Staff training/education for prevention.
-
Does the education of health and social care staff on prevention lead to a reduction in the incidence of pressure ulcers? If so, which are the most effective education programmes (at organisational and health/social care level)?
-
Ranking health professionals 3; patients/service users 12; carers 6.5.
-
Service models and healing outcomes.
-
What are the best service models (and are they sufficiently accessible) to ensure that patients with pressure ulcers receive the best treatment outcomes, for example awareness of and access to tissue viability nurses, and do people with specialist conditions (e.g. spinal injury) receive the best pressure ulcer treatment at specialist centres rather than at generalist services?
-
Ranking health professionals 9; patients/service users 4; carers 3.
-
Surgery for healing.
-
How effective are surgical operations to close pressure ulcers? What is the most appropriate surgical referral for people with MS and ulcers that will not heal?
-
Ranking health professionals 14; patients/service users 1; carers 14 (note highest for patients).
-
Bed rest for healing.
-
For wheelchair users sitting on a pressure ulcer, how effective is bed rest in promoting pressure ulcer healing? Is it safe for those with MS who have been advised to keep moving? Is it detrimental to mental health? How does its effectiveness compare with sitting on a pressure-relieving cushion? Is sitting for a small time (e.g. to eat a meal) likely to substantially increase pressure ulcer healing time?
-
Ranking health professionals 3 and 12; patients/service users 13, 17 and 18; carers 19.
-
Debridement for healing.
-
Does (surgical and non-surgical) debridement promote healing in patients with pressure ulcers? What is the most effective debridement strategy?
-
Ranking health professionals 11; patients/service users 14; carers 2 and 10.5.
-
Dressings for healing.
-
How effective are wound dressings in the promotion of pressure ulcer healing, including honey dressings? What is the optimal number of times that dressings should be changed to promote pressure ulcer healing?
-
Ranking health professionals 10 and 13; patients/service users 11; carers 7 and 8.
The following three topics were raised by all three groups but were ranked lower:
-
Dressings and zero friction products for prevention.
-
How effective are dressings and zero-friction fabric products in preventing pressure ulcers?
-
Ranking health professionals 26; patients/service users 16; carers 16.
-
Heel protectors for prevention.
-
How effective are heel protectors in preventing pressure ulcers?
-
Ranking health professionals 15; patients/service users 20; carers 9.
-
Topical products and skincare regimes for prevention.
-
How effective are topical skincare products at preventing pressure ulcers, for example the application of barrier creams or sprays [Sudocrem® (Allergan plc) and CavilonTM (3M)], methylated spirits and moisturisers? Does the use of skincare regimes diminish the likelihood of pressure ulcer occurrence, for example, for patients confined to bed, does treating with washing, surgical spirit and/or talcum powder at all pressure areas prevent the occurrence of pressure ulcers?
-
Ranking health professionals 24; patients/service users 25, 26 and 27; carers 12 and 17.
Nine topics were raised by just two groups and were lower ranked:
-
Exercise for prevention.
-
For people with limited mobility, does exercise coaching help in the prevention of pressure ulcers? Do community physiotherapy interventions change the incidence of pressure ulcers?
-
Ranking patients/service users 14; carers 10 and 15.
-
Risk assessment scale/tool.
-
Is using a pressure ulcer risk rating scale/tool better than clinical judgement in preventing pressure ulcers and is there a best scale?
-
Ranking health professionals 23; patients/service users 19.
-
Service models for prevention.
-
What impact do different service models have on the incidence of pressure ulcers? For people with MS or spinal injury, is working with specialist clinicians more effective for prevention than working with generalists? Does access to a specialist wound prevention service improve pressure ulcer prevention?
-
Ranking health professionals 15 and 22; patients/service users 11.
-
Incontinence products for prevention.
-
How effective are incontinence devices in the prevention of pressure ulcers? Do cheap incontinence pads cause more irritation than expensive pads?
-
Ranking health professionals 19.5; carers 13.
-
Involvement and healing outcomes.
-
Does getting people with pressure ulcers and their carers more involved in their own pressure ulcer management improve ulcer healing? If so, what are the most effective models of engagement, for example what is the most effective way for family carers to be involved to help ulcer healing and how effective is education for lay carers in promoting pressure ulcer healing including understanding and concordance to recommendations (like turning regimes, diet, fluids)?
-
Ranking health professionals 6 and 7; carers 6 (note patients raise this in terms of prevention not treatment).
-
Continuity of care and healing outcomes.
-
How does continuity of care impact on treatment of pressure ulcers?
-
Ranking patients/service users 8; carers 4.
-
Pain relief.
-
For people with pressure ulcers (including people with MS), which analgesics are effective and safe for the treatment of pressure ulcer-related pain?
-
Ranking patients/service users 3; carers 15.
-
Topical agents for healing.
-
What is the value of topical agents in promoting pressure ulcer healing (including honey-based treatments and the application of sugar)?
-
Ranking patients/service users 15 and 19; carers 16.
-
Negative pressure wound therapy for healing.
-
How effective is negative pressure wound therapy as a treatment for pressure ulcers?
-
Ranking patients/service users 16; carers 20.
These topics indicated shared levels of agreement between stakeholders. In addition, there were a number of uncertainties that had been identified and ranked by one group only.
In a voting sheet exercise (see Appendix 5) conducted at events on Worldwide STOP Pressure Ulcer Day, 60 stakeholders ranked the three most important prevention and three most important treatment uncertainties that had been submitted by one stakeholder group only. The events attracted more health professionals than patients or carers, with 50 votes cast by health professionals, six by patients and four by carers.
The most highly ranked uncertainties raised by one group but agreed by all three groups were:
-
Do staffing levels have an impact on the incidence of pressure ulcers (originally raised by health professionals and ranked joint 4th)?
-
Is staff training effective in improving pressure ulcer healing (originally raised by patients and ranked 5th)?
-
What is the most effective way of keeping clean (personal hygiene) to aid pressure ulcer healing (originally raised by patients and ranked 9th)?
-
For people in hospital at risk of developing pressure ulcers, is the current organisation of nursing care effective as best practice in the prevention of pressure ulcers (originally raised by patients and ranked 24th)?
-
How effective are pressure ulcer grading systems in determining the amount of skin damage and does the use of such scales improve ulcer healing (originally raised by health professionals and ranked joint 4th)?
There was no clear agreement across groups on the following uncertainty but it gained a high number of votes:
-
How does continuity of care impact on the prevention of pressure ulcers (originally raised by carers and ranked 5th)?
Eight members of PURSUN UK took part in a workshop at Leeds Centre for Independent Living to hear about the process so far, identify any gaps and rank the three most important prevention and three most important treatment uncertainties that had been submitted by only one stakeholder group. The most highly ranked treatment uncertainties were:
-
How effective are pressure ulcer grading systems in determining the amount of skin damage and does the use of such scales improve ulcer healing (originally raised by health professionals and ranked joint 4th)?
-
Is staff training effective in improving pressure ulcer healing (originally raised by patients and ranked 5th)?
-
What is the most effective way of keeping clean (personal hygiene) to aid pressure ulcer healing (originally raised by patients and ranked 9th)?
These had also been selected in the voting exercise above. Participants also ranked the following prevention uncertainties highly but did not agree on a whole group set of priorities:
-
How effective in preventing pressure ulcers is having a wound care champion (particular staff, managers or personal carers responsible for the prevention of pressure ulcers) (originally raised by health professionals and ranked 17th)?
-
For wheelchair users at risk of developing pressure ulcers, what is the most effective method of pressure mapping to assess pressure areas at risk to prevent the development of pressure ulcers (originally raised by patients and ranked 17th)?
-
For people in hospital at risk of developing pressure ulcers, is the current organisation of nursing care effective as best practice in the prevention of pressure ulcers (originally raised by patients and ranked 24th)?
-
Do staffing levels have an impact on the incidence of pressure ulcers (originally raised by health professionals and ranked joint 4th)?
-
How does continuity of care impact on the prevention of pressure ulcers (originally raised by carers and ranked 5th)?
Two uncertainties were identified by PURSUN UK that were different from those in the first exercise and the results were fed into the shortlisting process.
Home and bedside interviews
Although there had been success in engaging, as primary participants, specific patient/service user groups (including wheelchair users, those at home, living independently or supported by family or carers, and people aged 40–64 years), there had been less success in engaging with older people and those living in care settings. We knew from the Leeds prevalence survey (see Chapter 2) that older people and particularly those in nursing homes were under-represented in the prioritisation process. To address this, efforts were made to interview people at risk of pressure ulcers in care homes and a rehabilitation centre. Permission was granted to access three sites and two site visits were made. The rehabilitation centre could not identify anyone at risk who was able to participate. We also found that the formal consent processes required by the conditions of ethics approval were off-putting to residents of care homes, who were willing to speak informally. Although the steering group agreed that this work was important because of the effective disempowerment of people in care settings, the complexity of the task required more resources than available and the group felt that this was something that should be addressed in future research. Health-care professionals and carers had also taken part in the survey, generating uncertainties about all patient groups, and it was hoped that this would, at least to some extent, make up for these missing voices.
Final priority-setting meeting
The pre-workshop questionnaire can be found in Appendix 6. The voting scores from each round are available on request.
Top 12 uncertainties
The top 12 uncertainties chosen at the final meeting were ranked in order of decreasing priority as follows:
-
How effective is repositioning in the prevention of pressure ulcers?
-
How effective at preventing pressure ulcers is involving patients, family and lay carers in patient care?
-
Does the education of health and social care staff on prevention lead to a reduction in the incidence of pressure ulcers and, if so, which are the most effective education programmes (at organisational and health/social care level)?
-
What is the relative effectiveness of the different types of pressure-relieving beds, mattresses, overlays, heel protectors and cushions (including cushions for electric and self-propelling wheelchairs) in preventing pressure ulcers?
-
What impact do different service models have on the incidence of pressure ulcers including staffing levels, continuity of care (an ongoing relationship with the same staff members) and the current organisation of nursing care in hospitals?
-
What are the best service models (and are they sufficiently accessible) to ensure that patients with pressure ulcers receive the best treatment outcomes (including whether or not getting people with pressure ulcers and their carers more involved in their own pressure ulcer management improves ulcer healing and, if so, the most effective models of engagement)?
-
For wheelchair users sitting on a pressure ulcer, how effective is bed rest in promoting pressure ulcer healing?
-
How effective are wound dressings in the promotion of pressure ulcer healing?
-
Does regular turning of patients in bed promote healing of pressure ulcers?
-
Does improving diet (eating) and hydration (drinking) promote pressure ulcer healing?
-
How effective are surgical operations to close pressure ulcers?
-
How effective are topical skincare products and skincare regimens at preventing pressure ulcers?
The results of group votes in the final priority-setting meeting are available on request. Each uncertainty is made up of many individual broad and nuanced questions that have been condensed and combined throughout the process. A summary of the range of specific interventions, comparators and population groups behind each question, which may be useful for commissioning and designing specific research projects, is available on request.
Evaluation
Sally Crowe, the JLA representative and ‘honest broker’ for the process, told the steering group that she ‘thought that the process had demonstrated integrity and authenticity in what was a particularly difficult patient group to reach, and that it had used interesting innovative methodologies including the Dialogue model and social media’. The full steering group evaluation is available on request.
Discussion
Given the relative scarcity of high-quality research evidence for pressure ulcer management and finite funding for health research, competing research topics need to be prioritised to ensure investment yields maximum benefit. The JLAPUP produced an agreed, ranked list of condition-specific intervention uncertainties, which are being disseminated to funders and the wider research community. These were submitted to NICE as part of its pressure ulcers guideline consultation. Two of the five research recommendations made by NICE in the updated clinical guidelines,181 namely repositioning to prevent pressure ulcers and pressure-redistributing devices for prevention, were from the JLAPUP top 12 questions for research. The remaining three NICE research recommendations: pressure ulcer wound debridement, negative pressure wound therapy and risk assessment appeared in the JLAPUP top 30.
The majority of uncertainties (71.9%) related to the effectiveness of interventions. Of these, the most frequently submitted uncertainties related to methods of pressure and shear reduction and relief (30%) and the organisation of care (30%). In total, 61% of research questions generated focused on prevention as an investigative outcome and 32% focused on healing. Research into pressure ulcer prevention was therefore identified as an important research gap in the consultation phase. However, in the deliberative phase, research into treatment was also given high priority on the basis that some pressure ulcers are unavoidable and in light of the enormous level of uncertainty about treatment effectiveness in this area.
In addition to the effectiveness of treatment and prevention interventions, stakeholders also placed high priority on research concerned with causes, diagnosis and prognosis of pressure ulcers and other aspects of pressure ulcer care. In keeping with previous PSPs, the JLAPUP also raised questions about the effectiveness and harmonisation of NHS service models and the best means of supporting patient and family self-care within those models.
The JLAPUP faced the particular challenges in developing patient and public involvement because those affected by pressure ulcers are often elderly, immobile, unrepresented and unwell, with many living with concurrent long-term conditions. In the absence of patient groups and charities with a wound care focus, the JLAPUP provided a point of focus, developing ‘live’ and virtual networks with individuals and groups affected by, but not organised around, the topic of pressure ulceration (e.g. the Multiple Sclerosis Society and the Spinal Injuries Association) and with the newly emerging PURSUN UK. Participants in the process were generally younger and more likely to be living at home than the pressure ulcer population as a whole. People in more formal care settings (including people in nursing homes and hospital inpatients) were under-represented compared with the pressure ulcer population as a whole. These omissions may be offset to some extent by the inclusion of uncertainties from health professionals and carers for these groups.
Decision-making in the JLAPUP was carried out by patients, carers and clinicians working together but supported by researchers and a patient and public involvement officer with expertise in participatory research methods, systematic reviews, survey design and the conduct of RCTs. The JLAPUP process revealed the level of difficulty in structuring meaningful conversations with patients, carers and health professionals about uncertainty as a concept and also the difficulty for stakeholders in acknowledging that some strongly held beliefs about wound care are actually big research uncertainties. The strengths and weaknesses of the JLAPUP approach have been discussed in a separate methodological paper. 177 An important limitation of this approach to research prioritisation, however, is that it prioritises research within a narrow topic and only from the perspective of those directly affected (and relevant clinicians) rather than from wider societal perspectives. This approach also avoids any consideration of the economics of research and the comparative efficiencies of investing to reduce uncertainty in one area relative to another. Alternative approaches to research prioritisation, specifically value of information approaches, apply these other perspectives. 182
Overall conclusions from workstream 2
In-depth interviews with people with different complex wounds, carers and health professionals aimed to elicit the views of as wide a range of people as possible on the relative importance of different outcomes of wound treatments. We particularly wanted to hear the views of past and current intravenous drug users with complex wounds as their voices had not previously been heard. We heard from them that it can be a struggle coming to terms with the fact that they may have inflicted irreparable damage to the veins in their legs such that chronic ulceration may be a permanent feature.
Most patients and health professionals identified healing as a treatment outcome of primary importance. Patients and professionals were focused on achieving healing as quickly as possible while managing pain, infection and discomfort. Most also spoke about the difficulties that they were experiencing in achieving healing as well as concerns about preventing recurrence. Health professionals reported a lack of sense of achievement because they rarely saw the outcomes of their interventions, whereas many patients and carers reported feeling left ‘in limbo’ and as if their lives were ‘on hold’. They wanted to ‘get their lives back’. Wound care was largely targeted at trying to heal the wound rather than helping the patient live with a long-term condition and its consequences.
Our review of the funding source and quality of reports of trials of complex wound interventions was driven by several factors. First, we had a perception, but no evidence, that most of the trials in wound management were undertaken by the pharmaceutical and devices industry as part of marketing strategy. Knowing who funds wound treatment studies is important because then we can know who to influence to improve the overall quality of the research. We also wanted to know whether industry-funded research was of higher or lower methodological and reporting quality than non-industry-funded research. However, particularly important in the context of this workstream, we wanted to know whether or not the outcomes that wounds researchers are measuring and reporting in treatment trials bear any resemblance to those regarded as most important by patients, carers and health-care professionals.
Our review of RCTs of complex wound treatments indicated that, although 41% declared a commercial funding source, 26% did not report any funder or the commercial status of the funder was unclear. Trials of complex wound treatments suffer from a range of methodological deficiencies: they have small sample sizes (median of 60 participants), short follow-up periods (median of 2.8 months), widespread use of surrogate healing measures (28% of trial primary outcomes) and frequently no primary outcome specified (41% of trials). Of those trials that specified a primary outcome, 89% specified some measure of healing, although this was a surrogate measure in approximately half of cases. An astonishing 94% of the trials we examined were at unclear or high risk of bias for selection and/or detection bias. Funding source was not associated with any difference in study quality; commercially funded studies had similar sample sizes, durations of follow-up, outcomes and risk of bias.
The JLAPUP process revealed the extent of research uncertainty about pressure ulcer treatment and prevention. An existing, reliable answer in the research literature was found for only one of the 960 intervention uncertainties submitted in the process. A prioritised list of 12 uncertainties in pressure ulcer prevention and treatment was successfully determined by a collaborative and consultative process involving patients, carers and clinicians.
Given the relative absence of high-quality research in complex wound prevention and treatment, the research agenda is infinite. Complex wounds research is also an area of research without a rich history of patient and public involvement. We therefore conceptualised a JLA PSP as a good way of addressing these two deficiencies. We selected pressure ulcer prevention and treatment as the focus of this work because those affected (people at risk, not just people with existing pressure damage) are probably the hardest to reach group of people affected by complex wounds. The majority of people with, or at risk of, pressure ulcers tend to be very elderly, with multiple comorbidities and by definition with very limited mobility. Pressure ulcers have not been seen as a unifying force for patient allegiance, probably because, apart from the poor general health of those affected, they are a consequence of other health deficits rather than being the primary diagnosis. This became particularly apparent when a colleague on the JLAPUP steering group tried to discuss pressure ulcer research with people in a nursing home at high risk. These people, perhaps understandably, could not see the point of a conversation about something that had not yet happened to them when so many other things already had. These contextual factors mean that our research priorities for pressure ulcers largely reflect the views of younger disabled people, carers and health professionals rather than those of older people at high risk of pressure injury. They are nonetheless a great step forward.
Chapter 4 Evidence synthesis for clinical decision-making (workstream 3)
Abstract
Background
Up-to-date summaries of current research evidence are needed to inform clinical decision-making. A variety of methodologies is now available for synthesising the data from different primary intervention studies including traditional meta-analysis, IPD meta-analysis and mixed-treatment comparison meta-analysis. Our objectives were to identify areas of high decision uncertainty in collaboration with our NHS colleagues and summarise the best available evidence using appropriate techniques.
Methods
Methods included stakeholder consultation to identify decision uncertainties; a scoping review of the evidence for silver-containing wound dressings for treating venous leg ulcers; application of Cochrane methods of systematic review to 11 complex wound topics; and mixed-treatment comparison meta-analyses of dressings for diabetic foot ulcers and venous leg ulcers.
Results
Techniques involving facilitated, face-to-face contact with health professionals performed best in generating clinical uncertainties as topics for evidence synthesis. The relative effectiveness of different wound dressings for different wound types had high priority. There was no evidence that silver dressings were more effective than non-antimicrobial dressings for healing venous ulcers; however, variability in the existing trials precluded IPD meta-analysis. A series of Cochrane reviews in prioritised topics identified several wound treatments that appear to be more effective than others in different wound types. The matrix hydrocolloid dressing was associated with the highest probability (70%) of being the best dressing for diabetic foot ulcers, whereas a hyaluronan fleece dressing had the highest probability (35%) of being the best for venous ulcers; however, in both cases there was high uncertainty and poor-quality evidence.
Conclusions
A range of approaches to evidence synthesis was applied to complex wound treatments across a broad range of topics that had been prioritised by health-care professionals. This approach identified some treatments associated with the highest probability of effectiveness.
Background
To date, the Cochrane Wounds Group has identified around 9500 clinical trials in wound management and assembled them on its Specialised Wounds Register [see http://wounds.cochrane.org/ (accessed 11 May 2016)]. Evidence synthesis methods, such as systematic review, enable the management of information from multiple primary studies by identifying, summarising, appraising and pooling groups of primary studies in relation to a predefined research question. 183 The standard systematic review approach detailed in the Cochrane Handbook for Systematic Reviews of Interventions163 describes methods to manage information, reduce subjectivity, establish generalisability and consistency, improve power and precision and identify gaps in the research. The aim is usually to derive a summary estimate of effect from multiple primary studies (e.g. in terms of treatment effectiveness) while minimising bias in the review process. To date, systematic reviews conducted under the auspices of the Cochrane Wounds Group have contributed important information to the evidence base in wound management. For example, we know that compression is an effective intervention for healing venous leg ulcers184 and that higher-specification foam mattresses reduce the incidence of pressure ulceration compared with standard hospital foam mattresses. 180 This said, it should be acknowledged that the standard systematic review methods can be restrictive as they are based mainly on pairwise comparisons and group-level data. Other, more advanced, systematic review and meta-analysis methods are now available that may be more informative in areas of persisting uncertainty. These include those based on IPD and those involving mixed-treatment comparison meta-analysis.
Individual patient data meta-analysis involves the identification and retrieval of original patient-level data from the primary investigators, who are invited to become research collaborators along with the review team. Data are recoded, checked, cleaned and reanalysed. Advantages include the potential to conduct powerful time-to-event analyses adjusted for predictive patient-level covariates. This helps to obtain a more precise estimate of treatment effect and so reduce uncertainty. Other advantages include opportunities to conduct meaningful subgroup analyses based on patient-level factors, reinstate patients who the primary investigators have excluded from their own analyses, include updated data on the event in question from follow-up beyond the trial end point and combat poor reporting through close collaboration with the primary investigators. 185 As such, IPD meta-analysis has been proposed as the ‘gold standard’ among systematic reviews because of its potential to generate estimates on the most complete and clean data set possible with full adjustment for predictive covariates. 186 It is particularly useful when there is considerable uncertainty (imprecision) despite the existence of a good-quality systematic review of trial-level data, together with a suspicion that important patient-level covariates are contributing to the imprecision. One such example from wound care relates to the comparison of the effects of the four-layer compression bandage and the short-stretch bandage on the healing of venous leg ulcers. In this case, an earlier Cochrane review had not shown a difference in healing between the two bandages when trial-level data were pooled,184 whereas a subsequent IPD meta-analysis of the same comparison, involving estimation from an adjusted Cox proportional hazards model, showed that, on average, patients healed faster with the four-layer bandage. 187 The main issue with undertaking IPD meta-analysis is that the success of the endeavour is contingent on the review team being able to access the original data for each eligible primary study, although IPD meta-analysis may still be beneficial even if data can be obtained for only a subset of eligible studies. Access to the original patient-level data may not always be straightforward; for example, data may no longer be available for older studies. In addition, such reviews take longer and are more resource intensive than standard systematic reviews of group-level data.
Pairwise comparisons of several types of interventions within a review can provide some useful information, depending on the number of different interventions available. However, when there is a range of competing interventions, multiple pairwise comparisons may not be informative for decision-makers, particularly when active treatments are always compared with placebo or usual care. In such cases it can be impossible to draw valid conclusions regarding the relative effects of several competing technologies and standard meta-analysis does not lend itself to the ranking of treatments in terms of their estimated effectiveness. Wound dressing selection is an aspect of health care, like many others, in which there are many competing interventions for the same indication, in which strong claims are made in the marketing literature regarding relative treatment effects and in which clinicians understandably struggle to make informed choices. 188 Mixed-treatment comparison systematic review and meta-analysis enables more than two different interventions to be compared simultaneously by extending the standard method of meta-analysis using a Bayesian model to utilise both direct and indirect comparisons within a network of evidence. Mixed-treatment comparison meta-analysis also provides the opportunity to rank interventions according to their probability of being the best intervention relative to the others in the network. 189 Issues with the mixed-treatment comparison approach include the need to meet assumptions such as consistency between evidence derived from direct and indirect comparisons190 and how to deal with variation in risk of bias across different parts of the network. 191 As with standard meta-analysis, variation in risk of bias across trials is always a concern as is variation in any other characteristic that influences treatment effects.
With a choice of possible review methods in mind, workstream 3 began with a consultation exercise with our NHS partners to identify high-priority topics for evidence synthesis. This was followed by consideration of suitable topics for advanced methods of evidence synthesis, as well as earmarking those appropriate for new and updated Cochrane reviews. The aims of this were to provide some immediate evidence to inform practice by making maximum use of existing evidence as well as to identify gaps in the evidence to inform future research. In this chapter we report the methods and results of the following four pieces of work:
-
the identification and prioritisation of topics for evidence synthesis
-
selecting candidate topics for IPD meta-analysis
-
undertaking new and updating existing high-priority Cochrane systematic reviews
-
mixed-treatment comparison meta-analysis in high-priority topic areas.
The identification and prioritisation of topics for evidence synthesis
Introduction
Evidence synthesis methods such as systematic review facilitate information management and clinical decision-making through the selection, appraisal and synthesis of primary studies that address a common, predefined question. Advantages of evidence synthesis include the potential to derive a summary estimate of treatment effect from multiple primary studies, improved precision, reduction of bias and the identification of gaps in the evidence base. 183 Such review work is resource intensive and it is important therefore that systematic review topics are important and meaningful to health-care decision-makers. For the purposes of this programme grant it was essential that topic selection for evidence synthesis was service led (as opposed to researcher led) to provide the most pertinent research-based information to the NHS.
A key feature of workstream 3 was the early identification and prioritisation of evidence synthesis topics through detailed consultation with our NHS collaborators (Leeds Community Healthcare NHS Trust). The main focus was on identifying areas relating to treatment effectiveness but other types of research questions were also considered. Topics could relate to any type of complex wound. The purpose of this was to identify areas that were deemed important for informing clinical practice and implementation, and for which health professionals felt that there was current uncertainty in terms of the evidence for best practice.
Objective
The objective was to identify a clinician-led, prioritised list of topics for evidence synthesis across all complex wound types, particularly those in relation to treatment effectiveness.
Methods
The identification and prioritisation process was carried out in two stages: harvesting of topic ideas and matching of ideas against currently available literature.
Harvesting of topic ideas
Topic ideas were obtained in three ways: by talking to participants at the programme grant’s launch event; by consultation with community clinical teams; and from enquiries sent via the programme grant’s website.
Consultation with participants at the launch event
The launch event for this programme grant took place during September 2009 and consisted of presentations, dedicated stands for each workstream, refreshments and opportunities for informal networking. The stand for workstream 3 included demonstration of, and access to, The Cochrane Library where delegates could attempt searches on research questions of interest to them if they wished. This helped to stimulate informal discussion about what could be important topics for evidence synthesis and provided a valuable opportunity to discuss possible uncertainties relating to practice. Delegates were invited to leave their contact details indicating an interest in contributing to workstream 3 in terms of further follow up, discussion and consultation. During the launch day presentations, delegates were invited to put forward topic ideas for evidence synthesis; some ideas came to light in the discussion period immediately following the presentations, whereas others filtered through to the programme grant team after the launch day, either directly (e.g. by e-mail or via the website) or via the clinical teams.
Consultation with community clinical teams
During January 2010, SO’M attended two routine meetings of community clinical teams in Leeds: podiatry and nursing (consisting of members of the tissue viability nursing team, community nurses and intermediate care nurses). In both instances, clinicians had been provided with details of the consultation exercise in advance and had been asked to reflect on possible topic ideas and uncertainties for evidence synthesis prior to the meetings. During both meetings, SO’M explained the purpose of the consultation exercise, emphasising the importance of having a list of ideas that had originated from health practitioners rather than from researchers. She then explained that, in particular, questions and uncertainties relating to treatment effectiveness were sought, but that any type of research question could be considered in the wider context of the programme grant and future research agenda. She made a brief presentation in which the following were explained: the purpose of the programme grant; the collaboration between Leeds NHS and the University of York; the focus on complex wounds; and a brief overview of the three workstreams. Finally, a structure for research question formulation was presented, based on the key domains of PICO. 174 Participants were invited to use this if they wished, to help structure research ideas. Relevant examples were provided (Tables 51 and 52) and hard copies of similar information were tabled, having already been circulated prior to the meeting. There then followed a period of discussion during which participants raised and discussed ideas; all were noted for later collation and assessment against existing literature. A follow-up e-mail was sent to group members during February 2010 to capture ideas emerging later and to target those who had been unable to attend the team meetings. In addition, the leaders of each clinical team forwarded any other ideas expressed to them by team members individually that had not already been captured.
| PICO component | Definition | Example |
|---|---|---|
| P | Participants | Patients with venous leg ulceration |
| I | Intervention | Four-layer bandage |
| C | Comparator | Short-stretch bandage |
| O | Outcome | Complete healing of ulcer |
| PICO component | Definition | Example |
|---|---|---|
| P | Participants | Patients with diabetic foot ulcers |
| I | Intervention | Silver-impregnated dressing |
| C | Comparator | Povidone iodine dressing |
| O | Outcome | Complete healing of ulcer; resolution of ulcer infection |
Enquiries sent via the programme grant website
During February 2010, follow up e-mails were sent to delegates who had indicated an interest in workstream 3 during the launch event and also to members of the two clinical teams to invite submission of later ideas. Web pages were set up to enable submission of ideas online and information about PICO was posted on the website to assist with question formulation and focusing of uncertainties.
Matching of ideas against currently available literature
All ideas were summarised and tabulated, with grouping according to wound type. Database searches were then undertaken to seek relevant evidence addressing the uncertainties raised. The databases accessed included MEDLINE and those housed in The Cochrane Library; all were searched from inception to February 2010. Results were tabulated, with this then being circulated to the programme grant working team to discuss appropriate actions.
Results
Overall, 26 research questions were identified. Nine emerged from consultation with the community podiatry team, 10 from consultation with the nursing team and three from discussions with delegates during the launch event, and four came from a community dietitian. The research questions are listed in the following sections, grouped according to wound type.
Research questions relating to foot ulcers and other foot conditions
-
What is the best package of care for people with non-diabetic foot ulcers?
-
What is the impact of the time lag involved in obtaining the dressing, antibiotic or other treatment that the podiatrist feels is clinically indicated?
-
What is the relationship between debridement and healing in all types of foot ulcers?
-
What is the effectiveness of a silver dressing compared with a povidone iodine dressing for treating diabetic foot ulcers?
-
What are the best strategies for detecting and treating infection in diabetic foot ulcers?
-
What is the cost-effectiveness of different dressings for healing after nail surgery (silver, povidone iodine, non-adherent)?
-
What is the best way to diagnose osteomyelitis in patients with diabetic foot ulcers?
-
What is the acceptability of different dressings for those with foot ulcers who self-care?
-
What is the significance of locus of control issues regarding the patient’s view of his or her own adherence with foot ulcer treatment [e.g. ‘It doesn’t matter what I do, you (i.e. the podiatrist) have to make it heal’]?
Research questions relating to leg ulcers
-
What is the effectiveness and cost-effectiveness of ‘breathable’ foam film dressings (e.g. Allevyn®, Smith & Nephew, London, UK) compared with absorbent dressings used with leg ulcers?
-
What is the effectiveness of silver dressings used with venous leg ulcers?
-
How does obesity affect healing of leg ulcers?
Research questions relating to pressure ulcers
-
What is the best management of underweight patients deemed to be at risk of pressure ulceration in relation to support surfaces?
-
What are the best ways to measure shear force in wheelchair users in relation to preventing and treating pressure ulcers?
Research questions relating to surgical wounds
-
What is the effectiveness of topical negative pressure compared with conventional dressings such as hydrocolloid or alginates when treating dehisced abdominal wounds?
-
How does obesity affect healing of dehisced abdominal wounds?
-
What are the best strategies for post-operative care of pilonidal sinus? Is it better to pack or not to pack?
Research questions relating to all wound types
-
What role should Edinburgh University Solution of Lime (EUSOL) have in wound management?
-
Is direct application of steroids and antifungal preparations to the wound bed effective?
-
What is the role of protease-modulating matrix dressings (e.g. Promogran®, Systagenix, Gargrave, UK) in wound management?
-
What is known about overgranulation (or hypergranulation)?
-
What is the best way to manage wounds in mental health patients who have dementia?
-
How many of those with complex/chronic wounds also have nutritional risks (e.g. poor hydration, undernutrition, 10% unintentional weight loss, poorly controlled diabetes, artificial feeding and obesity)?
-
Do complex wounds have an impact on patients’ ability to shop, prepare, cook or eat all their meals and drink all their drinks throughout the day and hence their overall food and fluid intake, resultant nutritional status and quality of life?
-
Which nutritional interventions aid wound healing?
-
Could food fortification using dried milk powder promote wound healing?
Actions taken following identification of relevant literature
Several research questions were identified as being of high priority and these resulted in new reviews or review updates. These included questions relating to choice of dressings for diabetic foot ulcers and venous leg ulcers. The following new reviews and review updates were conducted:
-
a series of Cochrane systematic reviews on dressings for diabetic foot ulcers192–195
-
a systematic review and mixed-treatment comparison meta-analysis of all dressing types in patients with diabetic foot ulcers196
-
two Cochrane reviews of non-antimicrobial dressings in venous leg ulcers197,198 and a substantive update of a Cochrane review on antimicrobial interventions for venous leg ulcers199
-
a scoping review of silver-impregnated dressings used with venous leg ulcers (see Chapter 4, Discussion)
-
a substantive update of a Cochrane review on nutritional interventions for preventing and treating pressure ulcers200 (note: this publication is not claimed as an output of the programme per se; however, the high priority of the topic stimulated us to strongly encourage the Cochrane reviewers to update the review and we expedited the editorial process to facilitate this).
The question on whether or not complex wounds impact on patients’ quality of life was addressed in workstream 2. Relevant and informative literature was identified for two questions on foot ulceration (namely care of people with non-diabetic foot ulcers and debridement of all types of foot ulcers) and the pertinent documents were forwarded to the podiatry team of Leeds Community Healthcare NHS Trust.
Several other topics did not appear ripe for evidence synthesis because there was no, or very little, available primary research and no further action could be taken in terms of systematic review:
-
cost-effectiveness of different dressings for healing after toenail surgery
-
obesity as a risk factor for delayed healing in leg ulcers
-
management of underweight patients at risk of pressure ulceration
-
post-operative strategies for pilonidal sinus
-
the role of EUSOL in wound management
-
topical application of combined steroids and antifungal treatments to the wound bed
-
overgranulation
-
management of wounds in patients with dementia
-
prevalence of nutritional risk factors in people with complex wounds.
Several other research questions were considered to be outside of the remit of the programme and were not progressed further. Examples include locus of control issues for people with foot ulcers, measurement of shear forces in wheelchair users and treatment of dehisced surgical wounds (covered by another programme grant [see www.nihr.ac.uk/funding/funded-research/funded-research.htm?postid=2194 (accessed 2 June 2016)]. Further details are shown in Appendix 7.
Discussion
While working closely with our NHS partners to prioritise topics for evidence synthesis we found face-to-face contact with clinicians was the most fruitful approach. Participants were facilitated in formulating PICO questions within the context of team meetings and at the research programme’s launch event we provided opportunities to view and use The Cochrane Library, which provided a useful focus for discussion about uncertainties.
The research questions obtained reflected much uncertainty around the best choice of dressings for different wound types. These uncertainties were addressed through a series of new systematic reviews and systematic review updates undertaken during the course of the programme. Several topic areas emerged for which there was very little primary material and research funders may wish to consider these. Several would appear to be of importance given demographic changes and the increasing proportion of older people with multiple morbidities. 201 These could include the management of complex wounds in people with obesity or mental illness or both. A further potential area for future primary research is postoperative care of patients undergoing toenail surgery (most frequently for ingrown toenails). Such surgery is common and complications such as delayed healing, infection and poor cosmesis can occur. 202 There is no relevant national clinical guideline and much uncertainty remains in terms of optimum methods of avulsion and post-operative care, for example choice of dressings. 203 Finally, there remains much uncertainty around the relationship between nutritional status and wound healing. Evidence suggests that malnutrition is common in community-based patients with wounds;204 however, the effect of specific nutritional interventions remains unclear. 200,205 Future research should address the prognostic performance of different nutritional risk factors in predicting ulcer incidence and healing. Also, efforts should be made to identify specific nutrients most likely to promote effective wound prevention and healing.
Selecting candidate topics for individual patient data meta-analysis
Background
Venous leg ulcers are common, costly and impact adversely on patients’ health-related quality of life. 206–209 Many of these wounds are colonised by bacteria or show signs of clinical infection and the presence of infection may delay healing. 210,211 The classic signs of infection include local pain, heat, redness, swelling and purulence; however, it has been suggested that these may not always be present in patients with venous leg ulcers. In light of this, other signs and symptoms have been proposed as an alternative method of assessment. These include delayed healing, unexpected pain, abnormal odour, pocketing at the base of the wound, discoloured (i.e. unusually dark) granulation tissue, friable granulation tissue and devitalised (sloughy or necrotic) tissue. These characteristics are sometimes referred to collectively as the signs and symptoms of critical colonisation. 212,213
Strategies for treating infected wounds include systemic antibiotics and topical antimicrobial agents. Silver-impregnated dressings are examples of topical antimicrobial applications; this intervention emerged as a priority topic for systematic review during consultation with clinicians. The history of using silver applications to treat infected leg ulcers dates back to at least the early twentieth century. 214 Recent years have seen a resurgence of interest in these products, together with intensive marketing by manufacturers; this has coincided with increased prescription. Silver-impregnated dressings are used frequently in the NHS and are costly, accounting for approximately £26M in prescription costs (representing 22% of the total cost of advanced wound dressings) for primary care in England during 2008/9. 215
Most evidence from systematic reviews does not suggest a benefit of using silver compared with other types of dressings in terms of healing venous leg ulcers. 216–218 However, the current evidence is based on group-level data and it is possible that true treatment effects can be concealed when important patient-level prognostic factors are not controlled for. A more powerful analysis based on IPD may be more informative and therefore aid clinical decision-making. 186
Aim and objectives
This study had the aim of establishing the value and feasibility of conducting an IPD meta-analysis of RCTs of silver-containing wound dressings for the treatment of venous leg ulcers. To meet this aim we had the following objectives:
-
to estimate the numbers of available RCTs of silver dressings and trial participants
-
to characterise the comparisons made within existing trials
-
to analyse the group-level data from trial reports of silver dressings
-
to explore trial-level covariates
-
to consider the clinical heterogeneity between existing trials.
Methods
A scoping review of the literature was undertaken. A single reviewer undertook study selection, data extraction, risk-of-bias assessment and data synthesis.
Study selection criteria
Randomised controlled trials evaluating silver-impregnated dressings used in people with venous leg ulceration and which reported an objective assessment of healing were included. Evaluations of topical silver sulfadiazine were excluded because this is used mainly as a treatment for burns. Comparator interventions included non-antimicrobial dressings of any type including a policy of applying dressings chosen according to clinicians’ judgement, as may be used in pragmatic RCTs. RCTs involving comparisons with alternative antimicrobial products were excluded, as were comparisons between different silver applications. Evaluations involving concurrent therapies such as compression were included as long as there was no systematic difference in cointerventions across treatment arms. For the purposes of this review, trial outcomes relating to resolution of wound infection were noted but not explored in terms of meta-analysis. RCTs were included only when the full report was available to allow a meaningful assessment of comparisons, outcomes and risk of bias.
Search strategy
The Cochrane Central Register of Controlled Trials was searched from inception to October 2009. In addition, MEDLINE, EMBASE and CINAHL were searched from the start of 2006 to October 2009 to cover any gaps on CENTRAL caused by a time lag in indexing references derived from other databases. The search string for MEDLINE is shown in Appendix 8. The reference lists of existing reviews were also examined.
Data extraction
For each eligible trial the following data were extracted:
-
study identifier (first author and year of publication)
-
number of randomised patients/limbs/wounds
-
leg ulcer aetiology (e.g. purely venous or mixed venous/arterial)
-
other wound characteristics serving as patient selection criteria in trials
-
baseline ulcer infection status
-
whether or not predictive covariates for healing were reported (baseline wound area and duration)
-
number of patients/limbs/wounds per treatment arm
-
details of interventions in each arm
-
details of cointerventions used for all patients, for example compression
-
frequency of dressing changes
-
duration of treatment
-
healing outcomes (time to healing, complete healing at given time points, change in ulcer area, healing rate)
-
outcomes relating to resolution of infection
-
brief mention of other reported outcomes
-
number of withdrawals per group
-
funding source of trial.
Risk-of-bias assessment
The Cochrane risk-of-bias tool was used as a basis for assessment. 219 Assessment focused on domains that have been empirically demonstrated as having the potential to generate biased estimates of effect when improperly handled, including allocation concealment, blinding of outcome assessors and handling of incomplete outcome data. 148,220–223 Details of the following were recorded from each included RCT:
-
method of randomisation
-
method of allocation concealment
-
method of blinded outcome assessment
-
methods for handling incomplete outcome data.
For each domain, an assignment of low, high or unclear risk of bias was allocated, together with the rationale for the reviewer’s judgement. In addition, an overall risk-of-bias judgement was assigned to each RCT according to the following decision rules. RCTs were classified as being at overall high risk of bias if they were rated as having high risk in relation to at least one of the three key domains (allocation concealment, blinding of outcome assessors and handling of outcome data). If none of the key domains was rated as high risk but one or more were rated as having an unclear risk of bias, the RCT was rated overall as having an unclear risk of bias. To attain an overall low risk of bias, all three key domains had to be rated as low risk individually.
Data synthesis
For the purposes of this review, healing data only were analysed. The reporting of other outcomes was described and tabulated. Methods for estimating measures of effect from individual RCTs and for pooling data were based on standard meta-analysis methods, as described in the Cochrane Handbook for Systematic Reviews of Interventions. 224
For dichotomous data (e.g. complete healing during the trial period), the risk ratio (RR) with 95% CI was estimated for each RCT for the comparison of silver-impregnated dressing compared with non-antimicrobial dressings using the number of patients healed per treatment group and the total number randomised in the group. In instances in which more than one RCT reported similar outcomes at similar time points, the RRs were pooled using an inverse variance method to provide an overall estimation of treatment effect. For the pooled analysis, studies were grouped according to length of follow-up (4–6 weeks, 9–12 weeks, 6 months and 1 year). A test of statistical heterogeneity was generated for each pooled outcome. Statistical heterogeneity was defined as a chi-squared p-value of ≤ 0.1 and the I2 test was undertaken to estimate the percentage of the variability in estimates of effect due to heterogeneity rather than chance. When the I2 estimation was equal to zero, a fixed-effects model was undertaken. When I2 was greater than zero, both fixed-effects and random-effects analyses were undertaken and any difference in estimates was noted.
It was planned to pool hazard ratio (HR) estimates if available from RCT reports. Otherwise, any data relating to time-to-healing outcomes were recorded.
For continuous outcomes (e.g. absolute or percentage change in ulcer surface area, healing rate) the difference in means with 95% CI was calculated for each RCT individually using the mean and SD value per group for the reported measure, together with the number randomised to that treatment group. When appropriate, trials were pooled using the mean difference, weighted by the inverse of the variance. Statistical heterogeneity was assessed and managed as described above.
Exploration of potential data required for individual patient data meta-analysis
As is the case for other types of systematic review, IPD systematic review and meta-analysis requires a predefined protocol to set out methods and decision rules in advance of carrying out the research to minimise bias. 183 A protocol for an IPD meta-analysis describes the data that would be requested from each participating triallist in terms of baseline and outcome variables. Many of the important data requirements for venous leg ulcer research have already been established from prognostic research, experience with large RCTs and an existing example of IPD meta-analysis in wound management. 18,19,187,225,226 For example, larger ulcer area and longer ulcer duration at the outset of treatment are known to be independent and significant predictors of delayed healing. 225,226 In terms of outcome assessment, time to healing is considered as much more informative than frequency of complete healing at fixed time points or intermediate healing measures because it provides information not only on whether or not healing occurs but also on how long it takes to occur. This provides important insights for resource use and clinical and policy decision-making.
This review investigated further data that may be required that are more specific to the topic of silver, particularly given its role in the management of infection. The likely availability of patient-level data was considered in light of information from trial reports and through contact with the primary investigators. In addition, differential treatment effect was examined in relation to several trial-level covariates. This was an attempt to inform the set of variables required for an IPD meta-analysis by highlighting important clinical differences between and within trials. The following variables were explored within the limits of available data from trial reports.
Participant characteristics
Treatment effects were considered for the following subgroups:
-
RCTs restricting selection to patients with venous leg ulcers as opposed to those also allowing inclusion of individuals with mixed ulcer aetiology (venous and arterial)
-
baseline ulcer infection status (e.g. defined as clinically infected, critically colonised or colonised).
Intervention characteristics
Differential treatment effects were considered for:
-
different types of silver dressing
-
different types of comparator dressings
-
use of compression as part of the trial treatment regimen.
Methodological considerations
The following risk-of-bias domains were examined in terms of their impact on treatment effect:
-
use of a true method of randomisation compared with no specified method
-
implementation of a robust allocation concealment method compared with a high-risk or an unclear strategy
-
blinding of outcome assessors compared with non-blinded or an unclear method of assessment
-
analysis by intention to treat compared with a high-risk or unclear strategy for handling incomplete outcome data.
Funding source
Treatment effect was examined in relation to the funding source. The main distinction made was funding by a manufacturer of one of the products under investigation in the trial as opposed to funding by an independent sponsor.
Results
Results of the literature search
The search strategy generated 355 records. Following examination of titles and abstracts, seven RCTs were identified as being eligible for inclusion227–233 (Table 53). Two RCTs recruited people with other wound types in addition to venous leg ulcers: pressure ulcers230 and burns, donor sites, surgical wounds, pressure ulcers and diabetic foot ulcers. 232 All seven RCTs included two treatment groups and all recruited patients as the unit of randomisation. The total number of patients with venous leg ulcers was 1012. Data extraction of the trials is provided in Appendix 9, presented alphabetically by first author.
| Trial | Country | Number of patients (all) | Number of patients (leg ulcers only) |
|---|---|---|---|
| Dimakakos 2009227 | Greece | 42 | 42 |
| Jørgensen 2005228 | Canada, Denmark, Germany, Italy, The Netherlands, UK, USA | 129 | 129 |
| Lazareth 2008229 | France | 102 | 102 |
| Meaume 2005230 | France | 99 | 71 |
| Michaels 2009231 | UK | 213 | 213 |
| Münter 2006232 | Germany, UK, Denmark, Italy, Switzerland, Belgium, Slovenia, Brazil, Canada | 619 | 415 |
| Wunderlich 1991233 | Germany | 40 | 40 |
| Total | 1244 | 1012 |
Comparisons undertaken in the trials
An initial scrutiny of the trials revealed that there were differences between trials in terms of the silver dressings, comparator dressings and the use of compression (Table 54).
| Trial, duration of intervention | Silver application | Comparator | Compression |
|---|---|---|---|
| Dimakakos 2009,227 9 weeks | Silver foam dressing | Non-adhesive foam dressing | Yes (short-stretch bandage) |
| Jørgensen 2005,228 4 weeks | Silver foam dressing | Non-adhesive foam dressing | Yes (according to best practice at each study centre) |
| Lazareth 2008,229 4 weeks | Silver hydrocolloid dressing | Hydrocolloid dressing | Yes (according to clinicians’ choice) |
| Meaume 2005,230 4 weeks | Silver hydroalginate dressing | Calcium alginate dressing | Yes (system not defined but designed to deliver 15–35 mmHg ankle pressure) |
| Michaels 2009,231 12 weeks | Various silver dressings | Various non-adherent dressings | Yes (multilayer according to best practice at each study centre) |
| Münter 2006,232 4 weeks | Silver foam dressing | Local best practice (including silver) | Yes (according to best practice at each study centre) |
| Wunderlich 1991,233 6 weeks | Silver charcoal dressing | Various topical agents | Not mentioned |
The base used for the silver-impregnated dressings included foam, alginate, hydrocolloid and charcoal. One trial of pragmatic design allowed the choice of the silver-impregnated dressing to be in accordance with the clinicians’ judgement; options for the base material included foam, alginate, hydrocolloid, low-adherent and non-adherent dressings. 231
The comparator intervention also varied across trials. Four RCTs used the non-impregnated version of the silver dressing in the trial. 227–230 Two pragmatic trials allowed participating study centres to deliver care according to the clinicians’ choice. 231,232 In one instance, 17% of patients in the control group also received a silver-impregnated dressing. 232 Another trial used various topical applications administered according to the observed stage of healing (granulation or epithelialisation). 233
In all RCTs apart from one233 it was clear that compression had been used as a standardised concurrent therapy. In one RCT, compression was not mentioned. 233 Previous research has shown that different types of compression may perform differently in terms of promoting healing. 184,187 In the context of an IPD meta-analysis, it would be important to take account of clinically significant differences in treatment comparisons and to include type of compression as a covariate when modelling the estimate of treatment effect.
The duration of treatment varied across the RCTs, with four trials treating ulcers for 4 weeks,228–230,232 one for 6 weeks,233 one for 9 weeks227 and one for 12 weeks231 (see Table 54).
Analysis of healing outcomes
The following analyses are based on group-level data gleaned from trial reports. It was not feasible to analyse all seven RCTs together because of differences in outcome measurement and duration of follow-up. One RCT assessed the time taken for the wound to heal (time-to-event data),231 others measured the proportion of patients with healed wounds at defined time points (dichotomous data)227,228,231,233 and others assessed intermediate outcomes such as the change in wound surface area during the trial or the healing rate over time. 228–230,232,233 For some RCTs it was not possible to estimate measures of treatment effect because of a lack of data.
Time to healing: one randomised controlled trial (210 patients analysed)
One RCT presented data on time to healing. 231 The difference between treatment groups was not statistically significant according to estimates of median time to healing (based on Kaplan–Meier survival curves) or HR. The estimate for median days to healing was 67 days (95% CI 54 to 80 days) for the silver dressing and 58 days (95% CI 43 to 73 days) for various non-antimicrobial dressings (p = 0.408, derived from the Cox proportional hazards model). The reported HR estimate for the silver dressing compared with the comparators was 1.13 (95% CI 0.85 to 1.15). Details of analyses based on time to healing were not available for the other RCTs, despite several requests for details from the primary investigators.
Complete healing during the trial period: four randomised controlled trials (424 patients analysed)
Four RCTs reported complete healing at various time points ranging from 4 weeks to 1 year. 227,228,231,233 Data were grouped according to different periods of follow-up: 4–6 weeks, 9–12 weeks, 6 months and 1 year. There was no evidence for a difference between groups at any time point (Figure 12). Figure 12 shows estimates derived from fixed-effects models. Statistically significant heterogeneity was detected for the analysis of complete healing at 9–12 weeks and so a random-effects analysis was generated that showed a similar estimate to the fixed-effects model (RR 1.27, 95% CI 0.79 to 2.03). The observed heterogeneity may have arisen from participant, comparator or methodological differences, or may have occurred by chance because of the small number of RCTs included in the meta-analysis.
FIGURE 12.
Complete healing during the trial period: fixed-effects model. df, degrees of freedom; M–H, Mantel–Haenszel.
Intermediate measures of healing
Five RCTs reported intermediate healing outcomes, namely absolute change in ulcer area, percentage change in ulcer area and healing rate over time. 228–230,232,233 Two RCTs provided sufficient data for pooling all three outcomes at 4 weeks. 229,230 The estimate for mean absolute change in ulcer area was in favour of silver (difference in means –4.70 cm2, 95% CI –8.46 to –0.94 cm2) (Figure 13). Estimates for mean percentage change in ulcer area and healing rate did not suggest differences between treatment groups. Statistically significant heterogeneity was detected for one analysis (mean percentage change in ulcer area). The estimate from the random-effects model did not change the finding (difference in means –6.13 cm2, 95% CI –32.59 to 20.32 cm2). As before, this heterogeneity may have arisen by chance.
FIGURE 13.
Intermediate measures of healing at 4 weeks: fixed-effects model. df, degrees of freedom; IV, inverse variance.

A further three RCTs reported the percentage change in ulcer area but did not provide sufficient information to plot estimates. 228,232,233 Two reported a median percentage change in ulcer area at 4 weeks in favour of silver-impregnated dressings: –45% for silver and –25% for non-antimicrobial dressings (p = 0.034)228 and –46% and –27%, respectively (p = 0.0001). 232 Both p-values are as reported by the trial authors. The third RCT reported respective mean changes of –75% and –60% at 6 weeks, describing the between-group difference as not statistically significant (although no p-value was provided). 233
Exploration of potential data required for individual patient data meta-analysis
Two RCTs included patients with venous leg ulcers and those of mixed arterial/venous aetiology. 228,232 One RCT did not report the numbers with different types of leg ulcers and did not stratify results accordingly. 228 It was included in a meta-analysis with two other trials that recruited solely venous leg ulcer patients227,233 and there was no evidence of statistical heterogeneity between studies (see Figure 12 – complete healing at 4–6 weeks). The second trial recruited 415 patients with different types of leg ulcers, of which 297 had venous leg ulceration. 232 The primary outcome for this trial was the median percentage reduction in ulcer area at 4 weeks. As reported earlier, for those with venous leg ulcers the median change in ulcer area at 4 weeks was –46% for silver dressings and –27% for comparator dressings (p = 0.0001, as reported by the trial authors). The respective values were similar when all types of leg ulcers were taken into account (–46% vs. –29%, respectively, p = 0.0001). In an IPD meta-analysis, ulcer aetiology could be included as a patient-level covariate, perhaps by using the ABPI.
The included RCTs varied in terms of the patients’ baseline ulcer infection status (Table 55). One RCT recruited only those with clinically infected ulcers227 and another recruited patients with clinical infection or critical colonisation of the ulcer but did not explain the distribution. 232 Three RCTs stipulated presence of critical colonisation of the ulcer as an eligibility criterion but also stated that those with clinically infected ulcers were excluded. 228–230 One RCT stated that patients receiving systemic antibiotics at baseline were excluded; this presumably would also exclude those with any type of clinical infection, including ulcer infection. 231 The last RCT did not provide any information about baseline ulcer infection status. 233
| Trial | Assessment of wound infection at baseline | Assessment of wound infection as an outcome |
|---|---|---|
| Dimakakos 2009227 | All wounds infected and had signs of clinical inflammation | Reported some data on presence of specific bacterial species; nothing on resolution of infection |
| Jørgensen 2005228 | Recruited patients with critical colonisation of ulcer; excluded patients with clinical infection of the ulcer | No outcomes reported that related to resolution of critical colonisation |
| Lazareth 2008229 | Recruited patients with critical colonisation of ulcer; excluded patients with clinical infection of the ulcer | Local signs of heavy bacterial colonisation |
| Meaume 2005230 | Recruited patients with critical colonisation of ulcer; excluded patients using systemic antibiotics within 5 days of recruitment | Incidence of systemic antibiotic use during the trial; number of wounds developing clinical infection (determined by cultures) |
| Michaels 2009231 | Excluded patients receiving systemic antibiotics at baseline | None |
| Münter 2006232 | Recruited patients with critically colonised ulcers or clinically infected ulcers | Reported change in use of systemic antibiotics during the trial period |
| Wunderlich 1991233 | No information provided | Bacterial colony growth during the trial period |
With one exception,229 the RCTs recruiting patients with clinically infected ulcers, critically colonised ulcers or both did not report directly related outcomes, that is, the resolution of signs and symptoms of infection or critical colonisation. Lazareth et al. 229 recruited patients characterised as having ‘critical colonisation’ of their ulcers and reported the number of patients with remaining signs of heavy bacterial colonisation during follow-up. The other RCTs sometimes reported outcomes linked to infection status but these did not always relate directly to the baseline variable. For example, one RCT restricting inclusion to patients with clinically infected ulcers reported the presence of specific bacterial species on the wound bed during the trial but there was no report of resolution of clinical infection. 227 Other examples are shown in Table 55.
Critical colonisation
In wound care, wound infection is commonly conceptualised as being at the far end of an infection continuum. The continuum, as defined by Kingsley,234 starts with sterility (a brief period that might follow surgery), moving to contamination (defined as the presence of microbes but little active growth) and then colonisation (considered as the normal status quo with growth wound flora being managed by the host immune system) and finally to critical colonisation and infection. Kingsley234 defines critical colonisation as a point between colonisation and infection in which the ‘healthy’ balance of wound flora is no longer maintained by the host and the bacterial load and/or species present in the wound shifts away from a so-called safe level. More recently, others have conceptualised critical colonisation as invasion of the wound surface by micro-organisms. 235 There is currently no standard way to diagnose critical colonisation; rather, it is generally noted as being associated with delayed healing in the absence of overt signs of wound infection,234,236 possibly with other symptoms such as increased exudate (to a lesser extent than in infection) and hypergranulation/friable tissue. 235 There are no robust clinical research data to support associations between a state defined as critical colonisation and wound outcomes; critical colonisation in wound care is a concept that still requires definitive characterisation. 237
Given the variation in these group-level data and the small number of RCTs identified, it is difficult to determine any relationship between ulcer healing and presence of infection or critical colonisation at baseline or persisting during the trial period. IPD meta-analysis is often based on regression analysis incorporating adjustment for patient-level baseline covariates. 185 In this instance it would be useful to explore the influence of baseline ulcer infection status on healing. However, the information in Table 55 indicates that definitions of infection and colonisation vary enormously across trials and this would be problematic for coding and analysis. Ideally, each patient would be coded as having confirmed clinical infection (yes/no) at baseline, using standardised criteria. Definitions of infection-related outcome variables also differ across trials and this would cause difficulties in exploring resolution of infection as an outcome.
The variation in silver-impregnated dressings, comparators and use of compression has already been discussed (see Comparisons undertaken in the trials and Table 54). There were no apparent differences between trials in terms of estimated treatment effect that could have been accounted for by different types of silver dressing or comparator, or different use of compression.
The included RCTs varied in terms of overall risk of bias. Only one was classified as being at low risk of bias,231 one was at high risk of bias228 and the risk of bias in the others was unclear because of poor reporting228–230,232,233 (see Appendix 9).
In terms of individual domains, three RCTs were assigned a low risk of bias for the method of randomisation as they had described the use of computerised sequence generation;228,231,232 the remaining RCTs were assigned an unclear risk of bias as they had mentioned randomisation but not specified the actual methods used to generate the sequence. One RCT was allocated a low risk of bias for allocation concealment because it described the use of a centralised telephone service231 but all of the other RCTs were assigned an unclear risk of bias. Two trials included clear statements indicating that outcome assessors were masked to treatment allocation229,231 but the other trials had an unclear risk of bias for this domain. For handling incomplete outcome data, five RCTs had a low risk of bias as all had indicated that analyses were by intention to treat. 227,229–232 One trial was at high risk of bias for this domain because withdrawal rates were different across groups and analysis was clearly not by intention to treat. 228 The remaining trial did not provide any information about the method of analysis and was rated as having an unclear risk of bias. 233
Many risk of bias judgements resulted in an unclear rating because of poor reporting of the relevant details. When carrying out an IPD meta-analysis, there is scope to elicit further information because of the close collaboration with participating triallists. In our previous meta-analysis, it was possible to establish details of the methods of randomisation and allocation concealment, and whether or not outcome assessment was masked as information was available on request that had not been published. 187 There is also the opportunity to reinstate patients excluded from the primary investigators’ own analyses and to aim for an analysis by intention to treat for the full data set. 185
It was difficult to judge whether or not source of funding influenced the trial results because of the limited available information. For two trials the funding source was not reported. 227,233 A large independently funded trial (by the UK NIHR Health Technology Assessment programme) found no difference in healing between silver and non-silver dressings. 231 The remaining four trials were funded by the manufacturers of the study dressings228–230,232 and generated mixed results. Pooled data from two RCTs found a difference in favour of silver for the intermediate outcome of mean absolute change in ulcer area at 4 weeks229,230 (see Figure 13). Two further RCTs each reported results that suggested a greater reduction in ulcer area with silver dressings than with non-antimicrobial dressings;228,232 however, both of these trials reported limited data, which precluded our plotting of it. All other analyses did not indicate a difference between treatment groups (see Figures 12 and 13).
It is notable that the one trial at overall low risk of bias did not detect a difference between groups for longer-term complete healing (up to 1 year) or for time to healing. 231
Likely availability of data for individual patient data meta-analysis
Tables 56–58 summarise the variables required for a patient-level analysis and judgements about their potential availability from each of the seven identified trials. These judgements were made using information from the trial reports and from contact with the original investigators. These summaries indicate that there was uncertainty about the availability of some variables that would be essential to perform a meaningful IPD meta-analysis. A particular concern was the uncertainty relating to time-to-healing data, for which the date of randomisation, healing status and date of healing (or date of withdrawal) would be required for each patient (see Table 57).
| Variable | Dimakakos 2009227 | Jørgensen 2005228 | Lazareth 2008229 | Meaume 2005230 | Michaels 2009231 | Münter 2006232 | Wunderlich 1991233 |
|---|---|---|---|---|---|---|---|
| Sex | Y | Y | Y | Y | Y | Y | Y |
| Patient age | Y | Y | Y | Y | Y | Y | Y |
| Primary or recurrent ulcer | ? | ? | Y | Y | Y | ? | ? |
| Ulcer duration | Y | Y | Y | Y | Y | Y | Y |
| Ulcer surface area | Y | Y | Y | Y | Y | Y | Y |
| Ulcer infection statusa | Y | Y | Y | Y | Y | ? | ? |
| ABPI | Y | Y | Y | Y | Y | Y | ? |
| Comorbidities | ? | ? | Y | Y | Y | ? | ? |
| Variable | Dimakakos 2009227 | Jørgensen 2005228 | Lazareth 2008229 | Meaume 2005230 | Michaels 2009231 | Münter 2006232 | Wunderlich 1991233 |
|---|---|---|---|---|---|---|---|
| Healing status (yes/no) | Y | Y | ? | ? | Y | ? | Y |
| Date of healing or time to healing | ? | ? | ? | ? | Y | ? | ? |
| Resolution of clinical infection | ? | ? | ? | ? | ? | ? | ? |
| Development of clinical infection during trial | ? | ? | ? | Y | ? | ? | ? |
| Adverse events | Y | Y | Y | Y | Y | ? | ? |
| Variable | Dimakakos 2009227 | Jørgensen 2005228 | Lazareth 2008229 | Meaume 2005230 | Michaels 2009231 | Münter 2006232 | Wunderlich 1991233 |
|---|---|---|---|---|---|---|---|
| Patient identifier | Y | Y | Y | Y | Y | Y | Y |
| Centre identifier | Y | Y | Y | Y | Y | Y | Y |
| Date of randomisation | ? | ? | ? | ? | ? | ? | ? |
| Allocated treatment | Y | Y | Y | Y | Y | Y | Y |
| Use of compression | Y | Y | Y | Y | Y | Y | ? |
| Received allocated intervention | ? | Y | ? | Y | ? | ? | ? |
| Whether or not excluded from analysis | NA | Y | Y | Y | Y | Y | Y |
| Reasons for exclusion from analysis | NA | Y | Y | ? | Y | Y | ? |
| Date of last follow-up | ? | ? | ? | ? | ? | ? | ? |
Discussion
A scoping review identified seven RCTs comparing silver-impregnated dressings with non-antimicrobial dressings in 1012 patients with venous leg ulcers. The type of analysis varied across trials and included time to healing, complete healing during the trial period and intermediate measures such as change in ulcer area and healing rate over time. Apart from some short-term (4-week) intermediate measures (absolute and percentage change in ulcer area), the group-level data did not suggest a difference in healing between silver-impregnated dressings and non-antimicrobial dressings. These findings are broadly in agreement with those from relevant systematic reviews. 216–218 Only one systematic review considered infection-related outcomes218 despite silver-impregnated dressings being indicated for use in infected wounds. 238 This scoping review considered the reporting of infection-related variables and found considerable variation at baseline and in terms of outcome definition.
Individual patient data meta-analysis normally focuses on time-to-event outcomes. Use of statistical techniques such as the Cox proportional hazard model provides the advantage of being able to adjust estimates of treatment effect for the influence of predictive covariates, generating a more precise estimate. 185 The lack of reported data on time to healing is a cause for concern in this body of literature, with only one RCT reporting this outcome. 231 Of the other RCTs, three reported frequency of complete healing during the trial period227,228,233 and three reported an intermediate measure of healing as the trial’s primary outcome. 229,230,232 This includes the largest RCT, which recruited 415 patients with venous and venous/arterial leg ulcers and reported percentage change in ulcer area at 4 weeks as the main outcome, with no information on follow-up to complete healing. 232
Prescribing guidelines state that silver-impregnated dressings should be restricted to use in wounds with identified clinical infection. 238 Another source suggests that silver dressings could be of value in managing critically colonised wounds and that resolution of infection should be considered as the primary outcome rather than healing. 239 Restricting inclusion to trials recruiting patients with confirmed clinical infection or critical colonisation would have resulted in the exclusion of two RCTs and 253 patients from this scoping review. 231,233 Even allowing for this, analysis of infection-related outcomes in a meta-analysis would still be difficult because of the variation in measures used (identification of bacterial species, presence of signs of critical colonisation, use of systemic antibiotics and incidence of clinical infection) and this could not be easily resolved, even with the provision of IPD.
In light of these factors, it was decided that this topic was unlikely to be a suitable candidate for IPD meta-analysis, despite the clear importance of the question for clinical practice. A previous IPD meta-analysis in venous leg ulcers was initiated with knowledge that time-to-healing data were likely to be available for most of the included trials because the primary investigators had described their own time-to-event analyses in published trial reports. 187 In addition, most trial reports indicated that information on predictive covariates would also be available, later confirmed through the collaboration with the triallists. IPD meta-analyses entail considerable effort, not just on the part of those undertaking the systematic review and meta-analysis but also by those who agree to share primary data. Therefore, it is essential that the likely benefits of such research are established prior to undertaking the project. First, it needs to be a research question for which there is current uncertainty in clinical practice despite the availability of an up-to-date and good-quality systematic review of group-level data. Second, the proposed meta-analysis should have sufficient statistical power to detect a clinically important difference. Finally, the correct type and quality of data are required. Advanced methods of evidence synthesis such as IPD meta-analysis are likely to become more frequent as it becomes increasingly important to derive value for money from research expenditure. Those undertaking clinical trials should ensure that outcomes are meaningful and informative and that the relevant baseline data are collected for each patient, focusing on variables that may influence the outcome of treatment. The Cochrane Collaboration has described the method of prospective meta-analysis whereby a collaborative group undertakes several clinical trials, applying design features so that meaningful pooled analyses can be designed in advance of knowing the results of individual trials. 240 They are usually based on IPD. Prospective meta-analyses have been used in fields such as CVD241 and cancer,242 and are planned in childhood obesity. 243 It may be useful to consider the feasibility of this method for future wound management topics.
Undertaking new and updating existing high-priority Cochrane systematic reviews
Introduction
We earlier described the identification and prioritisation of topics for systematic review. As a result of this, several topics were pinpointed as suitable candidates for Cochrane reviews, either as new reviews or updates of existing reviews. Eleven reviews were undertaken overall on interventions for diabetic foot ulcers (five reviews192–195,244), venous leg ulcers (five reviews184,197–199,245) and surgical wounds (one review246). All used standard Cochrane systematic review methodology as described in the Cochrane Handbook for Systematic Reviews of Interventions163 and all were carried out under the auspices of the Cochrane Wounds Group (see http://wounds.cochrane.org/). These Cochrane reviews have already been published in full in The Cochrane Library [see www.thecochranelibrary.com (accessed 12 May 2016)] and we summarise them more concisely here.
Reviews of interventions used with diabetic foot ulcers
-
Alginate dressings for healing diabetic foot ulcers. 194
-
Foam dressings for healing diabetic foot ulcers. 195
-
Hydrocolloid dressings for healing diabetic foot ulcers. 192
-
Hydrogel dressings for healing diabetic foot ulcers. 193
-
Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. 244
Reviews of interventions used with venous leg ulcers
Review of interventions used with surgical wounds
-
Pre-operative skin antiseptics for preventing surgical wound infections after clean surgery. 246
Objective
Our objective for this research was to provide valid, reliable information for wound care decision-making by undertaking systematic reviews in aspects of wound care prioritised by our NHS collaborators. The specific objectives for each review are shown in Table 59.
| Review | Objective | Review status |
|---|---|---|
| Diabetic foot ulcer reviews | ||
| Alginate dressings for healing diabetic foot ulcers194 | To compare the effects of alginate wound dressings with those of no dressing or alternative dressings on the healing of foot ulcers in people with diabetes mellitus | New review |
| Foam dressings for healing diabetic foot ulcers195 | To determine the effects of foam wound dressings on the healing of foot ulcers in people with diabetes | New review |
| Hydrocolloid dressings for healing diabetic foot ulcers192 | To compare the effects of all types of hydrocolloid wound dressings with those of no dressing or alternative dressings on the healing of foot ulcers in people with diabetes | New review |
| Hydrogel dressings for healing diabetic foot ulcers193 | To assess the effects of hydrogel wound dressings compared with alternative dressings or no dressing on the healing of foot ulcers in people with diabetes | New review |
| Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus244 | To assess the effects of negative pressure wound therapy compared with those of standard care or other therapies in the healing of foot wounds in people with diabetes mellitus | New review |
| Venous leg ulcer reviews | ||
| Alginate dressings for venous leg ulcers198 | To determine the effects of alginate dressings compared with those of alternative dressings, non-dressing treatments or no dressing, with or without concurrent compression therapy, on the healing of venous leg ulcers | New review |
| Foam dressings for venous leg ulcers197 | To determine the effects of foam dressings on the healing of venous leg ulcers | New review |
| Compression for venous leg ulcers184 | To undertake a systematic review of all RCTs evaluating the effects on venous ulcer healing of compression bandages and stockings | Update |
| Antibiotics and antiseptics for venous leg ulcers199 | To determine the effects of systemic antibiotics and topical antibiotics and antiseptics on the healing of venous ulcers | Update |
| Topical agents or dressings for pain in venous leg ulcers245 | To determine the effects of topical agents or dressings for pain in venous leg ulcers | Update |
| Surgical wounds review | ||
| Pre-operative skin antiseptics for preventing surgical wound infections after clean surgery246 | To determine whether or not pre-operative skin antisepsis immediately prior to surgical incision for clean surgery prevents surgical site infection and to determine the comparative effectiveness of alternative antiseptics | Update |
Methods
Each review was preceded by the development, peer review and publication of a protocol that set out detailed methodology in advance (as per established Cochrane Collaboration methodology163).
Criteria for considering studies for the reviews
Each review included only RCTs that evaluated the intervention of interest in participants with the condition of interest, irrespective of publication status or language. The eligible comparisons and primary and secondary outcomes for each review are summarised in Table 60. Each review avoided imposing narrow participant eligibility criteria to ensure that the evidence reviewed was as broad and pragmatic as possible (i.e. studies were accepted on the basis of the primary study authors’ definitions of diabetic foot ulcers and venous leg ulcers).
| Review | Comparisons | Primary outcomes | Secondary outcomes |
|---|---|---|---|
| Diabetic foot ulcer reviews | |||
| Alginate dressings for healing diabetic foot ulcers194 | Any comparison with alginate dressings including no dressing | TTH, RH | HRQoL, amputations, adverse events, costs, recurrence, change in ulcer area |
| Foam dressings for healing diabetic foot ulcers195 | Foam dressings compared with other types of dressing or compared with non-dressing treatments | TTH, RH | HRQoL, amputations, adverse events, costs, recurrence |
| Hydrocolloid dressings for healing diabetic foot ulcers192 | Hydrocolloid dressings compared with other types of dressing or non-dressing treatments | TTH, RH | HRQoL, amputations, adverse events, costs, recurrence, change in ulcer area |
| Hydrogel dressings for healing diabetic foot ulcers193 | Any comparison with hydrogels including no dressing | TTH, RH | HRQoL, amputations, adverse events, costs, recurrence, change in ulcer area |
| Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus244 | Any negative pressure wound therapy vs. standard care or alternative treatments | TTH, RH, change in wound size, rate of change in wound size, amputation | HRQoL, adverse events, resource use, recurrence |
| Venous leg ulcer reviews | |||
| Alginate dressings for venous leg ulcers198 | Alginate dressings vs. other wound dressings (including alternative alginate dressings), non-dressing wound treatment (e.g. topical application) or no dressing | TTH, RH, change in wound size/rate of change | HRQoL, cost, pain, debridement, control of bleeding, dressing performance, adverse events |
| Foam dressings for venous leg ulcers197 | Foam dressings vs. other wound dressings (including alternative foam dressings), non-dressing treatments or no dressing | TTH, RH, change in wound size/rate of change | Adverse events, HRQoL, costs, pain, dressing performance |
| Compression for venous leg ulcers184 | Any form of compression bandage or stocking vs. no compression or alternative compression | TTH, RH, change in ulcer size, rate of change of size | Recurrence, costs, HRQoL, pain, adverse events, patient withdrawal |
| Antibiotics and antiseptics for venous leg ulcers199 | Antibiotics (via any route) or antiseptics (topical) vs. placebo, alternative antibiotic or antiseptic or none | Wound healing (preferably TTH, RH, change in size) | Change in symptoms of infection, change in microbiology, bacterial resistance, ulcer recurrence, adverse events, patient satisfaction, HRQoL, costs |
| Topical agents or dressings for pain in venous leg ulcers245 | Topical analgesic or anaesthetic or dressing applied for pain relief (vs. placebo or alternative treatment – this comparison is implied rather than explicit) | Pain | TTH, RH, HRQoL, adverse events |
| Surgical wounds review | |||
| Pre-operative skin antiseptics for preventing surgical wound infections after clean surgery246 | Antiseptic vs. control, antiseptic vs. another antiseptic, single application vs. multiple applications of antiseptic, multiple applications vs. multiple applications of antiseptic | Risk of SSI | HRQoL, adverse events, resource use |
Searching for eligible studies
The search strategies used in the reviews have been developed by the Cochrane Wounds Group (see http://wounds.cochrane.org/) and are based on access to several bibliographic databases as follows:
-
Cochrane Wounds Group Specialised Register
-
CENTRAL
-
MEDLINE
-
EMBASE
-
CINAHL.
In each instance, search strings were designed initially for CENTRAL and were then adapted to be used with the other databases. As study inclusion was restricted to RCTs, methodological search filters were employed for use with MEDLINE, EMBASE and CINAHL. 247,248 Ongoing research was sought from clinical trial registries as follows:
-
World Health Organization International Trial Registry Platform [see www.who.int/ictrp/en/ (accessed 12 May 2016)]
-
ISRCTN register [see www.controlled-trials.com/isrctn/ (accessed 2 June 2016)]
-
ClinicalTrials.gov [see www.clinicaltrials.gov (accessed 12 May 2016)].
In addition, reference lists of included trials and review articles were scanned to identify articles not retrieved by database searching. Attempts were made to contact trial authors and manufacturers (when relevant) to identify unpublished material.
Data extraction and management
Data to be extracted were specified in the protocol of each review and tailored to the requirements of each review. Attempts were made to contact study authors to obtain any missing data. Data from RCTs published in more than one paper were included once, using all associated documents to extract the maximum amount of information while ensuring that data were not duplicated in the review.
Assessment of risk of bias in included studies
Assessment of risk of bias in each included trial was carried out using the Cochrane Collaboration’s risk-of-bias tool. 219 This tool is based on the following domains: the randomisation sequence; allocation concealment; blinding of participants and care providers; blinding of outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias (to be predefined in the context of each review). A judgement of ‘high’, ‘low’ or ‘unclear’ risk of bias was assigned for each domain, together with evidence and the rationale relating to each judgement. Most of the reviews included an overall risk-of-bias rating for each trial, based on key domains, which varied slightly according to the review but could include, for example, domains that have been empirically demonstrated to be associated with biased estimates of effect when improperly handled. These included allocation concealment, blinding of outcome assessment and handling of incomplete outcome data. 148,220,223
Review processes
The processes of study selection, data extraction and assessment of risk of bias were performed by at least two review authors working independently. Any disagreements in study selection decisions, data extracted and risk-of-bias judgements were resolved by discussion.
Measures of treatment effect
The reviews considered a range of outcomes relating to different types of data: dichotomous, continuous and time to event. When appropriate data were available from trial reports or through contact with trial authors, estimates for dichotomous outcomes (e.g. number of patients with complete healing) were reported as RRs with associated 95% CIs. Estimates for continuous data outcomes (e.g. change in ulcer area) were calculated as the difference in means with associated 95% CI. Measures of time to healing and HR estimates were extracted from trial reports. In cases in which HRs were not available but the reports provided other pertinent information in relation to time to healing, estimates were extrapolated in accordance with published recommendations. 249
Unit of analysis issues
Each review noted cases in which the unit of randomisation and unit of analysis did not match (e.g. legs or ulcers randomised but patients analysed). In trials in which multiple limbs or ulcers on the same individual were studied, a note was made regarding whether the trial authors’ analysis was appropriate (i.e. correctly taking account of highly correlated data) or inappropriate (i.e. considering outcomes for multiple ulcers on the same participant as independent).
Dealing with missing data
Some reviews attempted analyses by intention to treat in instances in which the triallists had excluded patients from their own analyses. This was generally only feasible for the outcome of risk of healing for which, provided the number of patients randomised per group was known, a conservative assumption could be made that the excluded patients did not heal, so that they were included in the denominator but not the numerator. In other situations we used complete case analysis (i.e. measures of effect were based on patients who completed the trial) and this was highlighted in the review.
Assessment of heterogeneity
All reviews considered both clinical (the degree to which RCTs vary in terms of participant, intervention and outcome characteristics) and statistical (variation in estimates of effect across trials) heterogeneity. Statistical heterogeneity was assessed using the chi-squared test (a significance level of p < 0.10 was considered to indicate statistically significant heterogeneity) in conjunction with the I2 statistic. The I2 statistic examines the percentage of total variation across RCTs that is due to heterogeneity rather than chance. Different thresholds of the I2 statistic can be predefined to help describe different amounts of heterogeneity. Thresholds used in some reviews were as follows: I2 values of ≤ 40% indicated a low level of heterogeneity and values of ≥ 75% indicated a very high level of heterogeneity. 224
Assessment of reporting biases
Most reviews planned to present funnel plots for meta-analyses including ≥ 10 RCTs using RevMan 5.1 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). This proved to be unfeasible as all meta-analyses were based on small numbers of studies.
Data synthesis
All reviews presented a narrative synthesis of included RCTs, grouped by comparison. Statistical pooling of outcome data was undertaken on groups of RCTs with available data that were considered to be sufficiently similar in terms of design and characteristics of participants, interventions and outcomes. The decision to undertake meta-analysis depended on the availability of outcome data and assessment of heterogeneity. In the I2 threshold example explained in Assessment of heterogeneity, the choice of meta-analysis model was as follows. For comparisons in which there was no apparent clinical heterogeneity and the I2 value was ≤ 40%, a fixed-effects model was applied. When there was no apparent clinical heterogeneity and the I2 value was > 40%, a random-effects model was undertaken. However, data were not pooled when heterogeneity was very high (I2 values ≥ 75%). Pooled dichotomous outcomes were presented as a summary RR estimate with 95% CI. When continuous outcomes were measured in the same way across RCTs, a pooled difference in means was presented with 95% CI. When outcomes shared the same underlying concept but were measured using different instruments (e.g. quality-of-life scales), the standardised mean difference was the pooled summary measure of choice. For time-to-event data, HR estimates and 95% CIs were pooled as presented in the RCT reports using the generic inverse variance method in RevMan 5.1. When HRs were not provided, estimates were extrapolated, when possible, using other reported data. 249 Individual and pooled estimates of treatment effect were generated using RevMan 5.1.
Some reviews also planned to include subgroup analyses (e.g. to explore the impact of sources of heterogeneity) and sensitivity analyses (to investigate the effects of parameters such as risk of bias on estimates of treatment effect).
The above analyses relate to group-level data for pairwise comparisons. Two reviews included more advanced meta-analytical techniques to complement the existing standard meta-analyses. These included a meta-analysis of IPD for one comparison184 and a mixed-treatment comparison meta-analysis. 196 Several of the reviews incorporated summary of findings tables. 250 These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined and the sum of the available data for the main outcomes. 251 The summary of findings tables also include an overall grading of the evidence related to each of the main outcomes using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. 252 The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence (which might relate to indirect comparisons or differences between the populations, treatments or outcomes measured in studies and the populations, treatments or outcomes of interest), heterogeneity, precision of effect estimates and risk of publication bias. 251 Each review selected key outcomes to be represented in the summary of findings tables; these included the primary outcomes and selected secondary outcomes such as quality of life and adverse events.
Here, rather than reproduce each Cochrane review in full, we present a summary of the reviews in table format (Tables 61–63). It should be noted that some reviews include trials with more than two treatment arms and therefore more trials may be listed in the ‘Comparisons’ column than are indicated in the ‘Number of RCTs included’ column. We have emphasised estimates of proportions of wounds completely healed in these summaries; complete wound healing is the outcome that matters to patients (see Chapter 3) and was the primary outcome for the reviews. However, wound healing can (and is) captured in trials in different ways (including time to complete healing and proportion of wounds completely healed). We have emphasised the latter here as it is the most frequently measured and reported measure (although time to complete healing with survival analysis is the better approach). Durations of follow-up varied considerably across trials. The information on quality of the evidence was drawn directly from the summary of findings tables in reviews when available. For reviews without summary of findings tables, each GRADE criterion was assessed against information presented in the review report and a summary rating of overall quality derived from the available details.
| Review | Number of RCTs (participants) included, search date | Results for healing | Quality of the evidence | Review authors’ conclusions |
|---|---|---|---|---|
| Alginate dressings for healing diabetic foot ulcers194 | 6 (375), April 2013 | Alginate dressing vs. basic wound contact dressing (three RCTs): RR 1.09 (95% CI 0.66 to 1.80) (two RCTs pooled) Alginate dressing vs. foam dressing (two RCTs): RR 0.67 (95% CI 0.41 to 1.08) (two RCTs pooled) Alginate dressing vs. silver hydrocolloid dressing (one RCT) RR 1.40 (95% CI 0.79 to 2.47) |
Limitations in study design and implementation (i.e. RoB): all RCTs were at high or unclear RoB Indirectness of evidence: most RCTs recruited participants with non-complex foot ulcers, limiting generalisability to populations with harder-to-heal foot ulcers Unexplained heterogeneity: none Imprecision of results: all RCTs had small sample sizes Publication bias: no specific evidence but cannot be discounted Overall quality of the evidence: low |
No evidence that alginate dressings promote healing of diabetic foot ulcers compared with alternative dressings (basic wound contact, foam and silver hydrocolloid) |
| Foam dressings for healing diabetic foot ulcers195 | 6 (157), April 2013 | Foam dressing vs. basic wound contact dressing (three RCTs): RR 2.03 (95% CI 0.91 to 4.55) (two RCTs pooled) Foam dressing vs. alginate dressing (two RCTs): RR 1.50 (95% CI 0.92 to 2.44) (two RCTs pooled) Foam dressing vs. hydrocolloid dressing (one RCT): RR 0.88 (95% CI 0.61 to 1.26) (one RCT) Follow-up times ranged from 8 to 16 weeks |
Limitations in study design and implementation (RoB): all RCTs were at unclear RoB Indirectness of evidence: most RCTs recruited participants with non-complex foot ulcers, limiting generalisability to populations with harder-to-heal foot ulcers Unexplained heterogeneity: none Imprecision of results: all RCTs had small sample sizes Publication bias: no specific evidence, but cannot be discounted Overall quality of the evidence: low |
No evidence that foam dressings promote healing of diabetic foot ulcers compared with alternative dressings (basic wound contact, alginate and hydrocolloid) |
| Hydrocolloid dressings for healing diabetic foot ulcers192 | 5 (535), April 2013 | Hydrocolloid dressing vs. basic wound contact dressing (two RCTs): RR 1.01 (95% CI 0.74 to 1.38) (two RCTs pooled) Hydrocolloid dressing vs. foam dressing (one RCT): RR 1.14 (95% CI 0.80 to 1.64) Silver hydrocolloid dressing vs. alginate dressing (one RCT): RR 1.40 (95% CI 0.79 to 2.47) Hydrocolloid dressing vs. iodine-impregnated dressing (one RCT): RR 1.00 (95% CI 0.74 to 1.34) Hydrocolloid dressing vs. herbal cream topical application (one RCT): no difference in median percentage change in ulcer area at 2 weeks |
Limitations in study design and implementation (RoB): one RCT low, others unclear Indirectness of evidence: most RCTs recruited participants with non-complex foot ulcers, limiting generalisability to populations with harder-to-heal foot ulcers Unexplained heterogeneity: none Imprecision of results: all comparisons based on small numbers Publication bias: no specific evidence, but cannot be discounted Overall quality of the evidence: moderate for hydrocolloid vs. basic wound contact and hydrocolloid vs. iodine-impregnated dressing; low for hydrocolloid vs. foam, silver hydrocolloid vs. alginate and hydrocolloid vs. herbal cream |
No evidence that hydrocolloid dressings promote healing of diabetic foot ulcers compared with alternative dressings (basic wound contact, foam, alginate and iodine-impregnated dressings and a herbal topical application) |
| Hydrogel dressings for healing diabetic foot ulcers193 | 5 (446), April 2013 | Hydrogel dressing vs. larval therapy (one RCT): RR 0.40 (95% CI 0.08 to 1.99) Hydrogel dressing vs. platelet-derived growth factor (one RCT): RR 0.81 (95% CI 0.50 to 1.32) Hydrogel dressing vs. basic wound contact dressing (three RCTs): RR 1.80 (95% CI 1.27 to 2.56) (three RCTs pooled) but high heterogeneity including in follow-up times Hydrogel dressing vs. alternative hydrogel dressing (one RCT): no meaningful data |
Limitations in study design and implementation (RoB): all RCTs unclear Indirectness of evidence: some RCTs recruited participants with non-complex foot ulcers, meaning that not all data could be generalised to populations with harder-to-heal foot ulcers Unexplained heterogeneity: none Imprecision of results: all comparisons were based on small numbers Publication bias: no specific evidence, but cannot be discounted Overall quality of the evidence: moderate for hydrogel vs. basic wound contact; low for hydrogel vs. larval therapy, hydrogel vs. platelet-derived growth factor and hydrogel vs. alternative hydrogel |
Hydrogel dressings may heal more wounds than basic wound contact dressings; however, this finding should be viewed with caution because of high heterogeneity. There was no evidence for a difference between hydrogel and larval therapy, between hydrogel and platelet-derived growth factor and between different brands of hydrogel |
| Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus244 | 5 (605), July 2013 | NPWT vs. moist (non-gauze) dressings (one RCT for post-amputation wounds): RR 1.44 (95% CI 1.03 to 2.01) NPWT vs. moist dressings (two RCTs for diabetic foot ulcers): RR 1.49 (95% CI 1.11 to 2.01) (one trial); second trial only n = 27 in three arms and very little information NPWT vs. gauze dressings (three RCTs for diabetic foot ulcers): RR 0.38 (95% CI 0.05 to 2.59) (one trial); median healing time 3.9 weeks for NPWT vs. 4.4 weeks for gauze, very little information (second trial); the third trial reported very little information |
Limitations in study design and implementation (RoB): all unclear Indirectness of evidence: no issues of concern Unexplained heterogeneity: none Imprecision of results: some comparisons were based on small numbers Publication bias: there is evidence of publication bias Overall quality of the evidence: low to very low |
NPWT may be an effective treatment for healing debrided foot ulcers and post-operative amputation wounds in people with diabetes mellitus. However, these studies are at RoB and the evidence was judged to be of low quality |
| Review | Number of RCTs (participants) included, search date | Results for healing | Quality of the evidence | Review authors’ conclusions |
|---|---|---|---|---|
| Alginate dressings for venous leg ulcers198 | 5 (295), November 2012 | Alginate dressing vs. alternative alginate dressing (one RCT): RR 6.00 (95% CI 0.32 to 111.04) Alginate dressing vs. hydrocolloid dressing (three RCTs): RR 0.42 (95% CI 0.14 to 1.21) (6 weeks, two RCTs pooled), RR 1.02 (95% CI 0.57 to 1.81) (12 weeks, one RCT) Alginate dressing vs. non-adherent dressing (one RCT): RR 1.08 (95% CI 0.86 to 1.36) |
Limitations in study design and implementation (RoB): two RCTs high, three RCTs unclear Indirectness of evidence: no issues of concern Unexplained heterogeneity: none observed but some comparisons based on a single RCT Imprecision of results: all comparisons underpowered Publication bias: no specific evidence but cannot be discounted Overall quality of the evidence: very low |
There is no evidence to suggest any difference in terms of wound healing between different alginate dressings or between alginate dressings and other dressing types (hydrocolloid and non-adherent dressings). Ease of dressing removal may be better for hydrocolloid dressings than for alginate dressings |
| Foam dressings for venous leg ulcers197 | 12 (1023), October 2012 | Hydrocellular foam dressing vs. polyurethane foam dressing (three RCTs): no treatment differences in individual trial results, which were not pooled Foam dressing vs. paraffin gauze dressing (two RCTs): RR 1.34 (95% CI 0.61 to 2.92) (one RCT); the other RCT was a pilot study with no report of complete healing and only limited data on change in wound area Foam dressing vs. hydrocapillary dressing (one RCT): RR 0.78 (95% CI 0.50 to 1.21) Foam dressing vs. hydrocolloid dressing (five RCTs): RR 1.00 (95% CI 0.81 to 1.22) (five RCTs pooled) Foam dressing vs. knitted viscose dressing (one RCT): RR 1.35 (95% CI 0.89 to 2.05) Foam dressing vs. protease-modulating matrix dressing (one RCT): insufficient data reported |
Limitations in study design and implementation (RoB): six RCTs high, six RCTs unclear Indirectness of evidence: no issues of concern Unexplained heterogeneity: none observed; some estimates were based on a single RCT Imprecision of results: most comparisons underpowered (low precision) Publication bias: possible, as two studies reported as abstracts are awaiting assessment Overall quality of the evidence: moderate for the comparison of foam vs. hydrocolloid for the outcome of complete healing; otherwise, low-quality evidence |
There is no evidence that foam dressings are better or worse than any other type of dressing for the management of venous leg ulcers |
| Compression for venous leg ulcers184 | 48 including nine new RCTs for this update (4321), May 2012 | Compression vs. no compression (eight RCTs): overall there is some evidence that venous ulcers heal more quickly with compression than without but these eight trials could not be pooled because of heterogeneity Single-component compression bandage vs. alternative single-component compression bandage (two RCTs): no differences in healing but data not pooled because of heterogeneity Single-component compression bandage vs. multicomponent compression bandage (five RCTs): overall evidence suggests multicomponent bandages associated with more healing but meta-analysis not possible because of heterogeneity Two-component compression bandage vs. alternative two-component compression bandage (two RCTs): some evidence that those with elastic component associated with more healing Two-component compression bandage vs. four-layer bandage (four RCTs): RR 0.83 (95% CI 0.66 to 1.05) (three RCTs pooled) Three-component compression bandage vs. alternative three-component bandage (four RCTs): RR 1.83 (95% CI 1.26 to 2.67) (two RCTs pooled) Four-layer bandage vs. alternative four-layer bandage (three RCTs): data not pooled because of heterogeneity Four-layer bandage vs. short-stretch bandage (six RCTs): HR 1.31 (95% CI 1.09 to 1.58) (IPD meta-analysis for five RCTs, 797 participants) Four-layer bandage vs. paste bandages (five RCTs): RR 1.34 (95% CI 0.78 to 2.28) (two RCTs pooled) Adjustable compression boots vs. compression bandages (two RCTs): individual trials suggest no difference but meta-analysis not possible Compression stockings vs. paste bandages (two RCTs): no differences in healing but meta-analysis not possible Compression stockings vs. short-stretch bandage (four RCTs): RR 1.62 (95% CI 1.26 to 2.10) (four RCTs pooled) Compression stockings vs. multicomponent bandages (one RCT): no difference in healing between stockings and two-component system or four-layer bandage (three-arm trial) Tubular compression vs. short-stretch bandage (one RCT): RR 0.98 (95% CI 0.76 to 1.26) Tubular compression vs. elastic bandages (two RCTs): when one or two elastic bandages were added to a base three-component system that included an outer tubular layer, healing outcomes were better for the two groups receiving elastic bandages: RR for two additional elastic bandages 0.42 (95% CI 0.26 to 0.68) (one RCT) |
Limitations in study design and implementation (RoB): one RCT low, 24 RCTs high, 23 RCTs unclear Indirectness of evidence: some RCTs excluded patients with larger ulcers; this applied to six of 11 RCTs evaluating stockings and tubular devices; details of some interventions were lacking (e.g. the exact components of Unna’s boot – a type of paste bandage), making it difficult to apply evidence directly Unexplained heterogeneity: none observed but maximum number of RCTs in a meta-analysis was five and many analyses were based on a single RCT Imprecision of results: most analyses were underpowered (exceptions were four-layer bandage vs. short-stretch bandage and stockings vs. short-stretch bandage) Publication bias: likely as one unpublished RCT known, three studies reported as abstracts awaiting assessment and three ongoing studies Overall quality of the evidence: moderate for four-layer bandage vs. short-stretch bandage; low for other comparisons |
Compression increases healing compared with no compression Multicomponent compression systems are more effective than single-component systems Multicomponent systems containing an elastic bandage appear more effective than those composed mainly of inelastic constituents Two-component bandage systems appear equivalent to the four-layer bandage in terms of healing Variations of the four-layer bandage achieve similar outcomes The four-layer bandage heals ulcers faster and is more cost-effective than multicomponent systems consisting of a short-stretch bandage No difference was found between four-layer bandage and paste bandages There was no difference between adjustable compression boots and compression bandage systems or between single-layer stockings and paste-bandages Two-layer stockings appear more effective than the short-stretch bandage The relative effectiveness of compression stockings and the four-layer bandage is unclear The relative effectiveness of tubular compression and compression bandages is unclear |
| Antibiotics and antiseptics for venous leg ulcers199 | 45 including 18 new RCTs for this update (4486), May 2013 | Systemic antibiotics vs. placebo, standard care, alternative antibiotics and topical antiseptics (five RCTs): more participants healed on levamisolea than on placebo: RR 1.31 (95% CI 1.06 to 1.62) (one RCT); no other differences in healing identified for other comparisons Topical preparations: Cadexomer iodine vs. standard care (seven RCTs): RR 2.17 (95% CI 1.30 to 3.60) (four RCTs pooled) Cadexomer iodine vs. hydrocolloid dressing, paraffin gauze dressing, dextranomer and silver-impregnated dressing (five RCTs): all single trial comparisons except for dextranomer (two trials); no differences in healing identified Povidone iodine vs. dextranomer, growth factor, hydrocolloid dressing, paraffin gauze dressing and moist or foam dressings (according to ulcer status) (six RCTs): generally no good evidence of differences in healing identified Benzoyl peroxide vs. saline dressings (two RCTs) and hydrogen peroxide vs. placebo (two RCTs): findings inconclusive as small studies (total of 72 participants) and healing outcomes heterogeneous Honey-based products vs. usual care (two RCTs): RR 1.15 (95% CI 0.96 to 1.38) Silver sulfadiazine cream vs. non-antimicrobial dressings and topical applications (three RCTs): no differences in healing identified Silver-impregnated dressing vs. alternative silver-impregnated dressing (one RCT): no difference in healing identified Silver-impregnated dressings vs. non-antimicrobial dressings (eight RCTs): RR 1.17 (95% CI 0.95 to 1.45) (four RCTs pooled) Chloramphenicol vs. enzymatic wound cleanser (one RCT): RR 0.13 (95% CI 0.02 to 0.99) in favour of enzymatic cleanser Framycetin sulphate vs. enzymatic wound cleanser (one RCT): no difference in healing identified Chloramphenicol vs. framycetin sulphate (one RCT): no difference in healing identified Mupirocin vs. vehicle (one RCT): no difference in healing identified Topical antibiotics according to antibiogram vs. herbal ointment (one RCT): no difference in healing identified Chlorhexidine vs. usual care (one RCT): no difference in healing identified Ethacridine lactate vs. placebo (one RCT): greater treatment ‘response’ with ethacridine than placebo: RR 1.45 (95% CI 1.21 to 1.73) |
Limitations in study design and implementation (RoB): three RCTs low, 13 RCTs high, 29 RCTs unclear Indirectness of evidence: few RCTs recruited patients with clinically infected ulcers at baseline, hindering interpretation in relation to the correct target population Unexplained heterogeneity: none observed but maximum number of RCTs in a meta-analysis was four and many analyses were based on a single RCT Imprecision of results: most analyses were underpowered Publication bias: no specific evidence, but cannot be discounted Overall quality of the evidence: moderate for silver-impregnated dressings vs. non-antimicrobial dressings and for honey vs. usual care; low for other comparisons |
The evidence does not suggest that routine use of systemic antibiotics promotes healing of venous leg ulcers. Levamisole was the only systemic agent for which data showed a benefit in terms of healing. In terms of topical preparations, there is some evidence that cadexomer iodine may increase healing. However, cadexomer iodine is associated with more frequent adverse effects than standard care. Current evidence does not suggest that honey- and silver-based preparations improve venous ulcer healing. Further good-quality research is required before definitive conclusions can be made about the effectiveness of topical preparations such as povidone iodine, peroxide-based preparations, chloramphenicol, framycetin sulphate, mupirocin, topical antibiotics given according to antibiogram, ethacridine lactate and chlorhexidine in healing venous leg ulceration |
| Topical agents or dressings for pain in venous leg ulcers245 | Eight including one new RCT for this update (813), May 2012 | EMLA cream vs. placebo or no anaesthetic used for debridement-related pain (six RCTs): the between-group difference in pain measured at debridement using a 100-mm scale was statistically significant in favour of EMLA (difference in means –20.65 mm, 95% CI –29.11 mm to –12.19 mm) (six RCTs pooled) Low-dose topical ibuprofen-containing dressing vs. local best practice and non-ibuprofen dressing for persisting venous leg ulcer pain (two RCTs): RR of > 50% maximum pain relief score days 1–5 vs. local best practice 1.63 (95% CI 1.24 to 2.15) (one trial); no difference in risk of slight to complete pain relief on first evening of treatment compared with identical dressing without ibuprofen in the second trial (limited data) |
Limitations in study design and implementation (RoB): the review authors did not present overall RoB ratings for each RCT. Allocation concealment was unclear for all RCTs. Blinded outcome assessment was low in six RCTs and high in the other two. Incomplete outcome data were low in seven RCTs and unclear in one Indirectness of evidence: no apparent concerns Unexplained heterogeneity: some heterogeneity was observed but this was explored and explained Imprecision of results: some analyses were underpowered Publication bias: one unpublished RCT included; cannot discount the possibility of additional unpublished studies Overall quality of the evidence: moderate to low |
There is some evidence that foam dressings containing ibuprofen provide pain relief for some people with painful venous leg ulcers. EMLA reduces pain during debridement of venous leg ulcers compared with placebo or no anaesthetic |
| Review | Number of RCTs (participants) included, search date | Results for SSI | Quality of the evidence | Review authors’ conclusions |
|---|---|---|---|---|
| Pre-operative skin antiseptics for preventing surgical wound infections after clean surgery246 | 13 RCTS (2632 participants), August 2012 | Iodine in alcohol vs. alcohol (one RCT): no evidence of a difference in SSI rates Iodophor vs. alcohol (one RCT): no evidence of a difference in SSI rates Iodophor vs. alternative iodophor (six RCTs): no evidence of a difference in SSI rates Iodophor vs. chlorhexidine (five RCTs): no evidence of a difference in SSI rates in four RCTs (all different comparisons). In one RCT comparing 0.5% chlorhexidine paint in methylated spirit vs. povidone iodine in alcohol paint, there was a reduction in SSI in the chlorhexidine group (RR 0.47, 95% CI 0.27 to 0.82) Chlorhexidine vs. alternative chlorhexidine (one RCT): no evidence of a difference in SSI rates between 0.75% chlorhexidine and 1.5% cetrimide scrub vs. 0.75% chlorhexidine and 1.5% cetrimide paint Alcoholic solutions vs. aqueous solutions (six RCTs): no evidence of a difference in SSIs when six studies pooled: RR 0.77 (95% CI 0.51 to 1.17). A mixed-treatment comparison meta-analysis concluded that alcohol-containing products had the highest probability of being effective; however, the quality of evidence was low |
Limitations in study design and implementation (RoB): summarised by the review authors as unclear for the review overall Indirectness of evidence: indirect comparison of interventions addressed with a mixed-treatment comparison meta-analysis; no apparent concerns regarding indirectness in relation to the population or nature of interventions Unexplained heterogeneity: none observed Imprecision of results: some pairwise analyses were underpowered Publication bias: one study awaiting assessment appears to be a conference abstract; no other specific evidence but cannot discount the possibility of additional unpublished studies Overall quality of the evidence: low |
A comprehensive review of current evidence found evidence from a single study that a preoperative skin preparation with 0.5% chlorhexidine solution in methylated spirits was more effective in preventing SSIs following clean surgery than alcohol-based povidone iodine paint. However, poor reporting of this trial makes this finding difficult to act on. Practitioners may therefore elect to consider other characteristics such as costs and potential side effects when choosing between alternatives |
Discussion
Eleven Cochrane reviews were undertaken or updated: five in diabetic foot ulcers, five in venous leg ulcers and one in surgical wounds. The number of included RCTs ranged from 5 to 48. The evidence suggested that hydrogel dressings may heal more diabetic foot ulcers than basic wound contact dressings193 and that negative pressure wound therapy may be an effective treatment for healing debrided diabetic foot ulcers and post-operative amputation foot wounds in people with diabetes mellitus. 244 The review of compression in venous leg ulcers184 suggested that compression increases healing compared with no compression; multicomponent compression systems are more effective than single-components systems; and multicomponent systems containing an elastic bandage are more effective than those composed mainly of inelastic constituents. The four-layer bandage heals ulcers faster and is more cost-effective than the short-stretch bandage. Two-layer stockings (high compression) appear more effective than the short-stretch bandage. In terms of systemic antimicrobial agents,199 there is some evidence that levamisole may be effective for healing venous leg ulcers; this treatment is normally used to treat roundworm infection and has restricted availability. In terms of topical antimicrobials for venous leg ulcers,199 cadexomer iodine may heal more wounds than standard care. There was no evidence for a difference between honey- and silver-based products compared with non-antimicrobial interventions. Foam dressings impregnated with ibuprofen may reduce pain for some people with painful venous leg ulcers and Eutectic Mixture of Local Anaesthetics (EMLA) cream reduces pain during debridement of venous leg ulcers compared with placebo or no anaesthetic. 245 A review evaluating pre-operative skin antiseptics for preventing surgical wound infection after clean surgery found that 0.5% chlorhexidine may be more effective than alcohol-based povidone iodine paint. 246 Most findings should be viewed with caution because of the poor quality of much of the evidence base. This particularly related to risk of bias and imprecision. It was difficult to conduct a full risk-of-bias assessment for many RCTs because of poor reporting. In addition, the frequent lack of information on reported outcomes meant that some data could not be converted to the reviews’ pre-specified measures of treatment effect (and therefore could not be pooled).
In some cases, the patient characteristics of those recruited to included trials hindered generalisation to real-life clinical practice. The four reviews focusing on dressings for diabetic foot ulcers noted that most included trials recruited patients with non-complex foot ulcers and, therefore, the current evidence may be of limited utility to health professionals when treating patients with harder-to-heal foot ulcers. The two reviews on dressings in venous leg ulcers noted that many trials restricted the baseline ulcer area as a trial entry criterion, so that the wound fitted the dimensions of the study dressings. It is likely that patients with a wide range of wound sizes, including larger wounds, would be seen in clinical practice and the relevance of the evidence is unclear for patients with larger ulcers and, therefore, a less favourable prospect of healing. 226 This also applied to some trials in the compression review. 184 A major issue in the review of antimicrobial agents was that most trials did not recruit patient with infected wounds at baseline and, therefore, we cannot know if the interventions reviewed can promote healing in patients with clinically infected wounds. In light of the current global concern about the misuse of antibiotics and resistance to antimicrobial treatment, clinical and prescribing guidelines suggest that the application of both systemic and topical antimicrobial agents should be restricted to patients with confirmed clinical infection and, therefore, the relevance of this evidence is in question. 94,238 In the review of topical agents and dressings for treating pain in venous leg ulcers, it was noted that the trials evaluating relief of persisting ulcer pain were of short duration (a matter of a few weeks), whereas the nature of the wounds was chronic; therefore, it is uncertain how effective pain relief may be in the longer term. 245
Applying different techniques of evidence synthesis
Earlier in this chapter we showed how health professionals expressed uncertainties about the effectiveness of different dressings for healing foot ulcers in people with diabetes and venous leg ulcers.
In wound care, decision-makers have several treatment options to choose from, typically involving the choice of alternative devices, for example negative pressure wound therapy and dressings. When selecting a dressing there are not only several classes to choose from but, within these classes, there are several dressing types (as well as competing products within these types). Table 64 summarises the dressing choices available to decision-makers in the UK using information from the British National Formulary (BNF). 238
| Class of dressing | Number of different products |
|---|---|
| Basic wound contact dressings | |
| Low-adherence dressings | |
| Knitted viscose primary dressing | 4 |
| Paraffin gauze dressing | Several – not all listed |
| Atrauman® (Hartmann, Heywood, UK) | 1 |
| Absorbent dressings | |
| Absorbent perforated dressing with adhesive border | 12 |
| Absorbent perforated plastic film-faced dressing | 8 |
| Absorbent cellulose dressing with fluid-repellent backing | 6 |
| Absorbent dressings for heavily exuding wounds | 4 |
| Advanced wound dressings | |
| Hydrogel dressings | |
| Hydrogel sheet dressing | 9 |
| Hydrogel application (amorphous) | 9 |
| Sodium hyaluronate dressing | 1 |
| Vapour-permeable films and membranes | |
| Vapour-permeable adhesive film dressing | 15 |
| Vapour-permeable adhesive film dressing with absorbent pad | 10 |
| Soft polymer dressings | |
| Without absorbent pad | 9 |
| With absorbent pad | 10 |
| Cellulose dressings | 2 |
| Hydrocolloid dressings | |
| Without adhesive border | 11 |
| With adhesive border | 5 |
| Hydrocolloid fibrous dressings | 3 |
| Polyurethane matrix dressing | 1 |
| Foam dressings | |
| Polyurethane foam film dressing with adhesive border | 16 |
| Polyurethane foam dressing | 3 |
| Polyurethane foam film dressing without adhesive border | 26 |
| Cavi-Care® (Smith & Nephew) | 1 |
| Alginate dressings | 12 |
| Capillary-action dressings | 4 |
| Odour-absorbent dressings | 6 |
| Antimicrobial dressings | |
| Honey | |
| Sheet dressing | 7 |
| Topical | 5 |
| Iodine | 5 |
| Silver | |
| Low-adherence dressings | 2 |
| With charcoal | 1 |
| Soft polymer dressings | 4 |
| Hydrocolloid dressings | 3 |
| Foam dressings | 5 |
| Alginate dressings | 9 |
| Other antimicrobials | 8 |
| Specialised dressings | |
| Protease-modulating matrix dressings | 7 |
| Silicone keloid dressings | |
| Silicone sheets | 8 |
| Silicone gels | 7 |
Dressings as a treatment for chronic wounds
Arguably, wound dressings are perceived as cheap and ‘inert’ items and thus their use in relation to existing evidence receives limited attention. However, as dressing types grow in number and complexity, and with corresponding claims of promoting healing, expenditure also increases. Of the 201 BNF chapters in 2010,253 the community prescribing cost of the ‘Wound management and other dressings’ chapter was £136M, making it the 17th most costly (the most costly chapter being ‘Drugs for diabetes’ at > £700M). There is also considerable variation in the cost of dressing types. For example, in 2013 a silver low-adherent dressing (10 cm × 10 cm) cost £8.07, whereas a similar-sized standard low-adherent dressing cost £0.35. 238
When selecting from so many competing treatment options (with some having large differences in unit costs), the evidence base is an important decision-making resource. However, a difficulty in the current evidence base for dressings in wound care (as with several other areas) is that most of the trials in this field are pairwise, that is, they compare one treatment with another in a head-to-head comparison. Looking again at Table 64 we can see that with so many different treatments types available, the number of trials required to produce relative effectiveness estimates for all possible comparisons would be huge. Previously, systematic reviews of the evidence for the effects of wound dressings on healing have focused on the available pairwise comparisons, showing that data on some head-to-head comparisons are lacking. 254 Although useful, such analysis can be of limited value to decision-makers who, when comparative trial data are not available for specific comparisons, are, essentially, required to make qualitative relative treatment estimates indirectly using the data available to them.
Mixed-treatment meta-analysis
An extension to standard meta-analysis of relative effectiveness evidence (healing data in this case) is mixed-treatment meta-analysis (sometimes called network meta-analysis or mixed-treatment comparison). 189,255 This approach formally links data from multiple relevant trials via common comparators to form a network of evidence. The network allows the direct comparisons of treatments for which pairwise data exist (as would normally happen in a standard meta-analysis) but also allows indirect comparisons to be made for treatments that have not been compared in a trial (Figure 14).
FIGURE 14.
Example of a simple evidence network. The solid lines show pairwise RCT data on the effectiveness of dressing A compared with dressing B and dressing A compared with dressing C. Although no RCT data for dressing B compared with dressing C are available, a relative effect estimate can be calculated indirectly using dressing A as the common comparator.
Mixed-treatment meta-analysis is a powerful evidence synthesis tool although, as with standard meta-analysis, assumptions are required when combining data in this way. A key assumption for mixed-treatment meta-analysis is that of transitivity, that is (for a fixed-effects model), all networked trials would be expected to estimate the same relative treatment effect for a treatment had that treatment been included in the trial. Another way of conceptualising this is that we are assuming that the network represents one big trial with the missing trial arms missing at random. 256 Essentially, this assumption relies on there being no important differences between trials (e.g. characteristics of patient populations or treatments) that would impact on relative treatment effects. In mixed-treatment meta-analysis there is a particular focus on assessing whether or not the assumption of transitivity holds through assessment of consistency between direct and indirect data when possible; for a full overview of this issue see Dias et al. 190
Assessing the quality of pooled estimates
With any evidence synthesis it is important to present the output data with due consideration and communication of the data quality used in its calculation. Although this is recognised with pooling of pairwise data through GRADE,252 there has been less focus on systematic approaches to support the assessment of the relative effect estimates for the outputs of mixed-treatment meta-analysis that include indirect as well as direct estimates. However, this is an important area for further research as presenting network findings without an assessment of quality (especially in a Bayesian framework in which the probability of a treatment being ‘the best’ may be the output) may encourage an overly simplistic interpretation of the evidence.
Within this work we therefore planned to conduct mixed-treatment meta-analysis to fully synthesise evidence from RCTs in areas of clinical decision uncertainty identified by health professionals within the programme grant. These uncertainties were in the selection of dressings for treating people with diabetic foot ulcers and venous leg ulcers. We planned to situate the estimates of dressing effectiveness in the context of the quality of the evidence.
Dressings to heal ulcers of the foot in people with diabetes
(Adapted from the previously published paper by Dumville et al. 196)
Objectives
Our objectives were to assess the relative effectiveness of dressings for the healing of foot ulcers in people with diabetes using a mixed-treatment comparison meta-analysis and to assess the quality of relative effective estimates produced in the mixed-treatment meta-analysis.
Methods
Study selection
This review was based on a pre-specified protocol. We included published or unpublished reports of RCTs, in any language and conducted in any country or setting, that evaluated the effects of wound dressings on the healing of foot ulcers in people with diabetes. We accepted study authors’ definitions of what constituted a diabetic foot ulcer and included trials that recruited patients with any type of diabetic foot ulcer. There was no restriction in relation to participant age.
The interventions of interest were dressings (including those impregnated with antimicrobials or moistened with saline). All forms of hydrogel were considered a dressing. Dressings that consist of a topical agent being spread or poured onto a proprietary dressing fabric were not included nor were other topical treatments. We included only RCTs that compared dressings with other dressings or dressings with no dressing. We did not include trials comparing dressings with topical treatments or other adjuvant therapies, for example:
-
growth factors
-
skin replacements
-
extracts of placenta or amniotic sac
-
herbal preparations
-
negative wound pressure therapy
-
systemic interventions (e.g. antibiotics, pentoxifylline)
-
surgical interventions.
We also excluded trials that compared different brands of the same dressing type.
Our primary outcome was ulcer healing, measured using the number of ulcers completely healed within a specific time period (we assumed this period to be the trial follow-up time unless otherwise stated).
Data sources and searches
The search string for CENTRAL (see Appendix 10) was adapted for use in the following databases, which were all searched from inception to June 2011: Cochrane Wounds Group Specialised Register, MEDLINE, EMBASE and CINAHL. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity- and precision-maximising version (2008 revision). 247 The EMBASE and CINAHL searches were combined with the trial filters developed by SIGN. 248 Reference lists of included studies and previous systematic reviews were also searched. We contacted appropriate manufacturing companies for details of any unpublished studies. Two review authors independently assessed the titles and abstracts of retrieved studies for relevance. After this initial assessment, we obtained in full all studies felt to be potentially relevant. We attempted to contact researchers to obtain any additional information required but not contained in the trial report.
Data extraction
Details of the eligible studies were extracted and summarised using a standardised data extraction sheet. Two review authors extracted data independently and disagreements were resolved by discussion. If data were missing from reports then attempts were made to contact the study authors to obtain further information. Studies published in duplicate were included once but a comprehensive data set was compiled from all publications.
Risk-of-bias assessment (individual studies)
Two review authors independently assessed each included RCT using the Cochrane Collaboration tool for assessing risk of bias. 219 This tool includes the following domains: sequence generation, allocation concealment, blinding of participants and care providers, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. In line with recently published Cochrane reviews on foam and alginate dressings,198,199 we classified RCTs as being at overall high risk of bias if they were rated as high for any one of three key domains (allocation concealment, blinding of outcome assessors and completeness of outcome data). If none of the key domains was classified as high risk but at least was unclear, the RCT was judged to be at overall unclear risk of bias. To have an overall rating of low risk of bias, all three key domains needed to be classified as low risk.
Relative treatment effects on ulcer healing: statistical analysis of direct data
When pairwise (direct) treatment comparisons were reported in one trial only, ORs and 95% CIs were calculated. When direct comparisons of dressings were available from more than one trial, appropriate standard meta-analyses (using ORs) were undertaken using WinBUGS (version 1.4.3) [Medical Research Council Biostatistics Unit, Cambridge, UK; see www.mrc-bsu.cam.ac.uk/bugs (accessed 13 May 2016)]. Results were reported with 95% credible intervals (CrIs), the Bayesian equivalent of CIs reflecting the uncertainty surrounding estimates. Unlike 95% CIs, 95% CrIs can be interpreted as follows: the (posterior) probability these limits contain the parameter mean is 95%. Fixed- and random-effects models were considered and model fit was assessed using the posterior mean of the residual deviance and the deviance information criterion (DIC).
Quality assessment of evidence generated using direct data
The overall quality of evidence surrounding estimates of effect using direct evidence only was assessed using GRADE. 252 GRADE assessment focuses not on individual studies but on a body of evidence and considers issues wider than threats to interval validity, including imprecision, inconsistency, indirectness and publication bias. Problems in any category lead to the quality of the evidence being decreased (we did not consider increasing quality of evidence options). In reflecting the quality of an estimate drawn from multiple sources in a systematic review, GRADE aims to help the reader consider how confident we are that an effect estimate is correct. Quality of evidence can be rated as high, moderate, low or very low.
Direct and indirect data: mixed-treatment meta-analysis
To maximise the use of all available trial data and to facilitate decision-making regarding dressing choice we conducted a mixed-treatment meta-analysis. 189,257 The mixed-treatment meta-analysis used the OR as the measure of effectiveness and was conducted from a Bayesian perspective, again using WinBUGS (version 1.4.3). Fixed- and random-effects models were fitted to these data with model fit assessed using residual deviance and DIC as before.
The treatment with the highest OR estimate in the mixed-treatment meta-analysis is expected to have the highest likelihood of healing diabetic foot ulcers. However, it is important to fully comprehend the uncertainty around such estimates. In addition to presenting CrIs, we represented uncertainty regarding treatment choice as the probability that each dressing was the ‘best’ treatment in terms of being the most likely to heal diabetic foot ulcers (when compared with all other evaluated treatments). To provide a complete overview of the spread of decision uncertainty around the choice of a ‘best’ treatment we then presented the probability of each treatment being the second best treatment and the third best and so on. Alternatively, this can be conceptualised for each treatment as a cumulative probability at each rank, summarised numerically as surface under the cumulative ranking (SUCRA) for each treatment. 258 Thus, a SUCRA would be 1 (or 100%) when a treatment was certain to be the best and 0 (0%) when a treatment was certain to be the worst.
Consistency of evidence
When direct and indirect evidence exist (i.e. a loop of evidence in the network diagram), inconsistencies between the ORs and intervals of these two sources may arise. We formally assessed for inconsistencies using the back calculation method,190 which is an extension to the method suggested by Bucher et al. 259 Briefly, when direct and indirect values could be compared, these values were calculated for each treatment, compared statistically against a null hypothesis that there would be no difference between them and a p-value for this test was presented. We also extended the analysis to include an inconsistency model, which omitted consistency equations. 260 Finally, potential inconsistencies between our direct and mixed-treatment meta-analysis estimates were also assessed by qualitatively comparing estimates of standard meta-analysis (direct) and mixed-treatment meta-analysis (direct and indirect).
Sensitivity analysis
We evaluated the sensitivity of the network to individual trials; when links were informed by more than one trial, we removed each trial one at a time (therefore, n – 1 for each analysis) and investigated the impact on the probability of which treatment was ‘best’.
Quality assessment of evidence generated from the mixed-treatment meta-analysis
We were also keen to reflect the quality of the evidence provided by the mixed-treatment meta-analysis so that this quality was transparently reflected in the strength of the conclusions made, as would be expected in other forms of evidence synthesis; however, there is no established method for doing this in mixed-treatment meta-analysis. We therefore modified the GRADE approach (we called this ‘iGRADE’) to allow us to access and communicate the quality of this mixed-treatment meta-analysis-derived evidence. We worked with the five categories in GRADE that allow the quality of evidence to be decreased; however, we altered the focus of some categories so that they were relevant for assessing a mixed-treatment meta-analysis (see Appendix 11 for a full description of the iGRADE tool). Briefly, the approach assessed risk of bias for links observed as informing specific mixed-treatment comparison estimates. We explored heterogeneity using a sensitivity analysis approach; indirectness was assessed by looking at the type of direct and indirect data informing estimates; precision was assessed using the size of intervals around estimates; and publication bias was assessed by looking at the studies used to inform relevant links in the mixed-treatment comparison. Estimates could be classed at being of high, medium, low or very low quality. No formal down-weighting of evidence was undertaken based on this assessment.
Results
Study characteristics
Fifteen eligible studies were included. 261–275 A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart is presented in Appendix 12 and a summary of study characteristics for the included studies is presented in Table 65. All 15 included studies reported the number of ulcers healed, whereas only three262,264,266 reported median time to healing. We therefore focused our analyses on the proportion of ulcers healed (or risk of healing). In terms of ulcer severity, four studies263,268,269,273 reported the inclusion of people with Wagner grade 1 or 2 ulcers and one study264 specified that ulcers were superficial. A further three studies specified that people with ulcers involving tendons, joint spaces and/or bone were excluded. 261,267,268 Only one study275 specifically included people with more severe grade 3 and 4 ulcers. Eight studies262,264,265,267,269,271–273 clearly excluded people with arterial disease. Eight studies262,264–268,271,273 excluded participants with infected or sloughy ulcers. Only one study,274 comparing a basic wound contact dressing with a hydrogel dressing, clearly specified the inclusion of people with necrotic and infected wounds. The evidence base therefore overwhelmingly relates to people with less severe and less complex diabetic foot ulcers.
| Study | Treatment | Follow-up (weeks) | n | Durationa (weeks) | Sizea (cm2) | Agea (years) | Number healed | Risk of biasb |
|---|---|---|---|---|---|---|---|---|
| Ahroni 1993261 | Basic wound contact | 4 | 19 | 10.7 | 1.68 | 65.4 | 7 | U, U, H |
| Alginate | 4 | 20 | 19.0 | 1.93 | 61.2 | 5 | ||
| Donaghue 1998265 | Basic wound contact | 8 | 25 | 32.1 | 2.99 | 60 | 9 | U, U, H |
| Alginate | 8 | 50 | 20.9 | 2.60 | 59 | 24 | ||
| Blackman 1994263 | Basic wound contact | 8 | 7 | 28 | 1.81 | 51 | 0.5a | U, U, U |
| Alginate | 8 | 11 | 25 | 2.67 | 59 | 3.5a | ||
| Mazzone 1993270 | Basic wound contact | 8 | 8 | NA | NA | NA | 2 | U, U, U |
| Foam | 8 | 11 | NA | NA | NA | 7 | ||
| Roberts 2001272 | Basic wound contact | 13 | 16 | NA | NA | NA | 4 | U, U, U |
| Foam | 13 | 14 | NA | NA | NA | 6 | ||
| Jeffcoate 2009267 | Basic wound contact | 24 | 106 | NA | NA | 61.9 | 41 | L, L, L |
| Fibrous hydrocolloid | 24 | 103 | NA | NA | 58.8 | 46 | ||
| Iodine-impregnated dressing | 24 | 108 | NA | NA | 59.5 | 48 | ||
| Piaggesi 2001271 | Basic wound contact | 350 | 10 | 5.9 | NA | 61.3 | 10 | L, U, U |
| Fibrous hydrocolloid | 350 | 10 | 6.8 | NA | 63.1 | 9 | ||
| Jensen 1998268 | Basic wound contact | 16 | 17 | NA | NA | NA | 6 | U, U, U |
| Hydrogel | 16 | 14 | NA | NA | NA | 11 | ||
| Vandeputte 1996274 | Basic wound contact | 12 | 14 | NA | NA | 65.3 | 7 | U, U, H |
| Hydrogel | 12 | 15 | NA | NA | 62.6 | 14 | ||
| D’Hemecourt 1998275 | Basic wound contact | 20 | 68 | 42 | 3.5 | NA | 15 | U, U, L |
| Hydrogel | 20 | 70 | 52.8 | 3.2 | N | 25 | ||
| Veves 2002273 | Basic wound contact | 12 | 138 | NA | 3.1 | 59 | 39 | U, U, U |
| Protease-modulating matrix | 12 | 138 | NA | 2.5 | 58 | 51 | ||
| Baker 1994262 | Alginate | 12 | 10 | 26.3 | 0.82 | 54.1 | 4 | L, U, U |
| Foam | 12 | 10 | 19.8 | 0.89 | 58.9 | 9 | ||
| Foster 1995266 | Alginate | 8 | 15 | 24.3 | 0.79 | 70 | 8 | U, U, U |
| Foam | 8 | 15 | 15.3 | 0.88 | 61 | 9 | ||
| Jude 2007269 | Alginate | 8 | 67 | 62.4 | 3.1 | 58.9 | 15 | L, U, U |
| Silver fibrous hydrocolloid | 8 | 67 | 72.8 | 4.2 | 61.1 | 21 | ||
| Clever 1996264 | Foam | 16 | 20 | 23.6 | 2.78 | 53.2 | 14 | U, U, H |
| Hydrocolloid matrix | 16 | 20 | 23.2 | 2.51 | 58.9 | 16 |
In terms of risk of bias, four included studies261,264,265,274 were deemed to be at high risk of bias. Only one study267 was deemed to be at low risk of bias. The remaining 10 studies were rated as being at unclear risk of bias for one or more key domains (see Table 65).
Relative treatment effects on ulcer healing: direct data
A summary of the network of dressing trials that measured healing in participants with diabetic foot ulcers is provided in Figure 15. Ten direct treatment comparisons were made in the 15 included trials; only five comparisons were informed by more than one trial for which standard meta-analysis could be conducted (all fixed effect). The overall quality of evidence for each direct link was assessed using the GRADE quality of evidence scale (Table 66): four links were formed by low-quality evidence and six by moderate-quality evidence. It is important to note that three of these four links formed by low-quality evidence were informed by the same three-arm trial assessed as being at low risk of bias. 267
FIGURE 15.
A network summary of all comparisons informed by direct trial data comparing wound dressings for diabetic foot ulcer healing. All linked by one trial unless otherwise stated. One three-arm trial was included that randomised to hydrocolloid (fibrous) dressing, iodine-impregnated dressing and basic wound contact dressing. ALG, alginate; BWC, basic wound contact; HYDRO, hydrocolloid; HYDRO(F), fibrous hydrocolloid; HYDRO(M), hydrocolloid matrix; HYDRO(SF), silver fibrous hydrocolloid; II, iodine impregnated; PMM, protease-modulating matrix.
| Dressing type | Reference treatment, OR (95% CrI or 95% CI),a,b risk of biasc | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BWC | ALG | Foam | HYDRO(F) | II | Hydrogel | PMM | HYDRO(SF) | HYDRO(M) | ||||||||||
| D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | |
| ALG | 1.26 (0.55 to 2.46),d L | 1.29 (0.57 to 2.51), L | ||||||||||||||||
| Foam | 4.01 (1.07 to 10.7),d,e L | 4.32 (1.56 to 9.85),e VL | 2.94 (0.71 to 8.33),d L | 3.61 (1.30 to 8.30),e VL | ||||||||||||||
| HYDRO(F) | 1.28 (0.71 to 2.14),d Mf | 1.28 (0.72 to 2.13), M | – | 1.15 (0.41 to 2.57), L | – | 0.37 (0.11 to 0.93),e M | ||||||||||||
| II | 1.27 (0.74 to 2.19), Mf | 1.28 (0.71 to 2.12), M | – | 1.16 (0.42 to 2.60), L | – | 0.37 (0.11 to 0.93),e M | 0.99 (0.58 to 1.71), Mf | 1.05 (0.59 to 1.75), M | ||||||||||
| Hydrogel | 3.10 (1.51 to 5.50),d,e L | 3.33 (1.65 to 6.11),e VL | – | 2.99 (0.98 to 7.12), VL | – | 0.96 (0.26 to 2.53), VL | – | 2.81 (1.10 to 6.00),e VL | – | 2.79 (1.09 to 6.00),e VL | ||||||||
| PMM | 1.49 (0.90 to 2.47), M | 1.54 (0.89 to 2.47), M | – | 1.38 (0.51 to 3.05), VL | – | 0.45 (0.13 to 1.10), M | – | 1.30 (0.57 to 2.57), M | – | 1.29 (0.57 to 2.53), M | – | 0.52 (0.20 to 1.08), L | ||||||
| HYDRO(SF) | – | 2.22 (0.65 to 5.60), VL | 1.58 (0.73 to 3.43), M | 1.73 (0.73 to 3.53), M | – | 0.60 (0.15 to 1.66), M | – | 1.88 (0.46 to 5.27), L | – | 1.86 (0.46 to 5.22), L | – | 0.75 (0.17 to 2.16), L | – | 1.55 (0.39 to 4.31), L | ||||
| HYDRO(M) | – | 10.38 (1.19 to 42.1),e VL | – | 8.66 (1.02 to 34.71),e VL | 1.71 (0.40 to 7.34), L | 2.40 (0.40 to 8.40), VL | – | 8.81 (0.88 to 37.8), VL | – | 8.72 (0.87 to 37.3), VL | – | 3.47 (0.33 to 14.7), VL | – | 7.24 (0.75 to 30.5), VL | – | 5.88 (0.53 to 26.2), VL | ||
There was evidence that hydrogel dressings were associated with significantly higher odds of ulcer healing than basic wound contact dressings (OR 3.10, 95% CI 1.51 to 5.50) (see Table 66). However, this finding was driven by low-quality evidence, that is, two small studies (sample sizes of 31 and 29 participants), one with an unclear risk of bias and one at high risk of bias. 268,274 Foam dressings were also associated with higher odds of ulcer healing than basic wound contact dressings (OR 4.01, 95 CI% 1.07 to 10.7) (see Table 66); however, again, the estimate was considered to be of low quality. In the remaining five single-study comparisons there was no evidence of any difference between one dressing and another. In general, estimates had large uncertainty because of small sample sizes.
Mixed-treatment meta-analysis
Based on assessment of fit, a fixed-effects model was employed. There was a minimal difference in mean residual deviances and DIC between the different models tested (fixed effect and random effects, the latter accounting for correlation within the three-armed trial); thus, the least complex model, given the limited data available for analysis, was applied.
There was a high degree of uncertainty in the many links in the network, especially those that were not informed by direct data (see Table 66). Evidence remained that both foam and hydrogel dressings were expected to be associated with higher odds of ulcer healing than basic wound contact dressings, although uncertainty was high (see Table 66). Foam and hydrogel dressings were estimated to be more effective than fibrous hydrocolloid and iodine-impregnated dressings; these results were driven by the more certain finding from a large, three-arm trial that there was no difference in ulcer healing between fibrous hydrocolloid dressings and basic wound contact dressings and iodine-impregnated dressings and basic wound contact dressings. In this situation we must consider the quality of the evidence provided in these analyses (results of the iGRADE scale are presented in Table 66). In general, the network included several small studies leading to high imprecision; additionally, estimates were informed by studies with a high risk of bias. We stress that the research used for deriving the estimates for fibrous hydrocolloid and iodine-impregnated dressings was regarded as higher quality, whereas evidence on hydrogel and foam dressings was regarded as being of more limited quality (see Table 66). 263,268,270,272,274,275
A valuable feature of Bayesian methods is the ability to illustrate the impact of uncertainty on decision-making by assessing the probability that each dressing treatment included in the network is the best in terms of ulcer healing. Notably, the treatment associated with the greatest probability of healing was hydrocolloid matrix (70%; Table 67). This result reflects the high relative effect estimates generated by the mixed-treatment meta-analysis from available indirect evidence (OR 10.38, 95 CrI 1.19 to 42.1; see Table 66); hydrocolloid matrix had a higher odds of healing than foam and foam had a higher odds of healing than basic wound contact. Again, when interpreting the evidence its quality must also be considered; these results are drawn from low-quality evidence and this limits the confidence that we have in the strength of the conclusions that can be drawn from them. Estimates for the three dressings with the highest probability of being the best (hydrocolloid matrix, foam and hydrogel) were informed by low-quality evidence, whereas estimates for some dressings with a 0% probability of being the best were informed by moderate-quality evidence. SUCRA estimates reflect these findings considered cumulatively across the ranks 1–9: hydrocolloid matrix dressings had a SUCRA of 92%, foam dressings 83% and hydrogel 78%, whereas basic wound contact dressings had a SUCRA of 11% (see Table 67) (a SUCRA of 100% means that a treatment is certain to be the best and a SUCRA of 0% means that a treatment is certain to be the worse).
| Dressing | Probability of being the best treatment choice in terms of healing (%) | SUCRA value (%) |
|---|---|---|
| Matrix hydrocolloid | 70 | 92 |
| Foam | 14 | 83 |
| Hydrogel | 14 | 78 |
| Silver fibrous hydrocolloid | 2 | 55 |
| Protease modulating | 0 | 43 |
| Impregnated iodine | 0 | 30 |
| Fibrous hydrocolloid | 0 | 30 |
| Alginate | 0 | 27 |
| Basic wound contact | 0 | 11 |
Consistency of evidence
There was one data loop in which both direct and indirect data informed relative treatment effectiveness estimates and the possibility of inconsistency was investigated. Although there was no evidence of statistically significant discrepancies between the direct data and the indirect data, given the uncertainty in the data only very large differences were likely to result in statistical significance. However, the inconsistency model returned DIC estimates that were very similar to those in the base-case model. Assessing the data qualitatively we noted that only one link, that between basic wound contact dressings and foam dressings, had an indirect point estimate that differed considerably from the direct data; this was likely driven by the high and uncertain estimates in two of the three studies that contributed direct evidence. For the basic wound contact–alginate link, although the direct and indirect estimates were close, the indirect estimate was much more uncertain. Interestingly, this link was the only one to have conflicting direct evidence. The direct and indirect estimates for the alginate versus foam comparison were close, with the indirect link having slightly more uncertainty. The direct evidence in this link had very differently sized estimates, although this might be explained by the large uncertainty in one study, which had only 20 participants.
Sensitivity analysis
The sensitivity of the network to specific studies was also investigated. In total, 11 analyses with 14 included studies (rather than the total 15 studies) were performed and the probability of each dressing being the best was assessed. Basic wound contact, alginate, fibrous hydrocolloid, iodine-impregnated and silver fibrous hydrocolloid dressings continued to have a very low or zero probability of being the ‘best’ treatment in any sensitivity analysis. Hydrocolloid matrix remained the most likely ‘best’ treatment in 10 of the 11 analyses (probability of being ‘best’ ranging from 62% to 75%) (Table 68). The exception was when the largest study comparing hydrogel with a basic wound contact dressing was removed. 275 This resulted in the direct odds of healing with hydrogel (and the uncertainty around this estimate) increasing dramatically as the two remaining small trials both significantly favoured hydrogel, with hydrogel having the highest probability of healing (62% vs. 35% for hydrocolloid matrix).
| Dressing | Probability of being the best treatment choice in terms of healing (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahroni 1993261 removed | Donaghue 1998265 removed | Blackman 1994263 removed | Mazzone 1993270 removed | Roberts 2001272 removed | Piaggesi 2001271 removed | D’Hemecourt 1998275 removed | Vandeputte 1997274 removed | Jensen 1998268 removed | Baker 1993262 removed | Foster 1994266 removed | |
| Matrix hydrocolloid | 71 | 66 | 67 | 67 | 72 | 70 | 35 | 73 | 72 | 63 | 75 |
| Hydrogel | 9 | 21 | 17 | 18 | 10 | 14 | 62 | 8 | 9 | 21 | 6 |
| Foam | 14 | 11 | 12 | 12 | 16 | 13 | 2 | 14 | 15 | 9 | 18 |
| Silver fibrous hydrocolloid | 6 | 2 | 3 | 3 | 2 | 3 | 1 | 3 | 3 | 7 | 1 |
| Basic wound contact layer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alginate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fibrous hydrocolloid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Impregnated-iodine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Protease modulating | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Dressings to heal venous leg ulcers
Objectives
Our objectives were to assess the relative effectiveness of dressings for healing venous leg ulcers using a mixed-treatment meta-analysis and to assess the quality of relative effective estimates produced in the mixed-treatment meta-analysis.
Methods
Study selection
We included published and unpublished RCTs (i.e. must be described as randomised in the report) reported in any language and conducted in any country and setting. RCTs recruiting participants with venous leg ulcers were included, with venous leg ulcers defined as an inclusion criterion of ABPI > 0.8 and studies having a clear focus on populations with venous leg ulcers only. When the ABPI was not reported as an inclusion criterion, studies that clearly considered the included wounds to be only venous leg ulcers were also included.
The interventions of interest were dressings (including those impregnated with antimicrobials or moistened with saline). Trials of dressings that consisted of a topical agent being spread or poured onto a proprietary dressing fabric were excluded as were trials of topical treatments per se. We included only RCTs that compared dressings with other dressings or dressings with no dressing. We did not include trials comparing dressings with topical treatments or other adjuvant therapies, for example:
-
growth factors
-
skin replacements
-
extracts of placenta or amniotic sac
-
herbal preparations
-
negative wound pressure therapy
-
systemic interventions (e.g. antibiotics, pentoxifylline)
-
surgical interventions.
We also excluded trials that compared different brands of the same dressing type.
We excluded RCTs in which the dressing comparison was not the only systematic difference between treatment groups. This decision rule has been used in other recent systematic reviews of wound management. 197,198 As a result of this rule, we included only RCTs in which compression therapy was deemed to have been standardised across trial arms. Compression therapies of any level of pressure were included, that is, compression did not have to be high-level compression for studies to be included.
Our primary outcome was ulcer healing, measured using number of ulcers completely healed within a specific time period (we assumed this period to be the trial follow-up period unless otherwise stated).
Data sources and searches
The search string for CENTRAL (see Appendix 13) was adapted for use in the following databases, all being searched from inception to 31 May 2013: Cochrane Wounds Group Specialised Register, MEDLINE, EMBASE and CINAHL. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity- and precision-maximising version (2008 revision). 247 The EMBASE and CINAHL searches were combined with the trial filters developed by SIGN. 248 Reference lists of included studies and previous systematic reviews were also searched. Two review authors independently assessed the titles and abstracts of retrieved studies for relevance. After this initial assessment, we obtained all studies felt to be potentially relevant in full. These were then screened as before.
Data extraction
This was undertaken following the same methodology as in the previous section (dressings for healing foot ulcers in people with diabetes).
Risk-of-bias assessment (individual studies)
This was undertaken following the same methodology as in the previous section (dressings for healing foot ulcers in people with diabetes).
Relative treatment effects on ulcer healing: statistical analysis of direct data
Where direct comparisons of dressings were available from two or more trials, appropriate pairwise meta-analyses were undertaken using WinBUGS (version 1.4.3).
All evidence synthesis was conducted using an extended approach that allowed time of study follow-up to be taken into account in the analysis even though healing data were reported (and extracted) as proportion of ulcers healed. 276
This methodology utilises a binomial likelihood to estimate the probability of participants being healed. The approach then assumes that the probability of healing can be related (algebraically) to a distribution of time to healing, allowing proportion healed to be defined as time to ulcer healing (see Soares et al. 276 for a more detailed description of the method). Thus, this approach reports the HR as a measure of relative effectiveness. This synthesis model used the uniparametric exponential distribution, which imposes a constant healing hazard over time. For the direct comparisons informed by a single trial, HRs and 95% CrIs were estimated using the same approach.
Results were reported with 95% CrIs, the Bayesian equivalent of CIs, reflecting the uncertainty surrounding estimates. Unlike 95% CIs, 95% CrIs can be interpreted as follows: the (posterior) probability these limits contain the parameter mean is 95%. All direct meta-analyses were fixed-effects models.
Quality assessment of evidence generated using direct data
The overall quality of evidence surrounding estimates of effect using direct evidence only was assessed using GRADE. 252 The quality of evidence was rated as high, moderate, low or very low.
Direct and indirect data: mixed-treatment meta-analysis
Fixed- and random-effects models were considered and model fit assessed using the posterior mean of the residual deviance and the DIC.
As for the standard meta-analysis of direct data, the mixed-treatment meta-analysis used the modified approach in which time of study follow-up was accounted for and findings are presented as HRs. We compared the findings of this modified method against the findings of a more standard mixed-treatment meta-analysis approach (reporting the odds of healing).
An important feature of the Bayesian framework employed here is the ability to assess the probability that each treatment is the best (considering its HR), reflecting the impact of uncertainty on the relative treatment effects, and this is important in informing recommendations on which treatment to use.
Quality assessment of evidence generated from the mixed-treatment meta-analysis
The quality of the data included in any synthesis model is key in determining the validity of the results and of inferences made. The iGRADE tool196 was used to assess the overall quality of evidence surrounding estimates of effect from the mixed-treatment meta-analysis (see Appendix 11).
Contributions of direct evidence in the network
The contributions of direct estimates to each of the estimates in a network of evidence are not all the same. Rather, contribution is a function of an estimate’s statistical precision (which is an indication of the amount of information available) and of its relative position in the network. A recently published tool allows the contribution of each direct estimate to each overall mixed-treatment pairwise effect size to be determined, as well as allowing the contribution of each direct estimate to the network as a whole to be assessed. 258,277 Estimating the contributions is only relevant for links in a network informed by mixed evidence (direct and indirect) or when multiple loops of indirect evidence inform the same link. Estimated contributions may be interpreted as weights, providing percentage contributions of both direct and indirect estimates to particular network estimates. When possible we applied these methods to the evidence loops in our network. We acknowledge that this approach is based on a frequentist rather than a Bayesian approach and thus returns approximate weights.
Consistency of evidence
We formally evaluated statistical inconsistency by estimating the inconsistency factor, that is, the absolute difference between the direct and the indirect estimates on the log scale (or the logarithm of the ratio of the two ORs/HRs) for each of the comparisons in a loop. 278 A statistically low-powered z-test and a 95% CI of the inconsistency were computed.
Results
Data were obtained from 22 RCTs (see PRISMA diagram in Appendix 14). In compiling these studies into a network of evidence three studies279–281 were excluded as the treatments evaluated in them did not connect to the main network. Thus, the final data set included 19 RCTs evaluating 39 unique treatments (Table 69). 227,231,282–298 The evidence in the final data set was organised in the network, shown in Figure 16.
| Study | Treatment | Follow-up (weeks) | n | Durationa (weeks) | Sizea (cm2) | Agea (years) | Number healed | Risk of biasb |
|---|---|---|---|---|---|---|---|---|
| Backhouse 1987282 | Basic wound contact | 12 | 28 | NA | 3.1 | 67.5 | 22 | U, U, U |
| Hydrocolloid | 12 | 28 | NA | 3.4 | 69.9 | 21 | ||
| Blair 1988283 | Basic wound contact | 12 | 30 | 84 | 3.1 | 67.5 | 23 | L, U, U |
| Hydrocolloid | 12 | 30 | 88 | 3.4 | 69.9 | 22 | ||
| Nelson 2007284 | Basic wound contact | 24 | 118 | 59.2 | 9.1 | 69.7 | 69 | L, L, U |
| Hydrocolloid | 24 | 127 | 45.2 | 7.9 | 70.3 | 72 | ||
| Moffatt 1992285 | Basic wound contact | 12 | 30 | NA | NA | NA | 7 | L, U, U |
| Hydrocolloid | 12 | 30 | NA | NA | NA | 13 | ||
| Callam 1992286 | Basic wound contact | 12 | 66 | 44.8 | 8.4 | 63 | 23 | U, U, U |
| Foam | 12 | 66 | 46.8 | 10.9 | 64 | 31 | ||
| Michaels 2009231 | Basic wound contact | 12 | 106 | NA | NA | 72.4 | 59 | L, L, L |
| Silver-donating dressing | 12 | 107 | NA | NA | 68.8 | 62 | ||
| Moffatt 1992287 | Basic wound contact | 12 | 30 | NA | NA | NA | 24 | U, U, U |
| Alginate | 12 | 30 | NA | NA | NA | 26 | ||
| Bowszyc 1995288 | Hydrocolloid | 16 | 41 | 36.1 | 3.5 | 55 | 24 | U, U, U |
| Foam | 16 | 41 | 26.2 | 4.0 | 64.2 | 24 | ||
| Charles 2002289 | Hydrocolloid | 12 | 60 | 99.4 | 9.8 | 72 | 34 | L, U, U |
| Foam | 12 | 31 | 137 | 8.8 | 71 | 18 | ||
| Thomas 1997290 | Hydrocolloid | 13 | 50 | NA | 3.4 | 75.3 | 19 | U, U, U |
| Foam | 13 | 50 | NA | 4.3 | 73.4 | 17 | ||
| Vanscheidt 2004291 | Hydrocolloid | 12 | 55 | 171.6 | 11.7 | 62.8 | 20 | U, U, U |
| Foam | 12 | 52 | 67.6 | 10.9 | 68.4 | 20 | ||
| Zuccarelli 1992292 | Hydrocolloid | 12 | 19 | 37.4 | 6.9 | 77.3 | 9 | U, U, U |
| Foam | 12 | 19 | 48.9 | 9.8 | 70.1 | 9 | ||
| Arnold 1994293 | Hydrocolloid | 10 | 35 | 46.2 | 21.0 | 65 | 11 | U, U, U |
| Paraffin-impregnated gauze or betadine/saline-impregnated gauze | 10 | 35 | 47.8 | 19.8 | 60 | 14 | ||
| Dimakakos 2009227 | Foam | 9 | 21 | NA | NA | 61.2 | 10 | U, U, U |
| Silver foam | 9 | 21 | NA | NA | 58.7 | 17 | ||
| Mulder 1995294 | Foam | 16 | 19 | NA | NA | NA | 17 | U, U, U |
| Alginate | 16 | 20 | NA | NA | NA | 10 | ||
| Vin 2002295 | Paraffin gauze dressing | 12 | 36 | 39.6 | 9.5 | 71.7 | 12 | U, U, U |
| Protease-modulating matrix | 12 | 37 | 34 | 7 | 74.1 | 18 | ||
| Taddeucci 2004296 | Paraffin gauze dressing | 8 | 12 | NA | NA | NA | 1 | U, U, U |
| Hyaluronan fleece | 8 | 12 | NA | NA | NA | 2 | ||
| Schmutz 2008297 | Protease-modulating matrix | 12 | 60 | 48.4 | 10.4 | 71 | 8 | U, U, U |
| Nano-oligosaccahride technology lipido-colloid | 12 | 57 | 41.6 | 11.4 | 71.5 | 10 | ||
| Hansson 1998298 | Hydrocolloid | 12 | 48 | NA | 10.7 | NA | 5 | U, U, U |
| Cadexomer iodine | 12 | 56 | NA | 20.6 | NA | 8 | ||
| Paraffin gauze dressing | 12 | 49 | NA | 8.8 | NA | 7 |
This network encompassed 10 non-silver treatments and two trial arms of silver-containing dressings (various ‘silver-donating’ dressings and silver foam).
Within the network, the most populated comparisons were foam compared with hydrocolloid (five RCTs) and hydrocolloid compared with basic wound contact (four RCTs). The remaining 13 links were informed by summary estimates extracted from one trial in each case, except for the comparison (loop) between hydrocolloid, paraffin gauze dressing and cadexomer iodine, which was informed by information from a single, three-arm trial.
Statistical analysis
The analysis evaluated the network in Figure 16. There were two trials evaluating silver-containing dressings. One of these evaluated a treatment arm in which health professionals could select from a wide range of types of silver dressing. For this reason, we could not assess the effectiveness of individual silver-based treatments. Also, we did not feel that it would be interpretable if we combined these treatment arms as if they were homogeneous. The decision was made to model the treatments jointly as an incremental effect of silver. By incremental we mean that the effect of silver was estimated over and above any potential relative effects of dressing type when compared with basic wound contact dressings. For example, a silver foam dressing may be estimated to be more (or less) effective than a non-silver foam dressing, which may, in turn, still be considered more (or less) effective than basic wound contact dressings. Given the limited data available this effect was also assumed to be common across treatments; thus, the HR of silver was the same for all dressing types. This means that the incremental effect of silver for foam (i.e. silver foam vs. foam) is equivalent to the incremental effect of silver for alginates (silver alginate vs. alginate). This approach was considered to be conservative as it uses any effect of silver but also any effect of the dressing type (non-silver) over that of a basic wound contact dressing to contribute to the estimate.
FIGURE 16.
Network of RCTs of dressings for venous leg ulcers. In the network, a unique treatment category is indicated by an ellipse. Arrows between ellipses indicate that these treatments were compared in a trial. The number of trials is one unless otherwise stated. ALG, alginate; BWC, basic wound contact; CADEX, cadexomer iodine; HYALU, hyaluronan fleece; N-OLIG, nano-oligosaccahride technology lipido-colloid; PAR, paraffin gauze; PAR-BET, paraffin-impregnated gauze or betadine/saline-impregnated gauze; PMM, protease-modulating matrix; SILVER DD, silver donating.

Relative effectiveness results with quality assessment
For the mixed-treatment meta-analysis the fixed- and random-effects models provided very similar results. The between-study variation was estimated to be 0.05 (95% CrI 0.0 to 0.39). The DIC statistic was marginally lower for the fixed-effects than for the random-effects model (DICs of 221.6 and 222.5, respectively), indicating that the former model, being less complex, may be a more parsimonious representation of the data. Fixed-effect models were used throughout.
We also compared the modified approach that we adopted with the standard method used to model dichotomous (healed yes/no) data, that is, without taking time into account. From the results (see Appendix 15) we deemed the modified approach to be the most appropriate. Thus, the proportion healed was defined as time to ulcer healing and reported using the HR alongside the 95% CrI.
Table 70 shows the HR estimates for the analyses of direct and mixed data. Estimates are for each alternative non-silver dressing compared with basic wound contact and are presented alongside the results of quality assessment. Because of the large volume of information from small single trials there was high imprecision around point estimates for the majority of the relative treatment effects derived from the network meta-analysis. This was particularly notable in the treatment with the highest point estimate, that is, the treatment that was shown to be most effective (a hyaluronan fleece dressing vs. basic wound contact: HR 3.92, 95% CrI 0.24 to 175.2). This was also the case for the other two treatments that had high point estimates (nano-oligosaccharide technology lipido-colloid vs. basic wound contact: HR 3.55, 95% CrI 0.63 to 22.0; collagen/cellulose vs. basic wound contact: HR 2.6, 95% CrI 0.64 to 11.5).
| Dressing type | Reference treatment, HR (95% CrI or 95% CI),a,b risk of biasc | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BWC | HYDRO | FOAM | ALG | PAR-BET | CADEX | PAR | PMM | HYALU | N-OLIG | |||||||||||
| D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | D | MTM | |
| HYDRO | 1.01 (0.77 to 1.30), Md | 1.11 (0.88 to 1.41), M | ||||||||||||||||||
| FOAM | 1.49 (0.87 to 2.64), L | 1.27 (0.93 to 1.72), M | 1.00 (0.75 to 1.33), Md | 1.14 (0.88 to 1.47), M | ||||||||||||||||
| ALG | 1.26 (0.68 to 2.39), L | 0.82 (0.48 to 1.35), M | – | 0.73 (0.42 to 1.25), L | 0.30 (0.12 to 0.71),e M | 0.65 (0.37 to 1.1), M | ||||||||||||||
| PAR-BET | – | 1.52 (0.67 to 3.58), L | 1.38 (0.62 to 3.12), L | 1.36 (0.62 to 3.12), L | – | 1.20 (0.53 to 2.87), L | – | 1.87 (0.73 to 5.1), L | ||||||||||||
| CADEX | – | 1.56 (0.46 to 5.69), L | 1.48 (0.49 to 4.78), Lf | 1.40 (0.43 to 4.92), L | – | 1.24 (0.38 to 4.37), L | – | 1.93 (0.52 to 7.43), L | – | 1.03 (0.24 to 4.4), L | ||||||||||
| PAR | – | 1.54 (0.47 to 5.72), L | 1.42 (0.46 to 4.87), Lf | 1.39 (0.43 to 4.94), L | – | 1.22 (0.37 to 4.42), L | – | 1.90 (0.52 to 7.48), L | – | 1.02 (0.24 to 4.53), L | 1.00 (0.33 to 2.83), Lf | 0.99 (0.34 to 2.88), L | ||||||||
| PMM | – | 2.60 (0.64 to 11.49), L | – | 2.32 (0.59 to 10.01), L | – | 2.04 (0.5 to 8.98), L | – | 3.16 (0.73 to 14.99), L | – | 1.70 (0.34 to 8.98), L | – | 1.66 (0.46 to 6.27), L | 1.65 (0.79 to 3.54), L | 1.68 (0.8 to 3.58), L | ||||||
| HYALU | – | 3.92 (0.24 to 175.2), L | – | 3.50 (0.22 to 155.6), L | – | 3.10 (0.18 to 137.8), L | – | 4.85 (0.29 to 230.4), L | – | 2.56 (0.14 to 125.4), L | – | 2.46 (0.16 to 104.3), L | 2.49 (0.20 to 78.4) | 2.47 (0.19 to 88.9), L | – | 1.49 (0.1 to 55.54), L | ||||
| N-OLIG | – | 3.55 (0.63 to 21.98), L | – | 3.18 (0.58 to 19.46), L | – | 2.81 (0.51 to 16.98), L | – | 4.34 (0.7 to 28.16), L | – | 2.33 (0.33 to 16.52), L | – | 2.28 (0.43 to 11.59), L | – | 2.32 (0.67 to 7.91), L | 1.35 (0.54 to 3.62), L | 1.38 (0.53 to 3.67), L | – | 0.91 (0.02 to 16.32), L | ||
The most precise treatment effect was obtained for the treatment comparison populated by data from four trials (hydrocolloid vs. basic wound contact: HR 1.11, 95% CrI 0.88 to 1.41). The alginate dressing was expected to be less effective than basic wound contact (HR 0.82, 95% CrI 0.48 to 1.35), although the CrI did include 1.
However, it is important to consider the point estimates alongside the quality of the estimates provided by these analyses. In general, the network estimates were affected by high imprecision. No included studies were deemed to be at high risk of bias, but all were at unclear risk of bias for some or all domains (because of poor reporting). Because we took a conservative approach in the iGRADE assessment and studies at unclear risk of bias were assumed to be conferring no serious risk of bias, most studies were considered to be at moderate or low risk of bias.
Incremental effect of silver
The mixed-treatment meta-analysis model estimated the incremental effect of silver on ulcer healing as a HR of 1.24 (95% CrI 0.88 to 1.72). Thus, there was significant uncertainty regarding an effect of silver. A quality assessment was not conducted for this estimate; however, we noted that the two studies contributing to the silver effect produced very different estimates. The first study231 included 213 participants (with infected and non-infected ulcers) and was deemed to be at low risk of bias and had a HR of healing of 1.07 (95% CrI 0.73 to 1.55). The second study227 included 41 participants who all had an infected ulcer and was deemed to be at unclear risk of bias and reported a HR of healing of 2.6 (95% CrI 1.17 to 6.10).
Probability of treatments being best (non-silver dressings)
The treatment associated with the greatest probability of ulcer healing was the hyaluronan fleece dressing (48%), followed closely by the nano-oligosaccharide technology lipido-colloid dressing (35%) (Table 71). These results reflect the fairly high relative effect point estimates obtained for these two dressings but also the wide uncertainty around them. The GRADE and iGRADE assessment in Table 70 highlights that estimates of the relative effectiveness of these dressings were deemed to be of low quality.
| Dressing type | Probability of being the best treatment choice in terms of healing (%) |
|---|---|
| Hyaluronan fleece | 48 |
| Nano-oligosaccahride technology lipido-colloid | 35 |
| Collagen/cellulose | 7 |
| Paraffin-impregnated gauze or betadine/saline impregnated gauze | 5 |
| Cadexomer iodine | 3 |
| Foam | 1 |
| Alginate | 0 |
| Paraffin gauze dressing | 0 |
| Basic wound contact | 0 |
| Hydrocolloid | 0 |
Contributions of direct evidence (non-silver dressings)
Within our network of evidence we were able to assess the contributions of direct evidence for estimates within the two interlinked evidence loops of the network: basic wound contact versus foam versus hydrocolloid and basic wound contact versus foam versus alginate. The remaining loop was informed by data from a single three-arm trial and consequently assessment of consistency was not appropriate.
The varying contributions of the direct estimates (in columns) for each of the five relevant comparisons (in rows) are presented in Table 72. For example, the mixed-treatment meta-analysis estimate for the alginate vs. basic wound contact comparison was predominantly informed by indirect data, with the estimate from the alginate vs. foam comparison contributing most (32.1%) and direct evidence contributing only 9.7% to the overall estimate. The increased weighting of indirect compared with direct data occurred here because the effect estimate derived from the study populating the alginate vs. foam comparison294 was more precise than the estimate derived from the study populating the alginate vs. basic wound contact comparison. 287 The mixed-treatment estimate for alginate versus hydrocolloid was also mainly informed by direct evidence from other pairwise comparisons: the alginate versus foam and foam versus hydrocolloid comparisons.
| Loop | Direct estimates | ||||
|---|---|---|---|---|---|
| ALG vs. BWC (%) | ALG vs. foam (%) | BWC vs. foam (%) | BWC vs. HYDRO (%) | Foam vs. HYDRO (%) | |
| Mixed-treatment comparison | |||||
| ALG vs. BWC | 9.7 | 32.1 | 5.9 | 26.2 | 26.2 |
| ALG vs. foam | 7.6 | 78.6 | 1.4 | 6.2 | 6.2 |
| BWC vs. foam | 7.8 | 7.8 | 8.6 | 37.9 | 37.9 |
| BWC vs. HYDRO | 5.1 | 5.1 | 5.6 | 73.4 | 10.7 |
| Foam vs. HYDRO | 6.6 | 6.6 | 7.2 | 13.8 | 65.8 |
| Indirect estimate | |||||
| ALG vs. HYDRO | 8.2 | 40.0 | 3.6 | 11.8 | 36.4 |
Consistency of evidence
The results of the inconsistency assessment over the loops of the network of evidence are presented in Table 73. The estimated inconsistency factor values and 95% CIs show no evidence of statistical inconsistency for any of the two loops in the network, that is, as depicted from the 95% CIs, both loops are compatible with zero inconsistency between direct and indirect evidence.
| Loop | Inconsistency factor | 95% CI |
|---|---|---|
| BWC vs. foam vs. ALG | 0.528 | 0.0 to 1.80 |
| BWC vs. foam vs. HYDRO | 0.438 | 0.0 to 1.41 |
Discussion of mixed-treatment comparison meta-analyses
We used mixed-treatment comparison meta-analysis to comprehensively summarise all trial evidence regarding the use of dressings to heal (1) foot ulcers in people with diabetes and (2) venous leg ulcers. We conclude that more expensive ‘advanced’ dressings may offer no advantage in terms of healing than cheaper, basic dressings. The work also highlights the risk of bias in some studies and how this can impact on the interpretation of mixed-treatment meta-analyses.
The work also provides a platform from which to consider future research. Given the large number of dressing options available to clinicians (although a number of dressings have been evaluated here there are many more for which no RCT data exist), the design of future studies should be driven by those questions regarded as being of high priority by decision-makers and patients and should be guided by these data.
When we conducted our meta-analysis there was no published method of assessing the quality of evidence within a network of evidence or mixed-treatment comparison meta-analysis. We developed and employed a preliminary framework for evidence quality assessment within the framework of mixed-treatment meta-analysis, based on GRADE. 252 We aimed to assess the feasibility of such an approach and highlight the potential challenges of applying quality assessment to mixed-treatment meta-analysis evidence. We note, however, that our modified approach has not been validated and is not recognised by GRADE. It may be that tools other than GRADE would provide a better starting point for assessing the quality of mixed-treatment meta-analysis evidence. GRADE is primarily concerned with guiding clinical and policy-level decision-making, which is somewhat different from the challenge of summarising the quality of evidence within mixed-treatment meta-analysis. At the very least, within our modified approach there are still several aspects that need addressing, emphasising future challenges in developing such a scale, for example indirectness as a characteristic of the evidence is not easy to apply to mixed-treatment meta-analysis (as indirect data are a common feature that should not necessary result in downgrading) and is perhaps more appropriately termed ‘inconsistency’ in this context. Within the standard GRADE approach, the term ‘inconsistency’ relates to unexplained heterogeneity. Within iGRADE we considered unexplained heterogeneity and inconsistency (the mixed-treatment meta-analysis-related meaning) together in one category. We then added a separate category that assessed the impact of the sensitivity analysis on the results. Our aim here was to assess the stability of the network and thus its estimates. Finally, imprecision could perhaps be omitted from this tool as it may be more useful for the reader to use his or her own judgement regarding the width of CrIs and what they mean. We acknowledge that the quality assessment of mixed-treatment meta-analysis output is a complex area and further research is required; however, at the time our work was the only example of quality assessment of mixed-treatment meta-analysis outputs. 196
Within our mixed-treatment comparison meta-analysis of dressings for venous leg ulcers, we implemented an analysis that allowed us to use available data to assess the impact of silver dressings beyond that of their non-silver-containing counterparts. From these data we conclude that there is considerable uncertainty regarding the effect of silver on the healing of venous leg ulcers in people with either infected or uninfected ulcers at baseline. It was not possible to rule out a true effect in either direction, that is, in favour of silver or non-silver dressings, and there may be no differential effect. The relative effect estimate was drawn from two studies involving a total of 255 participants. One study in particular was small (41 participants) and poorly reported. Ultimately, the scarcity of available data limited the conclusions that we were able to draw. The methods that have been presented here offer the potential for further work, notably using IPD from studies when available (although the work described earlier in this chapter suggests that this is unlikely to be possible currently).
The findings presented result from the analysis of the most comprehensive evidence base available regarding the effect on healing of dressings to treat diabetic foot ulcers. Although some may argue that the presence of sparse data should preclude any statistical synthesis of the evidence, we counter that clinicians cannot postpone treatment selection until high-quality evidence has accumulated. Furthermore, comprehensive evidence synthesis highlights to researchers and clinicians the state of the current evidence base and its limitations as well as signposting where future research might focus. A further strength of this study is the application of an exploratory framework based on GRADE to undertake quality assessment of mixed-treatment meta-analysis estimates and the presentation of findings in light of this assessment.
We do acknowledge that there are limitations in synthesising sparse data, especially in preventing further exploration of the potential impact of heterogeneity, for example length of trial follow-up. However, it is important to note that this is an issue for several evidence synthesis projects in wound care for which data are sparse and follow-up times vary. In other networks this has been dealt with by assuming a constant hazard of healing over time, although this assumption is potentially also not valid. In general, the limited evidence base made a random-effects model difficult to fit; however, we acknowledge that in other situations this model type may be more appropriate than a fixed-effects approach. We also note that, besides characterising the potential for bias, it may be possible to adjust the treatment effect estimates for bias. Further methodological development would be required to inform the application of bias adjustment to this research and this was beyond the scope of our programme.
Overall conclusions from workstream 3
We used a range of strategies to identify and prioritise topics for evidence synthesis, including face-to face contact with local clinicians. The topic of the effectiveness of silver-containing dressings emerged as a priority in relation to both diabetic foot ulcers and venous leg ulcers. We explored the potential for a new IPD meta-analysis of the effects of silver-containing wound dressings on venous ulcer healing. We identified seven eligible RCTs (1102 participants) within which there was a high degree of heterogeneity in terms of participant eligibility, dressings, comparators, cointerventions, outcomes and duration of follow-up. Overall, the trial-level data were not suggestive of a difference in healing time with silver dressings. The heterogeneity of the existing trials led us to conclude that investing in the resources required for a new IPD meta-analysis would not be worthwhile at this time (because such an analysis would be unlikely to draw a different conclusion from the aggregate data).
We produced a series of 11 Cochrane reviews (either as updates or new reviews) in areas prioritised by participating clinicians (five in diabetic foot ulcer treatment, five in venous ulcer treatment and one in surgical wound treatment). Some treatments may be effective for the management of diabetic foot ulcers (hydrogel and negative pressure wound therapy) and there is good evidence that compression is effective for venous leg ulcers and EMLA cream reduces leg ulcer pain during debridement. Beyond these findings much uncertainty remains because of the scarcity and poor quality of the primary research. The lack of evidence in relation to some populations, for example people with diabetic foot ulcers, should influence the future research agenda. Our consultation with clinicians also identified many topics for which the prevailing uncertainty is a challenge to their daily clinical decision-making and new primary research is warranted.
One approach to evidence synthesis that arguably delivers the ‘answer’ or result in a more decision-ready format is mixed-treatment comparison meta-analysis. This approach has the advantage of delivering ranked probabilities that each treatment is the ‘best’ rather than merely presenting innumerable pairwise comparisons. We undertook such mixed-treatment comparison meta-analyses of the evidence for different wound dressings influencing the healing of diabetic foot ulcers and venous leg ulcers. As part of this work it became obvious that a method of accounting for the quality of the evidence within the evidence network was required (clearly more complex than accounting for the quality of evidence in a pairwise comparison). In the absence of any published method at the time, we developed a modification of the GRADE approach that we called iGRADE.
The mixed-treatment comparison meta-analysis led us to conclude that, for diabetic foot ulcers, matrix hydrocolloid dressings are associated with a 70% probability of being the best dressing, followed by hydrogel and foam (both having a 14% probability of being the best). Hydrogel was deemed to have the highest probability of being the best in one sensitivity analysis but this was when the largest study evaluating hydrogel was removed from the network. For venous leg ulcers a hyaluronan fleece dressing had a 50% probability of being the best treatment, followed by a lipido-colloid dressing (35% probability). It is important to emphasise, however, that when advanced dressings were found to be the likely best treatments the findings were driven by very uncertain, low-quality data. Although supported by moderate-quality evidence, the results did not demonstrate a treatment effect (greater healing) associated with hydrocolloids or alginates relative to basic wound care for either foot ulcers in people with diabetes or venous leg ulcers.
Chapter 5 Discussion
Summary of key findings across the programme
Within workstream 1 we derived an estimate of the prevalence of people affected by (and receiving health care for) a complex wound in the city of Leeds (population 751,487). This prevalence study makes a valuable contribution to the current literature for several reasons. First, we knew from our own literature review that there was no accurate contemporary estimate of the prevalence of complex wounds in the UK, with most previous surveys having focused on individual wound types such as leg ulcers or any kind of wound (including the non-complex). Many of the surveys were poorly reported and/or flawed. There have been major developments in wounds research and its implementation since some of the early, higher-quality studies were conducted. For example, since the early studies of Callam et al. 38 and Cornwall et al. 41 in the 1980s, leg ulcer care has been revolutionised in the UK and now most venous ulcer patients receive good first-line treatment (compression therapy). It is quite likely that the point prevalence of open venous ulcers is therefore lower now than it was in the 1980s. Pressure ulcer prevention has also become a national priority as part of Commissioning for Quality and Innovation [see www.england.nhs.uk/wp-content/uploads/2014/02/sc-cquin-guid.pdf (accessed 25 June 2016)] and so one would expect their number to have reduced.
Second, our survey was unique in its comprehensiveness (we included private hospitals, prisons and hospices as well as NHS providers). We had a broad focus while maintaining relevance for service providers so we included people with any kind of complex wound and excluded straightforward wounds such as surgical incisions that were healing normally. We were particularly focused on gaining good estimates of the prevalence of complex wounds in people who were current or previous intravenous drug users as this was perceived as a clinical challenge in Leeds when we began the research and there were no published estimates at the time. Our finding that 5.64 people per 1000 with a history of current or previous intravenous drug use have a complex wound (compared with 1.47 per 1000 people in the general population) is novel. Since we undertook our study there has been a study published reporting the prevalence and history of skin problems in intravenous drug users. 299 This survey purposively sampled known previous and current injecting drug users, 7% of whom had a current leg ulcer during the survey. These two prevalence estimates are very different, which is probably explained by the very different sampling approaches used, but what is clear is that injecting drug users are at a greatly increased risk of chronic and complex wounds.
The prevalence survey also told us that pressure ulcers were the most frequent wound type (0.31 per 1000 people) and that most people with a complex wound have at least one comorbidity, with 51% having two or more. The most common comorbidity was CVD (affecting 45%) and 23% had some form of incontinence. Most people with a complex wound were receiving care from NHS community services and receiving a median of two consultations per week for their wound, each lasting just under half an hour on average. The quality of care seemed to be generally high: 81% of people reported as having a venous leg ulcer were in receipt of compression therapy, 73% of people with at least one pressure ulcer had a pressure-relieving mattress and nearly 95% of all pressure ulcers had a documented EPUAP ulcer severity classification in line with NICE guidelines.
We set out with the intention of developing and piloting a complex wounds register and informed this with a comprehensive review of existing registers of wounds. We were particularly influenced by the reviews of Drolet and Johnson97 and Raftery et al. ,99 who together provided a framework for us to think about registers. We identified no UK-based current wounds registers but were able to design and implement one, albeit as a pilot. It was easy to see the potential advantages to patient care and HTA from such a register, not least because there is a complete absence of usable, routinely collected data on community nurses’ work and its associated clinical outcomes. As far as we know this is the first time a complex wounds register has been implemented in UK community services and there were very real difficulties associated with this, even on a small scale. For instance, the ethics committee required individual participant consent to collect health-related quality-of-life data. The very act of seeking consent for data collection dissuaded participation and our consent rate was only 25%, with a probable selection bias towards people who were in better overall health. The difficulties of getting a register off the ground were also compounded by community nurses’ working practices, which do not generally feature mobile IT (or feature it sporadically). It is difficult or impossible to obtain these data from anyone other than community nurses because wound care is not documented by GPs or hospitals. The data that we were able to collect enabled us to glimpse the huge potential value of a high-quality prospective register of people with complex wounds. Such a register would succeed only if the technology infrastructure improved in community nursing and the collection of good routine data became possible, and this would require considerable investment.
Workstream 2 was our conduit to the patient and carer voice, a voice that is heard relatively rarely in complex wounds research for reasons that are understandable. As far as we are aware this is the first research conducted that has aimed to understand which outcomes matter to those directly affected by complex wounds. Consequently, we feel that workstream 2 makes a major contribution to the international literature on patients’ experiences of living with a complex wound, including for the first time hearing the voices of people whose wounds are a direct consequence of intravenous drug use. We learned that some of them are struggling to come to terms with the fact that they may have inflicted irreparable damage to the veins in their legs such that chronic ulceration may be a permanent feature of their lives. We also heard from patients with wounds of all types that their most desired outcome is complete healing and the absence of a wound. Some carers acknowledged that this may be an unrealistic expectation. The interviews with health-care professionals were in some ways the most fascinating. Here, the tension was palpable between striving to get wounds completely healed (by trying multiple products in rapid succession) and helping people to live with a long-term condition. Nowhere is this tension better exemplified than in this quote from an experienced nurse:
. . . and we don’t have any concept of what the impact of despair has on healing and kind of feeling that everything that everyone has tried is useless and that they’re in a hopeless situation. We haven’t really got an understanding of how that affects us metabolically, physiologically but I think that’s ultimately what we put our patients through a lot of the time.
H12
Our review of complex wounds trials, the nature of their funding and their overall quality also makes a unique contribution in that for the first time we have profiled the funding of wound treatment research. It was notable that a large proportion of wounds research did not report a funder (26%), with 41% of studies having some commercial funding. We have highlighted for the first time that only around 24% of trials in complex wound treatments have complete healing (the outcome that matters most to patients) as their primary outcome. We encourage wounds researchers to respond to this finding and endeavour to capture complete healing as the primary outcome in wounds trials (which will mean extending follow-up beyond the current median of 2.8 months).
We had not originally intended to undertake a JLA research prioritisation exercise but this seemed an ideal way of bringing together the goals of greater patient involvement and research prioritisation. We selected pressure ulcers as the focus for this, the first PSP in complex wounds, because we thought that those with pressure ulcers would be the most difficult patient group to engage (because of age, overwhelming illness and social isolation) and we could learn the most from the process. Pressure ulcers were also the most common type of complex wound in our prevalence survey. It did prove to be enormously challenging (and ultimately impossible) to ensure that the profile of our JLA participants (in terms of age and comorbidities) mirrored the profile of the people affected by pressure ulcers in our prevalence survey. Nonetheless, we captured the patient, carer and health-care professional voice in a way that had not been done before and together we developed a list of research priorities that has face validity. A key feature of the uncertainties generated was the focus on pressure ulcer prevention, with the top five prioritised uncertainties having prevention as the aim. Our prevalence survey (workstream 1) reported pressure ulceration as the most prevalent complex wound and in workstream 2 we heard from patients about the huge impact of these wounds on their lives. Thus, the prevention of pressure ulcers and other wound types is a key area for future focus.
Finally, workstream 3 focused on evidence synthesis in areas of high priority for the service. We explored whether or not a meta-analysis of trials of silver dressings for venous leg ulcers, using IPD, would be feasible and likely to be valuable for decision-making and concluded not because of the heterogeneity of the existing trials. A review of the existing data at trial level concluded that there was no evidence that silver dressings were more effective than non-silver dressings for healing venous leg ulcers. Alongside several Cochrane reviews, covering pairwise comparisons of wound interventions in high-priority topics, we implemented two major mixed-treatment comparison meta-analyses in areas of high priority for patients and the health service, namely dressings for leg ulcers and diabetic foot ulcers. These are the first network meta-analyses of wound dressings and at the time they were conducted there was no published method for accounting for the quality of the evidence in a network. Consequently, we developed and implemented one, which we named iGRADE. 196 Subsequently, others have published a method that has increased focus on assessment of the overall network and the treatment rankings as well as assessment of each comparison. 260
Our evidence synthesis work enabled us to conclude that matrix hydrocolloid dressings are associated with the highest probability (70%) of being the best dressing for diabetic foot ulcers, whereas a hyaluronan fleece dressing had the highest probability of being the best dressing for venous ulcers (35%); however, in both cases there was high uncertainty and poor-quality evidence. Looking across the programme we can see how, collectively, this work highlights limitations in the RCT evidence in wound care. The review of wound trials in workstream 2 highlighted the limited use of complete healing as an outcome even though this is most important outcome for patients. We consistently found in workstreams 2 and 3 that the methodological quality of RCTs is unclear or low in the majority of cases and trials recruit small numbers of participants and have short durations of follow-up. In workstream 3 we observed how these limitations impact on evidence synthesis in systematic reviews and meta-analyses. These study limitations mean that even novel approaches to evidence synthesis cannot overcome the evidence deficits so any effect estimates have high levels of uncertainty. There are several important clinical questions in complex wound care with no relevant RCT data. Improvements in trial conduct and reporting is a key priority in wound care. Given the data collected in the complex wounds register pilot (workstream 2), there would be potential for routine data to provide valuable intelligence to support the planning and development of RCTs only with further investment in routine data collection and IT in community nursing (commensurate with that seen in hospitals and general practices).
Summary of patient and public involvement involvement
In this programme of work, as with all our previous wounds research, we strived for patient involvement to be integrated and sustained throughout the research; however, this has proved extremely difficult to achieve because of the age and health of those people affected by complex wounds. We had planned a patient advisory group for the whole programme but found it difficult to identify participants and ultimately focused on more targeted exercises, which worked well. Central to this endeavour was the exploration of which outcomes are important to people with wounds as there had been little if any exploration of this previously. Second, the JLAPUP engaged patients and carers in a research prioritisation exercise that was unique in wound care, albeit with rather younger and fitter service users than those generally at risk of pressure ulceration. We felt that these exercises were largely successful in facilitating detailed and meaningful patient and public insights and contributions to future research approaches and agendas, but will continue to explore other strategies for patient involvement in future wounds research.
Generalisability of the findings
Most of the primary research within this programme was conducted solely in one northern English city, which may limit the generalisability of the findings. Leeds has a population of 751,000, 20% of its population live in some of the most deprived areas of the country22 and there are urban, suburban and rural communities. The population of Leeds is ethnically diverse with approximately 17% of residents from black and minority ethnic groups. 23 Perhaps in the context of this research, what is particularly exceptional about Leeds is the record of nationally funded wound care research conducted there since the 1990s. This feature together with long-established, research-led clinical leadership may mean that the standard of wound care in Leeds will be higher than in many places, although there are no national data with which to explore this. If it is true then the prevalence of complex wounds in Leeds may be lower than is typical elsewhere. It is difficult to say whether or not we would have experienced similar challenges in implementing a comprehensive complex wounds register elsewhere but given the commitment to high-quality wounds research and care in Leeds it is likely to be at least as difficult elsewhere.
The primary research on patients’, carers’ and professionals’ preferences for wound care outcomes also reflects the views of people from one city; however, it is difficult to imagine why their views would not be representative of people elsewhere as there is nothing particularly different about the wounds in Leeds. It may be that patients’ and carers’ reflections on the care that they receive may be specific to Leeds for the reasons described above. The research priorities gathered from health-care professionals within workstream 3 are likely to be shared elsewhere and we checked that they were genuine uncertainties by comparing them with the international evidence base. Finally, the JLAPUP was a national initiative and the evidence synthesis work in workstream 3 involved scrutiny of the international literature.
Implications for practice and service delivery
The UK complex wounds population is largely managed by community nursing teams who see a diverse population of people with complex wounds of different underlying causes. NHS data systems typically capture community nursing activity in terms of the numbers of patient visits but not detailed reasons for the visits and, when we began this research, Leeds Community Healthcare NHS Trust had no valid or reliable data regarding the numbers of people currently receiving wound care, the types of wounds or the treatments delivered. We now have good estimates of the numbers of people with different wounds and different comorbidities who need wound care in a large UK city.
Research and service development initiatives that lead to a reduction in the frequency of necessary consultations while maintaining or improving patient outcomes should be a key target. Furthermore, this was also a priority expressed by the health professionals in the qualitative interviews in Chapter 3. The recent research finding that two-layer compression hosiery is as effective as bandages for venous ulcers but more cost-effective as it reduces the number of nurse consultations is pertinent here; if hosiery use increases as a result, the NHS will save money without any detriment to patient care or outcomes. 118 Although not suitable for everyone with a venous ulcer, in our prevalence survey we found only 33 instances of its use (11% of people with venous ulcers) and therefore there is a great deal of scope for implementation and positive impacts on patients and services. Those people with leg ulcers as a consequence of injecting drugs may also benefit from hosiery rather than bandages as they are typically younger and may have chaotic lifestyles.
The data also underline the importance of cost-effectiveness analysis with robust assessment of costs and effects in evaluations of new treatments. Too frequently wound treatment evaluations merely report prices rather than total health-care costs. The survey also indicated that wound management in Leeds is largely in accord with evidence-based guidelines; however, only one leg ulcer patient was receiving pentoxifylline (which increases healing). Subsequently, in Leeds, increasing the prescribing of pentoxifylline became a target for implementation and local prescribing guidance has been developed (stimulated by the survey findings). 300
A surprising finding was the large number of people with non-diabetic foot ulcers (43% of foot ulcers were not associated with diabetes). Non-diabetic foot ulceration, likely to be caused by arterial disease, is under-researched and this subpopulation would benefit from closer scrutiny (including careful diagnosis of the underlying problem and vascular referral when necessary). Another surprising finding for the clinical staff from the prevalence survey was the significant proportion of people with wound types other than pressure, leg and foot ulcers; this could not have been known without the survey and allows the Leeds Community Wound Prevention and Management Service to consider how best to guide staff in delivering care. The challenge here then is the even poorer evidence base for less-common wounds.
The picture that emerged from the patient interviews is overwhelmingly one of a desperate desire for complete healing and eradication of the wound. The carers on the other hand seemed rather more circumspect, expressing beliefs that the wound in question was unlikely to heal. There were sometimes feelings of ‘drifting along’ without a clear treatment plan and this clearly engendered frustration. Others expressed difficulties in terms of having honest communication with health-care professionals and/or concerns that the nurses were really ‘stretched’ by a demanding workload and therefore were not to be pressed too hard. Staff on the other hand felt that healing was frequently an unrealistic expectation and symptom management was often the main goal. Senior tissue viability nurses, who are wound specialists, acknowledged that there was no such thing as the magic dressing to heal many of these complex wounds. What is not clear is the extent to which honest conversations are happening with patients about prognosis but in questioning this we must also acknowledge that these conversations are difficult to have, given the absence in the international literature of good prognostic data. We know that venous ulcers that are < 10 cm2 in area and < 12 months old at the first consultation have a 29% chance of not healing by 24 weeks. 226 This compares with those that are > 10 cm2 in area and > 12 months old on presentation, which have a 78% chance of not healing. A similar model exists for diabetic neuropathic foot ulcers. 100 We also know that, when wound care centres in the USA were randomised to receive this prognostic information for individual patients (or not), those patients in centres receiving it were more likely to heal than those in control centres, despite no advice regarding management being given. 301 Unfortunately, we do not know why this information makes a difference or if particular management strategies can improve outcomes for patients with a poorer prognosis.
There are also clearly other challenges for health professionals in encouraging patients to change behaviours, whether concerning treatment adherence (e.g. compression) or healthier lifestyle behaviours related to diet, smoking and exercise. Very positive messages emerged from the staff interviews: the pleasure felt when a really tricky dressing procedure produced a good result or when a patient was referred to the tissue viability service and finally prescribed compression therapy, which resulted in rapid healing of a leg ulcer, are just two examples.
The messages for the service from these interviews are complex and not straightforward. The treatment model described in the interviews recalled the ‘specialist healing’ model described by Briggs and Flemming142 as opposed to a ‘chronic care route’. The treatments used are mainly aimed at healing the open wound; however, the wound itself is just usually an outward manifestation of an underlying disease. Health professionals reported frustration with unrealistic expectations from patients about short-term healing but it may be that referral to tissue viability services itself raises expectations of a speedy resolution. Tissue viability nurses themselves observed patients’ experiences of cycling hope and despair, and carers were often frustrated with treatment plans (or an apparent lack of them). Given that most complex wounds are due to underlying systemic disease there is almost certainly a need for more of a psychological approach to helping patients live with a long-term condition and a more social model of care.
Finally, there is probably scope to improve communication and ‘intelligence sharing’ in community nursing and there is clearly an important role for IT in this. The challenges faced when trying to implement research evidence and high standards of care across community nursing in a large city are easily underestimated. The responsibility for taking the clinical lead in a topic such as wound care will typically lie either with nobody or within a specialist clinical team such as a tissue viability or wound care service. These services often feel under-resourced and ill-equipped to take responsibility for implementing high-quality wound care across a city and involving many professions and settings. Many and diverse staff groups are involved in organising and delivering wound care (e.g. practice nurses, district nurses, podiatrists, specialist wound care/tissue viability nurses, community matrons, GPs, nursing home staff), all with sometimes conflicting priorities and different managers and key performance indicators. There is a great deal of ‘silo working’ within professional groups and within neighbourhoods and communication between (and within) groups can be poor. Patients and health-care professionals would be better served by a secure clinical information system that allows the monitoring of patient progress, the ongoing collection of treatment and outcome data, interprofessional communication and decision support. Our experience of embedding a wound assessment template in SystmOne was that, although this was quite straightforward for a patient with one wound at baseline, the system did not lend itself to prospective monitoring of wound progress combined with clinical decision-making. When patients had multiple wounds it proved impossible during the register pilot to track each one individually.
Implications for future research
Prioritised uncertainties in the prevention and treatment of pressure ulcers
The JLAPUP prioritised 12 genuine uncertainties that can be taken forward by the research community (presented in order of decreasing priority):
-
How effective is repositioning in the prevention of pressure ulcers?
-
How effective at preventing pressure ulcers is involving patients, family and lay carers in patient care?
-
Does the education of health and social care staff on prevention lead to a reduction in the incidence of pressure ulcers and, if so, which are the most effective education programmes (at organisational and health/social care level)?
-
What is the relative effectiveness of the different types of pressure-relieving beds, mattresses, overlays, heel protectors and cushions (including cushions for electric and self-propelling wheelchairs) in preventing pressure ulcers?
-
What impact do different service models have on the incidence of pressure ulcers, including staffing levels, continuity of care (an ongoing relationship with the same staff members) and the current organisation of nursing care in hospitals?
-
What are the best service models (and are they sufficiently accessible) to ensure that patients with pressure ulcers receive the best treatment outcomes (including whether or not getting people with pressure ulcers and their carers more involved in their own pressure ulcer management improves ulcer healing and, if so, the most effective models of engagement)?
-
For wheelchair users sitting on a pressure ulcer, how effective is bed rest in promoting pressure ulcer healing?
-
How effective are wound dressings in the promotion of pressure ulcer healing?
-
Does regular turning of patients in bed promote healing of pressure ulcers?
-
Does improving diet (eating) and hydration (drinking) promote pressure ulcer healing?
-
How effective are surgical operations to close pressure ulcers?
-
How effective are topical skincare products and skincare regimens at preventing pressure ulcers?
Each one of these questions is the result of pooling several or many submitted ‘raw’ uncertainties and they are not neat PICO questions. We came under some pressure from a research funder to turn these uncertainties into more focused PICO questions but resisted this as we felt strongly that, after such a collaborative and productive PSP that excluded academics who were not also active clinicians (as per the JLA process), it was not our place as academics to now take these uncertainties and turn them into ‘our kind of’ research questions. We think a more sensitive and appropriate approach would be for the research community in collaborative teams with patients and clinicians to decide what the top-priority PICO question is under each of these broad headings. It is then up to these partnerships to persuade research funders that they have the right question. It may also be the case that further research is required to focus these questions, which may be epidemiological or modelling work.
Prioritising and conducting future randomised controlled trials in wound care
Over the last 20 years there has been a concerted effort to increase the volume and quality of wounds research, with a great deal of this research being funded by the NIHR. 18–20,118,231,267,302 One of the challenges that researchers encounter, however, is the lack of basic epidemiological and service delivery ‘intelligence’ about wounds and it is this lack of information that stimulated the initiation of this research programme. For example, when making the case to potential funders for a large RCT of a wound treatment, we need to know the prevalence, typical treatments and estimates of healing rates. When designing a RCT of a wound treatment, we need to know typical healing times to plan duration of follow-up. To evaluate prevention strategies, we need to know typical incidence rates and risk factors. For cost-effectiveness modelling of treatments we are likely to need to know all of these things plus others including the natural histories of different wounds and the impacts on health-related quality of life of different wounds. Generally, and for most common wounds, we have none of this information. The lack of basic information therefore also contributes to a lack of momentum in wound care research: it is hard to make the case for new research if we do not know how many people are affected by a condition, what standard care is, what it costs or what the usual outcomes are. Moreover, our review of wounds research funding in workstream 2 shows that the evidence base is strongly influenced by the devices industry, which begs the question, ‘where do patients and clinicians turn for valid evidence for decision-making?’. Whatever RCTs are conducted it is imperative that the wound healing research community engages with international standards of research conduct and reporting for future research. 169
Synthesising evidence in the two mixed-treatment comparisons highlighted the weak evidence base available to decision-makers when choosing between different dressing types based on the outcome of complete healing (which we know is the most important outcome for patients). In the light of the weak evidence base it is not easy to identify which future research questions should be the highest priority. It does not necessarily follow that we should prioritise RCTs of matrix hydrocolloid dressings or hyaluronan fleece dressings because there is a risk that the existing small, poor-quality evaluations will have a disproportionately large influence on the future research agenda. We argue that the results of the mixed-treatment comparisons underline a more general point: there are many different dressing types for venous leg ulcers and diabetic foot ulcers (although a number of dressings have been evaluated here there are many more for which no RCT data exist) and no current evidence that any one dressing is better than another. Based on this uncertainty there is a huge number of possible RCTs that could be planned and prioritising future work in this area is challenging. We had originally planned to undertake decision-analytic modelling and value of information analysis to inform the prioritisation of the primary research questions in the dressings field. It was obvious to us, however, that it would be impossible to populate a model without either harvesting data from the wounds register or eliciting key data from professionals. In the past we used elicitation to evaluate the value of undertaking future research into the effectiveness of negative pressure wound therapy for category 3 and 4 pressure ulcers. 6,7 In this previous study an absence of data meant that we had to elicit data on relative healing times of different dressings, treatment trajectories including probabilities of patients receiving specific treatments and complication rates. 6,7 In this programme of research, unfortunately the wounds register pilot was unable to yield the number of data required for modelling at the time that it was required.
Asking health professionals and patients to prioritise dressings for evaluation is a possibility. In our work, health professionals highlighted uncertainty regarding silver-containing dressings, foam dressings and protease-modulating dressings and, based on current data, there is no evidence that these dressings increase the risk of healing compared with alternatives. In terms of silver-containing dressings we believe that a next step would be to combine IPD from the NIHR-funded VULCAN (Venous ULcer Cost-effectiveness of ANtimicrobial dressings) trial231 alongside the aggregate data in the network, which would allow for more detailed analysis. As those allocated to the silver-containing dressing group in the VULCAN trial received different types of dressings (e.g. some people received silver-containing foam dressings and others silver-containing alginate dressings and so on), using IPD we can start to cautiously examine these subgroups separately and explore incremental impacts of silver-containing dressings against their non-silver counterparts more fully.
There is a risk that prioritising research may continue to encourage a focus on single RCTs of two or maybe three dressing types. Given the inverse relationship between the number of dressing options for complex wounds and the size of the evidence base regarding their relative effectiveness, we believe that continued collection and application of large-scale observational data in wound care is extremely important. This is true for uncertainties regarding dressing treatments, for which in fact it seems potentially of value to explore a hypothesis that advanced dressings may have little to offer in terms of improved healing rates for complex wounds that are due to systemic disease (although they may offer benefits for symptom management).
Although RCTs are key to answering questions about relative treatment effects, real-time naturalistic data are essential for cost-effectiveness modelling and also help identify which might be the most important research questions. 7 These data could also inform important aspects of trial design, for example by providing good estimates of potential eligible participants and typical healing rates for different wounds.
Indeed, wound care is an area with a lot of treatment uncertainty and much robust RCT data come from publicly funded studies [e.g. PRESSURE (Pressure RElieving Support SUrfaces: a Randomised Evaluation)302 and VenUS (Venous leg Ulcer Study) I to IV18–20,118]. However, there is a limit to the number of RCTs that can be commissioned and huge opportunity costs if suboptimal RCTs are conducted because of a lack of understanding about the key needs of patients and the NHS. Larger-scale register data collection could be particularly useful in the timely identification of treatments that are in widespread use and/or are very costly given the rapid introduction of devices used in wound care to market. The collection of observational data on wounds, treatments and outcomes offers the potential for estimating the comparative effects of alternative treatments, if methods such as propensity scoring are used to address likely confounding. The register pilot and a recent publication303 also suggest that the application of propensity scoring would have been possible had it been possible to collect more data. We are pursuing this approach as applied to surgical wounds healing by secondary intention in a separate NIHR programme grant (‘Surgical wounds healing by secondary intention: characterising and quantifying the problem and identifying effective treatments’; RP-PG-0609–10171). 304
Focus on the development of routine data collection in wound care
The wounds register pilot study highlights the huge challenge associated with collecting high-quality data about complex wound care and outcomes routinely in community services. Further evolution of IT infrastructure and of methods to maximise recruitment and follow-up are required as well as refinement of the scope and quality of data collection, for example the collection of data for multiple wounds, sometimes of different aetiologies, on the same individual at the same time.
This is arguably an optimal time to think about capturing routinely collected data for research with the changing state of electronic patient records in the NHS and the increased focus on data linkage as well as the staff enthusiasm for these changes observed here. However, expanding and developing a wounds register would require extensive central support. Alternative sources of data on the prognosis and costs of wound care could also be considered. Such alternatives include more time-limited observational cohort studies, such as that being run as part of another NIHR programme grant (RP-PG-0609–10171). 304 Although moving away from embedding data collection into routine clinical care to more costly, short-term, research-focused data collection is appealing as a means to an end, it has limitations as a long-term approach. We also suggest that future exploration regarding the use of GP data collection systems by practice nurses should take place, given the increased use of these services by ambulatory patients with wounds in recent years.
The type of data collected in the complex wounds register pilot, once the issues of recruitment rate and tracking of individual wounds were solved, would be eminently suitable for use in decision-analytical modelling, especially around the cost-effectiveness of treatments from a NHS perspective. 305 Such methods can inform clinical decision-making and research prioritisation, especially when there is decision uncertainty. Our previous experience in cost-effectiveness modelling around complex wounds suggests that the availability of robust data on even a small number of the fields included in the pilot study, for example treatments received, healing outcomes and the EQ-5D, would be extremely useful. In previous work we have had to develop methods to deal with sparse data as well as relying on expert elicitation of data with which to populate models as there was so little basic UK-relevant complex wounds data available. 7
A further area of research relevant to a wounds register is the value of its data for prognostic modelling and the extension of this into individualised patients care (stratified medicine). 306,307 As we have noted, the generally crude data available to describe wound care populations means that we currently have very little prognostic information regarding the future likelihood of positive or negative outcomes for most complex wound types. Such limited insight into the future outcomes for those with specific person- and wound-level characteristics limits current clinical decision-making and also blinds us to potentially important research questions. For example, given the current limited data available we have almost no way to explore whether or not there are treatments that have effect modification for specific subgroups of patients (currently masked in studies with heterogeneous study populations) and which could be investigated further in focused RCTs. Importantly, the largest proportion of non-intervention uncertainties raised by patients, carers and clinicians in the JLAPUP concerned aetiological and prognostic issues (see Chapter 3).
Other research questions emerging from the stand-alone reviews (without mixed-treatment comparisons)
-
The relative cost-effectiveness of negative pressure wound therapy compared with usual care for treating post-amputation wounds and debrided foot ulcers in people with diabetes.
-
The relative cost-effectiveness of 0.5% chlorhexidine compared with povidone iodine in alcohol for preoperative skin antisepsis in clean surgery.
-
The cost-effectiveness of ibuprofen-containing dressings for reducing pain in people with painful skin ulcers.
Other uncertainties raised by clinicians for which evidence synthesis was not undertaken because of a lack of primary research were:
-
the cost-effectiveness of different dressings for healing after toenail surgery
-
obesity as a risk factor for delayed healing in leg ulcers
-
the management of underweight patients at risk of pressure ulceration
-
post-operative strategies for pilonidal sinus
-
the role of EUSOL in wound management
-
the topical application of combined steroids and antifungal treatments to the wound bed
-
the management of overgranulation
-
the management of wounds in patients with dementia
-
the prevalence of nutritional risk factors in people with complex wounds.
The legacy of a having a 5-year wounds research programme in a community health-care trust
The Leeds Community Wound Prevention and Management Service within what is now Leeds Community Healthcare NHS Trust has been in existence since 1988. Since 1999 the service has been actively engaged in NIHR-funded wounds research, with the clinical lead being a coinvestigator on several NIHR Health Technology Assessment programme-funded trials as well as this NIHR programme grant. Partnership in this programme of research has delivered many benefits that we think are worth drawing attention to as additional positive consequences of research investment in a community nursing service. One of the remarkable consequences of this longstanding emphasis on research in Leeds is the involvement of frontline community nursing staff in research; this was particularly the case for the wounds register pilot study in this research programme. Clinical staff could see the potential value of it and were keen to see any results.
The research time funded for the clinical lead enabled her to engage fully in the research and ensure that it was embedded in the service and that research evidence could be rapidly implemented into patient care. Research is now part of the culture of the Leeds Community Wound Prevention and Management Service, with several staff wholly or partly funded on NIHR-funded research projects.
As a consequence of the prevalence survey in workstream 1 (see Chapter 2), the service developed a strategic action plan for the prevention of pressure ulcers. The survey identified that pressure ulcers were the most frequent complex wound in Leeds. Recent Department of Health-led safety strategies such as the NHS Safety Thermometer92 were developed to support a ‘no harm’ culture within the NHS. However, the data accrued in our prevalence survey highlighted to the trust that pressure ulcers remained a significant problem and provided robust data to drive the pressure ulcer prevention agenda at both grass roots and board level. A new local strategy drew directly from the findings of the prevalence survey and received support from the Leeds Community Healthcare NHS Trust’s Quality Committee whose membership includes representatives from the trust board. The new pressure ulcer strategy includes a five-point action plan that addresses training, robust documentation, timely assessment and the implementation and generation of research evidence to continue to support clinical practice. All of these components are encapsulated in the local Your Skin: Our Priority campaign, which was developed by the Leeds Community Wound Prevention and Management Service and is supported by the trust chief nurse and chief executive. This campaign has recently also been adopted by the University of Leeds student nursing forum.
The prevalence survey also highlighted the number of leg ulcer patients in Leeds. At the start of the programme grant (2008) there were two community-based leg ulcer clinics serving a population of > 750,000 people. The prevalence survey results enabled the development of two successful business cases to increase the number of leg ulcer clinics. There are now seven community leg ulcers clinics across the city of Leeds, with funding having been awarded by industrial partners as well as the local clinical commissioning group. These new clinics have the specific aims of bringing care closer to patients’ homes and reducing the number of inappropriate referrals to specialist hospital services.
The wound assessment template in SystmOne, initiated in the lead-up to the complex wounds register pilot, continues to be used as a clinical tool. The development of electronic methods of data capture supported by the programme grant enabled the Wound Prevention and Management Service to become the only service in the trust and one of very few in the country to use mobile technology and mobile working and to be completely paper free. The routine data collection facilitated by the wounds register pilot also allowed us to realise that the assessment and documentation of wounds in the city was often incomplete and that wound assessments were often being conducted by junior staff. The service responded by developing an extended wound care skills programme for band 5 nurses. The programme releases community staff nurses from their clinical teams to spend dedicated time with the specialist wound care nurses, initially for a period of 1 week and then 1 day per month for an additional 11 months. The nurses work through a tailored training programme that assesses their skills on entry to the programme and measures what they have learned on completion. The programme also measures their impact on patient care from a service user perspective via the Make Yourself Medical Outcome Profile tool [see www.bris.ac.uk/primaryhealthcare/resources/mymop/ (accessed 25 June 2016)]. In addition to supporting best practice locally, these nurses also support the Wound Prevention and Management Service in research conduct and implementation.
As a direct result of the research prioritisation work undertaken in workstream 2 the Wound Prevention and Management Service developed its approach to service user engagement, including by enhancing staff skills in this area. This new approach was exemplified during the worldwide STOP Pressure Ulcer Days in 2012 and 2013. In 2012 the service managed to garner the support of British paralympic basketball player, Jon Pollock. Jon’s story was a shocking one, both in terms of the care he had received for his pressure ulcers and the risks he took to compete even when his skin was broken. Jon brought the impact of living with a pressure ulcer to life and his story led to the service lead developing an application for an URGO Foundation Award to work alongside the British Paralympic Association. The aim of the application was to explore the impact of pressure ulceration in sportsmen and women. This application was successful and the clinical lead received the £20,000 award in November 2013 to conduct this innovative work.
Acknowledgements
The contributions of the following are gratefully acknowledged:
Sally Crowe, who chaired the JLAPUP.
Ms Sue Bennett, Dr Stephen Compton, Dr Rupert Earl, Mr Ed Holloway, Ms Jackie Philip and Mr Munawar Mecci, steering group members of the JLAPUP.
Richard Allen, who contributed to the review of wound care trials in workstream 2.
Sally Bell-Syer, who contributed to the review of wound care trials in workstream 2 and greatly facilitated the completion and updating of the Cochrane reviews in workstream 3 in her role as managing editor of the Cochrane Wounds Group.
Ellen Broderick, who contributed to the review of wound care trials in workstream 2.
Professor Martin Bland, who contributed to the review of wound care trials in workstream 2.
Ruth Foxlee, who undertook literature searching for the evidence synthesis work in workstream 3 and the review of wound trials in workstream 2.
Dr Robert Hodgson, who contributed to the review of wound care trials and assisted in a ranking system for the priority-setting meeting in workstream 2.
Dr Marrissa Martyn-St James, who assisted with some of the systematic reviews.
Dorothy McCaughan, who assisted with gathering uncertainties from clinical staff in workstream 3 and with the priority-setting meeting in workstream 2.
Delia Muir, who assisted with the priority-setting meeting in workstream 2.
Paul Morrin of Leeds Community Healthcare NHS Trust, who was a former cochair of the Programme Management Board.
Richard Slough, Head of Informatics at Leeds Community Healthcare NHS Trust, who greatly assisted in workstream 1.
Edward Syal, who was a patient adviser to the JLAPUP.
Contributions of authors
Professor Dame Nicky Cullum (Professor of Nursing) was the chief investigator and led the programme of research, chaired the Programme Management Group and cochaired the Programme Management Board.
Hannah Buckley (Statistician) undertook the statistical analysis for workstream 1.
Dr Jo Dumville (Senior Lecturer) contributed to the design, conduct, analysis and reporting of all three workstreams, conducted several systematic reviews and contributed to the mixed-treatment comparison meta-analyses and development of iGRADE.
Jill Hall (Research Fellow) led workstream 1 and was involved in the design, conduct, analysis and reporting of all aspects of workstream 1. She also contributed to the review of trial quality in workstream 2.
Karen Lamb (Data Co-ordinator) was involved in the design, conduct and analysis of workstream 1 and was particularly responsible for NHS liaison. She also contributed to the review of trial quality in workstream 2.
Dr Mary Madden (Lecturer in Applied Health) led workstream 2, developed the protocol for participative priority setting and undertook, analysed, interpreted and reported all of the patient, carer and staff interviews in that workstream.
Richard Morley (Project Support Officer) assisted in the design, analysis, reporting and interpretation of the JLAPUP in workstream 2.
Dr Susan O’Meara (Senior Research Fellow in Wounds) led the consultation with NHS staff about evidence synthesis priorities, scoped the review of silver dressings and conducted several reviews in workstream 3. She also contributed to the James Lind Alliance priority-setting process by checking uncertainties against existing evidence and contributed to the review of trial quality in workstream 2. She contributed to the mixed-treatment comparison meta-analyses in workstream 3.
Dr Pedro Saramago Goncalves (Research Fellow) is a health economist and statistician who contributed to the design, conduct, analysis, interpretation and reporting of the mixed-treatment comparison meta-analyses in workstream 3.
Dr Marta Soares (Research Fellow) is a health economist and statistician who contributed to the design, conduct, analysis, interpretation and reporting of the mixed-treatment comparison meta-analyses in workstream 3 and development of iGRADE.
Nikki Stubbs (Clinical Pathway Lead, Leeds Community Wound Prevention and Management Service) was the clinical lead for the entire programme of research and contributed to all aspects of each workstream.
Publications
Briggs M, Nelson EA, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2012;11:CD001177.
Dumville JC, Soares MO, O’Meara S, Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia 2012;55:1902–10.
Madden M. Alienating evidence based medicine vs. innovative medical device marketing: a report on the evidence debate at a wounds conference. Soc Sci Med 2012;74:2046–52.
O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11:CD000265.
Dumville J, Deshpande S, O’Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;6:CD009111.
Dumville J, Deshpande S, O’Meara S, Speak K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;8:CD009099.
Dumville J, Hinchliffe R, Cullum N, Game F, Akesson N, Sweeting M. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2013;10:CD010318.
Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev 2013;3:CD003949.
Dumville J, O’Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;6:CD009110.
Dumville J, O’Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;7:CD009101.
Lockyer S, Hodgson R, Dumville JC, Cullum N. ‘Spin’ in wound care research: the reporting and interpretation of randomized controlled trials with statistically non-significant primary outcome results or unspecified primary outcomes. Trials 2013;14:371.
O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2013;12:CD003557.
O’Meara S, Martyn-St James M. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev 2013;4:CD010182.
O’Meara S, Martyn-St James M. Foam dressings for venous leg ulcers. Cochrane Database Syst Rev 2013;5:CD009907.
Hodgson R, Allen R, Broderick E, Bland JM, Dumville JC, Ashby R, et al. Funding source and the quality of reports of chronic wounds trials: 2004 to 2011. Trials 2014;15:19.
Data sharing statement
Data relating to the systematic reviews reported in Chapters 2–4 are contained either in this report or in the relevant Cochrane review (referenced). Please contact the corresponding author for access to other data.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44-5.
- Kaltenthaler E, Whitfield MD, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. J Wound Care 2001;10:530-5. http://dx.doi.org/10.12968/jowc.2001.10.1.26039.
- Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QJM 2004;97:431-7. http://dx.doi.org/10.1093/qjmed/hch075.
- Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg 1998;176:5-10. http://dx.doi.org/10.1016/S0002-9610(98)00181-0.
- Hunt DL. Diabetes: foot ulcers and amputations. BMJ Clin Evid 2011;2011.
- Soares MO, Bojke L, Dumville J, Iglesias C, Cullum N, Claxton K. Methods to elicit experts’ beliefs over uncertain quantities: application to a cost-effectiveness transition model of negative pressure wound therapy for severe pressure ulceration. Stat Med 2011;30:2363-80. http://dx.doi.org/10.1002/sim.4288.
- Soares MO, Dumville JC, Ashby RL, Iglesias CP, Bojke L, Adderley U, et al. Methods to assess cost-effectiveness and value of further research when data are sparse: negative-pressure wound therapy for severe pressure ulcers. Med Decis Making 2012;33:415-36. http://dx.doi.org/10.1177/0272989X12451058.
- Health and Social Care Information Centre . Prescription Cost Analysis, England (2012) n.d. www.hscic.gov.uk/searchcatalogue?productid=11412&topics=0%2fPrescribing&sort=Relevance&size=10&page=3#top (accessed December 2013).
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health-related quality of life in hospital inpatients with pressure ulceration: assessment using generic health-related quality of life measures. Wound Repair Regen 2009;17:797-805. http://dx.doi.org/10.1111/j.1524-475X.2009.00544.x.
- Jull A, Parag V, Walker N, Rodgers A. Responsiveness of generic and disease-specific health-related quality of life instruments to venous ulcer healing. Wound Repair Regen 2010;18:26-30. http://dx.doi.org/10.1111/j.1524-475X.2009.00556.x.
- Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705-18. http://dx.doi.org/10.1007/s11136-005-2751-9.
- De Meneses LC, Blanes L, Francescato Veiga D, Carvalho Gomes H, Masako Ferreira L. Health-related quality of life and self-esteem in patients with diabetic foot ulcers: results of a cross-sectional comparative study. Ostomy Wound Manage 2011;57:36-43.
- National Institute for Health and Care Excellence, Royal College of Nursing . Pressure Ulcers: The Management of Pressure Ulcers in Primary and Secondary Care n.d. www.nice.org.uk/guidance/cg179 (accessed June 2013).
- Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001;323:334-6. http://dx.doi.org/10.1136/bmj.323.7308.334.
- Scottish Intercollegiate Guidelines Network . SIGN Guideline 120: Management of Chronic Venous Leg Ulcers 2010. www.sign.ac.uk/guidelines/fulltext/120/contents.html (accessed March 2012).
- National Institute for Health and Care Excellence . Type 2 Diabetes Foot Problems: Prevention and Management of Foot Problems 2004. publications.nice.org.uk/type-2-diabetes-foot-problems-cg10/full-guideline (accessed September 2012).
- National Institute for Health Research . Research n.d. www.nihr.ac.uk/research/Pages/default.aspx (accessed December 2013).
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8290.
- Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al. VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13550.
- Watson JM, Kang’ombe AR, Soares MO, Chuang L-H, Worthy G, Bland JM, et al. VenUS III: a randomised controlled trial of therapeutic ultrasound in the management of venous leg ulcers. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15130.
- Ashby RL, Dumville JC, Soares MO, McGinnis E, Stubbs N, Torgerson DJ, et al. A pilot randomised controlled trial of negative pressure wound therapy to treat grade III/IV pressure ulcers (ISRCTN69032034). Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-119.
- Office for National Statistics . 2011 Census for England and Wales 2011. www.ons.gov.uk/ons/guide-method/census/2011/index.html (accessed November 2013).
- Leeds City Council . State of the City 2012 n.d. www.westyorkshireobservatory.org/resource/view?resourceId=2661 (accessed 9 December 2013).
- Porta MS. International Epidemiological Association. A Dictionary of Epidemiology. Oxford: Oxford University Press; 2008.
- Shamliyan TA, Kane RL, Ansari MT, Raman G, Berkman ND, Grant M, et al. Development quality criteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: pilot study of new checklists. J Clin Epidemiol 2011;64:637-57. http://dx.doi.org/10.1016/j.jclinepi.2010.08.006.
- Gupta N, Gupta SK, Shukla VK, Singh SP. An Indian community-based epidemiological study of wounds. J Wound Care 2004;13:323-5. http://dx.doi.org/10.12968/jowc.2004.13.8.26657.
- Lindholm C, Bergsten A, Berglund E. Chronic wounds and nursing care. J Wound Care 1999;8:5-10. http://dx.doi.org/10.12968/jowc.1999.8.1.25828.
- McDermott-Scales L, Cowman S, Gethin G. Prevalence of wounds in a community care setting in Ireland. J Wound Care 2009;18:405-17. http://dx.doi.org/10.12968/jowc.2009.18.10.44602.
- Pieper B, Templin TN, Dobal M, Jacox A. Wound prevalence, types, and treatments in home care. Adv Wound Care 1999;12:117-26.
- Rodrigues I, Mégie M-F. Prevalence of chronic wounds in Quebec home care: an exploratory study. Ostomy Wound Manage 2006;52:46-8.
- Srinivasaiah N, Dugdall H, Barrett S, Drew PJ. A point prevalence survey of wounds in north-east England. J Wound Care 2007;16:413-16. http://dx.doi.org/10.12968/jowc.2007.16.10.27910.
- Vowden KR, Vowden P. A survey of wound care provision within one English health care district. J Tissue Viability 2009;18:2-6. http://dx.doi.org/10.1016/j.jtv.2008.11.003.
- Vowden KR, Vowden P. The prevalence, management, equipment provision and outcome for patients with pressure ulceration identified in a wound care survey within one English health care district. J Tissue Viability 2009;18:20-6. http://dx.doi.org/10.1016/j.jtv.2008.11.001.
- Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability 2009;18:13-9. http://dx.doi.org/10.1016/j.jtv.2008.11.002.
- Vowden K, Vowden P, Posnett J. The resource costs of wound care in Bradford and Airedale primary care trust in the UK. J Wound Care 2009;18:93-4. http://dx.doi.org/10.12968/jowc.2009.18.3.39814.
- Baker SR, Stacey MC, Jopp-McKay AG, Hoskin SE, Thompson PJ. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864-7. http://dx.doi.org/10.1002/bjs.1800780729.
- Baker SR, Stacey MC. Epidemiology of chronic leg ulcers in Australia. Aust N Z J Surg 1994;64:258-61. http://dx.doi.org/10.1111/j.1445-2197.1994.tb02196.x.
- Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J Clin Res Ed 1985;290:1855-6. http://dx.doi.org/10.1136/bmj.290.6485.1855.
- Clarke-Moloney M, Keane N, Kavanagh E. An exploration of current leg ulcer management practices in an Irish community setting. J Wound Care 2006;15:407-10. http://dx.doi.org/10.12968/jowc.2006.15.9.26963.
- Clarke-Moloney M, Keane N, Kavanagh E. Changes in leg ulcer management practice following training in an Irish community setting. J Wound Care 2008;17. http://dx.doi.org/10.12968/jowc.2008.17.3.28669.
- Cornwall JV, Doré CJ, Lewis JD. Leg ulcers: epidemiology and aetiology. Br J Surg 1986;73:693-6. http://dx.doi.org/10.1002/bjs.1800730905.
- Forssgren A, Fransson I, Nelzén O. Leg ulcer point prevalence can be decreased by broad-scale intervention: a follow-up cross-sectional study of a defined geographical population. Acta Derm Venereol 2008;88:252-6.
- Franks PJ, Morton N, Campbell A, Moffatt CJ. Leg ulceration and ethnicity: a study in west London. Public Health 1997;111:327-9. http://dx.doi.org/10.1016/s0033-3506(97)00063-2.
- Freak L, Simon D, Kinsella A, McCollum C, Walsh J, Lane C. Leg ulcer care: an audit of cost-effectiveness. Health Trends 1995;27:133-6.
- Johnson M. The prevalence of leg ulcers in older people: implications for community nursing. Public Health Nurs 1995;12:269-75. http://dx.doi.org/10.1111/j.1525-1446.1995.tb00147.x.
- Lees TA, Lambert D. Prevalence of lower limb ulceration in an urban health district. Br J Surg 1992;79:1032-4. http://dx.doi.org/10.1002/bjs.1800791015.
- Marklund B, Sülau T, Lindholm C. Prevalence of non-healed and healed chronic leg ulcers in an elderly rural population. Scand J Prim Health Care 2000;18:58-60. http://dx.doi.org/10.1080/02813430050202587.
- O’Brien JF, Grace PA, Perry IJ, Burke PE. Prevalence and aetiology of leg ulcers in Ireland. Ir J Med Sci 2000;169:110-12. http://dx.doi.org/10.1007/BF03166911.
- Pina E, Furtado K, Franks PJ, Moffatt CJ. Leg ulceration in Portugal: prevalence and clinical history. Eur J Vasc Endovasc Surg 2005;29:549-53. http://dx.doi.org/10.1016/j.ejvs.2005.01.026.
- Walker NK, Vandal AC, Holden JK, Rodgers A, Birchall N, Norton R, et al. Does capture–recapture analysis provide more reliable estimates of the incidence and prevalence of leg ulcers in the community?. Aust N Z J Public Health 2002;26:451-5. http://dx.doi.org/10.1111/j.1467-842X.2002.tb00346.x.
- Walker N, Rodgers A, Birchall N, Norton R, MacMahon S. The occurrence of leg ulcers in Auckland: results of a population-based study. N Z Med J 2002;115:159-62.
- Jull A, Walker N, Hackett M, Jones M, Rodgers A, Birchall N, et al. Leg ulceration and perceived health: a population based case–control study. Age Ageing 2004;33:236-41. http://dx.doi.org/10.1093/ageing/afh087.
- Andersson E, Hansson C, Swanbeck G. Leg and foot ulcer prevalence and investigation of the peripheral arterial and venous circulation in a randomised elderly population. An epidemiological survey and clinical investigation. Acta Derm Venereol 1993;73:57-61.
- Andersson E, Hansson C, Swanbeck G. Leg and foot ulcers. An epidemiological survey. Acta Derm Venereol 1984;64:227-32.
- Dale JJ, Callam MJ, Ruckley CV, Harper DR, Berrey PN. Chronic ulcers of the leg: a study of prevalence in a Scottish community. Health Bull (Edinb) 1983;41:310-14.
- Ebbeskog B, Lindholm C, Ohman S. Leg and foot ulcer patients. Epidemiology and nursing care in an urban population in south Stockholm, Sweden. Scand J Prim Health Care 1996;14:238-43. http://dx.doi.org/10.3109/02813439608997091.
- Harrison MB, Graham ID, Friedberg E, Lorimer K, Vandevelde-Coke S. Regional planning study. Assessing the population with leg and foot ulcers. Can Nurse 2001;97:18-23.
- Lorimer KR, Harrison MB, Graham ID, Friedberg E, Davies B. Assessing venous ulcer population characteristics and practices in a home care community. Ostomy Wound Manage 2003;49:32-4.
- Lindholm C, Bjellerup M, Christensen OB, Zederfeldt B. A demographic survey of leg and foot ulcer patients in a defined population. Acta Derm Venereol 1992;72:227-30.
- Nelzén O, Bergqvist D, Lindhagen A, Hallböök T. Chronic leg ulcers: an underestimated problem in primary health care among elderly patients. J Epidemiol Community Health 1991;45:184-7. http://dx.doi.org/10.1136/jech.45.3.184.
- Nelzén O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg 1994;81:182-7. http://dx.doi.org/10.1002/bjs.1800810206.
- Nelzén O, Bergqvist D, Lindhagen A. The prevalence of chronic lower-limb ulceration has been underestimated: results of a validated population questionnaire. Br J Surg 1996;83:255-8. http://dx.doi.org/10.1002/bjs.1800830235.
- Öien RF, Håkansson A, Ovhed I, Hansen BU. Wound management for 287 patients with chronic leg ulcers demands 12 full-time nurses. Leg ulcer epidemiology and care in a well-defined population in southern Sweden. Scand J Prim Health Care 2000;18:220-5. http://dx.doi.org/10.1080/028134300448788.
- Wong I, Lee D, Thompson D. Lower limb ulcerations in older people in Hong Kong. J Clin Nurs 2005;14:118-19. http://dx.doi.org/10.1111/j.1365-2702.2004.00947.x.
- Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ, et al. Foot ulcer risk is lower in South-Asian and African-Caribbean compared with European diabetic patients in the UK: the North-West diabetes foot care study. Diabetes Care 2005;28:1869-75. http://dx.doi.org/10.2337/diacare.28.8.1869.
- Al-Mahroos F, Al-Roomi K. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: a nationwide primary care diabetes clinic-based study. Ann Saudi Med 2007;27:25-31. http://dx.doi.org/10.4103/0256-4947.51536.
- Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, et al. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med J Br Diabet Assoc 1994;11:480-4. http://dx.doi.org/10.1111/j.1464-5491.1994.tb00310.x.
- Reid KS, Martin BD, Duerksen F, Nicolle LE, Garrett M, Simonsen JN, et al. Diabetic foot complications in a northern Canadian Aboriginal community. Foot Ankle Int 2006;27:1065-73.
- De Sonnaville JJ, Colly LP, Wijkel D, Heine RJ. The prevalence and determinants of foot ulceration in type II diabetic patients in a primary health care setting. Diabetes Res Clin Pract 1997;35:149-56. http://dx.doi.org/10.1016/S0168-8227(97)01380-6.
- Tseng C-H. Prevalence and risk factors of diabetic foot problems in Taiwan: a cross-sectional survey of non-type 1 diabetic patients from a nationally representative sample. Diabetes Care 2003;26. http://dx.doi.org/10.2337/diacare.26.12.3351.
- Bristow I. Foot ulceration in a non-diabetic population: a cross-sectional audit of staff in one health district. J Wound Care 2008;17:445-8. http://dx.doi.org/10.12968/jowc.2008.17.10.31309.
- Firth J, Hale C, Helliwell P, Hill J, Nelson EA. The prevalence of foot ulceration in patients with rheumatoid arthritis. Arthritis Rheum 2008;59:200-5. http://dx.doi.org/10.1002/art.23335.
- Barbenel JC, Jordan MM, Nicol SM, Clark MO. Incidence of pressure-sores in the Greater Glasgow Health Board area. Lancet 1977;2:548-50. http://dx.doi.org/10.1016/S0140-6736(77)90676-6.
- Barbenel JC, Jordon MM, Nicol SM. Major pressure of sores. Health Soc Serv J 1980;90:1344-5.
- Bergquist S, Frantz R. Pressure ulcers in community-based older adults receiving home health care. Prevalence, incidence, and associated risk factors. Adv Wound Care 1999;12:339-51.
- Hallett A. Managing pressure sores in the community. J Wound Care 1996;5:105-7.
- Inman C, Firth JR. Pressure sore prevalence in the community. Prof Nurse 1998;13:515-20.
- Meehan M, O’Hara L, Morrison YM. Report on the prevalence of skin ulcers in a home health agency population. Adv Wound Care 1999;12:459-67.
- Raghavan P, Raza WA, Ahmed YS, Chamberlain MA. Prevalence of pressure sores in a community sample of spinal injury patients. Clin Rehabil 2003;17:879-84. http://dx.doi.org/10.1191/0269215503cr692oa.
- Torrance C, Maylor M. Pressure sore survey: part one. J Wound Care 1999;8:27-30. http://dx.doi.org/10.12968/jowc.1999.8.1.25829.
- Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64-122.
- Torrance C. Pressure Sores: Aetiology, Treatment and Prevention. London: Groom Helm; 1983.
- Prevention and Treatment of Pressure Ulcers: Clinical Practice Guidelines. Washington, DC: National Pressure Ulcer Advisory Panel; 2009.
- Shea JD. Pressure ulcer classification and management. Clin Orthop 1975;112:89-100. http://dx.doi.org/10.1097/00003086-197510000-00012.
- Waterlow J. Pressure Ulcer Prevention Manual. Taunton: Waterlow; 2005.
- Norton D, McLaren R, Exton-Smith AN. An Investigation of Geriatric Nursing Problems in Hospital. New York, NY: Churchill Livingstone; 1962.
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden scale for predicting pressure sore risk. Nurs Res 1987;36:205-10. http://dx.doi.org/10.1097/00006199-198707000-00002.
- Williams C. A comparative study of pressure sore prevention scores. J Tissue Viability 1992;2:64-6. http://dx.doi.org/10.1016/S0965-206X(14)80165-4.
- Moffatt CJ, Franks PJ, Doherty DC, Smithdale R, Steptoe A. Psychological factors in leg ulceration: a case–control study. Br J Dermatol 2009;161:750-6. http://dx.doi.org/10.1111/j.1365-2133.2009.09211.x.
- Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995;33:AS264-79.
- Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. J Wound Care 2009;18:154-61. http://dx.doi.org/10.12968/jowc.2009.18.4.41607.
- NHS . Delivering the NHS Safety Thermometer CQUIN 2013 14. A New Mindset in Patient Safety Improvement 2012. harmfreecare.org/wp-content/uploads/2012/06/NHS-ST-CQUIN-2012.pdf (accessed 17 May 2016).
- Clinical Practice Guideline. The Nursing Management of Patients with Venous Leg Ulcers. Recommendations. London: RCH Publishing; 2006.
- Scottish Intercollegiate Guidelines Network . SIGN Guideline 120: Management of Chronic Venous Leg Ulcers: Summary of Recommendations n.d. www.sign.ac.uk/guidelines/fulltext/120/recommendations.html (accessed December 2013).
- Braden BJ, Maklebust J, Maklebust J. Preventing pressure ulcers with the Braden scale: an update on this easy-to-use tool that assesses a patient’s risk. Am J Nurs 2005;105:70-2. http://dx.doi.org/10.1097/00000446-200506000-00031.
- Waterlow J. A risk assessment card. Nurs Times 1985;81:24-7.
- Drolet BC, Johnson KB. Categorizing the world of registries. J Biomed Inform 2008;41:1009-20. http://dx.doi.org/10.1016/j.jbi.2008.01.009.
- Newton J, Garner S. Disease Registers in England. Oxford: Institute of Health Sciences, University of Oxford; 2002.
- Raftery J, Roderick P, Stevens A. Potential use of routine databases in health technology assessment. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9200.
- Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med 2003;115:627-31. http://dx.doi.org/10.1016/j.amjmed.2003.06.006.
- Kantor J, Margolis DJ. The accuracy of using a wound care specialty clinic database to study diabetic neuropathic foot ulcers. Wound Repair Regen 2000;8:169-73. http://dx.doi.org/10.1046/j.1524-475x.2000.00169.x.
- Golinko MS, Margolis DJ, Tal A, Hoffstad O, Boulton AJM, Brem H. Preliminary development of a diabetic foot ulcer database from a wound electronic medical record: a tool to decrease limb amputations. Wound Repair Regen 2009;17:657-65. http://dx.doi.org/10.1111/j.1524-475X.2009.00527.x.
- Coerper S, Wicke C, Pfeffer F, Köveker G, Becker H-D. Documentation of 7051 chronic wounds using a new computerized system within a network of wound care centers. Arch Surg 2004;139:251-8. http://dx.doi.org/10.1001/archsurg.139.3.251.
- Taylor RJ, Taylor AD, Marcuson RW. A computerised leg ulcer database with facilities for reporting and auditing. J Wound Care 1999;8:34-8. http://dx.doi.org/10.12968/jowc.1999.8.1.25832.
- Öien RF. RUT – a winning concept for both patients and the health care sector. Eur Wound Manage Assoc 2009;2:41-4.
- Öien RF. The Register RUT Can Help in Wound Care. Helsinki: European Wound Management Association; 2009.
- European-Hospital . RUT – A National Quality Register for Ulcer Treatment n.d. www.european-hospital.com/en/article/6136-RUT_-.html (accessed 26 December 2013).
- Lundstrom M. Literature Review on Quality Registries n.d. www.eurequo.org/downloads/EUREQUO.pdf (accessed 26 December 2013).
- White Paper. Saving Lives: Our Healthier Nation. London: The Stationery Office; 1999.
- The Power of Information: Putting All of Us in Control of the Health and Care Information We Need. London: Department of Health; 2012.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Human Rights Act 1998. London: The Stationery Office; 1998.
- NHS Act 2006. London: The Stationery Office; 2006.
- Hodgson R, Allen R, Broderick E, Bland J, Dumville J, Ashby R, et al. Funding source and the quality of reports of chronic wounds trials: 2004 to 2011. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-19.
- The EuroQoL Group . EuroQoL – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. New York, NY: Lippincott-Raven; 1996.
- Dolan P, Gudex C, Kind P. A Social Tariff for EuroQol: Results From a UK General Population Survey. York: University of York, Centre for Health Economics; 1995.
- Ashby RL, Gabe R, Ali S, Adderley U, Bland JM, Cullum NA, et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet 2014;383:871-9. http://dx.doi.org/10.1016/S0140-6736(13)62368-5.
- Petherick ES, Cullum NA, Pickett KE. Investigation of the effect of deprivation on the burden and management of venous leg ulcers: a cohort study using the THIN database. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0058948.
- Equity and Excellence: Liberating the NHS. London: Department of Health; 2010.
- The NHS Outcomes Framework 2012–13. London: Her Majesty’s Stationery Office; 2011.
- Mease PJ, Arnold LM, Crofford LJ, Williams DA, Russell IJ, Humphrey L, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008;59:952-60. http://dx.doi.org/10.1002/art.23826.
- Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-38.
- Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5090.
- Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420-5. http://dx.doi.org/10.1046/j.1523-1747.2002.19629.x.
- Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen 2008;16:19-22. http://dx.doi.org/10.1111/j.1524-475X.2007.00328.x.
- Edsberg L, Wyffels J, Daniel S. Longitudinal study of stage III and stage IV pressure ulcer area and perimeter as healing parameters to predict wound closure. Ostomy Wound Manage 2011;57:50-62.
- Temple R. Are surrogate markers adequate to assess cardiovascular disease drugs?. J Am Med Assoc 1999;282:790-5. http://dx.doi.org/10.1001/jama.282.8.790.
- Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d7995.
- Tallon D, Chard J, Dieppe P. Relation between agendas of the research community and the research consumer. Lancet 2000;355:2037-40. http://dx.doi.org/10.1016/S0140-6736(00)02351-5.
- Griffiths KM, Jorm AF, Christensen H, Medway J, Dear KBG. Research priorities in mental health, part 2: an evaluation of the current research effort against stakeholders’ priorities. Aust N Z J Psychiatry 2002;36:327-39. http://dx.doi.org/10.1046/j.1440-1614.2001.01024.x.
- Chalmers I, Essali A, Rezk E, Crowe S. Is academia meeting the needs of non-academic users of the results of research?. Lancet 2012;380. http://dx.doi.org/10.1016/S0140-6736(13)60219-6.
- NHS Direct . Patient Safety – 10 for 2010: Pressure Ulcers n.d. www.nrls.npsa.nhs.uk/resources/collections/10-for-2010/pressure-ulcers/ (accessed 6 January 2014).
- Gottrup F, Apelqvist J, Price P. European Wound Management Association Patient Outcome Group . Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care 2010;19:237-68. http://dx.doi.org/10.12968/jowc.2010.19.6.48471.
- McIsaac C. Managing wound care outcomes. Ostomy Wound Manage 2005;51:54-6.
- European Wound Management Association . Patient Outcome Group n.d. ewma.org/?id=243 (accessed 3 January 2014).
- Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs 2007;57:494-50. http://dx.doi.org/10.1111/j.1365-2648.2006.04140.x.
- Guest G, MacQueen KM, Namey EE. Applied Thematic Analysis. Thousand Oaks, CA: Sage; 2011.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publishing Company; 1967.
- Kuzel A, Crabtree B, Miller W. Doing Qualitative Research. Newbury Park, CA: Sage; 1992.
- Charmaz K, Scambler G, Scambler S. New Directions in the Sociology of Chronic and Disabling Conditions: Assaults on the Lifeworld. Basingstoke: Palgrave Macmillan; 2010.
- Briggs M, Flemming K. Living with leg ulceration: a synthesis of qualitative research. J Adv Nurs 2007;59:319-28. http://dx.doi.org/10.1111/j.1365-2648.2007.04348.x.
- Corbin J, Strauss A, Roth JA, Conrad P. The Experience and Management of Chronic Illness. Greenwich, CT: JAI Press Limited; 1987.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. http://dx.doi.org/10.1016/S0140-6736(12)60240-2.
- Bower P, Hann M, Rick J, Rowe K, Burt J, Roland M, et al. Multimorbidity and delivery of care for long-term conditions in the English National Health Service: baseline data from a cohort study. J Health Serv Res Policy 2013;18:29-37. http://dx.doi.org/10.1177/1355819613492148.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-9. http://dx.doi.org/10.7326/0003-4819-135-11-200112040-00010.
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5. http://dx.doi.org/10.1136/bmj.39465.451748.AD.
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c365.
- Singh JA, Murphy S, Bhandari M. Trial sample size, but not trial quality, is associated with positive study outcome. J Clin Epidemiol 2010;63:154-62. http://dx.doi.org/10.1016/j.jclinepi.2009.05.007.
- Chan A-W, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005;365:1159-62. http://dx.doi.org/10.1016/S0140-6736(05)71879-1.
- Hui D, Arthur J, Dalal S, Bruera E. Quality of the supportive and palliative oncology literature: a focused analysis on randomized controlled trials. Support Care Cancer 2012;20:1779-85. http://dx.doi.org/10.1007/s00520-011-1275-9.
- Strippoli GFM, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 2004;15:411-19. http://dx.doi.org/10.1097/01.ASN.0000100125.21491.46.
- Danilla S, Wasiak J, Searle S, Arriagada C, Pedreros C, Cleland H, et al. Methodological quality of randomised controlled trials in burns care. A systematic review. Burns 2009;35:956-61. http://dx.doi.org/10.1016/j.burns.2009.04.031.
- Yaphe J, Edman R, Knishkowy B, Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract 2001;18:565-8. http://dx.doi.org/10.1093/fampra/18.6.565.
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2012;12. http://dx.doi.org/10.1002/14651858.mr000033.pub2.
- Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. J Am Med Assoc 1998;279:281-6. http://dx.doi.org/10.1001/jama.279.4.281.
- Mandelkern M. Manufacturer support and outcome. J Clin Psychiatry 1999;60:122-3. http://dx.doi.org/10.4088/JCP.v60n0210a.
- Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, et al. The uncertainty principle and industry-sponsored research. Lancet 2000;356:635-8. http://dx.doi.org/10.1016/S0140-6736(00)02605-2.
- Cho MK, Bero LA. The quality of drug studies published in symposium proceedings. Ann Intern Med 1996;124:485-9. http://dx.doi.org/10.7326/0003-4819-124-5-199603010-00004.
- Jones R, Younie S, Macallister A, Thornton J. A comparison of the scientific quality of publicly and privately funded randomized controlled drug trials. J Eval Clin Pract 2010;16:1322-5. http://dx.doi.org/10.1111/j.1365-2753.2009.01335.x.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 2011. www.cochrane-handbook.org (accessed 15 July 2013).
- Lockyer S, Hodgson R, Dumville JC, Cullum N. ‘Spin’ in wound care research: the reporting and interpretation of randomized controlled trials with statistically non-significant primary outcome results or unspecified primary outcomes. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-371.
- O’Meara SM, Bland JM, Dumville JC, Cullum NA. A systematic review of the performance of instruments designed to measure the dimensions of pressure ulcers. Wound Repair Regen 2012;20:263-76. http://dx.doi.org/10.1111/j.1524-475X.2012.00783.x.
- Berger VW. Does the Prentice criterion validate surrogate endpoints?. Stat Med 2004;23:1571-8. http://dx.doi.org/10.1002/sim.1780.
- Devereaux PJ, Choi PT-L, El-Dika S, Bhandari M, Montori VM, Schünemann HJ, et al. An observational study found that authors of randomized controlled trials frequently use concealment of randomization and blinding, despite the failure to report these methods. J Clin Epidemiol 2004;57:1232-6. http://dx.doi.org/10.1016/j.jclinepi.2004.03.017.
- Hopewell S, Dutton S, Yu L-M, Chan A-W, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c723.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28-55. http://dx.doi.org/10.1016/j.ijsu.2011.10.001.
- Broerse JEW, Zweekhorst MBM, van Rensen AJML, de Haan MJM. Involving burn survivors in agenda setting on burn research: an added value?. Burns 2010;36:217-31. http://dx.doi.org/10.1016/j.burns.2009.04.004.
- Brett J, Staniszewska S, Mockford C, Seers K, Herron-Marx S, Bayliss H. The PIRICOM Study: A Systematic Review of the Conceptualisation, Measurement, Impact and Outcomes of Patients and Public Involvement in Health and Social Care Research. University of Warwick; 2010.
- Viergever RF, Olifson S, Ghaffar A, Terry RF. A checklist for health research priority setting: nine common themes of good practice. Health Res Policy Syst 2010;8:1-9.
- Cowan K, Oliver S. The James Lind Alliance Guidebook n.d. www.jlaguidebook.org/pdfguidebook/guidebook.pdf (accessed 3 January 2014).
- Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7. http://dx.doi.org/10.1186/1472-6947-7-16.
- Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ 2007;26:171-89. http://dx.doi.org/10.1016/j.jhealeco.2006.04.002.
- Moore DC, Hanratty B. Out of sight, out of mind? A review of data available on the health of care home residents in longitudinal and nationally representative cross-sectional studies in the UK and Ireland. Age Ageing 2013;42:798-803. http://dx.doi.org/10.1093/ageing/aft125.
- Madden M, Morley R. Exploring the challenge of health research priority setting in partnership: reflections on the methodology used by the James Lind Alliance Pressure Ulcer Priority Setting Partnership. Res Involve Engage 2016;2. http://dx.doi.org/10.1186/s40900-016-0026-y.
- Madden M. Providing patient centred pressure ulcer care. Nurs Pract 2015;85.
- Ford S. List of 12 Priorities for Pressure Ulcer Study Identified by UK Researchers 2013.
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2011;4. http://dx.doi.org/10.1002/14651858.cd001735.pub4.
- National Institute for Health and Care Excellence . Pressure Ulcers: Prevention and Management n.d. www.nice.org.uk/guidance/CG179/chapter/introduction (accessed 27 June 2014).
- Claxton KP, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. PharmacoEconomics 2006;24:1055-68. http://dx.doi.org/10.2165/00019053-200624110-00003.
- Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11. http://dx.doi.org/10.1002/14651858.cd000265.pub3.
- Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 2002;25:76-97. http://dx.doi.org/10.1177/0163278702025001006.
- Simmonds MC, Higgins JPT, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials 2005;2:209-17. http://dx.doi.org/10.1191/1740774505cn087oa.
- O’Meara S, Tierney J, Cullum N, Bland JM, Franks PJ, Mole T, et al. Four layer bandage compared with short stretch bandage for venous leg ulcers: systematic review and meta-analysis of randomised controlled trials with data from individual patients. BMJ 2009;338:1054-7. http://dx.doi.org/10.1136/bmj.b1344.
- Kent DJ. Effects of a just-in-time educational intervention placed on wound dressing packages: a multicenter randomized controlled trial. J Wound Ostomy Continence Nurs 2010;37:609-14. http://dx.doi.org/10.1097/WON.0b013e3181f1826b.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. http://dx.doi.org/10.1002/sim.3767.
- Li T, Puhan MA, Vedula SS, Singh S, Dickersin K. Ad Hoc Network Meta-analysis Methods Meeting Working Group . Network meta-analysis highly attractive but more methodological research is needed. BMC Med 2011;9. http://dx.doi.org/10.1186/1741-7015-9-79.
- Dumville J, Deshpande S, O’Meara S, Speak K. Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;8. http://dx.doi.org/10.1002/14651858.cd009099.pub3.
- Dumville J, O’Meara S, Deshpande S, Speak K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;7. http://dx.doi.org/10.1002/14651858.cd009101.pub3.
- Dumville J, O’Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;6. http://dx.doi.org/10.1002/14651858.cd009110.pub3.
- Dumville J, Deshpande S, O’Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013;6. http://dx.doi.org/10.1002/14651858.cd009111.pub3.
- Dumville JC, Soares MO, O’Meara S, Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia 2012;55:1902-10. http://dx.doi.org/10.1007/s00125-012-2558-5.
- O’Meara S, Martyn-St James M. Foam dressings for venous leg ulcers. Cochrane Database Syst Rev 2013;5. http://dx.doi.org/10.1002/14651858.cd009907.pub2.
- O’Meara S, Martyn-St James M. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev 2013;4. http://dx.doi.org/10.1002/14651858.cd010182.pub2.
- O’Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2013;12. http://dx.doi.org/10.1002/14651858.cd003557.pub4.
- Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev 2014;6. http://dx.doi.org/10.1002/14651858.cd003216.pub2.
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009;374:1196-208. http://dx.doi.org/10.1016/S0140-6736(09)61460-4.
- Noël B. Surgical treatment of ingrown toenail without matricectomy. Dermatol Surg 2008;34:79-83. http://dx.doi.org/10.1097/00042728-200801000-00013.
- McIntosh CD, Thomson CE. Honey dressing versus paraffin tulle gras following toenail surgery. J Wound Care 2006;15:133-6. http://dx.doi.org/10.12968/jowc.2006.15.3.26877.
- Collins CE, Kershaw J, Brockington S. Effect of nutritional supplements on wound healing in home-nursed elderly: a randomized trial. Nutr Burbank Los Angel Cty Calif 2005;21:147-55. http://dx.doi.org/10.1016/j.nut.2004.10.006.
- Thomas DR. Improving outcome of pressure ulcers with nutritional interventions: a review of the evidence. Nutr Burbank Los Angel Cty Calif 2001;17:121-5. http://dx.doi.org/10.1016/S0899-9007(00)00514-1.
- Augustin M, Brocatti LK, Rustenbach SJ, Schäfer I, Herberger K. Cost-of-illness of leg ulcers in the community. Int Wound J 2014;11:283-92. http://dx.doi.org/10.1111/j.1742-481X.2012.01089.x.
- Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower-limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care 2003;16:305-16. http://dx.doi.org/10.1097/00129334-200311000-00013.
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes 2007;5. http://dx.doi.org/10.1186/1477-7525-5-44.
- Nelzén O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphology 2008;15:143-50.
- Trengove NJ, Stacey MC, McGechie DF, Mata S. Qualitative bacteriology and leg ulcer healing. J Wound Care 1996;5:277-80.
- Davies CE, Hill KE, Newcombe RG, Stephens P, Wilson MJ, Harding KG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair Regen 2007;15:17-22. http://dx.doi.org/10.1111/j.1524-475X.2006.00180.x.
- Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen 2001;9:178-86. http://dx.doi.org/10.1046/j.1524-475x.2001.00178.x.
- Cutting KF, White R. Defined and refined: criteria for identifying wound infection revisited. Br J Community Nurs 2004;9:S6-15. http://dx.doi.org/10.12968/bjcn.2004.9.Sup1.12495.
- White R. An historical overview of the use of silver in wound management. Br J Community Nurs 2001;6:3-8. http://dx.doi.org/10.12968/bjcn.2001.6.Sup1.12619.
- NHS National Prescribing Centre . Evidence-based prescribing of advanced wound dressings for chronic wounds in primary care. MeReC Bull 2010;21:1-7.
- Chambers H, Dumville JC, Cullum N. Silver treatments for leg ulcers: a systematic review. Wound Repair Regen 2007;15:165-73. http://dx.doi.org/10.1111/j.1524-475X.2007.00201.x.
- Lo S-F, Chang C-J, Hu W-Y, Hayter M, Chang Y-T. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs 2009;18:716-28. http://dx.doi.org/10.1111/j.1365-2702.2008.02534.x.
- Vermeulen H, van Hattem JM, Storm-Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.cd005486.pub2.
- Higgins J, Altman D, Sterne J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 2007;36:847-57. http://dx.doi.org/10.1093/ije/dym087.
- Schulz KF. Subverting randomization in controlled trials. JAMA 1995;274:1456-8. http://dx.doi.org/10.1001/jama.1995.03530180050029.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. http://dx.doi.org/10.1001/jama.1995.03520290060030.
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta-analysis. Int J Epidemiol 2005;34:79-87. http://dx.doi.org/10.1093/ije/dyh300.
- Deeks J, Higgins J, Altman D, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages?. Am J Med 2000;109:15-9. http://dx.doi.org/10.1016/S0002-9343(00)00379-X.
- Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163-8. http://dx.doi.org/10.1111/j.1067-1927.2004.012207.x.
- Dimakakos E, Katsenis K, Kalemikerakis J, Arkadopoulos N, Mylonas S, Arapoglou V, et al. Infected venous leg ulcers: management with silver-releasing foam dressing. Wounds Compend Clin Res Pract 2009;21:4-8.
- Jørgensen B, Price P, Andersen KE, Gottrup F, Bech-Thomsen N, Scanlon E, et al. The silver-releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J 2005;2:64-73. http://dx.doi.org/10.1111/j.1742-4801.2005.00084.x.
- Lazareth I, Meaume S, Sigal-Grinberg M, Combemale P, Le Guyadec T, Zagnoli A, et al. The role of a silver releasing lipido-colloid contact layer in venous leg ulcers presenting inflammatory signs suggesting heavy bacterial colonization: results of a randomized controlled study. Wounds Compend Clin Res Pract 2008;20:158-66.
- Meaume S, Vallet D, Morere MN, Téot L. Evaluation of a silver-releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care 2005;14:411-19. http://dx.doi.org/10.12968/jowc.2005.14.9.26835.
- Michaels JA, Campbell B, King B, Palfreyman SJ, Shackley P, Stevenson M. Randomized controlled trial and cost-effectiveness analysis of silver-donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br J Surg 2009;96:1147-56. http://dx.doi.org/10.1002/bjs.6786.
- Münter KC, Beele H, Russell L, Crespi A, Gröchenig E, Basse P, et al. Effect of a sustained silver-releasing dressing on ulcers with delayed healing: the CONTOP study. J Wound Care 2006;15:199-206. http://dx.doi.org/10.12968/jowc.2006.15.5.26909.
- Wunderlich U, Orfanos CE. Treatment of venous ulcera cruris with dry wound dressings. Phase overlapping use of silver impregnated activated charcoal xerodressing. Hautarzt Z Für Dermatol Venerol Verwandte Geb 1991;42:446-50.
- Kingsley A. A proactive approach to wound infection. Nurs Stand 2001;15:50-4. http://dx.doi.org/10.7748/ns2001.04.15.30.50.c3012.
- Position Document of the Australian Wound Management Association: Bacterial Impact on Wound Healing: From Contamination to Infection. West Leederville, WA: Cambridge Media; 2011.
- White RJ, Cutting KF. Critical colonization – the concept under scrutiny. Ostomy Wound Manage 2006;52:50-6.
- Identifying Criteria for Wound Infection: EWMA Position Document. London: EWMA; 2005.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Leaper D, Drake R. Should one size fit all? An overview and critique of the VULCAN study on silver dressings. Int Wound J 2011;8:1-4. http://dx.doi.org/10.1111/j.1742-481X.2010.00766.x.
- Ghersi D, Berlin J, Akie L, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011.
- The PPP Investigators . Design, rational, and baseline characteristics of the Prospective Pravastatin Pooling (PPP) project – a combined analysis of three large-scale randomized trials: Long-term Intervention with Pravastatin in Ischemic Disease (LIPID), Cholesterol and Recurrent Events (CARE), and West of Scotland Coronary Prevention Study (WOSCOPS). Am J Cardiol 1995;76:899-905. http://dx.doi.org/10.1016/S0002-9149(99)80259-8.
- Marty M, Coombes R, Peckitt C, Perez Lopez F, Tres A, Morvan F, et al. Prospective meta-analysis of randomised trials of cyclophosphamide, methotrexate and fluorouracil (CMF) vs. fluorouracil, epirubicin & cyclophosphamide (FEC) chemotherapy in early breast cancer. J Clin Oncol 2004;22.
- Steinbeck KS, Baur LA, Morris AM, Ghersi D. A proposed protocol for the development of a register of trials of weight management of childhood overweight and obesity. Int J Obes (Lond) 2006;30:2-5. http://dx.doi.org/10.1038/sj.ijo.0803169.
- Dumville J, Hinchliffe R, Cullum N, Game F, Akesson N, Sweeting M. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2013;10. http://dx.doi.org/10.1002/14651858.cd010318.
- Briggs M, Nelson EA, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2012;11. http://dx.doi.org/10.1002/14651858.cd001177.pub3.
- Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev 2013;3. http://dx.doi.org/10.1002/14651858.cd003949.pub3.
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Scottish Intercollegiate Guidelines Network . Search Filters 2011. www.sign.ac.uk/methodology/filters.html#random (accessed 3 June 2016).
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. http://dx.doi.org/10.1002/(SICI)1097-0258(19981230)17:24%3C2815::AID-SIM110%3E3.0.CO;2-8.
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles – continuous outcomes. J Clin Epidemiol 2013;66:173-83. http://dx.doi.org/10.1016/j.jclinepi.2012.08.001.
- Schünemann H, Oxman A, Vist G, Higgins J, Deeks J, Glasziou P, et al. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Guyatt G, Oxman A, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. http://dx.doi.org/10.1016/j.jclinepi.2010.04.026.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2010.
- Palfreyman SJ, Nelson EA, Lochiel R, Michaels JA. Dressings for healing venous leg ulcers. Cochrane Database Syst Rev 2014;5. http://dx.doi.org/10.1002/14651858.cd001103.pub3.
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012;3:80-97. http://dx.doi.org/10.1002/jrsm.1037.
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447-59. http://dx.doi.org/10.1198/016214505000001302.
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9260.
- Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. http://dx.doi.org/10.1016/j.jclinepi.2010.03.016.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8.
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0099682.
- Ahroni JH, Boyko EJ, Pecoraro RE. Diabetic foot ulcer healing: extrinsic vs. intrinsic factors. Wounds 1993;5:245-55.
- Baker NR, Creevy J, Baker NR, Harding KG. Proceedings of the 3rd European Conference on Advances in Wound Management. London: Macmillan; 1994.
- Blackman JD, Senseng D, Quinn L, Mazzone T. Clinical evaluation of a semipermeable polymeric membrane dressing for the treatment of chronic diabetic foot ulcers. Diabetes Care 1994;17:322-5. http://dx.doi.org/10.2337/diacare.17.4.322.
- Clever HU, Dreyer M, Cherry GW. 5th European Conference on Advances in Wound Management Proceedings. London: Macmillan Magazines Ltd; 1996.
- Donaghue VM, Chrzan JS, Rosenblum BI, Giurini JM, Habershaw GM, Veves A. Evaluation of a collagen-alginate wound dressing in the management of diabetic foot ulcers. Adv Wound Care 1998;11:114-19.
- Foster AVM, Greenhill MT, Edmonds ME. Comparing two dressings in the treatment of diabetic foot ulcers. J Wound Care 1995;3:224-8.
- Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13540.
- Jensen JL, Seeley J, Gillin B. Diabetic foot ulcerations. A controlled, randomized comparison of two moist wound healing protocols: Carrasyn Hydrogel Wound dressing and wet-to-moist saline gauze. Adv Wound Care 1998;11:1-4.
- Jude EB, Apelqvist J, Spraul M, Martini J. Silver Dressing Study Group . Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non-ischaemic diabetic foot ulcers. Diabet Med 2007;24:280-8. http://dx.doi.org/10.1111/j.1464-5491.2007.02079.x.
- Mazzone T, Blackman JD. Evaluation of a new loaded foam membrane on the healing rate of diabetic foot ulcers. Wound Repair Regen 1993;1.
- Piaggesi A, Baccetti F, Rizzo L, Romanelli M, Navalesi R, Benzi L. Sodium carboxyl-methyl-cellulose dressings in the management of deep ulcerations of diabetic foot. Diabet Med 2001;18:320-4. http://dx.doi.org/10.1046/j.1464-5491.2001.00466.x.
- Roberts GH, Hammad LH, Baker N, Haggan G, Sandemann DD, Mani R. Hydrocellular against non-adherent dressings to treat diabetic foot ulcers – a randomised controlled study. Wound Repair Regen 2001;9.
- Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs. standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822-7. http://dx.doi.org/10.1001/archsurg.137.7.822.
- Vandeputte J, Gryson L. Diabetic foot infection controlled by immuno-modulating hydrogel containing 65% glycerine. Presentation of a clinical trial. J Wound Care 1996;5:50-3.
- D’Hemecourt PA, Smiell JM, Karim MR. Sodium carboxymethyl cellulose aqueous-based gel vs. becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds 1998;10:69-75.
- Soares MO, Dumville JC, Ades AE, Welton NJ. Treatment comparisons for decision making: facing the problems of sparse and few data. R Stat Soc Ser A Stat Soc 2014;177:259-79. http://dx.doi.org/10.1111/rssa.12010.
- Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol 2013;13. http://dx.doi.org/10.1186/1471-2288-13-35.
- Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G. Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. Int J Epidemiol 2013;42:1120-31. http://dx.doi.org/10.1093/ije/dyt074.
- Jull A, Walker N, Parag V, Molan P, Rodgers A. Honey as Adjuvant Leg Ulcer Therapy trial collaborators . Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br J Surg 2008;95:175-82. http://dx.doi.org/10.1002/bjs.6059.
- Harding K, Gottrup F, Jawień A, Mikosiński J, Twardowska-Saucha K, Kaczmarek S, et al. A prospective, multi-centre, randomised, open label, parallel, comparative study to evaluate effects of AQUACEL® Ag and Urgotul® Silver dressing on healing of chronic venous leg ulcers. Int Wound J 2012;9:285-94. http://dx.doi.org/10.1111/j.1742-481X.2011.00881.x.
- Wild T, Eberlein T, Andriessen A. Wound cleansing efficacy of two cellulose-based dressings. Wounds UK 2010;3:14-21.
- Backhouse CM, Blair SD, Savage AP, Walton J, McCollum CN. Controlled trial of occlusive dressings in healing chronic venous ulcers. Br J Surg 1987;74:626-7. http://dx.doi.org/10.1002/bjs.1800740732.
- Blair SD, Backhouse CM, Wright DDI, Riddle E, McCollum CN. Do dressings influence the healing of chronic venous ulcers?. Phlebology 1988;3:129-34.
- Nelson EA, Prescott RJ, Harper DR, Gibson B, Brown D, Ruckley CV. A factorial, randomized trial of pentoxifylline or placebo, four-layer or single-layer compression, and knitted viscose or hydrocolloid dressings for venous ulcers. J Vasc Surg 2007;45:134-41. http://dx.doi.org/10.1016/j.jvs.2006.09.043.
- Moffatt CJ, Franks PJ, Oldroyd MI, Greenhalgh RM. Randomized trial of an occlusive dressing in the treatment of chronic non-healing leg ulcers. Phlebology 1992;7:105-7.
- Callam MJ, Harper DR, Dale JJ, Brown D, Gibson B, Prescott RJ, et al. Lothian and Forth Valley Leg Ulcer Healing Trial, part 2: knitted viscose dressing versus a hydrocellular dressing in the treatment of chronic leg ulceration. Phlebology 1992;7:142-5.
- Moffatt CJ, Oldroyd M, Franks PJ. Assessing a calcium alginate dressing for venous ulcers of the leg. J Wound Care 1992;4:22-4.
- Bowszyc J, Bowszyc-Dmochowska M, Kazmierowski M, Ben-Amer HM, Garbowska T, Harding E. Comparison of two dressings in the treatment of venous leg ulcers. J Wound Care 1995;4:106-10.
- Charles H, Callicot C, Mathurin D, Ballard K, Hart J. Randomised, comparative study of three primary dressings for the treatment of venous ulcers. Br J Community Nurs 2002;7:48-54. http://dx.doi.org/10.12968/bjcn.2002.7.Sup1.12965.
- Thomas S, Banks V, Bale S, Fear-Price M, Hagelstein S, Harding KG, et al. A comparison of two dressings in the management of chronic wounds. J Wound Care 1997;6:383-6.
- Vanscheidt W, Sibbald RG, Eager CA. Comparing a foam composite to a hydrocellular foam dressing in the management of venous leg ulcers: a controlled clinical study. Ostomy Wound Manage 2004;50:42-55.
- Zuccarelli F. A comparative study of the hydrocellular dressing Allevyn and the hydrocolloid dressing Duoderm in the local treatment of leg ulcers. Phlébologie 1992;45:529-33.
- Arnold TE, Stanley JC, Fellows EP, Moncada GA, Allen R, Hutchinson JJ, et al. Prospective, multicenter study of managing lower extremity venous ulcers. Ann Vasc Surg 1994;8:356-62. http://dx.doi.org/10.1007/BF02132997.
- Mulder G, Jensen J, Seeley S. Use of hydrocellular dressing versus a calcium alginate in the treatment of venous leg ulcers. Wound Repair Regen 1995;3.
- Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11:335-41. http://dx.doi.org/10.12968/jowc.2002.11.9.26438.
- Taddeucci P, Pianigiani E, Colletta V, Torasso F, Andreassi L, Andreassi A. An evaluation of Hyalofill-F plus compression bandaging in the treatment of chronic venous ulcers. J Wound Care 2004;13:202-4. http://dx.doi.org/10.12968/jowc.2004.13.5.26613.
- Schmutz J-L, Meaume S, Fays S, Ourabah Z, Guillot B, Thirion V, et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. Int Wound J 2008;5:172-82. http://dx.doi.org/10.1111/j.1742-481X.2008.00453.x.
- Hansson C. The effects of cadexomer iodine paste in the treatment of venous leg ulcers compared with hydrocolloid dressing and paraffin gauze dressing. Cadexomer Iodine Study Group. Int J Dermatol 1998;37:390-6. http://dx.doi.org/10.1046/j.1365-4362.1998.00415.x.
- Coull AF, Atherton I, Taylor A, Watterson AE. Prevalence of skin problems and leg ulceration in a sample of young injecting drug users. Harm Reduct J 2014;11. http://dx.doi.org/10.1186/1477-7517-11-22.
- Jull AB, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2012;12. http://dx.doi.org/10.1002/14651858.cd001733.pub3.
- Kurd SK, Hoffstad OJ, Bilker WB, Margolis DJ. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen 2009;17:318-25. http://dx.doi.org/10.1111/j.1524-475X.2009.00487.x.
- Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332:1413-15. http://dx.doi.org/10.1136/bmj.38849.478299.7C.
- Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M, Glick HA, Thom SR, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care 2013;36:1961-6. http://dx.doi.org/10.2337/dc12-2160.
- National Institute for Health Research . Surgical Wounds Healing by Secondary Intention: Characterising and Quantifying the Problem and Identifying Effective Treatments n.d. www.nihr.ac.uk/funding/funded-research/funded-research.htm?postid=2194 (accessed 4 August 2016).
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.e5595.
- Steyerberg EW, Moons KGM, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLOS Med 2013;10. http://dx.doi.org/10.1371/journal.pmed.1001381.
- Podiatry Rheumatic Care Association . Standards of Care for People With Musculoskeletal Foot Health Problems 2008. www.prcassoc.org.uk/standards-project (accessed 21 November 2013).
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd003556.pub2.
- Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10120.
- Foley GB, Allen J. Wound healing after toenail avulsion. A comparison of Kaltostat and Melolin as postoperative dressings. Foot 1994;4:88-91. http://dx.doi.org/10.1016/0958-2592(94)90035-3.
- Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with diabetes have osteomyelitis of the lower extremity?. JAMA 2008;299:806-13. http://dx.doi.org/10.1001/jama.299.7.806.
- Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis 2008;47:519-27. http://dx.doi.org/10.1086/590011.
- Eckman MH, Greenfield S, Mackey WC, Wong JB, Kaplan S, Sullivan L, et al. Foot infections in diabetic patients. Decision and cost-effectiveness analyses. JAMA 1995;273:712-20. http://dx.doi.org/10.1001/jama.1995.03520330042035.
- Kapoor A, Page S, Lavalley M, Gale DR, Felson DT. Magnetic resonance imaging for diagnosing foot osteomyelitis: a meta-analysis. Arch Intern Med 2007;167:125-32. http://dx.doi.org/10.1001/archinte.167.2.125.
- Morrison WB, Schweitzer ME, Wapner KL, Hecht PJ, Gannon FH, Behm WR. Osteomyelitis in feet of diabetics: clinical accuracy, surgical utility, and cost-effectiveness of MR imaging. Radiology 1995;196:557-64. http://dx.doi.org/10.1148/radiology.196.2.7617877.
- LeukoScan. For Use in Diagnostic Imaging of the Long Bones and Feet in Patients with Suspected Osteomyelitis, Including those with Diabetic Foot Ulcers. Canberra, ACT: MSAC; 2003.
- Pakos EE, Koumoullis HD, Koumoulis HD, Fotopoulos AD, Ioannidis JPA. Osteomyelitis: antigranulocyte scintigraphy with 99mTC radiolabeled monoclonal antibodies for diagnosis – meta-analysis. Radiology 2007;245:732-41. http://dx.doi.org/10.1148/radiol.2452061877.
- Termaat MF, Raijmakers PGHM, Scholten HJ, Bakker FC, Patka P, Haarman HJTM. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am 2005;87:2464-71. http://dx.doi.org/10.2106/JBJS.D.02691.
- Wrobel JS, Connolly JE. Making the diagnosis of osteomyelitis. The role of prevalence. J Am Podiatr Med Assoc 1998;88:337-43. http://dx.doi.org/10.7547/87507315-88-7-337.
- Carter MJ, Tingley-Kelley K, Warriner RA. Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: a systematic review and meta-analysis. J Am Acad Dermatol 2010;63:668-79. http://dx.doi.org/10.1016/j.jaad.2009.09.007.
- Gilsdorf P, Patterson R, Fisher S. Thirty-minute continuous sitting force measurements with different support surfaces in the spinal cord injured and able-bodied. J Rehabil Res Dev 1991;28:33-8. http://dx.doi.org/10.1682/JRRD.1991.10.0033.
- Al-Khamis A, McCallum I, King PM, Bruce J. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd006213.pub3.
- Bergin S, Ross L, Thomas S, Royle P, Waugh N. Protease modulating dressings for treatment diabetic foot ulcers (protocol). Cochrane Database Syst Rev 2005;3.
- Lázaro-Martínez JL, García-Morales E, Beneit-Montesinos JV, Martínez-de-Jesús FR, Aragón-Sánchez FJ. Randomized comparative trial of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers. Cir Esp 2007;82:27-31. http://dx.doi.org/10.1016/S0009-739X(07)71657-3.
- Kakagia DD, Kazakos KJ, Xarchas KC, Karanikas M, Georgiadis GS, Tripsiannis G, et al. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Complications 2007;21:387-91. http://dx.doi.org/10.1016/j.jdiacomp.2007.03.006.
- Dunford C. Hypergranulation tissue. J Wound Care 1999;8:506-7. http://dx.doi.org/10.12968/jowc.1999.8.10.26222.
- Hampton S. Understanding overgranulation in tissue viability practice. Br J Community Nurs 2007;12:S24-30. http://dx.doi.org/10.12968/bjcn.2007.12.Sup4.43000.
- Nelson L. Wound care. Points of friction. Nurs Times 1999;95.
- Young T. Common problems in wound care: overgranulation. Br J Nurs 1995;4:169-70. http://dx.doi.org/10.12968/bjon.1995.4.3.169.
- Dovison R, Keenan AM. Wound healing and infection in nail matrix phenolization wounds. Does topical medication make a difference?. J Am Podiatr Med Assoc 2001;91:230-3. http://dx.doi.org/10.7547/87507315-91-5-230.
- Harris A, Rolstad BS. Hypergranulation tissue: a nontraumatic method of management. Ostomy Wound Manage 1994;40:20-2.
- Hofman D, Moore K, Cooper R, Eagle M, Cooper S. Use of topical corticosteroids on chronic leg ulcers. J Wound Care 2007;16:227-30. http://dx.doi.org/10.12968/jowc.2007.16.5.27047.
- Wang SQ, Goldberg LH. Pulsed dye laser for the treatment of hypergranulation tissue with chronic ulcer in postsurgical defects. J Drugs Dermatol 2007;6:1191-4.
- Russell L. The importance of patients’ nutritional status in wound healing. Br J Nurs 2001;10. http://dx.doi.org/10.12968/bjon.2001.10.sup1.5336.
Appendix 1 Prevalence survey proforma
Appendix 2 Prevalence of complex wounds by age and sex: prevalence survey data
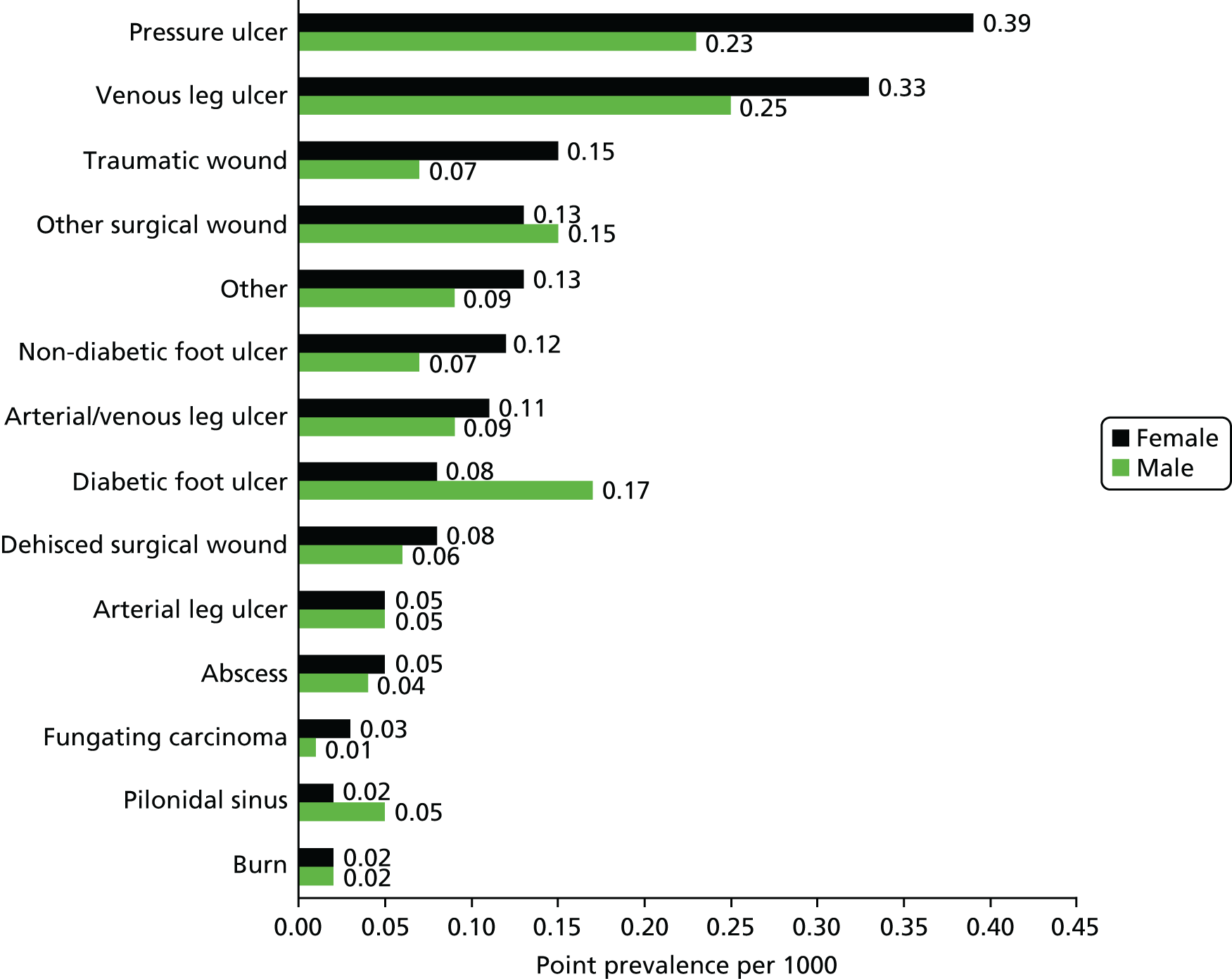
Mean age associated with individual wound types
| Wound type | Age (years), mean (SD) |
|---|---|
| Arterial/venous leg ulcer | 79.65 (11.32) |
| Arterial leg ulcer | 79.11 (13.79) |
| Non-diabetic foot ulcer | 78.89 (13.32) |
| Fungating carcinoma | 75.00 (17.69) |
| Pressure ulcer | 74.79 (14.51) |
| Traumatic wounds | 73.58 (22.10) |
| Diabetic foot ulcer | 70.68 (14.40) |
| Venous leg ulcer | 70.61 (17.10) |
| Other wounds | 70.48 (19.62) |
| Dehisced surgical wounds | 59.96 (17.72) |
| Burns | 58.07 (22.98) |
| Pilondial sinus | 37.48 (20.33) |
Appendix 3 Medical data registries of chronic diseases: review protocol
Background
One component of the NIHR programme grant is to assess the utility, feasibility and sustainability of a chronic wounds registry. We are not aware of any UK registries of chronic wound care; however, information from registries of other chronic diseases may prove insightful in the development of such a wounds registry and how data collection can be sustained and may provide insight into how the data generated from a registry can be used.
Objective
The objective is to identify, appraise and summarise reports of chronic disease registries.
Aims
Specific aims are:
-
to identify chronic diseases (and countries) in which a registry has been utilised
-
to identify the methods of data collection used
-
to summarise the uses of registry data
-
to describe how the registry (and data collection related to it) integrates with other data collection systems in the organisation
-
to examine the configuration of registries across multiple specialty teams or across health-care interfaces, for example primary and secondary care.
Methods
A scoping review/information gathering exercise will be undertaken.
Inclusion criteria
Any paper reporting a chronic disease registry will be included.
A chronic disease will be defined as a long-lasting or recurrent condition. Registries relating to surgical procedures will be excluded.
A medical registry will be defined as a database that meets the six characteristics that defined a medical database registry compiled by Drolet and Johnson:97
-
Mergeable data. Data stored in a format that allows a user/researcher to create a single aggregate and searchable data set for research and patient care purposes.
-
Data set standardised. The same data are collected for all patients/records in a registry.
-
Rules for data collection. A set of characteristics are defined prior to the collection of data. These data are collected in a systematic and prospective manner.
-
Observations associated over time. Database is designed so that each patient is identified in the registry as a single continuous record for storage of longitudinal data.
-
Knowledge of outcomes. Follow-up must be obtained to assess outcomes or manage patient care.
-
Inclusion principle. The characteristic that is common to all patients in a disease register, that is, disease.
General discussion articles about the design and implementation of disease registries will also be included for background information but data will not be extracted.
Search methods
Using OvidSP an electronic search on MEDLINE will be undertaken using the following search strategy:
-
exp Chronic Disease/ (182,090)
-
(chronic adj (illness$or disease$)).tw. (25,675)
-
1 or 2 (199,178)
-
exp Registries/ (35,210)
-
(registr$or register$1).tw. (77,603)
-
4 or 5 (92,049)
-
6 and 3 (1409)
Relevant information will also be gathered from other sources (known to the authors) who are experienced in the development and implementation of disease registries. We will also conduct a bibliographic search of the Drolet and Johnson publication. 97
Screening stage 1
All study titles and abstract (when available) will be reviewed. Full papers will be obtained when the title and/or abstracts suggests:
-
a review of a medical registries
-
a study in which a medical data registry relating to a chronic condition is discussed or explicitly used as a source of data.
This process will be carried out by two reviewers. Any disagreement will be taken to a third, senior reviewer.
Screening stage 2
Full articles will then be screened against the criteria of Drolet and Johnson97 and classed as medical data registries or not. When a paper provides very limited information, that is, a registry is mentioned only briefly as a source of data and does not provide enough data to make a decision using this criterion, we will follow the methods of Drolet and Johnson97 and search for further information on MEDLINE and the internet. We will also contact authors. When no further useful information can be obtained the study will be excluded for further data extraction. Again, all phases will be conducted by two reviewers.
Data collection
Medical registries will be extracted into a database according to predefined criteria. Studies describing the same registries will be grouped and extracted together so that each registry has one extraction record.
When possible, extracted data will include:
-
general information:
-
country
-
location
-
type of condition
-
duration of registry
-
-
data collection:
-
methods of data collection
-
summary of data collected
-
details of how registry data collection relates to data collection systems in the organisation, that is, routinely collected data
-
information on IT systems and storage of data
-
methods of data quality assurance
-
details of how data from multiple sources (i.e. health-care interfaces) is collected and collated
-
-
data use:
-
detail any outputs using registry data.
-
Analysis
Descriptive variables will be presented and a critical narrative report will be undertaken.
Appendix 4 Wound assessment form
Appendix 5 James Lind Alliance Pressure Ulcer Partnership voting sheet
Appendix 6 James Lind Alliance Pressure Ulcer Partnership pre-workshop questionnaire
Appendix 7 Collation and scoping of research ideas for workstream 3
Research questions relating to foot ulcers and other foot conditions
| Research question (origin) | Relevant literature and comments | Actions taken |
|---|---|---|
| What is the best package of care for people with non-diabetic foot ulcers? (Podiatry team) | A document outlining standards of care for people with musculoskeletal and rheumatological foot health problems was identified.308 This includes recommendations for the care of patients with inflammatory arthritis, osteoarthritis, back pain, metabolic bone disease and connective tissue disease. There are also sections covering the multidisciplinary team and suggestions for improving access to services, good practice examples and implementation materials including audit and educational resources | The podiatry team found this document useful for confirming standards of care for people with musculoskeletal foot disease and did not need to implement any changes to practice in relation to that patient group. The best strategy for managing non-diabetic patients with foot ischaemia, particularly those at risk of ulceration or amputation, remains uncertain |
| What is the impact of the time lag involved in obtaining the dressing, antibiotic or other treatment that the podiatrist feels is clinically indicated? (e.g. a time lag may ensue between identification of potential clinical infection and dispensing of antimicrobial dressings or systemic antibiotics if the podiatrist needs to refer the patient to the GP for prescription) (Podiatry team) | No literature was identified on different prescribing policies in relation to foot ulcers. This is a service delivery organisational issue rather than prescribing | None, as outside the remit of the programme grant |
| What is the relationship between debridement and healing in all types of foot ulcers? (Podiatry team) | One good-quality, up-to-date systematic review in diabetic foot ulcers was identified.309 Six RCTs were included (four evaluating hydrogel, one surgical debridement and one larval therapy). Conclusion: there is evidence to suggest that hydrogel increases the healing rate compared with gauze dressing/standard care and larval therapy resulted in a significantly greater reduction in wound area than hydrogel. More research is needed to evaluate the relative effects of all available debridement methods (including sharp debridement) and the effects of debridement per se. Frequency of the procedure was not addressed in any of the primary studies. No relevant references were found for non-diabetic foot ulceration | The podiatry team found this document useful for confirming good practice (i.e. use of hydrogel) in patients with diabetic foot ulcers |
| What is the effectiveness of silver dressing vs. povidone iodine dressing for treating diabetic foot ulcers? (Podiatry team) | At the time of scoping, there was no existing systematic review on dressings for diabetic foot ulcers but between 10 and 20 clinical trials of various dressing types were identified | Comparison of different dressing types for treating diabetic foot ulcers was selected as a priority topic for systematic review and network meta-analysis |
| What are the best strategies for detecting and treating infection in diabetic foot ulcers? (Podiatry team) | Previous systematic reviews of diagnostic and treatment strategies could not draw firm conclusions.310 An ongoing primary study is evaluating methods of diagnosing infection in diabetic foot ulcers [see www.nets.nihr.ac.uk/projects/hta/097501 (accessed 3 June 2016)] | Information was relayed to the Cochrane Wounds Group; this has helped to progress a review of systemic antibiotics, which now has a published protocol. Antimicrobial dressings were included in the systematic review and network meta-analysis of different dressing types for treating diabetic foot ulcers |
| What is the cost-effectiveness of different dressings for healing after nail surgery (silver, povidone iodine, non-adherent)? (Podiatry team) | No systematic reviews were identified. Two relevant RCTs were identified: honey dressing vs. paraffin tulle gras203 and alginate vs. non-adherent dry dressing.311 No economic evaluations were identified | Not selected for systematic review within the programme grant because of the small number of RCTs available |
| What is the best way to diagnose osteomyelitis in patients with diabetic foot ulcers? (Podiatry team) | Several systematic reviews, meta-analyses and economic evaluations were identified312–320 | This topic was undertaken as a MSc dissertation. A range of diagnostic tests was evaluated including probing to bone, erythrocyte sedimentation rate, plain radiographs, magnetic resonance imaging, bone scan and leucocyte scan. Overall, measures of diagnostic performance suggested that probing to bone and magnetic resonance imaging were the tests best able to discriminate between presence and absence of osteomyelitis |
| What is the acceptability of different dressings for those with foot ulcers who self-care? (Podiatry team) | No relevant literature identified | None, as not considered a high priority for the programme grant |
| What is the significance of locus of control issues regarding the patient’s view of his or her own adherence with foot ulcer treatment? [e.g. ‘It doesn’t matter what I do, you (i.e. the podiatrist) have to make it heal’] (Podiatry team) | No relevant literature identified | None, as not considered a high priority for the programme grant |
Research questions relating to leg ulcers
| Research question (origin) | Relevant literature and comments | Actions taken |
|---|---|---|
| What is the effectiveness and cost-effectiveness of breathable foam film dressings (e.g. Allevyn) compared with absorbent dressings used with leg ulcers? (Nursing team) | A systematic review of dressings for venous leg ulcers compared foam dressings with low-adherent dressings (three RCTs), alginate dressings (one RCT) and silicone dressings (one RCT) and did not detect any differences between foam and other types of dressing254 | New systematic review undertaken focusing on foam dressings in venous leg ulcers. All dressing types were included in the systematic review and network meta-analysis of dressings for venous leg ulcers |
| What is the effectiveness of silver-impregnated dressings when used with venous leg ulcers? (Nursing team) | Four relevant systematic reviews were identified.216–218,321 Most findings indicated no evidence to support the use of silver-impregnated dressings in venous leg ulcers | A scoping review of silver dressings used with venous leg ulcers was undertaken to explore the feasibility of IPD meta-analysis. There was no evidence to support the use of silver dressings except for some short-term (at 4 weeks) intermediate outcomes of healing (e.g. change in ulcer area) |
| How does obesity affect healing of leg ulcers? (Nursing team) | A search for prognostic studies investigating obesity as a predictor for delayed healing did not identify anything relevant to leg ulcers | None, because of lack of primary material |
Research questions relating to pressure ulcers
| Research question (origin) | Relevant literature and comments | Actions taken |
|---|---|---|
| What is the best management of underweight patients deemed to be at risk of pressure ulceration in relation to support surfaces? (Nursing team) | No literature identified that specifically targets this patient group | None, because of lack of primary material |
| What are the best ways to measure shear force in wheelchair users in relation to preventing and treating pressure ulcers? (Launch event participants) | One evaluation of measurement methods was identified322 | None – shear force is a surrogate outcome measure and evaluation of different measurement instruments would require methods comparison evaluations. This topic was outside of the scope of this programme grant |
Research questions relating to surgical wounds
| Research question (origin) | Relevant literature and comments | Actions taken |
|---|---|---|
| What is the effectiveness of topical negative pressure compared with conventional dressings such as hydrocolloid or alginates when treating dehisced abdominal wounds? (Nursing team) | No relevant literature identified | None – this is covered by a separate programme grant (see www.york.ac.uk/healthsciences/swhsi/) |
| How does obesity affect healing of dehisced abdominal wounds? (Nursing team) | A search for prognostic studies investigating obesity as a predictor for delayed healing did not identify anything relevant to abdominal wounds | None, because of lack of primary material |
| What are the best strategies for post-operative care of pilonidal sinus? Is it better to pack or not to pack? (Launch event participants) | Cochrane review of healing by primary vs. secondary intention after surgery suggested that both methods have pros and cons (26 RCTs).323 No new RCTs identified | None, because no new primary material |
Research questions relating to all wound types
| Research question (origin) | Relevant literature and comments | Actions taken |
|---|---|---|
| What role should EUSOL have in wound management? (Nursing team) | Findings from an existing systematic review indicated that there are no RCTs evaluating EUSOL in venous leg ulcers.199 No relevant RCTs were identified in other types of complex wounds | None, because of absence of primary material |
| Is direct application of steroids and antifungal preparations to the wound bed effective? (Nursing team) | No systematic reviews or RCTs identified | None, because of lack of primary material |
| What is the role of protease-modulating matrix dressings (e.g. Promogran) in wound management? (Nursing team) | A Cochrane review protocol324 and two clinical trials were identified.325,326 All three studies focus on diabetic foot ulceration | Protease-modulating dressings were included in both network meta-analyses: dressings for diabetic foot ulcers and dressings for venous leg ulcers |
| What is known about overgranulation (also known as hypergranulation)? This is a general question that includes natural history, prevalence, cause, relationship with other diseases (e.g. malignancy), types of wound most likely to be affected and best methods of management (Nursing team) | A small number of non-informative literature was identified including non-systematic topic overviews.327–330 One RCT of nail matrix ablation described hypergranulation as an adverse effect of hydrogel.331 Otherwise, individual case reports and uncontrolled case series were identified332–334 | None, because of lack of informative literature |
| What is the best way to manage wounds in mental health patients who have dementia? (Launch event participants) | No relevant literature identified | None, because no primary material |
| How many of those with complex/chronic wounds also have nutritional risks? Nutritional risks include poor hydration, undernutrition, evidence of 10% unintentional weight loss, poorly controlled diabetes, artificial feeding and obesity. (Community dietitian) | No relevant literature identified except for older, non-systematic topic overviews concluding that nutritional status is likely to be important for healing pressure ulcers205,335 | The wounds prevalence survey collected data on recent weight loss or gain (workstream 1). No further consideration of systematic review because of lack of suitable primary material |
| Do complex wounds have an impact on patients’ ability to shop, prepare, cook or eat all their meals and drink all their drinks throughout the day and hence their overall food and fluid intake, resultant nutritional status and quality of life? (Community dietitian) | No literature identified on whether or not complex wounds impact specifically on patients’ nutritional activities | The impact of complex wounds on quality of life is addressed through the qualitative interviews undertaken as part of workstream 2 |
| Which nutrition interventions aid wound healing? Can data from a wound register tell us about the effectiveness of nutritional interventions? (Community dietitian) | A Cochrane review concluded that there was no clear evidence that adding an oral nutritional supplement to standard diet reduced the incidence of pressure ulceration compared with standard diet alone.200 There were no firm conclusions for the treatment of existing pressure ulcers. The search was carried out in 2002 and the yield from CENTRAL suggested at least five additional recent trials that could be included | The wounds register captured data enabling evaluation of treatment effectiveness, but not specifically in relation to nutritional interventions. A previous review has undergone a substantive update and at the time of writing is in the final stages of editorial review. A new review on nutritional interventions for venous leg ulcers is in progress at the time of writing |
| Could food fortification using dried milk powder promote wound healing? (Community dietitian) | No relevant references found | None because of lack of primary material. Possible candidate topic for future research commissioning |
Appendix 8 Search string used for MEDLINE: silver dressings for venous leg ulcers
-
exp Leg Ulcer/
-
(varicose ulcer$or venous ulcer$or leg ulcer$or stasis ulcer$or (lower extremit$adj ulcer$) or crural ulcer$or ulcus cruris).ti,ab.
-
exp Silver/
-
silver.ti,ab.
-
1 or 2
-
3 or 4
-
5 and 6
Appendix 9 Data extraction tables: silver dressings for venous leg ulcers
| Study, methods and funding | Participants | Interventions | Outcomes |
|---|---|---|---|
| Dimakakos 2009;227 Greece; RCT No information about methods of randomisation, allocation concealment or blinding; healing data analysed by intention to treat Funder not stated |
42 patients with venous leg ulcers (confirmed by Doppler) randomised. All ulcers were infected at baseline. Baseline wound size and duration reported but no mention of primary/recurrent ulceration | Group 1: silver foam dressing (Contreet®, Coloplast) (n = 21; group 2: non-adhesive foam dressing (Biatain®, Coloplast) (n = 21) All patients received short-stretch bandage. Dressings were changed twice weekly. Some patients received antibiotics. Treatment duration was 9 weeks |
Complete healing at 5 weeks: group 1: 8/21 (38%); group 2: 4/21 (19%) Complete healing at 9 weeks: group 1: 17/21 (81%); group 2: 10/21 (48%) Healing also shown at 3, 4, 6, 7 and 8 weeks in a table Data on bacterial load were reported Other outcomes: pain, exudation, adverse events No withdrawals for healing data |
| Jørgensen 2005;228 Canada Denmark, Germany, Italy, The Netherlands, UK, USA; RCT Computer-generated block randomisation used; allocation concealment not described; outcome assessment non-blind; safety analysis was by intention to treat and efficacy analysis was per protocol Funded by Coloplast A/S, Humlebaek, Denmark |
129 patients with moderately or highly exuding venous or mixed venous/arterial leg ulcers were randomised. Minimum ulcer area was 2 cm2. Ulcers were ‘critically colonised’ at baseline (definition provided) but not clinically infected (this was an exclusion criterion). Baseline wound size and duration reported but no information about primary/recurrent ulceration | Group 1: silver-release foam dressing (Contreet) (n = 65); group 2: polyurethane foam dressing (Allevyn; S&N) (n = 64) Compression therapy described as ‘mandatory’ according to clinical practice of study centres (modification of compression for those with mixed disease not described). Some patients received topical steroids to peri-ulcer skin. Dressings and bandages were changed weekly. Treatment duration was 4 weeks |
Complete healing at 4 weeks (per protocol): group 1: 5/52 (10%); group 2: 5/57 (9%) Time to healing not reported Assessment of infection not reported (patients requiring systemic antibiotics were excluded from the per-protocol analysis) Other outcomes: absolute and relative change in ulcer area; odour; exudate; quality of life; dressing performance; adverse events Withdrawals: 20 in total (16%) |
| Lazareth 2008;229 France; RCT Block randomisation was used; allocation concealment by sealed envelopes; outcome assessment not blind but assessments retrospectively checked by blinded investigators; analysis was by intention to treat; population defined as randomised patients with at least one follow-up assessment for wound size Funded by URGO Laboratories, France |
102 patients with venous leg ulcers (ABPI > 0.8) of duration < 24 months and surface area 5–40 cm2 were randomised. In addition, leg ulcers had to have at least three of five clinical signs of ‘high bacterial load’ (pain, erythema, oedema, odour, exudation). At baseline, all patients had clinical signs of high bacterial load but clinical infection and current/recent antibiotic use were exclusion criteria. Baseline wound size, duration and ulcer status (primary or recurrent) were reported | Group 1: silver dressing (non-adhesive, non-occlusive dressing – proprietary name not stated) (n = 52); group 2: dressing as above but not containing silver (n = 50) All patients received compression (details not given). Dressings and bandages were changed every other day or less frequently. Treatment duration for randomised comparison was 4 weeks (all patients received the non-silver dressing between weeks 4 and 8) |
Dichotomous healing data not reported, nor time to healing Main outcome was mean ± SD (median) change in ulcer area at 4 weeks (cm2): group 1: –6.5 ± 13.4 (4.2); group 2: –1.3 ± 9.0 (1.1) (p = 0.023) Other outcomes: healing rate (cm2/day); probability of achieving 40% area reduction; local signs of heavy bacterial colonisation; adverse events Withdrawals at 8 weeks: group 1: 8/52 (15%); group 2: 20/50 (40%) |
| Meaume 2005;230 France; RCT Randomisation stratified according to wound type; allocation concealment not mentioned; outcome assessment not blind; analysis by intention to treat Funded by Johnson & Johnson Wound Management |
101 patients with venous leg ulcers and pressure ulcers were randomised (99 patients analysed). Wounds were colonised but not clinically infected at baseline; recent systemic antibiotic use was an exclusion criterion. Baseline wound area, duration and whether primary or recurrent ulcers were reported | Group 1: silver-releasing hydroalginate dressing (Silvercel®, Systagenix) (n = 51; 38 with leg ulcers and 13 with pressure ulcers); group 2: non-silver calcium alginate dressing (Algosteril®, Smith & Nephew) (n = 48; 33 with leg ulcers and 15 with pressure ulcers) All venous leg ulcer patients received compression. Dressings were changed every few days. Treatment duration was 4 weeks |
Dichotomous healing data not reported, nor time to healing Main outcome was mean ± SD change in ulcer area (cm2) – results for leg ulcers only: group 1: –9.5 ± 17.9; group 2: –6.0 ± 11.7 (p = 0.117) Other outcomes: percentage change in ulcer area; healing rate (cm2/day); use of systemic antibiotics during the trial; number of wounds developing clinical infection; adverse events Withdrawals: group 1: 10/51 (20%); group 2: 9/48 (19%) |
| Michaels 2009;231 UK; pragmatic RCT Computer-generated block randomisation with stratification according to ulcer size and treatment centre; allocation concealment not described; methods of outcome assessment not reported (but unlikely to be blind); analysis was by intention to treat Funded by UK NIHR Health Technology Assessment programme |
213 patients with venous leg ulcers (ABPI ≥ 0.8, wound duration > 6 weeks, maximum wound diameter < 1 cm) were randomised. Recent use of antibiotics was an exclusion criterion. Baseline ulcer size/duration and primary/recurrent ulceration were reported | Group 1: silver-releasing dressing according to clinicians’ choice [most commonly Urgotul SSD (Urgo), Acticoat 7 (S&N), Aquacel Ag (S&N) or Contreet foam] (n = 107); group 2: non-adherent, non-antimicrobial dressing from any manufacturer according to clinicians’ choice (most patients received low-adherence knitted viscose dressings) (n = 106) All patients received multilayer compression (choice of bandage according to local practice). Dressings/bandages were changed weekly or more often if required. Treatment duration was 12 weeks. After 12 weeks the randomised treatment could be changed or stopped, according to the clinicians’ judgement |
Complete healing at 12 weeks (n = 208 analysed): group 1: 62/104 (59.6%); group 2: 59/104 (56.7%) (p = 0.673) Complete healing at 6 months (n = 203 analysed): group 1: 87/102 (85.3%); group 2: 78/101 (77.2%) (p = 0.141) Complete healing at 12 months (n = 193 analysed): group 1: 95/99 (96.0%); group 2: 90/94 (95.7%) (p = 0.940) Median days to healing: group 1: 67 (95% CI 54 to 80); group 2: 58 (95% CI 43 to 73) (p = 0.408) (Cox proportional hazards model) Other outcomes: ulcer recurrence at 6 and 12 months (no difference between groups); adverse events; quality of life (EQ-5D and SF-36); costs; resource use; cost-effectiveness |
| Münter 2006;232 Germany, UK, Denmark, Italy, Switzerland, Belgium, Slovenia, Brazil, Canada; pragmatic RCT Computer-generated block randomisation; sealed envelopes; outcome assessment not blind; analysis by intention to treat (last observation carried forward) Funded by Coloplast A/S, Humlebaek, Denmark |
619 patients with various chronic wounds were randomised (burns, donor sites, surgical, leg ulcers, pressure ulcers, diabetic foot ulcers). Clinical infection of the wound was not an exclusion criterion. Baseline wound area and duration were reported but primary/recurrent ulceration not mentioned | Group 1: sustained silver-releasing foam dressing (Contreet Foam) (n = 326 overall; 218 with venous/mixed leg ulcers); group 2: local best practice (included various alternative dressings including silver) (n = 293 overall; 197 with venous/mixed leg ulcers) Compression given to leg ulcer patients in line with local practice. Dressings and bandages changed at least weekly. Treatment duration was 4 weeks |
No report of dichotomous healing or time to healing Median % reduction in ulcer area for all leg ulcers (n = 415 analysed): group 1: 45.5%; group 2: 28.8% (p = 0.0001); for venous leg ulcers (a subset of the above – n = 297 analysed): group 1: 46.2%; group 2: 26.9% (p = 0.0001) Other outcomes: % slough; wound progress; peri-ulcer skin/maceration; exudate; ease of dressing use; odour; pain; time spent on dressing change; mean wear time of dressing; quality of life Withdrawals not described |
| Wunderlich 1991;233 Germany; RCT Methods of randomisation and allocation concealment not stated; outcome assessment not blind; analysis not by intention to treat Funder not stated |
40 patients with venous leg ulcers were randomised. No mention of ulcer infection status at baseline. Baseline ulcer area and duration were reported; no mention of primary/recurrent ulceration | Group 1: silver-impregnated charcoal dressing (Actisorb Plus; J&J) used for all stages of wound healing (n = 20); group 2: various topical agents used; if wound in granulation phase, mineral oil or povidone iodine/sea salt paste was used, whereas if in epithelialisation phase, paraffin-impregnated gauze or oil and water emulsion was used (n = 20) All patients received debridement initially and throughout the trial as required. No mention of compression. Treatment duration was 6 weeks |
Complete healing at 6 weeks: group 1: 6/19 (32%); group 2: 2/19 (11%). Between-group difference reported as not significant Other outcomes: mean ulcer size at 6 weeks; percentage reduction in ulcer area at 2, 4 and 6 weeks Withdrawals: one patient per group |
Appendix 10 Dressings for foot ulcers in people with diabetes: search strategy
We searched CENTRAL using the following exploded medical subject heading (MeSH) headings and keywords:
#1 MeSH descriptor Occlusive Dressings explode all trees
#2 MeSH descriptor Biological Dressings explode all trees
#3 MeSH descriptor Alginates explode all trees
#4 MeSH descriptor Hydrogels explode all trees
#5 MeSH descriptor Silver explode all trees
#6 MeSH descriptor Honey explode all trees
#7 (dressing* or alginate* or hydrogel* or ‘foam’ or ‘bead’ or ‘film’ or ‘films’ or tulle or gauze or non-adherent or ‘non adherent’ or silver or honey or matrix):ti,ab,kw
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Foot Ulcer explode all trees
#10 MeSH descriptor Diabetic Foot explode all trees
#11 diabet* NEAR/3 ulcer*:ti,ab,kw
#12 diabet* NEAR/3 (foot or feet):ti,ab,kw
#13 diabet* NEAR/3 wound*:ti,ab,kw
#14 (#9 OR #10 OR #11 OR #12 OR #13)
#15 (#8 AND #14)
Appendix 11 Quality assessment of mixed-treatment comparison estimates using iGRADE: comparison with the GRADE tool
| GRADE category | GRADE definition and guidance | iGRADE category | iGRADE definitions and guidance | iGRADE issues |
|---|---|---|---|---|
| Limitations in design | Risk of bias:
|
Limitations in design | Use GRADE limitations in design rating for direct links to assess the mixed-treatment meta-analysis estimates these links clearly contributed to:
|
Qualitative assessment of risk of bias difficult for indirect evidence. When direct and indirect evidence are available this assessment may be subjective |
| Inconsistency | Unexplained heterogeneity of results:
|
Sensitivity of results | Judgement based on the impact of sensitivity analysis on the mixed-treatment meta-analysis network and thus estimates (e.g. removing each trial when there are two or more informing a link or sensitivity to alternative priors in random-effects analysis):
|
Does not address unexplained heterogeneity per se |
| Indirectness | Indirect comparison: if you think the evidence is direct choose no if you have serious doubts about directness choose serious if you have very serious doubts about directness choose very serious |
Indirectness/inconsistency Within GRADE the term ‘inconsistency’ is used to refer to unexplained heterogeneity. Within mixed-treatment meta-analysis inconsistency has a meaning specific to agreement between direct and indirect data. Furthermore, in GRADE the presence of indirectness is taken as a reason to downgrade evidence. However, in the context of a mixed-treatment meta-analysis in which indirect data are expected and which ideally add value, such an approach does not make sense. Thus, we merged these categories resulting in joint assessment of unexplained heterogeneity and/or assessment of inconsistency when possible |
Define the type of data available for each mixed-treatment meta-analysis comparison as follows:
Serious: 2 and 3 Very serious: not applicable |
Assessment of heterogeneity based in direct links is challenging. Cannot always assess for inconsistencies |
| Imprecision | CIs around estimates of treatment effect:
|
Imprecision | Judged by the size of the CrIs around the ORs. As ORs were used to analyse data with a relatively high number of events, a more conservative interval width was used than would have been employed were data presented using RRs:
|
|
| Publication bias | If you think there is no evidence of publication bias choose unlikely If there is high probability of publication bias choose likely If there is very high probability of publication bias choose very likely |
Publication bias | Use GRADE limitations in design rating for direct links to assess the mixed-treatment meta-analysis estimates these links clearly contributed to: Unlikely: GRADE publication bias category recorded as unlikely for links identified as informing the mixed-treatment meta-analysis estimate Likely: GRADE publication bias category recorded as likely for one or more links identified as informing the mixed-treatment meta-analysis estimate and none identified as very likely Very likely: GRADE publication bias category recorded as very likely for one or more links identified as informing the mixed-treatment meta-analysis estimate |
Qualitative assessment of publication bias difficult for indirect evidence. Again, in the presence of both direct and indirect evidence there is the need to consider potential publication bias in the indirect links as well as the direct links informing the same comparison. Yet, assessing potential bias in indirect comparison is complex. If, for example, AC is biased (missing studies) favouring A and BC is biased (missing studies) favouring B, then the AB indirect estimate will be unbiased if the bias in AC is similar to the bias in BC |
Appendix 12 Dressings for foot ulcers in people with diabetes: PRISMA flow chart
Appendix 13 Dressings for venous leg ulcers: search strategy
We searched CENTRAL using the following exploded MeSH headings and keywords:
#1 MeSH descriptor: [Leg Ulcer] explode all trees
#2 (varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or ((lower next extremit*) near/2 ulcer*) or (crural next ulcer*) or ‘ulcus cruris’:ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Bandages, Hydrocolloid] explode all trees
#5 MeSH descriptor: [Hydrogels] explode all trees
#6 MeSH descriptor: [Alginates] explode all trees
#7 (dressing* or hydrocolloid* or alginate* or hydrogel* or ‘foam’ or ‘bead’ or ‘film’ or ‘films’ or tulle or gauze or non-adherent or ‘non adherent’):ti,ab,kw
#8 MeSH descriptor: [Cellulose, Oxidised] explode all trees
#9 ((protease adj modulat*) or promogran):ti,ab,kw
#10 MeSH descriptor: [Silver Sulfadiazine] explode all trees
#11 silver*:ti,ab,kw
#12 MeSH descriptor: [Honey] explode all trees
#13 honey:ti,ab,kw
#14 #4 or #5 or #6 or #7 or #10 or #11 or #12 or #13
#15 #3 and #14
Appendix 14 Dressings for venous leg ulcers: PRISMA flow chart
Appendix 15 Alternative mixed-treatment meta-analysis models explored
The table shows the results from six synthesis models, divided into three sets. The first set (models A1 and A2) models the proportion of patients healed, thus ignoring the time component. The second set (models B1 and B2) considers the time component of the outcome but ignores the fact that an underlying silver effect may exist. The last set of results (models C1 and C2) takes into account the time component and considers the estimation of the incremental effect of silver. For each set of results, models 1 and 2 relate to the fixed-effects and random-effects approaches, respectively. All models use the same data and assume the same likelihood and, thus, the DIC is comparable across models.
| Dressing type | Model A1 FE Propa | Model A2 RE Propa | Model B1 FE Time Expa | Model B2 RE Time Expa | Model C1 FE Time Exp Silvera | Model C2 RE Time Exp Silvera | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR median (95% CrI) | Probability best (%) | OR median (95% CrI) | Probability best (%) | HR median (95% CrI) | Probability best (%) | HR median (95% CrI) | Probability best (%) | HR median (95% CrI) | Probability best (%) | HR median (95% CrI) | Probability best (%) | |
| BWC | – (–) | 0.0 | – (–) | 0.0 | – (–) | 0.0 | – (–) | 0.0 | – (–) | 0.0 | – (–) | 0.1 |
| HYDRO | 1.21 (0.85 to 1.73) | 0.0 | 1.27 (0.80 to 2.23) | 0.0 | 1.12 (0.88 to 1.42) | 0.0 | 1.16 (0.85 to 1.68) | 0.0 | 1.11 (0.88 to 1.41) | 0.1 | 1.17 (0.84 to 1.79) | 0.1 |
| Foam | 1.39 (0.9 to 2.14) | 0.0 | 1.45 (0.84 to 2.73) | 0.0 | 1.27 (0.95 to 1.73) | 0.0 | 1.32 (0.89 to 2.01) | 0.0 | 1.27 (0.93 to 1.72) | 0.7 | 1.32 (0.87 to 2.15) | 1.1 |
| SILVER DD | 1.09 (0.63 to 1.89) | 0.0 | 1.10 (0.45 to 2.70) | 0.2 | 1.06 (0.73 to 1.53) | 0.0 | 1.08 (0.58 to 2.01) | 0.1 | – (–) | – | – (–) | – |
| ALG | 0.57 (0.20 to 1.57) | 0.0 | 0.57 (0.17 to 1.83) | 0.0 | 0.82 (0.49 to 1.37) | 0.0 | 0.80 (0.41 to 1.50) | 0.0 | 0.82 (0.48 to 1.35) | 0.1 | 0.80 (0.40 to 1.55) | 0.2 |
| PAR-BET | 1.78 (0.61 to 5.11) | 0.9 | 1.86 (0.52 to 7.07) | 1.7 | 1.49 (0.65 to 3.59) | 1.5 | 1.57 (0.58 to 4.40) | 1.8 | 1.52 (0.67 to 3.58) | 5.5 | 1.57 (0.56 to 4.76) | 6.7 |
| CADEX | 1.77 (0.51 to 6.74) | 0.6 | 1.88 (0.44 to 9.09) | 1.2 | 1.64 (0.52 to 5.75) | 1.2 | 1.69 (0.47 to 6.56) | 2.0 | 1.56 (0.46 to 5.69) | 3.5 | 1.71 (0.50 to 6.78) | 4.6 |
| PAR | 1.76 (0.48 to 6.84) | 0.0 | 1.85 (0.42 to 9.15) | 0.1 | 1.63 (0.49 to 5.59) | 0.1 | 1.70 (0.47 to 6.83) | 0.1 | 1.54 (0.47 to 5.72) | 0.3 | 1.66 (0.46 to 6.79) | 0.5 |
| COLLA | 3.43 (0.67 to 18.04) | 3.0 | 3.60 (0.55 to 26.94) | 3.8 | 2.74 (0.65 to 11.88) | 4.3 | 2.82 (0.60 to 15.11) | 4.9 | 2.60 (0.64 to 11.49) | 6.8 | 2.81 (0.56 to 15.06) | 7.3 |
| HYALU | 4.73 (0.23 to 207.6) | 32.6 | 5.42 (0.23 to 241.3) | 32.5 | 4.18 (0.24 to 165.50) | 42.7 | 4.23 (0.19 to 191.90) | 41.1 | 3.92 (0.24 to 175.2) | 48.4 | 4.08 (0.22 to 137.6) | 47.7 |
| N-OLIG | 4.82 (0.69 to 34.1) | 19.9 | 5.02 (0.55 to 52.87) | 20.8 | 3.68 (0.66 to 22.4) | 26.0 | 3.88 (0.62 to 27.33) | 26.0 | 3.55 (0.63 to 21.98) | 34.7 | 3.80 (0.56 to 28.36) | 31.7 |
| Silver foam | 7.1 (1.66 to 34.52) | 42.8 | 7.41 (1.46 to 44.25) | 39.8 | 3.35 (1.40 to 8.31) | 24.3 | 3.47 (1.24 to 10.25) | 24.1 | – (–) | – | – (–) | – |
| Silver effect | – (–) | – | – (–) | – | – (–) | – | – (–) | – | 1.24 (0.88 to 1.72) | – | 1.34 (0.80 to 2.61) | – |
| Between-study variation | – (–) | – | 0.05 (0.00 to 0.70) | – | – (–) | – | 0.03 (0.00 to 0.32) | – | – (–) | – | 0.05 (0.00 to 0.39) | – |
| DIC | 220.1 | 221.8 | 220.4 | 221.4 | 221.6 | 222.5 | ||||||
The results show that the models represent the data equally well (the DICs are not different by > 5 units). We therefore pursued modelling approach C, in which follow-up time is taken into account and an effect of silver explored.
List of abbreviations
- ABPI
- ankle–brachial pressure index
- BNF
- British National Formulary
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CrI
- credible interval
- CVD
- cardiovascular disease
- DIC
- deviance information criterion
- EMLA
- Eutectic Mixture of Local Anaesthetics
- EPUAP
- European Pressure Ulcer Advisory Panel
- EQ-5D
- European Quality of Life-5 Dimensions
- EUSOL
- Edinburgh University Solution of Lime
- GP
- general practitioner
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluation
- HMP
- Her Majesty’s Prison
- HR
- hazard ratio
- HTA
- health technology assessment
- IPD
- individual patient data
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- IT
- information technology
- JLA
- James Lind Alliance
- JLAPUP
- James Lind Alliance Pressure Ulcer Partnership
- MS
- multiple sclerosis
- NETSCC
- NIHR Evaluation, Trials and Studies Coordinating Centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OMERACT
- Outcome Measures in Rheumatology
- OR
- odds ratio
- PICO
- population, intervention, comparison, outcome
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSP
- Priority Setting Partnership
- PURSUN UK
- Pressure Ulcer Research Service User Network for the UK
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SUCRA
- surface under the cumulative ranking
- UK DUETs
- UK Database of Uncertainties about the Effects of Treatments
- VULCAN
- Venous ULcer Cost-effectiveness of ANtimicrobial dressings