Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0707-10189. The contractual start date was in January 2009. The final report began editorial review in April 2014 and was accepted for publication in August 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Martin R Underwood is a member of the National Institute for Health Research Journals Library Editorial Group.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Taylor et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background to the study
Chronic conditions, especially musculoskeletal conditions, impose an increasing burden on society and the NHS. 1 Despite increased understanding of the factors contributing to the development of chronic pain, the population burden of chronic pain is rising, with more cases now than 40 years ago. 2
A key component of the UK Department of Health’s response to the perceived growing burden of chronic disease3 was to promote self-management4 and the most tangible aspect of this was the introduction and promotion of its flagship, lay-led (i.e. peer-led), self-management training course, the Expert Patients Programme. 5 This decision was made based on a belief and some limited evidence, mainly from the USA, that such self-management programmes for long-term conditions improve health status, slow disease progression and reduce health-care use. 6 The Expert Patients Programme was rolled out and implemented within the NHS from 2005 onwards. 3 By 2007 the Department of Health had invested £18M in the programme. 7 In 2008 the then prime minister, Gordon Brown, announced further expansion of the programme. 8
The Expert Patients Programme is a complex intervention. It is an anglicised version of the Stanford Chronic Disease Self-Management Program developed in the 1980s. 9 It is based on Bandura’s social cognitive theory of behaviour,10 which suggests that positive behaviour changes are more likely to occur if a person is confident of making the change and expects a good outcome. The Expert Patients Programme consists of a structured, 6-week course (2.5 hours per week) covering education, coping strategies such as relaxation, visualisation, positive thinking, action planning and goal-setting. It includes strategies to deal with anger, fear and frustration, and aims to promote better communication with health-care professionals (HCPs) and physical activity. The courses are led by trained and accredited laypeople who themselves have a chronic condition. The objectives of self-management programmes are to encourage participants to take responsibility for their own health, increase their knowledge about their health condition, identify positive or dysfunctional coping strategies, teach more effective management strategies, create networks for support and reduce isolation. 11 The UK Department of Health’s aims were to increase quality of life, reduce the demand for consultations and drugs and avoid unnecessary investigatory tests, thus generating longer-term cost savings and increasing patient satisfaction. 5 Despite a reduced emphasis on the Expert Patients Programme in recent years, the concept of supporting better self-management among people with chronic conditions remains central to the UK Department of Health agenda. 12
The optimal way to support people with chronic musculoskeletal pain to manage their condition is unclear. Systematic reviews report, at best, only modest benefits for lay-led self-management programmes compared with usual care for long-term musculoskeletal conditions such as low back pain and osteoarthritis (OA). 13,14 For OA self-management there is moderate-quality evidence (11 studies including 1706 participants) that indicates small benefits up to 21 months in terms of self-management skills, pain, OA symptoms and function, although the magnitudes of the effect sizes are of doubtful clinical importance. 13 The authors of this review found no improvement in positive and active engagement in life or quality of life. Similar findings were reported from lay-led self-management courses for low back pain. 14 Despite these modest effects there is still considerable popular support for these types of programmes. Some authorities argue that a small average benefit for many patients may still be worthwhile compared with a larger benefit for smaller numbers of patients with less common disorders. 15 Nevertheless, the Expert Patients Programme and other programmes of this nature need further research and validation. Squire and Hill16 have argued that a clear policy based on good research and evidence is required to guide clinicians, service delivery organisations and researchers in the content and delivery of self-management programmes for chronic pain patients.
A note on terminology
Although it is obvious that only the affected individual can ‘self-manage’ his or her condition, courses to promote better self-management are commonly referred to as ‘self-management courses’, whereas a more accurate term might be ‘self-management support courses’. For brevity we have used the term ‘self-management course’ throughout this report but it should be understood that by this we really mean ‘self-management support course’.
Chronic musculoskeletal pain
The focus of this research programme is on chronic, non-malignant musculoskeletal pain. The International Association for the Study of Pain (IASP) defines chronic pain as that which has persisted beyond normal tissue healing time, usually interpreted as 3 months. 17 Estimates of the prevalence of chronic pain vary, but one estimate is that 7.8 million people in the UK suffer moderate to severe pain that has lasted for > 6 months. 2
Musculoskeletal health care is costly. In 2011 in the UK, NHS trusts (secondary care services) spent around 10% of their patient care expenditure on musculoskeletal disorders (around £5.16B), and in 2009/10 there were around 21 million primary care consultations for musculoskeletal conditions in the UK. 6,18 Musculoskeletal pain is more commonly reported by women and those from socially or financially disadvantaged groups. 19 Chronic pain can cause considerable disruption to people’s lives. Around one-quarter of those who have chronic pain report severe disruption (> 2 weeks in the last 3 months) to their usual activities and chronic pain was associated with poorer mental health and well-being and lower levels of happiness and higher levels of anxiety and depression.
Psychology and chronic pain
The presence of chronic pain is strongly associated with adverse psychological factors. 20 European guidelines on the management and prevention of low back pain21 include psychological criteria to identify those at risk of poor outcomes, known as ‘psychosocial yellow flags’. These were developed to determine whether or not patients required more detailed assessment and to identify those for whom physical intervention could be less appropriate because of the dominance of psychological problems that would affect a successful outcome of the treatment. Psychological factors that consistently predict poor outcome include:
-
beliefs that back pain is harmful and potentially disabling
-
fear-avoidant behaviour and reduced activity levels
-
low mood and reduced social interaction
-
expectation that passive rather than active participation in treatment will help.
These factors can be identified during a consultation or with screening questionnaires. 22,23
Treatment approaches for people living with chronic low back pain that address some of the psychological issues include cognitive–behavioural therapy (CBT) and reactivation and reassurance strategies. 24 These encourage new behaviours and activity to overcome and change the psychological constructs limiting recovery and activity. Self-management courses incorporate some of the same strategies but more work is needed to maximise and quantify the clinical effectiveness and cost-effectiveness of these programmes. Redirecting resources to develop appropriate psychological interventions, with potentially more sustained benefits and fewer side effects than drug treatment, may have long-term clinical and economic advantages.
The biomedical model as opposed to the biopsychosocial model might be of limited benefit, especially for patients with high levels of health anxiety. 25 Linton et al. 24 suggest that a key component in establishing reassurance in patients is empathy and emotional support from the clinician. Emotional support outweighs the need for information and explanations in patients with unexplained pain. 26 A systematic review, however, found that cognitive reassurance was more effective in sustaining long-term improvements in patient outcomes than affective reassurance. 27 Cognitive reassurance includes objective information giving with clear explanations, whereas affective reassurance includes concepts such as empathy and warmth. The components of an effective self-management intervention should be designed in the knowledge that individual factors will determine different responses by patients to different components. Conceptually, the identification of groups of people for whom the same components will prove effective must be considered before implementing interventions. Indeed, the failure to adapt specific components that target the needs of different subgroups may explain the negative findings in some recent trials of behavioural interventions. 28
Self-management and evidence
We use the following broad definition of self-management throughout:
Self-management education programmes are distinct from simple patient education or skills training, in that they are designed to allow people with chronic conditions to take an active part in the management of their own conditions.
Foster et al. 14
The UK national evaluation of the original Expert Patients Programme for the self-management of chronic conditions reported a statistically significant increase in patients’ self-efficacy (which can be interpreted as self-confidence in relation to a specific context) and self-reported energy levels but no reduction in health-care utilisation. 15 Others found that the beneficial effects of lay-led self-management programmes for chronic conditions were modest in the short term and demonstrated a paucity of evidence on long-term benefit. 14 Two subsequent systematic reviews13,29 have reported improvements in patients’ confidence to manage their symptoms and small effects on pain and disability; both studies concluded that the benefits were too small to have any meaningful effect. Possible explanations for these findings included:
-
Suboptimal content of interventions.
-
Suboptimal delivery of interventions.
-
Programmes are effective only for some patients.
-
Measuring outcomes that may not be relevant to the intervention – the majority of studies measured change in pain symptoms, which are unlikely to change in chronic conditions. Self-management interventions may have a greater effect on confidence, positive outlook and coping methods than pain.
-
Poor targeting of the interventions (i.e. to those least likely to benefit).
-
Supporting self-management is an inherently ineffective approach.
Measuring outcomes
The fourth point above illustrates one of the key methodological challenges in measuring outcomes in populations experiencing persistent symptoms resulting from long-term conditions: selecting suitable outcomes. Typically, these will depend on the aim of the study. Measuring patient-centred outcomes, that is, those that are meaningful, relevant and important to patients, has already been recognised in both the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)30 and Multinational Musculoskeletal Inception Cohort Study (MMICS)31 recommendations. IMMPACT and MMICS were international consensus studies that recommended a list of outcome measures for research in chronic pain and back pain populations, respectively. Both made recommendations with regard to measures for pain, psychological states, patient satisfaction, disability, global health/well-being, health-care use, symptoms and adverse events, physical functioning, work-related outcomes, tests and examinations, financial issues, lifestyle, weight and social/demographic factors.
Predictors, moderators and mediators
In many areas of health-related research, attention has started to focus on better matching of subgroups of patients to interventions. This aims to improve the effectiveness of treatment by targeting those most likely to benefit and avoiding offering treatment to groups for whom treatment is neither acceptable nor beneficial.
For example, in research in the musculoskeletal pain and mental health population, identification of subgroups is considered a priority. 22 The terminology around subgroup effects can be confusing. We have adopted the distinctions between predictors, moderators and mediators to describe how participant factors affect outcomes in randomised controlled trials (RCTs) suggested by Kraemer et al.:32
-
Predictors are baseline variables that affect outcome (significant main effect only) but do not interact with treatment. Such factors significantly predict outcome equally for target interventions and control conditions.
-
Moderators are baseline variables (such as patient baseline characteristics) that interact with treatment to change outcome for each subgroup. These specify for whom and under what conditions treatment works.
-
Mediators are variables measured during treatment (factors that change during the intervention) that impact on outcome, with or without interaction with treatment, for example mood might be a mediator for a different outcome such as employment status. Mediators help inform the process and potential mechanisms (including causal mechanisms) through which treatment might work and therefore can be used to improve subsequent interventions through strengthening the components that best influence the identified mediators.
There is evidence from prospective cohort studies reporting predictors of outcomes for people with chronic musculoskeletal pain, but far less from RCTs reporting moderators and mediators. 20,33–35 Most of what is published, at least for low back pain, is of poor quality. 36 RCTs are the best study design to explore moderators and mediators but those that include planned subgroup analysis require very large samples. 37,38 Such subgroup analyses must also be based on good theoretical reasoning and previous evidence to support the hypothesis that the correct subgroups have been identified a priori. 39 In practice, these analyses are usually undertaken as secondary analyses and, consequently, are statistically underpowered, leading to a lack of robust data for mediators and moderators in self-management interventions.
Components of courses
Self-management involves undertaking tasks that enable a person to live with their chronic condition(s). Component tasks may include addressing the medical, social or role and emotional management of their condition(s). 40 This suggests that interventions aimed at improving self-management may require several different components. There are several meta-analyses of different treatments for those with chronic musculoskeletal pain. Psychological approaches (such as CBT),41–43 exercise and activity21 are beneficial, whereas patient education on its own has little or no effect. 44,45 The evidence for mind–body therapies (such as relaxation) is equivocal. 46–48 Self-management education courses or programmes for chronic musculoskeletal pain combine some or all of these approaches, but the evidence to date suggests that the overall effects of such courses are modest. 13,14
As the content and characteristics of interventions promoting self-management for chronic pain vary considerably, there is a need to determine which components and course characteristics of these complex interventions are most likely to be beneficial to participants. To date, there have been few attempts to dissect the functional details of multicomponent, self-management programmes for chronic pain.
Adherence and dose
Attendance rates are a barrier to the effectiveness of self-management programmes. A national evaluation of the Expert Patients Programme evaluated courses at different case study sites, with participation rates at each site (participants attending at least four out of six sessions) ranging from 62% to 88%. 49 The number of sessions attended at group self-management courses may impact on outcomes, with many factors influencing continued attendance or if participants attend at all. Participants’ physical and mental health, their confidence within a group environment and their expectations about the learning experience may affect whether or not they attend the first session. Expectations can be influenced to some extent by the recruitment process in terms of information giving beforehand, and appropriate screening may identify any serious physical or mental impediments to attendance. However, after attending the first session, other factors come into play. On an individual level, relevance of content and perceived difficulty of the material are important, as well as whether or not the participant’s own diagnosis and disease experience chimes with those of the rest of the group. One qualitative study sampling ‘completers’ of group CBT or group education for chronic pain found that motivation to attend was influenced by group cohesion and the actions of the facilitators for one group. 50 Facilitator competency during the delivery of courses is something that can be influenced by adequate training and preparation.
Reach and uptake
Identifying potential study participants with chronic pain from general practice is challenging. There are no universally acknowledged Read codes to identify chronic musculoskeletal pain and each general practice may have a different coding practice. Some UK-based studies have tackled this problem by sending out a blanket screening questionnaire to all registered patients over a certain age, excluding any major physical or psychiatric comorbidity. 51,52 Eligibility was then assessed from the screening questionnaires returned and suitable participants formally invited into the study. Enrolment rates for eligible patients were high at 53%51 and 50%,52 respectively, but the numbers of screening questionnaires sent out in the initial blanket mail-out were 12,44851 and 45,994,52 respectively. Another approach is to directly query the general practice patient record systems looking for indicator Read codes, prescriptions for analgesics and frequency of consultations and to send out invitations to potentially eligible patients, excluding any major physical or psychiatric comorbidity53–56 Once a potential participant has expressed an interest in the study, actual conversion to enrolment into the study can be influenced by a number of factors, for example some potential participants may not enrol because they think that the control arm is not a credible option or some may not be able to attend courses on weekdays because of other commitments such as work or child care.
Summary of evidence gaps: the need for research in this area
Treatment for chronic musculoskeletal conditions such as low back pain has done little to reduce their prevalence and health impact over the last two decades. 57,58 Chronic pain frequently coexists with other pain syndromes59 and traditional treatment approaches focus on conditions separately, which is unlikely to have a substantial impact on the population, or individual, burden of chronic pain. 59 There has been a shift towards a more biopsychosocial approach. 60 Two increasingly used psychosocial interventions are cognitive–behavioural approaches and self-management support programmes. Both approaches are rapidly expanding. However, the evidence has not been sufficient to justify such widespread and rapid introduction.
Some investigators have used RCTs to evaluate the effectiveness of self-management programmes but few have explored the effectiveness of different components of these programmes or courses. RCTs have found that self-management interventions may change attitudes but produce only modest improvements in clinical outcome. Overall, these data do not indicate whether specific aspects of the courses are effective or ineffective; it may be, for example, that social networking or the approach of the tutors is the most important factor.
We proposed to explore the components or elements of chronic pain self-management programmes that may be more effective than others and determine the most appropriate outcomes to measure. Without this work there are the twin hazards of continuing to spend NHS resources on ineffective interventions or failing to invest adequately in delivering an effective and cheap intervention. Even quite modest overall effects may be worth identifying because of the enormous personal, social and economic costs of chronic painful disorders.
Aims and objectives
The overall aim of this programme of research was to develop and test a self-management intervention for people living with chronic musculoskeletal pain.
The objectives were to develop a new self-management approach and provide evidence for, or against, its clinical effectiveness and cost-effectiveness. We proposed developing an intervention to promote individual independence, improve quality of life and reduce the level of need for health-care resources, thus lessening a proportion of the economic, personal and social burden of chronic pain conditions.
To achieve this we first needed to identify what was already known about good-quality self-management programmes for chronic pain by examining the existing scientific literature and evidence in a systematic manner. We also wanted to consult experts and patients to identify and explore best practice, theoretical underpinnings for self-management and ways to measure patient outcomes. Once this preliminary work was completed we devised and evaluated our new programme/intervention in a feasibility study to ensure that we had the best possible intervention and systems for measuring the effect of the intervention on patients.
Finally, we tested the new intervention in a pragmatic RCT. We collected information on both the clinical effectiveness and the cost-effectiveness of our intervention. The findings provide the information needed to decide whether or not the NHS should invest in such services in the future.
Overview of the study and the report
There are two parts to this report: the development of the intervention and testing the clinical effectiveness and cost-effectiveness of the intervention. Figure 1 illustrates the overall design of the study.
FIGURE 1.
The structure of the study and report.
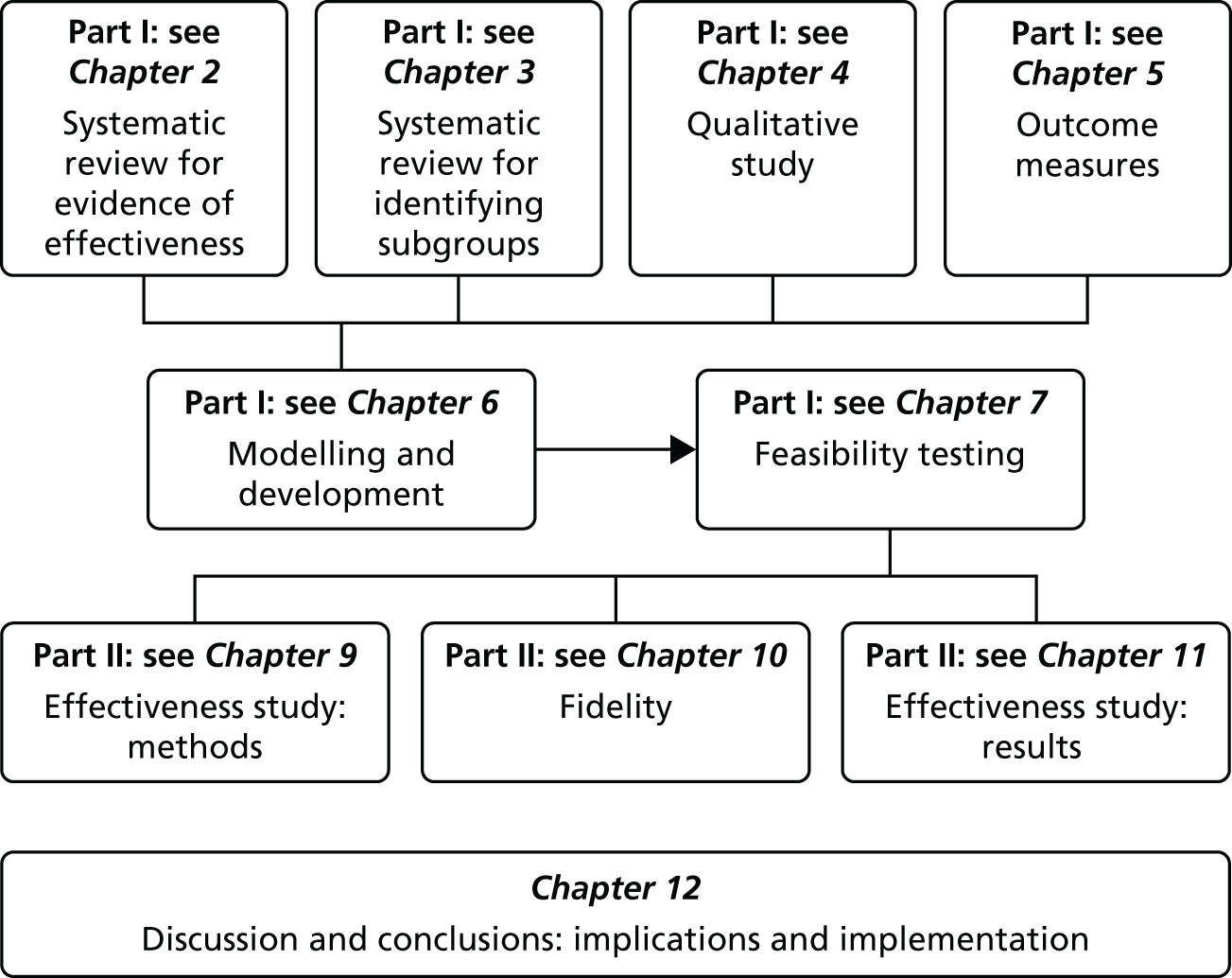
Part I: development, design and feasibility testing of the intervention
The development phase consisted of two systematic reviews, a qualitative study, modelling, the design of the intervention and a feasibility study. Chapter 8 summarises the findings from part I and is not shown as part of the study design illustrated in Figure 1.
Part II: clinical effectiveness and cost-effectiveness of the intervention
The testing phase consisted of a RCT and a cost-effectiveness analysis and study of the fidelity of the delivery of the intervention in the RCT.
At the end of part II we bring together the findings and discuss these in relation to current thinking in the field of chronic pain and self-management.
Patient and public involvement
We included patients and the public in phases 1 and 2 of the research. In phase 1 we recruited four people (one male and three females) with a chronic condition to a patient advisory group. These people gave advice and made comments on all of our patient-related documentation, resulting in substantial improvements to the documentation. In addition, they played a role in the outcomes study (see Chapter 5) by reviewing outcome measurement tools and commenting on their acceptability, brevity, comprehension and ease of completion (in retrospect we feel that we could have included more patient advisors in this phase of the study). People with experience of chronic pain were heavily involved in the development and refinement of the intervention, particularly in terms of their collaboration during the feasibility study when we collected data from all participants and from the lay facilitators on their experiences of every session (using a questionnaire) and at focus groups and interviews following the completion of the courses. The free and frank discussions at the focus groups enabled us to refine the intervention and the training to deliver the intervention. We also consulted extensively with the Bangladeshi community through interviews during the feasibility study of the intervention delivered in Sylheti. Two professional bilingual Bangladeshi advocates also provided extensive advice on patient-related material, running the courses in Sylheti and outcome measures in the Sylheti-speaking community.
In phase 2 we included two patient representatives with a chronic condition on the Trial Steering Committee (TSC), one female and one male. Both were experienced representatives who had previously sat on National Institute for Health Research (NIHR) research priorities panels. These people gave valuable advice to the TSC and the excellent recruitment rates and low attrition seen in the study are, in part, a reflection of their contribution.
In addition, Social Action for Health [see www.safh.org.uk (accessed 11 April 2016)] was a coapplicant in the study. Social Action for Health is a community interest company providing socially orientated services to the local community. Members of Social Action for Health were part of the trial study team and represented the patient perspective for decisions made throughout the progress of the study.
Finally, and perhaps most importantly, patients were integral to the design and delivery of the intervention as we recruited patients with experience of chronic pain to deliver our intervention for the feasibility study and the main trial.
Chapter 2 Systematic review: evidence for the effectiveness of components and characteristics of pain and self-management programmes
Abstract
Introduction: Evidence for self-management courses and course components that are beneficial for participants has not been established.
Aims: To systematically re-examine the overall effectiveness and determine the most successful course content and optimal delivery methods of self-management courses.
Methods: We searched 10 relevant electronic databases for RCTs and systematic reviews. RCTs were categorised according to the presence of psychological, mind–body therapy, physical, lifestyle and educational components; group or individual delivery; tutor; setting; and duration of the interventions studied. Outcomes analysed were pain intensity, global health, quality of life, physical function, self-efficacy, depression, anxiety and social function in the short term (< 4 months), medium term (4–8 months) and long term (> 8 months). Data were extracted comparing self-management with usual care or a waiting list control. Data were combined as a standardised mean difference (SMD) meta-analysis (random effects) with subgrouping. When statistical pooling was not possible we carried out a narrative synthesis.
Results: Forty-six RCTs published from 1994 to 2009 were included in the original meta-analyses and a further 18 RCTs were included in updated analyses to 2013. Heterogeneity between studies was generally high. Overall, the number of components or duration of the interventions did not influence effectiveness, but courses with a psychological component, courses delivered in groups and courses delivered by a HCP appeared to work well, showing significant effect sizes on several outcomes during post-course follow-up (short, medium and long term). Data were sparse on subgroup comparisons and on the detail of the components of individual interventions.
Conclusions: Our analysis provided useful information to inform the design of our intervention.
Introduction
The evidence for the effectiveness of self-management support courses61 (commonly known as ‘self-management courses’ and sometimes referred to as ‘pain management programmes’) for chronic musculoskeletal pain is limited. There is even less information on the effectiveness of specific components or on the content of courses and the way that they are delivered.
Aim
The aim of this review was to identify the types of courses (content and characteristics) that are most likely to be effective in helping those with chronic pain.
We sought to identify the evidence on:
-
the overall effectiveness of self-management courses
-
the key components and characteristics of potentially effective self-management programmes (including self-management education strategies) for people with chronic musculoskeletal pain.
We did this by comparing research on delivery characteristics (including setting) and components of self-management programmes for chronic musculoskeletal disorders that appear to have been successful or unsuccessful.
Method
We conducted a systematic review of RCTs examining the effectiveness of different types of self-management interventions (with and without individual components).
In addition, we systematically searched for systematic reviews to see if any other researchers had performed this type of work and used citation tracking from relevant reviews to supplement our searches.
We completed the initial systematic review of articles published between January 1994 and April 2009 to inform the design of the intervention study. At the end of the study we updated the review for selected outcomes [those measured in the COPERS (Coping with persistent Pain, Effectiveness Research into Self-management) study] to September 2013 to allow us to put our final results into context (see Chapter 12). The inclusion and exclusion criteria for the reviews are shown in Table 1.
| Definitions | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Type of study |
|
|
| Types of participants |
|
|
| Types of self-management interventions |
|
|
Outcome measures
The outcomes that we were interested in were extracted and grouped into the following categories:
-
global health measures
-
pain intensity
-
physical/functional capability
-
quality of life
-
self-efficacy
-
anxiety
-
depression
-
social role/function
-
Short Form questionnaire-36 items (SF-36)62 general mental health (excluded in update review)
-
number of visits to HCPs (excluded in update review)
-
fatigue (excluded in update review).
The mean and standard deviation (SD) of the final value and/or change scores for each group at each follow-up interval were extracted.
Search method
Electronic literature searches
The initial searches were conducted between January 1994 and April 2009 as self-management courses and the understanding of chronic pain have advanced considerably during this period. The following electronic databases were searched to identify all relevant studies: MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (Ovid), EMBASE (Ovid), PsycINFO (Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine Database (AMED) using the Health Information Resources [see www.library.nhs.uk (accessed 11 April 2016)], Web of Science Social Sciences Citation Index (SSCI) and The Cochrane Library [Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Cochrane Central Register of Controlled Trials].
Other sources
We also checked the citation lists of relevant systematic reviews and guidelines identified in our electronic database searches for any additional relevant studies.
Study selection
Two reviewers conducted the searches and independently screened all titles and abstracts for potentially eligible studies. Full texts of all potentially relevant articles were obtained. Inter-rater reliability for screening titles and abstracts was checked on a sample of the studies (approximately 10%). The full texts of all retrieved articles were scrutinised by both reviewers to determine whether or not to proceed to full data extraction. Disputed articles went to a third reviewer for arbitration.
Assessment of study bias
Two review authors independently assessed trial quality according to the following criteria modelled on The Cochrane Collaboration methods. 39 We asked:
-
Did the study have an adequate randomisation sequence?
-
Was allocation concealment carried out?
-
Was there a description (i.e. numbers provided) of withdrawals and dropouts?
-
Was outcome assessment blinded?
-
Was there an intention-to-treat (ITT) analysis?
Each question was rated as yes/no or unclear (see Table 3).
Data extraction
The two reviewers working independently each extracted data about country of origin, number randomised to each arm, who delivered the course (HCP or lay tutor or a combination), whether the course was delivered to groups or individuals or was self-delivered, setting [community, medical, occupational, remote (telephone/internet) or mixed], total number of sessions and contact hours, course duration, course components (see Table 2), description of control group and the description and results of any moderator analyses. Data were extracted, when possible, at four time points: baseline, short-term follow-up (< 4 months), medium-term follow-up (4–8 months), long-term follow-up (> 8 months) or a mixture of follow-up points.
Description of self-management components
To handle the vast number of data arising from the studies, we categorised self-management interventions into psychological, mind–body therapy, physical, lifestyle or medical education components, as described in Table 2. Each study was coded so that the intervention arm was described using two or more components from the list. We accept that these categorisations represent our interpretation of the published reports of studies and that some components may well have overlapped between our broad categories.
| Psychological | Mind–body therapies | Physical | Lifestyle management | Medical management |
|---|---|---|---|---|
|
|
|
|
|
Final study selection
Following data extraction, the RCTs were further selected to include only comparisons of the self-management intervention(s) with waiting list controls or usual care.
Meta-analysis methods
The meta-analyses were carried out using Review Manager v5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Calculations were based on final values as these were the most commonly reported data. Change score data were also collected when possible. When studies reported p-values for change from baseline for each group, this enabled a SD for the change score to be calculated. 39 Change scores were analysed in the same way as the final value data for the outcomes when there were sufficient data and compared with the final value results for the same outcomes as a sensitivity analysis. We used a random-effects model because of the heterogeneity in study populations and interventions.
When there was more than one measurement tool for an outcome we combined data across studies using a SMD meta-analytical approach (see section 9.2.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions39), where
For those instruments for which an increase in score indicates improvement we reversed the sign on the mean score to enable us to combine these as a pooled SMD with measures from instruments for which a decrease in score is beneficial.
The resulting SMDs were interpreted using Cohen’s d63,64 (where d is derived from the difference between two independent means divided by the within-population SD as above). The effect sizes were conventionally defined as follows: ‘minor’ < 0.2, ‘small’ ≥ 0.2, ‘moderate’ ≥ 0.5 and ‘large’ ≥ 0.8.
Meta-analysis comparisons
We tested:
-
Overall effectiveness. Total effect size or SMD for self-management interventions with regard to our prespecified outcome categories compared with the control.
-
The effect of course delivery mode. We grouped the studies at each follow-up interval into different course delivery modes [group, individual, mixed (group and individual) or remote (internet, mail, telephone)] and compared the pooled SMDs for each subgroup.
-
The effect of course leader. We grouped the studies and outcomes at each follow-up interval into those that were delivered by a HCP, those that were delivered by a lay tutor and those using a mix of delivery methods and compared the pooled SMDs for each subgroup.
-
The effect of course setting. We grouped the studies at each follow-up interval into those delivered in the community, those delivered in a medical setting (primary care or hospital) and those delivered in an occupational setting and compared the pooled SMDs for each subgroup.
-
The effect of course duration. We grouped the studies at each follow-up interval into those with courses of ≤ 8 weeks and those with courses of > 8 weeks and compared the pooled SMDs for each subgroup.
-
The effect of contributing self-management components. We tested whether or not the presence of a particular self-management component improved the effectiveness of the interventions. We compared the pooled SMDs of studies with psychological, mind–body therapy, physical, lifestyle and medical education components with the pooled SMDs of studies without these components.
-
The effect of number of components. We tested whether or not the number of components (two, three, four or five) influenced the effect size, comparing the pooled SMDs at short-, medium- and long-term follow-up intervals.
We produced forest plots of final value data for each comparison showing the pooled SMD for each subgroup.
Assessment of publication bias
Funnel plots were generated using Review Manager v5 for the outcomes with the most studies. The funnel plots were visually examined for symmetry about the y-axis and resemblance to an inverted funnel to denote absence of bias.
We present data from the original review meta-analysis and the updated review data.
Results
The results of the review of the effectiveness of self-management interventions are shown first followed by the effectiveness review of self-management courses with and without the different components and characteristics.
Literature search
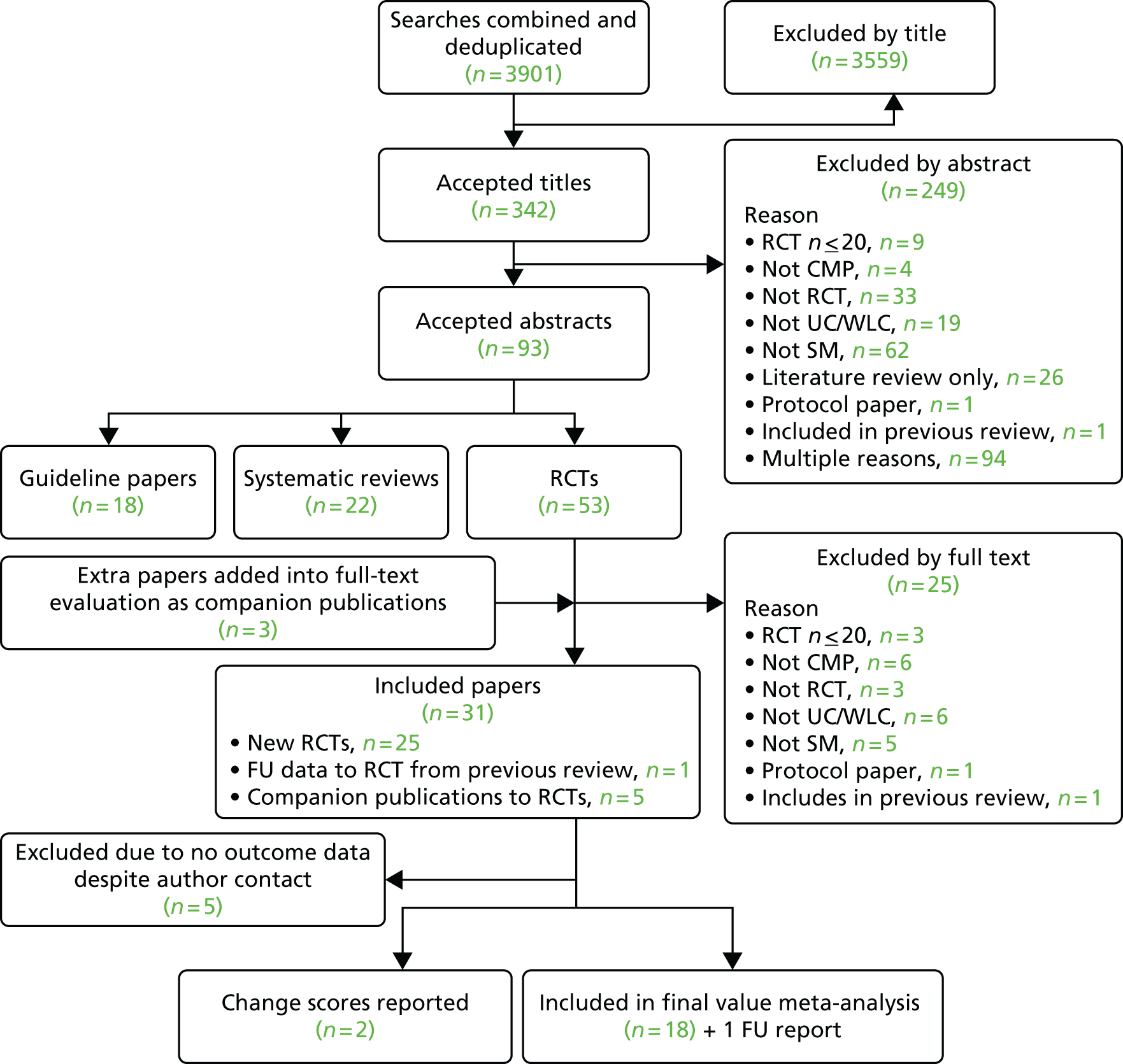
The initial search produced 4676 results and of these we included 46 RCTs. When we updated the search to September 2013 we included a further 18 trials in the meta-analyses for the overall effectiveness of self-management interventions.
Figure 2 shows the flow chart for the initial search and Figure 3 shows the flow chart for the updated search.
FIGURE 2.
Searches for the systematic review 1994–2009. CMP, chronic musculoskeletal pain; SM, self-management.
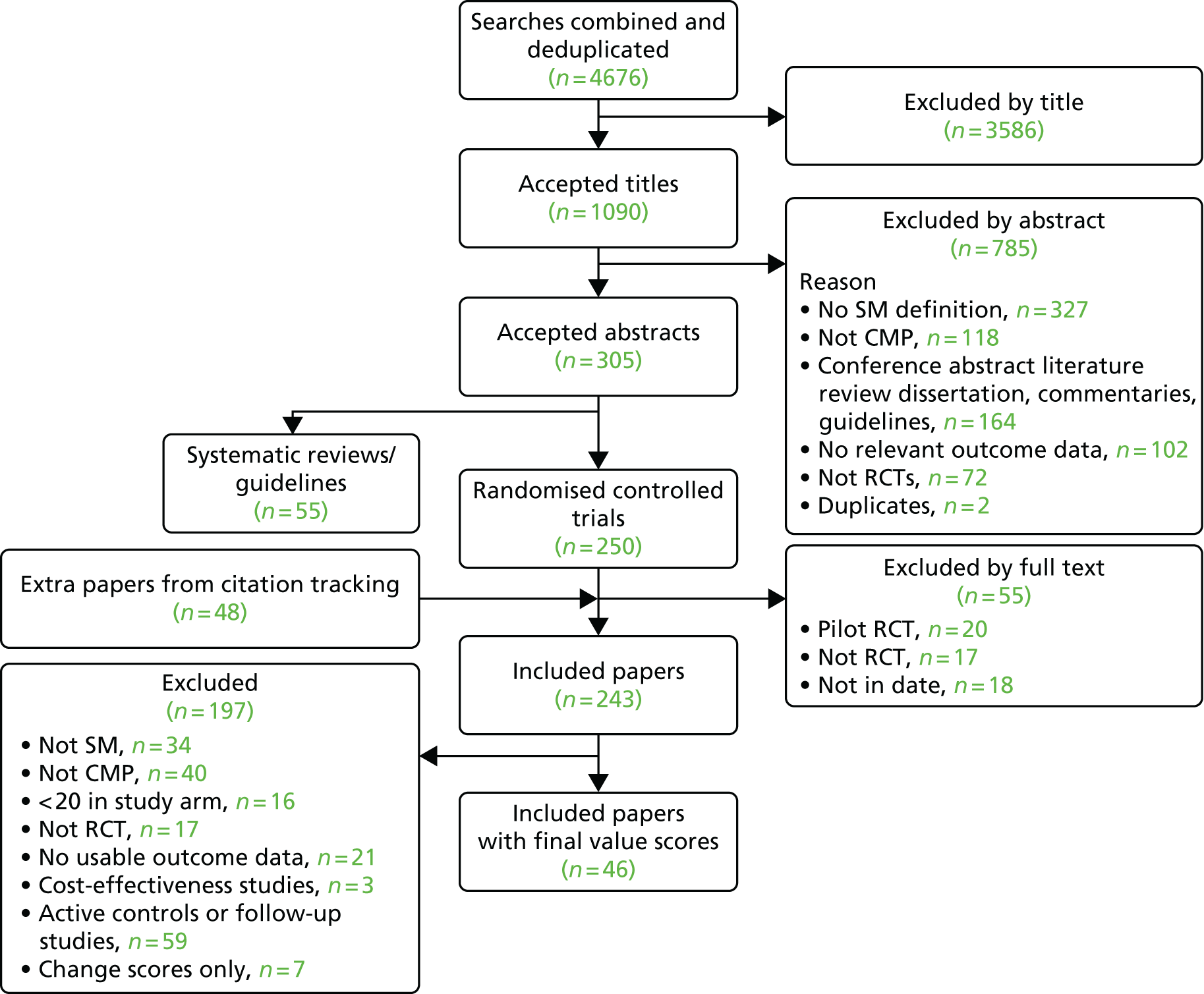
FIGURE 3.
Systematic review update June 2009–September 2013. CMP, chronic musculoskeletal pain; FU, follow-up; SM, self-management; UC, usual care; WLC, waiting list control.
Effectiveness analyses
For our original meta-analyses we included 46 RCTs54,65–111 with final-value data comparing self-management programmes with usual care or waiting list controls (n = 8539) (Table 3). Thirteen RCTs were conducted in the USA,72,74,77,83,85,86,91–93,95,104,106,108 seven in the Netherlands,69,81,94,96,98,102,110 five in the UK,54,67,70,88,97,103 five in Canada,68,76,80,87,107 three each in Sweden,82,89,99 Norway75,78,79,84 and China,100,105,111 two in Germany71,73 and one each in Turkey,65 Iran,66 Switzerland,90 Spain101 and Brazil. 109 Of these studies, 13 (28%) were on OA (hip or knee),74,81,88,91,92,94–97,101,104,105,111 12 (26%) were on low back pain,54,66,71,73,77–79,83,89,93,102,109,110 nine (20%) were on mixed pain,68,70,75,76,80,84,99,100,106 five (11%) were on fibromyalgia,69,82,85,87,90 three (7%) were on mixed arthritis (OA and rheumatoid arthritis)72,107,108 and one (2%) each was on temporomandibular joint disorder,86 osteoporosis,65 upper limb pain98 and knee pain. 67,103 The mean age of participants for the 44 studies reporting age was 55 years (range 38–82 years). In the 41 studies reporting gender breakdown, 72% were female participants, with two studies having exclusively female patients. Thirty-six studies were health care professionally led, six were mixed health care and lay led and four were lay led. Twenty-seven studies were conducted in a medical setting, sixteen in the community and three in occupational settings. Twenty-seven were delivered in groups, five were delivered remotely via the internet and five were delivered individually; nine used mixed group and individual delivery.
| Study | Country | Population | n | Self-management component details | Control arm | Course characteristics | Follow-up | Quality assessmenta | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delivery | Leader | Setting | Duration | QA1 | QA2 | QA3 | QA4 | QA5 | |||||||
| Corey 199668 | Canada | Mixed pain | 200 | P + MBT + PA + LS + ED | UC | Group | HCP | Medical | 4 weeks | L | U | U | Y | Y | N |
| Vlaeyen 199669 | The Netherlands | Fibromyalgia | 131 | P + MBT + PA + LS | WLC | Group | HCP | Medical | 6 weeks | S | U | U | Y | U | N |
| MBT + PA + LS | WLC | Group | HCP | Medical | 6 weeks | S | U | U | Y | U | N | ||||
| Williams 199670 | UK | Mixed pain | 78 | P + MBT + PA + LS + ED | WLC | Group | HCP | Medical | 8 weeks | S | Y | U | Y | Y | N |
| Basler 199771 | Germany | Low back pain | 94 | P + MBT + PA + LS + ED | UC | Group | HCP | Medical | 12 weeks | M | U | U | Y | U | N |
| Fries 199772 | USA | OA + RA | 809 | MBT + PA + LS + ED | WLC | Remote | HCP | Community | 52 weeks | M, L | Y | U | N | U | Y |
| Keller 199773 | Germany | Low back pain | 65 | P + MBT + PA + ED | WLC | Group + individual | HCP | Medical | 6 weeks | S | U | U | Y | U | N |
| Mazzuca 199774 | USA | OA | 211 | P + PA + LS + ED | UC | Individual | HCP | Medical | 6 weeks | M, L | U | Y | Y | U | N |
| Haldorsen 199875 | Norway | Mixed pain | 469 | P + MBT + PA + LS + ED | UC | Group + individual | HCP | Medical | 4 weeks | L | U | Y | Y | Y | N |
| LeFort 199876 | Canada | Mixed pain | 110 | P + MBT + PA + LS + ED | WLC | Group | HCP | Community | 6 weeks | S | U | Y | Y | U | Y |
| Von Korff 199877 | USA | Low back pain | 255 | P + PA + LS + ED | UC | Group | Lay | Medical | 4 weeks | S, M, L | U | U | Y | Y | Y |
| Glomsrod 2001,78 Lonn 199979 | Norway | Low back pain | 81 | PA + LS + ED | UC | Group | HCP | Medical | 13 weeks | L | Y | U | Y | U | Y |
| Currie 200080 | Canada | Mixed pain | 60 | P + MBT + LS | WLC | Group | HCP | Medical | 7 weeks | S | Y | U | Y | U | Y |
| Hopman-Rock 200081 | The Netherlands | OA | 120 | P + MBT + PA + LS + ED | UC | Group | HCP | Medical | 6 weeks | S, M | U | U | Y | Y | N |
| Mannerkorpi 200082 | Sweden | Fibromyalgia | 69 | P + MBT + PA + LS + ED | UC | Group | HCP | Community | 24 weeks | M | U | U | Y | Y | N |
| Moore 200083 | USA | Low back pain | 266 | P + PA + LS + ED | UC | Group + individual | HCP | Medical | 3 weeks | S, M, L | U | U | Y | U | Y |
| Haugli 200184 | Norway | Mixed pain | 174 | P + MBT + PA + ED | UC | Group | HCP | Occupational | 45 weeks | S, L | U | U | Y | Y | N |
| Oliver 200185 | USA | Fibromyalgia | 400 | P + MBT + PA + LS + ED | UC | Group | HCP + lay | Community | 52 weeks | L | U | U | Y | U | N |
| Dworkin 200286 | USA | TMD | 124 | P + MBT + LS + ED | UC | Individual | HCP | Medical | 6 weeks | S, M, L | U | U | Y | U | Y |
| King 200287 | Canada | Fibromyalgia | 124 | P + PA + LS + ED | WLC | Group | HCP | Community | 12 weeks | S | Y | U | Y | Y | Y |
| P + LS + ED | WLC | Group | HCP | Community | 12 weeks | S | Y | U | Y | Y | Y | ||||
| Quilty 200388 | UK | OA | 87 | PA + LS | UC | Individual | HCP | Community | 10 weeks | M, L | Y | Y | Y | U | Y |
| Buhrman 200489 | Sweden | Low back pain | 56 | P + MBT + PA + LS | WLC | Remote | HCP | Community | 8 weeks | S | Y | U | Y | U | N |
| Cedraschi 200490 | Switzerland | Fibromyalgia | 164 | P + MBT + PA + LS | WLC | Group | HCP | Medical | 6 weeks | M | Y | Y | Y | U | N |
| Hughes 200491 | USA | OA | 150 | P + PA | UC | Group | HCP + lay | Community | 8 weeks | S, M, L | Y | U | Y | U | N |
| Mazzuca 200492 | USA | OA | 186 | PA + LS + ED | WLC | Individual | HCP | Medical | 4 weeks | S, M, L | U | U | Y | U | N |
| Haas 200593 | USA | Low back pain | 109 | P + MBT + PA + LS + ED | WLC | Group | Lay | Community | 6 weeks | M | Y | Y | Y | U | Y |
| Heuts 200594 | The Netherlands | OA | 273 | P + MBT + PA + LS + ED | UC | Group | HCP | Medical | 6 weeks | S, L | Y | U | Y | Y | Y |
| Pariser 200595 | USA | OA | 92 | P + MBT + PA + LS + ED | UC | Remote | HCP | Community | 6 weeks | S | U | U | N | U | N |
| Tak 200596 | The Netherlands | OA | 109 | PA + LS | UC | Group + individual | HCP | Medical | 8 weeks | S | Y | U | Y | Y | Y |
| Victor 200597 | UK | OA | 193 | P + MBT + PA + LS + ED | WLC | Group + individual | HCP | Medical | 4 weeks | S, L | U | U | Y | Y | N |
| Bernaards 200698 | The Netherlands | Upper limb pain | 314 | P + PA + LS | UC | Group | HCP | Occupational | 24 weeks | M, L | Y | N | U | Y | N |
| Brattberg 200699 | Sweden | Mixed pain | 60 | P + LS | WLC | Remote | HCP + lay | Community | 20 weeks | S, L | U | U | Y | U | N |
| Li 2006100 | China | Mixed pain | 64 | P + LS + ED | WLC | Group + individual | HCP | Occupational | 3 weeks | S | Y | U | Y | U | Y |
| Núñez 2006101 | Spain | OA | 100 | P + PA + LS | UC | Group + individual | HCP | Medical | 12 weeks | L | Y | U | Y | U | N |
| Smeets 2006102 | The Netherlands | Low back pain | 111 | P + PA | WLC | Group + individual | HCP | Medical | 10 weeks | S | Y | Y | Y | Y | Y |
| Alp 200765 | Turkey | Osteoporosis | 50 | P + PA + LS + ED | UC | Group | HCP + lay | Medical | 5 weeks | S, M | Y | U | U | Y | N |
| Hurley 2007,67 Hurley 2012103 | UK | Knee pain | 418 | P + MBT + PA + LS + ED | UC | Group + individual | HCP | Medical | 6 weeks | M | Y | U | Y | Y | Y |
| Johnson 200754 | UK | Low back pain | 234 | P + MBT + PA + LS + ED | UC | Group | HCP | Community | 6 weeks | S, L | Y | U | Y | N | Y |
| Martire 2007104 | USA | OA | 143 | P + PA + ED | UC | Group | Lay | Community | 6 weeks | S, M | U | U | Y | U | Y |
| Tavafian 200766 | Iran | Low back pain | 102 | P + MBT + PA + LS + ED | UC | Group | HCP | Medical | 4 days | S | U | N | Y | U | Y |
| Yip 2007105 | China | OA | 182 | PA + LS + ED | UC | Group | HCP + lay | Medical | 6 weeks | S, M | Y | U | Y | Y | Y |
| Ersek 2008106 | USA | Mixed pain | 256 | P + MBT + PA + LS + ED | UC | Group | HCP | Community | 7 weeks | S, M, L | Y | U | Y | U | N |
| Laforest 2008107 | Canada | OA + RA | 113 | P + MBT + LS + ED | WLC | Individual | HCP | Community | 6 weeks | S | Y | U | Y | Y | N |
| Lorig 2008108 | USA | OA + RA | 866 | P + MBT + PA + LS + ED | UC | Remote | Lay | Community | 6 weeks | M, L | U | U | Y | U | Y |
| Ribeiro 2008109 | Brazil | Low back pain | 60 | PA + ED | UC | Group | HCP | Medical | 5 weeks | S, M | Y | Y | Y | Y | N |
| van der Hulst 2008110 | The Netherlands | Low back pain | 163 | P + PA | WLC | Group | HCP | Medical | 7 weeks | S, M | Y | U | Y | U | Y |
| Yip 2008111 | China | OA | 95 | PA + LS + ED | UC | Group | HCP + lay | Medical | 6 weeks | S, M, L | Y | U | Y | Y | Y |
| Studies included in the update | |||||||||||||||
| Crotty 2009112 | Australia | OA hip or knee | 152 | P + MBT + PA + LS + ED | UC | Group + individual | HCP + lay | Medical | 6 weeks | M | Y | Y | U | Y | Y |
| Jenkinson 200951 | UK | Knee pain | 389 | PA + LS | UC | Individual | HCP | Community | 104 weeks | L | Y | Y | Y | Y | Y |
| Kroenke 2009113 | USA | Mixed pain | 250 | P + MBT + PA + LS + ED | UC | Individual | HCP | Community | 52 weeks | M, L | Y | Y | U | Y | Y |
| Chiauzzi 2010114 | USA | Low back pain | 209 | P + PA + LS + ED | UC | Remote | Self | Community | 4 weeks | S, M | Y | U | Y | Y | Y |
| Glombiewski 2010115 | Germany | Low back pain | 128 | P + MBT + LS | WLC | Individual | HCP | Medical | 32 weeks | M | Y | U | U | U | Y |
| Hamnes 2012116 | Norway | Fibromyalgia | 150 | P + MBT + PA + LS + ED | WLC | Group | HCP | Medical | 1 week | S | Y | Y | U | U | U |
| Hansson 2010117 | Sweden | OA hip/knee/hand | 114 | PA + LS + ED | UC | Group | HCP | Medical | 5 weeks | M | Y | U | Y | Y | U |
| Hsu 2010118 | USA | Fibromyalgia | 45 | P + MBT + PA + LS + ED | WLC | Group | HCP | Medical | 3 weeks | S, M | Y | U | Y | Y | Y |
| Lamb 201053 | UK | Low back pain | 701 | P + MBT + PA + ED | UC | Group | HCP | Mixed | 6 weeks | S, M, L | Y | Y | Y | Y | Y |
| Williams 2010119 | USA | Fibromyalgia | 118 | P + MBT + PA + LS + ED | WLC | Remote | Self | Community | 24 weeks | M | Y | Y | Y | Y | Y |
| Luciano 2011120 | Spain | Fibromyalgia | 216 | P + MBT + PA + ED | UC | Group | HCP | Medical | 9 weeks | S | Y | U | U | Y | Y |
| Morone 2011121 | Italy | Low back pain | 73 | P + MBT + PA + LS + ED | UC | Group | HCP | Medical | 4 weeks | S, M | U | U | Y | Y | N |
| Brosseau 2012122 | Canada | OA knee | 222 | P + PA | UC | Group | HCP | Community | 52 weeks | L | Y | Y | U | Y | Y |
| Carpenter 2012123 | USA | Low back pain | 141 | P + MBT + PA + LS + ED | WLC | Remote | Self | Community | 3 weeks | S | Y | U | U | Y | U |
| Coleman 2012124 | Australia | OA knee | 146 | P + MBT + PA + LS + ED | WLC | Group | HCP | Community | 6 weeks | S, M | Y | Y | Y | Y | Y |
| Kao 2012125 | Taiwan | OA knee | 259 | P + PA + LS + ED | UC | Group | HCP | Medical | 4 weeks | S | Y | U | Y | U | N |
| Martin 2012126 | Spain | Fibromyalgia | 180 | P + MBT + PA + ED | WLC | Group | HCP | Medical | 6 weeks | M | Y | U | U | U | N |
| McBeth 201252 | UK | Mixed pain | 442 | P + PA + LS | UC | Remote | HCP | Community | 24 weeks | M, L | Y | Y | Y | Y | Y |
| P + LS | UC | Remote | HCP | Community | 24 weeks | M, L | Y | Y | Y | Y | Y | ||||
For the update review we included an additional 19 sets of data; one trial67 was included in the original meta-analyses but published longer-term follow-up data after our 2009 cut-off103 and we included this study in the update analyses. Of the 18 new studies, five were from the USA,113,114,118,119,123 three from the UK,51–53 two each from Australia112,124 and Spain120,126 and one each from Italy,121 Germany,115 Sweden,117 Norway,116 Taiwan125 and Canada122 (n = 3745). Five focused on low back pain,53,114,115,121,123 five on OA,112,117,122,124,125 five on fibromyalgia,116,118–120,126 two on mixed pain52,113 and one on knee pain only. 51 Fourteen were health care professionally led,51–53,113,115–118,120–122,124–126 one was mixed health care and lay led112 and three were self-led. 114,119,123 Nine were conducted in a medical setting,112,115–118,120,121,125,126 eight in the community51,52,113,114,119,122–124 and one in a mixed setting. 53 Ten were delivered in groups,53,116–118,120–122,124–126 four were delivered remotely via the internet52,114,119,123 and three were delivered individually;51,113,115 one used a mixed method of delivery. 112 The mean age of participants for 17 of the 18 studies reporting age was 54 years (range when specified 25–84 years) and 2509 (67%) of the 3745 participants were female (see Table 3).
Quality assessment
Around half of the original 46 studies (54%) reported an adequate randomisation sequence, and in the remainder of the studies this was unclear. Allocation concealment was present in nine studies (20%), absent in two (4%) and unclear in 35 (78%). Masked outcome assessment was reported in 19 studies (41%), with the remainder unclear. Nearly all studies (91%) reported reasons for dropping out of the study and, in the 44 studies reporting this, the mean attrition rate across all study arms was 18% (range 6–47%). One study reported a 100% completion rate and 11 studies had an attrition rate of > 20%. Only 22 studies (48%) reported that they had analysed their data on an ITT basis, using last observation carried forward or imputation methods to fill in missing data (see Table 3). Quality assessment in the updated articles showed that 17 out of 18 studies reported adequate randomisation procedures, 9 out of 18 used concealed allocation, 10 out of 18 reported withdrawals, in 14 out of 18 researchers were masked to outcomes and in 15 out of 18 ITT analyses were conducted (see Table 3).
Overall effectiveness of self-management programmes
We used the data from the original review (up to 2009) to inform our intervention design (Table 4). The final column of this table includes the updated meta-analyses. The addition of studies from the updated search made little difference to these findings with the exception that there are more studies reporting depression and those reporting medium- and longer-term results show small effects on most outcomes. The differences in results between 2009 and 2013 showed changes in effect sizes for anxiety (small significant medium effect size in the long term) and social function (in the medium term) (see Appendix 1 for forest plots). In summary, these data suggest that the interventions studied have small beneficial effects on global health, pain intensity, physical function, quality of life, anxiety and social function in the short term and sometimes the medium term but that these effects are much reduced in studies reporting longer-term follow-up (beyond 8 months). For quality of life and anxiety, the effects in studies reporting longer-term follow-up remain small rather than ‘minor’, but closer examination reveals that in each case there was only one small study supplying longer-term follow-up data, raising the possibility of publication bias. For depression the beneficial effects are ‘minor’ in the short term but small in the medium term; however, there are far fewer studies reporting medium-term (or long-term) effects. Unlike the other outcomes, there appears to be a small beneficial effect on self-efficacy in studies reporting short-, medium- and long-term follow-up.
| Outcome | Follow-up (months) | January 1994–April 2009 | January 1994–September 2013 | ||
|---|---|---|---|---|---|
| Number of participants (number of studies) | Effect size (95% CI) | Number of participants (number of studies) | Effect size (95% CI) | ||
| Global health | |||||
| Short term | < 4 | 632 (8) | –0.34 (–0.59 to –0.08) | 976 (10) | –0.33 (–0.52 to –0.13) |
| Medium term | 4–8 | 1082 (7) | –0.46 (–0.73 to –0.19) | 1575 (10)a | –0.33 (–0.51 to –0.15) |
| Long term | > 8 | 1101 (5) | –0.05 (–0.18 to 0.08) | 1818 (9)a | –0.10 (–0.23 to 0.03) |
| Pain intensity | |||||
| Short term | < 4 | 2810 (26) | –0.27 (–0.37 to –0.16) | 4723 (35) | –0.35 (–0.47 to –0.24) |
| Medium term | 4–8 | 3911 (20) | –0.25 (–0.38 to –0.12) | 6038 (32)a | –0.29 (–0.38 to –0.20) |
| Long term | > 8 | 3332 (18) | –0.18 (–0.28 to –0.07) | 5104 (25)a | –0.18 (–0.26 to –0.10) |
| Physical function | |||||
| Short term | < 4 | 2453 (19) | –0.26 (–0.40 to –0.12) | 4093 (26) | –0.31 (–0.44 to –0.18) |
| Medium term | 4–8 | 3759 (18) | –0.15 (–0.23 to –0.07) | 5546 (28)a | –0.19 (–0.25 to –0.13) |
| Long term | > 8 | 2482 (13) | –0.12 (–0.20 to –0.04) | 3980 (19)a | –0.14 (–0.22 to –0.06) |
| Quality of life | |||||
| Short term | < 4 | 258 (2) | –0.40 (–0.65 to –0.15) | 258 (2) | –0.40 (–0.65 to –0.15) |
| Medium term | 4–8 | 399 (2) | –0.11 (–1.05 to 0.82) | 665 (4) | –0.14 (–0.55 to 0.27) |
| Long term | > 8 | 170 (1) | –0.50 (–0.80 to –0.19) | 170 (1) | –0.50 (–0.80 to –0.19) |
| Self-efficacy | |||||
| Short term | < 4 | 1275 (12)a | –0.37 (–0.50 to –0.24) | 1173 (15)a | –0.38 (–0.52 to –0.25) |
| Medium term | 4–8 | 1214 (7) | –0.29 (–0.44 to –0.14) | 2030 (10) | –0.25 (–0.34 to –0.17) |
| Long term | > 8 | 1701 (7) | –0.25 (–0.35 to –0.15) | 2173 (8) | –0.23 (–0.31 to –0.14) |
| Depression | |||||
| Short term | < 4 | 1162 (13)a | –0.15 (–0.28 to –0.03) | 1743 (15)a | –0.15 (–0.24 to –0.05) |
| Medium term | 4–8 | 597 (4) | –0.25 (–0.47 to –0.03) | 1899 (12)a | –0.26 (–0.38 to –0.13) |
| Long term | > 8 | 641 (3) | –0.04 (–0.26 to 0.18) | 1516 (7)a | –0.20 (–0.44 to 0.03) |
| Anxiety | |||||
| Short term | < 4 | 282 (5) | –0.23 (–0.54 to 0.08) | 863 (7) | –0.16 (–0.33 to 0.01) |
| Medium term | 4–8 | 451 (3) | –0.28 (–0.56 to 0.00) | 878 (6) | –0.14 (–0.31 to 0.03) |
| Long term | > 8 | 50 (1) | –0.28 (–0.84 to 0.27) | 553 (3) | –0.41 (–0.58 to –0.24) |
| Social function | |||||
| Short term | < 4 | 555 (7) | –0.31 (–0.57 to –0.04) | 899 (9) | –0.33 (–0.53 to –0.12) |
| Medium term | 4–8 | 286 (4) | –0.19 (–0.61 to 0.22) | 931 (8)a | –0.24 (–0.40 to –0.09) |
| Long term | > 8 | 205 (2) | 0.19 (–0.09 to 0.47) | 922 (6)a | –0.11 (–0.26 to 0.05) |
Effectiveness of the different characteristics of self-management programmes
The data for these analyses come from the studies identified in the original search (1994–2009).
For ease of reading we present the statistically significant SMD effect sizes [with 95% confidence intervals (CIs)] only for data favouring self-management over waiting list control or usual care for each outcome subgroup comparison except for fatigue, SF-36 general mental health and visits to HCPs. Tables showing all of the results are available from the corresponding author.
In Tables 5–7 we present small (≥ 0.2), moderate (≥ 0.5) and large (≥ 0.8) effect sizes for different outcomes at different follow-up intervals. Results that favoured the control arm, favoured neither subgroup, were non-estimable or resulted in minor effect sizes are presented in the accompanying text for each outcome.
| Outcome | Course delivery mode (95% CI) | Course leader (95% CI) | Course setting (95% CI) | Course duration (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Individual | Mixed | Remote | HCP led | Lay led | Mixed | Medical | Community | Occupational | ≤ 8 weeks | > 8 weeks | |
| Global health | 0.45 (0.17 to 0.73) | 0.61a (0.07 to 1.15) | 0.56 (0.26 to 0.86) | 0.42 (0.05 to 0.80) | 0.30 (0.03 to 0.58) | 0.61a (0.07 to 1.15) | ||||||
| Pain intensity | 0.24 (0.12 to 0.35) | 0.59 (0.03 to 1.15) | 0.27 (0.14 to 0.39) | 0.28 (0.11 to 0.45) | 0.46 (0.11 to 0.81) | 0.24 (0.12 to 0.36) | 0.22 (0.03 to 0.42) | |||||
| Physical function | 0.25 (0.09 to 0.40) | 0.28 (0.10 to 0.47) | 0.24 (0.03 to 0.45) | 0.21 (0.07 to 0.34) | 0.78a (0.27 to 1.29) | 0.26 (0.10 to 0.41) | ||||||
| Quality of life | 0.40a (0.10 to 0.69) | 0.40 (0.15 to 0.65) | 0.40a (0.10 to 0.69) | 0.40 (0.15 to 0.65) | ||||||||
| Self-efficacy | 0.37 (0.25 to 0.50) | 0.38 (0.23 to 0.52) | 0.37a (0.03 to 0.71) | 0.37 (0.07 to 0.66) | 0.41 (0.26 to 0.57) | 0.39 (0.25 to 0.54) | ||||||
| Anxiety | 0.67a (0.17 to 1.18) | 0.67a (0.17 to 1.18) | ||||||||||
| Depression | 0.25 (0.04 to 0.46) | |||||||||||
| SF-36 social function | 0.38 (0.09 to 0.68) | 0.51 (0.11 to 0.91) | 0.54a (0.04 to 1.04) | |||||||||
| Outcome | Course delivery mode (95% CI) | Course leader (95% CI) | Course setting (95% CI) | Course duration (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Individual | Mixed | Remote | HCP led | Lay led | Mixed | Medical | Community | Occupational | ≤ 8 weeks | > 8 weeks | |
| Global health | 0.54 (0.21 to 0.88) | 0.67 (0.20 to 1.15) | 0.54 (0.22 to 0.87) | 0.36 (0.12 to 0.6) | 1.08a (0.52 to 1.64) | |||||||
| Pain intensity | 0.25 (0.02 to 0.47) | 0.20 (0.02 to 0.37) | 0.29a (0.06 to 0.51) | 0.22 (0.12 to 0.32) | 0.24 (0.01 to 0.47) | 0.25 (0.08 to 0.42) | ||||||
| Physical function | 0.26 (0.09 to 0.44) | |||||||||||
| Quality of life | 0.62a (0.09 to 1.15) | 0.62a (0.09 to 1.15) | 0.62a (0.09 to 1.15) | |||||||||
| Self-efficacy | 0.29 (0.08 to 0.50) | 0.29a (0.13 to 0.44) | 0.37 (0.16 to 0.59) | 0.30 (0.09 to 0.52) | 0.27 (0.11 to 0.43) | |||||||
| Anxiety | 0.25a (0.02 to 0.48) | 0.65a (0.12 to 1.19) | 0.65a (0.12 to 1.19) | |||||||||
| Depression | 0.76a (0.22 to 1.30) | |||||||||||
| SF-36 social function | ||||||||||||
| Outcome | Course delivery mode (95% CI) | Course leader (95% CI) | Course setting (95% CI) | Course duration (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Individual | Mixed | Remote | HCP led | Lay led | Mixed | Medical | Community | Occupational | ≤ 8 weeks | > 8 weeks | |
| Global health | ||||||||||||
| Pain intensity | 0.20 (0.04 to 0.36) | 0.26 (0.15 to 0.36) | 0.23 (0.03 to 0.42) | |||||||||
| Physical function | ||||||||||||
| Quality of life | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | ||||||||
| Self-efficacy | 0.23 (0.10 to 0.35) | 0.29a (0.13 to 0.44) | 0.25 (0.10 to 0.40) | 0.29a (0.13 to 0.44) | 0.26 (0.01 to 0.52) | 0.23 (0.11 to 0.36) | 0.39a (0.02 to 0.76) | 0.26 (0.14 to 0.37) | 0.23 (0.04 to 0.42) | |||
| Anxiety | ||||||||||||
| Depression | ||||||||||||
| SF-36 social function | ||||||||||||
Effect sizes for course delivery mode (see Tables 5–7)
Of the 46 studies, 27 (59%) involved group interventions, five (11%) remote interventions and five (11%) individually delivered interventions; the remaining nine (20%) used a mixture of both group delivery and individual delivery (see Table 3). We assessed the four types of delivery methods against eight outcomes over three time periods, giving a potential of 96 subgroup effect sizes. Twenty-three small to large beneficial effects favouring self-management were found. Only one effect size comparison favoured the control (mixed led, medium term, quality of life: –0.33, 95% CI –0.11 to –0.56). Forty of the subgroup effect sizes showed no difference between self-management interventions and the waiting list control/usual care, seven showed minor (< 0.2) benefits favouring self-management and 48 were non-estimable because of a lack of data. Courses delivered to groups appeared to have the most statistically significant beneficial effects compared with control groups in the short, medium and long term. Data on outcomes for courses delivered to individuals were very sparse. Mixed and remotely delivered interventions may be beneficial but not as much as courses delivered to groups.
Effect sizes for type of course leader (see Tables 5–7)
The majority of courses [36/46 (78%)] were delivered by HCPs, with six courses (13%) delivered by a combination of HCPs and laypeople and four courses (9%) delivered by laypeople only (see Table 3). Effect sizes were calculated for these three different types of leader combinations. A total of 90 comparisons were made. Small to large beneficial effects sizes were shown favouring self-management in 12 instances and minor benefits (< 0.2) were shown in nine. Thirty-nine subgroup comparisons showed no significant benefit in either study arm and 28 comparisons were non-estimable because of a lack of data. No comparisons favoured the control arm.
Health-care professional-led self-management courses showed beneficial effects in the short, medium and long term over a range of outcomes. Lay-led courses had a small, statistically significant beneficial effect on self-efficacy only. Mixed HCP- and lay-delivered courses showed moderate to large benefits for SF-36 social function scores and global health status, but data for several comparisons were sparse with some effect sizes obtained from only one study.
Effect sizes for course setting (see Tables 5–7)
Twenty-seven (59%) studies were conducted in medical settings, 16 (35%) in community settings and three (7%) in occupational settings. A total of 90 comparisons were made. Small to large beneficial effects favouring self-management were shown for 22 subgroup analyses, with one comparison favouring the control (medical setting, medium term, quality of life: –0.33, 95% CI –0.11 to –0.56). In 31 comparisons there was no difference between the study arms, five comparisons showed minor benefits (< 0.2) for self-management and 28 comparisons were non-estimable because of a lack of data.
Pain intensity was significantly improved at all three follow-up time points in the studies conducted in medical settings but not in the studies in community settings.
Self-efficacy showed small to moderate statistically significant improvements favouring self-management in medical and community settings at most time intervals. Data for self-efficacy for self-management courses in an occupational setting were sparse. Physical function appeared to show statistically significant effect sizes favouring self-management in medical, community and occupational settings in the short term. Overall, medical and community settings had better outcomes than occupational or remote settings but there were too few studies in occupational settings to draw firm conclusions.
Effect sizes for course duration (see Tables 5–7)
Two course duration periods were assessed: ≤ 8 weeks and > 8 weeks. A total of 60 comparisons were made. Around one-third of the comparisons (18/48) showed small to large beneficial effects favouring self-management. However, one comparison favoured the control (< 8 weeks, medium term, quality of life: –0.33, 95% CI –0.11 to –0.56). All other subgroup analyses showed no benefit for either arm or minor benefits for self-management (25/48), or were non-estimable because of a lack of data (5/48).
Small to moderate statistically significant beneficial effect sizes were shown for a mix of outcomes for both short and longer durations of self-management courses at all time intervals. Statistically significant effect sizes did not appear to be enhanced by increased duration of courses.
Effectiveness of the different components of self-management programmes
In Tables 8–13 we present small (≥ 0.2), moderate (≥ 0.5) and large (≥ 0.8) effect sizes for different outcomes at different follow-up intervals. Results that favoured the control arm, favoured neither subgroup, were non-estimable or resulted in minor effect sizes are presented in the accompanying text.
| Outcome | Psychological (95% CI) | Lifestyle (95% CI) | Medical education (95% CI) | Physical activity (95% CI) | Mind–body therapies (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | With | Without | With | Without | |
| Global health | 0.53 (0.18 to 0.88) | 0.29 (0.02 to 0.56) | 0.69a (0.15 to 1.24) | 0.30 (0.03 to 0.58) | 0.61a (0.07 to 1.15) | 0.34 (0.02 to 0.66) | 0.48 (0.24 to 0.72) | |||
| Pain intensity | 0.28 (0.16 to 0.41) | 0.20 (0.09 to 0.32) | 0.36 (0.10 to 0.62) | 0.21 (0.09 to 0.33) | 0.38 (0.17 to 0.59) | 0.23 (0.11 to 0.35) | 0.28 (0.04 to 0.51) | 0.21 (0.06 to 0.36) | 0.28 (0.12 to 0.44) | |
| Physical function | 0.34 (0.18 to 0.50) | 0.22 (0.04 to 0.39) | 0.36 (0.17 to 0.55) | 0.24 (0.10 to 0.38) | 0.22 (0.08 to 0.36) | 0.65 (0.28 to 1.02) | 0.24 (0.10 to 0.38) | |||
| Quality of life | 0.40a (0.10 to 0.69) | 0.40 (0.15 to 0.65) | 0.40a (0.10 to 0.69) | 0.40 (0.15 to 0.65) | 0.40a (0.10 to 0.69) | |||||
| Self-efficacy | 0.41 (0.25 to 0.56) | 0.41 (0.24 to 0.57) | 0.31 (0.09 to 0.52) | 0.35 (0.21 to 0.48) | 0.56 (0.18 to 0.94) | 0.39 (0.25 to 0.52) | 0.42 (0.17 to 0.67) | 0.35 (0.19 to 0.51) | ||
| Anxiety | 0.36 (0.04 to 0.67) | 0.51 (0.14 to 0.88) | 0.39 (0.09 to 0.70) | |||||||
| Depression | ||||||||||
| SF-36 social function | 0.35 (0.05 to 0.65) | 0.35 (0.05 to 0.65) | 0.48 (0.11 to 0.84) | 0.39 (0.12 to 0.66) | ||||||
| Outcome | Psychological (95% CI) | Lifestyle (95% CI) | Medical education (95% CI) | Physical activity (95% CI) | Mind–body therapies (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | With | Without | With | Without | |
| Global health | 0.45 (0.10 to 0.79) | 0.52 (0.10 to 0.95) | 0.42 (0.13 to 0.70) | 0.77a (0.22 to 1.32) | 0.51 (0.17 to 0.85) | 0.46 (0.19 to 0.73) | 0.33 (0.01 to 0.65) | 0.67 (0.26 to 1.09) | ||
| Pain intensity | 0.29 (0.11 to 0.48) | 0.22 (0.11 to 0.33) | 0.22 (0.09 to 0.35) | 0.22 (0.09 to 0.35) | 0.20 (0.08 to 0.33) | 0.30 (0.05 to 0.55) | ||||
| Physical function | 0.21 (0.12 to 0.30) | 0.25 (0.06 to 0.44) | ||||||||
| Quality of life | ||||||||||
| Self-efficacy | 0.30 (0.09 to 0.52) | 0.23 (0.06 to 0.40) | 0.46 (0.20 to 0.73) | 0.26 (0.12 to 0.40) | 0.58a (0.16 to 1.00) | 0.29 (0.14 to 0.44) | 0.36 (0.17 to 0.55) | |||
| Anxiety | 0.38 (0.01 to 0.74) | 0.31 (0.11 to 0.50) | 0.38 (0.01 to 0.74) | |||||||
| Depression | 0.25 (0.03 to 0.47) | 0.25 (0.03 to 0.47) | ||||||||
| SF-36 social function | 0.38 (0.09 to 0.67) | 0.38 (0.09 to 0.67) | 0.38 (0.09 to 0.67) | |||||||
| Outcome | Psychological (95% CI) | Lifestyle (95% CI) | Medical education (95% CI) | Physical activity (95% CI) | Mind–body therapies (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | With | Without | With | Without | |
| Global health | ||||||||||
| Pain intensity | 0.20 (0.07 to 0.34) | |||||||||
| Physical function | ||||||||||
| Quality of life | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | 0.50a (0.19 to 0.80) | |||||
| Self-efficacy | 0.25 (0.15 to 0.34) | 0.22 (0.12 to 0.33) | 0.45 (0.16 to 0.73) | 0.24 (0.14 to 0.33) | 0.52a (0.09 to 0.96) | 0.25 (0.15 to 0.35) | 0.23 (0.13 to 0.33) | 0.47 (0.13 to 0.81) | ||
| Anxiety | ||||||||||
| Depression | ||||||||||
| SF-36 social function | ||||||||||
| Outcome | Two components (95% CI) | Three components (95% CI) | Four components (95% CI) | Five components (95% CI) |
|---|---|---|---|---|
| Global health | 0.65 (0.27 to 1.04) | 0.77a (0.16 to 1.38) | ||
| Pain intensity | 0.37 (0.15 to 0.59) | 0.23 (0.03 to 0.42) | ||
| Physical function | 0.37 (0.19 to 0.55) | 0.37 (0.07 to 0.67) | ||
| Quality of life | 0.40a (0.10 to 0.69) | |||
| Self-efficacy | 0.42a (0.03 to 0.81) | 0.28 (0.10 to 0.47) | 0.50 (0.04 to 0.96) | 0.43 (0.15 to 0.72) |
| Anxiety | 0.67a (0.17 to 1.18) | |||
| Depression | ||||
| SF-36 social function | 0.54a (0.04 to 1.04) | 0.63a (0.03 to 1.23) |
| Outcome | Two components (95% CI) | Three components (95% CI) | Four components (95% CI) | Five components (95% CI) |
|---|---|---|---|---|
| Global health | 0.77a (0.22 to 1.32) | |||
| Pain intensity | 0.32 (0.06 to 0.59) | 0.63 (0.11 to 1.16) | ||
| Physical function | 0.21 (0.01 to 0.42) | |||
| Quality of life | ||||
| Self-efficacy | 0.58a (0.16 to 1.00) | 0.30 (0.08 to 0.52) | ||
| Anxiety | 0.38 (0.01 to 0.74) | |||
| Depression | ||||
| SF-36 social function |
| Outcome | Two components (95% CI) | Three components (95% CI) | Four components (95% CI) | Five components (95% CI) |
|---|---|---|---|---|
| Global health | ||||
| Pain intensity | 0.36 (0.20 to 0.52) | |||
| Physical function | ||||
| Quality of life | 0.5a (0.19 to 0.80) | |||
| Self efficacy | 0.52a (0.09 to 0.96) | 0.39a (0.02 to 0.76) | 0.22 (0.11 to 0.32) | |
| Anxiety | ||||
| Depression | ||||
| SF-36 social function |
Effectiveness of self-management courses that include a psychological component (see Tables 8–10)
Only eight (17%) of the interventions in the included studies did not have a psychological component. A total of 48 comparisons were made, 21 of which showed no differences or minor effect sizes of < 0.2 between self-management and control. Eleven of the comparisons were non-estimable because of a lack of data. For interventions that included a psychological component we found small statistically significant effect sizes favouring self-management for pain intensity, physical/functional capability, SF-36 social function and self-efficacy for both short- and medium-term follow-up and there was evidence of benefit in the long term for self-efficacy but not for the other outcomes. There was no evidence that depression improved significantly for interventions with a psychological component although at medium-term follow-up anxiety was improved compared with the control groups. There was little evidence to support self-management interventions without a psychological component but most comparisons, except for pain, physical/functional capability and self-efficacy, had only one study or none at all examining this subgroup.
Effectiveness of self-management courses that include a lifestyle component (see Tables 8–10)
We included a variety of elements for the lifestyle component such as sleep management, relationship advice, diet advice, ergonomic guidance for return to work and stress management. Seven (15%) of the included studies involved an intervention that did not include a lifestyle component. A total of 48 comparisons were made, 17 of which showed no differences or minor effect sizes of < 0.2 between self-management and control. Ten of the comparisons were not estimable because of a lack of data. Overall, there was no discernible difference in the effect on self-efficacy, physical/functional capability, depression or global health status between interventions with and those without a lifestyle component.
Effectiveness of self-management courses that include a medical education component (see Tables 8–10)
Thirty-five (76%) of the interventions included a medical education component. A total of 48 comparisons were made, 21 of which showed no differences or minor effect sizes of < 0.2 between self-management and control. Ten of the comparisons were not estimable because of a lack of data. There was some evidence in favour of a medical education component with regard to anxiety in the short term and pain intensity and depression in the medium to long term. Significant moderate benefits in terms of self-efficacy were noted compared with control groups in interventions without an educational component in the short term and in medium- and long-term single studies. Data for many comparisons were very sparse.
Effectiveness of self-management courses that include a physical activity component (see Tables 8–10)
A total of 48 comparisons were made, 18 of which showed no differences or minor effect sizes of < 0.2 between self-management and control. Twenty of the comparisons were not estimable because of a lack of data. Only six (13%) of the included studies involved an intervention that did not include a physical activity component. Interventions that included a physical activity component showed some small statistically significant effect sizes favouring self-management for the following outcomes: pain intensity (medium term), self-efficacy (short term), SF-36 general mental health (short term) and global health status (short term).
Most comparisons were limited by having only one study or no studies without a physical activity component.
Effectiveness of self-management courses that include a mind–body therapy component (see Tables 8–10)
Twenty-six (57%) of the included studies involved a mind–body therapy component. A total of 48 comparisons were made, 23 of which showed no differences or minor effect sizes of < 0.2 between self-management and control. Six of the comparisons were not estimable because of a lack of data. We found no discernible patterns with regard to the effect of including mind–body therapy in self-management interventions. In the short term, interventions that did not include mind–body therapy showed small significant benefits over a wide range of outcomes compared with control groups. In the medium term the picture was mixed, with small benefits over control groups seen for different outcomes both for interventions with a mind–body therapy component and those without. Depression consistently failed to improve with self-management, irrespective of whether or not the course included mind–body therapy.
Effect of the number of components included in self-management courses (see Tables 11–13)
We could potentially have estimated a total of 96 effects. No studies were available to estimate nine of these effects. Sixty-one of these comparisons showed no differences or minor effect sizes of < 0.2 between self-management and control. When the effect estimates were > 0.2, increasing the number of components present in self-management courses from two through to five did not appear to have an overall beneficial effect on any outcome measure.
Discussion
Effectiveness of self-management programmes
This review identified 64 studies (65 papers) providing usable final-value outcome data for meta-analysis. Overall, it showed ‘small’ statistically significant effect sizes in the short term (< 4 months) for pain intensity, physical/functional capability, self-efficacy, SF-36 general mental health, global health status and SF-36 social role. These effect sizes became ‘minor’ (SMD < 0.2) and/or statistically insignificant in the medium term (4–8 months) and long term (≥ 8 months) for all outcomes except for self-efficacy (small statistically significant effect size for all time intervals) and global health status (small statistically significant effect size for medium-term follow-up).
Course content and components
We examined the effect of individual course components by comparing effect sizes for interventions with certain components and interventions without these components. This approach was hampered by few interventions not having the component of interest, in particular psychological and physical components.
Overall, the evidence suggested that:
-
Increasing the number of components does not necessarily equate to improved effectiveness.
-
There is strong evidence for beneficial effects of psychological components but the content and mode of delivery to optimise effect is unknown.
-
Any exercise can be recommended as long as the exercise is carried out regularly and is within the capability of the individual. Initial exercise advice should be given on an individual basis but physical activity can then be conducted in a group and/or supervised setting to encourage adherence.
-
Education and mind–body therapies are better placed as adjunct rather than stand-alone therapies. Education should inform patients about their condition, managing their medication and self-management strategies that can have positive effects on lifestyle.
-
Of the mind–body therapies, the strongest positive evidence appears to be for relaxation.
Course delivery
The evidence suggested that group delivery was effective. Some cognitive–behavioural elements or personalised exercise plans may be more effectively deployed on an individual basis but should perhaps be discussed or practised as part of a group session.
Other reviews have found that individual classes127 were best or that individual, group and home-based exercises were all equally effective. 128 Guidelines on exercise129 recommend both individual structured and group approaches to exercise. In addition, another study suggested that a group lecture format was most effective for delivering education. 130
Course leader
The evidence suggested that HCP-led courses were most effective, although there was also some evidence for lay-led or mixed courses. We found limited evidence (three studies) to suggest that lay leaders are as effective as HCPs.
Our subgroup comparison between HCP-, lay- and mixed-led courses showed that HCP-led content produced small but significant effect sizes mainly in the short term There was some evidence for the effectiveness of lay-led courses for pain, physical/functional capability and self-efficacy but the effect sizes were either < 0.2 or the data were obtained from one study only.
Two other reviews52,127 have found that HCP-supervised or -monitored programmes were most effective.
Course setting
The evidence suggested that the effects of setting were varied. Our subgroup analysis showed that the medical setting and community settings were favoured compared with occupational and remote settings.
One review113 suggested that a ‘back school’ in an occupational setting was effective, whereas another review131 felt that programmes should be conducted in primary care (UK). One review examining web-based CBT132 found that ‘live’ internet sessions had the lowest dropout rate and that internet-delivered self-management material showed promise, especially for people with limited mobility. Self-management material available online may help to support participants during and after courses and provide a virtual forum to complement any friendships formed during the group sessions.
Course duration
The evidence suggested that shorter courses were as effective as longer ones (and were likely to be more cost-effective).
Other evidence from studies reviewing course duration is equivocal, with one review finding increased effectiveness for courses > 100 hours133 and another suggesting that longer courses (3–6 months) are too costly and impractical to implement134 and that, in a subgroup analysis, there is no evidence of a difference in effectiveness between courses of ≥ 30 hours and courses of < 30 hours.
In summary, therefore, we found that shorter courses were as effective as longer ones and that multicomponent courses were not necessarily more effective than those with two or three components; however, we found weak or no evidence that self-management courses reduce the number of health-care visits. Solely HCP-led courses were more beneficial for pain outcomes but would be more expensive to run than lay courses. A mixed-led approach is probably more viable. Using a digital versatile disk (DVD) of a pain consultant delivering an education component instead of being there in person, or perhaps having some psychological material delivered by a student psychologist rather than a consultant, may be a worthwhile alternative. Group and individual sessions were found to be effective, although individual sessions are more labour intensive and are therefore more likely to be expensive. Self-management courses delivered in a community setting may be less expensive than those delivered in a primary care venue. If any remotely delivered online material for use as a supporting or follow-up aid is included then the costs of website design and maintenance, website hosting and forum moderation would need to be considered.
Study limitations
Internal validity: study bias
Unfortunately, because of time constraints, it was not possible to write to the study authors to clarify their methods or seek more data.
Although the funnel plot and metabias test suggested that there was no publication bias, the low number of studies (n = 26) and uncertainty about the appropriateness of the metabias test for SMDs of continuous outcomes make these conclusions tentative. We also found very few studies that showed negative effects of self-management programmes and we did not consider literature in any other language than English and so there is a possibility of publication bias.
Treatment of the control groups
We acknowledge that ‘usual care’ (the control condition in most of the studies included in this review) can vary markedly from setting to setting and is often very poorly described in publications. Thus, there is likely to have been considerable heterogeneity in the treatment received across the control groups.
Multiple testing
We made no adjustment for multiple testing. We are aware that some of the positive associations that we report may have arisen by chance as a result of the large number of tests conducted.
Component analysis framework
We employed a subjective approach to categorising studies into component categories for our subgroup meta-analysis. It was sometimes difficult to distinguish the component elements from the intervention descriptions and so there is an amount of subjectivity involved. We found that descriptions for CBT or psychological interventions included elements from our psychological component framework and our mind–body therapies component framework. It may have been more meaningful to group psychological and mind–body therapies together.
We used the SMD to combine the results from different outcome measurement tools, using Cohen’s d63,64 as a measure of effect size. We are cognisant of the difficulties with interpretation of Cohen’s scale in a clinical setting.
We expected a high degree of heterogeneity because of the variation in self-management courses and particularly the variation between subgroups, with studies being subjectively grouped according to number and type of components. We found moderate to substantial heterogeneity for most outcomes, with a small statistically significant effect size, except for self-efficacy, which had < 25% heterogeneity across all time intervals.
Our subgroup analyses did not completely resolve heterogeneity for all subgroups. Although some subgroups showed an I2 value of 0%, some comparator subgroups had very few studies and so it was difficult to draw any conclusions from these patterns.
Overall conclusions
Our meta-analysis echoed the findings from previous systematic reviews, showing that self-management courses produce small statistically significant beneficial effects in the short term for outcomes such as pain intensity and physical/functional capability, but that these effects are not maintained into the longer term.
We found that increasing the number of self-management components and number of sessions did not necessarily result in increased effectiveness, which has implications for costs. We found strong evidence of effectiveness for courses including a psychological course component and encouraging evidence for courses delivered to groups. There was some limited evidence of beneficial effects for mind–body techniques and medical education, and these are best provided as an adjunct.
The ways in which the findings of the systematic review influenced the design of the intervention are described fully in Chapter 6.
Chapter 3 Identifying who is likely to respond to self-management programmes for chronic musculoskeletal pain
Abstract
Introduction: Establishing the characteristics of groups of people who are likely to gain the most benefit from self-management interventions is important but, as yet, this has not been accomplished.
Aim: The aim of this systematic review was to examine the evidence for predictors, moderators and mediators of patient outcomes, as reported in RCTs of self-management support for people with chronic musculoskeletal pain.
Methods: We searched relevant electronic databases for RCTs and systematic reviews that measured and reported baseline measures and analysed them in relation to interventions and outcomes. We assessed the evidence according to the methodological strengths of the studies. We carried out meta-regression analyses for age and gender, as potential moderators.
Results: Most of the studies were compromised by lack of power for moderator and mediator analyses. There was evidence that self-efficacy and depression at baseline predict outcome and evidence that pain catastrophising and physical activity can mediate outcome from self-management. There was no clear evidence on moderators.
Conclusions: Although the current evidence is scarce, it suggests that the development of interventions should include careful consideration of self-efficacy, depression, physical activity and catastrophising.
Introduction
In this chapter we review the available evidence indicating which type, or types, of people may benefit most from self-management courses for chronic pain. Specifically, we reviewed the literature identifying predictors, moderators and mediators of the effects of self-management interventions.
We used a systematic review approach to identify:
-
RCTs of self-management for chronic musculoskeletal pain that reported subgroup analyses looking at predictors, moderators and mediators in different subgroups of patients
-
RCTs of self-management for chronic musculoskeletal pain that included data on treatment moderation suitable for a meta-regression.
Definitions
The definitions of predictors, moderators and mediators have been refined in recent years. We adopted the approach of Kraemer et al. 32 in which three types of subgroups are described and clearly defined:
Predictors of treatment outcome are defined as baseline variables that affect outcome (significant main effect only) but do not interact with treatment. Such factors significantly predict outcome equally for target interventions and control conditions.
Effect moderators (or moderators) are variables measured at baseline (such as patient baseline characteristics) that interact with treatment to change outcomes. The interaction should be related to outcome in the linear model with or without a main effect. These specify for whom and under what conditions treatment works and can improve power in subsequent trials by better selection of target groups for stratification.
Mediators are variables measured during treatment (such as change-in-process factors) that impact on outcome, with or without interaction with treatment. Mediators help inform the process and potential mechanisms (including causal mechanisms) through which treatment might work. They can be used to improve subsequent interventions through strengthening the components that best influence the identified mediators. Mediators should not be a component of the treatment or outcome. There should be a clear distinction between the constructs measured by the proposed mediators and treatment outcome.
Predictors and moderators may include:31,32,42
-
demographic status [age, gender, education, marital status, lifestyle (alcohol consumption, exercise, smoking)]
-
clinical status (e.g. disability, duration of pain, pain intensity)
-
psychological status (e.g. catastrophising, depression, fear avoidance and beliefs)
-
work-related factors [e.g. employment status, type of work, reasons for not working, number of sick days taken over previous year, financial factors (pending compensation, sickness benefit, insurance and duration on current benefits), job satisfaction, social support at work, a sense of control at work]
-
medication history.
Methods
Studies were identified as an eligible subset of those identified in Chapter 2.
Quality assessment of included studies
For the overall quality of the papers we used the assessment carried out for the review of main effects. Early in the project we sought established methodological criteria for the assessment of subgroup analyses within RCTs. We were unable to identify any such criteria suitable for our purpose. Consequentially, as part of this programme of work we carried out a literature review and Delphi study to determine a consensus on methodological criteria for the evaluation of studies reporting moderator analyses within RCTs. We used these assessment criteria to assess the quality of our moderator studies and grade the evidence for moderators. 135
We applied the following criteria to included papers:
-
Was there an a priori specification of the subgroup?
-
Was there a theoretical or an evidence-based rationale for the selection of subgroup factors?
-
For moderator and predictor analyses only: was the measurement of subgroup factors carried out prior to randomisation?
-
Was the measurement of subgroup factors adequate (reliable and valid) and appropriate for the target population?
-
For moderator and mediator analyses only: does the analysis contain an explicit test of the interaction between subgroups and treatment?
Papers were classified as providing either confirmatory findings or exploratory findings. Confirmatory findings refer to studies that include a priori hypotheses in relation to subgroups, for which support was obtained through adequate statistical testing. Exploratory findings inform future research (hypothesis generating) and are the product of post hoc testing. Only studies that satisfied all of the above five criteria relevant to the analysis were regarded as providing confirmatory evidence. Papers satisfying criteria 3–5 were categorised as exploratory findings. Studies that did not satisfy these criteria were regarded as having insufficient findings. For this review we applied these same criteria to predictor and mediator analyses as appropriate.
We included any RCTs identified in the searches in Chapter 2 that reported predictor, moderator or mediator analyses. For selected RCTs that did not report any subgroup analyses but which had ≥ 80% completion rates for their primary analysis and > 200 participants in each arm, the authors were contacted and asked if they had carried out, or were now able to carry out, such analyses. We also contacted authors when any aspects of their reported subgroup analyses were unclear.
Data extraction
We extracted the following data from each RCT:
-
country and setting
-
population: brief description of participants, including size of eligible population identified and actual uptake
-
intervention/control
-
baseline factors measured
-
outcomes measured
-
description of predictor, moderator and mediator analyses
-
results of predictor, moderator and mediator analyses.
Finally, an independent statistician scrutinised the included studies to determine whether or not there were appropriate data to include in the meta-regression and whether any moderator or mediator analyses were carried out appropriately. The statistician checked for the presence of a reported power calculation; whether there were any statistician authors or whether the authors described seeking statistical advice; and the statistical methods used and the quality of reporting, paying particular attention to moderator and mediator analyses. We also conducted a meta-regression to identify potential moderators. We included any RCTs identified in our original search (see Chapter 2) whose final-value data were suitable for inclusion in a meta-regression. For all potential moderators reported in ≥ 10 studies we performed a random-effects meta-regression. 39 We collapsed outcomes into the following domains: pain intensity, physical/functional capability, self-efficacy, depression and global health status. When a variety of measurement tools had been reported for a domain, we calculated SMDs (difference in mean outcome between groups/SD of outcome among participants). 39 To make the best use of the available data, and reduce the possibility of making a type 1 error, we collapsed the different follow-up time points (early and late) to obtain one average effect size per outcome. We considered results from the meta-regression to be statistically significant if p < 0.10. We used this criterion because of a potential type II error as a result of the limited number of studies in these effect size calculations. 42,136 We used an I2 statistic to estimate the percentage of residual variation attributable to between-study heterogeneity and an adjusted R2 statistic to estimate the proportion of between-study variance explained by the covariate. We produced scatter diagrams using circles as plotting symbols in which the areas of the circles indicated the value of a third variable. 137 We fitted values and predicted random effects against age and gender separately, with 95% confidence.
Results
Of the 126 RCTs that looked at self-management programmes, a total of 20 articles67,75,93,104,107,110,138–151 covering 16 studies (n = 4047) met our inclusion criteria and included appropriate analyses of predictors, moderators or mediators. In addition, 46 of 126 studies met our criteria for meta-regression to identify potential treatment moderators.
Of the 16 studies, five were from the USA,93,104,138–140 four were from the Netherlands,93,141–143 two each were from Canada,107,144 Norway75,145 and Sweden146,147 and one was from the UK. 67
Methodological quality
In the first instance we applied quality assessment criteria to the trial methodology, using criteria adapted from The Cochrane Collaboration methods39 and described in Chapter 2. We independently analysed and coded each publication separately, even though some studies were further analyses of previously published RCTs; hence, in Table 14 we present the quality appraisal for 20 reports covering 16 studies. 67,75,93,104,107,110,138–151 Nine RCTs were of high quality, six were of medium quality104,110,138,139,145,148 and one was of low quality. 146 Eight of the subgroup studies provided confirmatory evidence, one study provided exploratory evidence93 and seven studies provided insufficient evidence. For subgroup analyses we applied the criteria described in Quality assessment of included studies. Only three studies67,107,142 used high-quality trial methodology and carried out preplanned theoretically driven subgroup analysis, using correct statistical analysis. However, there were no two trials in this category (for any predictors, moderators and mediators) that examined the same subgroup.
| Study | 1. Was the analysis a priori? | 2. Was selection of factors for analysis theory/evidence driven? | 3. Were subgroups measured prior to randomisation? | 4. Adequate quality of measurement of baseline factors? | 5. Contains an explicit test of the interaction between subgroup and treatment (e.g. regression)? | Strength of evidencea | Quality of underlying trial |
|---|---|---|---|---|---|---|---|
| Gallagher 1997138 | Yes | Yes | NA, gender as moderator | Yes | Yes | Confirmatory | Medium |
| Haas 200593 | No | No | Yes | Yes | Yes | Exploratory | High |
| Haldorsen 199875 | No | No | Yes | Yes | Unclear | Insufficient | High |
| Haugli 2000145 | Yes | Yes | Unclear | Yes | Yes | Confirmatory | Medium |
| Haugli 2003148 | Yes | Yes | Unclear | Yes | Yes | Confirmatory | Medium |
| Heapy 2005140 | Yes | Yes | Yes | Yes | No | Insufficient | High |
| Hurley 200767 | Yes | Yes | NA, cluster randomisation | Yes | Yes | Confirmatory | High |
| Jensen 2001147 | Yes | Yes | NA, gender as moderator | Yes | No | Insufficient | High |
| Jensen 2005149 | Yes | Yes | NA, gender as moderator | Yes | No | Insufficient | High |
| Kole-Snijders 1999141 | Yes | No | No | Yes | Yes | Insufficient | High |
| Laforest 2008107 | Yesb | Yes | Yes | Yes | Yes | Confirmatory | High |
| Lemstra 2005144 | No | No | Unclear | Yes | No | Insufficient | High |
| Lindh 1997146 | Yes | Yes | NA, demographic moderators | Yes | No | Insufficient | Low |
| Lorig 2002139 | Yes | Yes | Yes | Yes | No | Insufficient | Medium |
| Martire 2007104 | Yes | Yes | NA, demographic moderators | Yes | Yes | Confirmatory | Medium |
| Nour 2006150 | Yes | Yes | Yes | Yes | Unclear | Insufficient | High |
| Smeets 2006142 | Yes | Yes | Yes | Yes | Yes | Confirmatory | High |
| Spinhoven 2004151 | Yes | Yes | No | Yes | Yes | Insufficient | High |
| van der Hulst 2008110 | Yes | Yes | Yes | Yes | Yes | Confirmatory | Medium |
| Veenhof 2007143 | Yesb | Yes | NA, cluster randomisation | Yes | Yesc | Confirmatory | High |
We did not find any high-quality studies appropriately reporting moderator analyses.
Predictors
Hurley et al. 67 found that higher levels of depression at baseline predicted poorer physical functioning at 6 months in people with chronic knee pain (effect size 0.48; p = 0.011), whereas higher levels of self-efficacy at baseline, measured by positive exercise beliefs (effect size –0.24; p = 0.001) and confidence in the ability to exercise (effect size –0.62; p = 0.001), predicted better physical functioning at 6 months, regardless of intervention arm.
Mediators
Smeets et al. 142 found that reduced levels of pain catastrophising during treatment led to a post-treatment decrease in patient-specific complaints, disability and pain in people with chronic low back pain. Patients in the intervention group scored, on average, 1.3 points lower for disability (out of 24) than patients in the control arm, after adjusting for pain catastrophising. For current pain, the difference was 4.7 units on a visual analogue scale (out of 100). For patient complaints, the difference was 6.7 (out of 100).
Laforest et al. 107 reported that increases in physical activity mediated greater decreases in helplessness in people with arthritis; however, the data were not available to quantify this effect. This effect was defined by Laforest et al. 107 as a moderator, although from the authors’ description it is a mediator. Because of these limitations, we recommend that the findings for the mediating effects of physical activity be reviewed with caution.
Meta-regression to identify potential moderators
All RCTs identified from the original search that supplied full data on age and gender at baseline against at least one of our selected outcomes were included (n = 46/126).
We used bivariable meta-regression to determine whether or not the baseline characteristics (age and gender) explained the variation in treatment outcomes. Age and gender were selected because they are the most frequently reported demographic characteristics. 39 They were the only variables reported in at least 10 studies.
For one outcome, general mental health, a single measurement tool had been used, the SF-36. 62 We intended to combine the data as a weighted mean difference but because of anomalies in score values between studies we judged that SMD analyses would be more robust. A variety of measurement tools was reported for each domain; for these we also calculated SMDs.
Forty-six studies were included in the meta-regression and eight in the 16 subgroup meta-regressions. Gender was significantly associated with effect size for SF-36 general mental health and global health status, indicating that self-management interventions were more effective for groups with a higher proportion of females (all p < 0.10 and p ≥ 0.05) (Table 15). Inspection of bubble graphs (see Appendix 2, Meta-regression) suggested a positive association between studies with an increasing proportion of males (note that a fall in measures indicated improvement in our analyses). Gender was not statistically significantly associated with effect size for pain intensity, physical/functional capability, self-efficacy or depression. Age was associated with effect size for physical/functional capability and self-efficacy (all p < 0.10 and p > 0.05). The bubble graphs (see Appendix 2, Meta-regression) suggested a positive association between effect size and these outcomes, indicating that self-management interventions might be more effective in younger samples. Age was not associated with effect sizes for the other outcomes.
| Measure | Studies (sample size), n | Moderator | I2 (%) | Regression coefficient | 95% CI for regression coefficient | p-value |
|---|---|---|---|---|---|---|
| Pain intensity | 39 (6067) | Proportion male | 47.7 | –0.0006 | –0.0062 to 0.0051 | 0.840 |
| Functional capability | 27 (4790) | Proportion male | 51.8 | –0.0019 | –0.0084 to 0.0047 | 0.560 |
| Self-efficacy | 17 (2576) | Proportion male | 32.4 | –0.0017 | –0.0115 to 0.0082 | 0.732 |
| Depression | 16 (1902) | Proportion male | 24.2 | –0.0025 | –0.0108 to 0.0058 | 0.533 |
| SF-36 general mental health | 11 (1117) | Proportion male | 51.6 | 0.0097 | –0.0021 to 0.0214 | 0.095b |
| Global health status | 14 (1801) | Proportion male | 59.1 | 0.0114 | –0.0003 to 0.0230 | 0.055b |
| Pain intensity | 39 (6012) | Age | 43.1 | 0.0004 | –0.0114 to 0.0121 | 0.116 |
| Functional capability | 28 (4873) | Age | 45.8 | 0.0078 | –0.0008 to 0.0164 | 0.074b |
| Self-efficacy | 17 (2576) | Age | 17.3 | 0.0081 | –0.0004 to 0.0165 | 0.060b |
| Depression | 16 (1902) | Age | 13.3 | 0.0060 | –0.0025 to 0.0144 | 0.156 |
| SF-36 general mental health | 11 (1117) | Age | 53.2 | 0.0118 | –0.0082 to 0.0317 | 0.176 |
| Global health status | 14 (1801) | Age | 61.2 | 0.0159 | –0.0085 to 0.0402 | 0.223 |
Discussion
Summary of findings for predictors, moderators and mediators
There were only three studies with sufficient methodological rigour, including a priori planned subgroup analysis, to inform on predictors and mediators. The findings from these studies suggest that high levels of depression at baseline are associated with lower function and that self-efficacy, especially about the ability to exercise, is associated with improved function 6 months later, regardless of any intervention. 67 There was also evidence that reduction in catastrophic thinking directly after the intervention was associated with improved function 6 months later. 142 Finally, engaging in exercise during the intervention was associated with better function at outcome. 107
Findings from the meta-regression looking at age and gender in relation to outcomes showed no significant associations with pain intensity, physical/functional capability, self-efficacy, depression, SF-36 general mental health or global health status. As mentioned in the methods section, because of the small number of studies for each outcome, marginally significant associations (p < 0.10), were considered as hypothesis generating for future studies to explore possible relationships. 42
Recommendations
Based on the above findings we can make the following recommendations:
-
Findings for the predictive effect of self-efficacy on outcomes and the mediating effects of pain catastrophising on function indicate that interventions should incorporate strategies to improve self-efficacy and coping techniques.
-
Based on the currently available evidence, we do not recommend targeting the intervention to a particular age group or gender.
-
There is currently no evidence to support an association between duration of pain/complaint, pain intensity, work status, disability level or diagnosis of depression at baseline and outcome. Therefore, access to current interventions should not be limited based on these factors.
-
We recommend that study authors use the criteria that we identified for assessing the quality of moderator studies to guide their methodology.
Limitations/considerations of this review
Because of the small numbers of studies carrying out appropriate analyses, we were unable to quantitatively assess predictors, moderators and mediators of self-management programme outcome success, other than age and gender. Overall, the evidence is insufficient to inform on moderators, mediators and predictors of intervention success, other than for self-efficacy as a predictor and catastrophic thinking as a mediator. We have only considered RCT data here rather than including observational data. This is because we were specifically interested in factors to consider in the context of a RCT rather than an observational cohort study.
Because of the lack of consistent reporting of potential predictors, moderators and mediators in research, the meta-regression featured only two potential moderators, age and gender. These were selected as they are the most commonly reported characteristics in studies and data were readily available. Only when researchers report potential moderating variables as a standard can meta-analytical techniques be used to calculate a more accurate estimate of the variance between studies.
Studies reporting significant predictor, moderator or mediator effects of self-management interventions are more likely to be published than those studies that do not report significant effects. We did not include unpublished studies or dissertations in this review and therefore the studies identified for this review are likely to reflect those that escaped the ‘file drawer’ problem,152 which might be a result of publication bias.
An additional limitation, which most meta-analytical studies face, involves combining scores from different tests. For example, several different tests measuring self-efficacy were combined and it was unclear whether or not they were suitable for merging until an item analysis had been carried out on the measures. It could be argued that only constructs that are measured using the same instrument should be merged, to avoid this problem.
The analysis showed heterogeneity of findings for some outcomes. For example, a mild to moderate level of heterogeneity of findings across studies was reported in the meta-regression. Heterogeneity may result from the small number of studies available for each test. Other factors that have not yet been identified, or not yet included in moderator research, might also explain some of this heterogeneity. It would be premature to conclude that intervention effects do not vary by patient or study characteristics. We were unable to examine several moderator effects because of insufficient data (i.e. education, self-efficacy, pain duration, ethnicity, disability and depression).
We aimed to identify moderators, predictors and mediators of efficacy of self-management programmes for those with musculoskeletal pain. We believe that this systematic summary of the literature identifying moderators, predictors and mediators, and meta-analysis to examine influences of moderating factors provides useful recommendations based on evidence and we have also provided a checklist of methodological criteria for assessing moderator studies.
Chapter 4 Qualitative study
Abstract
Introduction: Our knowledge about the impact of self-management courses on individuals is limited. This study aimed to understand how different components and characteristics of self-management courses are perceived by people living with long-term conditions, course tutors and experts, to explore reasons why they might lead to different outcomes and to consider the implications for the content of a new intervention.
Methods: We used a qualitative approach with purposive sampling to maximise sample diversity. Face-to-face interviews were conducted with people with chronic musculoskeletal pain who had attended self-management courses. We then ran two focus groups, one with experts in the field of self-management for long-term conditions and one with self-management course lay tutors, to explore our interview findings. Topics discussed included referral, attendance, course content and character and outcomes.
Results: Sixteen previous course participants from Warwick and London were interviewed; in addition, six experts attended one focus group and five tutors attended the other. We identified two types of chronic pain behaviour: fixated and distracted. Promoters of positive change included support, motivation, engagement in the process, high-quality tutoring and identifying with the learning process. Sustainers of change included implementation of coping strategies, networking and socialising, distraction, support from others and new identity creation. Those who responded well to self-management courses moved away from absorption in their ‘pain world’ towards integrating into their social and/or work communities. We grouped outcomes into six domains: functional, physical, emotional, social, economic and medical.
Conclusions: Courses should involve good-quality facilitation, cognitive–behavioural approaches to promote change, medical education, a group/social setting and exposure to information about local activities to encourage long-term lifestyle behaviour change. Flexibility in course structure is required to accommodate social interaction and self-discovery to promote self-acceptance and the development of a new identity other than that of a ‘pain patient’.
Background
In this study we explored participant experiences of self-management programmes for chronic pain to identify and understand why some aspects of different courses may be more beneficial than others; the uptake, retention in and acceptability of self-management courses; and why some participants feel that they might do well on self-management courses, whereas others do not.
The findings are interpreted in the light of existing theory around concordance and living with chronic conditions and, when necessary, we propose new explanations for the findings. This study provided information for the development of the new self-management intervention. By exploring what people’s perceptions were of important components of self-management courses we were able to emphasise these components in our new course.
Method
We adopted qualitative methods, using an iterative approach (each stage informed the next). We used both in-depth interviews with participants and focus groups with experts in self-management of long-term conditions and lay tutors to explore the findings from the interviews. Interviews were conducted with participants in Tower Hamlets (inner-city London) and Warwick (a mix of urban and rural living in the Midlands). These represent two very socially and economically different areas, chosen to increase the range of views and opinions encountered. In Tower Hamlets there is a large Bengali population. First-generation Bengali migrants in Tower Hamlets have a particularly high prevalence of chronic pain. 153 As part of this overall programme of work we wanted to ensure that our intervention was suitable for this population.
First, we conducted face-to-face in-depth interviews with participants who had attended self-management programmes; the information from the interviews informed the questions and topic guide for the focus groups. We convened focus groups with experts from the field of self-management and lay tutors. Additionally, we used the focus groups to triangulate findings from the interviews.
Recruitment and sampling: individual interviews
We aimed for a diverse sample, with a total anticipated sample size of 20 (or fewer if data saturation was reached earlier). We recruited people living with persistent pain who had attended self-management courses. Two Expert Patients Programme community interest companies, one in London and one in Warwick, and Social Action for Health in London approached participants who had attended their self-management programmes and invited them to be interviewed by the study team. The invitation letter contained a consent to contact form and a reply-paid envelope so that the researchers could approach those willing to participate. We also invited people to participate via an internet chat forum for people who had attended self-management courses.
Participant inclusion and exclusion criteria are shown in Table 16.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
We used a purposive, diversity sampling method to obtain a good representation of different genders, age groups, socioeconomic areas (by postcode) and self-management course attendance record. Further data about the participants were collected after they had consented to participate in the study.
Conducting the interviews
Face-to-face interviews were arranged by the researchers at a time and place convenient to the participants. With permission, interviews were audio-recorded, transcribed and anonymised, with each interview lasting approximately 1 hour. The content of the topic guide was informed by knowledge of the literature, the specific needs of this study and the study team’s past experience of issues in other trials and evaluations of self-management programmes. The topic guide covered referral processes, motivation to attend courses, memorable aspects of courses, most effective and least effective components, positive and negative aspects of support/networking, follow-up, strategies for coping and long-term effects/outcomes of courses. Most interviews were conducted by a medical anthropologist; as a substantial proportion of first-generation Bangladeshi residents who live in Tower Hamlets are not fluent in English some interviews were carried out by a health researcher fluent in Bengali/Sylheti and English.
The focus groups
We convened two focus groups (maximum six participants in each), one with UK-based self-management academic researchers and opinion leaders in this field (the study team identified key personnel to approach based on their own knowledge of researchers and policy-makers in this field) and one with self-management course lay tutors and providers (a mix of programme leaders and tutors was approached). The focus groups were facilitated by a health researcher familiar with self-management research and a musculoskeletal clinician. We fed the findings from the individual interviews into the focus groups to test the ‘acceptability’ of our findings and to generate discussion about participant receipt of self-management courses and the issues raised by participants who attended courses.
Analysis
All focus group conversations and individual interviews were audio-recorded and transcribed. NVivo software version 9 (QSR International, Warrington, UK) was used to manage the analysis of the data. We carried out thematic analysis using the framework method; this involves familiarisation with the literature and the development of a framework based on emergent themes and subthemes. 154 A third experienced qualitative researcher was involved as an independent reviewer; to promote study validity, he commented on the framework of themes and subthemes based on a sample of interviews and independently coded a transcript. Three researchers coded the same three transcripts to test the framework and to compare the inter-rater reliability of our coding. We used this as a training exercise to improve the consistency and reliability of coding. The results from the focus groups with experts and lay tutors were also compared and contrasted with the findings from the interview study.
Ethical approval was granted by the East London and the City Research Ethics Committee Alpha (reference number 09/H0704/24).
Results
Sample
Twenty-six people participated in this study. We conducted 16 one-to-one interviews, at which point we felt that we had reached data saturation. Twelve interviews were conducted with English-speaking participants and four were conducted in Bengali/Sylheti. Four interviews were conducted in Warwickshire and the remainder were conducted in East London. Table 17 shows the characteristics of the interview sample.
| Participant | Male/female | Pain duration (years) | Age (years) | Previous self-management course attendance | Location rural/urban | Language fluency English/Bengali |
|---|---|---|---|---|---|---|
| P1 | M | 4 | > 45 | < 50% | U | E |
| P2 | F | 23 | > 45 | < 50% | U | E |
| P3 | F | 10 | ≤ 45 | 50% | R | E |
| P4 | M | 15 | > 45 | 50% | U | E |
| P5 | M | 7 | ≤ 45 | 50% | U | E |
| P6 | F | 20 | > 45 | 50% | R | E |
| P7 | F | 16 | > 45 | 50% | R | E |
| P8 | F | 6 | ≤ 45 | 50% | R | E |
| P9 | F | 18 | ≤ 45 | Unsure | U | E |
| P10 | F | 23 | > 45 | 50% | U | E |
| P11 | F | 20 | ≤ 45 | 50% | U | B and E |
| P12 | M | 16 | > 45 | 50% | U | B and E |
| P13 | F | 13 | ≤ 45 | < 50% | U | B |
| P14 | M | 17 | > 45 | < 50% | U | B |
| P15 | F | 10 | ≤ 45 | Unsure | U | B |
| P16 | F | 5 | ≤ 45 | ≥50% | U | B |
We ran two focus groups. The tutor focus group included five self-management course tutors or facilitators active in east London, south London and Essex (FG1.1–1.5). The expert focus group also consisted of five participants (one primary care trust service commissioner, three academic researchers and one self-management course service provider) (FG2.1–2.5). When the focus group discussion reflected the thoughts and beliefs of the interviewees we have added this into the text. We also report dissonant data from the focus group discussion when applicable.
Themes and subthemes from the interviews
We initially derived eight main themes (Table 18, first column). When we initially tested this framework we found a lot of duplication of data in themes 3 and 8. There were many references to social aspects of life (theme 3) but these were nearly always in the context of barriers to and/or promoters of change (theme 8); these themes were therefore merged. Additionally, the original themes 1 and 4 were collapsed to behaviour and thoughts, including empowerment and ‘negative’ behaviours, including self-absorption, anger and frustration. We also identified a new theme about outcome expectations. The last two columns in Table 18 show the final framework used for the analysis of the interviews.
| Original framework | Reasons for evolving framework | New framework | ||
|---|---|---|---|---|
| Theme | Subtheme | Theme | Subtheme | |
| Theme 1: personal attitude and behaviour | (a) Self-responsibility/empowerment | Overlap with theme 4. Merged 1(a) with 4(b) and 1(b) with 4(a) and 4(c) to create theme II | ||
| (b) Lack of self responsibility/empowerment | ||||
| Theme 2: identity | (a) Self (past, current and future) | This theme is robust: remains as theme I | Theme I: identity | (a) Self (past, current and future) |
| (b) Social (context of self with others, family and friends) | (b) Social (context of self with others, family and friends) | |||
| Theme 3: social support/sharing/networking | Overlap with theme 8. Merged 3 and 8(a) and 8(b) to create theme VI | |||
| Theme 4: psychological aspects of living with constant pain | (a) Self-absorption (with condition, self-justification, legitimisation, reinforcement, visual ‘badges’, carers and caring) | See theme 1 | Theme II: pain-related behaviours and thoughts | |
| (b) Positive emotions (distraction, looking beyond illness) | ||||
| (c) Negative emotions (anger, frustration, depression, boredom) | ||||
| Theme 5: knowledge and information | This theme is robust: remains as theme II | Theme III: knowledge and information | (a) Marketing, referrals and recruitment | |
| Theme 6: course-specific details | (a) Referrals | Renamed to more accurately represent data; expanded to create theme IV(a) | Theme IV: course characteristics | (a) Marketing, referrals and recruitment |
| (b) Relationship of course with general practitioner and treatment plans | Merged with theme IV(a) | |||
| (c) Expectations/long-term effects/outcomes, application of learning | A theme in itself and therefore classified accordingly as theme VII | |||
| (d) Tutor impact/behaviour/delivery | Expanded to include aspects of delivery. Renamed and now theme IV(b) | (b) Course delivery and impact of tutor | ||
| (e) Attendance | Unaltered | (c) Attendance | ||
| (f) Mix and character of attendees | Unaltered | (d) Mix and character of attendees | ||
| Theme 7: course components | (a) Pacing | Themes 7(a) and 7(b) inseparable and treated as one factor. Combined to form theme V(a) | Theme V: course components | (a) Pacing, action planning and goal-setting |
| (b) Goal-setting | ||||
| (c) Medication advice | Themes 7(c) and 7(d) combined to form theme V(b) | (b) Medical education | ||
| (d) Treatments | ||||
| (e) Other | Unaltered | (c) Other | ||
| Theme 8: change | (a) Promoters of change | Combined with theme 3 to form theme VI | Theme VI: change | (a) Promoters of change |
| (b) Barriers to change | (b) Barriers to change | |||
| Newly created theme VII from theme 6(c) | Theme VII: expectations and outcomes post course | |||
Findings
Theme I: identity
We noted a ‘persistent pain’ identity in some participants, some of whom had visual cues to help others identify their condition, such as bandages, walking aides or strapping. These participants’ lives were busy with being in pain, for example centring their days or weeks around medical appointments and/or personal health regimes. Referring back to one’s previous self, that is, before pain, was common. Views expressing boredom, social isolation, frustration and loneliness, predominantly caused by a lack of understanding from others, were not unusual. Examples of reframing one’s self-identity were evident in those who had adjusted well to their condition and who related positive stories about their quality of life. References to past selves were present in those who did not appear to be coping as well, whereas those with more positive attitudes to life described ways of adapting previous skills and experiences:
I was not like this before you know, they look at me and feel sorry for me, they say why is your health like this?
P11
I was very fit and active up until about 4 years ago, I started to get really bad pains in my joints, well I would say really chronic pains, it actually felt like my joints were being ripped apart.
P1
Because it’s like you revert back to like the young kid stage again; you have got to rely on your parents to do everything for you, you know, and that’s how I feel sometimes
P8
I feel defeated, because basically it brings a lot of failure and it can actually let you sit down and feel sorry for yourself, and because I was never that sort of person.
P4
So no wonder why I feel so bitter, absolutely bitter about all this, really – not a finger of help, nothing! The help had to come from outside and my daughter had to really . . .
P15
I do art now, it’s really helped me . . . I feel I’ve achieved something.
P1
I volunteer at the HIV [human immunodeficiency virus] centre, it gets me out and stops me thinking about myself and it’s good to go there.
P3
I’ve accepted I just have to do it [housework] differently now.
P16
Theme II: pain-related behaviours and thoughts
The behaviours and commentaries described by participants could be grouped into two areas: broadly ‘positive’ and broadly ‘negative’. Positive or adaptive behaviours were characterised by descriptions of engaging in social activities and evolvement of new concepts of living within the current capabilities of the individuals and not past ones; these participants did not overly dwell on their pain and described active lives, hobbies and social interactions, and had realistic expectations. We grouped self-absorption, pain fixation, dependency, isolation, frustration, anger, boredom and low mood and self-esteem into negative or pessimistic behaviours. These participants tended not to identify with the self-management concepts and not to incorporate self-management terminology in their speech. Some participants thought that they were coping already and that the course made them more confident about their coping skills and so reaffirmed their behaviour, others found it difficult to apply the techniques learned on the course to everyday life and some highly motivated participants continued their learning and had taken up new activities and adapted their lifestyles:
When I’m bad, I just sit and stare at the vase on the mantel piece, I feel really low, I do nothing.
P10
Some days I cannot get off the couch, I just watch TV [television].
P11
I pray, my pain it does not go but my mind is on my praying, it gives me strength.
P12
If I get really involved in it [painting] I can forget about the pain, but most of the time it’s too painful.
P1
The relaxation helps, yeah, really helps, and the breathing – it sounds stupid breathing – but it does.
P10
Theme III: knowledge and information
Most self-management courses involve giving participants a course manual or handbook. These manuals were generally well received and were used as reference tools and memory joggers and to supplement knowledge from missed sessions. They were also used as ‘proof’ for ‘significant others’ to justify behaviour and explain learning and new behaviours.
There was a strong suggestion from the interviews that a system of building up information and material related to the course gradually was preferable to receiving information all at once, as this could be overwhelming. Some participants took the manual at the first session and did not return to the course as they thought that they had all the information that they needed. It was also mentioned that the internet was underused. Some participants were computer literate; however, others were not and this form of communication would have been inappropriate, unfamiliar and impossible because of a lack of equipment.
Someone actually called it [a course handbook] their bible. I can still remember on the course, someone called it, it’s my bible.
FG 1.2
they take it, they take the handbook and then they do not come back!
FG 1.5
You cannot say, ‘right, I’ve got this terrible pain, now let me refer to the book and see how I can get rid of this pain!’
P6
I’m not going to say I’ve read it! [laughter] But, no, I just browse through it and bits and pieces. And certain things, I often turn round to the governor, and say, ‘You see look – told you! That’s what you should be doing!!‘
P7
we do not give them [course handbooks] because people just do not read them!
FG 1.3
But I think also to give them those in separate little . . . in stages [murmur of agreement] rather than give them a big manual at the end.
FG1.5
Theme IV: course characteristics
In theme IV, we separated the data into four categories: marketing, referrals and recruitment; course delivery and impact of tutor; attendance; and mix and character of attendees.
Theme IVa: marketing, referrals and recruitment
Two schools of thought emerged about recruitment and marketing. The participants did not want to be told or coerced to go on a course, but they did want legitimisation of the course through recommendation by their general practitioner (GP). They also felt that there was a need for a self-referral route, coupled with increased marketing and information for the public, as many GPs were not aware of the different courses. The tutors, however, were keen for recruitment to take place through GP registers and GPs themselves, as this allowed for better targeting of those with greater need and gave access to a ready source of potential participants.
Encouragement from those involved with the course from the initial point of contact was important. Building rapport with potential participants helped manage expectations and encouraged engagement with learning and the process. The presence of the recruiter at the first session was welcomed. The tutors thought that the recruitment process was very important for managing expectations. Both unrealistically high and very low expectations were seen by tutors:
And I think that’s a gap where some health care professionals will not understand what it is.
FG1.4
we are getting access to the GPs’ register, i.e. we have got a standard letter saying: a self management course is coming to the area, and so on, and we have asked the GP practice would they use that letter, you know, put their letterhead on it and also sign it and so on . . . And we are getting a really, really good response that way.
FG1.6
Mile End itself phoned up: a new unit’s opened up dealing with persistent pain, we wondered if you’d like to attend. So I thought, I’ll go anywhere where . . . there’s always ways of learning things.
P2
I must be honest, if that physiotherapist had not have put me forward, I would not have known about it.
P4
you cannot lose anything but you might gain. I can tell her, but that’s me telling her; if somebody else tells her, it’s totally different. It comes over different, you know what I mean?
P7
But I do think doctors need to know, because I do not think many doctors know that EPPs [Expert Patients Programmes] exist!
P8
I think you have to be ready to be open-minded about helping yourself, and if you have gone there because you have been referred, not because you want to go, I think that would be a bad reason.
P3
When they are sort of at the stage where they are asking: I really want to do something. I want to . . . I’m fed up of waiting for everything and I’d like to help. I think that would be when doctors should refer them to EPP [Expert Patients Programme].
P4
They are professional people, they would think and say whether you’re doing it wrong or right and advise you how else it would be good. We would comply with them.
P5
Because of the fact that some participants tend to be isolated in their everyday life, they do kind of look for somebody to kind of latch on to, you get me?
FG1.5
Theme IVb: course delivery and impact of tutor
Both professional and lay leadership was valued. The tutor was generally perceived as a strong role model, often with ‘more right’ to give advice and guidance than their GP:
Yeah, and the fact that the tutors themselves has an action plan as well. Yeah, the role modelling is important.
FG1.3
Well, by relating, they were more or less relating to . . . they definitely knew about pain themselves, that’s the main thing.
P1
The reason why because they know, they could understand the highs and lows.
P7
Participants generally had a need to feel ‘listened to’ and to be taken seriously. Some would have preferred more time to tell ‘their story’, whereas others felt that this would incur too much competition. The tutors felt that it was an important part of their job to contain comparisons between people. There were lengthy discussions about mixed-disease courses and disease-specific courses. There were pros and cons of both, with no clear conclusions possible.
Effective course tutors were described as friendly, open, honest and non-judgemental; they respected others’ opinions, made people laugh, enjoyed the sessions and were relaxed; they listened and were flexible, informal and engaging; they controlled the input of those who were very negative or those who were overdominant and curtailed those who talked too much about themselves; they related to the group culturally; and they had experience of chronic pain.
Conversely, the descriptions of poor tutoring involved reading from a crib sheet; rigidly sticking to timescales at the expense of learning; not having experience of chronic pain; and not managing disruptive, negative or self-absorbed people.
Theme IVc: attendance
A number of factors were mentioned with regard to attendance and non-attendance on courses. Attendance was influenced by personal motivation to attend the course, the quality of the tutors and receptiveness to information and participation in group activity and social bonding. When all or some of these were mentioned the participants demonstrated good attendance. Those with poor attendance were those with a low or depressed mood, a negative attitude to the course, lack of positive support and demanding friends and family. Other factors that affected attendance were medical appointments, sickness and holidays – these were common to those with both low and high rates of attendance (Figure 4). The interviewees and the tutors in the focus group indicated that people with low self-esteem and those who felt intimidated about talking in front of others were unlikely to fare well on courses:
They’d be negative against everything and would not give things a try, but it could be they are actually chronically, clinically depressed, but they actually left.
P9
but they have not got that something to help them, to drive, to push probably.
P9
It was nice to share ideas with other people and get ideas, get new ideas, fresh ideas, some of which I have taken on board and some I have not! I wanted it to stop my illnesses, to be beneficial for my body, that’s what I thought, it would be enough.
P10
There are seven of us in this house including my husband and I, it’s a two-bedroom house, and we are living in such difficulties [she goes on to describe the problems encountered getting to the course] . . . It was quiet a distance for me. I just went the one day.
P3
They do not get me to do exercise nor do they do it themselves. You have to do things yourself.
P4
FIGURE 4.
Factors influencing attendance.
Theme IVd: mix and character of attendees
Attending a course that was delivered to a group appeared to have an impact on several factors. It gave participants motivation to achieve goals as they had to feed back progress to the group and it reduced levels of isolation – some were inspired by others and some made friends and all appeared to learn from each other or the course itself. Conversely, some participants felt that the action planning and feedback could be false, that is, an exercise in creative storytelling, and others insinuated that the goal-setting could potentially set them up for failure if they consistently did not achieve in front of the group and indeed this was suggested as a reason for people not attending. ‘Eureka’ moments were described, such as the realisation that others felt the same and had the same issues to deal with, and some found this inspiring and motivating:
It is that proper group work and I think that might be where the gap is where . . . maybe more emphasis on forming as a group, like group activities, before just throwing at them the information, might help in that process.
P4
You get ideas when they ask you questions but now I can’t say, you get it when talking, you can learn things too. If you say your idea then that’s one way and I’ll think another way.
P8
Theme V: course components
There was a danger that self-management courses legitimised ‘the sick role’, especially pacing, as it justified resting and not doing things under the guise of ‘I’m pacing myself’. With pacing, patients are advised to schedule their activity evenly over a period of time; this may involve resting at points so that the person does ‘not overdo it’. Sometimes this was described as being used constructively to explain behaviour and at other times it was felt that pacing was used to manipulate circumstances to justify not doing some things:
I just tell her [his wife] ‘I’m pacing myself’ and watch telly.
P4
I can go back to the computer or I’ll just sit and chill out for the rest of the hour, that’s entirely up to me, but it’s pacing myself during the day.
P1
Encouragement of new activity and establishing contacts and generating new relationships were very helpful. Participants did want more information about local resources and even more courses. Additionally, participants liked being able to talk about their condition; however, the tutors actively discouraged this to avoid competition and self-absorption. Narratives of conditions were ‘permitted’ within the context of personal goal-setting, action planning and pacing:
The free-thinks [small amount of allocated time for discussion] I think there needs to be a little bit built in where discussion can take place.
FG1.4
Mmm. People are itching to talk, are not they?
FG1.3
One woman, to get into the bath, she needed a handrail. They said, ‘Well, have you been in touch with . . . some sort of organisation?’ ‘No!’ ‘Oh, get in touch with them and ask that . . .’. She came in, I think it was a week or two later, and she said, ‘They have put one in for me!’
FG3.1
I’ve learnt to cope with it on my own; it’s like it’s all right people saying, ‘Oh, do this, do that!’, but they are not with you all the time, they are not seeing you how you are.
P7
I’m really bitter about because no one helped me, no one advised me and I never got nothing.
P2
The main components of courses that participants liked were breathing techniques, relaxation, visualisation and social networking. They disliked the lack of involvement of ‘significant’ others and that feedback from the goal-setting could be daunting. Here, both tutors and participants admitted that there was scope for ‘stretching the truth’. The content of the courses was seen as useful, but its sustained application was considered difficult. The social networking and ‘doing something different’ was commented on and expanded on more than any other component of the courses.
Theme VI: change
From the data we identified three main explanations/models of behaviour about change: behaviours of those who remain fixated and absorbed in pain (Figure 5), factors that appear to promote and sustain beneficial change (Figure 6) and the process of change as lifestyles expand, broaden and evolve to include more things.
FIGURE 5.
Observed behaviours and attitudes towards pain.
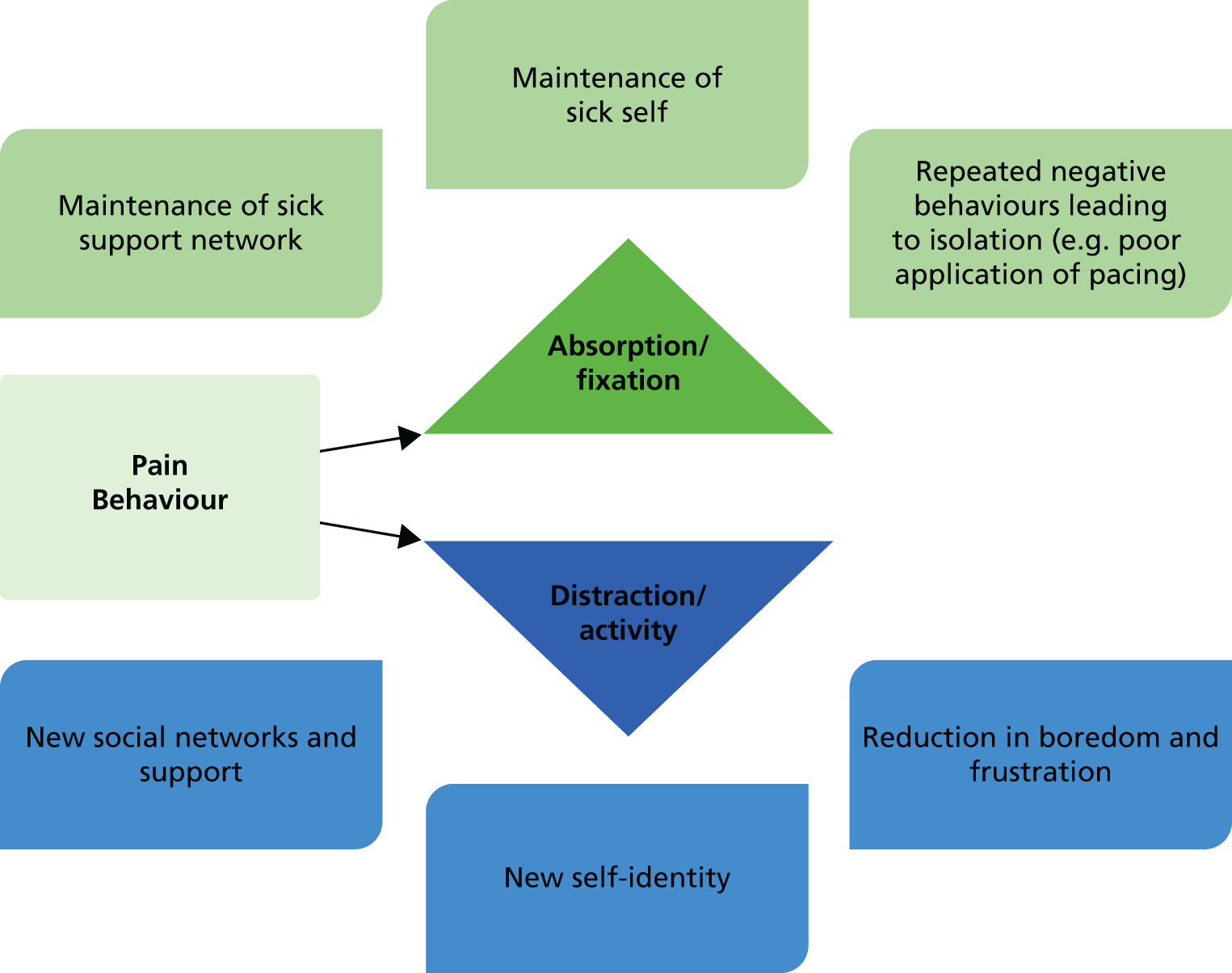
FIGURE 6.
Factors promoting and sustaining change behaviour.
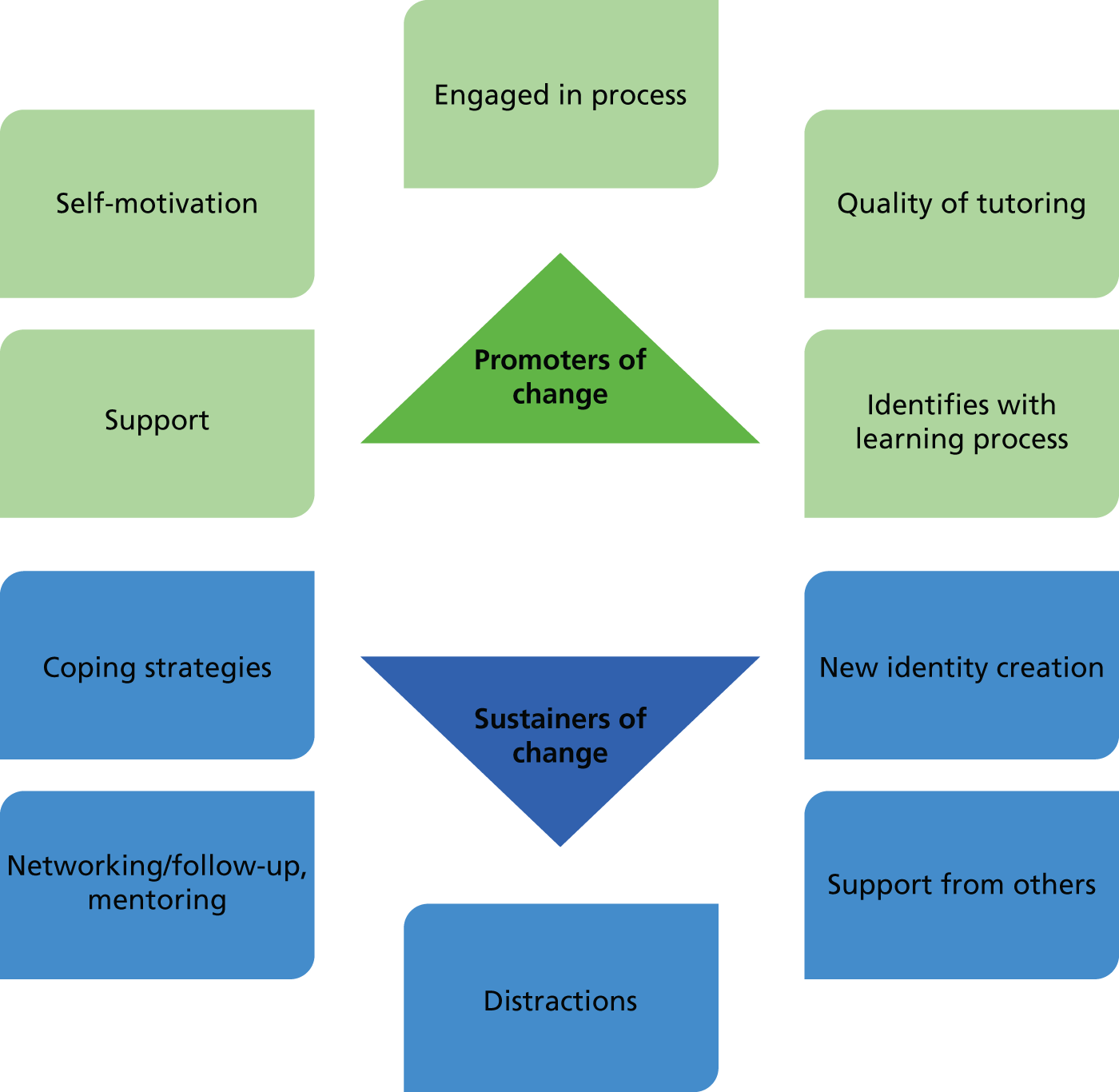
Figure 5 shows the behaviours associated with those who appeared to be fixated on their condition; their lives and their identity revolved around being in pain, with few non-pain-related activities. As a result, ‘sickness’-related activity and behaviours were maintained. These participants were generally isolated, bored and frustrated and they had a low mood and were dependent on family and friends. Conversely, those who were not fixated on their pain used distraction techniques to broaden their activity and life experience, often creating new activities and hobbies, leading to new social networks and a self-identity associated with the activity rather than with their pain.
Figure 6 shows the factors that we found to be associated with participants who had managed to change and sustain the changes that enhanced their quality of life. Those participants who had adapted and coped well were motivated, were engaged in the process, had support and reported having good, inspiring tutors.
Each figure illustrates the potential impact that group-related activities may have.
Barriers to change included depression, lack of motivation, physical capability/disability, change in benefit payments, change in family dynamics, stress/pressure/confusion and change in behaviour conflicting with a ‘sick role’.
Theme VII: expectations and outcomes post course
Those who were really engaged with the course enjoyed the social release it gave them and were inspired; they felt that there was a void at the end of the course. These participants were very vocal about continuing contact. Views expressed included a preference for ad hoc contact, such as top-up classes, and ‘buddying’ to maintain the social network and a need for local information about services, for example walking groups:
because after the six weeks, they kind of say, ‘Well what can we do now?’
P2
Some of us decided to meet up and carry on the goal-setting and action planning.
P3
There are course junkies, other courses to combine with are the healthy moves and healthy guides.
FG1.3
it was not between the tutor and the course people, but the people themselves, so they did a buddying up, mentoring thing.
P3
Outcomes
Outcomes that were seen as important to participants are summarised in Box 1. These are grouped into the following categories: functional, physical, emotional, social, economic and medical; examples of each are provided in parentheses in Box 1. Participants were rarely able to definitively outline success criteria unless they were based on a pain outcome, that is, no more pain, less pain, functional ability to start doing tasks that they used to be able to do. However, when asked what they wanted from the course the outcomes mentioned related to personal confidence in their ability to do things and these were not necessarily functional but also emotional and social. Confidence was inherent but rarely explicitly stated, for example ‘I want to be able to . . .’, ‘I am going to try and do . . .’, ‘I have learned to do “x”, which is great’ and ‘I feel more able to . . .’. Quotations were given in a positive context or the converse negative context: ‘I can’t . . .’, ‘I wish I could . . .’, ‘I’m just hopeless . . .’, ‘others could but not me’. Self-efficacy was not really an outcome but a means to achieving a better outcome.
Functional (practical daily living requirements).
Physical (equipment aides, support and practical help).
Emotional (dealing with frustration, anger, boredom, isolation, depression).
Social (social networking, relationships with partners, family and friends).
Economic (financial support, benefits, etc., work-related issues).
Medical (pain and drug-management related).
Additional findings from the expert focus group
A key consideration for the expert focus group was the legitimacy and credibility of findings from some studies and the transferability of some concepts in the Stanford self-management model9 to a UK audience:
It makes me wonder why people go on doing it [Expert Patients Programme] when there are better things available.
FG2.8
if we had a drug which on balance produced no effect, would we be working so hard to get GPs to put people on it?
FG2.5
Identifying those who do well and those who do not was discussed extensively but no firm conclusions were made about subgroups and screening. Selecting a ‘choose all’ strategy to include those with varying degrees of severity was, however, seen as a good way of recruiting:
Some people do benefit enormously . . ., it doesn’t make it unique to EPP [Expert Patients Programme] . . . a good regime of analgesics leaves some people self managing.
FG2.5
Rather than working hard to try and understand who does benefit and how we can target them better . . . what can we do about those who didn’t [benefit].
FG2.1
Cognitive reframing and linking pain and mood were flagged as lacking in current approaches to self-management. The group postulated that without personal identity reframing and acknowledgement of negative behaviours and cognitions no change would be made.
The involvement of GPs and other HCPs in referrals and recruitment lent credibility to the courses and was seen as integral to the success of any programme; additionally, medical staff involvement encouraged and motivated participants. However, the counter-argument that many chronic pain patients were disillusioned with their GP was not seen as detrimental to the self-management courses as GPs themselves were not running them.
the GP has assigned it, so it does make a difference.
FG2.5
All agreed that there was a need for aftercare or support. The Alcoholics Anonymous model was discussed, as were approaches to smoking cessation. Buddying and mentoring concepts were discussed and were thought to be helpful, but it was also thought that such schemes would be difficult to organise and maintain and that it would be difficult to provide support for the ‘buddies/mentors’. Longer-term thinking and support groups were discussed:
Education is an incredibly weak way to change behaviour . . . otherwise, you know, who would smoke?
FG2.4
Distraction is such a short-term thing . . . but developing interests and involvement which really compete for space in someone’s life.
FG2.1
it took about a year to get them to own it [the support group], it’s a very different dynamic with a key core of people.
FG2.5
Additional findings from the tutor/facilitator focus group
The tutor/facilitator focus group was characterised by the strength of the participants’ beliefs in the concept and process of the Expert Patients Programme. The terminology used and the phrases used from the Expert Patients Programme literature were common to all participants in this group:
We do not want cloned tutors . . . where you expand too big and you have got tutors for tutors sake, and the empathy is not there.
FG1.3
Tutors relayed stories relating to very practical considerations, such as not being able to access rooms because they were locked or centres were closed or had no disabled facilities. Participants had few criticisms of the Expert Patients Programme courses; however, they did suggest that providing more time for general discussion would be beneficial.
Discussion
Statement of principal findings
Our data indicate that those who got the most out of the courses were those who were motivated to change at the outset and who became engaged in the process. The quality of the tutoring influenced participants’ perceptions of the courses. Factors that helped people were social support (family, new and old friends) and undertaking new activities that extended beyond the course; conversely, factors that did not help were a low, depressed or negative mood at the outset and a reluctance to alter behaviour, activities or lifestyle.
Strengths and weaknesses of the study
Our study focused on participants with chronic pain who had untaken self-management courses as well as tutors and experts. Each group’s perspectives were ‘triangulated’ with the perspectives of the other groups to assess responses and reactions. Discordant data were mainly obtained from the ‘experts’, who questioned the overall evidence for effectiveness for lay-led self-management in general, and, conversely, the tutors, who provided very positive narratives about the effectiveness of the courses that they had run.
One-third of our sample was Bangladeshi, reflecting our London population. We were able to identify some cultural issues, for example running gender-specific courses and not organising sessions that coincided with Friday afternoon prayers for men, but we were not able to draw any conclusions about cognitive ethnic differences from this study as it was not designed for this purpose. A further limitation was that we were not able to interview those who were invited to attend a self-management course but who chose not to go. Our sampling strategy generated a range of views that enabled us to consider issues relevant to the design and delivery of self-management programmes and some speculative data about the traits of people who seem to respond or not to self-management courses. The model adds to the existing literature by consolidating common findings about social interaction and activity and depression and behaviour change.
Strengths and weaknesses in relation to other studies
Social interaction
Group cohesion was reported by those who had integrated self-management concepts and approaches into their everyday lives. The loss of ‘the group’ at the end of the course was felt by all those we interviewed. The development of support groups could provide a sort of continuity for participants. However, support groups can provide both positive and negative effects, such as reinforcing and maintaining dysfunctional pain behaviours. 155 The quality of support groups can be enhanced by training core members to facilitate groups, having a structure to support group meetings, having good facilitation and appreciating that this type of service will not suit all those with chronic pain. 156
Activity
New activities promoted self-esteem, distracted thoughts from pain and created a positive outlook. Guidelines for the management of chronic low back pain recommend activity and the focus of this activity is on exercise. 21 However, research does not recommend any one particular form of exercise over another, as any exercise would appear to have a benefit. We would argue from our findings that activity should include the uptake of hobbies and non-exercise-related activities. These tasks will normally involve mobilising the body and, although they would not typically be described as exercise for many people, for those with chronic pain these types of activity constitute a form of exercise that they would not necessarily consider doing. Activities and hobbies are more than a distraction or short-term solution; they normally engage people in the longer term, which potentially encourages a lifestyle change. 157 The mention of exercise was problematic with this group of people as the idea of undertaking an ‘exercise programme’ was beyond their capability and capacity.
Recruitment and attrition
Low uptake, recruitment and retention in group self-management programmes remains an issue. 158,159 The main barriers to attending self-management programmes were comorbidities, poor physical functioning, lack of finance, depression and health-care use (coinciding appointments). In addition to these factors we postulate that poor uptake may also result from the lack of credibility surrounding the courses, which could be enhanced by medical professional endorsement, and the fact that many chronic pain patients are isolated and find it difficult to make the transition to helping themselves and lack confidence to become social and group orientated.
Self-efficacy
Cognitive–behavioural therapy is a technique that is used to help enhance self-efficacy in the area of chronic pain treatment. 48,160 We too infer that cognitions are important for enhancing self-efficacy, but that they can be affected by levels of literacy and comprehension, cultural norms and past educational experience. These may unduly influence people’s perceptions of ‘educational’-type courses. Strong suggestions were expressed about the need for informality, lots of discussion and socialising. Conversely, our participants reported that some people did not cope well with the discussion part of the sessions on the Expert Patients Programme courses as they found them intimidating, especially when required to feed back on their goal-setting exercises. However, less formal teaching and learning techniques do involve reflection, discussion and investigation to embed learning, such as ‘problem-based learning’161 and ‘reflective learning’. 162
Social, cultural and ethnic grouping
Six of our sample were Bangladeshi. We were able to identify some culturally important issues but we are not able to say whether or not these are representative of particular ethnic communities, which is why we have not undertaken different cultural comparisons. The concept of somatisation (absorption and hypervigilance of pain) is ubiquitous,163 but in the south-east Asian populations physical symptoms are more legitimate than depressive feelings for visiting a physician. 164 Explanations or disease theories can differ between cultures and background and explanations of disease, healing and diagnoses will vary in their acceptability. Examples include shamanism, in which illness is explained by spirits and magic, chiropractic, in which it is explained by misalignment of the spine, psychotherapy, in which it is explained by conflict in the mind, and ‘new age’ rationale, which is about energy imbalances. 165 Understanding different explanations of disease and accommodating cultural traditions is an important part of self-management, especially if the culture of care rests within the family. Other more recent qualitative work in Tower Hamlets, focusing on the pain experience of the first-generation Bangladeshi immigrants, has, however, found remarkable similarities between this group’s experiences of pain and those found in studies focusing on white British populations. 152 There is a paradox of care in these circumstances between offending those who want and need to be seen to be caring and thus to be perceived, for example, as a good Christian or Muslim and the individual need to be dependent and self-sufficient. It is difficult for chronic pain patients to self-manage if others around them continue to care and provide for them. 166
Conclusion
From our study the most important factor that appeared to be associated with better coping and improved quality of life was shifting individual focus away from pain to other activities. However, this was not easy as chronic pain patients were often very occupied with their pain, which superseded other activities. Acceptance and self-efficacy appeared to be important factors in the process of positive change and enhanced quality of life.
Chapter 5 Outcome measures
Abstract
Aim: To develop a preferred list of patient-centred outcome measures for evaluating self-management programmes for chronic pain patients with musculoskeletal conditions.
Objectives: To review the literature for valid, reliable and appropriate outcome measures for evaluating the effects of self-management interventions that match the most important domains emerging from the work presented in Chapters 2–4 and discuss these with laypeople and pain sufferers to recommend a basket of appropriate and acceptable measurement tools.
Methods: We selected outcomes by using the data from the previously described systematic reviews to generate a draft preferred list of outcomes for which to identify patient-centred outcome measures. By ‘patient-centred’ outcome measures we mean those outcome measures that are the most meaningful, relevant and important to patients. We carried out a literature review to identify the most commonly used outcome measures for evaluating self-management interventions for chronic musculoskeletal pain and consulted patients, laypeople and experts. In the first instance we used the published IMMPACT and MMICS recommendations. To identify any relevant studies published after these consensus statements were published we reviewed papers published between 2004 (because the IMMPACT consensus review was published in January 2005, before the MMICS recommendations) and 2009 (the year the search was conducted) that had reported or reviewed clinimetric data on outcome measures in our list. We used these to inform three domains: pain and disability, depression and fear avoidance. When no recent review was identified (self-efficacy and social support), we carried out a systematic literature search and reviewed the measures’ clinimetrics. The clinimetric criteria applied to the questionnaires were based on published recommendations. Validated and reliable measures were presented to a panel of eight people (two laypeople, three study team members, one outcome measure expert, one GP and one psychologist). Data from our pilot study were also used to inform our decision-making. Consensus was sought for the most appropriate and valid methods.
Results: Seventy-eight questionnaires were considered and tools were chosen to evaluate responses to self-management interventions. We used these data to inform our final selection of outcome measures. The primary outcome selected was pain-related disability [subscale of the Chronic Pain Grade (CPG)]. Secondary outcomes were pain intensity (subscale of the CPG), quality of Life [European Quality of Life-5 Dimensions (EQ-5D)], perception of social support [social integration and support domain from the Health Education Impact Questionnaire (heiQ)], self-efficacy [Pain Self-Efficacy Questionnaire (PSEQ)], pain acceptance [Chronic Pain Acceptance Questionnaire (CPAQ)], depression and anxiety [Hospital Anxiety and Depression Scale (HADS)] and the general health question in the 2011 census.
Conclusions: A preferred battery of measures to evaluate responses to self-management courses was agreed, representing the most important domains to assess relevant outcomes.
Background
The importance of the selection of patient-based outcome measures when designing a clinical trial is well established. 167 Our aim was to develop a preferred list of patient-centred outcome measures for evaluating self-management programmes for chronic pain in patients with musculoskeletal pain for consideration for adoption in our pilot study and trial. By ‘patient-centred’ outcome measures we meant those outcome measures that are the most meaningful, relevant and important to patients. This project was directly informed by findings and data from Chapters 2–4.
At the time that we developed this study two international consensus studies had recommended a list of outcome measures that we considered to be the most informative in selecting our outcome measures.
The MMICS project aimed to improve the quality and completeness of measurement in prospective cohort studies of the transition from acute to persistent disabling low back pain. 31 It involved a collaboration of teams of back pain experts from 11 countries who had expertise in clinical practice, prospective cohorts, epidemiology, social sciences and health services. MMICS methodology included identifying preferred factors predicting back pain progression (using experts) followed by a systematic appraisal of published reviews and empirical studies of appropriate measurement instruments. Measurement instruments were assessed for clinimetric properties such as reliability, validity and responsiveness, and for practical considerations such as length and complexity of language.
The IMMPACT study aimed to develop consensus recommendations for specific measures of each of the core outcome domains in chronic pain trials. 30 The 35 participants were selected on the basis of their research, clinical or administrative expertise relevant to the design and evaluation of chronic pain treatment outcomes. Literature reviews of measures of the IMMPACT core outcome domains were carried out to identify measures that could be used across all chronic pain conditions and that were not specific to certain types of chronic pain. Again, the measurement tools were clinimetrically assessed and a list of outcome measures recommended. Although this project drew heavily on these two sources, it was also designed to address the use of measures that did not appear in these two sets of recommendations.
Objective
The objective of this study was to develop a preferred list of patient-centred outcome measures for evaluating self-management programmes for chronic pain patients with musculoskeletal conditions.
Methods
We reviewed the literature for valid, reliable and appropriate outcome measures for evaluating the effect of self-management interventions that matched the most important domains emerging from the systematic reviews and discussed these with laypeople and pain sufferers to recommend a basket of appropriate and acceptable measurement tools (costs of instruments and/or copyright issues were not among the practical aspects considered).
The project progressed through five stages (Figure 7).
FIGURE 7.
Stages of the study. SR, systematic review.
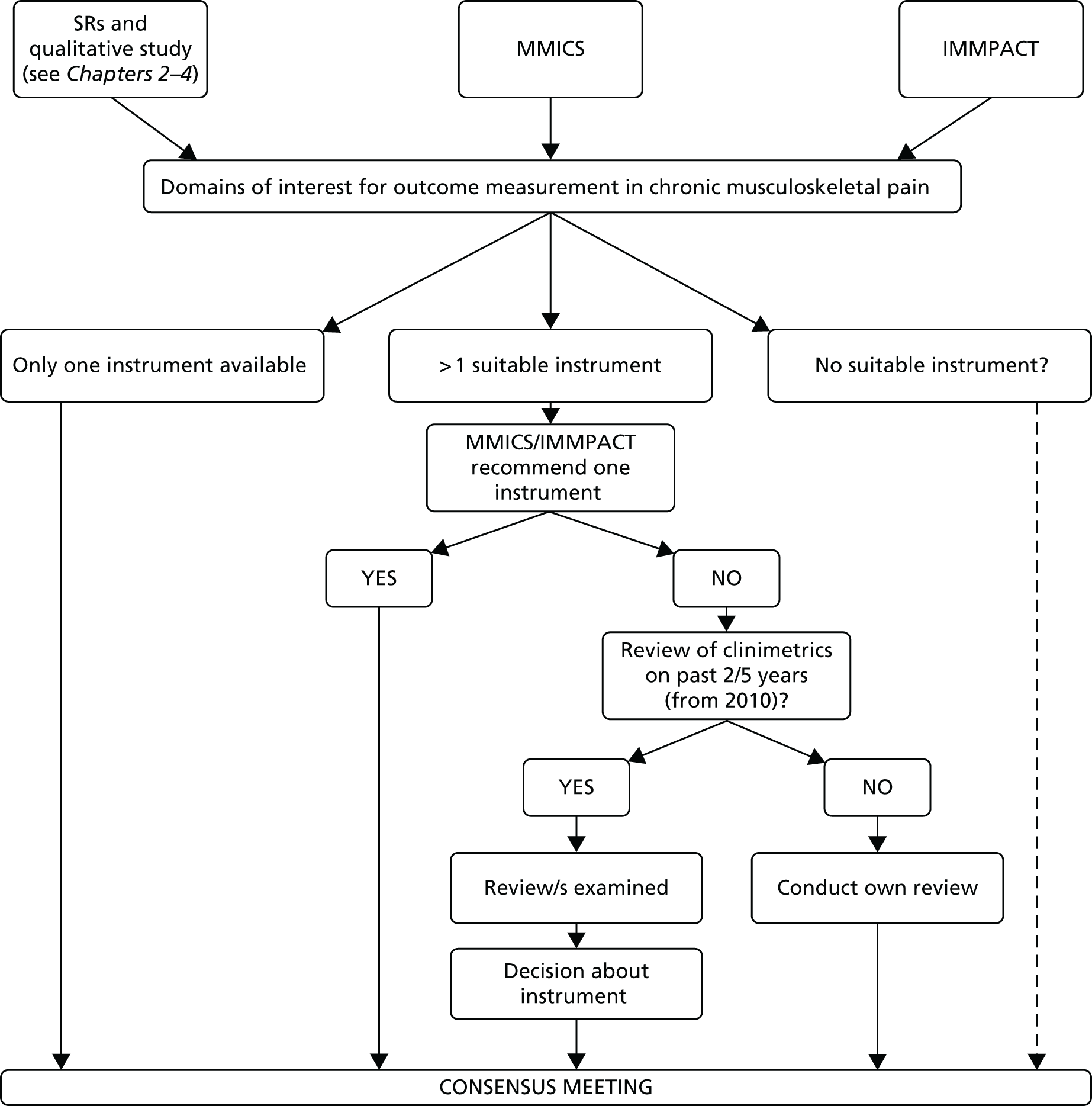
In the first instance we consulted two consensus statements on outcomes measures (MMICS31 and IMMPACT30 recommendations). We then examined reviews published in the previous 5 years of measures in each specific domain to identify reports of clinimetric quality. In one instance (depression) there were many candidate measures, all with strong clinimetric properties and a strong evidence base. For this domain alone, therefore, we conducted a Delphi study. 135 A list of 11 measures developed from the MMICS and IMMPACT recommendations and published reviews was presented to five international experts in the measurement of depression in pain populations. Experts selected the top two measures from the preprepared list during an interview (see Appendix 3) and gave their reasons for selection.
When no recent review of instruments to measure a domain had been carried out, original systematic literature searches were performed to:
-
ascertain which measures have been previously used in chronic pain research
-
seek out the psychometric and clinimetric data for the most commonly used measures.
For the domain of social integration and support we extracted common measures of social support, with good clinimetric properties, but the research team felt that these failed to capture the main components described by patients in our qualitative work. We therefore also searched for new, less well-established measures that seemed to have a better fit with the description of the domain. These, accompanied by published evidence on clinimetric properties, were presented to the user group.
Finally, all of the candidate instruments were presented at a meeting of experts, including members of the research team, and external clinicians, experts in outcomes research and patients. As well as clinimetric quality, ease of use, brevity, acceptability to patients and patient preference were also considered, with a final preferred list of outcome measures being identified.
Results
Selection of domains
The following measurement domains were included after brainstorming and consideration of the IMMPACT and MMICS recommendations:
-
pain and pain-related disability
-
pain intensity, interference
-
pain catastrophising
-
recovery
-
depression and anxiety
-
health-related quality of life
-
perception of social support/social integration
-
self-efficacy
-
fear avoidance
-
coping and acceptance
-
patient satisfaction at follow-up
-
health-care resource use.
Selection of candidate instruments
We were able to immediately select candidate measurement tools based on the IMMPACT and MMIC recommendations for the following domains (the tool chosen is given in brackets):
-
pain intensity (numerical rating scale)
-
pain interference (Brief Pain Inventory168)
-
recovery (numerical rating scale)
-
pain catastrophising (Pain Catastrophising Questionnaire170)
-
patient satisfaction at follow-up (the Patient Global Impression of Change171).
For the domain of fear avoidance, the only systematic review in this area looked at fear avoidance and prognosis in back pain. 34 The two main contenders for measuring fear avoidance from this review were the Tampa Scale of Kinesiophobia172 and the Fear Avoidance Beliefs Questionnaire (FABQ). 173 The MMICS study31 reported a slight advantage for the FABQ if work-related fear is considered important. These two measures were presented at the expert consensus meeting.
Another immediate selection was based on the fact that there was only one suitable measure for the domain of pain acceptance: the CPAQ. 174
For disability, the MMICS recommendations included only instruments specific to low back pain. The IMMPACT recommendations included the Brief Pain Inventory. The Brief Pain Inventory, however, measures both pain and pain-related disability. Furthermore, it is a measure principally designed for measuring acute pain. For this study we were interested in making a difference to long-term outcomes and so a measure that measures pain and its impact on just 1 day was not appropriate for our current purpose. For this reason we carried out a further search to identify measures of chronic pain and chronic pain-related disability. We identified the CPG as a well-established measure with good clinimetric properties when used to measure chronic pain and chronic pain-related disability. 175 We added this measure to our potential pool of measures to assess in the pilot study.
For depression, the Delphi study produced conflicting opinions from experts (see Appendix 3). The main reasons given for endorsing measures were absence of confounding somatic items, brevity and clarity, and widespread use in research. The consensus was around the use of four depression measures. The HADS176 was thought to be a good candidate, although there was some concern about responsiveness. Reasons for using the Beck Depression Inventory (BDI)177 were around compatibility with other studies in our population. The Center for Epidemiologic Studies Depression (CES-D) scale178 allows international comparisons and would be a useful measure. Finally, the Patient Health Questionnaire for Depression and Anxiety (PHQ)-4179 was thought to be a useful screening tool. The overall recommendation based on the Delphi study was to use the HADS as both a baseline descriptor and an outcome measure as the simplest option. The HADS, CES-D scale and PHQ-4 were presented to the expert consensus meeting for selection of a single measure.
For self-efficacy it was not possible to make a selection of outcome measures based on the MMICS and IMMPACT guidelines, and we could not identify a recent relevant systematic review. We conducted an original systematic review for self-efficacy and identified 37 different self-efficacy questionnaires (see Appendix 3 for methodological details and a description of the results). We selected the two most commonly used measures (where > 10 articles had used these measures): (1) the Arthritis Self-Efficacy Scale (ASES)180 with its four variants (for chronic disease and shorter versions) and (2) the PSEQ. 181 We searched for further information on the clinimetric and psychometric properties of these instruments. We reviewed the clinimetric properties of the questionnaires (full details are provided in Appendix 3). Construct validity was evident for most measures. There was evidence for content validity for the ASES-20,180 ASES-11183 and PSEQ181 questionnaires. There was some evidence of test–retest reliability for the ASES-20,180 Chronic Disease Self-Efficacy Scale-33 (CDSES-33)182 and PSEQ. There was (limited) evidence for responsiveness for the ASES-20, CDSES-33 and PSEQ.
For perceived social support there were no recommendations from the MMICS or IMMPACT guidelines, nor were we able to identify a systematic review comparing measures. We carried out a literature search to identify candidate instruments (see Appendix 3 for the methods and results). There were 30 social support questionnaires identified in the first broad search and a further nine additional social support measures identified from specialist text books (see Appendix 3). At this stage we created a reduced list of six measures that focused on our target population and which included items representing the domains that we had previously identified, with special emphasis on social (re)integration, while considering the psychometric properties of the questionnaires (see Appendix 3). Two of these six questionnaires were most closely aligned with our aims of tapping into use of both health resources and social relationships for social support. However, we decided to delay a full clinimetric assessment of the questionnaires because we felt that they did not quite capture our remit in reference to social (re)engagement. It was decided to present these two measures to the focus group meeting prior to clinimetric assessment.
Consensus meeting
The consensus meeting included five members from the project team (TP, ST, DC, CM and KH) and four other participants: two clinical psychologists (one from Mile End Persistent Pain Service and one from Whitechapel Health Centre), one researcher from Patient Reported Outcomes Measurement Group at Oxford University and one chronic pain patient and lay representative.
The measurement domains discussed and the decisions made with regard to recommending tools are described in the following sections.
Self-efficacy
The ASES (and its variants)180 and the PSEQ181 were presented. The PSEQ was the final measure selected because it had the most consistent evidence for reliability, validity and test–retest reliability. It was relevant for our population and the questions were comprehensive and comprehensible. It was also quick to administer, with 10 items, and was judged as easy to read, complete and score.
Depression
Findings and comments from the Delphi study were presented to the group, along with the shortlist of depression measures. The final selected measure was the HADS. 176 This was recommended because it has good clinimetric properties, is widely used and covers depression and anxiety in 14 items. The PHQ-4179 was rejected as it is a screening tool and more detail was needed. The Depression, Anxiety and Positive Outlook Scale,184 although good and covering positive outlook, was not widely used. In addition, the group felt that the HADS also covered positive outlook.
Quality of life
The EQ-5D169 and SF-3662 were potential candidates for this domain. The EQ-5D was chosen because it is shorter and simpler than the SF-36 and widely recognised and used in economic analysis. However, when this work was reported to the TSC, we were advised to use both measures in the pilot study.
Coping and fear
The only acceptable tool measuring catastrophic thinking was the Pain Catastrophising Questionnaire. 170 However, the group felt that the questions were quite disturbing and our patient representative expressed concern that some people might find it quite upsetting to complete. The discussion focused on prioritising the important domains for our target population while reducing the burden by not including too many measures. On reflection the group decided to exclude the domain of coping and fear, which is partially covered by the HADS.
Acceptance
The CPAQ174 was selected as it covered two domains independently in one questionnaire: activity engagement and pain and willingness, that is, engagement and avoidance. There were no other competing contenders for this domain.
Social support
Two measures were presented to the group members: the Chronic Illness Resources Survey (CIRS)185 and the Social Support Survey. 186 Discussion centred on eliciting the aspects of social (re)engagement. After a brainstorming session it was decided that the presented social support questionnaires failed to measure social integration and confidence to socially integrate adequately. We decided to seek guidance from others by exploring different questionnaires in the wider arena for non-pain health populations that might measure the construct that we had identified, that is, social integration. After a second search we found a relatively new measure, the heiQ,187 which included a domain called social integration and support that measured exactly our area of interest, that is, social engagement and integration in populations exposed to self-management interventions. Despite it being in the early stages of evaluation, there were no competing measures and we therefore decided that we would use this domain from the heiQ.
We also asked the group to consider which domain should be used for our primary outcome. An overall measure of health-related quality of life was deemed to be the most appropriate choice. Such a measure would synthesise the anticipated effects of our intervention on a wider range of aspects of participants’ lives. The EQ-5D169 was the consensus group’s preferred measure for the primary outcome. The five-level version of the EQ-5D was developed after our study was designed. When we refer to the EQ-5D we are referring to what might now be better identified as the EQ-5D-3L. We have, however, used the nomenclature current at the time that the study was developed.
Conclusion
The measures included in the feasibility study are provided in Table 19. Following the pilot study we reviewed the performance of the measures before making our final selection (see Chapter 8).
| Outcome measure | Description | Calculation | Score range |
|---|---|---|---|
| CPG overall175 | The CPG overall score is a composite of the CPG disability score, the CPG pain intensity score and the score for another question assessing the number of days off usual activities because of pain | The question assessing the number of days off usual activities because of pain has four categories: 0–6 days, 7–14 days, 15–30 days and ≥ 31 days. Categories are assigned 0 points for 0–6 days through to 3 points for ≥ 31 days. CPG pain intensity is categorised as < 50 vs. ≥ 50 and CPG disability is categorised as 0 (0–29 points), 1 (30–49 points), 2 (50–69 points) or 3 (70–100 points). An overall disability score is then formed by adding the points from the grouped CPG disability score (range 0–3) to the points assigned for the number of days off work (range 0–3), giving an overall range of 0–6 | Grade 0 – pain free: no pain problems in the last 6 months; grade I – low pain disability and low pain intensity: characteristic pain intensity < 50 and < 3 disability points; grade II – low pain disability and high pain intensity: pain intensity of ≥ 50 and < 3 disability points; grade III – high pain disability, moderately limiting: 3–4 disability points, regardless of pain intensity; grade IV – high disability, severely limiting: 5–6 disability points |
| CPG – pain-related disability175 | This is a composite of three questions that assess the participant’s pain-related disability at present and the maximum and average intensity over the past 6 months | Each question is scored on a scale of 0–10. The final score is the mean of the three questions, multiplied by 10 | Range 0–100, with higher scores indicating worse pain-related disability |
| CPG – pain intensity175 | This is a composite of three questions that assess the participant’s pain intensity at present and the maximum and average intensity over the past 6 months | Each question is scored on a scale of 0–10. The final score is the mean of the three questions, multiplied by 10 | Range 0–100, with higher scores indicating worse pain intensity |
| EQ-5D169 | Quality-of-life measure. This is a composite of five questions that ascertain whether the participant has any problems with mobility, self-care, performing their usual activities, pain or discomfort, or anxiety or depression | Each question has three answers ranging from ‘no problems’ (scored as 1) to the worst category (scored as 3) | Perfect health = 1.0. UK norms for healthy males/females: 40–49 years – 0.89/0.87; 50–59 years – 0.80/0.82188 |
| heiQ187 | This is a composite of five questions that ascertain the extent to which the participant is able to enjoy life | Each question has four answers ranging from ‘strongly agree’ (scored as 4) to ‘strongly disagree’ (scored as 1). The final score is the sum of the score for each question | Range 5–20, with higher scores indicating more enjoyment in life |
| CPAQ174 | This is a composite of 20 questions that ascertain the participant’s ability to cope with his or her pain | Each question is scored on a scale of 0–6, with 0 indicating that the statement is never true and 6 indicating that the statement is always true. There are two subscales: pain willingness and activities engagement. The statements in the pain willingness subscale are reverse scored so that an answer of ‘always true’ gives a score of 0 and an answer of ‘never true’ gives a score of 6. The statements in the activities engagement subscale are scored on a scale of 0–6, with 0 indicating that the statement is never true and 6 indicating that the statement is always true. The final score is the sum of the score for each question | Range 0–120, with higher scores indicating a better ability to cope |
| HADS depression score176 | This is a composite of seven questions that ascertain the extent of the participant’s depression (these are the even number questions of the HADS questionnaire) | Each question has four answers ranging from not experiencing a symptom at all, scored as 0, to experiencing a symptom nearly all of the time, scored as 3. The final score is the sum of the score for each question | Range 0–21, with higher scores indicating more severe depression |
| HADS anxiety score176 | This is a composite of seven questions that ascertain the extent of the participant’s anxiety (these are the odd number questions of the HADS questionnaire) | Each question has four answers ranging from not experiencing a symptom at all, scored as 0, to experiencing a symptom nearly all of the time, scored as 3. The final score is the sum of the score for each question | Range 0–21, with higher scores indicating more severe anxiety |
| PSEQ181 | This is a composite of 10 questions that ascertain the participant’s level of confidence to live a normal life despite his or her pain | Each question is scored on a scale of 0–6. The final score is the sum of the scores for all 10 questions | Range 0–60, with higher scores indicating higher levels of confidence |
Chapter 6 Development of the new self-management intervention
Abstract
Based on evidence from our previous work (see Chapters 2–5) we designed and manualised a psychologically orientated group course based on principles of CBT with elements covering acceptance, education about chronic pain, distraction, relaxation, visualisation, posture, social time, encouragement to buddy up and an introduction to new hobbies and activities. The course was underpinned by social learning theory and the theory of planned behaviour/reasoned action. The 24 different individual course components/sessions were delivered over 3 short days (10.00–14.45) with a single 2-hour follow-up session 2 weeks later. Teaching and learning modalities were varied and included a DVD featuring a medical expert addressing frequently asked questions, group discussion, role play and exercises. The course, for groups of up to 14 participants, was designed to be highly interactive and included experiential learning. Courses were facilitated by two trained facilitators: a lay individual with previous experience of small group facilitation and personal experience of chronic pain and a health professional with experience of treating people with chronic pain (chiropractor, GP, osteopath, psychologist or physiotherapist). We also designed a 2-day training programme for potential facilitators.
Introduction
The Medical Research Council (MRC)28 framework for developing and evaluating complex interventions describes three areas of activity involved in the actual design of an intervention:
-
identifying the evidence base
-
identifying or developing theory
-
modelling processes and outcomes.
We identified the relevant evidence through the systematic reviews reported in Chapters 2 and 3 and the qualitative study reported in Chapter 4. In this chapter we describe how the findings of these projects, together with relevant behaviour change theory, influenced the design of the COPERS intervention and our resulting conceptual model of the intervention. We then describe the intervention to be tested in the pilot and feasibility studies in detail and conclude by mapping the components of the intervention onto the behaviour change techniques taxonomy developed by Abraham and Michie and published in 2008189 (the taxonomy has since been further refined but this was the version available when we designed the intervention). Finally, we describe the training programme we developed to train facilitators to deliver the intervention in the pilot and feasibility studies.
Summary of the evidence base
The evidence identified from our reviews (see Chapters 2 and 3) indicated that group-based courses including psychological approaches with education about pain, undertaking activities and developing new interests were associated with better coping and improved quality of life. We also found that both lay- and HCP-led courses had some beneficial outcomes. From our qualitative work (see Chapter 4) we found that participants on courses enjoyed the social element and relaxation training. They had negative reactions to traditional exercise regimens. Good trainers with good facilitation skills made a difference to participants’ perceptions of courses and were associated with better course attendance. The ideal setting for courses was somewhere convenient and accessible and, if possible, familiar to participants. Table 20 explains in more detail how the evidence from Chapters 2–4 informed our course design.
| Key findings from Chapters 2–4 | How these findings influenced course designa |
|---|---|
| Group delivery appears to be effective (see Chapter 2); networking with others popular feature of self-management courses (see Chapter 4) | Group intervention |
| Most evidence to support professional tutors (see Chapter 2); mixed professional- and lay-led courses also effective (see Chapter 2) | Groups to be led by a combination of a lay tutor and a professional tutor |
| Medical and community settings both associated with effective courses (see Chapter 2); convenience of courses important to participants (see Chapter 4) | Courses to be held in convenient community or health centre setting |
| Courses > 8 weeks no more effective than courses < 8 weeks (see Chapter 2) | Short-duration course |
| Psychological components commonly used in self-management interventions for musculoskeletal pain evaluated in RCTs (see Chapter 2); self-management interventions with psychological components appear to be more effective than usual care (see Chapter 2); larger number of different components not associated with bigger effect sizes compared with usual care (see Chapter 2) | Principal component of new intervention to be psychological |
| Limited evidence to support mind–body therapy components (see Chapter 2) but relaxation popular with participants (see Chapter 4) | (Relaxation to be control intervention in the main trial) |
| Increasing self-efficacy may mediate intervention (see Chapter 3) | Course should aim to promote self-efficacy |
| There was evidence that pain catastrophising and physical activity can mediate outcome from self-management (see Chapter 3) | We decided to address this in the intervention |
| Increasing physical activity may mediate the intervention (see Chapter 3); patient resistance to concept of exercise but not general activity (see Chapter 4) | We decided against a large physical activity component in the course but instead to include taster activities (possible hobbies) |
| Depression at baseline may be a predictor for poorer outcomes (see Chapter 3) | Course covers depression and encourages people who feel that they may be depressed to discuss this with their GP. We considered screening people for depression at baseline and treating depression before enrolling people on the course but rejected this as we could not determine a suitable cut-off and many potential participants had depression |
| Few other predictors have been identified and no moderators (see Chapter 3) | Not possible to identify a subpopulation of chronic musculoskeletal pain patients who might particularly benefit from the intervention. Course to be offered to all eligible adult patients |
| Concerns of attendees about what happens after the course is completed (see Chapter 4) | Follow-up session at 2 weeks |
| Loss of activities common in chronic musculoskeletal pain patients; distraction from pain may be useful (see Chapter 4) | Inclusion of ‘taster’ sessions in the course |
| Isolation may arise in chronic musculoskeletal pain patients (see Chapter 4) | Introduction of ‘buddy’ system during the course |
The aim of the new programme was to facilitate and train people to acquire lifelong skills. We decided to use psychological, social and physical techniques to change perceptions and feelings about issues that influence behaviours and to promote accepting, adapting to and coping with life with chronic pain.
Identifying appropriate theories to underpin course design
At the same time as conducting the systematic reviews we searched the literature and spoke to experts about behaviour change theory and models of persisting pain. We considered psychological theoretical models and learning and behaviour modification techniques. We drew on social learning theory190 and cognitive–behavioural theory,191 including psychological flexibility (acceptance and commitment therapy), that is, the acceptance of internal experiences or things that cannot be changed countered by behaviour change techniques that are designed to reorientate people towards meaningful activity. 192 We reviewed the theory of planned behaviour and reasoned action193,194 (including emotional rationalisation) and health belief models. 195 Additionally, we looked at attention control techniques160 and techniques to promote posture and balance196 to underpin and inform our intervention. The theories that we considered were mapped onto the broad components of the intervention arising from the evidence base.
Social learning theory
Bandura’s10 model of social learning suggests that behaviour is learned through the process of observation of the environment and the social world to which we are exposed. Bandura identified the importance of learning from role models and peers. He suggested that imitation and social reactions to those imitations (positive and negative reinforcement) can influence future behaviour. Our systematic reviews and our qualitative research also reflected this, with group courses having better outcomes than individual and remote (web-based) courses and participants recalling other participants who coped well with their pain (and, conversely, those who coped particularly poorly). We decided that a group approach, in which participants could learn from each other and try techniques in the company of and with the support of others, was appropriate.
Theory of planned behaviour/reasoned action
The theory of planned behaviour and reasoned action suggests that a person’s behaviour is determined by an intention to perform the behaviour, which is based on an individual’s attitude towards the behaviour, his or her subjective norms and his or her readiness to perform the behaviour (i.e. whether or not the individual feels that he or she has control and the ability to perform the behaviour). 197
Typically, the more favourable the attitude and the subjective norm, and the greater the perceived control, the stronger the person’s intention to perform the behaviour in question should be. There are many coping behaviours that those with chronic pain adopt, and these behaviours may be beneficial or detrimental. Other behaviours that could be advantageous to pain management are not adopted at all. Raising an individual’s awareness of his or her own behaviour may change attitudes towards existing behaviours if he or she is exposed to, or becomes aware of, alternative behaviours that may be beneficial.
We wanted our self-management course to provide an environment that could promote positive attitudes, challenge and or change inappropriate subjective norms and empower and motivate people to realise that they have the ability to change.
Cognitive–behavioural concepts and acceptance
Cognitive–behavioural therapy evolved out of behaviour therapy and cognitive psychology research. Cognitive therapy has the premise that some behaviours are not simply influenced by rational thoughts but are also controlled by automatic thoughts. 191 In rational emotive therapy, developed by Ellis,198 it was proposed that once people are made aware of their thoughts and behaviours they can rationalise their emotions towards them and modify their behaviour accordingly. These two therapies informed the basis of CBT.
Cognitive–behavioural treatment focuses on individuals’ thoughts, images, beliefs and attitudes (cognitions) and how these impact on behaviour and emotions. The therapeutic process facilitates individual reflection on negative patterns of thinking or behaviour that may cause difficulties in living. Once these behaviours are addressed, this, in turn, is expected to change the way that individuals feel about their issues. In our study the focus was on pain-related behaviours, thoughts and emotions.
We used the fundamentals of modern CBT, which incorporates problem-solving (identifying problem behaviours), goal-setting and action planning, to underpin our approach to help individuals raise their consciousness about how they feel, think and behave towards their pain. Within the action planning stage we also considered ideas surrounding graded exposure used to overcome fear-avoidant behaviour. 173 Modern CBT also encompasses relaxation training, acceptance and commitment therapy, and mindfulness. 192 These are known as third-generation behavioural therapies, with the first- and second-generation therapies being traditional behaviour therapy and CBT. These third-generation therapies focus more on thoughts and feelings rather than behaviour. 199
Acceptance and commitment therapy has six core principles designed to help develop psychological flexibility:
-
cognitive defusion – learning methods to reduce the tendency to reify thoughts, images, emotions and memories (in other words when people ‘overvalue/prioritise’ thoughts and images and/or fit and fix them into misleading mental models)
-
acceptance – allowing thoughts to come and go without struggling with them
-
contact with the present moment – awareness of the here and now, experienced with openness, interest and receptiveness
-
observing the self – accessing a transcendent sense of self, a continuity of consciousness that is unchanging
-
values – discovering what is most important to one’s true self
-
committed action – setting goals according to values and carrying them out responsibly. 200
Pain catastrophising theory
Pain catastrophising describes a maladaptive thinking/cognitive style often seen in patients with chronic pain who have associated anxiety and depressive disorders. 201,202 It is characterised by the tendency to magnify the future threat of a predicted pain stimulus, regardless of whether or not it will occur. Chronic pain patients who catastrophise lose their ability to inhibit pain-related thoughts in anticipation of, during or following a painful encounter.
Figure 8 illustrates the relationships between the theories that we considered and our desired outcomes.
FIGURE 8.
Conceptual model.
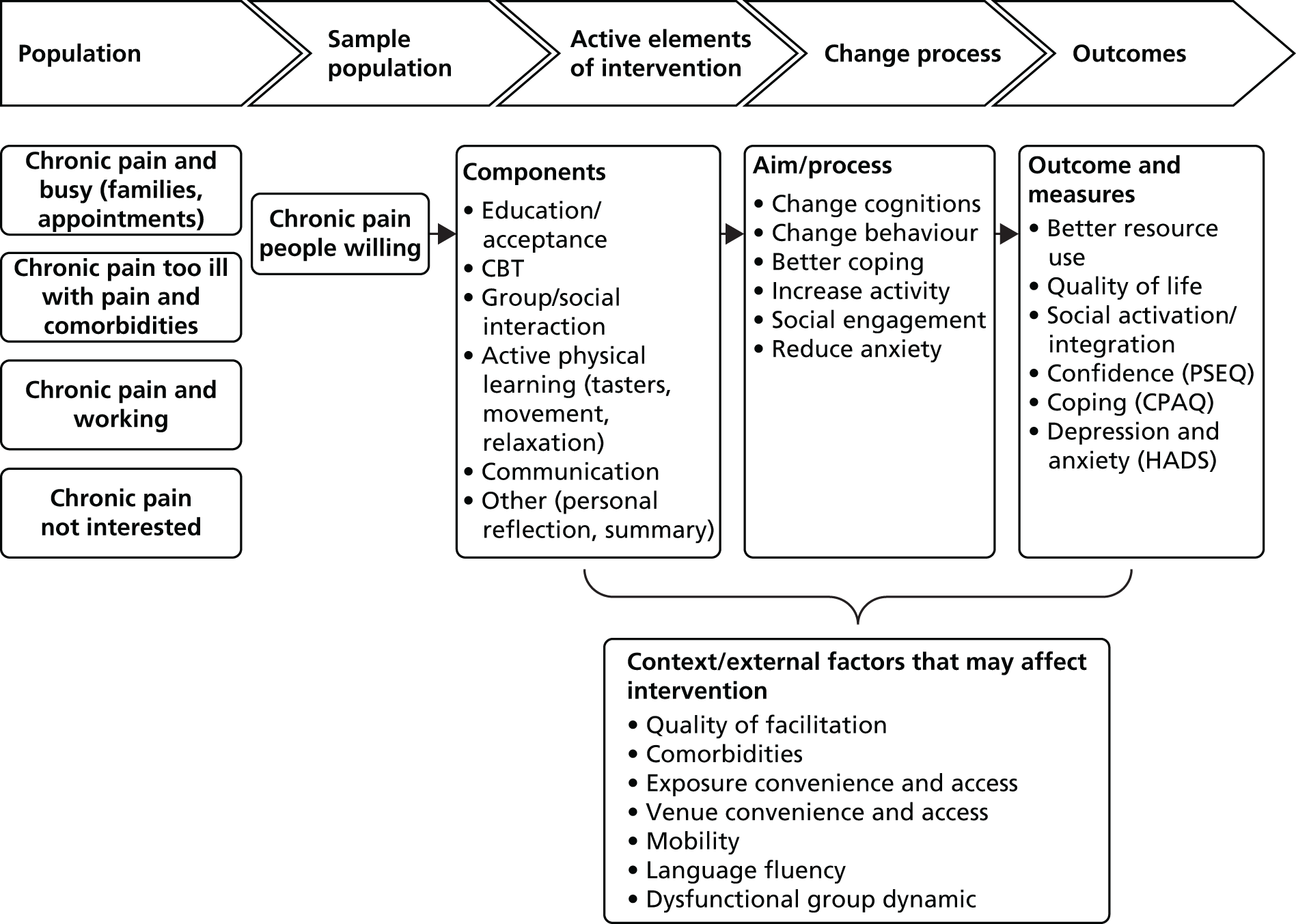
Modelling the potential course structure and function
As recommended by the MRC guidelines28 we considered patient pathways through the self-management programme and the likely action and interaction of the different components with regard to outcomes in an attempt to model the impact and effect of our intervention.
The first stage of modelling was to consider recruitment. We know that only a proportion of those with chronic pain will be interested in participating in the study and/or a self-management course (regardless of research). Once recruited, each participant will be exposed to various components of the intervention with the aim of affecting thoughts and behaviours to produce the desired outcomes.
Our conceptual model in Figure 8 diagrammatically represents the patients, the components of the intervention, the factors that may affect the intervention positively and negatively and the outcomes that we hope to affect. The first column in the model shows the chronic pain population and its potential to be involved in the study and the potential barriers that may affect uptake.
The model shows the patient pathways through the proposed self-management programme. We considered the likely action and interaction of the different components (e.g. sessions, materials, suggested behaviour changes) with regard to outcomes. This involved us elaborating the behaviour change theories relevant to the new intervention. However, it was not possible to develop any appropriate statistical models of the action of the intervention because of a lack of published data.
Mapping behaviour change techniques to course design
The outcomes that we decided that we might affect with our intervention were function despite pain, health-care resource use, social engagement, depression, anxiety, self-confidence in managing pain and coping. After assessing the relevant behaviour change theories we identified individual behaviour change techniques that might affect our chosen outcomes. We used Abraham and Michie’s189 taxonomy of behaviour change techniques to describe the techniques that we adopted to promote positive behaviour change in self-management groups.
Table 21 shows our rationale for mapping and modelling theory to behaviour change techniques and the methods used by facilitators throughout the courses. Some sessions required facilitators to employ techniques focusing on providing feedback; other sessions provided instruction to promote behaviour change; and some sessions allowed participants to try out techniques within the ‘safety’ of the learning environment and the group. The techniques employed by facilitators were dependent on the needs of the participants and the groups and therefore were utilised as required in each individual session. No negative or coercive behaviour change techniques were recommended or used as part of the course.
| Underlying theories and therapies | Influence on course design | Cognitive and behaviour change techniques used throughout the course |
|---|---|---|
| Biopsychosocial model of medicine: physiology, psychology and the social environment and society play a part in health | Whole course |
|
| Acceptance and commitment therapy: accepting the here and now and living with it | Pain information; acceptance: the uninvited guest; relaxation and mindfulness | |
| Fear avoidance and catastrophising: pain and fear lead to avoidance behaviour, which is not always beneficial | The pain cycle, goal-setting and action planning | |
| Attention management: keeping the brain occupied on things other than pain reduces pain perception | Attention control and distraction; relaxation, breathing, visualisation and imagery; taster sessions (e.g. art) | |
| Social cognitive theory: behaviour may be influenced by interaction between personal factors, environmental factors and own and others’ behaviour | Group work/discussion, reflection, listening skills | |
| Cognitive therapy: recognising the link between thoughts, emotions and behaviour; theory of planned behaviour: based on beliefs about the likely consequences of behaviour; rational emotive principles: logical unemotional rationalisation of events, thoughts, emotions | Identifying problems, goal-setting and action planning; barriers to change – unhelpful thinking; barriers to change – reframing negatives to positives; communicating with your GP; anger, irritability and frustration: managing emotion; follow-up – managing setbacks | |
| Mind–body therapies: muscle relaxation, biofeedback, visualisation and mindfulness techniques | Relaxation and breathing; relaxation and visualisation; relaxation and mindfulness of thoughts | |
| Physical theory and therapies: Alexander technique for posture and physical therapy practice of balancing and stretching | Posture, balance and stretch |
Overall course design
We appreciated that we would have to consider adult learning theory and a variety of modes of delivery. Our qualitative research indicated that an informal ‘non-lecture style’ was preferred, with plenty of opportunities for discussion and socialising. Cognitive and social approaches to behaviour change are often challenging as they require participants to reflect about themselves and confront issues that they may or may not be already aware of. Facilitating this process of change and learning requires skill. Facilitators needed training to motivate participants, actively listen, be non-judgemental, empathise and be patient.
A variety of educational, role-playing and discussion sessions that facilitate the learning and practice of behavioural change techniques, such as those used in CBT, have been shown to be effective within the group learning environment in other studies on chronic pain. 48,53 A short, intense group intervention has also been shown to be effective for depression. 203
There were two very important messages that we had to convey from the outset: (1) we promised no cure (to temper expectation and begin the process of acceptance) and (2) self-responsibility and personal action are important.
Social learning theory takes into account individual ability to learn by experience, either directly or indirectly through others. This was fundamental to the programme; it was the facilitator’s role to encourage learning through discussion and self-exploration, and to motivate participants to practise implementing techniques and use those that they felt might help them.
The theory of planned behaviour and emotional rationalisation are based on feedback from promoting, praising and practising positive behaviours, thoughts and feelings, to help generate confidence in personal ability and promote self-confidence. The facilitators and group participants were expected to contribute to this process.
Figure 9 shows how the course content was constructed and linked to aspects of chronic pain that were considered important by the people who we interviewed for the study, the theories that we assessed and the components that showed evidence of benefit.
FIGURE 9.
Mapping pain factors to the course content. Shaded box indicates course content.

Figure 10 shows the learning sequence that we adopted; this was informed from our interviews and by consulting educationalists in the School of Medicine and Dentistry at Queen Mary University of London. Our interviewees were relatively uninformed about their condition, knowledge about the persisting nature of pain was unclear and many had not accepted their condition or that there might not be a cure for it. We proposed to tackle these issues first and then build on the learning to encompass key behavioural change strategies and coping techniques. The educationalists recommended using a variety of different teaching techniques and we therefore used videos, didactic education (very little), brainstorming, role play and practice. We were also keen to make the setting as informal as possible and provide as much social time as possible.
FIGURE 10.
Learning sequence.
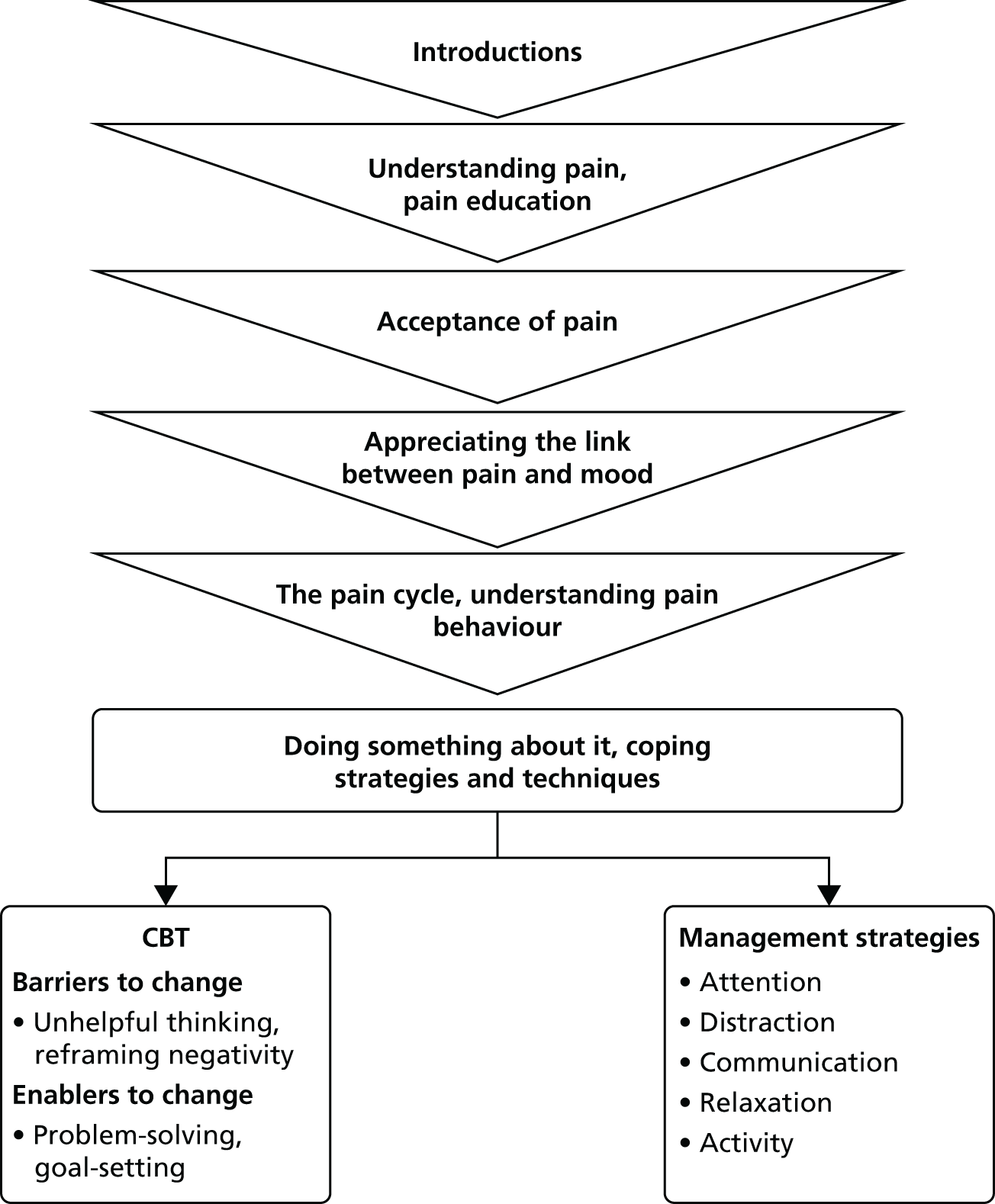
Key considerations from the evidence influencing course design have already been described; other key considerations are summarised in Table 22.
| Consideration | How this influenced course design |
|---|---|
| Adult educationalists advised that to be interesting and effective the course should employ a variety of media and modalities, be delivered in 20-minute bites and encourage experiential learning | Inclusion of role play, filmed material, small group exercises, exercises for pairs, active listening exercises, brain storming, etc. |
| Attrition from self-management courses running over 6–8 weeks known to be a problem | Course was run over 3 days in a single week |
| Expert professional input may be useful or appealing to participants | Expert professional input delivered by DVD for economy |
| Reproducibility and fidelity of the intervention | Development of a course manual and training package |
The course
We designed a group course to be facilitated by a lay person with chronic pain who had experience in small group facilitation (e.g. experience as a tutor on the Expert Patients Programme) and a HCP (GP, psychologist, physiotherapist, chiropractor or osteopath) to be delivered over 3 short days (10.00–14.45) with a 2-hour follow-up session 2 weeks later. We also designed a 2-day training programme for all potential facilitators. All courses were to be held in a convenient accessible location for the target population. A ‘buddying system’ was incorporated into the model.
The components of the course included psychological concepts using cognitive–behavioural approaches to managing chronic pain (these covered acceptance, attention control, goal-setting and action planning, recognising unhelpful thinking and behaviours). The course also covered communication skills, relationships, intimacy, promoting better sleep, education about chronic pain, social time, hobbies and activities, posture and movement, breathing, relaxation, and visualisation and guided imagery. The course included a bespoke educational DVD of a pain consultant answering common questions from patients with chronic pain; this was produced in both English and Bengali (which the Sylheti-speaking Bangladeshi community also understand).
A summary of the course aims, learning outcomes, rationale and teaching methods is provided in the following sections and Box 2 (in the box the numbers 1–24 are used to describe the individual sessions or components of the course).
-
Introductions
-
Aim: to introduce self succinctly and effectively.
-
Rationale: social awareness.
-
-
DVD on pain education
-
Aim: to increase understanding about chronic pain.
-
Rationale: introduce idea of acceptance.
-
Break
-
Acceptance
-
Aim: to relate the scenario about the unwanted guest to chronic pain.
-
Rationale: introduce idea of acceptance.
-
Taster activity (pain perception when thoughts not focused on pain).
Lunch break
-
Pain, bearable or not?
-
Aim: to start introducing the concept that pain and mood are linked.
-
Rationale: pain is not only physiological but also psychological, social and emotional.
-
-
The pain cycle
-
Aim: to explain the pain cycle and understand the process and the unhelpful things that we do that keep us in that cycle.
-
Rationale: behaviour theory and fear avoidance.
-
Break
-
Posture
-
Aim: to promote body awareness, posture and muscle weakness.
-
Rationale: evidence for exercise, physical therapy principles, Alexander technique.
-
-
Relaxation and breathing
-
Aim: to reduce muscle tension and introduce breathing as a relaxation technique.
-
Rationale: principles of third-generation CBT, mind–body therapies and biofeedback.
-
Summary of the day
-
Aim: reflection and embedding learning.
-
Reflections from day 1
-
Aim: to understand and empathise with the group.
-
Rationale: improve bonding and group cohesion, social cognitive theory.
-
-
Goal-setting and action planning
-
Aim: to help participants logically and systematically to identify problems, brainstorm solutions, set goals and devise action plans as a means of escaping the pain cycle.
-
Rationale: based on cognitive–behavioural techniques, change management principles, theory of reasoned action and theory of planned behaviour.
-
Break
-
Barriers: unhelpful thinking
-
Aim: to introduce ideas about unhelpful thoughts, automatic thoughts and errors in thinking.
-
Rationale: recognising errors in thinking can help with realistic assessment and more constructive/rational views of the world; based on the fundamentals of rational emotive therapy and cognitive–behavioural principles.
-
Taster activity.
Lunch break
-
Barriers: reframing ‘cons’ to ‘cans’
-
Aim: to identify reasons why people stay in the pain cycle and barriers to change.
-
Rationale: based on cognitive–behavioural techniques.
-
-
Attention control and distraction
-
Aim: to learn how to focus the mind away from pain thoughts.
-
Rationale: based on attention control and distraction techniques.
-
-
Making pain more manageable
-
Aim: to summarise the techniques learnt so far to manage pain.
-
Rationale: embedding learning from the day.
-
Break
-
Balance and stretch (as day 1).
-
Relaxation and visualisation (as day 1).
Summary of the day (as day 1).
Day 3 sessions-
Reflections from day 2
-
Aim: to understand and empathise with the group and ascertain current thoughts.
-
Rationale: improve bonding, group cohesion and understanding of learning, social cognitive theory.
-
-
Communicating with health professionals
-
Aim: to reflect on consulting behaviour, promote effective communication and constructive consultations.
-
Rationale: promote effective health-care utilisation; based on theories of reasoned action and planned behaviour.
-
Break
-
Listening skills
-
Aim: to improve listening and communication skills.
-
Rationale: to help with social integration, based on social cognitive theory.
-
-
Anger, irritability and frustration
-
Aim: to identify reasons for negative emotions and implement goal-setting and action planning.
-
Rationale: cognitive–behavioural principles.
-
Taster activity.
Lunch break
-
Stretch
-
Aim: to learn how to stretch muscles gently with low risk of injury and pain.
-
Rationale: manual therapy.
-
-
Relaxation and mindfulness of thoughts
-
Aim: to learn to apply guided imagery and detach emotion from reality to appreciate ‘the now’.
-
Rationale: evidence for focusing attention and separating emotion from sensation: mindfulness.
-
Break
-
Summary of the course
-
Aim: to clarify learning from the past 3 days and introduce the idea of buddying.
-
Rationale: embedding learning.
-
-
Reflections and narratives
-
Aim: to understand and empathise with the group and ascertain current thoughts.
-
Rationale: improve bonding, group cohesion and understanding of learning.
-
Break
-
Managing setbacks
-
Aim: to know what to do when experiencing a setback or a flare-up.
-
Rationale: cognitive–behavioural principles, attention control and re-embedding learning.
-
Day 1: Living with and dealing with pain
-
Aims: introduce aims of the course and concept of group work, and increase understanding of pain and reasons for it; introduce concept of acceptance and no cure; introduce the idea of recognising different moods and their effects on pain.
-
Learning outcomes: know where pain comes from and why we have it and, in chronic pain, why it persists; the participants should be able to be able to describe why they have persistent pain to somebody else and be able to identify some of their own beliefs about their pain and identify some negative thoughts and behaviours, for example when is pain OK/manageable and, when it is at its worst, how this can relate to mood.
-
Teaching methods: group introductions/presentation, facilitated discussion, watching a DVD of frequently asked questions, pain/mood exercise.
Day 2: Doing something about life with pain
-
Aim: identify opportunities to change and understand when change is possible and when it is not.
-
Learning outcomes: be able to break down issues into manageable chunks and set simple, measurable, achievable, realistic goals within a suitable time frame; be able to identify negative behaviours and thoughts and spot errors in thinking.
-
Teaching methods: group discussion, self-reflection and practice.
Day 3: Communication and relationships
-
Aim: promote effective utilisation of health-care services and improve communication skills.
-
Learning outcomes: moderate expectations; communicate effectively.
-
Teaching methods: group discussion and role play.
Follow-up at 2 weeks
-
Aim: managing setbacks: knowing what to do when experiencing a setback or a flare-up.
-
Learning outcomes: improve bonding, group cohesion and understanding of learning; embedding learning.
Facilitator recruitment and training
The recruitment criteria and training needs for facilitators were obtained from the qualitative study.
We were aware that courses facilitators who were highly regarded were those who managed difficult participants, included participants to the extent that they were comfortable with, were flexible and knew the course content well so that they could deviate when necessary and return to issues as appropriate. Facilitators who had good listening skills, who did not talk too much themselves and who made people laugh and relax were valued.
We recruited HCPs and laypeople. We recruited HCPs who had a specific interest in and understanding of chronic pain and we recruited laypeople who had experience of chronic pain and who had previous tutoring experience.
The course was designed to deliver:
-
facilitation skills training
-
trial procedures and protocol training
-
course content familiarisation and delivery
-
adverse event handling.
The course was spread over 2 days (Saturday and Sunday) and involved didactic education, role play and practice. Trainees were observed and evaluated throughout the course to assess their understanding, strengths and weaknesses (the training course structure is provided in Appendix 4).
Chapter 7 Feasibility study
Abstract
Introduction: In 2010 we conducted a feasibility study to inform us about the COPERS self-management course, in particular its delivery and content plus the trial procedures, in preparation for a definitive trial.
Method: We designed a pilot RCT with 100 chronic musculoskeletal pain patients randomised 3 : 1 to the intervention or usual GP care plus an advice leaflet. We included a non-randomised arm in which we delivered a version of the course translated into Sylheti to Bangladeshi patients not fluent in English. We used a mixed-methods evaluation including quantitative process information; qualitative feedback from course participants, facilitators and observers on their experience of the course; and quantitative data from participant self-report questionnaires at baseline and 3 months’ follow-up.
Results: Systematically identifying eligible participants from GP medical records proved difficult and spurred us to develop better search strategies for the main trial. Very uneven initial randomisation allocation led us to abandon the randomised design and offer all participants the intervention. In total, 167 (32%) of 526 potential participants, of whom 343 (65%) were female, expressed an initial interest in participating; 56 of these (34%) were recruited to the English-speaking courses and 41 (25%) to the Sylheti-speaking courses. We ran nine courses, six in English and three in Sylheti. Forty-two people attended the English-speaking courses and 26 attended the Sylheti-speaking courses. Nine facilitators were trained and seven facilitated a course. We sought written feedback from facilitators and participants and we also carried out 13 in-depth participant interviews. The course was regarded as beneficial by most participants, with the group experience being important. Key recommended changes before a definitive trial included:
-
better facilitator training
-
the provision of clear aims and summaries for each session of the course as well as links between sessions
-
audio-recording of each course to check quality and ‘treatment drift’
-
making the outcome questionnaires user friendly and shorter
-
adopting the pain-related disability subscale of the CPG as a future primary outcome
-
the provision of a more credible control arm
-
conducting the main trial only in English.
Conclusion: The feasibility study provided important information on the intervention and its delivery and on the design and conduct of a definitive trial of the intervention.
Introduction
Having designed the self-management support intervention for chronic musculoskeletal pain (see Chapter 6) we conducted a feasibility study to test the design of the new intervention and to inform a future definitive trial of the intervention. We called the intervention COPERS after the name of the study (COping with persistent Pain, Effectiveness Research into Self-management). The COPERS course aimed to improve the overall quality of life for people living with chronic pain.
Objectives
The objectives of the feasibility study were to evaluate the quality and appropriateness of the COPERS course and the trial processes, including the participant recruitment process, facilitator training, delivery of the course content and collection of outcome measures, in preparation for the main RCT.
Methods
We proposed a pilot RCT of 100 participants randomised to the intervention or usual GP care plus best practice patient advice on a 3 : 1 basis favouring the active intervention. In addition to this pilot RCT, to test the practicality of including participants from a wider range of backgrounds in our main study, we delivered the intervention separately in both English for the general population and in Sylheti for members of the Bengali community living in Tower Hamlets (Figure 11).
FIGURE 11.
Design of the feasibility study.
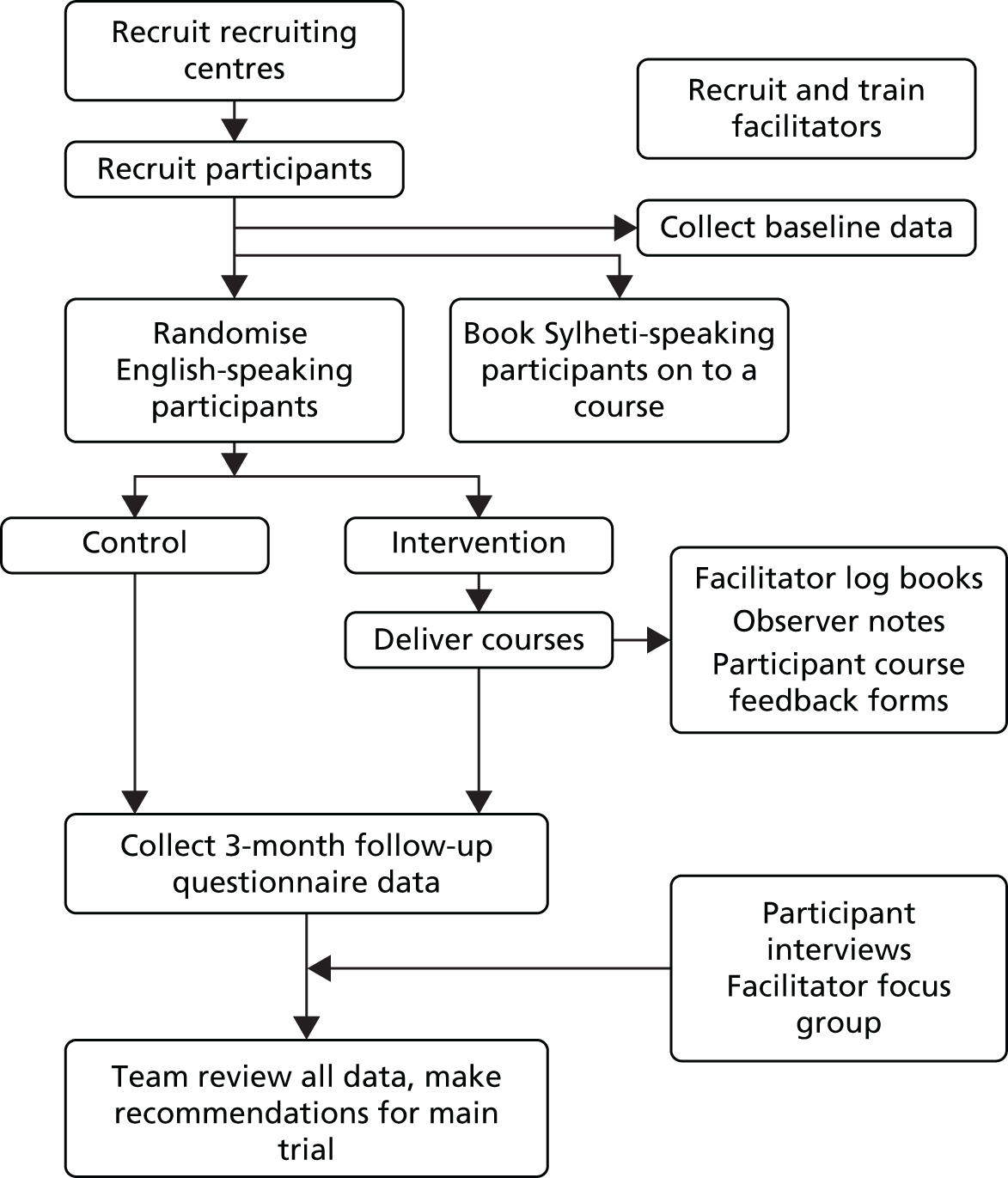
We used a mixed-methods approach including both qualitative feedback from course participants, facilitators and observers, and quantitative information from self-report questionnaires and activity data.
We chose randomisation on a 3 : 1 basis to enable us to run at least six to eight courses while testing the randomisation process and the acceptability of the control arm. This study was not designed to show an effect of the intervention but to test the feasibility of the intervention and the trial protocols. It was conducted in Tower Hamlets, London, from January to April 2010.
Recruitment
We aimed to recruit participants to the English-speaking courses by targeting patients with chronic pain who were registered with the local persistent pain service, physiotherapy department or two local general practices. We selected the community-based pain and physiotherapy services as they reported in excess of 1000 new chronic pain patients per year. We also involved general practices as clinicians estimated that around 10% of GP consultations in Tower Hamlets were for chronic pain. 204 One of the general practices was chosen because around 90% of patients were Sylheti speaking (in other local practices the equivalent figure was around 50%).
Recruitment of participants
Participant recruitment is key to any successful trial. We reviewed six studies that had also recruited participants to trials of group interventions for musculoskeletal pain (Table 23). Their mean uptake rate from invitations was 8.8%; we aimed for an uptake of around 10%.
| Study | Country | Pain condition | Setting | Duration of course (weeks) | Number invited | Number recruited | Uptake rate (%) |
|---|---|---|---|---|---|---|---|
| Bernaards 200698 | The Netherlands | Upper limb | Occupational | 24 | 8000 | 466 | 5.8 |
| Johnson 200754 | UK | Lower back | Community | 6 | 2068 | 234 | 11.3 |
| Lamb 201053 | UK | Lower back | Community | 6 | 9744 | 701 | 7.2 |
| Moore 200083 | USA | Lower back | Medical | 3 | 2582 | 226 | 8.8 |
| UK BEAM Trial Team 2004205 | UK | Lower back | Community | 6 | 11,341 | 1334 | 11.8 |
| Von Korff 199877 | USA | Lower back | Medical | 4 | 3292 | 255 | 7.7 |
Participant identification and participant recruitment materials
We anticipated inviting approximately 600 patients with chronic pain to participate in the feasibility study. We estimated a 60% response rate (around 360 people) based on previous studies that we have conducted and expected that around one-third of these would accept the invitation to participate, resulting in around 120 potential participants. Of these, we anticipated that about 10% would not be able to commit to the study or would not meet our inclusion criteria.
We asked the clinical staff at recruiting centres to search their computerised medical records to identify eligible patients. At this point we realised that there was no easy way to identify people with chronic pain from the patient electronic records in general practice using condition terms and/or Read codes, such as low back pain and OA combined with recent consultations for pain. This led to a separate study to explore the best way of identifying patients relevant to our trial. 206 We also encouraged face-to-face invitations at all recruitment sites as we anticipated that the personal interaction and authentication of the course by a HCP/patient adviser would increase interest (see Chapter 4). Potential participants were identified and reviewed by their clinician according to the inclusion and exclusion criteria described later in this chapter. Those deemed likely to be eligible were invited to participate in the trial by their clinic or general practice by letter. With the letter they also received a participant information leaflet and a consent to approach form to complete and return to the study team if they were interested in joining the study (the final versions of these documents as used in the main trial are contained in Appendix 6). We translated participant recruitment materials into Bengali script for the Bangladeshi participants (see Appendix 5). In addition, one of the recruiting general practices employed a bilingual patient advisor advocate who was able to translate and explain the information to any less literate Bangladeshi patients living with chronic pain identified for the study. Reminder invitation letters were sent after 10 days.
Sylheti-speaking groups
In addition to the English-speaking courses (proposed pilot RCT), we proposed three additional courses conducted in the Sylheti language for Bangladeshi participants. We ran separate Sylheti courses for male and female participants. The Bangladeshi community has a written language, Bengali, and a spoken language, Sylheti. Depending on the level of education potential participants may or may not have been able to read and understand the Bengali script but all could understand and speak Sylheti. Participants recruited to the Sylheti-speaking courses were not included in the randomisation process but were enrolled directly onto the courses. We were particularly keen to explore how this course could be made available to the Bangladeshi community of Tower Hamlets (where many of the study team were based), who, in common with other South Asian groups living in the UK, have a high prevalence of chronic musculoskeletal pain. 153 Delivering the course in a language other than English provided the specific experience needed to inform a decision for the main trial on whether to present all courses in English or to also offer the programme in minority languages.
Consent
The study team telephoned interested patients and spoke to them about the study to check that they were eligible and ensure that they had enough information. We were also able to establish language fluency during this initial telephone contact. If potential participants were still interested in the study, we attained informed verbal consent to participate and assigned them a study number. We then asked them to complete the baseline questionnaire and sign the study consent form and return both documents to the study team in the Freepost envelope provided. Another member of the COPERS team, fluent in Sylheti, made the initial contact telephone calls to interested Bangladeshi participants and sent out the consent form and questionnaire (Sylheti-speaking participants were given both a Bengali script and an English version of the questionnaire).
Participant inclusion criteria
-
Adults (aged > 18 years) with a diagnosis of chronic non-specific musculoskeletal pain.
Participant exclusion criteria
-
Not fluent in spoken English (except for Bangladeshi participants eligible for the Sylheti-speaking course).
-
Serious comorbidity such as cancer and not in remission.
-
Poorly controlled major psychological illness.
-
Terminal illness.
-
Unaddressed or poorly controlled addiction to drugs or alcohol or other substance misuse.
-
Inability to give valid consent.
Randomisation
On receipt of the signed study consent form the English-speaking participants were randomised to either the intervention or the control group. An independent statistician based at the Pragmatic Clinical Trials Unit (PCTU) at Queen Mary University of London performed the randomisation using minimisation, with gender as a minimisation factor, in a ratio of 3 : 1 favouring the intervention. Participants were then telephoned by the study team and informed of their allocation. Those randomised to the intervention group were enrolled on the earliest available convenient course and asked to complete a baseline questionnaire prior to the course. The Bangladeshi participants were not randomised but booked on to courses directly.
The intervention
The structure of the course and the rationale for its design are described in Chapter 6.
We aimed to book courses on alternating days when possible, for example Monday, Wednesday and Friday, in easily accessible, familiar and local places, with a large room, kitchen and toilet facilities. The course content was modified to be culturally appropriate for the local Bangladeshi population but remained as similar in design and structure as possible to test whether or not our approach was directly transferable to this particular ethnic group. The modifications included single-sex courses and avoiding a clash with Friday prayers.
Training facilitators to deliver the intervention
We recruited HCPs (chiropractors, GPs, osteopaths, psychologists and physiotherapists) and laypeople with chronic pain and experience of facilitating and/or teaching to deliver the intervention. Training took place over 2 days and consisted of familiarisation with the course content and structure, facilitation skills and trial procedures.
Control arm
Participants randomised to the control arm of the study received usual GP care plus best practice advice in the form of a 20-page booklet called The Pain Toolkit, developed by Frances Cole and Pete Moore. 207 We asked the control participants to refrain from attending any self-management courses for the duration of the study but encouraged them to contact their local Expert Patients Programme after the COPERS follow-up period had elapsed.
Data collection
We collected both quantitative and qualitative data as part of the feasibility study. Quantitative data included questionnaire-based self-reported outcomes and participant feedback. Qualitative data included observational field notes from the courses, free response questions from participant feedback forms, a focus group with team members and COPERS facilitators, and in-depth interviews with some of the participants.
Questionnaire for self-reported outcomes
Questionnaire-based self-reported outcomes were collected at baseline (just prior to randomisation) and 3 months after randomisation. We collected data from the Bangladeshi participants before the beginning of each course and 3 months after the course. The questionnaires were returned to the study team using reply-paid envelopes. Reminder letters and telephone calls were used to contact those who failed to respond.
We used the basket of outcome measures identified in Chapter 5. These were:
-
pain intensity using a 0–10 numerical rating scale
-
pain extent using a pain manikin drawing
-
heath-related quality of life using the EQ-5D169
-
self-efficacy using the PSEQ181
-
anxiety and depression using the HADS176
-
coping and acceptance using the CPAQ174
-
in addition, following a TSC meeting decision we used the SF-36. 208
At baseline we also collected self-reported demographic data: date of birth, gender, living arrangements (alone or with others), language fluency, ethnicity,209 employment status and education level, duration of pain and location of pain (the final questionnaires used in the main trial are in Appendix 6).
Facilitator log books
Each facilitator (two per course) was required to complete a structured log book. Facilitators were asked to reflect and comment on the following for each session/component of the course:
-
engagement of participants in the process
-
level of understanding shown by participants
-
types of questions asked
-
displays of discordance/expressed difficulties/dysfunctional elements
-
group dynamics
-
personal reflections and suggestions for improvements.
The facilitators returned their completed log books to the study team after each course was finished.
Observer field notes
Each course was observed by a member of the study team and notes were taken and prepared in a structured format for each session on:
-
the facilitation process
-
the content generated
-
the types of questions asked
-
displays of discordance/expressed difficulties/dysfunctional elements
-
the level of understanding shown
-
group dynamics/engagement of trainees in the process
-
personal reflections and suggestions for improvements.
Participant feedback questionnaires
Participants on the English-speaking courses were asked how satisfied they were, on a 5-point scale, with aspects of the course, together with some free-response questions (Box 3).
On a scale of 0–5, with 0 indicating least satisfied and 5 indicating most satisfied, how satisfied were you with the following:
-
the course today?
-
the teaching methods used?
-
the handouts?
-
the facilitators?
-
the group discussions process?
-
the amount of time for socialising?
-
the amount of time spent on each topic?
-
the taster session?
-
the facilities?
-
the amount of information given?
-
Name three things that you learned were important to you.
-
What parts of the course did you enjoy the most and why?
-
What parts of the course did you least enjoy and why?
-
How relevant was the course content to you?
-
Comments about facilitation.
-
Suggestions for improvements.
Questionnaires were handed out to participants by the course observers at the end of each course and questionnaire completion was optional and anonymous.
Participant interviews
Purposive sampling was used to identify participants to approach for interview. We invited a mix of genders and ages (≤ 40 years or > 40 years) and those who had good or poor attendance or who had chosen not to attend the course at all. The topic guide for the in-depth interviews was generated by the study team and aimed to cover:
-
the invitation process (recruitment and marketing)
-
randomisation (reasons for attending)
-
the baseline questionnaire
-
course content
-
course duration and facilities
-
the group process (‘buddying’ and mix of participants)
-
facilitation
-
post-course changes and activities.
All interviews were recorded and written up but were not transcribed verbatim.
Data analysis
Questionnaire for self-reported outcomes
The output from the baseline questionnaire was used to produce summary statistics on demographics and outcome measures for both the randomised participants and the Bangladeshi participants.
Facilitator feedback and observer data
Data collected from the observer notes, the facilitator focus group and facilitator log books were combined. Two members of the study team familiarised themselves with the data to identify themes and subthemes.
Participant data
Response rates and mean scores were calculated for the participant feedback questionnaire items. The qualitative data from the participant feedback questionnaire and the participant interviews were analysed using a framework approach. 154 Two researchers reviewed the data independently to derive a framework of themes and subthemes.
These data were used to inform the facilitator focus group and modifications to the course and the trial processes.
Results
Recruitment sources and participant identification
Recruitment and delivery of the intervention took place over 9 months (February to October 2010). We identified 526 potential participants, of whom 32% (n = 167) expressed an initial interest in participating. Of these, 42% (n = 70) were allocated a study ID number and sent a questionnaire and consent form to participate in the English-speaking study, and 24% (n = 40) were enrolled into the Sylheti-speaking feasibility study, representing an overall 21% recruitment rate (Table 24).
| Recruitment source | Number of invitations (letter or face to face) | Reminder letters | Initial interest | Enrolled | |
|---|---|---|---|---|---|
| Allocated study ID number | Booked onto Sylheti course | ||||
| Pain service | 423 | 342 | 124 | 59 | 26 |
| GP 1 | 68 | 5 | 24 | 0 | 12 |
| GP 2 | 23 | 19 | 12 | 7 | 2 |
| Physiotherapy | 12 | 0 | 7 | 4 | 1 |
| Total | 526 | 366 | 167 | 70 | 41 |
Reasons for declining to take part were:
-
a preference for ‘physical’ treatment such as physiotherapy or injections
-
inability to get time off work, especially for teachers
-
inability to attend because of childcare issues
-
language barriers (English fluency not sufficient to take part in a group process).
Consenting participants were randomised using minimisation with a 60 : 40 female/male split expected and a 3 : 1 ratio in favour of the intervention. However, we abandoned allocation to the control arm as the initial randomisation sequence was top heavy with control participants and did not yield sufficient participants in the intervention arm to fill the first prebooked course. We continued to run the randomisation sequence and eventually the randomisation sequence was as expected overall. We modified our randomisation strategy for the main study to include randomly permuted blocks to ensure that a steady flow of participants was randomised to the active intervention.
We ran nine courses in total starting in the last week of March 2010 and continuing to mid-July 2010, with around seven participants on each course. There were six mixed-gender English-speaking courses, one Sylheti-speaking male course and three Sylheti-speaking female courses. Fifty-six participants [n = 30/56 (54%) female participants] were booked onto the English-speaking courses. Fourteen participants (25%) did not attend any sessions. Four participants attended day 1 only and 38 attended ≥ 2 days (Table 25). Four male participants attended the Sylheti-speaking course, with three attending ≥ 3 days. The first Sylheti-speaking female course had 20 participants booked but four (20%) did not attend any sessions. Of the remaining 16 participants all attended at least 2 days. The second Sylheti-speaking female course had 14 participants booked but eight (57%) did not attend any sessions.
| Participants | Number contacted | Total number booked on a course (% of those contacted) | Attendance at day 1 (% of those approached/booked) |
|---|---|---|---|
| English-speaking females | 228 | 30 (13) | 23 (77) |
| English-speaking males | 134 | 26 (19) | 19 (73) |
| Sylheti-speaking females | 115 | 34 (30) | 22 (65) |
| Sylheti-speaking males | 46 | 7 (15) | 4 (57) |
There were 10 participants on the English-speaking courses who were not from a white British/Irish ethnic group and who spoke English with varied fluency. They coped with the course but those who were not very fluent did struggle to maintain full involvement with the discussions, often preferring to listen rather than talk.
There were more female than male participants interested in being part of the study (75 females vs. 34 males), particularly for the Sylheti-speaking group (33 females vs. 7 males) (Table 26). The mean number of participants attending each English-speaking course was seven and the mean number of participants attending each Sylheti-speaking courses was nine. The median age range of participants was 41–50 years (see Table 26).
| Age (years) | Sylheti speaking | English speaking | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| ≤ 20 | 0 | 0 | 0 | 0 |
| 21–30 | 0 | 1 | 0 | 2 |
| 31–40 | 2 | 9 | 7 | 5 |
| 41–50 | 3 | 8 | 6 | 17 |
| 51–60 | 0 | 13 | 7 | 12 |
| 61–70 | 0 | 2 | 3 | 4 |
| 71–80 | 2 | 0 | 3 | 0 |
| ≥ 81 | 0 | 0 | 1 | 2 |
| Total | 7 | 33 | 27 | 42 |
Questionnaire for self-reported outcomes
Of 56 potential participants allocated a study ID number and booked on a course, 48 (86%) returned usable baseline questionnaire and study consent forms. The response rate at 3 months was 52% (n = 25). Of 41 Bangladeshi participants booked onto courses, 18 (44%) returned their baseline questionnaire and three (7%) retuned their follow-up questionnaire.
The participants found the questionnaire extremely difficult to complete because it was long and repetitive; this was particularly so for the Bangladeshi group. We provided assistance and extra time prior to the courses being run to help participants complete the questionnaire but participants and the assistants found the task arduous and slow. Table 27 shows that, despite providing the questionnaire in both English and standard Bengali to the Bangladeshi group, more English questionnaires were returned. Our qualitative work explained that help to complete the questionnaire was often given by participants’ children who were fluent in English and but who did not read Bengali.
| Course | Questionnaires sent | Questionnaires returned (response rate, %) | Returned in English | Returned in Bengali | Fully completed (completion rate, %) | Partially completed (completion rate, %) |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| B1 | 4 | 3 (75) | 0 | 3 | 0 | 3 |
| B2 | 16 | 6 (38) | 5 | 1 | 3 | 3 |
| B3 | 6 | 6 (100) | 5 | 1 | 1 | 5 |
| Total | 26 | 15 (58) | 10 | 5 | 4 (27) | 11 (73) |
| Follow-up | ||||||
| B1 | 4 | 0 (0) | 0 | 0 | 0 | 0 |
| B2 | 16 | 2 (13) | 2 | 0 | 2 | 0 |
| B3 | 6 | 0 (0) | 0 | 0 | 0 | 0 |
| Total | 26 | 2 (8) | 2 | 0 | 2 (100) | 0 (0) |
There was a high proportion of missing data in the participant questionnaires, meaning that the data should be interpreted with caution (Table 28). At baseline, differences in employment were seen between the groups, with few of the Bangladeshi group being employed and the majority of responders being female home/family carers. The other between-group difference related to education: 7/14 (50%) of the Bangladeshi participants who supplied data on age completing education had either no formal education or were educated only to the age of 11 years, compared with 2% of the English-speaking group.
| Characteristic | Sylheti speaking (N = 26), n (%) | English speaking (N = 48), n (%) |
|---|---|---|
| Employment | ||
| Employed | 1 (4) | 13 (27) |
| Family/home keeper | 8 (31) | 5 (10) |
| Retired | 1 (4) | 8 (17) |
| Unable to work because of ill health | 4 (15) | 17 (35) |
| Unemployed | 3 (12) | 5 (10) |
| Missing | 9 (35) | 0 (0) |
| Education | ||
| None | 3 (12) | 1 (2) |
| Until < 12 years | 4 (15) | 0 (0) |
| Until 13–16 years | 4 (15) | 18 (38) |
| Until 17–19 years | 1 (4) | 9 (19) |
| Until ≥ 20 years | 2 (8) | 18 (38) |
| Still a student | 0 (0) | 2 (4) |
| Missing | 12 (46) | 0 (0) |
| Support | ||
| Living alone | 0 (0) | 17 (35) |
| Living with others | 6 (23) | 27 (56) |
| Missing | 20 (77) | 4 (8) |
| Pain | ||
| Duration > 4 years | 13 (50) | 36 (75) |
| Duration < 4 years | 4 (15) | 11 (23) |
| Missing | 9 (35) | 1 (2) |
| Mean extent (out of 13 sites) | 5 sites (n = 18) | 5 sites (n = 47) |
| Mean intensity (out of 10) | 7.7 (n = 18) | 7.0 (n = 44) |
The mean scores for pain extent, intensity, depression and social integration were similar between the groups at baseline (Table 29). EQ-5D, anxiety, self-efficacy and coping showed differences between the groups, indicating worse states in the Bangladeshi group. This is consistent with other evidence from Tower Hamlets. 153
| Outcome | Baseline English speaking (n = 43), mean score (SD) | Baseline Sylheti speaking (n = 26), mean score | Follow-up English speaking (n = 22), mean score (SD) |
|---|---|---|---|
| CPG pain intensity (scale 0–10)a | 6.7 (2.1) | 7.7 | 6.3 (2.2) |
| CPG pain extent (scale 0–13)b | 4.6 (2.4) | 5 | – |
| EQ-5D scorec | 0.23 (0.4) | 0.12 | 0.31 (0.4) |
| PSEQ score (scale 0–60)d | 22.5 (12.7) | 25.4 | 30.2 (13.1) |
| HADS anxiety score (scale 1–21)e | 11.3 (4.1) | 12.7 | 10.2 (3.8) |
| HADS depression score (scale 1–21)e | 9.4 (3.8) | 9.3 | 8.8 (4.1) |
| CPAQ score (scale 0–120)f | 46.7 (17.3) | 41.9 | 54.1 (18.02) |
| heiQ score (scale 5–20)g | 12.8 (3.1) | 12.4 | 13.1 (3.5) |
At follow-up, overall, almost all mean outcomes showed a tendency towards improvement but, when confined to those for whom we had longitudinal data, as anticipated, no statistically significant changes in scores were seen in these pilot data (see Table 29).
Recruitment and related issues are summarised in Table 30. These issues were identified from feedback from the facilitators and from observations by the study team. Detailed feedback data are provided in Appendix 5.
| Process | Issues identified | Potential solutions |
|---|---|---|
| Recruitment sources and patient identification | Time-consuming to secure collaborations with recruitment sources allowing for approvals | Allow time for recruitment of recruitment sources |
| Recruitment sources did not yield enough participants | Do not target all recruitment sources at the same time – phased recruitment; try to estimate number of participants expected compared with actual and flag up deficits early – allow for exclusions; boost recruitment by advertising in non-GP sites (e.g. local adverts in waiting rooms, pharmacy posters) | |
| More potential participants yielded from medical record searches than expected | Construct more specific search strategies for medical record systems to target the most appropriate participants | |
| Fewer participants yielded from secondary care registers than expected | ||
| GPs/clinicians too busy to screen lists of potential participants generated from medical record searches | Ensure participating GPs/clinicians are given enough time to check patient lists against exclusion criteria | |
| GP/clinic staff did not have dedicated resources to perform the searches of their record systems or have time to produce the mail-merge invitation letters and post the packs | Recruit a research assistant and allocate time to perform the initial searches of patient records to identify suitable participants and also to prepare the invitation packs and reminder letters | |
| Difficulty in finding a patient advocate who had time to assist in the recruitment of non-English-speaking participants | Provide specific funding for a patient advisor/advocate to help in face-to-face or over the telephone recruitment for non-English-speaking participants | |
| Randomisation and course booking | Freepost envelopes were sometimes delayed compared with stamped envelopes | Allow time for post office processing of the Freepost service |
| Need time to receive baseline questionnaire back prior to course dates | Allow at least 4 weeks for participants to return their baseline questionnaire and consent form; allow 2 months between the participant invitation mail-out and course booking | |
| Control group | It was difficult to ‘sell’ the control arm of usual care and an educational leaflet to potential participants | Provide a more credible control arm to motivate people to take part |
| Randomisation | Individual randomisation by minimisation yielded an unpredictable sequence of allocations | Use the block randomisation method to ensure that enough participants are allocated to the intervention to run a course |
| Cultural adaptation | Courses running on a Friday precluded the male Muslim population because they were attending prayer | Choose alternative day to Friday |
| Data collection | Questionnaire fatigue an issue | Shorten questionnaire |
| Response rate from non-English-speaking participants particularly poor | Use English-speaking population to test effectiveness to optimise the amount of data collected | |
| Low questionnaire response rate generally | Incentivise return with vouchers |
Table 31 shows the number of respondents recording each satisfaction score for each question. Overall, the average response rate was 72%.The mean overall satisfaction score was high (4.3). There was little variation in overall scores; however, the ranges indicate that there was less satisfaction with time allocated for discussion, socialising and each topic. At least four people found the ‘taster’ unsatisfactory.
| How satisfied were you with the . . .? | Level of satisfaction from least to most (0–5) | Number of questionnaires receiveda | Mean score | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||
| Course today | – | – | 3 | 7 | 30 | 46 | 86 | 4.4 |
| Teaching methods | – | – | – | 12 | 26 | 46 | 84 | 4.4 |
| Handouts | – | – | 1 | 14 | 33 | 38 | 86 | 4.3 |
| Facilitators | – | – | 1 | 4 | 21 | 61 | 87 | 4.6 |
| Group discussions | – | 1 | – | 6 | 26 | 52 | 85 | 4.5 |
| Time for socialising | 1 | 2 | 5 | 13 | 26 | 36 | 83 | 4.0 |
| Time on each topic | – | 2 | 7 | 13 | 26 | 37 | 85 | 4.0 |
| Taster session | – | 1 | 3 | 11 | 22 | 42 | 79 | 4.3 |
| Facilities | – | – | 2 | 10 | 24 | 50 | 86 | 4.4 |
| Information given | – | – | 2 | 6 | 27 | 50 | 85 | 4.5 |
| Mean score | 4.3 | |||||||
Participant interviews and feedback
The emergent themes and subthemes arising from the free response questions in the course feedback questionnaires related to knowledge and learning, course content, relevance to self, facilities and course structure and duration. These issues were also summarised and repeated in the participant interviews.
Sample
We approached 18 course participants who were potentially exposed to the intervention for interview; five declined [reasons included work commitments (n = 2); having an operation; bereavement; and ‘no time’] and we therefore interviewed 13 participants, including four males. Three interviews were conducted in Sylheti and the remainder were conducted in English. Four researchers conducted the interviews; all were members of the study team.
Themes
We organised the data into 12 themes: clarity of aims, motivation, positive aspects, negative aspects, learning, social interaction, effect of others, repercussions/outcomes post course, suggested changes, facilitation, ‘buddying’, course material; these themes are detailed in Appendix 6. There was no need to conduct second-order analysis to interpret the data in greater depth as the aim of the evaluation was to find out what modifications needed to be carried out to the intervention programme.
In summary, participants wanted not only the aims of course made clearer but also the rationale for each session. Participants greatly valued the social interaction and wanted more time for informal interaction. They valued good facilitation, which was seen as good group control, that is, managing difficult people and situations; this had implications for facilitator training. Different participants preferred different sessions but they really liked the relaxation sessions and requested audio-recordings of the scripts used. ‘Buddying’ was wanted by some and not others and those interviewed strongly recommended that it be left up to individuals in the groups to identify other participants to ‘buddy up’ with if they so wished.
Study team meeting for modifications and changes
Two study team meeting sessions were conducted because of the number of data, one for facilitator/observer feedback and one for participant feedback. The recommendations from the study team meetings are provided in Table 32.
| Facilitator/observer feedback | Participant feedback |
|---|---|
Course content:
|
Trial process:
|
Overall summary of the results of the feasibility study
The process evaluation of the feasibility study gave clear indications of areas for improvement for the main RCT. The key areas are summarised as follows:
-
The feasibility study demonstrated that it is feasible and acceptable to translate and deliver the intervention in another language; however, it also demonstrated that it was not feasible to collect sufficient outcome data from the non-English-speaking Bangladeshi population. The main trial should be conducted only among people fluent in English (but the results could probably be extrapolated to non-English-speaking people).
-
A credible control is required to encourage participants to enter into a definitive trial. Usual care and a pain education booklet were not sufficient to attract participants.
-
More time was required to secure recruitment sites. A phased approach would assist in the planning of recruitment to courses, along with recruitment monitoring against an expected rate per source to flag up problems early in the study.
-
A more specific search strategy needed to be developed for general practice medical record systems to find suitable participants. GPs do not have time to look through lists of hundreds of patients. General practices may need assistance from the study team in performing the searches and preparing invitation packs and reminder letters.
-
At least 8 weeks should be allowed between posting out participant invitation letters and the start of a local course to allow time for potential participants to complete and return the baseline questionnaire and complete the enrolment process.
-
A block system for randomisation would ensure that groups of participants are randomised in sufficient numbers to provide enough participants for a course.
-
The questionnaire was too long.
-
Good facilitators, and hence good facilitator training, were absolutely key to the successful delivery of the intervention and the retention and satisfaction of course participants.
Consideration of our outcome measures and choice of a primary outcome measure for the main trial
It is usual in trials of treatment for chronic painful disorders to see a substantial improvement between baseline and follow-up in the control arm. 210,211 This is because of a combination of two factors. First, the natural history of the disease is to improve as people will be more likely to be motivated to join a trial if they are in a period of relatively more severe pain; chronic musculoskeletal pain tends to wax and wane over time. Second, one would expect to see some regression to the mean in the results for any outcome measure, irrespective of any change in the underlying disorder. That the EQ-5D169 did not identify this expected change in our pilot study gave the study team serious concerns that it would not be sufficiently responsive to any change in participants’ underlying disorder. The team therefore considered that an alternative primary outcome was needed. The closest measure in the portfolio that we had evaluated (see Chapter 5) was the pain-related disability component of the CPG. This is a disorder-specific outcome that is directly related to overall quality of life. It has much greater disorder-specific clinical relevance than other measures in our portfolio. For this reason the study team chose to use the pain-related disability subscale of the CPG as the primary outcome in the main trial.
Results in the context of other research
Past research on self-management courses has shown that self-management can have small effects on self-efficacy14 and that group approaches can benefit participants. The group process encourages active involvement, cheering successes and offering and receiving advice and support. 49 Our findings support the ‘power’ of the group process as an active part of learning and behaviour change. Self-efficacy seems to enable and contribute to and/or evolve out of the group process. Participants could be empowered either by those who were seemingly worse off than themselves or by those who appeared to be proactive and/or worthy role models. We also found in our systematic review (see Chapter 2) that group-based self-management programmes provided more beneficial effects for participants than remote or individually delivered programmes.
Providing a meaning for learning about the complex interactions between the body, pain, mind and emotions, be it internal (via self) or external (via others), was observed in participants who went on to illustrate examples of better coping strategies. Heightened self-awareness potentially could lead to hypervigilance or, conversely, self-awareness could promote coping strategies that related to personal life situations and were therefore more meaningful to the participant.
Cognitive–behavioural approaches for pain management have been systematically reviewed48 and found to be effective for short-term pain relief. However, it is still unclear which types of cognitive approaches or which elements of the cognitive approaches are more effective than others. Based on our interview data we postulate that self-reflection on unhelpful behaviours and goal-setting have most impact on change. These elements of CBT are often linked with plans to carry out activities in the future; if these plans are carried out they can have an impact on physical and mental health. Other systematic reviews conclude that exercise (any type) is beneficial for persistent non-specific pain. 33 In the COPERS programme we encouraged activity as opposed to exercise to avoid raising existing negative cognitions about exercise, but ironically the posture and stretch sessions were very well received despite us trying to avoid the concept of exercise as much as we could.
Strengths and limitations
We specifically sought to deliver the intervention in a language other than English to a minority ethnic group. This research had its origins in a collaboration with Social Action for Health, a Tower Hamlets-based community organisation that seeks to promote health and well-being in the Bengali community of Tower Hamlets. Social Action for Health had identified the management of chronic musculoskeletal pain as a community priority. We had also demonstrated the higher prevalence of chronic musculoskeletal pain in first-generation Bengali migrants living in Tower Hamlets153 and were aware of the issues through our clinical work. Developing a programme to help this group was therefore a priority for both us and our patient partners.
The large Bengali community in Tower Hamlets meant that it was plausible to develop and a deliver an intervention tailored to this group. This information is locally important. However, we cannot necessarily extrapolate from these findings to other minority ethnic groups or establish how practical it might be to deliver such a programme in areas that do not have the same dominant minority ethnic group. Nevertheless, we have for the first time demonstrated that such an intervention is deliverable to such groups. We had no a priori expectation that this experience would not be transferable to other ethnic groups living in the UK.
Our evaluation was designed to gather information about the COPERS course and its content to determine whether or not any improvements needed to be made and how to improve delivery. We systematically collected data from participants, observers and facilitators. The data were gathered and analysed by different team members to try and avoid bias in interpretation; however, some bias may have occurred. We demonstrated that it was feasible to deliver the COPERS course in Sylheti, that the courses were acceptable to participants and that there was a relatively low rate of attrition. However, this pilot showed that it would be very difficult to determine the effectiveness of the Sylheti language course in a definitive effectiveness trial because most participants were unwilling or unable to complete questionnaires administered by post. An alternative would be to formally evaluate English-language courses for those fluent in English of all ethnicities and extrapolate the data to non-English-language courses. Delivering courses in multiple languages poses an issue in ethnically diverse areas because there is no one dominant language group; however, it is feasible in areas with predominantly one ethnic group, as we have shown. There is also the additional problem that some of our outcome measures have not been validated in other languages.
Recommended changes
The changes required to the COPERS course were not major changes. The suggestions for change could be categorised into three main areas: changes to the course content, changes to processes and changes to facilitator training. We believed that many of the issues that the participants flagged would be avoided by better facilitator training. The facilitator training course was therefore modified and run closer to the start of the courses; when this was not possible we decided to provide ‘top-up’ training to refamiliarise facilitators with the techniques and course material. Facilitators who wanted top-up training would be able to observe other courses and receive individual coaching on a one-to-one basis from the study team (in practice, two facilitators out of 39 (5%) requested additional training and observed a course). Additionally, we would endeavour to always pair a new facilitator with an experienced COPERS facilitator. We also needed to provide a more credible control. Relaxation does not have medium- to long-term effects on pain severity48 but our participants really enjoyed this element of the course. Relaxation can be carried out in groups or alone and so we decided to use this as part of our control as it has limited clinical effects and does not involve any interaction with others.
The use of observer notes for each course was useful to check for ‘treatment drift’ and fidelity. The observers also helped to debrief the facilitators after each day. An audio-recording of each course was used to check quality and ‘treatment drift’ in the main trial.
Conclusions
We demonstrated that it was feasible to deliver the COPERS course in a non-English-speaking population. However, because of the difficulties experienced with filling courses in multiple languages and collecting outcome data, there was an argument for conducting the main trial in English first and, if effective, then either simply extrapolating the results directly to non-English-speaking groups or perhaps evaluating the intervention in a true implementation study in these groups.
The qualitative evaluation of the feasibility study illustrated that the COPERS course was regarded as beneficial by most participants; however, ways to improve the course were suggested. As a result the course content was modified, the aims were made clearer and summarising and linking between sessions were incorporated as essential deliverables for each session. High-quality facilitation appeared to be the key to a successful course and therefore effective training for facilitators was prioritised.
Chapter 8 Phase 1: development of the intervention – discussion
Principal findings from phase 1
In phase 1 of this programme of work we carried out the substantial background work required to prepare for delivering a definitive trial of a self-management approach to managing chronic musculoskeletal pain. Following this we developed:
-
systems for recruiting our population of interest
-
an active intervention grounded in the best available evidence that was acceptable to participants, manualised and deliverable to a wide range of people living with chronic pain
-
an acceptable, manualised training programme to train facilitators to deliver the intervention
-
a credible and acceptable control treatment
-
a carefully selected package of outcome measures.
This put us in the best possible position to answer our original research question: ‘Does a self-management support programme improve outcomes for people living with chronic musculoskeletal pain?’.
The evidence
Group-delivered courses that had HCP input had better outcomes than other types of courses and longer courses did not appear to provide additional benefits over shorter courses. We found mixed evidence for the effectiveness of different proposed components of the intervention package. Courses with a psychological component, however, had more evidence of beneficial effects than those without psychological components and increasing the number of components did not appear to improve course effectiveness. We proposed that this indicated that other factors related to the group process, such as socialisation, may be as important as the content in the success of self-management courses.
Our qualitative work with chronic pain patients who had participated in courses also indicated that they gained benefit from the social aspects of courses and learning from others, as well as the need to focus their attention on things other than pain. People who had accepted their pain and had personal coping strategies that enabled them to lead fulfilling lives were most positive about their experience of pain management courses.
The psychological theories that we reviewed supported our qualitative study findings and underpinned the structure and content of the course. The theory underpinning the decisions to include a variety of sessions and behavioural change techniques worked well within the group learning environment; this has also shown to be effective in other studies of chronic pain. 48,53
Informed by the available theoretical and empirical evidence we developed a brief group intervention to be delivered jointly by a HCP and a lay expert.
The course
The course ran over 3 days in 1 week with a top-up session 2 weeks later. The target attendance was 10–12 participants.
The learning sequence that we adopted enabled each session to build on the previous session and in many cases the participants were able to predict the next phase of learning in advance. The learning and flow of information was pitched at a level at which participants could follow the structure and understand the content. This was shown in the daily feedback sheets that asked participants what they had learned: their learning mapped well onto the learning objectives.
In our feasibility study we demonstrated that the course was feasible, acceptable to participants and deliverable in a NHS context. Participants were positive about the course and the content appeared to be meaningful to them. Attrition was very low over the 3 main days: participants attended on average 85% of the course. Attrition has been reported as an issue in other trials. In one such trial of an Expert Patients Programme run over a 6-week period (intervention arm n = 313), one-quarter of participants failed to attend any session, only 60% were considered to have completed the course (attending at least four of the six sessions) and only one-third attended all six sessions. 15
We tested the feasibility of the intervention in a non-English-speaking Bangladeshi population. In Tower Hamlets in east London 32% of the community are of Bangladeshi origin,212 with some not speaking English or not speaking English well. The prevalence of chronic pain in the Bangladeshi population (arrived in UK after the age of 14 years) is higher than that in the white British and British Bangladeshi population living in Tower Hamlets, at around 75% compared with around 50%. 153 We demonstrated that the intervention can be delivered in Sylheti to a group who may find services hard to access. Although we could not collect validated participant-reported outcomes, our evidence from interviews and feedback from the courses indicated that the participants valued the courses and appreciated the effort made to accommodate their needs, for example running single-sex courses. From a national perspective the ethnic distribution varies, supporting our decision to run the trial in two areas. The non-white population in London makes up about 40% of the total population, whereas in the West Midlands it is around 17%. 212 In the census 8% report that English is not their main language and of these 21% do not speak English well (this equates to 1.7% of the total English and Welsh population). 213 Our experience running the non-English speaking courses was that they were feasible and well received but it was hard to recruit sufficient people onto the courses to make them viable and hard to collect outcome data, which may affect the analysis and sample size required for the main trial (attrition and incomplete data).
The facilitation and group process may have optimised the learning process as discussion embedded participant thinking. All of the course evaluation material suggested that good facilitation skills were crucial for positive participant perception. Comprehensive facilitator training was essential for courses to run effectively. We found that it was possible to train both laypeople and non-psychologists to facilitate the courses and deliver the cognitive–behavioural approach that we had developed. The facilitator training included four components: facilitation skills training, course content familiarisation and practice, trial management protocols and evaluation.
Delivery styles varied, indicating a need to embed fidelity assessment from the outset to measure both adherence and the competence of those delivering the intervention. Measuring whether or not an intervention has been delivered as efficiently and effectively as possible has been advocated by others;214,215 however, published reports of fidelity assessment, or the methods used to perform it, are sparse.
We found that the course withstood the inexperience of our facilitators in delivering an entirely new course; the content in terms of the discussions, information and handouts was robust enough to make an impression regardless of delivery style. We recommended that inexperienced personnel be partnered with experienced personnel in the main trial.
Recruitment and participants
Recruitment to the feasibility study was challenging; the conversion rate from invitation to course attendance was lower than we had hoped (approximately 13% of those invited) but was in line with other studies of this nature recruiting patients from primary care with chronic conditions. 53,83,98 Nevertheless, we had sufficient interest from patients to run nine courses and this demonstrated a demand for learning about non-pharmacological approaches to managing pain. Procedures for future recruitment need to be enhanced by increasing the number of invitations sent and devising and testing a comprehensive and inclusive electronic search strategy for patients with chronic pain. To address this need we developed and tested an electronic patient record search strategy using repeat prescription data for chronic pain-related medication, musculoskeletal Read codes and contact within the last 3 months. 206
In our feasibility study participants reported poor quality of life, low self-efficacy to manage their chronic pain, relatively high levels of social isolation, poor coping skills and a tendency towards anxiety and depression. This is consistent with the findings of a European survey carried out in 2005216 that asked respondents about the impact that pain had on their daily lives. In total, 32% reported that they were no longer able to work outside the home or attend social activities and 21% reported having a diagnosis of depression as a result of having chronic pain (24% in the UK); thus, our secondary outcome measures need to reflect these health and social states.
We noted the levels of depression and considered whether depression should be addressed with patients prior to, or in conjunction with, attending these types of courses.
The control
The pilot study indicated that the control arm needed to have more credibility; there was little motivation for people to join the trial if they perceived that they would receive no added benefit, that is, usual care. Our systematic review illustrated that mind–body approaches (mostly relaxation) may have some short-term beneficial outcomes but did not produce medium- or long-term benefits and this has also been shown by other reviews of relaxation. 217,218 Our qualitative work indicated that participants really enjoyed this part of the course that they attended and so we decided that if there was little evidence for effectiveness but positive appeal for relaxation this may be an incentive for recruiting people into the trial who might otherwise decline.
Strengths and weaknesses
We designed a novel approach to analyse the effectiveness of courses by content and characteristics by looking at interventions with and without prespecified components and characteristics. Although many studies were available, some subgroups had few data available and the conclusions drawn were tentative. However, looking at all of the studies, and the data indicating beneficial outcomes, we were able to identify characteristics and components that seemed to elicit better effects than others. This allowed us to translate the findings directly into our intervention design, enabling us to put together an evidence-based intervention at a level of detail that included content and delivery style.
A byproduct of our predictor, mediator and moderator study was that we found that there was no consensus about methodological standards for mediator studies. We carried out an additional expert consensus study that informed and enhanced our systematic review. 135 Additionally, we also tested and piloted an electronic search strategy for identifying chronic pain patients. 206 The strategy that we devised is applicable to other conditions but would need further testing. We involved patients in the selection of outcome measures but, in retrospect, as was pointed out by one of our reviewers at the end of the entire programme, we would have liked more patient involvement in this process. Following the recommendations in MRC guidance,28 as part of the feasibility phase of the project we started to design and develop a methodology for rigorously and systematically assessing and measuring the fidelity of our intervention delivery from the outset,207 so that the assessment was embedded into the training, delivery and implementation of the intervention. Importantly, we were able to demonstrate that we could deliver the course in Sylheti for the Bangladeshi community of Tower Hamlets. We were not able to include this group in the main trial; nevertheless, showing that the course can be simply translated and delivered to another ethnic group indicates that our findings are likely to be generalisable to other non-English-speaking groups in the UK. We suggest that running a parallel non-randomised arm of the study for groups who may find services hard to access may be a sustainable and credible option for researchers to demonstrate the generalisability of an intervention for groups who cannot be included in a randomised comparison.
Findings in relation to other studies
Optimal self-management of chronic pain can be achieved by addressing the reasons surrounding acceptance, negative cognitions and behaviour change to enhance quality of life. The course that we proposed is theoretically driven by behavioural approaches and techniques to address the emotional, psychological and social aspects of living with chronic pain. The Expert Patients Programme and the Chronic Disease Self-Management Programme are two interventions that have gone partway towards addressing the issues of living with chronic pain. In these programmes weekly sessions are held over 6 weeks, which has implications for attendance. 15 Our short intensive intervention appeared to overcome this problem because there was less scope for ‘life’ events to occur over the duration of a week, preventing attendance.
Our framework had a strong emphasis on utilising specific behaviour change techniques such as goal-setting and tailored education to address health beliefs. Our model is delivered to groups and addresses issues surrounding social integration. Our approach creates the opportunity for social support and integration shown to be important to this population and is likely to be more cost-effective than individually delivered programmes. We structured some group sessions to formally address and facilitate behaviour change through the application of well-established psychological theories, principally social cognitive theory.
Cognitive–behavioural therapy and the Expert Patients Programme have shown some evidence of effectiveness for improving function and pain and quality of life and self-efficacy. 13,14,53 Cognitive–behavioural approaches are typically delivered by HCPs and the Expert Patients Programme by laypeople. Our research shows that courses delivered by HCPs have a more beneficial impact on pain-related outcomes, whereas lay-led courses have a more beneficial impact on self-efficacy. Using both may optimise the potential for effectiveness but perhaps reduce cost–utility; this will need testing in the trial.
Conclusions
The MRC guidance for developing complex interventions28 enabled us to develop and test an evidence-based and theory-informed pain self-management course. 219 The process enhanced the intervention and gave the study team confidence in the modified intervention and trial procedures and processes necessary to run efficiently a full effectiveness and cost-effectiveness RCT.
The development phase of this study was comprehensive and thorough. Funding external feasibility or pilot studies has many ‘hidden’ benefits that may help ensure the success of large expensive trials. The feasibility study gave the team knowledge and experience and on-the-job training to deliver the intervention and run the trial. In addition, we were able to use existing contacts and peer networks and create new contacts and networks to help recruit general practices and use our already-trained facilitators to deliver the courses at the standard that we wanted.
Chapter 9 Randomised controlled trial of the clinical effectiveness and cost-effectiveness of the COPERS intervention: methods
Abstract
In part II of this report we describe how we tested the clinical effectiveness and cost-effectiveness of our self-management programme in a large RCT.
Aim
To establish the effectiveness and cost-effectiveness (expressed as the cost–utility) of the new self-management intervention (COPERS) for patients with chronic musculoskeletal pain when added to usual care plus a relaxation CD of simple relaxation exercises.
Study objective
To test the clinical effectiveness of a self-management course for chronic pain and usual care compared with usual care plus an education leaflet and a CD of simple relaxation exercises with respect to (1) the primary clinical outcome of pain-related disability and (2) the secondary outcomes: anxiety, depression, coping skills, chronic pain acceptance, social integration, self-efficacy, being prescribed analgesics and being prescribed weak and strong opioid drugs.
Methods: effectiveness study
Figure 12 illustrates the recruitment process and the different stages of the trial. This was a two-arm RCT with a follow-up period of 12 months to assess participant outcomes.
FIGURE 12.
Recruitment process and different stages of the trial.
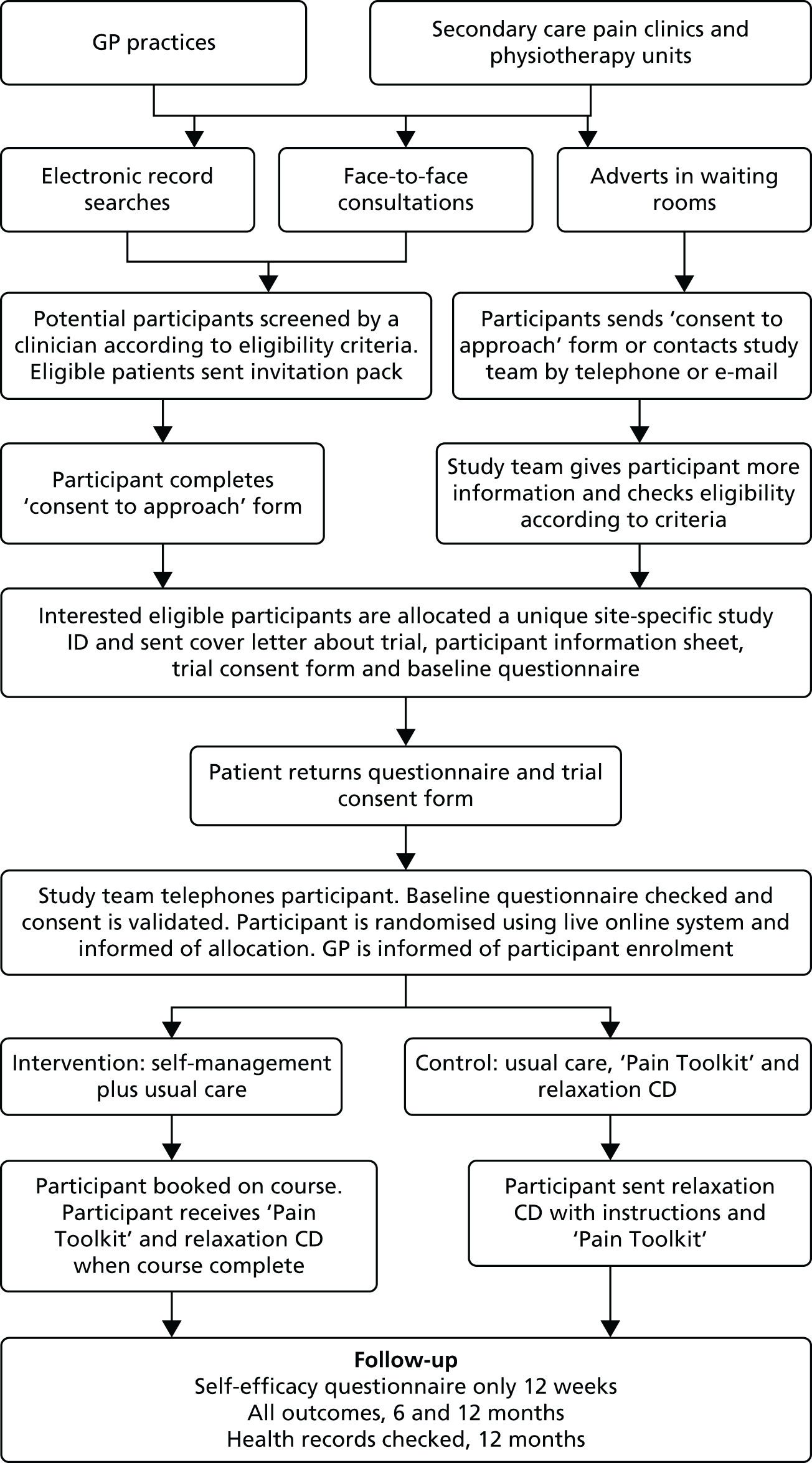
Ethical approval
The study was approved by the Cambridgeshire Ethics Committee on 18 March 2011 (reference no. 11/EE/046). Additional ethical approval was obtained to give participants unconditional £5 high-street shop vouchers with the 6- and 12-month questionnaires and, on the advice of the TSC, to add questions about non-NHS, pain-related personal costs at 12 months (7 October 2011). We sought further approval for additional questions at 12 months enquiring about attendance at other similar or pain-related courses, new hobbies or activities undertaken and any psychological help participants may have sought during the trial period (August 2011–July 2012).
Recruitment
Recruitment of recruiting centres
The study was conducted in two areas: inner east London (Tower Hamlets, City and Hackney and Newham Primary Care Trusts, which were coterminus with their respective London boroughs) and the Midlands (Warwickshire and Coventry Primary Care Trusts). Thus, the COPERS study population included residents of deprived inner-city areas and those living in affluent urban, suburban and rural settings.
Assisted by the primary care research networks in London and the Midlands we invited all general practices in both areas to recruit participants. We also invited intermediate and secondary care pain clinics, rheumatology services and musculoskeletal physiotherapy services to recruit participants. Interested practices and services signed a study agreement form and were reimbursed for all costs associated with recruiting patients.
Recruitment of participants
Participants were recruited in three ways:
-
searches of general practice/service electronic patient records
-
clinician referrals during face-to-face consultations (GPs, pain physicians, rheumatologists or physiotherapists)
-
through posters displayed in clinics advertising the study.
Recruitment of participants from general practice
Each practice designated a key contact to liaise with the study team. The contact was given a standard operating procedures booklet for the study and was responsible for maintaining the master list of those approached securely within the practice. The electronic searches were conducted by practice staff with the support of the study team. No single computer code for a diagnosis of chronic musculoskeletal pain is routinely used in primary care and so we developed a new search strategy to identify potential study participants. Full details of the development of our general practice search strategy and its rationale are described elsewhere. 206 In summary, during extensive consultation and piloting of searches we noted the different coding and prescribing ‘cultures’ in individual practices. Therefore, there was no definitive final search and each search was tailored to the individual practice but constructed using the same principles. Whenever possible searches followed three stages:
-
Stage 1. We selected patients who were aged ≥ 18 years and who were registered with the general practice and had consulted within the previous 6 months.
-
Stage 2. Within the population identified in stage 1 we searched for repeat prescribing information for a variety of drugs associated with chronic pain including low-dose tricyclic antidepressants, strong and weak opioids, non-steroidal anti-inflammatory drugs (NSAIDs), pregabalin and gabapentin. We chose the drugs most commonly prescribed within each individual practice and excluded opiates more commonly used in palliative care (e.g. slow-release morphine).
-
Stage 3. We searched the population identified in the first two stages by high-order Read (classification) codes for low back pain, back pain, OA and fibromyalgia and/or other codes commonly used by the practice staff for musculoskeletal conditions. 220
This strategy generated a list of potential participants, who were screened by their GP for suitability to ensure that no vulnerable people were approached (see inclusion and exclusion criteria). From previous searches and test runs we estimated that this search would yield around 5% of the registered patients, which is consistent with the National Pain Audit finding that 6.4% of the population have chronic pain (the exact figure varies by definition of chronic pain). 221
General practices sent all screened potential participants an invitation letter to the trial from each patient’s own GP. These were placed in preprepared envelopes that contained a consent to approach form, a patient information leaflet and a Freepost envelope for returning the consent to approach form to the study team. The general practices sent a postal reminder letter 10–14 days later if the study team had not received a response. The general practices provided us with an anonymised list of all of the patients invited into the study, which described only gender, age and ethnicity (if available).
General practitioners were also able to recruit patients directly into the study by offering an information pack or the study contact telephone number during a consultation. In addition, we placed posters about the study in practice waiting areas. This served as both a prenotification for participants approached by post or during a consultation and an opportunity for people to self-refer into the study. Those who wanted to refer themselves into the study could obtain information packs from the practice reception or by telephoning the study team.
We recruited from general practices in three waves, within sublocalities, to ensure that we had a sufficient flow of randomised participants living near enough to attend the intervention courses. At the time of the initial telephone contact with the study team potential participants were informed of the date and location of the course that they would attend if randomised to the active intervention. Completion of the baseline questionnaire and consent form could be deferred if a potential participant was unable to attend the next available course.
Recruitment of participants from other services
Recruitment processes in other services followed a similar general approach to that in general practices but we did not design a specific computer search for these services. When a service had a suitable electronic record identifying patients consulting for chronic musculoskeletal pain invitation packs were posted by the service with a covering letter signed by the treating clinician. Otherwise patients were recruited directly by the treating clinician or through self-referral.
Consent and withdrawal
We obtained participant consent in two stages. First, we sought consent for the study team to receive contact details to allow an approach (the consent to approach form). Second, on enrolment to the trial participants provided informed consent, including consent to access routine NHS records. Full details of the study procedures around consent are available in Appendix 6.
Participants were free to withdraw from the study at any time and without explanation; on formal withdrawal from the study we ceased to collect further questionnaire data but, when possible, we asked participants if we could still collect patient record data at 12 months. If we could not contact participants we assumed that this permission was withdrawn as well.
Inclusion criteria
-
People aged ≥ 18 years with musculoskeletal pain of > 3 months’ duration.
-
Available to participate in an intervention course if randomised to the intervention arm.
-
The IASP17 defines chronic pain as that which has persisted beyond normal tissue healing time, usually interpreted as 3 months. Causes/types of pain included, but were not restricted to, OA, back pain, chronic widespread pain and fibromyalgia.
Exclusion criteria
-
Unable to give informed consent.
-
Not fluent in English.
-
Life expectancy of < 6 months.
-
Presence of chronic pain arising from active malignant disease.
-
Presence of inflammatory arthritis such as rheumatoid arthritis.
-
Presence of a serious comorbidity that was more disabling than chronic pain.
-
Poorly controlled serious mental health illness that would make it difficult to participate in the intervention.
-
Misusing substances to an extent that would make it difficult to participate in the intervention.
We excluded people living with chronic pain arising from malignant disease because this requires specific management. However, chronic pain in people living with or beyond cancer may arise from non-malignant causes and such patients were eligible for our study.
We restricted the study to those who were fluent in spoken English for practical reasons:
-
The interactive, participatory nature of the intervention meant that it was unsuitable for delivery through an interpreter or advocate.
-
Our systematic review (see Chapter 3) identified that lack of fluency in the language of the programme was associated with lack of clinical effect.
-
The only language other than English that was sufficiently common to consider running courses in the study recruitment areas was Sylheti. We piloted delivering the intervention in Sylheti and found that it was not feasible to include a Sylheti language stream in the evaluation of the intervention (see Chapter 7).
-
The validity and reliability of the outcome measures translated into languages other than English have not always been established.
People with serious mental health problems or substance abuse problems were able to join the study. They were excluded only if their GP, or other screening clinician, judged that their current problems were so severe that they were likely to cause difficulties within the group sessions or if patients themselves felt that this might be the case.
Randomisation
The randomisation ratio between the intervention and control arms was 1.33 : 1 (see Sample size for an explanation). Randomisation was overseen and implemented by the PCTU at Queen Mary University of London. Participants were randomised after the study team had received signed informed consent and the completed baseline questionnaire. Strict allocation concealment was maintained through the use of Sheffield University’s Clinical Trials Research Unit’s web-based, real-time, randomisation programme,222 which was accessed by the study team while on the telephone to participants to avoid having to call them again. Randomisation was performed using random permuted blocks stratified by site of recruitment, with randomly varying block lengths of seven and 14. All randomisations were logged using an online audit trail of use according to each user ID.
Interventions
The active intervention
The intervention was a group-based facilitated learning course about chronic pain. The course had 24 distinct components (also called sessions) covering various aspects of pain education and pain management. At the end of the 3-day course participants received the same relaxation CD and self-help booklet as those in the control arm.
We aimed to include around 12 participants per course. The minimum number of participants required for a course to take place was eight and the maximum was 16. When a course was undersubscribed prior to the course running those registered were offered alternative courses. Our target was that all participants randomised to a course should start the course within 8 weeks of randomisation; whenever possible participants were recruited onto the next available course. This was not always possible when participants were booked on a course but were subsequently unable to attend; we gave participants a choice of up to three dates or venues.
Recruiting and training intervention course facilitators
Courses were led by two facilitators, a HCP and a lay person. We recruited facilitators from a variety of sources:
-
HCPs. Relevant HCPs included chiropractors, GPs, occupational therapists, osteopaths, physiotherapists and psychologists. Press releases were issued to professional magazines explaining that we were seeking people who might be interested in becoming study facilitators. We also used our own peer networks to recruit facilitators. Interested people submitted their curriculum vitae to the study team and were interviewed by telephone. The recruitment criteria were experience with chronic pain patients, articulate, empathic, having an interest in the psychological aspects of health care and available to run at least two courses.
-
Lay facilitators. Lay facilitators were recruited through an internet self-management news site [www.self-management.org.uk (accessed 2010)] and community interest companies providing expert patient self-management programmes. Interested people sent their curriculum vitae to the study team. The recruitment criteria for lay facilitators were interest and experience in facilitating self-management or self-help groups, current or past personal experience of chronic pain and available to run at least two courses.
Applicants meeting the recruitment criteria were invited to a local joint 2-day training course for lay and professional facilitators. We invited the trained and experienced facilitators from the pilot study to participate and paired these more experienced facilitators with the newly trained facilitators to optimise delivery of the intervention.
The training course covered the course content, how to facilitate, dealing with difficult situations and what to do if an adverse event occurred. During the course facilitators were required to demonstrate that they were good listeners, empathic, flexible, able to encourage equal participation, able to encourage laughter, able to manage difficult people and able to summarise sessions and put the course content into a chronic pain context. Those who were evaluated by the study team as competent by structured observation throughout the course and after a brief written ‘test’ at the end of the training were asked to facilitate future intervention courses.
The control intervention
Those in the control arm received usual care and a copy of The Pain Toolkit booklet,207 a relaxation CD and instructions for relaxation. During the study period 2011–12 The Pain Toolkit booklet was a free resource for people with chronic pain distributed by the Department of Health; from 2009 to 2012, 250,000 copies of the toolkit were distributed to health services. Because of its wide availability it can be viewed as a component of good usual care. Control participants also received a simple relaxation pack in the form of an audio CD with instructions for use and the rationale for the benefits of relaxation (see Appendix 6). Participants were asked to practise the techniques on the CD every day for at least 3 weeks (the same duration as the intervention) and as much as they liked thereafter. Both The Pain Toolkit booklet and the relaxation pack were posted to control patients following randomisation.
Quality control and fidelity of intervention delivery
To promote fidelity of intervention delivery the first day of each course was observed by a member of the study team experienced in delivering the intervention. In the case of first-time facilitators or facilitators who expressed a lack of confidence the course was observed in its entirety. When necessary, immediate feedback was given to facilitators during breaks in the course and at the end of each day, enabling them to modify and improve their performance.
Our assessment of the actual fidelity of delivery of the intervention is described in detail elsewhere223 and in Chapter 10. In summary, every course was audio-recorded. After all the courses had been delivered a random selection of audio-taped sessions was chosen for formal evaluation of fidelity. The evaluators used a checklist to record adherence to structure and content and facilitator competence.
Outcome measures
The rationale for our final choice of outcome measures is described in detail in Chapters 5 and 7. All selected outcome measures were used with approval and licence when required.
Primary outcome measure
Our primary outcome was pain-related disability as measured using the pain-related function subscale of the CPG175 at 12 months post randomisation. This measure has three items, each scored from 0 to 10, that assess the extent to which the participants’ pain has, in the previous 6 months: (1) interfered with their ability to perform their daily activities; (2) changed their ability to take part in recreational, social and family activities; and (3) changed their ability to work. The pain-related disability score is the mean score for these three items multiplied by 10 and is recorded on a scale from 0 to 100, with higher scores indicating worse pain-related disability.
Secondary outcome measures
-
CPG pain-related disability subscale175 at 6 months post randomisation.
-
CPG pain intensity subscale175 at 6 and 12 months post randomisation.
-
HADS depression subscale176 at 6 and 12 months post randomisation.
-
HADS anxiety subscale176 at 6 and 12 months post randomisation.
-
EQ-5D169 at 6 and 12 months post randomisation.
-
PSEQ181 at 12 weeks and 6 and 12 months post randomisation.
-
CPAQ174 at 6 and 12 months post randomisation.
-
heiQ social integration subscale187 at 6 and 12 months post randomisation.
-
Census global health question209 at 6 and 12 months post randomisation.
-
Total defined daily dose (DDD) consumed of psychotropic drugs up to 12 months post randomisation.
-
Total DDD consumed of analgesics (including all opioids and other central nervous system drugs) for pain up to 12 months post randomisation.
-
Total DDD consumed of weak opioids up to 12 months post randomisation.
-
Total DDD consumed of strong opioids up to 12 months post randomisation.
-
Proportion of participants using weak opioids at 12 months post randomisation (defined as having received a prescription for a weak opioid up to 12 weeks before the 12-month follow-up date).
-
Proportion of participants using strong opioids at 12 months post randomisation (defined as having received a prescription for a strong opioid up to 12 weeks before the 12-month follow-up date).
Additional baseline data collected
In addition, at baseline we collected:
-
demographic data from participants, that is, age, gender, ethnicity
-
pain duration
-
living arrangements
-
language fluency
-
age at completion of education
-
employment status.
Additional data collected at 6 and 12 months
Private health-care use was collected at 6 and 12 months:
-
number of and money spent on non-NHS consultations (including complementary and alternative consultations)
-
type and number of and money spent on tests and investigations
-
type of and money spent on medicines
-
type of and money spent on devices and aids
-
overnight admissions/stays in private hospitals
-
money spent on support and help at home as a result of pain.
Additional data collected at 12 months
We also collected data about other activities and interests undertaken in the last year to test whether or not participants were inspired to undertake other similar courses to help and/or manage their pain better.
We asked about:
-
attendance on other similar courses
-
psychological services accessed
-
hobbies and interests undertaken on a regular basis
-
regular relaxation undertaken.
At 12 months the study team also collected data from participants’ general practice records for the 12 months post randomisation on:
-
number and type of comorbidities
-
number of consultations (doctor and nurse consultations and other face-to-face, telephone and home visits)
-
hospital admissions
-
referrals to outpatient services
-
tests and investigations
-
all medications prescribed (in addition we collected data on all medications prescribed in the 3 months prior to randomisation).
We also collected secondary care information from the Secondary Uses Service (SUS) database. 224
Schedule of data collection
Participants received follow-up questionnaires by post at 12 weeks and 6 and 12 months post randomisation. Based on our systematic review225 (see Chapter 3), we hypothesised that improving self-efficacy was the principal mediator of the expected benefits from a self-management intervention. Improving self-efficacy was a key aim for the COPERS intervention. To ensure that we had evidence for any effect that our intervention had on this hypothesised mediator we sent participants the PSEQ at 12 weeks.
At baseline and 6 and 12 months we collected all of our primary and secondary outcomes from participants. We sent a postal reminder after 2 weeks if no response was received. If there was still no response we collected the primary clinical outcome and the EQ-5D data needed for the health economic analysis by telephone. We sent participants a £5 high-street shop voucher that was redeemable in multiple stores with their 6- and 12-month questionnaires to encourage a response. The vouchers were given on a non-conditional basis. This expression of appreciation has been shown to improve questionnaire return rates, with a systematic review reporting that ‘the odds of response were more than doubled when a monetary incentive was used (odds ratio 2.02; 95% confidence interval 1.79 to 2.27) and almost doubled when incentives were not conditional on response (1.71; 1.29 to 2.26)’. 226
At 12 months we collected health-care activity data from participants’ medical records for the 12 months post randomisation and the 3 months prior to randomisation for our health economic analyses.
Figure 13 illustrates the data collected at each time point and how the data were collected.
FIGURE 13.
Follow-up data collection.

Analysis of drug data
Medications used over a 15-month period were collected from participants’ medical records. We extracted drug names and strength used plus quantity and dates prescribed. We used the Prescription Cost Analysis (PCA) database227 to attach a cost to each individual preparation used. Using the World Health Organization (WHO) DDD228 for each drug we generated the number of days of each medication used organised by British National Formulary (BNF) chapter and subchapter. 229 The WHO does not provide DDDs for topical NSAIDs or rubefaciants and so we used a previously published report to define these. 56
The total DDD consumed for each drug was defined as:
The total DDD for a group of medications (e.g. the total DDD for opioids) was the sum of the total DDD for each drug within that medication group (e.g. each drug that was considered an opioid). For example, if there were three opioid drugs (drugs A, B and C), the total DDDopioid was defined as:
The DDD (used in the denominator of the calculation for the total DDD) was determined in the first instance using the WHO register, then by precedent in other trials56,230 and then by clinician consensus. For compound drugs, for example co-codamol, we separated out the components (paracetamol and codeine) and worked out the DDD for each component drug.
Medication outcomes
We considered the following outcomes:
-
Total DDD consumed of psychotropic drugs up to 12 months post randomisation.
-
Total DDD consumed of all analgesics up to 12 months post randomisation.
-
Total DDD consumed of weak opioids up to 12 months post randomisation (codeine, dihydrocodeine and meptazinol, as defined by BNF paragraph 4.7.2229).
-
Total DDD consumed of all NSAIDs (oral and topical combined) up to 12 months post randomisation.
-
Total DDD consumed of all central nervous system drugs for neuropathic pain up to 12 months post randomisation.
-
Total DDD consumed of strong opioids up to 12 months post randomisation (all opioids prescribed other than the ones listed above as weak, as defined by BNF paragraph 4.7.2229).
Calculations were based on BNF subchapters 4.1 and 4.3 for psychotropic drugs, BNF paragraph 4.7.2 for opioids and BNF paragraphs 4.7.1, 4.7.2 and 4.7.3 and paragraphs 10.1.1, 10.2.2 and 10.3.2 for analgesics including opioids229 (Table 33). These are drugs used for treating chronic pain. We excluded all drugs administered as injections but included topical preparations, patches and liquids.
| Drugs | BNF | Comments | ||
|---|---|---|---|---|
| Chapter | Subchapter | Paragraph | ||
| Psychotropic | 4. Central nervous system | 4.1 Hypnotics and anxiolytics | 4.1.1 Hypnotics | Not chloral and derivatives, clomethiazole or antihistamines |
| 4.1.2 Anxiolytics | ||||
| 4.3 Antidepressant drugs | 4.3.2 Monoamine oxidase inhibitors | |||
| 4.3.3 Selective serotonin reuptake inhibitors | ||||
| 4.3.4 Other antidepressant drugs | ||||
| Analgesic | 4.7 Analgesics | 4.7.1 Non-opioid analgesics | 4.8.1 Gabapentin and pregabalin feature as antiepileptics but also feature in 4.7.3 Neuropathic and functional pain. For this analysis 4.3.1 Tricyclic antidepressants are included in paragraph 4.7.3 | |
| 4.7.2. Opioid analgesics | ||||
| 4.7.3 Neuropathic and functional pain | ||||
| 10. Musculoskeletal and joint diseases (exclude steroids, DMARDs) | 10.1 Drugs used in rheumatic diseases and gout | 10.1.1 NSAIDs | Exclude aspirin; no steroids | |
| 10.2 Drugs used in neuromuscular disorders | 10.2.2 Skeletal muscle relaxants | |||
| 10.3 Drugs for the relief of soft tissue inflammation | 10.3.2 Rubefacients and other topical antirheumatics | Not enzymes | ||
Adverse event reporting
An adverse event was defined as any untoward physiological or psychological occurrence in a subject to whom the intervention or control intervention was administered.
We defined serious adverse events as:
-
death
-
life-threatening events
-
events necessitating hospitalisation
-
events resulting in persistent or significant disability or incapacity
-
events otherwise considered medically significant by the chief investigators.
Minor adverse incidents, for example a participant being tearful and distressed during a session, were logged and fed back to the study team by the end of the course.
If a course participant experienced an adverse event the facilitators were required to notify the study manager and, if necessary, the chief investigators immediately. The study team would then inform the Research Ethics Committee and the sponsor if, in the opinion of the chief investigators, the event was serious and (1) related to the COPERS intervention or (2) unexpected and possibly related to the COPERS intervention.
Details of any adverse events were recorded and stored in the trial master file.
Sample size
The sample size calculation was based on detecting a SMD of 0.3 in pain-related disability between the intervention group and the control group, with 80% power and at a 5% significance level. This effect size was commensurate with the largest change seen in a systematic review of Expert Patients Programmes14 and also with the sort of change effected by interventions for other chronic pain syndromes, such as low back pain, on any continuous outcome measure. An initial sample size calculation indicated that we would require data on 350 subjects (175 in each group). We inflated the sample size because of the possibility of a ‘clustering’ effect in the group intervention arm and chose a ratio between intervention participants and control participants to maximise statistical efficiency. 231 Using an intracluster correlation coefficient (ICC) of 0.1 and assuming an average of nine individuals in each group (from an average of 13 individuals recruited to each group, with a 30% loss to follow-up) meant that 480 individuals were needed, with 275 in the intervention group and 205 in control the control group (ratio of 1.33 : 1 between the intervention group and the control group). Allowing for this very conservative estimated 30% loss to follow-up we originally sought to randomise 685 participants (391 intervention participants and 294 control participants).
Previous research221 and electronic record searches in the pilot study indicated that around 5% of adults on GP registers consult with chronic musculoskeletal pain. Based on our pilot study we estimated that 10% of these may be interested in participating in the trial and around half of these would be recruited into the trial.
Around 80% of the population consists of adults aged > 18 years [www.statistics.gov.uk (accessed 19 April 2016)]. This meant that to recruit 685 participants we needed a population base of around 342,000 registered patients, assuming that 80% (approximately 274,000) would be adults. Of these, 5% would be identified from searches as having chronic pain (n = 13,700), of whom around 10% might express an interest in participating in the study (n = 1370). Half of these might be recruited and enrolled (n = 685). Using an average total practice list size of 7000, this equates to around 49 practices. We estimated that we would recruit between 12 and 14 patients per practice.
Blinding and protection from bias
All baseline data were collected by self-completed questionnaire prior to randomisation. After allocation, however, it was impossible to blind participants to treatment allocation because of the nature of the intervention. General practices and clinicians were informed of their patients’ enrolment into the trial but not their treatment allocation, although participants were free to divulge this information to their clinicians. We felt that this information in itself would have little impact on their care. Outcome data were collected using a participant-completed postal questionnaire. Complete blinding of the research team was not possible because of the need to manage course attendance and the unbalanced randomisation. Whenever possible we blinded the research team to treatment allocation during outcome data collection, for example, for those who did not return their questionnaire after the second reminder, primary outcome data and the EQ-5D instrument were collected over the telephone by research personnel blind to treatment allocation using a set script, asking participants not to divulge their allocation prior to collecting their data. To achieve this blinding London staff contacted Midlands patients and vice versa. Patient record data, such as GP consultations, hospital admissions and drug usage, were extracted by personnel blind to treatment allocation. All data were coded according to participants’ study ID and notes were anonymised as much as possible by clinical staff at the practices.
Data management
All data were managed in line with Queen Mary University of London’s PCTU standard operating procedures and were subject to review by PCTU staff audit and the Data Monitoring and Ethics Committee. All electronic participant data were stored in encrypted and/or password-protected files in a secure environment. A database was designed to manage the data input to ensure consistency of practice and coding, with a built-in audit trail that enabled us to track all entries and changes. Regular audit and double-checking of all primary outcome data was conducted to ensure the accuracy of data entry and a further random 10% of all data were double entered.
Statistical analysis
Our statistical analysis plan has been published elsewhere. 232 We briefly summarise the analytical approach used here.
The main analysis for each outcome followed ITT principles, meaning that all participants with a recorded outcome were included in the analysis233 and were analysed according to the treatment group to which they were randomised.
All analyses accounted for clustering by course in the intervention arm,234,235 Participants in the control arm (who did not attend courses) acted as their own cluster (i.e. we analysed the data as if each participant in the control arm belonged to a ‘course’ in which he or she was the only member).
Site of recruitment (London or Midlands), age, gender and HADS depression score at baseline were included as covariates in each analysis. 236–239 Additionally, for continuous outcomes (CPG pain-related disability, CPG pain intensity, PSEQ, HADS anxiety, HADS depression, CPAQ, heiQ and EQ-5D), the outcome measured at baseline was included in the analysis. Continuous covariates (age, HADS depression score at baseline) were assumed to have a linear relationship with the outcome.
Analysis of the primary outcome
The primary outcome (CPG pain-related disability at 12 months) was analysed using a mixed-effects linear regression model with ‘course’ as a random effect. Restricted maximum likelihood was used. The model included site of recruitment, age, gender, HADS depression score at baseline and CPG pain-related disability at baseline as covariates.
All participants who completed at least one of the three questions that form the CPG pain-related disability score at either 6 or 12 months were included in the analysis. Participants who did not fill out any portion of the CPG pain-related disability score at either 6 or 12 months were excluded from the analysis.
Multiple imputation (MI)240 was used to account for participants who had an observed outcome at 6 months but who were missing the outcome at 12 months, as well as participants who completed some but not all of the questions on the CPG pain-related disability scale at 12 months. Imputation was performed using REALCOM-IMPUTE version 1.0.20 (University of Bristol, Centre for Multilevel Modelling; www.bristol.ac.uk/cmm/software/realcom/). 241
Twenty imputations were performed and the results were combined using Rubin’s rules. 240 Only participants who were to be included in the analysis were included in the imputation model. Imputation was performed separately within each study arm. The imputation model included the three questions that form the CPG pain-related disability score at baseline and 6 and 12 months as well as site of recruitment, age, gender, the HADS depression score at baseline and employment status (employed or in full-time education vs. not employed or in full-time education) (14 variables in total). In the intervention arm, multilevel imputation was performed, with ‘course’ included in the imputation model as a random effect.
Missing data in any of the covariates to be adjusted for in the analysis (site of recruitment, age, gender, HADS depression score at baseline, CPG pain-related disability at baseline) were accounted for using the same MI model as above.
Sensitivity analyses for the primary outcome
We performed three sensitivity analyses242 for the primary outcome to assess the robustness of the results to other methods of accounting for missing data. The first sensitivity analysis involved specifying a different imputation model than that used in the primary analysis and the last two sensitivity analyses involved reanalysing the primary outcome using two approaches that are not based on MI.
Sensitivity analysis 1
We determined which baseline covariates are associated with loss to follow-up and included them in the imputation model. The analysis model was the same as above except for the inclusion of additional covariates in the imputation model.
Sensitivity analysis 2
We performed a complete case analysis in which all participants who did not complete all components of the CPG pain-related disability scale at 12 months were excluded from the analysis. The analysis model was the same as above except that missing baseline covariates were replaced using mean imputation.
Sensitivity analysis 3
We analysed the three components that form the CPG pain-related disability score at 12 months rather than the CPG pain-related disability score itself. This was carried out by performing a multivariate analysis in which each of the three components of the 12-month score was included in the model as an outcome (i.e. each participant had three outcomes). A three-level mixed-effects model was used, with random effects for ‘course’ and participant. Treatment–question interactions were included, allowing the treatment effect to vary for each of the three components. An overall treatment effect for CPG pain-related disability at 12 months was estimated using the lincom function in Stata version 14 (StataCorp LP, College Station, TX, USA) to combine the treatment estimates from the three separate components. As above, missing baseline covariates were replaced using mean imputation.
Participants with no completed follow-up data
The primary analysis assumed that the excluded participants (those not completing any questions on the CPG pain-related disability questionnaire at both 6 and 12 months) were missing at random (i.e. they were missing based on the covariates included in the analysis model). To assess the robustness to departures from this assumption, the primary outcome was assessed under a range of missing not at random scenarios. This was carried out using the formula ΔΔ = Δprimary + Y1P1 – Y2P2, where Δ is the treatment effect under the missing not at random scenario, Δprimary is the treatment effect from the primary analysis, Y1 and Y2 are the assumed mean responses for participants with missing data in treatment groups 1 and 2, respectively, and P1 and P2 are the proportion of participants who were excluded from the analysis in groups 1 and 2, respectively. The standard error for Δ was assumed to be approximately equal to the standard error for Δprimary. Y2 varied between 10, 25, 50, 75 and 90 and, for each value of Y2, Y1 was set to Y2 – 10, Y2 and Y2 + 10. For example, for Y2 = 25, Y1 varied between 15, 25 and 35.
Redefinition of the primary end point
The primary outcome was a composite of three questions. The first question (Q1) assessed to what extent the participant’s pain had interfered with daily activities in the previous 6 months. This was assessed on a scale of 0–10, with higher scores indicating more interference. The last two questions assessed to what extent the participant’s pain had changed their ability to take part in (1) recreational, social and family activities (Q2) and (2) work (Q3). Both of these questions were measured on a scale from 0 to 10, with higher scores indicating more extreme change.
For the last two questions, higher change scores are meant to represent a higher negative change; however, it is possible that some participants might have misinterpreted this and recorded a high score to indicate a large positive change. We therefore performed a sensitivity analysis by redefining the outcome for participants whose scores indicated that they might have misinterpreted the intended direction of the questions relating to change.
For participants with a score of ≤ 2 for Q1 (indicating very little interference in daily activities) and a score of ≥ 8 for either Q2 or Q3 (intending to indicate an extreme negative change in their ability to take part in social activities or to work), we assumed that the participant had misinterpreted the intended direction of the scale for Q2 or Q3 (as it is inconsistent for the pain to have had very little interference in daily activities and for there to have been an extreme negative change in the participant’s ability to take part in activities or work). We therefore rescored Q2 or Q3 based on a reverse scale (i.e. a score of 10 was rescored as 0, 9 was rescored as 1, 8 was rescored as 2, etc.). We reanalysed the outcome using the same method as for the main analysis.
Subgroup analyses for the primary outcome
Subgroup analyses were performed for the primary outcome (CPG pain-related disability at 12 months). All subgroup analyses were performed using the same analysis model as for the primary outcome but also included the subgroup of interest and a treatment–subgroup interaction. Interaction tests were considered significant at the 5% level. No correction was made for multiple tests.
The following a priori subgroups were assessed:
-
non-pain related:
-
comorbidities: three or fewer compared with more than three comorbidities, including musculoskeletal comorbidities
-
living arrangements: living alone compared with living with others
-
baseline self-efficacy: PSEQ score of 0–20 (not likely to be confident) compared with 21–39 (more likely to be confident and to self-manage) compared with ≥ 40 (confident)
-
socioeconomic status (based on the Index of Multiple Deprivation 2010243), calculated from participant postcodes via a geographical information system: lower social class (less than observed median in data) compared with higher social class (equal to or greater than observed median in data)
-
-
pain related
-
pain duration: 0–12 months compared with 13 months to 4 years compared with ≥ 5 years
-
baseline pain intensity: CPG intensity score of 0–3 (low) compared with 4–7 (medium) compared with 8–10 (high)
-
baseline pain-related disability: CPG disability score of 0–3 (low) compared with 4–7 (medium) compared with 8–10 (high)
-
baseline depression: HADS depression score of < 11 compared with ≥ 11.
-
Analysis of secondary outcomes
Chronic Pain Grade pain-related disability at 6 months
This outcome was analysed using the same methods as for CPG pain-related disability at 12 months.
Chronic Pain Grade pain intensity, Hospital Anxiety and Depression Scale anxiety and depression and Health Education Impact Questionnaire at 6 and 12 months
These outcomes were analysed using the same methods as for CPG pain-related disability at 6 and 12 months.
Pain Self-Efficacy Questionnaire at 6 and 12 months
We had prespecified in the statistical analysis plan that this outcome would be analysed using the same methods as for CPG pain-related disability at 6 and 12 months, except that the individual components of the PSEQ score at 12 weeks were also included in the imputation model. However, we were unable to perform the imputations because too many variables with missing data were included in the imputation model.
Therefore, rather than including the individual components of the PSEQ at baseline, we tried to include the overall score at baseline in the imputation model (setting scores to missing if participants had any missing components). However, there were still too many variables with missing data in the imputation model and the imputations did not work.
We therefore tried using mean imputation to replace missing baseline scores with the overall mean PSEQ score at baseline. This allowed us to include the baseline PSEQ score in the imputation model as an auxiliary variable (as it contained no missing data) rather than as a variable with missing data that needed to be imputed. This method allowed the imputations to proceed and so was the basis for the analyses of the PSEQ at 6 and 12 months.
Chronic Pain Acceptance Questionnaire at 6 and 12 months
We prespecified in the statistical analysis plan that this outcome would be analysed using the same methods as for CPG pain-related disability at 6 and 12 months, with the exception that we would include only the individual questions for CPAQ at 6 and 12 months in the imputation model and include the full CPAQ score at baseline (leading to 41 variables rather than 60). For participants who were missing the CPAQ score at baseline, we used mean imputation. However, we were unable to perform the imputations because too many variables with missing data were included in the imputation model.
To reduce the total number of variables, we combined the individual components into pairs, leading to 10 pairs of two components at each time point. For example, if Q1, Q2, . . ., Q20 are the 20 individual questions that form the overall CPAQ score at any time point, we generated 10 pairs as:
We set P to missing if either of the Qs involved was missing. We then included the Ps in the imputation model at 6 and 12 months, reducing the total number of variables from 40 to 20. This method allowed the imputations to proceed and so was the basis for the analyses of the CPAQ at 6 and 12 months.
European Quality of Life-5 Dimensions at 6 and 12 months
The EQ-5D was analysed using the same analysis model as for the primary outcome (i.e. mixed-effects linear regression model with ‘course’ as a random effect, adjusted for site of recruitment, age, gender, HADS depression score at baseline and EQ-5D at baseline).
All participants who fully completed the EQ-5D at either 6 or 12 months were included in the analysis. EQ-5D scores with missing components were regarded as completely missing.
Multiple imputation was used to account for participants who were missing the outcome at either 6 or 12 months. The MI strategy was the same as that for the primary and other secondary outcomes, except that, instead of imputing the individual components of the EQ-5D score, we imputed the whole score.
Census global health question at 6 and 12 months
This outcome was analysed using a mixed-effects ordered logistic regression model with ‘course’ as a random effect. Site of recruitment, age, gender, HADS depression score at baseline and the outcome at baseline were included as fixed covariates.
All participants who completed the census global health question at either 6 or 12 months were included in the analysis.
Multiple imputation was used to account for participants who were missing the outcome at either 6 or 12 months. The MI strategy was the same as that for the primary and other secondary outcomes, except that we imputed the whole score (as there are no individual components).
Total defined daily doses up to 12 months post randomisation for psychotropic drugs, drugs for pain, weak opioids and strong opioids
These outcomes were analysed using a mixed-effects linear regression model with ‘course’ as a random effect. Restricted maximum likelihood was used. The model included site of recruitment, age, gender, HADS depression score at baseline and total DDD in the 3 months before randomisation as covariates. All participants who had data on total DDD up to 12 months post randomisation were included in the analysis. Mean imputation was used for missing baseline covariates.
Proportion of participants using weak opioids and strong opioids at 12 months post randomisation
These outcomes were analysed using a mixed-effects logistic regression model with ‘course’ as a random effect. The model included site of recruitment, age, gender, HADS depression score at baseline and weak or strong (depending on outcome) opioid use at baseline (defined as a prescription for weak or strong opioids in the 12 weeks before randomisation) as covariates. All participants who had data on whether or not they had had a weak/strong opioid prescription at 12 months were included in the analysis.
Adherence-adjusted analyses
The primary complier average causal effect (CACE) analysis for each outcome was adjusted for all of the baseline covariates included in the primary analysis models, namely site of recruitment, age, gender and HADS depression score at baseline and the corresponding outcome at baseline. As a sensitivity analysis we also conducted CACE unadjusted analyses. We assumed:
-
that randomisation was a valid instrument for treatment received
-
that the intervention was not available outside the trial
-
the stable unit treatment value assumption
-
monotonicity
-
that exclusion restriction assumptions hold.
We used two-stage least-squares estimation on the multiply imputed data.
We predefined four levels of adherence (or exposure) to the intervention: none; low adherence – less than seven sessions (≤ 1 day); medium adherence – eight to 16 sessions; and adherent – ≥ 17 sessions attended. Day 1 included seven sessions, day 2 included eight sessions, day 3 included seven sessions and the follow-up included two sessions. We defined ‘compliers’ as those who attended at least half of the course (i.e. those present for at least 12 of the 24 course components).
Ethical considerations
There were few ethical concerns with this study. To maintain patient confidentiality only the clinical staff and primary care research network were able to search clinical records and invite suitable patients to participate. Research staff provided guidance and advice when needed and when confidentiality could be maintained. Only potential participants who had provided their contact details to the research team were approached. Patients received one reminder letter if they did not respond.
The risks to the participants in this study were low; however, the study team was aware that the course could trigger emotional reactions. We therefore ensured that the facilitator training course trained facilitators in distress management. Each course had two facilitators so if any participant became unduly distressed he or she was helped by a facilitator who, if necessary, and with the participant’s agreement, withdrew the participant from the group and helped him or her until he or she was ready to return to the group, go home or seek further help from a more suitable HCP. Under no circumstances was a participant left alone while distressed. If the facilitators felt that a participant was a danger to him- or herself or others, they sought permission to contact the participant’s GP or take him or her to an emergency department.
We ensured that another member of the study team was always available by mobile phone for the duration of any course should any emergency advice be needed.
Methods: health economic analysis
For the economic analysis we adopted a NHS perspective spanning primary, secondary and intermediate health-care sectors, given that the poor reporting of out-of-pocket expenses made it difficult to reflect a wider societal perspective. Economic evaluation followed the National Institute for Health and Care Excellence (NICE) Guide to the Methods of Technology Appraisal 2013. 244
Microcosting of the intervention
A microcosting of the self-management course for primary care patients with chronic musculoskeletal pain included a bottom-up construction of the costs associated with setting up and delivering the programme. The course running costs included salaries, room rental, course materials, facilitators’ travel expenses and administration costs. The cost of training facilitators included salaries and travel expenses (for both trainers and facilitators), room rental, course materials and administration costs. Courses were run in multiuse settings and the same daily rate was used for all venues. Trainers and facilitators were paid a fixed fee per session. Assumptions used in the microcosting are summarised in Appendix 6. The costs of the intervention were estimated as a cost per course and a cost per participant. Costs associated with usual care included the costs of the pain education booklet and the relaxation CD.
Cost of the intervention per participant
The estimated cost of the intervention per participant was based on the number of participants enrolled on the courses. The average cost per participant was estimated with and without the costs of staff training. The average cost of the intervention for each region (London and the Midlands) is reported without training, given that the two centres shared training costs. Sensitivity analyses were conducted using the minimum and maximum number of participants who could be enrolled on each course.
Use of health-care resources by participants
Service use data (all providers) were collected for each participant from GP electronic records at the 12-month follow-up. In addition, information about prescribed medication over the 3-month pre-randomisation period was requested to account for possible differences in baseline prescribing between the intervention group and the control group. To obtain these data, practices produced a printout of all prescriptions issued over a 15-month period for each participant. These data were then manually entered into a master database for analysis by product name and strength. This allowed both allocation of costs and the calculation of the number of days each medication was prescribed. Data relating to secondary care use over the 12 months since randomisation were downloaded from the SUS database224 after 15 months. This allowed for a 3-month ‘lag’ in the availability of SUS data. The primary care data included consultations, prescriptions, tests and investigations, and referrals to community care. Consultations included contacts with GPs, nurses, health-care assistants and other health professionals such as specialist nurses, physiotherapists, psychologists, counsellors, pharmacists, phlebotomists, dietitians, etc. Contacts with GPs and nurses also included telephone consultations, out-of-hours services and home visits. Referrals to community care included rehabilitation programmes, exercise programmes, community mental health teams, community diabetes teams and other health professionals. Secondary care services included inpatient stays, outpatient appointments and accident and emergency (A&E) admissions.
Cost of health-care services
Individual-level resource use data were combined with unit costs to calculate the total cost of health service use for each participant. Primary care consultations and referrals to community care were costed using the Unit Costs of Health and Social Care 2012. 245 Unit costs that were not available in this source were supplemented with costs from the National Schedule of Reference Costs 2011–2012. 246 Tests and investigations were costed using the National Schedule of Reference Costs 2011–2012, direct access diagnostic and pathology services. 246 The unit costs and assumptions used for costing primary health-care services is shown in Appendix 6.
Prescriptions were analysed using the PCA database 2011–12. 227 Some items, however, were missing in this database, whereas others had no cost per item. Costs missing in the PCA database were supplemented with costs from other sources, including the BNF,229,247 the NHS Drug Tariff,248 the Monthly Index of Medical Specialities249 and UK retailers’ price lists. 250–252 The flow chart depicting the costing process is shown in Appendix 6. Briefly, prescription items that were not found in the PCA database were first checked for spelling. If costs were not identified after the spelling check, they were taken from the BNF edition 62 published in 2011229 or from the BNF website in September 2013. 247 If costs were missing in the BNF, they were sourced from the NHS Drug Tariff, the Monthly Index of Medical Specialties or retailers’ price lists (for items other than drugs). Items missing in all of the above sources were substituted with alternative items from the PCA database that contained the same active ingredients/strengths. Generic products were selected when possible. Pack size was considered when indicated. When pack size was not indicated, the smallest pack size was assumed. For items that were included in the PCA database but for which there were no costs, the cost per item was taken from the BNF edition 62. 229 Costs missing in the BNF edition 62229 were supplemented with costs from the BNF 2013. 247 Costs obtained from UK retailers were used without value-added tax.
The costs of secondary health-care services used by participants were downloaded as part of the SUS database, and were used to cost the services received by participants due to the difficulties encountered in matching the SUS data set to unit cost databases. When costs were not provided, the National Schedule of Reference Costs 2011–2012246 was used. Outpatient costs were matched by specialty code. The average unit costs (all NHS trusts) were used given insufficient information about the type of outpatient appointment (consultant led/non-consultant led, face to face/non-face to face) provided by SUS. Inpatient and A&E department costs were matched by the Healthcare Resource Group (HRG) code. The average HRG costs (all NHS trusts) were used because of a lack of information about inpatient stay (elective/non-elective, short stay/long stay) provided by the SUS. A&E department costs were assumed to be ‘not leading to admitted’.
Health-related quality of life
Health-related quality of life was measured using the EQ-5D at baseline and 6 and 12 months’ follow-up. The EQ-5D is recommended by NICE244 and aims to measure the extent of problems across the domains of mobility, self-care, usual activities, pain/discomfort and anxiety/depression using three levels of severity (no problems, moderate problems and severe problems). The EQ-5D domain scores were converted to a preference-based score using a tariff derived from members of the general public. 253 EQ-5D scores at the three time points (baseline, 6 months and 12 months) were then aggregated to estimate the total quality-adjusted life-years (QALYs) for each participant over the 12-month period. More information about QALY calculation is provided in the following section.
Data analysis
Data analyses were conducted using Microsoft Excel® 2012 (Microsoft Corporation, Redmond, WA, USA) and Stata 12.1. 254 The base-case cost-effectiveness analysis was conducted for the ITT population on the imputed data set using a multilevel model (MLM). Sensitivity analyses were conducted using a generalised linear model (GLM) and a seemingly unrelated regression (SUR) model. The secondary analyses were performed on the non-imputed data set using a GLM, SUR and MLM. Per-protocol analysis was conducted on the imputed data set using a MLM. Subgroup analyses included participants with different levels of compliance and exposure to treatment. Additional subgroup analysis excluded participants with high service use costs (top 5%). All subgroup analyses were conducted on the imputed data set using a MLM. The intervention and follow-up period lasted for 12 months only so a discount factor of 1 was applied to the costs and benefits following standard discounting practice. 255
Missing data
The number of missing items in the health economics data set was analysed at baseline and 6 and 12 months’ follow-up. The proportion of missing data was reported for consultations, prescriptions, investigations, referrals to community care, secondary care service use data and the EQ-5D. Baseline data were collected for prescriptions (3 months pre-randomisation) and the EQ-5D. There were two types of missing data within the EQ-5D data set: missing items within a measure and missing measures at a particular time point (baseline or 6 or 12 months’ follow-up). EQ-5D data were considered missing if there were no data for at least one descriptive domain as this precludes calculation of total QALYs. Only data missing at a particular time point were imputed for the EQ-5D. Within GP records and the SUS database there was only one type of missing data, that is, when data were missing at a particular time point for all items. The aggregate costs were therefore imputed for primary and secondary care services.
Use of health-care resources by participants
The use of health-care services by participants from the intervention and control groups was analysed in quarters and over the entire duration of the trial. The number of contacts for each participant was extracted from the GP and SUS databases and arranged into quarters starting from the randomisation date. The following categories together accounted for approximately 95% of all primary care consultations: contacts with GPs (surgery and telephone), nurses (surgery and telephone) and health-care assistants (surgery). The category ‘other specialists’ included contacts with specialist nurses, physiotherapists, psychologists, counsellors, pharmacists, phlebotomists, dietitians and other health professionals, which together accounted for < 5% of all primary care contacts. It had been anticipated that the intervention would predominantly affect the use of primary care services, such as GP and nurse consultations. Other categories included in the resource use analysis were investigations, referrals to community care, inpatient stays, outpatient appointments and A&E admissions.
Cost of health-care services
The use of health-care services by participants from the intervention and control groups was analysed in quarters and over the entire duration of trial. Unit costs were assigned to each service category and multiplied by the number of contacts. Differences in costs between the intervention group and the control group, with 95% CIs, were estimated for consultations, investigations, prescriptions, referrals to community care, outpatient attendances, inpatient stays and A&E admissions.
Quality-adjusted life-years
The total QALYs over the 12-month period were estimated using the area-under-the-curve method. Two formulae for the area-under-the-curve calculation were compared: trapezoidal and Simpson’s rules. 256 The trapezoidal rule assumes that data for different time points (baseline and 6 and 12 months’ follow-up) are connected by a straight line, whereas Simpson’s rule applies a quadratic polynomial function (i.e. a parabola). Depending on the direction of QALY changes over time in the intervention and control groups, these methods may produce different incremental QALYs. The trapezoidal method was chosen as the more conservative method as Simpson’s method would potentially overestimate the difference in QALYs between the intervention group and the control group.
Imputations
Missing data for costs and QALYs were imputed using a MI procedure in Stata 12.1. Missing data were assumed to be missing at random. Patterns of missing data were not found to be related to patient characteristics. Imputed data included total primary care costs, total secondary care costs, baseline prescription costs and EQ-5D scores at baseline and 6 and 12 months’ follow-up. For each missing category five data sets were imputed. 257 Participants were excluded from imputations if they had more than one missing time point for the EQ-5D. We used a single-level imputation procedure as ICCs for both costs and QALYs were very low (< 0.001, 5–17 participants per cluster). Single-level imputations were carried out using the Stata chained imputation procedure. 258 Given the skewed distributions of cost and QALY data we used the predictive mean matching imputation method, which has been recommended for skewed distributions. 259 Covariates included in the imputation were age, gender, site of recruitment (London or Midlands), course and HADS depression score at baseline. Participants in the control arm were considered in the analysis as their own cluster (course).
Cost-effectiveness analysis
In the cost-effective analysis we assessed the incremental changes in costs and QALYs in the intervention group compared with the control group (a cost–utility analysis). In the base-case analysis, costs and QALYs were analysed using a mixed-effects linear regression model with ‘course’ as a random effect. Covariates included in the model as fixed effects were age, gender, site of recruitment, treatment group, EQ-5D score at baseline, prescription cost at baseline and HADs depression score at baseline. Sensitivity analyses were conducted using a GLM and a SUR model. SUR assumes that costs and QALYs are drawn from a bivariate normal distribution. 260 Covariates included in the GLM were age, gender, site of recruitment, course, treatment group, EQ-5D score at baseline, prescription cost at baseline and HADS depression score at baseline. The SUR model included regression equations for costs and QALYs, each regressed on the above variables.
The outcomes of the cost–utility analyses were an incremental cost-effectiveness ratio (ICER) and the probability of the intervention being cost-effective at the NICE threshold of £30,000 per QALY gained. The ICER was estimated as the difference in mean total costs between the intervention group and the control group divided by the difference in mean QALYs between the intervention group and the control group. A parametric approach was then used to address the uncertainty around ICER point estimates for the imputed data set. 261 Briefly, this method involved calculating the net monetary benefit (NMB) for each participant at different willingness-to-pay (WTP) thresholds,245 having first controlled for covariates using a MLM, GLM or SUR model. We then used Rubin’s rule240 to estimate the mean and standard error of the NMB for the intervention and control groups. The incremental net benefit (INB) was estimated for each WTP threshold (i.e. mean INB = mean NMB control – mean NMB intervention) and a normal distribution was assigned to the INB based on patient-level data. The probability of the intervention being cost-effective was estimated using 10,000 random samples from the above distribution.
Sensitivity analyses were conducted using the non-imputed data set (complete case analysis). Only participants with a complete health economics data set were included in this analysis. The ‘complete’ data set included EQ-5D data at baseline and 6 and 12 months’ follow-up, and resource use costs for primary and secondary care over 12 months. Total costs and QALYs were analysed using a mixed-effects linear model. Sensitivity analyses were conducted using a GLM and a SUR model. The non-parametric bootstrap method was used to address the uncertainty around the ICER point estimates. The probabilities of the intervention being cost-effective were estimated using both bootstrap and INB approaches.
The primary cost-effectiveness analysis was conducted on an ITT basis. Subgroup analyses were conducted using the per-protocol population. Additional subgroup analyses included participants with different rates of compliance and exposure to the intervention. Compliers were defined as individuals who attended 12–17 sessions and non-compliers were defined as those who attended ≤ 11 sessions. Full exposure to the intervention assumed 17 sessions, moderate exposure 9–16 sessions and no exposure eight sessions or fewer. The moderate-exposure subgroup was excluded from the cost-effectiveness analysis because of the small number of participants (n = 23).
Chapter 10 Fidelity
Abstract
Introduction: The fidelity of intervention delivery is crucial in any consideration of the results of the intervention. Fidelity has many components but this study was concerned with the fidelity of intervention delivery (intervention integrity), which was influenced by both the adherence of facilitators to the course content and their competence (or skill) in delivering the intervention.
Aim: To assess how well the COPERS intervention was delivered during the trial by measuring the adherence and competence of the facilitators delivering the intervention.
Methods: We identified seven of the 24 course components (or sessions) that we considered to be the most important in terms of effecting participant behaviour change. All of the courses were audio-recorded and intervention integrity was assessed by examination of the recordings of these seven components. Checklists to capture adherence and competence systematically were designed. Researchers also gave an overall impression rating for intervention delivery. We randomly selected four of the seven components on each of the 31 courses. Using the appropriate checklist one evaluator listened to each recorded component in its entirety and rated adherence, competence and overall impression. We checked the intra- and inter-rater reliability.
Results: Intra- and inter-rater reliability were excellent for adherence, very good for competence and less good for overall impression. Adherence was very good or excellent across the courses with competence being more variable across the courses, being excellent for some sessions and less good for others. The overall impression measure proved to be challenging to use and the data were difficult to interpret.
Conclusions: Overall, the results suggested that the COPERS course was delivered competently and as intended.
Background
Complex interventions such as the COPERS intervention are recognised in MRC guidance28 as having varied and challenging issues in terms of their design, evaluation and implementation. This guidance recognises that intervention fidelity is underevaluated. Intervention fidelity is defined as the use of methodological strategies to monitor and enhance the reliability and validity of behavioural programmes. 214
The construct of ‘intervention fidelity’ originated from concerns about the ‘treatment integrity’ of psychotherapeutic interventions expressed in the 1980s and 1990s. 262–264 The monitoring, measurement and assessment of intervention fidelity is important as it has been demonstrated that fidelity is a mediator of study outcomes. 265–268 For example, when interventions lack impact, this may reflect implementation failure rather than genuine ineffectiveness. One of the potential explanations for the small effect sizes generally seen in studies of self-management support14 may be the lack of intervention fidelity, but this is rarely reported.
In the last 20 years the notion of intervention fidelity has become increasingly differentiated and multilayered. 269–271 There is an ongoing debate about how core elements of fidelity should be defined and measured264,272 and a recognition of the need for reliable fidelity measurement instruments. 273 There is little consensus about the key elements that contribute to intervention fidelity, possibly because it is a multidimensional construct. 268 Some authorities have identified five domains of fidelity:
-
study design
-
training
-
intervention delivery, defined as the monitoring and assessment of behaviours at the point of intervention delivery
-
intervention receipt by participants
-
intervention enactment, defined as the extent to which participants apply the skills learned in their daily lives. 215,269
However, intervention enactment may be considered an outcome measure rather than an indicator of fidelity. Here we focus on the domain of intervention delivery or integrity.
The effectiveness of complex interventions may also be dependent on the ‘skills’ of those delivering them. ‘Skills’ can be characterised by the separate, but related, constructs of adherence and competence:214
-
adherence – the extent to which the intervention is delivered in the way that it was intended to be delivered (as per protocol and/or design)
-
competence – the level of ‘skill’ demonstrated by those delivering an intervention; this may include the ability to respond appropriately to a wide variety of contextual cues.
Competence is less likely to be assessed than adherence. This may be a reflection of the debate surrounding the definition of competence and ‘skill’,263 the methodological difficulties surrounding the monitoring and measurement of competence274 and the significant expenditure of time and resources required to collect and analyse competence data. 263
Aim
The overall aim of the fidelity study was to assess how well the COPERS intervention was delivered by measuring the adherence and competence of the facilitators delivering the intervention.
Methods
Setting and data collection
The research team identified seven of the 24 course components (or sessions) that they considered to be the most important in terms of effecting participant behaviour change (Table 34). These components focused on participant education and theoretically driven behaviour change techniques and strategies in contrast to other components, which encouraged social interaction, relaxation and postural awareness. All of the courses were audio-recorded and intervention integrity was assessed by examination of the recordings of the components listed in Table 35.
| Component | Component description |
|---|---|
| Component 2 (day 1): pain information | Participants watched a DVD aimed at educating them about chronic pain and introducing them, through facilitated discussion, to the notion of acceptance of their pain |
| Component 3 (day 1): acceptance | Participants were asked to consider a scenario about an uninvited/unwanted guest as a metaphor for their pain |
| Component 5 (day 1): the pain cycle | Groups were introduced to the pain cycle and the varied and individual emotions and behaviours that may perpetuate that cycle |
| Component 9 (day 2): identifying problems, goal-setting and action planning | Groups were introduced to strategies to enable them to systematically identify problems, brainstorm creative solutions, set goals and devise strategies to escape the pain cycle |
| Component 10 (day 2): barriers to change – unhelpful thinking | Groups were encouraged to consider that reflexive, automatic thinking patterns may prevent individuals from achieving their goals |
| Component 11 (day 2): barriers to change – reframing negatives to positives | Participants were asked to consider what they were able to do rather than what they were unable to do |
| Component 12 (day 2): attention control and distraction | Participants were introduced to techniques that might enable them to focus their minds away from thoughts about pain |
Developing the intervention integrity measures
We used the monitoring and assessment tools from three previously published trials to inform the development of the COPERS measures. 53,275,276 The learning outcomes outlined in the COPERS facilitator training course manual helped to design a provisional set of criteria to measure adherence and competence. To develop the measures we used a two-stage pilot testing process.
Our adherence measure was designed to assess the delivery of key elements of each component as described in the facilitators’ manual. The generic competence measure was designed to determine the extent to which the facilitators created an environment in which participants could share their experiences and learn new skills. An overall impression score was designed to reflect the extent to which the aims and objectives of the component were achieved and how the material was received by the group.
We tested a variety of scoring systems for adherence, competence and the overall impression score. We found that each method of assessment had its own strengths and weaknesses. Numerical scales and Likert scales seemed, intuitively, to be more suitable for measuring degrees of competence but they had low levels of intra- and inter-rater reliability. Frequency methods of assessment were resource intensive and time-consuming, had low levels of intra- and inter-rater reliability and were challenging to verify from audio recordings only. Dichotomous response categories (such as yes/no, present/absent or occurred/did not occur), when used to evaluate adherence items, were time efficient and had high intra- and inter-rater reliability.
The research team revised and amended the evaluation forms. The final agreed measures consisted of component-specific adherence forms, a generic competence form and an ‘overall impression’ scoring sheet (see Appendix 7).
The option to transcribe the audio recordings was unrealistic because of the volume involved and potential cost; evaluators were therefore asked to provide supportive quotations and or comments to justify their ratings.
Data analysis and presentation
Adherence measurement
The adherence form consisted of items that reflected the occurrence or non-occurrence of an event. Component-specific items, relating to the key elements prescribed in the COPERS facilitators’ manual, formed the basis of the assessments. The team added a third response of ‘unsure’ for cases when an item was unclear. The evaluation forms allowed the assessors to add explanatory notes if necessary to justify the categories chosen [these categories were ‘yes’, element occurred/was delivered (2 points); ‘no’, element did not occur/was not delivered (0 points); and ‘unsure’ (1 point)].
The number of adherence items evaluated for each component varied (Table 35). To ensure that all scores from the components were standardised to a consistent scale we summed the ‘raw scores’ for each component and divided them by the total number of items for that component. For example, component 2 (pain information) had six adherence items with a maximum ‘raw’ score of 12 (6 × 2). The total aggregate six-item score for this component was divided by 6. Thus, a maximum (100%) score was 2 and a minimum score was zero.
| Component | Number of components (sessions delivered) evaluated | Adherence: items evaluated | Adherence: maximum score | Competence: items evaluated | Competence: maximum score | Overall impression: items evaluated | Overall impression: maximum score |
|---|---|---|---|---|---|---|---|
| 2: Pain information | 16 | 6 | 12 | 4 | 8 | 1 | 4 |
| 3: Acceptance | 17 | 3 | 6 | 4 | 8 | 1 | 4 |
| 5: The pain cycle | 20 | 6 | 12 | 4 | 8 | 1 | 4 |
| 9: Identifying problems, goal-setting and action planning | 19 | 8 | 16 | 4 | 8 | 1 | 4 |
| 10: Barriers to change – unhelpful thinking | 18 | 6 | 12 | 4 | 8 | 1 | 4 |
| 11: Barriers to change – reframing negatives to positives | 18 | 5 | 10 | 4 | 8 | 1 | 4 |
| 12: Attention control and distraction | 14 | 6 | 12 | 4 | 8 | 1 | 4 |
Competence measurement
A competence evaluation form was designed to evaluate all of the course components. This generic measure consisted of items related to the extent to which the facilitators introduced the aims/rationale of each component, the success or failure of the facilitators to generate group discussion and individual disclosure, whether or not the facilitators consolidated and summarised the participant learning at the conclusion of each component and whether or not the facilitators linked that learning to other components in the course. Assessment was scored as ‘yes’/demonstrated (2 points), ‘no’/not demonstrated (0 points) and ‘unsure’ (1 point). The scores were also standardised by dividing the maximum ‘raw’ score of eight by the number of items (i.e. four); thus, the maximum score was 2 and the minimum score was zero.
Overall impression rating
We used an overall general impression rating scale ranging from 1 to 4, anchored at 1, ‘did not go well’, and 4, ‘excellent’.
As the scores were not normally distributed, the median and 25th and 75th percentiles are presented.
Selection and assessment of components
We used a random sampling grid to select four of the seven selected components from each course. Using the appropriate evaluation form one evaluator listened to each recorded component in its entirety and rated adherence, competence and overall impression. A number of components could not be analysed because of equipment failure, facilitator error, incomplete recording or poor sound quality; evaluators were instructed to substitute that component with the next available selected component from that course.
Three members of the COPERS research team (DE, TM, KH) evaluated/assessed the audio recordings.
Intra-/inter-rater reliability
A total of 10% of the assessed component recordings were tested for intra- and inter-rater reliability. Of this sample, a purposive sample of 10% of the evaluations that reflected a range of scores was used to assess both intra- and inter-rater reliability. For inter-rater reliability each reviewer was asked to code a session that had already been coded (they were blinded to the initial reviewer’s scores). For intrarater reliability a period of at least 2 weeks between the first and the second evaluations was adopted. We calculated the percentage agreement for each item rated on the evaluation forms.
Results
In total, 31 COPERS courses were delivered, 14 in London and 17 in Warwick. A total of 122 individual COPERS components were assessed (see Table 35) amounting to approximately 71 hours of intervention. Because of missing recordings two London courses were assessed on three rather than four components; all Warwick courses were assessed on four components.
Intra-/inter-rater reliability
Intrarater reliability was measured using assessments from 16 COPERS components consisting of 94 adherence item scores, 64 competence item scores and 16 overall impression scores. Intra-rater reliability was 91% for adherence items, 76% for competence items and 69% for overall impression scores.
Fifteen COPERS components were used to measure inter-rater reliability, consisting of 95 adherence item scores, 71 competence item scores and 15 overall impression scores. Inter-rater agreement was 80% for adherence items, 67% for competence items and 54% for overall impression scores.
Adherence
Both COPERS study centres achieved the maximum overall course delivery adherence score (median 2.00); however, there were some component score variations (Table 36). The lowest levels of adherence were observed for component 10 (unhelpful thinking) [median 1.67, interquartile range (IQR) 1.67–2.00] and component 2 (pain information) (median 1.75, IQR 1.42–2.00).
| Component | Warwick median | 25th–75th percentile | London median | 25th–75th percentile | Warwick/London median | 25th–75th percentile |
|---|---|---|---|---|---|---|
| 2: Pain information | 1.67 | 1.50–2.00 | 1.83 | 1.33–2.00 | 1.75 | 1.42–2.00 |
| 3: Acceptance | 2.00 | 1.92–2.00 | 2.00 | 1.67–2.00 | 2.00 | 1.83–2.00 |
| 5: The pain cycle | 2.00 | 1.75–2.00 | 2.00 | 2.00–2.00 | 2.00 | 2.00–2.00 |
| 9: Identifying problems, goal-setting and action planning | 2.00 | 2.00–2.00 | 2.00 | 1.91–2.00 | 2.00 | 2.00–2.00 |
| 10: Barriers to change – unhelpful thinking | 1.67 | 1.67–1.67 | 1.92 | 1.62–2.00 | 1.67 | 1.67–2.00 |
| 11: Barriers to change – reframing negatives to positives | 1.70 | 1.60–2.00 | 2.00 | 2.00–2.00 | 2.00 | 1.60–2.00 |
| 12: Attention control and distraction | 2.00 | 1.67–2.00 | 2.00 | 1.83–2.00 | 2.00 | 1.67–2.00 |
| Overall course adherence score | 2.00 | 1.67–2.00 | 2.00 | 1.83–2.00 | 2.00 | 1.67–2.00 |
Competence
Competence scores exhibited higher levels of variability than adherence scores (Table 37). The overall median course delivery competence score for both COPERS centres was 1.50 (IQR 1.25–2.00). In Warwick the highest level of competence was for component 11 (reframing negatives to positives) (median 1.75, IQR 1.25–2.00) and the lowest for component 12 (attention control and distraction) (median 1.13, IQR 1.00–1.88). In London the highest level of competence was observed for component 5 (the pain cycle) (median 2.00, IQR 1.56–2.00) and the lowest also for component 12 (attention control and distraction) (median 1.25, IQR 1.00–1.63).
| Component | Warwick median | 25th–75th percentile | London median | 25th–75th percentile | Warwick/London median | 25th–75th percentile |
|---|---|---|---|---|---|---|
| 2: Pain information | 1.50 | 1.13–2.00 | 2.00 | 1.25–2.00 | 1.75 | 1.25–2.00 |
| 3: Acceptance | 1.50 | 1.38–2.00 | 1.25 | 1.00–2.00 | 1.50 | 1.00–2.00 |
| 5: The pain cycle | 1.63 | 1.50–2.00 | 2.00 | 1.56–2.00 | 1.88 | 1.50–2.00 |
| 9: Identifying problems, goal-setting and action planning | 1.50 | 1.00–2.00 | 1.25 | 1.06–1.88 | 1.50 | 1.00–2.00 |
| 10: Barriers to change – unhelpful thinking | 1.50 | 1.50–2.00 | 1.38 | 1.00–1.56 | 1.50 | 1.00–1.81 |
| 11: Barriers to change – reframing negatives to positives | 1.75 | 1.25–2.00 | 1.50 | 1.06–2.00 | 1.63 | 1.25–2.00 |
| 12: Attention control and distraction | 1.13 | 1.00–1.88 | 1.25 | 1.00–1.63 | 1.13 | 1.00–1.63 |
| Overall course competence score | 1.50 | 1.31–2.00 | 1.50 | 1.00–2.00 | 1.50 | 1.25–2.00 |
Overall impression scores
The median overall impression score for all courses was 3.00 (IQR 2.00–3.00). There was some component score variability (Table 38). Component 12 (attention control and distraction) had an overall median impression score of 2.00, reflecting the low facilitator competence scores for this component. Component 11 (reframing negatives to positives) had a similarly low overall median impression score of 2 (IQR 2.00–3.25), although this component was delivered with the maximum score for adherence (median 2.00, IQR 1.60–2.00) and with good levels of competence (median 1.63, IQR 1.25–2.00).
| Component | Warwick median | 25th–75th percentile | London median | 25th–75th percentile | Warwick/London median | 25th–75th percentile |
|---|---|---|---|---|---|---|
| 2: Pain information | 3.00 | 2.00–3.00 | 3.00 | 3.00–4.00 | 3.00 | 3.00–3.00 |
| 3: Acceptance | 3.00 | 2.00–3.00 | 3.00 | 3.00–4.00 | 3.00 | 2.50–3.00 |
| 5: The pain cycle | 3.00 | 3.00–4.00 | 3.00 | 2.25–4.00 | 3.00 | 3.00–4.00 |
| 9: Identifying problems, goal-setting and action planning | 3.00 | 2.00–3.00 | 2.50 | 2.00–3.75 | 3.00 | 2.00–3.00 |
| 10: Barriers to change – unhelpful thinking | 2.50 | 2.00–3.00 | 3.00 | 2.75–3.25 | 3.00 | 2.00–3.00 |
| 11: Barriers to change – reframing negatives to positives | 2.00 | 2.00–3.25 | 2.50 | 2.00–3.75 | 2.00 | 2.00–3.25 |
| 12: Attention control and distraction | 2.50 | 2.00–3.00 | 2.00 | 1.00–3.00 | 2.00 | 1.75–3.00 |
| Overall course impressionistic score | 3.00 | 2.00–3.00 | 3.00 | 2.00–4.00 | 3.00 | 2.00–3.00 |
Discussion
The aim of this study was to develop a methodology and assess the level of intervention integrity achieved during the delivery of the COPERS self-management course in a RCT setting. Overall, the results suggested that the COPERS course was delivered competently and as intended. We were satisfied that intervention fidelity was acceptable and therefore that the results of the intervention are a reflection of an intervention that was delivered well.
As has been pointed out by others, this work suggests that effective adherence in complex interventions may involve not only the delivery of prescribed ‘surface’ content but also adherence to essential but non-content-related ‘core’ theoretical/structural elements. 270 Component 10 (unhelpful thinking) in the COPERS programme illustrates the challenges in defining adherence in complex interventions. This component was intended to help participants recognise and change patterns of automatic negative and self-limiting thoughts. The COPERS manual outlined the informational content of this component and the structure, sequence, timing and mode of delivery of the various elements to be used by the facilitators. To deliver this component as prescribed, a high level of adherence to both ‘surface’ content and ‘core’ elements was required. Component 10 had a relatively low adherence score, which was primarily caused by the facilitators’ difficulty in maintaining the complex structure of the component rather than a failure to deliver the prescribed content.
Component 10 also demonstrated that the constructs of adherence and competence are complex and may be seen to overlap. Competence, defined as the skilful delivery of content, implies some level of adherence. However, adherence, defined as the extent to which content is or is not delivered, does not imply any degree of skill or competence. High levels of content adherence may be associated with a mechanistic, inflexible or unresponsive delivery style and therefore with low levels of competence. 263 However, within component 10, facilitator ‘failure’ to order the component content as prescribed, that is, low structural adherence, was directly related to low levels of competence. Component content designed to promote group participation, if poorly sequenced or timed, resulted in a didactic/mechanistic delivery style that inhibited rather than encouraged group disclosure and discussion.
Seemingly low levels of adherence may not necessarily be associated with poor intervention delivery. In component 2 (pain information), some facilitators deviated from the prescribed content of the manual (and were by definition non-adherent) but these deviations could be reinterpreted positively as the facilitators had responded to individual or group need or intervention receipt. Some of the facilitators reframed questions and subtly changed delivery from the prescribed content in the manual but they still achieved the component’s overall aims and objectives. This may be a demonstration of high levels of facilitator competence despite them being rated as non-adherent. 270 There is, as yet, little empirical work that demonstrates the conditions that may influence adaptation or reinvention or whether, and in what circumstances, these deviations from prescribed protocol may enhance outcomes or decrease effectiveness.
The monitoring and assessment of competence within the COPERS study illustrated the difficulties associated with its measurement. Competence can be considered as a complex construct that includes the ability to establish collaborative relationships and form alliances with participants277 through the use of responsive tailoring of programme content,276 the pacing of delivery278 and the use of positive verbal and non-verbal behaviours. 279
The findings from the COPERS study support the view that competence is considered to be more contextually and/or externally or environmentally dependent than adherence. The greater variability in the competence scores than in the adherence scores reflects, in part, the diversity of facilitator skills required to deliver the COPERS programme and the recognised practical and methodological difficulties in measuring what may seem to be a subjective concept. 215,263
For example, facilitators were required to encourage participant reactions, elicit individual narratives and generate group discussion and debate. They were also required to deliver complex component structures, introduce their groups to new knowledge and skills and make the components individually relevant to ‘real-world’ situations, often while managing difficult situations, people and emotions. The COPERS study demonstrated how competence and effective course delivery may be influenced and moderated by many factors such as positive or negative individuals and/or groups, component content, facilitator and cofacilitator teamwork and skill, issues related to the use of computer hardware and software, the venue, the distribution of handouts, the use of flip charts, the co-ordination and organisation of group activities, feedback and time management. Experience also influences competence; we noted that our facilitators improved with each course that they conducted. Our ratings might also reflect the inexperience of the facilitators who were delivering a new initiative.
The overall impression measure was, in part, designed to reflect some of the ‘non-facilitator-determined’ factors not evaluated by the adherence and competence measures. This subjective measure assessed the extent to which the delivery of each component achieved its specific aims and was consistent with the goals of the wider programme. The overall impression measure proved to be challenging to use and the data difficult to interpret. Evaluators found it relatively straightforward to assess a component as either ‘excellent’ or ‘did not go well’ but the consistent use of the intermediate scores was problematic. The relationship between the level of adherence and competence, and the overall impression score was difficult to determine but generally low overall impression scores seemed to be associated with deficiencies in assessed competence rather than low levels of adherence.
Limitations
We tested only 10% of samples for both intra- and inter-rater reliability; although this sample size may be considered relatively small by some, it was felt to be reasonable and feasible, and indeed represented a higher degree of feasibility checking than has been reported in many other studies.
We used audio recordings to evaluate the components but it is doubtful if sound recordings alone can capture the subtleties of facilitator competence involving non-verbal behaviours and the dynamics of both facilitators and individual and group interactions. The component-specific adherence measures were designed to assess the fundamental requirements of course delivery; however, the use of a generic competence measure may not have reflected the range of skills required to deliver the various components. The absence of standardised definitions and the lack of valid and reliable measures of adherence and competence made assessments of the impact of either on outcomes difficult. There is a need for more empirical work to clarify how the findings from psychotherapeutic research may be applied to other similar interventions and populations free of mental health issues. 278
Conclusions
Complex interventions pose significant challenges for developing practical methods for assessing ‘treatment integrity’. Generic adherence and competence criteria seem inadequate to encompass the full complexity of interacting elements that occur in behaviour change interventions. The robust monitoring and assessment of treatment integrity requires the systematic collection of appropriate data, the formulation of programme- and component-specific measures and the comprehensive training of assessors. The explicit manualisation of programmes and their component competencies is necessary to ensure robust evaluation. We have proposed one model of assessing adherence and competence and demonstrated its use in a large pragmatic RCT. We recognise, however, that more work is necessary to develop valid, resource-efficient methods of evaluating intervention integrity. We are confident that the COPERS intervention was delivered with high levels of adherence and good levels of competence and that the programme aims were largely achieved. We therefore anticipate that our outcome data will not be influenced by poor intervention delivery.
Chapter 11 Randomised controlled trial of the clinical effectiveness and cost-effectiveness of the COPERS intervention: results
Recruitment
Recruitment of recruiting centres
We invited all of the general practices in our study areas (n = 282) to participate in the trial. We approached practices using the NIHR primary care research networks; we also used our own peer networks. Practices were paid service support costs to compensate them for the time spent on the study. We recruited 12 out of 141 (9%) practices in east London and 13 out of 141 (9%) practices in Warwick. Reasons given by general practices for not participating in the trial included lack of time or resources and/or that many of their registered patients with chronic pain did not speak fluent English. The practices that we recruited were based in areas with a wide range of deprivation (from the lowest to the second highest decile for deprivation) but generally all had very high Quality and Outcomes Framework scores (which might be a marker of good clinical care or organisation). We also recruited two secondary care pain services (one in London and one in Warwick) and one community musculoskeletal service in London to identify patients for the trial.
Recruitment of participants
Recruitment took place between August 2011 and July 2012. The total number of patients registered at the 25 recruited general practices was 223,425, of whom 8138 (3.6%) were identified by our searches. Screening by practice clinical staff led to 2278 (28.0%) of these patients being excluded. The remaining 5878 patients were invited by post to participate and 531 (9.0%) of these joined the study (recruitment rate 2.4/1000 registered patients). This represents a conversion rate from those identified by our searches of 6.5% (Table 39 and Figure 14). Although our approach was identical in both areas the proportion of potential participants approached by general practices who joined the study was higher in the Midlands (11%) than in London (7%). This may reflect the differences in socioeconomic and demographic factors between the two areas.
| General practice | Practice characteristics | Identified, n | Excluded by practice, n | Invited, n | Enrolled, n | ||
|---|---|---|---|---|---|---|---|
| List size, n | Deprivation decilea (1 = most deprived) | QOF scorea (out of 1000) | |||||
| 1 | 16,927 | 4 | 997.2 | 599 | 376 | 223 | 9 |
| 2 | 14,984 | 6 | 990.4 | 1058 | 211 | 847 | 60 |
| 3 | 14,147 | 5 | 984.9 | 390 | 30 | 360 | 36 |
| 4 | 14,000 | 1 | 962.1 | 546 | 34 | 512 | 6 |
| 5 | 12,600 | 8 | 961.9 | 568 | 285 | 283 | 50 |
| 6 | 12,500 | 9 | 997 | 218 | 83 | 135 | 21 |
| 7 | 12,190 | 2 | 990.1 | 602 | 439 | 163 | 12 |
| 8 | 12,181 | 1 | 995.7 | 312 | 74 | 238 | 22 |
| 9 | 12,051 | 4 | 986.4 | 309 | 87 | 222 | 11 |
| 10 | 10,878 | 8 | 988.7 | 372 | 104 | 268 | 38 |
| 11 | 10,500 | 5 | 917.6 | 428 | 254 | 174 | 33 |
| 12 | 10,000 | 8 | 983.9 | 375 | 44 | 331 | 40 |
| 13 | 9200 | 4 | 997.3 | 404 | 37 | 367 | 28 |
| 14 | 8300 | 1 | 962.7 | 350 | 10 | 340 | 26 |
| 15 | 8107 | 3 | 991 | 281 | 41 | 240 | 26 |
| 16 | 7300 | 1 | 982.4 | 166 | 6 | 160 | 7 |
| 17 | 7059 | 5 | 959.3 | 143 | 36 | 107 | 14 |
| 18 | 5700 | 1 | 961.9 | 300 | 37 | 263 | 24 |
| 19 | 5500 | 5 | 985.3 | 291 | 11 | 280 | 36 |
| 20 | 4300 | 2 | 889.5 | 38 | 0 | 38 | 6 |
| 21 | 3496 | 2 | 997.3 | 143 | 36 | 107 | 8 |
| 22b | 3093 | 5 | 988.6 | 0 | 0 | 0 | 3 |
| 23 | 3000 | 3 | 995.9 | 51 | 0 | 51 | 7 |
| 24 | 2900 | 2 | 951.7 | 175 | 25 | 150 | 8 |
| 25 | 2512 | 4 | 982.6 | 19 | 0 | 19 | 0 |
| Total London | 97,584 | – | – | 3365 (3.4%) | 825 (24.5%) | 2540 (75.5%) | 170 (6.7%) |
| Total Midlands | 125,841 | – | – | 4773 (3.8%) | 1453 (30.4%) | 3338 (69.9%) | 361 (10.8%) |
| Overall total | 223,425 | – | – | 8138 (3.6%) | 2260 (27.8%) | 5878 (72.2%) | 531 (9.0%) |
FIGURE 14.
Recruitment flow chart.
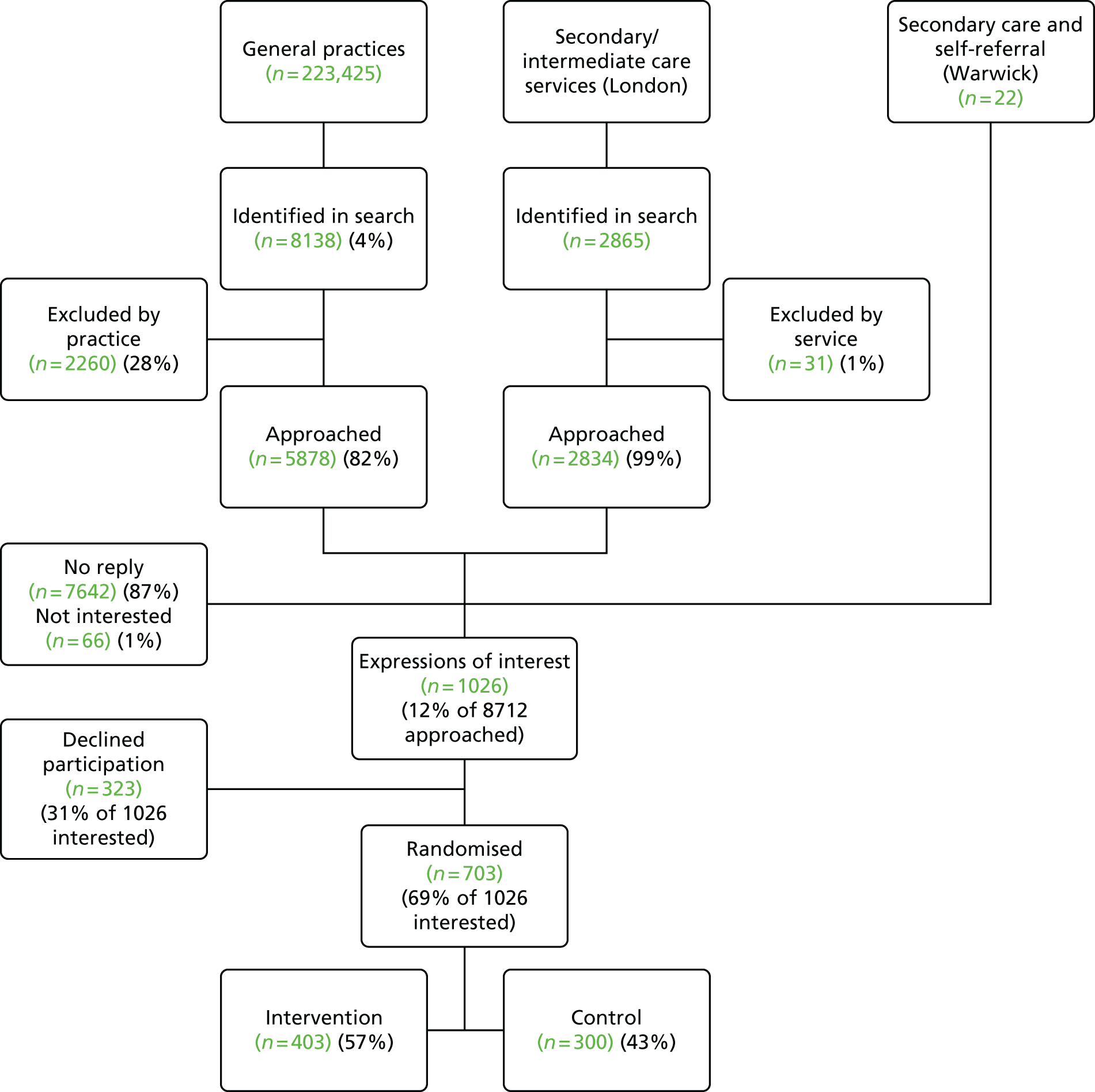
We recruited an additional 167 participants from secondary and intermediate care services. The Warwick centre was unable to provide the number of patients approached. A total of 2865 people attending the pain service or the musculoskeletal physiotherapy service in east London were identified from the service databases. Clinical staff excluded just 31 (1.1%) of these and 2834 (98.9%) were invited by their clinicians to join the study. In total, 150, or 5.2% of those originally identified, agreed to participate in the study. Five people in Warwick (and none in London) referred themselves to the trial giving a final total of 703 participants, with 383 participants recruited from Warwick and 320 recruited from London (see Figure 14).
To ensure that all of our intervention participants received the group intervention as intended it was important that every intervention patient was recruited to a group with at least eight participants. This meant that we had to recruit 18 participants more than our target of 685 participants. This necessary ‘over-recruitment’ was approved in advance by the TSC and the Research Ethics Committee.
As part of recruitment, general practices and secondary/intermediate services sent a total of 8712 letters inviting patients to join the trial. In response the trial offices received around 1500 informal telephone enquiries about the trial. Those callers who dropped out at this point could not meet the requirements to join the study, for example they could not take time off work to complete the course, they were no longer interested once the study had been explained to them or they did not meet our inclusion criteria; in London, this was principally because of a lack of fluency in English.
A total of 1026 people returned a consent to approach form or contacted us directly and met our inclusion criteria. These people were then sent a baseline questionnaire and a trial consent form. Of these, 323 people declined to join the study (Figure 15). Reasons for this included:
-
They could not commit or no longer wanted to commit to the course or the relaxation programme (unavailable because of holiday, work or family commitments; did not like being in groups or with strangers; too difficult to get to venues; did not think that they would benefit from being in the trial; had done other similar things in the past; or were no longer interested).
-
They had other more serious comorbidities and/or scheduled hospital visits.
-
They were not fluent in English.
FIGURE 15.
Consolidated Standards of Reporting Trials flow diagram. Total analysed is for primary outcome only. a, Loss to follow-up (moved or changed telephone number).

Description of invitees
Practices provided anonymous demographic data on 6182 (71%) of the 8712 people whom they invited to participate in the trial (not all practices were able to supply these data). The proportion of women invited was the same in each site (64%). The overall mean age was 59 years (range 18–101 years), but the mean age in Warwick (67 years) was higher than that in London (52 years). Describing the ethnicity of those invited was hampered by a lack of data. Of the 3151 invitees for whom ethnicity was recorded, 2122 (67%) were white British or European and 1029 (33%) were Asian or African (Table 40). The difference in ethnicity recording and reporting between east London and the Midlands might represent an underestimation of the proportion of white British/European invitees.
| Site | Female, n (%) | Age (years), mean (range) | Ethnicity, n (%) | ||
|---|---|---|---|---|---|
| White British, mixed or European | Asian or African or mixed | Unknown | |||
| London (N = 3187) | 2025 (64) | 52 (18–95) | 1029 (32) | 971 (30) | 1187 (37) |
| Warwick (N = 2995) | 1911 (64) | 67 (19–101) | 821 (27) | 83 (3) | 2091 (70) |
| Total (N = 6182) | 3936 (64) | 59 (18–101) | 1850 (30) | 1054 (17) | 3278 (53) |
Baseline characteristics of study participants
Of those recruited, 67% were female; the mean age was 60 years (range 19–94 years) and 81% were white British. Age and gender were similar between those recruited and those invited (with available data). Table 41 provides baseline characteristics of all participants.
| Characteristics | All participants (n = 703) |
|---|---|
| Age (years), mean (range) | 59.9 (19.3–94.4) |
| CPG pain-related disability score, mean (SD) | 63.3 (25.1) |
| CPG pain intensity score, mean (SD) | 71.3 (16.3) |
| PSEQ score, mean (SD) | 31.0 (13.9) |
| CPAQ score, mean (SD) | 56.6 (20.1) |
| HADS depression score, mean (SD) | 7.4 (4.1) |
| HADS anxiety score, mean (SD) | 9.2 (4.6) |
| hEIQ score, mean (SD) | 13.9 (3.5) |
| EQ-5D score, mean (SD) | 0.40 (0.34) |
| Comorbidities, median (range) | 2 (0–8) |
There were no important differences in baseline characteristics between the intervention arm and the control arm (Table 42). Just over half of the participants [381/703 (54%)] had completed formal education at or below the age of 16 years and only 169 out of 703 (24%) were in any form of employment, with 148 out of 703 (21%) unable to work because of long-term sickness and another 307 out of 703 (44%) being retired from work. In the previous 6 months nearly half of the participants [341/703 (49%)] had been prevented from engaging in their usual activities for ≤ 14 days because of their pain and 356 of 703 (51%) had been prevented from engaging in their usual activities ≥ 15 days because of their pain. In total, 40% (282/703) described their current health as good or very good at baseline (this compares with 83% of adults in Tower Hamlets, 83% in Hackney, 83% in Newham, 85% in Warwickshire and 81% in Coventry in the 2011 census280). Most of the participants had had pain for at least 3 years (85%), with 38% reporting pain for > 10 years.
| Characteristics | Control (N = 300), n (%) | Intervention (N = 403), n (%) | Number of participants with missing data (control, intervention) |
|---|---|---|---|
| Age (years), mean (SD) | 59.4 (13.8) | 60.3 (13.5) | 0, 0 |
| Male | 98 (33) | 132 (33) | 0, 0 |
| Lives alone | 101 (34) | 143 (36) | 4, 6 |
| Ethnicity | |||
| White | 239 (80) | 325 (81) | 0, 0 |
| Black | 36 (12) | 53 (13) | |
| Asian | 20 (7) | 13 (3) | |
| Mixed | 5 (2) | 9 (2) | |
| Other | 0 (0) | 3 (1) | |
| Self-rated English language fluency | |||
| Fluent | 259 (86) | 341 (85) | 0, 0 |
| Good | 36 (12) | 56 (14) | |
| Below average | 3 (1) | 6 (1) | |
| Poora | 2 (1) | 0 (0) | |
| Age at which formal education ended | |||
| No formal education received | 4 (1) | 1 (< 1) | 0, 0 |
| ≤ 12 years | 0 (0) | 1 (< 1) | |
| 13–16 years | 153 (51) | 222 (55) | |
| 17–19 years | 66 (22) | 68 (17) | |
| ≥ 20 years | 66 (22) | 102 (25) | |
| Still in full-time education | 3 (1) | 3 (1) | |
| Other | 8 (3) | 6 (1) | |
| Employment status | |||
| Employed, including self-employed (full- or part-time) | 78 (26) | 91 (23) | 0, 0 |
| Unemployed and looking for work | 10 (3) | 20 (5) | |
| Still in full-time education | 3 (1) | 1 (< 1) | |
| Unable to work because of long-term sickness | 62 (21) | 86 (21) | |
| Looking after home/family | 14 (5) | 23 (6) | |
| Retired from paid work | 132 (44) | 175 (43) | |
| Other | 1 (< 1) | 7 (2) | |
| Time kept from usual activities because of pain in the past 6 months | |||
| 0–6 days | 84 (28) | 136 (34) | 3, 3 |
| 7–14 days | 49 (16) | 72 (18) | |
| 15–30 days | 57 (19) | 71 (18) | |
| ≥ 31 days | 107 (36) | 121 (30) | |
| State of health | |||
| Very good | 17 (6) | 27 (7) | 0, 0 |
| Good | 100 (33) | 138 (34) | |
| Fair | 130 (43) | 159 (39) | |
| Bad | 45 (15) | 63 (16) | |
| Very bad | 8 (3) | 16 (4) | |
| Duration of pain | |||
| 0–3 months | 4 (1) | 1 (< 1) | 0, 0 |
| 4–12 months | 10 (3) | 15 (4) | |
| 13 months–2 years | 43 (14) | 45 (11) | |
| 3–4 years | 45 (15) | 55 (14) | |
| 5–6 years | 40 (13) | 49 (12) | |
| 7–10 years | 50 (17) | 81 (20) | |
| > 10 years | 108 (36) | 157 (39) | |
| CPG overall | |||
| 0 | 0 (0) | 0 (0) | 3, 5 |
| 1 | 18 (6) | 30 (8) | |
| 2 | 66 (22) | 99 (25) | |
| 3 | 81 (27) | 123 (31) | |
| 4 | 132 (44) | 146 (37) | |
| CPG pan-related disability score, mean (SD) | 63.8 (24.4) | 62.9 (25.7) | 0, 1 |
| CPG pain intensity score, mean (SD) | 70.9 (15.3) | 71.5 (17.0) | 1, 1 |
| PSEQ score, mean (SD) | 30.6 (14.1) | 31.2 (13.8) | 0, 5 |
| CPAQ score, mean (SD) | 55.3 (19.1) | 57.5 (20.7) | 7, 15 |
| HADS depression score, mean (SD) | 7.5 (4.0) | 7.4 (4.2) | 3, 2 |
| HADS anxiety score, mean (SD) | 9.3 (4.7) | 9.2 (4.6) | 3, 3 |
| HADS depression score | |||
| 0–7 | 159 (54) | 217 (54) | 3, 2 |
| 8–10 | 74 (25) | 95 (24) | |
| 11–21 | 64 (22) | 89 (22) | |
| heiQ score, mean (SD) | 13.8 (3.4) | 14.0 (3.6) | 5, 3 |
| EQ-5D score, mean (SD) | 0.39 (0.34) | 0.41 (0.34) | 1, 1 |
| Number of comorbidities, median (IQR) | 3 (2–4) | 2 (2–3) | 21, 32 |
| Total amount of drugs taken above the DDD in 3 months prior to randomisation, expressed in units of DDD | |||
| Psychotropic, median (IQR) | 0 (0–0) | 0 (0–0) | 4, 3 |
| Weak opioids, median (IQR) | 0 (0–7) | 0 (0–8) | 4, 3 |
| Strong opioids, median (IQR) | 0 (0–0) | 0 (0–0) | 4, 3 |
| Analgesics (including opioids, non-opioids, NSAIDs and other CNS drugs and oral and topical preparations), median (IQR) | 44 (0–136) | 49 (0–140) | 4, 3 |
| Drugs taken orally for neuropathic pain, median (IQR) | 0 (0–7) | 0 (0–0) | 4, 3 |
| NSAID analgesics (both oral and topical), median (IQR) | 0 (0 –44) | 0 (0–56) | 4, 3 |
| Proportion of participants prescribed weak opioids | 76 (26) | 107 (27) | 4, 3 |
| Proportion of participants prescribed strong opioids | 72 (24) | 90 (23) | 4, 3 |
Mean (SD) anxiety and depression scores on the HADS instrument at baseline were 9.2 (4.6) and 7.4 (4.1), respectively. The mean value exceeded the usual cut-off for caseness for anxiety (≥ 8) and was close to that for depression (≥ 8). 176 Overall, health utility as assessed by the EQ-5D instrument (commonly interpreted as quality of life) was very low, with a mean (SD) score of 0.4 (0.34). The median number of comorbidities (determined from primary care records) was two (range 0–8).
Overall, this was a group of with a high rate of medication use. Many individuals were taking multiple analgesic medications, meaning that a substantial minority were taking more than one DDD of analgesic medication per day. Similarly, it is notable that a substantial minority were prescribed no analgesic medication in the 3 months prior to randomisation. It is noteworthy that just under one-quarter (23%) were being prescribed strong opioids at baseline.
Overall attendance on the course was excellent, with little evidence of attrition; on average, participants attended 85% of the course.
Retention and follow-up rates
We obtained primary outcome data from 621 (88%) participants at 12 months. At 6 and 12 months’ follow-up 6% and 5% of responders, respectively, provided only primary outcome and quality of life (EQ-5D) data (Table 43).
| Site | Enrolled, n | Sent 6-month questionnaire, n | All responders, n | Telephone responders (CPG and EQ-5D only), n (%) | Response ratea (%) | Response rateb (%) |
|---|---|---|---|---|---|---|
| 6 months | ||||||
| London | 320 | 307 | 275 | 30 (11) | 90 | 86 |
| Warwick | 383 | 364 | 350 | 6 (2) | 96 | 91 |
| Total | 703 | 671 | 625 | 36 (6) | 93 | 89 |
| 12 months | ||||||
| London | 320 | 303 | 271 | 22 (8) | 89 | 85 |
| Warwick | 383 | 363 | 350 | 7 (2) | 96 | 91 |
| Total | 703 | 666 | 621 | 29 (5) | 93 | 88 |
A comparison of the characteristics of those retained in the study and the characteristics of those not included in the primary analysis is provided in Table 44. In general, the two groups were remarkably similar, the one exception being that people living alone were over-represented among those lost to follow-up (51% vs. 33%).
| Characteristics | Responder (N = 652), n (%) | Lost to follow-upa (N = 51), n (%) |
|---|---|---|
| Age (years), mean (SD) | 60.2 (13.4) | 56.8 (16.0) |
| Male | 215 (33) | 15 (29) |
| Living alone | 218 (33) | 26 (51) |
| Ethnicity | ||
| White | 523 (80) | 41 (80) |
| Black | 83 (13) | 6 (12) |
| Asian | 30 (5) | 3 (6) |
| Mixed or other | 16 (2) | 1 (2) |
| Fluent in or good at English | 642 (98) | 50 (98) |
| Age at which formal education ended | ||
| ≤ 12 years | 6 (1) | 0 (0) |
| 13–19 years | 472 (72) | 37 (73) |
| ≥ 20 years or still in full-time education or other | 174 (27) | 14 (27) |
| Employment or in full-time education | 157 (24) | 16 (31) |
| Time kept from usual activities because of pain in past 6 months | ||
| 0–14 days | 318 (49) | 23 (45) |
| ≥ 14 days | 328 (51) | 28 (55) |
| State of health | ||
| Very good, good or fair | 530 (81) | 41 (80) |
| Bad or very bad | 122 (19) | 10 (20) |
| Duration of pain | ||
| 0–2 years | 106 (16) | 12 (24) |
| 3–6 years | 171 (26) | 18 (35) |
| ≥ 7 years | 375 (58) | 21 (41) |
| CPG pain-related disability score, mean (SD) | 63 (25) | 69 (26) |
| CPG pain intensity score, mean (SD) | 71 (16) | 76 (16) |
| PSEQ score, mean (SD) | 31 (14) | 26 (13) |
| HADS depression score, mean (SD) | 7.4 (4.0) | 8.6 (4.6) |
| HADS anxiety score, mean (SD) | 9.2 (4.6) | 9.9 (5.1) |
| CPAQ score, mean (SD) | 57 (20) | 51 (19) |
| heiQ score, mean (SD) | 14.0 (3.5) | 12.8 (3.6) |
| EQ-5D score, mean (SD) | 0.41 (0.34) | 0.34 (0.37) |
| Number of comorbidities, mean (SD) | 2.7 (1.4) | 2.6 (1.3) |
Delivery of the intervention
Recruitment and training of facilitators
We identified 30 potential facilitators, 14 HCPs and 16 laypeople, who attended one of three 2-day training courses. Twenty-four (80%) of these were both available to deliver the course and assessed as being competent to deliver it. Eleven HCPs and 13 laypeople delivered courses; this included two members of the study team who delivered sessions on 10 courses when no other experienced qualified HCP was available.
The mean age of the HCPs delivering the intervention was 44.3 years (range 34–59 years), seven (64%) were female and the mean duration of practice was 13 years (range 3–29 years). They included one chiropractor, three osteopaths, four physiotherapists and three psychologists. All courses were facilitated by at least one experienced facilitator who had delivered the intervention before.
The mean age of the lay facilitators was 55 years (range 33–71 years), 10 (77%) were female and all had personal experience of living with chronic pain. The mean number of years of small group facilitation experience was 4 years (range 0–10 years); two had a background in teaching but considered that they had no previous facilitation experience of this nature. The characteristics of the facilitators are provided in Table 45. Overall, the median number of courses delivered was one (range 1–6) for the lay facilitators and three (range 1–5) for the HCPs.
| Facilitator | Site | Age (years) | Gender | Ethnicity | Type | HCP profession/experience | Years of professional experience (HCP) or facilitation (lay) | Number of courses facilitated |
|---|---|---|---|---|---|---|---|---|
| 1a | London | 60 | M | White British | HCP | Osteopath | 5 | 5 |
| 2a | London | 48 | F | White British | HCP | Osteopath | 14 | 5 |
| 3a | London | 50 | M | White British | Lay | EPP accreditation | 4 | 3 |
| 4a | London | 34 | M | Bangladeshi | Lay | EPP accreditation | 3 | 3 |
| 5 | London | 35 | F | British Pakistani | HCP | Psychologist (BABCP) | 9 | 2 |
| 6a | London | 36 | M | White British | HCP | Osteopath | 9 | 2 |
| 7 | London | 41 | F | Indian | Lay | EPP accreditation | 7 | 2 |
| 8a | London | 49 | M | White British | HCP | Chiropractor | 25 | 1 |
| 9 | London | 37 | F | White British | HCP | Clinical psychologist | 14 | 1 |
| 10 | London | 36 | M | White British | HCP | Clinical psychology assistant | 3 | 1 |
| 11 | London | 64 | F | British Pakistani | Lay | EPP accreditation | 4 | 1 |
| 12 | London | 55 | F | White British | Lay | EPP accreditation | 7 | 1 |
| 13 | London | 53 | F | White British | Lay | EPP accreditation | 0 | 1 |
| 14a | London | 69 | F | White British | Lay | Ex-nursing tutor | 30 | 1 |
| 15 | London | 42 | F | Black Caribbean | Lay | EPP accreditation | 9 | 1 |
| 16 | Warwick | 53 | F | Other white | Lay | EPP accreditation | 4 | 6 |
| 17 | Warwick | 53 | F | White British | HCP | Physiotherapist | 29 | 5 |
| 18 | Warwick | 61 | F | White British | Lay | EPP accreditation | 7 | 5 |
| 19 | Warwick | 37 | F | White British | HCP | Physiotherapist | 9 | 4 |
| 20 | Warwick | 51 | F | White British | HCP | Physiotherapist | 20 | 4 |
| 21 | Warwick | 72 | M | White British | Lay | EPP accreditation | 5 | 4 |
| 22 | Warwick | 53 | F | White British | HCP | Physiotherapist | 7 | 2 |
| 23 | Warwick | 69 | F | White British | Lay | Ex-teacher | 0 | 1 |
| 24 | Warwick | 57 | F | British Bangladeshi | Lay | CDSMP certification | 1 | 1 |
Courses run and attendance
Thirty-one courses were held in total, 14 in London and 17 in Warwick. The average number of participants booked on each course was 14 (London, n = 15; Warwick, n = 13) and the average number who attended on day 1 was 11 (London, n = 11; Warwick, n = 11) (Tables 46–49). Courses were delivered in accessible venues near the recruitment sites including community centres, hospitals, university premises and a hospice. The mean duration from randomisation to attending a course was 42 days (range 1–168 days).
| Course | Venue | Facilitator | Number enrolled (target 16) | Attended day 1 (% of enrolled) | Attendance rate (average number of sessions/24 × 100) (%) |
|---|---|---|---|---|---|
| L01 | University | Osteopath and lay person | 13 | 9 (69) | 82 |
| L03 | Community hospital | Osteopath and lay person | 12 | 7 (58) | 71 |
| L04 | Hospice community centre | Osteopath and lay person | 14 | 10 (71) | 88 |
| L05 | General practice | Osteopath and lay person | 14 | 11 (79) | 90 |
| L06 | Hospice community centre | Psychologist and lay person | 17 | 13 (76) | 87 |
| L07 | University | Chiropractor and lay person | 14 | 13 (93) | 83 |
| W04 | Community centre | Physiotherapist and lay person | 10 | 10 (100) | 95 |
| W06 | Community centre | Physiotherapist and lay person | 12 | 12 (100) | 91 |
| W07 | Community centre | Physiotherapist and lay person | 10 | 9 (90) | 93 |
| Overall | 116 | 94 (81) | 86 | ||
| London | 84 | 63 (75) | 84 | ||
| Warwick | 32 | 31 (97) | 92 |
| Course | Venue | Facilitator | Number enrolled (target 16) | Attended day 1 (% of enrolled) | Attendance rate (average number of sessions/24 × 100) (%) |
|---|---|---|---|---|---|
| L08 | Hospice community centre | Psychologist and lay person | 16 | 8 (50) | 70 |
| L09 | Community hospital | Osteopath and lay person | 16 | 12 (75) | 65 |
| L10 | Community hospital | Osteopath and lay person | 17 | 11 (65) | 84 |
| L11 | Community hospital | Psychologist and lay person | 16 | 10 (63) | 91 |
| L12 | University | Osteopath and lay person | 14 | 10 (71) | 98 |
| W08 | Community centre | Physiotherapist and lay person | 16 | 11 (69) | 99 |
| W09 | Community centre | Physiotherapist and lay person | 16 | 13 (81) | 90 |
| W10 | Community centre | Physiotherapist and lay person | 15 | 11 (73) | 89 |
| W13 | Community centre | Physiotherapist and lay person | 15 | 11 (73) | 96 |
| W14 | Community centre | Physiotherapist and lay person | 16 | 16 (100) | 70 |
| W15 | Community centre | Physiotherapist and lay person | 16 | 14 (88) | 65 |
| W16 | Hotel conference centre | Osteopath and lay person | 16 | 13 (81) | 84 |
| Overall | 189 | 140 (74) | 91 | ||
| London | 79 | 51 (65) | 98 | ||
| Warwick | 110 | 89 (81) | 99 |
| Course | Venue | Facilitator | Number enrolled (target 16) | Attended day 1 (% of enrolled) | Attendance rate (average number of sessions/24 × 100) (%) |
|---|---|---|---|---|---|
| L13 | Hospice community centre | Osteopath and lay person | 17 | 10 (59) | 93 |
| L14 | Community hospital | Osteopath and lay person | 16 | 12 (75) | 80 |
| L15 | University | Osteopath and psychologist and lay person | 17 | 14 (82) | 74 |
| W18 | Community centre | Osteopath and lay person | 16 | 16 (100) | 93 |
| W19 | Community centre | Physiotherapist and lay person | 14 | 8 (57) | 55 |
| W20 | Community centre | Physiotherapist and lay person | 9 | 6 (67) | 69 |
| W21 | Community centre | Physiotherapist and lay person | 11 | 10 (91) | 60 |
| W23 | Community centre | Physiotherapist and lay person | 12 | 11 (92) | 85 |
| W24 | Community centre | Physiotherapist and lay person | 5 | 5 (100) | 98 |
| W25 | Community centre | Physiotherapist and lay person | 11 | 10 (91) | 84 |
| Overall | 128 | 102 (80) | 80 | ||
| London | 50 | 36 (72) | 82 | ||
| Warwick | 78 | 66 (85) | 78 |
| Course | Number enrolled | Attended day 1 (% of enrolled) | Attendance rate (average number of sessions/24 × 100) (%) |
|---|---|---|---|
| Overall | 433 | 336 (78) | 86 |
| London | 213 | 150 (70) | 83 |
| Warwick | 220 | 186 (85) | 88 |
Adverse events
No serious adverse events occurred as a result of the study. Twenty-one incidents resulted in emotional upset, which was dealt with at the scene by the facilitators or later after follow-up contact with one of the study managers. One person in the control arm of the study died but the death was not related to the study.
Course adherence
Fifty-seven initial course non-attendees were booked onto a further course/courses, but most of these failed to attend any subsequent courses. Overall, 67 (17%) of those randomised to the intervention did not attend any course. The reasons given for not attending any course were that participants felt too unwell, had a preference for the control arm, had work commitments, had family issues, had been bereaved or considered the venues or times of courses offered unsuitable. Tables 46–49 show the number of courses that were run, the number of participants booked on the courses, the number attending on day 1 and the mean number of sessions attended per course per participant. There were 24 sessions/components on the course and overall the mean attendance broken down by sessions attended was 86% (20–21 sessions).
Overall, 282 (70%) intervention participants achieved our predefined criterion of adherence (≥ 17 sessions attended), whereas we considered 95 (24%) non-adherent to the intervention as they attended eight sessions or fewer. If we dichotomise participants into compliers and non-compliers based on our predefined criterion of attendance at more than half the course (at least 12 sessions), 76% were compliant. Attendance was consistently better in Warwick than in London (Table 50).
| Exposure | London, n (%) | Warwick, n (%) | Both, n (%) |
|---|---|---|---|
| Adherent (≥ 17 sessions) | 115 (63) | 167 (76) | 282 (70) |
| Moderate adherence (9–16 sessions) | 16 (9) | 10 (5) | 26 (6) |
| Non-adherent (≤ 8 sessions) | 52 (28) | 43 (20) | 95 (24) |
| Total | 183 (100) | 220 (100) | 403 (100) |
| Complier (≥ 12 sessions) | 130 (71) | 175 (80) | 305 (76) |
| Non-complier (≤ 11 sessions) | 53 (29) | 45 (20) | 98 (24) |
| Total | 183 (100) | 220 (100) | 403 (100) |
Primary outcome analyses
We included 652 participants in the analysis of the primary outcome of pain-related disability as determined by the CPG [278/300 (93%) control, 374/403 (93%) intervention]. Pain-related disability did not differ between groups at 12 months [intervention mean (SD) 52.9 (28.0) vs. control mean (SD) 53.3 (28.8); difference (intervention vs. control) –1.0, 95% CI –4.9 to 3.0] (Table 51). The results were similar at 6 months. This effectively excludes any possibility of a worthwhile effect on our primary outcome; the limit of the 95% CI is 0.22 SDs of its baseline value, well within our prespecified clinically importance benefit of 0.3 SDs.
| Outcome | Control (n = 300), mean (SD) | Intervention (n = 403), mean (SD) | Treatment effecta (95% CI) |
|---|---|---|---|
| CPG pain-related disabilityb | |||
| 6 months | 54.3 (26.7) | 53.2 (25.7) | –1.2 (–4.8 to 2.4) |
| 12 months | 53.3 (28.8) | 52.9 (28.0) | –1.0 (–4.9 to 3.0) |
| CPG pain intensityc | |||
| 6 months | 64.3 (19.4) | 65.0 (18.8) | 1.0 (–1.5 to 3.6) |
| 12 months | 64.4 (20.1) | 63.5 (20.3) | –0.9 (–3.7 to 1.9) |
| PSEQ scored | |||
| 6 months | 32.7 (15.0) | 35.5 (14.0) | 2.3 (0.6 to 4.1) |
| 12 months | 33.4 (15.1) | 35.4 (14.1) | 1.4 (–0.2 to 3.1) |
| HADS anxiety scoree | |||
| 6 months | 9.1 (4.8) | 8.2 (4.7) | –0.7 (–1.3 to –0.2) |
| 12 months | 8.4 (4.5) | 8.1 (4.5) | –0.4 (–0.9 to 0.1) |
| HADS depression scoree | |||
| 6 months | 7.0 (4.4) | 6.3 (4.1) | –0.7 (–1.2 to –0.2) |
| 12 months | 6.9 (4.6) | 6.2 (4.3) | –0.7 (–1.2 to –0.2) |
| CPAQ scoref | |||
| 6 months | 59.2 (19.7) | 64.4 (20.0) | 3.4 (1.3 to 5.5) |
| 12 months | 74.0 (14.4) | 73.1 (15.1) | –0.8 (–3.0 to 1.4) |
| heiQ scoreg | |||
| 6 months | 14.3 (3.6) | 14.9 (3.3) | 0.6 (0.1 to 1.0) |
| 12 months | 14.1 (3.6) | 14.9 (3.5) | 0.8 (0.4 to 1.2) |
| EQ-5D scoreh | |||
| 6 months | 0.41 (0.35) | 0.46 (0.34) | 0.03 (–0.01 to 0.08) |
| 12 months | 0.45 (0.35) | 0.46 (0.34) | 0.00 (–0.04 to 0.04) |
All sensitivity analyses for the primary outcome showed similar results.
Secondary outcomes: questionnaire items
The results for the secondary outcomes (except for the census global health question and the drug data) at 6 and 12 months’ follow-up are shown in Table 51. Self-efficacy (PSEQ score: difference 2.3, 95% CI 0.6 to 4.1), anxiety (HADS anxiety score: difference –0.7, 95% CI –1.3 to –0.2), depression (HADS depression score: difference –0.7, 95% CI –1.2 to –0.2), pain acceptance (CPAQ score: difference 3.4, 95% CI 1.3 to 5.5) and social integration (heiQ score: difference 0.6, 95% CI 0.1 to 1.0) were all significantly better in the intervention group than in the control group at 6 months’ follow-up.
At 12 months’ follow-up the differences favouring the intervention were sustained for depression (difference –0.7, 95% CI –1.2 to –0.2) and social integration (difference 0.8, 95% CI 0.4 to 1.2) and, although no longer statistically significant, the results for self-efficacy (difference 1.4, 95% CI –0.2 to 3.1) and anxiety (difference –0.4, 95% CI –0.9 to 0.1) tended to favour the intervention. The improvement in pain acceptance seen in the intervention group at 6 months was no longer present by 12 months (difference –0.8, 95% CI –3.0 to 1.4). Of the questionnaire items, only pain intensity (CPG pain intensity score at 6 months: difference 1.0, 95% CI –1.5 to 3.6) and EQ-5D score (at 6 months: difference 0.03, 95% CI –0.01 to 0.08) were not significantly better in the intervention group at either 6 or 12 months.
Table 52 shows the treatment effect sizes expressed as SMDs at 6 and 12 months using the adjusted SDs for centre, age, gender, baseline depression score and baseline value of the outcome.
| Outcome | Treatment effect (95% CI)a |
|---|---|
| CPG pain-related disability score | |
| 6 months | –0.06 (–0.24 to 0.12) |
| 12 months | –0.04 (–0.22 to 0.13) |
| CPG pain intensity score | |
| 6 months | 0.07 (–0.10 to 0.24) |
| 12 months | –0.06 (–0.23 to 0.12) |
| PSEQ score | |
| 6 months | 0.25 (0.07 to 0.43) |
| 12 months | 0.15 (–0.02 to 0.32) |
| HADS anxiety score | |
| 6 months | –0.24 (–0.41 to –0.06) |
| 12 months | –0.13 (–0.30 to 0.03) |
| HADS depression score | |
| 6 months | –0.25 (–0.44 to –0.06) |
| 12 months | –0.22 (–0.39 to –0.06) |
| CPAQ score | |
| 6 months | 0.27 (0.08 to 0.45) |
| 12 months | –0.03 (–0.20 to 0.13) |
| heiQ score | |
| 6 months | 0.25 (0.06 to 0.43) |
| 12 months | 0.32 (0.16 to 0.49) |
| EQ-5D score | |
| 6 months | 0.13 (–0.03 to 0.29) |
| 12 months | 0.01 (–0.16 to 0.17) |
Responses to the census global health question are summarised in Table 53. This table presents the results based on available data; those who did not provide data are not included. There was no difference between groups at either 6 or 12 months (odds ratio for being in a higher category at 6 months 1.09, 95% CI 0.77 to 1.54; odds ratio at 12 months 1.07, 95% CI 0.77 to 1.51).
| Response | Baseline, n (%) | 6 months, n (%) | 12 months, n (%) | |||
|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |
| Very good | 17 (6) | 27 (7) | 11 (5) | 20 (6) | 8 (3) | 14 (4) |
| Good | 100 (33) | 138 (34) | 81 (34) | 121 (35) | 84 (34) | 130 (38) |
| Fair | 130 (43) | 159 (39) | 100 (42) | 144 (42) | 115 (47) | 144 (42) |
| Bad | 45 (15) | 63 (16) | 39 (16) | 46 (13) | 32 (13) | 40 (12) |
| Very bad | 8 (3) | 16 (4) | 7 (3) | 11 (3) | 6 (2) | 14 (4) |
| Total | 300 (100) | 403 (100) | 238 (100) | 342 (100) | 245 (100) | 342 (100) |
Secondary outcomes: prescribed medicines
Differences in prescribed medicines between the groups at 12 months’ follow-up, expressed as DDD, are presented in Table 54. Intervention arm patients were prescribed significantly more DDDs of weak opioids in the 12 months following randomisation than those in the control arm, amounting to a difference of 18 days of medication at WHO standard dosing (95% CI 5 to 32 days). The proportion of intervention arm participants taking weak opioids at 12 months also tended to be higher than that in the control group, although the difference was not statistically significant (the odds of taking weak opioids was increased by 39% in the intervention arm, 95% CI 10% fewer to 114% more).
| Type of drug | Control (n = 258a) | Intervention (n = 350a) | Treatment effectb (95% CI) |
|---|---|---|---|
| DDD in 12 months post randomisation, median (IQR) | |||
| Psychotropics | 0 (0–21) | 0 (0–28) | –12 (–30 to 6) |
| Weak opioids | 0 (0–36) | 0 (0–64) | 18 (5 to 32) |
| Strong opioids | 0 (0–22) | 0 (0–24) | –1 (–12 to 11) |
| Analgesics (including opioids and other CNS drugs) | 232 (45–551) | 295 (57–648) | 98 (17 to 178) |
| Proportion of participants using opioids at 12 months post randomisation, n (%) | |||
| Weak opioids | 59 (23) | 103 (29) | 1.39 (0.90 to 2.14) |
| Strong opioids | 64 (25) | 82 (23) | 1.04 (0.59 to 1.85) |
Overall, intervention patients received considerably more analgesics than control arm patients in the 12 months after randomisation (98 DDDs, 95% CI 17 to 178 DDDs). However, there was no evidence of any difference in the prescription of strong opioids between study arms (–1 DDD, 95% CI –12 to 11 DDDs) nor in the proportions of those receiving strong opioids at 12 months (the odds of taking strong opioids was increased by 4% in intervention arm, 95% CI 41% fewer to 85% more).
Mediator analysis
The mediation analysis examining the potential role of self-efficacy at 12 weeks as a mediator is not presented because of the lack of effect seen on our primary outcome.
Preplanned subgroup analyses for the primary outcome
The results of our preplanned subgroup analyses for the primary outcome of CPG pain-related disability at 12 months are presented in Table 55. There is no evidence to support the intervention being more effective in those who live alone, who have four or more comorbidities or who have a lower socioeconomic status.
| Subgroup | Control, number included in analysis | Intervention, number included in analysis | Control, mean (SD) | Intervention, mean (SD) | Treatment effect (95% CI) | p-value for interaction |
|---|---|---|---|---|---|---|
| Non-pain related | ||||||
| Comorbidity | ||||||
| 0–3 | 192 | 269 | 50.2 (29.2) | 50.6 (27.7) | –0.6 (–5.1 to 4.0) | 0.72 |
| ≥ 4 | 76 | 90 | 59.8 (26.8) | 57.8 (28.0) | –2.1 (–9.4 to 5.3) | |
| Living arrangements | ||||||
| Living with others | 185 | 239 | 52.4 (28.1) | 50.9 (28.0) | –0.1 (–4.9 to 4.8) | 0.60 |
| Living alone | 89 | 129 | 54.5 (30.8) | 56.9 (27.4) | –2.2 (–8.9 to 4.5) | |
| PSEQ score | ||||||
| 0–20 | 72 | 83 | 71.7 (22.5) | 72.8 (23.6) | 0.5 (–7.0 to 7.9) | 0.78 |
| 21–39 | 121 | 184 | 56.5 (23.2) | 54.6 (24.2) | –2.2 (–7.6 to 3.3) | |
| 40–60 | 85 | 103 | 34.0 (29.1) | 34.6 (25.9) | 0.4 (–6.4 to 7.1) | |
| Socioeconomic status | ||||||
| Lower | 136 | 197 | 52.0 (29.3) | 48.5 (27.3) | –2.4 (–7.8 to 3.0) | 0.42 |
| Higher | 142 | 177 | 54.6 (28.4) | 57.9 (28.1) | 0.8 (–4.7 to 6.2) | |
| Pain related | ||||||
| Pain duration | ||||||
| 0–12 months | 13 | 13 | 40.0 (30.3) | 31.8 (29.4) | –5.5 (–23.5 to 12.6) | 0.88 |
| 13 months to 4 years | 80 | 93 | 51.7 (29.2) | 51.3 (26.6) | –1.7 (–8.9 to 5.4) | |
| ≥ 5 years | 185 | 268 | 54.9 (28.5) | 54.5 (28.1) | –0.8 (–5.5 to 3.8) | |
| CPG pain intensity score | ||||||
| 0–3 | 4 | 17 | 45.0 (42.6) | 21.6 (20.1) | –22.5 (–47.9 to 2.8) | 0.24 |
| 4–7 | 186 | 219 | 47.1 (28.1) | 46.1 (25.8) | –1.0 (–5.8 to 3.8) | |
| 8–10 | 87 | 138 | 66.7 (25.3) | 67.4 (25.1) | –0.2 (–6.6 to 6.3) | |
| CPG pain-related disability score | ||||||
| 0–3 | 51 | 70 | 31.7 (27.6) | 33.3 (27.4) | 0.5 (–8.1 to 9.1) | 0.60 |
| 4–7 | 138 | 187 | 51.1 (26.1) | 48.4 (23.7) | –2.8 (–8.2 to 2.5) | |
| 8–10 | 89 | 117 | 69.8 (23.8) | 71.5 (23.6) | 1.1 (–5.6 to 7.8) | |
| HADS depression score | ||||||
| 0–10 | 222 | 291 | 49.0 (28.7) | 49.0 (27.4) | –0.2 (–4.6 to 4.2) | 0.44 |
| 11–21 | 56 | 83 | 70.6 (22.5) | 67.1 (25.7) | –3.8 (–12.0 to 4.4) | |
There was a suggestion of a non-significant tendency for those with a shorter pain duration to show more benefit in terms of the primary outcome; however, interpretation is difficult as this subgroup analysis is hampered by the small number of participants as the vast majority of participants had long-standing pain. There was no evidence that treatment effects differed across subgroups.
No trend was seen in the association between pain-related self-efficacy and the primary outcome; however, there was an (inconclusive) suggestion that the effect size might be greatest in the group with an intermediate level of baseline self-efficacy.
Finally, there was a suggestion that those with a HADS depression score highly indicative of the likelihood of depression (scores of ≥ 11) may have shown a much greater improvement in pain-related disability at 12 months but, again, the numbers are relatively small and this finding is not statistically significant.
Compliers average causal effects
As a secondary analysis, the CPG pain-related disability, CPG pain intensity, PSEQ, HADS anxiety, HADS depression, CPAQ, heiQ and EQ-5D scores at 12 months were reanalysed to obtain a CACE of treatment, using our prespecified definition of ‘compliers’ (those who attended at least half of the course).
These analyses were performed on the same participants as the corresponding ITT analyses, so, for example, the CACE of the primary outcome excluded all participants who did not complete any CPG pain-related disability questions at 12 months and we assumed that the excluded participants were missing at random.
The CACE for the primary outcome of pain-related disability as determined by the CPG did not differ between treatment groups at 12 months (difference –1.0 intervention vs. control, 95% CI –5.9 to 3.9). This again excludes our prespecified worthwhile benefit of 0.3 SDs of the baseline score.
Only depression (HADS depression score –0.9, 95% CI –1.5 to –0.3) and social integration (heiQ score 1, 95% CI 0.5 to 1.5) were significantly better in the intervention group than in the control group at 12 months’ follow-up among the compliers.
Treatment effects on primary and secondary outcomes estimated from CACE adjusted and unadjusted analyses at 12 months’ follow-up are shown in Table 56.
| Outcome | Adjusted treatment effect (95% CI) | Unadjusted treatment effect (95% CI) |
|---|---|---|
| CPG pain-related disability score | –1.0 (–5.9 to 3.9) | –0.6 (–6.8 to 5.5) |
| CPG pain intensity score | –1.0 (–4.4 to 2.4) | –0.6 (–5.0 to 3.7) |
| PSEQ score | 1.7 (–0.3 to 3.7) | 2.7 (–0.4 to 5.9) |
| HADS anxiety score | –0.5 (–1.1 to 0.1) | –0.4 (–1.4 to 0.5) |
| HADS depression score | –0.9 (–1.5 to –0.3) | –0.8 (–1.7 to 0.2) |
| CPAQ score | –1.0 (–5.3 to 3.3) | –0.7 (–5.4 to 4.0) |
| heiQ score | 1.0 (0.5 to 1.5) | 1.1 (0.3 to 1.8) |
| EQ-5D score | 0.00 (–0.05 to 0.05) | 0.01 (–0.07 to 0.09) |
Participant exposure to other similar non-trial interventions
To make some assessment of performance bias, we collected data on participation in courses and activities other than the COPERS course during the 12-month follow-up period (Table 57). There were a considerable number of missing data, making interpretation of the data difficult. Overall, few respondents had attended any other courses and there appeared to be no differences in the proportions attending these courses between the groups. Reported practice of regular relaxation appeared to be somewhat higher in the control arm than in the intervention arm, with 32% of respondents in the control arm saying that they practised daily relaxation, compared with 21% in the intervention arm, and 26% of respondents in the control arm saying that they practised relaxation every week, compared with 18% in the intervention arm.
| Other activity/courses | Control (N = 300), n (%) | Intervention (N = 403), n (%) | Number of participants with missing data (control, intervention) |
|---|---|---|---|
| Courses or activities attended during the follow-up period outside of the COPERS trial | |||
| Pain management | 20 (9) | 26 (9) | 86, 109 |
| Expert Patient Programme/self-management course | 9 (4) | 11 (4) | 96, 118 |
| Other wellness or well-being courses | 15 (7) | 15 (5) | 96, 114 |
| Return to work courses | 7 (4) | 9 (3) | 101, 116 |
| Frequency of practising relaxation and/or meditation during the follow-up period | |||
| Daily | 75 (32) | 66 (21) | 69, 82 |
| Weekly | 59 (26) | 59 (18) | |
| Monthly | 14 (6) | 21 (7) | |
| Rarely | 56 (24) | 93 (29) | |
| Never | 27 (12) | 82 (26) | |
Changes from baseline
Our primary outcome decreased (i.e. improved) within both the control arm and the intervention arm between baseline and 6 months’ follow-up [mean (SD) difference between baseline and 6 months: control –8.8 (23.0), intervention –9.3 (23.3)] and these decreases were sustained at 12 months’ follow-up (Figure 16). CPG pain intensity followed a similar pattern. Indeed, all of the questionnaire variables improved to some extent between baseline and 6 months in both arms of the study and these improvements were sustained, but not generally increased, at 12 months. The one exception to this was the improvement in pain acceptance as measured by the CPAQ. In both study arms this appears to have improved a great deal in the second half of the follow-up period [mean (SD) difference between baseline and 6 months: control 3.0 (12.1), intervention 5.4 (14.6); between baseline and 12 months: control 17.2 (20.7), intervention 14.3 (22.7)] (Table 58).
FIGURE 16.
Reduction in pain-related disability over the 12-month trial period.
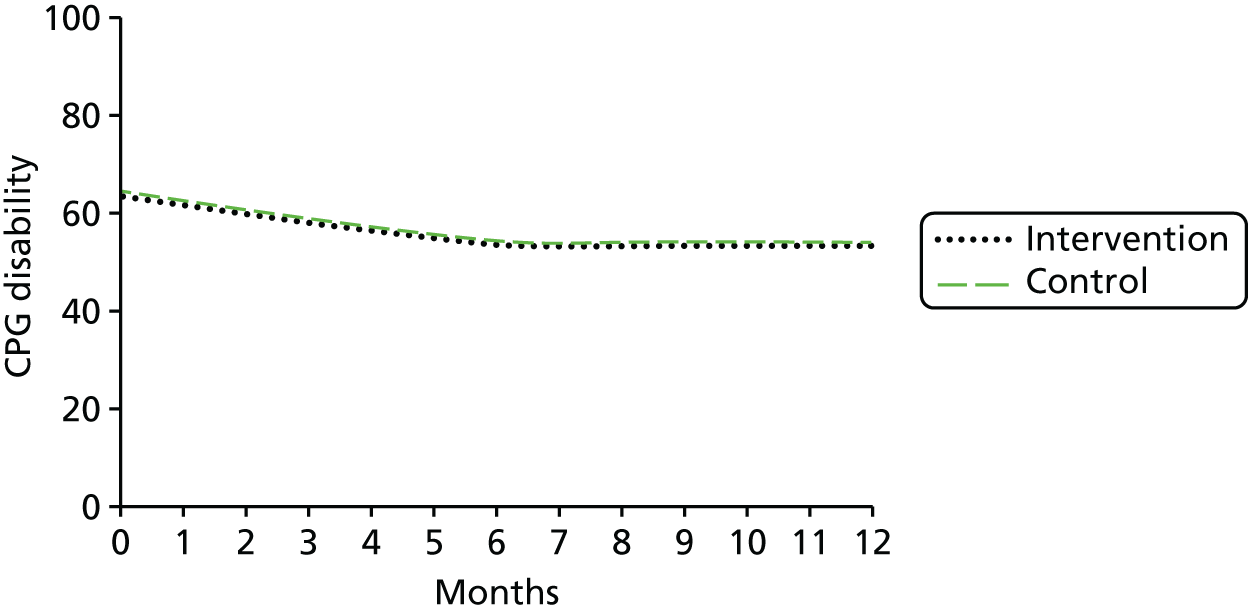
| Outcome | Control | Intervention | Number of participants (control, intervention) |
|---|---|---|---|
| CPG pain-related disability score | |||
| 6 months | –8.8 (23.0) | –9.3 (23.3) | 261, 356 |
| 12 months | –9.1 (26.4) | –9.5 (26.1) | 261, 355 |
| CPG pain intensity score | |||
| 6 months | –6.5 (16.2) | –5.4 (15.8) | 241, 337 |
| 12 months | –6.1 (17.2) | –7.2 (17.7) | 245, 341 |
| PSEQ score | |||
| 6 months | 1.9 (10.6) | 3.2 (11.0) | 240, 338 |
| 12 months | 2.2 (10.7) | 3.3 (10.6) | 244, 334 |
| HADS anxiety score | |||
| 6 months | –0.1 (3.2) | –0.7 (3.4) | 234, 333 |
| 12 months | –0.5 (3.2) | –0.9 (3.5) | 242, 338 |
| HADS depression score | |||
| 6 months | –0.3 (2.9) | –0.9 (3.0) | 238, 339 |
| 12 months | –0.3 (3.1) | –1.0 (3.4) | 242, 339 |
| CPAQ score | |||
| 6 months | 3.0 (12.1) | 5.4 (14.6) | 228, 321 |
| 12 months | 17.2 (20.7) | 14.3 (22.7) | 227, 323 |
| heiQ score | |||
| 6 months | 0.3 (2.5) | 0.7 (2.7) | 238, 337 |
| 12 months | 0.1 (2.7) | 0.7 (2.8) | 234, 340 |
| EQ-5D | |||
| 6 months | 0.02 (0.29) | 0.04 (0.28) | 255, 359 |
| 12 months | 0.04 (0.29) | 0.04 (0.28) | 258, 353 |
Post hoc analyses
As a result of the finding that mean levels of depressive symptoms were high at baseline we looked at the proportion of participants who might be depressed at each time point. We examined two cut-off points: a HADS depression score of ≥ 8, which is the most sensitive and specific cut-off for possible depression, and a HADS depression score of ≥ 11, which is often considered to be a cut-off for probable depression. 281 In total, 169 out of 698 (24%) participants scored 8–10 and 153 out of 698 (22%) participants scored ≥ 11 on the HADS depression subscale.
The statistically significant sustained reduction in depressive symptoms seen in the intervention group prompted us to conduct an exploratory post hoc subgroup analysis to assess whether or not the treatment differed between those who were depressed at baseline and those who were not depressed at baseline (HADS depression score 0–7 vs. 8–21). We hypothesised that the reduction in depressive symptoms had arisen in those who were likely to have been depressed at baseline and we also wanted to ascertain that people who were not depressed at baseline did not suffer psychologically through exposure to the intervention. This post hoc analysis revealed that the improvement in depressive symptoms seen in the intervention arm was indeed concentrated in those who were depressed at baseline, whereas those who were not depressed at baseline experienced no overall change in HADS depression score at 12 months (p-value for interaction = 0.004) (Table 59). Moreover, the SMD among those depressed at baseline was –0.50 (95% CI –0.74 to –0.25), which is highly likely to be clinically relevant. 282
| HADS depression score at baseline | HADS depression score at 12 months, mean (SD) | Treatment effect (95% CI) | p-value for interaction | |
|---|---|---|---|---|
| Control | Intervention | |||
| Original scale | ||||
| 0–7 | 4.2 (3.0) | 4.0 (3.0) | 0.0 (–0.7 to 0.6) | 0.004 |
| 8–21 | 9.4 (4.8) | 8.2 (4.7) | –1.5 (–2.3 to –0.8) | |
| SMD | ||||
| 0–7 | – | – | –0.01 (–0.23 to 0.21) | – |
| 8–21 | – | – | –0.50 (–0.74 to –0.25) | |
Sensitivity analyses
All of the sensitivity analyses produced similar results to those of the primary analysis and demonstrated that the primary outcome results were robust (Table 60 and Figure 17).
| Analysis | Treatment effect (95% CI) |
|---|---|
| Main analysis | –1.0 (–4.9 to 3.0) |
| Complete case analysis | –0.9 (–4.9 to 3.1) |
| Multivariate analysis | –0.1 (–5.5 to 5.2) |
| Different imputation model | –1.1 (–5.1 to 2.9) |
| Redefinition of primary outcome | –1.1 (–5.0 to 2.9) |
FIGURE 17.
Sensitivity analysis for CPG pain-related disability at 12 months (assuming that missing data are missing not at random).
The y-axis in Figure 17 shows the treatment effect for the CPG pain-related disability score at 12 months (e.g. a value of –2 indicates that the mean CPG disability score was 2 points less in the intervention group than in the control group). The x-axis shows the assumed CPG disability score in participants in the control group who were lost to follow-up (e.g. a value of 10 indicates that we set the average CPG disability score for participants in the control arm who were lost to follow-up to 10). In sensitivity analysis 1 we set the CPG disability score for participants in the intervention arm who were lost to follow-up to 10 points less than the score for participants in the control arm (e.g. if the value on the x-axis was 10, this would indicate that participants in the control arm who were lost to follow-up had a CPG disability score of 10 and participants in the intervention arm who were lost to follow-up had a CPG disability score of 0). In sensitivity analysis 2 we set the CPG disability score for participants in the intervention arm who were lost to follow-up to 10 points more than the score for participants in the control arm (e.g. if the value on the x-axis was 10, this would indicate that participants in the control arm who were lost to follow-up had a CPG disability score of 10 and participants in the intervention arm who were lost to follow-up had a CPG disability score of 20).
The primary analysis excluded all participants who did not complete any CPG pain-related disability questions at either 6 or 12 months. This analysis assumed that the excluded participants were missing at random, that is, the reason that these participants’ data were missing was based on variables that were included in the analysis (e.g. that older participants with a high baseline CPG pain-related disability score were more likely to be excluded from the analysis).
In the sensitivity analyses in Figure 17 we have made different assumptions regarding participants who were excluded from the analysis to assess how robust the primary analysis results are to departures from the missing at random assumption. Specifically, the analyses have assumed that the excluded participants were missing not at random, that is, the reason that these participants’ data were missing was actually based on their CPG pain-related disability scores at 6 and 12 months (e.g. participants with a higher CPG disability score at 6 and 12 months were more likely to be excluded from the analysis).
Figure 17 indicates that the results of the primary analysis for CPG pain-related disability are robust to departures from the missing at random assumption (i.e. even if the missing data from participants who were lost to follow-up were missing not at random, this would not alter the conclusions from our main analysis).
Health economics
Microcosting of the COPERS intervention
In total, 31 courses were delivered across two centres over the duration of the trial (14 in London and 17 in the Midlands). Each course consisted of three 1-day sessions delivered during 1 week and a 2-hour follow-up session delivered 2 weeks later. Each course was conducted by two specially trained facilitators. Facilitators were recruited from NHS staff (off-duty time) and self-employed health-care specialists. Facilitator costs included a daily fee of £100 plus £40 travel costs per person. Administrator costs associated with booking the venue, allocating facilitators and arranging the sessions were £20 per hour (14 hours per course). Courses were run in community, primary care, hospital and university premises. Meeting rooms were hired at £100 per day for 3.5 days (3-day course and half-day follow-up session). Each participant was supplied with an educational DVD (£1.20 each), a relax pack including an education booklet and a CD (£1.17 each), and printed handouts (£1.21 each). Facilitators were recruited by team members who were paid £20 per hour (2 hours per recruit). On recruitment, facilitators attended a 2-day training course run by two members of the research team. Trainers were paid £200 per day plus £20 travel expenses. Facilitators were paid £100 per day for attending the training course and £10 per day towards travel expenses. Facilitators were provided with DVDs, relax packs (including a pain education booklet and a CD) and training manuals. In total, 35 facilitators (divided into two groups) were trained.
Table 61 summarises the direct costs of delivering the 31 courses. The total cost of the intervention was £62,888, of which £48,184 was the cost of running the course and £14,704 was the cost of training the facilitators.
| Component | Cost per course (£) | Total cost (£) |
|---|---|---|
| Course running costs | ||
| Salary | ||
| Facilitators | 700.00 | 21,700.00 |
| Administrator | 347.20 | 10,763.20 |
| Subtotal (salary) | 1047.20 | 32,463.20 |
| Facilitators’ travel | 80.00 | 2480.00 |
| Course materials | ||
| DVD | 16.80 | 520.80 |
| Relax packs (including CD) | 16.38 | 507.78 |
| Handouts | 16.94 | 525.14 |
| Facility | 350.00 | 10,850.00 |
| Hospitality | 20.00 | 620.00 |
| Consumables | 7.00 | 217.00 |
| Subtotal (course) | 1554.32 | 48,183.92 |
| Training costs | ||
| Salary | ||
| Trainers | 992.00 | 1984.00 |
| Facilitators | 3600.00 | 7200.00 |
| Administrator (recruitment of facilitators) | 1562.40 | 3124.80 |
| Subtotal (salary) | 6154.40 | 12,308.80 |
| Trainers’ travel | 80.00 | 160.00 |
| Facilitators’ travel | 360.00 | 720.00 |
| Course materials | ||
| DVD | 24.00 | 48.00 |
| Relax packs (including CD) | 23.40 | 46.80 |
| Manuals | 180.00 | 360.00 |
| Facility | 200.00 | 400.00 |
| Hospitality | 320.00 | 640.00 |
| Consumables | 10.00 | 20.00 |
| Subtotal (training) | 7351.80 | 14,703.60 |
| Total cost of intervention | 62,887.52 | |
| Cost per participant (including training) | 145.24 | |
| Cost per participant (excluding training) | 111.28 | |
More than two-thirds of the total running cost was accounted for by staff-related costs, which included salaries (£32,463) and travel expenses (£2480). The majority of facilitators were freelancers paid a fixed fee (£100) to run a session. The salary cost for this group does not include any additional employment costs. The salary cost of the course administrator (university employee) included a fixed fee of £20 per hour plus 24% salary on-costs (employer’s pension and National Insurance contributions). Organisation overheads were not included and London multipliers were not used.
The second highest category of costs was venue costs. Rooms were hired at a flat rate of £100 per day (£350 per course). The total cost of venues over the duration of the programme, including hospitality costs, was £11,470.
Course materials and other consumables (e.g. letters and stamps) accounted for only a small proportion of the total running costs (£1771). Costs presented in this category reflect the direct costs incurred by the programme. Training costs were shared between the two centres and included all costing items described above: salaries (trainers, facilitators and administrator), travel, venue, course materials, etc. (see Table 61).
Given that trainers were university employees, 24% salary on-costs were added to a fixed pay rate of £200 per day. The total cost of training was £420 per facilitator and £14,704 per whole programme.
Costs associated with providing usual care for primary care patients with chronic musculoskeletal pain included the costs of a pain education booklet and a relaxation CD (£1.17 each).
Cost of the intervention per participant
Our base-case estimations of the cost of the intervention per participant were based on the following assumptions:
-
The cost of the intervention per participant was calculated by dividing the total cost of the intervention by the total number of participants enrolled on the courses across the two centres. Sensitivity analysis considered the maximum and minimum number of participants per course, observed in the trial.
-
The quantity of training materials was based on the average number of participants per course. Sensitivity analysis considered no wastage of course materials.
-
Training was included in the calculation of the average cost per participant across the two centres. The cost per participant for each centre was presented without training costs, given that centres shared training courses.
Different costing scenarios for the cost of the intervention per participant are shown in Table 62. The average number of participants per course across the two centres was 14 (London n = 15, Warwick n = 13). The minimum number of participants enrolled on a course was five and the maximum was 17.
| Costing scenario | Number of participants per course | Cost per participant (£) |
|---|---|---|
| All centres, including training | 14 | 145.24 |
| All centres, excluding training | 14 | 111.28 |
| London, excluding training | 14 | 102.16 |
| Warwick, excluding training | 14 | 120.11 |
| London, excluding training, no wastage | 15 | 102.43 |
| Warwick, excluding training, no wastage | 13 | 119.79 |
| Minimum number of participants, all centres, including training | 5 | 389.38 |
| Maximum number of participants, all centres, including training | 17 | 120.05 |
The total cost of the course per participant across the two centres was £145.24 with training and £111.28 without training. The cost without training was £102 for London and £120 for Warwick. In the cost-effectiveness analyses we used a conservative estimate of £145.24 per participant.
Two costing scenarios were considered to address the uncertainty around the number of participants enrolled on the course and the use of course materials. Given the maximum (n = 17) and the minimum (n = 5) number of participants enrolled on the courses, the minimum cost per participant including training was £120 and the maximum cost including training was £389. Based on the actual number of participants at each centre (in contrast to the average number across the two centres), we estimated that the recycling of course materials had only a very small impact on the total cost per participant (< £1) because of the low cost of the course materials (see Tables 61 and 62).
Use of health-care resources by participants
The use of health-care resources by participants was analysed at 12 months post randomisation. The analyses of contacts with primary and secondary health-care services by participants in the intervention and control groups are summarised in Table 63. To assess any changes in resource use over time we looked at the use of health-care services in 3-month periods, which is summarised in Table 64. Given that the intervention may affect the use of primary care resources by participants, in particular consultations with GPs or practice nurses; these categories are shown separately from other service use data in Table 64.
| Service | Intervention, mean (SD) | Control, mean (SD) | Difference in means | 95% CI |
|---|---|---|---|---|
| Primary care sector (intervention, n = 370; control, n = 276) | ||||
| Consultations | 12.32 (11.12) | 12.80 (12.50) | –0.48 | –2.31 to 1.35 |
| Investigation | 3.35 (3.09) | 3.43 (3.32) | –0.07 | –0.58 to 0.43 |
| Prescriptions | 49.62 (48.77) | 51.71 (55.01) | –2.09 | –10.19 to 6.01 |
| Referrals to community care | 0.40 (0.71) | 0.38 (0.72) | 0.02 | –0.09 to 0.13 |
| Secondary care sector (intervention, n = 383; control, n = 291) | ||||
| Outpatient | 4.08 (5.12) | 4.32 (6.52) | –0.24 | –1.15 to 0.67 |
| Inpatient | 0.65 (1.43) | 0.55 (1.04) | 0.11 | –0.08 to 0.29 |
| A&E | 0.57 (1.21) | 0.57 (1.29) | 0.01 | –0.19 to 0.20 |
| Service | Number of contacts, mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | |||||||
| 3 months | 6 months | 9 months | 12 months | 3 months | 6 months | 9 months | 12 months | |
| Primary care | ||||||||
| GP surgery | 1.87 (1.94) | 1.84 (1.84) | 1.94 (2.05) | 1.76 (2.22) | 1.81 (1.79) | 1.84 (1.91) | 1.95 (1.98) | 1.83 (1.80) |
| GP telephone | 0.32 (0.83) | 0.40 (0.99) | 0.41 (1.30) | 0.35 (0.98) | 0.31 (0.72) | 0.38 (1.03) | 0.36 (0.99) | 0.42 (1.13) |
| Nurse surgery | 0.45 (1.06) | 0.48 (1.02) | 0.52 (1.57) | 0.66 (1.59) | 0.68 (2.29) | 0.46 (0.92) | 0.60 (1.51) | 0.67 (1.64) |
| Nurse telephone | 0.06 (0.29) | 0.04 (0.26) | 0.04 (0.27) | 0.04 (0.19) | 0.08 (0.56) | 0.09 (0.72) | 0.08 (0.86) | 0.07 (0.49) |
| Health-care assistant | 0.13 (0.41) | 0.11 (0.39) | 0.14 (0.60) | 0.13 (0.42) | 0.15 (0.51) | 0.11 (0.47) | 0.14 (0.52) | 0.12 (0.40) |
| Other specialistsa | 0.15 (0.55) | 0.14 (0.54) | 0.18 (0.65) | 0.15 (0.67) | 0.18 (0.70) | 0.17 (0.62) | 0.17 (0.58) | 0.16 (0.65) |
| Investigations | 0.79 (1.24) | 0.82 (1.20) | 0.84 (1.23) | 0.91 (1.33) | 0.86 (1.32) | 0.79 (1.19) | 0.82 (1.31) | 0.95 (1.45) |
| Prescriptions | 13.43 (12.50) | 12.86 (12.18) | 14.32 (13.50) | 14.03 (13.26) | 14.39 (13.61) | 14.55 (14.05) | 15.34 (14.65) | 15.93 (16.24) |
| Referrals to community care | 0.10 (0.33) | 0.11 (0.34) | 0.09 (0.30) | 0.10 (0.36) | 0.09 (0.30) | 0.08 (0.33) | 0.11 (0.35) | 0.09 (0.32) |
| Secondary care | ||||||||
| Inpatient | 0.12 (0.35) | 0.16 (0.56) | 0.21 (0.68) | 0.16 (0.50) | 0.13 (0.45) | 0.15 (0.44) | 0.11 (0.38) | 0.15 (0.51) |
| Outpatient | 0.98 (1.66) | 1.00 (1.58) | 1.06 (1.75) | 1.04 (1.67) | 1.06 (2.06) | 1.09 (2.30) | 1.16 (2.13) | 1.01 (1.77) |
| A&E | 0.13 (0.50) | 0.16 (0.48) | 0.18 (0.53) | 0.10 (0.38) | 0.13 (0.42) | 0.17 (0.61) | 0.12 (0.37) | 0.14 (0.51) |
Over 12 months, participants in both the intervention group and the control group had, on average, 12 consultations with primary care, three investigations/tests, four outpatient appointments and less than one referral to community care, inpatient stay or A&E admission. The average number of prescriptions over 12 months was 50 and 52 per participant for the intervention and control groups, respectively. There were no significant differences between the groups in the use of health-care resources for any category (see Table 63).
Quarterly analyses of resource use did not find any statistically significant changes in the number of health-care contacts in either group over the duration of the trial (see Table 64). Consultations with GPs and nurses (both surgery and telephone) and health-care assistants (surgery) accounted for almost 95% of all primary care contacts, with consultations with all other specialists accounting for < 5%. The numbers of contacts were not significantly different between the groups at any time period.
We also collected information about the use of private care including:
-
number of and money spent on non-NHS consultations (including complementary and alternative consultations)
-
type and number of and money spent on tests and investigations
-
type of and money spent on medicines
-
type of and money spent on devices and aids
-
overnight admissions/stays in private hospital
-
money spent on support and help at home as a result of pain.
However, the private care costs were not well reported given that participants preferred not to answer questions about their personal expenses. Therefore we did not use these data in the health economics analysis.
Cost of health-care services
Resource use data were combined with unit costs to calculate the cost of health-care services for each quarter and over 12 months. The total costs of the health-care services used by participants are summarised in Table 65. The largest proportion of costs was associated with inpatient stay (approximately £1000 per participant). The next highest cost categories were prescriptions (approximately £580), followed by consultations (approximately £540) and outpatient appointments (approximately £480 per participant). There were no differences in the number of referrals to community care between the intervention group and the control group (see Table 63), although the participants from the intervention group were referred to more costly community rehabilitation programmes. Consequently, the mean referral costs for community care were higher for the intervention group (mean costs of £117 and £75, respectively), although this difference was not statistically significant. The breakdown of the costs of service use by quarters is shown in Table 66. There was no significant difference in the cost of health-care services between the intervention group and the control group for any category of primary or secondary care or any time period.
| Service | Intervention, mean (SD) (£) | Control, mean (SD) (£) | Difference in means (£) | 95% CI (£) |
|---|---|---|---|---|
| Primary care sector (intervention, n = 370; control, n = 276) | ||||
| Consultations | 539 (462) | 541 (428) | –2 | –70 to 66 |
| Prescriptions | 576 (873) | 585 (878) | –9 | –143 to 126 |
| Investigation | 56 (85) | 52 (92) | 4 | –10 to 17 |
| Referrals to community care | 117 (607) | 75 (383) | 42 | –34 to 118 |
| Secondary care sector (intervention, n = 383; control, n = 291) | ||||
| Outpatient | 484 (587) | 476 (750) | 8 | –96 to 113 |
| Inpatient | 1044 (2701) | 1000 (3021) | 45 | –395 to 484 |
| A&E | 61 (129) | 64 (166) | –3 | –26 to 20 |
| Service | Cost, mean (SD) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | |||||||
| 3 months | 6 months | 9 months | 12 months | 3 months | 6 months | 9 months | 12 months | |
| Primary care | ||||||||
| GP surgery | 108.63 (112.53) | 106.59 (106.68) | 112.71 (118.71) | 102.21 (128.85) | 105.07 (103.77) | 106.54 (110.80) | 112.85 (114.74) | 105.91 (104.44) |
| GP telephone | 7.72 (19.94) | 9.54 (23.86) | 9.79 (31.29) | 8.37 (23.44) | 7.39 (17.30) | 9.04 (24.68) | 8.52 (23.83) | 10.00 (27.19) |
| Nurse surgery | 10.03 (23.44) | 10.56 (22.54) | 11.52 (34.61) | 14.62 (35.11) | 15.12 (50.60) | 10.16 (20.22) | 13.28 (33.48) | 14.72 (36.16) |
| Nurse telephone | 0.61 (2.95) | 0.39 (2.69) | 0.44 (2.79) | 0.39 (1.96) | 0.78 (5.71) | 0.90 (7.41) | 0.78 (8.82) | 0.71 (5.01) |
| Health-care assistant | 1.32 (4.31) | 1.13 (4.05) | 1.46 (6.25) | 1.38 (4.42) | 1.59 (5.32) | 1.17 (4.85) | 1.43 (5.43) | 1.21 (4.18) |
| Other specialistsa | 3.79 (17.09) | 4.01 (19.56) | 6.78 (33.13) | 5.01 (32.83) | 3.89 (17.98) | 3.40 (15.56) | 3.83 (19.59) | 2.63 (11.66) |
| Investigations | 13.10 (34.53) | 13.15 (42.69) | 12.97 (36.34) | 16.67 (40.48) | 12.06 (37.94) | 13.05 (38.97) | 13.50 (38.52) | 13.74 (48.25) |
| Prescriptions | 153.53 (243.66) | 149.70 (231.06) | 162.78 (240.24) | 168.23 (254.68) | 159.25 (229.74) | 164.80 (240.45) | 174.82 (268.06) | 181.87 (251.09) |
| Referrals to community care | 37.03 (288.00) | 29.38 (253.48) | 13.26 (144.30) | 36.94 (285.43) | 15.12 (153.40) | 6.68 (31.20) | 28.18 (237.88) | 24.61 (235.31) |
| Secondary care | ||||||||
| Inpatient | 206.79 (1006.36) | 199.35 (935.18) | 320.43 (1193.87) | 318.12 (1523.05) | 244.97 (1584.35) | 331.96 (1663.91) | 213.61 (951.03) | 209.65 (1022.30) |
| Outpatient | 109.68 (178.62) | 123.71 (207.27) | 126.20 (206.14) | 124.44 (200.35) | 121.02 (255.63) | 119.62 (229.96) | 127.45 (255.25) | 107.51 (192.15) |
| A&E | 13.76 (49.62) | 16.37 (53.19) | 18.02 (53.98) | 12.68 (47.02) | 15.93 (56.15) | 19.54 (74.05) | 13.42 (42.21) | 15.11 (59.96) |
Missing data and multiple imputations
The complete health economics data set was obtained for 540 participants (intervention, n = 319; control, n = 221), 77% of all trial participants. Information about the completeness of the health economics data set is summarised in Table 67. The proportion of missing data varied from 3% to 24% for different data categories. The highest proportion of missing data was for baseline prescriptions, followed by the EQ-5D and primary care contacts.
| Service | Baseline, n (%) | 6-month follow-up, n (%) | 12-month follow-up, n (%) | |||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Consultations | NA | NA | 370 (92) | 276 (92) | 370 (92) | 276 (92) |
| Prescriptions | 332 (82) | 229 (76) | 350 (87) | 258 (86) | 350 (87) | 258 (86) |
| Investigations | NA | NA | 370 (92) | 276 (92) | 370 (92) | 276 (92) |
| Referrals | NA | NA | 370 (92) | 276 (92) | 370 (92) | 276 (92) |
| SUS data | NA | NA | 383 (95) | 291 (97) | 383 (95) | 291 (97) |
| EQ-5D | 402 (100) | 299 (100) | 360 (89) | 256 (85) | 354 (88) | 259 (86) |
The baseline characteristics of participants with complete and incomplete data are summarised in Table 68. This table demonstrates that participants with missing data had lower health-related quality of life and higher depression scores at baseline than participants with complete data. Among participants with complete data, those in the intervention group had a higher mean baseline EQ-5D score, whereas among participants with incomplete data those in the control group had a higher mean baseline EQ-5D score. Among participants with complete data, those in the intervention group had a lower mean baseline HADS depression score, whereas among participants with incomplete data those in the control group had a lower mean baseline HADS depression score. However, none of these reported differences was statistically significant.
| Parameter | Complete data (intervention n = 319, control n = 221) | Incomplete data (intervention n = 84, control n = 79) | ||
|---|---|---|---|---|
| Intervention, mean (SD) | Control, mean (SD) | Intervention, mean (SD) | Control, mean (SD) | |
| Baseline EQ-5D score | 0.4301 (0.3298) | 0.4014 (0.3321) | 0.3164 (0.3733) | 0.3617 (0.3546) |
| HADS depression score | 7.24 (4.05) | 7.31 (3.92) | 8.11 (4.49) | 8.02 (4.13) |
| Age (years) | 61 (13) | 61 (12) | 57 (14) | 54 (17) |
| Gender (%) | Male 33, female 67 | Male 30, female 70 | Male 32, female 68 | Male 41, female 59 |
| Site of recruitment (%) | London 41, Warwick 59 | London 39, Warwick 61 | London 61, Warwick 39 | London 63, Warwick 37% |
To address bias associated with missing data, MIs were conducted for 107 participants (intervention, n = 53; control, n = 54). MIs were conducted for primary and secondary care costs (12 months post randomisation), baseline prescriptions (3 months pre randomisation) and EQ-5D score (baseline and 6 and 12 months post randomisation). The 3-month pre-randomisation data were used for baseline adjustment in the sensitivity analyses. The complete baseline prescription data were obtained for 561 participants (intervention, n = 332; control, n = 229). MIs were conducted for 86 participants (intervention, n = 40; control, n = 46). The final number of participants in the imputed data set was 647 (intervention, n = 372; control, n = 275). This corresponds to 92% of the total number of participants in the trial and 99% of the trial population included in the statistical analyses of the primary outcome. The comparison of the imputed and complete data sets is shown in Table 69. Figure 18 shows the cost-effectiveness planes for the complete case and imputed data sets, generated using a non-parametric bootstrap.
| Parameter | Complete data set | Imputed data set | ||
|---|---|---|---|---|
| Intervention, mean (SD) | Control, mean (SD) | Intervention, mean (SD) | Control, mean (SD) | |
| Baseline prescription cost (£) | 154 (252) | 176 (253) | 148 (273) | 161 (257) |
| Baseline EQ-5D score | 0.4313 (0.3298) | 0.4014 (0.3321) | 0.4132 (0.3380) | 0.3970 (0.3381) |
| 6-month follow-up EQ-5D score | 0.4678 (0.3353) | 0.4096 (0.3461) | 0.4511 (0.3452) | 0.4102 (0.3542) |
| 12-month follow-up EQ-5D score | 0.4700 (0.3354) | 0.4491 (0.3423) | 0.4529 (0.3492) | 0.4445 (0.3551) |
| Primary care cost (£) | 1267 (1343) | 1317 (1232) | 1286 (1407) | 1285 (1271) |
| Secondary care cost (£) | 1453 (2545) | 1605 (3449) | 1614 (3126) | 1469 (3213) |
FIGURE 18.
Cost-effectiveness planes depicting 5000 bootstrapped estimates generated from complete case data and one imputed data set.
Health-related quality of life
Complete EQ-5D data (baseline and 6 and 12 months’ follow-up) were obtained for 298 participants in the intervention group and 205 participants in the control group. Health-related quality of life was low in the studied population compared with the UK national norms for the EQ-5D. 188 At baseline, the mean total EQ-5D scores were 0.41 for the intervention group and 0.40 for the control group. The difference between the two groups was not statistically significant (Table 70). Over the duration of the trial there was an increase in health-related quality of life in both groups. However, at 6 months the increase in the mean EQ-5D score in the intervention group was greater than that for the control group. Consequently, participants in the intervention group spent, on average, more time in a higher health state than participants in the control group. This resulted in higher mean total QALYs for the intervention group (0.44) than the control group (0.42) (see Table 70).
| Assessment | Intervention, mean (SD) | Control, mean (SD) | Difference in means | 95% CI |
|---|---|---|---|---|
| Non-imputed | ||||
| Baseline (intervention n = 371, control n = 274) | 0.4139 (0.3368) | 0.3976 (0.3376) | 0.0163 | –0.0364 to 0.0691 |
| 6-month follow-up (intervention n = 360, control n = 256) | 0.4572 (0.3391) | 0.4067 (0.3493) | 0.0505 | –0.0046 to 0.1057 |
| 12-month follow-up (intervention n = 354, control n = 259) | 0.4590 (0.3448) | 0.4506 (0.3459) | 0.0083 | –0.0471 to 0.0638 |
| Imputed (intervention n = 372, control n = 275) | ||||
| Baseline | 0.4132 (0.3380) | 0.3970 (0.3381) | 0.0162 | –0.0366 to 0.0690 |
| 6-month follow-up | 0.4511 (0.3452) | 0.4102 (0.3542) | 0.0409 | –0.0133 to 0.0952 |
| 12-month follow-up | 0.4529 (0.3492) | 0.4445 (0.3551) | 0.0084 | –0.0470 to 0.0638 |
| Total QALYs | 0.4421 (0.3058) | 0.4155 (0.3083) | 0.0266 | –0.0213 to 0.0745 |
Cost-effectiveness analysis
The results of the primary cost-effectiveness analysis for the imputed and complete case data sets are shown in Tables 71 and 72, respectively. The base-case cost-effectiveness analysis was conducted for the ITT population on the imputed data set using a multilevel mixed-effects model (see Table 71). The mean total cost was higher in the intervention group than in the control group (£2955 and £2767, respectively). The difference in mean costs was £188 (95% CI –£125 to £501). Total QALYs were higher in the intervention group (0.4475) than in the control group (0.4150). The difference in mean QALYs was 0.0325, which is equivalent to approximately 12 quality-adjusted days. This difference was not statistically significant because of the wide CIs (95% CI –0.0074 to 0.0724). The ICER point estimate was £5786 per QALY. The probability of the intervention being cost-effective at the NICE threshold of £30,000 per QALY was 87%. Figures 19 and 20 show the cost-effectiveness planes generated using bootstrapping of one imputed data set and the cost-effectiveness acceptability curve derived from five imputed data sets using a parametric approach to represent uncertainty, respectively (see the methods section). Sensitivity analyses conducted using the SUR model and GLM generated higher ICERs (£8995 and £9582, respectively), and lower probabilities of being cost-effective (80% and 79%, respectively) than the MLM (see Table 71).
| Group | Mean cost (95% CI) (£) | Mean QALYs (95% CI) | Difference in cost (95% CI) (£) | Difference in QALYs (95% CI) | ICER (£) | Probability of being cost-effective (%)a |
|---|---|---|---|---|---|---|
| Imputed MLM | ||||||
| Intervention | 2955 (2752 to 3159) | 0.4475 (0.4217 to 0.4733) | 188 (–125 to 501) | 0.0325 (–0.0074 to 0.0724) | 5786 | 87 |
| Control | 2767 (2539 to 2996) | 0.4150 (0.3844 to 0.4456) | ||||
| Imputed SUR | ||||||
| Intervention | 3041 (2847 to 3236) | 0.4417 (0.4157 to 0.4678) | 250 (–48 to 550) | 0.0279 (–0.0122 to 0.0680) | 8995 | 80 |
| Control | 2791 (2573 to 3008) | 0.4138 (0.3832 to 0.4446) | ||||
| Imputed GLM | ||||||
| Intervention | 3052 (2857 to 3247) | 0.4417 (0.4157 to 0.4678) | 267 (–32 to 566) | 0.0279 (–0.0122 to 0.0680) | 9582 | 79 |
| Control | 2785 (2568 to 3002) | 0.4139 (0.3832 to 0.4446) | ||||
| Imputed data set unadjusted | ||||||
| Intervention | 3045 (2657 to 3433) | 0.4421 (0.4109 to 0.4733) | 290 (–302 to 882) | 0.0266 (–0.0213 to 0.0745) | 10,891 | 72 |
| Control | 2755 (2308 to 3202) | 0.4155 (0.3789 to 0.4521) | ||||
| Group | Mean (SD) cost (£) | Mean (SD) QALYs | Difference in cost (95% CI) (£) | Difference in QALYs (95% CI) | ICER (£) | Probability of being cost-effective (%)a |
|---|---|---|---|---|---|---|
| Complete case MLM | ||||||
| Intervention | 2859 (771) | 0.4593 (0.2450) | –65 (–197 to 68) | 0.0418 (–0.0008 to 0.0844) | Dominant (–5160 to 6398) | 96 |
| Control | 2923 (772) | 0.4174 (0.2499) | ||||
| Complete case SUR | ||||||
| Intervention | 2865 (768) | 0.4593 (0.2452) | –57 (–189 to 74) | 0.0418 (–0.0003 to 0.0839) | Dominant (–5841 to 6367) | 97 |
| Control | 2923 (768) | 0.4174 (0.2497) | ||||
| Complete case GLM | ||||||
| Intervention | 2866 (768) | 0.4593 (0.2452) | –58 (–189 to 74) | 0.0418 (–0.0003 to 0.0839) | Dominant (–5841 to 6367) | 97 |
| Control | 2923 (768) | 0.4174 (0.2497) | ||||
| Complete case unadjusted | ||||||
| Intervention | 2866 (3290) | 0.4593 (0.2949) | –58 (–694 to 578) | 0.0418 (–0.0085 to 0.0922) | Dominant (–44,672 to 46,233) | 92 |
| Control | 2923 (3925) | 0.4174 (0.3010) | ||||
FIGURE 19.
Cost-effectiveness plane showing 5000 bootstrapped estimates generated from one imputed data set adjusted using the mixed-effects linear model.
FIGURE 20.
Cost-effectiveness acceptability curve estimated using the INB method from five imputed data sets adjusted using the mixed-effects linear model.
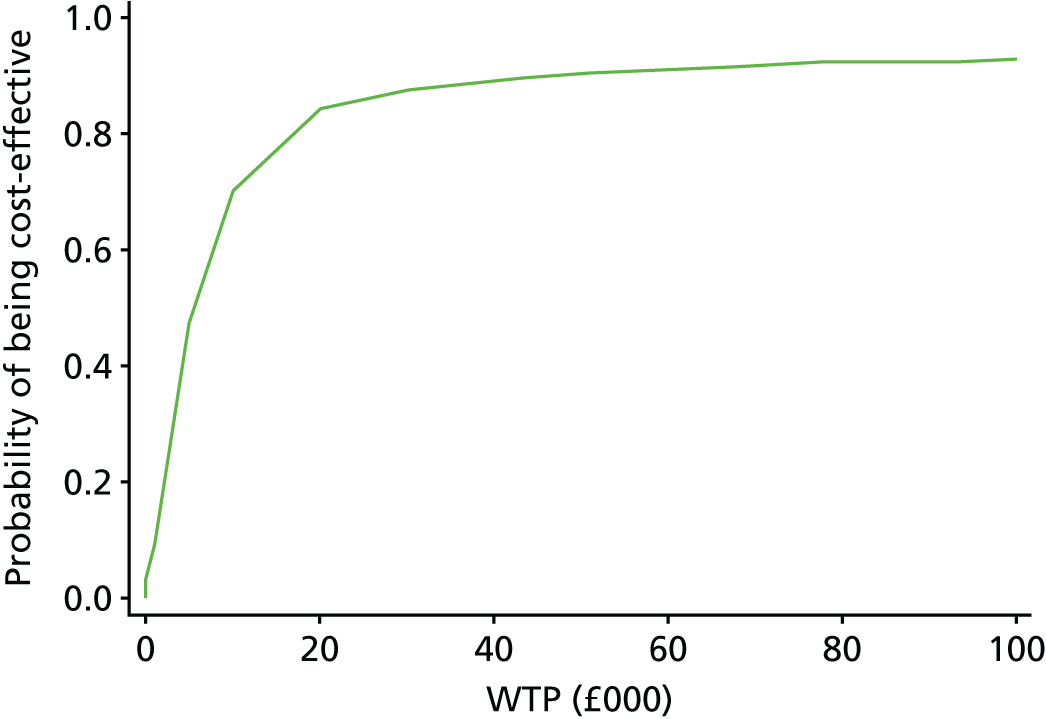
Complete case analyses were conducted for reference purposes only (see Table 72). The mean total costs for complete case were lower in the intervention group than in the control group; however, these differences were not statistically significant. The differences in mean QALYs between the intervention group and the control group were higher in the complete case analysis than in the imputed data analysis. The complete case analysis produced dominant ICERs as the intervention was less costly and more effective than the control. The probability of the intervention being cost-effective varied from 92% for unadjusted data to 97% for adjusted data. The results of the complete case analysis should be interpreted with caution because of potential bias associated with excluding non-responders from the analysis.
A secondary cost–utility analysis was conducted using the per-protocol population (Table 73). We excluded from the analysis 51 participants from the intervention group who, although randomised to the intervention group, did not receive any intervention. MIs were performed for this data set. Imputed data were analysed using the mixed-effects linear model. The difference in mean costs between the intervention group and the control group was £141, the difference in mean QALYs was 0.0351 and the ICER point estimate was £4033 per QALY. The probability of the intervention being cost-effective at £30,000 per QALY was 91%.
| Group | Mean cost (95% CI) (£) | Mean QALYs (95% CI) | Difference in cost (95% CI) (£) | Difference in QALYs (95% CI) | ICER (£) | Probability of being cost-effective (%)a |
|---|---|---|---|---|---|---|
| Per protocol | ||||||
| Intervention (n = 321) | 2887 (2814 to 2960) | 0.4523 (0.4254 to 0.4793) | 141 (30 to 253) | 0.0351 (–0.0048 to 0.0749) | 4033 | 91 |
| Control (n = 275) | 2745 (2661 to 2830) | 0.4173 (0.3878 to 0.4467) | ||||
| Compliance ≥ 12 sessions | ||||||
| Intervention (n = 295) | 2898 (2817 to 2980) | 0.4578 (0.4290 to 0.4867) | 143 (23 to 265) | 0.0412 (–0.0009 to 0.0833) | 3493 | 94 |
| Control (n = 275) | 2754 (2664 to 2845) | 0.4166 (0.3858 to 0.4474) | ||||
| Compliance < 12 sessions | ||||||
| Intervention (n = 77) | 3480 (3156 to 3804) | 0.4460 (0.3414 to 0.5106) | 729 (397 to 1063) | 0.0293 (–0.0402 to 0.0988) | 24,896 | 54 |
| Control (n = 275) | 2750 (2599 to 2901) | 0.4167 (0.3847 to 0.4486) | ||||
| Exposure 17 sessions (full) | ||||||
| Intervention (n = 275) | 2884 (2788 to 2981) | 0.4744 (0.4453 to 0.5035) | 141 (–2 to 284) | 0.0588 (0.0168 to 0.1008 | 2396 | 99 |
| Control (n = 275) | 2743 (2638 to 2849) | 0.4156 (0.3853 to 0.4460) | ||||
| Exposure ≤ 8 sessions (non-exposed) | ||||||
| Intervention (n = 74) | 3526 (3157 to 3896) | 0.4416 (0.3734 to 0.5099) | 767 (387 to 1147) | 0.0259 (–0.0466 to 0.0983) | 29,631 | 50 |
| Control (n = 275) | 2759 (2589 to 2930) | 0.4158 (0.3830 to 0.4485) | ||||
| Excluding high-cost participants (top 5%) | ||||||
| Intervention (n = 353) | 2293 (2217 to 2368) | 0.4560 (0.4295 to 0.4826) | 159 (42 to 277) | 0.0369 (–0.0414 to 0.0779) | 4326 | 92 |
| Control (n = 262) | 2133 (2042 to 2255) | 0.4191 (0.3876 to 0.4507) | ||||
Table 73 also summarises the results of the cost-effectiveness analyses for subgroups of participants with different levels of compliance and exposure to treatment. Analyses were conducted using the mixed-effects model. Between-group differences in cost were lower, and between-group differences in QALYs higher, among participants with high levels of compliance (≥ 12 sessions) than among those with low compliance (< 12 sessions). The ICER point estimate was £3493 for the high compliance subgroup and £24,896 for the low compliance subgroup. The probabilities of being cost-effective were 94% and 54%, respectively.
Similar analyses were conducted for subgroups with different levels of exposure to treatment (see Table 73). Participants who attended 17 sessions (full exposure) had a lower mean total cost and higher mean total QALYs than participants who attended eight or fewer sessions. A small number of participants attended 9–16 sessions (n = 23). Cost–utility analyses were not conducted because of the small size of this subgroup. The ICER point estimates were £2396 for participants with high exposure and £29,631 for those with an exposure of eight or fewer sessions. The probabilities of the intervention being cost-effective in these subgroups of participants were 99% and 50%, respectively.
We also conducted a subgroup analysis excluding the ‘high-cost’ participants. The top 5% of participants with total costs > £12,000 were excluded (intervention group, n = 19; control group, n = 13). Removing high-cost participants did not significantly affect the results of the cost-effectiveness analysis compared with the base case. The ICER was £4326 per QALY and the probability of the intervention being cost-effective was 92%.
In summary, our cost–utility analyses demonstrated that the intervention was more costly and more effective than the control. However, the differences in costs and QALYs were small and not statistically significant. The results of the analyses were robust to different analytical models. The mixed-effects linear model (accounting for clustering effects) produced better cost-effectiveness results than a SUR model and GLM. The results of the probabilistic analysis indicated that the intervention has a high probability (> 79%) of being cost-effective compared with usual care for patients with chronic pain. Subgroup analysis suggested that the intervention is more likely to be cost-effective in participants with a high compliance rate.
Chapter 12 Phase 2: evaluating the COPERS intervention – discussion
Summary and discussion of the principal findings
Our carefully designed evidence-informed chronic pain self-management intervention (COPERS) was relatively cheap to deliver at £111.28 per participant (excluding facilitator training costs), had a good uptake (336/403, 83%) with little attrition (85% of sessions attended) and was delivered as intended. The intervention had no impact on our primary outcome of pain-related disability at 12 months or on pain-related disability at 6 months. However, at 6 months’ follow-up the intervention led to improved psychological well-being compared with the control with regard to all of our psychological measures: anxiety, depression, chronic pain acceptance and pain-related self-efficacy. Across all of these outcomes the effect sizes were modest and their individual clinical importance is unclear. These beneficial effects appeared to be attenuated at 12 months except for depressive symptoms, which remained lower in the intervention group than in the control group. Social integration, as measured by the heiQ, was also significantly improved in the intervention group compared with the control group at both 6 and 12 months. Again, the effect size appeared to be modest but, as with depressive symptoms, there was no evidence of any attenuation at 12 months.
Pain intensity, as measured by the CPG pain intensity subscale, was not influenced by the intervention and nor was the overall response to the census global health question. The treatment group received significantly more analgesic medication and more weak opioids than the control group in the 12 months following the intervention but there was no difference in the prescription of strong opioids or psychotropic medication between the two groups. The intervention did not have any consistent pattern of effect on health service use: those in the intervention group had slightly few primary care and outpatient consultations and investigations; however, they had slightly more inpatient admissions. None of these differences approached conventional statistical significance. This resulted in an overall increase in base-case health-care costs in the intervention group of £188, that is, £76.72 more than the acquisition cost of the intervention. This does not support the notion that improved self-management reduces health-care costs. 283 These results proved robust in extensive sensitivity analyses and using different analytical approaches.
Both the intervention group and the control group improved between baseline and follow-up; the observation that there are improvements in both intervention and control groups over time is well recognised in intervention studies for chronic musculoskeletal pain. 210,211 This probably reflects the fluctuating course of chronic musculoskeletal pain, with people joining studies during periods of worsening symptoms. 210
We cannot exclude the possibility that, in this population, our control intervention may have had a beneficial effect or that those randomised to the control group consequentially sought additional care. However, the key component of our control intervention (relaxation) was selected because it had not been shown to be effective and The Pain Toolkit booklet is already a standard part of care. Our health service activity data do not support the notion that we have underestimated any treatment effects because of changed care-seeking behaviour. Any such effects are very unlikely to be of sufficient magnitude to change our overall conclusions.
There was a small gain in health utility of approximately 0.033 QALYs (12 quality-adjusted days) in the intervention group compared with the control group in our study. By way of comparison, the national evaluation of the Expert Patients Programme estimated that the programme generated 0.02 QALYs (approximately 7 quality-adjusted days) compared with usual care. 15 An evaluation of the Improving Access to Psychological Therapies (IAPT) demonstration sites, which compared the IAPT programme with usual care, found that the IAPT programme was associated with a gain of 0.013–0.014 QALYs (approximately 5 quality-adjusted days). 284 Thus, the QALY gain from the COPERS intervention is at least as good as that of comparable interventions already in common use.
Our cost–utility analyses demonstrated that the COPERS intervention is more costly and more effective than usual care. Our base-case analysis generated an ICER of £5786 per QALY with an 87% probability of the intervention being cost-effective at a threshold of £30,000 per QALY (see Table 71). The probability of the intervention being cost-effective was higher in the per-protocol population (91%) and in subgroups with high rates of compliance and exposure to the intervention (92–99%; see Table 73). This demonstrates that the COPERS programme is highly likely to be cost-effective compared with current care for people living with chronic musculoskeletal pain, with a cost per QALY that falls well within the usual NICE threshold range of £20,000–30,000 per QALY. Although the health gain observed in the COPERS study is typical of that of many patient self-management programmes, it is an extremely cost-effective intervention. 15,285–288
The findings of a long-term effect on depression are striking. Our exploratory post hoc analyses examined this further. Nearly half of our participants [322/703 (46%)] met criteria for possible depression at baseline. There was a clinically important sustained improvement in depressive symptoms at 12 months among these participants. The SMD in this group (–0.50, 95% CI –0.74 to –0.25) was of a similar size to that reported in Cochrane reviews of exercise for depression (–0.62, 95% CI –0.81 to –0.42)289 and tricyclic antidepressants in primary care (–0.49, 95% CI –0.67 to –0.32). 290 Our observed effect size exceeds that found in an individual patient data meta-analysis of selective serotonin reuptake inhibitors for mild/moderate depression (0.11, 95% CI −0.18 to 0.41) or severe depression (0.17, 95% CI −0.08 to 0.43). 291 Other reviews have found similar modest effect sizes from selective serotonin reuptake inhibitors; for example, Kirsch et al. 292 quote 2004 NICE guidance defining a SMD of 0.50 or a drug/placebo difference of 3 points on the scale as a threshold for clinical significance. However, it should be noted that studies focusing primarily on depression usually adopt different outcome measures from the one used in this study, (typically the 21-item Hamilton Rating Scale for Depression,293 which is completed by the researcher following a clinical interview and observation of the patient, or the 21-item participant-completed BDI177).
The commonly intractable nature of chronic pain was evident by the duration of pain experienced by COPERS participants. Our participants were predominantly older (mean age 60 years, range 19–94 years) women (67%). Our study population had a high level of morbidity and disability at baseline: EQ-5D scores were low compared with UK national norms188 and below those reported in studies of patients with other serious chronic conditions. 294 Participants’ mean HADS depression and anxiety symptom scores were high compared with norms for the general UK population [3.68 (SD 3.07) and 6.14 (SD 3.76), respectively281]. Compared with clinical populations with life-threatening illnesses, mean HADS depression and anxiety scores were similar to those found in patients with non-operable lung cancer [7.22 (SD 5.16) and 7.20 (SD 5.25), respectively] but better than those found in patients with end-stage chronic obstructive pulmonary disease [10.18 (SD 3.95) and 11.44 (SD 4.76), respectively]. 295 In total, 46% of the COPERS study population met criteria for possible depression on the HADS and nearly half of these (22% of the overall study population) were above the higher HADS threshold for probable depression; the equivalent UK norm figures are 7.8% and 2.9%, respectively. 281
General practice consultation rates (including doctor, nurse and any other health professional face-to-face or telephone contact in general practice) among the COPERS study population were very high, with the intervention and control group participants having a mean (SD) of 12.32 (11.2) and 12.80 (12.50) consultations in the 12-month follow-up period, respectively. This compares with 2008 data showing an average of around three general practice consultations per year for registered male patients aged 45–64 years and an average of four consultations per year for similarly aged women. 296 That one-quarter of our participants were using strong opioids gives some measure of the difficulties faced in managing their pain. This is a particularly striking observation because the COPERS study was run in localities with low opioid use compared with the rest of England; all participating primary care trust’s opioid prescribing rates were below regional and national median rates (Knaggs R, University of Nottingham, personal communication).
None of our prespecified moderator analyses achieved, or even approached, conventional statistical significance. This is to be expected in a trial powered on main effects rather than on interactions. Indeed, for a study of this nature in which there was no main effect, identifying a subgroup in which there was a large positive effect would inevitably mean commensurate harm in the rump of the trial population. It might be that these middle groups are worth targeting as key variables are more susceptible to meaningful change.
As we achieved a positive long-term effect on depression, the effect of the intervention in just those with depression at baseline is of greater interest post hoc than it was a priori. One would not expect a substantial improvement in depressive symptoms in those who were not depressed at baseline. It is reassuring that our post hoc analyses confirmed this and that we have not harmed those who were not depressed (see Table 59). The apparent effect size in the depressed group and the p-value for interaction are impressive. However, one cannot apply these findings in practice. There remains the possibility that this apparently statistically significant interaction may be no more than a chance finding because of multiple comparisons. Furthermore, it would not be possible to implement the COPERS intervention ‘as is’ because half of the participants were not depressed. The group dynamic might be radically different if only those with depression at baseline joined the group and the whole ethos of the intervention would change to a treatment for depression, in which case there are established psychological treatments for depression. Implementing the COPERS intervention just to help those with depression and including the whole population of those living with chronic pain, many of whom would not themselves gain any benefit, might be questionable.
Results in context
Once the COPERS analysis was completed we updated our original systematic review for the effectiveness of self-management interventions for chronic musculoskeletal pain. We reran our original searches to identify all studies published up to September 2013. When appropriate, we extracted outcome data and added these to our original meta-analyses. Finally, we added in the COPERS trial results (Table 74).
| Outcome | Follow-up (months) | Review January 1994–April 2009 | Review January 1994–September 2013 | |||
|---|---|---|---|---|---|---|
| Total n participants (number of studies) | Effect size (95% CI) | Total n participants (number of studies) | Effect size (95% CI) | Including COPERS results | ||
| Pain intensity | 4–8 | 3911 (20) | –0.25 (–0.38 to –0.12) | 6038 (32)a | –0.29 (–0.38 to 0.20) | –0.28 (–0.37 to 0.19) |
| > 8 | 3332 (18) | –0.18 (–0.28 to 0.07) | 5104 (25)a | –0.18 (–0.26 to 0.10) | –0.17 (–0.25 to 0.10) | |
| Physical function | < 4 | 2453 (19) | –0.26 (–0.40 to 0.12) | 4093 (26) | –0.31 (–0.44 to 0.18) | |
| 4–8 | 3759 (18) | –0.15 (–0.23 to 0.07) | 5546 (28)a | –0.19 (–0.25 to 0.13) | –0.18 (–0.23 to 0.12) | |
| > 8 | 2482 (13) | –0.12 (–0.20 to 0.04) | 3980 (19)a | –0.14 (–0.22 to 0.06) | –0.13 (–0.21 to 0.05) | |
| Quality of life | 4–8 | 399 (2) | –0.11 (–1.05 to 0.82) | 665 (4) | –0.14 (–0.55 to 0.27) | –0.13 (–0.40 to 0.15) |
| > 8 | 170 (1) | –0.50 (–0.80 to 0.19) | 170 (1) | –0.50 (–0.80 to 0.19) | –0.24 (–0.70 to 0.21) | |
| Self-efficacy | 4–8 | 1214 (7) | –0.29 (–0.44 to 0.14) | 2030 (10) | –0.25 (–0.34 to 0.17) | –0.24 (–0.32 to 0.16) |
| > 8 | 1701 (7) | –0.25 (–0.35 to 0.15) | 2173 (8) | –0.23 (–0.31 to 0.14) | –0.20 (–0.28 to 0.13) | |
| Depression | 4–8 | 597 (4) | –0.25 (–0.47 to 0.03) | 1899 (12)a | –0.26 (–0.38 to 0.13) | –0.24 (–0.35 to 0.13) |
| > 8 | 641 (3) | –0.04 (–0.26 to 0.18) | 1516 (7)a | –0.20 (–0.44 to 0.03) | –0.20 (–0.38 to 0.01) | |
| Anxiety | 4–8 | 451 (3) | –0.28 (–0.56 to 0.00) | 878 (6) | –0.14 (–0.31 to 0.03) | –0.16 (–0.28 to 0.04) |
| > 8 | 50 (1) | –0.28 (–0.84 to 0.27) | 553 (3) | –0.41 (–0.58 to 0.24) | –0.28 (–0.51 to 0.06) | |
| Social function | 4–8 | 286 (4) | –0.19 (–0.61 to 0.22) | 931 (8)a | –0.24 (–0.40 to 0.09) | –0.22 (–0.34 to 0.11) |
| > 8 | 205 (2) | 0.19 (–0.09 to 0.47) | 922 (6)a | –0.11 (–0.26 to 0.05) | –0.15 (–0.27 to 0.02) | |
Overall, despite our efforts to develop and test a more effective intervention, the effect sizes found with the COPERS intervention across a range of domains were broadly similar to those found in previous research. That in a large well-conducted study with a low risk of bias we found similar results to those found in multiple smaller studies lends credence to the notion that these are ‘true’ estimates of effect sizes.
Strengths of the study
A key strength of this study is that its pragmatic design means that our results directly relate to the real-world setting. 297 Another of its principal strengths lies in the robustness of the results. The study was adequately powered and recruited to target and attrition of follow-up data was very low. The statistical analysis plan was written and published before data were unblinded or any analysis was undertaken. We used MI to reduce bias, conducted extensive sensitivity analyses and adopted different analytical approaches to test the robustness of our statistical and economic analyses.
The study was designed and conducted to minimise bias: strict allocation concealment was maintained and usual health-care providers were aware that participants had joined the study but were not informed of their allocation arm. The intervention was delivered by trained facilitators who in the main were completely uninvolved in the collection or evaluation of study data; however, throughout the delivery of the intervention experienced members of the study team codelivered 10 of the 31 courses with new inexperienced facilitators. All outcome data collected by telephone were collected by researchers unaware of the allocation arm of the participants and a script was used asking respondents not to reveal their allocated treatment. Primary care patient record data were extracted by trained personnel blind to the allocation arm of participants. All questionnaire data, secondary care service use data and primary care record data were entered, checked and cleaned before any unblinding. All primary outcome data were double entered and checked and a further 10% random selection of other data were double entered and checked; accuracy was high, aided by sophisticated database construction limiting errors. Study participants were ethnically diverse (indeed our participants were more ethnically diverse than the UK overall) and lived in localities ranging from those characterised as very affluent to those characterised as very deprived. Our feasibility study indicated that the intervention could be successfully delivered in languages other than English and was acceptable to a non-English-speaking ethnic group. The study successfully identified the right patients – those significantly disabled by their chronic pain – as evidenced by their high levels of disability and morbidity at baseline. This population also consumed a considerable amount of health-care resources, including strong opioids. Thus, this is a highly appropriate group in which to study complex, non-pharmacological interventions such as the COPERS intervention, directed at improving health outcomes and potentially reducing health-care resource use.
The intervention tested was evidence informed and underpinned by behaviour change theory – the strengths in the design and nature of the intervention are described in the discussion to Chapter 8. In addition, the COPERS intervention was relatively cheap and, if successful, could plausibly be implemented within the NHS. Both the feasibility study and the main trail suggest that it was highly acceptable to participants.
The fidelity of intervention delivery in trials of complex interventions has not been routinely considered until recently14 but its importance has recently been underlined by the WISE (Whole System Informing Self-management Engagement) study, in which the failure of a complex, whole-system self-management support intervention may have arisen from a failure to implement the intervention as intended. 298 Our study included a detailed and comprehensive assessment of the fidelity of intervention delivery223 (see Chapter 10) and so we can be confident that the intervention tested here was delivered as intended. We intended the COPERS groups to include around 14 participants and we achieved this with the exception of just one course.
We were able to show a sustained effect of the intervention on our key target mediator, self-efficacy, indicating that the intervention worked as intended. That these changes failed to result in the desired effect on our primary outcome suggests that this is not, on its own, a sufficient change to result in changes in pain-related disability, but there may be other important unmeasured mediators that we were unable to investigate.
Limitations of the study
Our choice of primary outcome might be considered a limitation. Although we had identified pain-related disability as determined by the CPG as one of our preferred potential outcome measures (see Chapter 5), we did not use this as the primary outcome in the feasibility study (see Chapter 7). In designing the study we wished to move beyond the existing evidence that consistently showed benefits for intermediate outcomes such as self-efficacy without showing improvements in clinically relevant outcomes. In our development work we considered different candidate measures for our clinically relevant primary outcome. The mixture of disorders included in the trial meant that a generic outcome measure was needed. We had originally considered using the EQ-5D. In the feasibility study, however, it was clear that this was not sufficiently responsive to change from baseline for it to be a suitable outcome. For this reason we chose a measure with a narrower focus on pain-related disability (the main trial data confirmed that the EQ-5D was unresponsive to the intervention). Our choice of which pain-related disability outcome measure to use was constrained by the necessity of choosing an instrument suitable for pain at different musculoskeletal sites. The CPD pain-related disability subscale had been used with positive results in other studies of chronic pain populations, suggesting that it was an appropriate choice and sensitive to change. 53,83 Indeed, as anticipated from previous studies,208,210 CPG scores fell by more than one-third of a SD between baseline and 6 months (and then remained at similar levels at 12 months) in both the intervention arm and the control arm, suggesting that it is sensitive to change.
Another potential limitation of our study might have been our inclusion of a relaxation CD and leaflet along with usual care in the control arm. Our pilot study had demonstrated that recruiting people to trials in which one arm is usual care might be difficult and other researchers have described the possibility of ‘resentful demoralisation’, when patients with a strong preference for one arm of a study are assigned to the other arm. 299 To maximise recruitment and, perhaps, reduce the risk of resentful demoralisation we chose to add a very simple relaxation package to our control intervention (this package was also given to treatment arm patients at the end of the COPERS course). We chose the relaxation package because our systematic review (see Chapter 2) had suggested that, although relaxation was popular, it was unlikely to have an effect on our primary outcome of pain-related disability. A 2006 systematic review found that progressive muscle relaxation (the technique provided in our control arm) reduced chronic pain but in the two studies that reported longer-term follow-up the effect on pain was not sustained at 3 or 6 months. 300 However, one of the most important effects of our intervention was on the secondary outcome of depression and there is systematic review evidence that relaxation may improve participant-reported depression. 301 Moreover, there is a suggestion from the 12-month questionnaire that control arm patients might have practised regular relaxation more frequently than intervention arm patients, although the data are difficult to interpret because of poor response rates to the questions. (The questions were at the end of the questionnaire and we assumed that most missing responses meant that people did not practise any relaxation.) Thus, there is the possibility that the control arm intervention diminished the apparent effect of our intervention on depression and, by extension, potentially on our other secondary psychological outcomes. It might be argued in favour of our choice of control that, even if the relaxation CD had some efficacy, we would want to be able to demonstrate that our intervention was superior to such a simple (and very slightly effective) intervention. Control and intervention arm participants also received a copy of The Pain ToolKit booklet but we have already explained that we consider this to be good usual care (see Chapter 9).
The ethnic mix of our participants was not nationally representative (COPERS trial: 80% white, 13% black, 5% Asian; England and Wales: 86% white, 3.4% black, 7.5% Asian302). Unusually, we achieved an over-representation of minority ethnic groups compared with national norms. This reflects our decision to recruit in a very ethnically diverse locality. Nevertheless, our recruited population might still not have been representative of the ethnic mix of the communities from which we were recruiting. The overall uptake of the offer to join the study by patients recruited from primary care was low and differed between east London (6.7%) and the Midlands (10.8%), leading to concerns about the generalisability of the study and the feasibility of implementing the intervention (if it were successful). However, because of the nature of our electronic search many of the people identified for invitation from primary care records may not have been eligible. Even if invitees were eligible, it is not clear how inviting participants to participate in a research project, in which they are not guaranteed to receive an intervention and have to initiate contact with researchers themselves (data protection necessitated that they were invited by their GP, not the research team), relates to offering patients an intervention outside a research setting as part of usual care. Uptake (when reported) is generally low in studies of self-management. Overall, the low uptake seen in our study suggests that, although we can be confident that our results apply to the group included, we are less confident about extrapolating these results to the wider chronic pain population. These uptake rates are, however, comparable with those in other studies recruiting from primary care. 53,56,83 That the clinical course of low back pain is similar in RCTs and cohort studies provides some reassurance that the results from a trial of this nature do reflect real life. 210 The high levels of depressive symptoms seen in the COPERS study population are interesting in this context because there is evidence from other chronic conditions that depressed patients are less likely to participate in group self-management support and more likely to drop out. 303 If this is also true for chronic pain it suggests that levels of depressive symptoms could be even higher in eligible patients who did not participate in our study.
In common with other studies of self-management14 the majority of participants in our study were female (67%). A 2014 Cochrane review of self-management in OA included 29 studies and reported that overall 68% of participants were female13 [our chronic pain patients were younger (mean age 60 years) than those in the OA review (mean age 65 years)]. It is not clear why women are more attracted to self-management interventions than men although, in general, more women than men report chronic pain. In a previous study we found that the relative risk of women having chronic widespread pain was 1.3 (95% CI 1.2 to 1.4). 59
We originally wanted to identify subgroups who were most likely to benefit from a pain self-management intervention. In our systematic review (see Chapter 3) we failed to identify any groups who might benefit more than others and so we directed the intervention at all adult chronic musculoskeletal pain patients who met our inclusion criteria. Prespecified subgroup analyses (see Chapter 11) failed to identify any group in whom our primary outcome was significantly improved (although there was a suggestion that those with less intense pain and a shorter duration of pain and those with neither high nor low self-efficacy at baseline might benefit the most) but these analyses lacked statistical power. Thus, the issue of subgrouping remains unaddressed.
Strengths and limitations in relation to other studies
Measuring a primary outcome at 12 months’ follow-up is a strength of our study; most other studies of self-management in general, and of chronic pain self-management in particular, consider shorter follow-up periods. 13,14 Including self-efficacy among our choice of outcome measures is another strength. Although we regard increasing self-efficacy as a process outcome, it has been advocated as an important outcome that often goes unmeasured in studies of chronic pain self-management. 13
Our intervention was successful compared with other self-management support interventions. A recent RCT of the Arthritis Self-Management Programme in Australia, which also aimed to look at a real-world setting, was terminated because of a lack of enthusiasm from potential referrers and patients. 297 In contrast to our study, which principally recruited patients with very longstanding musculoskeletal pain from primary care, the Australian study recruited patients with knee and hip OA from secondary care, including private hospitals.
The results of the economic evaluation demonstrate that the COPERS self-management course is a relatively inexpensive intervention, with an average acquisition cost of approximately £145 per person, including staff training (see Chapter 11). This is a little over half of the cost of a year’s supply of duloxetine at a dose of 60 mg per day (£268.80),304 which is recommended for use for either fibromyalgia or knee OA. 305,306 It also compares favourably to the cost of the Expert Patients Programme, which is estimated to cost £250 per patient. 15 The estimated cost per participant for the COPERS course is also much lower than the average cost of £599 per patient for the IAPT programme283 (another study estimated that the cost of the IAPT programme per patient varied from £493 for low-intensity therapy to £1416 for high-intensity therapy307). Several factors suggest that the cost per person would be lower should the COPERS intervention become widely available. The first factor relates to training: although the training of facilitators in the trial was conducted by university staff, wider adoption of the intervention would be likely to reduce costs through using experienced facilitators in a training role. The second factor relates to venue costs: the COPERS team had to hire premises but courses could be delivered in NHS/primary care settings if the intervention were more widely adopted. Therefore, our cost estimate of £145 per participant per course may be regarded as conservative.
The COPERS intervention is not a true CBT intervention. However, it was never our intention to deliver CBT as such; we intended only to deliver an intervention informed by some of the principles of a cognitive–behavioural approach.
Meaning of the study: possible mechanisms and implications for clinicians or policy-makers
Our original conceptual model, adapted to include our revised primary outcome (Figure 21), suggested that we would influence a variety of outcomes. In fact, we influenced only the psychological outcomes and social integration and support, and a sustained effect at 12 months was seen only for depression and social integration and support. In other words, our psychologically based group intervention had marked psychological effects that were concentrated in those who were depressed at baseline, but did not appear to affect health-care resource use or disability. We are not in a position to say with certainty what the active elements of the intervention were for certain but it seems likely that these were the psychologically orientated components and the effect of being in a group of peers.
FIGURE 21.
The COPERS contextual model.
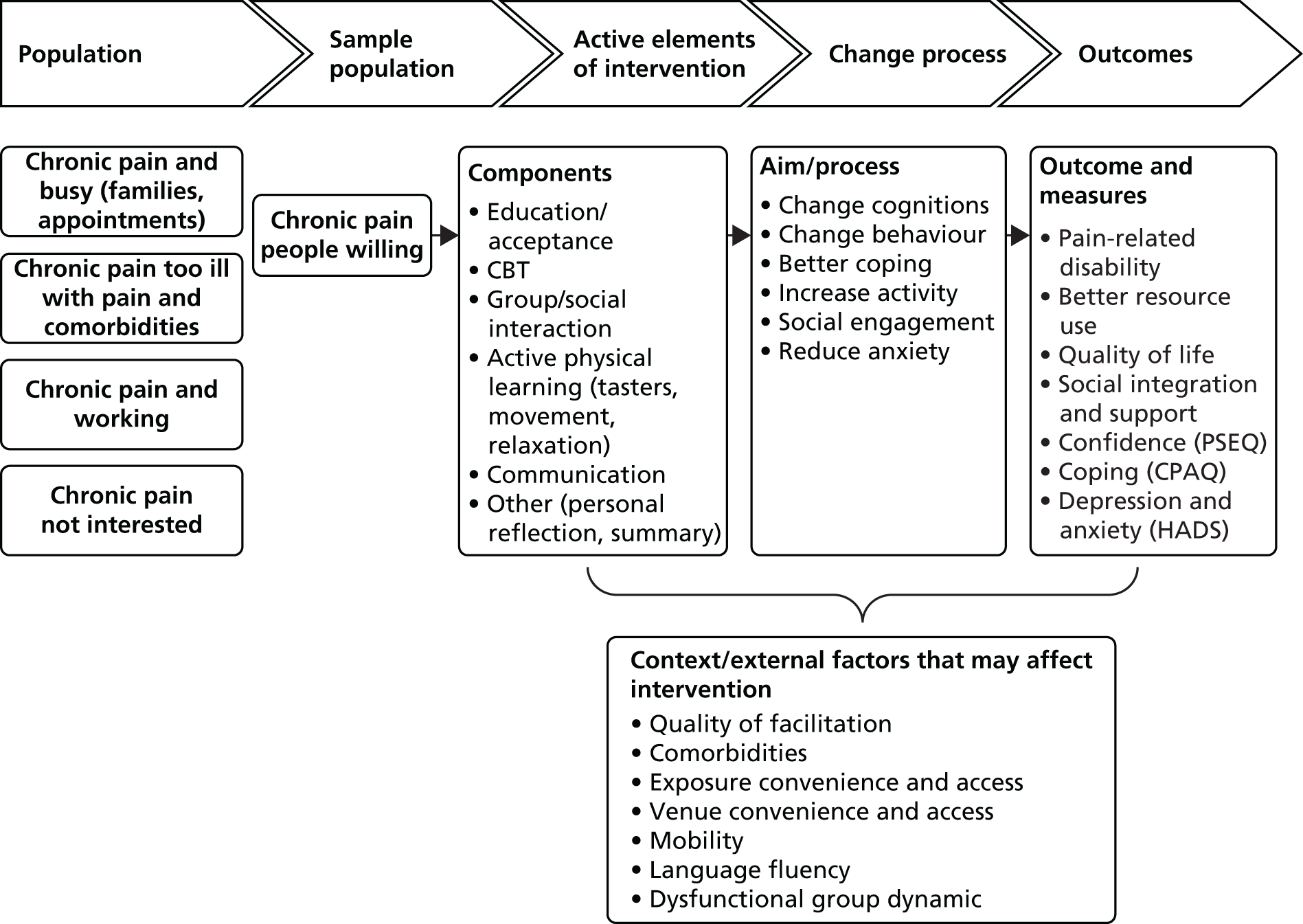
Implications
Overall, for patients with chronic pain, this rigorous, well-designed and well-conducted study suggests that we now know the limitations of self-management support interventions for chronic musculoskeletal pain.
First, the study demonstrates that research studies of these interventions appeal to a limited number of a particular type of chronic pain patient: those who are older, those who are likely to be retired or not working and predominantly female patients. Even if they are not representative of chronic pain patients as a whole, there may be merits in targeting this group as we also know that these patients are likely to be significantly disabled by their pain and have high levels of anxiety and depression, may well be on strong opioids, will be taking a lot of prescribed medications, attend their GP very frequently and have a long history of pain. Moreover, the feasibility study for the COPERS intervention showed that the intervention can actually be applied to a lot of other groups, for example the Sylheti-speaking group in Tower Hamlets could complete the intervention with the Bengali DVD, even though it was not feasible to include them in the main trial because of difficulties with collecting outcome data.
Second, the outcomes of these interventions appear to be modest and predominantly psychological. Such interventions do not appear to have a marked, if any, effect on health-care resource use, at least within the first 12 months. However, there is a strong suggestion in our post hoc analyses that this type of intervention may be very effective in the subgroup of chronic pain patients with depression with no deleterious effects on those who were not depressed at baseline.
Although some commissioners may interpret the modest effects of the COPERS intervention as justification not to fund this type of self-management support for chronic pain patients, the COPERS intervention is feasible and safe, can be delivered to large numbers of people, is evidence based and leads to levels of improvement that are at least as good as those seen with other similar interventions and meets NICE criteria for cost–utility. We suggest that the COPERS intervention could be a substitute for other less well-evidenced and more expensive chronic pain management programmes. Its potential role in the management of depression in chronic pain patients merits further research.
Research recommendations
Our work suggests that there is a need to address the following research questions (presented in order of importance).
Research questions related to chronic musculoskeletal pain
-
What can be done to improve the disability experienced by patients with chronic musculoskeletal pain?
-
Does the COPERS intervention have a role in the management of chronic pain patients with mild to moderate depression? Is it more effective and acceptable than alternative non-pharmacological treatments for depression in this population? Our post hoc analyses strongly suggest that this is an effective treatment for depression in this population. Nevertheless, this is based on a mixed clientele joining the groups. As the group dynamic may be different if only people with depression join the groups, it cannot be promoted as an intervention for this population. A new trial is needed to target this population and focus on depression. It may be appropriate to use a depression outcome that is more responsive to change than the HADS.
-
Could self-management interventions delivered before chronic musculoskeletal pain has become very longstanding be more effective than those delivered after the individual has experienced years of chronic pain? Would they be acceptable to patients? Would they have the potential to alter the disease trajectory and long-term outcomes?
Research questions related to methodology
-
Should complex interventions have single outcome measures? Work is needed to explore the interpretation of the results of trials of complex interventions in which multiple outcomes are reported. In common with many complex interventions, the COPERS intervention had multiple facets that might affect different outcomes in different ways. The traditional model whereby paramount importance is given to the primary outcome is well established. In this model any apparently important benefits in secondary outcomes are no more than hypothesis generating. A further full trial with these as the primary outcome would be needed to confirm the findings. This approach leads to a hazard that important beneficial effects, in our case a reduction in depressive symptoms, might be discarded as a chance finding when, in fact, it may be an important positive result. Research in this area may consider how such findings should be considered within the trial and also when data pooling.
-
Are large RCTs the best way to evaluate novel, complex, non-pharmacological interventions? There is a large opportunity cost in developing and testing interventions of this nature. The risk of harm from such interventions is small and a negative trial is unlikely to dissuade others from implementing similar intervention packages. Once we put the results of the COPERS trial into context they made little difference to the estimates of effects of such interventions on a wide range of outcomes and so our study has, arguably, not added to the evidence base commensurately with its size. Although a positive trial would support implementation it would strictly support the implementation only of the package tested. In reality, it is variants of the proven intervention that are delivered. For example, few physiotherapy departments offer more than six sessions for people with low back pain, whereas NICE guidance,129 based on the available evidence, suggests up to 12 sessions. This is in contrast to trials of pharmacological interventions in which fully understanding the benefits and harms of a particular preparation is critical. It may be worthwhile for the research community to explore whether or not other approaches might generate ‘good enough’ data to inform policy and practice across a wider range of topic areas for the same research cost.
Acknowledgements
This project was funded by the NIHR under its Programme Grants for Applied Research scheme (RP-PG-0707–10189). It also benefited from facilities funded through the Birmingham Science City Translational Medicine Clinical Research and Infrastructure Trials Platform, with support from Advantage West Midlands.
Other acknowledgements
Other researchers involved in the project
-
Naheedah Choudhury (project administrator).
-
Maria Ramirez (trial co-ordinator, Warwick).
-
Clare Miles (systematic reviewer).
-
David Ellard (senior research fellow) contributed to the design of the fidelity methods and fidelity assessment.
-
Anna Volkmann (researcher) conducted the English qualitative interviews for Chapter 4.
-
Yasmin Choudhury (researcher) conducted the Sylheti qualitative interviews for Chapter 4.
-
Geoff Harding (medical sociologist) contributed to the analysis of the qualitative data for Chapter 4.
Clinical Trials Units
-
Queen Mary University of London PCTU for the support given for the feasibility study and feasibility randomisation and for data management for the main trial and the quality audit: Stephen Bremner, Sandy Smith, Mike Waring and Amy Hoon.
-
Warwick Medical School Clinical Trials Unit: Sarah Duggan, Clare Daffern and Cheryl Ritchie.
-
Sheffield University Clinical Trials Unit for the use of the online randomisation service.
Clinical research networks
-
North and East London: Local Clinical Research Network.
-
London Primary Care Research Network: Jo Burns and Selina Gann.
-
North Central London Research Consortium: Lynis Lewis.
-
Warwick and Coventry: Local Clinical Research Network.
-
Warwick and Coventry Primary Care Research Network: Sue Ellwell.
Coapplicants
Coapplicants who have contributed to various aspects of the study: Jayne Gallagher (pain anaesthetist, Barts Health NHS Trust), Sally Hearne (primary care trust commissioner), Elizabeth Bayliss (Social Action for Health, Tower Hamlets) and Qasim Aziz (gastroenterologist, Queen Mary University of London).
Oversight committees
-
Trial Steering Committee: Mike Hurley (chairperson), Nadine Foster, Bart Koes, Lance McCracken, Jim Reece and Obi Ukoumunne.
-
Data Monitoring and Ethics Committee: Gene Feder (chairperson), Blair Smith and Rebecca Turner.
Patient advisors
We would like to thank our four patient advisors for their invaluable contribution to the project.
Secondary care user services data
-
North East London Commissioning Support Unit for the London secondary care user service data.
-
Arden Commissioning Support Unit for the Warwick and Coventry secondary care user service data.
Recruiting clinics
-
Dylan Morrisey and colleagues at Mile End Hospital Physiotherapy Department for recruiting patients for the feasibility study.
-
Angie Alamgir and colleagues at the Persistent Pain Service, Mile End Hospital, for involvement in both the feasibility study and the main trial.
-
Ingrid Bergson and colleagues at City and Hackney Locomotor Service.
-
Susie Crome from Social Action for Health for help with the recruitment facilitators.
General practices in London
Blithehale Health Centre, Chrisp Street Health Centre, Dr Gadhvi-Fountayne Road Health Centre, Elsdale Street Surgery, Elm-Fountayne Road Health Centre, Island Health, St Stephens Health Centre, Statham Grove Surgery, Shah Jalal Surgery, the Wapping Group Practice, Tredegar Health Centre and the Nightingale Practice.
General practices in Warwick and Coventry
Atherstone Surgery, Chancery Lane Surgery, Clarendon Lodge Medical Practice, Croft Medical Centre, Dr Chaudhuri Surgery, Manor Court Surgery, Old Mill Surgery, Rother House Medical Centre, Sherbourne Medical Centre, Sky Blue Medical Group, Stockingford Medical Centre and the Forum Health Centre.
Facilitators in London
Mohammad Anwar, Julie Atkins, Ingrid Bergson, Jonathan Field, Christine Hunter, Alex Irving, Zahida Kahn, Jonathan Liptich, Paul Montague, Samina Rashid, Sam Shakes and Angela Usher.
Facilitators in Warwick and Coventry
Malcolm Benny, Sarah Bridgewater, Sally Brown, Susanne Finnegan, Karen Keats, Jill Nussbaum, Cheryl Ritchie and Yvonne Rolston.
Participants
We would like to thank those who participated in the interviews and focus groups for the qualitative study and the outcome measures discussion group; those who participated in the feasibility study, including those at Whitechapel Health Centre who supported the recruitment of participants for the feasibility study for Sylheti-speaking participants; and the 703 study participants who took part in the main trial and gave their time to complete the questionnaires.
Film production
Chris Payne.
Report production
Antoinette Connelly.
Contributions of authors
Stephanie JC Taylor (Professor of Primary Care and Public Health, applicant, principal investigator) was primarily responsible for developing the proposal for funding and had overall responsibility for the conduct of the programme of work. She made major contributions to all aspects of the programme. She oversaw drafting of the final report and has reviewed it for crucial intellectual content.
Dawn Carnes (Senior Research Fellow, health services research, coapplicant) made substantial contributions to the design, organisation and conduct of the programme of work and was also responsible for collecting, analysing and interpreting data. She had primary responsibility for drafting of the final report and critiquing it for crucial intellectual content.
Kate Homer (Research Fellow and Systematic Reviewer, health services research) contributed substantially to the conduct of the study including the analysis and interpretation of data at each stage of the programme of work. She made a substantial contribution to the drafting of the report and critiqued it for crucial intellectual content.
Tamar Pincus (Professor in Psychology, coapplicant) contributed substantially to the design, conduct, analysis and interpretation of the moderator, mediator and predictor systematic review and the review that helped determine the outcome measures, contributed to the development of the intervention, drafted Chapters 3 and 5 and critiqued the whole report for crucial intellectual content.
Brennan C Kahan (Senior Statistician) made substantial contributions to the design of the statistical analysis plan, carried out the clinical effectiveness analyses and contributed to the interpretation of the findings. He helped draft the methods and results chapters for the main trial (see Chapters 9 and 11, respectively) and commented on these chapters, critiquing them for crucial intellectual content.
Natalia Hounsome (Senior Health Economist) made a substantial contribution to the design of the health economic analysis plan, carried out the cost-effectiveness analyses and contributed to the interpretation of the findings. She drafted the relevant text for the health economics methods, results and conclusions sections, critiquing them for crucial intellectual content.
Sandra Eldridge (Professor of Biostatistics, coapplicant) made a substantial contribution to the development of the intervention and the design, the statistical analysis and interpretation of the data and critiqued the whole report for intellectual content.
Anne Spencer (Professor of Health Economics, coapplicant) made a substantial contribution to the design of the health economic analysis plan, oversaw the cost-effectiveness analyses and contributed to the interpretation of the findings. She helped draft the relevant text for the health economics methods, results and conclusions sections, critiquing them for crucial intellectual content.
Karla Diaz-Ordaz (Statistician) made significant contributions to the design of the statistical analysis plan, carried out the case analyses and contributed to the interpretation of findings and helped draft the methods and results chapters (see Chapters 9 and 11, respectively) and commented on these chapters, critiquing them for crucial intellectual content.
Anisur Rahman (Professor of Rheumatology, coapplicant) contributed to the overall study design and made a major contribution to the conduct of the feasibility study, contributed to the development of the intervention and conduct of the main trial and critiqued the final report for crucial intellectual content.
Tom S Mars (Research Assistant, health services research) contributed substantially to the delivery of the intervention and the design of the methodology for the assessment and analyses of fidelity and drafted Chapter 10.
Jens Foell (General Practitioner) helped develop and tested the search strategy for identifying primary care chronic pain patients and helped with health economic data collection and extraction.
Chris J Griffiths (Professor of Primary Care, coapplicant) contributed to the development of the proposal and critiqued the report for intellectual content.
Martin R Underwood (Director, Warwick Clinical Trials Unit, coprincipal investigator) was the second principal investigator responsible for the Midlands recruiting site. He had major input into developing the proposal for funding and had overall responsibility for the conduct of the programme of work. He made substantial contribution to all aspects of the programme. He had major involvement in the drafting of the final report and has reviewed it for crucial intellectual content.
Publications
Miles CL, Pincus T, Carnes D, Homer KE, Taylor SJC, Bremner SA, et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of subgroup analysis within RCTs. Eur J Pain 2011;15:775e1–11.
Miles C, Pincus T, Carnes D, Underwood M, Taylor SJC. Measuring pain self-efficacy. Clin J Pain 2011;27:461–70.
Pincus T, Miles C, Froud R, Underwood M, Carnes D, Taylor S. Methodological criteria for the assessment of moderators in systematic reviews of randomised controlled trials: a consensus study. BMC Med Res Methodol 2011;11:14.
Carnes D, Homer KE, Miles CL, Pincus T, Underwood M, Rahman A, et al. Effective delivery styles and content for self-management interventions for chronic musculoskeletal pain: a systematic literature review. Clin J Pain 2012;28:344–54.
Carnes D, Homer K, Underwood M, Pincus T, Rahman A, Taylor SJC. Pain management for chronic musculoskeletal conditions: the development of an evidence based and theory informed pain self-management course. BMJ Open 2013;3:e003534.
Carnes D, Underwood M, Homer K, Bremner S, Eldridge S, Pincus T, et al. Effectiveness and cost-effectiveness of a novel, group self-management course for adults with chronic musculoskeletal pain: study protocol for a multi-centre, randomised controlled trial (COPERS). BMJ Open 2013;3:e002492.
Foell J, Carnes D, Homer K, Taylor SJC. Developing and implementing electronic search strategies to recruit patients with chronic musculoskeletal pain in primary care databases. Prim Health Care Res Dev 2013;15:234–43.
Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor SJ. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3:e003555.
Kahan BC, Diaz-Ordaz K, Homer K, Carnes D, Underwood M, Taylor SJC, et al. Coping with persistent pain, effectiveness research into self-management (COPERS): statistical analysis plan for a randomised controlled trial. Trials 2014;15:59.
Taylor SJC, Carnes D, Homer K, Kahan B, Eldridge S, Hounsome N, et al. Novel three-day community-based, non-pharmacological, group intervention for chronic musculoskeletal pain (COPERS): a randomized clinical trial. PLOS Med 2016;13:e1002040.
Data sharing statement
Please apply to our Data Access Committee via Dr Arouna Woukeu, Clinical Trials Information Systems (CTIS) manager at the PCTU.
Contact information:
Dr Arouna Woukeu
CTIS manager
PCTU
c/o Centre for Primary Care and Public Health
58 Turner Street
London
E1 2AB.
Telephone: + 44 (0)20 7882 3449
Facsimile: + 44 (0)20 7882 2552
Email: pctu-enquiries@qmul.ac.uk or a.woukeu@qmul.ac.uk
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Lim SS, Vos T, Flaxman AD, Danaei D, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of the burden of disease and injury to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2010;380:2224-60. http://dx.doi.org/10.1016/S0140-6736(12)61766-8.
- Bridges S. Health Survey for England 2011. Volume 1: Health, Social Care and Lifestyles. Chapter 9: Chronic Pain 2011. https://catalogue.ic.nhs.uk/publications/public-health/surveys/heal-surv-eng-2011/HSE2011-All-Chapters.pdf (accessed 10 December 2013).
- Self Care – a Real Choice. Self Care Support – a Practical Option. London: Department of Health, Stationery Office; 2005.
- Wanless D. Securing Our Future Health: Taking a Long-Term View. Final Report 2002. www.hm-treasury.gov.uk/Consultations_and_Legislation/wanless/consult_wanless_final.cfm (accessed 1 January 2008).
- Donaldson L. Expert patients usher in a new era of opportunity for the NHS. BMJ 2003;326:1279-80. http://dx.doi.org/10.1136/bmj.326.7402.1279.
- The Expert Patient: A New Approach to Chronic Disease Management for the 21st Century. London: Department of Health, Stationery Office; 2001.
- Griffiths C, Foster G, Ramsay J, Eldridge S, Taylor S. How effective are expert patient (lay led) education programmes for chronic disease?. BMJ 2007;334:1254-6. http://dx.doi.org/10.1136/bmj.39227.698785.47.
- Department of Health . Speech on the National Health Service, 7 January 2008 n.d. http://webarchive.nationalarchives.gov.uk/20100406111100/http://www.number10.gov.uk/Page14171 (accessed 10 December 2013).
- Lorig K, Feigenbaum P, Regan C, Ung E, Chastain RL, Holman HR. A comparison of lay-taught and professional-taught arthritis self-management courses. J Rheumatol 1986;13:763-7.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. http://dx.doi.org/10.1037/0033-295X.84.2.191.
- Expert Patients Programme n.d. www.expertpatients.co.uk/ (accessed 10 December 2013).
- The NHS Outcomes Framework 2014/15. London: Department of Health; 2013.
- Kroon FBP, van der Burg LRA, Buchbinder R, Osborne RH, Johnston RV, Pitt V. Self-management education programmes for osteoarthritis. Cochrane Database Syst Rev 2014;1. http://dx.doi.org/10.1002/14651858.cd008963.pub2.
- Foster G, Taylor SJC, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007;4. http://dx.doi.org/10.1002/14651858.cd005108.pub2.
- Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardsone G, et al. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254-61. http://dx.doi.org/10.1136/jech.2006.053538.
- Squire S, Hill P. The Expert Patients Programme. Clin Gov 2006;11:17-21. http://dx.doi.org/10.1108/14777270610646985.
- International Association for the Study of Pain . Pain terms: a current list with definitions and notes on usage. Pain 1986;24:215-21. http://dx.doi.org/10.1016/0304-3959(86)90113-2.
- NHS Networks . 2011–12 Programme Budgeting Data n.d. www.networks.nhs.uk/nhs-networks/health-investment-network/news/2011–12-programme-budgeting-data-now-available (accessed 10 December 2013).
- Croft P, Rigby AS. Socioeconomic influences of back problems in the community in Britain. J Epidemiol Community Health 1994;48:166-70. http://dx.doi.org/10.1136/jech.48.2.166.
- Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27:E109-20. http://dx.doi.org/10.1097/00007632-200203010-00017.
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 2006;15:S192-300. http://dx.doi.org/10.1007/s00586-006-1072-1.
- Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum 2008;59:632-41. http://dx.doi.org/10.1002/art.23563.
- Main CJ, Wood PL, Hollis S, Spanswick CC, Waddell G. The distress and risk assessment method. A simple patient classification to identify distress and evaluate the risk of poor outcome. Spine (Phila Pa 1976) 1992;17:42-5. http://dx.doi.org/10.1097/00007632-199201000-00007.
- Linton SJ, McCracken LM, Vlaeyen JW. Reassurance: help or hinder in the treatment of pain. Pain 2008;134:5-8. http://dx.doi.org/10.1016/j.pain.2007.10.002.
- Lucock MP, Morley S, White C, Peake MD. Responses of consecutive patients to reassurance after gastroscopy: results of self administered questionnaire survey. BMJ 1997;315:572-5. http://dx.doi.org/10.1136/bmj.315.7108.572.
- Salmon P, Ring A, Dowrick CF, Humphris GM. What do general practice patients want when they present medically unexplained symptoms, and why do their doctors feel pressurized?. J Psychosom Res 2005;59:255-60. http://dx.doi.org/10.1016/j.jpsychores.2005.03.004.
- Pincus T, Holt N, Vogel S, Underwood M, Savage R, Walsh DA, et al. Cognitive and affective reassurance and patient outcomes in primary care: a systematic review. Pain 2013;154:2407-16. http://dx.doi.org/10.1016/j.pain.2013.07.019.
- Developing and Evaluating Complex Interventions: New Guidance. London: MRC; 2008.
- Nuñez DE, Keller C, Ananian CD. A review of the efficacy of the self-management model on health outcomes in community-residing older adults with arthritis. Worldviews Evid Based Nurs 2009;6:130-49. http://dx.doi.org/10.1111/j.1741-6787.2009.00157.x.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. http://dx.doi.org/10.1016/j.pain.2004.09.012.
- Pincus T, Santos R, Breen A, Burton AK, Underwood M. A review and proposal for a core set of factors for prospective cohorts in low back pain: a consensus statement. Arthritis Rheum 2008;59:14-2. http://dx.doi.org/10.1002/art.23251.
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877-83. http://dx.doi.org/10.1001/archpsyc.59.10.877.
- Hayden JA, Chou R, Hogg-Johnson S, Bombardier C. Systematic reviews of low back pain prognosis had variable methods and results: guidance for future prognosis reviews. J Clin Epidemiol 2009;62:781-96. http://dx.doi.org/10.1016/j.jclinepi.2008.09.004.
- Pincus T, Vogel S, Burton AK, Santos R, Field AP. Fear avoidance and prognosis in back pain: a systematic review and synthesis of current evidence. Arthritis Rheum 2006;54:3999-4010. http://dx.doi.org/10.1002/art.22273.
- Patel S, Friede T, Froud R, Evans DW, Underwood M. Systematic review of randomised controlled trials of clinical prediction rules for physical therapy in low back pain. Spine (Phila Pa 1976) 2013;38:762-9. http://dx.doi.org/10.1097/BRS.0b013e31827b158f.
- Mistry D, Patel S, Hee SW, Stallard N, Underwood M. Evaluating the quality of subgroup analyses in randomized controlled trials of therapist delivered interventions for non-specific low back pain: a systematic review. Spine (Phila Pa 1976) 2014;39:618-29. http://dx.doi.org/10.1097/BRS.0000000000000231.
- Aguinis H, Beaty JC, Boik RJ, Pierce CA. Effect size and power in assessing moderating effects of categorical variables using multiple regression: a 30-year review. J Appl Psychol 2005;90:94-107. http://dx.doi.org/10.1037/0021-9010.90.1.94.
- Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychol Methods 2004;9:426-45. http://dx.doi.org/10.1037/1082-989X.9.4.426.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions v 5.1.0. The Cochrane Collaboration; 2011.
- Adams K, Greiner AC, Corrigan JM. The 1st Annual Crossing the Quality Chasm Summit – a Focus on Communities. Washington, DC: National Academic Press; 2004.
- Bernardy K, Fuber N, Klose P, Hauser W. Efficacy of hypnosis/guided imagery in fibromyalgia syndrome – a systematic review and meta-analysis of controlled trials. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-133.
- Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol 2007;26:1-9. http://dx.doi.org/10.1037/0278-6133.26.1.1.
- Savigny P, Watson P, Underwood M. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ 2009;338.
- Engers A, Jellema P, Wensing M, van der Windt D, Grol R, van Tulder MW. Individual patient education for low back pain. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd004057.pub3.
- Haines T, Gross A, Goldsmith CH, Perry L, Haines T, Gross A, et al. Patient education for neck pain with or without radiculopathy. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.cd005106.pub3.
- Cramer H, Haller H, Lauche R, Dobos G. Mindfulness-based stress reduction for low back pain. A systematic review. BMC Complement Altern Med 2012;12. http://dx.doi.org/10.1186/1472-6882-12-162.
- Hadhazy VA, Ezzo J, Creamer P, Berman BM, Hadhazy VA, Ezzo J, et al. Mind–body therapies for the treatment of fibromyalgia. A systematic review. J Rheumatol 2000;27:2911-18.
- Ostelo RW, van Tulder MW, Vlaeyen JWS, Linton SJ, Morley SJ, Assendelft WJJ. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev 2005;1. http://dx.doi.org/10.1002/14651858.cd002014.pub2.
- Kennedy A, Gately C, Rogers A. Process Evaluation of the EPP–Report II: Examination of the Implementation of the Expert Patients Programme within the Structures and Locality Contexts of the NHS in England. Manchester: University of Manchester, National Primary Care Research and Development Centre; 2005.
- Day MA, Thorn BE, Kapoor S. A qualitative analysis of a randomized controlled trial comparing a cognitive–behavioral treatment with education. Pain 2011;12:941-52. http://dx.doi.org/10.1016/j.jpain.2011.02.354.
- Jenkinson CM, Doherty M, Avery AJ, Read A, Taylor MA, Sach TH, et al. Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3170.
- McBeth J, Prescott G, Scotland G, Lovell K, Keeley P, Hannaford P, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Intern Med 2012;172:48-57. http://dx.doi.org/10.1001/archinternmed.2011.555.
- Lamb E, Hansen Z, Lall R, Castelnuovo E, Withers J, Nichols V, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. Lancet 2010;375:916-23. http://dx.doi.org/10.1016/S0140-6736(09)62164-4.
- Johnson RE, Jones GT, Wiles NJ, Chaddock C, Potter RG, Roberts C, et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine (Phila Pa 1976) 2007;32:1578-85. http://dx.doi.org/10.1097/BRS.0b013e318074f890.
- Buszewicz M, Rait G, Griffin M, Nazareth I, Patel A, Atkinson A, et al. Self management of arthritis in primary care: randomised controlled trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38965.375718.80.
- Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al. Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12220.
- Maetzel A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res Clin Rheumatol 2002;16:23-30. http://dx.doi.org/10.1053/berh.2001.0204.
- Palmer KT, Walsh K, Bendall H, Cooper C, Coggon D. Back pain in Britain: comparison of two prevalence surveys at an interval of 10 years. BMJ 2000;320:1577-8. http://dx.doi.org/10.1136/bmj.320.7249.1577.
- Carnes D, Parsons S, Ashby D, Breen A, Foster NE, Pincus T, et al. Chronic musculoskeletal pain rarely presents in a single body site: results from a UK population study. Rheumatology 2007;46:1168-70. http://dx.doi.org/10.1093/rheumatology/kem118.
- Foster NE, Pincus T, Underwood MR, Vogel S, Breen A, Harding G. Understanding the process of care for musculoskeletal conditions – why a biomedical approach is inadequate. Rheumatology 2003;42:401-4.
- Oliveira VC, Ferreira PH, Maher CG, Pinto RZ, Refshauge KM, Ferreira ML. Effectiveness of self-management of low back pain: systematic review with meta-analysis. Arthritis Care Res 2012;64:1739-48. http://dx.doi.org/10.1002/acr.21737.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988.
- Cohen J. A power primer. Psychol Bull 1992;112:155-9. http://dx.doi.org/10.1037/0033-2909.112.1.155.
- Alp A, Kanat E, Yurtkuran M. Efficacy of a self-management program for osteoporotic subjects. Am J Phys Med Rehabil 2007;86:633-40. http://dx.doi.org/10.1097/PHM.0b013e31806dd428.
- Tavafian SS, Jamshidi A, Mohammad K, Montazeri A. Low back pain education and short term quality of life: a randomized trial. BMC Musculoskelet Disord 2007;8. http://dx.doi.org/10.1186/1471-2474-8-21.
- Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Patel A, Williamson E, et al. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: a cluster randomized trial. Arthritis Care Res 2007;57:1211-19. http://dx.doi.org/10.1002/art.22995.
- Corey DT, Koepfler LE, Etlin D, Day HI. A limited functional restoration program for injured workers: a randomized trial. J Occup Rehabil 1996;6:239-50. http://dx.doi.org/10.1007/BF02110886.
- Vlaeyen JW, Teeken-Gruben NJ, Goossens ME, Rutten-van Molken MP, Pelt RA, van Eek H, et al. Cognitive-educational treatment of fibromyalgia: a randomized clinical trial. I. Clinical effects. J Rheumatol 1996;23:1237-45.
- Williams AC, Richardson PH, Nicholas MK, Pither CE, Harding VR, Ridout KL, et al. Inpatient vs. outpatient pain management: results of a randomised controlled trial. Pain 1996;66:13-22. http://dx.doi.org/10.1016/0304-3959(96)02996-X.
- Basler H, Jakle C, Kroner-Herwig B. Incorporation of cognitive–behavioral treatment into the medical care of chronic low back patients: a controlled randomized study in German pain treatment centers. Patient Educ Couns 1997;31:113-25. http://dx.doi.org/10.1016/S0738-3991(97)00996-8.
- Fries JF, Carey C, McShane DJ. Patient education in arthritis: randomized controlled trial of a mail-delivered program. J Rheumatol 1997;24:1378-83.
- Keller S, Ehrhardt-Schmelzer S, Herda C, Schmid S, Basler HD. Multidisciplinary rehabilitation for chronic back pain in an outpatient setting: a controlled randomized trial. Eur J Pain 1997;1:279-92. http://dx.doi.org/10.1016/S1090-3801(97)90037-9.
- Mazzuca SA, Brandt KD, Katz BP, Chambers M, Byrd D, Hanna M. Effects of self-care education on the health status of inner-city patients with osteoarthritis of the knee. Arthritis Rheum 1997;40:1466-74. http://dx.doi.org/10.1002/art.1780400815.
- Haldorsen EM, Kronholm K, Skouen JS, Ursin H. Multimodal cognitive behavioral treatment of patients sicklisted for musculoskeletal pain: a randomized controlled study. Scand J Rheumatol 1998;27:16-25. http://dx.doi.org/10.1080/030097498441128.
- LeFort SM, Gray-Donald K, Rowat KM, Jeans ME. Randomized controlled trial of a community-based psychoeducation program for the self-management of chronic pain. Pain 1998;74:297-306. http://dx.doi.org/10.1016/S0304-3959(97)00190-5.
- Von Korff M, Moore JE, Lorig K, Cherkin DC, Saunders K, González VM, et al. A randomized trial of a lay person-led self-management group intervention for back pain patients in primary care. Spine (Phila Pa 1976) 1998;23:2608-15. http://dx.doi.org/10.1097/00007632-199812010-00016.
- Glomsrod B, Lonn JH, Soukup MG, Bo K, Larsen S. Active back school: prophylactic management for low back pain: three-year follow-up of a randomized, controlled trial. J Rehabil Med 2001;33:26-30. http://dx.doi.org/10.1080/165019701300006506.
- Lonn JH, Glomsrod B, Soukup MG, Bo K, Larsen S. Active back school: prophylactic management for low back pain. A randomized, controlled, 1-year follow-up study. Spine (Phila Pa 1976) 1999;24:865-71. http://dx.doi.org/10.1097/00007632-199905010-00006.
- Currie SR, Wilson KG, Pontefract AJ, de Laplante L. Cognitive–behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol 2000;68:407-16. http://dx.doi.org/10.1037/0022-006X.68.3.407.
- Hopman-Rock M, Westhoff MH. The effects of a health educational and exercise program for older adults with osteoarthritis for the hip or knee. J Rheumatol 2000;27:1947-54.
- Mannerkorpi K, Nyberg B, Ahlmen M, Ekdahl C. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. J Rheumatol 2000;27:2473-81.
- Moore JE, Von Korff M, Cherkin D, Saunders K, Lorig K. A randomized trial of a cognitive–behavioral program for enhancing back pain self care in a primary care setting. Pain 2000;88:145-53. http://dx.doi.org/10.1016/S0304-3959(00)00314-6.
- Haugli L, Steen E, Laerum E, Nygard R, Finset A. Learning to have less pain – is it possible? A one-year follow-up study of the effects of a personal construct group learning programme on patients with chronic musculoskeletal pain. Patient Educ Couns 2001;45:111-18. http://dx.doi.org/10.1016/S0738-3991(00)00200-7.
- Oliver K, Cronan TA, Walen HR, Tomita M. Effects of social support and education on health care costs for patients with fibromyalgia. J Rheumatol 2001;28:2711-20.
- Dworkin SF, Huggins KH, Wilson L, Mancl L, Turner J, Massoth D, et al. A randomized clinical trial using research diagnostic criteria for temporomandibular disorders-axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain 2002;16:48-64.
- King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. J Rheumatol 2002;29:2620-7.
- Quilty B, Tucker M, Campbell R, Dieppe P. Physiotherapy, including quadriceps exercises and patellar taping, for knee osteoarthritis with predominant patello-femoral joint involvement: randomized controlled trial. J Rheumatol 2003;30:1311-17.
- Buhrman M, Faltenhag S, Strom L, Andersson G. Controlled trial of internet-based treatment with telephone support for chronic back pain. Pain 2004;111:368-77. http://dx.doi.org/10.1016/j.pain.2004.07.021.
- Cedraschi C, Desmeules J, Rapiti E, Baumgartner E, Cohen P, Finckh A, et al. Fibromyalgia: a randomised, controlled trial of a treatment programme based on self management. Ann Rheum Dis 2004;63:290-6. http://dx.doi.org/10.1136/ard.2002.004945.
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist 2004;44:217-28. http://dx.doi.org/10.1093/geront/44.2.217.
- Mazzuca SA, Brandt KD, Katz BP, Ragozzino LR, G’Sell PM. Can a nurse-directed intervention reduce the exposure of patients with knee osteoarthritis to nonsteroidal antiinflammatory drugs?. J Clin Rheumatol 2004;10:315-22. http://dx.doi.org/10.1097/01.rhu.0000147050.45377.df.
- Haas M, Groupp E, Muench J, Kraemer D, Brummel-Smith K, Sharma R, et al. Chronic disease self-management program for low back pain in the elderly. J Manipulative Physiol Ther 2005;28:228-37. http://dx.doi.org/10.1016/j.jmpt.2005.03.010.
- Heuts PH, de Bie R, Drietelaar M, Aretz K, Hopman-Rock M, Bastiaenen CH, et al. Self-management in osteoarthritis of hip or knee: a randomized clinical trial in a primary healthcare setting. J Rheumatol 2005;32:543-9.
- Pariser D, O’Hanlon A. Effects of telephone intervention on arthritis self-efficacy, depression, pain, and fatigue in older adults with arthritis. J Geriatr Phys Ther 2005;28:67-73. http://dx.doi.org/10.1519/00139143-200512000-00002.
- Tak E, Staats P, Van Hespen A, Hopman-Rock M. The effects of an exercise program for older adults with osteoarthritis of the hip. J Rheumatol 2005;32:1106-14.
- Victor CR, Triggs E, Ross F, Lord J, Axford JS. Lack of benefit of a primary care-based nurse-led education programme for people with osteoarthritis of the knee. Clin Rheumatol 2005;24:358-64. http://dx.doi.org/10.1007/s10067-004-1001-9.
- Bernaards CM, Ariëns GA, Hildebrandt VH. The (cost-)effectiveness of a lifestyle physical activity intervention in addition to a work style intervention on the recovery from neck and upper limb symptoms in computer workers. BMC Musculoskelet Disord 2006;7. http://dx.doi.org/10.1186/1471-2474-7-80.
- Brattberg G. Internet-based rehabilitation for individuals with chronic pain and burnout: a randomized trial. Int J Rehabil Res 2006;29:221-8. http://dx.doi.org/10.1097/01.mrr.0000210055.17291.f5.
- Li EJ, Li-Tsang CW, Lam CS, Hui KY, Chan CC. The effect of a ‘training on work readiness’ program for workers with musculoskeletal injuries: a randomized control trial (RCT) study. J Occup Rehab 2006;16:529-41. http://dx.doi.org/10.1007/s10926-006-9034-3.
- Núñez M, Núñez E, Segur JM, Macule F, Quinto L, Hernandez MV, et al. The effect of an educational program to improve health-related quality of life in patients with osteoarthritis on waiting list for total knee replacement: a randomized study. Osteoarthritis Cartilage 2006;14:279-85. http://dx.doi.org/10.1016/j.joca.2005.10.002.
- Smeets RJ, Vlaeyen JW, Hidding A, Kester AD, van der Heijden GJ, van Geel AC, et al. Active rehabilitation for chronic low back pain: cognitive-behavioral, physical, or both? First direct post-treatment results from a randomized controlled trial. BMC Musculoskelet Disord 2006;7. http://dx.doi.org/10.1186/1471-2474-7-5.
- Hurley MV, Walsh NE, Mitchell H, Nicholas J, Patel A. Long-term outcomes and costs of an integrated rehabilitation program for chronic knee pain: a pragmatic, cluster randomized, controlled trial. Arthritis Care Res 2012;64:238-47. http://dx.doi.org/10.1002/acr.20642.
- Martire LM, Schulz R, Keefe FJ, Rudy TE, Starz TW. Couple-oriented education and support intervention: effects on individuals with osteoarthritis and their spouses. Rehabil Psychol 2007;52:121-33. http://dx.doi.org/10.1037/0090-5550.52.2.121.
- Yip YB, Sit JW, Fung KKY, Wong DYS, Chong SYC, Chung LH, et al. Impact of an arthritis self-management programme with an added exercise component for osteoarthritic knee sufferers on improving pain, functional outcomes, and use of health care services: an experimental study. Patient Educ Couns 2007;65:113-21. http://dx.doi.org/10.1016/j.pec.2006.06.019.
- Ersek M, Turner JA, Cain KC, Kemp CA. Results of a randomized controlled trial to examine the efficacy of a chronic pain self-management group for older adults. Pain 2008;138:29-40. http://dx.doi.org/10.1016/j.pain.2007.11.003.
- Laforest S, Nour K, Gignac M, Gauvin L, Parisien M, Poirier MC. Short-term effects of a self-management intervention on health status of housebound older adults with arthritis. J Appl Gerontol 2008;27:539-67. http://dx.doi.org/10.1177/0733464808319712.
- Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Care Res 2008;59:1009-17. http://dx.doi.org/10.1002/art.23817.
- Ribeiro LH, Jennings F, Jones A, Furtado R, Natour J. Effectiveness of a back school program in low back pain. Clin Exp Rheumatol 2008;26:81-8.
- van der Hulst M, Vollenbroek-Hutten MM, Groothuis-Oudshoorn KGM, Hermens HJ. Multidisciplinary rehabilitation treatment of patients with chronic low back pain: a prognostic model for its outcome. Clin J Pain 2008;24:421-31. http://dx.doi.org/10.1097/AJP.0b013e31816719f5.
- Yip YB, Sit JW, Wong DY, Chong SY, Chung LH. A 1-year follow-up of an experimental study of a self-management arthritis programme with an added exercise component of clients with osteoarthritis of the knee. Psychol Health Med 2008;13:402-14. http://dx.doi.org/10.1080/13548500701584030.
- Crotty M, Prendergast J, Battersby MW, Rowett D, Graves SE, Leach G, et al. Self-management and peer support among people with arthritis on a hospital joint replacement waiting list: a randomised controlled trial. Osteoarthritis Cartilage 2009;17:1428-33. http://dx.doi.org/10.1016/j.joca.2009.05.010.
- Kroenke K, Bair J, Damush M, Wu J, Hoke S, Sutherland J, et al. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA 2009;301:2099-110. http://dx.doi.org/10.1001/jama.2009.723.
- Chiauzzi E, Pujol LA, Wood M, Bond K, Black R, Yiu E, et al. PainACTION-Back Pain: a self-management website for people with chronic back pain. Pain Med 2010;11:1044-58. http://dx.doi.org/10.1111/j.1526-4637.2010.00879.x.
- Glombiewski JA, Hartwich-Tersek J, Rief W. Two psychological interventions are effective in severely disabled, chronic back pain patients: a randomised controlled trial. Int J Behav Med 2010;17:97-107. http://dx.doi.org/10.1007/s12529-009-9070-4.
- Hamnes B, Mowinckel P, Kjeken I, Hagen KB. Effects of a one week multidisciplinary inpatient self-management programme for patients with fibromyalgia: a randomised controlled trial. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-189.
- Hansson EE, Jonsson-Lundgren M, Ronnheden A-M, Sorensson E, Bjarnung A, Dahlberg LE. Effect of an education programme for patients with osteoarthritis in primary care – a randomized controlled trial. BMC Musculoskelet Disord 2010;11. http://dx.doi.org/10.1186/1471-2474-11-244.
- Hsu MC, Schubiner H, Lumley MA, Stracks JS, Clauw DJ, Williams DA. Sustained pain reduction through affective self-awareness in fibromyalgia: a randomized controlled trial. J Gen Intern Med 2010;25:1064-70. http://dx.doi.org/10.1007/s11606-010-1418-6.
- Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DJ. Internet-enhanced management of fibromyalgia: a randomized controlled trial. Pain 2010;151:694-702. http://dx.doi.org/10.1016/j.pain.2010.08.034.
- Luciano JV, Martinez N, Penarrubia-Maria MT, Fernandez-Vergel R, Garcia-Campayo J, Verduras C, et al. Effectiveness of a psychoeducational treatment program implemented in general practice for fibromyalgia patients: a randomized controlled trial. Clin J Pain 2011;27:383-91. http://dx.doi.org/10.1097/AJP.0b013e31820b131c.
- Morone G, Paolucci T, Vulpiani MC, Matano A, Bureca I, Paolucci S, et al. Quality of life improved by multidisciplinary back school program in patients with chronic non-specific low back pain: a single blind randomized controlled trial. Eur J Phys Rehabil Med 2011;47:533-41.
- Brosseau L, Wells GA, Kenny GP, Reid R, Maetzel A, Tugwell P, et al. The implementation of a community-based aerobic walking program for mild to moderate knee osteoarthritis: a knowledge translation randomized controlled trial: part II: clinical outcomes. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-1073.
- Carpenter KM, Stoner SA, Mundt JM, Stoelb B. An online self-help CBT intervention for chronic lower back pain. Clin J Pain 2012;28:14-22. http://dx.doi.org/10.1097/AJP.0b013e31822363db.
- Coleman S, Briffa NK, Carroll G, Inderjeeth C, Cook N, McQuade J. A randomised controlled trial of a self-management education program for osteoarthritis of the knee delivered by health care professionals. Arthritis Res Ther 2012;14. http://dx.doi.org/10.1186/ar3703.
- Kao MJ, Wu MP, Tsai MW, Chang WW, Wu SF. The effectiveness of a self-management program on quality of life for knee osteoarthritis (OA) patients. Arch Gerontol Geriatr 2012;54:317-24. http://dx.doi.org/10.1016/j.archger.2011.05.018.
- Martin J, Torre F, Padierna A, Aguirre U, Gonzalez N, Garcia S, et al. Six and 12-month follow-up of an interdisciplinary fibromyalgia treatment programme: results of a randomised trial. Clin Exp Rheumatol 2012;30:S103-11.
- Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med 2005;142:776-85. http://dx.doi.org/10.7326/0003-4819-142-9-200505030-00014.
- Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2015;1. http://dx.doi.org/10.1002/14651858.cd004376.pub3.
- Low Back Pain in Adults: Early Management. London: NICE; 2009.
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292:2388-95. http://dx.doi.org/10.1001/jama.292.19.2388.
- Walsh NE, Mitchell HL, Reeves BC, Hurley MV. Integrated exercise and self-management programmes in osteoarthritis of the hip and knee: a systematic review of effectiveness. Phys Ther Rev 2006;11:289-97. http://dx.doi.org/10.1179/108331906X163432.
- Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med 2008;31:169-77. http://dx.doi.org/10.1007/s10865-007-9144-1.
- Guzman J, Esmail R, Karjalainen KA, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low-back pain. Cochrane Database Syst Rev 2002;1. http://dx.doi.org/10.1002/14651858.cd000963.
- Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Care Res 2009;61:216-25. http://dx.doi.org/10.1002/art.24276.
- Pincus T, Miles C, Froud R, Underwood M, Carnes D, Taylor S. Methodological criteria for the assessment of moderators in systematic reviews of randomised controlled trials: a consensus study. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-14.
- Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. Hoboken, NJ: Wiley-Blackwell; 2001.
- Upton G, Cook I. A Dictionary of Statistics. Oxford: Oxford University Press; 2002.
- Gallagher RA, Miller C, Cronan TA, Groessl E. Gender differences in participation and responsiveness to a health intervention for older Americans. Women Health 1997;25:63-82. http://dx.doi.org/10.1300/J013v25n03_05.
- Lorig KR, Laurent DD, Deyo RA, Marnell ME, Minor MA, Ritter PL. Can a back pain e-mail discussion group improve health status and lower health care costs? A randomized study. Arch Intern Med 2002;162:792-6. http://dx.doi.org/10.1001/archinte.162.7.792.
- Heapy A, Otis J, Marcus KS, Frantsve LM, Janke EA, Shulman M, et al. Intersession coping skill practice mediates the relationship between readiness for self-management treatment and goal accomplishment. Pain 2005;118:360-8. http://dx.doi.org/10.1016/j.pain.2005.09.004.
- Kole-Snijders AMJ, Vlaeyen JWS, Goossens MEJ, Rutten-van Mölken MPM, Heuts PHT, van Breukelen G, et al. Chronic low-back pain: what does cognitive coping skills training add to operant behavioral treatment? Results of a randomized clinical trial. J Consult Clin Psychol 1999;67:931-44. http://dx.doi.org/10.1037/0022-006X.67.6.931.
- Smeets RJE, Vlaeyen JWS, Kester ADM, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive–behavioral treatment in chronic low back pain. Pain 2006;7:261-72. http://dx.doi.org/10.1016/j.jpain.2005.10.011.
- Veenhof C, van den Ende CHM, Dekker J, Kiike AJA, Oostendorp RA, Bijlsma JWJ. Which patients with osteoarthritis of hip and/or knee benefit most from behavioral graded activity?. Int J Behav Med 2007;14:86-92. http://dx.doi.org/10.1007/BF03004173.
- Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clin J Pain 2005;21:166-74. http://dx.doi.org/10.1097/00002508-200503000-00008.
- Haugli L, Steen E, Laerum E, Finset A, Nygaard R. Agency orientation and chronic musculoskeletal pain: effects of a group learning program based on the personal construct theory. Clin J Pain 2000;16:281-9. http://dx.doi.org/10.1097/00002508-200012000-00002.
- Lindh M, Lurie M, Sanne H. A randomized prospective study of vocational outcome in rehabilitation of patients with non-specific musculoskeletal pain: a multidisciplinary approach to patients identified after 90 days of sick-leave. Scand J Rehabil Med 1997;29:103-12.
- Jensen IB, Bergström G, Ljungquist T, Bodin L, Nygren AL. A randomized controlled component analysis of a behavioral medicine rehabilitation program for chronic spinal pain: are the effects dependent on gender?. Pain 2001;91:65-78. http://dx.doi.org/10.1016/S0304-3959(00)00420-6.
- Haugli L, Steen E, Laerum E, Nygard R, Finset A. Psychological distress and employment status. Effects of a group learning programme for patients with chronic musculoskeletal pain. Psychol Health Med 2003;8:135-48. http://dx.doi.org/10.1080/1354850031000087519.
- Jensen IB, Bergström G, Ljungquist T, Bodin L. A 3-year follow-up of a multidisciplinary rehabilitation programme for back and neck pain. Pain 2005;115:273-83. http://dx.doi.org/10.1016/j.pain.2005.03.005.
- Nour K, Laforest S, Gauvin L, Gignac M. Behavior change following a self-management intervention for housebound older adults with arthritis: an experimental study. Int J Behav Nutr Phys Act 2006;3. http://dx.doi.org/10.1186/1479-5868-3-12.
- Spinhoven P, ter Kuile M, Kole-Snijders AMJ, Mansfeld MH, den Ouden DJ, Vlaeyen JWS. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. Eur J Pain 2004;8:211-19. http://dx.doi.org/10.1016/j.ejpain.2003.08.003.
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull 1979;88:638-41. http://dx.doi.org/10.1037/0033-2909.86.3.638.
- Choudhury Y, Bremner SA, Ali A, Eldridge S, Griffiths CJ, Hussain I, et al. Prevalence and impact of chronic widespread pain in the Bangladeshi and white populations of Tower Hamlets, East London. Clin Rheumatol 2013;32:1375-82. http://dx.doi.org/10.1007/s10067-013-2286-3.
- Ritchie J, Lewis J. Qualitative Research Practice. London: Sage Publications; 2003.
- Reigal B, Carlson B. Is individual peer support a promising intervention for persons with heart failure?. J Cardiovasc Nurs 2004;19:174-83. http://dx.doi.org/10.1097/00005082-200405000-00006.
- Subramaniam V, Stewart MW, Smith JF. The development and impact of a chronic pain support group: a qualitative and quantitative study. J Pain Symptom Manage 1999;17:376-83. http://dx.doi.org/10.1016/S0885-3924(99)00012-3.
- Eccleston C. Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther 1995;33:391-405. http://dx.doi.org/10.1016/0005-7967(94)00057-Q.
- Wootton IM, Wood V, Cook F. Who wants expert patient programmes for chronic mechanical spinal pain? An investigation into the value of, and recruitment to, an expert patient programme as part of the physiotherapy management of chronic spinal pain. Physiotherapy 2008;94:78-84. http://dx.doi.org/10.1016/j.physio.2006.12.012.
- Bayliss EA, Ellis JL, Steiner JF. Barriers to self-management and quality-of-life outcomes in seniors with multimorbidities. Ann Fam Med 2007;5:395-402. http://dx.doi.org/10.1370/afm.722.
- Morley S, Shapiro DA, Biggs J. Developing a treatment manual for attention management in chronic pain. Cogn Behav Ther 2004;33:1-11. http://dx.doi.org/10.1080/16506070310001794.
- Wood D. ABC of learning and teaching in medicine: problem based learning. BMJ 2003;326:328-30. http://dx.doi.org/10.1136/bmj.326.7384.328.
- Schön DA. The Reflective Practitioner. How Professionals Think in Action. London: Temple Smith; 1983.
- Kirmayer LJ, Young A. Culture and somatisation: clinical epidemiological and ethnographic perspectives. Psychosom Med 1998;60:420-30. http://dx.doi.org/10.1097/00006842-199807000-00006.
- Beiser M, Fleming JAE. Measuring psychiatric disorder among South East Asian refugees. Psychol Med 1986;16:627-39. http://dx.doi.org/10.1017/S0033291700010382.
- Kirmayer LJ. The cultural diversity of healing: meaning, metaphor and mechanism. Br Med Bull 2004;69:33-48. http://dx.doi.org/10.1093/bmb/ldh006.
- Cole F. Ethical Considerations in Chronic Pain Workshop. London: Barts and The London NHS Trust; 2009.
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2.
- Cleeland CS, Chapman CR, Loeser JD. Advances in Pain Research and Therapy, Volume 12: Issues in Pain Management. New York, NY: Raven Press; 1989.
- EuroQol Group . The EuroQol Instrument: EQ-5D n.d. www.euroqol.org/eq-5d (accessed 25 April 2016).
- Sullivan MJL. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524-32. http://dx.doi.org/10.1037/1040-3590.7.4.524.
- Guy W. ECDEU Assessment Manual for Psycholpharmacology. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Program; 1976.
- Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behavior. Pain Manag 1990;3:35-43.
- Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear avoidance in chronic low back pain and disability. Pain 1993;52:157-68. http://dx.doi.org/10.1016/0304-3959(93)90127-B.
- McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain 2004;107:159-66. http://dx.doi.org/10.1016/j.pain.2003.10.012.
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133-49. http://dx.doi.org/10.1016/0304-3959(92)90154-4.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol 1984;40:1365-7. http://dx.doi.org/10.1002/1097-4679(198411)40:6<1365::AID-JCLP2270400615>3.0.CO;2-D.
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401. http://dx.doi.org/10.1177/014662167700100306.
- Kroenke K, Spitzer RL, Williams JBW, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613-21.
- Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum 1989;32:37-44. http://dx.doi.org/10.1002/anr.1780320107.
- Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007;11:153-63. http://dx.doi.org/10.1016/j.ejpain.2005.12.008.
- Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J. Outcome Measures for Health Education and other Health Care Interventions. Thousand Oaks, CA: Sage Publications; 1996.
- Barlow JH, Williams B, Wright CC. The reliability and validity of the arthritis self-efficacy scale in a UK context. Psychol Health Med 1997;2:3-17. http://dx.doi.org/10.1080/13548509708400556.
- Pincus T, Williams ACD, Vogel S, Field A. The development and testing of the depression, anxiety, and positive outlook scale (DAPOS). Pain 2004;109:181-8. http://dx.doi.org/10.1016/j.pain.2004.02.004.
- Glasgow RE, Strycker LA, Toobert DJ, Eakin E. A social-ecologic approach to assessing support for disease self-management: the chronic illness resources survey. J Behav Med 2000;23:559-83. http://dx.doi.org/10.1023/A:1005507603901.
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705-14. http://dx.doi.org/10.1016/0277-9536(91)90150-B.
- University of Melbourne . Health Education Impact Questionnaire n.d. www.deakin.edu.au/research/cphr/our-research/public-health-innovation-unit/our-research (accessed 25 May 2016).
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health and Economics, University of York; 1999.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Bandura A. Self-efficacy: The Exercise of Control. New York, NY: Freeman; 1997.
- Beck JS. Cognitive Therapy. New York, NY: Guilford Press; 1995.
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behaviour Change. New York, NY: Guilford Press; 2004.
- Ajzen I, Fishbein M. Belief, Attitude, Intention and Behaviour: An Introduction to Theory and Research. Harlow: Longman Higher Education; 1976.
- Ajzen I, Fishbein M. Understanding Attitudes and Predicting Social Behaviour. Harlow: Pearson Education; 1980.
- Becker M, Cohen SJ. New Directions in Patient Compliance. Lexington, MA: Heath; 1979.
- Little P, Lewith G, Webley F, Evans M, Beattie A, Middleton K, et al. Randomised controlled trial of Alexander technique lessons, exercise and massage (ATEAM) for chronic recurrent back pain. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a884.
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179-211. http://dx.doi.org/10.1016/0749-5978(91)90020-T.
- Ellis A, Ellis A, Greiger R. Handbook of Rational-Emotive Therapy. New York, NY: Springer; 1977.
- Hayes S, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model processes and outcomes. Behav Res Ther 2006;44:1-25. http://dx.doi.org/10.1016/j.brat.2005.06.006.
- Robb H. Values as leading principles in acceptance and commitment therapy. Int J Behav Consult Ther 2007;3:118-23. http://dx.doi.org/10.1037/h0100170.
- Ellis A. Reason and Emotion in Psychotherapy. New York, NY: Lyle Stuart; 1962.
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1979.
- Brown J, Elliot S, Boardman J, Ferns J, Morrison J. Meeting the unmet need for depression services with psycho-educational self-confidence workshops; preliminary report. Br J Psychiatry 2004;185:511-15. http://dx.doi.org/10.1192/bjp.185.6.511.
- Carnes D, Gallagher J, Munday E, Ritchie S, Underwood M. Mapping care pathways and estimating the number and cost of musculoskeletal chronic pain patients to inform the development and implementation of a new service. Prim Health Care Res Dev 2008;9:241-7. http://dx.doi.org/10.1017/S1463423608000893.
- UK BEAM Trial Team . United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: effectiveness of physical treatments for back pain in primary care. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38282.669225.AE.
- Foell J, Carnes D, Homer K, Taylor SJC. Developing and implementing electronic search strategies to recruit patients with chronic musculoskeletal pain in primary care databases. Prim Health Care Res Dev 2013;15:234-43. http://dx.doi.org/10.1017/S1463423613000248.
- Cole F, Moore P. The Pain Toolkit n.d. www.paintoolkit.org (accessed 10 February 2014).
- Ware JE. SF-36® Health Survey Update n.d. www.sf-36.org/tools/sf36.shtml (accessed 25 April 2016).
- Office for National Statistics . Census 2011 n.d. www.ons.gov.uk/census/2011census (accessed 25 May 2016).
- Hauser W, Sarzi-Puttini P, Tolle TR, Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta-analysis. Clin Exp Rheumatol 2012;30:78-87.
- Artus M, van der Windt DA, Jordan KP, Hay EM. Low back pain symptoms show a similar pattern of improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology 2010;49:2346-56. http://dx.doi.org/10.1093/rheumatology/keq245.
- Office for National Statistics . Ethnicity and National Identity in England and Wales: 2011 2012. www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11 (accessed 22 June 2016).
- Nomis . UK Census 2011 n.d. www.nomisweb.co.uk/census/2011 (accessed 6 March 2014).
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. http://dx.doi.org/10.1037/0278-6133.23.5.443.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:S52-63. http://dx.doi.org/10.1111/j.1752-7325.2011.00233.x.
- Breivik H, Collett B, Ventrafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life and treatment. Eur J Pain 2006;10:287-333. http://dx.doi.org/10.1016/j.ejpain.2005.06.009.
- Dunford E, Thompson M. Relaxation, mindfulness in pain: a review. Rev Pain 2010;4:18-22. http://dx.doi.org/10.1177/204946371000400105.
- Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2009;2. http://dx.doi.org/10.1002/14651858.cd007407.pub2.
- Carnes D, Homer K, Underwood M, Pincus T, Rahman A, Taylor SJC. Pain management for chronic musculoskeletal conditions: the development of an evidence based and theory informed pain self-management course. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003534.
- Health and Social Care Information Centre . Standards n.d. www.connectingforhealth.nhs.uk/systemsandservices/data/uktc/readcodes (accessed 1 October 2013).
- The National Pain Audit . The National Pain Audit Final Report 2012 n.d. www.nationalpainaudit.org/media/files/NationalPainAudit-2012.pdf (accessed 11 February 2014).
- University of Sheffield . Clinical Trials Research Unit n.d. www.sheffield.ac.uk/scharr/sections/dts/ctru (accessed 29 March 2014).
- Mars T, Ellard D, Carnes D, Homer K, Taylor SJC. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003555.
- Health and Social Care Information Centre . Secondary Uses Service (SUS) n.d. www.hscic.gov.uk/sus (accessed 12 September 2016).
- Miles CL, Pincus T, Carnes D, Homer KE, Taylor SJC, Bremner SA, et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of sub-group analysis within RCTs. Eur J Pain 2011;15:775.e1-111.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7347.1183.
- NHS Business Services Authority . Prescription Cost Analysis (PCA) Data n.d. www.nhsbsa.nhs.uk/PrescriptionServices/3494.aspx (accessed September 2013).
- World Health Organization . Collaborating Centre for Drug Statistics Methodology n.d. www.whocc.no/ddd/defintion_and_general_considerations (accessed 11 May 2016).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2011.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther A, Spencer A, et al. Exercise for depression in care home residents: a randomised controlled trial with cost-effectiveness analysis (OPERA). Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17180.
- Moerbeek M, Wong WK. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat Med 2008;27:2850-64. http://dx.doi.org/10.1002/sim.3115.
- Kahan B, Diaz-Ordaz K, Homer K, Carnes D, Underwood M, Taylor SJC, et al. Coping with persistent pain, effectiveness research into self-management (COPERS) – statistical analysis plan for a randomised controlled trial. BMC Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-59.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d40.
- Kahan BC, Morris TP. Assessing potential sources of clustering in individually randomised trials. BMC Med Res Methodol 2013;13. http://dx.doi.org/10.1186/1471-2288-13-58.
- Pals SL, Murray DM, Alfano CM, Shadish WR, Hannan PJ, Baker WL. Individually randomized group treatment trials: a critical appraisal of frequently used design and analytic approaches. Am J Public Health 2008;98:1418-24. http://dx.doi.org/10.2105/AJPH.2007.127027.
- Kahan BC, Morris TP. Analysis of multicentre trials with continuous outcomes: when and how should we account for centre effects?. Stat Med 2013;32:1136-49. http://dx.doi.org/10.1002/sim.5667.
- Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome – when, why, and how?. BMC Med Res Methodol 2014;14. http://dx.doi.org/10.1186/1471-2288-14-20.
- Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5840.
- Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med 2012;31:328-40. http://dx.doi.org/10.1002/sim.4431.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley; 1987.
- Carpenter JR, Goldstein H, Kenward MG. REALCOM-IMPUTE software for multilevel multiple imputation with mixed response types. J Stat Softw 2011;45:1-14. http://dx.doi.org/10.18637/jss.v045.i05.
- Morris TP, Kahan BC, White IR. Choosing sensitivity analyses for randomised trials: principles. BMC Med Res Methodol 2014;14. http://dx.doi.org/10.1186/1471-2288-14-11.
- McLennan D, Barnes H, Noble M, Davies J, Garratt E, Dibben C. The English Indices of Deprivation 2010. Department for Communities and Local Government; n.d.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Department of Health . National Schedule of Reference Costs 2011–2012 – NHS Trusts and NHS Foundation Trusts 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed September 2013).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- NHS Business Services Authority . Drug Tariff n.d. www.nhsbsa.nhs.uk/924.aspx (accessed September 2013).
- Monthly Index of Medical Specialities (MIMS) n.d. www.mims.co.uk/ (accessed 25 May 2016).
- Boots Pharmaceuticals . Pharmacy and Health n.d. www.boots.com/en/Pharmacy-Health/ (accessed September 2013).
- NutriDrinks n.d. www.nutridrinks.co.uk (accessed September 2013).
- Charter Healthcare n.d. www.charterhealthcare.co.uk/ (accessed 12 September 2016).
- Szende A, Oppe M, Devlin NJ. EQ-5D Value Sets: Inventory, Comparative Review, and User Guide. London: Springer Verlag; 2006.
- StataCorp . Stata/IC/12.1 2012. www.stata.com// (accessed 12 September 2016).
- Morris S, Devlin N, Parkin D, Spencer A. Economic Analysis in Health Care 2012.
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations. London: Springer; 1987.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. http://dx.doi.org/10.1191/096228099671525676.
- White I, Royston P, Wood A. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Little RJA. Missing-data adjustments in large surveys. J Bus Econ Stat 1988;6:287-96.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Löthgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ 2000;9:623-30. http://dx.doi.org/10.1002/1099-1050(200010)9:7<623::AID-HEC539>3.0.CO;2-V.
- Yeaton WH, Sechrest L. Critical dimensions in the choice and maintenance of successful treatments: strength, integrity, and effectiveness. J Consult Clin Psychol 1981;49:156-67. http://dx.doi.org/10.1037/0022-006X.49.2.156.
- Moncher FJ, Prinz R. Treatment fidelity in outcome studies. Clin Psychol Rev 1991;11:247-66. http://dx.doi.org/10.1016/0272-7358(91)90103-2.
- Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: assessment of adherence and competence. J Consult Clin Psychol 1993;61:620-30. http://dx.doi.org/10.1037/0022-006X.61.4.620.
- Mihalic SF. The Importance of Implementation Fidelity. Boulder, CO: Centre for the Study and Prevention of Violence; 2002.
- Elliot DS, Mihalic S. Issues in disseminating and replicating effective prevention programs. Prev Sci 2004;5:47-53. http://dx.doi.org/10.1023/B:PREV.0000013981.28071.52.
- Noel P. The impact of therapeutic case management on participation in adolescent substance abuse treatment. Am J Drug Alcohol Abuse 2006;13:311-27. http://dx.doi.org/10.1080/00952990500328646.
- Thomas RE, Baker P, Lorenzetti D. Family-based programmes for preventing smoking by children and adolescents. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.cd004493.pub2.
- Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Wiley (Jossey-Bass); 2002.
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol 2005;73:852-60. http://dx.doi.org/10.1037/0022-006X.73.5.852.
- Durlak JA, Du Pre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol 2008;41:327-50. http://dx.doi.org/10.1007/s10464-008-9165-0.
- Mihalic SF, Argamaso S. Implementing the life skills training drug prevention program: factors related to implementation fidelity. Implement Sci 2008;3. http://dx.doi.org/10.1186/1748-5908-3-5.
- Parham LD, Roley SS, May-Benson TA, Koomar JA, Brett-Green B, Burke JP, et al. Development of a fidelity measure for research on the effectiveness of the Ayres Sensory Integration intervention. Am J Occup Ther 2011;65:133-42. http://dx.doi.org/10.5014/ajot.2011.000745.
- Stiles WB, Honos-Webb L, Surko M. Responsiveness in psychotherapy. Clin Psychol Sci Pract 1998;5:438-58. http://dx.doi.org/10.1111/j.1468-2850.1998.tb00166.x.
- Carroll KM, Nich C, Rounasville BJ. Use of observer and therapist ratings to monitor delivery of coping skills treatment for cocaine abusers: utility of therapist session checklists. Psychother Res 1998;8:307-20. http://dx.doi.org/10.1080/10503309812331332407.
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend 2000;57:225-38. http://dx.doi.org/10.1016/S0376-8716(99)00049-6.
- Creed TA, Kendall PC. Therapist alliance-building behavior within a cognitive-behavioral treatment for anxiety in youth. J Consult Clin Psychol 2005;73:498-505. http://dx.doi.org/10.1037/0022-006X.73.3.498.
- Davidson K, Scott J, Schmidt U, Tata P, Thornton S, Tyrer P. Therapist competence and clinical outcome in the prevention of parasuicide by manual assisted cognitive behaviour therapy trial: the POPMACT study. Psychol Med 2004;34:855-63. http://dx.doi.org/10.1017/S0033291703001855.
- Crowe TP, Grenyer BFS. Is therapist alliance or whole group cohesion more influential in group psychology outcomes?. Clin Psychol Psychother 2008;15:239-46. http://dx.doi.org/10.1002/cpp.583.
- Nomis . UK Census 2011, General Health by Ethnic Group by Sex by Age n.d. www.nomisweb.co.uk/census/2011/lc3206ew (accessed 13 March 2014).
- Crawford JR, Henry JD, Crombie C, Taylor EP. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol 2001;40:429-34. http://dx.doi.org/10.1348/014466501163904.
- Faraone SV. Interpreting estimates of treatment effects. PT 2008;33:700-3.
- Expert Patients Programme . Self Care Reduces Costs and Improves Health – The Evidence n.d. www.livinghealthynortheast.ca/Portals/0/Documents/self-care-reduces-cost-and-improves-health-evidence.pdf (accessed 25 May 2016).
- Mukuria C, Brazier J, Barkham M, Connell J, Hardy G, Hutten R, et al. Cost-effectiveness of an improving access to psychological therapies service. Br J Psychiatry 2013;202:220-7. http://dx.doi.org/10.1192/bjp.bp.111.107888.
- Schermer TR, Thoonen BP, van den Boom G, Akkermans RP, Grol RP, Folgering HT, et al. Randomized controlled economic evaluation of asthma self-management in primary health care. Am J Respir Crit Care Med 2002;166:1062-72. http://dx.doi.org/10.1164/rccm.2105116.
- Jowett S, Bryan S, Murray E, McCahon D, Raftery J, Hobbs FD, et al. Patient self-management of anticoagulation therapy: a trial-based cost-effectiveness analysis. Br J Haematol 2006;134:632-9. http://dx.doi.org/10.1111/j.1365-2141.2006.06243.x.
- Richardson G, Kennedy A, Reeves D, Bower P, Lee V, Middleton E, et al. Cost effectiveness of the expert patients programme (EPP) for patients with chronic conditions. J Epidemiol Community Health 2008;62:361-7. http://dx.doi.org/10.1136/jech.2006.057430.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4093.
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database Syst Rev 2013;9. http://dx.doi.org/10.1111/jebm.12076.
- Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.cd007954.
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton C, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010;303:47-53. http://dx.doi.org/10.1001/jama.2009.1943.
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson B. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLOS Med 2008;5. http://dx.doi.org/10.1371/journal.pmed.0050045.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. http://dx.doi.org/10.1136/jnnp.23.1.56.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20. http://dx.doi.org/10.1177/0272989X06290495.
- Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000;55:1000-6. http://dx.doi.org/10.1136/thorax.55.12.1000.
- Hippisley-Cox J, Vinogradova Y. Trends in Consultation Rates in General Practice 1995 to 2008: Analysis of the QResearch® Database. Q Research and the Information Centre for Heath and Social Care; 2009.
- Ackerman IN, Buchbinder R, Osborne RH. Challenges in evaluating an arthritis self-management program for people with hip and knee osteoarthritis in real-world clinical settings. J Rheumatol 2012;39:1047-55. http://dx.doi.org/10.3899/jrheum.111358.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self-management support for long term conditions in routine primary care settings: a cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2882.
- Dunn G, Maracy M, Dowrick C, Ayuso-Mateos JL, Dalgard OS. Estimating psychological treatment effects from a randomised controlled trial with both non-compliance and loss to follow-up. Br J Psychiatry 2003;183:323-31. http://dx.doi.org/10.1192/bjp.183.4.323.
- Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh 2006;38:269-77. http://dx.doi.org/10.1111/j.1547-5069.2006.00113.x.
- Jorm AF, Morgan AJ, Hetrick SE. Relaxation for depression. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.cd007142.pub2.
- Office for National Statistics . 2011 Census: Key Statistics for England and Wales, March 2011. Correction: 17 January 2014 n.d. www.ons.gov.uk/ons/dcp171778_290685.pdf (accessed 6 January 2014).
- Hogg L, Garrod R, Thornton H, McDonnell L, Bellas H, White P. Effectiveness, attendance, and completion of an integrated, system-wide pulmonary rehabilitation service for COPD: prospective observational study. COPD 2012;9:546-54. http://dx.doi.org/10.3109/15412555.2012.707258.
- Prescribing advice for GPs . BNF 62 2011. www.prescriber.org.uk/2011/10/bnf-62/ (accessed 28 June 2016).
- Rahman A, Underwood M, Carnes D. Fibromyalgia: clinical review. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1224.
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartilage 2014;22:363-88. http://dx.doi.org/10.1016/j.joca.2014.01.003.
- Radhakrishnan M, Hammond G, Jones PB, Watson A, McMillan-Shields F, Lafortune L. Cost of improving access to psychological therapies (IAPT) programme: an analysis of cost of session, treatment and recovery in selected primary care trusts in the East of England region. Behav Res Ther 2013;51:37-45. http://dx.doi.org/10.1016/j.brat.2012.10.001.
- Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63-70. http://dx.doi.org/10.1001/archpsyc.1965.01720310065008.
- Ware JE, Kosinski M. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. Lincoln, RI: QualityMetric Incorporated; 2001.
- Goldberg DP, Williams P. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci 1974;19:1-15. http://dx.doi.org/10.1002/bs.3830190102.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, NY: Lippincott-Raven; 1996.
- Nicholas MK, Wilson PH, Goyen J. Comparison of cognitive–behavioural group treatment and an alternative non-psychological treatment for chronic low back pain. Pain 1992;48:339-47. http://dx.doi.org/10.1016/0304-3959(92)90082-M.
- Bot SD. Clinimetric evaluation of shoulder disability questionnaires: a systematic review of the literature. Ann Rheum Dis 2004;63:335-41. http://dx.doi.org/10.1136/ard.2003.007724.
- Nunnally JC. Psychometric Theory. New York, NY: McGraw-Hill; 1978.
- Baheiraei A, Ritchie JE, Eisman JA, Nguyen TV. Psychometric properties of the Persian version of the osteoporosis knowledge and health belief questionnaires. Maturitas 2005;50:134-9. http://dx.doi.org/10.1016/j.maturitas.2004.05.001.
- Horan ML, Kim KK, Gendler P, Froman RD, Patel MD. Development and evaluation of the osteoporosis self-efficacy scale. Res Nurs Health 1998;21:395-403. http://dx.doi.org/10.1002/(SICI)1098-240X(199810)21:5<395::AID-NUR3>3.0.CO;2-I.
- Lomi C, Nordholm LA. Validation of a Swedish version of the Arthritis Self-Efficacy Scale. Scand J Rheumatol 1992;21:231-7.
- Mueller A, Hartmann M, Mueller K, Eich W. Validation of the arthritis self-efficacy short-form scale in German fibromyalgia patients. Eur J Pain 2003;7:163-71. http://dx.doi.org/10.1016/S1090-3801(02)00097-6.
- Gonzalez VM, Stewart A, Ritter PL, Lorig K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum 1995;38:1429-46. http://dx.doi.org/10.1002/art.1780381010.
- Sarda J, Nicholas MK, Pimenta CAM, Asghari A. Pain-related self-efficacy beliefs in a Brazilian chronic pain patient sample: a psychometric analysis. Stress Health 2007;23:185-90. http://dx.doi.org/10.1002/smi.1135.
- Lim HS, Chen PP, Wong TC, Gin T, Wong E, Chan IS, et al. Validation of the Chinese version of pain self-efficacy questionnaire. Anesth Analg 2007;104:918-23. http://dx.doi.org/10.1213/01.ane.0000255731.24092.a5.
- Shin Y, Jang H, Pender NJ. Psychometric evaluation of the exercise self-efficacy scale among Korean adults with chronic diseases. Res Nurs Health 2001;24:68-76. http://dx.doi.org/10.1002/1098-240X(200102)24:1<68::AID-NUR1008>3.0.CO;2-C.
- Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain 1995;63:77-84. http://dx.doi.org/10.1016/0304-3959(95)00021-J.
- Barlow JH, Shaw KL, Wright CC. Development and preliminary validation of a self-efficacy measure for use among parents of children with juvenile idiopathic arthritis. Arthritis Care Res 2000;13:227-35. http://dx.doi.org/10.1002/1529-0131(200008)13:4<227::AID-ANR7>3.0.CO;2-N.
- Bursch B, Tsao JC, Meldrum M, Zeltzer LK, Bursch B, Tsao JCI, et al. Preliminary validation of a self-efficacy scale for child functioning despite chronic pain (child and parent versions). Pain 2006;125:35-42. http://dx.doi.org/10.1016/j.pain.2006.04.026.
- Gard G, Rivano M, Grahn B. Development and reliability of the Motivation for Change Questionnaire. Disabil Rehabil 2005;27:967-76. http://dx.doi.org/10.1080/09638280500052682.
- Gibson L, Strong J. The reliability and validity of a measure of perceived functional capacity for work in chronic back pain. J Occup Rehabil 1996;6:159-75. http://dx.doi.org/10.1007/BF02110753.
- Matheson LN, Matheson M. Spinal Function Sort. Rancho Santa Margarita, CA: Performance Assessment and Capacity Testing; 1989.
- Sandborgh M, Lindberg P, Denison E. The Pain Belief Screening Instrument (PBSI): predictive validity for disability status in persistent musculoskeletal pain. Disabil Rehabil 2008;30:1123-30. http://dx.doi.org/10.1080/09638280701523200.
- Sandborgh M, Lindberg P, Denison E. Pain Belief Screening Instrument: development and preliminary validation of a screening instrument for disabling persistent pain. J Rehabil Med 2007;39:461-6. http://dx.doi.org/10.2340/16501977-0072.
- Vlaeyen JW, Geurts SM, Kole-Snijders AMJ. What do chronic pain patients think of their pain? Towards a pain cognition questionnaire. Br J Clin Psychol 1990;29:383-94.
- Lorig K, Sobel D, Ritter P, Laurent D, Hobbs M. Effect of a self-management program for patients with chronic disease. Eff Clin Pract 2001;4:256-62.
- Levin JB, Lofland KR, Cassisi JE, Poreh AM, Blonsky ER. The relationship between self-efficacy and disability in chronic low back pain patients. Int J Rehabil Health 1996;2:19-28. http://dx.doi.org/10.1007/BF02213561.
- McKay RT, Levin LS, Lockey JE, Lemasters GK, Medvedovic M, Papes DM, et al. Weight change and lung function: implications for workplace surveillance studies. J Occup Environ Med 1999;41:596-604.
- Nunnally JC, Bernstein IH. Psychometric Theory. New York, NY: McGraw-Hill; 1984.
- Asghari A, Nicholas MK. An investigation of pain self-efficacy beliefs in Iranian chronic pain patients: a preliminary validation of a translated English-language scale. Pain Med 2009;10:619-32. http://dx.doi.org/10.1111/j.1526-4637.2009.00623.x.
- Turner JA, Ersek M, Kemp C. Self-efficacy for managing pain is associated with disability, depression, and pain coping among retirement community residents with chronic pain. Pain 2005;6:471-9. http://dx.doi.org/10.1016/j.jpain.2005.02.011.
- Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A, Burckhardt CS, Mannerkorpi K, et al. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. J Rheumatol 1994;21:714-20.
- Williams AC, Nicholas MK, Richardson PH, Pither CE, Justins DM, Chamberlain JH. Evaluation of a cognitive behavioural programme for rehabilitating patients with chronic pain. Br J Gen Pract 1993;43:513-18.
- Adams JH, Williams AC. What affects return to work for graduates of a pain management program with chronic upper limb pain?. J Occup Rehabil 2003;13:91-106. http://dx.doi.org/10.1023/A:1022599731391.
- Cohen M, Nicholas MK, Blanch A. Medical assessment and management of work-related low back or neck-arm pain: more questions than answers. J Occup Health Saf Aust NZ 2000;16:307-17.
- Coughlan GM, Ridout KL, Williams AC, Richardson PH. Attrition from a pain management program. Br J Clin Psychol 1995;34:471-9. http://dx.doi.org/10.1111/j.2044-8260.1995.tb01481.x.
- Ahlstrom G, Wenneberg S. Coping with illness-related problems in persons with progressive muscular disease: the Swedish version of the ways of coping questionnaire. Scand J Caring Sci 2002;16:368-75. http://dx.doi.org/10.1046/j.1471-6712.2002.00099.x.
- Bell RA, Leroy JB, Stephenson JJ. Evaluating the mediating effects of social support upon life events and depressive symptoms. J Community Psychol 1982;10:325-40. http://dx.doi.org/10.1002/1520-6629(198210)10:4<325::AID-JCOP2290100405>3.0.CO;2-C.
- Orth-Gomer K, Unden AL. The measurement of social support in population surveys. Soc Sci Med 1987;24:83-94. http://dx.doi.org/10.1016/0277-9536(87)90142-0.
- Bennett SJ, Perkins SM, Lane KA, Deer M, Brater DC, Murray MD. Social support and health-related quality of life in chronic heart failure patients. Qual Life Res 2001;10:671-82. http://dx.doi.org/10.1023/A:1013815825500.
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol 1979;109:186-204.
- Blazer DG. Social support and mortality in an elderly community population. Am J Epidemiol 1982;115:684-94.
- Broadhead WE. A Proposal for Doctoral Research in the Department of Epidemiology, School of Public Health. Chapel Hill, NC: University of North Carolina at Chapel Hill; 1982.
- Cohen S, Mermelstein R, Kamarck T, Hoberman H, Sarason IG, Sarason BR. Social Support: Theory, Research and Applications. Dordrecht, the Netherlands: Martinus Nijhoff; 1985.
- Da Costa D, Dobkin PL, Fitzcharles MA, Fortin PR, Beaulieu A, Zummer M, et al. Determinants of health status in fibromyalgia: a comparative study with systemic lupus erythematosus. J Rheumatol 2000;27:365-72.
- Da Costa D, Dritsa M, Bernatsky S, Pineau C, Menard HA, Dasgupta K, et al. Dimensions of fatigue in systemic lupus erythematosus: relationship to disease status and behavioral and psychosocial factors. J Rheumatol 2006;33:1282-8.
- Sarason IG, Sarason BR, Shearin EN. A brief measure of social support: practical and theoretical implications. J Soc Pers Relat 1987;4:497-510. http://dx.doi.org/10.1177/0265407587044007.
- Dean A, Lin N, Ensel WM, Simmons R. Research in Community and Mental Health. Greenwich, CT: JAI Press; 1981.
- Doeglas D, Suurmeijer T, Briancon S, Moum T, Krol B, Bjelle A, et al. An international study on measuring social support: interactions and satisfaction. Soc Sci Med 1996;43:1389-97. http://dx.doi.org/10.1016/0277-9536(96)00036-6.
- Eakin EG, Reeves MM, Bull SS, Riley KM, Floyd S, Glasgow RE. Validation of the Spanish-language version of the chronic illness resources survey. Int J Behav Med 2007;14:76-85. http://dx.doi.org/10.1007/BF03004172.
- Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs 2009;18:1541-9. http://dx.doi.org/10.1111/j.1365-2702.2008.02648.x.
- Esteve MR, Ramirez C, Lopez AE. General versus specific indices in the assessment of chronic pain coping. Pyschol Spain 2005;9:49-56.
- Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive–behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain 2002;100:141-53. http://dx.doi.org/10.1016/S0304-3959(02)00274-9.
- Franks HM, Cronan TA, Oliver K. Social support in women with fibromyalgia: is quality more important than quantity?. J Community Psychol 2004;32:425-38. http://dx.doi.org/10.1002/jcop.20011.
- Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nurs Res 1981;30:264-9. http://dx.doi.org/10.1097/00006199-198109000-00003.
- Funch DP, Marshall JR, Gebhardt GP. Assessment of a short scale to measure social support. Soc Sci Med 1986;23:337-44. http://dx.doi.org/10.1016/0277-9536(86)90356-4.
- Garcia-Campayo J, Pascual A, Alda M, Ramirez MTG. Coping with fibromialgia: usefulness of the chronic pain coping inventory-42. Pain 2007;132:S68-76. http://dx.doi.org/10.1016/j.pain.2007.02.013.
- Glasgow RE, Toobert DJ, Barrera M, Strycker LA. The chronic illness resources survey: cross-validation and sensitivity to intervention. Health Educ Res 2005;20:402-9. http://dx.doi.org/10.1093/her/cyg140.
- Henderson S, Duncan-Jones P, Byrne DG, Scott R. Measuring social relationships. The Interview Schedule for Social Interaction. Psychol Med 1980;10:723-34. http://dx.doi.org/10.1017/S003329170005501X.
- Hesselink AE, Penninx BWJH, Schlosser MAG, Wijnhoven HAH, van der Windt DAWM, Kriegsman DMW, et al. The role of coping resources and coping style in quality of life of patients with asthma or COPD. Qual Life Res 2004;13:509-18. http://dx.doi.org/10.1023/B:QURE.0000018474.14094.2f.
- Van Eijk LM, Kempen GI, Sonderen V. Een korte schaal voor het meten van sociale steun bij ouderen de: SSL 12-I. [A short scale for measuring social support in the elderly: the SSL12-I.]. Tijdschr Gerontol Geriatr 1994;25:192-6.
- Marhold C, Linton SJ, Melin L. Identification of obstacles for chronic pain patients to return to work: evaluation of a questionnaire. J Occup Rehabil 2002;12:65-7. http://dx.doi.org/10.1023/A:1015056429505.
- McCormack LA, Williams-Piehota PA, Bann CM, Burton J, Kamerow DB, Squire C, et al. Development and validation of an instrument to measure resources and support for chronic illness self-management. Diabetes Educ 2008;34:707-18. http://dx.doi.org/10.1177/0145721708321021.
- McFarlane AH, Neale KA, Norman GR, Roy RG, Streiner DL. Methodological issues in developing a scale to measure social support. Schizophr Bull 1981;7:90-100. http://dx.doi.org/10.1093/schbul/7.1.90.
- Raleigh EH, Boehm S. Development of the Multidimensional Hope Scale. J Nurs Meas 1994;2:155-67.
- Savelkoul M, De Witte LP, Candel MJ, Van Der Tempel H, Van Den Borne B. Effects of a coping intervention on patients with rheumatic diseases: results of a randomized controlled trial. Arthritis Rheum 2001;45:69-76. http://dx.doi.org/10.1002/1529-0131(200102)45:1<69::AID-ANR86>3.0.CO;2-M.
- Suurmeijer TP, Doeglas DM, Briancon S, Krijnen WP, Krol B, Sanderman R, et al. The measurement of social support in the ‘European Research on Incapacitating Diseases and Social Support’: the development of the Social Support Questionnaire for Transactions (SSQT). Soc Sci Med 1995;40:1221-9. http://dx.doi.org/10.1016/0277-9536(94)00253-P.
- Tan G, Jensen MP, Robinson-Whelen S, Thornby JI, Monga TN. Coping with chronic pain: a comparison of two measures. Pain 2001;90:127-33. http://dx.doi.org/10.1016/S0304-3959(00)00395-X.
- Jensen MP, Turner JA, Romano JM, Strom SE. The Chronic Pain Coping Inventory: development and preliminary validation. Pain 1995;60:203-16. http://dx.doi.org/10.1016/0304-3959(94)00118-X.
- Tan G, Nguyen Q, Anderson KO, Jensen M, Thornby J. Further validation of the chronic pain coping inventory. Pain 2005;6:29-40. http://dx.doi.org/10.1016/j.jpain.2004.09.006.
- Thompson SC, Sobolew-Shubin A. Perceptions of overprotection in ill adults. J Appl Soc Psychol 1993;23:85-97. http://dx.doi.org/10.1111/j.1559-1816.1993.tb01053.x.
- Trief PM, Carnrike CL, Drudge O. Chronic pain and depression: is social support relevant?. Psychol Rep 1995;76:227-36. http://dx.doi.org/10.2466/pr0.1995.76.1.227.
- Weir R, Browne G, Tunks E, Gafni A, Roberts J. Gender differences in psychosocial adjustment to chronic pain and expenditures for health care services used. Clin J Pain 1996;12:277-90. http://dx.doi.org/10.1097/00002508-199612000-00007.
- Broadhead WE, Gehlbach SH, Degruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire – measurement of social support in family medicine patients. Med Care 1988;26:709-23. http://dx.doi.org/10.1097/00005650-198807000-00006.
- Yu DS, Lee DT, Woo J. Psychometric testing of the Chinese version of the medical outcomes study social support survey (MOS-SSS-C). Res Nurs Health 2004;27:135-43. http://dx.doi.org/10.1002/nur.20008.
- Barrera M, Gottlieb BH. Social Networks and Social Support. Beverly Hills, CA: Sage Publications; 1981.
- Procidano ME, Heller K. Measures of perceived social support from friends and from family – three validation studies. Am J Commun Psychol 1983;11:1-24. http://dx.doi.org/10.1007/BF00898416.
- Lubben JE. Assessing social networks among elderly populations. Fam Community Health 1988;11:42-5. http://dx.doi.org/10.1097/00003727-198811000-00008.
- Stokes JP. Predicting satisfaction with social support from social network structure. Am J Commun Psychol 1983;11:141-52. http://dx.doi.org/10.1007/BF00894363.
- Moos RH, Moos BS. Manual for Family Environment Scale. Palo Alto, CA: Consulting Psychologists Press; 1981.
- Vaux A, Phillips J, Holly L, Thomson B, Williams D, Stewart D. The social support appraisals (SS-a) scale – studies of reliability and validity. Am J Commun Psychol 1986;14:195-219. http://dx.doi.org/10.1007/BF00911821.
- Vaux A, Riedel S, Stewart D. Modes of social support – the social support behaviors (SS-B) scale. Am J Commun Psychol 1987;15:209-37. http://dx.doi.org/10.1007/BF00919279.
- Wenger GC. Support networks and dementia. Int J Geriatr Psychiatry 1994;9:181-94. http://dx.doi.org/10.1002/gps.930090303.
- Brandt PA, Weinert C. The RRQ – a social support measure. Nurs Res 1998;30:277-80.
- Weinert C. A social support measure – PRQ85. Nurs Res 1987;36:273-7. http://dx.doi.org/10.1097/00006199-198709000-00007.
Appendix 1 Systematic review (April 2009–September 2013): final value data by outcome and follow-up – forest plots
Global health
FIGURE 22.
Short-term follow-up (< 4 months).
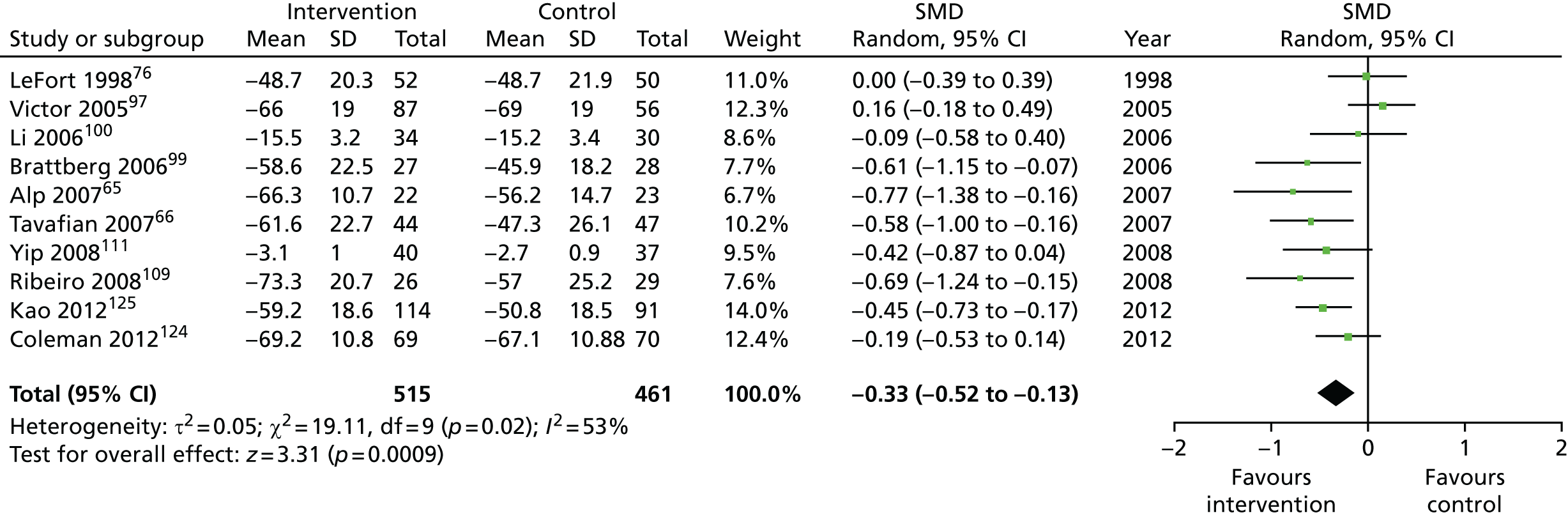
FIGURE 23.
Medium-term follow-up (4–8 months).
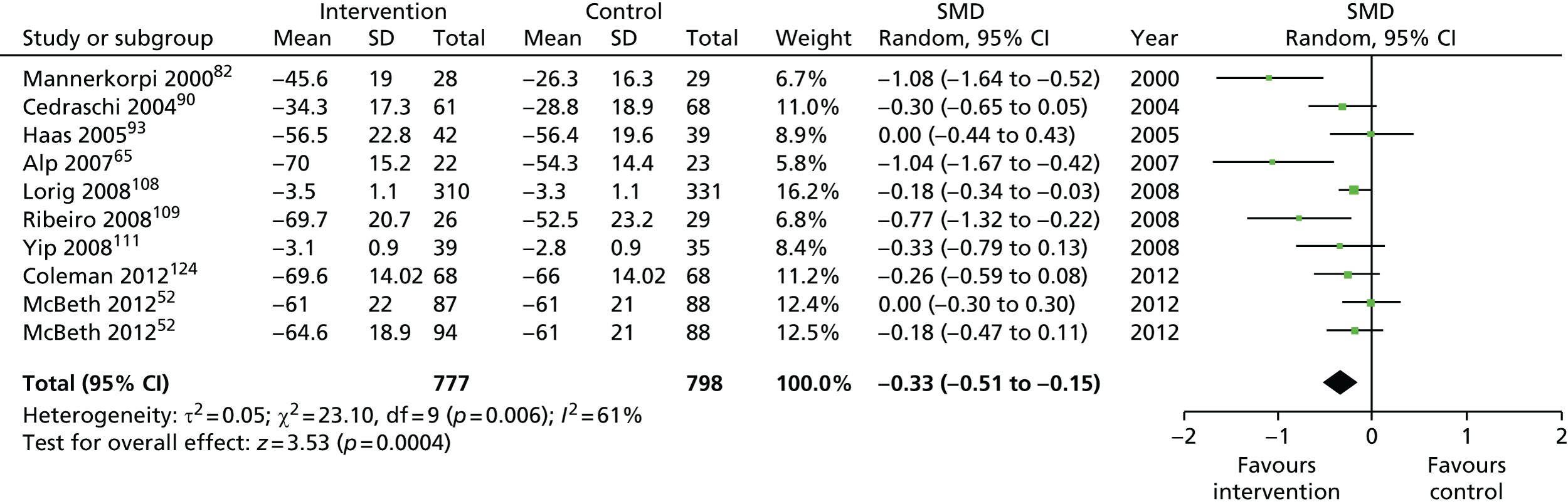
FIGURE 24.
Long-term follow up (> 8 months).
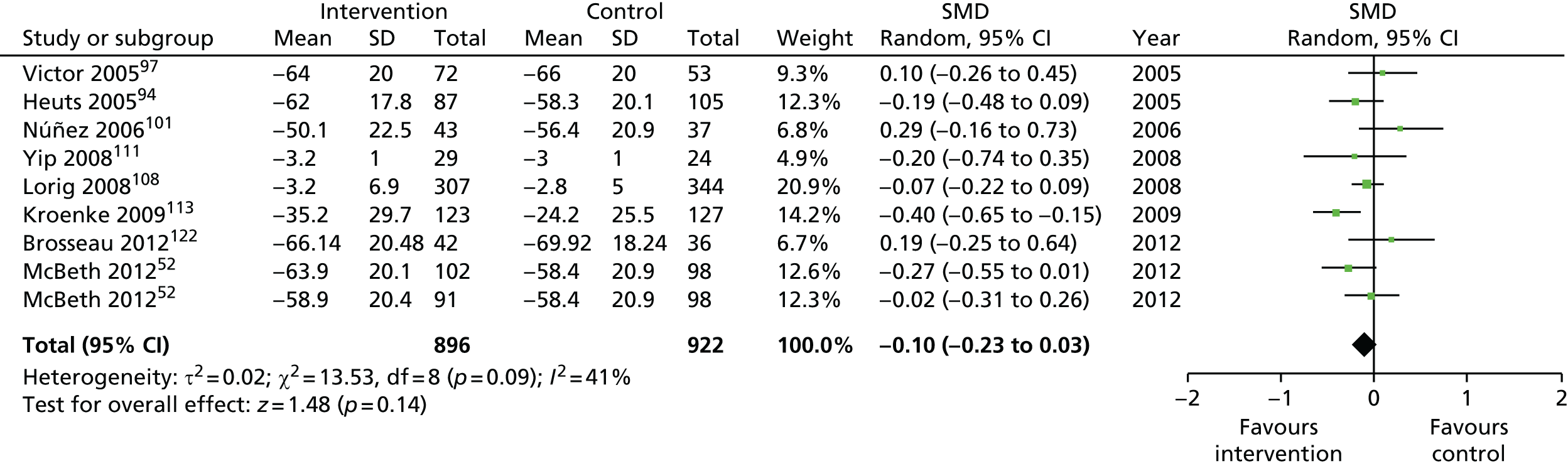
Pain intensity
FIGURE 25.
Short-term follow-up (< 4 months).
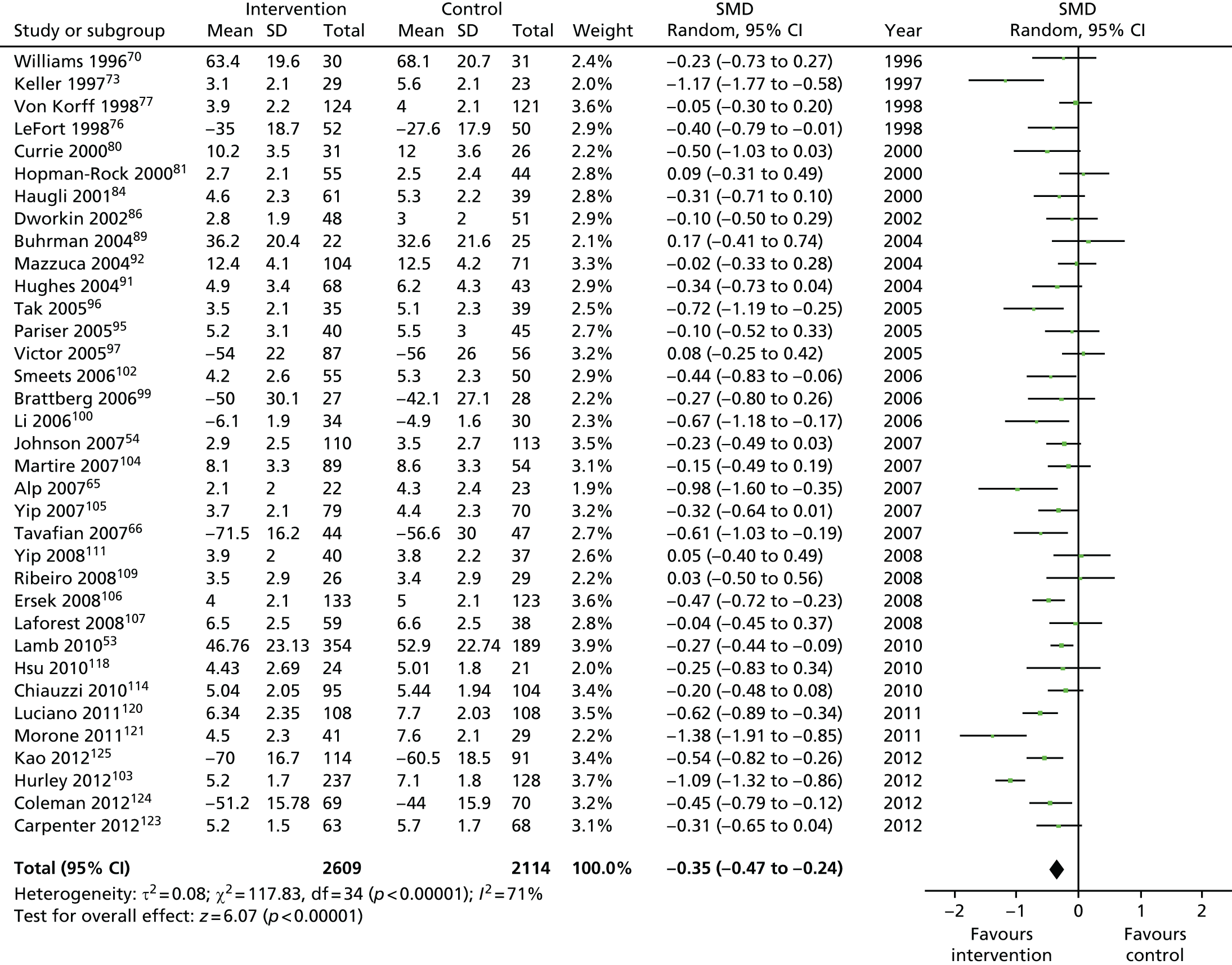
FIGURE 26.
Medium-term follow-up (4–8 months).
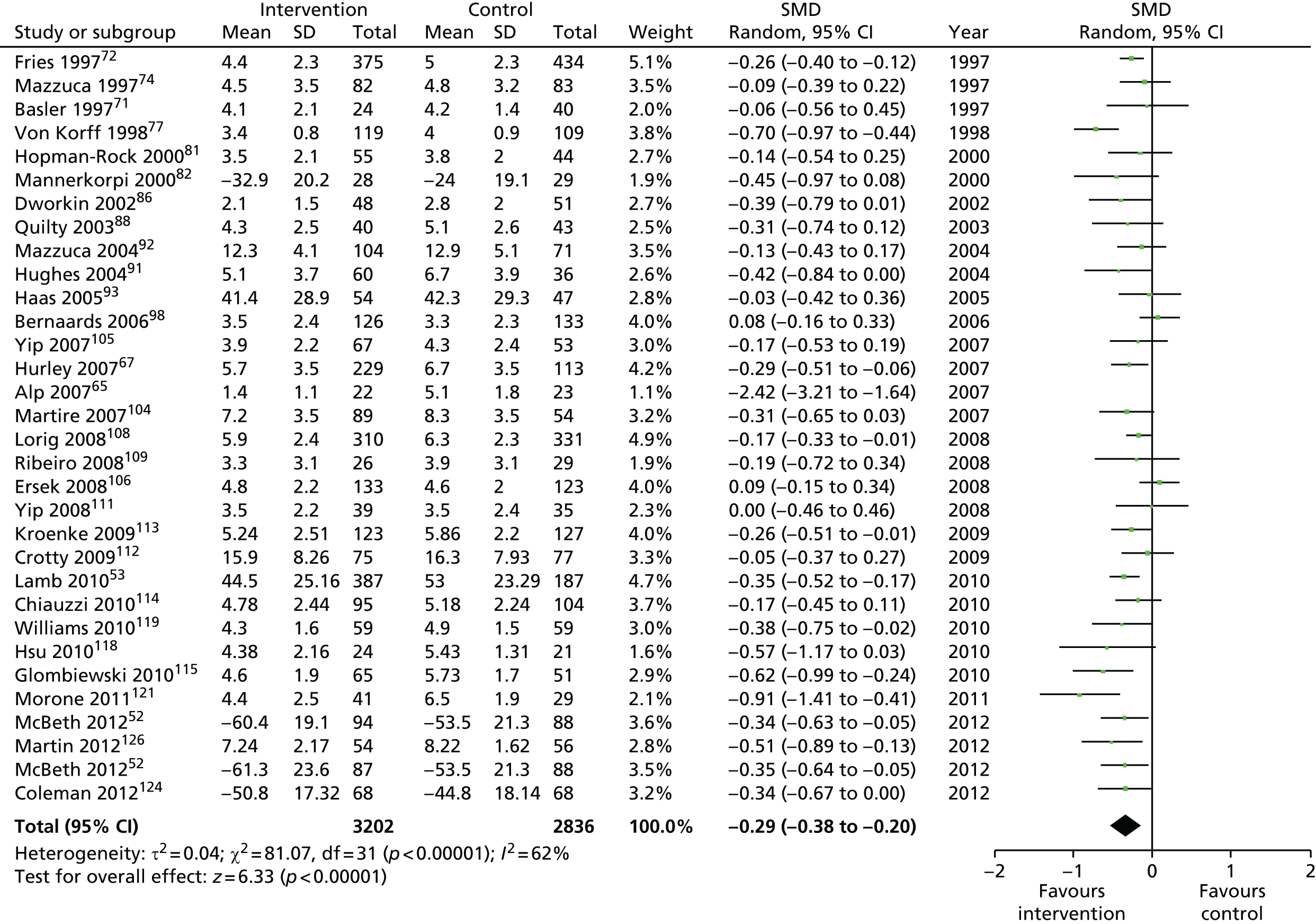
FIGURE 27.
Long-term follow-up (> 8 months).
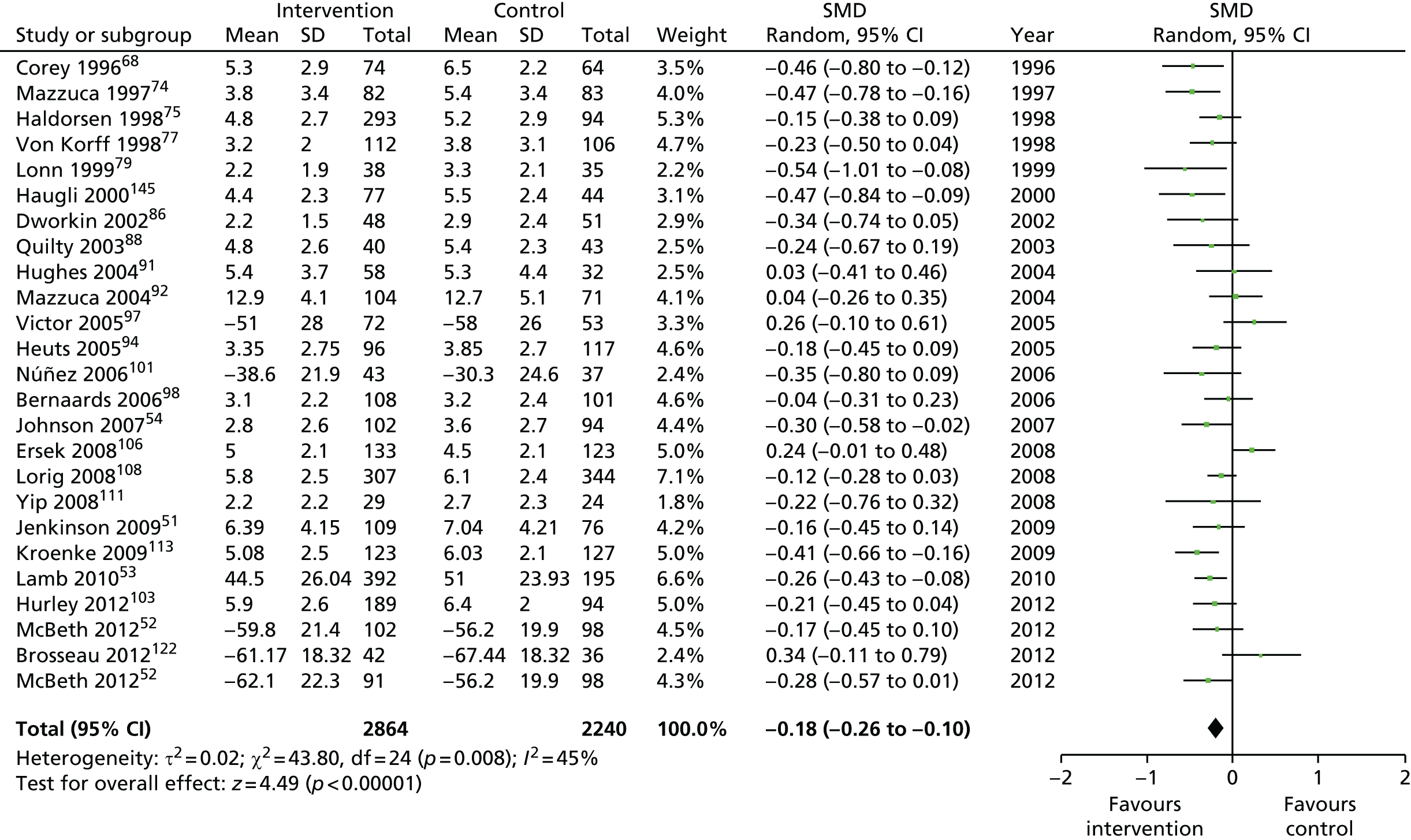
Physical function
FIGURE 28.
Short-term follow-up (< 4 months).
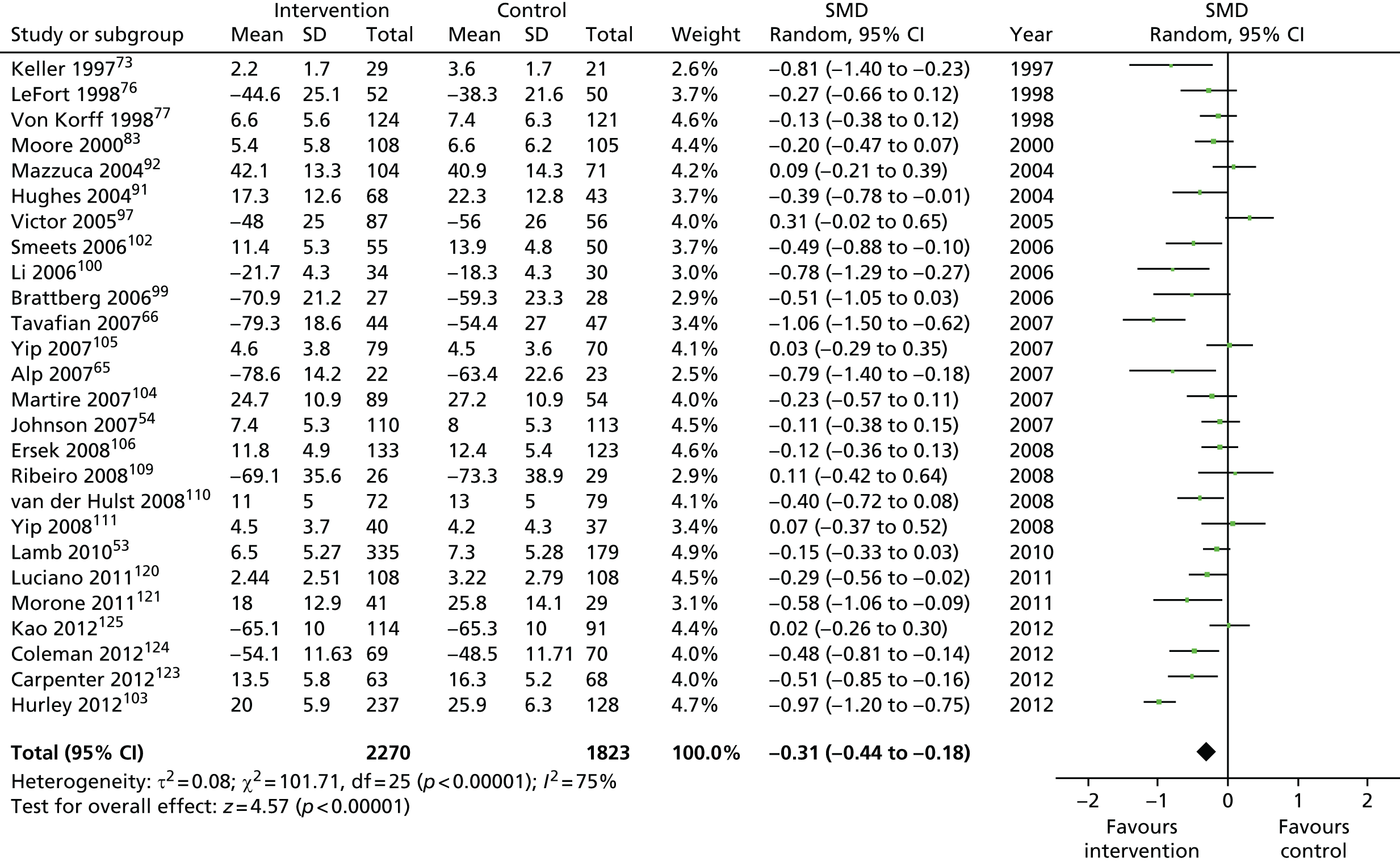
FIGURE 29.
Medium-term follow-up (4–8 months).
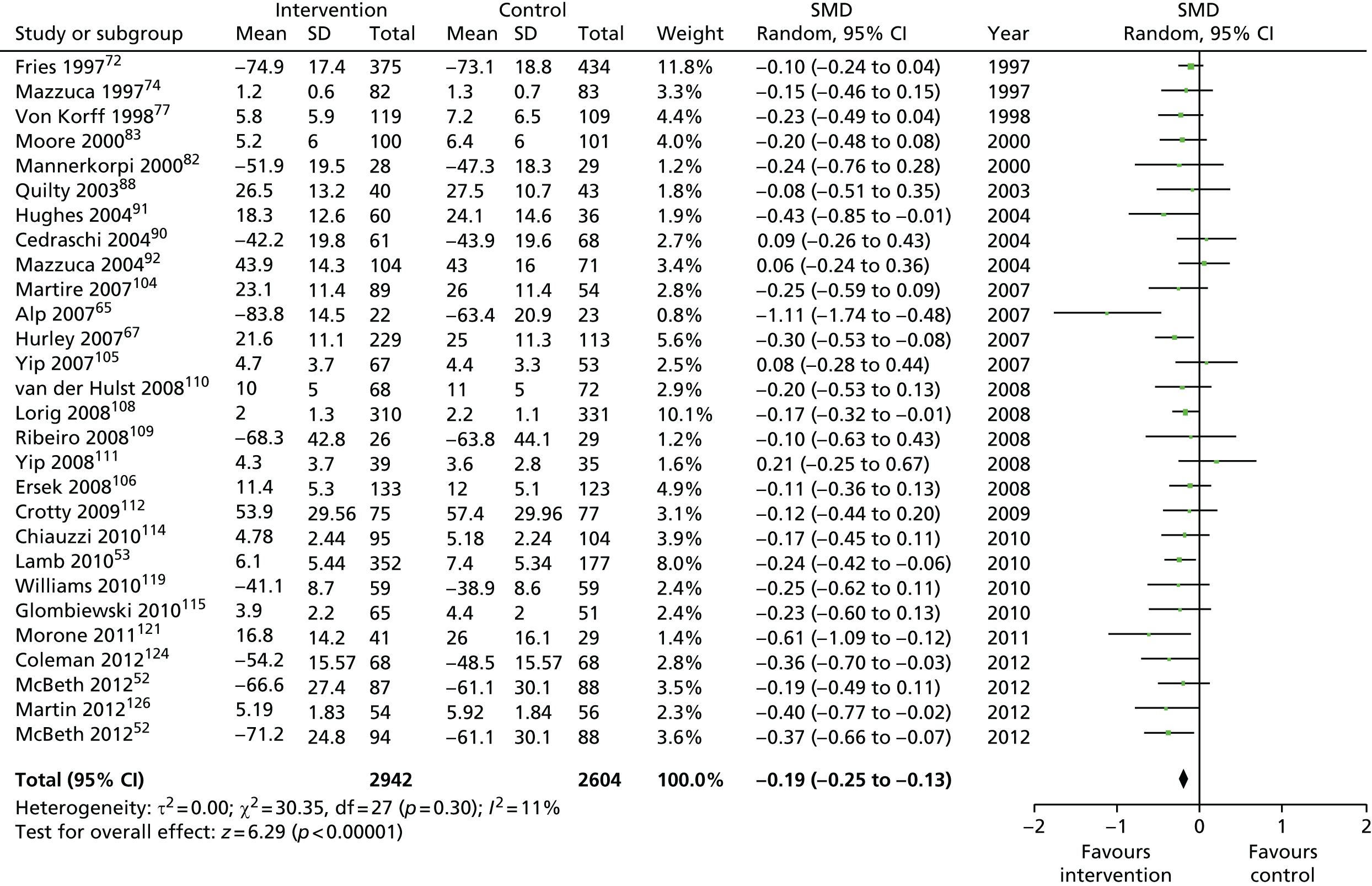
FIGURE 30.
Long-term follow-up (> 8 months).
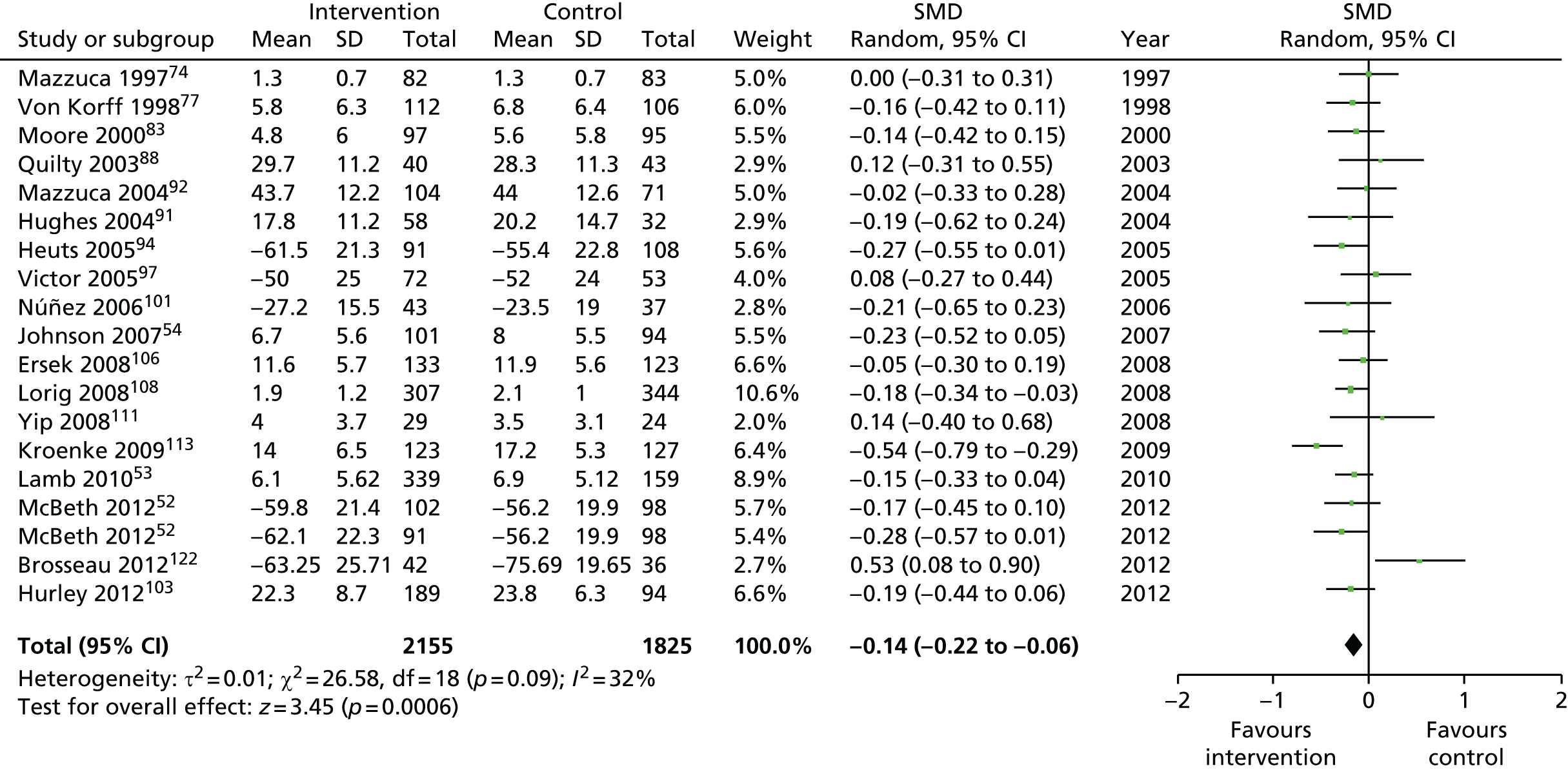
Quality of life
FIGURE 31.
Short-term follow-up (< 4 months).
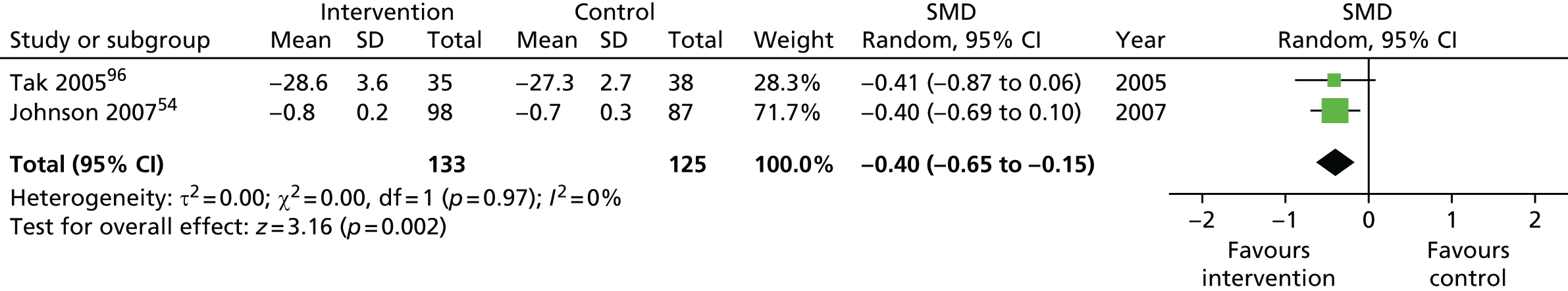
FIGURE 32.
Medium-term follow-up (4–8 months).
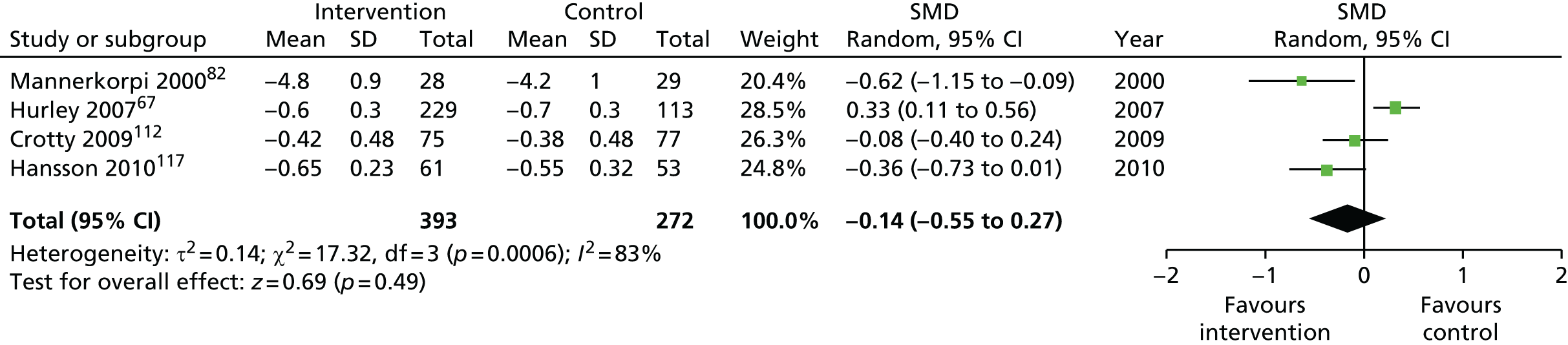
FIGURE 33.
Long-term follow-up (> 8 months).
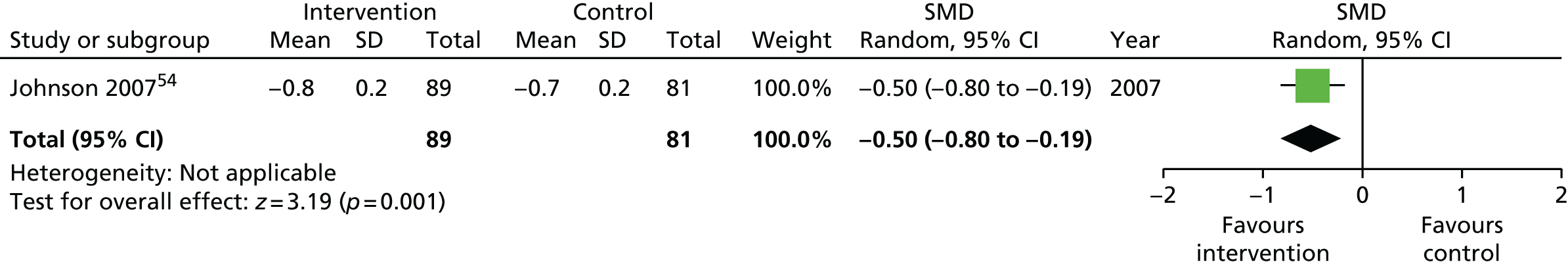
Self-efficacy
FIGURE 34.
Short-term follow-up (< 4 months).
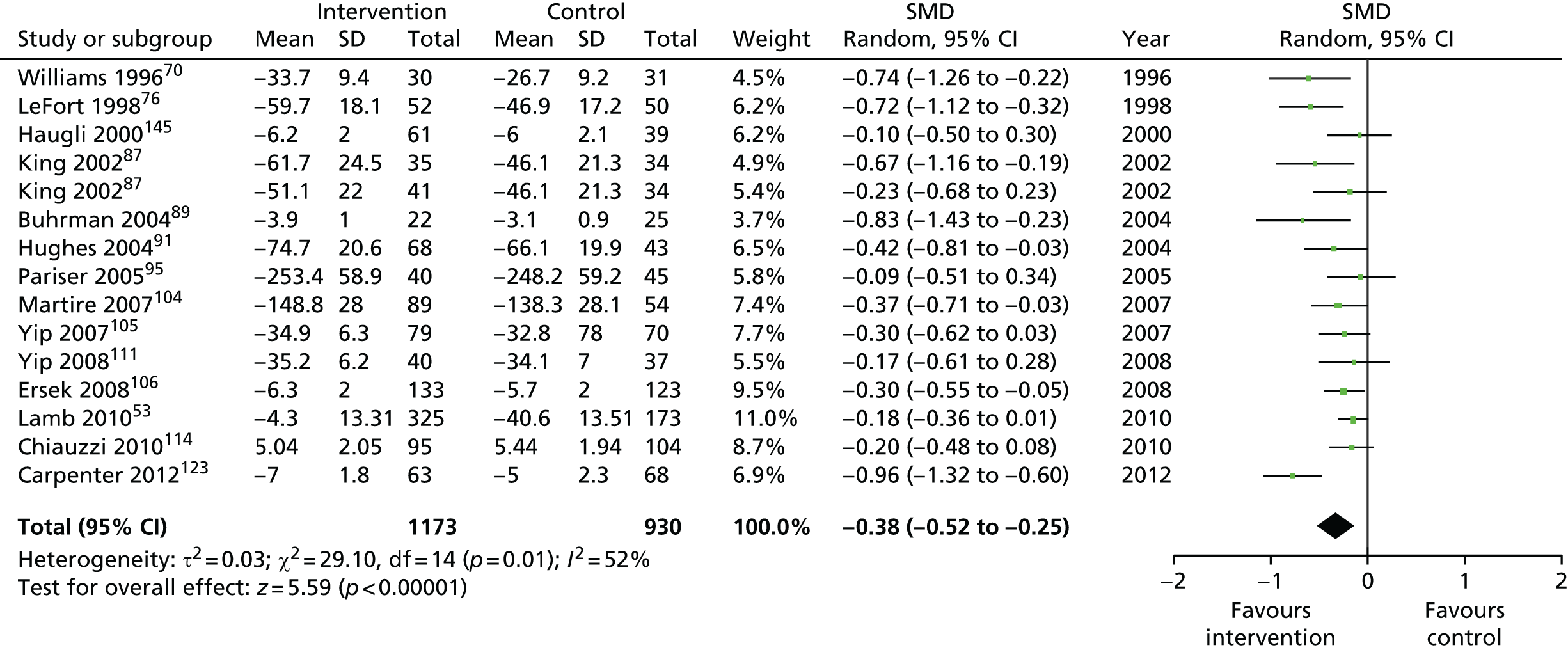
FIGURE 35.
Medium term follow-up (4–8 months).
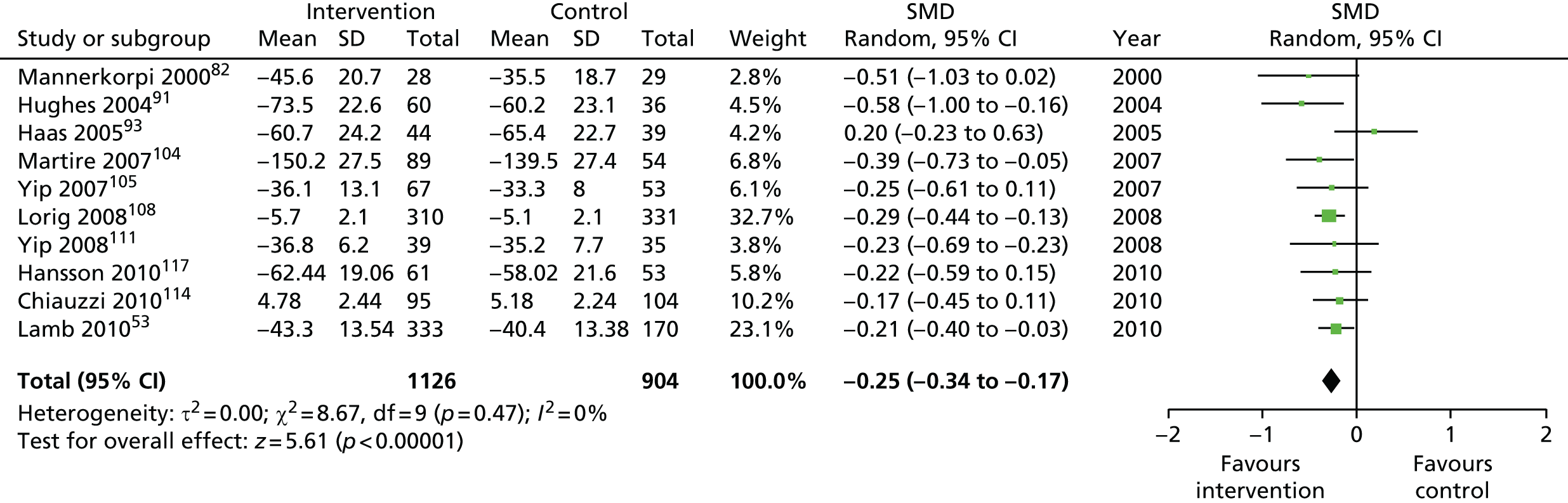
FIGURE 36.
Long-term follow-up (> 8 months).
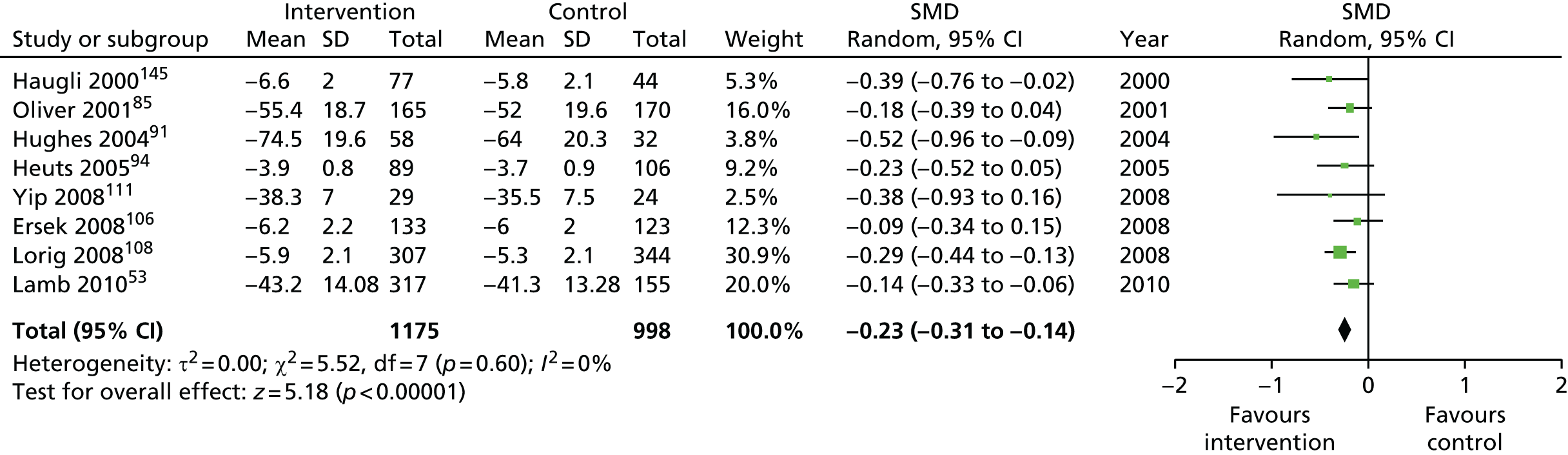
Depression
FIGURE 37.
Short-term follow-up (< 4 months).
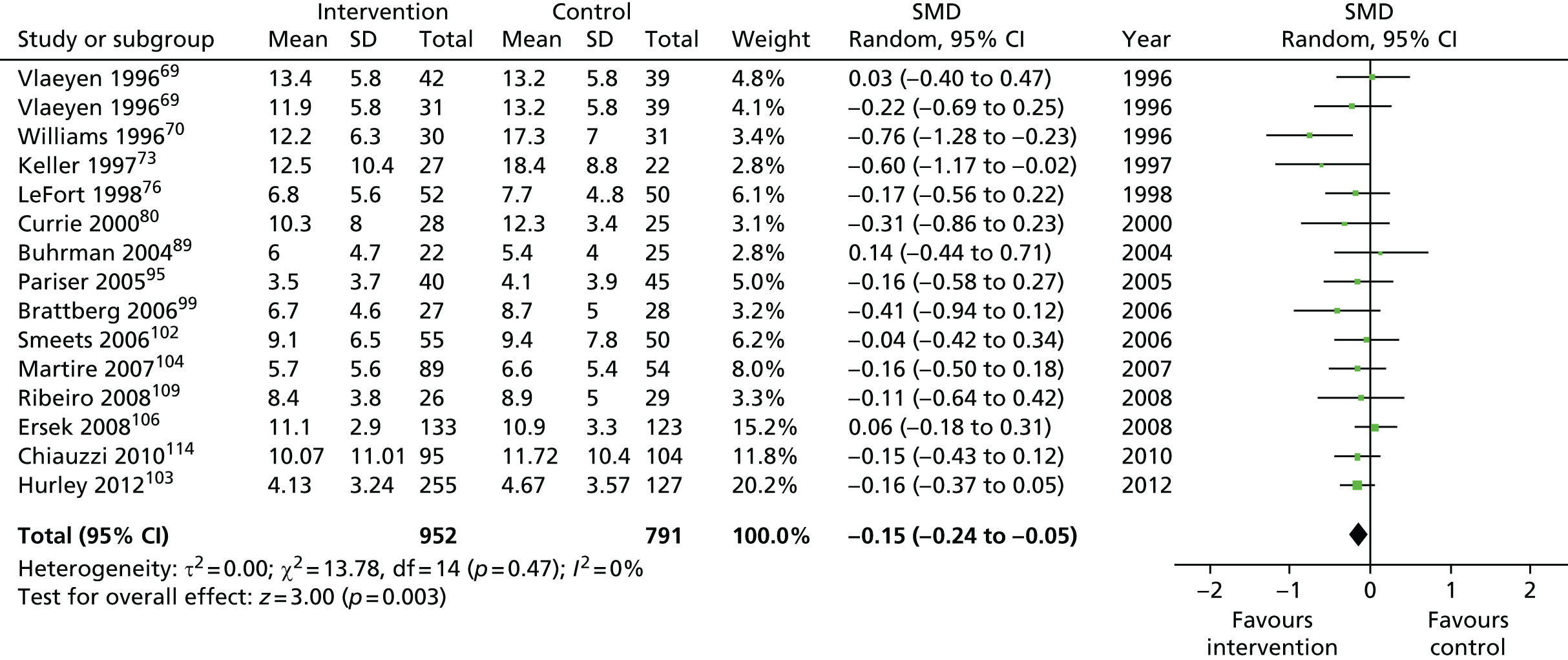
FIGURE 38.
Medium-term follow-up (4–8 months).
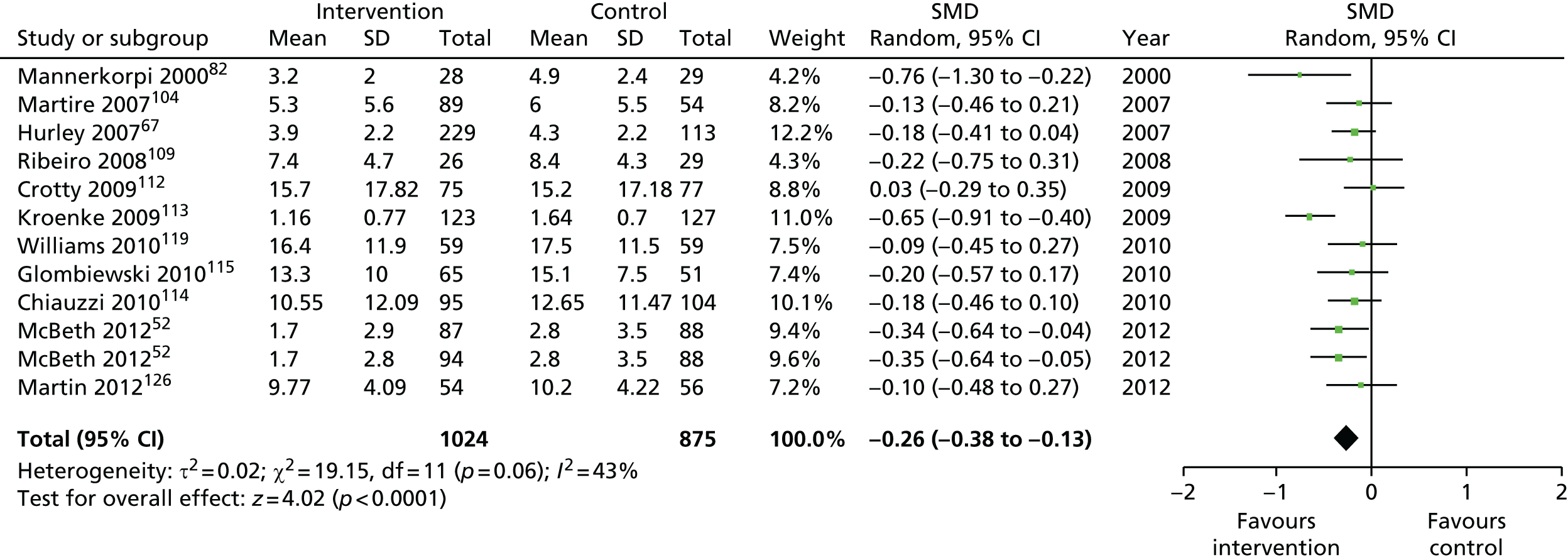
FIGURE 39.
Long-term follow-up (> 8 months).
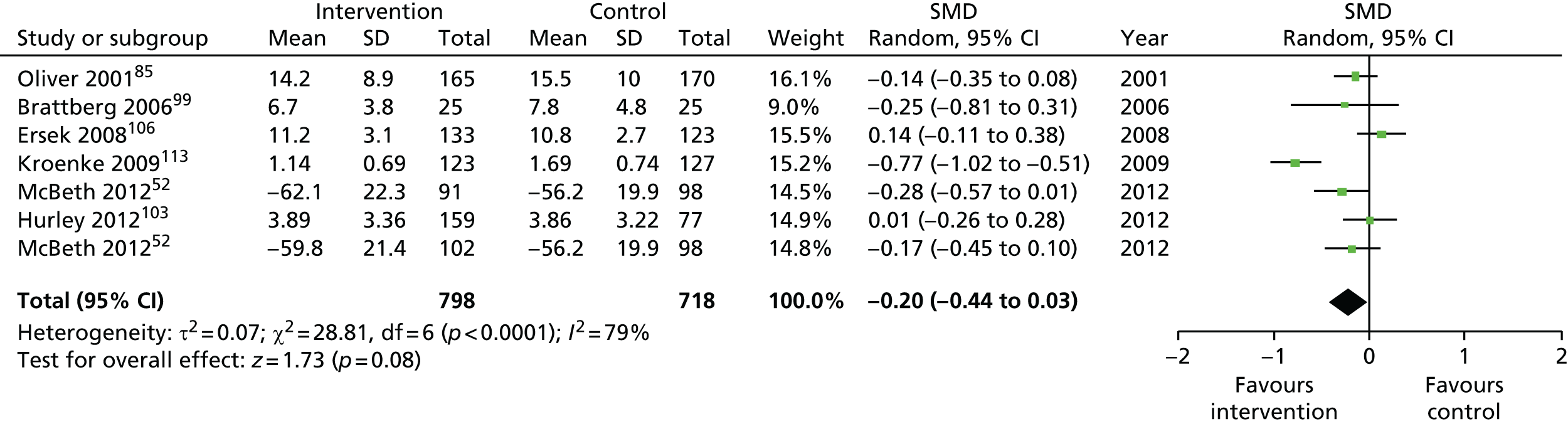
Anxiety
FIGURE 40.
Short-term follow-up (< 4 months).
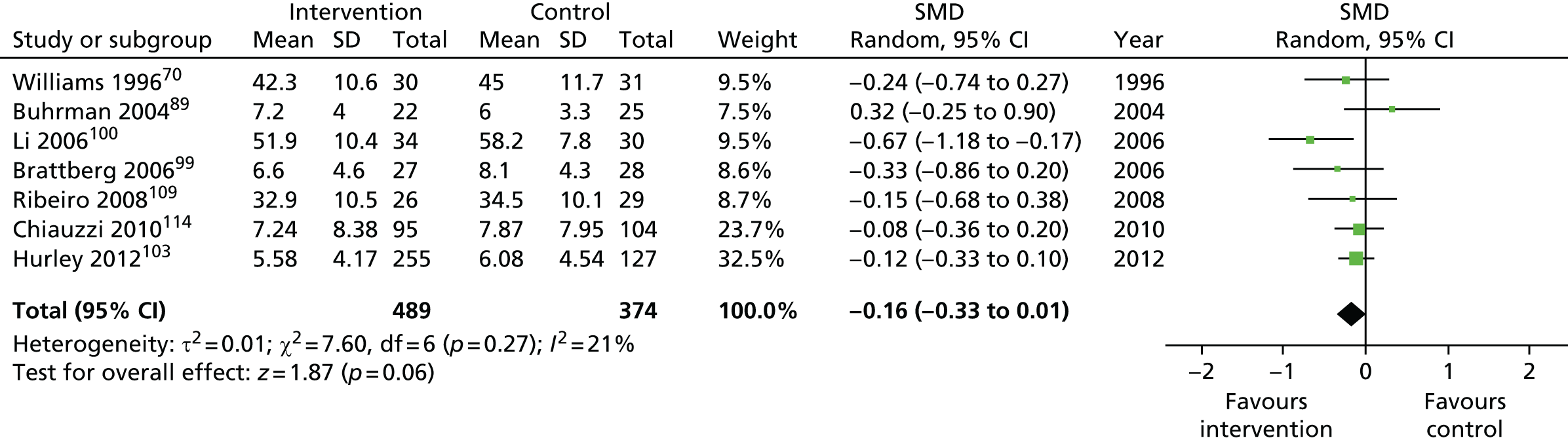
FIGURE 41.
Medium-term follow up (4–8 months).
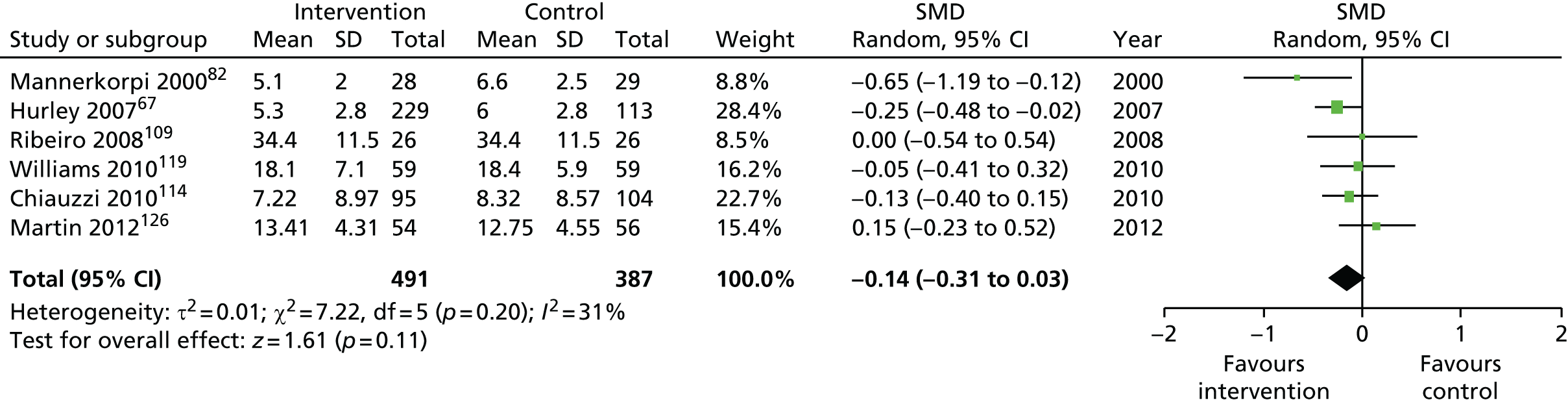
FIGURE 42.
Long-term follow-up (> 8 months).

Social function
FIGURE 43.
Short-term follow-up (< 4 months).

FIGURE 44.
Medium-term follow-up (4–8 months).
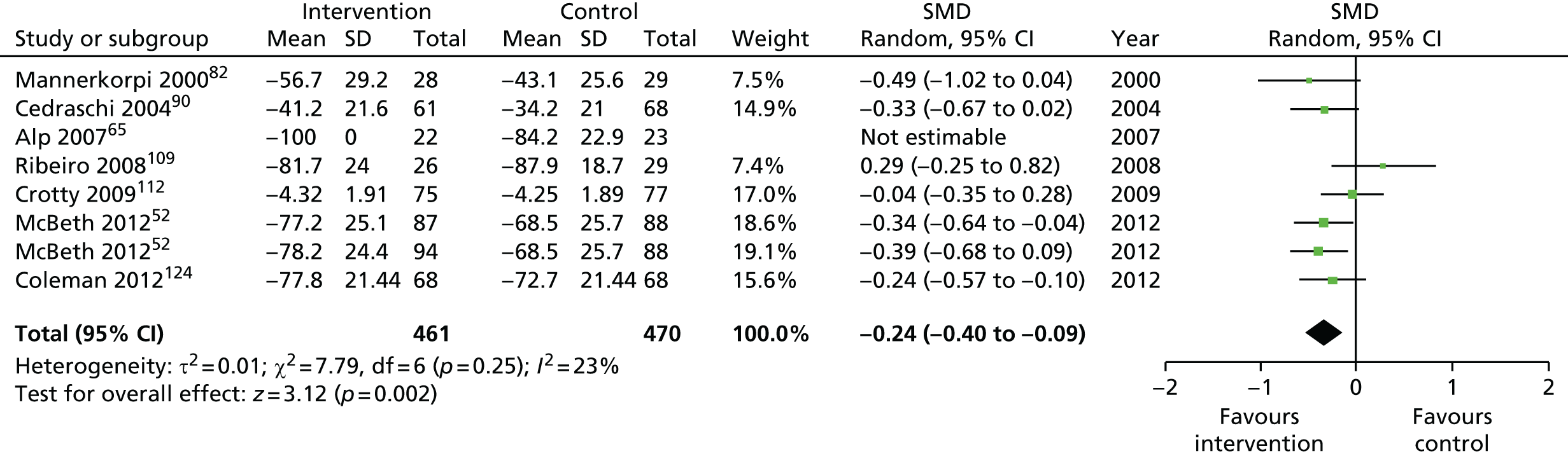
FIGURE 45.
Long-term follow-up (> 8 months).
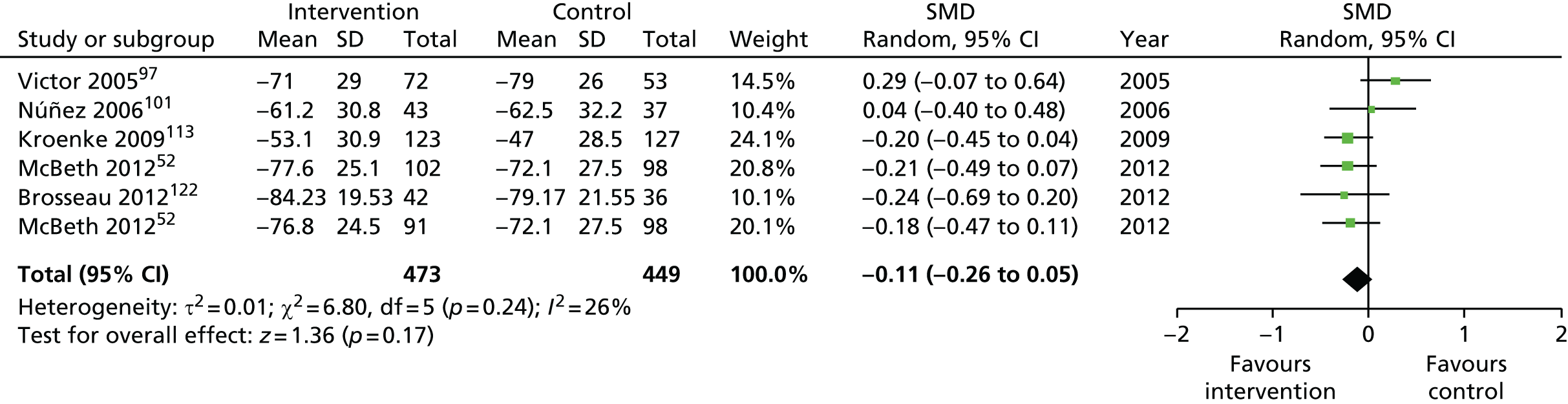
Appendix 2 Systematic review of predictors, mediators and moderators
Search strategies for the review
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
PATIENT EDUCATION AS TOPIC/
-
(patient$ adj (educat$ or train$ or teach$ or instruct$ or skill$ or participat$ or involv$)).ti,ab.
-
(expert adj patient).ti,ab.
-
SELF CARE/
-
SELF EFFICACY/
-
(self adj (manage$ or care or improve$ or develop$ or help or monitor$)).ti,ab.
-
(support adj group).ti,ab.
-
((computer or internet or web or telephone or online) adj base$).ti,ab.
-
((group or clinician or lay or volunteer or professional or expert or advisor or consultant or peer or tutor or educator) adj (led or run)).ti,ab.
-
or/1-9
-
CHRONIC DISEASE/ and PAIN/
-
MUSCULOSKELETAL DISEASES/ and PAIN/
-
LOW BACK PAIN/ or FIBROMYALGIA/ or NECK PAIN/ or SHOULDER PAIN/ or OSTEOARTHRITIS/
-
((chronic or persistent or long-term or wide-spread or recurrent or non-specific or musculoskeletal) adj pain).ti,ab.
-
((lower back or knee or neck or shoulder or hip or thoracic) adj pain).ti,ab.
-
osteoarthriti$.mp. or (osteo$ adj2 pain).ti,ab.
-
(osteoarthriti$ or (osteo$ adj2 pain)).ti,ab.
-
or/11-17
-
10 and 18
EMBASE (via Ovid)
-
PATIENT EDUCATION/
-
(patient$ adj (educat$ or train$ or teach$ or instruct$ or skill$ or participat$ or involv$)).ti,ab.
-
(expert adj patient).ti,ab.
-
SELF CARE/
-
SELF CONCEPT/
-
(self adj (manage$ or care or improve$ or develop$ or help or monitor$)).ti,ab.
-
(support adj group).ti,ab.
-
((computer or internet or web or telephone or online) adj base$).ti,ab.
-
((group or clinician or lay or volunteer or professional or expert or advisor or consultant or peer or tutor or educator) adj (led or run)).ti,ab.
-
or/1-9
-
CHRONIC PAIN/ and MUSCULOSKELETAL DISEASE/
-
MUSCULOSKELETAL PAIN/
-
SPINAL PAIN/ or LIMB PAIN/ or FIBROMYALGIA/ or NECK PAIN/ or SHOULDER PAIN/ or OSTEOARTHRITIS/
-
((chronic or persistent or long-term or wide-spread or recurrent or non-specific or musculoskeletal) adj pain).ti,ab.
-
((lower back or knee or neck or shoulder or hip or thoracic) adj pain).ti,ab.
-
osteoarthriti$.mp. or (osteo$ adj2 pain).ti,ab. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(osteoarthriti$ or (osteo$ adj2 pain)).ti,ab.
-
or/11-17
-
10 and 18
PsycINFO (via Ovid)
-
CLIENT EDUCATION/
-
(patient$ adj (educat$ or train$ or teach$ or instruct$ or skill$ or participat$ or involv$)).ti,ab.
-
(expert adj patient).ti,ab.
-
SELF MANAGEMENT/
-
SELF EFFICACY/
-
(self adj (manage$ or care or improve$ or develop$ or help or monitor$)).ti,ab.
-
(support adj group).ti,ab.
-
((computer or internet or web or telephone or online) adj base$).ti,ab.
-
((group or clinician or lay or volunteer or professional or expert or advisor or consultant or peer or tutor or educator) adj (led or run)).ti,ab.
-
or/1-9
-
MUSCULOSKELETAL DISORDERS/ and CHRONIC PAIN/
-
BACK PAIN/ or FIBROMYALGIA/
-
((chronic or persistent or long-term or wide-spread or recurrent or non-specific or musculoskeletal) adj pain).ti,ab.
-
((lower back or neck or knee or thoracic or shoulder or hip) adj pain).ti,ab.
-
(osteoarthriti$ or (osteo$ adj2 pain)).ti,ab.
-
or/11-15
-
10 and 16
Health Information Resources (www.nlh.nhs.uk) (Cumulative Index to Nursing and Allied Health Literature, Allied and Complementary Medicine Database)
-
(self AND (manage* OR help OR improve* OR care OR monitor* OR develop*)).ti,ab
-
(support group).ti,ab
-
(patient* AND (educat* OR train* OR teach* OR instruct* OR skill* OR participat* OR involv*)).ti,ab
-
(expert patient).ti,ab
-
((computer OR internet OR web OR telephone OR online) AND base*).ti.ab
-
((group OR clinician OR lay OR volunteer OR professional OR expert OR advisor OR consultant OR peer OR tutor OR educator) AND (led OR run)).ti,ab
-
OR (1-6)
-
(((chronic OR persistent OR long-term OR wide-spread OR recurrent OR non-specific OR musculoskeletal OR thoracic) AND pain)).ti,ab
-
((lower back OR neck OR knee OR shoulder OR spinal OR shoulder OR hip) AND pain).ti,ab
-
(osteoarthriti* OR (osteo* AND pain)).ti,ab
-
OR (8-10)
-
7 AND 11
The Cochrane Library (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects and Cochrane Central Register of Controlled Trials)
(self NEAR/1 (manage* OR help OR improve* OR care OR monitor* OR develop*)) OR support group OR (patient* NEAR/1 (educat* OR train* OR teach* OR instruct* OR skill* OR participat* OR involv*)) OR expert patient* (computer OR internet OR web OR telephone OR online) OR ((group OR clinician OR lay OR volunteer OR professional OR expert OR advisor OR consultant OR peer OR tutor OR educator) NEAR/1 (led OR run)) in Title, Abstract or Keywords
AND
(((chronic OR persistent OR long-term OR wide-spread OR recurrent OR non-specific OR musculoskeletal OR thoracic) NEAR/1 pain)) OR ((lower back OR neck OR knee OR shoulder OR spinal OR shoulder OR hip) NEAR/1 pain) OR osteoarthrit* in Title, Abstract or Keywords
Web of Science (Social Sciences Citation Index)
((self SAME (manage* OR help OR care)) OR “support group” OR (patient* SAME (educat* OR train* OR teach* OR instruct* OR skill*)) OR “expert patient” OR ((group OR clinician OR lay OR volunteer OR professional OR expert OR peer OR tutor OR educator) SAME (led OR run))) in Topic
AND
(((chronic OR persistent OR long-term OR wide-spread OR recurrent OR non-specific OR musculoskeletal OR thoracic) SAME pain) OR ((lower back OR neck OR knee OR shoulder OR spinal OR shoulder OR hip) SAME pain) OR osteoarthri*) in Topic
Meta-regression
To examine the impact of moderator variables, we used meta-regression analyses for the outcomes listed in Table 75. Age and gender were chosen as the moderators to assess as these are the most frequently reported demographics and we included at least six studies that reported these moderators for each outcome. All calculations were performed using Stata 11.
| Outcome | Short term (< 4 months), n | Medium term (4–8 months), n | Long term (> 8 months), n |
|---|---|---|---|
| Pain intensity | 26 | 20 | 18 |
| Physical/functional capability | 19 | 18 | 13 |
| Self-efficacy | 12 | 7 | 7 |
| Depression | 13 | 4 | 3 |
| SF-36 general mental health | 9 | 5 | 4 |
| Global health status | 8 | 7 | 5 |
There were few observations for some outcomes when considering only one time point. In addition, modelling each time point separately gave rise to multiplicity, increasing the type 1 error substantially. Therefore, the different time points were collapsed giving one average effect size to represent the different time points per outcome. Results from the meta-regression were considered statistically significant if p < 0.05, marginally significant if p < 0.10 and non-significant if p > 0.10. p-values between 0.05 and 0.10 were noted in light of potential type 2 errors as a result of the limited number of effect sizes in some of our pooled effect size calculations. 32
Table 76 presents the results for the meta-regression analyses with age and gender as moderators. Gender was not associated with effect sizes for pain intensity, physical/functional capability, self-efficacy and depression. Gender was marginally significantly associated with effect sizes for SF-36 general mental health and global health status (all p < 0.10), suggesting a positive association between effect sizes for these outcomes, that is, that self-management interventions favoured samples that included a lower percentage of males. Age was also marginally significantly associated with effect sizes for physical/functional capability and self-efficacy (all p < 0.10), suggesting a positive association between effect sizes for these outcomes, that is, that self-management interventions favoured younger samples.
| Measure | Studies, n | Moderator | Adjusted R2 (%) | I 2 | t | p-value | 95% CI |
|---|---|---|---|---|---|---|---|
| Pain intensity | 39 | Gender | –12.17 | 47.66 | –0.20 | 0.840 | –0.0062 to 0.0051 |
| Physical/functional capability | 27 | Gender | –3.57 | 51.84 | –0.58 | 0.560 | –0.0084 to 0.0047 |
| Self-efficacy | 17 | Gender | –20.19 | 32.36 | –0.44 | 0.732 | –0.0115 to 0.0082 |
| Depression | 16 | Gender | –18.67 | 24.23 | –0.64 | 0.533 | –0.0108 to 0.0058 |
| SF-36 general mental health | 11 | Gender | 35.48 | 51.60 | 1.86 | 0.095 | –0.0021 to 0.0214 |
| Global health status | 14 | Gender | 13.50 | 59.06 | 2.12 | 0.065 | –0.0003 to 0.0230 |
| Pain intensity | 39 | Age | 20.70 | 43.09 | –1.61 | 0.116 | –0.0114 to 0.0121 |
| Functional capability | 28 | Age | 28.27 | 45.84 | 1.86 | 0.074 | –0.0008 to 0.0164 |
| Self-efficacy | 17 | Age | 46.56 | 17.32 | 1.98 | 0.060 | –0.0004 to 0.0165 |
| Depression | 16 | Age | 42.30 | 13.27 | 1.62 | 0.156 | –0.0025 to 0.0144 |
| SF-36 general mental health | 11 | Age | 35.35 | 53.17 | 1.47 | 0.176 | –0.0082 to 0.0317 |
| Global health status | 14 | Age | 12.72 | 61.16 | 1.29 | 0.223 | –0.0085 to 0.0402 |
Heterogeneity
Study heterogeneity was generally in the mild to moderate range (I2 = 13.27–61.16%). Of the 12 comparisons, none exceeded 65%; five exceeded 50%, suggesting moderate to high levels of heterogeneity; four fell between 25% and 50%, suggesting low to moderate levels of heterogeneity; and three fell below 25%, suggesting low levels of heterogeneity and a high concordance between studies.
Meta-regression results
FIGURE 46.
Pain intensity, sex and effect size.
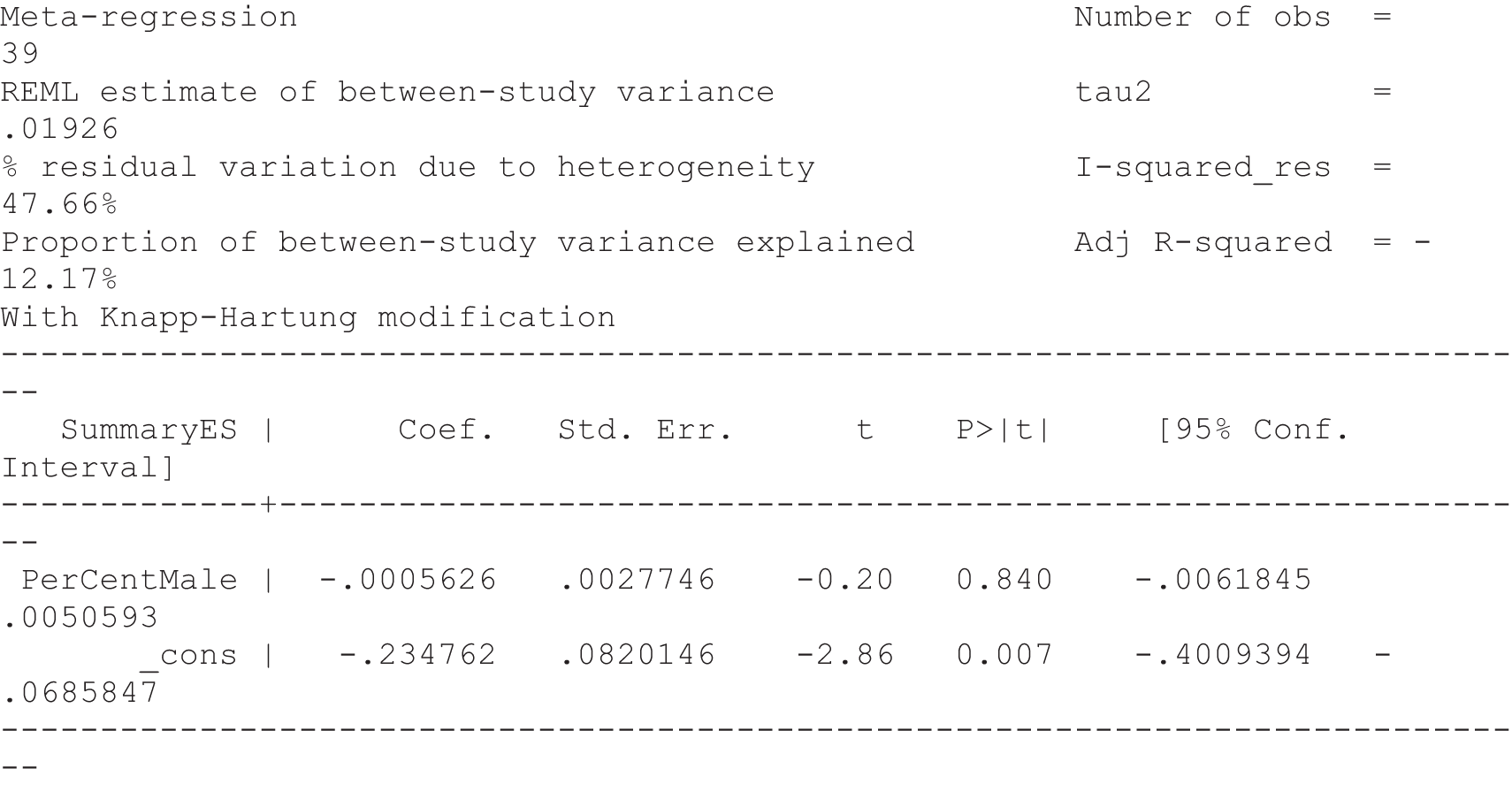
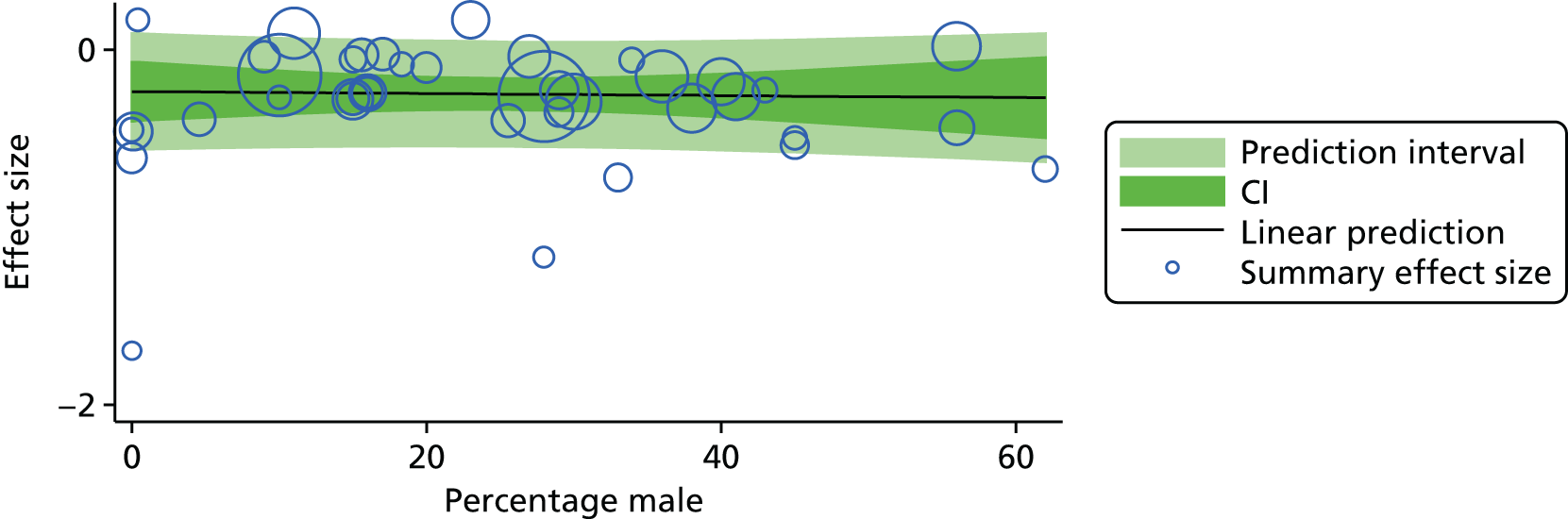
FIGURE 47.
Pain intensity, age and effect size.
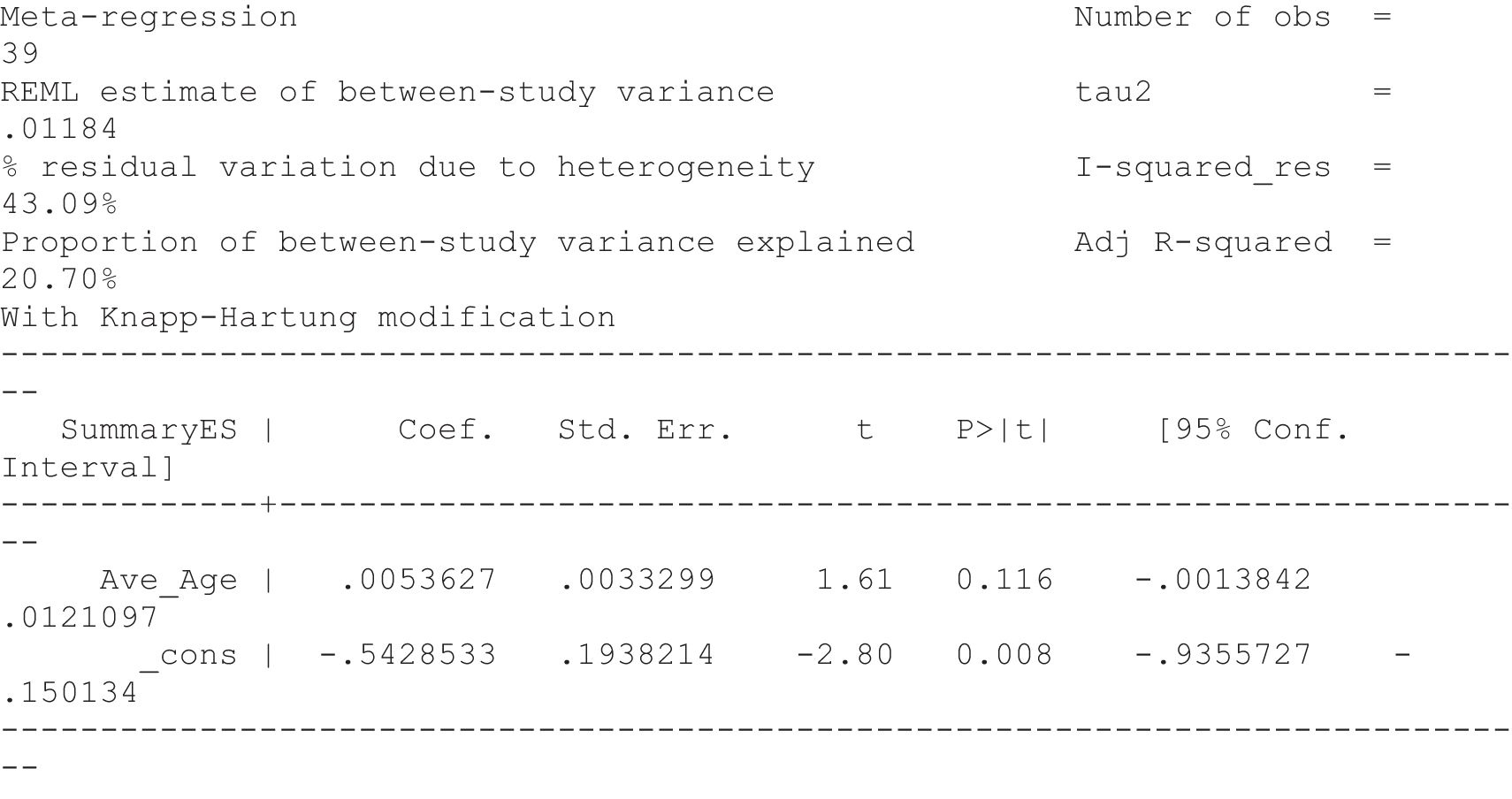
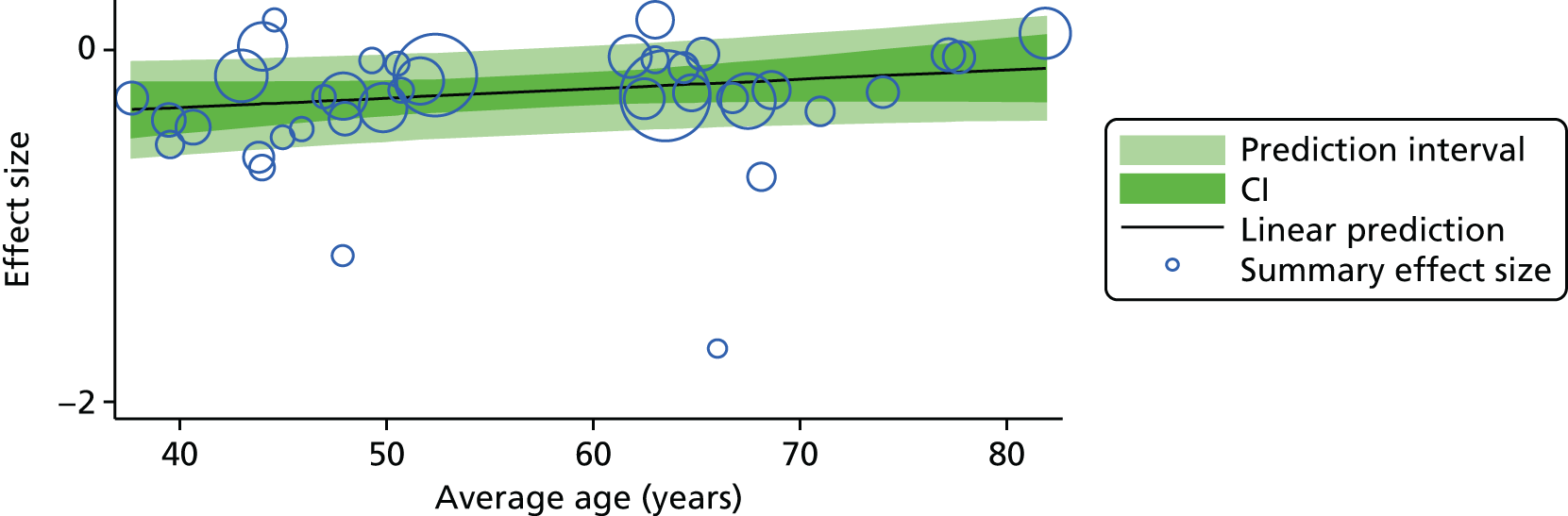
FIGURE 48.
Physical/functional capability, sex and effect size.
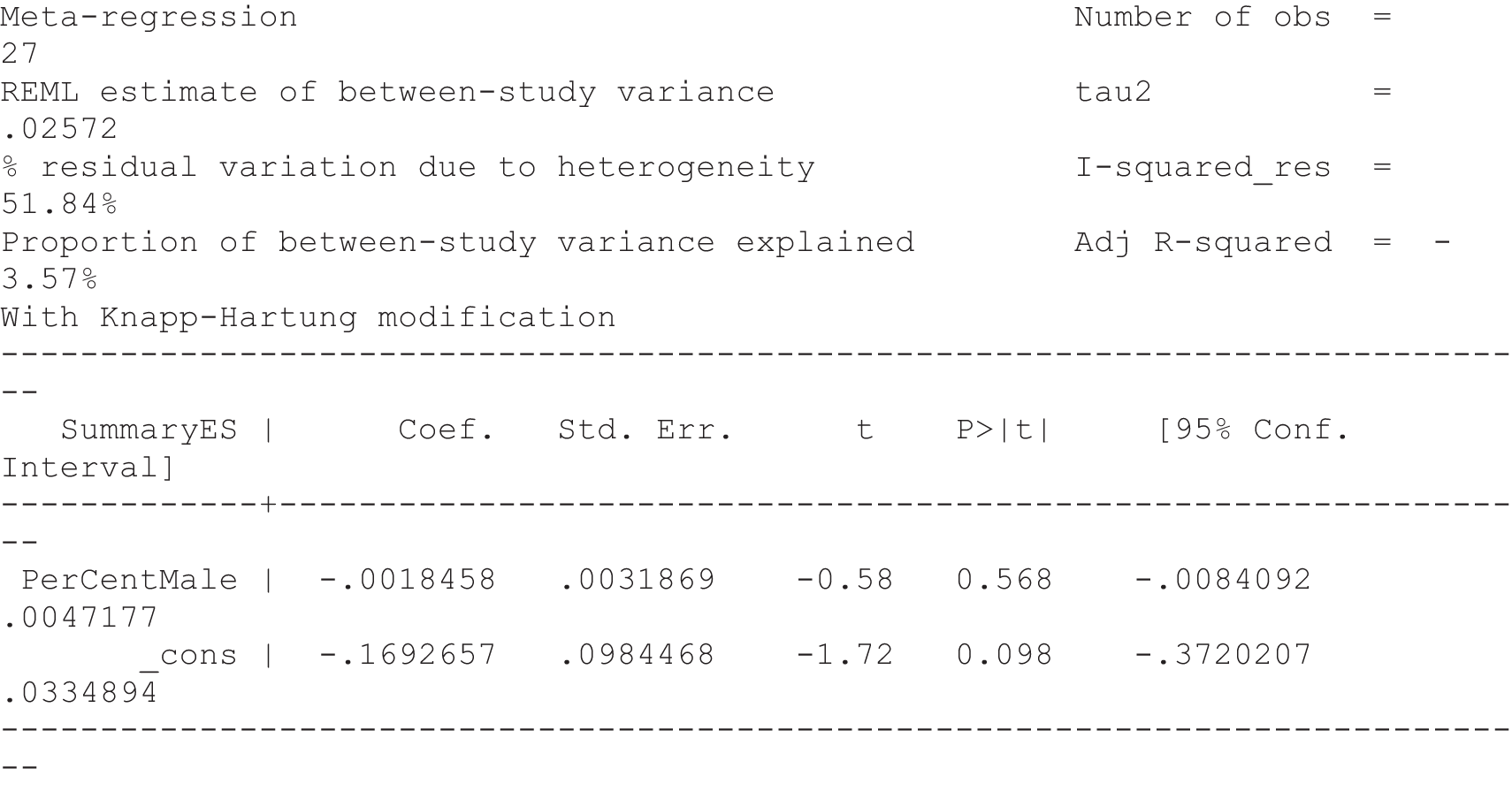
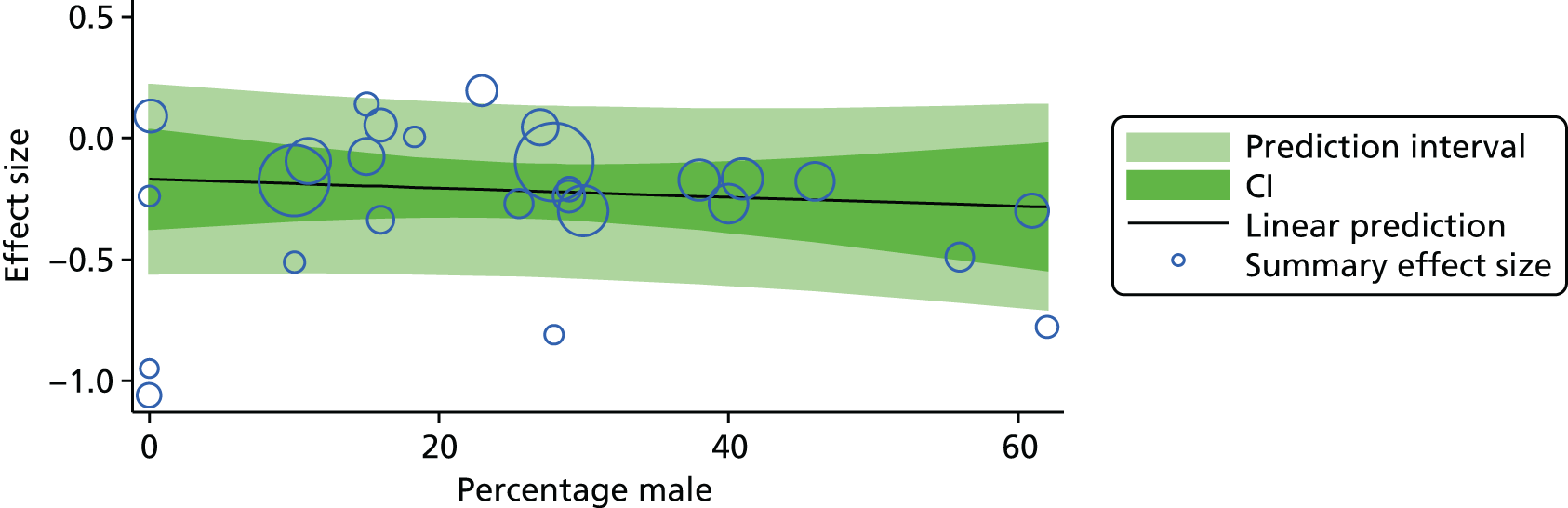
FIGURE 49.
Functional capability, age and effect size.
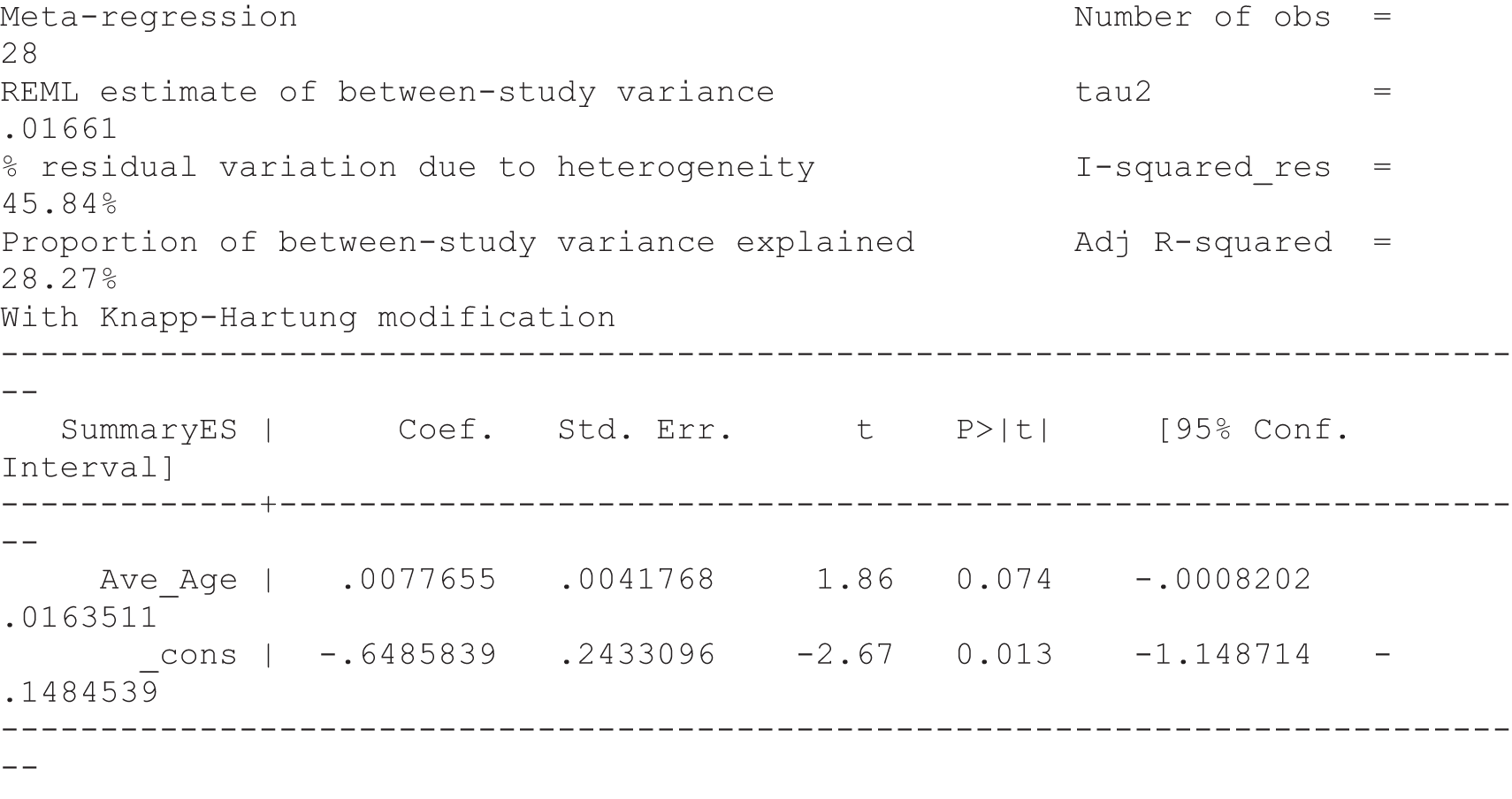
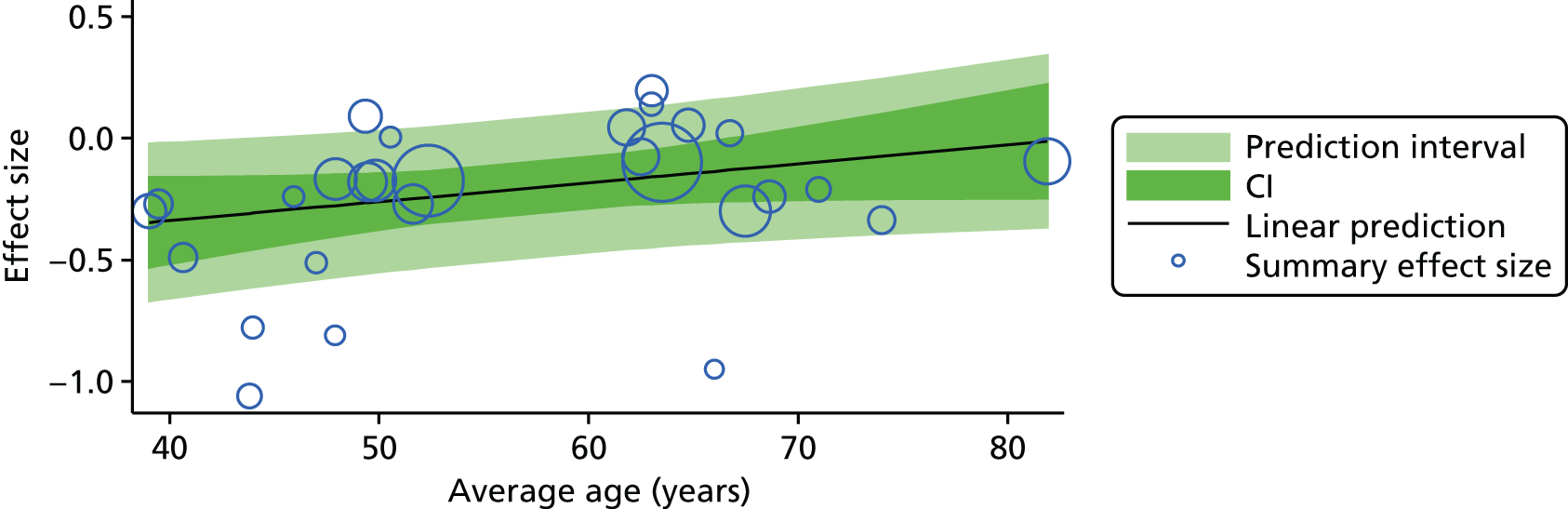
FIGURE 50.
Self-efficacy, sex and effect size.
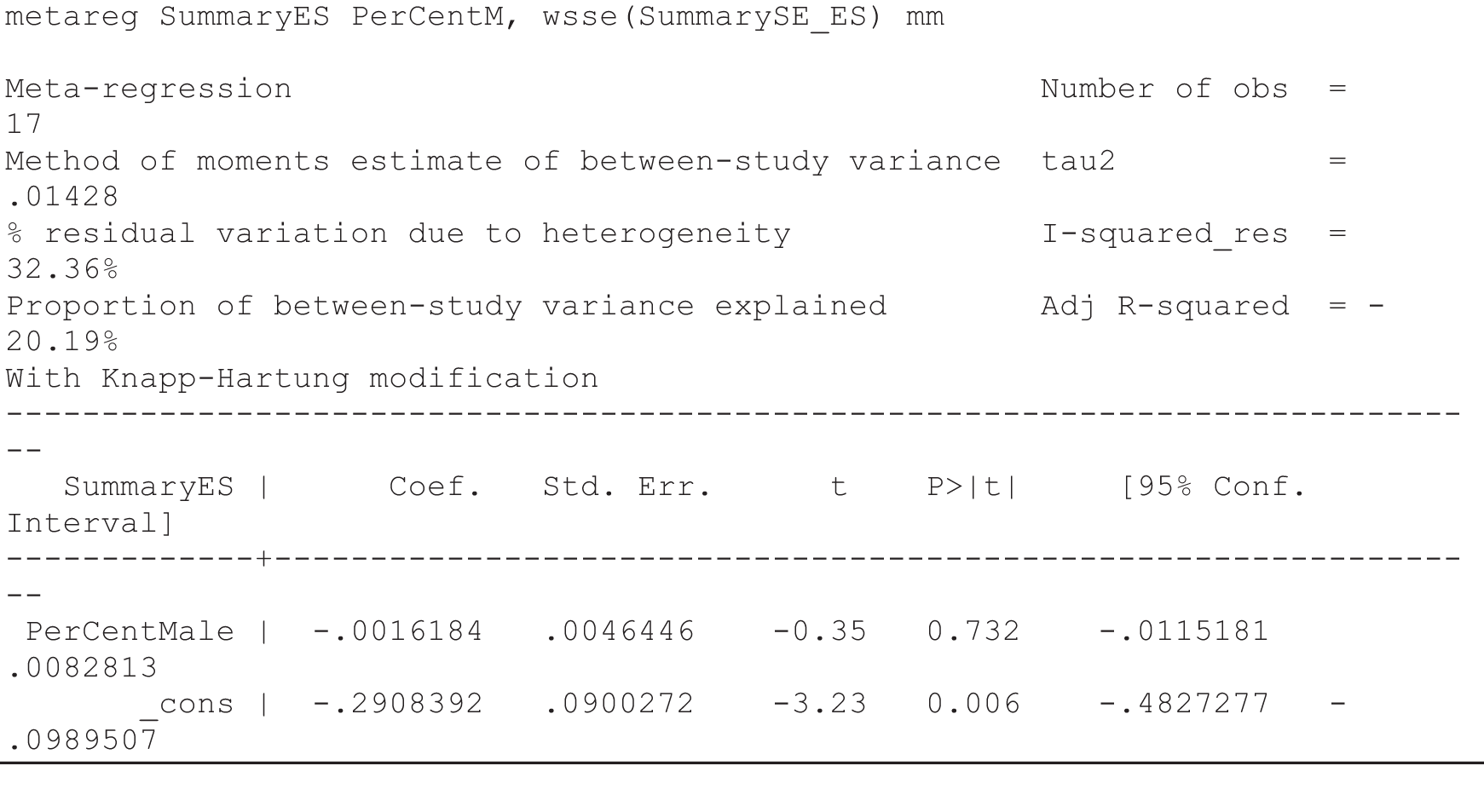

FIGURE 51.
Self-efficacy, age and effect size.
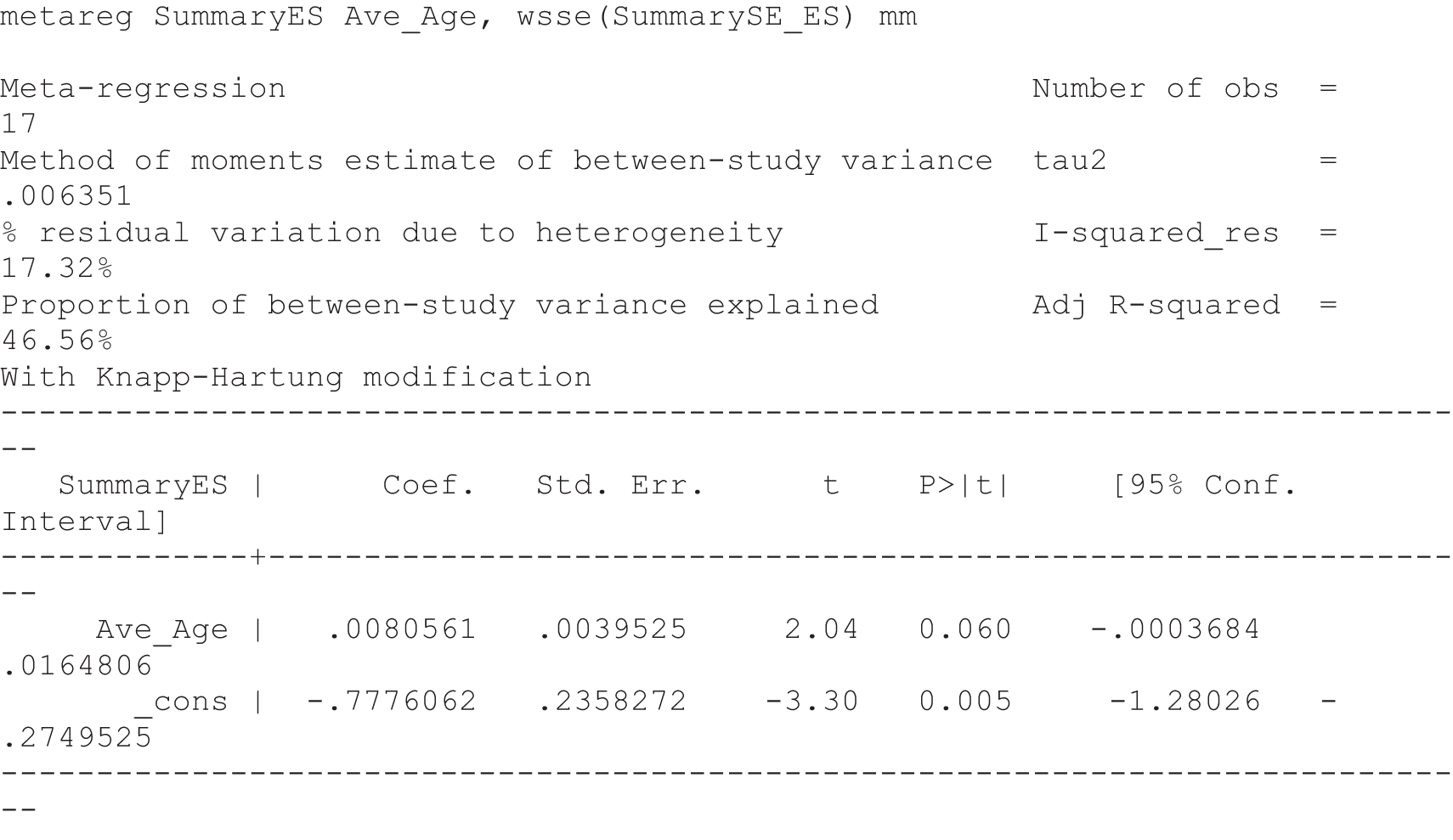
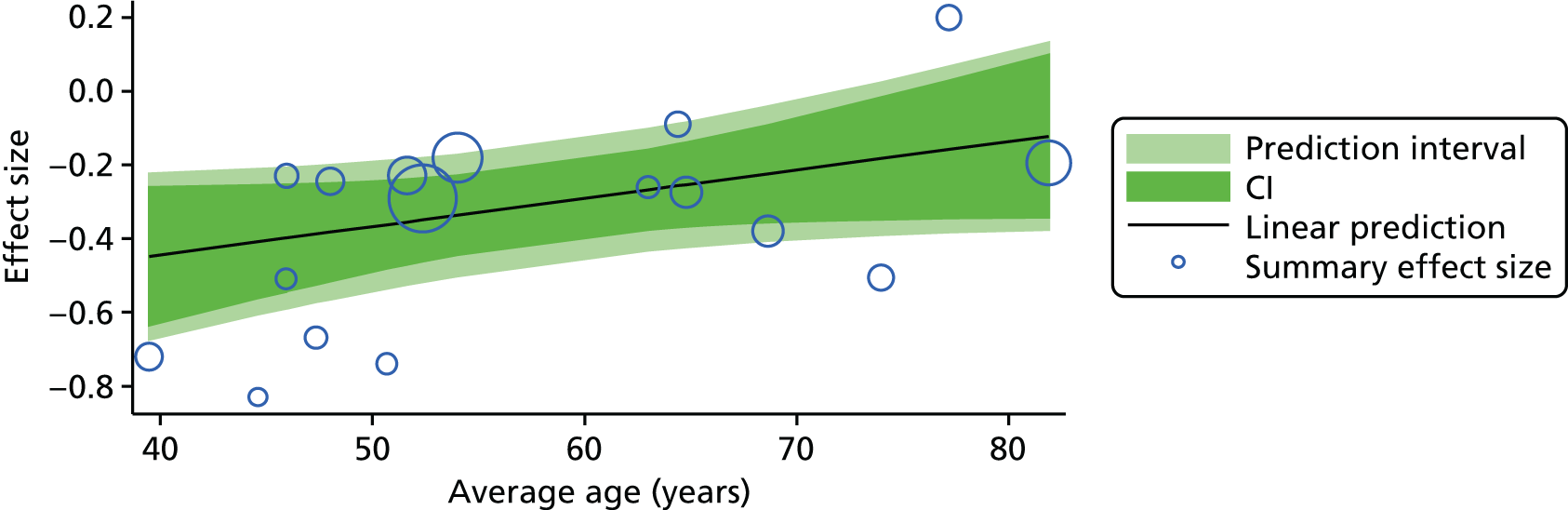
FIGURE 52.
Depression, sex and effect size.
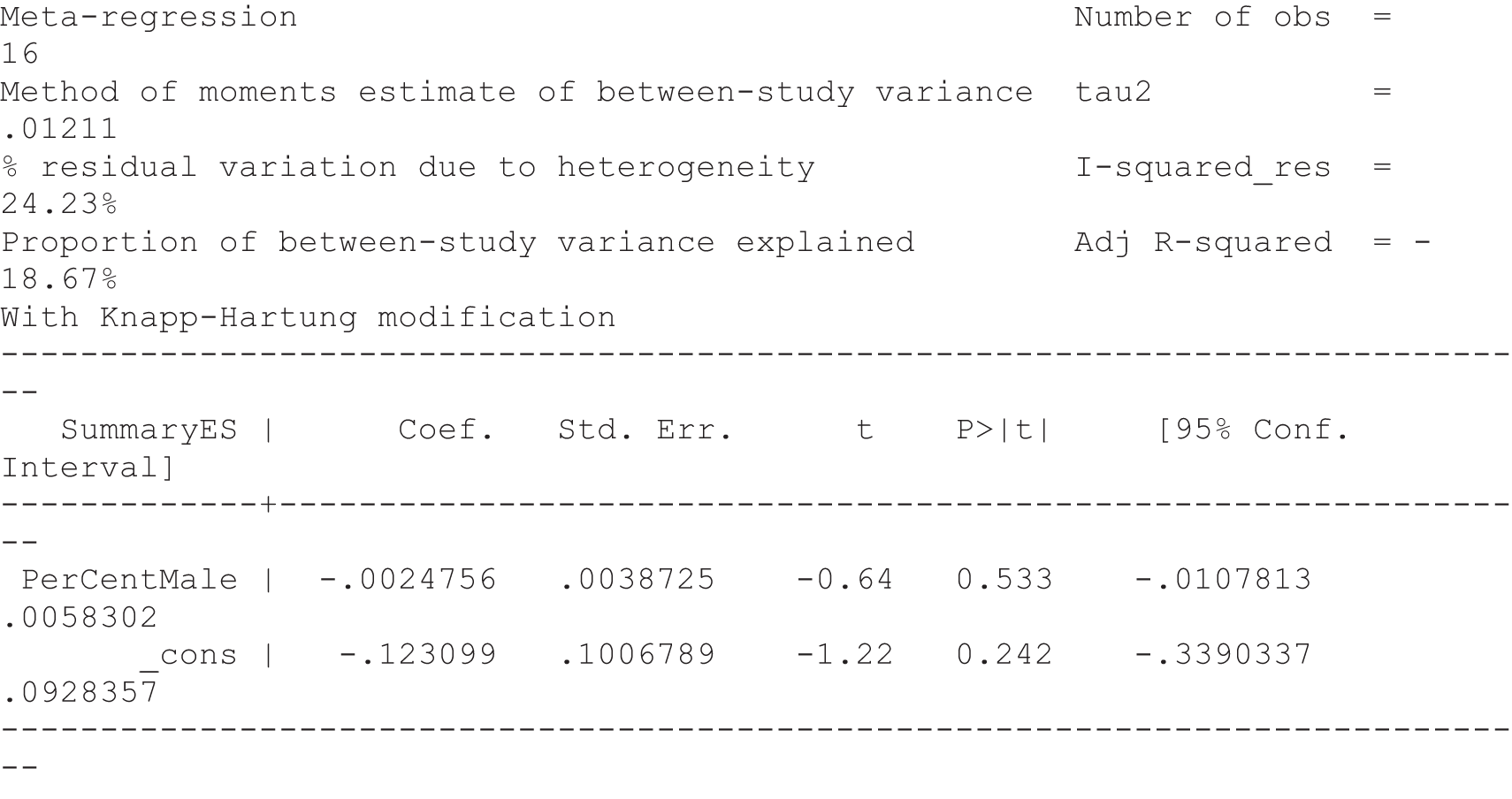
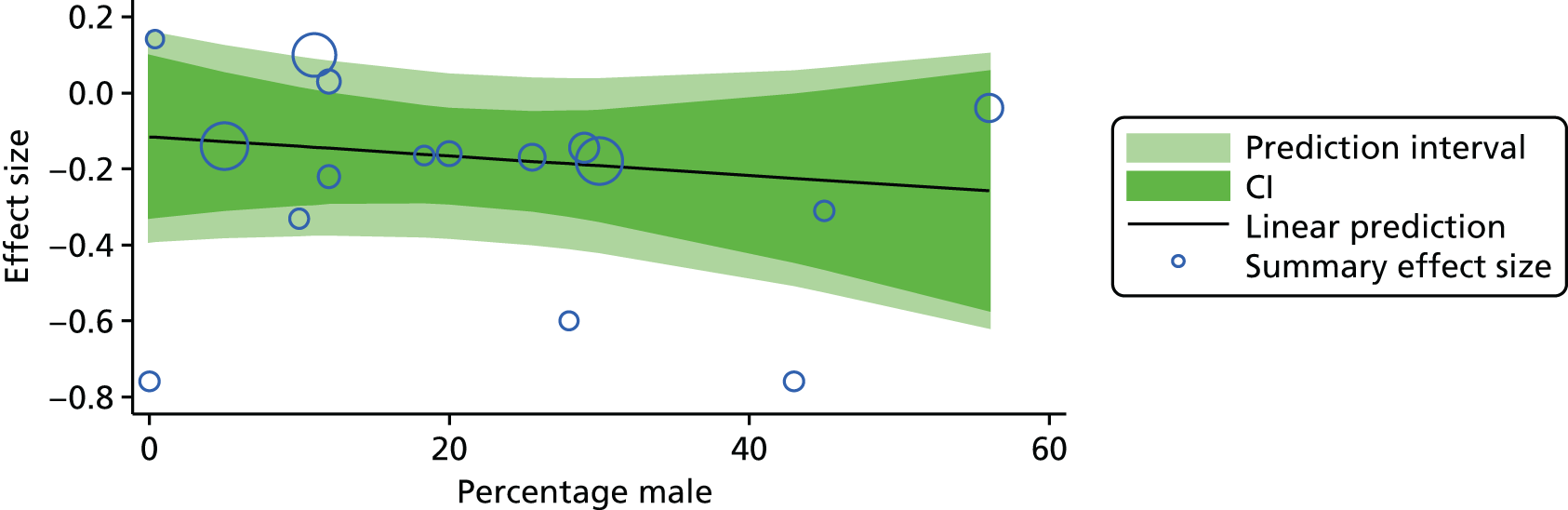
FIGURE 53.
Depression, age and effect size.
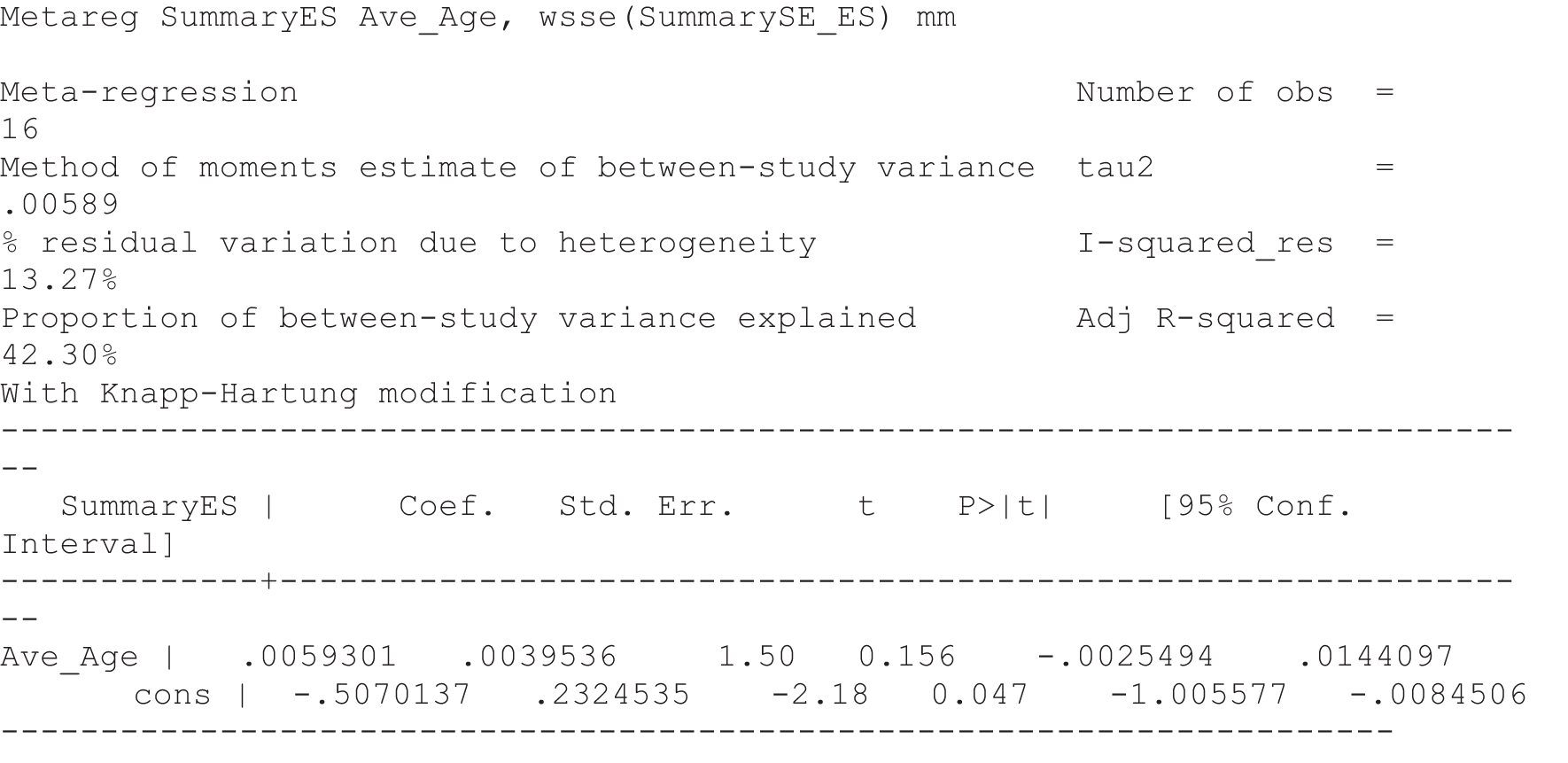
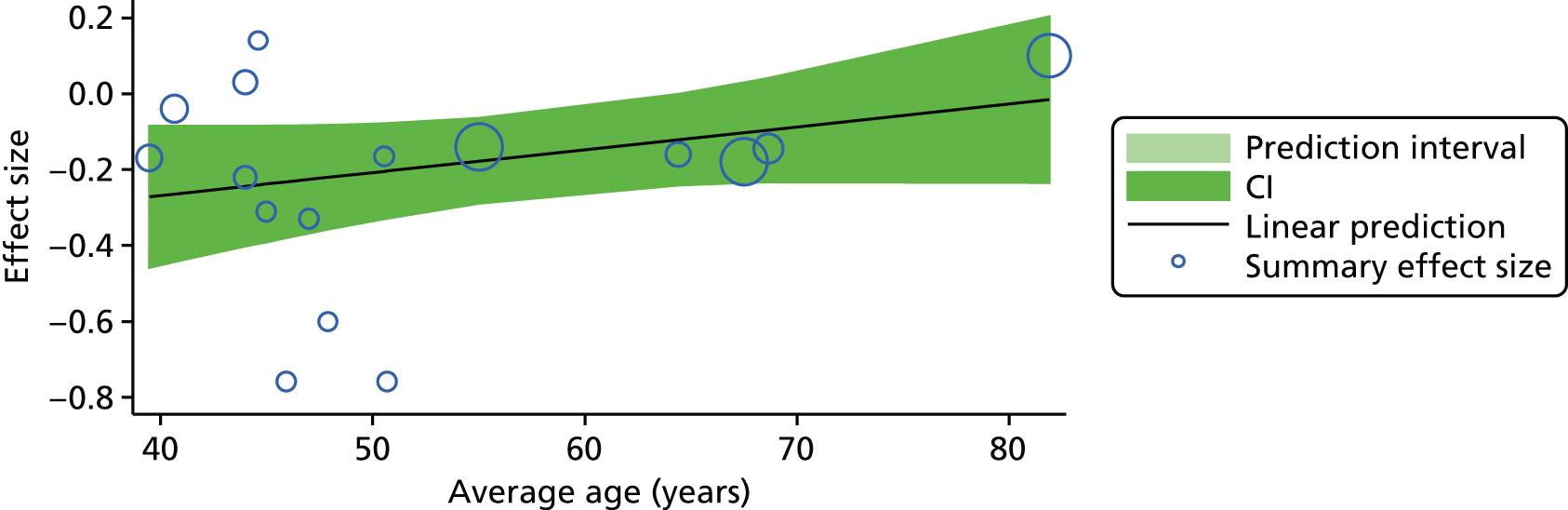
FIGURE 54.
General health score, sex and effect size.


FIGURE 55.
General health score, age and effect size.
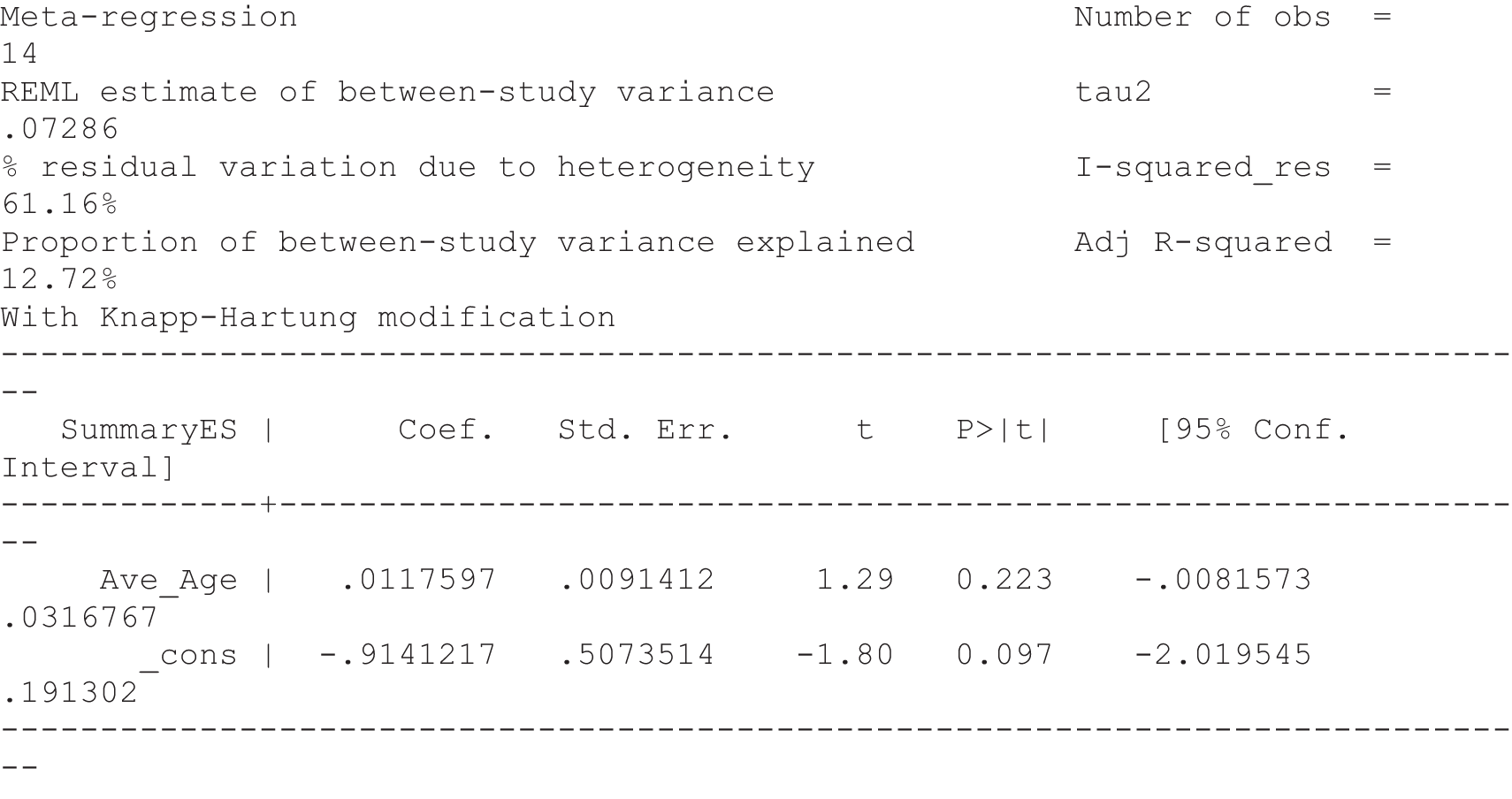
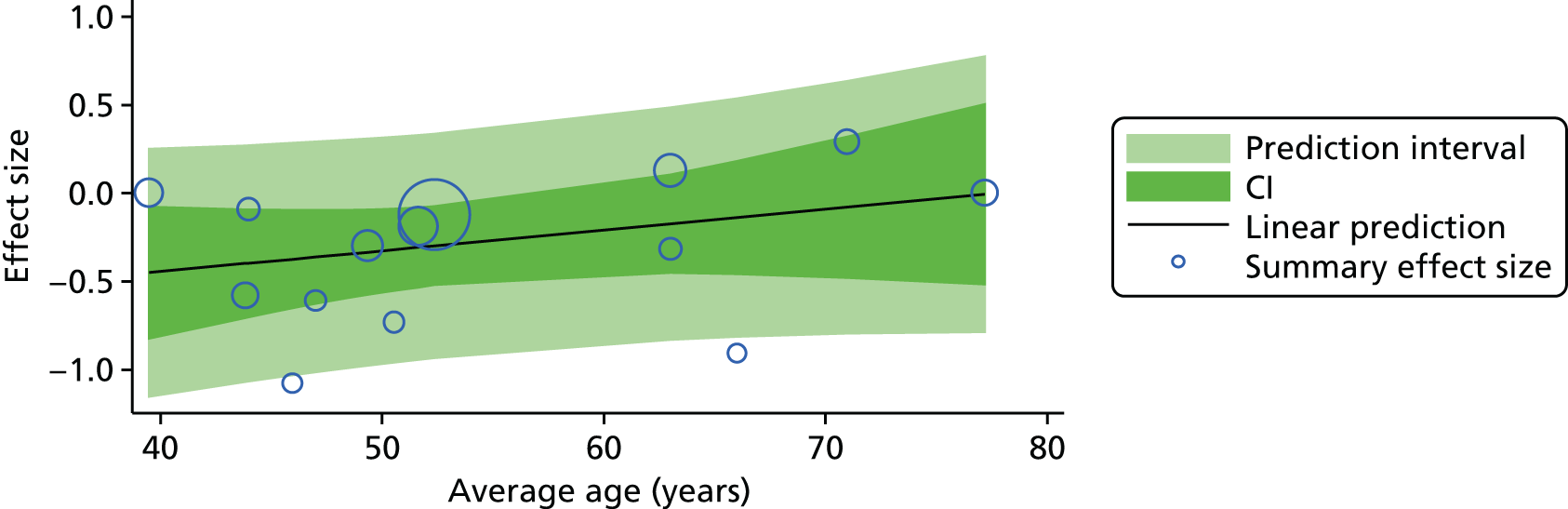
FIGURE 56.
General mental health, sex and effect size.
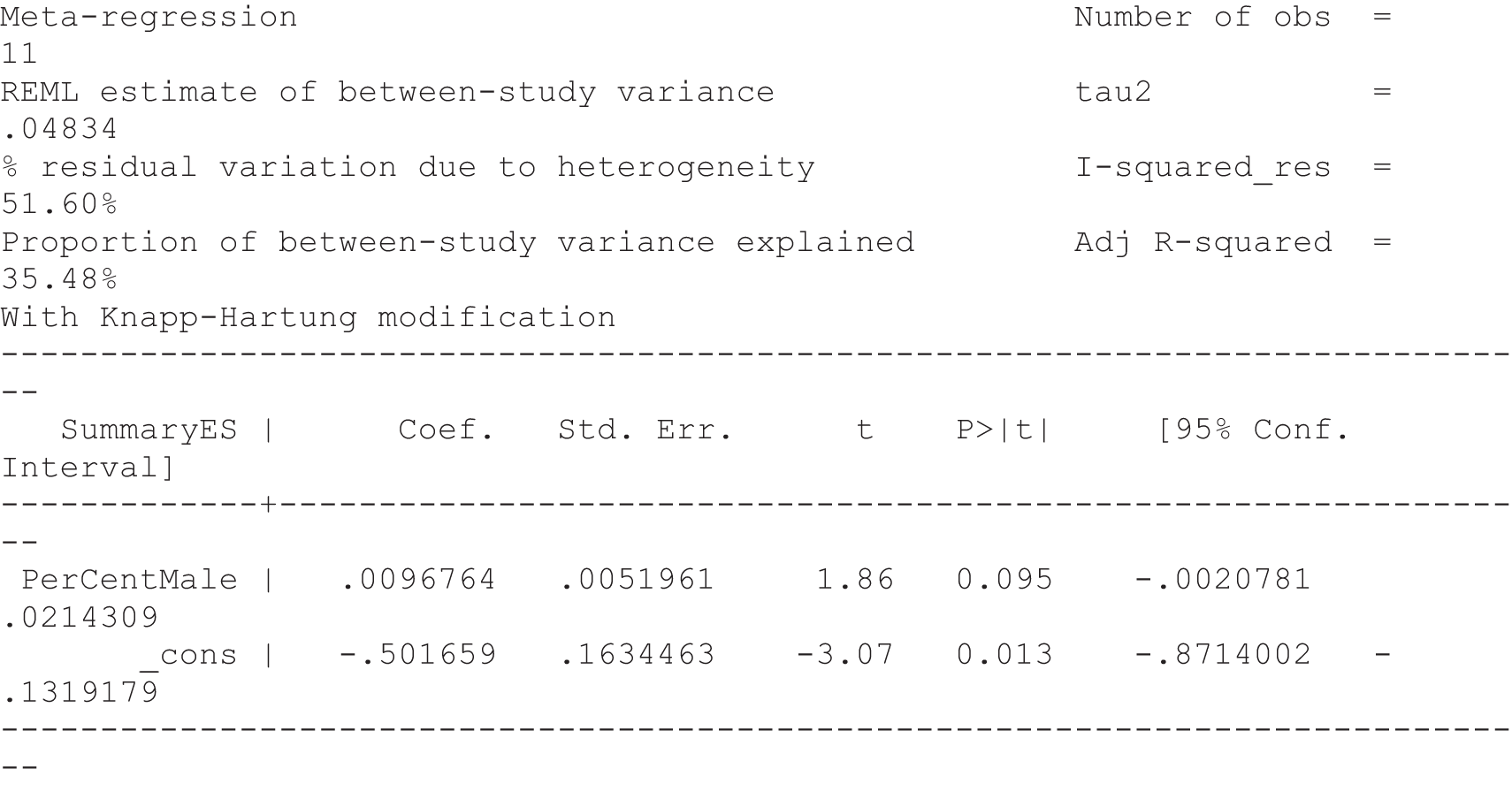
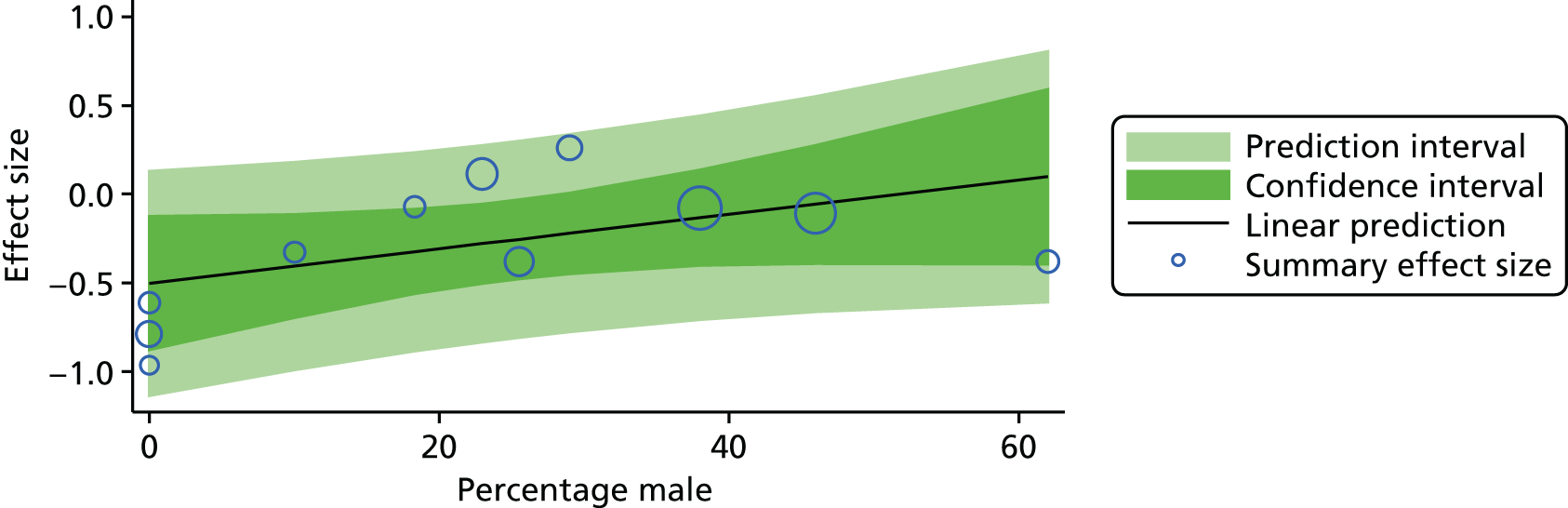
FIGURE 57.
General mental health, age and effect size.

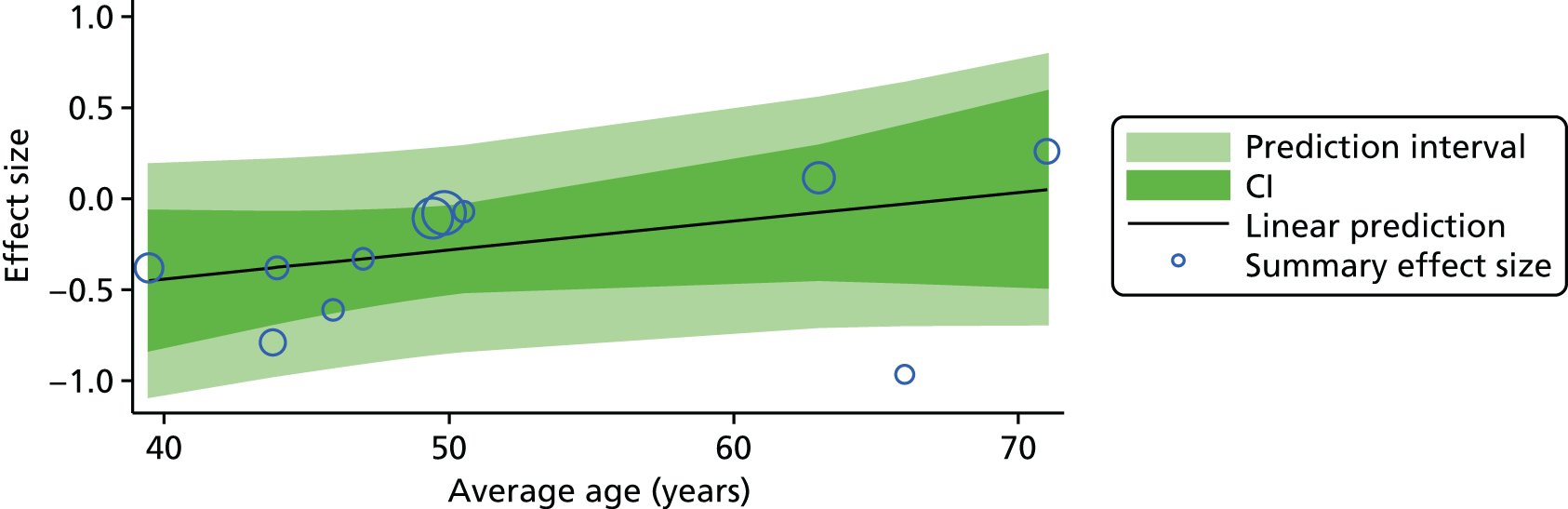
Appendix 3 Clinimetric study of outcome measures
Measures of depression in pain populations
| Measure | Description | Advantages | Limitations |
|---|---|---|---|
| HADS176 | 14 items, seven for depression and seven for anxiety | Designed for people with physical problems, commonly used, short, good psychometrics, responsive in our population, cut-points exist | One item (feeling slowed down) may reflect pain |
| DAPOS184 | 11 items, five for depression, three for anxiety and three for positive outlook | No somatic items, designed and validated in a chronic pain population, positive outlook dimension, a potentially useful subgroup, short, psychometrics OK, responsive in our population | No cut-points, not commonly used |
| BDI177 | 24 items, subgrouped into affect, cognitive and somatic items | Very commonly used | Contaminated by somatic items, quite depressing, developed for psychiatric population so may lack sensitivity in less depressed groups |
| Zung Depression Inventory308 | 24 items, similar to the BDI | Used in pain populations, designed and validated in back pain | Contaminated by somatic items |
| CES-D178 | 20 items, similar to BDI in structure | Very commonly used, good psychometrics | Contaminated items |
| SF-36 mental health309 | Five-item subscale, mixture of anxiety and depression items | Very common, good psychometrics, short if only those five items used | |
| GHQ-12310 (psychological) | 12 items, a mixed bag representing distress | Very common, properties OK, has been used in our population | Cut-points exist but they are very low: our entire population would become a case |
| PHQ-4179 (depression and anxiety) | Two items on each | Short | More screening than evaluation of mood |
| PHQ-9179 | Nine items | Commonly used in our population, cut-points exist | Some items may reflect pain: ‘Moving or speaking so slowly that other people could have noticed or being so fidgety or restless that you’ve been moving around a lot more than usual’ |
| HSCL-20311 | 20 items | Overeating, poor appetite and sexual interest items may reflect pain | |
| EQ-5D depression and anxiety subscale312 | Three items, simple self-classification as ‘very’, ‘moderate’ or ‘not’ | Essential for health economic analysis | Not sensitive, does not distinguish between depression and anxiety |
Self-efficacy systematic review methodology and results
Inclusion criteria
-
Studies were included if they were a published in a peer-reviewed journal and included adults with pain as a result of either chronic musculoskeletal disorders or chronic disease.
-
Studies that included some clinimetric or psychometric evaluation of the most commonly used self-efficacy and social support measures identified in the first search.
Search strategy for self-efficacy
-
Self-efficacy adj4 (scale or inventory or instrument or measure* or assess* or outcome).ti.ab
-
Self-efficacy.ti.ab
-
QUESTIONNAIRES/.ti.ab
-
OUTCOME ASSESSMENT (HEALTH CARE)/ti.ab
-
PSYCHOMETRICS/.ti.ab
-
3 OR 4 OR 5
-
2 AND 6
-
1 OR 7
-
CHRONIC DISEASE/ and PAIN/
-
MUSCULOSKELETAL DISEASES/ and PAIN/
-
LOW BACK PAIN/ or FIBROMYALGIA/ or NECK PAIN/ or SHOULDER PAIN/ or OSTEOARTHRITIS/
-
((chronic or persistent or long-term or wide-spread or recurrent or non-specific or ongoing or musculoskeletal) adj pain).ti,ab.
-
((lower back or knee or neck or shoulder or hip or thoracic) adj pain).ti,ab.
-
Osteoarthriti* or (osteo* adj2 pain).ti,ab.
-
9 OR 10 OR 11 OR 12 OR 13
-
8 AND 14
The most commonly used measures (ASES180 plus variants and PSEQ313) from the first search were selected for clinimetric evaluation. The names of these measures were used in the second search:
-
Arthritis self-efficacy or Chronic Disease self-efficacy or Stanford self-efficacy or Pain self-efficacy or PSEQ
-
Test Reliability/ or exp Psychometrics/ or exp Test Validity/ or exp Test Interpretation/
-
Validity or reliability or development or consistency or responsiveness or interpretability or psychometrics or clinimetrics
-
1 AND (2 or 3)
Clinimetric assessment
Clinimetric assessment was planned for the most commonly used measures obtained from both of the searches. To evaluate the clinimetrics of the questionnaires a checklist was used based on criteria developed in a former study. 314 The following information was extracted: name of the questionnaire, target population, domains measured, number of scales, number of questionnaire items; number of response options, range of scores, time to administer, ease of scoring and study population used in the clinimetric study.
Validity
Content validity here refers to the degree to which the items within a research instrument represent the domain of measurement. If a positive rating for readability and comprehension of the items was given by the studied population and/or experts, content validity was achieved.
The internal consistency of a questionnaire was rated as satisfactory when the Cronbach’s alpha was between 0.70 and 0.90 for each subscale (if more than one)315 and when the dimensional structure of the questionnaire was assessed by factor analysis or principal components analysis.
Construct validity refers to when a questionnaire demonstrates its ability to identify or measure the variables or constructs that it proposes to identify or measure. Construct validity is adequate when studies show correlations of the evaluated measure with other measures that the tool is hypothesised to be related to.
For floor and ceiling effects to be judged as adequate, authors were required to provide sufficient information regarding the distribution of scores.
Reproducibility (test–retest reliability)
This was evaluated as adequate if the interclass correlation coefficient was calculated and was > 0.70. The use of Pearson correlation coefficients to estimate test–retest reliability was viewed as doubtful.
An adequate assessment of the agreement of the questionnaire was the calculation of the 95% limits of agreement (the kappa coefficient or the standard error of measurement was viewed as an adequate measure of agreement).
Responsiveness
The aim here was to measure whether or not the questionnaire can measure change in the measurement domain in association with an intervention of some kind. If change scores were calculated and these were associated with changes in a reference measure that was based on predefined hypotheses then the measurement tool was given adequate responsiveness status.
Interpretability is when information is given that describes or explains a quantitative score obtained on a test. This information could be presented in various ways: (1) the authors had presented a minimal clinically important difference (MCID); (2) a report of means and SDs of patients scores before and after treatment; (3) comparative data on the distribution of scores in relevant subgroups; (4) information on the relationship of scores to well-known functional measures or to clinical diagnosis; or (5) relating changes in disability score to patients’ global ratings of the magnitude of change they have experienced. At least two of these types of information were needed for a positive rating of interpretability to be assigned.
Results
The electronic searches identified 1520 references, which were downloaded to an EndNote bibliographic database (version X2, Thomson Reuters, CA, USA). A search through the references within the EndNote database for the term ‘self-efficacy’ in either the title or the abstract resulted in 224 references being retained. Thirty-eight dissertations were then removed and 21 studies were excluded as they did not fit the inclusion criteria (chronic pain population including musculoskeletal conditions); in addition, three references were books and so these were removed. Of the 162 references remaining, five were discussion/review articles (data were extracted from three of these) (Table 77).
| Study | Measure | Description of psychometric evaluation |
|---|---|---|
| Baheiraei 2005316 | Osteoporosis Self-Efficacy Scale317 | Psychometric properties of the Persian version of the scale |
| Horan 1998317 | Osteoporosis Self-Efficacy scale317 | Development and evaluation |
| Barlow 1997183 | ASES180 | Reliability and validity of the scale in British people with arthritis |
| Lomi 1992318 | ASES, Swedish version | Evaluation of a Swedish version of the ASES with respect to factor structure and reliability on rheumatology and chronic pain patients |
| Mueller 2003319 | ASES,180,320 | Validation of the ASES short-form scale |
| Nicholas 2007181 | PSEQ313 | Reliability and validity in a low back pain population |
| Sarda 2007321 | PSEQ313 | The translation of the PSEQ into Portuguese. The Brazilian version of the PSEQ had a high concordance with the original version |
| Lim 2007322 | PSEQ313 Chinese version322 | Reliability and construct validity |
| Shin 2001323 | Exercise Self-Efficacy Scale190 | Reliability, face validity and factor analysis of the scale in a Korean population with chronic diseases |
| Anderson 1995324 | Chronic Pain Self-Efficacy Scale (CPSS)324 | Development and initial validation in chronic pain patients |
| Barlow 2000325 | Parent’s Arthritis Self-Efficacy (PASE)325 scale | Development and validation |
| Bursch 2006326 | Child Self-Efficacy Scale – parent and child version326 | Reliability and validity tested |
| Gard 2005327 | Motivation for Change questionnaire327 | Development and reliability |
| Gibson 1996328 | Spinal Function Sort Measure329 | Reliability and validity tested |
| Sandborgh 2008330 | Pain Belief Screening Instrument (PBSI)331 | Reliability and validity tested |
| Vlaeyen 1990332 | Liste des Cognitions de la Douleur332 | Development of the scale |
We identified the two most commonly used measures (> 10 articles had used these measures): (1) ASES-11 with its four variants (for chronic disease and shorter versions)180 and (2) PSEQ-5. 313 We searched for further information on the clinimetric and psychometric properties of these instruments.
The second search identified a further 21 articles for the ASES and its variants. The authors of this test were contacted for unpublished data and we were referred to the following website for psychometric data, where a further three references were retrieved [http://patienteducation.stanford.edu/research/ (25 April 2016)]. For the PSEQ-specific search, a further 20 articles were located. Three of these articles were relevant plus an additional eight studies identified from the reference lists of these papers. A description of these questionnaires and their properties is presented in Table 78.
| Questionnaire | Target population | Domains | Number of scales | Number of items | Number of response options | Range of scores | Time to administer | Ease of scoring | Study population(s) used in clinimetric studies |
|---|---|---|---|---|---|---|---|---|---|
| ASES-20180 | Arthritis patients | Self-efficacy pain, self-efficacy function, self-efficacy other symptoms | Three | 20 | 10 | 20–200 | ? | Easy | Arthritis patients |
| ASES-11180 | Arthritis patients | Self-efficacy pain, self-efficacy other symptoms | Two | 11 | 10 | Self-efficacy pain 5–50; self-efficacy other 6–60 | < 10 minutes | Easy | UK arthritis patients |
| ASES-8180 | Arthritis patients (short version) | Self-efficacy pain, self-efficacy other symptoms | Two | 8 | 10 | 8–80 | < 10 minutes | Easy | Arthritis patients |
| CDSES-33182 | Chronic disease patients | Self-efficacy to perform self-management behaviours, general self-efficacy, self-efficacy to achieve outcomes | 10 | 33 | 10 | 33–330 | ? | Easy | Chronic disease patients |
| CDSES-6333 | Chronic disease patients (short version) | Symptom control, role function, emotional functioning and communicating with physicians | 6 | 10 | 6–60 | < 10 minutes | Easy | Chronic disease patients | |
| PSEQ313 | Chronic pain? | Measures the strength and generality of patients’ beliefs about how confident they are that they can do each of the 10 activities or tasks at present despite the pain that they experience | 10 | 7 | 0–60 | 10 minutes | Easy | Chronic low back pain and heterogeneous chronic pain181 |
Content validity
Information regarding the content validity of the questionnaires is summarised in Table 79.
| Questionnaire | Content validity | |||||
|---|---|---|---|---|---|---|
| Item selection | Reading level examined | Item reduction | Dimensionality studied | Internal consistency | Confirmatory factor analysis | |
| ASES-20180 | Experts and patients182 | Items were removed that were not related to the total score180 | Yes, factor analysis revealed two factors (function and other symptoms)180 | Cronbach’s alpha coefficient: 0.76, 0.89 and 0.87 for pain, physical functioning and other symptoms, respectively;180 0.82, 0.91 and 0.92 for pain, physical functioning and other symptoms, respectively.334 Coefficients were 0.90 for FSE, 0.87 for OSE and 0.75 for PSE,180 McKay 1999335 | Confirmatory factor analysis revealed three subscales. Item loadings ranged from 0.59 to 0.90 for OSE, from 0.45 to 0.82 for PSE and from 0.59 to 0.75 for FSE180 | |
| ASES-11180 | Experts and patients183 | Comprehensibility was examined among 53 people with arthritis183 | Yes, factor analysis attempted and two factors resulted (pain and other symptoms)325 | Cronbach’s alpha coefficient: pain 0.84, 0.85 and 0.82; other symptoms 0.91, 0.90 and 0.89 for studies 2, 3 and 4, respectively.183 A high degree of consistency was demonstrated for both subscales across all three studies through interitem correlations (range 0.26–0.86, all correlations were significant) and corrected item total correlations (range 0.48–0.84, all significant)183 | ||
| ASES-8180 | Cronbach’s alpha coefficient: 0.89 | |||||
| CDSES-33182 | Experts and patients182 | Cronbach’s alpha coefficient: between 0.82 and 0.89 for eight out of 10 scales182 | ||||
| CDSES-6333 | Cronbach’s alpha coefficient: 0.91 | |||||
| PSEQ313 | Expert and patients181 | Readability and comprehension of the scale were assessed by patients181 | Yes, principal components analysis attempted and one factor resulted (Westmead and tertiary referral pain centre RNSH samples)181 | Cronbach’s alpha coefficient: 0.92 (Westmead sample),336 0.93 (RNSH sample),181 0.94328 and 0.92337 | ||
For perceived social support there were no recommendations from the MMICS31 or IMMPACT30 guidelines, nor were we able to identify a systematic review comparing measures. We carried out a literature search to identify candidate instruments. Items were selected by experts and patients for four of the questionnaires (PSEQ, ASES-20, CDSES-33 and ASES-11). Readability and comprehensibility were assessed in two questionnaires (PSEQ and ASES-11). Factor analysis and principal components analysis demonstrated the presence of factors for the ASES-20 (two factors), ASES-11 (two factors) and PSEQ (one factor). Internal consistency was studied in all of the questionnaires and Cronbach’s alpha ranged from 0.76 to 0.94 and was given a positive rating if it was > 0.70. 315 Therefore, content validity was most explored for the ASES-20, ASES-11 and PSEQ questionnaires. Item reduction and confirmatory factor analysis were carried out only for the ASES-20.
Construct validity
Construct validity was demonstrated for all measures except for the CDSES-6 through correlations of the self-efficacy measures with various outcomes. Hypotheses were given regarding expected relationships, although these were not always directional. Outcomes were depression, psychological well-being, reported pain and fatigue, positive effect, pain-related disability and pain coping strategies among the ASES scales. PSEQ scores were correlated with depression, anxiety, unhelpful coping strategies, pain ratings, somatic focusing and perceived capacity for work-related tasks (Table 80).
| Questionnaire | Construct validity | |||
|---|---|---|---|---|
| Hypothesis | (Main) results | FCE | Study size, n | |
| ASES-20180 | Yes – self-efficacy will be related to present health status and future health status (non-directional)180 | Pearson’s correlations were used. Baseline FSE with baseline pain (r = –0.29), disability (r = –0.76) and depression (r = –0.16). Baseline OSE with baseline pain (r = –0.27), disability (r = –0.25) and depression (r = –0.44). Baseline PSE with baseline pain (r = –0.29), disability (r = –0.21) and depression (r = –0.33).180 Predictive and concurrent validity also presented | 97180 | |
| ASES-11180 | Concurrent validity was examined through Pearson product–moment correlations of the ASES scores with demographic variables, physical status, psychological status and social well-being (no directional hypotheses). Congruence between specific arthritis self-efficacy and generalised self-efficacy beliefs was predicted, although the strength was expected to be modest (hypotheses). The predictive abilities of the two subscales of the ASES were examined using hierarchical regression analyses with psychological well-being (depression and positive affect) as the dependent variable (no directional hypotheses) | Greater self-efficacy results were associated with decreased physical functioning (in study 2 only). Greater self-efficacy beliefs tended to be associated with less reported pain and less fatigue. The strongest patterns of associations were in the expected directions, with correlation coefficients ranging from 0.30 to 0.61 for depression and from 0.25 to 0.63 for positive affect. Greater self-efficacy beliefs were associated with more positive psychological well-being.183 A consistent pattern of positive association was found between the ASES other symptoms subscale and the Generalised Self-Efficacy Scale.183 The ASES other symptoms subscale was influential in predicting depression (CES-D) and positive affect (B = –0.31, p = 0.024 and B = –0.34, p = 0.006, respectively). The ASES pain subscale was less predictive of depression or positive affect183 | Study 1 53, study 2 145, study 3 66, study 4 80183 | |
| ASES-8180 | Yes – self-efficacy will be associated negatively with pain, disability and depression (clear hypotheses) | Controlling for age, gender and pain intensity, self-efficacy was associated significantly and negatively with pain-related disability (r = –0.29, p < 0.001), pain (r = –0.34, p < 0.001) and depressive symptoms (r = –0.49, p ≤ 0.001) and positively with use of pain coping strategies (particularly task persistence, r = 0.48, p < 0.001)338 | The scale showed no floor effects (no one had the lowest possible score) and minimal (1.4%) ceiling effects in the sample338 | 140 chronic pain patients338 |
| CDSES-33182 | In addition to the item convergence and discriminant validity tests conducted as part of the multitrait scaling analyses, construct validity was examined by evaluating correlations among self-efficacy measures and their corresponding behaviour or outcome to ensure that self-efficacy was not highly correlated with the corresponding measure180 | The absolute magnitude of the correlations between self-management behaviours and self-efficacy to perform the behaviours ranged from 0.01 to 0.41. Therefore, the scales were sufficiently independent of the actual behaviours that they can be viewed as distinct scales. The absolute magnitude of the correlations between health outcomes and self-efficacy to achieve the outcomes ranged from 0.14 to 0.75. The largest correlation was between self-efficacy for managing depression and three of the psychological scales: depressive symptoms (–0.75), CES-D depression (–0.68) and psychological well-being/distress (0.72). However, multitrait scaling analysis in which these items were included showed that the items in these scales were discriminating sufficiently well to be used as distinct measures. The remaining correlations were of less concern, falling below 0.65182 | No floor or ceiling effects were observed182 | 1130182 |
| CDSES-6333 | ||||
| PSEQ313 | Validity was assessed by examination of the relationships between the PSEQ and validated measures of constructs that would be expected to have different types of relationship with self-efficacy.181 Self-efficacy theory would predict a strong relationship between the PSEQ and measures of activity181 (expected negative correlations with higher medication usage, pain coping strategies, pain-related activities and pain beliefs). Positive correlations would be expected with coping strategies (for active approaches) and negative correlations with passive approaches (these authors had hypotheses) | Pearson product–moment correlations between the PSEQ and the other assessment measures were examined. Because of the large number of intercorrelations, only correlations of r > 0.40 and p < 0.001 were considered significant. As expected, significant negative correlations were obtained between the PSEQ and total number of medications used, impact of pain on daily life (SIP-self-rated and SIP-significant-other-rated), mood (BDI, STAI) and unhelpful coping strategies and beliefs (catastrophising subscale of the CSQ, the PBQ) (between r = –0.45 and r = –0.60). Also as expected, significant positive correlations were obtained between the PSEQ and active coping strategies measured (ignoring pain, coping self-statements and increasing activity) (between r = –0.45 and r = –0.60). In contrast, no significant correlations were found between the PSEQ and measures of pain and somatic focusing (average pain ratings, MPQ subscales or MSPQ), but all were in the negative direction, as expected181 (all r > 0.40, p > 0.001).311 In a study with CLBP patients there were high correlations between PSEQ scores and perceived capacity for work-related tasks, as well as another self-efficacy measure (r = 0.78 and 0.63, respectively)328 | Westmead sample 103; tertiary referral pain centre (RNSH) sample 1306 | |
Information regarding floor/ceiling effects was available only for the ASES-8 and CDSES-33. Both questionnaires were free from floor effects, although minimal ceiling effects were reported for the CDSES-33. Such information was missing for the ASES-20, ASES-11, CDSES-6 and PSEQ.
Reproducibility
Test–retest reliability was assessed for three out of the six questionnaires (ASES-20, CDSES-33 and PSEQ) (Table 81). Time intervals between test administrations were between 9.4 days and 16.3 weeks. Test–retest correlations ranged from 0.68 to 0.88 across the three questionnaires. Pearson’s product correlations were used to assess test–retest reliability; however, the ICC is thought to be a more appropriate test of retest–reliability.
| Questionnaire | Test–retest reliability | Time interval between tests | Study size, n |
|---|---|---|---|
| ASES-20180 | The test–retest correlations (r) were 0.75 for pain self-efficacy, 0.84 for functional self-efficacy, 0.68 for other self-efficacy and 0.88 for the total score334 | 16.3 weeks334 | CLBP 59180 |
| ASES-11180 | |||
| ASES-8180 | |||
| CDSES-33182 | Test–retest reliability coefficients (r) ranged from 0.82 to 0.89 (method not specified)182 | 10 days182 | |
| CDSES-6333 | |||
| PSEQ313 | Carried out with Pearson correlations and analysis of chance. The test–retest correlation (r) from baseline to 3 months was 0.73 (p < 0.001). The mean scores for the two occasions were 26.7 (SD 12.5) and 26.9 (SD 12.6), respectively (i.e. no significant change). Interestingly, similar findings were reported by Williams 199670 with a waiting-list control group of mixed chronic pain patients (n = 31) tested 12 weeks apart. In that study, in which patients [mean age 51.1 (SD 10.7) years; mean pain duration 7.2 (SD 6.6) years; mean BDI 16.6 (SD 6.5); mean pain severity (0–100) 67.9 (SD 22.3)] continued with whatever treatments their doctors had prescribed, the mean (SD) PSEQ score at baseline was 26.3 (10.8) and at 12 weeks was 26.7 (6.2); again, no significant change was found (as well as no change in pain or disability)313 | 3 months | 245 chronic pain patients |
Responsiveness
The responsiveness of three of the questionnaires (ASES-20, CDSES-33 and PSEQ) was evaluated in five studies (Table 82). Hypotheses were provided in all of the studies except for that by Burckhardt et al. 339 regarding specific changes in self-efficacy in association with the intervention (note that a change was explored in Nicholas et al. ,313 not predicted). No data on responsiveness were found for the other three questionnaires (ASES-11, ASES-8, CDSES-6). The best way to analyse responsiveness is through receiver operating characteristic curve analysis and no study used this technique.
| Questionnaire | Responsiveness | Interpretability | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Time to follow-up | Hypothesis | (Main) results | Study size, n | Interpretability | Baseline and follow-up scores | Scores of relevant subgroups | MCID | |
| ASES-20180 | Patient education, Burckhardt 1994,339 | 4- to 8-month follow-up and 1 week after treatment, Burckhardt 1994,339 | Not specifically about self-efficacy. Burckhardt 1994 programme would improve psychological functioning339 | There were significant improvements on all three subscales of the ASES339 | 99 Burckhardt 1994339 | ? Burckhardt 1994,339 | Pre and post-treatment means for all measures, including self-efficacy. Burckhardt 1994 baseline mean, post-treatment means and change scores339 | ? | ? |
| ASES-11180 | |||||||||
| ASES-8180 | |||||||||
| CDSES-33182 | Self-management programme333 | 12 months333 | Programme improve self-efficacy333 | Participation in the Chronic Disease Self-Management Programme was associated with improvements in all health behaviours (exercise, cognitive symptom management, improved communication with physician and self-efficacy)333 | 613333 | ?333 | Baseline mean and change scores333 | ? | ? |
| CDSES-6333 | |||||||||
| PSEQ313 | Cognitive–behavioural programme;340 cognitive–behavioural programme and physiotherapy313 | 1- and 6-month follow-ups313,340 | Yes313,340 | There were significant improvements in all measures (physical and psychological), including self-efficacy | 243;340 181313 | ?313,340 | Mean and SDs provided for all measures including PSEQ | High PSEQ scores were highly associated with clinically significant functional levels and provide a useful gauge for evaluating outcomes in chronic pain patients.181 Scores of around 40, as found in injured workers who had returned to work341,342 were associated with return to work and maintenance of functional gains whereas lower scores after treatment (e.g. 30) tended to predict less-sustainable gains343 | ? |
Interpretability
Interpretability data were provided for three of the questionnaires (ASES-20, CDSES-33 and PSEQ) across seven studies (see Table 82). Baseline and post means were given for all three questionnaires; however, scores for relevant subgroups were described only for the PSEQ. MCIDs were not reported for any of the self-efficacy measures and there were no interpretability data available for the other three questionnaires (ASES-11, ASES-8 and CDSES-6).
Systematic review of social support measures in chronic pain populations with clinimetric properties
Search strategy for social support measures
The main aspects of social support that we wanted to measure were (1) friends and family and (2) health-care resources.
The studies in Table 83 either refer to psychometric evaluation of social support scales or are the actual psychometric studies.
| Study | Measure | Description of psychometric evaluation |
|---|---|---|
| Ahlstrom 2002344 | Swedish version of the Ways of Coping Questionnaire; 6/66 items focus on ‘seeking social support’ | Some psychometrics presented |
| Bell 1982345 | Social Support Index | Reliability and validity reported in Orth-Gomer and Unden346 |
| Bennett 2001347 | Social Support Survey186 | Reliability and validity has been established in previous research |
| Berkman 1979348 | Social Network Index | Reliability and validity reported in Orth-Gomer and Unden346 |
| Blazer 1982349 | Social support scale | Reliability and validity reported in Orth-Gomer and Unden346 |
| Broadhead 1982350 | Broadhead questionnaire | Reliability and validity reported in Orth-Gomer and Unden346 |
| Cohen 1985351 | Interpersonal Support Evaluation List (ISEL) | Reliability and validity reported in Orth-Gomer and Unden346 |
| Da Costa 2000352 | Short version of the Social Support Questionnaire (SSQ-6) | The SSQ-6 is psychometrically sound |
| Da Costa 2006353 | Social Support Questionnaire (SSQ) Sarason 1987354 was used to assess perceived satisfaction with social support | The SSQ is psychometrically sound and includes six items measuring satisfaction with social support, Sarason 1987354 |
| Dean 1981355 | The Instrumental-Expressive Social Support Scale | Reliability and validity reported in Orth-Gomer and Unden346 |
| Doeglas 1996356 | The Social Support Questionnaire (SSQ) consists of two parts: the Social Support Questionnaire for Transactions (SSQT) and the Social Support Questionnaire for Satisfaction with the supportive transactions (SSQS)374 | Yes |
| Eakin 2007357 | Spanish version of the CIRS357 | Validation |
| Edwards 2009358 | Social Support Survey186 | Reliability and validity has been established in other studies. Designed for chronically ill patients |
| Esteve 2005359 | The Vanderbilt Pain Management Inventory (VPMI) has a seeking social support scale (identified through confirmatory FA) | Some psychometrics presented (reliability) |
| Evers 2002360 | Social functioning in the past 6 months was measured with the IRGL social functioning scales | Good reliability and validity demonstrated elsewhere |
| Franks 2004361 | The Norbeck Social Support Questionnaire (NSSQ)362 | Reliability and validity demonstrated elsewhere |
| Funch 1986363 | Social support scale | Yes, reliability and validity |
| Garcia-Campayo 2007364 | The Norbeck Social Support Questionnaire (NSSQ)362 | Reliability and validity demonstrated elsewhere |
| Gard 2005327 | Social support is one of the scales in the Motivation for Change Questionnaire327 | Development and reliability |
| Glasgow 2005365 | CIRS185 | Cross validation and sensitivity to intervention data |
| Glasgow 2000185 | CIRS185 | Psychometrics presented |
| Henderson 1980366 | Interview Schedule for Social Interaction (ISSI) | Reliability and validity reported earlier, in this review |
| Hesselink 2004367 | Perceived social support was measured using a standardised 12-item questionnaire, the Social Support List – Interactions (SSL12-I), measuring ‘everyday social support’, ‘social support in problem situations’ and ‘esteem support’368 | Cronbach’s alpha given for the present study |
| Marhold 2002369 | Obstacles to Return-to-Work Questionnaire (ORQ). One scale identified by Confirmatory Factor Analysis as ‘social support at work’ | Development and validation |
| McCormack 2008370 | Resources and Support for Self-Management (RSSM) questionnaire | Development and validation |
| McFarlane 1981371 | Social relationship scale | Reliability and validity reported earlier, in this review |
| Raleigh 1994372 | A scale in the Multidimensional Hope Scale measures social support | Development and evaluation; reliability and validity |
| Savelkoul 2001373 | Action-directed coping and coping by seeking social support were measured with two subscales of a short version of the Utrecht Coping Questionnaire | Reliability and validity reported elsewhere as acceptable |
| Sherbourne 1991186 | Social Support Survey186 | Development and evaluation |
| Suurmeijer 1995374 | The Social Support Questionnaire (SSQ) consists of two parts: the Social Support Questionnaire for Transactions (SSQT) and the Social Support Questionnaire for Satisfaction with the supportive transactions (SSQS) | Development and validation |
| Tan 2001375 | The Chronic Pain Coping Inventory (CPCI) has 65 items that measure 11 coping strategies that patients might use to cope with or manage their chronic pain.376 One of the strategies measured is seeking social support | Psychometric data available elsewhere |
| Tan 2005377 | The CPCI has 65 items that measure 11 coping strategies that patients might use to cope with or manage their chronic pain.376 One of the strategies measured is seeking social support | Further validation of the CPCI |
| Thompson 1993378 | Short version of the Social Support Questionnaire (SSQ-6) | Discriminant validity. Adequate reliability and validity shown elsewhere |
| Trief 1995379 | Social Support Questionnaire (SSQ)354 | Discriminant validity |
| Weir 1996380 | Social support was measured using the Duke-UNC Functional Social Support Questionnaire381 | Validity established elsewhere |
| Yu 2004382 | Psychometric testing of the Chinese version of the Medical Outcomes Study Social Support Survey (MOS-SSS-C) against the Chinese version of the Multidimensional Perceived Social Support Survey | Yes, psychometric evaluation of this scale |
Table 84 provides a description of additional measures of social support extracted from specialist texts. Finally, Table 85 provides a list of measures that fitted most closely to our aims.
| Social support measure | What it measures | Length | Comments |
|---|---|---|---|
| Inventory of Socially Supportive Behaviours (ISSB)383 | Types of support: emotional, instrumental, information appraisal and socialising | 40 items/10 minutes | Not designed to provide information on the people who provided resources or appraisal of the support |
| Arizona Social Support Interview Schedule (ASSIS)383 | Measures several aspects of social support plus identifies social support network membership and satisfaction with social support | 15–20 minutes | Interview |
| Perceived social support from family and friends384 | Perceived social support | 8 minutes | Does not cover health care |
| Social Network Scale (SNS)385 | Network size, number of people respondent feels close to, number of relatives in network and network density | 8 items | Does not cover health care |
| Lubben Social Network Scale (LSNS)386 | Social network scale for use with older people | 10 items | Does not cover health care |
| Family Relationship Index387 | Social support within the family | ? | Does not cover health care |
| Social Support Appraisals Scale (SS-A) and Social Support Behaviours Scale (SS-B)388,389 | Social support from family and friends | ? | Does not cover health care |
| Network typology: the Network Assessment Instrument390 | Classifies a person into a network type | 8 questions | Those administering the questions need to go on a training course |
| Weinert and Brandt391 – part 2 of the Personal Resource Questionnaire392 | Family and social support | 25-item Likert scale | Lengthy |
| Measure | Description | Comment |
|---|---|---|
| Social Support Survey186 | 21 items | Measures both social and health support (n = 4 studies) |
| Chronic Pain Coping Inventory (CPCI)376 | 8-item scale | Only measures social interaction (n = 2 studies) |
| CIRS185 | 65 items (there is a 22-item version) | Lengthy and impractical. Shorter 22-item version includes social and health support (n = 3) |
| Norbeck Social Support Questionnaire362 | 9 items | Interview |
| Social Support Questionnaire for Transactions (SSQT) and the Social Support Questionnaire for Satisfaction with the supportive transactions (SSQS)374 | 23 items | Interview |
| Social Support Questionnaire (SSQ)354 | 27 items/15 minutes (also a short-form version including 6 items) | Does not cover health care |
Appendix 4 Development of the new intervention
Facilitator training course: outline
Saturday
| Time | Content |
|---|---|
| 09.30–10.00 | Introduction to selves and course |
| Evaluation sheets | |
| 1-minute introductions | |
| 10.00–10.30 | Background to project |
| Group facilitation (flip chart difficulties and what to do) | |
| Course overview and explanations | |
| 10.30–10.45 | Day 1 – the course Session 1: rules of group (practice facilitation, generate rules, use flip chart) Exercise group facilitation: ice-breaker with dominant person |
| 10.45–11.30 | Session 2: pain education DVD, discussion, DVD, discussion |
| Break | |
| 11.40–11.45 | Explain about lunch and tasters |
| 11.45–12.00 | Session 3: the unwanted guest (someone to read and facilitate) |
| 12.00–12.15 | Session 4: discuss pain – bad and not so bad (describe session only) |
| 12.15–12.30 | Session 5: pain cycle – show diagram. Allocate a facilitator, discuss why stay in cycle, make a list of unhelpful behaviours (including depression list). Ask group how to escape from cycle. Show diagram |
| 12.30–12.45 | Depressive symptoms – read out |
| Lunch | |
| 13.30–14.00 | Distress and suicidal intent (allocate facilitator and answer question). Go through protocol and questions |
| 14.00–14.15 | Session 6: posture (trainer to show) |
| 14.15–14.30 | Session 7: relaxation (allocate someone to read script) |
| 14.30–14.35 | Evaluation forms – end of day |
| 14.35–14.45 | Day 2 – session 8: reflections (allocate facilitator to carry out this) |
| 14.45–15.15 | Session 9: depressive symptom list, problems, brainstorm solutions, goals, actions (separate and carry out group discussions) |
| Break | |
| 15.30–16.00 | Session 10: pros and cons (allocate facilitator). Choose a con and reframe it |
| Brainstorm reasons that stop us doing things | |
Sunday
| Time | Content |
|---|---|
| 09.30–10.00 | Session 11: errors in thinking |
| Scenario 1: allocate person to read out statement, group to discuss it and why illogical and then try and reframe it | |
| Go through unhelpful thinking (based on session 10 discussions) | |
| Read scenario 2. Allocate facilitator to enable group to identify unhelpful ways of thinking | |
| 10.00–10.20 | Session 12: carry out exercises 1 and 3 (trainer) |
| 10.20–10.35 | Session 13: allocate facilitator to brainstorm ways to manage pain |
| 10.35–10.45 | Recap posture from last session and carry out balancing (trainer) |
| 10.45–11.00 | Breathing, relaxation and visualisation (allocate) |
| Break | |
| 11.10–11.20 | Session 16: reflections and feedback (allocate or skip if time short) |
| 11.20–11.30 | Session 17: run through and ask questions (trainer) |
| 11.30–12.15 | Session 18: role plays (assign parts), discuss each (allocate facilitators) |
| 12.15–12.45 | Session 19: listening skills (trainer to lead) |
| Lunch | |
| 13.30–13.45 | Session 20: sleep (allocate facilitator to generate ideas for solving sleep problems) |
| 13.45–14.00 | Session 21: intimacy – trainer to lead and discuss with them (can leave out if they wish) |
| 14.00–14.20 | Session 22: anger and frustration (allocate facilitator to read and lead discussion and to ask: ‘when was the last time you had fun?’) |
| 14.20–14.30 | Session 23: stretches (trainer to lead brief run through) |
| 14.30–14.45 | Session 24: mindfulness explanation and practice relaxation |
| 14.45–15.00 | Go through buddying idea, mention contract, suggest participants buddy up |
| 15.00–15.15 | Follow-up |
| 15.15–15.30 | Evaluation of the course, debrief, choose course dates |
Appendix 5 Feasibility study
Bengali questionnaire
Feedback from facilitators and participants
Facilitator and observer feedback on the intervention
| Session | Observer/facilitator notes/facilitator focus group | Study team discussion |
|---|---|---|
| Introduction |
|
|
| DVD |
|
|
| Unwelcome guest |
|
|
| Pain/mood |
|
|
| Pain cycle |
|
|
| Stretching, movement, posture |
|
|
| Relaxation visualisation |
|
|
| Reflections |
|
|
| CBT |
|
|
| CBT: goal-setting |
|
|
| CBT: pros and cons and barriers |
|
|
| CBT: unhelpful thoughts |
|
|
| CBT: attention control |
|
|
| CBT: coping strategies |
|
|
| Communication: GP consulting |
|
|
| Communication: role play |
|
|
| Communication: listening skills |
|
|
| Communication: intimacy |
|
|
| Communication: anger |
|
|
| Sleep |
|
|
| Tasters |
|
|
| Buddying up |
|
|
| Other general suggestions |
|
Facilitator and observer feedback about processes and training
| Session | Observer/facilitator notes/facilitator focus group | Study team discussion |
|---|---|---|
| Facilitation process |
|
|
| Facilitation training |
|
|
Emergent themes from participants’ feedback questionnaires
Q11: If you can, name three things you learned today that are important to you
| Themes | Illustrative quotations |
|---|---|
| Social aspects | |
| This was a recurrent theme. The social aspect was beneficial to different participants in different ways: the group experience, pain-specific interaction, several types of communication (e.g. family, health professionals) and the activities-based social interaction | there are people in the same situation as meDay 1, E7 |
| The social interaction between pain participants enabled group members to learn from each other’s coping strategies | Other people’s experiences can help me, meeting others in pain and discussing experiences and methodsDay 1, E7 |
| Knowledge and learning | |
| Participants valued three areas in particular, the mind–body and therapy components, the CBT components and the pain education (DVD) | Reminded distraction/absorption is something that can put pain in background rather than foregroundDay 1, E1I’ve learnt how to cope/change negative thinking about myself in relation to other people. (The unhelpful thoughts checklist is very useful here.)Day 1, E1 |
| The pain education covered general information about pain pathways, the link with mood and the limited impact that medicine has on chronic pain. Most participants found this useful; however, some found the content too simplistic and others found it too complex and the DVD did not cover diagnosed conditions very well | That acceptance of my pain is crucialDay 2, E1overcoming difficulties by acceptanceDay 1, E1 |
| Mood | |
| This theme was linked to pain education. Participants liked pain and mood being linked together. Several participants grasped the idea and wrote it on the evaluation form as a learnt theme. This suggested that the concept being taught was memorable | mood and pain go togetherDay 1, E4mood effects painDay 1, E1 |
Question 12: What parts of this course today, if any, did you enjoy or value the most? Please tell us why it was valuable for you
| Themes | Illustrative quotations |
|---|---|
| Discussion/social interaction | |
| Participants related personal accounts of how they manage their pain; they found that this was a very valuable aspect for group members | Discussing other people’s pain relief methods and what helps others that will also work for me. Just meeting other people who are similar to meDay 1, E1 |
| Participants appreciated that they were not alone and could seek ways of relieving themselves from their pain | group discussion, sharing, listening to people’s experienceDay 1, E7 |
| Relating learning to self/personal experience | |
| Relating the content taught in the course to personal experience was valuable for some participants who were able to relate things to themselves | planning/setting goals. This will help me with changing my life/become more activeDay 2, E7 |
| Participants were able to recognise methods of helping themselves. This demonstrated their willingness to take away what they had learnt and try to implement it | how to sleep. I picked a few points to use. No sleep and pain has been a big issue for meDay 3, E2 |
Question 13: What parts of this course today, if any, did you least enjoy? Please tell us why
| Themes | Illustrative quotations |
|---|---|
| Social content | |
| Participants mentioned a number of different contents that were less valuable. The intimacy session was mentioned by a few participants. It is difficult to know whether this is because they believed the content was irrelevant or because the discussion was awkward for some | talking about sex although I seemed to be the only one contributing. Was quite embarrassed afterwardsDay 3, E4 |
| Disruption | |
| Dominant group members were deemed to be disruptive to the group. Facilitators need to be aware of dominant/disruptive participants who steer away from the session topic and steer the group back into discussion or close off discussion to continue the session. Poor facilitation skills resulted in lost time and alienating participants | the cross talking. This group is easily distracted onto irrelevant topicsDay 2, E2 |
| Poor timing | |
| For some participants, the course appeared to be too short. They felt that this resulted in the course content being taught briefly and presented in a rushed manner | I found it a bit rushed due to the fact it has to be crammed into 3 daysDay 1, E1 |
| Many participants wanted a longer course. The timing allocated to each session was an issue too | too much time spent on personal historiesDay 1, E2 |
| Disclosure discomfort | |
| Not all participants liked disclosing information about themselves. For those who were shy about speaking out in a group, the discussions were nerve-racking for them | Introducing myself, not at ease with strangers but a good ice breakerDay 2, E3 |
| Lack of personal relevance | |
| The need for personal relevance was important for engaging participants in the course and optimising interest | some of the points made did not seem relevantDay 1, E3 |
Q14: Do you feel that the content was relevant and applicable to you? In what ways could relevance and applicability be improved?
| Themes | Illustrative quotations |
|---|---|
| Cause of pain | |
| Some participants felt that there should be more focus on known causes of pain. Some were quite sure of their own explanation for their pain and were resistant to learning | I still find my pain is purely from over-activity and not moodDay 2, E7 |
| Applicability of content | |
| A variety of responses with many people identifying with the scenarios in the video and others feeling that they were not in enough pain or that pain was more specific from a particular diagnosis | Wondered if I was slightly here under false pretences as not in serious pain all day every dayDay 1, E1Yes, very relevant. Charlie in the video was meDay 1, E7 |
| Relevance of learning from others (sharing) | |
| Most enjoyed learning from others | I really, really got a lot out of the other participants and would have loved to spend more time in facilitated discussionE1, Day 2 |
Q15: Do you have any comments about facilitation?
| Themes | Illustrative quotations |
|---|---|
| Listening, communication and empathy | |
| Participants highlighted the need for facilitators to be attentive and responsive towards participants. The importance of receiving participants well and responding to them accordingly, with understanding, reinforced the skills required as a facilitator. Facilitators need to deliver information to participants and listen to and counsel them | They are very good; they listen and respond appreciatively to everyoneDay 2, E6 |
| Tutors’ backgrounds | |
| For a few courses there were lay tutors who had experienced chronic pain. Participants picked up on this | Both are good communicators and it helps that they are also pain sufferers so we are not being lecturedDay 1, E1 |
| People management | |
| An important characteristic that facilitators need to attain is good people management. Handling dominant participants and off-topic discussions is vital for good facilitation as this can cause annoyance among other group participants | It was quite good. There was one attendee who took up a bit too much time talking and I think he could have been managed slightly more assertively by the facilitatorsDay 3, E4 |
Question 16: Suggestions
| Themes | Illustrative quotations |
|---|---|
| Whole course/structure | |
| Suggestions for the course included comments on the course length. Many participants mentioned that the course was too short | That perhaps longer need to be spent on the topics and 3 long days for people with chronic pain is not ideal. Maybe session every morning over 1 weekDay 2, E1 |
| For those who found the course beneficial and interesting, there was a desire for the course duration to be extended | whilst it was relevant and applicable, in a few weeks I will probably forget it. To maintain the benefits, further sessions at regular spaced out intervals would be helpfulDay 1, E6 |
| Discussion/social interaction | |
| The course provided a social platform for the participants. The theme social interaction/discussion emerged in many answers and illustrated the influence on the participants | Only encouraging more people to open up during discussion time, maybe moving around the room and asking people individually – start in a different position each time obviously within time framesDay 3, E1 |
| Flexibility (individual participant needs) | |
| There was a desire for course content to be personalised for participants to optimise relevance | Most of the day was about back trouble but I also have knee trouble and would have liked the course to touch on other areas of painDay 1, E1 |
| Content | |
| Participants wanted more exercise. They liked the practical side to the course, which included relaxation/breathing and stretching and posture exercises | If they can arrange more exercises handouts for the standing and posture, balance and movement, stretching exercises would be helpfulDay 2, E3 |
Themes from the participant interviews
| Themes | Illustrative quotations |
|---|---|
| Clarity of aims | |
| Overall, the aims were not clear | I do not know what the aim wasE2I did not know what to expectNon-attender |
| Motivation | |
| The main reason for attending the course was out of curiosity. Indifference characterised responses | I thought I’d give it a tryE3I wanted to learn and see how I can cope with my painE3I did not see much benefit for myselfNon-attender |
| Positive aspects | |
| The participants did learn from the DVD but they took away different things | it’s [pain] not damaging, but just more pain not damageE1Actually that video was brilliant, that guy was meE4 |
| Others commented on the unwelcome guest, listening skills session, the pain cycle, goal-setting, breathing and exercise, movement and the hand massage | |
| Negative aspects | |
| Sitting down for long periods, disclosure was too intense, the DVD was too much like school, the DVD talked about unknown pain not diagnosed conditions, the timing and work, the relevance of the tasters, too rushed, too chaotic, the relevance of photography | I did not see the relevance of photographyB2it was not well thought through, it was chaoticE3the course was pressurised, we moved on too quicklyE1 |
| Learning | |
| Overall, participants felt that they needed time to embed their learning | you can forget your pain if your mind is occupied by somethingB2I still have pain but I’m learning to deal with itE4 |
| Social interaction | |
| The discussion, meeting people and learning from others was by far the most strongly talked about aspect of the course. However, courses needed sufficient people (six or more) and good facilitation to let everyone talk and to be informal | we are all in it together, we did not have to pretend about our painE2It’s a forum to talk about pain without burdening other people who do not understandE4I was not the odd one outE1everyone offered suggestions, we learned from each otherB2we all self-helped, we came a component of one, instead of individualsE2 |
| Effect of others | |
| Negative, disruptive and dominant participants needed to be controlled by the facilitators | I felt overwhelmed by the intellectuals in the groupE3I know I’m not alone, there are others suffering like meB3he was so bad I felt sorry for him, he made me feel betterE3I would not have come if it had been mixed genderB2 |
| Repercussions/outcomes from the course | |
| Some reported no changes since the course; however, others reported that they had either gone back to work or were renegotiating working hours. One group had organised a trip and others had implemented action plans and goals. Distraction and relaxation techniques had been used since the course | the course has not changed me at allE3it put me on a pathE2I’m not moaning so muchE2It’s changed my life, every day I set my self a new goal like a new exerciseE4I thought all that stuff was mumbo jumbo and nonsense but that relaxation technique was goodE4I’m working from home nowE4I now go to workE3I do stuff despite my pain, I’m not panicking as muchE1 |
| Changes suggested | |
| These included extra time, less time, evenings and weekend courses for working people, more information prior to and during the course, more follow-up, better and clearer aims, changing the DVD so less didactic, more time spent on exercise and lifestyle advice such as financial advice | |
| Facilitation | |
| Good facilitation meant good ‘control’ of the group – managing conversation, disruption, etc. Laughing, joking and anecdotes were appreciated along with informality | the facilitators have to let everyone speakE3discussion could have been controlled a bit moreE2 |
| Buddying | |
| Buddying did not always work but there were examples of when it worked really well | I did not get a chance to get one person’s numberE3we all wrote our names down on a piece of paperE2we have kept in touchE2 |
| Material | |
| Although there were requests for more material, there was not much evidence from the interviewees that they referred to it afterwards | |
Appendix 6 Methods
Costing the intervention
| Item | Assumptions |
|---|---|
| Number of participants | The base-case costing scenario was based on the average number of participants enrolled on the course across the two centres (London and Midlands). Sensitivity analyses were conducted for each centre as well as for the minimum and maximum number of participants enrolled on the course |
| Facilitator costs | Facilitators were paid a fixed fee per session. Consequently, given that facilitators were either self-employed or conducted the courses during their free time, on-costs (pension and National Insurance contributions) were not included. The analysis assumed no overheads |
| Administrator costs | Costs were determined using a fixed daily rate; estimations include 24% salary on-costs (pension and National Insurance contributions).245 The analysis assumed no overheads |
| Trainer costs | Costs were determined using a fixed daily rate; estimations include 24% salary on-costs according to 1. The analysis assumed no overheads |
| Facility costs | Courses were run in multiuse settings. The same daily rate was used for all venues. The analysis assumed no overheads |
| Course running costs | It was assumed that the cost of course materials (relax packs, DVDs and handouts) depended on the number of participants. Other costs (facility, hospitality, facilitators’ fees and travel) were assumed to be independent of the number of participants enrolled. The base-case costing scenario included some wastage of course materials. Sensitivity analyses were conducted assuming no wastage of course materials |
Health economic costs
| Costing item | Unit cost (£) | Cost of contact (£) | Assumption | Reference |
|---|---|---|---|---|
| Acute medicine ambulatory assessment unit | 106 | 106.00 | Non-24-hour A&E/casualty department: not leading to admitted. Average cost of HRG codes B01Z, VB02Z, VB03Z, VB04Z, VB05Z, VB06Z, VB07Z, VB08Z, VB09Z and VB11Z246 | |
| Community pharmacy | 69 | 11.50 | Duration of contact 10 minutes | Per hour of patient-related activities including qualifications (p. 172)245 |
| Counselling | 59 | 59.00 | Per consultation (p. 53)245 | |
| Dietitian | 34 | 28.33 | Duration of contact 50 minutes | Per hour including qualifications (p. 216)245 |
| Dispensing assistant | 25 | 4.17 | Clinical support worker, duration of contact 10 minutes | Per hour of patient-related work (p. 179)245 |
| GP surgery | 58 | 58.00 | Per-patient contact lasting 17.2 minutes, excluding direct care staff costs, with qualification costs (p. 183)245 | |
| GP telephone | 24 | 24.00 | Per telephone consultation lasting 7.1 minutes, excluding direct care staff costs, with qualification costs (p. 183)245 | |
| Health-care assistant telephone | 21 | 5.25 | Clinical support worker, duration of contact 15 minutes | Per hour (p. 179)245 |
| Health-care assistant surgery | 25 | 10.42 | Clinical support worker, duration of contact 25 minutes | Per hour of patient-related work (p. 179)245 |
| Home visit nurse/GP | 70/101 | 85.50 | 50% nurse, 50% GP; duration of visit 1 hour nurse and 23.4 minutes GP | Per hour of home visiting nurse (including qualifications and travel) (p. 175) and per out-of-surgery GP visit lasting 23.4 minutes (including qualifications and travel) (p. 183)245 |
| New medicine service (pharmacist) | 69 | 11.50 | Duration of contact 10 minutes | Per hour of patient-related activities including qualifications (p. 172)245 |
| NHS walk-in service | 56 | 56.00 | A&E services: walk-in centres: not leading to admitted. Average cost of HRG codes VB01Z, VB02Z, VB03Z, VB04Z, VB05Z,VB06Z, VB07Z, VB08Z, VB09Z and VB11Z246 | |
| Nurse specialist surgery | 49 | 20.42 | Duration of contact 25 minutes | Per hour including qualifications (p. 178)245 |
| Nurse specialist telephone | 49 | 12.25 | Duration of contact 15 minutes | Per hour including qualifications (p. 178)245 |
| Nurse surgery | 53 | 22.08 | Duration of contact 25 minutes | Per hour of face-to-face contact including qualifications (p. 180)245 |
| Nurse telephone | 41 | 10.25 | Duration of contact 15 minutes | Per hour including qualifications (p. 180)245 |
| Out of hours | 70 | 70.00 | GP surgery contact cost plus 20% for out of hours including nights, weekends and bank holidays | Per patient contact lasting 17.2 minutes, excluding direct care staff costs, with qualification costs (p. 183)245 |
| A&E, no admission | 173 | 173.00 | Ambulance services – see and treat or refer: HRG code ASS01246 | |
| Phlebotomist surgery | 25 | 6.25 | Clinical support worker, duration of contact 15 minutes | Per hour of patient-related work (p. 179)245 |
| Physician’s assistant surgery | 91 | 30.33 | Duration of contact 20 minutes | Per hour of client contact, nurse advanced (includes lead specialist, clinical nurse specialist, senior specialist), including qualifications (p. 181)245 |
| Physiotherapy | 33 | 19.25 | Duration of contact 35 minutes | Per hour including qualifications (p. 167)245 |
| Psychologist | 136 | 136.00 | Duration of contact 1 hour | Per hour of client contact (p. 171)245 |
| Costing item | Unit cost (£) | Assumption | Reference |
|---|---|---|---|
| Complex echocardiogram | 85 | Direct access: diagnostic services. HRG code EA45Z246 | |
| Electrocardiogram monitoring and stress testing | 61 | Direct access: diagnostic services. HRG code EA47Z246 | |
| Minor cardiac procedures | 74 | Direct access: diagnostic services. HRG code EA44Z246 | |
| Complex oesophageal, stomach or duodenum procedures | 569 | Direct access: diagnostic services. HRG code FZ81B246 | |
| Minor therapeutic or diagnostic general abdomen procedures | 34 | Direct access: diagnostic services. HRG code FZ13C246 | |
| Minor lower genital tract procedures category 2 | 33 | Direct access: diagnostic services. HRG code MA23Z246 | |
| Other infections (genitourinary medicine) | 70 | Direct access: diagnostic services. HRG code WA10Z246 | |
| CT scan | 114 | Average cost for different types of CT scan, ≥ 19 years | Direct access: diagnostic imaging. HRG codes RA08A, RA09A, RA10Z, RA11Z, RA12Z,RA13Z and RA14Z246 |
| DEXA scan | 75 | Direct access: diagnostic imaging. HRG code RA15Z246 | |
| MRI scan | 185 | Average cost for different types of MRI scan, ≥ 19 years | Direct access: diagnostic imaging. HRG codes RA01A, RA02A, RA03Z, RA04Z, RA05Z, RA06Z and RA07Z246 |
| Nuclear medicine | 454 | Average cost for nuclear medicine categories 1–8 | Direct access: diagnostic imaging. HRG codes RA35Z, RA36Z, RA37Z, RA38Z, RA39Z, RA40Z and RA42Z246 |
| Simple echocardiogram | 62 | Direct access: diagnostic imaging. HRG code RA60A246 | |
| Ultrasound scan | 57 | Average cost for different types of MRI, ≥ 19 years | Direct access: diagnostic imaging. HRG codes RA23Z, RA24Z, RA25Z, RA26Z and RA27Z246 |
| Radiography plain film | 30 | Direct access: diagnostic services. HRG code DAPF246 | |
| Examination, follow-up or special screening, with complications | 26 | Direct access: diagnostic services. HRG code WA20Y246 | |
| Biochemistry | 1 | Direct access: pathology services. HRG code DAP841246 | |
| Cytology | 18 | Direct access: pathology services. HRG code DAP838246 | |
| Haematology | 3 | Direct access: pathology services. HRG code DAP823246 | |
| Histology/histopathology | 31 | Direct access: pathology services. HRG code DAP824246 | |
| Immunology | 8 | Direct access: pathology services. HRG code DAP830246 | |
| INR anticoagulant monitoring | 21 | All NHS trusts and NHS foundation trusts – outpatient attendances data246 | |
| Microbiology/virology | 8 | Direct access: pathology services. HRG code DAP831246 | |
| Other pathology | 6 | Direct access: pathology services. HRG code DAP842246 | |
| Phlebotomy | 3 | Direct access: pathology services. HRG code DAP839246 | |
| Full pulmonary function testing | 52 | Direct access: diagnostic services. HRG code DZ52Z246 | |
| Lung volume studies | 73 | Direct access: diagnostic services. HRG code DZ45Z246 | |
| Simple airflow studies (e.g. spirometry) | 54 | Direct access: diagnostic services. HRG code DZ44Z246 | |
| Other procedures for non-trauma | 26 | Direct access: diagnostic services. HRG code HB99Z246 | |
| Diagnostic vascular radiology or other transluminal diagnostic procedures | 69 | Direct access: diagnostic services. HRG code QZ16A246 | |
| Examination, follow-up or special screening, without complications | 32 | Direct access: diagnostic services. HRG code WA20W246 | |
| 24-hour blood pressure monitoring | 32 | Diagnostic services – examination, follow-up or special screening, without CC | Direct access: diagnostic services. HRG code WA20Y246 |
| 24-hour Holter ECG | 61 | Diagnostic services – electrocardiogram monitoring and stress testing | Direct access: diagnostic services. HRG code EA47Z246 |
| ECG | 61 | Diagnostic services – electrocardiogram monitoring and stress testing | Direct access: diagnostic services. HRG code EA47Z246 |
| Faecal microscopy: culture and sensitivity | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Faecal occult blood test | 6 | Pathology services – other | Direct access: pathology services. HRG code DAP842246 |
| Fasting glucose | 1 | Pathology services – biochemistry | Direct access: pathology services. HRG code DAP841246 |
| Gastroscopy | 31 | Diagnostic services – minor endoscopic or percutaneous, hepatobiliary or pancreatic procedures, ≥ 19 years | Direct access: diagnostic services. HRG code GB04D246 |
| Helicobacter pylori antigen test | 8 | Pathology services – immunology | Direct access: pathology services. HRG code DAP830246 |
| Histopathology | 31 | Pathology services – histology/histopathology | Direct access: pathology services. HRG code DAP824246 |
| Nail mycology | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Nasal swab | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Skin histology | 31 | Pathology services – histology/histopathology | Direct access: pathology services. HRG code DAP824246 |
| Sputum analysis | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Sputum culture | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Stool sample | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Stool sample H. pylori test | 8 | Pathology services – immunology | Direct access: pathology services. HRG code DAP830246 |
| Throat swab for microscopy: culture and sensitivity | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Urine microalbumin and creatinine | 1 | Pathology services – biochemistry | Direct access: pathology services. HRG code DAP841246 |
| Urine pregnancy test | 1 | Pathology services – biochemistry | Direct access: pathology services. HRG code DAP841246 |
| Vaginal swab microscopy: culture and sensitivity | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Wound swab culture | 8 | Pathology services – microbiology/virology | Direct access: pathology services. HRG code DAP831246 |
| Costing item | Unit cost (£) | Cost of consultation (£) | Assumption | Reference |
|---|---|---|---|---|
| Acupuncture | 33.00 | 19.25 | Duration of contact 35 minutes | Per hour including qualifications. Community physiotherapist (p. 167)245 |
| Audiology | 56.48 | 56.48 | Audiology – outpatient attendances. HRG code 840246 | |
| Chiropody | 30.00 | 30.00 | Duration of contact 60 minutes | Per hour, community chiropodist/podiatrist (p. 170)245 |
| Community diabetes team | 49.00 | 20.42 | Duration of contact 25 minutes | Per hour, nurse specialist surgery including qualifications (p. 178)245 |
| Community lymphoedema service | 49.00 | 20.42 | Duration of contact 25 minutes | Per hour, nurse specialist surgery including qualifications (p. 178)245 |
| Community mental health team | 2528.00 | 2528.00 | Average cost per case, community mental health team for adults with mental health problems (p. 200)245 | |
| Community physiotherapy | 33.00 | 67.38 | 3.5 sessions; duration of contact 35 minutes | Per hour including qualifications (p. 167)245 |
| Community rehabilitation | 2749.00 | 2749.00 | Per episode, community rehabilitation unit (p. 42)245 | |
| Continence service | 49.00 | 20.42 | Duration of contact 25 minutes | Per hour, nurse specialist surgery including qualifications (p. 178)245 |
| Dietetics | 34.00 | 28.33 | Duration of contact 50 minutes | Per hour including qualifications (p. 216)245 |
| District nursing | 58.00 | 101.50 | 3.5 contacts; duration of contact 30 minutes | Per hour of patient-related work, community nurse (district nursing sister, district nurse), including qualifications (p. 175)245 |
| Deep vein thrombosis service | 21.00 | 21.00 | Outpatient attendances. HRG code 324246 | |
| Exercise/weight loss/lifestyle programme | 174.00 | 174.00 | Per person, public health interventions, physiotherapy/physical activity (p. 117)245 | |
| GPSI dermatology | 58.00 | 58.00 | Per patient contact lasting 17.2 minutes, GP surgery excluding direct care staff costs, with qualification costs (p. 183)245 | |
| Home treatment team | 58.00 | 101.50 | 3.5 contacts; duration of contact 30 minutes | Per hour of patient-related work, community nurse (district nursing sister, district nurse) including qualifications (p. 175)245 |
| Minor ailments clinic | 69.00 | 11.50 | Duration of contact 10 minutes | Per hour of patient-related activities, community pharmacist including qualifications (p. 172)245 |
| Nursing care | 58.00 | 101.50 | 3.5 contacts; duration of contact 30 minutes | Per hour of patient-related work, community nurse (district nursing sister, district nurse) including qualifications (p. 175)245 |
| Occupational therapy | 33.00 | 16.50 | Duration of contact 30 minutes | Per hour including qualifications (p. 168)245 |
| Optometry | 61.01 | 61.01 | Optometry. Outpatient attendances. HRG code 662246 | |
| Orthotics | 93.00 | 93.00 | Unit cost taken from Secondary User Service database (London centre download) | |
| Other GP for minor surgery | 58.00 | 58.00 | Per patient contact lasting 17.2 minutes, excluding direct care staff costs, with qualification costs, (p. 183)245 | |
| Physiotherapy | 33.00 | 67.38 | 3.5 contacts; duration of contact 35 minutes | Per hour including qualifications (p. 167)245 |
| Podiatry | 30.00 | 30.00 | Duration of contact 60 minutes | Per hour, community chiropodist/podiatrist (p. 170)245 |
| Pulmonary rehabilitation | 2749.00 | 2749.00 | Per episode, community rehabilitation unit (p. 42)245 | |
| Psychology IAPT | 136/65 | 100.50 | 50% psychologist, 50% counselling; duration of contact 1 hour | Per hour of client contact, psychologist (p. 171), counselling (p. 53)245 |
| Rehabilitation | 2749.00 | 2749.00 | Per episode, community rehabilitation unit (p. 42)245 | |
| Social services | 214.00 | 107.00 | Duration of contact 30 minutes | Per hour of face-to-face contact, social worker (adult services) including qualification costs (p. 183)245 |
| Stop smoking clinic | 46–179 | 135.50 | Mid-range | Per person, public health interventions, drug therapies for smoking cessation (p. 117)245 |
| Bereavement care service | 59.00 | 59.00 | aCounselling, per consultation (p. 53)245 |
Prescription costing flow chart
FIGURE 58.
Prescription costing flow chart. MIMS, Monthly Index of Medical Specialties.
General practice and patient information sheets
Participant questionnaires
Detailed recruitment process
Participants were to be recruited in three ways:
-
electronic searches using the clinic databases
-
GP/clinician referrals during face-to-face consultations
-
advertisements in clinics.
The electronic searches were conducted by clinic staff with the support of a primary care research network research officer and/or the COPERS research study team pending appropriate NHS approvals.
We tested a search strategy to identify the most appropriate patients using GPs’ electronic patient registers. A general practice staff member conducted several searches of the practice electronic records to identify the most appropriate domains and search terms; these search results were reviewed by a clinician in the practice to check the appropriateness of the sample. Two people then independently searched the clinic records electronically using the same search instructions to test the reliability of the output and the search method and subsequent validity.
The first stage of the search was to identify registered patients who had consulted within the last 3 months; then, within this group, the second stage was to search for prescribing information about repeat prescriptions for antidepressant medication, hypnotics and analgesia. Finally, we searched by symptoms: low back pain, backache, musculoskeletal, connective tissue disorders and pain. This generated a list of potential participants. Each clinic designated a key contact to liaise with the primary care research network and the study team; these personnel were trained to conduct their own searches by the study team and were given a study manual outlining the standard protocols necessary for the study. They were given support and advice as required.
From previous searches and test runs we estimated that this type of search yielded around 5% of the registered patients, which supported other epidemiological research estimates that 5–10% of the population experience chronic pain.
A list of potential participants was produced and screened by the clinicians to check suitability; no vulnerable people were to be approached (see inclusion and exclusion criteria in Chapter 9). The study team was provided with a pooled anonymous data set to allow response rates to be calculated. This list contained gender, age (not date of birth) and ethnicity (if recorded). Once the list had been finalised the study representative printed off invitation letters from the patients’ GP or clinician. These were placed in preprepared envelopes that contained the consent to approach form, a patient information leaflet and a Freepost envelope to return the consent to approach form to the study team. A single postal reminder was sent after 10–14 days. Any interested patients were able to complete a consent to approach form and send this to the study team, or telephone or e-mail the study team directly to express interest and find out more about the study. Those who found out about the study from the waiting room advertisements contacted the study team directly or picked up an invitation pack from the GP receptionists. In these cases the study team screened and checked suitability to participate by using the inclusion and exclusion criteria as a checklist. GPs and clinicians were informed of all patients enrolled into the study but they were not informed of their allocation.
Informed consent procedures
Consent was requested for participation in the trial, audio-recording of the courses, the use of anonymised data and permission to check health records at 12 months (for extracting data about health-care resource use). The consent process was as follows: (1) the expression of interest, either by mailed form or by telephone or e-mail, triggered the mailing of a COPERS cover letter, the patient information sheet, the trial consent form and the baseline questionnaire; and (2) any patients who wanted to be part of the study returned their signed trial consent form and the baseline questionnaire.
Participants were then telephoned to:
-
introduce the study team
-
check that consent was valid and informed (at this point the consent form was countersigned by the study team member and confirmed as valid if appropriate)
-
check their questionnaire for completeness
-
It was at this point that participants were formally enrolled in the study.
Participants were then randomised and informed of their allocation. If allocated to the control group they were told about the process involved, were sent a relaxation CD with instructions and the Pain Toolkit booklet and were asked to continue with their usual GP care. They received further questionnaires at 12 weeks and 6 and 12 months. If allocated to the intervention they were offered the opportunity of participating in a course.
Criteria for withdrawal
All participants were free to withdraw from the study at any time and without having to give any explanation. On formal withdrawal from the study we ceased to collect further data.
Relaxation information
Statistical analysis plan
Appendix 7 Fidelity, adherence and competence
Adherence and competence assessment sheets
Day 1, session 2: pain information – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to increase understanding about chronic pain.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Was the DVD played? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) pose Q1 (What do you think about the consultant saying that pain comes from the muscles?) after 5 minutes 35 seconds of the DVD? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) pose Q2 (What do you think about this model of pain? Is it missing anything?) after 9 minutes 24 seconds of the DVD? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4 | Did the facilitator(s) pose Q3 (How do you feel about the consultant saying there is no cure?) after 12 minutes 13 seconds of the DVD? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 5 | Did the facilitator(s) pose Q4 (How do you feel about the Bert Trautmann example?) at the end of the DVD? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 6 | Did the facilitator(s) reiterate the aims of the course as explained at the end of the DVD? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage adherence score (total adherence score/12 × 100) |
Day 1, session 2: pain Information – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total competence score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 |
Day 1, session 3: acceptance: the uninvited guest – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to relate the scenario about the unwanted and uninvited guest to chronic pain.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Did the facilitator(s) read the street party story to the group? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) ask the group to discuss how the women handled the issue of the ‘uninvited guest’? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) encourage the group to relate to the story of the ‘uninvited guest’ as an analogy for their pain? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage adherence score (total adherence score/6 × 100) |
Day 1, session 3: acceptance: the uninvited guest – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total competence score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 |
Day 1, session 5: the pain cycle, unhelpful emotions and behaviours – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to explain the pain cycle and understand the process and the unhelpful things that we do to keep us in that cycle.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Did the facilitator(s) show and explain the persistent pain cycle to the group? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) ask the group to generate a list of unhelpful things that may keep them in the pain cycle? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) distribute and/or mention handout 1? (Unhelpful coping strategies.) Please refer to reviewer guidance below | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4 | Did the facilitator(s) distribute and/or mention handout 2? (Depressive symptom checklist.) Please refer to reviewer guidance below | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 5 | Did the facilitator(s) ask the group to generate a list of things that they could do to escape from the pain cycle? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 6 | Did the facilitator(s) distribute and/or mention handout 3? (Escape routes from the pain cycle.) Please refer to reviewer guidance below | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage adherence score (total adherence score/12 × 100) |
Reviewer guidance: if, on listening to the audio-recording, the score for this item is ‘unsure’ (1) or ‘no’ (0), please refer to the observation notes for this session to determine whether the handouts were/were not distributed and amend the adherence score accordingly.
Day 1, session 5: the pain cycle, unhelpful emotions and behaviours – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total competence score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 |
Day 2, session 9: identifying problems, goal-setting, action planning – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to help the participants identify problems, brainstorm solutions, set goals and devise action plans, as a means of escaping the pain cycle.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Did the facilitator(s) explain the process of identifying problems, brainstorming solutions, thinking about advantages/disadvantages to solutions and goal-setting? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) explain the SMART process to the group? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) distribute and/or mention handout 5 (SMART)? Please refer to reviewer guidance below | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4a | Did the facilitator(s) go through an example of the SMART process with the group? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4b | Group exercise: did the facilitator(s) divide the participants into smaller groups/pairs to tackle a chosen problem on their own? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 5 | Group exercise: were the groups given the opportunity to give feedback about the process of problem-solving? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 6 | Did the facilitator(s) distribute and/or mention handout 6 (goal-setting examples)? Please refer to reviewer guidance below | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 7 | Did the facilitator(s) distribute and/or mention handout 7 (tips for a good night’s sleep)? Please refer to reviewer guidance below. | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage score (total adherence score/16 × 100) |
Reviewer guidance: if, on listening to the audio-recording, the score for this item is ‘unsure’ (1) or ‘no’ (0), please refer to the observation notes for this session to determine whether the handouts were/were not distributed and amend the adherence score accordingly.
Day 2, session 9: identifying problems, goal-setting, action planning – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total competence score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 |
Day 2, session 10: barriers to change, unhelpful thinking – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to introduce ideas about unhelpful thoughts, automatic thoughts and errors in thinking.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Did the facilitator(s) distribute handout 8 (typical unhelpful/negative thoughts and thinking)? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) read the titles and describe the unhelpful thoughts in the list to the group for their consideration? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) use the unhelpful thought ‘flash cards’ to generate group discussion? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4 | Did the facilitator(s) read the ‘Sam’s morning’ scenario to the group with appropriate pauses for participants to ‘spot’ and name the negative thoughts? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 5 | Did the facilitator(s) distribute and/or mention handout 9 (unhelpful thoughts checklist)? Please refer to reviewer guidance below | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 6 | Did the facilitator(s) invite the group to consider ‘Sam’s morning’ again from an unemotional standpoint? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage adherence score (total adherence score/12 × 100) |
Reviewer guidance: if, on listening to the audio-recording, the score for this item is ‘unsure’ (1) or ‘no’ (0), please refer to the observation notes for this session to determine whether the handouts were/were not distributed and amend the adherence score accordingly.
Day 2, session 10: barriers to change, unhelpful thinking – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total compliance score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 |
Day 2, session 11: barriers to change, reframing negatives to positives – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to identify reasons why people stay in the pain cycle and barriers to change.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Did the facilitator(s) ask the group to consider the ‘cons’ of pain? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) ask the group to consider the ‘pros’ of pain? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) use a flip chart to encourage a consideration of the ‘pros’ and ‘cons’ of pain? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4 | Did the facilitator(s) use the example of ‘going to the gym’ (slide 16) to demonstrate reframing ‘cons’ to ‘cans’? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 5 | Did the facilitators(s) use the group-generated ‘cons’ of pain and ask the group to reframe them to ‘cans’ | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage adherence score (total adherence score/10 × 100) |
Day 2, session 11: barriers to change, reframing negatives to positives – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total competence score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 |
Day 2, session 12: attention control and distraction – adherence
COPERS course code:
Reviewer:
Review date:
Aim: to learn how to focus the mind away from pain thoughts.
| Item number | Item | Adherence measure | Comments |
|---|---|---|---|
| 1 | Did the facilitator(s) ask the group NOT to think about food for 30 seconds? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 2 | Did the facilitator(s) ask the group what happened when they tried not to think about food? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 3 | Did the facilitator(s) ask the group NOT to think about their pain for 30 seconds? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 4 | Did the facilitator(s) ask the group if their pain felt better or worse as they focused on it? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 5 | Did the facilitator(s) ask the group to close their eyes and recall a time when they were content calm and happy? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| 6 | Did the facilitator(s) ask the group what happened to their pain while they were doing this exercise? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total adherence score | |||
| Percentage adherence score (total adherence score/12 × 100) |
Day 2, session 12: attention control and distraction – competence
| Item | Competence measure | Comments | |
|---|---|---|---|
| Introduction | Did the facilitator(s) introduce the aims/rationale of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Discussion | Did the facilitator(s) create opportunities for discussion and encourage individual disclosure of narratives and participation? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Summary | Did the facilitator(s) consolidate/embed the group’s learning at the end of the session? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Linking | Did the facilitator(s) link the completed session to other sessions? | Yes (2) | |
| Unsure (1) | |||
| No (0) | |||
| Total competence score | |||
| Percentage competence score (total competence score/8 × 100) |
Overall session impression score (‘How well did you think the overall aims of the session were met?’)
| Excellent | Did not go well | Comments | ||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 |
Appendix 8 Results: number of participants in each analyses
| Analysisa | Control (N = 300), n (%) | Intervention (N = 403), n (%) |
|---|---|---|
| CPG pain-related disability score | 278 (93) | 374 (93) |
| CPG pain intensity score | 260 (87) | 364 (90) |
| PSEQ score | 270 (90) | 373 (93) |
| HADS anxiety score | 261 (87) | 364 (90) |
| HADS depression score | 261 (87) | 364 (90) |
| CPAQ score | 261 (87) | 364 (90) |
| heiQ score | 261 (87) | 363 (90) |
| EQ-5D score | 275 (92) | 372 (92) |
| Census global health question | 260 (87) | 364 (90) |
| Drug data up to 12 months post randomisation | 258 (86) | 350 (87) |
List of abbreviations
- A&E
- accident and emergency
- ASES
- Arthritis Self-Efficacy Scale
- BDI
- Beck Depression Inventory
- BNF
- British National Formulary
- CACE
- complier average causal effect
- CBT
- cognitive–behavioural therapy
- CDSES-33
- Chronic Disease Self-Efficacy Scale-33
- CES-D
- Center for Epidemiologic Studies Depression
- CI
- confidence interval
- CIRS
- Chronic Illness Resources Survey
- COPERS
- Coping with persistent Pain, Effectiveness Research into Self-management
- CPAQ
- Chronic Pain Acceptance Questionnaire
- CPG
- Chronic Pain Grade
- DDD
- defined daily dose
- DVD
- digital versatile disk
- EQ-5D
- European Quality of Life-5 Dimensions
- FABQ
- Fear Avoidance Beliefs Questionnaire
- GLM
- generalised linear model
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HCP
- health-care professional
- heiQ
- Health Education Impact Questionnaire
- HRG
- Healthcare Resource Group
- IAPT
- Improving Access to Psychological Therapies
- IASP
- International Association for the Study of Pain
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMMPACT
- Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials
- INB
- incremental net benefit
- IQR
- interquartile range
- ITT
- intention to treat
- MI
- multiple imputation
- MLM
- multilevel model
- MMICS
- Multinational Musculoskeletal Inception Cohort Study
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NSAID
- non-steroidal anti-inflammatory drug
- OA
- osteoarthritis
- PCA
- Prescription Cost Analysis
- PCTU
- Pragmatic Clinical Trials Unit
- PHQ
- Patient Health Questionnaire for Depression and Anxiety
- PSEQ
- Pain Self-Efficacy Questionnaire
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SMD
- standardised mean difference
- SUR
- seemingly unrelated regression
- SUS
- Secondary Uses Service
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
- WTP
- willingness to pay