Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0108-10011. The contractual start date was in November 2009. The final report began editorial review in June 2015 and was accepted for publication in March 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Chris Salisbury is a member of the National Institute for Health Research (NIHR) Health Services and Delivery Research Board and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West. He also had an honorary consultant contract with NHS Direct, the NHS trust that hosted this research, in order for him to act as principal investigator. Glyn Lewis is a member of the NIHR Efficacy and Mechanism Evaluation Board and Jon Nicholl is a member of the NIHR Clinical Trials Unit Standing Advisory Committee. Catherine Pope is a member of the NIHR CLAHRC Wessex, which is a partnership between Wessex NHS organisations and partners and the University of Southampton and is part of the NIHR.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Salisbury et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Open access to software developed for this research programme
The software developed to support the Healthlines Service is freely available to developers who wish to use the content under a GNU General Public License version 3.
Please note that we are not able to provide any further support for this software nor answer technical queries about it. The software is made freely available ‘as is’, so that other organisations can make use of our work and develop it further for patient benefit.
The Healthlines software is available from the following open access repository: https://github.com/Healthlines/Healthlines-Applications (accessed 26 September 2016).
This repository includes all the code relating to the following areas:
-
patient portal where patients can view previous interactions and add additional information
-
facility to support self-management of blood pressure, enabling patients to enter blood pressure readings and providing patients with graphical feedback about whether or not their blood pressure is within target limits
-
call handler management system (including call handling ‘scripts’ for each patient session, patient management)
-
administration tools (including importing patients, creating call handler scripts/flow)
-
general practitioner (GP) messaging solution (including ability to send details of patient interactions through to patients’ GPs by e-mail).
Content for the Healthlines depression intervention
Please note that the open access call handler protocols include ‘scripts’ that were designed to support the use of Living Life to the Full Interactive materials, which are licensed from Five Areas Limited. More information on the full range of Living Life to the Full Interactive materials can be obtained from www.fiveareas.com/ (accessed 26 September 2016).
Content for the Healthlines cardiovascular disease risk intervention
All code relating to call handling protocols for the cardiovascular disease risk intervention is not part of this repository as the intervention was largely based on material used under licence from Duke University (Durham, NC, USA). For further information about the content of the cardiovascular disease software, please contact Professor Hayden Bosworth at Duke University (hayden.bosworth@duke.edu).
Chapter 1 Introduction and background
Background to the development of this research programme
NHS Direct was established in 1998 as a nurse-led telephone advice service for patients with health problems, as part of a commitment in the White Paper The New NHS: Modern, Dependable1 to ‘modernise’ the NHS and to make its services more accessible and convenient. As the government stated in the White Paper, the main aim of NHS Direct was ‘to provide people at home with easier and faster advice and information about health, illness and the NHS so that they are better able to care for themselves and their families’ (p. 8). 1
During the decade beginning 2000, NHS Direct expanded its offering of services considerably, beyond the original telephone advice line. The NHS Direct website was introduced at the end of 1999 and provided health information and advice, including ‘symptom checkers’ and signposting to other local NHS services based on users’ postcodes. Services available from the website expanded and the number of users grew such that the number of visits to the website rose from 1.5 million a year in 2000 to approximately 18 million in 2009. 2 In addition, NHS Direct introduced an interactive digital television channel providing similar advice as the website. NHS Direct also provided a number of services commissioned nationally or by other NHS trusts, including additional telephone advice services during periods of excessive demand, such as flu epidemics or health scares, a dental nurse assessment service, telephone-based pre- and postoperative assessments for patients having surgery, and local contracts to provide care for people with long-term conditions (LTCs), such as the Birmingham OwnHealth® scheme [see www.england.nhs.uk/wp-content/uploads/2014/12/3million-lives-birmingham-ownhealth-cs.pdf (accessed 7 September 2016)], based on allocating care managers to provide telephone support for patients with conditions such as diabetes or heart failure. In 2003, NHS Direct3 published a strategy document which stated (with justification) that the organisation was ‘the largest and most successful healthcare provider of its kind, anywhere in the world’ (p. 3).
As part of its strategic planning, during the latter part of the decade NHS Direct decided to enhance its commitment to research and development. It approached Professor Chris Salisbury to explore possibilities for research, particularly in relation to topics in which they could develop and test new services, which they could then offer to commissioners. Several possible areas for research were considered, but it became clear that a key opportunity lay in the potential of NHS Direct to provide support for patients with LTCs. A team of academics with interest and expertise in this topic area was therefore brought together and this research programme was developed in partnership between NHS Direct and the Universities of Bristol, Sheffield, Manchester and Southampton, with Professor Salisbury as Chief Investigator.
Background to the research topic
As the population ages, the priority for the NHS is increasingly to help people manage LTCs. These individuals consume a high proportion of health-care resources, yet there is considerable variation in management. 4 There is a need to redesign services both to cope with the increasing number of people needing health care and to improve the standard of care being offered. This ‘requires wholesale change in the way health and social care services deliver care and support’ (p. 41). 5 In 2004, the Department of Health6 first published a strategy for improving the care of LTCs based on promoting better health by supporting self-care, providing responsive high-quality services and providing case management for those with the greatest needs.
One approach to meeting the need to support improved health care for LTCs is to make greater use of technologies such as text messaging, telephone support, the internet and remote monitoring. These approaches can be described as telehealth, which can be defined as the use of electronic and telecommunication technologies to support health care at a distance from the patient.
There is strong international interest in the use of telehealth to help patients with LTCs. 7 At the time that this programme was commissioned, a number of systematic reviews had been conducted. 8–23 These concluded that telehealth interventions were promising, but that further research was needed in relation to:
-
mechanisms of action
-
effectiveness in a wider range of conditions
-
clinical outcomes
-
economic effects
-
relevance to the NHS.
Within the NHS, many organisations were developing telehealth interventions, but formal evaluation had been relatively limited.
Importance of the research and its relevance to the priorities and needs of the NHS
Over 15 million people in England have a LTC and treatment of LTCs accounts for 70% of total health-care expenditure. Improvements in LTC management could have major benefits in terms of patient health, quality of life and use of NHS resources. 5
In this programme we decided to test a new telehealth approach, delivered by NHS Direct, within two exemplar conditions. These were (1) patients with depression and (2) those at high risk of cardiovascular disease (CVD). We chose these examples for several reasons. They are very different types of conditions, one affecting mental health and one physical health. However, both conditions are very common and have a major impact on quality of life. They also account for a high proportion of health-care resources and there was good reason to suggest that the potential need for care is considerably greater than the capacity of the NHS to deliver care. Furthermore, in both cases there was some evidence of effective telehealth interventions, but this evidence was inconclusive. Further discussion of these points in relation to the exemplar conditions is provided in the following sections.
Studying two very different LTCs in parallel, and developing management programmes for them based on a common theory-driven approach, would enhance generalisability. The vision was that, if these telehealth programmes were both effective, this would provide a framework and a justification for the future development of further programmes for other LTCs.
Depression
Mental health problems are very common: 11% of adults aged 16–74 years in England and Wales suffer from depression or mixed anxiety/depression. 24 These disorders account for 25% of primary care consultations25 and the cost to society is approximately £25B. 26 There is increasing evidence that therapies of the type that could be delivered by NHS Direct, including computerised or telephone-based cognitive–behavioural therapy (CBT), are effective. 27
Developing the role of NHS Direct in the management of LTCs was also consistent with NHS policy to design services around patients’ needs, improve convenience of access to care, encourage active involvement in care and provide information to support self-care. 5,28 NHS policy at the time envisaged telehealth becoming a mainstream aspect of NHS care delivery28,29 and this enthusiasm for the potential of telehealth to manage patients with LTCs has continued since this programme was commissioned. 30 This interest in the potential of telehealth is perhaps not surprising given that over the last 20 years there has been an explosion in the use of technology, particularly call centres, the internet, mobile devices and ‘apps’, that has revolutionised almost all other transactions that used to be conducted through face-to-face consultations, including banking, shopping and customer support in various industries.
High cardiovascular disease risk
Cardiovascular disease [coronary heart disease (CHD) and stroke] causes 36% of deaths in England and accounts for one-fifth of all hospital admissions. 31,32 In the past, hypertension, obesity and hyperlipidaemia were considered as separate LTCs, but there is increasing recognition that they should not be thought of as separate conditions but instead be considered in combination as factors that contribute to an individual’s overall CVD risk. Therefore, ‘raised CVD risk’ should be considered as a LTC, rather than treating hypertension, for example, as a LTC. To improve care for patients with a raised CVD risk it is important to manage all of their underlying risk factors through treatment of high blood pressure, weight loss, smoking cessation, cholesterol reduction and increased exercise. 31–33
One further factor in the decision to use raised CVD risk as an exemplar condition was the policy decision, announced in 2008, that the NHS was to begin a screening programme for CVD in those aged 40–74 years. This decision was based on predictions about the potential benefits of such as scheme, but the modelling used also suggested that screening was likely to identify a large number of people who would need to be offered intensive management of their CVD risk factors. 34 At the time that the Health Checks policy was announced, it was not clear how the NHS was going to provide the extra capacity to advise all of the extra people with raised risks who would be identified. Some new forms of help were, in time, introduced (e.g. health trainers), but it was apparent that the NHS needed to develop new approaches to meeting the extra needs identified through the Health Checks programme without overwhelming primary care services, which were already under pressure. Supporting people to use resources that were available online appeared to be a viable way to help meet this need and NHS Direct appeared to be ideally placed to both develop the online services and provide telephone support to signpost people to the most appropriate resources.
Need for research in this area
Lack of evidence about effectiveness and cost-effectiveness
At the time that this programme began, a number of systematic reviews of telehealth for a variety of LTCs had been carried out. 8–23 These showed that evidence of effectiveness was stronger for some conditions (e.g. heart failure) than for others (e.g. diabetes). There was good evidence that telehealth was feasible and could lead to improvements in specific health behaviours, but there was a lack of evidence about mechanisms of action, clinical outcomes, cost-effectiveness, patient satisfaction, impact on service utilisation and acceptability. 9,23
The need for theory
Much of the existing evidence about telehealth was inconsistent. This is unsurprising in view of the range of LTCs, interventions and health system contexts that had been considered. To address these inconsistencies, there was a need to develop a stronger theory about how and why certain types of intervention might be beneficial, then to develop interventions based on this theory and test them. In particular, evaluation was needed to provide robust evidence about clinical outcomes and economic impacts. Most previous research had focused on narrow intermediate outcomes and process measures rather than meaningful clinical outcomes.
The need to test wide-scale implementation alongside existing services
It was also important to learn how to implement telehealth interventions nationally and make them mainstream. Many telehealth studies have tested discrete ‘stand-alone’ technologies, such as a specific text messaging application or a specific website or home monitoring technology. These have been often tested in volunteer populations. However, evidence of efficacy in research populations does not provide evidence of effectiveness when the intervention is implemented on a wide scale in real-world application in the population who might benefit from it. Furthermore, telehealth interventions have often been developed without consideration of how they will integrate with other existing sources of health care.
This involves consideration not only of the technology but also of the organisational context. Most previous studies had been conducted in the USA, which has different problems of access to care, less well-developed primary care and different financial incentives. Research was needed on integration and mainstream implementation of telehealth in the NHS, particularly how a national telehealth provider such as NHS Direct could support local providers of primary care.
The promotion of telehealth-based long-term condition programmes by commercial providers
In the period around 2005, several commercial organisations were marketing telehealth programmes or telehealth applications to commissioners for the management of LTCs. This growth in telehealth has been encouraged by a succession of government policies. At the time that this research programme was being developed, NHS Direct was working with Birmingham East and North Primary Care Trust (PCT) and the pharmaceutical company Pfizer on the OwnHealth initiative to support patients with LTCs. This provided care for people with diabetes, heart failure or CHD. The intervention was based on a behavioural programme delivered by NHS Direct based on regular telephone support from nurses. Although evaluation was limited, patients enrolled in the project reported improvements in health behaviours and symptoms, improvements in some clinical outcomes, such as blood sugar control, and a reduction in primary and secondary care consultations. Patients had positive experiences of the service and found it accessible and easy to use. 35,36 This supports the feasibility of NHS Direct as a platform for delivering care for LTCs.
This programme grant was designed to build on these foundations to develop and test interventions to support patients with LTCs, including issues of implementation, so that (if successful) they could be rapidly rolled out nationally.
Target populations and generalisability
The introduction of NHS Direct reflected a policy drive to make the NHS more responsive to its users, for example by making it possible for people to seek advice at times and in ways that were convenient for them. A ‘one-size-fits-all’ approach to NHS provision was no longer considered appropriate. Similar considerations also applied to telehealth programmes for LTCs to be developed by NHS Direct. We did not consider it likely that telehealth support for LTCs was necessarily going to be acceptable or appropriate for all or even most individuals with a LTC. However, it may be a good solution for some people, particularly those who may have difficulty accessing care through conventional means based on face-to-face consultations. This might particularly apply, for example, to those who are at work during the day or those who are housebound. The number of people with LTCs is so large that even if telehealth provided help for only a minority of these individuals it could still be a worthwhile option if it was more accessible, acceptable and cost-effective for this group of people.
This has implications for generalisability. In conventional research designs there are concerns if studies do not recruit a large proportion of the eligible population because findings from the study may not be applicable to the wider population. However, in this research programme we were interested in identifying which groups of the population were most interested in and might benefit from a telehealth programme delivered via NHS Direct. If an intervention was beneficial for these people it would not necessarily need to be relevant to other people who would prefer to receive help in more conventional ways.
Combining technologies for which there is already proof of concept
In each of our exemplar conditions there was existing evidence that specific telehealth interventions may be effective, but there was a need to test organisational interventions to deliver them on a wide scale. The National Institute for Health and Care Excellence (NICE) had highlighted the need for large-scale trials of LTC management programmes for depression. 27 This programme set out to draw on the evidence on the most promising ‘active ingredients’ of telehealth for LTCs to develop integrated, comprehensive and theory-driven programmes of care to be delivered by NHS Direct.
Within our exemplar conditions there was some evidence of effectiveness for interventions that could be offered by NHS Direct. For depression, it included provision of CBT by telephone or online,37–40 online self-help41,42 and bibliotherapy. 43,44 For CVD risk, this included blood pressure telemonitoring,19 web-based hypertension management45 and telephone-based interventions to promote medication adherence and/or risk reduction. 46–48
However, in real-life management of patients with LTCs, specific interventions are not offered to patients in isolation. Patients with depression may well be treated with antidepressants and receive psychological therapy at the same time. For patients with a raised CVD risk, clinicians need to seek to improve multiple risk factors, such as blood pressure, weight and smoking behaviour. Attention to these risk factors may involve the provision of advice, medication, encouragement and signposting to a range of resources.
In this research programme we were not seeking to develop new ‘cutting-edge’ technology for use in LTCs. Instead, we were seeking to use existing technologies for which there was already some ‘proof of concept’ evidence to suggest a likely benefit for patients with our exemplar LTCs and to test how to deliver these interventions on a large scale in a co-ordinated way through a platform such as NHS Direct.
The Whole System Demonstrator project
At the time that this research was developed we were aware of and needed to take account of several other relevant ongoing projects, in particular the Whole System Demonstrator (WSD) programme. The Department of Health White Paper Our Health, Our Care, Our Say: a New Direction for Community Services28 set out a vision for preventative care services and set up the WSD programme to establish evidence in a UK context by deploying telecare and telehealth services covering a resident population of over 1 million across three areas of the country. 49 This was therefore a very large demonstrator programme that explored integrated health and social care supported by new technologies, based on radical systems redesign. The WSD programme was subject to a comprehensive evaluation based on a multicentre cluster randomised controlled trial (RCT) with accompanying economic evaluation. This was the largest robust evaluation of a telehealth programme ever attempted and it was important that our own research tested a different approach. The WSD programme focused on people with serious and life-threatening LTCs such as lung disease, heart failure and diabetes and, in particular, patients at high risk of hospital admission.
Focus on common long-term conditions and low-cost interventions
The rationale for the WSD programme is that some patients make very high use of expensive NHS resources and it may be possible using telehealth to support them in a more cost-effective way. However, the WSD approach was in itself also very resource intensive. Furthermore, those individuals with very high health-care needs, who are at the pinnacle of the ‘Kaiser pyramid’,50 represent only a very small proportion of patients with LTCs.
We therefore decided to think about telehealth in a different way and explored an approach to making self-management resources available via telehealth to large numbers of people with common LTCs. A core principle of epidemiology is that small shifts in the health of large numbers of people can have more impact on population health than large shifts in health in those with the greatest health problems. 51 The idea was that our intervention would be applicable to many people and so it was important to make use of inexpensive technologies that could potentially be widely available. Otherwise, the intervention would be unaffordable even if it was effective.
Furthermore, as we developed this research programme, our initial investigations suggested that most telehealth interventions being promoted were disproportionately expensive and were very unlikely to be cost-effective unless they had a big impact on hospital admission rates or led to big improvements in health. Previous research had not suggested that either was likely to be the case. 52–54 Because we were seeking to achieve small improvements in health in large numbers of people, it was important that the intervention could be delivered at minimum cost to maximise the likelihood that it would be both cost-effective and affordable at a population level.
The potential of NHS Direct
NHS Direct provided a unique opportunity to integrate a range of telehealth interventions for people with LTCs. It could reach people through several technological channels simultaneously, including an established network of telephone call centres, the NHS Direct website and a digital television service. No other service in the world offered this range of co-ordinated services or had the same potential to reach such a high proportion of the entire population.
NHS Direct had traditionally offered reactive services to people seeking health advice and information. But it also had the potential to provide proactive services, particularly for people who have difficulty accessing care through general practice, such as the housebound, commuters or those who do not speak English. For example, the nationally networked call centres of NHS Direct could, in theory, provide a nurse with expertise in diabetes who also spoke Hindi to patients anywhere in the country or could provide advice about smoking to patients at times convenient to them, such as in the evening.
NHS Direct was also a well-recognised and nationally recognised ‘brand’ and achieved high levels of satisfaction from its users. It had a national network of call centres, a cadre of well-trained staff with experience of providing health information and advice, well-established facilities for translation for people who could not speak English, availability of staff outside office hours and the ability to call on experienced nurses, pharmacists or information specialists who could research and provide specialised advice, for example about medication interactions. NHS Direct already had experience of testing case management-type approaches to the management of LTCs through the OwnHealth pilot projects. If it were possible to develop a cost-effective intervention for LTCs that could be delivered via NHS Direct in a research context, it would be possible to roll this out relatively quickly and easily to the entire population of England.
Postscript: closure of NHS Direct
This research programme ran from 2009 to 2015, with the trial of the intervention being conducted between 2013 and 2014.
NHS Direct was closed in March 2014 in favour of the less expensive ‘111’ telephone helpline and the NHS Choices website. The closure of NHS Direct had been announced in October 2013 but there had been widespread rumours of closure ever since the BBC leaked in August 2010 that the incoming coalition government planned to replace NHS Direct. 55
Although the potential of NHS Direct had provided the impetus for this research programme, the underlying research questions about the role of a comprehensive management programme for patients with LTCs based on telehealth remain entirely relevant. The closure of NHS Direct inevitably led to some difficulties in conducting the research programme and in particular meant some delays and pauses in the delivery of the intervention; however, we were able to complete the trial thanks to Solent NHS Trust community trust replacing NHS Direct as the host for the intervention and the research programme.
NHS Direct offered advantages because of its national reach and infrastructure. However, in practice, the NHS Direct intervention staff worked from one site (Nottingham) and the intervention that was developed through this programme could be delivered by other provider organisations and made available throughout the UK (as was demonstrated by our ability to successfully relocate the service to Solent NHS Trust). Therefore, we do not believe that the closure of NHS Direct has had an important impact on the relevance or findings of this research programme.
Aims and objectives
Aim
The aim of this programme of research was to develop, implement and evaluate new programmes of care delivered via NHS Direct for patients with LTCs and to provide evidence about the benefits and costs of these initiatives.
The intended benefits of these new forms of provision were to improve health outcomes for patients, facilitate self-management, improve the patient experience and improve the cost-effectiveness of care provision.
The programme focused on two exemplar conditions: depression and high CVD risk.
Objectives
-
To review evidence about telehealth interventions designed to improve health care for patients with LTCs in order to develop a theory about which types of interventions potentially delivered by NHS Direct are most likely to be effective.
-
Using qualitative methods, to critically examine how NHS Direct resources could best be incorporated into the LTC management of patients and integrated with current primary care professional practice.
-
To explore patient factors and access factors that are associated with unmet need and with willingness to use NHS Direct and specifically types of telehealth interventions that are most likely to be acceptable to different patient groups.
-
Based on the first three activities, to develop and optimise interventions offered by NHS Direct that are likely to be acceptable, effective and efficient.
-
To determine the clinical effectiveness and cost-effectiveness of LTC management provided by NHS Direct in the two exemplar conditions: depression and CVD risk.
Overview of the research plan
This programme consisted of five linked activities that address each of the objectives described in the previous section. Activities 1–3 were conducted in parallel and informed activities 4 and 5. Below is an overview of each of these activities:
-
Review and synthesis of quantitative and qualitative evidence about telehealth for patients with LTCs in order to develop a theory about which types of intervention potentially delivered by NHS Direct are most likely to be effective.
-
Qualitative research with patients and health professionals to examine how NHS Direct can best contribute to LTC management.
-
Survey of patients with two exemplar LTCs (depression, high CVD risk) to explore relationships between access to care, unmet need and willingness to use NHS Direct, to identify factors that are associated with interest in telehealth and the types of telehealth that are most likely to be acceptable to different patient groups.
-
Development of a theoretical model that could be used for intervention design and implementation, as well as development of the interventions for our exemplar LTCs to be offered by NHS Direct that, based on activities 1–3, are likely to be acceptable, effective and efficient.
-
Randomised controlled trial to determine the effectiveness of LTC management provided by NHS Direct in two exemplar conditions. We also used qualitative methods to study implementation and conducted an economic evaluation to assess cost-effectiveness and model future costs/benefits following national implementation.
The approach was based on Medical Research Council (MRC) guidance on the evaluation of complex interventions and included defining and understanding the problem, paying attention to context, developing and optimising the intervention based on a theory about how the intervention is intended to achieve its aims and conducting a definitive evaluation. 56 To develop the intervention, we used an intervention mapping approach to integrate the various research components. Intervention mapping involves focusing specifically on the behaviour of users of NHS Direct. A key process in intervention mapping is to first identify the relevant psychological determinants of behaviour and then systematically map these onto evidence-based strategies and techniques for changing behaviour. We made use of recent approaches to intervention mapping and theoretical modelling of behavioural interventions. 57–59
Chapter 2 An evidence synthesis of telehealth interventions for long-term conditions
Abstract
Background: The objective of the evidence synthesis was to review and synthesise evidence about telehealth interventions to provide guidance on the types of effective interventions that could be delivered by the Healthlines study.
Methods: This was a mixed-methods evidence synthesis consisting of six sequential studies: (1) a meta-review of systematic reviews of home-based telehealth for LTCs published between 2005 and 2010; (2) a review of systematic reviews of telehealth for depression published between 2005 and 2010; (3) an evidence synthesis of qualitative research on telehealth published between 2000 and 2010; (4) a realist synthesis based on studies in 1–3; (5) horizon scanning to ensure the inclusion of up-to-date evidence; and (6) a systematic review of the effectiveness of telehealth interventions to reduce overall CVD risk and/or CVD risk factors.
Findings: The evidence base addressing the effectiveness of home-based telehealth for LTCs and depression is large and generally positive. However, a number of systematic reviews recommend caution because of the poor quality of the studies (small sample sizes, weak study design and lack of adequate comparators). There is limited evidence on cost-effectiveness. Qualitative literature on patients’ views of telehealth are generally positive because of perceptions that it increases access to health care. Professionals are less accepting of telehealth than patients. Three mechanisms of action were identified in the realist synthesis: relationships between health professionals and patients; fit with patients’ needs and capabilities; and visibility through feedback. The systematic review found no evidence of an effect of telehealth on overall CVD risk, but weak evidence of a small reduction in systolic blood pressure and total cholesterol.
Conclusions: The evidence base shows that telehealth for LTCs is acceptable and can be effective. However, rigorous evaluation of telehealth interventions, including their cost-effectiveness, is needed.
Introduction
The objective of the evidence synthesis was to review and synthesise evidence about telehealth interventions to provide guidance on the types of effective interventions that could be delivered by NHS Direct. The aim was not to undertake a traditional systematic review with meta-analysis of the effect of different types of telehealth in different populations because a number of reviews had already been published. Rather, the aim was to undertake a mixed-methods review60 to offer an overview of the trial-based evidence and complement this with qualitative evidence to inform decisions about the Healthlines study intervention development.
Design
The mixed-methods evidence synthesis consisted of six complementary approaches undertaken in sequential order:
-
A meta-review of systematic reviews of home-based telehealth for LTCs published between 2005 and 2010, focusing on the breadth and quality of the evidence base, types of outcomes studied, acceptability, effectiveness and cost-effectiveness.
-
A review of systematic reviews of telehealth for depression published between 2005 and 2010.
-
Evidence synthesis of qualitative research published between 2000 and 2010 to develop an understanding of patient and organisational perspectives of telehealth.
-
Realist synthesis based on studies in 1–3 to draw out underlying mechanisms that might enable telehealth interventions.
-
Horizon scanning to ensure the inclusion of up-to-date evidence emerging during the 15 months of this mixed-methods review.
-
Because of resource constraints, we were unable to conduct a review of the evidence base for the effectiveness of telehealth interventions to reduce overall CVD risk concurrent with reviews 1 and 2. However, in 2013, an opportunity arose to allow us to undertake a review of RCTs and systematic reviews in this area. This evidence synthesis was published61 and used for interpretation of the trials in the later part of the programme rather than for developing the intervention.
Meta-review of systematic reviews
Introduction
The evidence base for home-based telehealth for LTCs is large, with numerous systematic reviews of a variety of telehealth interventions. Before commencing our evidence synthesis, there were already numerous systematic reviews of a variety of telehealth interventions and three relevant meta-reviews published. Meta-reviews synthesise the evidence from systematic reviews. The first meta-review focused on internet interventions only (including virtual communities, internet-based educational and behavioural programmes and internet-based CBT) and showed improved outcomes, particularly for patients with depression. 62 The second was a wider review encompassing all types of real-time telehealth and conditions, which included, but was not specific to, LTCs or home-based telehealth. 23 This concluded that the quality of the evidence base was poor, but indicated benefits of telehealth for the management of chronic diseases. These benefits included improving health outcomes (and mortality in heart failure), but there was no benefit for resource utilisation or processes of care. The third meta-review focused on telemedicine and included e-health, information communication technologies, internet-based interventions and telehealth for both diagnosis and management of a wide range of conditions, concluding that 21 of 80 reviews identified telemedicine as effective and 18 reviews identified telemedicine as promising. 63 Although these reviews offered important conclusions regarding the effectiveness of telehealth, they did not focus exclusively on home telehealth or the management of LTCs. Therefore, there was a need for a further meta-review of home-based telehealth for managing LTCs to inform the Healthlines study intervention.
Methods
Definitions
A key problem with this evidence base is inconsistent terminology; multiple definitions exist and are used interchangeably even within the same countries. The definition of telehealth used here included three types of intervention: (1) telephone-based interventions, including telecoaching, telephone counselling and telephone follow-up; (2) telemonitoring of patient symptoms and vital signs in which monitoring occurs at home and electronic data are sent to another site; and (3) computerised, internet- and web-based treatments with or without practitioner support. We included active (in which the patient interacts with the intervention or manually enters data) and passive (in which monitors transmit data remotely without patients manually entering data) interventions; real-time (synchronous) and asynchronous (i.e. e-mail) interventions; those with and without health-care professional (HCP) input; and those offering social support and feedback as well as monitoring of symptoms and vital signs only.
Our definition of LTCs was guided by the NHS National Service Framework for LTCs64 and other health-care guidance. 28,65,66 The LTCs included in the meta-review are listed in Box 1.
-
Chronic illness or chronic disease.
-
Asthma.
-
CHD or heart failure or coronary heart failure.
-
CVD.
-
Stroke and TIA.
-
Hypertension.
-
Diabetes mellitus.
-
COPD.
-
Epilepsy.
-
Thyroid disease (hypothyroidism or hyperthyroidism).
-
Cancer.
-
Dementia.
-
Depression (and anxiety).
-
Mental health including schizophrenia, psychosis, paranoia, obsessive–compulsive disorder, PTSD and agoraphobia.
-
Chronic kidney disease.
-
Atrial fibrillation.
-
Obesity.
-
Spinal cord injury.
-
Multiple sclerosis.
-
Motor neurone disease.
-
Parkinson’s disease.
-
Learning disabilities.
-
Arthritis.
-
Skin disease.
-
Hearing difficulty.
-
Headaches and migraine.
-
Visual problems.
-
Chronic liver disease.
-
Endocrine disorders (e.g. Addison’s disease, Cushing syndrome).
-
Bronchiectasis.
-
Cardiomyopathy.
-
Crohn’s disease/ulcerative colitis.
-
Glaucoma.
-
Haemophilia.
-
Hyperlipidaemia.
-
Systemic lupus erythematosus and other systemic autoimmune diseases.
-
Smoking (in relation to specific LTCs).
COPD, chronic obstructive pulmonary disease; PTSD, post-traumatic stress disorder; TIA, transient ischaemic attack.
Search strategy
We searched MEDLINE, EMBASE/Allied and Complementary Medicine Database (AMED), PsycINFO, Web of Science, Database of Abstracts of Reviews of Effects (DARE) and The Cochrane Library from 2005 to March 2010 for systematic reviews of telehealth and LTCs. Our search terms included ‘meta-review or meta review’, ‘quantitative review or overview’, ‘systematic review or systematic overview’, ‘methodologic* review or methodologic* overview’, ‘review’ ‘quantitative synthes*’, ‘clinical trial’, ‘randomised or randomised controlled trial’ and ‘controlled trial’ and ‘telemedicine’, ‘telehealth or tele-health’, ‘telenursing’, ‘telemonitoring’, ‘Ehealth or e-health’, ‘telehomecare’, ‘telehealthcare’, ‘home healthcare’ and ‘assisted homecare’.
Inclusion criteria
We included published systematic reviews (with or without meta-analysis) of telehealth for LTCs in English referring to home-based or mobile, synchronous (real-time) and asynchronous telehealth interventions. We focused on reviews of LTCs generally, not on reviews of specific LTCs, that is, we excluded reviews that focused exclusively on diabetes or depression, for example. We excluded reviews that focused exclusively on children, inpatient populations, service-to-service interventions, clinic-based interventions (i.e. not deliverable at home or on mobile technology, e.g. telecardiology) or smart home technology or that did not report outcomes. When reviews included some trials related to any of the above we included them, explicitly highlighting this and focusing on outcomes for patients relevant to our meta-review. We also excluded Cochrane reviews at protocol stage. Two reviewers independently reviewed abstracts to agree on papers for full-text retrieval. When there was doubt about a paper, the full-text paper was retrieved. Two independent reviewers reviewed full papers to ensure that they met the inclusion criteria.
Data extraction
Data were extracted from systematic reviews using a standardised form. All data were extracted by a member of the review team (AR) and checked by a second team member (AOC or CP). When there were any discrepancies, reviewers discussed this as a team to agree a resolution.
Quality appraisal
We assessed the quality of each systematic review using the five core quality questions from the Centre for Reviews and Dissemination for inclusion in DARE (see www.crd.york.ac.uk/CRDweb/html/helpdoc.htm). 67 Two reviewers examined and agreed on the quality of the reviews according to these five core quality questions. A review was included if it met at least the first three mandatory criteria and four out of the five criteria.
Results
The evidence base
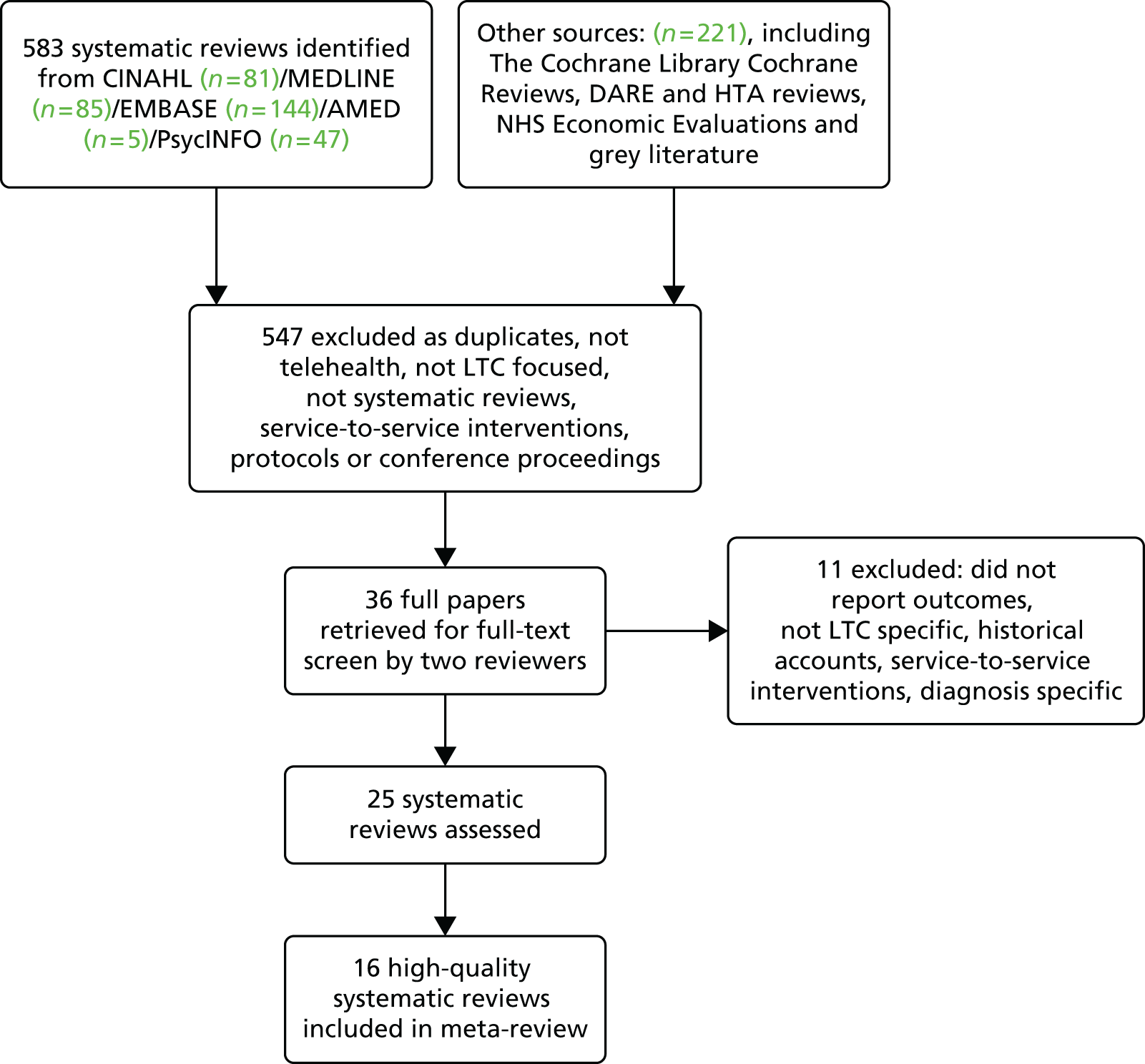
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram shows the stages that we went through to identify 36 reviews (Figure 1). Of these, 11 were excluded because they provided no outcomes, were not systematic or focused on acute care, a single condition or ‘smart home technology’. Twenty-one reviews satisfied the first three mandatory quality criteria and 16 of these met at least four criteria. Our meta-review includes these 16 high-quality systematic reviews,8,11,16,17,20,40,68–77 which cover 662 individual studies (Table 1). Six reviews included a meta-analysis. The reviews were undertaken by authors from Canada (n = 6), the USA (n = 5) and Europe (n = 5) and covered a range of telephone, mobile, internet-based and computer interventions. Details around data extraction are reported in Appendix 1.
FIGURE 1.
The PRISMA flow diagram for the meta-review of systematic reviews. CINAHL, Cumulative Index to Nursing and Allied Health Literature; HTA, Health Technology Assessment.
| Review | Inclusion/exclusion criteria reported? | Search adequate? | Included studies synthesised? | Validity of studies assessed? | Sufficient details about studies presented? |
|---|---|---|---|---|---|
| Barlow 200720 | Y | Y | Y | Y | U |
| Botsis 200870 | Y | Y | Y | Y | Y |
| Bowles 200717 | Y | Y | Y | N | Y |
| Cole-Lewis 201071 | Y | Y | Y | Y | Y |
| Cuijpers 200840 | Y | Y | Y | Y | Y |
| Dellifraine 200816 | Y | Y | Y | Y | Y |
| García-Lizana 20078 | Y | Y | Y | Y | Y |
| Hersh 200672 | Y | Y | Y | Y | U |
| Krishna 200973 | Y | Y | Y | N | Y |
| Murray 200511 | Y | Y | Y | Y | Y |
| Oake 200968 | Y | Y | Y | Y | Y |
| Paré 201069 | Y | Y | Y | N | Y |
| Polisena 200974 | Y | Y | Y | Y | Y |
| Rains 200975 | Y | Y | Y | Y | Y |
| Stinson 200976 | Y | Y | Y | Y | Y |
| Tran 200877 | Y | Y | Y | Y | Y |
Quality of the evidence base
We included only high-quality systematic reviews. Six of these 16 reviews urged caution regarding weak research designs of studies within them. 11,16,40,68,69,77
Effectiveness
Eleven of the 16 reviews concluded that telehealth was effective for some LTCs or improved some outcomes. 11,16,17,69–73,75–77 Meta-analyses tended to support telehealth, although effect sizes were often small or moderate. Reviews without meta-analyses produced more mixed conclusions, although none reported that telehealth was not effective at all.
Some specific conditions were highlighted by some systematic reviews. Positive effects of telehealth interventions were noted for diabetes in five out of 14 reviews17,70,72,73,77 and for asthma or chronic obstructive pulmonary disease (COPD) in four out of 12 reviews. 69,72,73,76 Three meta-analyses identified benefits for patients with heart failure or heart disease, including improved control of blood pressure in hypertension. 16,69,77 Two meta-analyses identified larger effect sizes for mental health than for other LTCs. 16,75
Specific outcomes
A range of positive outcomes, for example increased compliance or reduced burden of illness, was frequently reported. 16,17,69–71,73,77 Two reviews reported improved educational outcomes11,73 and seven reported significant positive behavioural change,11,17,20,68,71,73,75 particularly improved self-monitoring or management in patients with diabetes17 and better treatment adherence. 17,20,68 There were few firm conclusions about the impact of telehealth on quality of life, although one meta-analysis reported improvements associated with computer-mediated support groups. 75 The three reviews which suggested that telehealth improved social support11,73,75 were countered by three that reported inconsistent or insufficient evidence. 8,20,76
Resource utilisation
The evidence about the impact of telehealth on resource utilisation was mixed. Telehealth was shown to reduce admissions for heart failure, heart disease, diabetes and hypertension8,17,20,72 and reduce hospitalisations for elderly patients with LTCs. 70 However, other reviews showed limited impact on service utilisation11,76 and meta-analyses reported that the evidence for a positive impact of telehealth on resource utilisation was questionable. 11,68,77
Cost-effectiveness
Four reviews found some evidence for cost savings,20,70,74,77 but one was unable to determine cost-effectiveness. 11
Patient satisfaction
Three reviews commented on patient attitudes towards telehealth and indicated that patients find telehealth acceptable. 17,70,77
Types of technology
Telephone-based interventions worked well according to four out of the eight reviews that considered them alone or as part of more complex interventions. 8,20,70,77 The use of mobile phones and text messaging appeared to be effective,71,73 particularly for promoting behaviour change. Vital signs monitoring was reported as producing clinical benefits in approximately half of the trials in one review,20 but other reviews suggested that this might be limited to particular conditions, such as hypertension,69 diabetes,17,68,69,77 heart failure17,68 and respiratory conditions. 69 Vital signs monitoring was associated with increased mortality among COPD patients and appeared to offer no benefits for dementia,70 obesity or blood glucose control in diabetes. 72 Support for internet and computer technology was also mixed. Four out of the seven reviews addressing this showed some positive effects. 11,40,75,76 Text reminders appeared to work better than e-mail or internet reminders. 71 Videoconferencing was associated with improved outcomes across a range of LTCs in one review,16 but there was inconsistent evidence for its use in delivering support and education. 20
The role of health professionals
Few reviews compared different HCPs delivering the intervention or explored whether or not the presence of a professional was necessary. Professional care was not compared with lay or peer support. Telephone follow-up by nurses was shown to improve clinical outcomes and reduce service use. 20
Types of patients
Few reviews examined patient-specific characteristics. One review suggested that younger patients, male patients and possibly black ethnic groups benefited most from home telehealth,16 but another reported no differences in outcomes linked to age or sex. 71 Another identified that there was insufficient evidence with regard to disadvantaged groups benefiting, but some suggestion that computer interventions may benefit those living in rural communities. 11
Conclusions
The evidence base addressing the effectiveness of home-based telehealth for LTCs was extremely large and generally positive. However, a number of systematic reviews recommended caution when using this evidence base because of the poor quality of studies, citing small sample sizes, weak study design and lack of adequate comparators. There was also very limited evidence on cost-effectiveness. This was supported by a more recent evidence synthesis of the value of telemedicine in the management of five common LTCs: asthma, COPD, diabetes, heart failure and hypertension. 54 This concluded that most studies have reported positive effects but have measured outcomes in the short term only and that the evidence base is ‘on the whole weak and contradictory’ (p. 219). 54
The conclusion from this part of the evidence synthesis was that rigorous evaluation of telehealth interventions for LTCs, including their cost-effectiveness, is needed. The implications for the Healthlines study were that the evidence base was too diffuse to make a significant contribution to the development of the study intervention, although there was sufficient indication of positive effects from telehealth to make it worthwhile to develop a new intervention as proposed.
Review of depression
Introduction
After reading the meta-review, the Healthlines study team was interested in the evidence specific to telehealth interventions for the two exemplar LTCs in the study: depression and CVD risk factors. The review of CVD risk factors was not pursued at this stage because of resource constraints. Few trials or reviews had selected patients at risk of CVD and it was not feasible within the time available to conduct individual systematic reviews for the evidence in relation to each of the large number of factors that constitute raised CVD risk (hypertension, obesity, smoking, hyperlipidaemia, etc.). As we explain later (see Systematic review of telehealth interventions for primary prevention of cardiovascular disease), an opportunity did arise to carry out the CVD risk review at a later stage. However, we were able to review the evidence base for depression in the first phase of this programme, which is summarised here.
Methods
The focus of this review was on identifying systematic reviews of telehealth for depression and other mental health problems. We searched six databases for relevant systematic reviews that were published between January 2005 and March 2010. With regard to depression, we identified nine systematic reviews of telehealth and/or web-based interventions for depression,78–86 three of which provided a meta-analysis. 79,80,86 We also identified 11 reviews about a range of mental health problems or anxiety disorders15,40,87–95 and, of these, five provided a meta-analysis. 40,87–89,95 Most of these reviews included a small number of trials and small sample sizes, raising concerns about the quality of the evidence base.
Findings
When conducting this review we focused on five key questions that we sought to answer from the evidence base. We approached the evidence in this way because the main purpose of this work was to inform the development of the subsequent intervention to be tested later in the research programme. Each of the questions addressed by the review, as well as the available evidence derived from the depression review, is discussed in turn in the following sections.
Are mental health problems amenable to improved management using telehealth?
There was evidence of improved patient outcomes, including quality of life and medication adherence, for telehealth interventions for depression,78–80,85,94 anxiety-related disorders87,89 and post-traumatic stress disorder (PTSD). 88,95 Systematic reviews with meta-analyses for depression reported positive effects for telephone-based psychotherapy for depression,79 internet-based CBT80 and computerised psychological treatment. 86 Other reviews showed that telemedicine,78 internet-based interventions and support82 and computerised CBT81 improved symptoms. Positive effects were found in three of the four RCTs of computerised CBT for mild to moderate depression85 and improvements in mental health were linked to the MoodGYM and BluePages programmes in particular. 84 Three of the five systematic reviews with meta-analyses considering mental health problems or anxiety disorders reported moderate to large effects for mental health and/or anxiety. 87–89 For anxiety disorders, there were large effect sizes for computer-aided psychotherapy87 and remote communication technologies. 89 There were moderate effects overall for internet-based psychotherapeutic interventions for PTSD and panic disorder, but effects were small for depression and weight loss. 88
Which patient groups with mental health problems are most likely to use or benefit from telehealth?
Most RCTs of telehealth for mental health problems recruited adults, although some of the systematic reviews included trials involving youths, students or older school children. 78,84,86,88 There was some evidence that psychotherapeutic interventions delivered over the internet worked better for young adults (19–24 years) and adults (25–39 years) than for youths and older people. 88 Internet-based mental health programmes reduced anxiety in adults and students and performed similarly well in pilot studies with schoolchildren. 84 Patients with mild to moderate depression reported reduced symptoms after computerised CBT. 84,85,94 Telephone interventions for mental disorders were associated with significant symptom improvements for patients with mild depression. 15 One review reported that an internet-based programme (MoodGYM) may not be appropriate for people with low literacy levels. 84
Patients were generally satisfied with telehealth and internet or computerised treatments,83,91 especially computerised CBT, with some preference for therapist-led treatment. Therapists were generally less satisfied with telehealth than with usual care. 81
What kinds of technologies work best for mental health problems?
Telephone15,80,89,90 and internet79,82–84,86,88 interventions were associated with improvements in anxiety and produced large effect sizes with no difference in dropout rates compared with face-to-face care. 87 In contrast, computerised CBT with no or minimal therapist input was associated with high dropout rates. 81,85,87 Sub-analysis of technologies for computer-aided psychotherapies yielded non-significant differences between home computer and palmtop technologies. 87
How, where and by whom are telehealth interventions delivered?
In terms of how these interventions were delivered, internet-delivered interventions were largely found to be effective. 79,82–86,88,94 Telephone-administered psychotherapy interventions demonstrated significant benefits when delivered by mental health specialists. 80 There was some evidence that professional support for internet-based and computerised interventions resulted in larger effect sizes than unsupported interventions. 79,86
Individual therapy over the internet was more effective than group therapy, which suffered from greater attrition than individual therapy. 80 Interactive internet sites were significantly more effective than static (passive, information giving) sites in providing support for a range of mental health conditions, as were closed sites where participants were pre-screened. 88 Most systematic reviews reported on interventions in primary care settings. The duration of treatment was not related to symptoms or attrition rates80 and there was some evidence that effects could extend beyond the follow-up period. 78,87,88
What outcomes are associated with telehealth for mental health problems?
Most interventions were associated with moderate or large effects on depression and anxiety. 15,78–80,85–90,94 Other beneficial effects included the promotion of security and honesty and minimising potentially distracting behaviours91 and improved antidepressant medication adherence. 15 Most systematic reviews did not address cost-effectiveness, although there was some evidence of cost benefits of video conferencing in psychiatry91 and internet-based mental health programmes used by large numbers of patients. 84
Conclusions
The evidence base for telehealth for depression and other mental health problems was similar to the overall evidence base: although systematic reviews were available, these were based on a small number of trials with small sample sizes, creating an essentially weak evidence base. Nonetheless, the findings about effectiveness were generally positive. There was support for telephone-based and computerised- or internet-based interventions for depression. In fact, CBT interventions appeared to be very effective, with evidence that professional support could further improve the effectiveness of computerised CBT. There was little evidence of cost-effectiveness.
The implications of this part of the evidence synthesis for the Healthlines study were that further rigorous evaluation of interventions would be welcome, given the quality of the evidence base, but that there is support for a range of interventions, including computerised CBT. In addition, there was some indication that the provision of professional support for computerised interventions might be beneficial.
Synthesis of qualitative evidence
Introduction
In a third piece of work, we conducted a review of qualitative research into home-based telehealth for LTCs to develop an understanding of patient and organisational perspectives of telehealth.
Methods
Search strategy
We searched EMBASE, MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and PsycINFO for relevant articles between 2000 and 2010. In comparison to the other reviews described earlier, we included a longer time period here because we did not expect to find many papers. Our search terms included ‘meta-review or meta review’, ‘systematic review or systematic overview’, ‘qualitative exp’, ‘Review’, ‘meta-ethnograph*’, ‘meta-synthes*’, ‘observational method’, ‘focus group’, ‘narrative analysis’, ‘phenomenological research or phenomenology’ and ‘telemedicine’, ‘telehealth or tele-health’, ‘telenursing’, ‘telemonitoring’, ‘Internet’, ‘Ehealth or e-health’, ‘telehomecare’ and ‘telehealthcare’.
Inclusion criteria
We included any papers meeting our inclusion criteria for LTCs and telehealth as described earlier for the meta-review and also with a qualitative methodological focus, including qualitative interviews, focus groups and content analysis. We excluded opinion pieces, ‘data light’ studies, reports on service-to-service interventions, papers on generic internet use, papers looking exclusively at the content of an intervention and papers not meeting two of the 10 quality criteria (see Quality appraisal).
Data extraction
We identified 1876 references and retained 122 after application of inclusion and exclusion criteria to the abstracts. After removing duplicates, 69 papers were retained for full-text review. Two review team members then independently reviewed the 69 full-text papers in terms of the inclusion and exclusion criteria, as well as assessed the quality of these papers, as described in the following section.
Quality appraisal
The quality of qualitative papers was assessed by two reviewers (AR, CP) using the Critical Appraisal Skills Programme (CASP) quality criteria. 96 The 10 questions are shown in Box 2. We excluded papers that did not answer ‘yes’ to the first two CASP questions, which ask whether or not there were clearly stated research aims and whether or not appropriate qualitative methods were used. Very few studies satisfied all of the CASP quality criteria (see Appendix 2).
-
Was there a clear statement of the aims of the research?
-
Is a qualitative methodology appropriate?
-
Was the research design appropriate to address the aims of the research?
-
Was the recruitment strategy appropriate to the aims of the research?
-
Were the data collected in a way that addressed the research issue?
-
Has the relationship between researcher and participants been adequately considered?
-
Have ethical issues been taken into consideration?
-
Was the data analysis sufficiently rigorous?
-
Is there a clear statement of findings?
-
How valuable is the research?
The CASP quality criteria checklist was sourced from the Public Health Resource Unit, Institute of Health Science, Oxford. 96
Findings
The evidence base
Twenty-nine papers were included in the final review. 81,97–124 Details of the participants, methods and focus of these papers are provided in Appendix 3. There were two systematic reviews with qualitative and quantitative content81,97 and 27 primary studies of patient or stakeholder perspectives. Most papers originated in Europe (n = 18, including 11 in the UK), followed by the USA (n = 7), Canada (n = 3) and Australia (n = 1). The evidence base addressed a wide range of conditions, some of them relevant to the Healthlines study, particularly depression,98,99 hypertension100 and online counselling. 101
We identified three themes from these papers – perceived access, care and symptom management and technologies – and then focused on the question of which professionals or agencies should deliver services, as this was of particular interest to the Healthlines study in terms of designing the subsequent RCT. Evidence around each of these three themes is discussed in the following sections, followed by an examination of whether specific health professionals or agencies should deliver telehealth services.
Perceived access
Patients and professionals generally perceived that telehealth could lead to increased access and decreased emergency visits. 97,102–104 Rural patients with moderate depression perceived an improvement in access from using telehealth. 99 Patients reported advantages of online interventions for mental health and behavioural problems, including convenience, access and anonymity,98,99 but these were often balanced by concerns about lack of closeness and therapist trust, privacy and confidentiality fears and lack of visual cues. Patients shown a video of a Home Telecare Management System were positive, mentioning perceived benefits of less travel time and fewer medical visits, but there were some concerns about whether or not it could be used by some patients with disabilities. 105 Benefits of online support included the timing of sessions fitting into people’s routines. 98 Patients with severe symptoms from at least one LTC103 and patients with multiple chronic illnesses104 felt that they benefited from improved access. The perceived benefits of telehealth for older people included not having to travel99 and an ability of telehealth to reach an underserved elderly population. 106 Stakeholders believed that younger diabetic patients or those comfortable with technology would more likely benefit from virtual clinics. 107
It was suggested that adults with low literacy levels could benefit from e-health interventions, although concerns were also expressed around oversimplified interventions being perceived as too basic108 or that patients might be misunderstood and unable to express themselves adequately in relation to computerised CBT. 98 Online CBT was perceived as particularly beneficial for patients familiar with computers. 98 Conversely, patients with little or no information technology (IT) experience reported positively on technology for blood pressure control. 100 In a study of monitoring and messaging device alerts, clinicians reported that significant time, good knowledge and high engagement from patients was necessary, and patients with some health conditions (including tremors) could not use the technology. 109
Although there were benefits of telephone interventions overall, including mobile technology for asthma control and management,110 problems reaching transient populations with mental health problems were also cited. 111 However, internet programmes for people with chronic diseases reduced isolation and improved information sharing112 and patients with cancer involved in internet support groups appeared to benefit from empowerment and reduced isolation. 113
Care and symptom management
Professionals felt that telehealth aided diagnosis, could improve trust between patients and nurses and could lead to greater professional autonomy,103 but they were concerned about medico-legal implications. 104 Perceived benefits of a diabetes decision support system and telehealth included improved self-management, increased confidence and rapport with the diabetes team and increased patient openness. 106,114
A systematic review of internet-based CBT reported that there was an unintended consequence of an intervention in that it reinforced the health problem, rather than helped to address it. 97 Similarly concerning, a mobile technology for asthmatics was seen by professionals as engendering dependence on technology or the clinician. 110 Therapists, nurses and doctors were all less enthusiastic than patients about telehealth. 81,115
Improved self-management through monitoring in diabetes was noted,106,109,114,116 as was increased symptom awareness112,117 and better self-management for LTCs generally. 103 For example, middle-aged men with diabetes reported improved knowledge and management of symptoms linked to a diabetes decision support system, but the system was reported as not working for patients engaging in sport. 114 Reports also suggested that patients with poorly controlled diabetes benefited from frequent monitoring and medication adjustment109 and patients with good diabetic control benefited from telephone coaching,111 but a highly transient population had difficulties responding to telephone contact. 111 Patients of varied ethnic groups expressed improved peace of mind linked to home telecare. 105 Lastly, daily monitoring was perceived as promoting adherence. 118
Technologies
Some concerns were expressed about using technology, including technical difficulties and fear of technology. Although few technical problems related to online interventions were actually reported,98 there were some problems with imperfect technology. 106,109 For instance, nurses reported technology limitations of telehealth for COPD, including unpredictable equipment performance and poor picture quality, which impacted on the quality of care for these patients. 115 District nurses also experienced some technical problems with mobile technology (internet connections) for LTC care at home. 102
In terms of patient views, telehomecare technology for heart failure was perceived as positive, but spouses showed emotional responses when technology failed117 and the complexity of the technology was a concern for some heart failure patients. 118 The theme of ‘fear’ of technology, which was linked to older age, also emerged for some heart failure patients105 and training was advocated. Fears included computer anxiety and difficulties for older people in terms of understanding technology, expressed by participants in statements such as:
we have not grown up with computers . . . you only have to look at the level of resistance from older people using ATMs in the banks. A lot of old people when confronted with such a system, freeze up, as it is complicated for them . . . something they fear.
Rahimpour et al. (p. 492)105
However, patients with little or no IT experience reported positive attitudes towards technology for hypertension management. 100
Does it matter which professionals or agencies deliver telehealth services?
There was support in the literature for the view that health professional input was valued, particularly in the context of mental health problems. Psychologist delivery of internet CBT was viewed positively by patients98 and having a facilitator to improve the ‘personal’ experience was advocated. 99 Contact with a mental health clinician prior to telehealth was also seen as important. 99 Positive diabetes peer support was provided through virtual clinics116 and nurses were seen as good facilitators for providing online support by mothers of children with mental health problems. 119
Conclusions
Qualitative literature on patients’ views of telehealth suggests that it is generally accepted and appreciated because of perceptions that it increases access, particularly for remote and hard-to-reach patients or older patients, improves self-management if monitoring is involved and improves care or support, particularly in depression and mental health. However, the technology can be a barrier for some older patients, some patients with LTCs that involve physical disabilities and patients with low literacy levels. There were also some concerns about dependence on technology and health professional contact may be important for mental health interventions.
Professionals were less accepting of telehealth than patients, voicing concerns over loss of role, confidentiality, loss of face-to-face contact or the therapeutic relationship and loss of non-verbal communication. However, they also perceived some benefits of telehealth, such as increasing access and patient contact, improving communication and monitoring and facilitating self-management.
The implications for the Healthlines study were that there would be few problems with acceptability of interventions to patients with LTCs, there was potential to impact on self-management when monitoring was used, attention would need to be paid to directing computer-based interventions at people who were used to computers or technical support should be offered (particularly for older people) and health professional input might be of benefit for the intervention for depression.
Realist synthesis
Much of the text in this section is reproduced from Vassilev et al. 125 © Vassilev et al. ; licensee BioMed Central. 2015. This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Introduction
This part of the evidence synthesis used a realist synthesis to focus on the mechanisms that might be at play within telehealth interventions.
Methods
We followed Pawson’s seven stages for a realist synthesis: identify the question and clarify the purpose of the review; theory elicitation; search the evidence; appraisal; extract the results; synthesise findings; and draw conclusions and make recommendations. 126 This was an iterative rather than a linear process and in our study we had searched for much of our evidence prior to theory elicitation.
Identify the research question
Our research question was, ‘How does telehealth improve the health of people with LTCs?’ We focused on identifying the mechanisms by which telehealth interventions appear to change health behaviours or outcomes.
Searching the evidence
We used the literature reviews described earlier (see Meta-review of systematic reviews, Review of depression and Synthesis of qualitative evidence). We reread these papers to inductively identify features that appeared to contribute to successful interventions. We discussed and refined these features within our team and then returned to the evidence base to seek out confirmatory and disconfirmatory evidence about potential mechanisms that underpin successful telehealth interventions. When relevant, we read the individual primary studies that were included in the systematic reviews to further explore the relevant issues. In addition, we ran MEDLINE searches to identify other key papers that had been published subsequent to our earlier literature searches.
Theory elicitation
We focused on three possible explanations or theories that suggested how telehealth works to change health outcomes. These emerged from our team thinking about issues that might be important to the success of these types of interventions in the context of the evidence we had read about and synthesised in the three earlier literature reviews (see Meta-review of systematic reviews, Review of depression and Synthesis of qualitative evidence). We identified these explanations or theories with the intention of returning to the literature to help us draw conclusions about their importance:
-
Relationships – relationships or connections between people (patients, peer groups and/or lay and professional carers) are a necessary component of telehealth interventions.
-
Fit – the extent to which a telehealth intervention can be integrated within everyday life and health-care routines determines the success of deployment/adoption.
-
Visibility – systems that increase the visibility of symptoms or health problems to self or others impact positively or negatively on the adoption of telehealth interventions depending on whether or not patients want anonymity.
Appraisal
We had undertaken quality appraisal of all papers in our earlier reviews.
Data extraction
We developed the evaluative framework using our initial ‘theories’ to compare and examine the findings within the high-quality literature identified in the three literature reviews (see Meta-review of systematic reviews, Review of depression and Synthesis of qualitative evidence).
Synthesis of findings
We compared findings from different studies, looking for examples that challenged, refined or supported the theories identified.
Findings
Relationships
We examined the literature to see whether or not, why and how relationships were important for the success of a telehealth intervention. In particular, we focused on the relationship contexts, specific aspects of relationships (e.g. continuity, communication, rapport), differences between peer-to-peer and patient–professional relationships and whether telehealth technology augmented or substituted for face-to-face/personal contact.
Evidence about professional input
There was evidence to support the case that telehealth can work without professional input. For example, a RCT of an internet-based CBT programme, Beating the Blues, showed that computerised care outcomes were comparable to face-to-face care outcomes, nearly half of those completing the programme were reported to be clinically recovered and a computerised CBT programme for the management of mild to moderate depression was associated with clinically significant patient benefits. 127,128
Similarly, there was also evidence that interventions with minimal health professional input could deliver improved outcomes. First, internet CBT self-help for headache with online exercises was associated with significant reductions in headache-related symptoms and perceived stress and adding in therapist contact over the telephone did not influence the results. 129 Second, a meta-analysis of computer-tailored interventions for changing health behaviours (which relied completely on algorithms) had a clinically significant impact on behavioural risk factors. 130 Third, a trial of an internet behavioural weight loss programme, VTrim, showed no benefits of in-person therapy in addition to internet-only treatment, although the authors reported web-based therapist contact was important to maintain clinically significant weight loss over time. 131 Indeed, some telehealth interventions, such as telemonitoring, were designed to reduce professional input and contact. Automated and computer-based monitoring systems were sufficient to reduce blood pressure132 and also improved overall well-being and nutrition,133 despite minimal professional contact. Therefore, it is unlikely that professional support is essential to the success of telehealth.
However, professional contact appeared to be important in some cases. It enhanced outcomes for panic disorder134 and depression, with professional support alongside computerised programmes showing improved outcomes. 79,86 Although computer-based interventions alone were not able to change health behaviours (reported in all five studies in Saksena135), pharmacist assistance coupled with web-based education resulted in significant improvements in blood pressure control. 135 There was also some evidence of a sense of loss, expressed by both professionals and patients, associated with telehealth with reduced health professional contact93,98,99,136 and reductions in rapport,93 such that these interventions were ‘not preferable to seeing a doctor in person’ (p. 21). 136
In summary, professional relationships did not appear to be essential but may improve acceptability and may improve outcomes for mental health and computer-based interventions.
Evidence about peer support
There was little evidence to support the necessity of peer support for the success of an intervention. Sometimes the peer support was inherent within an intervention, that is, it was a peer support intervention. For example, computer-mediated support groups for depression and weight loss showed benefits. 75 For weight loss, logon rates were higher among those using an internet programme that included peer support than among those without this element of interaction. 131 Important aspects of this type of support appeared to be promotion of empowerment and a ‘sense of belonging, affiliation and social cohesion’ (p. 1875). 137 However, peer support was not always a positive experience: online support groups and peer-to-peer support included the risk of ‘trolling’, in which peers bully or disseminate negative comments or inaccurate information. 138
Fit
The reviews had demonstrated that ease of use could be important to users, which led to the possible mechanism that the extent to which a telehealth intervention can be integrated within everyday life and health-care routines determines the success of deployment or adoption. When we examined the literature to see how this mechanism might work, we found evidence of the importance of fit with a patient’s needs, skills and daily life.
Evidence of fit with patient needs
There was evidence that interventions that were perceived as fixing a problem from the patient’s point of view might fare better. Some telehealth interventions had the capacity to enhance accessibility of health care for those who might otherwise not access traditional face-to-face care or those who were geographically isolated,97,99,104,128 including underserved elderly106 and those who lived in remote areas or had mobility problems. 139 However, highly transient patients were found to be more likely to fail to respond to telephone communication. 111 The use of telehealth could also offer convenience in accessing care (e.g. not having to travel to appointments98), but this was not always perceived as important to patients. 136
Evidence of fit with patient skills
There was evidence that patients needed routine capability in technology to benefit from interventions. Simple technologies, including telephones, appeared effective,20,140,141 suggesting that technologies that are already used in everyday life may be easier to use to deliver telehealth. Internet programmes may require some level of basic training and web-based interventions were more accessible to patients who were familiar or comfortable with using the internet. 98,107 Less computer-literate users expressed concerns over being misunderstood105 and older people reported fear. 105
Evidence of fit with daily life
As well as being able to use the technology, patients with depression cited ease of fitting the intervention – in this case online CBT sessions – into daily life98 as important.
Visibility
We identified earlier that telemonitoring of symptoms and vital signs was perceived by patients to have positive impacts on outcomes. This led us to consider that this might have reinforcing and incentivising functions (e.g. reporting vital signs encouraged self-regulation; the belief that HCPs were monitoring information may encourage patients to follow instructions; telemonitoring of symptoms and vital signs enabled HCPs to respond to patients’ needs quickly). This ‘making visible’ to self or others seemed to have a powerful role to play yet was not always welcome because some patients valued the anonymity offered by some telehealth systems.
Telemonitoring improved a range of outcomes including reducing admissions and all-cause mortality for chronic heart failure,10 improving management of blood pressure106 and significantly improving glycated haemoglobin levels for patients with poor glycaemic control. 142 Web support and monitoring using a diabetes decision support system, which kept track of carbohydrate and blood glucose levels, also improved diabetes self-management in patients and increased awareness of carbohydrates and blood sugar regulation. Systems that encouraged accountability – the expectation that patients should check their blood glucose levels frequently, coupled with feedback from health-care staff facilitated through technology – also worked well. 114 Diabetes patients themselves reported that ‘when you have a date [upcoming videoconference] you are more likely to do something’ (p. 748)106 and transparency of recording blood glucose levels helped patients self-manage and enhanced the competence of nurses to make adjustments to insulin. 114
Displaying symptom records meant that ‘there was improved visualisation of blood sugar profiles; there was closer follow-up of diabetic patients; food became a matter of more pronounced interest to all members of the diabetes team’ (p. 73). 114 For hypertension, computer-based automated monitoring improved blood pressure and antihypertensive medication adherence; the study authors claimed that ‘it appears most likely that TLC [the intervention] exerted its effect on blood pressure by affecting patient medication-taking behaviour, and possibly by influencing physician counselling practice’ (p. 290). 132 For respiratory conditions, including asthma and COPD, visible telemonitoring of symptoms and vital signs was also associated with behaviour change143 and this type of visibility also seemed to enable success. Norman et al. 144 and Neve et al. 145 also showed that visible monitoring and self-monitoring of food and drink intake improved weight loss and a tailored telephone intervention for hypercholesterolaemia using goal-setting by nurses was associated with significant reductions in fat intake and cholesterol compared with usual care. 146 There was some evidence that involving hypertensive patients in monitoring promoted empowerment, resulting in the ‘activated patient’ and improved blood pressure control. 147 Visible self-monitoring of blood pressure was shown to improve blood pressure control,69,148–150 particularly when combined with remote monitoring by HCPs at a distance. 148,151
The visibility created by monitoring has been shown to enable reinforcement of information118,120,121 and of symptom and self-management106,109,114 and behaviour changes. 127,145,146,152 Visible reminders to record vital signs118 or to log on to web-based systems145 encouraged participation. Recording and monitoring of symptoms and behaviours also enabled feedback, for example telephone feedback for depression139 and hypertension,132 and it was suggested that both information relevance and depth of processing of the information could be heightened by updating feedback to reflect a person’s changes. 130
Support by peers or professionals also enhanced visibility and perceived personal accountability. For example, a RCT of a tailored telephone intervention to improve dietary cholesterol adherence showed being accountable to another person (at regular intervals) impacted positively on patient behaviour. 146 The telehealth programmes implemented by the Veterans Association in the USA, such as that described in Piette et al. ,142 used visibility to enable accountability-linked armed services hierarchy and authority relationships between health-care personnel and patients.
Conclusions
In terms of ‘relationships’, the evidence suggested two underpinning mechanisms by which relationships enable successful telehealth (or indeed any) interventions: relationships provide support (professional, peer, clinical and social) for behaviour change and relationships provide opportunities for professional feedback, which reinforces positive or required behaviour change. This suggests that interventions that enable connections and contact, notably between patients and professionals, can facilitate support and reinforcement necessary for behaviour change. When telehealth interventions limit or remove the relationship between patients and professionals, other opportunities to support and reinforce behaviour may be necessary. In terms of ‘fit’, the literature pointed to the importance of acceptability and ease of use of telehealth interventions for patients and professionals. Telehealth can increase accessibility of care for some populations. Simple technologies appeared to work as well or better than more complex ones and there are some patient groups who are less able to use some technologies (notably, the web). The third proposition centred on ‘visibility’. This had both positive and negative dimensions in that visible monitoring and surveillance appeared to be beneficial, but anonymity was valued by some patients. The evidence suggested that visibility operated in the following ways: by enabling feedback, which reinforces positive or required behaviour change; by providing incentives, reminders and behaviour prompts for action; and by inducing negative feelings (fear) regarding surveillance, stigma and punishment.
There were three implications for developing the Healthlines study intervention. First, relationships may be a necessary component of care interventions. For this reason, the research team needed to consider whether and how the telehealth intervention enables or limits the possibility for relationships with professionals and/or peers. If the intervention removes or replaces relationships, then other mechanisms for support and reinforcement may be necessary to effect behaviour change. Given the apparent importance of relationships, it seems likely that patients and professionals might resist or reject interventions that threaten or limit these. Second, successful telehealth interventions are well integrated into everyday life and health-care routines. Interventions that enhance or improve access to care (by enabling access or making it timelier) are more likely to be acceptable to patients. Ease of use is important for adoption of technologies. Our synthesis suggests that the intervention should be based on a comparatively simple technology (e.g. telephone), which is easy to access and use. The intervention should be designed so that it offers minimal disruption to patient lives and professional routines. Third, the design of the telehealth intervention should address the issue of visibility. How it does this may depend on the condition and patient group involved. Monitoring systems can offer opportunities for visible feedback and prompts to actions that serve as reinforcements of behaviour change. This may be especially important if relationships are not fostered by the intervention (i.e. monitoring may be used to mitigate the loss of a relationship with a HCP). This strategy is likely to work best for some physical conditions and diseases such as diabetes and heart failure. For some conditions, notably mental health, visibility may have negative connotations, as patients may wish to remain anonymous when using the system. The design of the telehealth intervention should consider if and how symptoms and signs are made visible by the system and how these are responded to by the technology, the patient and the HCP.
Horizon scanning
Introduction
In this fast-moving field, the horizon-scanning element considered new developments worldwide that might inform the Healthlines study intervention development.
Methods
A team member, Simon Brownsell, drew on his wide knowledge of developments in this field. This was complemented by systematic internet searches. Some documents were available in confidence and a detailed report of findings was circulated to the research team in June 2010. A summary is presented here because the horizon-scanning exercise was relevant only for intervention development. The summary draws on the World Health Organization survey of e-health activity, analysis of the Veterans Association home telehealth service model and the extensive knowledge of Dr Simon Brownsell, an expert in the field [Research Fellow in Health Services Research at the School of Health and Related Research (ScHARR) at University of Sheffield].
Results
An international perspective
The World Health Organization undertakes surveys of e-health activity, and reported the results of these in 2005 and 2009. Based on the 2009 survey it concluded that telehealth has yet to be consistently employed to deliver routine services in any health-care system in developed countries. 153 Barriers included resistance to change, lack of IT skills, a lack of evidence of economic benefit, the risk of medical liability for health professionals delivering services and the technical challenges of over-complex systems prone to malfunction. There are examples of large initiatives in other countries, with development of mHealth (use of mobile telephones to improve health). The US Veterans Association provides telehealth services to > 40,000 people, with evidence of a reduction in health-care resource utilisation for patients with a diagnosis of heart failure. 154 Some aspects of this initiative relate to findings from the four reviews that we conducted, which are described in the previous sections: patients’ acceptance of technology and motivation to use the service is part of the initial needs assessment; technology is carefully matched to the condition, needs and abilities of individual patients; and the new role of care co-ordinator was developed to continually monitor and manage the care of patients in the home telehealth programme across the spectrum of health services.
UK perspective
A number of planned telehealth initiatives were identified in local areas around the geographical boundaries of the Healthlines study and these were fed back to the team to ensure that we were up to date with changing local contexts. The most important ongoing initiative and evaluation in the UK at the time of developing the Healthlines study intervention was the WSD programme, which published its findings after we had developed the intervention. 155 This initiative took place in the context of a cluster RCT with > 3000 participants in nearly 200 general practices in three areas in England. The conditions included were complementary to those within the Healthlines study: diabetes, COPD and heart failure. Another important ongoing initiative was the Birmingham OwnHealth scheme,156 which was a telehealth programme that employed a care manager for patients with LTCs such as diabetes, CVD, COPD and heart failure. This initiative involved telephone sessions with the patients’ care manager, who used coaching and motivational interviewing to help patients to better self-manage their care in an informed manner.
Conclusions
The conclusions were that few mainstream telehealth services exist. Therefore, the Healthlines study intervention would be important because it was designed in partnership with a NHS organisation (NHS Direct) and would be delivered by NHS staff, with the aim of ensuring that it would be easier to implement within the NHS context. It was also the case that NHS Direct was a national service with the potential to provide telehealth routinely throughout England.
Systematic review of telehealth interventions for primary prevention of cardiovascular disease
The material in this section has been reprinted from Preventive Medicine, vol. 64, Merriel SW, Andrews V, Salisbury C, Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis, pp. 88–95, copyright 2015, with permission from Elsevier. 61
Introduction
During 2013, an opportunity arose to explore in more depth the evidence about the effectiveness of telehealth interventions in the primary prevention of CVD in adult patients in community settings, as the subject of a master’s thesis undertaken by Dr Sam Merriel under the supervision of Professor Chris Salisbury, Chief Investigator for The Healthlines Study. This was too late to influence the intervention development, but we have included it here to offer a more complete picture of the evidence base that we will draw on in our discussion chapter.
There are a number of systematic reviews assessing the effect of telehealth interventions on individual CVD risk factors, particularly in the areas of hypertension,9,143,150,157 tobacco use158–161 and obesity. 162–165 However, risk factors for CVD should not be considered in isolation, since it is the combination of factors that determine a person’s risk of CVD. 166 Similarly, since many people with high CVD risk have multiple risk factors, it seems appropriate to design interventions addressing all of their modifiable risks, since that is how patients are managed in primary care, rather than focusing on only one of their risk factors. Neubeck et al. 167 conducted a systematic review of the effectiveness of telehealth interventions in the secondary prevention of CHD and showed some evidence of lower all-cause mortality and reduction in multiple CVD risk factors. However, at the time of this search in 2013, there appeared to be no previous reviews of the evidence for telehealth interventions to reduce overall CVD risk in disease-free individuals (primary prevention).
Methods
Search strategy
Selected databases (MedLine via OVID, EMBASE Classic via OVID, Web of Science via Thomson Reuters, CINAHL Plus via EBSCOhost, PsycINFO via OVID, SCOPUS via SciVerse, BioMed Central, PLOS and The Cochrane Library via Wiley Online) were searched in June 2013. Key search terms were utilised for each database using Boolean operators, and combined with MeSH or subject terms specific to each database identified from initial search hits. RCT search filters created by the Scottish Intercollegiate Guidelines Network (SIGN) were utilised to refine searches in MedLine and EMBASE (www.sign.ac.uk/methodology/filters.html#random). Citation searching was also performed via Web of Science. Registers of incomplete systematic reviews and clinical trials (DARE, PROSPERO, ClinicalTrials.gov, and Current Controlled Trials) were searched utilising the key search terms. Reference lists of included papers were scanned by title for potentially relevant studies.
Inclusion/exclusion criteria
All search hits were assessed by title and abstract against the eligibility criteria. Trials utilising real-time or asynchronous telehealth interventions delivered to patients in at least one arm of the study to reduce overall CVD risk and/or addressing multiple (i.e. more than one) CVD risk factors were included. Studies were limited to those focused on adults (aged 18 years and above), with no history of CVD, based in the community. Non-randomised and observational studies were excluded in order to ensure that the best available evidence was used in the analysis. Studies of hospital inpatients, interventions only delivered in a clinic setting, decision support systems for clinicians, and interventions delivered between health services (not focused on patients) were excluded. Unpublished studies and grey literature were not included. Articles published in a language other than English were noted in the search but excluded from the review. No restrictions were placed on date of publication.
Change in overall CVD risk was the primary outcome of interest. A number of CVD risk scores have been created and validated. 166,168–171 Current guidelines for CVD prevention are not consistent in their recommendations of which CVD risk score to utilise in practice. 172–175 Therefore, a pre-specified risk score was not set for study inclusion. Similarly, specific CVD risk factor measures were not set as part of the eligibility criteria given the significant number of modifiable CVD risk factors that exist and the wide range of outcome measures used for each one.
Data extraction and quality assessment
One researcher (SM) and a second reviewer assessed search hits against the eligibility criteria independently. The Cochrane Risk of Bias tool was selected to assess the methodological quality of the included papers. The quality assessment was performed at the individual study level only. Data were extracted by the two reviewers using a standardised form that was developed based on two guides for systematic reviews. 176,177 The form was trialled with a sample of selected papers to allow iterative refinement. Data were extracted from the published articles in the following areas: study characteristics, participants, intervention, primary outcome and secondary outcome(s). Results of the independent data extraction were directly compared to ensure accuracy of data collection. When insufficient data were reported to perform the meta-analysis, study authors were contacted to request the relevant data. Figure 2 shows the PRISMA diagram.
FIGURE 2.
The PRISMA diagram for CVD risk review study selection. This figure is reprinted from Preventive Medicine, vol. 64, Merriel SW, Andrews V, Salisbury C, Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis, pp. 88–95, copyright 2015, with permission from Elsevier. 61
Statistical methods for meta-analysis
Data relating to changes in overall CVD risk and individual CVD risk factors in each study were converted into standard international units, allowing the standardised mean difference (SMD) between intervention and control groups to be determined. Analysis of individual CVD risk factors was limited to modifiable risk factors that are consistently used in overall CVD risk scores. Fixed- and random-effects models were used to generate an overall estimate of effect of the interventions on CVD risk and the individual risk factors. Study heterogeneity was assessed using the chi-squared test and I2 statistic, with an I2 > 70% being considered a high level of heterogeneity. Random-effects modelling was used in the case of high heterogeneity, and fixed-effects modelling was used when heterogeneity was low. Continuous variables were presented as the difference in means whilst dichotomous variables were summarised using odds ratios (ORs), both with 95% confidence intervals (CIs). The meta-analysis was conducted using Review Manager 5.2.5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark; 2012).
Results
The evidence base
The search for relevant studies in bibliographic databases, trial and review registries, and through citation searching returned 2268 hits. After removing duplicates, and applying the inclusion and exclusion criteria, 13 studies were included in this review. The 13 included studies are summarised in Appendix 4. They featured a diverse range of participants, with sample sizes ranging from 146 to 3382, for a total of 10,057 study subjects. Forty-one per cent of participants were male, with a mean age of 55.5 years. Seven studies recruited participants through general practitioner (GP) or specialist clinics; four trials recruited through the workplace; and the remaining studies gathered subjects through Health Maintenance Organisations, Veterans Affairs departments or the community. Follow-up periods for participants ranged from 3–96 months. However, only three trials followed participants for > 12 months. 178–180
Outcome measures
The trials used a broad selection of outcome measures (see Appendix 4), and the various studies utilised between 2–14 of these measures. Four studies reported a measure of overall CVD risk,48,180–182 with three of these studies using the Framingham 10-year CVD risk score48,181,182 and one using SCORE overall CVD mortality risk charts. 180
Quality
Quality varied markedly between studies and between different domains of the quality assessment. The majority of studies were assessed as being of moderate quality, with low risk of bias in three to five of the seven domains within the tool. However, incomplete reporting of outcome measures was a common problem and reporting on the blinding of participants and outcome assessors was particularly poor (see Appendix 5).
Impact on overall cardiovascular disease risk
Four of the included studies measured overall CVD risk. Bove et al. 181 compared a nurse management CVD risk reduction programme augmented with telemedicine communication with nurse management alone, in medically underserved urban and rural communities. The mean baseline Framingham CVD risk was relatively high in both study groups (17.50 vs. 17.80), and after 12 months follow-up the intervention (–2.50, 95% CI –3.38 to –1.61; p < 0.05) and control (–2.70, 95% CI –3.74 to –1.68; p < 0.05) groups had a similar reduction in overall risk.
The study by Nolan et al. 182 assessed the effects of a telehealth protocol using motivational interviewing on CVD risk factors for patients with existing diabetes or CHD, a Framingham 10-year absolute risk for CHD > 20%, or two or more modifiable CVD risk factors. Subgroup analysis of the participants without a history of CHD showed significant small reductions in Framingham 10-year CVD event risk for both the exposed (–1.12 95% CI –0.36 to –1.88) and active control (–1.77 95% CI –0.89 to –2.65) groups, with no statistically significant difference between the groups in adjusted analyses (0.65 95% CI –0.53, 1.83 p = 0.28).
Wister et al. 48 also utilised a telehealth counselling approach to deliver a ‘Heart health report card system’. This study was the only one that showed a significantly larger reduction in Framingham risk score in the intervention arm when compared with the control arm (difference = –1.97, 95% CI –2.85 to –1.09 p = 0.002). Claes et al. 180 utilised the European Society of Cardiology SCORE charts to assess 10-year risk of CVD mortality. This study evaluated the effect of a Medical + Lifestyle Programme, consisting of a CVD risk profile and personalised lifestyle follow-up via a website, e-mail and telephone, to a Medical Programme comprising just the CVD risk profile. Both groups had a small reduction in mean overall risk (–0.002 and –0.004 respectively) and the difference between groups was not statistically significant (p = 0.33, CIs not reported).
Combining the data from the three studies that used the Framingham 10-year CVD risk score in a random-effects model showed no evidence for a reduction in CVD risk (SMD –0.35, 95% CI –1.97 to 1.27) in the telehealth intervention groups compared with controls. Random-effects modelling was chosen given the high level of study heterogeneity (Figure 3).
FIGURE 3.
Random-effects modelling of differences in Framingham 10-year CVD risk between intervention and control groups. IV, inverse variance.
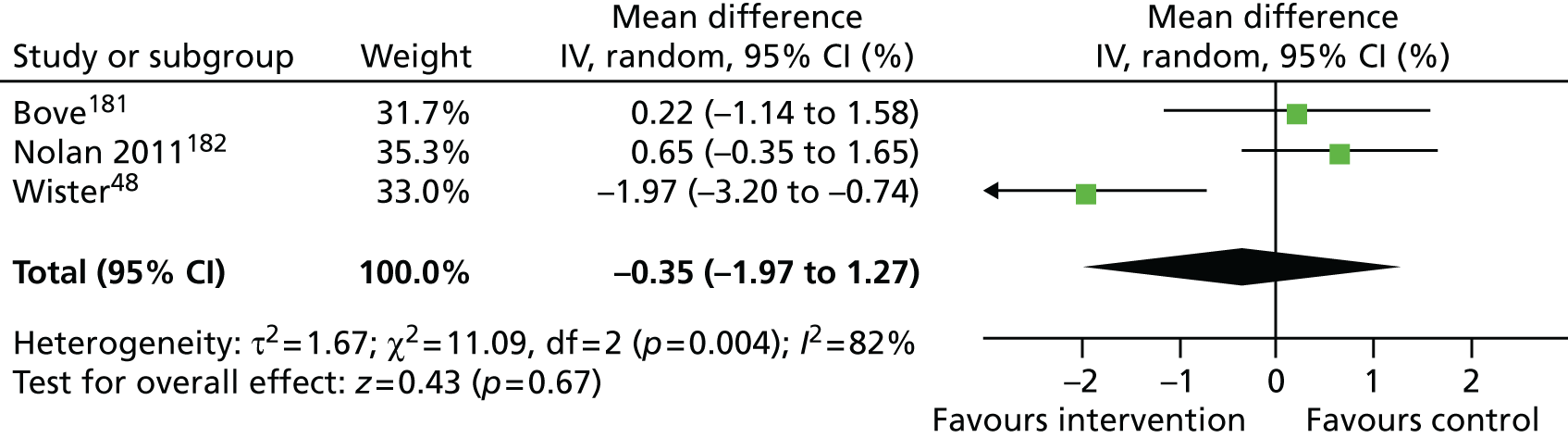
Impact on modifiable CVD risk factors
Eight studies measured systolic blood pressure at baseline (Figure 4). Four studies found a significantly larger reduction in systolic blood pressure in the intervention groups when compared to the control groups. 179,181–183 The trial by Dekkers et al. 179 featured two telehealth treatment arms, one delivered via telephone and the other via the internet. Summarising all included studies using a random-effects model suggests that multi-focal telehealth interventions have a small effect on reducing systolic blood pressure (SMD –1.22 mmHg, 95% CI –2.80 to 0.35 mmHg).
FIGURE 4.
Random-effects modelling of differences in systolic blood pressure between intervention and control groups. IV, inverse variance. This figure is reprinted from Preventive Medicine, vol. 64, Merriel SW, Andrews V, Salisbury C, Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis, pp. 88–95, copyright 2015, with permission from Elsevier. 61
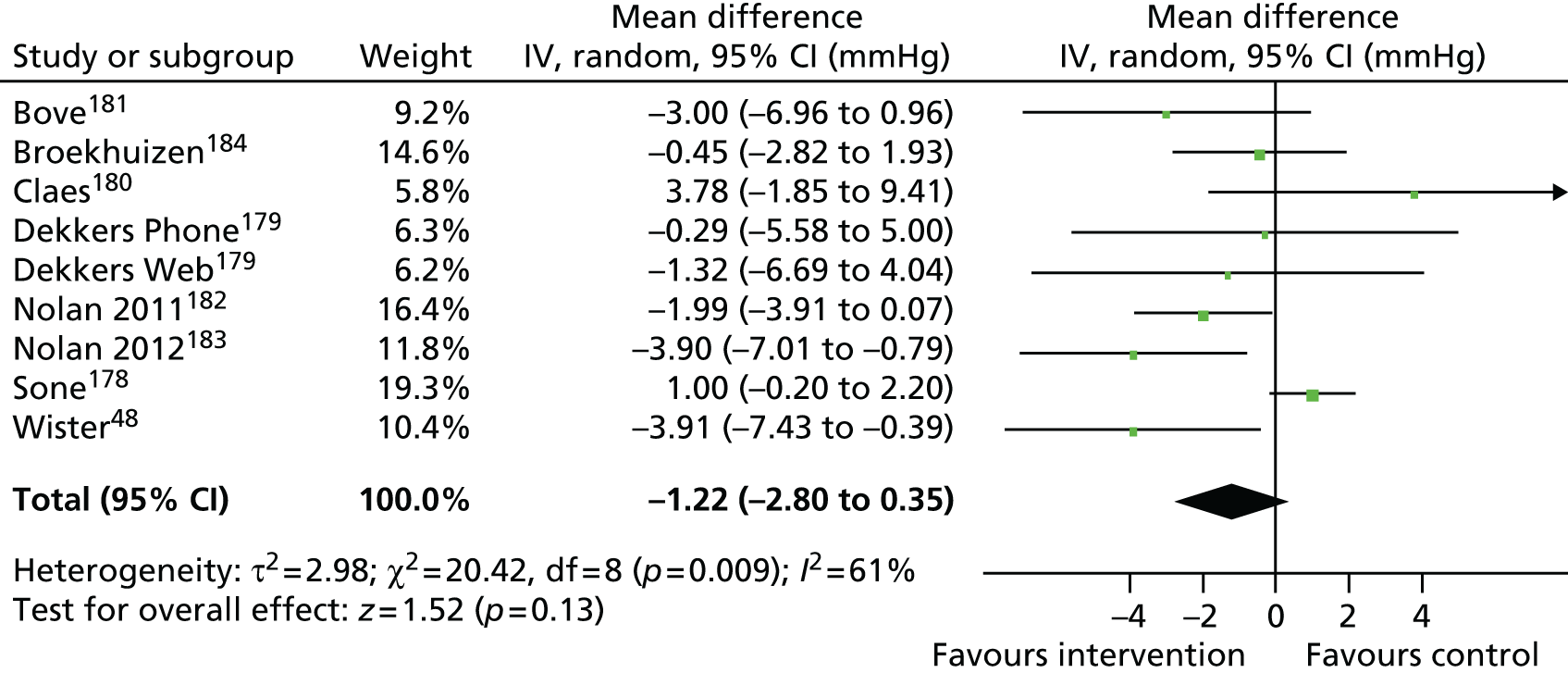
Six studies assessed total cholesterol. Three of the trials demonstrated a small but significant reduction in total cholesterol when the telehealth intervention groups were compared with control groups. 48,179,183 Overall, random-effects modelling showed that these telehealth interventions reduced total cholesterol by 0.07 mmol/l (95% CI –0.19 to 0.06). Figure 5 gives the full meta-analysis results.
FIGURE 5.
Random-effects modelling of differences in total cholesterol between intervention and control groups. IV, inverse variance. This figure is reprinted from Preventive Medicine, vol. 64, Merriel SW, Andrews V, Salisbury C, Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis, pp. 88–95, copyright 2015, with permission from Elsevier. 61

Four studies measured high-density lipoprotein (HDL) cholesterol. None of the individual studies demonstrated a larger change in the participants receiving telehealth interventions compared with their controls. Consequently, random-effects modelling (see Figure 6) calculated that these interventions did not affect HDL cholesterol levels (SMD –0.01 mmol/l, 95% CI –0.03 to 0.02).
FIGURE 6.
Random-effects modelling of differences in HDL cholesterol between intervention and control groups. IV, inverse variance. This figure is reprinted from Preventive Medicine, vol. 64, Merriel SW, Andrews V, Salisbury C, Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis, pp. 88–95, copyright 2015, with permission from Elsevier. 61
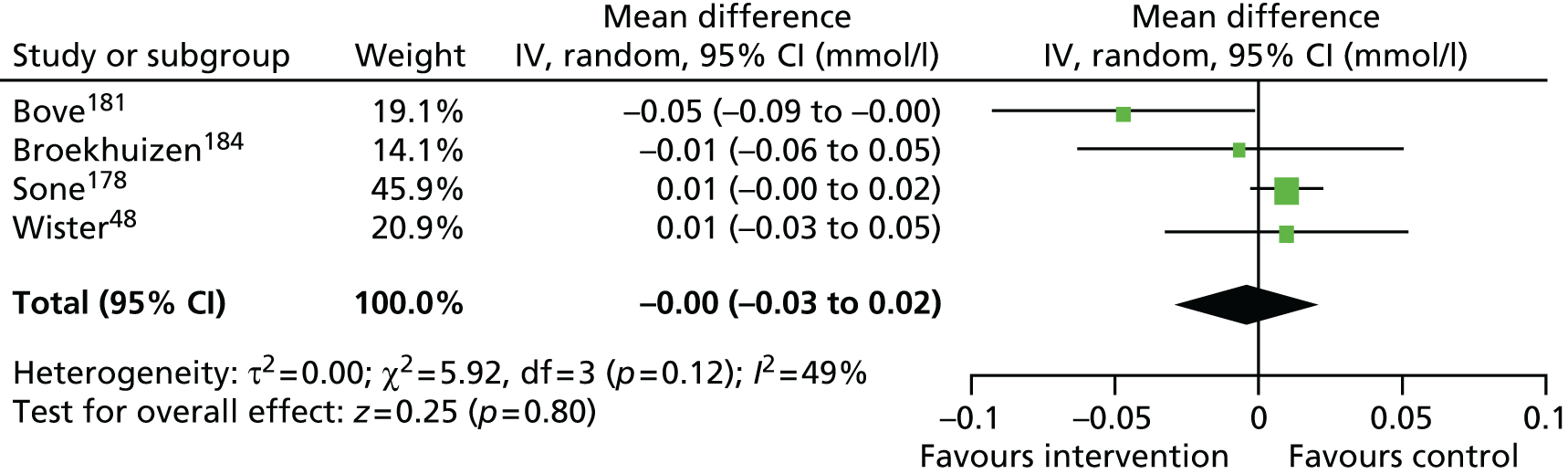
Four trials measured participants’ smoking status at baseline. There was no significant reduction in the number of current smokers in any of the relevant included trials. Study heterogeneity was very low for smoking, so a fixed-effects model was employed (Figure 7). The OR for smoking after receiving a telehealth intervention compared with no intervention was 1.09 (95% CI 0.82 to 1.44).
FIGURE 7.
Fixed-effects modelling of odds of smoking in intervention and control groups. IV, inverse variance. This figure is reprinted from Preventive Medicine, vol. 64, Merriel SW, Andrews V, Salisbury C, Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis, pp. 88–95, copyright 2015, with permission from Elsevier. 61
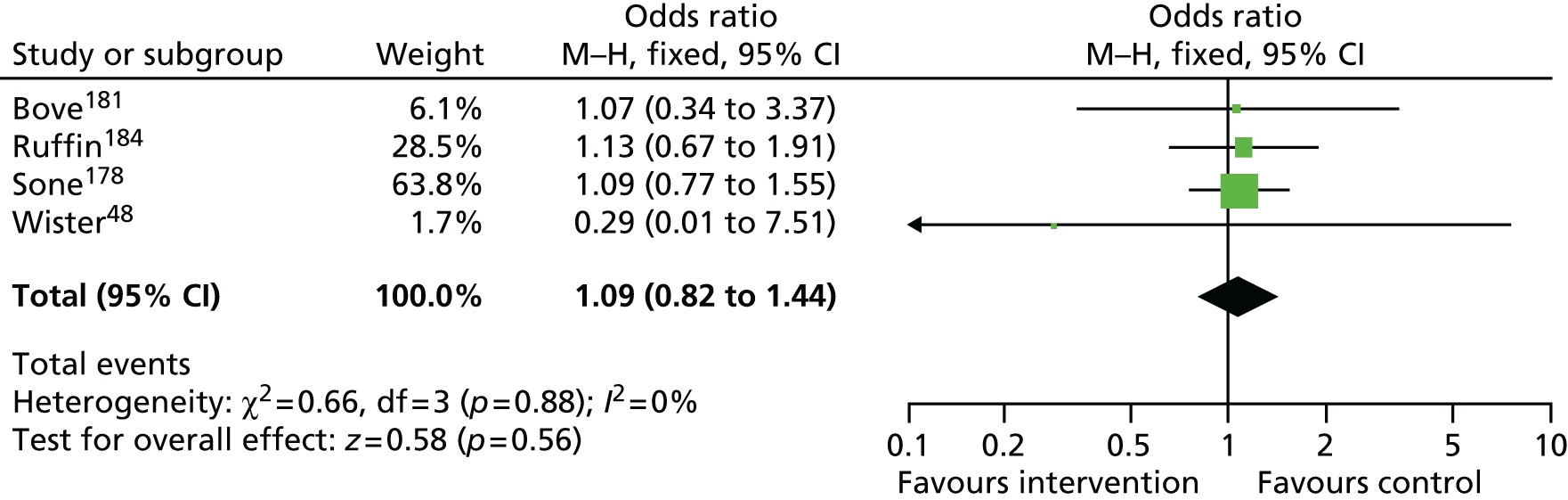
Meta-analyses and a summary of other modifiable CVD risk factors measured in the included trials was not performed due to lack of consistent inclusion in overall CVD risk score calculations, or the inability to convert reported outcomes into single measures for comparison and analysis.
Conclusions
We identified 13 trials that measured the impact of telehealth interventions on overall CVD risk and/or multiple CVD risk factors. Meta-analyses showed that these types of interventions have no effect on reducing overall CVD risk or cigarette smoking, or increasing HDL cholesterol. There was weak evidence of a small reduction in systolic blood pressure and total cholesterol. There are a number of possible reasons why this review failed to find any major effect for primary prevention telehealth interventions, given that some reviews focused on individual CVD risk factors (e.g. blood pressure) have shown more positive findings. It may be because of the quality of the included trials or the use of more appropriate methods when focusing on individual risk factors, or it could be that the effect of an intervention is diluted when addressing multiple CVD risk factors. The vast majority of studies included in this review had follow-up periods of 12 months or less, possibly affecting their ability to detect a true difference. Finally, it could be that small impacts on one risk factor intervention (e.g. to reduce blood pressure) do not translate to meaningful improvements in overall risk if other risk factors are not also improved.
This review had some limitations. The exclusion of non-randomised trials, unpublished studies, and articles not published in English could have introduced publication bias. There was no set minimum standard for study quality in assessing eligibility, resulting in the inclusion of some potentially low-quality studies. Some of these were inadequately analysed or reported, for example, not reporting 95% CIs or making between-group comparisons.
The implications for The Healthlines Study are that it is possible that the trial of an intervention may find evidence of an effect in reducing specific individual risk factors for CVD, but not overall CVD risk. The plan to assess cost-effectiveness will fill a current gap in the evidence.
Overall conclusions and implications for the Healthlines study
There was regular feedback to the Healthlines research team to ensure that the evidence described here was used to develop the intervention. The knowledge that there is a large amount of evidence on effectiveness but that the evidence base is of poor quality supported the need for a large-scale high-quality RCT and economic evaluation, making the Healthlines study a necessary addition to the evidence base.
Chapter 3 A cross-sectional survey of interest in telehealth: perspectives of patients with long-term conditions
The material in this chapter was adapted from Edwards L, Thomas C, Gregory A, Yardley L, O’Cathain A, Montgomery AA, et al. Are people with chronic diseases interested in using telehealth? A cross-sectional postal survey. J Med Internet Res 2014;16:e123. 185 Copyright © Louisa Edwards, Clare Thomas, Alison Gregory, Lucy Yardley, Alicia O’Cathain, Alan A Montgomery, Chris Salisbury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on www.jmir.org/, as well as this copyright and license information must be included.
Abstract
Background: There is growing interest in telehealth to support patients with LTCs, but little is known about what precipitates interest in using telehealth among these patients.
Objective: To explore the key factors that influence interest in using telehealth in two exemplar LTCs: depression and raised CVD risk.
Methods: Thirty-four general practices were recruited from two regions in England. Practice records were searched for patients with (1) depression (aged ≥ 18 years) or (2) 10-year risk of CVD of ≥ 20% and one or more modifiable risk factors (aged 40–74 years). Randomly selected patients were sent a postal questionnaire assessing sociodemographic characteristics, health needs, difficulties accessing health care, technology-related factors (availability, technology confidence, benefits and drawbacks of telehealth) and prior telehealth satisfaction. Multivariable regressions tested relationships between the key constructs and interest in telehealth via telephone, e-mail/internet and social media.
Results: Of the 3329 patients sent a questionnaire, 44.4% completed it (depression: 606/1589, 38.1%; CVD risk: 872/1740, 50.1%). There was moderate interest in telephone-based (854/1423, 60.0%) and e-mail-/internet-based (816/1425, 57.3%) telehealth, but not social media-based telehealth (243/1430, 17.0%). Sociodemographic characteristics largely had no association with interest in telehealth. The most important constructs related to interest in telehealth were confidence using the associated technology and perceiving greater advantages and fewer disadvantages from using telehealth.
Conclusions: Patients with LTCs are interested in using telephone- and e-mail-/internet-based telehealth, regardless of health status, access difficulties and sociodemographic characteristics. Interest could be increased by building technology confidence, highlighting benefits and addressing concerns about telehealth. At present, interest in social media telehealth is minimal.
Introduction
There is considerable international interest in telehealth as a possible alternative to face-to-face care for people with LTCs. 54,186 To realise the benefits of telehealth, patients must engage with and make use of it. 187 Some previous studies have suggested limited engagement with telehealth interventions in patients with LTCs81 and a refusal rate of up to 75% in those invited to join telehealth trials. 188 If telehealth is to make an important contribution to the health-care system for managing chronic diseases, it is imperative to identify, and then appropriately target, the factors that influence interest in telehealth, because people must be interested if they are going to make use of it. A systematic review of 52 studies on patient acceptance of computer-based health IT concluded that the majority of literature to date has focused on patient factors, such as sociodemographic variables. 189 For example, some previous research has suggested that interest in telehealth is highest in younger, educated and affluent patients,190,191 but these characteristics are inversely associated with the prevalence of LTCs. 5 A recent review commissioned by the NHS in England191 identified five categories of barriers to and facilitators of telehealth services: user characteristics, technological aspects, characteristics of services, social aspects of use and telehealth services in use. However, both this and the aforementioned review189 were not limited to patients with LTCs, nor did they aim to quantitatively assess the relative importance of factors influencing interest in telehealth. Nonetheless, in line with some of the findings from these reviews, we reasoned that both structural and evaluative technology-related factors would be key influences of interest in telehealth, namely, whether or not these patients have the technology readily available to use, their confidence in using technology and their attitude towards telehealth. Moreover, if those with the greatest health needs and greatest difficulties in accessing health care are indeed interested in using telehealth, a large gap in unmet need could be filled.
Equity of care is an important consideration for health-care systems. Telehealth has the potential to improve care for patients who have difficulty accessing traditional services, such as those who are housebound or those who live in rural areas. 192 These patients are also likely to be those who have the greatest health needs. 193 Additionally, as telehealth can enable patients to monitor their own vital signs at home (e.g. blood pressure), it may be more convenient and comfortable, enhance independence and empower patients. 9
We carried out a study to investigate the factors that are associated with interest in telehealth among patients with LTCs. This study was focused on two exemplar LTCs. The first was depression and the second was risk of a cardiovascular event (heart attack or stroke) of ≥ 20% over the next 10 years. This approach recognises that hypertension, obesity and hyperlipidaemia are risk factors for CVD, rather than conditions, and it is more appropriate to consider raised CVD risk as a LTC. 31 These two conditions were chosen to represent different types of LTCs, both of which are common, in which there is considerable unmet need and for which there is some evidence that particular forms of telehealth may be of clinical benefit. 90,147,167,194
This survey study was conducted as part of a larger research programme exploring the potential role of an existing health service in England, NHS Direct, in providing support for LTCs via telephone and the internet. For this reason, we did not name specific or existing telehealth services, but asked a large number of respondents about their interest in using several types of technology that could be used for telehealth. The aim of the current study was to determine whether or not interest in telehealth among patients with LTCs is related to the severity of patients’ health needs, difficulties in accessing health care or technology-related factors, including the availability of and attitudes to technology, while also considering the role of sociodemographic factors and taking account of previous experience of using NHS Direct.
Methods
Design
This was a cross-sectional postal survey.
Sampling and recruitment
General practices in two geographical areas of the UK, the south-west and the north-east, were invited to take part in the study. General practices were purposefully selected to represent a wide mix in terms of the socioeconomic characteristics of their patients. Between August 2010 and May 2011, a query was run on practice records to identify patients with either depression (aged ≥ 18 years, had consulted their doctor about a mental health issue and were prescribed an antidepressant medication within the last year) or raised risk of CVD (aged 40–74 years, QRISK2195 or Framingham196 10-year risk ≥ 20% and at least one modifiable risk factor, including hypertension, obesity or smoking). We calculated the QRISK2 score to assess CVD risk when possible but, as this score was not available through all general practice computer systems, we used Framingham risk scores in some practices. Patients were excluded if they were terminally ill, had cognitive impairment or had a severe mental health condition such as psychosis.
Fifty-four patients per practice from each of the two groups of eligible patients were selected using stratified random sampling. However, three practices had < 54 eligible patients with depression and so all eligible patients were selected from these practices. We sampled female and male patients in proportion to the number of eligible patients in each general practice. The CVD risk group was further stratified by age, such that equal proportions of young (aged 40–59 years) and older (60–74 years) participants were selected. This was because a CVD risk of ≥ 20% is more prevalent among older individuals, whereas access to technology is inversely associated with age. 190
Prior to invitation, GPs reviewed the patient lists and excluded any patients for whom it would be inappropriate to send a questionnaire (e.g. because of recent bereavement). The remaining patients were then mailed a letter by their general practice inviting them to take part in a study looking at new ways that the NHS could help people to improve their health, as well as a participant information leaflet and a questionnaire. The questionnaire could be completed and returned in the supplied prepaid envelope or there was the option to complete the questionnaire online or by telephone. A multi-language insert was included with instructions in 10 different languages on how to complete the questionnaire via telephone using a translator. Patients were asked to return a blank questionnaire if they did not want to take part. Those who did not respond were sent up to two postal reminders at approximately 2-weekly intervals. In an attempt to boost the rate of response, a ‘thank-you’ note with a teabag affixed to it was included in the first invitation questionnaire packages for the last 19 practices recruiting patients, representing half of the participating practices. All correspondence was sent by staff to the patients’ general practice and the researchers did not have access to patient-identifiable data at any point. Ethical approval was granted by the Southmead Research Ethics Committee (REC).
Sample size
Assuming an approximate 60% response rate, inviting 54 patients from each of 32 practices would provide around 960 respondents for each LTC group. This would provide 80% power to detect an absolute difference of ≤ 9.2% points in interest in using telehealth (binary outcome, equivalent OR ≤ 1.45), with a two-sided 5% alpha.
Measures
The questionnaire included questions about the key constructs that we hypothesised would predict interest in telehealth, namely sociodemographic variables, health needs, difficulties accessing health care, availability of and attitudes to technology and prior use of telehealth. To ensure the coherence of the questions included to assess these constructs and to reduce the questionnaire items to a smaller number of factors for data analysis, principal components analyses (PCAs) with orthogonal (varimax) rotation were carried out using Stata 11.2 (StataCorp LP, College Station, TX, USA) on constructed items. Decisions regarding the number of factors to extract were based on Kaiser’s criterion (eigenvalues > 1.0) by examining the scree plot and the subjective coherence of the factors. For each factor, items with an association of ≥ 0.3 were retained. 197 Next, the reliability of each factor was examined with Cronbach’s alpha, with coefficients > 0.70 indicating adequate reliability. Finally, mean summary scores for each reliable factor were calculated for individuals providing ratings for ≥ 50% of the relevant items. We treated each factor as a scale and labelled it according to the questions that it included.
Outcome variable
Interest in telehealth was assessed using questions about the participants’ interest in using a range of technologies. The item reduction techniques described above resulted in three summary scores for interest in telehealth, which related to interest in three types of technology: telephone (α = 0.82; landline or mobile telephone), e-mail/internet (α = 0.94; using e-mail or carrying out searches on the internet) and social media (α = 0.85; using chat rooms and social networking sites). These ‘interest’ summary scores were equal to the averaged sum of responses to three question items each (range 1–3), such that each corresponding summary score ranged from 1.0 (‘not at all interested’/’I don’t know what this is’) to 3.0 (‘very interested’), with scores of 2.0 equivalent to ‘fairly interested’.
Explanatory variables
Questions about sociodemographic characteristics of the respondents included those on sex, ethnicity,198 age group, employment status,199 educational qualifications200 and home ownership. 201 These questions were based on those used in previous validated surveys when possible.
Health needs were assessed using the Short Form questionnaire-12 items version 2 (SF-12v2). 202 Physical component summary (PCS) and mental component summary (MCS) scores were derived from the 12 items using proprietary scoring software (QualityMetric, Incorporated, Lincoln, RI, USA). These indexes of physical and mental health functioning are standardised with a mean of 50 and standard deviation (SD) of 10, such that lower scores indicate poorer health or greater needs.
The remainder of the questionnaire contained items constructed for the purposes of this research, although guided and informed by relevant literature and piloted with service users in advance. These are described below, with the specific questions that constituted each scale provided in Appendix 6.
Difficulty accessing health care was assessed using a series of questions that were based on themes identified through previous research. 203,204 Two ‘access difficulty’ summary scores resulted. Service delivery difficulties (seven items, α = 0.87) included questions about the convenience of accessing health care, as well as the nature or quality of the care itself (e.g. getting the right amount of care), whereas physical access difficulties (four items, α = 0.78) included questions about difficulties getting to appointments because of physical, psychological and transport problems, including costs. These summary scores ranged between 1.0 (‘no difficulty’) and 3.0 (‘lots of difficulty’).
Technology-related factors were assessed using questions on the availability of technologies and attitudes towards telehealth. Technology availability was assessed by asking respondents which of a range of technologies was easily available for them to use. Telephone availability (two items: landline, mobile) and e-mail/internet availability (two items: have e-mail address, have internet access) scores were formed by summing tallies (0 = absent, 1 = present) for these technologies.
Questions about attitudes towards telehealth were based on the theory of planned behaviour. 205 This theory suggests that perceived behavioural control – a concept capturing the extent to which one believes one is able to perform a behaviour – directly influences one’s intention to carry out a behaviour and may predict behaviour itself. Beliefs about one’s capability, which should be reflected in confidence levels, affect perceived behavioural control. Therefore, questions about confidence using different types of technology were devised. After item reduction, there were three clusters representing confidence in using telephone-based technologies (three items, α = 0.74), e-mail-/internet-based technologies (three items, α = 0.96) and social media-based technologies (three items, α = 0.88). Again, larger scores indicated greater technology confidence [range from 1.0 (‘not at all confident’/‘I have never tried this’/‘I don’t know what this is’) to 3.0 (‘extremely confident’)].
The theory of planned behaviour also states that positive or negative attitudes towards a behaviour predict one’s intention to perform that behaviour and are influenced by beliefs about the advantages and disadvantages of that behaviour. 205 Hence, items about the potential advantages and disadvantages of telehealth were generated based on previous qualitative research. 206 Summary scores for telehealth advantages (seven items, α = 0.87) and disadvantages (seven items, α = 0.90) were similarly formed [range from 1.0 (‘strongly disagree’) to 5.0 (‘strongly agree’)], with higher scores reflecting greater perceived advantages and disadvantages.
Finally, satisfaction with previous use of telehealth that was delivered by NHS Direct was evaluated by a single item. Respondents rated how satisfied they were with previous use of NHS Direct services on a scale from 1 (‘not at all’) to 5 (‘extremely’). At the time of the patient survey, NHS Direct provided health information and advice by telephone and through its interactive website throughout England. The rationale was that NHS Direct and other similar services could act as a provider of a wider range of telehealth services.
Patient and public involvement
To ensure that the questionnaire was as user-friendly as possible, we sought feedback from two service user groups. In both cases we asked volunteers to provide us with comments on the layout of the questionnaire and the wording used throughout, as well as whether or not the questions were clear and made sense. Service users could complete the questionnaire online or using a paper copy and could provide feedback by e-mail or telephone or as written comments. First, we contacted volunteers through the Mental Health Research Network, part of the National Institute for Health Research (NIHR), using a postal invitation letter. The other service user group was contacted via a general e-mail sent out from the service evaluation manager at NHS Direct. We received comments from a few members of both service user groups, which were collated and discussed among the research team. The questionnaire was then modified in accordance with this feedback and so the service user groups importantly contributed to the final version of the questionnaire used in this study.
Statistical analysis
We sought to examine two main questions in our analyses. First, we wanted to establish whether or not greater health needs were associated with greater difficulty accessing traditional health care. Then, the primary analysis investigated the extent to which interest in the use of telehealth was related to five key constructs: sociodemographic factors, health needs (including physical and mental health), access difficulties (including service delivery and physical access), technology-related factors (availability of technology and attitudes towards telehealth) and satisfaction with previous use of telehealth. We first used appropriate descriptive statistics (mean and SD or n and %) to summarise the sociodemographic characteristics of respondents and their needs, access difficulties, technology factors and interest in using telehealth. This included an exploration of how needs, health-care access and technology factors varied by age and LTC group. We then used multivariable regression models to examine (1) the relationship between health needs and access difficulties and (2) the associations between these variables and interest in telehealth, adjusting for the other variables in the model and taking into account the stratified survey design.
Results
Response rate
Thirty-four general practices took part in the survey. GPs excluded 11.2% (201/1790) of patients with depression and 5.2% (96/1836) of the CVD risk group prior to mailing questionnaires. Of the 3329 patients sent a study questionnaire, 1478 (44.4%) returned it. The majority of questionnaires, regardless of study location or patient group, were returned by post (89%, 1310/1478), with 11% (164/1478) completed online and four questionnaires completed by telephone (< 1.00%, 4/1478, all English language). None of the patients made use of the translator service. The response rate was higher for patients with a high risk of CVD (50.1%, 872/1740) than for patients with depression (38.1%, 606/1589). Separate logistic regression analyses for the two patient groups revealed that response rates for both depression and CVD risk were higher in older people, whereas the likelihood of responding did not differ by respondent sex or location (Table 2). The inclusion of the thank-you note and accompanying teabag had no significant effect on rate of response for patients with depression (OR 1.14, 95% CI 0.87 to 1.48) or CVD risk patients (OR 1.31, 95% CI 0.97 to 1.77) and so this variable was excluded from further analyses.
| Variable | Patient group | |||||
|---|---|---|---|---|---|---|
| Depression (n = 1497) | CVD risk (n = 1635) | |||||
| OR (95% CI) | Responded (n = 583), n (%) | No response (n = 914), n (%) | OR (95% CI) | Responded (n = 828), n (%) | No response (n = 807), n (%) | |
| Age (years) | ||||||
| 18–29 | Referent | 64 (11.0) | 218 (23.9) | – | – | – |
| 30–44 | 2.0 (1.4 to 2.8) | 166 (28.5) | 311 (34.0) | Referentb | 18 (2.2) | 39 (4.8) |
| 45–59 | 3.5 (2.5 to 5.0) | 197 (33.8) | 232 (25.4) | 1.6 (0.6 to 3.9) | 290 (35.0) | 391 (48.5) |
| 60–74 | 4.4 (3.0 to 6.4) | 112 (19.2) | 90 (9.8) | 3.0 (1.2 to 7.2) | 514 (62.1) | 377 (46.7) |
| 75+ | 2.8 (1.6 to 5.0) | 44 (7.5) | 63 (6.9) | – | 6 (0.7) | 0 (0) |
| Sexc | 1.4 (0.9 to 1.9) | 1.3 (0.9 to 1.7) | ||||
| Male | 148 (25.4) | 295 (32.3) | 620 (74.9) | 621 (77.0) | ||
| Female | 435 (74.6) | 619 (67.7) | 208 (25.1) | 186 (23.0) | ||
| Locationd | 1.0 (0.8 to 1.4) | 0.8 (0.6 to 1.0) | ||||
| Bristol | 282 (48.4) | 472 (51.6) | 438 (52.9) | 386 (47.8) | ||
| Sheffield | 301 (51.6) | 442 (48.4) | 390 (47.1) | 421 (52.2) | ||
Sample characteristics
Patients with CVD risk were older than those with depression [mean (SD) 61.9 (7.8) years vs. 49.1 (15.9) years], reflecting the inclusion criteria. Three-quarters of the depression group were female (452/606, 74.6%), whereas three-quarters of the CVD risk group were male (654/872, 75.0%). Both patient groups were predominantly white (depression 575/594, 96.8%; CVD risk 825/851, 96.9%), most patients were not currently employed (unemployed, studying, retired, etc.) (depression 317/597, 53.1%; CVD risk 498/861, 57.8%), only a minority had received higher education (depression 222/606, 36.6%; CVD risk 212/872, 24.3%) and the majority were home owners (depression 410/595, 68.9%; CVD risk 647/859, 75.3%).
Overview of health needs, access difficulties, technology-related factors and satisfaction with previous telehealth use
As expected, patients with CVD risk reported poorer physical than mental health whereas the reverse was true for patients with depression (Table 3). Whereas the reported physical health of patients with CVD risk was 0.5 SDs below the national average [UK mean (SD) 50.9 (9.4)], the reported mental health of patients with depression was more than 1.5 SDs below the national average [UK mean (SD) 50.9 (9.4)]. 207
| Explanatory variable | Patient group | |
|---|---|---|
| Depression | CVD risk | |
| Health needs | ||
| PCS score, mean (SD) (n) | 47.3 (13.8) (547) | 45.3 (11.8) (777) |
| MCS score, mean (SD) (n) | 37.7 (12.9) (547) | 49.8 (10.5) (777) |
| Access difficultiesa | ||
| Service delivery difficulties, mean (SD) (n)b | 1.5 (0.5) (595) | 1.3 (0.4) (848) |
| Physical access difficulties, mean (SD) (n)b | 1.2 (0.4) (594) | 1.1 (0.3) (854) |
| Technology-related factors | ||
| Telephone availability, % (n/N)c | 99.3 (595/599) | 98.4 (855/869) |
| E-mail/internet availability, % (n/N)c | 80.3 (481/599) | 67.2 (584/869) |
| Telephone confidence, mean (SD) (n)d | 2.5 (0.6) (596) | 2.5 (0.6) (861) |
| E-mail/internet confidence, mean (SD) (n)d | 2.3 (0.8) (595) | 2.0 (0.9) (851) |
| Social media confidence, mean (SD) (n)d | 1.6 (0.8) (594) | 1.3 (0.6) (847) |
| Telehealth advantages, mean (SD) (n)e | 3.7 (0.7) (588) | 3.6 (0.8) (853) |
| Telehealth disadvantages, mean (SD) (n)e | 3.3 (0.9) (593) | 3.5 (0.9) (860) |
| Satisfaction with previous telehealth use | ||
| NHS Direct satisfaction, mean (SD) (n)f | 3.4 (1.2) (336) | 3.4 (1.2) (247) |
Few patients reported access difficulties, with all summary scores approximating the ‘no difficulty’ response category (see Table 3). Despite these low mean summary scores, an important minority of participants indicated some difficulty in accessing health care and both patient groups were more likely to report having service delivery than physical access difficulties. For example, 27.9% (399/1432) of patients reported difficulties getting care when they need it most (service delivery) and 14.2% (206/1448) reported difficulties travelling to appointments because of their physical health (physical access).
Technology availability was high across both patient groups (see Table 3). Telephone technologies were more prevalent than computer-based technologies and markedly so for the patients with CVD risk. In fact, nearly all patients had access to telephone technologies. Across patient groups, age was associated only with the availability of computer-based technologies: 89.8% (115/128) of the two youngest age groups (18–44 years), 78.1% (400/512) of those aged 45–59 years and 60.5% (393/650) of those aged 60–74 years reported that they have these technologies readily available to use. It was only among the oldest, and proportionally smallest, age group (n = 49) that less than half of the respondents (n = 13, 26.5%) reported having easy access to computer technologies.
Technology confidence ratings were similar between patient groups but they varied somewhat across the technology types (see Table 3). In general, patients reported greatest confidence using telephone technologies, with mean summary scores approaching the ‘extremely confident’ response category and least confidence using social media technologies, with mean summary scores close to the ‘not at all confident’ response category. Respondents were ‘quite confident’ with regard to e-mail- and internet-based technologies.
Figure 8 shows the proportion of depression respondents reporting that they were confident using the various technologies across the different age groups. The pattern of findings was similar among the CVD risk group (Figure 9). Least associated with age were the telephone technologies, which received high confidence ratings by all age groups. The one exception was low confidence in text messaging by the oldest age group. Although confidence using e-mail/internet and social media technologies consistently decreased with age, more than half of the respondents in all age groups (except those aged > 75 years) reported confidence using e-mail/internet technologies. Conversely, confidence using social media technologies was strongly related to age, with only the younger age groups expressing high levels of confidence.
FIGURE 8.
Proportion of depression respondents within each age group reporting confidence using individual technologies. Source: This figure was adapted from Edwards et al. 185 © Louisa Edwards, Clare Thomas, Alison Gregory, Lucy Yardley, Alicia O’Cathain, Alan A Montgomery, Chris Salisbury. Originally published in the Journal of Medical Internet Research (www.jmir.org), 8 May 2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on www.jmir.org/, as well as this copyright and license information must be included.
FIGURE 9.
Proportion of CVD risk respondents within each age group reporting confidence using individual technologies. Source: This figure was adapted from Edwards et al. 185 © Louisa Edwards, Clare Thomas, Alison Gregory, Lucy Yardley, Alicia O’Cathain, Alan A Montgomery, Chris Salisbury. Originally published in the Journal of Medical Internet Research (www.jmir.org), 8 May 2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on www.jmir.org/, as well as this copyright and license information must be included.

Summary scores indicate similar levels of perceived advantages and disadvantages of using telehealth across patient groups (see Table 3). The most highly endorsed advantages were convenience and ability of telehealth to be delivered when and where one desires (Table 4). Dislike of non-face-to-face care and concerns over security issues emerged as the top disadvantages of telehealth (see Table 4). Of those respondents who had ever used NHS Direct (see Table 3), the majority were satisfied with that experience: 26.9% (157/583) were ‘moderately’ satisfied, 33.1% (193/583) were ‘quite a bit’ satisfied and 18.9% (110/583) were ‘extremely’ satisfied.
| Advantages and disadvantages | Patient group | |
|---|---|---|
| Depression, % (n/N) | CVD risk, % (n/N) | |
| Advantages | ||
| I would like being able to choose to get support at times that are best for me | 87.4 (514/588) | 81.7 (696/852) |
| I would find it reassuring to be able to get support when I feel that I need it most | 85.7 (504/588) | 81.0 (689/851) |
| I would like being able to get support in my own home | 71.2 (418/587) | 67.7 (573/846) |
| Getting support with my health by telephone or computer would be valuable to me | 60.8 (360/592) | 54.9 (466/849) |
| I could save money by not having to travel to appointments | 51.4 (299/582) | 50.7 (426/840) |
| Getting support in this way would help me to feel more independent | 54.2 (318/587) | 48.8 (413/847) |
| It would make me feel special to be getting ‘extra’ support when I feel that I need it most | 42.5 (247/581) | 41.9 (354/844) |
| Disadvantages | ||
| I would dislike being unable to see the person face-to-face | 60.2 (357/593) | 66.6 (571/858) |
| I would be concerned about the security of the information that I give | 60.3 (357/592) | 63.3 (544/860) |
| I would not want to discuss sensitive issues over the telephone or using a computer | 54.9 (325/592) | 61.9 (532/859) |
| I would dislike speaking to someone other than a doctor about my health | 45.8 (271/592) | 53.7 (462/860) |
| I would worry about relying too much on the technology | 42.2 (247/586) | 52.3 (447/854) |
| I would worry about the possibility of the equipment not working | 37.8 (222/588) | 45.4 (387/852) |
| Getting support in this way would make me feel anxious about my health | 26.0 (153/588) | 33.2 (284/855) |
Health needs and health-care access difficulties
The results clearly showed support for the first examined relationship, with greater physical (PCS) and mental (MCS) health needs reliably associated with greater service delivery and physical access difficulties for both patient groups (Table 5) after adjusting for sociodemographic factors (sex, ethnicity, age group, employment status, higher education status and study location). Although sociodemographic factors were mostly non-influential, two out of seven characteristics emerged as consistently related to access difficulties for both groups: those who were unemployed (depression: β = – 0.107, 95% CI –0.204 to –0.009, p = 0.03; CVD risk: β = –0.041, 95% CI –0.066 to –0.016, p = 0.002) and those not owning a house (depression: β = –0.133, 95% CI –0.210 to –0.055, p = 0.001; CVD risk: β = –0.050, 95% CI –0.098 to –0.003, p = 0.04) reported greater physical access difficulties.
| Health needs | Patient group | |||
|---|---|---|---|---|
| Service delivery difficulties, β (95% CI) | Physical access difficulties, β (95% CI) | |||
| Depression (n = 520) | CVD risk (n = 732) | Depression (n = 518) | CVD risk (n = 736) | |
| PCS | –0.011 (–0.015 to –0.008) | –0.008 (–0.011 to –0.005) | –0.013 (–0.016 to –0.010) | –0.008 (–0.010 to –0.006) |
| MCS | –0.012 (–0.017 to –0.006) | –0.007 (–0.009 to –0.004) | –0.008 (–0.012 to –0.004) | –0.009 (–0.012 to –0.005) |
Overview of interest in using telehealth
Regardless of patient group, there was moderate interest in telephone technologies [depression mean (SD) 1.9 (0.7); CVD risk mean (SD) 1.7 (0.6)] and e-mail/internet technologies [depression mean (SD) 1.9 (0.7); CVD risk mean (SD) 1.7 (0.7)]; these mean summary scores approximate the ‘fairly interested’ response category. In contrast, there was very little interest in social media technologies [depression mean (SD) 1.3 (0.5); CVD risk mean (SD) 1.2 (0.4)].
Figure 10 shows which of the individual technologies the respondents were more or less interested in using, with ratings of interest dichotomised into ‘some’ and ‘no interest’ for ease of interpretation. This shows that patients with depression were more interested than those with CVD risk in nearly every form of technology for telehealth. There was a clear preference for the landline telephone (1072/1428, 75.1% overall), followed by finding information on the internet (876/1427, 61.4% overall). Again, there was hardly any interest in the social media technologies. Averaging across the technology types and both patient groups (depression and CVD risk), there was moderate interest in using telephone-based (854/1423, 60.0%) and e-mail-/internet-based (816/1425, 57.3%) telehealth, but very little interest in social media (243/1430, 17.0%).
FIGURE 10.
Proportion of respondents interested in individual telehealth technologies by patient group. This figure was adapted from Edwards et al. 185 © Louisa Edwards, Clare Thomas, Alison Gregory, Lucy Yardley, Alicia O’Cathain, Alan A Montgomery, Chris Salisbury. Originally published in the Journal of Medical Internet Research (www.jmir.org), 8 May 2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on www.jmir.org/, as well as this copyright and license information must be included.
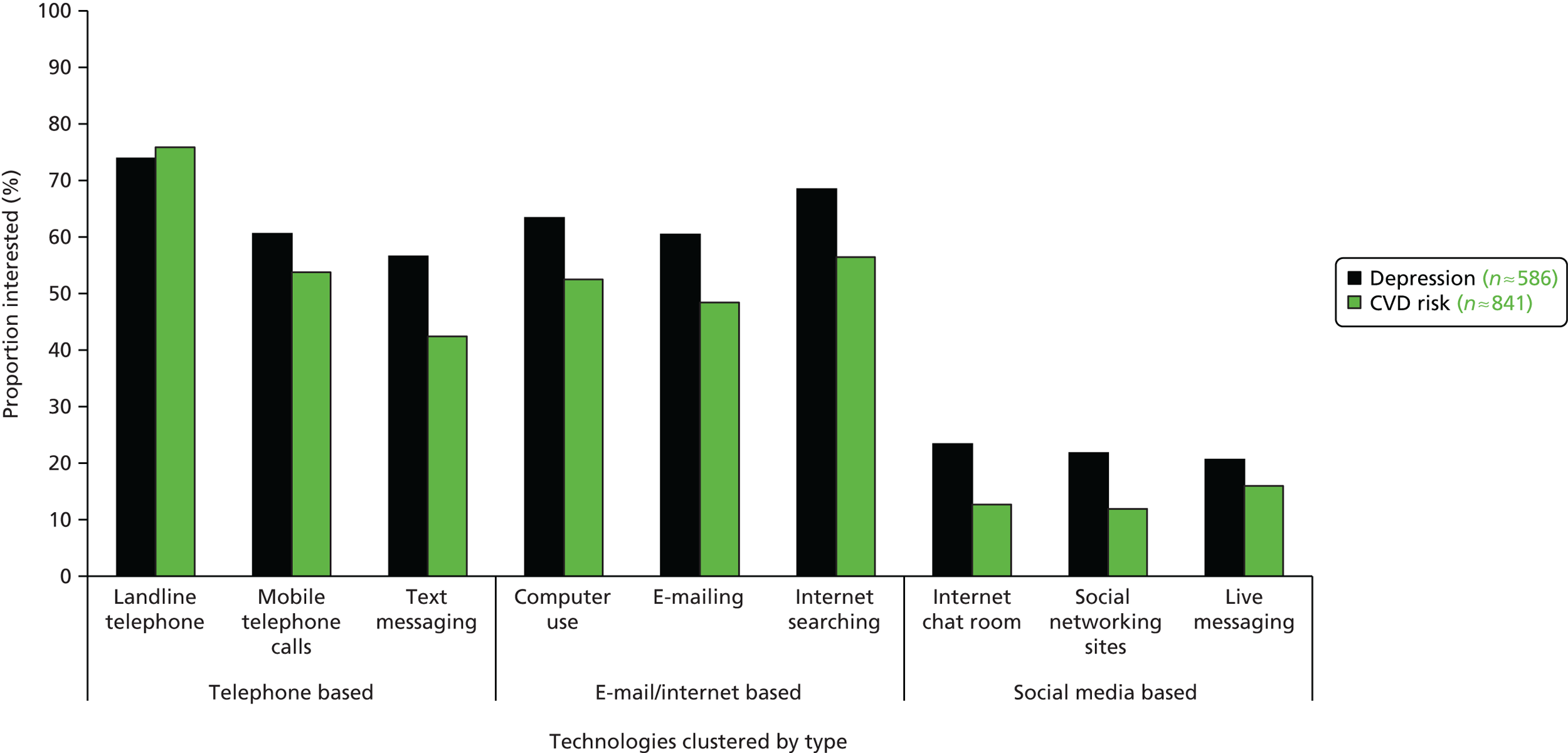
What factors are associated with interest in telehealth?
To address the main research question, sociodemographic factors, health needs, access difficulties, technology-related factors and satisfaction with past telehealth use were simultaneously regressed onto interest for each of the three telehealth mediums – telephone-, e-mail-/internet- and social media-based telehealth – separately for each patient group (depression and CVD risk). From these multivariable linear regression analyses, three variables were reliably related to interest in telehealth: greater technology confidence and perceiving both greater advantages and fewer disadvantages of telehealth (Tables 6–8). Moreover, these factors were consistently related to interest in each of the three telehealth mediums for both patient groups. Importantly, however, the technology confidence finding was modality specific. This means that greater telephone confidence was associated with greater telephone-based telehealth interest, greater e-mail/internet confidence was associated with greater e-mail-/internet-based telehealth interest and greater confidence using social media technologies was associated with greater interest in social media-based telehealth. Therefore, although confidence utilising a particular type of technology discriminated between interest in different modes of telehealth, perceiving greater benefits of and fewer drawbacks to using telehealth was uniformly related to greater interest. To aid interpretation of these multivariable regression results, Table 9 shows the unadjusted means and SDs for the level of interest in different forms of telehealth in relation to the explanatory variables (sociodemographic characteristics, health needs, access difficulties, technology-related factors, patient satisfaction).
| Variable | Patient group | |||
|---|---|---|---|---|
| Depression (n = 489) | CVD risk (n = 676) | |||
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Sociodemographic factors | ||||
| Age (years) | ||||
| 18–29 | Referent | – | – | |
| 30–44 | –0.054 (–0.187 to 0.079) | Referent (40–44 years) | ||
| 45–59 | –0.016 (–0.201 to 0.168) | –0.101 (–0.379 to 0.178) | ||
| 60–74 | –0.028 (–0.178 to 0.122) | –0.122 (–0.421 to 0.176) | ||
| 75+ | 0.087 (–0.303 to 0.476) | 0.79b | 0.301 (–0.484 to 1.086) | 0.35b |
| Sexc | –0.104 (–0.226 to 0.018) | 0.09 | –0.018 (–0.122 to 0.086) | 0.72 |
| Ethnicityd | 0.161 (–0.076 to 0.398) | 0.18 | –0.061 (–0.312 to 0.191) | 0.63 |
| Employede | 0.049 (–0.056 to 0.155) | 0.35 | 0.108 (0.015 to 0.201) | 0.02 |
| Higher educationf | –0.157 (–0.272 to –0.042) | 0.01 | –0.009 (–0.092 to 0.074) | 0.83 |
| Home ownerg | –0.173 (–0.281 to –0.065) | 0.003 | –0.088 (–0.206 to 0.030) | 0.14 |
| Locationh | –0.072 (–0.170 to 0.026) | 0.14 | –0.017 (–0.123 to 0.090) | 0.75 |
| Health needs | ||||
| PCS | 0.005 (0.001 to 0.009) | 0.02 | 0.001 (–0.003 to 0.005) | 0.67 |
| MCS | 0.004 (0.001 to 0.008) | 0.02 | –0.002 (–0.006 to 0.001) | 0.21 |
| Access difficultiesi | ||||
| Service delivery | 0.205 (0.069 to 0.340) | 0.004 | –0.093 (–0.232 to 0.046) | 0.19 |
| Physical access | –0.066 (–0.280 to 0.148) | 0.53 | 0.185 (–0.010 to 0.379) | 0.06 |
| Technology-related factors | ||||
| Telephone availabilityj | 0.203 (0.025 to 0.382) | 0.03 | 0.107 (–0.039 to 0.254) | 0.15 |
| E-mail/internet availabilityj | –0.089 (–0.178 to 0.0003) | 0.05 | –0.012 (–0.118 to 0.095) | 0.82 |
| Telephone confidencei | 0.164 (0.002 to 0.326) | 0.048 | 0.254 (0.151 to 0.358) | < 0.001 |
| E-mail/internet confidencei | –0.011 (–0.111 to 0.088) | 0.82 | –0.075 (–0.197 to 0.046) | 0.22 |
| Social media confidencei | –0.025 (–0.110 to 0.060) | 0.56 | 0.065 (–0.018 to 0.147) | 0.12 |
| Telehealth advantagesi | 0.308 (0.213 to 0.404) | < 0.001 | 0.296 (0.240 to 0.352) | < 0.001 |
| Telehealth disadvantagesi | –0.226 (–0.282 to –0.170) | < 0.001 | –0.201 (–0.261 to –0.140) | < 0.001 |
| Past telehealth satisfaction | ||||
| NHS Directi | 0.046 (0.001 to 0.090) | 0.045 | 0.088 (0.025 to 0.151) | 0.01 |
| Variable | Patient group | |||
|---|---|---|---|---|
| Depression (n = 488) | CVD risk (n = 681) | |||
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Sociodemographic factors | ||||
| Age (years) | ||||
| 18–29 | Referent | – | – | |
| 30–44 | –0.041 (–0.278 to 0.197) | Referent (40–44 years) | ||
| 45–59 | 0.006 (–0.198 to 0.209) | –0.184 (–0.373 to 0.005) | ||
| 60–74 | 0.045 (–0.160 to 0.250) | –0.132 (–0.346 to 0.082) | ||
| ≥ 75 | 0.032 (–0.299 to 0.364) | 0.89b | 0.371 (–0.120 to 0.863) | 0.01b |
| Sexc | 0.008 (–0.114 to 0.130) | 0.90 | –0.035 (–0.122 to 0.052) | 0.42 |
| Ethnicityd | –0.042 (–0.221 to 0.138) | 0.64 | 0.071 (–0.133 to 0.276) | 0.48 |
| Employede | –0.048 (–0.146 to 0.049) | 0.32 | 0.086 (–0.010 to 0.182) | 0.08 |
| Higher educationf | –0.037 (–0.155 to 0.080) | 0.52 | 0.070 (–0.068 to 0.207) | 0.31 |
| Home ownerg | –0.059 (–0.158 to 0.040) | 0.24 | –0.148 (–0.266 to –0.031) | 0.02 |
| Locationh | –0.090 (–0.164 to –0.016) | 0.02 | –0.002 (–0.096 to 0.092) | 0.97 |
| Health needs | ||||
| PCS | 0.003 (–0.001 to 0.008) | 0.16 | –0.002 (–0.007 to 0.003) | 0.44 |
| MCS | –0.005 (–0.010 to 0.0004) | 0.07 | –0.006 (–0.013 to 0.001) | 0.08 |
| Access difficultiesi | ||||
| Service delivery | 0.087 (0.005 to 0.168) | 0.04 | –0.013 (–0.124 to 0.098) | 0.81 |
| Physical access | –0.127 (–0.240 to –0.013) | 0.03 | 0.017 (–0.249 to 0.284) | 0.90 |
| Technology-related factors | ||||
| Telephone availabilityj | 0.093 (–0.095 to 0.282) | 0.32 | –0.099 (–0.211 to 0.012) | 0.08 |
| E-mail/internet availabilityj | 0.101 (–0.013 to 0.215) | 0.08 | 0.158 (0.091 to 0.225) | < 0.001 |
| Telephone confidencei | –0.199 (–0.367 to –0.032) | 0.02 | –0.021 (–0.121 to 0.079) | 0.68 |
| E-mail/internet confidencei | 0.403 (0.295 to 0.512) | < 0.001 | 0.304 (0.219 to 0.389) | < 0.001 |
| Social media confidencei | 0.003 (–0.075 to 0.080) | 0.95 | –0.002 (–0.157 to 0.154) | 0.98 |
| Telehealth advantagesi | 0.237 (0.150 to 0.324) | < 0.001 | 0.226 (0.165 to 0.286) | < 0.001 |
| Telehealth disadvantagesi | –0.211 (–0.287 to –0.134) | < 0.001 | –0.244 (–0.310 to –0.179) | < 0.001 |
| Past telehealth satisfactioni | ||||
| NHS Direct | 0.040 (–0.034 to 0.115) | 0.28 | 0.029 (–0.036 to 0.094) | 0.38 |
| Variable | Patient group | |||
|---|---|---|---|---|
| Depression (n = 489) | CVD risk (n = 680) | |||
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Sociodemographic factors | ||||
| Age (years) | ||||
| 18–29 | Referent | – | – | |
| 30–44 | 0.146 (–0.087 to 0.379) | Referent (40–44 years) | ||
| 45–59 | 0.187 (–0.036 to 0.411) | –0.092 (–0.301 to 0.117) | ||
| 60–74 | 0.174 (–0.033 to 0.382) | –0.030 (–0.246 to 0.186) | ||
| ≥ 75 | 0.061 (–0.163 to 0.285) | 0.31b | 0.047 (–0.286 to 0.380) | 0.25b |
| Sexc | 0.029 (–0.061 to 0.118) | 0.52 | –0.025 (–0.080 to 0.030) | 0.36 |
| Ethnicityd | 0.036 (–0.208 to 0.279) | 0.77 | –0.046 (–0.255 to 0.164) | 0.66 |
| Employede | –0.007 (–0.114 to 0.100) | 0.90 | 0.040 (–0.031 to 0.110) | 0.26 |
| Higher educationf | –0.033 (–0.147 to 0.080) | 0.55 | 0.018 (–0.057 to 0.093) | 0.63 |
| Home ownerg | –0.097 (–0.232 to 0.038) | 0.15 | –0.118 (–0.198 to –0.038) | 0.01 |
| Locationh | –0.005 (–0.082 to 0.073) | 0.91 | –0.015 (–0.061 to 0.032) | 0.53 |
| Health needs | ||||
| PCS | –0.001 (–0.006 to 0.004) | 0.67 | –0.001 (–0.004 to 0.001) | 0.28 |
| MCS | –0.001 (–0.007 to 0.004) | 0.63 | –0.002 (–0.007 to 0.003) | 0.44 |
| Access difficultiesi | ||||
| Service delivery | 0.029 (–0.098 to 0.156) | 0.64 | –0.079 (–0.161 to 0.002) | 0.06 |
| Physical access | –0.070 (–0.200 to 0.060) | 0.28 | 0.029 (–0.118 to 0.176) | 0.70 |
| Technology-related factors | ||||
| Telephone availabilityj | 0.077 (–0.096 to 0.250) | 0.37 | –0.109 (–0.256 to 0.039) | 0.14 |
| E-mail/internet availabilityj | 0.013 (–0.073 to 0.099) | 0.76 | 0.016 (–0.029 to 0.062) | 0.48 |
| Telephone confidencei | –0.068 (–0.218 to 0.082) | 0.36 | 0.012 (–0.071 to 0.095) | 0.78 |
| E-mail/internet confidencei | –0.038 (–0.118 to 0.041) | 0.33 | 0.001 (–0.056 to 0.057) | 0.98 |
| Social media confidencei | 0.361 (0.282 to 0.441) | < 0.001 | 0.243 (0.132 to 0.355) | < 0.001 |
| Telehealth advantagesi | 0.176 (0.106 to 0.245) | < 0.001 | 0.096 (0.045 to 0.146) | 0.001 |
| Telehealth disadvantagesi | –0.123 (–0.191 to –0.054) | 0.001 | –0.072 (–0.128 to –0.016) | 0.01 |
| Past telehealth satisfactioni | ||||
| NHS Direct | –0.006 (–0.051 to 0.040) | 0.80 | 0.033 (–0.006 to 0.072) | 0.10 |
| Variable | Patient group | |||||
|---|---|---|---|---|---|---|
| Depression (n = 489) | CVD risk (n = 676) | |||||
| Telephone, mean (SD) (n) | E-mail, mean (SD) (n) | Social media, mean (SD) (n) | Telephone, mean (SD) (n) | E-mail, mean (SD) (n) | Social media, mean (SD) (n) | |
| Sociodemographic factors | ||||||
| Age (years) | ||||||
| 18–29 | 1.95 (0.63) (65) | 2.17 (0.71) (65) | 1.41 (0.62) (65) | – | – | – |
| 30–44 | 2.00 (0.64) (171) | 2.09 (0.65) (171) | 1.42 (0.59) (171) | 2.04 (0.71) (18) | 2.06 (0.72) (18) | 1.43 (0.66) (18) |
| 45–59 | 1.86 (0.66) (200) | 1.85 (0.67) (200) | 1.26 (0.47) (200) | 1.80 (0.60) (303) | 1.82 (0.69) (305) | 1.21 (0.44) (304) |
| 60–74 | 1.72 (0.62) (112) | 1.57 (0.62) (111) | 1.13 (0.34) (112) | 1.70 (0.59) (514) | 1.60 (0.69) (515) | 1.13 (0.36) (519) |
| ≥ 75 | 1.51 (0.55) (41) | 1.25 (0.50) (40) | 1.03 (0.16) (40) | 1.92 (0.83) (4) | 1.60 (0.89) (5) | 1.13 (0.18) (5) |
| Sex: male | 1.87 (0.65) (151) | 1.87 (0.68) (149) | 1.29 (0.51) (149) | 1.75 (0.60) (636) | 1.72 (0.70) (638) | 1.17 (0.40) (640) |
| Sex: female | 1.86 (0.65) (438) | 1.86 (0.70) (438) | 1.28 (0.51) (439) | 1.73 (0.58) (203) | 1.61 (0.68) (205) | 1.18 (0.40) (206) |
| Ethnicity: Caucasian | 1.87 (0.65) (565) | 1.87 (0.70) (563) | 1.28 (0.50) (564) | 1.74 (0.60) (808) | 1.70 (0.70) (812) | 1.17 (0.39) (814) |
| Ethnicity: non-Caucasian | 1.67 (0.60) (19) | 1.81 (0.71) (19) | 1.44 (0.69) (19) | 1.76 (0.62) (25) | 1.53 (0.62) (25) | 1.23 (0.57) (25) |
| Employed | 1.96 (0.65) (276) | 2.02 (0.68) (276) | 1.34 (0.56) (276) | 1.83 (0.60) (357) | 1.84 (0.67) (358) | 1.20 (0.44) (360) |
| Not employed | 1.78 (0.65) (304) | 1.72 (0.69) (302) | 1.23 (0.46) (303) | 1.68 (0.58) (472) | 1.59 (0.70) (475) | 1.15 (0.36) (477) |
| Some higher education | 1.86 (0.65) (220) | 2.05 (0.66) (220) | 1.32 (0.55) (220) | 1.76 (0.60) (205) | 2.01 (0.68) (208) | 1.22 (0.46) (208) |
| No higher education | 1.86 (0.65) (369) | 1.75 (0.70) (367) | 1.26 (0.48) (368) | 1.74 (0.60) (634) | 1.59 (0.67) (635) | 1.15 (0.37) (638) |
| Home owner | 1.84 (0.65) (399) | 1.88 (0.69) (398) | 1.25 (0.48) (400) | 1.73 (0.59) (625) | 1.71 (0.70) (630) | 1.15 (0.36) (631) |
| Non-home owner | 1.92 (0.64) (179) | 1.86 (0.72) (178) | 1.36 (0.56) (177) | 1.76 (0.61) (202) | 1.64 (0.71) (201) | 1.24 (0.46) (204) |
| Location: Bristol | 1.85 (0.63) (287) | 1.90 (0.70) (285) | 1.27 (0.51) (287) | 1.76 (0.61) (444) | 1.71 (0.71) (447) | 1.18 (0.40) (448) |
| Location: Sheffield | 1.87 (0.67) (302) | 1.83 (0.70) (302) | 1.29 (0.51) (301) | 1.72 (0.59) (395) | 1.67 (0.69) (396) | 1.16 (0.39) (398) |
| Health needsa | ||||||
| PCS: high | 1.93 (0.67) (268) | 2.02 (0.68) (269) | 1.32 (0.53) (269) | 1.77 (0.60) (378) | 1.76 (0.70) (380) | 1.19 (0.43) (380) |
| PCS: low | 1.79 (0.63) (268) | 1.76 (0.69) (265) | 1.25 (0.49) (267) | 1.72 (0.59) (374) | 1.66 (0.70) (378) | 1.16 (0.38) (380) |
| MCS: high | 1.88 (0.66) (269) | 1.82 (0.69) (267) | 1.27 (0.50) (270) | 1.72 (0.58) (377) | 1.71 (0.70) (380) | 1.17 (0.41) (381) |
| MCS: low | 1.84 (0.64) (267) | 1.96 (0.69) (267) | 1.30 (0.53) (266) | 1.77 (0.62) (375) | 1.71 (0.70) (378) | 1.18 (0.40) (379) |
| Access difficultiesa | ||||||
| Service delivery: high | 1.88 (0.65) (285) | 1.96 (0.71) (285) | 1.35 (0.56) (284) | 1.75 (0.58) (349) | 1.73 (0.72) (352) | 1.18 (0.42) (351) |
| Service delivery: low | 1.84 (0.65) (294) | 1.77 (0.68) (292) | 1.22 (0.44) (294) | 1.73 (0.61) (470) | 1.67 (0.68) (473) | 1.16 (0.39) (474) |
| Physical access: high | 1.83 (0.66) (212) | 1.79 (0.71) (211) | 1.30 (0.53) (210) | 1.77 (0.58) (170) | 1.63 (0.71) (169) | 1.23 (0.47) (172) |
| Physical access: low | 1.88 (0.65) (365) | 1.91 (0.69) (365) | 1.28 (0.50) (367) | 1.73 (0.60) (654) | 1.71 (0.70) (659) | 1.16 (0.38) (660) |
| Technology-related factors | ||||||
| Some telephone availabilityb | 1.87 (0.65) (581) | 1.86 (0.70) (579) | 1.28 (0.51) (580) | 1.74 (0.59) (824) | 1.69 (0.70) (828) | 1.17 (0.39) (830) |
| No telephone availabilityb | 1.75 (0.96) (4) | 2.00 (1.15) (4) | 1.33 (0.67) (4) | 1.69 (0.73) (13) | 1.64 (0.85) (13) | 1.36 (0.63) (14) |
| Some e-mail/internet availabilityb | 1.89 (0.65) (474) | 2.00 (0.66) (475) | 1.32 (053) (475) | 1.76 (0.60) (572) | 1.92 (0.67) (575) | 1.21 (0.42) (574) |
| No e-mail/internet availabilityb | 1.74 (0.65) (111) | 1.26 (0.54) (108) | 1.14 (0.38) (109) | 1.69 (0.58) (265) | 1.19 (0.45) (266) | 1.08 (0.33) (270) |
| Telephone confidence: higha | 2.01 (0.68) (315) | 2.05 (0.66) (313) | 1.36 (0.56) (314) | 1.91 (0.65) (371) | 1.94 (0.69) (370) | 1.26 (0.48) (371) |
| Telephone confidence: lowa | 1.69 (0.57) (268) | 1.66 (0.68) (267) | 1.20 (0.43) (268) | 1.61 (0.51) (464) | 1.50 (0.64) (468) | 1.10 (0.30) (468) |
| E-mail/internet confidence: higha | 1.99 (0.66) (271) | 2.21 (0.63) (271) | 1.43 (0.62) (271) | 1.84 (0.64) (376) | 2.11 (0.67) (379) | 1.26 (0.47) (378) |
| E-mail/internet confidence: lowa | 1.76 (0.62) (310) | 1.58 (0.61) (310) | 1.16 (0.36) (310) | 1.66 (0.55) (451) | 1.36 (0.55) (453) | 1.10 (0.31) (453) |
| Social media confidence: higha | 2.02 (0.65) (245) | 2.18 (0.63) (245) | 1.55 (0.64) (244) | 1.96 (0.65) (233) | 2.18 (0.67) (234) | 1.43 (0.55) (233) |
| Social media confidence: lowa | 1.75 (0.62) (336) | 1.64 (0.66) (336) | 1.09 (0.25) (337) | 1.65 (0.55) (591) | 1.51 (0.61) (594) | 1.07 (0.27) (594) |
| Telehealth advantages: higha | 2.14 (0.62) (263) | 2.11 (0.72) (261) | 1.46 (0.62) (261) | 2.03 (0.60) (332) | 1.95 (0.75) (336) | 1.29 (0.49) (335) |
| Telehealth advantages: lowa | 1.63 (0.58) (316) | 1.67 (0.61) (316) | 1.14 (0.34) (317) | 1.55 (0.52) (494) | 1.52 (0.61) (495) | 1.09 (0.30) (496) |
| Telehealth disadvantages: higha | 1.61 (0.55) (279) | 1.59 (0.61) (278) | 1.13 (0.33) (279) | 1.59 (0.55) (411) | 1.40 (0.55) (415) | 1.07 (0.25) (415) |
| Telehealth disadvantages: lowa | 2.09 (0.65) (305) | 2.12 (0.69) (304) | 1.42 (0.60) (304) | 1.89 (0.61) (421) | 1.97 (0.72) (423) | 1.27 (0.48) (423) |
| Past telehealth satisfactionc | ||||||
| NHS Direct: high | 2.02 (0.64) (171) | 2.04 (0.70) (171) | 1.37 (0.58) (172) | 2.04 (0.64) (127) | 2.03 (0.78) (127) | 1.30 (0.53) (127) |
| NHS Direct: low | 1.78 (0.64) (400) | 1.80 (0.69) (399) | 1.25 (0.48) (398) | 1.69 (0.57) (684) | 1.64 (0.67) (689) | 1.15 (0.37) (691) |
Three other consistent findings emerged. First, for patients with depression but not those with CVD risk, greater difficulties with getting convenient, high-quality care (service delivery aspects of access) were related to more interest in telephone- and e-mail-/internet-based telehealth technologies. Second, as anticipated, greater satisfaction with previous use of NHS Direct was associated with heightened interest in future use of telephone-based telehealth and this was consistent across both patient groups. Third, there was more interest in e-mail-/internet-based and social media-based telehealth among those with CVD risk who were not home owners. Apart from these findings, the remaining variables in the model were unimportant to telehealth interest. After adjusting for the other variables in the model, health needs, access difficulties, technology availability and even sociodemographic factors did not reliably and consistently have an independent effect on interest in telehealth, either across patient groups or across more than one telehealth type within a patient group.
Discussion
Summary of the main results
The results from this patient survey show that patients with two very different LTCs are interested in using telephone- and e-mail- or internet-based telehealth, but not telehealth accessed through social media websites. Although greater health needs were associated with greater difficulties accessing traditional health care in these patient groups, neither factor was consistently related to interest in telehealth. Interest in all three forms of telehealth appears to stem from the perceived advantages and disadvantages of telehealth, as well as confidence in using the relevant technology. This is significant because beliefs and levels of confidence are far more malleable than most of the other constructs included in this study, such as technology availability or socioeconomic status. It is also noteworthy that these other constructs were not consistently and independently associated with interest in telehealth. First, interest in telehealth was not reliably related to health needs. This suggests that willingness to use telehealth spans across those with good and poor health. Furthermore, it is not only those who have difficulty accessing traditional health care who are motivated to use telehealth; by and large, those with and without access difficulties were interested. Sociodemographic factors were generally not, in themselves, systematically related to telehealth interest. Therefore, older people were just as interested as their younger counterparts after adjusting for other factors, such as confidence in using the technology. Although availability of technology was quite high, this factor also did not consistently relate to interest in telehealth. The ramifications of these findings are important for policy makers, researchers, health professionals and patients alike.
Results in the context of existing research
We examined whether those with greater health needs or greater difficulties accessing traditional health care were more interested in using telehealth but found only weak evidence for this in patients with depression. Our results revealed an association between greater service delivery access difficulties (getting the right amount of care, from the right health professional, at the right time) and heightened interest in both telephone- and e-mail-/internet-based telehealth among patients with depression. This finding aligns well with one aim of telehealth treatments for depression, namely, overcoming barriers to care. Given the results of systematic reviews which showed that telephone-administered psychotherapy attrition rates80 are far lower than those of face-to-face care,208 this level of engagement may suggest that telehealth meets this aim to some extent. It is important to note, however, that respondents in our survey reported very few difficulties with health-care access and did not report especially great health needs, except for the mental health needs of those with depression. Overall, this restriction in range may have made it difficult to detect an effect of need or a more widespread effect of access difficulties on telehealth interest.
Sociodemographic factors were found to be relatively unimportant after adjusting for attitudes towards telehealth and availability of technology, which suggests that telehealth appeals to a broader demographic than young, educated and affluent patients. Although this runs contrary to some previous literature,190,191 it might be explained by the fact that more proximal variables, such as technology confidence and beliefs about telehealth, had not been included in previous research. Indeed, when similar behavioural or motivational factors are assessed, other research is consistent with our findings in demonstrating the integral role,209 or even superiority,210 of these constructs over demographic variables, albeit in terms of using the internet alone. Furthermore, a systematic review concluded that focusing on patient factors alone, as the majority of research in this area has done, is probably not comprehensive enough to understand patient interest in using telehealth. 189 We agree with this review that future research must cut across a broader spectrum of factors, especially those at the level of human–technology interaction, the health-care system and other social or normative influences.
Technology confidence is an example of a human–technology interaction variable and a key finding of this and other189 research is that confidence is consistently associated with interest in using telehealth. Whereas we asked respondents about technology confidence, it is interesting to note that other studies have used negative framing and asked about technology anxiety211,212 or difficulty using the internet. 210 Nonetheless, these studies also showed the equivalent association between lower technology anxiety and heightened interest in using telehealth.
We drew on the theory of planned behaviour205 when devising some of the items in the survey instrument, in particular technology confidence (akin to perceived behavioural control), perceived benefits and drawbacks of using telehealth (akin to attitudes towards the behaviour) and interest in using telehealth (akin to behavioural intention). Our findings do show that these predictors of behavioural intention – technology confidence and perceived advantages and disadvantages of telehealth – were the only two sets of variables that consistently related to interest in using the three mediums of telehealth, lending some support for the applicability of the theory of planned behaviour in assessing the potential uptake of telehealth. It is important to note, however, that our intention was not to assess the suitability of this theory and we did not include other constructs from the theory of planned behaviour (namely, subjective norm) in our analysis. Nonetheless, a meta-review of applications of the theory of planned behaviour to a variety of health-related behaviours (e.g. exercising, smoking, health screening checks) found that both attitudes and perceived behavioural control were the most important predictors of intention to perform a health-related behaviour. 213 More recently, a systematic review and meta-analysis of the effectiveness of theoretically based internet interventions in promoting health behaviour change found that larger effect sizes were obtained in studies that made greater use of theory in intervention development. 214 Of the social cognitive theories examined, the authors note that only those interventions that drew on the theory of planned behaviour resulted in considerably larger effects. Therefore, it is not surprising that a measure of patient acceptance of telehealth that incorporates the constructs from the theory of planned behaviour has now been devised. 215 This measure was completed by a group of patients with LTCs (heart failure or COPD) who were current users of telehealth and the results showed that the theory of planned behaviour constructs were associated with greater motivation to continue using telehealth. 216 Taken together, our results agree well with other research which shows that greater perceived behavioural control and more positive attitudes are related to greater patient interest in telehealth use.
Strengths and weaknesses
The main strength of this research is that it is a broad exploration of patient interest in several general forms of telehealth. The findings, therefore, are not limited to a specific intervention, but highlight some of the key elements that we must pay particular attention to when designing and implementing future telehealth initiatives. This is also the only study to our knowledge that has gathered ratings about interest in using a variety of forms of telehealth from patients who are the most likely recipients of this type of care in the future – those with LTCs. If telehealth is going to have an important role in effectively supporting patients with LTCs, then knowing what interests patients in taking up different forms of telehealth is an important first step.
The primary limitation of this study is the response rate of 44.4%, although this compares favourably with response rates in other community surveys. 217 It is possible that those who did not respond had different characteristics from those who chose to respond, which could have implications for the findings. With respect to telehealth, non-response bias by age is important given the widely held perception that older people do not like or use technology much. In this survey, responders were actually older than non-responders and yet a fair amount of interest in telehealth was reported. There was also considerable variation in patient health and sociodemographics, as well as the other key variables of interest. Nevertheless, these findings may not be generalisable to other LTC patient groups. A second limitation is that the telehealth interest ratings are based on questions about hypothetical and general technologies, rather than existing or specifically named telehealth services. Although this approach was directly in line with the purpose of the research – to inquire about future interest in services that could be delivered by existing health-care providers – it is difficult to know what types of applications respondents were thinking about when they gauged their interest in telehealth delivered through social networking websites, for instance. Moreover, it is likely that there is a relationship between how frequently technology is used and technology confidence210,212 and this relationship should be controlled for in future research. Finally, the large number of variables analysed raises the possibility of type 1 error because of multiple comparisons. Therefore, we have conservatively drawn attention only to findings that were consistent across several analyses.
Implications of the results
There are several interesting implications of the finding that telehealth interest is most strongly associated with technology confidence and perceived advantages and disadvantages of telehealth. First, it suggests that telehealth interest is likely to increase over time as the population, as a whole, becomes more familiar with and comfortable using different forms of technology. This may be particularly true of social media-based telehealth,218 the newest type of technology included in the survey and also the technology that respondents reported least confidence and interest in using. Following on from this, as our results revealed that technology confidence was modality specific, whereby confidence using one type of technology was related only to interest in using that same form of telehealth, it suggests that willingness to use telehealth is not restricted to patients who are confident using technology in general. Third, if patients were provided with adequate training and support in using telehealth equipment, they might be more interested in using telehealth. Finally, as some of telehealth’s advantages are realised and other disadvantages are dispelled through telehealth use, the strength of this effect may increase. There is good reason to expect such positive experiences of telehealth as the majority of telehealth research that asks about patient satisfaction does report fairly high levels of satisfaction. 219 Our study is no exception: high levels of satisfaction with past NHS Direct use – a form of telehealth – were reported. Positive experiences of telehealth may stimulate interest in using additional forms of telehealth, in an upwards spiralling effect.
Conclusion
This research suggests that many people with LTCs are interested in using telehealth, regardless of their health status and age, and that they have the technology available to them. This interest can be increased by helping people gain confidence in using technologies, highlighting the perceived advantages of telehealth and dispelling or addressing concerns about perceived disadvantages. Based on our findings, future telehealth interventions would be best received by patients if delivered by telephone or e-mail and static forms of the internet, rather than through social media. This is because these forms of technology are readily available, patients are confident using them and patients are most interested in telehealth delivered via these means.
Chapter 4 A qualitative study of health-care professionals’ and patients’ views of telehealth to support long-term conditions in primary care
The material in this chapter was adapted with permission from Segar J, Rogers A, Salisbury C, Thomas C. Roles and identities in transition: boundaries of work and inter-professional relationships at the interface between telehealth and primary care. Health Soc Care Community 2013;21:606–13. 220 Copyright © 2013 by John Wiley & Sons Ltd.
Abstract
Introduction: Shifting the balance of care towards home and community primary care through technology-based interventions means that patients and health professionals are faced with new systems of delivery and working across boundaries of health care. Patients’ and primary care practitioners’ views of NHS Direct services were sought to explore a continuum of experiences of using systems to access online or telephone-based health-care support and the potential and actual use of NHS Direct.
Methods: Semistructured interviews were conducted between April 2010 and March 2011 with telehealth nurse care managers, practice nurses and GPs (n = 39), as well as 38 patients recruited from advertisements on the NHS Choices and NHS Direct websites. Observation was also undertaken at a UK telehealth call centre. Data were analysed using thematic analysis.
Results: Telehealth managers’ constructions of new roles and identities to fit telehealth were characterised by a holistic ideal of ‘traditional’ values of nursing and they distinguished their approach from that of practice nurses and GPs. The latter were ambivalent or sceptical about telehealth, expressing some protectiveness over maintaining boundaries around established LTC management and their positions as ‘gatekeepers’ to resources. Given waiting lists for talking therapies and variability in service access, patients were positive about the idea of telephone- or internet-based psychological therapies. Views of potential telehealth-based CVD risk management were less distinctively formulated.
Conclusion: Introducing telehealth interventions inevitably involves adapting professional roles and developing new ways of working. Considering professionals’ and patients’ understanding of complex, multifaceted roles and modes of delivery in advance is likely to facilitate telehealth service integration.
Introduction
Telephone and online support for LTCs has the potential to reach patients who may be marginalised from mainstream care. However, the design and deployment of a system of using telephones and computers to support LTC management and deliver health care is still not fully integrated into existing roles and expectations and remains relatively unfamiliar to both patients and primary care practitioners. 221,222
The provision of care at a distance necessarily alters the nature of relationships between HCPs and patients and researchers have observed a range of responses in different health-care spaces to these innovations. 221,223–225 Milligan et al. 226 make the point that care at a distance provides challenges for HCPs as the work of care shifts in both space and time. They refer to a ‘downward cascade of care-work’ (p. 352) as work is passed from doctors to nurses and from nurses to monitoring or call centre operator. Similar shifts have been noted in general practice where nurses are increasingly responsible for supporting LTC management. 227,228 The work of telehealthcare in the area of chronic illness management is increasingly protocol driven and routinised and incorporates a tendency to reduce work tasks to simplistic components that are readily delegated to others. 229 This has the potential to disrupt previously established patterns of work and skill mix arrangements. How professionals working in different contexts within primary care meet the challenge of the introduction of telehealthcare innovations and view their own role and those of others remains underexplored.
Advances in the area of LTC management, which includes the use of telehealth, highlight the importance of, and promote, self-management support. This in turn requires considerable efforts of mediation between service users and providers. 230 Primary care professionals are increasingly expected to become users of specific telemonitoring devices,231 which implicates them in a set of generated tasks at variance with conventional health work practices and divisions of labour. There is some evidence to suggest that, in relative terms, professionals are less accepting of telehealth than patients81 and that factors outside of the technologies themselves are implicated in their acceptability. These include a lack of co-ordination across social and primary care boundaries and financial incentives to include telehealth within primary care in a system increasingly driven by the principles of ‘pay for performance’. 222 The need to respond to telehealth innovations is also taking place at a time when nursing work in primary care has undergone a period of rapid transformation. This includes a newly expanded role in chronic condition management,232–235 which in turn has taken place in a context of increasing fragmentation of health- and social-care agency boundaries. 122,222 A mixed picture of adaptability to new roles, relationships and social change relevant to LTC management in primary care settings has emerged. 236 There has also been resistance to aspects of the taking on of new responsibilities and defensiveness against a threat to core identities. 237 McDonald et al. 234 also draw attention to the tensions involved for practice nurses, pulled between professionalising and medicalised roles on the one hand and caring ‘holistic emotion work’ (p. 1207) on the other.
The promotion of self-care and of telehealthcare forms part of a process of change in roles and expectations and the challenge of telehealthcare-driven services is one of introducing further new dimensions to a rapidly changing arena of work for health-care workers. In this context, exploring the nature and dynamics of interagency working and co-ordination implicates the reworking of existing professional roles, identities and notions of trust. For patients, changes are required, such as placing greater trust in themselves to carry out health-related actions that have previously lain with professionals. For professionals, there are new expectations of undertaking different tasks, as well as facilitating these for patients. 238 This reshaping of health care throws into relief the ways in which people ‘trust’ others and the need to work out new ways of doing things at the interpersonal and organisational levels. 239 This is evident in the way in which professionals in our study approached working together and working out new relationships of engagement.
The evidence review of telehealth trials and the qualitative literature review on telehealth (see Chapter 2) point to evidence for interventions using telephone and internet technology making a significant contribution to the support of patients with LTCs. They also demonstrate gaps in the literature. Particularly relevant to this qualitative study is the finding in the systematic review of an absence of consideration of the contextual factors that impact on the delivery of and also shape the reception of telehealth interventions. Knowledge about the social environment, the context within which telehealth takes place and the impact of ethnicity, health literacy, age, sex and other characteristics of those who use and deliver telehealth services is also largely missing from these reviews.
As described in Chapter 2, the qualitative literature review undertaken as part of this programme addresses some of these matters, highlighting themes that need to be considered in resource development and deployment. These included the value of telehealth in reaching and supporting depressed patients; the importance of maintaining human interaction and contact in the delivery of telehealth; the recognition of the significance of peer support and affirmation on health internet sites; the need to address the anxieties and doubts of HCPs; the utility of telehealth in reaching the vulnerable elderly; and the importance of trust in the sources of telehealth.
The aim of this research was to investigate ways in which NHS Direct resources and organisation could be used to support patients with LTCs, using depression and high risk of CVD as exemplars. The views of patients and primary care practitioners were sought using questions that covered a continuum of experiences of using an existing telehealth scheme, as well as views on accessing potential online or telephone-based health-care support. We were particularly interested in patient and practitioner perceptions and beliefs concerning the role function and utility of NHS Direct, as well as reported experiences of the actual use of NHS Direct services. We also wanted to explore opinions about gaps in support for LTCs and ways in which primary care providers could be assisted in filling such gaps.
The findings of this qualitative study helped shape the subsequent design of the telehealth intervention that we developed (see Chapters 5 and 6). It was focused, in particular, on contextually specific issues relating to the exemplar conditions, potential patient groups and NHS Direct capabilities. A number of the themes introduced in the evidence synthesis (see Chapter 2) are developed and built on further here. Additionally, new themes have been generated and explored.
Methods
Between April 2010 and March 2011, observational work and interviews were conducted to explore the experiences and perspectives of HCPs and patients in terms of telehealth. Observations were carried out over a 2-week period at a telehealth call centre, hereafter referred to as Telehealth Scheme (THS) to maintain anonymity. Calls were observed and listened to (with the permission of the patients) and care managers’ use of the software system was observed and discussed. Interviews were also a key method used in this qualitative project. Most were individual interviews although some were carried out in groups. Interviews were loosely structured around a set of topics but were open-ended, thereby allowing respondents to elaborate and move beyond the items specified in the initial topic guide. Interviews were conducted with:
-
‘Expert’ GPs. The views of academic GPs were sought on topics surrounding the treatment of LTCs in general practice and the management of exemplar conditions with the rapid development of telehealth. These discussions helped to shape the topic guides used in the subsequent patient and HCP interviews.
-
Telehealth Scheme patients and staff. Early on, we were able to take advantage of the opportunity to examine an existing telephone-based scheme run in part by NHS Direct. This provided us with an opportunity to explore a number of objectives with respect to the ways in which NHS Direct can havea potential impact on the management of LTCs and how such interventions come to be viewed by patients and primary care providers. Two weeks were spent at THS observing the work undertaken at the call centre and interviewing the care managers. Interviews were then carried out with THS patients and a range of primary care staff at general practices participating in the scheme. Finally, a focus group was conducted with Asian THS patients to discuss the low uptake of the scheme among Asian patients. Recruitment of THS patients was carried out by THS care managers. The general practices were purposefully sampled to include a wide range of sociodemographic groups, from economically deprived to affluent areas, as well as a high degree of ethnic diversity.
-
General practices in south Yorkshire and the wider Bristol area. A number of general practices already recruited to participate in the patient survey study (see Chapter 3) agreed to take part in the qualitative project. Staff members in these practices were interviewed individually or in groups. We included practices serving a variety of socioeconomic groups and those situated far from metropolitan centres. As with the THS practices, this was important in view of our objective of investigating ways of reducing inequalities of access to a potential new service.
-
Patients. At the time of the study, NHS Direct provided services over the telephone and an internet site, which in turn were linked to the broader NHS information site, NHS Choices. As THS provided telephone-based support, the patient interviews enabled interesting insights into the advantages and limitations of receiving support in this way. We were interested in investigating views and perceptions in relation to internet-based support for LTCs. To this end we were able to recruit patients through advertisements placed on the NHS Direct and NHS Choices websites. Invitations to participate in the research were placed on the research pages of the NHS Direct website and on the Depression and Lifestyle pages of the NHS Choices website. This enabled us to question active internet users about the kinds of online support already being used and the types of support that they would welcome from an expanded service. In addition, some interviews with patients recruited through the patient survey study (see Chapter 3) were also undertaken.
Table 10 shows the numbers of patient and HCP respondents interviewed or observed in each part of the qualitative project. Most of the patients were interviewed individually (apart from those involved with the focus group), as were the THS care managers. Most interviews at general practices took place with two or more interviewees, resulting in 39 interviews with a total of 62 participants. To provide more context, Table 11 shows the age and sex distribution of the patients who were interviewed according to each recruitment method.
| Recruitment method/source | Patients, n | HCPs, n |
|---|---|---|
| ‘Expert’ GPs | – | 6 |
| THS | 14 | 16 |
| THS focus group | 6 | – |
| THS practices (n = 11) | – | 17 |
| South Yorkshire practices (n = 5) | 3 | 21 |
| Bristol practices (n = 4) | – | 8 |
| Internet advertisement | 14 | – |
| Support group advertisement | 1 | – |
| Total | 38 | 68 |
| Recruitment method | Age group (years), n females (n males) | |||||
|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70+ | |
| THS | – | – | – | 1 (0) | 1 (2) | 6 (4) |
| THS focus group | – | – | 1 (0) | 1 (0) | 1 (1) | 1 (1) |
| South Yorkshire | – | – | – | – | 3 (0) | – |
| Internet and support group | 1 (0) | 3 (1) | 2 (2) | 4 (1) | 1 (0) | – |
Interviews were digitally recorded and fully transcribed and these, together with observation notes, were analysed with the help of Atlas.ti software (version 6; ATLAS.ti Scientific Software Development GmbH, Berlin, Germany). Themes were developed during repeated readings of and reflections on transcripts and discussions between researchers. In addition, discussions of the work took place at regular meetings of the broader project management group. These discussions helped to shape and modify emerging themes throughout the period of fieldwork and analysis.
Results
Patients
Telehealth in action: receiving long-term condition support from Telehealth Scheme
Telehealth Scheme was a partnership between NHS Direct, a local PCT and a private company. In existence since 2006, at the time of this research 65 care workers were providing self-care support to approximately 8000 people with LTCs such as diabetes, CHD, COPD, stroke and chronic kidney disease. At this time, 36 general practices had signed up to THS, with their patients invited to take part in it. In this way, THS was meant to complement and add to the health care and advice already being provided by the patients’ GPs and practice nurses. The work was telephone based and THS shared premises with a NHS Direct call centre. Most THS care managers were qualified nurses and many had years of experience in front-line nursing. Alongside the care managers was a smaller team of health coaches, who had sports degrees or similar non-clinical qualifications.
Each THS care manager had his or her own case load of patients, who he or she telephoned about once a month according to a pre-arranged appointment. Care managers were tasked with working through eight levels of a programme with their patients (known as members). These were knowing when to call for help; learning about your condition; taking medication correctly; getting tests and services that are needed; taking steps to control the condition; making lifestyle changes to feel better; learning ways to solve problems; and keeping appointments.
After a lengthy initial assessment (two or more telephone sessions), the aim of which was to build up a detailed case history and drug regimen, THS care managers were prompted by computer software to work through the eight areas of work. The care managers had a degree of freedom to use their discretion and judgement to work through other areas of concern with their patients. Often calls would cover a number of topics ranging from condition-specific topics to those concerning family and social life. Many THS members suffered from a range of comorbidities that were not formally supported by the scheme and care managers often spent time discussing broader issues relating to patients’ illness burdens. Consequently, the work that went on was broader, ‘messier’ and less clearly defined than is set out in THS literature. Often, it was the less focused aspects of care managers’ work that were most highly valued by patients.
On completion of the eight areas of work, care managers could ‘step down’ patients to one of the health coaches who would contact them less frequently and would focus primarily on monitoring LTCs and advising on lifestyle. Care managers aimed to telephone their members by appointment once a month, but had flexibility to increase the frequency of calls during periods of greater patient need or crisis. Health coaches phoned their members every 3 months. Members were also able to telephone into the call centre, which operated from Monday to Friday from 09.00 to 20.00 hours. However, THS experienced a low level of incoming calls.
Valuing the personal relationships and rapport forged with their care managers
Teleheath Scheme care managers aimed to build rapport with their members and to encourage behaviour change by employing non-confrontational techniques of motivational interviewing. 240 Almost all of the patients interviewed said that they valued the relationship that had been built with their care manager. Respondents mentioned, with approval, that care managers did not ‘nag’, used humour, fostered informality and familiarity via the telephone and took on the role of confidante. Some said that they were able to confide health-care worries to their care manager in a way that they were not able to with their own family and friends:
I feel as though I could sort of tell her if I’ve got a problem er that I probably could not tell anyone else you know. ‘Cos I do not tell people when I’m ill or feel ill ‘cos I think it worries [husband] if I do, so I do not tell anybody. And I’ve got a daughter, I never tell her either . . . . I feel I can tell her [care manager] and I know she’s going to put me on the right track.
THSPAT3
They felt able to raise issues with their care manager that they reported that they were not afforded the time to do, or lacked the confidence to discuss, with their GP or consultant. The following respondent, who has multiple LTCs and needed to attend primary care and outpatient facilities on a regular basis, illustrates the point when talking about how her care manager had the time to help her to understand her test results and changes in her drug regimen:
I can understand it then, so it’s easier for me. Because they [other HCPs] don’t tell you. And they use these words where you don’t understand what the words mean.
Yes. And sometimes you feel awkward don’t you?
Exactly! So . . . you don’t want to ask because you feel stupid not knowing. So when something like that happens I can turn round and say it to her [care manager] and she’ll . . . tell me, in plain English that I can understand.
In addition to time, ease of communication was central. All of those interviewed were positive about the telephone relationships that had been built up over months and years. Some interviewees who were widowed and living alone spoke about the significance of simple human contact and the interaction that these telephone calls brought them. A number said that they just liked having access to perceived support – having the knowledge that there was ‘someone there’ to contact if needed. THS care managers generally ended conversations by checking that members had the THS telephone number and encouraging them to telephone if they had a problem. Interestingly, THS received very few incoming calls from patients and, of those incoming calls that did occur, most were requests to change telephone appointment times:
They say, you know, ‘now do not forget . . . if you want anything ring this number and we’ll . . .’ Now this is good. You know, that’s good. You do not feel isolated in any way and I think it is the feeling of isolation that causes such a lot of depression amongst older people.
THSPAT 8
Some of the formal Telehealth Scheme goals were being achieved with patients interviewed
Some patients reported that the time spent with THS care managers and the positive relationships forged resulted in positive changes in their lives. Some said that they now had a better understanding of their drug regimen and of their conditions. Others mentioned benefiting from condition-specific literature and other information that had been posted to them by care managers. Some positive engagement around lifestyle issues was reported, particularly around diet – losing weight and eating better. Positive motivation, the use of humour and the absence of nagging or preaching were valued by patients:
She can be on the phone for 10, 15 minutes, just talking to him, yes, they have a bit of a laugh and she’s, you know, she makes you feel like you, you can tell her anything. And you have come off and you have . . . you know, and if she had not have said what she did do . . . it would not have w[orked] . . . Well, I mean he’s lost a stone since he’s, since he’s doing what he’s doing.
THSPAT10b (interview with husband and wife who are both THS members)
Yes, it’s just helping me but it’s my choice, and if I say I do not want to, she does not . . . you know, if I say I’m not interested, she’ll go, ‘Okay, fair enough’. But she will not nag.
THSPAT9
The relevance of articulation work
Telehealth Scheme care managers regarded themselves as being at the centre of a web of resources for their patients and pointed out that a proportion of their work entailed signposting patients to other services and information. Care managers stated that part of their work involved ‘empowering’ their patients to become better managers of their own care. To this end, they tried to emphasise the signposting part of their role. However, many THS patients were elderly and suffering from a number of comorbidities and care managers often spent time facilitating care from other services rather than pointing patients to these services.
Articulation work concerns planning and co-ordination work. 241 This aspect of THS was greatly appreciated by patients, who recounted how their care managers had helped them to get appointments with dieticians and chiropodists and emergency appointments at their own general practices, got them into exercise classes and assisted in getting a blue badge for parking, personal alarms and support railings in the home and even simple pieces of equipment such as pill sorters. These kinds of things had made a positive impact on their quality of life:
I’ve got a chiropodist now, I go and have me feet done because with the, this is with the diabetes, and with me weight and me breathing, I could not cope, you know. And the nurse at the surgery thought I was coping, but I was not. So [care manager] said, ‘we’ll get you a . . . into a chiropodist . . .’, which I do go now.
Right, so did she sort that for you?
She sorted that for me. Yeah.
They got the phone put in, the emergency phone, my smoke alarms, they had those changed, those go through to the fire brigade if they go off.
THSPAT1
Patients also mentioned that conversations with their care manager helped them when they were feeling depressed or down and they appreciated being able to discuss emotional issues:
You know, they ask you if you’ve been depressed, and . . . and sometimes that’s nice of them to ask. Erm, and I just feel . . . I don’t really know I think . . . it makes you keep on top of things because she rings regularly.
THSPAT2
Recruitment methods into the Telehealth Scheme may give rise to confusion
At the time of this research, recruitment into THS was undertaken in a number of steps. First, the names of patients were taken from the appropriate disease registers at participating general practices. Practice staff then checked the lists to exclude patients who, in their view, should not be asked to join the scheme (e.g. those with a hearing impairment). Next, letters were sent to patients telling them that their GP believed that they could benefit from THS membership and advising them that they would be contacted by THS. The THS recruitment team then contacted patients by telephone to explain the scheme and invite patients to become members of the scheme. Finally, patients were sent an information pack and an appointment was made for an initial assessment with a care manager.
During observational work at the THS call centre time was spent with a member of the recruitment team. Many of those called said that they did not remember receiving a letter about THS from their GP surgery. Further down the line, when interviewing patients, some were unclear about why or how they had been recruited into the scheme:
We’d never heard anything about it, it was a surprise when they rang, you know. You think, oh . . . oh it’s funny sort of thing but they’re very nice, you know. But, er, that’s about it I suppose.
Was it the doctors that put these people onto us?
THSPAT6a and b (husband and wife members)
Some patients said that they did not remember receiving a letter from either their GP or THS. They did not understand what the scheme was about or why they were being called. They carried on receiving calls and making future appointments because they did not want to hurt the feelings of the care manager:
He’s not actually doing anything for me, no. No, he just chats on the other end of the phone. And, er, he’s quite all right, I mean I don’t mind him chatting.
Well I haven’t said I don’t want you to ring me again because I feel that’s very, very rude.
THSPAT4
For some, the recruitment call from THS was akin to commercial cold calling, making them suspicious about the service. Having observed the system at THS, it seems that information packs were being sent out and THS was being introduced via the telephone. However, a proportion of the material posted out suffered the fate of much commercial ‘junk’ mail and the initial telephone explanation may not have been fully understood or appreciated. Some of the patients interviewed, including those in the focus group, mentioned that they would favour recruitment through verbal recommendation from their GP.
Speaking the same language
A small minority of THS staff were multilingual. When care managers were able to speak to patients in their own language, this was greatly appreciated. A language barrier was cited by the focus group as the most important obstacle to engagement with THS among the South Asian community. This was particularly true for older women who were first-generation immigrants, who had never learnt English and whose access to services was often mediated by spouses and other family members. Those who attended the focus group did so on the basis of their relationship with their care manager who was fluent in Punjabi, Urdu and English. However, trained nurses who were multilingual and working in telehealth were in short supply. THS staff were able to use the translation service, LanguageLine, but this service, although useful, hindered the building of a personal rapport between care manager and patient and made the task of motivational interviewing extremely difficult.
On the topic of communication, and connected to the previous point about recruitment, South Asian focus group participants discussed reasons for the reluctance of the South Asian community to engage with THS. Aside from the language issue, they discussed the strong sense of privacy in the community and the desire to keep personal matters, including health matters, within the family. It was suggested that the GP is trusted almost in the same way as a family member is trusted:
Well we give it a name as a family doctor . . . so that’s part of . . . the family. [Laughter] . . . Not that closely, but it’s the trust. It’s that’s who we trust in.
Yeah, it’s the trust thing, you know.
For this reason, it was strongly felt that recruitment into potential telehealth services should be personally and verbally recommended and endorsed by GPs:
Because if you’re just a random person then you think should I join or not? But if it’s coming from the nurse or the GP, I think yes, it’s a good thing, it’s going to help.
Focus group member 3
Telehealth potential: patient views
Most of the patients interviewed in this section of the study were recruited through the advertisements that appeared on the websites of NHS Direct and NHS Choices and so, by definition, these people were internet users. This was in contrast to the THS patient group who, with the exception of one patient, did not use the internet directly, although some did ask family members to access information for them on the internet. As can be seen from Table 11, these respondents were not elderly like the THS interviewees, but were spread across a wide age range.
Gaps in service
In terms of clear statements of need, those patients with depression articulated most plainly gaps in provision of care. They spoke about being ‘left alone’ with their condition between visits to their GP. They were generally positive about their GP, but mentioned that time with them was limited whereas the time between GP visits was long. During these periods (in other words, most of the time), they felt alone and adrift:
For years, for quite a while, my only contact with the services was via your GP, and you know, after a while, you think, ‘Well I might as well just bloody suffer on my own’ because it’s really a bit academic. I make an appointment with my GP, and you see somebody for like, you know, 10, 15 minutes, whatever the allocation is . . . it doesn’t really make a huge amount of difference. Do you know what I mean? It’s just . . . you know, I didn’t get a review for ages, and you just feel, like, on your own.
Patient 1
Another area of concern appeared to be related to drug treatment as a first line of treatment. Many felt uneasy about taking antidepressants; they worried about side effects and about becoming dependent on drugs (this has been documented elsewhere; see Grime and Pollock242 and Khan et al. 243). Some also reflected on the ease with which antidepressants were dispensed:
Anyway I did stay on the medication for a while but probably not very long, probably not much more than about 3 months and I didn’t like it, I didn’t like the way that it made me feel, stopped taking it um and a couple of times I’ve been to the doctor since then and my daughter’s 19 now and I’ve been to the doctor since and sort of broached the thing of feeling very stressed and very upset and all the rest of it, and each time it’s been well we can give you this medication – and I really don’t want to go down that route.
Patient 9
As for non-drug therapy for depression, seven patients spoke about their experiences of counselling. Five of them said that they had found counselling to be useful and worthwhile. However, only two of the seven patients received counselling through the NHS (one of them received seven sessions after being hospitalised for attempted suicide), with the rest receiving counselling through work, training or private practice.
He [GP] said basically don’t use NHS counselling, it’s not available, you’ll be waiting 6, 7 months on a waiting list, it’s too long. So obviously there was a counsellor at university, I went to see them.
Patient 11
In terms of CBT, only patient 1, who had a diagnosis of clinical depression and social phobia and had been a psychiatric service user for > 15 years, reported receiving any CBT. Two others spoke about being on a waiting list for CBT:
Um, and I don’t even know whether he’s sent off for it because we were on the subject of, you know, me the antidepressants and . . . I don’t even know if . . . he said it’s about a 16-week waiting list, but I don’t know whether he’s sent off for it, but I will ask him next time whether he did send off.
Patient 12
None of the others had been offered any form of CBT, either face-to-face or online, even though some lived in PCT areas where computerised CBT on prescription was available.
Unlike the patients living with depression, the gaps in service articulated by those considered at risk of CVD were not well defined. This was probably connected to the hypothetical nature of being ‘at risk’, with interviewees feeling that aspects of their lifestyle required change, but at the same time they felt healthy. As has been pointed out elsewhere,244,245 the often random occurrence of death from heart attack can contribute to a fatalistic approach to the idea of being ‘at risk of CVD’. Those who are in work and feeling well have little motivation to link identification of a problem, such as hypertension or high cholesterol, to lifestyle change. This is illustrated by patient 5, who was aged 45 years, was overweight, had a sedentary job, had a family history of hypertension, heart attack and stroke and was on medication for high blood pressure:
Well this is the problem with, um, with blood pressure. You do feel great, until you have a brain haemorrhage or a stroke or a heart attack. My doctor’s explained all this to me, you know. You will, you’ll feel fine, you won’t feel any different and that’s the problem with blood pressure. People don’t feel any different I suppose. But I keep taking the tablet again. There’s no side effects so far and I try to control my weight. I haven’t put any weight on for about 5 years, you know. I know that I could do with losing weight but . . .
Patient 5
Those in work and feeling well indicated that they would welcome initiatives that would fit in with their working lives, for example health screening in the workplace, which could then be linked to a telehealth intervention, including the use of mobile telephone applications. This is consonant with observations made by May et al. 246 that the treatment of LTCs should be aimed at minimising the burden and the disruption in the lives of patients.
It should also be mentioned that there are a number of examples in the interviews of how CVD risk and depression are intertwined. Patient 10 and patient 18 gave vivid accounts of how the loss of self that was associated with the onset of CVD led to depression. Patient 10 lived alone, led a solitary life and was dependent on sickness benefits. He explained how he felt that he had little reason to give up on the pleasures afforded by smoking, drinking alcohol and eating his favourite (high-cholesterol) foods:
If I give up smoking, and all drinking and all the rest of it, there’s nothing to replace it with. I mean, there’s no quality of life. I mean, you know, I mean that’s a part of my life sort of thing. Um, what do I replace it with? Um, what’s the point?
Patient 10
Likewise, for some of the THS patients, CVD and/or loneliness were intertwined with depression.
Finally, when discussing gaps in service that could be addressed by telehealth, it is probably useful to recognise that there is a hierarchy of need among patients. At the top of this hierarchy are those who have no or little family support and are socially isolated, as well as those patients with complex, multiple LTCs and those who live far from metropolitan centres or who have difficulties accessing mainstream health care.
Telephone-based support viewed as reducing isolation
The idea of receiving telephone-based support for depression was viewed positively by respondents. Depression patients liked the idea of having contact with a named care worker and of hearing a human voice. Some mentioned that, at times, being depressed made it difficult for them to leave the house, leading to social isolation and the feeling that they did not wish to be seen by anyone:
I think I would be better talking to somebody on the phone than in person, probably.
Patient 6
The notion of establishing a certain level of intimacy, but with a degree of distance via the telephone, was viewed as appealing, as was the idea that there was ‘someone there’ who cared and who could be contacted at times of crisis:
I think it’s good, I really do, because I know when I’m alone, the fact that somebody bothers to ring me, you know, I put out of my mind the fact that they might be paid to do that, the fact that somebody takes an interest in me is important, somebody cares.
Patient 4
Attitudes towards telephone support for lifestyle change were more ambivalent. There was the suggestion that this kind of contact could be useful when patients had already identified a specific goal:
There are certain types where it is appropriate, there are certain things if you’ve got a very clear objective like giving up smoking . . . or having a healthier lifestyle then yes you can address that by phone.
Patient 13
However, patients objected to being nagged or hectored and did not like the idea of being ‘nannied’ or ‘motivated’ to achieve lifestyle change.
Help from internet-based support
As mentioned earlier, most of the patients who were interviewed were, by definition, internet users as they had been recruited through an online advertisement (see Table 11). They were enthusiastic about the idea of receiving online support both for depression and for lifestyle change. The internet was seen as a powerful source of health information. For those living alone, the internet was described as a ‘window’ into the world, providing information, reassurance and a means of communication. In terms of information, a key issue was trust. Patients recognised that the internet could be a source of misinformation, of overwhelming volumes of material and of commercially biased information. Although respondents were eager to discover what fellow sufferers were posting on the internet, trepidation was expressed about exposing oneself by posting online. On the subject of reassurance, those sites that included accounts of sufferers – their experiences and how they had overcome them – were viewed as valuable. This accords with the findings of research on the significance of bibliotherapy in supporting self-care of LTCs. 247
Yeah it always helpful just seeing somebody else and you know that other people have the same thing but it is always, it’s good to see somebody who is like relatively normal in a sense, you know, because you sort of tend to ‘oh well I’m the only one and it’s me that’s there something wrong with’.
Patient 9
I think the NHS Choices site is generally very helpful, I like the fact that they’ve expanded it now and that you can get an expert’s view, I like the, sort of, videos, and things, they’re very helpful and, sort of, case studies, and things, so I think, you know, it’s become more real, the advice seems more real now.
Patient 6
The patients interviewed favoured ‘safe’ sites when seeking health-care information, sites that they trusted that were non-commercial, such as NHS sites and sites run by charitable organisations such as Mind [www.mind.org.uk (accessed 9 September 2016)].
I kind of started off with British, eh British Heart Foundation . . . cause they don’t really have an agenda as such, you know, they haven’t got an additional, I don’t feel like they’ve got any reason to misinform cause there’s so much misinformation and nonsense . . . on the internet as well.
Patient 7
For those in employment, the idea of accessing health care via the internet was viewed as being compatible with a busy lifestyle. Respondents were interested in the notion of computerised CBT, although some stressed that they would like some human interaction alongside computer work. Such interaction need not be face-to-face, but could take place over the telephone or by e-mail. It was perceived that such responses would be rapid, unlike lengthy waits associated with attending GP surgeries.
Trust in NHS Direct and NHS Choices
Most of those interviewed had used the NHS Direct telephone service, the NHS Direct online service or the other main NHS website for patients, NHS Choices. All responded positively about their experiences of using these services. Respondents felt that the telephone service had provided reassurance and good advice. The fact that these services were run by the NHS was seen as important:
I’ve phoned them, maybe once or twice myself, and my wife’s phoned them before . . . and it’s quite a good first port of call really. Because if it’s nothing to worry about, then you don’t want to waste the time of the doctor or the hospital.
Patient 3
It’s a very strong brand, you know, I know everybody sort of kicks the NHS but I don’t think really it can be beaten, when you come down to it and you see what happens in other countries.
Patient 2
NHS online resources were regarded as being safe and trustworthy and, by implication, the links from NHS sites were also trusted by patients:
I tend to go straight to the NHS one. Because I find quite a lot I look at, they’re just trying to sell you something anyway. So I don’t even bother now, now that I’ve found this, I would look at, straight at the NHS for information.
Patient 8
Health-care professionals
Telehealth Scheme care managers
The niche role of Telehealth Scheme care managers
A significant proportion of THS nursing staff had previously held senior NHS posts. Many had chosen to leave positions of significant responsibility, having taken cuts in pay and grade to work at THS. Reasons for career change ranged from feeling ‘burnt out’ by the stress of high-level front-line nursing and feeling worn out by years of work on wards, which included hard physical labour, and the desire to work regular hours to balance working with family life. Employment at THS provided an opportunity to continue a nursing career that otherwise might have ended. Nurses bring with them a wealth and diversity of experience.
Telehealth Scheme staff underwent in-house training and work at the call centre was monitored. As described in Telehealth in action: receiving long-term condition support from Telehealth Scheme, care managers worked through a programme of activity with their patients and, although they were prompted in this work by computer software, they had autonomy to move away from structured work to discuss other areas of concern with their patients. The shaping or ‘taming’ of technology in the practice of health care to suit the needs of patients and practitioners has been described elsewhere,225 but it does require a specific set of new working practices as well as building on existing health-care work. 229 This was evident here.
Telehealth Scheme care managers were trained in ‘motivational interviewing’240 and, when discussing their work, they referred to the technique of ‘rolling with resistance’. They noted that this non-confrontational approach is contrary to their traditional nursing training and that their THS training had obliged them to reorient their thinking.
When I first arrived here there was a collection of us who just thought, how is this going to work? How are we going to empower these patients from deprived areas that are used to the prescriptive approach, to using motivational interviewing; to encourage them to make informed decisions for their own well-being; health and well-being? And we did it. I just can’t believe it. I think a lot more motivational interviewing could take place on the shop floor. I think there’s still, as nurses we’re a culture where we do try and problem solve for people, that’s our make-up.
THS7
They also believed that much could be achieved over the telephone and that, given time, relationships of warmth and trust could be built between care managers and their patients. Some felt that the lack of visual clues and physical contact in their interactions with patients obliged them to become more careful and attentive listeners. Care managers worked hard at establishing and building rapport with their patients, carefully making notes about family relationships and personal events for reference in future conversations. These contextual notes would be read through alongside clinical notes before each new telephone appointment began. Humour was often a feature of these conversations, as well as an informal manner, so that care managers tended to speak in the same idiom as their patients. The importance of understanding lay knowledge and lay idiom for promoting health education is discussed by Hunt and Emslie. 245 This was particularly marked in those instances in which South Asian patients were served by a care manager who spoke their language and shared a cultural background with them.
When a new patient was recruited to THS, a lengthy ‘initial assessment’ took place that was guided and shaped by computer software templates. Most care managers chose to carry out this assessment over two or more telephone appointments and were careful to leaven the rather tedious assessment with general conversation and to work on relationship building with their patients. Although the work of care managers was strongly focused on the eight areas of support for certain LTCs described previously, care managers also emphasised the importance of ‘general’ caring work:
They want to feel safe, they want to feel that yes definitely you’re there for them, you’re concerned, not only about the diabetes, or you call them, ‘Oh, I’m having problems with the . . .’. The last member I had, ‘I’m having a problem with my son’, there are so many issues going on at home, so you step into that, trying to help.
THS6
Care managers saw themselves as being at the centre of a web of resources, in a position to signpost their patients to relevant social services and NHS services as well as charities and community organisations. Most importantly, care managers repeatedly mentioned that a key resource that they were able to offer patients was time. Time is important for individual conversations, but THS staff also stressed that building rapport and confidence to facilitate behaviour change takes place over a long period of time:
So you might not get a behavioural change and it might possibly be a pre-consultation for 6 months and for a GP to say: ‘Well what have they been doing with our patients?’ That could be rapport building and then suddenly – you just jot things down about the name of the dog, the name of the son or something just to get the rapport and the relationship building – and then suddenly out of the blue the patient will say, ‘Now what was it you said about cholesterol levels?’ And that could be 6 months; could be 12 months down the line.
THS7
Care managers believed that they had an important role to play in addressing depression with their patients. Both patients and THS staff voiced the opinion that GPs and practice nurses had limited time to spend with depressed patients. 248 Likewise, working on lifestyle changes required a lot of time. THS staff argued that (1) they were able to spend time on individual calls and (2) bringing about change in self-management and lifestyle behaviours was a long-term project:
It is a luxury I think this service is; we have got the time you know, we could be on the phone an hour, an hour-and-a-half and for us that is fantastic, the patient is fantastic. The surgeries out there haven’t got that luxury.
THS7
Professional intercommunications and other problems
Care managers ideally made quarterly visits to their assigned general practices with the aim of informing them about THS work, reviewing THS enrolment and airing problems. A recurrent theme from many care managers was that practice nurses and GPs were resistant to the work being carried out at THS and viewed THS as a threat. They felt that at times they were received with a certain amount of hostility when they went out on visits to practices and were of the opinion that many participating practices were either unaware or uninterested in the work of THS. Between care managers and practice nurses there appeared to be tensions over role and jurisdiction234 and between care managers and GPs there were tensions over the credibility of THS:
In my experience when I’ve gone to the surgery, they [practice nurses] don’t even want to see me because they think I’ve taken over their role, or I’m going to create more work for them because one of the things we educate the patient to do is go back and ask questions, you know, prepare them for their appointments, and also we send them with a hand-held record . . . So I try and encourage them to take that in and to fill in the record with any changes in the blood test result and the medication. Nurses don’t like that because I’m creating extra work for them. So I only ever get to see them the first time I went on the practice visit, and since then I only ever see the practice managers. I’ve yet to meet any of my doctors.
THS9
There was no formalised system of communication between THS and the participating general practices to exchange information about individual patients. Care managers had access to limited areas of their members’ patient record that pertained to cholesterol readings, blood pressure readings, body mass index (BMI) and other similar clinical measures and practices were sent updates on patient enrolment into the scheme. Concerns about the welfare of individual patients could be raised by care managers, but this may or may not have been welcomed in the participating practices. The latter could sometimes lead to conflicting health-care advice being given to patients, adding to the potential for tension between THS and practices.
The more the GPs’ surgeries understand about us . . . the better, it builds bridges really and things improve. But it is very difficult because sometimes we will phone the surgery and we will want to speak to a GP about a concern, but they are not always, you know, happy to discuss things . . . or you know: ‘Why are you phoning me for this reason?’
THS10
Linked to the issue of communication was the feeling that neither the public nor general practices were well informed about the service. Care managers felt frustration that both professionals and patients were ignorant about the work of THS, which had a bearing on recruitment into the scheme, which became more challenging if potential members (i.e. patients) had never heard of THS. The personal endorsement of GPs was thought to be important for successful recruitment:
I think that personal touch from their GP in the first instance is enough for a lot of people to say actually this sounds quite good, my GP’s recommended it then obviously I’m happy to join.
THS8
At the time of this research, patients were recruited into the scheme through the disease registers at participating general practices. After the practices had excluded the names of patients for whom recruitment into THS was not suitable (e.g. patients with hearing difficulties), a letter was sent to potential recruits. The recruitment team at THS subsequently telephoned potential members, explaining about the service, and, if patients were agreeable, sent out information packs and assigned them to a care manager. This system was designed to bring people with LTCs into the service in numbers, but did not discriminate between patients. Thus, it was possible to recruit into the scheme those who may be long-standing, well-controlled diabetics or those who were equivocal about membership or who did not fully understand why they had been recruited:
The word needs spreading, yeah, definitely. Because also you know, if it’s not a well-established and well aware . . . people are not well aware of this programme then all of a sudden out of the blue they’ve got this perhaps threatening figure wants to talk to them about their health and how to make changes. Why me, why have they picked me but if it was a well-known thing then it perhaps take away that sort of, intimidation factor as well.
THS13
These factors, in turn, contributed to a waste of resources when care managers spent time talking to people who were well informed, in control of their LTCs, or spent time pursuing patients who were not interested in the service.
Those care managers interviewed were positive about their role. They believed that they were making an important contribution to the support of patients with LTCs and enjoyed the convivial working atmosphere of the THS workplace. There were some frustrations about time wasted on missed appointments and as a result of cumbersome software. Many found ways to adapt their work to the computer software system, but pointed out that time spent doing this could be better spent talking to patients:
It’s so slow, and it wastes so much time in the day, while you’re watching that little thing go to and fro while it sorts itself out, and then you have to put the contacts into the diary and that takes ages.
THS4
I get a lot of frustration with the software itself. I think the majority of us do feel that, that slows us down quite a lot, and I don’t think it actually captures what we do in the day-to-day.
THS9
I’ll guarantee everybody said this, the software’s useless. The software’s not designed for the programme, is the problem, and the software is slow and cumbersome. I mean, we’re running four programmes, you know, to contact and notify and make note of all our conversations. It’s too slow. Too slow.
THS13
Finally, some respondents were concerned about the open-ended nature of their work. They noted that, at the start of the THS programme, patients were ‘graduated’ from the scheme once all eight areas of THS work had been completed. Subsequently, patients were kept on the scheme with the option to ‘step them down’ to health coaches, who contacted patients less frequently. Some care managers worried that such an arrangement could foster dependency rather than transitioning them to take charge of self-management of their LTCs.
Views from the practices
Practices participating in the Telehealth Scheme
Communication and evidence
The impact of THS services was not felt to be particularly salient in participating general practices. Those interviewed knew about the way that THS recruited patients because this required occasional access to the computer systems in practices. The frustrations voiced by THS care managers about the lack of communication with their practice-based counterparts were mirrored here. Staff in the general practices said that they were largely unaware of THS work and felt that it had little impact on patients. Attitudes to the service were indifferent or negative and the lack of communication about patient care was also highlighted by practice-based HCPs:
The meetings [with THS care managers] will come and say, ‘You’ve got X amount of patients to be looked at, if you want to put them in the system,’ which we do – end of story, don’t really know what goes on after that. You know, we’ve got no feedback from . . . patients.
THSPRACT4, practice nurse
We don’t want any contact with them, [laughter] with a service that we think is useless.
THSPRACT2, GP
Ambivalent or negative feelings about THS were related to unease over whether or not the service was actually working. Questions were asked about outcomes and evidence and whether or not expenditure on THS represented a good use of public money. Some, who had been keen on the scheme at its outset, now worried about the lack of published results and voiced unease about continuing support for a service for which they believed there was no hard evidence of its efficacy:
Our PCT recently wrote to us and said, what would you like THS to do next? And they were talking about arthritis, management of depression, other things. And although I’m a great supporter of the individual nurses, I think they’re, you know, they’re great, I haven’t seen any data, there is no data.
THSPRACT9, GP
So for us the outcomes are really important, the impact of using . . . THS people . . . or NHS Direct is really, really important. We would like to know erm, because if we’re in the future going to be in a position [to commission services], because the political scene’s changing . . . then er, we need to have that kind of confidence in them.
THSPRACT4, practice manager
Questioning the role of the Telehealth Scheme
In addition to concerns about the lack of evidence of the efficacy of THS, anxieties were raised about whether or not THS was duplicating services already being offered in general practices and other organisations. Practices indicated that they already run their own smoking cessation services, that practice nurses give dietary advice and that some practices make use of community psychiatric nurses. It was suggested that some patients, for example those with diabetes, may, at times, feel themselves to be bombarded with a plethora of services from both primary and secondary care providers. Practice nurses said that they already undertake monitoring for LTCs and care of LTCs, indicating that the THS overlaps or duplicates work that they are already doing:
THS, from what I can gather, they ring the patient up, say, ‘how have you been this month?’ From what I can gather, from what . . . what I’ve heard in the past, erm, and, ‘what’s your blood pressure reading? What’s your, your glucose or what’s your cholesterol?’ And they’re just asking these questions, and they’ve already had it done, because we’ve done it and we’ve monitored. So what else can they do for them?
THSPRACT4, practice nurse
Although none of the interviewees in the practices felt that THS had thus far made a significant impact on their patients, the future potential for the service was seen differently. Practices situated in the more affluent areas believed that THS was superfluous for their patients and tended to cater to the needs of the ‘worried well’. Conversely, some working in economically deprived areas felt overburdened and commented that they would welcome support in their work, but were, to date, not aware of any impact of THS activities on their workload:
When a patient comes and they’ve got problems, you’ve got so many things you need to do for them . . . it takes you away from other things or you end up working overtime . . . that you don’t get paid for . . . to sort these people out, . . . it’s time-consuming so it would be nice to have some kind of help . . . perhaps they’ve [THS] produced a plan when they’ve seen the patient, do a little plan, a care plan . . . and so that we know what’s happening.
THSPRACT3, practice nurse
The main areas identified by practice staff as gaps in service where help was needed were depression, alcohol dependency and dietary support.
Telehealth Scheme care managers attached importance to their caring role, believing in the value of time spent talking to their patients, building trusting and empathetic relationships. This aspect of their role was not necessarily valued in the participating general practices, where it tended to be dismissed as ‘just talk’, something that was ‘nice’ but not necessarily important. They questioned whether or not it was the job of qualified nurses to be ‘chatting’ to patients and whether or not this was the best use of NHS resources:
Every now and again they get a phone call from them saying, ‘how are you?’ And they go, ‘oh, fine thanks’. Or they might go, ‘well, I feel a bit low’. Oh, hang on, there’s somebody else I can talk to for an hour here, you know, I’ve got all these aches and pains and blah-blah-blah.
THSPRACT1, practice nurse
What benefit it’s actually giving, other than somebody is ringing them up and they are having a chat? Because they may be elderly, there may be some other system that they could get for these lonely people, but I think this a very expensive way of looking after these people.
THSPRACT2, practice nurse
You find that they [patients] love it, and they love it partly because it’s a voice on the other end of the line, that’s the other thing you’ve got to remember. Sometimes it’s the placebo effect is huge because somebody is phoning them, in their own home and they have maybe not seen somebody all day, or all week.
THSPRACT11, GP
This last quote was from a GP who was enthusiastic about THS. Nevertheless, there is a suggestion that the benefits gained from the social interaction over the telephone were just ‘placebo’ effects, in contrast to the ‘real’ work of HCPs.
Attitudes towards NHS Direct
When asked specifically about their views of NHS Direct (as distinct from THS), health professionals in the THS practices tended to be neutral or negative. Negative views of the service were often strongly held and positive views tended to be based on positive personal experiences, for example a practice nurse telephoning the service for advice about her baby. The sentiment that NHS Direct simply redirected patients to GP practices or hospital accident and emergency departments was strongly voiced. Views such as ‘it’s rubbish’, ‘it’s a waste of time’, ‘waste of money’ or ‘should be called NHS Redirect’ were forcefully expressed:
They just send you to hospital. They don’t want to know. It’s rubbish.
THSPRACT3, practice nurse
It might help some, but I haven’t come across an awful lot that it’s helped because they might have a problem, and they’ll say, ‘well, I rang NHS Direct and they told me to come and see you’. You know, that’s a very big problem.
THSPRACT4, practice nurse
Patients who’ve rang just to get a bit of advice . . . they said it, they’ve found it quite useful, ‘cause I think they find that more useful than the out of hours that we have because our out of hours is with XXX and they, they don’t like it.
THSPRACT5, practice manager
Practices in south Yorkshire and the wider Bristol area
Although interviews at THS practices focused on an existing scheme, the conversations in the South Yorkshire and wider Bristol practices concentrated on ideas of how telehealth could enhance future LTC care and management. HCPs were asked about current LTC care, perceived gaps in current care and views on telephone and internet support and on NHS Direct.
Co-ordination of care for long-term conditions
As was the case in the THS practices, the bulk of routine care for LTCs, as well as screening for LTCs, was undertaken by practice nurses. Commentators have noted the way in which practice nurses have carved out this particular niche of professional work for themselves234,237 and the practice nurses interviewed here stressed that LTC management was largely their domain of care:
I would say quite a large part of my role is long-term management now.
BrisPract4, practice nurse
So basically that’s the whole of my job is looking after people with chronic disease.
BrisPrac2, practice nurse
I think the vast majority of my work is lifestyle based quite frankly and chronic disease management.
SYPract4, practice nurse
In most practices, nurses tried to see their LTC patients twice a year for routine checks and were available for consultations in between these times. Some specialised in particular areas, such as COPD or diabetes, and some ran clinics aimed at targeting specific issues, such as smoking cessation or weight reduction. Some also undertook routine health checks and screening for CVD. In a few instances, they reported that some routine work was carried out over the telephone.
General practitioners, on the other hand, acknowledged that they no longer do much LTC work. In their view, the role of the GP was to concentrate on complex issues and to attend to patients with LTCs when they are experiencing problems or changes in their condition:
We, as GPs, maybe sort of only tend to get involved at the peripheries of some of these long-term conditions, erm, you know, when there’s treatment changes or difficult decisions to make about the treatments.
SYPract2, GP
We don’t, as GPs, do an awful lot of the long-term chronic conditions to be honest. If we’re talking about monitoring as opposed to managing, the nurses do a lot of monitoring for the, you know, diabetes, renal, ischaemic heart disease, you know, COPD. Erm, we tend to get involved if there are changes in the monitoring . . . if something in the monitoring shows there might be a change that’s medically needed, we don’t always get involved because the nurses often are very skilled and can make decisions.
BrisPract1, GP
General practitioners see themselves as being at the centre of patient care, describing themselves as ‘guardians’ of care or as ‘gatekeepers’. Most GPs interviewed were positive about the potential for telehealth interventions, but were keen to stress that general practices should remain at the centre of patient care. They felt that it was important that general practices be kept informed about and be part of a communication loop with any new telehealth intervention because they believe that, ultimately, the responsibility for patient care lies with them.
Both GPs and practices nurses were concerned that clear systems of communication be part of any new intervention, so that practice-based HCPs would be fully informed about the contents of a telehealth intervention and would provide treatment feedback for each patient (with regard to work carried out and referrals made). They were also concerned that care for patients should be ‘seamless’, in other words, that patients should receive consistent health-care messages from their general practice and from any new telehealth intervention:
What we don’t want is to develop different care tiers. We need to know exactly what is being delivered by other services involved in the care of those patients. And we need to be the ring holder of that care, so that everything comes back to us, and that we then know who’s doing what, when they’re doing it, and who’s accountable for what.
SYPract1, practice nurse
Concern was also raised about the potential of a new intervention to duplicate the work of existing services both inside and outside of general practices. Practice nurses, in particular, pointed out that they already undertake a great deal of support work for patients with LTCs and were anxious that resources should not be deflected away from existing services. This sentiment was expressed most bluntly by a practice nurse who felt that telehealth initiatives could potentially undermine the role of practice nurses:
Is it just you’re thinking that the practice won’t need practice nurses any more, they’ll just have people take blood and check blood pressures and then I’ll be made unemployed? Is that what you’re thinking? Is it a cost cutting exercise?
BrisPract2, practice nurse
In a similar vein, the GP quoted below wondered whether or not general practices would be interested in commissioning potential new telehealth services:
I think a lot of the stuff with ischaemic heart disease, and things, we do that anyway. And it’s all just, you know, part of what we’re already doing. Commissioning a further service to repeat it, I think is going to struggle to, you know, find the . . . come up with the money to do something that we already do.
BrisPract3, GP
Targeting a new intervention
From the concerns about duplication of services emerged a strong sense that any new proposed telehealth intervention needs to be carefully targeted to areas of greatest need. HCPs had three main suggestions for successfully introducing any new telehealth intervention into primary care. First, HCPs mentioned that they need to be convinced that a new telehealth intervention will be adding to existing services, rather than duplicating them:
So in terms of sort of supporting long-time conditions, what kind of things, on top of that, what added value would it be giving?
SYPract1, practice nurse
Second, the new intervention should be evidence based, cost-effective and clearly targeted:
Well I’d need to see evidence of effectiveness and, umm, and, cost-effectiveness, and so, umm [pause], umm, whatever the telehealth thing that was doing would have to be part of what we considered to be one of our objectives, and so you’d have to be relevant to our population.
BrisPract4, GP
This last point should be noted as ‘relevance’ to the general practice population in question, which certainly differs from one area to another and is linked to factors such as age distribution, wealth distribution and ethnic diversity.
The third suggestion provided was that any new intervention should be ‘QOF (Quality and Outcomes Framework) friendly’ [see http://digital.nhs.uk/qof (accessed 19 September 2016)], so that, at best, participation in a telehealth initiative should add to a practice’s QOF score or at least should not detract from a practice’s QOF rating:
I mean how would I know my patient had got the . . . primary prevention from you, and then I need to see them anyway ‘cos I need to get my QOF point and will . . . will I get my QOF point because you’ve seen them?
BristPract2, practice nurse
The majority of those interviewed mentioned the need for support for patients with depression and other mental health needs. Greater access to referral for talking therapies and CBT, both offline and online, support for patients with alcohol abuse problems and more options to be able to refer patients to exercise programmes were also mentioned. A number of interviewees said that their practices were already engaged in smoking cessation and weight loss support.
Attitudes towards telephone and internet-based support
Practices already carrying out telephone consultations were familiar with this mode of health-care delivery. Interviewees were generally comfortable with the idea of telephone-based health support, although there was a feeling that using the telephone is second best compared with face-to-face contact with a HCP:
We use telephone consultation a lot . . . in our practice, we have, er, one doctor doing the whole . . . doing a whole morning of telephone consultation, um, but I . . . I’m constantly being reminded that you don’t always get the full picture over the phone . . . you know, you’ve a gut instinct and you think, well I’d better just see this person, and they come in and you think, yeah, just as well.
SYPract5, GP
Issues that have been touched on earlier were also discussed here: the need to be careful not to confuse telehealth calls with cold calling, the need to build and maintain trusting relationships over the telephone in keeping with the relationships of trust between general practices and their patients and the importance of continuity of care, so that a patient speaks to the same telehealth-care worker over time.
Attitudes towards the idea of using internet resources to support patient care differed according to the type of population being served. In a small, rural practice serving a wealthy area, HCPs were using a number of computer-based aids and were directing patients to the internet:
I think we significantly, you know, patronise and underestimate the abilities of every patient, you know, person with a long-term condition. You know, they know they’re living with it and, you know, their grandchildren, you know, technology is absolutely part of all their lives. So I’ve no qualms at all about giving an iPad to a 75-year-old and asking them to create their care plan.
BrisPract1, GP
On the other hand, staff based in economically deprived areas were doubtful that their patients would have access to a computer:
The population is such that quite a number of them wouldn’t have access to that service [the internet] . . . in the first place, anyway, so . . . to some it would be useful, to a large number it wouldn’t.
SYPract3, GP
The idea of the importance of interpersonal contact between health-care supporter and patient was emphasised, with doubts being raised about the utility of internet-only support, particularly for those with depression.
Doubts about the NHS Direct ‘brand’
As with the THS practices, opinions voiced here about NHS Direct were generally negative. Some interviewees voiced the concern that NHS Direct is algorithm driven and rigid and wondered whether or not a new telehealth intervention would be similarly rigid and limited. It was also felt that NHS Direct has not been properly evaluated, is not cost-effective and is a waste of money. In particular, NHS Direct was associated in the minds of many interviewees with the burgeoning of a whole host of out-of-hours services, which, according to some, had led to duplication and ‘chaos’. The message was that associations of a new service with the name of NHS Direct would not necessarily be positive in the eyes of many HCPs:
Yeah, the name, I think the name would, personally, would have to change . . . yeah it has connotations.
SYPract2, GP
However, there were positive comments made about the online health information resources provided by NHS Direct as well as NHS Choices.
Discussion
A number of important themes emerged from the material presented here, which will hopefully be useful in the consideration and shaping of future telehealth interventions. These themes are identified in the following sections and supported by a discussion of the results obtained that underpin them.
Trust and confidence in proposed new systems
It became apparent that trust, as the basis of engagement, was important on a number of levels. THS care managers emphasised that their work was based on a foundation of trust. This was built over time as a consequence of careful nurturing of the relationships between care managers and their patients. THS patients appreciated the informal nature of the communications between them and their care manager and care managers were often placed in the role of confidante.
The telephone and internet are mediums of communication that are both associated with suspicion rather than trust. Telephone subscribers have become accustomed to cold calling and telesales and, likewise, the internet is associated not only with advertising but also with fraud, identity theft and the dissemination of misinformation. Any new telehealth initiative will need to allay anxieties and suspicions associated with its means of delivery. The idea that a new intervention should be personally endorsed by GPs and practice nurses seems to be a simple and practical suggestion in this regard.
It was very clear from the interviews that patients trust the NHS, NHS Direct, NHS Choices and internet sites that are linked to NHS sites. Patients felt reassured that NHS services would not be commercial and would be neutral sources of information. The idea of NHS Direct supporting the management of LTCs was positively viewed by patients. In contrast, HCPs’ views of NHS Direct tended to be negative. This clearly has implications for the branding of any new service. For patients, the inclusion of the name ‘NHS’ will be positive and will inspire confidence, whereas for many HCPs the association of a new service with NHS Direct is likely to be a deterrent.
Linked to trust is confidence that a proposed new service will actually work. Patients may need the endorsement of primary HCPs to begin the process of engagement with new services. HCPs need to be convinced that (1) there is evidence of efficacy of telehealth interventions and that telehealth will add something to patient care; (2) telehealth is cost-effective and will be compatible with the existing QOF scoring system; and (3) telehealth will not simply duplicate services that are already available. On this last point, it is important to note that practice nurses undertake much of the work associated with support of patients with LTCs. Telehealth interventions should therefore be orientated to the existing work of practice nurses. There is a potential for practice nurses to feel that areas of their work will be duplicated or replaced by a new telehealth service. In the changing landscape of the NHS, GPs will be the commissioners of telehealth services, illuminating questions about efficacy, cost-effectiveness and duplication.
An element for building trust, and one that was lacking in the THS operation, involves fostering clear channels of communication between staff in general practices and those running a telehealth intervention. At THS, the lack of such communication led to frustration on the part of care managers and to a mutual lack of appreciation and co-ordination of the work being carried out to support patients with LTCs. This was true both in the practices and at THS. The aims and contents of a potential telehealth intervention need to be apparent to those working in general practices, alongside clear channels of communication to avoid duplication and the giving of mixed messages to patients.
The exemplar conditions and areas of need
The clear message from both HCPs and patients was that support for patients with depression is a pressing need. Patients voiced anxiety about being on drug treatment for depression and felt that they needed time and non-drug therapy. There are long waiting lists for talking therapies and variability of access in different areas. Many interviewees were positive about the idea of delivering psychological therapies by telephone or via the internet, with the caveat that some personalised contact be included. It was clear in the case of the delivery of a package of care over the internet that both patients and HCPs thought that some kind of human interaction was necessary, whether this be face-to-face, over the telephone, using a webcam, using webchat or by e-mail.
The picture was less clear for those at risk of CVD and this was connected to the complex nature of understanding what it means to be ‘at risk’. Nevertheless, telehealth has the potential to promote lifestyle change. Diet and weight loss were identified by some patients as areas where support would be welcomed. HCPs also mentioned this and pointed to the lack of support for alcohol dependency, in contrast to a plethora of initiatives in the area of smoking cessation.
The promotion of lifestyle change risks encroaching on areas of life that many regard as being personal, such as dietary habits, daily routine and alcohol consumption. The idea of being ‘nagged’, ‘lectured’ or ‘nannied’ was not welcomed, but it was also identified as the basis of approaches and information currently on offer. THS patients pointed out that their care managers did not nag them and that they appreciated this. THS care managers were trained in motivational interviewing techniques and consciously avoided a lecturing style in their attempts to promote lifestyle change.
The importance of context and targeting
Telehealth has the potential to reach and support patients whose access to conventional health services may be difficult or limited. According to the Office for National Statistics, most households in the UK (88%) have a telephone and most (73%) have an internet connection (the most recent figures at the time of writing refer to 2010). 249 However, at the time of the study there was a disparity between the highest decile income group, with 97% accessing the internet, and the lowest decile income group, in which only 69% have an internet connection. 249 The Office for National Statistics reported in 2010 that 60% of those aged > 65 years had never accessed the internet, although this is rapidly changing. 249 These data correspond with the interview material cited here, with older patients and those living in less affluent areas not routinely using the internet. Thus, accessibility to the medium through which telehealth is to be delivered has to be an important consideration when developing a new intervention.
In addition to thinking about the means of delivering telehealth, it is also relevant to consider that the burden of LTCs and care and support for LTCs is not experienced uniformly. The patients and HCPs interviewed for this study raised a variety of context-specific issues. For those living far from metropolitan centres, telehealth offers the possibility of increased access to specialist services, such as psychological therapy. Likewise, telehealth can also increase access to care and support for isolated frail and elderly patients and the current THS service appeared to be particularly well received by older patients. However, the health-care challenges facing residents within metropolitan areas can vary dramatically from one residential area to the next. HCPs in some of the THS practices described problems of drug and alcohol dependency in areas where there was also a high dependency on income support. Incentives for maintaining claims to illness are shaped and reinforced by the current benefits system, thus providing a particular challenge for health-care interventions aimed at engaging people with an approach that runs counter to one demanding illness behaviour.
Within the Midlands and north-east of England, there are pockets of the population that are moderately and extremely affluent and THS care managers observed that many of their patients living in these areas had engaged particularly well with the service. They also observed a much lower level of uptake of the service and engagement with the service among the large South Asian populations in the same areas. Language and cultural sensitivities are relevant factors to be considered in terms of engaging ethnic minority patients with LTCs in new telehealth interventions.
A ‘one-size-fits-all’ telehealth intervention is unlikely to suit the needs of all patients when considering the complex and context specificity of lifestyle change. If the aim of a potential intervention is to address health inequalities, it needs to be sufficiently flexible to be tailored and targeted at particular areas of need.
Conclusion
In carrying forward this preliminary work to inform the creation and implementation of future telehealth interventions, the following key points should be borne in mind:
-
There should be clarity about what proposed telehealth interventions add to the existing support of LTCs. Patients are more likely to enrol in telehealth and to benefit from it if GPs and practice nurses know about interventions and endorse them. This would suggest that training of HCPs at the outset of a new programme is essential and care and attention needs to be exercised over the way that patients are recruited into a new THS.
-
Patients and HCPs identified gaps in service in respect of non-drug therapy for depression. Gaps in service for those at risk of CVD were more difficult to identify, but support around diet, exercise and weight loss was mentioned, as well as support to address alcohol dependency.
-
Health-care professionals were concerned that a telehealth intervention might duplicate services already being offered (such as smoking cessation services). A new service should not add to this duplication.
-
Telehealth interventions should be clearly targeted and, if aimed at certain groups with particular problems, then tailored accordingly.
-
Systems of communication between those running a telehealth programme and practice-based HCPs need to be in place from the outset.
-
The software that telehealth managers use needs to be user-friendly and developed for ease of communication between telehealth managers and practice staff.
-
There is a potential for practice nurses to feel undermined by telehealth initiatives taking over their areas of work and expertise. Telehealth should complement and support the work being carried out by practice nurses.
-
Practice-based HCPs who will be the future commissioners of telehealth need to be assured that such interventions are evidence based, cost-effective and QOF friendly.
-
Existing work at THS shows that support for LTCs can be delivered over the telephone and that this medium is acceptable to patients. The technique of motivational interviewing was highlighted by THS care managers, who believed it to be useful, and this approach was appreciated by patients. Motivational interviewing is a well-documented technique that can be used for interventions aimed at achieving lifestyle change.
-
Telehealth Scheme care managers and patients identified as important the relationships they built with each other. They also pointed out that these kinds of trusting relationships take time to build. THS care managers were of the opinion that working towards lifestyle changes is a slow process that can take months and years to achieve. This kind of slow work can be difficult to measure and assess in the short term.
This study had the advantage of being able to look at a THS in action, as well as canvas opinion from those not involved in any such scheme. This material gives a picture of a general acceptance of the idea of receiving health-care support by telephone or using the internet. Areas of concern have been highlighted by both patients and HCPs. Hopefully, these concerns will help to shape new telehealth interventions that address the needs of both HCPs and the patients that they serve.
Chapter 5 TElehealth in CHronic disease: mixed-methods study to develop the TElehealth in CHronic disease conceptual model for intervention design and evaluation
The material in this chapter was adapted from Salisbury C, Thomas C, O’Cathain A, Rogers A, Pope C, Yardley L, et al. TElehealth in CHronic disease: mixed-methods study to develop the TECH conceptual model for intervention design and evaluation. BMJ Open 2015;5:e006448. 250 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Abstract
Objective: To develop a conceptual model for the effective use of telehealth in the management of chronic health conditions and to use this to develop and evaluate an intervention for people with two exemplar conditions: depression and raised CVD risk.
Design: The model was based on several strands of evidence: a meta-review and realist synthesis of quantitative and qualitative evidence on telehealth for chronic conditions; a qualitative study of patients’ and health professionals’ experience of telehealth; a quantitative survey of patients’ interest in using telehealth; and a review of existing models of chronic condition management and evidence-based treatment guidelines. Based on these evidence strands, a model was developed and then refined at a stakeholder workshop. A telehealth intervention (the Healthlines Service) was then designed by incorporating strategies to address each of the model components. The model also provided a framework for the evaluation of this intervention within parallel RCTs in the two exemplar conditions and accompanying process and economic evaluations.
Setting: Primary care.
Results: The TElehealth in CHronic disease (TECH) model proposes that attention to four components will offer interventions the best chance of success: (1) engagement of patients and health professionals, (2) effective chronic disease management (including subcomponents of self-management, optimisation of treatment and care co-ordination), (3) partnership between providers and (4) patient, social and health system context. Key intended outcomes are improved health, access to care, patient experience and cost-effective care.
Conclusions: A conceptual model has been developed based on multiple sources of evidence that articulates how telehealth may best provide benefits for patients with chronic health conditions. It can be used to structure the design and evaluation of telehealth programmes, which aim to be acceptable to patients and providers and cost-effective.
Introduction
Evidence of benefits
In Chapter 1, we highlighted that, although the potential benefits of telehealth in chronic condition or LTC management have been rehearsed for at least 20 years, evidence to support these arguments is limited. 54,251,252 Systematic reviews have been conducted in specific chronic conditions, along with overviews that have combined findings from a range of conditions, and these have concluded that the evidence in favour of telehealth is weak and inconsistent. 11,20,23,54,69,74,251,253 Evidence of effectiveness is stronger for some conditions (e.g. heart failure) than it is for others (e.g. diabetes). Some studies report positive findings whereas others do not and it has been difficult to identify a pattern in terms of disease, type of technology or patient characteristics to explain these inconsistencies. There is a lack of evidence about mechanisms of action and about wider impacts of telehealth on the utilisation of other health-care services. 251 There is inconsistent reporting of outcomes, suggesting a lack of clarity about the intended benefits of telehealth and making it difficult to compare studies. Evidence about cost-effectiveness, or of successful large-scale implementation, is particularly limited.
The need for a conceptual model
Telehealth is a complex intervention252,254 involving a number of interacting components, such as the type of technology, the infrastructure, the human support available and the capabilities of the patient in relation to the technology. For any individual, telehealth is likely to be only one facet of the health care that they receive, so telehealth cannot be understood in isolation from the health-care system in which it is provided.
Over the last 15 years there has been increasing awareness of the importance of theory both in the development and in the evaluation of complex interventions. 56 Theory is needed to understand the relationship between context, mechanism of action and intended outcomes, but this has largely been neglected in the field of telehealth. 255–257 Although there are well-recognised theories in related topics, such as behaviour change (e.g. the theory of planned behaviour,205 the behaviour change wheel,258 the model of Ritterbrand et al. 259) and why technologies get used (e.g. the technology acceptance model260), there is no overarching theory that connects these and other elements (such as co-ordination between service providers) essential to LTC management in the context of telehealth.
What is needed is a clear conceptual model for how and why a telehealth intervention for patients with LTCs is intended to have specified beneficial effects. Making explicit the theoretical chain of causation by which an intervention is intended to lead to its effects focuses attention on the most important features of the intervention that need to be delivered for it to be effective. A conceptual model also provides a framework for evaluation by identifying the contextual factors, steps in the causal chain and most important outcomes that need to be assessed.
A useful theoretical model is one that is simple, clear and based on meaningful concepts, demonstrating lines of causation (including whether variables are proposed to have moderating or mediating effects), and which is sufficiently general to be tested in a range of settings. A model that applies to only one context is of little practical value as it has little explanatory value – the power of a model depends on the extent to which the relationships it proposes are demonstrable in a range of contexts and patient groups. In the context of telehealth for LTCs, this means that the model should ideally be applicable to a range of conditions, types of interventions and health-care settings.
This chapter describes the development of a conceptual model for the role of telehealth in the management of LTCs. This was developed to inform the design of an intervention to support people with two exemplar conditions: depression or raised CVD risk (because of risk factors such as hypertension, smoking, obesity and hyperlipidaemia). These exemplars were chosen to represent very different types of conditions, which would test the generalisability of the model but which are both common and for which there is existing evidence that some forms of telehealth could be effective. 16,69 By taking into account the views of patients and providers, and considerations about cost as well as evidence of effectiveness, the intention was to develop a model for interventions that are likely to be suitable for implementation at wide scale, acceptable to stakeholders and cost-effective.
Methods
Evidence review
The model was based on several sources of evidence. The methods and results for each strand of evidence are summarised below, but are described in more detail elsewhere in this report.
-
A meta-review and realist synthesis of existing quantitative and qualitative evidence on telehealth for LTCs. 253 This consisted of an overview of existing systematic reviews of telehealth interventions. We focused on reviews of LTCs generally, rather than in relation to specific conditions. Details of the methods are provided in Chapter 2. We supplemented the meta-review with a review of depression, as well as a new systematic review to look in more detail at studies of telehealth interventions focused on those for the prevention of CVD. 61 In addition, we identified and reviewed published qualitative studies of patients’ experiences of using telehealth interventions. In total, we included 16 systematic reviews (representing 662 quantitative studies) and 29 qualitative studies. We combined these sources of data in a realist synthesis in which we sought to identify mechanisms of action for telehealth in LTCs. Realist synthesis is an approach to reviewing research evidence on complex interventions in order to provide an explanatory analysis for how and why they work (or do not work) in particular contexts or settings. 261
-
A survey of patients to assess relationships between patient characteristics, health needs, difficulties with access to health care, attitudes towards and availability of various technologies and interest in using different types of telehealth (see Chapter 3). 185
-
A qualitative study of the potential role of telehealth in LTCs. 220 This involved interviews and observation with patients, doctors and nurses providing primary care for patients with LTCs and health information advisors (HIAs) who provided an existing telephone-based health coaching and care management service for patients with LTCs such as heart failure or diabetes. 156 Further details are provided in Chapter 4.
-
Comparison with other models of chronic disease management. To take account of and compare our emerging conceptual model with existing models and frameworks, we familiarised ourselves with other widely used models of chronic condition management, particularly (but not exclusively) those relating to the use of telehealth. We wanted to identify common factors in these models that appeared to be associated with improved care and benefits for patients.
-
Analysis of national guidelines. To apply the model to our exemplar conditions, we identified the main recommendations and priorities for treatment from the current UK guidelines and compared these with guidelines from the USA and Europe. We cross-referenced these recommendations with our meta-review to identify evidence for the effectiveness of telehealth interventions (e.g. the use of online programmes to deliver CBT for depression; the use of home monitoring of blood pressure in patients with hypertension).
Synthesis
We synthesised the findings from our evidence review in two stages. First, it was clear from the meta-review and the qualitative study that engagement from both patients and professionals appeared to be key to the success of a telehealth intervention. We therefore used a modified PRECEDE–PROCEED262 approach to intervention development in which we used the insights from our evidence sources to map the predisposing, enabling and reinforcing factors that determine engagement with telehealth, creating separate ‘maps’ for patients and health professionals. Predisposing factors provide the motivation to act in some way; enabling factors are those that make it possible to carry out the action; and reinforcing factors influence the likelihood that one will perform the behaviour in the future, based on positive or negative feedback. Through discussion within the research team, we listed and grouped themes from the literature reviews, qualitative research and patient survey, cross-referenced to the sources of evidence. Next, commonalities across these three sources of evidence were highlighted and key themes relating to engagement with telehealth were identified. These key themes were then independently organised into predisposing, enabling and reinforcing factors by members of the research team familiar with the PRECEDE–PROCEED262 definitions. As it is possible that the same information can serve first as a predisposing factor and then later as a reinforcing factor, differences in classification, although rare, were resolved through discussion. Nonetheless, the real importance of classifying information into these types of causal factors was to devise temporally appropriate strategies to enhance motivators of and mitigate barriers to the target behaviour.
Second, we developed a draft model for the use of telehealth to support the management of LTCs that encapsulated the main findings from the evidence review. We discussed the findings from the various studies within the research team, seeking to describe hypothesised relationships between different constructs in a schematic manner. Several different layouts and versions of the model were discussed iteratively in meetings of the research team as we critiqued and sought to improve the model. Finally, we convened an intensive 1-day workshop for a wide range of stakeholders (n = 38), including patients, care providers, managers, commissioners of services, independent academics and the research team. We presented the findings of the evidence review and the draft model to the stakeholders, who discussed it in small groups and provided feedback. We used this to refine the final model, which we labelled the TECH model. The term ‘chronic disease’ rather than ‘long-term condition’ was used as this is more widely used internationally and would enhance the international applicability of the model.
Using the model to design an intervention
The research team used the TECH conceptual model to design a telehealth intervention, which we called the Healthlines Service. In this chapter we describe the Healthlines Service model to support the management of patients with raised CVD risk, but a similar approach was used to develop an intervention for patients with depression. This was designed to be delivered by NHS Direct, which, at the time that the intervention was designed, provided health information and advice throughout England based on a network of telephone call centres and an associated website.
The intention was to design an intervention that would be likely to be cost-effective by maximising patient benefit at minimum cost and that could feasibly be rolled out quickly on a national scale if it proved to be effective. For these reasons, the design of the intervention sought to incorporate technologies that were already available and approaches for which there was already some evidence of effectiveness. We avoided cutting-edge technologies that were not already developed or tested and high-cost solutions that would be unlikely to be widely available or deliverable to large numbers of patients. To maximise population benefit, the aim was to focus on the large number of patients at moderate risk of health problems (e.g. patients with hypertension and other CVD risk factors), rather than the small number of patients at high risk (e.g. patients who have already had a stroke).
The research team used the patient and health professional ‘maps’ generated through the PRECEDE–PROCEED method to develop strategies to promote engagement with the telehealth intervention by addressing each of the predisposing, enabling and reinforcing factors previously identified.
The model as a framework for evaluation
The TECH conceptual model was used to provide a framework for evaluation by describing the extent to which each element of the model was successfully delivered and the intended outcomes were achieved. The Healthlines Service was evaluated within two pragmatic parallel RCTs and accompanying process and economic evaluations. Details of the methods for these trials are provided in Chapter 7. In brief, we recruited 43 general practices providing primary health care in three areas of England. Adult patients from these practices with either (1) depression or (2) a raised risk of a first cardiovascular event (10-year risk ≥ 20%) were recruited to take part and were individually randomised to receive either usual primary care plus extra support from the Healthlines Service or usual primary care alone.
Results
Evidence review
Meta-review, realist synthesis, qualitative study and quantitative patient survey
Key findings from these studies are summarised in Box 3.
-
Some evidence of improvements in clinical outcomes.
-
Much of the primary research is of poor quality and limited to short-term effects.
-
Evidence about impact on the wider health-care system and cost-effectiveness is sparse.
-
Inconsistent findings about effectiveness and resource utilisation, with few clear patterns in terms of types of patient, disease or technology associated with benefits.
-
Many telehealth interventions for LTCs have struggled to engage both patients and HCPs, with low uptake and high dropout rates.
-
Simple technologies, especially those based on telephone support, have at least as strong an evidence base as more sophisticated technologies, such as telemonitoring.
-
Telephone support seems to enhance the benefit of web-based technology.
This suggested three key mechanisms by which telehealth worked to improve health outcomes:
-
Relationships – good connections between patients, peer groups and/or professionals provide support.
-
Fit – acceptability, ease of use and integration into everyday routines are important to both patients and professionals.
-
Visibility – monitoring provides feedback, reinforcement and prompts to change behaviour, but can also have negative connotations of surveillance.
-
Nurses and doctors working in primary care were ambivalent about the contribution of telehealth to LTC management because of concerns about the lack of evidence of benefit, duplication of their own work and a threat to their role.
-
There is a need to take account of how new telehealth programmes integrate with existing health system structures.
-
Patients are more likely to trust a telehealth system if it is endorsed by their usual primary care providers.
-
Patients valued a personal approach based in human interaction.
-
There was moderately strong interest in telehealth support for LTCs across all age groups.
-
There was greatest interest in telephone- and internet-based interventions and minimal interest in social media, particularly among older patients with LTCs.
-
There was little relationship between health-care need or difficulties in accessing health care and interest in telehealth.
-
The most important constructs associated with interest in telehealth were confidence in using the technology and perceived advantages and disadvantages of telehealth.
-
Interest in telehealth was not related to patient sociodemographic variables after adjusting for modifiable factors such as access to and confidence in using the technology.
Source: adapted from Salisbury et al. 250 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Existing models of long-term condition management
We identified a number of existing models for the management of LTCs, but the dominant approach is the chronic care model (CCM). This describes a whole-system approach to transforming service delivery, with factors operating at the levels of community, organisation, practice and patient. 263 A number of studies have suggested that programmes based on the CCM can improve health outcomes for a range of LTCs, although it is uncertain which components of the model are most important or whether or not all are necessary. 50,264,265 The CCM includes elements that relate to national aspects of the health-care system and which cannot be addressed within a model of organisation delivered by one provider using telehealth, even in partnership with other providers. It therefore provides a useful basis but does not in itself provide a model for the design of telehealth interventions. Between 2003 and 2007, the Veterans Administration introduced a national home telehealth programme, Care Coordination/Home Telehealth (CCHT),154 which was strongly influenced by the CCM but applied the concepts more specifically to telehealth applications in a US context.
Review of national guidelines
To apply a conceptual model to a specific condition, the key health problems and care needs must be identified. For depression, the priorities for intervention included offering psychological therapies, such as CBT and/or antidepressant drug treatment, with the intensity of treatment tailored in relation to need, having relapse prevention strategies, ensuring medication adherence, offering peer support, avoiding alcohol misuse, encouraging exercise and assessing suicide risk. 266,267
For raised CVD risk, international guidelines suggested that the key health problems and care needs were the modifiable risk factors of hypertension, smoking, obesity, raised cholesterol and lack of exercise. 33,172,268–270 Evidence-based priorities for intervention included optimising drug treatment to achieve blood pressure targets, ensuring medication adherence, providing nicotine replacement therapy for smokers along with behavioural support, providing advice about diet and exercise and referral to weight management programmes for obesity and ensuring that statins were prescribed and taken.
Synthesis and development of the model
Figure 11 shows the final TECH model illustrating the key components, and relationships between them, that we hypothesise will deliver cost-effective improvements in long-term disease management using telehealth. In summary, this model proposes that interventions to promote self-management, optimisation of treatment and care co-ordination are all essential aspects of chronic disease management that are likely to lead to improved health outcomes, patient experience and access to care and more cost-effective delivery of care. These benefits are more likely to be achieved if the service is delivered in an integrated way with other health-care providers and the effectiveness of telehealth is likely to be moderated by the extent of patient and provider engagement and also by characteristics of patients and the health-care system. These components are described in more detail below.
FIGURE 11.
The TECH model for telehealth to support patients with LTCs. Source: adapted from Salisbury et al. 250 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
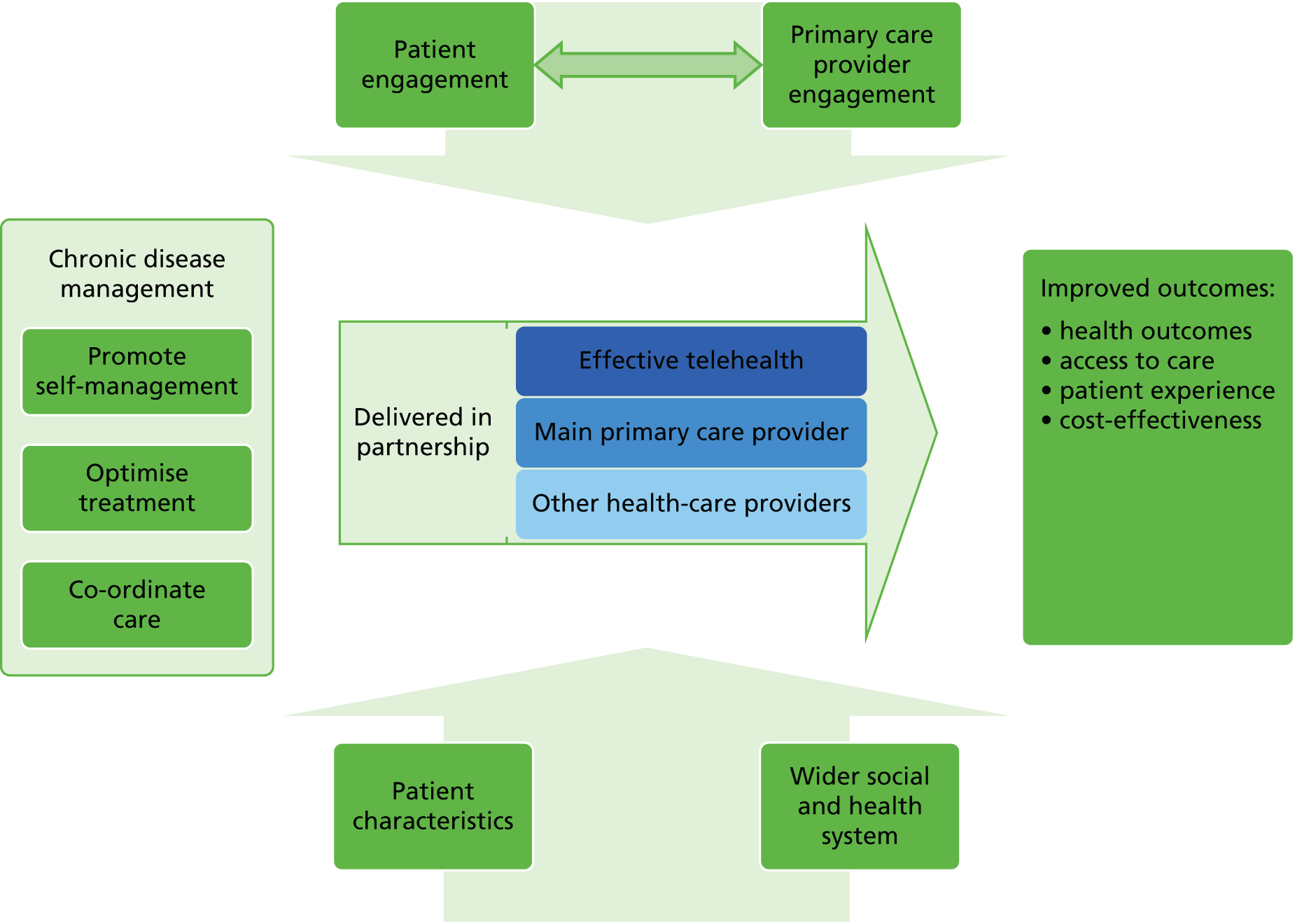
Engagement of patients and primary care providers
The literature meta-review highlighted that many telehealth interventions have been unsuccessful because of low uptake by patients and high rates of dropout. Both the patient survey and our qualitative research illustrated the range of factors that act as motivators or barriers to patients using telehealth, summarised in Box 4, based on our PRECEDE–PROCEED map of predisposing, enabling and reinforcing factors for patients.
-
Attraction of having support for health problems on demand, having more time, getting greater support.
-
Patients having a clear understanding of why they have been offered telehealth treatment.
-
Confidence in ability to use the technology.
-
Being reassured about privacy and confidentiality.
-
Good access to fast reliable internet connection.
-
Technology that is simple and inexpensive, not complicated to use.
-
Benefits of having a regular review.
-
Importance of self-monitoring, which promotes continued engagement.
-
Encouraging patient activation and involvement rather than passive reminders.
Source: adapted from Salisbury et al. 250 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
With regard to HCPs, our qualitative research indicated that many were unenthusiastic and, in some cases, resistant towards telehealth interventions. Our PRECEDE–PROCEED map for professionals identified several factors that were likely to influence engagement in telehealth. These included the belief that medicine should be evidence based and scepticism about the evidence for telehealth (predisposing factor), concerns about duplication of care (predisposing factor), the need for technology to be simple and reliable (enabling factor) and the importance of clarity of roles for conventional and telehealth providers and good communication between them (reinforcing factor).
The engagement of patients and professionals is likely to be symbiotic – doctors are unlikely to be supportive if their patients are not interested in using telehealth and patients are less likely to be interested if their doctors are dismissive of it. Therefore, a key priority for a telehealth intervention is to develop strategies to maximise the likelihood that patients will want and be able to use it.
Effective long-term disease management
Management provided through telehealth must be effective, with clear evidence that it is associated with improved patient outcomes. Our evidence synthesis and review of existing models of LTC management suggested that strategies that contribute to effective care and which could be delivered using telehealth can be summarised under three headings: promoting self-management, optimising treatment and care co-ordination. The various strategies that make up each of these headings are shown in Box 5, along with citations for specific studies or reviews that provide evidence of effectiveness for each element (not necessarily in the field of telehealth).
-
Behaviour change techniques, e.g. stimulus control, problem solving, cognitive restructuring, goal-setting. 271,272
-
Shared decision-making. 274
-
Risk stratification with case management for complex patients. 268,279
-
Share treatment recommendations with patients. 147
-
Interventions that include multiple reinforcing components. 272,274,278
-
Shared records, information and treatment recommendations between patients, primary care and the telehealth provider. 154,277
-
Communication (remote and face to face) between the telehealth provider and primary care. 154
-
Seek to support rather than duplicate primary care. 281
Source: adapted from Salisbury et al. 250 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Partnership
Our qualitative research highlighted that a telehealth intervention is just one aspect of the health care provided to a patient with a LTC. These patients are likely to continue to get the majority of their care from their family practitioner, with whom they may have had a long-term relationship and whom they will continue to consult for reasons apart from their LTC. In addition, many patients with LTCs are likely to be receiving help from hospital specialists and other health- and social-care agencies.
However, our evidence review suggested that many previous telehealth interventions appear to have failed because they were designed in isolation from the rest of the health-care system, leading to duplication of effort, lack of co-ordination between providers, inefficiency and confusion for patients. This is likely to reinforce the resistance expressed by other health-care providers. Our qualitative research showed that these other providers may perceive the telehealth intervention to be an unnecessary interference in their area of responsibility, possibly representing a threat to their future role.
Therefore, it is important for a model for telehealth interventions to emphasise that telehealth should be delivered in partnership, identifying the role that telehealth can play to support, rather than compete with, patients’ main primary health-care providers.
Context: characteristics of patients and wider social and health system
The patient survey and the literature review both indicated that the characteristics of patients are likely to have an impact on how telehealth affects outcomes. These include sociodemographic characteristics, particularly age, the nature of their LTC and the severity of their condition. The design of a telehealth intervention must also take account of the wider social and health system context. 222,282 For example, a programme designed to work within a health system context with a strong primary care foundation may need different features from one designed for a system in which patients consult different hospital specialists for each of their LTCs. Similarly, a system which assumes that patients have access to a fast and reliable internet connection will not work when this does not apply. Context is not only important in designing interventions, but also in interpreting evaluation. An intervention may have different effects in different contexts, but this does not necessarily undermine the theoretical basis for the intervention. Therefore, the telehealth LTCs model may be generalisable even if a specific intervention is not. Finally, different funding models for health care create different financial incentives for providers and patients, which may have a major influence on how telehealth systems are implemented.
Specifying outcomes
The TECH model depicted in Figure 11 seeks to capture the four components of the model in a way that is conceptually clear, simple and generalisable. It also proposes the improved outcomes that telehealth interventions are intended to deliver for patients with LTCs. These are improved health outcomes, access to care and patient experience and care provided in a way that is cost-effective. One criticism of earlier research on telehealth interventions has been the lack of consistency in reporting outcomes54 and this model provides a framework for the outcomes that should be assessed in future evaluations, as well as potential mediators to gain an understanding of the mechanism of action.
Using the model to develop a telehealth intervention
We used the conceptual model to develop telehealth intervention programmes to support the management of patients with (1) depression or (2) raised CVD risk. We used the same model to design interventions that were similar in concept but different in terms of detailed content to address each of the priority health and care needs for these two groups of patients, based on our review of national guidelines.
Table 12 provides examples of how we devised strategies to be delivered within the Healthlines Service to populate the conceptual model for the interventions to be used for depression and CVD risk.
| Model element | Strategies included in the interventions |
|---|---|
| Engagement | |
| Patient | Provide a ‘Welcome Pack’. Emphasise that support with technology will be provided |
| Healthlines advisors provide technical support, e.g. with getting logged in to websites | |
| Promote the advantages to patients of using Healthlines, based on perceived advantages identified in qualitative research and other literature, and address perceived disadvantages | |
| Encourage sense of personal care through seeking to maximise continuity of care from named Healthlines advisor | |
| Regular positive reinforcement through monthly telephone calls from Healthlines advisor | |
| Encourage sense of partnership between patient, Healthlines Service and GP through frequent communication | |
| Health professional | All communications seek to reinforce the message that the Healthlines Service is supporting and is delivered alongside primary care |
| Regular communication with primary care | |
| Messages to primary care continually emphasise evidence-based nature of interventions and guidance | |
| Promoting self-management | |
| Behaviour change techniques | Depression: intervention encounters support use of the Living Life to the Full Interactive cognitive behaviour course, with additional modules relating to alcohol consumption, encouraging exercise and preventing relapse |
| CVD risk: intervention adapted from the Duke self-management package,46,278 which uses scripts for advisors based on psychological principles of behaviour change (see also p. 95–96) | |
| In both cases, intervention is tailored to patients’ needs and goals | |
| Self-monitoring | Depression: patients using online Living Life to the Full Interactive course regularly monitor their progress with self-assessment modules, including score on the PHQ-9 questionnaire |
| CVD risk: provide patients with free blood pressure monitor and website to log readings | |
| Feedback | CVD risk: blood pressure website gives immediate feedback and graphical display about whether blood pressure is above or below target and next actions |
| Provide patient information | Healthlines advisor works with patients to identify goals and then e-mails them links to further resources available on the internet that have been quality assessed (e.g. diet advice, risk calculators, videos, patient forums) |
| Promote self-efficacy | Using motivational interviewing approach, identify motivating factors, encourage action plans and goal-setting |
| Motivational interviewing | All Healthlines advisors undertake motivational interviewing training |
| Shared decision making | Make targets (e.g. for blood pressure) explicit to patients, provide information about advantages and disadvantages of treatments, encourage patients to discuss options with GP, share letters to GPs with patients |
| Personal support from health professionals | As far as possible, provide continuity of care from one named Healthlines advisor rather than an anonymous ‘call centre’ approach |
| Peer support | Patients in depression intervention are offered option to access Big White Wall, an online forum for patients with depression |
| Treatment optimisation | |
| Risk stratification | Depression: assessment using PHQ-9 and advice about treatment in relation to severity. PHQ-9 also used to assess suicide risk with use of a protocol for escalation and more detailed risk assessment for patients at significant risk |
| CVD: calculate CVD risk using QRISK. Level of intervention guided by level of risk factor with escalation to GP for patients at high risk | |
| Treatment intensification | Depression: regular monitoring of PHQ-9 score and review and intensification of treatment if no improvement |
| CVD: monthly review of blood pressure using online log of blood pressure readings; protocol-driven advice to GP to intensify treatment each month if targets not met | |
| Evidence-based guidelines and protocols | Healthlines advisors’ scripts all based on careful review of national guidelines. Encourage compliance with guidelines by sending GPs a simple flow chart summary with each treatment recommendation |
| Regular review | Healthlines advisors telephone patients monthly, based on scripts that raise new topics each month and review progress against goals |
| Promote medication adherence | Monthly review of medication adherence; scripts use evidence-based strategies to improve adherence. Advice given to GPs by e-mail if patients are non-adherent |
| Share recommendations with patients | Patients are given online access to guidelines and treatment recommendations sent to GPs |
| Care co-ordination | |
| Multicomponent interventions | Intervention combines interactive patient web portal, self-monitoring and telephone support from health advisor |
| Shared records | At onset, Healthlines receives information about patients from primary care records. All treatment recommendations shared with both primary care provider and patient |
| CVD: a summary of recent blood pressure records from patient web portal is sent to GP when treatment change is recommended | |
| Communication between the telehealth provider and primary care | Ideally, Healthlines advisors would visit general practices to build relationships, facilitate engagement with telehealth and resolve problems, but this was not achieved in this trial |
| Regular monitoring of system performance | Reporting module that allows monitoring of management programme (e.g. of number of patients who have been telephoned, number actively self-monitoring blood pressure, number participating in online CBT) |
| Support rather than duplicate primary care | All communications with primary care providers and patients reiterate the message that Healthlines is designed to support GPs in their role of managing patients. All treatment recommendations are made to GPs and copied to patients |
| Partnership | |
| All communications are shared. Communication is two way: GPs can contact Healthlines, e.g. to change a patient’s blood pressure target | |
| GPs and service managers involved in designing the Healthlines intervention | |
| Context | |
| The nature and intensity of the intervention is tailored to the nature and severity of the patient’s health condition | |
| Patients are invited to participate only if they are above a specified severity threshold | |
| Recognise that, in the NHS, patients have an enduring relationship with their GP, which reinforces the importance of supporting rather than duplicating or undermining that role | |
| Not all patients have access to a reliable internet connection so this intervention is likely to be relevant only to a proportion of those in need. Provide technical support to help patients, e.g. to log in to the web portal. In evaluation, it is important to describe the characteristics of patients who take part | |
Use of the TElehealth in CHronic disease model for evaluation
The TECH model proposes four main outcomes resulting from telehealth interventions for long-term disease, the first of which is improved health outcomes. For depression, the primary outcome was a clinically significant improvement in depression. For the CVD trial, the primary outcome was CVD risk status 12 months following randomisation. Secondary outcomes for both trials included health-related quality of life, measures of access to health care and patient satisfaction with care. An economic analysis assessed cost-effectiveness over the 12 months of the trial and, for the CVD risk trial, modelled the long-term costs and benefits of the intervention after taking into account the predicted number of strokes and heart attacks over the next 10 years. 283
Alongside the RCT, a process evaluation explored the extent to which the intervention was delivered as intended and whether or not it led to the expected changes at each step of the causal chain hypothesised by the conceptual model. It assessed patient characteristics and health service context, patient and primary care engagement, patient self-management, treatment optimisation, care co-ordination and partnership with other health-care providers, as well as the primary and secondary outcomes described above. These were assessed using validated measures when possible. Qualitative research through interviews with patients, primary care health professionals and Healthlines information advisors were conducted to understand in greater detail how the service was delivered, barriers to and facilitators of implementation and how and why the intervention did or did not appear to be effective from the perspectives of those delivering and receiving it.
Discussion
Principal findings
This chapter describes the development of the TECH conceptual model for the effective use of telehealth among patients with LTCs and illustrates how it has been used to develop telehealth interventions for patients with either depression or raised risk of CVD, as well as design the evaluation of those interventions. If these evaluations in different LTCs are positive, this will provide support for the model with regard to how this type of telehealth intervention works, suggesting that it can then be applied to other LTCs.
Alternatively, if the intervention is unsuccessful, it will be possible to assess each of the processes in the hypothesised causal chain to determine if the intervention was not delivered as intended or if the assumed causal relationships were incorrect. For example, the model posits that one way in which telehealth works is by allowing people to monitor their own health, which will lead to changes in their behaviour and a positive impact on their health. Having a model highlights the need to assess the extent to which participants actually did self-monitor as intended, whether or not this was associated with behaviour change and whether or not this led to improved health outcomes. This kind of approach provides a framework for correction and adaptation of an intervention through understanding which intervention components are more or less effective at impacting proximal outcomes in the causal chain. 284
Strengths and limitations
The strength of this research is that we have used diverse sources of evidence to develop a conceptual model that creates a framework for intervention development and evaluation. The model suggests that, if patients engage with the intervention, and if the intervention promotes self-management that leads to behaviour change, along with optimised treatment in line with evidence-based guidelines, this will lead to improved health both now and in the future. Better co-ordination of services will reduce duplication and inefficiency, which may lead to more cost-effective care and may improve access to care. Each of the components of the model can be justified from our own research and evidence from previous literature.
Although it is arguable that the TECH model could be applicable not only to telehealth but also to all long-term disease management programmes, the model draws attention to topics that are particularly important for telehealth (such as the need for partnership with primary care providers and attention to patient engagement) but which have been neglected in many previous telehealth interventions.
Recognising that the simplest models have the greatest utility, we sought to provide a simple graphical depiction of the hypothesised causal chain in a successful telehealth intervention. However, we recognise that the model diagram oversimplifies the multiple potential mechanisms by which a telehealth intervention may have its effect. There are likely to be associations and interactions between different elements of the model and both recognised and unrecognised confounding factors. However, to indicate all of these potential relationships in the model would, in our view, reduce its usefulness in providing a framework.
A further limitation is that the strength of underlying evidence to support each of the components of the model is variable. For example, evidence of the benefit of patient self-monitoring is strong for some LTCs but not all and, although providing patient information and shared decision making are viewed as important aspects of LTC management in the CCM and other similar models, the evidence that these strategies lead to improved patient outcomes is limited. Nevertheless, we have sought to include components in the model for which the overall weight of evidence supports their value.
Relationship to previous studies
There are several existing models of behaviour change based on psychological theory that have been applied to, or are relevant to, telehealth. 205,258,259 However, behaviour change is only one aspect of the TECH model and this is not its main purpose. The TECH model is intended to provide a framework for the design and evaluation of telehealth services at scale within health-care systems, taking into account a much wider range of factors, such as the potential efficiencies gained through better co-ordination of services.
Several previous authors have argued for the importance of theory in designing telehealth interventions from a range of perspectives255,280,285 and there are also existing frameworks for the assessment (rather than the design) of telehealth for chronic conditions, such as the model for assessment of telemedicine (MAST). 286
The intervention that is most relevant to our study and well described in terms of its underlying theoretical basis is the Comprehensive Health Enhancement Support System (CHESS), an umbrella term for several e-health programmes combining information, adherence strategies, decision-making tools and support services. 287 Pingree et al. 284 used the example of CHESS to demonstrate how telehealth interventions are likely to be multifaceted and to depend on a complex interplay between patients, clinicians and the health-care system. Like the Healthlines intervention described here, CHESS was developed by combining several intervention features, each of which had some theoretical justification. However, CHESS was developed without any clear theory about how the programme features related to each other284 and the TECH model underpinning the Healthlines intervention is intended to address this limitation.
Catwell and Sheikh280 claimed that a telehealth programme should be viewed as an intervention in the same way as a new drug and that it should be evaluated in a similar way, while recognising the complexities of achieving this. Baker et al. 285 have highlighted the challenges of evaluating e-health because of the rapid state of change, but emphasise the need to focus on timeless universal principles and to develop models to elucidate treatment mechanisms. Kaplan255 has argued that the evaluation of telehealth interventions requires methodological pluralism to understand the range of social and organisational factors that determine how telehealth is implemented, used and has effects.
Greenhalgh et al. 288 have taken a more radical stance and argued against the quasi-experimental approach advocated by previous authors in favour of in-depth case studies, viewing programme evaluation not as experimentation but as social practice. They claim that there is a need to recognise the complex political dynamics and language games practised by different stakeholders and to question rationalist assumptions about ‘what works’. We need to ‘transcend . . . the linear and deterministic metaphors (“research into practice”) implying that the core task is determining what works, after which implementing an eHealth technology will be a straightforward exercise in project management’ (p. 558). 288
We recognise the importance of these political considerations in terms of how telehealth programmes are implemented and evaluated and in terms of how the findings from such evaluations are sometimes interpreted to fulfil a previous agenda. However, this does not undermine the need to develop interventions based on a understanding of how and in what ways telehealth programmes might be effective; indeed, a clear theoretical basis for interventions and clarity about intended outcomes might provide the most robust defence against selective use of findings and may allow a more nuanced understanding about why interventions are more or less effective in different contexts.
Implications for clinicians and policy makers
This chapter describes a clear conceptual model, based on several sources of evidence, that helps to articulate the theoretical basis for how, why and under what circumstances telehealth could provide specified benefits for patients with LTCs. Because it is based on evidence-based components and the views of stakeholders, the TECH model provides the basis for the design of telehealth interventions that are likely to be effective, cost-effective and acceptable to patients and health-care providers. Importantly, it also provides a framework for the evaluation of these interventions.
Chapter 6 The Healthlines Service: description of the telehealth intervention
The material in this chapter was adapted from Thomas CL, Man MS, O’Cathain A, Hollinghurst S, Large S, Edwards L, et al. Effectiveness and cost-effectiveness of a telehealth intervention to support the management of long-term conditions: study protocol for two linked randomized controlled trials. Trials 2014;15:36. 283 Copyright © Thomas et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The software developed to support the Healthlines Service is freely available to developers who wish to use it for the benefit of future patients under a GNU General Public License version 3 from the following open access repository: https://github.com/Healthlines/Healthlines-Applications (accessed 27 September 2016).
Overview
One issue with previous telehealth trials is that they largely lack a theoretical underpinning. Our aim was to utilise the TECH model that we developed to design a telehealth intervention, the Healthlines Service, for patients with LTCs. We therefore drew on the conceptual components of the TECH model, such as self-management and treatment optimisation, when developing the content and features of the Healthlines Service. Fundamentally, the intervention consisted of tailored telephone support and advice, as well as access to online resources, such as computerised CBT and graphical display of home blood pressure readings. This chapter outlines the development, content and mode of operation of the Healthlines Service.
Intervention overview
In Chapter 7 we describe the two linked parallel-arm RCTs in more detail. However, before describing the intervention, it is important to note that the trials involved two distinct patient groups: those with depression and those with raised risk of CVD. For this reason, the content of the intervention varied across the two trials, although the theoretical basis for the intervention and the intervention staff were common to both. Hereafter, these trials are referred to as the ‘depression trial’ and the ‘CVD risk trial’.
The Healthlines Service is the name given to the intervention in the depression and CVD risk trials. The core of the intervention was telephone support and advice delivered by NHS Direct HIAs through a series of scripted telephone encounters. Each telephone encounter could consist of a number of individual modules, which were condition-relevant health topics for discussion with the participant (e.g. taking up exercise). The intervention also consisted of access to a secure web portal where participants could enter clinical data (blood pressure readings for CVD risk), monitor their health condition, access health information, online apps and resources and request a call back from intervention staff, review previous summary letters sent to their own GP and update their personal details (e.g. address and telephone number). At prespecified points throughout the module scripts, letters were triggered and populated with summary clinical data [e.g. latest Patient Health Questionnaire-9 items (PHQ-9) score,289 average blood pressure readings] or advice about conducting a medication review with the participant (e.g. because of patient-reported side effects, uncontrolled blood pressure). These letters were e-mailed to the participants’ usual GP to keep them up to date with their patients’ health and well-being, as well as their patients’ progress through the intervention.
Development of the intervention
The content of the intervention was developed to reflect the conceptual TECH model (see Figure 11). The TECH model highlights the need to promote self-management (including using established approaches such as goal-setting, self-monitoring, information sharing, decision making, relapse prevention and regular review), optimisation of treatment (particularly titration of medications following protocols), co-ordination of care between providers and methods designed to enhance the engagement of patients and GPs. Additionally, appropriate and evidence-based priorities for the intervention were also identified by reviewing UK treatment guidelines for depression266,267 and CVD risk factors33,172,268–270 and relevant research literature, including Cochrane reviews. Furthermore, we compared our findings about important components of the intervention model with those of other related models such as the CCM. 263
In addition to reviewing the literature described above, development of the Healthlines Service also incorporated the findings of a usability and acceptability study conducted by NHS Direct. The overall aim of that substudy was to explore the utility, usability and acceptability of the proposed services for people with depression and raised CVD risk. Three deliberative panels including 11 patients were convened and 10 in-depth interviews were conducted with patients with either depression or raised CVD risk. The patients were recruited from one GP practice in the Southampton area. The study explored patient perspectives about the usefulness, benefits and drawbacks of the intervention and how the Healthlines Service could be used by patients to achieve specified goals within the context of its specified purpose. Accessibility issues related to the service were also discussed to help the research team understand potential barriers to using the service.
Content of the intervention
As mentioned above, the intervention was designed to address each of the components of the TECH model, with the particular types of help offered to each participant tailored to their health needs and preferences. The core of the intervention consisted of regular telephone calls from a NHS Direct HIA, who supported the participant in setting and addressing their health goals by using advice derived from computerised protocols and support scripts (see Appendices 7 and 8). These scripts frequently directed the participant to relevant online resources, including health and well-being information from reliable sources [particularly NHS Choices – see www.nhs.uk/Pages/HomePage.aspx (accessed 12 September 2016)], interactive programmes such as computerised CBT and relevant apps and widgets (e.g. for help with giving up smoking). Having identified a relevant resource, the HIA e-mailed the participant a link to the relevant website or resource or sent him or her the information by post. The HIAs also reviewed the participants’ progress with regard to each goal during subsequent telephone calls, in an effort to enhance their motivation.
Additionally, the intervention included access to an individualised password-protected page of the Healthlines Service web portal (see Appendix 9). This portal provided more information about the service, including a downloadable ‘Welcome Pack’ (see Appendix 10). The ‘Welcome Pack’ was designed to enhance patient engagement with the intervention and HIAs and so included details about the service and addressed participant expectations (benefits, drawbacks, hours of operation, security and confidentiality concerns); it also included information on the background of the HIAs and provided colour photographs of the HIAs (removed from Appendix 10 for anonymity). The web portal provided information about the participants’ health condition (depression or CVD risk) and contained links to other resources and relevant organisations [e.g. Mind for depression – see www.mind.org.uk/ (accessed 12 September 2016); British Heart Foundation for CVD risk – see www.bhf.org.uk/ (accessed 12 September 2016)]. As described in more detail below, the web portal also enabled CVD risk participants with raised blood pressure to record and display their blood pressure readings graphically over time, while all participants were able to request a telephone call from a HIA via the web portal.
In both the depression and the CVD risk trials, an initial telephone assessment was conducted with participants to provide further information about the Healthlines Service and the treatment options available. Relevant medical history and medication use were also recorded at this time. The next scheduled telephone appointment was then arranged and a report was sent to each participant’s GP to notify them of their patient’s participation in the service.
Depression trial
In the depression trial, the key resource that participants were offered was access to the Living Life to the Full Interactive (LLTTFi) programme, supported by regular telephone contact. LLTTFi is an online interactive multimedia programme that delivers CBT-based treatment for depression (or comorbid depression and anxiety; see Appendix 11). It was developed by Professor Chris Williams of the University of Glasgow, UK. 290,291 It involves six self-directed sessions, completed approximately every 2 weeks. The programme was offered to Healthlines participants either as an online interactive programme provided by Media Innovations Ltd [see www.llttfi.com/ (accessed 27 September 2016)] or, for those who preferred it, by giving them the corresponding Overcoming Depression and Low Mood: a Five Areas Approach workbook to use at home. 292 As part of the CBT package, participants completed the PHQ-9 (a nine-item questionnaire that assesses depression severity and response to treatment)289 at the start of the programme, midway through and then after the final session. This provided clinical data to monitor participants’ well-being, track progress through the CBT programme and assess whether or not participants were experiencing suicidal ideation. Moreover, these PHQ-9 scores were shared with the participants’ GPs, to keep GPs updated about their patients’ progress and health (see Appendix 12).
It is important to note that the intervention was not solely an online CBT programme, but also included a number of other intervention components in line with the TECH model. First, the self-directed CBT package was crucially supplemented with telephone support, which included motivational interviewing. Our research in the first phase of this research programme (see Chapters 2 and 4) indicated that online CBT alone would be less effective, less engaging and less valued by patients with depression if it did not also contain supportive, human contact. Therefore, the core of the depression intervention was regular telephone calls from a HIA, which were designed to motivate and support participants in making use of the mainly online resources and materials. Participants with depression received seven telephone calls at approximately fortnightly intervals over the course of 4 months and then two further contacts were made once every 3 months over the course of 6 months (see Appendix 13). Although the length of the calls and the topics discussed varied somewhat across the encounters, they each lasted around 15–20 minutes, with a mean duration of 18.5 minutes. The telephone scripts used during each contact were written by the NHS Direct Clinical Content Team and Professor Chris Salisbury and incorporated protocols for providing support to participants in using and applying LLTTFi or the book equivalent. In addition, they included modules covering monitoring of depression symptoms, optimising and stepping up treatment in cases of inadequate response, medication adherence, exercise and alcohol use (see Appendix 13).
Participants in the depression trial had access to the Healthlines web portal. Other than information about depression and links to support services, organisations and trusted online information resources, the web portal also included a link to the LLTTFi programme. Another resource offered through the web portal was Big White Wall (BWW) [see www.bigwhitewall.com (accessed 12 September 2016)]. BWW is a UK-based digital mental health network that is equipped with a 24/7 clinically moderated online forum involving other BWW members, a facility for posting ‘bricks’ on the wall (a form of creative self-expression using graphics or text displayed within a rectangular brick shape), information resources and several validated self-assessment tools [e.g. Generalised Anxiety Disorder-7 items (GAD-7)293 and PHQ-9289] that track scores and offer more information and pragmatic advice (see Appendix 14).
Cardiovascular disease risk trial
In the CVD risk trial, the software and telephone scripts were adapted from effective interventions developed by Professor Hayden Bosworth and colleagues at Duke University (Durham, NC, USA). 294,295 The content of these scripts was based on recognised behaviour change principles such as stimulus control, problem solving, cognitive restructuring and goal-setting. 271,272 The content and language of the original interventions were modified when appropriate to make them consistent with UK treatment guidelines and the NHS environment and to incorporate additional components written by the NHS Direct Clinical Content Team and Professor Chris Salisbury. The software guided the delivery of a series of modules during 12 telephone contacts approximately 4 weeks apart over a 12-month period, with each call lasting around 18 minutes. The modules covered a wide variety of topics associated with the management of raised CVD risk. The software was designed so that the intervention staff covered topics that were relevant to the participants during each telephone contact and the content of later encounters reflected the topics that were covered in earlier calls (see Appendix 15). Each telephone encounter included discussion of several topics (modules within the software) and some modules were covered repeatedly (e.g. weight loss was revised regularly in people trying to lose weight). The modules provided were:
-
knowledge about cardiovascular risk and healthy lifestyles
-
medication and side effects review
-
blood pressure medication optimisation
-
home blood pressure monitoring
-
statin medication review
-
support for medication adherence
-
smoking and nicotine replacement therapy
-
diet
-
weight loss and orlistat use
-
alcohol use
-
exercise.
The complete set of modules was not necessarily discussed with each participant. The intervention was tailored to each participant, such that the total number of modules covered partly depended on the particular CVD modifiable risk factors (high blood pressure, obesity or currently smoking) that a participant had, as well as the particular lifestyle changes the participant was interested in working on with his or her HIA. As will be covered in more detail in Chapter 7, and among other criteria, CVD risk participants were required to have one or more of these modifiable risk factors to be eligible to join the CVD risk trial. Therefore, if a participant did not smoke or was not obese, the smoking module or weight loss module, respectively, would not apply and would be skipped. Likewise, if a participant smoked but clearly indicated that he or she was not ready or willing to give up smoking, the HIA would not cover the smoking module. In that case, the HIA would not persist with the issue at that time, but return to it gently at a later date to see if the participant was now ready to contemplate quitting smoking. The rationale was that participants were likely to be at different stages of readiness to change,296 but through participating in the Healthlines Service – a lifestyle and behaviour change telehealth intervention with motivational interviewing – and developing rapport with their HIA, they might move into a state of readiness over time.
A key aspect of the CVD risk intervention was support for home blood pressure monitoring. Participants with a systolic blood pressure of ≥ 140 mmHg who did not have atrial fibrillation were offered an OMRON M3 home blood pressure monitor (OMRON Healthcare UK Ltd, Milton Keynes, UK) to take home and use for the duration of the 12-month study, if they wished to do so. This device is a basic arm cuff monitor, validated for home use by the European Society of Hypertension International Protocol. 297 Participants were given instructions on how and when to record their blood pressure and how to enter their readings on the Healthlines web portal. Participants were requested to take blood pressure readings twice daily for the first week and weekly thereafter. The web portal calculated average readings over the previous 6 days initially (if the participant had entered at least four readings) and then over the previous 6 weeks thereafter. After entering a set of readings, participants were automatically advised by the web portal on whether or not their blood pressure was above their target, when to take their blood pressure again and what to do if their blood pressure was too high or too low. Target blood pressure was based on UK NICE guidelines,268 but there was the option for a patient’s GP to modify the target if desired. The target blood pressure levels for different groups of participants were as follows: < 135/85 mmHg for participants aged < 80 years and without diabetes, < 125/75 mmHg for participants with type 1 diabetes (or for those with chronic kidney disease and type 1 or 2 diabetes) and < 135/75 mmHg for participants with type 2 diabetes. Regardless, the HIA reviewed each participant’s blood pressure readings during each monthly call. If the readings were above target, the HIA advised the participant to see his or her GP and also e-mailed the GP a letter asking the GP to review the participant’s treatment (see Appendix 16). Reinforcing the evidence-based nature of the intervention, the letter to the GP included a copy of the relevant NICE guidelines268 about recommended steps for intensifying treatment. A copy of this letter was available to the participant via the web portal.
Additional theoretical components incorporated into the intervention
In addition to the regular telephone calls and support for using resources available online, the intervention incorporated several other features designed to address components of the underlying theoretical model. For example, one recognised problem with previous telehealth interventions is low patient engagement and so intervention mapping was used to identify features that would act as barriers to or facilitators of patient engagement (see Chapter 5, Engagement of patients and primary care providers). As a result, the intervention was designed to address perceived barriers and highlight factors that would facilitate and reinforce engagement. One factor discovered during the developmental work that could enhance patient engagement was the importance of receiving consistent support from a named person, rather than from an impersonal call centre. Therefore, participants were offered follow-up calls from the same HIA as much as possible, knew the name of their HIA and had access to colour photographs of the individual HIAs.
Previous telehealth interventions have also had problems related to engagement of primary care clinicians. 220 The present research aimed to address this problem by framing the intervention as evidence based and complementary to primary care and by working with primary care clinicians, rather than duplicating their efforts or competing with them. Accordingly, letters were regularly e-mailed to the GPs about each participant’s progress during the course of the intervention, such as PHQ-9 scores (depression) or average monthly blood pressure readings (CVD risk). Letters making recommendations about treatment were also shared with participants via the web portal, which further reinforced participant engagement and could make it more likely that recommendations were acted on. Another problem with participant engagement experienced in other telehealth interventions has been lack of ability to use the software. Accordingly, the HIAs spent time in initial encounters helping participants to log in to the Healthlines portal and to gain confidence in using the software.
Intervention staff
As already mentioned, the Healthlines intervention was delivered by a team of NHS Direct HIAs. These staff were existing NHS Direct employees based within an existing NHS Direct call centre in Nottingham, UK. All of the HIAs had a minimum of a diploma-level qualification or equivalent experience in a health-care or social-care setting. They were also experienced with working for NHS Direct as HIAs, providing expert health and medication information. It is important to note that the HIAs were not nurses.
The HIAs received 3 weeks of training prior to delivering the Healthlines intervention. This training included classroom sessions, hands-on training and side-by-side call reviews with expert training staff. In addition, it covered health coaching skills, training on how to introduce and support the online or workbook CBT package for depression (delivered by Professor Chris Williams, who developed the CBT package) and how to use the CVD risk intervention software, as well as condition-specific knowledge and medicines training, with this being delivered by expert pharmacists. Therefore, the same HIAs were trained to deliver both the depression and the CVD risk content and they had access to clinical advice and management support as required throughout. Staff performance was also monitored using a rigorous call review process.
Initially, the intervention team consisted of six HIAs (two male, four female). However, about 6 months after implementing the Healthlines Service, which was an intensive period of participant recruitment, an additional six HIAs (one male, five female) were trained to deliver the intervention and allocated to the service. At this time, the service was operating under a ‘blended’ delivery model whereby the HIAs partly worked on the Healthlines Service and partly retained their role as call handlers within NHS Direct. With 12 HIAs on board who all worked part-time, it became challenging for one HIA to regularly maintain contact with the same participants throughout. Therefore, the decision was made to switch to a more ‘dedicated’ model whereby the intervention team was reduced to four HIAs (all female) who worked solely on the Healthlines Service and maintained regular contact with the same participants as much as possible. This change was fully implemented by August 2013, approximately 1 year after the service was first deployed.
Hours and mode of operation
To improve accessibility of care to participants, the Healthlines Service was available for extended hours: Monday to Friday from 1000 to 2000 and on Saturday from 1000 to 1400. At the outset, the service provided an initial assessment with participants, which was then followed by regular telephone contacts at prearranged appointment times that were convenient for the participants. When possible, all contacts with an individual participant were made by the same staff member to promote continuity of care and build rapport. The work of intervention staff was supported by computer software, which was used to guide the content of the telephone calls and create a record of what was discussed. In addition, participants were able to request a call back between their scheduled appointments via the intervention website or by leaving a telephone voicemail message.
To address early problems with participants forgetting their prearranged appointment with their HIA, automatic e-mail reminders were introduced and proved successful. Nevertheless, if a participant did not answer the HIA’s scheduled call, the HIA would reattempt telephone contact on six more occasions. This was later reduced to a more manageable three telephone attempts, followed by an e-mail requesting that the participant re-establish contact with the Healthlines Service if he or she wished. If the participant did not contact the service within approximately the next month after being sent this e-mail request, he or she was considered no longer interested in receiving support and classified as having discontinued the intervention.
Intervention completion
As detailed above, participants could remain in receipt of the intervention for up to 12 months. The final participant contact included a review of progress, information about other ongoing sources of support and creation of a final summary to be sent to their GP. On completion of the intervention, participants returned to the sole care of their GP. This transition was clearly communicated to both the participants and their GPs.
Closure of NHS Direct and transfer of the Healthlines Service to Solent NHS Trust
In October 2013, the government announced its intention to close NHS Direct on 31 March 2014. Following this announcement, negotiations were initiated between NHS Direct and Solent NHS Trust regarding the transfer of the study. Solent NHS Trust was the most suitable potential host because it was near to the NHS Direct site in Southampton where most of the NHS Direct staff involved in managing the research were based. Although the transfer of the research side of the study was agreed quite quickly, it was more difficult to finalise the transfer of the intervention to Solent NHS Trust. The complicating factors were the transfer of four intervention delivery staff (based in Nottingham) and a manager (based in Southampton), as well as making arrangements for ongoing clinical supervision, support for the IT infrastructure used to deliver the intervention and ensuring that contracts and finances were quickly organised. Further, as the building that the HIAs worked out of was no longer available with the closure of NHS Direct, the HIAs agreed to work from home for the remainder of the intervention period. Therefore, laptops had to be configured and mobile phones arranged, at the same time as transferring the IT platform from NHS Direct to NHS Solent Trust’s network. To allow for this IT work to be carried out and tested, it was necessary to institute a temporary break in intervention delivery between 1 April and 6 May 2014. Further details around the transfer of the service to Solent NHS Trust, as well as the impact on the intervention delivery schedule and participants, are described in Chapter 7 (see Transfer of the service from NHS Direct to Solent NHS Trust and Impact on intervention encounters).
Chapter 7 Randomised controlled trial methods
The material in the section Depression and cardiovascular disease risk trial methods was adapted from Thomas CL, Man MS, O’Cathain A, Hollinghurst S, Large S, Edwards L, et al. Effectiveness and cost-effectiveness of a telehealth intervention to support the management of long-term conditions: study protocol for two linked randomized controlled trials. Trials 2014;15:36. 283 Copyright © Thomas et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Overview
In the final phase of this research programme, we carried out two RCTs as well as an economic evaluation and an embedded qualitative study to explore the acceptability of the intervention (process evaluation). In this chapter, we begin by describing the trial methods, followed by the methods employed in the economic analysis and process evaluation.
Depression and cardiovascular disease risk trial methods
The Healthlines study is registered with the International Standard Randomised Controlled Trial Number registry [depression trial: ISRCTN14172341 (registered 26 June 2012); CVD risk trial: ISRCTN27508731 (registered 5 July 2012)].
Outline of the trial design
This phase of the programme involved two linked, multicentre, parallel-group, two-arm randomised superiority trials, with individual participants allocated on a 1 : 1 basis. The trials involved two different patient groups: those with depression and those with a raised CVD risk. The study infrastructure, including participating general practices, research staff and intervention staff, and the underlying theoretical basis for the intervention were common across both trials. However, the specific content of the intervention packages, data analysis and reporting were distinct. Once again, we refer to these trials as the depression trial and the CVD risk trial.
Figure 12 provides a flow chart summarising participant recruitment and follow-up procedures for each of the trials. The first two general practices recruited into the study formed part of a run-in phase, during which study procedures were tested and refined before patients were recruited from the remaining practices.
FIGURE 12.
Flow chart of participant recruitment procedures. CIS-R, Clinical Interview Schedule – Revised; DEP, depression. Source: Adapted from Thomas et al. 283 © Thomas et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
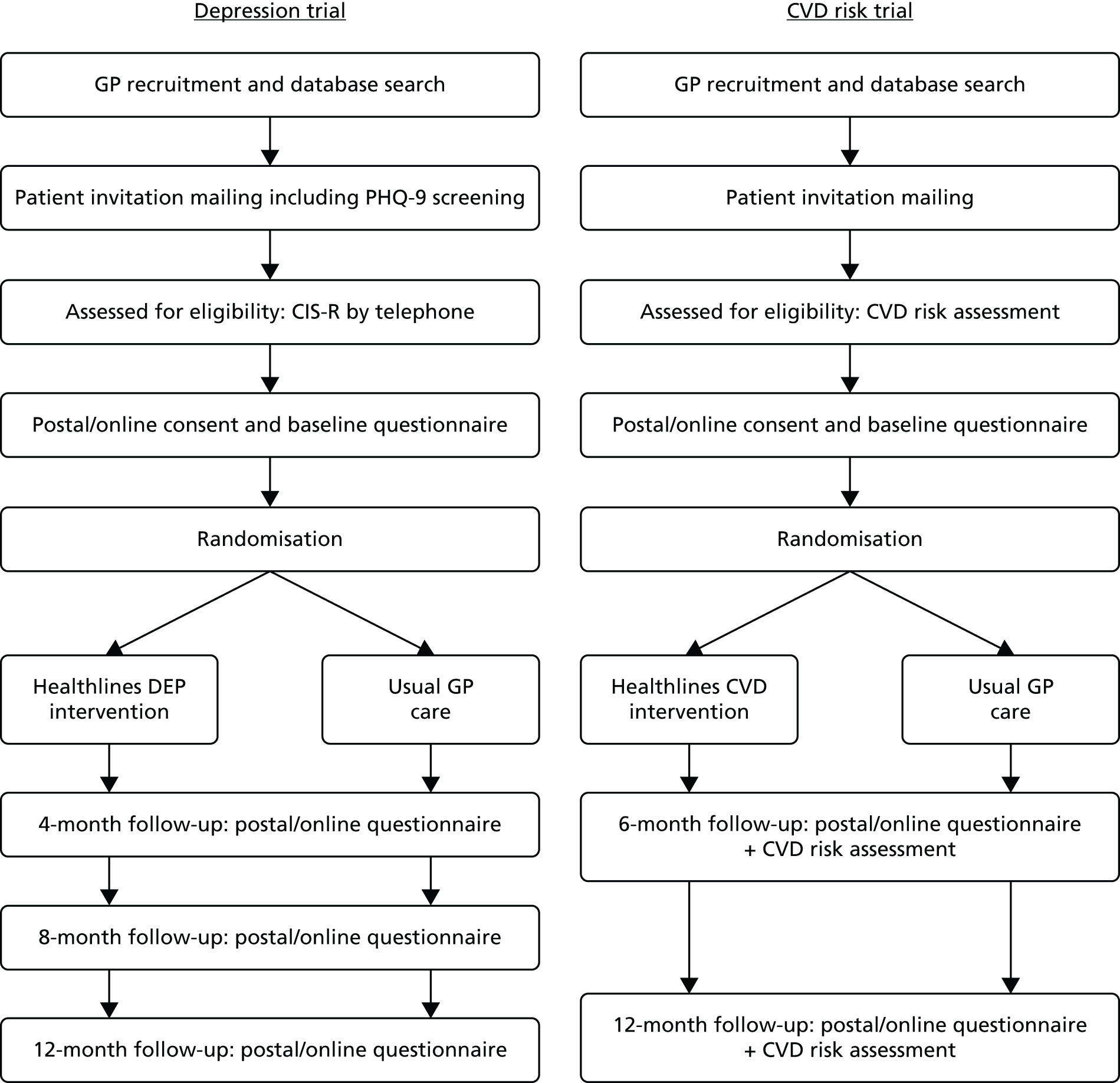
Aim and objectives of the trials
The aim of the trials was to determine the clinical effectiveness and cost-effectiveness of a NHS-delivered telehealth intervention to support patients with two exemplar LTCs: depression and raised CVD risk. Specifically, the study addressed the following research questions:
-
Does the intervention, in addition to usual primary care, improve condition-specific clinical outcomes compared with usual care alone?
-
Does the intervention have any effect on other patient outcomes, including quality of life and satisfaction with care?
-
What is the cost-effectiveness of the intervention in each condition?
-
What is the acceptability of and compliance with the intervention and what are the facilitators of and barriers to its delivery?
Settings
Forty-three general practices in the environs of Bristol (n = 20), Sheffield (n = 17) and Southampton (n = 6) took part in the study. Because we reached and surpassed our recruitment target for the CVD risk trial before invitations were sent to patients in the last practice (in the Southampton area), we invited patients to take part only in the depression trial in that practice and only 42 general practices were involved in the CVD risk trial. The 43 general practices taking part in the trials were recruited to represent a mix of sociodemographic characteristics, including urban and rural patient populations. Two practices participated in the run-in phase and the remainder were involved in the main trials. The two practices in the run-in phase were treated as an internal pilot and their patients were included in the main trial analysis as there were no changes in procedures between the run-in and the main trial phases.
Inclusion criteria
In both trials participants were required to have access to a telephone (landline or mobile), access to the internet and an e-mail address for personal use. It was not necessary for participants to have the internet in their own home but they had to have easy and available access to it. Therefore, it was permissible for participants to use the internet in a public place, such as the library or an internet café, or to use it at a friend’s or family member’s house, should they agree to this.
Additional inclusion criteria for the depression trial were:
-
aged ≥ 18 years on the date of invitation to participate
-
confirmed diagnosis of depression using the Clinical Interview Schedule – Revised (CIS-R) scale298
-
PHQ-9 score of ≥ 10. 289
Additional inclusion criteria for the CVD risk trial were:
-
aged between 40 and 74 years on the date of invitation to participate (the age range for which CVD risk scores have been validated and the same age range used for the NHS Health Checks vascular screening programme)
-
10-year risk of a cardiovascular event of ≥ 20%, calculated using the QRISK2 score195
-
at least one of the following modifiable risk factors: (1) current systolic blood pressure ≥ 140 mmHg (and suitability for home blood pressure monitoring, if this was the patient’s only modifiable risk factor); (2) BMI ≥ 30 kg/m2; and (3) currently smoking.
Patients who met the inclusion criteria for both the depression and the CVD risk trials were invited to take part in the depression trial only. Satisfying all inclusion criteria was essential to meet eligibility for the study.
Exclusion criteria
Exclusion criteria applying to both trials were:
-
bipolar disorder
-
psychotic illness
-
dementia or substantial cognitive impairment
-
severe learning disability
-
substance dependency (including alcohol)
-
receiving palliative care
-
significant suicide risk
-
GP’s determination that participation would cause distress (e.g. because of recent bereavement)
-
inability to communicate verbally in English sufficiently to receive telephone-based support delivered in English; patients who could communicate verbally in English but who were unable to read English were deemed eligible if they had a family member or friend who was willing and able to translate written materials, such as information sheets, consent forms and online material, for them.
Additional exclusion criteria for the depression trial were:
-
currently receiving case management from a specialist mental health worker
-
currently receiving face-to-face, telephone or computerised CBT or similar psychotherapy
-
given birth in the previous 12 months.
Patients were excluded from the study if any of the exclusion criteria were met.
Additional exclusion criteria for the CVD risk trial were:
-
established diagnosis of CVD, defined as history of heart attack, angina, heart failure, stroke or transient ischaemic attack (TIA)
-
currently pregnant or planning to become pregnant within the next 12 months
-
patients who will be invited to participate in the NHS Health Checks Programme during the period of the trial (only relevant in the Bristol recruitment area, in which the local PCT requested this)
-
patients with atrial fibrillation for whom high blood pressure was their only modifiable risk factor, as these patients were not suitable for home blood pressure monitoring.
Recruitment procedures
Initial identification and sampling
In the depression trial, potentially eligible patients were identified using two methods: query-based patient records search and direct HCP referral. Based on codes within general practice patient records, the computerised search included patients who had consulted the doctor for depression, low mood or other similar symptoms and/or who had been prescribed antidepressants within the previous 2 months. We accepted that this search strategy meant that some patients who were taking antidepressants for symptoms other than depression would be invited, but it was necessary to have a sensitive strategy because of the difficulty in identifying patients with depression from codes in the records. However, as GPs were asked to review the patient lists ahead of invitation, and because the study material clearly indicated that the study was recruiting patients with depression, we expected that most of the non-depressed patients taking antidepressants would be screened out in this way. The search also excluded individuals with any of the study exclusion criteria as far as possible, based on coded information within their medical records.
In the case of direct HCP referrals, the GP or other HCP identified potentially eligible participants during consultations. The GP or other HCP briefly described the study and provided the patient with an invitation letter and the participant information sheet. If the patient was interested in taking part, the GP or other HCP then completed a referral checklist and, with the patient’s permission, passed the patient’s contact details on to the local research team.
In the CVD risk trial, potentially eligible individuals were also identified using a computerised search of general practice patient records. Patients whose records suggested that they had a raised CVD risk and at least one modifiable risk factor were selected. For the purposes of initial sample selection, raised CVD risk was defined as a QRISK2 10-year risk score of ≥ 18, which was calculated from risk factor information (e.g. age, latest blood pressure reading, BMI) extracted from patients’ records. 299,300 Thus, the initial invitation recruitment threshold QRISK2 score of ≥ 18 was slightly lower than the trial recruitment threshold of 20. The rationale was that the initial QRISK2 scores obtained from screening medical records were often based on old, routinely entered data on variables such as weight and blood pressure and were therefore fairly approximate. We expected that some patients would have a higher QRISK2 score when calculated using complete, up-to-date risk factor data during assessment for eligibility for the trial.
In 19 out of 42 general practices, it was not possible to calculate QRISK2 scores because the query software either was not developed in time or did not function properly within a practice’s computer system. In these instances a Framingham risk score was similarly calculated using risk factor information drawn from patient records, which was then compared with a sex- and age-specific risk threshold. For men of any age, the threshold was set to Framingham risk of ≥ 25%. In women, the threshold varied by age: 40–49 years ≥ 22%, 50–59 years ≥ 24%, 60–69 years ≥ 22% and 70–74 years ≥ 17%. These thresholds reflect the published relationship between Framingham risk scores and QRISK2 scores301 and were conservative to allow for the fact that some patients were likely to have higher QRISK2 scores when they were fully assessed for eligibility. Whether initial risk was obtained using the Framingham risk score or the QRISK2 score to identify potentially eligible patients for invitation to the trial, eligibility was later confirmed with a CVD risk assessment and a recalculated QRISK2 score using the collected patient data. The CVD risk assessment and confirmation of eligibility is described in more detail in Confirmation of eligibility.
Searching practice records for potential participants took place between May 2012 and May 2013. In both trials, a simple random sample of the potentially eligible patients derived from the records search, or all of the identified patients, whichever was the smaller group, was selected from each general practice to receive an invitation to join the study. Potentially eligible patients were allocated a random number formula generated in Microsoft Excel® (2007; Microsoft Corporation, Redmond, WA, USA) and then the list of patients was sorted in ascending order by random number. The total required number of participants per general practice was then selected from the start of this list. Initially, we planned to invite 300 and 250 patients per general practice for the depression and CVD risk trials respectively. These sample sizes were determined prior to recruitment and were based on estimates of the recruitment rate. Following lower than expected recruitment rates during the run-in phase and from early main trial practices, these figures were revised to ensure that recruitment targets were met. Therefore, for the depression trial, up to 300 patients were randomly selected in the first 11 general practices, up to 500 in the next 25 practices and then up to 585 patients in the remaining practices. For the CVD risk trial, a maximum of 250 patients were selected from 36 general practices, with up to 285 selected from the remaining practices.
Invitations and reminders
Prior to invitation, one or more GPs from the practices reviewed the list of potentially eligible patients and excluded any who they felt to be unsuitable for invitation (e.g. because of recent bereavement). Subsequently, practice staff posted the remaining randomly selected list of patients a study invitation letter on practice-headed paper. Therefore, at no time through this process, nor during the patient searches, did the researchers have access to any patient-identifiable data. The invitation packs for the study were posted to potential participants between June 2012 and June 2013.
Also included with the invitation letter was a participant information sheet, an expression of interest form, a decline form and a reply-paid envelope. Patients interested in participating were asked to respond directly to the research team, providing contact details and consent for eligibility screening. Because of high levels of patient interest, but largely from those who were later found to have too low a PHQ-9 score or who lacked a current diagnosis of depression, patients in the depression trial were asked to complete and return a PHQ-9 questionnaire along with their initial expression of interest. This prescreening strategy was implemented from the ninth general practice onwards.
Those who did not wish to take part were asked to return an anonymised decline form. The decline form asked patients to select the reason(s) why they did not want to take part in the research from a list provided or write down their own reason(s) and also asked for sex, age and ethnicity. Some examples of the reasons provided on the decline form were, ‘I do not have regular access to the internet or an e-mail address’, ‘I am too busy at the moment’ and ‘I do not feel I need any more support with my health at this time’. Details on the proportions of patients declining for various reasons are reported in Appendix 17.
Practice staff sent one postal reminder to patients who had not responded with an expression of interest or decline form approximately 3 weeks after the initial mailing. The reminder pack included the same materials as in the initial invitation mailing, except for a modified invitation letter, which explained that the patient was being sent a reminder because his or her response had not yet been received.
Confirmation of eligibility
Patients who replied with an expression of interest were contacted by a member of the local research team by telephone usually between 1 and 14 days after their form was received. The researcher clarified with the patient what taking part in the study would involve, including an explanation of randomisation, what the intervention involved and the frequency and type of research-related activities (i.e. questionnaires and in-person CVD risk assessments) and answered any questions he or she had. Eligibility screening then proceeded in the two trials as described in the following sections.
Depression trial
From the 10th recruited general practice onwards, it is important to note that the PHQ-9 questionnaire completed by patients in the depression trial constituted a prescreening stage in the eligibility assessment. In this way, the research team telephoned only those patients who had a total PHQ-9 score of ≥ 10 to go through the study in more detail and to conduct further eligibility screening. During the 10- to 30-minute call, the researcher began by asking patients to confirm whether or not they were currently receiving case management from a specialist mental health worker, any form of CBT or any other psychological or talking therapy. If so, they were informed that the study would not be suitable for them. Patients receiving counselling or group therapy without a CBT element, and patients who were on a waiting list without having been given a start date for CBT or other formal psychological therapy, were deemed eligible. If patients were on a waiting list and knew that they would be starting CBT or another therapy within the next 2 months, to avoid conflicting with therapeutic styles for those who might be allocated to the intervention, they were not eligible to participate.
Provided that a patient was not already receiving the forms of support listed above, the researcher then conducted a computer-based CIS-R assessment to establish whether or not the patient had a confirmed diagnosis of depression. The CIS-R has an algorithmic structure so that the answer to one question determines which question is asked next, as well as how many questions are asked in total. Respondents are asked whether or not they have been experiencing various symptoms (e.g. tiredness, sleeping too much/too little, weight changes, suicidal ideation) in the past month or past week, with higher scores indicating greater severity. Although the CIS-R encompasses a number of sections, we modified the assessment such that only questions required to establish a depression diagnosis (rather than other psychiatric diagnoses) were asked of participants. This measure provides an ICD-10 (International Classification of Diseases, Tenth Revision) diagnosis of depression and has been validated for telephone completion. 302
After completing the CIS-R, the programme produced an output to enable the researcher to confirm whether or not patients met the inclusion criterion of having an ICD-10 diagnosis of mild, moderate, or severe depression. For these patients, the researcher went through the final set of eligibility criteria, namely confirming their ability to access the internet and e-mail, that they had the ability to communicate verbally in English sufficiently to receive telephone-based support delivered in English, that there were no literacy issues, that they had not had a baby in the last 12 months and that they did not plan to be away for more than 1 consecutive month in the next 4 months. As mentioned previously, patients who could communicate verbally in English but who were unable to read English were deemed eligible if they had a family member or friend who was willing and able to translate written materials (such as information sheets, consent forms and online material) for them. If a patient was unable to confirm each of these details, the study was unsuitable for him or her.
The procedure for confirming eligibility was similar to that described above for those participants who either were recruited prior to the inclusion of the prescreening PHQ-9 form in the study invitation packs or who failed to return a PHQ-9 form with their expression of interest. The main difference was that the researcher telephoned all patients registering interest in the trial, conducted the CIS-R assessment and then proceeded to go through the remaining eligibility criteria questions over the telephone, except for the PHQ-9. The final step in the procedure for these participants was to complete the full study questionnaire, which contained the PHQ-9, to confirm eligibility with a PHQ-9 score of ≥ 10.
Significant suicide risk was one of the exclusion criterion, which was determined by codes in patients’ electronic records, revision of the patient lists by GPs or in response to concerns raised with a patient’s GP by the research team. In terms of the last point, both the PHQ-9 and the CIS-R have one or more questions that assess potential suicidal ideation. We developed a suicide safety protocol which indicated that any concerns of suicidal ideation would be escalated to a patient’s GP with the patient’s permission. If the patient would not consent to this information being passed on to his or her GP, the protocol indicated that one of the trial clinicians would telephone the patient to assess risk. Regardless of the patient’s permission, the suicide protocol was activated if the patient’s response – whether by answers or comments written on the screening assessment questionnaires or a comment that was made during the telephone call with the patient – indicated suicidal ideation.
Cardiovascular disease risk trial
Unlike the depression trial, there was no prescreening instrument provided for CVD risk patients with the study invitation and so all of the patients who expressed an interest in participating were telephoned by a member of the local research team. The conversation lasted approximately 15–20 minutes. After explaining about the study, as described previously, the researcher began by confirming that patients met the basic inclusion criteria: ability to access the internet and e-mail for personal use, ability to communicate verbally in English sufficiently to receive telephone-based support delivered in English, no literacy issues and not pregnant or planning to become pregnant in the next 12 months. Again, translation of written materials or support with reading otherwise was permissible, provided that the patient had a friend or family member willing to do so. If relevant, the researcher confirmed that patients did not plan to be away for > 1 consecutive month in the next 6 months or for > 2 consecutive months in the last 6 months of the study.
Patients who met the basic inclusion criteria and wanted to proceed with the study were asked to attend a 15-minute appointment at their general practice with a practice nurse or health-care assistant (HCA) who had been trained by the local researcher on study procedures. The nurse or HCA took four blood pressure readings at 1-minute resting intervals using an OMRON M3 upper-arm blood pressure monitor. The first reading was always on the patient’s left arm, followed by the second reading on the right arm. Readings 3 and 4 were taken on the same arm, which was determined by the arm that gave a higher systolic result on readings 1 and 2. Using an electronic calculator within a Microsoft Excel spreadsheet provided by the researchers, the nurse or HCA then calculated the average systolic and diastolic result based on the final two readings and recorded this on an eligibility assessment form (see Appendix 18). Likewise, after measuring height and weight, the nurse or HCA used the researcher-provided electronic calculator to work out each patient’s BMI. Smoking status was assessed by patient self-report but also validated by measurement using a carbon monoxide monitor (COmpact Smokerlyzer, Bedfont Scientific, Maidstone, UK). Finally, other information necessary to calculate CVD risk using the QRISK2 algorithm was also collected (e.g. ethnicity, age, family history of CVD, diagnosis of diabetes).
At this point in the assessment, the nurse or HCA determined whether or not patients had one or more modifiable risk factors: systolic blood pressure of ≥ 140 mmHg, BMI of ≥ 30 kg/m2 or self-reported current smoker. If so, the nurse or HCA reviewed the patient records to look for the results of a full lipid profile test within the last 3 months. If a patient had had this test conducted within the previous 3 months, the existing result was recorded on the assessment form and the blood test was not repeated. If a patient had one or more modifiable risk factors but had not had a recent blood test, the nurse or HCA took a non-fasting blood sample for either finger prick or laboratory testing of the ratio of total cholesterol to HDL cholesterol. The CVD risk assessment form was then sent to the research team, who used this information to calculate a 10-year CVD risk score using QRISK2.
Patients with at least one modifiable risk factor and a QRISK2 score of ≥ 20 were deemed eligible and were contacted again by telephone to inform them of their eligibility status (patients who were not eligible were notified by e-mail). Additionally, patients were informed which modifiable risk factor(s) the intervention team would mainly work with them on improving if they were randomly allocated to the intervention group (i.e. getting blood pressure better controlled, becoming more active, eating a healthier diet and/or giving up smoking), depending on whether or not they had high blood pressure (systolic blood pressure ≥ 140 mmHg), were obese (BMI ≥ 30 kg/m2) and/or currently smoked. We decided that it was important to explain to patients what risk factors the intervention would primarily target so that they could make an informed choice about whether or not they were still interested in participating in the study. This second call typically took 5–10 minutes to complete.
Consent, baseline assessment and randomisation
After confirming eligibility as outlined in the previous section, patients in both trials were asked to complete a consent form and a baseline assessment questionnaire. These could be completed on a secure survey website or on a paper copy sent by post, according to patient preference. In total, the baseline questionnaire took about 40–45 minutes to complete. Once both were completed and received by the research team, patients were randomly allocated on a 1 : 1 basis to receive either (1) usual care plus the Healthlines Service (intervention group) or (2) usual care alone (control group). Participants were allocated using an automated web randomisation system to ensure concealment from research staff prior to allocation. This randomisation system was developed independently and hosted by the Bristol Randomised Controlled Trials Collaboration, a UK Clinical Research Collaboration-registered clinical trials unit. Randomisation was stratified by location of recruitment (Bristol, Sheffield or Southampton) and minimised by practice and baseline PHQ-9 depression score or QRISK2 score, retaining a probabilistic element by using a computer-generated random number sequence. The minimisation categories for PHQ-9 score were 10–14, 15–19 and ≥ 20,289 whereas the categories for QRISK2 score were 20.0–24.9, 25.0–29.9 and ≥ 30.0.
Participants in the depression trial were randomised into the study between July 2012 and July 2013. As explained in detail in Commencement of the depression trial and delay to the cardiovascular disease risk trial, there were delays to the start of the CVD risk intervention. Therefore, CVD risk participants were randomised into the study slightly later, between December 2012 and July 2013. For some participants, this meant that there was a gap of several months between their initial recruitment and beginning to receive the intervention. The implications of this are addressed in the discussion sections in Chapters 8, 9 and 12.
Communication following allocation
Participants in both trials were notified by e-mail from the research team of their allocation, as well as when the next study questionnaire would be sent to them. Their GP was also informed of their participation in the study, their allocation and their baseline PHQ-9 or QRISK2 score. For those allocated to the intervention, the notification e-mail also contained the same ‘Welcome Pack’ document that was available through the Healthlines web portal (see Chapter 6 and Appendix 10). As explained in more detail in Chapter 6, the ‘Welcome Pack’ included details about the service and information about and colour photographs of the HIAs delivering the intervention.
Subsequent to the e-mail, the local researcher telephoned participants in the intervention group in the CVD risk trial who had systolic blood pressure of ≥ 140 mmHg and who did not also have atrial fibrillation. These participants were offered a home blood pressure monitor to use during the study, but they could decline this option or use their own blood pressure monitor if they had one already. If participants wanted to use one of the blood pressure monitors provided by the study, they attended an appointment for a short training session at their own general practice with a practice nurse or HCA who had been instructed by the research team on study procedures. This session was to ensure that the participant knew how to use the monitor correctly and also to provide the participant with a set of detailed instructions and a booklet for recording their blood pressure readings ahead of entering them on the Healthlines web portal. Participants were instructed to wait until they had been contacted by their HIA before they began regularly taking their blood pressure. After this initial intervention encounter, participants were asked to take blood pressure readings twice daily for the first week and weekly thereafter. There are more details around the blood pressure monitoring component of the intervention in Chapter 6.
Following allocation, the local researcher passed the intervention participants’ details on to the intervention team via a secure NHS-based e-mail system (NHSmail), including participants’ age, sex, contact details and calling preferences. Referrals to the intervention team began in July 2012 and December 2012 for the depression and CVD risk trials respectively. In the depression trial, the researcher also provided the participants’ baseline PHQ-9 score, GAD-7 score and whether or not the participant was currently taking antidepressants. The following details were included in the referral to the intervention team for the CVD risk trial: baseline QRISK2 score, height, weight, BMI, average blood pressure, target blood pressure, whether or not the participant was taking blood pressure medication, smoking status, ratio of total to HDL cholesterol and whether or not the participant had diabetes, atrial fibrillation or chronic kidney disease. As detailed in Chapter 6, the participant referral information was passed on to the intervention team for those CVD risk participants who were self-monitoring their blood pressure only after they had picked up their blood pressure monitor. Regardless of trial arm, once these participant details were received and uploaded to the intervention patient management database, one of the HIAs contacted the participants by telephone to conduct an initial assessment and provide them with log-in details and a link to the Healthlines Service website.
Trial run-in phase
In the run-in phase of the trial recruitment commenced 1 month ahead of that in the main trials, with the patient searches starting in May 2012 and the first participants randomised into the trial in July 2012 (depression trial) and December 2012 (CVD risk trial). Two general practices were involved and each aimed to recruit 20 patients to the depression trial and 20 to the CVD risk trial. The purposes of the run-in phase were to test the study recruitment and follow-up procedures and to allow any required adjustments to be made before the main trials. These steps also allowed intervention staff the opportunity to develop their skills in using the intervention software and treatment protocols. To provide intervention staff with a larger number of participants to work with during the run-in phase, allocation in this phase was at a ratio of 3 : 1 in favour of the intervention group. Data collection, intervention schedules and intervention content were the same for run-in phase participants as in the main trials and so data provided by these participants were included in the final trial analysis. No substantial changes were made between the run-in phase and the main trials, other than increasing the number of patients sent an initial study invitation, as described earlier.
Commencement of the depression trial and delay to the cardiovascular disease risk trial
As covered in more detail in Chapter 6, after participants were randomised into the Healthlines Service group by the research team, it was anticipated that within the next 1–2 weeks the HIAs would have completed the initial assessment encounter with them. This was usually the case for the depression participants, but there was an unexpected delay to the start of the CVD risk trial. Although the first randomised depression participants began receiving the intervention by August 2012, the corresponding group of CVD risk participants was not randomised into the study until early December 2012. The delay was partly due to the IT infrastructure around the CVD patient management software. This required more work to adapt it to the NHS context and there were difficulties integrating the Duke software with the software used normally used within NHS Direct.
We believed that the IT infrastructure was in place by early December 2012 and began to randomise the CVD risk participants, but it then became apparent that there were several problems with the telephone scripts to support patients with high blood pressure, particularly patients who were not already taking blood pressure medication or who had not yet picked up their blood pressure monitors. To remedy this, revision to the scripts was required, which meant altering the patient management software.
The IT changes were not fully integrated until March 2013. In the meantime, the intervention progressed as planned from December 2012 for all other CVD risk patients not affected by the two blood pressure issues described above. Likewise, as soon as those patients already taking blood pressure medication had collected their home blood pressure monitor, they could be referred to the intervention team for their initial assessment telephone call. Nonetheless, some CVD risk patients were randomised and then experienced a significant delay of 2–3 months before they began receiving the intervention. We describe the effect of this on intervention delivery in the final subsection of this chapter.
Transfer of the service from NHS Direct to Solent NHS Trust
As covered in Chapter 6, we negotiated to transfer the study and intervention from NHS Direct to NHS Solent Trust following the closure of NHS Direct on 31 March 2014. To summarise, the main challenges in transferring the intervention across NHS organisations involved the reassignment of intervention staff and their manager in two different locations, making arrangements for ongoing clinical supervision, organising support for the IT infrastructure used to deliver the intervention and ensuring that contracts and finances were quickly organised. In terms of the main IT issues, laptops had to be configured and mobile telephones arranged for the HIAs who had agreed to work from home for the remainder of the study, at the same time as transferring the IT platform from NHS Direct to NHS Solent Trust’s network. To allow for this IT work to be carried out and tested, it was necessary to institute a temporary break in the intervention delivery between 1 April and 6 May 2014.
In preparation for the break in service delivery, all ongoing intervention participants and participating general practices were notified by e-mail that the NHS provider of the intervention was changing, but that other aspects of the intervention, including the staff delivering it, would remain unchanged. The possibility of a delay in some scheduled appointments and the new web portal address and telephone call-back number were all included in this notification. The web portal and resources available to participants otherwise remained the same. Ongoing control group participants were also notified by e-mail of the change of lead NHS organisation for the research.
At most, we estimate that the temporary break affected 39% (120/307) of depression and 67% (218/325) of CVD risk participants, the maximum number of participants remaining in the service at the time, but some of these participants would not have had a scheduled call during the service closure period. For those participants who did already have a scheduled telephone appointment during the temporary closure period, these appointments had to be rescheduled for after the break. As a result, some patients were expected to have a slightly longer period between some appointments for a time and then a slightly shortened period between other appointments thereafter. This adjustment to the usual call schedule was required to fit in their remaining appointments in the time available. Nevertheless, we did not anticipate that this would have a major effect on the total number of appointments that participants were due to receive over the entire study period, except for some patients whose 12-month follow-up period ended during the break.
As planned, the HIAs resumed patient telephone calls from 6 May 2014. However, because of technical difficulties surrounding the transfer of the IT infrastructure, the patient management software was not fully functional until 9 June 2014. As the patient management software included access to the telephone scripts and the facility for recording details around the telephone calls, including updating notes around the patients’ health, the intervention could not be delivered as planned until 9 June 2014. Likewise, CVD risk patients were unable to enter blood pressure readings on the web portal between 1 April and 16 June 2014. As these patients had also been supplied with a blood pressure recording booklet, they were able to keep track of their weekly blood pressure readings and enter these retrospectively when the web portal regained functionality after 16 June.
To carry out the scheduled telephone appointments between 6 May and 9 June 2014, and to do so without access to the patient management software, the research team developed a paper-based call log to record the details around calls and devised a ‘holding script’ for the HIAs to deliver. The ‘holding script’ explained about the unfortunate IT difficulties and asked how patients were getting on with the various resources (e.g. CBT package, blood pressure monitoring) they were using as part of the intervention, how they were doing in terms of their health needs and goals and whether or not they had any particular issues that they wanted to raise. Unless this was the final intervention call, the HIA then booked participants in for a further call, which would cover the next encounter that they were due to complete. If this appointment was the final intervention call at the end of the 12-month intervention period, the HIA thanked the participants for taking part and explained that they would return to the sole care of their GP from now on. Overall, the feedback from participants was that they liked the ‘holding script’ and it was a positive experience to have this kind of informal review. The details recorded in the paper-based call logs were later entered into the patient management software.
Impact on intervention encounters
The final research assessment for both depression and CVD risk participants was due 12 months after participants were randomised into the study. It was intended that participants’ intervention sessions would be delivered over the same 12-month period. However, as outlined in the previous two sections above, there were some delays between recruitment and the initial intervention session and delays in scheduling regular intervention calls, for example because of holidays or because participants had been difficult to contact or because of the necessary IT ‘down time’ as the service transferred from NHS Direct to Solent NHS Trust. This meant that many participants did not receive the full number of intervention sessions by the time that they were due for their final research assessment.
To remedy the situation and attempt to maximise the number of sessions that participants received ahead of their 12-month assessment, the frequency between intervention calls was reduced for all remaining participants from January 2014 as follows:
-
For depression participants, the fortnightly call schedule continued for encounters 1–8, but encounters 9 and 10 were scheduled at 6-weekly intervals instead of the 2- to 3-monthly intervals that were originally planned.
-
For CVD risk participants, encounters were scheduled every 3 weeks instead of monthly. If the date that occurred 3 weeks ahead was not possible (e.g. because of holiday), HIAs were encouraged to first try further reducing the appointment interval to 2 weeks, before considering dates ≥ 4 weeks ahead.
In addition to those procedures described above, the research team arranged one final scheduled session with the HIA for participants who had not received all of the intervention sessions by the 12-month research assessment, if participants wished. GPs were informed by e-mail when their patients completed the intervention.
Subsidiary studies
Within the Healthlines study, four subsidiary studies were embedded within the two RCTs. The first was an attempt to increase study uptake among invited participants in both trials by enhancing the participant information sheet, with the remaining three studies attempting to increase response rates to the follow-up questionnaire among participants in the depression trial alone.
First, the MRC Systematic Techniques for Assisting Recruitment to Trials (MRC START) study is funded by the MRC Methodology Research Programme. The purpose of the study is to develop the conceptual, methodological and logistical framework for nested studies and to assess their feasibility. 303 The Healthlines study acted as a host trial for one of the MRC START recruitment interventions. In particular, the aim was to test the impact of an enhanced patient information sheet and invitation letter on recruitment rates. 304 Through reduced use of jargon, simpler sentences and an altered layout of the materials, the MRC START materials sought to improve readability and ease of comprehension compared with the standard versions. The substudy was carried out in the final three general practices at the Bristol site only, with participants in both the depression and the CVD risk trials randomly allocated on a 1 : 1 basis to receive the optimised or standard study materials. After generating the list of participants to invite to the Healthlines study, it was sorted by a randomly generated number and the first half of the list was assigned the optimised study materials. There were no other differences in the information provided to these participants or the research procedures. The results of this MRC START substudy have now been published. 305
Next, as difficulties with motivation and concentration are at the heart of depression, this is a difficult patient group to keep engaged in research, especially over the course of a 12-month study. Therefore, we attempted three different methods to increase response rates with these participants at follow-up. This consisted of three individually nested studies within the depression trial alone. These studies aimed to investigate the impact on follow-up questionnaire response rates of pre-calling participants ahead of sending out the follow-up questionnaire or not (study 1), including a colour photograph of the research team with the questionnaire cover letter/e-mail or not (study 2) and using an urgent action subject line (‘ACTION REQUIRED’) in the e-mail reminder compared with one that simply states that this was a reminder (study 3). These three ideas were generated following discussion within the research team and in light of previous studies306 which suggested that such changes may have an effect on follow-up rates. Study 1 occurred partway through the 8-month follow-up time point, with participants in the Bristol location alternately allocated to receive the intervention or not. Based on an early and informal indication that there was a benefit from pre-calling these participants at 8 months’ follow-up, this procedure was adopted universally for all final 12-month follow-up appointments. Therefore, studies 2 and 3 included the manipulation from study 1 as an adopted standard procedure but examined the possibility of further boosting the 12-month follow-up response rates with two separate manipulations with participants from the different study locations, with the photo manipulation in study 2 conducted with all participants from the Bristol study centre only and the e-mail subject line manipulation in study 3 including respondents from the Sheffield and Southampton centres. Based on a random number generated using Microsoft Excel, participants in studies 2 and 3 were randomised on a 1 : 1 basis to receive the intervention using simple randomisation. Details of the methods and results of these studies have been published. 307
Outcome measurement
Primary outcomes
In both trials, the primary outcome was the proportion of participants responding to treatment. In the depression trial, response to treatment was defined as a PHQ-9 score of < 10 and a reduction in PHQ-9 score of ≥ 5 after 4 months. 289,308 A total PHQ-9 score is calculated as the sum of the responses to the nine questions that constitute the scale, with the response options ranging from 0 (‘not at all’) to 3 (‘nearly every day’). If one item was left unanswered on a particular PHQ-9 form, this was replaced with the mean response of the remaining eight questions for that participant. If more than one item was unanswered, then the PHQ-9 score was classed as missing data. Depression severity is given by the following total score ranges: 0–4 – none; 5–9 – mild; 10–14 – moderate; 15–19 – moderately severe; and 20–27 – severe. The stringent dual criterion for defining ‘response’ was chosen to take account of the fact that a PHQ-9 score of < 10 is conventionally taken as indicating recovery but a small reduction from 11 to 10 between baseline and follow-up may simply reflect random variation. Previous research suggested that a reduction of 5 points in PHQ-9 score at an individual level is a clinically important change. 308
Response in the CVD risk trial was defined as the maintenance or reduction of 10-year cardiovascular risk estimated on the basis of QRISK2 score195 after 12 months. As CVD risk increases with age, maintaining 10-year risk over 12 months requires an improvement in at least one modifiable risk factor. Throughout the study, the QRISK2 score was calculated using the same version of the QRISK2 algorithm, which was provided as a batch processor to the research team by the authors of the QRISK2. QRISK2 outcome scores at follow-up were calculated by changing only participant age and modifiable risk factor measurements (blood pressure, BMI and smoking status, as well as cholesterol values). The cholesterol level was measured at baseline and 12 months and so baseline cholesterol values were used in the 6-month QRISK2 score. Variables such as diagnosis of diabetes or atrial fibrillation and whether or not the patient was prescribed blood pressure-lowering medications were held constant across time points using baseline values. This was because it was possible that the extra support provided by the intervention might result in an increased number of patients in the intervention arm receiving a diagnosis or starting medication, which in turn would inflate risk scores in this group only. As mentioned earlier, smoking status was determined by participant self-report, although validated by a carbon monoxide reading using a Bedfont Scientific COmpact Smokerlyzer. Self-reported non-smokers and ex-smokers were recategorised as light smokers if the carbon monoxide device illuminated with three or more lights (11+ ppm); this affected four self-reported ex-smokers at baseline, one ex-smoker at 6 months and four non- and four ex-smokers at 12 months.
Finally, the QRISK2 score is highly sensitive to age. Exact age at baseline and follow-up was calculated based on date of birth and date of the baseline CVD risk assessment. However, age is included in QRISK2 as an integer and it was expected that all participants in the CVD risk trial would age 1 year between randomisation and the final 12-month follow-up. This was true for the vast majority of participants. Because of the timing of the assessments in relation to birthdays, some participants’ age either did not change or increased by 2 years. For the primary analysis age was analysed as calculated, but a sensitivity analysis was carried out in which all participants aged by exactly 1 year.
Primary outcome deviations from the protocol
During data cleaning, several problems were identified with how the QRISK2 scores had been calculated at baseline. These problems, and how they were rectified, are discussed in more detail in Appendix 19. As a consequence of these errors, six participants were identified who, on recalculation, were found to have a QRISK2 score of just under 20% and who therefore should not have been included in the trial. However, these participants were still included in the analyses for several reasons: (1) because of the intention-to-treat principle; (2) because the 20% high risk threshold is arbitrary and these six participants with a slightly lower risk still had the potential to benefit; and (3) because this approach was conservative, given that patients with a lower risk have less potential to benefit.
Secondary outcomes
Secondary outcomes are listed in Table 13. They include variables related to the primary outcome but dichotomised and/or at different time points; measures needed to examine components of our theoretical TECH model (e.g. self-management); and measures of constructs hypothesised to influence the effectiveness of the intervention (e.g. technology confidence). These measures were included in both trials unless otherwise indicated and were piloted in advance with 10 members of the research team, two patient and public involvement representatives affiliated with the research programme and eight members of the lay public approached by the service user representatives or study team.
| Outcomes | Measures used |
|---|---|
| Depression score (depression trial only) as a binary outcome at 8 and 12 months and as a continuous variable at 4, 8 and 12 months | PHQ-9289 |
| Ten-year CVD risk score (CVD risk trial only) as a binary outcome at 6 months and as a continuous variable at 6 and 12 months | QRISK2195 variable obtained by clinical assessment |
| Quality of life | EQ-5D-5L309 |
| Patient satisfaction | Subset of Y4Q1 GP Patient Survey,199 subset of CoBalT trial items310 and items constructed for present research |
| Patient perceived access to care | Items constructed for present research |
| Motivation for study participation | Subset of items adapted from TMQ-R311,312 |
| Physical activity | Health-directed behaviour domain of heiQ313 |
| Individual CVD risk factors, i.e. blood pressure, cholesterol, smoking status, weight, BMI (CVD risk trial only) | Clinical assessment |
| Diet (CVD risk trial only) | STC314 anglicised for present use |
| Use of telehealth | Items constructed for present research |
| Confidence using technology | Items constructed for present research |
| Self-management skills and self-efficacy | Self-monitoring and insight, constructive attitudes and approaches, skill and technique acquisition and health-care services navigation domains of heiQ313 |
| Medication adherence (depression trial asked about antidepressant medication; CVD trial asked about antihypertensive and cholesterol-lowering medications) | Morisky Medication Adherence Scale315 |
| Health literacy | Items adapted from eHEALS316 |
| Care co-ordination | Stand-alone indicators and two subscales (role clarity and co-ordination within GP surgery, evidence of a care plan) adapted from a generic measure of continuity of care questionnaire317 |
| Anxiety (depression trial only) | GAD-7293 |
The baseline questionnaire included sociodemographic characteristics (age, sex, ethnicity, employment status, educational attainment, house ownership), comorbidities (e.g. anxiety), current treatments (e.g. antidepressants, blood pressure medication) and all other secondary outcome measures listed in Table 13 (see Appendix 20). Follow-up questionnaires included primary and secondary outcome measures, as well as use of health-care resources (see Appendix 21). Details regarding frequency and dosage of medication prescriptions and primary care contacts were collected by the researchers from primary care medical records for all participants 12 months after randomisation. Patient-reported quality of life using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),309 use of health-care resources and data collected from primary care medical records relate to the economic evaluation and so are described in more detail in the next section of this chapter.
There were two additional sources of secondary outcome data. First, for those in the intervention groups in both trials, information about the type, number and length of contacts with intervention staff was collected from the Healthlines Service electronic records. Second, anonymised information, including age, sex and ethnicity (both trials) and CVD risk score and component data (CVD risk trial only), was obtained from practice records of invited patients who decided not to participate in the study.
As detailed in Table 13, most of the secondary outcome measures are from validated scales. In these cases, the individual response options to each scale were either summed to produce total scores (e.g. GAD-7 anxiety score) or were averaged to produce summary scores (e.g. role clarity and co-ordination subscale from the generic measure of continuity of care317) in accordance with the particular instrument in question. Additionally, two items from the generic measure of continuity of care317 are stand-alone indicators of care co-ordination and so a total score was produced from the responses to these single items.
For all secondary measures, scores were reversed as appropriate ahead of all analyses, such that higher numbers indicated a more positive response (i.e. greater satisfaction, less difficulty, more agreement and so on). Additionally, responses of ‘does not apply’, ‘did not use this’ or similar were excluded from the analyses.
The baseline questionnaire took around 45 minutes to complete, whereas the follow-up versions took approximately 1 hour. To reduce the burden on respondents as much as possible, subsets of items from larger validated scales were selected in some cases (see Table 13). When the larger measure consisted of multiple subscales, we selected all items from that subscale and created a subscale summary score as the average of the response options for the relevant items making up the subscale. However, in the case of the questions selected to assess motivation for study participation and health literacy, only a small proportion of the available items was included. Therefore, to ensure that the items we selected continued to group together as expected and had adequate psychometric properties, we conducted PCAs with orthogonal (varimax) rotation on these subset scale items using Stata 13.1. The items and resulting scales are presented in Table 14.
| Construct | Response format (reversed when appropriate) | Item or item content | Stand-alone or scale item | Scale reliability (n items, α or ra) |
|---|---|---|---|---|
| Patient satisfaction | ||||
| General GP satisfaction | Evaluative (1 = ‘extremely dissatisfied’ to 5 = ‘extremely satisfied’, 6 = ‘does not apply’) | ‘In general, how satisfied are you with the care you received in the last 4 [6 (CVD risk)] months from the doctor(s) at your GP surgery or health centre?’ | Stand-alone | – |
| General nurse satisfaction | Evaluative (1 = ‘extremely dissatisfied’ to 5 = ‘extremely satisfied’, 6 = ‘does not apply’) | ‘In general, how satisfied are you with the care you received in the last 4 [6 (CVD risk)] months from the nurse(s) at your GP surgery or health centre?’ | Stand-alone | – |
| General NHS Direct satisfaction | Evaluative (1 = ‘extremely dissatisfied’ to 5 = ‘extremely satisfied’, 6 = ‘does not apply’) | ‘In general, how satisfied are you with the care you received in the last 4 [6 (CVD risk)] months from NHS Direct health advisors?’ | Stand-alone | – |
| Detailed GP satisfactionb | Evaluative (1 = ‘very poor’ to 5 = ‘very good’, 6 = ‘does not apply’) | ‘Thinking about the doctor(s) you saw at your surgery in the last 4 [6 (CVD risk)] months, how good were they at doing each of the following: listening to you, explaining tests and treatments, involving you in decisions about your care, treating you with care and concern?’c | Scale (excluded ‘does not apply’) | 4 items; α = 0.96 for depression, α = 0.94 for CVD risk |
| Detailed nurse satisfactionb | Evaluative (1 = ‘very poor’ to 5 = ‘very good’, 6 = ‘does not apply’) | ‘Thinking about the nurse(s) you saw at your surgery in the last 4 [6 (CVD risk)] months, how good were they at doing each of the following: listening to you, explaining tests and treatments, involving you in decisions about your care, treating you with care and concern?’c | Scale (excluded ‘does not apply’) | 4 items; α = 0.96 for depression, α = 0.94 for CVD risk |
| Detailed HIA satisfactionb,d | Evaluative (1 = ‘very poor’ to 5 = ‘very good’, 6 = ‘does not apply’) | ‘Thinking about the NHS Direct Healthlines advisors that you had contact with in the last 4 [6 (CVD risk)] months, how good were they at doing each of the following: listening to you, explaining tests and treatments, involving you in decisions about your care, treating you with care and concern?’c | Scale (excluded ‘does not apply’) | 4 items; α = 0.96 for depression, α = 0.96 for CVD risk |
| Treatment satisfaction | Evaluative (1 = ‘strongly disagree’ to 5 = ‘strongly agree’, 6 = ‘does not apply’) | Extent of agreement that any treatment received for mental health in the last 4 months [heart health in the last 6 months (CVD risk)] was satisfactory; improved health, improved mood; good-quality advice and support was received; would recommend to others; would use again if needede | Scale (excluded ‘does not apply’) | 6 items; α = 0.92 for depression, α = 0.91 for CVD risk |
| Intervention website satisfactionb,d | Evaluative (1 = ‘strongly disagree’ to 5 = ‘strongly agree’, 6 = ‘does not apply’) | ‘Information on the NHS Direct Healthlines Service website was helpful’; ‘Information on the NHS Direct Healthlines Service website was easy to use’ | Scale (excluded ‘did not use this’) | 2 items; r = 0.60 for depression, r = 0.71 for CVD risk |
| Intervention CBT satisfactionb,d | Evaluative (1 = ‘strongly disagree’ to 5 = ‘strongly agree’, 6 = ‘does not apply’) | ‘The Living Life to the Full CBT programme was helpful’; ‘The Living Life to the Full CBT programme was easy to use’ (depression trial only) | Scale (excluded ‘did not use this’) | 2 items; r = 0.77 |
| Intervention blood pressure web page satisfactionb,d | Evaluative (1 = ‘strongly disagree’ to 5 = ‘strongly agree’, 6 = ‘does not apply’) | ‘The web page to record my blood pressure was helpful’; ‘The web page to record my blood pressure was easy to use’ (CVD risk trial only) | Scale (excluded ‘did not use this’) | 2 items; r = 0.86 |
| Access to care | ||||
| Service delivery access difficulties | Evaluative (1 = ‘extreme difficulty’ to 7 = ‘no difficulty at all’) | ‘In the past 4 [6 (CVD risk)] months, have you had any difficulty with getting support and advice at times that suit you? From the health professionals you want to see? When you feel you need it most? At a time that is convenient for you, according to your needs, lifestyle and preferences?’f | Scale | 4 items; α = 0.95 for depression, α = 0.96 for CVD risk |
| Amount of support | Evaluative (1 = ‘too little’ to 3 = ‘just about right’ to 5 = ‘too much’, 6 = ‘does not apply’) | Extent to which the amount of support and advice received for mental health in the last 4 months [heart health in the last 6 months (CVD risk)] was adequate | Stand-alone | – |
| Motivation for study participation | ||||
| Internal motivation | Evaluative (1 = .not at all true’ to 7 = ‘very true’) | ‘I joined this study because I want to make changes in my life’; ‘I joined this study because I am interested in getting help’g | Scale | 2 items; r = 0.69 for depression, r = 0.67 for CVD risk |
| External motivation | Evaluative (1 = ‘not at all true’ to 7 = ‘very true’) | ‘I joined this study because I felt under pressure to go for treatment’; ‘I joined this study because my doctor told me I should be in treatment’g | Scale | 2 items; r = 0.33 for depression, r = 0.57 for CVD risk |
| Health literacy | ||||
| Health literacy | Evaluative (1 = ‘strongly disagree’ to 5 = ‘strongly agree’) | ‘I know how to find helpful information about my health’; ‘I can tell high-quality from low-quality health information’; ‘I feel confident using health information to make health decisions’h | Scale | 3 items; α = 0.81 for depression, α = 0.81 for CVD risk |
| General web usage | ||||
| Internet use | Reporting (1 = ‘never’, 2 = ‘once a month’, 3 = ‘about every 2 weeks’, 4 = ‘once/twice a week’, 5 = ‘daily’) | Frequency of using e-mail and the internet (excluding e-mail) | Scale | 2 items; r = 0.69 for depression, r = 0.77 for CVD risk |
| Technology confidence | ||||
| Telephone confidence | Evaluative (1 = ‘not at all confident’ to 7 = ‘extremely confident’) | Level of confidence with using a telephone (landline), using a mobile telephone for telephone calls, using a mobile telephone to send and receive text messages | Scale | 3 items; α = 0.80 for depression, α = 0.64 for CVD risk |
| Internet confidence | Evaluative (1 = ‘not at all confident’ to 7 = ‘extremely confident’) | Level of confidence with searching for information on the internet (e.g. using Google), sending and receiving e-mails | Scale | 2 items; r = 0.74 for depression, r = 0.77 for CVD risk |
| Social media confidence | Evaluative (1 = ‘not at all confident’ to 7 = ‘extremely confident’) | Level of confidence with using a ‘chat room’ or forum on the internet, using social networking sites on the internet (e.g. Facebook) | Scale | 2 items; r = 0.67 for depression, r = 0.72 for CVD risk |
| Use of telehealth | ||||
| NHS Direct telephone | Reporting (1 = ‘never’, 2 = ‘once a month’, 3 = ‘about every 2 weeks’, 4 = ‘once/twice a week’, 5 = ‘daily’) | Frequency of using NHS Direct telephone services in the past 4 [6 (CVD risk)] months | Stand-alone | – |
| Online searching | Reporting (1 = ‘never’, 2 = ‘once a month’, 3 = ‘about every 2 weeks’, 4 = ‘once/twice a week’, 5 = ‘daily’) | Frequency of searching online for health information for oneself in the past 4 [6 (CVD risk)] months | Stand-alone | – |
| Online forum or group | Reporting (1 = ‘never’, 2 = ‘once a month’, 3 = ‘about every 2 weeks’, 4 = ‘once/twice a week’, 5 = ‘daily’) | Frequency of participating in an online forum or support group for physical or mental health in the past 4 [6 (CVD risk)] months | Stand-alone | – |
The following constructs were assessed with a collection of items constructed for this research: patient satisfaction, perceived access to health care, internet use, technology confidence and telehealth use. To ensure the coherence of the questions included to assess these constructs and to reduce the questionnaire items to a smaller number of factors for data analysis, PCAs with orthogonal (varimax) rotation were carried out on the constructed items using Stata 13.1. These analyses were conducted solely on the baseline data or at the first follow-up time point for questions asked only in the follow-up questionnaires. Decisions regarding the number of factors to extract were based on Kaiser’s criterion (eigenvalues > 1.0), by examining the scree plot and the subjective coherence of the factors. For each factor, items with an association of ≥ 0.3 were retained. 197 Next, the reliability of each factor was examined with Cronbach’s alpha and coefficients > 0.70 were taken to indicate adequate reliability. If the scale consisted of two items, a Pearson correlation coefficient was computed instead. Finally, mean summary scores for each reliable factor were calculated for individuals providing ratings for ≥ 50% of the relevant items. We treated each factor as a scale and labelled it according to the questions that it included (see Table 14).
The remaining baseline and follow-up questionnaire items were constructed for the present research based on previous literature, with the majority having been developed and pilot tested with service users for the patient survey (see Chapter 3). In these cases it was necessary to create our own measures because we were not aware of a validated measure that assessed the concept of interest or did so to the depth or breadth that was required by our research question. For example, we did not identify any validated scales that asked about qualitatively different types of difficulties accessing health care beyond concerns over waiting times for an appointment with a specifically named HCP.
Serious adverse events
Finally, serious adverse events (SAEs) in participants were monitored throughout the study. A SAE was defined as an untoward occurrence that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a congenital anomaly or birth defect or
-
was otherwise considered medically significant by the investigator.
Under the terms of the Standard Operating Procedures for RECs, any SAE that was considered ‘related’ and ‘unexpected’ had to be reported to the REC. 319 ‘Related’ was defined as resulting from the administration of any research procedures (e.g. as a result of the Healthlines Service intervention, completion of follow-up questionnaires, participation in the process evaluation) and ‘unexpected’ was defined as a type of event not listed in the protocol as an expected occurrence.
Data collection and follow-up
Data were collected from self-report questionnaires, primary care medical records, the Healthlines Service records and clinical measurement. Information was collected at baseline from all participants but the timing of follow-up data collection differed between the two trials (as detailed below). To maximise response rates, participants were offered the choice of completing the questionnaires on a secure survey website or on a paper copy sent in the post. The PHQ-9, which has been validated for telephone use, could also be completed by telephone with one of the researchers. The health economics quality of life measure (EQ-5D-5L) was not available for online completion because of licensing and technical restrictions and thus was completed on paper by all participants at each of the time points. If participants had opted to complete the questionnaire online, it was explained that the EQ-5D-5L would also be posted to them for completion. Up to five reminders, which were communicated by e-mail, telephone (twice) and post (twice in the depression trial, once in the CVD risk trial), were used if participants did not return their questionnaires. To maximise collection of the primary outcome, the second postal reminder in the depression trial contained the PHQ-9 alone. Likewise, participants in the depression trial were provided with the option to complete the PHQ-9 over the telephone in the second telephone reminder.
In the depression trial, self-report questionnaires were completed at four time points: at baseline and at 4, 8 and 12 months after randomisation. The PHQ-9 questionnaire was completed at each of these time points. At baseline it was completed as part of an initial screening questionnaire by post; at all other time points it was included with the follow-up questionnaire and therefore could be completed online or by post.
In the CVD risk trial, self-report questionnaires were completed at three time points: at baseline and at 6 and 12 months after randomisation. At each of these time points, participants were telephoned to ask them to attend an appointment with a nurse or HCA at their own general practice. As described in Primary outcomes, practice staff collected CVD risk factor information, including carrying out a blood test to measure cholesterol (at baseline and 12 months only), blood pressure, weight and smoking status (all time points). This information was used to calculate each participant’s QRISK2 score.
Statistical considerations
Sample size
This study was based on the anticipated analysis of data from 240 patients in each of the intervention and control groups for both the depression and CVD risk trials. The primary outcomes for both depression and CVD risk were binary, indicating response to the intervention. Assuming equal numbers in each trial arm for analysis and that all other parameters in a sample size estimate remain constant, the detectable between-group difference for a binary outcome is maximised when the overall proportion in the trial defined as responding is 50% (e.g. 57% responding to the Healthlines Service interventions and 43% responding to usual care). As the overall proportion responding increases or decreases from 50%, so does the detectable difference increase or decrease. Thus, with a sample size for analysis of 240 per arm, the absolute detectable difference between the trial arms with a 5% two-sided alpha level and 80% power is ≤ 14 percentage points (1.7 equivalent OR). With a 1% alpha level and 90% power, it is ≤ 18 percentage points (2.1 equivalent OR). For this trial there was no accepted minimum clinically important difference. Therefore, the sample sizes were chosen pragmatically, taking into account the size of effect that would be likely to influence policy and practice and which might be feasible using this intervention.
Regarding the depression trial, response to treatment using the PHQ-9 was defined as a score of < 10 and a reduction of at least 5 points at 4 months post randomisation. In a previous trial involving a similar patient group, the response in the control group was approximately 30%. 320 Regarding the CVD risk trial, a previous study reported that 38% of treated hypertensive patients aged 60–80 years in a control group had a reduced absolute CVD risk at the 12-month follow-up examination. 321 If the proportion responding in the control arm in either of our trials was in the 30–38% range, 240 participants per arm for analysis would have 80% power (5% alpha level) and 90% power (1% alpha level) to detect differences of 13 and 18 percentage points respectively.
Assuming 20% non-collection of primary outcome data at follow-up, we aimed to recruit 300 patients in each of the intervention and control groups for each trial or 1200 patients in total.
Descriptive analysis
A full statistical analysis plan was developed and agreed on with the Trial Management Group, the Data Monitoring Committee and the Trial Steering Committee prior to any comparative analyses. The analysis and presentation of each trial was carried out in accordance with CONSORT (Consolidated Standards of Reporting Trials) guidelines. 322 Appropriate descriptive statistics were used to compare summary characteristics of (1) invited patients who did or did not agree to take part, (2) eligible patients who were randomised or not randomised and (3) baseline characteristics of trial participants by allocated arm. When important imbalances were observed between intervention and control arms, these variables were included in regression models in sensitivity analyses.
Primary analysis
The null hypothesis for the depression trial was that there was no difference in response as measured by PHQ-9 score after 4 months between the intervention group and the control group. The null hypothesis for the CVD risk trial was that there was no difference in response as measured by maintenance or improvement in 10-year CVD risk, estimated using QRISK2, after 12 months between the intervention group and the control group. Each of these null hypotheses was tested using mixed-effects logistic regression models, adjusted for site, baseline value of the outcome and general practice (included as a random effect). Including a random effect at the practice level in the model measures the difference between the number of responses at each practice and the number of responses over all of the practices. This term accounts for practice-level differences in response to a treatment effect. Participants were analysed as randomised, regardless of how much intervention was received, and only participants with observed outcome data were included in these primary analyses. Effects are presented as ORs, 95% CIs and p-values.
Sensitivity analyses of the primary outcome
We conducted sensitivity analyses of the primary outcome in each trial as follows: (1) simple imputation of missing outcome data assuming no treatment response; (2) multiple imputation of missing data; (3) estimation of the treatment effect among compliers; (4) excluding general practice as a random effect; (5) calculation of QRISK2 score assuming that all participants aged by 1 year at the primary follow-up (CVD trial only); and (6) further adjustment by time between randomisation and follow-up and baseline variables imbalanced between arms (depression trial only).
Missing data were imputed using the multiple imputation by chained equation procedure, implemented using the ‘ice’ command in Stata 13.1. The imputation model for each trial followed recommended practice323 by including allocation, demographic variables and cost variables without missing data alongside outcome, cost and utility variables with missing data. The depression imputation model included the following trial outcome variables: baseline PHQ-9 and GAD-7 scores, as well as data on these variables for all follow-up time points; variables indicating whether or not participants were currently being prescribed antidepressants (at baseline and all subsequent follow-up time points); whether or not participants had a history of past depression; and depression status as measured by the CIS-R. Likewise, QRISK2 scores at baseline and all follow-up time points were included in the CVD imputation model.
For both trials, the imputation model was stratified by trial arm and the number of imputations was set to 60, which ensured that the number of imputations was greater than the proportion of missing data. 324 Predictive mean matching was used to account for non-Gaussian distributions in variables, particularly in the cost and utility variables included in the imputation model. 324 Passive imputation was performed for categorical outcome models that were functions of imputed variables, such as binary variables indicating QRISK2 responders in the CVD risk trial. Finally, analysis was performed on the imputed data set in a way that reflected the variation within and between the imputed data sets in accordance with Rubin’s rules. 323–325
To estimate the effect of the Healthlines intervention when received as intended, we estimated the complier average causal effect (CACE) using principal stratification. 326 Participants can be divided into always-takers, compliers and never-takers. We assumed that (1) the proportion of never-takers is the same in both arms because of randomisation and (2) the effect among never-takers is the same in both arms. The latter is equivalent to saying that being randomised to the intervention arm in itself has no effect and the intervention has an effect only if it is received. For simplicity, we ignored the small number of individuals who received the Healthlines intervention in error and treated them as if they had not received any intervention. Finally, we describe compliance with the Healthlines intervention as a three-level variable (none, partial, full), with number of completed sessions in each category different for the depression and CVD risk trials. The three resultant categories were composed of the following: contact initiated between zero and up to the first two intervention encounters (little or none); attempted at least the total number of scheduled encounters minus one (full; for depression, encounters 9 or 10, and for CVD risk, encounters 12 or 13); and began more than the first two intervention encounters but less than the total number of encounters minus one (partial; for depression, encounters 3–8, and for CVD risk, encounters 3–11). However, when estimating the CACE, we conducted two analyses for each trial, with compliance as a binary variable and partial compliers categorised as either non-compliers or full compliers in each case. This allowed estimation of crude ORs and 95% CIs of the intervention effect among compliers.
Subgroup analyses
We conducted subgroup analyses of the primary outcome for both trials by fitting an appropriate interaction term to the primary regression models. Subgroups of interest in both trials were categorical age (depression trial: < 40, 40–49, 50–59, 60–69, ≥ 70 years; CVD risk trial: 40–59, 60–69, ≥ 70 years) and sex, as well as baseline PHQ-9 score (10–14, 15–19, ≥ 20) in the depression trial and baseline QRISK2 score (17–24.9, 25–29.9, ≥ 30) in the CVD risk trial. For the depression trial, baseline CIS-R category (mild, moderate, severe) and self-reported prescription of antidepressant medication (no/yes) were also analysed as subgroups. For the CVD risk trial, each of the three modifiable risk factors at baseline (systolic blood pressure: < 140, ≥ 140 mmHg; BMI: < 30, ≥ 30 kg/m2; smoking: current smoker, not current smoker) were also analysed as subgroups.
A number of additional subgroup analyses of interest were identified during the process evaluation and are described in the following section. These were not specified a priori and are considered exploratory. They include treatment effects on the primary outcome according to baseline motivation to participate (internal motivation: ≤ 5, > 5; external motivation: ≤ 1, > 1) and effects on each of blood pressure, BMI and smoking at 12 months follow-up according to the presence/absence of each risk factor at baseline. As for the primary outcomes, these subgroup effects were estimated by fitting appropriate interaction terms to the regression models.
Secondary analyses
We conducted a repeated measures analysis of the primary outcome, both as binary and continuous outcomes, using appropriate mixed-effects regression models. We first investigated whether or not the between-group difference changed over time by fitting an interaction between trial arm and follow-up time point. In the absence of any such interactions, we report the average between-group effect across all of the follow-up occasions.
Secondary outcomes were analysed in a similar way to the primary analysis, that is, between-group effects were estimated using linear or logistic mixed-effects regression models, adjusted for stratification and minimisation variables and value of the outcome at baseline. Participants were analysed as randomised without imputation of missing data. No sensitivity analyses were conducted for secondary outcomes.
A range of process of care measures was collected from several different sources to explore the extent to which different components of the intervention may affect health outcomes in both trials. As covered in more detail in Chapter 5, the potential causal mechanisms of the intervention are complex in this study. The intervention is based on a model with several inter-related components, reflecting different concepts, and there may be multiple ways that any effect on the primary outcomes could be achieved. The focus of the process analysis was the extent to which the intervention was implemented as intended and whether or not the key concepts were achieved (and the extent to which this differed from the effects of care received by patients in the control arm). In this way, if the intervention was effective we may be able to develop hypotheses about whether or not different components may have been associated with effectiveness, without claiming any causal link. If the intervention was effective but there was no change in one of the components, that would suggest that it is not related to effectiveness. If the intervention was not effective, this analysis will aid understanding about whether this may have been because of implementation failure (the intervention was not delivered or did not have the desired intermediate effects) or intervention failure (the different components were achieved but there were no differences in primary or secondary outcomes). The reports of the main results of the trials in Chapters 8 and 9 describe the primary and secondary outcomes and the process measures when they are relevant to understanding the delivery of the intervention.
Finally, a descriptive analysis of patient safety was carried out. SAEs were categorised and presented by study arm and were described by number of SAEs reported and, of these, number of related SAEs and, of these, proportions that were expected/unexpected.
Minimising risk of bias
Allocation was concealed by use of a remote, automated web randomisation system. Blinding of participants, health professionals and researchers was not possible, although the trial statisticians who assessed the outcomes remained blinded to treatment allocation throughout. The primary outcome in the depression trial was self-reported by participants, whereas the primary outcome in the CVD trial was assessed by practice nurses or HCAs who were blinded to treatment allocation. Nevertheless, it is possible that the practice staff became unblinded through conversations with participants during the CVD risk assessments and often the same practice staff carried out the CVD risk assessments and distributed and collected back the blood pressure monitors allocated as part of the intervention. We acknowledge that patients in the usual care group might have been able to access similar information from other websites, including NHS Choices. However, we anticipated that such usage would be much less than in the intervention group because the latter was directed to these resources by the Healthlines Services staff. Nonetheless, we collected patient self-reported information about the use of other relevant web-based resources from all participants.
Research governance
Management, operational and academic aspects of the trials were the responsibility of the Trial Management Group, which met approximately every 6 weeks from the start of the trial until the main analyses were completed. Conduct of the trials was overseen by an independent Trial Steering Committee and an independent Data Monitoring Committee. The whole research programme was also overseen by the Programme Management Group, which included all of the co-applicants on the programme grant and two patient and public representatives. The University of Bristol was the study sponsor and the study was approved by the National Research Ethics Service Committee South West–Frenchay (reference no. 12/SW/0009). The trials were registered under Current Controlled Trials and allocated an ISRCTN as follows: depression trial ISRCTN14172341 and CVD risk trial ISRCTN27508731.
Patient and public involvement
Two patient and public representatives attended, and were active contributors at, the Programme Management Group and the independent Trial Steering Committee meetings throughout this phase of the research programme. Both representatives were recruited from a pool of NHS Direct volunteers. In addition to directly providing valuable feedback on the study questionnaires and recruiting additional members of the lay public to review these documents, the representatives provided feedback on study methods and procedures and the development of the dissemination plan and strategy for the trials.
Economic evaluation methods
Introduction to methods used for economic evaluation
The aim of the economic evaluation was to determine the cost-effectiveness of a telehealth intervention provided within an existing NHS organisation for LTC management in the two exemplar conditions of depression and raised CVD risk. The economic evaluation was conducted alongside the two RCTs.
Perspective
Estimates of direct and indirect costs incurred were calculated from the perspectives of (1) health- and social-care providers [the NHS and Personal Social Services (PSS)], (2) personal costs incurred by participants and (3) productivity losses because of time off work. Each type of cost was recorded and valued separately and costs were measured from randomisation until the completion of 12 months of follow-up.
Form of analysis
Costs were related to outcomes using the following forms of economic evaluation. A cost–consequences analysis was used to compare costs with a range of outcomes. In the depression trial these were response to treatment (defined as a PHQ-9 score of < 10 and a reduction of at least 5 points 4 months after randomisation); change in PHQ-9 depression score; EQ-5D-5L327 score at the 12-month follow-up; and quality-adjusted life-years (QALYs). In the CVD risk trial the outcomes included response to treatment (defined as maintenance of or reduction in 10-year risk at 12 months after randomisation); change in QRISK2 score; EQ-5D-5L score at the 12-month follow-up; and QALYs.
Two forms of cost-effectiveness analysis were undertaken for each trial. The first compared incremental NHS and PSS costs with the proportion of participants responding to treatment. The second analysis related incremental NHS and PSS costs to incremental QALYs, which permitted the cost-effectiveness of the interventions to be compared with estimates of society’s cost-effectiveness threshold (i.e. the opportunity costs that would result from health foregone elsewhere in the NHS if the Healthlines interventions were funded). This analysis used values for the cost-effectiveness threshold of £20,000–30,000, as suggested by NICE. 328
As the primary outcome of the CVD risk trial (risk of a CVD event) is not a clinical outcome, and the 12-month period of trial follow-up may not capture all relevant impacts of the intervention on clinical outcomes, we used cohort simulation modelling techniques to estimate the longer-term effect of the intervention on cost per QALY over the lifetime of CVD risk trial participants. This modelling is described in Introduction to long-term cardiovascular disease risk modelling.
Sources of data: outcomes
The sources for the clinical outcomes used in the cost–consequences analyses have been described previously in this chapter (see Primary outcomes). In the depression trial, participants reported responses on the EQ-5D-5L instrument at baseline and 4, 8 and 12 months after randomisation. In the CVD risk trial, responses were reported at baseline and 6 and 12 months. In the absence of a valuation set specific to the EQ-5D-5L at the time that this analysis was conducted, these responses were valued using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) value sets using the ‘cross-walk’ values available from EuroQol. 329
Quality-adjusted life-years were estimated using the area under the curve method. Imbalance between the intervention and usual care group values of the EQ-5D-5L at baseline were adjusted for in the cost-effectiveness analysis using the methods described in Manca et al. 330
Sources of data: resource use, personal costs and productivity
Cost of the Healthlines intervention
The resources used to develop the intervention fall into two categories: those that relate to the development of the Healthlines Service software, that is, the ‘product’, and those that relate to setting up the service to deliver the content and ongoing support. This distinction is relevant to the future roll-out of such a service; the analysis here focuses on the setting up and delivery of the service.
The resources used to deliver the intervention were extracted from the electronic systems used by NHS Direct and from trial records, such as the scheduling diaries of the HIAs. Per-participant resource items relating to the intervention included the number and length of all telephone calls and the number of unsuccessful attempts by a HIA to contact a participant. In the depression trial, other resources included use of the LLTTFi website, use of the designated CBT book Overcoming Depression and Low Mood: a Five Areas Approach292 and use of the BWW website. In the CVD risk trial, other resources included provision of a blood pressure monitor. The resources used in setting up the service were identified by NHS Direct and consisted of staff training and the purchase of licences for LLTTFi and the BWW.
Direct costs to the health- and social-care sectors: the NHS and Personal Social Services
Data on the use of primary care and community services were collected, when possible, from GP records. Data were collected either as direct downloads (such as for prescriptions) or using a proforma data collection sheet. Data collected included all face-to-face and telephone consultations and home visits by all practice staff, 24-hour blood pressure monitoring (in the CVD risk trial) and prescribed medication. Information on prescribed medication belonging to the following groups was collected in the depression trial: hypnotics and anxiolytics, drugs used in psychoses and related disorders and antidepressants. Information on prescribed medication belonging to the following groups was collected in the CVD risk trial: lipid-regulating drugs, antihypertensives, antiplatelet drugs, obesity treatments and treatments for nicotine dependence .
Follow-up questionnaires completed by participants at 4, 8 and 12 months for the depression trial and 6 and 12 months for the CVD risk trial included questions on the use of health- and social-care services not available from practice records, such as use of out-of-hours services, ambulances, accident and emergency services, walk-in centres, district nurses and hospital-based care (see Appendix 21, Section 12).
Participant out-of-pocket expenditure
The follow-up questionnaires administered during the trial collected data from participants about personal expenditure related to their LTC. This included items such as the use of private health care, complementary or alternative therapies and self-help materials. In the depression trial questionnaire this included queries related to consultations with private psychiatrists and the use of online or computerised CBT programmes and in the CVD risk trial questionnaire this included queries related to the purchase of exercise equipment and attendance at weight loss classes (see Appendix 21, Section 12).
Lost productivity and financial impacts
The follow-up questionnaires also asked participants to self-report any time off work as a result of their health condition or for health-care appointments/visits. The questionnaires included questions on working status (e.g. full-time, retired) and the amount of lost income incurred as a result of depression or CVD risk in the respective trials (see Appendix 21, Section 12).
Valuation of resources
The principal sources used in the valuation of resources were national reference cost sources and questionnaire responses. When information was not available from these sources, estimates were taken from published estimates and other literature. These sources are detailed in the text below and in Appendices 22 and 23. Costs are reported in 2012/13 UK pounds.
Costs of the Healthlines interventions
The elements of resource use that contributed to the costs of the interventions are summarised in Table 15. The principal cost involved in providing the interventions was the salary of the HIAs. All HIAs were remunerated at band 4 of the NHS salary scale. 331 Salary levels and related costs (such as on-costs and capital-related costs) were estimated using the methods of Curtis;332 these were adjusted for a 40-hour working week, to reflect the actual working hours of HIAs, and an appropriate estimate of non-contact time, to estimate the cost per hour of participant contact time.
| Element of intervention cost | Depression (£) | CVD risk (£) |
|---|---|---|
| Basic HIA annual salary (band 4) | 20,638.00 | 20,638.00 |
| HIA on-costs: National Insurance and superannuation | 4827.00 | 4827.00 |
| HIA overheads | 35,417.00 | 35,417.00 |
| HIA cost per hour | 23.61 | 23.61 |
| Training cost per HIA per hour | 0.53 | 0.61 |
| Ratio of contact to non-contact time | 2 : 1 | 2 : 1 |
| Adjusted cost per hour of HIA time | 36.22 | 36.34 |
| LLTTFi licence | 3000.00 | NA |
| CBT book per book | 52.85 | NA |
| BWW licence per participant using | 60.00 | NA |
| Blood pressure monitor | NA | 37.50 |
Non-contact time was estimated from anonymised HIA work diaries relating to the period 1 May to 30 June 2013. These 2 months were considered to be representative of workload and case mix during the period for which the intervention was operating. The diary entries classified the working days of each HIA on duty into the following categories: encounter completed, encounter incomplete/DNA (did not attend, meaning that the participant did not answer the telephone), paid break, unpaid lunch, appointment booked, meeting, auditing, administration, mentoring and training and sickness. The categories of encounter completed and encounter incomplete/DNA were taken to be participant contact time and the remainder were assumed to be non-contact time. The ratio of contact to non-contact time was estimated to be 2 : 1 based on an analysis of the diary data. The HIAs indicated that this ratio did not differ between the two participant groups (depression and CVD risk) and hence the basic cost per hour of participant time was estimated to be the same in both trials.
In addition to the basic cost per hour of participant time, differences between the costs of serving the two groups arose when considering the training of the HIAs, licences purchased and consumables. HIAs received initial and ongoing training from a nurse-grade trainer, the cost of which was incorporated into the cost per hour of HIA time. The initial training was assumed to last for 3 years. HIAs were trained in both the depression and the CVD risk software together. The CVD risk software was more complex and training for this took longer, so this was reflected in our cost estimates. Extra training was provided by a consultant psychiatrist in the use of the LLTTFi package, which was available to depression participants, and this was included in the analysis in the same way as was the initial nurse training. The purchase of a licence for LLTTFi was included by apportioning the cost across all depression encounters and the cost of the CBT book and DVD was allocated to those participants who chose to use them. Use of the BWW website was provided at no cost in the trial, but sources at the organisation provided a realistic cost, which was included in the analysis. Some CVD risk participants were provided with a blood pressure monitor to use at home, the costs of which were included on a per-patient basis as an upfront cost.
Primary care and community health and community services
The source for primary care consultation costs and community health services costs was Curtis,332 except when stated otherwise. The details of these services and associated costs are provided in Appendix 22.
Hospital-related services
The costs of hospital-related services, including accident and emergency and ambulance services, were based on NHS reference costs 2012/13333 and Curtis332 (see Appendix 23). Costs were reviewed by a clinician (CS) prior to analysis to ensure that only costs relevant to either mental health or CVD risk were included. For example, a patient in the CVD risk trial reported day care in a NHS hospital for eye complications associated with diabetes and blood pressure following a retinal scan and this resource use was retained in the analysis and costed as a non-admitted ophthalmology face-to-face consultation.
Personal costs of participants and carers
Personal expenditure
Categories of personal expenditure included over-the-counter purchases of medication and therapies and private health-care use. These were valued using participants’ responses to follow-up questionnaires.
Welfare benefits
Estimates of disability benefits provided as a consequence of mental health or emotional problems (depression trial) or having high blood pressure or high cholesterol, smoking or being overweight (CVD risk trial) were provided by participants in follow-up questionnaire responses.
Loss of earnings
Participants provided direct estimates of the impact of either depression or CVD risk on loss of income and time off work in answers to follow-up questionnaire.
Productivity
Productivity costs were calculated by valuing participant reports of work absence as a result of their condition at median hourly earnings (£11.59) using the Annual Survey of Hours and Earnings 2013 from the Office for National Statistics. 334
Prescribed medication
Medication costs were based on prescriptions issued during the year from randomisation to the end of follow-up. Prescription costs were related to the study data using Prescription Cost Analysis, England (PCAE). 335 Relevant drugs (those issued for treating and managing depression and CVD risk) were identified by the primary investigator (CS). The generic name for each drug was used, if available, as this was likely to reflect what would be prescribed by general practices. Brand name versions were used otherwise.
For each drug listed in the medication records, the net ingredient cost per quantity from PCAE was used to uniquely identify each entry. The net ingredient cost per quantity provided a single unit cost (e.g. for one tablet, one capsule or 1 ml) for each prescribed drug. This was multiplied by the unit quantity (e.g. 28 tablets, 150 ml) for each listed prescription to give a total cost for each prescription. All drug preparations in the form of sachets and liquids were checked against the British National Formulary (BNF). 336
Analysis
Per-participant costs and QALYs were estimated from the sources described in the previous sections. A cost–consequences matrix was constructed for each trial. The cost-effectiveness analysis was conducted from the perspective of the NHS and PSS.
The sensitivity of the results to the inclusion of certain costs was also tested in each trial. The base-case results used data generated through multiple imputation (see Missing data and imputation), but cost-effectiveness in complete cases was also calculated as a sensitivity analysis for the main results in each trial.
In both trials, the main cost-effectiveness analyses were rerun excluding NHS costs collected from participant questionnaires as a sensitivity check on the main results. These costs relate to resource use, such as district nurse home visits, overnight hospital stays and ambulance use. The rationale for the exclusion of these costs was to assess whether or not resource use based on patient recall had a material influence on the conclusions of the economic analysis. Additionally, elements of the cost of the intervention in the depression trial were subject to deterministic sensitivity analysis to account for potentially lower costs if the intervention was deployed throughout the NHS. All analyses were conducted using Stata 13.1.
Missing data and imputation
Missing data are important for cost-effectiveness analysis because cost and QALY data are constructed using cumulative sums of responses to cost and quality of life questionnaires and from sums of resource use from other data sources across all follow-up time points. Missingness in data relevant to the cost-effectiveness analysis arose in the trial for a number of reasons. Medical records, obtained from general practices, were incomplete in a very small number of cases (< 0.5% across both trials). Questionnaires were sometimes unavailable (because participants had not returned them) or were only partially completed.
Missing data were addressed in two ways. The first method used mean imputation for variables that had very low levels of missingness and were unlikely to be major cost drivers for the analysis overall. Mean imputation was used for the very small fraction of participants with incomplete primary care data (< 0.5% of all randomised participants in both trials, representing 10 patients overall). This incompleteness arose because patients declined consent for their records to be accessed or moved away from the practice with which they were associated at the time of randomisation.
Available cases, as reported in Chapter 10, were defined as cases for which it was possible to sum across all data sources, including questionnaires, for all follow-up time points for the resource use, cost or quality of life measure concerned. This meant that the number of available cases for a particular resource varied by question. Available cases were not used for cost-effectiveness inference, although they are referred to descriptively in cost–consequence matrices and elsewhere in summary tables in Chapter 10.
Complete cases for the purposes of economic analysis were defined as a subset of available cases. Complete cases, after the pre-imputation data cleaning described above, had complete data on all cost items and all health-related quality of life items, to enable full cost-effectiveness inference to be conducted. However, using only complete cases in analysis is equivalent to an assumption that data are missing completely at random and may lead to bias, whereas ignoring a proportion of available data is likely to be inefficient. Data from multiple imputation were therefore used as the second means to address missing data to inform the base-case cost-effectiveness results in each trial.
The same imputation models were used for the main trial analyses and for the economic analyses. Costs were imputed at the level of aggregate cost categories (primary care costs, medication costs, intervention costs, PSS costs and remaining NHS costs), rather than as individual resource use items. Health-related quality of life utility measures at each time point were imputed, which were then used to generate QALY measures.
Cost-effectiveness analysis was performed on the imputed data set in a way that reflected the variation within and between the imputed data sets in accordance with Rubin’s rules. 323–325 The economic analysis of cost-effectiveness on the imputed data was based on the methods and discussion in Faria et al. 324 – both seemingly unrelated regression analysis and bootstrapping (based on 5000 model iterations) were used to produce incremental cost-effectiveness ratios (ICERs) and cost-effectiveness acceptability curves (CEACs). The regression analysis was used as the basis for the main cost-effectiveness results.
Uncertainty
Both bootstrapping and regression analysis were used to characterise the level of uncertainty around point estimates of the ICER. Five thousand bootstrap replicates of the ICER were constructed by sampling, with replacement, from the original data and were used to generate cost-effectiveness planes. Cost-effectiveness statistics, such as net monetary benefit (NMB) mean estimates and associated CIs, were calculated parametrically from the regression output.
Discounting
Costs and outcomes in the trial-based analyses reporting at time horizons of up to 12 months were not discounted. Discounting for the long-term CVD risk modelling is described in Model perspective and discount rate.
Introduction to long-term cardiovascular disease risk modelling
The primary clinical outcome of the CVD risk trial was 10-year risk of a cardiovascular event, estimated using the QRISK2 prediction algorithm. A 10-year period is substantially longer than the follow-up period of the trial, but may be shorter than the actual duration of the effect of the intervention on patient health. The principles of economic evaluation337 and NICE guidance328 require that studies adopt a time horizon that is sufficient to reflect costs and effects that may differ between intervention and usual care groups. Data from the trial and from other sources were therefore used to develop and populate a state transition cohort simulation model to estimate the cost per QALY of the intervention over periods up to and including the lifetime of the CVD risk trial population. A similar model was not attempted for the depression trial in the absence of a comparably comprehensive set of economic and epidemiological data needed to populate a lifetime economic model.
The state transition cohort simulation model
The cohort simulated was a hypothetical population of patients with risk profiles that reflect those of the Healthlines CVD risk trial population. The extent to which the intervention was successful in altering modifiable risk factors between trial arms over the period of follow-up was used as a basis for simulating the costs, utility and mortality pathways experienced over the lifetime of the simulated cohort. The model is based on modified and updated versions of models used and described in previous health technology assessments in the area of CVD risk. 338,339
The model consists of a number of discrete and mutually exclusive health states, each of which is associated with the presence or absence of a particular CVD-related health condition or with death. Events, such as a stroke, establish the state to which an individual patient in the cohort is assigned at any moment in time. The probability of a primary, or initial, event was determined from incidence data, whereas the probability of moving from one state to another was determined by transition matrices calculated primarily from epidemiological data.
Sequences of particular types of health experienced by members of the cohort established the simulated clinical pathway of patients. Different sequences of events, averaged over the entire cohort modelled in each arm, give rise to differences in cost, utility and mortality. This probability-weighted averaging involves identifying how many patients are in a particular health state at a moment of time, for how long patients have been in particular states and the consequences of membership of each health state. This information permits the calculation of QALYs and costs for each arm and thus ICERs and net benefit statistics.
A total of 1000 iterations of the model were run in a Monte Carlo simulation in which values of input parameters were varied simultaneously. Each iteration took a value from the probability distributions assigned to the input parameters of the model. Probabilistic sensitivity analysis was used to quantify the uncertainty around the post-estimates of cost-effectiveness measured by the net benefit statistic. The model was implemented in Microsoft Excel 2013 and was based on an earlier Microsoft Excel model used in Ara et al. 338
Cohort size and characteristics
A cohort of 1000 patients was simulated, including one cohort for each arm of the trial and one for each sex. Baseline characteristics of the cohorts reflected those in the CVD risk trial participants. For each arm, the weighted average of male and female results was calculated to obtain an overall estimate of cost-effectiveness.
Definition of cardiovascular disease events and model states
Each patient enters the model in an ‘event-free’ state, meaning that no patient had a diagnosis of any CVD condition. This is consistent with the inclusion criteria of the trial and reflects the basis on which the QRISK2 algorithm was constructed. The output of the QRISK2 algorithm used in the trial is the risk of the first instance of CVD in a 10-year period. In practice, specific episodes of CVD differ in their impact on mortality, quality of life and cost, and may recur following an initial event, and these differences needed to be reflected in the simulation model.
It was necessary, therefore, to convert the 10-year predicted risk of any event into the risk for a specific first event in a single year. As a first step, 10-year risk was converted into an annual risk. The second step converted this risk into the risks of specific events as follows: CVD was defined for the purposes of constructing the QRISK2 algorithm as CHD (angina and myocardial infarction), stroke or TIA. These instances of CVD were used to define events in the simulation model, except that stable and unstable angina were distinguished as separate events. Data on the incidence of all of these conditions, described in Incidence rates for primary events and annual growth of QRISK2 scores, were used to define the probability of a first specific event by age and sex by distributing the overall age-adjusted risk of any first event outputted by the QRISK2 algorithm for each arm of the trial into its component parts.
The probability of moving between different states was initially determined by the interaction of baseline risk characteristics and age-dependent, time-dependent transition matrices. Data on the transition from a first (event-free) health state to subsequent health states were taken from the different sources described below. Together, the initial event-free state, the primary states (i.e. the first state after the event-free state) and subsequent or secondary health states (i.e. all states after a primary state) define the complete set of possible states into which patients may transition over the course of their simulated ‘life’. The set of states, and the permitted transitions between them, are informed by a combination of clinical feasibility and data availability (Figure 13).
Transitions differed by age and sex. The list of permitted transitions between states is described in Appendix 24 (see Box 6). Patients remained in each ‘state’ until a different state occurred.
Each patient was constrained to experience no more than three CVD ‘events’ over the course of their lifetime. This assumption was partly guided by model tractability and the consideration that, in practice, few of the 1000 patients simulated approached three events. Therefore, the assumption is not likely to influence cost-effectiveness conclusions. A similar assumption was used in the study by Ara et al. 338
Cycle length
The cycle length of the model was 1 year. A cycle distributes members of the simulated cohort to mutually exclusive states, each of which was defined by the events described above. Patients cycled at annual intervals through a finite number of health states until they died or reached age 100 years, at which point they were assumed to die. A half-cycle correction was applied to both costs and outcomes. 341
Model perspective and discount rate
A UK NHS perspective was adopted. The personal costs borne by patients in managing their condition or receiving treatment were not calculated, nor were wider impacts on society such as productivity effects. Modelled costs and outcomes were both discounted at 3.5%, in line with NICE recommendations. 328
Duration of the effect of the intervention
In the absence of information about the duration of effect of the intervention beyond the end of the 12-month follow-up, the difference in lifetime CVD risk between trial arms, estimated by the QRISK2 algorithm, was modelled in a number of scenarios and the implications for cost-effectiveness compared. The scenarios were defined by assumed durations of intervention effect of 1, 2 and 5 years and for the remaining life of the modelled patients up to and including age 100 years. For example, if the assumed duration of the intervention effect was 2 years, then after 2 years any benefit of the intervention would have ‘disappeared’ – in practical terms, this would mean that the intervention arm would have a lower risk for 2 years if indeed the intervention was shown to reduce the QRISK2 score, but the risk would be indistinguishable between arms (controlling for age and sex) once this 2-year period had expired. The same logic applies to different durations of effect: once the duration of the effect had expired, however long this duration of effect was modelled as persisting, it was assumed that there would be no difference in the risk faced by individuals in each arm of the trial, with intervention patients facing the same risk as usual care patients. The exception to this assumption was a permanent duration of effect persisting for the remaining lifetime of patients. To avoid sudden jumps at the point at which the intervention ends, the model used a smoothing adjustment that calculated the average score of the intervention risk and usual care risk for the year after which the duration of effect expired – this allowed for the intervention risk to more slowly adjust to the usual care arm risk, rather than jumping immediately to a higher risk. This approach to modelling different durations of effect is comparable to that taken by Mistry et al. ,342 who adopted a similarly ‘agnostic’ approach to the extrapolation of effect beyond the end of follow-up in a CVD-related intervention modelled using a similar type of simulation model.
Annual growth in QRISK2 score
Patients enter the model with a known age and QRISK2 score, corresponding to the average values of these parameters in each arm of the trial. These scores are incremented for each subsequent year of life (corresponding to the annual cycle length of the model) by a background growth rate in QRISK2 score. This background growth rate was obtained from the growth rates in annual risk in males obtained from the QRISK2 algorithm when all input factors other than age were held constant. Input values used for this exercise assumed that patients did not have any of the clinical conditions assessed during calculation of risk (such as atrial fibrillation) and were non-smokers and used values imputed by the algorithm itself for information on patient characteristics, such as cholesterol-to-HDL ratios, systolic blood pressure, BMI and ethnicity.
The growth risk in men was used for both men and women. This is because the 10-year or annual CVD risk is higher for men than for women, holding all other variables constant, at all ages. The growth rate in female risk at younger ages is higher than that for males at younger ages because the level of the former risk is lower than the level of the latter risk, but these differences narrow with age. It was observed in model development that risk levels in women exceeded those in men when extrapolating from the levels of risk estimated in the trial. The solution adopted to this issue was to use male growth rates for both men and women, which constrained female risk levels to below male risk levels at all ages. It is conceivable that risk grows more slowly when the level of risk is already high, or it may continue to grow more quickly than in the general population. It is also plausible that risk may change with age and with changes in the underlying risk behaviours that determine overall CVD risk. On balance, the approach adopted appeared to offer a reasonable and conservative compromise between these different considerations.
The QRISK2 algorithm is not validated for ages beyond 84 years. As a proportion of patients in the simulation will reach ages beyond 84 years, it was necessary to make an assumption about how risk would change beyond this age. A ‘healthy survivors’ effect would suggest that risk may have plateaued; alternatively, risk may continue to increase given how important age is to the level of risk at ages younger than 85 years. For the base-case analysis, risk was increased for ages beyond 84 years by extrapolating the 10-year risk by 1% per annum. This 1% figure was obtained from an ordinary least squares (OLS) regression of 10-year QRISK2 scores on ages between 45 and 84 years.
Incidence rates for primary events and annual growth of QRISK2 scores
Incidence rates for primary events were used in the model to calculate the probability of a primary CVD event – stable angina, unstable angina, acute myocardial infarction (AMI), stroke or TIA. They were used to disaggregate the risk of any such event – calculated for each trial arm by the QRISK2 algorithm – into the risk of one of the component events.
Incidence of stable and unstable angina
The incidence of angina by age decile and sex was taken from British Heart Foundation CHD statistics 2012,343 using the data reported for England. This incidence was split into incidences of stable (77%) and unstable (23%) angina using the incidence of exertional and unstable angina reported in the study by Sutcliffe et al. ,344 which reported incidences of varieties of CHD in people aged 25–74 years in the Bromley Health Authority. Applying this split assumes that the relative incidence of stable and unstable angina is the same for each age group.
Incidence of non-fatal and fatal acute myocardial infarction
The incidence of AMI was taken from British Heart Foundation CHD statistics 2012,343 using the data reported for England. Incidence is reported by age bands, as is the case fatality rate. Applying the case fatality rate allows fatal and non-fatal incidence to be distinguished.
Incidence of fatal and non-fatal stroke
The incidence of a first ever stroke was taken from the study by Wang et al. ,345 using the incidence rates in the white ethnicity group (approximately 99% of participants randomised to the CVD risk trial in the Healthlines study were of white ethnicity). Incidence rates were split into male and female rates using data from the same study. In the absence of specific information in the study by Wang et al. ,345 the mortality rates for strokes (11.3% for men and 18.6% for women) were taken from data on 56-day mortality after first stroke reported in the study by Lee et al. 346 and were assumed to apply equally to all of the age bands reported in the study by Wang et al. 345
Incidence of transient ischaemic attack
The incidence of TIA was taken from British Heart Foundation stroke statistics 2009,347 which are based on data from the Oxford Vascular Study. 348
Summary of incidence rates of primary events
The incidence rates of the seven primary CVD events per 1000 individuals by age band and sex are provided in Appendix 25 (see Tables 136 and 137). For each age band, the data in Tables 138 and 139 (see Appendix 25) show the proportion of the annual QRISK2 risk that is accounted for by the specific primary CVD events or conditions separately by sex.
Transitions and secondary events
Incidence rates for primary events describe the probability of transitioning from the event-free state, in which all patients enter the model, to a first CVD event. Subsequent transitions require estimates of the probability of experiencing secondary events after a primary event and after other secondary events.
The model used the probabilities from the study by Ara et al. ,338 one of the studies on which the model itself was based. The probabilities were obtained by Ara et al. 338 using a two-stage regression analysis. In the first stage, logistic regression was used to estimate the probability of experiencing any type of secondary event. In the second stage, this probability was disaggregated using multivariate regression to give a distribution across event types, conditional on an event occurring. The same methodology was used for all subsequent events. The transition probabilities differ by age and sex. The values of these probabilities, and the data sources used in their construction, are described in detail in the study by Ara et al. 338
Utility
Each health state in the model is associated with a specific level of utility. For the ‘event-free’ state, this utility equals the age- and sex-adjusted utility of the general population. 349 For other health states, this baseline utility is modified by the utility impact of the specific condition or event that defines one of the model’s mutually exclusive states. QALYs were adjusted to account for the difference between arms observed at the end of trial follow-up.
The work of Ara and Brazier350 was the principal source for most of the utility values used. Ara and Brazier350 used data from the Health Survey for England (HSE), an annual survey that uses random sampling of every private household address in England. The 2003 and 2006 surveys asked questions about history of CVD, with a random sample of respondents asked to complete the EQ-5D. Baseline utility was estimated by Ara and Brazier350 from individuals who reported no history of angina, heart attack or stroke (n = 25,080). An OLS regression was estimated to relate EQ-5D scores to age and sex. Results from the model so obtained (EQ-5D = 0.9454933 + 0.0256466 × male-0.0002213 × age + 0.0000294 × age2) are summarised in Figure 14.
FIGURE 14.
Baseline utility of health states in the CVD risk simulation model by age and sex.
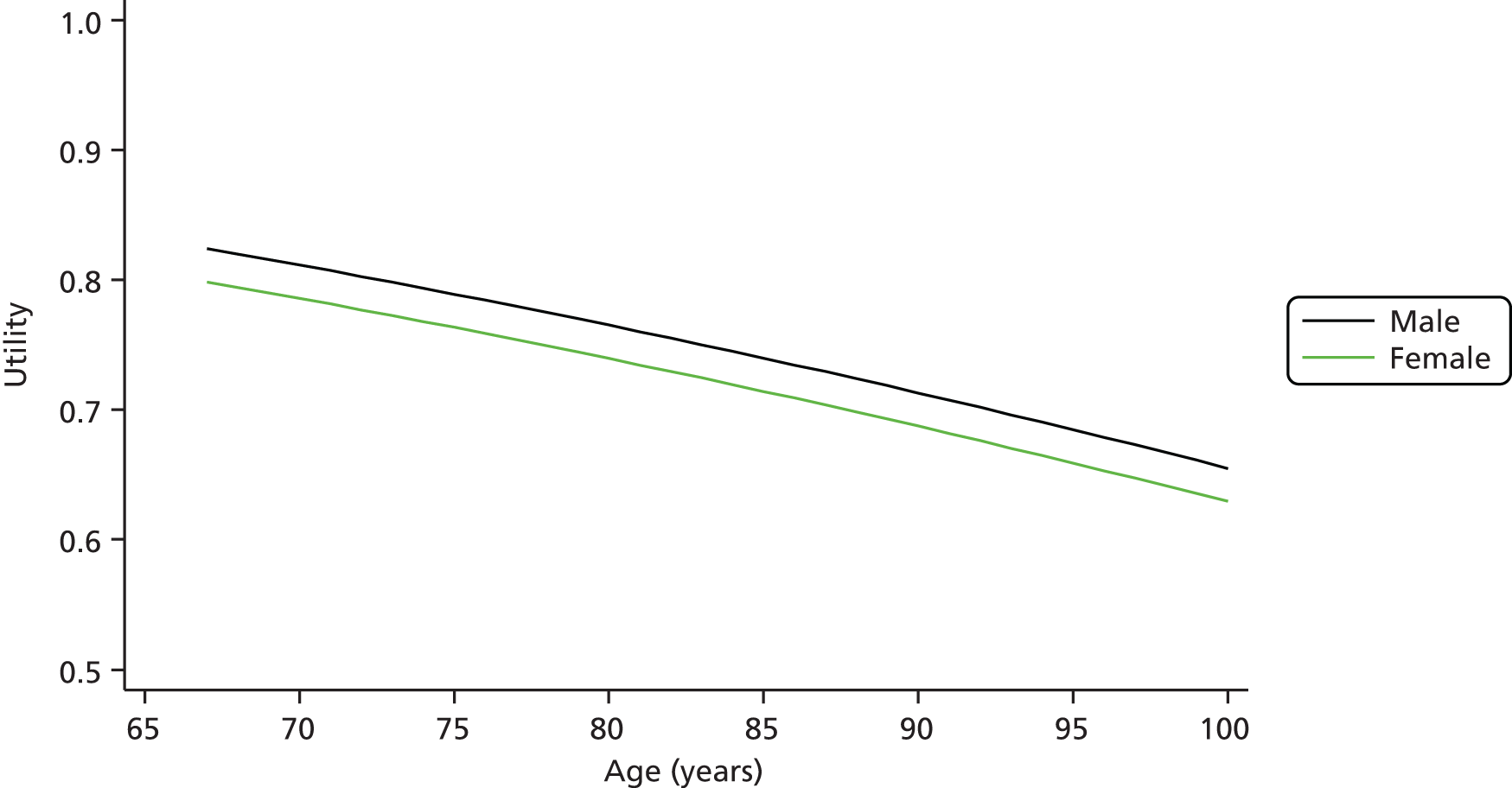
This baseline utility corresponds to the utility experienced by individuals in the ‘event-free’ health state. For example, a man aged 67 years is estimated to have a utility of 0.82 (out of a maximum possible utility of 1) in the event-free state, whereas a woman aged 69 years is estimated to have a utility of 0.80 in the event-free state.
Ara and Brazier350 calculated state utility values for angina, heart attack and stroke as follows. Data from individuals who reported a history of one of these CVD conditions was assumed to be representative of individuals who had a first ever primary CVD event. Individuals who reported more than one such condition were assumed to be representative of individuals who had more than one CVD event. Individuals who reported CVD events within the 12 months preceding the survey date were assumed to be representative of patients who recently experienced that event, whereas individuals who reported experiencing the event in question > 12 months before the survey were assumed to be representative of patients in the ‘post-event’ state. For example, patients who reported a stroke in the last 12 months were estimated to have a utility of 0.626 whereas those who experienced a stroke > 12 months before the survey date were estimated to have a utility of 0.668. The impact of histories of multiple CVD conditions was calculated in a similar way. In the absence of evidence to the contrary, the utility impact of a first secondary event was assumed to be the same as that of a subsequent event of the same type, for example a first secondary stroke was assumed to have the same utility impact as that of a subsequent stroke.
Ara and Brazier350 provided utility values for angina, heart attack, stroke and the baseline ‘event-free’ health state. Other sources were used to distinguish between stable and unstable angina and to provide estimates for TIA utilities. It was assumed that the angina utility value reported in Ara and Brazier350 related to stable angina. An estimate for unstable angina was obtained by assuming that the ratio of 90% for stable to unstable angina utilities was the same as that reported in Ara et al. 338 Data on TIA utilities were taken from the study by Luengo-Fernandez et al. 351 This study was based on the Oxford Vascular Study, a UK study that followed up stroke and TIA patients for 5 years and calculated EQ-5D utilities using the UK valuation. A table of health state utility values is provided in Appendix 24 (see Table 135).
Utility calculations were based on multiplicative adjustments to baseline utility. This assumes a constant proportional effect of specific conditions on baseline utility. This has been shown to be similar to additive models (which assume a constant additive effect of conditions on baseline utility) and appears to be more suitable than minimum models for the analysis of CVD-related health states. 350
To account for uncertainty in mean utility values, the condition-specific utility values (the numerator in the ratio used to create a multiplier) were drawn from univariate normal distributions in line with the suggestions of Ara and Wailoo. 349 Information on the variance structures associated with the baseline utility equations was not available. The utility of the health states was constrained to be no greater than that of the associated ‘post’ health state. For example, this meant that having an AMI in one model cycle was associated with a greater utility decrement than the utility decrement of the ‘post-AMI state’. Further information on utility sources and assumptions is provided in Appendix 24.
Mortality
Patients modelled in the simulation face an annual risk of death from non-CVD causes, as well as the CVD causes explicitly modelled. The risk of death from non-CVD causes was calculated for men and women from Office for National Statistics interim life tables. 352 The most recently available year for which data were available was 2012. A standardised mortality ratio was calculated by age and sex excluding CVD-related ICD-10 codes I20–I25 (ischaemic heart disease) and I60–I69 (cerebrovascular diseases). All-cause mortality data used in the model were based on draws from a univariate normal distribution.
Costs
A number of costs of treatments and health states were used in the cohort simulation model (see Appendix 26), as distinct from the costs described above concerning intervention-related costs collected as part of the Healthlines CVD risk trial. Many of the costs were based on estimates contained in the studies by Ward et al. 340 and Ara et al. 339 These technology assessments undertook systematic reviews to identify cost information. For the present study, more recent cost estimates were investigated, used and updated when appropriate and noted in Appendix 26.
Summary of health state costs
Health state costs were taken from a number of different sources and entered the model as random draws from the distributions described in Appendix 26. Mean costs are described in Table 16. To account for uncertainty, mean cost, information on variance and published CIs were used to parameterise a gamma distribution for each cost, from which the cost used in a single iteration of the model was a random draw.
| Health state | Mean cost (£) |
|---|---|
| Stable angina | 606.08 |
| Stable angina in subsequent years | 356.37 |
| Unstable angina | 4323.65 |
| Unstable angina in subsequent years | 452.55 |
| Non-fatal AMI | 3362.44 |
| Post non-fatal AMI | 356.37 |
| Fatal AMI | 1846.14 |
| TIA | 3963.12 |
| TIA in subsequent years | 1379.54 |
| Non-fatal stroke | 8989.26 |
| Non-fatal stroke in subsequent years | 1975.67 |
| Fatal stroke | 9493.40 |
The proportion of patients prescribed particular types of medication (see Appendix 26) were subject to random draws from a beta distribution for each iteration of the model, with the alpha scale parameter set equal to the proportion of patients described in each case.
Trial cost data measured during the 12 months of follow-up were used for the first year in which the simulation model was run. The model did not account for costs that fall outside the health system. This could be important for conditions such as stroke, for which the consequences of long-term disability can have material cost implications.
Sensitivity of the simulation model results to trial costs and quality-adjusted life-years
An alternative specification of the simulation model considered only the intervention cost from the trial, excluded other trial follow-up costs and did not include any adjustment for the QALY difference observed between arms in the trial. This sensitivity analysis was intended to investigate the effects of the intervention in modifying QRISK2 alone, at the cost of the intervention. This assumes no difference in costs between arms other than differences in intervention costs and disregards utility data from the trial in the initial year of the simulation. The analysis was intended to isolate the effect of the intervention on QRISK2.
Process evaluation methods
We undertook a process evaluation alongside both trials. At the start of the Healthlines programme, we viewed this process evaluation as an embedded qualitative component of the trial. 283 The design was a qualitative interview study of staff involved in delivering the intervention, staff involved in delivering primary care to trial participants and participants in the intervention arm of each trial. As the programme progressed, we took a wider view of the content of the process evaluation, based on new MRC guidance on process evaluations,353 that is, we drew on both quantitative and qualitative data that we had collected about processes related to the intervention and the trials to address the three themes of a process evaluation: implementation, mechanisms of impact and context. 353,354
Implementation considers what intervention is delivered in practice. 353 The focus includes fidelity (whether or not the intervention was delivered as intended), dose given (the quantity of intervention delivered) and dose received (uptake). Mechanisms of impact consider how the intervention, as delivered in practice, brings about change in the desired health outcome. Quantitative data can be used to test hypothesised causal pathways and qualitative methods can be used to better understand these pathways. This can help to identify evidence supporting the theory-based pathways, so that any effects might be replicated by similar future interventions; breaks in a proposed causal pathway, which challenge the original theory underlying the intervention development; or unanticipated mechanisms. Context focuses on how the setting affected either the implementation of the intervention or outcomes.
Qualitative interview study
Design and setting
The design was a qualitative interview study of staff involved in delivering either the intervention or primary care to trial participants and of participants in the intervention arm of each trial. Interviews were selected as the method of data collection because they allow in-depth exploration of how individuals think and feel about an issue. Interviews were undertaken in 13 of the 43 trial general practices sampled from two of the three larger areas in the trial (south-west and north of England).
Conceptual framework
We did not work with a single conceptual framework, but rather combined three that were relevant to the study. First, as we were undertaking this qualitative study as part of a process evaluation alongside a trial of a complex intervention, we considered the three key themes relevant to process evaluations of complex interventions: implementation, mechanisms of impact and context. 353 The complex intervention was telehealth for LTCs and so we were influenced by the findings of the evidence synthesis reported in Chapter 2. Finally, the intervention was theory based and thus we were influenced by components of that theory. 250
Sampling
We interviewed 21 staff and 24 trial participants. We sampled NHS Direct staff purposively to include those delivering the intervention to participants (HIAs, n = 4), those offering technical expertise for intervention development and refinement (n = 1) and those involved in team management and strategic management in the organisation (n = 3). We planned to sample six general practices, selected to include practices with populations with varying levels of deprivation. However, we had to widen our original sample from six to 13 general practices because it proved difficult to recruit primary care staff and trial participants from the original set of practices. Within these 13 practices, we sampled purposively to reflect the range of relevant professionals offering primary health care to participants within the intervention arm of the trials, including GPs (n = 6) and practice nurses or HCAs (n = 7). We sampled trial participants purposively from the intervention arm of the trials to ensure that half of the interviewees were in the depression trial (n = 12) and half were in the CVD risk trial (n = 12). We then used maximum variation sampling so that trial participants of different sex, age and levels of PHQ-9 and CIS-R scores or types of CVD risk factors were interviewed. We also interviewed a small number of trial participants who had withdrawn from the depression intervention (n = 4), because this withdrawal rate was higher than that for the CVD risk intervention.
We approached staff at NHS Direct whom senior managers from the organisation had identified as having experience of the intervention. We wrote to GPs and practice nurses in participating practices asking for consent for an interview. We contacted trial participants who had consented to participate in the interview study during the trial recruitment process. Written informed consent was received from all interviewees.
Data collection
We interviewed NHS Direct staff delivering the intervention in July 2013, around 12 months after the first depression participant was randomised and 6 months after the first CVD risk participant was randomised. This allowed staff to reflect back over early delivery of the intervention, as well as later stages of intervention delivery. We interviewed primary care staff at different times of the trial period to obtain a mix of views at an early and later stage of intervention delivery. We interviewed trial participants after at least 4 months (depression) or 6 months (CVD risk) of experiencing the intervention to allow us to obtain reflections on different stages of patient care. This was after the primary outcome measure had been collected in the depression trial (4 months) and after one of the secondary time points in the CVD risk trial (6 months). Trial participants who had withdrawn from the intervention were interviewed within 5 months of recruitment.
Interviews with NHS Direct and primary care staff took place at their workplace or by telephone according to convenience, whereas interviews with trial participants took place at their home or at their local university participating in the trial (Bristol or Sheffield), according to preference. In each case, the focus of the interviews was on the intervention: its perceived utility, problems arising and issues that enhanced or hindered its operation in practice (see topic guides in Appendices 27–29). In addition, some of the components of the theory underlying the intervention were explicitly explored: engagement, self-management, treatment optimisation and co-ordination. Interviews lasted on average 58 minutes for trial participants, ranging from 21 to 124 minutes, and 45 minutes for staff, ranging from 16 to 88 minutes.
Analysis
Interviews were digitally recorded and transcribed verbatim. The framework approach was used to analyse the data. 355 In accordance with stage 1 of this approach, we read some transcripts from each type of interviewee for familiarisation. Stage 2 involved constructing a thematic framework based on reading these transcripts and the three themes identified within the MRC guidance on process evaluation:353 implementation, mechanisms of impact and context. The thematic framework was also informed by a framework for the use of qualitative research alongside trials, which added themes concerning the trial, outcomes and the health conditions under study. 356 Subthemes of the theme mechanisms of impact were informed by the components of the TECH model: engagement, promoting self-management, treatment optimisation, care co-ordination, partnership and context. 250 Familiarisation with the transcripts highlighted the relevance of the realist synthesis mechanisms identified in Chapter 2 (relationship, fit and visibility)125 and these were added as subthemes. In stage 3 of the framework approach, we coded all transcripts to the thematic framework, adding emerging subthemes throughout this process. We then read all of the text within each subtheme, paying attention to which interviewees contributed to each subtheme. The final stage of the framework approach, mapping and interpretation, involved consideration of themes and subthemes in the context of learning from previous work packages of the programme and other literature.
As recommended, the analysis was undertaken prior to any team member knowing the outcomes of the trials. 357 In September 2014, a written draft of the findings was discussed by the wider team involved in the final phase of the programme. Once the trial outcomes were known in December 2014, we used the findings to help explain the outcomes of the trials.
Chapter 8 Results from the depression trial
Some of the material in this chapter was adapted from Salisbury C, O’Cathain A, Edwards L, Thomas C, Gaunt D, Hollinghurst S, et al. Effectiveness of an integrated telehealth service for patients with depression: a pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry 2016;3:515–25. 358 Copyright © Salisbury et al. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Abstract
Background: Many countries are exploring the potential of telehealth interventions to help manage the rising number of people with chronic conditions. However, evidence of effectiveness of telehealth is equivocal. Based on an evidence-based conceptual framework, we developed an integrated telehealth service (the Healthlines Service) for chronic conditions and tested it in patients with depression.
Methods: This pragmatic, multicentre RCT compared the Healthlines Service for depression plus usual care with usual care alone. The service consisted of regular telephone calls from non-clinical trained health advisors following standardised scripts generated by interactive software. Advisors supported participants to use a range of online resources (including computerised CBT) and sought to encourage a healthier lifestyle, optimise participants’ medication and improve treatment adherence. We recruited participants from 43 general practices in three areas of England. Eligible participants required access to the internet and e-mail, a PHQ-9 score of ≥ 10 and a confirmed diagnosis of depression. Participants were individually allocated in a 1 : 1 ratio using an automated randomisation system, stratified by site and minimised by practice and PHQ-9 score. Participants were aware of their allocation but outcomes were analysed masked. The primary outcome was the proportion of participants responding to the intervention 4 months after randomisation (defined as a PHQ-9 score of < 10 and a reduction in PHQ-9 score of ≥ 5 points), with further follow-up at 8 and 12 months. The primary analysis was based on the principle of intention to treat without imputation.
Findings: Between 24 July 2012 and 31 July 2013 we recruited 609 participants, randomly allocating 307 to the Healthlines Service and 302 to usual care. In total, 525 (86%) provided primary outcome data. Response to treatment at 4 months was higher in the intervention arm [27% (68/255)] than in the usual care arm [19% (50/270)] (adjusted OR 1.7, 95% CI 1.1 to 2.5; p = 0.02). A repeated measures analysis provided evidence of effectiveness over 12 months. Compared with usual care alone, intervention participants also reported improvements in anxiety, better access to support and advice, greater satisfaction with the support that they received and improvements in self-management and health literacy. There were no reported SAEs related to the intervention.
Conclusion: The Healthlines Service for depression was both acceptable and effective compared with usual care. This provides support for the development and testing of similar interventions in other chronic conditions to expand care provision.
Overview
In this chapter we begin by describing recruitment data and baseline characteristics of the participants in the depression trial. Next, we discuss the results of the primary and sensitivity analyses of the primary outcome, the compliance analyses and the prespecified subgroup analyses. We go on to present the results of the secondary outcomes and the process measures analysis, as well as explore potential mechanisms suggested by the TECH model and post hoc subgroup analyses suggested by the process evaluation.
Baseline characteristics
Between June 2012 and June 2013, 16,570 patients were invited to take part in the study (Figure 15). This total number of patients takes into account 2159 patients who were excluded by their GP (e.g. because of unsuitability or recent bereavement or because an invitation would otherwise cause distress) and the only two patients who had been directly referred into the study by their GP (both were randomised into the trial).
FIGURE 15.
Recruitment and retention in the depression trial.

Of the 16,570 patients invited, 2375 expressed an interest in participating and were further assessed for eligibility. These patients were similar in age, sex and deprivation to the 14,195 patients who were not further assessed for eligibility (Table 17). As can be seen in Figure 15, there were three main reasons why these patients were not assessed for eligibility. First, the majority of patients in this group did not respond at all to the study invitation. Next, a sizeable number declined participation in the trial either by returning a decline form or by informing the local researcher on the telephone prior to commencing eligibility assessments. The decline form contained a number of prespecified reasons for not wanting to participate in the trial (see Appendix 17 for the proportions of patients selecting each of these reasons). The final group of patients was not assessed for eligibility for a number of other reasons, such as failing to return a fully signed study acceptance form, not answering researcher telephone calls or an administrative error or because the recruitment target was reached and no further participants could be included in the trial.
| Characteristic | Did not want take part (n = 14,195) | Wanted to take part (n = 2375) |
|---|---|---|
| Age (years), mean (SD) | 50.6 (17.0) | 51.3 (13.9) |
| Female, n (%) | 9572 (67) | 1671 (70) |
| Index of Multiple Deprivation at practice level, mean (SD) | 18.2 (9.1) | 16.9 (8.5) |
The remaining 2375 patients were assessed for eligibility, with 69% of patients not meeting the eligibility criteria (e.g. PHQ-9 score < 10, did not have regular access to the internet, already participating in CBT or another established psychological therapy) (see Figure 15). A small proportion (1%) declined participation at this stage, with 4% excluded for other reasons (e.g. did not return a completed consent form and/or baseline questionnaire, did not respond to any further contact from the researchers, administrative error or the recruitment target was reached and no further participants could be randomised). Therefore, of the 726 eligible patients, 609 participants were randomised between July 2012 and July 2013 (302 to usual care and 307 to the intervention; see Figure 15). Compared with those who were eligible and not randomised, randomised patients were slightly older and less deprived but had similar levels of depression; there was also a similar proportion of women in both groups (Table 18).
| Characteristic | Not randomised (n = 117) | Randomised (n = 609) |
|---|---|---|
| Demographic data | ||
| Age (years), mean (SD) | 47.0 (14.3) | 49.3 (12.9) |
| Female, n (%) | 81 (69) | 417 (68) |
| Index of Multiple Deprivation at practice level, mean (SD) | 18.4 (7.9) | 16.9 (8.3) |
| Clinical data | ||
| PHQ-9 score, mean (SD) | 17.6 (5.2) (n = 100) | 16.9 (4.6) |
| Categorised with mild depression using CIS-R, n (%) | 16 (14) | 91 (15) |
| Categorised with moderate depression using CIS-R, n (%) | 56 (48) | 313 (51) |
| Categorised with severe depression using CIS-R, n (%) | 45 (38) | 205 (34) |
The baseline characteristics of randomised participants are presented in Table 19. The different arms were mostly well balanced, although there were small differences in work status, education qualifications, accommodation ownership, depression diagnosis using the CIS-R scale298 and use of antidepressants. These imbalances were addressed by including these variables as additional covariates in a sensitivity analysis of the primary outcome, as the research team considered these variables to be prognostically associated with depression. It is notable that most patients recruited to the trial had at least moderate depression, with 34% having severe depression at baseline according to the CIS-R. Of the randomised participants, 67% (409/609) completed the baseline questionnaire using the secure survey website and the remainder returned a postal copy (200/609).
| Characteristic | Usual care (n = 302)a | Intervention (n = 307)a | |||
|---|---|---|---|---|---|
| n or n/N | Mean (SD) or % | n or n/N | Mean (SD) or % | ||
| Demographic data | |||||
| Age (years) | 302 | 50.0 (12.8) | 307 | 49.1 (12.9) | |
| Female | 204/302 | 68 | 213/307 | 69 | |
| White | 292/301 | 97 | 300/306 | 98 | |
| Current employment situation | |||||
| Full-time employment | 92/299 | 31 | 88/303 | 29 | |
| Part-time employment | 39/299 | 13 | 56/303 | 18 | |
| Full-time education | 2/299 | 1 | 5/303 | 2 | |
| Unemployed | 13/299 | 4 | 14/303 | 5 | |
| Unable to work because of long-term illness/disability | 78/299 | 26 | 73/303 | 24 | |
| Unable to work because of carer responsibilities | 2/299 | 1 | 4/303 | 1 | |
| Fully retired from work | 44/299 | 15 | 40/303 | 13 | |
| Looking after the home | 10/299 | 3 | 13/303 | 4 | |
| Doing something else | 19/299 | 6 | 10/303 | 3 | |
| Occupation (most recent or current) | |||||
| Administrative or secretarial occupations | 51/262 | 19 | 49/270 | 18 | |
| Associate professional or technical occupations | 37/262 | 14 | 35/270 | 13 | |
| Elementary occupations | 21/262 | 8 | 19/270 | 7 | |
| Managers or senior officials | 32/262 | 12 | 41/270 | 15 | |
| Personal services | 28/262 | 11 | 27/270 | 10 | |
| Process, plant and machine operatives | 11/262 | 4 | 15/270 | 6 | |
| Professionals | 35/262 | 13 | 42/270 | 16 | |
| Sales and customer services | 35/262 | 13 | 29/270 | 11 | |
| Skilled trades | 12/262 | 5 | 13/270 | 5 | |
| Highest educational qualification achieved | |||||
| Degree or higher degree | 84/298 | 28 | 68/303 | 22 | |
| A levels or equivalent | 54/298 | 18 | 63/303 | 21 | |
| GCSEs/O levels or equivalent | 119/298 | 40 | 130/303 | 43 | |
| No qualifications | 41/298 | 14 | 42/303 | 14 | |
| Accommodation | |||||
| Own accommodation or buying with mortgage | 162/300 | 54 | 179/307 | 58 | |
| Part-rent or rent accommodation | 124/300 | 41 | 118/307 | 38 | |
| Live rent free | 14/300 | 5 | 10/307 | 3 | |
| Index of Multiple Deprivation | 301 | 18.0 (13.0) | 307 | 18.3 (12.8) | |
| Clinical data | |||||
| Previously treated for depression | 258/276 | 93 | 269/295 | 91 | |
| PHQ-9 score | 302 | 16.7 (4.7) | 307 | 17.1 (4.5) | |
| GAD-7 score | 298 | 12.4 (5.0) | 304 | 13.5 (4.6) | |
| Categorised with mild depression using CIS-R | 52/302 | 17 | 39/307 | 13 | |
| Categorised with moderate depression using CIS-R | 148/302 | 49 | 165/307 | 54 | |
| Categorised with severe depression using CIS-R | 102/302 | 34 | 103/307 | 34 | |
| Taking antidepressants | 258/288 | 90 | 251/289 | 87 | |
Participants could remain in the trial for up to 12 months and were asked to complete three follow-up questionnaires at 4, 8 and 12 months post randomisation. Follow-up data collection for the trial took place from November 2012 (first wave 4-month follow-up) until August 2014 (final wave 12-month follow-up). The baseline characteristics of patients who either did or did not complete the primary PHQ-9 outcome measure at 4 months post randomisation are reported in Appendix 30. Participants who completed the primary outcome were similar in most respects to those who did not, although they had higher mean depression scores and diagnoses of depression at baseline (see Appendix 30).
Primary outcome
As prespecified in the statistical analysis plan, the primary outcome was the proportion of participants responding to treatment. This is a binary outcome, defined as a PHQ-9 score of < 10 and a reduction in PHQ-9 score of ≥ 5 between baseline and the 4-month follow-up. According to this definition, the primary analysis suggested that the proportion of participants who reduced their PHQ-9 score at 4 months was greater in the intervention arm than in the usual care arm (27% vs. 19%; Table 20). The primary result remained largely unchanged in our sensitivity analyses (see Table 20), although the evidence for any effect was slightly weakened when missing outcome data were imputed as non-response. However, the effect estimate was similar to that in the primary analysis when missing data were imputed using multiple imputation.
| Analysis | Usual care, % (n/N) | Intervention, % (n/N) | Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Primary analysis | |||||
| PHQ-9 response to treatment | 19 (50/270) | 27 (68/255) | 1.7 | 1.1 to 2.5 | 0.019 |
| Sensitivity analyses | |||||
| PHQ-9 response to treatment: simple imputation (assuming missing binary outcome is non-response) | 17 (50/302) | 22 (68/307) | 1.5 | 1.0 to 2.2 | 0.063 |
| PHQ-9 response to treatment: multiple imputation | 19 (56/302) | 28 (86/307) | 1.7 | 1.1 to 2.6 | 0.010 |
| PHQ-9 response to treatment: not including general practice as a random effect | 19 (50/270) | 27 (68/255) | 1.7 | 1.1 to 2.5 | 0.019 |
| PHQ-9 response to treatment: adjusted by days since randomisation to completion of the primary outcome | 19 (50/270) | 27 (68/255) | 1.7 | 1.1 to 2.5 | 0.018 |
| PHQ-9 response to treatment: adjusted by days since randomisation to completion of the primary outcome and baseline outcomesa | 19 (50/270) | 27 (68/255) | 1.9 | 1.2 to 3.0 | 0.005 |
Complier average causal effect analysis
As there is currently no instrumental variable regression method available for estimating a treatment effect among compliers with three levels of compliance and a binary outcome, we decided to estimate the unadjusted odds of response among the compliers using the principal stratification method as suggested by Frangakis and Rubin. 326 This was carried out in two ways: first, by classifying the partial compliers (participants who started encounters three to eight) as non-compliers and, second, by classifying the partial compliers as full compliers.
The crude OR for the treatment response was 1.6 (95% CI 1.1 to 2.4). Table 21 shows the crude estimated effect among compliers, after classifying partial compliers in the intervention arm as either non-compliers or full compliers. The results suggest a large increase in effect when compliance is defined as completing most or all of the Healthlines encounters.
| Amount of intervention received | PHQ-9 response at 12 months’ follow-up, % (n/N) | Partial compliers classified as non-compliers | Partial compliers classified as full compliers | |||
|---|---|---|---|---|---|---|
| Usual care | Intervention | Unadjusted ORa | 95% CI | Unadjusted ORb | 95% CI | |
| None (0–2 encounters started) | 33 (86/261) | 31 (16/52) | ||||
| Partial (3–8 encounters started) | 38 (42/111) | |||||
| Full (9–10 encounters started) | 42 (36/86) | 1·9 | 0·99 to 3·48 | 1·3 | 0·87 to 1·95 | |
Subgroup analyses of the primary outcome
There was no evidence that the intervention was differentially effective for any subgroups defined by baseline characteristics (Table 22).
| Subgroup | PHQ-9 response at 4 months’ follow-up, % (n/N) | Adjusted ORa | 95% CI | Interaction p-value | |
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Age group (years) | |||||
| < 40 | 20 (11/54) | 23 (14/60) | 1.2 | 0.5 to 2.9 | |
| 40–49 | 17 (11/65) | 24 (16/66) | 1.7 | 0.7 to 4.0 | |
| 50–59 | 18 (15/85) | 29 (19/66) | 2.0 | 0.9 to 4.4 | |
| 60–69 | 19 (9/48) | 27 (14/52) | 1.6 | 0.6 to 4.1 | |
| ≥ 70 | 22 (4/18) | 45 (5/11) | 2.8 | 0.6 to14.6 | 0.880 |
| Sex | |||||
| Male | 17 (15/89) | 22 (18/81) | 1.4 | 0.7 to 3.1 | |
| Female | 19 (35/181) | 29 (50/174) | 1.7 | 1.1 to 2.9 | 0.673 |
| Baseline PHQ-9 score | |||||
| 10–14 | 21 (21/99) | 29 (26/91) | 1.6 | 0.8 to 3.0 | |
| 15–19 | 20 (19/93) | 32 (27/85) | 1.9 | 0.9 to 3.7 | |
| ≥ 20 | 13 (10/78) | 19 (15/79) | 1.6 | 0.7 to 3.9 | 0.933 |
| Baseline CIS-R category | |||||
| Mild | 35 (17/49) | 33 (11/33) | 0.9 | 0.4 to 2.3 | |
| Moderate | 15 (21/137) | 26 (36/136) | 2.0 | 1.1 to 3.7 | |
| Severe | 14 (12/84) | 24 (21/86) | 2.0 | 0.9 to 4.4 | 0.335 |
| Antidepressants at baseline | |||||
| Not prescribed | 13 (3/23) | 25 (8/32) | 2.4 | 0.5 to 10.3 | |
| Prescribed | 20 (46/233) | 28 (58/207) | 1.6 | 1.0 to 2.5 | 0.626 |
Secondary outcomes
The secondary outcome analyses are based on linear or logistic regression models (as appropriate) adjusted, as in the primary analysis, by site (Bristol, Sheffield or Southampton), baseline PHQ-9 score and the baseline outcome being analysed. General practice was included as a random effect.
Binary PHQ-9 response and repeated measures analysis
The proportion of participants who responded to treatment increased in both arms at 8 and 12 months (Table 23). Although the between-group difference appeared to diminish with increasing duration of follow-up, there was no statistical evidence of an interaction between treatment arm and time point (interaction p = 0.402). Therefore, we estimated an overall treatment effect using all follow-up data at 4, 8 and 12 months in a repeated measures analysis. This provided some evidence that the intervention had an average effect over 12 months in increasing the proportion of responders compared with usual care (see Table 23).
| Time point (months) | PHQ-9 binary response to treatment | Adjusted OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care, % (n/N) | Intervention, % (n/N) | ||||
| 4 | 19 (50/270) | 27 (68/255) | 1.7 | 1.1 to 2.5 | |
| 8 | 23 (61/263) | 30 (75/252) | 1.4 | 1.0 to 2.2 | |
| 12 | 33 (86/261) | 37 (95/255) | 1.2 | 0.9 to 1.8 | |
| Repeated measuresa | 1.6 | 1.0 to 2.6 | 0.035 | ||
Continuous PHQ-9 score
Mean PHQ-9 scores decreased over time in both groups (Table 24), with the adjusted between-group difference being greatest at 8 months. Although the between-group difference appeared to change over the duration of follow-up, there was no statistical evidence of an interaction between treatment arm and time point (interaction p = 0.345). Therefore, we estimated an overall treatment effect using all follow-up data at 4, 8 and 12 months in a repeated measures analysis, which provided some evidence that the intervention was effective in decreasing the PHQ-9 score compared with usual care (see Table 24).
| Secondary outcome | Usual care | Intervention | Adjusted difference in means | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| PHQ-9 score | |||||||
| Baseline | 16.7 (4.7) | 302 | 17.1 (4.5) | 307 | |||
| 4 months | 13.8 (6.2) | 270 | 13.3 (6.1) | 255 | –0.8 | –1.8 to 0.1 | |
| 8 months | 13.4 (6.2) | 263 | 12.4 (6.2) | 252 | –1.2 | –2.2 to –0.3 | |
| 12 months | 11.9 (6.4) | 261 | 11.6 (6.2) | 255 | –0.5 | –1.5 to 0.5 | |
| Repeated measuresa | –0.8 | –1.6 to 0.0 | 0.045 | ||||
| GAD-7 score | |||||||
| 4 months | 10.5 (5.9) | 250 | 10.5 (5.7) | 227 | –0.9 | –1.7 to 0.0 | 0.048 |
| 8 months | 10.2 (5.7) | 230 | 9.1 (5.4) | 212 | –1.6 | –2.6 to –0.7 | < 0.001 |
| 12 months | 9.2 (5.8) | 237 | 8.7 (5.5) | 223 | –1.1 | –2.0 to –0.2 | 0.020 |
Continuous GAD-7 score
Mean GAD-7 scores decreased over time for both groups and, as for the PHQ-9 mean scores, the greatest between-group difference was at 8 months (see Table 24).
Patient satisfaction
There was not much evidence that satisfaction with primary HCPs differed between the groups. However, satisfaction scores for NHS Direct and overall treatment were higher in the intervention arm (Table 25). Nonetheless, these satisfaction scores should be interpreted with caution. This is because participants in the intervention group may not have discriminated between NHS Direct as a public service and the Healthlines Service, which was delivered by NHS Direct staff, although they were asked to provide satisfaction with the two services separately. Satisfaction with GPs and nurses was not collected at baseline and therefore these analyses were not adjusted for any potential baseline differences between the two groups.
| Measure of satisfactiona | Usual care | Intervention | Adjusted difference in means | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| General GP satisfactionb | |||||||
| 4 months | 3.9 (0.9) | 232 | 3.9 (1.1) | 216 | 0.0 | –0.2 to 0.1 | 0.787 |
| 8 months | 3.9 (1.0) | 215 | 4.0 (1.0) | 188 | 0.1 | 0.0 to 0.3 | 0.162 |
| 12 months | 3.9 (1.0) | 218 | 4.0 (1.0) | 205 | 0.2 | 0.0 to 0.4 | 0.038 |
| Detailed GP satisfactionc | |||||||
| 4 months | 4.1 (1.0) | 217 | 4.1 (1.0) | 207 | 0.0 | –0.2 to 0.1 | 0.669 |
| 8 months | 4.0 (0.9) | 205 | 4.1 (0.9) | 176 | 0.1 | –0.1 to 0.3 | 0.185 |
| 12 months | 4.0 (0.9) | 203 | 4.2 (0.9) | 197 | 0.2 | 0.0 to 0.3 | 0.046 |
| General nurse satisfactionb | |||||||
| 4 months | 3.9 (0.8) | 154 | 4.1 (0.8) | 139 | 0.2 | 0.0 to 0.4 | 0.081 |
| 8 months | 4.0 (0.9) | 147 | 4.1 (0.9) | 127 | 0.1 | –0.1 to 0.3 | 0.376 |
| 12 months | 4.0 (0.9) | 142 | 4.1 (0.9) | 136 | 0.2 | 0.0 to 0.4 | 0.073 |
| Detailed nurse satisfactionc | |||||||
| 4 months | 4.2 (0.8) | 116 | 4.1 (0.8) | 112 | 0.00 | –0.2 to 0.2 | 0.746 |
| 8 months | 4.1 (0.9) | 125 | 4.2 (0.8) | 87 | 0.2 | –0.1 to 0.4 | 0.215 |
| 12 months | 4.0 (0.9) | 114 | 4.3 (0.8) | 101 | 0.2 | 0.0 to 0.5 | 0.033 |
| General NHS Direct satisfactionb | |||||||
| 4 months | 3.4 (0.9) | 71 | 4.0 (1.0) | 157 | 0.7 | 0.3 to 1.1 | 0.001 |
| 8 months | 3.5 (1.1) | 69 | 4.0 (1.0) | 128 | 0.9 | 0.4 to 1.3 | < 0.001 |
| 12 months | 3.5 (0.8) | 63 | 4.0 (1.0) | 121 | 0.4 | 0.0 to 0.8 | 0.070 |
| Treatment satisfactionb | |||||||
| 4 months | 3.2 (0.9) | 196 | 3.5 (0.9) | 207 | 0.3 | 0.1 to 0.4 | 0.004 |
| 8 months | 3.3 (0.9) | 182 | 3.6 (0.9) | 172 | 0.3 | 0.1 to 0.5 | 0.002 |
| 12 months | 3.3 (0.9) | 184 | 3.7 (0.9) | 193 | 0.5 | 0.3 to 0.6 | < 0.001 |
Intervention satisfaction
Satisfaction with aspects of the Healthlines Service among participants in the intervention group increased slightly over the follow-up period (Table 26). Although comparisons with the usual care group are not possible, these questions about intervention satisfaction used the same response scale as those questions about primary care and treatment satisfaction (see Table 25). Therefore, it is possible to make general comparisons about the level of satisfaction between the Healthlines Service and other forms of care among the intervention group. Participants in the intervention group appeared to be slightly more satisfied with the care that they received from the HIAs than with the care that they received from GPs and nurses in their own practice. They were also satisfied with the Healthlines website and CBT programme, LLTTFi.
| Measure of satisfactiona | Mean (SD) | n |
|---|---|---|
| Detailed HIA satisfaction | ||
| 4 months | 4.2 (0.9) | 180 |
| 8 months | 4.3 (0.9) | 136 |
| 12 months | 4.4 (0.8) | 126 |
| Intervention website satisfaction | ||
| 4 months | 3.7 (0.8) | 158 |
| 8 months | 3.8 (0.8) | 135 |
| 12 months | 3.9 (0.9) | 122 |
| Intervention CBT programme satisfaction | ||
| 4 months | 3.7 (0.9) | 175 |
| 8 months | 3.9 (0.9) | 150 |
| 12 months | 3.9 (0.9) | 142 |
Access to care
There is moderate to strong evidence that participants in the intervention group had fewer difficulties in accessing aspects of care related to service delivery (getting support and advice at times that suit the patient, when they need it most and from the HCPs they want to see) and they also reported greater satisfaction with the amount of support that they received (Table 27).
| Measure of access to care | Usual care | Intervention | Adjusted difference in means | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Service delivery access difficultiesa | |||||||
| 4 months | 3.9 (2.0) | 244 | 4.4 (1.9) | 226 | 0.6 | 0.4 to 0.9 | < 0.001 |
| 8 months | 4.2 (1.9) | 224 | 4.5 (1.8) | 206 | 0.4 | 0.0 to 0.7 | 0.024 |
| 12 months | 4.2 (1.9) | 232 | 4.5 (1.9) | 216 | 0.3 | 0.0 to 0.6 | 0.044 |
| Satisfaction with amount of supportb | |||||||
| 4 months | 2.1 (0.9) | 191 | 2.5 (0.9) | 200 | 0.5 | 0.3 to 0.6 | < 0.001 |
| 8 months | 2.2 (0.8) | 170 | 2.5 (0.8) | 170 | 0.3 | 0.1 to 0.4 | < 0.001 |
| 12 months | 2.1 (0.9) | 177 | 2.6 (0.8) | 185 | 0.5 | 0.4 to 0.7 | < 0.001 |
Process measures analyses
As shown in Table 28, there were only small differences between the groups in the numbers of participants using resources and services that were similar to those offered by the intervention. Early on during follow-up, participants in the intervention arm were more likely than those in the usual care arm to use self-help books and throughout follow-up they were more likely to use online/computerised CBT. This finding is largely expected, as these were components of the Healthlines Service. There were no reported differences in gym membership or numbers attending fitness or exercise classes.
| Service | Usual care, % (n/N) | Intervention, % (n/N) |
|---|---|---|
| Use of self-help books | ||
| 4 months | 18 (46/250) | 31 (70/227) |
| 8 months | 12 (28/228) | 24 (50/212) |
| 12 months | 17 (40/235) | 13 (29/219) |
| Use of online/computerised CBT | ||
| 4 months | 4 (10/247) | 31 (67/219) |
| 8 months | 3 (7/225) | 25 (51/208) |
| 12 months | 3 (7/232) | 16 (35/216) |
| Joined or renewed gym membership | ||
| 4 months | 14 (35/251) | 12 (27/228) |
| 8 months | 12 (27/229) | 13 (27/212) |
| 12 months | 11 (25/229) | 9 (19/221) |
| Attended exercise class or fitness activity | ||
| 4 months | 18 (46/250) | 20 (46/226) |
| 8 months | 18 (41/227) | 18 (38/213) |
| 12 months | 18 (42/236) | 19 (41/218) |
Amount of intervention received
Number of Healthlines encounters
Figure 16 shows the number of Healthlines encounters started by intervention participants out of a possible total of 10 encounters. Three participants did not receive the intervention because of an administrative error and another participant received only non-encounter calls and so these four participants were categorised as having no encounters. Data were also missing from the intervention database for 12 participants and so it was not possible to tell how many encounters they received. Out of the remaining 295 participants (307 randomised), 26% of participants received none or little of the intervention (started two or fewer encounters), 44% received some of the intervention sessions planned (three to eight encounters) and 29% received all or almost all of the planned encounters (nine or ten encounters). The median number of encounters started was five [interquartile range (IQR) 2 to 9, n = 295], the mean encounter duration was 18.5 minutes (SD 12.7 minutes) and there was a total of 1972 encounter calls. Figure 16 shows that one group of participants received two or three encounters before dropping out, whereas the other group continued for most or all of the course.
FIGURE 16.
Distribution of Healthlines encounters started in the depression trial.
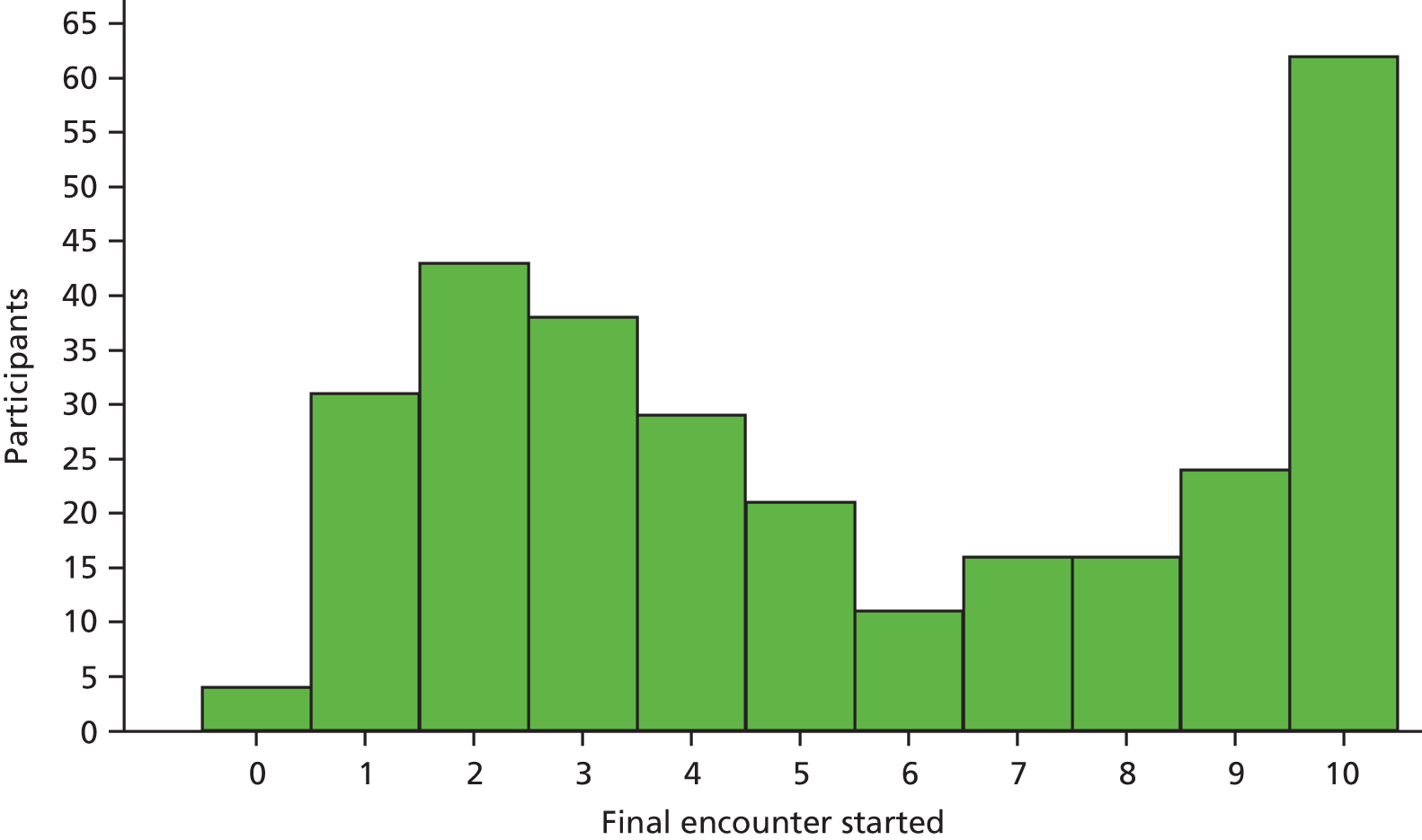
Letters sent by health information advisors to participants and general practitioners
The median number of e-mails sent to participants by the HIAs was zero (IQR 0 to 1, n = 283) and the median number of letters sent to participants’ GPs by e-mail was three (IQR 1 to 6, n = 283). The breakdown of these letters by their content is detailed in Table 29.
| Letter content | Number of letters (% of total letters sent) | Number of participants whose GP was sent at least one letter (% of 283) |
|---|---|---|
| Completion of CBT course | 84 (8) | 86 (30) |
| Generic (information for GP) | 34 (3) | 24 (8) |
| Intervention completion | 50 (5) | 49 (17) |
| Intervention introduction | 298 (27) | 278 (98) |
| Medication (information for GP) | 147 (13) | 92 (33) |
| PHQ-9 score | 374 (34) | 165 (58) |
| Potential suicide risk | 123 (11) | 64 (23) |
| Total number of letters sent | 1110 |
Use of cognitive–behavioural therapy
As described in Chapter 6 (see Depression trial), a central aspect of the Healthlines Service was CBT. Participants could access CBT through a book, Overcoming Depression and Low Mood: a Five Areas Approach,292 or online, using the LLTTFi programme. Table 30 presents the numbers of participants who chose to use the two different CBT formats.
| Form of CBT provided | n | % |
|---|---|---|
| Given the ODLM book | 45 | 16 |
| Given access to LLTTFi online | 229 | 80 |
| CBT declined | 3 | 1 |
| Given both LLTTFi access and the ODLM book | 8 | 3 |
| Total | 285 | 100 |
Living Life to the Full Interactive is operated by Media Innovations Ltd. In total, 204 participants logged onto the LLTTFi website and completed a median of two (IQR 0 to 5) out of the seven CBT modules each. Sixty-six participants who logged on did not complete the first module (Figure 17).
FIGURE 17.
Distribution of LLTTFi modules completed.
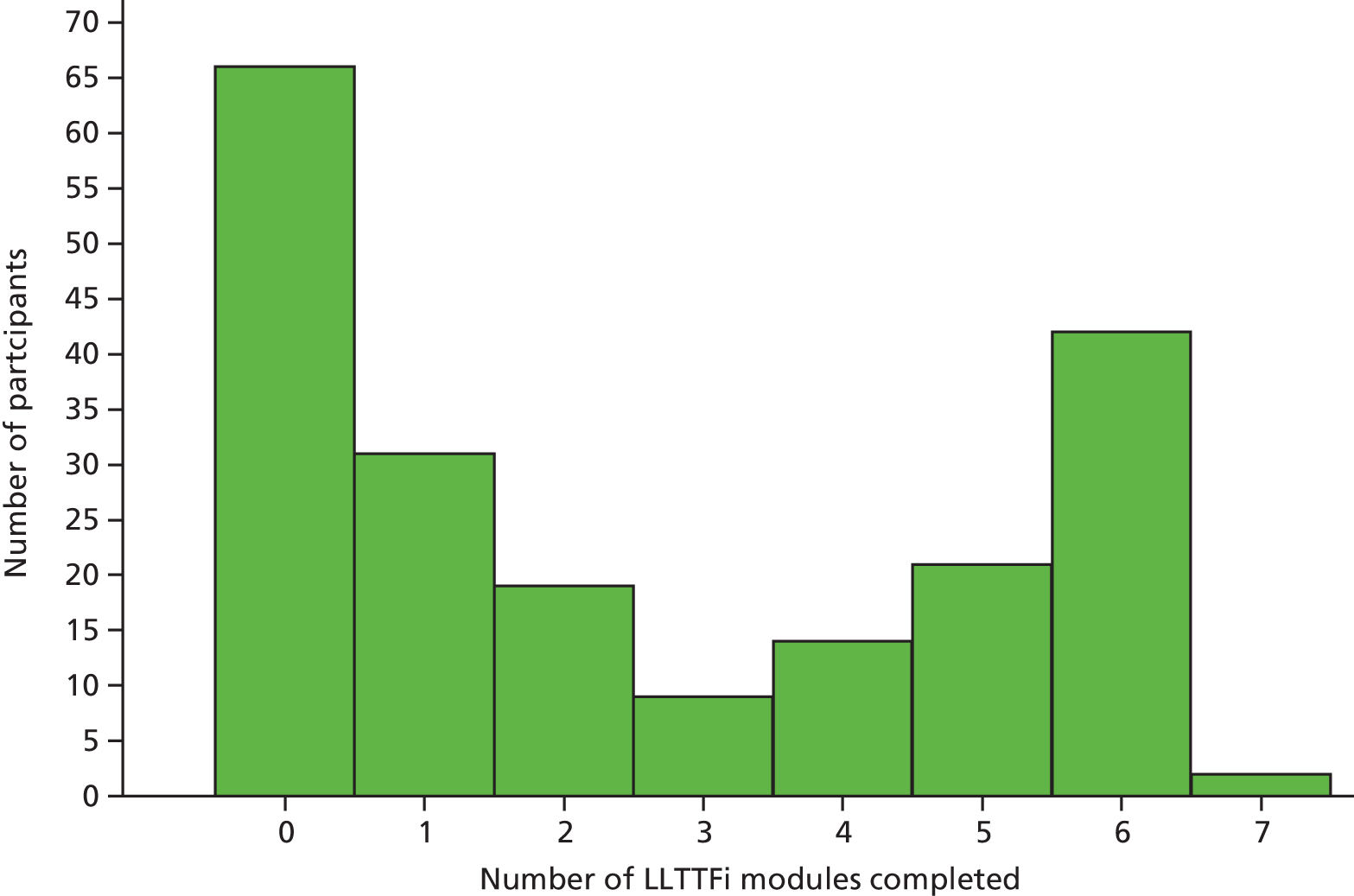
Use of Big White Wall
Another component of the intervention was access to BWW, an online mental health community and forum facility (see Chapter 6, Depression trial). In total, 105 participants started the registration process with BWW and 98 completed registration and logged on to the website at least once. Usage data are shown in Table 31.
| Usage of BWW | Median (range) |
|---|---|
| Number of logins | 2 (1–104) |
| Hours using site | 0.30 (0.00–11.17) |
| Number of ‘interactions’a | 0 (0–49) |
| Pages of ‘useful stuff’b | 1 (0–40) |
| All page views | 21 (0–991) |
Exploration of potential mechanisms
To explore the different components of the intervention, data were collected on the different concepts of the TECH model (see Chapter 5). These are presented descriptively within this report. Some of the explanatory variables from the TECH model were prespecified as secondary outcomes in the original protocol and so for these variables we have estimated between-group effects at the primary (4-month) and/or final (12-month) follow-up time -point.
Patient engagement
According to the TECH model, patient engagement with telehealth partly determines the success and effectiveness of such services. Few participants in either arm made use of the publicly available NHS Direct telephone services or an online forum or group for physical or mental health during the trial (Table 32). Around one-quarter of participants reported using online searching for health information, although there was no evidence that use differed between arms at 12 months.
| Telehealth service | Use of telehealth at least every 2 weeksa | Adjusted ORb | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care, % (n/N) | Intervention, % (n/N) | ||||
| NHS Direct telephone services | |||||
| 4 months | < 1 (1/248) | 4 (8/226) | |||
| 8 months | 1 (3/231) | 3 (6/213) | |||
| 12 months | < 1 (1/239) | 2 (4/222) | 4.0 | 0.4 to 36.5 | 0.216 |
| Online searching | |||||
| 4 months | 19 (47/247) | 26 (60/227) | |||
| 8 months | 24 (55/231) | 27 (57/213) | |||
| 12 months | 22 (53/237) | 23 (51/223) | 1.0 | 0.6 to 1.7 | 0.964 |
| Online forum or group | |||||
| 4 months | 6 (15/249) | 10 (23/225) | |||
| 8 months | 7 (16/231) | 7 (15/212) | |||
| 12 months | 8 (18/235) | 4 (10/223) | 0.5 | 0.2 to 1.1 | 0.072 |
Engagement with the intervention
There are some variables of relevance to patient engagement that were relevant only for those in the intervention group, as they pertain to engagement with the intervention. First, the median number of times that participants logged on the Healthlines Service web portal was one (IQR 0 to 6). Next, the continuity of care index359 in the present context measures the concentration of telephone calls with different HIAs. It measures the number of HIAs that each participant spoke to, such that a higher continuity score indicates a greater number of calls with fewer HIAs (range 0–1). As would be expected, the mean continuity score was lower when unscheduled calls were included (Table 33). Third, the usual practitioner care index359 is interpreted here as the percentage of Healthlines encounters that a participant had with their ‘usual’ HIA (i.e. the HIA with whom they spoke to most often; range 0–1). Over 70% of all encounters were with participants’ usual HIA (see Table 33) and, as for the continuity of care, usual practitioner care index was lower when unscheduled encounters were included.
| Measure of engagement | Mean (SD) | n |
|---|---|---|
| Continuity of care index | 0.56 (0.32) | 266 |
| Usual practitioner care index | 0.74 (0.23) | 291 |
| Continuity of care index including non-scheduled calls | 0.51 (0.30) | 269 |
| Usual practitioner care index including non-scheduled calls | 0.71 (0.23) | 292 |
General practitioner engagement
Based on the medication-related letters that the HIAs sent to GPs, a form of GP engagement in this trial was the number of medication changes, which would be expected to be greater in the intervention group. We defined a medication change as a change in drug name or dosage from baseline, which was captured from participants’ medical records. There was no difference in the number of changes in antidepressant medications between the two groups, with both groups experiencing a median of zero changes (IQR 0 to 1) (Figure 18).
FIGURE 18.
Distribution of changes in antidepressant medications: (a) control; and (b) intervention.
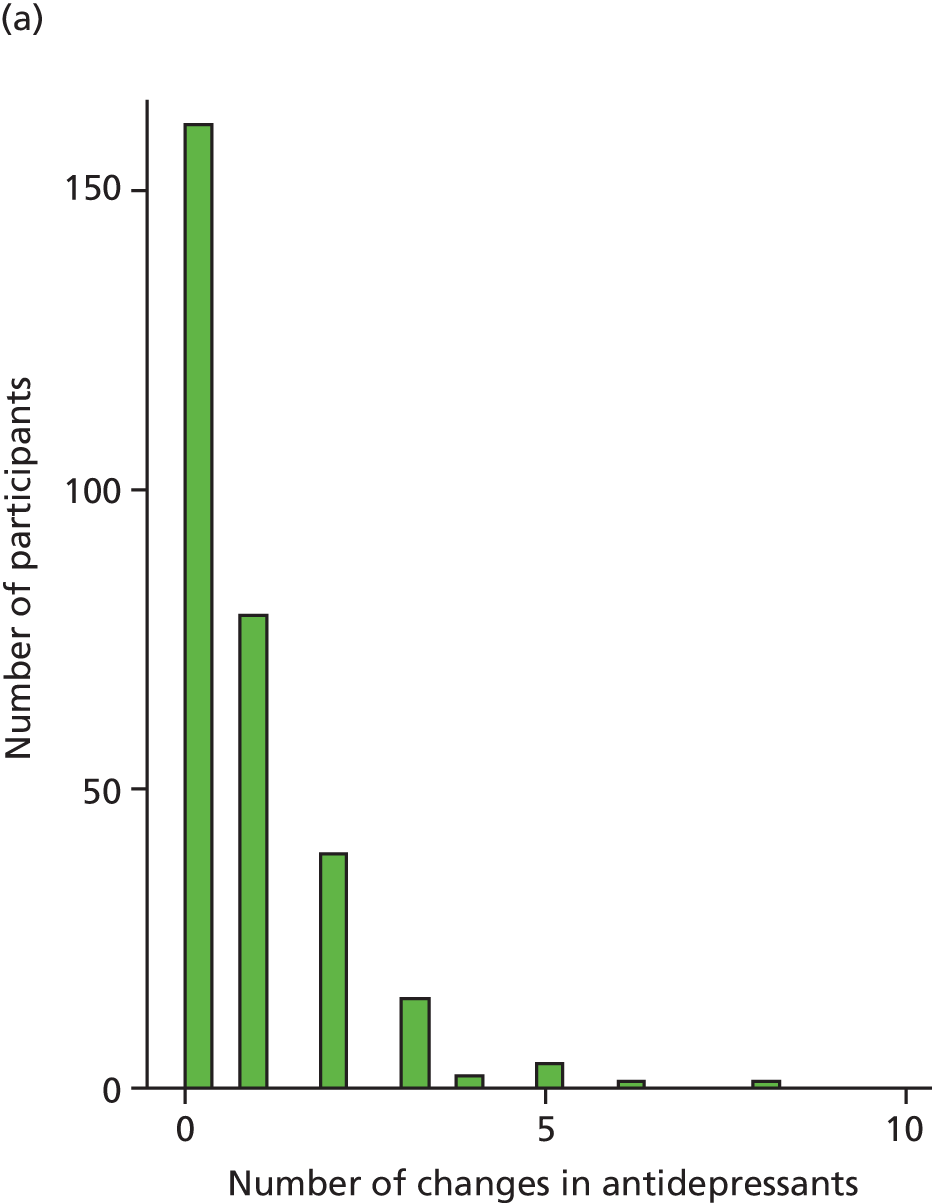

Self-management
Participants completed a number of the subscales from the Health Education Impact Questionnaire (heiQ),313 which measure different aspects of self-management. First, participants in the intervention group undertook slightly less physical activity than those in the usual care group at 8 months, but slightly more than those in the usual care group at 12 months (Table 34). However, there was no evidence of a difference between the groups at 12 months after adjusting for baseline physical activity. Next, Table 34 shows that the between-group unadjusted mean differences for the four subscales that tap aspects of self-management skills and self-efficacy were small. Nonetheless, there was a slight increase in these mean scores over the trial for both groups.
| Aspect of self-management | Usual care | Intervention | Adjusted difference in meansb | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Physical activity | |||||||
| 4 months | 2.4 (0.9) | 250 | 2.4 (0.9) | 228 | |||
| 8 months | 2.4 (0.9) | 228 | 2.3 (0.9) | 213 | |||
| 12 months | 2.4 (0.9) | 235 | 2.5 (0.9) | 221 | 0.1 | < 0.0 to 0.2 | 0.118 |
| Self-management skills and self-efficacy | |||||||
| Self-monitoring and insight | |||||||
| 4 months | 2.8 (0.4) | 249 | 2.9 (0.4) | 229 | |||
| 8 months | 2.8 (0.5) | 231 | 2.9 (0.4) | 212 | |||
| 12 months | 2.4 (0.9) | 237 | 3.0 (0.5) | 221 | 0.1 | 0.0 to 0.2 | 0.005 |
| Constructive attitudes and approaches | |||||||
| 4 months | 2.5 (0.6) | 250 | 2.6 (0.6) | 229 | |||
| 8 months | 2.5 (0.6) | 232 | 2.6 (0.6) | 231 | |||
| 12 months | 2.6 (0.6) | 238 | 2.7 (0.6) | 221 | 0.0 | –0.1 to 0.1 | 0.480 |
| Skill and technique acquisition | |||||||
| 4 months | 2.6 (0.5) | 250 | 2.6 (0.5) | 228 | |||
| 8 months | 2.6 (0.5) | 232 | 2.7 (0.5) | 212 | |||
| 12 months | 2.6 (0.5) | 239 | 2.8 (0.5) | 221 | 0.2 | 0.1 to 0.2 | 0.001 |
| Health services navigation | |||||||
| 4 months | 2.7 (0.6) | 250 | 2.8 (0.6) | 228 | |||
| 8 months | 2.8 (0.6) | 232 | 2.9 (0.6) | 212 | |||
| 12 months | 2.8 (0.6) | 238 | 2.9 (0.6) | 220 | 0.2 | 0.1 to 0.3 | < 0.001 |
Treatment optimisation
The proportions of participants who reported taking (Table 35) and adhering to (Table 36) antidepressants, as well as the proportions identified from practice records as being prescribed an antidepressant at least once during the trial (Table 37), were very similar in both arms. The adjusted difference in mean Morisky score315 for antidepressant medication adherence at 12 months’ follow-up was –0.1 (95% CI –0.2 to 0.1; p = 0.501). There was no evidence of a difference between groups in the proportions taking antidepressant medication at either 4 or 12 months (see Table 35).
| Follow-up point (months) | Usual care, % (n/N) | Intervention, % (n/N) | Adjusted ORa | 95% CI | p-value |
|---|---|---|---|---|---|
| 4 | 87 (202/233) | 87 (188/216) | 1.6 | 0.7 to 3.4 | 0.234 |
| 8 | 82 (176/214) | 79 (159/201) | NA | NA | NA |
| 12 | 78 (174/224) | 81 (172/213) | 1.6 | 0.9 to 2.8 | 0.103 |
| Follow-up point (months) | Usual care | Intervention | Adjusted difference in meansb | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| 4 | 3.2 (1.0) | 204 | 3.2 (1.1) | 192 | |||
| 8 | 3.4 (0.9) | 181 | 3.3 (1.0) | 163 | |||
| 12 | 3.4 (0.9) | 179 | 3.2 (1.1) | 173 | –0.1 | –0.2 to 0.1 | 0.511 |
| Antidepressant medication prescription | Usual care | Intervention | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| Prescribed at least one antidepressant medication | 273/302 | 90 | 277/307 | 90 |
| Had one or more changes in antidepressant medication or dose | 141/302 | 47 | 150/307 | 49 |
Care co-ordination
There were no meaningful differences in any of the scales that were intended to capture participants’ experiences of co-ordination of care (Table 38).
| Scale | Usual care | Intervention | Adjusted difference in meansb | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Role clarity and co-ordination (range 0–3) | |||||||
| 4 months | 2.7 (0.8) | 193 | 2.7 (0.7) | 194 | |||
| 8 months | 2.8 (0.6) | 183 | 2.8 (0.6) | 171 | |||
| 12 months | 2.8 (0.5) | 174 | 2.8 (0.6) | 181 | –0.1 | –0.2 to 0.1 | 0.361 |
| Evidence of a care plan (range 0–7) | |||||||
| 4 months | 2.9 (2.1) | 199 | 3.3 (2.1) | 197 | |||
| 8 months | 3.0 (2.2) | 185 | 3.3 (2.1) | 165 | |||
| 12 months | 3.1 (2.2) | 176 | 3.5 (2.4) | 179 | 0.3 | –0.1 to 0.8 | 0.173 |
| Overall experience of organisation of health care (range 1–5) | |||||||
| 4 months | 2.9 (1.0) | 251 | 3.1 (1.0) | 227 | |||
| 8 months | 3.0 (1.0) | 232 | 3.1 (1.1) | 213 | |||
| 12 months | 3.1(1.0) | 236 | 3.2 (1.0) | 219 | 0.1 | –0.1 to 0.3 | 0.247 |
| Self-organisation of health care (range 1–5) | |||||||
| 4 months | 2.9 (1.2) | 239 | 3.1 (1.3) | 215 | |||
| 8 months | 3.1 (1.2) | 224 | 3.1 (1.1) | 204 | |||
| 12 months | 3.2 (1.2) | 230 | 3.1 (1.2) | 210 | 0.0 | –0.2 to 0.2 | 0.841 |
Patient context
Motivation
Motivation to participate in the study was reported in the baseline questionnaire. As covered in Chapter 7 (see Table 14), internal motivation reflects participants’ inclination to make changes in their life, whereas joining the study because of external motivation reflects self-perceived external pressures for treatment (e.g. from one’s GP). As shown in Table 39, there was very little difference in internal or external motivation between the two groups and, in general, participants were more internally motivated than externally motivated.
| Motivation | Usual care | Intervention | ||
|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | |
| Internal motivation | 5.7 (1.5) | 299 | 5.9 (1.4) | 307 |
| External motivation | 1.5 (1.0) | 295 | 1.5 (1.0) | 305 |
Use of the internet
Participants were also asked about their general use of the internet, including e-mail. Table 40 shows the numbers of respondents who reported using the internet more than once a week. These results were balanced across the two groups, with slightly more intervention participants than usual care participants using the internet at 4 months and slightly fewer intervention participants than usual care participants using the internet at 8 months.
| Follow-up point (months) | Usual care, % (n/N) | Intervention, % (n/N) |
|---|---|---|
| 4 | 76 (189/250) | 75 (169/224) |
| 8 | 75 (170/227) | 76 (160/211) |
| 12 | 75 (175/234) | 75 (166/221) |
Health literacy and confidence using technology
Table 41 shows that participants in the intervention group had slightly better health literacy on average than participants in the usual care group at all time points. The intervention group reported slightly greater confidence in using the internet and social media at 8 and 12 months, as well as more confidence in using the telephone at all time points, than the usual care group (see Table 41). More detail on these subscales is provided in Table 14.
| Outcome | Usual care | Intervention | Adjusted difference in meansa | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Health literacyb | |||||||
| 4 months | 3.6 (0.9) | 243 | 3.7 (0.8) | 225 | |||
| 8 months | 3.7 (0.9) | 229 | 3.8 (0.8) | 212 | |||
| 12 months | 3.7 (0.8) | 235 | 3.9 (0.8) | 220 | 0.2 | 0.1 to 0.4 | < 0.001 |
| Confidence in using technologyc | |||||||
| Internet confidence | |||||||
| 4 months | 5.8 (1.7) | 251 | 5.8 (1.7) | 228 | |||
| 8 months | 5.8 (1.7) | 231 | 5.9 (1.6) | 214 | |||
| 12 months | 5.8 (1.7) | 237 | 5.9 (1.6) | 223 | |||
| Social media confidence | |||||||
| 4 months | 4.0 (2.2) | 251 | 4.0 (2.1) | 227 | |||
| 8 months | 3.9 (2.2) | 230 | 4.1 (2.2) | 212 | |||
| 12 months | 4.0 (2.1) | 236 | 4.3 (2.2) | 223 | |||
| Phone confidence | |||||||
| 4 months | 5.6 (1.6) | 251 | 5.8 (1.6) | 228 | |||
| 8 months | 5.7 (1.6) | 231 | 5.9 (1.6) | 214 | |||
| 12 months | 5.7 (1.6) | 236 | 5.9 (1.4) | 223 | |||
Adverse events
Over the course of the trial, and as defined in Chapter 7 (see Serious adverse events), there were 70 adverse events reported by participants, with no difference between the arms. Frequencies of adverse events are reported in Table 42.
| Adverse event | Usual care | Intervention | |
|---|---|---|---|
| Not related | Not related | Definitely related | |
| Not serious | 6 | 4 | 1 |
| Serious unexpected | 30 | 29 | 0 |
Post hoc subgroup analyses suggested by the embedded qualitative study
One hypothesis was generated by the embedded qualitative study (see Chapter 11, Testing hypotheses generated by the embedded qualitative study), which we have investigated in a post hoc exploratory subgroup analysis. We recognise the exploratory nature of this analysis and that any such analysis is likely to have low power to detect anything but very large interaction effects. The hypothesis was as follows:
-
Given concerns about whether or not the inclusion criteria should have been different, because some people had a low need or no desire to modify risk factors, undertake a subgroup analysis on the level of internal and external motivation for joining the study. Those who were highly motivated to join the study because they, personally, wanted to make changes to their life (i.e. internal motivation) would be expected to improve more than those with low internal motivation.
This hypothesis was tested using the internal and external motivation subscales314,315 (see Table 14). To categorise participants as having high or low motivation, these subscales were dichotomised at the overall median score (i.e. not by trial arm). The median for the internal motivation subscale was 6 and the median for the external motivation subscale was 1. The results are presented in Table 43, which shows that there is some evidence of a subgroup effect for external motivation. The intervention appears to be more effective among participants who reported lower external motivation at baseline, that is, participants who were less likely to have reported that they joined the study because of external pressures, such as from their doctor.
| Motivation | PHQ-9 response at 4 months’ follow-up, % (n/N) | Adjusted ORb | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Baseline internal motivation | |||||
| Low (≤ 6) | 16 (23/142) | 25 (33/131) | 1.8 | 1.0 to 3.3 | |
| High (> 6) | 21 (26/126) | 28 (35/124) | 1.5 | 0.9 to 2.8 | 0.707 |
| Baseline external motivation | |||||
| Low (≤ 1) | 17 (33/194) | 30 (53/176) | 2.2 | 1.3 to 3.7 | |
| High (> 1) | 22 (16/72) | 19 (15/78) | 0.8 | 0.4 to 1.8 | 0.036 |
Discussion
Interpretation of the findings
The Healthlines depression intervention was clinically effective, being associated with a greater response to treatment than usual care. The size of this effect was moderate (OR 1.7) and clinically important. This means that 12 participants would have to be treated for one of them to respond to treatment by 4 months, over and above the number who would respond in the usual care arm (number needed to treat 12). The criterion for response to treatment was based on previous research on the clinically important difference in the PHQ-9 primary outcome. 308 The difference in mean PHQ-9 score was small (0.8); however, the PHQ-9 suffers from considerable imprecision, which is why the primary outcome was stringent and defined as an improvement of at least 5 points in the PHQ-9 and a score of < 10. The discrepancy between the positive outcome using a binary outcome but the minimal difference seen in mean scores is discussed further later in this chapter.
The impact of the intervention was greatest after 4 months’ follow-up (the primary outcome) and the difference between the intervention and the usual care arms narrowed over time as both groups improved. After 12 months’ follow-up there was no evidence of a benefit from the intervention, although there was an average benefit over the whole 12-month follow-up period. Therefore, the main effect of the intervention in terms of depression was an increased speed of recovery, which is nevertheless important. However, only a minority of participants fulfilled our criterion for response to treatment, whether they received the intervention or usual care, and the mean PHQ-9 score was > 10 in both groups at all time points, indicating a substantial level of residual symptoms. It is also notable that many participants had severe and enduring depression when they were recruited to the trial and most were taking antidepressants at baseline. These findings suggest that many participants in the trial had ‘treatment-resistant depression’ in that they had enduring symptoms at the time of recruitment and most were still symptomatic at the end of the trial. This is consistent with the notion that depression is often a chronic disease.
The symptoms of depression and anxiety are strongly associated. There was clear evidence of an improvement in anxiety (GAD-7) scores associated with the intervention and this improvement was maintained over the 12-month follow-up period.
Intervention participants in the depression trial reported better access to health support and advice, better health literacy and greater satisfaction with the amount of help and support that they received than usual care participants. Intervention participants also reported improvements in various aspects of self-management, as measured by the heiQ, including self-monitoring and insight, skill and technique acquisition and health service navigation. However, these effects were all small.
In line with the TECH model, the proposed mechanism of action of the Healthlines depression intervention was based on the combined effects of improved patient self-management, optimisation of treatment and better care co-ordination. A key aspect of the intervention was support for participants to use CBT, either through an interactive online programme (LLTTFi) or by following a book based on similar material, with both groups receiving regular telephone support from a Healthlines HIA. It is notable that, although 204 (66%) of the intervention participants logged on to the LLTTFI website, use of the programme was limited, with the median number of completed sessions being two.
A further hypothesised mechanism of action was optimised treatment through more effective use of antidepressants and improved adherence to medication. However, there was no evidence that participants in the intervention arm were prescribed more antidepressants, had more changes in antidepressant type or dose or were more adherent to their medication than those in the usual care arm.
There was also no evidence that participants in the intervention arm experienced better co-ordination of care or reported more use of a care plan than usual care participants.
Therefore, although there were small benefits in the intervention arm, we could not detect change in most of the intended mechanisms. The improvements in the intervention arm were presumably related to the greater sense of support and self-management, and possibly the use of CBT by some participants, although the improvement could also have been related to other unmeasured factors.
Strengths and limitations
There are a number of strengths to the Healthlines depression trial, which also apply to the CVD risk trial, and these are discussed in more detail in Chapter 12. They include the large sample size, high level of participant retention and masked outcome assessment, which enhance internal validity. Additional shared benefits are the highly pragmatic nature of the intervention and the multicentre trial recruitment, both of which enhance external validity. Furthermore, basing the intervention on a conceptual model and conducting an embedded process evaluation helps to understand potential causal mechanisms, whereas the nested economic evaluation provides information about cost-effectiveness.
Limitations that apply to both the depression and the CVD risk trials include the difficulties and delays in service delivery, which occurred because of the closure of NHS Direct, and the relatively low level of recruitment as a percentage of all those sent information about the trial. Both of these issues are discussed in more detail in Chapter 12. A further limitation in the case of the depression trial was the relatively high proportion of participants who received less of the intervention than intended. This could have happened for several intervention-related reasons: they chose not to continue with the telephone calls, did not attend appointments, became uncontactable and/or stopped using the computerised CBT programme or were affected by the early closure of NHS Direct. Many participants who stopped receiving the intervention continued to participate in the trial by providing follow-up data, although 36 intervention participants also withdrew from the trial. The median number of encounters with HIAs in the depression trial was five (compared with a planned total of 10) and, for those participants using the online CBT package, the median number of modules completed was only two (out of a possible total of seven). A CACE analysis suggested a trend towards greater effectiveness of the intervention in participants who received more encounters.
These findings may help to explain the positive benefit of the Healthlines Service when response to treatment (based on the PHQ-9) was treated as a binary outcome, yet the minimal effect when the PHQ-9 score was treated as a continuous outcome. This could have occurred if a minority of individuals gained meaningful benefits from the Healthlines Service, but many people did not. This would be consistent with the finding that there appeared to be one group of individuals who dropped out of the service after just two or three intervention encounters and another group who completed the course and also with the finding of the CACE analysis that those who completed the course gained most benefit. The implication is that this type of intervention may be more effective if it is targeted at those who would be most interested in it.
The primary outcome measure in the depression trial, the PHQ-9 questionnaire, has limitations. It was designed as a screening rather than as a diagnostic measure and, being based on self-report, it is vulnerable to bias in an unblinded trial. Furthermore, participants in the intervention arm completed the PHQ-9 on several occasions as part of the intervention itself (to review their progress) and so there is a possibility that there could be practice effects. It is likely that participants in the control arm would also have been asked to complete the PHQ-9 as part of normal care, but we do not know how often this occurred. Although using a diagnostic measure such as the CIS-R at follow-up as well as at baseline may have been preferable, this would have added greatly to the resources needed for the trial and measures such as the CIS-R are still based on self-report. The PHQ-9 has reasonable sensitivity and specificity compared with clinical interview360,361 and has similar properties to the Beck Depression Inventory, which has been used in many previous studies. 362
Although the overall rate of retention was very high (86% and 85% after 4 and 12 months respectively), one potential limitation was the slight difference in retention rate at the primary 4-month outcome time point between the intervention arm and the usual care arm (83% and 89% respectively). There is evidence from the economic evaluation that those who were lost to follow-up had slightly different characteristics from those who were retained and it is quite likely that those who did not respond to treatment would be more likely to drop out. The imputation of missing data under the most conservative assumption (that all of those with missing data failed to respond to treatment) led to a slightly weaker estimate of an intervention effect (adjusted OR 1.5, 95% CI 1.0 to 2.2) whereas multiple imputation, which took account of a wide range of factors likely to be associated with missingness, provided a similar estimate of effect as the primary analysis. Therefore, we do not consider that taking account of differential attrition should change our conclusion about the effectiveness of the intervention.
How the findings compare with those of previous research
Telehealth services for depression
The Healthlines Service combined a range of interventions within one comprehensive case management approach based on telehealth support delivered by telephone and via the internet. There are therefore few directly comparable previous studies. The most similar previous study was conducted by Fortney et al. 363 in the Veterans Administration in the USA. This was based on a ‘collaborative care’ model, which applies the principles of the CCM to depression. Care managers were provided with a web-based clinical decision support system that guided them to deliver evidence-based clinical encounters through self-scoring instruments, scripts and clinical algorithms. 364 In a randomised trial with 395 participants, this intervention significantly improved medication adherence, treatment response, symptom remission, health-related quality of life and satisfaction with care. 363 The OR for treatment response was 1.9 (95% CI 1.09 to 3.45) at 6 months’ follow-up, which is very similar to the estimate of treatment response (1.7, 95% CI 1.1 to 2.5) observed in the Healthlines depression trial.
As described in Chapter 2, there have been several systematic reviews indicating positive effects on patient outcomes of a range of telehealth interventions for depression, including computerised CBT79,85 and telephone-administered psychotherapy,80 although the most recent systematic review described the evidence for internet-supported treatment for depression as promising but inconclusive. 78
Computerised cognitive–behavioural therapy
The largest body of evidence relates to computerised CBT. Although previous systematic reviews have been generally positive,79 Kaltenthaler et al. 85 have highlighted that all of the research published at the time of their review had been conducted on highly selected populations and by researchers who had a commercial interest in development of the intervention. A recent independent UK-based high-quality trial of two different computerised CBT packages has questioned the effectiveness of computerised CBT, showing a lack of effective results for both packages compared with usual GP care. 365 A qualitative study has highlighted the barriers to use of computerised CBT experienced by many potentially suitable participants81 and the rate of completion of internet-based CBT (65%) was lower than that of face-to-face CBT (85%). 366 Barriers to the use of online CBT include participants’ lack of identification with the applicability of CBT to their difficulties and problems using computers. 367 Several authors have suggested that internet-based therapy for mental health problems might be more effective if accompanied by low-intensity remote therapist support by telephone, e-mail or messaging. 79,367,368 In the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT)2 study, a follow-on trial to the negative REEACT trial of computerised CBT packages described above, Gilbody et al. have found that computerised CBT accompanied by telephone support is more effective than computerised CBT alone (S Gilbody, Hull York Medical School, University of York, 8 July 2015, personal communication). In the Healthlines Service for depression, participants were given telephone support to use computerised CBT and the results were similar to those of the REEACT2 trial.
Collaborative care
Although we have conceptualised the Healthlines Service as a telehealth intervention, the approach that we used has much in common with collaborative care interventions for depression. Collaborative care involves non-medical staff providing structured protocol-driven care according to chronic disease management principles in conjunction with medical support. 369 The concept of collaborative care arose and has mainly been tested in the USA as part of managed care programmes, although it has also been introduced in the UK. The Healthlines intervention reflects a collaborative care approach as it involves non-clinically qualified staff following evidence-based protocols and combines supporting participants to access online therapy with attention to treatment optimisation and medication adherence, as well as seeking to improve self-management behaviours.
A meta-analysis of trials of collaborative care published in 2006 showed moderately strong effect sizes. 369 However, these effects were more positive for studies conducted in the USA than for those conducted elsewhere and also for interventions in which case managers had specific mental health training or were supervised by a psychiatrist. 369 A more recent Cochrane review of collaborative care for depression included several meta-analyses,370 some of which provide data that can be compared with the findings of the Healthlines depression trial. The Cochrane review estimated that the risk ratio for response from collaborative care for depression in the short term (0–6 months) was 1.32 (95% CI 1.22 to 1.43). The risk ratio in the medium term (6–12 months) was similar. These results are very similar to the findings of the Healthlines depression trial, in which the observed OR of 1.7 (95% CI 1.1 to 2.5) at 4 months’ follow-up equates to a risk ratio of 1.5 (95% CI 1.1 to 1.9).
A recent high-quality UK-based trial of collaborative care involved care managers delivering support over the telephone, including medication management, behavioural activation, symptom assessment, relapse prevention and communication with participants’ GPs. 371 The Healthlines Service used very similar approaches but additionally provided care managers with web-based scripts and algorithms and participants with links to a range of internet resources, particularly computerised CBT. The findings of the Healthlines depression trial in terms of response to treatment (OR 1.7, 95% CI 1.1 to 2.5) are, however, extremely similar to those of the CADET trial (OR 1.7, 95% CI 1.2 to 2.4). 371
Conclusion
In this large pragmatic RCT of the Healthlines Service compared with usual care for patients with depression, we have demonstrated that the intervention was associated with a moderately positive effect on the primary outcome of response to treatment. The intervention was also associated with improvements in terms of anxiety symptoms, slightly improved self-management behaviours, better access to health support and advice and increased satisfaction with treatment. However, only a minority of patients completed all or most of the planned encounters or made full use of the computerised CBT provided, there was no evidence that the intervention led to better adherence to antidepressant medication and most of the positive effects listed above were small. These findings are largely consistent with previous research on collaborative care delivered by telephone or face-to-face by mental health workers. This study demonstrates that it is possible to deliver collaborative care for depression using mid-grade staff with limited specific training in mental health, if they are supported by computerised algorithms.
A unique aspect of the Healthlines Service was the way in which this telephone support was combined with the use of a range of resources available on the internet, including not only computerised CBT, but also social media (BWW) and applications to support healthy living. This use of bespoke and existing online resources, along with the use of staff without mental health qualifications, means that it would be possible to scale up this intervention to meet the pressing need to expand services for common mental health problems.
Chapter 9 Results from the cardiovascular disease risk trial
Some of the material in this chapter was adapted from Salisbury C, O’Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ 2016;353:i2647. 372 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
Abstract
Background: The aim of this trial was to determine whether or not the Healthlines Service for patients with high cardiovascular risk was more clinically effective and cost-effective than usual care, while also improving participants’ quality of life, risk behaviours and experiences of care.
Methods: This was a pragmatic, multicentre RCT. Participants were aged between 40 and 74 years with a 10-year CVD risk of ≥ 20%, no previous cardiovascular event, at least one modifiable risk factor (systolic blood pressure ≥ 140 mmHg, BMI ≥ 30 kg/m2 or current smoker) and access to a telephone, the internet and e-mail. Participants recruited from 42 general practices in England were individually allocated to the intervention group or the control group using automated randomisation stratified by site, minimised by practice and baseline risk score. Intervention group participants received the Healthlines Service (in addition to usual care) consisting of regular telephone calls from trained lay health advisors following scripts generated by interactive software. Advisors facilitated self-management by supporting participants to use online resources to reduce their risk factors and sought to optimise participants’ medication, improve treatment adherence and encourage healthier lifestyles. Control group participants received usual care alone. Participants were aware of treatment allocation. Outcomes were collected 6 and 12 months after randomisation and analysed masked. The primary outcome was the proportion of participants responding to treatment, defined as maintaining or reducing their cardiovascular risk (measured using QRISK2) after 12 months. Secondary outcomes included cardiovascular risk factors (blood pressure, weight, smoking, cholesterol), quality of life, physical activity, diet, patient experience, self-management skills, medication adherence and health literacy.
Findings: We recruited 641 participants, with 91% providing primary outcome data. Participants receiving the intervention had a modest response to treatment compared with usual care participants (50% vs. 43% respectively; adjusted OR 1.3, 95% CI 1.0 to 1.9). The intervention was associated with reductions in blood pressure (difference in mean systolic blood pressure –2.7 mmHg, 95% CI –4.7 to –0.6 mmHg) and weight (–1.0 kg, 95% CI –1.8 to –0.3 kg), but not in cholesterol or smoking status. It was also associated with improvements in diet, physical activity, medication adherence, access to care and satisfaction with treatment and care co-ordination. Only one-third of intervention participants received the full course of intended telephone encounters.
Conclusions: The Healthlines Service, a telehealth approach to care delivery based on a conceptual model and using tools that themselves had evidence of effectiveness, was associated with improvements in some but not all of the targeted cardiovascular risk factors. This led to marginal clinical benefits for people with high cardiovascular risk. The intervention was also associated with improvements in patients’ risk behaviours, access to care and perceptions of support.
Overview
In this chapter, we begin by describing the recruitment figures and baseline characteristics of the participants in the CVD risk trial. Next, we discuss the results of the primary and sensitivity analyses of the primary outcome, the compliance analyses and the prespecified subgroup analyses. We go on to discuss the secondary outcomes results and the process measures results, explore potential mechanisms and present the post hoc subgroup analyses as suggested by the embedded qualitative study.
Baseline characteristics
Between June 2012 and June 2013, 7582 patients were invited to take part in the study (Figure 19). This total number of patients takes into account 402 patients who were excluded by their GP (e.g. because of unsuitability or recent bereavement or because an invitation would otherwise cause distress) and 536 patients who were excluded by the research team because they were duplicates of depression patients (i.e. had depression and were also at risk of CVD and therefore were invited only into the depression trial). Of the 7582 patients who were posted a study invitation, a substantial number did not respond at all, with many others declining participation either by returning a decline form (see Appendix 17 for the proportion of patients selecting each of the pre-specified reasons on the decline form) or informing the researcher verbally; a final group was not assessed for eligibility for a variety of other reasons (e.g. because the recruitment target was reached and no further participants could be included in the trial, failure to return a fully signed study acceptance form, did not answer researcher telephone calls, administrative error or failed to attend an appointment at their surgery for a CVD risk assessment). The baseline demographics of patients who did and did not take part in the trial are presented in Table 44.
FIGURE 19.
Recruitment and retention in the CVD risk trial.

| Characteristic | Did not want to take part (n = 6377) | Wanted to take part (n = 1205) |
|---|---|---|
| Age (years), mean (SD) | 65.8 (6.2) | 66.4 (5.4) |
| Female, n (%) | 1766 (28) | 256 (21) |
| Index of Multiple Deprivation at practice level, mean (SD) | 18.1 (9.2) | 17.1 (8.4) |
| QRISK2 score, mean (SD) | 28.0 (8.6) | 27.4 (8.3) |
| Framingham score, mean (SD) | 27.6 (8.3) | 26.8 (8.4) |
| QRISK2 score from practice records, mean (SD)a | 26.7 (6.6) | 26.5 (7.5) |
In total, 1205 patients (16% of those invited) were screened for eligibility, of whom 641 participants were randomised between December 2012 and July 2013 (usual care, n = 316, and intervention, n = 325; see Figure 19). Figure 19 shows that 37% did not meet the eligibility criteria [e.g. did not have regular access to the internet, did not have at least one modifiable risk factor (high blood pressure, smoker or overweight), had a QRISK2 score of < 20), a small proportion (1%) declined participation at this stage and 8% were excluded for other reasons (e.g. did not return a completed consent form and/or baseline questionnaire, did not respond to any further contact from the researchers, administrative error or recruitment target was reached and no further participants could be randomised).
The baseline characteristics for the eligible patients who were and were not randomised are presented in Table 45. Compared with those who were eligible and not randomised, randomised patients were slightly older and less deprived, with higher Framingham scores; however, they had similar QRISK2 scores and there was a similar proportion of women in both groups.
| Characteristic | Not randomised (n = 115) | Randomised (n = 641) |
|---|---|---|
| Age (years), mean (SD) | 66.2 (5.2) | 67.2 (4.8) |
| Female, n (%) | 21 (18%) | 126 (20%) |
| Index of Multiple Deprivation at practice level, mean (SD) | 18.4 (7.9) | 16.9 (8.3) |
| QRISK2 score, mean (SD) | 28.1 (7.5) | 28.3 (8.7) |
| Framingham score, mean (SD) | 24.0 (9.2) | 27.1 (8.5) |
| QRISK2 score from practice records, mean (SD)a | 26.0 (8.0) | 27.8 (8.1) |
The baseline characteristics of the randomised participants are presented in Table 46. Overall, the participants were mainly retired white males and homeowners, although not all were highly educated. The treatment arms were mostly well balanced, although there were fewer smokers (15% vs. 20%) and more participants with diabetes (24% vs. 20%) in the intervention arm. However, smoking status and a diabetes diagnosis both contribute to the baseline QRISK2 score. As baseline QRISK2 score was already included as a covariate in all comparative analyses, we did not conduct additional sensitivity analyses of any primary or secondary outcomes that further adjusted for these baseline variables. Finally, of the randomised participants, 63% (403/641) completed the baseline questionnaire using the secure survey website and the remainder returned a postal copy (238/641).
| Characteristic | Usual care (n = 316) | Intervention (n = 325) | ||
|---|---|---|---|---|
| Mean (SD) or % | n or n/N | Mean (SD) or % | n or n/N | |
| Demographic data | ||||
| Age (years) at cardiovascular assessment | 67.3 (4.7) | 316 | 67.5 (4.9) | 325 |
| Female | 21 | 66/316 | 18 | 60/325 |
| White | 99 | 313/316 | 99 | 321/325 |
| Current employment situation | ||||
| Full-time employment | 13 | 39/311 | 17 | 54/316 |
| Part-time employment | 14 | 43/311 | 9 | 29/316 |
| Unemployed | 1 | 4/311 | 1 | 2/316 |
| Unable to work because of long-term illness/disability | 2 | 7/311 | 1 | 3/316 |
| Unable to work because of carer responsibilities | 1 | 3/311 | 1 | 2/316 |
| Fully retired from work | 63 | 196/311 | 66 | 210/316 |
| Looking after the home | 1 | 3/311 | 1 | 4/316 |
| Doing something else | 5 | 16/311 | 4 | 12/316 |
| Occupation (most recent or current) | ||||
| Administrative or secretarial occupations | 11 | 31/294 | 10 | 29/294 |
| Associate professional or technical occupations | 15 | 45/294 | 12 | 35/294 |
| Elementary occupations | 10 | 28/294 | 5 | 16/294 |
| Managers or senior officials | 19 | 55/294 | 22 | 65/294 |
| Personal services | 2 | 5/294 | 3 | 9/294 |
| Process, plant and machine operatives | 5 | 15/294 | 6 | 17/294 |
| Professionals | 19 | 57/294 | 22 | 64/294 |
| Sales and customer services | 4 | 11/294 | 4 | 13/294 |
| Skilled trades | 16 | 47/294 | 16 | 46/294 |
| Highest educational qualification achieved | ||||
| Degree or higher degree | 21 | 65/307 | 23 | 72/318 |
| A levels or equivalent | 19 | 58/307 | 17 | 53/318 |
| GCSEs/O levels or equivalent | 45 | 137/307 | 43 | 136/318 |
| No qualifications | 15 | 47/307 | 18 | 57/318 |
| Accommodation | ||||
| Own accommodation or buying with mortgage | 84 | 264/315 | 87 | 281/323 |
| Part-rent or rent accommodation | 15 | 46/315 | 12 | 40/323 |
| Live rent free | 2 | 5/315 | 1 | 2/323 |
| Index of Multiple Deprivation | 16.7 (12.6) | 316 | 15.5 (11.3) | 325 |
| Clinical data | ||||
| QRISK2 score | 30.8 (9.5) | 316 | 31.1 (10.2) | 325 |
| Systolic blood pressure (mmHg) | 148.1 (17.6) | 316 | 147.6 (16.2) | 325 |
| Diastolic blood pressure (mmHg) | 80.0 (10.4) | 316 | 81.2 (9.6) | 325 |
| Weight (kg) | 91.9 (18.9) | 316 | 93.2 (17.3) | 325 |
| BMI (kg/m2) | 30.9 (5.7) | 316 | 31.2 (5.4) | 325 |
| Total cholesterol (mmol/l) | 4.9 (1.2) | 315 | 4.9 (1.2) | 324 |
| Ratio of total cholesterol to HDL cholesterol | 4.2 (1.4) | 315 | 4.2 (1.5) | 323 |
| Non-smoker | 33 | 103/316 | 35 | 114/325 |
| Ex-smoker | 47 | 148/316 | 50 | 163/325 |
| Light smoker | 9 | 30/316 | 8 | 25/325 |
| Moderate smoker | 5 | 17/316 | 5 | 16/325 |
| Heavy smoker | 6 | 18/316 | 2 | 7/325 |
| Taking antihypertensives | 61 | 193/316 | 64 | 209/325 |
| Taking lipid-lowering medication | 49 | 153/312 | 49 | 158/322 |
| Diabetes | 20 | 62/316 | 24 | 77/325 |
| Chronic kidney disease | 11 | 34/316 | 6 | 20/325 |
| Atrial fibrillation | 6 | 20/316 | 7 | 23/325 |
| Rheumatoid arthritis | 3 | 8/316 | 2 | 6/325 |
Participants could remain in the trial for up to 12 months and were asked to complete two follow-up questionnaires at 6 and 12 months post randomisation. As such, the period of follow-up data collection for the trial was from June 2013 (first wave of 6-month follow-ups) to August 2014 (final wave of 12-month follow-ups). The baseline characteristics of participants who did or did not attend a CVD risk assessment at 12 months, from which the primary outcome measure (QRISK2 score) was computed, are reported in Appendix 31.
Primary outcome
As prespecified in the statistical analysis plan, the primary outcome was the proportion of participants responding to treatment. This is a binary outcome, defined as maintenance or reduction of 10-year CVD risk, which was estimated using the QRISK2 score at 12 months after randomisation. Follow-up QRISK2 scores were calculated by updating age and values for modifiable risk factors only. Other variables that contribute to calculation of the QRISK2 score, such as diagnosis of diabetes or atrial fibrillation or prescription of blood pressure-lowering medications, were held constant using baseline values (see Chapter 7, Primary outcomes).
The primary analysis suggested a modest increase in the proportion of participants in the intervention arm who maintained or reduced their CVD risk at 12 months (50% vs. 43%; Table 47). However, the 95% CI includes the null and therefore it is possible that the intervention has no real effect. The primary result remained largely unchanged in our sensitivity analyses (see Table 47), although the evidence for any effect was slightly weakened when missing outcome data were imputed.
| Analysis | Usual care, % (n/N) | Intervention, % (n/N) | Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Primary analysis | |||||
| QRISK2 response to treatment | 43 (124/291) | 50 (148/295) | 1.3 | 1.0 to 1.9 | 0.079 |
| Sensitivity analyses | |||||
| QRISK2 response to treatment: recalculated QRISK2 score at 12 months (assuming all participants were 1 year older) | 45 (130/291) | 52 (153/295) | 1.3 | 1.0 to 1.9 | 0.096 |
| QRISK2 response to treatment: simple imputation (assuming missing binary outcome is non-response) | 39 (124/316) | 46 (148/325) | 1.3 | 0.9 to 1.8 | 0.109 |
| QRISK2 response to treatment: multiple imputation | 44 (139/316) | 50 (163/325) | 1.3 | 0.9 to 1.8 | 0.115 |
| QRISK2 response to treatment: not including general practice as a random effect | 43 (124/291) | 50 (148/295) | 1.3 | 1.0 to 1.9 | 0.079 |
| QRISK2 response to treatment: adjusted by days since randomisation to primary outcome assessment | 43 (124/291) | 50 (148/295) | 1.3 | 1.0 to 1.9 | 0.094 |
Complier average causal effect analysis
There is currently no statistical method available for estimating a treatment effect among intervention compliers with three levels of compliance and a binary outcome. We decided to estimate the unadjusted odds of response among the compliers using the principal stratification method, as suggested by Frangakis and Rubin. 326 This was carried out in two ways: first, by classifying the partial compliers (participants who started encounters three to 11) as non-compliers and, second, by classifying the partial compliers as full compliers.
The crude OR for treatment response was 1.4 (95% CI 1.0 to 1.9). Table 48 shows the crude estimated effect among compliers, after classifying partial compliers in the intervention arm as either non-compliers or full compliers. The results suggest an increase in effect when compliance is defined as completing most or all of the Healthlines encounters.
| Amount of intervention received | QRISK2 response at 12 months’ follow-up, % (n/N) | Unadjusted ORa | 95% CI | Unadjusted ORb | 95% CI | |
|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||
| None (0–2 encounters started) | 43 (124/291) | 29 (4/14) | ||||
| Partial (3–11 encounters started) | 44 (77/177) | |||||
| Full (12 or 13 encounters started) | 65 (66/102) | 2.4 | 1.4 to 4.3 | 1.4 | 1.0 to 1.9 | |
Subgroup analyses of the primary outcome
There was no evidence that the intervention was differentially effective for any subgroups defined by baseline characteristics (Table 49).
| Subgroup | QRISK2 response at 12 months’ follow-up, % (n/N) | Adjusted ORa | 95% CI | Interaction p-value | |
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Baseline CVD assessment age group (years) | |||||
| 40–59 | 54 (7/13) | 61 (11/18) | 1.5 | 0.3 to 6.6 | |
| 60–69 | 44 (78/177) | 49 (75/152) | 1.2 | 0.8 to 1.9 | |
| ≥ 70 | 39 (39/101) | 50 (62/125) | 1.6 | 0.9 to 2.8 | 0.708 |
| Sex | |||||
| Male | 46 (105/227) | 51 (125/243) | 1.2 | 0.9 to 1.8 | |
| Female | 30 (19/64) | 44 (23/52) | 1.8 | 0.8 to 4.0 | 0.369 |
| Baseline QRISK2 score | |||||
| 17.3–24.9 | 37 (37/101) | 45 (44/98) | 1.4 | 0.8 to 2.5 | |
| 25.0–29.9 | 38 (26/68) | 44 (35/79) | 1.2 | 0.6 to 2.4 | |
| ≥ 30.0 | 50 (61/122) | 58 (69/118) | 1.4 | 0.8 to 2.4 | 0.947 |
| Baseline modifiable risk factor | |||||
| Systolic blood pressure < 140 mmHg | 33 (30/90) | 41 (35/85) | 1.5 | 0.8 to 2.8 | |
| Systolic blood pressure ≥ 140 mmHg | 47 (94/201) | 54 (113/210) | 1.3 | 0.9 to 1.9 | 0.726 |
| BMI < 30.0 kg/m2 | 50 (65/131) | 52 (67/129) | 1.1 | 0.6 to 1.8 | |
| BMI ≥ 30.0 kg/m2 | 37 (59/160) | 49 (81/166) | 1.7 | 1.1 to 2.6 | 0.200 |
| Current smoker | 51 (29/57) | 53 (23/43) | 1.1 | 0.5 to 2.5 | |
| Not current smoker | 41 (95/234) | 50 (125/252) | 1.4 | 1.0 to 2.1 | 0.546 |
Secondary outcomes
The secondary outcomes analyses were based on linear or logistic regression models (as appropriate) adjusted, as in the primary analysis, by site (Bristol, Sheffield or Southampton), baseline QRISK2 score and the baseline outcome being analysed. General practice was included as a random effect.
Binary QRISK2 response
The proportion of participants who responded to treatment at 6 months was slightly higher in the intervention group than in the usual care group (48% vs. 46%) but there was no evidence of any between-group effect (adjusted OR 1.1, 95% CI 0.8 to 1.5; p = 0.654).
Smoking
The proportion of non-smokers was greater in the intervention arm at both 6 and 12 months’ follow-up (Table 50). However, as non-smoking was also more prevalent in the intervention arm at baseline (see Table 46), the resulting ORs for non-smoking at follow-up were < 1 after adjustment for baseline smoking (see Table 50). The adjusted 95% CIs and p-values indicate no strong evidence of any effect on smoking at either 6 or 12 months’ follow-up.
| Outcome | Usual care, % (n/N) | Intervention, % (n/N) | Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Smoker at 6 months | |||||
| Yes | 18 (52/296) | 15 (45/301) | NA | NA | NA |
| No | 82 (244/296) | 85 (256/301) | 0.3 | 0.1 to 1.2 | 0.099 |
| Smoker at 12 months | |||||
| Yes | 18 (52/291) | 17 (49/295) | NA | NA | NA |
| No | 82 (239/291) | 83 (246/295) | 0.4 | 0.2 to 1.0 | 0.061 |
Continuous QRISK2 score
As anticipated, with participants’ increasing age, mean QRISK2 scores increased slightly at follow-up compared with baseline in both arms (Table 51). However, there was no evidence of any between-group difference in mean QRISK2 scores at either 6 months or 12 months. This can also be expressed in terms of change from baseline: mean (SD) change score from baseline to 6 months: usual care 0.1 (1.9), intervention 0.2 (1.8); mean (SD) change score from baseline to 12 months: usual care 0.4 (5.0), intervention 0.0 (5.2).
| Outcome | Usual care | Intervention | Adjusted difference in means | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| QRISK2 score | |||||||
| 6 months | 31.0 (9.5) | 296 | 31.4 (10.3) | 301 | 0.1 | –0.2 to 0.4 | 0.489 |
| 12 months | 31.2 (10.3) | 291 | 31.3 (10.7) | 295 | –0.4 | –1.2 to 0.3 | 0.269 |
| 12 months (sensitivity analysis: assuming 1-year age increase) | 31.1 (10.3) | 291 | 31.1 (10.7) | 295 | –0.4 | –1.2 to 0.3 | 0.259 |
| Systolic blood pressure (mmHg) | |||||||
| 6 months | 141.4 (15.4) | 296 | 141.0 (15.1) | 301 | 0.0 | –1.9 to 1.9 | 0.997 |
| 12 months | 142.2 (16.1) | 291 | 139.6 (14.0) | 295 | –2.7 | –4.7 to –0.6 | 0.011 |
| Diastolic blood pressure (mmHg) | |||||||
| 6 months | 78.0 (9.7) | 296 | 78.2 (9.9) | 301 | –0.6 | –1.8 to 0.6 | 0.337 |
| 12 months | 78.7 (9.9) | 291 | 76.6 (9.2) | 295 | –2.8 | –4.0 to –1.6 | < 0.001 |
| Total cholesterol (mmol/l) | |||||||
| 12 months | 4.7 (1.1) | 288 | 4.6 (1.2) | 295 | –0.1 | –0.2 to 0.0 | 0.167 |
| Ratio of total cholesterol to HDL cholesterol | |||||||
| 12 months | 4.0 (1.5) | 287 | 4.0 (1.7) | 294 | –0.1 | –0.2 to 0.1 | 0.451 |
| Weight (kg) | |||||||
| 6 months | 91.1 (18.4) | 296 | 91.7 (17.7) | 301 | –0.9 | –1.5 to –0.2 | 0.006 |
| 12 months | 91.2 (19.1) | 291 | 91.3 (17.5) | 293 | –1.0 | –1.8 to –0.3 | 0.008 |
| BMI (kg/m2) | |||||||
| 6 months | 30.6 (5.4) | 296 | 30.7 (5.5) | 301 | –0.3 | –0.5 to –0.1 | 0.006 |
| 12 months | 30.8 (5.7) | 291 | 30.5 (5.4) | 293 | –0.4 | –0.6 to –0.1 | 0.008 |
Although the trial period was 12 months, the timing and scheduling of primary outcome CVD assessments meant that some participants did not age a full year by the final follow-up, whereas others appeared to have aged by 2 years. Therefore, we conducted a sensitivity analysis which assumed that all participants had aged by 1 year during the trial period. Nevertheless, the result at 12 months was unchanged and there was still no between-group difference in mean QRISK2 score (see Table 51), with a mean (SD) change score from baseline of 0.3 (4.9) for usual care and –0.2 (5.2) for the intervention.
Blood pressure
There was no evidence of any between-group difference in mean systolic or diastolic blood pressure at 6 months follow-up (see Table 51). By the final follow-up at 12 months, however, there was strong evidence that mean blood pressure was lower in the intervention arm.
Cholesterol
There was no evidence that either meant total cholesterol or the ratio of mean total cholesterol to HDL cholesterol differed between the groups at 12 months (see Table 51). Cholesterol was not measured at 6 months (see Chapter 7, Primary outcomes).
Weight and body mass index
There was strong evidence of a modest but sustained effect of the intervention on weight loss. Mean weight was about 1 kg lower in the intervention arm at 6 months’ follow-up and this was maintained at 12 months (see Table 51). As would be expected, there was similar evidence of an effect on BMI (see Table 51).
Patient satisfaction
Participants’ satisfaction with various HCPs and the health care received over the trial period are reported in Table 52. General satisfaction relates to participants’ overall satisfaction with the care that they received from each type of HCP. Detailed satisfaction forms a scale that encompasses participant ratings of the HCPs’ listening skills, care and concern, explanations and involvement of patients in decisions. Treatment satisfaction forms a scale of six questions that relate to different aspects of participants’ perceptions of the quality and effectiveness of the care that they received from all of their care providers (usual care in the control group; usual care plus the Healthlines Service in the intervention group).
| Patient satisfaction | Usual care | Intervention | Adjusted difference in means | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| General GP satisfactiona | |||||||
| 6 months | 4.2 (0.9) | 284 | 4.4 (0.7) | 284 | 0.1 | –0.0 to 0.2 | 0.152 |
| 12 months | 4.2 (0.9) | 280 | 4.3 (0.7) | 283 | 0.0 | –0.1 to 0.1 | 0.815 |
| Detailed GP satisfactionb | |||||||
| 6 months | 4.3 (0.8) | 260 | 4.4 (0.7) | 258 | 0.1 | 0.0 to 0.2 | 0.078 |
| 12 months | 4.3 (0.8) | 250 | 4.4 (0.6) | 255 | 0.1 | 0.0 to 0.3 | 0.033 |
| General nurse satisfactiona | |||||||
| 6 months | 4.4 (0.7) | 284 | 4.5 (0.6) | 281 | 0.0 | –0.1 to 0.1 | 0.910 |
| 12 months | 4.5 (0.7) | 275 | 4.4 (0.6) | 281 | 0.0 | –0.1 to 0.1 | 0.338 |
| Detailed nurse satisfactionb | |||||||
| 6 months | 4.4 (0.7) | 261 | 4.4 (0.6) | 263 | 0.0 | –0.1 to 0.1 | 0.912 |
| 12 months | 4.4 (0.7) | 255 | 4.4 (0.6) | 262 | 0.0 | –0.1 to 0.1 | 0.950 |
| General NHS Direct satisfactiona | |||||||
| 6 months | 3.8 (1.0) | 88 | 4.3 (0.8) | 233 | 0.6 | 0.3 to 0.9 | < 0.001 |
| 12 months | 3.9 (1.0) | 78 | 4.3 (0.8) | 237 | 0.3 | 0.0 to 0.6 | 0.029 |
| Treatment satisfactiona | |||||||
| 6 months | 3.7 (0.8) | 208 | 3.9 (0.7) | 244 | 0.2 | 0.1 to 0.3 | 0.003 |
| 12 months | 3.7 (0.8) | 215 | 3.9 (0.7) | 244 | 0.1 | 0.0 to 0.3 | 0.038 |
As shown in Table 52, there was no evidence of any between-group differences in either general or detailed satisfaction with primary care HCPs (GPs and nurses). However, there was strong evidence of a difference in mean general satisfaction with NHS Direct, as well as overall satisfaction with the treatment that participants had received at both 6 and 12 months, in favour of the intervention group. Although participants in the intervention group were asked about their satisfaction with NHS Direct in addition to their satisfaction with NHS Direct intervention staff and the Healthlines Service, the participants may not have been able to distinguish between the two services (both were affiliated with NHS Direct).
Intervention satisfaction
Satisfaction with aspects of the Healthlines Service among participants in the intervention group remained both stable and strong over the follow-up period (Table 53). Although comparisons with the usual care group are not possible, these questions about intervention satisfaction used the same response scale as those questions about primary care and treatment satisfaction and so comparisons can be made between intervention satisfaction and primary care satisfaction. Participants in the intervention group appeared to be as least as satisfied with the care that they received from the HIAs as they were with the care that they received from GPs and nurses in their own practices (see Table 53). They were also satisfied with the Healthlines website in general and even more so with the blood pressure monitoring page on the website.
| Patient satisfaction | Mean (SD) | n |
|---|---|---|
| Detailed HIA satisfaction | ||
| 6 months | 4.5 (0.7) | 257 |
| 12 months | 4.5 (0.7) | 259 |
| Intervention website satisfaction | ||
| 6 months | 4.1 (0.7) | 236 |
| 12 months | 4.1 (0.7) | 235 |
| Satisfaction with blood pressure web page | ||
| 6 months | 4.3 (0.7) | 203 |
| 12 months | 4.3 (0.8) | 201 |
Access to care
There is strong evidence that participants in the intervention arm had fewer difficulties in accessing aspects of care related to service delivery (getting support and advice at times that suited them, when they needed it most and from the HCPs they wanted to see) and they also reported receiving a greater amount of support with their health care (Table 54).
| Measure of access to care | Usual care | Intervention | Adjusted difference in means | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Service delivery access difficultiesa | |||||||
| 6 months | 5.5 (1.6) | 291 | 5.8 (1.4) | 299 | 0.3 | 0.1 to 0.5 | 0.007 |
| 12 months | 5.5 (1.7) | 293 | 5.8 (1.3) | 287 | 0.3 | 0.0 to 0.5 | 0.016 |
| Amount of supportb | |||||||
| 6 months | 2.7 (0.7) | 206 | 3.0 (0.5) | 258 | 0.2 | 0.1 to 0.3 | < 0.001 |
| 12 months | 2.8 (0.6) | 207 | 3.1 (0.5) | 260 | 0.3 | 0.2 to 0.4 | < 0.001 |
Process measures analyses
With the exception of home blood pressure monitoring, there were no substantial differences between the arms in participants’ use of other interventions or services (Table 55). The difference in use of home blood pressure monitors in favour of the intervention group was expected, as this was a component of the Healthlines Service.
| Service | Usual care, % (n/N) | Intervention, % (n/N) |
|---|---|---|
| Use of lifestyle improvement books | ||
| 6 months | 6 (19/300) | 9 (26/305) |
| 12 months | 5 (14/296) | 8 (23/296) |
| Use of home blood pressure monitor | ||
| 6 months | 13 (40/303) | 51 (154/301) |
| 12 months | 13 (39/297) | 28 (83/293) |
| Use of internet-based smoking cessation group | ||
| 6 months | 0 (0/301) | 1 (2/306) |
| 12 months | 0 (0/298) | < 1 (1/306) |
| Use of GP/walk-in centre smoking cessation group | ||
| 6 months | 1 (2/303) | 1 (3/307) |
| 12 months | 0 (0/298) | < 1 (1/299) |
| Use of pharmacist advice for smoking cessation | ||
| 6 months | 1 (4/298) | 2 (7/308) |
| 12 months | 1 (4/296) | 1 (2/295) |
| Joined or renewed gym membership | ||
| 6 months | 8 (25/301) | 8 (23/306) |
| 12 months | 8 (25/299) | 6 (17/293) |
| Attended exercise class or fitness activity | ||
| 6 months | 18 (55/302) | 18 (54/306) |
| 12 months | 20 (60/297) | 16 (46/294) |
| Use of internet-based weight loss group | ||
| 6 months | 1 (3/301) | 1 (3/307) |
| 12 months | < 1 (1/297) | 1 (2/295) |
| Use of face-to-face weight loss group | ||
| 6 months | 1 (3/298) | 3 (8/308) |
| 12 months | 2 (5/299) | 1 (4/294) |
Amount of intervention received
Number of Healthlines encounters
Figure 20 shows the number of Healthlines encounters started by intervention participants out of a possible total of 13 encounters. The full intervention programme included an initial introduction and assessment encounter, followed by 12 scheduled encounters. Three participants did not receive the intervention because of an administrative error and two participants received only non-encounter calls and so these five participants were categorised as not having any encounters. Data were also missing from the intervention database for two additional participants and so it was not possible to tell how many encounters they received. Of the remaining 323 intervention participants (325 randomised), 8% received none or little of the intervention (started two or fewer encounters, including the initial encounter), 60% received some of the intervention sessions planned (3–11 encounters) and 32% received all or almost all of the planned encounters (12 or 13 encounters).
FIGURE 20.
Distribution of Healthlines encounters started in the CVD risk trial.
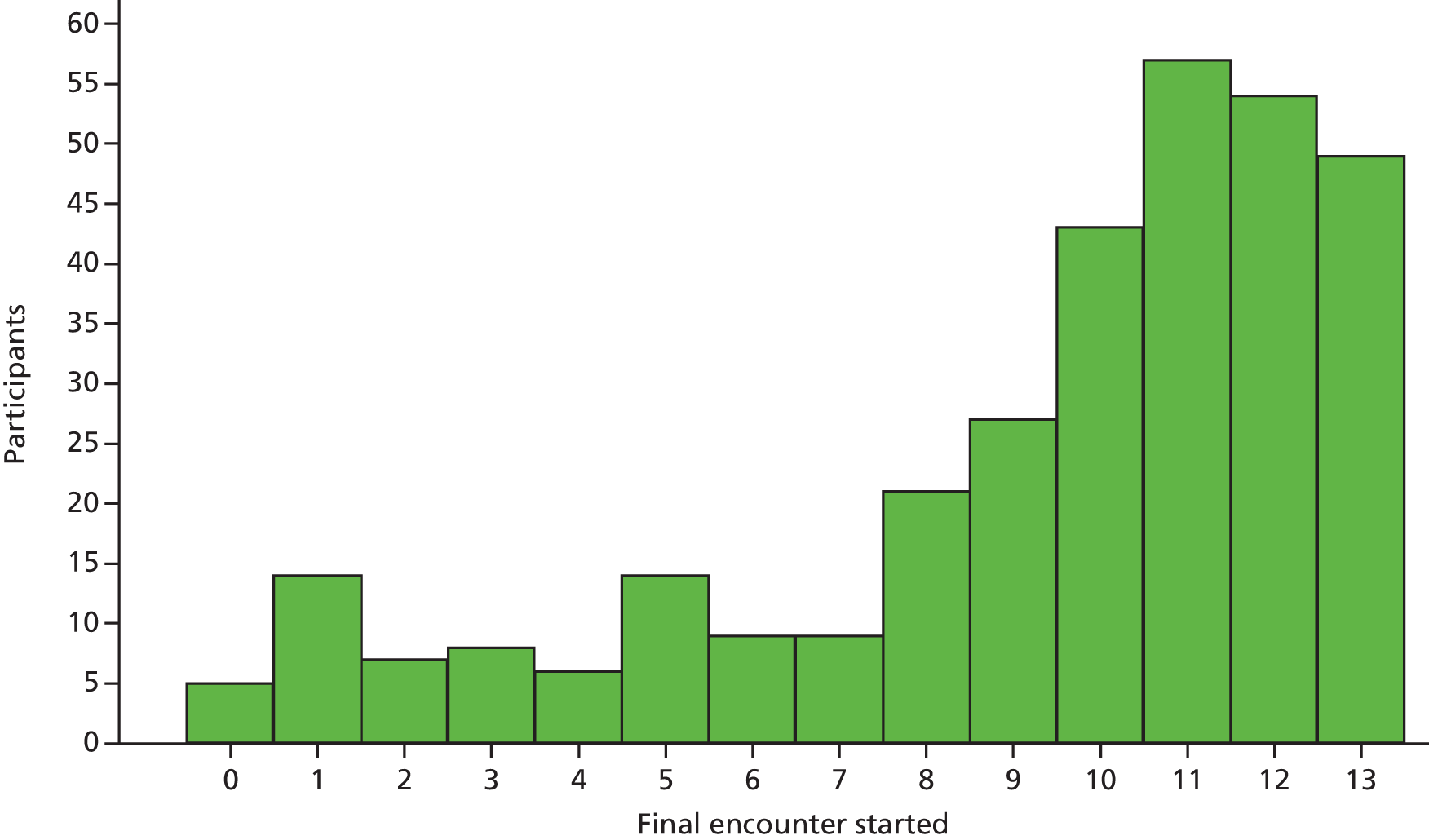
The median number of encounters started was 10 (IQR 8 to 12, n = 323), with a mean encounter duration of 18 minutes (SD 9.5 minutes), and there were 3247 encounter calls in total.
Letters sent by health information advisors to participants and general practitioners
The median number of e-mails sent to participants by the HIAs was 1 (IQR 0 to 3, n = 310). The median number of letters sent to participants’ GPs by e-mail was 5 (IQR 2 to 9, n = 310). The breakdown of these letters by their content is detailed in Table 56.
| Letter content | Number of letters (% of total letters sent) | Number of participants whose GP was sent at least one letter (% of 310) |
|---|---|---|
| Review of blood pressure medicationa | 257 (15) | 113 (36) |
| Blood pressure review (patient not on medication)a | 88 (5) | 37 (12) |
| Continuation note (monthly report) | 887 (51) | 233 (75) |
| Generic (information for GP) | 15 (1) | 14 (5) |
| Intervention introduction | 318 (18) | 304 (98) |
| Intervention conclusion | 56 (3) | 56 (18) |
| Side effects and medication issues | 70 (4) | 52 (17) |
| Referral for orlistat | 7 (< 1) | 7 (2) |
| Referral for smoking cessation therapy | 3 (< 1) | 3 (1) |
| Referral for statins | 39 (2) | 32 (10) |
| Uncontrolled blood pressurea | 1 (< 1) | 1 (< 1) |
| Total number of letters sent | 1741 |
Exploration of potential mechanisms
To explore the different components of the intervention, data were collected on different concepts of the TECH model (see Chapter 5). These are presented descriptively within this report. However, some of the explanatory variables from the TECH model were prespecified as secondary outcomes in the original protocol and so between-group effects at the primary (12-month) time point have been estimated.
Patient engagement
Few participants in either arm made use of telehealth interventions other than the Healthlines Service during the trial (Table 57) and there was no evidence that this differed between the two groups at 12 months.
| Telehealth service | Use of telehealth at least every 2 weeksa | Adjusted ORb | 95% CI | p-value | |
|---|---|---|---|---|---|
| Usual care, % (n/N) | Intervention, % (n/N) | ||||
| NHS Direct telephone services | |||||
| 6 months | < 1 (1/300) | 1 (3/307) | |||
| 12 months | < 1 (1/297) | 1 (2/299) | NAc | ||
| Online searching | |||||
| 6 months | 5 (15/300) | 8 (24/306) | |||
| 12 months | 7 (21/297) | 7 (22/296) | 1.2 | 0.6 to 2.5 | 0.533 |
| Online forum or group | |||||
| 6 months | 1 (4/297) | 1 (3/307) | |||
| 12 months | 1 (2/295) | 1 (2/298) | 1.3 | 0.1 to 17.1 | 0.838 |
Engagement with the intervention
There are some indicators relevant to patient engagement that applied only to those in the intervention group, as they pertain to engagement with the intervention. First, the median number of times that participants logged on to the Healthlines website was 14 (IQR 3 to 47), which equates to about once per month (Table 58). Most participants uploaded at least one blood pressure reading, with a median of 48 readings (IQR 0 to 87).
| Use of the web portal | Median (IQR) | n |
|---|---|---|
| Number of times participants logged onto the web portal | 14 (3 to 47) | 322 |
| Number of blood pressure readings participants uploaded onto the web portal | 48 (0 to 87) | 296 |
Second, to promote patient engagement with the Healthlines Service, every effort was made to ensure that participants spoke to the same HIA throughout the intervention. The continuity of care index359 in the current context measures the concentration of telephone calls with different HIAs. It measures the number of HIAs that each participant spoke to, such that a higher continuity score indicates a greater number of calls with fewer HIAs (range 0–1). As would be expected, the mean continuity score was lower when unscheduled calls were included (Table 59). Third, the usual practitioner care index359 is interpreted here as the percentage of encounters that a participant had with their ‘usual’ HIA, defined as the HIA with whom they spoke to most often (range 0–1). Around two-thirds of all encounters were with participants’ usual HIA and, like the continuity of care, the usual practitioner care score was lower when unscheduled encounters were included (see Table 59).
| Measure of engagement | Mean (SD) | n |
|---|---|---|
| Continuity of care index | 0.46 (0.19) | 307 |
| Usual practitioner care index | 0.65 (0.15) | 318 |
| Continuity of care index including non-scheduled calls | 0.43 (0.16) | 308 |
| Usual practitioner care index including non-scheduled calls | 0.61 (0.15) | 320 |
General practitioner engagement
A form of GP engagement in this trial was the number of medication changes, which would be expected to be greater in the intervention group because of the medication-related letters sent to GPs from the HIAs. The number of changes in medication during the trial, defined as a change in drug name or dose from baseline, was captured from participants’ medical records. There was no difference in number of changes in antihypertensive medication between the two groups, with a median of zero changes (IQR 0 to 1) in both groups (Figure 21). There was also no difference in the number of changes in cholesterol medication between the two groups (Figure 22), with a median of zero changes (IQR 0 to 1) in both groups.
FIGURE 21.
Distribution of changes in antihypertensive medication: (a) control; and (b) intervention.
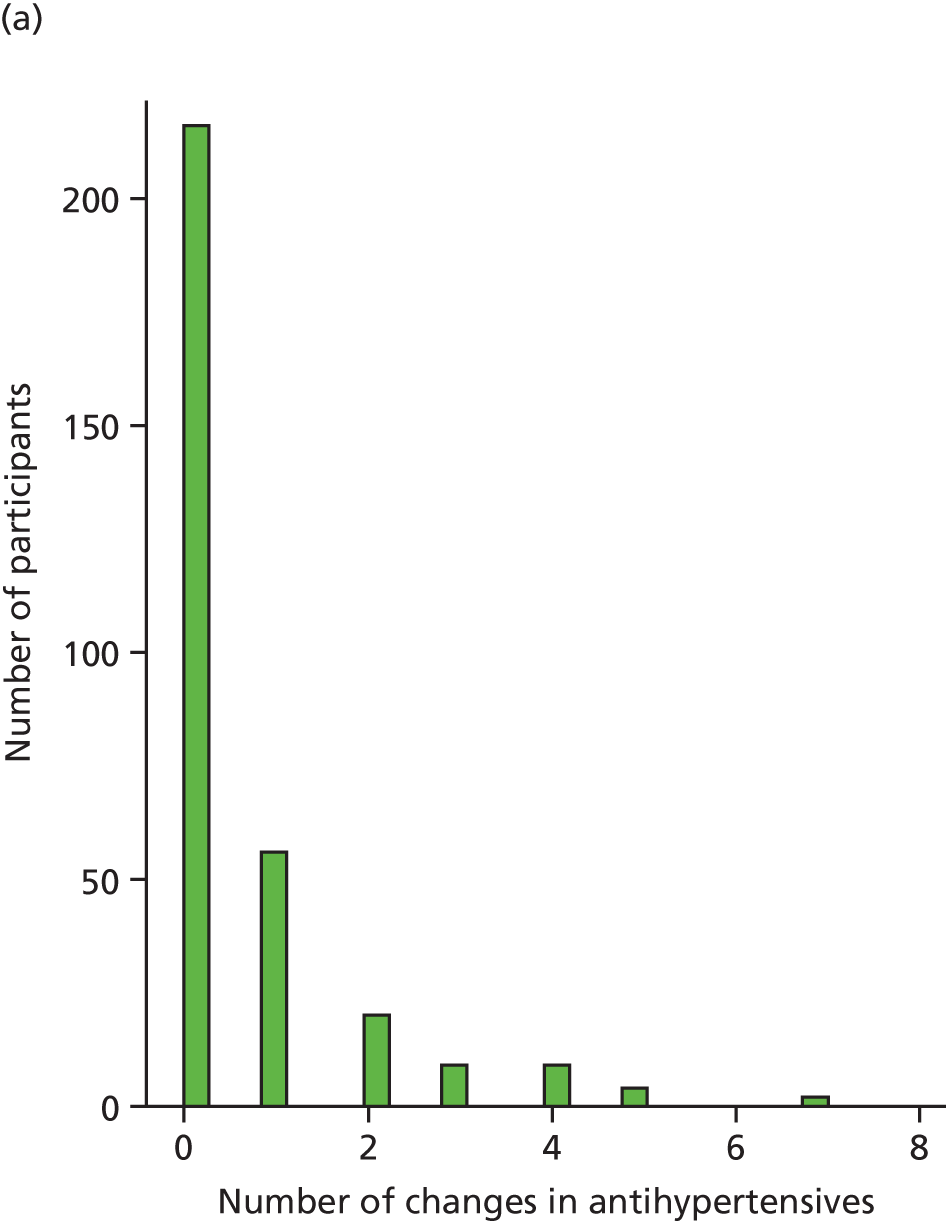
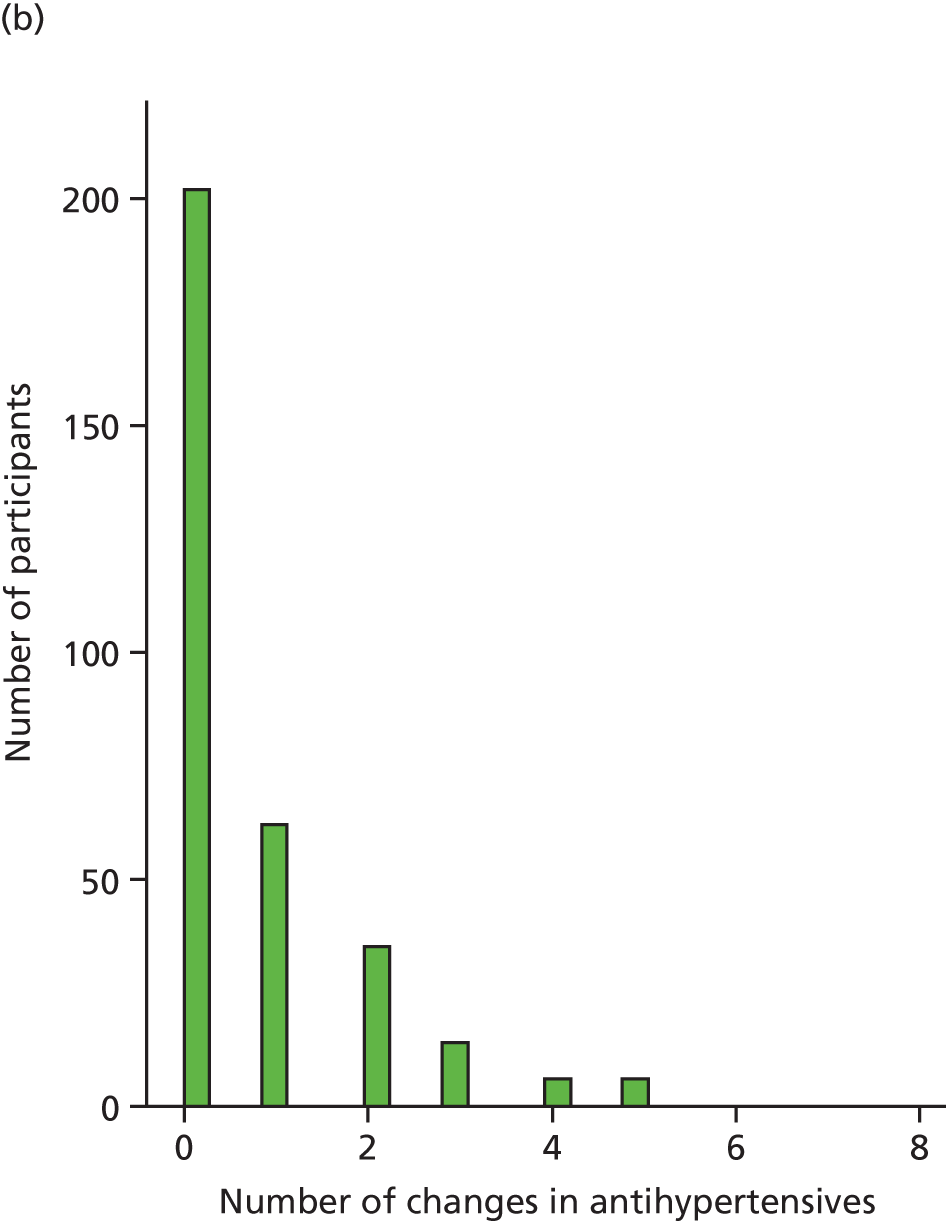
FIGURE 22.
Distribution of changes in cholesterol medication: (a) control; and (b) intervention.
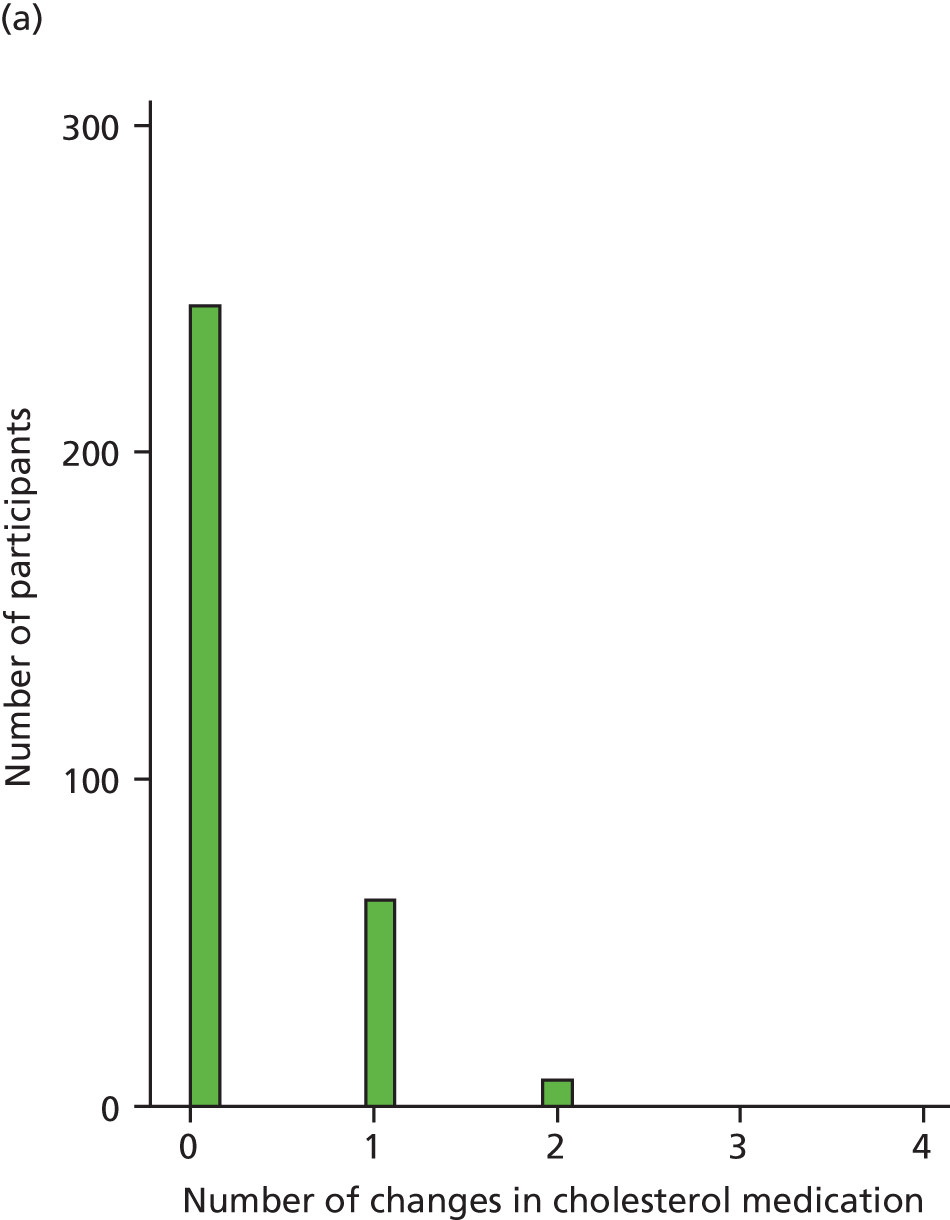
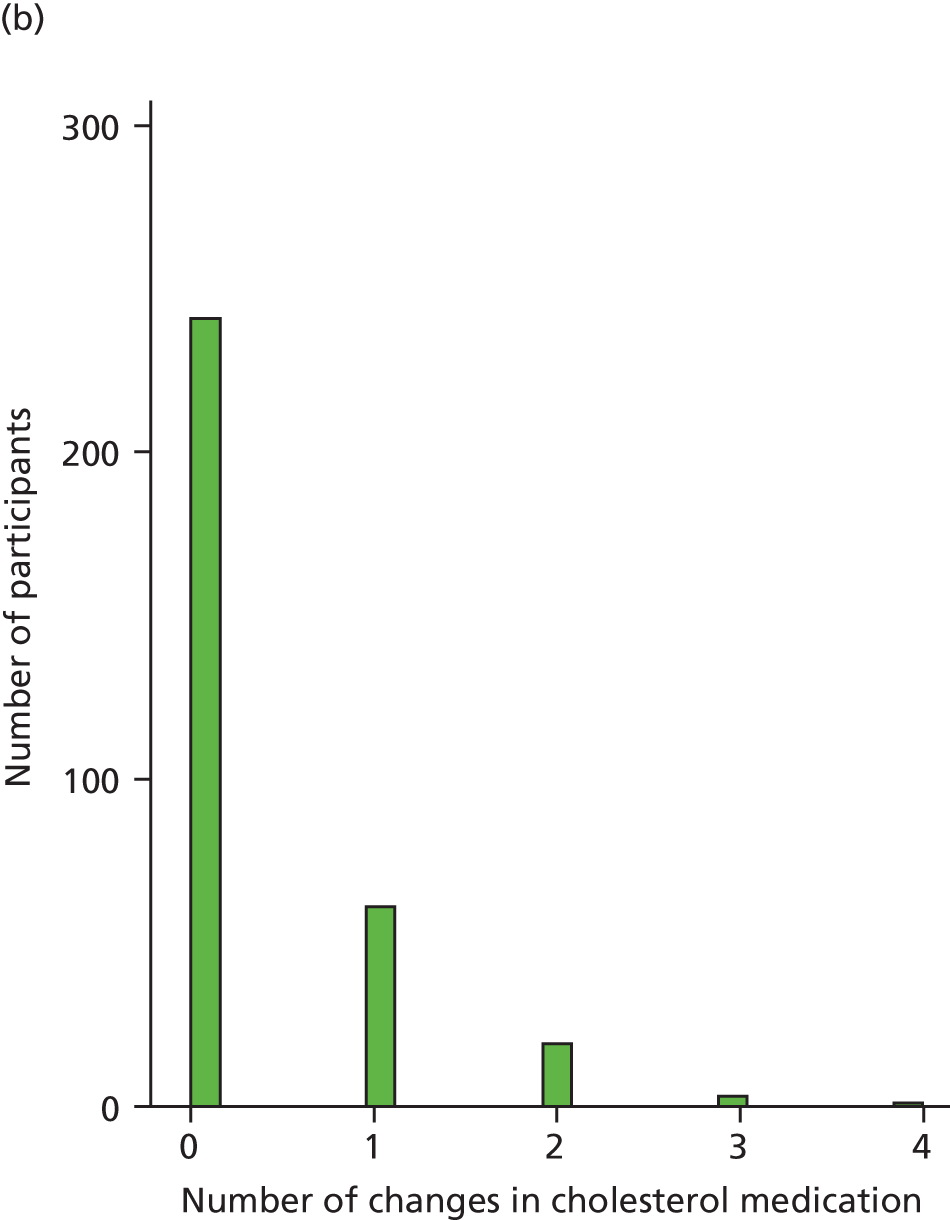
In terms of the results for those in the intervention group, these participants’ GPs rarely changed their blood pressure target after the initial target was set at the start of the study (Table 60). In fact, only 8% of participants suitable for home blood pressure monitoring had their target changed and no participants had their target changed more than once.
| Number of times blood pressure targets changed | Number of participants | Percentage |
|---|---|---|
| 0 | 272 | 92 |
| 1 | 24 | 8 |
| Total | 296 | 100 |
Self-management
Various aspects related to self-management in the CVD risk trial were measured by self-report in the follow-up questionnaires. The participants in the intervention group reported doing more physical activity and eating a healthier diet than the participants in the usual care group at 12 months (Table 61). It is worth noting that the unadjusted mean scores in these two areas of self-management were maintained or improved over the trial for both groups alike. In terms of the subscales from the Health Impact Education Questionnaire,313 and similar to the results from the depression trial, the between-group differences in unadjusted mean scores for aspects of self-management skills and self-efficacy are small (see Table 61). However, there is evidence of a difference between the two groups for ‘skill and technique acquisition’ at 12 months in favour of the intervention group, but almost no change in these mean scores over the trial for both groups.
| Aspect of self-management | Usual care | Intervention | Adjusted difference in meansa | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Physical activityb | |||||||
| 6 months | 2.9 (0.8) | 299 | 3.0 (0.8) | 304 | |||
| 12 months | 2.9 (0.8) | 294 | 3.0 (0.8) | 297 | 0.1 | 0.0 to 0.2 | 0.003 |
| Dietc | |||||||
| 6 months | 5.9 (2.2) | 303 | 5.2 (2.2) | 307 | |||
| 12 months | 5.7 (2.1) | 299 | 5.0 (2.1) | 300 | –0.7 | –0.9 to –0.4 | < 0.001 |
| Self-management skills and self-efficacyb | |||||||
| Self-monitoring and insight | |||||||
| 6 months | 3.2 (0.4) | 299 | 3.3 (0.4) | 298 | |||
| 12 months | 3.2 (0.4) | 295 | 3.3 (0.4) | 295 | 0.1 | 0.0 to 0.1 | 0.073 |
| Constructive attitudes and approaches | |||||||
| 6 months | 3.3 (0.5) | 299 | 3.4 (0.5) | 300 | |||
| 12 months | 3.3 (0.5) | 296 | 3.4 (0.5) | 295 | 0.0 | 0.0 to 0.1 | 0.628 |
| Skill and technique acquisition | |||||||
| 6 months | 3.1 (0.5) | 299 | 3.2 (0.5) | 299 | |||
| 12 months | 3.1 (0.5) | 297 | 3.2 (0.5) | 295 | 0.1 | 0.1 to 0.2 | < 0.001 |
| Health services navigation | |||||||
| 6 months | 3.1 (0.5) | 301 | 3.3 (0.5) | 301 | |||
| 12 months | 3.1 (0.6) | 296 | 3.2 (0.5) | 297 | 0.0 | 0.0 to 0.1 | 0.268 |
Treatment optimisation
The proportion of participants who reported taking blood pressure-lowering medication (antihypertensives) or cholesterol-lowering medication (statins) was very similar in both arms and there was no evidence of a difference between the two groups at 12 months (Table 62).
| Medication use | Usual care, % (n/N) | Intervention, % (n/N) | Adjusted ORa | 95% CI | p-value |
|---|---|---|---|---|---|
| Antihypertensives | |||||
| 6 months | 64 (184/288) | 68 (195/285) | NA | ||
| 12 months | 68 (196/289) | 70 (202/287) | 1.4 | 0.8 to 2.5 | 0.249 |
| Statins | |||||
| 6 months | 54 (158/294) | 53 (159/300) | NA | ||
| 12 months | 56 (165/297) | 57 (166/290) | 1.2 | 0.7 to 1.9 | 0.562 |
Both groups also self-reported similar adherence to their medications (Table 63), with generally greater adherence to antihypertensives than to statins at both 6 and 12 months. Nevertheless, there is evidence that the intervention group were slightly better than the usual care group at taking both types of medication as prescribed at 12 months.
| Medication adherence | Usual care | Intervention | Adjusted difference in meansb | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Antihypertensives | |||||||
| 6 months | 3.8 (0.6) | 187 | 3.9 (0.4) | 198 | |||
| 12 months | 3.8 (0.5) | 194 | 3.9 (0.3) | 203 | 0.1 | 0.0 to 0.2 | 0.013 |
| Statins | |||||||
| 6 months | 3.6 (0.7) | 161 | 3.8 (0.6) | 163 | |||
| 12 months | 3.6 (0.8) | 165 | 3.8 (0.5) | 169 | 0.2 | 0.1 to 0.3 | 0.005 |
The numbers of participants who were prescribed at least one medication related to CVD modifiable risk factors were similar for each drug type between the two groups (Table 64). The information shown in this table was extracted from participants’ medical records.
| Medication prescriptions | Usual care | Intervention | Intervention vs. usual care | ||||
|---|---|---|---|---|---|---|---|
| % | n/N | % | n/N | Adjusted OR | 95% CI | p-value | |
| Experienced at least one change in medication over the trial period | |||||||
| Antihypertensive | 32 | 100/316 | 38 | 123/325 | 1.3a | 0.9 to 1.8 | 0.117 |
| Cholesterol drugs including statins | 22 | 71/316 | 26 | 84/325 | 1.2a | 0.8 to 1.7 | 0.327 |
| Prescribed at least one medication over the trial period | |||||||
| Antiplatelet | 18 | 57/316 | 19 | 62/325 | |||
| Cholesterol drugs including statins | 61 | 192/316 | 62 | 201/325 | |||
| Smoking cessation | 1 | 3/316 | 2 | 5/325 | |||
| Obesity medication | 1 | 2/316 | 1 | 4/325 | |||
| Antihypertensive | 70 | 222/316 | 73 | 236/325 | |||
| Prescribed antihypertensive drug by drug class over the trial period | |||||||
| ACE inhibitors or ARBs | 50 | 159/316 | 52 | 170/325 | |||
| Beta-blockers | 18 | 58/316 | 16 | 52/325 | |||
| Calcium blockers | 36 | 114/316 | 40 | 129/325 | |||
| Diuretics | 28 | 90/316 | 29 | 93/325 | |||
| Other | 8 | 26/316 | 8 | 26/325 | |||
Care co-ordination
Despite quite similar unadjusted mean scores between the intervention group and the usual care group for the role clarity and co-ordination subscale from the generic measure of continuity of care,317 there was some evidence of a difference between the two groups in favour of the intervention group (Table 65). Second, those in the intervention group were more likely to report that their treatment involved a care plan at 6 months and there was strong evidence of a difference between the groups at 12 months. Additionally, there was some evidence that participants in the intervention group were more satisfied with the organisation of their health care than those in the usual care group. Finally, both groups had to organise their own health care to about the same degree at 6 and 12 months (see Table 65).
| Experiences of care co-ordination | Usual care | Intervention | Adjusted difference in meansb | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Role clarity and co-ordination (range 0–3) | |||||||
| 6 months | 2.9 (0.5) | 260 | 2.9 (0.4) | 270 | |||
| 12 months | 2.9 (0.5) | 247 | 3.0 (0.3) | 263 | 0.1 | 0.0 to 0.1 | 0.015 |
| Evidence of a care plan (range 0–7) | |||||||
| 6 months | 3.5 (2.1) | 227 | 4.9 (1.8) | 243 | |||
| 12 months | 3.8 (2.1) | 209 | 4.9 (2.0) | 236 | 1.2 | 0.8 to 1.5 | < 0.001 |
| Overall experience of organisation of health care (range 1–5) | |||||||
| 6 months | 3.6 (0.9) | 300 | 3.9 (0.7) | 302 | |||
| 12 months | 3.6 (0.9) | 296 | 3.8 (0.7) | 296 | 0.1 | 0.0 to 0.2 | 0.044 |
| Self-organisation of health care (range 1–5) | |||||||
| 6 months | 3.8 (1.1) | 286 | 3.8 (1.0) | 292 | |||
| 12 months | 3.9 (1.1) | 283 | 3.8 (1.0) | 287 | –0.1 | –0.2 to 0.1 | 0.368 |
Patient context
Motivation
Participants’ motivation to participate in the study was captured within the baseline questionnaire. As covered in Chapter 7 (see Table 14), internal motivation reflects a participant’s inclination to make changes in his or her life, whereas joining the study because of external motivation reflects self-perceived external pressures for treatment (e.g. from one’s GP). As illustrated in Table 66, there was very little difference between the scores across the two groups, but participants were more internally motivated than externally motivated.
| Motivation | Usual care | Intervention | ||
|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | |
| Internal motivation | 4.8 (1.8) | 313 | 4.7 (1.9) | 324 |
| External motivation | 1.5 (1.2) | 308 | 1.5 (1.0) | 319 |
Use of the internet
Participants were asked about their general use of the internet, including e-mail. Table 67 shows the numbers of participants who reported that they used the internet more than once week. These results seem balanced across the two groups, with slightly more using the internet at 6 months in the usual care group than in the intervention group.
| Follow-up point (months) | Usual care, % (n/N) | Intervention, % (n/N) |
|---|---|---|
| 6 | 79 (237/300) | 76 (230/303) |
| 12 | 79 (235/298) | 79 (236/299) |
Health literacy and technology confidence
Table 68 shows that participants in the intervention group had more health literacy than those in the usual care group at both 6 and 12 months, but there was no evidence of a reliable difference between groups. Interestingly, the between-group difference in mean health literacy score had decreased by 12 months.
| Outcome | Usual care | Intervention | Adjusted difference in meansa | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Unadjusted mean (SD) | n | Unadjusted mean (SD) | n | ||||
| Health literacyb | |||||||
| 6 months | 3.8 (0.7) | 300 | 4.0 (0.7) | 305 | |||
| 12 months | 3.9 (0.7) | 296 | 4.0 (0.7) | 295 | 0.1 | –0.0 to 0.2 | 0.128 |
| Confidence in using technologyc | |||||||
| Internet confidence | |||||||
| 6 months | 5.9 (1.8) | 298 | 5.7 (1.8) | 306 | |||
| 12 months | 5.9 (1.7) | 297 | 5.8 (1.8) | 297 | |||
| Social media confidence | |||||||
| 6 months | 3.0 (2.2) | 295 | 3.1 (2.1) | 301 | |||
| 12 months | 3.0 (2.1) | 294 | 3.0 (2.1) | 293 | |||
| Telephone confidence | |||||||
| 6 months | 6.2 (1.3) | 300 | 6.0 (1.4) | 306 | |||
| 12 months | 6.2 (1.3) | 297 | 6.1 (1.4) | 297 | |||
Finally, in terms of technology confidence, Table 68 illustrates that the usual care group had slightly greater confidence than the intervention group in using both the internet and the telephone across the trial period. Although the intervention group had greater social media confidence at 6 months, this decreased to the same level of mean confidence as the usual care group by 12 months. We note that hypothesis testing was not performed on the technology confidence mean differences as these variables were not prespecified secondary outcomes.
Adverse events
The frequencies of adverse events are reported in Table 69. Over the trial and as defined in Chapter 7 (see Serious adverse events), there was a total of 76 reported adverse events, with no difference between the arms. Although the majority of adverse events were either not serious or serious but unrelated to participation in the trial, there was one incident that was serious and possibly related to the intervention. In this case, the participant collapsed and was taken to hospital as a result of low blood pressure, which was deemed to have been caused by a combination of weight loss and the failure to then change the dosage of blood pressure medication he was taking. This participant was not eligible for blood pressure monitoring in the study and so the HIAs were not reviewing blood pressure readings with the participant, but it is possible that advice from the intervention may have contributed to the weight loss. The intervention team, however, was not responsible for changes in medication.
| Adverse event | Usual care | Intervention | ||||
|---|---|---|---|---|---|---|
| Not related | Definitely related | Not related | Unlikely to be related | Possibly related | Definitely related | |
| Not serious | 13 | 1 | 8 | 2 | 3 | 2 |
| Serious, unexpected | 24 | 0 | 19 | 2 | 1 | 0 |
| Serious, expected | 0 | 0 | 0 | 1 | 0 | 0 |
Post hoc subgroup analyses suggested by the embedded qualitative study
Two main hypotheses were generated by the embedded qualitative study (see Chapter 11, Testing hypotheses generated by the embedded qualitative study), which we have investigated in a post hoc exploratory subgroup analysis. We recognise the exploratory nature of these analyses and that any such analysis is likely to have low power to detect anything but very large interaction effects. The hypotheses, along with details of the analyses, were as follows:
-
As it might be expected that those who had more severe readings of their baseline CVD risk factors would improve these readings to a greater degree, a subgroup analysis was used to compare those with high and those with low baseline blood pressure in terms of their improvement in blood pressure at 12 months. Likewise, we also compared those with a high and those with a low BMI and those who smoked and those who did not smoke at baseline in terms of 12-month BMI and 12-month smoking status respectively.
This first hypothesis was tested using a linear or logistic regression model of the modifiable risk factor outcome as appropriate, adjusted by baseline QRISK2 score, site (Bristol, Sheffield or Southampton) and general practice as a random effect. The results are presented in Tables 70 and 71, which show that there is no evidence of a subgroup effect for any of the modifiable risk factors. Therefore, those with higher baseline systolic blood pressure did not reliably improve their blood pressure more than those with low baseline blood pressure, with similar results found for BMI and smoking status.
| Blood pressure/BMI | Outcome at 12 months’ follow-up | Adjusted difference in meansa | 95% CI | Interaction p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| Mean (SD) | n | Mean (SD) | n | ||||
| Systolic blood pressure (mmHg) | |||||||
| Low (< 140) | 133.9 (14.0) | 90 | 130.9 (12.5) | 85 | –2.9 | –6.9 to 1.1 | |
| High (≥ 140) | 146.0 (15.6) | 201 | 143.1 (13.1) | 210 | –3.1 | –5.7 to –0.5 | 0.942 |
| BMI (kg/m2) | |||||||
| Low (< 30.0) | 26.3 (2.6) | 131 | 26.2 (2.7) | 128 | –0.1 | –1.0 to 0.8 | |
| High (≥ 30.0) | 34.4 (4.8) | 160 | 33.8 (4.5) | 165 | –0.7 | –1.5 to 0.1 | 0.361 |
| Smoking status | Non-smokers at 12 months’ follow-up, % (n/N) | Adjusted ORa | 95% CI | Interaction p-value | |
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Current smoker | 18 (10/57) | 7 (3/43) | 0.4 | 0.1 to 1.6 | |
| Not current smoker | 98 (229/234) | 96 (243/252) | 0.6 | 0.2 to 1.8 | 0.642 |
-
Given concerns about whether or not the inclusion criteria should have been different, because some people had a low need or no desire to modify risk factors, we undertook a subgroup analysis on level of internal and external motivation for joining the study. Those who were highly motivated to join the study because they personally wanted to make changes in their life (i.e. internal motivation) would be expected to improve their cardiovascular risk more than those with low internal motivation.
This hypothesis was tested using the internal and external motivation subscales314,315 (see Table 14). Both scales were dichotomised at the overall median score (i.e. not by trial arm) to categorise the participants into two subgroups. The median for the internal motivation subscale was 5 and the median for the external motivation subscale was 1. The results are presented in Table 72, which show that there was no evidence of a subgroup effect for either internal or external motivation.
| Motivation | QRISK2 response at 12 months’ follow-up, % (n/N) | Adjusted ORb | 95% CI | Interaction p-value | |
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Baseline internal motivation | |||||
| Low (≤ 5) | 41 (67/165) | 47 (80/169) | 1.3 | 0.9 to 2.1 | |
| High (> 5) | 45 (55/123) | 54 (68/125) | 1.4 | 0.8 to 2.4 | 0.888 |
| Baseline external motivation | |||||
| Low (≤ 1) | 43 (91/214) | 50 (106/212) | 1.3 | 0.9 to 2.0 | |
| High (> 1) | 44 (31/70) | 49 (38/77) | 1.2 | 0.6 to 2.4 | 0.855 |
Discussion of the results of the cardiovascular risk trial
Interpretation of the findings
The CVD risk trial showed weak evidence of a modest benefit from the intervention on the primary outcome, which was response to treatment in terms of maintenance or reduction in CVD risk assessed using the QRISK2 over a 12-month period. Our point estimate of the effect was a 30% improvement in the odds of responding to treatment, which would be a meaningful clinical improvement. However, despite the large sample size, the 95% CIs for this estimate were wide and included the possibility of no effect (OR 1.0) or a 90% increase in the odds of improvement (OR 1.9). Furthermore, the secondary analysis treating QRISK2 as a continuous measure showed only a very small reduction in QRISK2 score in the intervention group. It is important to note that the QRISK2 as a continuous measure is strongly dominated by non-modifiable factors such as patient age and has a wide SD because of these factors. It is insensitive to change as an outcome measure in a trial, which is why it was not chosen as the primary outcome.
The QRISK2 is a composite measure, including the modifiable risk factors of blood pressure, smoking, cholesterol level and BMI (for which weight is the modifiable factor) as well as other non-modifiable factors. The Healthlines intervention was designed to address these modifiable risk factors by supporting participants to change their behaviour through techniques such as goal-setting and self-monitoring and by supporting them to access resources to help them with these behaviour changes. The Healthlines intervention also encouraged optimisation of drug treatment and co-ordination of efforts made by participants, their GP and Healthlines staff to improve their CVD risk factors. The individual risk factors that constitute the QRISK2 score are therefore key secondary outcomes. Our findings show that the intervention was associated with small but clinically meaningful reductions in participants’ blood pressure and weight, but no improvements in smoking status or cholesterol level. The combined effect of these factors means that there was only a modest benefit in terms of overall CVD risk reduction.
One further explanation for the limited benefit in terms of QRISK2 score is the effect of smoking, which makes a major contribution to overall CVD risk. 195 In this trial we observed a non-significant trend towards a greater chance of being a smoker in the intervention group than in the usual care group after 12 months. This could be because of regression to the mean, as there was a higher proportion of smokers in the usual care group than in the intervention group at baseline but a similar proportion at 12 months. However, this trend towards a negative impact of the intervention on smoking will have tended to cancel out any positive effect of improved blood pressure and weight in terms of overall CVD risk.
Although the intervention was associated with only small improvements in participants’ blood pressure, it is important to note that only 71% (210/295) of participants in the intervention arm had high blood pressure at baseline and so not all patients were treated to reduce their blood pressure. The mean baseline blood pressure in the intervention group was only slightly above target at 148/81 mmHg, so the scope for improvement was limited. Therefore, one would anticipate smaller reductions in blood pressure in this trial than in a trial of treatments for patients whom were selected because they all had hypertension.
For those with hypertension in this trial, the intervention was intended to improve blood pressure control through several mechanisms. Through self-monitoring, participants with hypertension should have been motivated to adhere to their medication, seek changes in their medication if their blood pressure was not well controlled and participate in other behaviours such as improved diet and exercise, which would, in turn, improve their blood pressure. In addition, the intervention was designed to optimise treatment by identifying patients with poor blood pressure control, informing participants’ GPs of this and encouraging them to escalate treatment to targets in line with NICE guidance. The GPs were sent details of the participants’ blood pressure readings, reminded of treatment targets and sent explicit guidance about recommended treatment changes.
The results demonstrate changes in some but not all of these mechanisms. There was evidence that participants in the intervention arm were more adherent to their blood pressure medication, improved their diet and undertook more physical activity. In line with the self-reported diet and physical activity improvements, the intervention was associated with small and sustained reductions in weight. It is important to note that only 56% (166/295) of participants in the intervention arm had a BMI ≥ 30 kg/m2 at baseline, which was the criterion for implementing more targeted weight loss interventions. However, there was no evidence that the GPs of participants in the intervention arm were more active in escalating drug treatment in terms of the proportion of people prescribed blood pressure treatment or the number of changes in blood pressure treatment dose or type. This is despite the fact that the Healthlines HIAs sent letters to the GPs of 138 (45%) patients in the intervention arm requesting a review of their blood pressure treatment. These letters were sent because the HIAs had reviewed the participants’ self-monitoring blood pressure readings and had identified that they were not being treated to the appropriate target.
There was no evidence that the intervention was associated with benefits in terms of total cholesterol level or ratio of total cholesterol to HDL cholesterol. Although the reduction in weight and improved diet associated with the intervention would be expected to have a small positive impact on cholesterol level, the main hypothesised mechanism of action was through greater prescription of statins and greater medication adherence. Almost all participants in this trial would be recommended prescription of statins according to NICE guidance, as they had a CVD risk of ≥ 20% as a criterion for entry to the trial. At baseline, however, only half (49%) of participants were being prescribed statins and at 12 months’ follow-up this had only slightly increased to 57% in both the intervention and the usual care arms. The topic of statins came up as an item for discussion in the HIA scripts at every Healthlines encounter and participants were encouraged to take their medication if it had been prescribed based on scripts exploring and addressing problems with adherence. If statins were not being prescribed, the scripts instructed the HIAs to explain about statins and to seek permission to contact participants’ GPs. GPs were sent a clear treatment recommendation, details of the participants’ CVD risk and a succinct summary of NICE guidelines. Our results provide evidence of improved patient adherence with regard to cholesterol-lowering medication, but relatively few letters about statins [39 letters relating to 32 (11%) patients] were sent by HIAs to GPs and there was no evidence of greater prescriptions of statins (above the increase also observed in the usual care group) or of more changes in dose or treatment.
Telehealth interventions such as the Healthlines Service have the potential to improve access to health care by making it easier to obtain care conveniently and by providing support for self-care. As with the depression trial, there was evidence from this trial that participants experienced better access to health care, felt that they had more support and advice and were more satisfied with the treatment that they received than those in the control arm. There was only limited evidence that participants gained more confidence in self-management, although they did express slightly more positive attitudes on the skills and technique scale of the heiQ.
The Healthlines Service was also designed to promote management of LTCs through a care plan (providing information about participants’ condition and discussing treatment options in line with their goals) and to improve co-ordination of the care and treatment available to participants from various sources including their GP and the internet. In contrast to the depression trial, participants in the intervention arm in the CVD risk trial were more likely to have discussed a care plan and were also more likely to have a positive experience of the organisation and co-ordination of their care than those in the usual care arm.
Strengths and limitations
There are a number of strengths to the CVD risk trial that also apply to the depression trial and these will be discussed in more detail in Chapter 12. These include the large sample size, high level of participant retention and masked outcome assessment, which enhance internal validity. Additional shared benefits are the highly pragmatic nature of the intervention and the multicentre trial recruitment method, both of which enhance external validity. Furthermore, basing the intervention on a conceptual model and conducting an embedded process evaluation help to understand potential causal mechanisms, whereas the nested economic evaluation provides information about cost-effectiveness.
There are also some shared limitations of both the depression and the CVD risk trials, which include the difficulties and delays in service delivery because of the closure of NHS Direct and the relatively low level of recruitment in terms of the percentage of all those sent information about the trial. Both of these issues will be discussed in more detail in Chapter 12.
There were several further limitations specific to the CVD risk trial, some of which relate to the implementation of the intervention and some of which relate to the research process. First, there were technical problems in adapting the Duke patient management software to suit the UK context and the NHS Direct software platform. This led to substantial delays both before and after participants were recruited. For some participants, there was a delay of several months between when their baseline measures were obtained and when they started receiving the intervention. This reduced the ‘dose’ of intervention that they received and, in addition, their risk factors may have changed in the intervention period and they may have lost interest in engaging with the intervention because of repeated delays. Second, and as will be discussed in Chapter 11, some participants appear to have taken part in the trial from an altruistic desire to support research rather than to make changes to their health. Third, the research process itself may have acted as part of the intervention, particularly the collection of CVD risk information at the baseline and outcome assessments. Finally, although randomised trials are intended to remove differences between the intervention and the treatment arms apart from the intervention, this is an oversimplification, particularly in trials involving behaviour change interventions, whereby being in one arm or the other may, in itself, act as an intervention and have an effect on motivation, behaviour and outcomes. 373
Use of the QRISK2 measure as the primary outcome also has limitations. The QRISK2 is a composite score calculated from a range of individual risk factors. In this trial, changes in risk depended on changes in the modifiable risk factors of blood pressure, ratio of total cholesterol to HDL cholesterol, smoking status and BMI. During the process of checking and cleaning the data for this trial, we found that some individuals had much larger changes in their QRISK2 scores between baseline and follow-up than we anticipated and this was often because of large fluctuations in blood pressure and cholesterol levels at different time points, as well as inconsistencies in the reporting of smoking even after validation using a carbon monoxide monitor. These variations may well be the result of day-to-day variability and/or measurement error rather than the effect of clinical management and this creates ‘noise’ in the measurement of the primary outcome, which reduces our ability to assess the impact of the intervention compared with usual care. In retrospect, it would have been better to use 24-hour ambulatory blood pressure monitoring to assess blood pressure, although the approach that we used was considered best practice at the time that the study was designed.
Furthermore, it is perhaps surprising that improvements in blood pressure and weight in the intervention arm did not lead to larger improvements in CVD risk as assessed using the QRISK2, as epidemiological studies suggest that a large reduction in risk is obtainable with small reductions in blood pressure, irrespective of baseline blood pressure. 374 The QRISK2 equation has not been published and so it is not clear how each component contributes to the overall risk estimate. In future sensitivity analyses we plan to explore whether or not our findings would have been different if we had used a different risk equation such as the Framingham risk score,196 which would help us to more fully understand the relationship between the risk factor changes observed and the overall estimate of risk.
Relationship to previous research
Two systematic reviews have been published recently, both of which are consistent with the findings of the Healthlines study. The first explored the effectiveness of multiple risk factor interventions delivered over the internet, with or without therapist support, in reducing cardiovascular risk. 375 Of nine studies included, three found benefits in terms of weight reduction, two found benefits for systolic blood pressure and one found benefits for cholesterol level. No studies found evidence of increased smoking cessation rates. Most of the studies were small (< 100 participants) and of moderate quality. The authors concluded that there was little evidence that these interventions provide benefit. 375 The second review was of trials of multifactorial telehealth interventions to reduce cardiovascular risk, with the findings reported in Chapter 2. The studies included in the review were heterogeneous in terms of context and the nature of the intervention and most had short periods of follow-up. Some (but not all) of the trials included in this review demonstrated a small improvement in systolic blood pressure and several showed very small and non-significant reductions in total cholesterol. There was no evidence that the multifactorial telehealth interventions were associated with reductions in smoking. Of the four studies that reported an outcome of overall CVD risk, one showed a small reduction in overall risk associated with the intervention,48 but this was not found in the other studies or a meta-analysis. 61
A more recent study from the Netherlands reported the findings of an online nurse practitioner-supported intervention for vascular risk management in patients with clinically manifest vascular disease. 376 Although the patients were recruited in secondary care and had an established disease (unlike Healthlines participants), the design of the study has similarities to that of the Healthlines CVD risk trial. The primary outcome was the relative change in Framingham heart risk score after 1 year, calculated as the difference between the groups in change in Framingham heart risk score from baseline to the 1-year follow-up divided by the mean Framingham heart risk score at baseline. The findings were of a decreased Framingham heart risk score after 12 months of –2.1 (95% CI –3.8 to –0.3), which equates to a relative change of –14% (95% CI –25% to –2%). However, there were baseline imbalances in the Framingham heart risk score and, after adjusting for these, there was no longer convincing evidence of a difference between arms (difference in Framingham score of –1.2, 95% CI –2.7 to 0.3; relative change –8%, 95% CI –18% to 2%).
Evidence about specific approaches used
The Healthlines Service for CVD risk targeted modifiable risk factors such as smoking, obesity and hypertension, as well as being designed to promote improved diet, exercise and medication adherence and reductions in alcohol consumption. Research on telehealth interventions to address these specific risk factors is therefore relevant.
Blood pressure self-monitoring
Several systematic reviews have demonstrated the effectiveness of home blood pressure monitoring. 148,150,377 In the review by Agarwal et al. ,148 compared with clinic-based measurements, self-monitoring of blood pressure was associated with small reductions in systolic blood pressure (–2.63 mmHg, 95% CI –4.24 to –1.02 mmHg) and diastolic blood pressure (–1.68 mmHg, 95% CI –2.58 to –0.79 mmHg). In a meta-regression analysis, interventions that included telemonitoring had slightly greater effects. In an overview of four systematic reviews of telemonitoring in patients with hypertension, all four showed benefits in terms of blood pressure of remote telemonitoring. 378 However, not all relevant trials show such benefits. For example, a recent UK-based trial of blood pressure telemonitoring and nurse-led telephone support for patients with hypertension and a history of stroke did not find any benefits for blood pressure. 379
The findings from the above systematic reviews are consistent with the findings of the Healthlines trial, showing small benefits in blood pressure from self-monitoring. Interestingly, a recent trial that included self-titration of blood pressure medication showed greater benefits in patients with hypertension at high CVD risk. 380 Some other studies have also shown large improvements in blood pressure. The CVD programme adapted for the Healthlines Service was based on that developed by Bosworth et al. 295 in the USA and a trial of this intervention in the USA found much larger reductions in blood pressure than we observed in the Healthlines CVD risk trial. Large reductions in blood pressure have also been observed in other North American studies of self-monitoring, online communication and pharmacist support. 45,381 The finding that studies from the USA consistently show greater benefits than trials of similar interventions in the UK may reflect differences in the usual care provided by primary care services to patients in the usual care arms of these trials or differences in how the interventions were delivered.
A recent Scottish study investigated blood pressure self-monitoring with remote transmission of findings to an online resource that provided automated feedback and clinician review. 382 Participants (n = 401) had uncontrolled high blood pressure. The intervention had similarities to the Healthlines approach to blood pressure monitoring and review. The benefits seen in the Scottish study were slightly greater than those observed in the Healthlines study, with a mean reduction of 4.3 mmHg and 2.3 mmHg in systolic and diastolic blood pressure, respectively, after 6 months’ follow-up. No differences between the intervention and the usual care groups were observed in weight, cholesterol medication adherence or quality of life. However, benefits in quality of life associated with blood pressure self-monitoring have been reported in other similar studies,383,384 which is of relevance to the findings on quality of life observed in the Healthlines CVD risk trial and reported in Chapter 10.
Smoking cessation
A recent systematic review of internet-based interventions to support smoking cessation identified 28 relevant trials. 385 The results suggest that some internet-based interventions can assist with smoking cessation, particularly those that are interactive and tailored to individuals. However, trials that compared internet interventions with usual care or self-help did not show consistent effects and were at risk of bias. On the other hand, a systematic review of five mobile telephone interventions, mainly based on text messaging, showed that these increased long-term smoking cessation rates compared with control programmes (risk ratio 1.71, 95% CI 1.47 to 1.99). 160 A Cochrane review found that telephone ‘quitlines’ can improve cessation rates in people who proactively contact them386 and a review of online and other electronic aids for smoking cessation demonstrated that these aids had small positive effects. 159
Given that these reviews were all positive, it is disappointing that the Healthlines Service had no positive impact on smoking, although this is also consistent with previous trials of multifactorial interventions. The most likely explanation is that smoking cessation aids may help people who specifically participate in an intervention trial designed to help them stop smoking, whereas those enrolled in a study to reduce overall CVD risk may not need to stop smoking (if they do not smoke at baseline) or be motivated to quit.
Weight loss
A recent Cochrane review of computer-based interactive interventions for weight loss in overweight individuals identified 14 studies. 164 A meta-analysis suggested that, compared with no intervention or minimal intervention, these interventions were associated with a mean weight loss of 1.5 kg (95% CI 0.9 to 2.1 kg). This is of a similar magnitude to the weight loss observed in the Healthlines CVD risk trial.
Conclusion
The Healthlines Service for raised CVD risk was associated with small improvements in blood pressure and weight, but no reductions in the ratio of total cholesterol to HDL cholesterol or smoking. Overall, there was weak evidence of a small beneficial effect of the intervention on the primary outcome of maintenance or reduction in CVD risk. Participants in the intervention group reported small improvements in the secondary outcomes of ease of access to health care, support and advice received and satisfaction with the treatment received. They were also more likely to have discussed a care plan and to have a positive experience of the organisation and co-ordination of their care than those in the usual care arm.
Chapter 10 Economic evaluation results
Some of the material in this chapter has been published in the following papers and is reproduced here with permission:
Dixon P, Hollinghurst S, Edwards L, Thomas C, Foster A, Davies B, et al. Cost-effectiveness of telehealth for patients with depression: evidence from the Healthlines randomised controlled trial. Br J Psychiatry Open 2016;2:262–9. 387 © The Royal College of Psychiatrists 2016.
Dixon P, Hollinghurst S, Edwards L, Thomas C, Gaunt D, Foster A, et al. Cost-effectiveness of telehealth for patients with raised cardiovascular disease risk: evidence from the Healthlines randomised controlled trial. BMJ Open 2016;6:e012352. 388
Dixon P, Hollinghurst S, Ara R, Edwards L, Foster A, Salisbury C. Cost-effectiveness modelling of telehealth for patients with raised cardiovascular disease risk: evidence from a cohort simulation conducted alongside the Healthlines randomised controlled trial. BMJ Open 2016;6:e012355. 389
These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Abstract
Background: The aim was to estimate the cost-effectiveness of the Healthlines depression and CVD risk interventions compared with usual care.
Methods: Cost–consequence and cost-effectiveness designs were used in the economic evaluation. Cost and quality of life data formed the basis of the within-trial evaluations. We undertook cost-effectiveness analysis from a health system perspective by comparing NHS costs with QALYs calculated from responses to the EQ-5D-5L questionnaire. A cohort simulation model was developed for the CVD risk trial to estimate the long-term impact of the intervention on participants’ lifetime health. Data sets obtained from multiple imputation were used for the base-case analysis to account for missingness (51% and 18% of data were missing in the depression and CVD risk trials respectively).
Findings: The depression intervention was not cost-effective, largely because of a very small mean between-arm difference in QALYs of 0.001, associated with an intervention incremental cost of £192. This was robust to a sensitivity analysis that tested alternative costs for the intervention and to the inclusion of NHS secondary and community care costs. The complete-case analysis, conducted as a sensitivity analysis on the main results, was biased by differential missingness in responses to health-related quality of life questionnaire items. The intervention was cost-effective for the CVD risk within-trial evaluation. A larger between-arms QALY difference (0.0132) was observed in this trial and the incremental cost associated with the intervention was lower (£138). Complete-case results were similar. Results from the simulation model were sensitive to cost and QALY data from the trial and to the assumed duration of the intervention’s effect.
Conclusion: Although the depression intervention was not cost-effective, the evidence suggests that the CVD risk intervention is probably cost-effective for long durations of effect (e.g. a permanent lifetime effect) but may not be cost-effective for short durations (e.g. effects that do not last much longer than 1 year).
Introduction
The aim of the economic evaluation was to investigate the cost-effectiveness of the Healthlines intervention for participants with depression and raised CVD risk. We undertook cost-effectiveness analysis from a health system perspective by comparing NHS costs with QALYs calculated from responses to the EQ-5D-5L questionnaire. Resource use and quality of life data are presented for available cases and complete cases and on an imputed basis (see Chapter 7, Economic evaluation methods). The number of available cases differed between information sources (e.g. primary care records, questionnaires) and within information sources (e.g. to specific questions within a questionnaire). Complete cases were defined in each trial for the purposes of economic analysis as participants with complete data on all NHS and PSS cost items and all health-related quality of life items at each measurement point. The depression trial had a lower proportion of complete cases (49% of those randomised) than the CVD risk trial (82%).
Results are reported in 2013 UK pounds.
Depression trial
NHS resource use in the depression trial
Intervention for depression participants
Information about the number of telephone calls made by the HIAs to depression participants and their duration is shown in Table 73. We also show the use of the other intervention resources, namely the LLTTFi CBT website, the designated CBT book (Overcoming Depression and Low Mood: a Five Areas Approach)292 and the BWW website. The results are also given for the subset of participants for whom we had complete NHS and PSS cost and QALY data (complete cases).
| Intervention element | All available data (n = 308),a mean (SD) | Complete cases (n = 145),a mean (SD) |
|---|---|---|
| Encounter calls | ||
| Number | 6.83 (4.64) | 8.11 (4.41) |
| Total duration (hours) | 1.98 (1.59) | 2.39 (1.56) |
| Non-scheduled calls | ||
| Number | 1.56 (2.18 | 1.18 (2.18) |
| Total duration (hours) | 0.09 (0.20) | 0.10 (0.18) |
| All calls | ||
| Number | 8.39 (6.03) | 9.92 (5.74) |
| Total duration (hours) | 2.07 (1.69) | 2.49 (1.63) |
| Use of LLTTFi website | ||
| Participants, n (%) | 237 (76.9) | 113 (77.9) |
| Use of ODLM book | ||
| Participants, n (%) | 53 (17.2) | 26 (17.9) |
| Use of BWW website | ||
| Participants, n (%) | 98 (31.8) | 46b (31.7) |
The mean total length of calls for all participants was 2 hours 4 minutes, with the maximum length being 8 hours 21 minutes (23 calls). The majority of participants used either the LLTTFi website or the CBT book (or both). Ninety-eight participants (32%) used the BWW website, although, because of data confidentiality, we have no information on which participants these were.
Participants included in the complete-case analysis had more telephone calls on average than the whole sample and these were, on average, slightly longer. A slightly higher percentage of complete cases accessed the LLTTFi website and CBT book.
The cost of the intervention is shown in Table 74 for all participants and for the complete cases (i.e. those for whom we had complete NHS and PSS cost and QALY data).
| Intervention element | All available data (n = 308),a mean (SD) (£) | Complete cases (n = 145),a mean (SD) (£) |
|---|---|---|
| Encounter calls | 71.84 (57.64) | 86.55 (56.39) |
| Non-scheduled calls | 3.26 (7.17) | 3.58 (6.51) |
| All calls | 75.11 (61.04) | 90.13 (59.18) |
| LLTTFi website | 9.74 (6.5) | 12.18 (6.39) |
| ODLM book | 9.09 (19.98) | 9.45 (12.53) |
| BWW | 19.09 (12.75) | 23.88 (12.53) |
| Total cost per participant | 113.03 (80.46) | 135.64 (77.93) |
For all participants, the cost of the telephone calls accounted for 66% of the total mean cost of £113 and the LLFFTi website and CBT book each contributed an additional 8%. The cost of the licence for BWW was reported as £60 per participant and so, with 98 participants accessing it, the mean overall cost was estimated as £19 or 17% of the total cost of the intervention. The cost of all components was higher for complete cases, giving an overall estimate that was £23 (20%) higher than for the whole sample. Again, calls accounted for 66% of the total cost.
Primary care consultations
The numbers and costs of primary care consultations are summarised in Tables 75 and 76 respectively. There was slightly higher usage of primary care services in the intervention group, hence costs were slightly higher for this group. The principal difference in the number and cost of consultations relates to the use of GP services.
| Primary care services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| GP consultations | 297 | 8.70 (6.51) | 305 | 9.68 (6.90) |
| Nurse consultations | 297 | 2.62 (3.78) | 305 | 2.81 (3.93) |
| Other primary care consultations | 297 | 3.28 (6.14) | 305 | 3.06 (3.78) |
| Complete cases | ||||
| GP consultations | 155 | 8.76 (6.93) | 144 | 9.35 (6.71) |
| Nurse consultations | 155 | 2.73 (3.33) | 144 | 2.76 (3.74) |
| Other primary care consultations | 155 | 3.68 (4.08) | 144 | 3.20 (4.00) |
| Primary care services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| GP consultations | 297 | 273.43 (198.62) | 305 | 303.52 (213.44) |
| Nurse consultations | 297 | 28.98 (41.99) | 305 | 30.75 (42.39) |
| Other primary care consultations | 297 | 58.88 (102.94) | 305 | 69.48 (136.95) |
| Total primary care cost | 297 | 361.29 (271.21) | 305 | 403.76 (292.72) |
| Complete cases | ||||
| GP consultations | 155 | 273.99 (206.14) | 144 | 295.12 (205.77) |
| Nurse consultations | 155 | 30.24 (36.88) | 144 | 30.65 (41.72) |
| Other primary care consultations | 155 | 64.57 (101.26) | 144 | 79.14 (165.22) |
| Total primary care cost | 155 | 368.80 (267.30) | 144 | 404.90 (298.18) |
Table 77 summarises the use and costs of prescribed medication, showing that the intervention group was prescribed fewer drugs. For complete cases, the cost of medication was higher in the usual care group than in the intervention group. However, using all available data estimated overall, medication costs were very similar for the two groups.
| Prescriptions | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Number of prescribed items | ||||
| All available data | 297 | 13.18 (20.82) | 305 | 12.18 (11.98) |
| Complete cases | 155 | 13.27 (11.75) | 144 | 12.26 (10.43) |
| Cost of prescribed medication (£) | ||||
| All available data | 297 | 89.13 (202.54) | 305 | 92.38 (179.82) |
| Complete cases | 155 | 86.92 (196.85) | 144 | 72.43 (127.03) |
Mean number of NHS community services accessed
Information was collected on contacts with a range of NHS community services (Table 78) and the associated costs (Table 79). Patterns of resource usage indicate that more of these resources were used by participants in the intervention group than by those in the usual care group. Additionally, the mean cost per participant of these services was higher in the intervention group than in the usual care group. Key drivers of this latter difference were higher costs associated with counsellor/psychologist consultations, psychiatrist clinic consultations and occupational therapist clinic consultations.
| Community services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| District nurse home visit | 200 | 0.15 (0.72) | 175 | 0.03 (0.31) |
| District nurse telephone consultation | 200 | 0.10 (0.52) | 175 | 0.06 (0.49) |
| Community mental health nurse home visit | 200 | 0.05 (0.41) | 175 | 0.07 (0.55) |
| Community mental health nurse telephone consultation | 200 | 0.05 (0.34) | 175 | 0.05 (0.35) |
| NHS counsellor/psychologist clinic consultation | 200 | 1.02 (2.69) | 175 | 1.38 (2.97) |
| NHS counsellor/psychologist telephone consultation | 200 | 0.17 (0.97) | 175 | 0.62 (1.93) |
| Psychiatrist clinic consultation | 200 | 0.16 (0.84) | 175 | 0.19 (0.96) |
| Psychiatrist home visit | 200 | 0.01 (0.07) | 175 | – |
| Psychiatrist telephone consultation | 200 | 0.03 (0.19) | 175 | 0.02 (0.24) |
| Occupational therapist clinic consultation | 200 | 0.19 (0.83) | 175 | 0.41 (1.60) |
| Occupational therapist home visit | 200 | 0.17 (0.80) | 175 | 0.05 (0.33) |
| Occupational therapist telephone consultation | 200 | 0.12 (0.53) | 175 | 0.10 (0.42) |
| NHS walk-in centre clinic consultation | 200 | 0.13 (0.46) | 175 | 0.22 (0.62) |
| NHS walk-in centre telephone consultation | 200 | 0.04 (0.25) | 175 | 0.09 (0.49) |
| GP out-of-hours service clinic consultation | 200 | 0.09 (0.39) | 175 | 0.14 (0.53) |
| GP out-of-hours service home visit | 200 | 0.01 (0.14) | 175 | 0.05 (0.34) |
| GP out-of-hours telephone consultation | 200 | 0.12 (0.49) | 175 | 0.19 (0.79) |
| Complete cases | ||||
| District nurse home visit | 155 | 0.16 (0.77) | 144 | – |
| District nurse telephone consultation | 155 | 0.08 (0.47) | 144 | 0.06 (0.53) |
| Community mental health nurse home visit | 155 | 0.03 (0.32) | 144 | 0.06 (0.51) |
| Community mental health nurse telephone consultation | 155 | 0.03 (0.27) | 144 | 0.05 (0.38) |
| NHS counsellor/psychologist clinic consultation | 155 | 1.01 (2.70) | 144 | 1.48 (3.08) |
| NHS counsellor/psychologist telephone consultation | 155 | 0.13 (0.65) | 144 | 0.67 (2.08) |
| Psychiatrist clinic consultation | 155 | 0.11 (0.53) | 144 | 0.19 (1.03) |
| Psychiatrist home visit | 155 | 0.01 (0.08) | 144 | – |
| Psychiatrist telephone consultation | 155 | 0.02 (0.14) | 144 | 0.02 (0.25) |
| Occupational therapist clinic consultation | 155 | 0.16 (0.82) | 144 | 0.40 (1.58) |
| Occupational therapist home visit | 155 | 0.20 (0.89) | 144 | 0.01 (0.12) |
| Occupational therapist telephone consultation | 155 | 0.09 (0.50) | 144 | 0.08 (0.38) |
| NHS walk-in centre clinic consultation | 155 | 0.13 (0.48) | 144 | 0.16 (0.50) |
| NHS walk-in centre telephone consultation | 155 | 0.03 (0.24) | 144 | 0.08 (0.51) |
| GP out-of-hours service clinic consultation | 155 | 0.09 (0.40) | 144 | 0.14 (0.56) |
| GP out-of-hours service home visit | 155 | 0.01 (0.16) | 144 | 0.03 (0.26) |
| GP out-of-hours telephone consultation | 155 | 0.11 (0.50) | 144 | 0.17 (0.76) |
| Community services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| District nurse home visit | 200 | 9.00 (43.27) | 175 | 1.71 (18.68) |
| District nurse telephone consultation | 200 | 1.00 (5.43) | 175 | 0.60 (5.12) |
| Community mental health nurse home visit | 200 | 3.25 (26.67) | 175 | 4.46 (35.93) |
| Community mental health nurse telephone consultation | 200 | 1.08 (7.45) | 175 | 0.99 (7.64) |
| NHS counsellor/psychologist clinic consultation | 200 | 59.16 (155.91) | 175 | 79.87 (172.05) |
| NHS counsellor/psychologist telephone consultation | 200 | 2.68 (15.32) | 175 | 9.81 (30.37) |
| Psychiatrist clinic consultation | 200 | 41.76 (219.60) | 175 | 49.22 (249.32) |
| Psychiatrist home visit | 200 | 1.30 (18.46) | 175 | – |
| Psychiatrist telephone consultation | 200 | 6.49 (48.39) | 175 | 5.97 (62.28) |
| Occupational therapist clinic consultation | 200 | 11.67 (52.57) | 175 | 25.96 (100.84) |
| Occupational therapist home visit | 200 | 10.87 (49.58) | 175 | 3.19 (20.27) |
| Occupational therapist telephone consultation | 200 | 2.15 (9.96) | 175 | 1.93 (7.79) |
| NHS walk-in centre clinic consultation | 200 | 4.26 (15.62) | 175 | 7.40 (21.25) |
| NHS walk-in centre telephone consultation | 200 | 0.78 (5.63) | 175 | 1.91 (10.88) |
| GP out-of-hours service clinic consultation | 200 | 2.89 (13.10) | 175 | 4.86 (18.12) |
| GP out-of-hours service home visit | 200 | 0.85 (12.02) | 175 | 3.89 (28.55) |
| GP out-of-hours telephone consultation | 200 | 2.30 (9.86) | 175 | 3.77 (15.81) |
| Total costs for available cases | 200 | 161.53 (346.84) | 175 | 205.52 (389.59) |
| Complete cases | ||||
| District nurse home visit | 155 | 9.68 (46.11) | 144 | – |
| District nurse telephone consultation | 155 | 0.88 (4.93) | 144 | 0.66 (5.58) |
| Community mental health nurse home visit | 155 | 1.68 (20.88) | 144 | 3.61 (33.31) |
| Community mental health nurse telephone consultation | 155 | 0.70 (5.75) | 144 | 1.05 (8.24) |
| NHS counsellor/psychologist clinic consultation | 155 | 58.75 (156.69) | 144 | 85.79 (178.60) |
| NHS Counsellor/psychologist telephone consultation | 155 | 2.03 (10.27) | 144 | 10.50 (32.69) |
| Psychiatrist clinic consultation | 155 | 28.63 (138.13) | 144 | 50.75 (267.81) |
| Psychiatrist home visit | 155 | 1.68 (20.96) | 144 | – |
| Psychiatrist telephone consultation | 155 | 5.05 (36.07) | 144 | 5.44 (65.25) |
| Occupational therapist clinic consultation | 155 | 10.18 (51.60) | 144 | 24.98 (99.90) |
| Occupational therapist home visit | 155 | 12.42 (54.99) | 144 | 0.86 (7.29) |
| Occupational therapist telephone consultation | 155 | 1.69 (9.39) | 144 | 1.56 (7.17) |
| NHS walk-in centre clinic consultation | 155 | 4.39 (16.33) | 144 | 5.44 (16.93) |
| NHS walk-in centre telephone consultation | 155 | 0.72 (5.33) | 144 | 1.85 (11.31) |
| GP out-of-hours service clinic consultation | 155 | 3.07 (13.63) | 144 | 4.72 (19.12) |
| GP out-of-hours service home visit | 155 | 1.10 (13.65) | 144 | 2.36 (22.35) |
| GP out-of-hours telephone consultation | 155 | 2.19 (10.08) | 144 | 3.47 (15.21) |
| Total costs for complete cases | 155 | 144.84 (278.06) | 144 | 203.05 (401.27) |
Hospital and ambulance use
Resource use associated with different types of hospital visits and ambulance use is reported in Table 80. Many of these resources were used infrequently. For example, there were no reported overnight hospital stays among available cases in the intervention group and only an average of 0.03 overnight hospital stays across all follow-up time points for the usual care group. By way of illustration, this figure of 0.03 was associated with a total of seven overnight stays undertaken by three different individuals. One participant had a total of three overnight hospital stays with the other participants having two overnight stays each.
| Services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| Overnight hospital stay | 204 | 0.03 (0.23) | 175 | – |
| Day care | 204 | 0.03 (0.25) | 175 | 0.02 (0.24) |
| Outpatient clinic | 201 | 0.13 (0.49) | 174 | 0.08 (0.29) |
| Accident and emergency | 246 | 0.03 (0.25) | 233 | 0.03 (0.18) |
| Other hospital services | 246 | 0.06 (0.24) | 233 | 0.05 (0.21) |
| Ambulance use | 199 | 0.03 (0.22) | 176 | 0.01 (0.11) |
| Complete cases | ||||
| Overnight hospital stay | 155 | 0.03 (0.23) | 144 | – |
| Day care | 155 | 0.03 (0.16) | 144 | 0.01 (0.08) |
| Outpatient clinic | 155 | 0.14 (0.50) | 144 | 0.04 (0.20) |
| Accident and emergency | 155 | 0.04 (0.30) | 144 | 0.02 (0.19) |
| Other hospital services | 155 | 0.06 (0.25) | 144 | 0.04 (0.20) |
| Ambulance use | 155 | 0.03 (0.20) | 144 | 0.01 (0.08) |
Resource use was higher in the usual care group and this is reflected in the mean cost per participant of hospital and ambulance care (Table 81).
| Services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| Overnight hospital stay | 204 | 12.70 (105.74) | 175 | – |
| Day care | 204 | 5.25 (41.70) | 175 | 3.34 (37.06) |
| Outpatient clinic | 201 | 52.12 (247.56) | 174 | 29.61 (208.31) |
| Accident and emergency | 246 | 5.84 (60.60) | 233 | 3.29 (27.93) |
| Other hospital services | 246 | 3.40 (21.12) | 233 | 1.00 (15.19) |
| Ambulance use | 199 | 7.68 (56.69) | 176 | 2.89 (27.06) |
| Mean cost for available casesa | 191 | 65.42 (282.74) | 170 | 34.05 (223.43) |
| Complete cases | ||||
| Overnight hospital stay | 155 | 9.79 (85.87) | 144 | – |
| Day care | 155 | 4.13 (33.33) | 144 | 0.72 (8.67) |
| Outpatient clinic | 155 | 41.21 (197.59) | 144 | 24.84 (220.28) |
| Accident and emergency | 155 | 8.65 (75.90) | 144 | 3.33 (32.85) |
| Other hospital services | 155 | 2.44 (14.26) | 144 | – |
| Ambulance use | 155 | 6.57 (49.81) | 144 | 1.77 (21.21) |
| Mean cost for complete cases | 155 | 72.78 (308.24) | 144 | 30.66 (235.14) |
Personal Social Services costs
Personal Social Services costs relating to services such as social work and community support work are summarised in Table 82. The difference between groups is driven by the higher costs associated with community support worker resource use in the usual care group.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | 206 | 36.36 (192.91) | 178 | 15.01 (75.42) |
| Complete cases | 155 | 44.69 (214.46) | 144 | 16.64 (81.54) |
Costs to participants and their friends and family
Private health-care costs and out-of-pocket expenditure
Participants were asked to report on their use of some private health-care services (not provided by NHS HCPs) for mental health and emotional problems (Table 83). There was limited use of some of these services in both groups and the mean total cost was similar in each group. Participants in the usual care group reported higher costs for private counselling/psychotherapy.
| Services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| Private counselling or psychotherapy | 246 | 28.35 (209.26) | 233 | 18.58 (163.14) |
| Private psychiatrist | 246 | – | 233 | 0.02 (0.17) |
| Complementary or alternative therapies | 246 | 13.72 (103.14) | 233 | 17.04 (152.15) |
| Over-the-counter medications or treatments | 246 | 5.47 (41.36) | 233 | 3.63 (23.99) |
| Mean cost for available cases | 246 | 47.54 (247.62) | 233 | 39.28 (222.36) |
| Complete cases | ||||
| Private counselling or psychotherapy | 155 | 35.97 (238.57) | 144 | 30.07 (206.95) |
| Private psychiatrist | 155 | – | 144 | 0.02 (0.19) |
| Complementary or alternative therapies | 155 | 9.35 (69.72) | 144 | 27.58 (193.05) |
| Over-the-counter medications or treatments | 155 | 5.72 (45.42) | 144 | 4.00 (25.34) |
| Mean cost for complete cases | 155 | 51.03 (267.90) | 144 | 61.67 (280.36) |
Participants were also asked to describe other out-of-pocket expenditure on goods or services intended to improve their mental health or emotional problems, such as self-help books, online or computerised CBT programmes, gym membership and exercise classes (Table 84).
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | 246 | 198.90 (522.93) | 233 | 177.11 (426.24) |
| Complete cases | 155 | 241.99 (624.96) | 144 | 162.02 (407.08) |
Participants were asked whether or not they received extra unpaid help from friends or relatives with childcare or household chores, help outside the house (e.g. shopping) or help in other categories because of mental health or emotional problems (Table 85).
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) hours | n | Mean (SD) hours | |
| All available data | 246 | 169.26 (900.72) | 233 | 146.05 (521.69) |
| Complete cases | 155 | 226.79 (1122.57) | 144 | 122.24 (386.11) |
Taken together, the evidence suggests that the intervention group had higher expenditure on private health care (complete cases), lower out-of-pocket expenditure and relied less on external help to support day-to-day activities. It is, however, important to note that, in most of these categories, estimates of variance associated with mean resource use and costs were large.
Loss of earnings and societal costs of lost production
The proportion of participants reporting employment at 4, 8 or 12 months was 46.3%, with a slightly higher proportion employed in the intervention group (48.2%) than in the usual care group (44.3%). Of those reporting employment, the proportion of participants reporting no impact of depression on their employment was 69.5% (SD 0.46%).
The mean number of working days lost is reported in Table 86. The mean number of days lost was higher in the intervention group, although the reported income losses were lower in the available cases for the intervention group (Table 87). A small number of individuals in the intervention arm reported a high number of working days lost. No participant in the usual care group reported > 120 working days lost, whereas 11 individuals in the intervention arm had missed this many days or more of work, with a mean number of days lost among this group of intervention arm participants of 187.4.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | 246 | 5.19 (15.73) | 233 | 12.42 (42.03) |
| Complete cases | 155 | 3.36 (10.58) | 144 | 13.78 (44.93) |
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | 246 | 408.13 (2138.58) | 233 | 334.23 (1547.38) |
| Complete cases | 155 | 253.03 (1691.64) | 144 | 386.67 (1792.54) |
Participants were asked if friends or relatives had taken time off work to care for them because of mental health or emotional problems (including depression). The total estimated time off work is summarised in Table 88.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) working hours | n | Mean (SD) working hours | |
| All available data | 246 | 2.81 (12.40) | 233 | 6.54 (46.66) |
| Complete cases | 155 | 3.25 (13.68) | 144 | 2.03 (10.01) |
Participants also estimated the amount of time taken off work to attend health-care appointments because of mental health or emotional problems (Table 89).
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) hours | n | Mean (SD) hours | |
| All available data | 246 | 2.90 (12.40) | 233 | 12.77 (86.83) |
| Complete cases | 155 | 2.93 (11.66) | 144 | 12.63 (87.59) |
The total time off from work associated with depression and its management can be estimated from the working days missed by trial participants, working hours missed by friends and relatives and time off work to attend appointments. Valuing total hours of work lost by participants and their friends and relatives at the median national wage implies a mean value of lost production of £74 in the usual care group and £242 in the intervention group. The difference in cost was jointly driven by the greater number of working days affected by depression and more hours taken off work to attend health-care appointments in the intervention group.
Disability payments
Participants were asked to disclose if they received disability benefits as a consequence of mental health or emotional problems (including depression). As shown in Table 90, payments were similar in each group.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | 246 | 120.51 (411.41) | 233 | 104.63 (409.24) |
| Complete cases | 155 | 134.06 (483.60) | 144 | 124.24 (486.38) |
Quality-adjusted life-years
Table 91 summarises quality of life and QALYs over the period of follow-up. Except for the data from available cases at baseline, mean quality of life values and QALYs were higher for the intervention group than the usual care group at all follow-up time-points.
| Measure | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| Baseline EQ-5D-5L score | 268 | 0.523 (0.30) | 273 | 0.514 (0.27) |
| EQ-5D-5L score at 4 months | 233 | 0.534 (0.29) | 220 | 0.559 (0.29) |
| EQ-5D-5L score at 8 months | 227 | 0.541 (0.30) | 210 | 0.556 (0.28) |
| EQ-5D-5L score at 12 months | 227 | 0.564 (0.30) | 219 | 0.569 (0.30) |
| Complete cases | ||||
| Baseline EQ-5D-5L score | 155 | 0.519 (0.30) | 144 | 0.535 (0.26) |
| EQ-5D-5L score at 4 months | 155 | 0.520 (0.31) | 144 | 0.591 (0.27) |
| EQ-5D-5L score at 8 months | 155 | 0.529 (0.29) | 144 | 0.592 (0.28) |
| EQ-5D-5L score at 12 months | 155 | 0.556 (0.29) | 144 | 0.580 (0.30) |
| QALYs, unadjusted for baseline difference | ||||
| All available data | 175 | 0.530 (0.27) | 158 | 0.571 (0.26) |
| Complete cases | 155 | 0.529 (0.28) | 158 | 0.580 (0.25) |
| QALYs, adjusted for baseline difference | ||||
| All available data | 175 | 0.536 (0.25) | 158 | 0.567 (0.22) |
| Complete cases | 155 | 0.535 (0.25) | 144 | 0.573 (0.21) |
Costs and quality-adjusted life-years from imputed data
The base-case estimates of cost-effectiveness use imputed data on costs and QALYs. Table 92 summarises imputed data on NHS costs. For reference, the intervention cost variable is reported in Table 93. This variable had no missingness but is reported to allow the sum of NHS costs and NHS/PSS costs to be formed from the total of NHS non-primary care costs, primary care drug costs and primary care consultation costs.
| Imputed costs | n a | Usual care, mean (SE) (£)b | Intervention, mean (SE) (£)b |
|---|---|---|---|
| Imputed mean primary care costs | 609 | 362 (15) | 404 (17) |
| Imputed mean drug costs | 609 | 88 (12) | 92 (10) |
| Imputed mean hospital, ambulance and other non-primary care NHS costs | 609 | 230 (34) | 263 (37) |
| Imputed mean NHS-related costs | 609 | 680 (41) | 872 (46) |
| Imputed mean NHS and PSS costs | 609 | 718 (45) | 886 (47) |
| Outcome | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£)a | n | Mean (SD) (£) | |
| Intervention cost | 302 | 0.38 (7) | 307 | 113 (81) |
Table 94 summarises the imputed unadjusted QALYs (the adjusted QALYs are reported in Table 97).
Summary of findings: depression
Cost–consequences
Table 95 presents a cost–consequence matrix in which costs related to available data in all of the respective categories are reported. Costs are reported to the nearest pound. Consequences are represented by primary and secondary outcomes.
| Costs and outcomes | n | Usual care | n | Intervention | Difference (95% CI) |
|---|---|---|---|---|---|
| Available data on costs (£) | |||||
| Mean cost of NHS resources, including intervention | 188 | 646 | 169 | 845 | –199 (–330 to –58)a |
| Mean cost of PSS resources | 193 | 36 | 171 | 15 | 21 (–2 to 61)a |
| Mean cost of NHS and PSS resources, including intervention | 188 | 683 | 169 | 860 | –177 (–326 to –41)a |
| Mean societal cost per patient of lost production | 246 | 74 | 233 | 242 | –168 (–407 to –60)a |
| Consequencesb | |||||
| PHQ-9 response to treatment (%) | 19 | 27 | OR 1.7 (1.1 to 2.5) | ||
| Adjusted mean PHQ-9 scorec | 11.99 | 11.47 | –0.5 (1.5 to 0.5) | ||
| EQ-5D-5L score at 12 monthsd | 0.564 | 0.569 | –0.005 (–0.062 to 0.050) | ||
| Adjusted QALYsd | 0.536 | 0.567 | –0.031 (–0.078 to 0.021) | ||
The purpose of the cost–consequence analysis is to present a number of difference costs and outcomes in the same table – it does not account for the relationships between costs and outcomes, nor the uncertainty associated with the joint distribution of these variables or the trade-offs between costs and consequences. Subsequent sections will take these considerations into account.
Cost-effectiveness
Cost-effectiveness results are presented from both a NHS and a PSS perspective. The base-case analysis uses the imputed data as the basis for inference, although the results from the complete-case analysis are also presented. Two forms of cost-effectiveness analysis were prespecified. The first was a comparison between costs and the proportion of participants responding to treatment (Table 96). Data in this table indicate that, for imputed NHS costs, an incremental cost of £192 is associated with an increase of 8% in the proportion of patients responding to treatment. These data alone cannot determine whether or not the intervention is cost-effective – they merely indicate the association between mean imputed NHS costs and the proportion of participants responding to treatment. A decision-maker cannot use this information alone to determine whether or not the intervention is cost-effective compared with other possible interventions for depression or whether or not money is better spent in some other area of health care.
| Cost and outcomes | Usual care | Intervention | Difference (95% CI) |
|---|---|---|---|
| Costs (£) | |||
| Per patient imputed NHS costs, mean (SE)a | 680 (41) | 872 (46) | 192 (71 to 313) |
| Per patient NHS/PSS costs, mean (SE)a | 718 (46) | 886 (46) | 168 (43 to 294) |
| Effect | |||
| Proportion responding to treatment, measured using PHQ-9 (%) | 19 | 27 | OR 1.7 (1.1 to 2.5) |
The second analysis therefore compares the NHS and PSS costs with gains in QALYs and presents estimates of the ICER and (incremental) NMB (Table 97). The NMB at a threshold of £20,000 is below zero, as are both bounds on the CI around this estimate. This suggests that the intervention is not cost-effective, a conclusion also evidenced in the probabilities that the intervention is cost-effective.
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) | 680 | 872 | 192 (71 to 313) |
| QALYs | 0.540 | 0.541 | 0.001 (–0.023 to 0.026) |
| Cost-effectiveness statistics | |||
| ICER: £152,590 | |||
| Probability that intervention cost-effective at cost-effectiveness threshold of £20,000: 0.27 | |||
| Probability that intervention cost-effective at cost-effectiveness threshold of £30,000: 0.35 | |||
| NMB at threshold of £20,000 (95% CI): –£167 (–£188 to –£146) | |||
Figure 23 illustrates the uncertainty around the estimates of the ICER and NMB presented in Table 97 by plotting 5000 bootstrap replicates of cost and QALY pairs on the cost-effectiveness plane, whereas Figure 24 illustrates the probability that the intervention is cost-effective at different levels of the cost-effectiveness threshold.
FIGURE 23.
Cost-effectiveness plane from a NHS perspective for the imputed depression model.
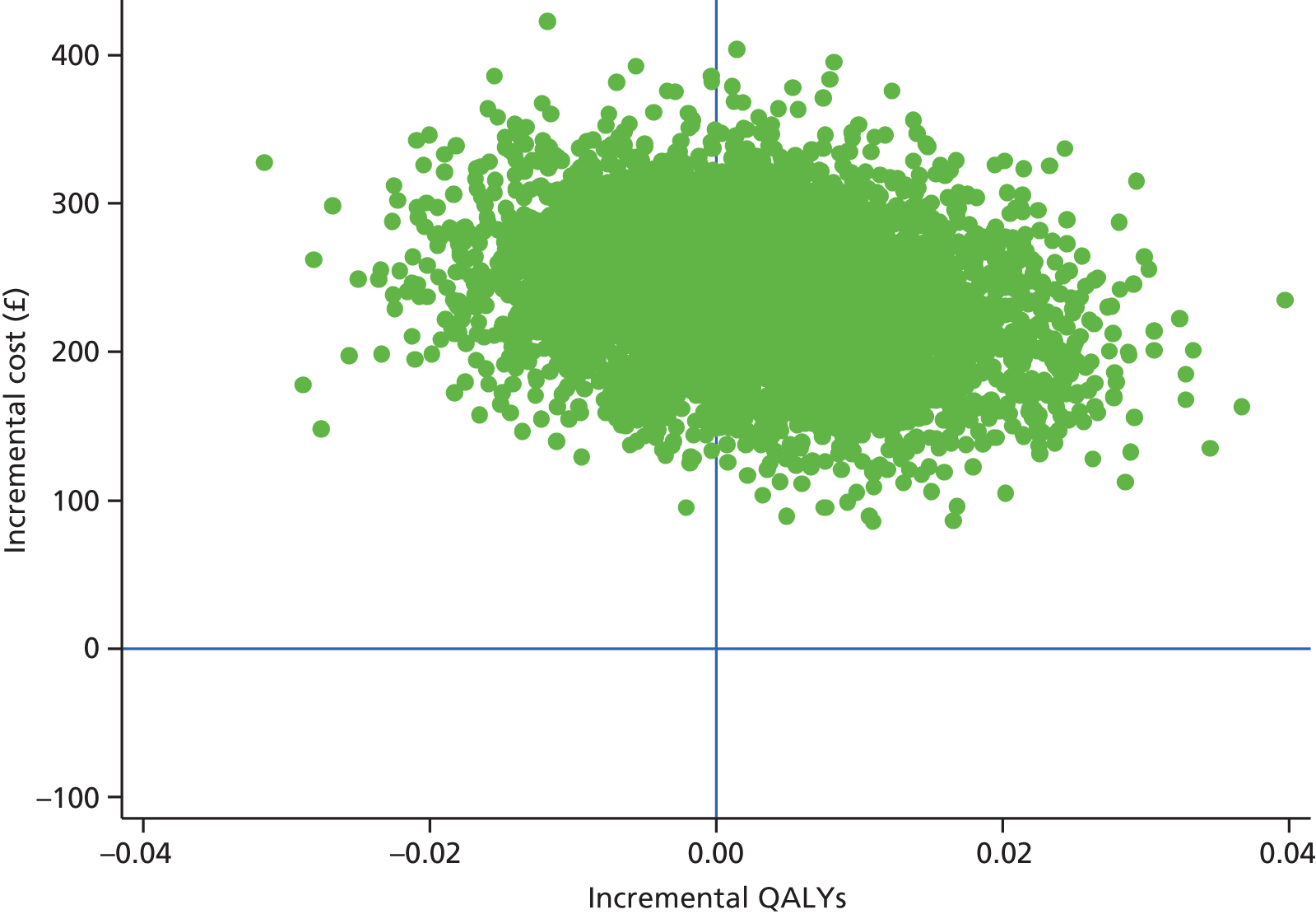
FIGURE 24.
Cost-effectiveness acceptability curve from a NHS perspective for the imputed depression model.
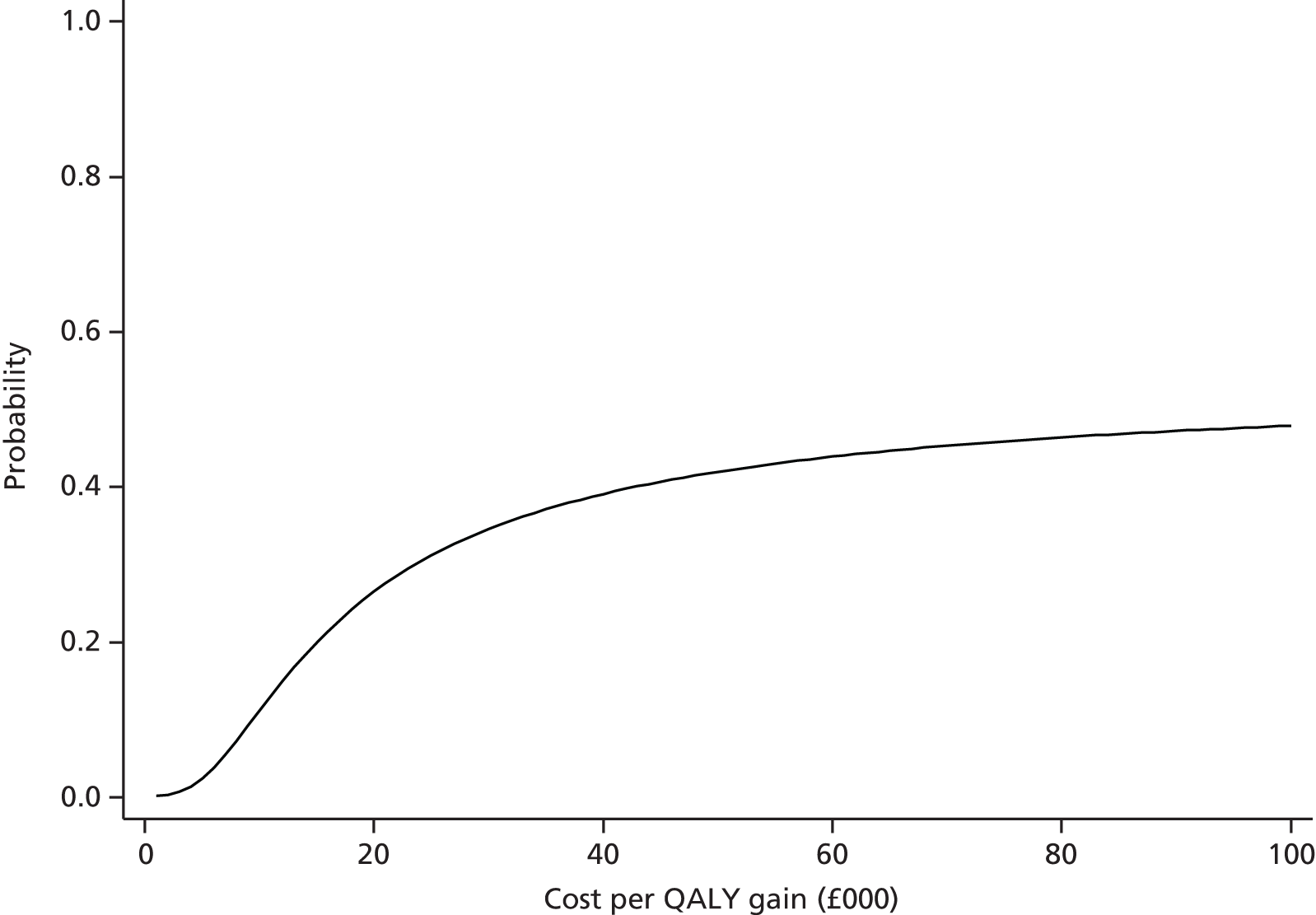
The analysis was also conducted with both PSS and NHS costs included. The results from the cost-effectiveness analysis are summarised in Table 98. The results again indicate that the intervention is not cost-effective. The ICER is slightly lower because the cost difference has narrowed in favour of the intervention, but QALYs remain the same as in the NHS costs-only model.
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS and PSS costs (£) | 718 | 886 | 168 (43 to 294) |
| QALYs | 0.540 | 0.541 | 0.001 (–0.023 to 0.026) |
| Cost-effectiveness statistics | |||
| ICER: £132,630 | |||
| Probability that intervention cost-effective at cost-effectiveness threshold of £20,000: 0.30 | |||
| Probability that intervention cost-effective at cost-effectiveness threshold of £30,000: 0.37 | |||
| NMB at threshold of £20,000 (95% CI): –£143 (–£164 to –£122) | |||
Figure 25 displays the CEAC from the NHS/PSS perspective and again indicates that the intervention is not likely to be cost-effective. Overall, the results from the analyses of imputed data indicate that the intervention is not cost-effective, whether a NHS or a NHS/PSS perspective is adopted.
FIGURE 25.
Cost-effectiveness acceptability curve from a NHS/PSS perspective for the imputed depression model.
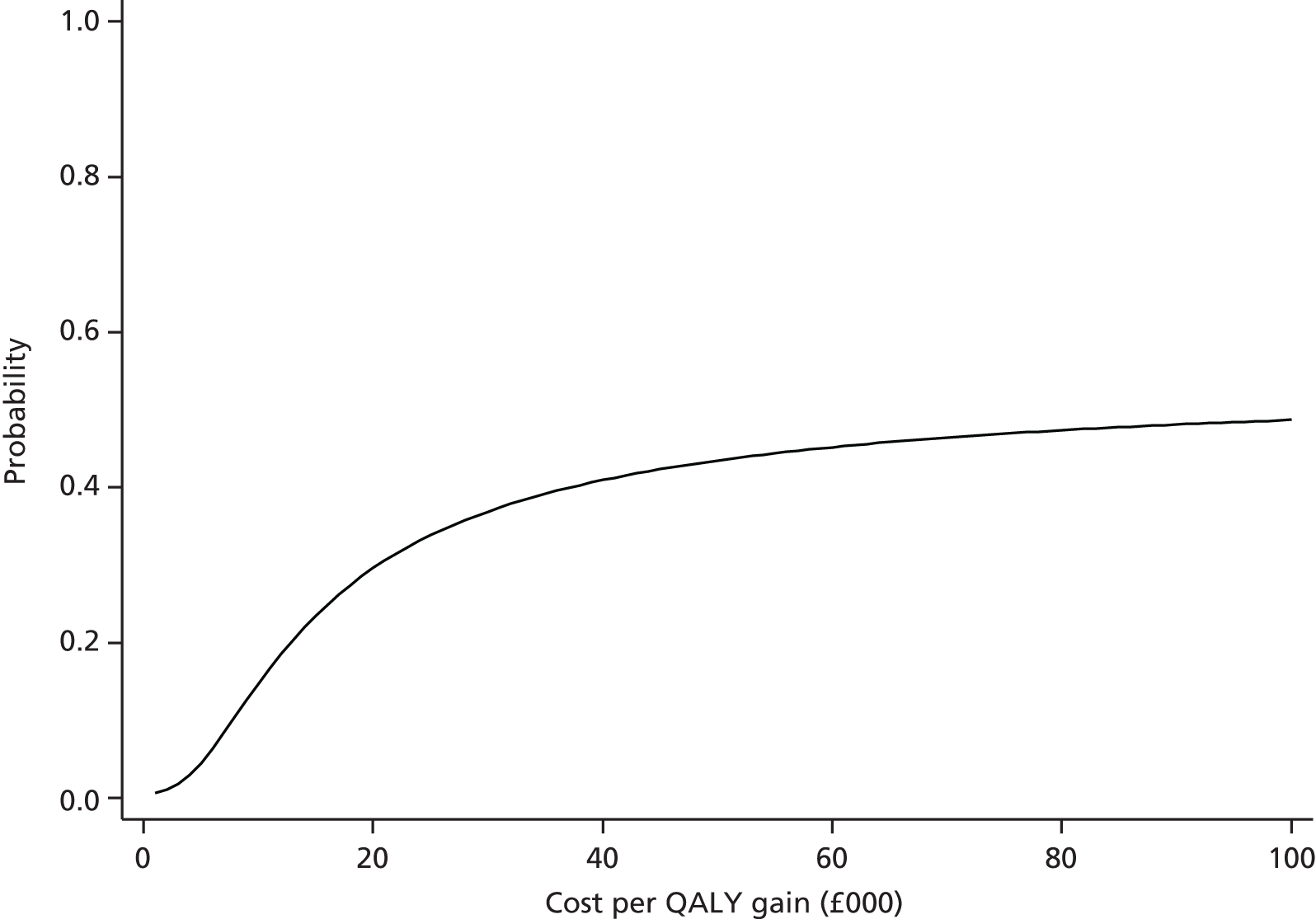
Sensitivity analysis
Complete-case analysis
The analysis was rerun on data from a NHS perspective on complete cases only and the results are summarised in Table 99. Uncertainty around these estimates is represented on the cost-effectiveness plane (Figure 26) and in the CEAC (Figure 27). The complete-case analysis suggests that the intervention is cost-effective with a high probability. It is clear that estimates of cost-effectiveness in the complete-case sample are starkly different from those in the imputed sample.
| Cost and outcomes | Usual care (n = 155), mean | Intervention (n = 144), mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) – complete cases | 674 | 847 | 173 (26 to 320) |
| QALYs – complete cases | 0.535 | 0.573 | 0.037 (0.009 to 0.066) |
| Cost-effectiveness statistics | |||
| ICER: £4593 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.97 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.98 | |||
| NMB at threshold of £20,000 (95% CI): £580 (£545 to £614) | |||
FIGURE 26.
Cost-effectiveness plane from a NHS perspective for the depression complete-case analysis.
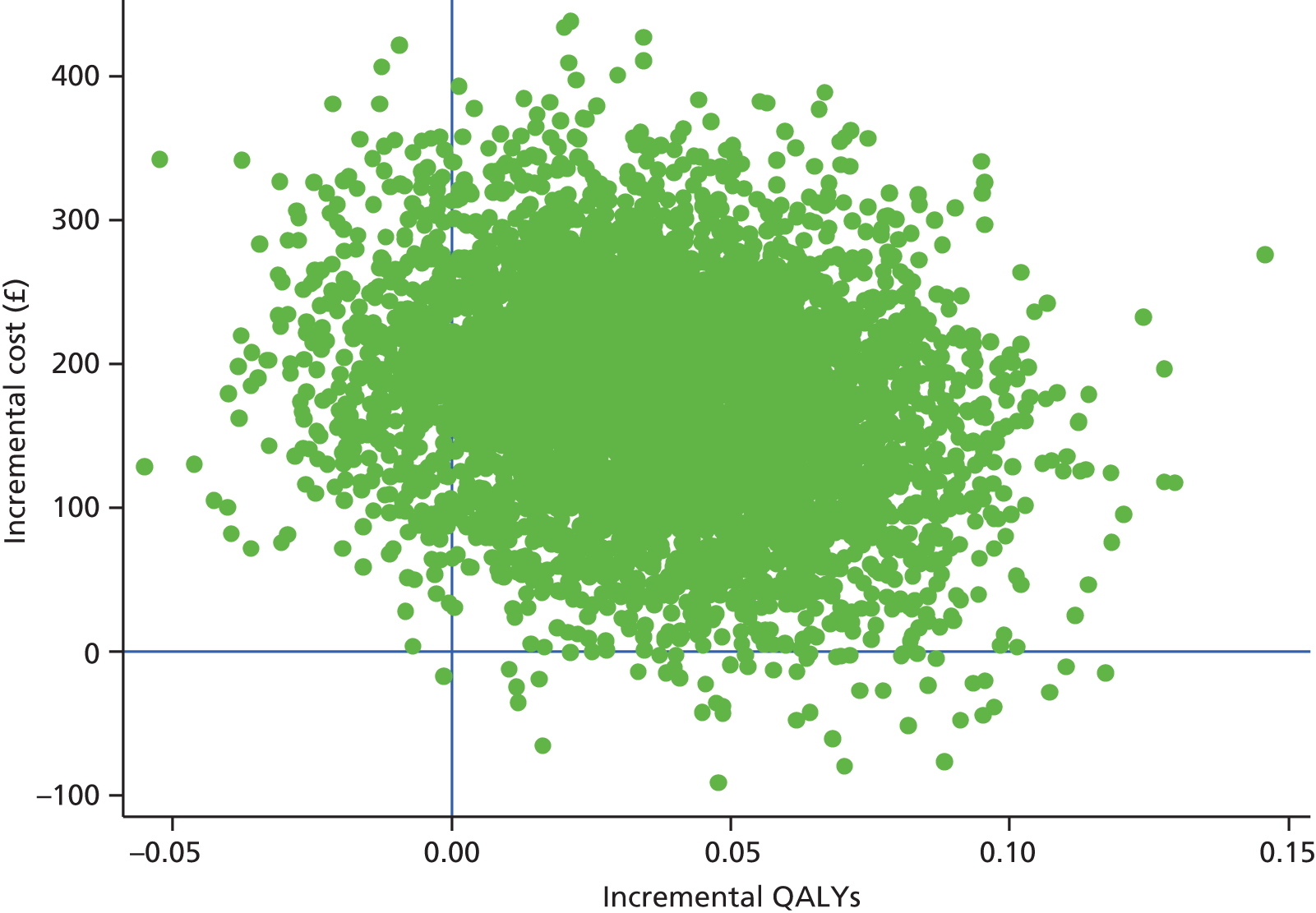
FIGURE 27.
Cost-effectiveness acceptability curve from a NHS perspective for the depression complete-case analysis.
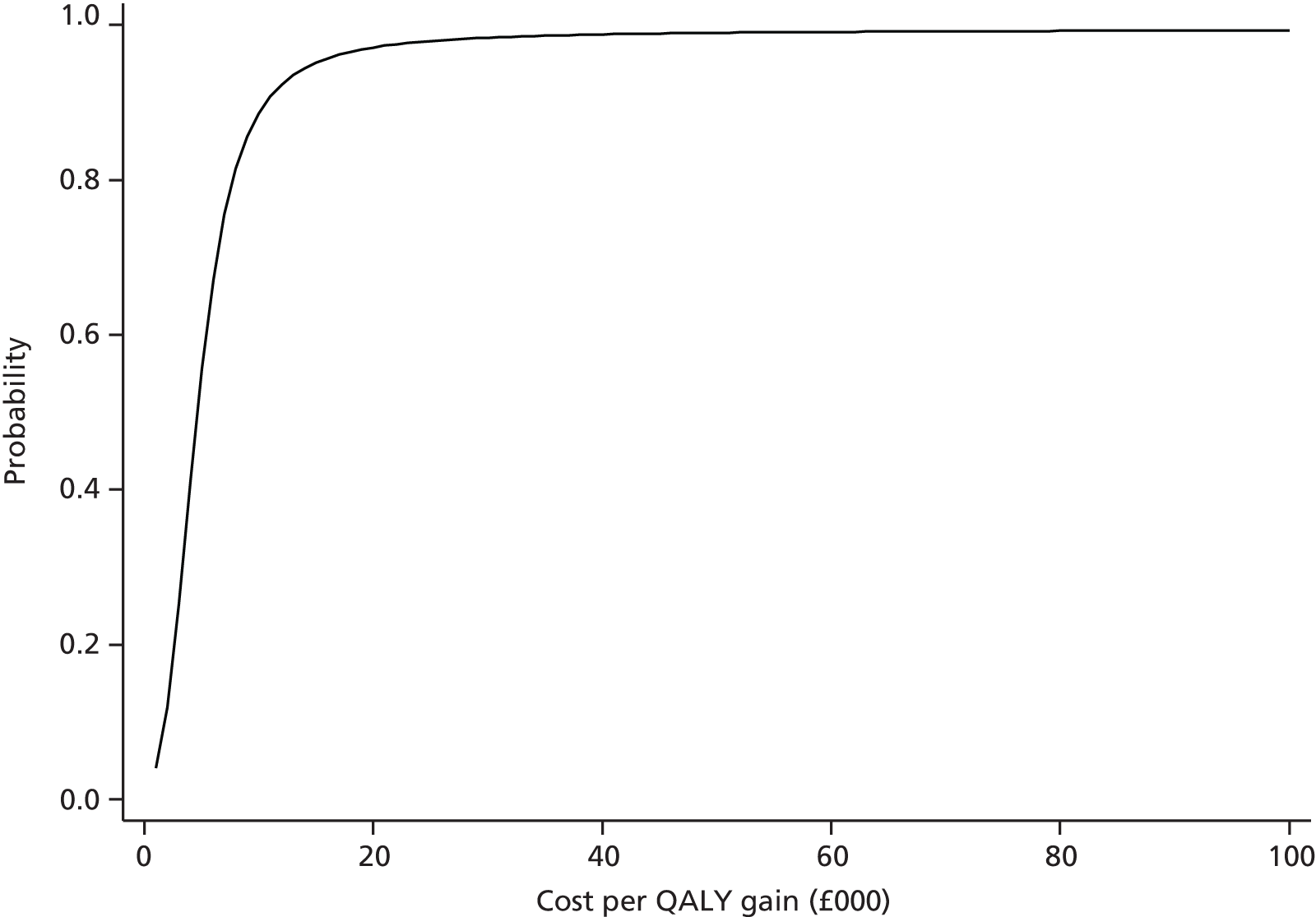
The complete-case analysis is likely to be biased, however, because of differential missingness in quality of life data. Table 100 illustrates how participants with complete utility data at follow-up time point T, but missing utility data at T + 1, have lower utility values at time T and vice versa. This suggests the presence of a substantial bias in the complete-case results. The effect of this bias is to exclude participants with low mean utility scores when conducting complete-case analysis and particularly those with low mean utility scores in the intervention arm. This can be seen in the pattern of utility scores for non-complete cases in Table 100. A complete-case analysis overlooks data from these participants and tends to make the intervention look much more cost-effective than it probably is. In addition to this bias, the complete-case analysis is likely to be inefficient as it uses data on less than half of the randomised participants, resulting in wider CIs around estimated cost-effectiveness statistics.
| Follow-up point | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean | n | Mean | |
| Baseline | ||||
| All available data | 268 | 0.52 | 273 | 0.51 |
| Complete cases | 155 | 0.52 | 144 | 0.54 |
| Non-complete cases | 113 | 0.53 | 129 | 0.49 |
| 4 months | ||||
| All available data | 233 | 0.53 | 220 | 0.56 |
| Complete cases | 155 | 0.52 | 144 | 0.59 |
| Non-complete cases | 78 | 0.56 | 76 | 0.50 |
| 8 months | ||||
| All available data | 227 | 0.54 | 210 | 0.56 |
| Complete cases | 155 | 0.53 | 144 | 0.59 |
| Non-complete cases | 72 | 0.57 | 66 | 0.48 |
| 12 months | ||||
| All available data | 225 | 0.57 | 218 | 0.57 |
| Complete cases | 155 | 0.56 | 144 | 0.58 |
| Non-complete cases | 70 | 0.60 | 74 | 0.56 |
Sensitivity of the results to the inclusion of certain secondary costs
The sensitivity of the imputed results to the inclusion of items of secondary care was tested by removing NHS costs that participants had self-reported (Table 101). These costs involve hospital and ambulance care as well as the use of services such as district nurses and walk-in centres. As these data were collected from participant responses to questionnaires, they may be liable to recall bias and include low-frequency, high-cost events that may not be related to depression but which may have high leverage on cost-effectiveness estimates. Questionnaire responses were reviewed by a clinician to ensure that only relevant resource use was included. Nevertheless, it is useful to examine the sensitivity of the base-case imputed results to these costs. Overall, the effect of removing certain NHS costs from each arm was to narrow the cost difference between arms relative to the base case but to leave QALYs unchanged. This slightly reduced the ICER but the intervention is again not cost-effective under this scenario.
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) | 450 | 608 | 158 (100 to 216) |
| QALYs | 0.540 | 0.541 | 0.001 (–0.023 to 0.026) |
| Cost-effectiveness statistics | |||
| ICER: £139,409 | |||
| Probability that intervention cost-effective at cost-effectiveness threshold of £20,000: 0.30 | |||
| Probability that intervention cost-effective at cost-effectiveness threshold of £30,000: 0.37 | |||
| NMB at threshold of £20,000 (95% CI): –£135 (–£156 to –£115) | |||
Sensitivity of results to certain intervention-related costs
The results were also assessed for their sensitivity to the licence costs for the BWW, which is a component of the intervention cost. This sensitivity analysis was run to establish whether or not a lower cost of this component would affect the cost-effectiveness results on the grounds that licences might be cheaper if the Healthlines Service was deployed throughout the NHS. A separate imputation model was run using a different intervention cost and, hence, there are differences in the costs and QALYs in Table 102 compared with those in Table 97. However, Table 102 again shows that the intervention still does not become cost-effective when this element of the intervention cost is reduced. The difference in costs narrows slightly as the cost of the intervention has become smaller, but the intervention is not cost-effective. In addition, the intervention is not cost-effective if the cost of the BWW licence is assumed to be zero (results not reported).
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) | 682 | 874 | 149 (91 to 207) |
| QALYs | 0.541 | 0.540 | 0.001 (–0.024 to 0.023) |
| Cost-effectiveness statistics | |||
| ICER: £276,302 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.22 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.29 | |||
| NMB at threshold of £20,000 (95% CI): –£206 (–£227 to –£185) | |||
Discussion of depression trial cost-effectiveness results
A total of 51% of participants in the depression trial had missing data on variables necessary to conduct the cost-effectiveness analysis. The base-case results, which use multiple imputation to account for this missingness, indicate that the intervention is not cost-effective. The cost of the intervention did not change drastically between the imputed and the complete-case analysis, indicating that the reason for the high ICERs is the low estimated QALY differences between arms. Estimated QALYs, adjusting for baseline differences and measured using imputed quality of life data, differed by 0.001 between groups. Quantitatively, this difference implies that the intervention group benefited by one-thousandth of a QALY relative to the usual care group, a difference equivalent to less than half a day in better health.
This is in spite of the evidence from the trial that the intervention is clinically effective (as measured by performance on the PHQ-9) and the evidence from the complete-case analysis that the intervention is (apparently) cost-effective. In relation to the former point, it may be that the EQ-5D-5L instrument, which we have used to calculate QALYs over the period of follow-up, is insufficiently sensitive to clinically relevant changes in depression, as measured by the PHQ-9 or the primary outcome in the trial. A recent systematic review390 of the validity and responsiveness of the EQ-5D in depression characterised the performance of the instrument as ‘adequate’ in distinguishing between known groups of patients, but it is less responsive to changes in depression over time. Nevertheless, it is not apparent that other generic measures of health-related quality of life would have offered greater responsiveness in calculating QALYs for the participants in the Healthlines depression trial.
Given the level of missing data in the trial, and evidence of differential missingness between arms with respect to completion of quality of life questions, the complete-case analysis seems likely to be biased by differences between groups in responses to the EQ-5D-5L. The complete-case results should not be used as a basis for claims about the cost-effectiveness of the intervention in the depression trial. In conclusion, there is no evidence available from the depression trial to suggest that the intervention is cost-effective.
Cardiovascular disease risk trial
This section presents findings from the CVD risk trial. Except when otherwise stated, the same methodologies were used as in the depression trial.
NHS resource use in the cardiovascular disease risk trial
Intervention for cardiovascular disease risk participants
Information about the number of telephone calls made by the HIAs to CVD risk participants and their duration, as well as the number of participants provided with a blood pressure monitor, is shown in Table 103.
| Intervention elements | All available data (n = 325), mean (SD) | Complete cases (n = 262), mean (SD) |
|---|---|---|
| Encounter calls | ||
| Number | 9.99 (3.99) | 10.45 (3.49) |
| Total duration (hours) | 2.99 (1.37) | 3.16 (1.27) |
| Non-scheduled calls | ||
| Number | 1.13 (1.07) | 1.18 (1.03) |
| Total duration (hours) | 0.04 (0.07) | 0.04 (0.07) |
| All calls | ||
| Number | 11.12 (4.40) | 11.64 (3.76) |
| Total duration (hours) | 3.03 (1.38) | 3.20 (1.27) |
| Blood pressure monitor | ||
| Participants, n (%) | 164 (50.5) | 132 (50.4) |
The mean total length of calls for all participants was 3 hours 2 minutes, with a maximum length of 7 hours 13 minutes (15 calls). Around half of the participants were provided with a blood pressure monitor, 10% of whom also needed an extra-large cuff. Participants in the complete-case analysis had more telephone calls on average than the whole sample and these were, on average, slightly longer. A similar percentage used a blood pressure monitor.
The cost of the intervention is shown in Table 104 for all participants and for the complete cases (i.e. those for whom we had complete NHS and PSS cost and QALY data). The cost of the telephone calls accounted for 85% of the total mean cost of £129 and the blood pressure monitors accounted for the remaining 15%. The mean cost for complete cases was £6 (5%) higher than that for the whole sample.
| Intervention elements | All available data (n = 325), mean (SD) (£) | Complete cases (n = 262), mean (SD) (£) |
|---|---|---|
| Encounter calls | 108.80 (49.75) | 114.68 (46.07) |
| Non-scheduled calls | 1.39 (2.57) | 1.47 (2.65) |
| All calls | 110.20 (50.13) | 116.15 (46.24) |
| Blood pressure monitor | 18.92 (18.78) | 18.89 (18.79) |
| Total cost per participant | 129.12 (56.33) | 135.04 (53.02) |
Primary care consultations
The number and costs of primary care consultations are summarised in Tables 105 and 106 respectively. There was slightly higher usage of primary care services other than GP consultations in the usual care group, but GP use was higher in the intervention group when considering all available data. Costs were similar between each group.
| Primary care services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Intervention mean (SD) | |
| All available data | ||||
| GP consultations | 313 | 5.30 (4.53) | 325 | 5.48 (4.38) |
| Nurse consultations | 313 | 3.03 (4.31) | 325 | 2.79 (2.93) |
| Other primary care consultations | 313 | 3.09 (3.91) | 325 | 3.01 (3.95) |
| Complete cases | ||||
| GP consultations | 266 | 5.21 (4.42) | 262 | 5.09 (3.79) |
| Nurse consultations | 266 | 3.06 (4.50) | 262 | 2.83 (3.05) |
| Other primary care consultations | 266 | 3.14 (3.87) | 262 | 3.05 (4.18) |
| Primary care services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| GP consultations | 313 | 168.92 (143.25) | 325 | 172.48 (131.77) |
| Nurse consultations | 313 | 33.82 (48.34) | 325 | 31.12 (32.68) |
| Other primary care consultations | 313 | 19.14 (26.68) | 325 | 19.13 (26.00) |
| Total primary care cost | 313 | 221.88 (166.20) | 325 | 222.73 (150.70) |
| Complete cases | ||||
| GP consultations | 266 | 165.46 (137.19) | 262 | 160.74 (116.66) |
| Nurse consultations | 266 | 34.27 (50.53) | 262 | 31.66 (34.06) |
| Other primary care consultations | 266 | 19.27 (25.94) | 262 | 19.35 (27.45) |
| Total primary care cost | 266 | 219.00 (164.17) | 262 | 211.76 (138.99) |
Prescribed medication
Table 107 summarises the use and costs of prescribed medication. Overall costs are similar between the groups.
| Community services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| No. of prescribed items | ||||
| All available data | 313 | 20.83 (21.41) | 325 | 20.52 (20.79) |
| Complete cases | 266 | 21.08 (21.40) | 262 | 20.91 (21.33) |
| Cost of prescribed medication (£) | ||||
| All available data | 313 | £67.38 (136.56) | 325 | £66.59 (102.38) |
| Complete cases | 266 | £67.78 (139.04) | 262 | £65.21 (102.51) |
As with the depression trial results, the following sections about resource use and costs relate primarily to data obtained from participant-reported questionnaire responses. Most of the NHS-related non-primary care services described henceforth are also based on self-report data.
NHS community-based services
Information was collected on contacts (Table 108) and costs (Table 109) associated with certain types of NHS community services. In general, slightly higher costs were associated with the use of these services in the intervention arm than in the usual care arm.
| Community services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| District nurse home visit | 290 | 0.07 (0.52) | 292 | 0.08 (1.18) |
| District nurse telephone consultation | 290 | 0.01 (0.08) | 292 | 0.09 (0.66) |
| NHS counsellor/psychologist clinic visit | 290 | 0.08 (0.61) | 292 | 0.10 (0.81) |
| NHS counsellor/psychologist telephone consultation | 290 | – | 292 | 0.16 (0.92) |
| NHS walk-in centre clinic visit | 290 | 0.21 (1.09) | 292 | 0.17 (1.33) |
| NHS walk-in centre telephone consultation | 290 | 0.02 (0.23) | 292 | 0.07 (0.69) |
| GP out-of-hours service clinic visit | 290 | 0.03 (0.25) | 292 | 0.05 (0.36) |
| GP out-of-hours service home visit | 290 | 0.01 (0.08) | 292 | 0.01 (0.13) |
| GP out-of-hours service telephone consultation | 290 | 0.05 (0.35) | 292 | 0.11 (0.70) |
| Complete cases | ||||
| District nurse home visit | 266 | 0.05 (0.45) | 262 | 0.01 (0.12) |
| District nurse telephone consultation | 266 | 0.00 (0.06) | 262 | 0.10 (0.69) |
| NHS counsellor/psychologist clinic visit | 266 | 0.08 (0.63) | 262 | 0.08 (0.77) |
| NHS counsellor/psychologist telephone consultation | 266 | – | 262 | 0.18 (0.97) |
| NHS walk-in centre clinic visit | 266 | 0.23 (1.13) | 262 | 0.16 (1.32) |
| NHS walk-in centre telephone consultation | 266 | 0.03 (0.24) | 262 | 0.06 (0.72) |
| GP out-of-hours service clinic visit | 266 | 0.03 (0.27) | 262 | 0.04 (0.29) |
| GP out-of-hours service home visit | 266 | 0.01 (0.09) | 262 | 0.01 (0.12) |
| GP out-of-hours service telephone consultation | 266 | 0.05 (0.36) | 262 | 0.10 (0.72) |
| Community services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| District nurse home visit | 285 | 4.21 (31.56) | 285 | 4.63 (71.41) |
| District nurse telephone consultation | 285 | 0.07 (0.88) | 285 | 0.96 (6.98) |
| NHS counsellor/psychologist clinic visit | 285 | 4.48 (35.48) | 285 | 5.90 (47.45) |
| NHS counsellor/psychologist telephone consultation | 285 | – | 285 | 2.65 (14.72) |
| NHS walk-in centre clinic visit | 285 | 7.29 (37.37) | 285 | 5.98 (45.88) |
| NHS walk-in centre telephone consultation | 285 | 0.55 (5.08) | 285 | 1.33 (15.38) |
| GP out-of-hours service clinic visit | 285 | 1.07 (8.73) | 285 | 1.67 (12.32) |
| GP out-of-hours service home visit | 285 | 0.60 (7.11) | 285 | 0.89 (11.24) |
| GP out-of-hours service telephone consultation | 285 | 0.91 (6.96) | 285 | 2.18 (14.22) |
| Total costs for available cases | 285 | 19.18 (63.69) | 285 | 26.19 (117.03) |
| Complete cases | ||||
| District nurse home visit | 266 | 3.16 (26.90) | 262 | 0.46 (7.41) |
| District nurse telephone consultation | 266 | 0.04 (0.64) | 262 | 1.04 (7.28) |
| NHS counsellor/psychologist clinic visit | 266 | 4.80 (36.71) | 262 | 4.87 (44.57) |
| NHS counsellor/psychologist telephone consultation | 266 | – | 262 | 2.83 (15.31) |
| NHS walk-in centre clinic visit | 266 | 7.68 (38.61) | 262 | 5.46 (44.89) |
| NHS walk-in centre telephone consultation | 266 | 0.59 (5.26) | 262 | 1.44 (16.04) |
| GP out-of-hours service clinic visit | 266 | 1.15 (9.03) | 262 | 1.30 (9.79) |
| GP out-of-hours service home visit | 266 | 0.64 (7.36) | 262 | 0.65 (10.50) |
| GP out-of-hours service telephone consultation | 266 | 0.98 (7.20) | 262 | 2.06 (14.34) |
| Total costs for complete cases | 266 | 19.03 (63.38) | 262 | 20.11 (93.07) |
Hospital and ambulance use
Resource use and costs associated with different types of hospital visit and ambulance use are reported in Tables 110 and 111 respectively. Resource use was higher in the usual care arm and this is reflected in the higher mean cost per participant of hospital and ambulance care in the usual care arm.
| Services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| Overnight hospital stay | 296 | 0.01 (0.10) | 297 | 0.01 (0.12) |
| Day care | 296 | 0.03 (0.18) | 297 | 0.01 (0.08) |
| Outpatient clinic | 293 | 0.04 (0.33) | 294 | 0.04 (0.26) |
| Accident and emergency | 298 | 0.01 (0.10) | 299 | 0.01 (0.14) |
| Other hospital services | 297 | 0.01 (0.12) | 299 | 0.01 (0.10) |
| Ambulance use | 294 | 0.01 (0.08) | 296 | 0.01 (0.10) |
| Complete cases | ||||
| Overnight hospital stay | 266 | 0.01 (0.11) | 262 | 0.02 (0.12) |
| Day care | 266 | 0.03 (0.19) | 262 | 0.01 (0.09) |
| Outpatient clinic | 266 | 0.04 (0.35) | 262 | 0.04 (0.27) |
| Accident and emergency | 266 | 0.01 (0.11) | 262 | 0.02 (0.15) |
| Other hospital services | 266 | 0.02 (0.12) | 262 | 0.01 (0.09) |
| Ambulance use | 266 | 0.01 (0.09) | 262 | 0.01 (0.11) |
| Services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| Overnight hospital stay | 296 | 6.31 (63.35) | 297 | 24.73 (333.73) |
| Day care | 298 | 15.68 (153.05) | 299 | 1.08 (13.46) |
| Outpatient clinic | 293 | 11.44 (143.49) | 295 | 4.82 (30.34) |
| Accident and emergency | 298 | 0.64 (7.83) | 299 | 1.28 (13.53) |
| Other hospital services | 297 | 1.12 (13.82) | 299 | 1.31 (15.98) |
| Ambulance use | 294 | 1.73 (21.00) | 296 | 2.58 (25.58) |
| Mean cost for available casesa | 283 | 38.61 (346.27) | 293 | 37.55 (352.79) |
| Complete cases | ||||
| Overnight hospital stay | 266 | 7.03 (66.80) | 262 | 28.03 (355.27) |
| Day care | 266 | 17.56 (161.92) | 262 | 1.23 (14.37) |
| Outpatient clinic | 266 | 12.60 (150.57) | 262 | 4.94 (31.27) |
| Accident and emergency | 266 | 0.72 (8.29) | 262 | 1.46 (14.45) |
| Other hospital services | 266 | 1.25 (14.60) | 262 | 0.75 (12.09) |
| Ambulance use | 266 | 1.92 (22.07) | 262 | 2.92 (27.18) |
| Mean cost for complete cases | 266 | 41.08 (357.06) | 262 | 39.34 (366.13) |
Costs to participants and their friends and family
Private health-care costs and out-of-pocket expenditures
Participants were asked to report on their use of certain types of private health-care services relating to CVD risk factors. Participants were asked to describe paid care not provided by NHS HCPs (Table 112). The difference between groups is largely attributable to costs pertaining to exercise equipment purchases in the usual care arm, in which large costs (> £1000) were reported by three individuals. In contrast, only one participant in the intervention group reported costs of > £1000.
| Services | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | ||||
| Private counselling or psychotherapy | 298 | 0.50 (8.69) | 299 | 0.43 (7.52) |
| Private nutritionist or dietitian | 298 | 0.13 (2.32) | 299 | 0.40 (6.94) |
| Complementary or alternative therapies | 298 | 12.23 (73.53) | 299 | 6.14 (35.63) |
| Over-the-counter treatments | 298 | 8.02 (34.42) | 299 | 6.86 (30.78) |
| Exercise equipment | 298 | 62.06 (447.04) | 299 | 23.14 (138.47) |
| Wii Fit or similar products | 298 | 1.17 (14.13) | 299 | 2.68 (32.20) |
| Other | 298 | 26.31 (165.64) | 299 | 19.83 (120.41) |
| Mean cost for available cases | 298 | 110.44 (518.78) | 299 | 59.48 (194.72) |
| Complete cases | ||||
| Private counselling or psychotherapy | 266 | 0.56 (9.20) | 262 | – |
| Private nutritionist or dietitian | 266 | – | 262 | 0.46 (7.41) |
| Complementary or alternative therapies | 266 | 8.49 (51.06) | 262 | 6.63 (37.71) |
| Over-the-counter treatments | 266 | 8.73 (36.24) | 262 | 7.21 (32.04) |
| Exercise equipment | 266 | 69.39 (472.73) | 262 | 25.80 (147.67) |
| Wii Fit or similar products | 266 | 0.56 (8.60) | 262 | 2.82 (34.21) |
| Other | 266 | 25.04 (172.02) | 262 | 21.52 (127.35) |
| Mean cost for complete cases | 266 | 112.78 (543.38) | 262 | 64.44 (206.38) |
Participants were also asked to describe other out-of-pocket expenditure intended to improve CVD risk factors on goods or services such as self-help books, quitting smoking groups, gym membership and exercise classes (Table 113).
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | 298 | 64.10 (166.15) | 299 | 78.76 (255.10) |
| Complete cases | 266 | 65.78 (168.58) | 262 | 84.57 (265.81) |
Loss of earnings and societal costs of lost production
The proportion of participants reporting employment at either 6 or 12 months was 25.2%, with a slightly higher proportion employed in the intervention arm (25.8%) than in the usual care arm (24.7%). Of those reporting employment, the proportion of participants reporting no impact of CVD risk factors on their employment was 95.1% (SD 0.22%). Working days lost because of CVD risk factors over the course of 12 months of follow-up are reported in Table 114.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | 298 | 0.79 (6.19) | 299 | 0.53 (5.06) |
| Complete cases | 266 | 0.78 (6.30) | 262 | 0.41 (4.47) |
The mean number of lost days was slightly higher in the usual care group, albeit the majority of participants in the trial were not in employment and only 14 participants (usual care, n = 9; intervention, n = 5) provided estimates greater than zero of the number of working days lost. Table 115 provides an estimate of the impact of CVD risk factors on lost income, which was again greater in the usual care group.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| All available data | 298 | 17.03 (197.66) | 299 | 3.01 (52.05) |
| Complete cases | 266 | 13.06 (185.16) | 262 | – |
Participants were also asked to report whether or not they took time off work to attend health-care appointments related to any of the CVD risk factors, such as hypertension (Table 116). When considering the complete cases, those in the intervention group reported slightly less time off work to attend such appointments than those in the usual care group.
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) hours | n | Mean (SD) hours | |
| All available data | 298 | 10.23 (68.47) | 299 | 12.46 (91.76) |
| Complete cases | 266 | 10.79 (72.02) | 262 | 9.87 (72.19) |
Disability payments
Participants were asked to disclose if they received benefits as a consequence of having high blood pressure, having a high cholesterol level, smoking or being overweight (Table 117).
| Analysis | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| Available data | 298 | 4.18 (30.09) | 299 | 0.67 (6.89) |
| Complete cases | 266 | 1.71 (16.80) | 262 | 0.77 (7.36) |
Quality-adjusted life-years
Table 118 summarises quality of life and QALY data for the 12 months of follow-up. The intervention group mean utility was higher at baseline and at all follow-up time points.
| Quality of life and QALYs | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| All available data | ||||
| Baseline EQ-5D-5L | 301 | 0.770 (0.189) | 309 | 0.806 (0.173) |
| EQ-5D-5L at 6 months | 301 | 0.774 (0.180) | 300 | 0.821 (0.177) |
| EQ-5D-5L at 12 months | 297 | 0.777 (0.200) | 295 | 0.811 (0.171) |
| QALYs, unadjusted for baseline difference | ||||
| All available data | 279 | 0.775 (0.170) | 295 | 0.817 (0.161) |
| Complete cases | 266 | 0.774 (0.172) | 262 | 0.815 (0.163) |
| QALYs, adjusted for baseline difference | ||||
| All available data | 279 | 0.788 (0.156) | 275 | 0.799 (0.135) |
| Complete cases | 266 | 0.788 (0.158) | 262 | 0.800 (0.135) |
Costs and quality-adjusted life-years from imputed data
The base-case estimates of cost-effectiveness use imputed data on costs and QALYs. Table 119 summarises imputed data on NHS costs. Total health-care costs are defined as the sum of primary care costs, medication costs and all hospital and NHS-related health-care costs, which were jointly provided from questionnaire and primary care record data, as well as the costs of the Healthlines intervention itself. For reference, the intervention cost variable is reported in Table 120 and the imputed unadjusted QALYs are provided in Table 121.
| Costs | n a | Usual care, mean (SE) (£)b | Intervention, mean (SE) (£)b |
|---|---|---|---|
| Imputed mean hospital, ambulance and other non-primary care NHS costs | 641 | 56 (19) | 65 (22) |
| Imputed mean drug costs | 641 | 67 (8) | 67 (6) |
| Imputed mean primary care costs | 641 | 241 (11) | 242 (9) |
| Imputed mean NHS-related costs | 641 | 364 (26) | 502 (27) |
| Outcome | Usual care | Intervention | ||
|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | |
| Intervention cost | – | – | 325 | 129 (56) |
Summary of findings: cardiovascular disease risk
Cost–consequences
Table 122 presents a cost–consequence matrix in which costs related to available data in all of the respective categories are reported. Costs are reported to the nearest pound. Consequences are represented by primary and secondary outcomes.
| Cost and outcomes | n | Usual care | n | Intervention | Difference (95% CI) |
|---|---|---|---|---|---|
| Costs and outcomes | |||||
| Mean cost of intervention | – | – | 325 | 129 | – |
| Mean cost of NHS resources, including intervention | 283 | 361 | 283 | 494 | 132 (–214 to –50)a |
| Mean societal cost per patient of lost production | 298 | 76 | 299 | 52 | 23 (–50 to 121)a |
| Consequencesb | |||||
| QRISK2 response to treatment (%) | 43 | 50 | OR 1.3 (1.0 to 1.9) | ||
| Adjusted difference in mean QRISK2 scorec | 31.41 | 30.98 | –0.4 (–1.2 to 0.3) | ||
| EQ-5D-5L at 12 monthsd | 0.776 | 0.812 | –0.037 (–0.066 to –0.007) | ||
| Adjusted QALYsd | 0.788 | 0.799 | –0.011 (–0.035 to 0.142) | ||
Cost-effectiveness
No resource use from the PSS sector was recorded in the CVD risk trial and hence the cost-effectiveness results are presented from a NHS perspective only. The base-case analysis uses imputed data although the results from the complete-case analysis are also presented as a sensitivity analysis to the main results. Two forms of cost-effectiveness analysis were prespecified. The first compares costs to the NHS with the proportion of participants responding to treatment (Table 123). Data in this table indicate that the expenditure of an additional £138 per participant in the intervention group is associated with an increase of 7% in the proportion of participants responding to treatment. Again, as in the depression trial, this does not account for the relationship between costs and effects and hence the second analysis compares incremental costs to the NHS with gains in QALYs and presents estimates of the ICER and NMB. The ICER and NMB from a health system perspective were obtained from a regression analysis (using seemingly unrelated regression) of the imputed trial data (Table 124).
| Cost and outcomes | Usual care | Intervention | Difference (95% CI) |
|---|---|---|---|
| Costs (£) | |||
| Per patient imputed NHS costs, mean (SE)a | 364 (26) | 502 (27) | 138 (66 to 211) |
| Effect | |||
| Proportion responding to treatment, measured using QRISK2 (%) | 43 | 50 | OR 1.3 (1.0 to 1.9) |
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) | 364 | 502 | 138 (66 to 211) |
| Adjusted QALYs | 0.786 | 0.798 | 0.012 (–0.001 to 0.026) |
| Cost-effectiveness statistics | |||
| ICER: £10,859 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.77 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.87 | |||
| NMB at threshold of £20,000 (95% CI): £116 (£105 to £128) | |||
Figure 28 illustrates the uncertainty around the estimates of the ICER and NMB presented in Table 124 by plotting 5000 bootstrap replicates of cost and QALY pairs on the cost-effectiveness plane, whereas Figure 29 illustrates the probability that the intervention is cost-effective at different levels of the cost-effectiveness threshold. The results indicate that the intervention is probably cost-effective. The estimated net benefit is positive and the estimated probabilities that the intervention is cost-effective at threshold values of £20,000–30,000 are well above 0.5.
FIGURE 28.
Cost-effectiveness plane from a NHS perspective for the imputed CVD risk model.
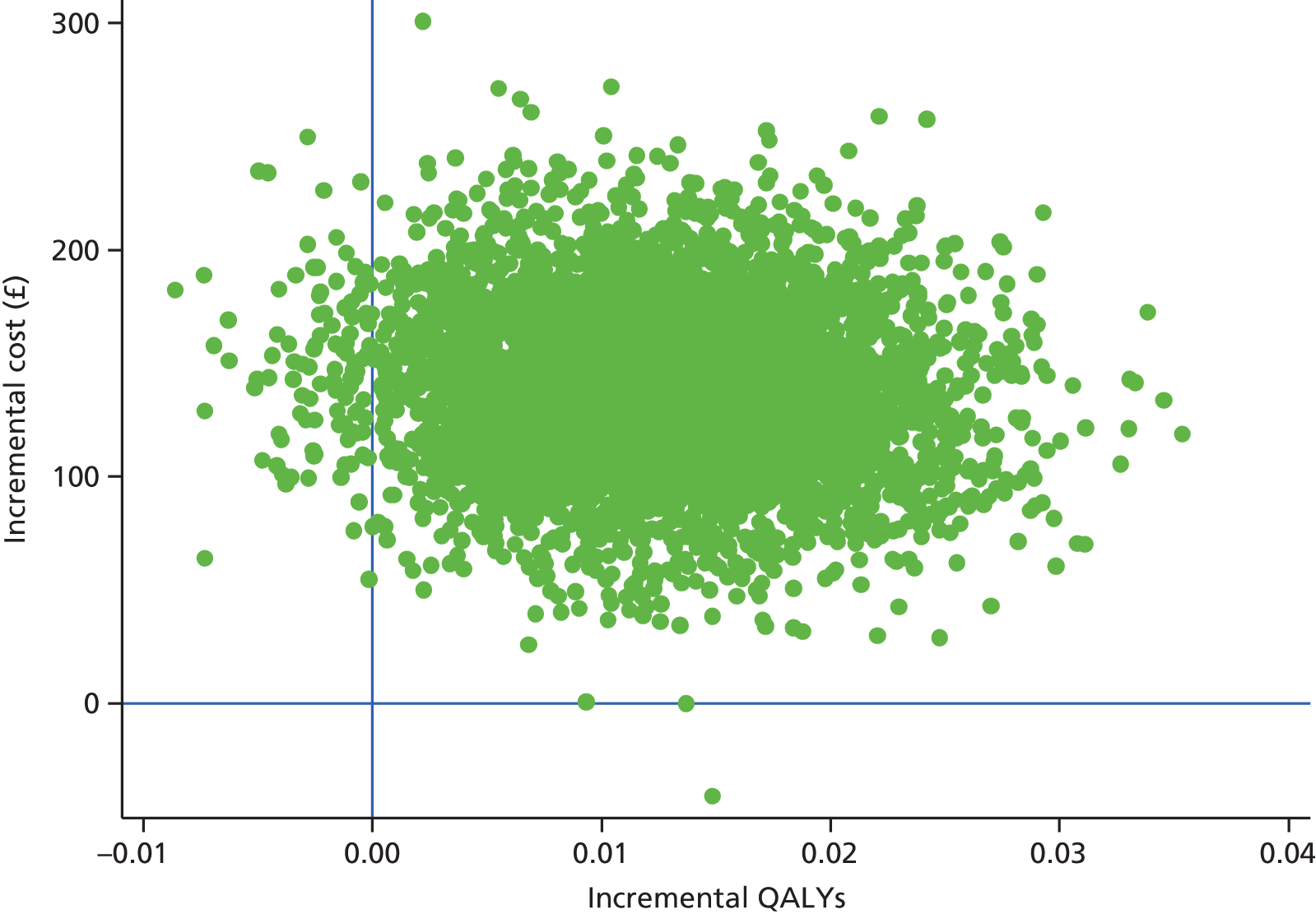
FIGURE 29.
Cost-effectiveness acceptability curve from a NHS perspective for the imputed CVD risk model.

Sensitivity analysis
Complete-case analysis
As in the base-case imputed data set, the costs of the intervention were compared with the proportion of participants responding to treatment (Table 125). The results in this table indicate that additional costs of £124 are associated with a 7% increase in the proportion of participants responding to treatment.
| Cost and outcomes | Usual care | Intervention | Difference (95% CI) |
|---|---|---|---|
| Costs (£) | |||
| Per patient NHS costs – complete cases, mean (SD) | 367 (489) | 490 (473) | 124 (42 to 206) |
| Effect | |||
| Proportion responding to treatment (%) | 43 | 50 | OR 1.3 (1.0 to 1.9) |
Next, the cost-effectiveness analysis was rerun using data from complete cases only (Table 126). The cost-effectiveness results from the complete-case sample are somewhat more favourable to the intervention than the imputed results. This is driven by both lower incremental costs and a similar incremental QALY gain. The complete-case results are similar to those using the imputed data, which is consistent with the relatively high proportion of complete cases in the CVD risk trial (82% of all participants randomised).
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) – complete cases | 367 | 490 | 124 (42 to 206) |
| QALYs – complete cases | 0.788 | 0.800 | 0.011 (–0.001 to 0.025) |
| Cost-effectiveness statistics | |||
| ICER: £10,366 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.79 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.87 | |||
| NMB at threshold of £20,000 (95% CI): £115 (£103 to £127) | |||
Uncertainty around these estimates is represented in the cost-effectiveness plane (Figure 30) and in the CEAC (Figure 31).
FIGURE 30.
Cost-effectiveness plane from a NHS perspective for the CVD risk complete-case analysis.
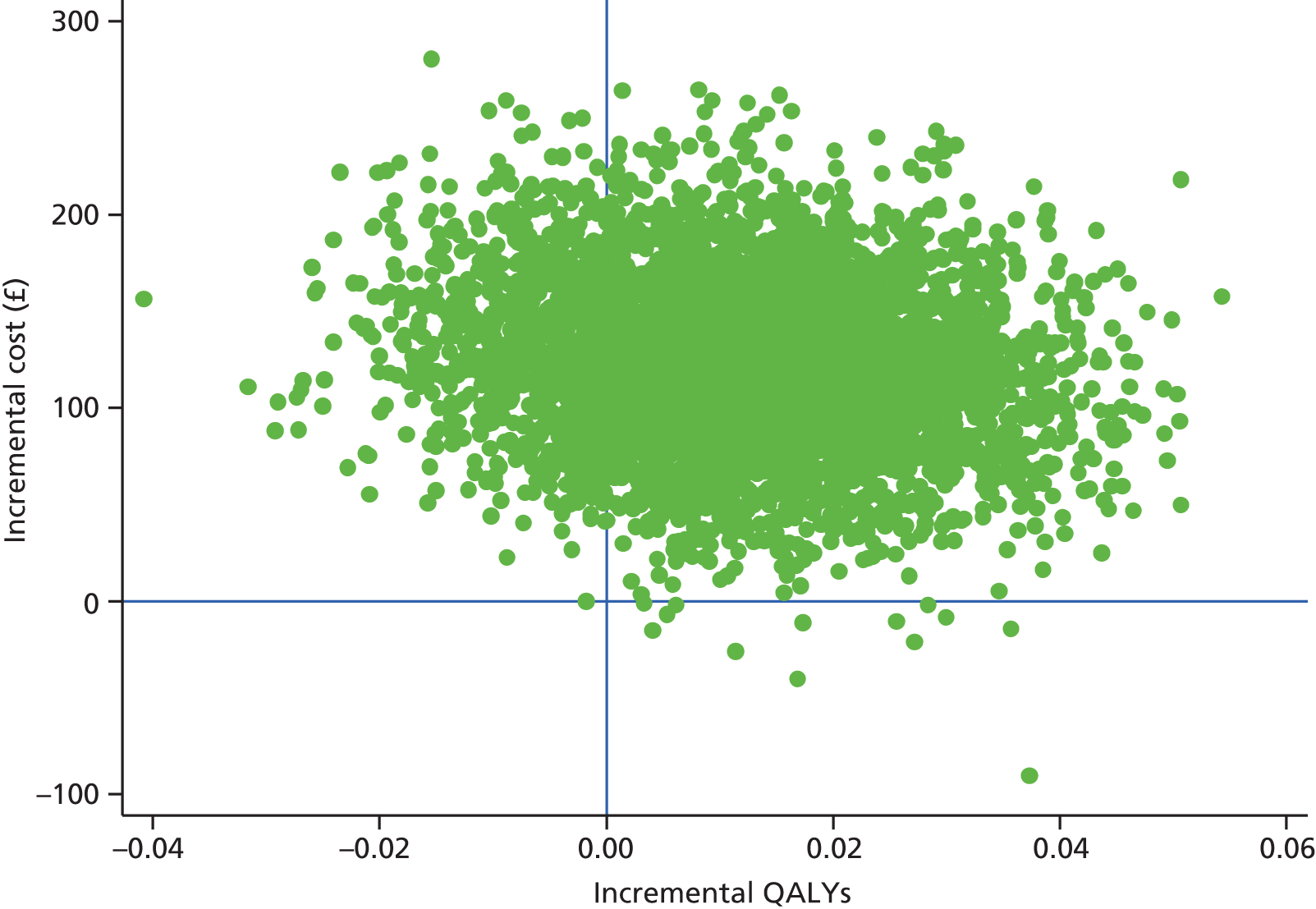
FIGURE 31.
Cost-effectiveness acceptability curve from a NHS perspective for the CVD risk complete-case analysis.
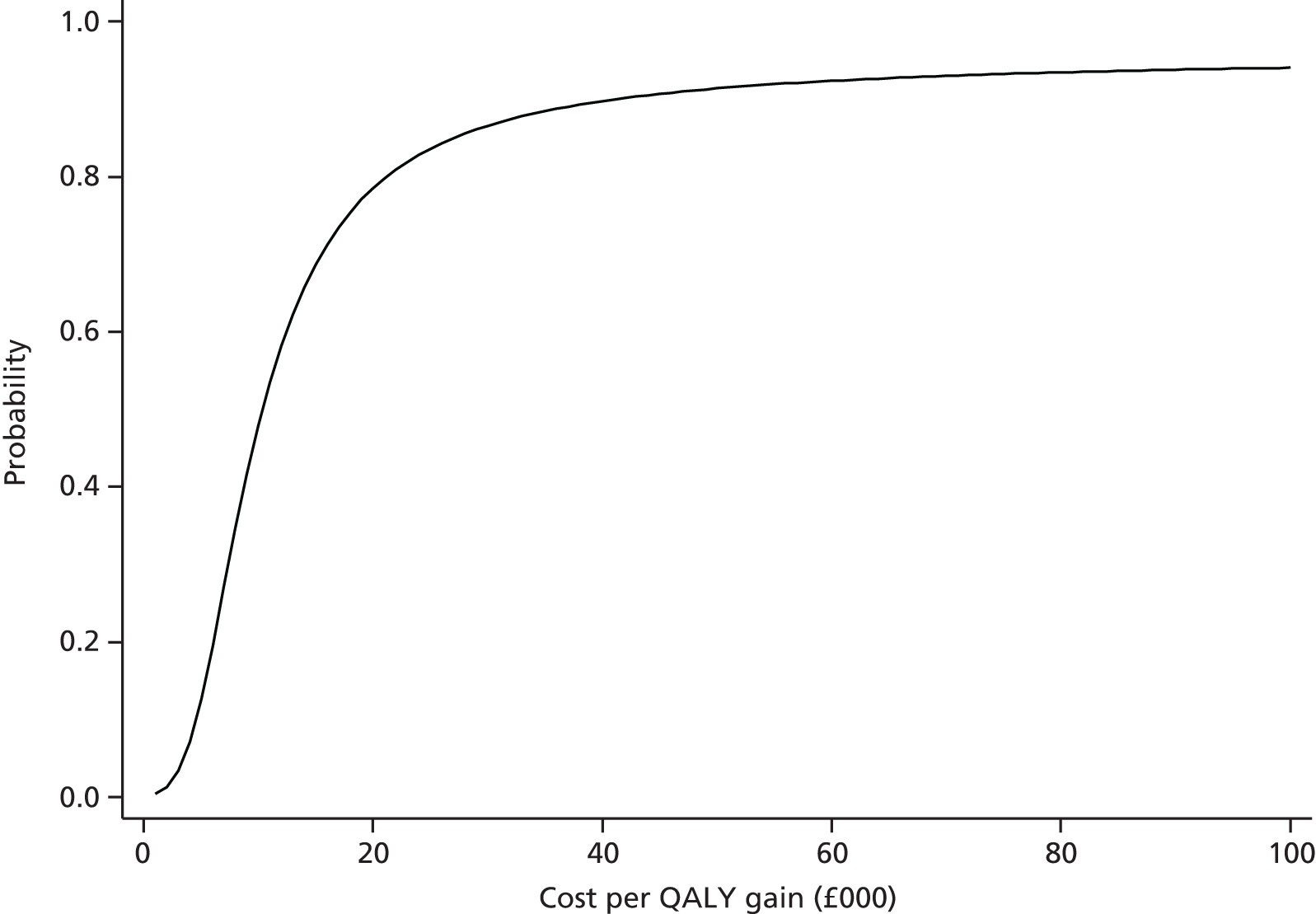
Sensitivity of the results to the inclusion of certain secondary costs
The sensitivity of the imputed results to the inclusion of items of NHS costs not related to the intervention or primary care costs (including the costs of prescribed medication) was assessed in the same way as for the depression trial (Table 127).
| Cost and outcomes | Usual care, mean | Intervention, mean | Incremental difference (95% CI) |
|---|---|---|---|
| Costs and QALYs | |||
| Total NHS costs (£) | 308 | 438 | 129 (93 to 166) |
| QALYs | 0.786 | 0.799 | 0.013 (–0.000 to 0.0262) |
| Cost-effectiveness statistics | |||
| ICER: £10,003 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.79 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.88 | |||
| NMB at threshold of £20,000 (95% CI): £129 (–£119 to £140) | |||
The effect of the sensitivity analysis is to leave the ICER almost unchanged and to slightly increase the probability of cost-effectiveness relative to the base case. This suggests that the costs excluded from this sensitivity analysis did not have an important impact on the base-case results.
Results from long-term modelling of cardiovascular disease risk
Introduction
The long-term state transition cohort simulation model used data from the 12 months of trial follow-up combined with simulated data on QRISK2 scores and costs and QALYs in future years to provide estimates of the cost-effectiveness of the intervention over the lifetime of trial participants. The model used imputed costs, QALYs and QRISK2 scores by sex and trial arm as estimated from trial data. ICERs and CEACs were calculated for different durations of intervention effect.
The base-case model specification used the 12-month data on QRISK2 scores adjusted for baseline CVD risk differences. The principal rationale for using the adjusted 12-month QRISK2 data was to account for the cumulative effects of the intervention on risk over the period of follow-up in a way that controls for baseline differences. Table 128 shows how QRISK2 scores, adjusted for baseline differences, differed between randomisation groups at 12 months using imputed data.
| Outcome | Usual care, mean | Intervention, mean |
|---|---|---|
| Adjusted QRISK2 score at 12 months | 31.26 | 30.86 |
Lifetime cost-effectiveness results
This section describes the results from the lifetime simulation cost-effectiveness analysis, which are presented for different durations of intervention effect. The effect at issue here is the difference between arms in QRISK2 score, constructed as a continuous variable. It was necessary to model different durations of effect because CVD risk levels after the end of the 12-month follow-up were (necessarily) not measured in the trial, whereas a lifetime perspective on cost-effectiveness that is driven by QRISK2 score requires an assumption of how participants in the intervention and usual care arms may be expected to differ once the intervention ends. At one extreme, one could assume that any differences in the trial are permanent, so that the intervention group will have lower QRISK2 scores than the usual care group over the remaining lifetime of trial participants. At the other extreme, it could be assumed that the effects of the intervention do not persist for > 1 year, after which time the intervention and usual care groups are modelled as having identical QRISK2 scores.
The ‘participants’ enter the simulation model on the day that they were randomised. Their CVD risk in this initial year (year 0) is their baseline QRISK2 score measured during the trial. Their CVD risk at the end of this year or start of the next year (year 1) is their (adjusted) QRISK2 score measured at 12 months’ follow-up. In practice, there are two reasons why these QRISK2 scores alone do not determine the costs and QALYs to which participants are assigned during the model’s initial year. The first reason is the use of a half-cycle correction, which means that simulated transitions do not occur only at the beginning or end of each cycle, and therefore the baseline QRISK2 score and the 12-month follow-up QRISK2 score, both of which were measured in the trial, determine events, states, costs and QALYs in the initial year of the model (year 0). The second reason is that data on both costs and effects are used in this first year of the simulation model. Simulated costs in this initial year are replaced with the mean per-participant cost measured during the trial. QALY values simulated during year 0 are adjusted to account for the between-arm differences in incremental QALYs observed during the trial. Thus, the simulated results are dependent on trial estimates of both QRISK2 scores and QALYs, along with observed costs over the 12 months.
Table 129 presents the cost-effectiveness results assuming a permanent difference in QRISK2 score between arms. Table 130 presents the cost-effectiveness results assuming a duration of effect of the intervention of 5 years, which means that for 5 years there is a difference between the intervention group and the usual care group in QRISK2 score that reflects the difference observed during the period of trial follow-up. However, once this period of effect has elapsed, then risk is modelled as being the same in each arm. Similar logic applies in Table 131 (for an assumed duration of effect of 2 years) and Table 132 (for an assumed duration of effect of 1 year).
| Cost and outcomes | Usual care, mean (SD) | Intervention, mean (SD) | Incremental difference (95% CI) |
|---|---|---|---|
| Lifetime duration of effect | |||
| Per participant lifetime discounted NHS costs (£) | 6602 (192) | 6657 (199) | 55 (49 to 61) |
| Per participant lifetime discounted QALYs | 8.572 (0.022) | 8.598 (0.026) | 0.026 (0.026 to 0.027) |
| Cost-effectiveness statistics for lifetime duration of effect | |||
| ICER: £2091 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.99 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 1.00 | |||
| NMB at threshold of £20,000 (95% CI): £472 (£197 to £728) | |||
| Cost and outcomes | Usual care, mean (SD) | Intervention, mean (SD) | Incremental difference (95% CI) |
|---|---|---|---|
| Lifetime duration of effect | |||
| Per participant lifetime discounted NHS costs (£) | 6608 (197) | 6714 (202) | 107 (101 to 112) |
| Per participant lifetime discounted QALYs | 8.573 (0.022) | 8.589 (0.022) | 0.016 (0.016 to 0.017) |
| Cost-effectiveness statistics for lifetime duration of effect | |||
| ICER: £6477 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.95 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.99 | |||
| NMB at threshold of £20,000 (95% CI): £223 (–£153 to £468) | |||
| Cost and outcomes | Usual care, mean (SD) | Intervention, mean (SD) | Incremental difference (95% CI) |
|---|---|---|---|
| Lifetime duration of effect | |||
| Per participant lifetime discounted NHS costs (£) | 6617 (206) | 6741 (214) | £124 (118 to 130) |
| Per participant lifetime discounted QALYs | 8.573 (0.021) | 8.586 (0.022) | 0.013 (0.012 to 0.013) |
| Cost-effectiveness statistics for lifetime duration of effect | |||
| ICER: £9886 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.84 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.93 | |||
| NMB at threshold of £20,000 (95% CI): £127 (–£144 to £382) | |||
| Cost and outcomes | Usual care, mean (SD) | Intervention, mean (SD) | Incremental difference(95% CI) |
|---|---|---|---|
| Lifetime duration of effect | |||
| Per participant lifetime discounted NHS costs (£) | 6595 (197) | 6726 (202) | 131 (125 to 138) |
| Per participant lifetime discounted QALYs | 8.573 (0.022) | 8.584 (0.022) | 0.011 (0.011 to 0.011) |
| Cost-effectiveness statistics for lifetime duration of effect | |||
| ICER: £11,776 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £20,000: 0.74 | |||
| Probability that intervention is cost-effective at cost-effectiveness threshold of £30,000: 0.87 | |||
| NMB at threshold of £20,000 (95% CI): £92 (–£172 to £352) | |||
The results indicate that the longer the duration of the effect of the intervention on QRISK2 score, the more likely it is that the intervention is cost-effective. When the duration of effect observed in the trial permanently differs between groups (see Table 129), the intervention is very likely to be cost-effective for low levels of the cost-effectiveness threshold. This scenario assumes a type of ‘free lunch’ – participants in the intervention arm receive the benefit of the intervention in terms of QRISK2 score for the rest of their lifetime, but the costs associated with the intervention are incurred only once. At the other extreme of duration of effect (1 year; see Table 132), the cost-effectiveness results are similar to those of the within-trial evaluation.
Except for very low levels of the cost-effectiveness threshold, the CEACs (Figure 32) indicate a monotonic relationship between assumed duration of effect and the probability that the Healthlines Service intervention is cost-effective.
FIGURE 32.
Cost-effectiveness acceptability curves from the lifetime simulation assuming different durations of effect.
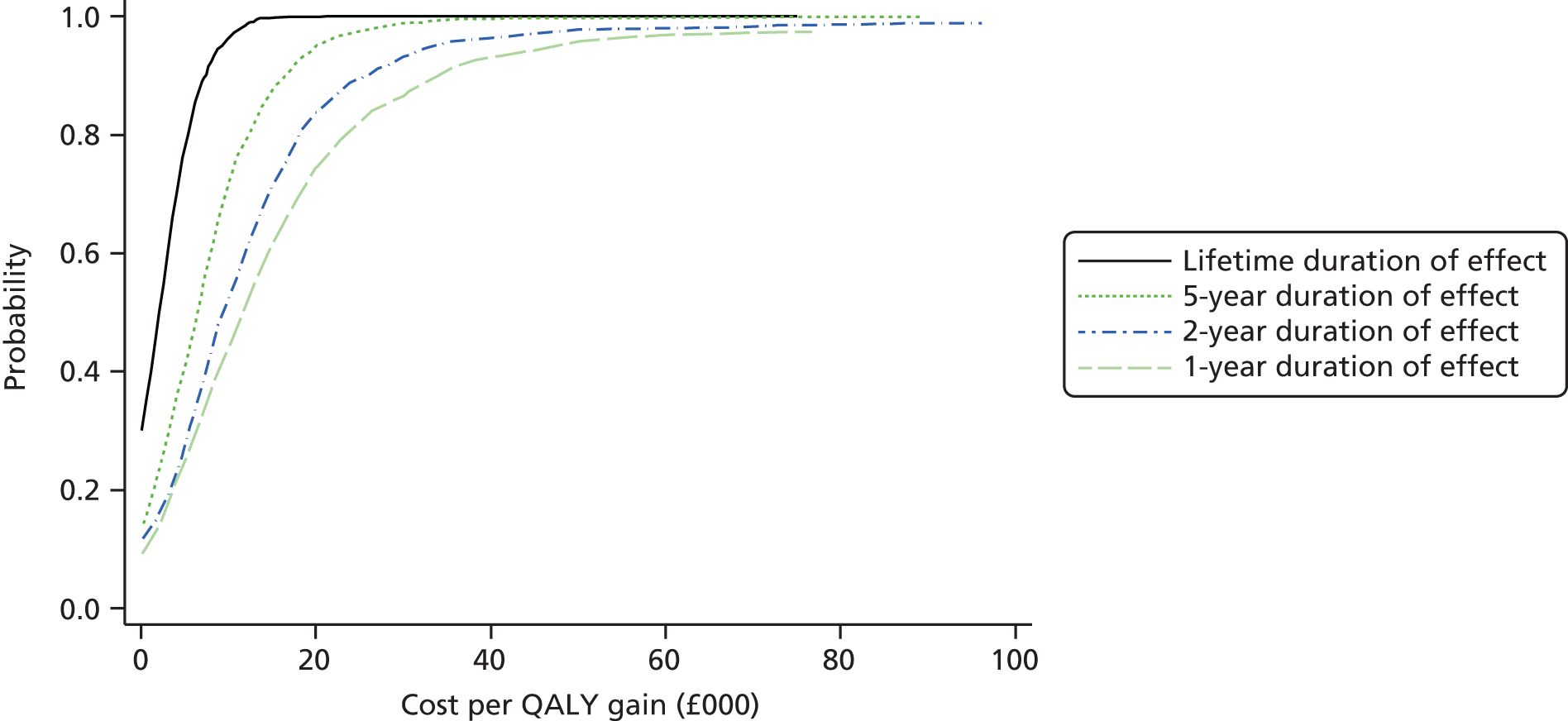
It is helpful to interpret Figure 32, and the simulation model cost-effectiveness findings more generally, by considering the extreme cases of a lifetime or permanent effect of the intervention in comparison with an intervention effect that lasts for just 1 year. Given the small absolute difference in QRISK2 score in favour of the intervention, the results from assuming a 1-year duration of effect are similar to the results from the cost-effectiveness analysis of the trial 12-month follow-up data – there is almost no difference in post-follow-up annual CVD risk between arms and the lifetime effects are driven by the inclusion of costs and QALYs from the trial in the first year of the model. The simulated clinical pathway experienced by participants in each arm is very similar once the assumed duration of effect expires and hence the cost-effectiveness results are primarily driven by data from the trial follow-up. The experiences of participants in each arm are not identical even once QRISK2 scores become the same in each arm because any events that occur when the risk is different will have an influence on future events (e.g. the effect of the occurrence of a TIA on the risk of future stroke). In essence, for a short duration of intervention effect, the differences in costs and QALYs observed during the trial are more influential than subsequent minor differences in costs and QALYs associated with the small difference between arms in simulated QRISK2 score.
In contrast, for a lifetime duration of effect, the data from the trial follow-up are important but less influential in determining cost-effectiveness. This is because the small but permanent reduction in risk leads to a greater impact on cost-effectiveness of the post-follow-up (simulated) QRISK2 scores, which are always lower in the intervention arm. This lower QRISK2 score gives rise to fewer strokes, fewer AMIs and fewer cases of angina and TIA and this has a cumulative effect on lifetime cost-effectiveness.
The sensitivity of the results to QALY differences observed during the trial follow-up is considered in the following section.
Sensitivity analysis of the simulation model
In the base-case analysis presented in the previous section, costs and QALYs observed during the period of trial follow-up have an important impact on estimates of lifetime cost-effectiveness. The sensitivity of the conclusions of the simulation model to data collected in the trial and included in the base-case analysis was assessed. This involved removing the QALY adjustment observed during the trial and removing costs observed during the trial, except for the cost of the intervention itself. Utility and costs are determined only by QRISK2 score, as simulated by the model. Therefore, this sensitivity analysis could be interpreted as capturing a type of direct or ‘pure’ effect of the intervention on QRISK2 score. Figure 33 shows the CEACs corresponding to this analysis.
FIGURE 33.
Cost-effectiveness acceptability curves from the lifetime simulation assuming different durations of effect: sensitivity to QALY differences.
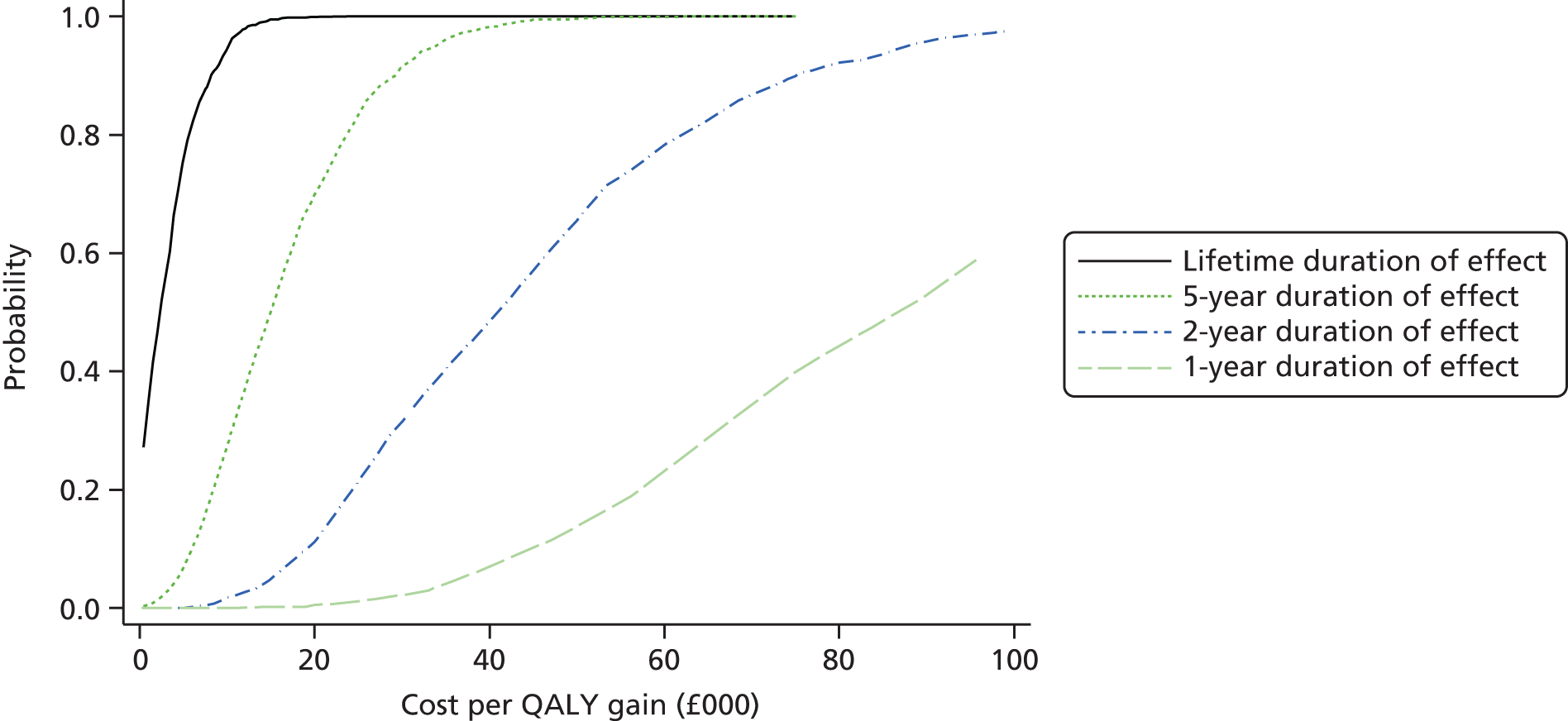
The results indicate that removing the QALY and cost data (other than the intervention cost) has a material impact on estimates of cost-effectiveness. The lifetime duration of effect is unambiguously cost-effective. The probability that the intervention is cost-effective for a 5-year duration of effect at a threshold value of £20,000 is 0.70, but the corresponding probabilities for 2-year and 1-year durations of effect are 0.11 and 0.01 respectively. This means that if the primary and secondary care costs measured during the trial are ignored, and no allowance is made for the health-related quality of life differences observed during the period of trial follow-up, then the intervention is not cost-effective for modest durations of effect. Put differently, in the scenario modelled, the small differences in continuous QRISK2 scores observed at the end of the intervention and used as the basis for simulation modelling are not sufficient to render the intervention cost-effective if the effects of the intervention are relatively short-lived.
Discussion of the cost-effectiveness findings from the within-trial evaluation and the lifetime simulation model
Two different but complementary sources of evidence were used to inform conclusions concerning the cost-effectiveness of the CVD risk intervention. The first was data collected during the trial, which were used to estimate the probability that the intervention was cost-effective at the end of the 12-month follow-up. The conclusion from this analysis is that there is a reasonable probability that the intervention is cost-effective under all modelled durations of effect. The second analysis used a simulation model to assess the probability that the intervention was cost-effective when evaluated over the remaining lifetime of trial participants. The results from the base-case simulation analysis suggest that the intervention is probably cost-effective, whatever duration of effect is assumed, although the evidence in support of this conclusion is stronger for longer durations of effect. The findings are sensitive, particularly at lower levels of duration of effect, to the higher QALY differences observed in the intervention arm during the period of trial follow-up. If the QALY differences are ignored, and non-intervention costs are omitted from the simulation model, then the intervention is not cost-effective under short durations of effect.
The simulation analysis is subject to a number of limitations. First, the model understates the uncertainty associated with QRISK2 score. In particular, information on the covariances between variables included in the QRISK2 algorithm is not published and the inability of the model to account for these covariances has the effect of understating uncertainty and therefore of narrowing CIs around cost-effectiveness statistics. Second, the analysis accounts only for health system costs and does not reflect the impact that CVD events may have on carers and the wider economy. For example, strokes are associated with a large391 economic burden (including productivity impacts), not all elements of which can be accounted for in a simulation model concerned with health system costs. Third, the simulation model does not identify which assumed duration of effect is most plausible. The best source of evidence on the duration of effect is long-term follow-up. An alternative to long-term follow-up would be to model the likelihood of reductions in modifiable risk factors observed in the trial (such as systolic blood pressure) by synthesising evidence from different sources, a task beyond the scope of this project.
The conclusions of the simulation model reflect the data used in its construction. The QRISK2 model is not validated for ages above 84 years and assumptions about growth rates in risk for the small number of simulated participants living to an advanced age were necessary. The model of health-related quality of life adopted a conservative assumption, in the absence of evidence, that second and third events had the same utility impact as each other. This rules out multiplicative effects, for example, that the impact on quality of life from having a third stroke is not made worse because someone has previously had a second stroke. Using an alternative to this assumption would tend to make the intervention appear more cost-effective, as participants with lower mean continuous QRISK2 scores (the Healthlines intervention group) tend to avoid, on average, more CVD events than those with higher QRISK2 scores (the usual care group).
In summary, the balance of evidence suggests that the intervention may be cost-effective for long durations of effect but may not be cost-effective for short durations of effect.
Chapter 11 Process evaluation results: embedded qualitative interview study
Much of this text has been reproduced from O’Cathain et al. 392 © Alicia O’Cathain, Sarah J Drabble, Alexis Foster, Kimberley Horspool, Louisa Edwards, Clare Thomas, Chris Salisbury. Originally published in the Journal of Medical Internet Research (www.jmir.org), 30 June 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on www.jmir.org/, as well as this copyright and license information must be included.
Abstract
Aim: To help explain the results of the trials by exploring the implementation, mechanisms of action and context of the Healthlines Service.
Methods: A qualitative interview study of 45 staff and trial participants with experience of the intervention consisting of face-to-face and telephone interviews with eight NHS Direct staff involved in developing and delivering the intervention, 13 health professionals in primary care whose patients used the intervention and 24 intervention participants.
Findings: There was evidence to support the importance of all components of the TECH model on which the intervention was based. The Healthlines Service was largely delivered as planned with the exception of two elements: continuity of the HIAs and tailoring of the intervention to participants’ needs. These were compromised in the early months of implementation until a smaller team of dedicated HIAs delivered the intervention in later months. This detrimentally affected participant engagement with the intervention, which was a key mechanism of action. In the depression trial, the intervention did not appear to meet the needs of some participants who were seeking a talking therapy rather than CBT. In the CVD risk trial, some participants were not motivated to change their behaviour at recruitment, reducing the potential of the intervention; in particular, smokers were identified as lacking motivation to change.
Conclusions: The TECH model appears to be a good basis for developing telehealth interventions for LTCs. The extent to which the intervention was implemented as planned varied during the course of the trials. This is likely to have impacted negatively on patient engagement and through this the effectiveness of the intervention. An intervention offered by a dedicated team of HIAs delivering continuity and tailoring has the potential to be more effective than the intervention tested here.
Introduction
In our trial protocol we stated that we would undertake qualitative research embedded within both trials to consider the acceptability of the intervention, as well as facilitators of and barriers to the delivery of, use of and compliance with the intervention. 283 As the trials progressed we viewed the qualitative data and quantitative process data collected as part of the trials as a process evaluation. As described in Chapter 7 (see Process evaluation methods), the aims of the process evaluation were to explore the implementation, mechanisms of impact and context of the intervention in practice. 353 In this chapter we explore the following research questions:
-
Was the intervention implemented as planned? (implementation)
-
How did the intervention produce outcomes? (mechanisms of impact)
-
How did the setting affect the intervention and the outcomes? (context)
Findings
Description of participants
We undertook 45 interviews in total: eight with NHS Direct staff, six with GPs, seven with nurses or HCAs in general practices, 12 with users of the depression intervention and 12 with users of the CVD risk intervention.
NHS Direct staff
We interviewed four HIAs who delivered the intervention. Two had worked on the Healthlines Service from the beginning, one was part of a second tranche of recruitment and one was new in terms of being in post for a month at the time of the interview. We also interviewed a strategic manager who had led the intervention development within NHS Direct, a technical manager who had helped to develop the intervention, a manager of the Healthlines Service team and a NHS Direct team manager not directly involved in the Healthlines Service. Although this sample was small, it was wide and reflected the small number of staff with experience of the Healthlines Service. There was considerable convergence of views within this sample, leading us to believe that data saturation had been obtained at the data collection stage. On reflection, interviewing some of the HIAs who had left the service might have offered a different perspective on the intervention.
Primary care staff
We interviewed six GPs whose practices had participated in the trials. We approached GPs who had screened lists of potential trial participants prior to recruitment because we thought that they would take more of an interest in the Healthlines Service than their colleagues. We approached 13 GPs to obtain the planned number of interviews because some GPs did not want to participate. Based on feedback we received when trying to recruit, we surmised that GPs felt too busy to be interviewed or felt that they had nothing to say about the intervention.
We had also planned to interview practice nurses but ended up interviewing a research nurse (who offered a perspective on the research, rather than the intervention) and a HCA, as well as practice nurses. We included all of these interviews in the analysis. We approached 11 practice nurses to obtain seven interviews. Some practice nurses did not want to be interviewed because they felt that they had nothing to say about the intervention. In terms of data saturation, there seemed little point in interviewing more primary care staff because those we interviewed had little to say about their experience of the intervention in practice.
Trial participants receiving the intervention
We approached 16 depression and 20 CVD risk trial participants to obtain 12 interviews with each group. Trial participants declined to participate because they were not interested (n = 5), were too busy (n = 3), could not be contacted (n = 1), had withdrawn from the intervention (n = 1) or did not show up for the arranged interview (n = 2). One depression participant was known to the interviewer and was excluded from the sample. Interviews took place over an 8-month period (Tables 133 and 134).
| ID | General practice | Deprivation level of general practicea | Withdrawn from intervention | Randomisation date | Monthb | Sex | Ethnicityc | Age (years) | Work status | PHQ-9 scored | Internal motivatione | External motivationf |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 101 | 22 | Y | July 2012 | 8 | M | 1 | 63 | Unable to work because of illness/disability | 19 | 6 | 1 |
| 2 | 101 | 22 | Y | July 2012 | 8 | M | 1 | 60 | Unable to work because of illness/disability | 18 | 4 | 1 |
| 3 | 201 | 30 | N | October 2012 | 8 | F | 1 | 53 | On sick leave because of depression/anxiety | 18 | 6 | 1 |
| 4 | 201 | 30 | N | September 2012 | 10 | F | 1 | 56 | Unable to work because of illness/disability | 10 | 3.5 | 1 |
| 5 | 203 | 8 | N | September 2012 | 6 | F | 1 | 47 | Looking after home | 12 | 5.5 | 1 |
| 6 | 203 | 8 | N | January 2012 | 6 | F | 1 | 49 | Unable to work because of illness/disability | 22 | 6 | 1 |
| 7 | 206 | 14 | N | November 2012 | 9 | F | 1 | 38 | Part-time paid | 14 | 6.5 | 1 |
| 8 | 206 | 14 | N | January 2013 | 8 | F | 1 | 66 | Fully retired | 10 | 7 | 1 |
| 9 | 207 | 17 | Y | February 2013 | 5 | M | 1 | 52 | Unemployed | 14 | 7 | 1 |
| 10 | 207 | 17 | Y | January 2013 | 6 | M | 1 | 51 | Full-time paid | 10 | 4 | 1 |
| 11 | 208 | 29 | N | January 2013 | 10 | M | 1 | 66 | Fully retired | 19 | 4.5 | 3 |
| 12 | 208 | 29 | N | January 2013 | 9 | F | 1 | 30 | Part-time work, part-time education | 10 | 7 | 1 |
| ID | General practice | Deprivation level of general practicea | Withdrawn from intervention | Randomisation date | Monthb | Sex | Ethnicityc | Age (years) | Work status | Blood pressure (mmHg) | BMI (kg/m2) | Smoker | Total cholesterol (mmol/l) | Blood pressure medication prescribed | QRISK2 scored | Internal motivatione | External motivationf |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 101 | 22 | N | December 2012 | 3 | M | 1 | 62 | Full-time paid | 155/77 | 30.2 | Y | 3.9 | N | 35.6 | 5 | 1 |
| 2 | 201 | 30 | N | November 2012 | 11 | M | 1 | 65 | Fully retired | 136/79 | 31 | N | 3.7 | Y | 28.2 | 7 | 1 |
| 3 | 202 | 12 | N | December 2012 | 7 | M | 1 | 75 | Fully retired | 119/86 | 32.8 | N | 3.7 | Y | 50.8 | 7 | 2 |
| 4 | 203 | 8 | N | December 2012 | 8 | F | 1 | 70 | Fully retired | 170/94 | 34.2 | N | 7.6 | Y | 20.7 | 5 | 1 |
| 5 | 206 | 14 | N | April 2013 | 10 | M | 1 | 64 | Part-time paid | 143/82 | 37.7 | N | 5.3 | Y | 58.1 | 6 | 1 |
| 6 | 206 | 14 | N | February 2013 | 6 | M | 1 | 70 | – | 160/79 | 17.8 | Y | 4.5 | N | 36.9 | 5 | 1 |
| 7 | 206 | 14 | N | January 2013 | 8 | M | 1 | 74 | Fully retired | 148/73 | 20.9 | N | 3.3 | N | 51.1 | 3.5 | 2 |
| 8 | 207 | 17 | N | January 2013 | 6 | M | 1 | 70 | Fully retired | 169/102 | 28.4 | N | 5.2 | N | 35.2 | 7 | 4 |
| 9 | 207 | 17 | N | January 2013 | 9 | F | 1 | 72 | Part-time paid | 159/81 | 37.9 | N | 5.1 | Y | 26.8 | 1 | 1 |
| 10 | 208 | 29 | N | May 2013 | 8 | M | 1 | 71 | Fully retired | 150/70 | 30.1 | N | 3.4 | Y | 31.7 | 7 | 1 |
| 11 | 210 | 49 | N | May 2013 | 10 | F | 1 | 64 | Fully retired | 122/78 | 42.6 | N | 3.9 | Y | 22.6 | 7 | 1 |
| 12 | 210 | 49 | N | April 2013 | 11 | M | 1 | 66 | Fully retired | 118/94 | 47.2 | N | 5.1 | Y | 34.5 | 6 | 1 |
Depression interviewees were identified from six general practices and were interviewed a median of 8 months after randomisation, varying between 5 and 10 months (see Table 133). Of the interviewees, five were male, all were white and most were middle-aged (age range 30–66 years). This generally reflected the demographics of participants in the depression trial. One interviewee had a very high score on the PHQ-9289 at baseline, indicating severe depression, and four interviewees had moderately severe depression. Four interviewees had formally withdrawn from the intervention at the time of the interview.
Cardiovascular disease risk interviewees were interviewed a median of 8 months after randomisation, varying between 3 and 11 months (see Table 134). They were recruited from eight general practices and there was little variation in their demographic characteristics: they were mainly male, all were white and all were older (age range 62–75 years). However, this largely reflected the participants in the CVD risk trial. They had a mix of modifiable CVD risk factors: two smoked, nine had a BMI of > 30 kg/m2 and eight had systolic blood pressure of > 140 mmHg. Eight were on blood pressure medication. The QRISK2 score195 was high for all interviewees, varying between a 21% and 58% chance of having a cardiovascular event in the next 10 years. All CVD risk interviewees were still using the intervention at the time of the interview. Therefore, our interview data included more participants who were engaged with the intervention than in the total population of CVD risk participants in the trial.
Overview of findings
The findings are organised around four themes: outcomes, implementation, mechanisms and context. Verbatim quotes are labelled to identify interviewees: HIA, NHS Direct managers (M), general practitioners (GP), practice nurses or HCAs (PN), depression trial participants (DEP) and CVD risk trial participants (CVD risk). For trial participants, the numbers relate to the first column in Tables 133 and 134 (e.g. DEP 1 refers to the first depression trial participant interviewee in Table 133).
Outcomes
The qualitative research could not determine whether or not the intervention had ‘worked’; this was the role and focus of the trials. The qualitative research could, however, identify the range of perceived benefits reported by the interviewees and explore why some interviewees felt that they had obtained benefit from the intervention and some did not. We interviewed depression participants at 5–10 months into the trial and so all of them had had their 4-month primary outcome (PHQ-9) measured. We interviewed CVD risk participants at 6–11 months and so none had had their 12-month primary outcome (QRISK2) measured.
As will be described in more detail in the following sections, half of the depression and CVD risk interviewees reported improvements in health, which they associated with the intervention. The remaining interviewees were clear that they had obtained no benefit or did not discuss any benefit during the interview. Primary care practice staff also described seeing improvements in some of the trial participants and the HIAs felt that they had helped some people to benefit but not others.
Perceived impact on primary outcomes in the trials
Reduction in depression
We selected four of the interviewees because they had withdrawn from the depression trial. One of these participants had withdrawn because the intervention had improved his mood so much that he felt that he no longer required it. Six of the remaining interviewees described benefits, such as feeling more positive, because they had been shown ways of coping, had learned to share problems with their family, had more energy, felt listened to, knew someone was there or felt that they meant something to someone:
What I needed was a way of dealing with the great sadnesses and a way of coming to terms with it, and I think I’ve got that from [pause], from the Healthlines Study.
DEP 8
Both primary care staff and trial participants described the potential for long-term effects of CBT because it gave people tools for the future and complemented other services. In contrast, those who felt that the intervention had not been helpful described the intervention as too superficial, not giving access to someone to talk to or the same as previous treatments that they had already tried.
Weight loss
Weight is one of the modifiable risk factors for CVD. Nine of our 12 CVD risk interviewees had a BMI of ≥ 30 kg/m2 at baseline. Three of these reported weight loss that they were happy with and which they attributed to the intervention. Trial participants who had lost weight were delighted with this and described positive consequences including reduced blood pressure, the ability to walk more easily and having more energy. Two practice nurses had noticed weight loss in their patients at the 6-monthly CVD assessment for the trial and one of the HIAs noted that this was a benefit apparent in her dealings with some participants. Weight loss was not always attributed to the intervention – one CVD risk interviewee had lost weight during the trial that she attributed to contact with a different service. Weight loss was described as occurring through educating people about good eating habits, setting goals and reviewing their weight each month.
Lowered blood pressure
High blood pressure is a risk factor for CVD. Eight of our 12 CVD risk interviewees had high blood pressure at baseline (systolic blood pressure > 140 mmHg). Four reported lowered blood pressure and one other reported reduced use of blood pressure medication related to the intervention. These interviewees were sometimes different from those who had lost weight, such that six of our 12 CVD risk interviewees reported an improvement in weight or blood pressure management and related this to the intervention. Improvements in blood pressure were attributed to weight loss or the introduction of blood pressure medication:
You’ve got high blood pressure I’m presuming?
Not any more.
Not any more, good [laugh].
Mainly thanks to this system.
CVD risk 8
Smoking cessation
Only two of our CVD risk interviewees smoked at baseline. Both of these reported no success with smoking cessation, with one interviewee quite clear that he would continue smoking: ‘don’t bother, I smoke’ (CVD risk 1). Indeed, one of the smokers probably increased his smoking from baseline because he talked about having an illness when the baseline outcome was measured, which caused him to cut down his smoking radically, but then later returned to his usual habits. HIAs noted that few participants had reported giving up or cutting down smoking and that this was a difficult lifestyle issue to impact on.
Changes in the outcomes pathways
Both HIAs and trial participants described making lifestyle changes that could affect the primary outcomes in the trials, in particular, increased exercise and raised awareness of health problems. Some depression interviewees reported exercising more, which could improve general well-being by making them ‘feel better’. Some CVD risk interviewees reported making lifestyle changes that could affect CVD risk factors, such as exercising more because the intervention had introduced them to different types of exercise. Other lifestyle changes undertaken were eating more healthily and reducing alcohol intake. HIAs described how they helped to make people more aware of their health problems and the behaviours that could affect them. For example, one depression interviewee did not understand that he was depressed before the intervention and was grateful for being made aware of it, as well as learning about ways of dealing with it.
Timing of measurements in the trials
NHS Direct managers felt that there had been delays in offering the CVD risk intervention to trial participants once they had been recruited. They worried that baseline measures were obtained a few months before the intervention started, resulting in the primary outcome being measured before some participants had experienced 12 months of the intervention. This was because QRISK2 scores were collected 12 months after randomisation, regardless of the amount of time that participants had received the intervention for.
Changes in resources
Some interviewees reported changes in use of primary care or medication brought about by the intervention. A GP perceived that the Healthlines Service had reduced the number of GP appointments because it monitored blood pressure; a CVD risk interviewee felt that he did not need to bother the GP for this reason. There were also reports of medication for both depression and high blood pressure being reduced. On the other hand, resource use could also increase if the HIAs prompted participants to check their health problems with their GP. For example, one CVD risk interviewee reported gaining the confidence to visit his GP from talking to the HIA. Trial participants also described changes in their own resource use related to the CVD risk intervention. These were usually given in jest within the interview. Examples were spending less on food and alcohol or spending more on new clothes if they had lost weight. One CVD risk interviewee expressed concern about increases in health insurance if the intervention identified any new health problems.
Unintended consequences
A positive unintended consequence of the intervention was that family and friends of trial participants were sometimes described as improving their lifestyles because they joined in with exercise and healthy eating programmes recommended by the Healthlines Service. Negative unintended consequences of the intervention were also described in the interviews. Some primary care staff and trial participants described problems that had occurred with the intervention, such as anxiety caused by people discovering that they had high blood pressure, anxiety caused by monitoring one’s blood pressure, confusion caused by different definitions of high blood pressure held by the Healthlines Service and some GPs, CVD risk participants suffering dizzy spells that turned out to be due to loss of weight without reduction in blood pressure medication and CBT making people feel worse because it reminded them of their depression.
Implementation of the intervention
The content delivered in practice
The amount of intervention received by participants in each trial, and the specific content that they engaged with, were described in Chapters 8 and 9. In this interview sample, the content of the depression intervention described most commonly by both HIAs and trial participants was CBT delivered by computer or book and supported by telephone calls with HIAs. Two interviewees from the depression trial described using the BWW (see Chapter 7, Depression trial) and two experienced medication changes. The main content of the CVD risk intervention described by both HIAs and trial participants in this sample was blood pressure monitoring with subsequent advice about medication and lifestyle advice about weight reduction. HIAs also reported offering some lifestyle advice about exercise and diet to people in the depression trial.
Implementation of the different components of the TElehealth in CHronic disease model
During our analysis, we coded data related to each of the components of the TECH model under a theme of ‘mechanisms of action’: engagement, promoting self-management, treatment optimisation, care co-ordination, partnership and context. 250 In practice, many of the data related to if and how these were implemented during the trials.
The TElehealth in CHronic Disease model: engagement
The TECH model emphasises the role of the Healthlines Service, particularly the HIAs, in engagement of the participant and engagement with primary care (see Table 12, Engagement).
To what extent did the Healthlines Service engage patients?
Primary care interviewees believed that CVD risk trial participants were engaged with the intervention. They based this view on the absence of negative feedback from participants, because they saw participants as keen to attend CVD risk assessments related to the trial or because they saw some participants gaining benefits such as weight loss. HIAs reported engagement of both CVD risk and depression participants. Some depression and CVD risk interviewees who had experienced the intervention expressed enthusiasm for it. However, some of the contextual issues related to individual participants’ lives, such as depressed participants being too busy to engage with the intervention or CVD risk participants being motivated to participate in research rather than change their behaviour (see Motivation and ability), made engagement of some participants hard and sometimes impossible for the HIAs to facilitate.
Support with technology
Health information advisors described how offering participants technical support and getting them logged on to the patient management system at the beginning of the process was time-consuming. Participants wanted advice about how to use their computer and appreciated the technical knowledge of the HIAs. Participants described how family and friends also helped them with the technological side of the intervention.
Continuity of health information advisors
An aim of the intervention was to offer participants continuity with a named HIA to enable engagement. When continuity occurred, both depression and CVD risk interviewees used the first name of the HIA when talking about the intervention and described the support of the HIA using words such as ‘caring’, ‘smashing’, ‘motivational’, ‘therapeutic’, ‘supportive’, ‘brilliant’, ‘intuitive’, ‘helpful’, ‘good’, ‘nice to talk to’, ‘easy-going manner’, ‘I’ve enjoyed talking to [advisor]‘, ‘pleasant’, ‘calming’ and ‘they listened’. Continuity of HIAs allowed a relationship to develop between the HIA and the participant in four cases for depression and five cases for CVD: ‘it has been good to build up some, kind of, relationship’ (CVD risk 11). Participants valued having someone to listen to them, someone who cared about them and who could help them: ‘it was just nice at the end of it, he said to me, you have helped me today [name] it’s just that I’ve got nobody else to actually speak this off to’ (HIA 3). This continuity appeared to be vital for some depression participants who felt that they needed to discuss difficult issues and to build up trust in the HIA to do this. The relationship with the HIA made the intervention attractive in that participants looked forward to talking to the advisor and wanted to do the CBT to show their commitment to their HIA.
When continuity was lost, participants often felt the loss of the relationship with the HIA and a reduction in engagement and perceived outcome. Continuity appeared to be more important for the depression interviewees than the CVD risk interviewees in our sample, indicated by the fact that half of the depression interviewees and two CVD risk interviewees spoke negatively about the effects of having no continuity with the HIA. Four CVD risk interviewees were not concerned about having the same HIA; one participant did not know if he had had the same HIA and expressed no particular preference, one had moved to a second HIA but had found continuity since then and two expressed no preference, one stating that it was ‘because the spiel was exactly the same’ (CVD risk 10).
Passive engagement from primary care
The plan was that the Healthlines Service would engage with primary care by sending letters about participants to GPs when action was needed, that is, there was no intention for HIAs to have personal contact with practice staff. The GPs interviewed described engaging with the intervention by taking action on written requests to consider the suicide risk of depression participants or review CVD risk participants’ medication. This engagement was one way in that GPs did not seek to engage proactively with the Healthlines Service around the care offered to individual patients. Practice nurses and HCAs described being involved in undertaking assessments on CVD risk participants for the trial but were otherwise unaware of what the intervention entailed beyond what participants told them: ‘to be honest I have absolutely no idea what goes on between the Healthlines study staff and the participant’ (PN 111). GPs interviewed also had little knowledge of the intervention and some participants were aware of this:
Has your general practice helped you with Healthlines at all, or has there been any interaction between Healthlines and your doctor?
No.
Nothing?
Nothing. I don’t even think he knows anything about it to be honest, no.
DEP 4
General practitioners and HIAs seemed happy with this limited level of engagement with primary care because GPs were described as busy. HIAs felt that they could encourage contact between participants and their GP through participants if necessary. Indeed, some participants described how they had contacted their GP after encouragement by a HIA or if their GP had not contacted them about a letter sent by the Healthlines Service. This passive engagement by GPs was identified as problematic by one participant who wanted his GP to ask him how the intervention was progressing; however, it was welcomed by another participant as a way of being independent of her GP:
I’m quite glad that there wasn’t, I think I would have felt more pressure if my GP had been, like, oh are you doing this, doing that, because I’d rather do something under my own steam.
DEP 12
There was also some evidence that communication between primary care and HIAs did not always reach the level of partnership intended by the TECH model, which could cause confusion for some patients:
There was this one particular patient who was constantly being, it was being suggested that he be reviewed by the GP. And the GP was reviewing him, but it was still the same, you know, it was a bit, you know, flogging a bit of a dead horse really, because she was, the GP was very happy with the blood pressure. Healthlines study staff were saying, oh, no, no, no you need to go and see the GP . . . and of course the patient is the one caught in the middle.
PN 111
The TElehealth in CHronic Disease model: self-management
An aim of the intervention was to promote self-management for participants. This was operationalised within the interventions in nine ways, differing somewhat for each condition (see Table 12, Promoting self-management). Some of the ways in which the intervention was planned to promote self-management were the same as those planned to promote engagement and have been discussed already (e.g. Continuity of health information advisors). Indeed, promotion of self-management required that the participants engaged with the intervention. Other aspects of the self-management components, including self-monitoring, motivational interviewing and review, are discussed in the following sections.
To what extent was self-management enhanced?
Most of the interviewees described how the intervention had helped them develop self-management through raising awareness of their health problems and educating them about ways of dealing with those problems. As one participant put it, the intervention was about ‘helping myself to help myself’ (DEP 2). Indeed, the intervention could have a profound effect on self-management. One participant with depression described how the intervention had changed her identity, which had previously been defined by her physical illness:
Do you think it has helped you to self-manage your problems?
Yes, because I got [pause], it’s very easy for physical symptoms to just overtake, take over every function, so, you know, having physical limitations, mobility limitations actually shouldn’t be to the fore all the time . . . it’s people define you, I hate being defined by my back [pause], but they do it if you define yourself [that way].
However, some participants (four depression interviewees and one CVD risk interviewee) were explicit that their self-management capacity was not enhanced because they felt that they already managed their health problems sufficiently themselves. One GP interviewee also felt that the intervention might be telling people what they already knew, limiting its ability to promote further self-management.
Self-monitoring and feedback
For CVD risk interviewees, self-monitoring and feedback of blood pressure readings occurred through graphs on the computer via the Healthlines web portal. Some CVD risk interviewees described how helpful it was to view their blood pressure results via the web portal or to discuss them with their HIA. A practice nurse particularly liked this aspect of the intervention because it could help participants to make a clear connection between their daily lifestyle behaviours and their blood pressure:
I think it makes people realise that there are things that you can do on a day-to-day basis . . . to bring it down, if they’re checking it that regularly for a purpose. You know, I went out for a walk this morning and my blood pressure was really good today, and things like that. It makes it very obvious in black and white right in front of them that the days when they are doing things, and being a bit more well behaved if you like, that it does make a difference.
PN 113
For depression interviewees, the monitoring and feedback mechanism was less tangible than for CVD risk interviewees in our sample. Participants could self-monitor through the PHQ-9 questionnaire and the BWW, with HIAs monitoring changes in depression scores and providing information to GPs if participants were feeling suicidal or their scores had changed dramatically. However, this information was not mentioned by the depression interviewees directly as useful feedback or a useful self-monitoring tool. For one interviewee, the lack of feedback from the remote telephone calls added to his dissatisfaction with the service.
Raising awareness
The HIA interviewees highlighted that the intervention raised awareness of participants’ health problems and the range of solutions available to them. Some depression and CVD risk interviewees described how they had been unaware of their depression or high blood pressure until they were invited to take part in the trial. Awareness of their weight could come as a shock to some participants. It was not that they had never been recommended to lose weight, but that someone other than a primary care professional was reinforcing this:
I think perhaps the participants that really haven’t quite got on board with it, so they’re not really accepting that you know taking a cholesterol-lowering tablet is going to be useful for them or losing weight’s really going to be useful and maybe just having a chance to talk to somebody and then think about it and then talk again about it.
GP 101
In fact, one GP interviewee felt that the Healthlines Service could be of most benefit for participants who were not self-aware.
Educating participants and building skills
Some depression and CVD risk interviewees were aware of their health problem or poor lifestyle and benefited from the educational aspects of the intervention. They felt that the intervention helped them to understand how their medications worked or the meaning of a blood pressure reading and taught them strategies and skills to manage their condition or improve their lifestyle. For CVD risk interviewees, the focus was on education and information provision. One HIA and some GPs felt that this was not necessary for the more educated CVD risk participants and should be focused on those with poor knowledge levels, that is, more tailoring to participants’ needs was necessary. For example, one of the CVD risk interviewees was a retired nurse who reported experiencing no gains in knowledge. For depression interviewees, the focus seemed more about building skills to cope with the condition: ‘whenever you suffer something like depression, you don’t immediately build up those skills for coping with things’ (DEP 12).
Promoting self-efficacy
The HIAs were trained in motivational interviewing techniques to help them to encourage participants to modify their behaviours. They valued this training and some of the techniques within it, such as goal setting, because they felt that these were important for empowering participants to take control of their health and lifestyle. Some depression and CVD risk interviewees believed that the intervention empowered them to take action to help themselves, rather than be fixed by someone else. This came about not simply through motivational interviewing, but through HIAs signposting participants to various other services.
Different definitions of empowerment appeared to be held by different types of interviewees. Primary care staff valued any intervention that helped participants to take responsibility for their care, rather than always looking to their GP for this. However, from the participant or the Heathlines Service perspective, empowerment might include giving the participant confidence to seek treatment from their GP. For example, a depression interviewee described the benefit of being encouraged to see her GP about access to further care, stating that it ‘makes me sort of keep on top of things’ (DEP 6).
At the time that we interviewed them, HIAs felt that motivational interviewing could not help when people came to the intervention unprepared to change their lifestyle. HIAs found this very frustrating and wondered if the inclusion criteria for the trial should have screened out people who were not motivated to change:
We’ve had some people come in where the only modifiable risk isn’t something that they want to change or they’re not prepared to change, so there’s really not much we can do with them.
HIA2
I’ve got one lady who swears she never eats that much, never eats anything unhealthy and she’s really active, but she’s, like, 10 stone overweight, . . . it’s got to come from them.
HIA1
Goal-setting
The CVD risk intervention was set up to identify individual modifiable risk factors for each participant so that HIAs could work with the participants to set goals to change relevant risk factors. Goals could be long term, such as giving up smoking or losing 10 stone, or short term and small, such as going for a walk or doing some gardening over the next few weeks. HIAs thought that these smaller goals were helpful and motivating. These goals created a feedback loop because some participants liked to report their achievements to the HIA since the previous call and took action to look good for these calls:
And then it just gives them something to work on and I make it clear to them all that they have to do the hard work themselves if they want to reach their target. And eight times out of ten, next time I speak to them they‘ve done it or the first thing they say to me is ‘well I’ve been eating off a smaller plate’ and it’s really nice to hear that.
HIA2
But I’m going to be honest with you now, I tend to ease off for a few days after she’s rung . . . and I’ve seen the wife, you know, she’s starved herself the day before [the HIA calls].
CVD risk 10
The HIAs described the need for flexibility in goal-setting, establishing goals that suited participants at times that suited them rather than following the CVD risk script. In particular, the CVD risk intervention addressed different risk factors each session so that the risk factor of most relevance to a participant might not be discussed for a number of sessions. The HIAs described working at making the intervention fit participants’ needs by offering them advice relevant to their key risk factor at an early stage of the intervention.
Peer support
Peer support was not raised within the interviews as a major aspect of the intervention for either condition. The BWW, an online community and forum for people with mental health issues including depression, suited only some of the depression interviewees because others were wary of placing too much information on social network sites, thought that they might receive abuse or found that it made them feel uncomfortable or that they were brought down by others’ negativity. It suited one depression interviewee who felt cared for by the service; however, this was because of her relationship with the site facilitators, rather than relationships with her peers.
Relationships with peers were described more as context rather than in relation to the intervention. For depression interviewees, their family and friends could be a source of support for the depression as well as a cause of it. An outcome of the depression intervention could be efforts to communicate with family and friends more to bring more support into their lives. As mentioned earlier, family or friends could also support weight loss by accompanying the participants in their exercise or with their dietary improvements.
The TElehealth in CHronic disease model: treatment optimisation
According to the TECH model, there were six elements of treatment optimisation (see Table 12, Treatment optimisation): risk stratification, treatment intensification, evidence-based guidelines and protocols, regular review, promote medication adherence and share recommendations with patients.
To what extent did treatment optimisation occur?
There was evidence of medication optimisation occurring. Some depression participants were on medication such as antidepressants and sleeping pills and some CVD risk participants were on blood pressure medication and statins. Interviewees reported that the intervention impacted on medication-taking through prompts from the HIAs to participants to discuss medication with their GP or through letters sent directly to GPs from HIAs. Some trial participant interviewees described being put on medication, having their medication altered or stopping medication as a result of the intervention. HIAs felt that medication adherence could be facilitated by explaining the role of any medicine in lay language, which helped participants to understand the importance of taking a particular medication, and telephoning participants to remind them about adherence:
I duly saw the GP and he started me off on ramipril [blood pressure medication] and we’ve been gradually increasing and increasing until we got up to, what did we get up to? I don’t know, anyway, all of a sudden we were getting nowhere so they doubled it, and that’s made a big difference.
CVD risk 8
I’ve had very positive comments from participants about good support, and these are my patients who I happen to see, coming in to see me who’ve said ‘I’m stopping my tablets now because actually I’ve improved on the, with doing Healthlines and I don’t feel I need them any more’.
GP 117
She always asks me, ‘do you ever forget to take your tablets?’
CVD risk 11
Medication was not always welcomed by service providers or patients. One HIA expressed concern about the emphasis of the CVD risk intervention on promoting the use of medication when participants themselves wished to avoid it. A couple of GPs and a participant questioned whether or not medication was the best treatment for depression:
There was one point where the Healthlines advisor suggested that I increase the dose and I didn’t, I was wary about it and then actually I’m kind of reluctant to increase the dose . . . because I just think I just go up and down anyway, you know. . . . it just seems like there’s nothing else. I just feel like now that the NHS doesn’t have anything else to offer.
DEP 5
Regular review helps general practitioners to monitor health
General practitioners found the information from the regular review letters sent from the Healthlines Service useful as an early warning of problems or confirmation that a problem was under control:
the information coming back has been very good as well, with the progress and the PHQ-9’s. Obviously . . . the people running the service also informed us if patients’ mood had dipped as well, get in touch, because often patients don’t tell us that, other than having a crisis, but actually we’ve had some early warning about people who have become more unwell, so that has been good. And from the hypertension side as well, we’ve been getting useful figures on their blood pressure and how that’s been controlled, so we’ve found it really useful.
GP 117
However, these reviews and subsequent feedback to GPs were not always seen as useful or appropriate if GPs disagreed with the information. For example, GPs might have different viewpoints from the Healthlines Service about the definition of high blood pressure and might not wish to change a patient’s medication. Similarly, the view of GPs about whether or not there was a real risk of suicide might differ from suicidal risk identified by a screening tool in the study:
We would get this, a hotline e-mail saying because the PHQ-9 response, this patient has contemplated suicidal tendencies, or suicidal thoughts, but, you know, rush round to see the GP. The GP invariably would phone the patient, and the patient says no I’m fine, and, you know, and then, dare I say, some of the GPs were a bit like, oh god here we go again, it’s the Healthlines people getting their knickers in a twist.
PN 111
What is an optimal treatment?
Despite the intervention being based on best available evidence and guidance, interviewees questioned the existence of an optimal treatment for depression. A GP described depression as ‘such a woolly area’ (GP 203), requiring different treatments depending on the individual, the cause of depression and how well participants responded to treatment. Blood pressure for CVD risk was perceived by some GP interviewees to be easier to optimise treatment for, because it was more visible and could be monitored and adjusted accordingly.
The TElehealth in CHronic Disease model: co-ordination of care
The topic guide was not clear on the meaning of co-ordination of care and so the interviewer did not offer the TECH model definition to interviewees. Therefore, interviewees addressed the issue of whether or not the Healthlines Service facilitated co-ordination of the different sources of care received by participants. However, the meaning of co-ordination of care in the TECH model was different, focusing on ensuring co-ordination of care provided by the Healthlines Service with that provided by primary care (see Table 12, Care co-ordination). When co-ordination of care was asked about during the interviews, participants were usually confused about what this meant. As a result, some aspects of this component of the model were not explored explicitly in the interviews.
To what extent did co-ordination of care occur?
Within the interviews, there was evidence of information sharing and understanding of the complementary nature of the Healthlines Service to primary care. However, the NHS Direct interviewees did not mention monitoring of the system performance. Participants and GPs saw co-ordination of their care as the responsibility of GPs because chronic disease is complicated, relies on prescriptions and needs knowledge of the whole patient and a relationship with him or her. They saw the Healthlines Service as a stand-alone service, complementing primary care and other care, rather than co-ordinating it, as had been the intention of the service:
It’s kind of, independent of what we’re doing in surgery to some extent. It runs alongside it, so, although we might get things saying check this or check that . . .
GP 209
During discussions about co-ordinating care, some primary care staff raised the potential of the intervention to fragment care because it introduced another service to communicate with. This appeared to be a hypothetical concern, rather than based on experience of using the Healthlines Service.
Sharing information
Given that GPs and participants felt that co-ordination of care was the domain of the GP, it was important for GPs to receive information from the Healthlines Service to help them manage their patients’ conditions. Two types of information were discussed by interviewees: information that went onto patient records, which GPs did not need to deal with immediately but which gave them background information about patients to refer to if an issue arose at a later date, and information about a change in circumstances that needed attention. The HIAs commented that they contacted GPs more about CVD risk participants than about depression participants. Practice staff valued the background information for CVD risk participants because it prevented them calling in participants for further blood pressure checks. GPs appreciated the feedback signalling a change in circumstances or requirement for escalation of treatment (see Regular review helps general practitioners to monitor health), seeing it as a safety net. GPs preferred this approach to monthly updates, because if no action was required it simply increased their workload to have to read this information. This information sharing was visible to participants because HIAs talked to them about the letters being sent to their GP and advised them to go and see their GP if necessary, as well as letters being available to participants on the web portal. One depression participant described how she could see copies of the letters under the ‘letters bit’ of the internet programme.
Interviewees described some problems with information sharing. One GP was unhappy about the extent to which the information helped him to manage his patients. He saw the PHQ-9 as a research rather than a management tool and also criticised the layout of the information sent to primary care, which made it difficult to quickly see how to manage the patient:
They’re not, I don’t think they’re laid out particularly well. There’s lots of information on them, so teasing out the sentences ‘so please bring them in and discuss their blood pressure treatment’ could be better, it can be better worded I think.
GP 109
The HIAs interviewed also thought that the letters produced by the system were repetitive and were worried about telling GPs what to do. It was also the case that all of this information exchange occurred between GPs and the Healthlines Service; practice nurses did not receive feedback and some of the nurses who we interviewed wanted this.
A service complementary to primary care
The consensus among our interviewees was that the Healthlines Service was complementary to any other service that the participants were using in primary care or the voluntary/private sector. Some depression interviewees discussed how they still accessed their GP, practice nurse or a counsellor for help while also using the Healthlines Service. Participants who had used, or were concurrently using, different services felt that they acquired something different from each of these services:
This [the Healthlines Service] is like the left arm, the doctors is the right arm and this is the left arm. That gives me that, something to lean on if you like, to prop, that’s how they help me. So it’s, it keeps my conscience clear that I’m not clogging up his surgery, they are giving me information and advice, plus, through them, again you don’t necessarily do the doctors, you can’t, you can’t go the doctors, he’s not there for that.
CVD risk 6
Even if a participant was using a private weight loss service such as Slimming World, he or she described how the Healthlines Service offered support to help him or her continue to engage with it. In fact, one GP was pleased that someone else was looking after patients as well as general practice, to help pick up on those who were not being well managed. In contrast, there were some expressions of concern among primary care staff who we interviewed that the Healthlines Service might be duplicating existing services. This concern may have been heightened by the detachment of practice nurses from the intervention and their role in collecting data for the trial using check-ups that were similar to those in the conventional practice nurse workload.
The TElehealth in CHronic Disease model: partnership
As covered in the previous section, we saw that, for our interviewees, the information flow from the Healthlines Service to GPs appeared to be occurring as planned. However, an element of the partnership component of the TECH model was that GPs could contact the Healthlines Service to change their patients’ blood pressure target, for example. However, there was no evidence in the interviews of proactive use of the Healthlines Service by GPs (see Passive engagement from primary care).
The TElehealth in CHronic Disease model: context
There were three elements to the context component of the TECH model (see Table 12, Context): tailoring the intensity of the intervention to the individual, inviting only individuals with health problems and being aware of the fact that the requirement for having an internet connection for inclusion in the trial would affect the representativeness of trial participants. Efforts made by HIAs to tailor the interventions have already been described. There was some indication of participant problems with internet access. One interviewee reported that they did not have internet access and several were using the CBT book rather than the online programme. A NHS Direct manager drew attention to the popularity of the CBT book, indicating the preference of some participants to avoid computers. A related issue was technical competence, as identified earlier. HIAs noted how much time they spent helping people get set up on their computers and dealing with computer issues. They felt that this function had not been allowed for within the time allocated to offer the intervention. Some participants were technically competent because they described how they monitored their health on the computer.
How implementation differed from planned
On the whole, it appeared from the interviews that the intervention was delivered as planned. However, a key element of the TECH model – patient engagement – was not delivered as planned because continuity of HIAs was not delivered consistently throughout the trial. This impacted detrimentally on patient engagement with the intervention, an aspect that appeared to be an essential part of the intervention. As we explain in the following sections, there was evidence that this affected both fidelity and dose of the intervention.
Fidelity: continuity of health information advisors did not occur throughout the trial
An important aspect of the TECH intervention was offering continuity of HIAs to enhance patient engagement. Continuity of HIAs occurred for large parts of the trial period (see Chapters 8 and 9) but was disrupted by a change in the Healthlines Service staffing model over the course of the trial. NHS Direct interviewees described how, at the start of the Healthlines Service, there were six HIAs working part-time. These HIAs had to deal with a number of ‘teething problems’ with the intervention during the early part of intervention delivery for the CVD risk trial. As the trial progressed, NHS Direct moved to a model whereby some HIAs worked partly in NHS Direct and partly in the Healthlines Service. This model was introduced to offer a more efficient and sustainable way of delivering the service. There were 12 HIAs working part-time, with the six new HIAs working on the Healthlines Service for only a few hours a week. The NHS Direct interviewees described the new HIAs as struggling to understand the intervention and keep up with the number of changes that were being made to it over this time period. Participants who we interviewed who described a protocolised approach to intervention delivery were likely to have experienced this staffing model. During the trials, discussions between the research team and the Healthlines Service staff revealed the difficulties of this staffing model and the detrimental effect on continuity of HIAs. Therefore, NHS Direct created a dedicated team of three full-time HIAs and one part-time HIA to deliver the intervention. The NHS Direct staff interviewed favoured this new model of a small dedicated team, which allowed HIAs passionate about the intervention to offer continuity to participants and build up knowledge of how to tailor the intervention to individuals. This final staffing model, focused only on delivering the Healthlines Service by a dedicated team, also meant that the service operated as a ‘stand-alone service from NHS Direct’ (M2), offering autonomy to tailor the service.
Fidelity to cardiovascular disease risk intervention was not possible
The HIAs described problems with the CVD risk intervention patient management software that had to be sorted out during, rather than before, the trial. The CVD risk intervention was described by NHS Direct managers as imported from elsewhere and not ideal for the purposes of the Healthlines Service. HIAs described the CVD risk software as cumbersome, not intuitive, not allowing them to document what they needed to document, asking questions that were hard to understand and not encouraging them to set goals for participants. They described having to get used to how to tailor the CVD risk intervention to reduce the amount of potential repetition built into it. By the time we interviewed HIAs in July 2013, they had been using the CVD risk intervention for around 6 months. They were happy with the CVD risk software at that point in time, although they described having to work hard to make it useable in practice, even with the improvements that had been made over time. HIAs felt that they did not follow the script within the intervention, but created their own way of making it work.
Dose: challenges of workload management affected the dose offered
The HIAs were concerned about the amount of workload involved in delivering the intervention and how this impacted on their ability to provide timely appointments. Appointments that should have taken place every 2 weeks ended up taking place every 4–5 weeks in practice. This was related to perceptions that there were not enough HIAs, the need to have annual leave and some aspects of delivery being more time-consuming than originally envisaged. For example, HIAs described how offering participants technical support and getting them logged on to the system at the beginning of the process took a lot of time, how some participants needed a lot of chasing up when they missed appointments and how there was administrative work, such as obtaining information requested during a call. Over time, HIAs had learnt to manage this ‘admin work’ by understanding that some sessions took longer or were shorter than the intervention stated and by squeezing administrative tasks in between telephone calls. Workload management was reported as being exacerbated by spikes in recruitment to the trial, particularly for CVD risk. It could be argued that these spikes in demand were specific to the trial context or that similar spikes might occur for the intervention operating in the real world.
Mechanisms of action
In this section we consider which aspects of the intervention appeared to be key to obtaining outcomes. The three mechanisms of telehealth for LTCs identified by our realist synthesis (see Chapter 2) were highly relevant: relationship, fit and visibility. 125
Engagement
Engagement with primary care
Engagement with GPs appeared to be essential because the Healthlines Service needed to feed back any health problems identified to GPs so that they could take action, particularly around changes in medication. The mechanism was ‘visibility’ in that the Healthlines Services helped to make patients’ health problems visible to their GPs.
Engagement with patients
Engagement with patients was also essential and depended to some extent on the mechanism of the ‘relationship’ offered by the HIAs. Some participants wanted telephone contact with the same HIA over the course of the intervention to build rapport and trust. Trust was important for some depression patients who were dealing with past traumatic life events. This ‘relationship’ with the HIA facilitated ‘visibility’, whereby some participants wanted to show their progress to their HIA during review telephone calls (e.g. their weight loss). Participants described how encouraging it was to engage with someone who took an interest in them and cared about them. This ‘relationship’ also facilitated ‘fit’ in that the HIAs could then more easily tailor the intervention to participants’ needs and help them to fit the intervention into the complexity of their lives in terms of balancing the intervention with work and family commitments.
Disengagement occurred when participants described HIAs as failing to tailor the intervention to meet their needs or as not interested in whether or not any CBT homework had been completed. Half of the depression and half of the CVD risk interviewees described the intervention as protocolised, with a detrimental effect on engagement. Indeed, three of the depression interviewees expressing concern about this had withdrawn from the intervention and the other three had largely disengaged from it at the time of the interview. Some participants called the intervention ‘repetitive’, ‘impersonal’, ‘a ‘script, ‘not a script, but a list of topics that have to be covered’, ‘going through the motions’ and ‘a regular spiel’. Participants found this frustrating and disengaged if they felt that the HIAs were just doing their job, were ticking boxes, didn’t care whether or not CBT homework was completed, were ‘clock watching’ or were going through the motions:
I could really I could see it in my head a checklist in front of them, we must get through this tick, tick, tick, tick, tick.
DEP 1, who had withdrawn from the intervention
It’s just like a call centre, giving you the same information every month.
CVD risk 3
The HIAs who we interviewed were aware of the need to adapt the intervention to stop it feeling robotic. They felt that the CVD risk patient management software, in particular, could be repetitive and did not set goals early enough in the process, so that HIAs had to make it work by ‘going off script’:
Definitely, definitely, I think the participants need to know that they’ve been listened to, and that’s a really big thing with CVD, that’s why we’ve managed to build rapport with them. If we hadn’t listened to them and stuck with the scripts I probably say a lot more would have quit by now, easily, because it’s not really a service that’s going to work for them if we’re not working with them. I think you have to be quite, you have to meet their needs and make sure they know that you want to help them. You’re not just delivering a generic service, and hoping they will take what they need from it, so you have to put more effort into it with CVD.
HIA2
The HIAs felt that the Duke patient management system, on which the CVD risk intervention was based, limited the extent to which the service could be tailored. The work that HIAs did by tailoring the intervention to people’s needs and knowledge levels (fit) was appreciated by some participants – ‘it is a bit more personal than a very set sort of script, if you like’ (CVD risk 11) – as was flexibility to make appointments fit around people’s lives.
Continuity of HIAs (relationship) or tailoring of the intervention (fit) could not solve all problems with engagement because some participants were looking for a different type of service from the one offered by the Healthlines Service (fit), that is, some participants struggled to engage with the intervention because it did not fit their expectations of the service or their perceived needs. Some participants who we interviewed viewed the Healthlines Service as too simplistic for treating depression caused by major life events, such as bereavement or abuse, and felt that they wanted a service based on counselling rather than CBT or additional access to HIAs beyond the scope of the intervention. In other words, they were looking for a talking therapy rather than self-management. For CVD risk participants who already had knowledge of the importance of lifestyle for their health, the service did not offer them anything new (fit). Indeed, one CVD risk participant felt that he knew everything he was being told: ‘I don’t think I’m thick’ (CVD risk 3). The fit could also be poor for participants who wanted help with another health problem that they had or who wanted in-depth conversations with the HIAs or a personal approach:
There never felt enough space to go deep enough into things there, and definitely with the telephone thing I don’t feel comfortable with that. When I can’t see somebody’s face, and feeling that they’re just going through set questions anyway, I don’t feel any sense of being able to get into what’s really going on.
DEP 5
Basically, the person who rings you up is a disembodied voice, so really doesn’t know you very well, and the amount of time they have to, to actually get to know you is very limited, so you wouldn’t expect much else with you really. I think in theory it’s a great idea, but I’m just wondering whether you can be personal enough.
CVD risk 5
Engagement of health information advisors
Helping the intervention to ‘fit’ participants through tailoring was facilitated by the high level of engagement of the HIAs with the Healthlines Service. The HIAs who we interviewed discussed how passionate and enthusiastic they were about their work. They had volunteered for the Healthlines Service because they believed in the work that it was doing. Their passion meant that they were committed to the work and reflected on ways of delivering the service well. They also pointed out how much they liked people and wanted to connect with them (relationship), which helped them to tailor the intervention (fit). The HIAs were aware that some of their colleagues in NHS Direct who had delivered the intervention during a period when the organisation had not offered a dedicated Healthlines Service team did not share this passion or did not have the time to practise using the software. The HIAs, a GP and some participants described the attributes of a good HIA, which supported the importance of engaged HIAs: listens, shows interest, does not lecture but suggests, does not tell you what you already know and gets to know you to address your concerns. The enthusiasm of the dedicated team of HIAs was likely to have encouraged them to enhance any ‘fit’ and ‘visibility’ built into the technological aspects of the intervention. Therefore, continuity of enthusiastic HIAs was very important for patient engagement.
Engagement of family and friends
‘Relationships’ outside the intervention were also important. Friends and family were mentioned by interviewees as facilitating patient engagement in terms of supporting participants through the intervention, encouraging increased activity such as indoor bowling, walking or swimming, altering their diet to help participants or helping with technological problems. A couple of the HIAs also talked about how, as wives often do the cooking among the older generation, their support was necessary for male participants to engage:
I’ve had to persuade the wife as well . . . and she, she keeps her system, her weights coming down as well, you know, now we’ve started, you know, sort of liaising with one another.
CVD risk 10
I do find that with the older generation, it’s normally the wives that do the cooking, so I have had a few people that have said, ooh yeah, will you tell this to my wife because I don’t do the cooking.
HIA 4
Raising awareness and education about health and health care
Depression and CVD risk interviewees discussed how the intervention helped them to become aware of their health problems and educated them about both strategies for dealing with those problems and how those strategies worked (e.g. education about medications), that is, it increased the ‘visibility’ of both their problems and potential solutions. The CBT and BWW technologies offered solutions for depression participants. The HIAs created a ‘fit’ between a participant’s world and the medical world by using simple language during communications. However, ‘fit’ could be a problem if HIAs followed CVD risk intervention modules as prescribed when some participants felt that they were already aware and educated or had no interest in the content of a module. For example, the HIAs believed that the CVD risk modules focused overly on medication when some participants had made it clear that they were not interested in taking medication. ‘Fit’ was also a problem when participants with experience of depression felt that they had used CBT before and they were looking for a more personal and in-depth approach.
Awareness and education were relevant throughout the intervention but were very important at the start. The transtheoretical stages of change model proposes six stages of change that a person must go through to change their behaviour: pre-contemplation (no intention to take action over the next 6 months), contemplation (intends to take action within the next 6 months), preparation (intends to take action within the next 30 days and has taken some behavioural steps), action (has changed behaviour for < 6 months), maintenance (has changed behaviour for > 6 months) and termination (no prospect of relapse and no need for intervention to continue). 394 The HIAs felt that raising awareness and education had brought about change only at the pre-contemplation, contemplation and preparation stages for some participants:
I think the most difficult bit is to get them to stop smoking, I find because, not stop but to say you are a smoker, this is a risk factor. Quite often you can only get them to prepare maybe, get it set in their mind to stop, because ultimately it’s up to them whether they’re going to.
HIA 3
We had a lot of participants who initially just didn’t want to, hadn’t really got the motivation to do that, to go through all that process, so it took a long time to start, to get the initial engagement going.
HIA 4
Feedback and review
Participants could monitor their weight or blood pressure and this increased the ‘visibility’ of both their health problems and the progress they were making. Feedback and review also helped with patient engagement (see The TElehealth in CHronic Disease model: self-management). Monthly telephone calls from the HIA could prompt participants to take action:
It brings it to the forefront, you know, specially every, when they’re phoning you up every month and what [laughter], they keep jabbing you like, but it’s good, it’s good, you know what I mean, it is good.
CVD risk 2
Self-efficacy
The intervention needed to help people to believe that they could manage their condition or make lifestyle changes. Some participants discussed how the intervention helped them to seek treatment from elsewhere, empowered them to return to their GP about a health problem (not necessarily the health problem they were participating in the Healthlines Service for) or changed their perspective on their control of their lives:
At the end of the day, nobody can make the changes for you, you’ve got to do it yourself, but having somebody to point you in the right direction is what you need.
DEP 7
However, there was also a sense that some participants relied on the intervention in terms of needing to be ‘visible’ to the HIA to encourage them to take action:
The fact that I know that I’m going to get a phone call [laughter] in a month, and she’s going to ask me how much I weigh, that does focus your mind.
CVD risk 11
The implication of this extrinsic motivation offered by the Healthlines Service was that progress would end once the HIA stopped telephoning. However, we did not interview any participants who had completed the intervention and the focus on self-efficacy within the intervention may have increased towards the end of intervention delivery.
Context
Four contexts emerged that were important for the intervention and outcomes achieved: the lives of individual participants, the landscape of care available to participants, NHS Direct as the intervention deliverer and delivery of the intervention within a RCT rather than the real world.
The lives of individual participants
Complexity of people’s lives
Some people declined to participate in the trial because they felt too busy or too ill. 395 The complexity of people’s lives could also be a major barrier to engaging with the intervention once they had agreed to participate. Younger women with depression were highlighted by the NHS Direct staff as having so much going on in terms of looking after children or working that they could not find the time to participate fully in the intervention. In particular, they could not find time to complete the sessions and CBT homework and often missed telephone appointments. The HIAs wondered whether or not some of this was the result of their depression as much as their busy lives:
The depression ones, a large, it seems to be a lot, to me, younger people, a lot more women, not all but they‘re rushing around, they don’t have time, they forget they’ve got appointments, and whether it’s part of depression or not I don’t know, but they don’t often, they don’t answer the phone.
HIA 1
Some depression interviewees described serious ongoing life events, such as threat of loss of disability and unemployment benefits, physical illnesses or family members and friends who were very ill or depressed. These issues caused stress on top of the depression, making engagement with the intervention difficult. Life events getting in the way of engaging with the intervention appeared to be less of an issue for CVD risk interviewees. This may have been because we did not interview people who had withdrawn from using the CVD risk intervention, but it was also the case that the HIAs who we interviewed saw this as less of an issue for CVD risk participants. The lack of complexity of people’s lives may have been related to age because the CVD risk participants were older and many were retired. Only one of our CVD risk interviewees was still in full-time paid employment and this interviewee did report finding it difficult to fit the intervention into his life.
Finally, our CVD risk interviewees tended to have a number of comorbidities, which occasionally could act as barriers to making lifestyle changes. For example, a CVD risk interviewee with severe arthritis found it difficult to exercise.
Complexity and longevity of depression
Even though we selected participants for interview with a range of depression scores, most of the depression interviewees described serious causes of their depression, some of which had affected them for decades or since childhood. Some depression interviewees had multiple issues that had accumulated over their lifetime or which were occurring simultaneously at the time of the interview. Examples included domestic violence, death of a close relative, a serious illness, job loss, children with severe health problems and a family break-up. Some had already tried CBT and counselling and were looking for something to help them understand their feelings rather than ‘be processed like McDonald’s’ (DEP 9). The HIAs understood this and sometimes found themselves in the role of counsellor because people had such difficult circumstances to deal with.
Depression could vary considerably by participant and over time. Our GP interviewees were aware that depression had a variety of causes and that therefore a variety of solutions was needed. Participants and HIAs were also aware that depression varied over time. This had implications for the intervention in that there was a need for it to adapt to this variation and implications for the trial in terms of when measures were taken in this variable cycle of depression.
Motivation and ability
An individual’s motivation to join the study appeared to be a key contextual issue. Of the 11 CVD risk and nine depression patients who we asked directly, three CVD risk participants and four depression patients had joined the trial solely because they were motivated to improve their health: ‘this is [a] chance now to make some sort of determined effort’ (CVD risk 11); two from each condition had both personal and altruistic motives; and six of the CVD risk interviewees and three depression interviewees had been recruited to the trial from research practices and took part in the research to be helpful or because their GP wanted them to participate:
Well I don’t know, I just, I just got asked and I thought well, I don’t have much trouble and I’m usually all right with everything, but, I suppose they’ve got to get things from different people who, people who are ill and people who are not really, so I just thought well, I hadn’t a lot on so may as well do it.
CVD risk 9
It was from an altruistic point of view rather than, I, I didn’t really know what it would entail for me.
CVD risk 11
There was the belief among all groups of interviewees that, if intrinsic motivation was absent, then participants, particularly those in the CVD risk trial, would find it difficult or be unable to make lifestyle changes. Depression participants may have lacked intrinsic motivation because of their condition. CVD risk participants may have lacked motivation to improve health because of the invisibility of high blood pressure, the lack of symptoms, the perception that they were managing their health problem already with medication or they were happy with their lifestyle.
Primary care interviewees described affluent populations as more willing and able to engage with the intervention. They were described as motivated to change as well as being technically competent and therefore more able to engage easily with the intervention:
So, from the technological point of view, they’re very good. And I think they probably would use something like Healthlines in that way because they would take advantage of it. They will often take advantage of stuff, of input, and they will understand. They usually are well educated and will understand advice that’s given them. So I think it would be, it is useful for them; they would find it helpful.
GP 203
The landscape of care available
The Healthlines Service: one of many services available . . .
The Healthlines Service did not offer an intervention in the context of a desert of care. Primary care interviewees described how services were already available for both people with depression and people with CVD risk factors. For depression patients, primary care staff described that they were not offered psychiatric services, which were reserved for more serious mental health problems, but did use their GP, Improving Access to Psychological Therapies (IAPT) and counselling from a range of sources, such as CRUSE, occupational health, private care and the NHS. Some participants themselves described past and current use of these services over the lifetime of their long-term or periodic depression. Indeed, one HIA saw it as her role to ensure that services in contemporaneous use for depression did not cause conflict for participants in the Healthlines Service. For CVD risk factors, practice nurses, in particular, pointed out how a number of checks and reviews were undertaken on this group, including NHS Health Checks, measurements for the QOF and medication reviews with the GP. Some practice nurses also described how they guided patients to other services, such as smoking cessation, health trainers and weight loss groups, as well as offering lifestyle advice themselves. In other words, they described their role in much the same way as HIAs described their role. Self-management was also described by participants and primary care staff as available through use of blood pressure monitors on loan from the practice or bought by patients:
I would normally, if I saw somebody for a general lifestyle advice, so weight management something like that, I would always check their blood pressure anyway. And if their blood pressure was high, then, yes, it would be part of their tailored programme, that they look to increase their activity, to monitor their blood pressure. Usually when they come back to see me for a weigh in I would take their blood pressure again at that stage.
PN 113
. . . but more needed
Having pointed out the availability of a range of services for the two exemplar conditions, primary care interviewees nonetheless saw that the Healthlines Service could address a need for help with low-level depression and lifestyle advice for CVD risk patients. They also pointed out that accessibility of care for depression could be an issue, with waiting times of 4–6 weeks for IAPT and 12 months for counselling for some general practices. Depression interviewees concurred with this sense of a lack of accessibility of appropriate services, identifying that waiting times were long for NHS services and the numbers of sessions limited and that they could not necessarily afford to fund counselling themselves. There was also some indication that accessibility of services could differ by general practice:
It does seem to be an area that is lacking in services. I know when I’ve spoken to people they’ve said even if there are things available it’s such a long waiting list.
DEP 5
Similarly, some CVD risk interviewees highlighted the need for the Healthlines Service, pointing out their lack of awareness about the availability of some services, that they had been offered advice but not known how to act on it and that they had been offered access to a service but it had not materialised. Some primary care interviewees identified that the Healthlines Service could make access to health checks more systematic and facilitate patient actions. Therefore, there was a perceived need for the intervention on offer by the Healthlines Service, supporting the findings of our earlier survey of interest in telehealth. 185 However, primary care interviewees’ views of the need for the Healthlines Service could be described as ‘mildly positive’, rather than ‘actively seeking’. A GP said they would want to know about cost-effectiveness and the ability of the intervention to save GP workload if they had to pay for it. This contrasted with participants’ views, with some interviewees with CVD risk factors and particularly those with depression actively wanting more services. In fact, one depression interviewee was delighted to have the intervention even though they were currently attending counselling, showing that there could be an expressed need for the intervention when access to services was good.
A threat to primary care?
Earlier qualitative research in our programme (see Chapter 4) identified that practice nurses may consider the management of LTCs as their domain, causing some scepticism in primary care about what telehealth could really offer. 220 However, there was little sign that GPs or practice nurses in this qualitative sample, who had experienced the intervention, held any scepticism about the Healthlines Service or sensed any threat. As detailed earlier, they seemed happy to see it in operation alongside the other services available. Only one practice nurse expressed a sense of threat and this was voiced as a joke, with the perceived threat to GPs rather than to practice nurses.
A surprising gap in our data was that interviewees had little or nothing to say about the context of primary care in which the trial was occurring. This may have been related to the separateness of the Healthlines Service from primary care in practice, in that GPs reacted to prompts from the Healthlines Service but did not take an interest in patients’ experiences of it.
NHS Direct for intervention delivery
Wider political changes to NHS Direct
Before the start of the trial, NHS Direct was an established national service offering health information and advice to the general public and triage for GP out-of-hours services. In 2010, a new national service – NHS 111 – was introduced to offer access to urgent care for the general public and NHS Direct delivered a number of these contracts around England as the service was rolled out. During the trial, a national announcement was made that NHS 111 would replace NHS Direct and, indeed, NHS Direct ceased to operate in March 2014, towards the end of the trial (see Chapter 6, Closure of NHS Direct and transfer of the Healthlines Service to Solent NHS Trust). This national change was not raised very much by our interviewees, even NHS Direct staff. Our interviews with NHS Direct staff mainly occurred after NHS 111 had been operating for a while and prior to the announcement of the closure of NHS Direct. It was also the case that the HIAs came from the information provision section of NHS Direct, rather than the urgent telephone line provision section, and so would not have been immediately affected by the introduction of NHS 111:
Not impacted on it at all, because it is, even though they are NHS Direct staff, actually man it that they’re health information advisors that actually run it, they have been working solely on it now for the last few months, it’s not changed it’s, it’s a service we deliver regardless of any organisation changes.
M2
This might explain why our NHS Direct interviewees, apart from those from senior management, had little to say about these contextual changes. Comments about the closure of NHS Direct came from managers in the organisation who were making efforts to ensure that another organisation took over the intervention and the research and allowed the trial to complete, with continuity of the quality of the intervention.
External views of NHS Direct
Two participants described how they had previously experienced NHS Direct as a telephone helpline in ways that had coloured their views of the service. For example, one participant had waited a long time for a nurse to call back, so when the HIA recommended calling NHS Direct if there were any problems the participant decided not to because they expected a long wait. However, this was not a common issue among our interviewees. According to one HIA, some participants did not know about NHS Direct and so had not formed an opinion about it.
The added value of NHS Direct for intervention delivery
NHS Direct may have delivered the intervention in ways that another service provider would not have. The HIAs felt that, although the work that they did in the Healthlines Service was different from their NHS Direct work because they were able to spend time building up a relationship with people in the intervention and worked more autonomously, embedding the service in NHS Direct had brought added value to the service. HIAs felt that they could draw on wider resources within NHS Direct to help participants, such as advice from clinical staff and information on medications. They also found it easier to fulfil the part of the intervention that required them to signpost participants to a range of information and voluntary services because of their experience of doing this within NHS Direct. Finally, they felt that NHS Direct had trained them to attend to clinical safety, handle difficult situations such as participants being upset because of their depression and communicate with people over the telephone.
A potential downside of being situated in NHS Direct was that participants sometimes assumed that the HIAs were clinically trained and would ask about their wider health problems. The HIAs reported dealing with this by being clear about their background and, if necessary, redirecting participants to clinicians within NHS Direct, their GP or other services. The added value of NHS Direct in delivering the intervention was also noted for intervention development. The NHS Direct interviewees felt that their organisation had developed other telehealth services for LTCs and built on this expertise to develop the technological platform for the Healthlines Service. This raises the issue of whether or not organisations other than NHS Direct could deliver the Healthlines Service. When NHS Direct managers discussed the search for an alternative NHS trust to deliver the intervention, the search was wide, encompassing different types of trusts with experience of delivering telephone-based care, indicating the availability of alternative options.
The trials
The intervention was delivered in the context of a trial and it is important to consider the extent to which the trial affected the implementation of the intervention and the outcomes achieved.
Inappropriate inclusion criteria?
The HIAs and NHS Direct managers expressed frustration at the perceived inappropriateness of some of the people recruited to the CVD risk trial. They felt that some people should not have been offered the intervention because they had relatively low levels of certain modifiable risk factors that they were willing to change but were unwilling to make lifestyle changes for other risk factors such as smoking. The HIAs also described some participants as wondering why they were being offered such an intervention. Indeed, our CVD risk interviewees expressed mixed motivations for joining the trial (see Motivation and ability), with some reporting altruistic reasons and others wanting to gain help with their health problems. Altruistic reasons included the common good, to help with research, to give back to the NHS or because they thought it sounded interesting:
People who want to feel that they are helping the research in some way . . . I’ve got some that are perfect weight, don’t smoke, don’t drink, exercise a lot, they’re fitter than I am some of them, and you think, gosh, I don’t understand why they’re in this service.
HIA 1
One HIA was not concerned about the inclusion of people who did not want to change because she recognised that convincing people of the need to change their lifestyle was important, although she acknowledged that this was a long-term endeavour.
The HIAs had less concerns about the inclusion criteria for the depression trial. They noted that some people with depression had busy lives so did not complete the intervention or had undertaken CBT previously and did not want to do so again. They felt that those who did complete the course got a lot of benefit from it and so wanted the inclusion criteria to focus on those who were really committed to the intervention. Again, participants reported a mix of altruism and expressed need as reasons for joining the depression trial. There was little evidence of low need in this participant group, with only one interviewee stopping using the depression intervention at an early stage because he no longer felt depressed.
The HIAs also expressed more general concerns about inclusion criteria for both trials related to people who were unable to participate because of a physical disability or a lack of computer competence. Some informal screening may have occurred in that a GP described having screened out people he felt could not benefit or who would be rude to those delivering the intervention.
Driven by research needs or real-world needs?
NHS Direct managers expressed concerns that, even though they had developed the intervention jointly with researchers, the researchers placed more emphasis on theory and research evidence than operating the intervention in the real world. They felt that there was a lack of research evidence available about important issues, such as how best to implement telehealth. There was a fear that, if the interventions were not shown to be effective, then the interpretation in the wider world would be that interventions delivered by NHS Direct were not effective, rather than that these specific interventions were not effective.
Another concern of NHS Direct managers was that there had been too much emphasis on the numbers recruited rather than on who was recruited. They felt that, if GPs had to select participants to receive an intervention in the real world, they would select people who had a need and motivation to change.
What is the research and what is the intervention?
The HIAs felt that some participants did not distinguish between the researchers and the staff delivering the intervention. Indeed, during our interviews, participants sometimes described the intervention as delivered by researchers. This has potential implications for the qualitative research and perhaps the completion of subjective measures in the trials in that participant views may have been tempered in order to offer a polite view of the service, such as ‘it was good, it helped’, when people did not necessarily mean this. Having said this, some participants appeared to have no difficulty pointing out problems with the intervention from their perspective during our interviews.
Some aspects of the trials and qualitative research may have acted as interventions. Health checks were undertaken on CVD risk participants at baseline and at follow-up points in the trial to measure blood pressure among other things. This was part of the trial measurement, rather than the intervention. Some CVD risk participants identified this check as the best thing about the intervention, even though it was not part of the intervention. A GP liked it too because it helped to identify people who needed better management. A practice nurse described this measurement session as giving them longer than usual with patients, which was beneficial for the relationship between patient and health professional. These checks occurred for the usual care group, too, so did not impact on the experimental design. However, they may have impacted on outcomes for both the intervention and the usual care arms, potentially causing improvement in both arms.
A similar issue may have occurred with the qualitative research. In a couple of participant interviews, participants who described themselves as having drifted away from the intervention said that they would get back in touch with the Healthlines Service. This is unlikely to have had any impact on trial outcomes because the numbers of interviews that we undertook were small compared with the numbers participating in the trials and these participants may have been being polite while having no intention of renewing contact with the intervention.
Finally, the HIAs had worked closely with the research team during the early implementation of the intervention and really enjoyed ironing out ‘teething problems’ during weekly meetings with the researchers. They were unhappy when the researchers withdrew from this process to leave NHS Direct to implement the service, although this was the most sensible thing to do from the perspective of the research.
Internal pilot
There had been an internal pilot for the trials and therefore some of the development work on the intervention was undertaken during the early months of the trials, particularly for the CVD risk trial. The possible implications of this are that participants in the early stage of the trials may have received an underdeveloped intervention, with those recruited later receiving the more definitive intervention.
Research as benefit or burden
Many of our interviewees wanted to know the results of the trials, including participants, primary care staff and NHS Direct staff. This highlighted the level of interest in the research and the importance of the research team feeding back results to all stakeholders. Participating in the research could also be burdensome: GPs found their research task of screening participants for the trials time-consuming and NHS Direct managers noted that it was costly to host the intervention.
Reach: who is not in the trials?
We interviewed participants receiving the interventions and therefore reflections on those who were not in the trials came from comments made by primary care and NHS Direct interviewees and our views of who we were not seeing in our interviews. We selected people from practices with a range of deprivation scores and went to visit council estates for our interviews. However, we were aware that participant interviewees were receiving welfare benefits for health rather than for social reasons and we did not feel that we had interviewed people in very socially deprived circumstances. There were few people from minority ethnic groups in our interviews. This was also the case for the trials, which has implications for the transferability of the trial results in that they may not be transferable to very deprived communities or ethnic minority communities.
Testing hypotheses generated by the embedded qualitative study
In our draft report of the qualitative study to the team in September 2014 we generated the following hypotheses, which were tested either as part of the original statistical analysis plan or because of the qualitative study reported here. These are described below, followed by a summary of the results of each analysis.
-
Some patients with depression had severe life situations and a long history of depression and had used many interventions to deal with their depression. There is an issue about whether or not CBT delivered by computer or book could impact on such difficult circumstances and that severity of baseline depression or length of history of depression might be useful to compare with size of effect. The hypothesis would be that those with a shorter history and less severity would be more likely to show improvement.
A prespecified subgroup analysis for the depression trial was carried out on baseline severity of depression. There was no evidence of any difference in effect for mild, moderate or severe depression (see Table 22).
-
Because some risk factors for CVD required behaviour change and others medication, with the former much harder to affect than the latter, in a subgroup analysis we expected people with high blood pressure to have better outcomes (i.e. 12-month QRISK2 treatment response) than those with weight issues and especially those who smoke.
This hypothesis was not supported as there was no evidence of a difference in treatment response between those who had high and those who had low systolic blood pressure at baseline nor between those who had a high BMI and those who had a low BMI or between those who were smokers and those who were non-smokers at baseline (see Tables 70 and 71).
-
As it might be expected that those who had more severe readings of their baseline CVD risk factors would improve these readings to a greater degree, another subgroup analysis compared those with high baseline blood pressure with those with low baseline blood pressure in terms of their improvement in blood pressure at 12 months. Likewise, we also compared those with high and those with low BMI and those who smoked and those who did not smoke at baseline in terms of 12-month BMI and 12-month smoking status respectively.
This hypothesis was also prespecified in our statistical analysis plan and was largely supported by the results. In particular, the secondary outcomes analyses did show that the intervention group was more likely to show improvements in blood pressure and BMI than those in the usual care group at 12 months, whereas there was no evidence of a difference between the trial arms in terms of 12-month smoking status (see Table 51).
-
Given concerns about whether or not the inclusion criteria should have been different, because some people had a low need or no desire to modify risk factors, undertake a subgroup analysis on the level of internal and external motivation for joining the study. Those who were highly motivated to join the study because they personally wanted to make changes in their life (i.e. internal motivation) would be expected to improve their cardiovascular risk more than those with low internal motivation.
There was no indication of any difference in effect between those with low and those with high baseline internal motivation in either of the trials nor any difference in effect for those with different levels of external motivation in the CVD risk trial (see Tables 43 and 72). However, there was some evidence of a subgroup effect for external motivation in the depression trial. In this case, the intervention appeared to be more effective among participants who had low external motivation at baseline, that is, who were less likely to have reported that they joined the study because of external pressures, such as from their doctor.
Summary of findings
There was evidence to support the contribution of all components of the TECH model to the effectiveness of the intervention to some extent. The Healthlines Service was largely delivered as planned with the exception of two elements: continuity of the HIAs for individual participants and tailoring of the intervention to participants’ needs. These were compromised in the early months of implementation until a small dedicated team of enthusiastic HIAs delivered the intervention in later months. This detrimentally affected patient engagement with the intervention, which appeared to be key to the effectiveness of the intervention. The context of the lives of individual participants may also have affected participant engagement with the intervention: some depression trial interviewees who had or who were experiencing severe depression felt that the intervention was too simplistic for their needs and some CVD risk trial interviewees who were not motivated to change their behaviour at the point of joining the trial may have struggled to engage with the intervention. There was also a suggestion that the context of delivering the intervention in a trial setting contributed to improvements in blood pressure control in the usual care group; this was supported by the trial data because there were reductions in blood pressure in both the intervention and the usual care groups.
Before the trial results were available, we reported the findings from this qualitative study to the wider team, suggesting that the intervention would be effective but that the size of effect would be small to moderate given some of the issues about implementation and context. We generated hypotheses that the intervention might be more effective for some subgroups, but there was little or no evidence to support these. In the following sections the findings are explored in more detail and in the context of related research.
Did the qualitative interview study indicate that the intervention would work?
Process evaluations are undertaken to help explain the results of trials. Oakley et al. 357 recommend that analysis is undertaken prior to the trial results being known in order not to bias the interpretation of the process evaluation. Based on interviewees’ perceptions of outcomes, the process evaluation team communicated in writing to the wider research team in September 2014 (the trial results were available in December 2014) that:
There is evidence of actions on the causal pathways, evidence of people improving greatly and associating this with the intervention, and evidence of people who have not needed the intervention or engaged with it. Our prediction is a small to moderate effect size, with wide variation in the amount of change seen in individuals. Indeed the spread of change in intervention individuals compared with controls would be useful to view. We would predict large change in a small group [of] participants.
Internal research team report
The process evaluators felt that it was unlikely that no improvements would be seen, as trial participants in our sample described improvements that they associated with the intervention. Usual care participants, who might also have described improvements in their depression and CVD risk factors, had not been interviewed but intervention participants directly related changes to the intervention and this gave us confidence that the intervention had had an effect for some participants. The process evaluation team felt that it was also unlikely that large improvements would be seen because the intervention had not always been successful at engaging patients and the trials had included participants who were unlikely to change their behaviour in the time period of the trials. Specifically, the process evaluators predicted that people with severe depression would be less likely to benefit and that some CVD risk factors would be affected more than others, namely, that smoking would not be affected because participants did not want to give up smoking and that blood pressure would be affected because the medication and lifestyle aspects of the CVD risk intervention appeared to work for some interviewees. This prediction was correct in terms of a small to moderate effect in both trials, affecting blood pressure rather than smoking, but not in terms of the prediction about which types of people would benefit. In the following sections we discuss how modest effects might have been gained and why they were smaller than the minimum clinically significant changes used in the sample size calculation.
Was the TElehealth in CHronic disease model a good basis for an intervention?
The TECH model was based on evidence about what works in telehealth for LTCs (see Chapter 5). In particular, it attended to ‘relationship’, ‘fit’ and ‘visibility’, which were identified as key mechanisms of change in telehealth for LTCs. 125 The process evaluation identified that the TECH model, in practice, appeared to have a number of active ingredients: HIAs facilitated patient engagement with the intervention by offering continuity and tailoring the intervention to patient needs (the mechanisms of ‘relationship’ and ‘fit’); the focus on medication optimisation in conjunction with communication with GPs facilitated reductions in blood pressure; HIAs helped patients to become aware of their problems and understand them (‘visibility’), as well as educated them about solutions; regular reviews through self-monitoring and telephone calls from HIAs encouraged patients to make changes (‘visibility’); and there was a focus on self-efficacy, which has been identified by others as key to successful digital interventions. 396 The intervention appeared to be person centred, as well as theory and evidence based, which has been identified as crucial for successful telehealth interventions. 396
Was the intervention implemented as planned?
The intervention was largely delivered as planned. At the beginning, however, there were problems with the CVD risk intervention and HIAs had to learn how to manage the patient management system and make up for its perceived problems. This was likely to have affected patient engagement as some participants viewed the intervention as protocolised rather than tailored to them. A more problematic issue was that the continuity of HIAs, a planned element of the patient engagement component of the TECH model, did not occur consistently throughout the trials, that is, there was a lack of fidelity to the intervention when it started. This lack of continuity of HIAs in the early months of the trial most likely contributed to withdrawals from the intervention and a lack of engagement with it in terms of doing CBT homework and making behaviour changes. There may also have been lower than planned doses of intervention received because of workload management issues in the early phases of the trials and because of disruption as a result of the closure of NHS Direct in the later phases of the trials.
The importance of continuity of advisor was included as a component of the intervention because it was identified as potentially important in our earlier evidence synthesis (see Chapter 2) and qualitative research (see Chapter 4). 125,220 The importance of this element of telehealth interventions has been emphasised by other researchers:
Tele-carers need to be flexible and recognise that participants vary in their knowledge, skills and psychological adaption to diabetes. Continuity of care and consistent contact is pivotal to participants being able to move through the various phases of their illness trajectory and make the transition towards improved self-care management.
Gambling and Long (p. 219)397
However, providing continuity of care from a particular advisor is not usually a feature of telehealth interventions based on a ‘call centre’ model. Engagement has been identified as problematic in a systematic review of computerised CBT, with only a median of 56% of participants completing a full course of it. 81 The personal circumstances of those using the intervention have been cited as a cause for withdrawal from the intervention, which supports our findings that the complexity of people’s lives in our depression trial was problematic. It is also interesting that significant staff time for supporting clients was highlighted in this review, supporting our study findings that dedicated HIAs were necessary. 81
The intervention was intended to promote self-efficacy, which has been considered essential for digital interventions to achieve longer-term effects. 396 Although some of the process evaluation interviewees described feeling empowered by the intervention, others appeared to rely on their relationship with the HIAs to stay engaged with the intervention and make progress with health improvements. This was highlighted in earlier qualitative research used to develop the intervention (see Chapter 4). 220 We did not interview participants who had completed the intervention and therefore cannot comment fully on the extent to which the intervention encouraged self-efficacy.
Was the intervention aimed at the right people?
A light intervention for severe depression?
Depression trial participants largely joined the trial to obtain treatment because they viewed access to services as limited. Some of our depression interviewees had tried a number of treatments over the life of their long-term depression, including CBT. They did not view CBT or the BWW as sufficient for their problems because they were looking for a more therapist-based approach to help them identify the causes of their condition or address deep trauma. However, the subgroup analysis of the trial data did not support the hypothesis that effectiveness would be related to severity of depression.
When participants were on their ‘stages of change’ journey
Some CVD risk trial participants were not motivated to change their behaviour when entering the trial. They had consented to participate in the trial for altruistic reasons or to gain health benefits without necessarily understanding that they would have to make an effort themselves to do so. HIAs had to focus on getting some participants ‘ready for change’. Although motivational interviewing was part of HIA training, the TECH model may have assumed that participants would be able to move to the action ‘stage of change’ relatively quickly and that, by the end of the intervention, they would have reached the maintenance or termination stages. The CVD risk trial was designed to measure clinical effectiveness, defined by change at the end of the stages of change journey, so success at shifting people along the early stages of change may not have been measured by the trial primary outcome.
Behaviour change was a large focus of the CVD risk intervention. The COM-B model combines a number of psychological behaviour change theories,258 proposing that capability, opportunity and motivation are required to affect behaviour change. Some of the CVD risk trial participants lacked motivation to change when they entered the trial and the HIAs dealt with this by trying to change awareness of risks and encourage motivation to change. They were equipped to do this because they had received training in motivational interviewing to deliver the intervention. However, the HIAs struggled to deal with this lack of motivation at first. One option for a future intervention might be to exclude people not ready to change or to ensure that tailoring the intervention to the right motivation level is built into the intervention.
The issue of participants lacking motivation to change was identified by the HIAs at an early stage in the internal pilot and the research team discussed the option of including only participants who expressed motivation to change. However, we decided that this would reduce generalisability, as referral from general practice to other services does not usually include an explicit assessment of motivation to change. Instead, we ensured that potential participants were fully informed about the purpose of the Healthlines Service, what sort of health issues and behaviours it addressed and what types of interventions it offered and then left them to decide if they wished to receive this intervention. This was felt to be more representative of how the Healthlines Service would function in normal operation.
Technological competence
Some people did not participate in the trial because they did not have the technology or technological competence to do so. 395 The WSD trial also identified a lack of technical competency as a reason for not joining the trial. 398 Even though technological support was built into the Healthlines intervention, and our interviewees described help from family and friends with technological issues, the extent of support needed possibly affected the dose of the intervention and the content delivered, in that a substantial minority of depression participants chose to use the CBT book rather than computerised CBT. A study of telehealth for LTCs found that technical problems can lead patients to question the benefits of telehealth and may lead patients to stop using it,399 concluding that good technical support is important so that any problems can be quickly resolved.
Did the intervention relate to primary care appropriately?
The TECH model paid attention to offering a complementary service to primary care. This was important because telehealth for LTCs can be seen as a threat to primary care. 220 In practice, both the GPs and practice nurses who we interviewed for the process evaluation were happy for the Healthlines Service to operate and they saw it as complementary rather than threatening. The intervention emphasised engagement with primary care. In practice, the relationship between the Healthlines Service and primary care was one-way, with GPs reacting to requests rather than being proactive in their contacts with the service. It is possible that the intervention could have been more effective if a proactive GP element was built into the TECH model, but it is probable that attempts to encourage proactive engagement might fail because of time pressures on GPs in the UK currently. The intervention could be improved from a primary care perspective by offering information about individual patients in a simpler format, stating what the Healthlines Service wanted GPs to do and why.
Did the research affect effectiveness?
As part of the research-related risk assessments in the CVD risk trial, practice nurses/HCAs measured the blood pressure of all participants at baseline. This is likely to have raised awareness of high blood pressure for both the health professional and the patient in both the intervention and the usual care groups. Health professionals may have taken action on this by referring participants in both groups to their GP, that is, the research process may have delivered the part of the Healthlines intervention that raised awareness of health problems by making them visible. This may explain why blood pressure fell in the usual care group as well as the intervention group over time. There are other possible explanations for the reduction in blood pressure in both groups, such as regression to the mean or guidelines encouraging increasingly aggressive treatment of high blood pressure in primary care in the UK. However, this change in the health of the usual care group as a result of the existence of the trial has been identified elsewhere for behavioural interventions373 and for a large telehealth trial. 155 The WSD trial found an increase in admissions in the control group occurring in the weeks after recruitment into the trial and suggested that research recruitment processes had caused this. 155
Another ‘trial effect’ that should be borne in mind when interpreting the CVD risk trial results is that a large minority of participants were recruited several months before the intervention started. For these participants, 12 months post baseline was not the same as after 12 months of receiving the intervention.
How should the intervention be used in the future?
The HIAs were a key part of the intervention. They were employed on band 4 of the NHS Agenda for Change331 but took a considerable amount of responsibility for the intervention that went beyond their grade. Their enthusiasm and dedication ensured that they worked hard to tailor the intervention. Some of their learning could be formally incorporated into the CVD risk intervention before it is used elsewhere. Even when this is completed, attention would need to be paid to employing enthusiastic and competent HIAs.
Strengths and limitations
A strength of this qualitative study was the inclusion of interviews with a wide range of stakeholders: managers and frontline staff delivering the intervention, staff in primary care offering care to participants and participants who had used the intervention and those who had and had not chosen to continue with it (depression only).
There were seven key limitations:
-
We did not use direct observation of the intervention (e.g. listening to telephone calls and observing HIAs in their daily work), which may have helped to further identify mechanisms of action.
-
We did not interview people from very deprived areas or from minority ethnic communities. This may have reflected the diversity of trial participants and so has implications for the interpretation of the trial results based on this qualitative research.
-
We did not interview those in the usual care group and, had we done so, we may have identified whether or not the measurements for the trial led to improvements in blood pressure management for them.
-
We misinterpreted the meaning of co-ordination of care in the TECH model during data collection.
-
In the process evaluation it is unlikely that we obtained data saturation for participants in the CVD risk trial because of the range of risk factors and because we did not interview those who had withdrawn from the intervention.
-
We interviewed trial participants in the middle of receiving the intervention and some interviews with those who had completed the intervention at 12 months might have offered more insights into issues such as self-efficacy.
-
We interviewed HIAs who were still working for NHS Direct and may have gained further insights from interviewing those who had left the service.
Conclusions
The TECH model appears to be a good basis for developing telehealth interventions for LTCs. The implementation of the intervention in the Healthlines Service could have been improved by having a dedicated team of HIAs delivering continuity and tailoring the intervention consistently throughout the trials. An intervention offered by a dedicated team of HIAs has the potential to be more effective than the intervention tested in this study.
Chapter 12 Discussion and conclusions of the Healthlines programme
Summary of main findings
In this research programme we used a series of mixed-methods studies to develop a telehealth-based intervention for patients with LTCs in primary care and evaluated this intervention through two pragmatic RCTs in patients with the exemplar conditions of depression and raised CVD risk.
As described in Chapter 2, we synthesised the findings from previous quantitative and qualitative research on telehealth for LTCs and found that, although there was a large volume of literature, much of the research was of low quality. There was evidence that telehealth interventions could sometimes be effective at improving a range of outcomes, although effect sizes were generally small. It was difficult to reach clear conclusions about which types of telehealth for which types of patients or conditions were most likely to be effective because the evidence was mixed and inconsistent. There were very few studies providing evidence of cost-effectiveness. A systematic review focusing on studies of telehealth interventions for depression and anxiety showed that these could have moderate to large effect sizes (particularly for computerised CBT) and some studies suggested that effectiveness could be enhanced by providing a moderator to support internet-based interventions. A systematic review of telehealth interventions for CVD risk found no evidence of an effect on overall risk. There was evidence of a small reduction in systolic blood pressure and weak evidence of reductions in total cholesterol, but no evidence of benefits in terms of promoting smoking cessation. A review of qualitative literature on patients’ views of telehealth suggested that it is generally appreciated because of perceptions of increased access to health care but that professionals are less accepting of telehealth than patients. The various sources of evidence were combined in a realist synthesis, which proposed three mechanisms of action that may be important for effective telehealth for LTCs: relationships between health professionals and patients; fit with patients’ needs and capabilities; and visibility through feedback.
In Chapter 3, we described a survey of almost 1500 patients (44% response rate) with depression or raised CVD risk to explore the key factors that influence interest in using telehealth. We found that there was moderate interest in telephone-based and internet-based telehealth, but little interest in social media-based telehealth. In multivariate analyses, these findings were largely unaffected by patients’ sociodemographic characteristics, health needs or difficulties with accessing health care. The most important constructs related to interest in telehealth were confidence using the technology and perceiving greater advantages and fewer disadvantages from using telehealth. This suggested that an effective intervention needed to clearly explain the advantages of telehealth and address perceived disadvantages, while providing support to use the technology.
In Chapter 4, we reported a qualitative study that explored the views of patients and practitioners in more detail, based on semistructured interviews with telehealth nurse care managers working for a telephone-based case management programme delivered partly by NHS Direct, patients who had used this service and practice nurses and GPs in nearby general practices. We found that patients were positive about telephone- and internet-based care for mental health problems, but less clear about the advantages for CVD risk management. GPs and practice nurses were ambivalent and sometimes sceptical about telehealth. Telehealth nurse managers tended to characterise their roles in terms of traditional nursing ideals of having time to develop personal caring relationships with patients, which the patients themselves also appreciated. This is consistent with the evidence synthesis and implies that telehealth interventions should be based on personal relationships rather than on an automated ‘call centre’ approach.
Building on the evidence collected in the three strands of research described above, we developed a conceptual model to provide a theoretical framework for the development and evaluation of a telehealth intervention for patients with LTCs, which we labelled the TECH model. This is described in Chapter 5. The TECH model proposes that effective telehealth interventions are most likely to be effective and acceptable if they pay attention to four components: (1) engagement of patients and health professionals; (2) effective chronic disease management (including subcomponents of self-management, optimisation of treatment and care co-ordination); (3) partnership between providers; and (4) patient, social and health system context. The model also suggests that the key intended benefits are improved health, access to care, patient experience and cost-effective care and these should therefore be the main outcomes included in an evaluation.
We used the TECH model to design telehealth interventions for our two exemplar conditions of depression and raised CVD risk. The intervention was named the Healthlines Service and is described in detail in Chapter 6. It was based on a series of telephone calls from a named HIA, who used motivational interviewing skills to encourage behaviour change and improve patient self-management. Participants were encouraged to identify goals and were then offered links to information about quality-assessed resources available from the internet. In particular, in the context of our exemplar conditions, participants with depression were offered an interactive computerised CBT programme and participants with hypertension and raised CVD risk were offered blood pressure self-monitoring with automated feedback via a web portal. Participants’ use of medication was reviewed using algorithms and, when they were not being treated in accordance with NICE guidelines, a treatment recommendation was sent to their GP (with recent PHQ-9 or blood pressure or cholesterol readings as appropriate along with a summary of the relevant guidelines) and copied to participants. Problems with medication adherence were identified and addressed. The intervention was designed to support and work in tandem with and complement primary care provided by general practice.
This intervention was tested in two pragmatic RCTs, which included a nested economic evaluation to assess cost-effectiveness and a nested process evaluation to assess implementation. In the Healthlines depression trial (see Chapter 8), we found that the intervention was associated with a small benefit in terms of response to treatment compared with usual care. The impact was greatest after 4 months of follow-up and reduced over 12 months, suggesting that the greatest benefit of the intervention was in a shorter time to recovery, which is nevertheless important to patients. The intervention was also associated with clear evidence of an improvement in anxiety. Participants receiving the Healthlines depression intervention reported better access to health support and advice and greater satisfaction with the amount of help and support that they received. They also reported improvements in various aspects of self-management, although the effects were generally small. There was no evidence of optimised medication and nor were participants more likely to report that their care was well co-ordinated. The Healthlines depression intervention was unlikely to be cost-effective according to the primary economic analysis, because it was associated with only small benefits in quality of life and because it incurred not only the costs of the intervention itself but also increased costs of other community-based health care services (see Chapter 10).
In the Healthlines CVD risk trial (see Chapter 9), there was weak evidence of a modest benefit from the intervention in terms of maintenance or reduction in CVD risk assessed using QRISK2 score over a 12-month period. This was defined as response to treatment. Although there were benefits in terms of small reductions in blood pressure and weight, there was no evidence of improvements in cholesterol or smoking cessation. In combination, these led to the minimal improvement in overall CVD risk that we observed. There was some evidence that participants in the intervention arm were more adherent to their blood pressure medication, improved their diet and undertook more physical activity. However, there was no evidence that the GPs of participants in the intervention arm were more active in escalating drug treatment for either hypertension or raised cholesterol. As in the depression trial, there was evidence that intervention participants in the CVD trial experienced better access to health care, felt that they received more support and advice and were more satisfied with the treatment that they received than those in the usual care arm, but little evidence that they gained more confidence in self-management. Nevertheless, intervention participants were more likely to have discussed a care plan and were more likely to have had a positive experience of the organisation and co-ordination of their care than usual care participants. Although the benefits of the Healthlines CVD risk intervention were small, there was evidence that the intervention could be cost-effective (see Chapter 10). This is because of improvements in quality of life observed during the year of the trial and because small reductions in the risk of a cardiovascular event are associated with big improvements in quality of life because of the severe adverse consequences of having a heart attack or stroke.
The process evaluation (see Chapter 11) provided support for all of the components of the TECH model and showed that the Healthlines Service was largely delivered as planned. There were some problems with the delivery of the intervention in the first few months, which may have detrimentally affected patient engagement, a key mechanism of action of the intervention. A lack of continuity of HIAs in the early weeks of implementation of the Healthlines Service was likely to have affected both relationship and fit, which were two mechanisms of action for telehealth for LTCs. Indeed, a substantial proportion of those receiving the intervention did not complete it. In the depression trial, some participants did not feel that the CBT approach was appropriate for their needs. In the CVD risk trial, some participants were more motivated by an altruistic wish to support research, rather than by a wish to change their behaviour, which may have reduced the potential for the intervention to deliver behaviour change.
What do the findings tell us about the utility of the TElehealth in CHronic disease model?
Both the depression and CVD risk interventions were based on the same underlying conceptual TECH model. Having a conceptual model helps to make explicit the intended mechanisms of action in a complex intervention such as this and also makes it possible to explore whether or not these mechanisms were associated with any impact of the intervention. If the intervention was effective in two very different clinical conditions, this would provide support for the underlying model and justify the development and testing of similar interventions in other LTCs. If the intervention was ineffective in both exemplar conditions, the model makes it possible to explore whether or not particular components of the intervention were achieved. If the intervention was effective in one exemplar condition but not the other, this may support the idea that different approaches may be necessary in different LTCs.
The Healthlines intervention was associated with small but clear benefits in terms of response to treatment for patients with depression and also led to improvements in anxiety that are clinically important. For patients with CVD risk, the Healthlines intervention was associated with improvements in some modifiable risk factors but not others and this translated into very modest and uncertain benefits with regard to the primary outcome of reduction or maintenance of overall CVD risk. Considering both exemplar conditions together suggests that the Healthlines Service is likely to be associated with improvements in response to treatment for LTCs, but that the size of these improvements is likely to be small.
Components of the TElehealth in CHronic disease model
Given these limited benefits, it is important to consider whether or not the different components of the TECH model (see Figure 11) were achieved as intended.
Self-management
The Healthlines intervention was designed to incorporate many well-recognised approaches to promoting self-management behaviours including self-monitoring, goal-setting, personal and individualised support and providing information. The telephone support scripts were based on behaviour change principles and were delivered by HIAs who were trained in motivational interviewing. There was evidence from both trials that the intervention was associated with improved skill and technique acquisition. Participants in the depression trial reported greater self-monitoring and insight and there was a similar trend in the CVD risk trial, but the effect was small in both cases. The overall conclusion is that the Healthlines approach had only a small impact on promoting self-management behaviours.
Treatment optimisation
A key way in which we hypothesised that the Healthlines Service would improve outcomes was through ensuring that patients received and adhered to the most appropriate treatment for their problems. It is well recognised that patients in normal clinical practice rarely achieve the same levels of disease control as can be achieved in clinical trials and this phenomenon has been labelled ‘clinical inertia’. 148,400 In an attempt to overcome this, we sought to replicate many of the strategies used in trials, particularly close monitoring of patients, feeding back the results to GPs, reminding GPs of the relevant targets for individuals and providing summaries of NICE guidance with clear treatment recommendations. We also shared these targets, guidelines and recommendations with the participants, on the basis that they might gain a sense of ‘shared ownership’ of the problem and expect their doctors to optimise their treatment. However, in neither the depression nor the CVD risk trial was there evidence of substantial changes in patients’ treatment. The process evaluation suggested that in some cases GPs felt that the blood pressure targets advised by the Healthlines Service were not appropriate for their patients. After the finding about the lack of increase in statin prescribing was known, we explored with the HIAs why so few letters had been sent to GPs to advise prescribing of statins, given that this topic would have been raised repeatedly during the intervention. They suggested that many participants had previously been offered statins by their GP but were reluctant to take them. This suggests that treatment inertia is related to patients’ as well as clinicians’ views.
Care co-ordination
The TECH model suggests that an important component of effective LTC management is well co-ordinated care. This was intrinsic to the design of the Healthlines Service, incorporating an organised recall system, regular monitoring and feedback, protocol-driven care based on clinical guidelines and good communication between providers. All treatment recommendations were channelled through the participants’ usual general practice and copied to patients to ensure good communication and avoid conflicting advice. However, there were few measures of care co-ordination within the evaluation, apart from an item in the patient questionnaire. Participants in the Healthlines CVD risk service but not the Healthlines depression service reported that their care was more co-ordinated. Unfortunately, this does not fully assess the impact of this component of the model.
Participant engagement
We identified wide variation in the extent of participant engagement in the intervention, which is reflected in the fact that only a minority of participants received all or almost all of the planned encounters (although, in some cases, this occurred because of delays in starting the intervention and the closure of NHS Direct; see Chapter 7). Particularly in the depression trial, one group (a minority) of participants received all or most of the intervention whereas another group dropped out after only a few encounters. Many participants who initially started to use the interactive online CBT programme did not continue after completing a few modules. In the process evaluation, some lack of participant engagement was attributed to a lack of continuity of HIAs during a period of intervention delivery, as well as the complexity of participants’ lives. However, the process evaluation also demonstrated that some participants did gain support from the regular calls from the HIAs and the CACE analysis demonstrated that participants who received more of the intervention tended to gain better outcomes. Engagement with some aspects of the CVD risk intervention was good, with many participants completing the encounters and enthusiastically providing self-monitored blood pressure readings. As with the depression intervention, completion of a greater number of modules in the CVD risk intervention was associated with greater effectiveness.
Engagement of primary care clinicians
There were no direct measures of clinician engagement in the evaluation and, in retrospect, this is a weakness. It was clear from the qualitative research reported in Chapter 4 that gaining clinician engagement was likely to be problematic. There are several indirect indicators that this was indeed a problem in the Healthlines trials. The process evaluation suggested that some primary care clinicians were unaware of the service being provided to their patients. The lack of optimisation of treatment in response to letters from the Healthlines Service may have been for several reasons including a lack of agreement of clinicians with the recommendations based on NICE guidelines made by the HIAs, clinicians not receiving or reading the e-mails from NHS Direct or participants not being convinced that a change in treatment was desirable or necessary. Clinicians were encouraged to contact the Healthlines Service if they wished to set individualised targets for specific patients, but very few such letters or e-mails were received. We cannot be sure whether or not the lack of treatment optimisation is necessarily related to a lack of clinician engagement, but it is very likely to be one reason why the Healthlines Service had less impact on clinical outcomes than was anticipated.
Contextual factors
Patient context refers to characteristics of participants who are most likely to benefit from a telehealth intervention such as the Healthlines Service. The subgroup analyses in the trials suggested that sociodemographic characteristics, such as participant age, were not associated with variation in effectiveness of the interventions. However, the process evaluation suggested that some participants in the depression trial did not engage with the intervention because they did not want CBT and that some of those in the CVD risk trial were not motivated to change their behaviour. Although interested participants were fully informed about the nature of the intervention before they agreed to take part, this suggests that this type of intervention is likely to be beneficial only if it meets participants’ perceived needs.
Context also relates to aspects of the health-care system. The Healthlines Service was designed for the NHS context, in which almost all patients have access to reasonably accessible and comprehensive primary care services from general practice and other community services. These provide access to face-to-face versions of most of the interventions offered by the Healthlines Service. The main potential advantages of a telehealth service such as the Healthlines Service are greater convenience and support, the interactive nature of the service to support self-management and the potential to reduce demands on other possibly more expensive primary care providers. Participants in the Healthlines trials did report more accessible and convenient support and advice and they were more satisfied with the amount of support they received and more satisfied overall with the treatment they received. Yet, in both cases, the intervention acted as an additional rather than a substitute service and was possibly associated with increased rather than decreased use of other health-care resources.
Did the trials provide support for the TElehealth in CHronic disease model?
In conclusion, the TECH model had a sound theoretical basis and provided a useful framework for intervention design and evaluation. However, although most of the specific components of the model were delivered as designed, they led to little or no change in the dimensions of care that they were intended to influence. These can be considered as intermediate outcomes. There were small improvements in some aspects of participant self-management, no evidence of better optimisation of treatment apart from slightly improved participant adherence to medication, variable participant engagement and little evidence of engagement from primary care clinicians. It is therefore unsurprising that the Healthlines approach was associated with only small benefits for participants.
This does not suggest a failure of the conceptual model, which would have been implied if the components of the model, such as optimisation of treatment, had occurred but did not lead to patient benefits. Nor does it imply a failure of implementation, as the intervention was mostly delivered as planned (although there were problems because of delays at the beginning of the trial and the closure of NHS Direct at the end of the trial). Instead, it suggests a failure of intervention design in that the telehealth approach chosen for the Healthlines Service did not lead to the changes in either patient or clinician behaviour that were intended.
The implication is that other approaches are needed to have an impact on the intermediate outcomes of engagement, self-management, treatment optimisation and care co-ordination described in the TECH model. We set out to devise a low-cost intervention that could be rapidly implemented, but it may be that a more intensive (and more costly) intervention would have been more effective. Alternatively, a similar intervention but delivered without the problems that we experienced and targeted more clearly at people who wanted to use it and outside the context of a research trial might be more effective.
Strengths and limitations of the research programme
Strengths
In the evidence synthesis (see Chapter 2) we identified that there were major limitations of earlier research on telehealth for LTCs. These included a lack of pragmatic research on real-world implementation in generalisable populations, with a clear theoretical basis for the intervention, and a lack of evidence about clinical outcomes and cost-effectiveness. Much previous research has also included small numbers of participants and been of low quality. This research programme was designed to address all of these limitations.
Pragmatic approach
This report provides findings from two of the largest RCTs of a telehealth intervention for LTCs ever conducted. A major strength of these trials is that they were highly pragmatic. Unlike most telehealth trials, which relate to studies of specific technological interventions in research volunteers, this study replicated as far as possible what would happen if the Healthlines Service was introduced in the NHS. The intervention was offered to eligible patients identified from general practices, which is the way that most health interventions are offered in the UK. Participants were recruited from a range of general practices in three areas of England, each with different contexts and different provision of other related local services, which enhances the generalisability of the findings. The intervention was designed in such a way that it could be immediately rolled out across the NHS and the research provided evidence about whether or not this was likely to be feasible, effective and cost-effective. The Healthlines Service was highly integrated with normal service delivery, being provided through existing NHS Direct infrastructure, and was designed to support mainstream service delivery in primary care, rather than delivery as a ‘stand-alone’ intervention.
Thorpe et al. 401 described a pragmatic–explanatory continuum indicator summary (PRECIS) diagrammatic tool, which provides a guideline for researchers seeking to design pragmatic trials. This has since been developed further into the PRECIS-2 tool,402 which specifies nine key domains that distinguish pragmatic from explanatory trials and proposes that evaluators map these on a PRECIS-2 diagram. We have done this for the Healthlines trials (Figure 34) and this highlights the pragmatic nature of these trials. The exceptions are the primary outcome measurement, which required a special visit to the practice nurse in the CVD risk trial, follow-up intensity (as many participants required reminder questionnaires to collect outcome data), the eligibility and recruitment criteria (as only those with access to the internet were eligible) and the flexibility of the intervention. This was constrained by the telephone scripts used by the HIAs, although in practice they adapted them and used them flexibly.
FIGURE 34.
Application of the PRECIS-2 tool to the Healthlines study. PRECIS diagram using the PRECIS-2 tool402 and marked up by us to show which components were included in the Healthlines trials. The wider the diagram on any dimension, the more explanatory the trial on the pragmatic–explanatory continuum. Reproduced from BMJ, Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M, vol. 350, pp. h2147, copyright 2015, with permission from BMJ Publishing Group Ltd. 402
Generalisability
Most research studies of telehealth interventions relate to specific technological innovations. Examples of relevance to the Healthlines programme include studies of remote monitoring and self-management of blood pressure or trials of online computerised CBT. Such studies can be characterised as efficacy or explanatory trials in that they demonstrate the effect of a well-defined intervention in a research context, usually in individuals with tightly defined inclusion and exclusion criteria and paying less attention to issues of local context and implementation. These studies may lead to estimates of effect that are exaggerated compared with the effects observed when the intervention is implemented on a wide scale in the real world.
Similarly, some of the claims for the benefits of telehealth interventions come not from research trials but from evaluations involving a small number of participants and using weak research designs such as before-and-after studies. These observational studies may provide exaggerated estimates of the effect of interventions. Very few studies have explored the effectiveness and cost-effectiveness of the real-world implementation of a telehealth- based service on a wide scale, one notable exception being the WSD evaluation in the UK. 403
Effectiveness and cost-effectiveness rather than efficacy
In contrast, the Healthlines study provides robust evidence about effectiveness rather than efficacy. We offered the intervention to the groups of people who would be likely to be offered it in practice. Rather than testing a specific telehealth technology, we tested a new approach to care based on remote support from a telehealth provider and using a range of telehealth-based resources that may be relevant to individual participants. Not all aspects of the intervention would be equally applicable to all participants. Because the intention was to provide practical evidence to inform commissioning decisions, it was vital to include an economic evaluation. The lack of information about the cost-effectiveness of telehealth was identified as a major deficiency at the time that this study was planned404 and this study has added to the limited evidence base about the economic impact of telehealth.
Evidence-based, theory-informed and patient-centred intervention development
The Healthlines Service was based on an underlying TECH conceptual model, tailored to each of the exemplar conditions. The TECH model was based on evidence from a series of studies using a range of methods and sources of evidence during the early phases of this research programme. The TECH model provided a theoretical basis for the different components and strategies used in the intervention. This is a major strength as the conceptual model helps to make explicit the intended causal pathway and ‘essential ingredients’ of the intervention. This makes it possible to consider whether or not the Healthlines Service was implemented as intended and whether or not this, in turn, was associated with benefits. Although the importance of basing interventions on an underlying theory is debated,405,406 a recent review confirmed that, in the case of internet interventions to promote behaviour change, greater use of theory in intervention development was associated with greater effectiveness. 214 Because this is a complex intervention, it was particularly important to explore the process of implementation and the mechanisms of action, which we did through the process evaluation.
Rigour
The internal validity of this study was enhanced by the size of the trials, attention paid to measuring the delivery of the intervention (both quantitatively and qualitatively), the excellent rates of retention in the trials and the masked outcome assessment. All outcomes have been reported based on a prespecified analysis plan or in response to clearly identified post hoc exploratory analyses. External validity was enhanced through the multicentre nature of participant recruitment, the wide participant inclusion criteria, the intervention being embedded as part of normal NHS service delivery and the relatively long patient follow-up for 12 months, with modelling of longer-term effects in the CVD risk trial.
In line with current policy
Since this work was planned, the NHS Five Year Forward View has been published, which sets out a strategic plan for the NHS. 407 This introduces the concept of ‘combinatorial innovation’ in which a range of technologies are used in combination with new ways of working:
Many of the innovation gains we should be aiming for over the next five or so years probably will not come from new standalone diagnostic technologies or treatments – the number of these blockbuster ‘silver bullets’ is inevitably limited. But we do have an arguably larger unexploited opportunity to combine different technologies and changed ways of working in order to transform care delivery. For example, equipping house-bound elderly patients who suffer from congestive heart failure with new biosensor technology that can be remotely monitored can enable community nursing teams to improve outcomes and reduce hospitalisations. But any one of these components by itself produces little or no gain, and may in fact just add cost. So instead we need what is now being termed ‘combinatorial innovation’.
Reproduced from NHS. 407 Contains public sector information licensed under the Open Government Licence v3.0
Following the publication of the NHS Five Year Forward View, the NHS has issued a challenge to innovators to evaluate such ‘combinatorial’ approaches in real-world test bed sites. 408 The Healthlines programme was effectively ahead of its time and has already tested and developed such a combined approach. It was based on exactly this philosophy, that technological innovations should not be considered in isolation but should be used in combination as part of a coherent care programme and need to be accompanied by a new way of working and new models of staffing rather than being ‘bolted on’ to existing models of service provision.
Limitations
The research programme had a number of limitations, which are discussed in the following sections.
Closure of NHS Direct
The closure of NHS Direct had a number of consequences. There were widespread rumours about the closure for months before the official announcement. This led to changes in staff and meant that decisions about the implementation of the Healthlines Service were made in the context of considerable uncertainty about the future of NHS Direct as a host organisation. This uncertainty may also have impacted on participant recruitment for the trials if potential participants had believed that NHS Direct was closing down. When the closure was finally announced the Healthlines Service had to be closed down and then transferred to a new host NHS organisation, Solent NHS Trust. In the process of doing this, delivery of the intervention was paused for about 2 months, which meant that some participants received less of the intervention than planned and others likely lost interest in the study. However, the fact that it was possible to recruit a new host organisation and to re-establish the service in a matter of weeks provides evidence about the potential for the Healthlines Service to be implemented quickly by a range of organisations and provides further support for generalisability.
A further complication relating to the closure of NHS Direct was that we had to obtain data about participants’ use of the intervention from a combination of databases held on NHS Direct servers. There were separate databases for the CVD risk and depression interventions and a further database that contained details of participants’ appointments and encounters. Although these were packaged up when NHS Direct closed and transferred to Solent NHS Trust, it was very difficult to reconstruct the details of encounters and in some cases there were discrepancies between the different sources of data. In a small number of cases (n = 12/307 randomised for depression; n = 2/325 randomised for CVD risk) data about participants’ encounters were missing. We have described how we handled these discrepancies in the relevant sections of the results chapters.
Constraints of timescale
Ideally, the TECH model would have been fully developed and aspects of it would have been tested in modelling studies before it was used to design the Healthlines intervention and the trials. However, the timeline for the research programme (5 years) and the length of time needed to plan an intervention and the RCTs meant that the TECH model was not utilised as far as it might have been in developing the intervention and planning the evaluation. This is because some of the planning for the intervention had to be carried out in parallel with finalising the TECH model. Furthermore, some aspects of the intervention were delivered when they were insufficiently developed and more time for refinement would have been beneficial. In real life, especially in a fast-moving field such as telehealth, the service would be continually modified and improved, but in a trial this would not be appropriate as it would make it impossible to define the intervention being tested.
Participant recruitment rate
One potential limitation of both trials is the low recruitment rate: only 16% (depression trial) and 20% (CVD risk trial) of those sent information about the trial expressed an interest in it and only 31% (depression trial) and 63% (CVD risk trial) of those assessed were found to be eligible to participate. In many trials, this would be seen as a threat to external validity if it implied that those participating in the research were not typical of those who would receive the intervention in real life. This is not necessarily the case in the Healthlines trials. First, the initial search for people to be sent information about the trial lacked specificity, because it was not possible to accurately identify people who were currently depressed or at high CVD risk. Some of those who did not reply probably knew that the study was not relevant to them. From information returned by those who declined to participate,395 it appears that about 40% of those who declined felt that they did not have health needs that required intervention, about 55% felt that they did not have access to or the skills to use a computer and about 15% said that they were not interested in taking part in the research (respondents could give more than one reason). The first explanation (lack of need) does not present a threat to validity as these people would not be offered the intervention in real life. The third explanation (lack of interest in the research) would represent a threat to external validity but this represented only a small proportion of respondents. The second explanation (people who were eligible but who were not interested in or able to take part in a telehealth intervention) is not a threat to validity. This type of telehealth intervention is not necessarily appropriate or acceptable to all or even most people with a LTC. However, these conditions are so common that if a telehealth approach is effective for some people it would still be a useful treatment option. When people did express an interest and were assessed, most of those excluded were excluded because they did not have the condition of interest (i.e. depression or CVD risk ≥ 20%). Once eligibility had been confirmed, the proportion of those eligible who agreed to participate was high (84% in the depression trial and 85% in the CVD risk trial).
Challenges to internal validity
There are several limitations that represent challenges to the reliability of the findings from the trials. These are discussed in detail in the concluding sections of Chapters 8 and 9. In summary, for the depression trial they include the potential for bias because the primary outcome (PHQ-9) was based on self-report and because participants in the intervention arm may have completed the PHQ-9 more often during the trial period than those in the control arm, leading to practice effects. There was also some differential attrition between the arms and under the most conservative assumption (that none of those with missing data had improved) the evidence of benefit from the intervention was reduced, although analysis based on multiple imputation showed the same effect as the analysis using complete data. For the CVD risk trial, there were technical problems at the beginning of the trial that may have affected engagement with the intervention and reduced the amount of treatment received. The use of the QRISK2 score as the outcome measure also had limitations because of day-to-day variability, measurement error and limited sensitivity to change given that the score is dominated by non-modifiable factors such as an individual’s age and sex.
Challenges to generalisability
Although the trials were highly pragmatic and applicable to a NHS context, this high level of contextual application also potentially reduces generalisability to other countries with different health-care systems. It is notable that most of the apparently successful telehealth interventions come from the USA and this may reflect the fragmented nature of health-care provision in that country.
The range of participants included in the trials also limits generalisability. There were very few participants from ethnic minority groups (reflecting the populations within the practices that took part in the study) and, to be eligible for the trial, participants had to have access to the internet, a telephone and an e-mail address.
Depression and cardiovascular disease risk as exemplar long-term conditions
One consideration is whether or not the exemplar LTCs that we chose were the most appropriate for this type of intervention. We chose these conditions because they are common and there was some proof of concept that specific telehealth interventions could be effective and to avoid overlap with previous research, which has mainly explored the effectiveness of intensive (and expensive) telehealth programmes for serious health conditions such as heart failure and diabetes.
However, the evidence supporting the benefit of telehealth interventions for depression was limited and equivocal at the outset. 85 Although at the time that we planned this study NICE recommended computerised CBT packages for depression,27,39,266 Kaltenthaler et al. 85 have highlighted that all of the research published at the time of her review had been conducted on highly selected populations and more recent research based on the findings of the REEACT trial has questioned the efficacy of computerised CBT. 409 A qualitative study has highlighted the barriers to the use of computerised CBT experienced by many potentially suitable participants. 81
With regards to participants at raised CVD risk, earlier research had demonstrated the effectiveness of telehealth interventions for specific risk factors such as hypertension150 and smoking cessation,385,386 but our systematic review of trials of telehealth interventions aimed at reducing multiple risk factors to reduce overall CVD risk found no evidence of effectiveness. 61 Earlier studies of primary prevention interventions to reduce CVD risk based on face-to-face nurse-led care had demonstrated small benefits410,411 and modelling conducted in advance of the NHS Health Checks programme has suggested that they could be cost-effective in the long term. 34 This conclusion, of limited short-term benefits but with the potential for long-term gains in cost-effectiveness, is consistent with the findings of the Healthlines CVD risk trial.
Relation to previous studies
Since the evidence synthesis was conducted (March 2010) and the Healthlines trials began, a number of further systematic reviews and primary studies have been published. The most relevant of these papers are summarised in Appendix 32. The recent systematic reviews concluded, as did the earlier reviews, that, despite a large number of studies of telehealth, much of the research is of low quality and the findings are inconsistent.
In a recent publication, Wootton54 undertook a high-level systematic review of trials and reviews of telehealth interventions for five common chronic diseases (asthma, COPD, diabetes, heart failure and hypertension). The methods for the review were similar to those used in our own evidence synthesis (see Chapter 2) and Wootton reached similar conclusions. He found that most studies had reported positive effects but a funnel plot suggested the likelihood of publication bias and more recent trials provided weaker evidence of benefit. Much of the research was short term and (like earlier reviews) there were few studies of cost-effectiveness. Wootton concluded that ‘the evidence base for the value of telemedicine in managing chronic diseases is on the whole weak and contradictory’ (p. 211). 54
Although there are a large number of studies of specific approaches (especially telemonitoring) for particular conditions (mainly heart failure and diabetes), there are few comparable studies of comprehensive telehealth programmes for patients with LTCs. These encompass a range of telehealth approaches and combine telephone support, use of internet resources and communication with other providers to optimise and co-ordinate treatment. The relevance of our findings to the previous research specific to depression and CVD is discussed in Chapters 8 and 9 respectively.
The findings from the two Healthlines trials suggest that telehealth for LTCs is likely to have small positive benefits for patients but we can conclude that it is unlikely that this form of telehealth will lead to marked improvements compared with usual care. This is consistent with Wootton’s54 conclusion that more recent and better-quality studies tend to demonstrate less strongly positive findings than earlier and lower-quality studies. It is also consistent with Rossi’s ‘iron law’ of evaluation that, as a new model is implemented widely across a broad range of settings, the effect will tend towards zero. 412
To some extent, the small impact of the Healthlines intervention was likely to be associated with limited patient engagement. This is consistent with studies of previous telehealth interventions, which have often struggled to recruit or retain participants. 413 This has been the case for broad-based interventions such as the WSD programme398 as well as studies of specific technologies such as computerised CBT. 367
The limited effectiveness of the Healthlines Service is disappointing, given that some of the specific interventions (such as telemonitoring for blood pressure,148,150 referrals to text-based smoking cessation programmes160 or computerised CBT414) that it incorporated have had more positive results in earlier studies. There are several possible explanations for this finding. It could be that seeking to change one specific risk factor using one telehealth approach is more effective than seeking to modify several risk factors simultaneously (as in the CVD trial) or seeking to combine several telehealth approaches (as in both trials). An alternative explanation is that the previous positive trials have been based on volunteers who were interested in the particular technology of interest and that pragmatic implementation in the broader population is less effective. A further likely explanation is that the impacts of any specific intervention in the Healthlines CVD risk trial are diluted by the fact that not all participants were eligible for reduction in any one risk factor and that benefits in one risk factor can be negated by lack of benefits in others. Yet another possible explanation is that the effectiveness of the Healthlines Service was compromised by the delays and difficulties that we experienced in delivering the service, both at the beginning (because of technical problems) and at the end (because of the closure of NHS Direct) of the study.
There are few comparable trials of telehealth that provide data about cost-effectiveness. In 2002, Whitten et al. 404 stated that there was ‘no good evidence that telemedicine was a cost-effective means of delivering health care’ (p. 1434) and there has been no convincing evidence since then to change that conclusion. 415 The most relevant study in the NHS context is the WSD trial, which was designed to save health-care resources by cutting avoidable hospital admissions but which in fact was not cost-effective because it increased health service costs with minimal benefits in quality of life. 53 The Healthlines trials, therefore, help to fill this evidence gap and suggest that this type of comprehensive approach to health care based on telehealth is likely to become cost-effective only if it can be provided very inexpensively (or more likely by replacing the use of other existing NHS resources), given that impacts on quality of life are small in the short term. However, the economic evaluation of the Healthlines CVD risk trial shows that it is possible for there to be long-term economic benefits if improvements in health as a result of telehealth are sustained beyond the intervention period.
Successes and challenges
Successes
Given the closure of NHS Direct, which closed more than three-quarters of the way through the period of intervention delivery of these two large and complex multicentre trials, we consider it a major achievement that we managed to complete the research programme at all. The main consequence of the closure of NHS Direct was a delay in delivery of the Healthlines Service for about 2 months, which involved disruption and may have had some adverse consequences with regard to the results, although we do not think that these consequences were major. It is important to note that the continuation of the research programme was possible only because Solent NHS Trust generously agreed to take over the project and because of the enormous determination and commitment of NHS Direct research management staff, IT staff and HIAs, as well as the research team.
A further success of this programme grant was the way in which it involved a large multidisciplinary collaboration between a range of academic and health service organisations. This included working with international partners (Duke University, USA) as well as private providers (Media Innovations Ltd, BWW) in the UK.
The success of this programme was facilitated by the fact that there is considerable interest in the topic of telehealth for LTCs from patients, health-care providers, academics and policy makers, as discussed in Implications for health care.
Challenges
The biggest challenge to this programme was the closure of NHS Direct, the consequences of which have been discussed previously (see Chapter 7). There could be a perception that this will have reduced the relevance of this research programme, but we would argue that the messages from the research are not specific to NHS Direct and that the Healthlines Service could be delivered by other organisations. With the help of NHS Direct IT staff, we have developed a demonstration system and a package that would enable other organisations to install all of the necessary infrastructure (as was achieved successfully when the intervention was transferred to Solent NHS Trust).
One strength of this research programme was the opportunity to conduct a series of linked studies over a 5-year period. However, despite the 5-year duration of a NIHR programme grant, it is still very challenging to conduct a systematic literature review and quantitative and qualitative research and then develop, pilot and evaluate an intervention using a RCT within 5 years.
Conversely, the length of time that is needed to conduct a RCT in relation to a topic such as telehealth means that, by the time the results are known, the technology will have changed considerably. If one designed an intervention today one would probably not use the same technologies that were available when the Healthlines Service was designed in 2011. This is a paradox for the evaluation of all health service innovations, but particularly in relation to telehealth, and a number of authors have discussed alternative approaches to timely evaluation. 254,255,280
The future of this type of initiative and the relationship with primary care
The closure of NHS Direct and the transfer of the service to Solent NHS Trust was disruptive for this research programme but did provide learning about the requirements of an organisation needed to host this type of telehealth-based intervention. Although part of the argument for basing the intervention within NHS Direct was its national scale, we managed to continue providing the intervention unaltered after it was transferred to a local organisation. This kind of service can be delivered from anywhere in the country. However, it would require a resilient infrastructure to provide expertise and support for both hardware and software. In the longer term it would also require resources to enable the continual updating of the content to reflect changes in clinical management guidelines. Furthermore it would need to be a large enough organisation to employ a range of health advisors, arranging their support and training from experienced health professionals and ensuring high standards of clinical governance.
The key questions include which type of organisation could provide this support and what would be the business model to make it viable. At the time that this project was planned a number of commercial organisations, often supported by pharmaceutical companies, were developing innovative support services and marketing them to NHS commissioners. One example was the Birmingham OwnHealth scheme,156 a partnership including Pfizer and NHS Direct, which was an initiative that helped to inform the Healthlines Service. There are still some examples of such initiatives [e.g. the Hurley Group is actively marketing webGP at present; see http://webgp.com/ (accessed 13 October 2016)] but generally the concept of a market for extra services alongside primary care has not grown as rapidly as might have been anticipated 5 years ago. Within the current NHS model, it would be very possible for a commercial or not-for-profit organisation to provide an intervention similar to the Healthlines Service and to make it available to be commissioned by local clinical commissioning groups. Whether or not this occurs will depend more on the incentives and structure of the NHS rather than on technical limitations.
It is clear from the research reported in Chapter 7 that clinicians in primary care were ambivalent about the potential of telehealth. Although primary care clinicians are always busy, instead of seeing telehealth as a way to reduce their workload they may feel threatened by the possibility of another organisation undermining their role. They may also believe that telehealth services will increase rather than decrease their work and there is little evidence from this or other research to counter that belief.
For telehealth services [whether for chronic disease management as in the Healthlines Service or for management of new problems, e.g. Skype™ (Microsoft Corporation, Redmond, WA, USA) consultations] to flourish in primary care, several conditions would need to be satisfied. First, there has got to be demonstrable benefit for clinicians, particularly by reducing their workload. Second, the system has to be integrated (technically, organisationally and philosophically) with general practice, which is overwhelmingly the main provider of primary health care, rather than being provided independently. This requires an in-depth knowledge of general practice culture and organisation. In this study of the Healthlines Service we failed to engage clinicians as we would have hoped and this would be easier outside the context of a trial. It would be important to invest in building partnerships with practices, for example by the Healthlines HIAs regularly visiting practices to meet the GPs and nurses, although this was beyond the resources of this programme. Third, this type of initiative needs to be sustainable. Restructuring service provision to include a substantial element of telehealth would mean significant service redesign and this would be wasteful and damaging to patient care if the telehealth services are not likely to be reliable partners over the longer term. It is arguable that some of the pressures on emergency departments at present can be traced to the introduction and subsequent dissolution of new services such as NHS walk-in centres, NHS Direct and GP out-of-hours co-operatives, which have confused the public about how to obtain urgent medical help. It is even more important that changes to services for chronic disease management based on telehealth are robust for the long term or they will undermine patients’ confidence in the general practice services they currently rely on.
Impact of patient and public involvement
Given the variety of technologies now available that can be used to support patient health and the accompanying new systems of delivering health care for these technologies, it is especially important to solicit the views and experiences of patients and the wider public when conducting telehealth research. Throughout the Healthlines programme there was strong and valuable patient and public involvement.
This was particularly relevant to the development of the survey instruments. As mentioned in Chapter 3, two different service user groups (volunteers from the Mental Health Research Network and those contacted through NHS Direct) provided feedback on the patient survey format and design, wording and comprehensibility of the questions. Based on this feedback, the questionnaire was modified and improved on before distribution to patients invited to take part in the survey. An indirect benefit of this process may have been achieving a fairly reasonable response rate to the postal survey, whereas a direct benefit was that few questions were left unanswered by respondents completing the survey. Similarly, two public contributors (Michelle McPhail and Anne Jacob), or patient and public representatives, affiliated with the programme provided valuable comments on the questionnaire developed for use in the RCTs (see Chapter 7), as well as contacted other members of the public for us to gain a broader range of feedback on the questionnaire. Again, based on the feedback we received, the questionnaire was revised and finalised ahead of use with participants in the trial and we achieved a high response rate for questionnaires throughout the RCTs.
Anne Jacob and Michelle McPhail were recruited from a volunteer pool of NHS Direct volunteers in early 2010 to join the Healthlines Programme Management Group. Therefore, from an early point in the research programme these representatives contributed to various aspects of the planning, development and implementation of both the patient survey (activity 3), the TECH model and subsequent intervention (activity 4) and the RCTs (activity 5). They participated in intervention development, which included small group discussions and workshops to discuss the original conception of the TECH model (see Chapter 5). Once again, we used this feedback to refine and finalise our TECH model, which was the basis of the Healthlines Service intervention. Moreover, throughout the RCT phase (activity 5) the public contributors also attended the biannual Programme Management Group and the independent Trial Steering Committee meetings and were active contributors during these meetings. Towards the end of the RCTs, they assisted with the brainstorming and development of the dissemination plan and strategy for the trials. In summary, the public contributors have been a fundamental component of the many phases of this research programme, from development to implementation and dissemination of the results, and so have greatly impacted and benefited this research. We have taken on board their comments and suggestions, which have improved the quality and reach of this programme.
Priorities for research
We have conducted two trials of telehealth interventions based on the same underlying approach, which have shown evidence of modest benefit. This is consistent with the results of several other studies. A key research priority is therefore to determine whether or not it is possible to make telehealth more effective. Specific questions and related priorities include:
-
Would the Healthlines approach be more effective if applied to conditions with greater consequences for quality of life, such as heart failure, cancer or rheumatoid arthritis?
-
Would it be more effective if targeted to people who have passed an initial screening process, in which motivation to change behaviour and interest in using a telehealth intervention or willingness to try CBT are explicitly assessed?
-
How can barriers to patient engagement with telehealth interventions be addressed? This may require qualitative work to understand the barriers to engagement, trials of different approaches to promote engagement or a focus on different groups of patients.
-
How can barriers to the engagement of general practice teams with telehealth services be overcome?
-
What type of organisational arrangement (how large an organisation; provided locally, regionally or nationally; what business model) would lead to the most cost-effective telehealth service and how could it be organised to integrate most effectively with other primary care services, particularly general practice?
-
Is it more effective to target interventions at one risk behaviour at a time, rather than to use a ‘healthy lifestyles’ approach that targets several different risk behaviours?
-
The Healthlines depression intervention was similarly as effective as collaborative care interventions based on telephone and face-and-face support from mental health workers, but these studies were carried out in different populations of patients. Further research should directly compare conventional collaborative care run by mental health workers with telehealth interventions such as the Healthlines Service provided by less highly trained generic health advisors with computer support.
-
A key problem of the Healthlines trials was the lack of escalation of treatment in both trials despite prompts from HIAs based on national guidelines. This requires further investigation in terms of why it is difficult to achieve the level of optimisation of medication in normal clinical practice that is achieved in clinical trials and ways to overcome this.
-
In our evidence synthesis we were unable to reach clear conclusions about whether or not particular types of technology were more likely to be effective. There is a need for a clearer framework to describe the nature and underlying theoretical basis of telehealth interventions. There should be more detailed descriptions of these aspects of interventions within reports of trials and other evaluations. This would enable examination of which aspects of telehealth interventions are associated with effectiveness.
-
What are the most effective methods for evaluation of a fast-moving field such as telehealth that balance rigour, pragmatic application, generalisability and timeliness?
Implications for health care
This research programme addressed a topic of great importance to health care. Considerable resources have been committed to implementing different forms of telehealth for chronic conditions in different countries. For example, the US-based Veterans Health Administration introduced a national home telehealth programme that had enrolled about 50,000 patients by 2011,154,416 the Renewing Health Consortium is developing and testing a telehealth programme in nine European countries417 and in the UK the WSD project was established to provide telehealth at scale for patients with conditions such as heart failure or chronic lung disease. 52,53,418
Telehealth services are currently being promoted and commissioned on a wide scale in line with a general policy enthusiasm for telehealth. In the UK, the Technology Enabled Care Services initiative30 encourages and supports commissioners to introduce telehealth services. The following quotation comes from the NHS England website describing this initiative:30
Technology enabled care services refers to the use of telehealth, telecare, telemedicine, telecoaching and self-care in providing care for patients with long term conditions that is convenient, accessible and cost-effective. We recognise the potential of these solutions to transform the way people engage in and control their own healthcare, empowering them to manage their care in a way that is right for them.
Our ambition through the Technology Enabled Care Services (TECS) programme is to create the right commissioning environment that supports and encourages the innovative use of technology to improve health outcomes for patients with long term conditions and deliver more cost-effective services. We believe that by embracing this sort of technology, we can empower millions of patients to own their own care and transform the way we plan and deliver services to create a sustainable NHS for the future.
Reproduced from NHS England. 30 Contains public sector information licensed under the Open Government Licence v3.0
The logic and appeal of this approach is clear, given the need to develop new approaches to support the increasing number of people with LTCs in sustainable ways within limited resources. However, it is notable that the argument in favour of telehealth as the best way to achieve this (as articulated in the quotation above) is built on a number of assumptions about the potential for telehealth to promote patient self-management and to be delivered cost-effectively. This argument has been rehearsed for many years, but in the absence of consistent evidence about either effectiveness or cost-effectiveness. 54
Indeed, the conclusion that can be drawn from the most recent, relevant and high-quality studies, including the Healthlines study and the WSD programme,52,53 is that this approach to comprehensive multifaceted health-care delivery based on telehealth is likely to have only modest benefits for health and providing it in a way that is cost-effective is likely to very challenging. Telehealth innovations have the potential to considerably increase, rather than decrease, NHS expenditure and so may not help the sustainability of health care. Although telehealth applications may have the potential to ‘transform the way we plan and deliver services’, there have been few examples of this being done successfully by widespread pragmatic implementation that have stood up to independent evaluation. The consistent message from research such as the Healthlines study is that considerable caution should be used before widespread implementation of telehealth services for patients with LTCs.
Part of the reason for the limited impact of these services appears to be the difficulty in achieving meaningful change in both patient and practitioner behaviour. It appears that only a minority of people are, at present, likely to be interested in this type of health care. However, our research suggests that this interest is not likely to be limited to particular groups in terms of age or other socioeconomic characteristics, but is determined more by confidence and attitudes to technology, and these may increase over time and with growing technology familiarity. Furthermore, those people who did participate in the Healthlines Service expressed positive views about perceptions of access, satisfaction with the care provided and a greater sense of support for self-management.
Second, despite the efforts used within the Healthlines Service to work in partnership with other NHS providers, these did not appear to be effective. It will be very important to ensure that telehealth services are fully integrated with other existing services, rather than ‘bolted on’. New approaches are needed to ensure this, which may include greater multisector involvement in planning service delivery at a whole-system level, as well as commissioning levers such as contracts and financial incentives.
Third, the issue of cost-effectiveness is crucial to the argument about the role of telehealth in providing a sustainable future for health care. However, there may be a lack of clarity about whether or not telehealth programmes are intended to provide (1) more effective care at extra cost or (2) equally effective care at lower cost than alternative approaches. Almost all telehealth programmes that have so far been evaluated (including the Healthlines Service) have been delivered at extra cost and whether or not they are cost-effective depends on the extent to which they provide greater health gain. The Healthlines depression intervention provided modest health benefits, similar to those from other interventions based on a collaborative care model, but the increased cost made it unlikely to be cost-effective. The Healthlines CVD risk intervention also had limited impacts in terms of the primary outcome of reducing cardiovascular risk but may be cost-effective because of the long-term benefits, assuming that the impact of the intervention is sustained. In neither example was there any evidence that this telehealth approach substituted for other NHS resource use. In fact, in both trials the intervention was associated with increases rather than decreases in the use of other primary care and community care services. It may be that the intervention made people more likely to engage with improving their health or more adherent to appointments, but this will have further increased the additional cost of the telehealth approach. Our findings are consistent with those from the WSD programme, which led to small benefits in quality of life at considerably greater cost, meaning that the intervention was unlikely to be cost-effective. 53
For a NHS telehealth service to become cost-effective, and assuming that such services are unlikely to lead to major health gains compared with usual face-to-face care (an assumption supported by most previous research), several considerations are important. First, telehealth services will have to be delivered at much lower cost than most services currently available. This may be possible with economies of scale and reductions in the cost of hardware over time, but the size of the overall cost reduction that will be necessary should be modelled before services are commissioned and should be based on assumptions using previous evidence, rather than being over-optimistic. This type of modelling was conducted in the evaluation of the WSD trial and showed that the intervention was likely to be cost-effective only if equipment prices were reduced by 80% and the service operated at twice the level of productivity observed in the trial. 53
Telehealth services could reduce health-care expenditure and therefore aid the sustainability of the NHS only if they substituted for the use of other more expensive health-care resources. We observed no reduction in the costs of primary or secondary care in the Healthlines trials. The WSD evaluation did identify reductions in the cost of hospital and community care,53 but the interpretation of this finding is debatable because of a short-term spike in hospital admissions in the control group when the trial began. 155 Other studies that have demonstrated cost savings from telehealth have generally either been conducted in the USA, with their very different health-care system, or been of low quality. 415
On the other hand, telehealth could aid the sustainability of health-care systems if it allows the use of less highly trained staff to provide equally good care at a similar cost as more conventional approaches. This was the case with the Healthlines depression intervention, which provided similar results to those of earlier studies that have relied on mental health workers. In this scenario, the argument is not about more cost-effective use of resources, but allowing the expansion of services to meet growing needs if there are shortages of trained health workers.
Despite these somewhat pessimistic conclusions, the underlying enthusiasm for the potential role of new technologies to transform health care is understandable, based on the way that most other services in other aspects of life have changed over the last 30 years. Within health care, there are examples of simple, low-cost technologies that have been shown to improve health and provide more convenient care, such as the txt2stop programme for smoking cessation419 and the use of home monitoring and self-titration of medication for hypertension. 147 In the Healthlines trials, we attempted to combine these and other interventions in a comprehensive new approach to supporting self-management based on telehealth, which was less successful. It may be that this approach to whole-system change based on telehealth is over-ambitious. Rather than attempting a step change in service delivery, telehealth may become normalised in an incremental fashion, with one new technology being introduced at a time for a specific purpose. Some of these innovations will find a place in the tapestry of health-care services and some that do not meet a need will be superseded. This kind of piecemeal development would parallel the way in which other innovations based on technology have come to be used in everyday life. However, there is a danger that even this kind of stepwise introduction of technology could lead to incremental increases in costs without concomitant benefits and so caution and independent evaluation will still be important.
Telehealth includes a wide range of technologies and approaches and is a rapidly evolving field in which the cost of technology usually drops rapidly as it becomes widespread. Although the approach used in the Healthlines programme had limited success, we still face the conundrum that some trials of specific telehealth interventions appear to be successful, yet without much of a clear pattern about why some interventions are effective whereas others are not. The challenge is therefore to continue to innovate and to try to understand what works, how, for which groups of patients and in what circumstances. The TECH model developed through this research programme will hopefully provide a useful framework to focus attention on areas for development (e.g. the importance of patient engagement, the need to optimise treatment and the importance of integration with primary care services) and also provide a framework for evaluation.
Conclusion
In the Healthlines research programme we used a range of methods to develop a comprehensive telehealth approach to support the management of LTCs, based on a clear conceptual framework. We evaluated this intervention through large pragmatic multicentre RCTs in two exemplar conditions of depression and raised CVD risk. Our evaluation provided some evidence that the intervention was associated with modest health benefits. Patients receiving the Healthlines Service had more positive experiences accessing health care, more support for self-management and higher levels of satisfaction with the treatment they received. The small benefits and extra cost of the Healthlines Service for depression suggested that it was unlikely to be cost-effective, but the Healthlines Service for raised CVD risk was likely to be cost-effective if the benefits observed are sustained over a long period.
Acknowledgements
The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the NIHR, the NHS or the Department of Health. The NIHR and Department of Health had no role in study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the paper for publication.
NHS Direct and Solent NHS Trust hosted the research programme.
We are very grateful to all of the participating general practices and patients recruited from these practices for taking part and making this research possible. In addition, we thank BigWhiteWall Ltd and Media Innovations Ltd (LLTTFi) for the use of these programmes, as well as providing anonymised data on participant usage. We are grateful to the Primary Care Research Network [now NIHR Clinical Research Network; see www.crn.nihr.ac.uk/ (accessed 26 September 2016)] for assisting us with general practice recruitment for the trials. Finally, we also extend our gratitude to the volunteers who were recruited through the Mental Health Research Network (part of the NIHR) and NHS Direct for providing valuable feedback on the patient survey.
We would also like to thank the following people for their invaluable contributions and help with undertaking components of this programme of work:
Patient and public representatives: Michelle McPhail and Anne Jacobs.
Health information advisors: Marie Porter, Helen Duszynski, Alice Osmond and Angela Harrow.
Research administrators: Frederika Collihole (Bristol) and Richard Campbell (Sheffield).
Additional Bristol researchers: Ben Davies (activity 5), Alison Gregory (activity 3) and Lorna Duncan (activity 5).
Southampton researchers: Janet Cooke and Diane Beck (participant recruitment for activity 5).
Co-ordination of study transfer to Solent NHS Trust and hosting the research: Dr Sarah Williams.
Trial Steering Committee members: Professor Brian McKinstry (Chairperson), Dr Peter Brindle, Professor David Richards, Professor Rod Taylor, Anne Jacob, Michelle McPhail, Dr Sarah Williams, Professor Chris Salisbury, Professor Alicia O’Cathain, Professor Alan Montgomery, Dr Louisa Edwards, Dr Clare Thomas and Dr Sarah Drabble.
Data Monitoring Committee members: Professor Elizabeth Murray (Chairperson), Dr Tim Holt, Professor Catherine Hewitt, Professor Alan Montgomery, Dr Clare Thomas and Dr Louisa Edwards.
NHS Direct staff involved in developing the intervention and content: Mark Symes, Christine McKinney, Ian James, Jacqui Jedrzejewski, Suzanne Nicholson, Susan Green and Bilkis Islam-Ali.
Use of the Duke CVD risk patient management software, including assistance with modification and implementation and advice and feedback on the CVD trial and results: Professor Hayden Bosworth and Felicia McCant.
Training HIAs and advice and feedback on the depression intervention: Professor Chris Williams.
Information technology development and support for the intervention: Steve Bellerby and his supporting team.
Research database support: David Carmichael.
Lead author of the CVD risk systematic review: Dr Sam Merriel.
Advice and assistance with the CVD risk simulation modelling: Roberta Ara.
Contributions of authors
Professor Chris Salisbury (Professor of Primary Health Care) was Chief Investigator on the project, led the grant application, co-conceived and designed the project, led the survey study (activity 3) and model development (activity 4) and coled the RCTs (activity 5), provided clinical input, contributed methodological and practical advice, was lead author of the Scientific summary and Plain English summary and Chapters 1, 5 and 12, coauthored Chapters 8 and 9 and finalised the report.
Professor Alicia O’Cathain (Professor of Health Services Research) was a co-applicant on the programme grant, co-conceived and designed the project, led the Sheffield-based research team and components of the research (process evaluation), contributed methodological and practical advice and was coauthor of Chapters 2, 7 and 11.
Dr Clare Thomas (Programme Manager) co-ordinated and managed the project from November 2009 to August 2012 and from September 2013 to June 2014, codeveloped the study protocols (activities 3 and 5), convened research meetings, contributed methodological and practical advice to the research and was coauthor on Chapters 6 and 7.
Dr Louisa Edwards (Research Associate; Programme Manager June 2014–May 2015) conducted the patient survey (activity 3), led the recruitment, retention and data collection process for the RCT participants in the Bristol area (activity 5) and devised one of the response rate subsidiary studies. She contributed methodological and practical advice to the research and was lead author on Chapter 3 and coauthor on Chapters 6 and 7.
Professor Alan A Montgomery (Professor of Medical Statistics and Clinical Trials) was a co-applicant on the programme grant, co-conceived and designed the project, coled the RCTs (activity 5), was the lead statistician throughout the programme and contributed methodological and practical advice to the research.
Dr Sandra Hollinghurst (Senior Lecturer in Health Economics) was a co-applicant on the programme grant, co-conceived and designed the project, supervised and led the economic evaluation of the trials (activity 5), contributed methodological and practical advice to the research and was coauthor of Chapters 7 and 10.
Shirley Large (formerly Head of Research and Clinical Audit, NHS Direct) was a co-applicant on the programme grant, co-conceived and designed the project, led the NHS Direct working groups and assisted with the management of intervention delivery staff, conducted the usability testing ahead of the trials (activity 4) and contributed methodological and practical advice to the research.
Professor Jon Nicholl (Dean of School) was a co-applicant on the programme grant, co-conceived and designed the project and contributed methodological and practical advice to the research.
Professor Catherine Pope (Professor of Medical Sociology) was a co-applicant on the programme grant, co-conceived and designed the project, coled the evidence synthesis (activity 1), contributed methodological and practical advice to the research and was coauthor of Chapter 2.
Professor Anne Rogers (Professor of Health Systems Implementation) was a co-applicant on the programme grant, co-conceived and designed the project, coled the qualitative research study (activity 2), contributed methodological and practical advice to the research and was coauthor of Chapter 4.
Professor Glyn Lewis (Professor of Psychiatric Epidemiology) was a co-applicant on the programme grant, co-conceived and designed the project, provided clinical input and guidance and contributed methodological and practical advice to the research.
Professor Tom Fahey (Professor of General Practice) was a co-applicant on the programme grant, co-conceived and designed the project, provided clinical input and guidance and contributed methodological and practical advice to the research.
Professor Lucy Yardley (Professor of Health Psychology) co-conceived and designed the project and contributed methodological and practical advice to the research.
Dr Simon Brownsell (formerly Senior Research Fellow, Mixed Methods) co-conceived and designed the project and led the horizon-scanning component (activity 1) reported in Chapter 2.
Dr Padraig Dixon (Research Associate, Health Economics) conducted the analysis of the economic evaluation of the trials (activity 5), contributed to the methodological and practical components of the economic evaluation and was coauthor of Chapters 7 and 10.
Dr Sarah Drabble (Qualitative Research Associate) was the lead researcher conducting the interviews and analysis for the process evaluation of the trials (activity 5). She contributed to the methodological and practical components of the process evaluation and was coauthor of Chapters 7 and 11.
Lisa Esmonde (formerly Research Associate) was the lead Sheffield-based researcher for the patient survey (activity 3) and contributed to the methodological and practical components of the survey.
Alexis Foster (Research Associate) was the lead researcher conducting the RCTs in the Sheffield area and contributed to patient follow-up in the Southampton area (activity 5). She also assisted with the interviews and analysis for the process evaluation (activity 5) and codevised one of the response rate subsidiary studies.
Dr Katy Garner (formerly Research Assistant) supported and contributed to the recruitment, retention and data collection process for the RCT participants in the Bristol area (activity 5). She also coconceptualised one of the response rate subsidiary studies.
Daisy Gaunt (Research Assistant, Medical Statistics) was the lead researcher conducting the analysis of the trial results (activity 5). She developed the statistical analysis plan and was coauthor of Chapters 8 and 9.
Kim Horspool (formerly Research Assistant) contributed to recruitment, retention and data collection process for the RCT participants in the Sheffield area and patient follow-up in the Southampton area (activity 5). She also assisted with the interviews and analysis for the process evaluation (activity 5) and codevised one of the response rate subsidiary studies.
Dr Mei-See Man (Trial Manager) provided maternity cover as Programme Manager for the trials from August 2012 to September 2013. She co-ordinated one of the subsidiary studies (MRC START) and contributed methodological and practical advice to the randomised trials (activity 5).
Alison Rowsell (Qualitative Research Fellow) coled the evidence synthesis (activity 1), contributed methodological and practical advice to the evidence synthesis and was coauthor of Chapter 2.
Dr Julia Segar (Research Fellow) coled the qualitative research study and carried out the fieldwork (activity 2), contributed methodological and practical advice to this component of the research and was coauthor of Chapter 4.
All authors contributed to publications arising from this research and to the writing of this report and have approved the final version of the report.
Publications
Segar J, Rogers A, Salisbury C, Thomas C. Roles and identities in transition: boundaries of work and inter-professional relationships at the interface between telehealth and primary care. Health Soc Care Community 2013;21:606–13.
Edwards L, Thomas C, Gregory A, Yardley L, O’Cathain A, Montgomery AA, Salisbury C. Are patients with chronic diseases interested in telehealth? A cross-sectional postal survey. J Med Internet Res 2014;16:e123.
Merriel SW, Andrews V, Salisbury C. Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis. Prev Med 2014;64:88–95.
Thomas CL, Man M, O’Cathain A, Hollinghurst S, Large S, Edwards L, et al. Effectiveness and cost-effectiveness of a telehealth intervention to support the management of long-term conditions: study protocol for two linked randomized controlled trials. Trials 2014;15:36.
Foster A, Horspool K, Edwards L, Thomas CL, Salisbury C, Montgomery AA, O’Cathain A. Who does not participate in telehealth trials and why? A cross-sectional survey. Trials 2015;16:258.
Man M-S, on behalf of the Healthlines Study Group, Rick J, Bower P, on behalf of the MRC-START Group. Improving recruitment to a study of telehealth management for long-term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16:309.
Salisbury C, Thomas C, O’Cathain A, Rogers A, Pope C, Yardley L, et al. TElehealth in CHronic disease: mixed-methods study to develop the TECH conceptual model for intervention design and evaluation. BMJ Open 2015;5:e006448.
Vassilev I, Rowsell A, Pope C, Kennedy A, O’Cathain A, Salisbury C, et al. Assessing the implementability of telehealth interventions for self-management support: a realist review. Implement Sci 2015;10:59.
Dixon P, Hollinghurst S, Edwards L, Thomas C, Foster A, Davies B, et al. Cost-effectiveness of telehealth for patients with depression: evidence from the Healthlines randomised controlled trial. Br J Psychiatry Open 2016;2:262–9.
Dixon P, Hollinghurst S, Edwards L, Thomas C, Gaunt D, Foster A, et al. Cost-effectiveness of telehealth for patients with raised cardiovascular disease risk: evidence from the Healthlines randomised controlled trial. BMJ Open 2016;6:e012352.
Edwards L, Salisbury C, Horspool K, Foster A, Garner K, Montgomery AA. Increasing follow-up questionnaire response rates in a randomized controlled trial of telehealth for depression: three embedded controlled studies. Trials 2016;17:107.
O’Cathain A, Drabble S, Foster A, Horspool K, Edwards L, Thomas C, et al. Being human: a qualitative interview study of why a telehealth intervention for chronic conditions had a modest effect. J Med Internet Res 2016;18:e163.
Salisbury C, O’Cathain A, Edwards L, Thomas C, Gaunt D, Hollinghurst S, et al. Effectiveness of an integrated telehealth service for patients with depression: a pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry 2016;3:515–25.
Salisbury C, O’Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ 2016;353:i2647.
Dixon P, Hollinghurst S, Ara R, Edwards L, Foster A, Salisbury C. Cost-effectiveness modelling of telehealth for patients with raised cardiovascular disease risk: evidence from a cohort simulation conducted alongside the Healthlines randomised controlled trial. BMJ Open 2016;6:e012355.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- The New NHS: Modern, Dependable. London: Department of Health; 1997.
- NHS Direct . What Is NHS Direct? History 2014. http://webarchive.nationalarchives.gov.uk/20140220132333/http://www.nhsdirect.nhs.uk/About/WhatIsNHSDirect/History (accessed 14 April 2015).
- Developing NHS Direct – Strategy Document for the Next Three Years. London: Department of Health; 2003.
- Long Term Conditions Compendium of Information. London: Department of Health; 2012.
- Raising the Profile of Long Term Conditions Care: A Compendium of Information. London: Department of Health; 2008.
- Long Term Conditions Model. London: Department of Health; 2008.
- Murray E. Providing information for patients. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a280.
- García-Lizana F, Sarría-Santamera A. New technologies for chronic disease management and control: a systematic review. J Telemed Telecare 2007;13:62-8. http://dx.doi.org/10.1258/135763307780096140.
- Paré G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc 2007;14:269-77. http://dx.doi.org/10.1197/jamia.M2270.
- Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39156.536968.55.
- Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive health communication applications for people with chronic disease. Cochrane Database Syst Rev 2005;4. http://dx.doi.org/10.1002/14651858.cd004274.pub4.
- Hersh WR, Helfand M, Wallace J, Kraemer D, Patterson P, Shapiro S, et al. Clinical outcomes resulting from telemedicine interventions: a systematic review. BMC Med Inform Decis Mak 2001;1. http://dx.doi.org/10.1186/1472-6947-1-5.
- Barlow J. Building an Evidence Base for Successful Telecare Implementation – Updated Report of the Evidence Working Group of the Telecare Policy Collaborative Chaired by James Barlow 2006. www.researchgate.net/publication/259533332_Building_an_evidence_base_for_successful_telecare_implementation._Updated_report_of_the_Evidence_Working_Group_of_the_Telecare_Policy_Collaborative_chaired_by_James_Barlow (accessed 28 May 2015).
- Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000;2. http://dx.doi.org/10.1002/14651858.cd002098.
- Leach LS, Christensen H. A systematic review of telephone-based interventions for mental disorders. J Telemed Telecare 2006;12:122-9. http://dx.doi.org/10.1258/135763306776738558.
- Dellifraine JL, Dansky KH. Home-based telehealth: a review and meta-analysis. J Telemed Telecare 2008;14:62-6. http://dx.doi.org/10.1258/jtt.2007.070709.
- Bowles KH, Baugh AC. Applying research evidence to optimize telehomecare. J Cardiovasc Nurs 2007;22:5-15. http://dx.doi.org/10.1097/00005082-200701000-00002.
- Farmer A, Gibson OJ, Tarassenko L, Neil A. A systematic review of telemedicine interventions to support blood glucose self-monitoring in diabetes. Diabet Med 2005;22:1372-8. http://dx.doi.org/10.1111/j.1464-5491.2005.01627.x.
- Artinian NT. Telehealth as a tool for enhancing care for patients with cardiovascular disease. J Cardiovasc Nurs 2007;22:25-31. http://dx.doi.org/10.1097/00005082-200701000-00004.
- Barlow J, Singh D, Bayer S, Curry R. A systematic review of the benefits of home telecare for frail elderly people and those with long-term conditions. J Telemed Telecare 2007;13:172-9. http://dx.doi.org/10.1258/135763307780908058.
- Martínez A, Everss E, Rojo-Alvarez JL, Figal DP, García-Alberola A. A systematic review of the literature on home monitoring for patients with heart failure. J Telemed Telecare 2006;12:234-41. http://dx.doi.org/10.1258/135763306777889109.
- Health Services Management Centre . Reducing Unplanned Hospital Admissions: What Does the Literature Tell Us? 2006. www.birmingham.ac.uk/Documents/college-social-sciences/social-policy/HSMC/publications/2006/Reducing-unplanned-hospital-admissions.pdf (accessed 26 September 2016).
- Deshpande A, Khoja S, McKibbon A, Jadad AR. Real-Time (Synchronous) Telehealth in Primary Care: Systematic Review of Systematic Reviews. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2008.
- Singleton R. Psychiatric Morbidity among Adults Living in Private Households, 2000. London: HMSO; 2001.
- Health and Social Care Information Centre . Prescriptions Dispensed in the Community: Statistics for 1994 to 2004 – England 2005. www.hscic.gov.uk/catalogue/PUB01237/pres-disp-com-eng-1994–2004-rep.pdf (accessed 13 February 2015).
- Mental Health Policy Group . The Depression Report: A New Deal for Depression and Anxiety Disorders 2006. http://eprints.lse.ac.uk/archive/00000818 (accessed 28 May 2015).
- Depression (Amended): Management of Depression in Primary and Secondary Care. London: NICE; 2007.
- Our Health, our Care, our Say: A New Direction for Community Services. London: Department of Health; 2006.
- Health Informatics Review. London: Department of Health; 2008.
- Technology Enabled Care Services (TECS). NHS England; 2015.
- Stroke Association . JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart 2005;91:1-52. http://dx.doi.org/10.1136/hrt.2005.079988.
- Davies M, Khunti K, Chauhan U, Stribling B, Goyder E, Farooqi A, et al. The Handbook for Vascular Risk Assessment, Risk Reduction and Risk Management. London: UK National Screening Committee; 2008.
- Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London: NICE; 2008.
- Putting Prevention First, Vascular Checks: Risk Assessment and Management. London: Department of Health; 2008.
- Pfizer Health Solutions . OwnHealth Birmingham: Successes and Learning from the First Year 2007. www.ehiprimarycare.com/img/document_library0282/BirminghamOwnHealth_-_successes_and_learning_from_the_first_year.pdf (accessed 20 April 2015).
- Azarmina P, Lewis J. Patient satisfaction with a nurse-led, telephone-based disease management service in Birmingham. J Telemed Telecare 2007;13:3-4. http://dx.doi.org/10.1258/135763307781645194.
- Christensen H, Griffiths KM, Jorm AF. Delivering interventions for depression by using the internet: randomised controlled trial. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.37945.566632.EE.
- Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA 2004;292:935-42. http://dx.doi.org/10.1001/jama.292.8.935.
- Computerised Cognitive Behaviour Therapy for Depression and Anxiety: Review of Technology Appraisal 51. London: NICE; 2006.
- Cuijpers P, van Straten A, Andersson G. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med 2008;31:169-77. http://dx.doi.org/10.1007/s10865-007-9144-1.
- Andersson G, Bergström J, Holländare F, Carlbring P, Kaldo V, Ekselius L. Internet-based self-help for depression: randomised controlled trial. Br J Psychiatry 2005;187:456-61. http://dx.doi.org/10.1192/bjp.187.5.456.
- van Straten A, Cuijpers P, Smits N. Effectiveness of a web-based self-help intervention for symptoms of depression, anxiety, and stress: randomized controlled trial. J Med Internet Res 2008;10. http://dx.doi.org/10.2196/jmir.954.
- Anderson L, Lewis G, Araya R, Elgie R, Harrison G, Proudfoot J, et al. Self-help books for depression: how can practitioners and patients make the right choice?. Br J Gen Pract 2005;55:387-92.
- Lewis G, Anderson E, Araya R, Elgie R, Harrison G, Proudfoot J, et al. Self-help Interventions for Mental Health Problems. London: Department of Health; 2003.
- Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857-67. http://dx.doi.org/10.1001/jama.299.24.2857.
- Bosworth HB, Olsen MK, Neary A, Orr M, Grubber J, Svetkey L, et al. Take Control of your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008;70:338-47. http://dx.doi.org/10.1016/j.pec.2007.11.014.
- Vale MJ, Jelinek MV, Best JD, Dart AM, Grigg LE, Hare DL, et al. Coaching patients On Achieving Cardiovascular Health (COACH): a multicenter randomized trial in patients with coronary heart disease. Arch Intern Med 2003;163:2775-83. http://dx.doi.org/10.1001/archinte.163.22.2775.
- Wister A, Loewen N, Kennedy-Symonds H, McGowan B, McCoy B, Singer J. One-year follow-up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. CMAJ 2007;177:859-65. http://dx.doi.org/10.1503/cmaj.061059.
- White Paper Pilots: Whole System Long Term Conditions (LTC) Demonstrator Programme. London: Department of Health; 2007.
- Singh D. Transforming Chronic Care: Evidence about Improving Care for People With Long-Term Conditions 2005. www.download.bham.ac.uk/hsmc/pdf/transforming_chronic_care.pdf (accessed 20 April 2015).
- Rose G. Sick individuals and sick populations. Int J Epidemiol 1985;14:32-8. http://dx.doi.org/10.1093/ije/14.1.32.
- Cartwright M, Hirani SP, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f653.
- Henderson C, Knapp M, Fernández J-L, Beecham J, Hirani SP, Cartwright M, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1035.
- Wootton R. Twenty years of telemedicine in chronic disease management – an evidence synthesis. J Telemed Telecare 2012;18:211-20. http://dx.doi.org/10.1258/jtt.2012.120219.
- BBC News . Government Confirms Plan to Scrap NHS Direct Helpline 29 08 2010 2010. www.bbc.co.uk/news/uk-11120853 (accessed 15 April 2015).
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-9. http://dx.doi.org/10.1136/bmj.39108.379965.BE.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Hardeman W, Sutton S, Griffin S, Johnston M, White A, Wareham NJ, et al. A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res 2005;20:676-87. http://dx.doi.org/10.1093/her/cyh022.
- Bartholomew LK, Parcel GS, Kok G, Gottlieb NH, Fernandez ME. Planning Health Promotion Programs: An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass; 2011.
- Mays N, Pope C, Popay J. Systematically reviewing qualitative and quantitative evidence to inform management and policy-making in the health field. J Health Serv Res Policy 2005;10:6-20. http://dx.doi.org/10.1258/1355819054308576.
- Merriel SW, Andrews V, Salisbury C. Telehealth interventions for primary prevention of cardiovascular disease: a systematic review and meta-analysis. Prev Med 2014;64:88-95. http://dx.doi.org/10.1016/j.ypmed.2014.04.001.
- Murray E. Internet-delivered treatments for long-term conditions: strategies, efficiency and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res 2008;8:261-72. http://dx.doi.org/10.1586/14737167.8.3.261.
- Ekeland AG, Bowes A, Flottorp S. Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform 2010;79:736-71. http://dx.doi.org/10.1016/j.ijmedinf.2010.08.006.
- The National Service Framework for Long Term Conditions. London: Department of Health; 2005.
- Disease Management Information Toolkit (DMIT). London: Department of Health; 2007.
- Improving the Health and Well-Being of People with Long Term Conditions. World Class Services for People with Long Term Conditions – Information Tool for Commissioners. London: Department of Health; 2010.
- Petticrew M, Song F, Wilson P, Wright K. Quality-assessed reviews of health care interventions and the Database of Abstracts of Reviews of Effectiveness (DARE). Int J Technol Assess Health Care 1999;15:671-8.
- Oake N, Jennings A, van Walraven C, Forster AJ. Interactive voice response systems for improving delivery of ambulatory care. Am J Manag Care 2009;15:383-91.
- Paré G, Moqadem K, Pineau G, St-Hilaire C. Clinical effects of home telemonitoring in the context of diabetes, asthma, heart failure and hypertension: a systematic review. J Med Internet Res 2010;12. http://dx.doi.org/10.2196/jmir.1357.
- Botsis T, Hartvigsen G. Current status and future perspectives in telecare for elderly people suffering from chronic diseases. J Telemed Telecare 2008;14:195-203. http://dx.doi.org/10.1258/jtt.2008.070905.
- Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev 2010;32:56-69. http://dx.doi.org/10.1093/epirev/mxq004.
- Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: update of a systematic review of telemedicine services. J Telemed Telecare 2006;12:3-31. http://dx.doi.org/10.1258/135763306778393117.
- Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health 2009;15:231-40. http://dx.doi.org/10.1089/tmj.2008.0099.
- Polisena J, Coyle D, Coyle K, McGill S. Home telehealth for chronic disease management: a systematic review and an analysis of economic evaluations. Int J Technol Assess Health Care 2009;25:339-49. http://dx.doi.org/10.1017/S0266462309990201.
- Rains SA, Young V. A meta-analysis of research on formal computer-mediated support groups: examining group characteristics and health outcomes. Hum Commun Res 2009;35:309-36. http://dx.doi.org/10.1111/j.1468-2958.2009.01353.x.
- Stinson J, Wilson R, Gill N, Yamada J, Holt J. A systematic review of internet-based self-management interventions for youth with health conditions. J Pediatr Psychol 2009;34:495-510. http://dx.doi.org/10.1093/jpepsy/jsn115.
- Tran K, Polisena J, Coyle D, Coyle K, Kluge E-HW, Cimon K, et al. Home Telehealth for Chronic Disease Management. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2008.
- García-Lizana F, Muñoz-Mayorga I. Telemedicine for depression: a systematic review. Perspect Psychiatr Care 2010;46:119-26. http://dx.doi.org/10.1111/j.1744-6163.2010.00247.x.
- Spek V, Cuijpers P, Nyklícek I, Riper H, Keyzer J, Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med 2007;37:319-28. http://dx.doi.org/10.1017/S0033291706008944.
- Mohr DC, Vella L, Hart S, Heckman T, Simon G. The effect of telephone-administered psychotherapy on symptoms of depression and attrition: a meta-analysis. Clin Psychol 2008;15:243-53. http://dx.doi.org/10.1111/j.1468-2850.2008.00134.x.
- Waller R, Gilbody S. Barriers to the uptake of computerized cognitive behavioural therapy: a systematic review of the quantitative and qualitative evidence. Psychol Med 2009;39:705-12. http://dx.doi.org/10.1017/S0033291708004224.
- Griffiths KM, Calear AL, Banfield M. Systematic review on internet support groups (ISGs) and depression (1): do ISGs reduce depressive symptoms?. J Med Internet Res 2009;11. http://dx.doi.org/10.2196/jmir.1270.
- Griffiths KM, Calear AL, Banfield M, Tam A. Systematic review on internet support groups (ISGs) and depression (2): what is known about depression ISGs?. J Med Internet Res 2009;11. http://dx.doi.org/10.2196/jmir.1303.
- Griffiths KM, Christensen H. Internet-based mental health programs: a powerful tool in the rural medical kit. Aust J Rural Health 2007;15:81-7. http://dx.doi.org/10.1111/j.1440-1584.2007.00859.x.
- Kaltenthaler E, Parry G, Beverley C, Ferriter M. Computerised cognitive-behavioural therapy for depression: systematic review. Br J Psychiatry 2008;193:181-4. http://dx.doi.org/10.1192/bjp.bp.106.025981.
- Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther 2009;38:196-205. http://dx.doi.org/10.1080/16506070903318960.
- Cuijpers P, Marks IM, van Straten A, Cavanagh K, Gega L, Andersson G. Computer-aided psychotherapy for anxiety disorders: a meta-analytic review. Cogn Behav Ther 2009;38:66-82. http://dx.doi.org/10.1080/16506070802694776.
- Barak A, Hen L, Boniel-Nissim M, Shapira N. A comprehensive review and a meta-analysis of the effectiveness of internet-based psychotherapeutic interventions. J Technol Hum Serv 2008;26:109-60. http://dx.doi.org/10.1080/15228830802094429.
- Bee PE, Bower P, Lovell K, Gilbody S, Richards D, Gask L, et al. Psychotherapy mediated by remote communication technologies: a meta-analytic review. BMC Psychiatry 2008;8. http://dx.doi.org/10.1186/1471-244X-8-60.
- Hailey D, Roine R, Ohinmaa A. The effectiveness of telemental health applications: a review. Can J Psychiatry 2008;53:769-78.
- Norman S. The use of telemedicine in psychiatry. J Psychiatr Ment Health Nurs 2006;13:771-7. http://dx.doi.org/10.1111/j.1365-2850.2006.01033.x.
- Postel MG, de Haan HA, De Jong CA. E-therapy for mental health problems: a systematic review. Telemed J E Health 2008;14:707-14. http://dx.doi.org/10.1089/tmj.2007.0111.
- Simpson S. Psychotherapy via videoconferencing: a review. Br J Guidance Couns 2009;37:271-86. http://dx.doi.org/10.1080/03069880902957007.
- Griffiths K, Farrer L, Christensen H. Clickety-click: e-mental health train on track. Australas Psychiatry 2007;15:100-8. http://dx.doi.org/10.1080/10398560601123716.
- Reger MA, Gahm GA. A meta-analysis of the effects of internet- and computer-based cognitive-behavioral treatments for anxiety. J Clin Psychol 2009;65:53-75. http://dx.doi.org/10.1002/jclp.20536.
- Public Health Resource Unit . Critical Appraisal Skills Programme (CASP) 2006. http://media.wix.com/ugd/dded87_29c5b002d99342f788c6ac670e49f274.pdf (accessed 30 March 2015).
- Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the internet? A systematic review of the published literature. J Med Internet Res 2006;8. http://dx.doi.org/10.2196/jmir.8.2.e10.
- Beattie A, Shaw A, Kaur S, Kessler D. Primary-care patients’ expectations and experiences of online cognitive behavioural therapy for depression: a qualitative study. Health Expect 2009;12:45-59. http://dx.doi.org/10.1111/j.1369-7625.2008.00531.x.
- Swinton JJ, Robinson WD, Bischoff RJ. Telehealth and rural depression: physician and patient perspectives. Fam Syst Health 2009;27:172-82. http://dx.doi.org/10.1037/a0016014.
- Lahdenperä TS, Kyngäs HA. Patients’ views about information technology in the treatment of hypertension. J Telemed Telecare 2000;6:108-13. http://dx.doi.org/10.1258/1357633001935130.
- Hanley T. The working alliance in online therapy with young people: preliminary findings. Br J Guidance Couns 2009;37:257-69. http://dx.doi.org/10.1080/03069880902956991.
- Nilsson C, Skar L, Soderberg S. Swedish district nurses’ experiences on the use of information and communication technology for supporting people with serious chronic illness living at home – a case study. Scand J Caring Sci 2010;24:259-65. http://dx.doi.org/10.1111/j.1471-6712.2009.00715.x.
- Lamothe L, Fortin JP, Labbé F, Gagnon MP, Messikh D. Impacts of telehomecare on patients, providers, and organizations. Telemed J E Health 2006;12:363-9. http://dx.doi.org/10.1089/tmj.2006.12.363.
- Liddy C, Dusseault JJ, Dahrouge S, Hogg W, Lemelin J, Humbert J, et al. Telehomecare for patients with multiple chronic illnesses: pilot study. Can Fam Physician 2008;54:58-65.
- Rahimpour M, Lovell NH, Celler BG, McCormick J. Patients’ perceptions of a home telecare system. Int J Med Inform 2008;77:486-98. http://dx.doi.org/10.1016/j.ijmedinf.2007.10.006.
- Sandberg J, Trief PM, Izquierdo R, Goland R, Morin PC, Palmas W, et al. A qualitative study of the experiences and satisfaction of direct telemedicine providers in diabetes case management. Telemed J E Health 2009;15:742-50. http://dx.doi.org/10.1089/tmj.2009.0027.
- Armstrong N, Hearnshaw H, Powell J, Dale J. Stakeholder perspectives on the development of a virtual clinic for diabetes care: qualitative study. J Med Internet Res 2007;9. http://dx.doi.org/10.2196/jmir.9.3.e23.
- Mackert M, Kahlor L, Tyler D, Gustafson J. Designing e-health interventions for low-health-literate culturally diverse parents: addressing the obesity epidemic. Telemed J E Health 2009;15:672-7. http://dx.doi.org/10.1089/tmj.2009.0012.
- Hopp FP, Hogan MM, Woodbridge PA, Lowery JC. The use of telehealth for diabetes management: a qualitative study of telehealth provider perceptions. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-14.
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Understanding the potential role of mobile phone-based monitoring on asthma self-management: qualitative study. Clin Exp Allergy 2007;37:794-802. http://dx.doi.org/10.1111/j.1365-2222.2007.02708.x.
- Kahn LS, Fox CH, Carrington J, Desai U, Bartlett DP, Lyle H, et al. Telephonic nurse case management for patients with diabetes and mental illnesses: a qualitative perspective. Chronic Illn 2009;5:257-67. http://dx.doi.org/10.1177/1742395309350229.
- Marziali E. E-health program for patients with chronic disease. Telemed J E Health 2009;15:176-81. http://dx.doi.org/10.1089/tmj.2008.0082.
- Høybye MT, Johansen C, Tjørnhøj-Thomsen T. Online interaction. Effects of storytelling in an internet breast cancer support group. Psychooncology 2005;14:211-20. http://dx.doi.org/10.1002/pon.837.
- Dinesen B, Andersen PE. Qualitative evaluation of a diabetes advisory system, DiasNet. J Telemed Telecare 2006;12:71-4. http://dx.doi.org/10.1258/135763306776084329.
- Hibbert D, Mair FS, May CR, Boland A, O’Connor J, Capewell S, et al. Health professionals’ responses to the introduction of a home telehealth service. J Telemed Telecare 2004;10:226-30. http://dx.doi.org/10.1258/1357633041424386.
- Armstrong N, Powell J. Patient perspectives on health advice posted on internet discussion boards: a qualitative study. Health Expect 2009;12:313-20. http://dx.doi.org/10.1111/j.1369-7625.2009.00543.x.
- Dinesen B, Nohr C, Andersen SK, Sejersen H, Toft E. Under surveillance, yet looked after: telehomecare as viewed by patients and their spouse/partners. Eur J Cardiovasc Nurs 2008;7:239-46. http://dx.doi.org/10.1016/j.ejcnurse.2007.11.004.
- LaFramboise LM, Woster J, Yager A, Yates BC. A technological life buoy: patient perceptions of the Health Buddy. J Cardiovasc Nurs 2009;24:216-24. http://dx.doi.org/10.1097/JCN.0b013e318199a60f.
- Scharer K, Colon E, Moneyham L, Hussey J, Tavakoli A, Shugart M. A comparison of two types of social support for mothers of mentally ill children. J Child Adolesc Psychiatr Nurs 2009;22:86-98. http://dx.doi.org/10.1111/j.1744-6171.2009.00177.x.
- Kerr C, Murray E, Stevenson F, Gore C, Nazareth I. Interactive health communication applications for chronic disease: patient and carer perspectives. J Telemed Telecare 2005;11:32-4. http://dx.doi.org/10.1258/1357633054461912.
- Kerr C, Murray E, Stevenson F, Gore C, Nazareth I. Internet interventions for long-term conditions: patient and caregiver quality criteria. J Med Internet Res 2006;8. http://dx.doi.org/10.2196/jmir.8.3.e13.
- Dinesen B, Toft E. Telehomecare challenge collaboration among healthcare professionals. Wirel Pers Commun 2009;51:711-24. http://dx.doi.org/10.1007/s11277-009-9767-3.
- Cornwall A, Moore S, Plant H. Embracing technology: patients’, family members’ and nurse specialists’ experience of communicating using e-mail. Eur J Oncol Nurs 2008;12:198-20. http://dx.doi.org/10.1016/j.ejon.2007.09.008.
- Overberg RI, Alpay LL, Verhoef J, Zwetsloot-Schonk JH. Illness stories on the internet: what do breast cancer patients want at the end of treatment?. Psychooncology 2007;16:937-44. http://dx.doi.org/10.1002/pon.1157.
- Vassilev I, Rowsell A, Pope C, Kennedy A, O’Cathain A, Salisbury C, et al. Assessing the implementability of telehealth interventions for self-management support: a realist review. Implement Sci 2015;10. http://dx.doi.org/10.1186/s13012-015-0238-9.
- Pawson R. Evidence-Based Policy: A Realist Perspective. London: Sage; 2006.
- Cavanagh K, Shapiro DA, Van Den Berg S, Swain S, Barkham M, Proudfoot J. The effectiveness of computerized cognitive behavioural therapy in routine care. Br J Clin Psychol 2006;452:499-514. http://dx.doi.org/10.1348/014466505X84782.
- Learmonth D, Rai S. Taking computerized CBT beyond primary care. Br J Clin Psychol 2008;472:111-18. http://dx.doi.org/10.1348/014466507X248599.
- Andersson G, Strömgren T, Ström L, Lyttkens L. Randomized controlled trial of internet-based cognitive behavior therapy for distress associated with tinnitus. Psychosom Med 2002;64:810-16.
- Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med 2010;51:214-21. http://dx.doi.org/10.1016/j.ypmed.2010.06.004.
- Micco N, Gold B, Buzzell P, Leonard H, Pintauro S, Harvey-Berino J. Minimal in-person support as an adjunct to internet obesity treatment. Ann Behav Med 2007;33:49-56. http://dx.doi.org/10.1207/s15324796abm3301_6.
- Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, Torgerson J, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996;91:285-92. http://dx.doi.org/10.1016/0895-7061(95)00353-3.
- Demiris G, Finkelstein SM, Speedie SM. Considerations for the design of a web-based clinical monitoring and educational system for elderly patients. J Am Med Inform Assoc 2001;8:468-72. http://dx.doi.org/10.1136/jamia.2001.0080468.
- Richards J, Klein B, Carlbring P. Internet-based treatment for panic disorder. Cogn Behav Ther 2003;32:125-35. http://dx.doi.org/10.1080/16506070302318.
- Saksena A. Computer-based education for patients with hypertension: a systematic review. Health Educ J 2010;69:236-45. http://dx.doi.org/10.1177/0017896910364889.
- May C, Gask L, Ellis N, Atkinson T, Mair F, Smith C, et al. Telepsychiatry evaluation in the north-west of England: preliminary results of a qualitative study. J Telemed Telecare 2000;6:20-2. http://dx.doi.org/10.1258/1357633001934618.
- Barak A, Boniel-Nissim M, Suler J. Fostering empowerment in online support groups. Comput Hum Behav 2008;24:1867-83. http://dx.doi.org/10.1016/j.chb.2008.02.004.
- Demiris G. The diffusion of virtual communities in health care: concepts and challenges. Patient Educ Couns 2006;62:178-88. http://dx.doi.org/10.1016/j.pec.2005.10.003.
- Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ 2000;320:550-4. http://dx.doi.org/10.1136/bmj.320.7234.550.
- Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001;323:715-18. http://dx.doi.org/10.1136/bmj.323.7315.715.
- Hunkeler EM, Meresman JF, Hargreaves WA, Fireman B, Berman WH, Kirsch AJ, et al. Efficacy of nurse telehealth care and peer support in augmenting treatment of depression in primary care. Arch Fam Med 2000;9:700-8. http://dx.doi.org/10.1001/archfami.9.8.700.
- Piette JD, Weinberger M, Kraemer FB, McPhee SJ. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs health care system: a randomized controlled trial. Diabetes Care 2001;24:202-8. http://dx.doi.org/10.2337/diacare.24.2.202.
- Jaana M, Pare G, Sicotte C. Hypertension home telemonitoring: current evidence and recommendations for future studies. Dis Manag Health Outcomes 2007;15:19-31. http://dx.doi.org/10.2165/00115677-200715010-00004.
- Norman GJ, Zabinski MF, Adams MA, Rosenberg DE, Yaroch AL, Atienza AA. A review of eHealth interventions for physical activity and dietary behavior change. Am J Prev Med 2007;33:336-45. http://dx.doi.org/10.1016/j.amepre.2007.05.007.
- Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev 2010;11:306-21. http://dx.doi.org/10.1111/j.1467-789X.2009.00646.x.
- Burke LE, Dunbar-Jacob J, Orchard TJ, Sereika SM. Improving adherence to a cholesterol-lowering diet: a behavioral intervention study. Patient Educ Couns 2005;57:134-42. http://dx.doi.org/10.1016/j.pec.2004.05.007.
- McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet 2010;376:163-72. http://dx.doi.org/10.1016/S0140-6736(10)60964-6.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011;57:29-38. http://dx.doi.org/10.1161/HYPERTENSIONAHA.110.160911.
- Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38121.684410.AE.
- AbuDagga A, Resnick HE, Alwan M. Impact of blood pressure telemonitoring on hypertension outcomes: a literature review. Telemed J E Health 2010;16:830-8. http://dx.doi.org/10.1089/tmj.2010.0015.
- Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2010;3. http://dx.doi.org/10.1002/14651858.cd005182.pub4.
- Christensen H, Griffiths KM, Farrer L. Adherence in internet interventions for anxiety and depression. J Med Internet Res 2009;11. http://dx.doi.org/10.2196/jmir.1194.
- Telemedicine: Opportunities and Developments in Member States. Report on the Second Global Survey on eHealth. Geneva: WHO; 2010.
- Darkins A, Ryan P, Kobb R, Foster L, Edmonson E, Wakefield B, et al. Care coordination/home telehealth: the systematic implementation of health informatics, home telehealth, and disease management to support the care of veteran patients with chronic conditions. Telemed J E Health 2008;14:1118-26. http://dx.doi.org/10.1089/tmj.2008.0021.
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3874.
- Steventon A, Tunkel S, Blunt I, Bardsley M. Effect of telephone health coaching (Birmingham OwnHealth) on hospital use and associated costs: cohort study with matched controls. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4585.
- Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FDR, Deeks JJ, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d3621.
- Mottillo S, Filion KB, Bélisle P, Joseph L, Gervais A, O’Loughlin J, et al. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur Heart J 2009;30:718-30. http://dx.doi.org/10.1093/eurheartj/ehn552.
- Chen YF, Madan J, Welton N, Yahaya I, Aveyard P, Bauld L, et al. Effectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysis. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16380.
- Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2012;7. http://dx.doi.org/10.1002/14651858.cd006611.pub3.
- Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2006;19. http://dx.doi.org/10.1002/14651858.cd002850.pub2.
- Bacigalupo R, Cudd P, Littlewood C, Bissell P, Hawley MS, Buckley Woods H. Interventions employing mobile technology for overweight and obesity: an early systematic review of randomized controlled trials. Obes Rev 2013;14:279-91. http://dx.doi.org/10.1111/obr.12006.
- Cohen GM, Irby MB, Boles K, Jordan C, Skelton JA. Telemedicine and pediatric obesity treatment: review of the literature and lessons learned. Clin Obes 2012;2:103-11. http://dx.doi.org/10.1111/j.1758-8111.2012.00050.x.
- Wieland LS, Falzon L, Sciamanna CN, Trudeau KJ, Brodney S, Schwartz JE, et al. Interactive computer-based interventions for weight loss or weight maintenance in overweight or obese people. Cochrane Database Syst Rev 2012;8. http://dx.doi.org/10.1002/14651858.cd007675.pub2.
- Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health 2010;16:931-8. http://dx.doi.org/10.1089/tmj.2010.0065.
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.699579.
- Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. Eur J Cardiovasc Prev Rehabil 2009;16:281-9. http://dx.doi.org/10.1097/HJR.0b013e32832a4e7a.
- Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: WHO; 2011.
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987-1003. http://dx.doi.org/10.1016/S0195-668X(03)00114-3.
- Hippisley-Cox J, Vinogradova Y. Trends in Consultation Rates in General Practice 1995/6 to 2008/9: Analysis of the QResearch® Database. Leeds: NHS Information Centre for Health and Social Care; 2009.
- Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007;93:172-6. http://dx.doi.org/10.1136/hrt.2006.108167.
- Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients without Coronary or other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002;106:388-91. http://dx.doi.org/10.1161/01.CIR.0000020190.45892.75.
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Funck-Brentano C, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur Heart J 2012;33:1635-701. http://dx.doi.org/10.1093/eurheartj/ehs092.
- Prevention of Cardiovascular Disease. London: NICE; 2010.
- Prevention of Cardiovascular Disease: Guideline for Assessment and Management of Cardiovascular Risk. Geneva: WHO; 2007.
- Systematic Reviews. York: Centre for Reviews and Dissemination, University of York; 2009.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Sone H, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010;53:419-28. http://dx.doi.org/10.1007/s00125-009-1622-2.
- Dekkers JC, van Wier MF, Ariëns GA, Hendriksen IJ, Pronk NP, Smid T, et al. Comparative effectiveness of lifestyle interventions on cardiovascular risk factors among a Dutch overweight working population: a randomized controlled trial. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-49.
- Claes N, Jacobs N, Clays E, Schrooten W, De Bourdeaudhuij I. Comparing the effectiveness of two cardiovascular prevention programmes for highly educated professionals in general practice: a randomised clinical trial. BMC Cardiovasc Disord 2013;13. http://dx.doi.org/10.1186/1471-2261-13-38.
- Bove AA, Santamore WP, Homko C, Kashem A, Cross R, McConnell TR, et al. Reducing cardiovascular disease risk in medically underserved urban and rural communities. Am Heart J 2011;161:351-9. http://dx.doi.org/10.1016/j.ahj.2010.11.008.
- Nolan RP, Upshur RE, Lynn H, Crichton T, Rukholm E, Stewart DE, et al. Therapeutic benefit of preventive telehealth counseling in the Community Outreach Heart Health and Risk Reduction Trial. Am J Cardiol 2011;107:690-6. http://dx.doi.org/10.1016/j.amjcard.2010.10.050.
- Nolan RP, Liu S, Shoemaker JK, Hachinski V, Lynn H, Mikulis DJ, et al. Therapeutic benefit of internet-based lifestyle counselling for hypertension. Can J Cardiol 2012;28:390-6. http://dx.doi.org/10.1016/j.cjca.2012.02.012.
- Broekhuizen K, van Poppel MN, Koppes LL, Kindt I, Brug J, van Mechelen W. No significant improvement of cardiovascular disease risk indicators by a lifestyle intervention in people with familial hypercholesterolemia compared to usual care: results of a randomised controlled trial. BMC Res Notes 2012;5. http://dx.doi.org/10.1186/1756-0500-5-181.
- Edwards L, Thomas C, Gregory A, Yardley L, O’Cathain A, Montgomery AA, et al. Are people with chronic diseases interested in using telehealth? A cross-sectional postal survey. J Med Internet Res 2014;16. http://dx.doi.org/10.2196/jmir.3257.
- Cruickshank J, Beer G, Winpenny E, Manning J. Healthcare Without Walls: A Framework for Delivering Telehealth at Scale n.d. www.2020health.org/dms/2020health/downloads/reports/2020telehealthLOW.pdf (accessed 22 December 2011).
- Donkin L, Christensen H, Naismith SL, Neal B, Hickie IB, Glozier N. A systematic review of the impact of adherence on the effectiveness of e-therapies. J Med Internet Res 2011;13. http://dx.doi.org/10.2196/jmir.1772.
- Subramanian U, Hopp F, Lowery J, Woodbridge P, Smith D. Research in home-care telemedicine: challenges in patient recruitment. Telemed J E Health 2004;10:155-61. http://dx.doi.org/10.1089/tmj.2004.10.155.
- Or CK, Karsh BT. A systematic review of patient acceptance of consumer health information technology. J Am Med Inform Assoc 2009;16:550-60. http://dx.doi.org/10.1197/jamia.M2888.
- Green BB, Anderson ML, Ralston JD, Catz S, Fishman PA, Cook AJ. Patient ability and willingness to participate in a web-based intervention to improve hypertension control. J Med Internet Res 2011;13. http://dx.doi.org/10.2196/jmir.1625.
- Hardiker NR, Grant MJ. Factors that influence public engagement with eHealth: a literature review. Int J Med Inform 2011;80:1-12. http://dx.doi.org/10.1016/j.ijmedinf.2010.10.017.
- Coulter A, Ellins J. Patient-Focused Interventions: A Review of the Evidence 2006. www.health.org.uk/sites/health/files/PatientFocusedInterventions_ReviewOfTheEvidence.pdf (accessed 12 October 2016).
- Fahey T, Montgomery AA, Barnes J, Protheroe J. Quality of care for elderly residents in nursing homes and elderly people living at home: controlled observational study. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7389.580.
- Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34. http://dx.doi.org/10.1016/S0140-6736(09)61257-5.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82. http://dx.doi.org/10.1136/bmj.39609.449676.25.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8. http://dx.doi.org/10.1016/0002-8703(91)90861-B.
- Kline P. An Easy Guide to Factor Analysis. London: Routledge; 1994.
- Office for National Statistics . 13 May Census Test 2007 (England &Amp; Wales) 2007. www.ons.gov.uk/ons/guide-method/census/2011/the-2011-census/collecting-the-information/developing-the-questionnaires/test-questionnaire-for-england-and-wales.pdf (accessed 22 December 2011).
- The GP Patient Survey – Y4Q1 April–June. London: Department of Health; 2009.
- Office for National Statistics . 29 April Census 2001 (England Household Form H1) 2001. www.ons.gov.uk/ons/guide-method/census/census-2001/about-census-2001/census-2001-forms/england-household-form-h1.pdf (accessed 17 October 2013).
- Health and Social Care Information Centre . The Health Survey for England 2007 – Household Questionnaire 2007. esds.ac.uk/doc/6112/mrdoc/pdf/6112interviewingdocs.pdf (accessed 22 December 2011).
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Gulliford M, Figueroa-Munoz J, Morgan M, Hughes D, Gibson B, Beech R, et al. What does ‘access to health care’ mean?. J Health Serv Res Policy 2002;7:186-8. http://dx.doi.org/10.1258/135581902760082517.
- Rosen R, Florin D, Dixon J. Access to Health Care – Taking Forward the Findings from the Scoping Exercise. Report of a Rapid Appraisal of Stakeholder Views and Review of Existing Literature. London: National Co-ordinating Centre for NHS Service Delivery and Organisation; 2001.
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179-211. http://dx.doi.org/10.1016/0749-5978(91)90020-T.
- Gately C, Rogers A, Kirk S, McNally R. Integration of devices into long-term condition management: a synthesis of qualitative studies. Chronic Illn 2008;4:135-48. http://dx.doi.org/10.1177/1742395308092484.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. http://dx.doi.org/10.1016/S0895-4356(98)00109-7.
- Wierzbicki M, Pekarik G. A meta-analysis of psychotherapy dropout. Prof Psychol Res Pract 1993;24:190-5. http://dx.doi.org/10.1037/0735-7028.24.2.190.
- Busselle R, Reagan J, Pinkleton B, Jackson K. Factors affecting internet use in a saturated-access population. Telem Inform 1999;16:45-58. http://dx.doi.org/10.1016/S0736-5853(99)00018-0.
- Mead N, Varnam R, Rogers A, Roland M. What predicts patients’ interest in the internet as a health resource in primary care in England?. J Health Serv Res Policy 2003;8:33-9. http://dx.doi.org/10.1258/13558190360468209.
- Kim J, Park H. Development of a health information technology acceptance model using consumers’ health behavior intention. J Med Internet Res 2012;14. http://dx.doi.org/10.2196/jmir.2143.
- Lai TY, Larson EL, Rockoff ML, Bakken S. User acceptance of HIV TIDES – Tailored Interventions for Management of Depressive Symptoms in persons living with HIV/AIDS. J Am Med Inform Assoc 2008;15:217-26. http://dx.doi.org/10.1197/jamia.M2481.
- Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot 1996;11:87-98. http://dx.doi.org/10.4278/0890-1171-11.2.87.
- Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res 2010;12. http://dx.doi.org/10.2196/jmir.1376.
- Gorst S, Armitage C, Coates L. Telehealth Acceptance Measure: To Assess Patient Motivation in the Use of Telehealth 2014. http://malt.group.shef.ac.uk/assets/files/MALT TAM Final.pdf (accessed 10 April 2015).
- Gorst S. User Experience: Findings from the Patient Telehealth Survey 2014. www.malt.group.shef.ac.uk/assets/files/Sarah%20Gorst 30.09.14.pdf (accessed 10 April 2015).
- Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol 2007;17:643-53. http://dx.doi.org/10.1016/j.annepidem.2007.03.013.
- Magnezi R, Bergman Y, Grosberg D. Online activity and participation in treatment affects the perceived efficacy of social health networks among patients with chronic illness. J Med Internet Res 2014;16. http://dx.doi.org/10.2196/jmir.2630.
- Williams TL, May CR, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E Health 2001;7:293-316. http://dx.doi.org/10.1089/15305620152814700.
- Segar J, Rogers A, Salisbury C, Thomas C. Roles and identities in transition: boundaries of work and inter-professional relationships at the interface between telehealth and primary care. Health Soc Care Community 2013;21:606-13. http://dx.doi.org/10.1111/hsc.12047.
- Finch TL, Mort M, Mair FS, May CR. Future patients? Telehealthcare, roles and responsibilities. Health Soc Care Community 2008;16:86-95. http://dx.doi.org/10.1111/j.1365-2524.2007.00726.x.
- May CR, Finch TL, Cornford J, Exley C, Gately C, Kirk S, et al. Integrating telecare for chronic disease management in the community: what needs to be done?. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-131.
- Mort M, Roberts C, Callén B. Ageing with telecare: care or coercion in austerity?. Sociol Health Illn 2013;35:799-812. http://dx.doi.org/10.1111/j.1467-9566.2012.01530.x.
- O’Cathain A, Goode J, Luff D, Strangleman T, Hanlon G, Greatbatch D. Does NHS Direct empower patients?. Soc Sci Med 2005;61:1761-71. http://dx.doi.org/10.1016/j.socscimed.2005.03.028.
- Pols J, Willems D. Innovation and evaluation: taming and unleashing telecare technology. Sociol Health Illn 2011;33:484-98. http://dx.doi.org/10.1111/j.1467-9566.2010.01293.x.
- Milligan C, Roberts C, Mort M. Telecare and older people: who cares where?. Soc Sci Med 2011;72:347-54. http://dx.doi.org/10.1016/j.socscimed.2010.08.014.
- Charles-Jones H, Latimer J, May C. Transforming general practice: the redistribution of medical work in primary care. Sociol Health Illn 2003;25:71-92. http://dx.doi.org/10.1111/1467-9566.t01-1-00325.
- Grant S, Huby G, Watkins F, Checkland K, McDonald R, Davies H, et al. The impact of pay-for-performance on professional boundaries in UK general practice: an ethnographic study. Sociol Health Illn 2009;31:229-45. http://dx.doi.org/10.1111/j.1467-9566.2008.01129.x.
- Rogers A, Kirk S, Gately C, May CR, Finch T. Established users and the making of telecare work in long term condition management: implications for health policy. Soc Sci Med 2011;72:1077-84. http://dx.doi.org/10.1016/j.socscimed.2011.01.031.
- Hudson J. Digitising the structures of government: the UK’s information age government agenda. Policy Polit 2002;30:515-31. http://dx.doi.org/10.1332/030557302760590431.
- Oudshoorn N. Diagnosis at a distance: the invisible work of patients and healthcare professionals in cardiac telemonitoring technology. Sociol Health Illn 2008;30:272-88. http://dx.doi.org/10.1111/j.1467-9566.2007.01032.x.
- Atkin K, Lunt N. Negotiating the role of the practice nurse in general practice. J Adv Nurs 1996;24:498-505. http://dx.doi.org/10.1046/j.1365-2648.1996.02179.x.
- Harrison S, Dowswell G, Wright J. Practice nurses and clinical guidelines in a changing primary care context: an empirical study. J Adv Nurs 2002;39:299-307. http://dx.doi.org/10.1046/j.1365-2648.2002.02277.x.
- McDonald R, Campbell S, Lester H. Practice nurses and the effects of the new general practitioner contract in the English National Health Service: the extension of a professional project?. Soc Sci Med 2009;68:1206-12. http://dx.doi.org/10.1016/j.socscimed.2009.01.039.
- Rashid C. Benefits and limitations of nurses taking on aspects of the clinical role of doctors in primary care: integrative literature review. J Adv Nurs 2010;66:1658-70. http://dx.doi.org/10.1111/j.1365-2648.2010.05327.x.
- Goodman C, Drennan V, Davies S, Masey H, Gage H, Scott C, et al. Nurses As Case Managers in Primary Care: The Contribution to Chronic Disease Management 2010. http://eprints.kingston.ac.uk/12331/1/Goodman_Drennan_et_al_122-final-report.pdf (accessed 01 January 2017).
- Macdonald W, Rogers A, Blakeman T, Bower P. Practice nurses and the facilitation of self-management in primary care. J Adv Nurs 2008;62:191-9. http://dx.doi.org/10.1111/j.1365-2648.2007.04585.x.
- Rogers A, Bury M, Kennedy A. Rationality, rhetoric, and religiosity in health care: the case of England’s Expert Patients Programme. Int J Health Serv 2009;39:725-47. http://dx.doi.org/10.2190/HS.39.4.h.
- Pilgrim D, Tomasini F, Vassilev I. Examining Trust in Healthcare: A Multidisciplinary Perspective. London: Palgrave Macmillan; 2011.
- Rollnick S, Miller WR. What is motivational interviewing?. Behav Cogn Psychother 1995;23:325-34. http://dx.doi.org/10.1017/S135246580001643X.
- Corbin J, Strauss A. Managing chronic illness at home: three lines of work. Qual Sociol 1985;8:224-47. http://dx.doi.org/10.1007/BF00989485.
- Grime J, Pollock K. Patients’ ambivalence about taking antidepressants: a qualitative study. Pharm J 2003;270:516-19.
- Khan N, Bower P, Rogers A. Guided self-help in primary care mental health: meta-synthesis of qualitative studies of patient experience. Br J Psychiatry 2007;191:206-11. http://dx.doi.org/10.1192/bjp.bp.106.032011.
- Davison C, Smith GD, Frankel S. Lay epidemiology and the prevention paradox: the implications of coronary candidacy for health education. Sociol Health Illn 1991;13:1-19. http://dx.doi.org/10.1111/1467-9566.ep11340301.
- Hunt K, Emslie C. Commentary: the prevention paradox in lay epidemiology – Rose revisited. Int J Epidemiol 2001;30:442-6. http://dx.doi.org/10.1093/ije/30.3.442.
- May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2803.
- Kennedy A, Robinson A, Rogers A. Incorporating patients’ views and experiences of life with IBS in the development of an evidence based self-help guidebook. Patient Educ Couns 2003;50:303-10. http://dx.doi.org/10.1016/S0738-3991(03)00054-5.
- Pollock K, Grime J. Patients’ perceptions of entitlement to time in general practice consultations for depression: qualitative study. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7366.687.
- Office for National Statistics . Internet Access 2010 – Households and Individuals 2010. www.ons.gov.uk/ons/rel/rdit2/internet-access---households-and-individuals/2010/index.html (accessed 22 December 2011).
- Salisbury C, Thomas C, O’Cathain A, Rogers A, Pope C, Yardley L, et al. TElehealth in CHronic disease: mixed-methods study to develop the TECH conceptual model for intervention design and evaluation. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2014-006448.
- Black AD, Car J, Pagliari C, Anandan C, Cresswell K, Bokun T, et al. The impact of eHealth on the quality and safety of health care: a systematic overview. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1000387.
- Car J, Huckvale K, Hermens H. Telehealth for long term conditions. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e4201.
- Pope C, Rowsell A, O’Cathain A, Brownsell S. For Want of Evidence: A Meta-Review of Home-Based Telehealth for the Management of Long-Term Conditions 2011. www.bristol.ac.uk/healthlines/documents/popeetal.pdf (accessed 25 March 2015).
- Lilford RJ, Foster J, Pringle M. Evaluating eHealth: how to make evaluation more methodologically robust. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000186.
- Kaplan B. Evaluating informatics applications – some alternative approaches: theory, social interactionism, and call for methodological pluralism. Int J Med Inform 2001;64:39-56. http://dx.doi.org/10.1016/S1386-5056(01)00184-8.
- Mackert M. Expanding the theoretical foundations of telemedicine. J Telemed Telecare 2006;12:49-50. http://dx.doi.org/10.1258/135763306775321425.
- Gammon D, Johannessen LK, Sørensen T, Wynn R, Whitten P. An overview and analysis of theories employed in telemedicine studies. A field in search of an identity. Methods Inf Med 2008;47:260-9.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Ritterband LM, Thorndike FP, Cox DJ, Kovatchev BP, Gonder-Frederick LA. A behavior change model for internet interventions. Behav Med 2009;38:18-27. http://dx.doi.org/10.1007/s12160-009-9133-4.
- Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q 1989;13:319-40. http://dx.doi.org/10.2307/249008.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist Synthesis: An Introduction 2004. www.ccsr.ac.uk/methods/publications/RMPmethods2.pdf (accessed 07 January 2011).
- Green L, Kreuter M. Health Program Planning: an Educational and Ecological Approach. New York, NY: McGrawhill; 2005.
- Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q 1996;4:12-25.
- Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag 2003;6:63-71. http://dx.doi.org/10.1089/109350703321908441.
- Ham C. The ten characteristics of the high-performing chronic care system. Health Econ Policy Law 2010;52:71-90. http://dx.doi.org/10.1017/S1744133109990120.
- Depression in Adults: The Treatment and Management of Depression in Adults. London: NICE; 2009.
- Practice Guideline for the Treatment of Patients with Major Depressive Disorder. Arlington, VA: American Psychiatric Association; 2010.
- Hypertension: Clinical Management of Primary Hypertension in Adults. London: NICE; 2011.
- Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: a guideline from the American Heart Association. Circulation 2011;123:1243-62. http://dx.doi.org/10.1161/CIR.0b013e31820faaf8.
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur Heart J 2007;28:2375-414. http://dx.doi.org/10.1093/eurheartj/ehm316.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-119.
- Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611-20. http://dx.doi.org/10.1056/NEJMoa1003955.
- Roumie CL, Elasy TA, Greevy R, Griffin MR, Liu X, Stone WJ, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006;145:165-75. http://dx.doi.org/10.7326/0003-4819-145-3-200608010-00004.
- Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. London: NICE; 2011.
- Lorig KR, Ritter PL, Laurent DD, Plant K. Internet-based chronic disease self-management: a randomized trial. Med Care 2006;44:964-71. http://dx.doi.org/10.1097/01.mlr.0000233678.80203.c1.
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1-7. http://dx.doi.org/10.1207/S15324796ABM2601_01.
- Solomon M, Wagner SL, Goes J. Effects of a web-based intervention for adults with chronic conditions on patient activation: online randomized controlled trial. J Med Internet Res 2012;14. http://dx.doi.org/10.2196/jmir.1924.
- Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009;151:687-95. http://dx.doi.org/10.7326/0000605-200911170-00148.
- Shea S, Weinstock RS, Starren J, Teresi J, Palmas W, Field L, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 2006;13:40-51. http://dx.doi.org/10.1197/jamia.M1917.
- Catwell L, Sheikh A. Evaluating eHealth interventions: the need for continuous systemic evaluation. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000126.
- Bodenheimer T. The future of primary care: transforming practice. N Engl J Med 2008;359:2086-9. http://dx.doi.org/10.1056/NEJMp0805631.
- McLean S, Protti D, Sheikh A. Telehealthcare for long term conditions. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d120.
- Thomas CL, Man MS, O’Cathain A, Hollinghurst S, Large S, Edwards L, et al. Effectiveness and cost-effectiveness of a telehealth intervention to support the management of long-term conditions: study protocol for two linked randomized controlled trials. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-36.
- Pingree S, Hawkins R, Baker T, duBenske L, Roberts LJ, Gustafson DH. The value of theory for enhancing and understanding e-health interventions. Am J Prev Med 2010;38:103-9. http://dx.doi.org/10.1016/j.amepre.2009.09.035.
- Baker LC, Macaulay DS, Sorg RA, Diener MD, Johnson SJ, Birnbaum HG. Effects of care management and telehealth: a longitudinal analysis using medicare data. J Am Geriatr Soc 2013;61:1560-7. http://dx.doi.org/10.1111/jgs.12407.
- Kidholm K, Ekeland AG, Jensen LK, Rasmussen J, Pedersen CD, Bowes A, et al. A model for assessment of telemedicine applications: mast. Int J Technol Assess Health Care 2012;28:44-51. http://dx.doi.org/10.1017/S0266462311000638.
- Gustafson D, Wise M, Bhattacharya A, Pulvermacher A, Shanovich K, Phillips B, et al. The effects of combining web-based eHealth with telephone nurse case management for pediatric asthma control: a randomized controlled trial. J Med Internet Res 2012;14. http://dx.doi.org/10.2196/jmir.1964.
- Greenhalgh T, Russell J, Ashcroft RE, Parsons W. Why national eHealth programs need dead philosophers: Wittgensteinian reflections on policymakers’ reluctance to learn from history. Milbank Q 2011;89:533-63. http://dx.doi.org/10.1111/j.1468-0009.2011.00642.x.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Pittaway S, Cupitt C, Palmer D, Arowobusoye N, Milne R, Holttum S, et al. Comparative, clinical feasibility study of three tools for delivery of cognitive behavioural therapy for mild to moderate depression and anxiety provided on a self-help basis. Ment Health Fam Med 2009;6:145-54.
- Williams C, Wilson P, Morrison J, McMahon A, Walker A, Andrew W, et al. Guided self-help cognitive behavioural therapy for depression in primary care: a randomised controlled trial. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0052735.
- Williams C. Overcoming Depression and Low Mood: A Five Areas Approach. London: Hodder Arnold; 2009.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- Bosworth HB, Olsen MK, McCant F, Harrelson M, Gentry P, Rose C, et al. Hypertension Intervention Nurse Telemedicine Study (HINTS): testing a multifactorial tailored behavioral/educational and a medication management intervention for blood pressure control. Am Heart J 2007;153:918-24. http://dx.doi.org/10.1016/j.ahj.2007.03.004.
- Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med 2011;171:1173-80. http://dx.doi.org/10.1001/archinternmed.2011.276.
- Prochaska J, DiClemente C. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5. http://dx.doi.org/10.1037/0022-006X.51.3.390.
- O’Brien E, Atkins N, Stergiou G, Karpettas N, Parati G, Asmar R, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit 2010;15:23-38. http://dx.doi.org/10.1097/MBP.0b013e3283360e98.
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 1992;22:465-86. http://dx.doi.org/10.1017/S0033291700030415.
- Marshall T. Identification of patients for clinical risk assessment by prediction of cardiovascular risk using default risk factor values. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-25.
- University of Birmingham . The Risk and Benefit Calculator n.d. www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/cardiovascular/calculator/index.aspx (accessed 2 April 2012).
- Collins GS, Altman DG. An independent external validation and evaluation of QRISK cardiovascular risk prediction: a prospective open cohort study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2584.
- Evans M, Kessler D, Lewis G, Peters TJ, Sharp D. Assessing mental health in primary care research using standardized scales: can it be carried out over the telephone?. Psychol Med 2004;34:157-62. http://dx.doi.org/10.1017/S0033291703008055.
- University of Manchester . Systematic Techniques for Assisting Recruitment to Trials (MRC START) n.d. www.medicine.manchester.ac.uk/mrcstart/ (accessed 5 July 2012).
- Knapp P, Raynor DK, Silcock J, Parkinson B. Can user testing of a clinical trial patient information sheet make it fit-for-purpose? A randomized controlled trial. BMC Med 2011;9. http://dx.doi.org/10.1186/1741-7015-9-89.
- Man M-S, Rick J, Bower P. behalf of the MRC-START Group . Improving recruitment to a study of telehealth management for long-term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0820-0.
- Edwards P, Roberts I, Clarke M, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;8. http://dx.doi.org/10.1002/14651858.mr000008.pub4.
- Edwards L, Salisbury C, Horspool K, Foster A, Garner K, Montgomery AA. Increasing follow-up questionnaire response rates in a randomized controlled trial of telehealth for depression: three embedded controlled studies. Trials 2016;17. http://dx.doi.org/10.1186/s13063-016-1234-3.
- McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord 2010;127:122-9. http://dx.doi.org/10.1016/j.jad.2010.04.030.
- EuroQol Group . EuroQol Group EQ-5D-5L n.d. www.euroqol.org/about-eq-5d/valuation-of-eq-5d/eq-5d-5l-value-sets.html (accessed 11 February 2015).
- Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84. http://dx.doi.org/10.1016/S0140-6736(12)61552-9.
- Ryan RM, Plant RW, O’Malley S. Internal motivations for alcohol treatment: relations with patient characteristics, treatment involvement, and dropout. Addict Behav 1995;20:279-97. http://dx.doi.org/10.1016/0306-4603(94)00072-7.
- Ferron JC, Elbogen EB, Swanson JW, Swartz MS, McHugo GJ. A conceptually based scale to measure consumers’ treatment motivation. Res Soc Work Pract 2011;21:98-105.
- Osborne RH, Elsworth GR, Whitfield K. The Health Education Impact Questionnaire (heiQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns 2007;66:192-201. http://dx.doi.org/10.1016/j.pec.2006.12.002.
- Paxton AE, Strycker LA, Toobert DJ, Ammerman AS, Glasgow RE. Starting the conversation performance of a brief dietary assessment and intervention tool for health professionals. Am J Prev Med 2011;40:67-71. http://dx.doi.org/10.1016/j.amepre.2010.10.009.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. http://dx.doi.org/10.1097/00005650-198601000-00007.
- Norman CD, Skinner HA. eHEALS: the eHealth Literacy Scale. J Med Internet Res 2006;8. http://dx.doi.org/10.2196/jmir.8.4.e27.
- Haggerty JL, Roberge D, Freeman GK, Beaulieu C, Bréton M. Validation of a generic measure of continuity of care: when patients encounter several clinicians. Ann Fam Med 2012;10:443-51. http://dx.doi.org/10.1370/afm.1378.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20. http://dx.doi.org/10.1016/0149-7189(79)90094-6.
- National Research Ethics Service . Standard Operating Procedures for Research Ethics Committees v4.1 2010. www.nres.npsa.nhs.uk/news-and-publications/publications/standard-operating-procedures/ (accessed 1 November 2010).
- Richards DA, Lovell K, Gilbody S, Gask L, Torgerson D, Barkham M, et al. Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med 2008;38:279-87. http://dx.doi.org/10.1017/S0033291707001365.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000;320:686-90. http://dx.doi.org/10.1136/bmj.320.7236.686.
- Schulz K, Altman D, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med 2010;152:726-32. http://dx.doi.org/10.7326/0003-4819-152-11-201006010-00232.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley; 1987.
- Frangakis CE, Rubin DB. Principal stratification in causal inference. Biometrics 2002;58:21-9. http://dx.doi.org/10.1111/j.0006-341X.2002.00021.x.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- EuroQol Group . Interim Scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L Value Sets n.d. www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Crosswalk_5L/EQ-5D-5L_Crosswalk_model_and__methodology.pdf (accessed 11 February 2015).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- NHS Health Education England . Agenda for Change – Pay Rates 2015. www.nhscareers.nhs.uk/working-in-the-nhs/pay-and-benefits/agenda-for-change-pay-rates/ (accessed 20 February 2015).
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Reference Costs 2012–13. London: Department of Health; 2013.
- Office for National Statistics . Annual Survey of Hours and Earnings, 2013 Provisional Results 2013. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition = tcm%3A77–328216 (accessed 13 February 2015).
- Stevenson LJ, Coody DK, Evans KD, Plumb SC, Montgomery DF, Yetman RJ. Providing better access to health care: a pediatric nurse practitioner WIC-based clinic for one-stop health care. J Pediatr Health Care 1994;8:168-72. http://dx.doi.org/10.1016/0891-5245(94)90029-9.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12210.
- Ara R, Pandor A, Stevens J, Rees A, Rafia R. Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13340.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11140.
- Gray A, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2010.
- Mistry H, Morris S, Dyer M, Kotseva K, Wood D, Buxton M. Cost-effectiveness of a European preventive cardiology programme in primary care: a Markov modelling approach. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-001029.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics 2012 Edition. London: British Heart Foundation; 2012.
- Sutcliffe SJ, Fox KF, Wood DA, Sutcliffe A, Stock K, Wright M, et al. Incidence of coronary heart disease in a health authority in London: review of a community register. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7379.20.
- Wang Y, Rudd AG, Wolfe CD. Age and ethnic disparities in incidence of stroke over time: the South London Stroke Register. Stroke 2013;44:3298-304. http://dx.doi.org/10.1161/STROKEAHA.113.002604.
- Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011;1. http://dx.doi.org/10.1136/bmjopen-2011-000269.
- Scarborough P, Peto V, Bhatnagar P, Kaur A, Leal J, Luengo-Fernandez R, et al. Stroke Statistics 2009 Edition. London: British Heart Foundation; 2009.
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773-83. http://dx.doi.org/10.1016/S0140-6736(05)67702-1.
- Ara R, Wailoo A. Using health state utility values in models exploring the cost-effectiveness of health technologies. Value Health 2012;15:971-4. http://dx.doi.org/10.1016/j.jval.2012.05.003.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology 2013;81:1588-95. http://dx.doi.org/10.1212/WNL.0b013e3182a9f45f.
- Office for National Statistics . Interim Life Tables, England and Wales, 2010–12 2013. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition = tcm%3A77–325699 (accessed 13 February 2015).
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Complex Interventions in Health: An Overview of Research Methods. Abingdon-on-Thames: Routledge; 2016.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Ritchie J, Spencer L, Huberman A, Miles M. The Qualitative Researcher’s Companion. Thousand Oaks, CA: Sage Publications; 2002.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002889.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. http://dx.doi.org/10.1136/bmj.332.7538.413.
- Salisbury C, O’Cathain A, Edwards L, Thomas C, Gaunt D, Hollinghurst S, et al. Effectiveness of an integrated telehealth service for patients with depression: a pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry 2016;3:515-25. http://dx.doi.org/10.1016/S2215-0366(16)00083-3.
- Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev 2006;63:158-88. http://dx.doi.org/10.1177/1077558705285294.
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE-OM. Br J Gen Pract 2007;57:650-2.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737-44. http://dx.doi.org/10.1001/jama.282.18.1737.
- Titov N, Dear BF, McMillan D, Anderson T, Zou J, Sunderland M. Psychometric comparison of the PHQ-9 and BDI-II for measuring response during treatment of depression. Cogn Behav Ther 2011;40:126-36. http://dx.doi.org/10.1080/16506073.2010.550059.
- Fortney JC, Pyne JM, Edlund MJ, Williams DK, Robinson DE, Mittal D, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med 2007;22:1086-93. http://dx.doi.org/10.1007/s11606-007-0201-9.
- Fortney JC, Pyne JM, Steven CA, Williams JS, Hedrick RG, Lunsford AK, et al. A web-based clinical decision support system for depression care management. Am J Manag Care 2010;16:849-54.
- Gilbody S, Littlewood E, Hewitt C, Brierley G, Tharmanathan P, Araya R, et al. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ 2015;351. http://dx.doi.org/10.1136/bmj.h5627.
- van Ballegooijen W, Cuijpers P, van Straten A, Karyotaki E, Andersson G, Smit JH, et al. Adherence to Internet-based and face-to-face cognitive behavioural therapy for depression: a meta-analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0100674.
- Gerhards SAH, Abma TA, Arntz A, de Graaf LE, Evers SMAA, Huibers MJH, et al. Improving adherence and effectiveness of computerised cognitive behavioural therapy without support for depression: a qualitative study on patient experiences. J Affect Disord 2011;129:117-25. http://dx.doi.org/10.1016/j.jad.2010.09.012.
- Berger T, Hämmerli K, Gubser N, Andersson G, Caspar F. Internet-based treatment of depression: a randomized controlled trial comparing guided with unguided self-help. Cogn Behav Ther 2011;40:251-66. http://dx.doi.org/10.1080/16506073.2011.616531.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. http://dx.doi.org/10.1001/archinte.166.21.2314.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.cd006525.pub2.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4913.
- Salisbury C, O’Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. BMJ 2016;353. http://dx.doi.org/10.1136/bmj.i2647.
- McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. J Clin Epidemiol 2014;67:247-53. http://dx.doi.org/10.1016/j.jclinepi.2013.09.004.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338.
- Vegting IL, Schrijver EJ, Otten RH, Nanayakkara PW. Internet programs targeting multiple lifestyle interventions in primary and secondary care are not superior to usual care alone in improving cardiovascular risk profile: a systematic review. Eur J Intern Med 2014;25:73-81. http://dx.doi.org/10.1016/j.ejim.2013.08.008.
- Vernooij JW, Kaasjager HA, van der Graaf Y, Wierdsma J, Grandjean HM, Hovens MM, et al. Internet based vascular risk factor management for patients with clinically manifest vascular disease: randomised controlled trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3750.
- Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med 2013;159:185-94. http://dx.doi.org/10.7326/0003-4819-159-3-201308060-00008.
- Purcell R, McInnes S, Halcomb EJ. Telemonitoring can assist in managing cardiovascular disease in primary care: a systematic review of systematic reviews. BMC Fam Pract 2014;15. http://dx.doi.org/10.1186/1471-2296-15-43.
- Kerry SM, Markus HS, Khong TK, Cloud GC, Tulloch J, Coster D, et al. Home blood pressure monitoring with nurse-led telephone support among patients with hypertension and a history of stroke: a community-based randomized controlled trial. CMAJ 2013;185:23-31. http://dx.doi.org/10.1503/cmaj.120832.
- McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014;312:799-808. http://dx.doi.org/10.1001/jama.2014.10057.
- Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310:46-5. http://dx.doi.org/10.1001/jama.2013.6549.
- McKinstry B, Hanley J, Wild S, Pagliari C, Paterson M, Lewis S, et al. Telemonitoring based service redesign for the management of uncontrolled hypertension: multicentre randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f3030.
- Madsen LB, Kirkegaard P, Pedersen EB, Madsen LB, Kirkegaard P, Pedersen EB. Health-related quality of life (SF-36) during telemonitoring of home blood pressure in hypertensive patients: a randomized, controlled study. Blood Press 2008;17:227-32. http://dx.doi.org/10.1080/08037050802433701.
- Parati G, Omboni S, Albini F, Piantoni L, Giuliano A, Revera M, et al. Home blood pressure telemonitoring improves hypertension control in general practice. The TeleBPCare study. J Hypertens 2009;27:198-203. http://dx.doi.org/10.1097/HJH.0b013e3283163caf.
- Civljak M, Stead LF, Hartmann-Boyce J, Sheikh A, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev 2013;10. http://dx.doi.org/10.1002/14651858.cd007078.pub4.
- Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013;8. http://dx.doi.org/10.1002/14651858.cd002850.pub3.
- Dixon P, Hollinghurst S, Edwards L, Thomas C, Foster A, Davies B, et al. Cost-effectiveness of telehealth for patients with depression: evidence from the Healthlines randomised controlled trial. Br J Psychiatry Open 2016;2:262-9. http://dx.doi.org/10.1192/bjpo.bp.116.002907.
- Dixon P, Hollinghurst S, Edwards L, Thomas C, Gaunt D, Foster A, et al. Cost-effectiveness of telehealth for patients with raised cardiovascular disease risk: evidence from the Healthlines randomised controlled trial. BMJ Open 2016;6. http://dx.doi.org/10.1136/bmjopen-2016-012352.
- Dixon P, Hollinghurst S, Ara R, Edwards L, Foster A, Salisbury C. Cost-effectiveness modelling of telehealth for patients with raised cardiovascular disease risk: evidence from a cohort simulation conducted alongside the Healthlines randomised controlled trial. BMJ Open 2016;6. http://dx.doi.org/10.1136/bmjopen-2016-012355.
- Brazier J, Connell J, Papaioannou D, Mukuria C, Mulhern B, Peasgood T, et al. A systematic review, psychometric analysis and qualitative assessment of generic preference-based measures of health in mental health populations and the estimation of mapping functions from widely used specific measures. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18340.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Costs of stroke using patient-level data: a critical review of the literature. Stroke 2009;40:e18-23. http://dx.doi.org/10.1161/STROKEAHA.108.529776.
- O’Cathain A, Drabble SJ, Foster A, Horspool K, Edwards L, Thomas C, et al. Being human: a qualitative interview study exploring why a telehealth intervention for management of chronic conditions had a modest effect. J Med Internet Res 2016;18. http://dx.doi.org/10.2196/jmir.5879.
- Department for Communities and Local Government . Estimates of Index of Multiple Deprivation (IMD) 2010 for GP Practices 2010. https://indicators.ic.nhs.uk/webview/index/en/MyServer/NHS-Information-Centre-indicators.c.MyServer/GP-Practice-data.d.48/Demography.d.77/Deprivation.d.202/Estimates-of-Index-of-Multiple-Deprivation-IMD-2010-for-GP-practices/fStudy/P01112 (accessed 28 November 2012).
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol 1992;47:1102-14. http://dx.doi.org/10.1037/0003-066X.47.9.1102.
- Foster A, Horspool KA, Edwards L, Thomas CL, Salisbury C, Montgomery AA, et al. Who does not participate in telehealth trials and why? A cross-sectional survey. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0773-3.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17. http://dx.doi.org/10.2196/jmir.4055.
- Gambling T, Long AF. The realisation of patient-centred care during a 3-year proactive telephone counselling self-care intervention for diabetes. Patient Educ Couns 2010;80:219-26. http://dx.doi.org/10.1016/j.pec.2009.11.007.
- Sanders C, Rogers A, Bowen R, Bower P, Hirani S, Cartwright M, et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-220.
- Gorst S, Armitage C, Coates L. MALT – Mainstreaming Assisted Living Technology n.d. http://malt.group.shef.ac.uk/patient-acceptance/patient-acceptance/ (accessed 07 April 2015).
- Salisbury C, Fahey T. Overcoming clinical inertia in the management of hypertension. CMAJ 2006;174:1285-6. http://dx.doi.org/10.1503/cmaj.060243.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009;180:E47-57. http://dx.doi.org/10.1503/cmaj.090523.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h2147.
- Bower P, Cartwright M, Hirani S, Barlow J, Hendy J, Knapp M, et al. A comprehensive evaluation of the impact of telemonitoring in patients with long-term conditions and social care needs: protocol for the whole systems demonstrator cluster randomised trial. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-184.
- Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S. Systematic review of cost effectiveness studies of telemedicine interventions. BMJ 2002;324:1434-7. http://dx.doi.org/10.1136/bmj.324.7351.1434.
- Prestwich A, Sniehotta FF, Whittington C, Dombrowski SU, Rogers L, Michie S. Does theory influence the effectiveness of health behavior interventions? Meta-analysis. Health Psychol 2014;33:465-74. http://dx.doi.org/10.1037/a0032853.
- De Silva MJ, Breuer E, Lee L, Asher L, Chowdhary N, Lund C, et al. Theory of change: a theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-267.
- NHS . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 11 October 2016).
- NHS England . Real World Testing of ‘Combinatorial Innovation’: A Global Invitation to Innovators 2015. www.england.nhs.uk/wp-content/uploads/2015/03/test-bed-prospectus.pdf (accessed 30 December 2015).
- Littlewood E, Duarte A, Hewitt C, Knowles S, Palmer S, Walker S, et al. A randomised controlled trial of computerised cognitive behaviour therapy for the treatment of depression in primary care: the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta191010.
- Wood DA, Kinmonth AL, Davies GA, Yarwood J, Thompson SG, Pyke SDM, et al. Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of British family heart study. Family Heart Study Group. BMJ 1994;308:313-20. http://dx.doi.org/10.1136/bmj.308.6924.313.
- Imperial Cancer Research Fund Oxcheck Study Group . Effectiveness of health checks conducted by nurses in primary care: final results of the OXCHECK study. Imperial Cancer Research Fund OXCHECK Study Group. BMJ 1995;310:1099-104. http://dx.doi.org/10.1136/bmj.310.6987.1099.
- Perla R, Reid A, Cohen S, Parry G. Health Care Reform and the Trap of the ‘Iron Law’ 2015. http://healthaffairs.org/blog/2015/04/22/health-care-reform-and-the-trap-of-the-iron-law/ (accessed 28 April 2015).
- Stellefson M, Chaney B, Barry AE, Chavarria E, Tennant B, Walsh-Childers K, et al. Web 2.0 chronic disease self-management for older adults: a systematic review. J Med Internet Res 2013;15. http://dx.doi.org/10.2196/jmir.2439.
- Andrews G, Cuijpers P, Craske MG, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0013196.
- Mistry H. Systematic review of studies of the cost-effectiveness of telemedicine and telecare. Changes in the economic evidence over twenty years. J Telemed Telecare 2012;18:1-6. http://dx.doi.org/10.1258/jtt.2011.110505.
- Cruickshank J. Telehealth: What Can the NHS Learn from Experience at the US Veterans Health Administration? 2012. www.2020health.org/dms/2020health/downloads/reports/Telehealth-VA/Telehealth%20VA.pdf (accessed 22 December 2011).
- Smith D, McMurray N, Disler P. Early intervention for acute back injury: can we finally develop an evidence-based approach?. Clin Rehabil 2002;16:1-11. http://dx.doi.org/10.1191/0269215502cr461oa.
- Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long- term conditions in primary care: trial protocol. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-7.
- Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49-55. http://dx.doi.org/10.1016/S0140-6736(11)60701-0.
- Bennett JB, Broome KM, Schwab-Pilley A, Gilmore P. A web-based approach to address cardiovascular risks in managers: results of a randomized trial. J Occup Environ Med 2011;53:911-18. http://dx.doi.org/10.1097/JOM.0b013e3182258bd8.
- Cook RF, Billings DW, Hersch RK, Back AS, Hendrickson A. A field test of a web-based workplace health promotion program to improve dietary practices, reduce stress, and increase physical activity: randomized controlled trial. J Med Internet Res 2007;9. http://dx.doi.org/10.2196/jmir.9.2.e17.
- Ruffin MT, Nease DE, Sen A, Pace WD, Wang C, Acheson LS, et al. Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Ann Fam Med 2011;9:3-11. http://dx.doi.org/10.1370/afm.1197.
- Verheijden M, Bakx JC, Akkermans R, van den Hoogen H, Godwin NM, Rosser W, et al. Web-based targeted nutrition counselling and social support for patients at increased cardiovascular risk in general practice: randomized controlled trial. J Med Internet Res 2004;6. http://dx.doi.org/10.2196/jmir.6.4.e44.
- Wakefield BJ, Holman JE, Ray A, Scherubel M, Adams MR, Hillis SL, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health 2011;17:254-61. http://dx.doi.org/10.1089/tmj.2010.0176.
- Scott A, Simoens S, Heaney D, O’Donnell CA, Thomson H, Moffat KJ, et al. What does GP out of hours care cost? An analysis of different models of out of hours care in Scotland. Scott Med J 2004;49:61-6.
- O’Dowd A. Cost of out of hours care was 22% higher than predicted in England. BMJ 2006;332. http://dx.doi.org/10.1136/bmj.332.7550.1113-c.
- Munro J, Nicholl J, O’Cathain A, Knowles E, Morgan A. Evaluation of NHS Direct First Wave Sites: Final Report of the Phase 1 Research. Sheffield: MCRU, University of Sheffield; 2001.
- Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS study no. 65). Diabet Med 2003;20:442-50. http://dx.doi.org/10.1046/j.1464-5491.2003.00972.x.
- Luengo-Fernandez R, Silver LE, Gutnikov SA, Gray AM, Rothwell PM. Hospitalization resource use and costs before and after TIA and stroke: results from a population-based cohort study (OXVASC). Value Health 2013;16:280-7. http://dx.doi.org/10.1016/j.jval.2012.10.013.
- Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003;21:43-50. http://dx.doi.org/10.2165/00019053-200321001-00005.
- Armfield NR, Edirippulige SK, Bradford N, Smith AC. Telemedicine – is the cart being put before the horse?. Med J Aust 2014;200:530-3. http://dx.doi.org/10.5694/mja13.11101.
- Eland-de Kok P, van Os-Medendorp H, Vergouwe-Meijer A, Bruijnzeel-Koomen C, Ros W. A systematic review of the effects of e-health on chronically ill patients. J Clin Nurs 2011;20:2997-3010. http://dx.doi.org/10.1111/j.1365-2702.2011.03743.x.
- Hutchison AJ, Breckon JD. A review of telephone coaching services for people with long-term conditions. J Telemed Telecare 2011;17:451-8. http://dx.doi.org/10.1258/jtt.2011.110513.
- Wennberg DE, Marr A, Lang L, O’Malley S, Bennett G. A randomized trial of a telephone care-management strategy. N Engl J Med 2010;363:1245-55. http://dx.doi.org/10.1056/NEJMsa0902321.
Appendix 1 Table of studies included in the meta-review of long-term conditions
| Study | Design | Country | Outcomes measured | Disease and patient characteristics | Intervention type and control group details | Conclusions |
|---|---|---|---|---|---|---|
| Barlow 200720 | 98 studies (68 RCTs; 30 observational studies) | UK | Health status (blood glucose, blood pressure), quality of life, satisfaction, utilisation, other (safety and security monitoring), cost-effectiveness, education | Asthma, arthritis, CHD/CHF, chronic back pain, COPD, dementia, depression, diabetes (31%), frail elderly, hypertension, assorted diseases Adults and elderly patients |
Telecare at home including telephone support, internet and e-mail, telemonitoring of vital signs, text messaging | More evidence is needed |
| Botsis 200870 | 54 studies (47 RCTs or CCTs, 7 qualitative studies) | Europe | Health status, clinical, quality of life, satisfaction, utilisation | CHF, CHD, chronic wounds, diabetes, Alzheimer’s disease or cognitive impairment, mobility disabilities Elderly patients |
Home telecare: telehealth, video conferencing, virtual visits, telemonitoring of vital signs, teleconsultations including real-time monitoring and communication | Could not replace nurse home visits |
| Bowles 200717 | 19 studies including RCTs, CCTs, observational studies and qualitative studies | USA | Health status, quality of life, satisfaction, clinical, support, utilisation, behaviour change | COPD, cancer, anxiety, CHF, CHD, CVD, hypertension, stroke (CVA), spinal cord injury, chronic wounds, diabetes Adults and elderly patient; focus on older adults |
Telehomecare (by nurses): interactive visits, video visits, transmission of vital signs | Telehomecare is best for patients requiring close monitoring and quick interventions (i.e. HF patients) |
| Cole-Lewis 201071 | 17 papers representing 12 studies (9 RCTs, 2 cross-over studies, 1 quasi-experimental study) | USA | Health status, clinical, behaviour change | Asthma, diabetes, weight loss/prevention, medication adherence, physical activity, smoking cessation All studies included adults; four targeted adolescents or young adults and one study included 10- to 19-year-olds |
Text messaging (only component in five studies – others also used internet and e-mail) | Evidence of a short-term effect on behavioural or clinical outcomes. Text messaging useful in disease prevention and management interventions for weight loss, smoking cessation and diabetes. Effective for adolescents and adults, minority and non-minority populations and across nationalities |
| Cuijpers 200840 | 12 RCTs | Europe | Health status, quality of life | Migraine/recurrent headache, other problems, pain Children, adults and elderly |
Online CBT with a strong psychoeducational element. Control group – waiting list or self-help only | Effective for some conditions but unable to draw definite conclusions because of small numbers of studies. Very few studies compared the online intervention with face-to-face treatment. Unable to draw definite conclusions about whether or not CBT delivered through the internet was effective – more research on online CBT needed |
| Dellifraine 200816 | 29 studies (25 RCTs, 2 not randomised, 2 unable to tell) | USA | Health status, utilisation, clinical | Asthma, arthritis, anxiety, CHF, coronary artery bypass, COPD, depression, diabetes, hypertension, MS, multiple conditions, obesity, schizophrenia Children, adults and elderly |
Home telehealth: video monitoring, telephone, internet, vital signs transmission | Effective for some conditions but limited information about intervention characteristics |
| Garcia-Lizana 20078 | 24 RCTs | Europe | Health status, quality of life, satisfaction, clinical, support, utilisation, behaviour change, education | Asthma, CHF, CHD, CVD, diabetes, hypertension Children and adults |
Information and communication technologies including interactive computer games, telemonitoring of vital signs, internet and web-based interventions for disease management, telephone, videophone | Some positive utilisation and clinical outcomes. Benefits for controlling and managing chronic diseases were limited. No agreement on appropriate features of interventions. Limitations of studies – lack of high-quality evidence and data on the ability of information and communication technologies to increase knowledge and social support for people with chronic disease |
| Hersh 200672 | 106 studies including RCTs (class I), CCTs (class II), case series (class III), cohort studies (class III), case–control studies (class III). One-quarter met class I criteria and 28 studies were of home-based interventions | Canada | Health status, utilisation | Home-based interventions reported on only: asthma, CAD, CHF, chronic disease, diabetes, hypertension, lung transplant, MS, obesity, psychiatry, spinal cord injury Mainly adult; one paediatric asthma study; four chronic disease in the elderly studies and one home-based study mentioning senior agencies. Two studies included veterans |
Telemedicine: web-based monitoring, transtelephonic monitoring, telemonitoring, Health Buddy, remote monitoring, telemedicine, uploading electronic diary, video monitoring | Home-based services used to enhance the care of patients who already receive conventional services. Limitations of studies included small sample sizes and poor designs |
| Krishna 200973 | 20 RCTs, 5 CCTs | USA | Health status, quality of life, satisfaction, behaviour change, education | Asthma, diabetes, general outpatient, HIV and AIDS, health promotion, hepatitis vaccinations, hypertension, physical disabilities, smoking cessation, stress management Adults and children |
Health information delivered by mobile telephone or text messaging | 80% showed significant differences between intervention and controls |
| Murray 200511 (updated 2009) | 24 RCTs | UK | Health status, clinical, utilisation, cost-effectiveness, education, support, behaviour change, other | Asthma, diabetes, breast cancer or leukaemia, Alzheimer’s disease or memory loss, eating disorders, encopresis (faecal soiling – children), HIV and AIDS, obesity, urinary incontinence (women) Children, adolescents and adults |
Interactive Health Communication Applications (IHCAs) including computers, modems, telephone lines, internet and CD ROMs | Significant positive effects on knowledge, social support and behavioural and clinical outcomes. Not likely to have positive effects on overall self-efficacy. No evidence of effects on health service utilisation. Unable to determine effects on emotional or economic outcomes. Insufficient evidence to determine if ICHAs can benefit disadvantaged groups. Overall lack of high-quality evidence |
| Oake 200968 | 32 RCTs, 8 CCTs | Canada | Clinical, health status, quality of life, utilisation, satisfaction, behaviour change | Appointment reminders, asthma, chronic pain, cervical cancer screening, CHF, diabetes, dyslipidaemia, hypertension, immunisations, mental health, smoking cessation Adults |
Interactive voice response systems to contact patients at home with reminders or to track patient-assessed parameters at home | Caution against interpretation that technology improves outcomes as currently insufficient data |
| Paré 201069 | 62 studies (46 RCTs) | Canada | Health status, quality of life, utilisation, satisfaction | Asthma, CHF, diabetes, hypertension Age not reported |
Telemonitoring | Positive effects reported for diabetes, asthma and hypertension (associated with telemonitoring allowing for more frequent follow-up of patients). Failed to show a reduction in either mortality or hospitalisation rates for HF, although some trend towards shorter lengths of stay in hospital. Better glycaemic control and improved control of asthma and blood pressure. Larger trials are needed to confirm the benefits of this technology for these patients |
| Polisena 200974 | 22 studies (14 RCTs, 4 CCTs, 4 pre–post studies) | Canada | Cost-effectiveness (including one cost unit analysis) | Cancer, CHF, CHD, COPD, diabetes, stroke, wound care Severe and moderate; adolescents and adults (one study looked at those aged > 65 years) |
Telehealth, telemanagement, Health Buddy devices, transmission of vital signs information | Quality of the studies in terms of economic evaluation was poor. Studies heterogeneous so difficult to make an informed decision on resource allocation. Potential of telehealth to be cost saving but cannot be sure until higher-quality studies are conducted |
| Rains 200975 | 28 pre- and post-test studies | USA | Health status, quality of life, satisfaction, behaviour change | Back pain, breast cancer, chronic pain, chronic illness, CVD, depression, diabetes, eating disorders, heart transplant, HIV/AIDS, panic disorder, Parkinson’s disease, weight loss maintenance, smoking cessation 80% adult population (non-student), remainder students or adolescents (focused on eating disorders and depression) |
Computer-mediated support groups (CMSGs) | Participation in a CMSG intervention was associated with perceptions of significantly improved social support, reduced levels of depression and increased quality of life and self-efficacy. These effects were observed across a variety of different health conditions |
| Stinson 200976 | 29 studies including 5 RCTs and 1 pilot RCT | Canada | Health status, quality of life, utilisation, satisfaction, behaviour change | Asthma (including persistent asthma), encopresis, obesity, recurrent headaches, traumatic brain injury Children and adolescents only (five studies targeted youth and parents; several used youth and parent dyads) |
Internet-based self-management including web-based interactive programmes, e-mail, telephone and e-mail support | Improvements in symptoms in four of the five health conditions. Limited evidence regarding impact on health-care utilisation, knowledge and quality of life outcomes. Unable to determine effectiveness of internet interventions for self-efficacy, social support and emotional well-being. No definite conclusions about whether or not self-management interventions delivered through the internet are as effective as face-to-face therapies as most of the studies used usual care or wait-list control comparison groups. Limited data in studies in the review |
| Tran 200877 | 78 studies (diabetes, chronic disease, COPD, CHF, diabetes, CHF, COPD, chronic disease | Canada | Health status, utilisation, cost-effectiveness, quality of life, satisfaction | CHF, chronic diseases, COPD, diabetes Adults and older people (> 65 years) |
Telehealth: telephone, videophone, telemonitoring, pager, mobile technology | Home telemonitoring and telephone support effective for improving glycaemic control for patients with diabetes and for reducing mortality rates among patients with HF. Higher mortality rate among patients with COPD using home telehealth interventions, but few studies and sample sizes small. Variability in the quality of studies – poor study designs or small sample sizes. Home telehealth generally clinically effective and no patient adverse events reported. Evidence on service utilisation is more limited but shows potential. Economic review – overall quality of the original research was low |
Appendix 2 Quality assessment of qualitative papers according to the Critical Appraisal Skills Programme criteria
| Study | CASP criteriaa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | |
| Armstrong 2007107 | Y | Y | Y | Y | Y | N | Y | Y | Y | Indicates the value of asynchronous communication to various stakeholder groups |
| Armstrong 2009116 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Provides limited evidence from a small sample on patient perspectives of perceived support available through internet sites |
| Beattie 200998 | Y | Y | Y | Y | Y | N | Y | Y | Y | Provides evidence on patients’ actual experiences of online CBT. Good design (before-and-after interviews). Weighted towards women. Further work targeting men needed and, therefore, questions about transferability |
| Cornwall 2008123 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Provides nurse and patient perceptions on e-mail communication. Restricted to small sample |
| Dinesen 2006114 | Y | Y | Y | N | Y | Y | N | U | U | Limited to three patients and one staff team. It does highlight some indicators for the use of DiasNet (diabetes decision support system) and some benefits of Diasnet for monitoring and improving patients’ understanding of their condition |
| Dinesen 2008117 | Y | Y | Y | Y | Y | N | Y | Y | Y | Reveals new aspects of home hospitalisation and perceived advantages of telehomecare |
| Dinesen 2009122 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Gives an indication of nurse fears about telehomecare in particular and the need to form new working relationships for success |
| Griffiths 200697 | Y | Y | Y | NA | Y | NA | NA | Y | Y | Identifies some advantages and disadvantages of internet interventions targeting a range of health issues. Limited to internet interventions |
| Hanley 2009101 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Presents preliminary findings that it is possible to create therapeutic relationships with young people online |
| Hibbert 2004115 | Y | Y | Y | U | Y | N | Y | N | Y | Provides limited evidence of nurses’ perceptions of telehealth (small sample). Particularly focuses on problems associated with telehealth implementation |
| Hopp 2007109 | Y | Y | Y | Y | Y | N | N | Y | Y | Suggests that monitoring and messaging devices not appropriate for all patients; useful with regard to identifying barriers |
| Høybye 2005113 | Y | Y | Y | Y | Y | U | Y | Y | Y | Limited to cancer. Women’s experiences of empowerment through a single internet site only |
| Kahn 2009111 | Y | Y | U | NA | Y | Y | N | Y | Y | Limited because progress notes were taken by one nurse only. No link between notes and clinical readings for diabetes. Gives some indication of how transient patients with complex needs might benefit from telephonic nurse case manager and describes practices in telephonic case management |
| Kerr 2005120 | Y | Y | Y | Y | Y | N | Y | U | N | Gives some indication that participants see the value of interactive health communication applications, but limited support/detail of findings provided (see Kerr et al.121 for further details) |
| Kerr 2006121 | Y | Y | Y | Y | Y | N | N | Y | Y | Looks at interactive health communication applications for a range of LTCs. Generates useful information regarding important features of interactive health communication applications |
| LaFramboise 2009118 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Provides useful information regarding the acceptability, ease of use and difficulties of a single system only – Health Buddy (Health Hero Inc.) – but small sample size (n = 13). Authors note comparison with other devices may better inform health-care providers’ decision making. Authors also note time between parent trial and interviews may have impacted on participants’ ability to recall components of the experience adequately |
| Lahdenperä 2000100 | Y | Y | Y | Y | Y | U | U | Y | Y | Explores some of the advantages of using IT as a tool in the treatment of hypertension (patient attitudes before taking part in a trial) |
| Lamothe 2006103 | Y | Y | Y | Y | Y | N | N | N | Y | Provides information on major impact of telehomecare on patients and providers and explains how this may be achieved. Identifies two models. Some methodological concerns about this study |
| Liddy 2008104 | Y | Y | Y | Y | Y | N | U | Y | Y | Gives preliminary evidence only of benefits and limitations of telehomecare linked to primary care |
| Mackert 2009108 | Y | Y | Y | U | Y | Y | N | Y | Y | Gives some indication of individual needs and preferences for e-health, particularly for adults with lower literacy skills |
| Marziali 2009112 | Y | Y | Y | Y | Y | N | Y | Y | Y | Provides feedback on single intervention only, but some indication of the value of internet-based support provided by facilitators |
| Nilsson 2010102 | Y | Y | Y | Y | Y | Y | N | Y | Y | Gives two district nurses’ perspectives on information and communication technology in nursing care. However, there is a lack of generalisability (important relationships revealed, but difficult to see if the same relationships would manifest themselves in other subjects; p. 264). Findings not applicable to all kinds of care |
| Overberg 2007124 | Y | Y | Y | Y | Y | Y | Y | U | Y | Gives evidence of requirements for internet-based applications, but represents only active information seekers because of recruitment strategy. Limited to cancer |
| Pinnock 2007110 | Y | Y | Y | Y | Y | N | Y | Y | Y | Gives initial data based on a small number of clinicians and patients that mobile telephone-based systems have the potential to support self-management |
| Rahimpour 2008105 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Adds to evidence base by identifying anxiety and self-efficacy as important variables that should be included in future Home Telecare Management System acceptance models. Informs the development of the Home Telecare Management System acceptance model, which is the theoretical framework for the next stage of the model |
| Sandberg 2009106 | Y | Y | Y | Y | Y | U | Y | Y | Y | Unique to this intervention – not generalisable to other settings or other types of telehealth case management |
| Scharer 2009119 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Shows the efficacy of chat room and telephone support, but limited to small sample size. Shows that the interventions provided social support to these mothers |
| Swinton 200999 | Y | Y | Y | U | Y | N | Y | Y | Y | Gives patients’ and primary care physicians’ views on telehealth. Limited by participants being from the state of Nebraska only and only 10–15 minutes of each focus group was spent on issues relating to telehealth. |
| Waller 200981 | Y | Y | Y | NA | Y | NA | NA | Yb | Y | Limited qualitative comments (only three high-quality studies reviewed), but suggest acceptability is high among those in included studies. Review suggests qualitative evidence is generally poor |
Appendix 3 Data extraction of qualitative evidence for long-term conditions and telehealth
| Study, country | Disease or setting, participants and methods | Intervention and focus |
|---|---|---|
| Armstrong 2007,107 UK | Diabetes (patients with type 1 diabetes who used insulin pumps). Patient consensus was gathered via email on the important and useful features of internet-based systems used for LTCs or chronic conditions Patients and experts for stakeholder opinion (three focus groups with three to five participants in each), five interviews with HCPs Expert workshop |
Proposed virtual clinic to ‘provide people living with diabetes and their healthcare professionals access to records of their condition (including uploaded blood glucose readings), a messaging facility, information and advice for patients, and peer-to-peer support area’ |
| Armstrong 2009,116 UK | Diabetes 12 patients took part in three focus groups; five patients took part in pretesting Patient focus groups and pretesting followed by focus group reported in this paper (6-month pilot reported elsewhere) |
Virtual clinic system |
| Beattie 2009,98 UK | Depression (at least moderate) 24 patients from five general practices in the UK, mostly female (17/24; 22–66 years of age). No discernible patterns in data linked to other sociodemographic backgrounds 24 interviews prior to therapy, 20 post therapy |
10 sessions of CBT delivered via the internet by a psychologist. Patients were given a manual on how to access therapy sessions, including booking and ‘attending’ appointments. The patient and therapist agreed an appointment time when they both logged onto the web page. Interaction was through questions and answers similar to instant messaging |
| Cornwall 2008,123 UK | Lung cancer 16 patients and family members (10 male, 6 female) Review of e-mail contact (44 e-mails by 16 patients and family members); 12/16 invited to complete a questionnaire. Focus group/reflective session with lung cancer nurse specialists |
E-mail contact between lung cancer nurse specialists and patients and family members |
| Dinesen 2006,114 Denmark | Diabetes (insulin dependent) Three patients (male, aged 45–57 years) and diabetes team members (two diabetes nurses, a consultant doctor, a medical secretary and a dietitian). Two stages of qualitative interviews with patients. Narrative approach used. Health professionals: documentary material and observation were used as data collection techniques to gain a basic understanding of characteristics, contexts and phenomena. In total, 10 qualitative interviews were carried out Qualitative interviews with each team member before and after implementation of DiasNet (diabetes decision support system). Focus group with diabetes team members |
DiasNet – keeps track of carbohydrate and blood glucose levels |
| Dinesen 2008,117 Denmark | Heart failure Four patients with heart failure, four with arrhythmia (six male, age 67 ± 9.3 years; two female, age 76 ± 11.3 years; six spouses). Had been admitted to hospital for 1–12 days (mean 4.4 days). Home hospitalised with telehomecare for 1–6 days (mean 3.75 days). Total number of admission days 8.1 Phenomenological hermeneutic approach. Triangulation of data from electronic patient records. Participation observation and qualitative semistructured interviews |
Home hospitalisation with telehomecare following acute event and treatment in hospital |
| Dinesen 2009,122 Denmark | Heart patients (past acute phase) HCPs Action research; triangulation of data – documents, participatory observations, qualitative interviews; focus group interviews |
Home hospitalisation with telehomecare following acute event and treatment in hospital |
| Griffiths 2006,97 UK | Patients with cancer, HIV infection, mental health disorders, eating disorders and back pain; health promotion issues such as smoking, physical activity and obesity; and interventions aimed to support carers of those with Alzheimer’s disease and stroke, parents of babies and parents of children in intensive care Qualitative systematic review of peer-reviewed evaluations of interventions (28 interventions, nine pilots) |
CBT for a range of mental health issues Provides a qualitative review of peer-reviewed health interventions delivered to a known client/patient group using networked features of the internet’ |
| Hanley 2009,101 UK | Various Seven individuals (young people) Mixed-methods study (qualitative element: interviews) |
Online counselling |
| Hibbert 2004,115 UK | COPD 12 nurses Ethnographic, with participant observation related to a 13-month period |
Telehealth |
| Hopp 2007,109 USA | Diabetes Clinicians including 10 registered nurses (four of whom were also nurse practitioners). Included those providing monitoring and messaging device services from outpatient clinic care and home care programmes In-depth semistructured interviews |
Monitoring and messaging devices as part of telehealth |
| Høybye 2005,113 Scandinavia | Breast cancer 15 women with breast cancer Ethnographic case study [12 face-to-face interviews and nine online interviews plus e-mail contributions and observations (virtual)] |
Internet support group/mailing list |
| Kahn 2009,111 USA | Diabetes and mental health problems 539 Gold Choice members and telephonic nurse case manager. Patients were middle-aged (8% were aged < 40 years) and all were Medicaid recipients (presumed to have a low socioeconomic status) Qualitative analysis of patient progress notes (853 de-identified progress notes) and interview with TNCM |
TNCM |
| Kerr 2005,120 UK (linked to Kerr 2006121) | LTCs (diabetes, ischaemic heart disease, hepatitis C, parents of children with asthma or diabetes mellitus, caregivers of people with Alzheimer’s disease) 40 participants including patients and carers Eight focus groups; thematic analysis |
ICHAs – interactive computer-based information packages |
| Kerr 2006,121 UK | Range of LTCs (adult patients with diabetes, ischaemic heart disease or hepatitis C; parents of children with asthma or diabetes mellitus; caregivers of people with Alzheimer’s disease) 40 patients and caregivers (mixed socioeconomic background, including ethnic minorities, rural, lower socioeconomic status, urban) 10 focus groups (two to eight participants in each) |
ICHAs or internet interventions – patients/carers accessed a networked personal computer preloaded with three interventions (participants used each internet intervention for up to 30 minutes) |
| LaFramboise 2009,118 USA | Heart failure (77% class II) 13 patients (eight Health Buddy only, five HealthBuddy and home visit; 61% female; mean age 68 years; 54% lived alone; 92% white; 62% high school diploma) Focus groups (three to four participants) and individual interviews. Content analysis used to analyse data |
Health Buddy (Health Hero Inc.) with display screen and buttons that attach to a telephone line. Participants using the Health Buddy were asked seven questions daily about their symptom status and ability to follow the prescribed regimen |
| Lahdenperä 2000,100 Finland | Hypertension, high blood pressure 21 patients (aged 32–63 years) Interviews |
IT Focus on experiences of technology and expectations of technology in the treatment of hypertension |
| Lamothe 2006,103 Canada | COPD, cardiac insufficiency, hypertension, unstable diabetes. Many patients had a severe condition in two of three pathologies 82 patients (26 Quebec, 45 urban Manitoba, 11 rural Manitoba) 82 individual interviews and five group interviews with managers, partners, professionals and patients |
Telehomecare including daily taking and sending of required measures (vital signs) |
| Liddy 2008,104 Canada | Multiple chronic illness identified as ‘at risk’ for functional decline, physical deterioration 22 patients aged 50+ years (from RCT) Pilot, questionnaires, focus group with nurse, pharmacist and doctors, interviews (including initial telephone survey) with patients and health professionals. Thematic analysis of qualitative data |
Telehomecare including monitoring devices. Nurse practitioners and pharmacist upload to web via telephone lines (run by primary care service, not commercial provider) |
| Mackert 2009,108 USA | Obesity and health 43 parents (mothers and fathers; African American, Hispanic and white; age > 18 years; had not completed a 4-year college degree or worked in the health-care field) Focus groups |
Discussion of internet use for health information and the demonstration of a website designed specifically for users with low levels of health literacy and asking participants about ideas under consideration for future interventions |
| Marziali 2009,112 Canada | Chronic disease 18 community-dwelling older adults Interviews |
Internet-based support group programme: participants provided with equipment (computers, webcams, headsets) and trained to access easy-to-use, password-protected website that uses videoconferencing to support group member–facilitator interactive communication |
| Nilsson 2010,102 Sweden | Serious chronic illness Highly qualified and experienced nurses caring for two patients with serious chronic illness living at home (who required extensive 24-hour care) Case studies with repeated semistructured interviews with nurses (interviewed before the implementation started, 2 months after the implementation and after the implementation period had finished) |
Information and communication technology at home: new technology in the form of an electronic messaging programme called Rexnet and mobile telephones with internet connection. Programme consisted of different virtual rooms and enabled the district nurses to communicate with patients by sending and receiving text messages |
| Overberg 2007,124 Netherlands | Breast cancer 26 patients (mean age 51 years) Qualitative interviews |
Internet |
| Pinnock 2007,110 UK | Asthma 48 participants (34 adults and teenagers with asthma and 14 asthma nurses and doctors); 39 participated in six focus groups. Patients were recruited in Lothian (central Scotland) and Kent and were of mixed age, sex and socioeconomic status Focus groups during which participants were shown an example of a mobile telephone-based monitoring system that had been trialled in asthma patients |
Potential role of mobile telephone-based monitoring for asthma self-management (including transmitting symptoms and peak flows, with immediate feedback of control and reminder of appropriate actions) |
| Rahimpour 2008,105 Australia | Chronic heart failure (NYHA classes II–IV), COPD or both 77 volunteers (64% of those approached) from seven different ethnic groups; age > 40 years (range 50–90 years, mean and median age 71 years) Focus group interviews (groups of 6–12 participants) |
HTMS: shown a video demonstrating the HTMS and its operation, followed by a demonstration of the HTMS prototype. Participants asked their perceptions in potentially real situations |
| Sandberg 2009,106 USA | Diabetes 10 certified diabetes educators [five registered nurses, two registered dieticians, two registered as both; three described themselves as Latina, one as bi-racial (Native and African American) and five as white]. Worked in their respective field for an average of 19 years (range 4–39 years) Semistructured interviews. Used grounded theory approach to qualitative research |
IDEATel (Informatics for Diabetes Education and Telemedicine) |
| Scharer 2009,119 USA | Child mental health/psychiatric problems 11 mothers of children with psychiatric problems. Educational attainment of mothers from grade school education to college graduates Qualitative description of mother and nurse interactions |
Qualitative description of mother and nurse interactions with web-based social support intervention (chat room available once a week in the late evening for an hour; nurse facilitator always available when chat room was open) compared with telephone social support (calls made every other week for 6 months) |
| Swinton 2009,99 USA | Depression – PHQ-9 used to assess severity of depression. Patients ranged from having severe depression to some who did not receive a score indicating depression Patients recruited from 15 different sites (sites were between 71 and 307 miles from an urban city with a population > 50,000; all sites were government-designated mental health professional shortage areas). A total of 17 primary care physicians in rural Nebraska and 28 patients (22 female, 6 male) were included 10 focus groups, qualitative multiple case study design; one individual interview |
Telehealth (use of therapy via the internet, satellite transmission or other technological means) |
| Waller 2009,81 UK | Psychiatry Systematic review (46 papers representing 36 studies) Integrative review |
Computerised CBT Focus on acceptability, accessibility and adverse consequences |
Appendix 4 Summary of included studies addressing changes in overall cardiovascular disease risk factors
| Study | Participants | Intervention | Follow-up | Comparison | Outcome measures |
|---|---|---|---|---|---|
| Bennett 2011420 | 145 managers from eight US organisations | Internet-based training programme | 6 months | Normal daily activities | Dietary attitudes and beliefs, exercise participation and beliefs, mental health symptom frequency and beliefs, weight, waist circumference, BMI, percentage body fat |
| Bove 2011181 | 465 participants from medically underserved and rural areas (USA) | Nurse management of CVD risk factors with telemedicine communication | 12 months | Nurse management of CVD risk factors | Framingham 10-year CVD event risk, waist circumference, BMI, SBP, DBP, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, blood glucose, HbA1c, smoking |
| Broekhuizen 2012184 | 340 Dutch adults with familial hypercholesterolaemia | Personalised health counselling using computer-generated tailored advice and face-to-face counselling complemented by telephone booster sessions | 12 months | Usual care | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, SBP, serum glucose, BMI, waist circumference |
| Claes 2013180 | 314 self-employed lawyers in Belgium | Personalised online and one-to-one coaching by mail, e-mail and telephone and face-to-face. Printed profile with individual risk factors and total CVD risk. Annual multidisciplinary screening | 3 years | Printed profile with individual risk factors and total CVD risk. Annual multidisciplinary screening | 10-year risk of a fatal CVD event, SBP, DBP, total cholesterol, BMI, physical fitness (heart rate in recovery post exercise) |
| Cook 2007421 | 419 US human resource company employees | Comprehensive multimedia health promotion programme | 3 months | Commercially available booklets | Eating practices and attitudes, stress management, physical activity |
| Dekkers 2011179 | 276 healthy overweight employees of Dutch companies | Distance counselling lifestyle intervention programme by via phone or internet called Leef je Fit (Live yourself Fit). Also received self-help materials | 2 years | Self-help materials | Body weight, waist circumference, sum of skinfolds, SBP, DBP, total cholesterol |
| Nolan 2011182 | 680 participants at high risk of CVD or history of CAD (Canada) | Small group lifestyle counselling via teleconference. Written summary of CVD risk factor profile with brief advice and educational handouts | 6 months | Written summary of CVD risk factor profiles with brief advice and educational handouts | Framingham 10-year CVD risk, SBP, DBP, ratio of total cholesterol to HDL cholesterol |
| Nolan 2012183 | 387 hypertensive patients (Canada) | E-counselling with motivational messaging | 4 months | Usual care and e-newsletter | SBP, DBP, pulse pressure, total cholesterol |
| Ruffin 2011422 | 3382 participants in Family Healthware Impact Trial (USA) | Interactive online tool for family history of six common diseases (including CHD and stroke). Provides risk assessment and tailored preventative health messages | 6 months | Standard prevention messages for common diseases of interest | Smoking, fruit and vegetable intake, physical activity, blood pressure, cholesterol, blood glucose |
| Sone 2010178 | 2033 Japanese type 2 diabetes patients | Individual telephone counselling sessions every 2 weeks | 8 years | Usual care | Incidence of macrovascular and microvascular complications, BMI, SBP, DBP, fasting plasma glucose, HbA1c, total cholesterol, triacylglycerol, HDL cholesterol, lipoprotein, food energy intake, exercise, smoking |
| Verheijden 2004423 | 146 general practice patients with chronic diseases (Canada) | Self-assessment tool for stage of behaviour change, providing targeted information packages | 8 months | Usual care | BMI, SBP, DBP, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides |
| Wakefield 2011424 | 302 US war veterans with hypertension and diabetes | Home telehealth device for blood pressure and blood glucose data input. High-intensity group received prompts from pre-programmed branching disease management algorithm. Low-intensity group received automated responses from device | 12 months | Usual care | HbA1c, SBP |
| Wister 200748 | 611 patients at high risk of CVD or history of CHD (Canada) | Health report card with telehealth counselling follow-up sessions | 12 months | Usual care | Framingham 10-year CVD risk, Framingham global risk score, total cholesterol, HDL cholesterol, glucose, SBP, smoking, physical activity, BMI |
Appendix 5 Cochrane risk of bias assessment
| Study | Sequence generation | Allocation concealment | Blinding participants and personnel | Blinded outcome assessment | Incomplete outcome data | Selective outcome reporting | ‘Other issues’ |
|---|---|---|---|---|---|---|---|
| Bennett 2011420 | ? | ? | – | – | – | – | – |
| Bove 2011181 | + | ? | – | ? | – | + | + |
| Broekhuizen 2012184 | + | + | – | ? | + | + | + |
| Claes 2013180 | – | + | ? | ? | + | + | ? |
| Cook 2007421 | ? | ? | – | ? | + | + | + |
| Dekkers 2011179 | + | + | – | ? | – | + | – |
| Nolan 2011182 | + | + | + | ? | + | + | + |
| Nolan 2012183 | + | + | + | + | – | + | – |
| Ruffin 2011422 | ? | ? | – | ? | + | + | + |
| Sone 2010178 | + | ? | – | ? | – | + | + |
| Verheijden 2004423 | + | + | + | + | + | ? | – |
| Wakefield 2011424 | – | + | ? | ? | – | + | + |
| Wister 200748 | + | + | – | + | – | + | – |
Appendix 6 Subset of constructed questionnaire items
Appendix 7 Depression and cardiovascular disease risk patient management software
Note: these depression system screenshots, as well as the content in Appendices 9, 12, 13, 15 and 16, have been reproduced with permission from Solent NHS Trust.
Depression management system: screenshot of first question in encounter 1

Depression management system: screenshot of example question during encounter 2
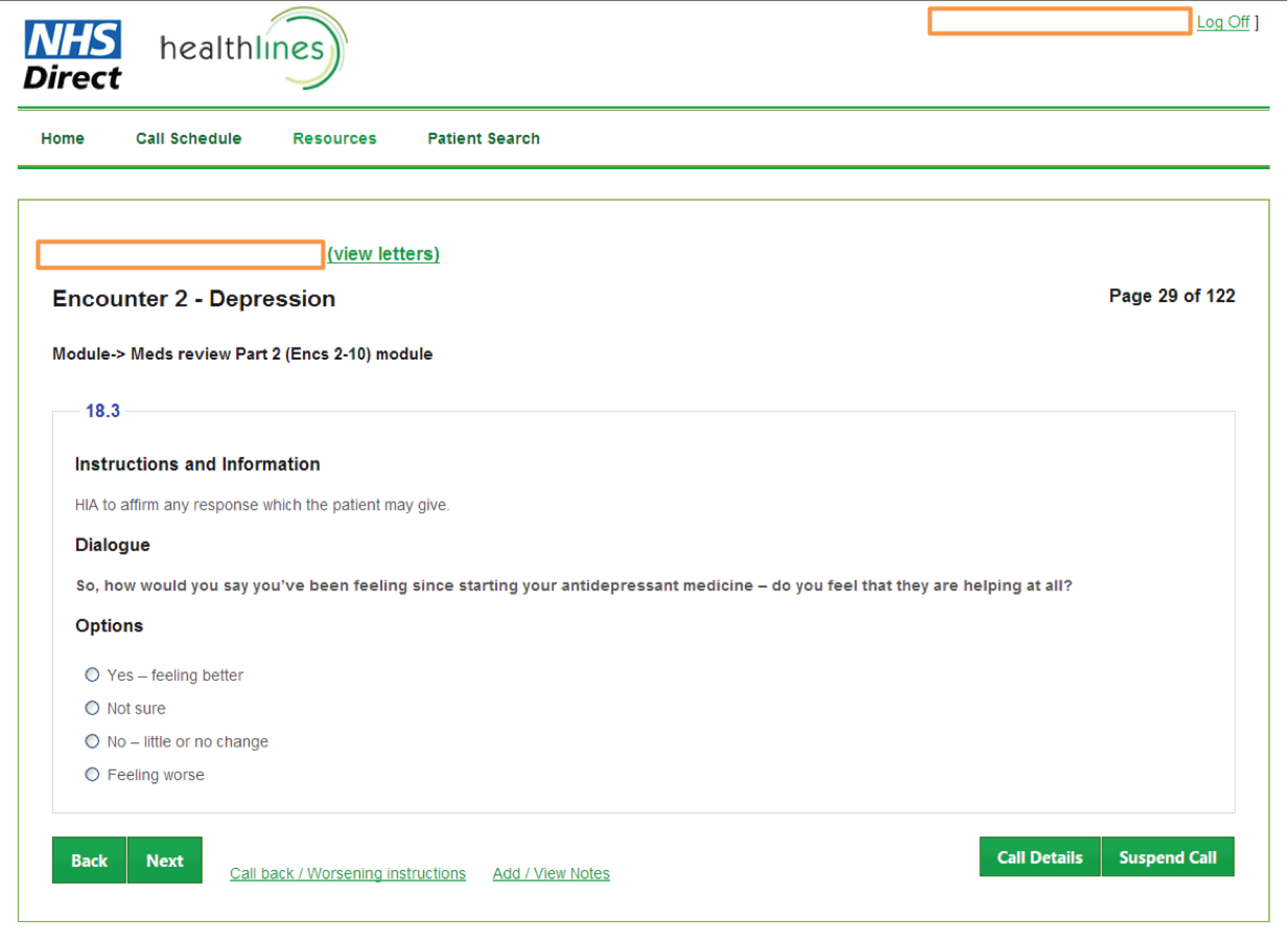
Note: the CVD risk system screenshots in this appendix have been reproduced with permission from Professor Hayden Bosworth (Duke University, Durham, NC, USA).
Cardiovascular disease risk management system: screenshot of intervention schedule tab
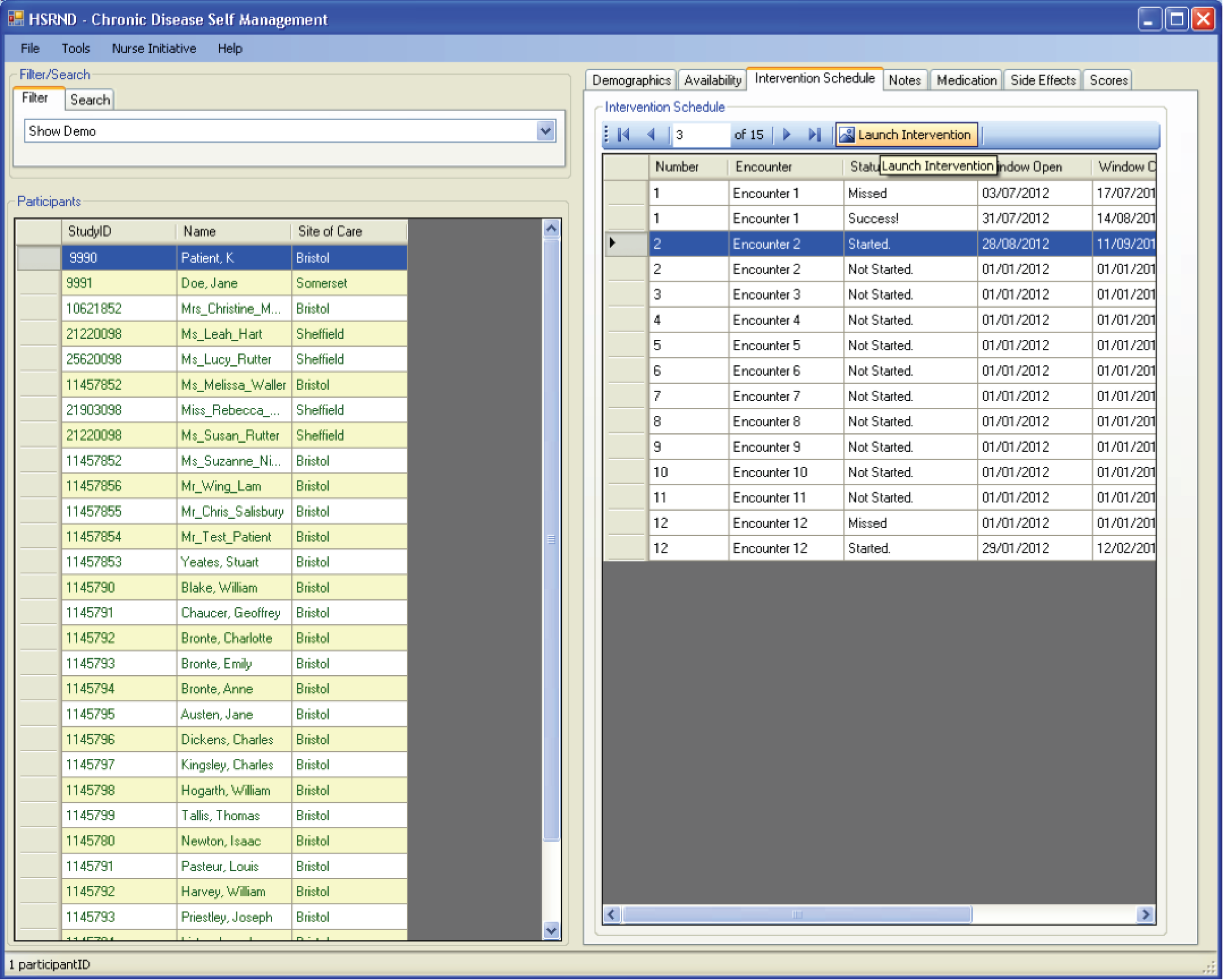
Cardiovascular disease risk management system: screenshot of first question in encounter 1
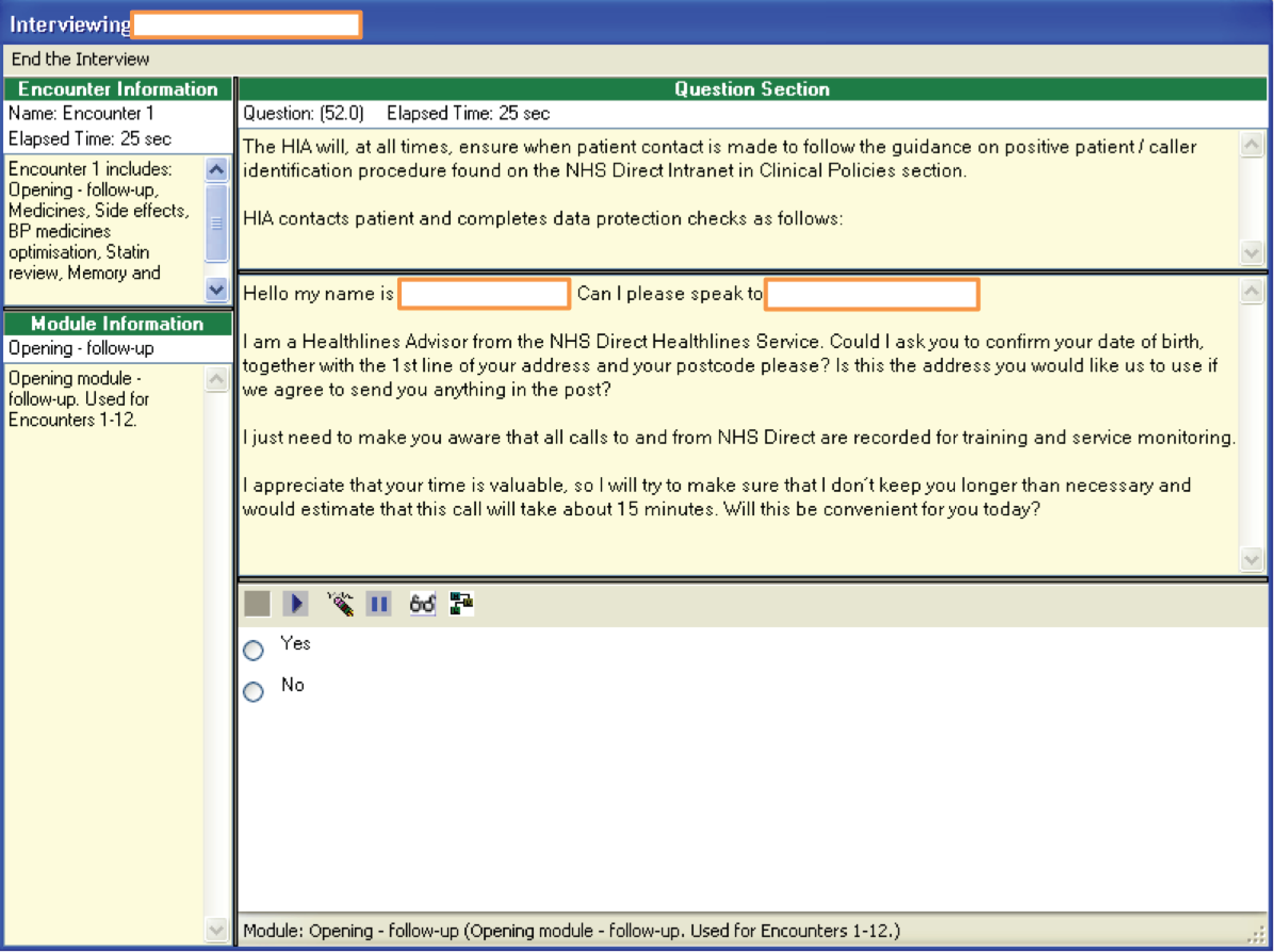
Cardiovascular disease risk management system: screenshot of example question during encounter 2
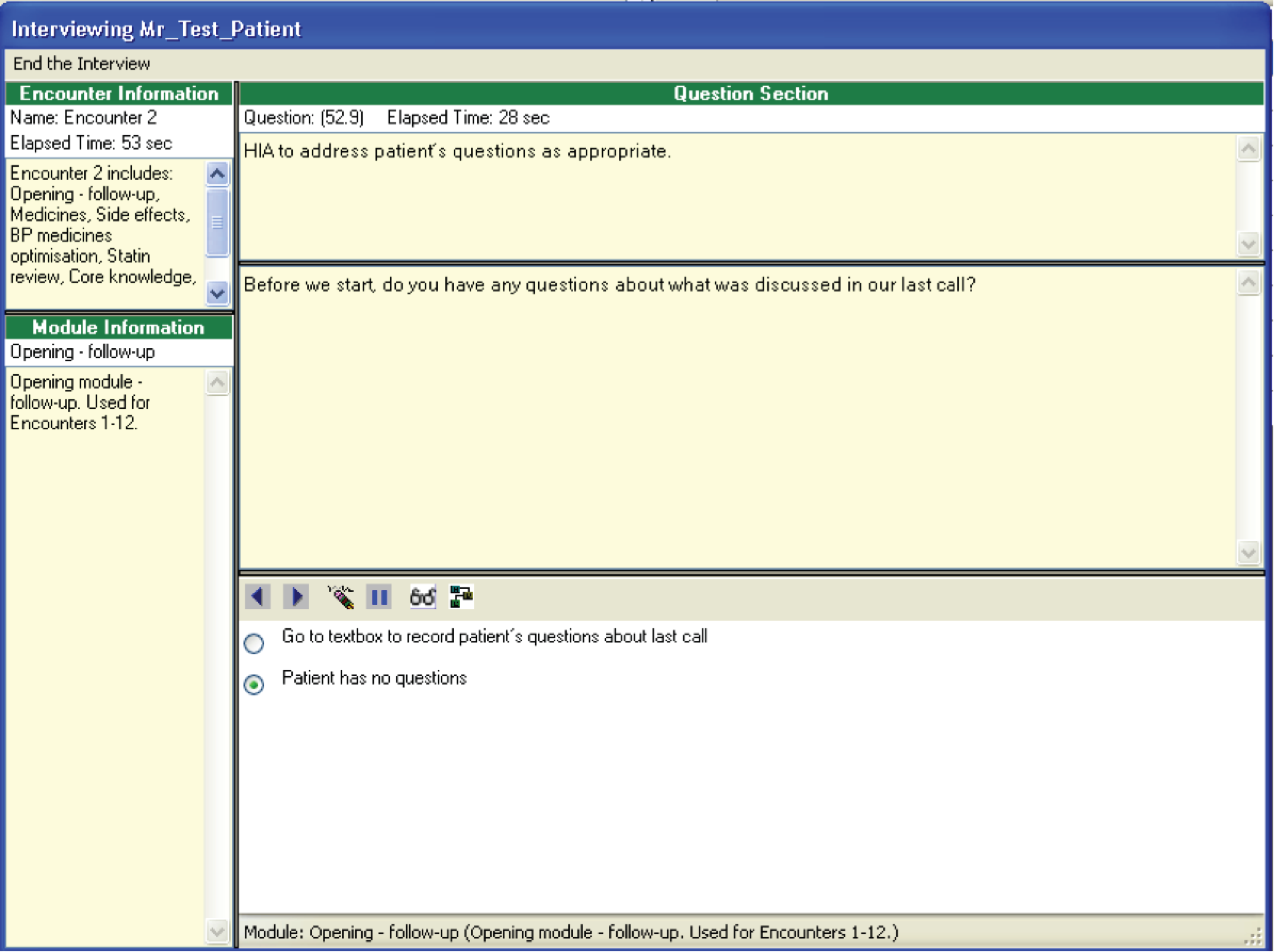
Cardiovascular disease risk management system: screenshot example of smoking cessation advice during encounter 2
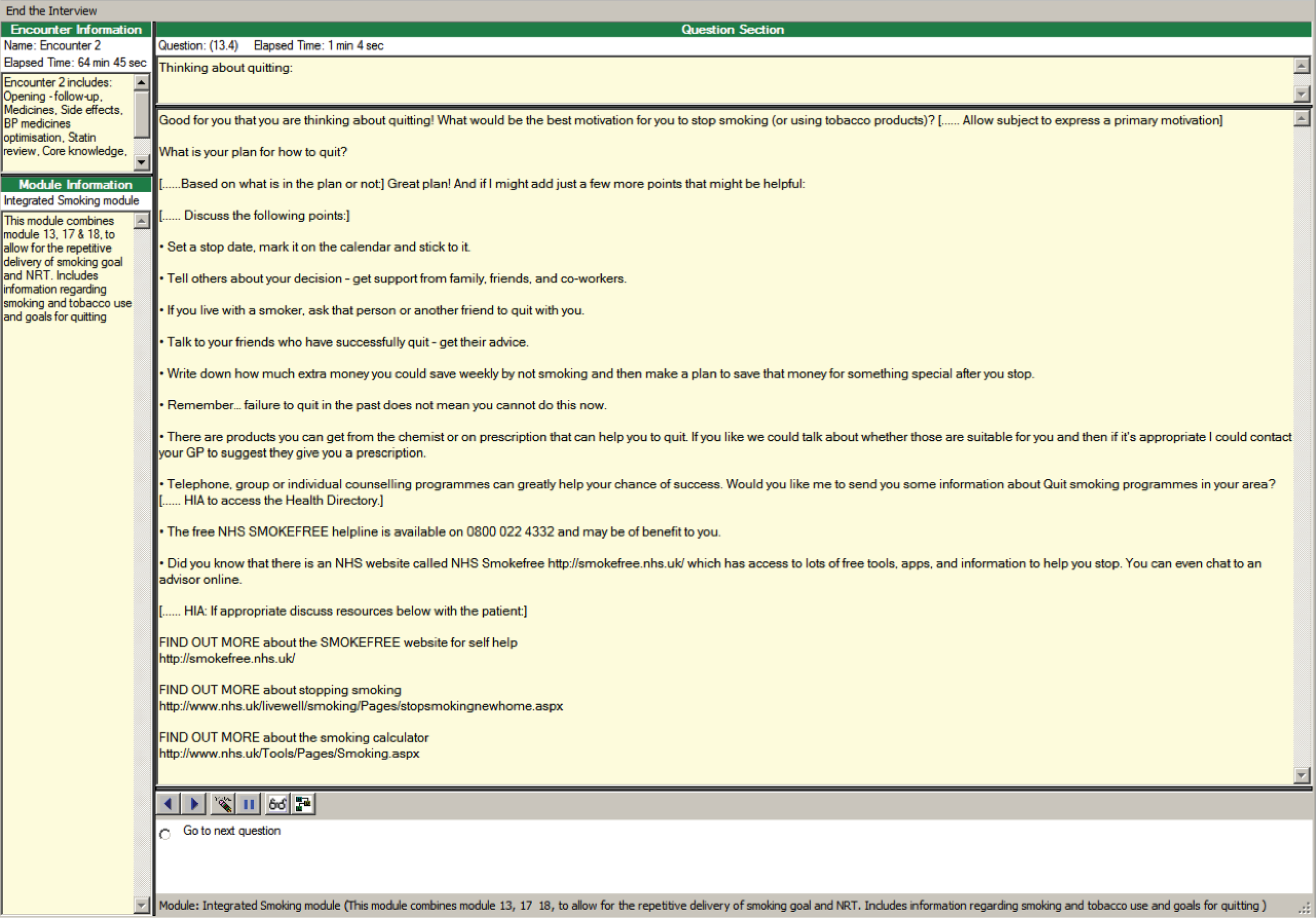
Cardiovascular disease risk management system: screenshot example of smoking cessation information to send to patient during encounter 2
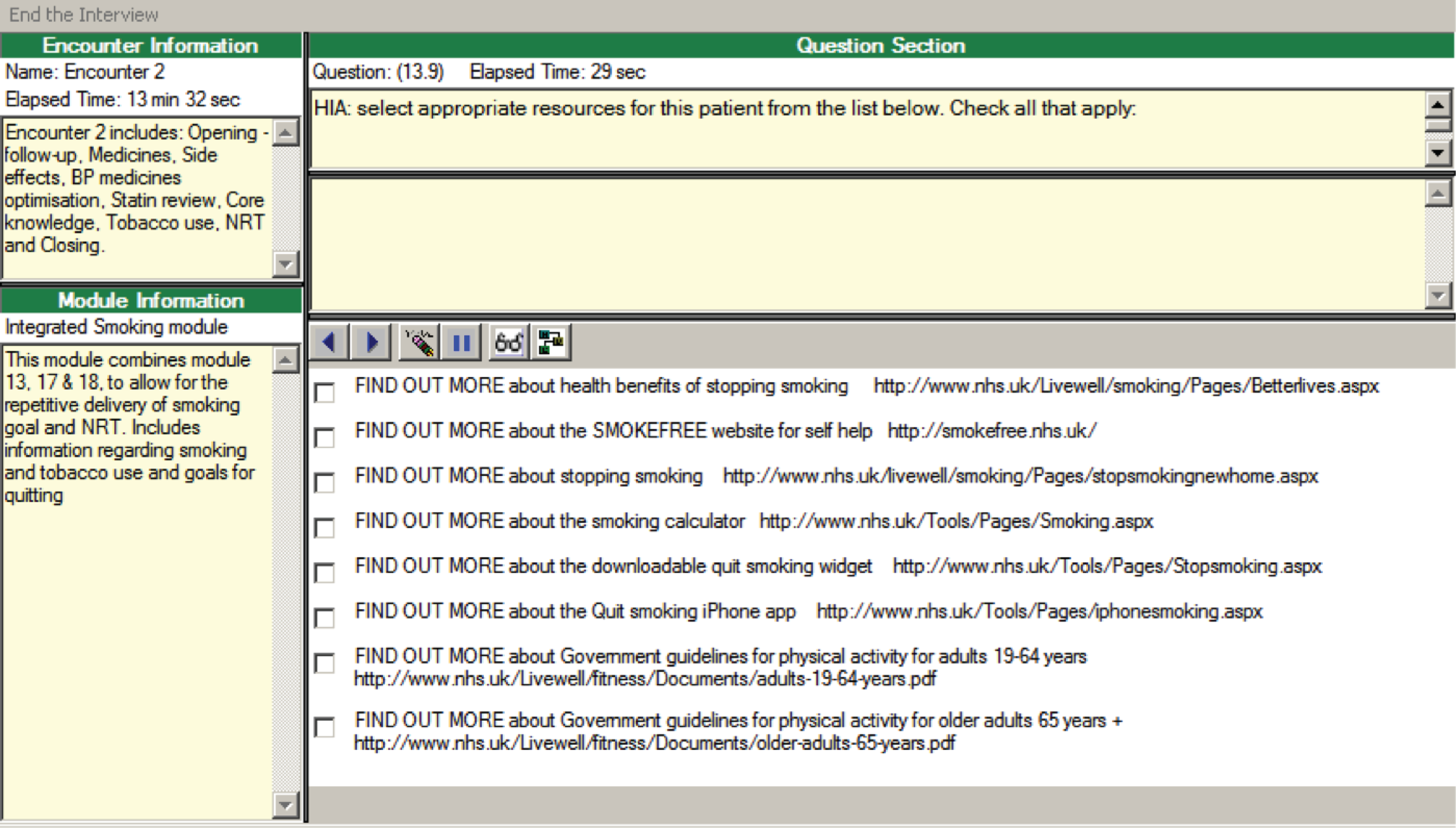
© Copyright 2012 by NHS Direct – Version: 1.1.1.24025.
Appendix 8 Excerpts of computerised scripts from the Healthlines Service for the depression and cardiovascular disease risk interventions
Note: this content has been reproduced with permission from Solent NHS Trust.
Extract from health information advisor script for depression: encounter 3
2b. For LLTTFi users
Is there anything about LLTTF you would like me to go over again with you?
HIA to respond accordingly.
How did you get on with session 1 in LLTTF – (Introduction and Why do I feel so bad?)?
HIA to allow patient to respond.
-
Problems encountered – go to 3.2b.1.
-
No problems encountered – go to 3.2b.2.
3.2b.1 If patient had encountered problems, e.g. not completed session 1
Not to worry; was there a reason for this; is there anything I can help you with?
If patient has been unable to complete session 1 or the assignment or has had other difficulties, encourage them to use the review sheet to help them reflect on what problems occurred and why and then to use the planner sheet to try a different approach.
If applicable, encourage the patient to resume or start session 1 as soon as possible and, if appropriate, get them started on it again, ensuring they know how to continue.
HIA to try to resolve session 1 issue before moving on to discussing status of assignment.
-
Problem with session 1 – where assignment attempted but not completed – go to 3.2b.3.
-
Problem with session 1 where assignment was completed – go to 3.2b.4.
-
Problem with session 1 where assignment not attempted prior to this call – go to 3.2b.3.
3.2b.2 If no problems encountered
Well done. I hope you are pleased with yourself.
You have achieved your first goal to complete session 1.
Did you also manage to complete your assignment?
HIA to allow patient to respond then HIA respond as appropriate.
-
Problems encountered – go to 3.2b.3.
-
No problems encountered – go to 3.2b.4.
3.2b.3 If patient had encountered problems
Not to worry, was there a reason for this; is there anything I can do to help you with this?
HIA to try to resolve issue and reassure the patient before moving on.
-
Go to next question – go to 3.2b.4.
3.2b.4
Ok; try to keep putting into practice what you have learned, and don’t be put off if you have any setbacks, it’s not always easy – just keep working at it – you’ve made a good start.
I think we can now move onto LLTTFi session 2.
In session 2 you can choose to concentrate on either:
-
unhelpful behaviour or
-
unhelpful thinking.
You are asked to choose a behaviour or a thought to work on that you find unhelpful.
Then your assignment will be to do a five areas assessment on your unhelpful thinking or behaviour so you can work towards changing it for the better.
Whichever one you don’t choose here in session 2, you get the chance to work on it in session 3.
Which area do you think you’d like to look at in session 2, thinking or behaviour?
HIA to allow patient to respond.
Remember to download/use the workbook for session 2, where you’ll find your DOs and DON’Ts card which you will need and don’t forget your Planner and Review sheets.
HIA to ask patient to summarise their understanding so far and deal with any misunderstanding or questions.
. . . and remember, you are not on your own, if you have any problems with any of the sessions or other aspects of LLTTF, you can call us and leave a voicemail message or e-mail us and we will get back to you to offer you assistance. I will give you the contact details and opening times of the service at the end of our call.
When you have completed session 2, spend the next week or so studying your workbook and homework tasks in time for our next call.
Good luck with session 2 – I hope you enjoy it!
We will see how you got on with it in our next call.
-
Go to next question – go to 3.3.0.
3.3.0
3. Big White Wall
HIA: Was this patient given an access code for Big White Wall on previous call?
-
Yes – go to 3.3.1.
-
No – go to 3.3.2.
3.3.1
If the patient was given an access code for BWW on previous call:
How are you getting on with Big White Wall?
Allow patient to respond, affirm response and HIA to give encouragement if necessary.
-
Go to next question – go to 3.4.
3.3.2
If the patient was not given an access code for BWW on previous call:
Have you thought about Big White Wall since our last call? Would you now like to join?
Check that they are interested in social media sites/forums, etc.
-
Yes – go to 3.3.2a.
-
No – go to 3.3.2b.
3.3.2a
Ok, I can send you a link to Big White Wall now. You will receive an e-mail with the link shortly – all you have to do then is to click the link and you will be presented with a page where you are asked to enter your e-mail address and a password and user name of your choice – then you are registered!
HIA access BWWProfessional (using password and user name allocated by BWW).
Enter patient’s e-mail address and trial ID number into the appropriate fields.
Inform patient that they will receive an e-mail shortly containing a link to BWW.
-
Go to next question – go to 3.4.
3.3.2b
That’s fine, social media sites are not for everyone, but if you change your mind you can always contact us and let us know.
-
Go to next question – go to 3.4.
3.4
4. Summary
Use notes section to record brief notes on session, e.g.:
-
risk questions asked (PHQ-9 questionnaire)
-
sessions completed/in progress
-
any difficulties encountered by patient
-
agreed actions planned between now and next session
-
worsening instructions – getting urgent help.
-
Go to closing script – depression.
The patient e-mail template will be generated at the end of this encounter.
Extract from the health information advisor script for cardiovascular disease risk: statin module 56
Note: this module discusses the risks and benefits of taking statins and explores a patient’s suitability for taking statins.
56.0 Script
HIA: Have you already delivered the statin module during this call?
-
1 = Yes I have – go to next module – 1(4.0Script), 2(6.0), 3(8.0), 4(4.0Script), 5(19.0Script), 6(21.0b/21.0/21.0a*), 7(8.0), 8(21.0b/21.0/21.0a*), 9(8.0), 10(20.0), 11(14.0), 12(21.0b/21.0bScript/21.0a_6*).
-
2 = No I have not – continue to statins (go to 56.0).
56.0 Are you currently taking a statin?
If No – stopped taking on own, ask subject WHY and WHEN. This can be added to the NOTES section of computer program.
If No – my doctor ordered it to be stopped or No – it was never prescribed, remove stopped medicine(s) from the medication list.
-
1 = Yes (go to next module) – 1(4.0Script), 2(6.0), 3(8.0), 4(4.0Script), 5(19.0Script), 6(21.0b/21.0/21.0a*), 7(8.0), 8(21.0b/21.0/21.0a*), 9(8.0), 10(20.0), 11(14.0), 12(21.0b/21.0bScript/21.0a_6*)].
-
2 = No – stopped taking on own (go to 56.0a).
-
3 = No – my doctor ordered it to be stopped (go to next module) – 1(4.0Script), 2(6.0), 3(8.0), 4(4.0Script), 5(19.0Script), 6(21.0b/21.0/21.0a*), 7(8.0), 8(21.0b/21.0/21.0a*), 9(8.0), 10(20.0), 11(14.0), 12(21.0b/21.0bScript/21.0a_6*).
-
4 = No – it was never prescribed (go to 56.1).
56.0a Is your doctor aware that you have changed/stopped this medicine(s)?
-
1 = Yes (go to 56.1).
-
0 = No (go to 56.0b).
-
777 = DK (go to 56.0b).
-
888 = Refused (go to 56.0b).
[statin0a]
56.0b
It is important to tell your doctor if you are having trouble with your cholesterol medicine. It’s best to discuss any changes with him/her before changing or stopping your medicine(s). You and your doctor should work together to find the right cholesterol medicine(s) for you. I encourage you to contact your doctor within this week to inform him/her about your decision.
-
Go to next module – 1(4.0Script), 2(6.0), 3(8.0), 4(4.0Script), 5(19.0Script), 6(21.0b/21.0/21.0a*), 7(8.0), 8(21.0b/21.0/21.0a*), 9(8.0), 10(20.0), 11(14.0), 12(21.0b/21.0bScript/21.0a_6*).
56.1
I will now discuss the risks and benefits of taking a statin. If you decide that you want to start taking a statin we will complete a brief assessment and send this to your GP so you can discuss this further.
Note to HIA: Use critical thinking to go through the risks and benefits of statins. Not all statements need be used depending on the patient’s reasons for not taking a statin.
Benefits of statins
-
Lowers your risk of having a heart attack or stroke. If you have certain risk factors including raised blood pressure, being a smoker and being overweight then you have a 2 in 10 chance or more of developing CVD in the next 10 years. Taking a statin can substantially reduce this risk.
-
Reduces the amount of cholesterol made by your liver. Excess cholesterol is deposited in the blood vessels (atherosclerosis).This narrows the blood vessels and limits the amount of blood and oxygen delivered to your body. If this happens to the blood vessels (coronary arteries) in the heart muscle, it can cause chest pain (angina) and in severe cases a heart attack (myocardial infarction). If this happens to the blood vessels in your brain, it can cause a stroke.
Risks of statins
-
Most people do not get side effects or just minor ones that getter better with time. About 1–3% of people get minor side effects. These include tiredness, vague aches and pains, headache, feeling sick, indigestion, diarrhoea or constipation.
-
Important but rare side effects are muscle problems and liver problems. Muscle problems (myopathy) – muscle and tendon pain, stiffness, muscle weakness or cramping. You should tell your doctor if you get any of these. Stopping the statin or reducing the dose will usually return your muscles to normal. Liver problems – if you take a statin, you will have regular blood tests to check on your liver. People with liver disease or persistently abnormal liver tests should not take a statin unless considered necessary by their doctor. If you get liver problems, stopping the statin or reducing the dose will usually return your liver to normal.
-
Statins have interactions with a number of other medicines. You will need to discuss this with your doctor. Always check the patient information leaflet that comes with your medicine before taking it.
If you would like more information I can e-mail you a leaflet (www.patient.co.uk/health/Statins-%28Cholesterol-Lowering-Medicines%29.htm).
-
1 = Information provided (go to 56.2).
-
0 = Information not provided (go to 56.2).
[statin1]
56.2 Does the patient want to be referred to their GP to discuss taking a statin?
-
1 = Yes – complete patient assessment/GP referral letter (go to 56.3a).
-
0 = No – go to next module (go to 56.2 script).
[statin2]
56.2 Script
HIA: Which module do you need to go to now?
If you need to complete the Medicines module choose the first option, otherwise choose ‘Go to next module’ option.
For a subject initiated call, ALWAYS choose the first option to return to Medicines module.
-
1 = Go back to Medicines module (go to 2.4e1).
-
0 = Go to next module – 1(4.0Script), 2(6.0), 3(8.0), 4(4.0Script), 5(19.0Script), 6(21.0b/21.0/21.0a*), 7(8.0), 8(21.0b/21.0/21.0a*), 9(8.0), 10(20.0), 11(14.0), 12(21.0b/21.0bScript/21.0a_6*).
*Programming information: The system will automatically go to one of three options for encounters 6, 8 and 12, depending on the conditions set as follows:
-
21.0b – if the patient’s BMI < 25
-
21.0 – for encounters 6 and 8 – if the patient’s BMI is 25 or more and they set a weight loss goal in encounter 3
-
21.0bScript – for encounter 12 – if the patient’s BMI is 25 or more and they set a weight loss goal in encounter 9
-
21.0a – for encounters 6 and 8 – if the patient’s BMI is 25 or more and they did not set a weight loss goal in encounter 3
-
21.0a_6 – for encounter 12 – if the patient’s BMI is 25 or more and they did not set a weight loss goal in encounter 9.
[56.2Script]
Appendix 9 Screenshots of depression and cardiovascular disease risk patient web portal
The Healthlines Service web portal: depression home page
(Note: only clickable tabs differed for CVD risk)
The Healthlines Service web portal: depression page
The Healthlines Service web portal: ‘Welcome Pack’ page
The Healthlines Service web portal: ‘Request a Call Back‘ page
The Healthlines Service web portal: ‘Letters to my GP‘ page
The Healthlines Service web portal: ‘Urgent Help‘ page
The Healthlines Service web portal: ‘My Heart Health‘ page (‘Information‘ tab)

The Healthlines Service web portal: ‘My Heart Health‘ page (‘Taking Your Blood Pressure‘ tab)
The Healthlines Service web portal: ‘My Heart Health‘ page (‘Enter New Blood Pressure Reading‘ tab)
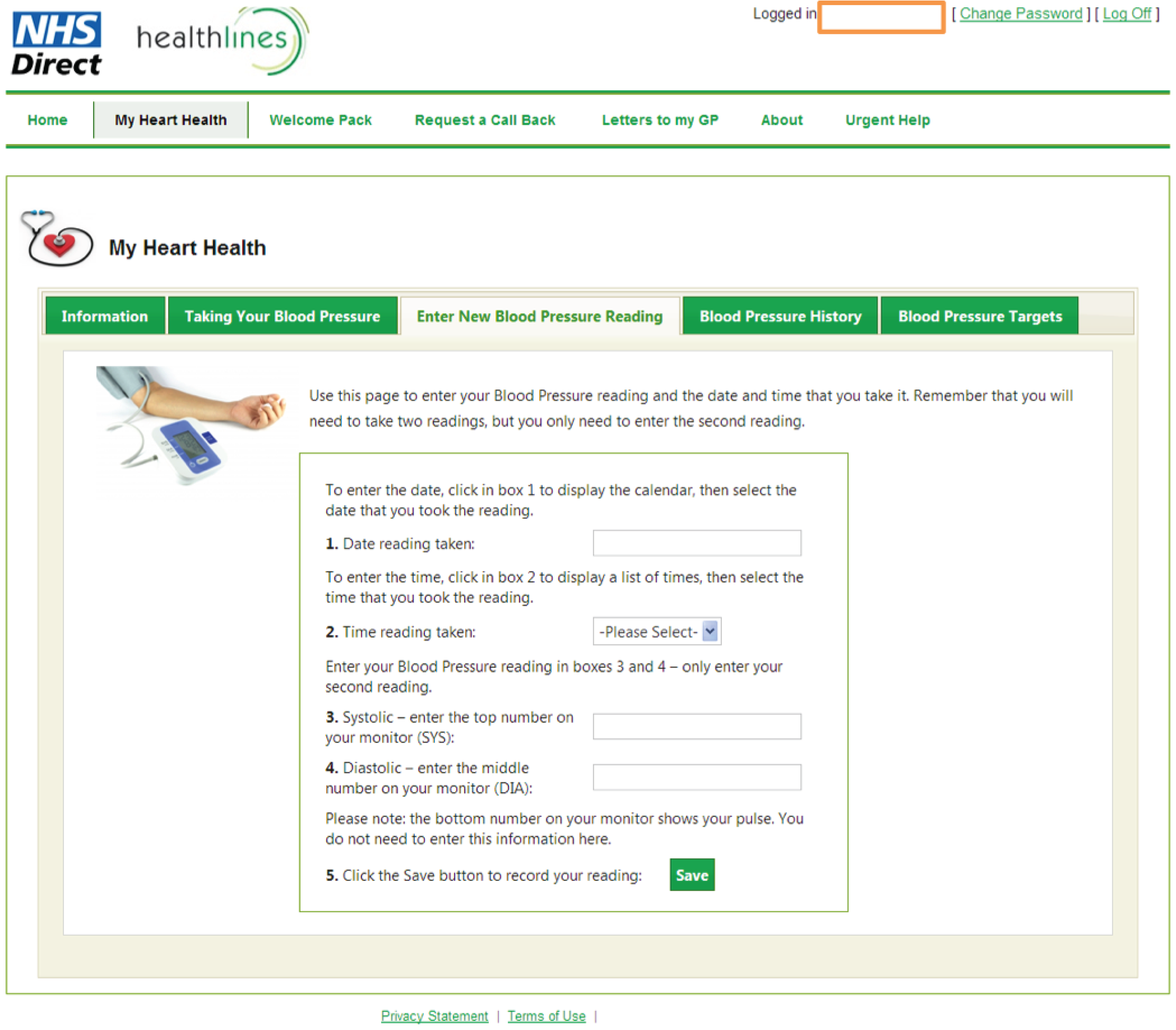
The Healthlines Service web portal: ‘My Heart Health‘ page (‘Enter New Blood Pressure Reading‘ tab, high reading warning)
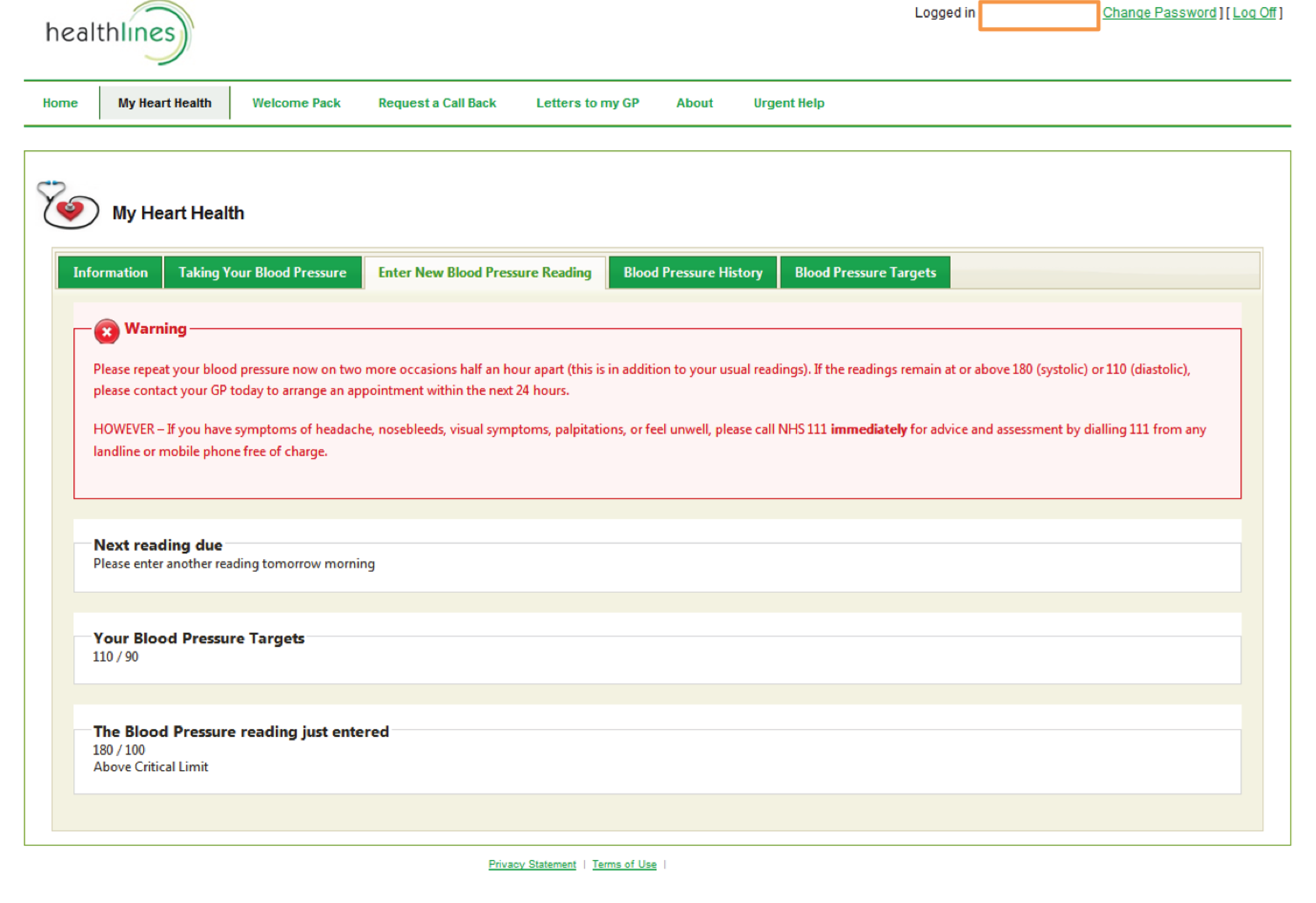
The Healthlines Service web portal: ‘My Heart Health‘ page (‘Blood Pressure History‘ tab)
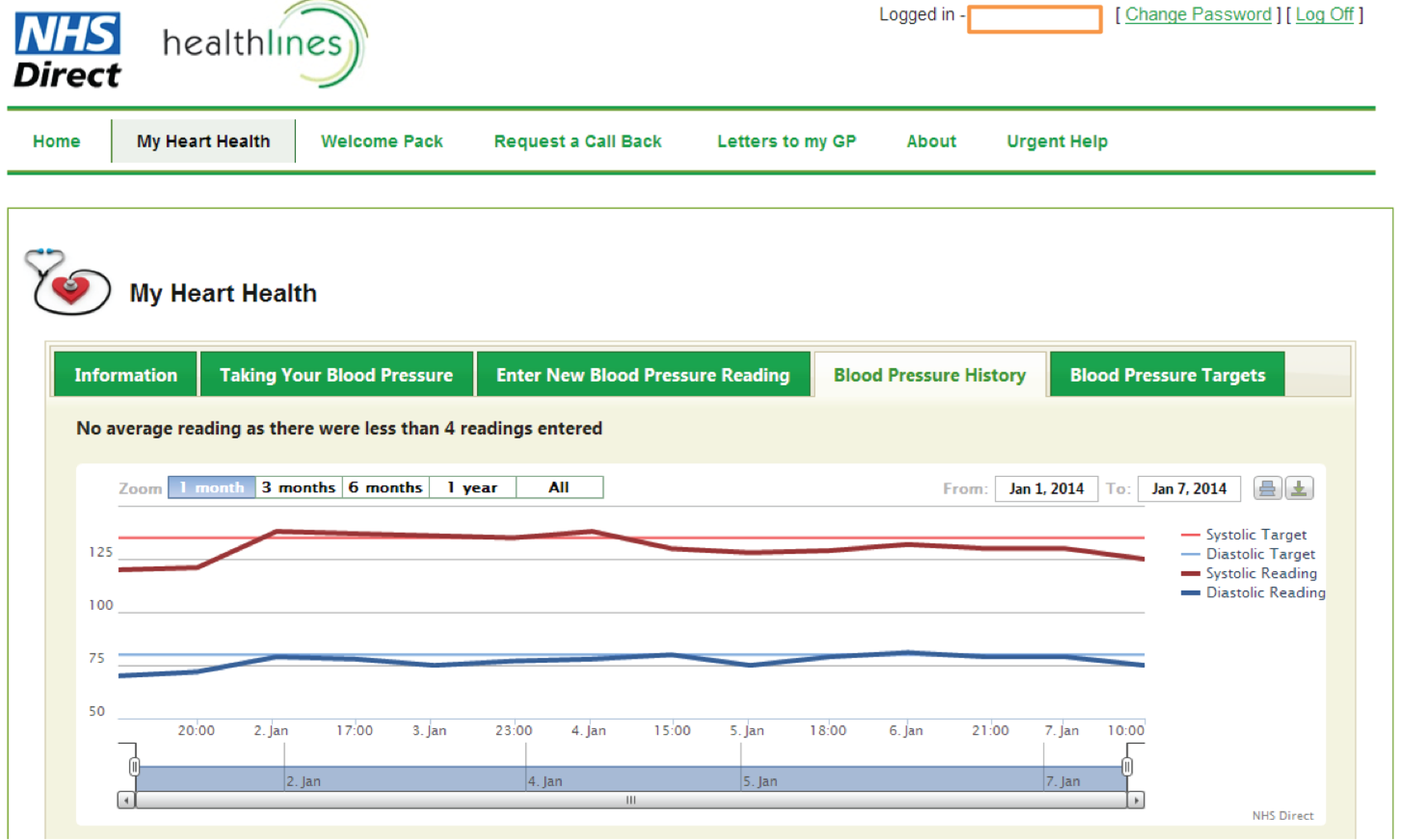
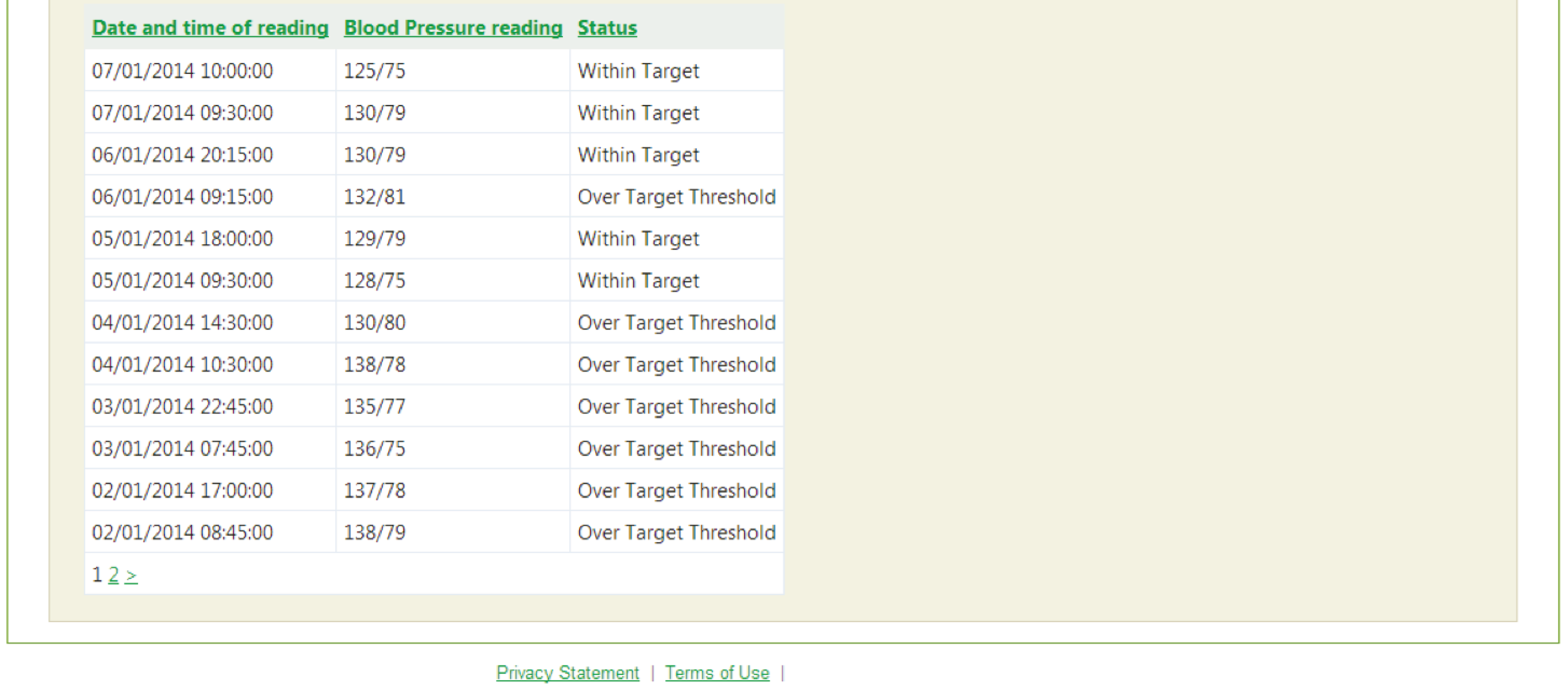
The Healthlines Service web portal: ‘My Heart Health’ page (‘Blood Pressure Targets’ tab)
© Copyright 2012 by NHS Direct.
Appendix 10 Welcome Pack (depression trial)
Appendix 11 Example screenshots from the Living Life to the Full Interactive programme
Note: All screenshots in this appendix have been reproduced with permission from Media Innovations Ltd.
Example of course content selection screen:

PHQ-9, GAD-7 and Work and Social Adjustment Scale data are collected and stored at the beginning of each session:
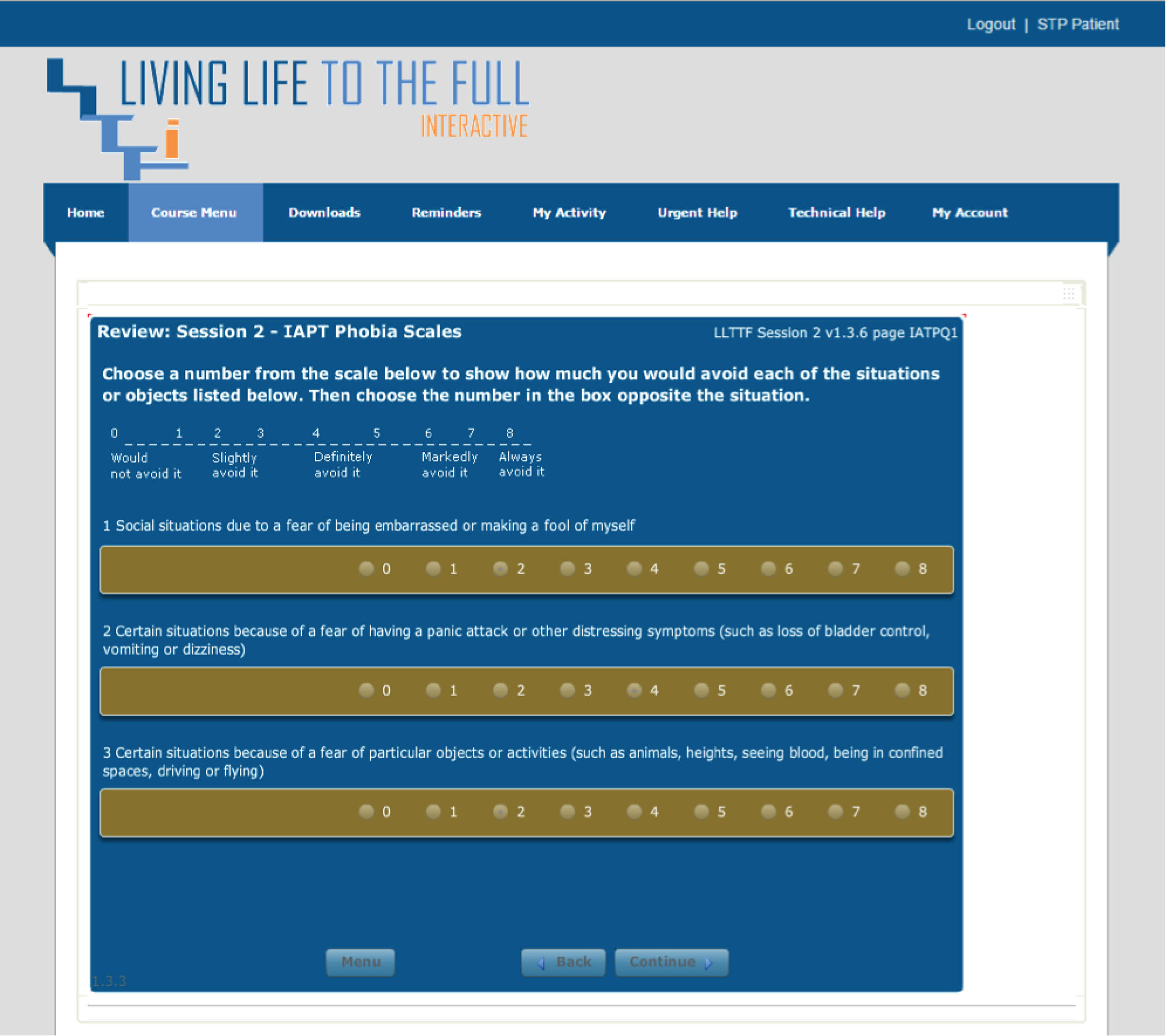
Living Life to the Full Interactive builds up a picture of the patients’ symptoms and contributory problems:
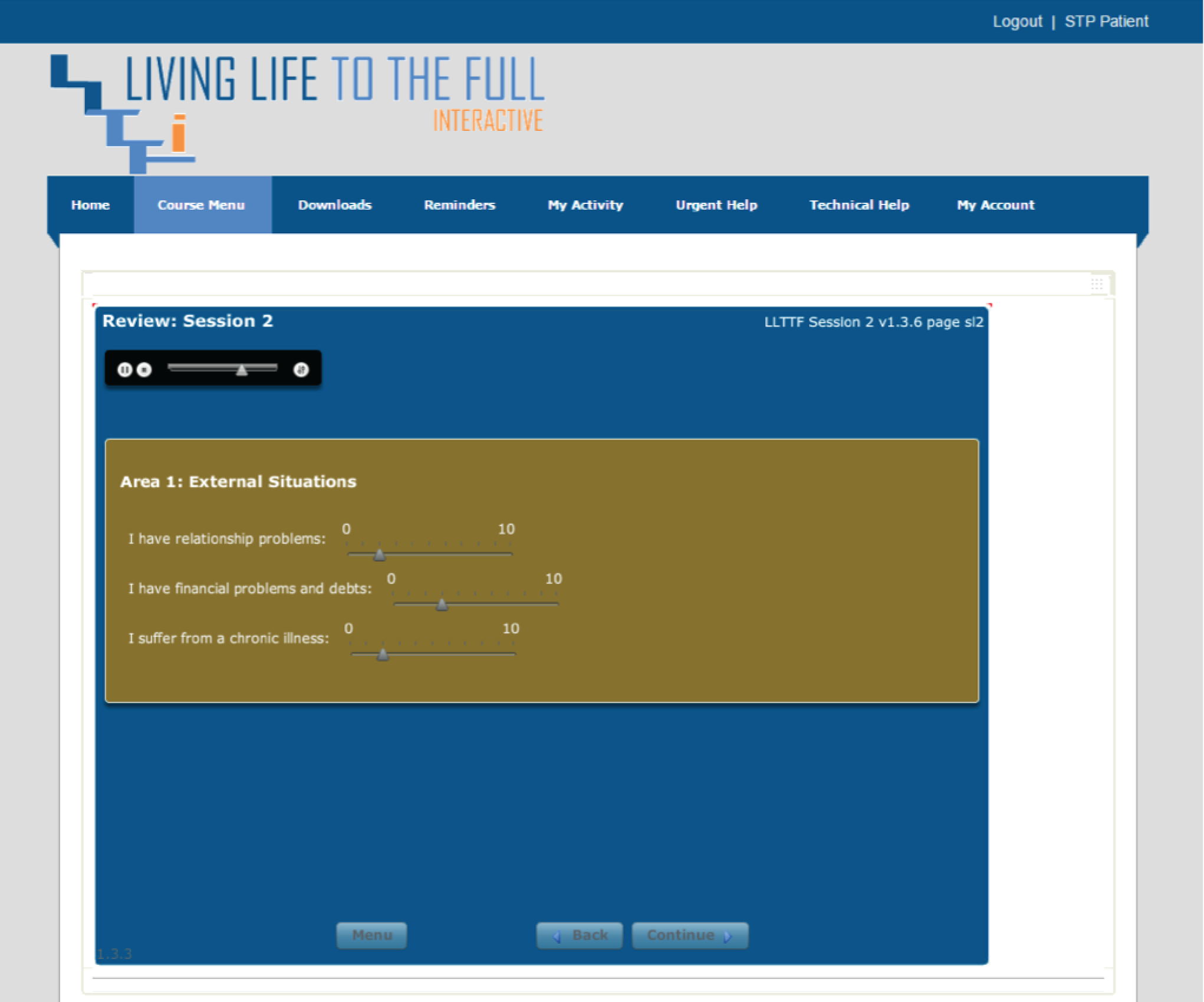
Interactive questions and responses lead the patient through content specific to both behaviour and thought problems:
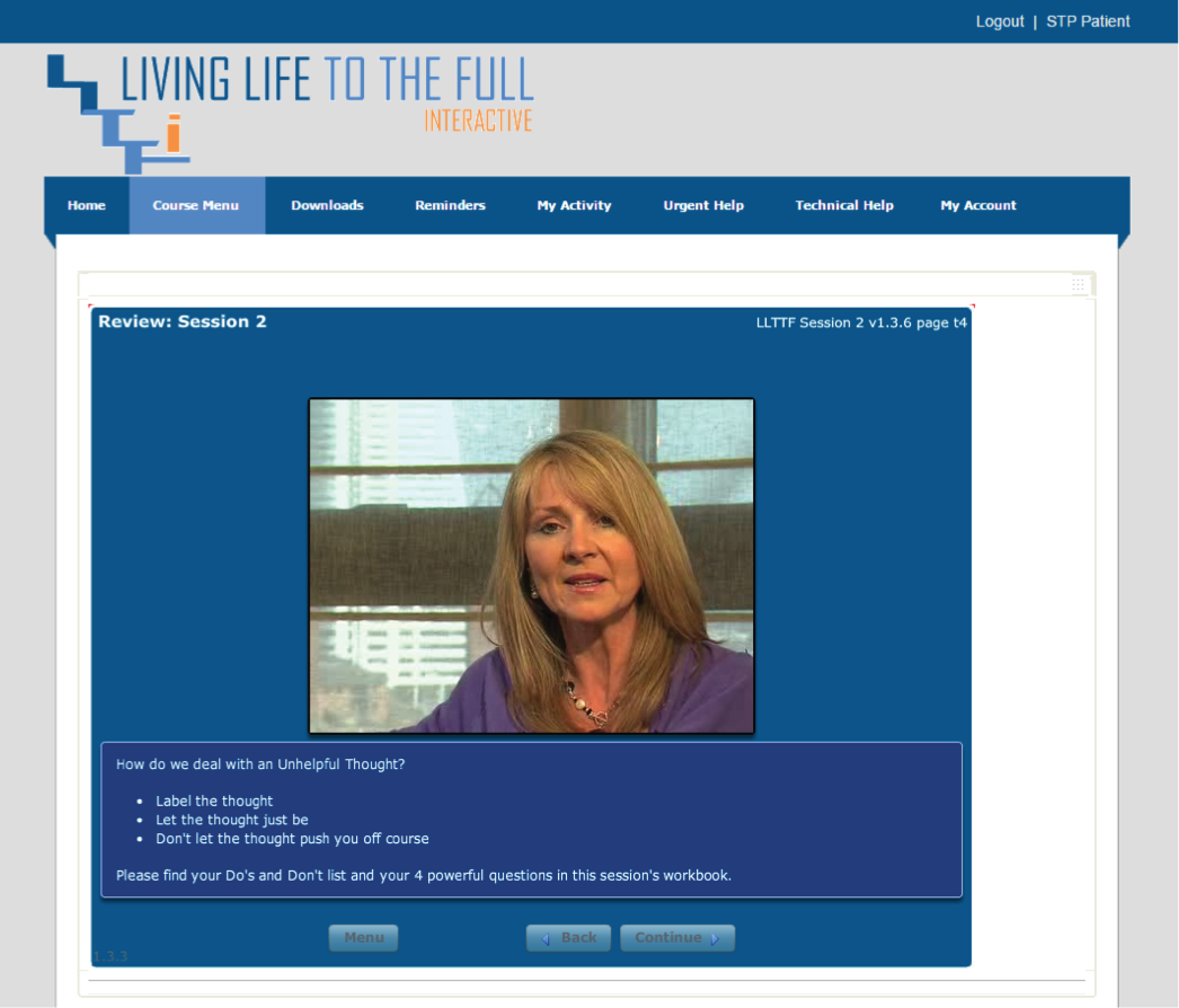
Each session sets a ‘homework’ task to be completed before the next session and responses can be reviewed by the HCP and patient:
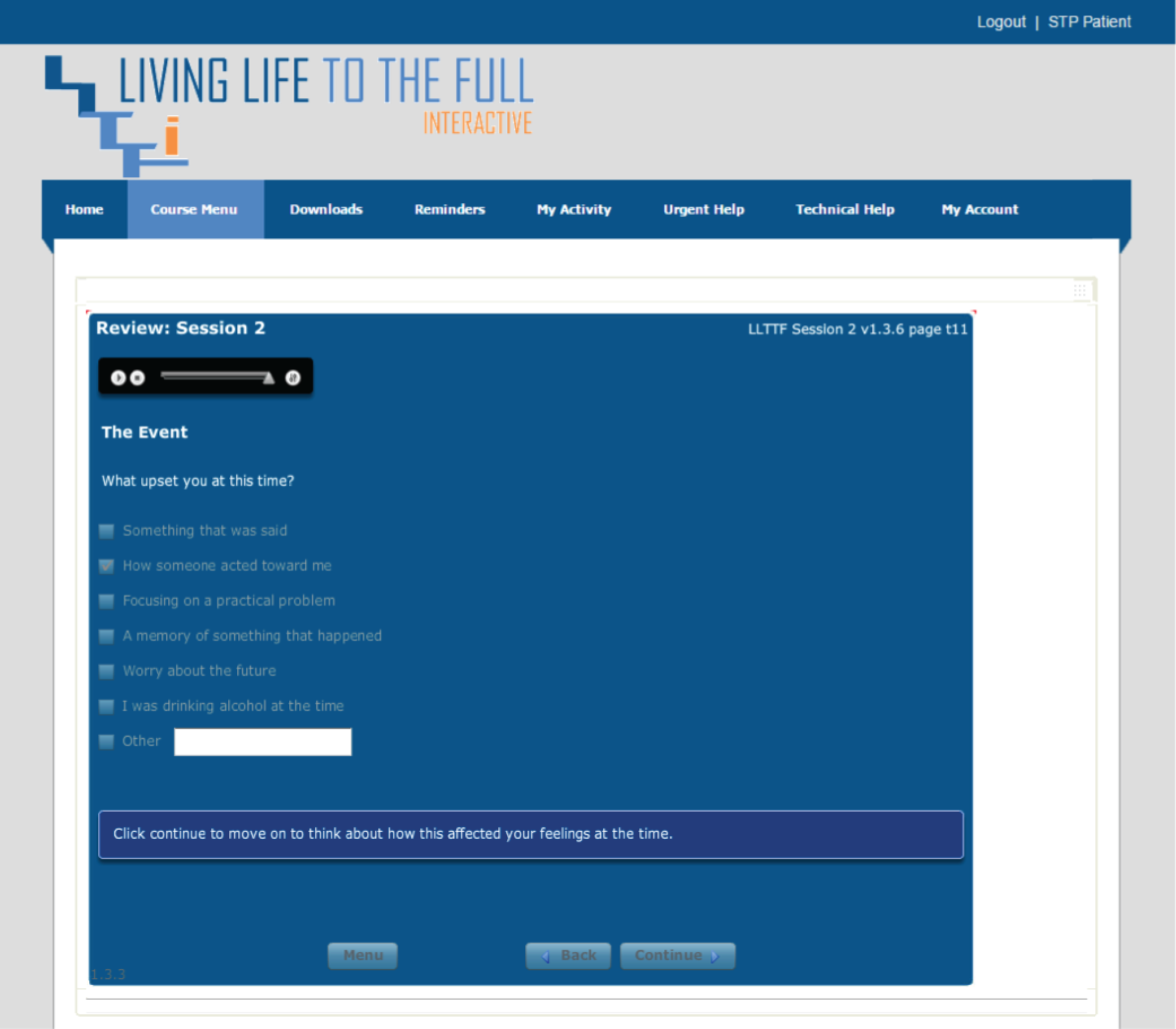
Alternative pathways through the content are offered to the patient to help address the most prevalent condition or symptom:

Once contributing thoughts or behaviours have been identified, positive coping strategies are explained and the patient is encouraged to implement these:
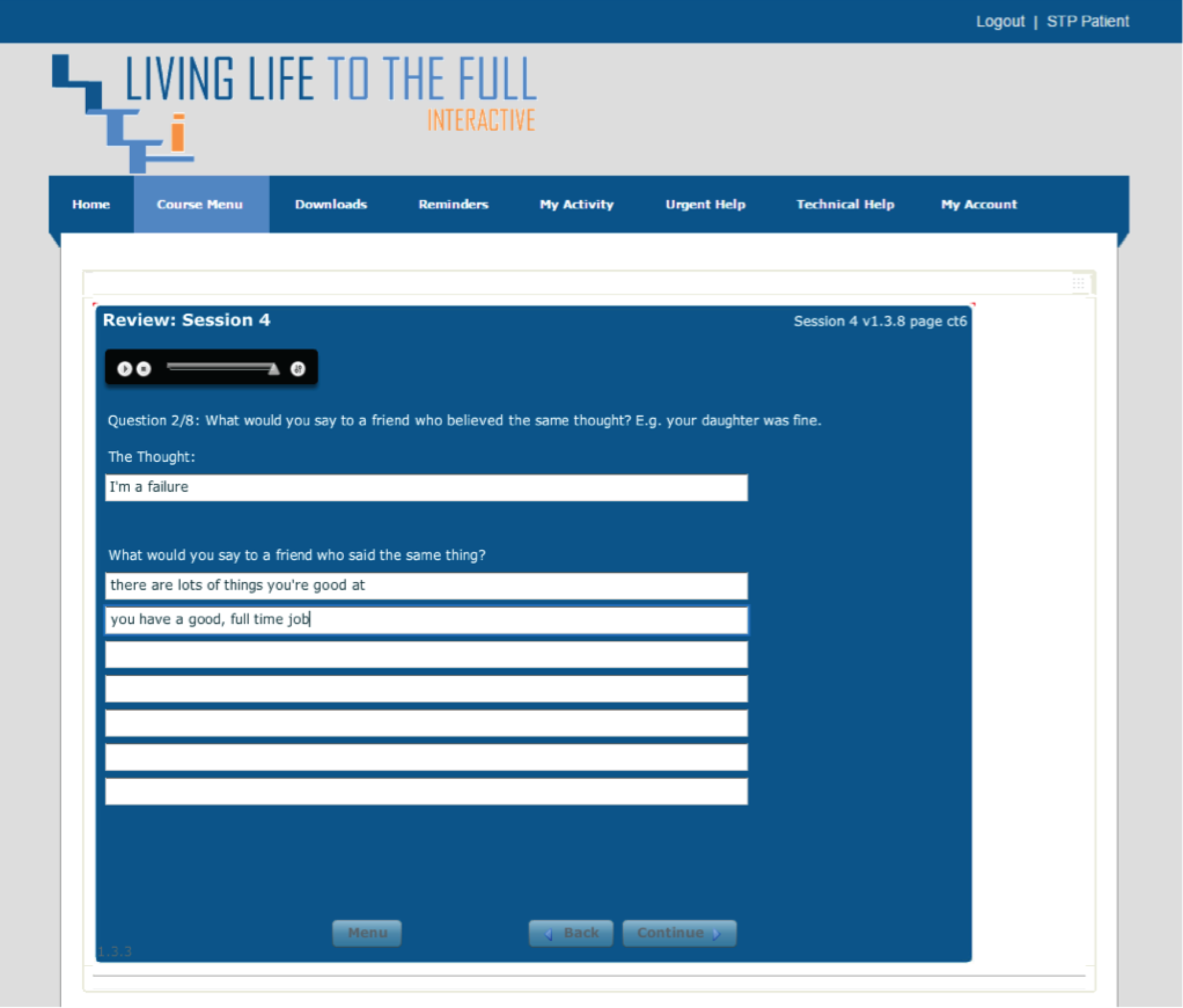
Self-assessment by the patient is taught and skills are developed to analyse and positively interpret situations and events:
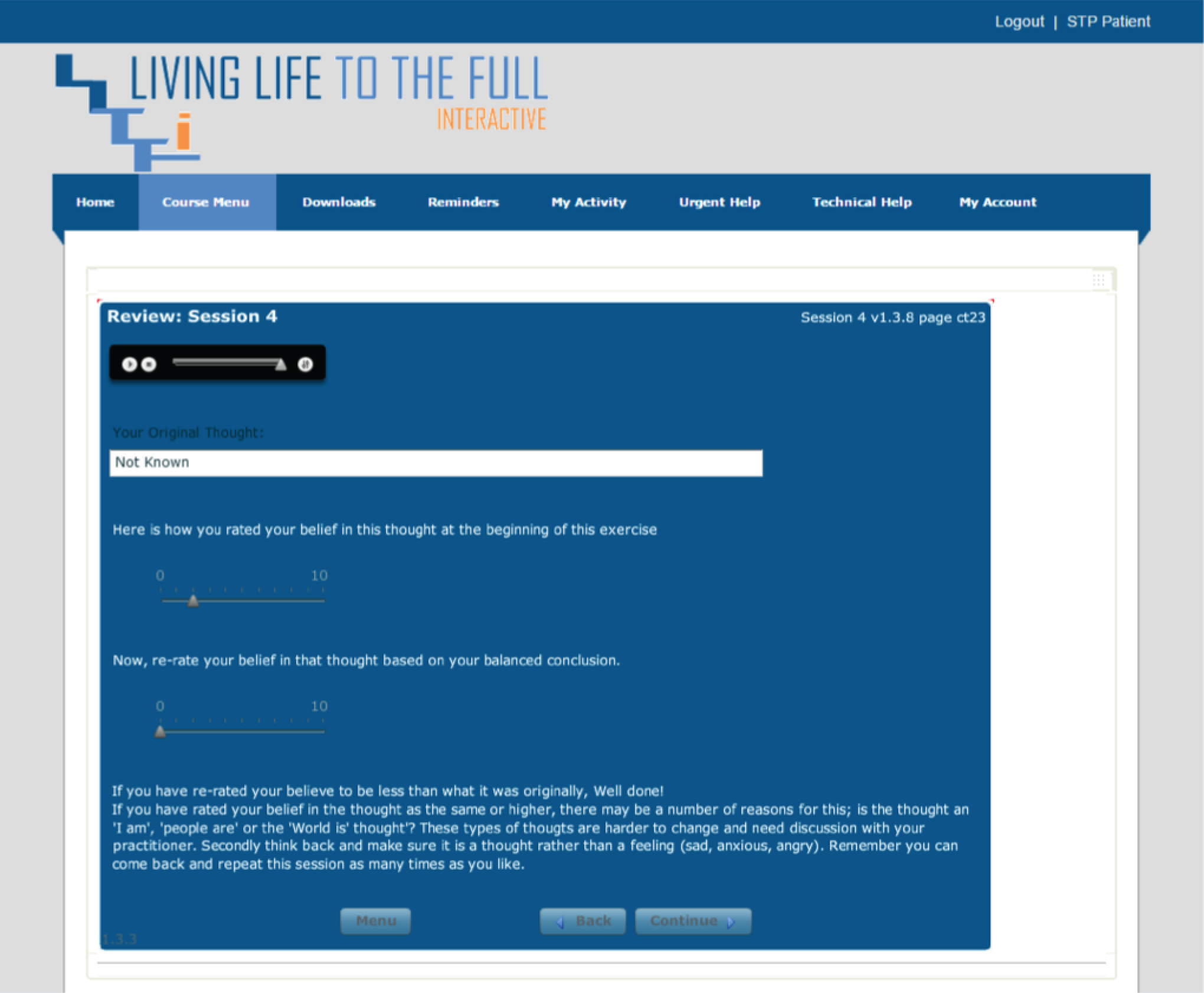
Appendix 12 Sample letter to depression patients’ general practitioner with update on PHQ-9 score

GP name The Healthlines Study
GP address line 1c/o Solent NHS Trust
Address line 2 Adelaide Health Centre
Address line 3Western Community Hospital Campus
Address line 4 William Macleod Way
Postcode Milbrook
Southampton
SO16 4XE
Telephone:
Today’s date
Patient’s name: (imported into note)
Patient’s date of birth: (imported into note)
Address: (imported into note)
NHS number: (imported into note)
Patient’s Healthlines study number: (imported into note)
PHQ-9 score
This patient is participating in the Healthlines Service. This is a Telecoaching Case Management programme designed to support patients with long-term health conditions.
This patient is taking part in the Healthlines Service because of depression.
This consists of regular phone calls from an advisor, helping the patient to make good use of resources available over the internet such as cognitive behaviour therapy.
As part of this service, we regularly assess the patient’s PHQ-9 score in order to monitor their progress. We inform you of these scores for your information.
Their latest PHQ-9 score was xxxxx on xxxx
Other important information for GP:
[free text for completion by HIA, if required]
Kind regards
[HIA name]
Healthlines Service
Appendix 13 Modular structure of intervention encounters in the depression trial
| Encounter 1 | Encounter 2 | Encounter 3 | Encounter 4 | Encounter 5 | Encounter 6 | Encounter 7 | Encounter 8 | Encounter 9 | Encounter 10 |
|---|---|---|---|---|---|---|---|---|---|
| Opening – initial patient contact and initial assessment | Opening – follow-up | Opening – follow-up | Opening – follow-up | Opening – follow-up | Opening – follow-up | Opening – follow-up | Opening – follow-up | ||
| PHQ-9 | PHQ-9 | PHQ-9 | PHQ-9 | ||||||
| Medicines review | Medicines review | Medicines review | Medicines review | Medicines review | Medicines review | Medicines review | Medicines review | Medicines review | |
| LLTTFi recap and introduction to session 1 For book users – discuss appropriate sections |
Review LLTTFi session 1 Introduce session 2 For book users – discuss appropriate sections |
Review LLTTFi session 2 Introduce session 3 For book users – discuss appropriate sections |
Review LLTTFi session 3 Introduce session 4 For book users – discuss appropriate sections |
Review LLTTFi session 4 Introduce session 5 For book users – discuss appropriate sections |
Review LLTTFi session 5 Introduce session 6 For book users – discuss appropriate sections |
Review LLTTFi session 6 For book users – discuss appropriate sections Maintenance Emergency plan |
Encourage patient to keep going with exercises learnt from CBT Discuss alternative sources of help if not improving. Maintenance Emergency plan |
Encourage patients to keep going with exercises learnt from CBT Discuss alternative sources of help if not improving Maintenance Emergency plan |
|
| Ask about BWW | Ask about BWW | Exercise | Alcohol | Revisit alcohol and exercise | |||||
| Closing script | Closing script | Closing script | Closing script | Closing script | Closing script | Closing script | End of course script (integral to encounter) | Closing script (integral to encounter) | Final closing script (integral to encounter) |
| GP letter –start of intervention | GP letter –PHQ-9 | GP letter – PHQ-9/end of course | GP letter – PHQ-9 | GP letter – PHQ-9/end of follow-up | |||||
| Next call in approximately 2 weeks | Next call in approximately 2 weeks | Next call in approximately 2 weeks | Next call in approximately 2 weeks | Next call in approximately 2 weeks | Next call in approximately 2 weeks | Next call in approximately 2 weeks | Follow-up call in approximately 2–3 months | Follow-up call in approximately 2–3 months | No further calls |
Appendix 14 Example screenshots of features available from the Big White Wall programme
Note: all screenshots in this appendix have been reproduced with permission from BigWhiteWall Ltd.
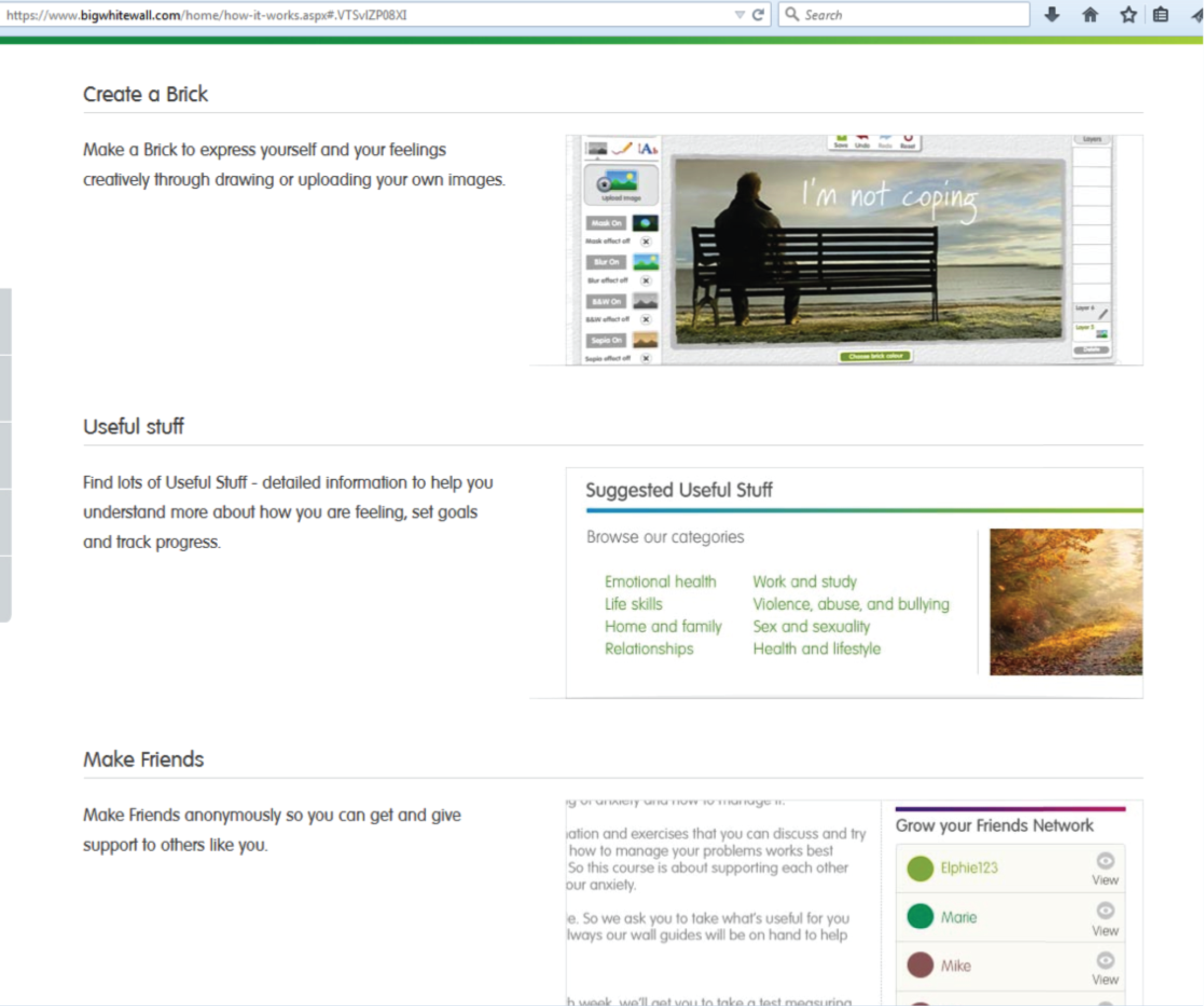
Appendix 15 Modular structure of intervention encounters in the cardiovascular disease risk trial
| Introductory encounter: 7–10 days before main modules | Encounter 13 – can occur any time | ||||||
|---|---|---|---|---|---|---|---|
| Module name | Module number | Module name | Module number | ||||
| Initial patient contact and initial assessment | 53 | Subject initiated | 26 | ||||
| Closing | 5 | ||||||
| Encounter 1 (note: introductory module comes before this) | Encounter 2 | Encounter 3 | Encounter 4 | ||||
| Module name | Module number | Module name | Module number | Module name | Module number | Module name | Module number |
| Opening | 52 | Opening | 52 | Opening | 52 | Opening | 52 |
| Medicines | 2 | Medicines | 2 | Medicines | 2 | Medicines | 2 |
| Side effects | 3 | Side effects | 3 | Side effects | 3 | Side effects | 3 |
| Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 |
| Review statins | 56 | Review statins | 56 | Review statins | 56 | Review statins | 56 |
| Memory | 4 | Core knowledge | 6 | Diet | 8 | Memory | 4 |
| Closing | 5 | Tobacco use | 13 | Weight loss | 9 | Exercise | 10 |
| (NRT module) | 55 | (Orlistat) | 54 | Weight loss 1 Mo FU | 21 | ||
| Closing | 5 | Tobacco 1 Mo FU | 17 | (Orlistat) | 54 | ||
| Closing | 5 | Tobacco 1 Mo FU | 17 | ||||
| Closing | 5 | ||||||
| Encounter 5 | Encounter 6 | Encounter 7 | Encounter 8 | ||||
| Module name | Module number | Module name | Module number | Module name | Module number | Module name | Module number |
| Opening | 52 | Opening | 52 | Opening | 52 | Opening | 52 |
| Medicines | 2 | Medicines | 2 | Medicines | 2 | Medicines | 2 |
| Side effects | 3 | Side effects | 3 | Side effects | 3 | Side effects | 3 |
| Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 |
| Review statins | 56 | Review statins | 56 | Review statins | 56 | Review statins | 56 |
| Exercise 1 Mo FU | 19 | Weight loss 1 Mo FU | 21 | Diet | 8 | Weight loss 1 Mo FU | 21 |
| Weight loss 1 Mo FU | 21 | (Orlistat) | 54 | Alcohol | 14 | (Orlistat) | 54 |
| (Orlistat) | 54 | Diet | 8 | Tobacco 1 Mo FU | 17 | Tobacco 6 Mo FU | 18 |
| Tobacco 1 Mo FU | 17 | Tobacco 1 Mo FU | 17 | Closing | 5 | Closing | 5 |
| Closing | 5 | Closing | 5 | ||||
| Encounter 9 | Encounter 10 | Encounter 11 | Encounter 12 | ||||
| Module name | Module number | Module name | Module number | Module name | Module number | Module name | Module number |
| Opening | 52 | Opening | 52 | Opening | 52 | Opening | 52 |
| Medicines | 2 | Medicines | 2 | Medicines | 2 | Medicines | 2 |
| Side effects | 3 | Side effects | 3 | Side effects | 3 | Side effects | 3 |
| Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 | Blood pressure medicines optimisation | 51 |
| Review statins | 56 | Review statins | 56 | Review statins | 56 | Review statins | 56 |
| Diet | 8 | Exercise 6 Mo FU | 20 | Alcohol | 14 | Weight loss 1 Mo FU | 21 |
| Weight loss 6 Mo FU | 22 | Weight loss 1 Mo FU | 21 | Weight loss 1 Mo FU | 21 | (Orlistat) | 54 |
| (Orlistat) | 54 | (Orlistat) | 54 | (Orlistat) | 54 | Tobacco 1 Mo FU | 17 |
| Tobacco 1 Mo FU | 17 | Tobacco 1 Mo FU | 17 | Tobacco 1 Mo FU | 17 | Closing | 5 |
| Closing | 5 | Closing | 5 | Closing | 5 | ||
Appendix 16 Sample letter to general practitioner of cardiovascular disease risk patient to request a review of blood pressure medication
Note: the NICE guidance contained within this sample letter was correct at the time of publication and comes from the following source: National Institute for Health and Clinical Excellence (2011) CG 127 Hypertension: Clinical Management of Primary Hypertension in Adults. London: NICE. 268 Available from www.nice.org.uk/CG127. Reproduced with permission. The NICE Bites summary contained within this sample letter is reproduced with permission from Editor: Banks L, North West Medicines Information Centre. NICE Bites; Hypertension, No 34: September 2011.
Appendix 17 Proportion of patients invited to the depression and cardiovascular disease risk trials who declined participation by selecting one of the prespecified reasons on the decline form
| Decline form reason | Depression, n (%) | CVD risk, n (%) | Total, n (%) |
|---|---|---|---|
| No internet access | 1834 (41.9) | 1491 (54.4) | 3325 (46.7) |
| No need for additional support with health issues | 1717 (39.3) | 1135 (41.4) | 2852 (40.1) |
| No computer confidence | 1580 (36.1) | 1225 (44.7) | 2805 (39.4) |
| Too busy | 1214 (27.8) | 718 (26.2) | 1932 (27.2) |
| Not interested | 648 (14.8) | 444 (16.2) | 1092 (15.3) |
| Do not understand what the research entailsa | 11/215 (5.1) | 5/91 (5.5) | 16/306 (5.2) |
| Other reason | 1021 (23.5) | 441 (16.1) | 1462 (20.5) |
Appendix 18 Baseline cardiovascular disease risk assessment form
Appendix 19 Further details on primary outcome deviations from the protocol
Every effort was made to collect the primary outcome on or as close as possible to a participant’s 12-month post-randomisation date. However, this was not always possible because of availability or other scheduling difficulties and so some of these assessments occurred > 30 days before or after their due date. Regardless, the data were included in the main analyses, although a sensitivity analysis was carried out to adjust for the length of time between randomisation and the actual assessment date.
Occasionally, one or more of the individual QRISK2 variables was not collected or provided on the assessment form (see Appendix 17). If it was not possible to collect the missing information from the participant, the QRISK2 algorithm imputed the missing variable(s).
Two main issues occurred with cholesterol data provided by general practices. First, practice staff could provide existing cholesterol results from the patient’s record if these results were within 3 months of the date of the baseline assessment or within 30 days of the 12-month assessment date. During a final visit to each general practice after completion of the 12-month follow-up, the local researcher checked the date and accuracy of the cholesterol results as recorded in each participant’s record. When cholesterol was measured prior to these threshold dates, or when it was not possible to calculate a ratio of total cholesterol to HDL cholesterol because only the total cholesterol result was provided, the cholesterol data were treated as missing and we allowed the QRISK2 algorithm to impute these data. Second, after internal data checking and cleaning carried out by the research team, a further baseline cholesterol reporting issue was discovered. In this case, the total cholesterol level at baseline was incorrectly provided in three of the general practices (hereafter, ‘the cholesterol issue’). In this instance, practice staff had been misreading the cholesterol result from the laboratory report and had been recording the ratio of total cholesterol to HDL cholesterol in place of the total cholesterol result on our assessment forms. This resulted in an artificially low ratio being included in the baseline QRISK2 score for patients from these practices, although only a small number of participants were affected (n = 6).
Two additional problems were identified with regard to the variables that are entered into the QRISK2 score. One was a calculation issue, in which the process for exporting data from the research database to the QRISK2 batch processor incorrectly labelled ex-smokers as ‘never smoked’ (hereafter, ‘the smoking issue’). Again, the effect of this error resulted in an underestimation of the QRISK2 score of ex-smokers. Finally, some data entry errors were also discovered, which affected the baseline QRISK2 score of six participants (hereafter, ‘the data entry issue’). Further detail on the scope of these issues and the actions taken are detailed below.
After discovering the issues described above, we recalculated the baseline CVD risk scores of all patients and identified that, for a small number of patients, this recalculation resulted in a shift in their eligibility for the trial. In total, we identified 29 patients of 961 with a baseline QRISK2 score (3%) who had a QRISK2 score of ≥ 20% but whom were incorrectly excluded as not eligible (QRISK2 score < 20%). Of these 29 patients, the error was due to the cholesterol issue in six patients, the smoking issue in 17 patients and the data entry issue in six patients. After their baseline assessment, these patients were told that their risk of having a heart attack or stroke in the next 10 years was less than one in five, so they were not at particularly high risk but should continue to follow a healthy lifestyle. They were told that the trial would not be suitable for them. After recalculation, most of these patients experienced a fairly small shift in their QRISK2 score, that is, the original (incorrect) QRISK2 scores were close to the threshold of 20% and the corrected scores were not much above this threshold. We also identified six patients who were randomised into the trial but who, on recalculation, were found to have a CVD risk of just under 20%. Consistent with an intention-to-treat principle, these participants were followed up and included in all analyses.
These issues were reported to each of the trial advisory committees (Trial Management Group, Programme Management Group, Data Monitoring Committee and the Trial Steering Committee), the ethics committee and the study sponsor. All were satisfied with the way in which these issues were managed and agreed with our proposed analyses. In addition, the GPs of those patients who, subsequent to discovering the smoking, cholesterol and data entry issues, were found to have a QRISK2 score of ≥ 20% were notified by letter. The letter explained the precipitating smoking, cholesterol or data entry issue, provided the GP with the patient’s recalculated QRISK2 score and asked the GP to review the patient’s notes in light of this information, should the patient require further treatment.
Appendix 20 Cardiovascular disease risk trial baseline questionnaire
Appendix 21 Cardiovascular disease risk trial 12-month follow-up questionnaire (intervention group)
Appendix 22 National average unit costs for primary care and personal social services
| Services | National average unit cost (£)a | Comments |
|---|---|---|
| GP clinic consultation | 34.00 | Based on a 11.7-minute consultation |
| GP telephone consultation | 20.00 | Based on a 7.1-minute consultation |
| GP home visit | 85.00 | Based on a 23.4-minute visit |
| Out-of-hours GP service face-to-face at clinic | 25.03 | Estimate derived from Scott et al.425 and increased by 22% as per O’Dowd,426 inflated to 2012/13 prices |
| GP out-of-hours service home visit | 62.56 | Adjusted for duration in line with GP consultations |
| GP out-of-hours service telephone consultation | 14.72 | Adjusted for duration in line with GP consultations |
| Practice nurse clinic consultation | 11.37 | Based on 15.5-minute consultation |
| Practice nurse telephone consultation | 6.69 | Based on 9.4-minute consultation |
| Other primary and community care services | ||
| Community mental health nurse home visit | 65.00 | Assume 1-hour consultation |
| Community mental health nurse telephone consultation | 21.67 | Assume average length of call 20 minutes |
| Community support worker clinic consultation | 24.50 | Assume 30-minute consultation |
| Community support worker home visit | 49.00 | Assume 1-hour consultation |
| Community support worker telephone consultation | 16.33 | Assume average length of call 20 minutes |
| Counsellor clinic consultation | 58.00 | Assume 55-minute consultation |
| Counsellor telephone consultation | 15.75 | Assume average length of call 15 minutes |
| Dietitian | 14.67 | Assume 20-minute consultation |
| District nurse home visit | 60.00 | Includes non-contact time related to the visit; assume visit plus these activities take 1 hour |
| District nurse telephone consultation | 10.50 | Assume average length of call 15 minutes |
| Health trainer | 14.67 | Same as practice nurse but assume appointment time of 20 minutes |
| Midwife | 12.75 | Assume 15-minute consultation |
| NHS walk-in centre | 34.06 | From Munro et al.,427 inflated to 2012/13 prices |
| NHS walk-in centre telephone consultation | 22.23 | From Munro et al.,427 inflated to 2012/13 prices |
| Occupational therapist clinic consultation | 34.00 | Assume 23-minute consultation |
| Occupational therapist home visit | 62.09 | Assume 42-minute visit |
| Occupational therapist telephone consultation | 18.72 | Assume average length of call 12.7 minutes |
| Phlebotomist consultation | 6.46 | Assume 15.5-minute consultation |
| Physician associate | 22.00 | Costed as per specialist nurses |
| Physiotherapist | 30.00 | Assume 1-hour consultation |
| Podiatry | 30.00 | Assume 1-hour consultation |
| Social worker clinic consultation | 79.50 | Assume 30-minute consultation |
| Social worker home consultation | 159.00 | Assume 1-hour visit |
| Social worker telephone consultation | 53.00 | Assume average length of call 20 minutes |
| Specialist nurse (e.g. asthma nurse) | 22.00 | Costed per consultation |
Appendix 23 National average unit costs of hospital-related services
| Services | National average unit cost (£)a |
|---|---|
| CVD risk | |
| Inpatient stays | |
| Arrhythmia or conduction disorders with a CC score of 4–6 | 571 |
| Major knee procedures for non-trauma, category 2, without CC | 5676 |
| Minor vascular interventional radiology procedures | 766 |
| Syncope or collapse with a CC score of 0–3 | 528 |
| Unspecified chest pain with a CC score of 0–4 | 533 |
| Day care and outpatient visits | |
| Arrhythmia or conduction disorders with a CC score of 0–3 | 818 |
| Cardiology: non-admitted face-to-face attendance, first | 167 |
| Cardiology: non-admitted face-to-face attendance, follow-up | 126 |
| Computerised tomography scan, one area, no contrast, 19 years and over | 104 |
| Diabetics: non-admitted face-to-face attendance, first | 189 |
| Diabetics: non-admitted face-to-face attendance, follow-up | 142 |
| Electrocardiogram monitoring and stress testing (day case) | 477 |
| Electrocardiogram monitoring and stress testing (outpatient) | 136 |
| General medicine: non-admitted face-to-face attendance, follow-up | 102 |
| Nephrology: non-admitted face-to-face attendance, follow-up | 116 |
| Nuclear medicine, category 3 | 196 |
| Ophthalmology: non-admitted face-to-face attendance, follow-up | 80 |
| Percutaneous standard ablation, with a CC score of 0–2 | 2367 |
| Simple echocardiogram, 19 years and over | 77 |
| Unspecified chest pain with a CC score of 0–4 | 690 |
| Accident and emergency | |
| Accident and emergency – type 01 non-admitted | 96 |
| Paramedic/ambulance | |
| See and treat or refer | 196 |
| See and treat and convey | 255 |
| Depression | |
| Inpatient stays | |
| Syncope or collapse with a CC score of 0–3 | 382 |
| Alcohol services, adult, admitted patient | 349 |
| All patients between 19 and 69 years with a mental health primary diagnosis, treated by a non-specialist mental health service provider | 376 |
| Syncope or collapse with a CC score of 0–3 | 528 |
| Unspecified chest pain with a CC score of 0–4 | 533 |
| Day care and outpatient visits | |
| Adult mental illness: non-admitted face-to-face attendance, non consultant-led, first | 34 |
| Adult mental illness: non-admitted face-to-face attendance, consultant-led follow-up | 232 |
| Alcohol services, adult, outpatient attendances | 104 |
| Counsellor | 58 |
| Drug and alcohol services, adult and elderly | 77 |
| Endocrinology: multiprofessional non-admitted face-to-face attendance, follow-up | 162 |
| General medicine: all patients between 19 and 69 years with a mental health primary diagnosis, treated by a non-specialist mental health service provider | 288 |
| General medicine: syncope or collapse with a CC score of 0–3 | 366 |
| Group therapy | 28 |
| IAPT, adult and elderly | 104 |
| Occupational therapist | 63 |
| Pain management: non-admitted face-to-face attendance, first | 171 |
| Pain management: non-admitted face-to-face attendance, follow-up | 136 |
| Psychiatrist | 261 |
| Specialist nurse | 123 |
| Urology: non-admitted face-to-face attendance, first | 123 |
| Weighted average of all adult outpatient attendances (excluding elderly people) | 100 |
| Accident and emergency | |
| Accident and emergency – type 01 non-admitted | 96 |
| Paramedic/ambulance | |
| See and treat and convey | 255 |
Appendix 24 Further information on elements of long-term simulation modelling of the Healthlines cardiovascular disease risk trial data
Transient ischaemic attack utility
Data on TIA utility were based on information contained in the study by Luengo-Fernandez et al. 351 In this study data were collected from 440 TIA patients and 748 stroke patients. Surviving patients were followed up at 1, 6, 12, 24 and 60 months after the event and completed the EQ-5D-3L questionnaire. Quality of life in these patients was compared with that in a set of control subjects taken from the 2006 HSE. These data were used in a slightly different way from other sources of utility data for the following reasons. First, using the difference between TIA cases and control subjects resulted in a utility ratio that was 90% of baseline utility, with baseline in this case calculated as the utility of the sex- and age-adjusted matched control subjects. This is bigger than the impact of stroke in the HSE data and is not a tenable assumption. Conversely, using the utility impact for stroke from this paper and expressing it as a ratio of baseline utility estimated by the equation from Ara and Brazier350 gave a utility for females that was > 1, which is an inadmissible utility value. The approach adopted was to calculate the ratio of TIA cases to control subjects in the study by Luengo-Fernandez et al. 351 and use this ratio as a multiplier. The same multiplier was used for men and women. This multiplier was not subject to uncertainty analysis in the absence of information on the covariance of utility. The post-primary TIA utility value was calculated as the average of the 24- and 60-month TIA values reported in the study by Luengo-Fernandez et al. 351
Multiplicative utility adjustments
An example of how the multipliers are used to adjust baseline utility for the impact of experiencing one of the CVD events is as follows: the mean age at which a first stroke was reported in the data presented in the study by Ara and Brazier350 was 67.9 years, corresponding to a reported average utility of 0.626. The baseline utility for a male without a stroke at this age was estimated to be 0.82. The ratio of utilities – stroke to baseline – gives a multiplier of 0.76, which can then be used to adjust the baseline utility for stroke patients of any age. The same process was used to calculate multipliers for females and for the other health states.
Utility values of model health states
Mean health state utility values are listed in Table 135. The primary source for all utilities other than those relating to TIA was Ara and Brazier. 350 Information on TIA health state utilities was based on Luengo-Fernandez et al. 351
| Health state | Mean utility value |
|---|---|
| Primary stable angina | 0.615 |
| Primary unstable angina | 0.556 |
| Primary AMI | 0.721 |
| Primary TIA | 0.760 |
| Primary stroke | 0.626 |
| Post-primary angina | 0.775 |
| Post-primary unstable angina | 0.701 |
| Post-primary AMI | 0.742 |
| Post-primary TIA | 0.78 |
| Post-primary stroke | 0.668 |
| Secondary stable angina | 0.541 |
| Secondary unstable angina | 0.489 |
| Secondary AMI | 0.431 |
| Secondary TIA | 0.760 |
| Secondary stroke | 0.479 |
| Post-secondary stable angina | 0.715 |
| Post-secondary unstable angina | 0.647 |
| Post-secondary AMI | 0.685 |
| Post-secondary TIA | 0.78 |
| Post-secondary stroke | 0.641 |
As discussed in Utility (see Chapter 7), any ‘third’ events, (i.e. those occurring after a secondary event) have the same utility as the ‘second’ event. Hence, a third stroke has the same utility impact as a second stroke.
Permitted transitions between states in the simulation model
Figure 13 summarised the list of permitted transitions between the states in the simulation model. Further detail on the list of permitted transitions is provided in Box 6. This is the same as the list of permitted transitions described in Ara et al. 338
Event free to primary SA
Event free to primary USA
Event free to primary AMI
Event free to primary TIA
Event free to primary Stroke
Event free to FCVD
Event free to DOC
Primary states to subsequent statesPrimary_SA to secondary_(USA or first AMI or first stroke or FCVD or DOC) else post SA
Primary_USA to secondary_(first AMI or first stroke or FCVD or DOC) else post USA
Primary_AMI to secondary_(first AMI or first stroke or FCVD or DOC) else post AMI
Primary_TIA to secondary_(first AMI or first stroke or FCVD or DOC) else post TIA
Primary_stroke to secondary_(first stroke or FCVD or DOC) else post stroke
Post-primary states to subsequent statesPost primary_SA to secondary_(USA or first AMI or first stroke or FCVD or DOC) else post SA
Post primary_USA to secondary_(first AMI or first stroke or FCVD or DOC) else post USA
Post primary_AMI to secondary_(first AMI or first stroke or FCVD or DOC) else post AMI
Post primary_TIA to secondary_(first AMI or first stroke or FCVD or DOC) else post TIA
Post primary_stroke to secondary_(first stroke or FCVD or DOC) else post stroke
Secondary state transitionsSecondary_SA to secondary_(USA or second AMI or second stroke or FCVD or DOC) else post SA
Secondary_USA to secondary_(second AMI or second stroke or FCVD or DOC) else post USA
Secondary_AMI to secondary_(second AMI OR second stroke OR FCVD OR DOC) else post AMI
Secondary_TIA to secondary_(second AMI or second stroke or FCVD or DOC) else post TIA
Secondary_stroke to secondary_(second stroke or FCVD or DOC) else post stroke
Post secondary_SA to secondary_(USA or second AMI or second stroke or FCVD or DOC) else post SA
Post secondary_USA to secondary_(second AMI or second stroke or FCVD or DOC) else post USA
Post secondary_AMI to secondary_(second AMI or second stroke or FCVD or DOC) else post AMI
Post secondary_TIA to secondary_(second AMI or second stroke or FCVD or DOC) else post TIA
Post secondary_stroke to secondary_(second stroke or FCVD or DOC) else post stroke
DOC, death other causes; FCVD, fatal cardiovascular disease event; SA, stable angina; USA, unstable angina.
Source: the information in this table was taken from Table 82 in Ara et al. 338 Contains information licensed under the Non-Commercial Government Licence v1.0.
Appendix 25 Primary cardiovascular disease event incidence rates used in the simulation model
| Age band (years) | Stable angina | Unstable angina | Non-fatal AMI | Fatal AMI | TIA | Non-fatal stroke | Fatal stroke |
|---|---|---|---|---|---|---|---|
| 55–64 | 0.84 | 0.25 | 0.28 | 0.04 | 0.74 | 0.65 | 0.08 |
| 65–74 | 1.16 | 0.35 | 0.42 | 0.11 | 1.45 | 1.27 | 0.16 |
| 75–84 | 0.75 | 0.23 | 0.73 | 0.29 | 3.27 | 2.73 | 0.35 |
| 85+ | 0.75 | 0.23 | 1.23 | 0.76 | 7.94 | 5.12 | 0.65 |
| Age band (years) | Stable angina | Unstable angina | Non-fatal AMI | Fatal AMI | TIA | Non-fatal stroke | Fatal stroke |
|---|---|---|---|---|---|---|---|
| 55–64 | 0.35 | 0.11 | 0.07 | 0.02 | 1.05 | 0.63 | 0.14 |
| 65–74 | 0.74 | 0.22 | 0.18 | 0.06 | 2.18 | 1.23 | 0.28 |
| 75–84 | 0.57 | 0.17 | 0.38 | 0.22 | 5.61 | 2.64 | 0.60 |
| 85+ | 0.57 | 0.17 | 0.75 | 0.64 | 9.14 | 4.95 | 1.13 |
| Age band (years) | Stable angina | Unstable angina | Non-fatal AMI | Fatal AMI | TIA | Non-fatal stroke | Fatal stroke |
|---|---|---|---|---|---|---|---|
| 55–64 | 29 | 9 | 10 | 2 | 26 | 22 | 3 |
| 65–74 | 24 | 7 | 9 | 2 | 29 | 26 | 3 |
| 75–84 | 9 | 3 | 9 | 3 | 39 | 33 | 4 |
| 85+ | 5 | 1 | 7 | 5 | 48 | 31 | 4 |
| Age band (years) | Stable angina | Unstable angina | Non-fatal AMI | Fatal AMI | TIA | Non-fatal stroke | Fatal stroke |
|---|---|---|---|---|---|---|---|
| 55–64 | 15 | 4 | 3 | 1 | 44 | 26 | 6 |
| 65–74 | 15 | 5 | 4 | 1 | 45 | 25 | 6 |
| 75–84 | 6 | 2 | 4 | 2 | 55 | 26 | 6 |
| 85+ | 3 | 1 | 4 | 4 | 53 | 29 | 7 |
Appendix 26 Costs of treatments and health states used in the cohort simulation model
Cost of stable angina
The cost of stable angina was based on the studies by Ward et al. 340 and Ara et al. 339 The cost of year 1 angina (i.e. the year in which the condition was diagnosed) was assumed to include the costs of three GP appointments, three outpatient appointments and medication costs. The cost of GP appointments was taken from Curtis. 332 Outpatient costs for stable angina were estimated from NHS reference costs (2012/13)333 as the costs of a first and subsequent appointment for non-admitted face-to-face outpatient attendances under the ‘Cardiology’ service description. This was calculated as a weighted average of consultant-led and non-consultant-led consultations. Medication costs were based on the following assumptions: 90% of patients receive glyceryl trinitrate spray, isosorbide mononitrate and verapamil, atenolol or diltiazem; 90% receive aspirin; 90% receive ramipril or angiotensin-converting enzyme inhibitors and the other 10% receive a generic angiotensin receptor blocker. Additionally, 90% of patients were assumed to use one statin daily, assumed to be 40 mg of simvastatin. Stable angina in subsequent years was costed in the same way, except that no outpatient appointments were assumed to take place.
Cost of unstable angina
The cost of unstable angina was based on the studies by Ward et al. 340 and Ara et al. 339 The cost of unstable angina in the year of diagnosis was assumed to include the costs of three GP visits, three outpatient visits (both costed in the same way as for stable angina) and the same medication as for stable angina with the additional assumption that 90% of patients receive clopidogrel. Additionally, it was assumed that 50% of patients undergo revascularisation, costed as 77% of the cost of percutaneous coronary intervention and 23% of the cost of coronary artery bypass graft. All patients were assumed to be hospitalised, the costs of which were assumed to be equal to half the cost of a cardiac arrest.
The costs of unstable angina in subsequent years were based on the three GP visits, 25% of patients receiving the same outpatient procedures as in the year of diagnosis and the same medication as for stable angina.
Cost of non-fatal acute myocardial infarction
The cost of a non-fatal AMI was based on the studies by Ward et al. 340 and Ara et al. 339 It was assumed that patients will have three GP appointments and receive the same medication as for year 1 of stable angina. It was assumed that 50% of patients will undergo revascularisation (costed as for unstable angina) and that 25% of patients will incur the cost of actual or suspected cardiac arrest. The cost of non-fatal AMI in the years after the initial event were based on three GP appointments, 25% of patients having an outpatient visit and the same medication costs as for year 1 stable angina.
Cost of fatal acute myocardial infarction
It was assumed that only 50% of fatal AMI cases incur a cost, an assumption similar to that in the study by Ara et al. 339 The costs of the 50% of cases who do incur a cost were based on the study by Clarke et al. ,428 inflated to 2012/13 prices using the Hospital and Community Health Service (HCHS) pay and inflation index. 332 Clarke et al. 428 used prospectively collected data from a large diabetes trial to obtain estimates of the costs of major diabetes-related complications.
Cost of non-fatal stroke
The cost of stroke was based on the study by Luengo-Fernandez et al. ,429 who reported cost estimates from a UK population-based cohort study of TIA and stroke patients over 5 years of follow-up up to 2010. The paper reports mean 5-year hospital costs for stroke survivors, noting the proportion of costs that were incurred in the first year after stroke. This proportion was assumed to represent costs in the year of diagnosis, whereas the remaining costs were averaged to give an estimate of costs in subsequent years. Costs were expressed in 2012/13 prices after inflating by the HCHS pay and inflation index332 and after converting the dollar estimates into UK pounds using the currency conversion factor reported in the paper.
Cost of fatal stroke
The cost of a fatal stroke was based on the study by Youman et al. 430 This source was used instead of the study by Luengo-Fernandez et al. 429 because the latter study did not separately report the costs of fatal and non-fatal stroke. The study by Youman et al. 430 described a burden of illness model developed using data from a stroke RCT and other sources. Costs were inflated from 2001/2 prices.
Cost of transient ischaemic attack
The cost of TIA was based on the study by Luengo-Fernandez et al. ,429 the same source used for stroke. The costs were calculated in the same way as for non-fatal stroke – the proportion of costs incurred in the first year was taken to equal costs in the year after diagnosis and subsequent year costs were obtained from the annual average of costs of survivors in subsequent years. Costs were converted to UK pounds and inflated to 2012/13 prices using the HCHS pay and inflation index. 332
Appendix 27 Topic guide for NHS Direct staff
-
Can you describe the role you have within the Healthlines study?
-
Do you think this is a useful intervention? Please explain why.
-
Prompt: (i) in general, (ii) for depression, (iii) for CVD risk factors.
-
-
Is it operating well in practice? Please explain why.
-
Prompt: Any differences for the two conditions?
-
-
How engaged are key people with the intervention?
-
Prompt: (i) the patients, (ii) primary care staff, (iii) anyone else important to the intervention.
-
-
Do you feel the intervention helps patients to self-manage? Please explain.
-
Do you feel the intervention helps to optimise treatment for patients? Please explain.
-
Do you feel you have been able to facilitate co-ordinated care? Please explain.
-
How has communication been between you and primary care staff?
-
How has the intervention fit with other services already provided by the practice or in the locality?
-
What helps the intervention to work well?
-
What prevents the intervention from performing well?
-
Which patient groups are most likely to benefit from this intervention? Why?
-
Which patient groups are least likely to benefit from the intervention? Why?
-
What benefits (if any) do you think it offers patients?
-
Any other comments.
Appendix 28 Topic guide for primary care staff
-
Can you describe your role in relation to the Healthlines study?
-
Do you think this is a useful intervention? Please explain why.
-
Prompt: (i) in general, (ii) for depression, (iii) for CVD risk factors.
-
-
Is it operating well in practice? Please explain why.
-
Prompt: Any differences for the two conditions?
-
-
How engaged are key people with the intervention?
-
Prompt: (i) the patients, (ii) primary care staff, (iii) anyone else important to the intervention.
-
-
Do you feel the intervention helps patients to self-manage? Please explain.
-
Do you feel the intervention helps to optimise treatment for patients? Please explain.
-
Do you feel it facilitates co-ordinated care? Please explain.
-
How has communication been between you and NHS Direct?
-
How has the intervention fit with other services already provided by your practice or in the locality?
-
What helps the intervention to work well?
-
What prevents the intervention from performing well?
-
Which patient groups are most likely to benefit from this intervention? Why?
-
Which patient groups are least likely to benefit from the intervention? Why?
-
What benefits (if any) do you think it offers patients?
-
Any other comments.
Appendix 29 Topic guide for trial participants
-
What types of things did NHS Direct recommend that you do?
-
Do you think you have had any benefit from this service?
-
If yes, what benefit and what about the service helped you to get this?
-
In particular, has the service:
-
– helped you to manage your health problems yourself?
-
– helped you get the right treatment for you?
-
– helped to ensure the care you get is co-ordinated?
-
-
If no, what has stopped you gaining benefit?
-
-
What was good about how the service has been delivered?
-
What needs to be improved?
-
How has your general practice helped you with this service?
-
How has the service fit with other services already provided by your practice or in your area?
-
Is this a good thing to do in general for people with depression (raised CVD risk)?
-
Anything else you’d like to say?
Appendix 30 Baseline characteristics of participants in the depression trial who completed or did not complete the primary outcome (PHQ-9) at 4 months
| Characteristic | Non-missing primary outcome, n (%) | Missing primary outcome, n (%) | ||
|---|---|---|---|---|
| Usual care (n = 270) | Intervention (n = 255) | Usual care (n = 32) | Intervention (n = 52) | |
| Demographic data | ||||
| Age (years), mean (SD) | 50.6 (12.9) | 49.7 (12.7) | 44.3 (11.2) | 46.1 (13.3) |
| Female | 181 (67) | 174 (68) | 23 (72) | 39 (75) |
| White | 261 (97) | 248 (98) | 31 (97) | 52 (100) |
| Current employment situation | ||||
| Full-time employment | 78 (29) | 74 (29) | 14 (44) | 14 (27) |
| Part-time employment | 36 (13) | 48 (19) | 3 (9) | 8 (16) |
| Full-time education | 2 (1) | 4 (2) | 0 (0) | 1 (2) |
| Unemployed | 12 (4) | 10 (4) | 1 (3) | 4 (8) |
| Unable to work because of long-term illness/disability | 69 (26) | 58 (23) | 9 (28) | 15 (29) |
| Unable to work because of carer responsibilities | 2 (1) | 3 (1) | 0 (0) | 1 (2) |
| Fully retired from work | 42 (16) | 34 (13) | 2 (6) | 6 (12) |
| Looking after the home | 10 (4) | 12 (5) | 0 (0) | 1 (2) |
| Doing something else | 16 (6) | 9 (4) | 3 (9) | 1 (2) |
| Occupation (most recent or current) | ||||
| Administrative or secretarial occupations | 45 (19) | 40 (18) | 6 (21) | 9 (20) |
| Associate professional or technical occupations | 37 (16) | 31 (14) | 0 (0) | 4 (9) |
| Elementary occupations | 17 (7) | 16 (7) | 4 (14) | 3 (7) |
| Managers or senior officials | 27 (12) | 29 (13) | 5 (18) | 12 (27) |
| Personal services | 25 (11) | 25 (11) | 3 (11) | 2 (4) |
| Process, plant and machine operatives | 11 (5) | 14 (6) | 0 (0) | 1 (2) |
| Professionals | 32 (14) | 35 (16) | 3 (11) | 7 (16) |
| Sales and customer services | 30 (13) | 23 (10) | 5 (18) | 6 (13) |
| Skilled trades | 10 (4) | 12 (5) | 2 (7) | 1 (2) |
| Highest educational qualification achieved | ||||
| Degree or higher degree | 75 (28) | 58 (23) | 9 (28) | 10 (19) |
| A levels or equivalent | 52 (20) | 49 (20) | 2 (6) | 14 (27) |
| GCSEs/O levels or equivalent | 102 (38) | 110 (44) | 17 (53) | 20 (38) |
| No qualifications | 37 (14) | 34 (14) | 4 (13) | 8 (15) |
| Accommodation | ||||
| Own accommodation or buying with mortgage | 150 (56) | 152 (60) | 12 (38) | 27 (52) |
| Part-rent or rent accommodation | 107 (40) | 97 (38) | 17 (53) | 21 (40) |
| Lives rent free | 11 (4) | 6 (2) | 3 (9) | 4 (8) |
| Index of Multiple Deprivation, mean (SD) | 17.7 (12.9) | 18.4 (13.1) | 21.1 (14.2) | 17.7 (11.4) |
| Clinical data | ||||
| Treated for depression | 230 (94) | 224 (91) | 28 (90) | 45 (90) |
| PHQ-9 score, mean (SD) | 16.6 (4.7) | 17.0 (4.5) | 17.7 (4.5) | 17.2 (4.3) |
| GAD-7 score, mean (SD) | 12.4 (5.0) | 13.8 (4.5) | 13.0 (5.4) | 12.4 (4.9) |
| Categorised with mild depression from CIS-R | 49 (18) | 33 (13) | 3 (9) | 6 (12) |
| Categorised with moderate depression from CIS-R | 137 (51) | 136 (53) | 11 (34) | 29 (56) |
| Categorised with severe depression from CIS-R | 84 (31) | 86 (34) | 18 (56) | 17 (33) |
| Taking antidepressants | 233 (91) | 207 (87) | 25 (78) | 44 (88) |
Appendix 31 Baseline characteristics of participants in the cardiovascular disease risk trial who completed or did not complete the primary outcome (QRISK2) at 12 months
| Characteristic | Non-missing primary outcome, n (%) | Missing primary outcome, n (%) | ||
|---|---|---|---|---|
| Usual care (n = 291) | Intervention (n = 295) | Usual care (n = 25) | Intervention (n = 30) | |
| Demographic data | ||||
| Age (years) at CVD assessment, mean (SD) | 67.5 (4.3) | 67.6 (5.0) | 64.9 (7.0) | 66.6 (3.8) |
| Female | 64 (22) | 52 (18) | 2 (8) | 8 (27) |
| White | 288 (99) | 292 (99) | 25 (100) | 29 (97) |
| Current employment situation | ||||
| Full-time employment | 36 (13) | 48 (17) | 3 (13) | 6 (20) |
| Part-time employment | 38 (13) | 27 (9) | 5 (21) | 2 (7) |
| Unemployed | 3 (1) | 2 (1) | 1 (4) | 0 (0) |
| Unable to work because of long-term illness/disability | 6 (2) | 1 (< 0) | 1 (4) | 2 (7) |
| Unable to work because of carer responsibilities | 3 (1) | 2 (1) | 0 (0) | 0 (0) |
| Fully retired from work | 183 (64) | 191 (67) | 13 (54) | 19 (63) |
| Looking after the home | 3 (1) | 4 (1) | 0 (0) | 0 (0) |
| Doing something else | 15 (5) | 11 (4) | 1 (4) | 1 (3) |
| Occupation (most recent or current) | ||||
| Administrative or secretarial occupations | 29 (11) | 29 (11) | 2 (8) | 0 (0) |
| Associate professional or technical occupations | 44 (16) | 32 (12) | 1 (4) | 3 (12) |
| Elementary occupations | 25 (9) | 15 (6) | 3 (13) | 1 (4) |
| Managers or senior officials | 48 (18) | 61 (23) | 7 (29) | 4 (15) |
| Personal services | 5 (2) | 7 (3) | 0 (0) | 2 (8) |
| Process, plant and machine operatives | 13 (5) | 15 (6) | 2 (8) | 2 (8) |
| Professionals | 53 (20) | 58 (22) | 4 (17) | 6 (23) |
| Sales and customer services | 9 (3) | 10 (4) | 2 (8) | 3 (12) |
| Skilled trades | 44 (16) | 41 (15) | 3 (13) | 5 (19) |
| Highest educational qualification achieved | ||||
| Degree or higher degree | 60 (21) | 66 (23) | 5 (20) | 6 (21) |
| A levels or equivalent | 52 (18) | 46 (16) | 6 (24) | 7 (24) |
| GCSEs/O levels or equivalent | 125 (44) | 127 (44) | 12 (48) | 9 (31) |
| No qualifications | 45 (16) | 50 (17) | 2 (8) | 7 (24) |
| Accommodation | ||||
| Own accommodation or buying with mortgage | 245 (84) | 259 (88) | 19 (76) | 22 (73) |
| Part-rent or rent accommodation | 40 (14) | 32 (11) | 6 (24) | 8 (27) |
| Lives rent free | 5 (2) | 2 (1) | 0 (0) | 0 (0) |
| Index of Multiple Deprivation, mean (SD) | 16.2 (11.9) | 15.6 (11.1) | 22.5 (17.8) | 14.9 (12.8) |
| Clinical data | ||||
| QRISK2 score, mean (SD) | 30.7 (9.4) | 31.3 (10.4) | 31.2 (10.1) | 28.9 (7.7) |
| Systolic blood pressure (mmHg), mean (SD) | 147.9 (17.6) | 147.7 (16.4) | 150.4 (18.6) | 146.8 (13.9) |
| Diastolic blood pressure (mmHg), mean (SD) | 79.8 (10.4) | 81.2 (9.4) | 82.2 (10.7) | 80.3 (11.6) |
| Weight (kg), mean (SD) | 92.2 (18.7) | 93.4 (17.6) | 88.9 (21.2) | 91.5 (14.9) |
| BMI (kg/m2), mean (SD) | 31.1 (5.6) | 31.1 (5.4) | 29.5 (6.6) | 32.0 (5.7) |
| Total cholesterol, mean (SD) | 4.9 (1.2) | 4.9 (1.2) | 4.9 (1.2) | 5.1 (1.5) |
| Ratio of total cholesterol to HDL cholesterol, mean (SD) | 4.2 (1.3) | 4.3 (1.5) | 4.6 (1.9) | 4.2 (1.3) |
| Non-smoker | 96 (33) | 106 (36) | 7 (28) | 8 (27) |
| Ex-smoker | 138 (47) | 146 (49) | 10 (40) | 17 (57) |
| Light smoker | 26 (9) | 24 (8) | 4 (16) | 1 (3) |
| Moderate smoker | 15 (5) | 12 (4) | 4 (16) | 1 (3) |
| Heavy smokers | 16 (5) | 7 (2) | 2 (8) | 0 (0) |
| Taking antihypertensives | 113 (39) | 110 (37) | 10 (40) | 6 (20) |
| Taking lipid-lowering medication | 142 (49) | 140 (48) | 11 (44) | 18 (60) |
| Diabetes | 58 (20) | 67 (23) | 4 (16) | 10 (33) |
| Chronic kidney disease | 33 (11) | 19 (6) | 1 (4) | 1 (3) |
| Atrial fibrillation | 19 (7) | 23 (8) | 1 (4) | 0 (0) |
| Rheumatoid arthritis | 8 (3) | 5 (2) | 0 (0) | 1 (3) |
Appendix 32 Relevant systematic reviews and randomised controlled trials published since March 2010
Recent relevant systematic reviews
In 2012, Wootton54 published a high-level systematic review of trials and reviews of telehealth interventions for five common chronic diseases (asthma, COPD, diabetes, heart failure and hypertension). The review was similar to our own evidence synthesis reported in Chapter 2 and reached similar conclusions. Wootton54 found that most studies had reported positive effects, but a funnel plot suggested the likelihood of publication bias and more recent trials provided weaker evidence of benefit. Much of the research was short term and there are still few studies of cost-effectiveness. Wootton54 concluded that ‘the evidence base for the value of telemedicine in managing chronic diseases is on the whole weak and contradictory’ (p. 211). Several other systematic reviews and meta-reviews have been published since March 2010. 63,282,415,431,432 These have continued to support our previous conclusion that the evidence for the benefit of telehealth is limited, although a systematic review of telephone health coaching for people with LTCs published in 2011 was generally positive. 433
A useful review focused on the evidence on the implementation as well as the effectiveness of interactive web-based programmes for older patients with LTCs. 413 This found that these programmes were associated with improvements in self-efficacy and self-management behaviours, but there was little evidence of benefits in terms of health outcomes. Early attrition because of discontinuing the intervention was a problem in several studies included in the review.
Recent relevant primary studies
The WSD study155 was a cluster randomised trial of a whole-system redesign of care for patients with diabetes, COPD or heart failure in three areas of England using a range of technological approaches, such as remote monitoring supported by telephone-based case management. The primary outcome was the proportion of people with an inpatient admission over a 12-month period and secondary outcomes included mortality and costs. The trial involved > 3000 participants from 179 general practices. The results showed a modest reduction in hospital admission rates but this appears to have been mainly related to a short-term increase in admission rates in the control group at the start of the trial, which may have been related to the process of participant recruitment. 155 An economic analysis suggested that the intervention was not cost-effective because of very small benefits in quality of life associated with high costs (incremental cost per QALY £92,000). 52,53
The design of the Healthlines Service was influenced by the previous experience of NHS Direct as a partner in Birmingham OwnHealth, which provided telephone-based health coaching for people with LTCs. The results of an evaluation of the impact of this intervention on hospital admission rates have now been published. 156 This was a cohort study with matched control subjects that involved 2698 intervention participants with heart failure, CHD, diabetes or COPD and a history of inpatient or outpatient hospital use. Contrary to expectations, the intervention was associated with increases, rather than reductions, in both hospital admissions and secondary care costs.
In contrast, a US-based RCT compared two groups of patients who were offered a telephone intervention with similarities to the Healthlines Service. 434 Health coaches telephoned participants to provide support for behavioural change and self-management, sending them links to internet-based resources and DVDs as appropriate. The trial compared one group of patients for whom predictive modelling software was used to offer health coaching to those with a high risk of hospital admission and another group of patients who had access to health coaching but without an outreach programme to offer it to them. The study showed that 10% of those in the intervention group and 3% of those in the control group received health coaching. The intervention group showed large reductions in costs and hospital admissions compared with the control group. However, this study design makes it difficult to be sure whether or not these benefits resulted entirely from the health coaching and the relevance of this US-based study to the NHS environment is also uncertain.
List of abbreviations
- AMI
- acute myocardial infarction
- BMI
- body mass index
- BWW
- Big White Wall
- CACE
- complier average causal effect
- CASP
- Critical Appraisal Skills Programme
- CBT
- cognitive–behavioural therapy
- CCM
- chronic care model
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CHESS
- Comprehensive Health Enhancement Support System
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised
- COPD
- chronic obstructive pulmonary disease
- CVD
- cardiovascular disease
- DARE
- Database of Abstracts of Reviews of Effects
- DNA
- did not attend
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GAD-7
- Generalised Anxiety Disorder-7 items
- GP
- general practitioner
- HCA
- health-care assistant
- HCHS
- Hospital and Community Health Service
- HCP
- health-care professional
- HDL
- high-density lipoprotein
- heiQ
- Health Education Impact Questionnaire
- HIA
- health information advisor
- HSE
- Health Survey for England
- IAPT
- Improving Access to Psychological Therapies
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IT
- information technology
- LLTTFi
- Living Life to the Full Interactive
- LTC
- long-term condition
- MCS
- mental component summary
- MRC
- Medical Research Council
- MRC START
- Medical Research Council Systematic Techniques for Assisting Recruitment to Trials
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OLS
- ordinary least squares
- OR
- odds ratio
- PCA
- principal components analysis
- PCS
- physical component summary
- PCT
- primary care trust
- PHQ-9
- Patient Health Questionnaire-9 items
- PRECIS
- pragmatic–explanatory continuum indicator summary
- PSS
- Personal Social Services
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- REEACT
- Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy
- SAE
- serious adverse event
- SD
- standard deviation
- SMD
- standardised mean difference
- TECH
- TElehealth in CHronic disease
- THS
- Telehealth Scheme
- TIA
- transient ischaemic attack
- WSD
- Whole System Demonstrator