Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0707-10186. The contractual start date was in April 2009. The final report began editorial review in February 2015 and was accepted for publication in January 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Mark Sculpher reports grants from the National Institute for Health Research (NIHR) during the conduct of the study and personal fees from various pharmaceutical and other life science companies outside the submitted work. Andrea Manca reports grants from NIHR during the conduct of the study. Beth Woods reports grants from the NIHR during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by MacPherson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Around 4 million acupuncture treatments are provided each year in the UK according to a national survey conducted in 2012. 1 Approximately one-third of the 4 million treatments were reported as being provided within the NHS and the remainder in the independent or not-for-profit sectors. 1 Reasons for consultation were dominated by musculoskeletal complaints (59%) and neurological conditions (9%), primarily headaches and migraine. Younger people predominantly consulted for back pain and headaches, whereas older people were proportionately more likely to consult for knee pain. These data reflect a steady increase over time in the utilisation of acupuncture: in 2001, a survey reported an estimate of the total number of acupuncture treatments in the UK per year of 3 million, with a similar proportion, namely one-third, provided within the NHS. 2 The provision of acupuncture within the NHS appears to be patchy, with limited access for patients with an interest in receiving acupuncture. Despite general practitioners (GPs) being, in general, supportive of the idea of wider acupuncture provision,3 the funding of acupuncture clinics in primary care has been difficult. 4 This situation has led to many patients turning to the independent sector for acupuncture treatment. 5,6 The common conditions treated by independent acupuncturists showed a marked correspondence with the conditions that GPs in primary care have acknowledged that they are not fully effective in treating, especially musculoskeletal conditions, depression and chronic pain. 7 Across Europe, a similar pattern has been reported whereby patients are most commonly seeking help for painful conditions. 8
The provision of acupuncture in the UK involves practitioners who are primarily members of the four main professional organisations that regulate acupuncture in the UK: the Acupuncture Association of Chartered Physiotherapists (AACP), British Acupuncture Council (BAcC), British Academy of Western Medical Acupuncture (BAWMA) and British Medical Acupuncture Society (BMAS). The AACP is a clinical interest group of the Chartered Society of Physiotherapy with membership (n = 5600) requiring a minimum of 80 hours of training. The BAcC is the leading self-regulatory body for independent acupuncturists in the UK, with membership (n = 2600) open to graduates of acupuncture courses based on 3 years of full-time study or equivalent, with most courses awarding a university degree. Most members (n = 300) of BAWMA are nurses or other health-care professionals, and they have received approximately 100 hours of training delivered over eight weekends, leading to an Academy Licentiate Certificate. Practising members (n = 2400) of the BMAS are primarily doctors with minimum training taking place over two weekends, which may be extended, with accreditation based on completion of 100 hours of training along with provision of a series of case histories.
Unlike the pharmaceutical agents within conventional medicine, acupuncture has had no regulatory gatekeeper controlling its therapeutic activity before being made available on the open market. Treatments have therefore been used widely before researchers have evaluated their effect, whether in terms of safety, efficacy, clinical effectiveness or cost-effectiveness. It has been argued that all of these issues are important in the context of the ‘uncontrolled’ provision of acupuncture, especially when widely used outside the national health-care system, and therefore it is the question of patient safety that needs to be most urgently addressed. 9 This concern about the risks of acupuncture was highlighted some time ago by a systematic review that documented case reports of six deaths that may have been caused by acupuncture. 10 However, case reports are a limited source of evidence on risk, as there is no sense of the frequency or rate with which such events occur. More robust research led to data on adverse event rates involving two prospective surveys of practitioners in the UK, with data involving members of three professional associations: one study involving practitioners of the BAcC11 and the other involving practitioners from both the BMAS and AACP. 12 These studies covered > 30,000 treatments each and reported no serious adverse events, leading to the conclusion that acupuncture is safe in qualified hands. 13 A subsequent prospective UK survey14 of patient reports of adverse events over a 3-month period found results that were largely consistent with the aforementioned practitioner surveys. For most patients it has been found that their experience of benefit following acupuncture appears to outweigh the perceived adverse reactions to treatment. 15 These data on safety have been reinforced by two independent prospective surveys in Germany. One covered adverse events associated with 760,000 acupuncture treatments, leading to reports of two cases of pneumothorax, one case of exacerbation of depression, one acute hypertensive crisis, one vasovagal reaction, and one asthma attack with hypertension and angina. 16 Another covered 2.2 million consecutive acupuncture treatments provided for 229,230 patients, with two patients found to have had a pneumothorax (life-threatening for neither patient) and one to have had a lower limb nerve injury that persisted for 180 days. 17 Taken together, these prospective surveys provide evidence that serious adverse events associated with acupuncture are extremely rare.
Although questions of safety have been largely addressed over the last decade or so, questions of the physiological mechanisms of acupuncture have been more widely debated. A feature of acupuncture research has been the concentrated effort to understand how acupuncture works. For example, numerous acupuncture-related biomarkers have been identified, including antinociceptive endogenous opioids,18,19 immune system markers,20,21 cardiovascular activity,22 gastrointestinal function23 and functional magnetic resonance imaging-detected brain activity. 24 Biomarker outcomes, however, are more revealing of correlations (i.e. when needling occurs, changes can be detected) than mechanisms or causal pathways. Reviews of acupuncture from China25 and the West26 continue to use the overarching term ‘mechanism’ but focus almost entirely on ‘correlates’.
Beyond correlations between acupuncture and biomarkers, research has focused on the search for acupuncture-related biochemical, physiological and anatomical mechanisms. These research efforts include a focus on elucidating the nature of acupuncture points and meridian pathways, and the neurological ‘signals’ that they may carry. For example, some researchers are exploring to what extent the stimulation of the underlying neural pathways accounts for the physiological effects of and clinical responses to acupuncture in patients. 27 The experimental recording of neural activity associated with needle insertion, along with correlations between acupuncture-induced pain and endogenous opioids, led to models that proposed a set of pathways of acupuncture analgesia involving both the peripheral and the central nervous systems. 18,19 These models showed how the effects of acupuncture could be mapped onto the nervous system,27,28 an understanding that has received some support from neuroimaging research. 24,29 The neural hypothesis has been used to explain the existence of meridians, given the observations that many acupuncture points and sections of meridians overlie the major peripheral nerves. 27
There is also an emerging view that loose connective tissue (fascia) provides an alternative biomedical explanation for the role of acupuncture points and meridians. 30 For example, a study has shown that a large proportion of traditional acupuncture points are located at sites where the underlying nerve–vessel bundles are wrapped in a loose sheath of connective tissue that penetrates the fascia to reach the outer dermal layers. 31 A subsequent study demonstrated a high correspondence between the sites of acupuncture points and the location of loose connective tissue planes. 30 Indications are that the superficial fascia provides an initial ‘response element’ to needle stimulation, which may explain the ‘needle grasp’ phenomenon of acupuncture practice. 32 These studies provide a viable alternative to the prevailing neurobiological models based on the emerging evidence on loose connective tissue anatomy and its relationship with the acupuncture system.
Another line of physiological research has explored electrodermal activity at acupuncture points. For example, in an early study, acupuncture points were found to be at the summits of individually contoured conductivity fields. 33 The experimental and physiological confounders to such measurements have been highlighted in recent reviews. 34 In a recent narrative review, the relation of acupuncture point electrodermal activity to pathology has been described. 35 In this review one blinded study found that electrodermal activity at auricular acupuncture points could be used to distinguish which patients had recent or prior cardiopathology and which were healthy control subjects. 36 The observation that traditional pathways of acupuncture meridians correspond to ultrasound images of connective tissue planes30 has been followed by the insight that these meridian-oriented collagenous structures are associated with lower electrical impedance. 37 Lower electrodermal activity along acupuncture meridians was also reported in seven of nine studies at both subcutaneous and intermuscular depths. 38
Our understanding of how acupuncture might have a pain-relieving effect has been informed by basic science research. One mechanism to explain acupuncture is called the ‘gate theory’. 39 This suggests that the pathway associated with acupuncture involves the A delta fibres entering the dorsal horn of the spinal cord, which inhibit pain impulses that are carried in the slower, unmyelinated C fibres. Descending inhibition of C fibre pain impulses is also enhanced through neural connections in the midbrain. 40 Additional mechanisms have been proposed for acupuncture’s effect on pain, which are not necessarily in contradiction to the gate theory; for example, acupuncture stimulates release of endogenous opioids and other neurotransmitters. Interest in acupuncture and endogenous opioids was sparked in the 1970s by research into acupuncture’s analgesic effects, for example research showing that acupuncture could induce analgesia in mice, which was blocked by naloxone. 18 After receiving acupuncture, levels of endogenous opioids in the cerebrospinal fluid have been directly observed to increase in humans. 41 Neuroimaging research has also provided insights into neurological changes associated with acupuncture when used to treat for pain. The research using functional magnetic resonance imaging has demonstrated that acupuncture elicits changes in the brain that appear to correlate with the presumed clinical effects of the points used. For example, a study of acupuncture for carpal tunnel syndrome has shown how acupuncture elicits neural plasticity in the somatosensory area of the brain that correlates with clinical benefits. 42
These physiological studies are important in the context of efficacy research and the need to design a sham acupuncture needle to be used as a control in clinical trials. The lack of a clear understanding of the physiological events that are initiated by acupuncture needling is problematic. This is compounded by confusion surrounding the concept and definition of a ‘placebo’, and the difficulty in interpreting ‘placebo effects’ in a clinical context. This arises because placebo effects vary with different treatments, different settings, different coloured pills, varying patient and/or practitioner expectations, whether a drug or a device is used as a placebo, how placebo effects are explained at the outset of a trial, the extent that placebo effects might interact with concurrent treatment and whether or not placebo effects can be satisfactorily separated out from within a complex intervention. 43 Separating out placebo effects from other effects, such as the natural history of the condition or regression to the mean, is not straightforward. In a Cochrane review, larger effects of placebo interventions were associated with physical placebo interventions, such as sham acupuncture, and with patient-reported outcomes, for example as commonly used to monitor pain-related conditions. 44 Moreover, sham needling is implemented in a number of ways, each with its associated physiological effects. Two approaches to sham needling are commonly used: either needles are used at ‘incorrect’ locations but to penetrate the skin or non-penetrating (stage dagger type) needles are used at the ‘correct’ locations. 45 Some argue that a placebo should be physiologically inert, whereas others suggest that a placebo intervention is acceptable as long as it looks and feels the same as the active intervention that it is controlling for but does not trigger any of the physiological activity elicited by the active intervention. One aspect of this debate has clearly emerged, namely that there is no agreement that the physiological activity of sham acupuncture has been fully characterised.
Beyond the uncertainty of what a sham needle actually triggers at a physiological level, there is considerable agreement that it is useful in principle to determine if an active intervention has a ‘specific’ effect over and above what are commonly called ‘non-specific effects’, a term often used synonymously with ‘placebo effects’. The use of the term ‘non-specific effect’ is preferred as it bypasses the confusion associated with the concept of the placebo that has been discussed above. In this programme of research, we have reviewed many trials that have designated ‘sham’ acupuncture arms and which have made the assumption that any difference in effect that we might observe between true and sham acupuncture in these trials provides the best available assessment of whether or not acupuncture outperforms a placebo.
A major focus in this programme was on questions regarding the efficacy and clinical effectiveness of acupuncture’s putative benefits. Understandably, these are essential questions for the field, whether for patients, practitioners or commissioners. First, the widespread utilisation of acupuncture, as discussed above, raises public health issues related to patient safety. In addition, concerns about safety are often linked to questions regarding the risk/benefit ratio; for example, it can be argued that ‘if there is no benefit, any risk is too much’. 46 Given that an overview of systematic reviews in 2010 concluded that ‘numerous reviews have produced little convincing evidence that acupuncture is effective in reducing pain’,47 this is an understandable concern. Another concern is over questions of bias, given the tendency of trials with a greater risk of bias to deliver more positive results. In this context, a 2009 review of trials of acupuncture for pain stated that ‘the effects of acupuncture cannot be clearly distinguished from bias and that it is unclear whether needling at acupuncture points relieves pain independently of the psychological impact of the treatment ritual’. 48 The question of interest here is whether or not true acupuncture, when adequately assessed, has an effect above and beyond that of a placebo or whether or not the effect becomes negligible as bias is reduced, potentially leading to all effect finally vanishing in the sands of placebodom. 49 As with many emerging fields, and especially those fields that are outside the many regulatory structures of modern medicine, the need for rigorous and unbiased research is an essential requirement so that fair judgements can be made regarding a role in our national health-care system. An unbiased assessment of the evidence base on acupuncture is necessary to inform decisions made by patients, practitioners and policy-makers.
It is useful to distinguish between randomised controlled trials (RCTs) of efficacy and RCTs of effectiveness. The term ‘effectiveness’ is used to measure the overall impact of an intervention on outcome, as would be expected to occur in usual care, with an emphasis on generalisability. The term ‘efficacy’ is used to measure the impact of an intervention on outcome in as ideal conditions as possible, with an emphasis on controlling for placebo effects. In both cases there is a need to limit bias as much as possible, although the challenges of doing so vary somewhat between the two types of trials. When evaluating effectiveness, a comparative or pragmatic design is commonly used in which acupuncture is compared with another active treatment, usual care or no treatment. It should be noted that many acupuncture trials have three-arm designs that include both a sham and another comparator treatment, because the researchers are attempting to address the questions of efficacy and effectiveness in the same trial. The effectiveness/efficacy dichotomy is a useful perspective as it provides a framework for understanding the different types of acupuncture trials in the field, the different questions that they seek to answer and the different ways that the results of these trials need to be interpreted. In reality, many trials are hybrids that contain features of both effectiveness and efficacy trials. 50
With regard to questions of the efficacy of acupuncture for a number of pain-related conditions, there has been considerable uncertainty, especially prior to the mid- to late 2000s. The state of the evidence to that time had been drawn together in a number of systematic reviews of acupuncture trials that included at least one sham-controlled arm, thereby enabling assessment of the difference between true and sham acupuncture. These reviews raised as many questions as answers, with authors identifying a range of mixed outcomes, some positive and some negative. These included data on chronic pain,51 osteoarthritis of the knee,52–54 headache and migraine,55,56 and lower back pain. 57,58 A common feature identified in these reviews was a concern about the relatively small numbers of patients in many of the included trials. As an example, a review of acupuncture for chronic pain, published in 2000,51 included 51 RCTs of acupuncture for a variety of conditions. Typical of these early reviews, there was a low sample size, with a median of 18 patients per trial arm, and weak methodology, with 68% of the trials defined as being of poor quality and only three of the 51 studies receiving a maximum quality score; in addition, there was a typical final conclusion of ‘inconclusive evidence’ on whether or not acupuncture is more effective than a placebo. 51
The state of the evidence when considering the question of the effectiveness (rather than efficacy) of acupuncture, and specifically the evidence on acupuncture compared with usual care, has appeared more clear-cut. The aforementioned reviews published during the 2000s were generally more positive, with effect sizes considerably larger than when acupuncture was compared with sham acupuncture and with the differences between acupuncture and usual care more commonly being statistically significant. Nevertheless, these trials were also subjected to criticism because of the absence of a sham control, the argument being that there might be bias introduced because of unblinded practitioners, increasing the relative effect of the acupuncture, or bias because of resentful demoralisation in participants in the usual care arm who enrolled into a trial because they wanted to receive acupuncture. 59 Two pragmatic trials of acupuncture for chronic pain conducted in the UK were of particular relevance to discussions of effectiveness. 60,61 Both trials were pragmatic in nature, recruiting everyday patients from within primary care and providing the approach to acupuncture that was as near as possible to normal practice, with one evaluating acupuncture for headache and migraine60 and one evaluating acupuncture for lower back pain. 61 The cost-effectiveness analyses of these two trials62,63 turned out to be of importance in terms of subsequent decisions on clinical guidance related to policy and practice. In reviewing these and other trials evaluating acupuncture against usual care for chronic pain in the mid- to late 2000s, authors of meta-analyses typically found that acupuncture was more effective than non-acupuncture controls (comprising waiting list, usual care or no treatment) for the conditions of lower back pain,58 migraine/headache64,65 and osteoarthritis of the knee. 52 Nevertheless, there remained some uncertainty about the clinical relevance of the effect size. As with the efficacy comparison between acupuncture and sham acupuncture, the studies that included an evaluation of effectiveness at this time were dominated by small trials of questionable methodological quality.
Towards the end of the 2000s it was becoming clear that the landscape of research into the efficacy and effectiveness of acupuncture was undertaking a remarkable shift in terms of the number of completed trials, the number of participants in the trials and the methodological quality of the trials. Of particular note was the funding by insurance companies of a series of trials in Germany, some of which had patient numbers in the thousands. These included the cluster of German Acupuncture Randomized Trials published in 2005 and early 2006,66–69 which recruited around 300 patients in each of four separate trials on osteoarthritis,69 chronic lower back pain,66 migraine68 and chronic tension headache. 67 Conducted at the same time were the GERman ACupuncture (GERAC) trials, including around 1000 patients with osteoarthritis,70 chronic lower back pain71 and migraine,72 and 400 patients with chronic tension headache. 73 The third group of trials, the Acupuncture in Routine Care (ARC) trials, had even larger sample sizes, with ≥ 3000 patients in each of three separate pragmatic trials of back pain,74 neck pain75 and chronic headache,76 and 700 patients in a trial of osteoarthritis arthritis. 77 The combination of this remarkable set of trials along with various other larger and higher-quality trials provided an extraordinary opportunity towards the end of the 2000s to provide a robust meta-analysis to provide the necessary clarity to supersede the prevailing uncertainty of the times.
Our programme of research consisted of a series of studies, some of which utilised this unusually large and recently completed set of acupuncture trial data related to chronic pain. The first of these was an individual patient data (IPD) meta-analysis, funded primarily by the US National Institutes of Health, which we report in Chapter 2. In this study we examined the clinical effectiveness of acupuncture for managing chronic pain in conditions including back and neck pain, osteoarthritis of the knee, and chronic headache and migraine. IPD meta-analysis is the most powerful method to synthesise research data. One of the founders of the Cochrane Collaboration, Ian Chalmers, has been quoted as saying that IPD meta-analysis is the ‘yardstick’ by which meta-analyses should be compared. 78 There are a number of advantages of using IPD compared with the summary methods of traditional meta-analyses, which analyse only the published summary data. 79 IPD meta-analysis allows for standardisation within the analysis of different types of outcome measures, for example allowing for the combination of continuous change scores with those reporting only percentage response rates. There is greater power to address subgroups within the population and explore if patient characteristics such as age, sex or baseline severity might impact on outcome. Prior to combining data sets, reanalysis of all trials should be carried out, which will ensure that the data are of a high quality. Finally, an IPD meta-analysis has greater statistical power and consequently more precision in estimating clinical effects. In Chapter 2 we use this method for the first time in acupuncture research to evaluate outcomes from the large number of high-quality trials of acupuncture for chronic pain.
Acupuncture has been recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of chronic headache and migraine,80 and lower back pain,81 but not chronic pain associated with osteoarthritis of the knee. 82 This last decision in part reflected concerns regarding the available evidence. 83 In Chapter 3 we address the question of how competing physical therapies compare for osteoarthritis of the knee, a condition for which there is some uncertainty with regard to the effectiveness of acupuncture. 84 A powerful method to compare the outcomes from a range of interventions for the same condition is a network meta-analysis, which has several advantages over comparing interventions using only pairwise meta-analysis. A network meta-analysis, which is also referred to as a multiple treatment comparison meta-analysis, can be used to compare interventions that may or may not have been evaluated directly against each other. Although direct evidence can come from head-to-head trials, a network meta-analysis can incorporate indirect evidence, which adds strength to the analysis as it allows the effects to be compared between interventions that have not been investigated head to head in RCTs and uses both direct and indirect evidence to inform estimates of effect. 85 For many comparisons, the network meta-analysis may yield more reliable and definitive results than a pairwise meta-analysis would. 86 Clinical practice guidelines that inform the decisions about optimal care need to rely on evidence-based evaluation of often many treatment options. As it provides comparisons across multiple interventions, a network meta-analysis is an approach that can inform a cost-effectiveness analysis, which in turn can inform clinical decision-making. As a step towards providing evidence on competing physical therapies, including acupuncture, for osteoarthritis of the knee, we report a network meta-analysis in Chapter 3.
Given that the current study has been designed to improve evidence around the costs and effects of acupuncture, in Chapter 4 we report a synthesis of the IPD from the RCTs that provided the data set in Chapter 2, which evaluated acupuncture for headache/migraine and musculoskeletal and osteoarthritis pain. We used a network meta-analysis to leverage all available evidence to inform estimates of relative treatment effects when acupuncture was compared with usual care or sham acupuncture, or when both control interventions were compared with each other. The availability of IPD for all studies expanded the set of feasible analyses and allowed development of de novo methods to fully exploit the benefits of access to these data. Although evidence of effectiveness is important, policy-makers faced with difficult resource allocation decisions require estimates of the costs and effects of alternative treatment options. These estimates should reflect all relevant data and treatments should be compared using a metric that can be used across clinical areas – in the UK the quality-adjusted life-year (QALY) is typically used. Synthesising all relevant evidence to produce comparable estimates of costs and effects generates a series of challenges as the available evidence base rarely captures all costs and effects of treatment (because of the nature of data collection or the duration of follow-up), and often requires evidence to be generalised from different populations. The available trial evidence may compare different sets of treatments and in many instances the health-related quality-of-life (HRQoL) data required to estimate QALYs directly are not available. The mapping to convert heterogeneous outcome data on to the EuroQol-5 Dimensions (EQ-5D) summary index scale forms a key component of Chapter 4. In turn, this enabled us to estimate the cost-effectiveness of acupuncture for chronic pain conditions, with the caveat that not all other possible interventions have informed this analysis.
In Chapter 5 we report a cost-effectiveness analysis that was conducted by systematically identifying and synthesising outcome data on a wide range of adjunct non-pharmacological interventions for osteoarthritis of the knee. This allowed an assessment of value for money to be made for all available alternatives at this point in the treatment pathway. We mapped the available HRQoL data to EQ-5D preference weights, producing a common statistic for synthesis. Network meta-analysis was used to synthesise data reported at both the individual-patient and aggregate level. Estimates of effect from a network meta-analysis of EQ-5D outcomes were used to estimate QALYs within a decision-analytic model, which also incorporated cost data from a range of sources. As well as allowing us to estimate the expected costs and effects associated with a wide range of treatments, the decision-analytic methods used allowed us to quantify both the nature and extent of uncertainty, and the value of further research. The synthesis in Chapter 5 therefore combines the use of the IPD related to osteoarthritis of the knee presented in Chapter 2, the data and network meta-analysis methods used to compare competing treatments in Chapter 3 and the mapping methods as set out in Chapter 4.
The focus on acupuncture for chronic pain conditions is central to this programme of research for two related reasons. First, much of the basic research into acupuncture is related to its pain-relieving effects. For example, acupuncture-induced analgesia caught the public imagination in the 1970s and led to research findings showing how acupuncture analgesia is mediated in part by endogenous opioids. 87 More recently, acupuncture neuroimaging research on pain has not only led to a better understanding of how acupuncture might work,88 but also has informed biomedical understanding of neuroplasticity. 89 Second, there has been a remarkable growth in the utilisation of acupuncture for chronic pain after its transmission from East Asia to the West. In part because of media attention and insurance-related reimbursement, the leading indication for acupuncture utilisation is for pain, whether provided in Europe,8 the USA90 or Australia. 91 Moreover, increased acceptance of acupuncture by physicians and allied health-care specialists is paralleled by proportionately more provision for chronic pain within biomedical settings, as is the case for the one-third of the 4 million annual acupuncture sessions that the UK provided within the NHS by doctors and physiotherapists. 1
The final study in this programme of research moved on from chronic pain and focused on acupuncture as a potential treatment for depression. In a previous study exploring the clinical areas in which GPs experience themselves to be not fully effective, described as ‘effectiveness gaps’, GPs reported that depression was the second most common effectiveness gap after musculoskeletal problems. 7 Moreover, patients with psychological problems, including depression, make up the second most common group treated by acupuncture practitioners after those with chronic pain, with much of the provision resulting from patients seeking help from independent acupuncturists. 1 Acupuncture is rarely available as a referral option within NHS mental health services or primary care. 2 Pain and depression often appear to be experienced concurrently, with around 50% of individuals who are diagnosed with and treated for depression also presenting with painful symptoms. 92 Although these data formed a basis for further investigation, the evidence base from systematic reviews in the mid-2000s suggested that there was insufficient evidence to draw conclusions. 93,94 The evidence for pharmacological antidepressant treatment also raised some concern at the time, with pharmacological antidepressant treatment being associated with up to 33% of patients not showing an adequate response. 95 Moreover, 30% of patients have been found to not adhere to their medication regime. 96 An over-reliance on prescribed antidepressant medications has also been identified by patients, who also report that they are interested in being offered more of a range of possible treatment choices. 97
The focus of the work in Chapter 6 was the evaluation of the clinical effectiveness and cost-effectiveness of acupuncture or counselling for depression when offered in primary care as an adjunct to usual GP care. It is accepted that the question of whether or not acupuncture is more than simply a placebo is important; however, we were reluctant to use a form of sham acupuncture as a control for reasons addressed above regarding the lack of an adequate understanding of the mechanism of acupuncture, leading to a difficulty in interpretation. Moreover, the feasibility of implementing a sham acupuncture arm would be challenging, given the lack of institutional support if acupuncture was to be delivered in the field. For these reasons we opted for a pragmatic design that built on our pilot RCT98 and used non-directive counselling as an active control. Our rationale for this was based on the following: (1) counselling is a credible and widely used intervention for patients with depression; (2) there is structural equivalence between acupuncture and non-directive counselling in terms of contact time (1-hour sessions) with empathetic practitioners and therefore if acupuncture performs better than counselling the difference is unlikely to be because of the effects of time or quality of attention; (3) this trial design would help inform patients, decision-makers and providers of the relative merits of counselling compared with acupuncture; and (4) the most recent Cochrane systematic review at the time proposed the wider use of non-placebo comparative designs when evaluating acupuncture for depression – future studies may need to consider the use of comparative designs using medication or structured psychotherapies (cognitive–behavioural therapy, psychotherapy, counselling) or standard care, due to the ethics of administering this intervention to this study population’. 94 It is this design that formed the basis for the RCT described in Chapter 6.
The overarching aim of this programme of research was to use high-quality methods, and innovative ones if necessary, to develop the evidence base on acupuncture. The widespread utilisation of acupuncture combined with insufficient confidence regarding outcomes and decision-making provides a research imperative that is in the public interest. An important question has been asked regarding the extent that acupuncture is simply a remarkably effective placebo as opposed to a physiologically active and scientifically proven intervention. This programme of research provides the latest evidence from high-quality trials that have been carefully designed to answer this question. Innovative research has been conducted, in particular the IPD meta-analysis (see Chapters 2, 4 and 5) and the network-meta-analysis (see Chapters 3–5). As for any research endeavour, not all of the concerns and questions are answered within this report. For example, we do not directly address the placebo question with regard to acupuncture for depression (see Chapter 6); however, we do address this question rigorously, and in some depth, when conducting reviews of the literature on the evaluation of acupuncture for chronic pain in IPD meta-analyses (see Chapter 2) and for osteoarthritis of the knee in a network meta-analysis (see Chapter 3). We did not take into account all competing therapies for the cost-effectiveness analyses of acupuncture for musculoskeletal pain and for headache and migraine (see Chapter 3), although we do so for osteoarthritis of the knee (see Chapter 5). Overall, our focus has been on delivering results that inform patients, providers and decision-makers, which required us to assess whether or not acupuncture outperforms a placebo, if there is a clinically relevant change in clinical status and whether or not there is a sufficiently robust economic case, ideally based on comparisons with all other competing therapies.
Chapter 2 Acupuncture for chronic pain: an individual patient data meta-analysis
Background
An estimated 4 million acupuncture treatments are provided each year in the UK and the most common reasons for consulting are related to chronic pain. 1 Despite this widespread use, there remains uncertainty regarding the clinical effectiveness of acupuncture, and particularly the effectiveness of acupuncture over and above that of sham acupuncture. Many RCTs of acupuncture for chronic pain have been conducted. Most of these trials are methodologically poor in quality, which in turn leads to difficulty in interpreting their results in meta-analyses. Moreover, there has been some controversy regarding the role of sham acupuncture and concerns have been raised that the differences found in these trials between acupuncture and sham acupuncture have been either negligible or sufficiently small to be of little value. Indeed, some commentators have suggested that acupuncture is entirely a theatrical placebo,99 whereas others claim that any putative differences between acupuncture and sham acupuncture are vanishingly small, tending to zero when issues of bias are fully addressed. 49 It is in this climate of uncertainty that the opportunity has arisen to resolve important questions regarding the true effect of acupuncture.
The recent growth in the number100 of RCTs of acupuncture, and improvement in quality, have provided a further rationale regarding the timing of this project to establish more robust evidence. Much of the clinical trial-based research has evaluated the effectiveness of acupuncture for typical chronic pain conditions that commonly occur in primary care. 101 Our method of choice to synthesise these data was an IPD meta-analysis, which is a superior method to conventional meta-analysis using summary data. In the words of Iain Chalmers, one of the founders of the Cochrane Collaboration, using IPD in a meta-analysis is the ‘yardstick’ by which all meta-analyses should be measured. 78 Compared with traditional reviews, which analyse summary data that have already been published, the advantages of using IPD are as follows:102
-
Standardisation is possible between different analytical approaches. Some trials of acupuncture have reported mean change in pain whereas others have reported ‘response rates’ relating to the proportion of patients who experienced a threshold reduction in pain (e.g. 33%). These results cannot be combined without access to the raw data, which allows conversion from one type of analysis to another.
-
Greater power is available in the application of statistical methods. In a typical meta-analysis, the investigator records the means and standard deviations (SDs) for the acupuncture and control groups separately. This does not allow the application of techniques such as analysis of covariance (ANCOVA), which have greater statistical power than unadjusted analysis. 103,104
-
When analysing associations between patient-level characteristics and outcomes, IPD analyses have far greater power to investigate questions such as whether age or baseline symptom severity influence outcome. As an example, if there were four trials with 250 patients in each, analysis of published data would attempt to correlate four values of a predictor (e.g. mean age in each trial) with four values of an outcome (e.g. difference between mean pain scores). Analysis of IPD would be able to create a model with 1000 data points.
-
With regard to data quality, the process of combining data from different sources requires careful data scrutiny by an independent investigator, which provides an opportunity to identify and correct errors in the data set.
The updating of results is an issue of particular importance for trials with longer-term outcomes as data continue to accrue on a daily basis after publication. It is possible that acupuncture triallists have data from long-term follow-up that have yet to be published. Because this method of meta-analysis uses superior statistical methods, it leads to greater precision in the results. In summary, we have optimised the synthesis of existing acupuncture trials by conducting an IPD meta-analysis, including only the highest-quality trials to enhance the quality of the resulting evidence.
To this end, the Acupuncture Triallists’ Collaboration (ATC) was established to manage this project. Collaborators included a group of triallists, statisticians and other researchers with the goal of sharing raw data and developing, in partnership, a set of research questions and associated analytical strategies. The group was motivated to work together to help break down the oppositional culture of competing triallists, to share data in a robust scientific collaboration and to help translate clinical trial findings into patient benefit. Lead investigators from each of the eligible trials contributed raw data, which then were combined into a single data set. This data set was then analysed to address questions concerning the management of chronic pain conditions. The full protocol of the meta-analysis has been published. 105 The study was conducted in three phases: (1) identification of eligible RCTs; (2) collection, checking and harmonisation of raw data; and (3) the IPD meta-analysis.
Our primary objectives, which are addressed in this chapter, were identified as follows:
-
To conduct a systematic review to identify high-quality trials of acupuncture for common chronic pain conditions and then establish a single individual patient-level database of raw data from these trials. This database provided the opportunity to address several key questions in acupuncture research. Our plan is to then publish the database for the benefit of the acupuncture research community.
-
To determine whether or not real acupuncture is superior to sham acupuncture for the treatment of common chronic pain conditions and, if so, to determine the effect size. ‘Real acupuncture’ was defined as the acupuncture intervention that is designed to have activity against pain. ‘Sham acupuncture’ was defined as a comparator intervention that is designed to mimic real acupuncture, with the patient not knowing whether he or she has received real acupuncture, and which ideally has no acupuncture-specific effects.
-
To determine whether or not real acupuncture is superior to non-acupuncture controls for the treatment of common chronic pain conditions and, if so, to determine the effect size. ‘Non-acupuncture controls’ were defined to include care, such as medication ‘as needed’, that is also received by the acupuncture group. Non-acupuncture controls were sometimes described as waiting-list controls, usual or standard care controls or controls receiving no additional treatment. Attention control, in which patients receive general education and advice, was also included in this category.
Within this chapter we also address two secondary objectives in two substudies. In the first substudy we determined the influence of the control group on the effect size of acupuncture. We first identified the variations in types of sham and non-sham controls used and then analysed their impact on effect size. This substudy will inform the design of trials that evaluate acupuncture, as the choice of control will help inform aspects of the design such as sample size. In the second substudy we analysed the data set to determine whether there are characteristics of acupuncture or acupuncturists that act as effect modifiers for treatment outcome.
By meeting both primary and secondary objectives, it is hoped that the evidence generated by this collaboration will have important implications for both clinical practice and research. IPD meta-analysis of high-quality trials provides the most reliable basis for treatment decisions. Analyses of the impact of different sham techniques, styles of acupuncture or frequency and duration of treatment sessions can be expected to guide future clinical trials of acupuncture.
Methods
The methods related to the primary objectives for this study are described in three phases below, after which the methods for the two substudies that address the secondary objectives are described.
Phase I: systematic review to identify eligible trials
Trial quality criteria for trial eligibility
In terms of methodological quality, unconcealed allocation is the most important source of bias in RCTs. 106,107 To be included in the study, a key criterion therefore was the requirement that RCTs of acupuncture for chronic pain conditions had to have unambiguously concealed allocation of the randomisation sequence. When this was not clear from the published paper, we contacted the trial authors for further information concerning the exact logistics of the randomisation process. We considered allocation to be adequately concealed if both of the following two conditions held: (1) the researchers were unable to predict the group to which a patient would be randomised until the patient was explicitly registered on study and (2) the researchers were unable to change a patient’s allocation after a patient was randomised. Allocation concealment was considered inadequate if participants or investigators enrolling participants could possibly foresee or modify assignments and, thus, introduce selection bias. Researchers had to have established clear procedures to ensure that these two conditions were met. For example, there should have been procedures to prevent investigators resealing and reusing an envelope after it was opened (e.g. envelopes were held by an independent party).
Patient criteria for trial eligibility
Trials were eligible if the patient population was recruited on the basis of pain conditions related to osteoarthritis, chronic or recurrent headaches (e.g. tension or migraine headaches), specific and non-specific shoulder pain, and non-specific back or neck pain. Trials were excluded when the back or neck pain was associated with specific pathologies (e.g. osteoporotic fracture). Trials of shoulder pain were included when the pain was associated with specific pathologies (e.g. rotator cuff tendonitis, frozen shoulder or bursitis). As the main analyses were conducted separately by indication, we did not expect to identify more than one or two eligible trials for other pain conditions. For osteoarthritis and headache pain we did not require a specific pain duration, as both are chronic in nature. Back, neck and shoulder pain are commonly episodic conditions and we used the frequently employed criterion for chronicity that the current episode must be of at least 4 weeks’ duration.
Intervention criteria for trial eligibility
Trials were included provided patients in at least one trial arm received acupuncture in the form of penetrating needles at either acupuncture points or trigger points. Trials were classed as ineligible if patients in the acupuncture group, but not the control group, were protocolled to receive medication (conventional or otherwise), surgery or physical therapy. With regard to control groups, eligible trials needed to have included at least one group receiving sham acupuncture or a non-acupuncture control intervention. Sham acupuncture was defined as any intervention designed to prevent the patient from knowing whether he or she received real acupuncture but which was thought by researchers to have minimal activity against pain. Variations of sham acupuncture included variations of superficial needle insertion; needle insertion at non-acupuncture points or at points not indicated for the condition under study; ‘placebo’ needles such as the Streitberger needle,108 which act like stage daggers, appearing to penetrate the skin but which do not do so; techniques such as tapping on a guide tube, designed to feel like needle penetration; and non-needle methods, such as detuned lasers or deactivated transcutaneous electrical nerve stimulation (TENS) devices. It is worth noting that we did not consider these controls to be equivalent a priori; possible differences between sham procedures were analysed as one of our objectives.
Trials with non-acupuncture control groups were included provided the care received in the control group was defined as any of the following: trials with a waiting-list control; trials in which patients received usual clinical care in both arms of the trial, for example a study in which the effects of a course of physiotherapy plus acupuncture were compared with the effects of physiotherapy alone; trials in which the intervention in the control group involved general advice, education and support (sometimes described as ‘attention control’); and trials in which the control group, but not the acupuncture group, received recommendations for guideline care, although no specific treatment plan was mandated and no treatment was provided by the trial. As with sham acupuncture, we did not expect these different types of non-acupuncture controls to have equivalent effects, but we included these different types of control in our analyses and we also investigated differences between them. Trials were excluded if the control groups received a specific programme of treatment such as medication, massage or physical therapy in addition to sham acupuncture or treatments also available in the true acupuncture group.
Outcome criteria for trial eligibility
For eligibility, trials were required to have a measure of pain. The primary end point must have been measured > 4 weeks after the end of the initial acupuncture treatment. There was no restriction on eligibility because of the type of end point.
Trial size and language for trial eligibility
For inclusion, there was no restriction on the size of the trial. We also had no language exclusions. All papers in languages other than English were translated into English and the English text made available to all collaborators.
Search strategy for identification of trials
We searched MEDLINE, Cochrane Central Register of Controlled Trials and the citation lists of systematic reviews. The search strategy used (detailed in Appendix 1) was the same as for the previous reviews of headache,109 back pain57 and osteoarthritis110 (each of which was coauthored by one or more members of the ATC) with the addition of the following terms: ‘neck’, ‘shoulder’, ‘cervical’ and ‘musculoskeletal’. Searching established databases for trials conducted in China or published in the Chinese language was expected to have involved very poor precision as few of these studies are of sufficient quality to merit inclusion. 111–114 Accordingly, Chinese trials were identified by a separate process: Jianping Liu of the Chinese Cochrane Centre in Beijing used that institution’s resources to identify trials of acupuncture for chronic pain that involved full allocation concealment.
Inclusion of trials
All retrieved references were scanned by one of two investigators to remove any clearly inappropriate titles. Hard copies of all remaining papers were then obtained and read by both investigators to remove any for which there was no possibility of eligibility. Inclusion criteria for the remaining papers were applied by two reviewers separately (no reviewer assessed a trial on which he or she was listed as a coauthor). Disagreements about study inclusion were resolved by consensus. Authors of trials were contacted, if necessary, to clarify details such as allocation concealment if this was not clear. All retrieved trials that were excluded from the review were given a reason for exclusion as follows: not a randomised trial; allocation unclear or inadequate; not acupuncture; inappropriate control; not pain; only short-term measurement of pain; or not an osteoarthritis, headache, back, neck or shoulder pain trial.
Quality assessment
With regard to potential bias, the most important quality criteria for a RCT concerned the quality of randomisation, blinding and exclusions and dropouts. 106,107 The quality of randomisation was an inclusion criterion for this study: only trials with full allocation concealment were included in the analysis. Exclusions and dropouts were dealt with by multiple imputation in the statistical analysis. Hence, our quality assessment focused on blinding. For all studies involving sham acupuncture, assessment of blinding followed that in previous Cochrane reviews, with grading as A, B or C. In this categorisation, A represented a low likelihood of bias: either the adequacy of blinding was checked by direct questioning of patients, for example with a credibility questionnaire, and no important differences were found between groups or a blinding method was used that had previously been validated as being able to maintain blinding (e.g. the Streitberger sham device108). A categorisation of C represented a high likelihood of bias: there were clear reasons to believe that blinding was broken, for example differential responses to a credibility questionnaire or an obviously non-credible sham technique was used. Between these two categorisations, category B represented an intermediate likelihood of bias: a trial that did not meet the criteria for a grade of either A or C. Quality assessment was conducted by two reviewers separately with disagreements resolved by consensus.
Phase II: collection, checking and harmonisation of data
Development of the database
We sought IPD for all of the included trials, which we entered into a single database. Data were obtained for all randomised patients, regardless of whether they received treatment or provided post-randomisation data. Trial-level data were then added to the individual patient records. For example, a data set for a trial might have an indicator variable for acupuncture compared with control. This was replaced by several variables indicating the type of acupuncture and control as described in the trial report. When raw data were not available for a trial, we conducted sensitivity analyses to determine if inclusion of the trial might alter the results.
Initial data manipulation
The raw data were saved in their original format and then converted to a Stata format (version 11; StataCorp LP, College Station, TX, USA). Three blank statistical programs were then saved: one to undertake preliminary checks on the data, one to rename and label the variables and one to replicate statistics reported in the trial publication. All files were saved using a standard notation: ‘raw data [descriptor]’, ‘initial import [descriptor]’, ‘initial set up [descriptor]’, ‘initial data checks [descriptor]’ and ‘replication [descriptor]’, where ‘[descriptor]’ is a unique label for each data set (e.g. ‘Linde 2005 migraine’).
Annotation checks
Statistical code was written for the ‘initial set up’ program. Each variable in the raw data set was renamed to a standard notation (e.g. ‘age_at_randomisation’ became ‘age’) and given a standard label (a label is a text description of the variable, such as ‘combined headache score at 60 days’, that is stored by the statistical software). Variables unique to a particular data set, for example a Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score115 in an arthritis trial, were then identified and labelled. Any variables that could not be identified, or which were ambiguous, were documented and appropriate clarification was sought from the original investigator.
Checking for erroneous or missing data
Statistical code was written for the ‘initial data checks’ program. First, the number of missing observations for each variable was calculated and checked against data available in the original publication. Any inconsistencies, or variables for which information on rates of missing data were not available in the trial publication, were brought to the attention of the original investigator for clarification. Second, ‘range’ checks were conducted on all variables to determine whether or not all values were reasonable. As a trivial example, a visual analogue scale (VAS) of 123, or an age of 567 years, immediately suggested an error. Third, we checked categorical variables by tabulation. For instance, if 200 patients were categorised as having stage I disease, 220 categorised as having stage II disease and one as having stage IIa disease, the investigator would be queried as to the accuracy of the IIa categorisation.
Replication
The third program ‘replication [descriptor]’ was then written. This replicated as far as possible every number reported in the trial publication. Replications included baseline characteristics such as age, sex and duration of disease within each group; outcome data such as pain scores within each group at each follow-up time; and comparisons, such as the difference in pain scores between groups at the post-treatment follow-up. In each case, we used the statistical methods reported by the authors and derived the statistics given in the publication. For example, if a mean and SD for baseline pain score were given in the trial publication, we similarly calculated the mean and SD and, if the difference between groups was calculated by linear regression with baseline score and duration of disease as covariates, we used exactly this method to see if we could obtain the same difference between groups, 95% confidence interval (CI) and p-value. Any discrepancies between our results and those reported in the published papers were brought to the attention of the investigators for clarification. We considered that any data set that had gone through these checks – independent labelling of every variable, assessment of prima facie errors and replication of all reported statistics – could be considered valid for inclusion in an independent patient data meta-analysis. Across all trials, the variable names were harmonised.
Phase III: statistical methods
Principal end point
For each trial we identified the primary outcome defined by the study authors in terms of both the scale (e.g. WOMAC) and time point (e.g. 6 months after randomisation). We kept end points on a continuous scale. For example, in some studies the primary end point was defined in terms of the proportion of patients who had at least a 35% reduction in the number of days with headache pain at 6 months’ follow-up. In this case, the primary end point was specified as the number of days with pain at 6 months. If multiple criteria were considered in the primary outcome, or if the primary outcome was inherently categorical, we used a continuous measure of pain measured at the same time point as the original primary end point. For example, if a trial’s primary outcome was a response to treatment defined as a given degree of improvement on a pain scale or a function scale, we selected the pain scale for inclusion in our primary analysis. If there were multiple pain measurements we selected one according to the outcome measures preference list (see Appendix 1). For analyses that included trials with different primary end points, we created a standardised primary end point by dividing by the SD.
Primary analysis: analysis of the effect size of acupuncture
Each trial was reanalysed by ANCOVA with the standardised principal end point as the dependent variable and the baseline principal end point and variables used to stratify randomisation as covariates. This approach has been shown to have the greatest statistical power for trials in general with baseline and follow-up measures,104 and also when specifically applied to acupuncture research. 103 For trials in which randomisation was stratified by centre or practitioner, this stratification was included in the analysis only if there were ≤ 20 sites and there was a mean of at least 20 patients per site, with at least one patient in each arm at each site. In trials in which there was more than one acupuncture group, for example trials in which patients were randomised to local points, distal points or sham points, results from all real acupuncture groups were combined (local points and distal points in this case). The standardised mean between-group difference (effect size) for acupuncture from each trial [i.e. the coefficient and standard error (SE)] was then entered into a meta-analysis; the meta-analytic statistics were created by weighting each coefficient by the reciprocal of the variance, summing and dividing by the sum of the weights. Meta-analysis was accomplished using the metan command in Stata 11.
Our primary analysis was a fixed-effects model. Our rationale for a fixed-effects analysis was that it constituted a valid test of the null hypothesis of no treatment effect. Moreover, we have taken the view that the use of a fixed-effects model does not imply an assumption that all trials are estimating the same effect, but that the robustness of the fixed-effects approach is likely to lead to a more accurate estimate. Nonetheless, we also report the results of the random-effects analysis. In addition, we report heterogeneity statistics. 116 We computed effect sizes separately for comparisons of acupuncture with sham and non-acupuncture controls. Comparisons between acupuncture and sham controls omitted trials graded as category C (high likelihood of bias) because of concerns regarding blinding. These analyses were conducted separately for each pain condition (specific shoulder conditions, musculoskeletal pain, osteoarthritis, headache) and then within each pain condition (neck pain and back pain or chronic tension headache and migraine).
Secondary analyses
We repeated the analyses of effect size for the secondary end points of pain intensity, pain frequency, functional impairment, combined measures of pain and functional impairment, mental well-being [e.g. Short Form questionnaire-36 items (SF-36) mental health], physical well-being (e.g. SF-36 physical health), overall quality of life (e.g. global assessment), range of motion or stiffness, health change, satisfaction with care and medication use. If a trial reported more than one end point that could be placed in a particular category, the outcome measures preference list (see Appendix 1) was consulted to select the most appropriate measure. On occasion, this could have involved taking a mean score of two end points. For example, if a trial reported both a daytime and a night-time VAS score, we calculated the average for each patient and the combined score was then entered into the analysis. Note that this demonstrates a key advantage of IPD meta-analysis: such a data manipulation would not be possible with summary-level data. As different measurement scales were used in the different trials, we used the standardised mean difference (SMD) as the meta-analytic statistic. Time was always measured from randomisation. For our data, we used end points of 1, 2, 3, 6 and 9 months and 1 year. For outcomes with these exact time points (or the equivalent in another unit of time: 13 weeks = 3 months), no time point standardisation was required. Otherwise, the time point closest to the selected scheme was adopted. For example, if for a trial there was no measurement at 6 months but there was for 24 weeks, this time point was selected and relabelled appropriately. Numerical rating scale (NRS) scores were converted to a 0–100 point scale by appropriate multiplication.
Sensitivity analyses
The first sensitivity analysis involved multiple imputation for missing data, following the approach used in the analysis of the NHS trial of acupuncture for headache. 60 The second sensitivity analysis was for publication bias. Although we did not believe that there were many unpublished adequately concealed acupuncture trials large enough to have an important weight in the meta-analysis, we included scenarios that could change the study results. For example, if we found a statistically significant difference between acupuncture and sham, we estimated the parameters for the following scenarios that, if added to the meta-analysis, would change the p-value to 0.05: (1) the number of trials with 50 patients per group and no differences between groups; and (2) the number of trials with 50 patients per group and an effect size of 0.25 in favour of the control. The third sensitivity analysis omitted subsets of trials based on trial quality. We omitted trials graded as category B for blinding from the comparison of acupuncture with sham. Our final sensitivity analysis involved adding the results of studies for which we did not receive individual patient-level data. We calculated an estimate of the difference between groups and the resulting SE from published summary data.
Methods for substudy 1: influence of control group on effect size
Types of sham acupuncture control
In the included trials with a sham acupuncture control group we assessed whether or not a sham needle was used, whether or not a sham needle that penetrated the skin was used, whether sham needling was performed on true acupuncture points or non-acupuncture points, and whether a sham needle insertion was deep or superficial. Information on acupuncture characteristics was obtained from the trial manuscript supplemented by a questionnaire sent to triallists.
When trial authors reported using either a penetrating or a non-penetrating needle for sham acupuncture, the trial was classified as using a ‘needle sham’. Trials using non-needle methods of sham acupuncture, such as an inactivated laser or a TENS device, were classified as ‘non-needle sham’. Needle sham trials were further classified as using penetrating needles, which were almost always inserted at locations away from true acupuncture points (thereby investigating point location), or using non-penetrating sham needles, which were applied either at the same points as in the true acupuncture group (testing exclusively skin penetration and not location) or at non-acupuncture points (investigating penetration and location simultaneously).
We had hoped to include two other features of sham controls: whether the depth of insertion for penetration was categorised by triallists as superficial or deep and whether sham acupuncture was applied at or away from true acupuncture points; however, only one trial reported using deep insertion in sham acupuncture. 117 For point location, there was strong collinearity with sham technique, with only techniques avoiding skin penetration using true acupuncture points. As a sensitivity analysis, we reanalysed the data excluding four trials that were determined by consensus among external reviewers to have an ‘intermediate likelihood of unblinding’. 72,117–119 However, after excluding these trials, only one remaining trial used non-needle sham acupuncture, limiting our ability to use metaregression.
Types of non-sham control
We categorised trials that included controls without sham acupuncture into two types: ‘routine care’ and ‘protocolled care’. In ‘routine care’ trials, both treatment and control groups had access to non-specified care as needed, such as rescue medications or other conventional care, but the use of such treatment was at the discretion of patients and doctors, with no specification in the protocol as to what treatments patients could receive. If protocols prescribed some treatments such as surgery but did not make specific recommendations as to allowable treatments, trials were defined as ‘routine care’. Control groups in which treatment consisted of information or education given to a patient (‘attention control’) were also considered to be routine care control groups.
In ‘protocolled care’ trials, the care in the control group was specified in the study protocol. This was typically when the acupuncture group and the usual care control group both received an additional non-acupuncture treatment that was specifically indicated as part of the trial protocol. For example, trials that studied the effect of acupuncture and physical therapy compared with physical therapy alone were categorised as protocolled care.
Statistical methods related to effect of control group
To test the effect of each characteristic of sham acupuncture on the main effect estimate, we used random-effects metaregression with the Stata command metareg. This command was also used to run a random-effects metaregression to test the effect of routine compared with protocolled care on the main effect estimate for usual care control groups. The main effect estimate of each trial was determined using linear regression, and the coefficient and SE for each trial were entered as the dependent variable in the random-effects metaregression.
All analyses were conducted using Stata 12. We excluded three trials by Vas et al. 120–122 in a sensitivity analysis. As described in Meta-analysis, these trials had very much larger effect sizes than average and their exclusion resulted in heterogeneity becoming non-significant in the comparisons between acupuncture and sham acupuncture. More detail on this substudy’s methods is reported separately. 45
Methods for substudy 2: characteristics of acupuncture
Data included at the trial and patient level
Data on the trial and patient-level characteristics of the acupuncture interventions were obtained directly from responding triallists by use of a questionnaire and are presented in Appendix 2. Characteristics investigated included the style of acupuncture, which was defined as based on traditional Chinese theory or contemporary Western acupuncture or a mixture of both approaches. Point prescriptions were defined as fixed, flexible or individualised. Trials were categorised as using a flexible needle formula if triallists indicated that acupuncture was semistandardised, a flexible formula with fixed points or both fixed and flexible formulas. Triallists reported whether or not their trial allowed electrical stimulation to be added to the needles during acupuncture sessions and whether or not moxibustion was allowed. Trial-level information was reported on whether or not acupuncturists attempted to elicit deqi and whether it was felt by the acupuncturist or the patient. Triallists reported on whether or not acupuncture-specific interactions between the patient and the acupuncturist were allowed, for example through explanations of treatment, advice, support and suggestions about helpful lifestyle changes. These interactions, when driven by acupuncture theory, are considered ‘specific’ to the acupuncture treatment. Triallists reported the minimum number of years of practice as an acupuncturist required to participate in their trial. The maximum number of acupuncture treatment sessions allowed during the trial period was reported by each triallist and these data were analysed per five-session increments. The frequency of sessions was recorded and analysed continuously as a weekly average (i.e. typical number of sessions per week). The duration of sessions was reported as the average length of a session in minutes among the patients receiving acupuncture. Patient-level data were used when available by taking the mean duration of patients’ sessions. Trials were not included in this analysis if treatment was individualised and no individual-level data were available. The duration of sessions was included as a continuous variable in the analyses and the results reported per 5-minute increments. Triallists were asked to report the average number of needles used per treatment session. Trials were excluded from this analysis if the number of needles used was unknown. If patient-level data were available an average was included. The average number of needles used was analysed as a continuous variable, with the coefficient reported per five-needle increments. The placement of acupuncture needles was categorised as local (at or near the location of pain), distal to the location of pain or both.
Statistical methods for analysis of characteristics
We identified the primary outcome as defined by the study authors in terms of both the scale and the time point. We kept end points on the continuous scale. For analyses that included trials with different primary end points, we created a standardised primary end point by dividing by the SD. We conducted analyses separately for sham and non-acupuncture controls using Stata 12.
We used random-effects metaregression for trial-level analyses to test the effect of each characteristic on the main effect estimate using the Stata command metareg. We first calculated the effect size and SE for each trial as described in Statistical methods related to effect of control group. For each documented treatment characteristic, we entered the effect size and SE for each trial into a metaregression along with the trial-level average for that characteristic. The coefficients obtained from these analyses are estimates, in SDs, of the effect of each acupuncture characteristic on the main treatment effect.
In the patient-level analyses we were able to use the number of sessions, the number of needles and the age and sex of the acupuncturist for a subset of the trials. For each trial we created a linear regression using random effects as for the main analysis of effect size, but included the characteristic and an interaction term between the characteristic and treatment allocation. The coefficient and SE for the interaction term represents the change in the outcome score in SDs associated with the acupuncture characteristic in the acupuncture treatment group. This was then entered into a meta-analysis using the Stata command metan.
We excluded a set of outlying trials, all by the same team120–122 and which had very much larger effect sizes than other trials, as a pre-planned sensitivity analysis. Further details of the methods are reported separately. 123
Results
Results from the main study
Eligible studies
In our initial search we identified and assessed 83 RCTs for eligibility (Figure 1), of which 31 were eligible (for further details of the 29 studies included in the patient-level meta-analysis, see Appendix 2). Eleven studies were sham controlled, 10 had non-acupuncture controls and 10 were three-armed studies with both sham and no-acupuncture control arms. A second search for studies was requested by the Archives of Internal Medicine prior to publication and therefore subsequent to conducting the meta-analysis; we identified an additional four eligible studies, which were used in a sensitivity analysis. 124–127
FIGURE 1.
Systematic review Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Data extraction and quality assessment
From the 31 eligible RCTs, usable raw data were obtained from 29 trials including a total of 17,922 patients from the USA, UK, Germany, Spain and Sweden (Table 1). For two studies the raw data were unavailable: the study database had become corrupted in one trial128 and in another, despite approval for data sharing being obtained from the principal investigator, the trial statisticians failed to respond to repeated enquiries. 129
| Indication (n = 35) | Pain type | Control group | Primary outcome measure | Time point |
|---|---|---|---|---|
| Chronic headache (n = 7) | Migraine (n = 268,72); tension-type headache (n = 367,73,128); both (n = 260,76) | Sham (n = 467,68,72,73); no acupuncture control (n = 6) – ancillary carea (n = 1128), usual careb (n = 460,67,68,76), guidelined carec (n = 168) | Severity score (n = 260,128); days with headache (n = 173); migraine days (n = 367,72,76); days with moderate to severe pain (n = 168) | 1 month (n = 1128); 3 months (n = 367,68,76); 6 months (n = 272,73); 12 months (n = 160) |
| Non-specific musculoskeletal pain (back and neck) (n = 15) | Back (n = 1061,66,71,74,117,119,124,129–131); neck (n = 575,118,121,132,133) | Sham (n = 1066,71,117–119,121,124,129,130,132); no acupuncture control (n = 9) – ancillary carea (n = 1129), usual careb (n = 661,66,74,75,124,133), non-specific adviced (n = 1131), guidelined carec (n = 171) | VAS (n = 766,117–119,121,129,132); Roland Morris Disability Questionnaire (n = 3124,130,131); neck pain and disability (n = 175); Hannover Functional Questionnaire (n = 174); Northwick Park Neck Pain Questionnaire (n = 1133); von Korff pain score (n = 171); SF-36 bodily pain (n = 161) | 1 month (n = 4117,121,131,132); 2 months (n = 366,124,131); 3 months (n = 574,75,129,130,133); 6 months (n = 271,119); 24 months (n = 161) |
| Osteoarthritis (n = 9) | Sham (n = 669,70,120,125,134,135); no acupuncture control (n = 8) – ancillary carea (n = 370,125,135), usual careb (n = 369,77,126), non-specific adviced (n = 2134,136) | Oxford Knee Score questionnaire (n = 1136); WOMAC (n = 269,77); WOMAC pain subscore (n = 670,120,125,126,134,135) | 2 months (n = 269,136); 3 months (n = 477,120,125,126); 6 months (n = 370,134,135) | |
| Shoulder pain (n = 4) | Sham (n = 4122,127,137,138); no acupuncture control (n = 1) – usual careb (n = 1127) | Constant–Murley score (n = 2122,137); VAS (n = 2127,138) | 1 month (n = 2122,137); 6 months (n = 2127,138) |
In terms of design, the 29 RCTs included 18 comparisons of acupuncture with non-acupuncture controls (14,597 patients) and 20 comparisons of acupuncture with sham acupuncture controls (5230 patients). Patients in all RCTs had access to analgesics and other standard treatments for pain. Four sham-controlled RCTs were determined to have an intermediate likelihood of bias from unblinding;72,117–119 the 16 remaining sham RCTs were graded as having a low risk of bias from unblinding. Dropout rates were low on average (weighted mean of 10%), with rates being > 25% for only four RCTs. For the trials by Molsberger et al. 127,129 (dropout rates 27%129 and 33%129), the raw data were not received and neither RCT was included in the main analysis; the other two trials with a dropout rate of > 25% were those by Carlsson and Sjölund119 (46%, RCT excluded in a sensitivity analysis for blinding) and Berman et al. 134 (31%). This RCT had a high dropout rate among non-acupuncture control subjects (43%); dropout rates were close to 25% in the acupuncture and sham groups. The RCT by Kerr et al. 117 had a large difference in dropout rates between groups (acupuncture 13%, control 33%) but was excluded in the sensitivity analysis for blinding.
Clinical heterogeneity between studies was identified as being related to the control groups. For sham RCTs, the type of sham control varied, including acupuncture needles inserted superficially,72 sham acupuncture devices with needles that retract into the handle rather than penetrate the skin,137 and non-needle approaches such as deactivated electrical stimulation132 or a detuned laser. 118 The cointerventions also varied, with no additional treatment other than analgesics in some RCTs66 and both acupuncture and sham groups receiving a course of additional treatment, such as exercise led by physical therapists, in other RCTs. 135 For the trials with non-acupuncture control groups, there was variation in usual care, for example control group patients being merely advised to ‘avoid acupuncture’;62 an attention control, such as group education sessions;134 and guidelined care, in which, for example, patients were given advice on specific drugs and doses. 72
Meta-analysis
The comparisons of acupuncture against no acupuncture controls and against sham acupuncture are shown separately for each of the four pain conditions in forest plots (Figures 2 and 3, respectively). Meta-analytic statistics are shown in Table 2. Acupuncture was found to be statistically superior to all types of control intervention for all analyses (p < 0.001). Effect sizes were larger for the comparison between acupuncture and non-acupuncture controls than for the comparison between acupuncture and sham controls (0.37, 0.26 and 0.15 for comparison with sham controls vs. 0.55, 0.57 and 0.42 for comparison with non-acupuncture controls for musculoskeletal pain, osteoarthritis and chronic headache, respectively).
FIGURE 2.
Forest plots comparing acupuncture with no acupuncture controls (see Table 2 for references): (a) osteoarthritis; (b) chronic headache; and (c) musculoskeletal pain. No forest plot is available for shoulder pain as there were fewer than three trials and therefore no meta-analysis was performed. TTH, tension-type headache.
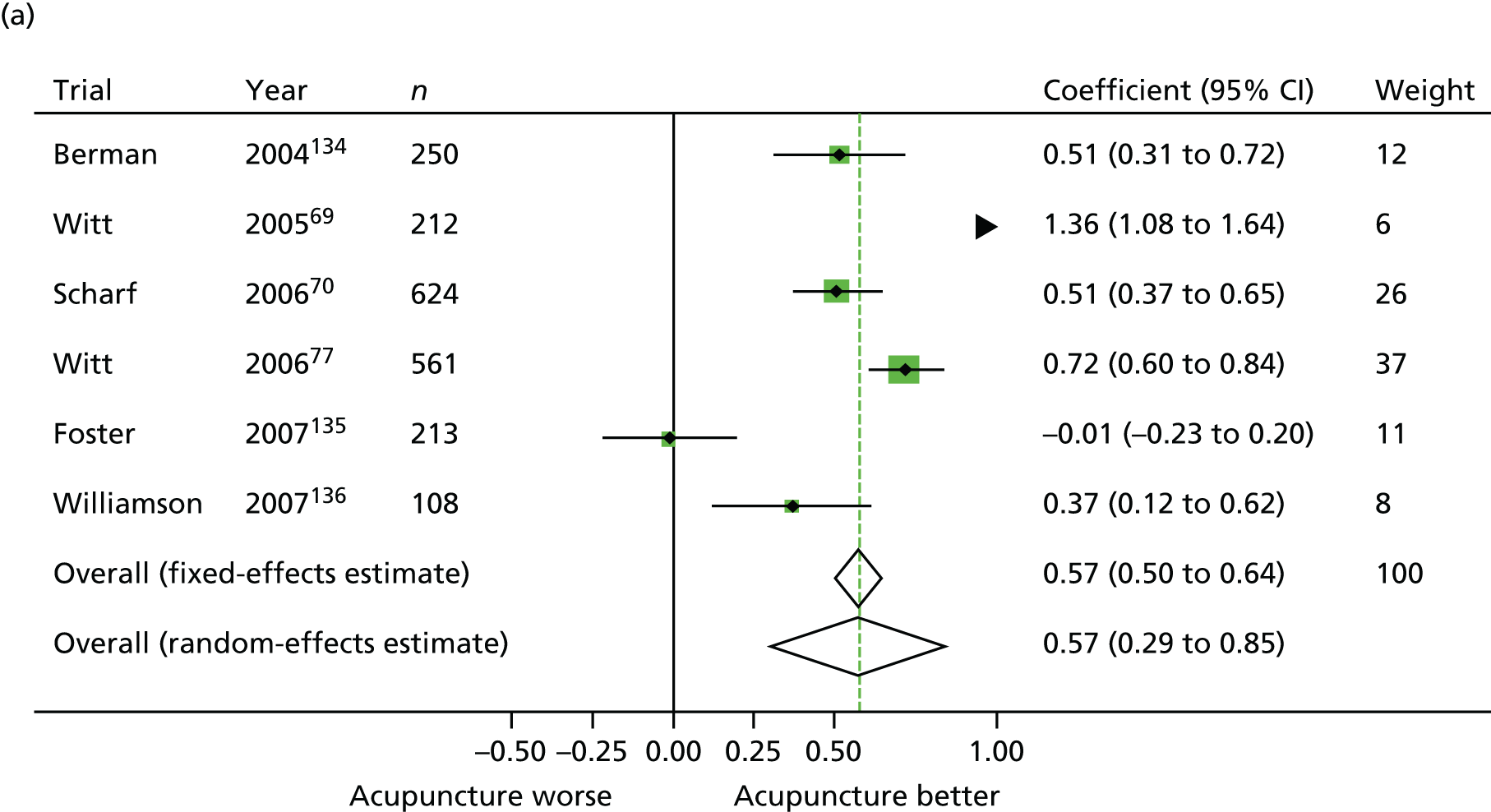

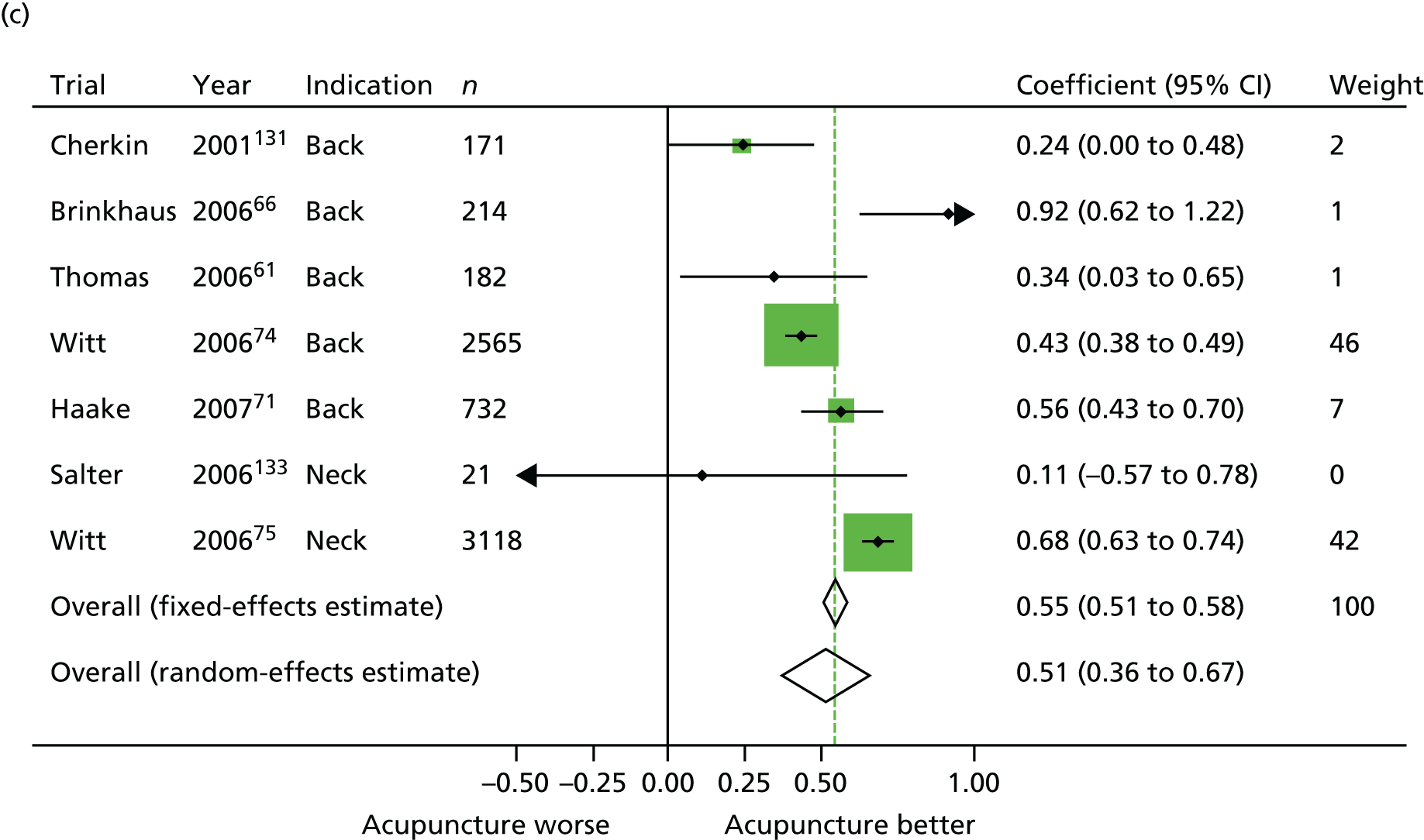
FIGURE 3.
Forest plots comparing acupuncture with sham acupuncture (see Table 2 for references): (a) osteoarthritis; (b) chronic headache; (c) musculoskeletal pain; and (d) shoulder pain. TTH, tension-type headache.

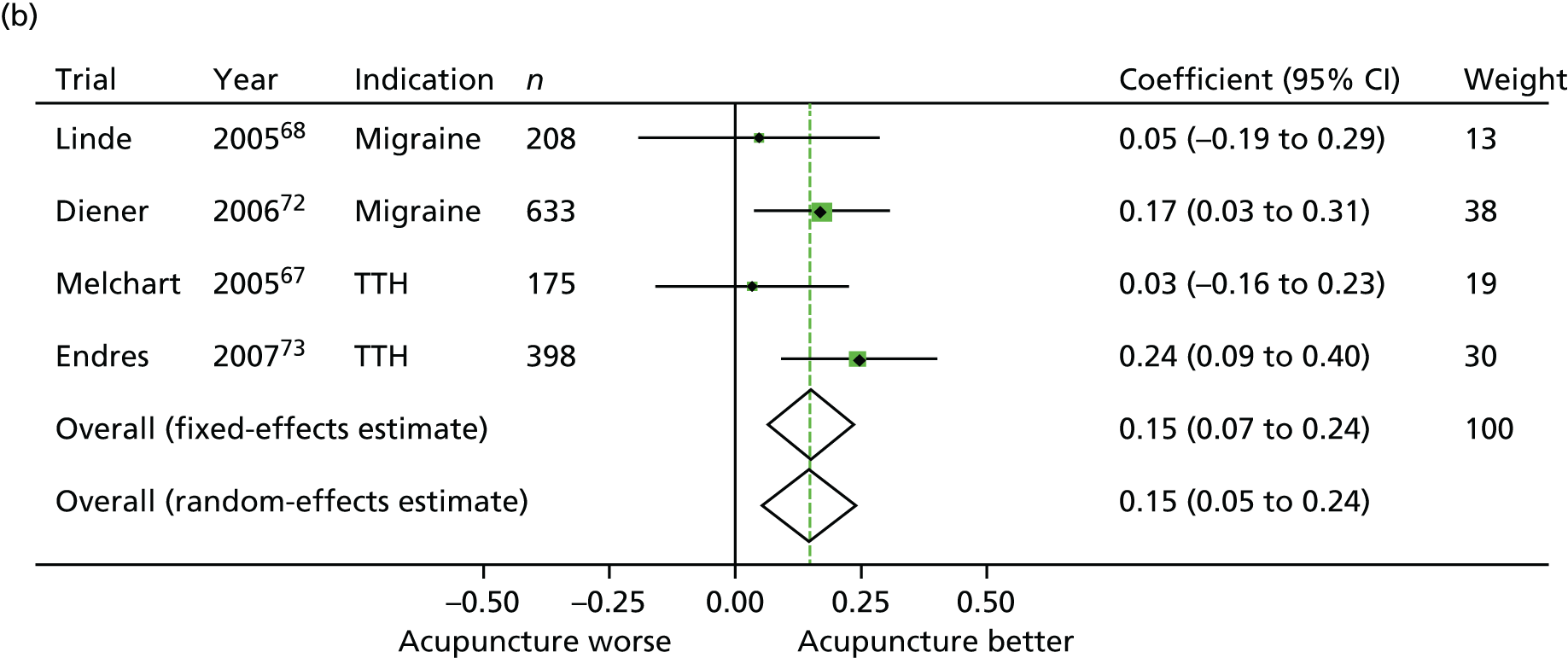
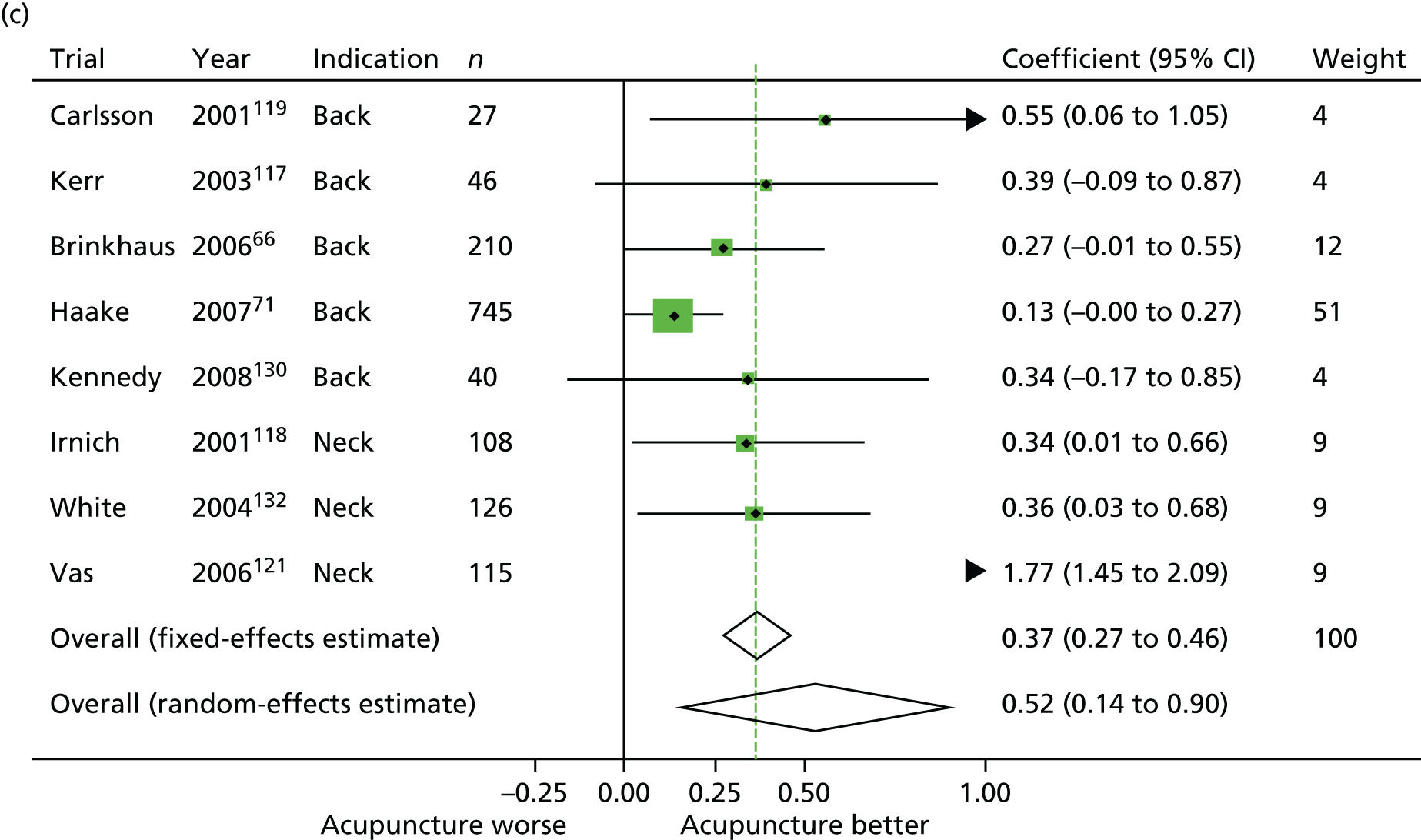

| Indication | n | Fixed effects (95% CI) | Random effects (95% CI) | p-value for overall effect |
|---|---|---|---|---|
| Acupuncture vs. sham acupuncture | ||||
| Non-specific musculoskeletal pain (back and neck)66,71,117–119,121,130,132 | 8 | 0.37 (0.27 to 0.46); heterogeneity: p < 0.001 | 0.52 (0.14 to 0.90) | 0.001 |
| Osteoarthritis69,70,120,134,135 | 5 | 0.26 (0.17 to 0.34); heterogeneity p < 0.001 | 0.37 (0.03 to 0.72) | 0.001 |
| Chronic headache67,68,72,73 | 4 | 0.15 (0.07 to 0.24); heterogeneity: p = 0.3 | 0.15 (0.05 to 0.24) | 0.001 |
| Shoulder pain122,137,138 | 3 | 0.62 (0.46 to 0.77); heterogeneity: p = 0.4 | 0.62 (0.46 to 0.77) | 0.001 |
| Acupuncture vs. no acupuncture control | ||||
| Non-specific musculoskeletal pain (back and neck)61,66,71,74,75,131,133 | 7 | 0.55 (0.51 to 0.58); heterogeneity: p < 0.001 | 0.51 (0.36 to 0.67) | 0.001 |
| Osteoarthritis69,70,77,134–136 | 6 | 0.57 (0.50 to 0.64); heterogeneity: p < 0.001 | 0.57 (0.29 to 0.85) | 0.001 |
| Chronic headache60,67,68,72,76 | 5 | 0.42 (0.37 to 0.46); heterogeneity: p < 0.001 | 0.38 (0.22 to 0.55) | 0.001 |
| Shoulder pain | 0 | No trials | ||
The test for heterogeneity was statistically significant for five of the seven analyses. In the case of comparisons with sham acupuncture, the RCTs by Vas et al. 120–122 are clear outliers. For example, the effect size in the RCTs by Vas et al. for neck pain is about five times greater than the meta-analytic estimate. One effect of excluding these RCTs in a sensitivity analysis (Table 3) was that there was no significant heterogeneity in the comparisons between acupuncture and sham acupuncture.
| Sensitivity analysis | Indication | n | Fixed effects (95% CI) | Random effects (95% CI) | p-value for overall effect |
|---|---|---|---|---|---|
| Acupuncture vs. sham acupuncture | |||||
| Exclusion of Vas et al.120–122 trials | Non-specific musculoskeletal pain | 7 | 0.23 (0.13 to 0.33); heterogeneity: p = 0.51 | 0.23 (0.13 to 0.33) | 0.001 |
| Osteoarthritis | 4 | 0.16 (0.07 0.25); heterogeneity: p = 0.15 | 0.17 (0.00 to 0.35) | 0.001 | |
| Shoulder pain | Fewer than three trials | ||||
| Separate pain types | Back pain | 5 | 0.20 (0.09 to 0.31); heterogeneity: p = 0.4 | 0.20 (0.09 to 0.32) | 0.001 |
| Neck pain | 3 | 0.83 (0.64 to 1.01) heterogeneity: p < 0.001 | 0.82 (–0.11 to 1.75) | 0.001 | |
| Acupuncture vs. no acupuncture control | |||||
| Separate pain types | Back pain | 5 | 0.46 (0.40 to 0.51); heterogeneity: p = 0.004 | 0.49 (0.33 to 0.64) | 0.001 |
| Neck pain | 0 | No trials | |||
Moreover, the effect size for acupuncture became relatively similar for the different pain conditions: 0.23, 0.16 and 0.15 against sham control and 0.55, 0.57 and 0.42 against non-acupuncture control for back and neck pain, osteoarthritis and chronic headache, respectively (fixed effects; results similar for the random-effects analysis).
To understand what these effect sizes mean in real terms, a baseline pain score on a 0–100 scale for a typical RCT might be 60, for example. Given a SD of 25, follow-up scores would average 43 in a no-acupuncture group, 35 in a sham-acupuncture group and 30 in patients receiving true acupuncture. If average responses were defined in terms of a pain reduction of ≥ 50%, response rates would be approximately 30%, 42.5% and 50%, respectively.
There is evidence of heterogeneity in the comparisons with non-acupuncture controls, which can be explained by the differences in the types of control groups used. In the case of osteoarthritis, the largest effect size was in the study by Witt et al. ,69 in which patients in the waiting-list control group received only rescue pain medication, and the smallest was in the study by Foster et al. ,135 which involved a programme of exercise and advice led by physical therapists. For the musculoskeletal analyses, heterogeneity was driven by two very large RCTs74,75 (n = 256574 and n = 311875) for back and neck pain. If only back pain is considered, heterogeneity is dramatically reduced and is again driven by one RCT, that by Brinkhaus et al. 66 with a waiting-list control. In the headache meta-analysis, the study by Diener et al. 72 reported much smaller differences between groups. This RCT involved providing drug therapy according to national guidelines in the non-acupuncture control group, including initiation of beta-blockers as migraine prophylaxis. There was disagreement within the collaboration about whether or not this constituted an active control. Excluding this RCT reduced evidence of heterogeneity (p = 0.04) but had little effect on the effect size (0.42–0.45).
Pre-specified sensitivity analyses found no substantive effect on our main estimates of either restricting the sham RCTs to those with a low likelihood of unblinding or adjusting for missing data. There was also little impact of including summary data from RCTs for which raw data were not obtained (two RCTs)128,129 or which were published recently (four RCTs),124–127 either for the primary analysis or the analysis with the outlying RCTs by Vas et al. 120–122 excluded (data not shown).
To address whether or not publication bias might be involved, we entered all RCTs into a single analysis and compared the effect sizes from small and large studies. 139 We found some evidence that small studies had larger effect sizes overall for the comparison with sham acupuncture (p = 0.02) but not for the comparison with non-acupuncture controls (p = 0.72). However, these analyses were influenced by the outlying RCTs by Vas et al. ,120–122 which were smaller than average, and by indication, because the shoulder pain RCTs were small and had large effect sizes. Tests for asymmetry were non-significant when we excluded the RCTs by Vas et al. 120–122 and shoulder pain studies (n = 15; p = 0.07), and when small studies were also excluded (n < 100 and n = 12; p = 0 30). Nonetheless, we repeated our meta-analyses excluding the RCTs with a sample size of n < 100. This had essentially no effect on our results. We also considered the possible effect on our analysis if we had failed to include high-quality unpublished studies in terms of publication bias. There would need to be 47 unpublished RCTs with n = 100 patients showing an advantage of acupuncture over sham acupuncture of 0.25 SDs before the difference between acupuncture and sham acupuncture would lose its significance.
The effect of pooling different end points measured at different periods of follow-up was the focus of a further sensitivity analysis. We repeated our analyses including only pain end points measured at 2–3 months after randomisation. We found no material effect on the results: effect sizes increased by 0.05–0.09 SDs for musculoskeletal and osteoarthritis RCTs, and were otherwise stable.
We compared sham control with no-acupuncture control in an exploratory analysis. In a meta-analysis of nine RCTs,66–72,134,135 the effect size for sham control was 0.33 (95% CI 0.27 to 0.40) and 0.38 (95% CI 0.20 to 0.56) for fixed- and random-effects models, respectively (for tests of both effect and heterogeneity, p < 0.001).
Results for substudy 1: influence of the control group on effect size
Sham acupuncture controls
Twenty trials with 5230 patients included a control arm in the form of sham acupuncture (Table 4) and the trial-level characteristics for these trials are described in Table 5. The majority of sham-controlled trials (80%) used needle-based sham acupuncture. The number of trials using penetrating or non-penetrating needles was similar: seven trials used non-penetrating needles and nine trials used penetrating needles. All trials using penetrating needles placed these outside the true acupuncture points, whereas only one of seven trials using non-penetrating needles did so.
| Needle used? | Penetrating? | True acupuncture points? | Depth of insertion? | Trials |
|---|---|---|---|---|
| Yes | Yes | No | Superficial | Linde et al.,68 Melchart et al.,67 Diener et al.,72 Scharf et al.,70 Haake et al.,71 Endres et al.,73 Witt et al.69 and Brinkhaus et al.66 |
| Yes | Yes | No | Deep | aBerman et al.134 |
| Yes | No | No | N/A | Vas et al.122 |
| Yes | No | Yes | N/A | Foster et al.,135 Guerra de Hoyos et al.,138 Kennedy et al.,130 Kleinhenz et al.,137 Vas et al.120 and Vas et al.121 |
| No | No | No | N/A | Carlsson and Sjölund119 and Kerr et al.117 |
| No | No | Yes | N/A | Irnich et al.118 and White et al.132 |
| Characteristic | n (%) |
|---|---|
| Needle used | |
| Yes | 16 (80) |
| No | 4 (20) |
| Penetrating needle used | |
| Yes | 9 (45) |
| No | 7 (35) |
| Non-needle | 4 (20) |
| True acupuncture points used | |
| Yes | 8 (40) |
| No | 12 (60) |
| Superficial or deep sham | |
| Superficial | 8 (40) |
| Deep | 1 (5) |
| Non-penetrating sham | 11 (55) |
| Pain type | |
| Lower back pain | 5 (25) |
| Migraine | 2 (10) |
| Neck | 3 (15) |
| Osteoarthritis | 5 (25) |
| Shoulder | 3 (15) |
| Tension-type headache | 2 (10) |
The effect sizes for sham-controlled acupuncture trials, as categorised by the type of sham, are shown in Table 6. Acupuncture was significantly superior to sham irrespective of the type of sham control, both in the main analysis and in a sensitivity analysis excluding outlying studies. This table also includes the results of the primary sensitivity analysis that excluded the Vas et al. 120–122 trials as outliers. Overall, we found that larger effect sizes were associated with acupuncture compared with non-penetrating sham needles (0.43, 95% CI 0.01 to 0.85) than with penetrating sham needles (0.17, 95% CI 0.11 to 0.23), although the difference between groups did not reach conventional levels of statistical significance.
| Type of sham control | Main analysis | Excluding Vas et al.120–122 trials | ||
|---|---|---|---|---|
| Number of trials | Effect size (95% CI) | Number of trials | Effect size (95% CI) | |
| Needle sham | 16 | 0.42 (0.19 to 0.66) | 13 | 0.22 (0.11 to 0.33) |
| Non-needle sham | 4 | 0.38 (0.19 to 0.57) | 4 | 0.38 (0.19 to 0.57) |
| Non-penetrating needle | 7 | 0.76 (0.31 to 1.21) | 4 | 0.43 (0.01 to 0.85) |
| Penetrating needle | 9 | 0.17 (0.11 to 0.23) | 9 | 0.17 (0.11 to 0.23) |
| Non-needle and non-penetrating needle | 11 | 0.63 (0.33 to 0.94) | 8 | 0.40 (0.18 to 0.62) |
Comparisons between types of sham control are provided in Table 7, which shows the results of the random-effects metaregression for sham-controlled trials. Although trials that used needles did not differ significantly from trials that used a non-needle sham control (p ≥ 0.2 for all comparisons), there was clear evidence of a greater effect size when acupuncture was compared against non-penetrating sham than when compared against penetrating sham. Trials using a penetrating needle had an effect size that was –0.21 (95% CI –0.41 to –0.01) SDs lower than trials that did not use a needle sham (p = 0.036). Trials that used penetrating needles for sham control had smaller effect sizes than those with a non-penetrating sham control or sham control without needles. The difference in effect size was –0.45 (95% CI –0.78 to –0.12; p = 0.007). For the sensitivity analysis that excluded the Vas et al. 120–122 trials, this effect size reduced to –0.19 (95% CI –0.39 to 0.01; p = 0.058). There were no significant differences between non-penetrating needles and sham techniques that did not involve needling. Details of further sensitivity analyses are reported separately. 45
| Sham control | Main analysis | Excluding Vas et al.120–122 trials | ||||
|---|---|---|---|---|---|---|
| Number of trialsa | Change in effect size (95% CI) | p-value | Number of trialsa | Change in effect size (95% CI) | p-value | |
| Needle vs. non-needle sham | 16 | 0.02 (–0.49 to 0.53) | 0.9 | 13 | –0.17 (–0.43 to 0.09) | 0.2 |
| 4 | 4 | |||||
| Non-penetrating needle vs. non-needle sham | 7 | 0.35 (–0.28 to 0.99) | 0.3 | 4 | 0.01 (–0.45 to 0.47) | 1 |
| 4 | 4 | |||||
| Penetrating needle vs. non-penetrating needle | 9 | –0.57 (–0.96 to –0.18) | 0.004 | 9 | –0.19 (–0.47 to 0.08) | 0.2 |
| 7 | 4 | |||||
| Penetrating needle vs. non-needle sham | 9 | –0.21 (–0.41 to –0.01) | 0.036 | 9 | –0.21 (–0.41 to –0.01) | 0.036 |
| 4 | 4 | |||||
| Penetrating needle vs. non-needle or non-penetrating needle | 9 | –0.45 (–0.78 to –0.12) | 0.007 | 9 | –0.19 (–0.39 to 0.01) | 0.058 |
| 11 | 8 | |||||
Non-sham acupuncture controls
Eighteen trials including 14,597 patients used a non-sham control, with trial-level characteristics for these trials described in Table 8. The majority of these control groups (72%) were classified as ‘routine care’, with the rest classified as ‘protocolled care’. Table 9 provides details of the non-sham control groups by pain type. The effect size for acupuncture in trials with routine care control arms (0.55, 95% CI 0.40 to 0.70) was larger than when acupuncture was compared with protocolled care (0.29, 95% CI 0.01 to 0.58). Although the difference in effect size was large, it was not significant (difference in effect size 0.26, 95% CI –0.05 to 0.57; p = 0.1). Details of further sensitivity analysis are reported separately. 45
| Trial | Control group | Type of control group |
|---|---|---|
| Foster et al. (2007)135 | Advice and exercise: all three arms of the trial received advice and exercise. Patients received a leaflet with information on knee osteoarthritis. Patients on non-steroidal anti-inflammatory drugs were allowed to continue with a stable dose. Individualised exercises of progressive intensity for lower limb stretching, strengthening and balance (up to six 30-minute sessions over 6 weeks). Patients in the control arm did not receive verum or sham acupuncture | Protocolled |
| Linde et al. (2005),68 Melchart et al. (2005)67 | Waiting list control: control patients were not permitted to undergo prophylactic treatment for 12 weeks. All patients were allowed to treat acute headache as necessary (following current guidelines) | Routine |
| Thomas et al. (2006),61 Salter et al. (2006),133 Vickers et al. (2004)60 | GP care: all patients received NHS treatment according to GPs’ assessment and recommendations. Control patients did not receive acupuncture or any other specified interventions | Routine |
| Berman et al. (2004)134 | Education/attention control: patients in this arm attended six 2-hour group sessions based on arthritis self-management and received periodic educational materials by mail. Patients in the acupuncture and sham-acupuncture arms did not participate in this intervention | Routine |
| Cherkin et al. (2001)131 | Self-care education: patients in this group received a book with information about back pain, treatment, improving quality of life and coping with emotional and interpersonal issues surrounding back pain. Patients also received two professionally produced videos that addressed self-management of back pain and demonstrated exercises. Patients in the acupuncture and massage groups did not receive this educational material | Routine |
| Scharf et al. (2006)70 | Conservative therapy: patients in the conservative therapy group had 10 visits with a physician and received prescriptions for either diclofenac (up to 150 mg/day) or rofecoxib (25 mg/day) up to week 23. Patients in this group who had ‘partially successful’ results were given the option of attending an additional five visits. Patients in the verum acupuncture and sham acupuncture groups were permitted to take up to 150 mg/day of diclofenac for the first 2 weeks and a total of 1 g of diclofenac during the rest of the study. Patients in both acupuncture groups and in the conservative management group received up to six sessions of physiotherapy. All patients were prohibited from taking any analgesics other than diclofenac and rofecoxib and any corticosteroids | Protocolled |
| Diener et al. (2006)72 | Standard migraine treatment: control group patients were treated according to the guidelines of the German Migraine and Headache Society. Patients had six to seven visits in which standard treatment was established. First choice of treatment was beta-blockers, followed by flunarizine and then valproic acid. Acute medication use was permitted in all groups | Protocolled |
| Haake et al. (2007)71 | Conventional therapy: patients in the conventional therapy group were treated according to German guidelines. Conventional therapy patients had 10 visits with a physician or physiotherapist at which physiotherapy, exercise and/or similar treatments were offered. Patients in all three arms were permitted to take non-steroidal anti-inflammatory drugs up to the maximum daily dose | Protocolled |
| Williamson et al. (2007)136 | Education and exercise: patients in the control group were told that they were in the ‘home exercise’ group and received an exercise and advice leaflet | Routine |
| Witt et al. (2005),69 Brinkhaus et al. (2006)66 | Waiting list control: patients in the waiting list control group received no acupuncture treatment for 8 weeks after randomisation. All patients were allowed oral non-steroidal anti-inflammatory drugs for pain as rescue medication. All patients were prohibited from taking corticosteroids or pain medication that acted on the central nervous system | Routine |
| Witt et al. (2006)77 (osteoarthritis), Witt et al. (2006)74 (lower back pain) | Conventional treatment: patients in the control group were not allowed to use any kind of acupuncture during the first 3 months. All patients were allowed to use additional conventional treatments as needed | Routine |
| Jena et al. (2008),76 Witt et al. (2006)75 (neck pain) | Conventional treatment: patients in the control group were not allowed to use any kind of acupuncture during the first 3 months. All patients were allowed to use additional conventional treatments as needed | Routine |
| Pain type | Routine care | Protocolled care | Total |
|---|---|---|---|
| Headache | 2 | 0 | 2 |
| Migraine | 1 | 1 | 2 |
| Tension-type headache | 1 | 0 | 1 |
| Osteoarthritis | 4 | 2 | 6 |
| Lower back pain | 3 | 2 | 5 |
| Neck pain | 2 | 0 | 2 |
| Total | 13 (72%) | 5 (28%) | 18 (100%) |
Results for substudy 2: characteristics of acupuncture
Table 10 provides a summary of the trial-level acupuncture characteristics and Table 11 provides a summary of the patient-level acupuncture characteristics. Fuller details of the characteristics of each individual trial are presented in Appendix 2. The acupuncture in the majority of trials was based on traditional Chinese acupuncture (59%) and had a flexible point prescription (55%). In all 29 trials manual needle stimulation was used in the acupuncture group, whereas only about one-quarter of trials allowed the addition of electrical stimulation (n = 7) and 14% allowed moxibustion (n = 4). Attempts to elicit deqi in the acupuncture group were made in all 25 trials that provided this information. The mean session frequency ranged from one session every 8 days to two sessions per week. The maximum number of sessions varied widely, from three to 30, as did the mean number of needles used (range 1–18) and the mean session duration (range 15–32 minutes).
| Characteristics | n (%) |
|---|---|
| Style of acupuncture | |
| Traditional Chinese techniques | 17 (59) |
| ‘Western’ | 4 (14) |
| Combination of traditional Chinese techniques and Western | 8 (28) |
| Point prescription | |
| Fixed needle formula | 4 (14) |
| Flexible formula | 16 (55) |
| Individualised | 9 (31) |
| Location of needles | |
| Local points only | 0 (0) |
| Distal points only | 1 (3) |
| Both local and distal points | 28 (97) |
| Electrical stimulation allowed | 7 (24) |
| Moxibustion allowed | 4 (14) |
| Deqi attempted (n = 25) | 25 (100) |
| Acupuncture-specific patient–practitioner interactions | 12 (41) |
| Minimum years of experience required | |
| No requirement specified (0 years) | 12 (41) |
| 6 months to 2 years | 5 (17) |
| 3 years | 9 (31) |
| 5 years | 2 (7) |
| 10 years | 1 (3) |
| Maximum number of sessions | |
| 3–5 | 3 (10) |
| 6–10 | 14 (48) |
| 11–15 | 7 (24) |
| 16–20 | 3 (10) |
| 21–30 | 2 (7) |
| Frequency of sessions (mean number of sessions per week) | |
| 0.88 | 1 (3) |
| 1 | 14 (48) |
| 1.5 | 7 (24) |
| 1.67 | 1 (3) |
| 2 | 6 (21) |
| Mean duration of sessions (minutes), rounded to whole numbers | |
| 15–19 | 1 (4) |
| 20–24 | 4 (16) |
| 25–29 | 6 (24) |
| 30+ | 14 (56) |
| Mean number of needles used, rounded to whole numbers | |
| 1–4 | 1 (4) |
| 5–9 | 6 (25) |
| 10–14 | 9 (38) |
| 15–20 | 8 (33) |
| Characteristics | n (%) |
|---|---|
| Number of sessions | |
| 0 | 383 (2) |
| 1–5 | 402 (2) |
| 6–10 | 7161 (39) |
| 11–15 | 1998 (11) |
| 16–20 | 45 (< 1) |
| 21–30 | 16 (< 1) |
| Missing | 1806 (10) |
| Not reported | 6623 (36) |
| Average session duration (minutes) | |
| 2–15 | 166 (1) |
| 16–30 | 2552 (14) |
| 31–45 | 406 (2) |
| 46–60 | 60 (< 1) |
| 60+ | 3 (< 1) |
| Missing | 1257 (7) |
| Not reported | 13,990 (76) |
| Average number of needles | |
| 2–5 | 20 (< 1) |
| 6–10 | 610 (3) |
| 11–15 | 717 (4) |
| 16–20 | 627 (3) |
| 21–25 | 177 (1) |
| 26+ | 27 (< 1) |
| Missing | 2529 (14) |
| Not reported | 13,727 (74) |
| Age of physician/acupuncturist (years) | |
| 30–35 | 298 (2) |
| 36–40 | 2119 (11) |
| 41–45 | 2630 (14) |
| 46–50 | 2407 (13) |
| 51–55 | 1701 (9) |
| 56–60 | 872 (5) |
| 60+ | 303 (2) |
| Missing | 368 (2) |
| Not reported | 7736 (42) |
| Physician/acupuncturist sex | |
| Male | 7002 (38) |
| Female | 3626 (20) |
| Missing | 0 (0) |
| Not reported | 7806 (42) |
None of the acupuncture characteristics evaluated in the trial-level analysis, including style of acupuncture, number or placement of needles, number, frequency or duration of sessions, patient–practitioner interactions or experience of the acupuncturist significantly modified the effect of acupuncture on pain (all p > 0.05) in sham-controlled trials (Table 12). Compared with non-sham controls, there was little evidence that these characteristics modified the effect of acupuncture (see Table 12). The exception was that acupuncture effects increased in comparison to non-sham controls when more needles were used.
| Characteristic | Acupuncture vs. sham control (n = 20) | Acupuncture vs. non-acupuncture control (n = 18) | ||||
|---|---|---|---|---|---|---|
| βa | 95% CI | p-value | βa | 95% CI | p-value | |
| Style of acupuncture | ||||||
| Some traditional Chinese medicine vs. Western only | 0.05 | –0.52 to 0.63 | 0.9 | 0.13 | –0.51 to 0.77 | 0.7 |
| Traditional Chinese medicine only vs. some Western | 0.20 | –0.20 to 0.61 | 0.3 | –0.10 | –0.38 to 0.19 | 0.5 |
| Point prescription | ||||||
| Fixed needle formula | Reference | Reference | ||||
| Flexible formula | –0.08 | –0.58 to 0.43 | 0.8 | 0.02 | –0.64 to 0.68 | > 0.9 |
| Individualised | –0.15 | –1.16 to 0.86 | 0.8 | –0.08 | –0.74 to 0.59 | 0.8 |
| Electrical stimulation allowed | 0.34 | –0.13 to 0.80 | 0.15 | –0.19 | –0.56 to 0.17 | 0.3 |
| Manual stimulation allowed | All allowed | All allowed | ||||
| Moxibustion allowed | All did not allow | –0.28 | –0.63 to 0.06 | 0.11 | ||
| Deqi attempted | All allowed | All allowed | ||||
| Acupuncture-specific patient–practitioner interactions allowed | –0.22 | –0.70 to 0.26 | 0.4 | 0.06 | –0.23 to 0.35 | 0.7 |
| Minimum experience required (years) | 0.01 | –0.08 to 0.10 | 0.8 | 0.05 | –0.05 to 0.16 | 0.3 |
| Maximum number of sessions (per five sessions) | –0.14 | –0.37 to 0.08 | 0.2 | 0.02 | –0.07 to 0.12 | 0.6 |
| Frequency of sessions (per week) | –0.19 | –0.66 to 0.27 | 0.4 | 0.09 | –0.31 to 0.49 | 0.7 |
| Duration of sessions (per 5 minutes)b | –0.10 | –0.30 to 0.11 | 0.4 | –0.01 | –0.26 to 0.24 | 0.9 |
| Number of needles used (per five needles)c | –0.17 | –0.37 to 0.03 | 0.095 | 0.33 | 0.08 to 0.58 | 0.01 |
The results of the sensitivity analysis that excluded the three outlying trials,120–122 all sham controlled and by the same team and with very much larger effect sizes than the other trials, are presented in Table 13. This showed that trials allowing electrical stimulation showed a significantly stronger effect of acupuncture than of sham controls and those with a longer average treatment session duration had a smaller effect than sham controls.
| Characteristic | Acupuncture vs. sham control (n = 17) | |||
|---|---|---|---|---|
| n | βa | 95% CI | p-value | |
| Style of acupuncture | 17 | |||
| ‘Western’ only | Reference | |||
| Traditional Chinese medicine | –0.14 | –0.46 to 0.17 | 0.4 | |
| Point prescription | 17 | |||
| Fixed needle formula | Reference | |||
| Flexible formula | –0.25 | –0.50 to 0.00 | 0.054 | |
| Individualised | –0.11 | –0.58 to 0.36 | 0.6 | |
| Electrical stimulation allowed | 17 | 0.27 | 0.03 to 0.51 | 0.027 |
| Manual stimulation allowed | 17 | All allowed | ||
| Moxibustion allowed | 17 | All did not allow | ||
| Deqi elicited (n = 26) | 17 | All allowed | ||
| Acupuncture-specific patient–practitioner interactions allowed | 17 | –0.04 | –0.28 to 0.20 | 0.8 |
| Minimum experience required (years) | 17 | 0.00 | –0.05 to 0.05 | > 0.9 |
| Maximum number of sessions (per five sessions) | 17 | –0.05 | –0.18 to 0.08 | 0.4 |
| Frequency of sessions (per week) | 17 | –0.04 | –0.29 to 0.21 | 0.8 |
| Duration of sessions (per 5 minutes) | 17 | –0.14 | –0.22 to –0.06 | 0.001 |
| Number of needles used (per five needles) | 17 | –0.08 | –0.22 to 0.05 | 0.2 |
In the patient-level analysis, we found that the direction of the effect of the acupuncture characteristics was unchanged (Table 14). As expected, the CIs were much tighter around the patient-level estimates despite the fact that fewer trials could be included in each analysis. There were no more than six trials with patient-level data available for each of the specific predictors being analysed. The patient-level analysis suggested that a higher number of acupuncture treatment sessions improves the effect of acupuncture. Further details of the results are reported separately. 123
| Characteristic | Sham control | Non-acupuncture control | ||||||
|---|---|---|---|---|---|---|---|---|
| n a | βb | 95% CI | p-value | n a | βb | 95% CI | p-value | |
| Number of sessions (per five sessions) | 3 (646/648) | –0.76 | –1.75 to 0.22 | 0.13 | 5 (8292/9321) | 0.11 | 0.01 to 0.21 | 0.0007 |
| Duration of sessions (per 5 minutes) | 5 (2444/2482) | –0.03 | –0.08 to 0.03 | 0.3 | Fewer than three trials | |||
| Number of needles used (per five needles) | 5 (1769/2484) | –0.11 | –0.35 to 0.14 | 0.4 | Fewer than three trials | |||
| Age of acupuncturist (per 5 years) | Fewer than three trials | 6 (9127/9446) | –0.01 | –0.04 to 0.02 | 0.5 | |||
| Male acupuncturist | Fewer than three trials | 6 (9384/9446) | –0.07 | –0.16 to 0.02 | 0.084 | |||
Discussion
Our principal finding is that there are statistically significant differences between acupuncture and sham acupuncture, and between acupuncture and non-acupuncture controls for all of the pain types studied. The meta-analytic effect sizes were similar across pain conditions for both of these comparisons after excluding an outlying set of studies. The difference between acupuncture and sham acupuncture was of a lesser magnitude, ranging from 0.15 to 0.23 SDs depending on condition. For acupuncture compared with non-acupuncture control, there was a larger effect size of around 0.5 SDs, although there was some variation in the effect size for individual RCTs, a variation that could be partly explained in terms of the type of control used. For example, acupuncture had a smaller benefit in patients who received a programme of ancillary care involving physiotherapist-led exercise135 than in patients who continued to be treated with usual care. Nevertheless, the average effect, as expressed in the meta-analytic estimate of approximately 0.5 SDs, was of clear clinical relevance either considered as a standardised difference or when converted back to a pain scale.
This meta-analysis has not been compromised by study quality or sample size, on the basis that only high-quality studies were included and the total sample size was large. In addition, we saw no evidence that publication bias, or failure to identify published eligible studies, might affect our conclusions. With regard to limitations, the comparisons between acupuncture and non-acupuncture controls cannot be blinded and therefore both performance and response bias are possible. Although we considered the risk of bias from unblinding to be low in most studies comparing acupuncture and sham acupuncture, health-care providers could not but be aware of the treatment that they provided and therefore a certain degree of bias of our effect estimate for specific effects is possible. However, it should be kept in mind that this problem applies to almost all studies on non-pharmacological interventions. We would argue that the risk of bias in the comparison between acupuncture and sham acupuncture was low compared with the risk of bias for other non-drug treatments for chronic pain, such as cognitive therapies, exercise or manipulation, which are rarely subject to placebo control. The meta-analyses combined different end points, such as pain and function, measured at different times. However, when we restricted the analysis to pain end points measured at a specific follow-up time, 2–3 months after randomisation, our results did not change.
Many previous systematic reviews of acupuncture for chronic pain have included RCTs of low methodological quality because of liberal eligibility criteria. As a result, the authors have come to the circular conclusion that weaknesses in the data did not allow conclusions to be drawn. 57,140 Because of variation in the study end points, other reviews have not included meta-analyses. 110,141 Both limitations have been avoided by including only high-quality RCTs and obtaining raw data for IPD meta-analysis. Some more recent systematic reviews have published meta-analyses48,64,65,142 and reported findings that are broadly comparable with ours, with clear differences between acupuncture and non-acupuncture controls and smaller differences between true and sham acupuncture. Our findings have greater precision: all previous reviews have analysed summary data, an approach of reduced statistical precision compared with IPD meta-analysis. 105,143 In particular, we have demonstrated a robust difference between acupuncture and sham acupuncture that can be distinguished from bias. This is a novel finding that moves the evidence base beyond the existing literature.
These findings are important both clinically and scientifically. The total effects of acupuncture, as experienced by the patient in routine clinical practice, are clinically relevant as a result of our determination of an effect size of around 0.5. Our finding that acupuncture also has statistically significant effects over and above those of sham acupuncture, with an effect size of around 0.2, is therefore of major importance for clinical practice. We have found the effect size of sham acupuncture compared with non-acupuncture controls to be around 0.3. Accordingly, there appears to be no evidence to support the claim that it makes little difference whether one receives real or sham acupuncture. The size of this effect may be associated with sham acupuncture’s potentially potent placebo or context effects,144–146 or with the additional physiological effects associated with needle penetration even when at the wrong acupuncture points. 147 If one accepts that, on average, the differences in effect sizes between acupuncture and sham acupuncture are small, the clinical decision made by physicians and patients is not between true and sham acupuncture but between a referral to an acupuncturist or avoiding such a referral. The total effects of acupuncture, as experienced by the patient in routine practice, include the specific effects associated with correct needle insertion according to acupuncture theory, non-specific physiological effects of needling and non-specific psychological (placebo) effects related to the patient’s belief that treatment will be effective.
Further research will include the ongoing addition of new trials to the database subject to eligibility. This will require further literature searches and then updating of the main meta-analysis. Although six substudies were planned as part of the original programme of work, we report in detail on only two here. 45,123
Discussion for substudy 1: influence of control group on effect size
This substudy has extended the primary findings of the main study, confirming that acupuncture is significantly superior to either sham acupuncture or non-sham controls by also showing that this result holds irrespective of which type of sham or non-sham control is used. This substudy has produced robust data on the differences in effect sizes between trials with different control conditions. We found that the acupuncture in trials with sham controls involving penetrating needles had smaller effect sizes than the acupuncture in trials that did not use a needle control or in which the needles in the control group did not penetrate the skin. An important implication is that the central estimates from our primary meta-analysis may have underestimated the effects of acupuncture compared with sham acupuncture. Although differences did not reach statistical significance, we have also found evidence that the effect size of acupuncture when compared with protocolled care is smaller than when compared with the less intensive routine care.
With regard to the impact of placebo controls for non-pharmacological therapies, one approach has been to investigate trials that included both a placebo arm and a no-treatment arm and then compare outcomes between these two, and in this way explore variations in the impact of the different types of placebo. An example of this is a Cochrane review of placebo controls covering a wide range of trials for different conditions, including some acupuncture trials. 44 In a subgroup analysis the authors found that trials using ‘physical placebos’ (including sham acupuncture) were associated with greater placebo effects than trials using pharmacological placebos. This finding is consistent with the results of a trial that was specifically designed to compare a sham device (sham acupuncture) with an inert pill, the sham device being associated with a greater reduction in self-reported pain. 144 Our finding that different types of sham control led to different estimates of treatment effects is consistent with these findings.
By combining patient data from 29 high-quality trials in a single database, we have had, for the first time, sufficient power to explore the role of controls in trials of acupuncture for chronic pain. This is because the power of metaregression is strongly influenced by the number of trials and their variation. However, even with this large data set we are not able to obtain a full understanding of the different physiological and psychological effects of sham acupuncture. One limitation within the field generally is that the mechanisms for a persistent effect of acupuncture on chronic pain are incompletely understood and therefore we have no clear idea of whether or not a sham control inadvertently activates these mechanisms. This lack of understanding about the physiological mechanisms of acupuncture limits any firm conclusions that we can draw regarding the extent that any of the sham controls discussed above can be considered as a true ‘placebo’. Moreover, when implementing sham acupuncture trials the outcome may also be influenced by factors not included in our analysis. These include practical implementation issues such as how carefully the ring that comes with the Streitberger needle108 is taped in place, the believability of the control, patients’ prior knowledge of acupuncture, whether or not the true acupuncture group is treated identically and the extent to which the acupuncturists are able to maintain equipoise.
Although sham acupuncture involving penetrating needles may well have a place when addressing questions of point specificity in explanatory trials, our results provide support to the contention that needle penetration should be avoided as a sham technique when controlling for non-specific effects associated with acupuncture for chronic pain. We are more cautious with regard to recommending the use of non-penetrating needles. Many forms of Japanese acupuncture use shallow insertion or non-insertion (the toya hari method). 148 Using non-penetrating needles in controlled trials is not without its challenges: although apparently less active than other types of sham control, we cannot assume that non-penetrating needles have complete physiological inactivity. Furthermore, there are practical and generic questions regarding the use of sham acupuncture, for example whether or not to enrol only acupuncture-naive patients and whether or not practitioners can maintain equipoise in large trials over reasonable periods of time.
The choice of control needs to be driven by the research question. For instance, in the UK NHS trial of acupuncture for chronic headache, the study question of Vickers et al. 60 was related to the effects of making acupuncture more widely available in primary care, a pragmatic comparison of ‘use acupuncture’ and ‘avoid acupuncture’. On the other hand, Foster et al. 135 were interested in the impact of acupuncture when added to an existing rehabilitation programme. Our findings have clear implications for sample size calculations, with larger sample sizes needed in trials in which care in the control arm is carefully specified. Further discussion has been reported separately. 45
Discussion for substudy 2: characteristics of acupuncture
Our results from high-quality trials of acupuncture for chronic pain provide robust data on treatment effect modifiers related to treatment characteristics. Across the many characteristics that might have been commonly expected to modify outcomes, such as style of acupuncture, use of electrical stimulation, addition of moxibustion, experience of the acupuncturist and the frequency and duration of sessions, we found no evidence of a modifying effect on pain outcomes in trial-level analyses. We also found in patient-level analyses, involving a subset of only five trials, that more treatment sessions were associated with better pain outcomes in acupuncture treatment groups compared with non-acupuncture controls (p < 0.001). It appears that the ‘dose’ of acupuncture has an impact on treatment outcomes. We need to be careful when interpreting these results in the context of testing multiple hypotheses, which increases the risk of falsely rejecting at least one null hypothesis. We did not feel that formal statistical correction to account for multiple testing was justified given the largely null results.
Our observations on the minimal impact of style of acupuncture practice on outcomes contrasts with findings from qualitative studies asserting the importance of the theoretical affiliation and institutional context of the acupuncturists. 149,150 For example, theoretical affiliation has been linked to impact on ‘almost all aspects of treatment’, with ‘demonstrable implications for the practice and research of acupuncture’. 149 Similarly, others have argued that without a theoretical approach to acupuncture that is ‘holistic’, with an emphasis that includes the process of care, there are likely to be reduced or absent outcomes. 150
In a study that pooled data from four German-based trials of acupuncture for chronic pain,151 all of which are included in our study, we see some concordance with our results, as might be expected. The only physician characteristic that the authors found had a significant influence on outcome in these four trials was that internists performed slightly better than an average physician and ‘orthopaedists’ slightly worse.
Given the results of the primary analyses in this study, which showed small differences between real and sham acupuncture, it is perhaps unsurprising that this substudy showed little evidence of substantial differences between alternative approaches to, or characteristics of, acupuncture. It is likely that we had insufficient power to determine the extent, if any, of the contribution of each individual component of acupuncture to outcome, even with the large data set at our disposal.
As for any meta-analysis, the main limitation was data availability. The total number of trials was relatively modest and analyses with IPD included no more than five trials. Consequently, many of our analyses had relatively low power, with wide CIs around central estimates. Furthermore, heterogeneity of treatment characteristics was relatively limited. For example, nearly 75% of trials involved between six and 15 treatments, and in no trial was acupuncture administered more than twice a week. It is not unusual in China for acupuncture to be given four or five times a week. A feature that characterises acupuncture when practised according to the principles of traditional Chinese medicine involves syndrome differentiation, but we had no data on which to assess the resulting impact on outcome.
The quality of the current evidence supports the case that contemporary acupuncture trials do not systematically underestimate treatment effects. Although acupuncturists have long been concerned about what constitutes ‘correct’ practice,152,153 it can be argued that the consensus methods that are often used to determine acupuncture characteristics – number of treatment sessions, duration of sessions, needle prescriptions and training and experience of acupuncturists – are appropriate. This is because the variations in outcome associated with these factors are likely to be small. This study would suggest that the most useful characteristics to test would be the number of needles and number of treatment sessions.
Few characteristics of acupuncturists were reported sufficiently consistently by triallists, namely age, sex and minimum experience as an acupuncture practitioner, and consequently our results on related effects on outcome were compromised. We know that some practitioners have better results than others, yet we do not have the data from this substudy to understand why, a concern also raised in relation to other therapist-led interventions. 154,155 It is likely that more sophisticated measures are needed, such as a measure of the patients’ perception of a practitioner’s empathy, which has been shown to correlate with enablement and in turn with outcome. 156
Recommendations for future research
Four additional substudies are planned by the ATC, as well as an update to the database by adding in a set of more recently published trials. The first additional substudy will explore the time course of acupuncture, establishing for how long the effects of acupuncture last beyond the end of treatment. Another will explore the relationship between characteristics of the patients and variations in outcome, for example whether or not there any baseline characteristics of patients, such as age, psychological distress or baseline severity, that might influence the effects of acupuncture. In the third substudy we will determine if there is a certain type of patient who could be classed as a super-responder. Finally, a substudy will explore whether or not the effects of acupuncture are significantly influenced by the acupuncture practitioner.
An important recommendation for future research is that trials need to be designed with sufficient power to detect the small differences in outcome that might be associated with differences between acupuncture and sham acupuncture. Given our analyses involving acupuncture trials for chronic pain, an effect size of 0.2 requires a sample size in a two-arm trial of > 1000 patients, a number rarely reached in almost all sham-controlled trials. To determine differences between two different styles of acupuncture, for example when one might hypothesise that the difference in outcome between two individual acupuncture characteristics of treatment is associated with a smaller effect size of say 0.10 or 0.05, would require > 2000 or > 8000 patients, respectively, assuming 90% power and a 0.05 significance level.
To explore contextual factors, further research into empathy and enablement as well as other measures such as the therapeutic alliance or success of patient–practitioner interactions may be useful lines of enquiry. Interestingly, a widely accepted principle underlying traditional Chinese medicine is that it is not the techniques and methods used but the cultivation of the practitioner that is the key to effective practice. 157 A qualitative study might provide a useful way of exploring the key factors which explain the observation that some practitioners consistently have better patient outcomes than others.
Conclusion
Acupuncture is superior to both non-acupuncture controls and sham acupuncture for the treatment of chronic pain and is therefore a reasonable referral option for patients with chronic pain. The data indicate that acupuncture is more than a placebo; however, the differences between true and sham acupuncture are relatively modest. Other factors, in addition to the specific effects of needling, are important contributors to therapeutic effects. Given that the results are from IPD meta-analyses of nearly 18,000 randomised patients in high-quality RCTs, it can be concluded that they provide the most robust evidence to date on acupuncture for chronic pain.
Moreover, acupuncture is significantly superior to control, irrespective of the subtype of control. With regard to sham acupuncture, non-penetrating needles appear to be a more effective sham control. In addition, the effect size of acupuncture is greater when compared with routine care than when compared with protocol-guided care. Our findings can help inform study design in acupuncture, particularly with respect to sample size, in the context that the choice of control should be driven by the research question.
We found little evidence that different characteristics of acupuncture or acupuncturists were effect modifiers. There was modest evidence that more needles and more sessions were associated with better outcomes when comparing acupuncture with non-sham controls, suggesting that the dose of acupuncture is important. Trials designed to evaluate the potentially small differences in outcome associated with different acupuncture or acupuncturist characteristics are likely to require large sample sizes.
Chapter 3 Comparison of acupuncture with other physical treatments for pain caused by osteoarthritis of the knee: a network meta-analysis
Background
Osteoarthritis is a degenerative condition involving the progressive wearing down of (joint) bone and cartilage, normally resulting in pain, stiffness and functional disability. These symptoms usually worsen according to how much the affected joint is used. In adults aged ≥ 45 years, the knee represents the most common site of peripheral joint pain and the prevalence of painful, disabling knee osteoarthritis in people aged > 55 years is 10%. 158 Risk factors for knee osteoarthritis include age, sex, obesity, bone density, genetic factors and injury.
Diagnosis is usually made using clinical features of knee osteoarthritis, by radiological assessment of the knee or by a combination of the two. Radiographic features – the severity of which are commonly summarised using the Kellgren and Lawrence score159 – have been significantly associated with knee pain. 160
The WOMAC index is a self-administered disability status measure for knee (or hip) osteoarthritis. 115 Its individual components assess pain, stiffness and function, with the summed scores producing an overall measure of disability (WOMAC index). As a standardised and comprehensive assessment of disability and its components, the WOMAC index increases transparency and comparability within clinical research.
The treatment of knee osteoarthritis should be tailored according to knee risk factors (obesity, adverse mechanical factors, physical activity), general risk factors (age, comorbidity, polypharmacy), level of pain intensity and disability, sign of inflammation, and location and degree of structural damage. 161 The main objective of a GP treating a patient with knee osteoarthritis is normally alleviation of pain; failure to control pain may result in reduced mobility and daily activities, leading to a reduction in quality of life. 161 The more sedentary lifestyle that might follow may, in turn, exacerbate the symptoms of knee osteoarthritis through lack of exercise and joint movement, and weight gain.
In clinical practice, treatment often begins with analgesia [paracetamol and/or topical non-steroidal anti-inflammatory drugs (NSAIDs)] and, when these are ineffective, a cyclooxygenase-2 inhibitor is recommended. GP advice about exercise and weight loss, which NICE guidelines82 recommend as part of core therapy, is often given in addition to (rather than instead of) analgesic drugs. The regular and long-term use of pharmacological agents such as NSAIDs for pain may be associated with side effects, such as gastrointestinal bleeding, without necessarily resulting in worthwhile pain reduction. 162 A UK review of qualitative studies of medicine taking163 revealed considerable reluctance to take drugs and a preference to take as little as possible; many knee osteoarthritis patients want non-pharmacological treatments for pain relief. 164 The use of physical (i.e. non-pharmacological) treatments such as acupuncture is therefore likely to be attractive for patients seeking alternatives, particularly for a condition such as osteoarthritis of the knee for which there is currently no cure.
In patients for whom insufficient pain relief has been provided by the core interventions mentioned above (as recommended by NICE), coupled with paracetamol and/or topical NSAIDs, GPs may consider a range of physical treatments as the next step in the treatment pathway. The NICE guidelines82 list muscle-strengthening and aerobic exercise, manual therapy, TENS, braces and insoles, weight loss, and heat and cooling treatments as being among such alternatives, but acupuncture was not recommended.
Other non-pharmacological interventions used for osteoarthritis of the knee, but which would not be considered as alternatives to acupuncture, include surgery, an intervention that would be considered at a later stage in the treatment pathway. Similarly, structured psychosocial/educational interventions are generally considered for a different group of patients, that is, when pain-reducing therapies have failed and the emphasis is on a need for pain-coping skills, rather than pain reduction. 165
Many reviews have been undertaken of the varying types of physical therapies for osteoarthritis of the knee, but evaluation of a single therapy for a single condition provides only a limited basis for decision-making. Few randomised trials have directly compared physical therapies and no review has attempted to address the question of how effective such treatments are relative to each other using statistical methods. The focus of interest within our study was on acupuncture, as this review was funded as part of this programme of projects on acupuncture and chronic pain, and because of the uncertainty within the NICE decision-making process with regard to the level of evidence on acupuncture for osteoarthritis relative to other physical treatments. 84 The purpose of this systematic review, therefore, was to comprehensively synthesise both the direct and the indirect evidence – using mixed-treatment comparison methods in a network meta-analysis – to compare the effectiveness of different physical therapies used for the alleviation of knee pain caused by osteoarthritis.
In a separate substudy within this project we summarise the reporting methods of the WOMAC pain subscale and the WOMAC index from the trials identified for the main study, and make recommendations to improve reporting in future studies.
Methods
Using the methods recommended by the Centre for Reviews and Dissemination (CRD)166 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,167 the systematic review and network meta-analysis was first conducted in 2010. A report based on this study is available on the CRD website. 168 This chapter reports an update of this systematic review and network meta-analysis conducted in 2013 and published in Osteoarthritis and Cartilage. 169
Literature search
We searched 17 electronic databases from inception to June 2013. A combination of relevant free-text terms, synonyms and subject headings relating to osteoarthritis of the knee and named physical treatments were included in the strategy. A search filter was used to limit retrieval of studies to RCTs. No language or date restrictions were applied.
The base search strategy developed in MEDLINE was translated to run on the databases listed in Appendix 3. Adaptations to the search strategy were necessary for certain databases: Manual, Alternative and Natural Therapy Index System (MANTIS), PASCAL (database of the Institut de l’Information Scientifique et Téchnique), Inside Conferences, Physiotherapy Evidence Database (PEDro), CAMbase (Complementary and Alternative Medicine), Latin American and Caribbean Health Sciences Literature (LILACS) and ClinicalTrials.gov. Supplementary internet searches of websites relating to osteoarthritis were undertaken to locate any additional studies not found from the database searches. The bibliographies of all relevant reviews and guidelines were checked for further potentially relevant studies. The base MEDLINE search strategy can be found in Appendix 3.
Study selection and definitions of interventions and outcome
All abstracts were screened by two reviewers independently, followed by all relevant full papers. Disagreements were resolved by discussion or, when necessary, by a third reviewer. We included RCTs in adults with osteoarthritis of the knee (in which the mean age of the population was ≥ 55 years) that assessed pain as an outcome. Studies with mixed populations (e.g. including both patients with osteoarthritis of the knee and those with osteoarthritis of the hip) that presented results by site of osteoarthritis were eligible for inclusion. Trials of acute knee pain or trials in which the mean age of the population was < 55 years were excluded.
We included treatment with the following: acupuncture, balneotherapy, braces, aerobic exercise, muscle-strengthening exercise, heat treatment, ice/cooling treatment, insoles, interferential therapy, laser/light therapy, manual therapy, neuromuscular electrical stimulation (NMES), pulsed electrical stimulation (PES), pulsed electromagnetic fields (PEMFs), static magnets, t’ai chi, TENS and weight loss. We aimed not to be restrictive with regard to selecting the types of intervention within these categories. However, exercise interventions that were predominantly home based and unsupervised were excluded as being too similar to standard care. Trials evaluating surgery or medication were also excluded, as were studies evaluating the combination of two or more physical treatments and studies comparing only different regimens/durations/modalities of the same type of intervention.
When considering the electrotherapy interventions, we classed studies using ‘pulsed short-wave’ interventions as being PES. Although interferential therapy works in a similar way to TENS, we classed it as a distinct intervention. Similarly, NMES was considered separately from TENS as it is commonly used to elicit muscle contraction, as opposed to TENS, which stimulates nerves with the aim of blocking pain inputs to the brain. PEMFs (in which an electric current is generated in the treated area by means of a magnetic field) was also classed separately.
We classified adjunctive components to the main intervention into five categories, based on what was reported in the trials: (1) treatment as usual, (2) treatment as usual plus specified home exercise or education, (3) treatment as usual plus specified (trial-specific) analgesics, (4) no medication and (5) no medication plus specified home exercise or education. Using this coding we explored the impact of different adjunctive components on the main interventions and on variations in standard care.
Eligible comparators included any form of standard or usual care, including waiting-list control (which could incorporate one or more of analgesics, education and exercise advice), all of which we classed as being ‘standard care’. Placebo interventions, no intervention and sham acupuncture were also eligible. Because of evidence suggesting that sham acupuncture is more active than an inert ‘placebo’, it was treated as a separate comparator. 145 Commonly, sufficient details about standard care or usual treatment were not reported.
Data extraction
Using a standardised data extraction form created using EPPI-Reviewer software (version 4.0; Evidence for Policy and Practice Information and Co-ordinating Centre, University of London, London, UK), data were extracted on population characteristics [population type, method of diagnosis, age, sex, weight, body mass index (BMI) and Kellgren and Lawrence score], intervention parameters and study quality. Data on pain assessment at baseline, at the end of treatment and at all subsequent time points were extracted onto a Microsoft Excel® 2010 spreadsheet (Microsoft Corporation, Redmond, WA, USA). Data extraction was performed by one reviewer and independently checked by a second reviewer. Any disagreements were resolved by discussion or by a third reviewer when necessary. Data from non-English-language papers were extracted by one reviewer together with a native speaker. Multiple publications of the same study were extracted as one study, using all of the information available.
Assessment of trial quality
Trial quality was assessed using 14 questions adapted from a checklist used in a previous review by researchers at the CRD. 170 Based on the number of criteria satisfied, studies were then graded as excellent, good, satisfactory or poor. To be of satisfactory quality studies had to report the number of randomised participants; have groups with comparable baseline characteristics for important variables, such as pain; adequately report eligibility criteria; clearly report on losses to follow-up; report data for the intention-to-treat population; and use an appropriate placebo (if relevant). Poor-quality trials were those that failed to satisfy one or more of the criteria required for satisfactory study quality. Beyond questions relating to the above grading, other questions in the assessment covered methods of randomisation and allocation concealment, level of blinding, use of a power calculation and level of losses to follow-up. A further quality assessment was conducted using the Cochrane risk-of-bias tool. 171 Quality assessments were performed by one reviewer and independently checked by a second reviewer. Any disagreements were resolved by discussion or by a third reviewer when necessary.
Outcomes and data transformations
It was anticipated that pain – our primary outcome – would be measured using a variety of measures, for example a VAS, Likert scale, WOMAC pain subscale and Arthritis Impact Measurement Scale (AIMS), with all scales accepted.
The WOMAC is a widely used, self-administered health status measure that assesses the dimensions of pain, stiffness and physical function in patients with osteoarthritis of the hip or knee. It is available in five-point Likert, 11-point numerical rating and 100-mm VAS formats. Under each dimension there are a number of questions designed to assess the clinical severity of the disease (five questions for pain, two questions for stiffness and 17 questions for physical function). The patient’s response to each question produces a score, with the scores summed to derive an aggregate score for each dimension. There are three subscale scores (pain, stiffness and physical function) and a total score (WOMAC index), which reflects disability overall.
The WOMAC pain score range has been reported in various ways: a VAS 0–10 scale (commonly reported across a 0–50 range), a VAS 0–100 scale (commonly reported as a 0–500 range) or a Likert scale (commonly reported as a 0–20 range). The overall WOMAC score (index) is determined by summing the scores across the three dimensions and the score range includes the following: a VAS 0–10 scale (commonly reported as a 0–240 range), a VAS 0–100 scale (commonly reported as a 0–2400 range) and a 0–4 Likert scale (commonly reported as a 0–96 range). A number of transformations and modifications are reported in the literature.
The preferred measure of pain was the WOMAC pain scale (using either a VAS or a Likert scale). Another pain scale was included in the analysis when a trial did not measure the WOMAC pain scale, with prioritisation of pain scales made on a clinical, or prevalence, basis. The secondary outcome was the WOMAC index. Studies that did not report a pain outcome were excluded from the review. Outcome data were extracted for different time points: baseline, end of treatment and any follow-up time point.
As a variety of pain scales were used, Hedges’ g SMDs between treatment groups were calculated for the meta-analyses (studies reporting medians could not be analysed). Different doses/regimens of the same type of treatment within a study were pooled. Final values were used in the analysis to maximise the evidence available and to avoid the need to make assumptions about within-patient correlation between baseline and final values, which the use of change from baseline data would have necessitated. For trials reporting change from baseline but not final values, we calculated final values provided baseline data were reported along with variance estimates (e.g. SDs). When the number of patients included in a trial’s analysis was not reported, but the number of patients randomised was, we estimated the number of analysed patients by multiplying the number of patients randomised by the average proportion of patients included in an analysis across trials. SEs or 95% CIs were used to derive SDs when they were not reported. When this was not possible, trials that used the same or a similar scale as that used in the trial with missing SDs were identified and their SDs pooled, with this imputed estimate being used. We present results as SMDs, as well as SMDs converted to the WOMAC VAS 0–100 pain scale, to provide more clinically meaningful results.
Evidence synthesis
A network meta-analysis draws on both direct (treatments compared in the same trial) and indirect evidence (different treatments studied in separate trials, but compared when they share the use of a common comparator treatment). The summary treatment effect from each study is utilised, so the benefit of randomisation in each study is retained. To conduct a meta-analysis of trials, study characteristics must be similar within a comparison. For indirect and direct evidence to be consistent, study characteristics must be similar across comparisons. 85,86,172–175
We planned analyses for three different time points to assess both the immediacy and the durability of effects: (1) end of treatment, which was our primary time point, as defined in the individual studies; (2) 3 months from the start of treatment, which was the time point closest to 3 months from the start of treatment (excluding outcomes recorded at < 4 weeks from the start of treatment); and (3) 3 months after the end of treatment, which was the time point closest to 3 months, but between 8 and 16 weeks, from the end of treatment. However, there was a paucity of long- and medium-term data across the trials, and for the 3 months after the end-of-treatment time point no connected network incorporating acupuncture existed and this time point was evaluated by only 21 trials. Data for the 3 months from the start of treatment analysis were very similar to data for the end-of-treatment analysis because for around two-thirds of trials the two time points were the same. Furthermore, in most studies, the primary time point specified by investigators was the end of treatment and this time point produced the largest network, incorporating more interventions, and studies, than the other time points. We therefore report the results for the end-of-treatment time point.
To evaluate the impact of study quality on the results, two sets of analyses were performed: one included all studies regardless of quality (labelled ‘any quality’) and one was a primary sensitivity analysis including only studies of satisfactory, or better, quality (labelled ‘better quality’). Studies with atypical populations, interventions or results were excluded in a second sensitivity analysis. These studies were identified from pairwise meta-analyses conducted (in RevMan 5.0; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) using outcomes recorded at the end of treatment only. These studies were not intended as a comprehensive stand-alone synthesis, but as a means of informing and complementing the network meta-analysis. In particular, they were used to investigate the within-intervention clinical and statistical heterogeneity. We assessed for possible publication bias using a funnel plot when enough studies within individual treatments were available. This was deemed to be appropriate only for muscle-strengthening exercise and the funnel plot provided no evidence to suggest publication bias. 168
Analyses were conducted using WinBUGS software (version 1.4; MRC Biostatistics Unit, Cambridge, UK), which uses Markov chain Monte Carlo (MCMC) simulation to estimate model parameters and follows a Bayesian approach in which prior probabilities are specified for parameters (these were specified to be vague throughout the analysis). The treatment difference was assumed to be normally distributed and a random-effects network meta-analysis model was selected as clinical and methodological heterogeneity within the treatment definitions appeared likely. 176 A common between-study variance was modelled to allow a between-study variance to apply for comparisons with few data points.
Convergence of the MCMC chains was assessed by observing the history of the traces of the starting values for selected priors, the Brooks–Gelman–Rubin statistic and posterior distributions. 177 The first 10,000 iterations were discarded and then a further 50,000 iterations were conducted on which parameter estimates were based. The model fit was evaluated using the residual deviance, with this being approximately equal to the number of data points if the fit was good. 174,178–180
Inconsistency in the treatment effect estimates derived separately from direct and indirect evidence was assessed for many of the comparisons distributed across the networks using the node-splitting method where a p-value is 2 × min(prob, 1 – prob), where ‘prob’ is the probability that the direct estimate is higher than the indirect estimate. 172,179
Uncertainty in all estimates is presented using the upper and lower limits of the 95% credible intervals (CrIs) of these estimates. These credible limits describe the boundaries within which it is believed that there is a 95% chance that the true value lies. The median rank of each intervention and the 95% CrIs of the rank are presented to summarise the uncertainty across all of the treatment effect estimates. 181
To present more clinically meaningful network meta-analysis results, we present both SMDs and the SMDs converted to the WOMAC VAS 0–100 pain scale (although it is acknowledged that back-transformation can be of limited value in heterogeneous populations). 182 A pooled SD for the WOMAC VAS 0–100 pain scale was calculated from all of the arms of the six trials in the analysis that utilised this scale. The SMDs were then multiplied by this pooled SD (16.49) to produce a difference in WOMAC VAS 0–100 score.
In the substudy within this study that explored reporting of the WOMAC pain subscale and WOMAC index, further details were extracted for those trials that utilised the WOMAC: scale used (Likert/VAS 0–10, VAS 0–100/NRS); whether the WOMAC pain subscale or the WOMAC index was used; and whether any modifications were reported. In the light of inconsistencies and lack of clarity identified during the review, the WOMAC outcome details were re-examined by a third reviewer (NFW) and further information was extracted as necessary to address the following four questions:
-
Was it clear that all assessments had been conducted?
-
Was the score range clear?
-
Were details reported on how the final score had been calculated (sum, average or transformation to 0–100 scale)?
-
Were baseline scores reported (and approximate baseline score)?
In addition, the scale used and the score range that could be deduced from the information provided in each paper was recorded and the ease of identification of these was categorised as clearly stated in the paper (stated), required assumptions to be made (assumed) or unclear. All information that could support any assumptions, including baseline score (or, when not reported, follow-up scores), was also recorded. Further details of this substudy’s methods are reported elsewhere. 183
Results
In total, 3820 references were retrieved from searches, of which 156 trials (detailed in Appendix 3) including 18 distinct interventions and four comparators met the inclusion criteria. Four of 10 foreign-language papers that appeared eligible based on their English abstracts could not be translated and so had to be excluded. Thirty-eight trials reported data in ways that meant they could not be incorporated in the network meta-analyses. One study was found to have been retracted and was subsequently removed from all analyses. A study flow diagram is presented in Figure 4.
FIGURE 4.
Study flow chart.
Study characteristics
An overview of all eligible studies is presented in Table 15, regardless of whether or not they reported data suitable for network meta-analysis. The mean treatment duration (and timing of the end-of-treatment assessment) varied widely, from just a single session (TENS) to 69 weeks (weight-loss interventions), although the majority of interventions were administered over a 2- to 6-week period. Most studies were classified as having recruited a general knee osteoarthritis population, although weight-loss trials (as expected) recruited only overweight or obese participants. The mean BMI in some studies recruiting a general population fell into the overweight or obese classification, although most studies did not report BMI.
| Intervention | Number of trials eligible for the review (number of patientsb) | Type of population recruitedc (number of studies) | Mean age (years), range | Female (%), range | Comparators (number of treatment armsd) |
|---|---|---|---|---|---|
| Acupuncture | 25 (2794) | General (23), both knees affected (1), awaiting surgery (1) | 58–85 | 50–96 | Sham acupuncture (15), standard care (13), TENS (3), muscle-strengthening exercise (1), ice/cooling (1) |
| Balneotherapy | 14 (1008) | General (12), both knees affected (2) | 54–70e | 47–100 | Placebo (8), standard care (6), heat treatment (1) |
| Braces | 1 (24) | General (1) | 59.5 | 63 | Insoles (1) |
| Aerobic exercise | 13 (1136) | General (9), both knees affected (2), overweight or obese (2) | 54–75e | 50–100 | Standard care (13), muscle-strengthening exercise (2), weight loss (1) |
| Muscle-strengthening exercise | 34 (3013) | General (26), both knees affected (5), awaiting surgery (2) | 53–77e | 31–100 | Standard care (22), placebo (4), no treatment (2), aerobic exercise (2), heat treatment (1), TENS (1), acupuncture (1), PES (1), manual therapy (1), NMES (2) |
| Heat treatment | 7 (412) | General (7) | 61–74 | 63–100 | Placebo (4), standard care (1), TENS (1), muscle-strengthening exercise (1), balneotherapy (1), ice/cooling (1) |
| Ice/cooling treatment | 4 (211) | General (4) | 56–61 | 48–91 | TENS (2), acupuncture (1), standard care (1), heat treatment (1), placebo (1), no treatment (1) |
| Insoles | 6 (893) | General (6) | 58–68 | 54–100 | Placebo (5), braces (1) |
| Interferential therapy | 5 (240) | General (5) | 59–67 | 67–80 | Placebo (3), TENS (1), no treatment (1) |
| Laser/light therapy | 9 (379) | General (6), both knees affected (3) | 58–74 | 68–90 | Placebo (8), standard care (1) |
| Manual therapy | 6 (486) | General (6) | 56–68 | 63–78 | Standard care (4), placebo (2), muscle-strengthening exercise (1) |
| NMES | 3 (78) | General (3) | 60–71 | 42–79 | Standard care (2), muscle-strengthening exercise (2) |
| PES | 8 (392) | General (8) | 55–70 | 46–100 | Placebo (7), standard care (1), muscle-strengthening exercise (1), no treatment (1) |
| PEMF | 6 (521) | General (6) | 60–69 | 28–80 | Placebo (6) |
| Static magnets | 3 (131) | General (3) | 63–65 | 60–79 | Placebo (3) |
| T’ai chi | 4 (307) | General (4) | 65–70 | 75–93 | Standard care (4) |
| TENS | 18 (805) | General (17), awaiting surgery (1) | 56–85 | 48–97 | Placebo (12), standard care (3), acupuncture (3), ice/cooling (2), heat treatment (1), interferential (1), no treatment, muscle-strengthening exercise (1) |
| Weight loss (dieting) | 5 (870) | Overweight or obese (5) | 61–70 | 26–89 | Standard care (5), aerobic exercise (1) |
Around three-quarters of the studies (110/152) were classed as ‘poor-quality’ studies. Of the remainder, 33 studies were classed as ‘satisfactory’ and nine studies were classed as ‘good’, which together were classed as ‘better quality’. Only 12 trials were considered to be at low risk of bias in the network meta-analysis. Trial quality was commonly compromised by a lack of adequate blinding and small sample sizes, which limited the effectiveness of randomisation, resulting in baseline imbalances. Quality assessment data are presented in Appendix 3. Study quality did vary by intervention, making the evidence base more robust in some areas than in others. No evidence was found for publication bias (only assessable for muscle-strengthening exercise). Individual study characteristics of all studies included in the systematic review can be found in Appendix 3.
Network meta-analysis
Suitable data for the end-of-treatment analyses were reported in 114 trials (9709 patients) (detailed in Appendix 3). This includes data from the 22 new studies identified from the search update conducted in 2013 and nine studies that had been excluded from the original review analyses but which were now included by calculating final values from the change from baseline data. In our original analyses (based on searches up to 2010) there was no indication that the majority of the adjunctive components of the experimental interventions were associated with a treatment effect difference. 169 The one exception was that standard care incorporating active analgesia was more effective than standard care with ‘treatment as usual’ (with or without home exercise/education). However, as analgesic adjuncts were used in only eight trials, and most studies were classified as using the ‘treatment as usual’ adjunct, with little adjunct detail defined, the focus of this study was on comparing the interventions categorised without adjuncts. The resulting network for any-quality studies, with analysis at the end of treatment and interventions categorised without adjuncts, is illustrated in Figure 5.
FIGURE 5.
Network diagram for the end-of-treatment analysis of any-quality studies (with interventions categorised without adjuncts). In this figure, each solid arrow indicates that there is a data point for that comparison entered into the analysis. The thickness of the arrow reflects the number of trials. The dotted line reflects an extra comparison in a multiarm trial. The numbers are a measure of inconsistency: 0 is no inconsistency; 1 is complete inconsistency.
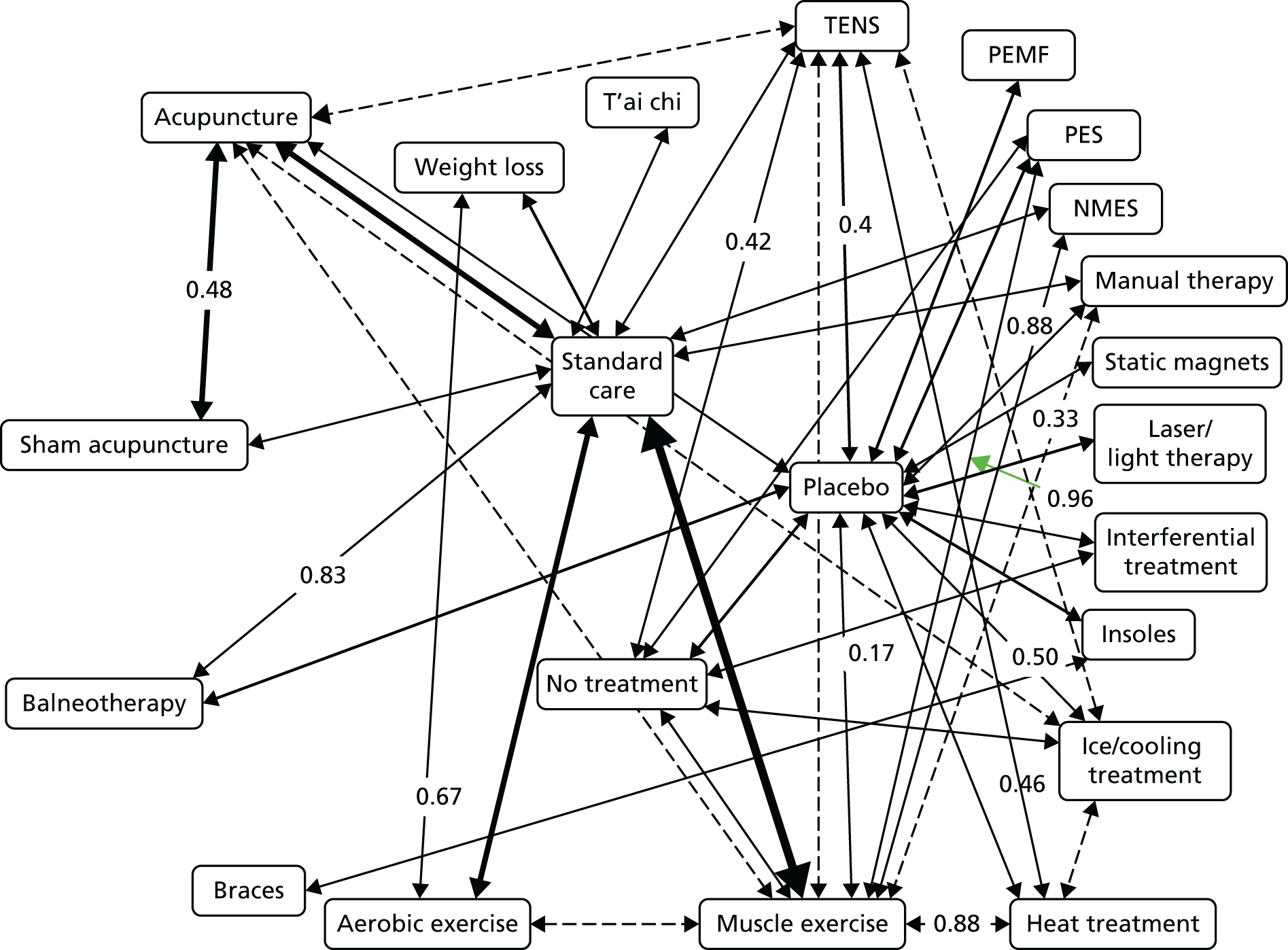
The interventions drawn from the any-quality trials were compared with standard care and acupuncture (Tables 16 and 17, respectively), with caterpillar plots shown in Figures 6 and 7, respectively, and interventions ordered by treatment effect. Across all comparisons, inconsistency at a p-value of < 0.05 was identified only for the two comparisons involving PES. Eight physical treatments had a statistically significant mean beneficial effect compared with standard care, namely interferential therapy, acupuncture, TENS, PES, balneotherapy, aerobic exercise, sham acupuncture and muscle-strengthening exercise (see Table 16 and Figure 6). When acting as a comparator, acupuncture was statistically significantly better at reducing pain than sham acupuncture, muscle-strengthening exercise, weight loss, PEMF, placebo, insoles, NMES and no intervention (see Table 17 and Figure 7).
| Intervention | Number of trials (patientsa) | SMD (95% CrI) | Difference expressed on a WOMAC VAS 0–100 pain scale (95% CrI) |
|---|---|---|---|
| Standard care (comparator) | – (–) | – (–) | |
| Interferential therapy | 3 (98) | –1.63 (–2.39 to –0.87) | –26.90 (–39.39 to –14.40) |
| Acupuncture | 24 (1219) | –0.89 (–1.18 to –0.59) | –14.69 (–19.52 to –9.80) |
| TENS | 12 (285) | –0.65 (–1.06 to –0.25) | –10.77 (–17.50 to –4.05) |
| PES | 6 (180) | –0.65 (–1.19 to –0.10) | –10.65 (–19.59 to –1.66) |
| Balneotherapy | 9 (275) | –0.60 (–1.04 to –0.15) | –9.87 (–17.15 to –2.48) |
| Aerobic exercise | 11 (428) | –0.55 (–0.89 to –0.21) | –9.02 (–14.68 to –3.51) |
| T’ai chi | 4 (159) | –0.51 (–1.03 to 0.01) | –8.39 (–16.98 to 0.13) |
| Static magnets | 2 (41) | –0.50 (–1.34 to 0.33) | –8.27 (–22.08 to 5.43) |
| Sham acupuncture | 14 (892) | –0.47 (–0.84 to –0.09) | –7.76 (–13.89 to –1.52) |
| Manual therapy | 4 (166) | –0.44 (–0.96 to 0.09) | –7.21 (–15.90 to 1.49) |
| Muscle-strengthening exercise | 28 (1254) | –0.40 (–0.61 to –0.19) | –6.54 (–9.99 to –3.11) |
| Ice/cooling treatment | 3 (51) | –0.35 (–1.03 to 0.33) | –5.81 (–16.94 to 5.44) |
| Heat treatment | 5 (123) | –0.31 (–0.86 to 0.24) | –5.14 (–14.20 to 3.98) |
| Laser therapy | 5 (155) | –0.27 (–0.86 to 0.32) | –4.53 (–14.19 to 5.20) |
| Weight loss | 5 (436) | –0.26 (–0.67 to 0.15) | –4.25 (–10.97 to 2.43) |
| PEMF | 5 (238) | –0.15 (–0.71 to 0.42) | –2.43 (–11.76 to 6.90) |
| Placebo | 42 (1077) | –0.07 (–0.42 to 0.29) | –1.15 (–6.98 to 4.70) |
| Braces | 1 (12) | 0.00 (–1.39 to 1.39) | 0.07 (–22.84 to 22.94) |
| Insoles | 3 (197) | 0.10 (–0.65 to 0.85) | 1.64 (–10.71 to 13.97) |
| NMES | 2 (28) | 0.22 (–0.62 to 1.05) | 3.58 (–10.26 to 17.33) |
| No intervention | 5 (87) | 0.44 (–0.15 to 1.04) | 7.25 (–2.51 to 17.12) |
| Intervention | Number of trials (patientsa) | SMD (95% CrI) | Difference expressed on a WOMAC VAS 0–100 pain scale (95% CrI) |
|---|---|---|---|
| Acupuncture (comparator) | – (–) | – (–) | |
| Interferential therapy | 3 (98) | –0.74 (–1.54 to 0.05) | –12.21 (–25.33 to 0.84) |
| TENS | 12 (285) | 0.24 (–0.22 to 0.70) | 3.92 (–3.70 to 11.50) |
| PES | 6 (180) | 0.25 (–0.35 to 0.84) | 4.04 (–5.78 to 13.87) |
| Balneotherapy | 9 (275) | 0.29 (–0.22 to 0.81) | 4.82 (–3.60 to 13.28) |
| Aerobic exercise | 11 (428) | 0.34 (–0.11 to 0.79) | 5.67 (–1.84 to 13.00) |
| T’ai chi | 4 (159) | 0.38 (–0.22 to 0.98) | 6.30 (–3.58 to 16.12) |
| Static magnets | 2 (41) | 0.39 (–0.48 to 1.25) | 6.41 (–7.86 to 20.61) |
| Sham acupuncture | 14 (892) | 0.42 (0.15 to 0.70) | 6.93 (2.50 to 11.46) |
| Manual therapy | 4 (166) | 0.45 (–0.14 to 1.05) | 7.47 (–2.30 to 17.23) |
| Muscle-strengthening exercise | 28 (1254) | 0.49 (0.15 to 0.84) | 8.14 (2.41 to 13.83) |
| Ice/cooling treatment | 3 (51) | 0.54 (–0.16 to 1.25) | 8.88 (–2.70 to 20.61) |
| Heat treatment | 5 (123) | 0.58 (–0.02 to 1.18) | 9.55 (–0.30 to 19.44) |
| Laser therapy | 5 (155) | 0.62 (–0.02 to 1.25) | 10.16 (–0.28 to 20.61) |
| Weight loss | 5 (436) | 0.63 (0.13 to 1.14) | 10.44 (2.13 to 18.72) |
| PEMF | 5 (238) | 0.74 (0.13 to 1.36) | 12.26 (2.22 to 22.36) |
| Placebo | 42 (1077) | 0.82 (0.40 to 1.25) | 13.53 (6.58 to 20.53) |
| Standard care | 53 (2308) | 0.89 (0.59 to 1.18) | 14.69 (9.80 to 19.52) |
| Braces | 1 (12) | 0.89 (–0.51 to 2.31) | 14.76 (–8.49 to 38.01) |
| Insoles | 3 (197) | 0.99 (0.21 to 1.78) | 16.33 (3.41 to 29.30) |
| NMES | 2 (28) | 1.11 (0.22 to 1.98) | 18.27 (3.57 to 32.72) |
| No intervention | 5 (87) | 1.33 (0.69 to 1.97) | 21.95 (11.30 to 32.52) |
FIGURE 6.
Standardised mean differences of each treatment compared with standard care for the analysis including studies of any quality. Numbers in parentheses indicate the numbers of studies. ACU, acupuncture; AE EX, aerobic exercise; BAL, balneotherapy; BRA, braces; HEA, heat treatment; ICE, ice/cooling treatment; INS, insoles; INT, inferential therapy; LAS, laser therapy; MAG, static magnets; MAN, manual therapy; MU EX, muscle-strengthening exercise; NO INT, no intervention; PLA, placebo; SH ACU, sham acupuncture; TAI, t’ai chi; WEI, weight loss.
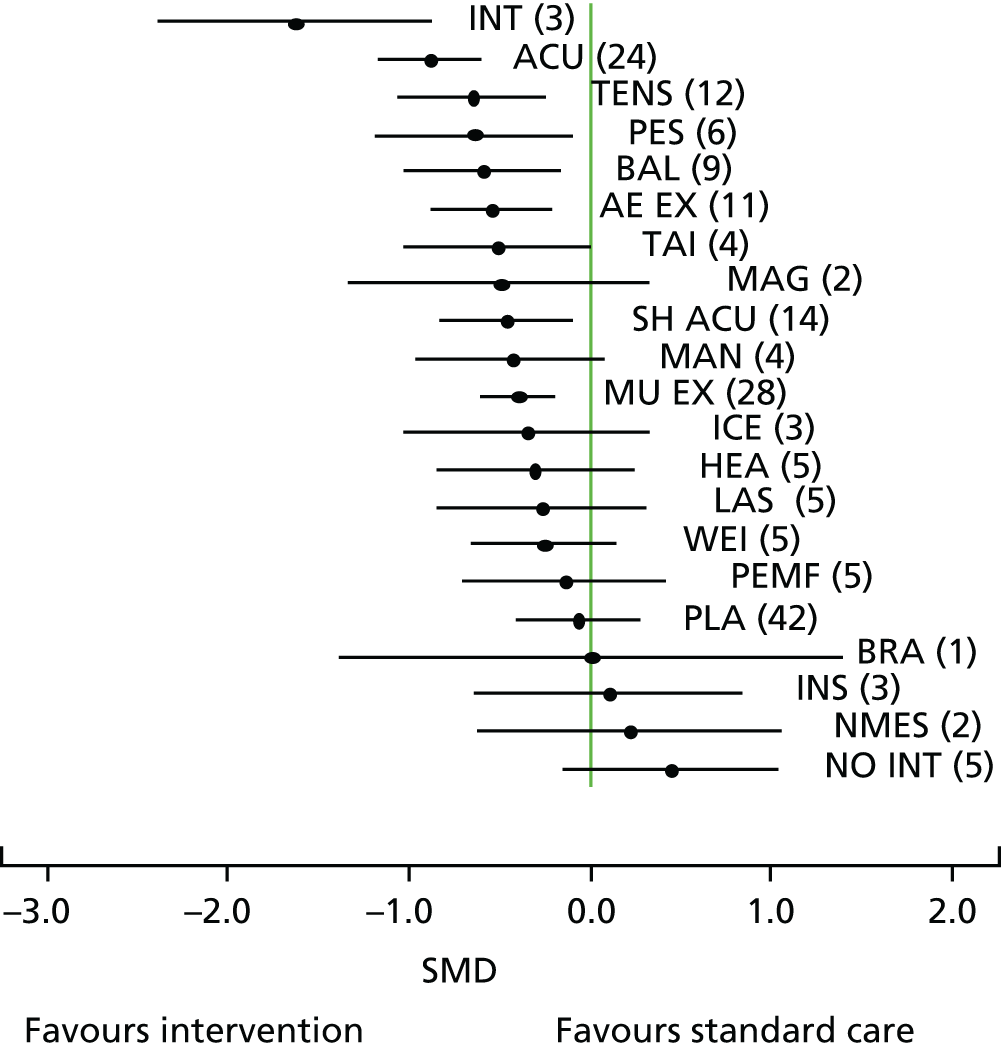
FIGURE 7.
Standardised mean differences of each treatment compared with acupuncture for the analysis including studies of any quality. Numbers in parentheses indicate the numbers of studies. AE EX, aerobic exercise; BAL, balneotherapy; BRA, braces; HEA, heat treatment; ICE, ice/cooling treatment; INS, insoles; INT, inferential therapy; LAS, laser therapy; MAG, static magnets; MAN, manual therapy; MU EX, muscle-strengthening exercise; NO MED, no intervention; PLA, placebo; SH ACU, sham acupuncture; ST CARE, standard care; TAI, t’ai chi; WEI, weight loss.
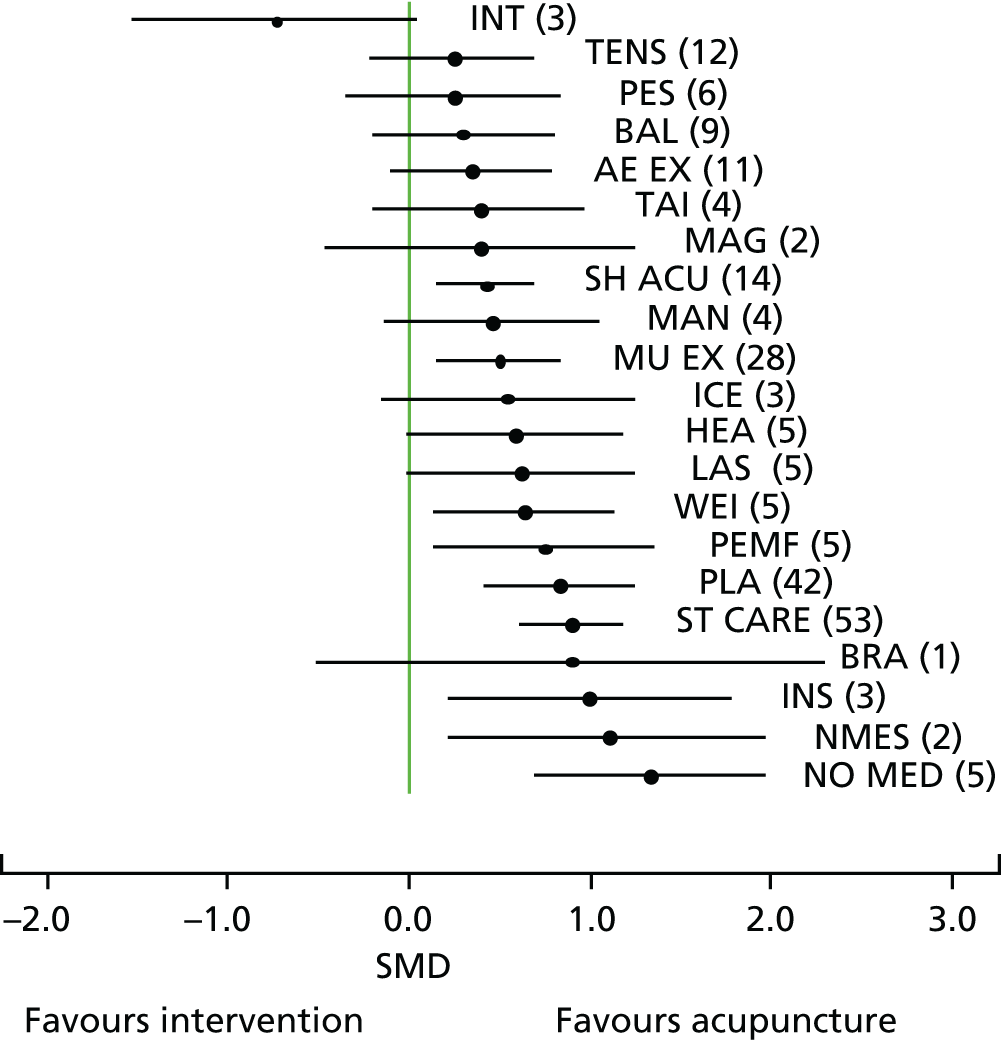
Effect sizes for each intervention are presented in terms of both SMDs and the WOMAC VAS 0–100 pain scale. To help evaluate these conversions, one study reported the minimal clinically important change as –15 mm (on a VAS 0–100 scale and derived from a prior Delphi exercise)184 and the minimal perceptible clinical improvement (MPCI, the smallest change detectable by the patient) as –9.7 mm (on a WOMAC VAS 0–100 scale). 185 Another study estimated the minimal clinically important improvement (MCII), although only for pain on movement, as –19.9 mm on a VAS 0–100 scale; this figure varied by baseline pain score, with patients with less pain having a smaller MCII (10.8 mm) and patients with severe pain having a larger MCII (36.6 mm). 186
When analysing only the better-quality studies (see Appendix 3) in the primary sensitivity analysis, 35 trials were included, with nine types of intervention and 3499 patients. One study was identified as causing inconsistency in the main analysis (a small study of muscle-strengthening exercise vs. PES) and was therefore excluded. 187 The network is illustrated in Figure 8, in which the analysis is at the end of treatment and interventions are categorised without adjuncts. Uncertainty around the true between-study variance increased because of the reduction in the number of studies per comparison, as well as loops in the network. Most studies were of acupuncture (n = 11) or muscle-strengthening exercise (n = 9), with some interventions represented by few studies.
FIGURE 8.
Network diagram for the end-of-treatment analysis of better-quality studies (with interventions categorised without adjuncts).
When compared with standard care, there was a statistically significant reduction in pain for acupuncture, balneotherapy, sham acupuncture and muscle-strengthening exercise (Table 18 and Figure 9). When acupuncture was the comparator, it was statistically significantly better at a 95% level of credibility than sham acupuncture, muscle-strengthening exercise, weight loss, aerobic exercise and no intervention (Table 19 and Figure 10).
| Intervention | Number of trials (patientsa) | SMD (95% CrI) | Difference expressed on a WOMAC VAS 0–100 pain scale (95% CrI) |
|---|---|---|---|
| Standard care (comparator) | – (–) | – (–) | |
| Acupuncture | 11 (878) | –1.01 (–1.43 to –0.61) | –16.70 (–23.61 to –10.07) |
| Balneotherapy | 1 (40) | –1.01 (–1.92 to –0.11) | –16.65 (–31.73 to –1.74) |
| Sham acupuncture | 8 (685) | –0.68 (–1.17 to –0.19) | –11.14 (–19.29 to –3.16) |
| Muscle-strengthening exercise | 9 (450) | –0.52 (–0.84 to –0.22) | –8.62 (–13.92 to –3.58) |
| T’ai chi | 2 (51) | –0.26 (–0.96 to 0.44) | –4.29 (–15.87 to 7.23) |
| Weight loss | 3 (357) | –0.08 (–0.55 to 0.39) | –1.34 (–9.10 to 6.41) |
| Aerobic exercise | 1 (80) | 0.07 (–0.69 to 0.84) | 1.23 (–11.30 to 13.78) |
| No intervention | 1 (30) | 0.19 (–0.77 to 1.14) | 3.11 (–12.72 to 18.77) |
FIGURE 9.
Standardised mean differences of each treatment compared with standard care for the analysis including better-quality studies. Numbers in parentheses indicate the numbers of studies. ACU, acupuncture; AE EX, aerobic exercise; BAL, balneotherapy; MU EX, muscle-strengthening exercise; NO TREAT, no intervention; SH ACU, sham acupuncture; TAI, t’ai chi; WEI, weight loss.
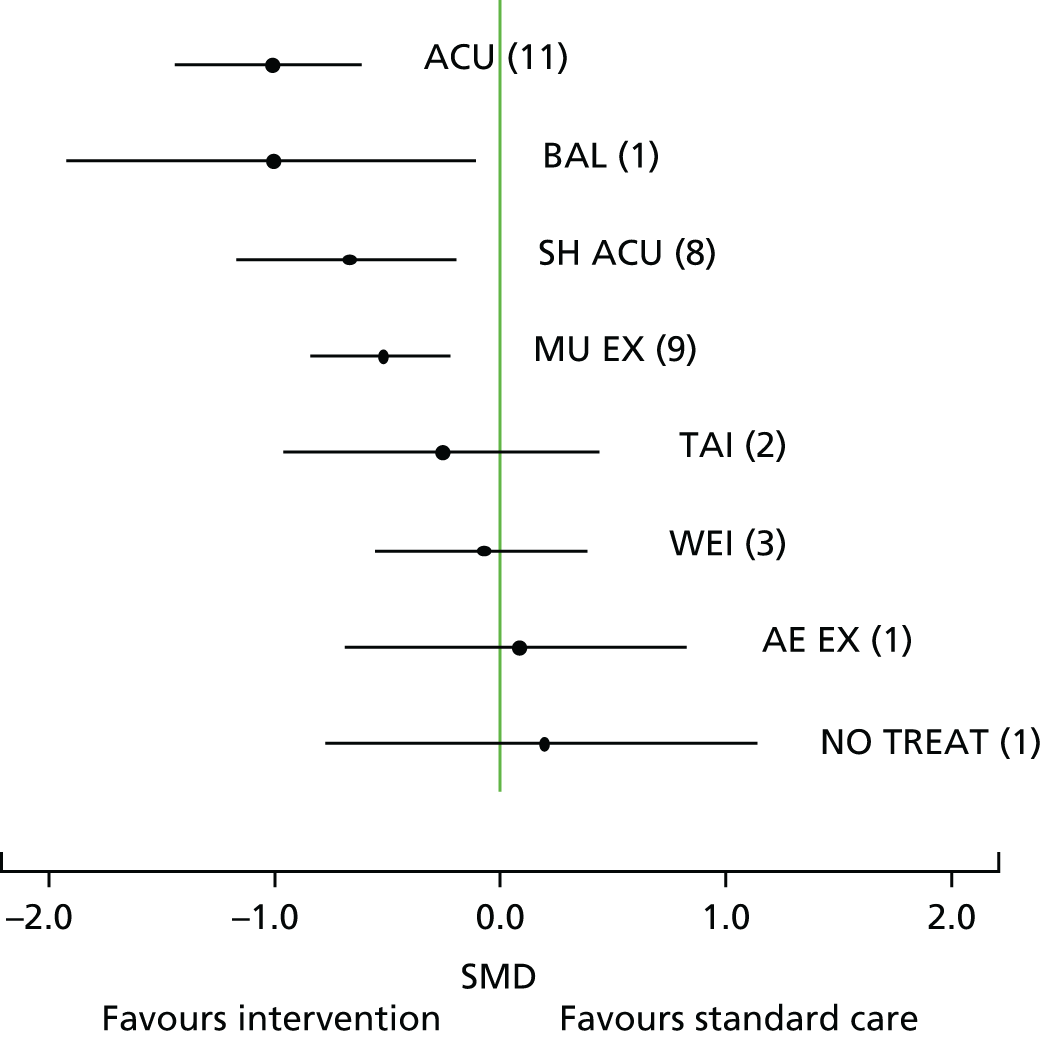
FIGURE 10.
Standardised mean differences of each treatment compared with acupuncture for the analysis including better-quality studies. Numbers in parentheses indicate the numbers of studies. AE EX, aerobic exercise; BAL, balneotherapy; MU EX, muscle-strengthening exercise; NO TREAT, no intervention; SH ACU, sham acupuncture; ST CARE, standard care; TAI, t’ai chi; WEI, weight loss.
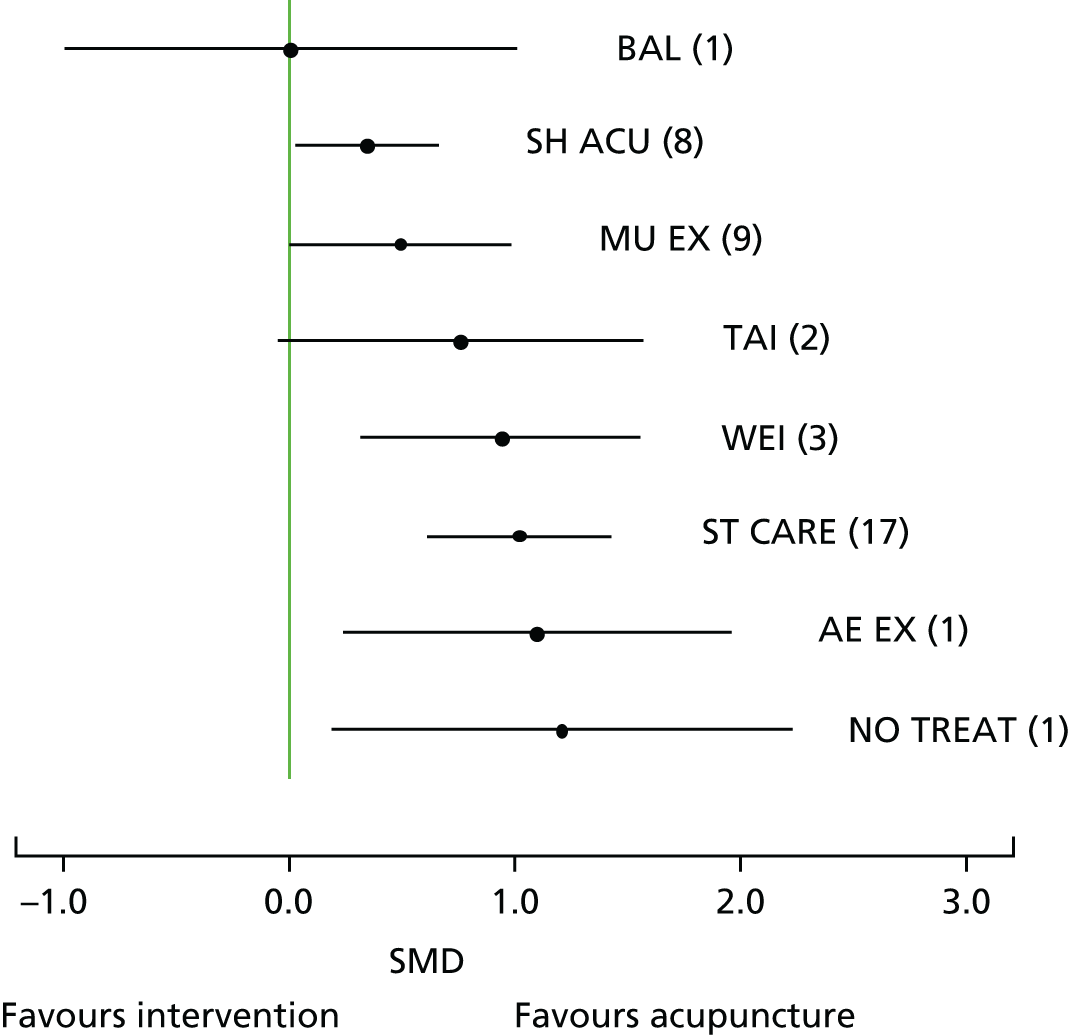
| Intervention | Number of trials (patientsa) | SMD (95% CrI) | Difference expressed on a WOMAC VAS 0–100 pain scale (95% CrI) |
|---|---|---|---|
| Acupuncture (comparator) | – (–) | – (–) | |
| Balneotherapy | 1 (40) | 0.00 (–0.99 to 1.01) | 0.05 (–16.36 to 16.62) |
| Sham acupuncture | 8 (685) | 0.34 (0.03 to 0.66) | 5.57 (0.42 to 10.86) |
| Muscle-strengthening exercise | 9 (450) | 0.49 (0.00 to 0.98) | 8.08 (0.02 to 16.21) |
| T’ai chi | 2 (51) | 0.75 (–0.05 to 1.57) | 12.42 (–0.81 to 25.84) |
| Weight loss | 3 (357) | 0.93 (0.31 to 1.57) | 15.36 (5.18 to 25.81) |
| Standard care | 17 (928) | 1.01 (0.61 to 1.43) | 16.70 (10.07 to 23.61) |
| Aerobic exercise | 1 (80) | 1.09 (0.23 to 1.96) | 17.94 (3.82 to 32.27) |
| No intervention | 1 (30) | 1.20 (0.18 to 2.23) | 19.80 (2.94 to 36.81) |
In terms of ranking, a probability statistic calculated from the treatment effect distributions showed that acupuncture and balneotherapy were the two interventions with the highest rank (Table 20). Because of overlapping CrIs for sham acupuncture, muscle-strengthening exercise and t’ai chi, there is some uncertainty around these rankings.
| Intervention | Number of trials | Median rank | 95% CrI |
|---|---|---|---|
| Acupuncture | 11 | 2 | 1 to 3 |
| Balneotherapy | 1 | 2 | 1 to 6 |
| Sham acupuncture | 8 | 3 | 2 to 6 |
| Muscle-strengthening exercise | 9 | 4 | 2 to 6 |
| T’ai chi | 2 | 5 | 2 to 9 |
| Weight loss | 3 | 6 | 4 to 9 |
| Standard care | 17 | 7 | 6 to 9 |
| Aerobic exercise | 1 | 8 | 3 to 9 |
| No intervention | 1 | 8 | 3 to 9 |
In a secondary sensitivity analysis several trials were excluded based on population or intervention differences, or on extreme data;136,188–193 the results were not sensitive to these changes, although the model fit improved, as reported elsewhere. 169 No network link could be made with the placebo-controlled studies in the analysis of better-quality studies. We therefore conducted a separate network meta-analysis for these studies. Both interferential therapy and heat treatment were statistically significantly more effective than placebo, but laser therapy, PES and insoles were not; these data are also reported elsewhere. 169
In the substudy on the reporting of the WOMAC pain subscale and WOMAC index, the former was reported in 60 (45%) trials and the latter in 31 (23%) trials. Reporting of the exact method used in administering the WOMAC pain subscale scoring was poor in many cases. Overall, only 15 (25%) trials reported unambiguously both the scale and the score range for their use of the WOMAC pain subscale. Only four (13%) trials reported unambiguously both the scale and the score range for their use of the WOMAC index. Further details of the results of this substudy are reported elsewhere. 183
Discussion
Principal findings
In the comprehensive network meta-analysis that we report here we compared all physical treatments for osteoarthritis of the knee with each other within a coherent framework. This analysis provides the first estimate of the relative effect of these treatments, which can be viewed as essential for decision-makers when comparing treatment effects. By providing a basis for synthesising all of the available evidence in a consistent framework, a network meta-analysis obviates the need to make decisions based on subjective inferences from disconnected data.
Compared with standard care, eight of the 22 interventions that we evaluated produced a statistically significant reduction in pain: interferential therapy, acupuncture, TENS, PES, balneotherapy, aerobic exercise, sham acupuncture and muscle-strengthening exercise. Of these eight, only two interventions were represented by more than three trials in the sensitivity analysis of better-quality studies: acupuncture (11 trials) and muscle-strengthening exercise (nine trials), with acupuncture having statistically significantly better outcomes. Acupuncture and balneotherapy (only one trial) were the two interventions with the highest rank, although there is some uncertainty around this. For the better-quality placebo-controlled studies, interferential therapy (one trial) showed a strong effect compared with placebo.
Strengths and limitations
Numerous systematic reviews, some summarised in a review of reviews,194 have evaluated the interventions (or classes of interventions) included in this review. However, our analysis represents the use of the most practical methods currently available to compare a large number of different types of treatment, enabling a fair comparison to be made of competing physical treatments (including acupuncture) with each other.
A network meta-analysis requires an assumption of exchangeability between the trials in the same way as is required for a standard meta-analysis. With regard to concerns that might arise from within- or between-intervention heterogeneity, we sought to minimise these by using an age restriction as part of our inclusion criteria and by excluding interventions consisting of more than one physical treatment. We found that patient characteristics appeared to be broadly comparable across interventions. Inevitably, there will be some clinical heterogeneity in a wide-ranging study such as this, but as far as it was possible to tell, given the wide variation of scales used, baseline pain did not appear to vary systematically between interventions.
We used a random-effects model to incorporate heterogeneity and we evaluated levels of inconsistency and model fit. We also conducted sensitivity analyses excluding trials causing heterogeneity. Although heterogeneity is accounted for in our results with the CrIs, it is possible that unknown confounding factors may be affecting the results of indirect comparisons. With regard to trials of placebo interventions, the majority used electrical or electromagnetic interventions and so it is not unreasonable to assume that the placebo effects were similar (as the interventions were similar). In our review the trials, covering a diverse range of interventions, were all assessed using the same quality assessment tools, which enabled equivalence in the comparisons and better interpretation of the evidence base for each intervention.
Our sensitivity analysis of the better-quality studies resulted in fewer trials per comparison and fewer network loops. This led to greater uncertainty about the true heterogeneity and about the differences between the direct and the indirect evidence. The uncertainty associated with inconsistency may not be fully captured in the results because fewer loops in relation to the size of the network meant that there were fewer data to quantify inconsistency.
We were not able to include all of the studies in our analyses because of the variable reporting of pain results. Moreover, our analyses focused only on the end-of-treatment data and these were available mostly for short-term time periods. Of the trials that investigated effectiveness over medium- or long-term time periods, only a few provided the data required for our analyses. However, a comparison of the maximum effect of interventions is not without merit, given that the treatments under consideration are not intended as being cures and that any treatment effect might be expected to attenuate over time.
It is important that our results are evaluated in context. Most of the studies in our review were rated as being of poor quality. Many of the better-quality studies were pragmatic trials in which blinding of patients was not possible, that is, most studies are likely to have been subject to some form of bias. For this reason there can be methodological limitations in clinical trials of physical treatments that are often inherent and unavoidable. For the trials in which patients were not blinded and treatments were compared with standard care, the overall treatment effect is likely to incorporate non-specific (placebo) effects. We assumed that such non-specific effects were similar across all interventions, but variation may in fact be present. However, there were also limitations that could have been avoided by triallists using better methodology and reporting practices. For example, in our substudy on WOMAC reporting,183 we found poor reporting of both the WOMAC pain subscale and the WOMAC index, which in turn resulted in significant uncertainty in the interpretation of the results of individual trials and limited their contribution to our evidence synthesis.
Comparison with the wider literature
In light of our results, it is worth considering what might be the true (or specific) effect of acupuncture. In a Cochrane review,142 a statistically significant, clinically relevant, short-term improvement in pain was reported (acupuncture vs. waiting list control: SMD –0.96, 95% CI –1.19 to –0.72), a similar finding to what we have reported. A similar effect to ours was also observed in the comparison of acupuncture with sham acupuncture (SMD –0.35, 95% CI –0.55 to –0.15). It is worth noting that the largest study70 in this Cochrane analysis, which showed no statistically significant difference between acupuncture and sham acupuncture, was one of two trials that used an intensive sham needling technique, which may have had physiological effects. Also, our analysis included a recent large trial125 that used what appeared to be a very active sham. Therefore, the inclusion of trials with sham controls that might be more active than an inert placebo control could lead to pooled results that underestimate the short-term effect of acupuncture. It is of interest to note that the effect size of acupuncture compared with sham acupuncture is of the same order as that seen for NSAIDs compared with placebo (SMD 0.32, 95% CI 0.24 to 0.39), a difference that has also been described as being too small to be clinically significant. 195
An IPD meta-analysis that included an evaluation of acupuncture for patients with knee osteoarthritis was recently reported196 (see Chapter 2). All included studies were deemed be of high quality because the allocation concealment methods were assessed to be unambiguously adequate. This study also found acupuncture to be more effective than sham acupuncture and with a smaller effect size than when acupuncture was compared with no-acupuncture (usual care) controls. These findings indicate that non-specific effects provide a partial contribution to the pain-alleviating effects of acupuncture. Non-specific effects will also be contributing to the effectiveness of other (non-acupuncture) interventions in our network meta-analysis. When interventions were not controlled by a placebo or a relevant sham, commonly when blinding was not possible, the contribution of non-specific effects to the overall effect cannot be estimated. However, given that there are inherent problems with identifying non-specific effects in interventions involving physical treatments, it is reasonable to assume that fair comparisons between treatments have been made.
There is some evidence to suggest that larger treatment effects are associated with sham acupuncture than with pharmacological or other physical placebos. 145,197 However, one of two contrasting factors may impact on the effect of sham acupuncture in a given trial: either there is inadequate patient blinding because of using unsuitable shams or there is the use of physiologically active shams. The former may lead to an overestimation of the true effect of acupuncture, whereas the latter may lead to an underestimation of the true effect. In the trials that we have reviewed in this study we found that important details about sham acupuncture (e.g. depth of insertion) were sometimes poorly reported or were not reported at all. As with the variations in styles of acupuncture, so too were there variations in the types of sham acupuncture, both contributing to the possibility of clinical heterogeneity.
Implications for clinical practice
Five guidelines82,161,198–200 have evaluated treatment effects on key outcomes of knee osteoarthritis (including pain, function and disability). Only the Osteoarthritis Research Society International (OARSI) guideline200 is unequivocal in its recommendation to offer acupuncture for knee osteoarthritis. The American College of Rheumatology (ACR)198 conditionally recommended acupuncture but only for patients with moderate to severe pain who are unable or unwilling to undergo total knee arthroplasty. The American Academy of Orthopaedic Surgeons (AAOS)199 found the acupuncture evidence to be inconclusive and the European League Against Rheumatism (EULAR)161 and NICE82 did not recommend the use of acupuncture. Our analyses of the better-quality studies suggest that acupuncture should be considered as one of the short-term physical treatment options for relieving pain caused by osteoarthritis of the knee.
Guidance from all organisations recommended treatment with muscle-strengthening and aerobic exercise, education, weight loss (if required) and, when necessary, paracetamol and/or topical NSAIDs; when these are ineffective, a choice of one or more options from a range of pharmacological and non-pharmacological treatments is sometimes recommended, including TENS, thermal (heat/cooling) treatments, insoles and braces. Some of our results on effectiveness do not concur with existing guidance on the (non-acupuncture) physical treatments: our evidence differs from the EULAR guidelines161 with regard to insoles, braces and weight loss; from the NICE guidelines82 with regard to TENS, insoles, braces, weight loss, manual therapy and heat or cooling treatment; from the ACR guidelines198 with regard to weight loss, insoles, thermal agents and t’ai chi; from the AAOS guidelines199 with regard to weight loss; and from the OARSI guidelines200 with regard to insoles, braces, heat or cooling treatment, TENS and weight loss. Our analyses found little evidence (of significant differences from standard care, let alone clinically relevant differences) to support such guidance with respect to treating pain, other than for TENS, for which the evidence was of poor quality and likely to be unreliable. It should be remembered, however, that our review was focused on pain outcomes rather than on function, disability or cost-effectiveness.
The clinical relevance of improvements in knee pain scores has been quantified in several ways. In this context, our better-quality trial results appear to indicate that acupuncture produces both a MPCI185 and quite possibly a minimum clinically important change,184,185 but may yield a MCII only for patients with low levels of pain. 186 A MPCI remains a possibility for muscle-strengthening exercise (with evidence from nine trials). Our better-quality results suggest that few physical treatments are likely to have a clinically relevant pain-relieving effect. The exceptions were balneotherapy, interferential therapy and heat treatment for which we found evidence of effectiveness compared with standard care. However, the results for these three interventions were informed by single small studies and so a cautious interpretation is warranted.
When interpreting effectiveness results, other factors to consider beyond effectiveness are acceptability, safety, rapidity and durability of benefit, convenience, cost and likelihood of patient adherence to treatment. 201 Given the diverse range of interventions that we studied, these factors will clearly differ between interventions, as well as in relation to pharmacological and other treatments.
Recommendations for future research
To comprehensively assess the value of many of these interventions, larger RCTs, with risk of bias reduced and with longer treatment periods, are needed. Given the stronger evidence on acupuncture and muscle-strengthening exercise in the better-quality trials, there is a need in future studies to determine the optimum timing and parameters of treatment. Ideally, trials should examine the effectiveness of retreatment following treatment cessation (to evaluate durability and attenuation effects), which would match the way that these physical treatments are often delivered in practice.
In the substudy on standards of reporting of WOMAC scales,183 we found that in general the reporting of methods and results in RCTs using the WOMAC assessment tool lacked clarity. Poor reporting of WOMAC scales limits the interpretation of trial results and their useability for evidence synthesis. Given that the various versions of WOMAC available are clearly defined and have all been validated, full descriptions by researchers are needed. Adherence to the standard WOMAC scoring system should be encouraged. As an absolute minimum, the type of WOMAC used and the score range must be reported. Clear reporting is important and should not be sacrificed to reduce word count.
Conclusion
The evidence available for our network meta-analyses, in which physical interventions for osteoarthritis of the knee were compared equally with each other within a coherent framework, suggests that the evidence of effectiveness for most interventions is weak. However, when comparing all interventions, whether based on the any-quality or the better-quality trials, acupuncture can be considered as one of the more effective physical treatments for alleviating pain in the short term. Despite the large evidence base found, the methodological limitations associated with many of the trials indicate that high-quality trials of many of the physical treatments are still required.
Chapter 4 Towards a cost-effectiveness analysis of acupuncture for chronic pain: developing methods in a case study
Background
Evidence synthesis in health technology assessment
Cost-effectiveness analyses of health technologies have a number of key requirements. 202 These analyses should entail (1) a clear definition of the decision problem, which should include all relevant comparators; (2) an appropriate time horizon for the analysis; (3) the systematic identification and consideration of all relevant evidence;203 (4) an appropriate characterisation of all sources of uncertainty; and (5) an assessment of the value of acquiring additional research. It is extremely rare for the evidence base informing a cost-effectiveness analysis to come from a single study. 204 Data (typically summary study level) are derived from multiple sources and are often available in multiple formats, for example using different instruments for measurement and using different measures of effect reported at different time points. Evidence synthesis and decision modelling are used extensively in health technology appraisal to meet the challenge of reflecting these disparate sources of evidence within a coherent framework.
Synthesis tools are increasingly used to obtain pooled estimates of the parameters of interest to inform economic decision models. This is particularly the case for treatment effect estimates when multiple relevant RCTs may be available. In many circumstances the synthesis of treatment effect evidence considers only pairwise comparisons through the use of standard meta-analysis. However, frequently there are more than two treatment choices. Network meta-analysis (also known as mixed-treatment comparisons) is a tool that extends standard pairwise meta-analysis, allowing the estimation of the relative effectiveness of multiple treatments by simultaneously synthesising all relevant evidence. This statistical method is a well-established technique and its methods have been described extensively in the literature. 85,86,178,205–208 As is the case for standard meta-analysis, most published work using network meta-analysis focuses on the synthesis of aggregate data. These data are usually obtained from published literature and consist of an estimate of treatment effectiveness (e.g. mean difference in the case of continuous outcomes) and an appropriate measure of uncertainty (e.g. the variance or SE).
Network meta-analysis and the use of individual patient data
With the increasing availability of IPD for economic evaluation, together with considerable support for utilising this type of evidence,209,210 meta-analytic methods have emerged to address the challenges of IPD study synthesis. 211,212 Most progress has been made in the area of the statistical synthesis of clinical effectiveness, whereas little work has been undertaken to address the challenges of synthesising information on other important decision model parameter types. 213 Techniques for pairwise meta-analysis of individual-level evidence exist for most outcome types including binary,214 continuous210,215 and time-to-event data. 216,217 Use of IPD to inform decisions creates added value by offering the potential to reduce network heterogeneity, tackle existing evidence inconsistencies172 and examine subgroup effects in patients in whom interventions might have an effectiveness and cost-effectiveness profile which differs from that of the wider population. 218 Few methodological studies on the synthesis of IPD in network meta-analysis are available in the published literature and even fewer examples of its use within cost-effectiveness analysis exist. 213
Objectives and structure
Given the potential benefits of IPD network meta-analysis as a basis for informing cost-effectiveness modelling and the paucity of examples of this approach in the literature, we present a case study of using IPD network meta-analysis to inform a cost-effectiveness analysis of acupuncture for chronic pain. The objectives of this research were to both develop novel methods for IPD network meta-analysis and demonstrate the application of IPD network meta-analysis for use in economic evaluation.
To our knowledge the current synthesis methods literature does not offer modelling tools for continuous data within an IPD network meta-analysis framework. Using a pairwise meta-analysis framework, Riley et al. 219 discuss different approaches to the synthesis of continuous outcome data when IPD are available. Riley et al. 219 highlight that modelling the follow-up result, adjusted for the baseline value, commonly called ANCOVA,219,220 is the preferred approach. The availability of IPD is crucial for such models. If IPD are not available, the use of ANCOVA would require all original study authors to have reported appropriate treatment effect estimates, ideally at the same follow-up time. These requirements, in most circumstances, make this option unfeasible. Flexibility is introduced with the availability of IPD as the analyst can apply the same modelling approach across trials and derive consistent outputs. This report describes a novel, methodological framework for IPD network meta-analysis of continuous data within the Bayesian framework, which builds on the work described in Riley et al. 219
Two approaches to synthesising data on heterogeneous continuous outcomes are explored. The first involves standardising outcomes by dividing primary outcome scores by study-specific SDs. This creates a dimensionless measure of treatment effect usually termed the SMD. 221–223 Although commonly used, this approach does not produce results that can directly feed into cost-effectiveness analysis models, as absolute treatment effect estimates are required. Furthermore, health-care policy-makers require a common health outcome measure to be able to make decisions across different conditions and clinical areas. In many jurisdictions, including England and Wales,224 this measure is the QALY. 225 The QALY is a composite measure and provides an estimate of an individual’s remaining life expectancy weighted by a preference-based measure of HRQoL. The most popular HRQoL measure for generating quality-of-life weights is the EQ-5D. 226 These considerations motivate the second synthesis approach used, which involves translating (or ‘mapping’) the available HRQoL data from the trials to EQ-5D values and synthesising the resulting data.
After describing the motivating data set in Motivating case study: the cost-effectiveness of acupuncture for chronic pain in primary care, the core of Methods outlines the novel statistical models for the IPD network meta-analysis. A variety of modelling approaches are described and discussed. This section also describes how comparable end points suitable for synthesis were obtained, the estimation of costs and the cost-effectiveness modelling methods. The section Application provides the results of the IPD network meta-analysis and cost-effectiveness analysis. The discussion section offers concluding remarks and discusses relevant issues, including extensions to the current work.
Motivating case study: the cost-effectiveness of acupuncture for chronic pain in primary care
Background to acupuncture and acupuncture guidance
There is currently a lack of agreement about the effectiveness of acupuncture as a treatment for chronic pain, as reflected in debates about recent UK guidance surrounding its value. 83,84,227 Acupuncture received a positive recommendation from NICE for its use in back pain81 and headache/migraine,80 whereas a negative recommendation was given for its use in osteoarthritis in 2008228 and 2014. 82 The methods in this chapter were developed as part of a project to improve evidence regarding the clinical effectiveness and cost-effectiveness of acupuncture for chronic non-specific pain to inform decision-making in the UK NHS.
Data description and network of evidence
Data for the current study were made available by the ATC. To address the lack of good-quality evidence in acupuncture, the ATC undertook a systematic review in which relevant high-quality trials were identified and, for a large proportion, IPD were obtained. 196 From 31 eligible RCTs from the ATC database, IPD were obtained from 29. However, data from Cherkin et al. 131 were not available to us because of sharing restrictions. The data set analysed here included 28 high-quality RCTs60,61,66–77,117–122,130,132–138 that assessed the effectiveness of acupuncture for three pain conditions: osteoarthritis of the knee (seven trials), headache [including tension-type headache (TTH)] and migraine (six trials), and musculoskeletal pain, encompassing lower back, shoulder and neck pain (15 trials), totalling approximately 17,500 patients from the USA, UK, Germany, Spain and Sweden. These studies are summarised in Table 21.
| ID | Study | Location | Pain group (type) | Age (years), mean (SD) | Follow-up/time point used (months) | Treatment | Observations | HRQoL outcome mapped | Pain outcome standardised |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Diener 200672 | Germany | Headache (migraine) | 37.62 (10.4) | 6/3 | Usual care | 328 | SF-12 | Migraine days |
| Sham acupuncture | 202 | ||||||||
| Acupuncture | 305 | ||||||||
| 2 | Endres 200773 | Germany | Headache (TTH) | 38.44 (11.77) | 6/3 | Sham acupuncture | 200 | SF-12 | TTH days |
| Acupuncture | 209 | ||||||||
| 3 | Jena 200876 | Germany | Headache (headache) | 43.66 (12.69) | 6/3 | Usual care | 1613 | SF-36 | Headache days |
| Acupuncture | 1569 | ||||||||
| 4 | Linde 200568 | Germany | Headache (migraine) | 42.55 (11.35) | 6/3 | Usual care | 76 | SF-36 | Days of moderate to severe pain |
| Sham acupuncture | 81 | ||||||||
| Acupuncture | 145 | ||||||||
| 5 | Melchart 200567 | Germany | Headache (TTH) | 42.68 (13.18) | 6/3 | Usual care | 75 | SF-36 | Headache days |
| Sham acupuncture | 62 | ||||||||
| Acupuncture | 132 | ||||||||
| 6 | Vickers 200460 | UK | Headache (headache) | 46.34 (10.39) | 12/3 | Usual care | 161 | SF-36 | Severity score |
| Acupuncture | 140 | ||||||||
| 7 | Brinkhaus 200666 | Germany | Musculoskeletal (lower back) | 58.81 (9.13) | 12/2 | Usual care | 79 | SF-36 | VAS pain score |
| Sham acupuncture | 73 | ||||||||
| Acupuncture | 146 | ||||||||
| 8 | Carlsson 2001119 | Sweden | Musculoskeletal (lower back) | 49.84 (15.4) | 6/3 | Sham acupuncture | 16 | VAS pain | VAS pain score |
| Acupuncture | 34 | ||||||||
| 9 | Guerra de Hoyas 2004138 | Spain | Musculoskeletal (shoulder) | 59.19 (11.37) | 6/3 | Sham acupuncture | 65 | VAS pain | VAS pain score |
| Acupuncture | 65 | ||||||||
| 10 | Haake 200771 | Germany | Musculoskeletal (lower back) | 50.15 (14.68) | 6/3 | Usual care | 388 | SF-12 | von Korff pain intensity score |
| Sham acupuncture | 387 | ||||||||
| Acupuncture | 387 | ||||||||
| 11 | Irnich 2001118 | Germany | Musculoskeletal (neck) | N/A | 3/3 | Sham acupuncture | 61 | VAS pain | VAS pain score |
| Acupuncture | 56 | ||||||||
| 12 | Kennedy 2008130 | Northern Ireland | Musculoskeletal (lower back) | 45.58 (11.1) | 3/3 | Sham acupuncture | 24 | VAS pain | Roland Morris disability score |
| Acupuncture | 24 | ||||||||
| 13 | Kerr 2003117 | Northern Ireland | Musculoskeletal (lower back) | N/A | 6/1 | Sham acupuncture | 20 | VAS pain | VAS pain score |
| Acupuncture | 26 | ||||||||
| 14 | Kleinhenz 1999137 | Germany | Musculoskeletal (shoulder) | N/A | 3/1 | Sham acupuncture | 27 | CMS and predicted VAS pain | CMS |
| Acupuncture | 25 | ||||||||
| 15 | Salter 2006133 | UK | Musculoskeletal (neck) | 47.71 (16.51) | 3/3 | Usual care | 14 | No mapping – EQ-5D available | Northwick Park pain score |
| Acupuncture | 10 | ||||||||
| 16 | Thomas 200661 | UK | Musculoskeletal (neck) | 42.62 (10.71) | 24/3 | Usual care | 80 | No mapping – EQ-5D available | SF-36 bodily pain score |
| Acupuncture | 159 | ||||||||
| 17 | Vas 2006121 | Spain | Musculoskeletal (neck) | 46.73 (13.2) | 6/1 | Sham acupuncture | 62 | SF-36 | VAS pain score |
| Acupuncture | 61 | ||||||||
| 18 | Vas 2008122 | Spain | Musculoskeletal (shoulder) | 55.68 (11.37) | 12/3 | Sham acupuncture | 220 | VAS pain | CMS |
| Acupuncture | 205 | ||||||||
| 19 | White 2004132 | UK | Musculoskeletal (neck) | 53.36 (15.61) | 12/3 | Sham acupuncture | 65 | VAS pain | VAS pain score |
| Acupuncture | 70 | ||||||||
| 20 | Witt 200675 | Germany | Musculoskeletal (neck) | 50.57 (12.93) | 6/3 | Usual care | 1698 | SF-36 | Neck pain and disability score |
| Acupuncture | 1753 | ||||||||
| 21 | Witt 200674 | Germany | Musculoskeletal (lower back) | 52.83 (13.33) | 6/3 | Usual care | 1390 | SF-36 | Hanover functional ability score |
| Acupuncture | 1451 | ||||||||
| 22 | Foster 2007135 | UK | Osteoarthritis of the knee | 63.23 (8.81) | 12/1 | Usual care | 116 | VAS pain | WOMAC pain score |
| Sham acupuncture | 119 | ||||||||
| Acupuncture | 117 | ||||||||
| 23 | Berman 2004134 | USA | Osteoarthritis of the knee | 65.46 (8.62) | 6/2 | Usual care | 189 | No mapping – EQ-5D available | WOMAC pain score |
| Sham acupuncture | 191 | ||||||||
| Acupuncture | 190 | ||||||||
| 24 | Scharf 200670 | Germany | Osteoarthritis of the knee | 62.81 (10.07) | 6/3 | Usual care | 316 | SF-12 | WOMAC total score |
| Sham acupuncture | 365 | ||||||||
| Acupuncture | 326 | ||||||||
| 25 | Vas 2004120 | Spain | Osteoarthritis of the knee | 67.04 (10.09) | 3/3 | Sham acupuncture | 49 | WOMAC total | WOMAC total score |
| Acupuncture | 48 | ||||||||
| 26 | Williamson 2007136 | UK | Osteoarthritis of the knee | 70.67 (8.94) | 3/3 | Usual care | 61 | WOMAC total | Oxford Knee Score |
| Acupuncture | 60 | ||||||||
| 27 | Witt 200569 | Germany | Osteoarthritis of the knee | 64.01 (6.49) | 12/2 | Usual care | 70 | SF-36 | WOMAC total score |
| Sham acupuncture | 75 | ||||||||
| Acupuncture | 149 | ||||||||
| 28 | Witt 200677 | Germany | Osteoarthritis of the knee | 61.2 (10.39) | 6/3 | Usual care | 310 | SF-36 | WOMAC total score |
| Acupuncture | 322 |
Nine of these studies were three-arm trials, assessing the three treatments simultaneously, 11 evaluated acupuncture and sham acupuncture only, and eight considered acupuncture and usual care only. Thus, the comparison of acupuncture with usual care is informed by 17 studies, acupuncture with sham acupuncture by 20 studies and sham acupuncture with usual care by nine studies. The resulting evidence network is presented in Figure 11.
FIGURE 11.
Network of RCTs. In the network, a unique treatment category is indicated by a box. Arrows between boxes indicate that these treatments had been compared in a trial. Pain groups: H, headache/migraine; MSK, musculoskeletal; OAK, osteoarthritis of the knee (as set out in Table 21).

Resource use information in the data set was limited to five60,61,66,69,71 of the 28 studies. Of these, three are specific to the German health-care system66,69,71 and, given the jurisdiction-specific nature of health-care resource use data, non-UK studies are of limited value to inform decision-making in the UK. 229 The remaining two studies that provided resource use evidence60,61 were carried out in the UK, although only one of these60 collected resource use for time points that matched the 3-month time frame of the effectiveness assessment. The study by Thomas et al. 61 recorded resource use at 12 and 24 months only. The study by Vickers et al. 60 focused on one of the three clinical areas of interest (headache/migraine). It was decided to seek additional external data sets outside the ATC data set instead of assuming that health-care resource use data from headache/migraine trials could be generalised to musculoskeletal conditions and osteoarthritis of the knee. Therefore, IPD were obtained from the UK Back pain Exercise And Manipulation (BEAM) study230 for musculoskeletal pain and the UK Topical or Oral IBuprofen (TOIB) study231 for knee osteoarthritis pain.
Methods
This section describes how individual-level comparable values for the two end points of interest were generated, that is, EQ-5D index values and standardised pain scores. The section then goes on to describe the Bayesian IPD network meta-analysis synthesis modelling framework for both end points. Extensions to the modelling approach are then considered. Following this, methods used to analyse resource use and costs, and to generate cost-effectiveness results, are described.
Overview of the analysis
The ATC data set includes trials comparing acupuncture with sham acupuncture or usual care or comparing all three comparators. All trials were included in the synthesis to maximise use of the available data. In the context of clinical or other health-care decision-making, a sham comparator is not a clinically meaningful intervention as it would not be prescribed; the focus of the cost-effectiveness analysis was therefore on acupuncture compared with usual care. The range of possible treatment options for chronic non-specific pain in primary care is much wider than those considered here. The cost-effectiveness results presented in this report should not therefore be interpreted as providing a definitive answer to the question of whether or not acupuncture is cost-effective for the treatment of chronic non-specific pain. Instead, the cost-effectiveness analysis provides an illustration of how IPD network meta-analysis can be used.
Two outcome measures were used to value benefit in the present analysis: pain and EQ-5D index values. Given the spectrum of pain conditions considered, the availability of multiple instruments with which to measure pain, the lack of agreement about the preferred outcomes with which to measure pain and variable quality in reporting, pain measurement was highly heterogeneous across trials. SMDs were used to synthesise the pain outcomes. The HRQoL data were also obtained using a variety of instruments – some generic [e.g. Short Form questionnaire-12 items (SF-12) and SF-36] and others disease specific (e.g. the WOMAC index). These data were therefore mapped to EQ-5D values using a series of statistical mapping algorithms, which are described below.
End points were measured at a variety of follow-up times across trials. To consistently assess the effect of the treatments of interest across trials, the analysis focused on the time point closest to 3 months from the start of treatment. The 3-month time point was used as this was typically the measurement taken after the end of an acupuncture treatment course in the trials forming the evidence base and was reported for the majority (21/28) of the trials (see Appendix 1).
Generating homogeneous health-related quality-of-life scores and pain outcomes
The EQ-5D index score was the preferred end point for our analysis because of its importance for cost-effectiveness analysis. The conventional three-level version of the EQ-5D questionnaire includes five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each of which can be at one of three severity levels (no problems, some or moderate problems, or extreme problems), to generate a health status descriptor of one of 243 (35) health states (245 states in total when also considering the ‘unconscious’ and ‘dead’ states). The descriptor is quality adjusted using a score derived from analysing the preferences of approximately 3400 members of the UK public. 232 Bounded by full health and by the worst imaginable health state, the score ranges from 1 to –0.594. The distribution of EQ-5D health-state utility data is commonly non-normal. This, among other features, makes statistical modelling of the EQ-5D particularly challenging. 233 Only a small number of trials in the data set (see Table 21) provided EQ-5D data.
When EQ-5D data were not available they were predicted using other generic and disease-specific measures (see Table 21) through published mapping algorithms. Mapping algorithms were identified using the University of Oxford’s Health Economics Research Centre (HERC) database of studies mapping from HRQoL or clinical measures to the EQ-5D. 234,235 When using this tool and when multiple mapping algorithms were available for a given instrument, the preferred algorithm was selected on the basis of the sample size, adequacy of statistical modelling and relevance of study population. The selection of the outcome to be mapped was not at random. Preference was given to generic health status-based instruments (i.e. SF-12 and SF-36) and, in their absence, to condition-specific instruments [i.e. WOMAC, VAS pain and Constant–Murley Score (CMS)], conditional on the existence of a valid and published algorithm. The WOMAC was used in preference to VAS pain and CMS as it covers a broader definition of HRQoL. In 50% of the trials (n = 14) (see Appendix 4 for trial details), well-established published algorithms were used to map from SF-36 dimensions and SF-12 summary scores to the EQ-5D61,236,237 [a random-effects generalised least-squares algorithm considering dimensions, dimensions squared and interactions from Rowen et al. 236 was used (model R2 = 0.71); a multinomial logit using physical component summary (PCS) and mental component summary (MCS) scores, summary scores squared and interaction terms (mean square error = 0.021) from Gray et al. 237 was used to map the SF-12 to the EQ-5D. In 10 of the 28 trials, published algorithms that map VAS pain scores [an ordinary least squares (OLS) regression including VAS pain and VAS pain squared as covariates from Maund et al. 238 was used (R2 = 0.101)] and WOMAC scores [an OLS regression including total WOMAC score, total WOMAC score squared, age and sex as covariates from Barton et al. 239 (R2 = 0.313) was used] to the EQ-5D were used. For one trial,137 a double mapping was necessary as, to our knowledge, no direct mapping algorithm exists to obtain EQ-5D values from the CMS. Thus, an in-house unpublished mapping algorithm240 was used to derive VAS pain estimates from the CMS, which were used to obtain individual-level EQ-5D predictions using the algorithm mentioned above (available on request from Kamran Khan – K.A.Khan@warwick.ac.uk). For further details on mapping to the EQ-5D, see Chapter 5 (Health-related quality of life for cost-effectiveness analysis).
A high level of unexplained variation was found in the majority of the mapping algorithms used, that is, the proportion of total variation of the outcome(s) explained in these models (quantified by the coefficient of determination, R2, in most cases) was low. To account for this source of uncertainty in the mapping process, an additional variance component was included in the EQ-5D predictions. 241 A mapping process involves additional sources of uncertainty – the uncertainty in the mapping function regression coefficients and the structure of the mapping model. These additional sources of uncertainty are not accounted for in this analysis. This was achieved by drawing from a normal distribution with a mean of zero and variance equal to the study-specific residual variance. The residual variance was calculated as the difference between the total variance (calculated by dividing the variance of the mapped data by the R2 for the mapping algorithm) and the mapped outcome variance. Each random draw was then added to each individual-level EQ-5D prediction.
The second outcome measure assessed was standardised pain. Across the 28 trials, the primary outcome of each study was used to generate patient-level standardised pain estimates. Pain measures varied from days with headache in the headache/migraine pain condition to VAS pain in the musculoskeletal group or WOMAC pain in the osteoarthritis of the knee group, as reported in Table 21. Individual-level standardised pain estimates were obtained for each trial by dividing the primary outcome scores by the study-specific SD. Note that, although these estimates were used as inputs in the synthesis models, the outputs of the synthesis are in the SMD format, as differences between treatments were estimated within the modelling [considering stxt as the standardised value of the pain measurement p made at the time point t in patients under treatment tx, it can be demonstrated that (stx1t1−stx1t0)−(stx0t1−stx0t0)=(stx1t1−stx0t1)−(stx1t0−stx0t0)=ΔSMD].
Health-related quality of life and standardised pain estimates were obtained at baseline and at the follow-up point closest to 3 months following the start of treatment. Changes from baseline were obtained by calculating the difference between values for these two time points.
Statistical methods
This section describes the IPD network meta-analysis models. All analyses were conducted from a Bayesian perspective. Bayesian methods can be considered an alternative to the classical (frequentist) approach to statistical modelling and have been frequently used in the data synthesis and the economic evaluation of heath-care technologies. 202,207 They provide a more appealing, intuitive and flexible modelling framework as both the data and the model parameters are considered as random quantities. The key feature in this framework is the likelihood function, which defines how reasonable the data are given values of those model parameters. A key feature of this approach is that it allows the model to incorporate external information alongside available data in the format of prior distributions. When very little or no information is accessible, or when wanting the data to dominate, the posterior, subjective or ‘vague’ beliefs are set as priors. 242 This framework also allows the uncertainty in the relative effect estimates to be translated into probabilities of decision uncertainty, that is, the probability of which treatment is best (most efficacious) out of all treatments being compared. This explicit consideration of decision uncertainty leads naturally into a decision theory framework, which usually also considers costs and utilities, typically used in health-care decision-making. 202
A one-step IPD network meta-analysis modelling approach was preferred as, together with relative treatment effect estimates, the estimation of treatment–covariate interactions for patient-level covariates were of interest. 210,212 In the following model descriptions a random-effects approach was taken because of the expected between-study heterogeneity. Nonetheless, a fixed-effect framework could be attained with straightforward simplifications. 86,243 The models described apply both to the EQ-5D and to the standardised pain outcome.
The main modelling approach considered (model 1) is a variation of the ANCOVA approach, modelling the change score but also adjusting for baseline outcome values. 219,220,243,244 Model 1 was used as changes from baseline more closely approximated a normal distribution than absolute outcomes at 3 months. The model included interaction effects for pain type as it was expected that the impact of acupuncture (and sham acupuncture) may differ across pain types. Interaction effects for each pain type were modelled as exchangeable and related245 as it was expected that the impact of each pain type on the treatment effect of acupuncture may be related to the impact of each pain type on sham acupuncture effects.
Individual patient data network meta-analysis considering pain type as a treatment effect modifier
The model considers a set of J studies for which IPD were available. These studies included patients with a specific pain condition, with the pain conditions being headache/migraine, musculoskeletal pain and osteoarthritis of the knee. The set of treatments included in these trials are labelled [A,B,C], where A is the reference treatment, and there are K (= 3) treatments in total. At baseline, patient i in study j allocated to treatment k provides a baseline measurement Yijk0, where 0 indicates time t at baseline). Each patient provides a follow-up measurement (the assessment closest to 3 months) Yijk3. The change from baseline (Yijk3 – Yijk0) is denoted ΔYijk.
Model 1: analysis of covariance variation – change score modelling, adjusted for baseline
This model can be written as:
where Vj represents the study-level variance, the quantity µjb represents the outcome for the treatment b in study j for a patient with a baseline utility of 0, the parameter β0j represents the impact of the (outcome) baseline on the change outcome for each study j, the term δjbk represents the study-specific treatment effect for treatment k relative to treatment b and Xjp are p – 1 dummy variables representing pain type p in the jth study. Pain × treatment interaction effects βAkp were considered different for each treatment but exchangeable and were assumed to be drawn from a random distribution with a common mean (Bp) and between-treatment variance (σBp2).
Independent prior distributions were defined as follows: 1/Vj ∼ Gamma(0.001,0.001); µjb ∼ N(0,106); β0j ∼ N(0,106); dAk ∼ N(0,106); σ ∼ Unif(0,2); Bp ∼ N(0,106); σBp ∼ Unif(0,2). Correlations in the random effects from trials with three or more arms were accounted for following published methodology. 86,222 In this report, k > b indicates that k is after b in the alphabet.
Modelling extensions
Model 1 can be extended to consider covariates. Age and BMI were identified as potential treatment effect modifiers, with the clinical expectation being that older age or higher BMI may make patients more difficult to treat (i.e. reduce the effect of treatment). BMI data were, however, rarely reported and were available in only 10 of the 28 studies. This covariate was not therefore adjusted for in the modelling.
Age was assumed to modify outcomes by the same amount across pain types and to modify treatment effects by the same margin for acupuncture and sham acupuncture (i.e. a single interaction term is assumed to apply to all comparisons with usual care). Squared terms were included for main effects and treatment interaction effects as a non-linear impact of age on outcomes and treatment effects was expected a priori. Age was centred prior to inclusion in the model.
The following model (model 2) extends model 1 by considering the effects of the covariate Z:
Coefficients on the main covariate effect and the effect squared are represented by ϕ0 and φ0, respectively. Coefficients on the treatment–covariate interaction term and the interaction between treatment and the squared covariable term are represented by ϕ and φ, respectively. No interaction term for comparisons of k and b was included when b ≠ A because the common regression coefficient cancels out.
Because of the possibility of missing covariate information for some individuals, Zijk was represented as a normally distributed random variable with mean m and precision prec, common across all IPD studies. This represents a multiple imputation technique and assumes that the covariable data were missing at random. Additional priors were required for this model: ϕ0, ϕ, φ0, φ, ∼ N(0,106); m ∼ Unif(–50,50), 1/prec∼Unif(0,30).
Analysis in the presence of restricted evidence
Although model 1 is the preferred choice, this model would not be feasible in the absence of information at the individual level at the baseline and follow-up time points. Models that do not rely on the availability of IPD were therefore run for comparison purposes. Two options219 are typically available to the analyst when only aggregate data are available: modelling the change score (model 3) or modelling the final outcome score (model 4), both without baseline adjustment. These models represent simplifications of model 1 in which the baseline outcome variable is omitted.
Model selection and implementation
Data management was performed in the freely available software package, R version 3.0.0 (The R Foundation for Statistical Computing, Vienna, Austria). The network meta-analysis was undertaken in WinBUGS version 1.4.3,246 linked to the R software through the packages R2WinBUGS246 and CodaPkg. 247 Code for the network meta-analysis is provided in Appendix 4.
In all models the MCMC Gibbs sampler was initially run for 10,000 iterations and these were discarded as ‘burn-in’. Models were run for a further 5000 iterations, on which inferences were based. Chain convergence was checked using autocorrelation and Gelman and Rubin248 diagnostics. Within the network meta-analysis, goodness of fit was assessed using the deviance information criterion (DIC) and residual deviance. 180 The DIC is a measure that balances fit and complexity, allowing parsimony to be considered in model choice. The DIC is often used for model comparison when smaller DIC models should be preferred. The residual deviance of each data point may be viewed as a measure of the data point’s contribution to the total residual deviance (or lack of fit) of the model. A posterior mean for the total residual deviance similar to the number of data points will imply that model predictions fit well to the observed data.
Results are presented as EQ-5D index scores and SMD treatment effect estimates (and associated 95% CrIs), and also the probability of treatment being the ‘best’ treatment in terms of being the most clinically effective. 207
Modelling resource use
Acupuncture was assumed to be administered during 10 sessions with a physiotherapist. Ten sessions of acupuncture have been recommended by NICE in the context of lower back pain81 and headache/migraine,80 and it was assumed that this duration of therapy could be generalised to other musculoskeletal conditions and osteoarthritis of the knee. The first session was assumed to last for 40 minutes and subsequent sessions for 30 minutes. All sessions were costed using a unit cost for a physiotherapist (£36 per hour; Schema 9.1, with qualifications249).
The NICE recommendations, alongside the above assumptions regarding appointment durations, equate to a total of 5.2 hours of therapist time. A sensitivity analysis using a weighted average of the therapist time observed in the trials was conducted. Data were obtained from the data extractions conducted by Vickers et al. 105 Therapist time was calculated as the duration of sessions multiplied by the number of sessions and included only sessions that occurred within the 3-month time horizon considered for efficacy. The sensitivity analysis used total therapist interaction times of 5.6 hours for headache/migraine, 3.9 hours for musculoskeletal and 4.7 hours for osteoarthritis of the knee chronic pain.
The potential impact of improved health outcomes on resource use was explored using the three data sets described in Data description and network of evidence. 60,230,231 EQ-5D predictions (mapped from the available SF-36 physical and mental summary scores) together with the number of primary care (i.e. GP) and secondary care (i.e. specialist) visits from Vickers et al. 60 were used to estimate the relationship between change in HRQoL and change in health resource utilisation for the headache pain group. The relationship estimated from Vickers et al. 60 was assumed to apply for the entire headache group of patients (which includes patients with TTH and migraine pain). A simple OLS analysis was used to regress the change in resource use from 0–3 months to 3–12 months on the change in EQ-5D scores between month 3 and month 12. Primary and secondary care visits were analysed separately. Although not aimed at evaluating acupuncture, the UK BEAM study230 (with approximately 1300 patients) and the TOIB study231 (with approximately 280 patients) were used to estimate this relationship for lower back pain and osteoarthritis of the knee patients, respectively, using the same approach. Data from the UK BEAM study were assumed to be applicable to the other patients within the musculoskeletal pain category (i.e. those with neck and shoulder pain).
Resource utilisation at baseline was not collected in these studies (and is generally not collected in clinical trials). Changes in resource use were preferred to absolute resource use estimates as their relationship with EQ-5D changes is less likely to be confounded. To estimate the change in resource use it was therefore necessary to use the change from 0–3 months to 3–12 months. Use of the change from 0–3 months to 3–12 months to infer change in resource use over the 0- to 3-month time horizon of the economic model, however, assumes that a given utility change would drive a given change in resource use regardless of the time frame. Given that this is a strong assumption a secondary analysis was conducted using the absolute resource use in the period 0–3 months and regressing this on the change in EQ-5D score over this period.
The statistical software Stata 13 was used to model resource use for each pain condition.
The average cost of non-intervention resources used for each pain condition was calculated as the product of the EQ-5D estimates derived from the synthesis models, the coefficients on the EQ-5D estimates from the resource use regressions and the relevant unit costs [primary care visits were costed at £46.8 – this represents a weighted average of GP (£45 per consultation) and nurse (£49 per hour) visits with weights taken from the UK BEAM study and unit costs taken from the Curtis249 – and secondary care visits at £135 – this is the weighted average NHS reference cost for all outpatient procedures taken from Curtis249). Costs are reported in UK pounds for the financial year 2012–13. Other treatments and health-care interactions that may form a package of ‘usual care’ were assumed to have been provided equally to all patients regardless of comparator. These costs were therefore omitted from the analysis.
Estimation of cost-effectiveness outcomes
Quality-adjusted life-years were estimated assuming that the benefit of acupuncture over usual care estimated from the network meta-analysis of EQ-5D index scores was achieved instantaneously, with benefit maintained from 0 to 3 months, and was then lost instantaneously, illustrated in Figure 12 by the accrued benefit 2. This is equivalent to assuming that the full benefit was gradually achieved over a specified period and then lost linearly over the same period, which may be viewed as a more realistic scenario (see Figure 12, accrued benefit 1). For example, the benefit could be linearly achieved from the start of treatment until 12 weeks and gradually lost over the 12 weeks following treatment completion. Costs and effects beyond the 3-month time horizon were not considered in the current model and given the short time horizon no discounting was applied.
FIGURE 12.
Illustrative diagram of the treatment benefits over the period of assessment.
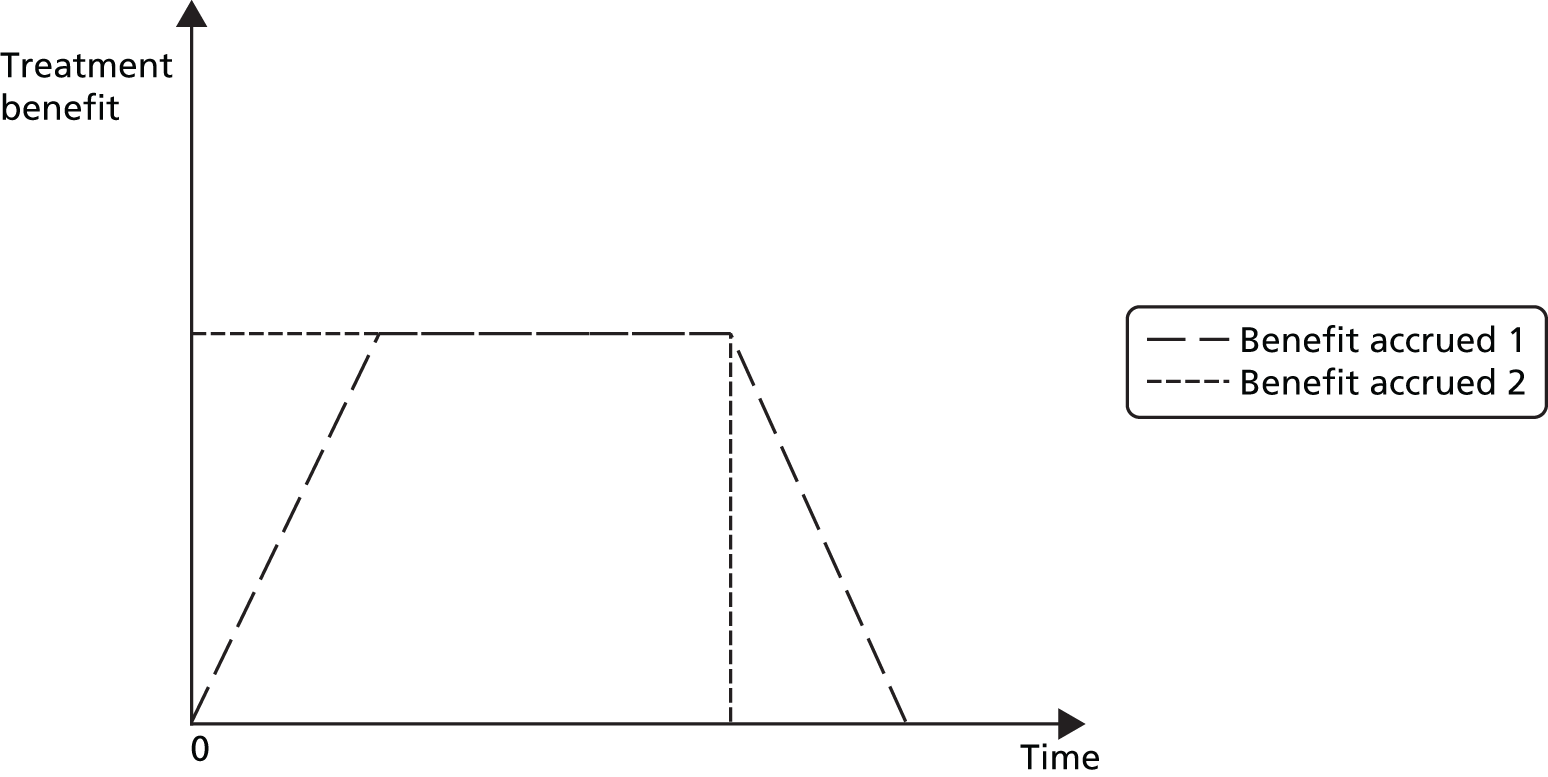
Incremental QALY estimates were compared with incremental cost estimates (intervention costs and non-intervention costs) to calculate incremental cost-effectiveness ratios (ICERs). These can be compared with a threshold value of £20,000–30,000 per QALY as conventionally applied in England and Wales. 203
Uncertainty in the estimates was quantified through the use of probabilistic analysis. The 5000 posterior samples from the synthesis of effectiveness (extracted from the Convergence Diagnostic and Output Analysis WinBUGS output) were used together with 5000 samples of the cost parameters, generated through Monte Carlo simulation. Uncertainty surrounding the decision to accept/reject acupuncture on the basis of cost-effectiveness was illustrated through cost-effectiveness acceptability curves. The cost-effectiveness modelling was also implemented in R.
Application
Results of generating homogeneous health-related quality-of-life scores and pain outcomes
Appendix 4 presents the (mapped) EQ-5D data and standardised pain outcomes. In general, patients’ HRQoL increased from baseline to 3 months. Similarly, standardised pain estimates decreased from baseline to 3 months. For both time points, it appears that osteoarthritis of the knee patients had, on average, lower HRQoL (and higher mean values of standardised pain) than patients suffering from headache/migraine or musculoskeletal pain.
For both end points, baseline imbalances between trial arms were observed within trials. For the EQ-5D end point, the biggest within-trial differences at baseline were found in the studies by Carlsson et al. 119 and Salter et al. 133 For the SMD end point, the largest differences were found in the same two trials and also in the studies by Kleinhenz et al. 137 and White et al. 132 These large imbalances are not surprising as most of these trials included only a small number of patients (around 50 or fewer). These observations supported the use of a modelling framework that allows for baseline adjustment,219 involving the use of either model 1 or 2 as the appropriate tool to synthesise this evidence.
Analysis of covariance (model 1)
Table 22 shows the parameter estimates obtained from model 1 applied to the EQ-5D and the standardised pain outcome data. For each parameter estimate the median of the MCMC posterior sample and 95% CrI are shown. Relative treatment effect estimates are shown, adjusted for baseline and treatment–pain interaction effects, together with measures of model fit (total residual deviance and DIC). The osteoarthritis of the knee pain group is the reference category for the pain interaction effects.
| IPD NMA results | Comparison | Model 1 (ANCOVA extension, change in outcome score, adjusted for baseline), median MCMC posterior sample (95% CrI) | ||
|---|---|---|---|---|
| Change EQ-5D | Change standardised pain | |||
| Relative treatment effects for OAK | SHAM vs. UC | 0.057 (0.013 to 0.095) | 0.271 (–0.007 to 0.537) | |
| ACU vs. UC | 0.079 (0.042 to 0.114) | 0.703 (0.399 to 0.984) | ||
| ACU vs. SHAM | 0.022 (–0.014 to 0.060) | 0.438 (0.121 to 0.715) | ||
| Pain exchangeable interactions (vs. OAKa) | Headacheb,c | SHAM (vs. UC) | –0.005 (–0.060 to 0.054) | 0.057 (–0.351 to 0.485) |
| ACU (vs. UC) | –0.023 (–0.071 to 0.029) | –0.121 (–0.467 to 0.254) | ||
| Musculoskeletalb,c | SHAM (vs. UC) | 0.002 (–0.052 to 0.062) | –0.199 (–0.595 to 0.218) | |
| ACU (vs. UC) | 0.003 (–0.046 to 0.054) | –0.108 (–0.465 to 0.307) | ||
| Between-study variance | 0.001 (0 to 0.003) | 0.090 (0.049 to 0.170) | ||
| Total residual devianced | 15,850 (15,480 to 16,230) | 17,060 (16,660 to 17,450) | ||
| DICe | –6420.4 | 37,394.2 | ||
For both end points, model 1 indicates that acupuncture treatment increases the HRQoL of patients and/or reduces pain more than usual care and sham acupuncture treatments, irrespective of the pain group they belong to. For the EQ-5D end point, the median treatment effect of acupuncture compared with usual care in the osteoarthritis of the knee population is 0.079 (95% CrI 0.042 to 0.114); for headache/migraine and musculoskeletal pain patients the comparable median treatment effects are 0.056 (95% CrI 0.021 to 0.092) and 0.082 (95% CrI 0.047 to 0.116), respectively. The results also favour acupuncture over sham acupuncture, although with a greater degree of uncertainty, as reflected by the fact that the CrIs include zero for all pain types (osteoarthritis of the knee 0.022, 95% CrI –0.014 to 0.060; headache/migraine 0.004, 95% CrI –0.035 to 0.042; musculoskeletal pain 0.023, 95% CrI –0.007 to 0.053). The probability that acupuncture is the best treatment at improving HRQoL is 0.89 for osteoarthritis of the knee, 0.64 for headache/migraine and 0.95 for musculoskeletal pain.
For the SMD end point the median treatment effect of acupuncture compared with usual care in the osteoarthritis of the knee population is 0.703 (95% CrI 0.399 to 0.984); for headache/migraine and musculoskeletal pain patients the comparable median treatment effects are 0.588 (95% CrI 0.311 to 0.869) and 0.588 (95% CrI 0.334 to 0.863), respectively. The results also favour acupuncture over sham acupuncture. In contrast to the EQ-5D analysis, the CrIs do not include zero in the standardised pain analysis for osteoarthritis of the knee (0.438, 95% CrI 0.121 to 0.715) and musculoskeletal pain (0.527, 95% CrI 0.323 to 0.735), although the CrI for headache/migraine does (0.256, 95% CrI –0.073 to 0.560). The probability that acupuncture is the best treatment at improving standardised pain is 0.96–1.00, depending on pain type. These results are presented as a forest plot in Figure 13.
FIGURE 13.
Forest plot showing the network meta-analysis results for the standardised pain and EQ-5D outcomes: (a) acupuncture vs. usual care; (b) acupuncture vs. sham acupuncture; and (c) sham acupuncture vs. usual care. OAK, osteoarthritis of the knee.
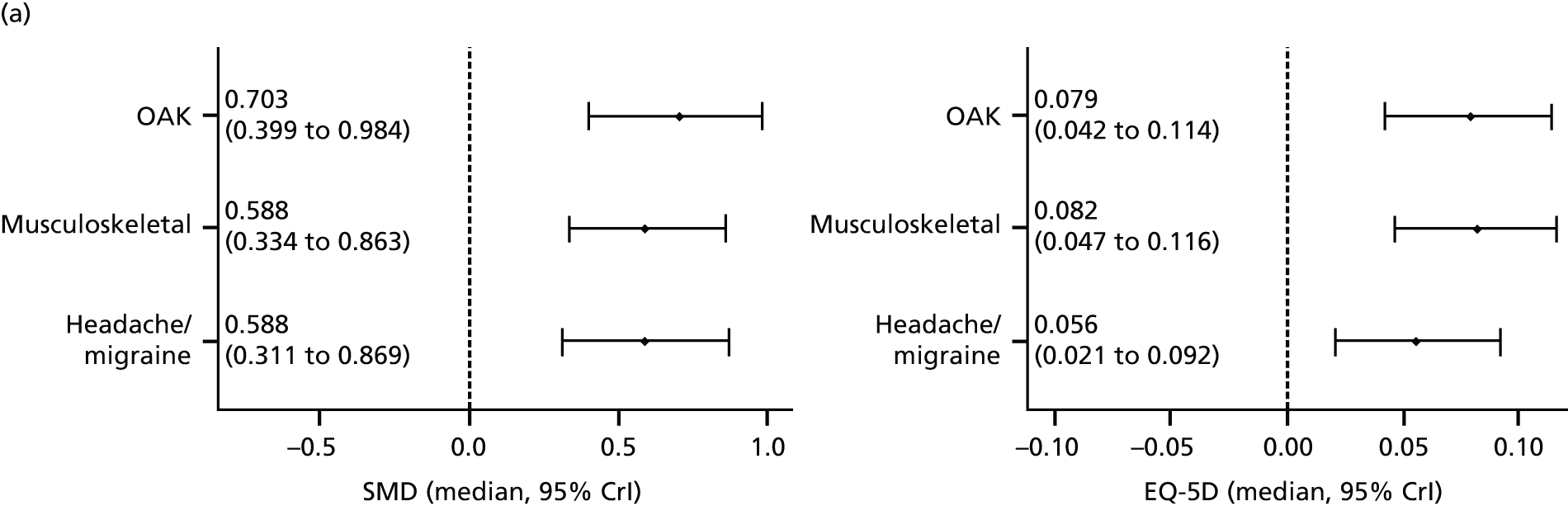
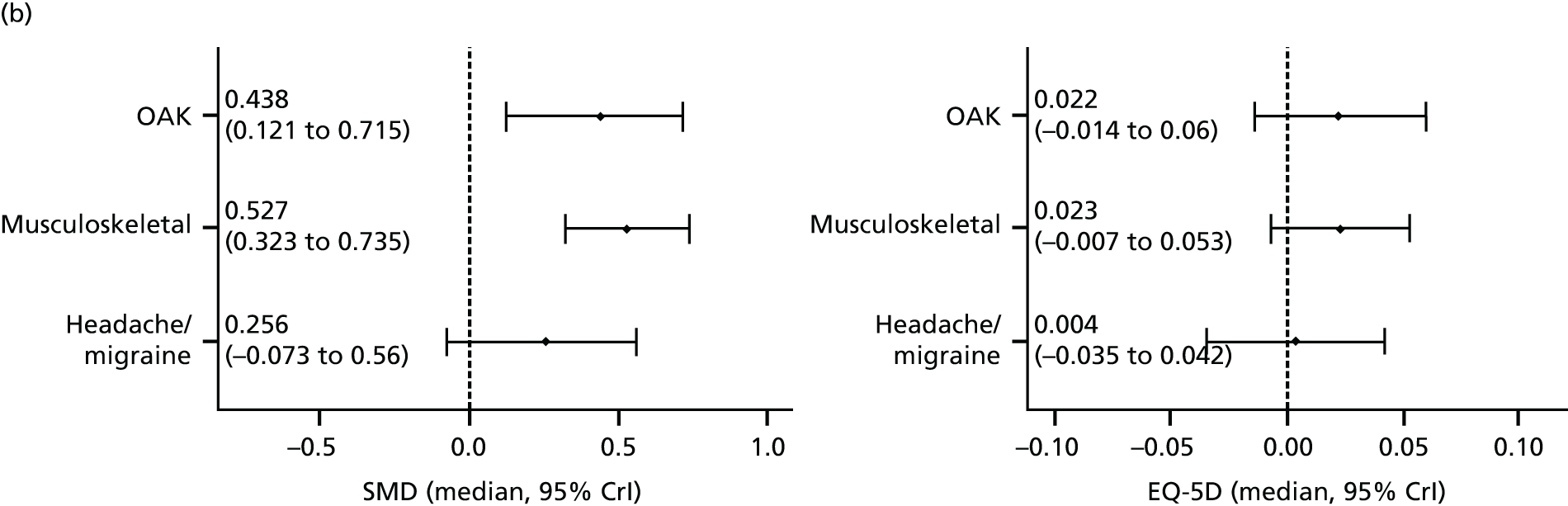
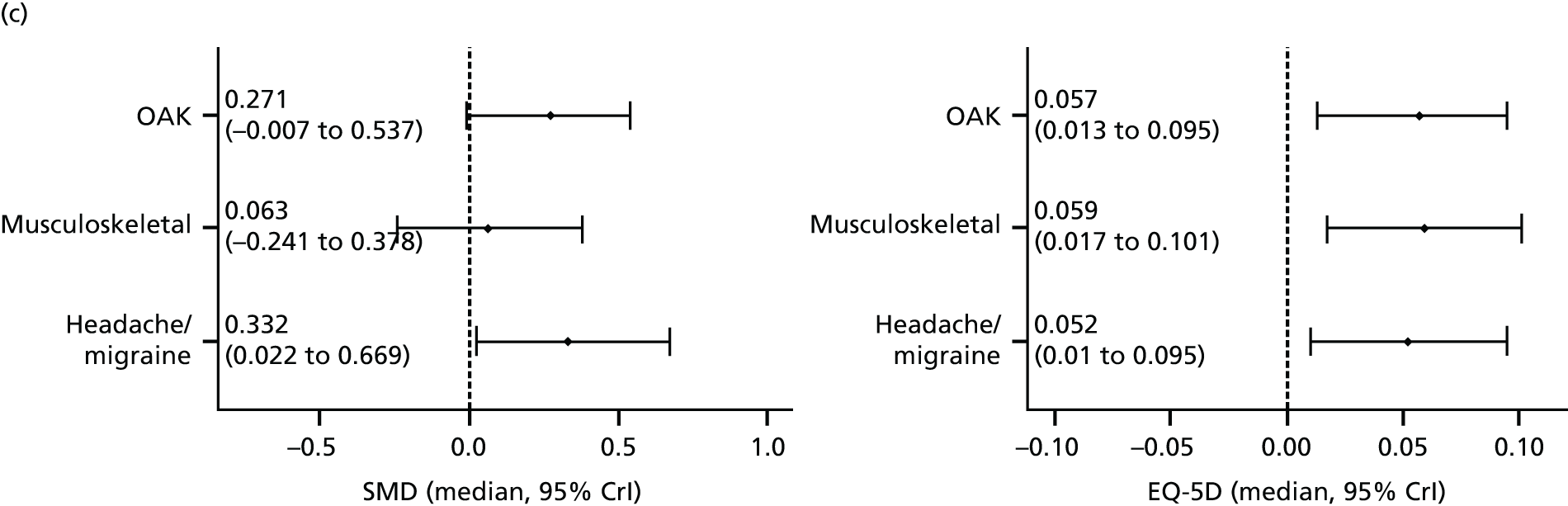
The expectations were that some level of heterogeneity existed between trials. Possibly as a consequence of the mapping work performed, this expectation was not fulfilled for the EQ-5D end point (the between-study variance estimate is 0.001). For the standardised pain end point, the between-study variance was also small relative to the magnitude of the treatment effects (the between-study variance estimate is 0.09). The total residual deviance suggests that the models provide an adequate fit to the data (see Table 22).
Controlling for patient-level characteristics
Table 21 provides information on age of participants for each of the trials included in the data set. On average, age was lower in the headache/migraine pain group than in the musculoskeletal and osteoarthritis of the knee groups.
Using the change in EQ-5D as the outcome for synthesis, Table 23 presents the results of applying model 2 (an extension of model 1) to include patient-level information on age, age being a potential treatment effect modifier. The model fit statistics show that this adjusted by age model is marginally better than model 1, providing lower DIC statistics and reduced posterior residual deviance. The results of this model are very similar to those of model 1 and do not suggest that age is a strong effect modifier or that non-linear effects of age on the effect of treatments exist.
| IPD NMA results | Comparison | Model 2 (with adjustment for baseline age and treatment by age interactions), median MCMC posterior sample (95% CrI) | |
|---|---|---|---|
| Relative treatment effects | SHAM vs. UC | 0.040 (–0.006 to 0.084) | |
| ACU vs. UC | 0.066 (0.025 to 0.105) | ||
| ACU vs. SHAM | 0.026 (–0.012 to 0.066) | ||
| Main effects | Age | –0.002 (–0.002 to –0.001) | |
| Age2 | 0.000 (0.000 to 0.000) | ||
| Pain exchangeable interactions (vs. OAKa) | Headacheb,c | SHAM (vs. UC) | 0.016 (–0.044 to 0.079) |
| ACU (vs. UC) | –0.006 (–0.059 to 0.047) | ||
| Musculoskeletalb,c | SHAM (vs. UC) | 0.006 (–0.057 to 0.070) | |
| ACU (vs. UC) | 0.008 (–0.045 to 0.062) | ||
| Age common interactions | Age | 0.000 (0.000 to 0.001) | |
| Age2 | 0.000 (0.000 to 0.000) | ||
| Between-study variance | 0.001 (0.000 to 0.003) | ||
| Total residual devianced | 15,590 (15,210 to 15,970) | ||
| DICe | –6462.0 | ||
Analysis with restricted evidence (models 3 and 4)
Results for models 3 and 4 are presented in Table 24, together with the model 1 results for comparison. Generally, all three models convey the same message in relation to which treatment provides higher increases in patients’ HRQoL, that is, acupuncture is found to be better than sham acupuncture and usual care treatments. Nevertheless, given the presence of baseline imbalance, models 3 and 4 (but model 3 in particular) provide very different and potentially inappropriate summary results of treatment effects when compared with model 1. These two models show also a fit to the data that is worse than model 1 (higher DIC of –6420 in model 1 compared with –69 and –3824 in models 3 and 4, respectively). In the absence of baseline outcome data, if the choice was between modelling change (model 3) or modelling follow-up scores (model 4), results from the latter model indicate that this would be a better option as the relative treatment effect estimates and pain interaction effects are closer to those in model 1.
| IPD NMA results | Comparison | Model | |||
|---|---|---|---|---|---|
| 1 (ANCOVA extension, change in EQ-5D scores, adjusted for baseline), median MCMC posterior sample (95% CrI) | 3 (change in EQ-5D scores without baseline adjustment), median MCMC posterior sample (95% CrI) | 4 (follow-up EQ-5D scores without baseline adjustment), median MCMC posterior sample (95% CrI) | |||
| Relative treatment effects | SHAM vs. UC | 0.057 (0.013 to 0.095) | 0.077 (0.033 to 0.118) | 0.051 (0.008 to 0.094) | |
| ACU vs. UC | 0.079 (0.042 to 0.114) | 0.093 (0.054 to 0.129) | 0.074 (0.035 to 0.113) | ||
| ACU vs. SHAM | 0.022 (–0.014 to 0.060) | 0.016 (–0.022 to 0.054) | 0.023 (–0.014 to 0.065) | ||
| Pain exchangeable interactions (vs. OAKa) | Headacheb,c | SHAM (vs. UC) | –0.005 (–0.060 to 0.054) | –0.032 (–0.089 to 0.029) | 0.001 (–0.059 to 0.064) |
| ACU (vs. UC) | –0.023 (–0.071 to 0.029) | –0.035 (–0.082 to 0.014) | –0.021 (–0.074 to 0.032) | ||
| Musculoskeletalb,c | SHAM (vs. UC) | 0.002 (–0.052 to 0.062) | –0.016 (–0.074 to 0.045) | 0.003 (–0.056 to 0.064) | |
| ACU (vs. UC) | 0.003 (–0.046 to 0.054) | –0.009 (–0.059 to 0.042) | 0.005 (–0.048 to 0.058) | ||
| Between-study variance | 0.001 (0 to 0.003) | 0.001 (0 to 0.003) | 0.001 (0 to 0.003) | ||
| Total residual devianced | 15,850 (15,480 to 16,230) | 16,990 (16,570 to 17,420) | 15,370 (15,010 to 15,730) | ||
| DICe | –6420.4 | –69.9 | –3823.7 | ||
Results of analysing resource use
Table 25 shows the results from regressing change in primary and secondary care health resources on change in EQ-5D index score for each study. Generally, an increase in EQ-5D score over time implies a reduction in health-care resource use. The analysis of secondary care resource use for osteoarthritis of the knee was an exception, with improvements in EQ-5D score being associated with increased secondary care attendances; however, this result was not statistically significant.
| Study | Pain group (type) | Analysis | |||
|---|---|---|---|---|---|
| Main 3–12 months of EQ-5D on type of resource use, mean (95% CI) | Secondary 0–3 months of EQ-5D on resource use, mean (95% CI) | ||||
| Primary carea | Secondary careb | Primary carea | Secondary careb | ||
| Vickers 200460 | Headache (headache) | –1.247 (–2.409 to –0.085) | –0.17 (–0.63 to 0.289) | –0.719 (–1.849 to 0.412) | –0.057 (–0.503 to 0.390) |
| UK BEAM Trial Team230 | Musculoskeletal (lower back) | –0.296 (–0.988 to 0.393) | –0.193 (–0.58 to 0.193) | –0.414 (–0.925 to 0.098) | –0.19 (–0.863 to 0.483) |
| Underwood 2008 (TOIB)231 | Osteoarthritis of the knee | –0.885 (–1.93 to 0.16) | 0.47 (–0.638 to 1.579) | –0.294 (–0.966 to 0.378) | 0.069 (–0.421 to 0.559) |
Results of the illustrative cost-effectiveness analysis
Illustrative cost-effectiveness results are presented in Table 26. The ICERs in each indication are well below the threshold of £20,000–30,000 per QALY generally considered acceptable in the UK. Results using the 0- to 3-month data for the resource use regressions were very similar to the results in the base case and are therefore not shown here. Acupuncture has close to a 100% probability of being cost-effective in patients with osteoarthritis of the knee and musculoskeletal pain types, and an 86% probability of being cost-effective for the headache/migraine indication, assuming a threshold of £20,000 per QALY. The sensitivity analysis using trial data with a weighted average of the therapist time observed in the trials provided fairly similar results, with musculoskeletal pain now obtaining the lowest estimated ICER compared with the other two pain groups, as shown in Table 26. The results of the probabilistic sensitivity analysis are presented as cost-effectiveness acceptability curves in Figure 14.
| Analysis | Pain group | Treatment | Incremental QALYs, mean (95% CrI)a | Incremental costs, mean (95% CrI) (£)a | ICER (£ per QALY) | Probability cost-effective at £20,000 per QALY |
|---|---|---|---|---|---|---|
| Main analysis (NICE guidance treatment regimen) | Osteoarthritis of the knee | Usual care | – | – | – | 0.02 |
| Acupuncture | 0.0196 (0.0101 to 0.0287) | 189 (176 to 202) | 9673 | 0.98 | ||
| Headache/migraine | Usual care | – | – | – | 0.14 | |
| Acupuncture | 0.0140 (0.0053 to 0.0231) | 183 (176 to 188) | 13,076 | 0.86 | ||
| Musculoskeletal | Usual care | – | – | – | 0.01 | |
| Acupuncture | 0.0205 (0.0118 to 0.0291) | 184 (179 to 189) | 8997 | 0.99 | ||
| Sensitivity analysis (trial-based treatment regimen) | Osteoarthritis of the knee | Usual care | – | – | – | 0.01 |
| Acupuncture | 0.0196 (0.0101 to 0.0287) | 169 (156 to 182) | 8651 | 0.99 | ||
| Headache/migraine | Usual care | – | – | – | 0.18 | |
| Acupuncture | 0.0140 (0.0053 to 0.0231) | 197 (191 to 202) | 14,110 | 0.82 | ||
| Musculoskeletal | Usual care | – | – | – | 0.00 | |
| Acupuncture | 0.0205 (0.0118 to 0.0291) | 138 (132 to 143) | 6745 | 1.00 |
FIGURE 14.
Cost-effectiveness acceptability curves for each pain condition. The curves indicate the probability that acupuncture is cost-effective at different values of the ceiling ratio. Separate curves are shown for osteoarthritis of the knee (OAK), headache/migraine and musculoskeletal chronic pain types.
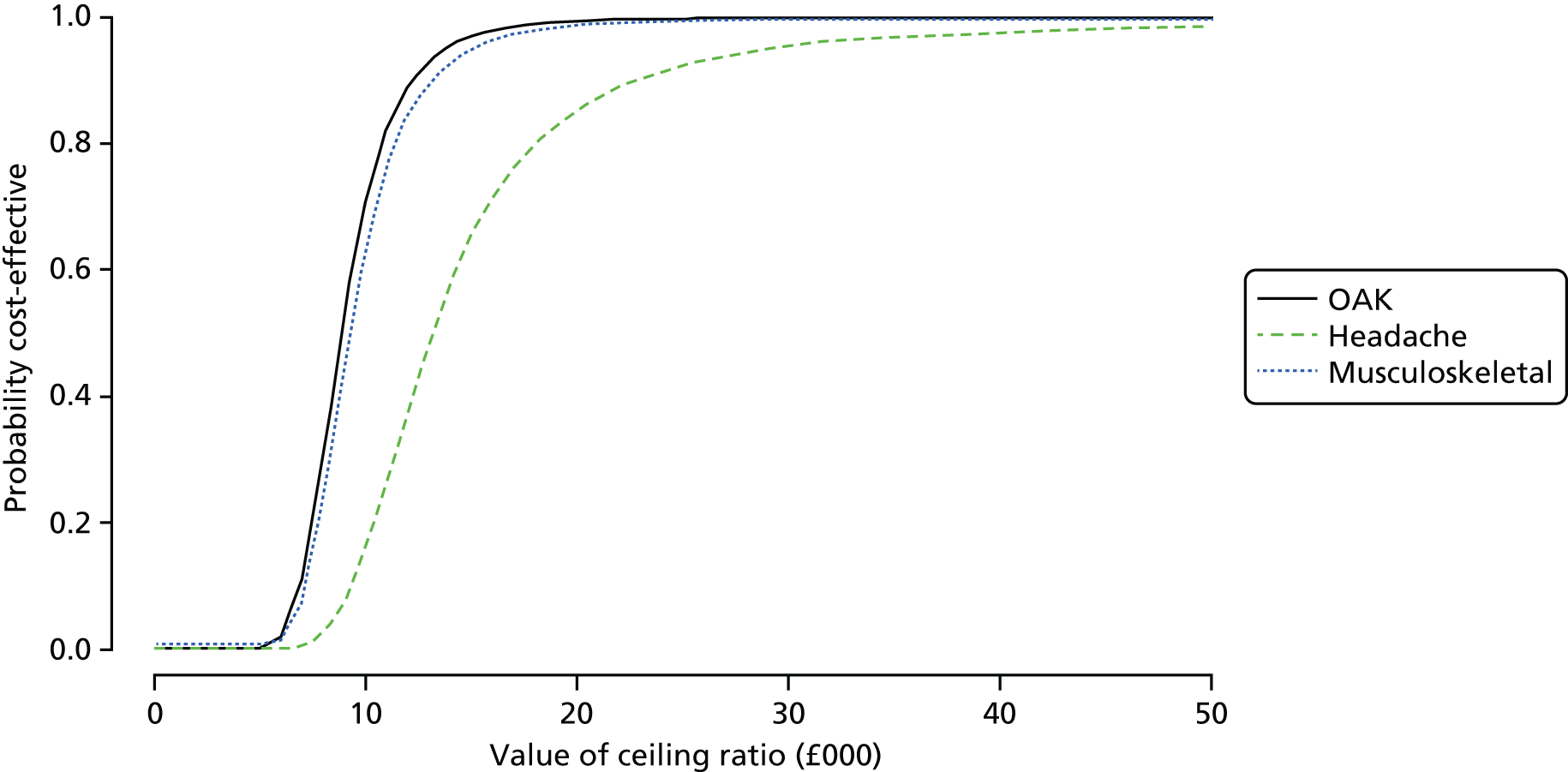
Discussion
Principal findings
Policy-makers faced with difficult resource allocation decisions require estimates of the costs and effects of alternative treatment options. These estimates should reflect all relevant data and compare treatments using a metric that can be used across clinical areas – in the UK the QALY is typically used. Synthesising all relevant evidence to produce comparable estimates of costs and effects generates a series of challenges as the available evidence base rarely captures all costs and effects of treatment (because of the nature of data collection or the duration of follow-up), and often requires evidence to be generalised from different populations. The available trial evidence may compare different sets of treatments and in many instances the HRQoL data required to estimate QALYs directly are not available.
The National Institute for Health and Care Excellence has recommended acupuncture for the treatment of chronic headache and musculoskeletal pain but not in the context of chronic pain associated with osteoarthritis of the knee and headache/migraine. 80–82 This decision in part reflected concerns regarding the available evidence. The current study was commissioned as part of a programme intended to improve evidence around the costs and effects of acupuncture. This study synthesised IPD from RCTs of acupuncture in headache/migraine, musculoskeletal and osteoarthritis of the knee chronic pain. Trials compared acupuncture with usual care, sham acupuncture or both control interventions. Bayesian network meta-analysis synthesis modelling was therefore used in this study to leverage all available evidence to inform estimates of relative treatment effects. The studies reported heterogeneous and distinct outcome sets. Methods to homogenise outcomes for synthesis were therefore used. The availability of IPD for all studies expanded the set of feasible analyses and allowed development of de novo methods to fully exploit the benefits of access to these data.
Novel methods for network meta-analysis of IPD on continuous outcomes were developed, building on previous work on ANCOVA models for pairwise meta-analysis. 219 Analysis of the pain outcome required development of methods for conducting SMD analysis with IPD. Analysis of the EQ-5D data required an extensive mapping exercise whereby separate mapping functions were applied to each study, with choice of mapping dependent on the available outcome data. Access to IPD allowed ANCOVA models to be applied, thus improving precision and adjusting for baseline imbalance. Access to IPD also avoided the use of any assumptions regarding the distribution of HRQoL instrument scores, thus allowing the observed distributions to be adequately reflected in the mapped utilities. Finally, access to IPD provided the opportunity to adjust for covariates based on within- and across-trial information. Given the demonstrable benefits of access to IPD, more effort should be made to share and develop repositories for data. A recent survey indicated a high level of support from reviewers affiliated with Cochrane Collaboration’s IPD Meta-analysis Methods Group for the development of a central repository for storing IPD. 250
Analyses were conducted to explore the importance of modelling change scores in the presence of non-normally distributed outcome data and to explore the implications of using non-ANCOVA models, as would be necessary in the absence of IPD. The results showed that modelling final scores or change scores without baseline adjustment produced estimates of treatment effect that differed by up to 26% compared with the baseline adjusted model, emphasising the importance of baseline adjustment and therefore of having access to IPD.
The results of the network meta-analysis show acupuncture to be more effective than usual care with respect to reducing pain and improving HRQoL. There remains uncertainty regarding whether or not the benefit of acupuncture varies across the pain types analysed. The analysis of EQ-5D preference scores suggests that patients with the headache/migraine pain type may benefit from acupuncture, but less so than patients with osteoarthritis of the knee or musculoskeletal pain, although interaction effects are relatively uncertain. A reduced benefit in patients with headache/migraine-related chronic pain could be caused by ceiling effects as individuals with chronic headache/migraine pain had higher baseline EQ-5D index values. Results for the standardised pain analysis were more consistent across indications. Differences between acupuncture and sham acupuncture were relatively small. The large effect of the sham acupuncture intervention compared with usual care may reflect the potency of the sham comparators in the higher-quality trials included in the ATC systematic review. In contrast to the NICE guidelines, our results suggest that if anything the evidence base for acupuncture is stronger in the osteoarthritis of the knee and musculoskeletal conditions (for which acupuncture is recommended only for lower back pain) than in the headache/migraine pain group (for which acupuncture is recommended). The recommendations in the NICE osteoarthritis guidelines were heavily driven by comparisons with sham acupuncture. The network meta-analysis found strong evidence of an effect of acupuncture when compared with sham acupuncture in osteoarthritis of the knee for the standardised pain outcome but not the EQ-5D outcome (for which the CrI contained zero).
Considerable commonalities exist between the methodologies and the results presented in Chapter 2 and this chapter; however, there are some differences. Across pain types, the two chapters report minor differences in effect between acupuncture and usual care, and acupuncture and sham acupuncture. Nevertheless, the results were broadly consistent across the two chapters. The exact magnitude of the treatment effects and their precision inevitably varied given that there are differences in the data and methods being used. The current analysis used 28 trials (rather than 29) and consistently used the 3-month end point rather than the primary end point as in Chapter 2. For example, in Chapter 2 the two headache trials used the primary end point, which was at 6 months. Additionally, a different methodology was used. Chapter 2 used IPD pairwise meta-analysis based on a frequentist approach. In contrast, in this chapter, IPD network meta-analysis was implemented using a Bayesian random-effects framework. The synthesis model implemented in the current analysis considered all evidence and all available treatments of interest in a single analysis, simultaneously deriving relative treatment effects for all comparisons. Finally, Chapter 2 focused on the standardised pain outcome whereas this chapter analysed standardised pain and HRQoL (EQ-5D) estimates.
The cost-effectiveness results suggest that, compared with usual care alone, acupuncture is cost-effective with ICERs ranging from £9000 to £13,000 per QALY. These values fall within both the NICE plausible threshold range (i.e. between £20,000 and £30,000 per QALY gained) and a more recent empirical threshold estimate of £13,000 per additional QALY obtained. 251 These values are comparable to those in other studies in the UK comparing acupuncture with usual care for the same pain indications, which have estimated ICERs of £4000–17,000. 62,63,227 These ICERs were derived from individual studies, whereas the ICERs presented here reflect the synthesis of a large number of studies.
Limitations
The study has a series of limitations. First, synthesis of heterogeneous outcomes relied on imperfect standardisation processes (which assume that any differences in within-trial outcome variability result from the use of different instruments) and mappings, which are typically able to explain only a minority of variation in EQ-5D scores. Clearly, the use of any mapping tool is considered a second best approach to directly eliciting relevant preference-based measures from study participants. The magnitude of bias introduced by using standardisation processes and mapping functions (and different mapping functions across trials) is unknowable. The availability of key outcomes across trials would have reduced these concerns, as would the collection of generic preference-based measures of HRQoL in all trials. A ‘core outcome set’ for osteoarthritis is available, along with the recommendation that future Phase III trials of knee, hip and hand osteoarthritis should evaluate the following domains: pain, physical function, patient global assessment and, for studies of ≥ 1 year, joint imaging. 252 Other recommendations have tended to focus on domains rather than specific instruments. Recommendations that go beyond Phase III regulatory trials, and which define the instruments that should be used to measure outcomes in these domains, are warranted.
Second, outcome data closest to 3 months were selected for synthesis. The synthesis therefore requires the assumption that, in the minority of trials not reporting at 3 months, the available data are reflective of the 3-month time point. Some trials reported outcomes at months 1 and 2. If the effect of acupuncture is gradual, these effects may underestimate 3-month outcomes. For the cost-effectiveness analysis, the HRQoL effects observed at 3 months were applied from 0 to 3 months to generate QALYs. Other quality-of-life trajectories may, however, be more plausible. For example, quality of life may increase gradually during treatment and reduce gradually following treatment completion. Moreover, there is some evidence of benefits increasing for some time after the first 3 months when treatment was provided, for example at 12 months for headache/migraine60 and at 24 months for lower back pain. 61 Depending on the nature and magnitude of these effects, the incremental benefit of sham acupuncture and acupuncture could be larger or smaller than presented here. Further work analysing repeated outcome measurements in a network meta-analysis could be used to evaluate the importance of these effects.
All sham interventions were assumed to be equivalent in the analysis, as were the usual care controls. Evidence from work recently conducted by the ATC suggests that the effect of sham acupuncture may vary depending whether penetrating or non-penetrating needles are used and that the effect of usual care may depend on whether or not a treatment protocol for usual care is specified. 45 Exploration of a network including more refined comparator definitions may, therefore, be of value.
The impact of each pain condition on treatment effects was assumed to be exchangeable;173 this assumption could be explored further by comparing the fit of models assuming a common pain–treatment effect interaction and models assuming completely separate pain–treatment effect interactions.
The studies analysed here are from a range of countries, which may differ in terms of the method and intensity with which acupuncture is administered. For instance, following NICE recommendations for lower back pain, we assumed that acupuncture treatments are fixed at 10 sessions, irrespective of the pain condition. This assumption might be questionable as the optimum number of treatment sessions may vary according to setting and pain type. Also, acupuncture sessions were costed using a unit cost for a physiotherapist of £36 per hour. This is also an assumption of the current work as unit costs will depend on how the NHS will provide the service. In addition, differences in the nature of health care for chronic pain more generally could have impacted on outcomes.
The analysis of non-intervention resource use assumed that only primary care and specialist visits are impacted on by changes in outcomes following acupuncture, and that the impact of treatment on resource use can be captured through changes in the EQ-5D. It is possible, however, that this did not capture the full impact of treatment on resource use.
Our analysis of standardised pain included the primary end point for each study and, therefore, the outcomes on which we would expect the trials to have been powered. The outcomes included in the analysis ranged from pure pain measures to wider measures of HRQoL (e.g. total WOMAC score). Both pain and functioning outcomes have been highlighted in previous NICE Guidance Development Groups to be of critical importance to decision-making. 80–82 Our analysis suggests that, based on the standardised pain outcome, acupuncture is better than usual care and sham acupuncture for all indications, although CrIs include zero for the headache group when acupuncture is compared with sham acupuncture.
Recommendations for future research
First, a key limitation of this work is the use of imperfect standardisation processes to adequately combine available heterogeneous evidence. Thus, we consider it a research priority to identify key outcomes for the conditions considered here and improve reporting so that consistency exists across the body of evidence. Second, in the impossibility of achieving complete homogeneity of outcomes across the relevant evidence resulting in mapping tools being required, a worthwhile methodological extension of the current work would be to develop a model that would map the existing evidence to the desired outcome and simultaneously synthesise it together with other relevant evidence. Finally, it was highlighted in this work how important it is to have access to, and analyse, evidence at the individual level. It showed that IPD has clear value over summary data for both synthesis and decision modelling aspects of the analysis. Thus, continuing efforts to share this data type across the research community is highly commended.
Although results from this analysis provide robust estimates of the incremental costs and effects of acupuncture compared with usual care, they are unlikely to provide a suitable basis for decision-making. There is a wide range of alternative treatments for chronic pain and the relative value of these alternatives should be appraised alongside the costs and effects of acupuncture and usual care to reliably inform decision-making. In the context of osteoarthritis of the knee, an evaluation of a broader set of treatment options has been conducted and is presented in the following chapter.
Conclusions
This study presents methods for conducting IPD network meta-analysis of continuous outcomes when the instruments used to measure outcomes differ between trials. Using the example of acupuncture for the treatment of chronic pain, our novel methods show how heterogeneous outcomes can be analysed using standardisation and mapping approaches, and how the resulting outcomes can be translated into cost-effectiveness results to inform resource allocation decisions.
The methods developed allowed all available trials to inform the synthesis. Availability of IPD allowed the true distribution of outcome measures to be reflected in the mapping to EQ-5D and avoided the use of non-baseline-adjusted models, which produced quite different results. Use of baseline-adjusted change score models produced better results than non-adjusted models, suggesting the superiority of the ANCOVA framework in the context of treatment effect estimation.
The analysis found acupuncture to be more effective than usual care with respect to reducing pain and improving EQ-5D preference scores in patients with chronic pain of osteoarthritis of the knee, musculoskeletal and headache/migraine origin. The benefits of acupuncture over sham acupuncture are smaller than when compared with usual care. The probability that acupuncture is associated with better pain outcomes than sham acupuncture and usual care is high (> 0.96) across indications. The probability that acupuncture is associated with higher EQ-5D preference scores than sham acupuncture and usual care is high in osteoarthritis of the knee (0.89) and musculoskeletal chronic pain (0.95). For headache/migraine this probability is 0.64, reflecting the smaller benefit of acupuncture compared with sham acupuncture for this indication. The methods used provide outputs in a format that can be used to directly inform cost-effectiveness considerations once the full set of relevant comparators is considered.
Chapter 5 Cost-effectiveness of non-pharmacological adjunct treatments for patients with osteoarthritis of the knee
Introduction
Health policy-makers worldwide are under increasing pressure to provide the best and most affordable care to their fellow citizens to maximise population health given existing constraints (e.g. budgetary, ethical, structural). Cost-effectiveness analysis is now being used in many jurisdictions to support ‘value for money’ appraisals as part of the health technology assessment of competing interventions. 253 Examples of national agencies that use cost-effectiveness analysis for health technology assessment to inform their deliberation process include NICE in England and Wales, the Pharmaceutical Benefits Advisory Committee in Australia and the Common Drugs Review in Canada.
Many other similar agencies exist around the world253 and, although the methods they use may vary slightly across jurisdictions, there are a number of essential information requirements, common to all, that must be met for these decision-makers to be able to formulate their funding recommendations. These requirements include the systematic consideration and quantification of the clinical effectiveness, quality of life and health-care cost implications associated with each competing treatment strategy relevant to the decision problem in the jurisdiction of interest.
Unfortunately, the above information is often either not available at all or not available in the format required by the decision-maker. For instance, the evidence base may (1) lack (or be informed by a limited set of) studies comparing head to head all of the relevant treatment strategies, (2) present a fragmented picture with different studies reporting different sets of outcomes and summary statistics, (3) include clinical studies with too short a follow-up duration to directly inform questions about the long-term (cost) effectiveness of the technologies, (4) reflect large variations in clinical practice (between and within jurisdictions) and (5) provide little or no health-care resource utilisation data relevant to the jurisdiction of interest. 204,254
In all of these cases, to use the existing evidence base to inform health policy inevitably requires the application of statistical evidence synthesis and decision-analytic (cost-effectiveness) models. These types of models facilitate the organisation and synthesis of the available information within a coherent mathematical framework developed to evaluate the outcomes of interest (e.g. long-term costs and effects), identify and quantify their key drivers, and appropriately reflect all sources of uncertainty surrounding the decision problem. 255
In many clinical areas and for many interventions, particularly those not subject to strict regulation, the evidence base may include a significant proportion of poor-quality studies, making decision-making challenging. For example, not all interventions that come under the category of complementary and alternative medicine (CAM) therapies256 are subject to rigorously conducted RCTs. However, there is growing public interest in (and demand for) the use of these therapies, with patients often making direct contact with practitioners, with or without their primary care physician’s referral. 257,258 Health policy-makers are therefore responsible for assessing the role and position of CAM therapies within the management of patients with certain conditions and health-care staff need to be familiar with the various treatment options, their possible benefits and risks, and potential interactions with more conventional medical therapies. 259
The limitations of the evidence base mean that there is considerable uncertainty surrounding the (cost) effectiveness of some of the CAM therapies, so much so that, in a recent review of UK clinical guidelines that discussed CAM, 62% did not reach a conclusive recommendation for or against treatment. 260
In its guideline on the care and management of osteoarthritis, NICE228 acknowledges the limitations in the published evidence base and advises people with osteoarthritis of the knee to use a range of core treatments (access to appropriate information, exercise and weight loss for people who are overweight or obese) and a range of pharmacological and non-pharmacological (e.g. manipulation and stretching, electrotherapy) adjunct treatments. The guideline stated that acupuncture should not be offered for the management of osteoarthritis. This recommendation spurred reactions from patient groups and practitioners,84 particularly because it is in contrast to the fact that, of the various forms of CAM, acupuncture is one of the most popular referrals (based on a review of three different surveys) and approximately 4 million sessions are provided annually in the UK each year. 1,261,262
In this chapter we address some of the concerns about the limitations in the published evidence base on CAM, particularly with regard to the role of acupuncture for osteoarthritis of the knee. The study featured in this chapter builds on the IPD meta-analysis of Chapter 2 in which we found that acupuncture was superior to both usual care and sham acupuncture for the treatment of back and neck pain, osteoarthritis of the knee, and chronic headaches and migraine. In Chapter 3 we conducted a comprehensive evidence synthesis (i.e. systematic literature review and network meta-analysis) of physical therapies for patients with pain related to osteoarthritis of the knee in which we found acupuncture to be one of the more effective therapies for alleviating knee pain. However, the study did not attempt to quantify the wider quality-of-life benefits of the included interventions or their value for money. In Chapter 4, we reanalysed the IPD of Chapter 2 in which pain outcome measures and measures of HRQoL mapped to EQ-5D preference weights were analysed and a number of methodological challenges were addressed.
This chapter tackles the methods issues identified in Chapter 4 and builds on the work of Chapter 3 with its focus on osteoarthritis of the knee. We report on novel methods and results of a network meta-analysis of multiple sources of aggregate data and IPD, and a cost-effectiveness (or ‘value for money’) assessment of non-pharmacological adjunct interventions in patients with knee pain caused by osteoarthritis in the UK NHS primary care setting. The study compares the costs and effects of 13 therapies that could be (and in some cases already are being) used within the UK NHS as adjunct treatments for knee osteoarthritis. The evidence base and economic decision problem associated with osteoarthritis of the knee describes the decision problem and the evidence base. The methods section describes the network meta-analysis models developed for the simultaneous synthesis of continuous aggregate data and IPD, and the cost-effectiveness model methods. The results section presents the results of the application of the methods to the decision problem and data at hand. The discussion section discusses the study findings, its strengths and limitations, and recommendations for future applied and methodological research. This is followed by the final conclusions.
The evidence base and economic decision problem associated with osteoarthritis of the knee
The decision problem
Osteoarthritis is most commonly located in the knee and is a major cause of pain, activity limitation and health-care utilisation, especially among older people. 228 There appears to be an unmet need for treatment for this condition. 263,264 Although acupuncture has been advocated as a potentially effective therapy to manage osteoarthritis-related pain,265 NICE does not recommend its use because of questions over its (cost) effectiveness. 228 Other international guidelines have varied in their recommendations. EULAR161 and AAOS266 do not recommend the use of acupuncture, the ACR198 recommends acupuncture for those with moderate to severe pain who are unwilling, unable or ineligible to undergo total knee arthroplasty and OARSI267 makes an ‘uncertain’ recommendation regarding acupuncture. The Scottish Intercollegiate Guidelines Network268 recommendations regarding the management of chronic pain recommend acupuncture for short-term pain relief in osteoarthritis and guidance on the management of pain in older people has deemed acupuncture worthy of further investigation. 269 In addition, a range of other adjunct treatments have been recommended and/or are available that could be used to manage osteoarthritis-related pain.
This evaluation aimed to assess the cost-effectiveness of alternative non-pharmacological adjunct interventions in patients experiencing pain attributable to osteoarthritis of the knee. The setting of the study was UK primary care and the costing perspective was that of the NHS and Personal Social Services. The outcome measure used was the QALY. The comparators included in the economic evaluation are documented in Table 27. The exact delivery of comparators reflects the delivery (and heterogeneity in delivery) of the underlying trials (see Appendix 3).
| Intervention | Intervention subtype |
|---|---|
| Acupuncture | |
| Appliances | Braces |
| Insoles | |
| Electrotherapy | Interferential therapy |
| Laser/light therapy | |
| NMES | |
| PES | |
| PEMFs | |
| TENS | |
| Manual therapy | |
| Static magnets | |
| Heat treatment | |
| Usual care |
The evidence base
To inform estimates of the effectiveness of the alternative interventions, we conducted an extensive systematic literature review, presented in Chapter 3, synthesising the network of available RCT data using network meta-analysis methods, to assess the effectiveness of acupuncture and other relevant physical treatments for alleviating osteoarthritis-related knee pain. Of the 22 main interventions in the studies forming this evidence base, we found that muscle-strengthening exercise, acupuncture, TENS and balneotherapy were the interventions most commonly investigated. Studies typically recruited from general populations with osteoarthritis of the knee, although weight-loss trials (as expected) recruited only overweight or obese participants. Mean ages in the studies ranged from 53 to 85 years and the proportion of female patients ranged from 26% to 100%. Usual care and placebo were the most frequently studied comparators, with ‘no intervention’ being used rarely. There was considerable variation in the average treatment duration across the interventions, although a majority of interventions were administered over a 2- to 6-week period. For five of the studies included in this review, IPD were available from the repository prepared by the ATC for the IPD meta-analysis study presented in Chapter 2.
The evidence base in the context of the economic decision problem
The NICE clinical pathway for the management of osteoarthritis distinguishes between core treatments (i.e. information, exercise and weight loss) and a number of non-core treatments that may be given to patients as needed, depending on preferences, needs and risk factors. 228 These additional treatments are classified by NICE as pharmacological adjunct, non-pharmacological adjunct and possible joint surgery following referral. The 22 interventions included in the systematic literature review and network meta-analysis presented in Chapter 3 included core and non-pharmacological adjunct treatments. Furthermore, NICE will undertake a full review of evidence on the pharmacological management of osteoarthritis, which will be carried out after a review of the safety of over-the-counter analgesics is completed by the Medicines and Healthcare products Regulatory Agency. Therefore, the decision problem addressed in this chapter is, ‘which is the most cost-effective non-pharmacological adjunct treatment for individuals in England and Wales with pain of the knee caused by osteoarthritis’? It follows that information provision, activity and exercise and interventions to achieve weight loss are excluded from the cost-effectiveness comparisons on the grounds that they are core treatments and the decision about which to use is expected to be independent of the choice of adjuvant therapy. Activity and exercise interventions were retained in the network meta-analysis as they provide indirect evidence regarding the relative efficacy of the interventions of interest in the cost-effectiveness analysis. Trials of weight-loss interventions were excluded from the network meta-analysis as these interventions were trialled only in overweight osteoarthritis of the knee patients and inclusion of these data was expected to increase heterogeneity in the network.
Usual care and no intervention were pooled in the current analysis as information available from the trials did not allow these comparators to be clearly distinguished. Furthermore, balneotherapy was included in the network meta-analysis but not the cost-effectiveness analysis as it seemed unlikely that the NHS in England and Wales would invest in provisions for mineral bathing. Ice/cooling treatment was included in the network meta-analysis but not the cost-effectiveness analysis as use of local cold application (packs, massage) is widely used as part of self-management at no (or minimal) cost and no known risk. 228
Comparator interventions were classified as in Chapter 3. This included classifying sham acupuncture separately from other placebo interventions but otherwise considering all sham interventions in a single placebo category. The potential for this to increase heterogeneity in the network is addressed in the discussion.
Data included in the synthesis model
All studies from the systematic review in Chapter 3 that provided data suitable for the network meta-analysis were initially considered for inclusion in the synthesis (see Appendix 5 for a description of the data required for the network meta-analysis). The characteristics of the studies included in this study have been reported in detail in Appendix 3 as well as in a previous report168 and are briefly summarised in Appendix 5. The network meta-analysis included 88 studies (out of a possible 152) and 7507 patients. The remaining 64 studies were not included because of limitations in the collection and reporting of data. IPD were available for five of the 88 trials,69,77,120,134,136 including 1329 patients. As IPD were made available by the ATC, IPD were available only for acupuncture trials. The systematic review in Chapter 3 identified 25 trials including acupuncture as a comparator; of these, 16 provided data suitable for inclusion in the current analysis and five were included by the ATC and therefore contributed IPD.
The studies were generally small, with only 15 of the 88 studies including > 50 patients per trial arm. It was felt that, because of the heterogeneity in follow-up assessment and duration of treatment, the analysis should focus only on data reported while patients were on treatment (or within 2 weeks of treatment discontinuation). Based on feedback from clinical experts (a GP and a physiotherapist), patients in the NHS are typically offered 6–10 weeks of treatment to alleviate pain related to osteoarthritis of the knee. The analysis therefore included data reported closest to the 8-week time point. This time point was also used in the review of acupuncture conducted as part of the development of the NICE osteoarthritis guideline. 228 The time points available for analysis ranged from < 1 day to 1 year, as determined by the nature of the intervention, treatment duration, trial-specific design and planned follow-up assessment. Figure 15 provides a visual representation of the network of all included trial data.
FIGURE 15.
Network of evidence for interventions in osteoarthritis of the knee.
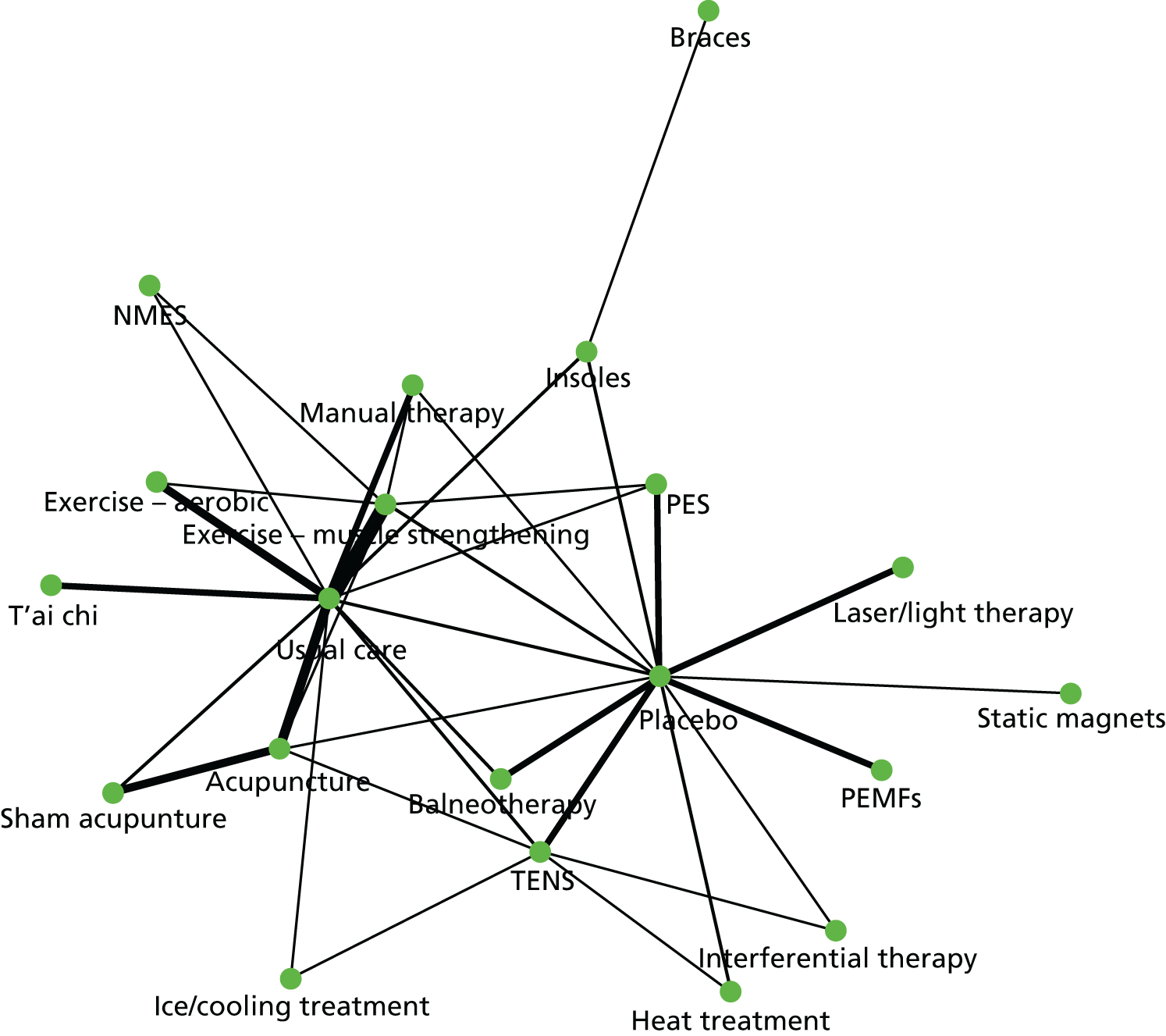
Here, points represent competing treatment strategies and solid lines describe treatment comparisons for which direct trial evidence exists. Each line’s thickness is proportional to the number of studies informing a comparison between two strategies, thus, the thicker the line the larger the number of trials available for that comparison. The largest numbers of trials were available for the comparisons between muscle strengthening exercise and usual care (n = 14), acupuncture and sham acupuncture (n = 8), aerobic exercise and usual care (n = 7), and acupuncture and usual care (n = 7).
Given the variable and often poor quality of the underlying evidence base, the appropriate data set to inform decision-making is uncertain. Three different networks of evidence were therefore used in the primary analysis: first, a network meta-analysis in which all trials were included as described above; second, a subset of trials that restricted the network meta-analysis to studies with a low risk of bias for allocation concealment (39 trials); and, third, a network meta-analysis that used this criterion as well as further restricting the data set to those studies that reported outcomes between 3 and 13 weeks (31 trials). Figures 16 and 17 show the network of evidence available for the second and third scenarios, respectively. Appendix 5 documents which studies were included within each network.
FIGURE 16.
Network of evidence: trials with a low risk of bias for allocation concealment.
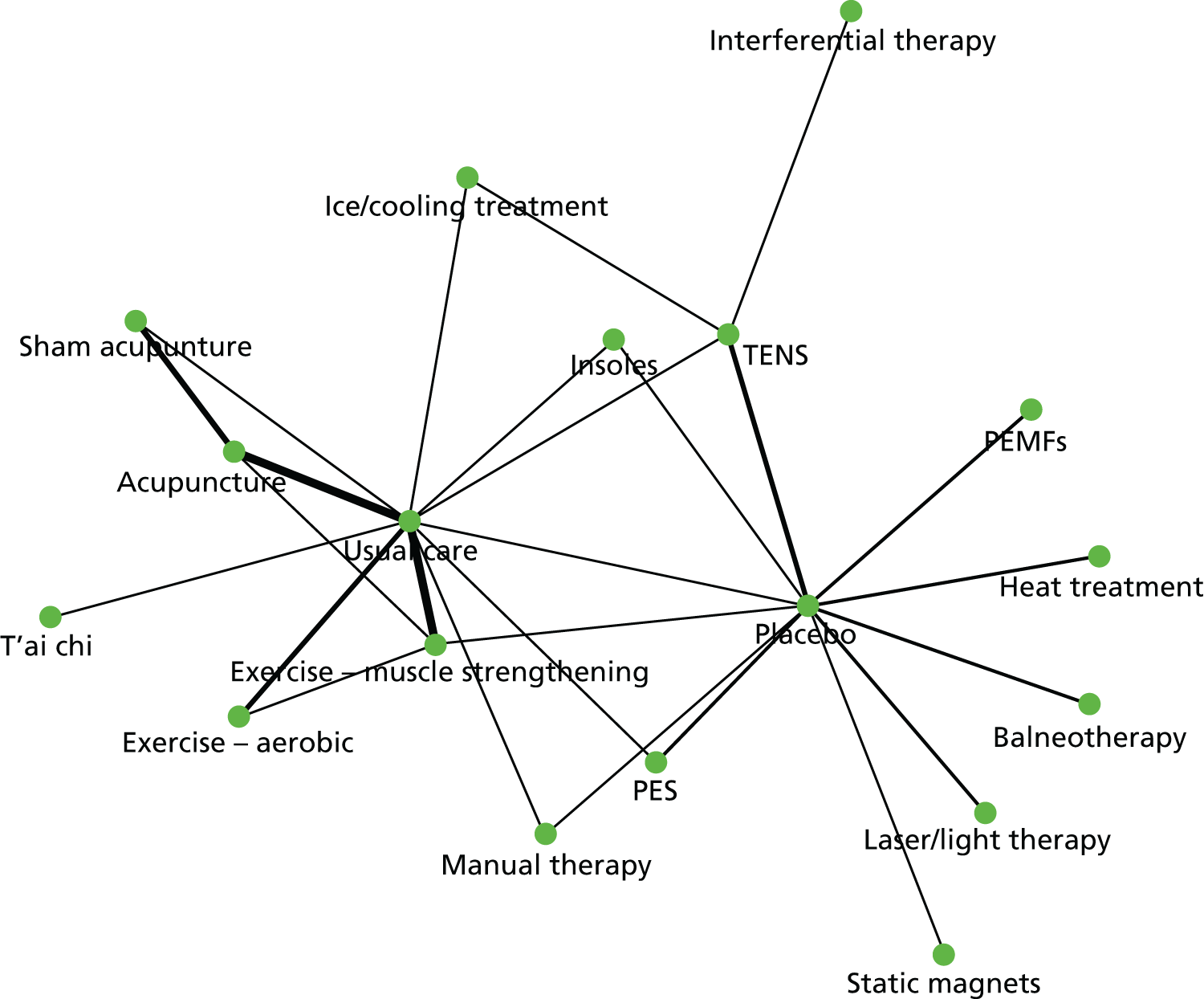
FIGURE 17.
Network of evidence: trials with a low risk of bias for allocation concealment and reporting at 3–13 weeks.
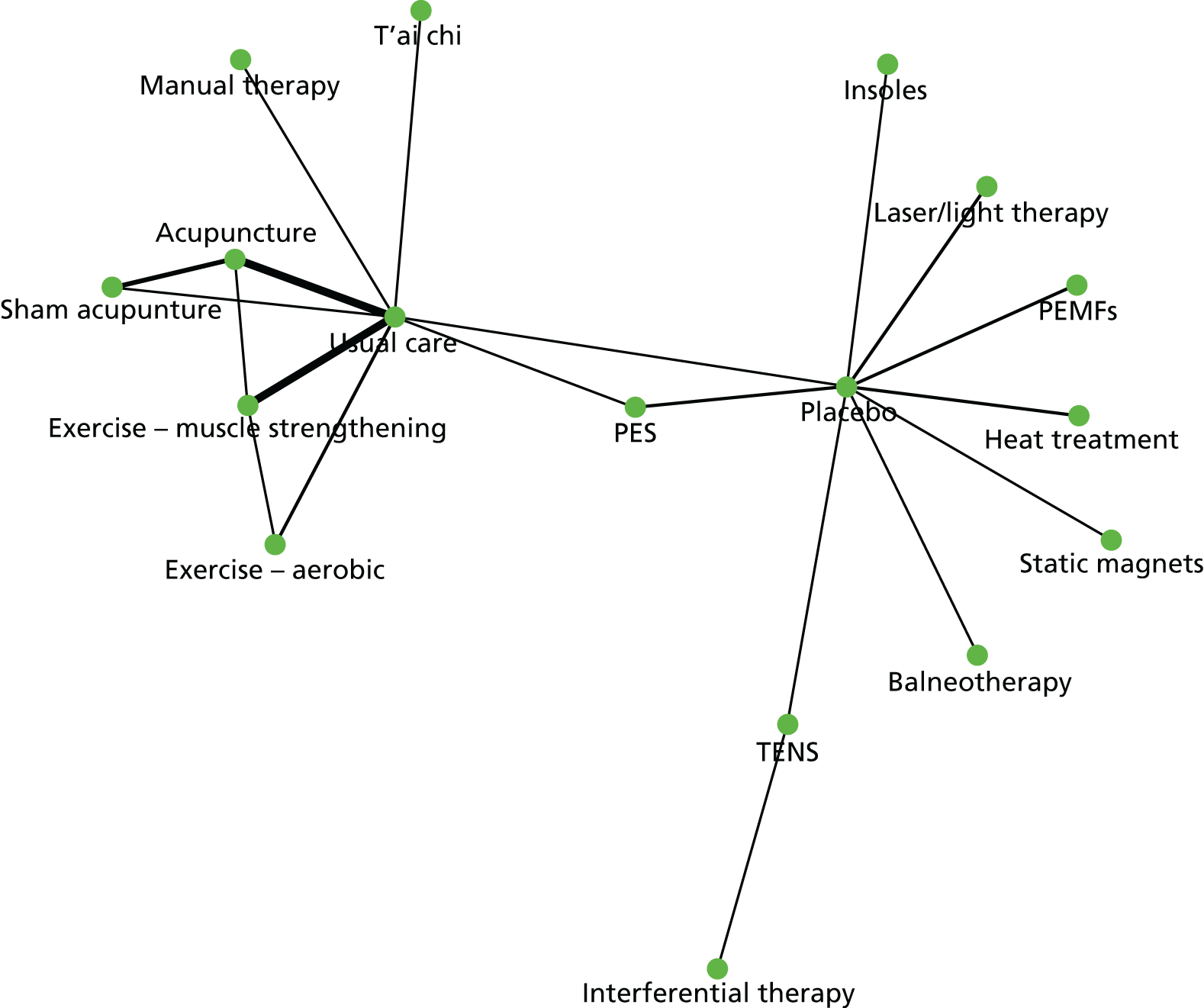
Health-related quality of life
Instruments designed to measure patient HRQoL can be classified according to whether they are generic or condition specific and whether they are preference based or not preference based (depending on whether or not the values used to score them have been derived using methods consistent with economic theory). 270,271 Examples of generic preference-based HRQoL instruments include the EQ-5D,272 Health Utilities Index-3273 and Short Form questionnaire-6 Dimensions,274 whereas examples of generic non-preference-based instruments include the SF-36275 and the SF-12. 276 Similarly, the WOMAC115 and the Health Assessment Questionnaire (HAQ)277 are examples of condition-specific non-preference-based HRQoL instruments in osteoarthritis, whereas an example of a condition-specific preference-based HRQoL instrument is the HAQ preference-based measure. 278
The HRQoL instrument(s) reported in each study varied considerably across the 88 trials and many trials reported more than one instrument. The EQ-5D was our preferred end point for the economic assessment given the preferences of UK decision-makers, such as NICE, for this HRQoL measure. 224 We therefore focused on HRQoL instruments for which a mapping algorithm to the EQ-5D was available. The instruments for which a mapping algorithm to the EQ-5D was available, and the mapping algorithms themselves, were identified using the University of Oxford’s HERC database of studies mapping from HRQoL or clinical measures to EQ-5D as reported by Dakin. 235 When multiple mapping algorithms were available for a given instrument, the preferred algorithm was selected on the basis of the sample size, adequacy of statistical modelling and relevance of the study population.
In a number of studies data for multiple HRQoL instruments for which mappings were available were reported. The preferred instrument was selected from these studies based on the extent to which the instrument was expected to reflect all dimensions of the EQ-5D. This resulted in the following hierarchy: EQ-5D preference values; SF-36 dimension scores; SF-36 MCS and PCS scores; SF-12 MCS and PCS scores; WOMAC total score; VAS measures of pain; and NRS measures of pain.
The HRQoL instrument used for each study is presented in Appendix 5. Generic HRQoL instruments were available for 19 studies (EQ-5D, n = 3; SF-36 dimensions, n = 6; SF-36 MCS and PCS, n = 9; SF-12 MCS and PCS, n = 1). The remaining 69 studies provided WOMAC (n = 33); pain VAS (n = 33) or pain NRS (n = 3) data. The distribution of instruments used according to treatment comparison and study size is shown in Figure 18. Those studies that included generic HRQoL data tended to be larger and of higher quality. Generic HRQoL instruments were used in studies including muscle-strengthening or aerobic exercise, acupuncture, PES, t’ai chi and NMES comparators.
FIGURE 18.
Best available quality-of-life data by comparison. Note: shape size is proportional to study size. Shapes are used to represent different HRQoL instruments. Studies informing multiple comparisons (because of the presence of three or more trial arms) appear for each comparison.

Health-care resource use
Only one study in the IPD data set provided resource use data. 69 This study was conducted in Germany and the only resource use item provided was the number of acupuncture sessions. It was, thus, deemed appropriate to bring in external study data to inform resource use. Resource use associated with administering each intervention was obtained using information from the clinical trials regarding the intensity of treatment and information from clinical experts (a GP and a physiotherapist) regarding the typical method of administering the treatment and equipment required.
It was anticipated that the frequency of primary care and outpatient specialist visits might be impacted on by interventions that reduce individuals’ chronic pain. Resource use data from the TOIB RCT231 was therefore used to relate changes in health outcomes (measured by the EQ-5D) to changes in these resource use items.
Methods
This section describes the methods developed to carry out the synthesis of IPD and aggregate data, the rationale and methods for mapping generic and condition-specific HRQoL into EQ-5D scores, the approach used to derive health-care resource use and cost estimates for each treatment strategy being compared and the methods used to carry out the cost-effectiveness analysis. Given the complexity of the evidence base at hand and the range of methods used to address the challenges described in the previous section, Figure 19 provides a visual representation of the relationships between data, models and outputs to facilitate exposition in this section.
FIGURE 19.
Visual map of the model integrating synthesis, mapping and economic analysis. AD, aggregate data; HCRU, health-care resource use; NMA, network meta-analysis.

Health-related quality of life for cost-effectiveness analysis
As mentioned in the previous section, the 88 studies included in the base-case analysis used a range of different HRQoL instruments, which were also reported in a fragmented way (see Appendix 5 for more details). To overcome this problem, in Chapter 3 we synthesised study-level standardised mean (pain) differences as a measure of effectiveness. This is one of the possible solutions when combining treatment effect estimates from studies that measured the same outcome using a variety of different instruments. 279,280 However, the method has limitations and these are important when the SMD outcome is to be used to inform cost-effectiveness considerations. This is because health-care policy-makers require a common health outcome measure to use as a yardstick to be able to make decisions across different conditions and clinical areas. In many jurisdictions, including England and Wales,224 this yardstick is the QALY. 225 The QALY is a composite measure that combines mortality and morbidity into a single numeraire, thus providing an estimate of an individual’s remaining life expectancy weighted by some measure of HRQoL. In England and Wales, HRQoL is typically measured using a preference-based instrument such as the EQ-5D. 272
The EQ-5D has been developed by the EuroQol Group [see www.euroqol.org (accessed 20 July 2016)] as a standardised instrument for describing and valuing HRQoL. It describes an individual’s health state on five domains – mobility, self-care, usual activity, pain/discomfort, anxiety and depression – each of which has three levels of severity (no problem, some problems, extreme problems), giving rise to 243 possible health states. Studies in the general population have been used to derive societal preference values for each of the EQ-5D health states in several countries, including the UK. 232 The UK survey estimated the EQ-5D index score using the time trade-off method to elicit preference values for 42 of the 243 health states in the EQ-5D using a representative sample (n = 3395) of the UK population, and regression methods to predict the values of the remaining health states. The EQ-5D index value (also referred to as ‘tariff’ or ‘social tariff’) for the UK ranges from –0.594 (health state 33333, i.e. the worst possible state) to 1 (health state 11111, i.e. full health).
As the metric of interest for quantifying health benefits in cost-effectiveness analysis is the (absolute) difference in mean QALYs between treatment groups, a series of network meta-analysis models were developed to synthesise the EQ-5D data. These models were designed to include data available at both the study and the patient level. Unfortunately, most studies had not reported EQ-5D data. Therefore, the synthesis was preceded by a series of ‘mapping’ exercises used to predict EQ-5D scores from observed generic or condition-specific HRQoL data. Details of the mapping are provided in the next section.
Synthesis of multiple heterogeneous outcomes
As described in the previous section, a range of mapping algorithms was used to predict EQ-5D estimates from the available HRQoL data. This generated a series of challenges. First, the mapping algorithms used were all non-linear and frequently used multiple correlated input dimensions. When IPD are available, these non-linearities and correlations between dimensions are appropriately reflected simply by applying the algorithm to the data reported for each patient. However, when only summary statistics for the HRQoL dimension scores are available, methods were required to appropriately reflect the non-linear nature of the mapping algorithms and the correlation between the dimension scores. This was addressed by generating simulated data sets for each aggregate data study’s HRQoL instrument dimension scores, applying the relevant mapping algorithm and then estimating the statistics required for the synthesis from the resulting simulated EQ-5D data set. The statistics required for synthesis were baseline EQ-5D score and follow-up EQ-5D score as well as their variances. As the distribution of HRQoL dimensions was unknown, individual dimensions from the HRQoL instrument were assumed to follow a multivariate normal distribution. This required an estimate of the variance–covariance matrix for the HRQoL instrument dimensions at baseline and follow-up. Variances were obtained directly from the aggregate data studies. Covariances were estimated using these variances combined with correlations estimated from the IPD studies using standard formulae for the variance–covariance matrix. Correlations between EQ-5D index values at baseline and follow-up were estimated from one study. 134 Correlations between SF-36 dimension scores (and SF-36 PCS and MCS scores) at baseline and follow-up were estimated from three studies. 69,77,134 Correlations between SF-12 PCS and MCS scores were obtained from a study not included in the systematic review reported in Chapter 3, but made available by the ATC. 70 Correlations between baseline and follow-up WOMAC scores were obtained from four studies. 69,77,120,136 Correlations between baseline and follow-up VAS scores were estimated from four studies120,134,136 and an additional trial obtained from the ATC. 135 No IPD were available for the pain NRS end point; correlations between baseline and follow-up NRS data were therefore assumed to be the same as for the VAS data. Samples of HRQoL measures that fell outside of the feasible range were truncated to the minimum or maximum possible values for each HRQoL instrument. Mapped utilities generated by both the IPD and the aggregate data were truncated using the minimum and maximum EQ-5D tariff values.
A second challenge faced in all analyses of mapped HRQoL data was that simply applying a mapping algorithm does not adequately reflect the uncertainty in the mapping process. This is because of both the presence of unmeasured predictors (reflected in the residual error of the mapping algorithm) and the fact that the coefficients of the mapping algorithm are also random variables. Recent methodological developments241 were therefore applied to capture residual error in the mapping algorithms. As recommended in Chan et al. ,241 variances for the mapped aggregate data were inflated by the inverse of the R2 statistic from the corresponding mapping algorithm. For the IPD studies the observed variances were inflated in the same way. As data entered the model at the individual patient level, the additional variance was incorporated by adding a random deviate to the observed outcomes. This deviate was drawn from a normal distribution with a mean of zero and variance equal to the difference between the original and the inflated variances. Uncertainty in the regression coefficients from the mapping algorithm and in the valuation of the EQ-5D preference scores was not captured.
As reported in Appendix 5, the HRQoL measures reported in the evidence base of knee pain related to osteoarthritis included several different instruments, including the SF-36 (sometimes reported in terms of its eight dimensions and sometimes reported in terms of its PCS and MCS scores), SF-12 (MCS and PCS scores), WOMAC and pain (pain VAS or pain NRS). The following published mapping algorithms were applied to data collected using these instruments to derive EQ-5D estimates.
SF-36 to EQ-5D
Those studies that used the SF-36 questionnaire reported the results either in terms of the score for the eight dimensions of the instrument or in terms of its PCS and MCS scores. The model used the mapping algorithm published by Rowen et al. 236 for those studies that reported results for the eight dimensions of the SF-36 questionnaire and the mapping algorithm published by Maund et al. 238 for those studies that reported the SF-36 PCS and MCS scores. Rowen et al. 236 mapping coefficients were obtained from a generalised least-squares regression of individual patient-level EQ-5D scores against the values of the eight dimensions of the SF-36, their squares and their interactions, as this was identified as the preferred model by the authors. Rowen et al. 236 analysed data from a wide range of inpatients and outpatients at Cardiff and Vale NHS Hospitals Trust. Maund et al. 238 mapping coefficients were obtained from an OLS regression of individual patient-level EQ-5D scores against the values of the SF-36 PCS and MCS scores, using data from patients with rotator cuff disease in primary care recruited in the SAPPHIRE (Stenting and Angioplasty With Protection In Patients at High Risk for Endarterectomy) trial. 281 The authors estimated five models: three OLS regressions (one with main effects for PCS and MCS scores only, another adding squared terms and a third adding both squared and interaction terms), one Tobit regression and one censored least absolute deviations (CLAD) model (both the Tobit and CLAD models included main effects, squared terms and interaction terms). All models performed similarly (with mean absolute errors of 0.18–0.19). Given this, for simplicity OLS models were preferred and the model including the main effects, their squares and their interaction was used on the basis of marginal improvements in explanatory power and model fit. The analysis of 1-, 3- and 12-month data was used as these analyses had slightly improved explanatory power and model fit compared with the 3-month analysis.
SF-12 to EQ-5D
Those studies that used the SF-12 questionnaire reported its results in terms of its PCS and MCS scores. The model used the mapping algorithm published by Gray et al. ,237 which was obtained from a multinomial logistic regression model of individual patient-level EQ-5D scores against the values of the two summary scores of the SF-12, their squares and interaction terms. This model is based on an analysis of data from the Medical Expenditure Panel Survey [see https://meps.ahrq.gov/mepsweb/ (accessed 13 October 2016)], which reflects the HRQoL outcomes of non-institutionalised US civilians.
WOMAC to EQ-5D
Barton et al. 239 developed a series of algorithms to map the WOMAC instrument on to EQ-5D scores. Using IPD from patients who participated in the Lifestyle Interventions for Knee Pain study,282 the authors conducted a series of OLS regressions, relating the EQ-5D index value to various possible ways in which the WOMAC questionnaire may be reported. Five models were estimated in total: the first included total WOMAC score as the only explanatory variable; the second used the WOMAC pain, stiffness and functioning subscales; the third used the total WOMAC score and total WOMAC score squared; the fourth included pain, stiffness and functioning, their interactions and their squares; and the final model was the best fitting of the previous four plus age and sex. Access to the five different models proved useful as the WOMAC had been reported in different ways in the literature forming the evidence base. The model preferred by Barton et al. 239 included total WOMAC score, total WOMAC score squared, age, age squared and sex. This model was therefore applied to the IPD. Because of variable reporting of age and sex across studies, for aggregate data studies the model including only total WOMAC score and total WOMAC score squared was used.
Pain to EQ-5D
A final set of studies included in the analysis reported pain, measured using either a NRS or a VAS. The report by Maund et al. 238 that provided the mapping algorithm for the SF-36 MCS and PCS scores also provided an algorithm to map pain VAS to EQ-5D scores. The authors estimated four models: two OLS regressions (one with the pain VAS and another with the pain VAS and its squared term), one Tobit regression and one CLAD model, using the pain VAS and its squared term as explanatory variables. All models performed similarly (with mean absolute errors of 0.18–0.20). Given this, for simplicity OLS models were preferred and the model including the main effects and their squares was used as there were no observed differences in model fit or explanatory power between the model with and without the squared term, and it seemed plausible that the relationship between pain VAS and EQ-5D scores was non-linear. The analysis of 1-, 3- and 12-month data was used as these analyses had slightly improved explanatory power and model fit compared with the 3-month analysis.
A mapping algorithm from the 11-point Pain Intensity Numerical Rating Scale (PI-NRS-11), ranging from 0 (‘no pain’) to 10 (‘pain as bad as you can imagine’), to the EQ-5D was available from Gu et al. 283 To estimate two mapping algorithms, the authors used survey data from a US sample of patients who had at least 3 months of neuropathic pain, either painful diabetic peripheral neuropathy or post-herpetic neuralgia, and were receiving medications treating neuropathic pain. The first related the EQ-5D index score to a set of pain NRS dummy variables using OLS and the second used an ordered logistic regression model to predict the response levels (i.e. 1, 2, 3) for each of the EQ-5D dimensions using the same explanatory variables. Models were run with and without patient age, sex and pain duration as independent variables. The reduced models, which excluded age, sex and disease duration, were used given variable reporting of these variables across studies. The OLS model was used in the current analysis as it had a better fit than the ordered logistic model.
Synthesis of individual-level and aggregate reporting of continuous outcomes
The second challenge that the analysis reported here had to address is common to the synthesis of all continuous end points when only summary statistics are available. ANCOVA is the preferred method for analysing continuous outcome data from RCTs. 219 In an ANCOVA analysis the post-baseline outcome of interest is regressed on both a treatment indicator and the baseline value of the outcome of interest. ANCOVA is preferred as it offers improved precision and lower bias than other available methods when there is imbalance in baseline outcomes. However, ANCOVA analyses are rarely reported in clinical publications, particularly for secondary end points such as HRQoL. Meta-analyses therefore typically analyse either post-baseline (or ‘final’) values or change from baseline values. An analysis of final scores would be expected to be biased in favour of the treatment with a higher baseline value. In the current data set, patients with higher EQ-5D values experienced lower changes in values; a change score analysis would therefore be expected to bias against the treatment with higher baseline values. Existing proposals to handle this have significant limitations. Fu et al. 284 recommend sensitivity analyses using final scores and change scores. It is not clear how the results of this analysis would be interpreted, as there is no guarantee that, at the (network) meta-analysis level, the true treatment effects will be bounded by the results of these scenarios. Riley et al. 219 propose dropping studies with significant imbalance; however, this results in a loss of information and requires a demarcation of what constitutes ‘significant imbalance’. Methods were therefore developed to address this concern, given the significant imbalances observed in this data set.
Differences in study designs, study populations and the implementation of individual interventions seemed likely to generate heterogeneity in the underlying true effects. We therefore developed random-effects models as in Chapter 3. This model extends the IPD model presented in Chapter 4 to the more commonly faced situation in which IPD are available for only a subset of studies, with only aggregate data reporting available for the remainder.
The outcome variable, absolute EQ-5D score at follow-up, was assumed to be normally distributed. Although this assumption is unlikely to characterise EQ-5D scores,233 simulation results have shown treatment effect estimates from ANCOVA to be very close to the true treatment effects for a range of non-normal outcome distributions. 285
Individual patient data were included in the model using an ANCOVA network meta-analysis model:
where Yijkt and Yijk0 are the values of the (continuous) outcome at time point t and baseline (t = 0), respectively, for participant i in treatment arm k of study j; σj2 represents the study-level variance; the quantity αbj represents the outcome for the baseline treatment k = b in study j for a patient with an EQ-5D score Yijk0 of zero at time 0 (this is study specific to respect within-study randomisation); the parameter βj0 represents a study-specific estimate of the impact of baseline EQ-5D score on the final outcome; and δkbj represents the treatment effect for treatment k relative to treatment b in trial j.
The aggregate data are included in a final outcomes model that has been modified to adjust for the potential bias caused by differences in outcomes between treatments at baseline (Ykbj0 = Ykb0 – Ybj0):
where Ykjt and σkj2 are the mean and variance for the final outcome for treatment arm k of study j at time t, respectively.
This model adjusts the treatment effects to emulate the ANCOVA model results. This is achieved by considering the reported unadjusted results as being subject to omitted variable bias, where the omitted variable is baseline EQ-5D score. Omitted variable bias can be estimated as the product of the coefficient on the baseline EQ-5D score from the ‘correctly’ specified model (the ANCOVA model) multiplied by the coefficient from a regression of the omitted variable (baseline EQ-5D score) on the included variable (treatment). 286 The coefficient on the baseline EQ-5D score from the ANCOVA model (βj0) is obviously unknown for the aggregate data studies. This coefficient is assumed to be exchangeable with the coefficients obtained from the studies for which IPD were available and can therefore be estimated as:
β0 and σβ0 are estimated by assuming that the coefficient on the baseline outcome measure for each IPD trial (βj0) is drawn from a normal distribution with mean β0 and variance σβ0.
The covariate from the regression of baseline EQ-5D score on treatment is simply the difference in baseline EQ-5D score between the treatment and the control for the comparison of interest (i.e. Ykbj0).
The estimated study-specific treatment effects (δkbj) from the IPD and aggregate data inform a common random-effects consistency model:
where dkb represents the treatment effects for the comparison of treatment k with treatment b; these are assumed to be derived from a set of basic parameters (dk1), which estimate the treatment effect of treatment k relative to the reference treatment (k = 1), where d11 = 0.
Priors were defined to be vague and were specified as follows: αbj ∼ N(0,104); dk1 ∼ N(0,103); σk ∼ Unif(0,2); β0 ∼ N(0,106); σβ0∼Unif(0,2);σ∼Unif(0,2).
If outcome data were missing for patients within the IPD, these cases were dropped from the analysis (this occurred in 21% of all cases). If baseline data were missing for the aggregate data studies, the difference at baseline (Ykbj0) was assumed to be zero.
Correlation in the random effects from trials with three or more arms were accounted for using previously published methods. 222 When SEs were not reported and could not be derived from the reported data, they were imputed using the methods described by Dakin et al. ,234 which allow for the uncertainty in the imputation process.
Model fit was assessed using the posterior residual deviance and the DIC. In the IPD each patient (data point) should contribute approximately 1 to the residual deviance, whereas for the aggregate data each study arm (data point) should contribute approximately 1 to the residual deviance. Residual deviance contributions from each patient in each IPD study arm were therefore summed and divided by the total number of patients in the study arm to allow an aggregate measure of mean posterior residual deviance to be estimated and to allow deviance contributions to be compared across studies.
Inconsistency was assessed using the methods set out in Dias et al. ,287 whereby the fit of the consistency model is compared with the fit of an inconsistency model. As recommended in Dias et al. ,287 changes in the model fit associated with using the inconsistency model were assessed using ‘omnibus’ diagnostics (DIC; posterior mean residual deviance). Residual deviance contributions for each data point were also reviewed for both consistency and inconsistency models. 180
Convergence was assessed by inspecting trace plots, density plots and Brooks–Gelman–Rubin plots as well as autocorrelation plots. 177 Efficiency was assessed by comparing the Monte Carlo error to the posterior SD. All models were run for 100,000 ‘burn-in’ iterations and a further 100,000 samples.
Data manipulation and analysis was carried out using the software packages R version 3.0.2 and WinBUGS246 version 1.4.3. The network meta-analyses were undertaken in WinBUGS, linked to the R software through the package R2WinBUGS. 247,288 The statistical software Stata 12.0 was used to analyse the relationship between changes in resource use and changes in EQ-5D score. The annotated WinBUGS code is provided as Appendix 5.
Study heterogeneity
Three sources of heterogeneity were anticipated to be potential treatment effect modifiers based on a review of the trial data and clinical opinion: bias associated with trial quality, variable reporting times across trials and differences in patients across trials.
Study quality has been found to be a study-level treatment effect modifier in the context of osteoarthritis trials. 289 A meta-epidemiological study by Nüesch et al. 289 of 16 meta-analyses comparing active with control interventions (or placebo) in patients with hip or knee osteoarthritis found effect sizes to be less beneficial in trials with adequate allocation concealment than in those with inadequate or unclear allocation concealment according to the Cochrane risk-of-bias tool. 171 The Cochrane risk-of-bias tool assesses allocation concealment based on whether or not the method used to conceal the allocation sequence is sufficient to avoid intervention allocations being foreseen before or during enrolment. The risk-of-bias score (low, unclear or high) is assigned based on the risk of selection bias because of inadequate concealment of allocations prior to assignment.
The difference in effect size was most pronounced when the trial data set was restricted to trials of complementary medicine interventions, which included acupuncture, chondroitin, glucosamine, PEMFs and static magnets. The impact of patient blinding was also explored; however, observed differences in treatment effects were less consistent and disappeared after accounting for allocation concealment. We therefore ran analyses restricting the study set to those with adequate allocation concealment according to the Cochrane risk-of-bias tool. 171
It was expected that trials reporting at very short or very long time points may have both different characteristics (e.g. very short trials may be of a more experimental nature) and reflect different points on a patients’ HRQoL change trajectory. An additional analysis was therefore conducted further restricting the analysis of trials with adequate allocation concealment to only those trials reporting within the 3- to 13-week period (note that originally an analysis restricted to trials reporting in the 4- to 12-week range was planned; however, this resulted in the network becoming disconnected).
Increased age and BMI were potentially expected to reduce the efficacy of the interventions considered in this synthesis. Given the low number of studies available for many of the contrasts we used the common interaction effect model outlined in the study by Dias et al. 245 A common interaction term βxk1 was assumed to apply to all treatment effects relative to treatment 1 (usual care). This required modifications to Equations 3 and 4 as shown in Equations 7 and 8, where Xikj represents the centred age (BMI) of patient i in treatment arm k of study j in the IPD studies, and Xj represents the centred mean age (BMI) of study j in the aggregate data studies. The coefficient βxj represents the study-specific impact of centred age (BMI) on the outcome measure. The logical operator I(u) takes the value 1 if the condition u is met and 0 otherwise.
The following additional priors were required for the covariate modelling: βxj ∼ N(0,104); βxk1 ∼ N(0,104). Missing covariate values were imputed assuming an ignorable missing covariate mechanism using the methods described in Lunn. 290 Missing data for BMI and age were assumed to be drawn from independent normal distributions, and separate models were assumed for missing IPD and aggregate data covariates.
Methods for estimating the total cost
The total cost included the costs of providing the interventions, primary care visits and outpatient hospital visits. The cost year was 2012–13.
Intervention costs
The main purpose of the cost-effectiveness analysis was to estimate the costs and QALYs associated with each treatment and identify which strategy provided ‘value for money’ in the NHS for England and Wales. Unfortunately, most of the studies in the data set either did not collect health-care resource use data to inform a costing exercise relevant to the NHS for England and Wales or did not collect resource use data at all. Health-care resource use information is typically considered not transferable from country to country as it is a function of country-specific health-care system factors (e.g. the incentive structure faced by providers and patients, relative prices of available health-care resource use, the treatment strategies available in the country), which are difficult to control for even when the analyst has access to IPD.
To estimate the resource use associated with delivering each intervention, clinical experts were consulted, as well as the published literature (including the published trial data) and UK NHS data, such as NHS foundation trust information available on the internet. The resulting intervention resource use and cost data are summarised in Table 28.
| Interventions | Resource use | Trials (weekly attendance in minutes), mean (range) | ||
|---|---|---|---|---|
| All | With adequate allocation concealmenta | |||
| Acupuncture | Administered weekly by a physiotherapist over 8 weeks | 37 (18–80) | 40 (20–50) | |
| Biomedical appliances | Braces | Initial 40-minute visit to a physiotherapist to prescribe brace and a subsequent 30-minute visit to fit brace. Assume brace lasts for 6 months | N/A | N/A |
| Insoles | Initial 40-minute visit to a podiatrist to prescribe insole and a 30-minute follow-up visit. Assume use of a ready-made insole, which lasts for 1 year | N/A | N/A | |
| Electrotherapy | Interferential therapy | Administered weekly by a physiotherapist over 8 weeks | 159 (40–245) | 245 (N/Ab) |
| Laser/light therapy | 105 (25–210) | 60 (N/Ab) | ||
| NMES | 100 (N/Ab) | N/Ac | ||
| PES | 82 (57–114) | 85 (57–114) | ||
| PEMFs | 303 (80–600) | 120 (N/Ab) | ||
| TENS | Initial 40-minute visit to a physiotherapist to provide intervention, which is subsequently self-administered. Assume TENS machine lasts for 1 year | N/A | N/A | |
| Thermotherapy | Heat treatment | Short-wave diathermy administered weekly by a physiotherapist over 8 weeks. In one trial, heat treatment consisted of a heat-retaining knee sleeve. This is assumed to be prescribed by a physiotherapist (40-minute consultation) and then used as required for 6 months. Total cost for heat treatment is a weighted average of diathermy and heat-retaining sleeve costs, with weights of 73%/27% in line with the number of patients enrolled in the heat treatment trial arms | 84 (60–143) | 60 (N/Ab) |
| Manual therapy | Administered weekly by a physiotherapist over 8 weeks | 63 (30–90) | 57 (30–90) | |
| Static magnets | Initial 40-minute visit to a physiotherapist to prescribe static magnet. Assume static magnets last for 2 years and 50% of patients require one replacement wrist strap | N/A | N/A | |
For interventions assumed to be administered via regular physiotherapy attendance (acupuncture, electrotherapy excluding TENS, thermotherapy and manual therapy), weekly attendance durations were estimated from the clinical trial data. Acupuncture is administered in the UK by a range of practitioners. Recent survey data suggest that, of acupuncture administered within the NHS, 59% is administered by physiotherapists with the remainder administered by medical doctors (29%), nurses (4%), other health-care professionals (5%) or independent practitioners (4%). 1 For the purpose of estimating costs, we therefore assume that acupuncture is carried out by a physiotherapist. Unless a marked difference in outcomes was expected between delivery by a physiotherapist and delivery by a medical doctor, it seems unlikely that provision by medical doctors would be cost-effective given the large difference in hourly costs. 249
Weekly attendance was estimated for each trial as the total number of sessions multiplied by the number of minutes per session divided by the number of weeks of treatment. A weighted average for weekly attendance was then estimated from the trials included in the network meta-analysis. This was carried out separately for the analysis of all trials and the analysis restricted to trials with adequate allocation concealment to ensure that in each cost-effectiveness analysis scenario the resource use was aligned with the efficacy data. Equipment administered by physiotherapists was not included in the costing, as the associated per-patient costs were expected to be small given the high throughput of individuals experiencing osteoarthritis of the knee and chronic pain of other aetiologies.
Insoles, braces and static magnets would be typically used for longer durations, beyond the 8-week cost-effectiveness model time horizon. Therefore, it was assumed that for these therapies, the (upfront) costs could be spread across their useable lifetimes and that the effects observed within the network meta-analysis would be maintained throughout their useable lifetimes. For TENS we assumed that the patient used the TENS machine at home for 8 weeks and then returned it to the physiotherapy unit for use by other patients.
Unit costs were obtained from published national sources when possible and are reported in Table 29. The financial year 2012–13 was used to value resource use relevant to each intervention. Costs reported for previous financial years were inflated using the Hospital and Community Health Services prices index. 249 Total costs were calculated by multiplying resource use by the unit cost. Other treatments and health-care interactions that may form a package of ‘usual care’ were assumed to have been provided equally to all patients regardless of comparator. These costs were therefore omitted from the analysis.
| Health-care professional/appliance | Unit costa (£) | Unit | Source |
|---|---|---|---|
| Hospital physiotherapistb | 36 | Per hour | Schema 13.1291 |
| Community podiatrist | 30 | Per hour | Schema 9.4291 |
| GP visit | 45 | Per 11.7-minute consultation | Schema 10.8b291 |
| Secondary care specialist visit | 135 | Per consultation | Schema 7.1291 |
| Brace | 88 | Brace | Bauerfeind GenuTrain Knee Support [www.healthandcare.co.uk (accessed 9 May 2014)] |
| Insole | 50 | Insole | Ready-made lateral wedge foot insole [www.rcht.nhs.uk/DocumentsLibrary/PeninsulaCommunityHealth/OperationsAndServices/Podiatry/PodiatryDischargeLetter.pdf (accessed 9 June 2014)] |
| Heat-retaining sleeve | 11 | Sleeve | www.amazon.co.uk/TITANIUM-ADJUSTABLE-KNEE-HEATING-STRAP/dp/B007EBTXDS/ref=sr_1_3?ie=UTF8&qid=1401871047&sr=8–3&keywords=heat+knee (accessed 9 June 2014) |
| Static magnets | 35 | Per static magnet | www.magnetsforall.com/category/40397320 (accessed 8 July 2014) |
| 15 | Per additional strap | ||
| TENS | 35 | Per TENS machine | www.boots.com/en/Boots-TENS-Digital-Pain-Relief-Unit_1405593/ (accessed 22 May 2014) |
Other health-care costs
Non-treatment-specific health-care resource utilisation was assumed to be a function of any change in EQ-5D over the 8 weeks of the treatment period and was derived from the TOIB trial. 231,292 Briefly, the TOIB trial collected EQ-5D and health-care resource utilisation data at baseline and follow-up; having gained access to the TOIB trial IPD, it provided an opportunity to estimate a series of simple models that regressed changes in health-care resource utilisation (i.e. GP visits, nurse practitioner visits, outpatient hospital specialist visits) against changes in EQ-5D score. A simple OLS analysis was used to regress the change in resource use from 0–3 months to 3–12 months on the change in EQ-5D score between month 3 and month 12. Primary and secondary care visits were analysed separately. The estimated regression coefficients were then used (in combination with the unit costs from Table 29) to derive changes in cost components and, following aggregation, change in total costs as a function of changes in EQ-5D score over the 8-week time period of the base-case analysis. The regression coefficients estimated that a 1-unit increase in the EQ-5D resulted in a 0.89 (SE 0.53) decrease in primary care visits and a 0.47 (SE 0.56) increase in specialist visits.
Cost-effectiveness analysis
Linking predicted changes in EQ-5D score from the network meta-analysis for each of the competing strategies, and changes in total costs (treatment and non-treatment related) based on the analysis described in the previous section, facilitated the value for money assessment of competing adjunct non-pharmacological interventions for pain of the knee in individuals with osteoarthritis in the NHS. Direct HRQoL benefits were the focus of the evaluation as the interventions under appraisal are expected to impact on pain and functioning but not on disease progression. QALYs were therefore estimated from the EQ-5D estimates using an area-under-the-curve (AUC) approach.
The time horizon for the analysis was 8 weeks, in line with the preferred time point for the synthesis. There is limited evidence regarding whether or not the effects of the therapies appraised continue beyond the treatment period. For example, an earlier report by Corbett et al. 168 found that only 23% of studies reporting data suitable for synthesis reported data 8–16 weeks from the end of treatment. The possibility that interventions may offer longer-term benefits was explored in scenario analyses. Some studies did, however, report results for multiple on-treatment time points. This could have provided information on interim on-treatment outcomes. However, it was not expected that there would have been sufficient data to provide robust treatment-specific estimates of the HRQoL profile from baseline to 8 weeks.
Quality-adjusted life-years were therefore estimated assuming that the benefit of treatment was achieved instantaneously, was maintained for 8 weeks and was then lost instantaneously. This is equivalent to assuming that the full benefit was gradually achieved over a specified period and then lost linearly over the same period, which may be viewed as a more realistic scenario for some interventions. For example, the benefit could be linearly achieved from the start of treatment until 8 weeks and gradually lost over the 8 weeks following treatment completion. The model is therefore expected to reasonably approximate a range of alternative HRQoL profiles (Figure 20). For example, expert opinion suggested that electrotherapy, static magnets and heat treatment may be more likely to follow an instant gain/instant loss-type profile, whereas acupuncture, manual therapy and the appliances may be associated with a gradual gain/gradual loss-type profile. These issues are explored further in the sensitivity analyses.
FIGURE 20.
Health-related quality-of-life profiles compatible with base-case assumptions.
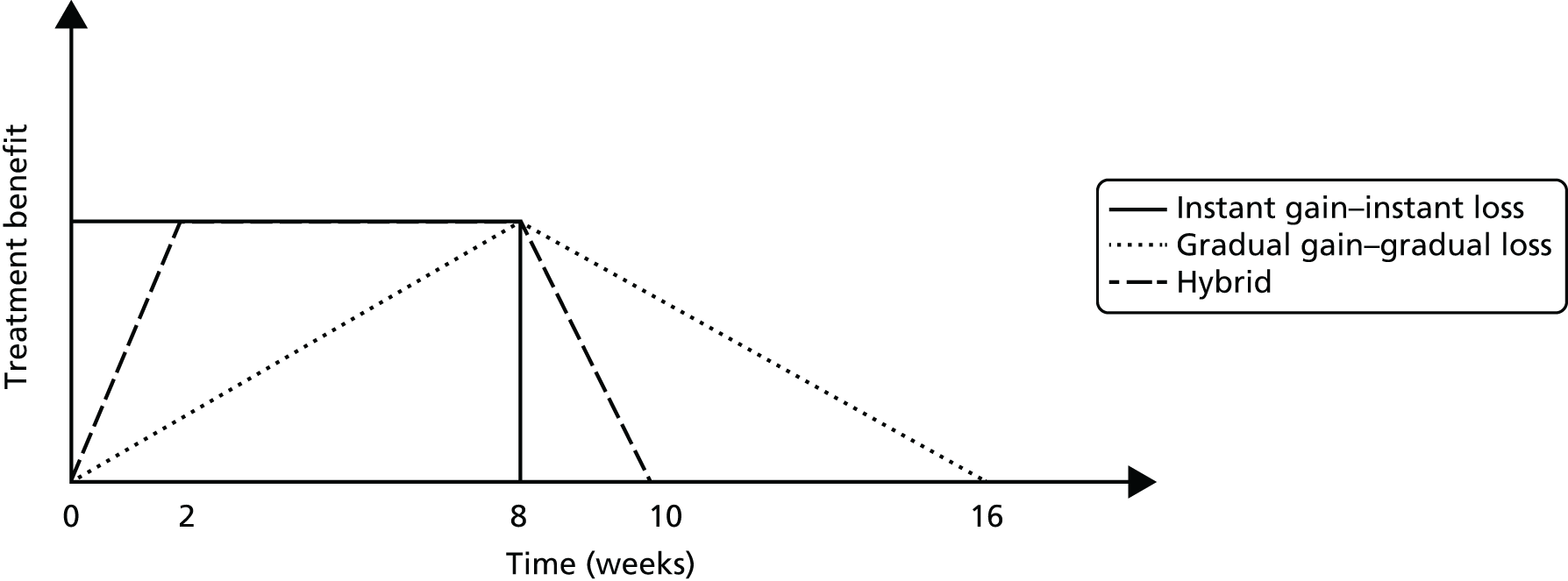
Placebo and sham acupuncture were not included as comparators in the cost-effectiveness analysis as it is not expected that the NHS would be willing to prescribe these as treatments. The analysis therefore controls for regression to the mean effects (as all improvements are relative to usual care) but not patient expectation effects. 293 The underlying assumption made here is therefore that the patient expectation effects observed in the trials would also be observed in clinical practice.
The model was run probabilistically by assigning the posterior distribution from the network meta-analysis to the effect sizes and reflecting the uncertainty in the relationship between the EQ-5D and primary care/outpatient resource use using a normal distribution (parameterised using data described in Other health-care costs).
After obtaining changes in expected costs and QALYs for each strategy, these were ranked by mean change in costs, starting from the least costly. ICERs were then calculated by dividing incremental (change in) costs by incremental (change in) QALYs. Treatment strategies that were dominated and those subject to extended dominance were subsequently excluded and ICERs recalculated if necessary. A treatment is dominated if it generates worse health outcomes and has equal or higher costs when compared with an alternative treatment. Extended dominance occurs when a treatment is less effective and has a higher ICER than an alternative treatment.
Derived posterior distributions for the incremental costs and incremental QALYs were used to calculate the probability that each strategy is cost-effective at the conventional cost-effectiveness threshold levels used by NICE (i.e. between £20,000 and £30,000 per QALY gained224). Given the large number of treatment strategies, it was considered more appropriate to plot the study results as a cost-effectiveness acceptability frontier,294 which provides a direct indication of both the optimal strategy and the probability of that strategy being cost-effective for a given threshold value. Given that the time horizon was < 1 year, costs and QALYs were not discounted.
The cost-effectiveness model was analysed using outputs of the synthesis of all trials; of trials that had a low risk of bias for adequate allocation concealment according to the Cochrane risk-of-bias tool;171 and of trials with a low risk of bias for allocation concealment and reporting end-point data between weeks 3 and 13 post baseline.
For each scenario a series of sensitivity analyses were run. For treatments delivered via regular physiotherapy attendance, sensitivity analyses were run assuming that each session lasted as long as the shortest and the longest duration observed in the trial data for each intervention (as shown in Table 28). In the case of therapies for which the cost was driven by both labour and equipment costs (braces, insoles, static magnets and TENS), the costs were varied by ±50%. We also explored the impact of assuming that acupuncture was delivered by a private practitioner at the higher cost of £47.50 for the first session and £37.50 for subsequent sessions (for data sources see Chapter 6, Methods for substudy 4: cost-effectiveness analysis).
The intensity of therapy (hours per week) varied considerably across trials, with many therapies being administered much more intensively than would typically be expected in the context of a UK musculoskeletal outpatient physiotherapy service. Given the small number of studies for many of the interventions it was not possible to explore the impact of treatment intensity on treatment efficacy within the synthesis. We therefore conducted sensitivity analyses using appointment times more typical in the NHS for the purposes of costing [based on clinical opinion: 40-minute consultation followed by 20-minute treatment sessions (or 30 minutes for acupuncture and manual therapy)] and exploring different assumptions with respect to how the efficacy of treatment might vary when the session duration was reduced. Four assumptions with respect to the dose–response relationship of session duration and EQ-5D outcome were explored: (1) outcomes increase linearly to achieve a maximum benefit with the mean session duration, as listed in Table 28; (2) outcomes increase linearly up to the maximum value for 1 hour of session time per week, beyond which no further benefit is observed; (3) 75% of outcomes are achieved using 30-minute sessions with the remaining 25% of benefit achieved by moving from 30- to 60-minute sessions; and (4) the full benefit of treatment observed is obtained with 20-minute sessions (or 30 minutes for acupuncture), that is, no adjustment to outcomes. Moving from scenario (1) through to scenario (4), the benefits of increased session duration are assumed to be reduced. Scenarios (1) and (4) probably represent quite extreme scenarios. As an example, Figure 21 shows the method for estimating outcomes under each scenario assuming that the weighted average session duration for the treatment is 80 minutes, which we wish to extrapolate to a 20-minute session duration.
FIGURE 21.
Methods for treatment appointment duration sensitivity analysis.
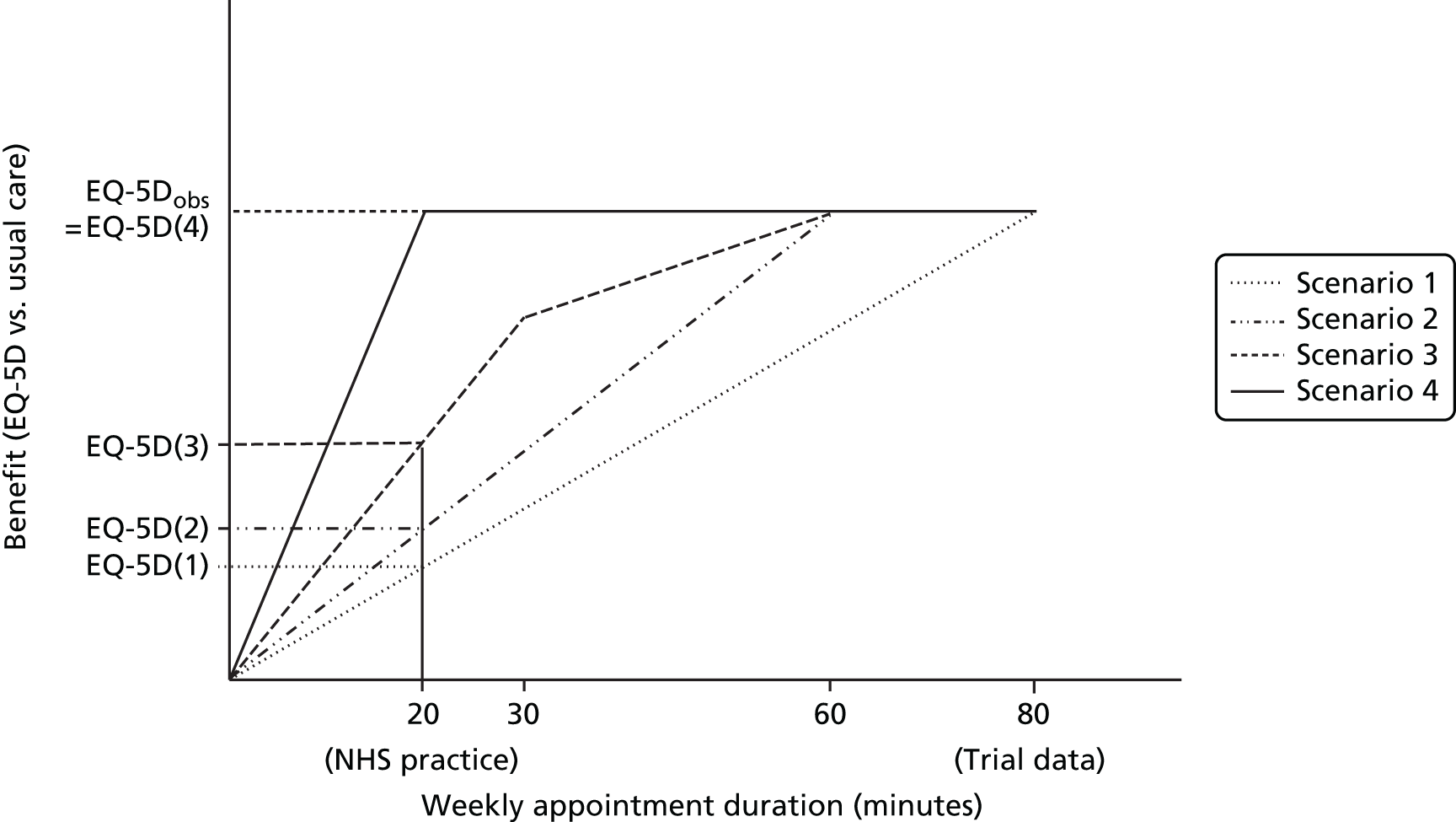
Sensitivity analyses using the upper and lower values of the 95% CrIs from the network meta-analysis were also conducted. The threshold value treatment effect at which the decision would change, as well as the probability of observing a value at least this extreme, were calculated. These estimates provide an indication of the likelihood of observing an outcome sufficiently extreme for the conclusions of the analysis to alter. However, they are univariate analyses and do not account for other sources of uncertainty or the correlation between treatment effects estimated from the network meta-analysis.
In the base case the benefit of treatment is assumed to be instantaneous at time zero and to disappear immediately at the end of week 8. Two sensitivity analyses were conducted: the first was a threshold analysis estimating the duration of benefit beyond the 8-week period of treatment that would be required to alter the decision, assuming that all interventions delivered benefit over this period; the second extended the benefit of each intervention individually by 50% (equivalent to 4 weeks at full benefit or 8 weeks linear decline).
Given the large number of scenarios and comparators, the results are summarised by stating the intervention that is cost-effective at a cost-effectiveness threshold of £20,000 per QALY under each scenario.
The expected value of perfect information (EVPI) was also calculated for the decision problem. The EVPI provides the upper bound on the value of reducing uncertainties in the decision problem. The EVPI associated with each instance of a decision was calculated using established methods. 202 It is important that the EVPI is calculated for the total population eligible to benefit from additional information. This requires an assessment of the period over which information will be useful and the estimated incidence of the decision during this period.
Studies of new GP referrals for knee pain and of incident radiographic osteoarthritis suggest that among older patients (typically aged > 45 years or > 50 years) incidence is 2–4% per year. 158,295 The most recent data suggest that the incidence of consultations for knee pain in those aged > 50 years is 4% per year. 295 We estimate that 56% of consultations for knee pain are given a diagnosis of osteoarthritis. 296 Applying these data to UK population data on the number of people aged ≥ 50 years in the UK (22.773 million297) yields an estimate of 490,721 new consultations per year. Some data are available in the literature regarding the proportion of individuals with osteoarthritis of the knee (or with knee pain) who receive non-pharmacological treatments. Estimates of the proportion of patients receiving physiotherapy range from 13% to 41%. 296,298 One study reported the use of complementary therapy (21%)296 and another reported the use of TENS (13%), acupuncture (8%), wedged insoles (6%) and appliances (5%). 298 Based on this information we estimate that approximately 50% of patients will be considered for adjunct non-pharmacological interventions. We assume that patients are considered for non-pharmacological interventions only once during their entire treatment for osteoarthritis of the knee; this may therefore underestimate the number of instances in which the decision of interest is faced.
We used the value of information analysis to compare three possible policy options that are available for cost-effective interventions:299
-
adopt the cost-effective intervention (adopt)
-
adopt the cost-effective intervention and commission research or ‘adopt with research’ (AWR)
-
delay adoption until new research is available or ‘only in research’ (OIR).
Given the nature of the uncertainties in the model we anticipate that any further research would take the form of a clinical study, which would take approximately 5 years to be planned, conducted and reported. We also assume that current practice is usual care. In reality there is likely to be some use of the appraised interventions in current practice. This assumption will not impact on the difference between the adopt and the AWR policies but will increase (reduce) the benefit of OIR relative to these policies if the net health benefit of current usage is positive (negative) relative to usual care.
We therefore consider the net health benefits associated with adoption (the population net health benefits generated by the cost-effective technology, which we assume would be used for 10 years by which point new interventions may be available and the management pathway for osteoarthritis of the knee may have changed dramatically), the health benefits associated with AWR (the health benefits generated by adoption for 5 years followed by a revised decision based on perfect information from year 6 to year 10) and the health benefits associated with OIR (the health benefits generated by no adoption from year 1 to year 5 and a decision based on perfect information from year 5 to year 10). The AWR and OIR estimates should be considered upper bounds as they are based on an assumption that research will resolve all uncertainties, which will not be the case for real research designs that reduce but do not remove uncertainty.
Adopt with research will always offer the highest health benefits if net health benefits are constant over time. There are two reasons why AWR may not, however, be the preferred policy choice. First, it may not be feasible, for instance if adoption removes incentives for health-care professionals to conduct research or patients to participate in research. Second, if there are high upfront costs OIR may offer higher health benefits as under this policy such upfront (or ‘sunk’) costs are incurred only if the intervention turns out to be cost-effective.
The main source of sunk costs is likely to be the cost of training physiotherapists to deliver acupuncture. Our estimates suggest that 245,360 patients would be eligible for treatment each year. Assuming that these patients receive eight sessions of treatment and that physiotherapists who deliver acupuncture typically deliver 208 sessions per year,1 then 9437 physiotherapists would require training. We assume that training consists of an 80-hour course (40 hours taught) costing £495. 300 The opportunity cost of this course is unclear as many accredited courses run during weekends and require out-of-hours study. We therefore conservatively assume an opportunity cost of 40 hours of time (at £36 per hour) or £1440. The total cost of training would therefore be £18.3M, which we assume would be incurred in the first year of adoption. Spreading this across all treated patients over 10 years (or more if training provides benefits over a career) would have a minimal impact on the cost-effectiveness results. However, if this cost is incurred in the first year, this may increase the benefit of OIR over adoption or AWR, and is therefore included in our comparison of the three policies.
Results
Mapping and synthesis
Appendix 5 presents the results of the mapping from individual HRQoL measures to the EQ-5D. Figure 22 displays the results of the network meta-analysis of (1) all trials, (2) trials that were assessed to be at low risk of bias with respect to the adequacy of allocation concealment and (3) trials that were assessed to be at low risk of bias with respect to the adequacy of allocation concealment and which reported in the restricted 3- to 13-week range.
FIGURE 22.
Results of the network meta-analysis with 95% CrIs (results presented as values corresponding to 2.5%, 50% and 97.5% of the posterior distribution): (a) all trials; (b) trials with adequate allocation concealment; and (c) trials with adequate allocation concealment and reporting in the range 3–13 weeks.

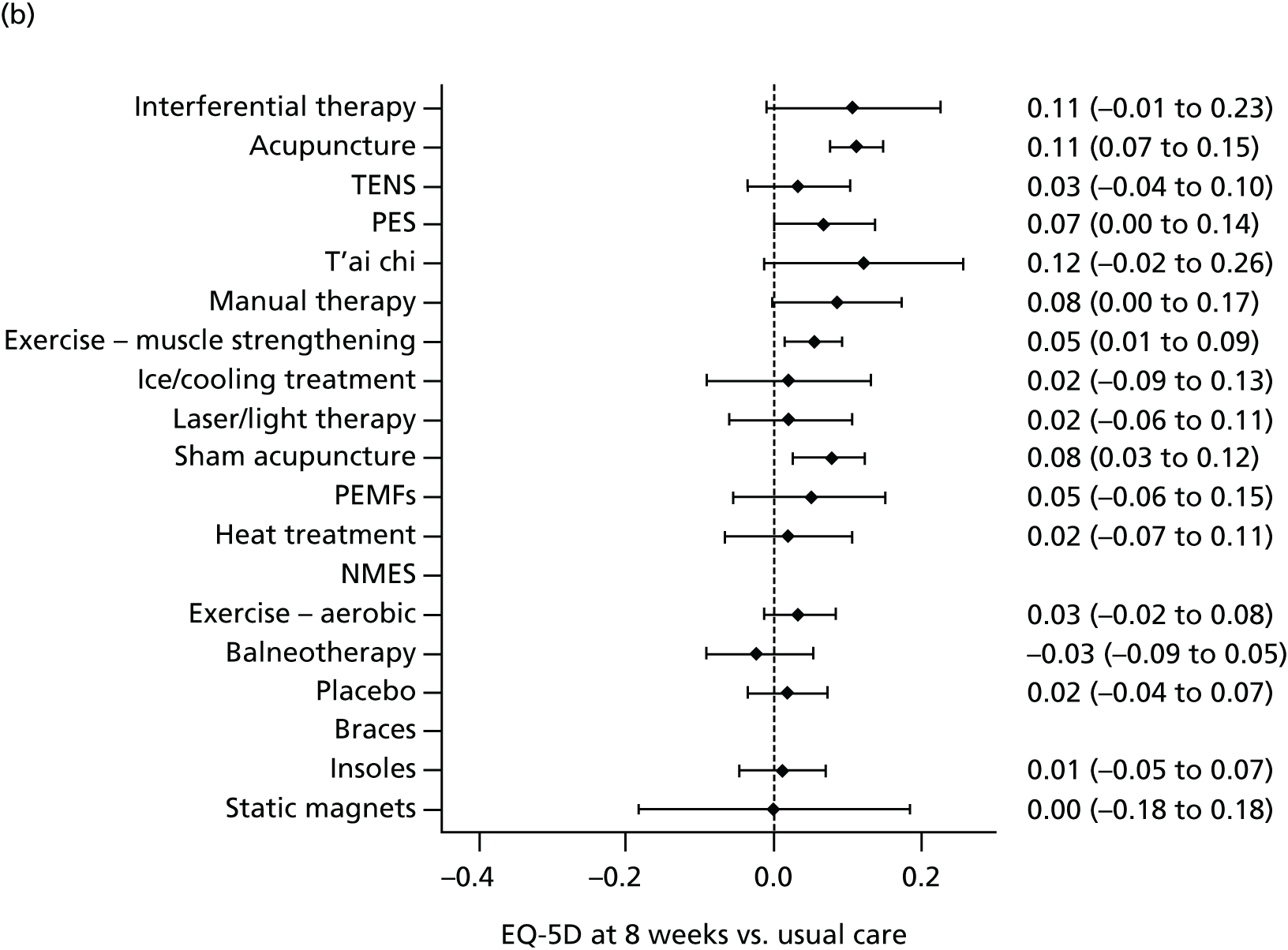

In the all-trials analysis, interferential therapy followed by acupuncture, TENS, PES and t’ai chi offered the largest benefit in terms of the EQ-5D, based on point estimates of the effect size alone. However, the CrI crossed zero for t’ai chi. The least uncertainty, as reflected by the narrowest CrIs, was associated with acupuncture and muscle-strengthening exercises. In the analysis of trials with a low risk of bias for allocation concealment, t’ai chi followed by acupuncture, interferential therapy, manual therapy and sham acupuncture offered the largest benefits, again based on point estimates of effect. However, the CrIs touched or crossed zero for t’ai chi, interferential therapy and manual therapy. When this analysis was restricted to trials reporting between week 3 and week 13, t’ai chi followed by manual therapy, acupuncture, interferential therapy and sham acupuncture offered the largest benefit. In this case the CrIs touched or crossed zero for t’ai chi, manual therapy and interferential therapy. The magnitude of the effect sizes differed between analyses. The effects of interferential therapy and TENS diminished when the analysis was restricted to trials with a low risk of bias with respect to allocation concealment. The effects of t’ai chi and sham acupuncture were stronger in the analyses restricted to trials with a low risk of bias with respect to allocation concealment, as was the effect of manual therapy, particularly when the analysis was restricted to trials reporting in between 3 and 13 weeks.
The results are associated with a high degree of uncertainty for most but not all of the interventions as indicated by the wide CrIs around many of the treatment effect estimates. Muscle-strengthening exercise and acupuncture are associated with the least uncertainty, as they have the narrowest CrIs across analyses, and static magnets and NMES are associated with the most uncertainty. The treatment effects associated with TENS and interferential therapy are considerably more uncertain when the evidence is restricted to trials with adequate allocation concealment reporting in between 3 and 13 weeks.
Forest plots are unable to represent the full set of information regarding treatment effect uncertainty as they do not reflect the correlation in treatment effects. Figure 23 therefore presents a ‘rankogram’181 showing the probability that each treatment ranks as the first, second, third etc. best of the treatments considered in each analysis. The majority of the rankograms are fairly flat, indicating high uncertainty with respect to where the treatment would rank. Exceptions to this are acupuncture, for which there is a peak at ranks 2–3 (depending on analysis) with the majority of the density covering ranks 1–5, and interferential therapy, which peaks at rank 1 in all analyses but which is subject to considerable uncertainty in the analyses restricted to trials with adequate allocation concealment. Static magnets is likely to be one of the worst treatments across analyses, with balneotherapy likely to be one of the worst treatments in the analyses restricted to trials with adequate allocation concealment. T’ai chi peaks at rank 1 in the analyses restricted to trials with adequate allocation concealment. For NMES, the rankogram displays a ‘bucket’ shape as the posterior density is spread over a very wide range and at the lowest and highest extremes it competes with few treatments to be the ‘best’ and ‘worst’ of those considered.
FIGURE 23.
Results of the network meta-analysis (results presented as the probability that each treatment is ranked first, second, third etc.): (a) acupuncture; (b) balneotherapy; (c) braces; (d) insoles; (e) NMES; (f) placebo; (g) TENS; (h) usual care; (i) sham acupuncture; (j) muscle-strengthening exercise; (k) aerobic exercise; (l) heat treatment; (m) ice/cooling treatment; (n) inferential therapy; (o) laser/light therapy; (p) manual therapy; (q) PES; (r) PEMF; (s) static magnets; and (t) t’ai chi. The solid line represents the all-trials analysis; the small dots the analysis of trials with adequate allocation concealment and the long dashes the analysis of trials with adequate allocation concealment reporting at 3–13 weeks.

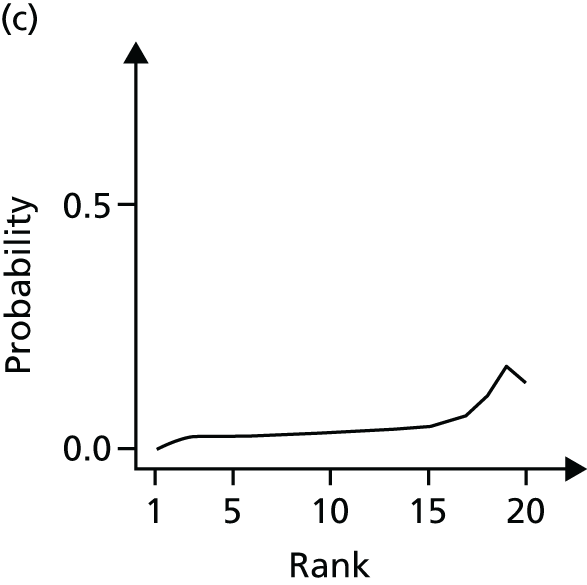

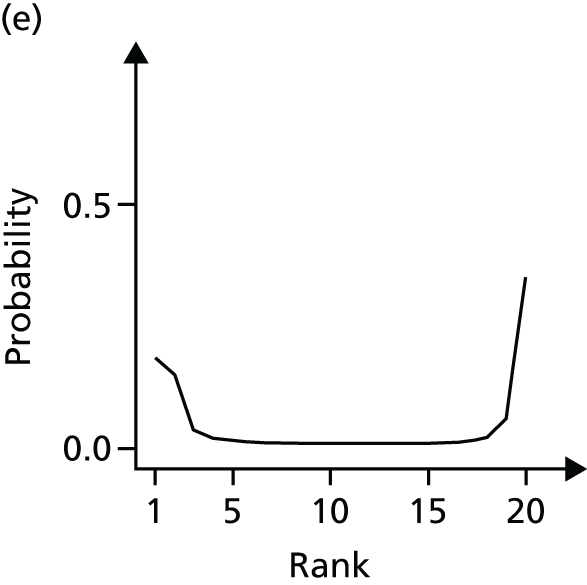

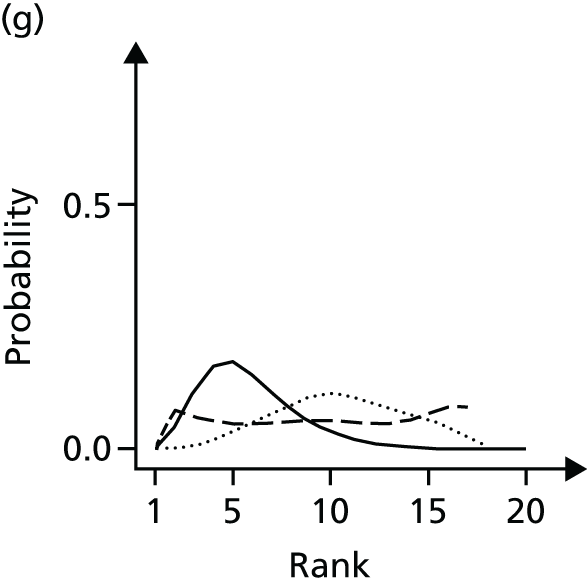
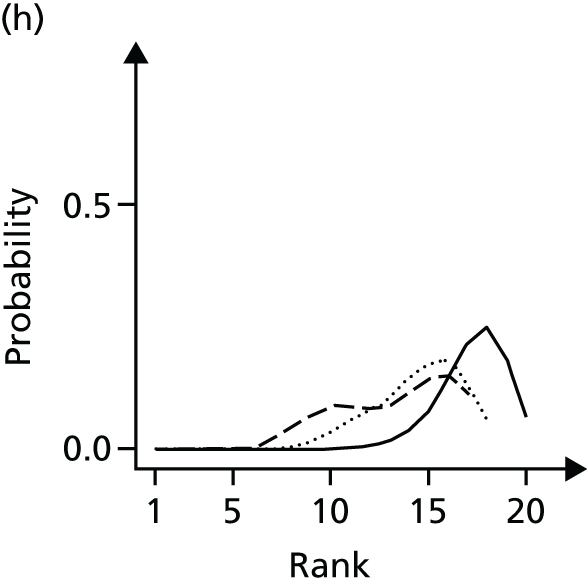

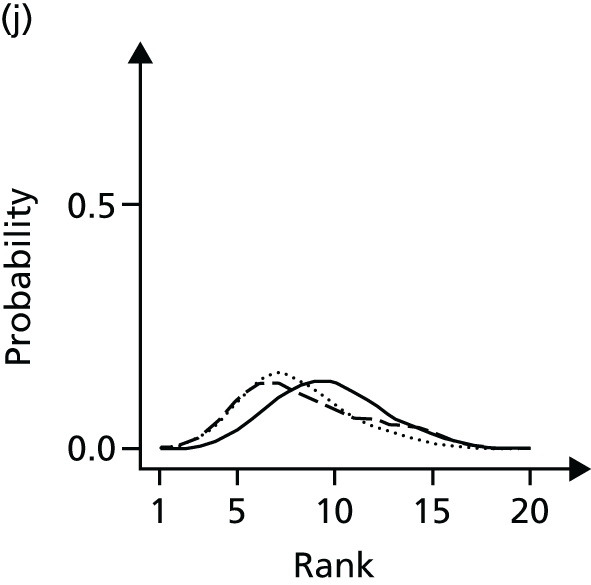
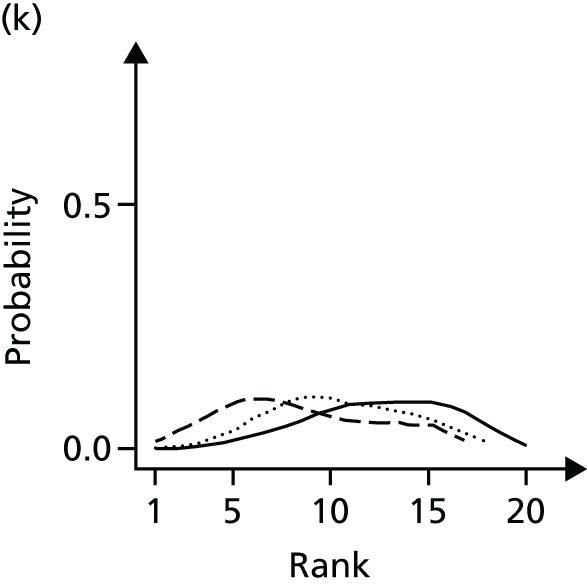
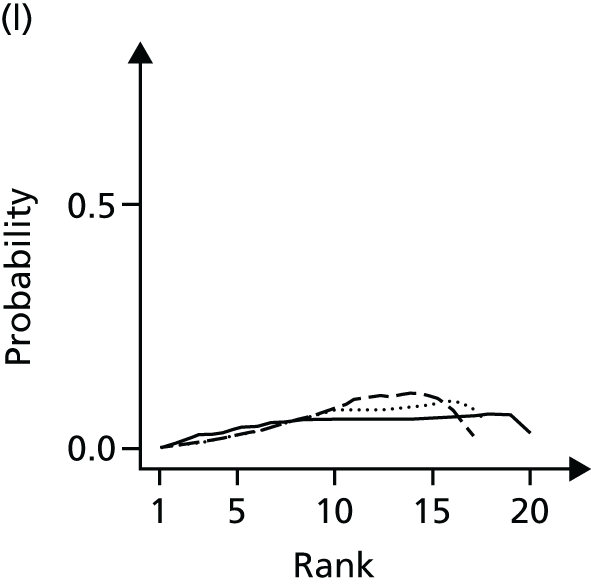
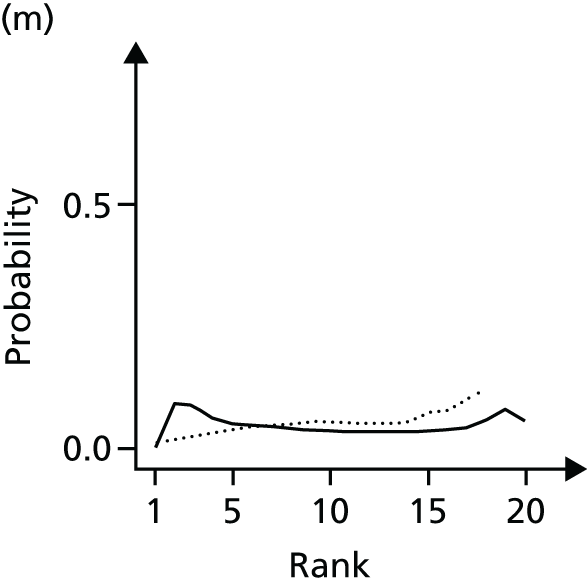

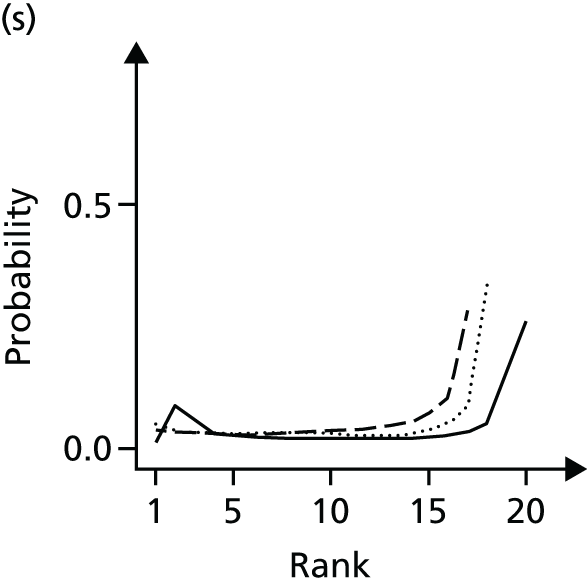
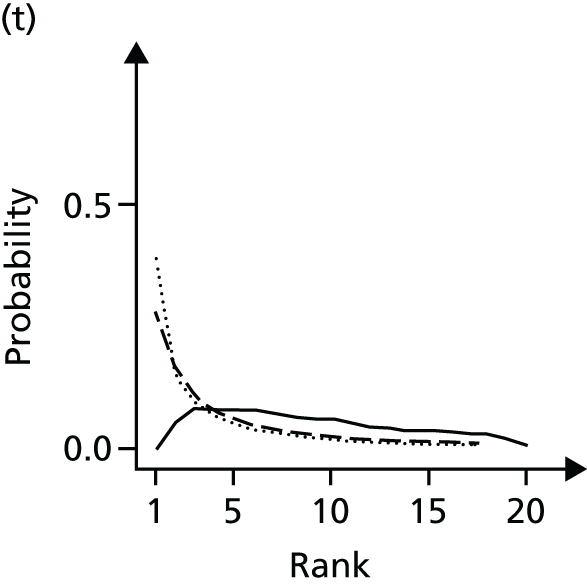
The level of uncertainty regarding the effect of some comparators was very high, particularly for NMES and static magnets. The 95% CrIs cross zero for many interventions, with the exception of muscle-strengthening exercise and acupuncture in all three analyses; interferential therapy, PES and TENS in the all-trials analysis only; and sham acupuncture in both analyses involving a low risk of bias for allocation concealment.
Inclusion of age as a potential treatment effect modifier did not improve the model fit according to the residual deviance or DIC measures and had a very minimal impact on results. The results of this analysis are therefore not shown here. The model analysing the role of BMI as a treatment effect modifier did not converge, which was likely because of the high volume of missing data for BMI. This was not addressed through more advanced imputation approaches as the available data were unlikely to provide a useful basis for imputation (weight was rarely reported in the studies that did not report BMI).
Model fit statistics are provided in Table 30. The mean residual deviance suggests an adequate fit of each model to the data. Comparison of mean residual deviance and DIC statistics between the inconsistency and the consistency models for each analysis suggests no global evidence of inconsistency. However, inspection of individual data points’ posterior mean deviance contributions suggests that the model is a poor fit for some data points. In the all-trials analysis, four data points had very high deviance contributions (3.4–6.9) in both the consistency and the inconsistency models. Two of the points were from trials comparing interferential therapy with placebo. 301,302 These studies are clearly inconsistent with each other. The difference in EQ-5D scores for interferential therapy compared with placebo is 0.07 (no variance measure reported) in the study by Adedoyin et al. 301 and 0.30–0.33 (95% CrI 0.19 to 0.42), depending on interferential therapy arm, in the study by Gundog et al. 302 These effects are also quite different from the indirect data on interferential therapy from Burch et al. 303 (interferential therapy vs. TENS 0.07, 95% CrI –0.01 to 0.16); thus, although all studies suggest that interferential therapy offers benefit, the magnitude of this benefit is inconsistent across studies and therefore more uncertain than the analysis of all trials suggests. No clear source of the uncertainty was identified, although all studies were assigned a high or unclear risk-of-bias score. One potential source of heterogeneity is the treatment intensity: patients in the Adedoyin et al. 301 study were delivered the equivalent of a 40-minute weekly dose, whereas patients in the Gundog et al. 302 study were delivered 100 minutes of treatment and patients in the Burch et al. 303 study were delivered 245 minutes of treatment (some of this period involved patterned muscle stimulation rather than interferential therapy). The two other points contributing high residual deviances were from a study comparing aerobic exercise with usual care. 189 This study is considered to be at high risk of bias and has been identified as an outlier in previous analyses. 168 The benefit from treatment estimated in this study may also be viewed as implausibly large (the difference in EQ-5D scores for aerobic exercise vs. usual care is 0.37, 95% CI 0.20 to 0.54). As aerobic exercise does not form part of a closed loop, any impact of this study will be limited to the aerobic exercise–usual care contrast. Residual deviance contributions for all other data points in the all-trials consistency model were < 2.3. For the other models, residual deviance contributions for all data points were < 2.5.
| Statistic | Trials | |||||
|---|---|---|---|---|---|---|
| All | With adequate allocation concealment | With adequate allocation concealment, end point reporting 3–13 weeks | ||||
| Consistency model | Inconsistency model | Consistency model | Inconsistency model | Consistency model | Inconsistency model | |
| Mean residual deviance (data points) | 206 (210) | 214 (210) | 84 (92) | 87 (92) | 69 (73) | 68 (73) |
| pD | 173 | 182 | 87 | 92 | 77 | 77 |
| DICa | 12,872 | 12,891 | 12,814 | 12,821 | 12,787 | 12,787 |
| Random effect SD (95% CrI) | 0.03 (0.02 to 0.05) | 0.03 (0.01 to 0.05) | 0.02 (0.00 to 0.04) | 0.02 (0.00 to 0.04) | 0.02 (0.00 to 0.05) | 0.01 (0.00 to 0.04) |
Cost-effectiveness analysis
The results of the cost-effectiveness analysis are presented in Table 31. Intervention costs represented the vast majority of costs. Expected outcomes calculated using the posterior distributions of costs and effects are presented; uncertainty around these results is discussed in the remainder of the results section. In the analysis including all trials, TENS is the cost-effective intervention assuming a willingness-to-pay threshold in the range £20,000–30,000 per QALY gained, with an ICER of £2690 per QALY compared with usual care. In the analysis restricted to trials with adequate allocation concealment, acupuncture is the cost-effective intervention, with an ICER of £13,502 per QALY compared with TENS. When the analysis is restricted to trials with adequate allocation concealment and which reported in between 3 and 13 weeks, acupuncture is the cost-effective intervention, with an ICER of £14,275 per QALY compared with TENS.
| Intervention | Trials | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | With adequate allocation concealment | With adequate allocation concealment, end point reporting 3–13 weeks | |||||||
| Cost (£) | QALYs | ICER (£ per QALY) | Cost (£) | QALYs | ICER (£ per QALY) | Cost (£) | QALYs | ICER (£ per QALY) | |
| Usual care | 0 | 0.000 | Referent | 0 | 0.000 | Referent | 0 | 0.000 | Referent |
| Static magnets | 5 | 0.001 | ED | 5 | 0.000 | Dominated | 5 | –0.001 | Dominated |
| Insoles | 13 | 0.001 | ED | 13 | 0.002 | ED | 14 | 0.004 | 3540 |
| TENS | 31 | 0.011 | 2690 | 30 | 0.005 | 6142 | 30 | 0.006 | 9750 |
| Braces | 40 | 0.001 | Dominated | NA | NA | NA | NA | NA | NA |
| Acupuncture | 179 | 0.014 | ED | 192 | 0.017 | 13,502 | 192 | 0.017 | 14,275 |
| Heat treatment | 297 | 0.005 | Dominated | 214 | 0.003 | Dominated | 213 | 0.002 | Dominated |
| Manual therapy | 304 | 0.008 | Dominated | 276 | 0.013 | Dominated | 277 | 0.018 | 86,964 |
| PES | 396 | 0.011 | Dominated | 410 | 0.010 | Dominated | 410 | 0.010 | Dominated |
| NMES | 481 | 0.005 | Dominated | NA | NA | NA | NA | NA | NA |
| Laser light therapy | 503 | 0.007 | Dominated | 288 | 0.003 | Dominated | 288 | 0.003 | Dominated |
| Interferential therapy | 770 | 0.033 | 33,866 | 1179 | 0.016 | Dominated | 1179 | 0.017 | Dominated |
| PEMF | 1453 | 0.007 | Dominated | 577 | 0.008 | Dominated | 577 | 0.007 | Dominated |
Cost-effectiveness acceptability frontiers for the three primary analyses are presented in Figure 24. The probability that each intervention would be cost-effective at decision thresholds of £20,000 and £30,000 per QALY gained is presented in Table 32. These summaries provide the probability that each intervention is cost-effective in the context of a fully incremental analysis comparing all interventions. The probability is calculated as the proportion of simulations in which the intervention has the highest ICER (under a given threshold) or, equivalently, the highest incremental net benefit. At a cost-effectiveness threshold of £20,000 per QALY, the probability that TENS is cost-effective in the all-trials analysis is 49%. In the analysis of trials with adequate allocation concealment the probability that acupuncture is cost-effective is 47%. In the analysis of trials with adequate allocation concealment reporting between 3 and 13 weeks the probability that acupuncture is cost-effective is 25%.
FIGURE 24.
Cost-effectiveness acceptability frontiers: (a) all trials; (b) trials with adequate allocation concealment; and (c) trials with adequate allocation concealment and reporting in the range 3–13 weeks.


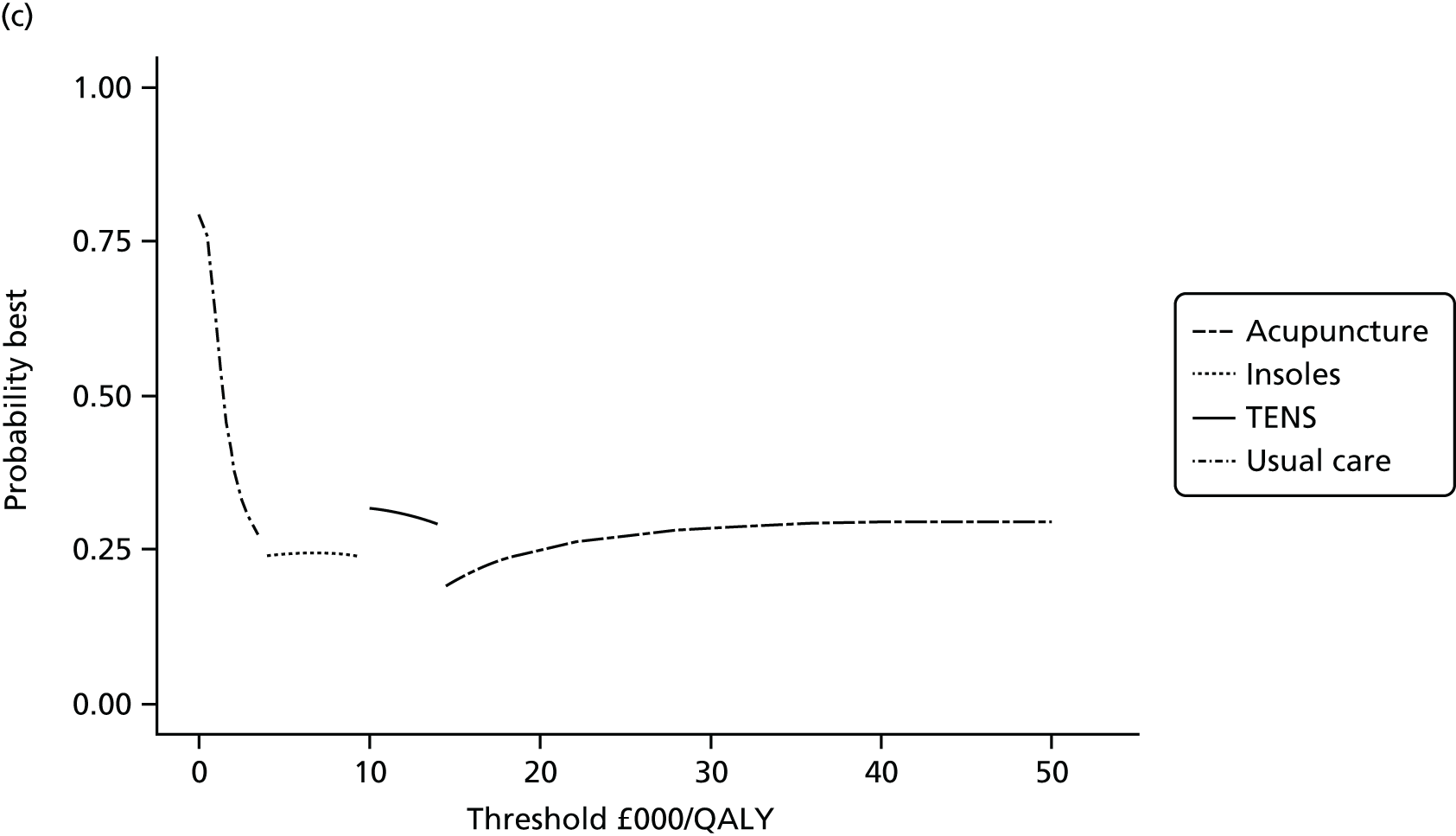
| Intervention | Trials | |||||
|---|---|---|---|---|---|---|
| All | With adequate allocation concealment | With adequate allocation concealment 3–13 weeks | ||||
| £20,000 | £30,000 | £20,000 | £30,000 | £20,000 | £30,000 | |
| Usual care | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Static magnets | 0.22 | 0.18 | 0.26 | 0.20 | 0.17 | 0.13 |
| Insoles | 0.00 | 0.00 | 0.04 | 0.02 | 0.13 | 0.09 |
| TENS | 0.49 | 0.28 | 0.15 | 0.08 | 0.25 | 0.20 |
| Braces | 0.06 | 0.05 | NA | NA | NA | NA |
| Acupuncture | 0.06 | 0.12 | 0.47 | 0.57 | 0.25 | 0.28 |
| Heat treatment | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 |
| Manual therapy | 0.00 | 0.00 | 0.07 | 0.12 | 0.20 | 0.28 |
| PES | 0.00 | 0.00 | 0. 00 | 0.00 | 0.00 | 0.00 |
| NMES | 0.16 | 0.21 | NA | NA | NA | NA |
| Laser light therapy | 0.00 | 0.00 | 0. 00 | 0.00 | 0.00 | 0.00 |
| Interferential therapy | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| PEMF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Sensitivity analyses
A summary of all sensitivity analyses that altered the optimal choice of intervention at a cost-effectiveness threshold of £20,000 per QALY is provided as Table 33. Detailed results of the sensitivity analyses are provided in Appendix 5.
| Data set | Scenario | Cost-effective intervention at threshold of £20,000 per QALYa |
|---|---|---|
| All trials | Base case | TENS |
| Shortest session duration used for acupuncture costing | Acupuncture | |
| Shortest session duration used for interferential therapy costing | Interferential therapy | |
| Shortened sessions – 75% of benefit in first 30 minutes, remainder by 1 hour | Interferential therapy | |
| Shortened sessions – all benefit achieved within 20–30 minutes | Interferential therapy | |
| Increase in duration of benefit for all interventions by 6 weeks | Interferential therapy | |
| Increase in duration of benefit of acupuncture by 31% | Acupuncture | |
| Increase in duration of benefit of interferential therapy by 45% | Interferential therapy | |
| Upper 95% CrI from NMA for acupuncture | Acupuncture (0.05) | |
| Upper 95% CrI from NMA for braces | Braces (0.08) | |
| Upper 95% CrI from NMA for NMES | NMES (0.18) | |
| Upper 95% CrI from NMA for static magnets | Static magnets (0.25) | |
| Lower 95% CrI from NMA for TENS | Acupuncture (0.15) | |
| Trials with low risk of bias for allocation concealment | Base case | Acupuncture |
| Use of private cost for acupuncture sessions | TENS | |
| Shortened sessions – all benefit achieved within 20–30 minutes | Interferential therapy | |
| Lower 95% CrI from NMA for acupuncture | TENS (0.05) | |
| Upper 95% CrI from NMA for insoles | Insoles (0.09) | |
| Upper 95% CrI from NMA for manual therapy | Manual therapy (0.10) | |
| Upper 95% CrI from NMA for static magnets | Static magnets (0.29) | |
| Upper 95% CrI from NMA for TENS | TENS (0.21) | |
| Trials with low risk of bias for allocation concealment, end point reporting 3–13 weeks | Base case | Acupuncture |
| Use of private cost for acupuncture sessions | Manual therapy | |
| Shortest session duration used for manual therapy costing | Manual therapy | |
| Shortened sessions – all benefit achieved within 20–30 minutes | Interferential therapy | |
| Increase in duration of benefit for all interventions of 27 weeks | Manual therapy | |
| Increase in duration of benefit of manual therapy by 18% | Manual therapy | |
| Lower 95% CrI from NMA for acupuncture | TENS (0.14) | |
| Upper 95% CrI from NMA for heat treatment | Heat treatment (0.35) | |
| Upper 95% CrI from NMA for insoles | Insoles (0.35) | |
| Upper 95% CrI from NMA for manual therapy | Manual therapy (0.35) | |
| Upper 95% CrI from NMA for static magnets | Static magnets (0.30) | |
| Upper 95% CrI from NMA for TENS | TENS (0.40) |
Costs
Varying the costs of treatment as specified in Cost-effectiveness analysis altered the optimal decision at a decision threshold of £20,000 per QALY gained in only three of the analyses (out of the 70 conducted; see Appendix 5 for full results). In the analysis of all trials, using the shortest duration of session time for acupuncture resulted in this intervention becoming cost-effective (ICER £19,033 per QALY gained) and using the shortest duration for interferential therapy resulted in this intervention becoming cost-effective (ICER £7626 per QALY gained). In the remainder of the all-trials analyses, TENS remained the cost-effective treatment, with all ICERs < £4000 per QALY gained. In the analysis restricted to trials with adequate allocation concealment, acupuncture remained cost-effective across all analyses, with ICERs ranging from £6000 to £18,000 per QALY gained. In the analysis restricted to trials with adequate allocation concealment and reporting within 3–13 weeks, using the shortest duration for manual therapy resulted in this intervention becoming cost-effective (ICER £9500 per QALY gained). In the other analyses acupuncture was cost-effective, with ICERs ranging from £7000 to £19,000 per QALY gained.
Assuming that acupuncture was delivered by private practitioners resulted in acupuncture no longer being cost-effective in the analysis restricted to trials with adequate allocation concealment and the analysis restricted to trials with adequate allocation concealment reporting within 3–13 weeks. In these analyses TENS and manual therapy became cost-effective, respectively.
As described in Cost-effectiveness analysis a series of scenarios explored shorter treatment sessions and a range of assumptions about the response of intervention effects to session duration. In the all-trials analysis, interferential therapy became cost-effective if we assumed that 75% of the benefit of treatment occurs in the first 30 minutes and the remainder of the benefit occurs between 30 minutes and 1 hour. In both analyses restricted to trials with adequate allocation concealment, the preferred treatment choice switched only under the extreme scenario whereby all of the benefit observed within the trials is achieved within the first 20 minutes (or 30 minutes in the case of acupuncture or manual therapy).
Effectiveness
The optimal choice of intervention at a cost-effectiveness threshold of £20,000 per QALY gained using 95% CrI values was altered in our sensitivity analyses for the following interventions: acupuncture, braces, heat treatment, insoles, manual therapy, NMES, static magnets and TENS. The values required to alter decisions were generally at the extremes of the posterior distributions (see Table 33), although there were exceptions to this.
In the analysis of all trials the probability that the treatment effect for TENS would be sufficiently small for acupuncture to become cost-effective was 0.15. In the analyses of trials with adequate allocation concealment, the probabilities that the treatment effect for TENS would be sufficiently large for TENS to become cost-effective were 0.21 and 0.40 (analysis restricted to trials reporting between 3 and 13 weeks). Conversely, in the analysis of trials with adequate allocation concealment reporting in the 3- to 13-week period the probability that the treatment effect for acupuncture would be sufficiently small for TENS to become cost-effective was 0.14. In the analyses of trials with adequate allocation concealment, the probabilities that the treatment effect for manual therapy would be sufficiently large for manual therapy to become cost-effective were 0.10 and 0.35 (analysis restricted to trials reporting between 3 and 13 weeks). In the analysis of trials with adequate allocation concealment reporting in the 3- to 13-week period, insoles and heat treatment had a probability of 0.35 of being sufficiently effective to become the cost-effective treatment. Across analyses, the probability of observing a treatment effect for static magnets sufficiently large for it to become cost-effective was 0.25–0.30, whereas for NMES this probability was 0.18. In both cases this reflected the high levels of uncertainty around the efficacy of these comparators.
In the analysis of all trials the extension of benefit would have to be 6 weeks for interferential therapy to become cost-effective. In the analysis restricted to trials with adequate allocation concealment no extension of the duration of benefit impacted on the decision. In the analysis restricted to trials with adequate allocation concealment and which reported between 3 and 13 weeks, the extension of benefit would have to be 27 weeks for manual therapy to become cost-effective. Extending the duration of benefit of each intervention individually (by up to 50%) altered the decision in only three cases. In the all-trials analysis, increasing the benefit of acupuncture and interferential therapy by 50% resulted in these interventions becoming cost-effective. In the analysis of trials with adequate allocation concealment reporting in the 3- to 13-week period, increasing the benefit of manual therapy resulted in this intervention becoming cost-effective. Further threshold analyses indicated that the extension of benefit required to alter these decisions was 31%, 45% and 18%, respectively.
Value of further information
Assuming a decision threshold of £20,000 per QALY, the per-decision EVPI for the all-trials scenario is £113, for the analysis of trials with adequate allocation concealment is £74 and for the trials with adequate allocation concealment reporting in the 3- to 13-week period is £164. The EVPI for the total population eligible to benefit from improved decision-making is estimated as £239M, £156M and £346M, respectively, over a 10-year period.
Figure 25 shows the cumulative net health benefits (in QALYs) generated under the policies of adoption, AWR and OIR. This shows that AWR is always the preferred policy. If AWR was not available, adoption is the preferred option in the all-trials analysis and the analysis of trials with adequate allocation concealment. In the analysis restricted to trials with adequate allocation concealment reporting between 3 and 13 weeks, there is higher uncertainty and the adoption and OIR policies deliver very similar health benefits. Given that this analysis reflects the maximum value of further research and that the real value will be lower (as research will not deliver perfect information and is associated with research costs), adoption is the preferred policy.
FIGURE 25.
Cumulative health generated under the adoption, AWR and OIR policies: (a) all trials; (b) trials with adequate allocation concealment; and (c) trials with adequate allocation concealment reporting at 3–13 weeks.
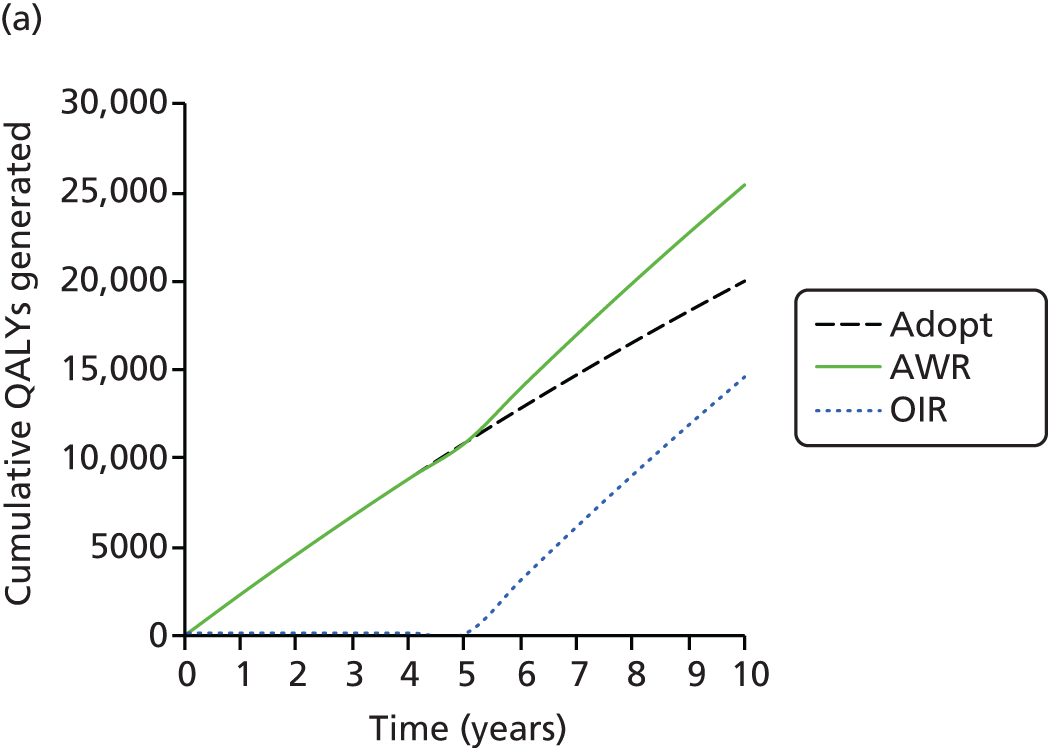

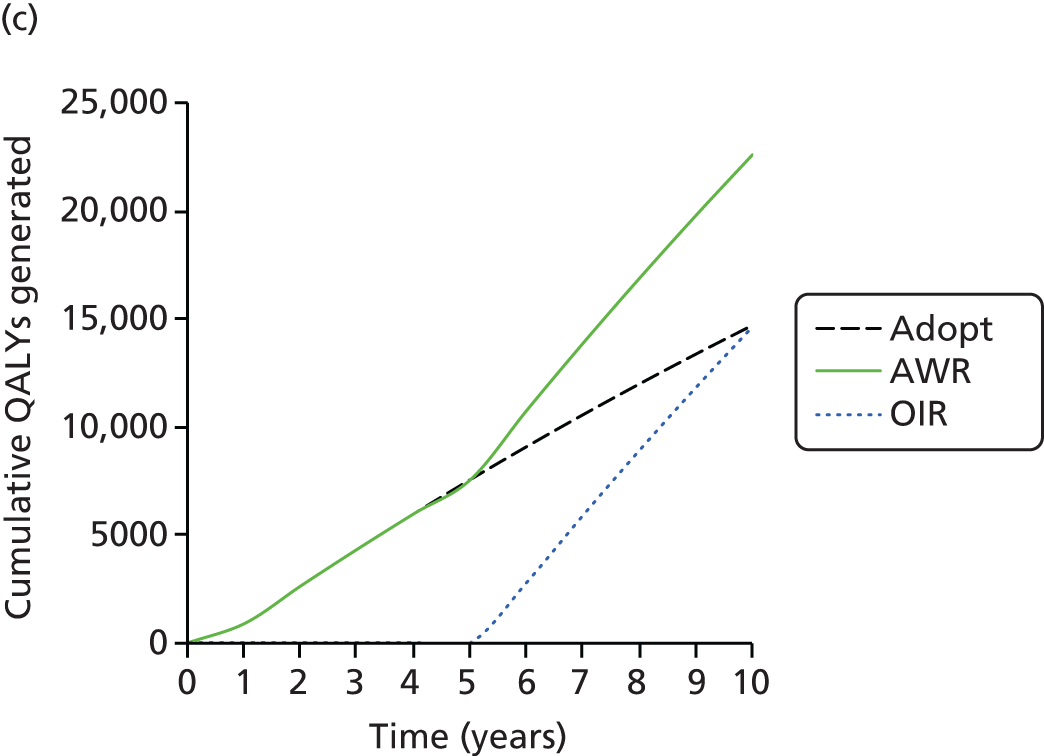
Discussion
Principal findings
Health-care decision-makers, aiming to maximise population health, require evidence of the costs and effects of competing treatment strategies. In many cases the available information is not in a format that readily facilitates comparisons of costs and effects. RCTs rarely compare all of the treatments of interest and rarely provide sufficiently long or detailed follow-up for all costs and outcomes of treatment to be observed. In addition, evidence may need to be generalised across different settings (e.g. countries, disease subpopulations). In the case of complementary therapies and other non-pharmaceutical interventions for which there are more limited regulatory processes, there is an increased potential for heterogeneous conduct of trials and data availability, especially when combined with a focus on subjective outcome measures. However, regardless of the limitations and uncertainties in the evidence base, policy-makers must make decisions.
The National Institute for Health and Care Excellence has recommended acupuncture for the treatment of chronic headache and migraine,80 and for musculoskeletal pain,81 but not in the context of chronic pain associated with osteoarthritis of the knee. 82 This decision in part reflected concerns regarding the available evidence. The current study was commissioned as part of a programme intended to improve evidence around the costs and effects of acupuncture. The work presented in this chapter builds on work presented in Chapter 3 to systematically identify and synthesise outcome data on a wide range of interventions for osteoarthritis of the knee. The work extends the synthesis in Chapter 3 to the EQ-5D end point, allowing calculation of QALYs and a cost-effectiveness analysis. The available trials were of varying quality, reporting distinct outcomes at different time points. IPD were available for a minority of studies with the rest providing only summary statistics. Novel Bayesian network meta-analysis models were developed to synthesise continuous outcome data reported at both the individual patient level and the aggregate level. Mapping of the available HRQoL data to EQ-5D preference weights was conducted to produce a common statistic for synthesis and one that could inform the cost-effectiveness analysis. The simulation outputs from the network meta-analysis informed a decision-analytic cost-effectiveness model that compared the costs and QALYs of the competing interventions, and was used to reflect the degree of uncertainty surrounding the optimal treatment decision under different assumptions concerning quality of the evidence, treatment effectiveness, duration of treatment effectiveness and costs.
Network meta-analyses were conducted for three data sets: (1) all trials, (2) trials with a low risk of bias for allocation concealment and (3) trials with a low risk of bias for allocation concealment and reporting within the 3- to 13-week window. In the analysis of all trials, interferential therapy was the most effective treatment based on the point estimate of effect, followed by acupuncture, TENS, PES and t’ai chi. These results are broadly consistent with the work conducted in Chapter 3 on the standardised pain outcome, which found that the least uncertainty, as reflected by the narrowest CrIs, is associated with acupuncture and muscle-strengthening exercises. Although this analysis of all trials suggested a high probability that interferential therapy was the most effective treatment, analyses of model fit suggested that the model estimates were inconsistent with the observed trial-level treatment effect estimates because of large differences in the effects observed in different studies. No obvious sources of heterogeneity were observed, although differences in treatment intensity may have played a role. Results from this analysis should therefore be interpreted with caution.
The analysis including trials with a low risk of bias for allocation concealment found that t’ai chi was the most effective treatment based on the point estimate of effect, followed by acupuncture, interferential therapy, manual therapy and sham acupuncture. The analysis including trials with a low risk of bias for allocation concealment and reporting within the 3- to 13-week window found that t’ai chi was the most effective treatment based on the point estimate of effect, followed by manual therapy, acupuncture, inferential therapy and sham acupuncture. The analyses suggested an incremental effect of acupuncture over usual care that ranged from 0.09 (95% CrI 0.06 to 0.13) to 0.11 (95% CrI 0.07 to 0.15), depending on the analysis. These results for acupuncture are similar to those reported for osteoarthritis of the knee in Chapter 4 (0.08, 95% CrI 0.05 to 0.12). The level of uncertainty regarding the treatment effect of some comparators was very high. By contrast, in both the analyses of higher-quality trials, acupuncture and muscle-strengthening exercises stand out as being associated with the narrowest CrIs and therefore the least uncertainty. The 95% CrIs cross zero for all interventions with the exception of muscle-strengthening exercise and acupuncture in all three analyses; for interferential therapy, PES and TENS in the all-trials analysis only; and for sham acupuncture in the two analyses involving trials with a low risk of bias for allocation concealment.
The cost-effectiveness results varied according to the data set considered relevant for decision-making. The analysis of all trials found that TENS was cost-effective (ICER £2690 per QALY vs. usual care) whereas analyses (2) and (3) found that acupuncture was cost-effective (ICER £13,502–14,275 per QALY vs. TENS) as an adjunct treatment for knee osteoarthritis in the UK NHS. The difference between these results is attributable to the reduced effect of TENS in analyses (2) and (3), in which trials with a high risk of bias were excluded.
The sensitivity analyses conducted suggested that the optimal intervention with respect to cost-effectiveness was uncertain. In analysis (1), plausible reductions in the cost of acupuncture, reductions in the efficacy of TENS and increases in the duration of benefit of acupuncture resulted in acupuncture becoming the cost-effective treatment. Using the shortest duration of interferential therapy sessions observed in the studies included in the synthesis resulted in this intervention becoming cost-effective, as was the case in analyses assuming that the duration of interferential therapy sessions could be reduced without proportionate reductions in efficacy. In analyses (2) and (3) plausible increases in the efficacy of TENS resulted in TENS becoming the cost-effective treatment and plausible increases in the efficacy of manual therapy resulted in this comparator becoming cost-effective. In analysis (3) using the shortest duration of manual therapy sessions observed in the studies included in the synthesis resulted in this comparator becoming cost-effective, as did carrying out plausible increases to the duration of benefit of manual therapy. Analysis (3) was particularly uncertain and use of the upper and lower CrIs from the network meta-analysis altered the cost-effective intervention in six instances.
There is considerable uncertainty in terms of both the effects of many of the interventions and the probability that each intervention is cost-effective. The latter reflects both uncertainty in the effect of treatment and the large number of interventions appraised. Despite these uncertainties the value of information analysis suggested that adoption (of TENS or acupuncture, depending on the analysis) will generate more health to the NHS than delaying adoption until further research is conducted. There may be value to conducting further research alongside adoption if this is feasible and possible avenues for this research are discussed later in this section.
The conclusions of the current work are based on a cost-effectiveness threshold of £20,000–30,000 per QALY as this has been used historically by NICE. A cost-effectiveness threshold is used to assess if the health benefits offered by an intervention are greater than the health likely to be lost because the additional resources required are not available to fund other effective treatments in the NHS. Research conducted during the course of this study suggests that cost-effectiveness thresholds of £20,000–30,000 per QALY may be too high as it was estimated that £13,000 of NHS resources adds 1 QALY to NHS patients. 251 In the context of this study, using this lower estimate of the cost-effectiveness threshold would result in TENS being the cost-effective choice in the base-case analyses.
Strengths and limitations
This study has a number of strengths and limitations. The study methods and the presentation of the results are consistent with the requirements of funding agencies, which need to ensure efficient allocation of resources across a wide range of programmes and diseases. The analysis was based on evidence identified by the previous systematic literature review presented in Chapter 3, all decisions with respect to inclusion/exclusion of studies and evidence are made transparent and the assumptions required to make comparisons across therapies are made explicit. The analysis was able to include a large number of studies and interventions despite a lack of head-to-head trial data, access to IPD and many studies not collecting (or reporting) the outcome of interest (EQ-5D preference weights).
The synthesis of heterogeneous outcomes relied on imperfect mappings, which are typically able to explain only a minority of variation in EQ-5D scores. The magnitude of bias introduced by using mapping functions (and different mapping functions across trials) is unknowable. The availability of key outcomes across trials would have reduced these concerns, as would the collection of generic preference-based measures of HRQoL in all trials. Mapping is always a second-best approach compared with directly collecting data on preference-based measures, such as the EQ-5D, as it leads to increased uncertainty and error around HRQoL estimates. 304 A ‘core outcome set’ for osteoarthritis is available. This recommended that future Phase III trials of knee, hip and hand osteoarthritis should evaluate the following domains: pain, physical function, patient global assessment and, for studies of ≥ 1 year, joint imaging. 252 Recommendations that go beyond Phase III regulatory trials and which define the instruments that should be used to measure outcomes in these domains are warranted to foster consistency.
Our analysis accounted for the limited explanatory power of the mapping algorithms; however, it did not explicitly account for measurement error around the HRQoL measures or the EQ-5D. None of the mapping algorithms used reflected measurement error and methods to reflect measurement error in this context have only recently been piloted. 305 Future research should explore how measurement error can be adequately reflected in mapping algorithms and synthesis of multiple outcomes (some work on this has begun – see, for example, Lu et al. 306).
Individual patient data were available only for a minority of studies. Means and variances for the EQ-5D data mapped from aggregate data on HRQoL were required for the synthesis. The HRQoL instrument dimension scores were therefore assumed to be distributed according to a multivariate normal distribution. This may not have been a good approximation in some cases, thus increasing uncertainty regarding the resulting mean or variance estimates. In addition, samples from the HRQoL instruments that were outside the feasible range for each instrument were truncated at the minimum or maximum value. This will have altered the means and variances from those reported in the publications. Checks were performed to identify any trials for which the direction of treatment effect on utility was not as would have been expected using the mean values of the HRQoL instrument. This found discrepancies for five of the 88 trials.
The relationship between baseline and final outcomes observed in the IPD studies was assumed to generalise to the aggregate data studies. This allowed potential baseline imbalances in studies for which only summary data were available to be adjusted for. This assumption may not have been appropriate in all cases, particularly when follow-up time points in the aggregate data studies were particularly short or long. Missing data from the IPD studies were excluded from the analysis, which may have biased the results. Development of methods to account for missing data in the context of IPD and aggregate data network meta-analysis is warranted.
Outcome data closest to 8 weeks’ follow-up were selected for synthesis. The synthesis therefore required the assumption that, in the many trials not reporting outcomes at 8 weeks, the available data are reflective of the 8-week time point. Sensitivity analyses around this assumption suggested that the results were not particularly sensitive to the inclusion of studies with very short or very long follow-up periods, with the exception of manual therapy. For the cost-effectiveness analysis, the HRQoL effects observed at 8 weeks were applied from 0 to 8 weeks to generate QALY estimates. Sensitivity analyses were conducted to assess the impact of extending the duration of benefit of the interventions. These analyses found that the model was sensitive to the duration of benefit of acupuncture, interferential therapy and manual therapy. Further work to understand the long-term effects of therapies would be of value.
All usual care interventions were assumed to be equivalent in the analysis, as were all sham acupuncture interventions and all other placebo interventions. Evidence from work recently conducted by the ATC suggests that the effect of sham acupuncture may depend on whether it involves the use of penetrating or non-penetrating needles and that the effect of usual care may depend on whether or not usual care is delivered according to a pre-specified protocol. 45 It seems plausible that other non-acupuncture sham procedures may also vary in their effects. Exploration of a network including more refined comparator definitions may therefore be of value.
There was substantial variation in the duration of therapy course, frequency of sessions and duration of sessions administered. Further research should explore the impact of these attributes of intervention delivery on outcomes and cost-effectiveness.
The studies analysed here are from a range of countries, which may differ in terms of the method by which and intensity with which interventions are administered. In addition, differences in the nature of health care for chronic pain more generally could have impacted on outcomes.
The analysis of non-intervention resource use assumed that only primary care and specialist visits are impacted on by changes in outcomes following interventions and that the impact of treatment on resource use can be captured through changes in EQ-5D scores. It is possible that this did not capture the full impact of treatment on resource use. Intervention costs were derived using a combination of expert opinion on required resource use and trial data on the intensity with which interventions were administered. Empirical resource use data for each intervention would have increased the robustness of the intervention costs. In addition, the costing involved a number of strong assumptions. Insoles, braces and static magnets were assumed to deliver the benefits estimated from the network meta-analysis for their usable lifespan. Costs of equipment used by physiotherapists were not included in the analysis as per-use costs were expected to be small. Inclusion of these costs is unlikely to change the conclusions of the analysis as equipment costs for acupuncture and manual therapy are likely to be small and many musculoskeletal outpatient physiotherapy departments are likely to already have interferential therapy devices. 307 We assumed that acupuncture would be administered by physiotherapists. Survey data suggest that a non-negligible proportion of acupuncture sessions administered within the NHS are administered by medical doctors. We did not evaluate this as, unless a marked difference in outcomes was expected between delivery by a physiotherapist and delivery by a medical doctor, it seemed unlikely that provision by medical doctors would be cost-effective given the large difference in hourly costs. Survey data also suggest that independent acupuncturists currently deliver the majority of acupuncture sessions per annum in the UK, primarily for musculoskeletal pain. 1 However, a large majority of this is delivered outside of the NHS.
The analysis presented here evaluated adjunct treatments assuming that their benefit was independent of any concomitant core treatments. Further work could explore interactions between core and adjunct therapies. The analysis also assumes that health-care professionals are faced with a single opportunity to select treatment for an individual, whereas in reality individuals with chronic pain are likely to be treated with a series of interventions. Further work could explore the costs and effects of different sequences of interventions.
Recommendations for future research
The EVPI in this area is relatively high, suggesting that additional research may be cost-effective. Further analysis is required to identify the most cost-effective and clinically appropriate specifications for further research. Such analyses could explore the cost-effectiveness of research to address the following key areas of uncertainty:
-
The impact of the exact attributes of the treatment approach on HRQoL outcomes. This could include exploring variation in attributes such as the treatment protocol, overall duration of the course of therapy and frequency and duration of sessions.
-
The time profile of HRQoL benefits of interventions both within the treatment period and beyond the treatment period.
The value of new clinical research to inform these areas of uncertainty should ideally be assessed by extending the existing model to include data (and when necessary expert opinion) that quantify our current understanding of these uncertain quantities. This would allow the uncertainties that are most important in determining cost-effectiveness to be identified. Clinical research to reduce these key uncertainties could take a number of forms including prospective trials or observational studies.
More generally, there would be value in developing a set of recommendations defining which HRQoL instruments should be used when evaluating therapies aimed at improving the HRQoL of knee osteoarthritis patients. Development of these recommendations would help to ensure that appropriate instruments are used in future trials and foster consistency in outcomes collected across trials. This in turn would improve the reliability of future meta-analyses and network meta-analyses.
Conclusion
When all trials are included in the network meta-analysis irrespective of quality, TENS is the cost-effective non-pharmacological adjunct treatment for patients with chronic pain related to osteoarthritis of the knee. However, the effect of TENS may be exaggerated because of biases associated with poor trial conduct. When the network meta-analysis is restricted to trials with adequate allocation concealment, an attribute known to be associated with treatment effect magnitude in osteoarthritis, the effect of TENS diminishes and is associated with wide CRIs that include zero. In this analysis acupuncture becomes cost-effective.
Chapter 6 Acupuncture, Counselling or Usual Care for Depression (ACUDep): a randomised controlled trial
Introduction
A substantial proportion of the global disease burden involves depression,308 which is a major cause of suicide. Depression involves more than just everyday mood fluctuations; it also involves feelings of severe sadness, anxiety, hopelessness and worthlessness. Those affected individuals lose interest in the activities that they used to enjoy and often have physical symptoms such as chronic pain, fatigue and insomnia.
The front-line treatment for depression in primary care usually involves antidepressants; however, they do not work well for more than half of patients. 95 Moreover, the effectiveness of newer antidepressants has recently come into question for patients with mild to moderate depression. 309 Non-pharmacological treatment options for depression are of interest to many patients, in part because of concerns about dependency on antidepressants. 97
Among non-pharmacological treatments, acupuncture and counselling show some promise in the treatment of depression, yet further evidence is needed. A Cochrane review of acupuncture for depression was inconclusive on whether or not acupuncture is an effective intervention for depression, in part because of the high risk of bias in the majority of studies. 310 Counselling is a widely used intervention for patients with depression and is provided in approximately half of the 9000 primary care practices in England. 311 A Cochrane review of counselling for mental health and psychosocial problems in primary care found short-term but not long-term benefits from counselling. 312 Both Cochrane reviews recommended extending the evidence base to include comparisons not just with usual care but also with other interventions, as this would have the potential to increase patient choice.
The aims of this study were to determine the clinical effectiveness and cost-effectiveness of short courses of either acupuncture or counselling compared with usual care for patients with depression. We also planned to compare acupuncture and counselling on the basis that there would be structural equivalence regarding time and attention. Additional exploratory studies nested within the trial focused on (1) the experience of treatment from the patient perspective; (2) the impact of comorbid pain on depression outcomes; (3) the approaches that practitioners use to enhance longer-term benefits; and (4) a health-economic analysis to investigate HRQoL and the costs of these treatments, and understand whether or not they should be considered a good use of limited health resources.
Methods
Design
Patients were randomised by the York Trials Unit to one of three arms in a pragmatic RCT (registration number ISRCTN63787732), as detailed in a published protocol. 313 An allocation ratio of 2 : 2 : 1 was used for the randomised groups: acupuncture plus usual care, counselling plus usual care and usual care alone, respectively. The York Trials Unit ensured that the allocation was securely concealed from the researchers who subsequently informed patients of their allocation. The unit recorded patient details prior to using Structured Query Language software for computer-generated block randomisation, with block sizes of five and 10. Randomisation was conducted by an investigator with no clinical involvement in the trial. The York NHS Research Ethics Committee (reference number 09/H1311/75) provided ethical approval on 21 September 2009.
Population
Patients were recruited from a primary care population who had consulted with depression within the past 5 years, who were continuing to experience depression and who were aged ≥ 18 years. We identified potential participants from general medical practice databases. Signed consent forms and baseline questionnaires were returned by patients. Patients were not eligible if they were receiving acupuncture or counselling at the time, had a terminal illness, significant learning disabilities, haemophilia, hepatitis or human immunodeficiency virus infection, were pregnant or had confounding psychiatric conditions (bipolar disorder, post-partum depression, adjustment disorder, psychosis, dementia or personality disorder). Patients who had suffered a close personal bereavement or given birth during the previous 12 months were also excluded. Spoken English was a requirement. Patients were eligible if they scored ≥ 20 on the Beck Depression Inventory-II (BDI-11),314 which is classified within this scale as ‘moderate’ or ‘severe’ depression.
Interventions
Up to 12 sessions were offered, usually on a weekly basis, to patients allocated to the acupuncture or counselling groups. Practitioners providing acupuncture were registered with the BAcC, with at least 3 years of post-qualification experience. In consultation with participating acupuncturists, an acupuncture treatment protocol was developed, which allowed for customised treatments within a standardised theory-driven framework. 315 Practitioners providing counselling were members of the British Association for Counselling and Psychotherapy with accreditation or who were eligible for accreditation having completed 400 supervised hours post qualification. Counselling competences using a humanistic approach drawn from those independently developed for Skills for Health316 were incorporated into a manualised protocol. All practitioners recorded in logbooks the number and length of sessions, treatment provided and adverse events. Further details of the two interventions are presented in Appendix 6. In all three patient groups, usual care, both NHS and private, was available according to need and was monitored for all for the purposes of comparison.
Outcome measures
The Patient Health Questionnaire-9 items (PHQ-9)317 at 3 months was the primary outcome measure. We also evaluated the overall impact over the 12-month period. PHQ-9 scores range from 0 to 27, with depression considered mild (5–9), moderate (10–14), moderately severe (15–19) or severe (≥ 20). As a preference-based measure of health outcome, we used the EQ-5D. 226 Medication use was ascertained by asking patients if they had taken any prescribed medication for depression or any prescribed analgesics/painkillers. Patients reported health service use, including the number of times that they had consulted a health professional because of their depression. Patients also reported out-of-pocket spending on acupuncture, counselling or psychotherapy, including cognitive–behavioural therapy. Data were collected at baseline and by postal questionnaire at 3, 6, 9 and 12 months. Because these data were self-reported by patients independently of the research team, we minimised potential bias associated with unblinded researchers or clinicians measuring outcomes. We also collected demographic data and patients’ prior preferences and expectations of the interventions at baseline and BDI-II data at baseline and 12 months. We measured patients’ perceptions of their practitioners’ empathy at 3 months using the Consultational and Relational Empathy (CARE) measure14 in both the acupuncture group and the counselling group. A payment of £5 as reimbursement was enclosed with the final questionnaire to enhance the response rate. 15
Sample size
We sought an effect size of 0.39 on the PHQ-9 when comparing either acupuncture with usual care alone or counselling with usual care alone, an effect size that was taken to be both moderate and realistic. We used the allocation ratio of 2 : 2 : 1 to increase the power to detect statistically significant differences between acupuncture and counselling. A smaller effect size of 0.32 was sought when comparing acupuncture and counselling, although the anticipated difference between these two treatments was expected to be small and not clinically meaningful. With a two-sided significance level of 5% and 90% power the required group sizes were 204, 204 and 102 in the acupuncture, counselling and usual care alone arms, respectively. The total sample size required was 640 (i.e. groups of 256, 256 and 128 respectively) when allowing for 20% attrition.
Analysis
Primary comparisons were between acupuncture plus usual care and usual care alone, and between counselling plus usual care and usual care alone. A secondary comparison was between acupuncture plus usual care and counselling plus usual care. The PHQ-9 was the primary outcome measure and 3 months was the primary end point, and we used ANCOVA, with baseline PHQ-9 score as covariate. For missing data we used multiple imputation by chained regression using treatment group, baseline measures (PHQ-9, BDI-II, SF-36, EQ-5D anxiety/depression) and demographics (age and sex). We used imputed rather than raw data for the primary analysis to take account of the profile of non-responders.
In a secondary analysis we assessed the overall clinical impact over 12 months, using the AUC method for the PHQ-9 by linear regression, predicting the average AUC while controlling for baseline PHQ-9. We also explored in more detail the PHQ-9 outcomes across all time points using random intercept linear mixed models with fixed effects for treatment arm, time and arm–time interaction for each treatment comparison. The models nested time points within patients and controlled for baseline PHQ-9 score and potential mediators including patients’ prior expectations and preferences regarding the treatments. Potential mediators were identified by univariate regressions (p < 0.1) of the PHQ-9 at 3 months for the whole patient sample, controlling for baseline PHQ-9 score. We controlled for treatment time (combined length of sessions) and quality of attention (CARE score) when comparing acupuncture and counselling. We used ANCOVA to evaluate treatment differences between BDI-II depression scores at 12 months, while controlling for BDI-II baseline scores and covariates for depression as identified above. We used multiple imputation for missing data as for the PHQ-9.
Using an intention-to-treat basis, all analyses were carried out in Stata 12.1. Statistical tests were at a two-sided 0.05 significance level. To assess model assumptions, analysis of residuals was undertaken for all regression models.
Patient and public involvement
In preparation for conducting this research into acupuncture for depression, we conducted a feasibility study that included a prospective case series of 10 patients who received acupuncture treatment for their depression and a focus group with six patients with experience of depression. We used a focus group because as a research team we wanted to learn from patients about their depression and experiences of treatment. We also needed guidance on how best to address the question of whether or not acupuncture is a suitable treatment option for people with depression.
A local patient-centred mental health group, York & District Mind, agreed to work with us on this research and became a long-term partner in its support of research into acupuncture for depression. As a first step we drew on this support in forming a focus group, which met once for 2 hours. Participants in the group were two researchers and six volunteers, identified through York & District Mind, who had experienced depression and were either staff or users of mental health services. A topic guide provided structure for the meeting, covering personal experiences of depression, experiences of both conventional and alternative treatments for depression, and feedback on our research plans. It is the last of these that is most relevant to the patient and public impact on our research.
With regard to feedback on our research plans, participants were concerned about recruitment through GPs on the basis that people who do not consult their GP could not be part of the study. It was agreed that reaching out to those not consulting their GP was a good idea; however, the methods of recruitment would be challenging as there was no way of identifying this hard-to-reach group in sufficient numbers for a RCT.
Another issue raised was to do with the definition of depression amid concerns that there might be some cut-off in severity, for example having a cut-off point so that only people with mild to moderate depression could be recruited. There was a strong feeling among participants that people with severe depression, particularly those who had exhausted all other treatment options, would want to be included in the offer to participate in a trial. The feelings regarding discrimination against mental health users were clearly resonating in this discussion; nobody wanted to be excluded.
This point about not including more severely depressed patients in the trial had a major impact on our trial design when finalising the eligibility criteria. The resulting design included a cut-off to exclude mild depression; however, there was no cut-off for the more severely depressed. This was operationalised with the BDI-II, which we used to recruit patients with both moderate (score of 20–28) and severe (score 29–63) depression. This eligibility requirement led to 62% of patients recruited being categorised as having severe depression and the remaining 38% of patients being categorised as having moderate depression.
York & District Mind continued to be involved with our research into acupuncture for depression. Two lay members and their chief executive joined our independent steering group, which met roughly every 6 months throughout the duration of the trial. Beyond the steering group, their contributions also involved editing the wording in documentation related to the questionnaires that were to be filled in by patients. The chief executive took responsibility for consulting members of his organisation, including users of mental health services, and then aggregated the feedback, which led to improvements in the wording of the questionnaires. Once we had agreed the documentation, and received ethics approval, there was less scope for the York & District Mind participants to contribute, as the trial became primarily a logistic task for the next 3 years. In 2011 York & District Mind merged with another charity called Our Celebration to become York Mind. This change also brought a new chief executive, a transition that took some time to be completed. The new chief executive attended one independent steering committee meeting in June 2011. The combination of this change of personnel and the associated participation gap led to a reduced involvement by patients and the public in the trial from this point on.
Methods for substudy 1: experience of treatment from the patient perspective
The aim of this substudy was to explore patients’ experiences of depression, both with and without comorbid pain, and the perceived changes as a result of receiving the treatments of acupuncture or counselling and usual care. A secondary aim was to report the aspects of treatment that patients reported might have had a positive influence on any long-term change.
In terms of methods, this was a nested qualitative substudy within the Acupuncture, Counselling or Usual Care for Depression (ACUDep) trial. To recruit patients for interview, we selected only from those who had already consented to interview. We used a purposive sampling frame and recruited in the same 2 : 2 : 1 proportions as in the main trial from the treatment groups of acupuncture, counselling and usual care, respectively. We balanced the proportions of men and women and whether or not in pain at baseline. On receipt of their final 12-month postal questionnaire, and based on a sampling frame, a sample of participants who had previously consented to be contacted for an interview was invited to engage in a telephone interview of approximately 30 minutes’ duration. Altogether, 52 people consented to a one-to-one, audio-recorded, semistructured telephone interview for this study.
A researcher (AH) interviewed all participants and all interviews were conducted between February and May 2012. The researcher was unknown to the participants prior to the interview and therefore the interview opened with an introduction designed to set the participant at ease, reveal the context for their depression and draw out their account of treatment received as part of the trial. Prompts from a prepared topic guide were used to elicit the participants’ experiences of depression and treatment. On average, the interviews lasted approximately 25 minutes (range 11–46 minutes). To encourage participants to relax, participants were asked to introduce themselves by speaking about things they like to do or hoped to do and then how depression had entered their lives, before moving on to the research-related questions within the topic guide. Interviews were audio taped, transcribed verbatim and checked for accuracy. All recordings were of sufficient clarity and content that no repeat interviews were necessary. Each transcription was checked to remove any names and was then assigned a participant identification number.
Across the data set of 52 transcribed interviews we used an inductive thematic analysis. 318 With a constructivist approach to grounded theory,319 data were analysed initially by AH. Coding was performed sequentially on each transcript by working systematically through the entire data set. Coding and extractions were checked by JE to verify that the participants’ experiences were reflected and summarised accurately. Coding was conducted within each interview and across interviews resulting in themes associated with the experience of depression and further themes associated with the experience of the treatment. Further details of the methods are provided in a separate publication. 320
Methods for substudy 2: impact of comorbid pain on depression outcomes
The aim of this substudy was to find out whether people with depression who were also in pain had better or worse depression outcomes than those without pain. We documented the prevalence of pain in this trial population, the demographic profile of patients in pain and the relationship between pain and depression. We determined depression and pain outcomes following treatment with acupuncture or counselling compared with usual care alone. A full report has been published elsewhere. 321
In terms of methods, the participants were first divided into two groups according to their response to the EQ-5D pain statements at baseline. People who reported either ‘moderate’ or ‘extreme pain’ were considered together as the ‘pain group’; the remainder were assigned to a ‘no-pain comparator group’.
Analysis of variance (ANOVA) was used to compare the mean baseline PHQ-9 scores between the pain group and the no-pain comparator group. For the pain group alone, a Kendall’s tau correlation was used to test the association between the baseline scores of the PHQ-9 and SF-36 bodily pain score. A series of regression models was applied to determine the influence of baseline pain and other demographic variables on the PHQ-9 depression outcome at 3 months, the primary end point of the trial. To take into account the additional impact of other demographic predictors of PHQ-9 depression at 3 months, a systematic univariate analysis of demographic variables was conducted on the BDI-II depression items and the five EQ-5D items while controlling for PHQ-9 scores at baseline. For this scoping exercise, the level of significance was set at p < 0.1 to maintain consistency with the analysis within the main ACUDep trial. The variables identified by univariate analysis were then included in a linear regression model (p < 0.05); the odds ratios and 95% CIs of the variables identified by the regression models are presented within the results section.
Using any remaining significant predictors and controlling for baseline PHQ-9 depression scores, ANCOVA was used to test if baseline pain affected treatment outcomes measured by the PHQ-9 at 3 months. An interaction term between treatment and pain group in this model was used to establish whether or not patients in pain responded differently to the treatments with regard to their depression from patients reporting no pain. Also, using any remaining significant predictors and controlling for baseline PHQ-9 depression scores, ANCOVA was used to test whether or not baseline pain affected treatment outcome measured by the number of depression-free days at 3 months; an approximate summary measure was derived from PHQ-9 cut-off scores averaged over the period between measurements. 322
An ANCOVA model controlling for baseline depression and baseline SF-36 bodily pain was used to assess the impact of treatment for depression on the bodily pain scores at 3 months. Descriptive analysis of the depression and pain scores was conducted over the 12-month follow-up period. Finally, a comparison of the adverse events reported between baseline and 12 months’ follow-up was conducted using proportions and odds ratios.
Methods for substudy 3: approaches that practitioners used to enhance longer-term benefits
The aim of this qualitative substudy was to explore practitioners’ experiences of providing treatment within the trial and to specifically identify the strategies that practitioners reported using to promote longer-term benefits for their patients.
Nested within the trial, this substudy involved in-depth interviews and focus groups with acupuncturists and counsellors who provided treatments to at least two patients within the trial. Our aim was to recruit up to 30 counsellors and acupuncturists (approximately 15 in each group). All participants provided written consent. The interviews and focus groups were informed by topic guides, which were developed by the research team to address the substudy aims. Along with questions, the topic guides also included prompts.
The interviews and focus group were conducted by two experienced health services researchers from the research team who were not involved in the conduct of the trial itself (Liz Newbronner and Ruth Chamberlain). The interviews commonly lasted around 45 minutes (range 40–90 minutes) and the focus group 90 minutes. They were all audio-recorded (with participants’ permission) and transcribed verbatim.
The transcripts were analysed thematically. 318 A framework approach facilitated the thematic content analysis, which involved analysing the data across the two groups of practitioners contributing to the study. 323 An inductive process was used to identify themes, drawing on shared experiences or points of difference identified from the data. A full report of the methods has been published separately. 324
Methods for substudy 4: cost-effectiveness analysis
The main outcome of the cost-effectiveness analysis was the cost per QALY, which takes into account the treatment differences in HRQoL and mortality. HRQoL was measured using the EQ-5D325 at baseline and at months 3, 6, 9 and 12. The cost perspective of the NHS was used, although out-of-pocket expenses were also identified. Using patient questionnaires in the trial, resource use data were collected at 3, 6, 9 and 12 months. The sum of the resource use collected during each 3-month period was used to determine the total annual resource use. The total annual cost was calculated by multiplying the total annual resource use by the unit costs using publicly available 2012 national unit costs (see Appendix 6). We used the costs of acupuncture as estimated previously326 and the average of the ranges reported by NHS Choices:327 £47.50 for an initial session and £37.50 for subsequent sessions. The costs of counselling were those currently used in the NHS: £65 per hour of client contact. 291
Multiple imputation methods were used to manage the uncertainty caused by the missing data. Chained imputation using predictive mean matching was undertaken using resource use data, PHQ-9 and BDI scores, QALYs and patient characteristics such as age, sex and education. EQ-5D data were analysed using ordered logit models on each of the five dimensions of the instrument. Analysis at 3 months controlled for the baseline response and analysis over 12 months used random-effects models and controlled for the baseline response and the timing of each response (i.e. the day from randomisation). HRQoL weights on the 0 (equivalent to death) to 1 (full health) scale, including negative values (health states worse than death), were calculated using an independent pre-defined algorithm obtained by the elicitation of societal preferences for the health dimensions in a random population sample through a time trade-off technique. 328 QALYs were calculated by applying an individual’s HRQoL weights and the time between EQ-5D measures using the AUC approach. 329 For all cost-effectiveness analyses, seemingly unrelated regressions were used to account for the correlation between costs and QALYs. 330 QALYs were regressed on baseline HRQoL and treatment arm, and costs were regressed on the treatment arm only. We estimated ICERs using fully incremental analysis.
Using total costs we calculated base-case results taking into account the uncertainty from the multiple imputation and the seemingly unrelated regression. Probabilistic sensitivity analysis was used to reflect uncertainty in mean total costs and QALYs, and we estimated the probability of cost-effectiveness conditional on alternative cost-effectiveness thresholds. Further exploratory scenario analyses were undertaken to understand the influence on cost-effectiveness of (1) the differential cost of the acupuncture and counselling interventions, (2) depression-related resource use, (3) the complete case and (4) a population for whom acupuncture is not appropriate or unavailable. All analyses considered the published NICE cost-effectiveness thresholds of £20,000 and £30,000 per QALY gained. 224
Results
Participants
We exceed our recruitment target of 640, recruiting 755 patients in total. Patients registered with 27 general medical practices were recruited between December 2009 and April 2011 in Yorkshire and north-east England. These practices recruited an average of 28 patients (range 0–122 patients). The baseline characteristics of patients were balanced between the trial arms and are presented in Table 34. Notably, baseline expectations of treatment effectiveness were lowest for acupuncture yet, by contrast, a majority (58%) expressed a baseline preference to be allocated to acupuncture. These baseline variations were taken into account in the linear mixed-model analysis. Comparison of baseline data between patients with and without missing data at 3 months is presented in Appendix 6. Figure 26 presents the patient flow in the trial.
| Characteristic | Intervention | Total (N = 755) | ||
|---|---|---|---|---|
| Acupuncture + usual care (N = 302) | Counselling + usual care (N = 302) | Usual care (N = 151) | ||
| Age (years) | ||||
| Mean (SD) | 43.4 (13.24) | 43.5 (13.26) | 43.5 (13.93) | 43.5 (13.37) |
| Median (min., max.) | 43 (18, 86) | 43 (18, 93) | 42 (18, 89) | 43 (18, 93) |
| Interquartile range | 34–52 | 33–52 | 32–54 | 33–53 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sex, n (%) | ||||
| Male | 88 (29.1) | 69 (22.8) | 44 (29.1) | 201 (26.6) |
| Female | 214 (70.9) | 233 (77.2) | 107 (70.9) | 554 (73.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age left education (years) | ||||
| Mean (SD) | 18.0 (4.69) | 18.0 (3.92) | 18.1 (4.62) | 18.0 (4.37) |
| Median (min., max.) | 16 (13, 54) | 16 (14, 48) | 16 (14, 54) | 16 (13, 54) |
| Interquartile range | 16–18 | 16–19 | 16–19 | 16–19 |
| Missing, n (%) | 17 (5.6) | 7 (2.3) | 7 (4.6) | 31 (4.1) |
| Employment, n (%) | ||||
| Full-time education | 13 (4.4) | 5 (1.7) | 5 (3.3) | 23 (3.1) |
| Working full-time | 112 (38.0) | 107 (36.4) | 62 (41.3) | 281 (38.0) |
| Working part-time | 57 (19.3) | 59 (20.1) | 28 (18.7) | 144 (19.5) |
| Unable to work | 38 (12.9) | 42 (14.3) | 15 (10.0) | 95 (12.9) |
| Looking after home | 37 (12.5) | 32 (10.9) | 14 (9.3) | 83 (11.2) |
| Retired | 23 (7.8) | 30 (10.2) | 12 (8.0) | 65 (8.8) |
| Other | 15 (5.1) | 19 (6.5) | 14 (9.3) | 48 (6.5) |
| Missing | 7 (2.3) | 8 (2.6) | 1 (0.7) | 16 (2.1) |
| Depression, n (%) | ||||
| In last 2 weeks | 224 (75.7) | 235 (78.6) | 115 (77.7) | 574 (77.3) |
| Missing | 6 (2.0) | 3 (1.0) | 3 (2.0) | 12 (1.6) |
| Not first major episode | 196 (89.5) | 217 (93.5) | 100 (87.7) | 513 (90.8) |
| Missing | 5 (2.2) | 3 (1.3) | 1 (0.7) | 9 (1.6) |
| Four or more previous episodes | 143 (73.0) | 165 (76.7) | 81 (82.7) | 389 (76.4) |
| Missing | 0 (0.0) | 2 (0.9) | 2 (2.0) | 4 (0.8) |
| Age at first major depressive episode (years) | ||||
| Mean (SD) | 25.8 (12.69) | 24.9 (11.73) | 24.4 (12.55) | 25.2 (12.28) |
| Median (min., max.) | 23 (3, 79) | 22 (6, 71) | 20 (0, 78) | 22 (0, 79) |
| Interquartile range | 16–33 | 16–31 | 16–30 | 16–31 |
| Missing, n (%) | 9 (3.0) | 3 (1.0) | 4 (2.6) | 16 (2.1) |
| Medication, n (%) | ||||
| Depression medication in last 3 months | 189 (62.6) | 220 (72.8) | 110 (72.8) | 519 (68.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Analgesic medication in last 3 months | 147 (48.8) | 126 (42.3) | 86 (57.3) | 359 (47.9) |
| Missing | 1 (0.3) | 4 (1.3) | 1 (0.7) | 6 (0.8) |
| EQ-5D anxiety/depression, n (%) | ||||
| Not anxious/depressed | 8 (2.7) | 8 (2.6) | 5 (3.3) | 21 (2.8) |
| Moderately anxious/depressed | 219 (73.0) | 221 (73.2) | 114 (75.5) | 554 (73.6) |
| Extremely anxious/depressed | 73 (24.3) | 73 (24.2) | 32 (21.2) | 178 (23.6) |
| Missing | 2 (0.7) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| PHQ-9 score | ||||
| Mean (SD) | 15.3 (5.33) | 16.6 (5.27) | 16.2 (5.09) | 16.0 (5.29) |
| Median (min., max.) | 15 (3, 27) | 17 (4, 27) | 16 (5, 27) | 16 (3, 27) |
| Interquartile range | 11–19 | 13–21 | 13–20 | 12–20 |
| Missing, n (%) | 1 (0.33) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| PHQ-9 group, n (%) | ||||
| None (0–4) | 4 (1.3) | 2 (0.7) | 0 (0.0) | 6 (0.8) |
| Mild (5–9) | 44 (14.6) | 29 (9.6) | 14 (9.3) | 87 (11.5) |
| Moderate (10–14) | 97 (32.2) | 74 (24.5) | 46 (30.5) | 217 (28.7) |
| Moderately severe (15–19) | 88 (29.2) | 96 (31.8) | 47 (31.1) | 231 (30.6) |
| Severe (20–27) | 68 (22.6) | 101 (33.4) | 44 (29.1) | 213 (28.2) |
| Missing | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| BDI-II score | ||||
| Mean (SD) | 32.0 (8.54) | 33.3 (9.11) | 31.8 (8.17) | 32.5 (8.72) |
| Median (min., max.) | 31 (20, 57) | 32 (20, 60) | 30 (20, 56) | 31 (20, 60) |
| Interquartile range | 25–37 | 26–39 | 25–37 | 26–38 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BDI-II group, n (%) | ||||
| Moderate (20–28) | 124 (41.1) | 104 (34.4) | 56 (37.1) | 284 (37.6) |
| Severe (29–63) | 178 (58.9) | 198 (65.6) | 95 (62.9) | 471 (62.4) |
| SF-36 bodily pain | ||||
| Mean (SD) | 58.8 (27.99) | 58.0 (29.17) | 54.4 (27.83) | 57.6 (28.44) |
| Median (min., max.) | 62 (0, 100) | 62 (0, 100) | 51 (0, 100) | 52 (0, 100) |
| Interquartile range | 41–84 | 31–84 | 31–74 | 32–84 |
| Missing, n (%) | 1 (0.3) | 3 (1.0) | 0 (0.0) | 4 (0.5) |
| Expectation acupuncture, n (%) | ||||
| Very ineffective | 10 (3.3) | 6 (2.0) | 1 (0.7) | 17 (2.3) |
| Fairly ineffective | 9 (3.0) | 11 (3.7) | 6 (4.0) | 26 (3.5) |
| Can’t decide | 187 (61.9) | 204 (68.0) | 103 (68.2) | 494 (65.6) |
| Fairly effective | 66 (21.9) | 44 (14.7) | 29 (19.2) | 139 (18.5) |
| Very effective | 30 (9.9) | 35 (11.7) | 12 (7.9) | 77 (10.2) |
| Missing | 0 (0.0) | 2 (0.7) | 0 (0.0) | 2 (0.3) |
| Expectation counselling, n (%) | ||||
| Very ineffective | 23 (7.7) | 18 (6.0) | 15 (9.9) | 56 (7.5) |
| Fairly ineffective | 50 (16.7) | 43 (14.3) | 21 (13.9) | 114 (15.2) |
| Can’t decide | 101 (33.8) | 100 (33.2) | 39 (25.8) | 240 (32.0) |
| Fairly effective | 95 (31.8) | 97 (32.2) | 65 (43.0) | 257 (34.2) |
| Very effective | 30 (10.0) | 43 (14.3) | 11 (7.3) | 84 (11.2) |
| Missing | 3 (1.0) | 1 (0.3) | 0 (0.0) | 4 (0.5) |
| Expectation of usual care, n (%) | ||||
| Very ineffective | 29 (9.6) | 34 (11.3) | 6 (4.1) | 69 (9.2) |
| Fairly ineffective | 60 (19.9) | 62 (20.7) | 44 (29.7) | 166 (22.1) |
| Can’t decide | 108 (35.8) | 92 (30.7) | 50 (33.8) | 250 (33.3) |
| Fairly effective | 95 (31.5) | 97 (32.3) | 46 (31.1) | 238 (31.7) |
| Very effective | 10 (3.3) | 15 (5.0) | 2 (1.4) | 27 (3.6) |
| Missing | 0 (0.0) | 2 (0.7) | 3 (2.0) | 5 (0.7) |
| Expectation of actual treatment randomised to, n (%) | ||||
| Very ineffective | 10 (3.3) | 18 (6.0) | 6 (4.1) | 34 (4.5) |
| Fairly ineffective | 9 (3.0) | 43 (14.3) | 44 (29.7) | 96 (12.8) |
| Can’t decide | 187 (61.9) | 100 (33.2) | 50 (33.8) | 337 (44.9) |
| Fairly effective | 66 (21.9) | 97 (32.2) | 46 (31.1) | 209 (27.8) |
| Very effective | 30 (9.9) | 43 (14.3) | 2 (1.4) | 75 (10.0) |
| Missing | 0 (0.0) | 1 (0.3) | 3 (2.0) | 4 (0.5) |
| Treatment preference, n (%) | ||||
| Acupuncture | 177 (58.8) | 171 (57.6) | 82 (54.7) | 430 (57.5) |
| Counselling | 55 (18.3) | 75 (25.3) | 34 (22.7) | 164 (21.9) |
| Usual care | 2 (0.7) | 7 (2.4) | 1 (0.7) | 10 (1.3) |
| No preference | 67 (22.3) | 44 (14.8) | 33 (22.0) | 144 (19.3) |
| Missing | 1 (0.3) | 5 (1.7) | 1 (0.7) | 7 (0.9) |
| Treatment concordance, n (%) | ||||
| Randomised to preferred treatment | 177 (58.8) | 75 (25.3) | 1 (0.7) | 253 (33.8) |
| Randomised to non-preferred treatment | 57 (18.9) | 178 (59.9) | 116 (77.3) | 351 (46.9) |
| No preference | 67 (22.3) | 44 (14.8) | 33 (22.0) | 144 (19.3) |
| Missing | 1 (0.3) | 5 (1.7) | 1 (0.7) | 7 (0.9) |
FIGURE 26.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. HIV, human immunodeficiency virus.

Interventions
Of the patients allocated to acupuncture, 266 (88.1%) received one or more treatment sessions (mean 10.3, SD 3.14 treatment sessions) with one of 23 acupuncturists. The average number of sessions received at 3 months was 8.7 (SD 3.34), with 133 patients (50.0%) having completed all of their sessions. Of the patients allocated to counselling, 231 (76.5%) received one or more treatment sessions (mean 9.0, SD 3.74 treatment sessions) with one of 37 therapists. The average number of sessions received at 3 months was 7.5 (SD 3.60), with 114 patients (49.4%) having completed all of their sessions. The mean time from randomisation to last treatment was 117 days in both treatment arms (SD 47.0 and 51.2 days, respectively), a period that included time to first appointment. An average of 13 patients were allocated to each acupuncturist (range 2–45 patients) and an average of eight patients were allocated to each counsellor (range 1–27 patients).
We collected data on acupuncturists’ and counsellors’ self-reports of intervention protocol violations in logbooks. There were reports of four cases of violation of the acupuncture protocol (which incidentally did not involve counselling), one of which was deemed to be a true case involving prescription of a herbal lotion application to reduce swelling and pain. There were seven reports of violation of the counselling protocol, of which two were deemed to be true cases; one case involved goal setting and in another the counsellor reported being analytical and interpretative beyond the scope of humanistic counselling.
Details of all three interventions are provided in Appendix 6, with usual care documented in the following categories: patients seeing health professionals; patients attending hospital accident and emergency departments; patients admitted to hospital; patients paying for private health care. Medication use is detailed later in this chapter. A more complete report on acupuncture provision has been published331 as well as a report reflecting on experiences of providing the counselling. 332
Clinical outcomes
For the PHQ-9, unadjusted mean scores at all time points are presented in Table 35 and Figure 27. Table 36 provides the primary outcome results for between-group differences in PHQ-9 depression at 3 months using ANCOVA. With regard to missing data at 3 months, patients for whom no data were available tended to be younger with higher levels of baseline depression. Imputed data were used to take their profile into account. Compared with usual care, patients in the acupuncture arm experienced an average additional reduction in depression of –2.46 points on the PHQ-9 (95% CI –3.72 to –1.21 points; p < 0.001), an observed effect size equivalent to a Cohen’s d of –0.39 (95% CI –0.58 to –0.19). Compared with usual care, patients allocated to the counselling arm experienced an average additional reduction in depression of –1.73 points (95% CI –3.00 to –0.45 points; p = 0.008), equivalent to a Cohen’s d of –0.27 (95% CI –0.47 to –0.07). There was no statistically significant difference between acupuncture and counselling (–0.76 points on the PHQ-9, 95% CI –1.77 to 0.25 points; p = 0.41). These data are conservative as non-imputed data showed slightly larger treatment effects (see Appendix 6).
| Outcome measure | Intervention | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Acupuncture + usual care | Counselling + usual care | Usual care | ||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| PHQ-9 | ||||||||
| Baseline | 301 | 15.3 (5.33) | 302 | 16.6 (5.27) | 151 | 16.2 (5.09) | 754 | 16.0 (5.29) |
| 3 months | 249 | 9.4 (6.33) | 237 | 10.9 (6.45) | 128 | 12.7 (6.47) | 614 | 10.7 (6.51) |
| 6 months | 235 | 9.1 (6.51) | 228 | 10.1 (6.87) | 120 | 12.0 (6.85) | 583 | 10.1 (6.80) |
| 9 months | 234 | 9.7 (6.90) | 215 | 10.1 (7.03) | 120 | 11.9 (7.04) | 569 | 10.3 (7.02) |
| 12 months | 233 | 9.3 (6.68) | 220 | 10.1 (6.86) | 119 | 11.5 (6.98) | 572 | 10.1 (6.85) |
| BDI-II | ||||||||
| Baseline | 302 | 32.0 (8.54) | 302 | 33.3 (9.11) | 151 | 31.8 (8.17) | 755 | 32.5 (8.72) |
| 12 months | 226 | 20.4 (13.19) | 211 | 21.4 (13.64) | 151 | 23.8 (12.63) | 588 | 21.4 (13.29) |
FIGURE 27.
Unadjusted PHQ-9 mean scores from baseline to 12 months.
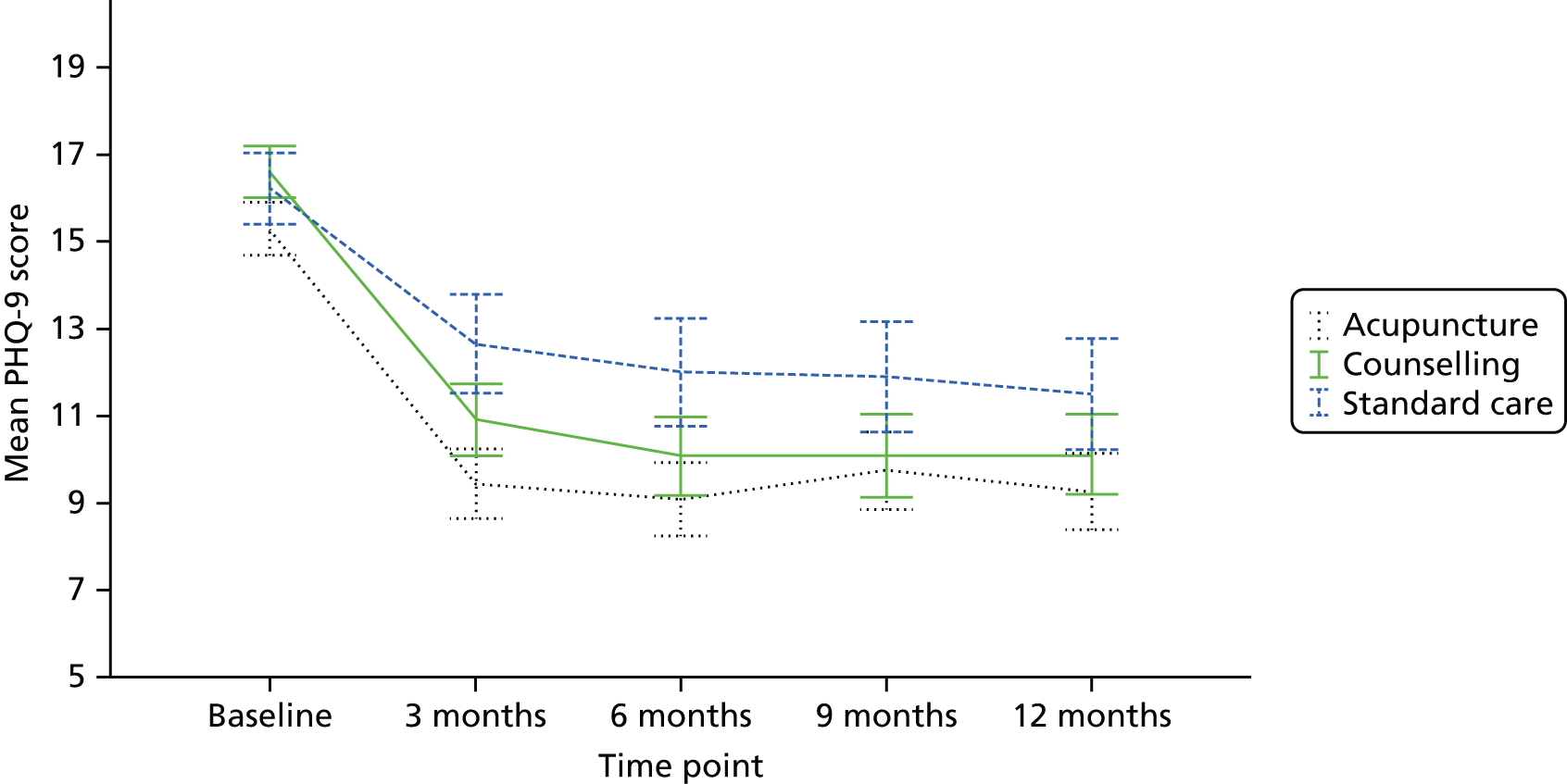
| Analysis | Month | n | Group | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | |||||||||
| Mean | SE | Mean | SE | Mean | SE | 95% CI | p-value | |||
| Acupuncture | Usual care | |||||||||
| ANCOVAa | 3 | 452 | 9.8 | 0.41 | 12.3 | 0.58 | –2.46 | 0.636 | –3.72 to –1.21 | < 0.001 |
| Mixedb | 3 | 372 | 9.8 | 0.27 | 12.1 | 0.38 | –2.29 | 0.475 | –3.22 to –1.36 | – |
| 6 | 350 | 9.6 | 0.33 | 11.5 | 0.46 | –1.90 | 0.569 | –3.02 to –0.79 | – | |
| 9 | 348 | 10.2 | 0.39 | 11.1 | 0.54 | –0.83 | 0.671 | –2.15 to 0.49 | – | |
| 12 | 347 | 9.7 | 0.45 | 10.7 | 0.64 | –0.99 | 0.785 | –2.53 to 0.55 | – | |
| AUCc | 3–12 | 407 | 10.9 | 0.25 | 12.5 | 0.35 | –1.55 | 0.435 | –2.41 to –0.70 | – |
| Counselling | Usual care | |||||||||
| ANCOVAa | 3 | 453 | 11.1 | 0.40 | 12.8 | 0.58 | –1.73 | 0.648 | –3.00 to –0.45 | 0.008 |
| Mixedb | 3 | 362 | 10.9 | 0.28 | 12.8 | 0.38 | –1.83 | 0.477 | –2.76 to –0.90 | – |
| 6 | 345 | 10.4 | 0.33 | 12.2 | 0.47 | –1.78 | 0.576 | –2.91 to –0.65 | – | |
| 9 | 332 | 10.5 | 0.40 | 11.8 | 0.55 | –1.26 | 0.688 | –2.61 to 0.08 | – | |
| 12 | 336 | 10.4 | 0.47 | 11.4 | 0.65 | –1.00 | 0.805 | –2.58 to 0.57 | – | |
| AUCc | 3–12 | 402 | 11.6 | 0.28 | 13.1 | 0.38 | –1.50 | 0.470 | –2.43 to –0.58 | – |
| Acupuncture | Counselling | |||||||||
| ANCOVAa | 3 | 603 | 10.0 | 0.41 | 10.8 | 0.40 | –0.76 | 0.514 | –1.77 to 0.25 | 0.140 |
| Mixedb | 3 | 402 | 9.5 | 0.29 | 9.4 | 0.32 | 0.11 | 0.439 | –0.75 to 0.97 | – |
| 6 | 371 | 9.6 | 0.35 | 9.1 | 0.38 | 0.45 | 0.527 | –0.58 to 1.49 | – | |
| 9 | 360 | 10.0 | 0.41 | 9.0 | 0.45 | 0.97 | 0.621 | –0.25 to 2.19 | – | |
| 12 | 361 | 9.6 | 0.48 | 9.0 | 0.53 | 0.59 | 0.721 | –0.82 to 2.01 | – | |
| AUCc | 3–12 | 531 | 11.1 | 0.27 | 11.2 | 0.27 | –0.06 | 0.378 | –0.81 to 0.68 | – |
In the AUC analysis over the 12-month period, the statistically significant benefit of acupuncture and counselling over usual care alone in terms of PHQ-9 score reduction seen at 3 months remained: acupuncture reduced the PHQ-9 score by –1.55 (95% CI –2.41 to –0.70) and counselling by –1.50 (95% CI –2.43 to –0.58) (see Table 36).
In a secondary analysis exploring potential mediators, PHQ-9 scores at 3 months were found to be associated with two factors: expectations of counselling (p = 0.064) and expectations about the treatment that patients were allocated to (p = 0.015). These factors were included as potential mediators of the effect of trial arm in further analyses. Related to the comparison of the acupuncture and counselling arms, additional significant factors were total session time at 3 months (p < 0.001) and perceived empathy of practitioners (p < 0.001), which were added as further covariates.
From the linear mixed modelling, PHQ-9 depression scores in the acupuncture and counselling groups were reduced compared with usual care at 3 and 6 months (see Table 36). The scores in the usual care group continued to reduce over time, such that differences were no longer statistically significant at 9 and 12 months. There was no evidence of significant differences between acupuncture and counselling throughout.
Using ANCOVA, depression scores on the BDI-II were found to be reduced in the acupuncture arm (–2.88, 95% CI –5.68 to –0.08) and the counselling arm (–2.74, 95% CI –5.50 to 0.02) to a greater extent than by usual care alone at 12 months, but with no statistically significant difference between acupuncture and counselling (Table 37).
| Analysis | Month | n | Group | Group difference | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||||
| Mean | SE | Mean | SE | Mean | SE | 95% CI | |||
| Acupuncture | Usual care | ||||||||
| ANCOVAa | 12 | 445 | 22.8 | 1.34 | 25.7 | 1.82 | –2.88 | 1.419 | –5.68 to –0.08 |
| Counselling | Usual care | ||||||||
| ANCOVAa | 12 | 449 | 22.7 | 1.47 | 25.4 | 1.74 | –2.74 | 1.399 | –5.50 to 0.02 |
| Acupuncture | Counselling | ||||||||
| ANCOVAa | 12 | 401 | 22.5 | 0.92 | 21.9 | 1.02 | 0.59 | 1.281 | –1.93 to 3.11 |
Prescribed medication
Among all patients, the majority (68.7%) were taking antidepressants at baseline (Table 38) and prescribed antidepressant utilisation decreased steadily by an average of 12% over the 12-month study period, a rate comparable between trial arms. Around half of patients (47.9%) were taking analgesics at baseline, which decreased on average to 41.0% over 12 months. Patients in the acupuncture arm showed a marked decrease in the use of analgesics in the first 3 months.
| Prescribed medication | Intervention | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture + usual care | Counselling + usual care | Usual care | ||||||||||
| n | %a | Change from baseline (%) | n | %a | Change from baseline (%) | n | %a | Change from baseline (%) | n | %a | Change from baseline (%) | |
| Antidepressants | ||||||||||||
| Baseline | 189 | 62.6 | – | 220 | 72.9 | – | 110 | 72.9 | – | 519 | 68.7 | – |
| 3 months | 147 | 60.7 | –0.9 | 155 | 69.2 | –3.7 | 80 | 66.7 | –6.2 | 382 | 65.2 | –3.5 |
| 6 months | 126 | 54.6 | –8.0 | 145 | 65.3 | –7.6 | 75 | 63.6 | –9.3 | 346 | 60.6 | –8.1 |
| 9 months | 123 | 54.0 | –8.6 | 124 | 60.8 | –12.1 | 74 | 66.1 | –6.8 | 321 | 59.0 | –9.7 |
| 12 months | 121 | 52.4 | –10.2 | 124 | 58.2 | –14.7 | 69 | 61.6 | –11.3 | 314 | 56.5 | –12.2 |
| Analgesics | ||||||||||||
| Baseline | 147 | 48.8 | – | 126 | 42.3 | – | 86 | 57.3 | – | 359 | 47.9 | – |
| 3 months | 73 | 30.8 | –18.0 | 93 | 42.5 | 0.2 | 57 | 48.3 | –9.0 | 223 | 38.9 | –9.0 |
| 6 months | 99 | 43.4 | –5.4 | 84 | 38.5 | –3.8 | 52 | 46.4 | –10.9 | 235 | 42.1 | –5.8 |
| 9 months | 82 | 36.8 | –12.0 | 71 | 34.8 | –7.5 | 55 | 50.0 | –7.3 | 208 | 38.7 | –9.2 |
| 12 months | 84 | 36.8 | –12.0 | 87 | 41.0 | –1.3 | 54 | 49.5 | –7.8 | 225 | 41.0 | –6.9 |
Adverse events
Over the 12 months, the number of patients experiencing a serious adverse event, as judged by a clinician (IW), was 16 out of 302 (5.3%) in the acupuncture group, 26 out of 302 (8.6%) in the counselling group and nine out of 151 (6.0%) in the usual care group, of whom nine had more than one serious adverse event (range 2–4). None of the serious adverse events, including three deaths, was known to be related to treatment. Over the 12 months, the number of patients experiencing a non-serious adverse event was 56 (18.5%), 47 (15.6%) and 40 (26.5%), respectively, of whom 17 had more than one non-serious adverse event (range 2–4).
Results for substudy 1: experience of treatment from the patient perspective
Of the 61 participants invited, four declined participation and three did not respond; in addition, two recordings failed for technical reasons. In total, 52 participants, 24 men and 28 women, with an age range of 22–89 years (mean 46 years, SD 13.8 years), were interviewed. At baseline, 26 of these participants had reported having moderate or extreme pain or discomfort on the EQ-5D questionnaire; these people formed the pain group, with the remainder forming the no-pain comparator group. As part of the ACUDep trial, 22 of the 52 had been randomised to receive acupuncture, 20 had been randomised to receive counselling and 10 had been randomised to receive usual care alone. A summary is presented in the sampling frame in Appendix 6. On average, those allocated to acupuncture attended 11 sessions (range 4–12 sessions) and those allocated to counselling attended 10 sessions (range 6–12 sessions).
Participants’ experiences of depression varied considerably between those with comorbid pain and those with depression alone. Those participants with depression and comorbid pain commonly experienced a number of other physical symptoms concurrently such as fatigue, low energy and sleep problems. For some this meant withdrawing from social and day-to-day activities. This reduced ability to engage in social activity was one of the factors that led to this group having less in the way of internal and external resources available to manage their depression effectively. The majority in the no-pain comparator group were in full- or part-time employment, or were relatively affluent retired professional people. For many, their experience of depression concerned feelings of low self-esteem brought about by high expectations of themselves within their working life or hectic social schedules.
Those with physical symptoms who were receiving acupuncture commonly reported that these broader symptoms were usually addressed as part of the treatment. As treatment progressed, many participants reported that their acupuncturist began guiding them to make changes to their lifestyle to engender beneficial long-term outcomes. For most people with pain, fear of pain and potential injury posed a barrier to engaging in physical activity. Nevertheless, the majority of the pain group reported being encouraged to take up gentle exercise for their overall health. The advice given to the no-pain comparator group more often focused on dietary change and a reduction in caffeine and alcohol intake, as well as encouragement of some form of relaxation. Overall, longer-term improvement in depression was developed through the participants’ active engagement in health-promoting behaviours, supported by a positive therapeutic relationship.
For those receiving counselling there was less emphasis on physical symptoms and more on help with gaining an understanding of themselves and their situation. Despite many having low expectations of counselling based on their previous experiences, the majority of participants engaged with the counselling process. Most reported feeling relieved to have someone to talk to in confidence. For both the pain and the no-pain groups, the process of change followed a common pathway, beginning with the participants’ disclosure of personal information and being listened to and leading to an exploration of their past, which helped to clarify their understanding of themselves and their situation. The final stage in the process of change for many was directed towards enabling them to maintain their progress and recovery independently.
In summary, longer-term change was encouraged by most practitioners over the course of treatment. This was facilitated by strong support to cope with depression and pain independently of treatment, with a focus on relevant lifestyle and behaviour changes. Participants identified as important components of treatment the therapeutic relationship with their practitioner and their active engagement in the treatment process, whether they were receiving acupuncture or counselling, A full report on the experiences of treatment has been published separately. 320
Results for substudy 2: impact of comorbid pain on depression outcomes
Patients reporting moderate and extreme pain or discomfort on the EQ-5D questionnaire were merged together to form a single pain group (n = 384, 51%) with the remainder forming the no-pain comparator group (n = 371, 49%). A summary of the demographic variables in the subgroups allocated to the pain and no-pain groups at baseline is presented in Appendix 6. The variables were comparable for most items with the following notable exceptions: (1) the pain group members tended to be older than the no-pain comparator group members (mean 47 years vs. 40 years); and (2) in terms of health and employment, 56% of the pain group (vs. 9% of the no-pain group) reported a painful health condition or illness that predated the onset of depression, for which 64% (vs. 31%) used analgesic medication regularly, and 32% (vs. 9%) were unable to work or were retired.
The baseline PHQ-9 depression scores indicate that the pain group reported higher levels of depression at baseline (mean 17.0, SD 5.2) than the no-pain comparator group (mean 14.9, SD 5.2). Results of the ANOVA confirmed the difference to be highly significant (mean difference 2.02, 95% CI 1.28 to 2.76). For the pain group alone, the correlation between the PHQ-9 scores and SF-36 bodily pain scores was weak but highly significant (Kendall’s tau –0.172; p < 0.001).
Using the average across all treatment groups, participants in the pain group showed a smaller reduction in depression score at 3 months compared with baseline (mean reduction from 16.70 to 12.06 at 3 months) than the no-pain comparator group (mean reduction from 14.06 to 9.10 at 3 months). A linear regression model found that the presence of moderate or extreme pain at baseline predicted a poorer outcome of depression treatment at 3 months (mean difference –1.72, 95% CI –2.64 to –0.80; p < 0.001) when controlling for baseline depression scores. An ANCOVA model controlling for significant predictors and baseline PHQ-9 score revealed that the effect of pain group remained significant, with patients with baseline pain having poorer depression outcomes (mean difference –1.16, 95% CI –2.2 to –0.12; p = 0.028).
Regarding PHQ-9 depression scores by pain group and by trial arm at baseline and all follow-up time points, relevant data are shown in Figure 28. Controlling for baseline depression and covariates, an ANCOVA model including a pain group–treatment interaction term showed that, in the pain group, participants showed a larger reduction in depression with acupuncture at 3 months (mean reduction in PHQ-9 score from baseline 6.0, 95% CI 5.07 to 7.11), with smaller reductions associated with counselling (mean reduction 4.3, 95% CI 3.3 to 5.4) and usual care (mean reduction 2.7, 95% CI 1.50 to 4.06). In comparison, no notable differences were seen between treatment arms within the no-pain comparator group.
FIGURE 28.
Mean PHQ-9 scores by treatment, comparing the pain and no-pain groups: (a) patients with moderate to extreme pain at baseline (n = 384); and (b) patients pain free at baseline (n = 371).
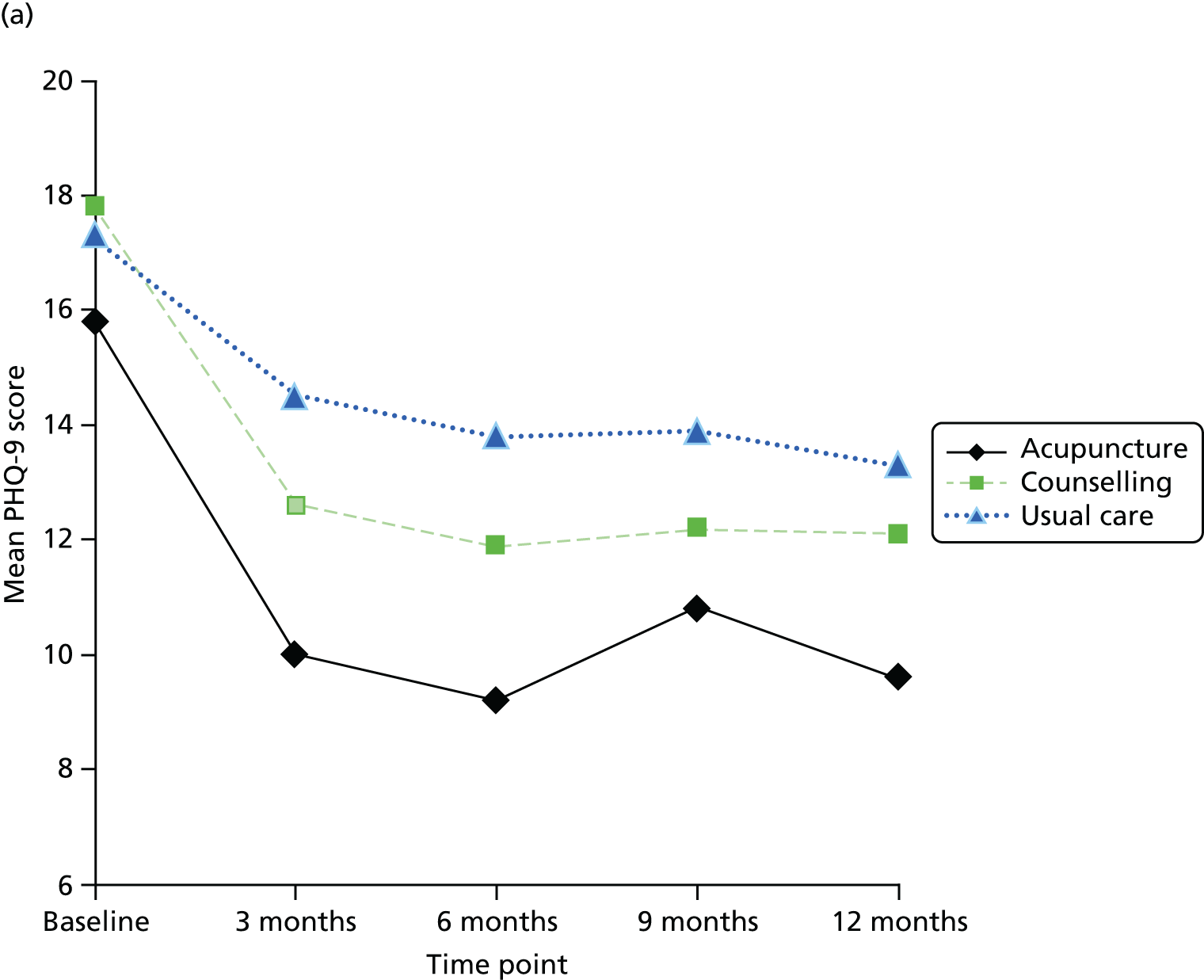
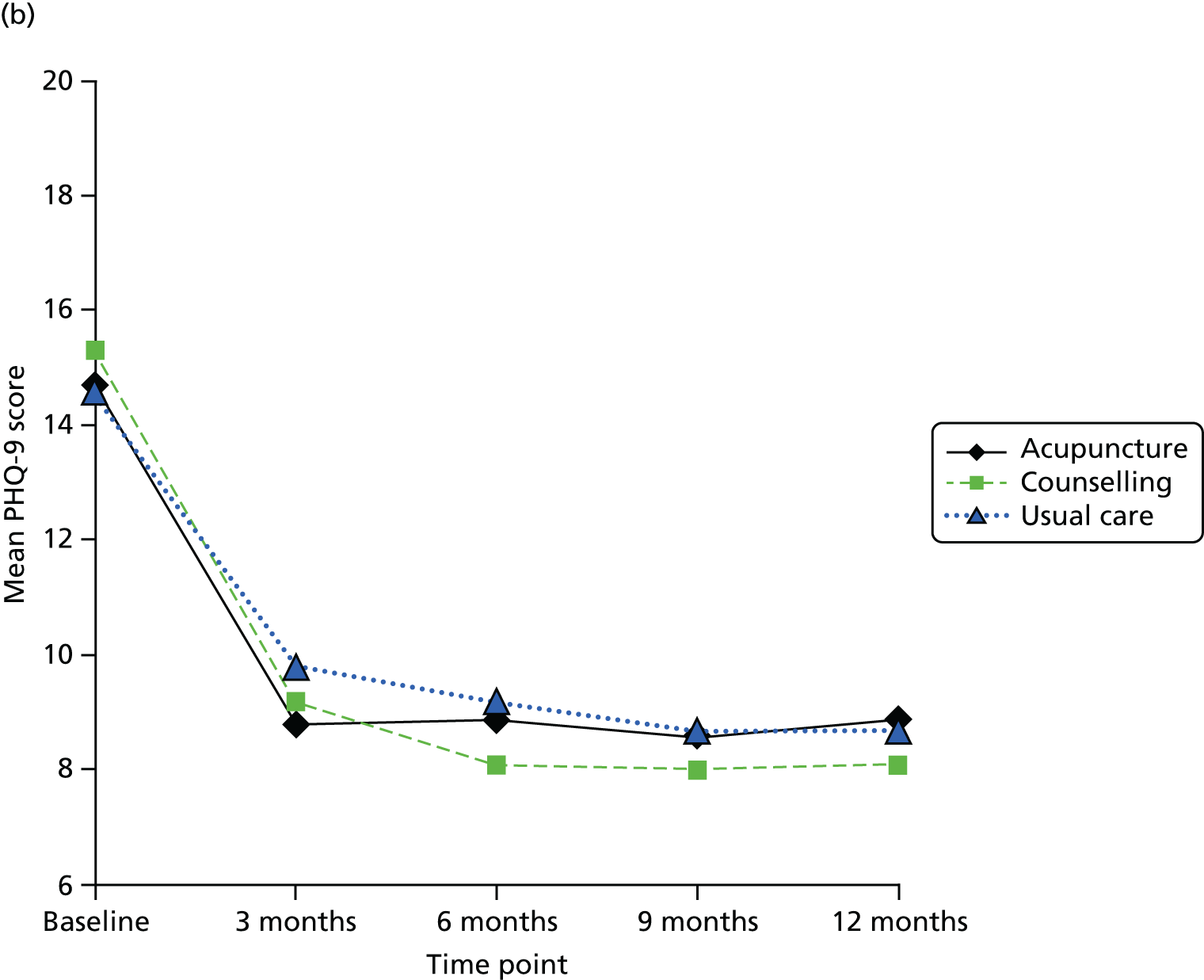
Using the SF-36 bodily pain score at 3 months’ follow-up as the end point, and controlling for baseline SF-36 bodily pain and baseline PHQ-9 depression scores, the results of the ANCOVA show that the pain group continued to experience significantly worse pain after treatment for depression compared with the no-pain comparator group (mean difference 14.57, 95% CI 9.73 to 19.40). There was also a significant interaction between pain group and treatment arm (F2,1 = 3.3; p = 0.036), with pain group patients who received acupuncture for depression experiencing a greater reduction in SF-36 bodily pain (represented by an increase in scores) between baseline and 3 months’ follow-up (mean reduction 11.2, 95% CI 7.1 to 15.2) than those who received counselling (mean reduction 7.6, 95% CI 3.6 to 11.6) or usual care (mean reduction 7.2, 95% CI 2.3 to 12.1). The reduction in pain at 3 months persisted through to the 12-month follow-up point (Figure 29); however, the median score in the pain group after 12 months (median 41, interquartile range 1 = 31, interquartile range 3 = 62) remained below the trial baseline median score of 52 on the SF-36 bodily pain scale. Further details of the results have been published separately. 321
FIGURE 29.
Mean SF-36 bodily pain scores by treatment over the course of the trial, comparing the pain and no-pain groups: (a) patients with moderate to extreme pain at baseline (n = 384); and (b) patients pain free at baseline (n = 371).
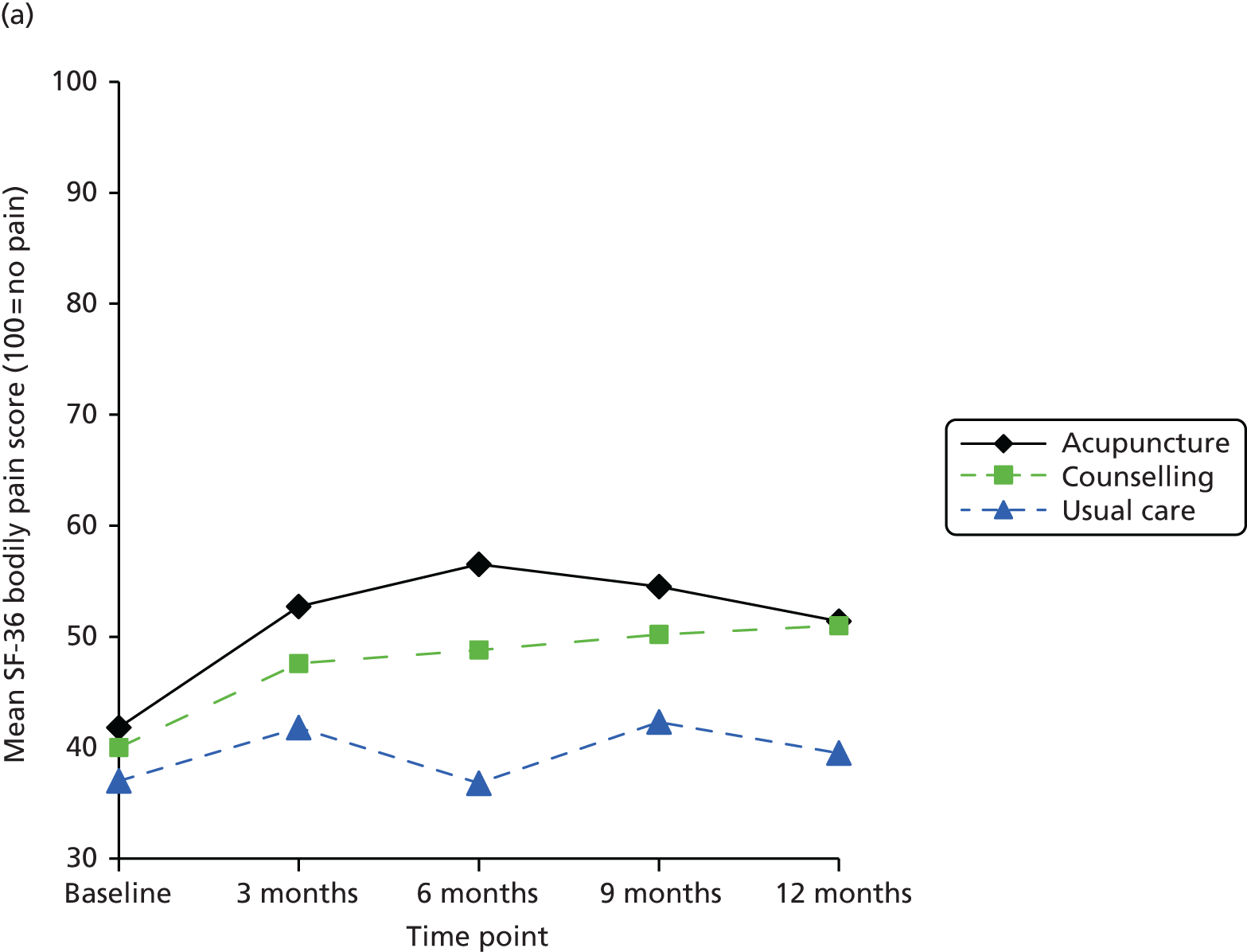
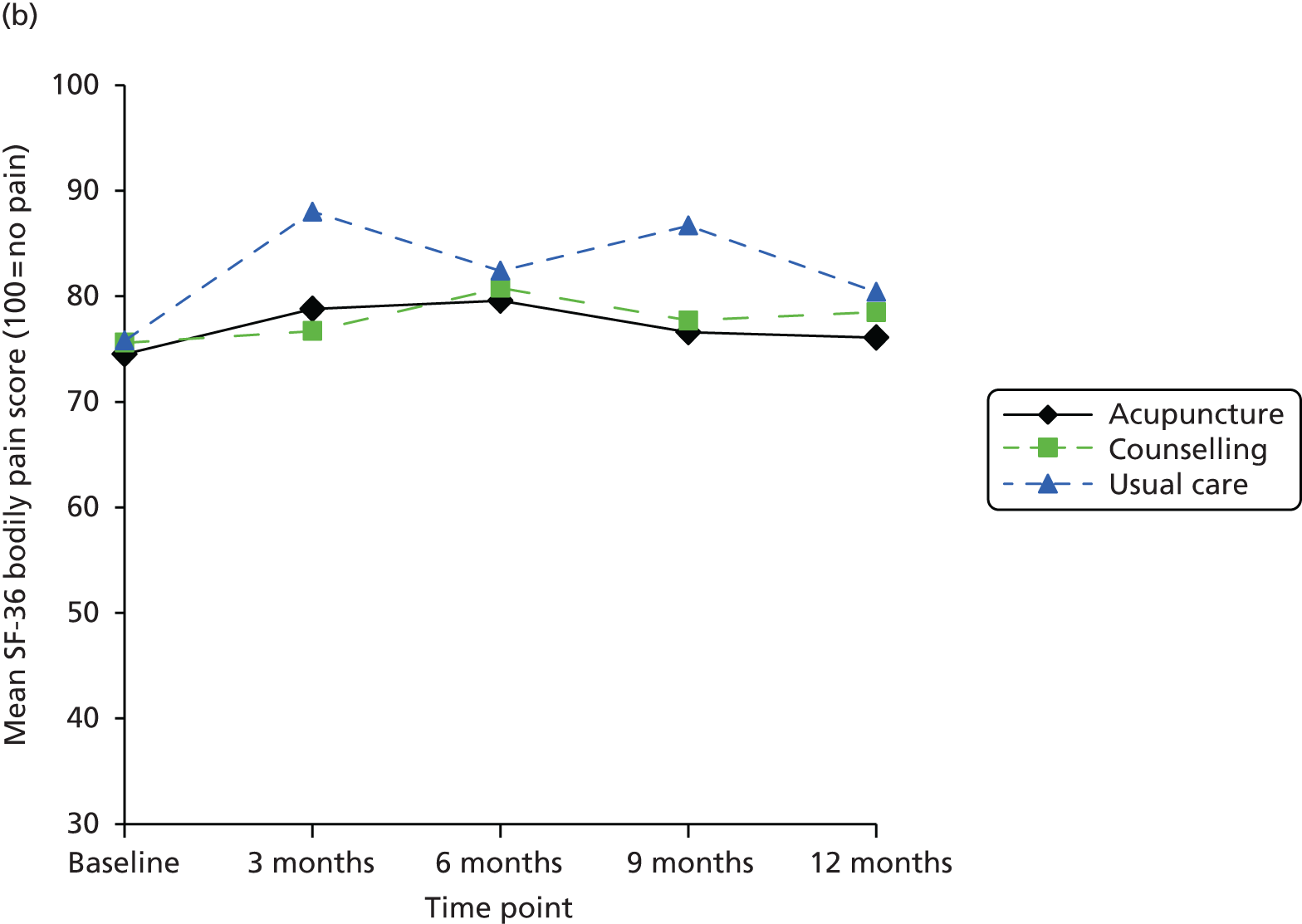
Results for substudy 3: approaches that practitioners used to enhance longer-term benefits
Forty-one therapists consented to be involved in the study but five therapists later withdrew or did not respond when the fieldwork was being set up. The substudy included individual telephone interviews with 15 counsellors and 13 acupuncturists (n = 28), and one focus group with four counsellors and four acupuncturists (n = 8) (see Appendix 6). The sample for the qualitative substudy included over half (56%) of the total number of therapists involved in the main trial. The 17 acupuncturists were predominantly male (39% were female), with an average duration of practice of 12 years. In the trial they treated on average nine patients, who attended on average for 10 sessions. The 19 counsellors were predominantly female practitioners (79%), with an average duration of practice of 7 years. In the trial they treated on average seven patients, who attended on average for nine sessions.
A cluster of eight themes emerged from the framework analysis. Almost all of the acupuncturists and counsellors stressed the importance that they attached to promoting longer-term benefits.
-
Importance of a long-term focus. Almost all of the practitioners mentioned the long-term perspective as inherent to the way they worked. For longer-term impact, both acupuncturists and counsellors encouraged insight into root causes of depression on an individual basis and saw small incremental changes as precursors to sustained benefit.
-
Identifying root causes. The commitment to identifying and addressing the root causes of depression within the treatment process was a commonly expressed factor among both acupuncturists and counsellors. Acupuncturists commonly used the theoretical concept of ‘root’ (ben) and ‘branch’ (biao). The counsellors were more interested in ‘going deeper’ and ‘further back (in time)’ with their clients as a way of getting to the root causes.
-
Individualisation. When practised within the theoretical framework of traditional Chinese medicine, acupuncture treatment has been customised to the individual, such that treatment varies not only between patients but also, for the same patient, over time. Likewise for the counsellors in the trial, all of whom were committed to working within the humanistic tradition, a person-centred approach was provided.
-
Valuing incremental change. Practitioners reported that they did not expect change to happen all at once. Rather than seeking cathartic experiences, ideally improvement in symptoms would develop slowly with small incremental changes.
-
Addressing concurrent physical symptoms. Acupuncturists generally stressed the importance of addressing concurrent physical symptoms, for example helping patients relax or sleep better to be more receptive to change. Counsellors were less focused on the physical symptoms and worked more on the assumption that by treating the underlying causes of depression there should be a knock-on effect in terms of reducing physical symptoms.
-
Lifestyle changes. Acupuncturists tended to highlight the importance of giving advice about lifestyle change that was relevant to the Chinese medicine perspective. By contrast, the counsellors had a more non-directive style, consistent with the humanistic tradition.
-
Therapeutic relationship. More often than the acupuncturists, the counsellors stressed the importance of the therapeutic relationship as a facilitator of change.
-
Careful ‘pacing’ based on ‘readiness to change’. Many of the counsellors also emphasised the need for careful ‘pacing’ such that the processes and tools that they employed were tailored and timed for each individual, depending on the ‘readiness’ to change. A number of acupuncturists reflected on the best way of sustaining benefit over the longer term, suggesting that ideally appointments should be spread out towards the end of the course of treatment and beyond.
The above themes capture both similarities and differences in approaches between acupuncture and counselling, with most practitioners of both interventions having a shared sense of the prerequisites for sustained long-term benefit. The impression formed from the interviews is that the various approaches do not operate in isolation. The themes appear to be integrated into a coherent combination that uniquely informs the practice of each practitioner, although the emphasis may vary. A full report of the results has been published separately. 324
Results for substudy 4: cost-effectiveness analysis
At 3 months patients treated with acupuncture or counselling were less likely than patients treated with usual care to report that they were moderately or extremely anxious or depressed rather than not anxious or depressed (Table 39). The 3-month improvement in anxiety and depression was sustained over the trial period to 12 months. Combining the EQ-5D dimension results with the UK population health state preferences resulted in the HRQoL scores over time and by treatment presented in Figure 30. For all treatment arms HRQoL increased between baseline and 3 months, with the acupuncture and counselling arms having a higher HRQoL than usual care and remaining higher at 12 months. QALYs were estimated to be 0.604, 0.663 and 0.666 for the usual care, acupuncture and counselling arms, respectively, using imputed data and seemingly unrelated regression and controlling for baseline HRQoL.
| EQ-5D dimension | At 3 monthsb | Over 12 monthsb | ||
|---|---|---|---|---|
| Acupuncture OR (95% CI) | Counselling OR (95% CI) | Acupuncture OR (95% CI) | Counselling OR (95% CI) | |
| Anxiety and depression | 0.63 (0.40 to 0.98) | 0.66 (0.42 to 1.02) | 0.40 (0.23 to 0.70) | 0.40 (0.23 to 0.70) |
| Pain | 0.77 (0.48 to 1.23) | 0.96 (0.60 to 1.53) | 0.87 (0.49 to 1.54) | 0.88 (0.5 to 1.55) |
| Usual activities | 1.14 (0.48 to 2.71) | 1.05 (0.44 to 2.54) | 0.57 (0.34 to 0.95) | 0.72 (0.43 to 1.21) |
| Self-care | 0.81 (0.52 to 1.27) | 0.85 (0.54 to 1.33) | 0.40 (0.15 to 1.09) | 0.58 (0.22 to 1.53) |
| Mobility | 1.29 (0.64 to 2.61) | 1.19 (0.59 to 2.41) | 0.89 (0.41 to 1.94) | 0.74 (0.35 to 1.59) |
FIGURE 30.
Health-related quality-of-life scores over time and by treatment.
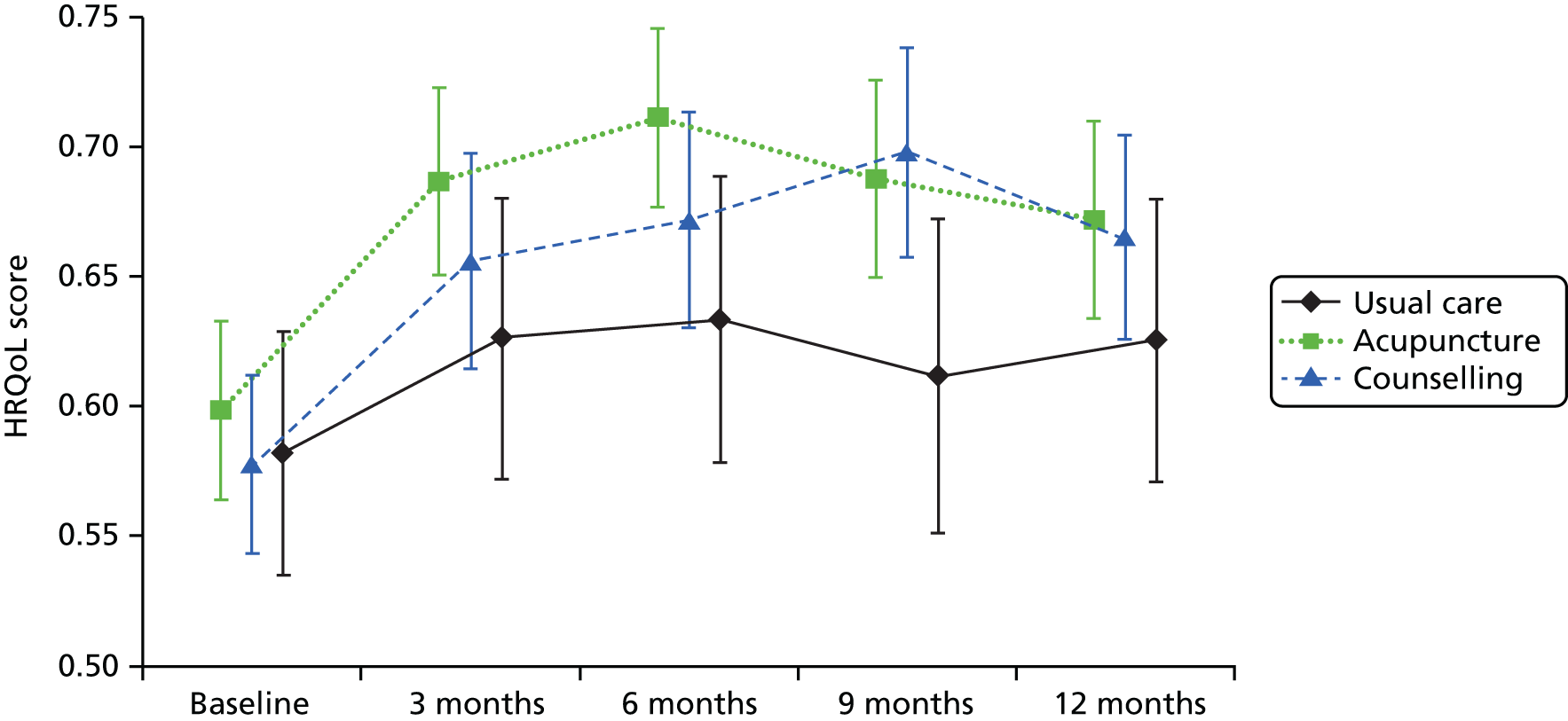
Mean NHS resource use is reported in Table 40. Total costs and depression-related costs are reported in Table 41. Patients reported the amount spent on out-of-pocket acupuncture, counselling or usual care. Patients in the acupuncture arm reported spending a mean of £32 (SD £93) on acupuncture, whereas those in the counselling and usual care arms reported spending £7 (SD £41) and £6 (SD £57), respectively. Patients in the counselling arm reported spending a mean of £42 (SD £173) on counselling, whereas those in the acupuncture and usual care arms reported spending £6 (SD £42) and £5 (SD £32), respectively. Patients spent on average £2 (SD £33), £15 (SD £87) and £3 (SD £23) on psychotherapy in the acupuncture, counselling and usual care arms, respectively. The mean number of days off work over 12 months was similar across arms: 238 (SD 115), 240 (SD 112) and 231 (SD 113) for the acupuncture, counselling and usual care arms, respectively.
| Resource | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care | Acupuncture | Counselling | |||||||
| n | Complete case mean (95% CI) | Imputed mean (95% CI) | n | Complete case mean (95% CI) | Imputed mean (95% CI) | n | Complete case mean (95% CI) | Imputed mean (95% CI) | |
| GP | 69 | 6.48 (5.16 to 7.80) | 6.56 (5.37 to 7.75) | 145 | 5.57 (4.78 to 6.37) | 5.66 (4.88 to 6.43) | 127 | 4.94 (4.19 to 5.7) | 5.06 (4.38 to 5.73) |
| Practice nurse | 60 | 1.40 (0.86 to 1.94) | 1.53 (1.05 to 2.02) | 140 | 1.16 (0.84 to 1.48) | 1.25 (0.95 to 1.54) | 127 | 1.25 (0.95 to 1.55) | 1.36 (1.05 to 1.66) |
| Other health professional | 54 | 1.39 (0.74 to 2.04) | 1.76 (0.93 to 2.6) | 133 | 1.24 (0.7 to 1.79) | 1.37 (0.85 to 1.89) | 116 | 1.4 (0.78 to 2.01) | 1.54 (1 to 2.07) |
| NHS hospital outpatient clinic | 82 | 1.55 (0.99 to 2.1) | 2.01 (1.19 to 2.83) | 175 | 1.4 (0.95 to 1.85) | 1.52 (1.05 to 1.98) | 151 | 1.69 (1.17 to 2.21) | 1.87 (1.35 to 2.39) |
| Hospital ward | 79 | 0.29 (–0.01 to 0.60) | 0.32 (0.04 to 0.6) | 166 | 0.15 (0.02 to 0.28) | 0.21 (0.05 to 0.38) | 138 | 0.27 (0.06 to 0.47) | 0.35 (0.12 to 0.59) |
| Hospital ICU | 76 | – | 0 (0 to 0) | 160 | 0.04 (–0.04 to 0.11) | 0.03 (–0.03 to 0.1) | 136 | – | 0 (0 to 0) |
| Hospital mental health unit | 76 | 0.53 (–0.48 to 1.53) | 0.48 (–0.34 to 1.3) | 160 | 0.04 (–0.04 to 0.13) | 0.12 (–0.18 to 0.43) | 134 | – | 0.42 (–0.13 to 0.97) |
| Other hospital unit | 75 | 0.01 (–0.01 to 0.04) | 0.02 (–0.02 to 0.07) | 152 | 0.05 (–0.04 to 0.14) | 0.04 (–0.02 to 0.09) | 132 | 0.05 (0 to 0.11) | 0.04 (0 to 0.08) |
| Accident and emergency | 84 | 0.37 (0.17 to 0.57) | 0.4 (0.15 to 0.64) | 191 | 0.21 (0.13 to 0.29) | 0.25 (0.13 to 0.37) | 157 | 0.35 (0.11 to 0.59) | 0.35 (0.16 to 0.53) |
| Community mental health nurse | 74 | 0.43 (–0.04 to 0.91) | 0.42 (0.04 to 0.79) | 164 | 0.15 (0.02 to 0.29) | 0.2 (0.04 to 0.37) | 145 | 0.1 (–0.02 to 0.23) | 0.18 (0.01 to 0.35) |
| Psychologist or psychiatrist | 72 | 0.75 (0.04 to 1.46) | 0.73 (0.16 to 1.3) | 164 | 0.21 (0.08 to 0.34) | 0.31 (0.09 to 0.54) | 135 | 0.35 (0.09 to 0.61) | 0.55 (0.22 to 0.88) |
| NHS counsellor not involved in the study | 68 | 0.34 (0.07 to 0.60) | 0.38 (0.07 to 0.68) | 161 | 0.29 (0.08 to 0.49) | 0.28 (0.11 to 0.45) | 142 | 0.17 (0.02 to 0.32) | 0.23 (0.06 to 0.4) |
| Resource | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care | Acupuncture | Counselling | |||||||
| n | Complete case mean (95% CI) | Imputed mean (95% CI) | n | Complete case mean (95% CI) | Imputed mean (95% CI) | n | Complete case mean (95% CI) | Imputed mean (95% CI) | |
| Total costs (£) | 22 | 621 (365 to 877) | 958 (739 to 1180) | 69 | 1110 (930 to 1291) | 1227 (1103 to 1350) | 59 | 1355 (1082 to 1627) | 1450 (1305 to 1592) |
| Depression-related costs (£) | 18 | 226 (92 to 360) | 496 (288 to 704) | 54 | 769 (644 to 894) | 913 (764 to 1061) | 48 | 962 (759 to 1166) | 1006 (761 to 1251) |
When comparing acupuncture, counselling and usual care, acupuncture was found to be the cost-effective alternative with an ICER of £4560 per additional QALY compared with usual care alone, with probabilities of being cost-effective of 0.68, 0.62 and 0.56 at thresholds of £13,000, £20,000 and £30,000 per QALY, respectively (Table 42). Counselling resulted in higher costs and benefits than acupuncture, with an ICER of £71,757 per additional QALY compared with acupuncture.
| Treatment | QALYs | Total costs (£) | ICER (£ per QALY) | Probability of cost-effectiveness | ||
|---|---|---|---|---|---|---|
| Threshold £13,000 per QALY | Threshold £20,000 per QALY | Threshold £30,000 per QALY | ||||
| Usual care | 0.604 | 958 | – | 0.07 | 0.03 | 0.02 |
| Acupuncture | 0.663 | 1227 | 4560 | 0.68 | 0.62 | 0.56 |
| Counselling | 0.666 | 1450 | 71,757 | 0.26 | 0.36 | 0.42 |
In a scenario analysis which assumed that each session of acupuncture was the same price as each session of counselling (£65), counselling had higher QALYs and lower costs than acupuncture, that is, acupuncture was dominated (Table 43). Restricting the analysis to the complete case data resulted in an ICER for acupuncture of £10,979 per QALY and counselling having higher costs and lower QALYs than acupuncture. For patients in whom acupuncture is inappropriate or unavailable, the incremental cost-effectiveness of counselling compared with usual care was £7935 per additional QALY.
| Scenario analysis | QALYsa | Total costs (£) | ICER (£ per QALY) | Probability of cost-effectiveness | ||
|---|---|---|---|---|---|---|
| Threshold £13,000 per QALY | Threshold £20,000 per QALY | Threshold £30,000 per QALY | ||||
| 1. Assuming acupuncture has the same cost as counselling (£65) | ||||||
| Usual care | 0.558 | 524 | – | 0.15 | 0.06 | 0.03 |
| Counselling | 0.620 | 1050 | 8497 | 0.50 | 0.55 | 0.56 |
| Acupuncture | 0.617 | 1073 | Dominated | 0.35 | 0.39 | 0.42 |
| 2. Using depression-related costs | ||||||
| Usual care | 0.601 | 513 | – | 0.08 | 0.03 | 0.02 |
| Acupuncture | 0.659 | 853 | 5819 | 0.61 | 0.58 | 0.54 |
| Counselling | 0.663 | 1025 | 50,612 | 0.32 | 0.39 | 0.44 |
| 3. Complete case analysis | ||||||
| Usual care | 0.638 | 648 | – | 0.43 | 0.29 | 0.20 |
| Acupuncture | 0.682 | 1121 | 10,979 | 0.57 | 0.70 | 0.79 |
| Counselling | 0.643 | 1378 | Dominated | 0.01 | 0.01 | 0.01 |
| 4. Population for whom acupuncture is not appropriate | ||||||
| Usual care | 0.604 | 958 | – | 0.21 | 0.09 | 0.05 |
| Counselling | 0.666 | 1450 | 7935 | 0.79 | 0.91 | 0.95 |
Discussion
Principal findings
For patients continuing to experience depression in primary care, we found statistically significant benefits at 3 months associated with both acupuncture and counselling interventions when provided as adjuncts to usual care. Prior to recruitment to the trial, these patients experienced recurring bouts of depression (76% having had four or more episodes), with the first episode on average occurring at age 25 years, some 19 years previously, and 69% were on antidepressant medication. We also found statistically significant benefits for patients over the 12-month period as a whole in an AUC analysis. At 12 months the benefits of acupuncture and counselling were no longer significantly better than those of usual care for our primary outcome measure, the PHQ-9, but statistically significant differences remained when depression was measured by the BDI-II. No serious adverse events related to treatment were reported.
Strengths and limitations
This study design of a pragmatic RCT had a clear and practical research question with an appropriate trial design to model closely what would happen if patient referrals to acupuncturists and counsellors were routine. With an emphasis on external validity, our pragmatic trial was designed to have findings that are generalisable to typical patients and settings. We recruited patients in primary care from among those who had consulted with depression and who continued to be depressed, thereby excluding patients whose symptoms had been alleviated sufficiently by other treatment.
The attrition of patient-reported data between randomisation at baseline and the follow-up time-points was typical of trials that recruit through primary care databases. We used multiple imputation to compensate in part for the limitations related to the loss of follow-up data. Our design controlled for temporal effects, such as the natural history of depression, and other factors across all patients that might have influenced outcomes beyond the treatment itself through the randomisation into groups. The standardised treatment protocols for the acupuncturists and counsellors were designed to reflect routine practice, allowing individualisation to match patient variability while ensuring that all practitioners met appropriate standards of qualification and experience. No attempt was made to standardise usual care; however, we were careful to document the usual care that was provided to patients in all arms of the trial. The majority of patients continued with antidepressants and differences between groups in usual care at all time points were minimal (see Appendix 6). For this reason we can assume that the differences in outcomes between arms can largely be ascribed to the treatments provided by the acupuncturists and the counsellors.
Our study was not designed to determine which aspects of the interventions might be most or least beneficial. In contrast to pragmatic trials, explanatory trials are designed to separate out the relative contributions of specific or non-specific components of treatment. Nevertheless, we took into account in our regression model the expectations and preferences reported by all patients in the trial, components that are often considered non-specific effects. Moreover, for two treatment-related components in the acupuncture and counselling groups of our trial that are often considered ‘non-specific’, namely session time and quality of therapist attention, we found that specific treatment effects remained when these were accounted for. Despite the limitation that a pragmatic approach has in ascribing outcomes to different treatment components, a pragmatic trial design provides a useful estimate of the overall effect, an estimate of most interest to patients, practitioners and providers.
Relationship to the literature
In a recent Cochrane review of acupuncture for depression,310 no studies focused on the BDI-II-related categories of moderate to severe depression, unlike patients in our trial who, for eligibility, had to have a score of ≥ 20 on the BDI-II. Moreover, only two trials in the Cochrane review, by the same research team, used a usual care comparator, which was based on a wait list. 333,334 A meta-analysis of these two trials involving 94 patients showed a reduction in the SMD of –0.73 (95% CI –1.18 to –0.29). 310 This is a larger effect size than we found (we found a point estimate of –0.39); however, we need to be cautious in the interpretation of the result, as the patients in these studies were less depressed and the patient numbers were small.
From a Cochrane review of counselling for mental health and psychosocial problems in primary care,312 there is evidence from six trials of counselling for mild to moderate depression including 772 patients that counselling is more effective than usual care in terms of mental health outcomes over 1–6 months (SMD –0.28, 95% CI –0.43 to –0.13). These advantages were not shown to endure over the longer term from 7 to 12 months (SMD –0.09, 95% CI –0.27 to 0.10). Their number needed to treat for short-term benefit was six, somewhat less than the 10 we found for moderate to severely depressed patients. We found no counselling trial equivalent to the one that we report here, namely one based in primary care that evaluated counselling for moderate to severe depression. In a review of psychological interventions for depression, four cognitive–behavioural therapy studies based in primary care covering 259 patients found a similar short-term effect (SMD –0.33, 95% CI –0.60 to –0.06). 335
In this report we present the first evidence on acupuncture and counselling for patients in primary care who are representative of those continuing to experience symptoms of moderate to severe depression. At 3 months, which is approximately at the end of the course of 12 treatment sessions, both acupuncture and counselling were shown to be effective treatments compared with usual care alone. There was also evidence that there are benefits over the 12-month period in terms of clinical symptoms.
Implications for clinical practice
To interpret the results for clinical practice, we found that 33% of acupuncture patients, 29% of counselling patients and 18% of usual care patients achieved a successful treatment outcome when this is defined as improvement from a depressed PHQ-9 score (≥ 10) to a non-depressed score (≤ 9), with an improvement of at least 50%. 336 These percentages apply to the total number of patients with a baseline PHQ-9 score of ≥ 10 and for whom data were available at 3 months. The number needed to treat is also a useful way to interpret results. We found that for one additional treatment success, as defined above, the number needed to treat was 7 for acupuncture (95% CI 4.3 to 17.4) and 10 for counselling (95% CI 5.3 to 47.3). A further illustration of the impact on patient experience can be provided in terms of ‘depression-free days’,322 which is a summary measure derived from PHQ-9 cut-off scores averaged over the period between measurements. The mean number of depression-free days over 3 months was 34 (95% CI 31 to 38) for the acupuncture group, 27 (95% CI 24 to 30) for the counselling group and 23 (95% CI 19 to 27) for the usual care group.
We used the BDI-II as a screening tool, with patients having to score ≥ 20 for eligibility, a score that is classified by this measure as moderate or severe depression. Other classification systems, such as the Diagnostic and Statistical Manual of Mental Disorders [see www.psychiatry.org/psychiatrists/practice/dsm (accessed 13 October 2016)], have different categories of severity. Our trial did not determine whether acupuncture or counselling performed better or worse for the more mild forms of depression. Our trial also did not determine whether or not patients not receiving antidepressant medication (one-third of our sample) do better or whether fewer sessions would be sufficient for mild to moderate depression or more sessions would improve outcomes for those with severe depression (approximately 60% of our sample). It is not clear from our trial whether or not there was any impact on outcomes because, for those allocated to acupuncture, it was usually their first experience of this intervention, whereas for many randomised to counselling, it was an intervention they had received before.
Discussion for substudy 1: experience of treatment from the patient perspective
Patients commonly reported that their acupuncturists appeared to have a more physical perspective on treatment, with a focus on directly relieving the symptoms of depression as well as concurrent physical symptoms. This was particularly welcomed by patients who reported comorbid pain at baseline, who appreciated having their physical symptoms treated alongside their depression. In conjunction with the acupuncture sessions, the acupuncturists often helped patients engage in health behaviours that had a positive influence on long-term change. In contrast, patients typically reported that their counsellors helped them identify and confront underlying causes of depression and then helped them find their own way forward. For both treatment modalities, most participants reported that the establishment of a therapeutic relationship and their active engagement as patients helped them develop coping strategies, which in turn helped them be more effective in reducing their depression in the longer term.
These qualitative findings are concordant with, and supplement, the quantitative data on depression and comorbid pain within the trial. 321 Notably, the patients with moderate or extreme pain at baseline (as reported on the EQ-5D) had worse outcomes at 3 months for depression than the no-pain comparator group in all three treatment arms. Our findings extend the findings of the trial’s quantitative data in two ways. First, there are identifiable differences in the experiences of depression when comorbid pain is present. Second, acupuncture appears to involve an approach that more directly addresses physical symptoms, including the comorbid pain, whereas counselling more directly addresses the underlying psychological causes of depression, which may or may not be linked to comorbid pain.
Our study has some limitations. Participants may have attributed changes directly to treatment rather than concurrent, coincidental contextual changes. We accept that there is a possibility of recall bias as it has long been known that there is a significant, stable association between depression and memory impairment,337 which may have altered what was recalled and how it was recalled. The lack of face-to-face contact during the telephone interviews prevented the interviewer gathering non-verbal contextual information, such as social cues, body language, appearance and setting, to supplement the verbal answers of the interviewees. A full report of the discussion related to this substudy has been published separately. 320
Discussion for substudy 2: impact of comorbid pain on depression outcomes
Participants with moderate to extreme pain at baseline had worse outcomes for depression than the no-pain comparator group in all three treatment arms after controlling for baseline depression. Participants in the pain group had greater reductions in depression symptoms with acupuncture from baseline to 12 months than those who received counselling or usual care, whereas those who were pain free did relatively well whichever group they were assigned to. Participants in the pain group receiving acupuncture found that their pain reduced markedly in the first 3–6 months compared with those in the other two groups, but by the end of the 12-month trial these differences disappeared. It should be noted that this study, as a substudy of a larger trial, was not powered to detect differences between the subgroups with moderate to extreme pain and no pain.
The estimated prevalence of moderate to extreme pain within our study population of depressed patients was 51%, which is comparable to the 50% identified in previous literature. 92 Evidence from an English longitudinal study of ageing identified pain and mobility disability at baseline as predictors of comorbid pain and depression. 338 In a large European study, a higher number of pain locations, pain of the joints and longer duration of pain (for ≥ 90 days), daily use of pain medication and more severe pain at baseline were found to be associated with a significantly increased risk of still having a depressive or anxiety disorder after 2 years. 338 Together, these factors are known to adversely affect the outcomes of treatment for depression. 338 Consistent with previous research, the majority of painful complaints within the study sample were of musculoskeletal origin and accompanied by poor mobility and a loss of energy. Pain from osteoarthritis is known to determine subsequent depressed mood through its effect on fatigue and disability. 339
That patients reported reduced pain following acupuncture is not surprising given that 32% of patients had chronic musculoskeletal pain and acupuncturists within the trial were encouraged to work how they normally would, incorporating treatment for pain alongside treatment for the symptoms of depression. Moreover, there is a growing body of evidence supporting the efficacy of acupuncture for chronic pain. 196 Patients who received counselling reported a more gradual reduction in pain over the 12-month follow-up period. This finding is consistent with a Cochrane review340 which reports that psychological therapies, primarily cognitive–behavioural therapy, can help people with chronic pain reduce negative mood (depression and anxiety), disability and, to a lesser degree, pain over a 6-month period.
Frequently patients with comorbid pain and depression will attribute their condition to one or other of these two symptoms and seek help accordingly. 341 For treatment success, our trial provides some evidence that both pain and depression should be both recognised and treated from the outset. Overall, the evidence emerging in the current study is that both acupuncture and counselling appear to have the potential to reduce symptoms associated with pain and depression when treated concurrently, with the potential to relieve symptoms of depression and reduce the intensity of pain in both the short and the longer term. Further discussion related to this substudy can found elsewhere. 321
Discussion for substudy 3: approaches that practitioners used to enhance longer-term benefits
Encouraging longer-term change was integral to the work of both acupuncturists and counsellors. Although both types of practitioners reported on the need to address the root causes, the approach differed. For the acupuncturists there was a focus drawn from Chinese medicine theory on treating the root cause as well as the manifesting symptoms, with the precise details of the intervention customised to their patients at an individual level. By contrast, the approach of the counsellors was to get below the surface of the clients’ problems and to ‘go deeper’ and ‘further back (in time)’ as a way of getting a handle on the root cause. For both types of practitioners, this required an individualised approach.
Further differences in approach to facilitating more sustained benefits were noted, in that acupuncturists were more focused on physical symptoms, on whether or not these could be resolved by acupuncture to speed up the improvements in the symptoms of depression and on providing lifestyle advice linked to the Chinese medicine diagnosis. Meanwhile, counsellors were more explicit about the importance of a strong therapeutic relationship accompanied by a careful consideration of what might be a manageable pace of change.
The methods used in this substudy, involving interviews and focus groups with verbatim transcripts and thematic analysis, were consistent with many of the markers of quality in qualitative research. 342 By involving an independent research team, we have helped establish the credibility and dependability of the results. With regard to the transferability of the results, we have provided details of the practitioners as well as the patients who were the focus of this substudy, such that readers can draw conclusions regarding relevance for other areas. In terms of limitations, our data are limited to patients receiving acupuncture as practised by those using the theories of traditional Chinese medicine and to clients receiving counselling as provided in the humanistic and non-directive and person-centred style.
Within the wider literature there is a dearth of evidence on the acupuncture treatment factors that might be associated with longer-term change in the symptoms of depression. The findings from a small study involving interviews with six practitioners in a trial of acupuncture for back pain found that these acupuncturists had a goal of a positive long-term outcome and developed a therapeutic partnership to support the active engagement of patients in their own recovery. 343 Consistent with some of the findings we report here, the authors reported that the key elements were establishing a rapport, using an interactive diagnostic process, matching treatment to the patient and using explanatory models from Chinese medicine to aid a shared understanding and motivate lifestyle changes to reinforce the potential recovery. 343
There is also limited evidence in the counselling literature from qualitative studies on the factors that improve the long-term effects of counselling. One report contains client interview data drawn from 15 clients who had received counselling from between 1 and 3 years previously. 344 The authors’ interpretation of the interview data led them to describe a model of the change process and mechanisms that were perceived as essential to produce a lasting benefit. They identified as key elements of the counselling process the active engagement of the client during and between sessions, and the acquisition of a ‘box of skills’ to be built on further after the counselling was finished. 344 A full report of the discussion related to this substudy has been published separately. 324
Discussion for substudy 4: cost-effectiveness analysis
This economic analysis demonstrated that the HRQoL results are consistent with the clinical results. The cost-effectiveness results, taking into account the uncertainty in the estimates, suggest that acupuncture is the cost-effective option. In the base-case analysis, acupuncture had an ICER of £4560 per additional QALY and was cost-effective with a probability of 0.62 at a cost-effectiveness threshold of £20,000 per QALY.
Currently, acupuncture for depression is not provided by the NHS. To understand the cost-effectiveness of counselling in a population for whom acupuncture is not appropriate (e.g. those who are needle phobic), a scenario analysis of counselling compared with usual care, excluding acupuncture as a comparator, was undertaken. In this population counselling had an estimated ICER of £7935 and a 0.91 probability of being cost-effective.
It is possible that the regulation of acupuncture may increase the per-session costs. A sensitivity analysis was undertaken assuming that each acupuncture session costs £65, the same as the cost of counselling. In this scenario counselling was preferred to acupuncture because not only were the expected benefits higher but the expected costs were lower. This demonstrates that the cost-effectiveness of acupuncture in this study is reliant on it having a lower cost than that of counselling.
Recommendations for future research
Further research could usefully identify the optimal populations for acupuncture and counselling. It would also be useful to explore the impact of different recruitment methods on the characteristics of those recruited. For example, patients recruited at the point of consultation will be different from those recruited through databases, as we did in this trial, and different again from those with depression who do not consult their GP at all. What was important in the trial that we report here was that all patients had consulted in primary care, all continued to be depressed and all were seeking other interventions that might reduce their depression. Other recruitment methods could be used, for example to assess whether the interventions would be more effective for patients at the time of consulting their GP or for patients who were experiencing their first episode of depression, rather than after many episodes, as was the case in the present study, in which 76% of participants had four or more previous episodes, or for patients who have given up consulting in primary care yet continue to be depressed.
Further research into optimal treatment regimens would also be useful. With regard to acupuncture provision, there is some evidence from the literature that a uniform combination of points can work for depressed patients. 345,346 However, a large-scale head-to-head trial or extensive synthesis in an IPD meta-analysis would be required to determine if a uniform approach for all acupuncture provision might be more beneficial than a style of acupuncture in which treatment is individualised for each patient and with changes over time, as was the case with the trial that we report here.
Although our findings are that both acupuncture and counselling for depression appear to be associated with longer-term benefits, it would be useful to explore the different ways that the interventions work. For example, we found that acupuncturists include in their treatment of patients with depression a focus on physical symptoms, yet it is not clear how important this is for outcomes related to depression. Further research is needed into the patient perspective on the treatment of depression with comorbidities and specifically the value that patients place on their comorbid symptoms being addressed concurrently. Further research is also needed from the perspective of patients on their experiences of treatment from these two modalities. To assist referral, a clearer understanding is needed of which type of person with depression would benefit from acupuncture and which from counselling. When taking into account patient preference, such a typology would provide referring clinicians with valuable guidance on suitability for referral.
Further analyses are needed to explore variations in the time horizon and the related impact on the cost-effectiveness of acupuncture and counselling. In this trial patients were followed up for 12 months and our analysis considered only this 12-month time frame, which assumed no differences between treatment arms beyond 12 months. This is a conservative assumption as there would be no further intervention costs, but our trial results suggest that a continued difference in treatment outcomes after 12 months is plausible, even though these treatment differences seem to be converging (see Figure 30). Extrapolating these differences beyond 12 months would result in a lower ICER for acupuncture. Further evaluation of the cost-effectiveness of acupuncture and counselling when compared with other physical and psychological interventions as well as with different levels of usual care will provide a better understand of how to best allocate scarce health-care resources.
Conclusion
In this report we present what is to our knowledge the first study to rigorously evaluate the clinical and economic impacts of acupuncture and counselling for patients who are representative of those who continue to experience depression in primary care. Our evidence on acupuncture compared with usual care and counselling compared with usual care shows that both treatments are associated with a statistically significant reduction in symptoms of depression in the short to medium term, with no reported serious adverse events related to treatment. Acupuncture is cost-effective compared with counselling or usual care alone, although the ranking of counselling and acupuncture depends on the relative costs of delivering these interventions. For patients in whom acupuncture is unavailable or perhaps inappropriate, counselling has an ICER that is less than most cost-effectiveness thresholds.
Chapter 7 Conclusions
In this programme of research we have addressed several key questions regarding the evidence base on acupuncture for chronic pain and depression. Our focus has been on assessing the clinical effectiveness of acupuncture compared with usual care as well as the efficacy of acupuncture for chronic pain beyond a placebo. Moreover, we have evaluated acupuncture in terms of cost-effectiveness and value for money. Our questions have led to a range of different methods, some innovative and some not, all of which have been appropriate for the questions being considered. For example, among the more innovative methods, for the first time in acupuncture research we have used an IPD meta-analysis, which has provided more power to explore treatment effects and to explore subgroup variations. We have used network meta-analyses to compare acupuncture with other physical therapies for the first time, leading to new evidence on both clinical impact and cost-effectiveness. We have also used more standard methods, for example in a RCT, in which acupuncture or counselling were compared with usual care for depression. These studies within the programme have provided a comprehensive evidence synthesis on acupuncture for a number of chronic pain conditions (lower back pain and neck pain, osteoarthritis of the knee and headache and migraine), as well as a substantive trial of acupuncture and counselling for depression in primary care.
In the IPD meta-analysis, we determined the effect size of acupuncture for chronic pain based on direct evidence, both when acupuncture is compared with sham acupuncture and when it is compared with a non-acupuncture control. Based on data from 29 of 31 high-quality trials, including a total of 17,922 patients, we found that patients receiving acupuncture had less pain than those receiving sham control treatment, with effect sizes for the different pain conditions being in the order of 0.2 (p < 0.001). When comparing acupuncture with non-acupuncture controls, effect sizes were larger for all conditions, with effect sizes in the order of 0.5 (p < 0.001). The difference between these two comparisons, which is of the order of 0.3, can be ascribed to ‘placebo’ effects, sham needle-related effects and other context effects. Given the highly statistically significant effect sizes, these data are relevant to the debate regarding the proportion that ‘placebo’ effects contribute to the overall effect of acupuncture for chronic pain.
In a network meta-analysis, we addressed the question of how effective physical treatments for osteoarthritis of the knee are, when compared with each other on an equal basis, for relieving pain. When synthesising the data from 114 trials involving 22 treatments and 9709 patients, we found that eight interventions statistically significantly outperformed standard (usual) care, including acupuncture and sham acupuncture. The intervention with the most higher-quality studies was acupuncture, which was also one of the more effective physical treatments for alleviating osteoarthritis knee pain in the short term. The caveat for this study was that much of the evidence on physical therapies was of poor quality, which made it difficult to draw conclusions on the effectiveness of many of them.
In another network meta-analysis, we used innovative methods to analyse IPD, drawn from the study discussed above. HRQoL data are required for a cost-effectiveness analysis. To synthesise heterogeneous outcomes we have made use of standardisation of pain measurements and of published mapping algorithms to convert and compare HRQoL evidence on to the EQ-5D summary index scale. This enabled us to analyse HRQoL data across trials in which these data were not collected. Our analysis used the same evidence base as the IPD pairwise meta-analysis. This included approximately 17,500 patients from 28 trials in which we compared acupuncture, sham acupuncture and usual care with each other. Standardised pain pooled results were broadly similar to the ones obtained from the above-mentioned pairwise meta-analysis. The synthesis of mapped EQ-5D estimates found that acupuncture was effective compared with usual care. However, the EQ-5D benefit of acupuncture over sham acupuncture was found to be smaller and less certain. When combined with resource use and cost estimates, these EQ-5D data can be used to generate estimates of cost-effectiveness. Cost-effectiveness results suggested that acupuncture was cost-effective when compared with usual care alone, with ICERs ranging from £7000 to £14,000 per QALY across pain types. Because this was primarily a methods exercise, not all relevant comparators were included and aggregate data beyond the IPD from the 28 trials evaluated were not included. These are, therefore, not robust estimates of cost-effectiveness and are not an adequate basis for resource allocation decision-making.
To address the question of the cost-effectiveness of acupuncture for osteoarthritis of the knee, we conducted an economic evaluation of non-pharmacological adjunct interventions. We again used network meta-analysis methods, and IPD when available, this time to synthesise RCT evidence on 17 active interventions and three control interventions for osteoarthritis of the knee. The network meta-analysis included 88 eligible studies and 7507 patients. IPD were available for five of the studies including 1329 patients. Data from HRQoL instruments were mapped to EQ-5D preference weights prior to synthesis using network meta-analysis. We estimated resource use associated with the interventions from trial data, expert opinion, the literature and information obtained from NHS trust websites. When all trials were included in the synthesis, TENS was cost-effective at conventional cost-effectiveness threshold values with an ICER of £2690 per QALY compared with usual care. The effectiveness of TENS may be exaggerated because of biases associated with poor-quality trials. When the analysis was restricted to trials with adequate allocation concealment, acupuncture was cost-effective with an ICER of £13,502 per QALY compared with TENS. The EVPI in this area is relatively high, suggesting that additional research may be cost-effective. Further analysis is required to identify the most cost-effective and clinically appropriate specification of further research. Given the likely ‘up-front’ training costs associated with expansion of acupuncture services in the NHS, investment in further research may need to precede the widespread availability of acupuncture services.
In a RCT of acupuncture or counselling for patients with depression in primary care, we recruited 755 patients from 27 primary care practices in the north-east of England. This was the largest trial to date evaluating acupuncture for depression. Allocation was to acupuncture (n = 302), counselling (n = 302) or usual care alone (n = 151). A mean of 10 sessions was attended for acupuncture and nine sessions for counselling. We found a statistically significant reduction in mean PHQ-9 depression score at 3 months for both acupuncture and counselling compared with usual care, which was largely sustained at 12 months. When controlling for time and attention, we found no significant differences in clinical outcome between acupuncture and counselling. No serious adverse events were reported that were both unexpected and related to treatment. Acupuncture and counselling were found to have higher mean QALYs and costs than usual care. Acupuncture had an ICER of £4560 per additional QALY in the base-case analysis and was cost-effective with a probability of 0.62 at a cost-effectiveness threshold of £20,000 per QALY.
There are limitations to what we report here for two major reasons. First, in our systematic reviews we were limited by the available literature. The history of acupuncture research, as with much of the literature for physical therapies, has been littered with small trials of generally low quality. The higher the risk of bias, the less certainty we have in drawing conclusions. This programme of research has been possible only because of the number of high-quality acupuncture trials with large sample sizes conducted in the 2000s. Moreover, for all physical therapies, including acupuncture, the difficulty in having available a scientifically acceptable control for non-specific effects means that attempts to delineate the proportion of any effect that is non-specific are inevitably limited. At least in acupuncture trials for chronic pain, in which the research question has been related to efficacy rather than effectiveness, the sham needle has been widely used as a control for ‘placebo’ effects. Remarkably, across all physical therapies for osteoarthritis, there are more high-quality trials of acupuncture with a sham comparator than of any other physical therapy. Therefore, the limitations in the existing literature on physical therapies for chronic pain are less in acupuncture trials, allowing us to draw conclusions with more certainty than for other physical therapies.
The second broad limitation of the programme is that we have not addressed all of the aspects of the evidence base that continue to be associated with uncertainty. In part, this is because we have yet to complete a number of substudies that were planned as integral to individual projects. For example, the IPD meta-analysis had six substudies planned as part of the original mission, yet we have only managed to report on two at this stage; a further four will be completed and published soon. These are a study of the time course of acupuncture, a study exploring the relationship between patient characteristics and variations in outcome, a study of practitioner effects and a study to identify whether or not there are ‘super-responders’. We have recently published two substudies related to the acceptability, feasibility and validity of using text messaging scores of depression,347,348 and there remains a further substudy exploring the variation in practitioner outcomes that has yet to be published. In part, this is because some of the much-needed associated research fell outside the scope of the programme at the outset. A case in point here is the absence of relevant comparators when we evaluated the cost-effectiveness of acupuncture for musculoskeletal pain and headache and migraine. There are limited data regarding the long-term effects of many non-pharmacological interventions used to treat osteoarthritis of the knee and sensitivity analyses suggested that the cost-effectiveness model results may be sensitive to the magnitude of these effects. The active and control interventions in the trials informing the cost-effectiveness analysis were subject to heterogeneity in the methods, duration and intensity with which they were administered. Another example is that, because of our trial design, we did not control for context effects in our depression trial when comparing acupuncture with usual care, although we did control for time and attention when comparing acupuncture with counselling. In part, further trials of acupuncture would usefully expand the generalisability of data on effectiveness, for example with different populations, such as only patients with mild to moderate depression or only patients on antidepressants. Similarly, different outcomes could be addressed, for example finding out whether or not acupuncture reduces the relapse rate and sustains remission. Further research is merited in these areas related to acupuncture for chronic pain and depression.
The results of this programme of research are important on several counts. First, the data on acupuncture for chronic pain are particularly relevant as pain is the condition for which acupuncture is most commonly used. Moreover, chronic pain and depression are known to be areas in which patients and GPs consider conventional medical treatments have their limitations, whether because of perceived limitations of routinely prescribed medication or because of concerns regarding side effects and dependency. Second, we have used robust and rigorous methods throughout the studies to provide a high level of evidence. Indeed, in the systematic reviews we have purposely sought to identify and synthesise the higher-quality studies as they are known to be affected less by bias than data drawn from trials with a high risk of bias. Third, we have asked questions and used methods that have led to the synthesis of trials that between them have large numbers of participants and, when analysed, these large data sets have provided results with more precision than would be otherwise possible. Fourth, our results are relevant to important questions about resource use in a climate of limited funding within the NHS. By comparing acupuncture in an unbiased way with other ‘competing’ physical or psychological therapies, we are providing the very evidence on clinical effectiveness and cost-effectiveness that is of most value to policy-makers and commissioners. Finally, it is in the interests of patients to have available well-informed results based on high-quality evidence to make decisions about their health care.
Acknowledgements
We wish to acknowledge Mike Bennett, Richard Blackwell, Peter Bower, Matthew Bowes, Sally Brabyn, Stephen Brealey, Ann Burton, Ruth Chamberlain, Ben Cross, Ben Elliot, David Geddes, Christina Giannopoulou, Simon Gilbody, Peter Hall, Pauline Holloway, Victoria Hurtado-Menses, Kamran Khan, Harriet Lansdown, Hillary Marshall, Peter Morley, Liz Newbronner, Joanne O’Conner, Karen Overend, Sara Perren, Lucy Revell, Steve Rice, Mark Roman, Micah Rose, Trevor Sheldon, Eleftherios Sideris, David Smith, Lesley Stewart, Alex Sutton, Val Wadsworth, Kerry Wheeler and Nerys Woolacott.
Acknowledgement of related funding
The ATC is funded by a R21 (AT004189I) from the National Center for Complementary and Alternative Medicine at the National Institutes of Health to Andrew Vickers and by a grant from the Samueli Institute. Andrea Manca’s contribution was made under the terms of a career development research training fellowship issued by the NIHR (grant CDF-2009–02–21).
Contributions of authors
All authors made a substantial contribution to at least one area within the programme of research.
Hugh MacPherson (Professor of Acupuncture Research) was lead applicant and principal investigator. He designed the programme of research and was involved in all projects and their presentation in the chapters of this report.
Andrew Vickers (Statistician) conducted the IPD meta-analysis in Chapter 2 and contributed to the interpretation of the results in Chapters 4 and 5.
Martin Bland (Professor of Health Statistics) was involved in the conduct of the depression trial in Chapter 6.
David Torgerson (Professor, Director of the York Trials Unit) had oversight on the conduct of the depression trial in Chapter 6.
Mark Corbett (Research Fellow, Systematic Reviewer) wrote the first draft of Chapter 3 and contributed to writing Chapters 1, 5 and 7.
Eldon Spackman (Research Fellow, Health Economist) took the lead in the health economic analysis of the depression trial presented in Chapter 6.
Pedro Saramago (Research Fellow, Statistician) was the lead analyst for and author of Chapter 4. He contributed to all aspects of development of the health economic methods, the analysis and the write-up for Chapters 4 and 5.
Beth Woods (Research Fellow, Health Economist) was the lead analyst and author of Chapter 5. She contributed to all aspects of development of the health economic methods, the analysis and the write-up for Chapters 4 and 5.
Helen Weatherly (Senior Research Fellow) supported and co-ordinated the research for Chapters 4 and 5. She contributed to all aspects of development of the health economic methods, the analysis and the write-up for Chapters 4 and 5.
Mark Sculpher (Professor, Health Economist) advised on all aspects of development of the health economic methods and analysis for Chapters 4 and 5. He was co-investigator of the overall research and he participated in the design of the study, the methods development and the write-up for Chapters 4 and 5.
Andrea Manca (Professor, Health Economist) advised on all aspects of development of the health economic methods and analysis for Chapters 4 and 5. He participated in the design of the study, the methods development and the write-up for Chapters 4 and 5.
Stewart Richmond (Research Fellow) was the trial manager for the depression trial in Chapter 6.
Ann Hopton (Research Fellow) had a management role across the programme of research and was directly involved in the conduct of the depression trial in Chapter 6.
Janet Eldred (Research Administrator across the programme of research) was directly involved in the conduct of the depression trial in Chapter 6.
Ian Watt (Professor of Primary Care) was involved in the conduct of the depression trial in Chapter 6, including the monitoring of adverse events.
Publications
Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Victor N, et al. Individual patient data meta-analysis of acupuncture for chronic pain: protocol of the Acupuncture Triallists’ Collaboration. Trials 2010;11:90.
Corbett M, Rice S, Slack R, Harden M, Madurasinghe V, Sutton A, et al. Acupuncture and Other Physical Treatments for the Relief of Chronic Pain due to Osteoarthritis of the Knee: A Systematic Review and Network Meta-Analysis. CRD Report 40. York: CRD, University of York; 2011.
MacPherson H, Richmond S, Bland MJ, Lansdown H, Hopton A, Kang’ombe A, et al. Acupuncture, Counseling, and Usual care for Depression (ACUDep): study protocol for a randomised controlled trial. Trials 2012;13:209.
Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med 2012;172:1444–53.
Woolacott NF, Corbett MS, Rice SJ. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology 2012;51:1440–6.
Corbett MS, Rice SJC, Madurasinghe V, Slack R, Fayter DA, Harden M, et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthr Cartil 2013;2:1290–8.
MacPherson H, Elliot B, Hopton A, Lansdown H, Richmond S. Acupuncture for depression: patterns of diagnosis and treatment within a randomised controlled trial. Evid Based Complement Alternat Med 2013;2013:286048.
MacPherson H, Maschino AC, Lewith G, Foster NE, Witt C, Vickers AJ, et al. Characteristics of acupuncture treatment associated with outcome: an individual patient meta-analysis of 17,922 patients with chronic pain in randomised controlled trials. PLOS ONE 2013;8:e77438.
MacPherson H, Richmond S, Bland, Brealey S, Gabe R, Hopton A, et al. Acupuncture and counselling for depression in primary care: a randomised controlled trial. PLOS Med 2013;10:e1001518.
Vickers AJ, Maschino AC, Lewith G, MacPherson H, Sherman KJ, Witt CM; Acupuncture Triallists’ Collaboration. Responses to the Acupuncture Triallists’ Collaboration individual patient data meta-analysis. Acupunct Med 2013;31:98–100.
Hopton A, Eldred J, MacPherson H. Patients’ experiences of acupuncture and counselling for depression and comorbid pain: a qualitative study nested within a randomised controlled trial. BMJ Open 2014;4:e005144.
Hopton A, MacPherson H, Keding A, Morley S. Acupuncture, counselling or usual care for depression and comorbid pain: secondary analysis of a randomised controlled trial. BMJ Open 2014;4:e004964.
MacPherson H, Newbronner L, Chamberlain R, Richmond SJ, Lansdown H, Perren S, et al. Practitioner perspectives on strategies to promote longer-term benefits of acupuncture or counselling for depression: a qualitative study. PLOS ONE 2014;9:e104077.
MacPherson H, Vertosick E, Lewith G, Linde K, Sherman KJ, Witt CM, et al. Acupuncture Triallists’ Collaboration. Influence of control group on effect size in trials of acupuncture for chronic pain: a secondary analysis of an individual patient data meta-analysis. PLOS ONE 2014;9:e93739.
Spackman E, Richmond S, Sculpher M, Bland M, Brealey S, Gabe R, et al. Cost-effectiveness analysis of acupuncture, counselling and usual care in treating patients with depression: the results of the ACUDep trial. PLOS ONE 2014;9:e113726.
Keding A, Böhnke JR, Croudace TJ, Richmond SJ, MacPherson H. Validity of single item responses to short message service texts to monitor depression: an mHealth sub-study of the UK ACUDep trial. BMC Med Res Methodol 2015;15:56.
Perren S, Richmond S, MacPherson H. The human face of an RCT: reflections on providing counselling for clients with moderate to severe depression in a randomised controlled trial. Healthcare Couns Psychother J January 2015, pp. 8–13.
Richmond SJ, Keding A, Hover M, Gabe R, Cross B, Torgerson D, et al. Feasibility, acceptability and validity of SMS text messaging for measuring change in depression during a randomised controlled trial. BMC Psychiatry 2015;15:68.
Data sharing statement
All available data can be obtained on request from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Hopton AK, Curnoe S, Kanaan M, Macpherson H. Acupuncture in practice: mapping the providers, the patients and the settings in a national cross-sectional survey. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000456.
- Thomas KJ, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med 2001;9:2-11. http://dx.doi.org/10.1054/ctim.2000.0407.
- Lipman L, Dale J, MacPherson H. Attitudes of GPs towards the provision of acupuncture on the NHS. Complement Ther Med 2003;11:110-14. http://dx.doi.org/10.1016/S0965-2299(03)00042-6.
- Lim JH. Provision of medical acupuncture service in general practice under practice-based commissioning. Acupunct Med 2010;28:103-4. http://dx.doi.org/10.1136/aim.2010.002287.
- Dale J. Acupuncture practice in the UK. Part 1: report of a survey. Complement Ther Med 1997;5:215-20. http://dx.doi.org/10.1016/S0965-2299(97)80032-5.
- MacPherson H, Sinclair-Lian N, Thomas K. Patients seeking care from acupuncture practitioners in the UK: a national survey. Complement Ther Med 2006;14:20-3. http://dx.doi.org/10.1016/j.ctim.2005.07.006.
- Fisher P, van Haselen R, Hardy K, Berkovitz S, McCarney R. Effectiveness gaps: a new concept for evaluating health service and research needs applied to complementary and alternative medicine. J Altern Complement Med 2004;10:627-32. http://dx.doi.org/10.1089/acm.2004.10.627.
- Robinson N, Lorenc A, Ding W, Jia J, Bovey M, Wang XM. Exploring practice characteristics and research priorities of practitioners of traditional acupuncture in China and the EU-A survey. J Ethnopharmacol 2012;140:604-13. http://dx.doi.org/10.1016/j.jep.2012.01.052.
- Fønnebø V, Grimsgaard S, Walach H, Ritenbaugh C, Norheim AJ, MacPherson H, et al. Researching complementary and alternative treatments – the gatekeepers are not at home. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-7.
- Ernst E, White A. Life-threatening adverse reactions after acupuncture? A systematic review. Pain 1997;71:123-6. http://dx.doi.org/10.1016/S0304-3959(97)03368-X.
- MacPherson H, Thomas K, Walters S, Fitter M. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ 2001;323:486-7. http://dx.doi.org/10.1136/bmj.323.7311.486.
- White A, Hayhoe S, Hart A, Ernst E. Adverse events following acupuncture: prospective survey of 32 000 consultations with doctors and physiotherapists. BMJ 2001;323:485-6. http://dx.doi.org/10.1136/bmj.323.7311.485.
- Vincent C. The safety of acupuncture. BMJ 2001;323:467-8. http://dx.doi.org/10.1136/bmj.323.7311.467.
- Macpherson H, Scullion A, Thomas KJ, Walters S. Patient reports of adverse events associated with acupuncture treatment: a prospective national survey. Qual Saf Health Care 2004;13:349-55. http://dx.doi.org/10.1136/qshc.2003.009134.
- Hopton AK, Thomas KJ, MacPherson H. Willingness to try acupuncture again: reports from patients on their treatment reactions in a low back pain trial. Acupunct Med 2010;28:185-8. http://dx.doi.org/10.1136/aim.2010.002279.
- Melchart D, Weidenhammer W, Streng A, Reitmayr S, Hoppe A, Ernst E, et al. Prospective investigation of adverse effects of acupuncture in 97 733 patients. Arch Intern Med 2004;164:104-5. http://dx.doi.org/10.1001/archinte.164.1.104.
- Witt CM, Pach D, Brinkhaus B, Wruck K, Tag B, Mank S, et al. Safety of acupuncture: results of a prospective observational study with 229,230 patients and introduction of a medical information and consent form. Forsch Komplementmed 2009;16:91-7. http://dx.doi.org/10.1159/000209315.
- Pomeranz B. Scientific research into acupuncture for the relief of pain. J Altern Complement Med 1996;2:53-60. http://dx.doi.org/10.1089/acm.1996.2.53.
- Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003;26:17-22. http://dx.doi.org/10.1016/S0166-2236(02)00006-1.
- Kim SK, Bae H. Acupuncture and immune modulation. Auton Neurosci 2010;157:38-41. http://dx.doi.org/10.1016/j.autneu.2010.03.010.
- Cabioğlu MT, Cetin BE. Acupuncture and immunomodulation. Am J Chin Med 2008;36:25-36. http://dx.doi.org/10.1142/S0192415X08005552.
- Zhou W, Fu L-W, Tjen-A-Looi SC, Li P, Longhurst JC. Afferent mechanisms underlying stimulation modality-related modulation of acupuncture-related cardiovascular responses. J Appl Physiol 2005;98:872-80. http://dx.doi.org/10.1152/japplphysiol.01079.2004.
- Noguchi E. Acupuncture regulates gut motility and secretion via nerve reflexes. Auton Neurosci 2010;156:15-8. http://dx.doi.org/10.1016/j.autneu.2010.06.010.
- Dhond RP, Kettner N, Napadow V. Neuroimaging acupuncture effects in the human brain. J Altern Complement Med 2007;13:603-16. http://dx.doi.org/10.1089/acm.2007.7040.
- Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 2008;85:355-75. http://dx.doi.org/10.1016/j.pneurobio.2008.05.004.
- Li P, Longhurst JC. Neural mechanism of electroacupuncture’s hypotensive effects. Auton Neurosci 2010;157:24-30. http://dx.doi.org/10.1016/j.autneu.2010.03.015.
- Longhurst JC. Defining meridians: a modern basis of understanding. J Acupunct Meridian Stud 2010;3:67-74. http://dx.doi.org/10.1016/S2005-2901(10)60014-3.
- Kagitani F, Uchida S, Hotta H. Afferent nerve fibers and acupuncture. Auton Neurosci 2010;157:2-8. http://dx.doi.org/10.1016/j.autneu.2010.03.004.
- Lewith GT, White PJ, Pariente J. Investigating acupuncture using brain imaging techniques: the current state of play. Evid Based Complement Alternat Med 2005;2:315-19. http://dx.doi.org/10.1093/ecam/neh110.
- Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec 2002;269:257-65. http://dx.doi.org/10.1002/ar.10185.
- Heine H. The morphological basis of the acupuncture points. Acupunct Sci Int J 1990;1:1-6.
- Langevin HM, Churchill DL, Wu J, Badger GJ, Yandow JA, Fox JR, et al. Evidence of connective tissue involvement in acupuncture. FASEB J 2002;16:872-4. http://dx.doi.org/10.1096/fj.01-0925fje.
- Becker RO, Reichmanis M, Marino AA. Electrophysiological correlates of acupuncture points and meridians. Psychoenerg Syst 1976;1:105-12.
- Ahn AC, Martinsen OG. Electrical characterization of acupuncture points: technical issues and challenges. J Altern Complement Med 2007;13:817-24. http://dx.doi.org/10.1089/acm.2007.7193.
- Colbert AP, Spaulding KP, Ahn AC, Cutro JA. Clinical utility of electrodermal activity at acupuncture points: a narrative review. Acupunct Med 2011;29:270-5. http://dx.doi.org/10.1136/acupmed-2011-010021.
- Saku K, Mukaino Y, Ying H, Arakawa K. Characteristics of reactive electropermeable points on the auricles of coronary heart disease patients. Clin Cardiol 1993;16:415-19. http://dx.doi.org/10.1002/clc.4960160509.
- Ahn AC, Park M, Shaw JR, McManus CA, Kaptchuk TJ, Langevin HM. Electrical impedance of acupuncture meridians: the relevance of subcutaneous collagenous bands. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0011907.
- Ahn AC, Colbert AP, Anderson BJ, Martinsen OG, Hammerschlag R, Cina S, et al. Electrical properties of acupuncture points and meridians: a systematic review. Bioelectromagnetics 2008;29:245-56. http://dx.doi.org/10.1002/bem.20403.
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971-9. http://dx.doi.org/10.1126/science.150.3699.971.
- Carlsson C. Acupuncture mechanisms for clinically relevant long-term effects – reconsideration and a hypothesis. Acupunct Med 2002;20:82-99. http://dx.doi.org/10.1136/aim.20.2-3.82.
- Clement-Jones V, McLoughlin L, Tomlin S, Besser GM, Rees LH, Wen HL. Increased beta-endorphin but not met-enkephalin levels in human cerebrospinal fluid after acupuncture for recurrent pain. Lancet 1980;2:946-9. http://dx.doi.org/10.1016/S0140-6736(80)92106-6.
- Napadow V, Liu J, Li M, Kettner N, Ryan A, Kwong KK, et al. Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Hum Brain Mapp 2007;28:159-71. http://dx.doi.org/10.1002/hbm.20261.
- Birch S. A review and analysis of placebo treatments, placebo effects, and placebo controls in trials of medical procedures when sham is not inert. J Altern Complement Med 2006;12:303-10. http://dx.doi.org/10.1089/acm.2006.12.303.
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd003974.pub3.
- MacPherson H, Vertosick E, Lewith G, Linde K, Sherman KJ, Witt CM, et al. Influence of control group on effect size in trials of acupuncture for chronic pain: a secondary analysis of an individual patient data meta-analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0093739.
- Hall H. Acupuncture’s claims punctured: not proven effective for pain, not harmless. Pain 2011;152:711-12. http://dx.doi.org/10.1016/j.pain.2011.01.039.
- Ernst E. Acupuncture – a treatment to die for?. J R Soc Med 2010;103:384-5. http://dx.doi.org/10.1258/jrsm.2010.100181.
- Madsen MV, Gøtzsche PC, Hróbjartsson A. Acupuncture treatment for pain: systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.a3115.
- Ernst E, Lee MS, Choi TY. Acupuncture: does it alleviate pain and are there serious risks? A review of reviews. Pain 2011;152:755-64. http://dx.doi.org/10.1016/j.pain.2010.11.004.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464-75. http://dx.doi.org/10.1016/j.jclinepi.2008.12.011.
- Ezzo J, Berman B, Hadhazy VA, Jadad AR, Lao L, Singh BB. Is acupuncture effective for the treatment of chronic pain? A systematic review. Pain 2000;86:217-25. http://dx.doi.org/10.1016/S0304-3959(99)00304-8.
- Manheimer E, Linde K, Lao L, Bouter LM, Berman BM. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med 2007;146:868-77. http://dx.doi.org/10.7326/0003-4819-146-12-200706190-00008.
- Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology 2006;45:1331-7. http://dx.doi.org/10.1093/rheumatology/kel207.
- White A, Foster NE, Cummings M, Barlas P. Acupuncture treatment for chronic knee pain: a systematic review. Rheumatology 2007;46:384-90. http://dx.doi.org/10.1093/rheumatology/kel413.
- Davis MA, Kononowech RW, Rolin SA, Spierings EL. Acupuncture for tension-type headache: a meta-analysis of randomized, controlled trials. J Pain 2008;9:667-77. http://dx.doi.org/10.1016/j.jpain.2008.03.011.
- Sun Y, Gan TJ. Acupuncture for the management of chronic headache: a systematic review. Anesth Analg 2008;107:2038-47. http://dx.doi.org/10.1213/ane.0b013e318187c76a.
- Furlan AD, van Tulder M, Cherkin D, Tsukayama H, Lao L, Koes B, et al. Acupuncture and dry-needling for low back pain: an updated systematic review within the framework of the Cochrane Collaboration. Spine 2005;30:944-63. http://dx.doi.org/10.1097/01.brs.0000158941.21571.01.
- Manheimer E, White A, Berman B, Forys K, Ernst E. Meta-analysis: acupuncture for low back pain. Ann Intern Med 2005;142:651-63. http://dx.doi.org/10.7326/0003-4819-142-8-200504190-00014.
- Ernst E, Lee MS. A trial design that generates only ‘positive’ results. J Postgrad Med 2008;54:214-16. http://dx.doi.org/10.4103/0022-3859.41806.
- Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al. Acupuncture for chronic headache in primary care: large, pragmatic, randomised trial. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38029.421863.EB.
- Thomas KJ, MacPherson H, Thorpe L, Brazier J, Fitter M, Campbell MJ, et al. Randomised controlled trial of a short course of traditional acupuncture compared with usual care for persistent non-specific low back pain. BMJ 2006;333:623-6. http://dx.doi.org/10.1136/bmj.38878.907361.7C.
- Wonderling D, Vickers AJ, Grieve R, McCarney R. Cost effectiveness analysis of a randomised trial of acupuncture for chronic headache in primary care. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38033.896505.EB.
- Ratcliffe J, Thomas KJ, MacPherson H, Brazier J. A randomised controlled trial of acupuncture care for persistent low back pain: cost effectiveness analysis. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38932.806134.7C.
- Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR. Acupuncture for tension-type headache. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.cd007587.
- Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.cd001218.pub2.
- Brinkhaus B, Witt CM, Jena S, Linde K, Streng A, Wagenpfeil S, et al. Acupuncture in patients with chronic low back pain: a randomized controlled trial. Arch Intern Med 2006;166:450-7. http://dx.doi.org/10.1001/archinte.166.4.450.
- Melchart D, Streng A, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, et al. Acupuncture in patients with tension-type headache: randomised controlled trial. BMJ 2005;331:376-82. http://dx.doi.org/10.1136/bmj.38512.405440.8F.
- Linde K, Streng A, Jürgens S, Hoppe A, Brinkhaus B, Witt C, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA 2005;293:2118-25. http://dx.doi.org/10.1001/jama.293.17.2118.
- Witt C, Brinkhaus B, Jena S, Linde K, Streng A, Wagenpfeil S, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet 2005;366:136-43. http://dx.doi.org/10.1016/S0140-6736(05)66871-7.
- Scharf HP, Mansmann U, Streitberger K, Witte S, Krämer J, Maier C, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med 2006;145:12-20. http://dx.doi.org/10.7326/0003-4819-145-1-200607040-00005.
- Haake M, Müller HH, Schade-Brittinger C, Basler HD, Schäfer H, Maier C, et al. German Acupuncture Trials (GERAC) for chronic low back pain: randomized, multicenter, blinded, parallel-group trial with 3 groups. Arch Intern Med 2007;167:1892-8. http://dx.doi.org/10.1001/Archinte.167.17.1892.
- Diener HC, Kronfeld K, Boewing G, Lungenhausen M, Maier C, Molsberger A, et al. Efficacy of acupuncture for the prophylaxis of migraine: a multicentre randomised controlled clinical trial. Lancet Neurol 2006;5:310-16. http://dx.doi.org/10.1016/S1474-4422(06)70382-9.
- Endres HG, Böwing G, Diener HC, Lange S, Maier C, Molsberger A, et al. Acupuncture for tension-type headache: a multicentre, sham-controlled, patient-and observer-blinded, randomised trial. J Headache Pain 2007;8:306-14. http://dx.doi.org/10.1007/s10194-007-0416-5.
- Witt CM, Jena S, Selim D, Brinkhaus B, Reinhold T, Wruck K, et al. Pragmatic randomized trial evaluating the clinical and economic effectiveness of acupuncture for chronic low back pain. Am J Epidemiol 2006;164:487-96. http://dx.doi.org/10.1093/aje/kwj224.
- Witt CM, Jena S, Brinkhaus B, Liecker B, Wegscheider K, Willich SN. Acupuncture for patients with chronic neck pain. Pain 2006;125:98-106. http://dx.doi.org/10.1016/j.pain.2006.05.013.
- Jena S, Witt CM, Brinkhaus B, Wegscheider K, Willich SN. Acupuncture in patients with headache. Cephalalgia 2008;28:969-79. http://dx.doi.org/10.1111/j.1468-2982.2008.01640.x.
- Witt CM, Jena S, Brinkhaus B, Liecker B, Wegscheider K, Willich SN. Acupuncture in patients with osteoarthritis of the knee or hip: a randomized, controlled trial with an additional nonrandomized arm. Arthritis Rheum 2006;54:3485-93. http://dx.doi.org/10.1002/art.22154.
- Chalmers I. The Cochrane Collaboration: preparing, maintaining, and disseminating systematic reviews of the effects of health care. Ann NY Acad Sci 1993;703:156-63. http://dx.doi.org/10.1111/j.1749-6632.1993.tb26345.x.
- Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 2002;25:76-97. http://dx.doi.org/10.1177/0163278702025001006.
- Headaches: Diagnosis and Management of Headaches in Young People and Adults. London: NICE; 2012.
- Low Back Pain: Early Management of Persistent Non-Specific Low Back Pain. London: NICE; 2009.
- Osteoarthritis: Care and Management in Adults. London: NICE; 2014.
- Latimer N. NICE guideline on osteoarthritis: is it fair to acupuncture? Yes. Acupunct Med 2009;27:72-5. http://dx.doi.org/10.1136/aim.2009.000802.
- Cummings M. Why recommend acupuncture for low back pain but not for osteoarthritis? A commentary on recent NICE guidelines. Acupunct Med 2009;27:128-9. http://dx.doi.org/10.1136/aim.2009.001214.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. http://dx.doi.org/10.1136/bmj.331.7521.897.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Han JS. Acupuncture and endorphins. Neurosci Lett 2004;361:258-61. http://dx.doi.org/10.1016/j.neulet.2003.12.019.
- Napadow V, Webb JM, Pearson N, Hammerschlag R. Neurobiological correlates of acupuncture: November 17–18, 2005. J Altern Complement Med 2006;12:931-5. http://dx.doi.org/10.1089/acm.2006.12.931.
- Napadow V, Maeda Y, Audette J, Kettner N, Knotkova H, Cruciani RA, et al. Neural Plasticity in Chronic Pain. New York, NY: Nova Publishers; 2011.
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 2008;12:1-23.
- Xue CC, Zhang AL, Lin V, Myers R, Polus B, Story DF. Acupuncture, chiropractic and osteopathy use in Australia: a national population survey. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-105.
- Katona C, Peveler R, Dowrick C, Wessely S, Feinmann C, Gask L, et al. Pain symptoms in depression: definition and clinical significance. Clin Med 2005;5:390-5. http://dx.doi.org/10.7861/clinmedicine.5-4-390.
- Mukaino Y, Park J, White A, Ernst E. The effectiveness of acupuncture for depression – a systematic review of randomised controlled trials. Acupunct Med 2005;23:70-6. http://dx.doi.org/10.1136/aim.23.2.70.
- Smith CA, Hay PP. Acupuncture for depression. Cochrane Database Syst Rev 2005;2.
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003;53:649-59. http://dx.doi.org/10.1016/S0006-3223(03)00231-2.
- Gilbody S, Whitty P. Improving the Recognition and Management of Depression in Primary Care. York: NHS Centre for Reviews and Dissemination, University of York; 2002.
- Mind . My Choice: A Survey 2002. www.mind.org.uk/News+policy+and+campaigns/Press+archive/Mind+launches+campaign+for+more+choice+of+mental+health+services+at+GP+level.htm (accessed 13 January 2008).
- Schroer S, Macpherson H. Acupuncture, or non-directive counselling versus usual care for the treatment of depression: a pilot study. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-3.
- Colquhoun D, Novella SP. Acupuncture is theatrical placebo. Anesth Analg 2013;116:1360-3. http://dx.doi.org/10.1213/ANE.0b013e31828f2d5e.
- Han JS, Ho YS. Global trends and performances of acupuncture research. Neurosci Biobehav Rev 2011;35:680-7. http://dx.doi.org/10.1016/j.neubiorev.2010.08.006.
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333. http://dx.doi.org/10.1016/j.ejpain.2005.06.009.
- Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference?. Lancet 1993;341:418-22. http://dx.doi.org/10.1016/0140-6736(93)93004-K.
- Vickers AJ. Statistical reanalysis of four recent randomized trials of acupuncture for pain using analysis of covariance. Clin J Pain 2004;20:319-23. http://dx.doi.org/10.1097/00002508-200409000-00006.
- Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 1992;11:1685-704. http://dx.doi.org/10.1002/sim.4780111304.
- Vickers AJ, Cronin AM, Maschino AC, Lewith G, Macpherson H, Victor N, et al. Individual patient data meta-analysis of acupuncture for chronic pain: protocol of the Acupuncture Trialists’ Collaboration. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-90.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?. Lancet 1998;352:609-13. http://dx.doi.org/10.1016/S0140-6736(98)01085-X.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. http://dx.doi.org/10.1001/jama.1995.03520290060030.
- Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet 1998;352:364-5. http://dx.doi.org/10.1016/S0140-6736(97)10471-8.
- Melchart D, Linde K, Fischer P, Berman B, White A, Vickers A, et al. Acupuncture for idiopathic headache. Cochrane Database Syst Rev 2001;1. http://dx.doi.org/10.1002/14651858.cd001218.
- Ezzo J, Hadhazy V, Birch S, Lao L, Kaplan G, Hochberg M, et al. Acupuncture for osteoarthritis of the knee: a systematic review. Arthritis Rheum 2001;44:819-25. http://dx.doi.org/10.1002/1529-0131(200104)44:4<819::AID-ANR138>3.0.CO;2-P.
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials 1998;19:159-66. http://dx.doi.org/10.1016/S0197-2456(97)00150-5.
- Tang JL, Zhan SY, Ernst E. Review of randomised controlled trials of traditional Chinese medicine. BMJ 1999;319:160-1. http://dx.doi.org/10.1136/bmj.319.7203.160.
- Wang G, Mao B, Xiong ZY, Fan T, Chen XD, Wang L, et al. The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China. Clin Ther 2007;29:1456-67. http://dx.doi.org/10.1016/j.clinthera.2007.07.023.
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-46.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. http://dx.doi.org/10.1002/sim.1186.
- Kerr DP, Walsh DM, Baxter D. Acupuncture in the management of chronic low back pain: a blinded randomized controlled trial. Clin J Pain 2003;19:364-70. http://dx.doi.org/10.1097/00002508-200311000-00004.
- Irnich D, Behrens N, Molzen H, König A, Gleditsch J, Krauss M, et al. Randomised trial of acupuncture compared with conventional massage and ‘sham’ laser acupuncture for treatment of chronic neck pain. BMJ 2001;322:1574-8. http://dx.doi.org/10.1136/bmj.322.7302.1574.
- Carlsson CP, Sjölund BH. Acupuncture for chronic low back pain: a randomized placebo-controlled study with long-term follow-up. Clin J Pain 2001;17:296-305. http://dx.doi.org/10.1097/00002508-200112000-00003.
- Vas J, Méndez C, Perea-Milla E, Vega E, Panadero MD, León JM, et al. Acupuncture as a complementary therapy to the pharmacological treatment of osteoarthritis of the knee: randomised controlled trial. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38238.601447.3A.
- Vas J, Perea-Milla E, Méndez C, Sánchez Navarro C, León Rubio JM, Brioso M, et al. Efficacy and safety of acupuncture for chronic uncomplicated neck pain: a randomised controlled study. Pain 2006;126:245-55. http://dx.doi.org/10.1016/j.pain.2006.07.002.
- Vas J, Ortega C, Olmo V, Perez-Fernandez F, Hernandez L, Medina I, et al. Single-point acupuncture and physiotherapy for the treatment of painful shoulder: a multicentre randomized controlled trial. Rheumatology (Oxford) 2008;47:887-93. http://dx.doi.org/10.1093/rheumatology/ken040.
- MacPherson H, Maschino AC, Lewith G, Foster NE, Witt CM, Witt C, et al. Characteristics of acupuncture treatment associated with outcome: an individual patient meta-analysis of 17,922 patients with chronic pain in randomised controlled trials. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0077438.
- Cherkin DC, Sherman KJ, Avins AL, Erro JH, Ichikawa L, Barlow WE, et al. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch Intern Med 2009;169:858-66. http://dx.doi.org/10.1001/archinternmed.2009.65.
- Suarez-Almazor ME, Looney C, Liu Y, Cox V, Pietz K, Marcus DM, et al. A randomized controlled trial of acupuncture for osteoarthritis of the knee: effects of patient-provider communication. Arthritis Care Res 2010;62:1229-36. http://dx.doi.org/10.1002/acr.20225.
- Lansdown H, Howard K, Brealey S, MacPherson H. Acupuncture for pain and osteoarthritis of the knee: a pilot study for an open parallel-arm randomised controlled trial. BMC Musculoskelet Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-130.
- Molsberger AF, Schneider T, Gotthardt H, Drabik A. German Randomized Acupuncture Trial for chronic shoulder pain (GRASP) – a pragmatic, controlled, patient-blinded, multi-centre trial in an outpatient care environment. Pain 2010;151:146-54. http://dx.doi.org/10.1016/j.pain.2010.06.036.
- Coeytaux RR, Kaufman JS, Kaptchuk TJ, Chen W, Miller WC, Callahan LF, et al. A randomized, controlled trial of acupuncture for chronic daily headache. Headache 2005;45:1113-23. http://dx.doi.org/10.1111/j.1526-4610.2005.00235.x.
- Molsberger AF, Mau J, Pawelec DB, Winkler J. Does acupuncture improve the orthopedic management of chronic low back pain: a randomized, blinded, controlled trial with 3 months follow up. Pain 2002;99:579-87. http://dx.doi.org/10.1016/S0304-3959(02)00269-5.
- Kennedy S, Baxter GD, Kerr DP, Bradbury I, Park J, McDonough SM. Acupuncture for acute non-specific low back pain: a pilot randomised non-penetrating sham controlled trial. Complement Ther Med 2008;16:139-46. http://dx.doi.org/10.1016/j.ctim.2007.03.001.
- Cherkin DC, Eisenberg D, Sherman KJ, Barlow W, Kaptchuk TJ, Street J, et al. Randomized trial comparing traditional Chinese medical acupuncture, therapeutic massage, and self-care education for chronic low back pain. Arch Intern Med 2001;161:1081-8. http://dx.doi.org/10.1001/archinte.161.8.1081.
- White P, Lewith G, Prescott P, Conway J. Acupuncture versus placebo for the treatment of chronic mechanical neck pain: a randomized, controlled trial. Ann Intern Med 2004;141:911-19. http://dx.doi.org/10.7326/0003-4819-141-12-200412210-00007.
- Salter GC, Roman M, Bland MJ, MacPherson H. Acupuncture for chronic neck pain: a pilot for a randomised controlled trial. BMC Musculoskelet Disord 2006;7. http://dx.doi.org/10.1186/1471-2474-7-99.
- Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med 2004;141:901-10. http://dx.doi.org/10.7326/0003-4819-141-12-200412210-00006.
- Foster NE, Thomas E, Barlas P, Hill JC, Young J, Mason E, et al. Acupuncture as an adjunct to exercise based physiotherapy for osteoarthritis of the knee: randomised controlled trial. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39280.509803.BE.
- Williamson L, Wyatt MR, Yein K, Melton JT. Severe knee osteoarthritis: a randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology (Oxford) 2007;46:1445-9. http://dx.doi.org/10.1093/rheumatology/kem119.
- Kleinhenz J, Streitberger K, Windeler J, Güssbacher A, Mavridis G, Martin E. Randomised clinical trial comparing the effects of acupuncture and a newly designed placebo needle in rotator cuff tendinitis. Pain 1999;83:235-41. http://dx.doi.org/10.1016/S0304-3959(99)00107-4.
- Guerra de Hoyos JA, Andrés Martín Mdel C, Bassas y Baena de Leon E, Vigára Lopez M, Molina López T, Verdugo Morilla FA, et al. Randomised trial of long term effect of acupuncture for shoulder pain. Pain 2004;112:289-98. http://dx.doi.org/10.1016/j.pain.2004.08.030.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. http://dx.doi.org/10.1136/bmj.315.7109.629.
- Green S, Buchbinder R, Hetrick S. Acupuncture for shoulder pain. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.cd005319.
- White AR, Ernst E. A systematic review of randomized controlled trials of acupuncture for neck pain. Rheumatology 1999;38:143-7. http://dx.doi.org/10.1093/rheumatology/38.2.143.
- Manheimer E, Cheng K, Linde K, Lao L, Yoo J, Wieland S, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd001977.pub2.
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c221.
- Kaptchuk TJ, Stason WB, Davis RB, Legedza AR, Schnyer RN, Kerr CE, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 2006;332:391-7. http://dx.doi.org/10.1136/bmj.38726.603310.55.
- Linde K, Niemann K, Meissner K. Are sham acupuncture interventions more effective than (other) placebos? A re-analysis of data from the Cochrane review on placebo effects. Forsch Komplementrmed 2010;17:259-64. http://dx.doi.org/10.1159/000320374.
- Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance?. Ann Intern Med 2002;136:817-25. http://dx.doi.org/10.7326/0003-4819-136-11-200206040-00011.
- Lundeberg T, Lund I, Sing A, Näslund J. Is placebo acupuncture what it is intended to be?. Evid Based Complement Alternat Med 2011;2011.
- Birch S, Felt R. Understanding Acupuncture. Edinburgh: Churchill Livingstone; 1999.
- Hughes JG, Goldbart J, Fairhurst E, Knowles K. Exploring acupuncturists’ perceptions of treating patients with rheumatoid arthritis. Complement Ther Med 2007;15:101-8. http://dx.doi.org/10.1016/j.ctim.2006.09.008.
- Paterson C, Britten N. Acupuncture as a complex intervention: a holistic model. J Altern Complement Med 2004;10:791-80. http://dx.doi.org/10.1089/acm.2004.10.791.
- Witt CM, Lüdtke R, Wegscheider K, Willich SN. Physician characteristics and variation in treatment outcomes: are better qualified and experienced physicians more successful in treating patients with chronic pain with acupuncture?. J Pain 2010;11:431-5. http://dx.doi.org/10.1016/j.jpain.2009.08.010.
- Birch S. Reflections on the German acupuncture studies. J Chinese Med 2007;83:12-7.
- Hanfileti L. What Licensed Acupuncturists Need to Know about the Training and Qualifications of Physicians Providing Medical Acupuncture 2013. www.insights-for-acupuncturists.com/medical-acupuncture.html (accessed 16 September 2013).
- Wampold BE, Brown GS. Estimating variability in outcomes attributable to therapists: a naturalistic study of outcomes in managed care. J Consult Clin Psychol 2005;73:914-23. http://dx.doi.org/10.1037/0022-006X.73.5.914.
- Lewis M, Morley S, van der Windt DA, Hay E, Jellema P, Dziedzic K, et al. Measuring practitioner/therapist effects in randomised trials of low back pain and neck pain interventions in primary care settings. Eur J Pain 2010;14:1033-9. http://dx.doi.org/10.1016/j.ejpain.2010.04.002.
- Price S, Mercer SW, MacPherson H. Practitioner empathy, patient enablement and health outcomes: a prospective study of acupuncture patients. Patient Educ Couns 2006;63:239-45. http://dx.doi.org/10.1016/j.pec.2005.11.006.
- Scheid V, Scheid V, MacPherson H. Integrating East Asian Medicine into Contemporary Healthcare. Edinburgh: Churchill Livingstone; 2012.
- Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91-7. http://dx.doi.org/10.1136/ard.60.2.91.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502. http://dx.doi.org/10.1136/ard.16.4.494.
- Lethbridge-Cejku M, Scott WW, Reichle R, Ettinger WH, Zonderman A, Costa P, et al. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore Longitudinal Study of Aging. Arthritis Care Res 1995;8:182-8. http://dx.doi.org/10.1002/art.1790080311.
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145-55. http://dx.doi.org/10.1136/ard.2003.011742.
- Tramèr MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain 2000;85:169-82. http://dx.doi.org/10.1016/S0304-3959(99)00267-5.
- Pound P, Britten N, Morgan M, Yardley L, Pope C, Daker-White G, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med 2005;61:133-55. http://dx.doi.org/10.1016/j.socscimed.2004.11.063.
- Osteoarthritis Nation: The Most Comprehensive UK Report of People with Osteoarthritis. London: Arthritis Care; 2004.
- Devos-Comby L, Cronan T, Roesch SC. Do exercise and self-management interventions benefit patients with osteoarthritis of the knee? A metaanalytic review. J Rheumatol 2006;33:744-56.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: CRD, University of York; 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2535.
- Corbett M, Rice S, Slack R, Harden M, Madurasinghe V, Sutton A, et al. Acupuncture and Other Physical Treatments for the Relief of Chronic Pain due to Osteoarthritis of the Knee: A Systematic Review and Network Meta-Analysis. York: CRD, University of York; 2011.
- Corbett MS, Rice SJ, Madurasinghe V, Slack R, Fayter DA, Harden M, et al. Acupuncture and other physical treatments for the relief of pain due to osteoarthritis of the knee: network meta-analysis. Osteoarthritis Cartilage 2013;21:1290-8. http://dx.doi.org/10.1016/j.joca.2013.05.007.
- Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al. Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12340.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. http://dx.doi.org/10.1002/sim.3767.
- Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med 2009;28:1861-81. http://dx.doi.org/10.1002/sim.3594.
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol 2009;169:1158-65. http://dx.doi.org/10.1093/aje/kwp014.
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. http://dx.doi.org/10.1177/0962280207080643.
- Ades AE, Welton N, Lu G. Introduction to Mixed Treatment Comparisons. Bristol: Bristol University; 2007.
- Brooks SP, Gelman AJ. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55.
- Lu G, Ades AE, Sutton AJ, Cooper NJ, Briggs AH, Caldwell DM. Meta-analysis of mixed treatment comparisons at multiple follow-up times. Stat Med 2007;26:3681-99. http://dx.doi.org/10.1002/sim.2831.
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447-59. http://dx.doi.org/10.1198/016214505000001302.
- Spiegelhalter DJ, Best NG, Carlin BP, Van der Linden A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B 2002;64:583-639. http://dx.doi.org/10.1111/1467-9868.00353.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. http://dx.doi.org/10.1016/j.jclinepi.2010.03.016.
- Scholten RJ, de Beurs E, Bouter LM. From effect size into number needed to treat. Lancet 1999;354. http://dx.doi.org/10.1016/S0140-6736(05)77952-6.
- Woolacott NF, Corbett MS, Rice SJ. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology 2012;51:1440-6. http://dx.doi.org/10.1093/rheumatology/kes043.
- Bellamy N, Carette S, Ford PM, Kean WF, le Riche NG, Lussier A, et al. Osteoarthritis antirheumatic drug trials. III. Setting the delta for clinical trials – results of a consensus development (Delphi) exercise. J Rheumatol 1992;19:451-7.
- Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27:2635-41.
- Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005;64:29-33. http://dx.doi.org/10.1136/ard.2004.022905.
- Dias RC, Dias JM, Ramos LR. Impact of an exercise and walking protocol on quality of life for elderly people with OA of the knee. Physiother Res Int 2003;8:121-30. http://dx.doi.org/10.1002/pri.280.
- Weiner DK, Rudy TE, Morone N, Glick R, Kwoh CK. Efficacy of periosteal stimulation therapy for the treatment of osteoarthritis-associated chronic knee pain: an initial controlled clinical trial. J Am Geriatr Soc 2007;55:1541-7. http://dx.doi.org/10.1111/j.1532-5415.2007.01314.x.
- Aglamiş B, Toraman NF, Yaman H. Change of quality of life due to exercise training in knee osteoarthritis: SF-36 and WOMAC. J Back Musculoskelet Rehabil 2009;22:43-8. http://dx.doi.org/10.3233/BMR-2009-0219.
- Börjesson M, Robertson E, Weidenhielm L, Mattsson E, Olsson E. Physiotherapy in knee osteoarthrosis: effect on pain and walking. Physiother Res Int 1996;1:89-97. http://dx.doi.org/10.1002/pri.6120010205.
- Cheing GL, Tsui AY, Lo SK, Hui-Chan CW. Optimal stimulation duration of TENS in the management of osteoarthritic knee pain. J Rehabil Med 2003;35:62-8. http://dx.doi.org/10.1080/16501970306116.
- Lu TW, Wei IP, Liu YH, Hsu WC, Wang TM, Chang CF, et al. Immediate effects of acupuncture on gait patterns in patients with knee osteoarthritis. Chin Med J 2010;123:165-72.
- Yip YB, Sit JW, Fung KK, Wong DY, Chong SY, Chung LH, et al. Impact of an arthritis self-management programme with an added exercise component for osteoarthritic knee sufferers on improving pain, functional outcomes, and use of health care services: an experimental study. Patient Educ Couns 2007;65:113-21. http://dx.doi.org/10.1016/j.pec.2006.06.019.
- Jamtvedt G, Dahm KT, Christie A, Moe RH, Haavardsholm E, Holm I, et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys Ther 2008;88:123-36. http://dx.doi.org/10.2522/ptj.20070043.
- Bjordal JM, Ljunggren AE, Klovning A, Slørdal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38273.626655.63.
- Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med 2012;172:1444-53. http://dx.doi.org/10.1001/archinternmed.2012.3654.
- Lund I, Näslund J, Lundeberg T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: a physiologist’s perspective. Chin Med 2009;4. http://dx.doi.org/10.1186/1749-8546-4-1.
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465-74. http://dx.doi.org/10.1002/acr.21596.
- Treatment of Osteoarthritis of the Knee (Non-Arthroplasty). Rosemont, IL: AAOS; 2008.
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137-62. http://dx.doi.org/10.1016/j.joca.2007.12.013.
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146:238-44. http://dx.doi.org/10.1016/j.pain.2009.08.019.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Guide to the Methods of Technology Appraisal. London: NICE; 2012.
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making?. Health Econ 2006;15:677-87. http://dx.doi.org/10.1002/hec.1093.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8.
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313-24. http://dx.doi.org/10.1002/sim.1201.
- Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1-19. http://dx.doi.org/10.2165/00019053-200624010-00001.
- Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 2008;26:753-67. http://dx.doi.org/10.2165/00019053-200826090-00006.
- Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med 1995;14:2057-79. http://dx.doi.org/10.1002/sim.4780141902.
- Higgins JP, Whitehead A, Turner RM, Omar RZ, Thompson SG. Meta-analysis of continuous outcome data from individual patients. Stat Med 2001;20:2219-41. http://dx.doi.org/10.1002/sim.918.
- Riley RD, Dodd SR, Craig JV, Thompson JR, Williamson PR. Meta-analysis of diagnostic test studies using individual patient data and aggregate data. Stat Med 2008;27:6111-36. http://dx.doi.org/10.1002/sim.3441.
- Riley RD, Lambert PC, Staessen JA, Wang J, Gueyffier F, Thijs L, et al. Meta-analysis of continuous outcomes combining individual patient data and aggregate data. Stat Med 2008;27:1870-93. http://dx.doi.org/10.1002/sim.3165.
- Saramago P, Manca A, Sutton AJ. Deriving input parameters for cost-effectiveness modeling: taxonomy of data types and approaches to their statistical synthesis. Value Health 2012;15:639-49. http://dx.doi.org/10.1016/j.jval.2012.02.009.
- Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta-analysis of clinical trials with binary outcomes. Stat Med 2000;19:3417-32. http://dx.doi.org/10.1002/1097-0258(20001230)19:24<3417::AID-SIM614>3.0.CO;2-L.
- Goldstein H, Yang M, Omar R, Turner R, Thompson S. Meta-analysis using multilevel models with an application to the study of class size effects. J R Stat Soc Series C (Applied Stat) 2000;49:399-412. http://dx.doi.org/10.1111/1467-9876.00200.
- Smith CT, Williamson PR, Marson AG. An overview of methods and empirical comparison of aggregate data and individual patient data results for investigating heterogeneity in meta-analysis of time-to-event outcomes. J Eval Clin Pract 2005;11:468-78. http://dx.doi.org/10.1111/j.1365-2753.2005.00559.x.
- Welton NJ, Willis SR, Ades AE. Synthesis of survival and disease progression outcomes for health technology assessment of cancer therapies. Res Synth Methods 2010;1:239-57. http://dx.doi.org/10.1002/jrsm.21.
- Saramago P, Sutton AJ, Cooper NJ, Manca A. Mixed treatment comparisons using aggregate- and individual-participant level data: an efficient use of evidence for cost-effectiveness modelling. Value Health 2011;14:A237-8. http://dx.doi.org/10.1016/j.jval.2011.08.024.
- Riley RD, Kauser I, Bland M, Thijs L, Staessen JA, Wang J, et al. Meta-analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Stat Med 2013;32:2747-66. http://dx.doi.org/10.1002/sim.5726.
- Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123-4. http://dx.doi.org/10.1136/bmj.323.7321.1123.
- Dias S, Welton N, Sutton A, Ades A. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. Sheffield: Decision Support Unit, School of Health and Related Research, University of Sheffield; 2011.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. http://dx.doi.org/10.1177/0272989X12458724.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford: Wiley-Blackwell; 2011.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2012.
- Williams A. EuroQol – a new facility for the measurement of health-related quality-of-life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Latimer NR, Bhanu AC, Whitehurst DG. Inconsistencies in NICE guidance for acupuncture: reanalysis and discussion. Acupunct Med 2012;30:182-6. http://dx.doi.org/10.1136/acupmed-2012-010152.
- Osteoarthritis: the Care and Management of Osteoarthritis in Adults. London: National Collaborating Centre for Chronic Conditions; 2008.
- Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al. Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8490.
- UK BEAM Trial Team . United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: effectiveness of physical treatments for back pain in primary care. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38282.669225.AE.
- Underwood M, Ashby D, Cross P, Hennessy E, Letley L, Martin J, et al. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomised controlled trial and patient preference study. BMJ 2008;336:138-42. http://dx.doi.org/10.1136/bmj.39399.656331.25.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Hernández Alava M, Wailoo AJ, Ara R. Tails from the peak district: adjusted limited dependent variable mixture models of EQ-5D questionnaire health state utility values. Value Health 2012;15:550-61. http://dx.doi.org/10.1016/j.jval.2011.12.014.
- Dakin HA, Welton NJ, Ades AE, Collins S, Orme M, Kelly S. Mixed treatment comparison of repeated measurements of a continuous endpoint: an example using topical treatments for primary open-angle glaucoma and ocular hypertension. Stat Med 2011;30:2511-35. http://dx.doi.org/10.1002/sim.4284.
- Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes 2013;11. http://dx.doi.org/10.1186/1477-7525-11-151.
- Rowen D, Brazier J, Roberts J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship?. Health Qual Life Outcomes 2009;7. http://dx.doi.org/10.1186/1477-7525-7-27.
- Gray AM, Rivero-Arias O, Clarke PM. Estimating the association between SF-12 responses and EQ-5D utility values by response mapping. Med Decis Making 2006;26:18-29. http://dx.doi.org/10.1177/0272989X05284108.
- Maund E, Craig D, Suekarran S, Neilson A, Wright K, Brealey S, et al. Management of frozen shoulder: a systematic review and cost-effectiveness analysis. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16110.
- Barton GR, Sach TH, Jenkinson C, Avery AJ, Doherty M, Muir KR. Do estimates of cost–utility based on the EQ-5D differ from those based on the mapping of utility scores?. Health Qual Life Outcomes 2008;6. http://dx.doi.org/10.1186/1477-7525-6-51.
- Khan K. Mapping Outcomes Data to Estimate Health State Utilities: An Analysis of Individual Patient Level Data from 15 Acupuncture Trials. York: University of York; 2011.
- Chan KKW, Willan AR, Gupta M, Pullenayegum E. Underestimation of uncertainties in health utilities derived from mapping algorithms involving health-related quality-of-life measures: statistical explanations and potential remedies. Med Decis Making 2014;34:863-72. http://dx.doi.org/10.1177/0272989x13517750.
- O’Hagan A, Luce B. A Primer on Bayesian Statistics in Health Economics and Outcomes Research. Sheffield: Centre for Bayesian Statistics in Health Economics; 2003.
- Senn S. Change from baseline and analysis of covariance revisited. Stat Med 2006;25:4334-44. http://dx.doi.org/10.1002/sim.2682.
- Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies. J Clin Epidemiol 2006;59:920-5. http://dx.doi.org/10.1016/j.jclinepi.2006.02.007.
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity –subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 2013;33:618-40. http://dx.doi.org/10.1177/0272989X13485157.
- Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Computing 2000;10:325-37. http://dx.doi.org/10.1023/A:1008929526011.
- Plummer M, Best N, Cowles K, Vines K. CODA: convergence diagnosis and output analysis for MCMC. R News 2006;6:7-11.
- Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci 1992;7:457-72. http://dx.doi.org/10.1214/ss/1177011136.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Tudur Smith C, Dwan K, Altman DG, Clarke M, Riley R, Williamson PR. Sharing individual participant data from clinical trials: an opinion survey regarding the establishment of a central repository. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0097886.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19140.
- Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol 1997;24:799-802.
- Drummond M. Twenty years of using economic evaluations for drug reimbursement decisions: what has been achieved?. J Health Polit Policy Law 2013;38:1081-102. http://dx.doi.org/10.1215/03616878-2373148.
- Urdahl H, Manca A, Sculpher MJ. Assessing generalisability in model-based economic evaluation studies: a structured review in osteoporosis. Pharmacoeconomics 2006;24:1181-97. http://dx.doi.org/10.2165/00019053-200624120-00004.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices – overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health 2012;15:796-803. http://dx.doi.org/10.1016/j.jval.2012.06.012.
- Complementary, Alternative, or Integrative Health: What’s In a Name?. Bethesda, MD: National Center for Complementary and Alternative Medicine; 2013.
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 1998;280:1569-75. http://dx.doi.org/10.1001/jama.280.18.1569.
- Fairfield KM, Eisenberg DM, Davis RB, Libman H, Phillips RS. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med 1998;158:2257-64. http://dx.doi.org/10.1001/archinte.158.20.2257.
- Report of the Select Committee on Science and Technology: Complementary and Alternative Medicine. London: The Stationery Office; 2000.
- Lorenc A, Leach J, Robinson N. Clinical guidelines in the UK: do they mention complementary and alternative medicine (CAM) – are CAM professional bodies aware?. Eur J Integrative Med 2014;6:164-75. http://dx.doi.org/10.1016/j.eujim.2013.11.003.
- Acupuncture. York: CRD, University of York; 2001.
- Posadzki P, Alotaibi A, Ernst E. Prevalence of use of complementary and alternative medicine (CAM) by physicians in the UK: a systematic review of surveys. Clin Med 2012;12:505-12. http://dx.doi.org/10.7861/clinmedicine.12-6-505.
- Laufer S. Osteoarthritis therapy – are there still unmet needs?. Rheumatology 2004;43:i9-15. http://dx.doi.org/10.1093/rheumatology/keh103.
- Chang J, Kauf TL, Reed SD, Friedman JY, Omar M, Kahler KH, et al. Productivity loss as an indicator of unmet needs for osteoarthritis patients. Arthritis Rheum 2003;48:S292-3.
- Selfe TK, Taylor AG. Acupuncture and osteoarthritis of the knee: a review of randomized, controlled trials. Fam Community Health 2008;31:247-54. http://dx.doi.org/10.1097/01.FCH.0000324482.78577.0f.
- Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. Rosemont, IL: AAOS; 2013.
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil 2014;22:363-88. http://dx.doi.org/10.1016/j.joca.2014.01.003.
- SIGN 136: Management of Chronic Pain. A National Clinical Guideline. Edinburgh: Scottish Intercollegiate Guidelines Network; 2013.
- Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. Guidance on the management of pain in older people. Age Ageing 2013;42:i1-57. http://dx.doi.org/10.1093/ageing/afs199.
- Brazier JE, Harper R, Munro J, Walters SJ, Snaith ML. Generic and condition-specific outcome measures for people with osteoarthritis of the knee. Rheumatology 1999;38:870-7. http://dx.doi.org/10.1093/rheumatology/38.9.870.
- Brazier J, Deverill M, Green C, Harper R, Booth A. A review of the use of health status measures in economic evaluation. Health Technol Assess 1999;3.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-54.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Kirwan JR, Reeback JS. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol 1986;25:206-9. http://dx.doi.org/10.1093/rheumatology/25.2.206.
- Versteegh MM, Leunis A, Uyl-de Groot CA, Stolk EA. Condition-specific preference-based measures: benefit or burden?. Value Health 2012;15:504-13. http://dx.doi.org/10.1016/j.jval.2011.12.003.
- Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health-related quality of life outcomes in meta-analysis – a tutorial and review of methods for enhancing interpretability: enhancing interpretability in continuous meta-analysis. Res Synthesis Methods 2011;2:188-203. http://dx.doi.org/10.1002/jrsm.46.
- Cummings P. Arguments for and against standardized mean differences (effect sizes). Arch Pediatr Adolesc Med 2011;165:592-6. http://dx.doi.org/10.1001/archpediatrics.2011.97.
- McKenna C, Bojke L, Manca A, Adebajo A, Dickson J, Helliwell P, et al. Shoulder acute pain in primary health care: is retraining GPs effective? The SAPPHIRE randomized trial: a cost-effectiveness analysis. Rheumatology 2009;48:558-63. http://dx.doi.org/10.1093/rheumatology/kep008.
- Barton GR, Sach TH, Jenkinson C, Doherty M, Avery AJ, Muir KR. Lifestyle interventions for knee pain in overweight and obese adults aged > or = 45: economic evaluation of randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2273.
- Gu NY, Bell C, Botteman MF, Ji X, Carter JA, van Hout B. Estimating preference-based EQ-5D health state utilities or item responses from neuropathic pain scores. Patient 2012;5:185-97.
- Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Vickers AJ. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med Res Methodol 2005;5. http://dx.doi.org/10.1186/1471-2288-5-35.
- Wooldridge JM. Introductory Econometrics: A Modern Approach. Cincinnati, OH: South-Western College Publishing; 2003.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641-56. http://dx.doi.org/10.1177/0272989X12455847.
- Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Software 2005;12:1-16. http://dx.doi.org/10.18637/jss.v012.i03.
- Nüesch E, Reichenbach S, Trelle S, Rutjes AW, Liewald K, Sterchi R, et al. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta-epidemiologic study. Arthritis Rheum 2009;61:1633-41. http://dx.doi.org/10.1002/art.24894.
- Lunn D. The BUGS Book: A Practical Introduction to Bayesian Analysis. Boca Raton, FL: CRC Press; 2013.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Castelnuovo E, Cross P, Mt-Isa S, Spencer A, Underwood M. Cost-effectiveness of advising the use of topical or oral ibuprofen for knee pain; the TOIB study [ISRCTN: 79353052]. Rheumatology 2008;47:1077-81. http://dx.doi.org/10.1093/rheumatology/ken128.
- Hawkins N, Scott DA. Cost-effectiveness analysis: discount the placebo at your peril. Med Decis Making 2010;30:536-43. http://dx.doi.org/10.1177/0272989X10362106.
- Barton GR, Briggs AH, Fenwick EAL. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Jordan K, Jinks C, Croft P. A prospective study of the consulting behaviour of older people with knee pain. Br J Gen Pract 2006;56:269-76.
- Mitchell HL, Carr AJ, Scott DL. The management of knee pain in primary care: factors associated with consulting the GP and referrals to secondary care. Rheumatology 2006;45:771-6. http://dx.doi.org/10.1093/rheumatology/kei214.
- Taxonomy. ONS; 2010.
- Mitchell HL, Hurley MV. Management of chronic knee pain: a survey of patient preferences and treatment received. BMC Musculoskelet Disord 2008;9. http://dx.doi.org/10.1186/1471-2474-9-123.
- Claxton K, Palmer S, Longworth L, Bojke L, Griffin S, McKenna C, et al. Informing a decision framework for when NICE should recommend the use of health technologies only in the context of an appropriately designed programme of evidence development. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16460.
- AACP . AACP Homepage n.d. www.aacp.org.uk/ (accessed 20 December 2015).
- Adedoyin RA, Olaogun MO, Fagbeja OO. Effect of interferential current stimulation in management of osteo-arthritic knee pain. Physiotherapy 2002;88:493-9. http://dx.doi.org/10.1016/S0031-9406(05)60851-6.
- Gundog M, Atamaz F, Kanyilmaz S, Kirazli Y, Celepoglu G. Interferential current therapy in patients with knee osteoarthritis: comparison of the effectiveness of different amplitude-modulated frequencies. Am J Phys Med Rehabil 2012;91:107-13. http://dx.doi.org/10.1097/PHM.0b013e3182328687.
- Burch FX, Tarro JN, Greenberg JJ, Carroll WJ. Evaluating the benefits of patterned stimulation in the treatment of osteoarthritis of the knee: a multi-center, randomized, single-blind, controlled study with an independent masked evaluator. Osteoarthr Cartil 2008;16:865-72. http://dx.doi.org/10.1016/j.joca.2007.11.013.
- Longworth L, Rowen. NICE DSU Technical Support Document 10: The Use of Mapping Methods to Estimate Health State Utility Values. Sheffield; 2011.
- Lu G, Brazier JE, Ades AE. Mapping from disease-specific to generic health-related quality-of-life scales: a common factor model. Value Health 2013;16:177-84. http://dx.doi.org/10.1016/j.jval.2012.07.003.
- Lu G, Kounali D, Ades AE. Simultaneous multioutcome synthesis and mapping of treatment effects to a common scale. Value Health 2014;17:280-7. http://dx.doi.org/10.1016/j.jval.2013.12.006.
- Shah S, Farrow A, Esnouf A. Availability and use of electrotherapy devices: a survey. Int J Ther Rehabil 2007;14:260-4. http://dx.doi.org/10.12968/ijtr.2007.14.6.23895.
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med 1998;4:1241-3. http://dx.doi.org/10.1038/3218.
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLOS Med 2008;5. http://dx.doi.org/10.1371/journal.pmed.0050045.
- Smith CA, Hay PP, MacPherson H. Acupuncture for depression. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd004046.pub3.
- Mellor-Clark J, Simms-Ellis R, Burton M. National survey of counsellors working in primary care: evidence for growing professionalisation?. Occas Pap R Coll Gen Pract 2001;79:vi-7.
- Bower P, Knowles S, Coventry PA, Rowland N. Counselling for mental health and psychosocial problems in primary care. Cochrane Database Syst Rev 2011;9. http://dx.doi.org/10.1002/14651858.cd001025.pub3.
- MacPherson H, Richmond S, Bland JM, Lansdown H, Hopton A, Kang’ombe A, et al. Acupuncture, Counseling, and Usual care for Depression (ACUDep): study protocol for a randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-209.
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
- MacPherson H, Schroer S. Acupuncture as a complex intervention for depression: a consensus method to develop a standardised treatment protocol for a randomised controlled trial. Complement Ther Med 2007;15:92-100. http://dx.doi.org/10.1016/j.ctim.2006.09.006.
- Roth A, Hill A, Pilling S. The Competences Required to Deliver Effective Humanistic Psychological Therapies. London: University College London; 2009.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London: Sage; 2006.
- Hopton A, Eldred J, MacPherson H. Patients’ experiences of acupuncture and counselling for depression and comorbid pain: a qualitative study nested within a randomised controlled trial. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005144.
- Hopton A, Macpherson H, Keding A, Morley S. Acupuncture, counselling or usual care for depression and comorbid pain: secondary analysis of a randomised controlled trial. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-004964.
- Vannoy SD, Arean P, Unützer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatr Serv 2010;61:160-3. http://dx.doi.org/10.1176/ps.2010.61.2.160.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- MacPherson H, Newbronner L, Chamberlain R, Richmond SJ, Lansdown H, Perren S, et al. Practitioner perspectives on strategies to promote longer-term benefits of acupuncture or counselling for depression: a qualitative study. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0104077.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- MacPherson H, Tilbrook H, Bland JM, Bloor K, Brabyn S, Cox H, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterol 2012;12. http://dx.doi.org/10.1186/1471-230X-12-150.
- NHS Choices . Acupuncture n.d. www.nhs.uk/Conditions/acupuncture/Pages/Introduction.aspx (accessed 16 July 2016).
- Dolan P, Gudex C. Time preference, duration and health state valuations. Health Econ 1995;4:289-99. http://dx.doi.org/10.1002/hec.4730040405.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45. http://dx.doi.org/10.1002/sim.1794.
- Macpherson H, Elliot B, Hopton A, Lansdown H, Richmond S. Acupuncture for depression: patterns of diagnosis and treatment within a randomised controlled trial. Evid Based Complement Alternat Med 2013;2013. http://dx.doi.org/10.1155/2013/286048.
- Perren S, Richmond S, MacPherson H. The human face of an RCT: reflections on providing counselling for clients with moderate to severe depression in a randomised controlled trial. Healthc Counsell Psychother J 2015:8-13.
- Allen JJB, Schnyer R, Hitt SK. The efficacy of acupuncture in the treatment of major depression in women. Psychol Sci 1998;9:397-401. http://dx.doi.org/10.1111/1467-9280.00074.
- Allen JJ, Schnyer RN, Chambers AS, Hitt SK, Moreno FA, Manber R. Acupuncture for depression: a randomized controlled trial. J Clin Psychiatry 2006;67:1665-73. http://dx.doi.org/10.4088/JCP.v67n1101.
- Cape J, Whittington C, Buszewicz M, Wallace P, Underwood L. Brief psychological therapies for anxiety and depression in primary care: meta-analysis and meta-regression. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-38.
- McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord 2010;127:122-9. http://dx.doi.org/10.1016/j.jad.2010.04.030.
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995;117:285-30. http://dx.doi.org/10.1037/0033-2909.117.2.285.
- Hawker GA, Gignac MA, Badley E, Davis AM, French MR, Li Y, et al. A longitudinal study to explain the pain–depression link in older adults with osteoarthritis. Arthritis Care Res 2011;63:1382-90. http://dx.doi.org/10.1002/acr.20298.
- White DK, Wilson JC, Keysor JJ. Measures of adult general functional status: SF-36 Physical Functioning Subscale (PF-10), Health Assessment Questionnaire (HAQ), Modified Health Assessment Questionnaire (MHAQ), Katz Index of Independence in activities of daily living, Functional Independence Measure (FIM), and Osteoarthritis-Function-Computer Adaptive Test (OA-Function-CAT). Arthritis Care Res 2011;63:S297-307. http://dx.doi.org/10.1002/acr.20638.
- Eccleston C, Williams A de C, Morley S. Psychological Therapies for the Management of Chronic Pain (Excluding Headache) in Adults. Chichester: John Wiley; 2009.
- Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. Front Biosci (Landmark Ed) 2009;14:5031-51. http://dx.doi.org/10.2741/3585.
- Hannes K, Noyes J, Booth A, Hannes K, Harden A, Harris J, et al. Supplementary Guidance for Inclusion of Qualitative Research in Cochrane Systematic Reviews of Interventions. Cochrane Collaboration Qualitative Methods Group; 2011.
- MacPherson H, Thorpe L, Thomas K. Beyond needling – therapeutic processes in acupuncture care: a qualitative study nested within a low-back pain trial. J Altern Complement Med 2006;12:873-80. http://dx.doi.org/10.1089/acm.2006.12.873.
- Perren S, Godfrey M, Rowland N. The long-term effects of counselling: the process and mechanisms that contribute to ongoing change from a user perspective. Couns Psychother Res 2009;9:241-9. http://dx.doi.org/10.1080/14733140903150745.
- Yeung AS, Ameral VE, Chuzi SE, Fava M, Mischoulon D. A pilot study of acupuncture augmentation therapy in antidepressant partial and non-responders with major depressive disorder. J Affect Disord 2011;130:285-9. http://dx.doi.org/10.1016/j.jad.2010.07.025.
- Mischoulon D, Brill CD, Ameral VE, Fava M, Yeung AS. A pilot study of acupuncture monotherapy in patients with major depressive disorder. J Affect Disord 2012;141:469-73. http://dx.doi.org/10.1016/j.jad.2012.03.023.
- Richmond SJ, Keding A, Hover M, Gabe R, Cross B, Torgerson D, et al. Feasibility, acceptability and validity of SMS text messaging for measuring change in depression during a randomised controlled trial. BMC Psychiatry 2015;15. http://dx.doi.org/10.1186/s12888-015-0456-3.
- Keding A, Böhnke JR, Croudace TJ, Richmond SJ, MacPherson H. Validity of single item responses to short message service texts to monitor depression: an mHealth sub-study of the UK ACUDep trial. BMC Med Res Methodol 2015;15. http://dx.doi.org/10.1186/s12874-015-0054-6.
- Abrahams S, Demetriou P. A comparison of the benefits of physiotherapy and anti-inflammatory drugs for osteoarthritis of the knee. J Orthop Med 2002;24:79-85. http://dx.doi.org/10.1080/1355297X.2002.11736170.
- Alcidi L, Beneforti E, Maresca M, Santosuosso U, Zoppi M. Low power radiofrequency electromagnetic radiation for the treatment of pain due to osteoarthritis of the knee. Reumatismo 2007;59:140-5.
- An B, Dai K, Zhu Z, Wang Y, Hao Y, Tang T, et al. Baduanjin alleviates the symptoms of knee osteoarthritis. J Altern Complement Med 2008;14:167-74. http://dx.doi.org/10.1089/acm.2007.0600.
- Arman MI. Therapeutischer Effekt der Kryotherapie bei der aktivierten Gonarthrose. Eine untersucherblinde, kontrollierte Studie. Z Phys Med Baln Med Klim 1988;17:368-9.
- Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol 2001;28:1655-65.
- Baker K, Goggins J, Xie H, Szumowski K, LaValley M, Hunter DJ, et al. A randomized crossover trial of a wedged insole for treatment of knee osteoarthritis. Arthritis Rheum 2007;56:1198-203. http://dx.doi.org/10.1002/art.22516.
- Bálint GP, Buchanan WW, Adám A, Ratkó I, Poór L, Bálint PV, et al. The effect of the thermal mineral water of Nagybaracska on patients with knee joint osteoarthritis – a double blind study. Clin Rheumatol 2007;26:890-4. http://dx.doi.org/10.1007/s10067-006-0420-1.
- Bao F, Wu Z. Observation on therapeutic effect of knee osteoarthritis treated by electroacupuncture. Int J Clin Acupuncture 2007;16:191-5.
- Berman BM, Singh BB, Lao L, Langenberg P, Li H, Hadhazy V, et al. A randomized trial of acupuncture as an adjunctive therapy in osteoarthritis of the knee. Rheumatology 1999;38:346-54. http://dx.doi.org/10.1093/rheumatology/38.4.346.
- Bezalel T, Carmeli E, Katz-Leurer M. The effect of a group education programme on pain and function through knowledge acquisition and home-based exercise among patients with knee osteoarthritis: a parallel randomised single-blind clinical trial. Physiotherapy 2010;96:137-43. http://dx.doi.org/10.1016/j.physio.2009.09.009.
- Bilgici A, Akdeniz O, Kuru O, Ulusoy H. The effect of a home-based exercise therapy versus an aerobic exercise programme on pain and functional disability in patients with knee osteoarthritis. Ann Rheum Dis 2004;63:364-5.
- Brismée JM, Paige RL, Chyu MC, Boatright JD, Hagar JM, McCaleb JA, et al. Group and home-based tai chi in elderly subjects with knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2007;21:99-111. http://dx.doi.org/10.1177/0269215506070505.
- Bülow PM, Jensen H, Danneskiold-Samsøe B. Low power Ga-Al-As laser treatment of painful osteoarthritis of the knee. A double-blind placebo-controlled study. Scand J Rehabil Med 1994;26:155-9.
- Callaghan MJ, Oldham JA, Hunt J. An evaluation of exercise regimes for patients with osteoarthritis of the knee: a single-blind randomized controlled trial. Clin Rehabil 1995;9:213-18. http://dx.doi.org/10.1177/026921559500900306.
- Callaghan MJ, Whittaker PE, Grimes S, Smith L. An evaluation of pulsed shortwave on knee osteoarthritis using radioleucoscintigraphy: a randomised, double blind, controlled trial. Joint Bone Spine 2005;72:150-5. http://dx.doi.org/10.1016/j.jbspin.2004.03.010.
- Cantarini L, Leo G, Giannitti C, Cevenini G, Barberini P, Fioravanti A. Therapeutic effect of spa therapy and short wave therapy in knee osteoarthritis: a randomized, single blind, controlled trial. Rheumatol Int 2007;27:523-9. http://dx.doi.org/10.1007/s00296-006-0266-5.
- Cheing GL, Hui-Chan CW, Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain?. Clin Rehabil 2002;16:749-60. http://dx.doi.org/10.1191/0269215502cr549oa.
- Chen CY, Chen CL, Hsu SC, Chou SW, Wang KC. Effect of magnetic knee wrap on quadriceps strength in patients with symptomatic knee osteoarthritis. Arch Phys Med Rehabil 2008;89:2258-64. http://dx.doi.org/10.1016/j.apmr.2008.05.019.
- Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthr Cartil 2005;13:20-7. http://dx.doi.org/10.1016/j.joca.2004.10.008.
- Clarke GR, Willis LA, Stenners L, Nichols PJ. Evaluation of physiotherapy in the treatment of osteoarthrosis of the knee. Rheumatol Rehabil 1974;13:190-7. http://dx.doi.org/10.1093/rheumatology/13.4.190.
- Defrin R, Ariel E, Peretz C. Segmental noxious versus innocuous electrical stimulation for chronic pain relief and the effect of fading sensation during treatment. Pain 2005;115:152-60. http://dx.doi.org/10.1016/j.pain.2005.02.018.
- Durmuş D, Alayli G, Cantürk F. Effects of quadriceps electrical stimulation program on clinical parameters in the patients with knee osteoarthritis. Clin Rheumatol 2007;26:674-8. http://dx.doi.org/10.1007/s10067-006-0358-3.
- Ettinger WH, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA 1997;277:25-31. http://dx.doi.org/10.1001/jama.1997.03540250033028.
- Fioravanti A, Iacoponi F, Bellisai B, Cantarini L, Galeazzi M. Short- and long-term effects of spa therapy in knee osteoarthritis. Am J Phys Med Rehabil 2010;89:125-32. http://dx.doi.org/10.1097/PHM.0b013e3181c1eb81.
- Fischer G, Pelka RB, Barovic J. Adjuvant treatment of knee osteoarthritis with weak pulsing magnetic fields. Results of a placebo-controlled trial prospective clinical trial. Z Orthop Ihre Grenzgeb 2005;143:544-50. http://dx.doi.org/10.1055/s-2005-836830.
- Flusser D, Abu-Shakra M, Friger M, Codish S, Sukenik S. Therapy with mud compresses for knee osteoarthritis: comparison of natural mud preparations with mineral-depleted mud. J Clin Rheumatol 2002;8:197-203. http://dx.doi.org/10.1097/00124743-200208000-00003.
- Forestier R, Desfour H, Tessier JM, Françon A, Foote AM, Genty C, et al. Spa therapy in the treatment of knee osteoarthritis: a large randomised multicentre trial. Ann Rheum Dis 2010;69:660-5. http://dx.doi.org/10.1136/ard.2009.113209.
- Fukuda TY, Ovanessian V, Cunha RAD, Filho ZJ, Cazarini C, Rienzo FA, et al. Pulsed short wave effect in pain and function in patients with knee osteoarthritis. J Applied Res 2008;8:189-98.
- Garland D, Holt P, Harrington JT, Caldwell J, Zizic T, Cholewczynski J. A 3-month, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a highly optimized, capacitively coupled, pulsed electrical stimulator in patients with osteoarthritis of the knee. Osteoarthr Cartil 2007;15:630-7. http://dx.doi.org/10.1016/j.joca.2007.01.004.
- Grimmer K. A controlled double blind study comparing the effects of strong burst mode TENS and high rate TENS on painful osteoarthritic knees. Aust J Physiother 1992;38:49-56. http://dx.doi.org/10.1016/S0004-9514(14)60551-1.
- Gür H, Cakin N, Akova B, Okay E, Küçükoğlu S. Concentric versus combined concentric-eccentric isokinetic training: effects on functional capacity and symptoms in patients with osteoarthrosis of the knee. Arch Phys Med Rehabil 2002;83:308-16. http://dx.doi.org/10.1053/apmr.2002.30620.
- Gür A, Cosut A, Sarac AJ, Cevik R, Nas K, Uyar A. Efficacy of different therapy regimes of low-power laser in painful osteoarthritis of the knee: a double-blind and randomized-controlled trial. Lasers Surg Med 2003;33:330-8. http://dx.doi.org/10.1002/lsm.10236.
- Hasegawa R, Islam MM, Nasu E, Tomiyama N, Lee SC, Koizumi D, et al. Effects of combined balance and resistance exercise on reducing knee pain in community-dwelling older adults. Phys Occup Ther Geriatr 2010;28:44-56. http://dx.doi.org/10.3109/02703180903381086.
- Hay EM, Foster NE, Thomas E, Peat G, Phelan M, Yates HE, et al. Effectiveness of community physiotherapy and enhanced pharmacy review for knee pain in people aged over 55 presenting to primary care: pragmatic randomised trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38977.590752.0B.
- Hinman MR, Ford J, Heyl H. Effects of static magnets on chronic knee pain and physical function: a double-blind study. Altern Ther Health Med 2002;8:50-5.
- Huang MH, Lin YS, Lee CL, Yang RC. Use of ultrasound to increase effectiveness of isokinetic exercise for knee osteoarthritis. Arch Phys Med Rehabil 2005;86:1545-51. http://dx.doi.org/10.1016/j.apmr.2005.02.007.
- Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Patel A, Williamson E, et al. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: a cluster randomized trial. Arthritis Rheum 2007;57:1211-19. http://dx.doi.org/10.1002/art.22995.
- Itoh K, Hirota S, Katsumi Y, Ochi H, Kitakoji H. A pilot study on using acupuncture and transcutaneous electrical nerve stimulation (TENS) to treat knee osteoarthritis (OA). Chin Med 2008;3. http://dx.doi.org/10.1186/1749-8546-3-2.
- Itoh K, Hirota S, Katsumi Y, Ochi H, Kitakoji H. Trigger point acupuncture for treatment of knee osteoarthritis – a preliminary RCT for a pragmatic trial. Acupunct Med 2008;26:17-26. http://dx.doi.org/10.1136/aim.26.1.17.
- Jacobson JI, Gorman R, Yamanashi WS, Saxena BB, Clayton L. Low-amplitude, extremely low frequency magnetic fields for the treatment of osteoarthritic knees: a double-blind clinical study. Altern Ther Health Med 2001;7:54-6.
- Jan MH, Lin JJ, Liau JJ, Lin YF, Lin DH. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: a randomized controlled trial. Phys Ther 2008;88:427-36. http://dx.doi.org/10.2522/ptj.20060300.
- Jenkinson CM, Doherty M, Avery AJ, Read A, Taylor MA, Sach TH, et al. Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3170.
- Jubb RW, Tukmachi ES, Jones PW, Dempsey E, Waterhouse L, Brailsford S. A blinded randomised trial of acupuncture (manual and electroacupuncture) compared with a non-penetrating sham for the symptoms of osteoarthritis of the knee. Acupunct Med 2008;26:69-78. http://dx.doi.org/10.1136/aim.26.2.69.
- Kang T, Kim S, Kim S. The efficacy of low power laser therapy in patients with knee osteoarthritis. Ann Rheum Dis 2006;65.
- Kang RW, Lewis PB, Kramer A, Hayden JK, Cole BJ. Prospective randomized single-blinded controlled clinical trial of percutaneous neuromodulation pain therapy device versus sham for the osteoarthritic knee: a pilot study. Orthopedics 2007;30:439-45.
- Karagülle M, Karagülle MZ, Karagülle O, Dönmez A, Turan M. A 10-day course of spa therapy is beneficial for people with severe knee osteoarthritis. A 24-week randomised, controlled pilot study. Clin Rheumatol 2007;26:2063-71. http://dx.doi.org/10.1007/s10067-007-0618-x.
- Keefe FJ, Blumenthal J, Baucom D, Affleck G, Waugh R, Caldwell DS, et al. Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study. Pain 2004;110:539-49. http://dx.doi.org/10.1016/j.pain.2004.03.022.
- Keogan F, Gilsenan C, Hussey J, O’Connell P. Open or closed chain quadriceps exercises in treatment of osteoarthritis of the knee; which is more effective? A blinded randomised controlled trial. Physiother Ire 2007;28:47-8.
- Kovács I, Bender T. The therapeutic effects of Cserkeszölö thermal water in osteoarthritis of the knee: a double blind, controlled, follow-up study. Rheumatol Int 2002;21:218-21. http://dx.doi.org/10.1007/s00296-001-0167-6.
- Kovar PA, Allegrante JP, MacKenzie CR, Peterson MG, Gutin B, Charlson ME. Supervised fitness walking in patients with osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med 1992;116:529-34. http://dx.doi.org/10.7326/0003-4819-116-7-529.
- Kuptniratsaikul V, Tosayanonda O, Nilganuwong S, Thamalikitkul V. The efficacy of a muscle exercise program to improve functional performance of the knee in patients with osteoarthritis. J Med Assoc Thai 2002;85:33-40.
- Law PP, Cheing GL. Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis. J Rehabil Med 2004;36:220-5. http://dx.doi.org/10.1080/16501970410029834.
- Lee HJ, Park HJ, Chae Y, Kim SY, Kim SN, Kim ST, et al. Tai Chi Qigong for the quality of life of patients with knee osteoarthritis: a pilot, randomized, waiting list controlled trial. Clin Rehabil 2009;23:504-11. http://dx.doi.org/10.1177/0269215508101746.
- Lewis B, Lewis D, Cumming G. The analgesic efficacy of transcutaneous electrical nerve stimulation (TENS) compared with a non-steroidal anti-inflammatory drug (naprosyn) in painful osteoarthritis (OA) of the knee. Aust NZ J Med 1988;18.
- Lewis B, Lewis D, Cumming G. The comparative analgesic efficacy of transcutaneous electrical nerve stimulation and a non-steroidal anti-inflammatory drug for painful osteoarthritis. Br J Rheumatol 1994;33:455-60. http://dx.doi.org/10.1093/rheumatology/33.5.455.
- Lewis D, Lewis B, Sturrock RD. Transcutaneous electrical nerve stimulation in osteoarthrosis: a therapeutic alternative?. Ann Rheum Dis 1984;43:47-9. http://dx.doi.org/10.1136/ard.43.1.47.
- Lim BW, Hinman RS, Wrigley TV, Sharma L, Bennell KL. Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Rheum 2008;59:943-51. http://dx.doi.org/10.1002/art.23823.
- Lin D-H, Lin C-HJ, Lin Y-F, Jan M-H. Efficacy of 2 non-weight-bearing interventions, proprioception training versus strength training, for patients with knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther 2009;39:450-7. http://dx.doi.org/10.2519/jospt.2009.2923.
- Lund H, Weile U, Christensen R, Rostock B, Downey A, Bartels EM, et al. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med 2008;40:137-44. http://dx.doi.org/10.2340/16501977-0134.
- Mahboob N, Sousan K, Shirzad A, Amir G, Mohammad V, Reza M, et al. The efficacy of a topical gel prepared using Lake Urmia mud in patients with knee osteoarthritis. J Altern Complement Med 2009;15:1239-42. http://dx.doi.org/10.1089/acm.2009.0304.
- Maillefert JF, Hudry C, Baron G, Kieffert P, Bourgeois P, Lechevalier D, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis: a prospective randomized controlled study. Osteoarthr Cartil 2001;9:738-45. http://dx.doi.org/10.1053/joca.2001.0470.
- Maurer BT, Stern AG, Kinossian B, Cook KD, Schumacher HR. Osteoarthritis of the knee: isokinetic quadriceps exercise versus an educational intervention. Arch Phys Med Rehabil 1999;80:1293-9. http://dx.doi.org/10.1016/S0003-9993(99)90032-1.
- Mazzuca SA, Page MC, Meldrum RD, Brandt KD, Petty-Saphon S. Pilot study of the effects of a heat-retaining knee sleeve on joint pain, stiffness, and function in patients with knee osteoarthritis. Arthritis Rheum 2004;51:716-21. http://dx.doi.org/10.1002/art.20683.
- McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al. Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8460.
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004;50:1501-10. http://dx.doi.org/10.1002/art.20256.
- Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity 2006;14:1219-30. http://dx.doi.org/10.1038/oby.2006.139.
- Miranda-Filloy JA, Barbazán AC, Monteagudo Sánchez B, Graña GJ, Galdo Fernández F. Effect of transcutaneous electrical quadriceps muscle stimulation in knee osteoarthritis symptomatology. Rehabilitación 2005;39:167-70. http://dx.doi.org/10.1016/S0048-7120(05)74340-1.
- Molsberger A, Böwing G, Jensen KU, Lorek M. Acupuncture treatment for the relief of gonarthrosis pain – a controlled clinical trial. Schmerz 1994;8:37-42. http://dx.doi.org/10.1007/BF02527508.
- Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther 2007;12:109-18. http://dx.doi.org/10.1016/j.math.2006.02.009.
- Ng MML, Leung MCP, Poon DMY. The effects of electro-acupuncture and transcutaneous electrical nerve stimulation on patients with painful osteoarthritic knees: a randomized controlled trial with follow-up evaluation. J Altern Complement Med 2003;9:641-9. http://dx.doi.org/10.1089/107555303322524490.
- Nguyen M, Revel M, Dougados M. Prolonged effects of 3 week therapy in a spa resort on lumbar spine, knee and hip osteoarthritis: follow-up after 6 months. A randomized controlled trial. Br J Rheumatol 1997;36:77-81. http://dx.doi.org/10.1093/rheumatology/36.1.77.
- Ni GX, Song L, Yu B, Huang CH, Lin JH. Tai chi improves physical function in older Chinese women with knee osteoarthritis. J Clin Rheumatol 2010;16:64-7. http://dx.doi.org/10.1097/RHU.0b013e3181cf344f.
- Nigg BM, Emery C, Hiemstra LA. Unstable shoe construction and reduction of pain in osteoarthritis patients. Med Sci Sports Exerc 2006;38:1701-8. http://dx.doi.org/10.1249/01.mss.0000228364.93703.53.
- Nivbrant B, Friberg S. Therapeutic laser treatment in gonarthrosis. Acta Orthop 1989;60.
- Péloquin L, Bravo G, Gauthier P, Lacombe G, Billiard JS. Effects of a cross-training exercise program in persons with osteoarthritis of the knee a randomized controlled trial. J Clin Rheumatol 1999;5:126-36. http://dx.doi.org/10.1097/00124743-199906000-00004.
- Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med 2006;166:2533-8. http://dx.doi.org/10.1001/archinte.166.22.2533.
- Perrot S, Marty M, Kahan A, Menkes CJ. Efficacy of Pulsed Electromagnetic Therapy in Painful Knee Osteoarthritis n.d.
- Petrou P, Winkler V, Genti G, Balint G. Double blind trial to evaluate the effect of acupuncture treatment on knee osteoarthritis. Scand J Acupunc Electrother 1988;3:112-15.
- Pietrosimone BG, Hart JM, Saliba SA, Hertel J, Ingersoll CD. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med Sci Sports Exerc 2009;41:1175-81. http://dx.doi.org/10.1249/MSS.0b013e3181982557.
- Pipitone N, Scott DL. Magnetic pulse treatment for knee osteoarthritis: a randomised, double-blind, placebo-controlled study. Curr Med Res Opin 2001;17:190-6. http://dx.doi.org/10.1185/03007990152673828.
- Pollard H, Ward G, Hoskins W, Hardy K. The effect of a manual therapy knee protocol on osteoarthritic knee pain: a randomised controlled trial. J Can Chiropr Assoc 2008;52:229-42.
- Rapp W, Boeer J, Albrich C, Heitkamp HC. Efficiency of vibration or strength training for knee stability in osteoarthritis of the knee. Aktuelle Rheumatol 2009;34:240-5. http://dx.doi.org/10.1055/s-0029-1225366.
- Rattanachaiyanont M, Kuptniratsaikul V. No additional benefit of shortwave diathermy over exercise program for knee osteoarthritis in peri-/post-menopausal women: an equivalence trial. Osteoarthr Cartil 2008;16:823-8. http://dx.doi.org/10.1016/j.joca.2007.10.013.
- Røgind H, Bibow-Nielsen B, Jensen B, Møller HC, Frimodt-Møller H, Bliddal H. The effects of a physical training program on patients with osteoarthritis of the knees. Arch Phys Med Rehabil 1998;79:1421-7. http://dx.doi.org/10.1016/S0003-9993(98)90238-6.
- Rosemffet MG, Schneeberger EE, Citera G, Sgobba ME, Laiz C, Schmulevich H, et al. Effects of functional electrostimulation on pain, muscular strength, and functional capacity in patients with osteoarthritis of the knee. J Clin Rheumatol 2004;10:246-9. http://dx.doi.org/10.1097/01.rhu.0000141831.40350.91.
- Sangdee C, Teekachunhatean S, Sananpanich K, Sugandhavesa N, Chiewchantanakit S, Pojchamarnwiputh S, et al. Electroacupuncture versus diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. BMC Complement Altern Med 2002;2. http://dx.doi.org/10.1186/1472-6882-2-3.
- Schilke JM, Johnson GO, Housh TJ, O’Dell JR. Effects of muscle-strength training on the functional status of patients with osteoarthritis of the knee joint. Nurs Res 1996;45:68-72. http://dx.doi.org/10.1097/00006199-199603000-00002.
- Selfe TK, Bourguignon C, Taylor AG. Effects of noninvasive interactive neurostimulation on symptoms of osteoarthritis of the knee: a randomized, sham-controlled pilot study. J Altern Complement Med 2008;14:1075-81. http://dx.doi.org/10.1089/acm.2008.0305.
- Shen X, Zhao L, Ding G, Tan M, Gao J, Wang L, et al. Effect of combined laser acupuncture on knee osteoarthritis: a pilot study. Lasers Med Sci 2009;24:129-36. http://dx.doi.org/10.1007/s10103-007-0536-9.
- Sherman G, Zeller L, Avriel A, Friger M, Harari M, Sukenik S. Intermittent balneotherapy at the Dead Sea area for patients with knee osteoarthritis. Isr Med Assoc J 2009;11:88-93.
- Smith CR, Lewith GT, Machin D. TNS and osteo-arthritic pain. Preliminary study to establish a controlled method of assessing transcutaneous nerve stimulation as a treatment for the pain caused by osteo-arthritis of the knee. Physiotherapy 1983;69:266-8.
- Stelian J, Gil I, Habot B, Rosenthal M, Abramovici I, Kutok N, et al. Improvement of pain and disability in elderly patients with degenerative osteoarthritis of the knee treated with narrow-band light therapy. J Am Geriatr Soc 1992;40:23-6. http://dx.doi.org/10.1111/j.1532-5415.1992.tb01824.x.
- Sukenik S, Flusser D, Codish S, Abu-Shakra M. Balneotherapy at the Dead Sea area for knee osteoarthritis. Isr Med Assoc J 1999;1:83-5.
- Takeda W, Wessel J. Acupuncture for the treatment of pain of osteoarthritic knees. Arthritis Care Res 1994;7:118-22. http://dx.doi.org/10.1002/art.1790070304.
- Talbot LA, Gaines JM, Ling SM, Metter EJ. A home-based protocol of electrical muscle stimulation for quadriceps muscle strength in older adults with osteoarthritis of the knee. J Rheumatol 2003;30:1571-8.
- Tascioglu F, Armagan O, Tabak Y, Corapci I, Oner C. Low power laser treatment in patients with knee osteoarthritis. Swiss Med Wkly 2004;134:254-8.
- Taylor P, Hallett M, Flaherty L. Treatment of osteoarthritis of the knee with transcutaneous electrical nerve stimulation. Pain 1981;11:233-40. http://dx.doi.org/10.1016/0304-3959(81)90008-7.
- Thamsborg G, Florescu A, Oturai P, Fallentin E, Tritsaris K, Dissing S. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthr Cartil 2005;13:575-81. http://dx.doi.org/10.1016/j.joca.2005.02.012.
- Thorstensson CA, Roos EM, Petersson IF, Ekdahl C. Six-week high-intensity exercise program for middle-aged patients with knee osteoarthritis: a randomized controlled trial [ISRCTN20244858]. BMC Musculoskelet Disord 2005;6. http://dx.doi.org/10.1186/1471-2474-6-27.
- Tishler M, Rosenberg O, Levy O, Elias I, Amit-Vazina M. The effect of balneotherapy on osteoarthritis. Is an intermittent regimen effective?. Eur J Intern Med 2004;15:93-6. http://dx.doi.org/10.1016/j.ejim.2004.01.002.
- Toda Y, Tsukimura N. Influence of concomitant heeled footwear when wearing a lateral wedged insole for medial compartment osteoarthritis of the knee. Osteoarthr Cartil 2008;16:244-53. http://dx.doi.org/10.1016/j.joca.2007.06.010.
- Topp R, Woolley S, Hornyak J, Khuder S, Kahaleh B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Arch Phys Med Rehabil 2002;83:1187-95. http://dx.doi.org/10.1053/apmr.2002.33988.
- Trans T, Aaboe J, Henriksen M, Christensen R, Bliddal H, Lund H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee 2009;16:256-61. http://dx.doi.org/10.1016/j.knee.2008.11.014.
- Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials. J Rheumatol 1994;21:1903-11.
- Tucker M, Brantingham JW, Myburg C. Relative effectiveness of a non-steroidal anti-inflammatory medication (Meloxicam) versus manipulation in the treatment of osteo-arthritis of the knee. Eur J Chiropractic 2003;50:163-84.
- Tukmachi E, Jubb R, Dempsey E, Jones P. The effect of acupuncture on the symptoms of knee osteoarthritis – an open randomised controlled study. Acupunct Med 2004;22:14-22. http://dx.doi.org/10.1136/aim.22.1.14.
- Wang C, Schmid CH, Hibberd PL, Kalish R, Roubenoff R, Rones R, et al. Tai Chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1545-53. http://dx.doi.org/10.1002/art.24832.
- Wigler I, Elkayam O, Paran D, Yaron M. Spa therapy for gonarthrosis: a prospective study. Rheumatol Int 1995;15:65-8. http://dx.doi.org/10.1007/BF00262710.
- Wolsko PM, Eisenberg DM, Simon LS, Davis RB, Walleczek J, Mayo-Smith M, et al. Double-blind placebo-controlled trial of static magnets for the treatment of osteoarthritis of the knee: results of a pilot study. Altern Ther Health Med 2004;10:36-43.
- Wu ZH, Bao F. Observation on therapeutic effects of electroacupuncture for the treatment of knee osteoarthritis. Zhongguo Gu Shang 2008;21:170-2.
- Young SL, Woodbury MG, Fryday-Field K, Donovan T, Bellamy N, Haddad R. Efficacy of interferential current stimulation alone for pain reduction in patients with osteoarthritis of the knee: a randomized placebo control clinical trial. Phys Ther J Am Phys Ther Assoc 1991;71.
- Yurtkuran M, Kocagil T. TENS, electroacupuncture and ice massage: comparison of treatment for osteoarthritis of the knee. Am J Acupunct 1999;27:133-40.
- Yurtkuran M, Yurtkuran M, Alp A, Nasircilar A, Bingöl U, Altan L, et al. Balneotherapy and tap water therapy in the treatment of knee osteoarthritis. Rheumatol Int 2006;27:19-27. http://dx.doi.org/10.1007/s00296-006-0158-8.
- Zizic TM, Hoffman KC, Holt PA, Hungerford DS, O’Dell JR, Jacobs MA, et al. The treatment of osteoarthritis of the knee with pulsed electrical stimulation. J Rheumatol 1995;22:1757-61.
- Arazpour M, Bani MA, Maleki M, Ghomshe FT, Kashani RV, Hutchins SW. Comparison of the efficacy of laterally wedged insoles and bespoke unloader knee orthoses in treating medial compartment knee osteoarthritis. Prosthet Orthot Int 2013;37:50-7. http://dx.doi.org/10.1177/0309364612447094.
- Bennell KL, Bowles KA, Payne C, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d2912.
- Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis 2011;70:1798-803. http://dx.doi.org/10.1136/ard.2010.142018.
- Brosseau L, Wells GA, Kenny GP, Reid R, Maetzel A, Tugwell P, et al. The implementation of a community-based aerobic walking program for mild to moderate knee osteoarthritis: a knowledge translation randomized controlled trial: part II: clinical outcomes. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-1073.
- Bruce-Brand RA, Walls RJ, Ong JC, Emerson BS, O’Byrne JM, Moyna NM. Effects of home-based resistance training and neuromuscular electrical stimulation in knee osteoarthritis: a randomized controlled trial. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-118.
- Fary RE, Carroll GJ, Briffa TG, Briffa NK. The effectiveness of pulsed electrical stimulation in the management of osteoarthritis of the knee: results of a double-blind, randomized, placebo-controlled, repeated-measures trial. Arthritis Rheum 2011;63:1333-42. http://dx.doi.org/10.1002/art.30258.
- Foroughi N, Smith RM, Lange AK, Baker MK, Fiatarone Singh MA, Vanwanseele B. Lower limb muscle strengthening does not change frontal plane moments in women with knee osteoarthritis: a randomized controlled trial. Clin Biomech 2011;26:167-74. http://dx.doi.org/10.1016/j.clinbiomech.2010.08.011.
- Fukuda TY, Alves da Cunha R, Fukuda VO, Rienzo FA, Cazarini C, Carvalho Nde A, et al. Pulsed shortwave treatment in women with knee osteoarthritis: a multicenter, randomized, placebo-controlled clinical trial. Phys Ther 2011;91:1009-17. http://dx.doi.org/10.2522/ptj.20100306.
- Fukuda VO, Fukuda TY, Guimarães M, Shiwa S, de Lima Bdel C, Martins RÁ, et al. Short-term efficacy of low-level laser therapy in patients with knee osteoarthritis: a randomized placebo-controlled, double-blind clinical trial. Rev Bras Ortop 2011;46:526-33. http://dx.doi.org/10.1590/S0102-36162011000500008.
- Giombini A, Di Cesare A, Di Cesare M, Ripani M, Maffulli N. Localized hyperthermia induced by microwave diathermy in osteoarthritis of the knee: a randomized placebo-controlled double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc 2011;19:980-7. http://dx.doi.org/10.1007/s00167-010-1350-7.
- Hsieh R-L, Lo M-T, Lee W-C, Liao W-C. Therapeutic effects of short-term monochromatic infrared energy therapy on patients with knee osteoarthritis: a double-blind, randomized, placebo-controlled study. J Orthop Sports Phys Ther 2012;42:947-56. http://dx.doi.org/10.2519/jospt.2012.3881.
- Imoto AM, Peccin MS, Trevisani VF. Quadriceps strengthening exercises are effective in improving pain, function and quality of life in patients with osteoarthritis of the knee. Acta Ortop Bras 2012;20:174-9. http://dx.doi.org/10.1590/S1413-78522012000300008.
- Lim JY, Tchai E, Jang SN. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PM R 2010;2:723-31. http://dx.doi.org/10.1016/j.pmrj.2010.04.004.
- Mavrommatis CI, Argyra E, Vadalouka A, Vasilakos DG. Acupuncture as an adjunctive therapy to pharmacological treatment in patients with chronic pain due to osteoarthritis of the knee: a 3-armed, randomized, placebo-controlled trial. Pain 2012;153:1720-6. http://dx.doi.org/10.1016/j.pain.2012.05.005.
- Miller E, Maimon Y, Rosenblatt Y, Mendler A, Hasner A, Barad A, et al. Delayed effect of acupuncture treatment in OA of the knee: a blinded, randomized, controlled trial. Evid Based Complement Alternat Med 2011;2011. http://dx.doi.org/10.1093/ecam/nen080.
- Perlman AI, Ali A, Njike VY, Hom D, Davidi A, Gould-Fogerite S, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0030248.
- Salacinski AJ, Krohn K, Lewis SF, Holland ML, Ireland K, Marchetti G. The effects of group cycling on gait and pain-related disability in individuals with mild-to-moderate knee osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther 2012;42:985-95. http://dx.doi.org/10.2519/jospt.2012.3813.
- Salli A, Sahin N, Baskent A, Ugurlu H. The effect of two exercise programs on various functional outcome measures in patients with osteoarthritis of the knee: a randomized controlled clinical trial. Isokinet Exerc Sci 2010;18:201-9.
- Vance CG, Rakel BA, Blodgett NP, DeSantana JM, Amendola A, Zimmerman MB, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther 2012;92:898-910. http://dx.doi.org/10.2522/ptj.20110183.
- Wang TJ, Lee SC, Liang SY, Tung HH, Wu SF, Lin YP. Comparing the efficacy of aquatic exercises and land-based exercises for patients with knee osteoarthritis. J Clin Nurs 2011;20:2609-22. http://dx.doi.org/10.1111/j.1365-2702.2010.03675.x.
- MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLOS Med 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000261.
- Humanistic Psychological Therapies Competences Framework. University College London Division of Psychology and Language Sciences; 2013.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Department of Health . NHS Reference Costs 2011–2012 2012.
Appendix 1 Review data related to Chapter 1
MEDLINE search strategy
Acupuncture
acupuncture OR electro-acupuncture OR electroacupuncture
Back pain
back pain OR backache OR Intervertebral disk OR lumbar* OR sciatica
Neck pain
neck OR cervic* OR spinal OR torticollis OR whiplash
Shoulder pain
shoulder OR rotator cuff OR bursitis OR tendinitis OR tendonitis OR adhesive capsulitis
Osteoarthritis knee pain
Knee OR Arthralgia* OR Arthriti* OR Osteoarthrit* OR Hip
Headache pain
headache OR migrain* OR cephalgi* OR hemicrania
Randomised trials of acupuncture for pain
Pain with “Randomized Controlled Trial” as a limit
Outcome measure preferences
This section is to guide the selection of end points to be included in the meta-analyses. End points are classified by domain (e.g. WOMAC pain is in the ‘pain intensity’ domain; days of headache is in the ‘pain frequency’ domain). This section specifies which end point should be chosen if a trial has data on more than one end point per domain. In general, we have given preference to measures that are specific to pain types, then to the most widely used measures.
Pain frequency
Headache
-
For trials with only migraine patients:
-
days with migraine
-
days with migraine symptoms
-
have you had a migraine during the last 4 weeks (1 = yes/0 = no)?
-
migraine attacks
-
list as below.
-
-
For trials including non-migraine headache patients:
-
days with moderate/strong headache
-
days with headache
-
days with strong headache
-
total headache hours
-
days with headache from other causes
-
during the past 4 weeks have you experienced a TTH (1 = yes/0 = no)?
-
do you have other pain than a TTH (1 = yes/0 = no)?
-
days with impaired activity
-
during the past 4 weeks have you had migraine as well (as a TTH) (1 = yes/0 = no)?
-
do you have other pain in addition to a migraine headache (1 = yes/0 = no)?
-
days with at least one accompanying symptom
-
days with a minimum of one accompanying symptom.
-
Lower back pain
-
Days with pain in the past month (diary 31 days maximum).
Knee and/or hip osteoarthritis
-
Days with pain in the past week (diary 7 days maximum).
Shoulder
-
Pain persistent at time of assessment.
-
New episode of pain at time of assessment.
Pain intensity
-
Diary scores (if pain diaries use more than one measure of pain, then preference is as below).
-
VAS and NRS will be combined by multiplication (NRS × 10 = VAS).
-
– Average pain measured by the VAS or NRS will be first choice
-
– If a trial measures:
-
– if worst, day and night scores, we will average day and night scores
-
– if day and night scores are given, we will take the average
-
– if good and bad knee scores are given, we will average the two scores
-
– if average, highest intensity, lowest and current are given, we will use the average
-
– if pain severity in the past week, unpleasantness in the past week and severity of main problem are given, we will use severity in the past week.
-
-
-
WOMAC pain.
-
Self-Efficacy Scale pain affective/sensoric.
-
Arthritis Self-Efficacy Scale – pain subscale.
-
Shoulder Pain and Disability Index (SPADI) pain component (0–50).
-
Quality of life (SF-36): bodily pain.
-
McGill Pain Questionnaire.
-
Global headache severity.
-
CMS pain component.
-
von Korff scale Q1–3 (pain intensity).
Functional impairment
-
WOMAC function.
-
CMS activity component.
-
Disability: Oswestry Disability Index.
-
Disability: Roland Morris Disability Questionnaire.
-
Back function score [FFbH-R (Hannover Functional Ability Questionnaire) 0–100%].
-
SPADI disability (0–80).
-
Days with impairment because of pain:
-
– days with limited function because of pain (180 days maximum)
-
– days unable to do household work
-
– days with inability to work because of pain.
-
-
Disability: Pain Disability Index.
-
von Korff scale Q5–7 (impairment).
-
Walking ability.
Combined pain and functional impairment
-
WOMAC index/combined.
-
Neck pain and disability score.
-
CMS total.
-
Northwick Park Neck Pain Questionnaire (measures pain and disability).
-
OARSI-OMERACT Responder Index (pain structure function scale).
-
SPADI global (0–130).
-
Oxford Knee Score.
Mental quality of life
-
Quality of life (SF-36): MCS score.
-
Average quality of life (SF-36): social functioning, role emotional and mental health components.
-
SF-12 mental health summary score.
-
Average of Profile of Quality of Life in the Chronically Ill psychological, positive mood, negative mood, social functioning and social well-being components.
-
Average of Hospital Anxiety and Depression Scale anxiety and depression components.
-
Worry about neck pain compared with worry felt at previous questionnaire.
-
Other depression scores.
-
Other anxiety scores.
Physical quality of life
-
Quality of life (SF-36): PCS score.
-
Average quality of life (SF-36): physical activity and role physical.
-
Profile of Quality of Life in the Chronically Ill physical component.
-
SF-12 physical health summary score.
Overall quality of life
-
SF-36 total score (1–100).
-
Quality of life (SF-36): general health perception.
-
Quality of life: COOP/WONCA (World Organization of Colleges, Academies and Academic Associations of General Practitioners/Family Physicians) charts (30–0) (lower scores mean higher quality of life).
-
Quality of life (SF-36): vitality.
-
EQ-5D.
Range of motion/stiffness
-
WOMAC stiffness.
-
Average of CMS range of motion, abduction, flexion, external rotation, internal rotation, strength.
-
Average of active and passive cervical mobility.
-
Range of movement.
Health change
-
Self-evaluated improvement (NRS 0–10).
-
Assessment of treatment effect (1–5).
-
Global assessment of change (1–5).
-
Quality of life (SF-36): health change over the past year (0–10).
-
Globally improved, judged by clinician (yes/no).
-
Physician-evaluated improvement (NRS 0–10).
Satisfaction
-
Satisfaction with treatment outcome.
-
Satisfaction with care.
-
Satisfaction with information.
Medication
-
Days taking medication.
-
Medication quantification scale.
-
Consumption of drugs (none; less than, equal to, greater than or extra than what was prescribed).
-
Any use of pain medication.
-
Amount of trial drug taken (diclofenac) measured by weight of remaining pills.
-
Other.
Trial-level information
Trials evaluating acupuncture for osteoarthritis pain (n = 9)
| Trial | Patient counts for those included in primary analysis | Primary outcome | Time point | Results reported by authors | Dropout rate (in parentheses) | Assessment of blindinga |
|---|---|---|---|---|---|---|
| Berman 2004134 | Total n = 391: acupuncture, n = 142; sham – both penetrating and non-penetrating needles, n = 141; no acupuncture control – non-specific advice, n = 108 | WOMAC pain subscore | 6 months | Mean (SE): acupuncture –3.79 (0.33); sham –2.92 (0.30); no acupuncture –1.69 (0.33) Difference between groups (95% CI): acupuncture vs. sham 0.87 (0.16 to 1.58; p = 0.003); acupuncture vs. no acupuncture (not given) |
Total 391/570 (31%): acupuncture 142/190 (25%); sham 141/191 (26%); non-specific advice 108/189 (43%) | A |
| Vas 2004120 | Total n = 88: acupuncture, n = 47; sham – non-penetrating needles, n = 41 | WOMAC pain subscore | 3 months | Mean (SD): acupuncture 1.7 (2.6); sham 6.4 (5.8) Difference between groups (95% CI): acupuncture vs. sham 4.7 (2.9 to 6.5; p < 0.001) |
Total 88/97 (9%): acupuncture 47/48 (2%); sham 41/49 (16%) | A |
| Witt 200569 | Total n = 285: acupuncture, n = 145; sham – penetrating needles, n = 73; no acupuncture control – usual care, n = 67 | WOMAC index | 2 months | Mean (SE): acupuncture 26.9 (1.4); sham 35.8 (1.9); no acupuncture 49.6 (2.0) Difference between groups (95% CI): acupuncture vs. sham 8.8 (4.2 to 13.5; p < 0.001); acupuncture vs. no acupuncture 22.7 (17.9 to 27.5; p < 0.001) |
Total 285/300 (5%): acupuncture 145/150 (3%); sham 73/76 (4%); usual care 67/74 (9%) | A |
| Scharf 200670 | Total n = 985:b acupuncture, n = 318; sham – penetrating needles, n = 360; no acupuncture control – ancillary care, n = 307 | WOMAC pain subscore | 6 months | Mean (95% CI): acupuncture 2.9 (2.65 to 3.17); sham 3.2 (2.93 to 3.43); no acupuncture 4.0 (3.69 to 4.22) Difference between groups (95% CI): acupuncture vs. sham 0.3 (–0.05 to 0.59; no p-value given); acupuncture vs. no acupuncture 1.0 (0.71 to 1.38; no p-value given) |
Total 985/1039 (5%): acupuncture 318/330 (4%); sham 360/367 (2%); usual care 307/342 (10%) | A |
| Witt 200677 | Total n = 579:b acupuncture, n = 300; no acupuncture control – usual care, n = 279 | WOMAC index | 3 months | Mean (SEM): acupuncture 30.5 (1.0); no acupuncture 47.3 (1.0) Difference between groups (SEM): acupuncture vs. no acupuncture 16.7 (1.4; p < 0.001) |
Total 579/712 (19%): acupuncture 300/357 (16%); usual care 279/355 (21%) | NA |
| Foster 2007135 | Total n = 325: acupuncture, n = 108; sham – non-penetrating needles, n = 112; no acupuncture control – ancillary care, n = 105 | WOMAC pain subscore | 6 months | Mean (SD): acupuncture 7.07 (4.4); sham 6.50 (4.8); no acupuncture 6.78 (4.5) Difference between groups (95% CI): acupuncture vs. sham: not given; acupuncture vs. no acupuncture 0.08 (–1.0 to 0.9; p = 0.9) |
Total 325/352 (8%): acupuncture 108/117 (8%); sham 112/119 (6%); usual care 105/116 (9%) | A |
| Williamson 2007136 | Total n = 181:c acupuncture, n = 60; no acupuncture control – non-specific advice, n = 61; physiotherapy, n = 60 (not analysed) | Oxford Knee Score | 2 months | Mean (SD): acupuncture 36.8 (7.20); no acupuncture 40.3 (8.48) Difference between groups (95% CI): acupuncture vs. no acupuncture 3.5 (0.66 to 6.33; Bonferroni p = 0.016) |
Total 161/181 (11%): acupuncture 59/60 (2%); usual care 49/61 (20%); physiotherapy 53/60 (12%) | NA |
| Lansdown 2009126 | Total n = 30: acupuncture, n = 15; no acupuncture control – usual care, n = 15 | WOMAC pain subscore | 3 months | Mean (SD): acupuncture 3.6 (2.92); no acupuncture 6.57 (4.54) Difference between groups (95% CI): acupuncture vs. no acupuncture –2.62 (–0.77 to –4.47; p = 0.007) |
Total 28/30 (7%): acupuncture 14/15 (7%); usual care 14/15 (7%) | (Not evaluated) |
| Suarez-Almazor 2010125 | Total n = 494: acupuncture, n = 139; sham – penetrating needles, n = 283; no acupuncture control – ancillary care, n = 72 | WOMAC pain subscore | 3 months | Mean (SD): acupuncture 30.8 (17.9); sham 31.0 (19.1); no acupuncture 42.4 (16.8) Difference between groups: acupuncture vs. no acupuncture p = 0.0002 (no estimate given); acupuncture vs. sham p > 0.20 (no estimate given) |
Acupuncture 139/153 (91%); sham 283/302 (94%); usual care 72/72 (100%) | (Not evaluated) |
Trials evaluating acupuncture for chronic headache pain (n = 7)
| Pain type | Trial | Patient counts for those included in primary analysis | Primary outcome | Time point | Results reported by author | Dropout rate | Assessment of blindinga |
|---|---|---|---|---|---|---|---|
| Migraine n = 2 | Linde 200568 | Total n = 272: acupuncture, n = 132; sham – penetrating needles, n = 76; no acupuncture control – usual care, n = 64 | Moderate to severe pain days | 3 months | Mean (SD): acupuncture 2.8 (2.3); sham 2.6 (2.4); no acupuncture 4.3 (2.2) Difference between groups (95% CI): acupuncture vs. sham 0.0 (–0.7 to 0.7; p > 0.9); acupuncture vs. no acupuncture 1.4 (0.8 to 2.1; p < 0.001) |
Total 272/302 (10%): acupuncture 132/145 (9%); sham 76/81 (6%); usual care 64/76 (16%) | A |
| Diener 200672 | Total n = 794:b acupuncture, n = 290; sham – penetrating needles, n = 317; no acupuncture control – guidelined care, n = 187 | Migraine days | 6 months | Change from baseline, mean (SD): acupuncture –2.8 (3.8); sham –2.0 (3.9); no acupuncture –2.7 (4.2) Difference between groups (95% CI): acupuncture vs. sham 0.57 (0.09 to 1.05; p = 0.021); acupuncture vs. no acupuncture 0.50 (–0.06 to 1.05; p = 0.4) |
Total 794/960 (7%): acupuncture 290/313 (7%); sham 317/339 (6%); guidelined care 187/308 (39%) | B | |
| TTH (n = 3) | Coeytaux 2005128 | Total n = 71: acupuncture, n = 34; no acupuncture control – ancillary care, n = 37 | Headache Impact Test | 1 month | Change from baseline, mean (95% CI): acupuncture –3.9 (–6.5 to –1.2); no acupuncture –0.4 (–1.8 to 1.0) Difference between groups (95% CI): acupuncture vs. no acupuncture 3.0 (1.0 to 4.9; no p-value given) |
Total 71/74 (4%): acupuncture 34/35 (3%); usual care 37/39 (5%) | NA |
| Melchart 200567 | Total n = 238: acupuncture, n = 118; sham – penetrating needles, n = 57; no acupuncture control – usual, care n = 63 | Migraine days | 3 months | Mean (SD): acupuncture 9.9 (8.7); sham 10.8 (8.3); no acupuncture 16.3 (7.4) Difference between groups (95% CI): acupuncture vs. sham 0.06 (–1.2 to 2.4; p = 0.5); acupuncture vs. no acupuncture 5.8 (4.0 to 7.6; p < 0.001) |
Total 238/270 (12%): acupuncture 118/132 (11%); sham 57/63 (10%); usual care 63/75 (16%) | A | |
| Endres 200773 | Total n = 398: acupuncture, n = 204; sham – penetrating needles, n = 194 | Headache days | 6 months | Mean (SD): acupuncture 6.0 (6.2); sham 8.4 (7.9) Difference between groups (95% CI): acupuncture vs. sham 1.94 (0.69 to 3.18; p = 0.002) |
Total 398/413 (3%): acupuncture 204/209 (2%); sham 194/200 (3%) | A | |
| Both (n = 2) | Vickers 200460 | Total n = 301: acupuncture, n = 161; no acupuncture control – usual care, n = 140 | Severity score | 12 months | Mean (SD): acupuncture 16.2 (13.7); no acupuncture 22.3 (17.0) Difference between groups (95% CI): acupuncture vs. no acupuncture 4.6 (2.2 to 7.0; p = 0.0002) |
Total 301/401 (25%): acupuncture 161/205 (21%); usual care 140/196 (29%) | NA |
| Jena 200876 | Total n = 2871:c acupuncture, n = 1447; no acupuncture control – usual care, n = 1424 | Headache days | 3 months | Percent reduction, mean (95% CI): acupuncture 43.0 (41.0 to 45.1); no acupuncture 15.2 (13.3 to 17.0) Difference between groups (95% CI): acupuncture vs. no acupuncture 27.9 (25.1 to 30.6; p < 0.001) |
Total 2871/3404 (16%): acupuncture 1447/1711 (15%); usual care 1424/1693 (16%) | NA |
Trials evaluating acupuncture for non-specific musculoskeletal pain (n = 15)
| Pain type | Trial | Patient counts for those included in primary analysis | Primary outcome | Time point | Results reported by author | Dropout rate | Assessment of blindinga |
|---|---|---|---|---|---|---|---|
| Back (n = 10) | Carlsson 2001119 | Total n = 27: acupuncture, n = 21; sham – non-needle, n = 6 | Pain VAS | 6 months | Mean weekly VAS in percent of baseline, mean (SD):b acupuncture – morning VAS 75 (33), night VAS 68 (31); sham – morning VAS 132 (76), night VAS 101 (48) Difference between groups: acupuncture vs. sham – morning VAS p = 0.13 (no estimate given), night VAS p = 0.056 (no estimate given) |
Total 27/50 (46%): acupuncture 21/34 (38%); sham 6/16 (63%) | B |
| Cherkin 2001131 | Total n = 249:c acupuncture, n = 89; no acupuncture control – non-specific advice, n = 83; massage, n = 77 (not analysed) | Roland Morris Disability Questionnaire | 2 months | Mean (95% CI): acupuncture 7.9 (6.5 to 9.3); no acupuncture 8.8 (7.4 to 10.2) Difference between groups: acupuncture vs. no acupuncture – adjusted p = 0.75 (no estimate given) |
Total 249/262 (7%): acupuncture 89/94 (5%); usual care 83/90 (8%); massage 77/78 (1%) | NA | |
| Molsberger 2002129 | Total n = 124: acupuncture, n = 47; sham – penetrating needles, n = 41; no acupuncture control – ancillary care, n = 36 | Pain VAS | 3 months | Mean (SD): acupuncture 23 (20); sham 43 (23); no acupuncture control 52 (19) Difference between groups:d acupuncture vs. sham p < 0.001 (no estimate given); acupuncture vs. no acupuncture control p < 0.001 (no estimate given) |
Total 124/186 (33%): acupuncture 47/65 (28%); sham 41/61 (33%); usual care 36/60 (40%) | (Not evaluated) | |
| Kerr 2003117 | Total n = 46: acupuncture, n = 26; sham – non-needle, n = 20 | Pain VAS | 1 month | Mean (SD): acupuncture 51.3 (22.4); sham 61.7 (30.6) Difference between groups: acupuncture vs. sham p = 0.2 (no estimate given) |
Total 46/60 (23%): acupuncture, 26/30 (13%); sham 20/30 (33%) | B | |
| Brinkhaus 200666 | Total n = 284: acupuncture, n = 140; sham – penetrating needles, n = 70; no acupuncture control – usual care, n = 74 | Pain VAS | 2 months | Mean change from baseline (SD): acupuncture 28.7 (30.3); sham 23.6 (31.0); no acupuncture 6.9 (22.0) Difference between groups (95% CI): acupuncture vs. sham 5.1 (–3.7 to 13.9; p = 0.3); acupuncture vs. no acupuncture 21.7 (13.9 to 30.0; p < 0.001) |
Total 284/301 (6%): acupuncture 140/147 (5%); sham 70/75 (7%); usual care 74/79 (6%) | A | |
| Thomas 200661 | Total n = 182: acupuncture, n = 123; no acupuncture control – usual care, n = 59 | SF-36 bodily pain | 24 months | Mean (SD): acupuncture 67.8 (24.1); no acupuncture 59.5 (23.4) Difference between groups (95% CI): acupuncture vs. no acupuncture 8.0 (2.8 to 13.2; p = 0.003) |
Total 182/241 (24%): acupuncture 123/160 (23%); usual care 59/81 (27%) | NA | |
| Witt 200674 | Total n = 2594:e acupuncture, n = 1350; no acupuncture control – usual care, n = 1244 | Back function measured with the Hannover Functional Ability Questionnaire | 3 months | Mean (SE): acupuncture 12.1 (0.4); no acupuncture 2.7 (0.4) Difference between groups (95% CI): acupuncture vs. no acupuncture 9.4 (8.3 to 10.5; p < 0.001) |
Total 2594/3093 (16%): acupuncture 1350/1549 (13%); usual care 1244/1544 (19%) | A | |
| Haake 200771 | Total n = 1117:f acupuncture, n = 377; sham – penetrating needles, n = 376; no acupuncture control – guidelined care, n = 364 | von Korff chronic pain scale | 6 months | Mean (SD) of von Korff chronic pain scale: acupuncture 40.2 (22.5); sham 43.3 (23.0); no acupuncture 52.3 (21.2) Difference between groups in treatment success (95% CI) (%):g acupuncture vs. no acupuncture 20.2 (13.4 to 26.7; p < 0.001); acupuncture vs. sham 3.4 (–3.7 to 10.3; p = 0.4) |
Total 1117/1162 (4%): acupuncture 377/387 (3%); sham 376/387 (3%); usual care 364/388 (6%) | A | |
| Kennedy 2008130 | Total n = 40: acupuncture, n = 22; sham – needle, non-penetrating, n = 18 | Roland Morris Disability Questionnaire | 3 months | Mean (SEM): acupuncture 5.0 (1.0); sham 7.7 (1.5) Difference between groups (95% CI): acupuncture vs. sham 2.6 (–0.7 to 5.9; p = 0.12) |
Total 40/48 (17%): acupuncture 22/24 (8%); sham 18/24 (25%) | A | |
| Cherkin 2009124 | Total n = 606: acupuncture, n = 299h (individualised n = 147, standardised n = 152); sham – non-needle, n = 159; no acupuncture control – usual care, n = 148 | Roland Morris Disability Questionnaire | 2 months | Mean (SD): individualised acupuncture 6.4 (5.3), standardised acupuncture 6.3 (5.7); sham 5.4 (4.9); no acupuncture 8.9 (6.0) Difference between groups (95% CI): individualised vs. standardised 0.16 (–0.90 to 1.22; p ≥ 0.05); individualised vs. sham 0.45 (–0.61 to 1.50; p ≥ 0.05); individualised vs. no acupuncture –2.47 (–3.53 to –1.40; p < 0.05) |
Total 606/638 (5%): individualised acupuncture 147/157 (6%); standardised acupuncture 152/158 (4%); sham 159/162 (2%); usual care 148/161 (8%) | (Not evaluated) | |
| Neck (n = 5) | Irnich 2001118 | Total n = 108: acupuncture, n = 51; sham – non-needle, n = 57; massage, n = 57 (not analysed) | Pain VAS | 1 month | Change from baseline, mean (95% CI): acupuncture 24.22 (16.5 to 31.9); sham 17.28 (10.0 to 24.6) Difference between groups (95% CI): acupuncture vs. sham 6.9 (–5.0 to 18.9; p = 0.3) |
Total 165/177 (8%): acupuncture 51/56 (9%); sham 57/61 (7%); massage 57/60 (5%) | B |
| White 2004132 | Total n = 124:i acupuncture, n = 63; sham – non-needle, n = 61 | Pain VAS | 1 month | Mean (SD): acupuncture 20.39 (20.26); sham 30.69 (22.00) Difference between groups (95% CI): acupuncture vs. sham 6.3 (1.4 to 11.3; p = 0.012) |
Total 124/135 (8%): acupuncture 63/70 (10%); sham 61/65 (6%) | A | |
| Salter 2006133 | Total n = 21: acupuncture, n = 9; no acupuncture control – usual care, n = 12 | Northwick Park Neck Pain Questionnaire | 3 months | Mean (SD): acupuncture 22.73 (18.64); no acupuncture 25.72 (16.29) Difference between groups: acupuncture vs. no acupuncture 1.75 (no CI given; p = 0.8) |
Total 21/24 (13%): acupuncture 9/10 (10%); usual care 12/14 (14%) | NA | |
| Vas 2006121 | Total n = 123:j acupuncture, n = 61; sham – non-needle, n = 62 | Pain VAS | 1 month | Change from baseline, mean (SD): acupuncture 42.1 (21.1); sham 14.0 (15.7) Difference between groups (95% CI): acupuncture vs. sham 28.1 (21.4 to 34.7; p < 0.001) |
Total 115/123 (7%): acupuncture 58/61 (5%); sham 57/62 (8%) | A | |
| Witt 200675 | Total n = 3162:e acupuncture, n = 1618; no acupuncture control – usual care, n = 1544 | Neck Pain and Disability Scale | 3 months | Change from baseline, mean (SE): acupuncture 16.2 (0.4); no acupuncture 3.9 (0.4) Difference between groups (95% CI): acupuncture vs. no acupuncture 12.3 (11.3 to 13.3; p < 0.001) |
Total 3162/3766 (16%): acupuncture 1618/1880 (14%); usual care 1544/1886 (18%) | NA |
Trials evaluating acupuncture for specific shoulder pain (n = 4)
| Trial | Patient counts for those included in primary analysis | Primary outcome | Time point | Results reported by author | Dropout rate | Assessment of blindinga |
|---|---|---|---|---|---|---|
| Kleinhenz 1999137 | Total n = 45: acupuncture, n = 22; sham – non-penetrating needles, n = 23 | CMS | 1 month | Mean change from baseline (SD): acupuncture 19.2 (16.1), sham 8.4 (14.6) Difference between groups (95% CI): acupuncture vs. sham (no estimate given) (2.3 to 19.4; p = 0.001) |
Total 45/52 (13%): acupuncture 22/25 (12%); sham 23/27 (15%) | A |
| Guerra de Hoyos 2004138 | Total n = 110: acupuncture, n = 55; sham – non-penetrating needles, n = 55 | Pain VAS | 6 months | Mean (SD): acupuncture 3.5 (3.0); sham 1.2 (1.9) Difference between groups (95% CI): acupuncture vs. sham 2.0 (1.2 to 2.9; p < 0.0005) |
Total 110/130 (15%): acupuncture 55/65 (15%); sham 55/65 (15%) | A |
| Vas 2008122 | Total n = 425:b acupuncture, n = 205; sham – non-needle, n = 220 | CMS | 1 month | Mean change from baseline (SD): acupuncture 16.6 (15.6); sham 10.6 (13.5) Difference between groups (95% CI): acupuncture vs. sham 6.0 (3.2 to 8.8; p < 0.001) |
Total 409/425 (4%): acupuncture 202/205 (1%); sham 207/220 (6%) | A |
| Molsberger 2010127 | Total n = 308: acupuncture, n = 128; sham – non-penetrating needles, n = 74; no acupuncture control – usual care, n = 106 | VAS | 6 months | Mean (SD): acupuncture 19 (23.3); sham 33 (29.6); no acupuncture 33 (26.6) Difference between groups (95% CI): acupuncture vs. sham 14 (7.87 to 20.13; p < 0.001); acupuncture vs. no acupuncture 14 (8.22 to 19.78; p < 0.001) |
Total 308/424 (27%): acupuncture 128/154 (17%); sham 74/135 (45%); usual care 106/135 (21%) | (Not evaluated) |
Appendix 2 Acupuncture characteristics related to trials in Chapter 2
Trial- and patient-level information when analysed
| Pain type | Trial | Patient counts for those included in primary analysis | Results and data reported by author |
|---|---|---|---|
| Chronic headache/migraine (n = 6) | |||
| Migraine (n = 2) | Linde 200568 | Total n = 272: acupuncture, n = 132; sham – penetrating needles, n = 76; no acupuncture control – usual care, n = 64 | Difference between groups (95% CI): acupuncture vs. sham 0.0 (–0.7 to 0.7; p > 0.9); acupuncture vs. no acupuncture control 1.4 (0.8 to 2.1; p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 3 Maximum number of sessions: 12 Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 29 Number of needles used: 17 |
| Diener 200672 | Total n = 794:a acupuncture, n = 290; sham – penetrating needles, n = 317; no acupuncture control – guidelined care, n = 187 | Difference between groups (95% CI): acupuncture vs. sham 0.57 (0.09 to 1.05; p = 0.021); acupuncture vs. no acupuncture control 0.50 (–0.06 to 1.05; p = 0.4) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 2 Maximum number of sessions: 10 Frequency of sessions (per week): 2 Duration of sessions (minutes): 32 (patient-level data analysed) Number of needles used: 16 (patient-level data analysed) |
|
| TTH (n = 2) | Melchart 200567 | Total n = 238: acupuncture, n = 118; sham – penetrating needles, n = 57; no acupuncture control – usual care, n = 63 | Difference between groups (95% CI): acupuncture vs. sham 0.06 (–1.2 to 2.4; p = 0.5); acupuncture vs. no acupuncture control 5.8 (4.0 to 7.6; p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 3 Maximum number of sessions: 12 Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 30 Number of needles used: 15 |
| Endres 200773 | Total n = 398: acupuncture, n = 204; sham – penetrating needles, n = 194 | Difference between groups (95% CI): acupuncture vs. sham 1.94 (0.69 to 3.18; p = 0.002) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 0 Maximum number of sessions: 10 Frequency of sessions (per week): 2 Duration of sessions (minutes): 32 Number of needles used: 16 |
|
| Both migraine and headache (n = 2) | Vickers 200460 | Total n = 301: acupuncture, n = 161; no acupuncture control – usual care, n = 140 | Difference between groups (95% CI): acupuncture vs. no acupuncture control 4.6 (2.2 to 7.0; p = 0.0002) Style of acupuncture: traditional Chinese Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: yes Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Maximum number of sessions: 24 (patient-level data analysed) Frequency of sessions (per week): 1 Duration of sessions (minutes): 30 Number of needles used: not provided |
| Jena 200876 | Total n = 2871:b acupuncture, n = 1447; no acupuncture control – usual care, n = 1424 | Difference between groups (95% CI): acupuncture vs. no acupuncture control 27.9 (25.1 to 30.6; p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: not provided Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Age of acupuncturists: yes (patient-level data analysed) Sex of acupuncturists: yes (patient-level data analysed) Maximum number of sessions: 30 (patient-level data analysed) Frequency of sessions (per week): 1 Duration of sessions (minutes): not provided Number of needles used: not provided |
|
| Non-specific musculoskeletal (n = 13) | |||
| Back (n = 8) | Carlsson 2001119 | Total n = 27: acupuncture, n = 21; sham – non-needle, n = 6 | Difference between groups: acupuncture vs. sham – morning VAS p = 0.13 (no estimate given), night VAS p = 0.056 (no estimate given) Style of acupuncture: combination of traditional Chinese and Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 5 Maximum number of sessions: 10 Frequency of sessions (per week): 1 Duration of sessions (minutes): 20 Number of needles used: 16 |
| Cherkin 2001131 | Total n = 172:c acupuncture, n = 89; no acupuncture control, non-specific advice, n = 83 | Difference between groups: acupuncture vs. no acupuncture control – adjusted p = 0.75 (no estimate given) Style of acupuncture: traditional Chinese Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: yes Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 3 Maximum number of sessions: 10 Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 28 Number of needles used: 12 |
|
| Kerr 2003117 | Total n = 46: acupuncture, n = 26; sham – non-needle, n = 20 | Difference between groups: acupuncture vs. sham p = 0.2 (no estimate given) Style of acupuncture: Western Point prescription: fixed formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Maximum number of sessions: 6 Frequency of sessions (per week): 1 Duration of sessions (minutes): 30 Number of needles used: 11 |
|
| Brinkhaus 200666 | Total n = 284: acupuncture, n = 140; sham – penetrating needles, n = 70; no acupuncture control – usual care, n = 74 | Difference between groups (95% CI): acupuncture vs. sham 5.1 (–3.7 to 13.9; p = 0.3); acupuncture vs. no acupuncture control 21.7 (13.9 to 30.0; p < 0.001) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 3 Age of acupuncturists: yes (patient-level data analysed) Sex of acupuncturists: yes (patient-level data analysed) Maximum number of sessions: 12 (patient-level data analysed) Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 28 (patient-level data analysed) Number of needles used: 17 (patient-level data analysed) |
|
| Thomas 200661 | Total n = 182: acupuncture, n = 123; no acupuncture control – usual care, n = 59 | Difference between groups (95% CI): acupuncture vs. no acupuncture control 8.0 (2.8 to 13.2; p = 0.003) Style of acupuncture: traditional Chinese Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: yes Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 3 Maximum number of sessions: 10 Frequency of sessions (per week): 1 Duration of sessions (minutes): 25 Number of needles used: 10 |
|
| Witt 200674 | Total n = 2594:b acupuncture, n = 1350; no acupuncture control – usual care, n = 1244 | Difference between groups (95% CI): acupuncture vs. no acupuncture control 9.4 (8.3 to 10.5; p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: not provided Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Age of acupuncturists: yes (patient-level data analysed) Sex of acupuncturists: yes (patient-level data analysed) Maximum number of sessions: 25 (patient-level data analysed) Frequency of sessions (per week): 1 Duration of sessions (minutes): not provided Number of needles used: not provided |
|
| Haake 200771 | Total n = 1117:d acupuncture, n = 377; sham – penetrating needles, n = 376; no acupuncture control – guidelined care, n = 364 | Difference between groups in treatment success (95% CI):e acupuncture vs. no acupuncture control 20.2% (13.4% to 26.7%; p < 0.001); acupuncture vs. sham 3.4% (–3.7% to 10.3%; p = 0.4) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 2 Maximum number of sessions: 10 Frequency of sessions (per week): 2 Duration of sessions (minutes): 32 (patient-level data analysed) Number of needles used: 16 (patient-level data analysed) |
|
| Kennedy 2008130 | Total n = 40: acupuncture, n = 22; sham – non-penetrating needles, n = 18 | Difference between groups (95% CI): acupuncture vs. sham 2.6 (–0.7 to 5.9; p = 0.12) Style of acupuncture: Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 10 Maximum number of sessions: 12 Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 30 Number of needles used: 11 |
|
| Neck (n = 5) | Irnich 2001118 | Total n = 108: acupuncture, n = 51; sham – non-needle, n = 57 | Difference between groups (95% CI): acupuncture vs. sham 6.9 (–5.0 to 18.9; p = 0.3) Style of acupuncture: combination of traditional Chinese and Western Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 0 Maximum number of sessions: 5 Frequency of sessions (per week): 2 Duration of sessions (minutes): 30 Number of needles used: 9 |
| White 2004132 | Total n = 124:f acupuncture, n = 63; sham – non-needle, n = 61 | Difference between groups (95% CI): acupuncture vs. sham 6.3 (1.4 to 11.3; p = 0.012) Style of acupuncture: Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 5 Maximum number of sessions: 8 Frequency of sessions (per week): 2 Duration of sessions (minutes): 30 Number of needles used: 6 |
|
| Salter 2006133 | Total n = 21: acupuncture, n = 9; no acupuncture control – usual care, n = 12 | Difference between groups: acupuncture vs. no acupuncture control 1.75 (no CI given; p = 0.8) Style of acupuncture: traditional Chinese Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: yes Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 3 Maximum number of sessions: 10 Frequency of sessions (per week): 1 Duration of sessions (minutes): 25 Number of needles used: 13 |
|
| Vas 2006121 | Total n = 123:g acupuncture, n = 61; sham – non-needle, n = 62 | Difference between groups (95% CI): acupuncture vs. sham 28.1 (21.4 to 34.7; p < 0.001) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 3 Maximum number of sessions: 5 Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 30 Number of needles used: 10 |
|
| Witt 200675 | Total n = 3162:b acupuncture, n = 1618; no acupuncture control – usual care, n = 1544 | Difference between groups (95% CI): acupuncture vs. no acupuncture control 12.3 (11.3 to 13.3; p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: not provided Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Age of acupuncturists: yes (patient-level data analysed) Sex of acupuncturists: yes (patient-level data analysed) Maximum number of sessions: 30 (patient-level data analysed) Frequency of sessions (per week): 1 Duration of sessions (minutes): not provided Number of needles used: not provided |
|
| Osteoarthritis (n = 7) | |||
| Berman 2004134 | Total n = 391: acupuncture, n = 142; sham – both penetrating and non-penetrating needles, n = 141; no acupuncture control – non-specific advice, n = 108 | Difference between groups (95% CI): acupuncture vs. sham 0.87 (0.16 to 1.58; p = 0.003); acupuncture vs. no acupuncture control not given Style of acupuncture: traditional Chinese Point prescription: fixed formula Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 2 Maximum number of sessions: 23 Frequency of sessions (per week): 0.88 Duration of sessions (minutes): 20 Number of needles used: 11 |
|
| Vas 2004120 | Total n = 88: acupuncture, n = 47; sham – non-penetrating needles, n = 41 | Difference between groups (95% CI): acupuncture vs. sham 4.7 (2.9 to 6.5; p < 0.001) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 3 Maximum number of sessions: 13 Frequency of sessions (per week): 1 Duration of sessions (minutes): 30 Number of needles used: 8 |
|
| Witt 200569 | Total n = 285: acupuncture, n = 145; sham – penetrating needles, n = 73, no acupuncture control – usual care, n = 67 | Difference between groups (95% CI): acupuncture vs. sham 8.8 (4.2 to 13.5; p < 0.001); acupuncture vs. no acupuncture control 22.7 (17.9 to 27.5; p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 3 Age of acupuncturists: yes (patient-level data analysed) Sex of acupuncturists: yes (patient-level data analysed) Maximum number of sessions: 12 (patient-level data analysed) Frequency of sessions (per week): 1.5 Duration of sessions (minutes): 29 (patient-level data analysed) Number of needles used: 17 (patient-level data analysed) |
|
| Scharf 200670 | Total n = 985:b acupuncture, n = 318; sham – penetrating needles, n = 360; no acupuncture control – ancillary care, n = 307 | Difference between groups (95% CI): acupuncture vs. sham: 0.3 (–0.05 to 0.59; no p-value given); acupuncture vs. no acupuncture control 1.0 (0.71 to 1.38; no p-value given) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 2 Maximum number of sessions: 10 Frequency of sessions (per week): 1.67 Duration of sessions (minutes): 30 (patient-level data analysed) Number of needles used: 13 (patient-level data analysed) |
|
| Witt 200677 | Total n = 579:b acupuncture, n = 300; no acupuncture control – usual care, n = 279 | Difference between groups (SEM): acupuncture vs. no acupuncture control 16.7 (1.4) (p < 0.001) Style of acupuncture: combination of traditional Chinese and Western Point prescription: fully individualised Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: not provided Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Age of acupuncturists: yes (patient-level data analysed) Sex of acupuncturists: yes (patient-level data analysed) Maximum number of sessions: 15 (patient-level data analysed) Frequency of sessions (per week): 1 Duration of sessions (minutes): not provided Number of needles used: not provided |
|
| Foster 2007135 | Total n = 325: acupuncture, n = 108; sham – non-penetrating needles, n = 112; no acupuncture control – ancillary care, n = 105 | Difference between groups (95% CI): acupuncture vs. sham not given; acupuncture vs. no acupuncture control 0.08 (–1.0 to 0.9; p = 0.9) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: yes Minimum years of experience required: 0 Maximum number of sessions: 9 (patient-level data analysed) Frequency of sessions (per week): 1 Duration of sessions (minutes): 30 Number of needles used: 8 |
|
| Williamson 2007136 | Total n = 121:g acupuncture, n = 60; no acupuncture control – non-specific advice, n = 61 | Difference between groups (95% CI): acupuncture vs. no acupuncture control 3.5 (0.66 to 6.33; Bonferroni p = 0.016) Style of acupuncture: Western Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 0 Maximum number of sessions: 6 Frequency of sessions (per week): 1 Duration of sessions (minutes): 20 Number of needles used: 9 |
|
| Specific shoulder (n = 3) | |||
| Kleinhenz 1999137 | Total n = 45: acupuncture, n = 22; sham – non-penetrating needles, n = 23 | Difference between groups (95% CI): acupuncture vs. sham no estimate given (2.3 to 19.4; p = 0.001) Style of acupuncture: traditional Chinese Point prescription: flexible formula Location of needles: both local and distal points Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 0 Maximum number of sessions: 8 Frequency of sessions (per week): 2 Duration of sessions (minutes): 20 Number of needles used: 10 |
|
| Guerra de Hoyos 2004138 | Total n = 110: acupuncture, n = 55; sham – non-penetrating needles, n = 55 | Difference between groups (95% CI): acupuncture vs. sham 2.0 (1.2 to 2.9; p < 0.0005) Style of acupuncture: traditional Chinese Point prescription: fixed formula Location of needles: both local and distal points Electrical stimulation allowed: yes Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 0 Maximum number of sessions: 7 Frequency of sessions (per week): 1 Duration of sessions (minutes): 15 Number of needles used: 7 |
|
| Vas 2008122 | Total n = 425:g acupuncture, n = 205; sham – non-needle, n = 220 | Difference between groups (95% CI): acupuncture vs. sham 6.0 (3.2 to 8.8; p < 0.001) Style of acupuncture: traditional Chinese Point prescription: fixed formula Location of needles: distal points only Electrical stimulation allowed: no Manual stimulation allowed: yes Moxibustion allowed: no Deqi elicited: yes Acupuncture-specific patient–practitioner interactions: no Minimum years of experience required: 0.5 Maximum number of sessions: 3 Frequency of sessions (per week): 1 Duration of sessions (minutes): 30 Number of needles used: 1 |
|
Appendix 3 Review data related to Chapter 3
Databases searched
-
MEDLINE.
-
MEDLINE In-Process & Other Non-Indexed Citations.
-
EMBASE.
-
Allied and Complementary Medicine Database.
-
Cochrane Database of Systematic Reviews.
-
Database of Abstracts of Reviews of Effects.
-
Health Technology Assessment database.
-
Cochrane Central Register of Controlled Trials.
-
Cumulative Index to Nursing and Allied Health Literature.
-
MANTIS.
-
PASCAL.
-
Inside Conferences.
-
Conference Proceedings Citation Index – Science.
-
PEDro.
-
CAMbase.
-
LILACS.
-
ClinicalTrials.gov.
MEDLINE search strategy
The following search strategy was developed in Ovid MEDLINE and adapted for use in the other databases searched. The strategy incorporates the sensitivity-maximising version of the Cochrane Highly Sensitive Search Strategy for randomised trials in MEDLINE.
-
Osteoarthritis, Knee/ (8978)
-
(gonarthrosis or gonarthritis).ti,ab. (912)
-
1 or 2 (9707)
-
Osteoarthritis/ (27,166)
-
(Osteoarthriti$ or OA or osteo arthriti$ or osteoarthros$ or osteo arthros$ or arthropath$ or arthrosis or arthroses).ti,ab. (50,583)
-
degenerative arthriti$.ti,ab. (969)
-
degenerative joint disease.ti,ab. (1515)
-
4 or 5 or 6 or 7 (61,760)
-
Knee/ (10,013)
-
Knee Joint/ (36,003)
-
(knee$ or patella$ or knee cap$ or kneecap$ or femorotibial or femoro tibial or tibiofemoral or tibio femoral or patellofemoral or patello femoral).ti,ab. (87,341)
-
9 or 10 or 11 (98,496)
-
8 and 12 (16,792)
-
Arthralgia/ (4180)
-
(arthralgi$ or (joint$ adj3 pain$)).ti,ab. (11,723)
-
Chronic Pain/ (1255)
-
chronic pain$.ti,ab. (17,043)
-
14 or 15 or 16 or 17 (31,979)
-
12 and 18 (2629)
-
3 or 13 or 19 (21,357)
-
Acupuncture/ (1115)
-
exp Acupuncture Therapy/ (15,218)
-
acupuncture$.ti,ab. (12,290)
-
(electroacupuncture$ or electro acupuncture$).ti,ab. (2514)
-
(osteopuncture$ or osteo puncture$).ti,ab. (2)
-
(perioste$ adj3 (stimulati$ or therap$ or needling)).ti,ab. (74)
-
exp Physical Therapy Modalities/ (115,890)
-
(physiotherap$ or physio therap$ or physical therap$ or manual therap$).ti,ab. (24,353)
-
(massage$ or acupressure or shiatsu or shiatzu or zhi ya or chih ya).ti,ab. (6745)
-
Chiropractic/ (2970)
-
Traction/ (5704)
-
(chiropractic or manipulat$ or traction or kinesiolog$ or mobilis$ or mobiliz$).ti,ab. (165,625)
-
Osteopathic medicine/ (2472)
-
osteopath$.ti,ab. (3568)
-
(hydrotherap$ or hydro therap$ or water therap$ or pool therap$).ti,ab. (801)
-
exp Exercise/ (99,442)
-
exp Sports/ (101,842)
-
Physical Fitness/ (20,358)
-
(exercise$ or workout$ or work out$ or train$ or physical$ activ$ or kinesiotherap$ or keep$ fit or aerobics).ti,ab. (450,237)
-
(muscle$ adj3 (stretch$ or strengthen$)).ti,ab. (3418)
-
(walk$ adj3 (fitness or aerobic or program$ or intervention$ or session$ or regime$)).ti,ab. (1398)
-
pedometer$.ti,ab. (1102)
-
(bicycl$ or cycle$ or cycling).ti,ab. (373,541)
-
(run$ or jog$ or treadmill$).ti,ab. (122,114)
-
(swim$ or water sport$ or aquatic$ or water aerobic$ or aqua aerobic$ or water gymnastics).ti,ab. (41,769)
-
(tai ji or taiji or taijiquan or tai chi or t ai chi or taichi or shadow boxing).ti,ab. (666)
-
(yoga or yogic or pilates or danc$).ti,ab. (4740)
-
(qigong or qi gong or chi kung or chikung or ch i kung).ti,ab. (347)
-
(CPM or (passive adj (motion or movement))).ti,ab. (5468)
-
vibration/ (18,338)
-
(vibrati$ or mechanical stimul$).ti,ab. (32,959)
-
(balneology or balneotherap$ or balneo therap$ or bath$ or crenobalneotherap$ or thalassotherap$ or spa or spas).ti,ab. (42,994)
-
(thermotherap$ or thermo therap$ or hypertherm$ or hyper therm$ or diatherm$ or short wave or shortwave or ultrasonic or cryotherap$ or cryo therap$).ti,ab. (64,704)
-
(heat or hot or ice or cold).ti,ab. (227,320)
-
exp Cryotherapy/ (19,170)
-
exp Orthotic Devices/ (9055)
-
(brace$ or bracing or orthotic$ or orthoses).ti,ab. (8251)
-
(insert$ or insole$).ti,ab. (180,523)
-
(TENS or ALTENS).ti,ab. (6246)
-
(transcutaneous adj2 nerve stimulation).ti,ab. (1425)
-
(electroanalgesia or electro analgesia).ti,ab. (215)
-
(electric$ nerve stimulation or electrostimulation or electro stimulation).ti,ab. (4261)
-
EMS.ti,ab. (7219)
-
((muscle or electric$) adj3 stimulat$).ti,ab. (55,060)
-
(neuromodulation or neuro modulation or neurostimulation or neuro stimulation).ti,ab. (3051)
-
interferential.ti,ab. (256)
-
Electromagnetic Fields/ (13,738)
-
Magnetic Field Therapy/ (516)
-
PEMF.ti,ab. (308)
-
((electromagnetic$ or magnetic$) adj3 field$).ti,ab. (20,877)
-
(biomagnetic$ or bio magnetic$ or pulsed signal).ti,ab. (391)
-
Laser Therapy, Low-Level/ (2660)
-
laser.ti,ab. (124,281)
-
phototherapy/ (5215)
-
(light or phototherap$ or photo therap$).ti,ab. (347,805)
-
or/21-75 (2,221,346)
-
20 and 76 (4608)
-
exp Obesity/ (125,953)
-
Overweight/ (9213)
-
body mass index/ (69,416)
-
(obese or obesity).ti,ab. (141,323)
-
(overweight or over weight).ti,ab. (29,555)
-
Weight Loss/ (21,542)
-
Weight Gain/ (20,613)
-
(weight adj3 los$).ti,ab. (52,781)
-
(weight adj3 reduc$).ti,ab. (20,417)
-
(weight adj3 decreas$).ti,ab. (13,520)
-
(weight adj3 gain$).ti,ab. (41,860)
-
(weight adj3 increas$).ti,ab. (27,215)
-
(weight adj3 chang$).ti,ab. (14,373)
-
(BMI or body mass index).ti,ab. (102,658)
-
or/78-91 (367,063)
-
Bariatrics/ (209)
-
exp Diet/ (178,778)
-
exp Diet Therapy/ (38,718)
-
(diet$ or slim or slimming).ti,ab. (336,098)
-
(weight adj3 control$).ti,ab. (10,820)
-
(weight adj3 manage$).ti,ab. (2468)
-
low calorie$.ti,ab. (1958)
-
calorie control$.ti,ab. (64)
-
(calorie adj3 count$).ti,ab. (79)
-
(caloric adj3 restrict$).ti,ab. (2805)
-
(calorie$ adj3 restrict$).ti,ab. (1998)
-
(energy adj3 restrict$).ti,ab. (1894)
-
(protein adj3 restrict$).ti,ab. (3973)
-
(weight watchers or weightwatchers or slimfast or nutrition class$ or meal replacement$).ti,ab. (470)
-
or/93-106 (436,733)
-
exp Anti-Obesity Agents/ (10,515)
-
((anti obes$ or antiobes$) adj3 (agent$ or drug$ or therap$ or medicine$)).ti,ab. (877)
-
appetite suppressant$.ti,ab. (492)
-
appetite depressant$.ti,ab. (108)
-
(orlistat or xenical).ti,ab,rn. (1224)
-
phentermine.ti,ab,rn. (794)
-
Phentermine/ (659)
-
(sibutramine or reductil).ti,ab,rn. (1067)
-
(rimonabant or acomplia).ti,ab,rn. (2109)
-
or/108-116 (13,527)
-
107 or 117 (447,143)
-
20 and 92 and 118 (119)
-
77 or 119 (4647)
-
randomized controlled trial.pt. (342,334)
-
controlled clinical trial.pt. (85,694)
-
randomized.ab. (244,919)
-
placebo.ab. (136,550)
-
drug therapy.fs. (1,588,363)
-
randomly.ab. (175,193)
-
trial.ab. (253,825)
-
groups.ab. (1,145,730)
-
121 or 122 or 123 or 124 or 125 or 126 or 127 or 128 (2,960,405)
-
exp animals/ not humans/ (3,812,817)
-
129 not 130 (2,515,366)
-
120 and 131 (1614)
Methods for rating study quality and risk of bias
Method for rating overall study quality
See column headings in following table for description of questions.
-
Excellent – answers ‘yes’ for questions 1–3, 5, 6, 8–14 and ‘yes’ or ‘not applicable’ for question 4.
-
Good – answers ‘yes’ for questions 1, 5, 8, 9, 11–14 and ‘yes’ or ‘unclear/not stated’ for questions 2–4 (or ‘not applicable’ for question 4).
-
Satisfactory – answers ‘yes’ for questions 1, 5, 11–13 and not ‘no’ for question 4.
-
Poor – the answer is not ‘yes’ for one of the criteria required for ‘satisfactory’.
Comparability of quality assessment and risk of bias
In applying the risk-of-bias tool we included the level of patient blinding when assessing the blinding of the outcome assessment. This was necessary given that patients recorded their pain level and response to treatment. Therefore, even in studies in which the outcome assessor was reported as blinded, if patient were not blinded the risk of bias was recorded as high. Note that both patients and outcome assessor had to be blinded for a trial to be rated as being of ‘good’ quality.
All of the studies rated as being of ‘poor’ quality were found to have a high or unclear risk of bias. Of the 27 trials rated ‘satisfactory’, 21 were found to have a high risk of bias, three an unclear risk of bias and three a low risk of bias. Of the 21 rated as being at high risk of bias, the explanation in most cases was that the study was not blinded: the above method for rating study quality made allowances for the difficulties of blinding treatments in trials of physical therapies, so a trial could be ‘satisfactory’ (but not ‘good’) if it was not blinded. In addition, this method accounted for poor reporting of randomisation and allocation concealment methods, meaning that a study could be rated as ‘satisfactory’ (but not ‘good’) if these were unclear, providing the study groups appeared to have similar baseline characteristics (i.e. the aim of randomisation/allocation concealment appeared to have been achieved). Of the four ‘good’ studies, two had an unclear risk of bias and two a low risk of bias.
Study quality assessment/risk-of-bias details for original review in 2011
For algorithm to produce overall study quality ratings see previous section.
| Study | 1. Number of patients randomised to treatment stated? | 2. Method of randomisation appropriate? | 3. Appropriate allocation concealment? | 4. Appropriate type of placebo used? | 5. Group baseline characteristics comparable? | 6. Described as double blind? | 7. Treatment giver blinded? | 8. Patient blinded? | 9. Outcome assessor blinded? | 10. Use of a power calculation reported? | 11. Eligibility criteria adequately reported? | 12. Clear reporting of losses to follow-up? | 13. Intention-to-treat data reported/analysed? | 14. At least 90% full follow-up achieved? | Overall study quality/risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrahams 2002349 | Yes | UC/NS | UC/NS | N/A | No | Yes | No | No | UC/NS | No | Yes | No | UC | UC | Poor |
| UC | UC | High | UC | UC | High | ||||||||||
| Adedoyin 2002301 | Yes | No | UC/NS | Yes | Yes | No | No | Yes | Yes | No | Yes | No | UC | UC | Poor |
| High | UC | Low | Low | UC | High | ||||||||||
| Aglamiş 2009189 | Yes | Yes | UC/NS | N/A | No | No | UC/NS | No | No | No | Yes | Yes | No | No | Poor |
| Low | UC | High | High | High | High | ||||||||||
| Alcidi 2007350 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | No | No | No | No | No | No | UC | UC | Poor |
| UC | UC | UC | High | High | High | ||||||||||
| An 2008351 | Yes | UC/NS | UC/NS | N/A | No | No | No | No | No | No | Yes | Yes | No | No | Poor |
| UC | UC | High | High | High | High | ||||||||||
| Arman 1988352 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | No | No | No | No | No | No | UC | UC | Poor |
| UC | UC | UC | High | UC | High | ||||||||||
| Baker 2001353 | Yes | Yes | Yes | No | Yes | No | No | No | No | No | Yes | Yes | No | No | Poor |
| Low | Low | low | High | High | High | ||||||||||
| Baker 2007354 | Yes | Yes | UC/NS | Yes | Yes | Yes | UC/NS | UC/NS | UC/NS | Yes | Yes | Yes | No | Yes | Poor |
| Low | UC | Low | UC | UC | UC | ||||||||||
| Bálint 2007355 | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No | Poor |
| High | Low | Low | Low | High | High | ||||||||||
| Bao 2007356 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | UC/NS | No | Yes | No | Yes | UC | Poor |
| UC | UC | Low | UC | UC | UC | ||||||||||
| Berman 1999357 | Yes | Yes | Yes | N/A | No | No | No | No | No | Yes | Yes | Yes | Yes | No | Poor |
| Low | Low | High | High | Low | High | ||||||||||
| Berman 2004134 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Bezalel 2010358 | Yes | Yes | UC/NS | N/A | No | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Poor |
| Low | UC | High | High | Low | High | ||||||||||
| Bilgici 2004359 | No | UC/NS | UC/NS | N/A | UC/NS | No | UC/NS | UC/NS | UC/NS | No | No | No | UC | UC | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Börjesson 1996190 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Satisfactory |
| Low | UC | Low | High | Low | High | ||||||||||
| Brismée 2007360 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | UC | Low | High | Low | High | ||||||||||
| Bülow 1994361 | Yes | UC/NS | Yes | Yes | UC/NS | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Poor |
| UC | Low | UC | Low | Low | UC | ||||||||||
| Burch 2008303 | Yes | Yes | Yes | N/A | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Poor |
| Low | Low | High | Low | High | High | ||||||||||
| Callaghan 1995362 | Yes | UC/NS | UC/NS | N/A | No | No | No | No | No | No | No | No | UC | UC | Poor |
| UC | UC | High | High | UC | High | ||||||||||
| Callaghan 2005363 | Yes | UC/NS | UC/NS | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | No | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Cantarini 2007364 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Poor |
| UC | UC | UC | High | Low | High | ||||||||||
| Cheing 2002365 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | No | No | No | No | Yes | Yes | No | Yes | Poor |
| UC | UC | UC | High | High | High | ||||||||||
| Cheing 2003191 | Yes | UC/NS | UC/NS | No | No | UC/NS | No | No | UC/NS | No | Yes | Yes | No | Yes | Poor |
| UC | UC | High | High | High | High | ||||||||||
| Chen 2008366 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| Christensen 2005367 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | Yes | Yes | Yes | No | No | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Clarke 1974368 | Yes | UC/NS | UC/NS | Yes | Yes | No | UC/NS | UC/NS | UC/NS | No | No | Yes | Yes | Yes | Poor |
| UC | UC | Low | UC | Low | UC | ||||||||||
| Defrin 2005369 | Yes | UC/NS | UC/NS | Yes | No | No | No | Yes | UC/NS | No | Yes | No | UC | UC | Poor |
| UC | UC | High | UC | UC | High | ||||||||||
| Dias 2003187 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | No | No | No | No | No | Yes | No | Yes | Poor |
| UC | UC | UC | High | High | High | ||||||||||
| Durmuş 2007370 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | No | Yes | Yes | Yes | Yes | Satisfactory |
| UC | UC | Low | High | Low | High | ||||||||||
| Ettinger 1997371 | Yes | Yes | Yes | N/A | UC/NS | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Poor |
| Low | Low | UC | High | Low | High | ||||||||||
| Fioravanti 2010372 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | UC/NS | No | Yes | Yes | Yes | Yes | Satisfactory |
| Low | UC | Low | High | Low | High | ||||||||||
| Fischer 2005373 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Poor |
| High | High | Low | Low | High | High | ||||||||||
| Flusser 2002374 | Yes | UC/NS | UC/NS | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | UC | UC | Poor |
| UC | UC | Low | Low | UC | UC | ||||||||||
| Forestier 2010375 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | UC/NS | Yes | Yes | Yes | No | No | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Fukuda 2008376 | Yes | UC/NS | Yes | UC | Yes | No | No | UC/NS | Yes | No | Yes | Yes | No | No | Poor |
| UC | Low | Low | Low | High | High | ||||||||||
| Garland 2007377 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Poor |
| Low | Low | High | Low | High | High | ||||||||||
| Grimmer 1992378 | Yes | Yes | Yes | Yes | UC/NS | Yes | No | Yes | Yes | No | Yes | Yes | UC | Yes | Poor |
| Low | Low | UC | Low | UC | UC | ||||||||||
| Gür 2002379 | Yes | UC/NS | UC/NS | N/A | Yes | No | UC/NS | UC/NS | UC/NS | No | No | No | UC | Yes | Poor |
| UC | UC | Low | UC | UC | UC | ||||||||||
| Gür 2003380 | Yes | UC/NS | UC/NS | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Satisfactory |
| UC | UC | Low | High | Low | High | ||||||||||
| Hasegawa 2010381 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | No | No | Yes | Yes | Yes | Poor |
| UC | UC | Low | High | Low | High | ||||||||||
| Hay 2006382 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | No | No | Poor |
| Low | Low | Low | High | High | High | ||||||||||
| Hinman 2002383 | No | UC/NS | Yes | Yes | UC/NS | Yes | Yes | UC/NS | Yes | No | Yes | Yes | No | Yes | Poor |
| UC | Low | UC | UC | High | High | ||||||||||
| Huang 2005384 | Yes | No | Yes | N/A | Yes | No | No | UC/NS | Yes | No | No | Yes | UC | Yes | Poor |
| High | Low | Low | UC | UC | High | ||||||||||
| Hurley 2007385 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| UC | UC | Low | High | Low | High | ||||||||||
| Itoh 2008386 | Yes | Yes | UC/NS | N/A | UC/NS | No | No | No | No | No | Yes | Yes | No | No | Poor |
| Low | UC | UC | High | High | High | ||||||||||
| Itoh 2008387 | Yes | UC/NS | No | No | Yes | No | No | No | Yes | No | Yes | Yes | No | No | Poor |
| UC | High | Low | High | High | High | ||||||||||
| Jacobson 2001388 | No | UC/NS | Yes | Yes | UC/NS | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Poor |
| UC | Low | UC | Low | High | High | ||||||||||
| Jan 2008389 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | No | Yes | Yes | Yes | No | Yes | Satisfactory |
| Low | UC | Low | High | High | High | ||||||||||
| Jenkinson 2009390 | Yes | Yes | Yes | N/A | Yes | No | No | No | No | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Jubb 2008391 | Yes | Yes | UC/NS | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | UC | High | Low | High | High | ||||||||||
| Kang 2006392 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | UC/NS | UC/NS | UC/NS | No | No | No | UC | Poor | |
| UC | UC | UC | UC | UC | |||||||||||
| Kang 2007393 | Yes | UC/NS | UC/NS | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| UC | UC | Low | Low | High | High | ||||||||||
| Karagülle 2007394 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | No | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Keefe 2004395 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | No | Yes | Yes | UC | Yes | Poor |
| UC | UC | Low | High | UC | High | ||||||||||
| Keogan 2007396 | Yes | UC/NS | UC/NS | N/A | Yes | No | UC/NS | UC/NS | UC/NS | No | No | No | Yes | UC | Poor |
| UC | UC | Low | UC | UC | UC | ||||||||||
| Kovács 2002397 | No | Yes | UC/NS | Yes | UC/NS | Yes | UC/NS | Yes | Yes | No | No | Yes | UC | No | Poor |
| Low | UC | UC | Low | UC | UC | ||||||||||
| Kovar 1992398 | Yes | Yes | UC/NS | N/A | No | No | No | No | No | No | Yes | Yes | No | Yes | Poor |
| Low | UC | High | High | High | High | ||||||||||
| Kuptniratsaikul 2002399 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | UC/NS | No | No | Yes | No | Yes | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Lansdown 2009126 | Yes | Yes | Yes | N/A | UC/NS | No | No | No | No | No | Yes | Yes | Yes | Yes | Poor |
| Low | Low | UC | High | Low | High | ||||||||||
| Law 2004400 | No | Yes | UC/NS | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | UC | UC | Poor |
| Low | UC | Low | Low | UC | UC | ||||||||||
| Lee 2009401 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Satisfactory |
| Low | UC | Low | High | Low | High | ||||||||||
| Lewis 1988402 | Yes | UC/NS | UC/NS | UC/NS | UC/NS | Yes | UC/NS | UC/NS | UC/NS | No | No | No | UC | No | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Lewis 1994403 | Yes | UC/NS | UC/NS | Yes | UC/NS | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Poor |
| UC | UC | UC | Low | High | High | ||||||||||
| Lewis 1984404 | Yes | UC/NS | UC/NS | Yes | UC/NS | Yes | Yes | Yes | UC/NS | No | Yes | Yes | No | Yes | Poor |
| UC | UC | UC | UC | High | High | ||||||||||
| Lim 2008405 | Yes | Yes | Yes | N/A | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Lin 2009406 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Lu 2010192 | Yes | UC/NS | UC/NS | Yes | Yes | No | No | Yes | No | No | Yes | Yes | Yes | Yes | Satisfactory |
| UC | UC | Low | High | Low | High | ||||||||||
| Lund 2008407 | Yes | UC/NS | Yes | N/A | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Poor |
| UC | Low | High | High | Low | High | ||||||||||
| Mahboob 2009408 | Yes | UC/NS | Yes | Yes | UC/NS | Yes | Yes | Yes | Yes | No | No | No | Yes | UC | Poor |
| UC | Low | UC | Low | UC | UC | ||||||||||
| Maillefert 2001409 | Yes | UC/NS | UC/NS | Yes | Yes | No | No | UC/NS | UC/NS | Yes | Yes | Yes | Yes | Yes | Satisfactory |
| UC | UC | Low | UC | Low | UC | ||||||||||
| Maurer 1999410 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | Yes | Yes | Yes | No | UC | No | Poor |
| Low | UC | Low | High | UC | High | ||||||||||
| Mazzuca 2004411 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| McCarthy 2004412 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | UC | No | Satisfactory |
| Low | Low | Low | High | UC | High | ||||||||||
| Messier 2004413 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Miller 2006414 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | No | Yes | Yes | No | No | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Miranda-Filloy 2005415 | Yes | UC/NS | UC/NS | Yes | UC/NS | No | UC/NS | Yes | UC/NS | No | No | No | UC | UC | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Molsberger 1994416 | Yes | UC/NS | UC/NS | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | UC | Yes | Poor |
| UC | UC | Low | Low | UC | UC | ||||||||||
| Moss 2007417 | Yes | UC/NS | UC/NS | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| UC | UC | Low | Low | Low | UC | ||||||||||
| Ng 2003418 | Yes | Yes | No | N/A | Yes | No | No | No | Yes | No | Yes | No | UC | UC | Poor |
| Low | High | Low | High | UC | High | ||||||||||
| Nguyen 1997419 | Yes | Yes | UC/NS | N/A | UC/NS | No | No | No | No | No | Yes | Yes | Yes | Yes | Poor |
| Low | UC | UC | High | Low | High | ||||||||||
| Ni 2010420 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | No | Yes | Yes | No | No | Poor |
| Low | Low | Low | High | High | High | ||||||||||
| Nigg 2006421 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | No | No | Yes | Yes | Yes | Yes | Satisfactory |
| Low | UC | Low | High | Low | High | ||||||||||
| Nivbrant 1989422 | Yes | UC/NS | UC/NS | Yes | UC/NS | No | UC/NS | UC/NS | UC/NS | No | No | No | UC | Yes | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Péloquin 1999423 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | UC | Low | High | High | High | ||||||||||
| Perlman 2006424 | Yes | Yes | UC/NS | N/A | No | No | No | No | No | Yes | Yes | Yes | Yes | No | Poor |
| Low | UC | High | High | Low | High | ||||||||||
| Perrot 1998425 | Yes | UC/NS | UC/NS | UC/NS | UC/NS | Yes | UC/NS | UC/NS | UC/NS | No | No | Yes | UC | No | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Petrou 1988426 | Yes | UC/NS | UC/NS | UC/NS | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Poor |
| UC | UC | Low | Low | Low | UC | ||||||||||
| Pietrosimone 2009427 | Yes | UC/NS | Yes | N/A | Yes | No | No | No | No | No | No | Yes | Yes | Yes | Poor |
| UC | Low | Low | High | Low | High | ||||||||||
| Pipitone 2001428 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Poor |
| Low | Low | High | Low | Low | High | ||||||||||
| Pollard 2008429 | Yes | Yes | Yes | Yes | UC/NS | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Poor |
| Low | Low | UC | Low | Low | UC | ||||||||||
| Rapp 2009430 | Yes | UC/NS | UC/NS | N/A | UC/NS | No | No | No | No | No | Yes | No | UC | UC | Poor |
| UC | UC | UC | High | UC | High | ||||||||||
| Rattanachaiyanont 2008431 | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | Low | High | Low | High | High | ||||||||||
| Røgind 1998432 | Yes | Yes | UC/NS | N/A | UC/NS | No | No | No | Yes | No | Yes | Yes | No | Yes | Poor |
| Low | UC | UC | High | High | High | ||||||||||
| Rosemffet 2004433 | Yes | Yes | UC/NS | N/A | No | No | No | No | No | No | Yes | No | No | No | Poor |
| Low | UC | High | High | High | High | ||||||||||
| Sangdee 2002434 | Yes | UC/NS | UC/NS | No | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Schilke 1996435 | Yes | Yes | UC/NS | N/A | No | No | No | No | No | No | No | No | Yes | No | Poor |
| Low | UC | High | High | UC | High | ||||||||||
| Selfe 2008436 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| Shen 2009437 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Sherman 2009438 | No | Yes | UC/NS | No | No | No | UC/NS | No | Yes | No | Yes | Yes | No | Yes | Poor |
| Low | UC | High | High | High | High | ||||||||||
| Smith 1983439 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No | Yes | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| Stelian 1992440 | Yes | UC/NS | UC/NS | Yes | UC/NS | Yes | Yes | Yes | UC/NS | No | No | Yes | UC | Yes | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Sukenik 1999441 | Yes | UC/NS | UC/NS | Yes | Yes | No | No | No | Yes | No | No | Yes | No | Yes | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Takeda 1994442 | Yes | UC/NS | UC/NS | Yes | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes | Poor |
| UC | UC | Low | Low | UC | UC | ||||||||||
| Talbot 2003443 | Yes | UC/NS | UC/NS | N/A | No | No | No | No | No | No | Yes | Yes | No | No | Poor |
| UC | UC | High | High | High | High | ||||||||||
| Tascioglu 2004444 | Yes | UC/NS | UC/NS | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Satisfactory |
| UC | UC | Low | High | Low | High | ||||||||||
| Taylor 1981445 | Yes | UC/NS | UC/NS | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Poor |
| UC | UC | Low | Low | High | High | ||||||||||
| Thamsborg 2005446 | Yes | UC/NS | UC/NS | UC/NS | Yes | Yes | UC/NS | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| UC | UC | Low | Low | High | High | ||||||||||
| Thorstensson 2005447 | Yes | No | UC/NS | N/A | Yes | No | No | No | No | Yes | Yes | Yes | No | Yes | Poor |
| High | Low | High | High | High | |||||||||||
| Tishler 2004448 | Yes | UC/NS | No | N/A | Yes | No | UC/NS | No | No | No | Yes | Yes | No | Yes | Poor |
| UC | High | Low | High | High | High | ||||||||||
| Toda 2008449 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Topp 2002450 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | UC/NS | No | Yes | No | Yes | Yes | Poor |
| UC | UC | Low | High | UC | High | ||||||||||
| Trans 2009451 | Yes | UC/NS | Yes | N/A | Yes | No | No | UC/NS | UC/NS | Yes | Yes | Yes | Yes | No | Satisfactory |
| UC | Low | Low | UC | Low | UC | ||||||||||
| Trock 1994452 | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | UC | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| Tucker 2003453 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | No | Yes | Yes | No | Yes | Poor |
| UC | UC | Low | High | High | High | ||||||||||
| Tukmachi 2004454 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Vas 2004120 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Wang 2009455 | Yes | Yes | Yes | N/A | No | No | No | No | UC/NS | No | Yes | Yes | Yes | Yes | Poor |
| Low | Low | High | UC | Low | High | ||||||||||
| Weiner 2007188 | Yes | UC/NS | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Good |
| UC | Low | Low | Low | Low | Low | ||||||||||
| Wigler 1995456 | Yes | UC/NS | UC/NS | Yes | UC/NS | Yes | Yes | Yes | UC/NS | No | Yes | No | UC | Yes | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Williamson 2007136 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Witt 200569 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | UC/NS | Yes | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | UC | Low | UC | ||||||||||
| Witt 200677 | Yes | Yes | Yes | N/A | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | High | Low | High | ||||||||||
| Wolsko 2004457 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Poor |
| Low | Low | High | Low | Low | High | ||||||||||
| Wu 2008458 | Yes | No | UC/NS | N/A | Yes | No | No | No | No | No | Yes | No | UC | UC | Poor |
| High | Low | High | UC | High | |||||||||||
| Yip 2007193 | Yes | Yes | UC/NS | N/A | UC/NS | No | No | No | No | Yes | Yes | Yes | Yes | No | Poor |
| Low | UC | UC | High | Low | High | ||||||||||
| Young 1991459 | No | UC/NS | UC/NS | Yes | UC/NS | UC/NS | UC/NS | UC/NS | UC/NS | No | Yes | No | UC | UC | Poor |
| UC | UC | UC | UC | UC | UC | ||||||||||
| Yurtkuran 1999460 | Yes | UC/NS | UC/NS | Yes | UC/NS | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Poor |
| UC | UC | UC | Low | Low | UC | ||||||||||
| Yurtkuran 2006461 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| Zizic 1995462 | Yes | UC/NS | UC/NS | UC/NS | Yes | Yes | UC/NS | UC/NS | UC/NS | No | Yes | Yes | No | Yes | Poor |
| UC | UC | Low | UC | High | High |
Study quality assessment/risk-of-bias details for the 22 studies identified for the 2013 update
For algorithm to produce overall study quality rating see earlier.
| Study | 1. Number of patients randomised to treatment stated? | 2. Method of randomisation appropriate? | 3. Appropriate allocation concealment? | 4. Appropriate type of placebo used? | 5. Group baseline characteristics comparable? | 6. Described as double blind? | 7. Treatment giver blinded? | 8. Patient blinded? | 9. Outcome assessor blinded? | 10. Use of a power calculation reported? | 11. Eligibility criteria adequately reported? | 12. Clear reporting of losses to follow-up? | 13. Intention-to-treat data reported/analysed? | 14. At least 90% full follow-up achieved? | Overall study quality/risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arazpour 2013463 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | No | No | Yes | No | UC | UC | Poor |
| UC | UC | Low | High | UC | High | ||||||||||
| Bennell 2011464 | Yes | Yes | Yes | Yes | Yes | Yes | UC/NS | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Bliddal 2011465 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Brosseau 2012466 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | Yes | No | Yes | Yes | No | No | Poor |
| Low | UC | Low | Low | High | High | ||||||||||
| Bruce-Brand 2012467 | Yes | Yes | UC/NS | N/A | No | No | No | No | Yes | No | Yes | Yes | No | No | Poor |
| Low | UC | High | Low | High | High | ||||||||||
| Fary 2011468 | Yes | Yes | Yes | Yes | Noa | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Satisfactorya |
| Low | Low | UC | Low | Low | Low/UCa | ||||||||||
| Foroughi 2011469 | Yes | Yes | UC/NS | UC/NS | Yes | No | No | Yes | UC/NS | Yes | Yes | Yes | No | No | Poor |
| Low | UC | Low | UC | High | High | ||||||||||
| Fukuda 2011470 | Yes | UC/NS | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| UC | Low | Low | Low | High | High | ||||||||||
| Fukuda 2011471 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Good |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Giombini 2011472 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Gundog 2012302 | Yes | Yes | UC/NS | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Low | UC | Low | Low | Low | UC | ||||||||||
| Hsieh 2012473 | Yes | UC/NS | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| UC | Low | Low | Low | Low | UC | ||||||||||
| Imoto 2012474 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Lim 2010475 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | Low | Low | Low | High | High | ||||||||||
| Mavrommatis 2012476 | Yes | Yes | UC/NS | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Poor |
| Low | UC | Low | Low | High | High | ||||||||||
| Miller 2011477 | Yes | Yes | Yes | Yes | Yes | UC/NS | No | Yes | Yes | No | Yes | Yes | Yes | No | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Perlman 2012478 | Yes | Yes | Yes | N/A | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Satisfactory |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Salacinski 2012479 | Yes | Yes | Yes | N/A | No | No | No | No | No | Yes | Yes | Yes | No | No | Poor |
| Low | Low | High | High | High | High | ||||||||||
| Salli 2010480 | Yes | UC/NS | UC/NS | N/A | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Satisfactory |
| UC | UC | Low | Low | Low | UC | ||||||||||
| Suarez-Almazor 2010125 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Low | Low | Low | Low | Low | Low | ||||||||||
| Vance 2012481 | Yes | UC/NS | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Poorb |
| UC | Low | High | Low | Low | High | ||||||||||
| Wang 2011482 | Yes | Yes | UC/NS | N/A | Yes | No | No | No | No | Yes | Yes | Yes | No | Yes | Poor |
| Low | UC | Low | High | High | High |
List of individual studies included in the systematic review by intervention
Acupuncture studies
| Study | Treatments (if suitable for NMA, adjunct code in parenthesesa) | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean unless stated | Weight (kg) | BMI (kg/m2) | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Number of points needled | Depth of insertion | Electrical stimulation used? | Pain outcomes assessed | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bao 2007356 | Acupuncture (2), standard care (4) | 40, yes | China | 63 | 62 | NR | NR | Clinical | NR | Poor | 20 | 12 | 4 | 7 | Unclear | Yes | Lysholm scores | No |
| Berman 1999357 | Acupuncture (2), standard care (2) | 73, yes | USA | 60 | 65 | NR | Mean 32 | Clinical and radiological | ≥ 2 | Poor | 20 | 16 | 8 | 5 | 0.4–0.6 inches | Yes | WOMAC pain 5-point Likert scale | Yes |
| Berman 2004134 | Acupuncture, sham acupuncture | 330, yes | USA | 63 | 66 | NR | NR | Clinical and radiological | ≥ 2 | Satisfactory | 20 | 23 | 26 | 9 (11 for sham: 9 knee, 2 abdomen) | 0.3–1.0 inches; unclear for sham | Yes; no for sham | WOMAC pain 5-point Likert scale, range 0–20 | Other overall score: patient global assessment |
| Itoh 2008386 | Acupuncture (1), TENS (1), standard care (2) | 18, yes | Japan | 66 | Range 62–83 | NR | NR | Clinical and radiological | ≥ 2 | Poor | 15–25; extra 10 minutes when deqi achieved | 5 | 5 | 6 | 10 mm | No | Other pain VAS, 10-cm VAS scale (0–100) | Yes |
| Itoh 2008387 | Acupuncture (2), sham acupuncture (2) | 24, yes | Japan | 77 | 73 | NR | NR | Clinical and radiological | ≥ 2 | Poor | 30 | 5 | 5 | 6 for standard acupuncture; mean of 3.3 for trigger acupuncture; 3.1 for sham | 10 mm for standard acupuncture; 10–30 mm for trigger acupuncture; 0 mm for sham | No | Other pain VAS, 100 mm | Yes probably using Likert scale |
| Jubb 2008391 | Acupuncture, sham acupuncture | 62, yes | UK | 81 | 65 | NR | Mean 32 | Clinical and radiological | 2 or 3 | Poor | Manual acupuncture: 10; electrical acupuncture: anterior part 10, then posterior part 10 | 10 | 5 | 9 | Varied between 1 and 1.5 cm; needle not inserted for sham | Yes; no for sham | WOMAC pain subscale VAS 0–100; other pain VAS, overall knee pain (0–100) | Individual WOMAC subscores reported |
| Lansdown 2009126 | Acupuncture (2), standard care (2) | 30, yes | UK | 60 | 64 | NR | NR | Clinical | NR/unclear | Poor | 10–30 | Up to 10 (as necessary) | Around 10 | Varied from 4 to 24 | Varied from 3 to 30 mm | Unclear/not stated | WOMAC pain 5-point Likert scale | Yes |
| Lu 2010192 | Acupuncture (2), sham acupuncture (2) | 20, yes | China | NR | 64 | Mean 66 | NR | Clinical and radiological | 2 or 3 | Satisfactory | 30 | 1 | Unclear/not stated | 5 | 1–1.5 cm for both acupuncture and sham | Yes; no for sham | Other pain VAS 1–10 (note: SD for post-treatment pain read off graph) | No |
| Mavrommatis 2012 476 | Acupuncture, sham acupuncture, standard care | 117, yes | Greece | 76 | 62 | NR | Mean 31 | Clinical and radiological | ≥ 2 | Poor | Unclear/not stated | 16 | 8 | 10 | Unclear/not stated; sham: retractable needles (Streitberger) | Yes | WOMAC pain subscale VAS 0–100; other pain VAS 0–100 | Yes |
| Miller 2011 477 | Acupuncture, sham acupuncture | 55, yes | Israel | 69 | 71 | NR | NR | Unclear/not stated | NR/unclear | Satisfactory | 20; manually manipulated every 5 minutes | 16 | 8 | 7 | Unclear/not stated; sham: not inserted into skin | No | Other pain VAS: Knee Society knee pain score | No |
| Molsberger 1994416 | Acupuncture, sham acupuncture | 97, nob | Germany | 63 | 60 | NR | NR | Clinical and radiological | NR (Wirth classification) | Poor | 20 | 10 | 5 | 9 | 0.5–1.5 cm; 0.5 cm for sham | Unclear/not stated | Other pain VAS 0–10 | No |
| Ng 2003418 | Acupuncture (3), TENS (3), standard care (3) | 14 (imputed), yes (only vs. TENS) | China | 96 | 85 | NR | NR | Clinical | NR/unclear | Poor | 20 | 8 | 2 | 2 | 10–15 mm | Yes | NRS of pain | No |
| Petrou 1988426 | Acupuncture (1), sham acupuncture (1) | 31, yes | Hungary | 74 | 62 | Mean 80 | NR | Clinical | NR/unclear | Poor | 20 | 8 | 2 | 12 | Unclear/not stated; ‘superficially’ for sham | No | Graded standard pain scale (4 points = severe, 3 points = moderate, 2 points = mild, 1 point = none) | No |
| Sangdee 2002434 | Acupuncture, sham acupuncture, standard care | 186, yes | Thailand | 78 | 63 | Mean 60 | NR | Clinical and radiological | ≥ 1 | Poor | 20 | 12 | 4 | 4 | 0.5 inches; patched electrodes used for sham | Yes; sound generated for sham | WOMAC pain 5-point Likert scale; other pain VAS; patient’s global pain as 100-mm VAS | Yes |
| Suarez-Almazor 2010 125 | Acupuncture, sham acupuncture, standard care | 455, yes | USA | 64 | 64 | NR | NR | Clinical and radiological | NR/unclear | Good | 20 | 12 | 6 | 7 | 0.7–4.0 cm (0.2–1.2 cun); sham: 0.7–1.7 cm (0.2–0.5 cun) | Yes | WOMAC pain subscale VAS 0–100 | No |
| Takeda 1994442 | Acupuncture (2), sham acupuncture (2) | 40, yes | Canada | 50 | 62 | Mean 90 | Mean 33 | Clinical and radiological | NR/unclear | Poor | 30 | 9 | 3 | 5 | 30 mm; sham: ‘just enough to puncture skin’ | No | WOMAC pain subscale VAS 0–10; Pain Rating Index of McGill Pain Questionnaire | Individual WOMAC subscores reported: pain, stiffness and function indices |
| Tukmachi 2004454 | Acupuncture (1 and 2), standard care (2) | 29, yes | UK | 83 | 61 | NR | NR | Clinical and radiological | 2 or 3 | Satisfactory | 20–30 | 10 | 5 | 9 | 1–1.5 cm | Yes | WOMAC pain, 5-point Likert scale; other pain VAS 0–10 cm | Other overall score: global assessment VAS 0–10 |
| Vas 2004120 | Acupuncture (4), sham acupuncture (4) | 88, yes | Spain | 84 | 67 | NR | Mean 33 | Clinical and radiological | NR - used Ahlbäck grade 1 or higher | Good | 20 | 12 | 12 | 8 | Unclear/not stated | Yes for acupuncture and sham | WOMAC pain, 5-point Likert scale; other pain VAS 0–100 | Yes |
| Weiner 2007188 | Acupuncture (2), sham acupuncture (2) | 88, yes | USA | 55 | 71 | NR | Mean 32 | Clinical and radiological | ≥ 2 | Good | 30 | 6 | 6 | 6 | Until just touched the bone | Yes, four needles for 30 minutes, two for 2 minutes; sham: two needles for 1 minute | WOMAC pain 5-point Likert scale | Individual WOMAC subscores reported: WOMAC function |
| Williamson 2007136 | Acupuncture (2), MSE (2), standard care (3) | 181, yes | UK | 54 | 71 | NR | Mean 32 | Clinical and radiological (patients awaiting arthroplasty) | NR/unclear | Satisfactory | 20 | 6 | 6 | 7–10 | Unclear/not stated | No | Other pain VAS; VAS pain 0–10 cm | Yes |
| Witt 200569 | Acupuncture (2), sham acupuncture (2), standard care (2) | 285, yes | Germany | 66 | 64 | NR | Mean 29 | Clinical and radiological | ≥ 2 | Good for acupuncture vs. sham; satisfactory for acupuncture vs. standard care | 30 | 12 | 8 | At least 8 for unilateral pain and at least 16 for bilateral pain | Unclear/not stated; ‘superficial’ for sham | No | WOMAC pain subscale VAS 0–100 | Yes |
| Witt 200677 | Acupuncture (2), standard care (2) | 342, yes | Germany | 60 | 61 | NR | NR | Clinical and radiological | NR/unclear | Satisfactory | Individually prescribed | On average 11 | 12 | Individually prescribed | Unclear/not stated | No | WOMAC pain subscale VAS 0–10 | Yes |
| Wu 2008458 | Acupuncture (2), standard care (4) | 34 (imputed), yes | China | 63 | 62 | NR | NR | Clinical and radiological | NR/unclear | Poor | 20 | 12 | 4 | At least 8 | Unclear/not stated | Yes | Lysholm score | No |
| Yurtkuran 1999460 | Acupuncture (2), ice/cooling treatment (2), TENS (2), placebo TENS (2) | 100, yes | Turkey | 91 | 58 | NR | NR | Clinical and radiological | NR/unclear | Poor | Unclear | 10 | 2 | 4 | 0.5–1.0 inches | Yes | 5-point Likert scale | No |
Muscle-strengthening exercise studies
| Study | Treatments (if suitable for NMA, adjunct code in parenthesesa) | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg) | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Intervention also incorporated home exercise? | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrahams 2002349 | MSE, standard care | 56, nob | UK | NR | Range 22–83 | NR | NR | Clinical and radiological | NR/unclear | Poor | Unclear/NR | Unclear/NR | 12 | Unclear/NR | Other pain VAS, VAS slide indicator version (0-–100) but no data reported | No |
| An 2008351 | MSE (2), standard care (2) | 21, yes | China | 100 | Mean 65 | NR | 26 | Clinical | Other method used to classify osteoarthritis severity: ACR criteria | Poor | 30 | 40 | 8 | No | WOMAC pain subscale VAS 0–100 | No |
| Baker 2001353 | MSE (2), placebo (2) | 38, yes | USA | 78 | Mean 69 | NR | 32 | Clinical and radiological | NR/unclear, median 3 | Poor | Unclear/NR | 48 (patients exercised three times per week for 16 weeks at home, with 12 supervised visits) | 16 | Yes | WOMAC pain subscale VAS 0–100 | Pain and physical function subscores reported |
| Bezalel 2010358 | MSE (2), heat treatment (2) | 50, yes | Israel | 74 | Mean 74 | NR | NR | Unclear/NR | NR/unclear | Poor | 45 | 4 | 4 | Yes | WOMAC pain five-point Likert scale; data for post treatment and follow-up read off graph – not very precise | Yes |
| Börjesson 1996190 | MSE (2), standard care (2) | 68, yes | Sweden | 50 | Mean 64 | Mean 83 | NR | Clinical and radiological | Other method used to classify osteoarthritis severity: Ahlbäck grades I–III | Satisfactory | 40 | 15 | 5 | Yes | Borg scale (11-grade category scale, 0 = no pain to 10 = worse pain) | No |
| Bruce-Brand 2012 467 | MSE, NMES, standard care | 26, yes | Republic of Ireland | 42 | Mean 64 | NR | 33 | Clinical and radiological | ≥ 3 | Poor | 30 | 18 | 6 | Yes | WOMAC pain five-point Likert scale | No |
| Callaghan 1995362 | MSE, standard care, placebo | 27, nob | UK | 31 | Median 53 | NR | NR | Radiological | NR/unclear | Poor | 20 | 8 | 4 | No | Other pain VAS 0–10 scale | No |
| Cheing 2002365 | MSE (2), TENS (2), placebo TENS (2) | 47, yes | China | 89 | Mean 63 | Mean 67 | 28 | Clinical and radiological | ≥ 2 | Poor | 20 | 20 | 4 | No | Other pain VAS 0–100, with baseline score standardised to 100 | No |
| Durmuş 2007370 | MSE (2), PES (2) | 50, yes | Turkey | 100 | Mean 55 | NR | 33 | Clinical and radiological | ≤ 3 | Satisfactory | 20 | 20 | 4 | No | WOMAC pain subscale VAS 0–10; other pain VAS 0–10 | All three individual WOMAC subscores reported |
| Ettinger 1997371 | MSE (2), aerobic exercise (2), standard care (3) | 364, yes | USA | 70 | Mean 69 | NR | 53% > 30 kg/m2 | Clinical and radiological | NR/unclear | Poor | 60 | 216 | 72 | Yes | Disability score five-point Likert scale; pain score: 1 (no pain) to 6 (excruciating pain) | No |
| Foroughi 2011 469 | MSE, placebo | 45, yes | Australia | 100 | Mean 66 | Mean 83 | 32 | Clinical | Other method used: modified Outerbridge classification | Poor | Not stated | 78 | 26 | No | WOMAC pain five-point Likert scale | Yes |
| Gür 2002379 | MSE (1), no treatment (1) | 23, yes | Turkey | NR | Mean 56 | Mean 79 | NR | Radiological | 2 or 3 | Poor | Unclear/NR | 24 | 8 | No | Other pain VAS, NRS 0–10 (10 unbearable pain); score is the sum of scores for pain at night, after inactivity, on sitting, on rising from a chair, climbing stairs, descending stairs | No |
| Hasegawa 2010381 | MSE (2), standard care (2) | 28, yes | Japan | 64 | Mean 77 | Mean 55 | 24 | Clinical diagnosis was of knee joint pain | NR/unclear | Poor | 80 (60 exercise plus 20 warm-up/-down) | 12 (also a minimum of 24 home exercise sessions) | 12 | Yes | Other pain VAS; 0–10 NRS (pain on movement) | No |
| Hay 2006382 | MSE, standard care | 217, yes | UK | 65 | Mean 68 | NR | NR | Clinical patients with knee pain – osteoarthritis of the knee not specified | NR/unclear | Poor | 20 | 3–6 | 10 | Yes | WOMAC pain Likert 5; other pain VAS; pain severity over previous 7 days on scale 0–10 | No |
| Huang 2005384 | MSE (2), standard care (2) | 98, yes | Taiwan | 81 | Mean 62 | NR | NR | Clinical and radiological | NR; patients with Altman grade II were included | Poor | Unclear/NR | 24 | 8 | Yes, but only after 8 weeks of treatment (i.e. during post-treatment follow-up) | Other pain VAS 1–10 | No |
| Hurley 2007385 | MSE, standard care | 53, nob | UK | 70 | Mean 67 | Mean 81 | 30 | Clinical | NR/unclear | Satisfactory | 35–45 | 12 | 6 | No, but did include self-management and coping skills | WOMAC pain five-point Likert scale | Yes |
| Imoto 2012 474 | MSE, standard care | 100, yes | Brazil | 92 | Mean 60 | NR | 30 | Clinical and radiological | ≥ 2 | Satisfactory | 30–40 | 16 | 8 | No | WOMAC pain five-point Likert scale; NRS 0–10 | No |
| Jan 2008389 | MSE (1), no treatment (1) | 98, yes | Taiwan | 81 | Mean 63 | Mean 63 | NR | Clinical and radiological | ≤ 3 | Satisfactory | 30 high-resistance exercise, 50 low-resistance exercise | 24 | 8 | No | WOMAC pain five-point Likert scale | Only physical subscale reported |
| Keogan 2007396 | MSE, aerobic exercise, standard care | 80, noc | Republic of Ireland | 64 | Mean 66 | NR | 30.6 | Clinical and radiological | NR/unclear | Poor | Unclear/NR | Unclear/NR | 6 | Unclear/NR | Other pain VAS, NR – likely 0–10 | No |
| Kuptniratsaikul 2002399 | MSE (2), standard care (2) | 366, yes | Thailand | 78 | Mean 68 | NR | 11% were obese | Radiological | 2 or 3 | Poor | 60 | 16 | 8 | Unclear/NR | Other AIMS pain subscale (0–10) | No |
| Lim 2008405 | MSE (2), standard care (2) | 55, yes | Australia | 62 | Mean 62 | Mean 78 | 29 | Clinical and radiological | ≥ 2 | Satisfactory | Unclear/NR | 60 (7 with physiotherapist at weeks 1–5, 7 and 10) | 12 | Yes, exercise was home based but participants also visited the physiotherapist seven times during the 12-week period | WOMAC pain five-point Likert scale (transformed to a 0–100 scale) | No |
| Lin 2009406 | MSE (2), standard care (2) | 72, yes | Taiwan | 69 | Mean 63 | Mean 62 | NR | Clinical and radiological | ≤ 3 | Satisfactory | Unclear/NR | 24 | 8 | No, told to cease other exercises | WOMAC pain five-point Likert scale | Pain and function subscores reported |
| Lund 2008407 | MSE (2), standard care (2) | 79, yes | Denmark | 78 | Mean 68 | Mean 75 | NR | Clinical and radiological | Other method used to classify osteoarthritis severity: Lequesne (1 –26) score. Mean score of participants was 11 | Poor | 50 | 16 | 8 | Unclear/NR | Other pain VAS 0–100 | No |
| Maurer 1999410 | MSE (2), standard care (3) | 98, yes | USA | 42 | Mean 65 | Mean 85 | NR | Clinical and radiological | ≤ 3 | Poor | Unclear/NR | 24 | 8 | Unclear/NR | WOMAC pain subscale VAS 0–100 | Pain and function subscores reported |
| McCarthy 2004412 | MSE (3), standard care (3) | 172, yes | UK | 58 | Mean 65 | NR | 30 | Clinical and radiological | Satisfactory | 45 | 16 | 8 | Yes | WOMAC pain five-point Likert scale; other pain VAS, VAS pain score 0–100 | No (only at baseline) | |
| Péloquin 1999423 | MSE (3), standard care (3) | 124, yes | Canada | 70 | Mean 66 | NR | 30 | Clinical and radiological | ≤ 3 | Poor | 60 | 36 | 12 | No | Other AIMS2 subscale for pain | No |
| Rapp 2009430 | MSE (2), manual therapy (2), standard care (2) | 39, yes | Germany | 64 | Mean 60 | Mean 83 | NR | Clinical and radiological | ≥ 2 | Poor | 45 | 16 | 8 | Unclear/NR | Other pain VAS 0–10 | No |
| Røgind 1998432 | MSE, standard care | 23, nob | Denmark | 91 | Mean 71 | Mean 71 | 27 | Clinical and radiological | ≥ 3 | Poor | Unclear/NR | 24 or 26 (two per week for 3 months) | 12 | Yes | Other pain VAS, pain on an 11-point scale. Separate scores for pain at night, at rest and on weight bearing | No |
| Rosemffet 2004433 | MSE, NMES | 18, nob | Argentina | 77 | Median 60 | NR | 30.9 | Clinical and radiological | ≥ 2 | Poor | 75 | 16 | 8 | Unclear/NR | Other pain VAS 20–80 mm | Yes |
| Salli 2010 480 | MSE, standard care | 71, yes | Turkey | 82 | Mean 57 | NR | 32 | Clinical and radiological | ≤ 2 | Satisfactory | Not stated | 24 | 8 | No | Other VAS 0–10 | No |
| Schilke 1996435 | MSE (2), standard care (2) | 20, yes | USA | 85 | Mean 66 | NR | NR | Clinical | NR/unclear | Poor | Unclear/NR | 24 | 8 | No | Other pain VAS: Osteoarthritis Screening Index 10-cm VAS | No |
| Topp 2002450 | MSE (2), standard care (2) | 102, yes | USA | 73 | Mean 63 | Mean 89 | NR | Clinical and radiological | NR/unclear | Poor | 50 | 48 | 16 | Yes | WOMAC pain five-point Likert scale | No |
| Trans 2009451 | MSE (2), standard care (2) | 52, yes | Denmark | 100 | Mean 60 | Mean 81 | 30 | Clinical and radiological | NR/unclear | Satisfactory | Up to 10.5 | 16 | 8 | No, both interventions | WOMAC pain subscale VAS 0–100 | All three individual WOMAC subscores reported |
| Williamson 2007136 | MSE (2), acupuncture (2), standard care (3) | 181, yes | UK | 54 | Mean 71 | NR | 32 | Clinical and radiological, patients on NHS arthroplasty waiting list | NR/unclear | Satisfactory | 60 | 6 | 6 | No | Other pain VAS; VAS pain 0–10 cm | Yes |
Aerobic exercise studies
| Study | Treatments (if suitable for NMA, adjunct code in parenthesesa) | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg) | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Individual or group sessions? | Also incorporated home exercise? | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglamiş 2009189 | Aerobic exercise (2), standard care (2) | 25, yes | Turkey | 100 | Mean 56 | NR | 33 | Clinical and radiological | ≥ 2 | Poor | 20 | 36 | 12 | Unclear/NR | No | WOMAC pain subscale VAS 0–10 | Yes |
| Bilgici 2004359 | Aerobic exercise, standard care | NR, nob | Turkey | NR | Mean 54 | NR | NR | Clinical | NR/unclear | Poor | 60 | 16 | 8 | Unclear/NR | Unclear/NR | WOMAC pain subscale VAS 0–10; unclear what form of WOMAC was used: no data reported | No |
| Brosseau 2012 466 | Aerobic exercise, standard care | 84, yes | Canada | 69 | Mean 63 | Mean 82 | 30 | Clinical and radiological | NR/unclear | Poor | 65 | Approximately 18 | 52 | Group | No | WOMAC pain five-point Likert scale | Yes |
| Dias 2003187 | Aerobic exercise, standard care | NR, nob | Brazil | 88 | Median 75 | NR | NR | Clinical and radiological | NR/unclear | Poor | 40 | 12 | 6 (followed by 6 weeks of home exercise) | Sessions given to groups | Yes for 6 weeks, following the 6-week intervention | SF-36 bodily pain; insufficient pain outcome data reported for data extraction | No |
| Ettinger 1997371 | Aerobic exercise (2), MSE (2), standard care (3) | 364, yes | USA | 70 | Mean 69 | NR | 53% > 30 | Clinical and radiological | NR/unclear | Poor | 60 | 216 | 72 | Sessions given to groups | Yes | Other pain 5-point Likert scale; Likert scale 1 (no pain) to 6 (excruciating pain) | No |
| Keefe 2004395 | Aerobic exercise (2), standard care (2) | 30 (imputed), yes | USA | 50 | Mean 59 | NR | NR | Clinical | NR/unclear | Poor | 60 | 36 | 12 | Sessions given to groups | No | Other; AIMS four-item pain scale | No, but Arthritis Self-efficacy Scale used |
| Keogan 2007396 | Aerobic exercise, MSE, standard care | 80, noc | Republic of Ireland | 64 | Mean 66 | NR | 31 | Clinical and radiological | NR/unclear | Poor | Unclear/NR | Unclear/NR | 6 | Unclear/NR | Unclear/NR | Other pain VAS; VAS 0–10 most likely to have been used | No |
| Kovar 1992398 | Aerobic exercise (2), standard care (3) | 92, yes | USA | 83 | Mean 69 | Mean 77 | NR | Clinical and radiological | NR/unclear | Poor | 90 | 24 | 8 | Sessions given to groups | No | Other: AIMS VAS 10 | No |
| Lim 2010 475 | Aerobic exercise, standard care | 66, yes | Korea | 87 | Mean 66 | NR | 28 | Clinical and radiological | ≥ 2 | Poor | Aquatic group 40; land group 40 | Aquatic 24; land NR | Both 8 | Groups | Unclear/NR | Brief pain inventory (0–10) | Yes |
| Messier 2004413 | Aerobic exercise (2), weight loss (2), standard care (3) | 240, yes | USA | 72 | Mean 69 | Mean 94 | 34 | Clinical and radiological | ≤ 3 | Satisfactory | 60 | 234 | 78 | Sessions given to individuals | Yes | WOMAC pain five-point Likert scale | Pain and physical function subscores reported |
| Salacinski 2012 479 | Aerobic exercise, standard care | 28, yes | USA | 73 | Mean 58 | NR | 24 | Clinical and radiological | ≤ 3 | Poor | 40–60 | 24 | 12 | Groups | No | WOMAC pain five-point Likert scale | Yes |
| Thorstensson 2005447 | Aerobic exercise, standard care | 56, noc | Sweden | 51 | Mean 56 | NR | 30 | Clinical and radiological | ≥ 3 | Poor | 60 | 12 | 6 | Sessions given to groups | Yes | Other pain VAS: KOOS | No |
| Wang 2011 482 | Aerobic exercise, standard care | 78, yes | Taiwan | 86 | Mean 68 | NR | 26 | Clinical and radiological | NR/unclear | Poor | 60 | 36 | 12 | Groups | Unclear | KOOS pain (0–100) | No |
T’ai chi studies
| Study | Treatments (if suitable for NMA, adjunct code in parenthesesa) | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brismée 2007360 | T’ai chi (2); standard care (3) | 41, yes | USA | 83 | 70 | 73 | 28 | Clinical | NR | Satisfactory | 40 | 36 (18 classes and 18 home sessions) | 12 | WOMAC pain subscale VAS 0–100 (reported as 7–35); knee pain VAS 0–10 | Yes (scale 26–130) |
| Lee 2009401 | T’ai chi (2); standard care (2) | 44, yes | Korea | 93 | 69 | 61 | 26 | Clinical and radiological | ≥ 2 | Satisfactory | 60 | 16 | 8 | WOMAC used but reported as 26–130 | Yes (scale 26–130); all three individual WOMAC subscores reported |
| Ni 2010 420 (retracted study) | T’ai chi (2); standard care (3) | 29, yes (but excluded – retracted study) | China | 100 | 63 | 66 | 27 | Clinical | NR | Poor | 40 | 72 | 24 | WOMAC pain five-point Likert scale | Yes |
| Wang 2009455 | T’ai chi; standard care | 40, yes | USA | 75 | 65 | NR | 30 | Clinical and radiological | ≥ 2 | Poor | 60 | 24 | 12 | WOMAC pain subscale VAS 0–100; global knee pain VAS 0–10 | All three individual WOMAC subscores reported |
| Yip 2007193 | T’ai chi (1); standard care (2) | 182, yes | Hong Kong | 84 | 65 | NR | NR | Clinical | NR | Poor | 15 (within a 2-hour self-management session) | 6 | 6 | VAS 0–100 | No |
Weight-loss studies
| Study | Treatments (if suitable for NMA, adjunct code in parenthesesa) | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg) | BMI (kg/m2) | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of dieting period (weeks) | Difference in weight loss between treatment groups (kg) | Weight-loss cut-off used to analyse effect on pain or WOMAC (e.g. 10% of body weight) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bliddal 2011 465 | Weight loss, standard care | 89, yes | Denmark | 89 | 63 | Mean 96 | Mean 36 | Clinical and radiological | 2 or 3 | Satisfactory | 52 | 7.3 | 8% | WOMAC pain subscale VAS 0–100 | Yes |
| Christensen 2005367 | Weight loss, standard care | 78, yes | Denmark | 89 | 63 | Mean 97 | Mean 36 | Clinical and radiological | 2 or 3 | Poor | 8 | 6.6 (95% CI 5.3 to 7.9; p < 0.0001); percentage of patients achieving 10% body weight loss was 50% in weight-loss group and 0% in standard care group | 10% | WOMAC pain subscale VAS 0–100 | Yes |
| Jenkinson 2009390 | Weight loss (2 and 3), standard care (3) | 389, yes | UK | 66 | 61 | Median 93 | Median 33 | Clinical | ≤ 4; 41% had a score of 0 | Satisfactory | 104 | NR/unclear | Cut-off used; dietary interventions aimed for a weight loss of 0.5–1.0 kg per week; weight loss NR | WOMAC pain five-point Likert scale | No |
| Messier 2004413 | Weight loss (2), aerobic exercise (2), standard care (3) | 240, yes | USA | 72 | 69 | Mean 94 | Mean 34 | Clinical and radiological | ≤ 3 | Satisfactory | 78 | Mean weight loss by group: weight-loss group 4.61 kg, exercise group 3.46 kg, standard care group 1.1 kg | Cut-off used; average weight-loss goal was 5% | WOMAC pain five-point Likert scale | Individual WOMAC subscores reported: pain, physical function |
| Miller 2006414 | Weight loss (3), standard care (3) | 74, yes | USA | 26 | 70 (all ≥ 60) | Mean 98 | Mean 35 | Clinical | NR/unclear | Poor | 26 | 8 kg | Cut off used; 10% weight loss was goal for intervention group | WOMAC pain five-point Likert scale | Yes |
Balneotherapy studies
| Study | Treatments (if suitable for NMA, adjunct code in parenthesesa) | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Number of sessions | Duration of treatment period (weeks) | Temperature (°C) | Type of treatment | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bálint 2007355 | Balneotherapy (3), placebo (3) | 52, yes | Hungary | 63 | Range 50–75 | NR | NR | Clinical | NR | Poor | 30 | 20 | 4 | 36 | Bathing mineral water | WOMAC pain subscale | Yes |
| Cantarini 2007364 | Balneotherapy, heat treatment, standard care | 74, nob | Italy | 63 | Mean 64 | 71 | NR | Clinical and radiological | ≤ 3 | Poor | 35 | 15 | 3 | Water 38, mud packs 45 | Bathing mineral water and mud packs | Pain VAS 0–100 | No |
| Fioravanti 2010372 | Balneotherapy (2), standard care (2) | 80, yes | Italy | 75 | Mean 70 | NR | 26 | Clinical and radiological | ≤ 3 | Satisfactory | 35 | 12 | 2 | Mud packs 45, mineral bath 38 | Bathing mineral water and mud packs | WOMAC pain subscale VAS 0–100 | No |
| Flusser 2002374 | Balneotherapy (2), placebo (2) | 58, yes | Israel | 85 | Mean 65 | 76 | NR | Clinical and radiological | 2 or 3 | Poor | 20 | 15 | 3 | 30–35 | Mud packs | Pain VAS 0–10 | Lequesne index |
| Forestier 2010375 | Balneotherapy, standard care | 309, nob | France | 47 | Mean 64 | NR | 30 | Clinical and radiological | ≥ 1 | Poor | 65 | 18 | 3 | Mineral hydrojet 37, massages by physiotherapist 38, mineral matured mud 45, collective mineral water pool 32 | Bathing mineral water and mud packs | WOMAC pain subscale VAS 0–100; other pain VAS 0–100 | WOMAC function |
| Karagülle 2007394 | Balneotherapy, standard care | 20, nob | Turkey | 85 | Mean 60 | NR | NR | Clinical and radiological | ≥ 2 | Satisfactory | 30 | 20 | 10 days | 38 ± 1 | Bathing mineral water | Pain VAS 0–10 | Lequesne Algofunctional Index |
| Kovács 2002397 | Balneotherapy, placebo | 68, nob | Hungary | Range 58–78 | NR | NR | NR | Clinical and radiological | NR/unclear | Poor | 30 | 15 | 15 days | 36 | Bathing mineral water | Pain VAS | No |
| Mahboob 2009408 | Balneotherapy (2), placebo (2) | 50, yes | Iran | 100 | Range 44–79 | NR | NR | Clinical | NR | Poor | 20 | 30 | 30 days | NR | 50 g of mud gel | WOMAC pain subscale VAS | Individual WOMAC subscores for pain, function and stiffness |
| Nguyen 1997419 | Balneotherapy, standard care | 64, nob | France | 81 | NR | NR | 61% > 24 | Clinical | NR | Poor | Unclear | Unclear | 3 | NR | Bathing mineral water | Pain VAS 0–100 | No |
| Sherman 2009438 | Balneotherapy (2), placebo (2) | 44, yes | Israel | 80 | Mean 67 | NR | NR | Clinical and radiological | ≥ 1 | Poor | 20 | 12 | 6 | 35–36 | Bathing mineral water | WOMAC pain subscale VAS 0–10; pain VAS 0–100 | Individual WOMAC subscores for pain, function and stiffness |
| Sukenik 1999441 | Balneotherapy (2), placebo (2) | 36, yes | Israel | 89 | Mean 63 | NR | NR | Clinical and radiological | Lequesne index of severity | Poor | 20 | 14 | 2 | Dead sea sulphur pools 37, sweet water control 24–25 | Bathing mineral water | VAS 0–10 | No |
| Tishler 2004448 | Balneotherapy, standard care | 68, yes | Israel | 78 | Mean 64 | NR | NR | Clinical | NR | Poor | 30 | 6 | 6 | 37 | Bathing mineral water | WOMAC pain five-point Likert scale; other pain VAS 0–100 | WOMAC subscores reported for pain, function and stiffness |
| Wigler 1995456 | Balneotherapy (2), placebo (2) | 33, yes | Israel | 88 | Mean 65 | NR | NR | Clinical and radiological | NR | Poor | 40 | 7 | 2 | Water 38, mud pack 45 | Bathing mineral water and mud packs | Pain VAS 0–10 | No |
| Yurtkuran 2006461 | Balneotherapy (5), placebo (5) | 52, yes | Turkey | 97 | Mean 54 | 77 | 32 | Clinical and radiological | 2 or 3 | Poor | 20 | 10 | 2 | 37 | Bathing mineral water | WOMAC pain five-point Likert scale; pain VAS 0–100 | Yes |
Insoles studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Time spent wearing (per day) (minutes) | Number of days worn per week | Duration of treatment period (weeks) | Ankle support also worn? | Pain outcome | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arazpour 2013 463 | Insoles, braces | 24, yes | Iran | 63 | 59.5 | NR | 27 | Clinical and radiological | ≤ 2 | Poor | 7.6 hours | Unclear | 6 weeks | No | Other pain VAS 0–10 | No |
| Baker 2007354 | Insoles, placebo | 172, nob | USA | 59 | 68 | NR | 33 | Clinical or radiological | ≥ 1 | Poor | Approximately 420 (7 hours) | Unclear | 6; phase 1: 6, phase 2: 6, washout period: 4 | No | WOMAC pain subscale VAS 0–100 | No |
| Bennell 2011 464 | Insoles, placebo | 200, yes | Australia | 59 | 64 | NR | 29 | Clinical and radiological | 2 or 3 | Good | Unclear | 7 | 12 months | Unclear | WOMAC pain five-point Likert scale | No |
| Maillefert 2001409 | Insoles (4), placebo (4) | 156, yes | France | 74 | 65 | NR | 29 | Clinical and radiological | ≥ 2 | Satisfactory | Wear permanently | Wear permanently | Unclear: up to 24 months | No | WOMAC pain subscale VAS 0–100 | No |
| Nigg 2006421 | Insoles, placebo | 123, noc | Canada | 54 | 58 | 85 | 30 | Clinical and radiological | ≥ 2 | Satisfactory | As much as possible | 7 | 12 | No | WOMAC pain subscale VAS 0–100 | Yes |
| Toda 2008449 | Insoles, placebo | 122, noc | Japan | 88 | 64 | NR | 25 | Radiological | ≤ 4 | Satisfactory | 300–600 | 7 | 12 | Sock-type ankle support | Other pain VAS 0–100% | No |
Static magnets studies
| Study | Treatments | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Strength of magnetic field (gauss or tesla) | When to wear magnets | Duration of treatment period (weeks) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2008366 | Static magnets, placebo | 42, noa | Taiwan | 79 | Mean 65 | 64 | 27 | Clinical and radiological | Ahlbäck classification (grade I) | Poor | 35 mT, as measured with a Lakeshore 430 gaussmeter | Only during waking hours | 12 | HAQ pain scale 0–100 | No |
| Hinman 2002383 | Static magnets, placebo | 43, yes | USA | 60 | Mean 63 | NR | NR | Clinical | NR | Poor | 1.08 T | Magnet group: 1.0–23.5 hours per day, mean number of hours magnet worn 116.33 (range 25–235); placebo group: 1–24 hours per day, the mean number of hours magnet worn 85.12 (range 6.5–213) | 2 | WOMAC pain subscale VAS 0–10 | No |
| Wolsko 2004457 | Static magnets, placebo | 46, yes | USA | 69 | Median 63 | NR | 30 | Clinical and radiological | ≥ 1 | Poor | 40–850 G | At least 6 hours a day | 6 | WOMAC pain subscale VAS 0–100 and VAS 0–100 with five scales (maximum 500) | No |
Braces studies
| Study | Treatments | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Time spent wearing (per day) (hours) | Number of days worn per week | Duration of treatment period (weeks) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arazpour 2013 463 | Braces, insoles | 24, yes | Iran | 63 | 59.5 | NR | 27 | Clinical and radiological | ≤ 2 | Poor | 7.3 | Unclear | 6 | Other pain VAS 0–10 | No |
Transcutaneous electrical nerve stimulation studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Number of sessions | Duration of treatment period (weeks) | Frequency of current (Hz) | Intensity of current (mA) | Pulse width/duration (microseconds) | Electrodes placed at acupuncture points? | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcidi 2007350 | TENS (2), heat treatment (2) | 40, yes | Italy | 85 | Mean 66 | NR | NR | Clinical and radiological | NR | Poor | 20 | 5 | 1 | 50 | ‘Well tolerated tingling’ | 0.5 | No | Other pain VAS 1–100 | No |
| Burch 2008303 | TENS, interferential therapy | 116, nob | USA | 72 | Mean 62 | 86 | 31 | Clinical and radiological | ≥ 1 | Poor | 35 | 56 | 8 | 0.2 | 60 | 300 | Unclear/not stated | WOMAC pain five-point Likert scale | All three individual WOMAC subscores reported |
| Cheing 2002365 | TENS (2), MSE (2), placebo (2) | 47, yes | China | 89 | Mean 63 | 67 | 28 | Clinical and radiological | ≥ 2 | Poor | 60 | 20 | 4 | 80 | To achieve a tingling sensation | 140 | Yes | Other pain VAS 0–100 | No |
| Cheing 2003191 | TENS (2), placebo (2) | 38, yes | China | 89 | Mean 66 | 66 | NR | Clinical and radiological | ≥ 2 | Poor | 20–60 | 10 | 2 | 100 | NR | 200 | Yes | Other pain VAS 0–10 | No |
| Grimmer 1992378 | TENS (1), placebo (1) | 60, yes | Australia | 62 | Mean 67 | NR | NR | Clinical and radiological | NR | Poor | 30 | 1 | 1 session | 80 | NR | NR | Yes | Other pain VAS | No |
| Itoh 2008386 | TENS (1), acupuncture (1), standard care (2) | 12, yes (only one study) | Japan | 66 | Range 62–83 | NR | NR | Clinical and radiological | ≥ 2 | Poor | 15 | 5 | 5 | 4 | NR | NR | No | Other pain VAS 0–100 | Yes |
| Kang 2007393 | TENS (2), placebo (2) | 63, yes | USA | 71 | Mean 57 | NR | NR | Clinical and radiological | NR | Poor | 30 | 1 | 1 session | NR | 16% at start, increased to 23% at 15 minutes | NR | No | Other pain VAS 0–10 | All three individual WOMAC subscores reported |
| Law 2004400 | TENS, placebo | 34, noc | Hong Kong | 97 | Mean 83 | 57 | 26 | Clinical and radiological | ≥ 2 | Poor | 40 | 10 | 2 | 2, 100 or alternating 2/100 | 25–35 | 2 Hz: 576,100 Hz: 200 | Yes | Other pain VAS 0–100 | No |
| Lewis 1988402 | TENS, placebo | Unknown, noc | Australia | NR | NR | NR | NR | Unclear/not stated | NR | Poor | NR | NR | 9 | Unclear/not stated | Unclear/not stated | Unclear/not stated | Unclear/not stated | McGill Pain Questionnaire, Pain Index | No |
| Lewis 1994403 | TENS (1), standard care (4) | 56, yes | Australia | 58 | Mean 66 | NR | NR | Clinical and radiological | NR | Poor | 30–60 | 63 | 3 | 70 | Adjusted until comfortable | 100 | Spleen 9 and 10, stomach 34 and 35 | VAS 0–100 | No |
| Lewis 1984404 | TENS, placebo | 30, noc | UK | 73 | Median 61 | NR | NR | Unclear/not stated | NR | Poor | 30–60 | 63 | 7 | 70 | NR | NR | Yes | VAS | No |
| Ng 2003418 | TENS, electroacupuncture, standard care | 14, yes | China | 96 | Mean 85 | NR | NR | Clinical | NR | Poor | 20 | 8 | 2 | 2 | NR | 200 | Yes | NRS | No |
| Pietrosimone 2009427 | TENS (1), ice/cooling treatment (1), no treatment (1) | 33, yes | USA | 48 | Mean 56 | 88 | 30 | Radiological | NR | Poor | 45 | 1 | 1 day | 150 | NR | 150 | No | VAS | No |
| Selfe 2008436 | TENS (2), placebo (2) | 37, yes | USA | 68 | Mean 67 | NR | 31 | Clinical and radiological | NR | Poor | 20–30 | 17 | 8 | NR | NR | NR | Sometimes | WOMAC pain VAS 0–10; NRS | Yes |
| Smith 1983439 | TENS, placebo | 30, noc | UK | 67 | Mean 68 | NR | NR | Clinical | NR | Poor | 20 | 8 | 4 | 32–50 | Adjusted until comfortable | 80 | Yes | Other significant pain reliefd | No |
| Taylor 1981445 | TENS, placebo | 20, nob | USA | 90 | 72 | NR | NR | Clinical and radiological | NR | Poor | 30 | NR | 2 | NR | NR | NR | No | Five-point Likert scale | No |
| Vance 2012 481 | TENS, placebo | 75, yes | USA | 61 | 56 | NR | 36 | Clinical and radiological | NR/unclear | Poor | 40–50 | 1 | 3 hours | 100 | High frequency 27, low frequency 24 | 100 | No | Other pain VAS 0–100 | No |
| Yurtkuran 1999460 | TENS (2), acupuncture (2), ice/cooling (2), placebo (2) | 100, yes | Turkey | 91 | 58 | NR | NR | Clinical and radiological | NR | Poor | 20 | 10 | 2 | 4 | 0.4–2.5 V | 1000 ms | Yes | Five-point Likert scale | No |
Pulsed electrical stimulation studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Frequency of current (Hz) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Callaghan 2005363 | PES (2), placebo (2) | 21, yes | UK | 48 | 60 | NR | 28 | Radiological | ≥ 3 | Poor | 20 | 6 | 2 | High-frequency group 27 MHz; low-frequency group 27 MHz | VAS 0–10 and pain subscale of AIMS | No |
| Durmuş 2007370 | PES (2), MSE (2) | 50, yesb | Turkey | 100 | 55 | NR | 33 | Clinical and radiological | ≤ 3 | Satisfactory | 20 | 20 | 4 | 50 | WOMAC pain subscale VAS 0–10; pain VAS 0–10 | All three individual WOMAC subscores reported |
| Fary 2011 468 | PES, placebo | 70, yes | Australia | 47 | 70 | NR | 28.1 | Clinical and radiological | ≤ 4 | Satisfactory c | 7 hours | 182 | 26 | 100 | Other pain VAS | Yes |
| Fukuda 2008 376 | PES, standard care, placebo | NR, no d | Brazil | 100 | 61 | 67 | NR | Clinical and radiological | NR; grade II or III using Gupta criteria | Poor | 38 for 33-KJ dose group; 19 for 17-KJ dose group and placebo group | 39 (3 per week for 3 months) (13 weeks) | 13 | 33 kJ vs. 17 kJ (27.12 Hz with a pulse frequency of 145 Hz) | Other pain VAS; VAS (unclear what scoring) | Other overall score: Lequesne |
| Fukuda 2011470 | PES, placebo, no treatment | 112, yes | Brazil | 100 | 60 | 71 | 28 | Radiological | NR/unclear | Poor | Low dose 19; high dose 38 | 9 | 3 | 145 | NRS 0–10 | No |
| Garland 2007377 | PES (2), placebo (2) | 58, yes | USA | 66 | 66 | NR | 31 | Clinical and radiological | ≥ 3 | Poor | ≥ 6 hours | Unclear/NR; each day | 13 | 100 | WOMAC pain subscale VAS 0–100 | Yes |
| Miranda-Filloy 2005415 | PES (2), placebo (2) | 18 (imputed), yes | Spain | 80 | > 40 | NR | NR | Clinical and radiological | ≥ 3 | Poor | 20 | 24 | 8 | 35 | WOMAC pain subscale VAS 0–100; other pain VAS; VAS 0-100 | No |
| Zizic 1995462 | PES, placebo | 71, nod | USA | 46 | NR | NR | NR | Clinical and radiological | NR/unclear | Poor | 6–10 hours a day | Unclear/NR | 4 | 100 | Other pain VAS 0–10 | No |
Pulsed electromagnetic fields studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Frequency of pulse (Hz) | Strength of magnetic field (gauss or tesla) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fischer 2005373 | PEMF (2), placebo (2) | 69, yes | Slovenia | 72 | Mean 60 | NR | 29.3 | Radiological | NR/unclear | Poor | 16 | 30 | 6 | Unclear/not stated; low frequency | Varied between 3.4 and 13.6 μT | VAS 0–100 and 5-point Likert scale | No (Knee Society score) |
| Jacobson 2001388 | PEMF (1), placebo (1) | 176, yes | USA | NR | NR | NR | NR | Unclear/not stated | NR/unclear | Poor | 6 | 8 | 2 | 0.976–7.7 | 2.74 × 10–7–3.4 × 10–8 G | Scale from 1 to 10 (no further details) | No |
| Perrot 1998425 | PEMF (2), placebo (2) | 40, yes | France | 80 | Mean 69 | NR | NR | Unclear/not stated | NR | Poor | 60 | 9 | 1.3 | Unclear/not stated | Unclear/not stated | Other Pain VAS 0–100 | No |
| Pipitone 2001428 | PEMF, placebo | 69, nob | UK | 28 | Median around 63 (range 40–84) | NR | NR | Clinical and radiological | NR/unclear | Poor | Unclear/not stated | 147 | 6 | 7.8 in morning and afternoon, 3 in the evening | < 0.5 G | WOMAC pain five-point Likert scale | Yes |
| Thamsborg 2005446 | PEMF (2), placebo (2) | 83, yes | Denmark | 54 | Mean 60 | NR | 27 | Clinical and radiological | ≥ 1 | Poor | 120 | 30 | 6 | 50 | Unclear/not stated | WOMAC pain five-point Likert scale | All three individual WOMAC subscores reported |
| Trock 1994452 | PEMF, placebo | 84, yes | USA | 70 | Mean 67 | 80 | NR | Clinical and radiological | NR/unclear | Poor | 30 | 18 | Around 4–6 | 5 for 10 minutes, 10 for 10 minutes and 12 for 10 minutes | 10–15 G for 10 minutes and 15–25 G for 20 minutes | Other pain VAS 0–10 | No |
Neuromuscular electrical stimulation studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Frequency of current (Hz) | Intensity of current | Pulse width/duration (microseconds) | Electrodes placed at acupuncture points? | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruce-Brand 2012 467 | MSE, NMES, standard care | 26, yes | Republic of Ireland | 42 | Mean 64 | NR | 33 | Clinical and radiological | ≥ 3 | Poor | 20 | 30 | 6 | 50 | 18 mA | 100–400 | No | WOMAC pain five-point Likert scale | No |
| Rosemffet 2004433 | NMES, MSE | 18, nob | Argentina | 77 | Median 60 | NR | 30.9 | Clinical and radiological | ≥ 2 | Poor | 30 | 24 | 8 | 25 | According to patient tolerance | Unclear/NR | Unclear/NR | 20–80 VAS scale | Yes |
| Talbot 2003443 | NMES (3), standard care (3) | 34, yes | USA | 79 | Mean 71 | NR | 30 | Clinical and radiological | ≥ 1 | Poor | 15 | 36 | 12 | Unclear/NR | Unclear/NR | 300 | Unclear/NR | McGill Pain Rating Index | No |
Interferential therapy studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of individual sessions (minutes) | Total number of sessions | Duration of treatment period (weeks) | Frequency (Hz) | Pulse width/duration | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adedoyin 2002301 | Interferential therapy (5), placebo (5) | 26 (imputed), yes | Nigeria | 67 | Mean 59 | 78 | 28 | Clinical and radiological | NR/unclear | Poor | 20 | 8 | 4 | 100 for 15 minutes, reduced to 80 for a further 5 minutes | 0.033 seconds | VAS 0–10 | No |
| Burch 2008303 | Interferential TENS | 106, nob | USA | 72 | Mean 62 | 86 | 31 | Clinical and radiological | ≥ 1 | Poor | 15 of interferential followed by 20 of patterned stimulation | 56 | 8 | Base of 5000, and a pre-modulated beat frequency sweeping between 1 and 150 | Between 3 and 102 microseconds | WOMAC pain five-point Likert scale | All three individual WOMAC subscores reported |
| Defrin 2005369 | Interferential therapy (2), placebo (2), no treatment (2) | 55 (imputed), yes | Israel | NR | Mean 67 | NR | NR | Clinical | NR/unclear | Poor | 20 | 12 | 4 | Constantly ranged between 30 and 60 | NR | VAS 0–10 | No |
| Gundog 2012 302 | Interferential therapy, placebo | 60, yes | Turkey | 80 | Mean 60 | NR | 29 | Clinical and radiological | 2 or 3 | Good | 20 | 15 | 3 | Group 1 40, group 2 100, group 3 180 | NR | WOMAC Likert scale | No |
| Young 1991459 | Interferential therapy, placebo | NR, noc | Canada | NR | Range 18–75 | NR | NR | Clinical and radiological | NR/unclear | Poor | 25 | 10 | 1.7 | NR/unclear | NR/unclear | Reported WOMAC but unclear how assessed | No |
Heat treatment studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of sessions (minutes) | Number of sessions | Duration of treatment period (weeks) | Type of treatment | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcidi 2007350 | Heat (2), TENS (2) | 40, yes | Italy | 85 | 66 | NR | NR | Clinical and radiological | NR | Poor | 20 | 5 | 0.7 | Radiofrequency electromagnetic radiation | VAS 1–100 | No |
| Bezalel 2010358 | Heat (2), MSE (2) | 50, yes | Israel | 74 | 74 | NR | NR | NR | NR | Poor | 20 | 6 | 4 | Short-wave diathermy | WOMAC pain five-point Likert scale | Yes |
| Cantarini 2007364 | Heat, balneotherapy, standard care | 74, nob | Italy | 63 | 64 | 71 | NR | Clinical and radiological | ≤ 3 | Poor | 15 | 10 | 3 | Short-wave diathermy | VAS 0–100 | No |
| Clarke 1974368 | Heat (2), ice/cooling treatment (2), placebo (2) | 30, yes | UK | 69 | 61 | NR | NR | Radiological | NR | Poor | NR | 9 | 3 | Short-wave diathermy | Likert scale 0–3; maximum score 17 | No |
| Giombini 2011 472 | Heat treatment, placebo | 63, yes | Italy | 67 | 67 | NR | 27 | Clinical and radiological | 2 or 3 | Satisfactory | 30 | 12 | 4 | Short-wave diathermy | WOMAC pain | Yes |
| Mazzuca 2004411 | Heat (2), placebo (2) | 51, yes | USA | 77 | 63 | NR | NR | Clinical and radiological | ≥ 2 | Poor | N/A | N/A | 4 | Heat-retaining knee sleeve | WOMAC pain five-point Likert scale | Individual WOMAC subscores |
| Rattanachaiyanont 2008431 | Heat, placebo | 104, noc | Thailand | 100 | 63 | NR | 26 | Clinical | NR | Poor | 20 | 9 | 3 | Short-wave diathermy | WOMAC pain VAS 0–10 | Yes |
Ice/cooling studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years) | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of sessions (minutes) | Number of sessions | Duration of treatment period (weeks) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arman 1988352 | Ice/cooling, standard care | 33, nob | Turkey | NR | NR | NR | NR | Clinical and radiological | NR | Poor | 10 | 40 | 3 | NR | No |
| Clarke 1974368 | Ice/cooling (2), heat treatment (2), placebo (2) | 45, yes | UK | 69 | Mean 61 | NR | NR | Radiological | NR | Poor | NR | 9 | 3 | Likert scale 0–3 (maximum score 17) | No |
| Pietrosimone 2009427 | Ice/cooling (1), TENS (1), no treatment (1) | 33, yes | USA | 48 | Mean 56 | 88 | 30 | Radiological | NR | Poor | 20 | 1 | 1 day | VAS | No |
| Yurtkuran 1999460 | Acupuncture (2), ice/cooling (2), TENS (2), placebo TENS (2) | 100, yes | Turkey | 91 | Mean 58 | NR | NR | Clinical and radiological | NR | Poor | 20 | 10 | 2 | Likert scale 1–5 | No |
Laser/light therapy studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of sessions (minutes) | Number of sessions | Duration of treatment period (weeks) | Light dose | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bülow 1994361 | Laser, placebo | 29, nob | Denmark | 83 | ≥ 74 | NR | NR | Clinical and radiological | NR | Poor | 15 | 9 | 3 | Accumulated dose 202.5 J | Likert scale 0–3 (maximum score 126) | No |
| Fukuda 2011 471 | Laser/light therapy, placebo | 47, yes | Brazil | 72 | 63 | 75 | 29 | Clinical and radiological | ≥ 2 | Good | NR | 9 | 3 | 27 J per session | Visual numerical pain scale 0–10 | No |
| Gür 2003380 | Laser, placebo | 90, nob | Turkey | 80 | 60 | NR | 30 | Clinical and radiological | ≥ 2 | Satisfactory | 5 | 10 | 2 | Group 1, 30 J accumulated dose; group 2, 20 J accumulated dose | VAS 0–10 | Yes |
| Hsieh 2012 473 | Laser/light therapy, placebo | 72, yes | Taiwan | 86 | 60 | NR | 26 | Clinical and radiological | ≥ 2 | Good | 40 | 6 | 2 | 2.08 J/cm 2 /minute per session | Knee injury and Osteoarthritis Outcome Score VAS | No |
| Kang 2006392 | Laser, standard care | Unclear, noc | Korea | NR | NR | NR | NR | Clinical and radiological | ≥ 2 | Poor | 30 | 56 | 8 | 402 laser diodes, 650 nm, 50 mW | WOMAC pain subscale VAS 0–10 | Individual WOMAC subscores reported |
| Nivbrant 1989422 | Laser, placebo | Unclear, noc | Sweden | NR | NR | NR | NR | Clinical and radiological | Osteoarthritis severity, radiographic grades 2–4 | Poor | 20 | 6 | 6 | NR | VAS | No |
| Shen 2009437 | Laser (2), placebo (2) | 40, yes | China | 90 | 58 | NR | NR | Clinical and radiological | ≥ 2 | Satisfactory | 20 | 12 | 4 | 283 J | WOMAC pain five-point Likert scale | All three individual WOMAC subscale scores |
| Stelian 1992440 | Laser (2), placebo (2) | 50, yes | Israel | 68 | 68 | NR | NR | NR | NR | Poor | 30 | 20 | 10 days | Red light 10.3 J; infrared light 11.1 J | Other pain VAS 0–10; pain Likert scale 0–5 | No |
| Tascioglu 2004444 | Laser (4), placebo (4) | 60, yes | Turkey | 70 | 62 | NR | 29 | Clinical and radiological | 2 or 3 | Satisfactory | 10 minutes for first group, 5 minutes for second group | 10 | 2 | Group 1, 3 J per tender joint (five joints in all), dose per treatment 15 J; group 2, 1.5 J per tender joint (five joints in all), dose per treatment 7.5 J | WOMAC pain five-point Likert scale; other pain VAS 0–100 | All three individual WOMAC subscale scores |
Manual therapy studies
| Study | Treatments (if suitable for NMA, adjunct code in parentheses)a | Number analysed at end of treatment, data suitable for pain analyses? | Country | % Female | Age (years), mean | Weight (kg), mean | BMI (kg/m2), mean | Method of diagnosis | Kellgren and Lawrence score | Overall study quality | Duration of sessions (minutes) | Number of sessions | Duration of treatment period (weeks) | Pain outcomes used | Overall WOMAC score reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moss 2007417 | Manual therapy, placebo | 114, nob | Australia | 66 | 65 | NR | NR | Clinical and radiological | NR | Good | 10 | 1 | 10 minutes | WOMAC five-point Likert scale; VAS 0–10 | No |
| Perlman 2006424 | Manual therapy, standard care | 68, nob | USA | 78 | 68 | NR | 29 | Clinical and radiological | NR | Poor | 60 | 12 | 8 | WOMAC VAS 0–100; pain VAS 0–100 | Yes |
| Perlman 2012 478 | Manual therapy, standard care | 119, yes | USA | 70 | 64 | NR | 32 | Clinical and radiological | NR/unclear | Satisfactory | Varied across four groups (30 or 60) | 8 or 12 depending on group | 8 | WOMAC VAS 0–100 | Yes |
| Pollard 2008429 | Manual therapy (2), placebo (2) | 43, yes (only study) | Australia | NR | 56 | NR | NR | Clinical and radiological | NR | Poor | NR | 6 | 2 | VAS 0–10 | No |
| Rapp 2009430 | Manual therapy (2), MSE (2), standard care (2) | 39, yes | Germany | 64 | 60 | 83 | NR | Clinical and radiological | ≥ 2 | Poor | 20 | 16 | 8 | VAS 0–10 | No |
| Tucker 2003453 | Manual therapy (2), standard care (4) | 103, yes | South Africa | 63 | 59 | 82 | NR | Clinical and radiological | NR | Poor | NR | 8 | 3 | VAS 0–100; NRS | No |
Appendix 4 Review data related to Chapter 4
Mapping and standardised pain descriptive results
| ID | Study | Pain group (type) | Treatment | HRQoL mapped outcome | Score, mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Predicted EQ-5D | Pain standard outcome | Standardised pain | |||||||
| t0 – baseline | t1 – 3 months | t0 – baseline | t1 – 3 months | ||||||
| 1 | Diener 200672 | H | Usual care | SF-12 | 0.68 (0.268) | 0.759 (0.213) | Migraine days | 1.795 (1.142) | 1.222 (1.114) |
| Sham acupuncture | 0.696 (0.248) | 0.76 (0.218) | 1.629 (0.948) | 1.08 (1.036) | |||||
| Acupuncture | 0.683 (0.251) | 0.783 (0.203) | 1.705 (0.95) | 0.996 (0.865) | |||||
| 2 | Endres 200773 | H | Sham acupuncture | 0.666 (0.263) | 0.765 (0.237) | TTH days | 2.863 (1.065) | 1.258 (1.103) | |
| Acupuncture | 0.633 (0.281) | 0.757 (0.219) | 2.728 (0.931) | 0.943 (0.865) | |||||
| 10 | Haake 200771 | MSK | Usual care | 0.633 (0.216) | 0.648 (0.231) | von Korff pain intensity score | 4.877 (1.051) | 2.809 (0.944) | |
| Sham acupuncture | 0.656 (0.208) | 0.666 (0.212) | 4.86 (0.946) | 2.485 (1.001) | |||||
| Acupuncture | 0.631 (0.236) | 0.668 (0.199) | 4.87 (1.002) | 2.325 (0.995) | |||||
| 24 | Scharf 200670 | OAK | Usual care | 0.533 (0.309) | 0.536 (0.325) | WOMAC total score | 3.609 (0.992) | 2.148 (0.999) | |
| Sham acupuncture | 0.494 (0.34) | 0.593 (0.318) | 3.646 (1.021) | 1.697 (0.978) | |||||
| Acupuncture | 0.514 (0.323) | 0.596 (0.32) | 3.591 (0.986) | 1.566 (0.933) | |||||
| 3 | Jena 200876 | H | Usual care | SF-36 | 0.725 (0.198) | 0.754 (0.191) | Headache days | 1.171 (0.97) | 1.225 (1.033) |
| Acupuncture | 0.726 (0.202) | 0.826 (0.159) | 1.2 (1.029) | 0.765 (0.911) | |||||
| 4 | Linde 200568 | H | Usual care | 0.741 (0.175) | 0.744 (0.13) | Days of moderate to severe pain | 2.082 (1.132) | 1.797 (0.9) | |
| Sham acupuncture | 0.767 (0.128) | 0.815 (0.133) | 1.933 (0.927) | 1.099 (1.018) | |||||
| Acupuncture | 0.737 (0.142) | 0.809 (0.138) | 2.001 (0.97) | 1.178 (0.963) | |||||
| 5 | Melchart 200567 | H | Usual care | 0.737 (0.164) | 0.721 (0.159) | Headache days | 2.534 (1.017) | 1.883 (0.855) | |
| Sham acupuncture | 0.75 (0.167) | 0.846 (0.126) | 2.59 (0.982) | 1.248 (0.954) | |||||
| Acupuncture | 0.766 (0.135) | 0.834 (0.144) | 2.56 (1.006) | 1.145 (1.001) | |||||
| 6 | Vickers 200460 | H | Usual care | 0.834 (0.148) | 0.806 (0.182) | Severity score | 1.732 (1.088) | 1.48 (1.05) | |
| Acupuncture | 0.789 (0.171) | 0.809 (0.189) | 1.593 (0.915) | 1.125 (0.927) | |||||
| 7 | Brinkhaus 200666 | MSK | Usual care | 0.612 (0.185) | 0.635 (0.24) | VAS pain score | 4.714 (0.97) | 1.979 (0.847) | |
| Sham acupuncture | 0.598 (0.168) | 0.728 (0.187) | 4.749 (1.121) | 1.474 (1.005) | |||||
| Acupuncture | 0.616 (0.192) | 0.763 (0.196) | 4.508 (0.944) | 1.165 (0.963) | |||||
| 17 | Vas 2006121 | MSK | Sham acupuncture | 0.556 (0.226) | 0.611 (0.203) | VAS pain score | 4.842 (1.033) | 2.326 (0.724) | |
| Acupuncture | 0.605 (0.271) | 0.752 (0.215) | 4.603 (0.959) | 0.918 (0.697) | |||||
| 20 | Witt 200675 | MSK | Usual care | 0.663 (0.217) | 0.684 (0.22) | Neck pain and disability score | 3.394 (1.004) | 2.698 (0.966) | |
| Acupuncture | 0.65 (0.209) | 0.769 (0.188) | 3.46 (0.995) | 2.079 (0.937) | |||||
| 21 | Witt 200674 | MSK | Usual care | 0.596 (0.232) | 0.638 (0.241) | Hanover functional ability score | –3.027 (0.994) | –3.051 (1.012) | |
| Acupuncture | 0.585 (0.235) | 0.732 (0.225) | –2.957 (1.005) | –3.453 (0.95) | |||||
| 27 | Witt 200569 | OAK | Usual care | 0.564 (0.185) | 0.59 (0.2) | WOMAC total score | 2.675 (1.042) | 2.084 (0.813) | |
| Sham acupuncture | 0.561 (0.2) | 0.626 (0.218) | 2.751 (0.991) | 1.57 (1.001) | |||||
| Acupuncture | 0.564 (0.218) | 0.71 (0.19) | 2.664 (1.032) | 1.119 (0.921) | |||||
| 28 | Witt 200677 | OAK | Usual care | 0.56 (0.213) | 0.57 (0.225) | WOMAC total score | 2.057 (1.002) | 1.876 (0.921) | |
| Acupuncture | 0.576 (0.234) | 0.682 (0.234) | 2.062 (0.999) | 1.185 (0.956) | |||||
| 8 | Carlsson 2001119 | MSK | Sham acupuncture | VAS pain | 0.604 (0.222) | 0.434 (0.314) | VAS pain score | 2.386 (1.022) | 2.251 (1.154) |
| Acupuncture | 0.481 (0.197) | 0.685 (0.246) | 2.79 (0.978) | 1.684 (0.911) | |||||
| 9 | Guerra de Hoyos 2004138 | MSK | Sham acupuncture | 0.526 (0.229) | 0.632 (0.256) | VAS pain score | 2.857 (0.871) | 1.134 (1.07) | |
| Acupuncture | 0.525 (0.202) | 0.666 (0.229) | 2.732 (1.118) | 0.488 (0.814) | |||||
| 11 | Irnich 2001118 | MSK | Sham acupuncture | 0.551 (0.236) | 0.642 (0.259) | VAS pain score | 2.381 (1.113) | 1.527 (1.055) | |
| Acupuncture | 0.562 (0.225) | 0.636 (0.233) | 2.256 (0.913) | 1.277 (0.918) | |||||
| 12 | Kennedy 2008130 | MSK | Sham acupuncture | 0.61 (0.25) | 0.653 (0.242) | Roland Morris disability score | 2.382 (1.033) | 1.265 (1.155) | |
| Acupuncture | 0.568 (0.22) | 0.562 (0.247) | 2.359 (0.988) | 0.813 (0.825) | |||||
| 13 | Kerr 2003117 | MSK | Sham acupuncture | 0.523 (0.178) | 0.548 (0.204) | VAS pain score | 3.981 (0.924) | 2.324 (1.154) | |
| Acupuncture | 0.5 (0.132) | 0.514 (0.266) | 4.176 (1.065) | 1.943 (0.849) | |||||
| 18 | Vas 2008122 | MSK | Sham acupuncture | 0.556 (0.171) | 0.585 (0.254) | CMS | –3.123 (0.971) | –3.346 (0.975) | |
| Acupuncture | 0.563 (0.155) | 0.623 (0.249) | –3.281 (1.026) | –4.102 (0.874) | |||||
| 19 | White 2004132 | MSK | Sham acupuncture | 0.548 (0.129) | 0.679 (0.202) | VAS pain score | 3.971 (1.073) | 1.18 (1.039) | |
| Acupuncture | 0.572 (0.133) | 0.653 (0.228) | 3.64 (0.906) | 0.871 (0.943) | |||||
| 22 | Foster 2007135 | OAK | Usual care | 0.543 (0.214) | 0.566 (0.279) | WOMAC pain score | 2.463 (1.004) | 1.626 (0.999) | |
| Sham acupuncture | 0.531 (0.205) | 0.621 (0.234) | 2.428 (0.903) | 1.418 (1.029) | |||||
| Acupuncture | 0.576 (0.213) | 0.559 (0.266) | 2.52 (1.092) | 1.513 (0.969) | |||||
| 25 | Vas 2004120 | OAK | Sham acupuncture | WOMAC total | 0.37 (0.381) | 0.565 (0.352) | WOMAC total score | 3.308 (1.068) | 1.589 (1.037) |
| Acupuncture | 0.345 (0.366) | 0.76 (0.233) | 3.273 (0.937) | 0.333 (0.452) | |||||
| 26 | Williamson 2007136 | OAK | Usual care | 0.424 (0.304) | 0.487 (0.314) | Oxford Knee Score | 4.886 (1.039) | 5.104 (1.02) | |
| Acupuncture | 0.405 (0.278) | 0.508 (0.262) | 4.841 (0.922) | 4.774 (0.861) | |||||
| 14 | Kleinhenz 1999137 | MSK | Sham acupuncture | CMS and VAS pain | 0.558 (0.127) | 0.577 (0.071) | CMS | –3.986 (1.037) | –3.462 (0.965) |
| Acupuncture | 0.566 (0.101) | 0.578 (0.109) | –4.483 (0.909) | –4.417 (0.795) | |||||
| 15 | Salter 2006133 | MSK | Usual care | EQ-5D available – no mapping necessary | 0.546 (0.322) | 0.67 (0.254) | Northwick Park pain score | 2.395 (1.156) | 1.518 (0.961) |
| Acupuncture | 0.614 (0.268) | 0.734 (0.269) | 2.138 (0.73) | 1.342 (1.1) | |||||
| 16 | Thomas 200661 | MSK | Usual care | 0.532 (0.284) | 0.655 (0.274) | SF-36 bodily pain score | –1.815 (1.075) | –2.316 (1.064) | |
| Acupuncture | 0.534 (0.293) | 0.753 (0.19) | –1.839 (0.964) | –2.545 (0.962) | |||||
| 23 | Berman 2004134 | OAK | Usual care | 0.65 (0.196) | 0.628 (0.24) | WOMAC pain score | 2.577 (1.059) | 1.851 (1.098) | |
| Sham acupuncture | 0.615 (0.246) | 0.693 (0.169) | 2.547 (0.969) | 1.587 (0.981) | |||||
| Acupuncture | 0.608 (0.232) | 0.682 (0.184) | 2.552 (0.979) | 1.345 (0.892) | |||||
WinBUGS code (models 1 and 2)
The WinBUGS modelling code is provided in the following section followed by a summary table of all variables included in the data sets and the R-code describing the specification of initial values for two chains.
WinBUGS model code
Description of data sets and variables
| Data set | Variable | Description |
|---|---|---|
| Data set descriptors, constants | n.subjects | Number of subjects in data set |
| n.arms | Number of arms in studies in data set | |
| n.trials | Number of trials in data set | |
| NT | Number of treatments in data set | |
| Data at individual patient level | study | Study code |
| treat | Treatment code | |
| baseline | Baseline (lowest index) treatment in study | |
| y | EQ-5D or standardised pain at follow-up | |
| bl_y | EQ-5D or standardised pain at baseline | |
| index | Sequential numbering of study arms in data set | |
| Data at study arm level | pain1 | Dummy variable indicating whether or not study was conducted in patients with headache pain |
| pain2 | Dummy variable indicating whether or not study was conducted in patients with musculoskeletal pain | |
| treat1 | Treatment code | |
| baseline1 | Baseline (lowest index) treatment in study | |
| m | Code for each study arm indicating whether it is the first, second, third, fourth or fifth arm in the study | |
| Data at study level | start | Number of the first line of IPD data set including study data |
| end | Number of the last line of IPD data set including study data |
R-code used to generate initial values (two sets)
Appendix 5 Review data related to Chapter 5
Data requirements
Studies were included in the data set if they provided post-baseline mean outcomes for all dimensions of any of the following HRQoL instruments: EQ-5D, SF-36 (individual dimensions or PCS and MCS scores), SF-12 (individual dimensions or PCS or MCS scores), WOMAC, pain VAS and pain NRS. Although a wide range of additional HRQoL data was reported, mapping algorithms were not available to translate these data to EQ-5D preference scores. [Note: for the Nottingham Health Profile (NHP) an algorithm was available but this was estimated from a small sample of patients in a very different disease area (hepatitis C). Data from the NHP were not therefore included in the analysis. One study reported Assessment of Quality of Life preference scores but as these were reported to only one decimal place these data were not included.]
Absolute values and change from baseline values were included. When reported, baseline data were also extracted. When change from baseline data were reported studies were included only if baseline data were also reported, to enable calculation of absolute scores at follow-up time points. SEs were extracted directly or derived from reported SDs or CIs. Studies for which no measure of variance was reported were retained within the analysis and the missing SDs were imputed (see Chapter 5, Health-related quality of life for cost-effectiveness analysis). Methods for deriving final value variances from baseline value and change score variances are not currently available. In cases in which change from baseline data were reported the variance at follow-up was therefore assumed to be equal to the variance at baseline.
The WOMAC instrument can be administered in a variety of ways resulting in a range of possible scoring ranges. Mapping from the WOMAC score to the EQ-5D, however, requires the WOMAC score range to be 0–96. Reporting of the WOMAC score range in the data was often ambiguous;183 only data in which the scoring range was sufficiently clear to allow the data to be transformed onto the 0–96 range were therefore included in the data set. Where all subcomponents of the WOMAC (pain, stiffness and physical function) were reported, they were aggregated to produce a total WOMAC score. Calculation of the variance for the resulting total WOMAC score required the covariance of the subcomponents to be accounted for. The covariance for each study was therefore imputed using correlations between subcomponents derived from the IPD studies.
Study characteristics for the analysis data set
| Study | Intervention | n analysed | Quality-of-life instrument | Analysis time point (weeks) | IPD available | Adequate allocation concealment | Adequate allocation concealment 3–13 weeks |
|---|---|---|---|---|---|---|---|
| Adedoyin 2002301 | Interferential therapy | 15 | Pain VAS | 4 | No | No | No |
| Adedoyin 2002301 | Placebo | 15 | Pain VAS | 4 | No | No | No |
| Aglamiş 2009189 | Exercise – aerobic | 16 | SF-36 dimensions | 6 | No | No | No |
| Aglamiş 2009189 | Usual care | 9 | SF-36 dimensions | 6 | No | No | No |
| Alcidi 2007350 | Heat treatment | 20 | Pain VAS | 0.71 | No | No | No |
| Alcidi 2007350 | TENS | 20 | Pain VAS | 0.71 | No | No | No |
| An 2008351 | Exercise – muscle strengthening | 11 | WOMAC | 8 | No | No | No |
| An 2008351 | Usual care | 10 | WOMAC | 8 | No | No | No |
| Arazpour 2013463 | Braces | 12 | Pain VAS | 6 | No | No | No |
| Arazpour 2013463 | Insoles | 12 | Pain VAS | 6 | No | No | No |
| Baker 2001353 | Exercise – muscle strengthening | 19 | SF-36 dimensions | 16 | No | Yes | No |
| Baker 2001353 | Placebo | 19 | SF-36 dimensions | 16 | No | Yes | No |
| Bálint 2007355 | Balneotherapy | 27 | WOMAC | 4 | No | Yes | Yes |
| Bálint 2007355 | Placebo | 25 | WOMAC | 4 | No | Yes | Yes |
| Bennell 2011464 | Insoles | 90 | WOMAC | 52 | No | Yes | No |
| Bennell 2011464 | Usual care | 89 | WOMAC | 52 | No | Yes | No |
| Berman 1999357 | Acupuncture | 36 | WOMAC | 8 | No | Yes | Yes |
| Berman 1999357 | Usual care | 37 | WOMAC | 8 | No | Yes | Yes |
| Berman 2004134 | Acupuncture | 154 | EQ-5D | 8 | Yes | Yes | Yes |
| Berman 2004134 | Sham acupuncture | 145 | EQ-5D | 8 | Yes | Yes | Yes |
| Brismée 2007360 | Usual care | 19 | WOMAC | 9 | No | No | No |
| Brismée 2007360 | T’ai chi | 22 | WOMAC | 9 | No | No | No |
| Brosseau 2012466 | Exercise – aerobic | 44 | SF-36 dimensions | 52.18 | No | No | No |
| Brosseau 2012466 | Exercise – aerobic | 44 | SF-36 dimensions | 52.18 | No | No | No |
| Brosseau 2012466 | Usual care | 41 | SF-36 dimensions | 52.18 | No | No | No |
| Bruce-Brand 2012467 | Exercise – muscle strengthening | 10 | SF-36 MCS and PCS | 8 | No | No | No |
| Bruce-Brand 2012467 | NMES | 10 | SF-36 MCS and PCS | 8 | No | No | No |
| Bruce-Brand 2012467 | Usual care | 6 | SF-36 MCS and PCS | 8 | No | No | No |
| Burch 2008303 | Interferential therapy | 52 | WOMAC | 8 | No | Yes | Yes |
| Burch 2008303 | TENS | 53 | WOMAC | 8 | No | Yes | Yes |
| Callaghan 2005363 | Placebo | 9 | Pain VAS | 2 | No | No | No |
| Callaghan 2005363 | PES | 9 | Pain VAS | 2 | No | No | No |
| Callaghan 2005363 | PES | 9 | Pain VAS | 2 | No | No | No |
| Cheing 2003191 | Placebo | 8 | Pain VAS | 1.43 | No | No | No |
| Cheing 2003191 | TENS | 10 | Pain VAS | 1.43 | No | No | No |
| Cheing 2003191 | TENS | 10 | Pain VAS | 1.43 | No | No | No |
| Cheing 2003191 | TENS | 10 | Pain VAS | 1.43 | No | No | No |
| Durmuş 2007370 | Exercise – muscle strengthening | 25 | Pain VAS | 4 | No | No | No |
| Durmuş 2007370 | PES | 25 | Pain VAS | 4 | No | No | No |
| Fary 2011468 | Placebo | 36 | SF-36 MCS and PCS | 4 | No | Yes | Yes |
| Fary 2011468 | PES | 34 | SF-36 MCS and PCS | 4 | No | Yes | Yes |
| Fischer 2005373 | Placebo | 35 | Pain VAS | 6 | No | No | No |
| Fischer 2005373 | PEMFs | 34 | Pain VAS | 6 | No | No | No |
| Flusser 2002374 | Balneotherapy | 40 | Pain VAS | 3 | No | No | No |
| Flusser 2002374 | Placebo | 18 | Pain VAS | 3 | No | No | No |
| Foroughi 2011469 | Exercise – muscle strengthening | 20 | WOMAC | 26.09 | No | No | No |
| Foroughi 2011469 | Placebo | 25 | WOMAC | 26.09 | No | No | No |
| Fukuda 2011470 | Placebo | 21 | Pain NRS | 3 | No | Yes | Yes |
| Fukuda 2011470 | PES | 30 | Pain NRS | 3 | No | Yes | Yes |
| Fukuda 2011470 | PES | 29 | Pain NRS | 3 | No | Yes | Yes |
| Fukuda 2011470 | Usual care | 32 | Pain NRS | 3 | No | Yes | Yes |
| Fukuda 2011471 | Laser/light therapy | 25 | Pain VAS | 6 | No | Yes | Yes |
| Fukuda 2011471 | Placebo | 22 | Pain VAS | 6 | No | Yes | Yes |
| Garland 2007377 | Placebo | 19 | WOMAC | 13.04 | No | Yes | Yes |
| Garland 2007377 | PES | 39 | WOMAC | 13.04 | No | Yes | Yes |
| Grimmer 1992378 | Placebo | 20 | Pain VAS | 0.14 | No | Yes | No |
| Grimmer 1992378 | TENS | 20 | Pain VAS | 0.14 | No | Yes | No |
| Grimmer 1992378 | TENS | 20 | Pain VAS | 0.14 | No | Yes | No |
| Gundog 2012302 | Interferential therapy | 15 | WOMAC | 4.35 | No | No | No |
| Gundog 2012302 | Interferential therapy | 15 | WOMAC | 4.35 | No | No | No |
| Gundog 2012302 | Interferential therapy | 15 | WOMAC | 4.35 | No | No | No |
| Gundog 2012302 | Placebo | 15 | WOMAC | 4.35 | No | No | No |
| Hasegawa 2010381 | Exercise – muscle strengthening | 14 | Pain NRS | 12 | No | No | No |
| Hasegawa 2010381 | Usual care | 14 | Pain NRS | 12 | No | No | No |
| Huang 2005384 | Exercise – muscle strengthening | 25 | Pain VAS | 8 | No | Yes | Yes |
| Huang 2005384 | Usual care | 28 | Pain VAS | 8 | No | Yes | Yes |
| Imoto 2012474 | Exercise – muscle strengthening | 38 | SF-36 dimensions | 8 | No | Yes | Yes |
| Imoto 2012474 | Usual care | 43 | SF-36 dimensions | 8 | No | Yes | Yes |
| Itoh 2008386 | Acupuncture | 6 | WOMAC | 5 | No | No | No |
| Itoh 2008386 | Usual care | 6 | WOMAC | 5 | No | No | No |
| Itoh 2008386 | TENS | 6 | WOMAC | 5 | No | No | No |
| Itoh 2008387 | Acupuncture | 9 | WOMAC | 5 | No | No | No |
| Itoh 2008387 | Acupuncture | 8 | WOMAC | 5 | No | No | No |
| Itoh 2008387 | Sham acupuncture | 7 | WOMAC | 5 | No | No | No |
| Jubb 2008391 | Acupuncture | 31 | WOMAC | 5 | No | No | No |
| Jubb 2008391 | Sham acupuncture | 31 | WOMAC | 5 | No | No | No |
| Kang 2007393 | Placebo | 28 | Pain VAS | 0.29 | No | No | No |
| Kang 2007393 | TENS | 35 | Pain VAS | 0.29 | No | No | No |
| Kovar 1992398 | Exercise – aerobic | 47 | Pain VAS | 8 | No | No | No |
| Kovar 1992398 | Usual care | 45 | Pain VAS | 8 | No | No | No |
| Kuptniratsaikul 2002399 | Exercise – muscle strengthening | 173 | Pain VAS | 8 | No | No | No |
| Kuptniratsaikul 2002399 | Usual care | 193 | Pain VAS | 8 | No | No | No |
| Lansdown 2009126 | Acupuncture | 15 | EQ-5D | 13.04 | No | Yes | Yes |
| Lansdown 2009126 | Usual care | 15 | EQ-5D | 13.04 | No | Yes | Yes |
| Lee 2009401 | Usual care | 15 | SF-36 MCS and PCS | 8 | No | No | No |
| Lee 2009401 | T’ai chi | 29 | SF-36 MCS and PCS | 8 | No | No | No |
| Lewis 1994403 | Placebo | 28 | Pain VAS | 3 | No | No | No |
| Lewis 1994403 | TENS | 28 | Pain VAS | 3 | No | No | No |
| Lim 2010475 | Exercise – muscle strengthening | 22 | SF-36 MCS and PCS | 8 | No | Yes | Yes |
| Lim 2010475 | Exercise – muscle strengthening | 24 | SF-36 MCS and PCS | 8 | No | Yes | Yes |
| Lim 2010475 | Usual care | 20 | SF-36 MCS and PCS | 8 | No | Yes | Yes |
| Lu 2010192 | Acupuncture | 10 | Pain VAS | 0.14 | No | No | No |
| Lu 2010192 | Sham acupuncture | 10 | Pain VAS | 0.14 | No | No | No |
| Lund 2008407 | Exercise – aerobic | 25 | Pain VAS | 8 | No | Yes | Yes |
| Lund 2008407 | Exercise – muscle strengthening | 27 | Pain VAS | 8 | No | Yes | Yes |
| Lund 2008407 | Usual care | 27 | Pain VAS | 8 | No | Yes | Yes |
| Maillefert 2001409 | Insoles | 82 | WOMAC | 4.35 | No | No | No |
| Maillefert 2001409 | Placebo | 74 | WOMAC | 4.35 | No | No | No |
| Maurer 1999410 | Exercise – muscle strengthening | 49 | WOMAC | 8 | No | No | No |
| Maurer 1999410 | Usual care | 49 | WOMAC | 8 | No | No | No |
| Mavrommatis 2012476 | Acupuncture | 39 | SF-36 MCS and PCS | 8 | No | No | No |
| Mavrommatis 2012476 | Sham acupuncture | 40 | SF-36 MCS and PCS | 8 | No | No | No |
| Mavrommatis 2012476 | Usual care | 38 | SF-36 MCS and PCS | 8 | No | No | No |
| Mazzuca 2004411 | Heat treatment | 26 | WOMAC | 4 | No | Yes | Yes |
| Mazzuca 2004411 | Placebo | 25 | WOMAC | 4 | No | Yes | Yes |
| McCarthy 2004412 | Exercise – muscle strengthening | 80 | EQ-5D | 4 | No | Yes | Yes |
| McCarthy 2004412 | Usual care | 71 | EQ-5D | 4 | No | Yes | Yes |
| Messier 2004413 | Exercise – aerobic | 69 | SF-36 MCS and PCS | 39.13 | No | Yes | No |
| Messier 2004413 | Usual care | 68 | SF-36 MCS and PCS | 39.13 | No | Yes | No |
| Miranda-Filloy 2005415 | Placebo | 10 | WOMAC | 8 | No | No | No |
| Miranda-Filloy 2005415 | PES | 10 | WOMAC | 8 | No | No | No |
| Ng 2003418 | Acupuncture | 8 | Pain NRS | 4 | No | No | No |
| Ng 2003418 | TENS | 8 | Pain NRS | 4 | No | No | No |
| Nguyen 1997419 | Balneotherapy | 91 | Pain VAS | 4 | No | No | No |
| Nguyen 1997419 | Usual care | 97 | Pain VAS | 4 | No | No | No |
| Nigg 2006421 | Insoles | 57 | WOMAC | 12 | No | No | No |
| Nigg 2006421 | Usual care | 66 | WOMAC | 12 | No | No | No |
| Perlman 2006424 | Manual therapy | 34 | WOMAC | 8 | No | No | No |
| Perlman 2006424 | Usual care | 34 | WOMAC | 8 | No | No | No |
| Perlman 2012478 | Manual therapy | 22 | WOMAC | 8 | No | Yes | Yes |
| Perlman 2012478 | Manual therapy | 24 | WOMAC | 8 | No | Yes | Yes |
| Perlman 2012478 | Manual therapy | 24 | WOMAC | 8 | No | Yes | Yes |
| Perlman 2012478 | Manual therapy | 25 | WOMAC | 8 | No | Yes | Yes |
| Perlman 2012478 | Usual care | 24 | WOMAC | 8 | No | Yes | Yes |
| Perrot 1998425 | Placebo | 19 | Pain VAS | 1.29 | No | No | No |
| Perrot 1998425 | PEMFs | 21 | Pain VAS | 1.29 | No | No | No |
| Pietrosimone 2009427 | Ice/cooling treatment | 11 | Pain VAS | 0.14 | No | Yes | No |
| Pietrosimone 2009427 | Usual care | 12 | Pain VAS | 0.14 | No | Yes | No |
| Pietrosimone 2009427 | TENS | 10 | Pain VAS | 0.14 | No | Yes | No |
| Pipitone 2001428 | Placebo | 35 | WOMAC | 6 | No | Yes | Yes |
| Pipitone 2001428 | PEMFs | 34 | WOMAC | 6 | No | Yes | Yes |
| Pollard 2008429 | Manual therapy | 26 | Pain VAS | 2 | No | Yes | No |
| Pollard 2008429 | Placebo | 17 | Pain VAS | 2 | No | Yes | No |
| Rapp 2009430 | Exercise – muscle strengthening | 9 | Pain VAS | 8 | No | No | No |
| Rapp 2009430 | Manual therapy | 15 | Pain VAS | 8 | No | No | No |
| Rapp 2009430 | Usual care | 15 | Pain VAS | 8 | No | No | No |
| Rattanachaiyanont 2008431 | Heat treatment | 50 | WOMAC | 3 | No | Yes | Yes |
| Rattanachaiyanont 2008431 | Placebo | 54 | WOMAC | 3 | No | Yes | Yes |
| Salacinski 2012479 | Exercise – aerobic | 13 | WOMAC | 12 | No | Yes | Yes |
| Salacinski 2012479 | Usual care | 15 | WOMAC | 12 | No | Yes | Yes |
| Salli 2010480 | Exercise – muscle strengthening | 24 | SF-36 MCS and PCS | 8 | No | No | No |
| Salli 2010480 | Exercise – muscle strengthening | 23 | SF-36 MCS and PCS | 8 | No | No | No |
| Salli 2010480 | Usual care | 24 | SF-36 MCS and PCS | 8 | No | No | No |
| Sangdee 2002434 | Acupuncture | 46 | WOMAC | 4 | No | No | No |
| Sangdee 2002434 | Acupuncture | 46 | WOMAC | 4 | No | No | No |
| Sangdee 2002434 | Placebo | 45 | WOMAC | 4 | No | No | No |
| Sangdee 2002434 | Usual care | 49 | WOMAC | 4 | No | No | No |
| Selfe 2008436 | Placebo | 19 | WOMAC | 8 | No | Yes | Yes |
| Selfe 2008436 | TENS | 18 | WOMAC | 8 | No | Yes | Yes |
| Shen 2009437 | Laser/light therapy | 18 | WOMAC | 4 | No | Yes | Yes |
| Shen 2009437 | Placebo | 9 | WOMAC | 4 | No | Yes | Yes |
| Sherman 2009438 | Balneotherapy | 24 | WOMAC | 7 | No | No | No |
| Sherman 2009438 | Placebo | 20 | WOMAC | 7 | No | No | No |
| Stelian 1992440 | Laser/light therapy | 15 | Pain VAS | 1.43 | No | No | No |
| Stelian 1992440 | Laser/light therapy | 18 | Pain VAS | 1.43 | No | No | No |
| Stelian 1992440 | Placebo | 17 | Pain VAS | 1.43 | No | No | No |
| Suarez-Almazor 2010125 | Acupuncture | 153 | SF-12 MCS and PCS | 6 | No | Yes | Yes |
| Suarez-Almazor 2010125 | Sham acupuncture | 302 | SF-12 MCS and PCS | 6 | No | Yes | Yes |
| Sukenik 1999441 | Balneotherapy | 10 | Pain VAS | 2 | No | No | No |
| Sukenik 1999441 | Balneotherapy | 10 | Pain VAS | 2 | No | No | No |
| Sukenik 1999441 | Balneotherapy | 7 | Pain VAS | 2 | No | No | No |
| Sukenik 1999441 | Placebo | 9 | Pain VAS | 2 | No | No | No |
| Tascioglu 2004444 | Laser/light therapy | 20 | WOMAC | 3 | No | No | No |
| Tascioglu 2004444 | Laser/light therapy | 20 | WOMAC | 3 | No | No | No |
| Tascioglu 2004444 | Placebo | 20 | WOMAC | 3 | No | No | No |
| Thamsborg 2005446 | Placebo | 41 | WOMAC | 6 | No | No | No |
| Thamsborg 2005446 | PEMFs | 42 | WOMAC | 6 | No | No | No |
| Thorstensson 2005447 | Exercise – aerobic | 28 | SF-36 MCS and PCS | 6 | No | No | No |
| Thorstensson 2005447 | Usual care | 28 | SF-36 MCS and PCS | 6 | No | No | No |
| Tishler 2004448 | Balneotherapy | 44 | Pain VAS | 6 | No | No | No |
| Tishler 2004448 | Usual care | 24 | Pain VAS | 6 | No | No | No |
| Toda 2008449 | Insoles | 43 | Pain VAS | 12 | No | Yes | Yes |
| Toda 2008449 | Insoles | 41 | Pain VAS | 12 | No | Yes | Yes |
| Toda 2008449 | Placebo | 38 | Pain VAS | 12 | No | Yes | Yes |
| Topp 2002450 | Exercise – muscle strengthening | 35 | WOMAC | 16 | No | No | No |
| Topp 2002450 | Exercise – muscle strengthening | 32 | WOMAC | 16 | No | No | No |
| Topp 2002450 | Usual care | 35 | WOMAC | 16 | No | No | No |
| Trock 1994452 | Placebo | 44 | Pain VAS | 5 | No | Yes | Yes |
| Trock 1994452 | PEMFs | 40 | Pain VAS | 5 | No | Yes | Yes |
| Tucker 2003453 | Manual therapy | 30 | Pain VAS | 3 | No | No | No |
| Tucker 2003453 | Usual care | 30 | Pain VAS | 3 | No | No | No |
| Tukmachi 2004454 | Acupuncture | 9 | Pain VAS | 5 | No | Yes | Yes |
| Tukmachi 2004454 | Acupuncture | 10 | Pain VAS | 5 | No | Yes | Yes |
| Tukmachi 2004454 | Usual care | 10 | Pain VAS | 5 | No | Yes | Yes |
| Vance 2012481 | Placebo | 25 | Pain VAS | 0.14 | No | Yes | No |
| Vance 2012481 | TENS | 25 | Pain VAS | 0.14 | No | Yes | No |
| Vance 2012481 | TENS | 25 | Pain VAS | 0.14 | No | Yes | No |
| Vas 2004120 | Acupuncture | 47 | WOMAC | 12 | Yes | Yes | Yes |
| Vas 2004120 | Sham acupuncture | 43 | WOMAC | 12 | Yes | Yes | Yes |
| Wang 2009455 | Usual care | 20 | SF-36 MCS and PCS | 12 | No | Yes | Yes |
| Wang 2009455 | T’ai chi | 20 | SF-36 MCS and PCS | 12 | No | Yes | Yes |
| Wigler 1995456 | Balneotherapy | 11 | Pain VAS | 4 | No | No | No |
| Wigler 1995456 | Balneotherapy | 10 | Pain VAS | 4 | No | No | No |
| Wigler 1995456 | Placebo | 12 | Pain VAS | 4 | No | No | No |
| Williamson 2007136 | Acupuncture | 59 | WOMAC | 8 | Yes | Yes | Yes |
| Williamson 2007136 | Exercise – muscle strengthening | 53 | WOMAC | 8 | Yes | Yes | Yes |
| Williamson 2007136 | Usual care | 49 | WOMAC | 8 | Yes | Yes | Yes |
| Witt 200569 | Acupuncture | 145 | SF-36 dimensions | 8 | Yes | Yes | Yes |
| Witt 200569 | Sham acupuncture | 73 | SF-36 dimensions | 8 | Yes | Yes | Yes |
| Witt 200569 | Usual care | 67 | SF-36 dimensions | 8 | Yes | Yes | Yes |
| Witt 200677 | Acupuncture | 250 | SF-36 dimensions | 12 | Yes | Yes | Yes |
| Witt 200677 | Usual care | 244 | SF-36 dimensions | 12 | Yes | Yes | Yes |
| Wolsko 2004457 | Placebo | 13 | WOMAC | 6 | No | Yes | Yes |
| Wolsko 2004457 | Static magnets | 13 | WOMAC | 6 | No | Yes | Yes |
| Yip 2007193 | Usual care | 70 | Pain VAS | 1 | No | No | No |
| Yip 2007193 | T’ai chi | 79 | Pain VAS | 1 | No | No | No |
| Yurtkuran 2006461 | Balneotherapy | 27 | WOMAC | 2 | No | Yes | No |
| Yurtkuran 2006461 | Placebo | 28 | WOMAC | 2 | No | Yes | No |
| Zizic 1995462 | Placebo | 33 | Pain VAS | 4 | No | No | No |
| Zizic 1995462 | PES | 38 | Pain VAS | 4 | No | No | No |
WinBUGS code
The WinBUGS modelling code is provided in the following section followed by a summary table of all variables included in the data sets and the R-code describing the specification of initial values for two chains.
WinBUGS model code
Description of data sets and variables
| Data set | Variable | Description |
|---|---|---|
| Data set descriptors, constants | n.subjects.ipd | Number of subjects in IPD |
| n.arms.ipd | Number of arms in IPD trials | |
| n.trials.ipd | Number of IPD trials | |
| n.trials.ad | Number of AD trials | |
| n.arms.ad | Number of arms in AD trials | |
| n.treatments | Total number of treatments | |
| alpha | Alpha and beta parameters for gamma distribution, used to impute missing SDs | |
| beta | ||
| mod1 | Dummy variable indicating whether or not to include age covariate | |
| mod2 | Dummy variable indicating whether or not to include BMI covariate | |
| Data at individual patient level (IPD studies) | study.ipd | Study code |
| treat.ipd | Treatment code | |
| baseline.ipd | Baseline (lowest index) treatment in study | |
| y.ipd | EQ-5D at follow-up | |
| basey.ipd | Baseline EQ-5D | |
| age.ipd | Centred baseline age | |
| bmi.ipd | Centred baseline BMI | |
| index.ipd | Sequential numbering of study arms in IPD set | |
| Data at study arm level (IPD studies) | study.ipd.sum | Study code |
| treat.ipd.sum | Treatment code | |
| baseline.ipd.sum | Baseline (lowest index) treatment in study | |
| start.ipd.sum | Number of the first line of data including study arm | |
| end.ipd.sum | Number of the last line of data including study arm | |
| m.ipd.sum | Code for each study arm indicating whether it is the first, second, third, fourth or fifth arm in the study. Labelled as m = 2, 2, 3, 4, 5, respectively | |
| Data at study arm level (AD studies) | study.ad | Study code |
| treat.ad | Treatment code | |
| baseline.ad | Baseline (lowest index) treatment in study | |
| y.ad | Mean EQ-5D at follow-up | |
| sd.y.ad | SD for EQ-5D at follow-up (set to zero if missing) | |
| n.ad | Number of patients | |
| imb.ad | Difference in baseline EQ-5D from baseline treatment (imbalance) | |
| m.ad | Code for each study arm indicating whether it is the first, second, third, fourth or fifth arm in the study. Labelled as m = 2, 2, 3, 4, 5, respectively | |
| Data at study level (AD studies) | age.ad | Centred baseline age |
| bmi.ad | Centred baseline BMI |
R-code used to generate initial values
Mapped EQ-5D values
| Study | Intervention | Quality-of-life instrument | EQ-5D score, mean (SE) | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | Change in | |||
| Adedoyin 2002301 | Interferential therapy | Pain VAS | 0.48 (N/A) | 0.69 (N/A) | 0.22 (N/A) |
| Adedoyin 2002301 | Placebo | Pain VAS | 0.49 (N/A) | 0.63 (N/A) | 0.13 (N/A) |
| Aglamiş 2009189 | Usual care | SF-36 dimensions | 0.41 (0.08) | 0.50 (0.08) | 0.08 (0.07) |
| Aglamiş 2009189 | Exercise – aerobic | SF-36 dimensions | 0.68 (0.05) | 0.87 (0.03) | 0.19 (0.04) |
| Alcidi 2007350 | Heat treatment | Pain VAS | 0.53 (0.04) | 0.59 (0.06) | 0.06 (0.05) |
| Alcidi 2007350 | TENS | Pain VAS | 0.56 (0.05) | 0.60 (0.05) | 0.03 (0.05) |
| An 2008351 | Usual care | WOMAC | 0.70 (0.03) | 0.62 (0.08) | –0.08 (0.07) |
| An 2008351 | Exercise – muscle strengthening | WOMAC | 0.66 (0.06) | 0.68 (0.06) | 0.02 (0.06) |
| Arazpour 2013463 | Insoles | Pain VAS | 0.52 (0.02) | 0.61 (0.02) | 0.09 (0.02) |
| Arazpour 2013463 | Braces | Pain VAS | 0.54 (0.02) | 0.61 (0.02) | 0.07 (0.02) |
| Baker 2001353 | Placebo | SF-36 dimensions | 0.76 (0.04) | 0.75 (0.05) | –0.02 (0.03) |
| Baker 2001353 | Exercise – muscle strengthening | SF-36 dimensions | 0.69 (0.05) | 0.78 (0.04) | 0.09 (0.04) |
| Bálint 2007355 | Balneotherapy | WOMAC | 0.56 (0.01) | 0.60 (0.01) | 0.04 (0.01) |
| Bálint 2007355 | Placebo | WOMAC | 0.56 (0.01) | 0.65 (0.01) | 0.10 (0.01) |
| Bennell 2011464 | Usual care | WOMAC | 0.61 (0.02) | 0.64 (0.02) | 0.03 (0.02) |
| Bennell 2011464 | Insoles | WOMAC | 0.60 (0.02) | 0.63 (0.02) | 0.03 (0.02) |
| Berman 1999357 | Acupuncture | WOMAC | 0.47 (0.05) | 0.64 (0.03) | 0.17 (0.05) |
| Berman 1999357 | Usual care | WOMAC | 0.46 (0.04) | 0.46 (0.05) | 0.00 (0.04) |
| Berman 2004134 | Acupuncture | EQ-5D | 0.61 (0.02) | 0.68 (0.01) | 0.07 (0.02) |
| Berman 2004134 | Sham acupuncture | EQ-5D | 0.62 (0.02) | 0.69 (0.01) | 0.07 (0.02) |
| Brismée 2007360 | T’ai chi | WOMAC | 0.34 (0.08) | 0.48 (0.08) | 0.14 (0.08) |
| Brismée 2007360 | Usual care | WOMAC | 0.41 (0.07) | 0.38 (0.07) | –0.02 (0.07) |
| Brosseau 2012466 | Usual care | SF-36 dimensions | 0.81 (0.02) | 0.82 (0.02) | 0.02 (0.02) |
| Brosseau 2012466 | Exercise – aerobic | SF-36 dimensions | 0.82 (0.02) | 0.81 (0.02) | –0.01 (0.02) |
| Brosseau 2012466 | Exercise – aerobic | SF-36 dimensions | 0.79 (0.02) | 0.81 (0.02) | 0.03 (0.02) |
| Bruce-Brand 2012467 | Usual care | SF-36 MCS and PCS | N/A (N/A) | 0.42 (0.26) | 0.42 (0.26) |
| Bruce-Brand 2012467 | Exercise – muscle strengthening | SF-36 MCS and PCS | N/A (N/A) | 0.46 (0.17) | 0.46 (0.17) |
| Bruce-Brand 2012467 | NMES | SF-36 MCS and PCS | N/A (N/A) | 0.44 (0.15) | 0.44 (0.15) |
| Burch 2008303 | TENS | WOMAC | 0.48 (0.04) | 0.57 (0.04) | 0.09 (0.04) |
| Burch 2008303 | Interferential therapy | WOMAC | 0.49 (0.04) | 0.64 (0.02) | 0.15 (0.03) |
| Callaghan 2005363 | PES | Pain VAS | 0.54 (0.08) | 0.56 (0.09) | 0.03 (0.08) |
| Callaghan 2005363 | PES | Pain VAS | 0.57 (0.11) | 0.58 (0.12) | 0.01 (0.11) |
| Callaghan 2005363 | Placebo | Pain VAS | 0.55 (0.02) | 0.54 (0.07) | –0.01 (0.06) |
| Cheing 2003191 | TENS | Pain VAS | 0.59 (0.04) | 0.67 (0.04) | 0.08 (0.04) |
| Cheing 2003191 | TENS | Pain VAS | 0.57 (0.03) | 0.71 (0.02) | 0.14 (0.03) |
| Cheing 2003191 | TENS | Pain VAS | 0.56 (0.04) | 0.68 (0.03) | 0.12 (0.03) |
| Cheing 2003191 | Placebo | Pain VAS | 0.58 (0.03) | 0.59 (0.03) | 0.01 (0.03) |
| Durmuş 2007370 | PES | Pain VAS | 0.57 (N/A) | 0.68 (N/A) | 0.10 (N/A) |
| Durmuş 2007370 | Exercise – muscle strengthening | Pain VAS | 0.55 (N/A) | 0.67 (N/A) | 0.12 (N/A) |
| Fary 2011468 | Placebo | SF-36 MCS and PCS | 0.58 (0.04) | 0.64 (N/A) | 0.06 (0.04) |
| Fary 2011468 | PES | SF-36 MCS and PCS | 0.59 (0.04) | 0.66 (N/A) | 0.07 (0.04) |
| Fischer 2005373 | PEMFs | Pain VAS | 0.56 (0.02) | 0.58 (0.03) | 0.02 (0.03) |
| Fischer 2005373 | Placebo | Pain VAS | 0.55 (0.02) | 0.62 (0.03) | 0.07 (0.03) |
| Flusser 2002374 | Balneotherapy | Pain VAS | 0.53 (0.03) | 0.55 (0.03) | 0.02 (0.03) |
| Flusser 2002374 | Placebo | Pain VAS | 0.54 (0.05) | 0.53 (0.05) | –0.02 (0.04) |
| Foroughi 2011469 | Exercise – muscle strengthening | WOMAC | 0.65 (0.04) | 0.70 (0.02) | 0.05 (0.03) |
| Foroughi 2011469 | Placebo | WOMAC | 0.62 (0.04) | 0.66 (0.03) | 0.04 (0.04) |
| Fukuda 2011470 | Usual care | Pain NRS | 0.58 (0.04) | 0.60 (0.04) | 0.02 (0.04) |
| Fukuda 2011470 | Placebo | Pain NRS | 0.49 (0.04) | 0.54 (0.05) | 0.05 (0.04) |
| Fukuda 2011470 | PES | Pain NRS | 0.53 (0.05) | 0.68 (0.03) | 0.15 (0.04) |
| Fukuda 2011470 | PES | Pain NRS | 0.55 (0.05) | 0.64 (0.04) | 0.10 (0.04) |
| Fukuda 2011471 | Placebo | Pain VAS | 0.54 (0.02) | 0.57 (0.04) | 0.03 (0.04) |
| Fukuda 2011471 | Laser/light therapy | Pain VAS | 0.54 (0.04) | 0.59 (0.02) | 0.05 (0.03) |
| Garland 2007377 | PES | WOMAC | 0.46 (0.05) | 0.54 (0.06) | 0.08 (0.05) |
| Garland 2007377 | Placebo | WOMAC | 0.53 (0.05) | 0.52 (0.07) | –0.01 (0.06) |
| Grimmer 1992378 | TENS | Pain VAS | 0.52 (0.06) | 0.65 (0.05) | 0.13 (0.05) |
| Grimmer 1992378 | TENS | Pain VAS | 0.55 (0.05) | 0.68 (0.03) | 0.13 (0.04) |
| Grimmer 1992378 | Placebo | Pain VAS | 0.54 (0.05) | 0.62 (0.06) | 0.08 (0.05) |
| Gundog 2012302 | Interferential therapy | WOMAC | 0.31 (0.06) | 0.73 (0.01) | 0.42 (0.05) |
| Gundog 2012302 | Interferential therapy | WOMAC | 0.24 (0.08) | 0.74 (0.01) | 0.50 (0.07) |
| Gundog 2012302 | Interferential therapy | WOMAC | 0.22 (0.06) | 0.71 (0.03) | 0.48 (0.05) |
| Gundog 2012302 | Placebo | WOMAC | 0.27 (0.06) | 0.41 (0.05) | 0.14 (0.05) |
| Hasegawa 2010381 | Exercise – muscle strengthening | Pain NRS | 0.64 (0.05) | 0.72 (0.05) | 0.08 (0.05) |
| Hasegawa 2010381 | Usual care | Pain NRS | 0.68 (0.05) | 0.67 (0.06) | –0.01 (0.05) |
| Huang 2005384 | Exercise – muscle strengthening | Pain VAS | 0.58 (0.03) | 0.61 (0.01) | 0.04 (0.02) |
| Huang 2005384 | Usual care | Pain VAS | 0.58 (0.03) | 0.60 (0.03) | 0.02 (0.03) |
| Imoto 2012474 | Usual care | SF-36 dimensions | 0.52 (0.04) | 0.60 (0.04) | 0.09 (0.03) |
| Imoto 2012474 | Exercise – muscle strengthening | SF-36 dimensions | 0.52 (0.03) | 0.66 (0.04) | 0.14 (0.03) |
| Itoh 2008386 | Acupuncture | WOMAC | 0.45 (0.08) | 0.59 (0.05) | 0.14 (0.06) |
| Itoh 2008386 | TENS | WOMAC | 0.44 (0.13) | 0.60 (0.04) | 0.16 (0.11) |
| Itoh 2008386 | Usual care | WOMAC | 0.49 (0.06) | 0.52 (0.06) | 0.03 (0.06) |
| Itoh 2008387 | Acupuncture | WOMAC | 0.39 (0.08) | 0.54 (0.03) | 0.16 (0.07) |
| Itoh 2008387 | Acupuncture | WOMAC | 0.46 (0.08) | 0.63 (0.05) | 0.17 (0.06) |
| Itoh 2008387 | Sham acupuncture | WOMAC | 0.45 (0.06) | 0.48 (0.05) | 0.03 (0.05) |
| Jubb 2008391 | Sham acupuncture | WOMAC | 0.41 (0.07) | 0.48 (0.06) | 0.07 (0.06) |
| Jubb 2008391 | Acupuncture | WOMAC | 0.37 (0.06) | 0.54 (0.05) | 0.17 (0.05) |
| Kang 2007393 | TENS | Pain VAS | 0.56 (N/A) | 0.59 (N/A) | 0.02 (N/A) |
| Kang 2007393 | Placebo | Pain VAS | 0.55 (N/A) | 0.57 (N/A) | 0.01 (N/A) |
| Kovar 1992398 | Exercise – aerobic | Pain VAS | 0.57 (0.03) | 0.61 (0.02) | 0.04 (0.02) |
| Kovar 1992398 | Usual care | Pain VAS | 0.58 (0.03) | 0.58 (0.03) | 0.00 (0.03) |
| Kuptniratsaikul 2002399 | Exercise – muscle strengthening | Pain VAS | 0.56 (0.01) | 0.60 (0.02) | 0.04 (0.01) |
| Kuptniratsaikul 2002399 | Usual care | Pain VAS | 0.55 (0.01) | 0.57 (0.02) | 0.02 (0.01) |
| Lansdown 2009126 | Usual care | EQ-5D | 0.67 (0.04) | 0.66 (0.06) | –0.01 (0.06) |
| Lansdown 2009126 | Acupuncture | EQ-5D | 0.61 (0.06) | 0.71 (0.07) | 0.10 (0.08) |
| Lee 2009401 | Usual care | SF-36 MCS and PCS | 0.58 (0.10) | 0.56 (0.12) | –0.02 (0.06) |
| Lee 2009401 | T’ai chi | SF-36 MCS and PCS | 0.51 (0.09) | 0.49 (0.09) | –0.01 (0.10) |
| Lewis 1994403 | TENS | Pain VAS | N/A (N/A) | 0.58 (N/A) | 0.58 (N/A) |
| Lewis 1994403 | Placebo | Pain VAS | N/A (N/A) | 0.56 (N/A) | 0.56 (N/A) |
| Lim 2010475 | Usual care | SF-36 MCS and PCS | 0.51 (0.07) | 0.54 (0.08) | 0.04 (0.02) |
| Lim 2010475 | Exercise – muscle strengthening | SF-36 MCS and PCS | 0.57 (0.04) | 0.65 (0.03) | 0.08 (0.02) |
| Lim 2010475 | Exercise – muscle strengthening | SF-36 MCS and PCS | 0.53 (0.06) | 0.63 (0.03) | 0.10 (0.03) |
| Lu 2010192 | Acupuncture | Pain VAS | 0.56 (0.03) | 0.60 (0.03) | 0.03 (0.03) |
| Lu 2010192 | Sham acupuncture | Pain VAS | 0.57 (0.04) | 0.58 (0.04) | 0.02 (0.04) |
| Lund 2008407 | Usual care | Pain VAS | 0.58 (0.05) | 0.55 (0.04) | –0.03 (0.04) |
| Lund 2008407 | Exercise – muscle strengthening | Pain VAS | 0.54 (0.03) | 0.56 (0.04) | 0.01 (0.03) |
| Lund 2008407 | Exercise – aerobic | Pain VAS | 0.57 (0.06) | 0.57 (0.04) | 0.00 (0.05) |
| Maillefert 2001409 | Insoles | WOMAC | 0.48 (0.04) | 0.45 (0.04) | –0.02 (0.04) |
| Maillefert 2001409 | Placebo | WOMAC | 0.47 (0.04) | 0.49 (0.04) | 0.01 (0.04) |
| Maurer 1999410 | Usual care | WOMAC | 0.60 (N/A) | 0.64 (N/A) | 0.04 (N/A) |
| Maurer 1999410 | Exercise – muscle strengthening | WOMAC | 0.62 (N/A) | 0.67 (N/A) | 0.05 (N/A) |
| Mavrommatis 2012476 | Usual care | SF-36 MCS and PCS | 0.46 (0.04) | 0.60 (0.02) | 0.14 (0.02) |
| Mavrommatis 2012476 | Sham acupuncture | SF-36 MCS and PCS | 0.43 (0.05) | 0.61 (0.02) | 0.18 (0.03) |
| Mavrommatis 2012476 | Acupuncture | SF-36 MCS and PCS | 0.48 (0.03) | 0.72 (0.02) | 0.23 (0.02) |
| Mazzuca 2004411 | Heat treatment | WOMAC | 0.46 (0.07) | 0.52 (0.06) | 0.06 (0.06) |
| Mazzuca 2004411 | Placebo | WOMAC | 0.47 (0.07) | 0.53 (0.05) | 0.07 (0.06) |
| McCarthy 2004412 | Usual care | EQ-5D | 0.50 (N/A) | 0.52 (N/A) | 0.02 (N/A) |
| McCarthy 2004412 | Exercise – muscle strengthening | EQ-5D | 0.54 (N/A) | 0.60 (N/A) | 0.06 (N/A) |
| Messier 2004413 | Usual care | SF-36 MCS and PCS | 0.55 (0.03) | 0.61 (0.02) | 0.06 (0.01) |
| Messier 2004413 | Exercise – aerobic | SF-36 MCS and PCS | 0.58 (0.02) | 0.63 (0.02) | 0.06 (0.01) |
| Miranda-Filloy 2005415 | PES | WOMAC | 0.51 (N/A) | 0.69 (N/A) | 0.18 (N/A) |
| Miranda-Filloy 2005415 | Placebo | WOMAC | 0.46 (N/A) | 0.55 (N/A) | 0.09 (N/A) |
| Ng 2003418 | Acupuncture | Pain NRS | 0.65 (0.05) | 0.70 (0.04) | 0.05 (0.05) |
| Ng 2003418 | TENS | Pain NRS | 0.67 (0.04) | 0.69 (0.04) | 0.02 (0.04) |
| Nguyen 1997419 | Usual care | Pain VAS | 0.58 (0.02) | 0.58 (0.02) | 0.00 (0.02) |
| Nguyen 1997419 | Balneotherapy | Pain VAS | 0.57 (0.02) | 0.62 (0.02) | 0.05 (0.02) |
| Nigg 2006421 | Insoles | WOMAC | 0.61 (0.03) | 0.66 (0.02) | 0.05 (0.03) |
| Nigg 2006421 | Usual care | WOMAC | 0.60 (0.03) | 0.65 (0.03) | 0.06 (0.03) |
| Perlman 2006424 | Manual therapy | WOMAC | 0.49 (0.06) | 0.65 (0.04) | 0.16 (0.05) |
| Perlman 2006424 | Usual care | WOMAC | 0.42 (0.06) | 0.47 (0.06) | 0.05 (0.06) |
| Perlman 2012478 | Manual therapy | WOMAC | 0.44 (0.08) | 0.60 (0.05) | 0.16 (0.07) |
| Perlman 2012478 | Manual therapy | WOMAC | 0.47 (0.07) | 0.62 (0.05) | 0.15 (0.06) |
| Perlman 2012478 | Manual therapy | WOMAC | 0.44 (0.07) | 0.64 (0.04) | 0.20 (0.06) |
| Perlman 2012478 | Manual therapy | WOMAC | 0.49 (0.07) | 0.66 (0.04) | 0.17 (0.06) |
| Perlman 2012478 | Usual care | WOMAC | 0.45 (0.06) | 0.52 (0.05) | 0.06 (0.05) |
| Perrot 1998425 | PEMFs | Pain VAS | 0.52 (N/A) | 0.60 (N/A) | 0.08 (N/A) |
| Perrot 1998425 | Placebo | Pain VAS | 0.50 (N/A) | 0.54 (N/A) | 0.05 (N/A) |
| Pietrosimone 2009427 | TENS | Pain VAS | 0.68 (0.04) | 0.70 (0.02) | 0.03 (0.04) |
| Pietrosimone 2009427 | Ice/cooling treatment | Pain VAS | 0.67 (0.04) | 0.69 (0.04) | 0.02 (0.04) |
| Pietrosimone 2009427 | Usual care | Pain VAS | 0.67 (0.04) | 0.67 (0.05) | 0.00 (0.04) |
| Pipitone 2001428 | Placebo | WOMAC | 0.44 (0.05) | 0.46 (0.05) | 0.01 (0.05) |
| Pipitone 2001428 | PEMFs | WOMAC | 0.44 (0.07) | 0.49 (0.06) | 0.05 (0.06) |
| Pollard 2008429 | Manual therapy | Pain VAS | 0.63 (0.04) | 0.67 (0.03) | 0.04 (0.03) |
| Pollard 2008429 | Placebo | Pain VAS | 0.62 (0.06) | 0.63 (0.05) | 0.01 (0.05) |
| Rapp 2009430 | Exercise – muscle strengthening | Pain VAS | N/A (N/A) | 0.67 (0.05) | 0.67 (0.05) |
| Rapp 2009430 | Manual therapy | Pain VAS | N/A (N/A) | 0.66 (0.04) | 0.66 (0.04) |
| Rapp 2009430 | Usual care | Pain VAS | N/A (N/A) | 0.64 (0.05) | 0.64 (0.05) |
| Rattanachaiyanont 2008431 | Heat treatment | WOMAC | 0.60 (0.03) | 0.66 (0.02) | 0.07 (0.02) |
| Rattanachaiyanont 2008431 | Placebo | WOMAC | 0.58 (0.03) | 0.66 (0.02) | 0.07 (0.03) |
| Salacinski 2012479 | Usual care | WOMAC | 0.66 (0.04) | 0.64 (0.05) | –0.02 (0.04) |
| Salacinski 2012479 | Exercise – aerobic | WOMAC | 0.65 (0.04) | 0.71 (0.02) | 0.06 (0.04) |
| Salli 2010480 | Usual care | SF-36 MCS and PCS | 0.54 (0.07) | 0.59 (0.05) | 0.04 (0.03) |
| Salli 2010480 | Exercise – muscle strengthening | SF-36 MCS and PCS | 0.55 (0.08) | 0.62 (0.05) | 0.07 (0.08) |
| Salli 2010480 | Exercise – muscle strengthening | SF-36 MCS and PCS | 0.53 (0.09) | 0.60 (0.08) | 0.07 (0.11) |
| Sangdee 2002434 | Acupuncture | WOMAC | 0.42 (0.06) | 0.65 (0.03) | 0.23 (0.05) |
| Sangdee 2002434 | Acupuncture | WOMAC | 0.42 (0.06) | 0.65 (0.03) | 0.23 (0.05) |
| Sangdee 2002434 | Usual care | WOMAC | 0.44 (0.05) | 0.63 (0.03) | 0.19 (0.04) |
| Sangdee 2002434 | Placebo | WOMAC | 0.44 (0.05) | 0.60 (0.03) | 0.16 (0.04) |
| Selfe 2008436 | Placebo | WOMAC | 0.45 (0.08) | 0.63 (0.05) | 0.18 (0.07) |
| Selfe 2008436 | TENS | WOMAC | 0.44 (0.10) | 0.64 (0.05) | 0.21 (0.08) |
| Shen 2009437 | Laser/light therapy | WOMAC | 0.58 (0.06) | 0.69 (0.03) | 0.11 (0.06) |
| Shen 2009437 | Placebo | WOMAC | 0.66 (0.03) | 0.73 (0.01) | 0.07 (0.04) |
| Sherman 2009438 | Placebo | WOMAC | 0.40 (0.09) | 0.50 (0.08) | 0.10 (0.08) |
| Sherman 2009438 | Balneotherapy | WOMAC | 0.36 (0.08) | 0.49 (0.08) | 0.13 (0.08) |
| Stelian 1992440 | Laser/light therapy | Pain VAS | 0.53 (0.06) | 0.62 (0.06) | 0.09 (0.05) |
| Stelian 1992440 | Laser/light therapy | Pain VAS | 0.51 (0.05) | 0.63 (0.05) | 0.11 (0.05) |
| Stelian 1992440 | Placebo | Pain VAS | 0.54 (0.05) | 0.54 (0.05) | 0.00 (0.05) |
| Suarez-Almazor 2010125 | Sham acupuncture | SF-12 MCS and PCS | 0.61 (0.02) | 0.69 (0.02) | 0.08 (0.02) |
| Suarez-Almazor 2010125 | Acupuncture | SF-12 MCS and PCS | 0.61 (0.02) | 0.71 (0.02) | 0.10 (0.02) |
| Sukenik 1999441 | Balneotherapy | Pain VAS | 0.54 (0.05) | 0.55 (0.04) | 0.01 (0.04) |
| Sukenik 1999441 | Balneotherapy | Pain VAS | 0.53 (0.03) | 0.56 (0.05) | 0.03 (0.04) |
| Sukenik 1999441 | Balneotherapy | Pain VAS | 0.52 (0.03) | 0.61 (0.06) | 0.09 (0.05) |
| Sukenik 1999441 | Placebo | Pain VAS | 0.54 (0.05) | 0.54 (0.04) | 0.00 (0.04) |
| Tascioglu 2004444 | Laser/light therapy | WOMAC | 0.46 (0.05) | 0.48 (0.05) | 0.02 (0.04) |
| Tascioglu 2004444 | Laser/light therapy | WOMAC | 0.42 (0.05) | 0.45 (0.05) | 0.03 (0.05) |
| Tascioglu 2004444 | Placebo | WOMAC | 0.43 (0.06) | 0.46 (0.05) | 0.03 (0.05) |
| Thamsborg 2005446 | PEMFs | WOMAC | 0.46 (0.04) | 0.54 (0.03) | 0.08 (0.04) |
| Thamsborg 2005446 | Placebo | WOMAC | 0.42 (0.05) | 0.48 (0.05) | 0.05 (0.05) |
| Thorstensson 2005447 | Usual care | SF-36 MCS and PCS | 0.72 (N/A) | 0.73 (N/A) | 0.01 (N/A) |
| Thorstensson 2005447 | Exercise – aerobic | SF-36 MCS and PCS | 0.71 (N/A) | 0.74 (N/A) | 0.02 (N/A) |
| Tishler 2004448 | Balneotherapy | Pain VAS | 0.53 (0.03) | 0.66 (0.03) | 0.13 (0.03) |
| Tishler 2004448 | Usual care | Pain VAS | 0.52 (0.04) | 0.53 (0.04) | 0.01 (0.04) |
| Toda 2008449 | Placebo | Pain VAS | 0.59 (0.03) | 0.58 (0.02) | –0.01 (0.03) |
| Toda 2008449 | Insoles | Pain VAS | 0.61 (0.03) | 0.60 (0.03) | –0.01 (0.03) |
| Toda 2008449 | Insoles | Pain VAS | 0.59 (0.03) | 0.60 (0.04) | 0.01 (0.03) |
| Topp 2002450 | Exercise – muscle strengthening | WOMAC | 0.35 (0.06) | 0.40 (0.09) | 0.05 (0.08) |
| Topp 2002450 | Exercise – muscle strengthening | WOMAC | 0.40 (0.06) | 0.45 (0.05) | 0.04 (0.05) |
| Topp 2002450 | Usual care | WOMAC | 0.41 (0.06) | 0.39 (0.06) | –0.01 (0.05) |
| Trock 1994452 | Placebo | Pain VAS | 0.53 (0.03) | 0.57 (0.03) | 0.04 (0.03) |
| Trock 1994452 | PEMFs | Pain VAS | 0.51 (0.03) | 0.59 (0.03) | 0.08 (0.03) |
| Tucker 2003453 | Manual therapy | Pain VAS | 0.62 (0.03) | 0.69 (0.02) | 0.07 (0.02) |
| Tucker 2003453 | Usual care | Pain VAS | 0.63 (0.04) | 0.69 (0.02) | 0.06 (0.03) |
| Tukmachi 2004454 | Acupuncture | Pain VAS | 0.54 (0.06) | 0.63 (0.08) | 0.08 (0.07) |
| Tukmachi 2004454 | Acupuncture | Pain VAS | 0.54 (0.05) | 0.67 (0.06) | 0.12 (0.05) |
| Tukmachi 2004454 | Usual care | Pain VAS | 0.51 (0.04) | 0.52 (0.06) | 0.01 (0.05) |
| Vance 2012481 | TENS | Pain VAS | 0.65 (0.04) | 0.68 (0.04) | 0.02 (0.04) |
| Vance 2012481 | TENS | Pain VAS | 0.64 (0.04) | 0.66 (0.04) | 0.02 (0.04) |
| Vance 2012481 | Placebo | Pain VAS | 0.64 (0.04) | 0.68 (0.03) | 0.04 (0.04) |
| Vas 2004120 | Acupuncture | WOMAC | 0.40 (0.06) | 0.75 (0.04) | 0.35 (0.08) |
| Vas 2004120 | Sham acupuncture | WOMAC | 0.35 (0.06) | 0.56 (0.06) | 0.21 (0.07) |
| Wang 2009455 | Usual care | SF-36 MCS and PCS | 0.50 (0.06) | 0.56 (0.05) | 0.06 (0.02) |
| Wang 2009455 | T’ai chi | SF-36 MCS and PCS | 0.58 (0.05) | 0.71 (0.04) | 0.13 (0.03) |
| Wigler 1995456 | Balneotherapy | Pain VAS | 0.51 (N/A) | 0.55 (N/A) | 0.04 (N/A) |
| Wigler 1995456 | Balneotherapy | Pain VAS | 0.51 (N/A) | 0.56 (N/A) | 0.05 (N/A) |
| Wigler 1995456 | Placebo | Pain VAS | 0.51 (N/A) | 0.54 (N/A) | 0.03 (N/A) |
| Williamson 2007136 | Acupuncture | WOMAC | 0.44 (0.04) | 0.43 (0.04) | –0.01 (0.05) |
| Williamson 2007136 | Exercise – muscle strengthening | WOMAC | 0.43 (0.05) | 0.46 (0.05) | 0.03 (0.06) |
| Williamson 2007136 | Usual care | WOMAC | 0.43 (0.04) | 0.36 (0.04) | –0.06 (0.05) |
| Witt 200569 | Acupuncture | SF-36 dimensions | 0.57 (0.02) | 0.70 (0.02) | 0.14 (0.02) |
| Witt 200569 | Sham acupuncture | SF-36 dimensions | 0.55 (0.02) | 0.62 (0.03) | 0.07 (0.02) |
| Witt 200569 | Usual care | SF-36 dimensions | 0.56 (0.03) | 0.57 (0.02) | 0.01 (0.02) |
| Witt 200677 | Acupuncture | SF-36 dimensions | 0.57 (0.01) | 0.67 (0.02) | 0.10 (0.02) |
| Witt 200677 | Usual care | SF-36 dimensions | 0.57 (0.01) | 0.56 (0.02) | –0.01 (0.01) |
| Wolsko 2004457 | Static magnets | WOMAC | 0.47 (0.12) | 0.64 (0.07) | 0.17 (0.10) |
| Wolsko 2004457 | Placebo | WOMAC | 0.48 (N/A) | 0.66 (N/A) | 0.18 (N/A) |
| Yip 2007193 | Usual care | Pain VAS | 0.59 (0.02) | 0.59 (0.03) | 0.00 (0.03) |
| Yip 2007193 | T’ai chi | Pain VAS | 0.57 (0.02) | 0.61 (0.02) | 0.04 (0.02) |
| Yurtkuran 2006461 | Placebo | WOMAC | 0.44 (0.04) | 0.58 (0.04) | 0.14 (0.04) |
| Yurtkuran 2006461 | Balneotherapy | WOMAC | 0.47 (0.06) | 0.64 (0.03) | 0.17 (0.05) |
| Zizic 1995462 | PES | Pain VAS | 0.53 (N/A) | 0.59 (N/A) | 0.06 (N/A) |
| Zizic 1995462 | Placebo | Pain VAS | 0.54 (N/A) | 0.58 (N/A) | 0.04 (N/A) |
Results of sensitivity analysis using upper and lower values for intervention costs and private costs for acupuncture sessions
(See Chapter 5, Cost-effectiveness analysis for values used.)
All trials
| Treatment | Cost-effective intervention at threshold of £20,000 per QALY (ICER, £ per QALY) | |
|---|---|---|
| Use of minimum cost | Use of maximum cost | |
| Standard care | TENS (2691) | TENS (2695) |
| Acupuncture | Acupuncture (19,033) | TENS (2694) |
| Braces | TENS (2693) | TENS (2691) |
| Heat treatment | TENS (2694) | TENS (2691) |
| Insoles | TENS (2690) | TENS (2694) |
| Interferential therapy | Interferential therapy (7626) | TENS (2687) |
| Laser light therapy | TENS (2690) | TENS (2694) |
| Manual therapy | TENS (2695) | TENS (2692) |
| NMES | TENS (2689) | TENS (2691) |
| PES | TENS (2690) | TENS (2693) |
| PEMFs | TENS (2688) | TENS (2695) |
| Static magnets | TENS (2696) | TENS (2689) |
| TENS | TENS (1426) | TENS (3959) |
Trials with a low risk of bias for allocation concealment
| Treatment | Cost-effective intervention at threshold of £20,000 per QALY (ICER, £ per QALY) | |
|---|---|---|
| Use of minimum cost | Use of maximum cost | |
| Usual care | Acupuncture (13,501) | Acupuncture (13,500) |
| Acupuncture | Acupuncture (5842) | Acupuncture (17,690) |
| Heat treatment | Acupuncture (13,499) | Acupuncture (13,500) |
| Insoles | Acupuncture (13,501) | Acupuncture (13,501) |
| Interferential therapy | Acupuncture (13,502) | Acupuncture (13,500) |
| Laser light therapy | Acupuncture (13,503) | Acupuncture (13,499) |
| Manual therapy | Acupuncture (13,500) | Acupuncture (13,503) |
| PES | Acupuncture (13,503) | Acupuncture (13,502) |
| PEMFs | Acupuncture (13,498) | Acupuncture (13,500) |
| Static magnets | Acupuncture (13,499) | Acupuncture (13,501) |
| TENS | Acupuncture (14,701) | Acupuncture (12,297) |
Trials with a low risk of bias for allocation concealment reporting at 3–13 weeks
| Treatment | Cost-effective intervention at threshold of £20,000 per QALY (ICER, £ per QALY) | |
|---|---|---|
| Use of minimum cost | Use of maximum cost | |
| Usual care | Acupuncture (14,271) | Acupuncture (14,272) |
| Acupuncture | Acupuncture (6518) | Acupuncture (18,697) |
| Heat treatment | Acupuncture (14,278) | Acupuncture (14,275) |
| Insoles | Acupuncture (14,271) | Acupuncture (14,272) |
| Interferential therapy | Acupuncture (14,274) | Acupuncture (14,275) |
| Laser light therapy | Acupuncture (14,273) | Acupuncture (14,280) |
| Manual therapy | Manual therapy (9500) | Acupuncture (14,276) |
| PES | Acupuncture (14,272) | Acupuncture (14,276) |
| PEMFs | Acupuncture (14,276) | Acupuncture (14,276) |
| Static magnets | Acupuncture (14,273) | Acupuncture (14,269) |
| TENS | Acupuncture (15,544) | Acupuncture (13,704) |
When the private cost was used for acupuncture sessions the conclusions of the analysis of all trials were unaffected. The results of the analysis restricted to trials with adequate allocation concealment were affected and TENS became the cost-effective comparator (ICER £6142 per QALY vs. usual care). The results of the analysis restricted to trials with adequate allocation concealment reporting between 3 and 13 weeks were also affected and manual therapy became the cost-effective comparator (ICER £19,942 per QALY vs. TENS).
Results of sensitivity analysis using shorter treatment durations and a range of assumptions around efficacy
| Relationship between EQ-5D treatment effect and session duration | Cost-effective intervention at threshold of £20,000 per QALY (ICER, £ per QALY) | ||
|---|---|---|---|
| All trials | Trials with adequate allocation concealment | Trials with adequate allocation concealment, reporting at 3–13 weeks | |
| Scenario 1: linear relationship | TENS (2695) | Acupuncture (17,669) | Acupuncture (19,253) |
| Scenario 2: linear up to 60 minutes followed by plateau | TENS (2693) | Acupuncture (17,664) | Acupuncture (19,240) |
| Scenario 3: 75% benefit in 0–30 minutes, remaining benefit 30–60 minutes | Interferential therapy (18,159) | Acupuncture (13,516) | Acupuncture (14,423) |
| Scenario 4: all benefit achieved in first 20/30 minutes | Interferential therapy (4324) | Interferential therapy (8150) | Interferential therapy (8356) |
Results of sensitivity analysis using upper and lower credible intervals from network meta-analysis
Bold text indicates scenarios in which the optimal decision remains as per the base case (assuming a cost-effectiveness threshold of £20,000 per QALY). Black text indicates scenarios in which the optimal decision switches. The last column indicates the effect size required to alter the decision and the univariate posterior probability of observing a value of the treatment effect this extreme. This was calculated only when use of the upper or lower 95% CrI altered the decision.
All trials
| Intervention | Cost-effective treatment at threshold of £20,000 per QALY (ICER, £ per QALY) | Parameter value required to alter decision (probability) | |
|---|---|---|---|
| Lower 95% CrI | Upper 95% CrI | ||
| Acupuncture | TENS (2691) | Acupuncture (£19,154) | Effect > 0.12 (0.05) |
| Braces | TENS (2692) | Braces (2509) | Effect > 0.08 (0.08) |
| Heat treatment | TENS (2689) | TENS (2692) | – |
| Insoles | TENS (2693) | TENS (5515) | – |
| Interferential therapy | TENS (2689) | TENS (2687) | – |
| Laser/light therapy | TENS (2690) | TENS (2689) | – |
| Manual therapy | TENS (2689) | TENS (2692) | – |
| NMES | TENS (2693) | NMES (8043) | Effect > 0.22 (0.18) |
| PES | TENS (2686) | TENS (2694) | – |
| PEMFs | TENS (2692) | TENS (2697) | – |
| Static magnets | TENS (2694) | Static magnets (325) | Effect > 0.07 (0.25) |
| TENS | Acupuncture (15,070) | TENS (1740) | Effect < 0.05 (0.15) |
Trials with adequate allocation concealment
| Intervention | Cost-effective treatment at threshold of £20,000 per QALY (ICER, £ per QALY) | Parameter value required to alter decision (probability) | |
|---|---|---|---|
| Lower 95% CrI | Upper 95% CrI | ||
| Acupuncture | TENS (6143) | Acupuncture (9273) | Effect < 0.08 (0.05) |
| Heat treatment | Acupuncture (13,507) | Acupuncture (13,504) | – |
| Insoles | Acupuncture (13,500) | Insoles (1389) | Effect > 0.05 (0.09) |
| Interferential therapy | Acupuncture (13,500) | Acupuncture (13,500) | – |
| Laser/light therapy | Acupuncture (13,499) | Acupuncture (13,501) | – |
| Manual therapy | Acupuncture (13,501) | Manual therapy (11,583) | Effect > 0.14 (0.10) |
| PES | Acupuncture (13,504) | Acupuncture (13,500) | – |
| PEMFs | Acupuncture (13,502) | Acupuncture (13,501) | – |
| Static magnets | Acupuncture (13,500) | Static magnets (335) | Effect > 0.05 (0.29) |
| TENS | Acupuncture (11,687) | TENS (2002) | Effect > 0.06 (0.21) |
Trials with adequate allocation concealment reporting at 3–13 weeks
| Intervention | Cost-effective treatment at threshold of £20,000 per QALY (ICER, £ per QALY) | Parameter value required to alter decision (probability) | |
|---|---|---|---|
| Lower 95% CrI | Upper 95% CrI | ||
| Acupuncture | TENS (9784) | Acupuncture (9568) | Effect < 0.09 (0.14) |
| Heat treatment | Acupuncture (14,271) | Heat treatment (11,425) | Effect > 0.12 (0.05) |
| Insoles | Acupuncture (14,274) | Insoles (676) | Effect > 0.05 (0.35) |
| Interferential therapy | Acupuncture (14,272) | Acupuncture (14,274) | – |
| Laser/light therapy | Acupuncture (14,274) | Acupuncture (14,272) | – |
| Manual therapy | Acupuncture (14,271) | Manual therapy (8484) | Effect > 0.14 (0.35) |
| PES | Acupuncture (14,278) | Acupuncture (14,277) | – |
| PEMFs | Acupuncture (14,272) | Acupuncture (14,273) | – |
| Static magnets | Acupuncture (14,271) | Static magnets (315) | Effect > 0.05 (0.30) |
| TENS | Acupuncture (13,709) | TENS (1034) | Effect > 0.06 (0.40) |
Results of sensitivity analysis of extending the duration of treatment benefit by 50%
| Intervention | Cost-effective treatment at threshold of £20,000 per QALY (ICER, £ per QALY) | ||
|---|---|---|---|
| All trials | Trials with adequate allocation concealment | Trials with adequate allocation concealment, reporting at 3–13 weeks | |
| Standard care | TENS (2693) | Acupuncture (13,500) | Acupuncture (14,276) |
| Acupuncture | Acupuncture (14,732) | Acupuncture (8000) | Acupuncture (8373) |
| Braces | TENS (2691) | NA | NA |
| Heat treatment | TENS (2689) | Acupuncture (13,499) | Acupuncture (14,270) |
| Insoles | TENS (2691) | Acupuncture (13,501) | Acupuncture (16,061) |
| Interferential therapy | Interferential therapy (19,286) | Acupuncture (13,503) | Acupuncture (14,270) |
| Laser/light therapy | TENS (2689) | Acupuncture (13,502) | Acupuncture (14,277) |
| Manual therapy | TENS (2690) | Acupuncture (13,507) | Manual therapy (11,678) |
| NMES | TENS (2689) | NA | NA |
| PES | TENS (2694) | Acupuncture (13,499) | Acupuncture (14,270) |
| PEMFs | TENS (2692) | Acupuncture (13,494) | Acupuncture (14,280) |
| Static magnets | TENS (2697) | Acupuncture (13,505) | Acupuncture (14,269) |
| TENS | TENS (1849) | Acupuncture (16,857) | Acupuncture (18,794) |
Appendix 6 Trial data related to Chapter 6
Details of acupuncture treatment based on the Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) reporting guidelines,483 an official extension to the Consolidated Standards of Reporting Trials (CONSORT)
| STRICTA item | Details of acupuncture treatment within the trial (n = 266) |
|---|---|
| 1. (a) Style | Traditional Chinese medicine |
| 1. (b) Reasoning for treatment provided | Systematic Cochrane review312 and consensus methods315 were used to develop a treatment protocol in a pragmatic trial designed to evaluate acupuncture as provided in routine care |
| 1. (c) Variation | Individualised treatments using common theoretical frameworks of TCM as reported separately |
| 2. (a) Number of needles per treatment | On average, 13 needles were inserted per session (range 3–26) |
| 2. (b) Names | 246 different points were used. Common points were SP-6, LIV-3, ST-36 and LI-4, which were used within a course of treatment on 91%, 89%, 83% and 74% of patients, respectively |
| 2. (c) Depth of insertion | Range of minimum mean depths was 0.49 cm, maximum 1.41 cm. Total range 0.05–2.5 cm |
| 2. (d) Response sought | The response sought varied, most commonly deqi by 96% of acupuncturists |
| 2. (e) Needle stimulation | Manual methods: tonifying (68%), reducing (43%) and even method (55%) |
| 2. (f) Retention | Average 22.5 minutes (range 15–45 minutes) |
| 2. (g) Needle type | Needle length ranged from 5 mm to 40 mm and needle diameter ranged from 0.13 mm to 0.35 mm |
| 3. (a) Number of sessions | Patients were offered 12 sessions and completed an average of 10.3 sessions (median 12, range 1–12) |
| 3. (b) Frequency and duration | Average number of sessions per week: 0.74. Average number of weeks between sessions: 1.36. Average duration of session: 53 minutes (range 28–95 minutes) |
| 4. (a) Other components of treatment | Acupuncturists were allowed to use moxa, electroacupuncture, ear seeds, cupping, acupressure and heat lamps. Most commonly used were acupressure (used with 13% of patients), moxa (12%) and electroacupuncture (7%). Acupuncturists were allowed to provide acupuncture theory-based lifestyle advice. In total, 66% of patients received lifestyle advice, most commonly diet (42%), exercise (30%) and relaxation (25%) advice. Advice unrelated to acupuncture theory as well as herbs and magnets were proscribed |
| 4. (b) Setting and context | Provision or treatments in independent clinics. Acupuncturists encouraged to practice as closely as possible to their normal practice and 96% reported applying acupuncture exactly the same way or similarly as they did routinely |
| 5. Participating acupuncturists | BAcC members with > 3 years of post-qualification experience |
| 6. Control or comparator interventions | Patients continued to receive usual care as an adjunct from their GP, as well as over-the-counter treatments according to need, based on a need to evaluate the impact of acupuncture plus usual care compared with the impact of usual care alone |
Details of the competences that underpinned the delivery of humanistic counselling
The counselling was designed to be humanistic in approach and based on relevant competences. These competences were developed independently by Anthony D Roth, Andrew Hill and Stephen Pilling,316 as commissioned by Skills for Health, supported by an Expert Reference Group and now documented by the Centre for Outcomes Research and Effectiveness at University College London. 484 A manualised protocol was developed using an evidence-based methodology. These competences for the delivery of high-quality humanistic psychological therapies covered the following three domains:
-
Basic competences in humanistic psychological therapies. Humanistic counselling interventions are underpinned by a range of more specific humanistic techniques. These basic competences set out a range of activities that humanistically oriented therapists should be able to acknowledge as fundamental to their practice and that should be delivered to patients within the trial.
-
Metacompetences. These are the competences needed to implement higher-order links between theory and practice and will adapt the implementation of the humanistic approach to the needs of individual patients. These competences are more abstract than those in other domains; however, they will guide the procedures and practice used by counsellors within the trial.
-
Specific humanistic psychological therapies competences. These are technical interventions employed by some (although not all) forms of humanistic psychological therapies. These include, for example, competences related to approaches to working with emotions and emotional meaning.
Baseline characteristics for patients with and without missing data at 3 months
| Characteristic | Patients with | Total (n = 755) | |
|---|---|---|---|
| Available data at 3 months (n = 616) | Missing data at 3 months (n = 139) | ||
| Age (years) | |||
| Mean (SD) | 44.6 (13.01) | 38.2 (13.42) | 43.5 (13.37) |
| Median (min., max.) | 43.5 (18, 89) | 35.0 (18, 93) | 43 (18, 93) |
| Interquartile range | 35–54 | 27–46 | 33–53 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sex, n (%) | |||
| Male | 164 (26.6) | 37 (26.6) | 201 (26.6) |
| Female | 452 (73.4) | 102 (73.4) | 554 (73.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Depression, n (%) | |||
| In last 2 weeks | 457 (74.2) | 117 (84.2) | 574 (76.0) |
| Missing | 11 (1.8) | 1 (0.7) | 12 (1.6) |
| Not first major episode | 408 (89.4) | 105 (89.7) | 513 (89.4) |
| Missing | 8 (1.8) | 1 (0.9) | 9 (1.6) |
| Four or more previous episodes | 309 (75.7) | 80 (76.2) | 389 (75.8) |
| Missing | 4 (1.0) | 0 (0.0) | 4 (0.8) |
| Age at first major depressive episode (years) | |||
| Mean (SD) | 25.7 (12.5) | 22.9 (10.8) | 25.2 (12.28) |
| Median (min., max.) | 22 (0, 79) | 20 (3, 78) | 22 (0, 79) |
| Interquartile range | 16.5–32 | 15–30 | 16–31 |
| Missing, n (%) | 12 (1.9) | 4 (2.9) | 16 (2.1) |
| Medication, n (%) | |||
| Depression medication in last 3 months | 426 (69.2) | 93 (66.9) | 519 (68.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Analgesic medication in last 3 months | 301 (48.9) | 58 (41.7) | 359 (47.5) |
| Missing | 4 (0.7) | 2 (1.4) | 6 (0.8) |
| EQ-5D anxiety/depression, n (%) | |||
| Not anxious/depressed | 17 (2.8) | 4 (2.9) | 21 (2.8) |
| Moderately anxious/depressed | 465 (75.7) | 89 (64.0) | 554 (73.6) |
| Extremely anxious/depressed | 132 (21.5) | 46 (33.1) | 178 (23.6) |
| Missing | 2 (0.3) | 0 (0.0) | 2 (0.3) |
| PHQ-9 score | |||
| Mean (SD) | 15.7 (5.32) | 17.3 (4.94) | 16.0 (5.29) |
| Median (min., max.) | 16 (3, 27) | 17 (6, 27) | 16 (3, 27) |
| Interquartile range | 12–20 | 14–21 | 12–20 |
| Missing, n (%) | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| PHQ-9 group, n (%) | |||
| None (0–4) | 6 (1.0) | 0 (0.0) | 6 (0.8) |
| Mild (5–9) | 78 (12.7) | 9 (6.5) | 87 (11.5) |
| Moderate (10–14) | 182 (29.6) | 35 (25.2) | 217 (28.8) |
| Moderately severe (15–19) | 184 (29.9) | 47 (33.8) | 231 (30.6) |
| Severe (20–27) | 165 (26.8) | 48 (34.5) | 213 (28.2) |
| Missing | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| BDI-II score | |||
| Mean (SD) | 32.0 (8.62) | 34.5 (8.91) | 32.5 (8.72) |
| Median (min., max.) | 31 (20, 60) | 34 (20, 53) | 31 (20, 60) |
| Interquartile range | 25–37.5 | 28–41 | 26–38 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BDI-II group, n (%) | |||
| Moderate (20–28) | 248 (40.3) | 36 (25.9) | 284 (37.6) |
| Severe (29–63) | 368 (59.7) | 103 (74.1) | 471 (62.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| SF-36 bodily pain | |||
| Mean (SD) | 57.2 (28.54) | 59.6 (28.01) | 57.6 (28.44) |
| Median (min., max.) | 56.5 (0, 100) | 52 (0, 100) | 52 (0, 100) |
| Interquartile range | 31–82 | 41–84 | 32–84 |
| Missing, n (%) | 4 (0.6) | 0 (0.0) | 4 (0.5) |
| Treatment preference, n (%) | |||
| Acupuncture | 343 (55.7) | 87 (62.6) | 430 (57.0) |
| Counselling | 137 (22.2) | 27 (19.4) | 164 (21.7) |
| Usual care | 10 (1.6) | 0 (0.0) | 10 (1.3) |
| No preference | 122 (19.8) | 22 (15.8) | 144 (19.1) |
| Missing | 4 (0.7) | 3 (2.2) | 7 (0.9) |
Details of the usual care provided to patients in all three arms
(Except medication, which is set out in Table 38.)
Number of patients seeing a health professional and average number of visits (total and specifically for depression) for these patients in the preceding 3 months
| Health professional | Time point (months) | Intervention | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture + usual care | Counselling + usual care | Usual care | |||||||||||||||
| Patients | Mean number of visits | Patients | Mean number of visits | Patients | Mean number of visits | Patients | Mean number of visits | ||||||||||
| n | % | Total | Depressiona | n | % | Total | Depressiona | n | % | Total | Depressiona | n | % | Total | Depressiona | ||
| GP | 3b | 106 | 45.1 | – | 2.2 | 108 | 50.2 | – | 2.3 | 70 | 58.8 | – | 2.2 | 284 | 49.9 | – | 2.2 |
| 6 | 153 | 73.6 | 2.8 | 0.8 | 134 | 68.0 | 2.5 | 0.8 | 73 | 74.5 | 2.6 | 1.1 | 360 | 71.6 | 2.7 | 0.9 | |
| 9 | 135 | 67.2 | 2.2 | 0.7 | 121 | 66.5 | 2.2 | 0.8 | 81 | 75.7 | 2.3 | 0.9 | 337 | 68.8 | 2.3 | 0.8 | |
| 12 | 127 | 63.2 | 2.4 | 1.0 | 131 | 68.2 | 2.3 | 0.7 | 73 | 69.5 | 2.4 | 0.7 | 331 | 66.5 | 2.4 | 0.8 | |
| Practice nurse | 3b | 7 | 3.7 | – | 1.4 | 12 | 6.9 | – | 2.0 | 8 | 9.1 | – | 1.8 | 27 | 6.3 | – | 1.8 |
| 6 | 58 | 26.1 | 1.7 | 0.0 | 63 | 29.9 | 1.5 | 0.1 | 36 | 33.0 | 1.4 | 0.0 | 157 | 29.0 | 1.6 | 0.0 | |
| 9 | 55 | 25.0 | 1.7 | 0.1 | 50 | 25.1 | 1.9 | 0.0 | 28 | 25.5 | 1.8 | 0.3 | 133 | 25.1 | 1.8 | 0.1 | |
| 12 | 54 | 25.0 | 1.6 | 0.0 | 63 | 30.7 | 1.4 | 0.0 | 39 | 35.5 | 1.8 | 0.0 | 156 | 29.4 | 1.6 | 0.0 | |
| Outpatient clinic | 3b | 11 | 4.6 | – | 2.0 | 13 | 5.9 | – | 2.1 | 9 | 7.4 | – | 3.3 | 33 | 5.7 | – | 2.6 |
| 6 | 46 | 20.6 | 2.3 | 0.1 | 60 | 28.4 | 2.2 | 0.1 | 25 | 23.1 | 1.3 | 0.0 | 131 | 24.2 | 2.1 | 0.1 | |
| 9 | 50 | 22.4 | 1.8 | 0.2 | 44 | 22.3 | 2.0 | 0.2 | 27 | 25.2 | 1.5 | 0.1 | 121 | 23.0 | 1.8 | 0.2 | |
| 12 | 61 | 28.1 | 1.7 | 0.3 | 57 | 28.4 | 1.8 | 0.2 | 35 | 32.4 | 1.7 | 0.1 | 153 | 29.1 | 1.8 | 0.2 | |
| Mental health nurse | 3b | 5 | 2.2 | – | 1.6 | 1 | 0.5 | – | 5.0 | 4 | 3.5 | – | 4.5 | 10 | 1.8 | – | 3.1 |
| 6 | 4 | 1.8 | 4.0 | 2.3 | 1 | 0.5 | 8.0 | – | 2 | 1.8 | 3.5 | 3.5 | 7 | 1.3 | 4.4 | 2.8 | |
| 9 | 7 | 3.2 | 4.1 | 2.9 | 3 | 1.5 | 5.3 | 6.5 | 4 | 3.8 | 3.0 | 1.8 | 14 | 2.7 | 4.1 | 3.1 | |
| 12 | 11 | 5.1 | 3.5 | 2.3 | 4 | 2.1 | 2.3 | 1.8 | 3 | 2.9 | 4.0 | 2.3 | 18 | 3.5 | 3.3 | 2.2 | |
| Psychologist or psychiatrist | 3b | 4 | 1.8 | – | 1.8 | 5 | 2.4 | – | 6.2 | 6 | 5.4 | – | 2.7 | 15 | 2.7 | – | 3.6 |
| 6 | 10 | 4.5 | 1.7 | 1.2 | 9 | 4.5 | 4.7 | 1.7 | 7 | 6.5 | 2.9 | 1.6 | 26 | 4.9 | 3.0 | 1.5 | |
| 9 | 7 | 3.3 | 2.3 | 2.1 | 7 | 3.6 | 1.9 | 1.3 | 4 | 3.8 | 5.5 | 5.3 | 18 | 3.5 | 2.8 | 2.5 | |
| 12 | 11 | 5.1 | 3.5 | 3.5 | 7 | 3.7 | 2.9 | 2.0 | 5 | 4.8 | 2.2 | 1.4 | 23 | 4.5 | 3.0 | 2.6 | |
| NHS counsellor | 3b | 5 | 2.2 | – | 3.0 | 4 | 1.9 | – | 3.0 | 5 | 4.4 | – | 2.4 | 14 | 2.5 | – | 2.8 |
| 6 | 9 | 4.2 | 2.7 | 1.0 | 5 | 2.5 | 3.2 | 3.8 | 5 | 4.8 | 1.4 | 0.5 | 19 | 3.6 | 2.5 | 1.6 | |
| 9 | 5 | 2.3 | 3.0 | 1.8 | 2 | 1.0 | 3.5 | 3.5 | 4 | 3.9 | 3.8 | 3.0 | 11 | 2.1 | 3.4 | 2.6 | |
| 12 | 10 | 4.7 | 3.8 | 3.1 | 3 | 1.5 | 3.3 | 4.0 | 5 | 5.0 | 2.2 | 2.2 | 18 | 3.5 | 3.3 | 2.9 | |
| Other NHS health professional | 3b | 16 | 8.9 | – | 3.9 | 10 | 6.1 | – | 2.5 | 15 | 19.0 | – | 3.1 | 41 | 9.7 | – | 3.2 |
| 6 | 20 | 9.1 | 2.3 | 0.3 | 26 | 12.6 | 2.7 | 0.0 | 14 | 13.3 | 2.1 | 0.0 | 60 | 11.3 | 2.4 | 0.1 | |
| 9 | 24 | 11.2 | 2.8 | 0.6 | 26 | 13.3 | 2.5 | 0.3 | 16 | 14.5 | 2.2 | 0.5 | 66 | 12.7 | 2.5 | 0.4 | |
| 12 | 25 | 11.5 | 2.3 | 0.5 | 32 | 16.2 | 2.7 | 0.0 | 20 | 18.7 | 3.1 | 0.2 | 77 | 14.8 | 2.7 | 0.2 | |
Number of patients attending accident and emergency departments and number of attendances in the preceding 3 months
| Time point (months) | Intervention | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture + usual care | Counselling + usual care | Usual care | ||||||||||||||
| Patients | Number of attendances | Patients | Number of attendances | Patients | Number of attendances | Patients | Number of attendances | |||||||||
| n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | |
| 3 | 1 | 0.4 | 3.0 | – | 3 | 1.3 | 1.7 | 1.15 | 3 | 2.4 | 1.3 | 0.58 | 7 | 1.2 | 1.7 | 0.95 |
| 6 | 17 | 7.5 | 1.1 | 0.33 | 20 | 8.8 | 1.8 | 2.51 | 11 | 9.7 | 1.1 | 0.30 | 48 | 8.5 | 1.4 | 1.65 |
| 9 | 11 | 4.8 | 1.3 | 0.47 | 13 | 6.3 | 1.4 | 0.96 | 12 | 10.7 | 1.5 | 1.00 | 36 | 6.6 | 1.4 | 0.84 |
| 12 | 21 | 9.2 | 1.2 | 0.40 | 11 | 5.3 | 1.3 | 0.47 | 12 | 10.5 | 1.1 | 0.29 | 44 | 8.0 | 1.2 | 0.39 |
Number of patients admitted to hospital and number of overnight stays in the preceding 3 months
| Time point (months) | Intervention | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture + usual care | Counselling + usual care | Usual care | ||||||||||||||
| Patients | Number of overnight stays | Patients | Number of overnight stays | Patients | Number of overnight stays | Patients | Number of overnight stays | |||||||||
| n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | |
| Hospital ward | ||||||||||||||||
| 3 | 2 | 0.8 | 3.5 | 0.71 | 0 | 0.0 | – | – | 0 | 0.0 | – | – | 2 | 0.3 | 3.5 | 0.71 |
| 6 | 6 | 2.8 | 2.0 | 0.89 | 10 | 4.8 | 5.6 | 6.59 | 2 | 1.8 | 6.0 | 1.41 | 18 | 3.4 | 4.4 | 5.15 |
| 9 | 4 | 1.9 | 3.8 | 3.20 | 4 | 2.0 | 6.8 | 4.79 | 7 | 6.1 | 3.1 | 3.76 | 15 | 2.9 | 4.3 | 3.95 |
| 12 | 6 | 2.7 | 2.0 | 0.89 | 7 | 3.4 | 1.9 | 1.46 | 8 | 7.1 | 2.5 | 2.00 | 21 | 3.9 | 2.1 | 1.53 |
| Intensive care | ||||||||||||||||
| 3 | 0 | 0.0 | – | – | 0 | 0.0 | – | – | 0 | 0.0 | – | – | 0 | 0.0 | – | – |
| 6 | 0 | 0.0 | – | – | 1 | 0.5 | 2.0 | – | 0 | 0.0 | – | – | 1 | 0.2 | 2.0 | – |
| 9 | 0 | 0.0 | – | – | 1 | 0.5 | 4.0 | – | 0 | 0.0 | – | – | 1 | 0.2 | 4.0 | – |
| 12 | 1 | 0.5 | 6.0 | – | 0 | 0.0 | – | – | 0 | 0.0 | – | – | 1 | 0.2 | 6.0 | – |
| Mental health unit | ||||||||||||||||
| 3 | 0 | 0.0 | – | – | 0 | 0.0 | – | – | 1 | 0.8 | 1.0 | – | 1 | 0.2 | 1.0 | – |
| 6 | 1 | 0.5 | 7.0 | – | 1 | 0.5 | 3.0 | – | 0 | 0.0 | – | – | 2 | 0.4 | 5.0 | 2.82 |
| 9 | 0 | 0.0 | – | – | 1 | 0.5 | 3.0 | – | 1 | 0.9 | 18.0 | – | 2 | 0.4 | 10.5 | 10.61 |
| 12 | 0 | 0.0 | – | – | 1 | 0.5 | 2.0 | – | 1 | 0.9 | 21.0 | – | 2 | 0.4 | 11.5 | 13.44 |
| Other | ||||||||||||||||
| 3 | 0 | 0.0 | – | – | 1 | 0.5 | 3.0 | – | 1 | 0.8 | 1.0 | – | 2 | 0.3 | 2.0 | 1.41 |
| 6 | 0 | 0.0 | – | – | 1 | 0.5 | 1.0 | – | 0 | 0.0 | – | – | 1 | 0.2 | 1.0 | – |
| 9 | 2 | 0.9 | 3.5 | 3.54 | 3 | 1.5 | 1.0 | 0.00 | 0 | 0.0 | – | – | 5 | 1.0 | 2.0 | 2.24 |
| 12 | 1 | 0.5 | 7.0 | – | 1 | 0.5 | 1.0 | – | 0 | 0.0 | – | – | 2 | 0.4 | 4.0 | 4.24 |
Number of patients paying for private health care and money spent in the preceding 3 months
| Time point (months) | Intervention | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture + usual care | Counselling + usual care | Usual care | ||||||||||||||
| Patients | Money spent (£) | Patients | Money spent (£) | Patients | Money spent (£) | Patients | Money spent (£) | |||||||||
| n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | |
| Private acupuncture | ||||||||||||||||
| 3 | 3 | 1.2 | 60.0 | 21.79 | 1 | 0.4 | 300.0 | – | 2 | 1.6 | 117.5 | 116.67 | 6 | 1.0 | 119.2 | 107.49 |
| 6 | 27 | 11.5 | 96.8 | 73.29 | 3 | 1.3 | 146.7 | 80.83 | 3 | 2.5 | 63.3 | 49.33 | 33 | 5.7 | 98.4 | 72.48 |
| 9 | 27 | 11.6 | 91.5 | 68.32 | 2 | 0.9 | 130.0 | 70.71 | 2 | 1.7 | 750 | 63.64 | 31 | 5.5 | 93.0 | 66.68 |
| 12 | 22 | 9.6 | 108.1 | 68.75 | 2 | 0.9 | 129.5 | 43.13 | 2 | 1.7 | 75.0 | 63.64 | 26 | 4.6 | 107.2 | 65.80 |
| Private counselling | ||||||||||||||||
| 3 | 2 | 0.8 | 162.5 | 53.03 | 3 | 1.3 | 453.3 | 646.71 | 2 | 1.6 | 152.5 | 137.89 | 7 | 1.2 | 284.3 | 409.96 |
| 6 | 4 | 1.7 | 116.7 | 57.74 | 13 | 5.8 | 198.5 | 103.35 | 1 | 0.8 | 130.0 | – | 18 | 3.1 | 180.0 | 98.04 |
| 9 | 0 | 0.0 | – | – | 10 | 4.7 | 162.5 | 112.18 | 0 | 0.0 | – | – | 10 | 1.8 | 162.5 | 112.18 |
| 12 | 3 | 1.3 | 175.0 | 238.48 | 10 | 4.6 | 170.8 | 107.65 | 1 | 0.9 | 65.0 | – | 14 | 2.5 | 164.1 | 132.63 |
| Psychotherapy | ||||||||||||||||
| 3 | 0 | 0.0 | – | – | 7 | 3.2 | 175.7 | 218.26 | 2 | 1.9 | 40.0 | – | 9 | 1.6 | 158.8 | 207.69 |
| 6 | 0 | 0.0 | – | – | 2 | 0.9 | 152.5 | 67.18 | 1 | 0.8 | 160.0 | – | 3 | 0.5 | 155.0 | 47.70 |
| 9 | 0 | 0.0 | – | – | 2 | 10 | 291.5 | 362.75 | 0 | 0.0 | – | – | 2 | 0.4 | 291.5 | 362.75 |
| 12 | 1 | 0.4 | 450.0 | – | 2 | 0.9 | 182.5 | 166.17 | 1 | 0.9 | 65.0 | – | 4 | 0.7 | 220.0 | 189.16 |
Effect of trial arm on PHQ-9 depression score at 3 months and BDI-II depression score at 12 months (non-imputed data)
| Analysis | n | Group | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||||
| Mean | SE | Mean | SE | ||||||
| Acupuncture | Usual care | Mean | SE | 95% CI | p-value | ||||
| PHQ-9a | 376 | 9.8 | 0.35 | 12.4 | 0.48 | –2.58 | 0.598 | –3.76 to –1.40 | < 0.001 |
| BDI-IIb | 335 | 21.3 | 1.25 | 24.6 | 1.70 | –3.27 | 1.462 | –6.14 to –0.39 | 0.026 |
| Counselling | Usual care | ||||||||
| PHQ-9a | 365 | 11.0 | 0.38 | 12.9 | 0.52 | –1.97 | 0.639 | –3.22 to –0.71 | 0.002 |
| BDI-IIb | 321 | 23.9 | 1.37 | 27.2 | 1.62 | –3.37 | 1.445 | –6.21 to –0.52 | 0.021 |
| Acupuncture | Counselling | ||||||||
| PHQ-9a | 485 | 10.0 | 0.36 | 10.7 | 0.37 | –0.65 | 0.516 | –1.67 to 0.36 | 0.207 |
| BDI-IIb | 354 | 21.7 | 1.21 | 21.1 | 1.37 | 0.62 | 1.300 | –1.95 to 3.18 | 0.636 |
FIGURE 31.
Sampling frame of the patients interviewed in nested substudy 1 (experience of treatment from the patient perspective).

Demographics and variables of interest at baseline comparing the pain and the no-pain groups in substudy 2
| Characteristic | Group | Total (n = 755) (100%) | |
|---|---|---|---|
| No pain (n = 371) (49%) | Moderate to extreme pain (n = 384) (51%) | ||
| Age (years) | |||
| Mean (SD) | 39.9 (11.58) | 46.83 (14.11) | 43.5 (13.37) |
| Median (min., max.) | 39 (18, 75) | 46 (18, 93) | 43 (18, 93) |
| Interquartile range (25%, 75%) | 31, 48 | 38, 56 | 33, 53 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sex, n (%) | |||
| Male | 88 (23.7) | 113(29.4) | 201 (26.6) |
| Female | 283 (76.3) | 271(70.6) | 554(73.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age left education (years) | |||
| Mean (SD) | 18.3 (3.90) | 17.53 (4.61) | 18.0 (4.37) |
| Median (min., max.) | 17.0 (13, 52) | 16 (14, 54) | 16 (13-54) |
| Interquartile range (25%, 75%) | 16, 21 | 16, 18 | 16, 19 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Employment, n (%) | |||
| Full-time education | 13 (3.5) | 10 (2.6) | 23 (3.1) |
| Working full-time | 167 (45.0) | 114 (29.7) | 281 (37.2) |
| Working part-time | 83 (22.4) | 61 (15.9) | 144 (19.5) |
| Unable to work | 19 (5.1) | 76 (19.8) | 95 (12.9) |
| Looking after home | 41 (11.1) | 42 (10.9) | 83 (11.0) |
| Retired | 14 (3.8) | 51 (13.3) | 65 (8.8) |
| Other | 26 (7.0) | 22 (5.7) | 48 (6.5) |
| Missing | 8 (2.2) | 8 (2.1) | 16 (2.1) |
| Painful health or medical condition, n (%) | |||
| Current painful health or medical condition | 41 (12.7) | 299 (77.9) | 346 (45.8) |
| Onset before depression | 32 (8.6) | 216 (56.2) | 248 (32.8) |
| Missing | 2 (0.5) | 4 (1) | 6 (0.8) |
| Type of health problem, n (%) | |||
| Musculoskeletal | 21 (6) | 221 (58) | 242 (32) |
| Other | 27 (7) | 81 (21) | 108 (14) |
| No health problem | 321 (87) | 81 (21 | 402 (53) |
| SF-36 bodily pain | |||
| Mean (SD) | 76.97 (22.53) | 39.03(22.5) | 57.6 (28.44) |
| Median (min., max.) | 77.5 (12, 100) | 41 (0, 100) | 52 (0, 100) |
| Interquartile range (25%, 75%) | 62, 100 | 22, 51 | 32, 84 |
| Missing, n (%) | 3 (0.39) | 1 (1.3) | 4 (0.5) |
| Depression, n (%) | |||
| In last 2 weeks | 264 (71.2) | 310 (80.7) | 574 (76.1) |
| Missing | 2 (0.5) | 10 (2.6) | 12 (1.6) |
| Not first major episode | 240 (64.7) | 273 (71.1) | 513 (67.9) |
| Missing | 108 (29.1) | 82 (21.3) | 190 (25.2) |
| Four or more previous episodes | 162 (43.7) | 227 (59.1) | 389 (51.5) |
| Missing | 133 (35.8) | 113 (29.4) | 246 (32.6) |
| Age at first major depressive episode | |||
| Mean (SD) | 24.89 (11.56) | 25.47 (12.9) | 25.2 (12-28) |
| Median (min., max.) | 21 (6, 72) | 23 (0, 79) | 22 (0, 79) |
| Interquartile range (25%, 75%) | 16, 32 | 16, 31 | 16, 31 |
| Missing, n (%) | 7 (0.9) | 9 (1.2) | 16 (2.1) |
| Medication, n (%) | |||
| Depression medication in last 3 months | 250 (67.3) | 269 (70) | 519 (68.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Analgesic medication in last 3 months | 114 (30.7) | 245 (63.8) | 359 (47.5) |
| Missing | 4 (1) | 2 (0.52) | 6 (0.8) |
| EQ-5D pain, n (%) | |||
| No pain | 371 (49.1) | 0 (0.0) | 371 (49.1) |
| Moderate pain or discomfort | 0 (0.0) | 298 (77.6) | 298 (39.5) |
| Extreme pain or discomfort | 0 (0.0) | 86 (22.4) | 86 (11.4) |
| Missing | 3 | 4 | 7 (0.9) |
| EQ-5D anxiety/depression, n (%) | |||
| Not anxious/depressed | 13 (3.5) | 8 (2.1) | 21 (2.8) |
| Moderately anxious/depressed | 282 (76.0) | 272 (70.8) | 554 (73.4) |
| Extremely anxious/depressed | 74 (19.9) | 104 (27.1) | 178 (23.6) |
| Missing | 2 (0.5) | 0 (0.0) | 2 (0.3) |
| EQ-5D mobility, n (%) | |||
| No problems walking about | 361 (31) | 234 (31) | 595 (79.0) |
| Some problems walking about | 9 (1.2) | 148 (19.7) | 157 (20.8) |
| Confined to bed | 0 (0) | 1 (0.1) | 1 (0.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| EQ-5D self-care, n (%) | |||
| No problems with self-care | 358 (47.5) | 314 (41.7) | 672 (89.2) |
| Some problems with self-care | 11 (1.5) | 68 (9) | 79 (10.5) |
| Unable to wash or dress | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Missing | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| EQ-5D usual activities, n (%) | |||
| No problems performing usual activities | 251 (33.2) | 111 (14.7) | 362 (48.1) |
| Some problems performing usual activities | 115 (15.3) | 247 (32.8) | 115 (48.1) |
| Unable to perform usual activities | 5 (0.7) | 24 (3.2) | 29 (3.9) |
| Missing | 2 (0.3) | 0 (0) | 2 (0.3) |
| PHQ-9 score | |||
| Mean (SD) | 14.97 (5.15) | 17.0 (5.23) | 16.0 (5.29) |
| Median (min., max.) | 15 (3, 27) | 17 (3, 27) | 16 (3-27) |
| Interquartile range (25%, 75%) | 11, 19 | 13, 21 | 12, 20 |
| Missing, n (%) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| BDI-II score | |||
| Mean (SD) | 31.3 (8.3) | 33.6 (8.9) | 32.5 (8.7) |
| BDI-II group, n (%) | |||
| Moderate (20–28) | 155 (41.8) | 129 (33.6) | 284 (37.6) |
| Severe (29–63) | 216 (52.8) | 255 (66.4) | 471 (62.4) |
| Trial arm allocation, n (%) | |||
| Acupuncture | 156 (20.7) | 146 (19.3) | 302 (40) |
| Counselling | 151 (20) | 151 (20) | 302 (40) |
| Usual care | 64 (8.5) | 87 (11.5) | 151 (20) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Expectation of treatment allocated, n (%) | |||
| Very ineffective | 13 (3.5) | 21 (9.6) | 34 (4.5) |
| Fairly ineffective | 39 (10.5) | 57 (14.8) | 96 (12.8) |
| Can’t decide | 159 (42.9) | 178 (46.4) | 337(44.9) |
| Fairly effective | 122 (32.9) | 87 (22.7) | 209 (27.8) |
| Very effective | 38 (10.2) | 37 (9.6) | 75 (10.0) |
| Missing | 0 (0.0) | 4 (0.5) | 4 (0.5) |
| Treatment preference, n (%) | |||
| Acupuncture | 196 (52.8) | 234 (60.9) | 430 (57.5) |
| Counselling | 94 (25.3) | 70 (18.2) | 164(21.9) |
| Usual care | 3 (0.8) | 7 (1.8) | 10 (1.3) |
| No preference | 76 (20.5) | 68 (17.7) | 144 (19.3) |
| Missing | 2 (0.5) | 5 (1.3) | 7 (0.9) |
Details of acupuncturists and counsellors participating in an interview or focus group in nested substudy 3
| Group | Number involved in | Total number participating | ||
|---|---|---|---|---|
| Main trial | One-to-one interviews | Focus group | ||
| Acupuncturists | 23 | 13 | 4 | 17 |
| Counsellors | 41 | 15 | 4 | 19 |
| Total | 64 | 28 | 8 | 36 |
Unit costs 2012
| Resource | Unit cost (£) | Description | Source |
|---|---|---|---|
| Depression medication | 0.047 | Per dose of fluoxetine | BNF485 |
| Pain medication | 0.330 | Per dose of co-codamol | BNF485 |
| GP | 43 | Appointment of 11.7 minutes | Curtis 2012291 |
| Practice nurse | 22 | Band 5, appointment of 15.5 minutes | Curtis 2012291 |
| Other health professional | 43 | Appointment of 11.7 minutes | Curtis 2012291 |
| NHS hospital outpatient clinic | 131 | Community setting mental health services | Curtis 2012291 |
| Hospital ward | 254 | Non-elective inpatient (long stay) excess bed day HRG data | NHS Reference Costs 2011–2012 486 |
| Hospital ICU | 654 | Mental health services inpatient attendances, intensive care – adult | Curtis 2012291 |
| Hospital mental health unit | 338 | Weighted average of all adult mental health inpatient bed-days | Curtis 2012291 |
| Accident and emergency | 122 | Weighted average of ‘leading to admission’ and ‘not leading to admission’ | NHS Reference Costs 2011–2012 486 |
| Community mental health nurse | 85 | Band 5, per hour of client contact | Curtis 2012291 |
| Psychologist or psychiatrist | 136 | Band 8a, per hour of client contact | Curtis 2012291 |
| NHS counsellor | 65 | Band 7, per hour of client contact | Curtis 2012291 |
| Acupuncturist (initial session) | 47.5 | Average of initial sessions costs of £35 and £60 | MacPherson et al.326 |
| Acupuncturist (further session) | 37.5 | Average of further session costs of £25 and £50 | MacPherson et al.326 |
List of abbreviations
- AACP
- Acupuncture Association of Chartered Physiotherapists
- AAOS
- American Academy of Orthopaedic Surgeons
- ACR
- American College of Rheumatology
- ACUDep
- Acupuncture, Counselling or Usual Care for Depression
- AIMS
- Arthritis Impact Measurement Scale
- ANCOVA
- analysis of covariance
- ANOVA
- analysis of variance
- ATC
- Acupuncture Triallists’ Collaboration
- AUC
- area under the curve
- AWR
- adopt with research
- BAcC
- British Acupuncture Council
- BAWMA
- British Academy of Western Medical Acupuncture
- BDI-II
- Beck Depression Inventory-II
- BEAM
- Back pain Exercise And Manipulation
- BMAS
- British Medical Acupuncture Society
- BMI
- body mass index
- CAM
- complementary and alternative medicine
- CARE
- Consultational and Relational Empathy
- CI
- confidence interval
- CLAD
- censored least absolute deviations
- CMS
- Constant–Murley Score
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- DIC
- deviance information criterion
- EQ-5D
- EuroQol-5 Dimensions
- EULAR
- European League Against Rheumatism
- EVPI
- expected value of perfect information
- GP
- general practitioner
- HAQ
- Health Assessment Questionnaire
- HERC
- Health Economics Research Centre
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- MCII
- minimal clinically important improvement
- MCMC
- Markov chain Monte Carlo
- MCS
- mental component summary
- MPCI
- minimal perceptible clinical improvement
- NICE
- National Institute for Health and Care Excellence
- NMES
- neuromuscular electrical stimulation
- NRS
- numerical rating scale
- NSAID
- non-steroidal anti-inflammatory drug
- OARSI
- Osteoarthritis Research Society International
- OIR
- only in research
- OLS
- ordinary least squares
- PCS
- physical component summary
- PEMF
- pulsed electromagnetic field
- PES
- pulsed electrical stimulation
- PHQ-9
- Patient Health Questionnaire-9 items
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SMD
- standardised mean difference
- TENS
- transcutaneous electrical nerve stimulation
- TOIB
- Topical or Oral IBuprofen
- TTH
- tension-type headache
- VAS
- visual analogue scale
- WOMAC
- Western Ontario and McMaster Universities Osteoarthritis Index