Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1209-10057. The contractual start date was in January 2012. The final report began editorial review in January 2016 and was accepted for publication in September 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Mike Gillett has undertaken consultancy work for NHS England and Public Health England (PHE), for the National Diabetes Prevention Programme. Thomas Yates has been a member of National Institute for Health and Care Excellence (NICE) public health guidance on preventing type 2 diabetes. Sabyasachi Bhaumik has been a member of the Health Services and Delivery Research (researcher-led) panel for the last 3 years and before that he was a member of the Community and Psychological Therapies panel of the National Institute for Health Research (NIHR) for 3 years. He is the chairperson of the Diaspora Committee of the Royal College of Psychiatrists and was the chairperson of the Faculty of Psychiatry of Learning Disability for 4 years. He is also a co-editor of the only prescribing guidelines in intellectual disability nationally, and the third edition of this book, The Frith Prescribing Guidelines for People with Intellectual Disability, was published in 2015 by Wiley. Chloe Thomas has undertaken consultancy work for NHS England and PHE, for the National Diabetes Prevention Programme. Susannah Sadler has undertaken consultancy work for NHS England and PHE, for the National Diabetes Prevention Programme. Sally-Ann Cooper has received grants from NIHR during the conduct of the study, and grants from NIHR and from the Scottish Government outside the submitted work. Melanie Davies is a member of NICE public health guidance on preventing type 2 diabetes and an advisor to the UK Department of Health for the NHS Health Check Programme. She has acted as a consultant, an advisory board member and a speaker for Novo Nordisk, Sanofi Aventis, Eli Lilly and Company, Merck Sharp & Dohme Corp., Boehringer Ingelheim, AstraZeneca and Janssen Pharmaceutica, and as a speaker for Mitsubishi Tanabe Pharma Corp. She has received grants in support of investigator and investigator-initiated trials from Novo Nordisk, Sanofi Aventis and Eli Lilly and Company. She received grants and support from NIHR during the conduct of this study. Kamlesh Khunti (chairperson) is a member of the NICE public health guidance on preventing type 2 diabetes and an advisor to the UK Department of Health for the NHS Health Check Programme. He has acted as a consultant, served on advisory boards and been a speaker for Novartis, Novo Nordisk, Sanofi Aventis, Eli Lilly and Company, Janssen Pharmaceutica, Boehringer Ingelheim and Merck Sharp & Dohme. He has received grants in support of investigator and investigator-initiated trials from Novartis, Novo Nordisk, Sanofi Aventis, Eli Lilly and Company, Roche, Boehringer Ingelheim and Merck Sharp & Dohme. He also received grants and support from NIHR during the conduct of this study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Dunkley et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Easy-read summary
The images and symbols used in the easy-read summary are reproduced with permission from the following organisations.
Change Picture Bank. © CHANGE www.changepeople.org

Somerset Total Communication. © STC 2016. All rights reserved. These symbols may not be reproduced as a whole by any means.
Somerset Total Communication (STC), c/o Resources for Learning, Parkway, Bridgwater, Somerset, TA6 4RL. Telephone: 01278 444949. E-mail: stc@somerset.gov.uk. www.somersettotalcommunication.org.uk; www.SupportServicesforEducation.co.uk.
People First.

Widget. Widgit Symbols © Widgit Software 2002–2017. www.widgit.com
Chapter 1 Introduction
Rationale
The focus of this research programme was to estimate the prevalence of type 2 diabetes mellitus (T2DM) and impaired glucose regulation (IGR) among people with intellectual disability (ID) and to develop and test a lifestyle education programme for the prevention of T2DM that would be suitable for use in this population.
This research programme was developed to address gaps in the evidence base with regard to determining the prevalence of T2DM and IGR in adults with ID, and the lack of suitable prevention programmes that are specially tailored for people with ID. Since this research began, priorities set out in the 2015–16 UK NHS England Business Plan1 have highlighted the need to improve services for people with ID and to establish a national ID mortality review, with both diabetes and obesity identified as health priorities. 2 An additional health priority that was identified for all patients is the prevention of obesity and T2DM via a national ‘evidence-based lifestyle management programme’ to support people to make healthy lifestyle changes. 1
The current evidence base for screening and successfully managing those at risk of diabetes – through diet, exercise and behaviour therapy – relates to the general population. It is not currently known whether or not screening for T2DM and IGR or prevention strategies through lifestyle education can be successful in people with ID.
Aims and objectives
The aims of the programme were to:
-
establish a programme of research that significantly enhances the knowledge and understanding of IGR and T2DM in people with ID
-
test strategies for the early identification of IGR and T2DM in people with ID
-
develop a lifestyle education programme and educator training protocol to promote behaviour change in a population with ID and IGR [or high risk of T2DM/cardiovascular disease (CVD) based on elevated body mass index (BMI) score].
Overview of the programme of research
To achieve these aims, three distinct work packages (WPs) were developed (Figure 1).
FIGURE 1.
Programme of work.
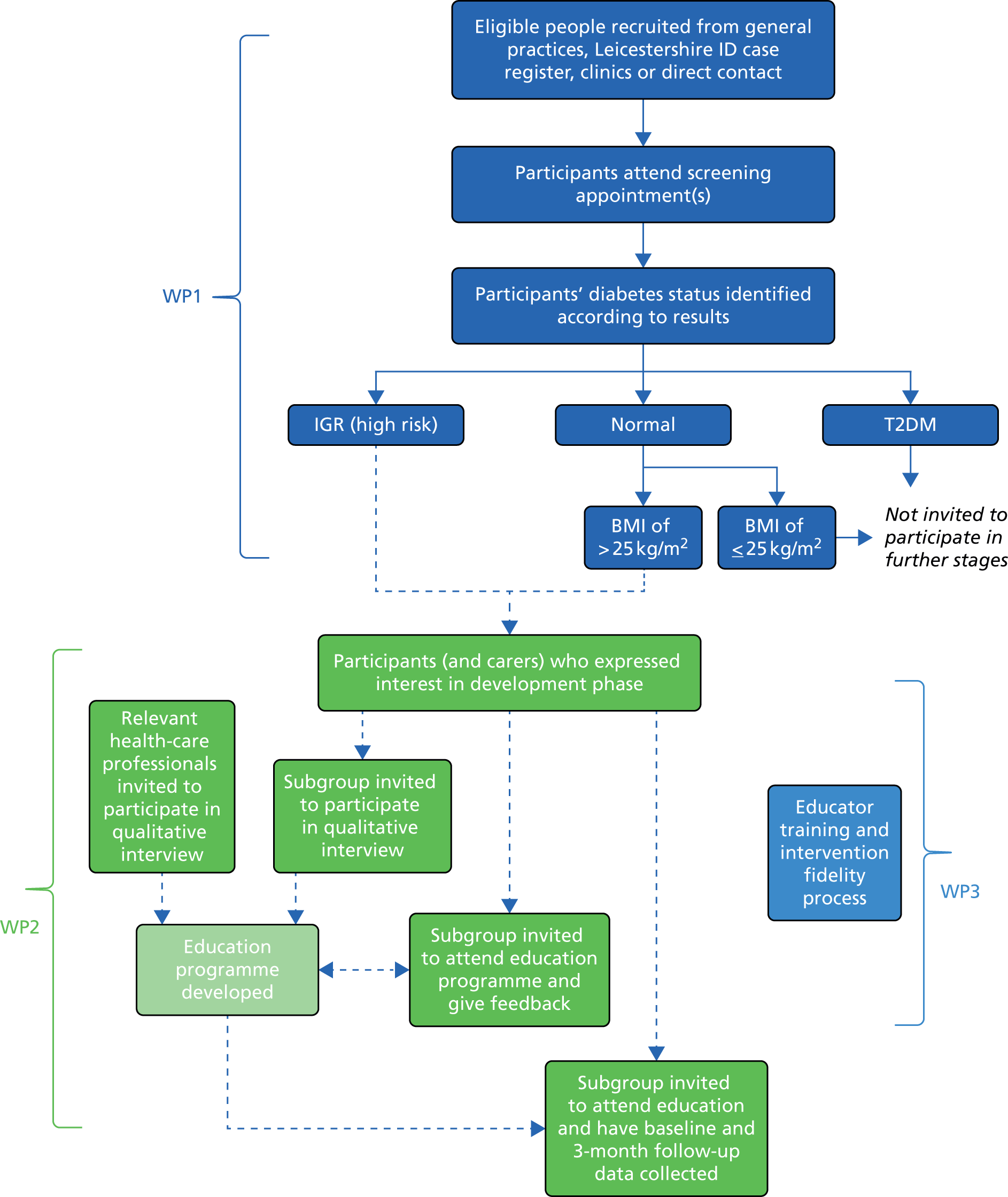
Work package 1
The aims of WP1 were to:
-
develop and assess the feasibility of a diabetes screening programme in a community setting for adults with ID (see Chapters 5 and 6)
-
determine the prevalence and demographic risk factors for T2DM and IGR in people with mild to profound ID (see Chapters 5 and 6)
-
validate the Leicester Self-Assessment diabetes risk score in people with ID (see Chapters 5 and 6)
-
determine the cost-effectiveness of lifestyle intervention (see Work package 2), compared with current care (see Chapter 12)
-
establish data linkage to Hospital Episode Statistics and the Office for National Statistics (ONS) (see Chapters 5 and 6).
Work package 2
The aims of WP2 were to:
-
develop a lifestyle education programme for people with ID and IGR (or high risk of T2DM/CVD based on elevated BMI) (see Chapters 8 and 9)
-
assess the feasibility of collecting outcome measures before and 3 months after attendance at lifestyle education (see Chapter 10).
Work package 3
The aim of WP3 was to:
-
develop a quality assurance (‘intervention fidelity’) process for the assessment of educators who are delivering the education (see Chapter 11).
Scope of the report
The remainder of this chapter provides a brief overview of the ethics and governance arrangements, and provides the detailed background for this research programme.
Subsequent chapters contain individual summaries, but, briefly, comprise:
-
a systematic review of the prevalence/incidence of T2DM in people with ID (see Chapter 2)
-
a systematic review of multicomponent behaviour change interventions in people with ID (see Chapter 3)
-
details of the involvement of people with ID throughout the programme of research (see Chapter 4)
-
methods for the screening programme (see Chapter 5)
-
results from the screening programme (see Chapter 6)
-
methods and results from a physical activity substudy (see Chapter 7)
-
details of the development of the lifestyle education programme (see Chapters 8 and 9)
-
methods and findings from a feasibility phase collecting pre- and post-intervention outcome measures (see Chapter 10)
-
details of the development of the intervention fidelity process (see Chapter 11)
-
methods and results for the economic analysis undertaken (see Chapter 12)
-
discussion of findings and conclusions (see Chapter 13).
Ethics and governance
Approvals
The University of Leicester acted as sponsor for the programme of research. NHS research ethics approval was obtained from the East of England – Cambridge Central Research Ethics Committee (reference 12/EE/0340). Research and development approval was obtained for the research sites from Leicestershire Partnership NHS Trust (LPT); Leicester City Clinical Commissioning Group (CCG), East Leicestershire & Rutland CCG and West Leicestershire CCG; and the University Hospitals of Leicester NHS Trust.
Adherence to mental capacity legislation
Obtaining consent was the largest ethics consideration for this programme. Strict standard operating procedures needed to be established to ensure that valid consent was obtained in accordance with English capacity legislation,3 while taking into account the heterogeneity in capacity of individuals. More details on the assessment of capacity and taking consent are contained in the methodology section for the screening programme (see Chapter 4). This included providing people with all of the information that was relevant to making the decision on whether or not to participate in the research, and communicating this information in a way that was appropriate to them (such as using simple language and visual aids).
The process for those who lacked capacity involved talking to a ‘consultee’, whose role was to consider the study from the participant’s perspective (see Appendix 1, Figure 26). Regardless of whether or not the person with ID had the capacity to decide on participation, the research was discussed with the individual to help him or her to understand the project as far as he or she had the capacity to do so, and to ascertain any opinion that he or she had on participation. For example, if a person without decision-making capacity appeared even slightly anxious or reluctant to take part, then this was respected, and he or she was not recruited to the study.
Programme steering group
Strategic oversight and direction of the research programme was provided by the programme steering group (Figure 2), which comprised the chief investigator (KK), the lead researcher/project manager (AD) and co-applicants listed in the application, with ad hoc attendance from service users. The meetings were held four times per year and were independently chaired by Dr Colin Greaves, University of Exeter (see Figure 2). The meetings involved discussion of contractual issues, staffing, protocol and ethical amendments, public involvement (a rolling agenda item), recruitment progress, economic analysis, education development, anticipated timelines and progress against project aims.
FIGURE 2.
Governance structure of the STOP Diabetes programme. NETSCC, NIHR Evaluation, Trials and Studies Coordinating Centre.

Operational groups
The research team (researchers, ID research nurses and research administrator) met frequently throughout the programme to plan the individual components of the programme and to discuss progress. The details of these meetings were fed back to the steering group.
The education development team [a multidisciplinary team of health-care professionals (HCPs) and researchers with expertise in the field of both ID and developing diabetes and CVD prevention programmes] met regularly to oversee and facilitate the development of the lifestyle education programme (WP3). Progress and key decisions were fed back at steering group meetings.
Service user groups
A number of service users were involved in the research programme, but two service user self-advocacy groups were particularly influential. The groups met regularly, facilitated by an experienced supporter, and their comments were fed back to the steering group. More information about these and other service users’ involvement is detailed in Chapter 4.
Data protection
A six-digit study code was used to identify all of the study participants. This code was used for all hard and electronic copies of data that were collected for this programme (including questionnaires, anthropometric data and blood samples), which were retained in a secure setting.
The Leicester Clinical Trials Unit (UK Clinical Research Collaboration registration number 43) was responsible for the development of a secure database for the data that were collected as part of this research programme.
Background
Definition of intellectual disability and case identification
Intellectual disability, also known as learning disability, is a lifelong condition with onset before adulthood, characterised by a reduced ability to understand new or complex information and learn new skills, and a reduced ability to cope independently. 4 Severity levels for ID are typically categorised by broad intelligence quotient (IQ), alongside the required deficits in independent living skills, into mild (IQ 50–69), moderate (IQ 35–49), severe (IQ 20–34) and profound (IQ < 20) ID. 5 Acknowledging the wide variation that exists between individuals with ID, typical abilities suggested for each category are outlined in Table 1 [based on World Health Organization (WHO) International Classification of Diseases, Tenth Edition (ICD-10)]. 5 More recently in the UK, the Learning Disabilities Public Health Observatory has offered a ‘working definition’, which includes brief practical guidance to improve the recognition of ID and to assist agencies in targeting services. 6
| Severity of ID | Suggested abilities and skills | IQ level |
|---|---|---|
| Mild |
|
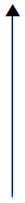
|
| Moderate |
|
|
| Severe |
|
|
| Profound |
|
The aetiology of ID can be broadly divided into problems that occur in the antenatal, perinatal or postnatal periods, or as a result of multiple factors. Common causes of ID include genetic and chromosomal disorders – both non-inherited (e.g. Down syndrome)7 and inherited (e.g. fragile X syndrome) – and non-genetic factors, such as infection and environmental factors. However, in the majority of cases no specific cause is found. 8
A recent meta-analysis of population-based studies suggests that, overall, around 1% of people worldwide have ID, with wide variation dependent on age group and the income of the country (lower, middle and higher); for adults, the proportion is around 0.5%. 9 The evidence from existing ID registers and general practice lists in England suggests that the prevalence of ID is approximately 3–5 per 1000 individuals. 10,11 However, it is thought that the true prevalence could be as high as 2% of the adult population, as people with mild ID are generally under-represented. 11
For the current research programme, cases were identified via (1) records held on adults with ID in general practices and (2) a register of adults attending ID services owned by the local mental health trust (LPT), the Leicestershire Learning Disability Register (LLDR; see below).
General practices in the UK are now incentivised to maintain a register of people with ID. 12,13 Locally, for practices within Leicester City, East Leicestershire and Rutland, and West Leicestershire CCGs, the total number of adults (aged ≥ 18 years) on general practice registers with an identified ID is estimated to be around 4300 (based on figures provided by LPT).
The LLDR comprises adults with ID (aged ≥ 19 years) who live in the unitary authorities of Leicester city, Leicestershire and Rutland. 14 The register was established in 1987 to help facilitate the provision and monitoring of services, and to enable the collection of public health data. It is currently a joint venture between LPT and Leicester City CCG. Enrolment is via a large network of service providers, including specialist ID services, social services and primary care. Currently, there are ≈3900 people with mild to profound ID on the register. However, as the learning disability register is based on service use, some adults, particularly those with mild ID who have little or no support from services, may not currently be identified. This potentially accounts for some of the differences between the number of people identified on this register and the number on local general practice registers.
Type 2 diabetes and impaired glucose regulation
Type 2 diabetes is a serious chronic disease, characterised by prolonged hyperglycaemia. 15 Its symptoms can reduce quality of life and lead to serious health complications, including blindness, renal failure and amputation; 50% of new cases have demonstrable atherosclerosis at diagnosis. 15–17 The prevalence of diabetes in England is estimated to be 6.2%,18 rising to 8.0% [95% confidence interval (CI) 5.7% to 11.7%] when undiagnosed cases are included. 19 T2DM accounts for around 85–90% of diabetes cases; it creates a huge economic burden on NHS resources, at a cost of £8.8B annually (≈10% of total NHS expenditure). 20
Impaired glucose regulation is a condition in which blood glucose concentrations are elevated above the normal range but do not satisfy the criteria for T2DM. 21,22 Approximately 12% of the UK adult population have IGR, of which an estimated 5–12% go on to develop T2DM each year. Observational studies show a consistent and continuous association between glycaemia and CVD risk, whereby people with IGR have a significantly elevated risk of CVD. 23–25 Given the economic burden associated with this condition and its related comorbidities, this group represents an important target for preventative strategies. 26 Other commonly used terms to describe IGR include pre-diabetes, non-diabetic hyperglycaemia and high risk of diabetes; throughout the report, this high-risk group will be referred to as having IGR.
Previously in clinical practice, T2DM and IGR were identified using the ‘gold standard’ oral glucose tolerance test. 22 However, since the publication of updated WHO guidance in 2011 and subsequent National Institute for Health and Care Excellence (NICE) guidance in 2012, there has been a shift away from the use of the oral glucose tolerance test to the glycated haemoglobin (HbA1c) test. 27,28 The potential benefits of the HbA1c test include it being a non-fasting blood test, less inter-test variability and its ability to provide an indication of longer-term hyperglycaemia (over 6–8 weeks). 29 A HbA1c level of ≥ 48 mmol/l (6.5%) is suggestive of T2DM, whereas a level of 42–47 mmol/l (6.0–6.4%) is suggestive of IGR or a high risk of diabetes. 27 Further details on the methods used to identify T2DM and IGR for this programme of research are provided in Chapter 5 (see Outcomes) and Figure 15.
Risk factors for type 2 diabetes and cardiovascular disease in people with intellectual disabilities
In the general population, increasing levels of obesity and sedentary lifestyles have been associated with a rise in non-communicable diseases, including T2DM and CVD. 30–33
Chronic conditions are becoming increasingly important for people with ID as their life expectancy increases. 34 There are a number of risk factors for T2DM that are known to be highly prevalent in people with ID, suggesting that T2DM and CVD may be more prevalent in this group. These risk factors include:
-
increased antipsychotic drug use for the management of challenging behaviour40,41 and psychosis,42 which are associated with weight gain, hyperglycaemia and worsening of other metabolic CVD risk factors43–45
-
genetic conditions associated with obesity (e.g. Prader–Willi syndrome). 46
Physical inactivity and sedentary behaviour are both common among people with ID, with only a minority (18–33%) achieving the recommended 30 minutes of moderate to vigorous physical activity (MVPA) daily,47,48 and < 15% of people with ID complete the recommended 10,000 steps per day. 49 Furthermore, < 10% of adults with ID who live in supported accommodation have an intake of fruit and vegetables that is sufficient for a balanced diet. 50 The evidence suggests that paid carers know little about public health recommendations on dietary intake. 50
However, little is known about T2DM, CVD and associated risk factors in the population with ID. UK-based data on the prevalence of T2DM are currently unclear. 32 Current estimates for diabetes prevalence in the UK are based on routinely reported data rather than on population-based studies. The suggested prevalence of diagnosed diabetes in people with ID in England is around 6–7%, but estimates are unable to distinguish between T2DM and other forms of diabetes. 13,51 Similarly, the prevalence of CVD among people with ID is reported to be greater than that among the general population, but the overall prevalence is unclear. 52
Further information on the current prevalence of T2DM, CVD and related risk factors in the ID population is presented in the systematic review in Chapter 2.
Diabetes screening
Given the increasing prevalence of diabetes, and the conferred risk of developing CVD, early identification and intervention through screening has been shown to be a useful approach in the general population. 53,54 The value of screening for IGR has also been demonstrated.
It is currently unknown if screening for asymptomatic glucose disorders is viable within UK populations with ID; there is a lack of evidence on feasibility, acceptability, outcomes and benefits. People with ID have been recommended by NICE as being an important group to consider in terms of diabetes prevention strategies, given their supposed high risk of developing diabetes. 27
General practitioners (GPs) in England have been incentivised to provide annual health checks to adults with ID since 2008–9 (for those aged ≥ 14 years since 2014). Recent data suggest that, nationally, the uptake of checks is around 44%. 55 However, the proportion who additionally have bloods taken as part of the health check, including HbA1c (7%) and cholesterol (30%), is extremely low. 13
Risk scores for the early identification of impaired glucose regulation and type 2 diabetes
A staged approach to screening is recommended by NICE for those at risk of diabetes in the general population. 56 This involves using a risk score to pre-screen for individuals at the greatest risk of T2DM followed by a blood test in those at the highest risk. However, this approach has not been tried with populations with ID.
Risk scores are a non-invasive way of stratifying a population for targeted screening. They use information data from non-invasive risk factors to calculate an individual’s score: a higher score reflects a higher risk. Risk scores can be applied to (1) an individual as a questionnaire (these scores generally require data from only non-invasive risk factors, which would be known by members of the public) or (2) a population (e.g. in primary care, for which software is used to calculate the score using routine data from electronic medical records) and then screening invitations can be sent to those at highest risk. A number of diabetes risk scores have been developed and validated for use in the UK general population. 56–60 One such score is the Leicester Self-Assessment risk score (see Appendix 2), which allows people to easily assess their own risk of having undiagnosed IGR or T2DM and then self-refer for screening with a HCP. 58 The score contains seven questions that ask about age, sex, ethnicity, BMI, waist circumference, family history of diabetes and high blood pressure (BP). The score has been validated for use in a multiethnic UK population58,61 and is specifically recommended by NICE for identifying people who are at risk opportunistically. 56
To date, we are not aware of any risk scores that have been specifically assessed for use in populations with ID. However, it cannot be assumed that a risk score that is developed for a specific population will work well in another;62 for people with ID, there may be different risk factors, or weightings for specific risk factors may change, when compared with the general population. Therefore, this programme of work will seek to validate the Leicester Self-Assessment risk score in a population with ID (this work is presented as part of the screening study; see Chapters 5 and 6).
Diabetes prevention in adults with intellectual disabilities
People with ID experience a disproportionate burden of health inequalities compared with the general population, including poorer mental and physical health and higher rates of mortality. 63–66 Despite their increased health needs, they often find it difficult to access primary care services and to participate in health promotion activities. 67–69
Given the health inequalities among people with ID, and the possible increased risk of developing diabetes, people with ID have the potential to benefit from lifestyle changes (with appropriate support) that are addressed in lifestyle education programmes. However, the evidence base for diabetes prevention relates to the general adult population; literature focusing on ID is scarce. Details of the key literature on lifestyle behaviour change interventions aimed at modifying risk factors for T2DM and CVD in people with ID are presented in the systematic review in Chapter 3.
Current evidence from studies conducted in the general population suggest that intensive multicomponent lifestyle interventions aimed at weight loss, a healthy diet and increased physical activity can successfully reduce the risk of diabetes by 30–60% in those with IGR, and are likely to be cost-effective in the long term. 54,70
Increasing physical activity is fundamental to diabetes prevention initiatives, as research suggests that inactivity may have more impact than increased body weight in the development of insulin resistance. 71
For both obesity management72 and prevention of T2DM,27 NICE recommends that lifestyle interventions should be multicomponent, involving both dietary and physical activity advice, and incorporating behaviour change techniques. However, at present there are no national prevention programmes that suitable for people with ID, despite ongoing recommendations to make ‘reasonable adjustments’ to health-care services to address inequities in provision. 73
Education, exercise and leisure pursuits are often determined or influenced by carers (paid or family carers), who may have a range of competing time demands and a number of people for whom to provide support. For people with limited carer support, difficulties in understanding health risks could also influence motivation to change lifestyles. Therefore, there is the potential for this group to benefit from the development of a lifestyle education programme that is targeted at both people with ID and their carers in order to encourage changes in lifestyle behaviours that could reduce the long-term chances of this high-risk group developing diabetes.
Concluding remarks
This chapter has provided the rationale and aims for the research programme, and an overview of the programme of work undertaken. The next chapter presents a systematic review conducted to consolidate the evidence on rates of T2DM, CVD and associated risk factors in adults with ID.
Chapter 2 Systematic review and meta-analysis: rates of type 2 diabetes, cardiovascular disease and associated risk factors in populations with intellectual disability
Overview
In this chapter, we describe the first of two systematic reviews carried out for the research programme. We present the existing evidence in relation to the prevalence of T2DM, CVD and associated risk factors among people with ID. We have used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist74 as a guide to reporting the methods and findings from the review.
Rationale
It is recognised in the literature that ID populations may be at increased risk of developing T2DM and subsequent CVD through increased risk factors, such as obesity. The global increase in the prevalence of obesity, CVD and T2DM, and current discrepancies between studies focusing on prevalence of such conditions in those with ID, suggested a need for a systematic review of literature in this area.
Two recent reviews75,76 have focused on diabetes prevalence among people with ID. The reviews were unable to distinguish between T2DM and other types of diabetes. Similarly, the prevalence of CVD among people with ID is reported to be greater than that among the general population, but the overall prevalence is unclear. 52
The overall aim of this component of the research programme was to consolidate the evidence for current rates of T2DM, CVD and associated risk factors, restricting to population-based studies of adults with ID. If sufficient data were available, we also intended to conduct a meta-analysis. A secondary aim was to compare these data with the general population, when possible.
Objectives
The objectives of this review were to establish the prevalence of:
-
T2DM in a population with ID
-
CVD in a population with ID
-
risk factors for T2DM and/or CVD (obesity, adverse lipid profiles, IGR and hypertension) in a population with ID.
Methods
Protocol and registration
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42015019048). 77
Eligibility criteria
The review was guided by the population, intervention, comparison, outcomes, study design (PICOS) model. 78 We defined the population as adults (aged ≥ 18 years) with ID (whole study population or a defined subsample). The items of interest were defined as T2DM, CVD and their associated risk factors. Context was defined as population-based studies. We defined the outcomes as prevalence and/or incidence rates (or data to enable this calculation). Study designs included cross-sectional, retrospective and prospective cohort studies (Table 2).
| PICOS elements | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Whole study population or defined subsample of adults (aged ≥ 18 years)a | Restrictively selected cohort, based on outcome (e.g. all of the participants were obese at time of data collection) |
| Items of interest | T2DM/diabetes | |
| CVD (atherosclerotic) | ||
| Overweight/obesity | ||
| Hypertension | ||
| Hyperlipidaemia | ||
| Elevated glucose level/IGR | ||
| Metabolic syndrome | ||
| Context | Population-based studies | |
| Outcomes | Prevalence | |
| Incidence | ||
| Study designs | Cross-sectional | |
| Retrospective cohort | ||
| Prospective cohort |
All studies published since 1 January 2000 (until 21 April 2015) and in the English language were eligible. We contacted lead authors for further information when inclusion/exclusion could not be determined.
We chose to limit studies to those published in and after the year 2000 so that the current prevalence of T2DM and CVD could be estimated accurately; it is known that the prevalence of both of these conditions has increased substantially in recent decades.
Information sources
For this review, we searched the databases EMBASE, MEDLINE and PsycINFO. The last date of the search was 21 April 2015. We also searched the reference lists of relevant articles for possible additional studies.
Search
We combined medical subject heading terms and key words for T2DM, CVD, overweight/obesity, hypertension, hyperlipidaemia, elevated glucose level/impaired glucose tolerance, metabolic syndrome and ID (MEDLINE search strategy; Box 1). The search was limited to English-language studies with cohorts of adults aged ≥ 18 years, depending on the database.
-
exp Diabetes Mellitus, Type 2/
-
(diabet* adj3 type adj ‘2’).ti,ab.
-
T2DM.ti,ab.
-
(diabet* adj3 type adj ii).ti,ab.
-
niddm.ti,ab.
-
(non-insulin-dependent adj2 diabet*).ti,ab.
-
(adult-onset adj2 diabet*).ti,ab.
-
Or/1–7
-
exp Hypertension/
-
hypertens*.ti,ab.
-
(blood adj pressure adj3 (high or elevated or increased or raised)).ti,ab.
-
or/9–11
-
exp Metabolic syndrome x/
-
(metabolic adj syndrome).ti,ab.
-
(cardiometabolic adj syndrome).ti,ab.
-
(Insulin adj resistance adj syndrome).ti,ab.
-
MetSyn.ti,ab.
-
MetS.ti,ab.
-
or/13-18
-
exp. Hyperlipidemias/
-
Hyperlipid*.ti,ab.
-
dyslipid*.ti,ab.
-
hypercholes*.ti,ab.
-
hypertriglycer*.ti,ab.
-
(cholesterol* adj2(high or elevated or raised or increased)).ti,ab.
-
(triglcerid* adj2(high or elevated or raised or increased)).ti,ab.
-
(lipid adj profile adj2(adverse or abnormal)).ti,ab.
-
or/20-27
-
exp. Glucose intolerance/
-
(impaired adj glucose adj(tolerance or regulation)).ti,ab.
-
(impaired adj fasting adj glucose).ti,ab.
-
IGT.ti,ab.
-
IFG.ti,ab.
-
IGR.ti,ab.
-
exp Prediabetic state/
-
prediabet*.ti,ab.
-
pre-diabet*.ti,ab.
-
or/29–37
-
(cardiovascular adj diseas*).ti,ab.
-
CVD.ti,ab.
-
CHD.ti,ab.
-
exp. MI/
-
(infarct* adj2 myocardial).ti,ab.
-
exp Coronary disease/
-
(coronary adj2 diseas*).ti,ab.
-
(acute adj coronary adj syndrom*).ti,ab.
-
exp angina pectoris/
-
angina.ti,ab.
-
exp myocardial ischemia/
-
(isch* adj2 heart adj2 diseas*).ti,ab.
-
(Myocardial adj2 isch*).ti,ab.
-
exp. Stroke/
-
strok*.ti,ab.
-
(cerebrovascular adj2 diseas*).ti,ab.
-
(cerebrovascular adj2 accident*).ti,ab.
-
(cerebral adj2 diseas*).ti,ab.
-
(cerebral adj2 accident*).ti,ab.
-
CVA.ti,ab.
-
TIA.ti,ab.
-
(brain adj1 infarc*).ti,ab.
-
(brainstem adj1 infarc*).ti,ab.
-
exp ischemic attack, transient/
-
(isch* adj2 attac* adj2 transient).ti,ab.
-
exp Atherosclerosis/
-
atheroscle*.ti,ab.
-
(arteriosclerotic adj vascular adj diseas*).ti,ab.
-
exp Peripheral Arterial Disease/or exp Peripheral Vascular Diseases/
-
(peripheral adj2 arter* adj2 diseas*).ti,ab.
-
(peripheral adj2 vascular adj2 diseas*).ti,ab.
-
(peripheral adj1 angiopath*).ti,ab.
-
or/39-70
-
exp obesity/
-
obes*.ti,ab.
-
overweight.ti,ab.
-
(body adj weight adj2 (high or elevated or increase*)).ti,ab.
-
(bodyweight adj2 (high or elevated or increase*)).ti,ab.
-
(body adj mass adj3 (high or elevated or increase*)).ti,ab.
-
(waist adj2 (large or elevated or increas*)).ti,ab.
-
exp body mass index/
-
(BMI adj2 (high or elevated or increase*)).ti,ab.
-
or/72-80
-
exp Intellectual disability/
-
(learning adj1 disabilit*).ti,ab.
-
(developmental adj1 disabilit*).ti,ab.
-
(intellectual adj1 disabilit*).ti,ab.
-
(impair* adj2 intellectual adj2 function*).ti,ab.
-
(mental* adj1 impair*).ti,ab.
-
(mental* adj1 handicap*).ti,ab
-
exp mentally disabled persons/
-
(mental* adj1 disabl*).ti, ab
-
(mental* adj2 retard*).ti, ab
-
Or/82-91
-
8 or 12 or 19 or 28 or 38 or 71 or 81
-
92 and 93
-
limit 94 to yr=2000-current
-
limit 95 to English language
-
(animals not humans.mp) [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
96 not 97
Study selection
Full texts were identified after titles and abstracts were read separately by two investigators (TC and AD) who discussed discrepancies in selection at a later meeting. Only full-length articles were included; review articles were removed after being examined for references. Once we had retrieved the full texts of the articles, these were examined separately (by TC and AD) to check their suitability for inclusion.
Data collection process
We designed a data extraction form specifically for this review. Data were extracted by one investigator (TC) and verified for accuracy by another investigator (AD).
Data items
For each study, the first author’s name, title of the paper, year of publication, country of the cohort, study type, sampling method, dates of data collection and inclusion/exclusion criteria were extracted. We also extracted total sample size or subpopulation size, mean ages, proportion of male/female, severity of ID and ethnicity. For each of the outcomes, we also extracted how it was defined, how it was measured and the total number, and proportion, of people for whom it was measured. We extracted data separately for males and females, when reported. When framing the research question and designing the search strategy, we did not consider physical activity/sedentary behaviour, dietary factors or smoking; however, we extracted this information for studies that reported it. We also extracted information on general population data.
Risk of bias in individual studies
We used funnel plots79 and the Egger’s test80 to examine potential publication bias in the literature for the outcomes T2DM, ischaemic heart disease, obesity, hypertension and undefined CVD.
Summary measures
The main outcome measure for the meta-analysis was the prevalence of T2DM and CVD. The secondary outcome measures were the prevalence of:
-
overweight/obesity
-
hypertension
-
hyperlipidaemia
-
elevated glucose level/impaired glucose tolerance
-
metabolic syndrome.
Synthesis of results
Owing to the variation in reporting of outcomes, we extracted descriptions and definitions of each outcome for analytic purposes and subcategorised for meta-analyses. We subcategorised circulatory disease outcomes as ischaemic heart disease, cerebrovascular disease and undefined CVD. We subcategorised diabetes outcomes as T2DM and pooled diabetes. BMI outcomes were labelled as obese (BMI of > 30 kg/m2) and overweight (BMI of 25–29.9 kg/m2). In some articles, overweight and above (BMI of > 25 kg/m2) was used as an outcome. We combined papers reporting both obese and overweight data to create an overweight and above outcome. The outcome definitions can be seen in Appendix 3 (see Table 60).
Owing to the large amount of variability between studies, we used a random-effects model to pool the point prevalence for each outcome. We conducted a secondary meta-analysis including data from a subset of 10 papers,81–90 which additionally reported general population comparison data (from the same population and time period). We assessed heterogeneity using the I2 test. 80
Additional analysis
After the meta-analysis, metaregression was used to determine if study characteristics could explain heterogeneity (as measured using the I2 test). These study characteristics were severity of ID, mean age and method of data collection (self-/carer reported, researcher collected, retrospective records/database). We conducted all of the analyses using Stata® statistical software, version 14 (StataCorp LP, College Station, TX, USA). Significance was set at the 5% level (p < 0.05) and 95% CIs are presented throughout.
Results
Study selection
In total, we identified 4513 articles via the literature searches. After duplicates were removed, 3645 articles remained to be screened. We reviewed the full texts of 148 articles once seven articles from other sources had been added (Figure 3). The authors of seven studies91–97 were contacted for information regarding their studies; five authors replied and two studies94,95 were deemed suitable to be included in the systematic review and meta-analysis. We also included a study93 by one of the authors who did not reply, after we had reread and discussed the article collectively in more depth.
FIGURE 3.
Study selection.
After review, 62 articles50,81–90,93–95,98–145 were included. Four of these articles90,102–104 reported findings from the same study and a further two articles109,125 reported findings from the same study, leaving 58 studies (see Table 3) remaining for the final systematic review and meta-analysis.
Study characteristics
The 58 studies included in the quantitative synthesis presented data on > 47,000 individuals. The characteristics of each of the studies are presented in Table 3.
| First author (year) | Country | ID severity | Data source/collection method | Total n | Male (%) | Mean age (years) | Outcomes reported | Study design |
|---|---|---|---|---|---|---|---|---|
| Molteno (2000)131 | South Africa | MILD 0.3%, MOD 18.7%, SEV 37.7%, PROF 33.5%, missing data | Researcher-collected data | 615 | 51 | NR | Overweight, obese | Cross-sectional observational |
| Robertson (2000)50 | UK | NR | Secondary data analysis | 500 | 60.3 | 44.4 | Overweight, obese | Cross-sectional retrospective |
| Janicki (2002)114 | USA | MILD 1.3%, MOD 50.3%, SEV/PROF 47% | Postal questionnaire | 1373 | 53.0 | 53.5 | CVD, undiagnosed diabetes, overweight, obese, hypertension, hyperlipidaemia | Cross-sectional observational |
| Lewis (2002)119 | USA | MILD 37.1%, MOD 16.4%, SEV 14.7%, PROF 15.3% | Medical review/researcher-collected data | 353 | 49.9 | 35.8 | Overweight, obese, hypertension, hypercholesterolaemia | Cross-sectional observational |
| Marshall (2003)124 | UK | NR | Baseline data from health screening programme | 728 | NR | NR | Overweight, obese, hypertension, hypercholesterolaemia | Cross-sectional observational (baseline) |
| Havercamp (2004)83 | USA | MILD 39.4%, MOD 26.6%, SEV 14.7%, PROF 10.6% | Database/medical records | 477 | 56.1 | NR | CVD, undefined diabetes, overweight, obese, hypertension | Cross-sectional, retrospective |
| Hove (2004)111 | Norway | MILD 39.2%, MOD 42.1%, SEV 15.5% | Postal questionnaire | 274 | 52.0 | NR | Overweight, obese | Cross-sectional observational |
| Merrick (2004)129 | Israel | NR | Postal questionnaire | 2282 | 51 | 49.8 | Heart disease, T2DM, overweight+, hypertension, hyperlipidaemia | Cross-sectional observational |
| Moore (2004)132 | Australia | NR | Researcher-collected data | 93 | NR | 32.5 | Overweight, obese | Cross-sectional observational |
| Emerson (2005)105 | UK | NR | Secondary data analysis | 1304 | 54.0 | 49.3 | Overweight, obese | Cross-sectional retrospective |
| Yen (2005)144 | Taiwan | MILD 22.2%, MOD 34.9%, SEV 28.1%, PROF 14.8% | Secondary data analysis | 516 | NR | NR | Overweight, obese | Cross-sectional retrospective |
| Ito (2006)89 | Japan | NR | Database/medical records | 526 | NR | NR | Overweight, obese | Cross-sectional retrospective |
| Lennox (2006)116 | Australia | NR | Medical history chart/GP examination | 25 | NR | 45.0 | Overweight, obese, hypertension | Cross-sectional observational |
| Levy (2006)117 | USA | MILD 47.6%, MOD 31.1%, SEV 14.6%, PROF 6.8% | Database/medical records | 103 | 52.4 | 38.2 | Hypertension, hypercholesterolaemia, overweight, obese, undefined diabetes | Cross-sectional retrospective |
| McDermott (2006)86 | USA | NR | Database/medical records | 618 | NR | NR | Ischaemic heart disease, cerebrovascular disease, hypertension, obese, T1&T2DM | Cross-sectional retrospective |
| Rurangirwa (2006)93 | USA | NR | Secondary data analysis | 173 | 58.0 | 23.3 | Overweight+ | Cross-sectional retrospective |
| Shah (2006)135 | UK | NR | Postal questionnaire | 119 | NR | NR | Undefined diabetes | Cross-sectional observational |
| Van Den Akker (2006)140 | Netherlands | MILD 11%, MOD 53%, SEV 28%, PROF 8% | Database/medical records | 436 | 52 | NR | Ischaemic heart disease, cerebrovascular disease, hypertension | Cross-sectional retrospective |
| Levy (2007)118 | USA | SEV 65.4%, PROF 34.6% | Database/medical records | 52 | 52.0 | NR | Overweight+ hypercholesterolaemia, hypertension, undefined diabetes | Cross-sectional retrospective |
| McDermott (2007)87 | USA | NR | Database/medical records | 585 | NR | NR | Undefined diabetes | Cross-sectional retrospective |
| McGuire (2007)127 | Ireland | MILD 14.1%, MOD 63.5%, SEV 12.8%, PROF 9% | Postal questionnaire | 155 | 53.5 | 37.0 | Overweight, obese | Cross-sectional observational |
| Wang (2007)142 | Taiwan | NR | Face-to-face interview questionnaire | 1128 | 57.6 | NR | Heart disease, overweight+ | Cross-sectional observational |
| Bhaumik (2008)99 | UK | NR | Questionnaire data register | 1119 | 59.0 | NR | Overweight, obese, hypertension | Cross-sectional, retrospective |
| Henderson (2008)84 | USA | NR | Database/medical records | 100 | NR | T2DM, overweight, obese hypertension, dyslipidaemia | Cross-sectional, Retrospective | |
| Melville (2008)128 | UK | MILD 40.9%, MOD 25.1%, SEV 18.2%, PROF 15.8% | Researcher-collected data | 945 | 55.6 | NR | Overweight, obese | Cross-sectional observational |
| Wallace (2008)141 | Australia | NR | Database/medical records | 155 | 52 | NR | CVD, elevated glucose level, T1&T2DM, overweight, obese, hypertension, hypercholesterolaemia | Cross-sectional retrospective |
| de Winter (2009)81 | Netherlands | MILD 12.1%, MOD 33.2%, SEV 34.3%, PROF 20.4% | GP screened/medical chart/structured interview | 470 | NR | NR | MI, cerebrovascular disease, hypertension, diabetes, elevated glucose level, obese, hypercholesterolaemia | Cross-sectional observational |
| Gale (2009)107 | UK | NR | GP survey data | 1097 | 58.0 | NR | Overweight, obese | Cross-sectional retrospective |
| Henderson (2009)110 | USA | MILD/MOD 53%, SEV/PROF 47% | Health questionnaire data | 1196 | 53.0 | NR | Overweight+ | Cross-sectional observational |
| Maaskant (2009)123 | Netherlands | NR | Database/medical records (2007 data) | 336 | 55.1 | NR | Overweight, obese | Cross-sectional retrospective (2007 data) |
| Moss (2009)134 | South Africa | NR | Questionnaire/researcher-collected data | 100 | 47 | NR | Elevated glucose level, overweight+, hypertension, hypercholesterolaemia | Cross-sectional observational |
| Sohler (2009)136 | USA | NR | Database/medical records | 5930 | NR | NR | Undefined diabetes, overweight, obese, hypertension, hypercholesterolaemia | Cross-sectional retrospective |
| Van de Louw (2009)139 | Netherlands | MILD 10%, MOD 38%, SEV/PROF 52% | Researcher-collected data | 258 | 51.6 | 47 | Hypertension | Cross-sectional observational |
| Shireman (2010)95 | USA | NR | Database/medical records | 291 | 52.6 | NR | Undefined diabetes | Cross-sectional retrospective |
| Stedman (2010)138 | New Zealand | NR | Database/medical records | 98 | NR | 43 | Overweight, obese | Cross-sectional retrospective |
| Tyler (2010)88 | USA | NR | Database/medical records | 1267 | 53.8 | 38.8 | Ischaemic heart disease, undiagnosed diabetes, obese, hypertension, hyperlipidaemia | Cross-sectional retrospective |
| Chen (2011)101 | China | NR | Physical examination (2008) | 117 | NR | NR | Heart disease, diabetes, hypertension, elevated glucose level, hypercholesterolaemia | Cross-sectional observational |
| Frighi (2011)106 | UK | MILD 48%, MOD 30.2%, SEV/PROF 21.8% | Researcher-collected data | 202 | 52.0 | 42.1 | Overweight+, T2DM | Cross-sectional observational |
| Haveman (2011) POMONA II study109 | 14 European countries | MILD 22.7%, MOD 28.2%, SEV 20.7%, PROF 11.8% | Interview survey data | 1253 | 51.0 | 41.0 | Undefined diabetes, hypertension, MI, cerebrovascular disease | Cross-sectional observational |
| Lee (2011)115 | Australia | MILD 33%, MOD 22%, SEV 23%, PROF 21% | Database/medical records | 162 | 52.0 | 44.0 | Ischaemic heart disease, overweight, obese, undefined diabetes, hypertension | Cross-sectional retrospective |
| Martínez-Leal (2011) POMONA II study125 | 14 European countries | MILD 21.8%, MOD 27.7%, SEV 19.7%, PROF 11.4% | Interview survey data | 1257 | 50.5 | 41.4 | Overweight, obese | Cross-sectional observational |
| Stancliffe (2011)137 | USA | NR | Consumer survey interview | 8911 | NR | 43.5 | Overweight, obese, overweight+ | Cross-sectional observational |
| Wong (2011)143 | Hong Kong | MILD 4.9%, MOD 41.8%, SEV/PROF 51.9% | Postal questionnaire | 811 | 53.3 | 44 | Heart disease, cerebrovascular disease, undefined diabetes, overweight+, hypertension, hypercholesterolaemia | Cross-sectional observational |
| Chang (2012)100 | Taiwan | MILD 65%, MOD 16%, SEV 9%, PROF 10% | Annual health checks | 129 | 56.6 | 33.0 | Overweight, obese, hypertension, elevated glucose level, hypercholesterolaemia, metabolic syndrome | Cross-sectional observational |
| De Winter (2012)_1 HA-ID study103 | Netherlands | MILD 24.8%, MOD 48%, SEV 16%, PROF 8.9% | Medical records/physical examination | 945 | 51.0 | 61.5 | Overweight, obese | Cross-sectional observational |
| De Winter (2012)_2 HA-ID study102 | Netherlands | MILD 24.5% MOD 48.6%, SEV 16%, PROF 8.7% | Medical records/physical examination | 980 | 51.3 | 61.5 | Hypertension, hypercholesterolaemia, metabolic syndrome, diabetes | Cross-sectional observational |
| Gazizova (2012)82 | UK | MILD 61%, MOD 24%, SEV 15% | Routine health assessment of people within a service | 100 | 67.0 | NR | Overweight, obese | Cross-sectional observational |
| Hsu (2012)113 | Taiwan | MILD/MOD 47%, SEV/PROF 53% | Health examination charts | 164 | NR | 33.0 | Overweight+, metabolic syndrome | Cross-sectional retrospective |
| Lin (2012)122 | Taiwan | NR | Annual health examination chart | 184 | 62.5 | NR | Hypertension | Cross-sectional retrospective |
| Morin (2012)133 | Canada | MILD 32.9%, MOD 46.4%, SEV 11.2%, PROF 5.2% | Postal questionnaire | 789 | NR | NR | Heart disease, undefined diabetes | Cross-sectional observational |
| Bégarie (2013)98 | France | NR | Questionnaire data | 255 | NR | NR | Overweight, obese | Cross-sectional observational |
| De Winter (2013)104 HA-ID study | Netherlands | MILD 24.9%, MOD 53%, SEV 13.4%, PROF 4.6% | Medical records/physical examination | 629 | 53.6 | 61.5 | Peripheral arterial disease | Cross-sectional observational |
| Haider (2013)108 | Australia | NR | Telephone interview | 897 | NR | 38.4 | Heart disease, cerebrovascular disease, T2DM, overweight, obese | Cross-sectional observational |
| Jansen (2013)85 | Netherlands | MILD 6.9%, MOD 37.8%, SEV 29%, PROF 26.3% | Database/medical records | 510 | 55.7 | 65.5 | MI, cerebrovascular disease | Cross-sectional retrospective |
| Lin (2013)120 | Taiwan | NR | Annual health examination chart | 215 | NR | NR | Hypercholesterolaemia hypertension, elevated glucose level | Cross-sectional retrospective |
| McCarron (2013)126 | Ireland | NR | Face-to-face questionnaire – first wave data for a longitudinal study | 753 | 45.0 | 54.8 | Ischaemic heart disease, cerebrovascular disease, hypertension | Cross-sectional observational (first wave) |
| Vacek (2013)94 | USA | NR | Database/medical records | 3079 | NR | NR | Hypertension | Cross-sectional retrospective |
| Hsieh (2014)112 | USA | MILD 44.9%, MOD 23.7%, SEV/PROF 8.4% | Secondary data analysis | 1450 | 55.2 | 37.1 | Overweight, obese | Cross-sectional retrospective |
| Mikulovic (2014)130 | France | NR | Face-to-face interview questionnaire | 570 | NR | 38.1 | Overweight, obese | Cross-sectional observational |
| de Winter (2015)90 | Netherlands | MILD 21.3%, MOD 47.6%, SEV 16.7%, PROF 9.0% | Medical records/physical examination | 990 | 51.3 | 61.1 | Hypertension, hypercholesterolaemia, T1DM, T2DM, diabetes, peripheral arterial disease, elevated glucose level, obese, metabolic syndrome | Cross-sectional observational |
| Lin (2015)121 | Taiwan | MILD 6.5%, MOD 32.6%, SEV 34.8%, PROF 26.1% | Researcher-collected data | 67 | NR | NR | Overweight, obese | Cross-sectional observational |
| Zaal-Schuller (2015)145 | Netherlands | MILD/MOD 51.1%, SEV/PROF 48.9% | Researcher-collected data | 407 | NR | NR | Peripheral arterial disease | Cross-sectional observational |
Ten of the studies81–89,102,103 included in the systematic review also presented as general population comparison data for inclusion in the secondary meta-analysis.
The studies represented 23 countries on five continents. One study109 covered 14 European countries. Most studies were conducted in the USA/Canada (n = 1783,84,86–88,93–95,110,112,114,117–119,133,136,137). The remaining studies were conducted in Europe [the Netherlands (n = 781,85,102,123,139,140,145), the UK (n = 950,82,89,105–107,124,128,135), France (n = 298,130), Norway (n = 1111) and Ireland (n = 2126,127)], Israel (n = 1129), Asia (n = 1089,100,101,113,120–122,142–144), Australia/New Zealand (n = 6108,115,116,132,138,141) and South Africa (n = 2131,134). Primarily, the included studies were cross-sectional observational (n = 3181,82,98,100–102,104,106,109–111,114,116,119,121,124,126–135,137,139,142,143,145). The remaining studies involved retrospective database or medical records data (n = 2283–89,94,95,99,107,113,115,117,118,120,122,123,136,138,140,141) or secondary data analysis (n = 550,93,105,112,144).
All studies were published in the years 2000–15. The average mean age of participants was 42.8 years, with an average mean age range of 23.3–65.5 years. The average mean percentage of male participants was 52.4%. The number of people included in the studies ranged from 25 to 8911, with a mean of 824.
Risk of bias within studies
The funnel plots did not show any obvious asymmetry, and Egger’s test was not statistically significant for any of the outcome measures (specifically T2DM, t = –0.22; p = 0.84; ischaemic heart disease, t = –0.13; p = 0.91; cerebrovascular disease, t = 0.35; p = 0.58) (see Appendices 4–6, Figures 27–29, for funnel plots).
Results of individual studies and synthesis of results
Prevalence of type 2 diabetes
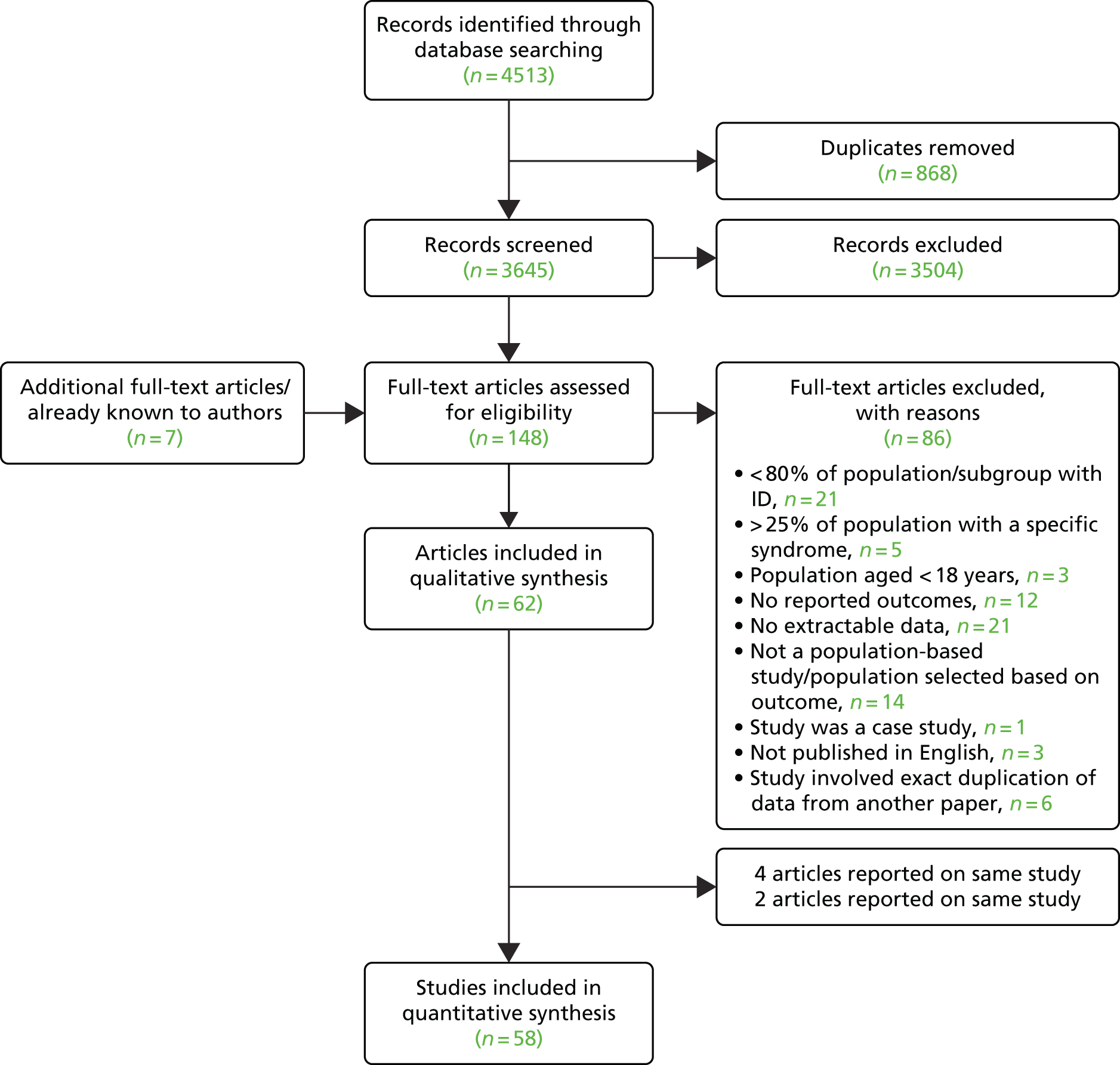
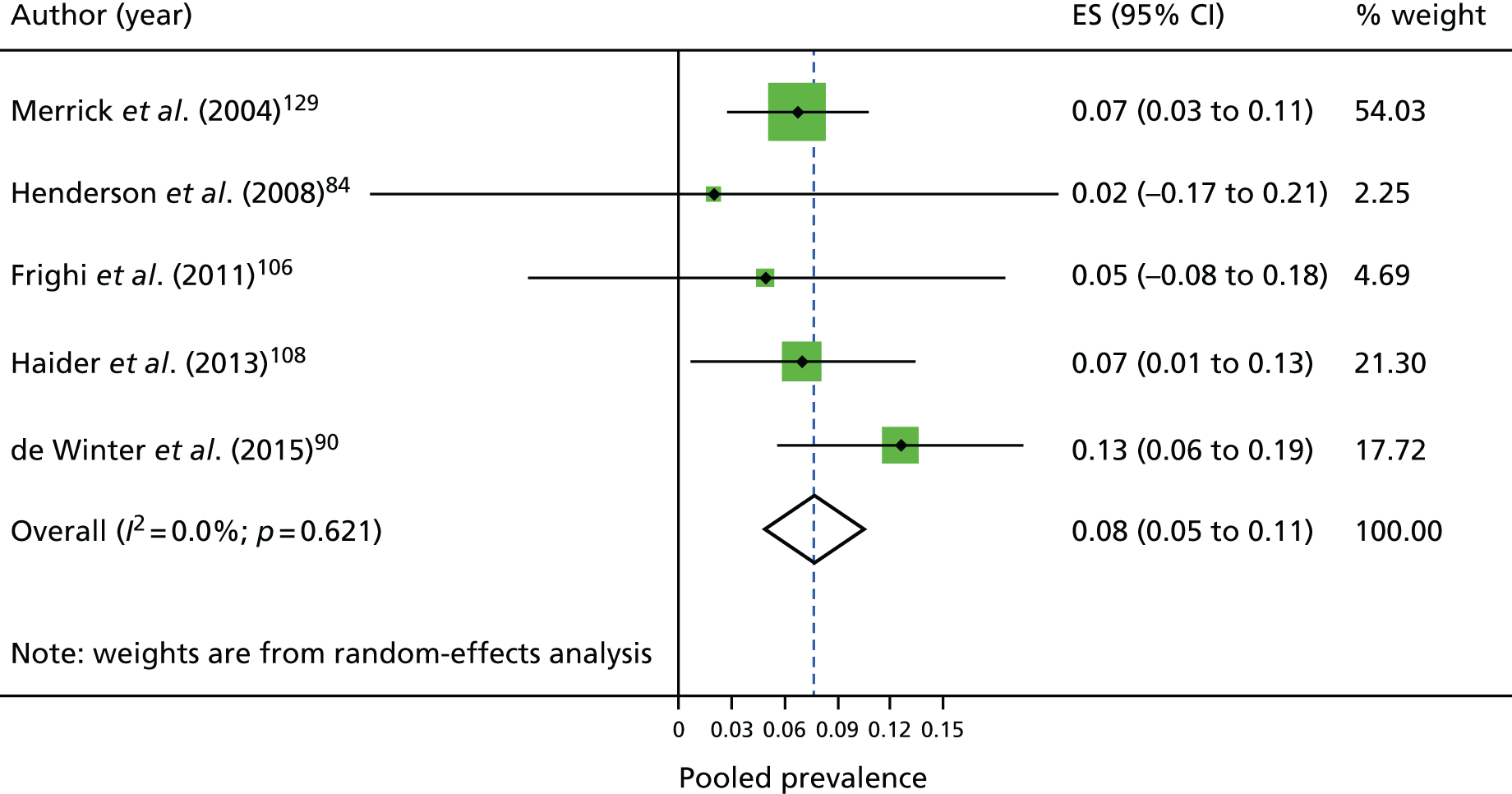
Figure 4 shows the individual studies reporting on the prevalence of T2DM and overall pooled prevalence. The prevalence estimates ranged from 2%84 to 13%. 90 The pooled prevalence of T2DM was 7.6%. The prevalence of any diabetes was 8.7%; this ranged from 2%84 to 11%95,102,117 (data not presented).
FIGURE 4.
Individual studies and pooled prevalence of T2DM. ES, effect size.
Prevalence of cardiovascular disease
Figure 5 shows the individual studies reporting on the prevalence of ischaemic heart disease. The prevalence estimates for ischaemic heart disease ranged from 0%140 to 12%. 126 The pooled prevalence of ischaemic heart disease was 3.7%.
FIGURE 5.
Individual studies and pooled prevalence of ischaemic heart disease. ES, effect size.

Similarly, Figure 6 shows the individual studies reporting on the prevalence of cerebrovascular disease. The estimates were fairly consistently in the < 1–4% range. The pooled prevalence of cerebrovascular disease was 2.2%. The pooled prevalence for undefined CVD was 10.6%, but ranged by individual study from 4%143 to 22%,114 reflecting the diverse case definitions.
FIGURE 6.
Individual studies and pooled prevalence of cerebrovascular disease. ES, effect size.
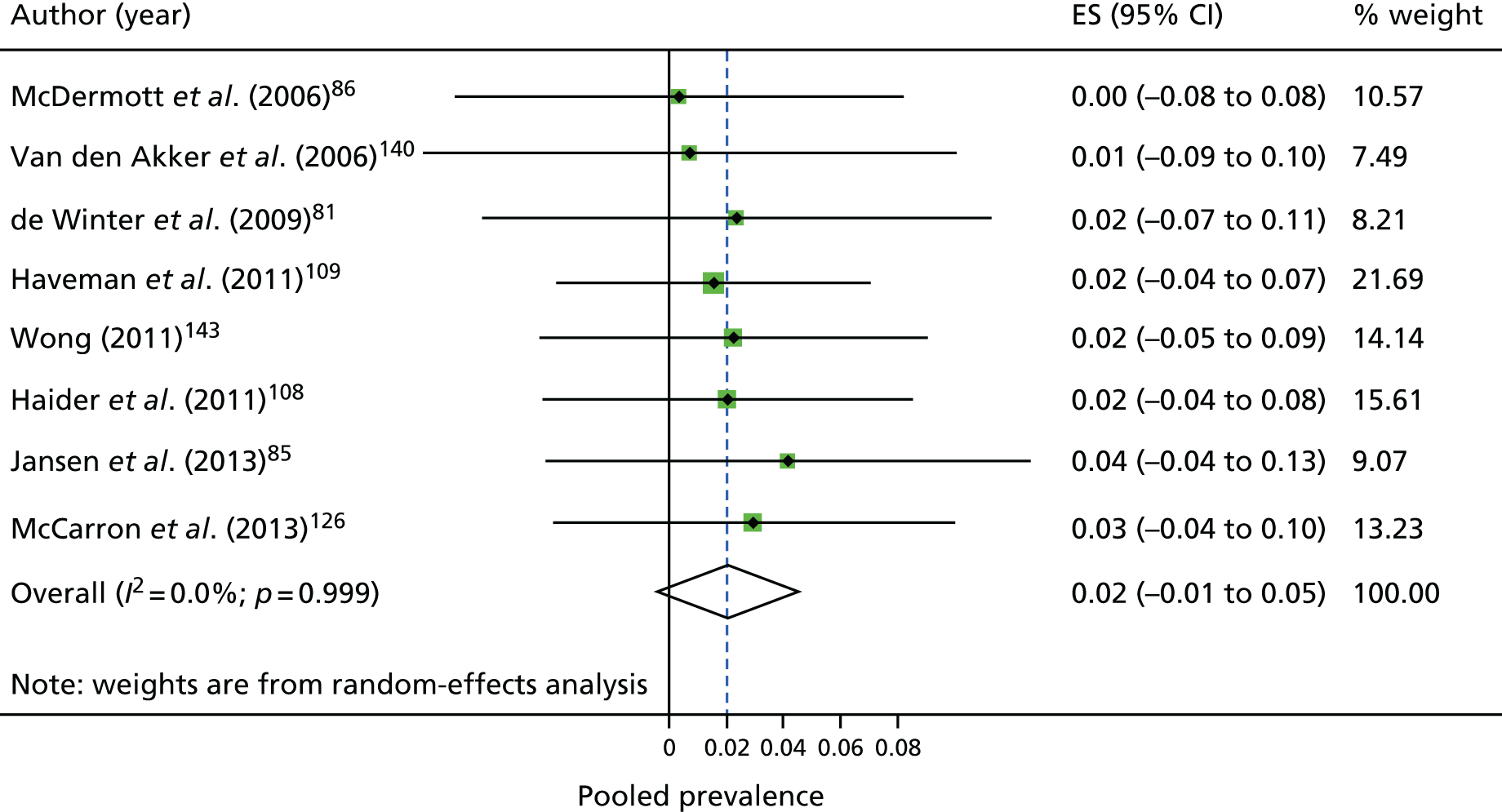
Prevalence of other risk factors
Table 4 summarises the findings from the individual meta-analyses. The overall estimated prevalence of hypertension was 18.5%. The estimated prevalence of overweight was 29.2%, the prevalence of obesity was 27.3% and the prevalence of BMI of ≥ 25 kg/m2 was 53.4%.
| Outcome | Study, n | Total, N | Total n with outcome | Pooled estimate (95% CI) | I2 (%) |
|---|---|---|---|---|---|
| Ischaemic heart disease | 9 | 5586 | 200 | 0.04 (0.01 to 0.06) | 0 |
| Cerebrovascular disease | 8 | 5748 | 114 | 0.02 (0.00 to 0.05) | 0 |
| Undefined CVD | 8 | 7773 | 881 | 0.10 (0.05 to 0.15) | 77.5 |
| T2DM | 5 | 4183 | 317 | 0.08 (0.05 to 0.11) | 0 |
| Any diabetes | 23 | 19,133 | 1636 | 0.09 (0.07 to 0.10) | 0 |
| Hypertension | 28 | 17,161 | 3008 | 0.19 (0.13 to 0.24) | 93.2 |
| Overweight | 32 | 24,923 | 7434 | 0.29 (0.26 to 0.33) | 89.5 |
| Obese | 35 | 27,274 | 7741 | 0.27 (0.23 to 0.32) | 93.6 |
| Overweight and above | 41 | 31,172 | 16,525 | 0.53 (0.49 to 0.58) | 96.4 |
| Hypercholesterolaemia | 9 | 3892 | 491 | 0.17 (0.08 to 0.26) | 86.9 |
| Metabolic syndrome | 3 | 821 | 287 | 0.23 (0.00 to 0.50) | 91.7 |
On making comparisons with the general population, we found that the population with ID had decreased odds of having ischaemic heart disease [odds ratio (OR) 0.44, 95% CI 0.34 to 0.58; p < 0.01]. No other statistically significant results were found, but we observed high heterogeneity for the other outcomes (Table 5).
| Outcomes | Study, n | ID total, N | ID total, n with outcome | GP total, N | GP total, n with outcome | OR (95% CI) | I2 (%) |
|---|---|---|---|---|---|---|---|
| Ischaemic heart disease | 3 | 2395 | 67 | 5441 | 335 | 0.44 (0.34 to 0.58)* | 0 |
| Any diabetes | 6 | 4014 | 411 | 13,404 | 1371 | 0.96 (0.61 to 1.5) | 92.2 |
| Hypertension | 6 | 3588 | 1097 | 14,262 | 4598 | 0.76 (0.58 to 0.99) | 86.9 |
| Overweight | 4 | 1487 | 477 | 17,819 | 5986 | 1.31 (0.47 to 3.66) | 96.5 |
| Obese | 7 | 3838 | 1004 | 23,230 | 6824 | 1.09 (0.65 to 1.82) | 95.3 |
Discussion
Summary of evidence
In this systematic review, we found that the prevalence of T2DM was 8% and of any diabetes was 9%. For CVD, the prevalences of ischaemic heart disease, cerebrovascular disease and undefined CVD were 4%, 2% and 10%, respectively.
The current prevalences of T2DM and CVD and associated risk factors in the population with ID were found to be similar to those in the general population. However, we found that ischaemic heart disease was significantly lower in the ID population. The metaregression showed that the method of data collection had minor effects on pooled diabetes and obesity. Mean age had minor effects on hypertension.
Strengths and limitations
A particular strength of this review is that we used robust methods. We wrote to authors to clarify and obtain additional data rather than excluding the articles. To our knowledge, this is the first systematic review and meta-analysis of prevalence of T2DM, CVD and associated risk factors in adults with ID. In addition, it is the first review of its kind to make comparisons with the general population.
However, we had limited data to separate T2DM from other diabetes, and we were sometimes restricted to unclear or poorly defined outcome measure definitions.
There were also limited data available to make comparisons with the general population. We would have benefited from additional general population data alongside the population with ID data to make more valid, generalisable comparisons.
Findings in relation to other studies
Two recent reviews75,76 have been conducted that have focused on diabetes prevalence among people with ID. The reviews found mean prevalences of 8.7%75 and 8.3%76 for combined gestational, type 1 diabetes mellitus and T2DM, respectively, but the reviews were unable to report on specific types of diabetes. The overall prevalence of CVD among people with ID is unclear. 52 However, our finding that ischaemic heart disease was significantly lower in the population with ID differs somewhat from the literature, which suggests that the prevalence of CVD among people with ID is greater than the general population. 52
Conclusions
Findings from the systematic review and meta-analysis presented in this chapter suggest that T2DM is at least as common in people with ID as in the general population. The findings also identify a gap in knowledge in relation to the prevalence of T2DM as many studies did not report this separately. In addition, none of the studies in our review reported on screen-detected T2DM in the population with ID.
Chapter 3 Systematic review of the effectiveness of multicomponent behaviour change interventions aimed at reducing modifiable risk factors
Overview
In this chapter, we describe the second of two systematic reviews conducted as part of the research programme. We present the existing evidence in relation to multicomponent behaviour change interventions that modify risk factors for T2DM and CVD in people with ID. The PRISMA checklist74 has been used to guide the reporting of this systematic review.
Rationale
Non-communicable diseases are on the rise globally and there is increasing demand for lifestyle behaviour change interventions to reduce morbidity, mortality and rising health costs. 146 The suggested mechanisms for this rise are increased availability of energy-rich foods and more sedentary lifestyles. 147 T2DM and CVD, and shared associated risk factors, are major contributors to morbidity and mortality. 148
Conditions such as CVD and T2DM share similar risk factors, including dyslipidaemia, hypertension, obesity and IGR. In the general population, these risk factors can be effectively lowered through interventions focusing on changes in nutrition and physical activity. 149–151 With a suggested increased risk of non-communicable diseases within populations with ID, special attention needs to be paid to the efficacy and effectiveness of multicomponent behaviour change interventions to reduce this disparity. However, there is a lack of quality evidence on the health and health care of people with ID, including the effectiveness of health interventions. 152 Previous systematic reviews of lifestyle behaviour change interventions in ID153–155 have generally been unable to make specific recommendations because of inadequacies in study design and conduct, a lack of theory basis for intervention and/or unclear reporting.
For the current review, we aimed to consolidate the evidence for the reduction of risk of T2DM and/or CVD through the delivery of multicomponent behaviour change interventions in the population with ID.
Objectives
The objectives were to establish the effectiveness of multicomponent behaviour change interventions:
-
in promoting weight loss in the population with ID
-
in reducing other modifiable risk factors for T2DM and/or CVD in the population with ID
-
aimed at primary prevention of T2DM or CVD, or reducing associated risk factors in the population with ID.
Methods
Protocol and registration
The systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO 2015: CRD42015020758). 156
Eligibility criteria
The review was guided by the PICOS model. 78 We defined the population as adults (aged ≥ 18 years) with ID (whole study population or a defined subsample). We defined the intervention as any multicomponent lifestyle behaviour change intervention aimed at primary prevention of T2DM or CVD, or a reduction in associated risk factors for people with ID and/or their carers. We included studies with and without comparison groups. We defined outcome measures as changes in anthropometric measures (weight, BMI, waist circumference), BP, lipid levels, glucose levels, physical activity levels, sedentary behaviour and dietary habits. The study design was defined as an experimental study [before-and-after study, randomised controlled trial (RCT) or non-RCT] with a follow-up period of at least 24 weeks or 6 months from baseline (to allow for the initiation and maintenance of medium- and longer-term behaviour change)157 (Table 6).
| PICOS elements | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Whole study population or defined subsample of adults (aged ≥ 18 years)a | |
| Intervention | Multicomponent lifestyle behaviour change intervention aimed at primary prevention of T2DM or CVD, or a reduction in associated risk factors (weight management, increasing physical activity/reducing sedentary behaviour, dietary improvement) | Interventions involving meal replacements or those aimed at increasing physical fitness (in isolation) as opposed to changes in levels of physical activity |
| Comparison | Studies without comparison groups were included | |
| Outcomes | Changes in anthropometric measures (e.g. weight, BMI, body fat, waist circumference), BP, lipid levels, physical activity, sedentary behaviour, dietary habits | |
| Study designs | Before-and-after study, RCT, non-RCT | Follow-up period of < 24 weeks/< 6 months from baseline |
All studies that had been published on or after 1 January 2000 (until 21 April 2015) and in the English language were eligible. Studies were limited to those published in or after the year 2000, when most large diabetes prevention trials were first published in the general population. 70
We contacted lead authors for further information when inclusion/exclusion could not be determined.
Information sources
We searched the electronic databases EMBASE, MEDLINE, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials and PsycINFO for this systematic review. The last date of the search was 21 April 2015. We searched the references lists of relevant systematic reviews and included papers within those for additional studies.
Search
We combined medical subject heading terms and key words for multicomponent lifestyle interventions and outcome measures and ID. The search was limited to English-language studies with cohorts of adults aged ≥ 18 years, depending on the database. Box 2 shows the MEDLINE search strategy.
-
(Behav* adj1 (Modif* or therap*)).ti,ab.
-
Cognitive* therap*.ti,ab.
-
(Health* adj2 (Educat* or promot* or behav*)).ti,ab.
-
Educat* adj2 program*.ti,ab.
-
(Diet* adj2 (Intervention* or modif* or therap*)).ti,ab.
-
(Health* adj2 Eating).ti,ab.
-
(Nutrition* adj2 (intervention* or modif* or counsel* or therap*)).ti,ab.
-
(Exercis* adj2 (intervention* or therap*)).ti,ab.
-
(Physical adj (education or fitness or activit* or training or exercise)).ti,ab.
-
(Lifestyle adj2 (advice or guidance or modif* program* or interven*)).ti,ab.
-
(Weight adj2 (control* or los* or reduc* or maintenance or management)).ti,ab.
-
Weight adj loss adj program*.ti,ab.
-
Exercise*.ti,ab.
-
Sport*.ti,ab.
-
exp Health Promotion/
-
exp Nutrition Therapy/
-
exp Exercise Therapy/
-
(Sedentary adj (behav* or lifestyle* or individual* or population*)).ti,ab.
-
or/1-18
-
exp Intellectual disability/
-
((learning or development* or intellectua* or mental*) adj1 disabilit*).ti,ab.
-
(impair* adj2 intellectual adj2 function*).ti,ab.
-
(mental* adj1 (impair* or handicap*)).ti,ab.
-
Exp mentally disabled persons/
-
(mental* adj2 retard*).ti,ab.
-
Or/20–25
-
19 and 26
-
animal/not (animal/and human/)
-
27 not 28
-
limit 29 to english language
-
limit 30 to yr=2000-current
Study selection
Full texts were identified after titles and abstracts were read separately by two investigators (TC and AD), who discussed discrepancies in selection at a later meeting.
After retrieval of the full-text articles, the papers were again examined separately by two investigators (TC and AD) to check their suitability for inclusion.
Data collection process
We created a data extraction form for this review. The data were extracted by one investigator (TC) and verified for accuracy by another investigator (AD).
Data items
For each study, we collected the first author’s name, title of paper, year of publication, country of the cohort, study design, sampling method, intervention details, dates of data collection and the intended recipient of the intervention. For the whole study population (and for each group, if applicable), we extracted data on total sample size or subpopulation size, mean ages, proportion of males/females, severity of ID, ethnicity and withdrawals.
For each reported outcome, we extracted information on how outcomes were defined and measured, the total number measured for each outcome, length of follow-up, mean baseline and post-intervention value, mean between-group change and/or change baseline to follow-up along with a measure of variability [standard deviation (SD), standard error (SE), etc.]. We extracted data separately for males and females, when reported.
Quality assessment
The NICE quality appraisal checklist for quantitative intervention studies158 was used to assess the quality of the selected studies. The checklist included criteria for assessing the internal and external validity of experimental and observational quantitative studies (RCTs, non-RCTs, before-and-after studies) and allowed assignment of an overall quality grade (categories ++, + or –). Studies were assessed by one reviewer (TC) and verified for accuracy by a second reviewer (RS).
Risk of bias in individual studies
Prior to carrying out this systematic review, we anticipated using funnel plots79 and the Egger’s test80 to examine potential publication bias in the literature for the collected outcomes. However, owing to the small number of studies included in this review resulting in low power to detect bias, these methods were not used.
Data synthesis
Data synthesis for this review involved describing the study characteristics (country, population size, age, percentage male, ethnicity, severity of ID, eligibility criteria, outcomes report and follow-up period) of the included articles. We then described the details and behavioural strategies of each of the multicomponent lifestyle behaviour change interventions, including their structure and delivery, and the underlying theory behind each of the interventions. Finally, we described the outcome measures and study findings. Given the low number of studies included, a formal evidence synthesis was not undertaken.
Results
Study selection
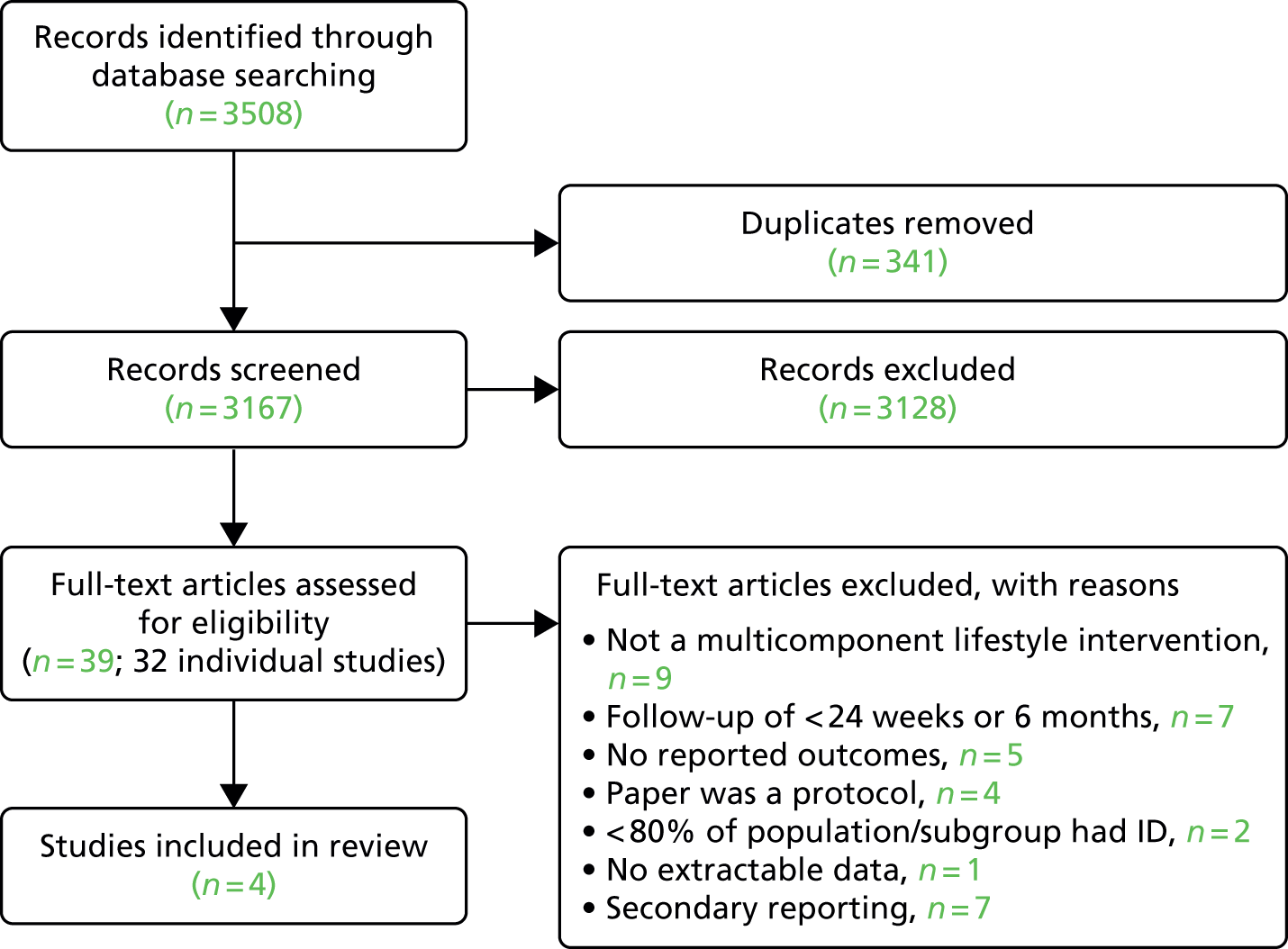
The literature searches yielded 3508 articles. After duplicates were removed, 3167 articles remained to be screened. We retrieved and reviewed the full text of 39 articles for 32 studies (Figure 7). Most potentially relevant studies were noted to have small sample size, short follow-up, high attrition rates and/or incomplete data for key outcomes. We contacted the authors of four study protocols159–162 for further information. One of the authors did not reply, and we were informed by the remaining three that their study results were still awaiting publication and could not be included in the review. In total, we identified only four studies163–166 for inclusion in this review.
FIGURE 7.
Study selection.
Study characteristics
The four studies163–166 included in the systematic review presented data on 700 individuals. The characteristics of each of the studies163–166 are presented in Table 7.
| First author (year) | Country | N (n after dropout) | Mean age (SD), years | Male (%) | Ethnic group (%) | Mean BMI (kg/m2) | Eligibility criteria | Severity of ID | Key outcomes reported | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|---|
| Bazzano (2009)163 ‘Healthy Lifestyle Change Program (HLCP)’ | USA | 68 (44) | 44.0 | 38.6 | White (64), black African (21), other (16) | 33.3 | Aged 18–65 years High-functioning ID, BMI of ≥ 25 kg/m2, diabetes or risk factors for diabetes [including hypertension, hyperlipidaemia, family history, hyperglycaemia, ethnicity (non-white), aged > 45 years] |
NR | BMI, weight, waist circumference, physical activity (frequency and duration) | 7 months |
| Melville (2011)164 ‘TAKE-5 STUDY’ | UK | 54 (47) | 48.3 | 40.7 | White (97), Pakistani (2), other Asian (2) | 40.0 | Aged ≥ 18 years, BMI of ≥ 30 kg/m2, ambulatory Excluded: Prader–Willi syndrome |
MILD 31.5%, MOD 31.5%, SEV 35.2%, PROF 1.9% | BMI, weight, waist circumference, sedentary and, physical activity (minutes/day, accelerometer) | 24 weeks |
| McDermott (2012)165 ‘Steps To Your Health (STYH)’ | USA | 443 (196) | 38.8 | 49.2 | White (42), black (57), Hispanic (1), other (1) | 32.4 | Aged 18–65 years, voluntary participation, ambulatory and communicative, mild to moderate ID, residence in independent or supported settings Excluded: underweight (BMI of < 18.5 kg/m2) |
NR | BMI, MVPA (accelerometer) | 12 months |
| Bergström (2013)166 | Sweden | 130 (129) | Intervention, 36.2 (10.1); control, 39.4 (11.3) | 43.1 | NR | Intervention, 30.0; control, 28.5 | Adults: mild to moderate ID; three or more residents | NR | BMI, weight, waist circumference, physical activity (steps/day pedometer) | 12–16 months |
The studies163–166 covered three countries (USA, UK and Sweden). Two studies163,164 were single arm, and two studies165,166 had a control group. All of the four studies163–166 reported data for physical activity and/or sedentary behaviour.
The mean age was 42.2 years and the mean percentage of male participants was 42.9%. The mean group size was 174 before dropout and 104 after dropout. Group sizes ranged from 54 to 443 before dropout, and from 44 to 196 after dropout. The majority of participants were white (68% where known); approximately one-quarter (26%) were black and the remaining 7% were from other ethnic groups. Only one study164 provided information on severity of ID, but, based on eligibility criteria, the remaining studies were likely to comprise adults in the mild to moderate ID range. Other descriptive information for each study is presented in Table 7.
Study quality
A breakdown of study quality is presented in Table 8. The studies163–166 were generally of high quality; in particular, all of the studies achieved at least a good quality rating for internal and external validity. However, two163,165 of the four studies failed to account for all of the participants when concluding the study, and three163–165 of the four studies did not report on whether or not the studies were sufficiently powered to detect differences.
| Section | Bazzano (2009)163 | Melville (2011)164 | McDermott (2012)165 | Bergström (2013)166 |
|---|---|---|---|---|
| 1. Population | ||||
| Source population/area well described? | + | ++ | ++ | ++ |
| Eligible population/area representative of source population/area? | ++ | + | ++ | ++ |
| Selected participants/areas represent eligible population? | + | ++ | ++ | + |
| 2. Method of allocation to intervention (or comparison) | ||||
| Allocation to intervention (or comparison). Was selection bias minimised? | NA | NA | ++ | ++ |
| Interventions (and comparisons) well described and appropriate? | ++ | ++ | ++ | ++ |
| Was allocation concealed? | NA | NA | NR | ++ |
| Participants or investigators blind to exposure and comparison? | NA | NA | NA | NA |
| Exposure to intervention appropriate? | ++ | ++ | ++ | ++ |
| Contamination acceptably low? | NA | NA | ++ | ++ |
| Other interventions similar in both groups? | NA | NA | ++ | ++ |
| Participants accounted for at study conclusion? | – | ++ | – | ++ |
| Did setting reflect usual UK practice? | ++ | ++ | ++ | ++ |
| Did intervention or control comparison reflect usual UK practice? | ++ | + | + | ++ |
| 3. Outcomes | ||||
| Outcome measures reliable? | + | + | ++ | + |
| All outcome measurements complete? | ++ | ++ | + | – |
| All important outcomes assessed? | ++ | ++ | ++ | ++ |
| Outcomes relevant? | ++ | ++ | ++ | + |
| Similar follow-up times in exposure and comparison groups? | NA | NA | ++ | + |
| Follow-up time meaningful? | + | ++ | ++ | ++ |
| 4. Analyses | ||||
| Exposure and comparison groups similar at baseline? If not, were these adjusted? | NA | NA | NR | ++ |
| ITT analysis conducted? | – | – | ++ | ++ |
| Sufficiently powered to detect an intervention effect (if one exists)? | NR | NR | NR | ++ |
| Estimates of effect size given or can be calculated? | NR | ++ | ++ | + |
| Analytical methods appropriate? | + | – | ++ | + |
| Precision of intervention effects given or able to be calculated? Were they meaningful? | + | ++ | ++ | ++ |
| 5. Summary | ||||
| Study results internally valid? (i.e. unbiased) | + | + | ++ | + |
| Findings generalisable to the source population? (i.e. externally valid) | ++ | + | ++ | + |
Results of individual studies and descriptive data synthesis
Table 9 summarises the multicomponent lifestyle behaviour change interventions evaluated in the individual studies.
| First author (year) | Key elements of intervention | Structure of intervention (number and length of sessions) | Who delivered intervention | Where intervention delivered | Goal-setting | Theory |
|---|---|---|---|---|---|---|
| Bazzano (2009)163 |
Diet, exercise and behaviour modification
|
Twice-weekly, 2-hour sessions (for 7 months) Each class included 50 minutes of health education and 1 hour of supervised physical activity Outcomes assessed at baseline and at 7 months |
Professionals with ID expertise with assistance from peer mentors | Community organisation | NR | Based on Bandura’s social cognitive theory of health behaviour change167 |
| Melville (2011)164 |
Weight loss, diet and exercise
|
Nine sessions, every 2–3 weeks (40–60 minutes each) 24-week follow-up |
Two health professionals (dietitian and sports medicine graduate) with experience working with individuals with ID | Participant’s home | Individual goal-setting regarding weight loss, dietary change and physical activity | NR |
| McDermott (2012)165 |
Diet, exercise and stress reduction
|
Eight weekly sessions (90 minutes each) Data collected at baseline, 9 weeks after completion, 6 months and 1 year |
Health educator with experience working with adults with ID | Community venue | NR | Based on Bandura’s social cognitive theory of health behaviour change167 |
| Bergström (2013)166 | Diet and exercise modification Three components:
|
12–16 months to complete programme Health ambassadors: six network meetings (3 hours each) Study circle: 10 sessions (90 minutes each) Health course: 10 sessions Outcomes assessed at baseline and at end of intervention (12–16 months from baseline) |
Health ambassador Member of staff from residence Course leader from national educational association for adults |
Community residential homes | NR | Based on Bandura’s social cognitive theory of health behaviour change167 |
Bazzano et al. 163 conducted a single-arm before-and-after intervention in already overweight or obese individuals (BMI of ≥ 25 kg/m2). The intervention involved peer mentoring, one-to-one health education, supervised physical activity and clinical support aimed at reducing weight, improving diet and increasing physical activity.
Melville et al. 164 also conducted a single-arm study in already-obese individuals (BMI of ≥ 30 kg/m2) who had been referred to a dietitian by their GP. Nine lessons, every 2–3 weeks, were provided for participants and their carers. The lessons were aimed at increasing physical activity and better diet, as well as weight loss. Interventions also consisted of personalised diet plans with calorie restrictions [600 kilocalories (kcal) per day].
Bergström et al. 166 conducted a two-armed trial in community residential homes, targeting both people with ID and their carers. The intervention offered a ‘study circle’ for carers and also an appointed health ambassador at each residential home. An educational health course for the residents was also provided. The community residences in the control arm received the option to take part in the intervention after study completion (wait list control). The primary outcome for this trial was increasing physical activity; the secondary outcomes were decreasing weight and BMI.
Finally, McDermott et al. 165 conducted a two-arm RCT. Intervention participants were assigned to eight weekly lessons in nutrition, exercise and changing ways of thinking. The lessons focused on stress management, complications of obesity and behaviour management. The classes emphasised MVPA, healthy eating and BMI reduction. The control group was assigned to eight weekly lessons on safety and hygiene.
Table 10 summarises the components of the individual behaviour change interventions. All of the interventions used both dietary and exercise components.
| Component | Bazzano (2009)163 | Melville (2011)164 | McDermott (2012)165 | Bergström (2013)166 |
|---|---|---|---|---|
| Dietary | ||||
| Energy restriction | 600 kcal/day | |||
| Weight loss target | 5% of initial body weight | |||
| Nutrition advice | ✓ | ✓ | ||
| Try healthy foods in session | ✓ | ✓ | ✓ | |
| National recommendations | ✓ | |||
| Healthy dietary habits | ✓ | |||
| Portion sizes | ✓ | |||
| Individualised diet plan | 50% carbohydrates < 35% fats < 20% protein |
|||
| Individualised diet goals | Set one goal per week | |||
| Exercise | ||||
| Individualised exercise goals | Walking targets (using pedometer) Set one goal per week Minimum 30 minutes of moderate intensity physical activity at least 5 days per week |
|||
| Advice regarding time and intensity | Advice on replacing sedentary behaviour for activities in the home (e.g. housework) | |||
| Supervised activity in session | 1 hour during each session Use of local parks and fitness facilities Exercise video created by peer mentors |
Sessions followed by optional brisk walk | Physical activities in sessions | |
| Information provided regarding local leisure facilities | ✓ | |||
Of the four included studies,163–166 the two single-arm studies,163,164 with follow-ups of 7 months163 and 24 weeks,164 indicated significantly improved outcomes; reductions in weight, BMI and waist circumference were demonstrated after the implementation of a behaviour change intervention programme aimed at increasing physical activity and improving diet. Additionally, both studies163,164 demonstrated a significant improvement in physical activity outcomes, specifically for ‘minutes per week’ and ‘frequency of sessions’163 and ‘reduction in sedentary behaviour’. 164 Both cohorts163,164 were overweight to obese when they were enrolled into the study. For the further two studies,165,166 for which the cohort was not recruited based on health status, one of the studies166 showed significant positive improvements in waist circumference, BMI and steps per day in those who received the intervention compared with the control subjects; the second study165 did not show any significant differences between control and intervention arms (Table 11). 165
| Author and year | BMI (kg/m2) | Weight (kg) | Waist circumference (cm) | Vegetable intake (servings per day) | Physical activity/sedentary behaviour |
|---|---|---|---|---|---|
| Bazzano (2009)163 | |||||
| (a) Minutes per week;a (b) sessions per weeka | |||||
| Baseline | 33.3, n = 44b | 88, n = 44b | 104.9, n = 39 | 2, n = 44 | (a) 133; (b) 3.2; n = 44 |
| Follow-up, 7 months from baseline) | 32.8, n = 44 | 86.8, n = 44 | 102.6, n = 39 | 2.2, n = 44 | (a) 206.4; (b) 3.9; n = 44 |
| Intervention group change | –1.5%* | –1.34%* | –2.18%** | 10% | (a) 54.89%;** (b) 21.88%** |
| Melville (2011)164 | |||||
| (a) Sedentary minutes per day; (b) low physical activity minutes per day; (c) MVPA minutes per day (accelerometer) | |||||
| Baseline (SD) | 40 (8.03), n = 47 | 100.6 (26.8), n = 47 | 122.1 (15.7), n = 47 | NR | (a) 623.3 (121.5); (b) 73.4 (46.8); (c) 14.2 (17.5); n = 45 |
| Follow-up (24 weeks from baseline) (SD) | 39.2 (8.2), n = 47 | 96.1 (26.9), n = 47 | 115.8 (16.7), n = 47 | NR | (a) 581.9 (116.4); (b) 81.3 (45.6); (c) 17.8 (17.3); n = 33 |
| Intervention group change | –4.45%** | –4.55%** | –5.15%** | NR | (a) –6.64%;* (b) 10.76%; (c) 25.42% |
| McDermott (2012)165 | |||||
| MVPA ratio: minutes performed/minutes worn (accelerometer) | |||||
| Baseline (SD) | 32.38 (6.85), n = 437 | NR | NR | NR | 3.24 (3.93), n = 401 |
| Follow-up, 12 months from baseline) (SD) | 32.13 (6.59), n = 195 | NR | NR | NR | 4.62 (3.27), n = 118 |
| Intervention group change | –0.78% | NR | NR | NR | –4.18% |
| Bergstrom (2013)166 | |||||
| Steps per day (pedometer)a | |||||
| Baseline (SD) | 30 (7.6), n = 126 | NR | 94.5 (16.5), n = 124 | 1.4 (0.6), n = 101 | 8042 (5524), n = 99 |
| Follow-up (12–16 months from baseline) | 29.7, n = 108 | NR | 92.8, n = 103 | 1.6, n = 66 | 9650, n = 69 |
| Intervention group change | –1%a | NR | –1.8%a | 14.29% | 19.99%* |
Discussion
Summary of evidence
This review contributes to the existing knowledge on the effectiveness of multicomponent lifestyle behaviour change interventions in adults with ID. Three of the interventions included in this review led to some reductions in BMI, weight and waist circumference,163,164,166 but inferences are limited owing to small sample sizes, missing data, selected populations and/or lack of control groups.
Strengths and limitations
To our knowledge, this is the first systematic review and meta-analysis focusing on long-term multicomponent behaviour change interventions for people with ID in order to reduce CVD and/or T2DM risk. We used robust methods and sought additional information from authors where relevant. However, only four papers163–166 met our inclusion criteria. Significant findings were observed only for the single-arm studies, which are known to overestimate effect sizes. 168 We were unable to test for publication bias or to carry out meta-analytical work to explore combined effects, particularly as the interventions that were evaluated were so diverse. Similarly, research has shown that improvements in health can be difficult to sustain in the longer term;169 only two of the included studies165,166 had a follow-up period of at least 12 months, and even this may not be enough to indicate long-term sustained benefits.
Findings in relation to other studies
In line with previous systematic reviews in this area,153–155 findings from this systematic review demonstrate a lack of quality evidence on the effectiveness of multicomponent behaviour change interventions in people with ID. In 2010, Jinks et al. 153 focused a systematic review on qualitative studies of behavioural change approaches in people with ID to aid weight loss and health. The review found 12 papers, of which only one was qualitative. The authors noted an overall lack of research on behavioural approaches and using qualitative methods. Similarly, in 2013, Spanos et al. 154 reviewed 22 papers that assessed interventions for weight loss in people with ID. They noted that many of the interventions did not meet the recommended duration in clinical guidelines and were too specific. Brooker et al. 155 also reviewed interventions with a primary focus on physical activity in people with ID. Again, the review noted small sample sizes and invalid measurement tools, and recommended further longer-term intervention studies.
Implications of findings
This systematic review informed the evidence base for the development of the STOP Diabetes educational programme, which is described in Chapters 8 and 9. The studies also revealed a high rate of missing follow-up data for participants who completed the multicomponent lifestyle behaviour change interventions, which helped to inform further development work on feasibility testing (see Chapter 10). The wider implications for research and practice are discussed in Chapter 13.
Conclusions
The findings from this systematic review have provided some evidence that multicomponent behaviour change interventions may be beneficial in modifying risk factors for T2DM and CVD in people with ID. However, there is a paucity of literature on their long-term effects in this population. In keeping with existing recommendations,154 we highlight the need for robust RCTs to evaluate the long-term effects of multicomponent behaviour change interventions, informed by current guideline recommendations, for people with ID.
Chapter 4 Service user involvement
Overview
This chapter details the service user involvement throughout the STOP Diabetes research programme. Involvement was integrated into the research from the early stages.
Introduction
The involvement of service users in research is central to UK policies170,171 and is becoming increasingly common, both nationally and internationally. 172–175 The benefits of such involvement in health and social care research are manifold. Service users can provide valuable knowledge and insights to research,176–181 encourage recruitment through publicity,177,179,180 improve quality, relevance and impact of research,182–186 and potentially help to meet recruitment targets. 172 Service users in England contribute financially to publicly funded research, so, arguably, have a right to be involved186,187 and can personally benefit from their involvement. 183,185,188 However, challenges to the successful involvement of service users in research include contrasting priorities,183,189,190 understanding of research methods,189 use of language and jargon,189 and lack of time and resources. 183,189
The involvement of people with ID in research can pose additional challenges to those outlined above and include the need to plan ahead, allow time for effective communication and regular breaks, and ensure that meeting locations are accessible to all. 191–194 Such challenges can be at odds with researchers’ own demands and priorities,195 and they often resort to seeking the proxy views of ‘sympathetic others’, such as parents or carers,196 which is disappointing given that people with ID have a lot to say and can improve the quality and relevance of research. 195
Involvement prior to submitting the research proposal
Before submitting the research proposal, members of the team visited three local ID partnership boards to discuss the study, invite feedback, discuss how adults with ID could be involved in the research process, and advise on reasonable adjustments and practical considerations. The boards comprised a mix of professionals and public members, including councillors, commissioners, clinicians, charity representatives, police officers, family carers, paid carers and people with ID. The boards provided useful advice on tailoring information sheets to service users (e.g. using pictures as well as text, using a larger font, and modifying the size and colour of the paper for those with visual impairment) and on communication issues (e.g. using a staged, step-by-step approach to delivering information). The team also began forming links with two local self-advocacy groups for people with ID: both groups met at least monthly in a central location and were led by an experienced facilitator whose role was to ensure that members understood what was being discussed; they had every opportunity to give their views and contribute to the discussion, and only one person spoke at a time.
Involvement during the research programme
Selection of service users for involvement
Service users were approached from different sources to encourage a diverse range of views and to minimise burden. Members from the two self-advocacy groups approached the Speaking up for Health Group and the Charnwood Action Group, who agreed to help the team with the study. In addition, the manager and residents of a communal care establishment were approached through the lead nurse’s contacts, and they agreed to help us with the study. Service users who entered the poster competition (see next section) were indirectly involved by providing publicity materials for the team (Figure 8).
FIGURE 8.
Service users involved in the research programme. Charnwood logo from Charnwood Action Group Partnership Board.
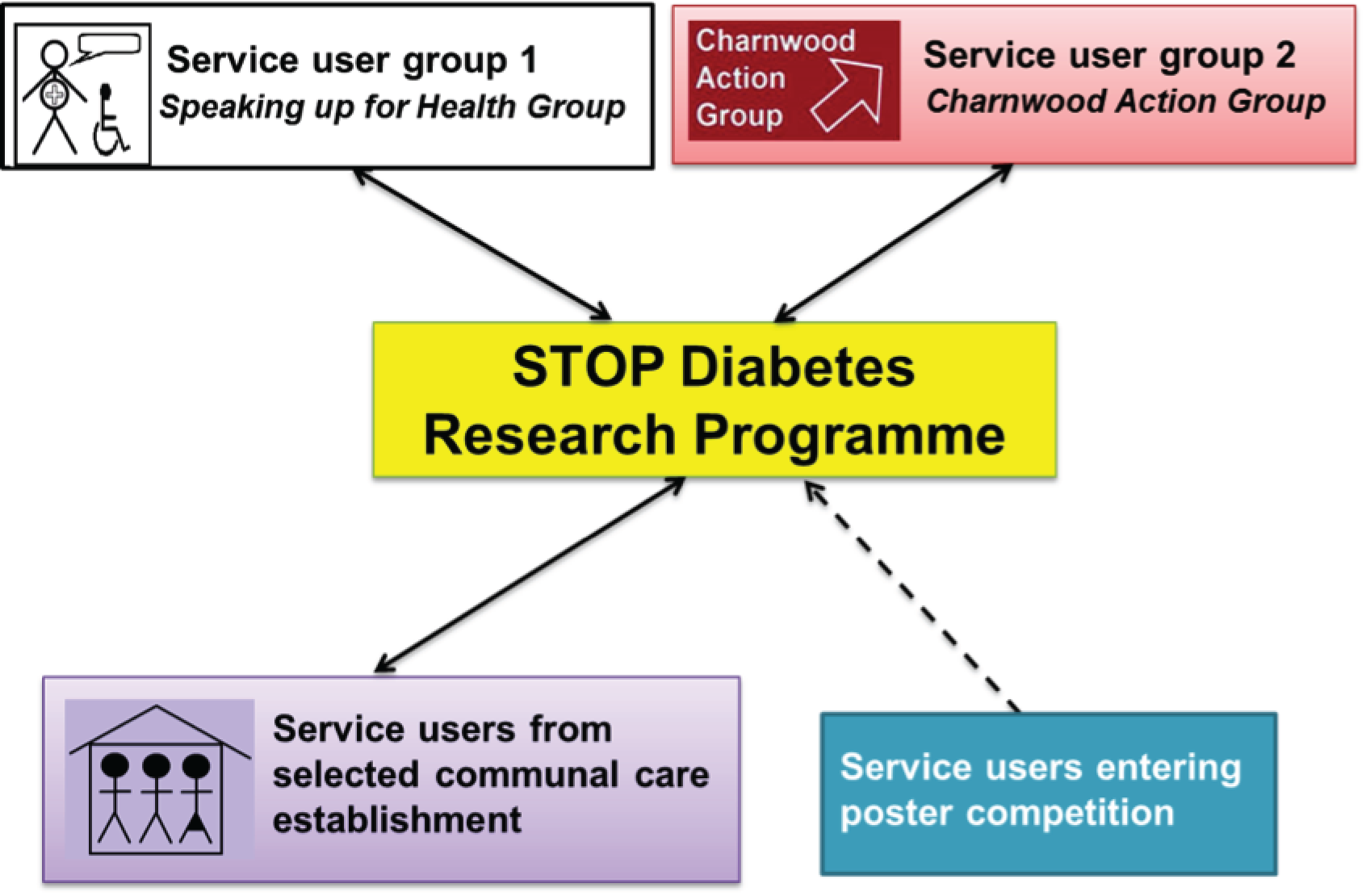
Service user involvement in the programme management
A common way of involving service users in research is through representation in steering group meetings,197 and we discussed the potential for this with the service users. We considered tailoring these meetings to make them more accessible, but past experience suggested that they could be lengthy, involving complex discussions about procedures, accelerometer data, health economics and statistical methodologies, and often used conference call facilities. We were concerned that the meetings would be isolating for the service users and their supporters so, instead, we agreed to feed back key points from the meetings and that service users could attend on an ‘ad hoc’ basis.
Service user involvement in promoting the research programme
The study involved a Leicestershire-wide screening programme and it was important to promote the research as widely as possible. Among other considerations, the study logo and publicity materials needed to be suitable and appropriate for the target population. Both of the service user groups that we approached used ‘word police’ cards, which were shown whenever another member of the group or visitor used an acronym or abbreviation that they did not understand. Therefore, the proposed use of an acronym for the programme was not received favourably and, instead, the team opted to call the programme the ‘STOP Diabetes study’. The creative director subsequently devised four corresponding logos (Figure 9) and asked the Charnwood Action Group for their preference, using a feedback form with a scale and pictures. The preferred logo (see Figure 9, option 4) was shown to the Speaking up for Health Group, and there was discussion about ways in which the logo and other publicity materials, such as posters and fliers, could be used to publicise the programme. Service users recommended printing the STOP Diabetes logo on notepads, pens and fridge magnets. When directly asked, they also thought that the logo should be printed on the study documentation, such as information leaflets and consent forms. Members of the group also suggested holding a poster competition as a means of publicising the programme.
FIGURE 9.
Proposed logos for the STOP Diabetes programme. Option 4 was the service users’ preferred logo.

All of the ideas were collated and reported back to the research team for further discussion and to determine if sufficient resources were available to action them. All of the suggestions were taken up by the research team.
Service users were invited to enter the poster competition using brief easy-read information distributed by staff at local day centres, health clinics and other organisations. People who entered the competition were given a certificate and a small award of art and craft materials. Four of the pictures were chosen for the promotional materials (Figure 10); this decision was made by both service users and members of the research team.
FIGURE 10.
Some of the artwork submitted for the poster competition. The four pictures on the left were used for the publicity materials.

The research programme was publicised via the Leicestershire Diabetes Centre website and was also published in the National Institute for Health Research INVOLVE Summer 2013 newsletter,198 both as means of raising awareness about the programme and sharing our experiences of service user involvement (Figure 11). Similarly, having attended one of the programme steering group meetings, one of the co-chairpersons of the Charnwood Action Group contacted the media and was consequently interviewed about the programme (radio and newspaper199), which helped with recruitment.
FIGURE 11.
Service users’ involvement in promoting the research programme. Image of article ‘Volunteers needed for study’ reproduced with permission of Leicester Mercury. Copyright © 2016 Local World. All rights reserved. Image of article ‘STOP Diabetes study’ reproduced with permission of INVOLVE. Copyright © INVOLVE. All rights reserved 2015.

Service user involvement in study documentation and process development
It was important that information about the programme should be available in simple language, free from jargon and acronyms, and using pictures and symbols, so that potential participants had every opportunity to understand what the research team were doing and reach an informed decision about whether or not to take part. Prior to submission to the research ethics committee, the research team, with support from local ID services, drafted an easy-read (symbols and words) information sheet and consent form to partnership boards, local ID services and service user groups for feedback on the symbols, text and whether or not additional communication aids might be necessary.
The service users who we asked to read the easy-read documents did not report any difficulties in understanding them, but recommended additional modes of communication, such as flash cards and story cards. A member of the Speaking up for Health Group assisted the team by taking photographs to illustrate the information leaflet, flash cards and story cards; three service users modelled for the photographs (Figure 12).
FIGURE 12.
Service users’ involvement in assisting with study documentation.

Service user involvement in staff recruitment
Research nurses were integral to successful recruitment to the study, needing to be patient, sensitive and responsive to participants’ needs (e.g. seeing participants outside typical working hours), as well as able to communicate effectively with people with ID. Two members from the Speaking up for Health Group offered to help with the interview process for recruiting nurses into the research programme. Supported by their group facilitator, they created two questions to assess how good the nurses were at communicating with people with ID, and how they might adapt their style of communication if that person did not understand them. On the day that the nurses were interviewed, the service users asked these questions in a separate room, with the facilitator present. They then rated the nurses’ responses on a 4-point scale (Figure 13). Their input helped to reinforce the panel’s decision on who to recruit and was particularly valuable in helping the panel to decide between two similar applicants.
FIGURE 13.
Service users’ rating form for recruitment of nurses.
Service user involvement in training staff and assessing acceptability of measures
Service users at the participating communal care establishment helped to train staff by allowing them to practise communication-based interactions, consent-taking and measurement collection. Service users from one of the self-advocacy groups were invited to attend a follow-on staff training session so that nurses could put their new skills into practice. They gave feedback on nurses’ skills and discussed what they liked and what they did not like, enabling staff to gain confidence and develop competency in various procedures. The mock clinics also helped the research team to determine how long the appointments might take and how many visits may be needed. The service users reported that some of the questionnaires were too lengthy and complex. The team met to discuss the issues raised and made changes to reduce participant burden: these included swapping one of the questionnaires [Psychiatric Assessment Schedules for Adults with Developmental Disabilities (PAS-ADD) mini200 for the PAS-ADD checklist200] and removing two questionnaires (Dietary Instrument for Nutrition Education201 and International Physical Activity Questionnaire202) entirely.
For the research programme, and to help with the design of the anticipated future trial, two members of the Speaking up for Health Group and one member of the Charnwood Action Group wore the activity monitors (both wrist- and waist-worn monitors) and provided feedback on their ease of use.
Service user involvement during final stages of programme
The service user groups were involved in the discussions around disseminating the findings and identifying relevant conferences. During the consent process, participants were asked if they wished to be informed of the findings. As a means of supporting this, and to acknowledge the group homes that had allowed residents to take part in the study, two of the research nurses visited 27 homes to present the findings in an easy-read format. Other participants received an easy-read report posted to them.
Discussion
This chapter discusses the involvement of service users with ID into our research programme and draws on our own published work arising from this study. 203 In line with previous service user initiatives, the impact of involving service users in the research study is difficult to quantify. 204,205 We feel that involvement of service users improved the quality of, and recruitment for, our study, but we do not know what would have happened had we not involved them, and there are no similar studies in the UK on which to draw comparisons.
We can say with certainty that the team benefited from the involvement, developing a greater understanding of the health and personal issues faced by people with ID. The team also received positive comments from the service users, particularly in relation to being involved in the interview panel process and visiting our study offices. In line with previous research,206 we found that people with ID valued the opportunity to discuss health issues. Unusually for service user involvement initiatives, our service users were also allowed to take part in the research (because it was a screening programme); the fact that many also chose to be participants in the programme is testament to their commitment.
The service users’ involvement in the research programme was collaborative and not participatory (or ‘emancipatory’), which is favoured by many disability academics. 207 The agenda was set by the researchers and final decisions were always made by the lead investigator. Established self-advocacy groups contributed hugely to the success of the involvement because there was an established group dynamic and all of the service users were keen to discuss health issues and voice their own opinions. As involvement initiatives expand, there is a danger that self-advocacy groups will become inundated with requests for support,208 so we need to ensure that we widen our approach to involvement for future studies. We also encountered problems when we discussed paying the service users for their contribution, because they were concerned about loss to their benefits, and we came across organisational restrictions. For future work, we aim to consider more innovative group payments, such as water coolers or coffee machines, with prior organisational approval.
We reiterate the recommendations from INVOLVE: that involvement should commence at the early stage of the research process when identifying and prioritising topics for research. 209 We had limited involvement at this stage of the programme and further involvement is likely to have improved the quality of our application and reduced the need to make changes once the study had started. When involving people with ID, it is important to allow extra time for communication and consider their physical and/or psychosocial needs, which may include working outside normal hours, travelling to different locations, making suitable venue arrangements and considering the need for carers, advocates or supporters to be present. We also recommend approaching service users through a number of sources to minimise the burden of their involvement.
Chapter 5 Screening programme: methods
Overview
This chapter describes the methods used for the screening component of the STOP Diabetes research programme included in WP1. The background and rationale are presented in Chapter 1. The methodology for the cost-effectiveness, which also formed part of this WP, is described in Chapter 12. An additional physical activity substudy, which was conducted alongside the screening, is described in Chapter 7.
Aims and objectives
The primary aim of the screening component of the research programme was to evaluate the feasibility and effectiveness of a diabetes screening programme for identifying undiagnosed T2DM and IGR in people with ID.
The specific objectives were to:
-
develop and assess the feasibility of a diabetes screening programme in a community setting for adults with ID
-
determine the prevalence and demographic risk factors for T2DM, IGR and CVD in people with mild to profound ID
-
validate the Leicester Self-Assessment diabetes risk score in people with ID
-
establish data linkage to Hospital Episode Statistics and the ONS.
Study design
Cross-sectional, population-based screening study.
Study setting
The screening study was conducted between February 2013 and September 2015, in a variety of community locations within the unitary authorities of Leicester city, Leicestershire and Rutland (see Chapter 1, Approvals). Based on assumed familiarity and acceptability to service users, the locations initially chosen included day centres, community hospitals, primary care venues and group residential/nursing homes, which were identified through existing service listings. This was subsequently widened to include family homes and independent housing, to maximise recruitment.
Participants
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria were adults who:
-
had ID
-
were aged 18–74 years inclusive
-
were registered with a general practice in Leicester city, Leicestershire or Rutland
-
had (or had a carer with) sufficient English-language skills to enable fully informed consent to be obtained.
Exclusion criteria
The exclusion criteria were adults who:
-
had previous diagnosis of T2DM or type 1 diabetes mellitus
-
had a disability not confirmed to be ID
-
had malignancy or life-limiting terminal illness
-
had severe systemic disease that could interfere with the measurement and interpretation of HbA1c level.
Participant recruitment process
Eligible participants were invited to take part in the screening programme using a four-pronged approach (summarised in Figure 14):
-
approach via general practice registers
-
approach via the LLDR
-
approach via specialist ID psychiatric service clinics
-
direct contact with the research team.
FIGURE 14.
Recruitment pathway to screening programme. LDR, learning disability register.
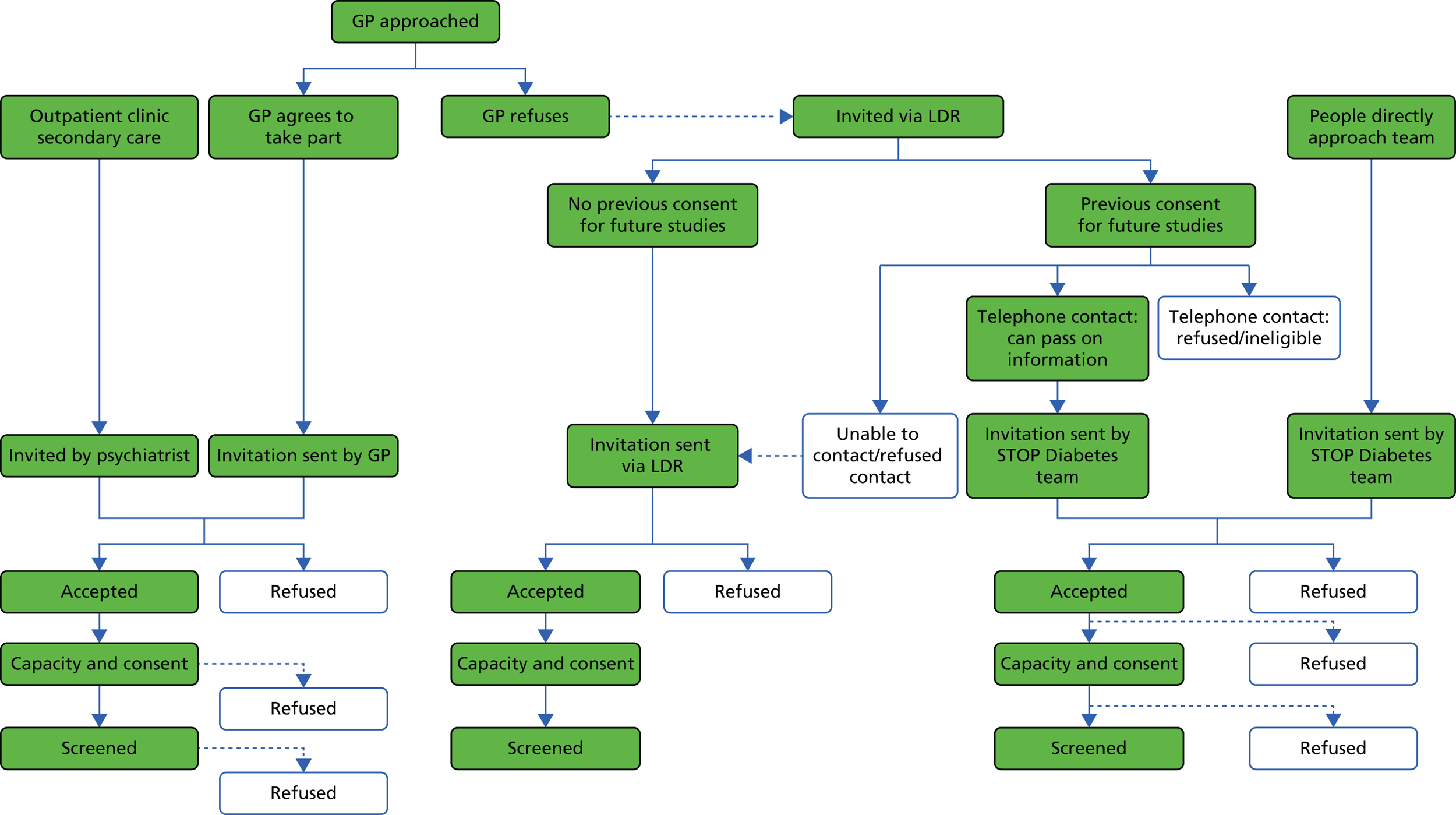
Approach via general practice registers
All of the general practices in Leicester City, Leicestershire and Rutland that had patients with ID on their practice register were sent a letter of invitation about the study. This was followed up, if necessary, by a postal reminder and/or telephone call. Practices were asked to return a reply slip to the research team to indicate their willingness to participate. The research team visited interested practices to explain the study in more detail, answer any questions and confirm their willingness to participate. General practice staff were then asked to identify people who were eligible to take part in the study from their practice ID register and to send out a postal invitation.
To adhere to the requirements and underlying principles of the Mental Capacity Act,210 information about the research was provided in stages. First, practices sent potential participants an easy-read invitation letter and a brief easy-read information leaflet, outlining the study. Potential participants were then asked to notify the research team of their willingness to participate (assisted by carers) using an easy-read reply slip and a Freepost addressed envelope, or via the telephone. To be equitable to people with ID who could not read, lived alone or lived with carers who also had reading difficulties, those who did not respond were followed up with a telephone call. The aim of the call was to check if the invitation had been received, to briefly explain what the information was about and to establish if the person or their carer wished to find out more about the research programme. The telephone calls were initially made by practice staff; however, owing to difficulties with practices prioritising the time to undertake them, approval was later sought for an ID research nurse to be employed on the study to make these telephone calls from the relevant general practice site. See Appendices 7–9 for examples of easy-read documentation used in the research programme.
Following this initial approach, a member of the research team telephoned interested people to discuss the study further. We anticipated that carers would play an important role in supporting the person with ID with their choice about participation; individuals were encouraged to talk to someone whom they trusted about whether or not to participate. Potential participants received verbal explanations about the study, had the opportunity to ask questions and received a preliminary assessment of their decision-making capacity to consent to participate in the research. Any indication of reluctance or anxiety about taking part was taken as a refusal. Full study information, in an appropriate format, was then sent to volunteers and/or an identified consultee. If a personal consultee (i.e. a person who had an interest in the potential participant’s welfare but not doing so for remuneration, such as a parent) could not be identified then a nominated consultee (e.g. a key worker) was identified and consulted. Alternatively, for some people, a face-to-face visit was arranged to facilitate the provision of further/full information, supplemented by additional communication aids/methods. See Appendices 10 and 11 for examples of consultee information leaflets used.
Approach via the Leicestershire Learning Disability Register
When general practices declined to take part in the study, potential participants were approached via the LLDR. 14 Adults who were known to the LLDR were invited to participate following the pathways described in Figure 14.
-
The register operates a rolling programme of home interviews,211 and those who agreed to be contacted for research purposes at their most recent interview were contacted by the custodian of the register to confirm that these people were happy to be contacted by the research team. Their contact details were passed on directly to the research team for invitation.
-
People who did not agree to direct contact for research purposes (because of either a lack of agreement at previous home interview or a refusal when approached by the custodian of the register, as above) were invited by the principal investigator in LPT.
For both methods outlined above, potential participants were approached in the same way as for those approached through general practices (easy-read invitation letter, brief information leaflet and reply slip to be returned to the research team). To avoid duplicates, invitations were cross-checked with those that had already been sent via general practices.
Non-responders were also followed up in a similar manner as previously described for general practices; follow-up telephone calls were made by either the custodian of the learning disability register or ID research nurses working on the research programme. A restricted-access database held on a Microsoft SQL server (Microsoft Corporation, Redmond, WA, USA) was used to record and track whether or not the individual had received the letter of invitation, and whether or not they would like any more information about the project.
Capacity assessment and provision of full study information, including involvement of carers and/or consultees, also followed the same process as previously described.
The sending of study invitations, via general practices and the LLDR, commenced in December 2012 and January 2013, respectively. Approval to utilise two further ways was obtained in February 2014 (see Approach via specialist intellectual disability psychiatric service clinics and Direct contact with the research team).
Approach via specialist intellectual disability psychiatric service clinics
An additional approach to potential participants was made via specialist ID psychiatric service clinics. For patients attending a planned appointment, the consulting psychiatrist briefly described the research programme and issued an easy-read invitation letter, a brief information leaflet and a reply slip. Service users were given the opportunity to take the information leaflet and reply slip away with them (to return in the post) or to have their details passed on to the research team. Those who agreed to pass on their details were contacted by a member of the research team to provide further information and make an initial assessment of capacity.
Recruitment and capacity assessment then followed the same procedure as for general practices and the LLDR. As before, all of the potential participants were cross-checked against those who were already invited to ensure that they were not invited to take part in the study more than once.
Direct contact with the research team
In some cases, direct contact was made by eligible individuals with ID (and/or their carers) who had heard about the study via publicity materials or through other people who had taken part in the study. The STOP Diabetes team provided them with the same initial brief written information as described for the other recruitment sources. Recruitment, capacity and cross-checking procedures were similarly undertaken.
Screening process
Following the invitation stage, volunteers were asked to attend an initial screening appointment for consent (see Informed consent) and data collection (see Data collection). Appointments were arranged by the research team at a time and location that was convenient to the participants (and carers), often early morning or late afternoon/evening in their own homes, but also in day centres, residential homes and primary care settings. The number and length of appointments was flexible to allow for participants’ individual needs.
Informed consent
At the participant’s first appointment, a final face-to-face capacity assessment was undertaken by a trained ID research nurse and informed consent was obtained; appropriate mental capacity legislation (see Chapter 1, Adherence to mental capacity legislation) was followed. Consent was taken only when it had been established that the person understood the consent form and information sheet, and that they had been given the opportunity to ask questions.
People with capacity to consent were asked to sign a consent form. For those who could decide, but were unable to read, the consent form was read to them in the presence of an independent witness. For people who did not have capacity to consent, an appropriate consultee was identified and consulted about the person’s potential participation. The consultee was asked to sign a consultee declaration form confirming that they had been consulted, had their questions answered and had considered the study from the participant’s perspective.
The participant and/or personal/nominated consultee (if appropriate) were asked to confirm that:
-
they understood the study and were happy with what taking part would mean for them
-
they understood that they could withdraw from the study at any time, without giving a reason (and that this would not affect their care)
-
they had been given a chance to discuss their questions with the research team
-
they agreed for their GP to be notified about their participation and of their screening results
-
the research team could access their medical records or records held at their residential home or day centre for additional information, if unable to obtain from the participant or carer
-
relevant sections of their medical notes and/or study data could be looked at by responsible people/regulatory authorities for purposes of auditing the research.
Participants provided their consent for screening to be undertaken (see Informed consent and Box 3), including a blood test (if the participant was willing). Additional optional consent items that participants could chose to agree to, or not, included:
-
being contacted to take part in further phases of the study if they screened positive for IGR or high risk of developing T2DM (based on elevated BMI)
-
having an additional blood sample taken for storage and future anonymised genetic analyses
-
allowing access to medical records for long-term follow-up
-
contact details being stored by the research team so that participants could be informed of the study findings and be contacted about future research studies.
At the end of the appointment, the nurse highlighted the office’s telephone number on the participant information sheet, which could be used if the participant decided to withdraw from the study or had any queries.
At the start of any subsequent appointments, participants’ retention and understanding of the study was reconfirmed.
Following the final study appointment, a photocopy of the signed consent form/consultee advice form (as appropriate) was sent to both the participant and the general practice; the copy of the form accompanied the screening results, which were subsequently sent (see Informing of screening results). The original consent and advice forms were retained at the research offices. See Appendices 12–14 for examples of the consent/advice forms.
Data collection
Data collection was usually undertaken over two appointments but could be longer (the maximum was five). The data collection process is summarised in Box 3. All of the data were collected in a standardised way by specially trained research nurses, following study-specific standard operating procedures. Full details of the assessment of outcomes are described below (see Assessment of outcomes).
Bloods:
-
Plasma glucose (2.7-ml fluoride bottle). a
-
HbA1c (2.7-ml EDTA bottle).
-
Lipids (total cholesterol, LDL, HDL, triglyceridesb). c
-
Urea and electrolytes (sodium, potassium, creatinine). c
-
Liver function tests (Bili, ALT ALP, GGT). c
-
Thyroid function (TSH, free T4). c
-
Genetic sample – whole blood (9-ml EDTA bottle). d
-
ACR (urine).
Anthropometric:
-
Height (cm).
-
Weight (kg).
-
BMI (kg/m2).
-
Waist and hip circumference (cm).
BP (mmHg).
QuestionnairesDepression:
-
Glasgow Depression Scale (GDS) and Carer Supplement.
Problem behaviour:
-
Aberrant Behaviour Checklist (ABC).
-
Health-related quality of life – EQ-5D.
-
Psychiatric disorders – PAS-ADD checklist.
Age.
Sex.
Residential circumstances; level of support.
Ethnic background.
Deprivation score.
Medical and family historyCause of ID; severity of ID.
Medical history (physical, mental health, ID related).
Family history of diabetes (first degree).
Current medication.
Smoking status.
LifestylePhysical activity:
-
Brief questions on mobility, walking, sitting and exercise.
Diet and nutrition:
-
Brief questions on eating, food preparation, food groups, portions of fruit and vegetables.
Activity and sedentary behaviour:
-
Accelerometer – worn for 7 days. e
ACR, albumin-to-creatinine ratio; ALT, alanine aminotransferase; ALP, alkaline phosphatase; Bili, bilirubin; EDTA, ethylenediaminetetraacetic acid; EQ-5D, EuroQol-5 Dimensions; GGT, gamma glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; T4, free thyroxine (FT4); TSH, thyroid-stimulating hormone.
a, Glucose, fasting (8 hours) or non-fasting; b, triglycerides, only requested if fasting; c, one bottle (4.9 ml serum gel) used for all 4; d, only if provided optional consent; e, only for a subgroup.
Anthropometric measurements, BP and demographic and lifestyle data were frequently obtained at the first appointment, after consent was obtained, and usually took between 1 hour and 90 minutes. Questionnaires were completed via interview during the initial screening visit (or at a subsequent appointment) or were given to carers to be completed outside the appointment, as applicable (see Box 3). These typically took between 30 and 60 minutes to complete. Venous blood samples were usually taken during a separate appointment after deciding with participants (and their carers, where relevant) whether a fasting or non-fasting sample would be more appropriate; this decision was based on potential behavioural difficulties and/or cognitive understanding of participants. This appointment lasted about 30 minutes. Medical history and prescribed medication were collected during screening or at a later date from medical records. Other additional information was extracted by a researcher from the LLDR or from records held at residential homes or day centres.
Informing of screening results
All participants were informed of their key biomedical screening results in an easy-read format, supplemented by verbal explanations as appropriate. Anthropometric measures and BP readings were presented to participants on the day that they were taken. Results of blood tests taken to determine diabetes status were provided within 7–10 days.
Participants with normal results were informed by post and given the option to contact the research team and discuss further if they wished. For participants who were screen positive for IGR or T2DM, a research nurse telephoned them to explain their results and answer any questions, prior to a letter being sent in the post. In some cases, this also involved a face-to-face visit by the nurse to support the participant and/or their carer. In accordance with consent taken, these participants were then referred to their general practice for usual care.
As agreed at the time of consent, participants’ GPs were provided with full details of the screening results, including diabetes status. Additionally, for all of the participants who were identified as meeting the criteria for IGR or T2DM, a member of the research team contacted their general practice and informed their GP, prior to any results letters being sent.
See Appendices 15 and 16 for example letters that were used to inform participants and GPs of the results.
Outcomes
Primary and secondary outcomes
The primary outcomes for the screening study were the prevalence of T2DM, IGR and abnormal (T2DM or IGR) blood glucose level.
Diagnosis of T2DM was made following the most recent WHO criteria,28 more specifically a HbA1c level of ≥ 48 mmol/l or 6.5%. IGR was defined as impaired fasting glucose, following the WHO criteria of a HbA1c level of 42–47 mmol/l or 6.0–6.4% (Figure 15).
FIGURE 15.
Diagnosis of T2DM and IGR for participants in the screening programme. FPG, fasting plasma glucose.
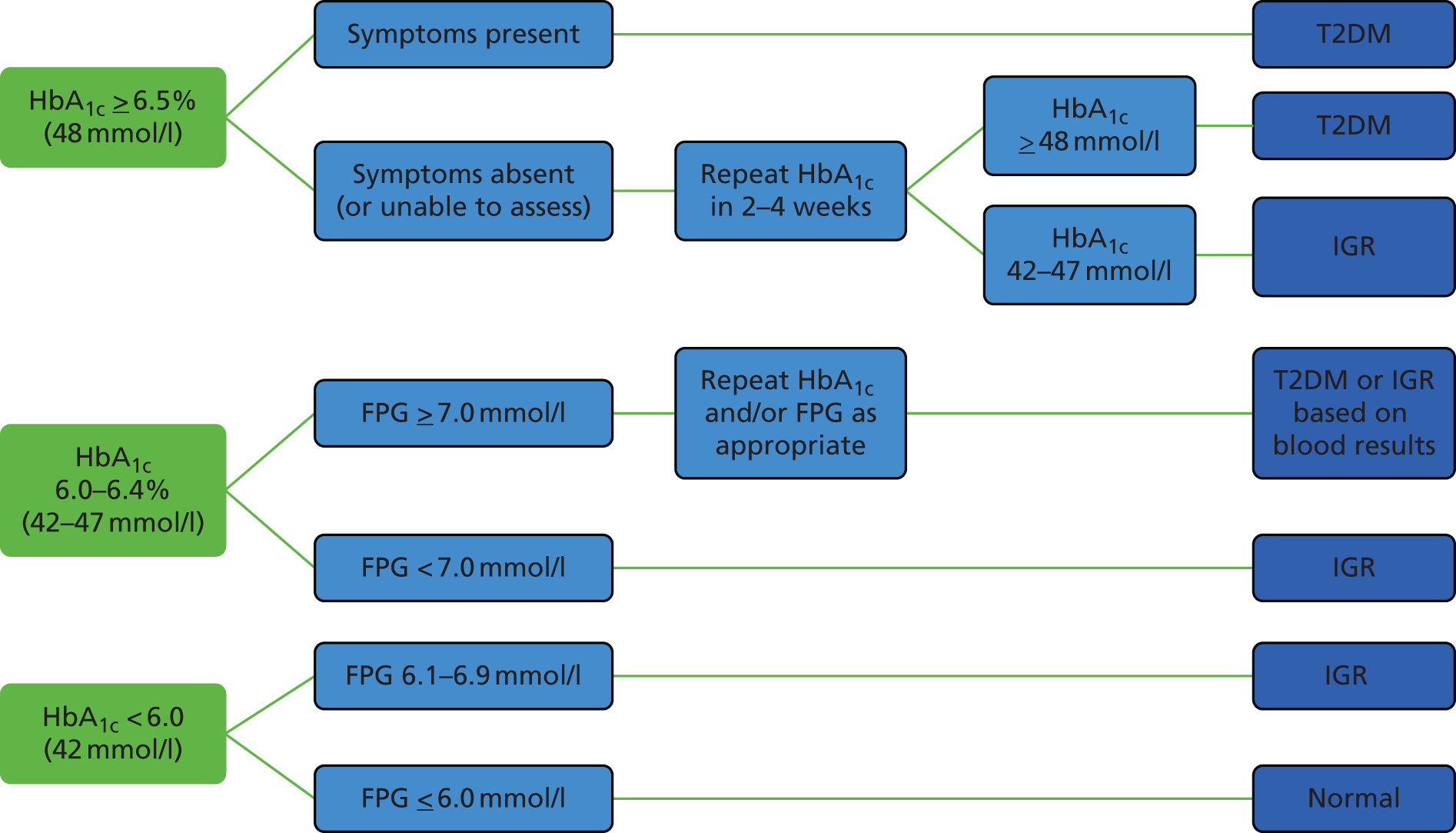
The secondary outcomes included:
-
physical activity levels, including sedentary behaviour, measured by brief questions and accelerometer (for a small subgroup)
-
lipid levels [triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol]
-
BP (systolic, diastolic)
-
cardiovascular risk, as measured by the Framingham Risk Score212,213
-
health-related quality of life, as measured by the EuroQol-5 Dimensions (EQ-5D) questionnaire214
-
dietary/nutritional intake (food groups and fruit and vegetable intake)
-
behavioural disorders, as measured by the Aberrant Behaviour Checklist (ABC)215,216
-
psychiatric disorders, as measured by the PAS-ADD checklist217
-
depression, as measured using the Glasgow Depression Scale (GDS) and Carer Supplement. 218
Assessment of outcomes
All blood and urine samples were analysed at by the University Hospitals of Leicester NHS Trust laboratory services, using stable methodology standardised to external quality assurance reference values. HbA1c level was measured using an ARKRAY ADAMS HA-8180T analyser (ARKRAY, Kyoto, Japan). Plasma glucose level (fasting and non-fasting); serum total cholesterol, HDL cholesterol and triglycerides, and urine albumin and creatinine were measured using a Siemens Advia 2400 analyser (Siemens Healthcare Diagnostics, Camberley, UK). The Friedewald equation was used to estimate LDL cholesterol. 219
Resting BP was assessed in a seated position on the brachial artery, using an Omron M5-I automatic BP monitor (Omron Healthcare UK, Milton Keynes, UK); a series of three measurements was recorded, with a mean value calculated from the final two. Waist circumference was measured to the nearest millimetre over minimal clothing, midway between the costal margin and the iliac crest, and in the mid-axillary line; hip circumference was measured to the nearest millimetre at the widest point over the buttocks; a soft tape was used for both anthropometric measures (WM02 Body Tape; Chasmors Ltd, London, UK). Weight was assessed in light clothing and no shoes to the nearest 0.1 kg, using a seca 875 digital floor scale (seca, Birmingham, UK); and height to the nearest centimetre using a Leicester portable height measure (Chasmors Ltd, London, UK) and with head placed in the Frankfurt plane.
Additional data on health-related quality of life (EQ-5D)214 and depression (GDS and Carer Supplement),218 were collected face to face via interview-administered questionnaires at an appointment. To assess problem behaviour (ABC)215,216 and psychiatric disorders (PAS-ADD checklist),217 questionnaires were taken away by carers and self-completed following the appointment. The validated questionnaires used are described in detail in Appendix 17. Deprivation was assessed according to the 2015 Index of Multiple Deprivation. 220
Ambulatory activity and sedentary behaviour were measured for a small subsample of participants; full details of the physical activity substudy undertaken are presented in Chapter 7.
Uptake of screening was measured by recording the number of (1) invitations sent, (2) people responding and refusing at each stage and (3) people attending for screening.
If BP, anthropometric measures and/or bloods were unable to be assessed, then details of the reason were recorded (refused, physical/behavioural difficulty, equipment error, other). For demographic, lifestyle, medical history and prescribed medication, additional details were recorded on how the data were obtained, for example from the volunteer, carer/relative or a combination of both, or if personalised records such as a health action plan4 were used.
Sample size
We aimed to screen 1000 adults with ID, which would measure the overall prevalences of T2DM and IGR with 1.49% and 2.01% precision (95% CI), respectively, assuming similar prevalence rates of T2DM and IGR in people with ID as in the general population (6.2% and 12%, respectively). 18,21,22
Data analysis
Feasibility of diabetes screening in adults with intellectual disability
The feasibility of conducting a diabetes screening programme in a community setting for adults with ID was assessed using a flow diagram of the screening process and summarising the number of dropouts and those for whom data were unobtainable at each step of the screening process. Particular outcomes of interest in terms of the feasibility are the proportions of people who (1) were invited and who complete the screening programme (including the blood tests) and (2) attended the screening session but did not have a blood test. We also assessed the completeness of key data items from the case report form and questionnaire to assess the feasibility of data collection for future research projects in this group.
Characteristics
The characteristics of those who were screened were summarised using means (SDs for continuous variables) and ‘n (%)’ for categorical variables.
Additional analyses were conducted to compare the representativeness of the STOP Diabetes study cohort with the LLDR. 14
Prevalence of type 2 diabetes and impaired glucose regulation
The overall prevalence of IGR, T2DM and any abnormal glucose regulation was calculated with 95% CI.
Cardiovascular risk
Cardiovascular risk was calculated for participants aged 35–75 years with no previous history of CVD. The Framingham CVD risk score212,213 was used to assess risk in white European participants and ETHRISK (a modified version for British black and minority ethnic groups) was used for South Asians. 221 Participants with incomplete data for key variables (total and HDL cholesterol, systolic BP, smoking status) were unable to be included in analyses. The overall mean risk at 10 years and the level of risk (high, intermediate, low), based on thresholds determined by National Cholesterol Education Program,222 were calculated.
Factors associated with abnormal glucose regulation
Logistic regression was used to assess the association between key biomedical and anthropometric characteristics and the outcome – abnormal glucose regulation. ORs and 95% CIs were calculated.
Validation of Leicestershire self-assessment risk score
Our initial analysis plan was to update the Leicester Self-Assessment risk score,58 described in Chapter 1 (see Risk scores for the early identification of impaired glucose regulation and type 2 diabetes), for use in a population with ID. This may have included adding or removing risk factors and updating the relative weighting given to risk factors. However, given the low prevalence of IGR/T2DM that we found in our screening study (see Prevalence of type 2 diabetes and impaired glucose regulation), this was not considered feasible. There are no formal sample size requirements for developing risk scores, although it has been suggested that data sets that are used to develop risk scores should contain between 10 and 20 events for each risk factor being assessed. 223,224 Therefore, our data set would be very underpowered to develop a risk score.
Hence, it was decided that instead of updating the original Leicester Self-Assessment risk score, alternatively, we would assess the risk score’s performance to detect undiagnosed IGR/T2DM. Although this validation would also be underpowered (studies suggest that external validation data sets should have at least 100 events and 100 non-events),225 this analysis should provide some preliminary results to suggest if the score is sensitive and specific in our cohort with ID.
The Leicester Self-Assessment risk score contains seven risk factors (age, sex, ethnicity, family history of diabetes, waist circumference, BMI and high BP). 58 To maximise the number of people included in the analysis, the data were analysed in two ways: (1) complete case basis (including only those with all seven risk factors recorded and the outcome) and (2) imputing missing data for family history of diabetes and high BP. For both a family history of diabetes and high BP, the imputed data set assumed a negative response if these items were missing. In both data sets the sensitivity, specificity, positive predictive value and negative predictive value were calculated for a cut-off point of ≥ 16 points. This is the cut-off point that was used in the general population for invitation to screening. 58
All of the analysis was conducted using Stata; statistical significance related to p < 0.05 and 95% CIs are presented throughout.
Establish data linkage to Hospital Episode Statistics and the Office for National Statistics
An additional optional consent item about which participants were approached at their initial screening appointment (see Chapter 5, Informed consent) included consent for follow-up for health issues in the longer term.
Genetic markers
A supplementary component of WP1 involved collecting blood samples for future genetic studies in individuals who had provided consent (optional). For this, an extra whole blood sample was taken and stored at –80 °C. These samples will be analysed in a batch at the end of the study. Future work will involve extracting deoxyribonucleic acid and testing biologically plausible interactions between genetic markers and T2DM to determine T2DM susceptibility. The analysis of genetic markers does not form part of the work described in this report.
Concluding remarks
This chapter has described the methods for the screening component of the STOP Diabetes research programme. The next chapter presents the results of the screening study.
Chapter 6 Screening programme: results
Overview
This chapter reports the results of the diabetes screening programme that was undertaken for WP1. The methods for the screening study were reported in Chapter 5. An additional physical activity substudy, which was conducted alongside the screening, is described in Chapter 7.
Feasibility of conducting a diabetes screening programme in adults with intellectual disability
Participant recruitment
Initial approach
Participants were recruited to the STOP Diabetes screening study between February 2013 and September 2015. In total, 3201 adults with ID were invited to take part via the four different routes (Figure 16).
FIGURE 16.
Invitation flow chart. LDR, learning disability register.

Fifty-one per cent (n = 73) of all general practices in Leicester City, Leicestershire and Rutland (with adults with ID on their practice list) agreed to be involved with the study. Subsequently, 1736 potentially eligible people were identified and sent an invitation letter by their practice (median 19, range 3–116). People who were invited via this route accounted for the majority of study invitations (54%).
For practices refusing, 1595 people were identified for possible approach via the LLDR. Of these, contact details for 418 people (who had previously agreed to be contacted for research purposes) were passed directly to the research team so that they could be invited (13% of the overall study total). A further 864 (27%) people were invited directly by the principal investigator in LPT.
A much smaller proportion of people were invited via specialist ID psychiatric service clinics or after making direct contact with the research team: 2% (n = 52) and 4% (n = 131), respectively.
Full-stage invitation
From the initial invitation, approximately 30% of people refused, 29% were classed as non-responders and 40% expressed an interest in participating in the study (Figure 17). Following a preliminary assessment of volunteers’ decision-making capacity, 1209 individuals (38% of those initially invited) were then provided with full study information (postal invitation or a face-to-face visit). Subsequently, 984 (31%) proceeded to the screening stage.
FIGURE 17.
Recruitment.
For people who refused (or agreed) at the initial or full invitation stages, details relating to the method of recruitment and reasons for refusal are presented in Table 12.
| Characteristics and method | Stage | |||
|---|---|---|---|---|
| Initial invitation (or chasing non-responders) | Full invitation (or capacity 1 or 2) | |||
| Refuse | Agree | Refuse | Agree | |
| Total number | N = 918 | N = 1259 | N = 233 | N = 984 |
| Male, n | 53a | 58 | 62a | 58 |
| Age in years, mean | – | 43 | 40a | 44 |
| Resided in Leicester City | 40a | 41 | 48a | 40 |
| Recruitment method, n | ||||
| GP | 50 | 47 | 67 | 41 |
| Learning disability register | 33 | 18 | 12 | 19 |
| Previous consent to research | 15 | 19 | 17 | 20 |
| Direct invite | 1 | 12 | 2 | 14 |
| Psychiatrist clinic | 1 | 5 | 3 | 6 |
| Refusal/acceptance method, n | ||||
| Reply slip | 28 | 28 | 24 | 28 |
| Telephone call | 14 | 20 | 21 | 20 |
| Chasing person via telephone | 55 | 33 | 55 | 28 |
| GP notified team | 2 | 0 | 0 | 0 |
| In person | 1 | 18 | 1 | 24 |
| Via e-mail | 0 | 1 | 0 | 1 |
| Reason for refusing, n | ||||
| Not known | 77 | – | 72 | – |
| Behavioural issues | 7 | – | 6 | – |
| Carer would not agree consent | 7 | – | 5 | – |
| Health issues | 3 | – | 4 | – |
| Recent health check | 3 | – | 6 | – |
| Too busy | 2 | – | 2 | – |
| Other | 1 | – | 6 | – |
Screening: consent and data collection
At the consent stage, 930 people (29% of those originally approached) agreed to participate and were recruited to the screening study; 54 people either refused (n = 19) or were ineligible (n = 35) (see Figure 17). Thirty-eight per cent of participants (n = 350) were able to consent for themselves; the others required a nominated (39%) or personal (23%) consultee.
The availability of data for the key screening outcomes is presented in Table 13. The full details regarding the availability of data for all study variables are reported in Appendix 18, Table 61. Anthropometric measures and BP were obtained for most participants: approximately 86% and 89%, respectively. In the majority of cases, the documented reason for not obtaining anthropometric measures was physical or behavioural difficulties; for BP, the main reason was participant refusal.
| Screening outcomes | Outcome measured, n (%) |
|---|---|
| Anthropometric | |
| Height | 800 (86.0) |
| Weight | 799 (86.0) |
| BMI | 782 (84.1) |
| Waist circumference | 796 (85.6) |
| Hip circumference | 789 (84.8) |
| BP | |
| Diastolic/systolic | 826 (88.8) |
| Blood tests | |
| Agreed to blood test | 700 (75) |
| Fasted for test – yes | 491 (70) |
| Bloods obtained | 648 (70) |
| Blood results available | Taken for study; obtained from medical records |
| HbA1c | 648 (69.7); 27 (2.9) |
| Plasma glucose: fasting | 417 (44.8); 8 |
| Plasma glucose: non-fasting | 223 (24.0); 16 |
| Total cholesterol | 614 (66.0); 39 |
| HDL cholesterol | 615 (66.1); 29 |
| LDL cholesterol | 605 (65.1); 26 |
| Triglyceridesa | 404 (43.4); 3 |
| Diabetes status assessed | |
| Normal, high risk, abnormal | 675 (72.6) |
| Validated questionnaires | |
| EQ-5D score | 872 (93.8) |
| EQ-5D visual analogue scale | 877 (94.3) |
| GDS: volunteers with capacity | 317 (34.4) |
| GDS: Carer Supplement | 464 (50.2) |
| ABC | 341 (36.7) |
| PAS-ADD Checklist Section 2 | 325 (34.9) |
A high proportion of participants agreed to attend for a blood appointment (n = 825); 700 (75% of those recruited) proceeded to have a blood test, and bloods to allow screening were successfully obtained for 648 (70%). For a few additional participants, when a blood test was refused or a sample was not obtained, recent results were available from their medical records (HbA1c test, n = 27; for other tests the number varies) (see Figure 17 and Table 13). For a further five participants, HbA1c results were not included because of potential unreliability in assessing diabetes status (n = 4) and poor kidney function (n = 1, possible haemoglobin variant). Therefore, we were able to assess diabetes status for a total of 675 participants.
Validated questionnaires administered via interview were successfully completed for a high number of participants (EQ-5D ≈94%; GDS or Carer Supplement ≈85%). Carer completion of questionnaires outside the appointment (for the ≈80% of participants who had an identified carer) was less successful; approximately 45% of carers completed the ABC and/or the PAS-ADD.
Characteristics of the screened cohort
The key characteristics for the study population are presented in Tables 14–17. The full details for all of the screening variables are reported in Appendix 18, Table 61.
| Demographic | N | Mean (± SD), unless stated otherwise |
|---|---|---|
| Age (years) | 930 | 43.3 (± 14.2) |
| Gender, male, n (%) | 930 | 537 (57.7) |
| Ethnicity, n (%) | 930 | |
| White | 748 (80.4) | |
| Asian | 147 (15.8) | |
| Black | 14 (1.5) | |
| Mixed | 13 (1.4) | |
| Other | 8 (0.9) | |
| Residential circumstances, n (%) | 929 | |
| Alone | 51 (5.5) | |
| Lives with family | 338 (36.4) | |
| Shared house or supported living | 157 (16.9) | |
| Shared care | 16 (1.7) | |
| Residential home or nursing home | 350 (37.7) | |
| Other | 17 (1.8) | |
| Level of support, n (%) | 929 | |
| Independent | 69 (7.4) | |
| Some support | 205 (22.1) | |
| 24-hour support | 655 (70.5) | |
| Current status,a n (%) | ||
| Paid employment | 928 | 71 (7.7) |
| Voluntary work | 927 | 152 (16.4) |
| College | 925 | 170 (18.4) |
| Day opportunities or private day centre | 928 | 431 (46.4) |
| Shared lives (day placement) | 928 | 19 (2.1) |
| Attending meetings | 926 | 122 (13.2) |
| Other | 924 | 385 (41.7) |
| Biomedical measurements | N total (from medical record) | Mean (± SD), Unless stated otherwise |
|---|---|---|
| Bloods | ||
| Plasma glucose (mmol/l) | ||
| Fasting | 425 (8) | 4.7 (± 0.7) |
| Non-fasting | 239 (16) | 5.3 (± 1.5) |
| HbA1c | 675 (27) | |
| HbA1c (mmol/mol) | 35.0 (± 5.1) | |
| Derived HbA1c (%) | 5.4 (± 0.5) | |
| Lipids (mmol/l) | ||
| Total cholesterol | 653 | 4.9 (± 1.0) |
| HDL cholesterol | 644 | 1.3 (± 0.4) |
| LDL cholesterol | 631 | 2.9 (± 0.9) |
| Triglyceridesa | 407 | 1.4 (± 0.9) |
| Anthropometric | ||
| Height (m) | 800 | 1.6 (± 0.1) |
| Weight (kg) | 799 | 76.4 (± 20.7) |
| BMI (kg/m2) | 782 | 28.7 (± 7.1) |
| BMI categories, n (%) | ||
| Underweight | 30 (3.8) | |
| Normal | 223 (28.5) | |
| Overweight | 241 (30.8) | |
| Obese | 288 (36.8) | |
| Waist circumference (cm) | 796 | 100.4 (± 16.5) |
| BP measurements (mmHg) | ||
| BP | 826 | |
| Systolic | 121.4 (± 16.9) | |
| Diastolic | 78.2 (± 11.1) | |
| Medical history and current medication | n (%) |
|---|---|
| Severity of ID (N = 865) | |
| Not known | 49 (5.7) |
| Known | 816 (84.3) |
| Mild | 260 (30.1) |
| Moderate | 244 (28.2) |
| Severe | 279 (32.3) |
| Profound | 33 (3.8) |
| Cause of ID (N = 866) | |
| Not known | 581 (67.1) |
| Known | 285 (32.9) |
| Down syndrome | 133 (15.4) |
| Fragile X | 8 (0.9) |
| Cerebral palsy | 58 (6.7) |
| Hydrocephalus | 6 (0.7) |
| Phenylketonuria | 5 (0.6) |
| Prader–Willi syndrome | 4 (0.5) |
| Medical or health problems (N = 929) | |
| None | 117 (12.6) |
| Yes | 812 (87.4) |
| Physical health | |
| Stroke | 13 (1.4) |
| Peripheral arterial disease | 0 |
| CHD | 7 (0.8) |
| Congenital heart disease | 19 (2.1) |
| Other heart problems | 15 (1.6) |
| High BP | 63 (6.8) |
| High cholesterol | 62 (6.7) |
| Hypothyroidism | 93 (10.0) |
| Polycystic ovary syndrome | 1 (0.1) |
| Gestational diabetes | 0 |
| Pre-diabetes | 1 (0.1) |
| Chronic breathing problems | 88 (9.5) |
| Sleep apnoea | 3 (0.3) |
| Epilepsy | 262 (28.2) |
| Mental health | |
| Dementia | 18 (1.9) |
| Schizophrenia, schizotypal and delusional | 35 (3.8) |
| Mood (affective) disorders | 152 (16.4) |
| Neurotic, stress related and somatoform | 143 (15.4) |
| Personality disorders | 13 (1.4) |
| Drug/alcohol problems | 0 |
| ADHD | 8 (0.9) |
| ID related | |
| Autistic spectrum disorders | 165 (17.8) |
| Behavioural problems | 128 (13.8) |
| Current medication (N = 928) | |
| None | 172 (18.5) |
| Yes | 756 (81.5) |
| Anti-psychotic | 240 (25.9) |
| Depression/anxiety/OCD or related | 258 (27.8) |
| For ADHD | 4 (0.4) |
| Antiepileptic | 311 (33.5) |
| Antithrombotic | 36 (3.9) |
| Lipid lowering | 74 (8.0) |
| Statin | 72 (7.8) |
| Fibrate | 1 (0.1) |
| Statin and fibrate | 1 (0.1) |
| Antihypertensive | 85 (9.2) |
| Thyroid medication | 93 (10.0) |
| Steroids | 80 (8.6) |
| Oral | 5 (0.5) |
| Inhaled | 62 (6.7) |
| Topical | 9 (1.0) |
| More than one type of steroid | 3 (0.3) |
| Not known | 1 (0.1) |
| Anti-obesity | 1 (0.1) |
| Other | 571 (61.5) |
| Smoking status (N = 929) | |
| Current smoker | 76 (8.2) |
| Ex-smoker | 38 (4.1) |
| Never smoked | 815 (87.7) |
| Family history of diabetes (N = 592) | 180 (30.4) |
| Lifestyle and well-being | n (%) |
|---|---|
| Physical activity/exercise | |
| Able to walk (N = 927) | |
| No | 57 (6.2) |
| Yes (with or without walking stick, aid) | 787 (84.9) |
| Yes, with assistance from person(s) | 83 (9.0) |
| Amount of walking per day (N = 927) | |
| None | 74 (8.0) |
| A short distance | 259 (27.9) |
| Some | 359 (38.7) |
| Lots | 235 (25.4) |
| Frequency of physical activity, per week, (N = 928) | |
| None | 184 (19.8) |
| 1–2 times | 360 (38.8) |
| 3–4 times | 259 (27.9) |
| ≥ 5 times | 125 (13.5) |
| Time spent sitting per day (N = 928) | |
| All/most | 180 (19.4) |
| A lot | 252 (27.2) |
| Sometimes | 475 (51.2) |
| Never | 21 (2.3) |
| Nutrition and diet | |
| Problems relating to eating and drinking | |
| Difficulties with chewing or swallowing (N = 929) | 227 (24.4) |
| Needs help or assistance to feed self (N = 926) | 118 (12.7) |
| Use specialist equipment | 95 (10.3) |
| Fed via an nasogastric tube or gastrostomy | 7 (0.8) |
| Food shopping (N = 922) | |
| Independently | 89 (9.7) |
| With support | 230 (25.0) |
| Relative or carer | 297 (32.2) |
| Purchased by residential home | 306 (33.2) |
| Preparing meals (N = 921) | |
| Relative or carer | 561 (60.9) |
| With supervision | 117 (12.7) |
| Without supervision | 145 (15.7) |
| Without supervision can prepare variety of meals | 98 (10.6) |
| Daily portions of fruit and vegetables (N = 920) | |
| None | 33 (3.6) |
| 1 | 57 (6.2) |
| 2 | 130 (14.1) |
| 3 | 230 (25.0) |
| 4 | 199 (21.6) |
| 5 | 213 (23.2) |
| 6 | 36 (3.9) |
| ≥ 7 | 22 (2.4) |
Demographic characteristics
The mean age of those screened was 43.3 (SD 14.2) years, 58% were male and the majority were of white ethnicity (80%) (see Table 14).
Most participants lived either with family (36%) or in a residential/nursing home (38%), with 6% living alone. A high proportion required 24-hour support (71%) and only 7% reported to be independent.
The majority of individuals was able to access the community to undertake regular daytime activities. Common activities included attending college (18%), voluntary work (16%) or involvement in service user/advocacy meetings (13%). Around half of the participants attended day opportunities/day placements. Only a small number of people were in regular paid employment (8%).
Anthropometric and biomedical measures
Among those screened, the mean waist size was 100.4 (SD 16.5) cm, weight 76.4 (SD 20.8) kg and BMI 28.7 (SD 7.1) kg/m2 (see Table 15). Based on their BMI, 31% of participants were classed as overweight and 37% obese. Mean values for systolic and diastolic BP were 121.4 (SD 16.9) mmHg and 78.2 (SD 11.1) mmHg, respectively.
Among participants for whom blood results were available, the mean HbA1c level was 35.0 mmol/mol (SD 5.1 mmol/mol) (5.3%; SD 1.5%), FPG was 4.7 mmol/mol (SD 0.7 mmol/mol) and non-FPG was 5.3 mmol/mol (SD 1.5 mmol/mol). For lipids, mean total cholesterol was 4.9 mmol/mol (SD 1.0 mmol/mol), HDL cholesterol was 1.3 mmol/mol (SD 0.4 mmol/mol), LDL cholesterol was 2.9 mmol/mol (SD 0.9 mmol/mol) and triglycerides were 1.4 mmol/mol (SD 0.9 mmol/mol).
Current medication and medical history
When details on severity of ID were available (n = 816, 88%), similar proportions of participants were classified as mild, moderate or severe (≈30% each), and 4% were classified as profound ID (see Table 16). Most participants had no confirmed diagnosis or identified cause of their ID (≈70%); when the causes were known, the most common were Down syndrome (n = 133, 14%) and cerebral palsy (n = 58, 6%).
The overall prevalence of existing CVD was 2% (n = 19). A history of stroke was reported for 12 (1.3%) people and coronary heart disease (CHD) for six (0.6%) people, and one person had a history of both conditions.
Congenital heart disease (2%) and other heart problems (2%) were less frequently reported.
Seventy-four participants (8%) had a history of high cholesterol and/or were prescribed a lipid-lowering medication, 85 (9%) had a history of previously diagnosed hypertension and/or were prescribed an antihypertensive drug, and 36 (4%) were prescribed an anti-thrombotic drug. A minority of participants were either current smokers (8%) or ex-smokers (4%).
When known, approximately one-third of participants had a first-degree family history of diabetes. Only one participant reported a previous diagnosis of pre-diabetes and one reported polycystic ovary syndrome. Nine per cent were currently being prescribed a steroid medication (the majority of these medications were inhaled).
Overall, the most commonly reported diagnosed physical health problems were epilepsy (n = 262, 28%), hypothyroidism (n = 93, 10%) and chronic breathing problems (n = 88, 9%). Thirteen per cent of participants had no significant medical history and 19% were not currently prescribed any medication.
For mental health-related problems, 152 participants (16%) had a history of a mood spectrum disorder (ICD-10 codes F30–F39), 35 (4%) a psychotic spectrum disorder (ICD-10 codes F20–F29) and 52 (6%) had a history of both; 143 people (15%) had neurotic, stress-related or somatoform disorders (ICD-10 codes F40–F48). Additionally, 28% of participants were prescribed antipsychotic medication and 32% were prescribed depression- or anxiety-related medication. Other frequently reported problems included autistic spectrum disorders (18%) and a recognised behavioural problem (14%).
When comorbidities (two or more diagnosed health problems) were considered, 121 (13%) participants had co-occurring physical health problems, 182 (20%) had co-occurring mental health problems and 286 (31%) had multiple physical and/or mental health problems.
Lifestyle and well-being
Eighty-five per cent of those screened were able to walk independently (without the help/support of another person), but including 6% who required a walking aid (see Table 17). The data reported directly by participants and/or carers indicated that most people did at least ‘some’ walking on a typical day, but only 25% achieved ‘a lot’ of walking. Additionally, around half of the participants reported spending ‘a lot’ or ‘most/all’ of the day sitting.
Sport/exercise or other physical activities that individuals reported undertaking in a typical week included dance (25%), swimming (20%) or walking (21%). Around half of the participants reported doing housework (such as dusting/hovering) and ≈20% gardening. A small number of people (7%) did regular chair-based exercise.
Problems with eating and drinking were reported for some people: 24% had difficulties in chewing or swallowing and 13% needed help to feed themselves (< 1% were tube fed). For food shopping and preparation, overall ≈35% of participants did their own food shopping (either independently or with some support) and a similar number were able to prepare at least simple hot and cold food (with or without supervision). Reported daily intake of fruit, vegetables or salad indicated that only around 30% of participants were eating the recommended five or more portions a day.
When questionnaire data were available, the proportion of participants identified with possible depression (using a cut-off point of 13) by the GDS or GDS Carer Supplement was 22% and 16%, respectively. 218 For health-related quality of life, the mean EQ-5D descriptive score was 0.8 (SD 0.3) and for the visual analogue scale was 78.1 (SD 19.4). The mean scores for the five problem behaviour subscales measured by the ABC (for participants with carers) were ≈4 for irritability, lethargy and hyperactivity, and ≈1 for stereotyped behaviour and inappropriate speech. The prevalences of mental health problems for organic, affective/neurotic and psychotic disorders (as measured by PAS-ADD checklist) were 6%, 9% and 5%, respectively.
Comparison with the Leicestershire Learning Disability Register
Participant demographic characteristics from this study were compared with adults with ID on the Leicester Learning Disability Register. Comparison of age, sex and ethnicity suggests that the STOP Diabetes cohort is a representative sample of the population with ID, known to services within the Leicester, Leicestershire and Rutland area (Table 18).
| Characteristic | STOP Diabetes (N = 930), n (%) | LLDR < 80 years (N = 3867), n (%) |
|---|---|---|
| Age (years) | ||
| < 30 | 207 (22.3) | 1012 (26.2) |
| 30–39 | 195 (21.0) | 856 (22.1) |
| 40–49 | 211 (22.7) | 776 (20.1) |
| 50–59 | 185 (19.9) | 659 (17.0) |
| 60–69 | 107 (11.5) | 416 (10.8) |
| 70–79 | 25 (2.7)a | 148 (3.8) |
| Male | 537 (57.7) | 2222 (57.5) |
| Ethnicity | Of n = 3571 known | |
| White | 748 (80.4) | 2893 (81.0) |
| South Asian | 147 (15.8) | 553 (15.5) |
| Black/mixed | 27 (2.9)b | 80 (2.2) |
| Other | 8 (0.9) | 45 (1.3) |
Prevalence of type 2 diabetes and impaired glucose regulation
Outcome data to establish the prevalence of IGR/T2DM were available for 675 participants. Screening results indicated that, overall, 44 (6.5%) participants had abnormal glucose regulation, a prevalence of 0.07 (95% CI 0.05 to 0.08); nine participants (1.3%) were found to have undiagnosed T2DM, a prevalence of 0.01 (95% CI 0.005 to 0.02); and 35 (5.2%) had IGR, a prevalence of 0.05 (95% CI 0.04 to 0.07) (Table 19).
| Outcome | n (%) | Prevalence (95% CI) |
|---|---|---|
| Normal glucose | 631 (93.5) | 0.93 (0.92 to 0.95) |
| IGR | 35 (5.2) | 0.05 (0.04 to 0.07) |
| T2DM | 9 (1.3) | 0.01 (0.01 to 0.02) |
| Abnormal glucose | 44 (6.5) | 0.07 (0.05 to 0.08) |
Factors associated with abnormal glucose regulation
Table 20 shows the association of anthropometric and biomedical characteristics with having screen-detected abnormal glucose regulation. Participants of non-white ethnicity were almost four times more likely to have abnormal glucose levels than white European participants (OR 3.93, 95% CI 2.10 to 7.33); those with a first-degree family history of diabetes were over three times more likely (OR 3.35, 95% CI 1.64 to 6.86). In addition, abnormal glucose tolerance was associated with increasing weight, waist circumference, hip circumference, BMI, diastolic BP and triglycerides, and decreasing HDL cholesterol.
| Characteristic | Normal glucose (n = 631) | Abnormal glucose (n = 44) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 43.0 (± 14.3) | 45.4 (± 13.5) | 1.01 (0.99 to 1.03) | 0.27 |
| Male, n (%) | 377 (59.8) | 28 (63.6) | 1.18 (0.63 to 2.22) | 0.61 |
| Non-white ethnicity, n (%) | 119 (18.9) | 21 (47.7) | 3.93 (2.10 to 7.33) | < 0.0001 |
| Weight (kg), mean (SD) | 76.6 (± 20.2) | 91.7 (± 27.3) | 1.03 (1.01 to 1.04) | < 0.0001 |
| Waist circumference (cm), mean (SD) | 100.1 (± 16.2) | 114.0 (± 19.0) | 1.04 (1.03 to 1.07) | < 0.0001 |
| Hip circumference (cm), mean (SD) | 107.4 (± 13.5) | 115.6 (± 19.1) | 1.03 (1.01 to 1.06) | 0.001 |
| BMI (kg/m2), mean (SD) | 28.6 (± 6.9) | 34.1 (± 10.2) | 1.08 (1.04 to 1.13) | < 0.0001 |
| Current smoker, n (%) | 56 (8.9) | 6 (13.6) | 1.62 (0.66 to 4.00) | 0.30 |
| Family history of diabetes, n (%) | 132 (29.9) | 20 (58.8) | 3.35 (1.64 to 6.86) | 0.001 |
| Systolic BP (mmHg), mean (SD) | 121.8 (± 17.3) | 126.5 (± 14.4) | 1.01 (1.00 to 1.03) | 0.09 |
| Diastolic BP (mmHg), mean (SD) | 78.0 (± 11.2) | 83.7 (± 10.0) | 1.04 (1.02 to 1.07) | 0.002 |
| Total cholesterol (mmol/l), mean (SD) | 4.9 (± 1.0) | 4.7 (± 0.9) | 0.78 (0.56 to 1.10) | 0.15 |
| HDL cholesterol (mmol/l), mean (SD) | 1.4 (± 0.4) | 1.2 (± 0.3) | 0.14 (0.05 to 0.43) | 0.001 |
| LDL cholesterol (mmol/l), mean (SD) | 2.9 (± 0.9) | 2.7 (± 0.8) | 0.71 (0.48 to 1.07) | 0.10 |
| Triglycerides (mmol/l), mean (SD) | 1.4 (± 0.9) | 1.9 (± 1.0) | 1.53 (1.11 to 2.11) | 0.01 |
Validation of the Leicester Self-Assessment risk score
Overall, 365 (54%) of the 675 participants with the outcome obtained had complete data for the seven risk factors assessed by the Leicester Self-Assessment risk score. This was increased to 595 (88.1%) when imputing family history and high BP (Table 21). Similar percentages of participants fall into the four risk categories based on the complete case and imputed data. In the complete case data, 43.1% would be referred for screening based on their risk score (≥ 16 points) and 41.4% based on the imputed data.
| Variables | All (N = 675), n (%) | Complete case (N = 365), n (%) | Imputed (N = 675), n (%) |
|---|---|---|---|
| Age (years) | |||
| ≤ 49 | 445 (65.9) | 263 (72.1) | 445 (65.9) |
| 50–59 | 136 (20.2) | 69 (18.9) | 136 (20.2) |
| 60–69 | 75 (11.1) | 30 (8.2) | 75 (11.1) |
| ≥ 70 | 19 (2.8) | 3 (0.8) | 19 (2.8) |
| Sex | |||
| Male | 405 (60.0) | 207 (56.7) | 405 (60.0) |
| Female | 270 (40.0) | 158 (43.3) | 270 (40.0) |
| Ethnicity | |||
| White European | 535 (79.3) | 278 (76.2) | 535 (79.3) |
| Other ethnic group | 140 (20.7) | 87 (23.8) | 140 (20.7) |
| Family history of T2DM | |||
| No | 324 (48.0) | 248 (68.0) | 523 (77.5) |
| Yes | 152 (22.5) | 117 (32.1) | 152 (22.5) |
| Unable to assess | 199 (29.5) | 0 | 0 |
| Waist circumference (cm) | |||
| < 90 | 153 (22.7) | 90 (24.7) | 153 (22.7) |
| 90–99.9 | 153 (22.7) | 92 (25.2) | 153 (22.7) |
| 100–109.9 | 153 (22.7) | 83 (22.7) | 153 (22.7) |
| ≥ 110 | 157 (23.3) | 101 (27.4) | 157 (23.3) |
| Unable to assess | 59 (8.7) | 0 | 59 (8.7) |
| BMI (kg/m2) | |||
| < 25 | 188 (27.9) | 111 (30.4) | 188 (27.9) |
| 25–29 | 182 (27.0) | 116 (31.8) | 182 (27.0) |
| 30–34 | 123 (18.2) | 71 (19.5) | 123 (18.2) |
| ≥ 35 | 109 (16.2) | 67 (18.4) | 109 (16.2) |
| Unable to assess | 73 (10.8) | 0 | 73 (10.8) |
| Antihypertensive medication or high BP | |||
| No | 525 (77.8) | 325 (89.0) | 609 (90.2) |
| Yes | 66 (9.8) | 40 (11.0) | 66 (9.8) |
| Unable to assess | 84 (12.4) | 0 | 0 |
| Complete data for Leicester Self-Assessment risk score | |||
| Total | 365 | 365 | 595 |
| Final score | |||
| Low (0–6) | – | 63 (17.3) | 112 (18.8) |
| Medium (7–15) | – | 145 (39.7) | 237 (39.8) |
| High (16–24) | – | 121 (33.2) | 193 (32.4) |
| Very high (25–47) | – | 36 (9.9) | 53 (8.9) |
Table 22 presents the validation of the Leicester Self-Assessment risk score in this population with ID. The complete case and imputed data have similar results; therefore, only the complete case data are interpreted here. Of the 22 participants with abnormal glucose regulation and full risk score data, 18 are correctly classified as high or very high risk by the risk score. This gives a sensitivity of 81.8%. Given the low number of events, the 95% CI around this estimate is wide: 59.7% to 94.8%. Of the 344 participants with normal glucose regulation, 204 are correctly identified as being of low or medium risk and therefore would not be referred on for further screening. One hundred and forty participants would be referred for unnecessary screening, that is to say that, of those with a high or very high risk score, only 11.4% have undiagnosed IGR/T2DM. The findings suggest that the score may be useful for ruling out disease; 98.1% of those with a low or medium risk score are correctly identified and do not have undiagnosed IGR or T2DM.
| Analysis | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Complete case (n = 365) | 81.8 (59.7 to 94.8) | 59.5 (54.1 to 64.7) | 11.5 (6.9 to 17.5) | 98.1 (95.1 to 99.5) |
| Imputed (n = 595) | 83.3 (67.2 to 93.6) | 61.4 (57.2 to 65.4) | 12.2 (8.4 to 16.9) | 98.3 (96.3 to 99.4) |
Cardiovascular risk
Cardiovascular risk, based on Framingham risk score,212,213 or on ETHRISK221 for participants of South Asian ethnicity, was able to be calculated for 376 (40.4%) participants. The mean risk of CHD in 10 years was 5.9% (SD 4.9%) and of CVD was 7.3% (SD 6.2%). Most participants were at a low future risk of both CHD (83.5%) and CVD (75.3%) (Table 23). However, 16% of participants were at an intermediate or high risk of developing CHD in the next 10 years, and 25% of participants were at an intermediate or high risk of developing CVD in the next 10 years.
| Risk | CHD, n (%) | CVD, n (%) |
|---|---|---|
| Low (< 10%) | 314 (83.5) | 283 (75.3) |
| Intermediate (10–20%) | 54 (14.4) | 78 (20.7) |
| High (> 20%) | 8 (2.1) | 15 (4.0) |
Establish data linkage to Hospital Episode Statistics and the Office for National Statistics
Of the 930 people who were recruited to the main study, 883 (95%) gave additional consent for the research team to follow up their health in the longer term. Preliminary work to establish data linkage is currently being conducted.
Discussion
Summary of main findings
Utilising a variety of approaches to identify/invite potential volunteers, 930 adults with ID (29% of those approached) participated in the screening programme; 38% were able to consent for themselves, whereas other participants required a consultee. Anthropometric measures (≈86%) and BP (89%) were obtained for most participants. A high proportion of participants agreed to attend for a blood test and, subsequently, prevalence of T2DM/IGR was assessed for 675 participants (73%).
The mean age of participants was 43.3 years, 58% were male and the majority were of white ethnicity (80%). Most lived either with family (36%) or in a residential/nursing home (38%); a high proportion required 24-hour support (71%). Most participants were either overweight or obese; 2% had a history of existing CVD.
Screening results indicated the overall prevalence of undiagnosed T2DM was 1.3% (95% CI 0.5% to 2%) and IGR was 5.2% (95% CI 4% to 7%). Participants of non-white ethnicity were almost four times more likely to have abnormal glucose levels than white European participants; those with a first-degree family history of diabetes were over three times more likely.
Comparison with previous evidence
The prevalence of previously undiagnosed T2DM detected in the screening programme is much lower than previously reported. 90 Combined evidence from other studies, as presented in the meta-analysis in Chapter 2, suggests a prevalence rate of 8% for T2DM in adults with ID. Data to enable comparison of rates for T2DM in the UK population with ID are scarce (it is suggested that 85–90% of diabetes is T2DM20). Current estimated prevalence of diabetes (type not specified) in England, based on combined data reported by partnership boards, is 6.8% (range 6.2–8.4%) for people with ID of any age. 51 Based on current data supplied by 40 (55%) of the general practices that took part in the STOP Diabetes study, the suggested prevalence of diagnosed diabetes (type not specified) locally is 9.5% (n = 148 of 1553 adults aged 18–74 years with ID).
The estimates above are based on previously diagnosed diabetes. Our study aimed to screen adults with ID to identify undiagnosed T2DM. The rates suggested by data supplied by local general practices, alongside the higher recorded uptake of health checks locally (57–66% across the three CCGs)226 than the national average (44%),55 suggests that at a local level the lower rate may simply reflect a successful annual health checks programme. In the general population, estimated prevalence of diabetes rises from 6.2% to 8.0% when including undiagnosed cases. 18 However, it is acknowledged that the proportion of adults with ID who currently have bloods checked, including for diabetes, as part of their annual health check is unclear.
Strengths and limitations
To our knowledge, this is the first diabetes screening study that has been conducted in adults aged 18–74 years with mild to severe/profound ID. The successful integration of a multidisciplinary team, consisting of experienced researchers and ID HCPs, enabled the successful development and conduct of the STOP Diabetes screening programme. This multidisciplinary approach allowed for sharing of knowledge and best practice, and was complemented by service user involvement, particularly in the early stages of developing and trialling study procedures/processes.
The screening programme developed utilised robust methods. All of the data were collected by staff who had undertaken study-specific training and were following standard operating procedures. Minimal exclusion criteria were applied to the study, and reasonable adjustments to facilitate inclusion – such as staged invitation, easy-read documents, flexible appointments and carer involvement – maximised participation. This ensured that as many people as possible participated rather than being arbitrarily excluded. Additionally, we applied a staged approach to invitation and made efforts to contact/chase all people when possible.
It is acknowledged that we were unable to establish any contact with approximately 30% of people who were non-responders. We therefore do not know if they are different in any way from those who were included in the screening programme; evidence suggests that people with mild ID may be at increased risk as a result of unhealthier lifestyles and are less likely to access services. 11 However, similarities in the demographic characteristics (age, sex, ethnicity) between participants in this study and adults with ID on the Leicester Learning Disability Register suggest that the STOP Diabetes cohort is a representative sample of the population with ID that is known to services within the Leicester, Leicestershire and Rutland area.
The validation of the Leicester Self-Assessment risk score in the population with ID was successful despite the limited number of events and wide 95% CI. Estimates suggest that the Leicester Self-Assessment risk score works as well in populations with ID as in the general population: sensitivity 81.8%. Based on this, 140 participants would be referred for unnecessary screening. However, the tool is designed for use in a multistage screening programme and we would rather send more people through the first stage than falsely reassure.
Implications for clinical practice and future research
The screening uptake of those approached, at 29%, was relatively low, but it was favourable compared with two previous screening/prevention studies conducted locally in the general population, for which 22% and 19% of those invited took part. 227 These relatively low rates of uptake might reflect the fact that participants were invited to screening as part of a research project. If the intervention was rolled out in clinical practice, higher rates would be expected; for example, uptake rates to the NHS Health Check Programme,228 which is not a research project, are double those reported in this and other research screening studies. Future research should focus on increasing uptake to screening in all groups.
Bloods to enable diabetes screening were successfully obtained for a high proportion of participants. However, future research may want to consider allowing for separate consent for blood tests so as to not deter people at the initial recruitment stage. Very few people directly expressed ‘the blood test’ as a reason for refusal to participate in the screening study, but anecdotal evidence suggests that this may have deterred some. Alternatively, a staged approach to screening, involving risk stratification as recommended by NICE, might be considered. 27
Our findings suggest that the Leicester Self-Assessment risk score is statistically effective at identifying people with ID who are at a high risk of undetected IGR/T2DM. However, the feasibility of using it in practice with people with ID – given the levels of heterogeneity within the population with ID – needs to be considered. It may not be practical or acceptable for people with ID to calculate their own score, with or without added support from carers. Future research could involve developing an easy-read version (plus a carer supplement) and additional supportive material/communication aids, such as digital audio/visual materials; qualitative research would be needed to supplement this work. Alternatively, a better way may be to integrate the risk score at practice level and incorporate it into the Learning Disability Health Check (the learning disability Annual Health Check scheme).
Concluding remarks
This chapter presented the main results of the screening programme for WP1. The methods and results of the physical activity substudy are presented in Chapter 7.
Chapter 7 Physical activity substudy
Overview
This chapter describes the physical activity substudy, which was conducted alongside the screening component in WP1. The main methods and results of the screening stage are described in Chapters 5 and 6, respectively.
Aims and objectives
The aim of this substudy was to assess the feasibility of collecting physical activity data with the use of a waist-worn accelerometer (ActiGraph, Pensacola, FL, USA) (Figure 18). However, given the poor uptake to this initial measurement tool, we extended our aim to also include the feasibility of collecting physical activity via a wrist-worn device (GENEActiv, Activinsights Ltd, Cambridge, UK).
FIGURE 18.
Waist-worn accelerometer (ActiGraph) and wrist-worn (GENEActiv) accelerometer.


Methods
Participants
Participants who met the eligibility criteria, as outlined below, were asked to wear an accelerometer as part of the main screening component of WP1.
Inclusion criteria
-
Consented to take part in the main screening component.
-
Able to walk without assistance (stick or similar walking aid permissible).
Participant recruitment process
Initial assessment of eligibility to participate in the physical activity substudy commenced during the capacity assessment process (outlined in Chapter 5) and was subsequently confirmed once consent to the main screening study had been obtained. Eligible participants were then approached about wearing an accelerometer. For most people, this was usually at the end of their first screening appointment.
Data collection
Participants were asked to wear an accelerometer for 7 consecutive days, not including the appointment day. The procedure for wearing the accelerometer was explained to the participant and/or carer by an ID research nurse. Participants were also provided with a brief accelerometer information leaflet/diary in an easy-read format, which explained how to use the accelerometer; this diary was also used to log when participants had worn the accelerometer, with a page for each of the 7 days.
After wearing the accelerometer, participants were asked to return it at their next appointment. If a participant was not having another planned appointment, a member of the research team would contact them to arrange a convenient time for the accelerometer to be collected. If an accelerometer was not returned or unsuccessfully collected, the research team made repeated attempts (at least three) to try and retrieve it.
Two different accelerometers were used to collect data. Initially, physical activity data were recorded using a waist-worn accelerometer. Later, it was decided to also trial a wrist-worn accelerometer, given the poor compliance that was emerging with the waist-worn device (see Results) and following discussion with service users (who were assisting with patient and public involvement activities). The wrist-worn accelerometer was anticipated to encourage greater compliance, as it is waterproof and can also be worn when sleeping; therefore, participants could wear it continuously over the 7-day period.
Full details of the assessment of outcomes are described in Assessment of physical activity outcomes.
Outcomes
Physical activity levels were included as one of the secondary outcomes for the main screening study (see Chapter 5). Other anthropometric (BMI, waist circumference), demographic (ethnicity and social deprivation) and biochemical (fasting blood glucose and HbA1c levels) outcomes assessed are described in Chapter 5.
Sample size
Initially, we aimed to include at least 50 participants who were wearing the waist-worn accelerometer. This was updated to include a comparable number with the wrist-worn device.
Assessment of physical activity outcomes
The participants who were attending screening were offered the option of having their physical activity levels assessed by a waist-worn accelerometer. Once we had achieved our initial aim of at least 50 individuals with data, the remaining cohort were offered an alternative wrist-worn accelerometer. Details of the two accelerometers and analytical methods used are presented below.
Waist-worn accelerometer
ActiGraph waist-worn triaxial accelerometers were attached to the trunk (placed on the right anterior axillary line) using an elasticated belt. The participants were asked to wear the accelerometer during waking hours for 7 days, taking it off only at night when going to bed or when participating in water-based activities, such as showering or swimming. Participants (and carers) were shown how to reattach the accelerometer after sleep, and carers were asked to provide reminders. Data were set to record at 100 Hz and analysed using a commercially available software package, KineSoft version 3.3.76 (KineSoft, SK, Canada; www.kinesoft.org). Data were converted into 60-second epochs and count-based format. Time spent sedentary, in light-intensity physical activity and in MVPA was estimated by applying commonly used thresholds for adults. 229 Non-wear time was classified as 60 minutes of continuous zero counts.
Wrist-worn accelerometer
The GENEActiv original wrist-worn triaxial waterproof accelerometer was worn continuously on the participant’s non-dominant wrist for a minimum of 7 days. Data were captured in 100 Hz and processed using two methods.
Data analysis method 1
Raw acceleration data were converted to 60-second epochs using the GENEActiv Post-Processing PC Software version 2.2 (Activinsights). Next, the 60-second epoch data files were entered into an open-source Excel macro v2 (Activinsights) in order to classify activity. Subsequently, time spent in sedentary, light-intensity and MVPA activities was calculated for each participant-day using validated cut-off points. 230 Sleep time was estimated using a defined algorithm (Activinsights) and subtracted from total sedentary time in order to calculate time spent sedentary while awake.
Data analysis method 2
Given that standard definitions for physical activity categories are lacking for wrist-worn devices, we also included an alternative approach that was reported in the literature using the Euclidian Norm Method. 231 Data were processed in a freely available R package (GGIR version 1.2–0, http://cran.r-project.org, The R Foundation for Statistical Computing, Vienna, Austria) using the previously described methodology to include time spent in sedentary, light-intensity physical activity and MVPA. 231,232 In additional, total physical activity levels were reported in mg, where g = gravity.
Inclusion of physical activity data
Physical activity data were included from a device if there was a minimum of 8 hours’ wear per day for at least 3 days.
Data analysis
Data are presented as means (SDs). Analysis of covariance models were used to compare differences in levels of assessed physical activity between monitors, adjusted for age, sex, social deprivation and wear time.
Results
Feasibility of using accelerometers to assess physical activity in adults with intellectual disabilities
Participants were recruited to take part in the physical activity substudy between October 2013 and August 2015 (Figure 19).
FIGURE 19.
Collection of accelerometer data. (Data validity was based on a minimum of 8 hours’ wear per day for at least 3 days.)
Overall, 203 participants were approached to wear the ActiGraph waist-worn accelerometer. Subsequently, 97 participants (48%) agreed to wear the ActiGraph, and valid data (≥ 8 hours per day for 3 days) were obtained for 55 participants (57%). Reasons for attrition included 14 participants (14%) not returning their accelerometer and 28 participants (29%) not having enough valid days of wear for analysis.
A total of 76 participants were asked to wear the GENEActiv wrist-worn accelerometer and 47 participants (62%) agreed. Valid data were obtained for 39 participants (83%). Two individuals (4%) did not return their accelerometer and six participants did not have enough valid days of wear (13%).
Characteristics of participants in physical activity substudy
The characteristics of those who agreed to wear an accelerometer, on either the wrist or the waist, and those with valid physical activity data stratified by accelerometer type are shown in Table 24. Characteristics were similar between those who had valid physical activity data and those who did not. Characteristics were also similar between those who had valid waist-worn and and those who had wrist-worn accelerometer data. Overall, 54% of participants were male, their mean age was 39.9 (SD 13.0) years and 85% were of white ethnicity. Thirteen per cent lived alone, 42% lived in supported living and 46% lived with family; the majority (88%) had support from a carer for at least some of the time.
| Characteristics | Total agreed (N = 144) | Total valid (N = 94) | Waist agreed (N = 97) | Waist valid (N = 55) | Wrist agreed (N = 47) | Wrist valid (N = 39) |
|---|---|---|---|---|---|---|
| Sex, male, n (%) | 78 (54.2) | 50 (53.2) | 53 (54.6) | 30 (54.6) | 25 (53.2) | 20 (51.3) |
| Age (years), mean (SD) | 39.9 (± 13.0) | 41.9 (± 13.7) | 40.8 (± 13.6) | 43.6 (± 14.7) | 38.1 (± 11.8) | 39.3 (± 11.8) |
| Ethnicity, white, n (%) | 122 (84.7) | 82 (87.2) | 82 (84.5) | 48 (87.3) | 40 (85.1) | 34 (87.2) |
| HbA1c (%), mean (SD) | 5.3 (± 0.4) | 5.3 (± 0.3) | 5.3 (± 0.4) | 5.3 (± 0.3) | 5.4 (± 0.3) | 5.4 (± 0.3) |
| FPG (mmol/l), mean (SD) | 4.7 (± 0.5) | 4.6 (± 0.5) | 4.8 (± 0.5) | 4.6 (± 0.4) | 4.6 (± 0.5) | 4.6 (± 0.5) |
| Waist circumference (cm), mean (SD) | 98.9 (± 17.1) | 98.4 (± 16.6) | 98.7 (± 17.6) | 97.9 (± 16.2) | 99.4 (± 16.3) | 99.1 (± 17.3) |
| BMI (kg/m2), mean (SD) | 28.4 (± 7.5) | 28.3 (± 7.1) | 28.1 (± 7.4) | 27.7 (± 6.2) | 29.1 (± 7.7) | 29.0 (± 8.2) |
| IMD 2015 rank, median (IQR) | 16,280 (7859.5–24,227.5) | 16,280 (7734–23,871) | 16,292 (7546–24,572) | 16,456 (7351–24,572) | 16,086 (8815–23,871) | 15,279 (7734–21,525) |
| Accommodation, n (%) | ||||||
| Alone | 18 (12.5) | 10 (10.6) | 13 (13.4) | 6 (10.9) | 5 (10.6) | 4 (10.3) |
| Lives with family | 66 (45.8) | 46 (48.9) | 44 (45.4) | 27 (49.1) | 22 (46.8) | 19 (48.7) |
| Supported environment | 60 (41.8) | 38 (40.5) | 40 (41.3) | 22 (40.0) | 20 (42.5) | 16 (41.0) |
| Support, n (%) | ||||||
| Independent | 17 (11.8) | 11 (11.7) | 10 (10.3) | 5 (9.1) | 7 (14.9) | 5 (12.8) |
| Need support | 127 (88.2) | 83 (88.3) | 87 (89.7) | 50 (91.0) | 40 (85.2) | 34 (87.2) |
| Severity of ID, n (%) | ||||||
| Mild | 62 (46.6) | 39 (44.3) | 38 (42.7) | 17 (34.0) | 24 (54.6) | 22 (57.9) |
| Moderate | 39 (29.3) | 27 (30.7) | 29 (32.6) | 19 (38.0) | 10 (22.7) | 8 (21.1) |
| Severe/profound | 23 (17.3) | 16 (18.2) | 17 (19.2) | 11 (22.0) | 6 (13.6) | 5 (13.2) |
| Not known | 9 (6.8) | 6 (6.8) | 5 (5.6) | 3 (6.0) | 4 (9.1) | 3 (7.9) |
Main findings
The estimates of time spent in MVPA, light-intensity physical activity time and sedentary time are presented across the different monitors and physical methods used (Table 25). Estimates for MVPA and sedentary time were significantly higher with the wrist-worn device, whereas estimates of light-intensity physical activity were lower. The total physical activity volume measured by the wrist-worn device was 26.7 mg (SD 8.7 mg).
| Physical activity measures | Waist worn | Wrist worn | |||
|---|---|---|---|---|---|
| Method 1 | Method 2 | Method 1 vs. waist differencea | Method 2 vs. waist differencea | ||
| Time in MVPA (minutes/day) | 33.6 (30.8) | 136.9 (79.9) | 95.8 (51.8) | p < 0.001 | p < 0.001 |
| Time in light-intensity physical activity (minutes/day) | 269.1 (72.7) | 105.7 (47.1) | 195.1 (73.7) | p < 0.001 | p < 0.001 |
| Time spent sedentary (minutes/day) | 499.2 (96.7) | 632.5 (136.4) | 790.8 (116.1) | p < 0.001 | p < 0.001 |
| Ambulatory activity (steps/day) | 6761 (3483) | N/A | N/A | ||
Discussion
The key finding from this substudy was that the objective measurement of physical activity is likely to be challenging in adults with ID with high levels of non-compliance; however, compliance can be substantially improved and loss of accelerometers reduced with wrist-worn monitors. Overall, < 50% of participants agreed to wear the waist-worn device, with valid data collected for only 57% of the sample. In contrast, 62% agreed to wear the wrist-worn device, with 83% providing valid data.
To our knowledge, this is the first study to access the feasibility of collecting objectively assessed physical activity data in those with ID. However, other studies have reported high levels of missing data when using objectively measured physical activity within their study protocol. 164,233 These results suggest that studies including accelerometers may have poor uptake unless the participants are allowed to consent separately for this element. These factors will need to be taken into account and considered carefully in future physical activity intervention studies within this population.
To assist with compliance in our study, participants (and carers) were provided with a physical activity diary (instructions) in an easy-read format. Service users were involved with the development and initial testing of the diary; however, no formal assessment was conducted to see if the diary increased compliance for participants (and carers). Given the heterogeneity in capacity levels and support needs of individuals, further work is needed to explore possible ways to improve compliance with accelerometer wear in people with ID.
Based on estimates from the waist-worn device, our population engaged in more MVPA than several other studies conducted in those with ID. For example, studies from Scotland and the USA have reported between 7 and 14 minutes per day of MVPA. 164,233 Estimates for MVPA from the waist-worn device were also slightly higher than levels reported in a primary care cohort from Leicestershire, UK. 234 Similarly, estimates for total physical activity from the wrist-worn device were consistent with those reported for healthy non-obese adults and higher than those reported for obese or unhealthy populations within the UK. 235 However, in the UK a previous research study on those with ID reported similar levels to those found in our study. 236 This suggests that, in the UK, those with ID are not less active than the general population. This is despite institutional barriers that have been hypothesised to inhibit physical activity engagement in those with ID. 237
An important finding from this substudy was the difference in activity levels gained from wrist- and waist-worn devices. Although waist-worn devices have been widely used in research over the last decade, with established methods of categorising collected data, which allows for comparisons between studies, wrist-worn devices are newer and lack standardised approaches to data analysis. Although the underlying raw acceleration data between waist- and wrist-worn monitors are likely to be highly correlated, commonly used methods of converting these data into meaningful outputs, such as time spent in MVPA, are likely to be monitor and placement specific. This has important implications for future trials and suggests that intervention effects, SDs and population means should be estimated using data gained from the same tool that will be used in the study.
Concluding remarks
This chapter has described a physical activity substudy, which formed part of WP1. Chapter 8 describes the first phase of the education development process that was carried out as part of WP2, to develop an initial curriculum for a lifestyle education programme for adults with ID.
Chapter 8 Development of initial curriculum for structured education programme
Chapter overview
This chapter describes the work undertaken for WP2 to develop an educational programme for a population with ID and IGR or a high risk of developing diabetes and/or CVD (based on increased BMI level). A brief overview of the complete development process is presented below (see Overview of the development process). The remainder of this chapter details the work conducted to develop an initial curriculum. Further development work, including two pilot cycles of testing, evaluation and modification, is described in Chapter 9. An additional feasibility phase, which formed part of WP2, is presented in Chapter 10.
Aims and objectives
The aim of WP2 was to develop a structured lifestyle education programme for the prevention of T2DM would be suitable for use in a population with ID.
The specific objectives were to:
-
develop a lifestyle education programme for a population with ID who have IGR or are at a high risk of developing T2DM and/or CVD based on increased BMI level (see Chapters 8 and 9)
-
assess the feasibility of collecting outcome measures for participants with ID before and 3 months after they attend the education programme (see Chapter 10).
Overview of the development process
A multidisciplinary team with expertise in ID and in the development of nationally recognised diabetes and CVD prevention programmes developed the intervention. A systematic approach was used (Figure 20), based on the current Medical Research Council framework for developing and evaluating complex interventions238 and intervention mapping. 239 This included reviewing the relevant published evidence from existing programmes and the behaviour change literature. The curriculum was informed by previous prevention programmes that our research group has developed. 240–242 Additional qualitative work was undertaken to further inform the content, process and style of delivery.
FIGURE 20.
How phases of the development work fit together.
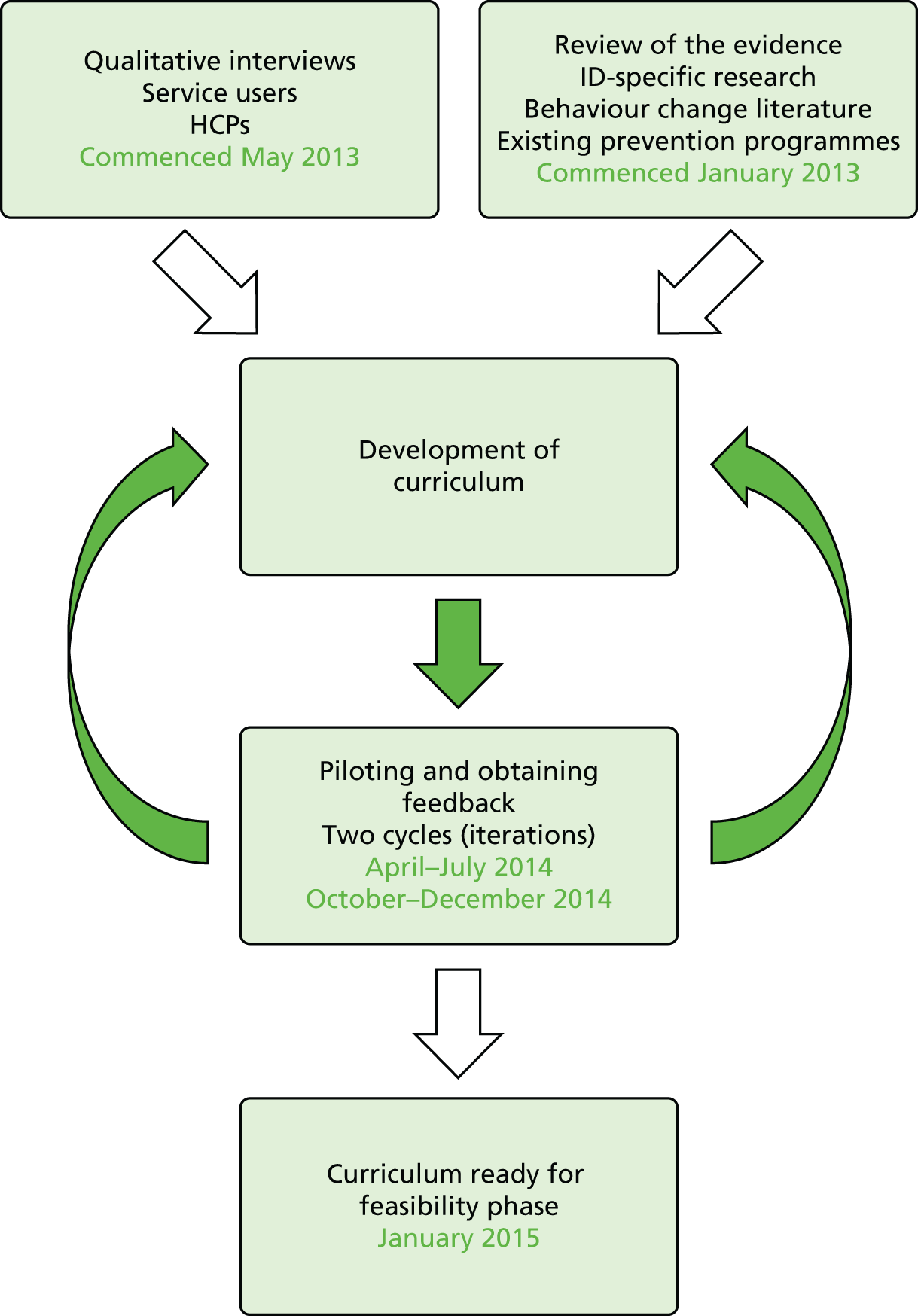
Following the development of an initial curriculum, two cycles of testing, evaluation, modification and retesting were conducted during the pilot phase (presented in Chapter 9) prior to the programme being used in a third iteration, in which the feasibility of collecting before-and-after data was explored (presented in Chapter 10). This iterative and reflective process, supplemented by qualitative research methodology, is an approach that our group has previously used, successfully, to adapt patient education modules for different groups. 240,243,244
The core multidisciplinary team – including ID nurses, education team members, a qualitative researcher and the lead study researchers – met monthly throughout all stages of the development, supplemented by more frequent meetings at key points in the process. The purpose of these meetings was to decide on the key elements relating to the content, process and style of the initial curriculum, and, subsequently, reach agreement on any modifications required. This collaborative multidisciplinary approach allowed the expertise of all of the members to be used and facilitated the iterative and reflective process.
This development work occurred over a period of approximately 27 months, commencing in October 2012 and ending in January 2015, when the final refinements were made to the curriculum (ready for use in the feasibility phase described in Chapter 10).
Participants
People invited to engage in WP2 (qualitative interview, see Chapter 8; pilot education sessions, see Chapter 9; or feasibility testing, see Chapter 10) were service users with mild to moderate ID who had taken part in the screening stage (see Chapter 5) and screened positive for IGR or had a BMI of ≥ 25 kg/m2, and, at that time, consented to being approached to assist with later phases of the research programme. Carers were also approached. An invitation pack, including easy-read documents, was sent directly by the research team. For people volunteering, the capacity assessment and consent followed a similar process to previous stages (see Chapter 5).
For people with ID who were invited to assist with the qualitative exploratory interviews (see Qualitative work to inform development: methods), no further eligibility criteria applied. Additional inclusion criteria for invitation to attend the pilot education sessions and give feedback (see Chapter 9) or the feasibility phase (see Chapter 10) included:
-
having the ability to stand and walk at least short distances
-
having the ability to attend group education sessions
-
not taking part in any other intervention study.
Intellectual disability HCPs were also invited to contribute/assist with development of the initial curriculum by agreeing to a qualitative interview. Further details are provided below (see Recruitment for interviews).
Qualitative work to inform development: methods
To help inform development of the initial curriculum, semistructured qualitative exploratory interviews were conducted with service users (and carers) and with HCPs providing services to adults with ID. This qualitative work was carried out between May 2013 and June 2014.
Recruitment for interviews
To enable a range of views to be captured, a provisional quota was set of conducting up to 25 interviews with the various stakeholders (HCPs and service users with ID).
The recruitment of HCPs commenced in May 2013. A variety of HCPs were identified through ID services at LPT. The invited HCPs all had previous experience of working with adults with ID. Purposive sampling was used to ensure the inclusion of HCPs who could offer a range of perspectives based on their occupation/professional background. Potential interviewees were sent an invitation pack.
For service users (and carers) the recruitment began in January 2014. The eligibility criteria and method of approach are described previously (see Participants).
Data collection and recording
Topic guides were developed to ensure that relevant issues were captured (see Appendix 19 for service user example). The interviews were semistructured and based on open questioning to elicit issues surrounding knowledge, understanding and experience of T2DM and modifiable risk factors, relevance of IGR, perceived barriers to behaviour change and support needs for people with ID. The practical aspects of the delivery of an education programme were also explored, for example whether or not to develop separate interventions for carers (family members and/or key workers) and people with ID, the inclusion of follow-up sessions and the length of the programme.
Questions were asked appropriately depending on who was being interviewed. Additional communication tools were used when interviewing service users, such as prompt cards depicting images of various activities (e.g. swimming, bowling, walking) to help with eliciting contributions.
The interviews with HCPs were conducted between June and August 2013. All of the interviews were conducted by an experienced qualitative researcher at the HCP’s normal place of work.
The interviews with service users were conducted from January to June 2014. There was an initial delay in finding service users who were either eligible to be invited or willing to be approached/interviewed. All of the interviews were conducted by the same qualitative researcher, with assistance from an ID research nurse. The researcher had expertise in developing and modifying diabetes prevention programmes for different populations; prior to commencing the interviews, the researcher had undertaken additional training within the research team to increase his or her knowledge and skills in the area of ID. To suit the needs and preferences of individual participants, the interviews were conducted in a variety of community settings, including at a participant’s family home, a residential/care home, an assisted independent-living flat and a community clinic.
Data analysis
Audio-recordings of interviews were transcribed verbatim and a thematic analysis was conducted using NVivo version 7 (QSR International, Warrington, UK), a qualitative software programme. Subsequently, themes that were relevant to the development of the intervention were identified.
Qualitative interviews: findings
Characteristics of participants
Service users
Eighteen service users were invited to participate. A total of seven service users were subsequently interviewed (Table 26). In two of the interviews, carers were present (one was a family carer and the other was a care worker). Three of the service users who participated were male; the median age of participants was 47 (range 28–68) years and all participants were of white European ethnicity. Six participants lived in a supported environment with family or carers and one lived independently. One of the participants was in paid employment (and did voluntary work), two attended college, two others carried out voluntary work and the remaining participants undertook other activities in the community on a regular basis.
| Characteristics of service users (N = 7) | n |
|---|---|
| Age (years) | |
| 18–39 | 3 |
| 40–59 | 3 |
| 60–74 | 1 |
| Sex | |
| Male | 3 |
| Female | 4 |
| Accommodation | |
| Alone | 1 |
| With family/carers | 3 |
| Residential home | 3 |
| Level of support | |
| Independent | 2 |
| Some support | 2 |
| 24-hour support | 3 |
Health-care professionals
Twenty HCPs were invited to participate. Subsequently, 14 HCPs were interviewed. All of the HCPs currently worked with adults with ID as all, or part, of their job. Professionals included ID psychiatrists, people in nurse-related roles (acommunity/primary care ID nurse, a practice nurse, an acute liaison nurse, a nursing assistant), allied HCPs (a clinical psychologist, an occupational therapist, a speech and language therapist) and a day centre manager (Table 27).
| Characteristics of HCPs (N = 14) | n |
|---|---|
| Age (years) | |
| 20–39 | 5 |
| 40–59 | 7 |
| Unknown | 2 |
| Sex | |
| Male | 2 |
| Female | 12 |
| Profession | |
| ID psychiatrist | 2 |
| Allied HCP | 5 |
| Nurse related | 6 |
| Other | 1 |
| Length of time working with adults with ID (years) | |
| < 5 | 1 |
| 6–10 | 2 |
| ≥ 10 | 9 |
| Unknown | 2 |
Key points from interviews with service users and carers
The interviews conducted with service users ranged between 9 and 15 minutes in duration.
In a few of the interviews it was possible to explore awareness of diabetes. Service users related this to ‘sugar’; they also spoke about family members who had diabetes and recalled them being on tablets and having injections. Attempts to gauge service users’ knowledge about healthy lifestyles elicited that some were able to describe basic health messages, such as eating vegetables, eating a high-fibre diet and exercising.
The interviews did yield some useful insights into the lives of service users, for example the types of food that they enjoyed and the degree of choice and control they had in relation to foods consumed; discussions about commonly consumed foods ultimately influenced the food images and food models in the dietary sections of the curriculum. For a few participants, the additional use of prompt cards enabled useful discussion around the types of physical activities undertaken; for those who were more independent, walking appeared to be the most preferred and accessible form of physical activity.
It was difficult to explore service users’ preferences towards learning as part of a group or learning on an individual basis. However, the majority of participants spoke about going to some form of group activity sessions, such as sessions held at a local day care centre or a college; activities included arts and crafts, and learning ‘life skills’ to facilitate independence. Further discussion about participants’ preferences for photographs or pictorial images (on educational resources) suggested that most preferred photographs.
More general points arising from the interviews included practical considerations to be taken into account. First, education sessions needed to be held locally (minimal travelling distance/time for participants) in a setting that was familiar and in a venue that was easily accessible via public transport or similar. Second, it was important to include carers in the education sessions to help (1) support participants and make them feel at ease during sessions and (2) facilitate service users in making changes to their diet and physical activity outside the sessions. It was clear from the interviews that both professional and family carers currently fulfilled this role in the daily lives of participants.
Key points from interviews with health-care professionals
All of the HCPs were enthusiastic to share their knowledge and experience of working with people with ID. Most HCPs had previous or current experience of promoting positive behaviour change with people with ID for behaviour management and/or health promotion.
Pre assessment
The majority of HCPs stressed the importance of undertaking a pre-assessment prior to embarking on delivery of an education session. Frequently stated reasons relating to carrying out a pre-assessment included:
-
enabling cognition matching, which would involve the assessment of preferred communication styles, reading and writing abilities, and preferences for working with pictures and/or written sheets/flip charts
-
ensuring that any differences in severity of ID (mild to moderate) between individuals in the group are not too wide
-
identifying and supporting people who may face challenges or difficulties with verbal communication (e.g. some people may be able to say only ‘yes’ or ‘no’)
-
preparing people for taking part in a programme and working in a group setting
-
gaining a measure of the level of insight that a person may have about their own health and the perceived relevance of the programme to themselves
-
assessing a participant’s ability to identify and engage with their own priorities, and reflect on their own skills for undertaking change or wanting to change
-
assessing how best to support individuals with decision-making.
Suggested activities relating to what pre-assessment could involve were:
-
speaking to the person with ID [and their carer(s), as appropriate] and carrying out an assessment via discussion/interview using established tools (questionnaires and checklists) or observations
-
extracting relevant information from health action plans and core information
-
eliciting relevant information and knowledge from staff teams involved with the person with ID.
Process and delivery of the programme
Preparing the group for learning
Ensuring that participants are in the right frame of mind or in the ‘best place to learn’ (HCP 07) requires some thought and preparation; one HCP described some of the strategies that they used to promote this during a ‘healthy living’ course. These included participants having two or three short breaks over every 1-hour period, or undertaking physical activity, or being encouraged to be physically mobile during the education sessions:
Because if you get them in the wrong place or they’re not in at the right level of arousal or even in the right mood, this can impact on their willingness and their ability to take in information.
HCP 07
Other ways in which participants’ receptiveness to learn was developed were through watching videos or taking part in practical fun activities.
Choosing methods to promote healthy choices
To deliver knowledge and promote healthy food choices, HCPs described using visual aids, including photographs and pictures of foods from magazines. They also discussed the importance of undertaking practical activities, such as preparing healthy foods. The rationale for these kinds of sessions was to show alternatives in a very literal way. In addition, to try to convey that too much of a particular food was bad for health, it would be necessary to show actual or pictorial images from real life, rather than cartoon images:
. . . rather than saying ‘too many biscuits’, which are words. You want to show pictures of biscuits and you want to show one pack, plus two pack, plus three pack is this much. Stuff like that. So real pictures, or even better real objects.
HCP 04
In relation to the number and type of messages during a session, the advice from HCPs was to keep the messages simple and not to give too many during one session.
Ways of promoting physical activity
Some HCPs suggested that giving an opportunity for participants to experience some of the activities during the education sessions (e.g. swimming or going for a walk) would be an effective way to convey messages about increasing levels of physical activity. If this was not possible, another suggestion was using pictorial images to stimulate discussion about how physical activity could be integrated into someone’s life. This would need to take into account individual needs, such as restrictive budgets, physical ability and level of independence. If going for a walk was not possible for some people, alternatives could be skipping or dancing to music.
The use of open-ended questions
When asked specifically whether or not the use of open-ended questions was appropriate for adults with ID, most HCPs went on to describe using this style of questioning with service users. However, they emphasised the need to follow up this approach with specific and direct questions. This helped to ensure that questions were not ‘too open’ or in danger of being misinterpreted. For example, as one HCP explained:
So sometimes open-ended questions can be too open. You have to be more specific, like . . . for ground rules – ‘What is going to keep us all safe amongst ourselves?’ – Not talking about slips, trips. Do you see what I mean? That you probably do have to tailor it a little bit . . .
HCP 03
Some HCPs also suggested that educators should not assume that commonly used words will always be understood by participants. They emphasised the importance of eliciting understanding on a frequent basis throughout the session and checking for consistency of responses. Other recommended strategies (particularly for those with autism) included giving two options or choices and changing the order of these to check that the participant’s selection is based on informed understanding.
One suggested disadvantage of asking open-ended questions was that it could place undue pressure on some individuals and invoke feelings of distress if they do not know the answer; instead, educators may need to use pictures to encourage a response.
Retention and recall
To aid the retention and recall of messages, the general advice was to use a combination of visual and verbal communication, with opportunities to experientially learn. The need to cater for differences in attention span, types and levels of abilities, and styles of learning, was emphasised. The key message of the interviews was that a flexible approach is needed, including educators (1) gauging understanding at regular intervals and addressing appropriately, and (2) using different methods to facilitate delivery to cater for diversity within a group.
Health beliefs and behaviours
When specifically asked, the majority of HCPs felt that the exploration of health behaviours may be challenging. For some adults with ID, the ability to process thoughts and associate them with behaviours, or to make causal links at a more complex level, may be lacking. The latter, they believed, may partly be influenced by the environment in which people live; service users may have limited or restricted opportunities to be in contact with (or be aware of) other people with a health condition. There may also be a lack of control about dietary choices and/or their association with health conditions. One participant felt that simple associations could be made, such as ‘too much sugar is not good’, and that these have the potential to inform changes in health behaviour (HCP 04).
A divergent view was that people with ID are not any different from the general population in relation to holding health beliefs. It may just be that the communication of these beliefs is different, necessitating educators taking different approaches, or that their beliefs may be more unusual/idiosyncratic.
Understanding the concept of future health risk of developing diabetes and self-reflection
When HCPs were asked whether or not people with ID are likely to understand the concept of risk, there was variation in the responses. This appeared to be related to views on the heterogeneous nature of the population with ID and also possible perspectives that were linked to the professional backgrounds of HCPs.
One view was that people with mild ID may understand the concept of risk but, generally, people with autism would have difficulties. However, it was also felt that this would depend on an individual’s attitude or motivation:
You’re going to have some people who do understand that things change in the future, and things may deteriorate. Then you may have other people who wouldn’t have that concept at all. Particularly you know if you’ve got somebody with autism and the future doesn’t really mean a great deal, because it’s not concrete enough for their understanding and perception quite often. So I think it would depend on the level of learning disability and many other conditions that the individual might have.
HCP 06
A few HCPs discussed the idea that people with ID may have difficulty understanding risk, as they may have a cognitive impairment that challenges their ability to conceptualise, including projecting into the future:
I think those sorts of things are more difficult. A lot of the time we probably are used to working in the here and now. So yes, projecting that this might happen to somebody, I think a lot of people find that difficult, don’t they, to understand.
HCP 03
However, the following participant acknowledged that there was also evidence to show that it is:
. . . possible for people with a learning disability to be able to handle abstract information, reflect on it, appraise it and therefore bring change, but that is probably best done by people skilled in offering those interventions.
HCP 04
Some HCPs suggested ways to explain the concept of future risk, but among these suggestions was a view that this had to be a balancing act between alerting and not scaring:
It’s really tricky ‘cause you don’t want to scare people, and people can get fixated on something and worry about it, and worry about it. And it could become a bit of an obsession, and they could be really worried and scared about that.
HCP 02
The potential dangers of the above approach were discussed with another HCP, who suggested that anxieties could be allayed by discussing the future with ‘positive bits’ (HCP 06).
One of the suggestions put forward for helping to promote self-reflection included using ‘DVD clips’ to show alternative scenarios and facilitate non-threatening reflection (i.e. that it was not about them). Nevertheless, even with this approach, it was acknowledged that it would take many weeks of guided discussion and support to facilitate this process.
Drafting of initial curriculum
Key points for initial curriculum from the literature
At the start of the curriculum development process described in this chapter, a structured literature review was conducted with a focus on existing lifestyle interventions for adults with ID, aimed at the primary prevention of T2DM and/or CVD or modification of risk factors. This was supplemented by reviewing relevant published guidelines, consensus statements, interventions currently in practice and service evaluations.
Later, this was formalised by conducting a systematic review to consider evidence on the effectiveness of multicomponent lifestyle behaviour change interventions for reducing risk factors for T2DM and/or CVD. The methods and findings of the systematic review are presented in Chapter 3.
Key findings from the literature that directly informed the content, theory and process of the programme are described below. There were only a small number of studies with a focus on people with ID and behaviour change lifestyle interventions. Few of these studies163–166 provided a description of their theoretical underpinning, although they did recommend the use of social cognition models, such as the Theory of Planned Behavior245 and Reasoned Action. 246,247
The Healthy Lifestyle Change Program, which was developed by Bazzano et al. ,163 was the only published intervention at this initial stage in the development of the STOP Diabetes programme that outlined a conceptual model. Thus, the STOP Diabetes theoretical conceptual framework, as shown in Figure 21, was influenced by this approach.
FIGURE 21.
Theoretical framework for the education programme. PA, physical activity.
The STOP Diabetes framework that was developed then informed all aspects of the education programme; the framework highlights the importance of an individual’s beliefs about health, ill health and its consequences, specifically the impact on them as individuals and their life. In terms of ‘attitude’, outcome expectancies were explored, that is to say ‘what would happen if I engage in a particular behaviour and how important is the outcome for me?’. Methods of learning, specifically vicarious, observational and concrete kinaesthetic were also highlighted in the literature, as was the importance of social support and peer norms. 163,167,245–247
Self-efficacy is a key component of behaviour change,167 that is, the person’s belief that they can perform the behaviour. However, there are many real barriers to behaviour change in this population, such as disability and/or a lack of control over the physical environment, for instance not being the person who buys or cooks the food. Therefore, the concept of actual behavioural control was included in the theoretical framework to ensure that these issues were addressed in the programme. The influences of strong intentions and a detailed action plan were also acknowledged. Intrinsic motivation and the power of reinforcing feedback loops were also highlighted via distal and proximal reinforcers, and the positive impact on quality of life and psychological well-being. These specific components of the programme can be viewed below (see Table 28).
Additional key lessons from the literature were the need to (1) maximise carer involvement; (2) recognise that people with ID have extremely heterogeneous needs and any intervention would require a multimodal approach; and (3) acknowledge that pre-group preparation is essential.
Key points from the multidisciplinary development group
The overarching framework, content, process and learning methods for the programme were formed at a large multidisciplinary meeting following a systematic process; this meeting was additional to the regular monthly meetings that were held throughout the development process. The qualitative findings from HCP interviews, relevant literature and core theoretical constructs were presented and debated, and a consensus was formed. This was later supplemented by findings from the qualitative interviews with service users (and carers), once available.
The core multidisciplinary team – which included ID nurses, education team members, a qualitative researcher and the lead study researchers – met monthly throughout all stages of the development, supplemented by more frequent meetings at key points in the process.
Key points agreed included:
-
using a concrete kinaesthetic learning style
-
ensuring that the resources developed and methods used to convey messages allowed for tailoring to different levels of intellectual ability
-
developing a specific carer session to engage and promote involvement
-
ensuring that participants were appropriately prepared prior to attendance and at the start of each session
-
reflecting on their own levels of risk
-
self-monitoring diaries and pedometers
-
goal-setting and action planning
-
exploring of barriers and individualised solutions.
The specific methods used to ensure that the themes highlighted above were operationalised are detailed below.
Preparation and grounding
The team agreed that all of the service users (and carers/family members) would need to meet the educator prior to commencing on the education programme. This would allow the educators to confirm suitability to attend the education sessions, make an assessment of any specific needs that would need to be met, and briefly describe the sessions and check willingness to attend.
A specific carer session was to be held prior to the participant education sessions.
To provide familiarity and consistency, the sessions would be delivered in a familiar community facility on the same day and time, with the room set out in the same way each week. Additionally, the same core group of educators would carry out the pre-assessment visits and deliver the programme to ensure continuity and develop rapport.
Style and principles of the education programme
Gaining an understanding of each participant’s emotional and physical well-being prior to each session was seen as essential to allow facilitators the flexibility to meet the needs of individual participants. Therefore, educators were to make time to meet and greet both the participants and carers before the start of each session. Additionally, educators would establish a set of mutually agreed guidelines for the group at the outset of the education session in order to support group functioning.
To meet learning styles within the group and tailor the content to meet individual needs, educators would use multiple methods (to check understanding) and multiple modalities; the additional support of an experienced ID health-care assistant would be utilised during the sessions.
Regular breaks would be taken, as indicated by the group’s expressed need or level of engagement. Educators would use the session material to create concrete examples and develop activities that create movement. In addition, the curriculum would be designed to support the use of recall and repetition to support learning; resources developed by participants – such as posters, postcards, cue/prompt cards – would support individuals to maintain behavioural and lifestyle changes.
Curriculum content and activities
The main behavioural goals and content (see Key behavioural goals of the education programme and Table 28) were to be drawn from previous prevention studies. 240–242 In addition, abstract concepts such as risk and future self were to be developed as activities, games or stories using a concrete kinaesthetic modality. To promote participant engagement in the programme, the educators would use practical and participatory methods, such as food models and images, visual memory aids and short walks using a pedometer. The educators would also use reinforcement methods, including certificates of attendance and attendance cards, as a regular activity within the programme. Self-monitoring activities/opportunities (such as diaries to record food and physical activity outside the session) would be promoted if the participant chose to do these; the opportunity to use a pedometer and scales would also be available to monitor weight when attending sessions. The curriculum would include action planning and goal-setting opportunities (in most sessions) around activity, food and other behavioural goals, supported by individualised resources. The educators would create opportunities through activities to explore barriers and solutions on an individual basis and in group activities.
Key behavioural goals of the education programme
For the STOP Diabetes programme, the key behavioural goals and lifestyle messages incorporated into the education sessions were based on those of the Let’s Prevent programme (nutritional)240 and PREPARE programme (physical activity). 241,242 Specific goals included losing weight, reducing consumption of total and saturated fat, increasing dietary fibre consumption, and increasing physical activity and/or reducing sedentary behaviour (Table 28). However, the emphasis of the STOP Diabetes programme was on enabling the individual tailoring of goals, based on a participant’s needs and abilities, including potential mobility restrictions, level of independence with food shopping and preparation, potential dietary restrictions, opportunities to access the community, cognitive level, and availability and level of carer support required. Therefore, more generalised behavioural goals were emphasised, rather than setting specified targets (see Table 28).
| Specific nutritional and physical activity goals | STOP Diabetes key behavioural goals |
|---|---|
| Weight reduction; sustained weight reduction of > 5% body weight | Choose smaller portions |
| Reduce fat intake from all sources | |
| Reduce sugary drinks and foods | |
| Choose healthier cooking methods | |
| Choose healthier snacks and treats | |
| Increase physical activity/reducing sedentary | |
| Reduce total fat consumption; moderate reduction in total fat to < 30% energy intake | Reduce fat from all sources |
| Choose lower-fat options | |
| Reduce processed foods and ready meals | |
| Choose healthier snacks and treats | |
| Low saturated fat intake; reduce saturated fat intake to < 10% energy intake | Reduce fat from all sources |
| Reduce processed and ready meals | |
| Choosing healthier snacks and treats | |
| Higher fibre intake; increase fibre intake to > 15 g per 1000 calories | Increase fruit and vegetable intake to five-a-day minimum |
| Choose healthier snacks and treats | |
| Increase physical activity/reduce sedentary behaviour; a minimum recommendation of 30 minutes of moderate intensity physical activity per day | Increase moderate intensity activity by increasing steps or adding extra physical activity |
| Reduce sitting time |
Discussion
This chapter describes the first phase of the development of a lifestyle behaviour change programme for adults with ID. We took a pragmatic approach to intervention development, using the Medical Research Council framework for developing complex interventions238 to combine existing prevention programme,240–242 intervention mapping,239 evidence reviews, stakeholder interviews and expert advice. This systematic process allowed us to make the following underlying assumptions for the programme:
-
People with ID have limited knowledge of healthy lifestyle messages.
-
People with ID generally have poorer diet and exercise less often than the general population.
-
Health beliefs, knowledge, motivation and social support are key in promoting behaviour change among people with ID.
-
People with mild and moderate ID need a specially tailored intervention to promote behaviour change; mainstream interventions are not suitable for this population.
Our qualitative findings largely support the literature47–50 in finding that people with ID had limited knowledge about healthy lifestyle messages and experienced barriers in undertaking physical activity. However, we acknowledge that findings from the qualitative exploratory interviews with service users may be limited because of the short interview length (average 9–10 minutes), although this length does not include the additional time taken to explain the study, assess capacity, obtain consent and allow for breaks. We recognise that people with ID are not a homogeneous group; some people found it difficult to concentrate and for other people several visits to allow trust to be built up may have been a better approach. Additionally, in some circumstances, carers (personal and care workers) were not able to be present throughout the whole interview or, if there, they did not agree to participate. It would have been beneficial to purposively seek the views of a larger number of carers (both personal and care workers) at the development stage. However, we were able to obtain valuable feedback from carers during the piloting phases (reported in Chapter 9) and modify the programme accordingly.
Concluding remarks
This chapter has described the first phase of the education development process that was carried out to develop an initial curriculum for a lifestyle education programme for adults with ID. Chapter 9 details a pilot testing and evaluation phase. Chapter 10 outlines a feasibility study that was conducted following development of the education programme. Chapter 11 describes development of an intervention fidelity process that was undertaken for WP3.
Chapter 9 Pilot testing and evaluation of an educational curriculum for prevention of type 2 diabetes
Overview
This chapter describes a pilot testing and evaluation phase, which follows on from work conducted to develop an initial education curriculum (presented in Chapter 8). An additional feasibility phase, which formed part of WP2, is presented in Chapter 10.
Aims and objectives
The aim of this further phase of the development work was to conduct two pilot cycles of testing, evaluation and modification of the initial education programme.
Methods
Following development of an initial curriculum, a pilot phase, which involved two cycles of testing, evaluation, modification and retesting, was conducted (see Figure 20) The first pilot cycle was conducted between April and July 2014, and the second cycle from October to December 2014.
Participants and recruitment
The inclusion/exclusion criteria for WP2 were described in Chapter 8 (see Participants). Those invited to engage in the pilot phase were service users who had taken part in the screening stage (see Chapter 5), had screened positive for IGR or had a BMI of ≥ 25 kg/m2, and at that time consented to being approached to assist with later phases of the research programme. Recruitment followed a similar process to that in the earlier development phase (see Chapter 8, Participants). An initial telephone call was made to potential participants, followed by further information sent in the post or provided at a face-to-face visit.
Delivery of the education
Potential volunteers with ID were approached about attending the education programme, approximately 4–6 weeks prior to the planned programme start date. Carers were invited to an initial session held 1 week before the delivery of the main education sessions. The aim of the carer session was to provide carers with an overview of the education programme, and explore their role in supporting individuals with ID, both within and between the sessions.
Subsequently, the initial curriculum was delivered to a group of individuals with ID. Carers were also invited to attend the sessions to support the service users. Following feedback and refinement of the curriculum (see Refinement), the modified curriculum was then delivered to a second separate group, which, again, was followed by feedback and refinement.
Three educators were involved with delivering the programme at each session: a registered ID nurse, a diabetes specialist with an education background, and an additional ID nurse or health-care assistant in a supporting role. The educator training process for the study is described in Chapter 11.
Data collection
A range of methods was used to evaluate the education sessions and collect feedback. These included observations recorded during the sessions by an experienced researcher, reflections from the educators leading the programme and qualitative interviews with people who received the programme (those with ID and carers). Additionally, subsequent to the first pilot phase, educators were also interviewed to explore their views about the content and style of delivery, experiences from delivering the programme and perceived practical issues. Feedback and reflection on educator training are described in Chapter 11.
Participants were approached to take part in a feedback interview prior to the last session of the education programme. Interviews were held as soon as possible after the final session. Written consent was obtained immediately prior to the interview. Participant interviews took place in July 2014 for the first cycle and December 2014 for the second cycle.
The purpose of these interviews was to explore participant and carer views about the education sessions, to identify whether or not the education sessions resulted in changes to participants’ diet and physical activity, and to inform changes to the next iteration of education sessions based on participant feedback.
Interviews were conducted and analysed by the same qualitative researcher that carried out the previous interviews (see Findings: second pilot phase). Key areas and topics that were explored included experiences of receiving the education programme, ease of understanding, views about the content and style of delivery, usefulness, relevance and practical issues (including duration, provision of support and suggestions for improvement).
Refinement
At the end of each pilot cycle, modifications were made to the curriculum prior to it being used in the next iteration. Modifications and refinements were informed by findings from participant and carer interviews, observations made during the education sessions, and the ongoing reflection and feedback of the educators.
Findings: first pilot phase
Uptake and attendance at education sessions
The first iteration of the education programme was held in a community resource centre. A total of 21 participants were invited to take part in the education programme. Five participants (four of whom had carers) initially agreed to attend the programme. Following the carers’ session, one person (and their carer) withdrew completely. Subsequently, four participants (and three carers) took part in the main education programme. The overall attendance at the education sessions (7 weeks, one session per week) was very good, with one participant (and carer) attending all of the 7 days and three participants attending 6 days (Figure 22).
FIGURE 22.
Uptake and attendance at first and second testing phases of pilot cycle. a, Participants were supported by various care workers who were present for part (or some) of the session(s).
Characteristics of participants
Of the four participants taking part in the first iteration of the education programme, two (50%) were male, the median age was 35 (range 29–60) years, three (75%) lived in a supported environment with family or carers, and one lived independently. None of the participants was in paid employment, one attended college and did voluntary work, and all four participated in other activities within the community.
Feedback interviews: first pilot cycle
Following on from the first iteration of the education programme, all of the participants (n = 4) and carers (n = 3) agreed to be interviewed. One participant was interviewed independently. For the remaining three interviews, carers and participants were interviewed together; in two of these interviews the carers made major contributions to the interviews.
Interviews with participants
Key learning and behaviour changes
One of the participants had previous experience of attending health-related courses/groups and, initially during the interview, suggested that she had not learnt anything new. However, through further exploration of the impact of specific activities/games, it was possible to identify that the programme had reinforced key health messages, including types of healthy/unhealthy foods, portion sizes, and the link between food eaten and body weight:
Mm, just be careful what . . . if you do eat any unhealthy [food] not to have so much of it . . . yeah, makes you think, doesn’t it, er, if you don’t control what you eat, you do put . . . [Interviewer: Weight] . . . Yeah, because they say this country’s, don’t they, obese?
Participant 2, female
The carer of another participant cited the education sessions as helping the participant to become more aware of the changes that he needed to make, although the participant was also receiving a form of therapy (hypnotherapy) that he believed was helping him to make lifestyle changes:
But since we’ve been on the course and you’ve attended the course and they’ve told you this, you’ve become much more aware of it, haven’t you?
I have, yes.
Examples of the dietary changes made by this participant included reducing the portion sizes of less healthy foods, swapping/replacing some foods for healthier alternatives and moderating the amount of alcohol that he drank:
Um, I’ve also learned that, um, alcohol is, kind of, fattening, but as long as you don’t drink it too much . . .
Participant 4, male
Changes to physical activity included doing more walking and going to the gym. Consequently, this participant (and carer) reported that he had initially lost 10 lbs in weight, which was maintained at 7 lbs (immediately following the intervention). However, this participant also reflected that, as he grew older, he was paying more attention to his lifestyle:
I just didn’t really care so much, to be quite honest, in the past. But now that I’m a lot more older, I’m starting to take things more . . . more wrong, aren’t I, Mumsy?
Participant 4, male
Sustaining changes
One participant discussed the wearing of a pedometer (optional) to measure activity; this had helped to quantify her existing level of activity, and to her surprise it was a lot higher than she had thought. However, there was a sense of despondency that could be observed during her interview about the perceived lack of support or encouragement she had in carrying out and sustaining lifestyle changes to her diet and activity levels outside the sessions/in the future. This issue, coupled with her concerns about her personal safety, featured in her decision not to seek more opportunities to go out walking:
[B]ut it’s just someone to go with, you know, encouragement . . . sometimes I give up easy with them sort of things. But I’ve started taking a friend’s dog a walk once a week with my other friend and her dog, so round [park], that’s nice. I go on the walks with the church and that . . . but I’m not walking to [town] or [town] because it’s not safe.
Participant 2, female
She also questioned her ability to make changes. When probed further, she identified that she could make changes if she had encouragement:
But sometimes I can’t believe that I can change it . . . I think I could with a bit of encouragement, you know.
Participant 2, female
For two of the participants who had expressed a desire and commitment to make lifestyle changes, the support and encouragement from carers and others in their lives assisted them with making changes and possibly helping them to sustain these changes. In the case of one participant, the people in his workplace were actively trying to support him to make changes (specifically changes to his diet). He acknowledged that this support helped to keep him on track:
. . . and so the girls there are trying to help and support you, aren’t they . . . in every way they possibly can.
It helps a lot, ‘cause then if I do it all by myself, I’ll be ending up having burgers or something.
Asked if he could sustain the changes he had made to his lifestyle, the participant responded by saying that he felt he could if he focused:
. . . if I put my mind to it.
Participant 4, male
The family and friends of another participant had helped by taking an interest in the the participant’s take-home activities and resources (completed outside the education sessions) and by buying her a bicycle. These were in addition to the dietary and physical activity changes that the family was making. They talked about sustaining these changes even when on holiday:
And she kept going . . . I says, no we’re walking, and it’s very early, but we did it didn’t we, [name]? From the beach right up to the hotel? Yes? Yeah, even though you wanted to sit down!
Carer for participant 3
Symbols and images used to support learning
For some activities, green smiley faces and red sad faces were used to indicate concepts such as healthy and less healthy. However, some participants found these images/symbols unhelpful and confusing, as they were open to misinterpretation or could have meaning that were different from those originally attended in the curriculum. Two of the participants took the red sad face to depict foods that they did not like as opposed to indicating less healthy food:
Now, if you gave him a bowl of salad and said, does that go in the red or the green, he’d put it in the red because he don’t like it.
Carer for participant 1
Another participant placed an unhealthy food on the green sticker because she liked the food.
Reasons for continuing to attend the education sessions
One participant wanted to continue with the education sessions, saying that these had helped him by giving him enjoyment and freedom from work:
. . . to actually carry on with it. ‘Cause I’ve got that much out of the . . . out of the sessions than I would normally do.
Participant 4, male
Other reasons people gave for continuing to attend included meeting other people and learning new activities, enjoying the games/activities and having a sense of achievement on completion.
Only one participant discussed that he would have liked a shorter session, about an hour and a half, and felt that there was too much information, with too much emphasis on food. Another participant felt that the venue may deter some people from attending, as it was one that cared for a range of people with ID (including people with behavioural difficulties) and participants may see other people behaving in a challenging way.
Interviews with carers
Carers benefited from the education sessions in various ways and at different levels. For some, the sessions reinforced prior learning, and for others the sessions motivated and encouraged them to make changes. These are described in more detail below.
Support and encouragement
It was evident from the contributions made by participants and carers during the education sessions that many of them had existing knowledge and understanding of key healthy lifestyle messages. These messages had been gained from their attendance at previous health education/promotion courses and from the media.
We were able to explore this issue with one of the carers, who was asked why participation in the education sessions appeared to have made such a difference to their life when there were so many health messages in the media that they could have acted on (e.g. in relation to physical activity). The carer attributed the impact of the sessions to the person-centred approach, which facilitated their understanding of ‘how’ to make and sustain lifestyle changes:
Well I think how you explained things to us really. I think that were very helpful, it was . . . on a level with me . . . you know? And it was as though you were speaking to each individual . . . Not just a great big party or you got a book . . . you were telling us and explaining more to us how to do it. Yeah, I think that’s what helped me anyway the most.
Carer for participant 3
When specifically asked whether or not she had heard about the benefits of walking before coming to the programme, her response confirmed that she had. However, the education sessions contributed by providing encouragement to put into practice her knowledge about walking:
Oh yes, yes, but we didn’t do it. I think you gave us the encouragement to do it.
Carer for participant 3
Motivation and focus to make changes
On a different level, another carer felt that her levels of knowledge were already higher than those of other people because, as a paid carer, she had previously attended other continuing development sessions. The STOP education sessions had helped to jog and refresh her memory. However, she felt that the sessions had had little impact on her or the participant making further changes, largely because the participant did not really want to make any changes to their lifestyle:
I don’t think you’ve helped [referring to participant], if I’m honest . . . because [referring to participant] you’re a bit stuck in your ways as to what you have and what you don’t want, aren’t you. For me, yes, it sort of jogged my memory and made me think, oh yeah we’ll do this and we’ll do that . . . not just for me with [participant], for me with me other service users as well. But I think, yeah, she can do that and what have you. So, although I’m a bit stumped with you [referring to participant].
Carer for participant 1
Another carer described how the experience of attending the education sessions had motivated and focused her efforts to support the participant in making and sustaining lifestyle changes. Underlying this motivation was a sense of fear for the participant’s future health, namely in case he needed to go on medication, and her concern about this if she was not around in the future:
. . . it’s made us focus. You’ve showed us the little smiley faces. We’ve had to put the smiley faces on the right things and the wrong things, and we’ve focused in with them, you know . . . Um, it makes us focus in to what he’s doing . . . because I don’t want him to take medication, because I know that eventually it’ll be insulin, and if I’m not around, goodness, you know . . .
Carer for participant 4
Additionally, carers identified a number of dietary changes that they had made for the whole family as a result of attending the education sessions, which included reducing sugary foods and fats and increasing fruit intake.
Carers’ session
We tried to ascertain how far the carers’ session had contributed to their understanding of what the education sessions would involve prior to them attending. Unfortunately, very little information was gleaned, as it was difficult for them to remember. One carer explained that they appreciated the carer session because it helped to prepare her and the participant for what the programme would involve:
So it were a bit, sort of, rather than being just chucked in, I had an idea of what we were going to be doing . . . So that I could explain to [name] what we were going to be doing . . . You see, I suppose really, the carers’ session is for my side of things, and other carers coming in who have to do the meals and have a bit of input.
Carer for participant 1
When asked what they would say to a new group of carers to motivate them to attend the next round of education sessions, one of the carers stated:
I’d stand up and I’d say go for it because you learn an awful lot that you think you know, and you don’t until it’s put down on these. I really would.
Carer for participant 3
Interviews with educators
Six educators, who were involved with delivering some or all of the education sessions for the first iteration, were invited for interview. Subsequently, face-to-face semistructured interviews were conducted with five educators, who comprised three registered ID nurses, one diabetes specialist with an education background and one health-care assistant. One educator was male. Four of the educators had ≥ 10 years’ experience in their professional area; the fifth had ≤ 5 years’ experience.
Overall, the educators felt that the education sessions had been positively received by people with ID and their carers. The key findings regarding the process, curriculum content and delivery of the education sessions are presented below.
Self- and peer reflection
The educators reported the process of self- and peer reflection after delivering the education sessions as invaluable for their role. Discussions with educator colleagues at the end of each session helped to identify what worked well and areas for improvement, in terms of adapting resources and identifying any sections of the curriculum or facilitation that required adjustment to meet individual needs on an ongoing basis. This, in turn, helped to iteratively refine and modify aspects of the curriculum, such as resources, and explore different strategies to respond to participant group dynamics.
Venue
The overall view was that a ‘day centre’ was a good environment in which to hold the education sessions because it was familiar to the participants.
Size of the group
Educators felt that the key issue was to balance the need for positive interaction with ensuring that enough support was given to participants to enable them to learn. The minimum number of participants suggested was four, and there was a preference for avoiding ‘double’ figures.
Views about resources
Educators highlighted that some of the resources needed to be modified to promote greater visibility and accessibility for all participants, as they were often used with the group sitting around a table. Suggested solutions included placing posters on a frame (so they could be displayed upright or flat) or using larger sizes of all images, including photographs. The need to avoid shiny paper/laminating was also advocated.
Educators also discussed in detail which participant resources worked well and which might need to be adapted. They highlighted the possibility of reducing the size, content or number of resources so that participants (and carers) were not overwhelmed. Some key issues identified were:
-
Attendance certificates – reduce to one card for the whole programme instead of individual sheets for each week.
-
The participant folder (handbook of resources) needed to be simplified and the overall amount of paperwork needed to be reduced.
-
Sections and inserts needed to be differentiated (e.g. by using colours).
-
Aiding the use of stickers in participant resources – clearer labelling was needed, as were boxes in which to place the stickers.
-
Food diary worked well for some people, but consideration of alternative ways of recording food intake may be needed.
-
Pedometer worked extremely well for some people, but not for others.
-
When discussing ‘health checks’ in the programme, actual equipment rather than images is needed to promote discussion/illustrate.
Views about the overall curriculum, style of delivery and group dynamics
Educators contributed a number of things that were perceived to have worked well within the group:
-
Participants had some prior knowledge that they wanted to apply for themselves; the education sessions contributed towards enabling/supporting this.
-
A ‘happy’ and ‘keen’ group, with ‘characters that complemented each other’.
-
The sessions were perceived to be ‘pitched‘ correctly, although it was acknowledged that for group education it may not be possible achieve this for everyone; a flexible approach (altering language, using different resources) and skilled facilitation helped to address this.
-
A lot of participant (and carer) interest in food and weight reduction.
-
There were visible changes to a participant’s level of confidence over the course of 7 weeks.
-
The bingo (game/activity) was a useful ‘recap’ tool.
-
Allowing time to complete ‘homework’ during the first session was perceived to be a better approach; participants could have been overwhelmed if they were required to take something away to complete on their first day.
There was a general perception that the short walking activity within sessions worked really well on several different levels:
-
It helped to break up sessions.
-
It was energising and helped concentration.
-
It sent a ‘massive message’, particularly to carers, to show how a short walk can result in a lot of steps.
-
It was a huge motivational tool that sparked discussion.
Educators felt that carer involvement had contributed to a positive learning experience, and that ‘carer’ dynamics in the group had worked well. Some of the suggested ways that carers had helped were:
-
identifying difficulties or challenges that participants may have at home and that could impact/affect making lifestyle change
-
helping to support challenging behaviours within the group
-
playing a crucial role in supporting and motivating participants to undertake behavioural changes.
What did not work so well
The primary issue underpinning the education sessions was perceived to be keeping the balance between maintaining the motivation of participants to attend each session and not overwhelming them. A few educators felt that there were too many messages within the curriculum and that these could be reduced, with an emphasis in a future iteration on linking and building on messages. Similar views were expressed about the amount of resources used and a recommendation to review the amount and timing of their introduction at different points within the education sessions.
Some specific difficulties raised that related to the second week of the main programme were that:
-
There was a lot of repetition (but this might have been linked to educators following the curriculum too rigidly).
-
There was too much discussion when pedometers were introduced, and participants found it difficult to make the connection about their results.
Other points for consideration were:
-
The dominance of one participant highlighted a need to explore different ways of addressing this, should it arise again in the future.
-
At times there was too much talking, during which some people were lost within the discussions.
-
A conceptual exercise/activity called ‘Big Daddy’ did not work well with some participants.
-
The pace of sessions was too fast earlier in the programme; this was adjusted in later sessions and was subsequently viewed as working better.
Finally, a few educators recognised that it was difficult to convey the concept of future risk of developing diabetes, and recommended that the next iteration emphasise the importance of providing foundational learning to help motivate and understand healthy eating. Educators also perceived that future follow-up sessions would be an essential part of the education programme and of particular importance in this population. Future sessions were seen as helping with retaining focus, reinforcing positive behaviour changes, recapping learning and identifying progress through practical measures (e.g. weighing on scales).
Findings: second pilot phase
Uptake and attendance at education sessions
The second iteration of the education programme was held in a residential setting. A total of nine participants were invited to take part in the education programme. Several staff (care workers) from the residential home attended the initial carers’ session, and seven participants agreed to take part in the education programme. In general, attendance at the education sessions was good, with three participants attending on all 7 days, one attending on 6 days and the remainder attending on at least 4 days. Care workers from the residential home also attended at various points during the seven sessions (see Figure 22).
Characteristics of participants
Of the seven participants who took part in the second iteration, three (43%) were male; the median age was 43 (range 29–50) years and all seven lived in a residential home supported by carers. One of the participants had paid employment, two did voluntary work and all seven participated in other community activities.
Feedback interviews: second pilot cycle
After the final education session, a total of five participants with ID were interviewed, along with two members of staff (carers) who had attended the education sessions. A care support worker who had attended some of the education sessions provided support to one of the participants during the interview, helping the participant to feel at ease and assisting her to recall and discuss some of the lifestyle changes that she had made. The remaining four participants chose to be interviewed in pairs with their partners. This arrangement worked well in terms of facilitating participants’ recall and support of each other, although it was challenging to ensure that the contribution of both participants to the interview was maximised and balanced.
The presentation of quotations to support the summary of findings includes singular quotations as well as sections of the discussion with the researcher to help contextualise some of the responses to the questions.
Interviews with participants
Enjoyable sessions
It was fairly evident that the education sessions had been an enjoyable experience for all of the participants. One participant was particularly happy about achieving weight loss and being able to share that achievement with his family:
The steps I’ve done, I’m amazed about the certificates. I tell my mum about it, she is very happy . . . They’re pleased about it, and my weight has gone down with it.
Participant 1, male
Other participants talked about the specific things that they had enjoyed, such as being part of a team/group, the resources (‘stickies’) they had used or, for one participant, being enthusiastic about all aspects of the programme:
Group activities . . . and working well as a team.
Participant 4, male
And sticking pictures on the posters, as well.
Participant 5, female
Everything!
Participant 3, female
For three participants, when asked further to expand on what they enjoyed the most, they described sessions and resources relating to physical activity. Their enjoyment appeared to be linked to group walking within sessions, using a pedometer (given to them as part of the programme) to record how many steps they were achieving and recording steps/activity in their physical activity diary in between the education sessions:
That walking around, kept going and going and going . . .
Participant 3, female
Plus the pedometers. Count how many steps . . . Shows how many steps.
Participant 4, male
Um, actually writing about my miles.
Participant 5, female
Key things learnt
In response to a question about what they had learnt from the education sessions, two participants cited physical activity and weight loss:
I’ve learnt a lot.
What kinds of things have you learnt?
To lose more weight.
And exercise.
And exercise more.
And you said exercise, what is it about exercise that you learnt?
It keeps you healthy.
And your heart . . .
And your heart beating.
Establishing if participants associated key dietary and physical activity messages with specific parts of the education sessions proved difficult to elicit, as recall about more detailed aspects of individual sessions was low. Therefore, drawing on observational data that were collected during the seven sessions, the researcher took the opportunity to explore two sessions (storytelling and bingo) that had noticeably demonstrated a high level of engagement and participation, to identify whether or not participants could link these sessions to specific messages.
Both the bingo session and the storytelling session were remembered as enjoyable. One participant’s comments also conveyed the key message he took from the storytelling session:
And the storybooks were absolutely fantastic . . . Yes, I enjoyed it, there’s nobody stopping me reading that, because [name] was showing it, or [name] was showing it, what the whole people were eating, lots of cakes. That’s not good, that’s bad you know.
Participant 1, male
Behaviour changes
All of the participants who were interviewed had discussed during the education sessions that they had lost some weight (ranging from 2 kg to 5 kg). A few of the participants were motivated to lose weight for personal goals that they had set for themselves. During the interviews, participants (or carers) described some of the dietary changes made to help them achieve their goals. These included cutting out fizzy (sugary) drinks, reducing alcohol, eating smaller portions, replacing chips with jacket potatoes, cutting down on puddings and eating more salad:
We used to drink loads of fizzy drinks and we don’t now.
Participant 3, female
You’re not having such big portions . . . you have been trying hard for quite a while to eat better, haven’t you? You don’t have chips; you have a jacket potato on Friday.
Care worker, participant 5
You went down by 2 (kgs)? Your weight went down didn’t it?
Yes.
[Name], what did you do to change things?
Salad.
Every Monday I don’t have puddings.
The feedback also suggested that some participants were consciously focused on undertaking physical activity as a consequence of attending the education sessions:
Not to too many sweets, go for a walk, try to get some more miles down, steps.
So, that’s something that’s changed for you, you’ve increased your steps?
Yes, I’m proving them right, you see.
I’ve done . . . so far I’m going on the walking group and making new friends.
Participant 4, male
I was starting riding my bike long time ago . . . So I’m starting it again.
Participant 5, female
Sustaining changes
In response to a question about whether making the changes had been easy or difficult, two participants acknowledged that making healthier choices was challenging with respect to reducing portion sizes and overcoming the temptation of sweets, which were available in the flat they shared with other residents:
Just getting used to the amount you want and stuff.
Participant 3, female
It’s hard; it’s tempting to have sweets in the flat all the time, that’s what tempting.
Participant 1, male
Care workers appeared to play a key role in helping to motivate and support participants to make and sustain changes to their diet and physical activity. This was illustrated by one participant who had lost a considerable amount of weight; he described how staff (and his partner) had helped him with making healthy food choices and eating smaller portions:
We do [help each other], because my link worker is helping me with my diet. She’s got all these healthy eating in my flat, see what I’ve got in the cupboard. That’s like salad sandwich and wraps as well, and coffee and oranges as well, squash.
Participant 1, male
When asked for examples of how staff had helped him, he responded by describing the following changes:
Eating less, eat salad, eat fresh fruit, coffee, or a sandwich, or something.
Is there anyone else who can help you to carry on with the changes?
Link workers.
Interviews with carers
Sustaining changes
Care staff had already considered ways to sustain the changes made and the motivation of residents after the education sessions stopped:
I’ve said, it’s important we keep it up. So while it’s still fresh and you can run with it, because the weight loss thing, for their only to be one person who hasn’t actually lost weight and even though it’s a little bit . . . We very often get the talk about how from your little acorns grow the big trees.
Care worker 1
Things currently being discussed by care workers were a healthy living course and a weekly physical activity session. According to staff, following residents’ participation in the STOP Diabetes study there was a general increased level of interest in ‘healthy living’, and a few residents had indicated recently that they would like to do more exercise:
Yes, it’s [putting on a health living course] come about because you’ve come here, because of how the residents have responded, but also because I know they’ve made that request about having more exercise.
Care worker 1
Suggestions to facilitate the recall of food messages and to sustain changes outside sessions included having images of breakfast alternatives that could be stuck on to a fridge door. This idea was being tried with one of the participants because he found it difficult to remember.
Amount of information covered in sessions
When carers were asked about what they thought of the education sessions, and specifically about the amount of information, two conflicting views were evident.
One carer stated:
Well, just right, yeah. That was fine for what they were . . . And because it was the mix of those that could write, could write things, but they have their stickers and their pictures.
Care worker 2
Another carer felt, based on her observation of one of the sessions, that there was too much information and that participants might find it difficult to retain all of it:
I think in one session I was at there was a lot of information being given and maybe just simplify a little bit. Maybe just doing very small steps, and even if it’s just one piece of information they learn that session, at least that might have more chance of sticking.
Care worker 1
To help with retention, this carer felt that concentrating on one aspect of diet, such as drinks, might have helped to focus efforts and facilitate discussion about alternatives.
Carer involvement
Carers were asked what educators needed to consider for any future programmes held in residential homes, and they highlighted the need to allow for variation in care worker attendance at sessions to support participants, as a result of organisational pressures (including low staff numbers).
The above discussions also elicited further suggestions for encouraging carer involvement, including the need to:
-
educate staff about what they will need to consider for people for whom they are responsible
-
enthuse and engage staff to help with practical support, such as completing the diaries and resetting the pedometer
-
provide information about alternative (healthier) food choices/options and portion sizes.
Modifications made to curriculum after the pilot cycles
A number of modifications were made to the programme based on feedback from participants and carers, observations made during the education sessions, and the ongoing reflection and feedback of the educators. Modifications consisted of refinement of resources together with adaptations to educator facilitation within the sessions.
Revisions made after the first cycle
The main revisions made after the first cycle included the following.
Modifications to the carer session
-
Reducing the amount of information provided at this session.
Modifications to education sessions
Participant resources
-
Reducing the amount of worksheets given out at any one time. For the first pilot phase, all of the activity sheets that were developed for the 7-week programme were given to participants in week 1 in a folder format. This caused distraction for some participants and impacted on the delivery of the session. Subsequently, this was changed to allow the work sheets to be provided directly at the point at which they were required in the programme, and for participants to add them to their programme folder on a weekly basis.
-
Simplifying the physical activity diary to a single sheet of A4, with a table to record the date, steps/activity and new goal, from a multipaged booklet.
-
Making the image cards (used to facilitate and support learning, recognition, recall and summaries) a much larger size.
-
Using realistic images and/or photographs in resources. Images were sourced and checked with service user groups prior to being changed.
Session content
-
Reducing/simplifying the content of some sessions. Providing too much information led to participants becoming disengaged.
-
Changing the symbols used to illustrate healthy and less healthy foods, as these were not universally understood by participants. Possibly use a menu of symbols tailored to individual cognitive needs. Ensure that educators explain and check understanding when symbols are used.
Maintaining and maximising engagement
-
Educators to create opportunities for movement, both within the room to engage in different activities and a short walk during each session to address participants becoming disengaged when sitting for longer periods. Additionally, to use the walking activity to highlight the number of steps achieved in 5–10 minutes of walking.
Communication aids
-
Using communication cards (with symbols/pictures) as an aid to manage discussions in the group and facilitate engagement of people who experience difficulty communicating.
Revisions made after the second cycle
Modifications to education sessions
Maintaining and maximising engagement
-
Allowing for educator flexibility to adjust the timetable and breaks to suit the needs of individuals, the group dynamic, energy levels and engagement.
-
Educators and supporting staff to be aware of the diversity and dynamics in the group, and to arrange the seating and positioning of participants to support engagement and one-to-one support when required.
-
Including more interactive games/activities, such as bingo and board games, to promote engagement.
Participant resources
-
Including a menu of options to encourage prompts and motivation towards goals. For example, fridge magnets may not be useful to those in a residential setting if they do not have their own fridge.
-
Incorporating photographs of participants into their ‘health checklist’, as a way of personalising documents, and helping individuals to relate this information to themselves.
Outline of the STOP education programme
An overview of the final education programme that was developed, prior to using in the feasibility phase (see Chapter 10), is outlined.
First, the initial carer session, which is held prior to the main education programme, is presented (Table 29). Second, in Table 30, the outline structure of a typical session in the main programme is outlined. Finally, for each individual session (weeks 1–7), the topic areas, the main aims and the key activities/resources that are designed to support learning and behavioural changes, both within and between sessions, are also presented (Tables 31–33).
| Session name | Overview and main aims of activities | Time |
|---|---|---|
| Welcome and introductions | To introduce the educators | 15 minutes |
| To understand the role of any observers | ||
| To be aware of the style and aims of the course | ||
| To ask questions related to the course | ||
| Outline of education course | To be aware of practical aspects (venue, times, number of sessions) | 10 minutes |
| To be aware that carers can attend with participants | ||
| What is different for people with ID? | To have an opportunity to share their thoughts about learning needs of the person for whom they care | 10 minutes |
| To share their thoughts about supporting people with ID to make lifestyle and behavioural changes | ||
| Course content | To be aware of course content and resources | 60 minutes |
| To be aware of course activities and the support that participants may need between sessions to complete | ||
| What is my role as a carer? | To explore the benefits of attending the course with the participant | 15 minutes |
| To explore the potential health benefits for the person they are supporting, and themselves, of attending | ||
| To be aware of their potential role in supporting the participant who chooses to make lifestyle and behavioural change | ||
| Questions and concerns | To have an answer to any questions | 10 minutes |
| To have concerns explored and addressed | ||
| Total | 2 hours | |
| Session | Aims and activities | Time |
|---|---|---|
| Welcome: ‘Welcome and getting to know you – week 1’ or ‘Welcome back – weeks 2–7’ | To ground and settle participants | 15 minutes |
| To outline the aims and style of the course | ||
| To outline the topic areas for the day | ||
| To reflect on actions from previous sessions – celebrate achievements and identify/explore barriers | ||
| To develop a good working relationship between educator and participants | ||
| Topic area 1 | Explore a different topic area each week | 30–45 minutes |
| Break | 15-minute break allocated within the session | 15 minutes |
| Breaks to be taken flexibly according to the expressed needs of the group or as indicated by educators’ assessment of engagement in the session | ||
| Topic area 2 | New topic area or may build on/consolidate learning from the earlier session | 30–45 minutes |
| Questions and preparation for next week | To provide an opportunity to express concerns, ask questions relating to the session | 10 minutes |
| To provide information and prepare participants/carers for activities between sessions | ||
| Total | Minimum 100 minutes, maximum 130 minutes |
| Week | Overview of main aims | Activities and resources | Theory |
|---|---|---|---|
| Week 1 | |||
| Topic area 1: ‘What is health? Being healthy and unhealthy’ | To explore what the concept of being healthy means to the individual | Healthy and unhealthy character poster Images to prompt recognition and recall Main message summary cards |
SRT, TPB |
| To explore the behaviours linked to health | |||
| Develop images that represent healthy and unhealthy characters that are used as a learning tool throughout the programme | |||
| Topic area 2: ‘What can go wrong with my health?’ | To explore the health consequences of lifestyle and behavioural choices | Images to prompt recognition and recall | SCT, SRT, TPB |
| To explore lifestyle and behavioural choices that promote health | |||
| To have an opportunity to express their emotional response to the different lifestyle choices the characters make | |||
| Week 2 | |||
| Topic area 1: ‘This is me’ and ‘Health checks my doctor or nurse will do’ | To create an image that represents the individual, their lifestyle and behavioural choices | Personal lifestyle and behaviours activity sheet Images to prompt recognition and recall Health profile with photograph of individual Biomedical data Coloured stickers |
SCT, SRT, TPB |
| To be aware of the health checks that a doctor or nurse will do and be provided with their own biomedical data and risk factors | |||
| To be aware of which results may be a problem to their health by placing a sticker on profile | |||
| Plot results on a health profile | |||
| Topic area 2: ‘What can I do to stay healthy?’ | To explore the impact of the biomedical results and risk factors on their own health | Images to prompt recognition and recall Confidence activity sheet |
SCT, SRT, TPB |
| To express any concerns/emotions relating to their results | |||
| Recall the consequences of lifestyle and behavioural choices | |||
| To explore lifestyle or behavioural choices relating to their risk factors | |||
| Have the opportunity to choose and record lifestyle or behavioural changes on a personal poster | |||
| Record level of confidence to make this change | |||
| Week | Overview of main aims | Activities and resources | Theory |
|---|---|---|---|
| Week 3 | |||
| Topic area 1: Being active | To explore what being active means | Images to prompt recognition and recall Physical activity record Pedometer Walking activity |
SCT, SRT, TPB |
| To be aware of the consequences to health of being inactive | |||
| To explore the benefits to health of being active, moving more and sitting less | |||
| To have an experience of using a pedometer to measure steps | |||
| Topic area 2: Me and my activity | To have an experience of a short walk and recording steps or activity in a diary | Images to prompt recognition and recall Confidence activity sheet Create prompt cards, send a postcard or create a fridge magnet to promote engagement with their goal within the session and between sessions |
II, SCT, SRT, TPB |
| To identify ways to increase activity by adding an activity, increasing step count and/or reducing sitting time | |||
| To record personal confidence to carry out their chosen goal | |||
| To create a prompt or reminder for their chosen goal | |||
| To record activity in a diary | |||
| Week 4 | |||
| Topic area 1: How did I do with my activity? | To reflect on current level of activity | Interactive dice game to explore barriers Physical activity record Walking activity (optional) |
SCT, SRT, TPB |
| Reflect on feelings related to level of activity | |||
| To explore own and listen to other group members’ barriers to physical activity | |||
| To explore strategies for overcoming barriers | |||
| To experience a short walk to highlight the increase in steps from short periods of activity | |||
| To identify a new steps or activity goal for the coming week | |||
| Topic area 2: Changes I can make to be healthy | Recall the lifestyle and behavioural changes that influence risk factors | Personal lifestyle and behaviours activity sheet Storybook Food diary |
II, SCT, SRT, TPB |
| To be aware of the impact of unhealthy lifestyle and behavioural choices over many years – this is facilitated by using a storybook | |||
| Recall the personal lifestyle and behavioural choices recorded in session 2 | |||
| Reflect on progress with these choices | |||
| Participants prepared and facilitated to explore sources of support for recording food, drinks and snacks over the next week | |||
| Week | Overview of main aims | Activities and resources | Theory |
|---|---|---|---|
| Week 5 | |||
| Topic area 1: How did I do with my activity? Eating well, eating healthy | To reflect on activity levels over the last week | Physical activity record Walking activity (optional) Food models and images to support recognition and recall Food sort task Stickers |
II, SCT, SRT, TPB |
| Generate ideas for overcoming barriers | |||
| Plan a new activity/step goal | |||
| Walking activity (optional – decision made collaboratively by the group) | |||
| Recall the main messages relating to health | |||
| Identify foods that relate to a healthy lifestyle | |||
| Identify foods that contribute to being unhealthy | |||
| Have an awareness of the consequences of high fat, sugar and large portions on health | |||
| Be aware of the consequences of lower fat, sugar and smaller portions on health | |||
| Sorting activity with food models and images | |||
| Topic area 2: Changes I can make to eat well and eat healthy | Recall the food messages from the earlier session | Food models and images Food diary Create prompt cards, send a postcard or create a fridge magnet to promote engagement with their goal within the session and between sessions |
II, SCT, SRT, TPB |
| Record personal confidence to make a change to food choices | |||
| Identify one or two small changes to make to personal food choices based on their food diary | |||
| Create personal prompts to behaviour change | |||
| Week 6 | |||
| Topic area 1: Where am I with my activity? | To reflect on activity levels over the last week | Physical activity record Food diary Food bingo activity Bingo prize for the winner |
II, SCT, SRT, TPB |
| Generate ideas for overcoming barriers | |||
| Plan a new activity/step goal | |||
| Walking activity (optional – decision made collaboratively by the group) | |||
| Recall the main food messages related to health and being unhealthy by participating in an interactive game | |||
| Recall the consequences of food choices | |||
| Topic area 2: How am I doing with my eating well, eating healthy? | Reflect on the food diary | Food diary Barriers board game Create or amend prompt cards, send a postcard or create a fridge magnet to promote engagement with their goal within the session and between sessions |
II, SCT, SRT, TPB |
| Identify successes and barriers to making changes to food choices | |||
| To explore own and listen to other group members barriers to making changes to food choices | |||
| To explore strategies for overcoming barriers with an interactive game | |||
| Explore how to reward personal success | |||
| Identify sources of support to reach goals | |||
| Plan a new food goal | |||
| Week 7 | |||
| Topic area 1: What have I learnt? | Reflect on activity levels and the food diary over the last week | Food diary Activity diary Healthy and unhealthy character posters Personal lifestyle and behaviours activity sheet Images to prompt recognition and recall |
II, SCT, SRT, TPB |
| Review the overall programme and raise any outstanding concerns or questions | |||
| Recall main points of the programme and revisit associated activities | |||
| Identify successes and barriers to making changes | |||
| Topic area 2: What can help me to keep going with changes to my food and activity levels? | Record personal changes on activity worksheet | Worksheet to record personal changes that have been made Postcards, fridge magnets, flash cards and stickers Prompt card to give to carers to ask for help Confidence activity sheet Course attendance certificates |
RP, SCT, SRT, TPB |
| Explore possible solutions to barriers | |||
| Explore strategies to support the maintenance of changes | |||
| Set new goals and use strategies to help such as writing a postcard to themselves to be sent in 3 months’ time or creating fridge magnets | |||
| Record personal confidence to carry out their chosen goal | |||
| Explore sources of support to help achieve these goals | |||
| Celebrate success | |||
Concluding remarks
This chapter and the previous chapter (see Chapter 8) has described the development and pilot phases (testing, evaluation, modification and retesting) that were carried out in order to develop a lifestyle education programme for adults with ID.
The STOP programme development benefited from a systematic process. 238,239 The theoretical underpinning was developed and expanded on from the limited evidence in the literature. This informed the content and style of approach, alongside the qualitative findings from people with ID, their carers and HCPs with expertise in working with people with ID. The whole programme was then tailored further to the specific needs of this group by more user feedback, and adaptation by a multidisciplinary team with expertise in ID and the development of education programmes.
From the initial phases the programme has been well received and is acceptable to the people it is trying to support. The initial feedback via qualitative interviews has suggested that some of the elements of treatment receipt that were initially hypothesised may have been achieved via reported changes in beliefs and health behaviours.
Chapter 10 details the feasibility phase. Chapter 11 details the intervention fidelity of the education programme.
Chapter 10 Feasibility study of STOP Diabetes programme
Overview
This chapter describes a feasibility phase, which follows on from the education development work described in Chapters 8 and 9, and forms part of WP2.
Aims and objectives
The aim of this substudy was to assess the feasibility of collecting outcome measures for participants with ID before and 3 months after they attend the lifestyle education programme.
Methods
Study design
Following initial development, testing and refinement of the curriculum (see Chapters 8 and 9), the education programme was delivered to another group of participants to assess the feasibility of collecting pre- and post-intervention outcome measures.
Study setting
The feasibility phase was conducted between January and June 2015.
Participants
Inclusion criteria
Eligibility criteria for the feasibility phase were the same as those for the two pilot phases described in Chapter 8, that is, service users who:
-
had participated in the screening stage (see Chapter 5)
-
screened positive for IGR or had a BMI of ≥ 25 kg/m2
-
indicated a willingness to assist with later phases of the research programme
-
had mild to moderate ID
-
were able to stand and walk at least short distances
-
were able to attend group education sessions
-
were not taking part in any other intervention study.
Participant recruitment and consent
Participants were recruited following a similar process to earlier phases (see Chapter 8, Participants). Potential participants received an initial telephone call to gauge their interest, and this was followed up by further information sent in the post or provided at a face-to-face visit.
Volunteers were invited to attend an initial appointment at a convenient time for them, in which information was provided about the study and informed consent was obtained. Consent was obtained following a similar process to that described previously (see Chapter 5, Informed consent). People were asked to consent to the collection of baseline data, attendance at the education programme and collection of 3-month follow-up data.
Data collection
Baseline data collection
Baseline data were collected using the same schedule as in the screening study (WP1, see Chapter 5). If participants had taken part in the screening stage within the last 3 months, and valid measurements had been successfully obtained, these data were used for baseline values. If a participant took part in the screening stage > 3 months ago then the measurements were repeated.
Data collected included:
-
weight
-
height
-
BMI
-
waist circumference
-
BP
-
dietary intake (fruit, vegetables and salad).
Physical activity (time spent in light, moderate and vigorous activity) and sedentary behaviour (time spent sedentary) were measured using the wrist-worn accelerometer (GENEActiv). The process for measuring activity using this accelerometer has previously been described in Chapter 7.
Three-month data collection
At 3 months following the end of the education programme, participants were recalled for repeat data collection, as per at baseline.
Outcomes
Particular outcomes of interest in terms of the feasibility included (1) the proportion of people invited who attend the baseline appointment, education programme, individual sessions within the programme and 3-month follow-up; and (2) the completeness of key data items at baseline and 3 months’ follow-up.
Problems encountered during data collection appointments and implementing the education were also considered.
Delivery of intervention
Following on from the pilot phase of testing, evaluation and modification described in Chapter 9, the final modified education programme (see Chapter 9, Outline of the STOP education programme) was delivered to another group of adults with ID, and their carers, at a local community venue.
Sample size
We aimed to conduct the feasibility study with at least one group of participants (four to eight people with ID, plus carers).
Data analysis
The feasibility of recruiting adults with ID to attend for baseline data collection, education sessions and 3-month follow-up data collection was assessed using a flow diagram to summarise dropouts at each stage. Completeness of outcome data collected at each stage was summarised using counts and proportions.
Findings
Study timelines for the feasibility phase enabled us to run one iteration of the education programme, with collection of before-and-after measurements.
Recruitment and consent
In total, 19 participants were invited to take part in the feasibility phase, of whom five (26%) agreed to attend an initial appointment at the end of February 2015, at which consent and baseline data were obtained. All of the participants had the capacity to give consent for themselves, without the need to involve a consultee.
Feasibility of collecting baseline data
At baseline, all of the participants required measurements to be taken, as it was > 3 months since they had originally taken part in the screening study. Baseline data were obtained for all five participants, with the exception of physical activity data that were collected for only four (80%) participants, as one person refused to wear the accelerometer (Table 34).
| Measurements | Baseline, n (%) | 3 months’ follow-up, n (%) |
|---|---|---|
| Weight (kg) | 5 (100) | 5 (100) |
| Height | 5 (100) | 5 (100) |
| BMI (kg/m2) | 5 (100) | 5 (100) |
| Waist circumference (cm) | 5 (100) | 5 (100) |
| Systolic BP (mmHg) | 5 (100) | 4 (80) |
| Diastolic BP (mmHg) | 5 (100) | 4 (80) |
| Portions of fruit and vegetables | 5 (100) | 5 (100) |
| Physical activity and sedentary behaviour (time spent in light, moderate, vigorous intensity activity; time sedentary) | 4 (80) | 3 (60) |
Uptake of and attendance at education programme
Following the baseline appointment, all five of the participants took part in the education programme, which was held from March to April 2015. A total of four carers (two paid care workers and two family members) attended at least some of the sessions (three regularly). Overall attendance at the education sessions was good: four (80%) participants attended on ≥ 5 days and two participants attended on all 7 days (Figure 23). However, one participant attended on only 3 days. It is important to note that the most common reason for not attending sessions was other existing commitments such as appointments and holidays.
FIGURE 23.
Uptake of and attendance at data collection and education sessions.
Feasibility of collecting data at 3-month follow-up
All of the participants agreed to attend a 3-month follow-up appointment at the end of June 2015 to obtain repeat measures. Four of the participants (80%) attended the appointment as arranged; one participant needed to have a second appointment arranged because they did not attend the first (this was the same participant who attended only 3 days of the education programme).
Anthropometric measures (weight, BMI, waist circumference) were obtained for all of the participants (see Table 34). BP readings were successfully obtained for four (80%) participants. Accelerometer data were obtained for three of the four participants who wore one at baseline; four participants initially agreed to wear the accelerometer, but one participant later telephoned the research team to inform them that he had changed his mind.
Characteristics of participants
The key demographic characteristics for the five participants are presented in Table 35. Four (80%) participants were male, the median age was 40 (range 20–51) years and all were of white ethnicity. Two participants lived alone, one lived in supported living accommodation and two lived in a family setting; the majority (n = 4, 80%) had support from a carer for at least some of the time. None of the participants was in paid employment, two did voluntary work on a regular basis, and two attended college and other community-related activities,
| Characteristic | n (%) |
|---|---|
| Age (years), median (range) | 40 (20–51) |
| Sex, male (%) | 80 |
| Ethnicity | |
| White | 5 (100) |
| South Asian | 0 |
| Other | 0 |
| Accommodation | |
| Alone | 2 (40) |
| With family/carers | 2 (40) |
| Residential/supported living | 1 (20) |
| Support from carer | |
| Yes, at least some of the time | 4 (80) |
| No | 1 (20) |
Baseline data
Biomedical and lifestyle characteristics, from data collected at baseline, are presented in Table 36. The median values for weight, BMI and waist circumference were 110.2 kg, 36.1 kg/m2 and 114.3 cm, respectively. For BP, the median systolic value was 123 mmHg and the mean diastolic value was 85 mmHg. Reported daily intake of fruit, vegetables or salad indicated that only two (40%) participants were eating the recommended five or more portions a day (median 5). For physical activity, the median minutes per day for MVPA and light-intensity activity were 107.5 and 93.0, respectively; the median time spent sedentary was 555.0 minutes per day.
| Participant | Pre/post | Systolic BP (mmHg) | Diastolic BP (mmHg) | Weight (kg) | BMI (kg/m2) | Waist (cm) | Fruit and vegetables (portions) | Physical activity – time in MVPA (minutes/day) | Time in light-intensity physical activity (minutes/day) | Sedentary behaviour – time spent sedentary (minutes/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pre | 117 | 87 | 133.0 | 36.1 | 136.8 | 7 | 153.0 | 126.0 | 708.0 |
| Post | 115 | 80 | 136.4 | 37.0 | 137.0 | 5 | 51.0 | 18.0 | 150.0 | |
| 2 | Pre | 101 | 66 | 84.4 | 36.1 | 112.3 | 3 | 106.0 | 102.0 | 672.0 |
| Post | 106 | 68 | 82.6 | 35.3 | 113.5 | 5 | 115.0 | 120.0 | 708.0 | |
| 3 | Pre | 124 | 83 | 110.2 | 36.4 | 117.5 | 1 | 109.0 | 84.0 | 438.0 |
| Post | 116 | 84 | 106.0 | 35.0 | 117.0 | 5 | 154.0 | 96.0 | 402.0 | |
| 4 | Pre | 123 | 86 | 112.4 | 37.6 | 114.3 | 6 | n/a | n/a | n/a |
| Post | n/a | n/a | 130.0 | 43.4 | 137.4 | 0 | n/a | n/a | n/a | |
| 5 | Pre | 146 | 85 | 88.6 | 28.3 | 108.1 | 3 | 91.0 | 60.0 | 366.0 |
| Post | 130 | 83 | 87.6 | 28.0 | 102.5 | 3 | n/a | n/a | n/a | |
| Overall | ||||||||||
| Median (range) | Pre | 123 (101–146) | 85 (66–87) | 110.2 (84.4–133.0) | 36.1 (28.3–37.6) | 114.3 (108.1–136.8) | 3 (1–7) | 107.5 (91.0–153.0) | 93.0 (60.0–126.0) | 555.0 (366.0–708.0) |
| Post | 115.5 (106–130) | 81.5 (68–84) | 106.0 (82.6–136.4) | 35.3 (28.0–43.4) | 117 (102.5–137.4) | 5 (0–5) | 115.0 (51.0–154.0) | 96.0 (18.0–120.0) | 402.0 (150.0–708.0) | |
Three months’ follow-up data
Data collected at 3 months’ follow-up are also presented in Table 36. The main aim of the work conducted was the feasibility of collecting data at two time points, and not looking for change.
However, on an individual basis, three participants lost weight (range 1.0–4.2 kg), but two participants gained weight. The participant who had gained a lot of weight (17.6 kg) had poor attendance at the education programme (three out of seven sessions); anecdotal evidence at 3 months’ follow-up suggested that significant changes had occurred in this participant’s personal circumstances since baseline, including moving out of the family home to live independently in his/her own flat.
When data were available, participants showed improvements in physical activity levels and sedentary behaviour at 3 months’ follow-up compared with baseline.
Discussion
Overall, the findings suggest that it is feasible to collect outcome measures at two time points: before and 3 months after attendance at a lifestyle behaviour modification intervention.
Twenty-six per cent (n = 5) of people who were invited to take part in the feasibility phase agreed to participate. At baseline, anthropometric measures and BP were obtained for all of the participants and accelerometer data for 80%. Attendance at the education programme was good overall, with 80% of participants attending on ≥ 5 days (out of seven sessions for the main programme). At 3 months’ follow-up, repeat data were successfully collected for a high proportion of participants (anthropometric measures 100%, BP 80%, accelerometer data 60%).
It is acknowledged that the feasibility phase involved only a very small number of participants (n = 5) and carers. Owing to time restrictions we were able to conduct only one feasibility cycle (delivery of intervention, plus pre- and post-intervention measures). However, the feasibility phase used robust processes, which were informed by the preliminary findings from the screening phase and lessons learnt from the earlier phases of education development and delivery.
It is recognised that the feasibility phase was not aimed at seeing significant findings from baseline to post-intervention follow-up. However, at 3 months there were some beneficial changes for most participants.
These preliminary findings are positive, but we are unable to assess whether or not it is possible to collect longer-term data, beyond 3 months post intervention or at repeated time points. The 8-week educational intervention developed as part of WP2 appears to be both feasible and acceptable to people with ID (and their carers), who are identified as being at high future risk of T2DM and/or CVD. However, the intervention is yet to be tested more robustly via a RCT, including possible follow-up maintenance education sessions. Findings from the development and feasibility phases provide valuable data to help inform future research.
Concluding remarks
This chapter has described a feasibility phase that was carried out to assess the feasibility of collecting outcome measures for participants with ID before and 3 months after they attend a lifestyle education programme. Development of the education programme was described in Chapters 8 and 9. Chapter 11 details the intervention fidelity of the education programme.
Chapter 11 Intervention fidelity process
Overview
The methods and results of the intervention fidelity process for WP3 are described below.
Rationale
Intervention fidelity relates to how an intervention is delivered in practice and whether or not the delivery of the intervention varies according to the context. It is now recognised as a key component in the evaluation of complex interventions,238,248,249 enabling the assessment of reliability and validity of an intervention and the process factors both advancing the study aims and reducing premature abandonment of future interventions. 250
The assessment of intervention fidelity is seen as particularly important when interventions are evaluated using multicentre RCTs because there is risk of delivering and/or receiving the intervention differently between sites. 249 It was anticipated that findings from the current research programme would inform a future multicentre RCT.
The Diabetes Education and Self Management for Ongoing and Newly Diagnosed (DESMOND)251 model of structured education,251 which underpins work conducted in this chapter, draws on theoretical and philosophical perspectives from both health psychology and education;252–254 patient empowerment is at its centre. DESMOND programmes meet nationally agreed quality criteria255 for patient education, including delivery by trained and accredited educators, and quality assurance.
One of the key components of quality assurance is intervention fidelity. In practice, assessment of intervention fidelity involves appraising education delivery, with particular emphasis on the assessment of educator behaviours. The DESMOND251 education approach purports that individuals, in the main, are responsible for making their own choices around self-management. Barriers outside the person’s control are acknowledged, but the role of the facilitator or educator is to encourage the participant to explore his/her motivations for self-management or engaging in health promoting behaviours, rather than telling them what to do; the former set of behaviours can be attributed to being ‘DESMOND’251 and the later are non-DESMOND. Further details of the methods used to assess educator behaviour and the DESMOND251 and non-DESMOND251 approach, are outlined below (see Preliminary assessment of educator behaviour, and Table 38).
The original programme of work included a pilot RCT to assess the effectiveness of the lifestyle education programme (intervention) developed. However, amendments to the programme of research requested by the National Institute for Health Research (NIHR) determined that a feasibility phase was conducted (described in Chapter 10) rather than a RCT. Thus, data collection from multiple iterations of the programme and across different educators was not practicable. The amended aims and objectives, as outlined in the next chapter (see Chapter 12, Methods), take the above into account.
Aims and objectives
The primary aim of this component of the research programme was to conduct preliminary work towards developing an intervention fidelity process and tool that is specifically tailored to this population.
The specific objectives were to:
-
develop an outline educator training programme
-
conduct a preliminary assessment of educator behaviour using an existing quality development tool
-
identify key important adaptations to the existing tool for use in a programme developed for the population with ID in the future.
Methods
Educator training
The educators for delivery of the intervention (outlined in Chapter 10) were a registered ID nurse and a diabetes specialist with an education background, with support from an ID nurse or health-care assistant.
The process of training the educators involved, first, professional development around the DESMOND251 education programme. This stage took place between January and March 2014, and included observations of DESMOND251-based programmes and attendance at a core DESMOND251 training day. This provided an introduction to the theoretical basis, philosophy and core skills of DESMOND. 251
Second, the educators attended an initial study-specific training day in April 2014, when they were introduced to the theoretical component, content, structure and delivery of the STOP Diabetes education programme (see Table 37). This was followed by a second study-specific day in June 2014. A staged approach was used for delivering the educator training to take into account any necessary changes to the programme (curriculum content, delivery and structure) based on the delivery of the early sessions.
The training was delivered by two members of the development team, a consultant clinical health psychologist and diabetes specialist nurse with an education background. Training included detailed information on the content, structure and delivery of the programme, and incorporated additional support and considerations around working with people with ID. Guidance around indirect approaches for educating participants (e.g. role-playing behaviour change techniques) and direct approaches (e.g. presentations) were included.
Educators used a specific training curriculum to equip them to support the delivery of education sessions and were encouraged to use personal reflection and peer review tools to reflect on their delivery. They were also supported with mentorship from trainers attached to the research team.
An outline of the training programme is presented in Table 37. The first training day covered the first five sessions of the education programme (the carers’ session and weeks 1–4).
| Session name | Overview and aims | Time (minutes) |
|---|---|---|
| Welcome and introductions | Explore expectations and concerns about training | 15 |
| Outline the style and aims of the training | ||
| What is different about group self-management education for people with ID | Exploring participants’ experience of delivering education to people with ID | 30 |
| Exploring how working with a group may be different from one to one | ||
| Methods to promote learning in this programme | ||
| Prevention and health messages | Exploring the key messages for CVD and diabetes prevention | 30 |
| Explore the key messages for the education programme | ||
| Key messages for each session | Provide an opportunity to review the curriculum and identify the key messages and activities for each session | 30 |
| Development and theoretical underpinning | Recap on the theories that underpin this programme | 60 |
| Providing an opportunity to explore their own health behaviours and making a plan | ||
| Lunch | ||
| Carer session | Review curriculum and aims of session | 45 |
| Explore how to engage carers | ||
| Sessions: weeks 1–3 | Review curriculum and resources for sessions 1–3 | 60 |
| Identify any challenges to delivering these sessions | ||
| Session: week 4 and resources | Review curriculum and resources for week 4 | 40 |
| Be aware of other resources available for use with the programme | ||
| Identify any challenges to delivering the session and using the resources | ||
| Overcoming challenges to delivering the sessions | Recall the challenges identified | 50 |
| Explore options for overcoming the challenges | ||
| Reflection and feedback tools | Explore the purpose of the reflection and feedback tools | 15 |
| Explore strategies to increase confidence to deliver the programme | ||
| Next steps | Give out food diaries, pedometers and activity diaries to provide experience of self monitoring and behaviour change | 15 |
| Explore the benefits of experiencing using the tools and self-monitoring that participants will use | ||
Day 2 educator training, which covered the curriculum and resources for the final three sessions (weeks 5–7), followed a similar format to the first training day, taking into account feedback relating to the initial training. In addition, educators were able to feed back their experiences on delivery of the first four sessions, and to reflect on their own self-monitoring and behaviour change experiences.
Evaluation of educator training
Subsequent to completion of their training, educators completed an evaluation form (see Appendix 20) for the STOP Diabetes training sessions. Following their first delivery of the education programme, educators involved in delivering the sessions were interviewed by a qualitative researcher to explore their views about the content and style of delivery, experiences from delivering the programme and perceived practical issues; feedback from these interviews is presented as part of the first pilot phase (see Chapter 9).
Preliminary assessment of educator behaviour
The quality development tool used to conduct the preliminary assessment of educator behaviour was based on current assessment tools developed by the DESMOND collaborative;251 which recognises that training of health professionals to deliver education programmes does not always result in appropriate or consistent delivery of the programmes. 256
The tool was developed in 2015 and consists of five ‘global’ categories, each containing specific items to evaluate programme delivery; it is a revision of a previous DESMOND tool. 257 The tool is designed to be used by a separate observer who assesses the educator’s behaviours when they teach/deliver education. Each item in the tool represents a discrete DESMOND251 behaviour, which is paired with an ‘opposite’ item (labelled as a non-DESMOND251 behaviour), which are coded when observed. The observer is asked to rate which behaviour (i.e. DESMOND251 or non-DESMOND251) is most commonly seen during the training session. For example, the first item requires the observer to assess whether or not the educator uses open body language to support engagement of participants (Table 38). DESMOND251 behaviours for this item include nodding and smiling at the participants, and non-DESMOND251 behaviours include avoiding eye contact and the educator turning their back on the participants when asking a question. By noting down these behaviours when they occur, the observer can determine which behaviour was most common during the training session.
| DESMOND251 behaviour | Non-DESMOND251 behaviour |
|---|---|
| Facilitating non-judgemental engagement of all participants | |
| Uses a range of open body language to support engagement of participants | Tends to use more closed body language behaviours |
| Uses non-judgemental statements regarding participant verbal utterances | Uses judgemental statements in response to participant verbal utterances |
| Seeks answers from a number of participants before discussing further, including right and wrong answers | Accepts the first (right) answer and/or immediately providers correct or up-to-date information |
| Seeks clarification of participants’ contribution | Rarely seeks clarification of participants’ contribution |
| Avoids giving general healthy messages | Provides general healthy messages |
| Avoids giving their own opinion | Gives their own opinion |
| Eliciting and responding to emotions/feelings (empathetic responding) | |
| Prompts participants to express and explore their feelings about diabetes | Avoids actively engaging participants in emotional discussion |
| Acknowledges and/or prompts exploration of participant emotional response | Retreats from/ignores/denied participant emotional response |
| Facilitating reflective learning | |
| Uses analogiesa | Avoids the use of analogiesa |
| Uses visual tools and resources | Does not use visual tools and resources |
| Uses and refers to participants’ comments and quotations | Uses his/her own words when working through session content |
| Encourages group to discuss/answer their own questions | Answers most questions asked by the group |
| Prompts participants to explore misconceptions and gaps in knowledge | Immediately provides correct information to fill apparent gaps in knowledge |
| Notices and prompts discussion of personal health beliefs | Avoids discussion of health beliefs within the group |
| Prompts all participants to ask questions about issues discussed | Rarely invites (more than once) participants to ask questions |
| Prompts group to summarise key messages | Tends to summarise key messages |
| Prompts group to summarise their own understanding | Tends to summarise what she/he thinks is the group’s understanding |
| Prompts ‘self-talk’ about how the key messages from the session applies to them | Does not ask participants to reflect on how the key messages apply to them |
| Provides new information only after group discussion | Regularly provides new information without group discussion |
| Behavioural change, planning and goal setting | |
| Acknowledges when participants decide not to make any future changes to self-care behaviours or beliefs | Expects participants to make necessary changes |
| Prompts participants to discuss their thoughts about possible changes | Avoids generating discussion about possible changes |
| Prompts participants to review the impact of possible choices on their future health | Avoids generating discussion about range of options/impact |
| Prompts participants to talk about what they are going to do as a result of the session | Rarely asks participants what they are going to do as a result of the session |
| Prompts problem-solving of possible barriers to change | Avoids ‘active’ problem-solving support |
| Prompts participants to reflect on their goals/plans | Avoids reflective discussion regarding goals/plans |
| Facilitates sharing of stories about positive attempts to manage their health | Avoids use of participant stories of positive success |
| Supports participants to plot their results on the health profile/action plan | Provides little support to assist participants with the completion of their health profile/action plan |
| Prompts reflection of changes already made | Does not prompt reflection of changes made |
| Overall group management | |
| Uses strategies to manage time | Avoids using strategies to manage time |
| Notices tone/dynamics within the group and uses these to manage the group | Tends to ignore issues within the group |
| Prompts engagement of quieter participants | Avoids seeking engagement of quieter members of the group |
| Uses co-educator to support delivery | Appears to work alone despite opportunities that may be assisted by co-educator |
| Manages group to provide time and space to complete tasks | Avoids managing group to allow time and space |
| Provides overviews of the sessions/day | Does not provide overviews of the sessions/day |
| Outlines the style of the sessions | Does not outline style of sessions |
| Facilitates full engagement in interactive tasks | Tends to facilitate interactive tasks with only a few participants |
| Engages participants using rapport-building skills | Avoids using rapport-building skills |
Prior to using the tool to assess educator behaviour for the STOP Diabetes programme, three members of the education development team reviewed the existing quality development tool and its application in the STOP Diabetes programme. As a result, only one of the items in the reflective learning category was changed (see Table 38) because it is recognised that people with ID are more amenable to concrete concepts and visual imagery than to more abstract concepts, such as analogies165 (see also Chapters 8 and 9).
Between March and April 2015, a member of the research team attended the final iteration of the education programme using the tool. The researcher was an experienced member of the research team, had attended specific STOP Diabetes educator training and was involved in the development of the education programme. The tool was used to describe how the educators interacted with the group, to identify differences between educators’ behaviours and to assess appropriateness to this client group. The researcher used the quality development tool to conduct assessments during six out of seven of the education sessions. During these sessions the researcher positioned themselves outside the group and completed the tool separately for each educator.
In addition to noting educator behaviour, an assessment of participant–educator interaction was undertaken during one observation visit, using a 10-second event coding to estimate the amount of time during which the educators were speaking. When the beep sounded, the coder indicated on a response sheet who was talking at that point in time (whether an educator or a participant), with other activity classed as ‘miscellaneous’ (indicating silence, laughter or multiple conversations during learning activities). The 10-second event coding is an objective measure and an established method of measuring talk time-to-educator ratio. 258,259
Identifying key important educator behaviours
Once the preliminary assessment had been conducted, members of the research team met to explore the items within the tool, primarily to distinguish between educator behaviours that were seen to be important compared with those that appeared less core or essential when facilitating adult learners with ID.
Results
Educator training
The STOP Diabetes specific training was attended by two diabetes education specialists, four ID HCPs and other members of the research team. It is important to note that not all of these members of the team went on to deliver the education programme.
Based on evaluation forms completed by six of the educators following the two training days, the training was universally well evaluated. Using a standard evaluation self-report measure, all of the educators agreed with the statement that they had learned new skills and believed they could apply these skills in practice. The most helpful aspects of training cited by educators included going through the curriculum and resources; learning about the development and theoretical underpinning of the programme; and group discussions and learning from colleagues.
Preliminary assessment of educator behaviour
This was based on findings from one observation visit that was conducted to assess participant–educator interaction. In general, the observer noted that the diabetes specialist (who was the trained educator) generally displayed more of the facilitation of learning behaviours, such as using open body language, seeking clarification of participants’ contributions and providing overviews of the sessions; the ID nurse displayed more engagement behaviours, prompting exploration of feelings, involvement of quieter participants and facilitating full engagement in interactive tasks. The DESMOND251 behaviour of prompting the group to summarise the key messages was rarely observed.
Discussion
Based on initial feedback, the educator training was universally well evaluated. Educators believed that they had learnt new skills and could apply them in practice. However, we acknowledge training of only one group of educators.
The preliminary assessment of educator behaviours has identified that different behaviours may be delivered depending on the educator. The small number of noted differences between educators in terms of behaviours may be due to professional background and training. The ID nurse may have had more specialist expertise in engaging people with greater communication and engagement needs. However, this difference may have also been due to differing roles in the delivery of the programme, as the diabetes educator had been the original developer of the programme and may have been focused on exploring the delivery and the content of the programme further. This work has identified what needs to be focused on for future training in this area.
This work has been a useful first step in the development of a tool that could be used valuably in this area. Further work is required, as the tool, thus far, has been used on only one programme by one observer. The first two iterations of the development of the STOP Diabetes programme could not be assessed using the tool, as the programme was still in development. However, despite this, the tool provided a structure for the preliminary assessment of intervention fidelity and the variance found between educators at this early stage will provide a benchmark for future work.
Recommendations
The findings from this chapter have provided a positive starting point and highlighted a number of recommendations for future work in this area. The tool is now ready for further adaptation to optimise its relevance for the target population. Possible adaptions include (1) shortening the tool for simplicity and (2) omission of some items that are not relevant or appropriate for this client group, such as items that relate to abstract concepts and that require participants to remember or summarise the training points. Items essential to this group have also been identified, such as teaching at the group’s pace and being flexible. In any future work the tool would also be specifically tailored to follow the structure of the STOP Diabetes programme. The tool would then need to be tested with a wider group of educators across the delivery of a larger number of programmes.
The health-care assistant and the nurses who supported the STOP Diabetes programme were not included in the intervention fidelity observations. It is suggested that they be included in future assessments, as they are also key to the delivery of the programme and need to be demonstrating the same set of behaviours. In addition, it is recommended that in the future more than one researcher completes the intervention fidelity tool to increase the reliability of the findings.
Furthermore, the findings from this work can also feed in to recommendations for future educator training, particularly the importance of being aware of quieter participants and encouraging engagement to avoid participants feeling isolated.
Chapter 12 Economic analysis
Overview
In this chapter, we describe the economic work undertaken as part of WP1, in order to estimate the cost-effectiveness of the STOP Diabetes lifestyle intervention. Development of the lifestyle intervention is described in Chapters 8 and 9.
Rationale and aims
The overall aim of the economic work undertaken was to estimate the cost-effectiveness of the STOP Diabetes lifestyle intervention, compared with current care, in reducing cardiometabolic comorbidities among individuals with ID.
The objectives of the economic analysis reflect the revised protocol for the STOP Diabetes study. The economic analysis focused on the purpose of the intervention; that is, to increase physical activity (and to a lesser extent change in dietary behaviours) among overweight or obese individuals with ID. The context of the analysis reflects the likely real-world implementation of the intervention, including a screening phase, which could be appended to the established health checks for people with ID that are carried out annually within current NHS practice, in order to identify individuals who are suitable for the intervention. This screening can therefore be viewed as a potential additional component of the Learning Disability Health Check (see below for more details of the Learning Disability Health Check). If the STOP Diabetes intervention was to be rolled out, it would target only those with mild or moderate ID, as the intervention is not suitable for those with severe or profound ID. We therefore restrict the economic evaluation to the subset of individuals in STOP Diabetes with mild/moderate ID.
The economic analysis does not attempt to estimate the cost-effectiveness of screening individuals for IGR and T2DM because of a lack of evidence and poor clarity of context within current clinical care and the proposed STOP Diabetes intervention. This is explained more fully in Appendix 21.
Context of proposed screening with existing intellectual disability care pathways
Learning Disability Health Check
The Royal College of General Practitioners guidelines state that NHS general practices should identify individuals who are a high priority for health checks, expressing that mild cases of ID are a lesser priority. 260 However, the current 2015–16 NHS General Medical Services contract states that all individuals with ID who are aged > 14 years should be offered a Learning Disability Health Check and we therefore do not exclude those with mild ID from the analysis.
It was assumed that the model starts at the point following attendance of a Learning Disability Health Check by all baseline individuals.
Figure 24 shows how the screening and intervention elements of the STOP Diabetes study would fit into the Learning Disability Health Check and the scope of the economic evaluation.
FIGURE 24.
How screening for suitability for the intervention fits within Learning Disability Health Check. LD, Learning Disability.
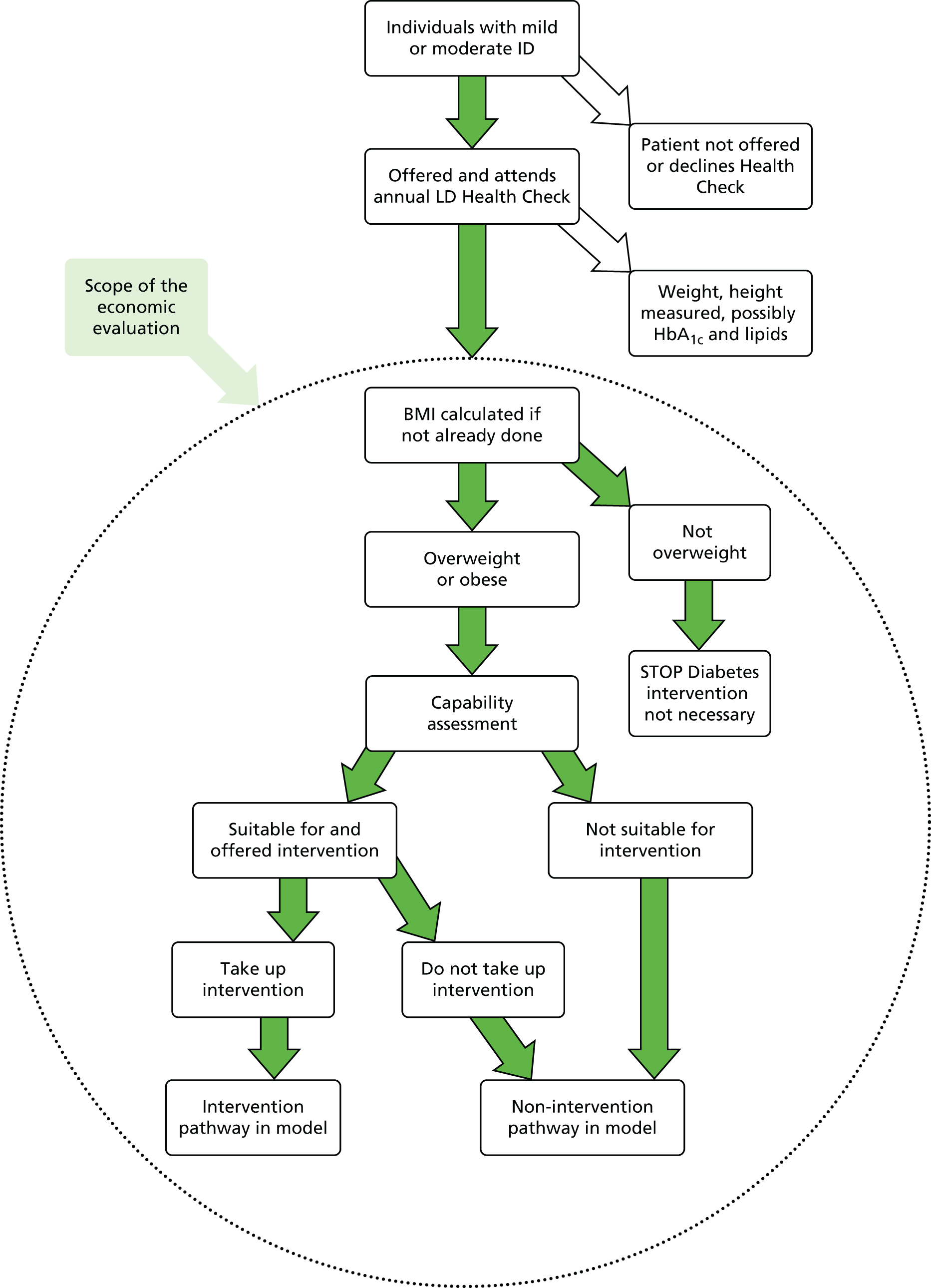
There is no need to explicitly model the Learning Disability Health Check process, as the baseline characteristics from the STOP Diabetes study are assumed to reflect characteristics post health check and the resulting baseline risks of CVD and T2DM.
Attending a Learning Disability Health Check obviates the need to participate in the general population NHS Health Check programme for those aged > 40 years.
In 2013–14, nationally, 44% of those on an ID register attended a Learning Disability Health Check; this varies greatly by geographical area, ranging from 10% to 60%. Specifically, 29% missed a check, implying an offer rate of 73% and an uptake rate (among those offered) of 44%/73% = 60%. 261
During the Learning Disability Health Check, BP, weight and height are measured260 [unless it is not possible because of physical disability (meaning that the patient is unable to stand for height and/or weight measurement) or behavioural difficulties] so that their BMI can be calculated. Some blood tests may also be carried out during a Learning Disability Health Check (or in advance of their Health Check, or as a follow-up test recommended by patients in their Health Action Plan). There is also scope to screen for hyperglycaemia (and dyslipidaemia) and assess cardiovascular risk as part of routine care, although there is much variation in practice as to whether or not such blood tests are deemed to be necessary or a priority within a Learning Disability Health Check, or indeed uptake of blood tests by individuals. For the purpose of the modelling, we assume that these blood tests would be carried out as part of the Learning Disability Health Check.
The model starting point is an individual having been offered, and attending, a Learning Disability Health Check, that is, one of the 44% of individuals noted above.
Methods
Overview of approach
This section provides an overview of the economic work before more details are described in subsequent sections.
The first phase of work involved obtaining all necessary parameter inputs and assumptions for the economic model, specifically:
-
data from the STOP Diabetes study to provide the baseline patient characteristics
-
data collected, and quotations and input from the study team to provide the details required to calculate the cost per participant of the STOP Diabetes lifestyle intervention
-
assumptions on uncertain inputs such as rate of uptake of the intervention and the durability of the benefits of the intervention through discussion with the clinical team.
The modelling itself comprised:
-
Model development An existing economic model of cardiovascular and diabetes risk, driven by characteristics such as BMI, total cholesterol, HDL cholesterol and HbA1c level, was adapted to incorporate the relationship between changes in physical activity (steps) and changes in the above risk factors.
-
Modelling the screening process Determining those individuals from the STOP Diabetes study who are suitable for, and take up, the intervention. The modelling takes account of individuals’ BMI and capacity to participate in the intervention and rates of uptake of the intervention.
Modelling the screening process involved an adaptation of the prevention ‘Sheffield School for Public Health Research (SPHR) Type 2 Diabetes Model’. The model is used to simulate the lifetime patient clinical pathways, incidence of complications and associated cost, and health utility impacts arising from an intervention compared with routine care. The assumptions for the modelling are described in detail later in the report, but the key ones are listed here:
-
The STOP Diabetes intervention was estimated to cost £1097 for the initial intervention and eight maintenance sessions, delivered within a 1-year time frame.
-
The benefits of increases in physical activity could be mapped to changes in BMI, systolic BP, and total and HDL cholesterol, using a relationship identified in the literature.
-
The durability of the intervention effects was uncertain so, for the modelling, two scenarios were adopted with the effects lasting (but decreasing linearly) for 3 and 5 years (from the start of the intervention), respectively.
The usual approach to economic evaluation is to estimate the incremental lifetime discounted costs and quality-adjusted life-years (QALYs) of an intervention compared with usual (routine) care. From this, the incremental cost-effectiveness ratio (ICER) can be calculated and compared with usual acceptability thresholds (£20,000–30,000 per QALY). However, because the clinical effectiveness of the STOP Diabetes intervention is not known, the economic modelling was primarily based on threshold analysis. Under this approach, the requisite clinical effect size needed for the intervention to be just cost-effective (given the cost of the intervention) was estimated. Because the STOP Diabetes intervention promotes physical activity and dietary change, the output of the threshold analyses was not a single effect size, but various permutations of these that would be adequate to make the intervention cost-effective.
Uncertainty around the results was analysed primarily using one-way sensitivity analysis. Owing to the computational demands of undertaking threshold analyses, probabilistic sensitivity analysis (PSA) was restricted to one scenario to give an indication of the degree of uncertainty around such an intervention.
Data sources: STOP Diabetes study
Baseline population
The baseline population in the model reflected, as far as possible, the patient-level baseline data from the STOP Diabetes study, during which data from 930 individuals with ID from the Leicester area were gathered. Other risk factors (left ventricular hypertrophy, heart rate and valve disease) were based on general population prevalence as data were not available from the STOP Diabetes study.
Only individuals with mild/moderate ID would be targeted with the STOP Diabetes intervention; thus, those recorded as having severe/profound ID were removed from the sample, leaving a total of 618 individuals in the modelled cohort. Summary statistics of these individuals are shown in Table 39.
| Parameter | Number (N = 618) | Percentage |
|---|---|---|
| Male | 337 | 54.5 |
| White | 537 | 86.9 |
| IMD 1 (least deprived) | 109 | 17.6 |
| IMD 2 | 107 | 17.3 |
| IMD 3 | 125 | 20.2 |
| IMD 4 | 142 | 23.0 |
| IMD 5 (most deprived) | 135 | 21.8 |
| Non-smoker | 512 | 82.8 |
| Antihypertensive treatment | 62 | 10.0 |
| Statin treatment | 55 | 8.9 |
| CVD | 12 | 1.9 |
| Depression/anxiety | 171 | 27.7 |
| Congenital heart disease | 12 | 1.9 |
| Capable of taking up intervention | 484 | 78.3 |
| Eligible for intervention by BMI criteriaa | 458 | 74.1 |
| Eligible for and capable of intervention | 384 | 62.1 |
| Mean (SD) | Median | |
| Age (years) | 43.07 (14.15) | 42.32 |
| BMI (kg/m2) | 29.25 (7.36) | 28.10 |
| Total cholesterol (mmol/l) | 4.91 (1.02) | 4.80 |
| HDL cholesterol (mmol/l) | 1.35 (0.49) | 1.30 |
| HbA1c (%) | 5.37 (0.49) | 5.35 |
| Systolic BP (mmHg) | 121.60 (17.67) | 120.00 |
| Diastolic BP (mmHg) | 78.10 (11.07) | 78.00 |
| EQ-5D | 0.838 (0.219) | 0.850 |
| Baseline physical activity in mean steps per day (N = 46) | 6892 (3556) | 6453 |
The initial Learning Disability Health Check itself is not simulated to prevent modification of baseline characteristics (such as diabetes diagnosis or statin treatment) before initiation of the intervention.
Data imputation
Many individuals were lacking responses to some questions in the baseline questionnaire but had data for others. The SPHR Diabetes Prevention Model uses imputation models based on Health Survey for England (HSE) 2011 data262 to impute missing anthropometric and metabolic measures. Full details of imputation models can be found elsewhere in an online discussion document. 263
Clinical effectiveness
The feasibility study was intended to assess the practicality of implementing the intervention in the target group. Sample sizes were, however, too small to quantitatively assess the effectiveness of the intervention (n = 4 with before-and-after accelerometer data reporting step count); therefore, there were no estimates of clinical effectiveness available (see Use of threshold analysis).
Assessing suitability for intervention
Process
All of the individuals at the start of the model were assumed to be attending the Learning Disability Health Check in the first year and therefore potentially eligible for the intervention. However, not all individuals were deemed to be at sufficiently high risk or capable of receiving a lifestyle intervention. Therefore, in the intervention arm of the model, suitable individuals needed to be identified as part of the Health Check process using pragmatic selection and capacity criteria. The selection criteria for clinical need for the intervention was being overweight or obese; that is, having a BMI of > 25 kg/m2 (or BMI of > 23 kg/m2 for individuals from black or minority ethnic groups). It was assumed that individuals were capable of taking part in the intervention if they could walk (without aids); did not have behaviour problems; and if their ID was mild, moderate or unknown. The capabilities assessment itself was assumed to occur in all of the baseline individuals at the start of the model.
These criteria resulted in 62.1% of individuals with mild or moderate disability being eligible for intervention.
The proportion of individuals receiving the intervention was further reduced by taking account of the willingness of suitable individuals to participate in the programme of intervention sessions, so the model also incorporates this rate of uptake.
Screening cost
In order to assess the real-world impact of the intervention if it was rolled out at scale, we assume that the above assessments would be carried out at the same time as their routine Learning Disability Health Check, as opposed to a separate appointment that occurred for the screening component of the programme. Therefore, the additional screening-related costs attributable to STOP Diabetes were simply those to assess an individual’s (1) need for the intervention and (2) capacity to undertake the intervention (whenever increased activity is recommended). Recruitment costs from the STOP Diabetes study were excluded, as these would be covered by the existing recruitment activity for the Learning Disability Health Check.
It was estimated that the BMI calculation, capabilities assessment and time taken to explain, and potentially gain, consent for the intervention would take on average an extra 15 minutes of health-care assistant time during the NHS Health Check compared with current care (£5.10). This cost is incurred by all individuals in the intervention arm of the model.
The process of risk assessment and any associated screening for diabetes or IGR, and any overall assessment of CVD risk, is assumed to fall within the existing remit of their annual Health Check and therefore outside the scope of this economic evaluation.
Intervention form, cost, clinical effectiveness and uptake
Form
The costs of the intervention were divided into three phases:
-
Development phase The costs of setting up the intervention, for example the upfront costs of training the educators and equipping them to deliver the intervention.
-
Delivering the initial intervention The key components are:
-
seven sessions for patients plus an additional one for their carers
-
each session lasting 2.5 hours, plus 30 minutes’ set-up/pack-up time
-
three educators per group – one band 8a, one band 7 and one band 3.
-
-
Maintenance sessions Eight monthly sessions starting after the initial intervention, as it is recognised that for lifestyle interventions to have sustained benefits, some ongoing education is needed to reinforce behaviour change.
Cost
A microcosting exercise was undertaken by colleagues at Leicester, assisted by the economics team, in order to obtain a cost-per-patient of receiving the intervention. As STOP Diabetes was a feasibility study, not all elements of the full cost of its delivery are known with precision. Furthermore, some cost would be different if incurred in a real-world setting. As the economic analysis is essentially a threshold analysis to inform any further study of the STOP Diabetes intervention within a trial setting, it was decided that there was little point in separate costings of the intervention so a single costing was undertaken based on actual resources incurred during the study where available, but modified where appropriate to reflect the costs that would be incurred in a real-world setting and using price quotations obtained by the clinical team for some aspects of the intervention’s development.
Within the STOP Diabetes research study, the average number of patients per group was six, but for the costing an average number of eight people per group was assumed, as this would be an acceptable maximum number in the ‘real world’.
‘Research costs’, such as recruiting patients to the study and initial development of the intervention, were excluded because the intervention had already been developed and it was assumed that recruitment costs were part of the existing annual health checks process for patients with ID.
Costs of the components of the intervention were obtained from several sources. Unit costs of some nurse grades are available from the Unit Costs of Health and Social Care. 264 Costs for other nurse bands were obtained by combining salary costs provided by the study team with overhead adjustment in line with the Unit Costs of Health and Social Care. 264 The costs of non-staff items were provided by the study team.
The individual cost elements of the STOP Diabetes intervention are shown in Table 40.
| Details | Cost apportioned to | Cost (£) |
|---|---|---|
| One-off costs of intervention | ||
Trainer costs for educator training (one-off): initial DESMOND + intervention specific:
|
‘Educator team set-up’a | 2118 + 1752 |
| In theory, up to a maximum of 15 educators possible per group training session | ||
| Trainer time for quality development intervention fidelity (i.e. sit in on educator delivering session): band 7 trainer – 1.5 days needed per educator × three educators @ £353/day | ‘Educator team set-up’a | 1588 |
Educator costs: attendance at training (time and travel) – initial DESMOND + intervention specific:
|
‘Educator team set-up’a | 519 + 1059 + 1314 |
| (This mix reflects a group of educators that can deliver a course together) | ||
Educator costs: preparation time to deliver curriculum – each educator time to prepare before delivery of their few courses:
|
‘Educator team set-up’a | 346 + 706 + 876 |
| (This mix reflects a group of educators who can deliver a course together) | ||
Educator time for quality development intervention fidelity: quality development and mentorship visits (1.5 days per educator)
|
‘Educator team set-up’;a assume this is required over a cycle of 3 years (as in DESMOND) | 260 + 530 + 657 |
| Developing training package, resources and intervention fidelity tools | ‘Trainer team one-off’ | |
| What trainers needed to train up educators | 1000 | |
| Some elements could be reused but some could be consumed (e.g. food during training) | ||
| Assume 50% of the estimated total £2000 costs could be attributable to a single team of educators (there may be a few training providers around the country, so cannot spread the cost over lots and lots of educator teams) | ||
| Delivery materials: education curriculum: three educators × £100 | ‘Educator team set-up’a | 300 |
| Delivery materials: education resources and resources/food models per set: initial set £100, more substantive set up to £2000 | ||
| Per team of educators – assume non-reusable | ‘Educator team set-up’a | 1000 |
| Venue costs for educator training (one-off): initial DESMOND + intervention specific – £100 per day × 3 days | ||
| In theory, up to maximum of 15 educators possible per group training session | ‘Educator team set-up’a | 300 |
| Initial educational intervention | ||
| Administrative time and co-ordinator time (combined) | ||
| Telephone calls to confirm suitability and willingness: 15 minutes per person, but would not be needed in real world as would be part of nurse assessment | ||
|
Per participant | 5.25 |
| Booking/confirming venue (30 minutes per group) | Per eight-session course | 10.50 |
| Co-ordinating educators and resources (60 minutes per session × 8) | Per eight-session course | 168 |
| Assuming £21 per hour average salary costs | ||
| Delivery | ||
| Seven sessions to patients plus one carer session = eight sessions | ||
| Each session = 2.5 hours’ delivery + 0.5 hour set-up/pack-up time per session = 3 hours per sessionb | Per eight-session course | |
| Each session run by two educators plus one health-care assistant (1 × band 3 plus 1 × band 7 plus 1 × band 8a) | ||
| Band 3 × 3 hours @ £23 × 8 sessions | 552 | |
| Band 7 × 3 hours @ £47 × 8 sessions | 1128 | |
| Band 8a × 3 hours @ £58 × 8 sessions | 1392 | |
| Participant handbook | Included under cost of course materials, below | |
| Pedometer: one per person + 50% for carers, at £8 each (assume need 10 per group + 20% for loss), total per group if seven, allow 13 pedometers | Per eight-session course | 104 |
| Refreshments: £1 per person, plus 50% for carers, for 8 weeks, total per group if seven | Per eight-session course | 84 |
| Postage/telephone calls: total per group = £10 | Per eight-session course | 10 |
| Stationery and reprographics: letters/information/course materials – £10 per person, total per group | Per eight-session course | 70 |
| Venue hire cost: could be NHS premises (in which case costs absorbed into Curtis264 rates) or non-NHS community (local authority) – some would charge | Per eight-session course | 360 |
| Assume 50% of venues not in NHS and incur a charge | ||
| 3 hours × 8 weeks, £10–20 per hour (assume £15) | ||
| Travel costs: staff – 45p per mile for three educators × eight sessions, return mileage estimated at 10–30 miles, depending on distance from base | Per eight-session course | 36.00 |
| Travel costs: in STOP Diabetes, participants [taxi travel or reimbursement of bus fare, assuming 50% of people (three or four per group) need travel costs paying, estimated £10–20 taxi and £2.00–3.00 bus fare per journey]. In real world, however, it can be assumed that such travel costs would all be accounted for with the patient’s free bus pass and/or mobility allowance | – | 0 |
| Monthly ongoing support sessions | ||
| Eight monthly sessions: the cost of delivering these sessions is uncertain. They could be delivered one-to-one or in a group. Although group-based delivery would normally be cheaper for lifestyle interventions, for individuals with ID this might not be the case. Delivering maintenance might be achievable through a single educator visiting the patient’s home (thereby avoiding venue costs) and provision of a shorter hour-long one-to-one session might be sufficient. It was therefore judged to be a conservative approach to allow for maximum potential costs by costing on the assumption of group-based delivery and cost of a maintenance session was assumed to be the same as an initial session | As in Initial educational intervention section, above | |
To obtain an overall cost per patient, a sequential process of apportioning ‘Educator team set-up’ to educators, then educator costs to a cost per course, then to course costs to patients was undertaken, giving a cost per patient of £1097 (combined cost for the initial and maintenance intervention).
Potentially, there might be some scope for either reducing the cost of the intervention or actual costs incurred being lower than estimated above. For example, the mode or frequency of delivery of maintenance sessions could be revisited, a different mix of grades of educators may be possible in the real world, some individuals who attend the initial course may not continue to the maintenance sessions and the method of apportioning overhead costs within Curtis264 is somewhat arbitrary (appears to load greater overhead costs to more senior staff). Through a cost-specific threshold analysis, we explored to what degree the cost of the intervention would need to be reduced in order to make it cost-effective.
Clinical effectiveness
Owing to the small number of individuals in the feasibility study with data on physical activity (as measured using an accelerometer) before and after the programme (n = 4), effectiveness estimates were not available from the STOP Diabetes programme itself. Therefore, a threshold analysis approach (see Use of threshold analysis) was used to estimate the threshold for the effect size needed to make the intervention marginally cost-effective in the £20,000–30,000 cost per QALY range as recommended by NICE. 265 As the intervention includes dietary advice as well as the physical activity element, we report the threshold in terms of possible permutations of the number of steps and the additional diet-attributable BMI and systolic BP changes that would be needed to be able to demonstrate that the intervention is cost-effective.
Durability of effect
The initial intervention-related changes in BMI, systolic BP and HDL cholesterol within the year of intervention were subsequently assumed to wear off linearly such that, after 3 years from the start of the intervention, individuals have returned to the BMI, systolic BP and HDL cholesterol trajectories that they would have followed in the absence of intervention. Alongside the above base-case assumption, an additional scenario analysis was carried out to test the response of the results to 5-year duration of effect.
Intervention uptake
Of individuals who are both eligible for, and capable of taking up, the intervention, we assumed that 55% do so and thereby incur the full costs of the intervention, based on advice for a previous lifestyle intervention evaluation. 266
Modelling the benefits of changes in physical activity (steps)
The economic model chosen for the modelling (the SPHR Diabetes Prevention model, described later) does not include physical activity as a risk factor for CVD or diabetes. However, the primary measure of interest, in terms of informing any future follow-on trial from the STOP Diabetes study, is change in steps per day [and this is a key output of the threshold-based economic analyses (see Use of Threshold analysis), and any subsequent full trial would be based around change in physical activity measured in steps using a pedometer]. It was therefore necessary to identify a mechanism to map changes in physical activity to changes in the above risk factors that already exist in the model (BMI, systolic BP, total and HDL cholesterol, and glucose-related risk factors) and vice versa.
Rationale for surrogate-based model
A surrogate-based approach was chosen because:
-
The SPHR Diabetes Prevention model uses metabolic trajectories to model long-term progression of risk factors and incidence of comorbidities.
-
A meta-analysis was identified, which linked changes in physical activity to changes in systolic BP, total and HDL cholesterol.
-
We are unaware of any meta-analyses of hazard ratios for the effect of changes in physical activity on incidence of CVD and diabetes.
-
Although we were made aware of a study linking physical activity to CVD (Yates et al. , NAVIGATOR Trial267), the analysis was undertaken assuming a constant change in steps over a 6-year period, whereas the effect of the STOP Diabetes intervention would decline over time. So any hazard ratio adjustments to the risks of CVD and diabetes would need to have been analysed in a time-dependent way. The behavioural changes in this study were also based on dietary advice, but it is unknown how intensive this was compared with the STOP Diabetes intervention (which contained some dietary advice, but no weight loss target). The reported hazard ratio may therefore include significant risk reduction that is attributable to dietary changes. Additionally, this study recruited a cohort of individuals with IGR at baseline and reported only CVD (not diabetes).
We did believe, however, that it would be useful to compare the predictions of the model (modified to reflect a constant changes in risk factors for 6 years) with the 6-year results reported in the Yates et al. study. 267 For an increase in activity of 2000 steps, Yates et al. 267 reported a hazard ratio of experiencing a cardiovascular event over the following 6 years of 0.92 (95% CI 0.86 to 0.99), that is a risk reduction of 8%. The hazard ratio from our adapted model was 0.95 (5% risk reduction). The details of the method behind this comparison are provided in Appendix 22.
There are various reasons why a slightly lower reduction in risk might be expected from the model compared with that observed in the NAVIGATOR trial. First, patients in the NAVIGATOR trial had IGR but were not necessarily overweight, whereas the baseline population in the model were mostly normally glucose tolerant but with BMI values above defined thresholds. BMI and diabetes both input into cardiovascular risk, but the relative effect this population difference might have on cardiovascular outcomes is unclear. Second, the trial participants received both exercise and dietary advice, but potential differences in diet between individuals with different step counts was not accounted for in the trial analysis. It is possible that individuals who follow exercise advice are more likely to follow dietary advice too and so may show a greater effect on metabolic trajectories than accounted for in our analysis based on steps alone. This would result in the model underestimating the results of the trial as is indeed observed. Third, there are some differences in the events picked up in the trial and those accounted for in the model (e.g. transient ischaemic attack and stable angina are in the model but not the trial), although it is unclear what effect there would be on the hazard ratio from restricting outcomes to those used in the trial. Finally, it cannot be ruled out that exercise may impact positively on factors that are not included in the model, which in turn result in reduced cardiovascular risk.
It should be noted that as the Yates et al. study267 reports hard outcomes in terms of cardiovascular events, but not changes in biomarkers of risk, the results could not be used to parameterise the SPHR model (which estimates a range of outcomes, including CVD, based on cardiometabolic risk factors and their projected trajectory over time).
Review of relationship between physical activity (steps) and risk factors for cardiovascular disease and diabetes in the model
To identify a suitable mapping between steps and model risk factors, a targeted review of the published literature was carried out using a search of the online PubMed database, a subsequent citation search and advice from clinicians. This evolved into a three-step process as described below.
Step 1: search strategy for physical activity studies reporting step as an outcome
The search strategy using the PubMed publications database is detailed in Appendix 23, Box 4. The search yielded 153 results, from which 54 abstracts were selected based on titles. Of these, 19 were relevant, of which full text was available for 14. One of these studies, by Stuckey et al. ,268 discussed a meta-analysis undertaken by Bravata et al. ,269 which is discussed below.
Step 2: citation search for review papers citing Bravata et al.269
This search identified 22 abstracts for review. Abstract/title sifting led to seven full-text papers being checked. Of these seven papers, four were excluded as narrative reviews only; one was a review of reviews, but focused on diet only or diet and physical activity interventions and therefore was not relevant; one was excluded because it was a primary trial not a review or meta-analysis; and one was excluded because it reported only weight and no metabolic outcomes. This search identified two relevant studies of potential use for the modelling: Murphy et al. 270 and Qui et al. ,271 which were reviewed in full text and are discussed below.
Step 3: conversation with clinical advisors
In discussion with clinicians it was determined that cholesterol changes were also very likely to be observed as a result of the intervention. In this respect, we were referred to a study by Camhi et al. ,272 which is described below. This conversation also highlighted the Yates et al. study,267 discussed earlier.
Description of key studies
Bravata et al. 269 reviewed and combined the results of studies that used a pedometer to measure physical activity and reported health outcomes (mean duration of studies = 18 weeks). The overall step change induced by intervention studies was 2491 additional steps per day. Significant reductions were reported in BMI (–0.38 kg/m2) when 18 studies (n = 562) were combined and in systolic BP (–3.8 mmHg) when 12 studies (n = 468) were combined. There were also non-significant reductions in lipids with a reduction in total cholesterol of 0.09 mmol/l and an increase in HDL cholesterol of 0.06 mmol/l.
Murphy et al. 270 was a review of walking interventions on metabolic risk factors, based on minutes per week rather than steps. It reported very similar outcomes to Bravata et al. 269 (188 additional minutes per week were associated with 0.95 kg of weight loss, a 0.28-kg/m2 BMI reduction and a 1.54-mmHg diastolic BP reduction).
Qui et al. 271 was a recent meta-analysis of step counting and its effects on HbA1c control. 271 Although it found evidence of significant increases in steps it found no strong evidence for changes in HbA1c level. This backs up the results of the previous Bravata et al. review,269 which only found significant changes in BMI and BP.
The Camhi et al. 272 study was cross-sectional, based on the US NHANES survey (National Health and Nutrition Examination Survey). This study272 assessed the relationship between activity (steps per day) and cardiometabolic risk factors, including cholesterol. Data for 1371 adults were analysed and significant changes were observed in triglycerides, HDL cholesterol and waist circumference for each 1000 additional steps achieved, but does not report BMI changes or BP. The study272 reported results as an OR of 0.91 (95% CI 0.81 to 0.96) of having HDL cholesterol above the cut-off point of 1.03 mmol/l (men) or 1.2 mmol/l (women).
For the purpose of parameterising the model, this study272 was not preferred over the evidence from the Bravata et al. study269 because this is only a single study and is cross-sectional, whereas the Bravata et al. study269 is a meta-analysis of intervention studies.
Implementation of mapping steps to risk factors
Physical activity and the mapping are not contained within the simulation model itself. The estimation of the number of steps needed is calculated manually after the model has been run several times and a threshold analysis has identified the degree of BMI change needed for the intervention to be marginally cost-effective (see Use of threshold analysis for more details).
Table 41 shows the mapping between physical activity and risk factors obtained from the Bravata et al. study. 269
| Risk factor | Mean change (95% CI) |
|---|---|
| BMI (kg/m2) | –0.38 (–0.72 to –0.05) |
| Systolic BP (mmHg) | –3.8 (–5.9 to –1.7) |
| Total cholesterol (mmol/l) | –0.09 (–0.32 to 0.15) |
| HDL cholesterol (mmol/l) | 0.06 (–0.012 to 0.14) |
Model: overview and structure
The SPHR Diabetes Prevention Model is an individual patient simulation model, written in the programming language R, which was built to enable evaluation of a wide range of different diabetes prevention and weight loss interventions in the general population. The model was originally developed using a new conceptual modelling framework for complex public health models,273 in collaboration with a project stakeholder group comprising health economists, public health specialists, research collaborators from other SPHR groups, diabetologists, local commissioners and lay members. A review of existing diabetes prevention models was undertaken to inform conceptual model development,274 resulting in the model including multiple diabetes risk factors (in particular both BMI and impaired fasting glucose) and complications of diabetes and obesity.
The model has been adapted to evaluate the outcomes of an intervention to promote physical activity in high-risk subgroups of a population with ID. Owing to limited data about care pathways, disease risk and utility values in populations with ID, much of the model is based on general population data; however, when possible, data from populations with ID have been used.
The model is based on individual longitudinal trajectories of metabolic risk factors (BMI, systolic BP, cholesterol and HbA1c). For each individual, yearly changes in these risk factors occur, dependent on the individual’s baseline characteristics.
Illustrated in Figure 25 is the sequence of updating clinical characteristics and clinical events that are estimated within a cycle of the model. This sequence is repeated for every annual cycle of the model. The first stage of the sequence updates the age of the individual. The second stage estimates how many times the individual attends the GP. The third stage estimates the change in BMI of the individual from the previous period. In the fourth stage, the change in glycaemia is estimated using different statistical models depending on whether or not they have been diagnosed with diabetes (see below). In stages 5 and 6, the individual’s BP and cholesterol are updated. In stage 7, the individual may undergo assessment for diabetes, hypertension and dyslipidaemia during a GP consultation. From stage 8 onwards the individual may experience cardiovascular outcomes, diabetes-related complications, cancer, osteoarthritis or depression. Individuals with a history of CVD follow a different pathway in stage 8 to those without a history of CVD. Individuals with a HbA1c level of > 6.5% are assumed to be at risk of diabetes-related complications: individuals who do not have a history of cancer are at risk of cancer diagnosis, whereas those with a diagnosis of cancer are at risk of mortality because of cancer; individuals without a history of osteoarthritis or depression may develop these conditions; and, finally, all individuals are at risk of dying from causes other than cardiovascular or cancer mortality. Death from renal disease is included in the estimate of other-cause mortality. The time horizon of the model is the lifetime of all baseline individuals.
FIGURE 25.
Risk of comorbidities. Osteo, osteoarthritis.

Cardiovascular events are modelled using the QRISK2 algorithm (more details are provided in Appendix 24, Tables 62 and 63). The model uses risk equations from the UK Prospective Diabetes Study (UKPDS) outcomes model to estimate the occurrence of major events relating to microvascular complications, including renal failure, amputation, foot ulcer and blindness. 275,276
Routine care: components of cardiovascular risk reduction
Both intervention and comparator arms of the model need to include any screening for hyperglycaemia and high CVD risk that is carried out routinely in clinical practice. This may be through the Learning Disability Health Check or opportunistic screening.
Learning Disability Health Checks
Individuals with ID should be invited to an annual health check during which they undergo screening for hypertension, high cardiovascular risk and diabetes among other conditions. Although uptake of health checks among people with ID is only 44%, it was assumed that, at baseline, all individuals had been identified through attending a Learning Disability Health Check and would therefore be very likely to attend future health checks. It was therefore assumed that all of the eligible individuals would attend annual health checks. Individuals who have been diagnosed with diabetes or CVD, or who are taking statins or antihypertensive drugs, do not continue to receive Health Checks, as they receive extra GP care that is specific to their diagnosis.
Not all individuals consent to blood tests as part of their Learning Disability Health Check. It was assumed that 33% of individuals would never consent to blood tests during a health check (based on uptake of blood tests and availability of results for the screening programme, presented in Chapter 5); therefore, they could not be screened for CVD risk or diabetes by this method. However, it was assumed that if such individuals met the criteria for opportunistic diagnosis (see below), they would consent to blood tests and so could be diagnosed through this means. A different cost for a Learning Disability Health Check was used for individuals who do or do not consent to blood tests (see Screening cost).
General practitioner attendance and opportunistic screening
Frequency of GP visits (separate from NHS health checks) was simulated in the data set for two reasons: first, to estimate the health-care utilisation for the population with ID without diabetes and CVD, and, second, to predict the likelihood that individuals participate in opportunistic screening for diabetes and elevated risk factors for CVD. This is important, as many individuals in the model cannot be diagnosed through annual health checks either due to ineligibility or because they do not consent to blood tests as part of their Learning Disability Health Check.
It was assumed that GP attendance in the population with ID occurs at the same frequency as in the general population. However, for cost purposes, consultations were assumed to take 40% longer than the general population average (see Screening cost). A model of GP attendance conditional on age, sex, BMI, ethnicity and health outcomes was derived from analysis of wave 1 of the Yorkshire Health Study263 and is described elsewhere.
Long-term longitudinal trajectories of metabolic factors
The SPHR Diabetes Prevention model263 is based on individual longitudinal trajectories of metabolic risk factors (BMI, latent blood glucose, total and HDL cholesterol, and systolic BP), derived from statistical modelling of the data set from the Whitehall II cohort study. 277 The statistical modelling uses parallel latent growth modelling to incorporate correlations and associations between risk factors that impact on long-term risk profiles. An advantage of the parallel growth analysis is that it is possible to estimate the effect of growth in BMI on the other metabolic risk factors so, for example, a change in an individual’s BMI will result in an indirect change in their HbA1c trajectory. Growth factors are also conditional on several individual characteristics including age, sex, ethnicity, smoking, family history of CVD and family history of T2DM. It is also possible to estimate correlation between changes in underlying glycaemia (measured by HbA1c level), systolic BP, and total and HDL cholesterol. Full details of this analysis are described elsewhere. 278
The characteristics of the Whitehall II cohort277 (civil servants living in London) are likely to differ significantly from that of the STOP Diabetes population. However, there are, to our knowledge, no available longitudinal surveys of populations with ID on which to base a similar analysis, and no other analysis of metabolic trajectories takes into account the correlations between risk factors that make the Whitehall model277 so powerful. Importantly, the baseline values for the metabolic risk factors do come from the STOP Diabetes population, with the Whitehall-based trajectories277 being used simply to describe the expected changes in metabolic values over time.
If an individual in the model is diagnosed with diabetes, or starts treatment with antihypertensive drugs or statins, trajectories alter to reflect the expected changes due to treatment. The criteria for opportunistic screening and diagnosis of diabetes, hypertension and high CVD risk can be found in Appendix 25, Table 64, together with details of changes in metabolic trajectories.
Risks of mortality: raised risk in individuals with intellectual disability
In every model cycle, individuals within the model are evaluated to determine whether or not they experience a fatal event or mortality. The evidence for risk of mortality in individuals with ID compared with the general population is described below, analysed by cause, that is, CVD, cancer and other causes.
Cardiovascular mortality
Cardiovascular disease mortality is included as an event within the estimated CVD risks calculated by the QRISK2 score as described below (see Cardiovascular disease). There is some evidence for an increased risk of CVD mortality in individuals with ID,279 but other studies report no difference or even reduced risk compared with the general population (see results of meta-analysis presented in Chapter 2). It is also unclear whether these differences in mortality risk are due to differences in risk factors included in the QRISK or due to other factors. It was assumed for the purposes of the model that any differences in cardiovascular mortality between individuals with ID and individuals in the general population occur simply due to differences in risk factors.
Cancer mortality
It was assumed that risk of cancer and subsequently cancer mortality would be the same in a population with ID as in the general population, taking into account individual differences in risk factors. This is supported by several studies looking at mortality rates from various causes in populations with ID. 279,280
Other-cause mortality
This describes the risk of death from any cause except CVD and cancer. This was derived from all-cause mortality rates by age and sex, extracted from the ONS. 281 The mortality statistics report the number of deaths by ICD codes for 5-year age groups. To obtain other-cause mortality, the number of CVD, breast cancer- and colorectal cancer-related deaths were subtracted from the all-cause mortality total.
There is good evidence from various sources that the rate of all-cause mortality is higher in individuals with ID,63,279,282 particularly due to excess deaths from respiratory disorders, neurological diseases, congenital abnormalities and accidents. Standardised mortality ratios (SMRs) of 2.28 (95% CI 2.02 to 2.56) for men with ID and 3.24 (95% CI 2.83 to 3.69) for women with ID, compared with the general population, reported in a 14-year study of individuals from the Leicester area279 were applied to the other-cause mortality rates that were derived from the ONS data. The SMRs were not adjusted upwards to take into account the minimal increase and decrease in cancer and CVD mortality, respectively, in populations with ID, as it was unclear how large this adjustment should be and it was expected to make little difference to the outcomes.
The rate of other-cause mortality by age and sex was treated as the baseline hazard. An increased risk of mortality was assigned to individuals with diabetes, using data from a published meta-analysis. 283 This study283 used data from 820,900 people from 97 prospective studies to calculate hazard ratios for cause-specific death, according to baseline diabetes status. Cause of death was separated into vascular disease, cancer and other-cause mortality. From this study283 it was estimated that individuals with a diagnosis of diabetes have a fixed increased risk of other-cause mortality (hazard ratio 1.8, 95% CI 1.71 to 1.9). The estimates reported in the meta-analysis include increased risk of death from renal disease; therefore, mortality from renal disease was not simulated separately to avoid double counting of benefits.
Comorbid outcomes with no excess risk in individuals with intellectual disability
In every model cycle, individuals within the model are evaluated to determine whether or not they have a clinical event. In each case within the simulation, risk equations estimate the probability that an individual has an event, and a random number is drawn to determine whether or not the event occurred.
Cardiovascular disease
The QRISK2 model was chosen to estimate cardiovascular risk and incidence as it is a validated model based on a UK population. 284 Probability of a first cardiovascular event in the next year (including cardiovascular mortality) is calculated, being conditional on ethnicity, smoking status, age, BMI, ratio of total cholesterol to HDL cholesterol, deprivation score, atrial fibrillation, rheumatoid arthritis, renal disease, hypertension, diabetes and family history of CVD. Coefficients for the QRISK2 model can be found in Appendix 24, Tables 62 and 63. The QRISK2 assumptions regarding the relationship between diabetes and CVD were modified to reflect observations from the UKPDS and the European Prospective Investigation into Cancer and Nutrition (EPIC) that HbA1c (rather than diabetes) increases the risk of myocardial infarction (MI) and stroke in a linear manner. 275,285
The STOP Diabetes baseline data did not include any information about atrial fibrillation but did include a category for unspecified other heart conditions, which was recorded for 10 individuals (1.6% of total population). A diagnosis of atrial fibrillation was randomly assigned to seven of these individuals in line with an audit of patients with ID in North Essex, which found that 1.1% of individuals had atrial fibrillation. 286 The STOP Diabetes baseline data also had no specific questions about rheumatoid arthritis or renal disease. However, one individual was noted to suffer from rheumatoid arthritis using the other health problems variables, and no individuals were noted to suffer from renal disease so we assumed ‘no renal disease’ at baseline.
There is conflicting evidence about whether or not individuals with ID have a greater risk of CVD than is seen in the general population, and if so, whether or not this can be accounted for through the risk factors already incorporated into the QRISK2 model. Although some studies have found an increase in prevalence of CVD83 or CVD mortality279 in individuals with ID, other studies report no difference or reduced risk compared with the general population (see Chapter 2, Results). Lower CVD mortality than in the general population could partially be explained through competing risks, given that individuals with ID have higher mortality from other causes, particularly respiratory illnesses, congenital abnormalities, neurological disorders and accidental injury. 279,280
The model estimates that individuals recruited into the STOP Diabetes study have a much lower incidence of CVD than unmatched (for age) individuals from the HSE 2011262 due to their baseline characteristics (Table 42). This cannot be explained solely by the lower (by 5 years) mean age of the ID cohort (e.g. compare year 10 in the ID cohort with year 5 in the general population cohort). Given the lack of a clear consensus over CVD risk in individuals with ID, it was assumed that the QRISK2 equations were suitable for use in the population with ID, and that any differences in CVD risk compared with the general population would be accounted for through the differences in baseline risk factors.
| CVD incidence (per 10,000) | Year | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | |
| Cohort with ID | 37 | 56 | 63 | 83 | 113 | 124 | 146 | 162 |
| General population cohort | 77 | 91 | 101 | 123 | 144 | 158 | 169 | 195 |
| Relative risk with ID | 0.48 | 0.61 | 0.62 | 0.68 | 0.78 | 0.78 | 0.86 | 0.83 |
Relationships between risk factors and different types of CVD (e.g. hypertension being more of a risk for stroke) are not incorporated into the model.
All of the CVD events modelled using QRISK2 are assigned to a specific diagnosis according to age- and sex-specific distributions of cardiovascular events reported in a previous Health Technology Assessment (HTA) for statins. 287 Events are also split into ‘fatal’ compared with ‘non-fatal’ ones.
Congestive heart disease
Congestive heart failure was coded as a separate cardiovascular event using the Framingham risk equation288 because it is not included as an outcome of the QRISK2. The Framingham equation is not ideal, as it is based on a US population from the 1990s and there is no evidence for its accuracy in representing risk in a population with ID. However, it was thought to be the best option in the absence of data that were specific to a population with ID. The equation includes age, diabetes diagnosis (either formal diagnosis or a HbA1c level of > 6.5%), BMI, systolic BP, congenital heart disease, left ventricular hypertrophy, heart rate and valve disease to adjust risk based on individual characteristics. Full details of the equation coefficients can be found in an online discussion paper. 263
No baseline information was available for three of these risk factors (left ventricular hypertrophy, resting heart rate and valve disease); therefore, these factors could not be included in the model to predict congestive heart disease. The baseline odds of congestive heart disease were adjusted to reflect the expected prevalence of these symptoms; this was done using general population data, as data that were specific to individuals with ID could not be identified. The heart rate for men was assumed to be 63.0 beats per minute and for women 65.6 beats per minute based on data from previous Whitehall II cohort analyses. 289 The proportion of the UK population with left ventricular hypertrophy was assumed to be 5%, in line with previous analyses of the Whitehall II cohort. 290 The prevalence of valve disease was estimated from the Echocardiographic Heart of England Screening study. 291 Twelve baseline individuals from the STOP Diabetes study suffered from congenital heart disease (1.9%). This is higher than the prevalence of congenital heart disease in the general population (0.80% of live births),292 and is unsurprising given the high proportion of individuals with Down and Williams syndromes, as people with these conditions are known to suffer from congenital heart defects. 293 This means that the risk of congestive heart failure is higher in individuals with learning difficulties than in the general population.
Microvascular complications of diabetes
Data from UKPDS, derived from a UK diabetic population,275,276 were used to estimate the incidence of major microvascular complications including ulcer, amputation, renal failure and blindness in individuals with a HbA1c level of ≥ 6.5%, whether or not they are diagnosed with diabetes. Earlier stages of microvascular complication were not included in the model, as they have less of an impact on costs and utilities. It was assumed that risk of microvascular complications would be the same in a population with ID as in the general population, taking into account individual differences in risk factors.
The UKPDS outcomes model version 2275 includes four statistical models to predict foot ulcers, amputation with no prior ulcer, amputation with prior ulcer and a second amputation. In order to simplify the simulation of neuropathy outcomes, the models for first amputation with and without prior ulcer were consolidated into a single equation. UKPDS outcomes model v2 was also used to estimate the incidence of blindness, whereas the UKPDS outcomes model v1 was used to estimate the incidence of renal failure. 276 Early validation analyses identified that the UKPDS v2 model implemented in the SPHR model substantially overestimated the incidence of renal failure. Details of the models used are reported elsewhere in an online discussion paper. 263
All of the equations incorporate a coefficient for age at diabetes diagnosis. This was multiplied by age in the current year if the individual had not been diagnosed with diabetes or by the age at diagnosis if the individual had received a diagnosis. The expected values for the risk factors not included in the SPHR model (heart rate, white blood cell count, micro-/macroalbuminuria, peripheral vascular disease and atrial fibrillation) were taken from figure 3 of the UKPDS publication in which these are described,275 with the exception of peripheral vascular disease, which was assumed to affect 2% of the population. The baseline risk was modified to take account of these mean values.
Cancer
Breast cancer and colorectal cancer risk are related to BMI and so were included in the SPHR model.
Incidence rates for breast and colorectal cancer in the UK were estimated from the EPIC cohort. This is a large, multicentre cohort study looking at diet and cancer. In 2004, the UK incidence of breast cancer by menopausal status was reported in a paper from this study investigating the relationship between body size and breast cancer. 294 A second paper from EPIC reported the UK incidence of colorectal cancer by sex. 295 Incidence rates in the model for breast and colorectal cancer are shown in Table 43.
| Cancer type and subgroup | Number of cases | Person-years | Mean age (years) | Mean BMI (kg/m2) | Incidence rate per person-year |
|---|---|---|---|---|---|
| Breast | |||||
| UK pre menopause | 102 | 103,115 | n/a | 24 | 0.00099 |
| UK post menopause | 238 | 84,215 | n/a | 24 | 0.00283 |
| Colon | |||||
| Male | 125 | 118,468 | 53.1 | 25.4 | 0.00106 |
| Female | 145 | 277,133 | 47.7 | 24.5 | 0.00052 |
A large meta-analysis that included 221 prospective observational studies has reported relative risks of cancers per unit increase in BMI, including breast cancer and colorectal cancer. 296 A risk adjustment was included in the model so that individuals with a higher BMI have a higher probability of pre- and post-menopausal breast cancer or colon cancer (Table 44). In the simulation, the incidence of breast and colorectal cancer was adjusted by multiplying the linear relative risk by the difference in the individual’s BMI and the average BMI reported in the EPIC cohort.
| Cancer type and subgroup | Mean relative risk | 2.5th CI | 97.5th CI |
|---|---|---|---|
| Breast | |||
| UK pre menopause | 0.89 | 0.84 | 0.94 |
| UK post menopause | 1.09 | 1.04 | 1.14 |
| Colon | |||
| UK pre menopause | 1.21 | 1.18 | 1.24 |
| UK post menopause | 1.04 | 1 | 1.07 |
Evidence suggests that mortality from breast and colon cancer occurs at a similar rate in populations with ID as in the general population. 279,280 Cancer mortality rates were obtained from the ONS. 297 The ONS reports 1- and 5-year net survival rates for various cancer types, by age group and sex. Net survival was an estimate of the probability of survival from the cancer alone. It can be interpreted as the survival of cancer patients after taking into account the background mortality that the patients would have experienced if they had not had cancer.
The age-adjusted 5-year survival rates for breast cancer and colorectal cancer were used to estimate an annual risk of mortality assuming a constant rate of mortality. It was assumed that the mortality rate does not increase due to cancer beyond 5 years after cancer diagnosis. The 5-year survival rate for breast cancer is 84.3%, which translated into a 3.37% annual probability of death from breast cancer. The 5-year survival rate for persons with colorectal cancer is 55.3%, which translated into an 11.16% annual probability of death from colorectal cancer.
Osteoarthritis
Osteoarthritis is related to BMI and diabetes, and so was included in the SPHR model. It was assumed that risk of osteoarthritis would be the same in a population with ID as in the general population, taking into account individual differences in risk factors. The Bruneck cohort,298 a longitudinal study of inhabitants of a town in Italy, reported diabetes and BMI as independent risk factors for osteoarthritis. The data used to estimate the incidence of osteoarthritis are reported in Table 45.
| Risk factor | Hazard ratio | 2.5th CI | 97.5th CI |
|---|---|---|---|
| Diabetes | 2.06 | 1.11 | 3.84 |
| BMI | 1.076 | 1.023 | 1.133 |
Depression
The SPHR Diabetes model includes depression as a health state due to its relationship with diabetes, but does not model its severity. Further details are available online. 263
Economic impact: utilities
Baseline utility
Baseline utilities for all of the individuals in the model were extracted from the STOP Diabetes study. The tariffs for the responses to the three-level EQ-5D were derived from a UK population study. 299 Baseline utility was assumed to decline because of ageing, as has been found in general population studies. An absolute decrement of 0.004 per year is applied in the model; this is based on previous HTA modelling in CVD. 287
Body mass index and utility
It was assumed that changes in BMI will impact on the utility of an individual with ID in the same way as for an individual in the general population. In a previous modelling of diabetes prevention, weight loss from education interventions was associated with an increase in utility of 0.0025 per kilogram change in weight. 300 This estimate was derived from weight loss trial data for which all of the participants were overweight or obese. In the population with ID a large proportion of individuals are normal weight or underweight, so it would not be appropriate to extrapolate the effects of weight loss on utility to these individuals. A change in utility due to a change in BMI was added to an individual’s EQ-5D if they had a BMI of > 25 kg/m2. As a consequence, obese individuals who reduce their BMI as a result of the intervention will experience an increase in EQ-5D.
Utility decrements
Utility decrements for long-term chronic conditions were applied to the age- and BMI-adjusted EQ-5D score. It was assumed that a diagnosis of diabetes was not associated with a reduction in EQ-5D independent of the utility decrements associated with complications, comorbidities or depression. CVD, renal failure, amputation, foot ulcers, blindness, cancer, osteoarthritis and depression were all assumed to result in utility decrements. The utility decrements are measured as a factor that is applied to the individual’s age- and BMI-adjusted baseline. If individuals have multiple chronic conditions then the utility decrements are multiplied together to give the individual’s overall utility decrement from comorbidities and complications, in line with current NICE guidelines for combining comorbidities. 301
Owing to the number of health states it was not practical to conduct a systematic review to identify utility decrements for all health states. Furthermore, there are very few or no data to inform utility decrements for comorbid conditions specifically in individuals with ID. A pragmatic approach was taken to search for health states within existing HTA for the relevant disease area in the general population or by considering studies that were used in previous economic models for diabetes prevention. Discussions with experts in health-economic modelling were also used to identify prominent sources of data for health-state utilities.
Two sources of data were identified for diabetes-related complications. A 2014 study from the UKPDS302 estimated the impact of changes in health states from a longitudinal cohort. These data were used to estimate the utility decrement for amputation and congestive heart failure. The absolute decrement for amputation was converted into utility decrement factors that could be multiplied by the individual’s current EQ-5D to estimate the relative effect of the complication. Utility decrements for renal failure and foot ulcers were not available from the UKPDS study described above, so were obtained from a different study303 of 2048 subjects with type 1 diabetes mellitus and T2DM.
Utility decrements for the variety of cardiovascular events were taken from a HTA assessing statins to reflect the utility decrements in the general population. 287 A burden-of-illness study with a broad utility decrement for cancer was identified and used to define utility decrements for breast and colon cancer. 304 A utility decrement for osteoarthritis was taken from a HTA,305 and a utility decrement for depression was calculated from a trial that had used the EQ-5D. 306
The multiplicative utility factors that are used in the model to describe health utility decrements from comorbid complications are shown in Table 46. The mean absolute decrement estimated in each study is reported alongside the baseline utility for each study. The utility factor was estimated by dividing the implied health utility with the comorbidity by the baseline utility.
| Event/comorbidity | Mean absolute decrement | SE for absolute decrement | Baseline utility | Multiplicative utility factor | Source |
|---|---|---|---|---|---|
| Foot ulcer | –0.099 | 0.013 | 0.689 | 0.856 | Coffey et al. (2002)303 |
| Amputation | –0.172 | 0.045 | 0.807 | 0.787 | UKPDS (2014)302 |
| Blind | 0.033 | 0.027 | 0.807 | 1.041 | UKPDS (2014)302 |
| Renal failure | –0.078 | 0.026 | 0.689 | 0.887 | Coffey et al. (2002)303 |
| Stable angina | 0.801 | Ward et al. HTA (2007)287 | |||
| Unstable angina, year 1 | 0.770 | Ward et al. HTA (2007)287 | |||
| Unstable angina, year 2 | 0.770 | Ward et al. HTA (2007)287 | |||
| MI, year 1 | 0.760 | Ward et al. HTA (2007)287 | |||
| MI, year 2 | 0.760 | Ward et al. HTA (2007)287 | |||
| TIA | 1.000 | Ward et al. HTA (2007)287 | |||
| Stroke, year 1 | 0.629 | Ward et al. HTA (2007)287 | |||
| Stroke, year 2 | 0.629 | Ward et al. HTA (2007)287 | |||
| Breast cancer | –0.060 | 0.800 | 0.913 | Yabroff et al. (2004)304 | |
| Colorectal cancer | –0.060 | 0.800 | 0.913 | Yabroff et al. (2004)304 | |
| Osteoarthritis | –0.101 | Black et al. HTA (2009)305 | |||
| Depression | –0.116 | 0.7905 | 0.875 | Benedict et al. (2010)306 | |
| Congestive heart failure | –0.101 | 0.032 | 0.875 | UKPDS (2014)302 |
Economic impact: costs
At any given time period of the model individuals can have multiple health complications that incur direct health-care costs. Some of the health states are mutually exclusive; however, an individual can accrue multiple complications within the model. Each health state is associated with an average cost, which is accrued by all of the individuals for every time period for which the state is indicated. Resource use for each comorbidity is added together and no savings are assumed to be made from the use of the same resources for two or more comorbidities for an individual. An exception to this is an assumed adjustment to the utilisation of GP services for individuals with chronic diseases. In the majority of cases it is assumed that the unit costs of health care for someone with ID would be the same as the unit costs for an individual in the general population.
The exception was cost for a GP appointment, which was expected to be 40% higher than in the general population as a result of increased length of consultation. All costs were inflated to 2014–15 values using the retail price index where necessary, from the Personal Social Services Research Unit sources of information. 264
At the present time, the following costs incorporated are:
-
costs of GP appointments
-
costs of hypertension/dyslipidaemia/diabetes diagnosis and treatment with statins and antihypertensive drugs; statins have a 65% uptake rate
-
diabetes costs [three-stage treatment regimen incorporating metformin (Glucophage®, Merck Serono Ltd, Feltham, UK) monotherapy (HbA1c level of > 6.5%), metformin plus a dipeptidyl peptidase-4 inhibitor (DPP-IV) (when HbA1c level is > 7.4%) and insulin plus antidiabetic drugs (when HbA1c level is > 8.5%)], together with associated costs such as blood tests and extra GP visits
-
CVD and heart failure costs (including hospital and primary care costs, medications and ongoing care costs for people with stroke)
-
microvascular costs (including renal dialysis and transplant, treatment costs for amputation, ulcer and blindness)
-
cancer costs (including screening and treatment by cancer stage)
-
osteoarthritis costs (including extra primary care, medications and joint replacement)
-
depression costs (including nurse costs, medication and emergency care).
A summary of all of the unit costs used in the model and their sources are shown in Table 47. 264,307–326
| Drug, treatment, care and resource costs | Cost per year/incident in 2014–15 prices (£) | Source |
|---|---|---|
| STOP Diabetes intervention per person | 1097 | Microcosting |
| Screening and intervention costs | ||
| Standard Learning Disability Health Check | 43.48 | Department of Health314 |
| Learning Disability Health Check without blood tests | 18.67 | |
| Health Check capabilities assessment and explanation of intervention (10 minutes of health-care assistant time) | 3.40 | PSSRU264 |
| First-line diabetes treatment: low-cost diabetes monotherapy | ||
| Ongoing costs of diabetes monotherapy | 79.06 | |
| Metformin 500 mg b.i.d. standard (85% of patients) or modified-release (15%) tablets | 18.83 | BNF313 |
| Nurse at GP (consultation) | 25.52 | PSSRU264 |
| Health-care assistant (10 minutes) | 3.40 | PSSRU264 |
| Urine sample | 1.00 | Department of Health324 |
| Eye screening | 24.31 | Burr et al.318 |
| Laboratory tests | 6.00 | |
| HbA1c test | 3.00 | Department of Health324 |
| Lipids test | 1.00 | Department of Health324 |
| Liver function test | 1.00 | Department of Health324 |
| B12 test | 1.00 | Department of Health324 |
| Additional first year costs of diabetes monotherapy: | 103 | |
| Nurse at GP (2 × consultations) | 51.03 | PSSRU264 |
| Health-care assistant (2 × 10 minutes) | 6.80 | PSSRU264 |
| Urine sample (×2) | 2.00 | Department of Health324 |
| Laboratory tests as above (×2) | 12.00 | Department of Health324 |
| Smoking cessation (central estimate of cost of nicotine replacement therapy) taken up by 50% of the assumed 20% of population who smoke | 30.90 | PSSRU264 |
| Second-line diabetes treatment | ||
| Metformin and a DPP-IV inhibitor | 529 | |
| Sitagliptina 100 mg daily | 434 | BNF313 |
| Metformin 500 mg b.i.d. standard (85% of patients) or modified-release (15%) tablets | 85 | BNF313 |
| Self-monitoring strips (82 per annum)320 | 16.36 | BNF313 |
| Nurse at GP (consultation) | 25.52 | PSSRU264 |
| Health-care assistant (10 minutes) | 3.40 | PSSRU264 |
| Urine sample | 1.00 | Department of Health324 |
| Eye screening | 24.31 | Burr et al.318 |
| Laboratory tests as for first-line treatment | 6.00 | Department of Health324 |
| Third-line diabetes treatment | ||
| Insulin and oral antidiabetic drugs, weighted composite | 1503 | |
| Nurse at GP (3 × consultations) | 76.55 | PSSRU264 |
| Health-care assistant (3 × 10 minutes) | 10.21 | PSSRU264 |
| Urine sample (×3) | 3.00 | Department of Health324 |
| Eye screening | 24.31 | Burr et al.318 |
| Laboratory tests as for first-line treatment (×3) | 18.00 | Department of Health324 |
| Insulin treatment costs: | 1376 | |
| Glargine | 830.83 | Poole et al.310 |
| Oral antidiabetic drugs | 57.75 | Poole et al.310 |
| Reagent test strips | 292.74 | Poole et al.310 |
| Hypoglycaemic rescue | 30.98 | Poole et al.310 |
| Pen delivery devices | 72.44 | Poole et al.310 |
| Sharps | 90.98 | Poole et al.310 |
| Other primary care costs | ||
| GP visit (17 minutes) | 68.38 | PSSRU264 |
| Diagnosis of hypertension (including ambulatory BP monitoring) | 56.51 | NICE325 |
| Annual treatment with statins (simvastatinb 20 mg b.i.d.) | 26.59 | BNF313 |
| Annual treatment with antihypertensive drugs | 195.94 | Blak et al.319 |
| CVD costs | ||
| Unstable angina, year 1: secondary care costs – 100% hospitalisation, 50% revascularisation procedure, three outpatient appointments); primary care costs (three GP visits) and medications | 4674 | Ara et al.322 |
| MI, year 1: secondary care costs – 100% hospitalisation, 50% revascularisation procedure, three outpatient appointments); primary care costs (three GP visits) and medications | 4813 | Ara et al.322 |
| Subsequent ACS care costs: secondary care costs (one outpatient appointment); primary care costs (three GP visits) and medications | 410 | Ara et al.322 |
| Stroke, year 1 (NHS costs): costs of acute events reported in Youman et al.307 weighted by the distribution of severity of stroke21 | 9716 | Youman et al.307 |
| Social care costs of stroke in subsequent years: the costs of ongoing care at home or in an institution weighted by the distribution of severity of stroke and discharge locations | 2730 | Ara et al.322 |
| Fatal CHD: assumed that 50% of fatalities incurred cost | 713 | Palmer et al.311 |
| Fatal non-cardiovascular event: assumed that 50% of fatalities incurred cost | 4443 | Youman et al.307 |
| Congestive heart failure | 3091 | UKPDS316 |
| Other complications of diabetes costs | ||
| Renal failure: weighted composite | 25,046 | |
| Haemodialysis with overheads | 42,049 | Baboolal et al.321 |
| Automated peritoneal dialysis | 27,217 | Baboolal et al.321 |
| Continuous ambulatory peritoneal dialysis | 19,742 | Baboolal et al.321 |
| Transplant (year 1) | 23,660 | NHS Blood and Transplant308 |
| Immunosuppressant (10 years) | 6959 | NHS Blood and Transplant308 |
| Foot ulcers | 216 | Gordois et al.315 |
| Amputation first year | 10,101 | UKPDS323 |
| Amputation subsequent years | 1896 | UKPDS323 |
| Blindness first year | 1434 | UKPDS323 |
| Blindness subsequent years | 479 | UKPDS323 |
| Breast cancer | 13,818 | Madan et al.312 |
| Colorectal cancer | 18,729 | Tappenden et al.309 |
| Osteoarthritis | 962 | NICE326 |
| Depression: made up of – | 137 | Chalder et al.317 |
| Practice nurse at surgery | 13.70 | |
| Practice nurse at home visit | 0.54 | |
| Practice nurse telephone | 0.99 | |
| Health visitor | 1.94 | |
| District nurse | 0.38 | |
| Other nurse | 1.17 | |
| Health-care assistant/phlebotomist | 1.05 | |
| Other primary care | 4.85 | |
| Out of hours | 6.18 | |
| NHS Direct | 2.28 | |
| Walk-in centre | 8.15 | |
| Prescribed medications | 74 | |
| Secondary care | 21 | |
Diabetes costs
A three-stage diabetes treatment regimen is applied in the model as a trade-off between model simplicity and capturing key cost differences between the interventions. At diagnosis, all patients are prescribed low-cost treatments, represented by metformin (weighted average of standard and modified release) to describe the average cost of these medications. If the HbA1c level increases above a threshold, the individual is prescribed one of the more expensive DPP-IV inhibitors in addition to metformin. The individual continues to receive metformin plus the DPP-IV inhibitor for a period of time until he/she requires insulin. The cost of diabetes in the year of diagnosis is assumed to be greater than subsequent years because the individual will receive more contact time while their diabetes is being controlled.
Simulated individuals experience an annual increase in HbA1c level. It is assumed that individuals switch to dual treatment in the first annual cycle in which HbA1c level increases above 7.4%, based on a 2014 HTA. 327 For costing purposes, the second drug to be added to metformin was assumed to be sitagliptin. The second major treatment change is assumed to be initiation of insulin. Within the model the individual is switched to insulin in the first annual cycle at which HbA1c levels exceed 8.5%. 327 The insulin glargine was chosen to represent insulin treatment in the UK.
Health checks
The cost of a health check in the population with ID was derived from the Department of Health Economic Modelling for vascular checks. 314 This study estimated the cost of a health check in the general population to be £23.70 in 2009, including blood tests, HCP time, follow-up and administration costs. For individuals with ID, it was assumed that all staff costs would double, as the health check would take twice as long to perform (Professor K Khunti, Diabetes Research Centre, University of Leicester, October 2015, personal communication). All of the other costs were assumed to stay the same. Costs were inflated to 2014–15 prices, giving a final value of £43.48 for a full Learning Disability Health Check.
Some individuals refuse to have blood taken as part of the health check. For these individuals a modified health check cost was derived, removing the cost of blood tests (consumables and laboratory costs) and the cost of nurse follow-up from the total. After inflation this came to £18.67.
Other primary care costs
Individuals with ID are assumed to visit their GP with the same frequency as individuals in the general population; however, each consultation is estimated to take 40% longer than the average consultation (based on personal communication, Professor K Khunti). Personal Social Services Research Unit unit costs were used to estimate the cost of a 17.2-minute consultation at £67,264 which was then inflated to 2014–15 prices. Individuals who are prescribed statins receive a daily dose of 40 mg of generic simvastatin. The individual remains on statins for the rest of their life. A unit cost of antihypertensive treatments was obtained from a 2004 study319 and inflated to 2014–15 prices. Owing to the number of different antihypertensive treatments available and possibilities for combination therapies, using the cost from this study of prescriptions was preferred to using costs directly from the British National Formulary.
Cardiovascular costs
Costs for CVD were obtained from a 2009 HTA for high-dose, lipid-lowering therapy. 322 The costs included are shown in Table 47. The costs of fatal stroke and MI were obtained from two separate studies,307,311 and it was assumed that 50% of individuals would incur these costs. The costs of congestive heart failure were estimated from the UKPDS costing study316 for complications related to diabetes.
Costs of other comorbidities
More details of the costs of microvascular complications of diabetes, cancers, osteoarthritis and depression are available online. 263
Other model inputs
-
Perspective The model adopts a NHS and social care perspective. Societal costs are not included.
-
Horizon The time horizon of the model is the lifetime of all baseline individuals.
-
Discount rates Costs and QALYs are discounted at 1.5% per annum, in line with NICE guidance for economic evaluations of public health interventions. 158
Reporting outcomes of the economic modelling
The model compares the outcomes of an identical baseline population undergoing the screening (and possible intervention) with the outcomes if current care was followed. The model allows a variety of different clinical outcomes to be gathered, as well as costs and QALYs. The model also allows a range of other incremental outcomes to be collected including life-years saved and diabetes/cardiovascular cases prevented.
Use of threshold analysis
The usual output of an economic evaluation, for a prespecified intervention with known clinical effectiveness and cost per patient, is the ICER (see below for formulae), which can then be compared with the £20,000–30,000 cost per QALY acceptability threshold set out by NICE.
However, as the clinical effectiveness of the STOP Diabetes intervention was not tested as part of the current programme of research (only its feasibility), a different approach for this analysis was needed. The output was the change in effectiveness needed for the intervention to be marginally cost-effective, that is, the ICER equals the cost per QALY acceptability threshold. As the primary clinical outcome of interest to the STOP Diabetes study investigators is change in steps per day, the economic analysis deals with the change in physical activity (steps) and associated risk factors (BMI, SBP, and total and HDL cholesterol) needed for the intervention to be marginally cost-effective. The STOP Diabetes intervention also contains dietary advice (but no specific weight goal), so the threshold analysis needs to take account of benefits attributable to physical activity and diet. The results tables for the threshold analyses therefore show alternative permutations of step changes together with the additional benefit from dietary change that would be necessary for the intervention to be cost-effective overall.
Analyses, scenarios and sensitivity analyses undertaken
All statements herein about an increase in the number of steps refer to the increase in steps per day.
Exploratory analysis
An initial analysis was undertaken to assess how cost-effective the STOP Diabetes intervention would be if it achieved the average 2491 change in daily steps reported in the Bravata meta-analysis. 269 Initially, it was assumed that the intervention would increase mean daily step count by 2491 steps as detailed in Bravata et al. ,269 leading to:
-
a mean reduction in BMI of 0.38 kg/m2
-
a mean reduction in systolic BP of 3.8 mmHg
-
a mean reduction in total cholesterol of 0.09 mmol/l
-
a mean increase in HDL cholesterol of 0.06 mmol/l.
The intervention effect was assumed to decline linearly such that by 3 years (from the start of the intervention), the risk factors have reverted back to their trajectory had there been no intervention.
The ICER is obtained using the incremental costs and QALYs gained from implementing the intervention rather than current care, calculated using the following formulae:
Although total costs and QALYs can be assessed at any year in the model, allowing estimation of both short- and long-term cost-effectiveness, we report the long-term cost-effectiveness, as this is what regulatory bodies are primarily interested in.
In addition, a simple budget impact was calculated, as follows:
-
The number of adults in England with moderate to critical needs using social care was taken from 2015 estimates by Public Health England’s learning disabilities observatory for adults (546,489). 328
-
The percentage of the above who have IGR was based on the percentage in the STOP Diabetes screening study who were found to have IGR after screening, recruitment and blood testing (5%).
-
The percentage that was likely to take up an intervention was based on the percentage of those in the feasibility study who were invited to take part on the STOP Diabetes programme, who actually attended sessions (26%).
-
The resulting number of likely STOP Diabetes users for the whole of England (7104) was multiplied by the intervention cost per user to give the total budget impact of implementing the STOP Diabetes programme.
Scenarios for duration of effect
For all of the analyses undertaken, we model two fundamental alternative scenarios to test the sensitivity of the cost-effectiveness to two alternative durations of effect of the intervention, that is, after starting to reduce after year 1, by which time point the benefits of intervention have worn off. The first scenario, assuming a 3-year duration, is considered most likely given that the proposed maintenance sessions finish at the end of the first year and the alternative 5-year scenario is presented as a ‘what if’ scenario.
Deterministic one-way sensitivity analysis
Previous analyses using the model and of economic evaluations of lifestyle interventions suggested which parameters are likely to have the largest effect on model results, so the following were considered for one-way sensitivity analysis.
Increased clinical effects
There is significant uncertainty around the relationship reported by Bravata et al. ,269 so we explored the impact of a more beneficial impact by taking the 65th percentile for the possible magnitude of beneficial change in BMI, systolic BP, total cholesterol and HDL cholesterol from the CIs reported by Bravata et al. 269 (assuming that the distributions are normally distributed). In this case, an increase in mean daily step count of 2491 steps, as detailed by Bravata et al. ,269 is estimated to lead to:
-
a mean reduction in BMI of 0.45 kg/m2
-
a mean reduction in systolic BP of 4.21 mmHg
-
a mean reduction in total cholesterol of 0.14 mmol/l
-
a mean increase in HDL cholesterol of 0.07 mmol/l.
This alternative mapping was explored in the context of both the 3- and 5-year duration of effect scenarios above.
Uptake rates
These were considered not to be a key driver because individuals who do not uptake the intervention incur no costs other than the very small cost of screening relative to the cost of the intervention.
Discount rates
Alternative rates were considered, but testing out rates that were < 1.5% seems implausible and higher rates would not have altered the conclusions.
Subgroup analyses
As initial analyses suggested that the intervention would be unlikely to be cost-effective in the overall population with ID, further work was set out in order to identify the most beneficial subgroups. Three subgroups were identified to explore if the cost-effectiveness of the intervention might be improved if screening were more targeted, by:
-
Age band Based on the distribution of age in the STOP Diabetes study, the following age bands were chosen: < 35 years; ≥ 35 and < 40 years; ≥ 40 and < 45 years; ≥ 45 and < 50 years; and ≥ 50 years. Selecting individuals aged ‘≥ 35 years’ would include 65% of the STOP Diabetes cohort, and those aged ‘≥ 40 years’ and ‘≥ 45 years’ and ‘≥ 50 years’ would include 55%, 45% and 35%, respectively. Age cut-off points of ≥ 55 and ≥ 60 years would have covered only 24% and 14% of the cohort only, respectively.
-
BMI We carried out a subgroup analysis in which everyone was screened, but only obese individuals were eligible for intervention.
-
Baseline cardiovascular risk We calculated the baseline 10-year CVD risk using the QRISK score and excluded any individuals with a risk of < 5%. This is a low cut-off point but using a higher cut-off point would have meant fewer than 25% of the cohort being screened, and a very low proportion actually receiving and benefiting from intervention.
Probabilistic sensitivity analysis
Owing to the exploratory nature of the analysis (described in Exploratory analysis), full PSA was carried out on only one of the threshold scenarios, with the aim of illustrating the extent of non-linearity in the model (i.e. by comparing the results of the PSA with the corresponding deterministic results). PSA, which describes the uncertainty in model parameter inputs, is not suitable for describing the decision uncertainty in this analysis, that is, the current research was leading to the stage at which the intervention could be considered for implementation into clinical practice. Instead, analyses were more exploratory to inform potential future research and intervention refinement.
In addition, the true uncertainty around the effectiveness estimates is much wider than that around the parameters available from the Bravata et al. 269-based relationship. There is also uncertainty around the effectiveness of the planned intervention in increasing physical activity and uncertainty around whether or not increasing the number of steps increases metabolic benefits in a linear way. This uncertainty cannot be accurately quantified (although it could potentially be estimated through a time-consuming expert elicitation, which is outside the scope of this investigation), but PSA analysis without it would vastly underestimate the uncertainty in the cost-effectiveness estimates.
For the single PSA completed, a suitable distribution was selected for each parameter, based on its mean and SE, and, within the simulations, random sampling across all input parameter distributions was undertaken. A total of 2000 different random samples of parameter values were selected, and each was applied to a different random cohort of 5000 individuals who were randomly sampled with replacement from the baseline STOP Diabetes population. For each PSA sample, the model was run and results ompiled.
More details of the distributions around key model parameters are shown in Appendix 26, Tables 67 and 68.
Results
This section presents a series of results for the scenarios and sensitivity analyses described earlier. First, is an analysis of how cost-effective the STOP Diabetes intervention would be if it achieved the average change for a physical activity intervention, 2491 steps, as reported in the Bravata et al. 269 meta-analysis. Next, results of the threshold analyses for the necessary risk factor changes needed to achieve cost-effectiveness are presented under a variety of scenarios and subgroups. Finally, an alternative threshold analysis explores what the maximum budget for the intervention would be given certain changes in risk factors.
Whenever results are stated as the ‘base case’, these reflect the base-case assumption for the effects a change in steps has on risk factors for CVD (BMI, systolic BP, and total and HDL cholesterol). Most analyses present results for both 3- and 5-year durations of intervention effect, but, if not specified, the base case of 3 years applies.
It should be noted that effects lasting to year 3 means that they have worn off 2 years from the end of year 1 (which is close to when the last monthly maintenance session occurs). Similarly, effects that have worn off by year 5 effectively last for 4 years from the end of the maintenance sessions.
Cost per quality-adjusted life-year results based on Bravata step count
Before the threshold analyses were undertaken, an exploratory analysis was undertaken to see how cost-effective the STOP Diabetes intervention would be, assuming an increase in steps in line with that calculated in the meta-analysis by Bravata et al. 269 This analysis assumes no dietary intervention.
The estimated ICER (cost per QALY gained) under the base case is £275,000 compared with a usual acceptability threshold (what funders are willing to pay) in the range £20,000–30,000 per QALY. Savings in lifetime costs of CVD and primary care, and savings in treating diabetes and its complications are far outweighed by the £1097 intervention cost per person. A much greater intervention effect in terms of either physical activity, diet or both, or a reduction in intervention cost would be required to make the intervention cost-effective.
The estimated budget impact for delivering the STOP Diabetes programme to 7104 adults with ID and IGR across England was estimated at £7.8M. If the programme were taken up by all adults with ID and IGR in England (> 27,000) then the total cost could be as high as £30M. There is uncertainty around the true prevalence of IGR among adults with ID. If this is actually 10% (rather than 5%), the above budget impacts would rise to £15.6M and £60M, respectively.
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis was carried out for the above Bravata et al. 269-based analysis (an increase of 2491 steps), giving a probabilistic central estimate of the ICER of £253,000, which is lower than the deterministic estimate by about 6%, demonstrating a small degree of non-linearity in the model. Given how high the deterministic ICER was compared with the acceptability threshold of £30,000 per QALY, this is clearly a negligible difference for the overall conclusion about the intervention.
The probability that such an intervention is cost-effective compared with current care at a threshold of £30,000 per QALY is almost zero, at 0.15%. However, as described above (see Methods), the uncertainty around intervention effectiveness is much higher than the uncertainty described in the Bravata et al. study269 and used in the PSA, meaning that the PSA will underestimate the total uncertainty.
The cost-effectiveness plane for the intervention compared with current care at £30,000 per QALY is shown in Appendix 27 (see Figure 30).
Threshold analyses for effect sizes needed
A series of model simulations was performed in order to determine the thresholds required for the intervention to be cost-effective at acceptability thresholds of £20,000 and £30,000 per QALY. The presented thresholds are rounded to the nearest 500 steps.
At the calculated thresholds, the ICER for the intervention is £20,000 (or £30,000) per QALY and the greater savings from CVD and diabetes treatment and primary care costs, together with the value of the health gain (QALYs), are just enough to outweigh the additional cost of the intervention.
Ascertainment of the thresholds relies on the assumption of a linear relationship between change in steps and risk factors (that observed in the Bravata et al. study269) being maintained over the wide range of steps inherent within the calculation of the thresholds.
It is recognised that under many, if not most, scenarios, the magnitude of the additional diet-related changes in BMI and systolic BP (that are necessary to attain cost-effectiveness) are implausible in terms of their achievability. They are nevertheless genuine estimates from the threshold approach that is fundamental to this economic analysis.
Summary of key results from threshold analyses
In the subsequent section (see Full threshold analysis results tables), detailed results are presented for a number of alternative levels of increase in steps per day, up to 15,000. However, there are many tables, each containing many permutations of the magnitude of physical activity- and dietary-related BMI change that could achieve cost-effectiveness (see Table 52). To aid digestion of the results, in this section the results are summarised for some mid-range levels of change in steps: 3000, 5000 and 7000 per day.
In Table 48, the necessary diet-related effects that would need to be achieved, in addition to the effects arising from an increase of 5000 steps, are shown across various scenarios and subgroups (see Use of threshold analysis for the rationale of the threshold analyses as presented).
| Population group | £20,000 per QALY | £30,000 per QALY | ||
|---|---|---|---|---|
| 3 years | 5 years | 3 years | 5 years | |
| Base case | –4.2 (–42) | –2.6 (–26) | –2.9 (–29) | –1.5 (–15) |
| Increased effectiveness intervention | –4.0 (–40) | –2.0 (–20) | –2.4 (–24) | –1.2 (–12) |
| Obese subgroup | –4.0 (–40) | –2.0 (–20) | –2.6 (–26) | –1.3 (–13) |
| 45–49 years subgroup | –1.8 (–18) | –1.1 (–11) | –1.4 (–14) | –0.9 (–9) |
| ≥ 50 years age group | –3.4 (–34) | –2.0 (–20) | –1.8 (–18) | –0.9 (–9) |
| High CVD risk group (≥ 5% 10-year risk) | –2.7 (–27) | –1.4 (–14) | –1.5 (–15) | –0.8 (–8) |
As an example, consider the base-case row of Table 48, assuming an acceptability threshold of £30,000 per QALY and 3-year duration of effects. BMI and systolic BP reductions of 2.9 kg/m2 and 29 mmHg, respectively, would be needed, in addition to the BMI and systolic BP benefits of 5000 additional steps, in order for the intervention to be cost-effective. An increase of 5000 steps corresponds to a BMI reduction of 0.76 kg/m2, a systolic BP reduction of 7.6 mmHg and a reduction in the lipid ratio of 0.22 (all purely through physical activity without diet). So the overall (steps plus dietary) BMI and systolic BP reductions needed for a cost-effective intervention would be 3.66 kg/m2 and 36.6 mmHg, which are clearly unachievable in practice.
Tables 49 and 50 present similar analyses for changes in steps of 3000 and 7000, respectively.
| Population group | £20,000 per QALY | £30,000 per QALY | ||
|---|---|---|---|---|
| 3 years | 5 years | 3 years | 5 years | |
| Base case | –4.5 (–45) | –2.9 (–29) | –3.2 (–32) | –1.8 (–18) |
| Increased effectiveness intervention | –4.3 (–43) | –2.3 (–23) | –2.7 (–27) | –1.5 (–15) |
| Obese subgroup | –4.3 (–43) | –2.3 (–23) | –2.9 (–29) | –1.6 (–16) |
| 45–49 years subgroup | –2.1 (–21) | –1.4 (–14) | –1.7 (–17) | –1.2 (–12) |
| ≥ 50 years age group | –3.7 (–37) | –2.3 (–23) | –2.1 (–21) | –1.2 (–12) |
| High CVD risk group (≥ 5% 10-year risk) | –3.1 (–31) | –1.7 (–17) | –1.8 (–18) | –1.1 (–11) |
| Population group | £20,000 per QALY | £30,000 per QALY | ||
|---|---|---|---|---|
| 3 years | 5 years | 3 years | 5 years | |
| Base case | –3.9 (–39) | –2.3 (–23) | –2.6 (–26) | –1.2 (–12) |
| Increased effectiveness intervention | –3.7 (–37) | –1.7 (–17) | –2.1 (–21) | –0.9 (–9) |
| Obese subgroup | –3.7 (–37) | –1.7 (–17) | –2.3 (–23) | –1.0 (–10) |
| 45–49 years subgroup | –1.5 (–15) | –0.8 (–8) | –1.1 (–11) | –0.6 (–6) |
| ≥ 50 years age group | –3.1 (–31) | –1.7 (–17) | –1.5 (–15) | –0.6 (–6) |
| High CVD risk group (≥ 5% 10-year risk) | –2.4 (–24) | –1.1 (–11) | –1.2 (–12) | –0.5 (–5) |
Intervention cost threshold analysis
As an alternative type of threshold analysis, we explored the maximum intervention cost that could be afforded for a given increase in steps per day. At a value of £30,000 per QALY, for the subgroup of individuals aged > 50 years, with an assumed duration of effect of 3 years, the maximum intervention budget (combined initial plus maintenance cost) to achieve cost-effectiveness is around £280 for an increase of 4000 steps per day (0.61-kg/m2 BMI reduction and 6-mmHg systolic BP reduction), and around £420 for an increase of 6000 steps per day (0.91-kg/m2 BMI reduction and 9-mmHg systolic BP reduction). For the subgroup of individuals with increased CVD risk, the maximum budget for a cost-effective intervention is around £500 for an increase of 4000 steps per day and around £700 for an increase of 6000 steps per day.
Interpretation of threshold analysis results
First, we summarise the implications of aiming to achieve cost-effectiveness through step changes alone. For the overall STOP Diabetes cohort, to achieve cost-effectiveness at a value of £20,000 per QALY, the results suggest that in excess of 30,000 additional steps per day would be required (around 5-kg/m2 reduction in BMI and 50-mmHg reduction in systolic BP), which is biologically implausible. At £30,000 per QALY the threshold was around 24,000 additional steps per day (3.7-kg/m2 reduction in BMI and 37-mmHg reduction in systolic BP). If the intervention effect is assumed to last (decreasing linearly) until year 5, the threshold reduces to around 22,000 steps (2.4-kg/m2 reduction in BMI and 34-mmHg reduction in systolic BP) at £20,000 per QALY and 15,000 steps (2.3-kg/m2 reduction in BMI and 23-mmHg reduction in systolic BP) at £30,000 per QALY. Note that as all of these values represent effect sizes that are more than five times those achieved in the source study,269 they are inherently reliant on extrapolations of the Bravata relationship269 well outside the range in which extrapolation can be done with a desirable level of reliability.
Cost-effective effect sizes could, alternatively, be achieved through a combination of risk factor changes through physical activity and risk factor changes through dietary intervention. Clearly, there are many permutations of the magnitude of physical activity- and dietary-related changes that could achieve cost-effectiveness. A necessary increase of 13,000 steps under an assumption of increased effectiveness and 5 years’ duration (at £30,000 per QALY) equates to a BMI change of –2.75 kg/m2 based on Bravata et al. 269 This overall BMI change could be achieved, for example, through the effect of an additional 7000 steps per day together with further BMI reduction achieved through dietary intervention of –1.68 kg/m2.
Alternative body mass index and systolic blood pressure equivalents
A limitation of the threshold results presented above is that the necessary BMI and systolic BP changes attributed to diet exclusively use the Bravata et al. study269 in their calculation. This results in the small combined (steps plus diet) BMI changes relative to the systolic BP changes reflecting the ratio of benefits that could be expected from a physical activity rather than a dietary intervention.
To aim to address this, as an exploratory analysis, Table 51 shows an alternative set of permutations, the difference being that the diet-related BMI and systolic BP changes are now ‘realigned’ to reflect more realistically the relative ratio of BMI and systolic BP changes likely through dietary change. The calculations are underpinned by the BMI and systolic BP hazard ratios in the QRISK score for CVD, and thereby the analysis relies on the assumption that most of the economic benefits of intervention accrue through CVD risk reduction. We estimated that a reduction in systolic BP of 1 mmHg gives approximately the same benefit as 0.6 kg/m2 of BMI reduction.
| Population group | £20,000 per QALY | £30,000 per QALY | ||
|---|---|---|---|---|
| 3 years | 5 years | 3 years | 5 years | |
| Base case | –11.8 (–29) | –7.3 (–18) | –8.1 (–20) | –4.2 (–11) |
| Increased effectiveness intervention | –11.2 (–28) | –5.6 (–14) | –6.7 (–17) | –3.4 (–8) |
| Obese subgroup | –11.2 (–28) | –5.6 (–14) | –7.3 (–18) | –3.6 (–9) |
| 45–49 years subgroup | –5 (–13) | –3.1 (–8) | –3.9 (–10) | –2.5 (–6) |
| ≥ 50 years age group | –9.5 (–24) | –5.6 (–14) | –5 (–13) | –2.5 (–6) |
| High CVD risk group (≥ 5% 10-year risk) | –7.6 (–19) | –3.9 (–10) | –4.2 (–11) | –2.2 (–6) |
The analysis was undertaken just in the context of a change of 5000 steps, that is, a reworking of results presented earlier in Table 48.
For the high-CVD-risk groups, if the effects could be maintained such that the effect is not completely lost until year 5 then the effect sizes needed are becoming closer to those achievable in practice.
Full threshold analysis results tables
Here we present the full set of model results for a wide range of changes in step count. For disadvantaged groups, there is a higher likelihood than normal of NICE recommending a treatment at the upper end of the usual £20,000–30,000 per QALY bracket, so the results presented in Table 52 are for a willingness to pay of £30,000 per QALY. The diet-related improvements in BMI, systolic BP and cholesterol that would be needed at £20,000 per QALY would be even more challenging (or implausible) than those presented here, so the results for £20,000 per QALY are presented in Appendix 28, Tables 67–72.
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –3.7 | –37 | –1.54 | –2.3 | –23 | –1.08 |
| 1000 | –0.2 | –2 | –0.09 | –3.5 | –35 | –1.49 | –2.1 | –21 | –1.03 |
| 3000 | –0.5 | –5 | –0.26 | –3.2 | –32 | –1.40 | –1.8 | –18 | –0.90 |
| 5000 | –0.8 | –8 | –0.42 | –2.9 | –29 | –1.30 | –1.5 | –15 | –0.78 |
| 7000 | –1.1 | –11 | –0.57 | –2.6 | –26 | –1.19 | –1.2 | –12 | –0.64 |
| 9000 | –1.4 | –14 | –0.71 | –2.3 | –23 | –1.08 | –0.9 | –9 | –0.50 |
| 11,000 | –1.7 | –17 | –0.84 | –2.0 | –20 | –0.97 | –0.6 | –6 | –0.34 |
| 13,000 | –2.0 | –20 | –0.97 | –1.7 | –17 | –0.84 | –0.3 | –3 | –0.18 |
| 15,000 | –2.3 | –23 | –1.08 | –1.4 | –14 | –0.71 | 0.0 | 0 | 0.00 |
Base-case results
The systolic BP and cholesterol ratio effects shown in the sixth and seventh columns of Table 52 are the total (step-related) effects for these parameters, that is, effects mediated indirectly through BMI reduction and direct effects of physical activity.
A more detailed breakdown of the base-case events, costs and QALYs is shown in Table 53. The mean QALY gain per person screened equates to an average of < 1 day of additional life (in full health).
| Incremental outcomes per person | Base-case effectiveness | Increased effectiveness | ||
|---|---|---|---|---|
| 3-year duration | 5-year duration | 3-year duration | 5-year duration | |
| Total costs (£) | 329 | 326 | 328 | 322 |
| Total QALYs | 0.0012 | 0.0018 | 0.0014 | 0.0021 |
| ICER (£) | 273,000 | 183,000 | 231,000 | 154,000 |
| Cardiovascular cases (per 1 million) | –130 | –187 | –153 | –239 |
| STOP Diabetes intervention cost (£) | 336 | 336 | 336 | 336 |
| Diabetes treatment costs (£) | 0 | 1 | 0 | 0 |
| Cardiovascular costs (£) | –5 | –6 | –5 | –7 |
| Costs: other diabetes complications (£) | –6 | –8 | –7 | –10 |
Sensitivity analyses: increased clinical effects
In this analysis, it was assumed that effects on BMI, systolic BP and the lipid ratio of a given increase in steps would be greater than the base case, as described above (see Methods).
Under this modified assumption, and assuming 3-year duration of effects, the estimated incremental cost per QALY gained for an increase of 2491 steps (with no dietary intervention) was £228,000.
To achieve cost-effectiveness at a value of £20,000 per QALY, threshold analysis suggests that in excess of 30,000 additional steps per day would be required (some 4.7-kg/m2 reduction in BMI and 47-mmHg reduction in systolic BP), which is still biologically implausible. At £30,000 per QALY the threshold is around 21,000 additional steps per day (3.2-kg/m2 reduction in BMI and 32-mmHg reduction in systolic BP). If the intervention effect is assumed to last (decreasing linearly) until year 5, the threshold reduces to around 18,000 steps (2.8-kg/m2 reduction in BMI and 27-mmHg reduction in systolic BP) at £20,000 per QALY, and 13,000 steps (2.0-kg/m2 reduction in BMI and 20-mmHg reduction in systolic BP) at £30,000 per QALY. The combination of step change and additional dietary change needed to reach cost-effectiveness (assuming that all risk factors change together) is shown in Table 54 (see Appendix 28, Table 68, for £20,000 per QALY acceptability).
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –3.2 | –32 | –1.40 | –2.0 | –20 | –0.97 |
| 1000 | –0.2 | –2 | –0.09 | –3.1 | –31 | –1.35 | –1.8 | –18 | –0.90 |
| 3000 | –0.5 | –5 | –0.26 | –2.7 | –27 | –1.25 | –1.5 | –15 | –0.78 |
| 5000 | –0.8 | –8 | –0.42 | –2.4 | –24 | –1.14 | –1.2 | –12 | –0.64 |
| 7000 | –1.1 | –11 | –0.57 | –2.1 | –21 | –1.03 | –0.9 | –9 | –0.50 |
| 9000 | –1.4 | –14 | –0.71 | –1.8 | –18 | –0.90 | –0.6 | –6 | –0.34 |
| 11,000 | –1.7 | –17 | –0.84 | –1.5 | –15 | –0.78 | –0.3 | –3 | –0.18 |
| 13,000 | –2.0 | –20 | –0.97 | –1.2 | –12 | –0.64 | 0.0 | 0 | 0.00 |
| 15,000 | –2.3 | –23 | –1.08 | –0.9 | –9 | –0.50 | 0.0 | 0 | 0.00 |
Subgroup analysis: obese
Separate results are reported for a subgroup of the population only (using the base-case clinical effects) who were defined as obese (Table 55).
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –3.4 | –34 | –1.45 | –2.1 | –21 | –1.00 |
| 1000 | –0.2 | –2 | –0.09 | –3.2 | –32 | –1.40 | –1.9 | –19 | –0.94 |
| 3000 | –0.5 | –5 | –0.26 | –2.9 | –29 | –1.30 | –1.6 | –16 | –0.81 |
| 5000 | –0.8 | –8 | –0.42 | –2.6 | –26 | –1.19 | –1.3 | –13 | –0.68 |
| 7000 | –1.1 | –11 | –0.57 | –2.3 | –23 | –1.08 | –1.0 | –10 | –0.53 |
| 9000 | –1.4 | –14 | –0.71 | –2.0 | –20 | –0.97 | –0.7 | –7 | –0.38 |
| 11,000 | –1.7 | –17 | –0.84 | –1.7 | –17 | –0.84 | –0.4 | –4 | –0.22 |
| 13,000 | –2.0 | –20 | –0.97 | –1.4 | –14 | –0.71 | –0.1 | –1 | –0.05 |
| 15,000 | –2.3 | –23 | –1.08 | –1.1 | –11 | –0.57 | 0.0 | 0 | 0.00 |
The estimated incremental cost per QALY gained for a 2491 increase in steps under this scenario was £276,000.
To achieve cost-effectiveness at a value of £20,000 per QALY, threshold analyses suggest that in excess of 30,000 additional steps per day would be required, and at £30,000 per QALY the threshold is around 22,000 additional steps per day. If the intervention effect is assumed to last (decreasing linearly) until year 5, the threshold reduces to around 18,000 steps at £20,000 per QALY, and 13,500 steps at £30,000 per QALY. These values are very similar to the whole population results.
Subgroup analysis: age subgroups
The results in Table 56 show the effect sizes needed for subgroups by age (for both the base-case clinical effects and increased effects sensitivity assumption).
| Population group | Base-case clinical effects | Increased clinical effects | ||||||
|---|---|---|---|---|---|---|---|---|
| £20,000 per QALY | £30,000 per QALY | £20,000 per QALY | £30,000 per QALY | |||||
| Base case (3 years) | 5 years | Base case (3 years) | 5 years | Base case (3 years) | 5 years | Base case (3 years) | 5 years | |
| All ages | –5.8/–58 | –3.4/–34 | –3.8/–38 | –2.3/–23 | –5.6/–56 | –2.9/–29 | –3.5/–35 | –1.9/–19 |
| Age < 35 years | –18.3/–183 | –4.7/–47 | –7.9/–79 | –3.4/–34 | –13/–130 | –4/–40 | –6.7/–67 | –2.8/–28 |
| Age 35–39 years | –4.3/–43 | –2.7/–27 | –3.6/–36 | –2.2/–22 | –2.8/–28 | –2.4/–24 | –2.4/–24 | –2/–20 |
| Age 40–44 years | –5.3/–53 | –3.1/–31 | –3.7/–37 | –2.2/–22 | –5.2/–52 | –2.7/–27 | –3.4/–34 | –1.9/–19 |
| Age 45–49 years | –2.6/–26 | –1.9/–19 | –2.1/–21 | –1.7/–17 | –2.8/–28 | –2.1/–21 | –2.2/–22 | –1.6/–16 |
| Age ≥ 50 years | –4.1/–41 | –2.7/–27 | –2.6/–26 | –1.7/–17 | –4.6/–46 | –2.1/–21 | –2.5/–25 | –1.3/–13 |
The estimated ICER (cost per QALY gained) in the base case across the whole baseline population was £276,000. For age-based subgroups, this ICER varied from £172,000 (ages ≥ 50 years) to £482,000 (ages 35–39 years). In the base case with 5-year durability, the ICER varied from £107,000 (ages ≥ 50 years) to £301,000 (ages < 35 years). In the increased effectiveness scenario with 5-year durability, the ICER ranged from £92,000 (ages ≥ 50 years) to £262,000 (ages < 35 years).
To achieve cost-effectiveness at a value of £30,000 per QALY in the overall cohort with ID, threshold analyses suggest that 25,000 additional steps per day would be required. For age subgroups, this threshold varied from 14,000 additional steps per day (ages 45–49 years) to 52,000 (ages < 35 years). If the durability was extended to 5 years, the threshold ranges from 11,000 additional steps (ages ≥ 45 years) to 22,000 additional steps (ages < 35 years) and if effectiveness is increased the threshold ranges from 8500 additional steps (ages ≥ 50 years) to 18,500 steps (ages < 35 years). The combinations of steps and additional dietary changes needed to reach cost-effectiveness for people aged 45–49 years and aged ≥ 50 years at £30,000 (£20,000) per QALY are shown in Tables 57 and 58 (and Appendix 28, Tables 70 and 71).
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –2.1 | –21 | –1.03 | –1.7 | –16.8 | –0.8 |
| 1000 | –0.2 | –2 | –0.09 | –2.0 | –20 | –0.97 | –1.5 | –15.3 | –0.8 |
| 3000 | –0.5 | –5 | –0.26 | –1.7 | –17 | –0.84 | –1.2 | –12.2 | –0.6 |
| 5000 | –0.8 | –8 | –0.42 | –1.4 | –14 | –0.71 | –0.9 | –9.2 | –0.5 |
| 7000 | –1.1 | –11 | –0.57 | –1.1 | –11 | –0.57 | –0.6 | –6.1 | –0.3 |
| 9000 | –1.4 | –14 | –0.71 | –0.8 | –8 | –0.42 | –0.3 | –3.1 | –0.2 |
| 11,000 | –1.7 | –17 | –0.84 | –0.5 | –5 | –0.26 | 0.0 | 0.0 | 0.0 |
| 13,000 | –2.0 | –20 | –0.97 | –0.2 | –2 | –0.09 | 0.0 | 0 | 0.00 |
| 15,000 | –2.3 | –23 | –1.08 | 0.0 | 0 | 0.00 | 0.0 | 0 | 0.00 |
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –2.6 | –26 | –1.19 | –1.7 | –16.8 | –0.8 |
| 1000 | –0.2 | –2 | –0.09 | –2.4 | –24 | –1.14 | –1.5 | –15.3 | –0.8 |
| 3000 | –0.5 | –5 | –0.26 | –2.1 | –21 | –1.03 | –1.2 | –12.2 | –0.6 |
| 5000 | –0.8 | –8 | –0.42 | –1.8 | –18 | –0.90 | –0.9 | –9.2 | –0.5 |
| 7000 | –1.1 | –11 | –0.57 | –1.5 | –15 | –0.78 | –0.6 | –6.1 | –0.3 |
| 9000 | –1.4 | –14 | –0.71 | –1.2 | –12 | –0.64 | –0.3 | –3.1 | –0.2 |
| 11,000 | –1.7 | –17 | –0.84 | –0.9 | –9 | –0.50 | 0.0 | 0.0 | 0.0 |
| 13,000 | –2.0 | –20 | –0.97 | –0.6 | –6 | –0.34 | 0.0 | 0.0 | 0.0 |
| 15,000 | –2.3 | –23 | –1.08 | –0.3 | –3 | –0.18 | 0.0 | 0.0 | 0.0 |
Subgroup analysis: high cardiovascular risk subgroup
We ran an additional analysis looking at effectiveness of intervening in a subgroup of the population with a 10-year CVD risk of at least 5% using the base-case assumptions about clinical effects. A 5% cut-off point was chosen because if the cut-off point had been ≥ 10% then this would have resulted in around only 10% of the population being screened (before factoring in eligibility, suitability and willingness, so probably < 5% would have actually received the intervention).
The estimated ICER (cost per QALY gained in the base case across the whole baseline population) was £177,000. With 5-year durability of effects, the ICER falls to £133,000.
To achieve cost-effectiveness in the base case at a value of £30,000 per QALY, threshold analyses suggest that 15,000 additional steps per day would be required with 3-year durability, and 10,000 if the durability was extended to 5 years. The combinations of steps and additional dietary changes needed to reach cost-effectiveness for people with a high CVD risk at £30,000 per QALY are shown in Table 59 (see Appendix 28, Table 72, for £20,000 per QALY).
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | Systolic BP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –2.3 | –23 | –1.08 | –1.5 | –15 | –0.78 |
| 1000 | –0.2 | –2 | –0.09 | –2.1 | –21 | –1.03 | –1.4 | –14 | –0.71 |
| 3000 | –0.5 | –5 | –0.26 | –1.8 | –18 | –0.90 | –1.1 | –11 | –0.57 |
| 5000 | –0.8 | –8 | –0.42 | –1.5 | –15 | –0.78 | –0.8 | –8 | –0.42 |
| 7000 | –1.1 | –11 | –0.57 | –1.2 | –12 | –0.64 | –0.5 | –5 | –0.26 |
| 9000 | –1.4 | –14 | –0.71 | –0.9 | –9 | –0.50 | –0.2 | –2 | –0.09 |
| 11,000 | –1.7 | –17 | –0.84 | –0.6 | –6 | –0.34 | 0.0 | 0 | 0.00 |
| 13,000 | –2.0 | –20 | –0.97 | –0.3 | –3 | –0.18 | 0.0 | 0 | 0.00 |
| 15,000 | –2.3 | –23 | –1.08 | 0.0 | 0 | 0.00 | 0.0 | 0 | 0.00 |
Discussion
Statement of principal findings
Using a threshold analysis approach, the base-case results indicate that the STOP Diabetes intervention that we have evaluated, costing £1097 per patient, would need to result in a very large overall increase in steps, systolic BP, BMI and cholesterol for it to be cost-effective at a threshold in the £20,000–30,000 cost per QALY range that is usually adopted by NICE. These increases are much more than could be expected to be achievable in practice, even for the general population. Specifically for steps, an increase of 3000–5000 per day appears to be much more commonly reported for an intervention.
If we adopt two very favourable assumptions, that (1) the benefits of the intervention would not be fully lost until 4 years after the intervention (5 years from the start) and (2) commissioners/payers would be willing to fund the intervention up to a threshold of £30,000 per QALY, then some of the scenarios begin to show more favourable results to some extent.
Targeting screening at individuals who are either aged > 45 years or obese, or at those at relatively high baseline risk of CVD, improves the cost-effectiveness of the intervention, but it is still not cost-effective at readily achievable combinations of steps and diet-attributable changes in risk factors (unless the cost of the intervention could be reduced).
Strengths and limitations
A strength of the analysis is that it was based on a relatively large set of baseline data for a cohort with ID, so the baseline risks of the cohort were well evidenced. However, there are large uncertainties around the intervention cost, and the precise relationship between changes in physical activity and cardiovascular risk factors. ‘Number of steps’ per day was the primary outcome of interest from the modelling, based on the fact that it is the primary measure of interest to the STOP Diabetes study investigators. The available evidence linking steps to biomarkers is somewhat limited, and comes from very diverse studies. Each intervention is unique and therefore estimates of effectiveness are unlikely to predict exactly the outcomes of the intervention in question. In addition, identified studies were undertaken in the general population, not a population with ID, and it is unknown whether differences in either behavioural or physiological response to intervention vary in the community with ID.
Despite the fact that most diabetes prevention interventions combine dietary and physical activity elements, in order to model the threshold level of the primary measure of interest (steps) we had to use steps as the key variable in our estimates, with its effects mediated through changes in BMI, systolic BP and cholesterol. This complicates interpretation because it then becomes necessary to show what permutations of combined diet and activity intervention may achieve the necessary magnitude of changes to BMI, systolic BP and cholesterol. The fact that the intervention is not cost-effective with 2491 additional steps (in line with the effect size observed by Bravata et al. 269) means that, in order to determine the cost-effective threshold, extrapolation is required. The extrapolation makes an assumption of a linear relationship between step increase and change in biomarkers; however, the true relationship may be non-linear.
The Bravata et al. study269 was considered to provide the most suitable data for the mapping between steps and risk factors. This study269 reported a seemingly large change in systolic BP (–3.8 mmHg) relative to the change in BMI (–0.38) for an increase of 2491 steps. Further evidence confirming this relationship is desirable. If this ratio of systolic BP change relative to BMI is overstated then the true results may be less favourable than presented. A further limitation is the inevitable disparity between the STOP Diabetes intervention and the heterogeneous mix of interventions (in terms of number of sessions, delivery period, end-point timing) that were pooled in the Bravata et al. meta-analysis. 269 The effects of uncertainty around the effectiveness and other parameter estimates were assessed using an illustrative PSA; however, it is not possible to incorporate the full uncertainty around the applicability of pooled estimates from Bravata et al. 269 to this specific intervention in the target population. Further uncertainty analysis would be required once the clinical effectiveness of the STOP Diabetes programme has been quantitatively assessed.
There is a lack of good evidence on the durability of effects of an intervention such as STOP Diabetes, especially when the maintenance sessions all take place within the same year as the initial intervention. Additionally, the STOP Diabetes cohort does not overall appear to be a particularly unhealthy one at baseline, perhaps due to recruitment criteria or self-selection, or just purely due to the average age of individuals recruited. There may be some obscure mechanisms that are not captured within the model structure and that drive the reported reduced life expectancy for individuals with ID. If such mechanisms exist, and can be modified by lifestyle intervention, then the results will not capture the economic impact of such benefits.
Comparison with related studies
There are several factors concerning the form of the intervention that lead to a high intervention cost per patient compared with other preventative lifestyle interventions:
-
Relative small group size of eight that the clinical team considered most appropriate for educating those with ID.
-
Longer sessions for those with ID than the general population: 2 hours, compared with 1 hour and 15 minutes for the NICE prevention modelling. 300
-
The need for three educators rather than two, and more advanced, at a higher grade.
-
The need for maintenance sessions to be spaced fairly close together, such as monthly, for information to be retained by individuals with ID. This compares with less frequent sessions, every 4 months in years 2–4 in the case of the modelling undertaken for the NICE diabetes prevention guidance. 300
Implications
The purpose of the economic analysis was to provide a reasonable estimate of the cost-effectiveness of the STOP Diabetes intervention with a view to a subsequent full trial to assess effectiveness. The results of the current analysis suggest that, in the likely range of effectiveness achievable, the STOP Diabetes intervention in its current form would not be cost-effective at a £20,000–30,000 cost/QALY threshold.
Risk profile of STOP Diabetes and the population with intellectual disability in general
The large effect sizes needed for the intervention to be cost-effective are partly a result of the low risk profile of the STOP Diabetes cohort, in particular the average age being 43 years.
The intervention primarily reduces risks of CVD and cancer. The evidence suggests that excess risks in cohorts with ID compared with the general population are attributable primarily to respiratory disorders, neurological diseases, congenital abnormalities and accidents. These risks are unlikely to be reduced through an intervention such as STOP Diabetes. The mortality aspects of these risks may be captured through the increased other-cause mortality for individuals with ID (see Other-cause mortality), but other-cause mortality is not linked to the risk factors modified by the STOP Diabetes intervention (BMI, systolic BP, and total and HDL cholesterol). It is therefore rational that an intervention targeting CVD risks, costing £1097 (three to four times the cost of diabetes prevention lifestyle interventions), will necessitate our reported very large reductions in risk factors in order to fall within usual NICE thresholds for cost-effectiveness.
The low risk profile is reflected in the mean QALY gain per person screened (with a subsequent increase in steps of 2491 for suitable individuals) equating to an average of < 1 day of additional life (in full health).
Equity is a factor that decision-makers take into account when deciding whether or not to recommend an intervention. Given that the population with ID is a disadvantaged group, decision-makers might be prepared to pay more per QALY than for the general population. 265 In practice, this means that there is a greater likelihood of recommending an intervention at the upper end of the usual £20,000–30,000 per QALY range than for the general population; £30,000 per QALY tends to be the upper limit except in the context of end of life.
Although the average person with ID is overweight (and nearly obese), the 10-year CVD risk using cohort averages (mild/moderate severity only) is around only 2%. This is because risk factors seem to be well controlled in STOP Diabetes: the average systolic BP was 121 mmHg, the lipid ratio was a healthy 3.63 and it was a relatively young cohort.
A limitation of the STOP Diabetes intervention is that the reduction in CVD risk is likely to be confined to a few years after intervention. Combined with the average baseline age of 43 years and associated low average CVD risk, this explains why the intervention has such as high cost per QALY for the overall group. An alternative more cost-effective structuring of the intervention sessions may be possible, such that a smaller initial benefit is achieved but sustained for more years, possibly through additional maintenance sessions beyond the first year.
A minority – although a significant one – of individuals who are identified as suitable for intervention may not make the desired progress towards reducing their risk factors for CVD. This could be due to an individual’s physiological response to the intervention or the numbers of intervention sessions that he/she actually attends, or a combination of both factors. For such patients, continuing with the maintenance session may be reducing the overall effectiveness of the intervention that might be more cost-effective, potentially at favourable cost-per-QALY levels, in those who achieve a good initial response.
Unanswered questions and further research
Further subgroup analyses could be undertaken in groups at a 10%, 15% or 20% 10-year risk of CVD (diabetes risk could also be factored in), although such subgroups would result in a very small proportion of the STOP Diabetes cohort actually receiving the intervention.
Further research is needed to identify the optimal mix of initial and maintenance sessions for physical activity programmes, together with a better understanding of how long benefits are likely to last. Modelling may also help to inform primary research into the optimal trade-off between investment in initial intervention and maintenance sessions.
Weight loss can be difficult to maintain even with ongoing maintenance, as seen in the Finnish Diabetes Prevention Study. 329 However, physical activity can help to maintain weight loss so it is unknown if benefits of physical activity can be sustained for longer than dietary-induced weight loss (hence why we explored 3 and 5 years as scenarios for durability of effect).
Further analysis may help to identify optimal permutations of the magnitude/intensity of the physical activity and dietary advice components to maximise the cost-effectiveness.
Chapter 13 Discussion and conclusions
Overview
In this chapter, we summarise and discuss the research programme’s findings against each of its objectives. We also summarise the findings in terms of outputs and implications for practice, make research recommendations, and discuss dissemination activities and plans for the research programme.
Development and assessment of the feasibility of a diabetes screening programme in adults with intellectual disability
Main findings
Adults with ID were identified for the screening programme through general practices, specialist ID services (through the LLDR), specialist ID clinics and through direct contact with the research team. In total, 930 (29% of those originally approached) took part in the screening programme, of whom 38% were able to consent for themselves; other participants required a consultee. There were slightly more men than women among those screened (58%) and participants were relatively young (mean age 43.3 years), mainly of white ethnicity (80%) and most were overweight (31%) or obese (37%). We were able to collect data on anthropometric measures for most participants (≈ 86%), and BP (89%) and outcome data for 675 participants (73%) to assess the prevalence of IGR/T2DM.
Physical activity substudy
We found that the objective measurement of physical activity is likely to be challenging in adults with ID, given that there are high levels of non-compliance. However, compliance could be substantially improved using wrist-worn monitors. Of 203 people approached, fewer than half (n = 97; 48%) consented to wear the waist-worn device, compared with 62% (47 of 76 approached) of those consenting to wear the wrist-worn device. Similarly, valid data were obtained from 57% (n = 55) of the sample who wore the waist-worn devices compared with 83% (n = 39) of those wearing the wrist-worn devices.
Other studies among adults with ID have found a high proportion of missing data when using objectively measured physical activity data. 164,233 However, to our knowledge, this is the first time the feasibility of collecting objectively measured physical activity data in those with ID has been formally assessed. The results suggest that poor compliance needs to be considered when conducting studies of physical activity interventions in this population. Researchers may also need to explore the potential for allowing separate consent in their study design for proposed accelerometer components.
Another somewhat unexpected finding was the high level of physical activity that was observed in our study population. We found that adults with ID engaged in similar amounts of physical activity as the general population, whereas most,164,233 but not all,236 studies have found that people with ID generally engage less. This might reflect the current policy drives to improve health and fitness in this population, but may also indicate selection bias (i.e. active people preferentially choosing to wear the monitors) or behaviour change as a result of accelerometer wear.
Prevalence and demographic risk factors for type 2 diabetes and impaired glucose regulation in people with intellectual disability
The overall prevalence of screen-detected (previously undiagnosed) T2DM was 1.3% (95% CI 0.5% to 2%) and IGR was 5% (95% CI 4% to 7%) among people with ID, which is lower than previously reported. Our systematic review (see Chapter 2) found that the prevalence of diagnosed T2DM was approximately 8% (95% CI 5% to 11%), similar to that found in the general population. None of the studies in the review reported on screen-detected T2DM (they included prevalent known cases, we excluded, so it is not possible to make direct comparisons. Our lower-than-expected rates of T2DM may simply reflect a successful annual health check programme, at least in the study’s geographical location, and the younger age of participants.
Abnormal glucose levels were associated with non-white ethnicity (OR 3.93, 95% CI 2.10 to 7.33), a first-degree family history of diabetes (OR 3.35, 95% CI 1.64 to 6.86), increasing weight, waist circumference, BMI, diastolic BP, triglycerides and decreasing HDL cholesterol.
Validation of the Leicester Self-Assessment diabetes risk score in people with intellectual disability
When the seven risk factors in the Leicester Self-Assessment risk score were used to explore risk of having undiagnosed IGR/T2DM among people with ID (with data available), the risk score achieved a sensitivity of 82% in identifying those with abnormal glucose regulation. High sensitivity is generally considered most important for screening tools because the priority is to ‘rule out’ the disease without missing true cases. Ninety-eight per cent of participants with a low-/medium-risk score were correctly identified as being at low risk. Our findings suggest that the Leicester Self-Assessment risk score is statistically effective at identifying people with ID who are at risk of undiagnosed IGR/T2DM and does not require modification if it should be integrated at practice level. However, it may not be practical or acceptable for people with ID to calculate their own score; development of an easy-read version (plus a carer supplement) and additional supportive material would need to be explored.
Cost-effectiveness
Findings from the health-economic analysis showed that, in its current form, the STOP Diabetes multicomponent intervention would need to result in a very large overall increase in steps, systolic BP, BMI and lipid levels for it to be cost-effective at a threshold in the £20,000–30,000 cost-per-QALY range usually adopted by NICE.
The results would be favourable under the assumptions that:
-
the benefits of the intervention would not be fully lost until 4 years after the intervention (5 years from the start)
-
commissioners/payers would be willing to fund the intervention up to a threshold of £30,000 per QALY.
The cost-effectiveness of the intervention would be improved by targeting screening at the following groups, that is, individuals:
-
aged > 45 years
-
with BMI in the obese range
-
with a relatively high baseline risk of CVD.
However, it is still not cost-effective at readily achievable levels of change in steps and diet-attributable risk factors, unless the cost of the intervention could be reduced.
The relatively high cost of the STOP Diabetes intervention compared with other similar multicomponent behaviour change interventions is due to a number of factors:
-
small samples needed for each group session
-
longer sessions
-
the need for three educators rather than two
-
the need for more experienced educators
-
the need for regular, monthly refresher sessions.
Many of these factors were identified in advance as being important for the interventions to be appropriate and relevant for people with ID. It is known that the high support needs of this population – including co-existing challenging behaviour,64 psychiatric disorders,330 physical health problems32 and communication difficulties – make this a challenging group for behavioural interventions. We aim to explore other ways in which the intervention may be adapted to minimise resources, such as the potential to target carers under certain circumstances.
Finally, the findings also revealed a lack of good-quality evidence for the durability of effects of multicomponent behaviour change interventions, such as that developed for the STOP Diabetes programme.
Data linkage to Hospital Episode Statistics and the Office for National Statistics
In total, 883 (95%) participants gave consent for the research team to follow up their health in the longer term via data linkage.
Development of a lifestyle education programme for people with intellectual disability and impaired glucose regulation
The research involved the development of a structured lifestyle education programme for a population with ID with IGR or at high risk of developing T2DM and/or CVD based on a high BMI. This was a complex process encompassing initial curriculum development, two cycles of testing, evaluation, modification and retesting, prior to final refinement of the programme.
The STOP Diabetes programme development benefited from a systematic process. 238,239 The theoretical underpinning was developed and expanded on from the limited evidence in the literature. This informed the content and style of approach, alongside the qualitative findings from people with ID, their carers and HCPs with expertise in working with people with ID. The whole programme was then tailored further to the specific needs of this group by more user feedback and adaptation by a multidisciplinary team with expertise in ID and the development of education programmes (with psychological underpinning).
From the initial phases, the programme has been well received and is acceptable to the people it is trying to support. The initial feedback via qualitative interviews has suggested that some of the elements of treatment receipt initially hypothesised may have been achieved via reported changes in beliefs and health behaviours.
The research also involved an assessment of feasibility of collecting outcome measures from participants with ID before and 3 months after delivering the intervention programme. For this component, our findings suggest that it is both acceptable and feasible to collect outcome measures for weight, height, BMI, waist circumference, BP and dietary intake (portions of fruit and vegetables), and objective measures for physical activity and sedentary behaviour, using wrist-worn accelerometers, both before and after (3 months) attending the programme. At baseline, anthropometric measures and BP were obtained for all of the participants and accelerometer data for 80%. Attendance at the education programme was overall good, with 80% of participants attending for ≥ 5 days (out of seven sessions for the main programme). At 3 months’ follow-up, repeat data were successfully collected for a high proportion of participants (anthropometric measures 100%; BP 80%; accelerometer data 60%). Owing to time restrictions, we were able to conduct only one feasibility cycle and were also unable to assess whether or not it is possible to collect longer-term data, but these preliminary findings are overall positive.
Only four of the five participants who took part in the intervention agreed to wear the wrist-worn accelerometers at baseline, and this suggests that an assessment of willingness to wear the accelerometer is an important component of any future evaluation work. Furthermore, the feasibility component of our work suggested that lifestyle circumstances could play an important role in adhering to the education programme and this needs to be considered for future work.
Development of an intervention fidelity process for the assessment of educators delivering the intervention
As part of this research, we successfully completed the first step in developing a tool for assessing intervention fidelity of the STOP Diabetes educational programme. Preliminary findings using the tool already suggest some variance between educators, which will provide a benchmark for future work. One of the key considerations for this component of the research involved reconsidering existing learning methods that are known to be effective in the general population to meet the needs of people with ID. This included removing abstract concepts, avoiding abbreviations and jargon, teaching at the group’s pace and, above all, avoiding isolating the learners by ‘putting them on the spot’ to summarise key messages.
Main findings and outputs
The main findings and outputs arising from this extensive research programme are summarised as follows.
-
We developed and assessed the feasibility of a diabetes screening programme for adults with ID.
-
In total, 930 (29% of those originally approached) people with ID took part in the screening programme; 58% were men and the average (mean) age of participants was 43 years.
-
Most people who took part in the screening programme (68%) were overweight or obese.
-
We were able to collect blood samples from 73% of participants and anthropometric measures on > 85% of participants.
-
To measure physical activity, we found that wrist-worn accelerometers were more acceptable to participants with ID than waist-worn accelerometers.
-
We found that 1.3% of people with ID had undiagnosed T2DM and 5% of people with ID had IGR (screen detected).
-
We found that abnormal glucose tolerance was associated with non-white ethnicity, first-degree family history of diabetes, increasing weight, waist circumference, hip circumference, BMI, diastolic BP, triglycerides and lower HDL cholesterol.
-
We developed a lifestyle intervention programme for a population with ID with IGR or at a high risk of developing T2DM and/or CVD based on a high BMI.
-
Using concrete messages and visual aids facilitated learning in this group; abstract and conceptual examples tended to be less well received.
-
We found that the collection of outcome measures prior to, and after (3 months), delivering the intervention was both acceptable and feasible.
-
We identified that for the intervention to be cost-effective (£20,000–30,000 cost-per-QALY range), the required change in steps and diet-attributable risk factors may be more than is achievable in practice.
-
We found that if commissioners were willing to fund the intervention up to a higher threshold, cost-effectiveness may improve by targeting specific individuals (aged > 45 years, obese, high CVD risk).
-
We developed a preliminary quality development tool to assess intervention fidelity of the educational programme for people with ID.
Limitations
We have found that conducting a programme of research to enhance the knowledge and understanding of IGR and T2DM in people with ID, including development of a lifestyle education programme, is feasible, but not without challenges. We acknowledge the following limitations:
-
With regard to the systematic review of the evidence in relation to prevalence of T2DM and IGR, we acknowledge that limited data were available on T2DM in people with ID and that reported outcomes were sometimes poorly defined or unclear. We would also have benefited from more general population data for comparison.
-
Similarly, for the systematic review of long-term multicomponent behaviour change interventions for the prevention of CVD and T2DM in people with ID, we acknowledge that only four papers met our inclusion criteria, which limited our ability to draw meaningful conclusions. However, our findings do highlight the lack of work in this area and the need for robust interventions, such as that developed for this programme of work.
-
Despite highlighting a number of achievements in involving service users in our research programme, we acknowledge that we could have done more to involve them in the design and dissemination phases of our programme.
-
We acknowledge that the recruitment approach utilised for the screening study may not be transferable to other geographical areas in England. Recruitment was facilitated by the LLDR14 (either via direct invitation from the register or for people previously agreeing to be approached about future research), which accounted for 40% of people invited (≈39% of participants). The register is only one of three adult ID case registers in England and has a strong research tradition. However, we approached people via this route only for general practices that declined to take part in the study and we feel that approaches such as direct invitation and invitation via ID psychiatric service clinics could be replicated in other areas.
-
We acknowledge some difficulties in recruiting services users to the development phase (WP2) despite using a direct approach to people who had already participated in the screening phase. For the qualitative development interviews, low recruitment was largely due to an initial lack of people who were either ineligible (based on severity of ID and/or a BMI of ≥ 25 kg/m2 or IGR) or unwilling. Additionally, for the later phases, for which participation involved attending a course of education sessions held over several weeks (with little flexibility in scheduling), reported barriers were largely linked to the regular daytime commitments (social activities/work/education) of service users that they were either unwilling or unable to change. The ‘busy schedules’ of potential participants has previously been identified as a barrier to recruitment for people with ID. 331 Unfortunately, within the constraints of this research study there was no flexibility to offer alternative dates to attend the programme. However, for the second pilot education cycle, which was held in a residential setting, the day and timing of sessions were arranged as much as possible to suit the needs of both service users and care workers, and recruitment levels were much higher.
-
For the economic evaluation, we acknowledge the exploratory nature of the work, given that data on clinical effectiveness for the STOP Diabetes programme were not available. In particular, the analysis involved extrapolating data outcomes, which assumed a linear relationship between step increase and changes in biomarkers (BMI, systolic BP and cholesterol), which may not reflect their true relationship.
-
We further acknowledge limitations with using the EQ-5D for the economic evaluation, as this has not been validated in people with ID. We look forward to the outputs from current work to validate the EQ-5D in this population. 159
Implications for practice
We have found that, at least in Leicester/Leicestershire, there is a low prevalence of previously undiagnosed (screen detected) IGR/T2DM. However, we also found that a significant proportion of people with ID are overweight or obese and are likely to be at risk of developing T2DM and/or CVD in the future. Our non-invasive risk score might also help to identify people at risk of undiagnosed IGR/T2DM. The development of the STOP Diabetes educational programme is the first stage in identifying preventative strategies for future research.
Research recommendations
We make the following recommendations for further research:
-
The recruitment rate for the screening study was relatively low (29%). In some cases the use of gatekeepers, including GPs, residential home managers and family carers presented a barrier to recruitment. We recommend utilising a multipronged/multilayered approach, actively engaging with both intermediaries and service users, and following up all of the potential participants to ensure that people are given an equitable chance to participate.
-
In order to be truly inclusive, we highlight the importance of making reasonable adjustments, including offering appointments whenever and wherever is most appropriate for the person, minimising disruption to their routine and ensuring that appropriate support is in place. Given limited resources, it is likely that researchers and funders need to lower the threshold for an ‘acceptable’ response in this population, so that adults with ID are not excluded altogether from taking part in research.
-
We recommend a staggered consent process when recruiting people with ID into research to enable them to opt out of some components, such as having blood tests or wearing accelerometers.
-
We have demonstrated that adults with ID can be meaningfully involved in the research process; we recommend exploring further ways in which people with ID can be involved in research and be recompensed for their time.
-
We recommend further work to explore ways in which compliance with accelerometer wear can be improved in people with ID.
-
We recommend ongoing monitoring of the participants in our study to identify longer-term health and mortality outcomes.
-
Finally, we have found preliminary evidence that the STOP Diabetes education programme is acceptable and feasible. We recommend further work to evaluate its clinical effectiveness and cost-effectiveness in a RCT informed by the Medical Research Council framework for evaluating complex interventions,238 with a view to integrating the programme into national preventative strategies and reducing health inequalities among people with ID.
Dissemination activities and plans
During the consent process, participants were asked if they wished to be informed of the findings. Between September and December 2015 we disseminated the results to participants (and carers). Two of the ID research nurses visited 57 homes (group homes, supported living, and residential and nursing homes) to present the findings to participants in an easy-read format, supplemented by verbal explanations/presentations. Other participants received a brief easy-read report that was sent in the post. We have begun to disseminate the findings to HCPs locally, both in primary care and within ID services.
The work from the service user involvement component of this research has been published in one of the NIHR INVOLVE newsletters198 and in the academic literature. 203 The initial education development work has previously been presented at the Diabetes UK Professional Conference in March 2015. 332 Similarly, the screening study was presented at the 2016 Diabetes UK Professional Conference. 333
The next steps will involve writing up and submitting academic articles in relation to the individual components of the research programme. This will include the screening study, risk score validation, cost-effectiveness component, intervention development and updated versions of the two systematic reviews. We will continue to present the findings both locally, through existing collaborations with NIHR Collaboration for Leadership in Applied Health Research and Care East Midlands and the East Midlands Academic Health Services Network, and nationally. We have been invited to present our work at a meeting of the Royal Society for Medicine Intellectual Disability Forum (Managing Diabetes in People with Intellectual Disabilities: Recent Advances) in November 2016.
Summary
Results from this programme of work have significantly enhanced existing knowledge and understanding of T2DM and IGR in people with ID, and enabled us to test strategies for the early identification of IGR and T2DM in people with ID. This is the first large diabetes screening study in people with ID in the UK and, to our knowledge, the largest screening study globally. We have also developed a lifestyle education programme and educator training protocol to promote behaviour change in a population with ID who are at risk of developing T2DM. Further work is now needed to evaluate the intervention we have developed and identify cost-effective strategies for its implementation.
Acknowledgements
The authors gratefully acknowledge the people with ID, their families and health professionals who took part in the STOP Diabetes study. We would especially like to thank the contribution from service users and facilitators from the ‘Speaking up for Health’ and the ‘Charnwood Action’ self-advocacy groups who helped us throughout the research process.
We acknowledge help from key people throughout the STOP Diabetes research programme: Colin Greaves for his excellent chairing of the steering group meetings; research nurses Ella Bailey, Clare Makepeace, Kay Massey, Elaine Perkins and Paul Underwood; administrative expertise from Yvette Walters; educational programme support from Marian Carey, Director of the DESMOND programme; and support from Lesley Green (LLDR).
Alan Brennan, Professor of Health Economics and Decision Modelling at the School of Health and Related Research, University of Sheffield, contributed to the planning of the economic analysis and to addressing comments from referees.
We also thank the following people who provided useful help and support at various stages of the programme: Navneet Aujla, Kiran Bains, Michael Bonar, Lesley Bryan, Mandy Clarkson, Heather Daly, Karen Davis, Charlotte Edwardson, Jules Galbraith, Panna Mandalia-Wilson, Jacqui Troughton and Shelley Winterton.
The work by Thomas Chalk was supported by a PhD studentship funded jointly by the University of Leicester and LPT.
Contributions of authors
Dr Alison Dunkley (Research Associate in Nursing) was the lead researcher/project manager for the programme and was responsible for its design and conduct. She also authored chapters of the manuscript and sat on the steering group.
Ms Freya Tyrer (Research Fellow in Epidemiology) led on recruitment from the LLDR, authored a chapter of the manuscript and sat on the steering group.
Ms Rebecca Spong (Research Assistant) contributed to all components of the programme and chapters of the manuscript.
Dr Laura Grey (Senior Lecturer of Population and Public Health Sciences) designed and analysed the quantitative results, and oversaw their reporting and interpretation. She also authored a chapter of the manuscript and sat on the steering group.
Mr Mike Gillett (Research Fellow) designed and led on the health economics component of the programme, authored a chapter of the manuscript and sat on the steering group.
Dr Yvonne Doherty (Consultant Clinical Psychologist) co-led on the development of the education programme, contributed to chapters of the manuscript and sat on the steering group.
Ms Lorraine Martin-Stacey (Senior Research Associate) co-led on the development of the education programme and contributed to chapters of the manuscript.
Ms Naina Patel (Research Associate) conducted the interviews with HCPs, service users and carers for the research programme, analysed the qualitative results, contributed to chapters of the manuscript and sat on the steering group.
Dr Tom Yates (Reader in Physical Activity, Sedentary Behaviour and Health) contributed to the initial grant application, advised on evidence relating to physical activity and cardiovascular risk for the health economics chapter and authored a chapter of the manuscript.
Professor Sabyasachi Bhaumik (Consultant Psychiatrist) was the principal investigator from LPT, contributed to the initial grant application, facilitated recruitment from specialist ID clinics, sat on the steering group and reviewed the final manuscript.
Mr Thomas Chalk (PhD student) led on the two systematic reviews for the programme and reviewed the final manuscript.
Ms Yogini Chudasama (Research Assistant in Medical Statistics) analysed the quantitative results and led on their reporting and interpretation under the supervision of Laura Grey. She reviewed the final manuscript.
Dr Chloe Thomas (Research Assistant) made a substantial contribution to the health economics component of the programme (under the supervision of Mike Gillett) and contributed to a chapter of the manuscript.
Ms Susannah Sadler (Research Associate) made a substantial contribution to the health economics component of the programme (under the supervision of Mike Gillett) and contributed to a chapter of the manuscript.
Professor Sally-Ann Cooper (Professor of Learning Disabilities) contributed to the initial grant application, sat on the steering group and reviewed the final manuscript.
Dr Satheesh K Gangadharan (Medical Director of LPT) contributed to the initial grant application, sat on the steering group and reviewed the final manuscript.
Professor Melanie Davies (Professor of Diabetes Medicine) contributed methodological and practical advice to the research programme and reviewed the final manuscript.
Professor Kamlesh Khunti (Professor of Primary Care Diabetes & Vascular Medicine) was the chief investigator for the study and conceived the idea for the study, and contributed methodological and practical advice to all of the components of the research programme and publications arising from the research.
Publications
Dunkley A, Clarkson M, Tyrer F. STOP Diabetes Study. INVOLVE Summer 2013 Newsletter. Eastleigh: NIHR; 2013.
Martin-Stacey L, Doherty Y, Makepeace C, Patel N, Spong R, Dunkley AJ. The systematic development and theoretical framework of a lifestyle educational programme for prevention of type 2 diabetes in a population with intellectual disabilities: P338. Diabet Med 2015;32(Suppl. 1):132.
Dunkley AJ, Spong R, Gray LJ, Tyrer F, Bhaumik S, Gangadharan SK, et al. Screening for people at high risk of type 2 diabetes in a population with intellectual disabilities: P474. Diabet Med 2016;33(Suppl. 1):177.
Tyrer F, Dunkley AJ, Spong R, Gangadharan SK, Bhaumik S, Khunti K. Involving service users with intellectual disability in research: experiences from the STOP Diabetes study [published online ahead of print 18 September 2016]. J Pol Pract Intellect Disabil 2016.
Dunkley AJ, Tyrer F, Gray LJ, Bhaumik S, Spong R, Chudasama Y, et al. Type 2 diabetes and glucose intolerance in a population with intellectual disabilities: the STOP Diabetes cross-sectional study. J Intellect Disabil Res 2017; in press.
Data sharing statement
All of the available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Building the NHS of the Five Year Forward View: The NHS England Business Plan 2015–16. London: NHS England; 2015.
- Rodway C, Windfur K, Kapur N, Shaw J, Appleby L. National Learning Disability Review Development Project. Stage 1 – Options Development Report. Manchester: University of Manchester; 2014.
- Mental Capacity Act 2005 Code of Practice. London: The Stationery Office; 2007.
- Valuing People: A New Strategy for Learning Disability for the 21st Century. London: DH; 2001.
- International Classification of Diseases (ICD-10) Guide for Mental Retardation. Geneva: WHO; 1996.
- Emerson E, Heslop P. A Working Definition of Learning Disabilities. Durham: Improving Health & Lives: Learning Disability Observatory; 2010.
- Wu J, Morris JK. The population prevalence of Down’s syndrome in England and Wales in 2011. Eur J Hum Genet 2013;21:1016-19. http://dx.doi.org/10.1038/ejhg.2012.294.
- Atlas: Global Resources for Persons with Intellectual Disabilities. Geneva: WHO; 2007.
- Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 2011;32:419-36. http://dx.doi.org/10.1016/j.ridd.2010.12.018.
- Emerson E, McGrother C. The Use of Pooled Data From Learning Disabilities Registers: A Scoping Review. Durham: Learning Disability Observatory, Improving Health & Lives; 2011.
- Hatton C, Emerson E, Glover G, Robertson J, Baines S, Christie A. People with Learning Disabilities in England 2013. London: Public Health England; 2014.
- British Medical Association, NHS Employers, NHS England . 2015/16/General/Medical/Services/(GMS)/Contract/Quality/and/Outcomes/Framework/(QOF)./Guidance/for/GMS/Contract/2015/16 2015. www.nhsemployers.org/∼/media/Employers/Documents/Primary%20care%20contracts/QOF/2015%20-%2016/2015%2016%20QOF%20guidance%20for%20stakeholders.pdf (accessed September 2015).
- Buszewicz M, Welch C, Horsfall L, Nazareth I, Osborn D, Hassiotis A, et al. Assessment of an incentivised scheme to provide annual health checks in primary care for adults with intellectual disability: a longitudinal cohort study. Lancet Psychiatry 2014;1:522-30. http://dx.doi.org/10.1016/S2215-0366(14)00079-0.
- Watson JM. Valuing people: valuing resources: the Leicestershire Learning Disabilities Register. Frontline 2003;53:24-5.
- National Service Framework for Diabetes: Standards. London: DH; 2001.
- UK Prospective Diabetes study (UKPDS) VIII . Study design, progress and performance. Diabetologia 1991;34:877-90.
- Turning the Corner: Improving Diabetes Care. London: DH; 2006.
- The Quality and Outcomes Framework – Prevalence, Achievements and Exceptions Report. England, 2013–2014. Leeds: HSCIC; 2014.
- APHO Diabetes Prevalence Model: Key Findings for England. York: Diabetes Health Intelligence, YHPHO; 2010.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, et al. Diagnosis, Prognosis, and Treatment of Impaired Glucose Tolerance and Impaired Fasting Glucose. Hamilton, ON: McMaster University Evidence-based Practice Center; 2005.
- Definition and Diagnoses and Classification of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva: WHO; 2006.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004;141:413-20. https://doi.org/10.7326/0003-4819-141-6-200409210-00006.
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 1997;14:1-85. https://doi.org/10.1002/(SICI)1096-9136(199712)14:5+<S7::AID-DIA522>3.3.CO;2-I.
- Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev 2000;16:230-6. https://doi.org/10.1002/1520-7560(2000)9999:9999<::AID-DMRR122>3.0.CO;2-W.
- Counting the Cost: The Real Impact of Noninsulin Dependent Diabetes Mellitus. UK: British Diabetic Association; 1997.
- Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. London: NICE; 2012.
- Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: WHO; 2011.
- Gholap NN, Davies MJ, Mostafa SA, Khunti K. Diagnosing type 2 diabetes and identifying high-risk individuals using the new glycated haemoglobin (HbA1c) criteria. Br J Gen Pract 2013;63:e165-7. http://dx.doi.org/10.3399/bjgp13X663244.
- Preventing Chronic Diseases. A Vital Investment. Geneva: WHO; 2005.
- Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–20. Geneva: WHO; 2013.
- Emerson E, Baines S, Allerton L, Welch V. Health Inequalities & People with Learning Disabilities in the UK: 2012. Durham: Learning Disabilities Observatory, Improving Health & Lives; 2012.
- Melville CA, Hamilton S, Hankey CR, Miller S, Boyle S. The prevalence and determinants of obesity in adults with intellectual disabilities. Obes Rev 2007;8:223-30. https://doi.org/10.1111/j.1467-789X.2006.00296.x.
- Krahn GL, Fox MH. Health disparities of adults with intellectual disabilities: what do we know? What do we do?. J Appl Res Intellect Disabil 2014;27:431-46. http://dx.doi.org/10.1111/jar.12067.
- Temple V, Walkley J. Physical inactivity of adults with intellectual disability. J Intellect Dev Disabil 2003;28:323-34. https://doi.org/10.1080/13668250310001616380.
- Messent P, Cooke C, Long J. Physical activity, exercise and health of adults with mild and moderate learning disabilities. Br J Learn Disabil 1998;26:17-22. https://doi.org/10.1111/j.1468-3156.1998.tb00041.x.
- Frey G. Comparison of physical activity levels between adults with and without mental retardation. J Phys Activ Health 2004;1:235-45. https://doi.org/10.1123/jpah.1.3.235.
- Draheim CC, Williams DP, McCubbin JA. Prevalence of physical inactivity and recommended physical activity in community-based adults with mental retardation. Ment Retard 2002;40:436-44. http://dx.doi.org/10.1352/0047-6765(2002)040<0436:POPIAR>2.0.CO;2.
- Samele C, Seymour L, Morris B, Cohen A, Emerson E. Central England People First . A Formal Investigation into Health Inequalities Experienced by People With Learning Difficulties and People With Mental Health Problems 2006. www.leeds.ac.uk/disability-studies/archiveuk/central%20england/Area_Studies_Final_Report.pdf (accessed December 2015).
- Nøttestad JA, Linaker OM. Psychotropic drug use among people with intellectual disability before and after deinstitutionalization. J Intellect Disabil Res 2003;47:464-71. https://doi.org/10.1046/j.1365-2788.2003.00511.x.
- Linaker OM. Frequency of and determinants for psychotropic drug use in an institution for the mentally retarded. Br J Psychiatry 1990;156:525-30. https://doi.org/10.1192/bjp.156.4.525.
- Cooper SA, Smiley E, Morrison J, Allan L, Williamson A, Finlayson J, et al. Psychosis and adults with intellectual disabilities. Prevalence, incidence, and related factors. Soc Psychiatry Psychiatr Epidemiol 2007;42:530-6. http://dx.doi.org/10.1007/s00127-007-0197-9.
- Levitt Katz LE, Swami S, Abraham M, Murphy KM, Jawad AF, McKnight-Menci H, et al. Neuropsychiatric disorders at the presentation of type 2 diabetes mellitus in children. Pediatr Diabetes 2005;6:84-9. https://doi.org/10.1111/j.1399-543X.2005.00105.x.
- North American Association for the Study of Obesity . Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatr 2004;65:267-72. https://doi.org/10.4088/JCP.v65n0219.
- Henderson DC. Schizophrenia and comorbid metabolic disorders. J Clin Psychiatry 2005;66:11-20.
- Nagai T, Mori M. Prader–Willi syndrome, diabetes mellitus and hypogonadism. Biomed Pharmacother 1999;53:452-4. https://doi.org/10.1016/S0753-3322(00)88102-0.
- Stanish HI, Temple VA, Frey GC. Health-promoting physical activity of adults with mental retardation. Ment Retard Dev Disabil Res Rev 2006;12:13-21. https://doi.org/10.1002/mrdd.20090.
- Barnes TL, Howie EK, McDermott S, Mann JR. Physical activity in a large sample of adults with intellectual disabilities. J Phys Act Health 2013;10:1048-56. https://doi.org/10.1123/jpah.10.7.1048.
- Peterson JJ, Janz KF, Lowe JB. Physical activity among adults with intellectual disabilities living in community settings. Prev Med 2008;47:101-6. https://doi.org/10.1016/j.ypmed.2008.01.007.
- Robertson J, Emerson E, Gregory N, Hatto C, Turner S, Kessissoglou S, et al. Lifestyle related risk factors for poor health in residential settings for people with intellectual disabilities. Res Dev Disabil 2000;21:469-86. https://doi.org/10.1016/S0891-4222(00)00053-6.
- Joint Health and Social Care Self-Assessment Framework 2014. London: PHE; 2015.
- Draheim CC. Cardiovascular disease prevalence and risk factors of persons with mental retardation. Ment Retard Dev Disabil Res Rev 2006;12:3-12. http://dx.doi.org/10.1002/mrdd.20095.
- Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11170.
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5. http://dx.doi.org/10.1136/bmj.39545.585289.25.
- The Uptake of Learning Disability Health Checks: 2013 to 2014. London: PHE; 2014.
- Chatterton H, Younger T, Fischer A, Khunti K. Programme Development Group. Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4624.
- Gray LJ, Davies MJ, Hiles S, Taub NA, Webb DR, Srinivasan BT, et al. Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia 2012;55:959-66. https://doi.org/10.1007/s00125-011-2432-x.
- Gray LJ, Taub NA, Khunti K, Gardiner E, Hiles S, Webb DR, et al. The Leicester Risk Assessment score for detecting undiagnosed type 2 diabetes and impaired glucose regulation for use in a multiethnic UK setting. Diabet Med 2010;27:887-95. http://dx.doi.org/10.1111/j.1464-5491.2010.03037.x.
- Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev 2000;16:164-71. https://doi.org/10.1002/1520-7560(200005/06)16:3<164::AID-DMRR103>3.0.CO;2-R.
- Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b880.
- Gray LJ, Khunti K, Wilmot EG, Yates T, Davies MJ. External validation of two diabetes risk scores in a young UK South Asian population. Diabetes Res Clin Pract 2014;104:451-8. http://dx.doi.org/10.1016/j.diabres.2014.03.018.
- Glümer C, Vistisen D, Borch-Johnsen K, Colagiuri S. DETECT-2 Collaboration. Risk scores for type 2 diabetes can be applied in some populations but not all. Diabetes Care 2006;29:410-14. https://doi.org/10.2337/diacare.29.02.06.dc05-0945.
- Tyrer F, Smith LK, McGrother CW. Mortality in adults with moderate to profound intellectual disability: a population-based study. J Intellect Disabil Res 2007;51:520-7. https://doi.org/10.1111/j.1365-2788.2006.00918.x.
- Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. Mental ill-health in adults with intellectual disabilities: prevalence and associated factors. Br J Psychiatry 2007;190:27-35. https://doi.org/10.1192/bjp.bp.106.022483.
- Heslop P, Blair PS, Fleming P, Hoghton M, Marriott A, Russ L. The confidential inquiry into premature deaths of people with intellectual disabilities in the UK: a population-based study. Lancet 2014;383:889-95. http://dx.doi.org/10.1016/S0140-6736(13)62026-7.
- Havercamp SM, Scott HM. National health surveillance of adults with disabilities, adults with intellectual and developmental disabilities, and adults with no disabilities. Disabil Health J 2015;8:165-72. https://doi.org/10.1016/j.dhjo.2014.11.002.
- Recognising the Importance of Physical Health in Mental Health and Intellectual Disability: Achieving Parity of Outcomes. London: BMA; 2014.
- Ali A, Scior K, Ratti V, Strydom A, King M, Hassiotis A. Discrimination and other barriers to accessing health care: perspectives of patients with mild and moderate intellectual disability and their carers. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0070855.
- Alborz A, McNally R, Glendinning C. Access to health care for people with learning disabilities in the UK: mapping the issues and reviewing the evidence. J Health Serv Res Policy 2005;10:173-82. http://dx.doi.org/10.1258/1355819054338997.
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334. https://doi.org/10.1136/bmj.39063.689375.55.
- Telford RD. Low physical activity and obesity: causes of chronic disease or simply predictors?. Med Sci Sports Exerc 2007;39:1233-40. http://dx.doi.org/10.1249/mss.0b013e31806215b7.
- Identification, Assessment and Management of Overweight and Obesity in Children, Young Adults and Adults. London: NICE; 2014.
- Obesity and Disability. London: PHE; 2013.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- McVilly K, McGillivray J, Curtis A, Lehmann J, Morrish L, Speight J. Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabet Med 2014;31:897-904. http://dx.doi.org/10.1111/dme.12494.
- MacRae S, Brown M, Karatzias T, Taggart L, Truesdale-Kennedy M, Walley R, et al. Diabetes in people with intellectual disabilities: a systematic review of the literature. Res Dev Disabil 2015;47:352-74. http://dx.doi.org/10.1016/j.ridd.2015.10.003.
- Chalk TEW, Dunkley AJ, Gray L, Khunti K, Spong R. Systematic Review and Meta-Analysis: Rates of Type 2 Diabetes, Cardiovascular Disease, and Associated Risk Factors in Intellectual Disability Populations n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015019048 (accessed December 2015).
- Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. University of York: CRD; 2009.
- Light RL, Pillemer DB. Summing Up: The Science of Reviewing Research. Cambridge, MA: Harvard University Press; 1984.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. http://dx.doi.org/10.1002/sim.1186.
- de Winter CF, Magilsen KW, van Alfen JC, Penning C, Evenhuis HM. Prevalence of cardiovascular risk factors in older people with intellectual disability. Am J Intellect Dev Disabil 2009;114:427-36.
- Gazizova D, Puri BK, Singh I, Dhaliwal R. The overweight: obesity and plasma lipids in adults with intellectual disability and mental illness. J Intellect Disabil Res 2012;56:895-901. http://dx.doi.org/10.1111/j.1365-2788.2011.01468.x.
- Havercamp SM, Scandlin D, Roth M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public Health Rep 2004;119:418-26. https://doi.org/10.1016/j.phr.2004.05.006.
- Henderson CM, Robinson LM, Davidson P, Haveman M, Janicki MP, Albertini G. Overweight status, obesity and risk factors for coronary heart disease in adults with intellectual disability. J Pol Pract Intellect Disabil 2008;5:174-7. https://doi.org/10.1111/j.1741-1130.2008.00170.x.
- Jansen J, Rozeboom W, Penning C, Evenhuis HM. Prevalence and incidence of MI and cerebrovascular accident in ageing persons with intellectual disability. J Intellect Disabil Res 2013;57:681-5. http://dx.doi.org/10.1111/j.1365-2788.2012.01567.x.
- McDermott S, Moran R, Platt T, Dasari S. Variation in health conditions among groups of adults with disabilities in primary care. J Community Health 2006;31:147-59. https://doi.org/10.1007/s10900-005-9008-y.
- McDermott S, Moran R, Platt T, Dasari S. Prevalence of diabetes in persons with disabilities in primary care. J Dev Phys Disabil 2007;19:263-71. https://doi.org/10.1007/s10882-007-9058-4.
- Tyler C, Schramm S, Karafa M, Tang AS, Jain A. Electronic health record analysis of the primary care of adults with intellectual and other developmental disabilities. J Policy Pract Intellect Disabil 2010;7:204-10. http://dx.doi.org/10.1111/j.1741-1130.2010.00266.x.
- Ito J. Obesity and its related health problems in people with intellectual disabilities. J Pol Pract Intellect Disabil 2006;3:129-32. https://doi.org/10.1111/j.1741-1130.2006.00064.x.
- de Winter CF, Hermans H, Evenhuis HM, Echteld MA. Associations of symptoms of anxiety and depression with diabetes and cardiovascular risk factors in older people with intellectual disability. J Intellect Disabil Res 2015;59:176-85. http://dx.doi.org/10.1111/jir.12049.
- Van Schrojenstein Lantman-De Valk HM, Metsemakers JF, Haveman MJ, Crebolder HF. Health problems in people with intellectual disability in general practice: a comparative study. Fam Pract 2000;17:405-7. https://doi.org/10.1093/fampra/17.5.405.
- Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. An epidemiological investigation of affective disorders with a population-based cohort of 1023 adults with intellectual disabilities. Psychol Med 2007;37:873-82. https://doi.org/10.1017/S0033291707009968.
- Rurangirwa J, Van Naarden Braun K, Schendel D, Yeargin-Allsopp M. Healthy behaviors and lifestyles in young adults with a history of developmental disabilities. Res Dev Disabil 2006;27:381-99. https://doi.org/10.1016/j.ridd.2005.01.003.
- Vacek JL, Hunt SL, Shireman T. Hypertension medication use and adherence among adults with developmental disability. Disabil Health J 2013;6:297-302. http://dx.doi.org/10.1016/j.dhjo.2013.02.003.
- Shireman TI, Reichard A, Nazir N, Backes JM, Greiner KA. Quality of diabetes care for adults with developmental disabilities. Disabil Health J 2010;3:179-85. http://dx.doi.org/10.1016/j.dhjo.2009.10.004.
- Yamaki K. Body weight status among adults with intellectual disability in the community. Ment Retard 2005;43:1-10. http://dx.doi.org/10.1352/0047-6765(2005)43<1:BWSAAW>2.0.CO;2.
- Reichard A, Stolzle H. Diabetes among adults with cognitive limitations compared to individuals with no cognitive disabilities. Intellect Dev Disabil 2011;49:141-54. http://dx.doi.org/10.1352/1934-9556-49.2.141.
- Bégarie J, Maïano C, Leconte P, Ninot G. The prevalence and determinants of overweight and obesity among French youths and adults with intellectual disabilities attending special education schools. Res Dev Disabil 2013;34:1417-25. http://dx.doi.org/10.1016/j.ridd.2012.12.007.
- Bhaumik S, Watson JM, Thorp CF, Tyrer F, McGrother CW. Body mass index in adults with intellectual disability: distribution, associations and service implications: a population-based prevalence study. J Intellect Disabil Res 2008;52:287-98. http://dx.doi.org/10.1111/j.1365-2788.2007.01018.x.
- Chang YW, Lin JD, Chen WL, Yen CF, Loh CH, Fang WH, et al. Metabolic syndrome and short-term heart rate variability in adults with intellectual disabilities. Res Dev Disabil 2012;33:1701-7. http://dx.doi.org/10.1016/j.ridd.2012.04.005.
- Chen G, Tan BK, Sun X, Meng X, Jiwa M. A preliminary report on the medical profile of disabled persons living in Zhabei District, Shanghai, Mainland China. Qual Prim Care 2011;19:233-44.
- De Winter CF, Bastiaanse LP, Hilgenkamp TI, Evenhuis HM, Echteld MA. Cardiovascular risk factors (diabetes, hypertension, hypercholesterolemia and metabolic syndrome) in older people with intellectual disability: results of the HA-ID study. Res Dev Disabil 2012;33:1722-31. https://doi.org/10.1016/j.ridd.2012.04.010.
- De Winter CF, Bastiaanse LP, Hilgenkamp TI, Evenhuis HM, Echteld MA. Overweight and obesity in older people with intellectual disability. Res Dev Disabil 2012;33:398-405. http://dx.doi.org/10.1016/j.ridd.2011.09.022.
- De Winter CF, Bastiaanse LP, Kranendonk SE, Hilgenkamp TI, Evenhuis HM, Echteld MA. Peripheral arterial disease in older people with intellectual disability in the Netherlands using the ankle-brachial index: results of the HA-ID study. Res Dev Disabil 2013;34:1663-8. http://dx.doi.org/10.1016/j.ridd.2013.02.007.
- Emerson E. Underweight, obesity and exercise among adults with intellectual disabilities in supported accommodation in Northern England. J Intellect Disabil Res 2005;49:134-43. https://doi.org/10.1111/j.1365-2788.2004.00617.x.
- Frighi V, Stephenson MT, Morovat A, Jolley IE, Trivella M, Dudley CA, et al. Safety of antipsychotics in people with intellectual disability. Br J Psychiatry 2011;199:289-95. http://dx.doi.org/10.1192/bjp.bp.110.085670.
- Gale L, Naqvi H, Russ L. Asthma, smoking and BMI in adults with intellectual disabilities: a community-based survey. J Intellect Disabil Res 2009;53:787-96. http://dx.doi.org/10.1111/j.1365-2788.2009.01192.x.
- Haider SI, Ansari Z, Vaughan L, Matters H, Emerson E. Health and wellbeing of Victorian adults with intellectual disability compared to the general Victorian population. Res Dev Disabil 2013;34:4034-42. http://dx.doi.org/10.1016/j.ridd.2013.08.017.
- Haveman M, Perry J, Salvador-Carulla L, Walsh PN, Kerr M, Van Schrojenstein Lantman-de Valk H, et al. Ageing and health status in adults with intellectual disabilities: results of the European POMONA II study. J Intellect Dev Disabil 2011;36:49-60. http://dx.doi.org/10.3109/13668250.2010.549464.
- Henderson CM, Rosasco M, Robinson LM, Meccarello J, Janicki MP, Turk MA, et al. Functional impairment severity is associated with health status among older persons with intellectual disability and cerebral palsy. J Intellect Disabil Res 2009;53:887-97. http://dx.doi.org/10.1111/j.1365-2788.2009.01199.x.
- Hove O. Weight survey on adult persons with mental retardation living in the community. Res Dev Disabil 2004;25:9-17. https://doi.org/10.1016/j.ridd.2003.04.004.
- Hsieh K, Rimmer JH, Heller T. Obesity and associated factors in adults with intellectual disability. J Intellect Disabil Res 2014;58:851-63. http://dx.doi.org/10.1111/jir.12100.
- Hsu SW, Yen CF, Hung WJ, Lin LP, Wu CL, Lin JD. The risk of metabolic syndrome among institutionalized adults with intellectual disabilities. Res Dev Disabil 2012;33:615-20. http://dx.doi.org/10.1016/j.ridd.2011.09.005.
- Janicki MP, Davidson PW, Henderson CM, McCallion P, Taets JD, Force LT, et al. Health characteristics and health services utilization in older adults with intellectual disability living in community residences. J Intellect Disabil Res 2002;46:287-98. https://doi.org/10.1046/j.1365-2788.2002.00385.x.
- Lee L, Rianto J, Raykar V, Creasey H, Waite L, Berry A, et al. Health and functional status of adults with intellectual disability referred to the specialist health care setting: a five-year experience. Int J Family Med 2011. http://dx.doi.org/10.1155/2011/312492.
- Lennox N, Rey-Conde T, Cooling N. Comprehensive health assessments during de-institutionalization: an observational study. J Intellect Disabil Res 2006;50:719-24. https://doi.org/10.1111/j.1365-2788.2006.00835.x.
- Levy JM, Botuck S, Damiani MR, Levy PH, Dern TA, Freeman SE. Medical conditions and healthcare utilization among adults with intellectual disabilities living in group homes in New York City. J Pol Pract Intellect Disabil 2006;3:195-202. https://doi.org/10.1111/j.1741-1130.2006.00079.x.
- Levy JM, Botuck S, Rimmerman A. Examining outpatient health care utilization among adults with severe or profound intellectual disabilities living in an urban setting: a brief snap shot. J Soc Work Disabil Rehabil 2007;6:33-45. https://doi.org/10.1300/J198v06n03_02.
- Lewis MA, Lewis CE, Leake B, King BH, Lindemann R. The quality of health care for adults with developmental disabilities. Public Health Rep 2002;117:174-84. https://doi.org/10.1016/S0033-3549(04)50124-3.
- Lin JD, Wu TY, Lin LP, Hsu SW, Liu CT, Wu CL. An exploratory study of health behaviors and the risks for triple H (hypertension, hyperlipidemia, and hyperglycemia) in young adults with disabilities between 20 and 39 years of age. Res Dev Disabil 2013;34:3211-17. http://dx.doi.org/10.1016/j.ridd.2013.06.044.
- Lin LP, Hsu SW, Yao CH, Lai WJ, Hsu PJ, Wu JL, et al. Risk for osteopenia and osteoporosis in institution-dwelling individuals with intellectual and/or developmental disabilities. Res Dev Disabil 2015;36C:108-13. https://doi.org/10.1016/j.ridd.2014.09.022.
- Lin LP, Liu CT, Liou SW, Hsu SW, Lin JD. High blood pressure in adults with disabilities: influence of gender, body weight and health behaviors. Res Dev Disabil 2012;33:1508-15. http://dx.doi.org/10.1016/j.ridd.2012.03.027.
- Maaskant MA, van Knijff-Raeven AGM, van Schrojenstein Lantman-de Valk HMJ, Veenstra MY. Weight status of persons with intellectual disabilities. J Applied Res Intellect Disabil 2009;22:426-32.
- Marshall D, McConkey R, Moore G. Obesity in people with intellectual disabilities: the impact of nurse-led health screenings and health promotion activities. J Adv Nurs 2003;41:147-53. https://doi.org/10.1046/j.1365-2648.2003.02522.x.
- Martínez-Leal R, Salvador-Carulla L, Linehan C, Walsh P, Weber G, Van Hove G, et al. The impact of living arrangements and deinstitutionalisation in the health status of persons with intellectual disability in Europe. J Intellect Disabil Res 2011;55:858-72. http://dx.doi.org/10.1111/j.1365-2788.2011.01439.x.
- McCarron M, Swinburne J, Burke E, McGlinchey E, Carroll R, McCallion P. Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS-TILDA). Res Dev Disabil 2013;34:521-7. https://doi.org/10.1016/j.ridd.2012.07.029.
- McGuire BE, Daly P, Smyth F. Lifestyle and health behaviours of adults with an intellectual disability. J Intellect Disabil Res 2007;51:497-510. https://doi.org/10.1111/j.1365-2788.2006.00915.x.
- Melville C, Cooper S-A, Morrison J, Allan L, Smiley E, Williamson A. The prevalence and determinants of obesity in adults with intellectual disabilities. J Applied Res Intellect Disabil 2008;21:425-37. https://doi.org/10.1111/j.1468-3148.2007.00412.x.
- Merrick J, Davidson PW, Morad M, Janicki MP, Wexler O, Henderson CM. Older adults with intellectual disability in residential care centers in Israel: health status and service utilization. Am J Ment Retard 2004;109:413-20. http://dx.doi.org/10.1352/0895-8017(2004)109<413:OAWIDI>2.0.CO;2.
- Mikulovic J, Vanhelst J, Salleron J, Marcellini A, Compte R, Fardy PS, et al. Overweight in intellectually-disabled population: physical, behavioral and psychological characteristics. Res Dev Disabil 2014;35:153-61. http://dx.doi.org/10.1016/j.ridd.2013.10.012.
- Molteno C, Smit I, Mills J, Huskisson J. Nutritional status of patients in a long-stay hospital for people with mental handicap. S Afr Med J 2000;90:1135-40.
- Moore K, McGillivray J, Illingworth K, Brookhouse P. An investigation into the incidence of obesity and underweight among adults with an intellectual disability in an Australian sample. J Intellect Dev Disabil 2004;29:306-18. https://doi.org/10.1080/13668250400014483.
- Morin D, Mérineau-Côté J, Ouellette-Kuntz H, Tassé MJ, Kerr M. A comparison of the prevalence of chronic disease among people with and without intellectual disability. Am J Intellect Dev Disabil 2012;117:455-63. http://dx.doi.org/10.1352/1944-7558-117.6.455.
- Moss SJ. Changes in coronary heart disease risk profile of adults with intellectual disabilities following a physical activity intervention. J Intellect Disabil Res 2009;53:735-44. http://dx.doi.org/10.1111/j.1365-2788.2009.01187.x.
- Shah A, Bruce M, Willson C, Malik M, Gaffney K. The care of people with diabetes in care homes within a primary care trust. J Diabet Nurs 2006;10:289-96.
- Sohler N, Lubetkin E, Levy J, Soghomonian C, Rimmerman A. Factors associated with obesity and coronary heart disease in people with intellectual disabilities. Soc Work Health Care 2009;48:76-89. http://dx.doi.org/10.1080/00981380802451160.
- Stancliffe RJ, Lakin KC, Larson S, Engler J, Bershadsky J, Taub S, et al. Overweight and obesity among adults with intellectual disabilities who use intellectual disability/developmental disability services in 20 U.S. States. Am J Intellect Dev Disabil 2011;116:401-18. http://dx.doi.org/10.1352/1944-7558-116.6.401.
- Stedman KV, Leland LS. Obesity and intellectual disability in New Zealand. J Intellect Dev Disabil 2010;35:112-15. http://dx.doi.org/10.3109/13668251003717928.
- Van de Louw J, Vorstenbosch R, Vinck L, Penning C, Evenhuis H. Prevalence of hypertension in adults with intellectual disability in the Netherlands. J Intellect Disabil Res 2009;53:78-84. http://dx.doi.org/10.1111/j.1365-2788.2008.01130.x.
- Van Den Akker M, Maaskant MA, van der Meijden RJ. Cardiac diseases in people with intellectual disability. J Intellect Disabil Res 2006;50:515-22. https://doi.org/10.1111/j.1365-2788.2006.00797.x.
- Wallace RA, Schluter P. Audit of cardiovascular disease risk factors among supported adults with intellectual disability attending an ageing clinic. J Intellect Dev Disabil 2008;33:48-5. http://dx.doi.org/10.1080/13668250701858463.
- Wang KY, Hsieh K, Heller T, Davidson PW, Janicki MP. Carer reports of health status among adults with intellectual/developmental disabilities in Taiwan living at home and in institutions. J Intellect Disabil Res 2007;51:173-83. https://doi.org/10.1111/j.1365-2788.2006.00819.x.
- Wong CW. Adults with intellectual disabilities living in Hong Kong’s residential care facilities: a descriptive analysis of health and disease patterns by sex, age and presence of Down syndrome. J Pol Pract Intellect Disabil 2011;8:231-8. https://doi.org/10.1111/j.1741-1130.2011.00318.x.
- Yen CF, Lin JD, Li CW, Wu JL, Lee JT. Body mass index for adults with intellectual disabilities: a survey of caregivers in Taiwan. J Med Sci 2005;25:131-8.
- Zaal-Schuller IH, Goorhuis AE, Bock-Sinot A, Claassen IH, Echteld MA, Evenhuis HM. The prevalence of peripheral arterial disease in middle-aged people with intellectual disabilities. Res Dev Disabil 2015;36C:526-31.
- Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. Lancet 2011;377:1438-47. http://dx.doi.org/10.1016/S0140-6736(11)60393-0.
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804-14. http://dx.doi.org/10.1016/S0140-6736(11)60813-1.
- Mortality and Burden of Disease Estimates for WHO Member States in 2004. Geneva: WHO; 2009.
- Caro JJ, Ward AJ, O’Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 2002;25:476-81. https://doi.org/10.2337/diacare.25.3.476.
- Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472-7. https://doi.org/10.1001/jama.1997.03540300040031.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-53. https://doi.org/10.2337/diacare.27.5.1047.
- Robertson J, Hatton C, Baines S, Emerson E. Systematic reviews of the health or health care of people with intellectual disabilities: a systematic review to identify gaps in the evidence base. J Appl Res Intellect Disabil 2015;28:455-523. https://doi.org/10.1111/jar.12149.
- Jinks A, Cotton A, Rylance R. Obesity interventions for people with a learning disability: an integrative literature review. J Adv Nurs 2011;67:460-71. http://dx.doi.org/10.1111/j.1365-2648.2010.05508.x.
- Spanos D, Melville CA, Hankey CR. Weight management interventions in adults with intellectual disabilities and obesity: a systematic review of the evidence. Nutr J 2013;12. http://dx.doi.org/10.1186/1475-2891-12-132.
- Brooker K, van Dooren K, McPherson L, Lennox N, Ware R. A systematic review of interventions aiming to improve involvement in physical activity among adults with intellectual disability. J Phys Act Health 2015;12:434-44. http://dx.doi.org/10.1123/jpah.2013-0014.
- Chalk TEW, Dunkley AJ, Gray L, Khunti K, Spong R. Evidence for Primary Prevention of Diabetes and Cardiovascular Disease in People With Intellectual Disabilities: A Systematic Review of the Effectiveness of Lifestyle Interventions Aimed at Reducing Modifiable Risk Factors n.d. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015019048 (accessed December 2015).
- Behaviour Change: Individual Approaches. London: NICE; 2014.
- Methods for the Development of NICE Public Health Guidance. London: NICE; 2012.
- Beeken RJ, Spanos D, Fovargue S, Hunter R, Omar R, Hassiotis A, et al. Piloting a manualised weight management programme (Shape Up-LD) for overweight and obese persons with mild-moderate learning disabilities: study protocol for a pilot randomised controlled trial. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-71.
- Van Schijndel-Speet M, Evenhuis HM, van Empelen P, van Wijck R, Echteld MA. Development and evaluation of a structured programme for promoting physical activity among seniors with intellectual disabilities: a study protocol for a cluster randomized trial. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-746.
- Pérez-Cruzado D, Cuesta-Vargas AI. Improving adherence physical activity with a smartphone application based on adults with intellectual disabilities (APPCOID). BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-1173.
- Harris L, Melville C, Jones N, Pert C, Boyle S, Murray H, et al. A single-blind, pilot randomised trial of a weight management intervention for adults with intellectual disabilities and obesity: study protocol. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/2055-5784-1-5.
- Bazzano AT, Zeldin AS, Diab IR, Garro NM, Allevato NA, Lehrer D. WRC Project Oversight Team. The Healthy Lifestyle Change Program: a pilot of a community-based health promotion intervention for adults with developmental disabilities. Am J Prev Med 2009;37:201-8. http://dx.doi.org/10.1016/j.amepre.2009.08.005.
- Melville CA, Boyle S, Miller S, Macmillan S, Penpraze V, Pert C, et al. An open study of the effectiveness of a multi-component weight-loss intervention for adults with intellectual disabilities and obesity. Br J Nutr 2011;105:1553-62. http://dx.doi.org/10.1017/S0007114510005362.
- McDermott S, Whitner W, Thomas-Koger M, Mann JR, Clarkson J, Barnes TL, et al. An efficacy trial of ‘Steps to Your Health’, a health promotion programme for adults with intellectual disability. Health Educ J 2012;71:278-90. http://dx.doi.org/10.1177/0017896912441240.
- Bergström H, Hagströmer M, Hagberg J, Elinder LS. A multi-component universal intervention to improve diet and physical activity among adults with intellectual disabilities in community residences: a cluster randomised controlled trial. Res Dev Disabil 2013;34:3847-57. http://dx.doi.org/10.1016/j.ridd.2013.07.019.
- Bandura A. Self-efficacy: The Exercise of Control. New York, NY: WH Freeman; 1997.
- Bland JM, Altman DG. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-264.
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med 2007;147:41-50. https://doi.org/10.7326/0003-4819-147-1-200707030-00007.
- Research Governance Framework for Health and Social Care. London: DH; 2005.
- Equity and Excellence. Liberating the NHS. London: The Stationery Office; 2010.
- Ennis L, Wykes T. Impact of patient involvement in mental health research: longitudinal study. Br J Psychiatry 2013;203:381-6. http://dx.doi.org/10.1192/bjp.bp.112.119818.
- Holmes W, Stewart P, Garrow A, Anderson I, Thorpe L. Researching Aboriginal health: experience from a study of urban young people’s health and well-being. Soc Sci Med 2002;54:1267-79. https://doi.org/10.1016/S0277-9536(01)00095-8.
- Mosavel M, Simon C, van Stade D, Buchbinder M. Community-based participatory research (CBPR) in South Africa: engaging multiple constituents to shape the research question. Soc Sci Med 2005;61:2577-87. https://doi.org/10.1016/j.socscimed.2005.04.041.
- Schneider B, Scissons H, Arney L, Benson G, Derry J, Lucas K, et al. Communication between people with schizophrenia and their medical professionals: a participatory research project. Qual Health Res 2004;14:562-77. http://dx.doi.org/10.1177/1049732303262423.
- Beresford P. Developing the theoretical basis for service user/survivor-led research and equal involvement in research. Epidemiol Psichiatr Soc 2005;14:4-9. https://doi.org/10.1017/S1121189X0000186X.
- Chalmers I. What do I want from health research and researchers when I am a patient?. BMJ 1995;310:1315-18. https://doi.org/10.1136/bmj.310.6990.1315.
- Entwistle VA, Renfrew MJ, Yearley S, Forrester J, Lamont T. Lay perspectives: advantages for health research. BMJ 1998;316:463-6. https://doi.org/10.1136/bmj.316.7129.463.
- Goodare H, Smith R. The rights of patients in research. BMJ 1995;310:1277-8. https://doi.org/10.1136/bmj.310.6990.1277.
- Oliver M. Changing the social relations of research production?. Disabil Handicap Soc 1992;7:101-14. https://doi.org/10.1080/02674649266780141.
- Smith E, Ross F, Donovan S, Manthorpe J, Brearley S, Sitzia J, et al. Service user involvement in nursing, midwifery and health visiting research: a review of evidence and practice. Int J Nurs Stud 2008;45:298-315. https://doi.org/10.1016/j.ijnurstu.2006.09.010.
- Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy 2002;61:213-36. https://doi.org/10.1016/S0168-8510(01)00214-7.
- Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient 2014;7:387-95. http://dx.doi.org/10.1007/s40271-014-0065-0.
- Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.CD004563.pub2.
- Staley K. Exploring Impact: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2009.
- Thompson J, Barber R, Ward PR, Boote JD, Cooper CL, Armitage CJ, et al. Health researchers’ attitudes towards public involvement in health research. Health Expect 2009;12:209-20. http://dx.doi.org/10.1111/j.1369-7625.2009.00532.x.
- Martin GP. ‘Ordinary people only’: knowledge, representativeness, and the publics of public participation in healthcare. Sociol Health Illn 2008;30:35-54. http://dx.doi.org/10.1111/j.1467-9566.2007.01027.x.
- Thompson J, Bissell P, Cooper CL, Armitage CJ, Barber R. Exploring the impact of patient and public involvement in a cancer research setting. Qual Health Res 2014;24:46-54. http://dx.doi.org/10.1177/1049732313514482.
- Boote J, Baird W, Beecroft C. Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy 2010;95:10-23. http://dx.doi.org/10.1016/j.healthpol.2009.11.007.
- Stewart D, Wilson R, Selby P, Darbyshire J. Patient and public involvement. Ann Oncol 2011;22:vii54-6. http://dx.doi.org/10.1093/annonc/mdr427.
- Booth T, Booth W. Sounds of silence: narrative research with inarticulate subjects. Disabil Soc 2003;11:55-69. https://doi.org/10.1080/09687599650023326.
- Garbutt R, Tattersall J, Dunn J, Boycott-Garnett R. Accessible article: involving people with learning disabilities in research. Br J Learn Disabil 2010;38:21-34. https://doi.org/10.1111/j.1468-3156.2009.00556.x.
- Palmer R, Paterson G. To what extent can people with communication difficulties contribute to health research?. Nurse Res 2013;20:12-6. http://dx.doi.org/10.7748/nr2013.01.20.3.12.c9491.
- Rapley M. Quality of Life Research: A Critical Introduction. London: Sage; 2003.
- Stalker K. Some ethical and methodological issues in research with people with learning difficulties. Disabil Soc 1998;13:5-19. https://doi.org/10.1080/09687599826885.
- Atkinson D. An Auto/biographical Approach to Learning Disability Research. Aldershot: Ashgate; 1997.
- Barber R, Boote JD, Cooper CL. Involving consumers successfully in NHS research: a national survey. Health Expect 2007;10:380-91. https://doi.org/10.1111/j.1369-7625.2007.00457.x.
- Dunkley A, Clarkson M, Tyrer F. STOP Diabetes Study 2013. www.invo.org.uk/wp-content/uploads/2013/07/INVOLVEsummer2013newsletter.pdf (accessed December 2015).
- Buss C. Volunteers needed for diabetes study. Leicester Mercury 2015. www.leicestermercury.co.uk/Volunteers-needed-diabetes-study/story-26495896-detail/story.html (accessed December 2015).
- Moss S. PAS-ADD Schedules. Brighton: Pavilion Publishers; 2002.
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375-81. https://doi.org/10.1093/fampra/11.4.375.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sport Exercise 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Tyrer F, Dunkley AJ, Spong R, Gangadharan SK, Bhaumik S, Khunti K. Involving service users with intellectual disability in research: experiences from the STOP Diabetes Study [published online ahead of print September 18 2016]. J Pol Pract Intellect Disabil 2016.
- Mockford C, Staniszewska S, Griffiths F, Herron-Marx S. The impact of patient and public involvement on UK NHS health care: a systematic review. Int J Qual Health Care 2012;24:28-3. http://dx.doi.org/10.1093/intqhc/mzr066.
- Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect 2014;17:637-50. http://dx.doi.org/10.1111/j.1369-7625.2012.00795.x.
- Rodgers J. Trying to get it right: undertaking research involving people with learning disabilities. Disabil Soc 1999;14:421-33. https://doi.org/10.1080/09687599926046.
- Farmer M, Macleod F. Involving Disabled People in Social Research. London: Office for Disability Issues; 2011.
- Apsis S. Self-advocacy for people with learning disabilities: does it have a future?. Disabil Soc 1997;12:647-54. https://doi.org/10.1080/09687599727182.
- Briefing Notes for Researchers: Involving the Public in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2012.
- Mental Capacity Act. London: Her Majesty’s Stationery Office; 2005.
- Smith S, Branford D, Collacott RA, Cooper SA, McGrother C. Prevalence and cluster typology of maladaptive behaviors in a geographically defined population of adults with learning disabilities. Br J Psychiatry 1996;169:219-27. https://doi.org/10.1192/bjp.169.2.219.
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47. https://doi.org/10.1161/01.CIR.97.18.1837.
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.699579.
- The Euroqol Group . Euroqol: a new facility for the measurement of health-related quality-of-life. Health Pol 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic 1985;89:485-91.
- Aman MG, Singh NN, Turbott SH. Reliability of the Aberrant Behavior Checklist and the effect of variations in instructions. Am J Ment Defic 1987;92:237-40.
- Taylor JL, Hatton C, Dixon L, Douglas C. Screening for psychiatric symptoms: PAS-ADD Checklist norms for adults with intellectual disabilities. J Intellect Disabil Res 2004;48:37-41. https://doi.org/10.1111/j.1365-2788.2004.00585.x.
- Cuthill FM, Espie CA, Cooper SA. Development and psychometric properties of the Glasgow Depression Scale for people with a learning disability. Individual and carer supplement versions. Br J Psychiatry 2003;182:347-53. https://doi.org/10.1192/bjp.182.4.347.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
- The English Indices of Deprivation 2015. London: DCLG; 2015.
- Brindle P, May M, Gill P, Cappuccio F, D’Agostino R, Fischbacher C, et al. Primary prevention of cardiovascular disease: a web-based risk score for seven British black and minority ethnic groups. Heart 2006;92:1595-602. https://doi.org/10.1136/hrt.2006.092346.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) . Final Report. Circulation 2002;106.
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. https://doi.org/10.1016/0895-4356(95)00048-8.
- Feinstein AR. Multivariable Analysis: An Introduction. New Haven, CT: Yale University Press; 1996.
- Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol 2005;58:475-83. https://doi.org/10.1016/j.jclinepi.2004.06.017.
- Public Health England . Learning Disability Health Checks Report Spreadsheet 2014. www.improvinghealthandlives.org.uk/publications/1239/The_Uptake_of_Learning_Disabilities_Health_Checks,_2013_to_2014 (accessed December 2015).
- Gray LJ, Khunti K, Edwardson C, Goldby S, Henson J, Morris DH, et al. Implementation of the automated Leicester Practice Risk Score in two diabetes prevention trials provides a high yield of people with abnormal glucose tolerance. Diabetologia 2012;55:3238-44. http://dx.doi.org/10.1007/s00125-012-2725-8.
- Dalton AR, Bottle A, Okoro C, Majeed A, Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: cross-sectional study. J Public Health 2011;33:422-9. http://dx.doi.org/10.1093/pubmed/fdr034.
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777-81. https://doi.org/10.1097/00005768-199805000-00021.
- Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA Accelerometer. Med Sci Sports Exerc 2011;43:1085-93. http://dx.doi.org/10.1249/MSS.0b013e31820513be.
- Hildebrand M, van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816-24. http://dx.doi.org/10.1249/MSS.0000000000000289.
- Da Silva IC, van Hees VT, Ramires VV, Knuth AG, Bielemann RM, Ekelund U, et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol 2014;43:1959-68. http://dx.doi.org/10.1093/ije/dyu203.
- Bodde AE, Seo DC, Frey GC, Van Puymbroeck M, Lohrmann DK. Correlates of moderate-to-vigorous physical activity participation in adults with intellectual disabilities. Health Promot Pract 2013;14:663-70. http://dx.doi.org/10.1177/1524839912462395.
- Yates T, Henson J, Edwardson C, Bodicoat DH, Davies MJ, Khunti K. Differences in levels of physical activity between White and South Asian populations within a healthcare setting: impact of measurement type in a cross-sectional study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-006181.
- Bell JA, Hamer M, van Hees VT, Singh-Manoux A, Kivimäki M, Sabia S. Healthy obesity and objective physical activity. Am J Clin Nutr 2015;102:268-75. http://dx.doi.org/10.3945/ajcn.115.110924.
- Phillips AC, Holland AJ. Assessment of objectively measured physical activity levels in individuals with intellectual disabilities with and without Down’s syndrome. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0028618.
- Messent PR, Cooke CB, Long J. Daily physical activity in adults with mild and moderate learning disabilities: is there enough?. Disabil Rehabil 1998;20:424-7. https://doi.org/10.3109/09638289809166104.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Bartholomew LK, Parcel GS, Kok G, Gottlieb NH, Fernandez ME. Planning Health Promotion Programs: An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass; 2011.
- Troughton J, Chatterjee S, Hill SE, Daly H, Martin Stacey L, Stone MA, et al. Development of a lifestyle intervention using the MRC framework for diabetes prevention in people with impaired glucose regulation. J Public Health 2016;38:493-501. https://doi.org/10.1093/pubmed/fdv110.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Rationale, design and baseline data from the Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: a randomized controlled trial. Patient Educ Couns 2008;73:264-71. http://dx.doi.org/10.1016/j.pec.2008.06.010.
- Yates T, Davies M, Gorely T, Bull F, Khunti K. Effectiveness of a pragmatic education program designed to promote walking activity in individuals with impaired glucose tolerance: a randomized controlled trial. Diabetes Care 2009;32:1404-10. http://dx.doi.org/10.2337/dc09-0130.
- Stone M, Patel N, Daly H. Using qualitative research methods to inform the development of a modified version of a patient education module for non-English speakers with type 2 diabetes: experiences from an action research project in two south Asian populations in the UK. Divers Health Social Care 2008;5:119-206.
- Dunkley AJ, Davies MJ, Stone MA, Taub NA, Troughton J, Yates T, et al. The Reversal Intervention for Metabolic Syndrome (TRIMS) study: rationale, design, and baseline data. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-107.
- Ajzen I. The theory of planned behaviour. Organ Behav Human Dec Proc 1991;50:179-211. https://doi.org/10.1016/0749-5978(91)90020-T.
- Fishbein M, Ajzen I. Belief, Attitude, Intention, and Behavior. Reading, MA: Addison-Wesley; 1975.
- Ajzen I, Fishbein M. Understanding Attitudes and Predicting Social Behavior. Englewood Cliffs, NJ: Prentice-Hall; 1980.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. Ripple Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Nigg CR, Allegrante JP, Ory M. Theory-comparison and multiple-behavior research: common themes advancing health behavior research. Health Educ Res 2002;17:670-9. https://doi.org/10.1093/her/17.5.670.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5. http://dx.doi.org/10.1136/bmj.39474.922025.BE.
- Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice-Hall; 1977.
- Leventhal H, Meyer D, Nerenz D, Rachman S. . Contributions to Medical Psychology. Oxford: Pergamon Press; 1980.
- Eagly AH, Chaiken S. Process Theories of Attitude Formation and Change: The Elaboration Likelihood and Heuristic-systematic Models. The Psychology of Attitudes. Orlando, FL: Harcourt Brace; 1993.
- Structured Patient Education in Diabetes: Report from the Patient Education Working Group. London: DH; 2005.
- Pill R, Rees ME, Stott NC, Rollnick SR. Can nurses learn to let go? Issues arising from an intervention designed to improve patients’ involvement in their own care. J Adv Nurs 1999;29:1492-9. https://doi.org/10.1046/j.1365-2648.1999.01037.x.
- Cradock S, Stribling B, Dallosso H, Daly H, Carey M, Cullen M, et al. The need for assessing reliability of quality development (QD) tools in structured self management education (SSME) programmes in diabetes. Diabet Med 2010;27.
- Skinner TC, Carey ME, Cradock S, Dallosso HM, Daly H, Davies MJ, et al. ‘Educator talk’ and patient change: some insights from the DESMOND (Diabetes Education and Self Management for Ongoing and Newly Diagnosed) randomized controlled trial. Diabet Med 2008;25:1117-20. http://dx.doi.org/10.1111/j.1464-5491.2008.02492.x.
- Flanders N. Analysing Teacher Behaviour. Reading, MA: Addison-Wesley; 1970.
- Hoghton M. A Step by Step Guide for GP Practices: Annual Health Checks for People with a Learning Disability. London: RCGP; 2010.
- The Uptake of Learning Disabilities Health Checks, 2013 to 2014. Durham: Learning Disabilities Observatory; 2015.
- Health Survey for England. London: Health and Social Care Information Centre; 2012.
- Breeze P, Thomas C, Squires H, Brennan A, Greaves CJ, Diggle PJ, et al. School for Public Health Research (SPHR) Diabetes Prevention Model: Detailed Description of Model Background, Methods, Assumptions and Parameters 2015. www.shef.ac.uk/polopoly_fs/1.474948!/file/1501.pdf (accessed October 2015).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Gillett M, Brennan A, Watson P, Khunti K, Davies M, Mostafa S, et al. The cost-effectiveness of testing strategies for type 2 diabetes: a modelling study. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19330.
- Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet 2014;383:1059-66. http://dx.doi.org/10.1016/S0140-6736(13)62061-9.
- Stuckey M, Russell-Minda E, Read E, Munoz C, Shoemaker K, Kleinstiver P, et al. Diabetes and Technology for Increased Activity (DaTA) study: results of a remote monitoring intervention for prevention of metabolic syndrome. J Diabetes Sci Technol 2011;5:928-35. https://doi.org/10.1177/193229681100500416.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. https://doi.org/10.1001/jama.298.19.2296.
- Murphy MH, Nevill AM, Murtagh EM, Holder RL. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomised, controlled trials. Prev Med 2007;44:377-85. https://doi.org/10.1016/j.ypmed.2006.12.008.
- Qiu S, Cai X, Chen X, Yang B, Sun Z. Step counter use in type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Med 2014;12. http://dx.doi.org/10.1186/1741-7015-12-36.
- Camhi SM, Sisson SB, Johnson WD, Katzmarzyk PT, Tudor-Locke C. Accelerometer-determined moderate intensity lifestyle activity and cardiometabolic health. Prev Med 2011;52:358-60. http://dx.doi.org/10.1016/j.ypmed.2011.01.030.
- Squires H. A Methodological Framework for Developing the Structure of Public Health Economic Models. Sheffield: White Rose E-theses Online, University of Sheffield; 2014.
- Watson P, Preston L, Squires H, Chilcott J, Brennan A. Modelling the economics of type 2 diabetes mellitus prevention: a literature review of methods. Appl Health Econ Health Policy 2014;12:239-53. http://dx.doi.org/10.1007/s40258-014-0091-z.
- Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 2013;56:1925-33. http://dx.doi.org/10.1007/s00125-013-2940-y.
- Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59. http://dx.doi.org/10.1007/s00125-004-1527-z.
- Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251-6. https://doi.org/10.1093/ije/dyh372.
- Breeze P, Squires H, Chilcott J, Stride C, Diggle PJ, Brunner E, et al. A statistical model to describe longitudinal and correlated metabolic risk factors: the Whitehall II prospective study. J Public Health (Oxf) 2015;38:679-87. https://doi.org/10.1093/pubmed/fdv160.
- Tyrer F, McGrother C. Cause-specific mortality and death certificate reporting in adults with moderate to profound intellectual disability. J Intellect Disabil Res 2009;53:898-904. http://dx.doi.org/10.1111/j.1365-2788.2009.01201.x.
- Lauer E, Heslop P, Hoghton M. Identifying and addressing disparities in mortality: US and UK perspectives. Int Rev Res Dev Disabil 2015;48:195-24.
- ONS . Mortality Statistics: Deaths Registered in England and Wales (Series DR), 2011 2013. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=t cm%3A77-277727 (accessed December 2015).
- Florio T, Trollor J. Mortality among a cohort of persons with an intellectual disability in New South Wales, Australia. J Appl Res Intellect Disabil 2015;28:383-93. http://dx.doi.org/10.1111/jar.12190.
- Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829-41. http://dx.doi.org/10.1056/NEJMoa1008862.
- QRISK 2-2015 Cardiovascular Disease Risk Calculator. Leeds: ClinRisk; 2015.
- Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 2001;322:15-8. https://doi.org/10.1136/bmj.322.7277.15.
- Warn J, Guy J. Audit of Patients with a Learning Disability: Executive Summary 2011–2012. London: Royal College of Psychiatrists’ Centre for Quality Improvement; 2012.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11140.
- Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med 1999;159:1197-204. https://doi.org/10.1001/archinte.159.11.1197.
- Johansen NB, Vistisen D, Brunner EJ, Tabák AG, Shipley MJ, Wilkinson IB, et al. Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0037165.
- Kaffashian S, Dugravot A, Brunner EJ, Sabia S, Ankri J, Kivimäki M, et al. Midlife stroke risk and cognitive decline: a 10-year follow-up of the Whitehall II cohort study. Alzheimers Dement 2013;9:572-9. http://dx.doi.org/10.1016/j.jalz.2012.07.001.
- Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001;358:439-44. https://doi.org/10.1016/S0140-6736(01)05620-3.
- Dadvand P, Rankin J, Shirley MD, Rushton S, Pless-Mulloli T. Descriptive epidemiology of congenital heart disease in Northern England. Paediatr Perinat Epidemiol 2009;23:58-65. http://dx.doi.org/10.1111/j.1365-3016.2008.00987.x.
- Emerson E, Baines S. Health Inequalities and People with Learning Disabilities in the UK: 2010. Durham: Improving Health & Lives, Learning Disabilities Observatory; 2010.
- Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC). Int J Cancer 2004;111:762-71. https://doi.org/10.1002/ijc.20315.
- Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2006;98:920-31. https://doi.org/10.1093/jnci/djj246.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. http://dx.doi.org/10.1016/S0140-6736(08)60269-X.
- ONS . Cancer Survival in England: Patients Diagnosed 2006–2010 and Followed up to 2011 2012. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=t cm%3A77-277733 (accessed December 2015).
- Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403-9. http://dx.doi.org/10.2337/dc12-0924.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQoL: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Gillett M, Chilcott J, Goyder E, Payne N, Thokala P, Freeman C, et al. Prevention of Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk: Economic Review and Modelling. Report for National Institute for Clinical Excellence (NICE). Sheffield: School of Health and Related Research, University of Sheffield; 2012.
- Ara R, Wailoo A. NICE DSU Technical Support Document 12: The Use of Health State Utility Values in Decision Models. Sheffield: School of Health and Related Research, University of Sheffield; 2011.
- Alva M, Gray A, Mihaylova B, Clarke P. The effect of diabetes complications on health-related quality of life: the importance of longitudinal data to address patient heterogeneity. Health Econ 2014;23:487-500. https://doi.org/10.1002/hec.2930.
- Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238-43. https://doi.org/10.2337/diacare.25.12.2238.
- Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst 2004;96:1322-30. http://dx.doi.org/10.1093/jnci/djh255.
- Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13520.
- Benedict A, Arellano J, De Cock E, Baird J. Economic evaluation of duloxetine versus serotonin selective reuptake inhibitors and venlafaxine XR in treating major depressive disorder in Scotland. J Affect Disord 2010;120:94-104. http://dx.doi.org/10.1016/j.jad.2009.04.017.
- Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. PharmacoEconomics 2003;21:43-50. https://doi.org/10.2165/00019053-200321001-00005.
- NHS Blood and Transplant . Cost-Effectiveness of Transplantation 2013. http://researchbriefings.files.parliament.uk/documents/POST-PN-441/POST-PN-441.pdf (accessed December 2015).
- Tappenden P, Eggington S, Nixon R, Chilcott J. Colorectal Cancer Screening Options Appraisal Report to the English Bowel Cancer Screening Working Group 2004. www.cancerscreening.nhs.uk/bowel/scharr.pdf (accessed October 2015).
- Poole C, Tetlow T, McEwan P, Holmes P, Currie C. The prescription cost of managing people with type 1 and type 2 diabetes following initiation of treatment with either insulin glargine or insulin determir in routine general practice in the UK: a retrospective database analysis. Curr Med Res Opin 2007;23:S41-8. https://doi.org/10.1185/030079907X167589.
- Palmer S, Sculpher M, Philips Z, Robinson M, Ginnelly L, Bakhai A, et al. A Cost-effectiveness Model Comparing Alternative Management Strategies for the Use of Glycoprotein IIb/IIIa Antagonists in Non-ST-elevation Acute Coronary Syndrome. York: York Centre for Health Economics; 2008.
- Madan J, Rawdin A, Stevenson M, Tappenden P. A rapid-response economic evaluation of the UK NHS Cancer Reform Strategy breast cancer screening program extension via a plausible bounds approach. Value Health 2010;13:215-21. https://doi.org/10.1111/j.1524-4733.2009.00667.x.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Economic Modelling for Vascular Checks. A Technical Consultation on the Work Undertaken to Establish the Clinical and Cost Effectiveness Evidence Base for the Department of Health’s Policy of Vascular Checks. London: DH; 2008.
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003;26:1790-5. https://doi.org/10.2337/diacare.26.6.1790.
- Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003;20:442-50. https://doi.org/10.1046/j.1464-5491.2003.00972.x.
- Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Searle A, Haase AM, et al. A pragmatic randomised controlled trial to evaluate the cost-effectiveness of a physical activity intervention as a treatment for depression: the treating depression with physical activity (TREAD) trial. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16100.
- Burr JM, Mowatt G, Hernández R, Siddiqui MA, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11410.
- Blak BT, Mullins CD, Shaya FT, Simoni-Wastila L, Cooke CE, Weir MR. Prescribing trends and drug budget impact of the ARBs in the UK. Value Health 2009;12:302-8. http://dx.doi.org/10.1111/j.1524-4733.2008.00423.x.
- Belsey JD, Pittard JB, Rao S, Urdahl H, Jameson K, Dixon T. Self blood glucose monitoring in type 2 diabetes. A financial impact analysis based on UK primary care. Int J Clin Pract 2009;63:439-48. http://dx.doi.org/10.1111/j.1742-1241.2008.01992.x.
- Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. The cost of renal dialysis in a UK setting: a multicentre study. Nephrol Dial Transplant 2008;23:1982-9. http://dx.doi.org/10.1093/ndt/gfm870.
- Ara R, Pandor A, Stevens J, Rees A, Rafia R. Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13340.
- Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;32:459-66. http://dx.doi.org/10.1111/dme.12647.
- Department of Health . NHS Reference Costs 2012–13 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed December 2015).
- NICE . Hypertension: Clinical Management of Primary Hypertension in Adults 2011. http://guidance.nice.org.uk/CG127/CostingTemplate/xls/English (accessed December 2015).
- NICE . Osteoarthritis Costing Report: Implementing NICE Guidance 2008. www.nice.org.uk/nicemedia/live/11926/39712/39712.pdf (accessed October 2015).
- Gillett M, Royle P, Snaith A, Scotland G, Poobalan A, Imamura M, et al. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: a systematic review and economic evaluation. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16330.
- Emerson E, Hatton C. Estimating Future Need for Social Care among Adults with Learning Disabilities in England: An Update. Durham: Improving Health & Lives, Learning Disabilities Observatory; 2011.
- Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. Finnish Diabetes Prevention Study Group . The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care Dec 2003;26:3230-6. https://doi.org/10.2337/diacare.26.12.3230.
- Bhaumik S, Tyrer FC, McGrother C, Ganghadaran SK. Psychiatric service use and psychiatric disorders in adults with intellectual disability. J Intellect Disabil Res 2008;52:986-95. http://dx.doi.org/10.1111/j.1365-2788.2008.01124.x.
- Nicholson L, Colyer M, Cooper SA. Recruitment to intellectual disability research: a qualitative study. J Intellect Disabil Res 2013;57:647-56. http://dx.doi.org/10.1111/j.1365-2788.2012.01573.x.
- Martin-Stacey L, Doherty Y, Makepeace C, Patel N, Spong R, Dunkley AJ. The systematic development and theoretical framework of a lifestyle educational programme for prevention of type 2 diabetes in a population with intellectual disabilities: P338. Diabet Med 2015;32.
- Dunkley AJ, Spong R, Gray LJ, Tyrer F, Bhaumik S, Gangadharan SK, et al. Screening for people at high risk of type 2 diabetes in a population with intellectual disabilities. Diabet Med 2016;33.
- Dixon-Woods M, Angell EL. Research involving adults who lack capacity: how have research ethics committees interpreted the requirements?. J Med Ethics 2009;35:377-81. http://dx.doi.org/10.1136/jme.2008.027094.
- Hypertension: Clinical Management of Primary Hypertension in Adults. London: NICE; 2011.
- Statins for the Prevention of Cardiovascular Events in Patients at Increased Risk of Developing Cardiovascular Disease or Those with Established Cardiovascular Disease. London: NICE; 2006.
Appendix 1 Assessment of capacity and consent
FIGURE 26.
How capacity and consent were assessed in the study. Adapted from Dixon-Woods and Angell. 334
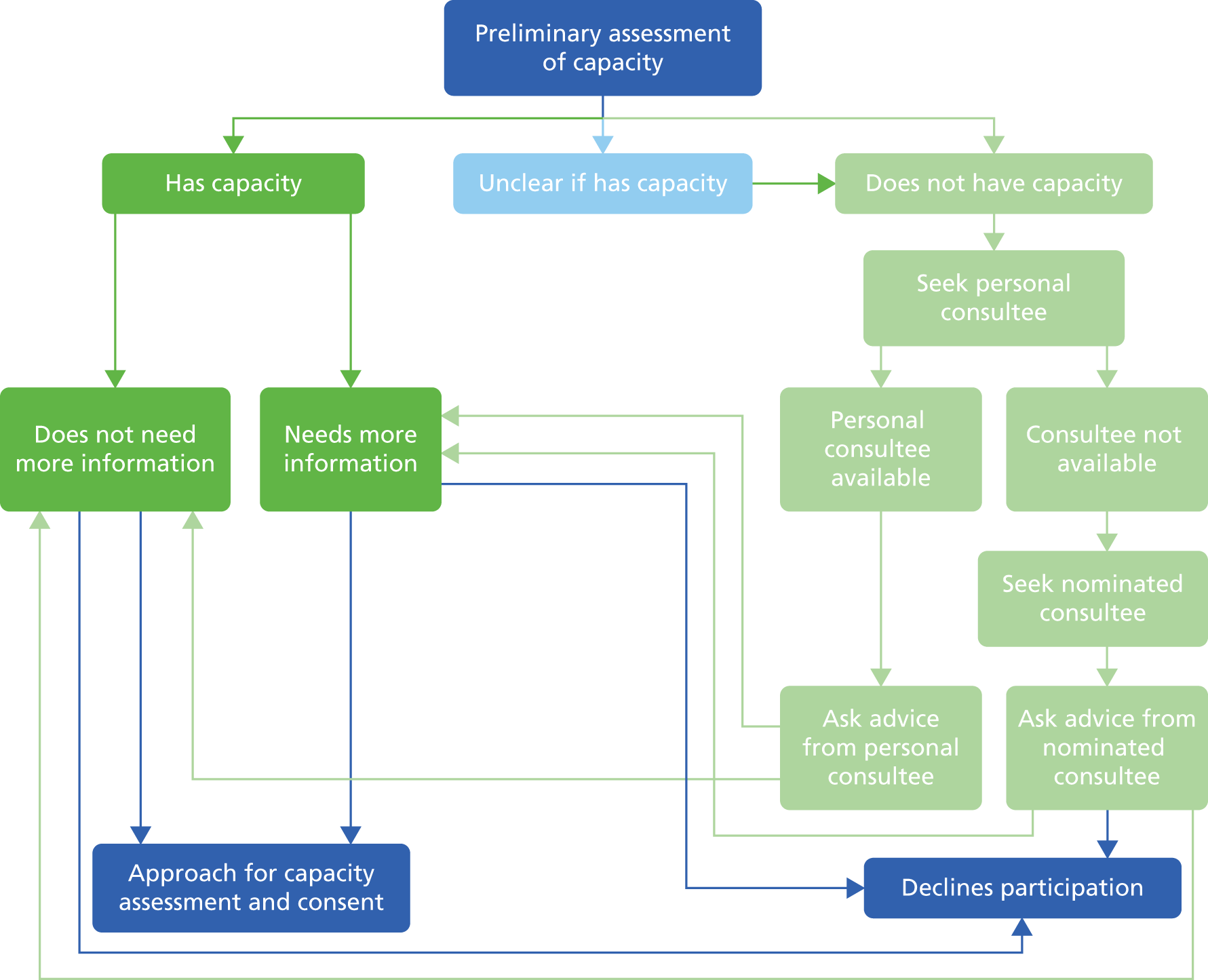
Appendix 2 Example from Leicester Self-Assessment risk score
Appendix 3 Outcome definitions for type 2 diabetes and cardiovascular disease prevalence and risk factors
(See main report for reference list.)
| First author (year) | CVD outcomes | Diabetes/blood sugar outcomes | Obesity/overweight outcomes | BP outcomes | Lipid outcomes | Metabolic syndrome | Split by ID severity |
|---|---|---|---|---|---|---|---|
| Molteno (2000)131 | Obese: BMI of > 30 kg/m2 | MILD 0.3%, MOD 18.7%, SEV 37.7%, PROF 33.5%, MISSING DATA | |||||
| Overweight: BMI of 25 to < 30 kg/m2 | |||||||
| Robertson (2000)50 | Obese: BMI of > 30 kg/m2 | ||||||
| Overweight: BMI of 25.1–30 kg/m2 | |||||||
| Janicki (2002)114 | CVD:a NR | Diabetes:a adult onset | Obese:a BMI of > 27 kg/m2 | Hypertension:a NR | Hyperlipidaemia:a NR | MILD 1.3%, MOD 50.3%, SEV/PROF, 47% | |
| Overweight:a BMI of 22–27 kg/m2 | |||||||
| Lewis (2002)119 | Obese: BMI of ≥ 30 kg/m2 | Elevated BP: SBP > 140 mmHg or DBP > 90 mmHg | Hypercholesterolaemia: total cholesterol ≥ 13.3 mmol/l | MILD 37.1%, MOD 16.4%, SEV 14.7%, PROF 15.3% | |||
| Overweight: BMI of 25–29.9 kg/m2 | |||||||
| Marshall (2003)124 | Obese: BMI of ≥ 31 kg/m2 | Hypertension: SBP > 140 mmHg | Elevated cholesterol definition NR | ||||
| Overweight: BMI of 26–30 kg/m2 | |||||||
| Havercamp (2004)83 | CVD:a definition NR | Diabetes:a definition NR | Obese: definition NR – BMI data were collected | Elevated BP:a definition NR | MILD 39.4%, MOD 26.6%, SEV 14.7%, PROF 10.6% | ||
| Overweight: definition NR – BMI data were collected | |||||||
| Hove (2004)111 | Obese: BMI of ≥ 30 kg/m2 | MILD 39.2%, MOD 42.1%, SEV 15.5% | |||||
| Overweight: BMI of 25–29.9 kg/m2 | |||||||
| Merrick (2004)129 | Heart disease:a definition NR | T2DM:a definition NR | Overweight and above:a BMI of > 27 kg/m2 | Hypertension:a definition NR | Hyperlipidaemia:a definition NR | ||
| Moore (2004)132 | Obese: BMI of ≥ 30 kg/m2 | ||||||
| Overweight: BMI of 25 to < 30 kg/m2 | |||||||
| Emerson (2005)105 | Obese:b BMI of > 30 kg/m2 | ||||||
| Overweight:b BMI of 25.1–30 kg/m2 | |||||||
| Yen (2005)144 | Obese:a BMI of ≥ 27 kg/m2 | MILD 22.2%, MOD 34.9%, SEV 28.1%, PROF 14.8% | |||||
| Overweight:b BMI of 24 to < 27 kg/m2 | |||||||
| Ito (2006)89 | Obese:a BMI of > 30 kg/m2 | ||||||
| Overweight: BMI of 25–30 kg/m2 | |||||||
| Lennox (2006)116 | Obese: BMI of > 30 kg/m2 | Elevated BP: SBP > 140 mmHg | |||||
| Overweight: BMI of 25–30 kg/m2 | |||||||
| Levy (2006)117 | Diabetes:b definition NR | Obese:b BMI of ≥ 30 kg/m2 | Elevated BP:b definition NR | Hypercholesterolaemia:b definition NR | MILD 47.6%, MOD 31.1%, SEV 14.6%, PROF 6.8% | ||
| Overweight: BMI of 25–29.9 kg/m2 | |||||||
| Obese/overweight: BMI of ≥ 25 kg/m2 | |||||||
| McDermott (2006)86 | Coronary artery disease:b ICD-9 codes | T1DM and T2DM:b ICD-9 codes | Obese:b NR | Hypertension and elevated BP:b ICD-9 codes | |||
| TIA:b ICD-9 codes | |||||||
| Rurangirwa (2006)93 | Overweight/obese:a BMI of ≥ 25 kg/m2 | ||||||
| Shah (2006)135 | Diabetes:a definition NR | ||||||
| Van Den Akker (2006)140 | CHD:b ICD-10 codes | Hypertension:b ICD-10 codes | MILD 11%, MOD 53%, SEV 28%, PROF 8% | ||||
| Cerebrovascular disease:b ICD-10 codes | |||||||
| Levy (2007)118 | Diabetes:b definition NR | Overweight and above: BMI of ≥ 25 kg/m2 | Elevated BP:b definition NR | Hypercholesterolaemia:b definition NR | SEV 65.4%, PROF 34.6% | ||
| McDermott (2007)87 | Diabetes:b although a detailed description is given, it is not possible to define the type of diabetes is used as an outcome | ||||||
| McGuire (2007)127 | Obese:a BMI of > 30 kg/m2 | MILD 14.1%, MOD 63.5%, SEV 12.8%, PROF 9% | |||||
| Overweight:a BMI of > 25 kg/m2 | |||||||
| Wang (2007)142 | Heart disease:a ICD-9 codes; specific codes in manual for the Rochester health status survey (includes some non-CVD codes) | Overweight and above a | |||||
| Bhaumik (2008)330 | Obese:b BMI of ≥ 30 kg/m2 | Hypertension:b SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg | |||||
| Overweight: BMI of 25.1 to < 30 kg/m2 | |||||||
| Henderson (2008)84 | T2DM:b derived from medical problem lists | Obese:b BMI of > 30 kg/m2 | Hypertension:b derived from medical problem lists | Dyslipidaemia:b derived from medical problem lists | |||
| Overweight:b BMI of ≥ 25 ≤ 30 kg/m2 | |||||||
| Melville (2008)128 | Obese: BMI of ≥ 30 kg/m2 | MILD 40.9%, MOD 25.1%, SEV 18.2%, PROF 15.8% | |||||
| Overweight: BMI of 25 to < 30 kg/m2 | |||||||
| Wallace (2008)141 | CVD:b history of peripheral vascular disease, stroke or CHD | Elevated glucose:b > 6.1 mmol/l (fasting and non-fasting tests grouped together in results) | Obese:b BMI of ≥ 30 kg/m2 | Hypertension:b SBP > 140 mmHg | Elevated cholesterol:b > 5.5 mmol/l (fasting and non-fasting tests grouped together in results) | ||
| T1DM and T2DM | Overweight: BMI of 25–29.9 kg/m2 | ||||||
| de Winter (2009)81 | Cerebrovascular disease:b diagnosed by CT scan | Diabetes: glucose ≥ 7.0 mmol/l or use of antidiabetic drugs | Obese: BMI of ≥ 30 kg/m2 | Hypertension: SBP ≥ 140 mmHg or use of drugs | Hypercholesterolaemia: total cholesterol > 5.1 mmol/l to ≥ 6.5 mmol/l (depending on laboratory reference values) or use of cholesterol-lowering drugs | MILD 12.1%, MOD 33.2%, SEV 34.3%, PROF 20.4% | |
| MI:b diagnosed by ECG changes | Elevated LDL: ≥ 3.5 mmol/l | ||||||
| Gale (2009)107 | Obese: BMI of 30 to < 40 kg/m2 | ||||||
| Severely obese: BMI of ≥ 40 kg/m2 | |||||||
| Overweight: BMI of 25 to < 30 kg/m2 | |||||||
| Henderson (2009)110 | Overweight or above:a BMI of ≥ 25 kg/m2 | MILD/MOD 53%, SEV/PROF, 47% | |||||
| Maaskant (2009)123 | Obese: BMI of ≥ 30 kg/m2 | ||||||
| Overweight: BMI of 25 to < 30 kg/m2 | |||||||
| Moss (2009)134 | Elevated glucose: non-fasting test – definition NR | Overweight and above: BMI of > 25 kg/m2 | Hypertension: definition NR | Elevated total cholesterol: non-fasting test – definition NR | |||
| Sohler (2009)136 | Diabetes:b definition NR | Obese:b BMI of ≥ 30 kg/m2 | Hypertension:b definition NR | Hypercholesterolaemia:b total cholesterol > 13.3 mmol/l | |||
| Overweight: BMI of 25–29.9 kg/m2 | |||||||
| Van de Louw (2009)139 | Hypertension: SBP > 140 mmHg | MILD 10%, MOD 38%, SEV/PROF 52% | |||||
| Shireman (2010)95 | Diabetes:b ICD-9 codes | ||||||
| Stedman (2010)138 | Obese:b BMI of ≥ 30 kg/m2 | ||||||
| Overweight:b BMI of 25–29.9 kg/m2 | |||||||
| Tyler (2010)88 | CHD:b ICD-9 codes | Diabetes:b ICD-9 codes | Obese:b ICD-9 codes | Hypertension:b ICD-9 codes | Hyperlipidaemia:b ICD-9 codes | ||
| Chen (2011)101 | Heart disease: such as cardiac arrhythmias and coronary atherosclerosis. Diagnoses based on clinical manifestations or ECG findings | Elevated blood glucose: exceeding normal range 3.9–6.1 mmol/l | Hypertension: SBP ≥ 140 mmHg or DBP ≥ 90mmHg | Elevated total cholesterol: ≥ 6.2 mmol/l | |||
| Diabetes: FPG ≥ 7 mmol/l or 2-hour plasma glucose ≥ 11.1 mmol/l or 2-hour OGTT > 11.1 mmol/l | Elevated triglycerides: ≥ 2.26 mmol/l | ||||||
| Frighi (2011)106 | T2DM: NR | Overweight or above: definition NR – BMI data and WC were collected | MILD 48%, MOD 30.2%, SEV/PROF 21.8% | ||||
| POMONA II study: Haveman (2011)109 plus Martinez-Leal (2011)125 (obesity data) | Heart attack:a definition NR | Diabetes:a definition NR | Obese: definition NR – BMI data were collected | Hypertension:a definition NR | Haveman:109 MILD 22.7%, MOD 28.2%, SEV 20.7%, PROF 11.8%; Martinez-Leal:125 MILD 21.8%, MOD 27.7%, SEV 19.7%, PROF 11.4% | ||
| Cerebrovascular disease:a definition NR | Overweight: definition NR – BMI data were collected | ||||||
| Hsu (2012)113 | Overweight or above:b BMI of ≥ 24 kg/m2 | 3/5 criteria, NCEP-ATPII | MILD/MOD, 47%, SEV/PROF 53% | ||||
| Lee (2011)115 | Cardiac illness:b history of CHD or congestive cardiac failure | Diabetes:b implied by prescription of hypoglycaemic drugs | Obese:b BMI of ≥ 31 kg/m2 | Hypertension:b definition NR | MILD 33%, MOD 22%, SEV 23%, PROF 21% | ||
| Overweight:b BMI of 26–30 kg/m2 | |||||||
| Stancliffe (2011)137 | Obese: BMI of ≥ 30 kg/m2 | ||||||
| Overweight: BMI of ≥ 25 to < 30 kg/m2 | |||||||
| Overweight and above: BMI of ≥ 25 kg/m2 | |||||||
| Wong (2011)143 | Heart disease:a definition NR | Diabetes:a definition NR | Overweight and above:a BMI of > 23 kg/m2 | Hypertension:a definition NR | Hypercholesterolaemia:a definition NR | MILD 4.9%, MOD 41.8%, SEV/PROF 51.9% | |
| Cerebrovascular disease:a definition NR | |||||||
| Chang (2012)100 | Elevated blood sugar: FPG ≥ 5.6 mmol/l or use of drugs | Obesity: BMI (definition NR) | Hypertensive SBP: ≥ 130 mmHg or use of drugs | Elevated triglycerides: ≥ 8.3 mmol/l (or use of drug) | 3/5 criteria NCEP-ATPIII and MetS criteria for Taiwanese people | MILD 65%, MOD 16%, SEV 9%, PROF 10% | |
| Overweight: BMI (definition NR) | Hypertensive DBP: ≥ 85 mmHg or use of drugs | Reduced HDL: HDL – male < 2.2 mmol/l, female < 2.8 mmol/l (or use of drugs) | |||||
| Central overweight: FWC ≥ 80 cm/MWC ≥ 90 cm | |||||||
| De Winter (2012)-1, HA-ID study103 | Obesity: BMI of ≥ 30 kg/m2 | MILD 24.8%, MOD 48%, SEV 16%, PROF 8.9% | |||||
| Overweight: BMI of ≥ 25 kg/m2 | |||||||
| Central obese: FWHR ≥ 88 cm/MWHR ≥ 102 cm | |||||||
| Central overweight: FWHR ≥ 80 cm/MWHR ≥ 94 cm | |||||||
| De Winter (2012)-2, HA-ID study102 | Diabetes: FSG ≥ 6.1 mmol/l or use of drugs | Hypertension: SBP ≥ 140 mmHg or DBP ≥ 90 mmHg and/or medication | Hypercholesterolaemia: fasting serum total cholesterol > 6.5 mmol/l or use of drugs | Defined separately by: 3/5 criteria (joint interim statement) and 3/5 criteria NCEP-ATPIII | MILD 24.5%, MOD 48.6%, SEV 16%, PROF 8.7% | ||
| Gazizova (2012)82 | Obese: BMI of > 30 kg/m2 | MILD 61%, MOD 24%, SEV 15% | |||||
| Overweight: BMI of 25.1–30 kg/m2 | |||||||
| Lin (2012)122 | Hypertension: SBP ≥ 140 mmHg or DBP ≥ 90 mmHg | ||||||
| Morin (2012)133 | Heart disease:a ICD-10 codes | Diabetes:a ICD-10 codes | MILD 32.9%, MOD 46.4%, SEV 11.2%, PROF 5.2% | ||||
| Bégarie (2013)98 | Obese: BMI of ≥ 30 kg/m2 | ||||||
| Overweight: BMI of ≥ 25 to < 30 kg/m2 | |||||||
| De Winter (2013),104 HA-ID study | Peripheral arterial disease: ankle-brachial pressure index ≤ 0.9 (measured only in subjects with > 1 CVD risk) | MILD 24.9%, MOD 53%, SEV 13.4%, PROF 4.6% | |||||
| Haider (2013)108 | Heart disease:a ever diagnosed by a doctor/relevant HCP | T2DM:a In the paper it groups T1DM and T2DM together, but in a separate report outcomes are available separately, it also says if been told by doctor | Obese:a BMI of > 30 kg/m2 | ||||
| Stroke:a ever diagnosed by a doctor/relevant HCP | Overweight:a BMI of 25 to < 30 kg/m2 | ||||||
| Jansen (2013)85 | Cerebrovascular accident:b acute disruption of cerebral circulation with focal neurological symptoms ≥ 24 hours | MILD 6.9%, MOD 37.8%, SEV 29%, PROF 26.3% | |||||
| MI:b clinical signs and ECG diagnosis and/or laboratory results | |||||||
| Lin, JD (2013)120 | Hyperglycaemia:b FPG ≥ 7 mmol/l | Hypertension:b SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or use of drugs | Hyperlipidaemia:b triglyceride ≥ 11.1 mmol/l or total cholesterol ≥ 13.3 mmol/l | ||||
| McCarron (2013)126 | Heart disease:a history of angina, heart attack, coronary heart failure, open heart surgery (ever diagnosed by a doctor/relevant HCP) | Hypertension:a ever diagnosed by a doctor/relevant HCP | |||||
| Stroke/TIA:a ever diagnosed by a doctor/relevant HCP | |||||||
| Vacek (2013)94 | Hypertension:b ICD-9 codes | ||||||
| Hsieh (2014)112 | Obese:a BMI of ≥ 30 kg/m2 | MILD 44.9%, MOD 23.7%, SEV/PROF 8.4% | |||||
| Overweight: BMI of 25 to < 30 kg/m2 | |||||||
| Mikulovic (2014)130 | Obese: BMI of > 30 kg/m2 | ||||||
| Overweight: BMI of ≥ 25 kg/m2 | |||||||
| de Winter (2015)90 | T1DM, T2DM | Central obese: FWHR ≥ 88 cm/MWHR ≥ 102 cm | Hypertension: SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg and/or medication | Hypercholesterolaemia: fasting serum total cholesterol > 6.5 mmol/l or use of drugs | Defined separately by: 3/5 criteria (joint interim statement), and 3/5 criteria NCEP-ATPIII | MILD 24.5%, MOD 48.6%, SEV 16%, PROF 8.7% | |
| Diabetes: FSG ≥ 6.1 mmol/l or use of drugs | Central overweight: FWHR ≥ 80 cm/MWHR ≥ 94 cm | ||||||
| Lin, LP (2015)121 | Obese: BMI of ≥ 27 kg/m2 | MILD 6.5%, MOD 32.6%, SEV 34.8%, PROF 26.1% | |||||
| Overweight: BMI of 24–26.9 kg/m2 | |||||||
| Zaal-Schuller (2014)145 | Peripheral arterial disease: ankle–brachial pressure index < 0.9 | MILD/MOD 51.1%, SEV/PROF 48.9% |
Appendix 4 Funnel plot for type 2 diabetes
FIGURE 27.
Funnel plot with pseudo 95% confidence limits for T2DM.
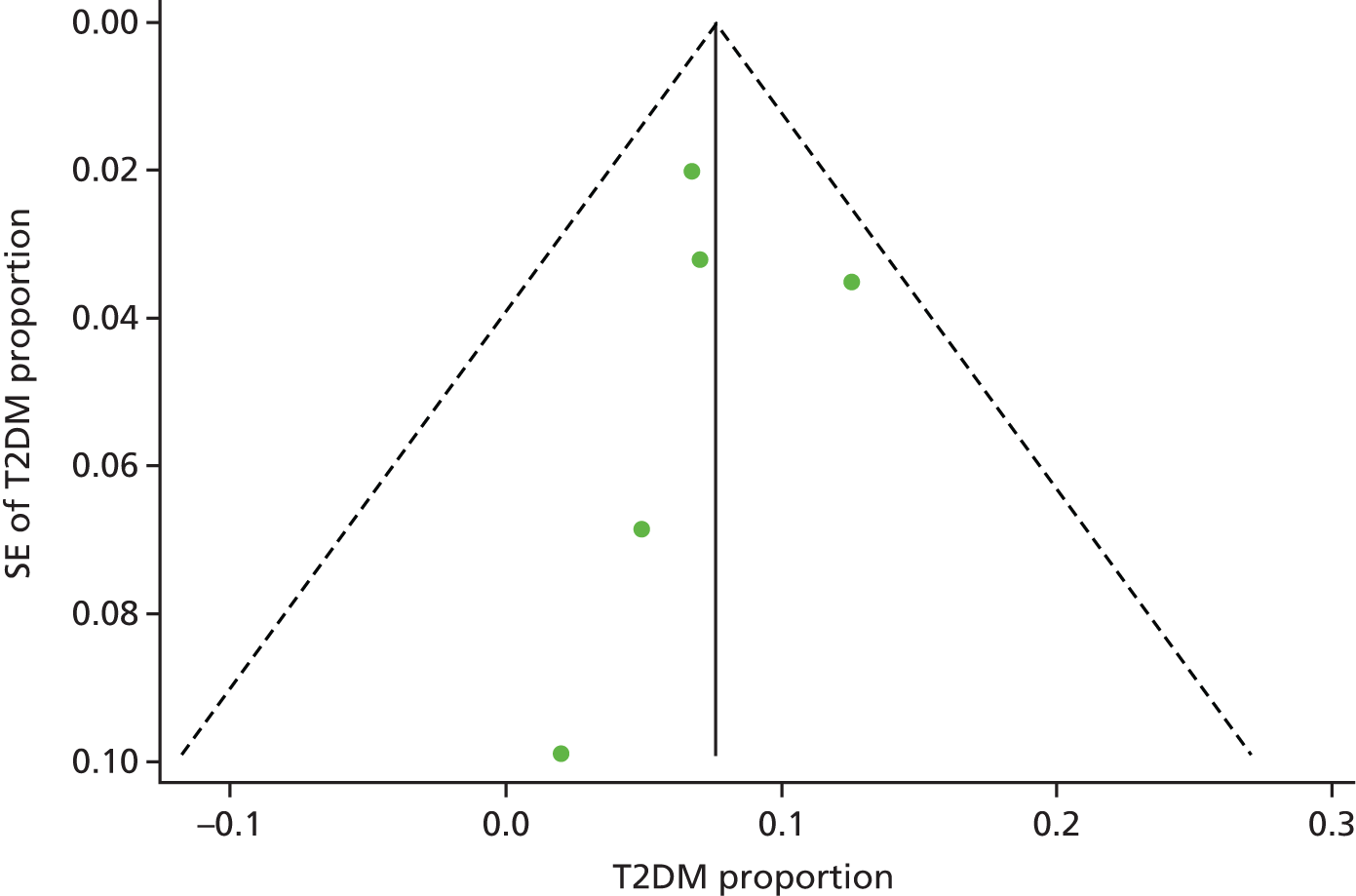
Appendix 5 Funnel plot for ischaemic heart disease
FIGURE 28.
Funnel plot with pseudo 95% confidence limits for ischaemic heart disease.
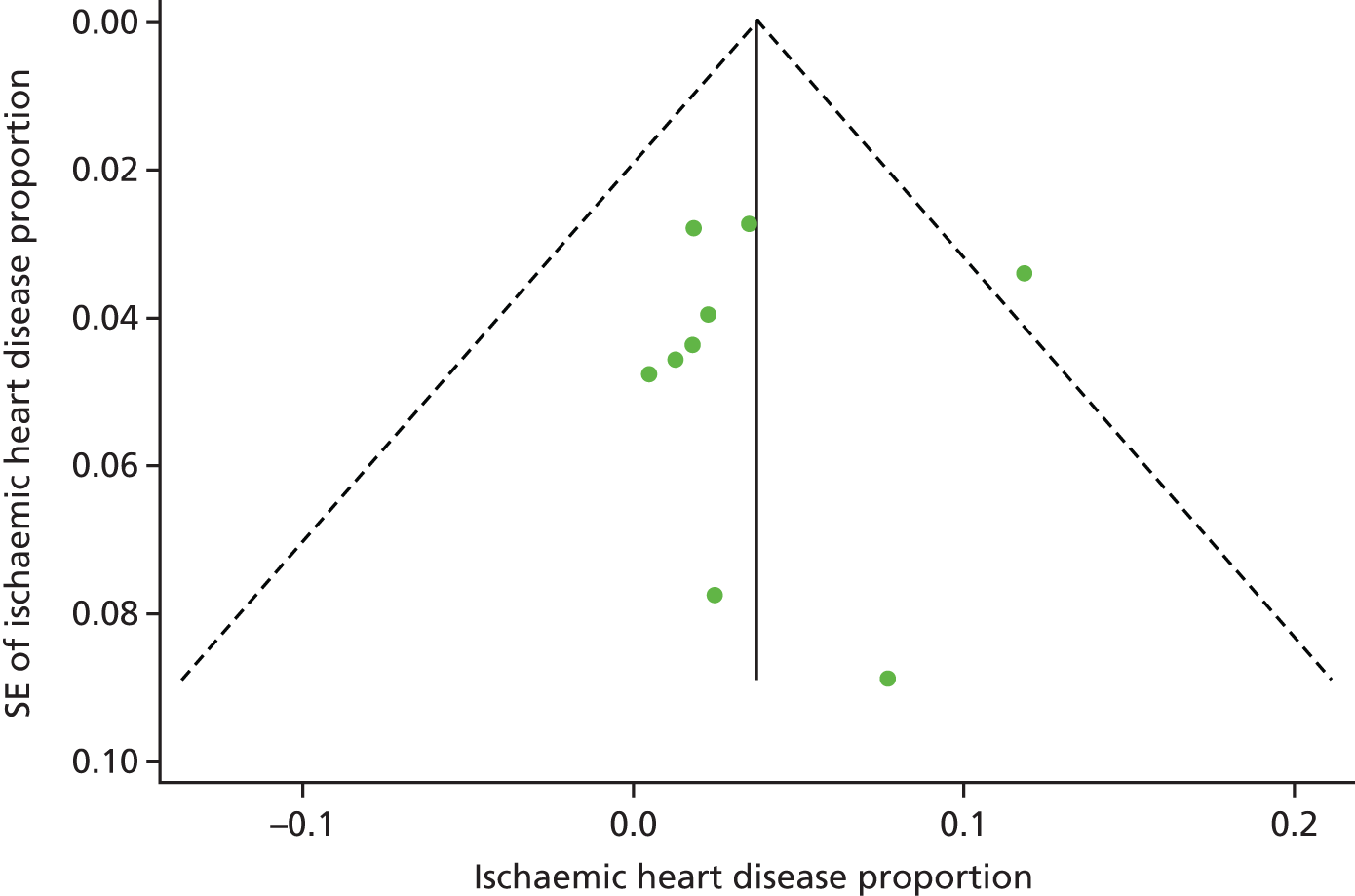
Appendix 6 Funnel plot for cerebrovascular disease
FIGURE 29.
Funnel plot with pseudo 95% confidence limits for cerebrovascular disease.
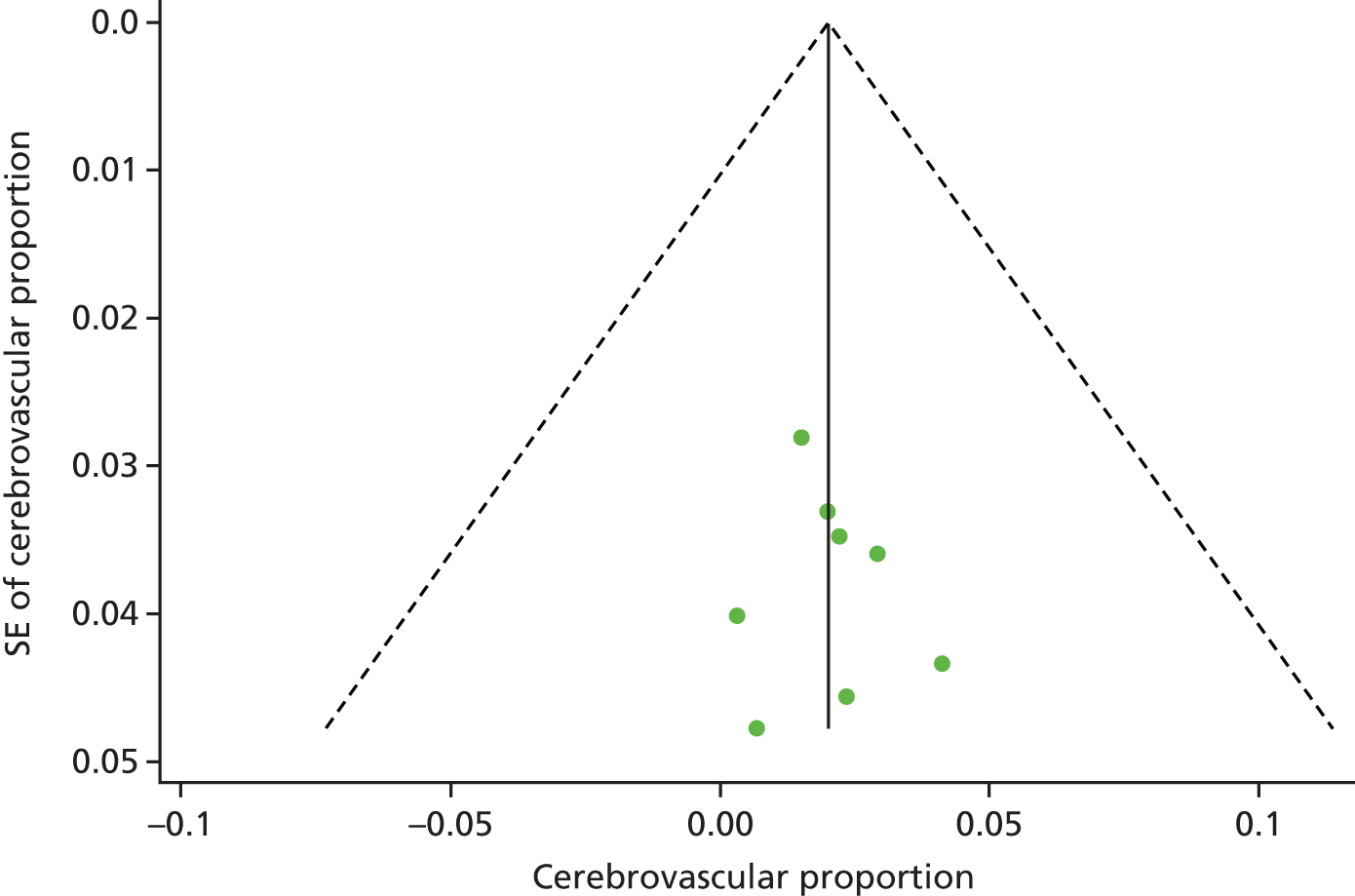
Appendix 7 Example easy-read invitation letter
Appendix 8 Full easy-read information sheet
Appendix 9 Full easy-read reply form
Appendix 10 Personal consultee information leaflet
Appendix 11 Nominated consultee information leaflet
Appendix 12 Easy-read consent form
Appendix 13 Personal consultee advice form
Appendix 14 Nominated consultee advice form
Appendix 15 Example of letter to inform participants of results
Appendix 16 Example letter to inform general practice of results
Appendix 17 Questionnaires used in the research programme
EuroQol-5 Dimensions (EQ-5D)
A generic instrument for the measurement of health-related quality of life. 214 It provides a simple descriptive profile in five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each with three levels. This instrument can be used in the clinical and economic evaluation of health care and to analyse changes in the health status of individuals or groups of individuals over time.
Aberrant Behaviour Checklist (ABC)
An informant-based problem behaviour rating scale, which assesses a wide range of behavioural disorders and has been shown to be a reliable and valid behaviour rating instrument. 216 The questionnaire consists of 58 items, scored on a four-point scale. 215 The subcategories are (1) irritability, agitation, crying; (2) lethargy, social withdrawal; (3) stereotypic behaviour; (4) hyperactivity, noncompliance; and (5) inappropriate speech.
Psychiatric Assessment Schedules for Adults with Developmental Disabilities (PAS-ADD) Checklist
A 25-item questionnaire and can be used to make an initial assessment for mental illness/psychiatric disorders in people with ID. 217 The instrument generates threshold scores, which are then used as a measure to indicate the likely absence or presence of possible psychiatric problems. The scores produced relate to (1) affective or neurotic disorder, (2) possible organic condition (including dementia) and (3) psychotic disorder.
Glasgow Depression Scale (GDS)
An established measure of depression among people with ID. 218 The GDS for people with learning disability (GDS-LD) differentiates depression and non-depression groups, correlates with the Beck Depression Inventory II (r = 0.88), has good test–retest reliability (r = 0.97) and internal consistency [Cronbach’s alpha (α) = 0.90], and a cut-off score of 13 yielded 96% sensitivity and 90% specificity. The Carer Supplement is also reliable (r = 0.98; α = 0.88), correlating with the GDS-LD (r = 0.93).
Appendix 18 Summary of baseline characteristics
| Characteristic | N (medical record) | Mean (±SD) unless stated otherwise |
|---|---|---|
| Biomedical measurements | ||
| Plasma glucose, mmol/l | ||
| Fasting | 425 (8) | 4.7 (± 0.7) |
| Non-fasting | 239 (16) | 5.3 (± 1.5) |
| HbA1c | 675 (27) | |
| HbA1c (mmol/mol) | 35.0 (± 5.1) | |
| Derived HbA1c (%) | 5.4 (± 0.5) | |
| Lipids, mmol/l | ||
| Total cholesterol | 653 | 4.9 (± 1.0) |
| HDL cholesterol | 644 | 1.3 (± 0.4) |
| LDL cholesterol | 631 | 2.9 (± 0.9) |
| Triglycerides (only if fasted) | 407 | 1.4 (± 0.9) |
| Urea and electrolytes | ||
| Sodium (mmol/l) | 713 (84) | 139.6 (± 3.1) |
| Potassium (mmol/l) | 701 (80) | 4.3 (± 0.5) |
| Urea (mmol/l) | 712 (83) | 5.4 (± 1.9) |
| Creatinine (mmol/l) | 714 (84) | 69.0 (± 22.7) |
| eGFR (ml/minute), n (%) | 603 (80) | |
| ≥ 90 | 476 (78.9) | |
| 60–89 | 110 (18.2) | |
| 45–59 | 10 (1.7) | |
| 30–44 | 4 (0.7) | |
| ≤ 29 | 3 (0.5) | |
| Liver function tests | ||
| Bilirubin (µmol/l) | 683 (52) | 9.6 (± 5.9) |
| Alanine transaminase (IU/l) | 691 (61) | 24.8 (± 15.8) |
| Alkaline phosphatase (IU/l) | 694 (67) | 86.8 (± 27.6) |
| GGT (IU/l) | 621 (3) | 32.5 (± 32.2) |
| Thyroid function | ||
| Thyroid-stimulating hormone (mIU/l) | 637 (22) | 2.6 (± 2.1) |
| Free thyroxine (T4) (pmol/l) | 621 (10) | 14.0 (± 2.4) |
| Urine ACR | ||
| Urine ACR (mg/mmol) | 569 (1) | 2.5 (± 12.5) |
| Anthropometric measurements | ||
| Height (m) | 800 | 1.6 (± 0.1) |
| Weight (kg) | 799 | 76.4 (± 20.7) |
| BMI (kg/m2) | 782 | 28.7 (± 7.1) |
| BMI categories, n (%) | ||
| Underweight | 30 (3.8) | |
| Normal | 223 (28.5) | |
| Overweight | 241 (30.8) | |
| Obese | 288 (36.8) | |
| Waist circumference (cm) | 796 | 100.4 (± 16.5) |
| Hip circumference (cm) | 789 | 107.6 (± 14.0) |
| BP measurements | ||
| BP (mmHg) | 826 | |
| Systolic | 121.4 (± 16.9) | |
| Diastolic | 78.2 (± 11.1) | |
| Demographic and lifestyle | ||
| Age in years | 930 | 43.3 (± 14.2) |
| Sex (male), n (%) | 930 | 537 (57.7) |
| Ethnicity, n (%) | 930 | |
| White | 748 (80.4) | |
| Asian | 147 (15.8) | |
| Black | 14 (1.5) | |
| Mixed | 13 (1.4) | |
| Other | 8 (0.9) | |
| Residential circumstances, n (%) | 929 | |
| Alone | 51 (5.5) | |
| Lives with family | 338 (36.4) | |
| Shared house or supported living | 157 (16.9) | |
| Shared care | 16 (1.7) | |
| Residential home or nursing home | 350 (37.7) | |
| Other | 17 (1.8) | |
| Level of support, n (%) | 929 | |
| Independent | 69 (7.4) | |
| Some support | 205 (22.1) | |
| 24-hour support | 655 (70.5) | |
| Current status,a n (%) | ||
| Paid employment | 928 | 71 (7.7) |
| Voluntary work | 927 | 152 (16.4) |
| College | 925 | 170 (18.4) |
| Day opportunities or private day centre | 928 | 431 (46.4) |
| Shared lives (day placement) | 928 | 19 (2.1) |
| Attending meetings | 926 | 122 (13.2) |
| Other | 924 | 385 (41.7) |
| Deprivation (IMD 2015),b median (IQR) | 930 | 16,353 (7351–23,606) |
| Medical history | ||
| Severity of ID, n (%) | 865 | |
| Not known | 49 (5.7) | |
| Known | 816 (84.3) | |
| Mild | 260 (30.1) | |
| Moderate | 244 (28.2) | |
| Severe | 279 (32.3) | |
| Profound | 33 (3.8) | |
| Cause of ID, n (%) | 866 | |
| Not known | 581 (67.1) | |
| Known | 285 (32.9) | |
| Down syndrome | 133 (15.4) | |
| Fragile X | 8 (0.9) | |
| Cerebral palsy | 58 (6.7) | |
| Angelman syndrome | 4 (0.5) | |
| Cytomegalovirus | 1 (0.1) | |
| Fetal alcohol syndrome | 0 | |
| Homocystinuria | 0 | |
| Hydrocephalus | 6 (0.7) | |
| Hurler syndrome | 0 | |
| Klinefelter syndrome | 3 (0.4) | |
| Lesch–Nyan syndrome | 0 | |
| Neurofibromatosis | 2 (0.2) | |
| Phenylketonuria | 5 (0.6) | |
| Prader–Willi syndrome | 4 (0.5) | |
| Rett syndrome | 1 (0.1) | |
| Sturge–Weber syndrome | 1 (0.1) | |
| Tay–Sachs disease | 0 | |
| Triple X syndrome | 0 | |
| Trisomy 13 | 0 | |
| Trisomy 18 | 0 | |
| Tuberous sclerosis | 2 (0.2) | |
| Turner syndrome | 0 | |
| Other cause | 57 (6.6) | |
| Medical or health problems, n (%) | 929 | |
| None | 117 (12.6) | |
| Yes | 812 (87.4) | |
| Physical health | ||
| Stroke | 13 (1.4) | |
| Peripheral arterial disease | 0 | |
| CHD | 7 (0.8) | |
| Congenital heart disease | 19 (2.1) | |
| Other heart problems | 15 (1.6) | |
| High BP | 63 (6.8) | |
| High cholesterol | 62 (6.7) | |
| Hypothyroidism | 93 (10.0) | |
| Polycystic ovary syndrome | 1 (0.1) | |
| Gestational diabetes | 0 | |
| Pre-diabetes | 1 (0.1) | |
| Chronic breathing problems | 88 (9.5) | |
| Sleep apnoea | 3 (0.3) | |
| Epilepsy | 262 (28.2) | |
| Mental health | ||
| Dementia | 18 (1.9) | |
| Schizophrenia, schizotypal and delusional | 35 (3.8) | |
| Mood (affective) disorders | 152 (16.4) | |
| Neurotic, stress-related and somatoform | 143 (15.4) | |
| Two or more disorders | 52 (5.6) | |
| Personality disorders | 13 (1.4) | |
| Drug/alcohol problems | 0 | |
| Attention deficit hyperactivity disorder | 8 (0.9) | |
| ID | ||
| Autistic spectrum disorders | 165 (17.8) | |
| Behavioural problems | 128 (13.8) | |
| Current medication, n (%) | 928 | |
| None | 172 (18.5) | |
| Yes | 756 (81.5) | |
| Anti-psychotic | 240 (25.9) | |
| Two or more medications | 24 (2.6) | |
| Depression/anxiety/OCD or related | 258 (27.8) | |
| Two or more medications | 43 (4.6) | |
| For ADHD | 4 (0.4) | |
| Antiepileptic | 311 (33.5) | |
| Antithrombotic | 36 (3.9) | |
| Lipid lowering | 74 (8.0) | |
| Statin | 72 (7.8) | |
| Fibrate | 1 (0.1) | |
| Statin and fibrate | 1 (0.1) | |
| Antihypertensive | 85 (9.2) | |
| Thyroid medication | 93 (10.0) | |
| Steroids | 80 (8.6) | |
| Oral | 5 (0.5) | |
| Inhaled | 62 (6.7) | |
| Topical | 9 (1.0) | |
| More than one type of steroid medication | 3 (0.3) | |
| Not known | 1 (0.1) | |
| Anti-obesity | 1 (0.1) | |
| Other | 571 (61.5) | |
| Smoking status, n (%) | 929 | |
| Current | 76 (8.2) | |
| Ex | 38 (4.1) | |
| Never | 815 (87.7) | |
| Family history of diabetes, n (%) | 592 | 180 (30.4) |
| Physical activity/exercise | ||
| Able to stand, n (%) | 929 | |
| No | 58 (6.2) | |
| Yes | 871 (93.8) | |
| Able to walk, n (%) | 927 | |
| No | 57 (6.2) | |
| Yes (with or without walking stick, aid) | 787 (84.9) | |
| Yes, with assistance from person(s) | 83 (9.0) | |
| Mobility aids, n (%) | 928 | |
| No | 703 (75.8) | |
| Yes | 225 (24.3) | |
| Uses a walking aid | 52 (5.6) | |
| Uses a wheelchair, all or most | 81 (8.7) | |
| Uses a wheelchair, some | 78 (8.4) | |
| Other | 12 (1.3) | |
| Not known | 2 (0.2) | |
| Amount of walking per day, n (%) | 927 | |
| None | 74 (8.0) | |
| A short distance | 259 (27.9) | |
| Some | 359 (38.7) | |
| Lots | 235 (25.4) | |
| Speed of normal walking (if can walk), n (%) | 850 | |
| Slow | 301 (35.4) | |
| Steady | 373 (43.9) | |
| Brisk or fast | 176 (20.7) | |
| Activities,a n (%) | ||
| Keep fit/aerobics | 928 | 83 (8.9) |
| Walking | 197 (21.2) | |
| Running/jogging | 929 | 39 (4.2) |
| Swimming | 190 (20.5) | |
| Dance | 233 (25.1) | |
| Bowling | 155 (16.7) | |
| Gym | 92 (9.9) | |
| Horse riding | 32 (3.4) | |
| Cycling | 62 (6.7) | |
| Gardening | 179 (19.3) | |
| Housework | 927 | 489 (52.8) |
| Chair-based exercise | 863 | 68 (7.9) |
| Other | 925 | 131 (14.2) |
| Amount of physical activity per week, n (%) | 928 | |
| None | 184 (19.8) | |
| 1–2 times | 360 (38.8) | |
| 3–4 times | 259 (27.9) | |
| 5 or more | 125 (13.5) | |
| Time spent sitting per day, n (%) | 928 | |
| All/most | 180 (19.4) | |
| A lot | 252 (27.2) | |
| Sometimes | 475 (51.2) | |
| Never | 21 (2.3) | |
| Nutrition and diet | ||
| Problems relating to eating and drinking, n (%) | ||
| Difficulties with chewing or swallowing | 929 | 227 (24.4) |
| Needs help or assistance to feed self | 926 | 118 (12.7) |
| Use specialist equipment | 95 (10.3) | |
| Fed via an nasogastric tube or a gastrostomy | 7 (0.8) | |
| Only included if not fed via tube | 922 | |
| Food shopping, n (%) | ||
| Independently | 89 (9.7) | |
| With support | 230 (25.0) | |
| Relative or carer | 297 (32.2) | |
| Purchased by residential home | 306 (33.2) | |
| Prepare meals, n (%) | 921 | |
| Relative or carer | 561 (60.9) | |
| With supervision | 117 (12.7) | |
| Without supervision | 145 (15.7) | |
| Without supervision and prepare variety of meals | 98 (10.6) | |
| Types of food daily eaten, n (%) | ||
| Starch | 919 | 916 (99.7) |
| Fruit/vegetables | 921 | 864 (93.8) |
| Milk/yoghurt | 920 | 896 (97.4) |
| Meat, fish, eggs/other vegetarian, alternative | 919 | 898 (97.7) |
| Daily proportion of fruit, vegetable, n (%) | 920 | |
| None | 33 (3.6) | |
| 1 a day | 57 (6.2) | |
| 2 a day | 130 (14.1) | |
| 3 a day | 230 (25.0) | |
| 4 a day | 199 (21.6) | |
| 5 a day | 213 (23.2) | |
| 6 a day | 36 (3.9) | |
| 7 or more a day | 22 (2.4) | |
| Questionnaires | ||
| Administered via interview | 930 | |
| Health-related quality of life | ||
| EQ-5D score | 872 | 0.8 (± 0.3) |
| EQ-5D scale | 877 | 78.1 (± 19.4) |
| Depression | ||
| GDS-LD | 317 | 7.5 (± 6.7) |
| Number depressed, n (%) | 67 (21.1) | |
| GDS-LD Carer Supplement | 464 | 5.5 (± 5.8) |
| Number depressed, n (%) | 71 (15.3) | |
| Carer completed outside appointment | 930 | |
| Behaviour problem | ||
| ABC | 341 | |
| 1. irritability, agitation, crying | 4.3 (± 6.7) | |
| 2. lethargy, social withdrawal | 3.5 (± 5.5) | |
| 3. stereotypic behaviour | 1.2 (± 2.6) | |
| 4. hyperactivity, noncompliance | 3.9 (± 6.0) | |
| 5. inappropriate speech | 1.3 (± 2.2) | |
| Total score | 14.0 (± 19.5) | |
| Psychiatric disorders | ||
| PAS-ADD Checklist Section 1 | 930 | |
| No events | 207 (22.3) | |
| Death of a first-degree relative | 34 (3.7) | |
| Death of a close friend, carer or relative | 36 (3.9) | |
| Serious illness or injury | 21 (2.3) | |
| Retirement form work | 1 (0.1) | |
| Serious illness of relative, carer or friend | 28 (3.0) | |
| Move of house or residence | 45 (4.8) | |
| Break-up of steady relationship | 10 (1.1) | |
| Separation or divorce | 1 (0.1) | |
| Alcohol problem | 1 (0.1) | |
| Drug problem | 1 (0.1) | |
| Serious problem with relative, carer/friend | 11 (1.2) | |
| Unemployed/seeking work | 4 (0.4) | |
| Breakdown of relationship with parent(s) | 4 (0.4) | |
| Laid off or sacked from work | 0 | |
| Something valuable lost or stolen | 4 (0.4) | |
| Problems with police or other authority | 7 (0.8) | |
| Major financial crisis | 1 (0.1) | |
| Sexual problem | 2 (0.2) | |
| Other event | 38 (4.1) | |
| PAS-ADD Checklist Section 2 | 325 | |
| Possible organic condition | 1.0 (± 1.7) | |
| Threshold score or above, n (%) | 20 (6.2) | |
| Affective or neurotic disorder | 1.4 (± 3.2) | |
| Threshold score or above, n (%) | 28 (8.6) | |
| Psychotic disorder | 0.2 (± 0.6) | |
| Threshold score or above, n (%) | 16 (4.9) | |
Appendix 19 Example topic guide for service users interviews: education development stage
Appendix 20 Example form for educator training
Appendix 21 Scope of the economic evaluation
The reasons for not attempting to estimate the cost-effectiveness of screening people with ID for diabetes (including T2DM)/IGR and overweight/obese) are listed below.
Lack of evidence
There is a dearth of good-quality evidence in relation to the costs and effects of diabetes prevention interventions in people with ID.
Number of pathways/screening strategies
The economic model needs to take account of all of the permutations of screening for diabetes only, screening for diabetes and IGR, and screening for overweight/obese. As screening cannot be considered in isolation (i.e. it depends on interventions), the economic model would need to take into account of how standard prevention interventions and the STOP Diabetes education programme would be implemented for people with ID. It is also unclear how such screening would fit into existing policy in relation to Learning Disability Health Checks.
Evaluation of screening outside the UK
Evaluating screening outside the UK in people with ID would lead to unreliable conclusions because:
-
we do not have estimates for the prevalence of undiagnosed diabetes and IGR, and the rates are likely to be different in other countries (even those within Europe)
-
there are different thresholds for HbA1c for diagnosing IGR
-
we do not know how effective prevention interventions would be
-
we would need to model different countries’ diagnostic and care pathways, use country-specific costs and use different thresholds for ‘willingness to pay’.
Appendix 22 Comparison of surrogate-based physical activity approach against Yates et al.267
When using biomarkers (e.g. changes in physical activity through BMI and SBP level) to predict clinical events, it is important, where possible, to undertake validation against a study that is reporting hard clinical outcomes. Potentially, surrogate-based modelling could overlook some other mechanism of reduction in risk of CVD. To the extent that any such other mechanisms are correlated with changes in BMI and systolic BP, these mechanisms would be captured within our mapping. It was decided to compare the model’s predicted impact on CVD outcomes with another study.
In consultation with clinical experts we were directed to the NAVIGATOR trial results,267 which could be used for the validation. In this study, all of the groups participated in a lifestyle modification programme that was designed to help them achieve and maintain a 5% weight loss, reduce the amount of saturated and total fats in their diet and increase physical activity to 150 minutes per week. The study reported the relationship between activity (steps) and CVD outcomes (events) in a cohort of 9306 people. The analysis controlled for changes in BMI.
For the validation, a model adaptation was created, which mimicked the NAVIGATOR trial267 by assuming that changes in daily step counts continued without declining for a period of 6 years (the study followed participants for 6 years, but it was not an intervention trial so we assumed that steps/day was stationary rather than declining). For an increase in activity of 2000 steps, Yates et al. 267 reported a hazard ratio of experiencing a cardiovascular event over the following 6 years of 0.92 (95% CI 0.86 to 0.99), that is, a risk reduction of 8%. The hazard ratio from our adapted model was 0.95 (5% risk reduction).
Appendix 23 Database search terms for health-economic analysis
(‘activity’[title] OR ‘sedentary’[title] OR ‘exercise’[title])
AND
(‘weight’[title] OR ‘diabetes’[title] OR ‘BMI’[title] or ‘cardio-metabolic’[title] or ‘glucose’[title])
AND
(‘steps’[All Fields] OR ‘step-counter’[All Fields] OR ‘accelerometer’[All Fields])
AND (‘weight’[All Fields] OR ‘diabetes’[All Fields] OR ‘BMI’[All Fields])
AND (‘blood glucose’[All Fields] OR ‘hba1c’[All Fields] OR ‘cholesterol’[All Fields] OR ‘BMI’[All Fields] OR ‘weight’[All Fields] OR ‘waist’[All Fields] OR ‘hip’[All Fields] OR ‘blood pressure’[All Fields] OR ‘glycated haemoglobin’[All Fields] OR ‘blood sugar’[All Fields])
NOT (‘school’[title] OR ‘child’[title] OR ‘children’[title] OR ‘childhood’[title])
Date of search: 23 October 2015.
Appendix 24 Modelling cardiovascular events
The QRISK2 risk equation can be used to calculate the probability of a cardiovascular event, including CHD (angina or MI), stroke, transient ischaemic attacks (TIAs) and fatality due to CVD.
The QRISK assumptions regarding the relationship between IGR, diabetes and CVD were modified for the model and are outlined below:
-
It was assumed that individuals with HbA1c levels of > 6.5 mmol/l have an increased risk of CVD, even if they have not received a formal diagnosis.
-
Risk of CVD was assumed to increase with HbA1c levels for test results of > 6.5% to reflect the observations from the UKPDS – that HbA1c increases the risk of MI and stroke. 275
-
Prior to T2DM (HbA1c level of > 6.5 mmol/l), HbA1c was assumed to be linearly associated with CVD. A study from the EPIC cohort found that a unit increase in HbA1c increases the risk of CHD by a hazard ratio of 1.25, after adjustment for other risk factors. 285 Individuals with a HbA1c level of greater than the mean HbA1c level that was observed in the HSE 2011 cohort were at greater risk of CVD than those with a HbA1c level that was lower than the HSE mean. 262
The QRISK algorithm identifies which individuals experience a cardiovascular event, but does not specify the nature of that event. The nature of the cardiovascular event was determined independently. A targeted search of recent health technology appraisals of CVD was performed to identify a model for the progression of CVD following a first event (Table 62).
| Covariates | Estimated coefficients adjusting for individual characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Interaction terms | Women | Men | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| White | 0.0000 | 0.0000 | 0.0000 | 0.0000 | Age1*former smoker | 0.1774 | 0.035 | –3.881 | 0.776 |
| Indian | 0.2163 | 0.0537 | 0.3163 | 0.0425 | Age1*light smoker | –0.3277 | 0.066 | –16.703 | 3.341 |
| Pakistani | 0.6905 | 0.0698 | 0.6092 | 0.0547 | Age1*moderate smoker | –1.1533 | 0.231 | –15.374 | 3.075 |
| Bangladeshi | 0.3423 | 0.1073 | 0.5958 | 0.0727 | Age1*Heavy smoker | –1.5397 | 0.308 | –17.645 | 3.529 |
| Other Asian | 0.0731 | 0.1071 | 0.1142 | 0.0845 | Age1*AF | –4.6084 | 0.922 | –7.028 | 1.406 |
| Caribbean | –0.0989 | 0.0619 | –0.3489 | 0.0641 | Age1*renal disease | –2.6401 | 0.528 | –17.015 | 3.403 |
| Black African | –0.2352 | 0.1275 | –0.3604 | 0.1094 | Age1*hypertension | –2.2480 | 0.450 | 33.963 | 6.793 |
| Chinese | –0.2956 | 0.1721 | –0.2666 | 0.1538 | Age1*Diabetes | –1.8452 | 0.369 | 12.789 | 2.558 |
| Other | –0.1010 | 0.0793 | –0.1208 | 0.0734 | Age1*BMI | –3.0851 | 0.617 | 3.268 | 0.654 |
| Non-smoker | 0.0000 | 0.0000 | 0.0000 | 0.0000 | Age1*family history CVD | –0.2481 | 0.050 | –17.922 | 3.584 |
| Former smoker | 0.2033 | 0.0152 | 0.2684 | 0.0108 | Age1*SBP | –0.0132 | 0.003 | –0.151 | 0.030 |
| Light smoker | 0.4820 | 0.0220 | 0.5005 | 0.0166 | Age1*Townsend | –0.0369 | 0.007 | –2.550 | 0.510 |
| Moderate smoker | 0.6126 | 0.0178 | 0.6375 | 0.0148 | Age2*former smoker | –0.0051 | 0.001 | 7.971 | 1.594 |
| Heavy smoker | 0.7481 | 0.0194 | 0.7424 | 0.0143 | Age2*light smoker | –0.0005 | 0.000 | 23.686 | 4.737 |
| Age 1a | 5.0327 | 47.3164 | Age2*moderate smoker | 0.0105 | 0.002 | 23.137 | 4.627 | ||
| Age 2a | –0.0108 | –101.2362 | Age2*Heavy smoker | 0.0155 | 0.003 | 26.867 | 5.373 | ||
| BMIa | –0.4724 | 0.0423 | 0.5425 | 0.0299 | Age2*AF | 0.0507 | 0.010 | 14.452 | 2.890 |
| Ratio of total cholesterol to HDL cholesterol | 0.1326 | 0.0044 | 0.1443 | 0.0022 | Age2*renal disease | 0.0343 | 0.007 | 28.270 | 5.654 |
| SBP | 0.0106 | 0.0045 | 0.0081 | 0.0046 | Age2*hypertension | 0.0258 | 0.005 | –18.817 | 3.763 |
| Townsend | 0.0597 | 0.0068 | 0.0365 | 0.0048 | Age2*Diabetes | 0.0180 | 0.004 | 0.963 | 0.193 |
| AF | 1.3261 | 0.0310 | 0.7547 | 0.1018 | Age2*BMI | 0.0345 | 0.007 | 10.551 | 2.110 |
| Rheumatoid arthritis | 0.3626 | 0.0319 | 0.3089 | 0.0445 | Age2*family history CVD | –0.0062 | 0.001 | 26.605 | 5.321 |
| Renal disease | 0.7636 | 0.0639 | 0.7441 | 0.0702 | Age2*SBP | 0.0000 | 0.000 | 0.291 | 0.058 |
| Hypertension | 0.5421 | 0.0115 | 0.4978 | 0.0112 | Age2*Townsend | –0.0011 | 0.000 | 3.007 | 0.601 |
| Diabetes | 0.8940 | 0.0199 | 0.7776 | 0.0175 | |||||
| Family history of CVD | 0.5997 | 0.0122 | 0.6965 | 0.0111 | |||||
All QRISK events are assigned to a specific diagnosis according to age- and sex-specific distributions of cardiovascular events used in a previous HTA. 287 The probability of cardiovascular outcomes by age and sex is shown in Table 63.
| Sex | Age (years) | Angina | MI rate | Fatal CHD | TIA | Stroke | Fatal CVD | |
|---|---|---|---|---|---|---|---|---|
| Stable | Unstable | |||||||
| Men | 45–54 | 0.307 | 0.107 | 0.295 | 0.071 | 0.060 | 0.129 | 0.030 |
| 55–64 | 0.328 | 0.071 | 0.172 | 0.086 | 0.089 | 0.206 | 0.048 | |
| 65–74 | 0.214 | 0.083 | 0.173 | 0.097 | 0.100 | 0.270 | 0.063 | |
| 75–84 | 0.191 | 0.081 | 0.161 | 0.063 | 0.080 | 0.343 | 0.080 | |
| ≥ 85 | 0.214 | 0.096 | 0.186 | 0.055 | 0.016 | 0.351 | 0.082 | |
| Women | 45–54 | 0.325 | 0.117 | 0.080 | 0.037 | 0.160 | 0.229 | 0.054 |
| 55–64 | 0.346 | 0.073 | 0.092 | 0.039 | 0.095 | 0.288 | 0.067 | |
| 65–74 | 0.202 | 0.052 | 0.121 | 0.081 | 0.073 | 0.382 | 0.090 | |
| 75–84 | 0.149 | 0.034 | 0.102 | 0.043 | 0.098 | 0.464 | 0.109 | |
| ≥ 85 | 0.136 | 0.029 | 0.100 | 0.030 | 0.087 | 0.501 | 0.117 | |
After an individual has experienced a cardiovascular event, it is not possible to predict the transition to subsequent cardiovascular events using QRISK2. Instead, as with assigning first CVD events, the statin HTA reports the probability of future events, conditional on the nature of the previous event. 287 More details on the probabilities within a year of transitioning from stable angina, unstable angina, MI, TIA or stroke for individuals in different age groups can be found in an online discussion paper. 263
Appendix 25 Assumptions made for diagnosis and treatment of diabetes, hypertension and cardiovascular disease risk for health-economic analysis
| Diabetes | Hypertension | High CVD risk |
|---|---|---|
| Diagnosis | ||
| At baseline, individuals are assigned a HbA1c threshold above which diabetes is detected opportunistically | Assumed that people who are eligible for antihypertensive treatment will be identified through opportunistic screening if they meet certain criteria and see the GP at least once during the simulation period | Assumed that people who are eligible for statins will be identified through opportunistic screening if they meet certain criteria and see the GP at least once during the simulation period |
| Individuals with HbA1c levels that are above their individual threshold will attend the GP to be diagnosed with diabetes | ||
| Treatment | ||
| Assumed that there are three, non-mutually exclusive outcomes from the vascular checks and opportunistic screening | ||
| Patient’s blood glucose test indicates T2DM as measured by HbA1c level of > 6.5 mmol/l (assumed that FPG and the 2-hour glucose test are not used for diabetes diagnosis, but future adaptations of the model could include these criteria) | Patient has high BP and should be treated with antihypertensive medication. Antihypertensive treatment initiated if:
|
Patient receives statins to reduce cardiovascular risk: |
A three-stage treatment regime is assumed (as a trade-off between model simplicity and capturing key cost differences between interventions):
|
||
Appendix 26 Distributions for key parameters within the probabilistic sensitivity analysis
Given the very large numbers of parameters in the model, many of which belong to complex forms of statistical modelling, it would not be helpful to present all of them in this report. In Table 65 we present distributions for parameters that are related to the intervention, the relationship between physical activity (steps)269 and BMI, and other risk factors. Details of distributions for the other model parameters are reported elsewhere. 263
| Parameter description | Distribution | Parameter | Central estimate | Source | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| BMI | Normal | –0.38 | 0.171 | –0.38 | Bravata et al. 2007269 |
| SBP | –3.8 | 1.071 | –3.8 | ||
| Total cholesterol | –0.09 | 0.120 | –0.09 | ||
| HDL cholesterol | 0.06 | 0.039 | 0.06 | ||
No uncertainty is included around uptake rates. As duration of effect is explored through scenario analyses, no uncertainty is included around this parameter.
Mortality
Mortality rates from other causes by age were assumed to be constant in the PSA. 281 The parameter distributions for the hazard ratio for other-cause mortality with diabetes and for the SMRs for other-cause mortality in males and females with ID are reported in Table 66. The table shows the probability distribution for each model parameter and the mean value (central estimate). Parameters 1 and 2 are arguments for the specific forms of statistical model, such as log-normal.
| Parameter description | Distribution | Distribution parameter | Central estimate | |
|---|---|---|---|---|
| 1 | 2 | |||
| Mortality hazard ratio for diabetes | Log-normal | 0.588 | 0.186 | 1.80 |
| SMR for ID in males | Normal | 3.24 | 0.219 | 3.24 |
| SMR for ID in females | Normal | 2.28 | 0.138 | 2.28 |
Appendix 27 Results: cost-effectiveness plane
FIGURE 30.
Cost-effectiveness plane for an increase of 2491 steps at £30,000 per QALY.
In Figure 30, each green dot represents a result from a sample run of the PSA. The green line represents the cost-effectiveness frontier; points below this line represent sample results, from the PSA, which lie in the cost-effective region. The spread of the points gives an indication, for this type of intervention, of how much uncertainty there is around the reported mean incremental costs and QALYs.
Appendix 28 Detailed threshold analysis results tables at £20,000 per quality-adjusted life-year
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –5.0 | –50 | –1.88 | –3.4 | –34 | –1.45 |
| 1000 | –0.2 | –2 | –0.09 | –4.8 | –48 | –1.85 | –3.2 | –32 | –1.40 |
| 3000 | –0.5 | –5 | –0.26 | –4.5 | –45 | –1.77 | –2.9 | –29 | –1.30 |
| 5000 | –0.8 | –8 | –0.42 | –4.2 | –42 | –1.69 | –2.6 | –26 | –1.19 |
| 7000 | –1.1 | –11 | –0.57 | –3.9 | –39 | –1.60 | –2.3 | –23 | –1.08 |
| 9000 | –1.4 | –14 | –0.71 | –3.6 | –36 | –1.52 | –2.0 | –20 | –0.97 |
| 11,000 | –1.7 | –17 | –0.84 | –3.3 | –33 | –1.42 | –1.7 | –17 | –0.84 |
| 13,000 | –2.0 | –20 | –0.97 | –3.0 | –30 | –1.32 | –1.4 | –14 | –0.71 |
| 15,000 | –2.3 | –23 | –1.08 | –2.7 | –27 | –1.22 | –1.1 | –11 | –0.57 |
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –4.7 | –47 | –1.83 | –2.7 | –27 | –1.25 |
| 1000 | –0.2 | –2 | –0.09 | –4.6 | –46 | –1.79 | –2.6 | –26 | –1.19 |
| 3000 | –0.5 | –5 | –0.26 | –4.3 | –43 | –1.71 | –2.3 | –23 | –1.08 |
| 5000 | –0.8 | –8 | –0.42 | –4.0 | –40 | –1.63 | –2.0 | –20 | –0.97 |
| 7000 | –1.1 | –11 | –0.57 | –3.7 | –37 | –1.54 | –1.7 | –17 | –0.84 |
| 9000 | –1.4 | –14 | –0.71 | –3.4 | –34 | –1.45 | –1.4 | –14 | –0.71 |
| 11,000 | –1.7 | –17 | –0.84 | –3.1 | –31 | –1.35 | –1.1 | –11 | –0.57 |
| 13,000 | –2.0 | –20 | –0.97 | –2.7 | –27 | –1.25 | –0.8 | –8 | –0.42 |
| 15,000 | –2.3 | –23 | –1.08 | –2.4 | –24 | –1.14 | –0.5 | –5 | –0.26 |
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –4.7 | –47 | –1.83 | –2.7 | –27 | –1.25 |
| 1000 | –0.2 | –2 | –0.09 | –4.6 | –46 | –1.79 | –2.6 | –26 | –1.19 |
| 3000 | –0.5 | –5 | –0.26 | –4.3 | –43 | –1.71 | –2.3 | –23 | –1.08 |
| 5000 | –0.8 | –8 | –0.42 | –4.0 | –40 | –1.63 | –2.0 | –20 | –0.97 |
| 7000 | –1.1 | –11 | –0.57 | –3.7 | –37 | –1.54 | –1.7 | –17 | –0.84 |
| 9000 | –1.4 | –14 | –0.71 | –3.4 | –34 | –1.45 | –1.4 | –14 | –0.71 |
| 11,000 | –1.7 | –17 | –0.84 | –3.1 | –31 | –1.35 | –1.1 | –11 | –0.57 |
| 13,000 | –2.0 | –20 | –0.97 | –2.7 | –27 | –1.25 | –0.8 | –8 | –0.42 |
| 15,000 | –2.3 | –23 | –1.08 | –2.4 | –24 | –1.14 | –0.5 | –5 | –0.26 |
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –2.6 | –26 | –1.19 | –1.9 | –19 | –0.94 |
| 1000 | –0.2 | –2 | –0.09 | –2.4 | –24 | –1.14 | –1.8 | –18 | –0.87 |
| 3000 | –0.5 | –5 | –0.26 | –2.1 | –21 | –1.03 | –1.4 | –14 | –0.74 |
| 5000 | –0.8 | –8 | –0.42 | –1.8 | –18 | –0.90 | –1.1 | –11 | –0.61 |
| 7000 | –1.1 | –11 | –0.57 | –1.5 | –15 | –0.78 | –0.8 | –8 | –0.46 |
| 9000 | –1.4 | –14 | –0.71 | –1.2 | –12 | –0.64 | –0.5 | –5 | –0.30 |
| 11,000 | –1.7 | –17 | –0.84 | –0.9 | –9 | –0.50 | –0.2 | –2 | –0.13 |
| 13,000 | –2.0 | –20 | –0.97 | –0.6 | –6 | –0.34 | 0.0 | 0 | 0.00 |
| 15,000 | –2.3 | –23 | –1.08 | –0.3 | –3 | –0.18 | 0.0 | 0 | 0.00 |
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –4.1 | –41 | –1.67 | –2.7 | –27.5 | –1.2 |
| 1000 | –0.2 | –2 | –0.09 | –4.0 | –40 | –1.63 | –2.6 | –25.9 | –1.2 |
| 3000 | –0.5 | –5 | –0.26 | –3.7 | –37 | –1.54 | –2.3 | –22.9 | –1.1 |
| 5000 | –0.8 | –8 | –0.42 | –3.4 | –34 | –1.45 | –2.0 | –19.8 | –1.0 |
| 7000 | –1.1 | –11 | –0.57 | –3.1 | –31 | –1.35 | –1.7 | –16.8 | –0.8 |
| 9000 | –1.4 | –14 | –0.71 | –2.7 | –27 | –1.25 | –1.4 | –13.7 | –0.7 |
| 11,000 | –1.7 | –17 | –0.84 | –2.4 | –24 | –1.14 | –1.1 | –10.7 | –0.6 |
| 13,000 | –2.0 | –20 | –0.97 | –2.1 | –21 | –1.03 | –0.8 | –7.6 | –0.4 |
| 15,000 | –2.3 | –23 | –1.08 | –1.8 | –18 | –0.90 | –0.5 | –4.6 | –0.3 |
| Initial increase in steps needed | Change attributable to the increase in steps | Additional change needed to be generated through diet | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Base case (3-year durability) | 5-year durability | ||||||||
| BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | BMI (kg/m2) | SBP (mmHg) | Total/HDL cholesterol ratio | |
| 0 | 0.0 | 0 | 0.00 | –3.5 | –35 | –1.49 | –2.1 | –21 | –1.03 |
| 1000 | –0.2 | –2 | –0.09 | –3.4 | –34 | –1.45 | –2.0 | –20 | –0.97 |
| 3000 | –0.5 | –5 | –0.26 | –3.1 | –31 | –1.35 | –1.7 | –17 | –0.84 |
| 5000 | –0.8 | –8 | –0.42 | –2.7 | –27 | –1.25 | –1.4 | –14 | –0.71 |
| 7000 | –1.1 | –11 | –0.57 | –2.4 | –24 | –1.14 | –1.1 | –11 | –0.57 |
| 9000 | –1.4 | –14 | –0.71 | –2.1 | –21 | –1.03 | –0.8 | –8 | –0.42 |
| 11,000 | –1.7 | –17 | –0.84 | –1.8 | –18 | –0.90 | –0.5 | –5 | –0.26 |
| 13,000 | –2.0 | –20 | –0.97 | –1.5 | –15 | –0.78 | –0.2 | –2 | –0.09 |
| 15,000 | –2.3 | –23 | –1.08 | –1.2 | –12 | –0.64 | 0.0 | 0 | 0.00 |
List of abbreviations
- ABC
- Aberrant Behaviour Checklist
- BMI
- body mass index
- BP
- blood pressure
- CCG
- Clinical Commissioning Group
- CHD
- coronary heart disease
- CI
- confidence interval
- CVD
- cardiovascular disease
- DESMOND
- Diabetes Education and Self Management for Ongoing and Newly Diagnosed
- DPP-IV
- dipeptidyl peptidase-4 inhibitor
- EPIC
- European Prospective Investigation into Cancer and Nutrition
- EQ-5D
- EuroQol-5 Dimensions
- GDS
- Glasgow Depression Scale
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HCP
- health-care professional
- HDL
- high-density lipoprotein
- HSE
- Health Survey for England
- HTA
- Health Technology Assessment
- ICD-9
- International Classification of Diseases, Ninth Edition
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- ID
- intellectual disability
- IGR
- impaired glucose regulation
- IQ
- intelligence quotient
- LDL
- low-density lipoprotein
- LLDR
- Leicestershire Learning Disability Register
- LPT
- Leicestershire Partnership NHS Trust
- MI
- myocardial infarction
- MVPA
- moderate to vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OR
- odds ratio
- PAS-ADD
- Psychiatric Assessment Schedules for Adults with Developmental Disabilities
- PICOS
- population, intervention, comparison, outcomes, study design
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SMR
- standardised mortality ratio
- SPHR
- School for Public Health Research
- T2DM
- type 2 diabetes mellitus
- UKPDS
- UK Prospective Diabetes Study
- WHO
- World Health Organization
- WP
- work package