Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10389. The contractual start date was in October 2008. The final report began editorial review in January 2016 and was accepted for publication in November 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Richard Morriss received personal fees for chairing the National Institute for Health and Care Excellence Guideline Development Group for bipolar disorder from 2012 to 2014.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Jones et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Bipolar disorder (BD) is a life-long condition that has an impact on the individual, carers and wider society. 1,2 As such, it presents a serious public health problem; it is the sixth leading cause of disability among people aged 15–44 years3 and costs the UK £5.2B per annum. 4 It affects approximately 2% of the population according to recent estimates5 and is typically characterised by a recurring relapse course. Although the number of episodes experienced varies substantially, many individuals experience 7–10 episodes, or more, over the course of their disorder. 6,7 There is also increasing evidence that many individuals experience a significant impact from mood symptoms between episodes of mania and depression. Studies over follow-up periods of > 13 years by Judd et al. 8,9 indicate that individuals with BD spend 47–54% of their time experiencing significant mood symptoms, in particular depression. Furthermore, a significant factor contributing to the cost of BD to society is the high rates of unemployment as a result of indaequate treament. 10
Bipolar disorder is also highly comorbid with anxiety disorders (93% lifetime11), with 32% experiencing current comorbid anxiety12 and substance misuse (40–60% comorbidity across psychiatric and community settings13–16). Both of these comorbidities are associated with significantly worse clincal course and outcomes, including negative impacts on engagement with care. For instance, comorbid BD and anxiety is associated with poor treatment response,17 increases in suicidality,18 earlier age at onset of BD19 (which is also associated with higher levels of suicidality)20 and greater relapse risk. 12 Similarly, substance use can have profound effects on mood and behaviour and may have an adverse impact on the course, outcome and morbidity of BD,21,22 including disrupting sleep and circadian rhythms. This, in turn, worsens prodromal symptoms, increases the risk of relapse23,24 and increases impulsive behaviour, including self-harm, aggression25 and suicide. 26
Bipolar disorder in general is further associated with a particularly high risk of suicide,1 a major public health problem and a leading cause of death among young people in England,25 with one-quarter of those dying by suicide having had recent contact with Mental Health Services (MHS). 26 In addition, during acute periods of mania or depression, individuals with BD can sometimes lose capacity under the Mental Health Act (MHA). 27 The Mental Capacity Act 2005 (MCA), implemented in October 2007,28 provides a statutory framework to assess whether or not a person has the capacity to make decisions, and defines how others can make decisions on behalf of someone who lacks capacity.
Psychological treatment to delay episodes and optimise function is efficacious, cost-effective and popular with service users (SUs),1 but difficult to implement owing to a lack of both experienced therapists and service structure in NHS trusts to deliver these treatments. 29 Recent evidence supports group psychoeducation (PEd) as a clinically effective and cost-effective approach30–33 that may be a cheaper, more sustainable and generalisable means to deliver effective psychological treatment of BD in the NHS. The provision of psychological treatment is a key National Institute for Health and Care Excellence (NICE) recommendation for BD and is cost-effective by preventing hospitalisation. 1,2 PEd increases return to work,34 a current Department of Health priority, and builds on the Expert Patients Programme, a current NHS priority. 35 SUs with BD have already delivered PEd successfully in some parts of the UK.
Effective time-limited interventions for anxiety already exist and would, therefore, have the potential to improve a range of adverse outcomes in BD. NICE guidelines for both BD and for substance misuse highlight the importance of psychosocial interventions in changing patterns of substance use and address comorbid affective symptoms. 1,23 Despite the evidence for the effectiveness of psychological interventions for anxiety,36 there have been no systematic efforts to apply these to those with BD. Similarly, BD is characterised by high rates of substance misuse. The investigation of psychological interventions for this comorbidity has been neglected, despite increasing evidence for effective integrated treatments in related disorders37 and for the efficacy of psychological interventions for substance misuse in non-bipolar populations. 23 A recent small trial of group cognitive–behavioural therapy (CBT) indicated some improvements in substance use but a deterioration in bipolar symptoms. 38 Uncontrolled data indicate that dual diagnosis BD patients who received generic dual disorders treatment (including motivational interventions) had increased remission from substance misuse compared with baseline, but the impact of this treatment on bipolar relapse was not assessed. 39
Suicide makes a major contribution to premature mortality:40 every year, 4500 people in England and Wales take their own life. Suicide is a major burden on health services and can devastate surviving family members. Suicide prevention is a health service priority,41 and a key goal of the English Suicide Prevention Strategy is to reduce suicide in high-risk groups, such as individuals with BD. 25 However, there is little current understanding of the factors associated with suicide risk in BD or how to ameliorate these. The MCA has been in effect since 1 October 2007. It is unclear what attempts NHS trusts have made to involve SUs and staff in ensuring that the Act is implemented. We have a unique opportunity to assess the extent and nature of its impact in BD, for which readmission, including temporary loss of capacity, is a key feature, and where mood can adversely affect decision-making,42 especially as research in BD indicates that joint planning in advance is clinically effective and cost-effective29 but is rarely implemented. 43
Key research areas for this programme
Bipolar disorder and suicide research have received only 1% of research spend, compared with 9% on schizophrenia, a disorder of similar prevalence and morbidity. 44 This programme encompasses five key areas neglected to date.
-
Group PEd jointly delivered by clinicians and service user facilitators (SUFs) has not been evaluated against group peer support (PS) controlling for the natural history of the condition, for duration and length of treatment, that both types of intervention have the same aim of treatment and for therapist effects in the NHS. The study will tell us whether the specific conduct and content of PEd provides added clinical effectiveness and cost-effectiveness or whether PS carried out for the same duration, time and conditions would be equally clinically effective and cost-effective. Such interventions may be particularly effective, as SUs and SUFs can share similar experiences. 35 In addition, joint working ensures that action plans are owned by SUs and services and are integrated into care plans, which is clinically effective and cost-effective in randomised controlled trials (RCTs) of implementing advanced directives for mental illness. 45,46
-
Although psychological interventions for anxiety are effective36 and NICE BD guidelines1 recommend psychological interventions for treatment of comorbid anxiety, no research has evaluated such interventions in those with BD. In addition, antidepressant therapies, prescribed to treat symptoms of anxiety may be problematic because of the risk of manic switch. 47
-
Research has yet to clarify relationships between BD, substance misuse and poor outcomes,48 although the ‘self-medication’ hypothesis indicates a role for amelioration of distressing symptoms. 49,50 Recent data indicate the complex and dynamic relationship between BD and substance use. 51 Reasons for substance use include attempting to manipulate mood changes and responding to triggers common to all substance users, such as stress, interpersonal conflict and social pressures. 52
-
Suicide occurs in 10% of individuals hospitalised with BD. 53 A number of risk factors for suicide attempts have been identified, but we understand little about the risk factors for completed suicide. 54 It is unclear how poor clinical management might influence suicide risk or how risk factors may link together to precipitate episodes of suicidal behaviour.
-
The MCA may promote SUs’ involvement in decision-making when they lose capacity, but, as such loss is temporary in those with BD, the assessment of their capacity may be poor,42 the implementation of the MCA may be over-ridden by the MHA, and SUs may experience difficulties regaining their rights once they have recovered capacity. Although early-warning sign interventions are effective for those with BD,29 the MCA applies when capacity is lost, so joint action plans might not be implemented until a crisis and harm have already occurred. 55
Aims
To conduct a series of linked projects to evaluate practical approaches to understanding recurrence, comorbidity, capacity and suicide risk, including the potential for enhancing SU involvement in care planning and delivery to improve clinical outcomes for SUs with BD.
Objectives and research questions
The Psychoeducation, Anxiety, Relapse, Advance Directive Evaluation and Suicidality (PARADES) programme comprised five linked workstreams (WSs) to achieve the aims described above. The corresponding objectives for each theme were to:
-
develop a service model for delivery of evidence-based PEd by:
-
evaluating whether or not group PEd is feasible and sustainable across different NHS sites
-
determining whether or not group PEd is clinically effective and cost-effective compared with group PS
-
identifying barriers, to and potential solutions of barriers, to the implementation of effective group PEd
-
-
evaluate the feasibility of individual psychological interventions to address comorbid anxiety to help enhance recovery and reduce relapse and morbidity by:
-
understanding more about the impact that anxiety has on the lives of people with BD
-
understanding what psychological help is required and by whom in relation to anxiety
-
developing and evaluating the feasibility of CBT-informed time-limited therapy for people with anxiety and BD
-
-
evaluate the feasibility of individual psychological interventions to address comorbid substance misuse to help enhance recovery and reduce relapse and morbidity by:
-
exploring the reasons for substance use and cessation and the links between substance use, stabilisation and impulsivity
-
developing a psychological therapy to reduce substance use, increase stabilisation and reduce impulsiveness informed by findings from the reasons-for-use study
-
conducting an exploratory trial to evaluate feasibility and acceptability of this therapy and to provide preliminary estimates of therapy effect size
-
-
identify and explore factors associated with risk of suicide and self-harm in BD by:
-
understanding the risk of suicide in BD in a NHS service setting
-
understanding the demographic-, clinical- and management-related risk factors for suicidal behaviour in BD
-
understanding how factors associated with suicide and self-harm in BD link together to precipitate episodes of suicidal behaviour
-
-
test the application and impact of advance directives on clinical practice by:
-
determining whether or not the MCA promotes joint care planning in the event of capacity being lost
-
examining the barriers to, and drivers of use of, the MCA in general at SU and staff levels, and specifically in relation to the assessment of capacity in affective disorder and to joint care planning in an emergency when capacity is lost for a period of time but then regained and whether or not advance directives are considered in SUs detained under the 1983 MHA.
-
Structure of this report
Workstreams 1–5 are presented in Chapters 2–6. Each chapter provides an introduction to the WS topic and an overview of methods across each phase (multiple phases for WSs 2–4, single phase for WSs 1 and 5). This is followed by methodology and results sections, and a discussion of the WS results. The final chapter integrates the findings across the programme as a whole and considers the significance of this work for future developments in clinical practice and clinical research.
Chapter 2a Pragmatic randomised controlled trial of group psychoeducation versus group peer support in the maintenance of bipolar disorder: main clinical outcomes
Abstract
Group PEd for BD is a cheap (£447 per participant) and easy-to-implement NICE-recommended treatment. This chapter reports the clinical effectiveness and cost-effectiveness of 21 sessions of group PEd versus group PS in which topics were selected by participants. The groups were matched for the duration, delivery and aim of treatment in a multicentre pragmatic RCT in England. Twenty-two groups facilitated by two health professionals and a SUF were delivered to 304 patients with BD, with a 24-month follow-up. The results showed that, overall, there was no significant difference in time to first bipolar episode [hazard ratio (HR) 0.83; p = 0.22], but group PEd reduced time to first bipolar episodes in those with seven or fewer previous bipolar episodes (HR 0.28; p = 0.034), and overall in relation to time to first mania episode (HR 0.66; p = 0.049) and interpersonal function (p = 0.012). There were no differences between the groups in bipolar symptoms, quality of life or other function. Group PEd was associated with better attendance (p = 0.026), while qualitative research showed that its structured approach was more acceptable to participants, although both group treatments increased knowledge and reduced isolation. Applying NICE thresholds for cost-effectiveness, group PEd was not more cost-effective than group PS using the EuroQol-5 Dimensions (EQ-5D), but was more cost-effective than using relapse-free years. Group PEd did not have the large clinical benefits first reported from Barcelona, but it has a small number of specific clinically important and economic benefits over group PS.
Chapter overview
This chapter describes the adaptation and evaluation of group PEd compared with group PS with a primary aim of reducing relapse in established BD. The chapter provides information on why PEd is a promising potential intervention for this group and then describes both PEd and PS interventions evaluated in the current research. Clinical and functional outcomes of the definitive RCT are described. This is followed by a discussion of the strengths, limitations and research and clinical implications of the trial. A health economic analysis of the trial is described in Chapter 2b.
Background
Bipolar disorder is a severe mental disorder characterised by recurrent episodes of mania and depression. It is the 19th leading cause of years lived with disability for any health problem. 56 The median age at onset is around 19 years. 5 Traditionally, medications such as lithium carbonate have been used to prevent episodes of illness and are still recommended for this purpose. 57 However, since 2006, clinical guidelines for the management of BD have also recommended psychological treatment for the prevention of relapse in addition to pharmacotherapy. 58,59 The most recently published guidelines from England and Wales recommend manualised evidence-based psychological interventions developed specifically for BD as a component of long-term management of BD. 57
Previous research in psychoeducation
Group PEd for BD is a manualised, evidence-based intervention. As relatively large groups of 10–18 people can undertake treatment together, group PEd is an efficient and easy-to-set-up option for MHS to deliver psychological treatment to improve longer-term outcomes. Although therapists need to be trained to run the groups, the training is less intensive than that for other psychological approaches such as individual CBT. 60 The first RCTs of group PEd versus group PS showed the clinical effectiveness and cost-effectiveness of group PEd for all types of bipolar relapse, with better outcomes maintained for 5 years. 33,61,62 However, these trials were carried out in a specialist BD service in Spain, which bears little resemblance to the UK NHS. Additionally, the relapse rates in the control arm of this Spanish trial were significantly higher than expected, with 92% of the sample who attended support groups experiencing a further bipolar episode within 2 years. This level of relapse was cited as an outlier in a recent meta-analysis of eight RCTs of PEd,32 which showed, in contrast, an unweighted mean of 70% relapse in treatment as usual (TAU) or attention control studies over follow-up periods ranging from 52 to 104 weeks. 32 Overall, in this meta-analysis, group PEd was effective in relation to all relapses [odds ratio (OR) 1.98, 95% confidence interval (CI) 1.09 to 3.58; p = 0.024; I2 = 54%; seven studies, 493 participants], almost significant for mania relapse (OR 1.68, 95% CI 0.99 to 2.85; p = 0.06; I2 = 55%; eight studies, 562 participants), but not significant for depression relapse (OR 1.39, 95% CI 0.78 to 2.48; p = 0.26; I2 = 63%; eight studies, 562 participants).
In another meta-analysis of RCTs for development of the NICE clinical guideline, the evidence base was reviewed for group psychological treatments overall, including CBT, mindfulness therapy, social cognition and interaction training, and dialectical behaviour therapy. 57,63 Up to 26 weeks, group psychological interventions showed a trend towards reducing overall relapse [relative risk (RR) 0.48, 95% CI 0.22 to 1.04; I2 = 59%; eight studies, 532 participants], reducing mania relapses (RR 0.48, 95% CI 0.28 to 0.82; I2 = 0%; six studies, 564 participants) and reducing depression relapses (RR 0.39, 95% CI 0.19 to 0.78; I2 = 0%; seven studies, 616 participants), compared with TAU or attentional controls. By 52 to 104 weeks’ follow-up, the benefits of group psychological treatments were confined to a reduction in depression relapses (RR 0.62, 95% CI 0.45 to 0.88; I2 = 44%; five studies, 333 participants), with no effects on mania relapses (RR 0.97, 95% CI 0.60 to 1.57; I2 = 69%; five studies, 328 participants) or on overall relapse (RR 0.86, 95% CI 0.61 to 1.20; I2 = 81%; five studies, 395 participants).
Limitations of research to date
As observed by NICE, the evidence base for group psychological treatments in preventing relapses for BD is inconsistent and imprecise. 63 The vast majority of RCTs were small (with around 100 participants or fewer), single-centre trials, sometimes in specialist settings and often with only 12-month follow-up, and the meta-analysis shows high heterogeneity. Some trials were not pre-registered on trial databases, few reported functional, quality-of-life and economic outcomes, and there was evidence of selective reporting of outcomes. Furthermore, most RCTs did not control for the clustering effect of group treatments in their analysis, thereby overestimating the effectiveness of their treatments. 64 Furthermore, none of the trials of group PEd to date has occurred within a UK setting. In addition, there are concerns in relation to RCTs of psychological interventions that they are often carried out by the developers of the treatment approach, and that there is lack of blinding and of appropriate control groups that control for expectancy of effect as well as frequency, length and duration of treatment. 65 Thus, there is a need for a large multicentre RCT of the clinical effectiveness and cost-effectiveness of group PEd carried out in non-specialist service settings in the UK that addresses all of these methodological issues.
In both the UK and other countries, people with BD often have the opportunity to access support groups, such as those run by Bipolar UK, as well as receiving medication and follow-up from health professionals. People with BD value such support,66 and PS groups run by expert patients improve self-efficacy and are effective for many health conditions. 67 The nature of the group PS in the first trials of PEd in Barcelona is unclear. 61,62 NICE57 could not find any RCT of support groups run by peers versus TAU for BD.
Aims of the current study
The primary aim of this RCT was to determine whether or not the content of a group PEd programme, as outlined by Colom et al. 60,68,69 but contextualised to UK practice, was clinically effective and cost-effective compared with group PS, the most frequently available form of group psychological treatment in the UK, with over 120 groups run by Bipolar UK (www.bipolaruk.org/find-a-support-group, accessed 9 October 2017). To ensure that groups were acceptable to participants and that participants benefited from both professional expertise and lived experience,70 both group interventions were co-facilitated by two health professionals and a trained SU. To ensure generalisability, health professionals currently working in NHS services were recruited to run the groups, and the inclusion and exclusion criteria for participants were designed to be as inclusive as possible.
To ensure that group PS was an effective control, both groups received the same number of sessions of the same duration; each set of sessions involved two health professionals and a SUF and started off with the same number of participants with BD, and each meeting was held at the same place but on a different day that was not disclosed to the blinded assessor of outcome. Each group intervention had a manual and had the same aim of treatment, which was to use the resources of the group to develop a plan to prevent further bipolar episodes and improve overall function. Both groups were supervised by senior clinical academics and both groups were closed. Therefore, although this group PS was similar to the group PS offered by Bipolar UK, in that the participants determined the content of the group, it was also a long-term supervised closed group with a more specific aim, so in many important ways it was quite different from the usual group PS offered in the UK. We also addressed many of the issues highlighted by NICE58,63 as key problems with previous group psychological treatment and PEd RCTs for BD by ensuring that our trial was multicentre and pre-registered, and had relapse, symptom, function, quality-of-life and economic outcomes (reported in the next chapter). The trial was carried out in routine secondary care mental health settings. (A protocol paper has already been published. 71)
Aims
-
To determine whether or not it was feasible to run group PEd for BD across many different settings in the NHS (urban and more rural, teaching and non-teaching hospital, different parts of England) to explore its generalisability.
-
To determine whether or not group PEd is clinically effective compared with group PS in increasing time to first bipolar relapse.
-
To determine whether or not group PEd is clinically effective compared with group PS in relation to secondary outcomes of time to the next mania and depression relapse, severity of mania and depression symptoms, function, quality of life, health status and costs.
-
To identify the barriers to, and potential solutions of barriers to, the implementation of effective group PEd.
Methods
Design
A single-blind RCT compared the effectiveness of:
-
21 weekly bipolar group PEd sessions delivered by two health professionals (nurse, psychiatrist, psychologist or occupational therapist) and a SUF, plus TAU
-
21 weekly unstructured bipolar group PS sessions delivered by two health professionals and a SUF plus TAU71 (see Table 3).
The trial was located in two centres in England (East Midlands and North West), with four clinical sites in each centre. Overall, 11 groups of PEd were compared with 11 groups of PS, with some sites running two waves of groups. The intervention was taken to different locations in each regional centre to improve access for those people who wanted to participate but for whom travelling distance was a practical barrier to engagement.
Consecutively eligible patients were individually randomised to either intervention, with stratification by clinical site and minimisation in terms of number of previous bipolar episodes (1–7, 8–19, ≥ 20). The latter was to control for the effect of rate of bipolar episodes: relapse is up to three times more likely in those with more than 20 episodes than in those with fewer than seven bipolar episodes. 72 There is also increasing evidence that psychological interventions may be particularly effective in people with fewer bipolar episodes or admissions to hospital,73,74 whereas one psychological intervention for unipolar depression, mindfulness CBT, may be more effective in people with three or more previous depression episodes than in those with fewer episodes. 75
Qualitative interviews were used to investigate the experiences of the group participants in order to identify facilitators of, and barriers to, the widespread delivery of the intervention across the NHS.
Setting and target population
The recruitment strategy was deliberately broad to ensure that targets were met and that the sample reflected a diversity of people with BD. Community mental health team bases at a number of NHS trusts located in two distinct geographical centres (East Midlands and North West) were encouraged to invite potentially relevant participants. The study was also promoted at a primary care level, with local family doctors being asked to display posters about the trial, and through SU-run local BD networks and the general media, allowing people to self-refer. The target population was patients with bipolar 1 or bipolar 2 affective disorder at increased risk of further relapse (defined as having had an episode in the last 24 months), as preventing relapse was the key aim of the intervention.
Inclusion/exclusion criteria
Participants were included if they:
-
had a Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-verified diagnosis of primary bipolar 1 or bipolar 2 disorder76,77
-
were at increased risk of relapse (at least one episode in the last 24 months)
-
were aged ≥ 18 years.
Participants were excluded if they had any of the following:
-
presence of a manic, hypomanic, mixed affective or major depressive episode currently or within the previous 4 weeks
-
current suicide plans or high suicide intent
-
inability or unwillingness to give written informed consent to the study
-
inability to communicate in written and verbal English to a sufficient level to allow them to complete the measures and take part in the groups.
Baseline and outcome measures
At baseline interview, the SCID was used to assess sociodemographic features, the presence of axis 1 comorbid psychopathology,76,77 the presence of borderline or antisocial personality disorder78 and the number of previous bipolar episodes. 79 Participants were also asked if they had any preference as to which group they were allocated to.
Primary outcome
The primary outcome measure was time to next bipolar episode using 16-weekly SCID Longitudinal Interval Follow-up Evaluation (LIFE) interviews80 to generate weekly scores of mania and depression on a 1–6 scale of severity, as we have previously used. 71,78 Specifically, we recorded the time from randomisation to the first week of recurrence of an episode of mania, hypomania, a mixed episode for 1 week, or a major depression for 2 consecutive weeks, satisfying DSM-Fourth Edition (DSM-IV) criteria76 (both coded as a 5 or 6 on SCID LIFE). The 16-weekly SCID LIFE interviews (alternating between telephone and face-to-face interviews) were used to generate weekly scores for mania and depression, both on a 1–6 scale of severity (1 = no symptoms; 2 = minor symptoms; 3 = partial remission; 4 = does not meet DSM-IV criteria but major symptoms of the disorder; 5 = DSM-IV definite, but no prominent psychotic symptoms and no extreme impairment in functioning; and 6 = DSM-IV definite, severe and either prominent psychotic symptoms or extreme impairment in functioning).
Those with a score of 5 or 6 on LIFE satisfied DSM-IV criteria. If a participant consented but did not complete the follow-up interview, the case notes (up to 96 weeks) were used to determine the time to recurrence, if applicable. Participants under the care of a NHS trust had their electronic and paper notes reviewed, whereas those participants seen only by their general practitioner (GP) had their GP notes reviewed. As previously outlined,71,78 a bipolar episode was recorded when there was a description of symptoms of a manic-type or a depression-type episode with symptoms of sufficient duration to meet episode criteria76 and there was a change of medication, care setting (inpatient or crisis team) or urgency of being reviewed because of these symptoms. When necessary, the same criteria were used to extract data from primary and secondary care records using assessors blinded to treatment group allocation.
Secondary outcomes
The secondary outcome measures were:
-
time to next manic-type episode (mania, hypomania or mixed affective episode) and time to next depressive episode72,79
-
assessment of mean weekly symptoms of mania-type symptoms and depression symptoms using the LIFE72,79
-
assessment of function using the Social Adjustment Scale (SAS)29 and Social and Occupational Functioning Assessment Scale (SOFAS)81
-
observer- and self-rated measures of mood: 17-item Hamilton Depression Rating Scale (HAM-D),82,83 Bech–Rafaelsen Mania Scale (MAS)84 and Hospital Anxiety and Depression Scale (HADS)85
-
the Short Form questionnaire-12 items (SF-12),86 a 12-item self-completed questionnaire evaluating eight domains of overall health (general health, role–physical, physical function, bodily pain, vitality, social functioning, role–emotional and mental health) in the preceding 4 weeks summarised as physical component scores and mental component scores.
The SCID LIFE, HAM-D and MAS efficacy outcome measures were recorded by interview at baseline and every 16 weeks up to 96 weeks. The remaining outcomes were recorded at baseline and at follow-up weeks 32, 64 and 96. Participants were given the option of returning self-rated outcomes by stamped, addressed envelope to the trial office.
Interventions
Group psychoeducation
The bipolar group PEd programme was run by three facilitators, two health professionals (usually one experienced and one in training as a facilitator), specially trained for the purpose, and a SUF with a diagnosis of BD35 and trained for the purpose. The SUF in the role of therapist working with a health professional was not part of the trials run in Spain60 (where they used three therapists), but was successfully piloted in Newcastle upon Tyne, England,70 before the start of the current trial. In the latter study, feedback suggested that the SUF ensures that the SU perspective is integral to the programme and provides additional credibility to the programme in the eyes of the participants. The SUF also served as a role model for the participants in tasks set out in the PEd manual. 70
In accordance with the trials of group PEd conducted in Spain,60 the PEd programme consisted of 21 sessions delivered over 26 weeks. 71 The group sessions used a closed group format; the sessions started with a minimum of 10 and a maximum of 18 participants in order to capture a variety of SU experience in relation to the topics covered at each session. A manual was produced, along with a handout for each session covering the content of that session. The groups were run as a collaborative workshop with a brief didactic introduction of the topic for the session, and the rest of the work taking the form of active interaction using the collective experience of the participants. 60 However, as recommended,60 the content of both the manual and the sessions themselves had been brought up to date to reflect recent research evidence,58,87 and the content was adapted to expectations of English SUs as determined by a panel of SUs working with the trial. For example, there was a greater emphasis on the role of family and the carer, there were changes to the wording of the manual and handouts so that they were written using more recovery-based language,57,88 and examples were given that were meaningful to English people and to the English context of service provision. Embedded in the PEd programme is the acquisition of specific skills by each individual, including life charting, recognition of early warning signs, problem-solving and other forms of coping, sleep hygiene and care planning, as well as general skills of actively participating and working collaboratively in the groups. Table 1 shows the sessional content of the programme. Any participant who missed a session was provided with printed materials for the session and an opportunity to discuss these before the next session.
| Session number | Topic |
|---|---|
| 1 | Introduction to the group and defining BD |
| 2 | What causes and triggers BD |
| 3 | Symptoms 1: mania and hypomania |
| 4 | Symptoms 2: depression and mixed episodes |
| 5 | Evolution of BD and the future |
| 6 | Treatment 1: mood stabilisers |
| 7 | Treatment 2: antimanic drugs |
| 8 | Treatment 3: antidepressants |
| 9 | Pregnancy, genetic counselling and effects on families |
| 10 | Prescribed drugs and alternative therapies |
| 11 | Risks associated with treatment withdrawal |
| 12 | Alcohol, smoking, diet and street drugs |
| 13 | Early detection of mania and hypomania 1 |
| 14 | Early detection of mania and hypomania 2 |
| 15 | Early detection of depression and mixed episodes 1 |
| 16 | Early detection of depression and mixed episodes 2 |
| 17 | What to do when a new phase is detected |
| 18 | Regularity of habits |
| 19 | Stress control techniques |
| 20 | Problem-solving strategies |
| 21 | Finalisation of stay well plan and closure |
At the point of consent to the study, and at the first and subsequent sessions, participants were told that the purpose of this intervention was to develop their own personalised and tailored ‘staying well plan’ using the information given by the group facilitators, their own experience made more explicit through life charting, and the collective experience of the group. They were told that the plan should fit into their personalised goals for recovery and had two elements: (1) those actions they take every day to stay well, such as taking medication and keeping a regular early morning routine, and (2) those actions that they would take if they started to become unwell with mania or depression. They were encouraged to share this information with the health professionals with whom they had continuing care relationships [e.g. community psychiatric nurse (CPN), psychiatrist, GP] and other people who were personally important to them. However, participants did not have to do this and were in control of how information was shared. Thus, many participants chose to write down detailed ‘staying well plans’ for themselves; some chose to share these with health professionals and other key significant people in their life, and others did not. Some groups chose to actively rehearse strategies for communicating this kind of information to health professionals and other close people.
Group peer support
The purpose of the PS intervention was to provide an active control for the bipolar PEd group, reflecting the most commonly available group PS offered by support groups for BD in England. The PS groups aimed to enable the group participants to devise ways of remaining well through discussion of collective experience, mutual information sharing and support. Although the groups were unstructured, participants collectively decided on an agenda for discussion at each session. Explicitly, participants were told at the point of consent to the study, and at the first and subsequent sessions, that the purpose of this intervention was that they used the resources of the group to generate their own personalised and tailored ‘staying well plan’. However, instead of having a session-by-session curriculum to follow, the group collectively decided what they would talk about and how they were going to help each other to stay well. They decided whether or not they told health professionals or other significant people in their life about their plans to stay well. Thus, these groups did not merely provide a meeting place where people with BD could meet other people with shared experiences, but also they gave people a shared sense of purpose to actively seek ways of learning from the group, and to remain well in the future. Therefore, the PS group provided a control for the processes of delivering a group intervention that was also consistent with the overall aim of the intervention for each participant, namely to stay well over time. As a result, the groups differ only in terms of the content and style of delivery. The two health professionals and one SUF met with the groups of up to 18 participants, but were there to facilitate discussion, encourage participation, prevent unhelpful group behaviour, such as bullying or scapegoating, prevent factual misinformation and, if directly asked, to clear up factual uncertainty. A manual on the conduct of the PS group was produced for the therapists and given as a handout in the first session. The supervision arrangements and recording of the conduct and content of the sessions were the same as for the group PEd sessions.
The content of the PS group sessions is shown in Table 2. In almost all PS groups participants chose to introduce themselves and discuss the causes and triggers of BD, mania, depression, prescribed drugs and alternative therapies, regularity of habits, stress control techniques, problem-solving strategies that overlapped with PEd, as well as services, benefits and welfare, emotions, relationships and personal experience that were not main topics in PEd. Just over half of the PS groups discussed early warning signs of mania and depression relapse, although they did so in one session, and also finalised a ‘staying well plan’. Hardly any PS groups discussed medication and the longitudinal course of BD in any detail.
| Topics covered | Number of groups (n = 11) (% groups) |
|---|---|
| Topic covered also by PEd programme | |
| Introduction to the group and defining BD | 10 (91) |
| What causes and triggers BD | 10 (91) |
| Symptoms 1: mania and hypomania | 11 (100) |
| Symptoms 2: depression and mixed episodes | 11 (100) |
| Evolution of BD and the future | 1 (9) |
| Treatment 1: mood stabilisers | 0 |
| Treatment 2: antimanic drugs | 0 |
| Treatment 3: antidepressants | 0 |
| Pregnancy, genetic counselling and effects on families | 5 (45) |
| Prescribed drugs and alternative therapies | 8 (73) |
| Risks associated with treatment withdrawal | 3 (27) |
| Alcohol, smoking, diet and street drugs | 5 (45) |
| Early detection of mania and hypomania | 6 (55) |
| Early detection of depression and mixed episodes | 6 (55) |
| What to do when a new phase is detected | 6 (55) |
| Regularity of habits | 8 (73) |
| Stress control techniques | 9 (82) |
| Problem-solving strategies | 9 (82) |
| Finalisation of stay well plan and closure | 7 (64) |
| Additional topics covered by PS | |
| Services | 9 (82) |
| Hospital | 5 (45) |
| Benefits and welfare | 9 (82) |
| Finances and debt | 4 (36) |
| Emotions | 8 (73) |
| Relationships (family and friends) | 8 (73) |
| Positivity | 5 (45) |
| The self (personal experience/life stories) | 8 (73) |
| The self (identity and perception) | 5 (45) |
| Stigma | 5 (45) |
| Anxiety (including panic attacks, social anxiety, health anxiety) | 4 (36) |
| Non-anxiety mental comorbidity and physical health | 4 (36) |
| Religion and spirituality | 4 (36) |
| Media | 4 (36) |
In both trial arms, participants received the trial group therapies in addition to their usual treatment. They did not attend any other group, family or individual psychological treatment for BD at the same time as attending the groups in the study. Otherwise, TAU was unconstrained and recorded through interviews and from case notes.
Randomisation and treatment allocation
Randomisation was conducted by a clinical trials unit that was given the participant information by the trial manager or by the trial administrator working under the direct supervision of the trial manager. Randomisation was undertaken only once a group of 20 participants in a wave had been identified at one geographical site close to where both groups would be held, as 10 was the minimum number of participants that would constitute a viable group size. The maximum number allocated to a group was 36. The allocation of participants to treatment was based on stochastic minimisation using number of previous episodes banded as 1–7, 8–19 and ≥ 20, and clinical site. The clinical trials unit conveyed the allocation to the trial manager, who then informed both the participants and the therapists conducting the group. The research assistants (RAs) conducting the interview assessments were blind to group allocation. All information regarding attendance at the groups was collected by the therapists so that RAs remained blind. Any instances of unblindings were recorded by the trial office. On the rare occasion that a RA was unblinded, an alternative RA at that site collected the remainder of that participant’s follow-up data. At baseline, before attending either group, participants were asked whether they had a preference for PEd or PS.
Ethics and research governance approval
The trial received national multicentre research ethics approval (09/H0408/33) and research governance approval and permission at each participating health service delivery organisation. The University of Nottingham sponsored and indemnified the trial. Each participant gave written informed consent to take part in the trial and, separately, gave written informed consent to take part in qualitative interviews, exploring in depth their experience of the trial. The trial was overseen by an independent Trial Steering Committee (TSC) and a Data Monitoring and Ethics Committee organised by the research investigators for the purpose. The members of the committees were drawn from outside the institutions in which the research team worked and were not involved in other currently funded research with the investigators to ensure their independence from the research team.
Sample size
When patients receive treatment in groups, interactions between patients may lead to correlation of outcomes of patients in the same group,89 sometimes referred to as cluster. As with cluster randomised trials, sample size calculation needs to consider the possibility of intracluster correlation. 64 As there are no previous trials of group interventions for BD that have considered clustering, we considered empirical evidence regarding the magnitude of clustering from cluster randomised trials. A previous trial90 found a very variable clustering effect, ranging from 0 to 0.27 with a median of 0, but this was from a small sample and so would be imprecisely estimated. We therefore assumed a small, but not zero, intracluster correlation coefficient (ICC) for group therapy of 5% for the purpose of sample size calculation. Based on the outcomes from the two previous Barcelona protocol PEd RCTs,61,62 a differential treatment effect of 0.22 was estimated (60% recurrence in the control group and 38% in the PEd group at 12-month follow-up, as one of these studies reported only 12-month follow-up). The power for 80% probability of detecting a difference at 0.05 level using two-tailed testing required 82 patients per arm. Assuming a mean group size of 18 and an ICC equal to 0.05, the design effect will be 1.85, giving a sample size of 152 in each arm. An 85% follow-up rate was assumed, giving a target of 360 SUs randomised, with 10 therapy groups planned in each treatment arm to achieve 80% power. During the initial waves of the study, the therapy group size was smaller than planned (approximately 14 participants per group owing to a slightly slower rate of accrual of each wave). In consultation with the Programme Steering Committee, the number of waves was increased from 10 to 11. Owing to the smaller therapy group size, with a reduced target sample size of 308, the study retained 80% power under the same assumptions about the ICC and follow-up rate.
Statistical methods
The quantitative trial outcome data included time-to-event data and longitudinal quantitative data that could be considered continuous.
Time-to-event analyses
A Cox model with robust standard errors for therapy group effect was fitted to the primary outcome measure incorporating the clinical notes data. To assess the clustering effect, a standard Cox model with the same baseline covariates without robust standard errors was fitted. When there is a clustering effect by therapy group, the robust standard errors will be larger. If the robust standard errors are smaller, this suggests no clustering and inference based on the robust standard errors will be non-conservative, and so the standard Cox model results are presented instead. The treatment effect (PEd compared with PS) was adjusted for sex, number of previous bipolar episodes and recruitment wave. The model assumptions were assessed and found to be acceptable. The same approach was used for the two secondary outcomes of time to first mania-type and depression episode.
Continuous longitudinal data
Longitudinal statistical models were fitted across follow-up time points to the secondary outcome scale data. The main statistical analysis used to compare the two interventions was a linear random-effects model (LME). This may be thought of as fitting regression lines of outcome against time for each patient, with variation between patients represented by differences in the intercept and gradient of these lines. It should be noted that these models incorporate time as a continuous variable rather than assuming a number of discrete assessment points. In the resulting statistical model, random effects are included to account for between-patient variation in the intercept and the gradient of the patient-specific lines. In addition, models were fitted that included random effects for wave and arm (nested within wave) to take account of therapy group clustering effects.
Fixed covariates were included to model systematic differences due to treatment, assessment time point and participant characteristics. Time since randomisation was calculated in months and was centred by subtracting the overall (grand) mean of assessment times for each outcome measure. Centring makes the intercept interpretable when there is a treatment by time interaction.
Rather than fitting a model across baseline and follow-up responses, the baseline value of the outcome measure was included as a covariate. This is usually more efficient than including the baseline as a response variable and simplifies interpretation when there are several follow-up assessments. Using baseline as a covariate also reduces the problem of non-linearity that can occur if the change from baseline to the first follow-up assessment tends to be large compared with the change between subsequent assessments. In addition to treatment group and baseline measure, models included the fixed-effect covariates: centred time from randomisation to each assessment at 16-week intervals, sex and number of previous bipolar episodes. In addition to the covariates specified in the statistical analysis plan, region and the interaction between baseline value and centred time were also included to increase the precision of the treatment effect. Including the fixed effects given above, up to four models were fitted to each outcome: both fixed-effect treatment by time interaction (F) and random effects for wave and arm (R) included for model 1; only R included for model 2; only F for model 3; and neither were used for model 4.
For those scales (or subscales) on which higher scores represent a ‘worse state’, a negative gradient over time represents improvement. When higher scores represent a ‘better state’, a positive gradient represents improvement over time. A non-zero time–treatment interaction corresponds to differences between treatments in the rate of improvement or worsening over time. To test for a time–treatment interaction, the selected model with the interaction (model 1 or 3) was compared with the corresponding model without the term (model 2 or 4, respectively) using a likelihood ratio test (LRT). If the interaction was not significant, then this term was omitted and differences in mean level over time were reported corresponding to a systematic difference between treatments in the follow-up.
The following sequence of steps was followed to select a model for each continuous outcome:
-
If the treatment by time interaction from model 1 was statistically significant at the 5% level using a LRT, then this model was selected.
-
Otherwise, model 2 was selected.
-
If model 2 could not be fitted (SCID mania), then models 3 and 4 were compared using a LRT; if the treatment by time interaction from model 3 was statistically significant at the 5% level using a LRT, then this model was selected.
For each outcome, the ICC was calculated using the random-effect variances and residual variance estimates. Based on the selected model, normal probability plots and histograms were used to check the distributional assumptions for residuals of within- and between-subject variance terms. Statistical analyses were carried out using Stata® release 13.1 (StataCorp LP, College Station, TX, USA).
Qualitative methods
A nested qualitative study was conducted to explore the experiences of group participants and identify facilitators of, and barriers to, the widespread delivery of the intervention. A maximum variance sample of SUs who had been randomised to either the PEd or PS groups was recruited, including participants who had taken part in the full programme and those who had dropped out at different stages. We sought to ensure that the final qualitative sample had a varied range of clinical and demographic variables (sex, age, number of previous bipolar episodes, geographical location of group), thereby likely to include the range of experiences from participants in the trial.
Service users were approached following the completion of the programme and invited to take part in a single semistructured interview at a time and location of their choice. A researcher then interviewed consenting participants. The interviews followed a topic guide that provided a flexible framework for questioning and explored a number of areas including participants’ experiences and views of the groups, their expectations and motivations for participating, group content and process, key points of learning or interest, and any issues or challenges experienced. The topic guide was developed following a literature search and discussion with the PARADES SU research group and amended following pilot interviews with members of this group.
The interviewing researcher used a combination of open questions to elicit free responses, and focused questions to probe and prompt as necessary. The interviews were digitally audio-recorded and transcribed verbatim by a professional transcriber. The interviewer checked the quality and accuracy of the transcripts against the original audio-recording. At this stage, any identifying information (e.g. names and places) was removed.
The analysis proceeded in parallel with the interviews. Coding was informed by accumulating data and continuing thematic analysis. 91,92 An inductive and emergent approach was taken to the analysis, guided by the study’s core questions about participants’ experiences of the groups. The interviewing researcher open-coded the interview transcripts as they became available using an inductive approach, and researchers from different backgrounds (health and clinical psychology, psychiatry and SU) discussed the developing coding scheme. All team members read and coded a selection of transcripts, and at least two members of the team read all of the transcripts. Hence, all authors undertook the interpretation and coding of data and agreed the themes through discussion. Using a multidisciplinary team is a recognised method for increasing the trustworthiness of the analysis. 93 The thematic categories identified in the initial interviews were then tested or explored in subsequent interviews in which disconfirmatory evidence was sought. Themes were continuously compared against the data using a constant comparative approach. 94 The final sample size was determined by thematic saturation: the point at which new data are no longer contributing to the findings owing to participants’ repetition of themes and comments. 95 At this point, data generation was terminated.
Of the 304 participants in the trial, 68 were identified for interview, of whom 37 consented and completed an interview (18 from the PEd arm and 19 from the PS arm). Of the 31 participants who were not interviewed, all initially agreed to be contacted, were in receipt of the participant information sheet and had the opportunity to discuss the interview with the interviewing researcher. Seven participants decided at this point not to take part, 20 were unable to be contacted and four did not attend their arranged interview. Twenty-eight interviews were completed face to face and eight interviews were completed by telephone. Owing to an unavoidable interruption, one participant was interviewed in two parts, initially by telephone and subsequently face to face. The mean duration of SU interviews was 47.2 minutes (range 29–103 minutes). Interviews were also conducted with health professional facilitators (n = 14/15) and SUFs (n = 9/10) to explore their experiences of the groups; however, these are not reported here.
Quantitative results
Recruitment into the trial
Table 3 and Figure 1 show the flow of participants through the trial, with six waves of recruitment in the North West (at four different geographical sites) and five in the East Midlands (at four different geographical sites). The additional wave in the North West was required to meet the total sample size of 308 participants estimated by our power calculation; we recruited 304 participants, four participants short of the revised target, between June 2009 and January 2012. In each wave the total numbers of participants were individually randomised so that they had an equal chance of attending PEd or PS, and the procedure for recruitment, treatment and assessment were the same in both the North West and the East Midlands. Recruitment was carried out in two inner-city sites, three rural sites and three urban sites.
| Wave | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 |
| East Midlands | |||||
| Group PEd (number randomised = 14) | Group PEd (number randomised = 11) | Group PEd (number randomised = 11) | Group PEd (number randomised = 14) | Group PEd (number randomised = 17) | |
| Group PS (number randomised = 11) | Group PS (number randomised = 10) | Group PS (number randomised = 13) | Group PS (number randomised = 14) | Group PS (number randomised = 17) | |
| Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | |
| North West | |||||
| Group PEd (number randomised = 12) | Group PEd (number randomised = 17) | Group PEd (number randomised = 10) | Group PEd (number randomised = 17) | Group PEd (number randomised = 16) | Group PEd (number randomised = 14) |
| Group PS (number randomised = 12) | Group PS (number randomised = 15) | Group PS (number randomised = 11) | Group PS (number randomised = 17) | Group PS (number randomised = 16) | Group PS (number randomised = 15) |
| Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) | Minimise for number of previous bipolar episodes (≤ 7, 8–19 and ≥ 20) |
| 49 recruited in total in wave 1 | 53 recruited in total in wave 2 | 45 recruited in total in wave 3 | 62 recruited in total in wave 4 | 66 recruited in total in wave 5 | 29 recruited in total in wave 6 |
FIGURE 1.
Flow chart of participants into the study from all sites and waves combined.
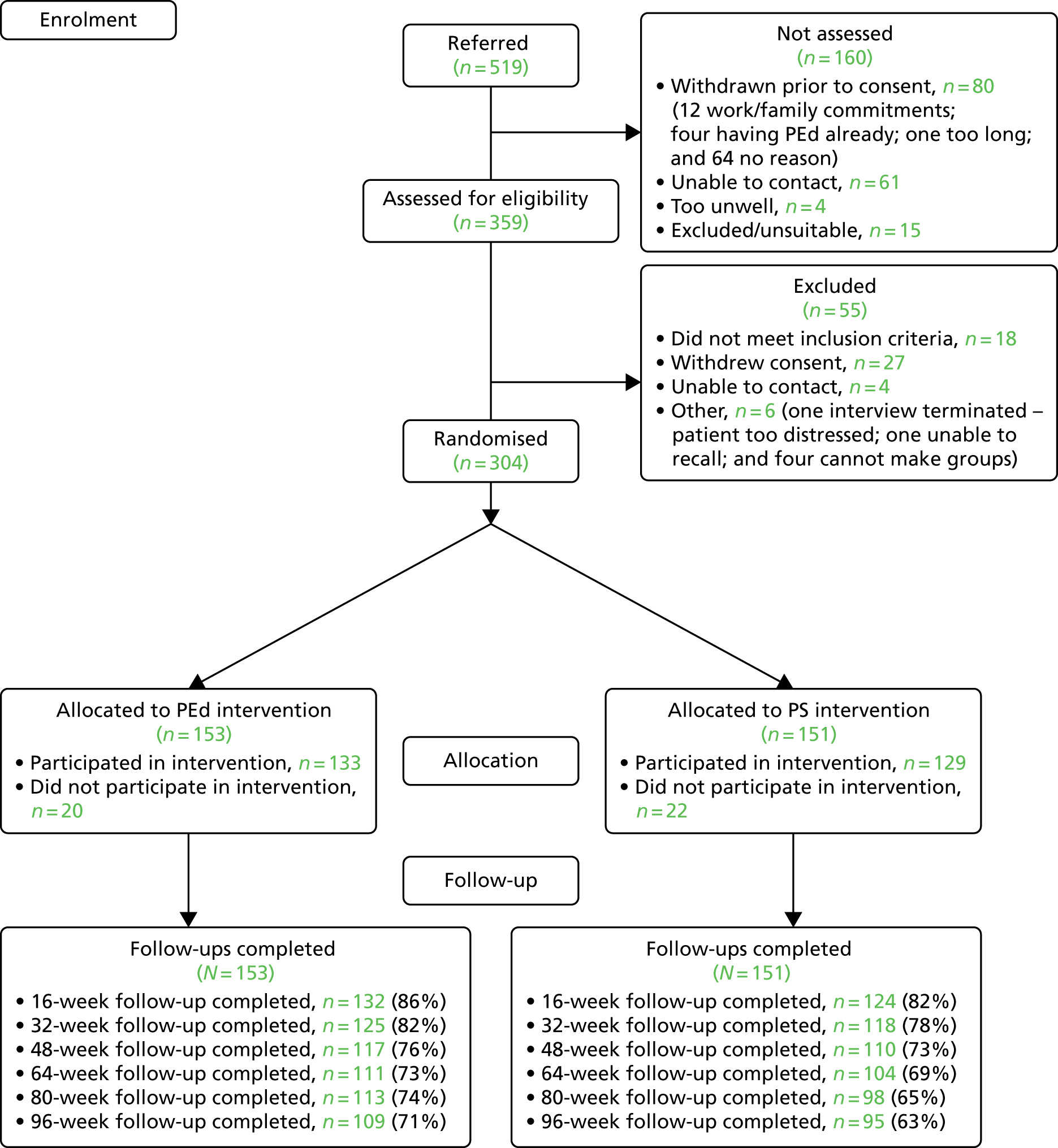
Three hundred and four (84.7%) participants were recruited into the study. In addition, 27 people who otherwise met the inclusion criteria of the study refused to give consent and six further people were unable to commit to the groups for practical reasons (e.g. time off work); 22 people were either not contactable or did not meet inclusion criteria on assessment. Two hundred and forty-three (79.9%) people completed the first follow-up point after completing treatment; and 204 (67.1%) completed interview assessments at the 96-week follow-up. Three participants were missing baseline assessments, although they had an assessment of eligibility and consented to take part. A further four subjects had baseline assessments taken after randomisation, so these data are not included in the summary statistics and analyses. Data on all bipolar relapses, mania and depression relapses were completed using primary care and secondary care records.
Four participants died during the follow-up period of the study from natural causes that were deemed by an independent TSC and Data Monitoring and Ethics Committee to be unrelated to the interventions or procedures in the trial.
Baseline characteristics of treatment groups
Table 4 shows the baseline characteristics by treatment group. The study recruited a predominantly white British sample that contained more women than men, and a wide age range from 21 to 73 years. There were some baseline differences between the groups, with the PEd group being, on average, slightly younger [PEd mean age = 44.2 years, standard deviation (SD) = 11.1 years; PS mean age = 46.5 years, SD = 11.4 years] and more likely to be married and including fewer people with bipolar 1 disorder. Only 24 (7.9%) participants were not white British; 10 were black, seven were of mixed race, three were South Asian, three were white non-British and one was Chinese. Ethnicity was unavailable for two participants. Also of note was that the majority of participants had children. Just over one-quarter of participants were employed. Forty per cent of the sample expressed no preference for either treatment group, but more people expressed a preference for PEd over PS, although all were willing to be randomised to either group. Table 4 also shows that the groups did not differ on psychiatric comorbidity, with around 70% reporting psychotic symptoms in their lifetime, 40% describing a current anxiety disorder, just over 40% reporting an alcohol abuse or dependence problem in their lifetime and just under 10% with a borderline or antisocial personality disorder. Nearly three-quarters of the participants in both groups were taking mood-stabilising medication, although the proportion doing so was slightly higher in the PS group than in the PEd group. Similar numbers from both groups were receiving antipsychotic (54–55%) and antidepressant (46–47%) medication.
| Characteristic | Treatment group, n (%) | |
|---|---|---|
| PEd (N = 153) | PS (N = 151) | |
| Sex: female | 92 (60.1) | 85 (56.3) |
| Number of previous bipolar episodes | ||
| 1–7 | 21 (13.7) | 18 (11.9) |
| 8–19 | 50 (32.7) | 45 (29.8) |
| ≥ 20 | 82 (53.6) | 88 (58.3) |
| Bipolar 1 | 114 (74.5) | 129 (85.4) |
| Marital status | ||
| Married/living as married | 63 (41.2) | 46 (30.5) |
| Never married | 46 (30.1) | 53 (35.1) |
| Divorced/separated/widowed | 44 (28.7) | 52 (34.4) |
| With children, one or more | 90 (58.8) | 93 (61.6) |
| Employment | ||
| In full-time/part-time work | 45 (29) | 37 (24) |
| Unemployed/sickness/retired | 108 (71) | 114 (76) |
| Ethnicity: white British | 140 (91.5) | 138 (91.4) |
| Region | ||
| North West | 86 (56.2) | 86 (56.9) |
| East Midlands | 67 (43.8) | 65 (43.1) |
| Group preference | ||
| Prefer PEd | 55 (35.9) | 54 (35.8) |
| No preference | 66 (43.1) | 57 (37.7) |
| Prefer PS | 29 (19.0) | 35 (23.2) |
| Missing | 3 (2.0) | 5 (3.3) |
| Psychosis, lifetime | 97 (63.3) | 114 (75.5) |
| Any anxiety disorder | ||
| Lifetime | 87 (56.9) | 75 (49.7) |
| Past month | 68 (44.4) | 57 (37.7) |
| Any alcohol abuse/dependence | ||
| Lifetime | 56 (36.6) | 61 (40.3) |
| Past month | 4 (2.6) | 12 (7.9) |
| Any drug abuse/dependence | ||
| Lifetime | 10 (6.5) | 21 (13.9) |
| Past month | 2 (1.3) | 2 (1.3) |
| Borderline/antisocial personality disorder | 12 (7.8) | 13 (9) |
| Baseline medication | ||
| Mood stabiliser | 108 (70.5) | 118 (78.1) |
| Antipsychotic drugs | 83 (54.2) | 84 (55.6) |
| Antidepressant drugs | 71 (46.4) | 71 (47.0) |
| Hypnotic/antianxiety | 21 (13.7) | 19 (12.6) |
| Baseline medication equivalences (mg/d) | ||
| Chlorpromazine | 188.2 | 182.8 |
| Imipramine | 83.6 | 80.4 |
| Diazepam | 2.25 | 2.43 |
Tables 5–7 summarise the trial secondary outcome measures at baseline and follow-up assessments. These show that, symptomatically and functionally, there were no clinically important differences between the groups at baseline. Symptomatically, participants scored on average just below the clinical threshold for observed depression symptoms on the HAM-D, slightly above the minimum clinical threshold for depression and anxiety symptoms on self-rated measures, with more severe anxiety than depression, and minimal mania symptoms at baseline. Consistent with the inclusion criteria, although a few participants scored above the symptomatic threshold score for depression for 1 week, the duration of symptoms meant that they did not meet the criteria for a major depressive episode. No participants met the criteria for a mania-type episode at baseline. On average, participants showed no more than slight impairment in social and occupational functioning overall (SOFAS and overall SAS) and across all domains (performance, interpersonal, friction and dependency).
| Time from baseline (weeks) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEd (n = 153) | PS (n = 151) | |||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | |
| SCID LIFE weekly mean score for depression over 16-week period | ||||||||||||
| 0 | 1.83 | 0.85 | 1.75 | 1.00 | 4.00 | 152 | 1.84 | 0.87 | 1.75 | 1.00 | 4.25 | 148 |
| 16 | 1.84 | 0.88 | 1.58 | 1.00 | 4.87 | 131 | 2.00 | 1.08 | 1.59 | 1.00 | 5.92 | 123 |
| 32 | 2.18 | 1.14 | 1.91 | 1.00 | 5.18 | 124 | 2.00 | 1.01 | 1.80 | 1.00 | 6.00 | 118 |
| 48 | 1.89 | 1.07 | 1.56 | 1.00 | 5.88 | 117 | 1.94 | 1.03 | 1.69 | 1.00 | 6.00 | 111 |
| 64 | 1.83 | 1.05 | 1.44 | 1.00 | 6.00 | 113 | 2.00 | 1.05 | 1.73 | 1.00 | 5.00 | 105 |
| 80 | 1.85 | 1.05 | 1.41 | 1.00 | 5.50 | 114 | 2.06 | 1.00 | 1.82 | 1.00 | 4.75 | 98 |
| 96 | 1.84 | 1.15 | 1.25 | 1.00 | 5.39 | 107 | 1.94 | 1.00 | 1.59 | 1.00 | 5.24 | 98 |
| SCID LIFE weekly mean score for mania over 16-week period | ||||||||||||
| 0 | 1.20 | 0.48 | 1.00 | 1.00 | 4.50 | 152 | 1.28 | 0.54 | 1.00 | 1.00 | 4.00 | 148 |
| 16 | 1.24 | 0.41 | 1.00 | 1.00 | 3.63 | 131 | 1.27 | 0.49 | 1.00 | 1.00 | 4.00 | 123 |
| 32 | 1.22 | 0.44 | 1.00 | 1.00 | 3.63 | 124 | 1.28 | 0.44 | 1.00 | 1.00 | 3.00 | 118 |
| 48 | 1.17 | 0.42 | 1.00 | 1.00 | 3.88 | 117 | 1.24 | 0.43 | 1.00 | 1.00 | 3.33 | 111 |
| 64 | 1.22 | 0.59 | 1.00 | 1.00 | 4.60 | 113 | 1.30 | 0.61 | 1.00 | 1.00 | 5.00 | 105 |
| 80 | 1.23 | 0.59 | 1.00 | 1.00 | 5.00 | 114 | 1.16 | 0.33 | 1.00 | 1.00 | 2.78 | 98 |
| 96 | 1.21 | 0.46 | 1.00 | 1.00 | 3.50 | 107 | 1.36 | 0.73 | 1.00 | 1.00 | 4.36 | 98 |
| HAM-D | ||||||||||||
| 0 | 6.59 | 5.18 | 7.00 | 0 | 27 | 152 | 6.17 | 5.00 | 5.00 | 0 | 26 | 145 |
| 16 | 7.04 | 7.15 | 4.50 | 0 | 30 | 131 | 7.58 | 6.29 | 6.63 | 0 | 24 | 123 |
| 32 | 7.00 | 7.17 | 5.00 | 0 | 35 | 122 | 8.18 | 7.26 | 6.00 | 0 | 33 | 119 |
| 48 | 6.09 | 6.91 | 4.00 | 0 | 32 | 117 | 7.19 | 7.71 | 5.00 | 0 | 36 | 111 |
| 64 | 6.73 | 6.71 | 4.38 | 0 | 29 | 111 | 7.62 | 6.49 | 6.00 | 0 | 34 | 104 |
| 80 | 5.69 | 6.09 | 3.63 | 0 | 32 | 112 | 7.08 | 7.25 | 5.00 | 0 | 30 | 98 |
| 96 | 6.39 | 6.04 | 5.00 | 0 | 28 | 107 | 7.07 | 7.33 | 5.00 | 0 | 36 | 98 |
| MAS | ||||||||||||
| 0 | 1.84 | 2.40 | 1.00 | 0 | 13 | 152 | 2.38 | 2.84 | 1.00 | 0 | 13 | 145 |
| 16 | 2.14 | 3.08 | 1.00 | 0 | 15 | 131 | 2.16 | 3.25 | 1.00 | 0 | 17 | 123 |
| 32 | 1.80 | 2.82 | 1.00 | 0 | 18 | 122 | 1.80 | 2.61 | 1.00 | 0 | 14 | 119 |
| 48 | 1.21 | 2.12 | 0.00 | 0 | 11 | 117 | 2.17 | 3.50 | 1.00 | 0 | 20 | 111 |
| 64 | 1.83 | 2.77 | 1.00 | 0 | 15 | 111 | 2.03 | 3.39 | 1.00 | 0 | 18 | 104 |
| 80 | 1.84 | 3.02 | 1.00 | 0 | 16 | 112 | 1.33 | 1.84 | 1.00 | 0 | 11 | 98 |
| 96 | 1.88 | 3.53 | 1.00 | 0 | 18 | 107 | 1.84 | 4.14 | 0.00 | 0 | 30 | 98 |
| Time from baseline (weeks) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEd (n = 153) | PS (n = 151) | |||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | |
| SOFAS | ||||||||||||
| 0 | 75.8 | 12.2 | 80 | 40 | 91 | 143 | 76.5 | 12.3 | 80 | 41 | 91 | 137 |
| 32 | 72.5 | 13.2 | 71 | 41 | 94 | 121 | 71.9 | 13.1 | 71 | 21 | 94 | 113 |
| 64 | 74.1 | 14.3 | 80 | 11 | 91 | 110 | 74.0 | 12.3 | 80 | 40 | 95 | 99 |
| 96 | 75.4 | 13.5 | 80 | 41 | 100 | 106 | 73.1 | 14.5 | 72 | 32 | 95 | 96 |
| SAS: overall | ||||||||||||
| 0 | 1.92 | 0.55 | 1.83 | 1.00 | 4.25 | 146 | 1.94 | 0.53 | 1.83 | 1.00 | 3.86 | 139 |
| 32 | 2.00 | 0.66 | 1.86 | 1.08 | 3.86 | 121 | 1.98 | 0.52 | 1.86 | 1.14 | 3.45 | 114 |
| 64 | 1.97 | 0.74 | 1.80 | 1.00 | 5.00 | 109 | 2.05 | 0.55 | 2.00 | 1.10 | 3.67 | 102 |
| 96 | 1.91 | 0.63 | 1.83 | 1.00 | 4.14 | 104 | 2.09 | 0.73 | 2.00 | 1.11 | 4.71 | 96 |
| SAS: performance | ||||||||||||
| 0 | 2.23 | 0.80 | 2.00 | 1.00 | 5.00 | 146 | 2.31 | 0.82 | 2.17 | 1.00 | 5.00 | 139 |
| 32 | 2.36 | 0.85 | 2.33 | 1.00 | 5.00 | 121 | 2.38 | 0.89 | 2.15 | 1.00 | 4.50 | 114 |
| 64 | 2.30 | 0.88 | 2.14 | 1.00 | 5.00 | 109 | 2.37 | 0.83 | 2.27 | 1.00 | 5.00 | 102 |
| 96 | 2.31 | 0.94 | 2.15 | 1.00 | 5.00 | 104 | 2.42 | 0.96 | 2.21 | 1.00 | 5.00 | 96 |
| SAS: interpersonal | ||||||||||||
| 0 | 1.70 | 0.60 | 1.62 | 1.00 | 3.67 | 146 | 1.77 | 0.58 | 1.67 | 1.00 | 3.33 | 139 |
| 32 | 1.81 | 0.70 | 1.67 | 1.00 | 5.00 | 121 | 1.73 | 0.69 | 1.67 | 1.00 | 4.00 | 114 |
| 64 | 1.64 | 0.61 | 1.50 | 1.00 | 4.67 | 109 | 1.73 | 0.63 | 1.57 | 1.00 | 3.33 | 102 |
| 96 | 1.52 | 0.55 | 1.33 | 1.00 | 3.67 | 104 | 1.75 | 0.76 | 1.43 | 1.00 | 5.00 | 96 |
| SAS: friction | ||||||||||||
| 0 | 1.51 | 0.59 | 1.33 | 1.00 | 4.00 | 146 | 1.54 | 0.54 | 1.50 | 1.00 | 3.50 | 139 |
| 32 | 1.59 | 0.72 | 1.33 | 1.00 | 4.00 | 121 | 1.57 | 0.61 | 1.33 | 1.00 | 3.50 | 114 |
| 64 | 1.56 | 0.78 | 1.33 | 1.00 | 5.00 | 109 | 1.61 | 0.58 | 1.50 | 1.00 | 3.00 | 102 |
| 96 | 1.50 | 0.67 | 1.25 | 1.00 | 5.00 | 104 | 1.70 | 0.75 | 1.50 | 1.00 | 4.50 | 96 |
| SAS: dependency | ||||||||||||
| 0 | 1.86 | 0.92 | 1.75 | 1.00 | 5.00 | 146 | 2.05 | 1.08 | 2.00 | 1.00 | 5.00 | 138 |
| 32 | 2.07 | 1.12 | 2.00 | 1.00 | 5.00 | 120 | 2.03 | 1.25 | 1.75 | 1.00 | 5.00 | 112 |
| 64 | 1.80 | 0.98 | 1.50 | 1.00 | 5.00 | 107 | 2.04 | 1.17 | 1.50 | 1.00 | 5.00 | 102 |
| 96 | 1.49 | 0.62 | 1.00 | 1.00 | 4.00 | 103 | 1.62 | 0.74 | 1.50 | 1.00 | 5.00 | 95 |
| Time from baseline (weeks) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEd (N = 153) | PS (N = 151) | |||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | |
| HADS-A | ||||||||||||
| 0 | 9.63 | 4.93 | 9.50 | 0.00 | 21.00 | 130 | 9.70 | 4.86 | 10.00 | 0.00 | 20.00 | 125 |
| 32 | 9.20 | 5.10 | 9.00 | 1.00 | 20.00 | 66 | 10.35 | 4.57 | 10.00 | 0.00 | 20.00 | 75 |
| 64 | 9.38 | 5.41 | 10.00 | 0.00 | 21.00 | 66 | 10.75 | 4.95 | 11.00 | 0.00 | 20.00 | 60 |
| 96 | 9.67 | 4.84 | 9.00 | 1.00 | 18.00 | 51 | 9.21 | 5.14 | 8.08 | 0.00 | 21.00 | 52 |
| HADS: depression | ||||||||||||
| 0 | 8.24 | 4.78 | 8.00 | 0.00 | 19.00 | 130 | 8.22 | 5.29 | 8.00 | 0.00 | 21.00 | 125 |
| 32 | 7.12 | 4.92 | 7.00 | 0.00 | 21.00 | 66 | 8.37 | 4.81 | 8.00 | 0.00 | 19.00 | 75 |
| 64 | 6.98 | 5.13 | 6.50 | 0.00 | 20.00 | 66 | 8.85 | 4.98 | 9.00 | 0.00 | 21.00 | 60 |
| 96 | 7.46 | 5.68 | 6.00 | 0.00 | 21.00 | 51 | 7.58 | 5.40 | 7.00 | 0.00 | 19.00 | 52 |
| SF-12: mental component score | ||||||||||||
| 0 | 37.0 | 12.1 | 34.8 | 13.6 | 63.2 | 128 | 36.1 | 12.1 | 33.4 | 12.8 | 63.7 | 120 |
| 32 | 37.5 | 11.7 | 37.6 | 15.3 | 62.8 | 66 | 36.6 | 10.9 | 34.0 | 14.1 | 57.3 | 75 |
| 64 | 37.9 | 13.2 | 35.4 | 13.0 | 66.7 | 66 | 35.0 | 11.1 | 33.5 | 16.1 | 61.0 | 60 |
| 96 | 38.9 | 12.8 | 36.6 | 12.1 | 60.3 | 49 | 37.1 | 11.4 | 34.1 | 19.2 | 60.6 | 52 |
| SF-12: physical component score | ||||||||||||
| 0 | 43.8 | 11.0 | 43.8 | 17.6 | 63.4 | 128 | 46.1 | 11.5 | 47.2 | 18.6 | 65.9 | 120 |
| 32 | 43.1 | 12.3 | 43.6 | 18.0 | 62.7 | 66 | 43.0 | 12.3 | 44.8 | 15.8 | 64.5 | 75 |
| 64 | 43.4 | 11.6 | 44.8 | 15.5 | 62.1 | 66 | 42.0 | 12.0 | 42.6 | 13.3 | 64.4 | 60 |
| 96 | 44.2 | 12.3 | 44.4 | 16.8 | 62.0 | 49 | 42.1 | 11.5 | 40.8 | 21.8 | 63.8 | 52 |
Attendance at the groups
The distribution of the number of sessions attended by PEd and PS group is presented in Table 8. The proportions who did not attend any group sessions of PEd and PS were similar (13% and 15%, respectively). The median number of sessions attended was 14 in the PEd group, compared with nine in the PS group. Overall, rates of attendance were higher for PEd groups than for the PS groups (Mann–Whitney U test z = 2.23; p = 0.026). With evidence of overdispersion, a negative binomial model for count data was used to investigate selected baseline factors that could potentially influence attendance. The following variables were included in the model (p-values given in brackets): sex (p = 0.888); number of previous episodes (1–7 vs. ≥ 20, p = 0.761; 8–19 vs. ≥ 20, p = 0.926); bipolar 1 disorder versus bipolar 2 disorder status (p = 0.289); group preference (none vs. prefer PEd; p = 0.779; prefer PS vs. prefer PEd p = 0.517); arm (p = 0.223); and wave (p-values ranged from 0.415 to 0.974). As shown from the above, none of these differences was significant.
| Sessions attended | Treatment group | |||||
|---|---|---|---|---|---|---|
| PEd (N = 153) | PS (N = 151) | |||||
| n | % each category | Cumulative % | n | % each category | Cumulative % | |
| 0 | 20 | 13.1 | – | 22 | 14.6 | – |
| 1–5 | 25 | 16.3 | 86.9 | 41 | 27.2 | 85.4 |
| 6–10 | 12 | 7.8 | 70.6 | 17 | 11.3 | 58.2 |
| 11–15 | 29 | 19.0 | 62.8 | 25 | 16.6 | 46.9 |
| 16–21 | 67 | 43.8 | 43.8 | 46 | 30.5 | 30.5 |
Response rates and missing data
Logistic models with a random effect for wave and arm (nested within wave) to take account of therapy group clustering effects were fitted to examine factors potentially predictive of missing primary depression outcome data at weeks 32 (over the treatment period) and 96 (over the whole follow-up period). The same covariates (i.e. sex, number of previous episodes, arm, baseline mania score and baseline depression score) were used for each model. None of these factors was predictive of non-response on the SCID LIFE at weeks 32 and 96. Sex, number of previous episodes and baseline score were specified as covariates in the statistical analysis plan for the LME analyses to increase the precision of estimates. No follow-up SCID LIFE data were available for a total of 14 (9%) participants in the PEd arm, compared with 21 (14%) in the PS arm.
Reliability and quality assurance
Ten unblindings occurred because the participant divulged their group allocation to their RA (three in PEd group and seven in the PS group). In seven cases this occurred during follow-up interview. One participant wrote their group allocation on self-completion paperwork, one telephoned the RA to change their assessment date and divulged their group allocation, and one RA overheard a conversation from the therapy team that unblinded them. When unblinding occurred during an interview, an alternative RA rated the SCID LIFE codings to avoid bias. For all seven participants, an unblinded RA was assigned for their remaining follow-ups.
An inter-rater reliability (IRR) study was carried out of the weekly SCID LIFE assessment in which scores of 1–6 were assigned for each of mania and depression. An ICC was determined for the depression and mania scores separately and percentile CIs were determined using a bootstrap resampling by subject. Six raters made 292 assessments of 17 subjects, with a mean of 17.2 assessments of each subject and 2.35 assessments per time point. The ICC was 0.71 (95% CI 0.64 to 0.85) for depression scores and 0.57 (95% CI 0.40 to 0.83) for mania scores, indicating a moderate level of inter-rater agreement. 96 There were 24 bipolar relapses in the IRR exercise (18 with depression-type and six with mania-type relapses). For the combined depression and mania outcomes, the ICC was 0.56 (95% CI 0.22 to 0.89).
Episode recurrence
Of the 153 participants in the PEd arm, 89 (58%) had a recurrence of an episode of depression or mania type during the 96-week follow-up. In the PS arm, 98 (65%) had a recurrent episode. Of the first recurrences, 75% in the PEd arm and 63% in the PS arm were depressive episodes. When the Cox models with and without robust standard errors were compared, there was no evidence of a clustering effect (as the ratio of the square of the standard errors of the models with and without a cluster term was less than one), and so the latter are presented. A Kaplan–Meier plot for time to first recurrence in the two groups is shown in Figure 2. The estimated median time to first bipolar relapse was 67.1 (95% CI 37.3 to 90.9) weeks in the PEd group, compared with 48.0 (95% CI 30.6 to 65.9) weeks in the PS group, a difference of 19.1 weeks. When the Cox model was fitted, the adjusted HR was 0.83 (95% CI 0.62 to 1.11; LRT p = 0.217). Male participants tended to show a greater improvement than female participants in terms of time to relapse (HR 0.74; p = 0.052). There was also a non-significant (p = 0.094) trend for greater beneficial effects among SUs, with few bipolar episodes prior to joining the trial (8–19 vs. ≥ 20, HR 0.74; p = 0.073; 1–7 vs. ≥ 20 HR, 0.67; p = 0.105). The moderating effect of episode pre trial is considered below.
FIGURE 2.
Kaplan–Meier estimates of time to first depression- or mania-type bipolar episode.

In a sensitivity analysis we additionally controlled for baseline differences between the groups in terms of the proportion of bipolar 2 versus bipolar 1, marital status and taking mood stabilisers (lithium, sodium valproate, carbamazepine and/or lamotrigine). When these covariates were included together, the intervention effect on relapse remained non-significant (LRT p = 0.294).
The proportion of participants with a first mania-type episode was lower in the PEd group than in the PS group [n = 39 (25%) vs. n = 53 (35%)]. As so few participants relapsed with mania over 2 years in both groups, we present the 15th centile time to relapse rather than the median time to relapse, which was 44.6 (95% CI 27.3 to 73.6) weeks after baseline in the PEd group and 21.7 (95% CI 13.1 to 39.3) weeks after baseline in the PS group. Figure 3 shows the Kaplan–Meier estimates for the two groups. There was evidence that the time to first relapse was delayed in the PEd arm compared with the PS arm (HR 0.66, 95% CI 0.44 to 1.002; LRT p = 0.049). When bipolar 1, marital status and the proportion taking mood stabilisers were added in a sensitivity analysis, the HR increased to 0.74 (95% CI 0.48 to 1.13; LRT p = 0.165); bipolar 1 and taking mood stabilisers increased the HR, whereas married/living as married decreased the risk of relapse, but all three were non-significant.
FIGURE 3.
Kaplan–Meier estimates of time to first mania-type bipolar episode.
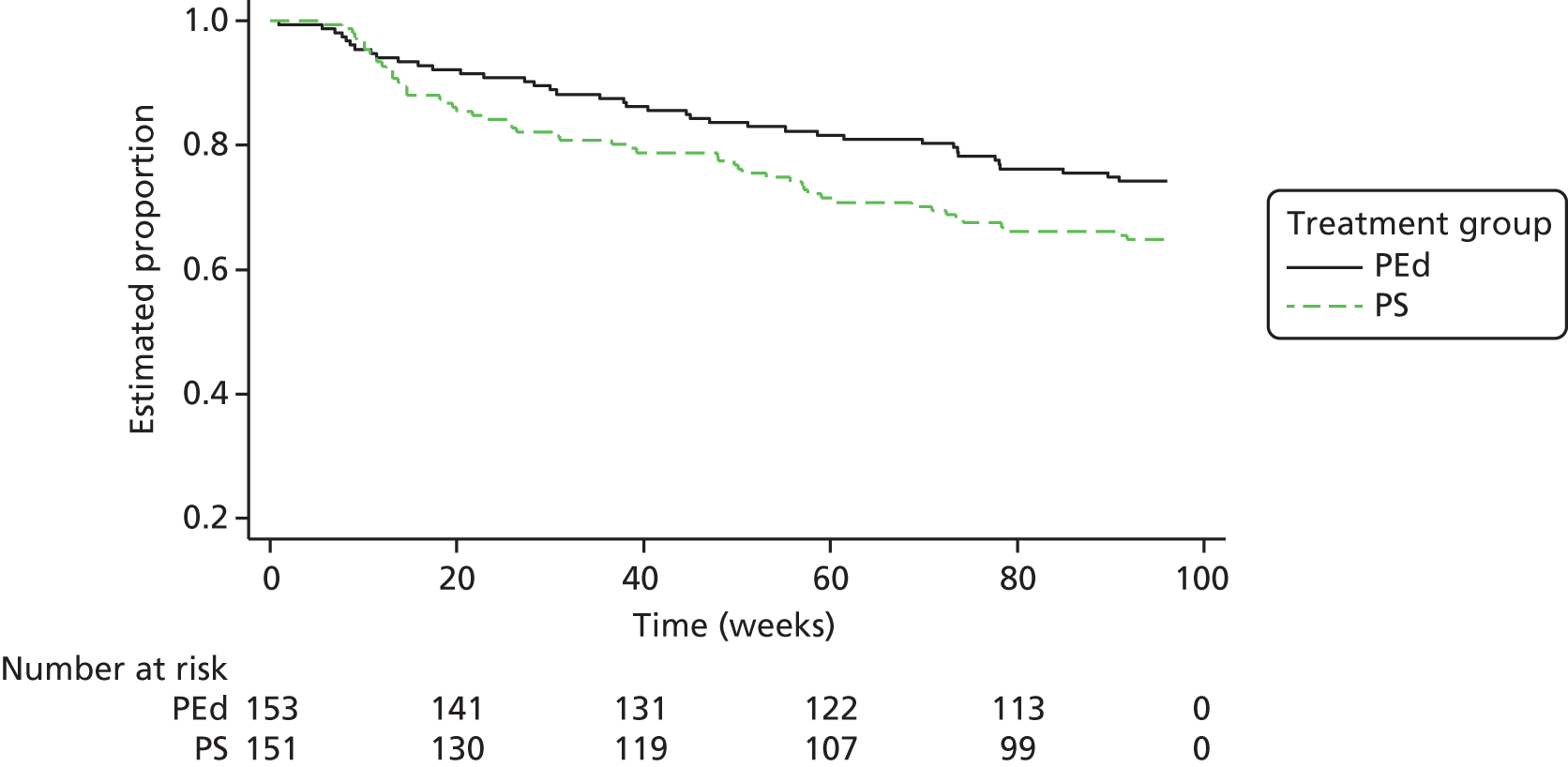
A total of 80 (52%) subjects in the PEd arm had a first depressive episode, compared with 82 (54%) in the PS arm. As so few participants relapsed with depression, we report the 25th centile time to relapse, which was 22.0 (95% CI 14.9 to 29.3) weeks after baseline in the PEd group and 19.4 (95% CI 14.6 to 27.6) weeks after baseline in the PS group. The Kaplan–Meier estimates comparing the two groups are shown in Figure 4. There was no evidence of a difference between the groups, with an estimated HR of 0.96 (95% CI 0.70 to 1.31; LRT p = 0.784). Adjusting for bipolar 1, marital status and the proportion taking mood stabilisers, covariates had little impact on the treatment effect (LRT p = 0.682).
FIGURE 4.
Kaplan–Meier estimates of time to first depression-type bipolar episode.
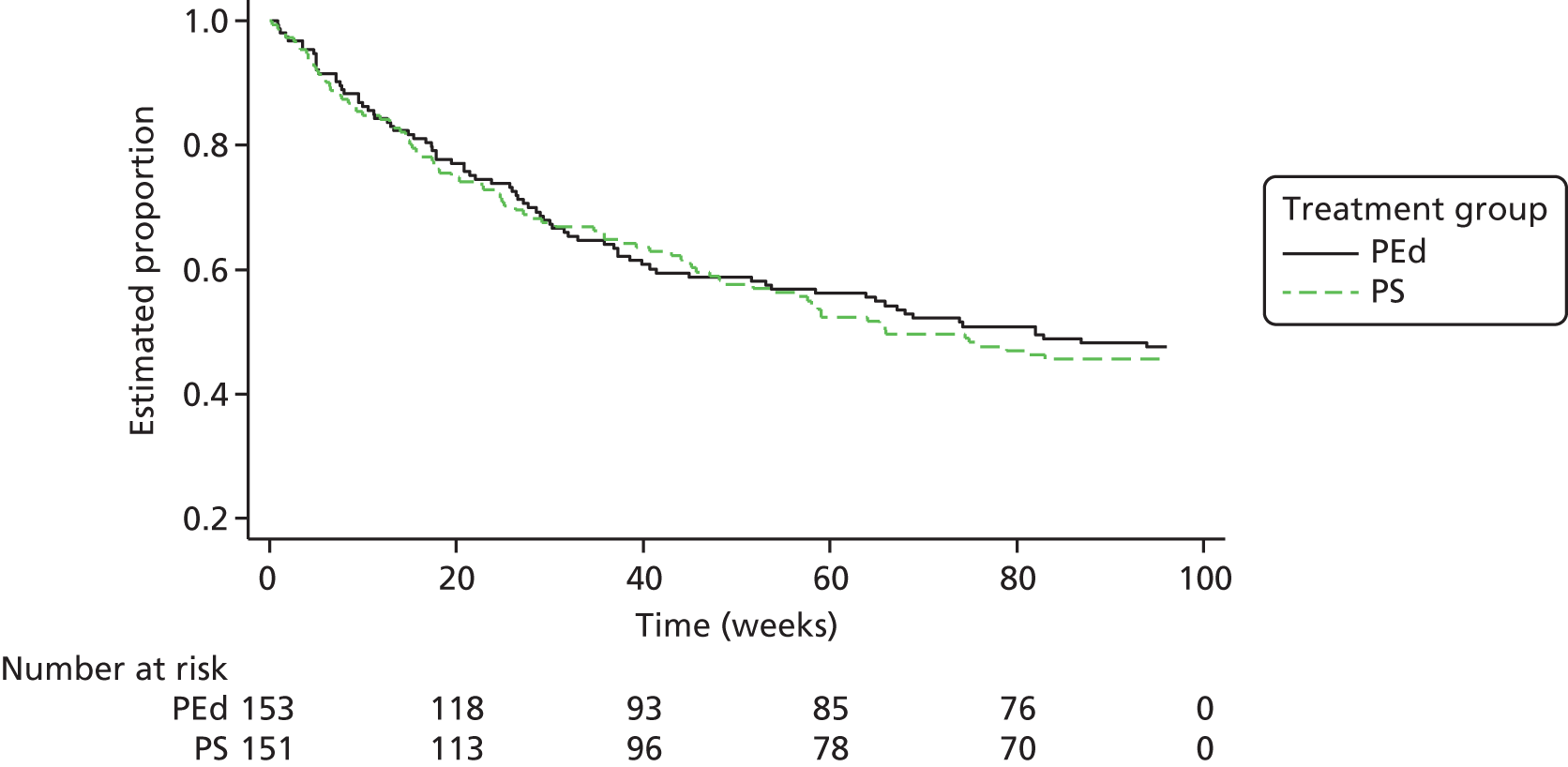
Post-treatment benefit
Theoretically, PEd may be more effective after the treatment period than PS because it explicitly develops a staying well plan for each participant, whereas both PEd and PS provide information and mutual support during the treatment period when the groups meet. PEd aims to teach participants in the group specific evidence-based methods to recognise and respond to the early stages of mania and depression relapse compared with PS with likely impact in the follow-up period after treatment is completed. Therefore, we decided to run an analysis separating the effects of the interventions during the treatment period and then those during the follow-up period. On inspection of the data on time to first relapse, we decided to carry out this analysis on time to the next bipolar episode and time to the next mania episode but not on time to the next depression episode, as overall it was clear that there were no differences between the two groups. For each wave we calculated the duration in weeks between the earliest randomisation date and the final date of the last session (i.e. session 21). The maximum was 26 weeks. We therefore used ≥ 27 weeks post randomisation as the start point for ‘off treatment’. A Kaplan–Meier plot and Cox analysis for the primary outcome (time to first depressive or mania relapse) were performed on data < 27 weeks. Excluding one subject who was censored at week 25, the data for the remaining 303 participants were then analysed from 27 weeks onwards (the ‘post-treatment’ period) up to 96 weeks. All failure (when a participant has a first relapse in the specified period) and censor times were rescaled by subtracting 27 weeks, so that the time origin was set to zero. The same covariates as for the main analysis were used for both Cox models. This was repeated for the mania outcome.
In the ‘on-treatment’ period, a total of 51 (33%) in the PEd arm had a first bipolar (mania-type or depression) relapse, compared with 60 (40%) in the PS arm. There was no evidence of a difference between the groups, with an estimated HR of 0.81 (95% CI 0.56 to 1.19; LRT p = 0.286). In the ‘off-treatment’ period, a total of 75 (49%) in the PEd arm had a ‘first’ bipolar relapse, compared with 81 (54%) on the PS arm. There was no evidence of a difference between the groups, with an estimated HR of 0.91 (95% CI 0.66 to 1.26; LRT p = 0.578).
Considering mania alone, during the in-treatment period, a total of 14 (9%) in the PEd arm had a first relapse, compared with 27 (18%) in the PS arm. There was evidence of a benefit for the PEd group, with an estimated HR of 0.49 (95% CI 0.26 to 0.94; LRT p = 0.028). In the off-treatment period, a total of 30 (20%) in the PEd arm had a first mania relapse, compared with 38 (25%) in the PS arm. There was no evidence of delaying relapse in the PEd arm, with an estimated HR of 0.75 (95% CI 0.47 to 1.22; LRT p = 0.246), suggesting that the differences between the groups largely emerged during the treatment period and did not continue to widen during the follow-up period.
Symptom and functioning outcomes
Table 5 gives summary statistics for the secondary outcome interview-measured symptom measures. Overall, 205 participants (67.4%) completed these measures by 96 weeks, with increasing attrition over follow-up from baseline. The mean depression and mania scores were based on each participant’s mean score over the previous 16 weeks (last 4 weeks for baseline). As shown in Table 5, the mean scores on SCID LIFE depression, mania, MAS, HAM-D and HADS depression and Hospital Anxiety and Depression Scale – anxiety subscale (HADS-A) were mostly flat over time. However, there was a small increase in the SCID LIFE depression score from 1.84 at 16 weeks to a maximum of 2.18 at week 32 when the group finished, and a fall back to 1.89 in the PEd arm. These changes were not seen in the PS group. However, all of these changes are too small to be of clinical importance and the increase was not mirrored in the HAM-D during the same time period.
Table 9 shows the LME analyses for observer-rated depression and mania symptom outcomes, with no statistically significant interaction between treatment arm and time, indicating that there was no significant difference between the two treatment groups in rate of improvement over time. There was little, if any, evidence of variation between therapy groups, with the ICC for therapy group within wave being either zero or negligible.
| Secondary outcome measure | Treatment effecta | 95% CI | p-value | ICC |
|---|---|---|---|---|
| SCID depression | ||||
| M1b time–treatment interaction | –0.004 | –0.020 to 0.012 | 0.652 | 0.000 |
| M2 treatment | –0.045 | –0.210 to 0.121 | 0.597 | 0.000 |
| SCID mania | ||||
| M3 time–treatment interaction | –0.002 | –0.010 to 0.007 | 0.697 | 0.000 |
| M4 treatment | –0.046 | –0.109 to 0.018 | 0.157 | 0.000 |
| SOFAS | ||||
| M1 time–treatment interaction | 0.082 | –0.178 to 0.342 | 0.536 | 0.007 |
| M2 treatment | 0.752 | –1.800 to 3.305 | 0.564 | 0.007 |
| SAS overall | ||||
| M1 time–treatment interaction | –0.008 | –0.020 to 0.004 | 0.176 | 0.000 |
| M2 treatment | –0.027 | –0.145 to 0.092 | 0.659 | 0.000 |
| SAS performance | ||||
| M1 time–treatment interaction | –0.004 | –0.019 to 0.011 | 0.612 | 0.000 |
| M2 treatment | –0.013 | –0.170 to 0.143 | 0.869 | 0.000 |
| SAS interpersonal | ||||
| M1 time–treatment interaction | –0.017 | –0.030 to –0.004 | 0.012 | 0.000 |
| M1 treatment | –0.037 | –0.156 to 0.082 | 0.544 | 0.000 |
| SAS friction | ||||
| M1 time–treatment interaction | –0.0097 | –0.022 to 0.004 | 0.174 | 0.000 |
| M2 treatment | –0.014 | –0.141 to 0.114 | 0.835 | 0.000 |
| SAS dependency | ||||
| M1 time–treatment interaction | –0.0143 | –0.035 to 0.006 | 0.172 | 0.005 |
| M2 treatment | –0.088 | –0.239 to 0.063 | 0.253 | 0.004 |
Tables 6 and 9 show the effects of PEd and PS on interview-rated function (SOFAS and SAS) over time with analysis using the LMEs. Overall, 200 (65.8%) participants completed these measures by 96 weeks with increasing attrition over time. Participants in the PEd group improved faster on the SAS interpersonal domain than those in the PS group (difference in gradient –0.017, 95% CI –0.030 to –0.004; p = 0.012). The estimated difference in means between PEd and PS was –0.037 (95% CI –0.156 to 0.082; p = 0.544). On fitting models without the treatment by time interaction to the remaining SOFAS and SAS (overall, friction and dependency) outcomes, there was no evidence of a difference between the PEd- and PS-adjusted means or in gradients. There were also no significant changes in function over time irrespective of treatment allocation.
Tables 7 and 10 show the results for the self-rated clinical outcomes. Unfortunately, only 141 (46.7%) and 103 (33.9%) of participants completed the secondary outcome measures by 32 and 96 weeks, respectively. In the analysis using the LMEs, there was a non-significant trend for a time by treatment group interaction (LRT p = 0.084) on HADS-A scores. From inspection of Table 7, this would appear to reflect the fact that levels of anxiety in the immediate post-treatment period were higher in the PS group than in the PE group, but equalised at the 96-week time point. Other secondary outcome measures, namely HADS depression score and scores on the SF-12 mental and physical component scales, did not differ between treatment arms. The effect of treatment on HADS-A was small, with a rise of just under 1 point in anxiety from baseline to 32 and 64 weeks in the PS group, and an even smaller drop in HADS-A at these time points in the PEd group, but in both groups these changes returned approximately to baseline scores by the final follow-up point. Therefore, these effects are probably not clinically important. There was rather more evidence of variation between therapy group, with the ICC for therapy group ranging from 0 to 0.048.
| Secondary outcome measure | Treatment effecta | 95% CI | p-value | ICC |
|---|---|---|---|---|
| HADS-A | ||||
| M1b time–treatment interaction | 0.085 | –0.011 to 0.180 | 0.084 | 0.035 |
| M2 treatment | –1.077 | –2.303 to 0.148 | 0.085 | 0.034 |
| HADS: depression | ||||
| M1 time–treatment interaction | 0.064 | –0.049 to 0.177 | 0.268 | 0.050 |
| M2 treatment | –1.196 | –2.607 to 0.215 | 0.097 | 0.048 |
| SF-12: mental component score | ||||
| M1 time–treatment interaction | 0.155 | –0.138 to 0.448 | 0.303 | 0.000 |
| M2 treatment | 1.181 | –1.412 to 3.773 | 0.372 | 0.000 |
| SF-12: physical component score | ||||
| M1 time–treatment interaction | 0.060 | –0.185 to 0.306 | 0.631 | 0.020 |
| M2 treatment | 1.462 | –1.420 to 4.345 | 0.320 | 0.021 |
Treatment moderators
We examined two prespecified treatment moderators: number of previous episodes (1–7, 8–19 and ≥ 20 episodes minimised in the randomisation as part of the design) and participant treatment preference at baseline. On adding two interaction terms for the number of previous bipolar episodes (1–7 and 8–19 relative to the ≥ 20 reference group) by treatment arm to the primary outcome model, the overall interaction was significant [χ2 = 6.80, degrees of freedom (df) = 2; p = 0.034]. The subgroup-specific HRs of the treatment effect were 0.28 (95% CI 0.12 to 0.68) for 1–7 previous episodes, 0.86 (95% CI 0.50 to 1.49) for 8–19 previous episodes and 1.01 (95% CI 0.70 to 1.46) for ≥ 20 previous episodes. Figure 5 shows the Kaplan–Meier for the three episode groups split by arm; those in the PEd group with 1–7 episodes had the longest delay in time to episode, whereas those with the same number of episodes in the PS arm had the shortest.
FIGURE 5.
Kaplan–Meier estimates of time to first mania-type or depressive bipolar episode by group and number of previous bipolar episodes.
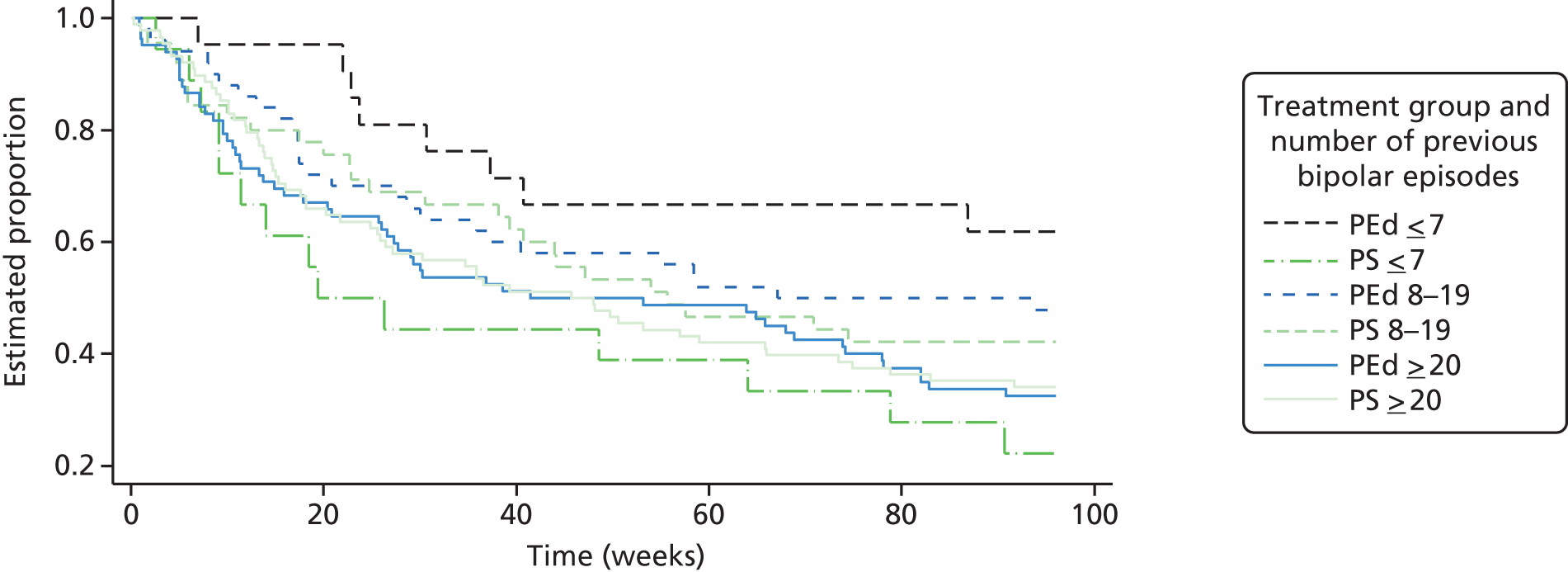
Similar plots for time to first mania- and depressive-type episodes are shown in Figures 6 and 7. Figure 7 shows that in the sample with 1–7 episodes, time to depression relapse was significantly delayed in the PEd group compared with the PS group (HR 0.24, 95% CI 0.08 to 0.68; p = 0.008), with five (22%) participants in the PEd group experiencing a depression relapse by 96 weeks, compared with 11 (61%) in the PS group. There were no other statistically significant interactions between number of episodes and time to depression relapse and none at all between number of episodes and time to mania-type relapse.
FIGURE 6.
Kaplan–Meier estimates of time to first mania-type bipolar episode by group and number of previous bipolar episodes.

FIGURE 7.
Kaplan–Meier estimates of time to first depressive bipolar episode by group and number of previous bipolar episodes.

The interaction between treatment and treatment preference (no preference or prefer PS vs. the reference category of ‘would prefer PEd’) was not statistically significant (χ2 = 1.95, df = 2; p = 0.378) when added to the primary outcome model.
Qualitative results
Thirty-seven participants were interviewed (Table 11).
| ID number | Group intervention | Number of sessions attended | Age (years) | Sex | Relationship status | Work status | Number of episodes | Interview length | Interview type |
|---|---|---|---|---|---|---|---|---|---|
| SU001 | PEd | 15 | 29 | F | Single | Employed | 8–19 | 51 minutes 53 seconds | F2F |
| SU002 | PEd | 13 | 47 | F | Divorced/separated | Unemployed | 1–7 | 57 minutes | F2F |
| SU003 | PEd | 14 | 49 | F | Married/cohabiting | Unemployed | ≥ 20 | 55 minutes 16 seconds | F2F |
| SU004 | PEd | 19 | 59 | M | Single | Unemployed | 1–7 | 53 minutes 17 seconds | TEL |
| SU005 | PEd | 18 | 42 | F | Married/cohabiting | Unemployed | ≥ 20 | 47 minutes 51 seconds | TEL |
| SU006 | PEd | 20 | 48 | M | Single | Unemployed | 8–19 | 46 minutes 48 seconds | F2F |
| SU007 | PS | 0 (dropout) | 42 | F | Divorced/separated | Employed | 1–7 | 34 minutes 39 seconds | TEL |
| SU051 | PEd | 20 | 63 | M | Married/cohabiting | Unemployed | ≥ 20 | 55 minutes 40 seconds | F2F |
| SU052 | PEd | 19 | 33 | F | Married/cohabiting | Employed | 1–7 | 49 minutes 25 seconds | F2F |
| SU053 | PEd | 20 | 59 | M | Married/cohabiting | Unemployed | ≥ 20 | 70 minutes 7 seconds | F2F |
| SU054 | PEd | 19 | 57 | M | Single | Unemployed | 8–19 | 57 minutes 34 seconds | F2F |
| SU055 | PEd | 21 | 60 | M | Married/cohabiting | Unemployed | ≥ 20 | 55 minutes 27 seconds | F2F |
| SU056 | PEd | 21 | 64 | F | Married/cohabiting | Unemployed | ≥ 20 | 42 minutes 37 seconds | F2F |
| SU057 | PEd | 7 (dropout) | 58 | M | Divorced/separated | Unemployed | 8–19 | 50 minutes 8 seconds | F2F |
| SU058 | PEd | 19 | 38 | F | Single | Unemployed | 8–19 | 31 minutes 1 second | F2F |
| SU059 | PEd | 1 | 55 | M | Married/cohabiting | Unemployed | ≥ 20 | 5 minutes 35 seconds and 25 minutes 49 seconds | TEL and F2F |
| SU060 | PEd | 2 (dropout) | 59 | F | Divorced/separated | Unemployed | ≥ 20 | 41 minutes 42 seconds | TEL |
| SU061 | PEd | 21 | 44 | F | Married/cohabiting | Employed | ≥ 20 | 35 minutes 56 seconds | F2F |
| SU062 | PEd | 18 | 47 | M | Divorced/separated | Unemployed | ≥ 20 | 49 minutes 49 seconds | F2F |
| SU101 | PS | 19 | 60 | M | Divorced/separated | Unemployed | 8–19 | 64 minutes 22 seconds | F2F |
| SU102 | PS | 18 | 30 | F | Single | Unemployed | 1–7 | 44 minutes 57 seconds | F2F |
| SU103 | PS | 15 | 26 | F | Single | Unemployed | 8–19 | 33 minutes 54 seconds | TEL |
| SU104 | PS | 17 | 54 | F | Married/cohabiting | Unemployed | 8–19 | 46 minutes 53 seconds | F2F |
| SU105 | PS | 14 | 29 | M | Single | Unemployed | ≥ 20 | 19 minutes 36 seconds | TEL |
| SU106 | PS | 17 | 43 | M | Single | Unemployed | ≥ 20 | 24 minutes 54 seconds | F2F |
| SU107 | PS | 17 | 67 | M | Married/cohabiting | Unemployed | 8–19 | 32 minutes 57 seconds | F2F |
| SU108 | PEd | 12 | 69 | M | Divorced/separated | Unemployed | 8–19 | 40 minutes 1 second | F2F |
| SU109 | PS | 3 (dropout) | 48 | F | Married/cohabiting | Unemployed | ≥ 20 | 33 minutes 6 seconds | TEL |
| SU151 | PS | 19 | 48 | F | Divorced/separated | Unemployed | ≥ 20 | 103 minutes 34 seconds | F2F |
| SU152 | PS | 11 | 52 | M | Married/cohabiting | Unemployed | ≥ 20 | 29 minutes 50 seconds | F2F |
| SU153 | PS | 4 (dropout) | 36 | F | Single | Unemployed | ≥ 20 | 45 minutes 27 seconds | F2F |
| SU154 | PS | 18 | 47 | M | Divorced/separated | Employed | 8–19 | 54 minutes 30 seconds | F2F |
| SU155 | PS | 3 (dropout) | 48 | M | Divorced/separated | Employed | ≥ 20 | 50 minutes 13 seconds | F2F |
| SU156 | PS | 19 | 44 | M | Married/cohabiting | Unemployed | ≥ 20 | 64 minutes 33 seconds | F2F |
| SU157 | PS | 18 | 50 | F | Divorced/separated | Unemployed | 1–7 | 35 minutes 17 seconds | TEL |
| SU158 | PS | 8 | 33 | F | Single | Unemployed | 8–19 | 69 minutes 37 seconds | F2F |
| SU159 | PS | 19 | 66 | M | Divorced/separated | Unemployed | ≥ 20 | 41 minutes | F2F |
The qualitative data are organised into two themes: ‘knowledge is power’ and ‘people like me’. In the reporting of the final analysis, data are presented to illustrate the range and commonality of meaning of each category of analysis. Participant ID numbers and whether they were allocated to the PEd or the PS group are provided.
Knowledge is power
Participants described having gained greater understanding about BD, the different forms it could take and ways of managing it, both in general and as it applied to them. Participants felt that this was an important outcome in its own right and that accessing information was a good reason for taking part in the trial. In particular, participants described how useful it had been to learn how the condition could affect them and their day-to-day living. Spending time reflecting on previous events (and particularly, for the PEd group, life charting; recording patterns of life events and mood experiences over time) helped them to understand their condition in different and more helpful ways. This included understanding the biological and genetic aspects of the illness, the function of medication and the inter-relationships of symptoms. Participants valued identifying triggers and early warning signs that were particular to them. Some described how this had led to their feeling more confident in managing future problems and talked about ways in which they had made use of the action plans in everyday life:
On holidays in the past I’ve had to come home because I’ve been so bad. This is way before the study. And now I can go away and think well if anything happens I can work on it, rather than get scared and just sort of abandon ship, and come to my home and curl up in bed . . . I have a little card which is in my handwriting about the early warning signs and strategies, just in my wallet, so I take that with me every day everywhere I go and it’s not there to show off it’s, well nobody sees it do they, well actually I have shown it to quite a few people and it’s a constant reminder to me.
SU053 PEd
Although the PS intervention had not been designed to cover the prescribed topics of the PEd, participants from the support groups also described that information exchange and learning was prevalent within their groups. Thus, many of the same topics (e.g. medication and early warning signs) were discussed in the PS and PEd groups:
We learnt probably the same if not more of what they were just teaching just from our experiences from the other people with their experience of the mental health sector and erm . . . using the services and also just their own personal experience how they get out of scrapes and how they, how they bring themselves out of depression or stuff.
SU102 PS
However, some participants commented that these groups could feel unstructured and lacking in focus, which meant they were more vulnerable to dominant group members:
I mean by the fifth week there was only about five or six of us left, so probably out of that group, probably only one or two you know that, erm . . . there was a couple of other people that I really wanted to listen to and I couldn’t because this other person took the whole hour-and-a-half up and I suppose that upset me.
SU153 PS
The newly acquired knowledge and understanding was described as being useful when interacting with others, such as carers. The materials were seen as useful resources to share and also helped individuals to be more confident to start conversations and explain the disorder to friends and family. Participants also talked about how the expertise they had acquired made them more confident about understanding and using medical ‘jargon’ and interacting with health-care professionals. Ultimately, having information that was tailored to them enabled individuals to feel that they had greater control over managing their condition in ways other than taking medication, and this was seen as empowering:
I’ve come down, and like halved my dose since . . . since I was on the programme. And I think that was contrary to the previous . . . you know, the line, if you like, for treating bipolar. It’s always definitely just drugs and that’s it and I think psychological therapy has a massive impact and I think having that information about what you can do to help yourself and to help manage your illness is power to you really.
SU052 PEd
People like me
Participants described having often felt isolated. Having BD had had an impact on their relationships as well as their ability to work, and people often felt unable to share their experiences and thoughts about the condition with friends, family members and colleagues. Many described having withdrawn as a way of coping or having lost confidence in social interaction, which had led some to become increasingly removed from other people. Many did not have regular contact with other people with BD, and meeting others who had similar experiences was raised as an important reason to take part and stay involved in the trial. Being able to associate with a group and share experiences with people they knew would understand was seen as comforting:
You feel very lonely when you go through this sort of experience, so if, you know, there is other people, you know, that have had bad times like yourself it helps to give you a bit of relief that you are not on your own.
SU004 PE
For some it was a very reassuring experience to learn that they were not unusual in the struggles they had encountered and to learn about how others managed their symptoms. It also allowed them to normalise some of their experiences, understand them and realise, therefore, that they were not abnormal. For some, attending the group was the first time that they had knowingly met someone with BD:
I think the first time to sit down in a room full of people who have bipolar and listen to what they are saying and how their condition affects them. You are thinking ‘wow, I am not alone . . . [laughs] it’s not just me’. So yes it was very beneficial at the time.
SU058 PE
Some participants described feeling wary of meeting people with BD and feeling fearful of being viewed as part of this group. Meeting and coming to know individuals helped them reconcile their own stigmatising views of people with BD and hence be more accepting of their own diagnosis:
If you are actually diagnosed and you are put with a group of people who are happy to talk about it, and their feelings, how they cope and actually they are normal human beings. You know, we had the good, the bad and the ugly there, so we had everyone, very glamorous young lady, and . . . [participant’s name] was very hippy. And a very big fat old man who was, well, there was just everybody and you didn’t, I didn’t feel alone. I felt for the first time I had somebody who really understood me, who didn’t judge me and, erm, and I wasn’t odd, so that is vitally important.
SU102 PS
Participants described feeling inspired by these encounters. It was encouraging to meet others who were able to function well and had recovered from severe episodes, particularly for individuals who had been diagnosed more recently:
I found that quite inspiring to know there were people who had been sectioned, had been full-blown woooo, and now have recovered to the degree that they can hold down some sort of work.
SU001 PEd
However, at the same time, this could be challenging:
Initially with the course, I’ll be really honest, I found it quite upsetting. And my friend said to me ‘I thought if anything it would comfort you. That you’d met other people that were in a similar situation, or actually worse’ . . . I found it really upset me to see all these nice normal people, suffering. And I know how it feels . . . I’d be alright in the group, and then I’d come home and get really upset, having heard people’s stories, and what they’d been through, and how relationships had been damaged, and they’d turned to drugs, and things like that. It just really . . . I sort of wanted to make it better and you can’t.
SU001 PEd
Taking part took effort in terms of both the amount of time participants had to dedicate to the 21-week programme and the emotional demands of thinking about and sharing dark times. In particular, some found it very challenging to create their life chart and talk about how they had felt and what they had done during episodes of illness. Although most felt safe and supported to do so, some individuals found the focus on difficult times too demanding and struggled to manage the feelings they were left with at the end of the session:
I left that group feeling worse . . . there was nothing positive that came along with it to me, it was negative, negative, negative.
SU153 PS (dropped out after four sessions)
As the intervention lasted 21 weeks, it is not surprising that some individuals experienced life events or illness during this period, and the groups could feel too demanding at these times:
I just couldn’t sit down for long enough to take in the subject matter. It wasn’t the subject matter or the way it was presented because it was presented very well and in an orderly and sensitive manner. What it was my own illness basically prevented me from attending all the sessions.
SU057 PEd
The relationships formed during the course of the groups were, for many, extremely important and even lasting; it was evident that this support function was just as important for participants in the PEd groups as for those in the PS groups:
I would say that what’s happened for us afterwards, for those that have retained the connection . . . it’s been almost as important as the course itself like . . . you’ve had that knowledge that you bring to each other, the relationships that you’ve established . . . [it’s] more important to staying stable than . . . that pile of hand-outs . . . these relationships hopefully are sustaining.
SU051 PEd
A novel feature of both interventions was that SUs co-facilitated the sessions. Participants found this extremely valuable and felt understood in a way that might not have been possible otherwise:
[SUF] was the only one that could have understood a couple of the symptoms I have . . . She had exactly the same symptom, and she went to the doctor loads of times and the doctor just brushed it off like ‘that is not a symptom of bipolar disorder’. And [health professional facilitator] had never heard of it, you know . . . and I said [SUF] I get exactly the same thing and I have been trying to explain it too and I can never describe it . . . and so without [SUF] there I would never really have been able to explore that or talk about that, or understand that in any way.
SU154 PS
They appreciated that at times SUFs might also feel challenged by the demands of the group and the role:
I think it was a hard role for her because, like me talking this morning, I’ve found it very hard now because it brings memories back, not about the good times, but about the bad times. I’m sure she had some, well she [said], you know, ‘I’m not too good this week’ and we were all very appreciative that she was able to share that and it made us understand she knows where we are coming from.
SU053 PEd
It appeared that what made the groups useful and engaging was the combination of gaining knowledge and techniques about BD alongside sharing experiences and forming part of a group with other SUs:
I’m not going too far when I say it has been life-changing, it really has. And I don’t think it would have been that massive a life-changing experience if it was just a set of lectures in a lecture theatre with no participation from many of the people who were listening . . . I think it’s that combination of having the right information given to you so that you can understand what’s happening, but also teach you some tools and techniques to be able to manage it properly as well as having all of the support of the people who have actually been through it.
SU052 PEd
Discussion
Summary of findings
The data show that both group PEd and group PS are feasible to deliver in routine NHS care across eight different inner-city urban and rural clinical sites in two regions of England. PEd was associated with improved attendance rates compared with PS, with the qualitative data indicating that participants who dropped out of PS did not like the lack of structure of the groups and the tendency of some individuals to dominate the sessions. Overall, the PEd and PS groups did not differ in terms of the primary outcome variable, namely time to the next bipolar relapse. However, there was evidence of a large effect favouring PEd over PS in delaying the time to relapse (HR 0.28, 95% CI 0.12 to 0.68) in the 13% (39/304) of participants with fewer than eight previous bipolar episodes. This result should be treated with caution, as only 13% of the sample had fewer than eight previous episodes. The level of attendance at groups, sex, recruitment centre and preference for one group over another at baseline did not significantly influence time to next bipolar relapse or differences in this outcome between the groups.
Among the secondary outcome variables, two favoured PEd over PS and none favoured PS over PEd. There was a significant but marginal delay in time to next mania episode and evidence of improved interpersonal function. However, the delay in time to next mania episode occurred during the treatment period with no further increases in effectiveness in the follow-up period after treatment, and was attenuated once baseline differences in the proportion with BD 1 to BD 2, marital status and treatment with mood-stabiliser medication were considered. Although there is little doubt that mood-stabilising drugs, such as lithium, sodium valproate and carbamazepine, reduce the risk of relapse with mania,57 it is less clear whether marital status or having BD 1 or BD 2 would necessarily change time to the next mania relapse (although BD 1 and BD 2 status would certainly be expected to affect the severity and duration of mania-type episodes once they had occurred). Therefore, controlling for these post hoc baseline differences may underestimate the relative effectiveness of PEd versus PS. There were no significant differences in time to the next depression relapse, although the proportion who relapsed with depression was more than halved in people with one to seven previous bipolar episodes compared with those with eight or more previous episodes. There were also no improvements in mania or depression symptoms, function (apart from interpersonal function) and self-rated physical health. Qualitative data indicated that, in both PEd and PS, participants valued gaining knowledge about BD and feeling less socially isolated by discussing their condition with other people who had BD. They also gained the confidence and knowledge to communicate with their friends, family and health-care professionals so that they could express their wishes and perspectives related to their condition and how it affected their life. People with fewer bipolar episodes and a more recent diagnosis of BD felt encouraged by meeting people with many previous episodes who were functioning well and leading productive lives. In terms of adverse effects, in both groups some people found the content and the reflection on the past distressing, particularly those who felt isolated or were experiencing life difficulties at that time.
Overall, there are no differences in effectiveness between PEd and PS groups except for those people who had fewer bipolar episodes. If only one treatment was to be offered, there would be some advantages in offering PEd over PS in terms of increased acceptability, some better clinical outcomes and better outcomes in people with few previous bipolar episodes. However, on the basis of this RCT, compared with an active control group, there is little evidence that PEd is more clinically effective than PS, except in people who have had up to seven previous bipolar episodes.
Strengths and limitations
The study achieved its recruitment target, with a shortfall of only four participants. It was designed to take clustering effects into account, thereby increasing generalisability. There was no evidence of a clustering effect of therapy group for the primary outcome. The study has, therefore, greater power than planned, as the sample size calculation assumed an ICC for group therapy of 5%. However, we aimed to detect a large differential treatment effect (0.22) found by Colom et al. 61,62 that was considered to be an outlier in a recent meta-analysis of RCTs of individual and group PEd versus TAU or group PS. 32 It is possible that a small differential treatment effect was not detected. Given that the study was designed as a superiority trial rather than a non-inferiority trial, and that there is no TAU group, we cannot conclude that group PEd and group PS are equally effective in BD on the basis of this RCT alone.
There was a high follow-up rate at the end of the treatment period, as reported in other group and individual psychological treatment trials for the long-term management of BD,73,80,97 and an acceptable follow-up for all interviewer-rated measures of 67% at 96 weeks. Few studies have reported interview follow-up rates at 96 weeks, but the follow-up rate in the current study at 12 and 18 months is similar to that in previously reported UK studies of psychological treatments using similar methods. 72,79 There was complete follow-up for the primary outcome variable. The IRR for measuring mania-type and depression symptoms scores was only moderate, but we believe that this is an acceptable level of agreement given that these were ordinal judgements over six levels of severity rather than binary assessments. Randomisation was carried out by a clinical trials unit, and there were only 10 instances of unblinding. Unusually for a psychological treatment study, there was very close matching of the PEd and PS groups for therapists, the existence of manuals and duration of treatment, so it was possible to blind assessors of outcome. Unblindings were rare, and in all cases independent unblinded assessment of outcome was achieved. Assessment at baseline occurred within a few weeks before the groups started, with the result that none of the participants started the groups while in a bipolar episode. The trial was registered and the protocol for the trial and its analysis has been published. 71
Unlike some previous studies of treatment in BD using time to next bipolar episode as the primary outcome, the study minimised at randomisation for the number of previous bipolar episodes that the participant had experienced. This is important, as time to next bipolar episode can increase threefold in people with ≥ 20 previous episodes, compared with those with ≤ seven episodes. 72 Not controlling for the natural history of illness is one of the most common failings of RCTs in mental health. 97 A further strength of the study is that the effects on function were measured in addition to symptoms and relapse; improvements in function are prioritised in the personal recovery of people with BD. 98 The analysis of the continuous data used a linear mixed modelling approach allowing for clustering by therapy group, unlike almost all previous group PEd trials in BD. We also explored the effects of missing non-response over time and baseline differences in the groups that might be clinically important. Overall, the study had high internal validity, and many methodological strengths compared with previous RCTs of group PEd and other psychological treatment in BD; it is also the largest RCT of its type.
The study recruited efficiently, suggesting that the participants welcomed the possibility of taking part in a study offering two types of group psychological interventions, including 85% of the sample who were contacted about taking part in the study following referral. Only 8% of participants withdrew their consent once they had received a full explanation of the study, and only 2% withdrew because they were unable to attend the groups. Overall, 22% of the sample never started or attended only one session of psychological treatment in this study, in comparison with 60% not attending or attending only one session of psychological treatment from the Improving Access to Psychological Treatment services routinely delivering NHS psychological treatment to adults with depression and anxiety in England. 99
The study successfully recruited from two regions in England, from inner-city, urban and rural sites, and from direct referral by primary care and secondary care and through self-referral. Participants were, on average, aged in their mid-forties, typical of samples in pragmatic RCTs in BD in the UK. 72,79,100 Similar to other samples recruiting a combination of participants with either BD 1 and BD 2, there was a slight excess of female participants in the sample. Also typical of previous studies in this field was that only around 40% of participants were currently married or cohabiting. The sample was predominantly white British and slightly above the average for England according to the 2011 census. 101 In particular, there were only three (1.0%) participants of South Asian origin. Similar to most previous studies of psychological treatments with a mixed BD 1 and BD 2 sample, 80% of participants had BD 1 and 80% of these had a lifetime history of psychotic symptoms. The current sample was similar to that recruited for another large UK study of psychological treatment in BD by Scott et al. 79 in the lifetime prevalence of anxiety disorder, alcohol and drug abuse or dependence and borderline or antisocial personality disorder. Overall, the representativeness of the sites and the participants, together with the study’s wide inclusion and exclusion criteria for comorbidity, suggests that it has high external validity and generalisability to NHS practice in England, except in relation to people of non-white British origin.
The main limitation of the study in terms of making inferences to routine clinical care and to other studies is the design, as there was no TAU group who were not receiving psychological treatment. This trial was always intended to be a rigorous examination of the clinical effectiveness of running group PEd using the structured Barcelona approach adapted to a UK NHS (public service) context compared with another well-matched and commonly available group psychological treatment alternative, rather than an examination of the clinical effectiveness of a group psychological approach to BD compared with not offering any structured psychological treatment. In the current study, the control group was an attentional control receiving the most widely available alternative group psychological treatment in the UK, namely PS. The control group was designed to control for everything except the content and structure of the intervention; it had the same aim of treatment, to develop a stay well plan, as well as the same duration of treatment sessions, the same number of treatment sessions, the use of therapists and the use of a manual. In contrast, the support group control for group PEd in the first two 21-session group PEd RCTs reported33,61,62 was not given the same purpose, but it did control for meeting as a group for the same number of sessions, the same duration of sessions and the number of therapists. In these studies, the control group did not necessarily discuss BD at all during their group sessions, and therefore the authors did not control for an expectancy of beneficial effect on outcome. 102
Another important limitation in psychological treatment studies is the experience level of the therapists offering the treatment. In both treatment arms, we utilised at least one therapist who had experience of delivering psychological treatment and running group interventions, and at least two therapists who had a great deal of experience of managing BD. The SUF was usually relatively new to the role. All therapists and SUFs were regularly supervised by experts who had extensive experience in conducting RCTs of psychological interventions in BD, including those involving PEd and psychological support. However, none of the therapists was experienced at delivering these interventions, and this relative inexperience could be a criticism of the study. On the other hand, a strength of the study was that none of the research team had developed these interventions and were not advocates for them, so they were independent researchers who were also experienced in running multicentre RCTs of psychological interventions in BD.
Our analysis of the content of the PS group shows that it had a clear focus on the task, namely to develop a stay well plan for participants. In terms of content, there was a considerable overlap, with participants in both groups almost always receiving sessions on causes and triggers of BD, mania, depression, prescribed drugs and alternative therapies, regularity of habits, stress control techniques and problem-solving strategies. In roughly half of the PS groups, the participants also discussed a method of preventing relapse into mania and depression episodes that has some evidence of effectiveness,34,72 but in one session only, rather than five sessions, as for PEd. Thus, the PS control group covered some of the content that is thought to be effective in reducing bipolar relapse,103 albeit not necessarily in a structured or intensive format . Qualitative data from the participants suggest that their experience in both groups was powerful in relation to information-giving and receiving support from people with similar experiences. There was no evidence of contamination between the groups of participants in the group PS who did not come together in any forum, or between facilitators, who allowed the participants to decide the content of their sessions and played a passive role in running the groups. Evidence in support of some effectiveness of the PS interventions is that the relapse rate for both PEd and TAU groups reported by Bond and Anderson32 was 60–70% overall at 1 year; in the current study, the relapse rate in the PS group was only 53% at 12 months and 65% at 24 months. Caution should therefore be applied before assuming that the rates of relapse in this study in the PS group are the same as would be found for TAU in future meta-analysis including this RCT. The results in the current RCT might underestimate the effectiveness of group PEd compared with TAU in NHS practice.
The PS group also differed in some other important ways from PS groups that are available routinely in NHS and non-NHS settings in the UK. PS groups are usually run by peers with BD rather than by health professionals. They often have an open format, so that new participants can join at any session, and sometimes are potentially long term and do not have any fixed number of sessions that a participant attends. They often do not have any fixed agenda or aim other than to broadly educate and support the people with BD who participate. Therefore, the results in this study may not be generalisable to other PS groups, particularly if these groups are open to new participants, do not have the same aim of developing a stay well plan and are open ended, and if the facilitators are not provided with peer and group supervision.
A limitation of the study may be the relatively poor attendance at the treatment groups. This, however, is, however, likely to be a reflection of what might happen in routine clinical care, given the pragmatic design of the RCT. Although attendance rates at sessions did not account for differences in outcome between the groups, it might have compromised the continuity in learning for those participants who attended intermittently and for the rest of the group as a whole in both treatment arms.
Finally, the study recruited people with only low levels of depression and mania symptoms at baseline, so floor effects limited a determination of whether or not group PEd may be effective against symptoms of mania or depression compared with PS groups. However, the study was designed primarily to test the effectiveness of group PEd for the prevention of future bipolar relapse and therefore deliberately did not recruit participants who were in a bipolar episode. Those in the sample did show mild anxiety, and around 40% of the sample had had anxiety disorders in the last month and over half had them in their lifetime. There was a trend towards a benefit of group PEd versus group PS on self-rated anxiety symptoms in the immediate post-treatment period, but this assessment was compromised by the low return rate of the HADS. The low return rate by participants who were given the option of returning questionnaires by post is a substantial limitation when considering self-rated outcomes in the current study.
Comparison with other psychological long-term treatment trials in bipolar disorder
Compared with the first studies of 21-session group PEd,33,61,62 the current RCT had a larger sample size and a larger number of groups, so it improved the generalisability of our findings to practice. Our trial also employed a control of using a credible alternative psychological treatment with a similar aim to remain well. We found that group PEd was more acceptable in terms of sessions attended and from qualitative interviews with participants from both treatment groups. However, group PEd was not more effective than PS in terms of time to the next bipolar episode, except for people with only one to seven previous bipolar episodes. We were able to replicate a more recent finding that group PEd was effective in improving interpersonal function compared with TAU. 104 Although there were some positive effects of group PEd on time to next mania episode, interpersonal function and quality of life, they do not support the effectiveness of PEd versus all other types of control treatment on overall relapse, as reported by Bond and Anderson,32 and provide only weak support for its effectiveness against mania relapse. The results are consistent with the lack of effectiveness of group psychological treatments versus control treatments against bipolar relapse of all types found by NICE, but unlike NICE we could find no evidence of effectiveness against depression episodes. 57,63 However, the latter meta-analysis included studies of group CBT, mindfulness CBT, dialectical behaviour therapy and social cognition and interaction training, not just PEd.
Given that sample size and other methodological limitations are unlikely to explain our results, there are a number of other possible reasons why the current RCT did not show the effectiveness of PEd compared with a control treatment. First, there is a tendency for large multicentre trials to show smaller effect sizes of treatments than smaller single-centre trials. Second, the PS group offered structure, provided information, promoted self-efficacy and provided interpersonal support over 26 weeks in addition to covering some approaches demonstrated to be effective against depression, such as problem-solving. 103 Thus, the PS group may have been effective in its own right. Bond and Anderson32 found that interventions offering > 20 hours of face-to-face group PEd were effective against depression relapse and those offering fewer hours were not effective. In the current study, only 24 (15.7%) participants in the PEd group and 15 (9.9%) participants in the PS group attended 20 or 21 group sessions, so it is possible that there were too few in either group receiving enough therapy time to show an effect on depression relapse. Overall, the proportion of participants experiencing a depression relapse in either group was comparable to the proportion relapsing in TAU or control groups in other RCTs of PEd; in contrast, the proportion experiencing mania relapse was low in both groups, but especially the PEd group, compared with other RCTs of PEd. 32 Group PEd is an intervention in which later sessions of treatment build on learning from earlier sessions, so it is possible that lower levels of attendance at key points might explain its overall lack of effectiveness against depression relapses versus PS.
However, another possible explanation is the case mix of participants in the groups in terms of both the course of their illness and sex. In the case of participants with one to seven previous bipolar episodes, the HR (0.28) for overall bipolar relapse was substantially lower in the PEd group than in the PS, with little or no difference between the groups with more bipolar episodes. Furthermore, the rate of relapse into depression in the PEd group was less than half that of the PS group. However, only 13% of the total sample in the current study had one to seven previous bipolar episodes. Other studies may have had a different case mix, with more participants in an earlier stage of their illness. RCTs of group PEd either vary in how they establish and report the number of previous bipolar episodes or have not reported this baseline variable. Colom et al. 61,62 reported a substantial effect of group PEd versus group PS, and the average number of previous bipolar episodes in the two treatment groups was between 8 and 11, suggesting that a higher proportion of their sample than of our study had few previous bipolar episodes. However, an assessment of the number of previous bipolar episodes is subject to recall bias, and little weight can be given to the precise number of previous bipolar episodes from study to study using different assessment methods; it is possible only to distinguish between those who have had relatively few bipolar relapses and those who have had many bipolar relapses. It is also unclear whether or not the number of previous bipolar episodes is a proxy measure for some other feature of BD that determines if people might benefit from PEd versus PS. Large effect sizes have been reported in people with BD of recent onset in RCTs of psychological interventions and service interventions incorporating group PEd compared with TAU. 73,74 A long-standing theory that has some empirical support is that psychosocial factors may be more associated with relapse earlier in the course of BD (a phenomenon known as behavioural sensitisation)105 than later. Our own qualitative data suggest that people with fewer previous bipolar episodes felt encouraged by meeting people with BD who functioned well, including the SUFs, although this was true of both groups and does not, therefore, explain why PEd was more effective than PS in people with fewer previous bipolar episodes.
The effects of individual and group PEd on function are mixed, although all except the smallest studies measuring function show a benefit of PEd on overall function,34,72,106 overall function in treatment completers107 or interpersonal function. 104 The benefits in this study with group PEd were seen only in relation to interpersonal function and not in overall function or in the performance, friction and dependency domains of the SAS. The participants in this study reported only mild impairments in these domains at the start of the study, although their marital and employment history suggested substantial damage to these aspects of their lives in the past. Therefore, floor effects on function are likely to be operating. The effects on interpersonal function are not necessarily a secondary benefit of PEd on time to bipolar relapse, given its effectiveness only in a subgroup compared with PS. Torrent et al. 104 proposed that forming new relationships and receiving repeated small group exercises in group PEd might also be plausible explanations for improvements in interpersonal function. In the current study, PS was characterised by poorer attendance and a more discursive approach to the content of the group.
There did not seem to be any differences across the treatment centres, suggesting that different types of mental health professional (MHP) can be utilised for this intervention (nurses and psychiatrists were used in the East Midlands, but nurses, occupational therapists and psychologists were used in the North West). Another important difference between group PEd in the current study and group PEd in other studies61,62 is that we utilised a SUF in the groups alongside health professionals. Some qualitative work has investigated this role, but feedback from the participants in both treatment arms in our qualitative study suggests that participants found the SUF role useful, a finding that resonated with a previous study exploring the feasibility of group PEd in another part of the UK. 70 The role of the SUF may be particularly welcome in the context of MHS in the UK, compared with some other countries. SUs now play an active role helping other SUs, particularly in the recovery phases of their mental health problems, so their role in groups promoting longer-term recovery seems culturally appropriate in the UK.
Implications for research
Overall, the current trial did not show that PEd was more clinically effective than PS when delivered in a very similar format and had the same expectations of benefit. The trial provides some limited support that the content of group PEd might provide greater clinical benefit over that of group PS in terms of acceptability, time to first bipolar episode in those with few previous bipolar episodes, time to first mania relapse and improvement in interpersonal function and quality of life. Ideally, another RCT would provide a precise estimate of the clinical and economic benefits of group PEd versus TAU, although this may become increasingly impractical in England and Wales given that psychological interventions for BD are a key NICE recommendation for implementation in the NHS. 57 The data suggest that there are important avenues to pursue both in terms of the stage of BD that a person is in (i.e. early or later in the course of BD) and related to the delivery of optimal psychological treatment such as the added benefit of an initial support group followed by group PEd coupled with optimal medication management. 108 A recent meta-analysis raises the question whether or not treatment lasting ≥ 20 completed sessions is required to improve depression outcomes. 32 It is unlikely that all people with BD who might benefit from group PEd or PEd in general can attend groups, so there are already attempts to deliver both individual PEd109 and group PEd110 through the internet. Further research is needed to refine and more thoroughly test such internet and video conferencing approaches to PEd and group PEd, as well as work on individual techniques to improve clinical outcomes further.
Implications for clinical practice
The National Institute for Health and Care Excellence has already recommended individual, family and group psychological interventions, including group PEd, in BD for implementation in the NHS. 57 Arguably, group PEd is one of the most efficient interventions to deliver because up to 18 participants can be treated at the same time by two health professionals and one SUF. Health professionals and SUFs can be trained quickly to deliver group PEd, provided that they have quite extensive clinical or personal experience of BD and knowledge of group processes, as well as training, access to manuals and supervision by health professionals with experience in both psychological treatment and BD. An account of the importance of these aspects of providing group PEd has been reported. 70 If NHS trusts are now looking to implement either group PEd or group PS, then group PEd appears more acceptable, and there are some clinical benefits, especially for people early in the course of their BD. Our qualitative data suggest that both group PEd and group PS help people to gain the knowledge required to self-manage their BD with more confidence, to discuss optimal treatment with health professionals and to feel less socially isolated. As the content of the groups could be distressing, particularly for those who are isolated or going through life stress, some consideration should be given to providing support after sessions if participants indicate that they have been distressed as a result. There is some evidence of sustained clinical improvement over 5 or 6 years at a reduced overall cost if group PEd is combined with specialist BD expertise in prescribing and ongoing care. 108,111 However, the evidence from the current trial suggests that there is still some doubt about the effectiveness of group PEd compared with providing closed group PS; if there are benefits from group PEd, these may be largely related to the nature of the support and information-sharing offered, rather than to the specific content of the PEd curriculum itself. As there was no TAU group (a group not receiving a group psychological intervention), the trial cannot draw any conclusions about the clinical effectiveness of group PEd versus TAU. Overall, the trial does not provide evidence to reverse recent NICE recommendations of group psychological treatments to prevent relapse, but the benefits of offering group PEd over the group PS offered in the trial are modest.
Chapter 2b Pragmatic randomised controlled trial of psychoeducation versus peer support in the maintenance of bipolar disorder: economic evaluation
Chapter overview
This chapter describes the methods and results of the economic evaluation conducted as part of the RCT of group PEd compared with group PS. The economic evaluation includes within-trial analyses of service use and health status data collected in the RCT. The within-trial analyses compared the costs and quality-adjusted life-years (QALYs) of group PEd and group PS, and estimated the cost per QALY gained. An economic model was used to explore the degree to which group PEd would need to reduce the probability of relapse and/or increase the time to relapse for it to be cost-effective compared with TAU.
The within-trial and economic model analyses estimated the costs and health benefits from the viewpoint of NHS health and social care service providers and patients for the 96-week follow-up period of the trial. All participants randomised to start treatment were included. The economic model used a combined decision tree and Markov model to reflect the trial pathways. Data for the economic model were estimated from the trial data. For all analyses, the probability that PEd was cost-effective was estimated.
The within-trial analyses suggest that group PEd could be cost-effective compared with PS if decision-makers are willing to pay at least £37,500 to gain 1 QALY. However, variance in the data and large numbers of missing data mean that the results are uncertain. The results of the economic model indicate that group PEd is cost-effective compared with TAU if it reduces the probability of relapse and/or increases the time to relapse.
Aims and objectives
The overall aim of the economic evaluation component was to determine the clinical effectiveness and cost-effectiveness of PEd (intervention) compared with PS (control). The specific objectives were to:
-
identify and compare the health and social care services used by participants in the intervention and control groups
-
identify and compare the overall health status of participants in the intervention and control groups at baseline and follow-up
-
estimate and compare the costs and QALYs of participants in the intervention and control groups at baseline and follow-up
-
explore the relative cost-effectiveness of the PEd intervention using within-trial primary and sensitivity analyses
-
explore whether or not PEd could be cost-effective compared with TAU using a probabilistic simulation model (economic model).
Methods
Approach
The framework of cost-effectiveness analysis was used to compare the relative costs and outcomes of the bipolar group PEd intervention with those of the bipolar group PS control. The primary measure of health benefit was the QALY. The primary economic outcome measure was the incremental direct cost per QALY gained. The direct costs of care and QALYs were estimated for each trial participant from baseline to the end of scheduled follow-up at 96 weeks. The perspective of the evaluation was that recommended by NICE and included health and social care agencies (direct costs of care) and patients (health benefits); these are the key components of a societal perspective. The target population for the economic evaluation (within-trial and economic model analyses) was adults with a primary diagnosis of BD 1 or BD 2 who were at increased risk of further relapse (an episode in the last 24 months). The within-trial analysis used an intent-to-treat approach and included all participants randomised to start therapy in both trial groups. Costs and outcomes were estimated over a 96-week time horizon and were not discounted.
Within-trial analyses
Direct costs
The range of costs included the costs of the following formal health and social care services:
-
inpatient psychiatric and non-psychiatric care (including intensive care, emergency and crisis admissions)
-
hospital outpatient and day hospital attendances
-
primary care contacts with the GP and general practice staff (including office and home visits)
-
prescription medicines
-
community mental health-care contacts
-
social care contacts.
Resource use data were collected from each patient using an Economic Patient Questionnaire at the baseline, 32-, 64- and 96-week assessments. At baseline, participants were asked to report health-care resources used in the preceding 6 months. At the 32-, 64- and 96-week assessments, participants were asked to report their health-care use since the previous assessment. The direct costs were estimated from resource use data combined with the most recently published national unit costs available at the time of data analysis. These were the Department of Health reference costs,112 the Unit Costs of Health and Social Care113 and the British National Formulary114 for the price year 2012–13. Each item of service use was costed by multiplying the quantity of service used with the average unit cost for that item.
The costs of the PEd and PS interventions included the costs of training staff and SUs to deliver the intervention (trainer and trainee time), the costs of delivering the intervention (time of staff and SUs to run the group sessions, plus materials) and the costs of supervision (time of supervisors and supervisees). This information was used to estimate the total cost of each of the interventions as planned. The cost per group was calculated by dividing the total cost by the number of groups run. The cost per participant was calculated by dividing the cost per group by the actual number of participants allocated to the group. This approach assumes that there are no savings associated with participants who did not attend one or more of the planned group sessions. The approach also assumes that there are no savings associated with the intervention requiring fewer than the 21 planned group sessions.
Quality-adjusted life-years
Quality-adjusted life-years were estimated from the EuroQol-5 Dimensions, three-level version (EQ-5D-3L),115 and published utility tariffs. The EQ-5D-3L has been used successfully for this purpose in previous trials of psychosis116,117 and BD. 118 The EQ-5D-3L was completed by interview with the participant at the scheduled baseline, 32-, 64- and 96-week assessments. The EQ-5D-3L gives a profile of the individual’s health state at the time of assessment. Each possible health state has a published utility weight. Using the published tariff for the UK,117 the health profile for each individual over the duration of follow-up was converted to a single utility value. QALYs were then estimated using an area under the curve approach:119
where U = utility value and t = time between assessments.
Missing data
The pattern of available cost and utility data across the different assessments is summarised in Table 12. The proportion of the sample for which it was possible to calculate costs or utilities was 91% and 85% at baseline and decreased at each subsequent assessment. The proportion of the sample with at least partial data recorded for resource use or utilities was marginally higher at baseline, at 98% and 88%, respectively. At the final assessment (week 96), 57% had full data recorded for resource use and 33% had full EQ-5D data allowing estimation of a utility value. Additionally, 65% (PS) and 71% (PEd) of participants had partial data about service use at the final assessment. Over half of the sample (54%) had both resource use and utility data at two or more time points, and one-third (34%) had these data at three or four time points. Around one-quarter (23%) of the sample had resource use and utility data at both baseline and final assessment. Overall, cost data were more complete than utility data, with similar proportions of available data for PEd and PS participants.
| Study time point | Treatment group | |||||
|---|---|---|---|---|---|---|
| PEd, n (%) [% partial data]a | PS, n (%) [% partial data]a | Both treatment arms (%) | ||||
| Cost | Utility | Cost | Utility | Cost | Utility | |
| Baseline | 142 (93) [99] | 133 (87) [88] | 135 (89) [98] | 125 (83) [88] | 91 | 85 |
| Week 32 | 112 (73) [80] | 75 (49) [49] | 100 (66) [77] | 86 (57) [57] | 70 | 53 |
| Week 64 | 96 (63) [71] | 66 (43) [44] | 85 (56) [68] | 60 (40) [40] | 60 | 41 |
| Week 96 | 91 (59) [71] | 49 (32) [33] | 82 (54) [65] | 50 (33) [34] | 57 | 33 |
| Number of assessments with complete cost and utility data | Treatment group | |||||
| PEd, n (%) | PS, n (%) | Both treatment arms (%) | ||||
| 0 | 23 (15) | 23 (15) | 15 | |||
| 1 | 41 (27) | 52 (35) | 31 | |||
| 2 | 37 (24) | 26 (17) | 21 | |||
| ≥ 2 | 89 (58) | 76 (50) | 54 | |||
| 3 | 25 (16) | 26 (17) | 17 | |||
| ≥ 3 | 52 (34) | 50 (33) | 34 | |||
| 4 | 27 (18) | 24 (16) | 17 | |||
| Baseline and week 96 | 39 (25) | 31 (21) | 23 | |||
Linear interpolation was originally planned as the imputation method to estimate missing follow-up, utility and QALY values for the primary analysis. 71 It was not possible to ascertain whether loss to follow-up was due to death or other reasons for 66 out of 304 participants (22%). There was an imbalance between the two intervention groups in the proportion of people for whom information on whether they were alive or dead at the end of follow-up was available (see Appendix 1, Table 76). There was also an imbalance in the proportion of people in each group for whom information about the last assessment was known, by survival status (see Appendix 1, Table 76). In addition, multiple imputation of both costs and QALYs is increasingly recognised as an appropriate approach to deal with missing observation and missing follow-up data. 120 For these reasons, it was decided to use multiple imputation for the primary analysis of both costs and QALYs.
All missing cost and utility data were treated as missing at random, and multiple imputation was used to impute values for missing utility values and costs (by type of service used) for each follow-up period. The imputations were conducted in Stata version 13.1 using predictive mean matching and sequential chained equations. Missing baseline data were imputed for participants who had data at subsequent assessments. Participants with missing data at all time points were excluded from the imputation and subsequent analyses. The variables included in the models were selected on the basis of descriptive and regression analyses of the pooled baseline and follow-up data. These were used to identify potential predictors of the utility, follow-up and cost measures. 120 The variables imputed were inpatient, outpatient, community, primary care and medication costs; total costs for each assessment; and EQ-5D utility scores.
Costs were imputed by category rather than as a total so that all available data were used to inform the imputed values. For example, participants may have been missing data relating to only a single category of health-care use, and so imputation was used to fill in the blanks. All available cost and outcome data for a particular participant were used to impute missing data, including baseline costs and outcomes. The following variables were used to inform the imputation models:
-
non-missing cost and utility data for all time points
-
treatment allocation
-
the PS or PEd group and wave that participants were treated in
-
whether or not the participant was currently working
-
sex
-
the number of previous episodes
-
whether or not the participant had further or higher education
-
type of bipolar diagnosis
-
whether or not the participant was white British
-
whether or not the participant was married
-
whether or not the participant lived alone
-
age
-
the SOFAS score, the SAS overall score, the HADS-A and HADS depression scores, the SCID depression and SCID mania scores
-
whether the participant was alive or dead (for those who did not complete follow-up)
-
the total number of days followed up (for those who did not complete follow-up)
-
Short Form questionnaire-6 Dimensions (SF-6D) utility, EQ-5D thermometer.
Missing total cost values (from baseline to end of 96-week follow-up) were estimated passively from the imputed costs at each follow-up assessment, and missing QALY values were estimated passively from the imputed days of follow-up and utility at each assessment.
As a sensitivity analysis, linear interpolation was used to impute missing health status data for participants who completed scheduled follow-up but had missing observations. For this analysis, missing values were imputed by linear interpolation (value of previous period plus value of next period divided by 2), if observations either side of the missing item were available. This was based on the assumption that utility values and time for one assessment period were linearly correlated with those of the previous and future assessment periods. If data for the baseline assessment were missing, but all subsequent assessments were completed, then the baseline value was imputed as the first observation carried backwards.
Linear interpolation was not used if patients did not complete follow-up. Any patients with one or more missing observations at the end of the period were treated as censored cases. For patients with censored data due to withdrawal or loss to follow-up, missing utility values and time between assessments were imputed from the mean values of those who completed scheduled follow-up or died, for each treatment allocation. Cox regression was used to estimate the survival function and probability of survival at each assessment point, using patient status (alive, dead) and treatment allocation. The QALYs for this group were estimated using an area under the curve approach,119 adjusting for censored data due to missing follow-up:
where U = utility value, S = probability of survival and t = time between assessments.
Economic analyses
Descriptive and regression analyses were used to identify key elements of service use and cost. Descriptive and regression analyses were also used to explore the potential impact of baseline participant and service characteristics on the costs and QALYs in order to identify covariates for the cost-effectiveness analysis.
The primary economic analysis was adjusted for the baseline covariates identified as potential predictors of future costs and outcomes. Average and incremental costs and QALYs were estimated, as was the incremental cost-effectiveness ratio (ICER).
The primary measure for the economic analysis was the ICER. Accordingly, no statistical tests of differences in mean costs or outcomes were conducted. The ICER was estimated as:
The estimates of incremental costs and outcomes from the regression were bootstrapped to simulate 10,000 pairs of net costs and net outcomes of the PEd group. These simulated pairs of net costs and net outcomes were used to generate cost-effectiveness acceptability curves, as is recommended by NICE for health technology appraisals. 119 The simulated data were also used to estimate the probability that the PEd intervention is cost-effective compared with PS. This takes a Bayesian approach to estimating the likelihood that the intervention is cost-effective and avoids hypothesis testing and the risk of a type II error.
The cost-effectiveness acceptability approach described above revalues effects or benefits in monetary terms. However, in the UK there is no universally agreed monetary value for the types of health benefit measures (such as QALYs or relapse-free years) typically used in cost-effectiveness analyses. An approach used in health care is to ask the question: what is the maximum amount decision-makers are willing to pay to gain one unit of health benefit? The simulated net utility values were revalued using a range of maximum willingness-to-pay values (WTPVs) from £1 to £50,000 to gain one unit of health benefit. This was based on the range of WTPVs historically implied by NICE decisions. 121
The data for the cost-effectiveness acceptability curve were derived by first revaluing each of the 10,000 net health benefit estimates from the bootstrap simulation by a single WTPV. This was repeated for each WTPV within the range used. A net benefit (NB) statistic for each pair of simulated net costs and net outcomes for each WTPV can then be calculated as:
where HB = net health benefit and C = net cost.
This calculation was repeated for each WTPV. Cost-effectiveness acceptability curves plotted the proportion of bootstrapped simulations when the NB of PEd is greater than zero for each WTPV. 122–125
Sensitivity and subgroup analyses were used to assess the robustness of the results to changes in the range of costs included in the total cost measure, the measure of health benefit and imputation method. The subgroup analyses explored whether or not the cost-effectiveness of PEd varied according to the type of BD, the number of previous episodes, the number of sessions attended and whether or not the participant completed the 96-week SCID assessment.
Decision tree and probabilistic simulation model
The clinical and economic trial was designed as a pragmatic evaluation. The economic within-trial sensitivity and subgroup analyses explore aspects of the trial design and participants that could affect generalisability. Economic models can be used to explore the cost-effectiveness of interventions over different time periods, settings and participants. The within-trial sensitivity and subgroup analyses explored the key issues discussed in Chapter 2a and that may occur when implementing PEd in routine practice.
A consistent issue raised by existing literature is that there is limited evidence about the relative effectiveness, costs or health benefits of psychological therapies,. As discussed in Chapter 2a, the existing clinical evidence is uncertain because of issues with the evaluation designs and participant samples used. 32,57 In addition, there was a lack of primary data on service use and costs, and measures of health benefits that could be used to estimate QALYs. This limits the extent to which further economic modelling could add to the information provided by the within-trial analyses about the relative cost-effectiveness of PEd compared with PS.
A key problem identified in the updated NICE guideline CG185 was that there was insufficient evidence to model the relative cost-effectiveness of psychological therapies compared with TAU. 57 This issue was also discussed in Chapter 2a of this report.
Accordingly, a simple economic model and a threshold analysis were used to explore the reduction in the probability of relapse and/or time to relapse associated with PEd that would be required for it to be cost-effective when compared with TAU. This approach provides additional information for settings or patient groups in which access to psychological therapies such as that provided by PS is limited. The key benefits of this approach are that it controls for heterogeneity in study design, participant samples, the range of service use measured and unit costs used to value resource use. However, the approach used means that the scope and relevance of the economic model results are limited to the participants, setting and time horizon of the clinical trial. The overall aim of these modelling analyses was to explore the circumstances in which PEd may be cost-effective. Key questions for the model based analyses were:
-
What is the relative reduction in the probability of relapse in PEd for it to be cost-effective compared with TAU?
-
What is the relative reduction in the time to relapse in PEd for it to be cost-effective compared with TAU?
-
What is the combined relative reduction in the probability of relapse and time to relapse in PEd for it to be cost-effective compared with TAU?
A simple economic model that combined an initial decision tree to map the early trial pathways and a Markov model to map relapse was constructed. The TAU arm of the model was defined as no psychological treatment.
The primary economic outcome measure for the model analyses was the incremental direct cost per QALY gained. The direct costs of care and QALYs were estimated from the start of treatment to 96 weeks, to mirror the trial scheduled follow-up. The perspective of the evaluation was that of health and social care agencies and patients. The target population for the economic model analyses was people with BD 1 or BD 2 at increased risk of further relapse (an episode in the last 24 months). Data from the trial sample of participants were used to represent this population.
The initial decision tree structure was based on the care pathways used in the trial design. The Markov section of the model was based on the main aim of the PEd and PS interventions, namely to prevent and/or delay the first relapse following treatment. The model structure is shown in Figure 8. The decision tree part of the model describes the distibution of participants in terms of attending the group sessions. The Markov section explores the probability and time to first relapse in the follow-up period. The time horizon is 96 weeks, split into three cycles of 32 weeks. An analysis of the trial data indicated that there were differences in the average costs per assessment period (32 weeks) between those participants who had a relapse and those who did not. This applied overall and by assessment. However, there did not appear to be differences in the average cost per assessment within those people who had a relapse, or for those people who did not have a relapse. This also applied to the utility data.
FIGURE 8.
Economic model structure.
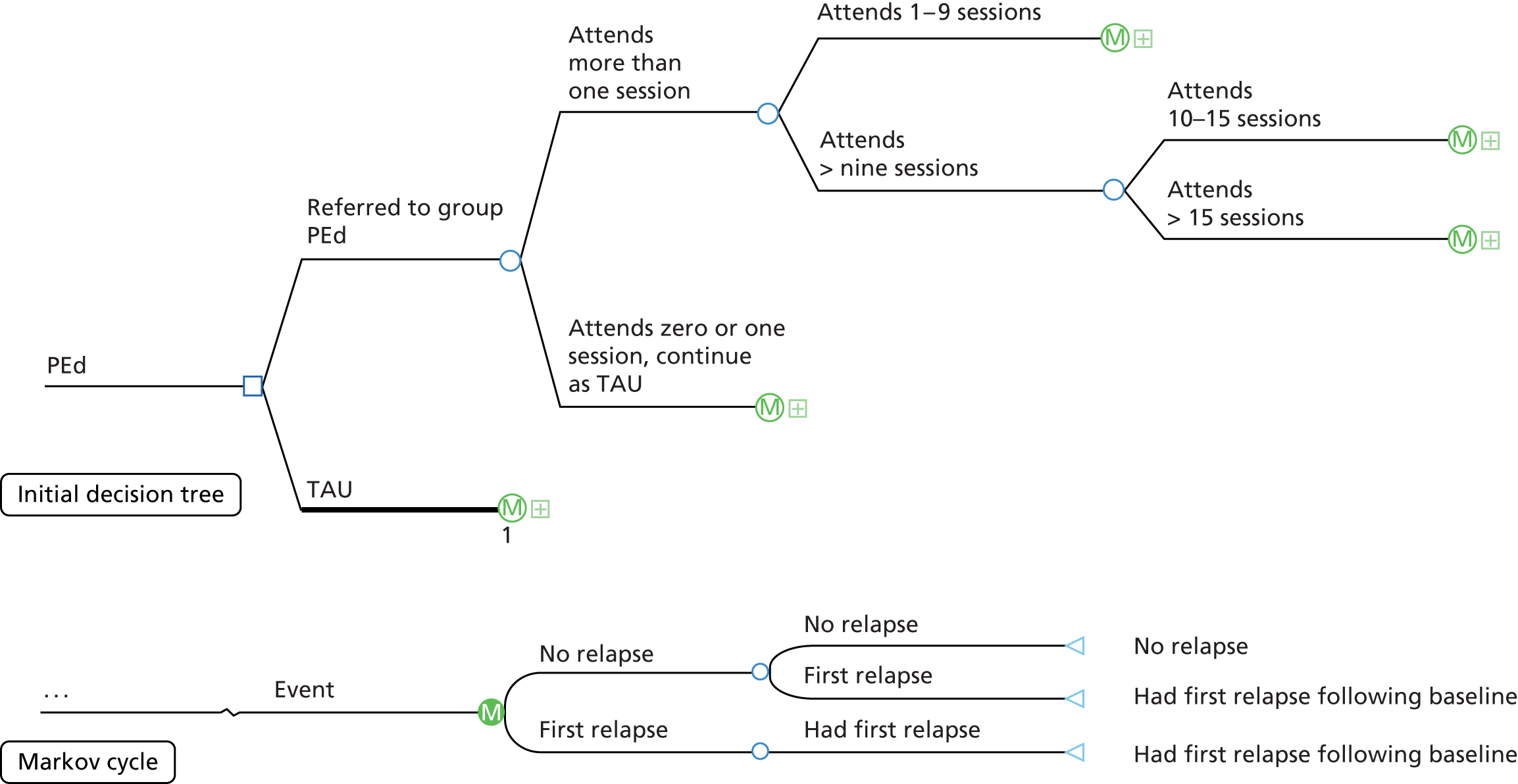
The following data were estimated from the PEd arm of the trial data set:
-
probability of attending group sessions
-
probability of first relapse in the follow-up period
-
the costs of the intervention
-
the cost per week of relapse/no relapse, estimated as the average cost per assessment divided by 32 weeks (the planned length of the period between assessments)
-
the average utility of relapse/no relapse
-
the time to relapse and time following relapse (weeks).
It was assumed that the cost per week of relapse/no relapse and the utility associated with the relapse and no relapse states would be the same for both PEd and TAU. All that differed between the two management strategies was the probability of relapse and time to relapse.
The probabilities of relapse and time to relapse for PEd were calculated from the predicted data generated for the clinical analysis. The utility and cost parameters for the relapse and no relapse states were estimated from the multiple imputation data generated for the economic evaluation. All data were entered into the model as distributions. Beta distributions for integers were used for the probability data. Gamma distributions were used for the cost, utility and time data. The cost and time distributions were constrained to a minimum value of zero.
Monte Carlo simulation in TreeAge software (10,000 iterations; TreeAge Software, Inc., Williamstown, MA, USA) was used to estimate the net costs, QALYs and ICER of PEd. A probabilistic sensitivity analysis was used to estimate the likelihood that PEd was cost-effective compared with TAU and to generate cost-effectiveness acceptability curves.
Results: within-trial analyses
Baseline clinical and demographic characteristics
The main sociodemographic characteristics of participants are reported in Table 4. Additional sociodemographic characteristics of participants were used as covariates for the economic analyses, and these are shown in Appendix 1, Table 77. Overall, there were no apparent differences between the groups on these additional characteristics, although fewer people in the PEd group (33%) than in the PS group (45%) lived alone.
The key baseline characteristics identified (by regression analysis and/or literature) as important for the analysis of costs and QALYs were:
-
which wave (PS or PEd group) participants were enrolled in
-
the number of previous episodes of BD
-
type of bipolar diagnosis (1 or 2)
-
the HADS-A and HADS depression scores
-
whether or not the participant was white British
-
whether or not the participant was currently working
-
whether or not the participant had further or higher education
-
sex and age
-
whether or not the participant lived alone.
These variables were used as covariates for all of the primary and secondary analyses of costs, QALYs and cost-effectiveness. In addition, the baseline SF-6D utility score and the EQ-5D thermometer were used as covariates in the analyses of QALY data and baseline costs were used in the analyses of cost data. Utility values were not correlated with the baseline SCID mania scores (Pearson’s coefficient: –0.004; p = 0.954).
Baseline utility scores were correlated with the following baseline clinical measures:
-
SOFAS (Pearson’s coefficient = 0.302; p = 0.000; n = 243)
-
SAS (Pearson’s coefficient = –0.163; p = 0.010; n = 246)
-
HADS-A (Pearson’s coefficient = –0.474; p = 0.000; n = 245)
-
HADS depression (Pearson’s coefficient = –0.481; p = 0.000; n = 245)
-
SCID depression (Pearson’s coefficient = –0.345; p = 0.000; n = 258).
In addition, utility values at the 96-week assessment were correlated with whether or not the participant had a relapse (Pearson’s coefficient = –0.298; p = 0.003; n = 99) and time to relapse (Pearson’s coefficient = 0.322; p = 0.001; n = 99). QALYs, which combine survival with utility, were similarly correlated with whether or not the participant relapsed (Pearson’s coefficient = –0.234; p = 0.048; n = 72) and time to relapse (Pearson’s coefficient = 0.227; p = 0.055; n = 72). Both utility values and QALYs were correlated with the 96-week clinical measures used as secondary outcomes (p < 0.02).
The trial participants had lower utility scores compared with the population norms for the UK (mean 0.68, 95% CI 0.65 to 0.72; n = 258) versus (95% CI 0.85 to 0.91126) for people of a similar age band (UK population norms, 35–54 years; trial participants, mean age 45 years; 95% CI 44 to 46 years). Overall, the baseline EQ-5D utility values indicate that this sample of participants was of similar health to the sample of participants enrolled in the Cost Utility of the Latest Antipsychotic drugs in Schizophrenia Study (CUtLASS) trials,116,127 who had severe schizophrenia and required a change in medication (mean 0.63, 95% CI 0.60 to 0.66; n = 362) (Linda M Davies, University of Manchester, 2015, unpublished analysis).
Descriptive analysis
Descriptive data are presented below for participants with complete cost and EQ-5D data. Tables reporting available data are summarised in Appendices 2 (utility and QALY data) and 3 (service use and costs) for interested readers.
Health status and quality-adjusted life-years at follow-up
Table 13 reports the percentage of people with no problems on each of the EQ-5D health domains at each follow-up assessment.
| EQ-5D health state | Treatment group | |
|---|---|---|
| PEd (N = 28/153), n (%) | PS (N = 27/151), n (%) | |
| Baseline | ||
| No problem with mobility | 21 (75) | 19 (70) |
| No problem with self-care | 27 (96) | 24 (89) |
| No problem with usual activities | 13 (46) | 13 (48) |
| No problem with pain/discomfort | 17 (61) | 18 (67) |
| No problem with anxiety/depression | 15 (54) | 14 (52) |
| 32-week assessment | ||
| No problem with mobility | 21 (75) | 18 (67) |
| No problem with self-care | 27 (96) | 21 (78) |
| No problem with usual activities | 14 (50) | 11 (41) |
| No problem with pain/discomfort | 16 (57) | 16 (59) |
| No problem with anxiety/depression | 12 (43) | 9 (33) |
| 64-week assessment | ||
| No problem with mobility | 21 (75) | 20 (74) |
| No problem with self-care | 26 (96) | 21 (78) |
| No problem with usual activities | 16 (57) | 12 (44) |
| No problem with pain/discomfort | 20 (71) | 15 (56) |
| No problem with anxiety/depression | 14 (50) | 9 (33) |
| 96-week assessment | ||
| No problem with mobility | 21 (75) | 18 (67) |
| No problem with self-care | 24 (86) | 22 (82) |
| No problem with usual activities | 12 (43) | 10 (37) |
| No problem with pain/discomfort | 18 (64) | 15 (56) |
| No problem with anxiety/depression | 11 (39) | 14 (52) |
Table 14 reports the overall utility index at each follow-up assessment. The values suggest some changes in utility between assessments for both groups. For the PEd group, utility appears to increase at the 32- and 64-week assessments, then decrease at the 96-week assessment. In contrast, the utility values for the PS group appear to decline at the 32- and 64-week assessments, and then improve at the 96-week assessment. However, a comparison of the 95% CIs suggests that these changes could be due to chance and are not, therefore, statistically significant.
| EQ-5D utility value | Treatment group | |
|---|---|---|
| PEd (n = 28/153), mean (SD) [95% CI] | PS (n = 27/151), mean (SD) [95% CI] | |
| Baseline | 0.73 (0.28) [0.62 to 0.83] | 0.80 (0.22) (0.72 to 0.88) |
| 32-week assessment | 0.80 (0.16) [0.74 to 0.86] | 0.70 (0.29) (0.59 to 0.81) |
| 64-week assessment | 0.78 (0.24) [0.69 to 0.87] | 0.64 (0.34) (0.51 to 0.77) |
| 96-week assessment | 0.73 (0.24) [0.64 to 0.82] | 0.77 (0.19) (0.69 to 0.84) |
A regression analysis was conducted to explore the association between baseline utility values, whether or not a person attended more than one group session and whether or not a participant completed the SCID assessment at week 96. This analysis indicates a trend towards lower baseline utility values for those participants who attended two or more sessions than for those who attended no sessions or only one session (β –0.05, 95% CI –0.15 to 0.04; p = 0.293; n = 258) and that those people who did not complete the 96-week SCID assessment had lower utility values at baseline and at follow-up than those who did complete the 96-week SCID assessment (β 0.10, 95% CI 0.017 to 0.183; p = 0.019; n = 258). The higher utility of people who did not engage with the PEd or PS interventions may indicate that they had better overall health, which may have influenced their decisions about whether or not to participate in the group interventions. Participants who did not complete the final follow-up assessment had lower overall health from the start of their involvement in the trial, which may have influenced whether or not they completed follow-up. It is important to note that there is a high level of uncertainty about these results, as the numbers of participants who either participated in fewer than two sessions or did not complete the 96-week assessment were low.
The length of follow-up (days) and QALYs (derived from the EQ-5D) for people with complete cost and QALY data are presented in Table 15. The 95% CIs suggest the PEd and PS groups have similar lengths of follow-up and QALYs.
| Assessment time point | Treatment group | |
|---|---|---|
| PEd (n = 28), mean (SD) [95% CI] | PS (n = 27), mean (SD) [95% CI] | |
| Days of follow-up | ||
| Baseline–32 weeks | 244 (13) [238 to 249] | 237 (11) [233 to 241] |
| 32–64 weeks | 228 (26) [218 to 238] | 228 (32) [216 to 241] |
| 64–96 weeks | 219 (19) [211 to 226] | 220 (25) [210 to 230] |
| Total days | 690 (17) [684 to 697] | 686 (12) [681 to 690] |
| QALYs | ||
| Baseline–32 weeks | 0.51 (0.12) [0.46 to 0.56] | 0.49 (0.12) [0.43 to 0.54] |
| 32–64 weeks | 0.49 (0.11) [0.45 to 0.53] | 0.42 (0.20) [0.35 to 0.50] |
| 64–96 weeks | 0.45 (0.14) [0.40 to 0.50] | 0.42 (0.14) [0.37 to 0.48] |
| Total QALYs | 1.45 (0.33) [1.33 to 1.58] | 1.33 (0.44) [1.16 to 1.50] |
Service use and costs
Table 16 reports the average use of different types of health and social care for people with complete cost and QALY data. Before baseline, people in the PEd group had an average of eight more contacts with community mental health services than those in the PS group. Otherwise, the average use of services before baseline was similar between the groups. Table 17 reports the ranges of unit costs of the different types of health and social care services used to calculate the costs of health and social care used by trial participants.
| Service type | Treatment group | |
|---|---|---|
| PEd (n = 28/153), mean (SD) [95% CI] | PS (n = 27/151), mean (SD) [95% CI] | |
| 6 months before baseline | ||
| Hospital inpatient admissiona | 6.70 (18.45) [0.00 to 13.83] | 7.74 (21.85) [0.00 to 16.17] |
| Hospital outpatient care | 3.71 (3.04) [2.56 to 4.87] | 4.08 (5.57) [1.84 to 6.32] |
| General practiceb | 4.64 (4.71) [2.86 to 6.43] | 3.63 (4.26) [1.99 to 5.27] |
| Other primary physical health care | 0.39 (0.63) [0.15 to 0.63] | 1.26 (4.59) [0.00 to 3.03] |
| Community mental health and social care | 18.04 (31.28) [6.18 to 29.89] | 10.46 (9.60) [6.69 to 14.24] |
| Baseline–week 32 | ||
| Hospital inpatient admissiona | 1.56 (8.08) [0.00 to 4.68] | 1.20 (6.00) [0.00 to 3.61] |
| Hospital outpatient care | 2.39 (2.00) [1.63 to 3.15] | 2.30 (2.09) [1.49 to 3.10] |
| General practiceb | 3.18 (3.14) [1.39 to 4.37] | 4.56 (4.98) [2.63 to 6.48] |
| Other primary physical health care | 1.18 (4.53) [0.18 to 2.89] | 1.93 (6.38) [0.00 to 4.39] |
| Community mental health and social care | 10.36 (12.71) [5.54 to 15.17] | 13.46 (14.57) [7.83 to 19.19] |
| Weeks 32–64 | ||
| Hospital inpatient admissiona | 0.18 (0.94) [0.00 to 0.54] | 2.07 (10.78) [0.00 to 6.23] |
| Hospital outpatient care | 1.79 (1.50) [1.22 to 2.35] | 3.19 (4.94) [1.28 to 5.09] |
| General practiceb | 3.46 (2.70) [2.44 to 4.49] | 3.67 (3.43) [2.34 to 4.99] |
| Other primary physical health care | 0.93 (4.53) [0.00 to 2.64] | 1.59 (4.70) [0.00 to 3.41] |
| Community mental health and social care | 8.43 (16.56) [2.12 to 14.74] | 12.33 (15.50) [6.35 to 18.31] |
| Weeks 64–96 | ||
| Hospital inpatient admissiona | 0.00 (0.00) [0.00 to 0.00] | 0.33 (1.73) [0.00 to 1.00] |
| Hospital outpatient care | 2.29 (2.31) [1.41 to 3.16] | 3.37 (5.23) [1.35 to 5.39] |
| General practiceb | 3.43 (1.85) [2.73 to 4.13] | 3.59 (4.19) [1.98 to 5.21] |
| Other primary physical health care | 0.39 (1.42) [0.00 to 0.93] | 0.30 (0.95) [0.00 to 0.66] |
| Community mental health and social care | 12.29 (17.92) [5.49 to 19.08] | 7.59 (11.84) [3.03 to 12.16] |
| Service type | Range of unit costs (£) | Source |
|---|---|---|
| Psychiatric hospital inpatient admission | 348 per day (acute psychiatric ward) | NHS reference costs113 |
| 334 per day (rehabilitation ward) | ||
| 736 per day (psychiatric intensive care ward) | ||
| Other hospital inpatient admission | 491 per day (general medical ward) | NHS reference costs113 |
| 1650 per day (ear, nose and throat; day case admission) | ||
| Psychiatric hospital outpatient care | 221 per visit (adult mental illness) | NHS reference costs113 |
| 102–216 per visit (other psychiatric outpatient visits) | ||
| 145–191 per visit (psychotherapy, psychology) | ||
| 184 per visit (crisis team) | ||
| 24.90 per visit (depot clinic) | ||
| Other hospital outpatient admissions | 108 per visit (type of visit not specified, costed as average of all outpatient visits) | NHS reference costs113 |
| 26–215 per visit (other physical health care) | ||
| Tests and diagnostics | 3–4 per test (haematology, phlebotomy) | NHS reference costs113 |
| 28–177 per test (radiographic scans, MRI, biopsy) | ||
| General practice | ||
| GP | 37.50 (per surgery visit) | PSSRU115 |
| 94.50 (per home visit) | ||
| Practice nurse | 12.40 per visit | PSSRU115 |
| Single estimate, no range | ||
| District nurse, health visitor, midwifery | 59.58 per visit | PSSRU115 |
| Single estimate, no range | ||
| Other primary physical health care | 25–115 per visit | NHS reference costs;113 PSSRU115 |
| Community mental health/social care | 13.50 per visit (home help/care worker/health-care assistant/clinical or care support worker/link mental health worker/support time and recovery worker) | NHS reference costs;113 PSSRU115 |
| 68–135 per visit (CPN/case manager, care co-ordinator/CMHT) | ||
| 58–123 per visit (counselling and therapy) | ||
| 204.50 per visit (psychiatrist) | ||
| 91–204 (other miscellaneous services) | ||
| 207 per visit (social worker) | ||
| 13.50–104 per visit (other miscellaneous services) | ||
Table 18 reports the costs of the different types of service used at each assessment point for participants with complete cost and QALY data. Overall, there appear to be few differences in the costs of health and social care services between the two intervention groups. An exception to this is the cost of prescribed medications, which appear lower in the PS group at each assessment point than in the PEd group. However, the 95% CIs overlap between the two groups, suggesting that this difference may be due to chance.
| Services used | PEd (n = 28/153), mean (£a) (SD) (95% CI) | PS (n = 27/151), mean (£a) (SD) (95% CI) |
|---|---|---|
| 6 months before baseline | ||
| Hospital inpatient admission | 2747 (8319) [0 to 5958] | 2694 (7603) [0 to 5629] |
| Hospital outpatient care | 617 (542) [411 to 822] | 611 (675) [340 to 882] |
| General practiceb | 182 (337) [55 to 310] | 114 (135) [62 to 166] |
| Other primary physical health care | 42 (71) [15 to 69] | 48 (84) [15 to 81] |
| Community mental health/social care | 435 (786) [137 to 733] | 215 (501) [15 to 417] |
| Prescription medications | 565 (1055) [165 to 965] | 383 (825) [65 to 702] |
| Baseline–week 32 | ||
| Intervention | 446 (78) [416 to 476] | 452 (88) [418 to 486] |
| Hospital inpatient admission | 660 (2831) [0 to 1732] | 492 (2024) [0 to 1273] |
| Hospital outpatient care | 339 (271) [236 to 442] | 466 (429) [300 to 631] |
| General practiceb | 71 (65) [46 to 96] | 176 (275) [70 to 282] |
| Other primary physical health care | 45 (94) [10 to 81] | 41 (102) [2 to 80] |
| Community mental health/social care | 334 (518) [138 to 531] | 313 (645) [64 to 562] |
| Prescription medications | 593 (732) [316 to 870] | 373 (532) [168 to 578] |
| Weeks 32–64 | ||
| Hospital inpatient admission | 62 (329) [0 to 187] | 722 (3750) [0 to 2169] |
| Hospital outpatient care | 283 (154) [224 to 341] | 557 (628) [315 to 800] |
| General practiceb | 95 (77) [66 to 124] | 95 (113) [5 to 139] |
| Other primary physical health care | 13 (61) [0 to 36] | 62 (119) [16 to 107] |
| Community mental health/social care | 286 (794) [0 to 587] | 302 (532) (97 to 507) |
| Prescription medications | 594 (1124) [168 to 1020] | 301 (532) (188 to 415) |
| Weeks 64–96 | ||
| Hospital inpatient admission | 0 (0) [0 to 0] | 116 (603) [0 to 349] |
| Hospital outpatient care | 400 (453) [228 to 571] | 529 (694) [261 to 797] |
| General practiceb | 84 (50) [65 to 103] | 111 (170) [45 to 176] |
| Other primary physical health care | 17 (54) [0 to 37] | 16 (54) [0 to 36] |
| Community mental health/social care | 468 (731) [191 to 745] | 196 (623) [0 to 436] |
| Prescription medications | 633 (1118) [209 to 1056] | 356 (364) [213 to 494] |
The total cost for each assessment period is shown in Table 19 for the sample of people with complete cost and QALY data. Again, these indicate the costs are similar for the PEd and PS groups. However, the results do indicate that costs following baseline were lower than those for the 6 months prior to baseline for each group. The average cost per month fell from £709–769 before baseline to £314–337 between baseline and the 32-week assessment, to £181–276 between the week 32 and week 64 assessments and to £179–217 between the week 64 and week 96 assessments.
| Assessment time point | Treatment group | |
|---|---|---|
| PEd (n = 28/153), mean (£a) (SD) [95% CI] | PS (n = 27/151), mean (£a) (SD) [95% CI] | |
| 6 months before baseline | 4612 (8612) [1283 to 7941] | 4253 (8401) [808 to 7697] |
| Baseline–week 32 | 2488 (3317) [1232 to 3745] | 2314 (2227) [1455 to 3173] |
| Weeks 32–64 | 1333 (1785) [657 to 2009] | 2039 (3899) [535 to 3543] |
| Weeks 64–96 | 1601 (1656) [974 to 2229] | 1321 (1335) [806 to 1836] |
| Total baseline–week 96 | 5422 (6511) [2956 to 7889] | 5674 (5060) [3722 to 7627] |
Table 20 reports the costs of delivering PEd and PS. Training, session delivery and supervision costs were equivalent for PEd and PS (total for each treatment: training, £14,896; sessions, £51,282; and supervision, £2080). PEd was more expensive than PS because of the manuals distributed to PEd participants (total cost £616). The cost to deliver 21 sessions was £6206 for PS and £6261 for PEd. It was assumed that the costs of each group were incurred whether or not all participants attended all sessions. The mean number of planned participants per wave was 14 and so a cost per participant can be estimated as the mean cost to deliver 21 sessions divided by the planned group size (i.e. £6261/14 = £447 for PEd and £6206/14 = £443 for PS). Table 20 also reports the number of people in each wave who actually attended one or more sessions. This ranged between 70% and 100% of invited participants.
| PEd or PS group and year started | Treatment group | |||||
|---|---|---|---|---|---|---|
| PEd | PS | |||||
| Planned number of participants | Number (%) attending one or more sessions | Cost per planned participant (£) | Planned number of participants | Number (%) attending one or more sessions | Cost per planned participant (£) | |
| Wave 1: Manchester 2009 | 12 | 10 (83) | 522 | 12 | 10 (83) | 517 |
| Wave 2: Manchester 2010 | 17 | 13 (76) | 368 | 15 | 12 (80) | 414 |
| Wave 3: Barrow 2010 | 10 | 9 (90) | 626 | 11 | 11 (100) | 564 |
| Wave 4: Carlisle 2010 | 17 | 15 (88) | 368 | 17 | 16 (94) | 365 |
| Wave 5: Preston 2010 | 16 | 12 (75) | 391 | 16 | 14 (88) | 388 |
| Wave 6: Preston 2011 | 14 | 14 (100) | 447 | 15 | 11 (73) | 414 |
| Wave 7: Nottingham 2009 | 14 | 13 (93) | 447 | 11 | 11 (100) | 564 |
| Wave 8: Mansfield 2010 | 11 | 11 (100) | 569 | 10 | 7 (70) | 621 |
| Wave 9: Boston 2010 | 11 | 8 (73) | 569 | 13 | 10 (77) | 477 |
| Wave 10: Chesterfield 2011 | 14 | 11 (79) | 447 | 14 | 12 (86) | 443 |
| Wave 11: Nottingham 2012 | 17 | 17 (100) | 368 | 17 | 15 (88) | 365 |
Primary analysis
As described in Methods, the primary analysis used multiple imputation to account for missing observations and missing follow-up data. The imputed mean costs and QALYs for each assessment are reported in Table 21. A regression analysis (using the Stata mi estimate command to account for multiple imputation data) was used to estimate the net costs and QALYs and to control for any impact of baseline covariates that may affect the cost and QALY values.
| Follow-up period | Unadjusted, mean (95% CI) | p-value for difference (PEd vs. PS) | ||
|---|---|---|---|---|
| PEd (n = 139/153a) | PS (n = 136/151a) | Unadjusted | Adjustedb | |
| Baseline–week 32 | ||||
| Days of follow-up | 227 (212 to 242) | 219 (203 to 235) | 0.48 | 0.68 |
| QALYs | 0.42 (0.38 to 0.46) | 0.39 (0.35 to 0.43) | 0.31 | 0.55 |
| Costs (£) | 2924 (2298 to 3549) | 2420 (1925 to 2914) | 0.17 | 0.15 |
| Weeks 32–64 | ||||
| Days of follow-up | 225 (218 to 231) | 227 (220 to 234) | 0.68 | 0.73 |
| QALYs | 0.40 (0.36 to 0.43) | 0.38 (0.34 to 0.42) | 0.50 | 0.78 |
| Costs (£) | 2379 (1432 to 3326) | 2383 (1556 to 3210) | 0.99 | 0.87 |
| Weeks 64–96 | ||||
| Days of follow-up | 224 (215 to 234) | 219 (209 to 230) | 0.46 | 0.51 |
| QALYs | 0.38 (0.35 to 0.42) | 0.37 (0.33 to 0.41) | 0.59 | 0.96 |
| Costs (£) | 2722 (1646 to 3799) | 2590 (1689 to 3491) | 0.85 | 0.59 |
| Baseline–week 96 | ||||
| Days of follow-up | 676 (658 to 694) | 665 (649 to 681) | 0.36 | 0.53 |
| QALYs | 1.20 (1.12 to 1.28) | 1.14 (1.04 to 1.23) | 0.33 | 0.67 |
| Costs (£) | 8025 (6001 to 10,049) | 7393 (5857 to 8929) | 0.61 | 0.42 |
Table 22 reports the net costs and QALYS for the two groups using the imputed data. As with the previous descriptive analyses, these results are characterised by relatively wide 95% CIs. This suggests that there are no statistically significant differences in costs or QALYs between the PEd and PS groups (after controlling for the effect of any baseline characteristics of participants or trial implementation, and treating costs and QALYs as independent of each other).
| Regression analysis with/without bootstrap simulation | Net cost (£) (standard error) [95% CI] | Net QALY (standard error) [95% CI] | Net cost (£) per QALY gained | Probability that PEd is cost-effective if willing to pay £30,000 to gain 1 QALY |
|---|---|---|---|---|
| No bootstrap (unadjusted) | 638 (1262) [–1848 to 3124] | 0.061 (0.062) [–0.064 to 0.185] | 10,484 | Not estimated |
| No bootstrap (adjusted) | 1042 (1276) [–1473 to 3557] | 0.025 (0.058) [–0.092 to 0.141] | 41,680 | Not estimated |
| Bootstrap | 1098 (431) [252 to 1943] | 0.023 (0.014) [0.001 to 0.056] | 47,739 | 0.35 |
The net cost and QALY data from the regressions were simulated (bootstrapped) 10,000 times to generate 10,000 pairs of net costs and QALYs. The results are reported in the last row of Table 22. In contrast to the results from the regression analysis alone, these simulated data suggest that PEd is associated with an overall net cost and gain in QALYs compared with the PS intervention. Given the similar costs of the two interventions, any additional cost incurred by the PEd participants indicates higher use of health and social care services. The point estimate of the net cost per QALY gained is relatively high at £47,739 (£1098/0.023 QALY), which is above the commonly reported NICE threshold of £20,000–30,000 per QALY gained. 119 There is some uncertainty associated with this cost per QALY estimate that reflects the variance in the costs and QALYs as well as uncertainty about the willingness-to-pay threshold.
This uncertainty is illustrated in Figure 9, which presents a scatterplot of the 10,000 pairs of net cost and QALY data in the form of a cost-effectiveness plane. Figure 9 illustrates that the net costs are mostly scattered above the horizontal axis, suggesting that, overall, PEd is likely to cost more than PS. The net QALY points on the scatterplot are mainly to the right of the y-axis, suggesting that PEd is more likely than PS to produce a health benefit in terms of QALYs.
FIGURE 9.
Cost-effectiveness plane of net cost and QALY pairs: primary analysis.

Figure 10 shows the cost-effectiveness acceptability plane. This indicates the likelihood or probability that the net cost per QALY gained by PEd (within the 10,000 simulated net cost and QALY pairs) is less than or equal to the amount (WTPV) that decision-makers would be willing to pay to gain 1 QALY. Compared with PS, the PEd intervention is likely to be cost-effective in ≥ 50% of cases if decision-makers are willing to pay ≥ £37,500 to gain 1 QALY.
FIGURE 10.
Cost-effectiveness acceptability curve: primary analysis.
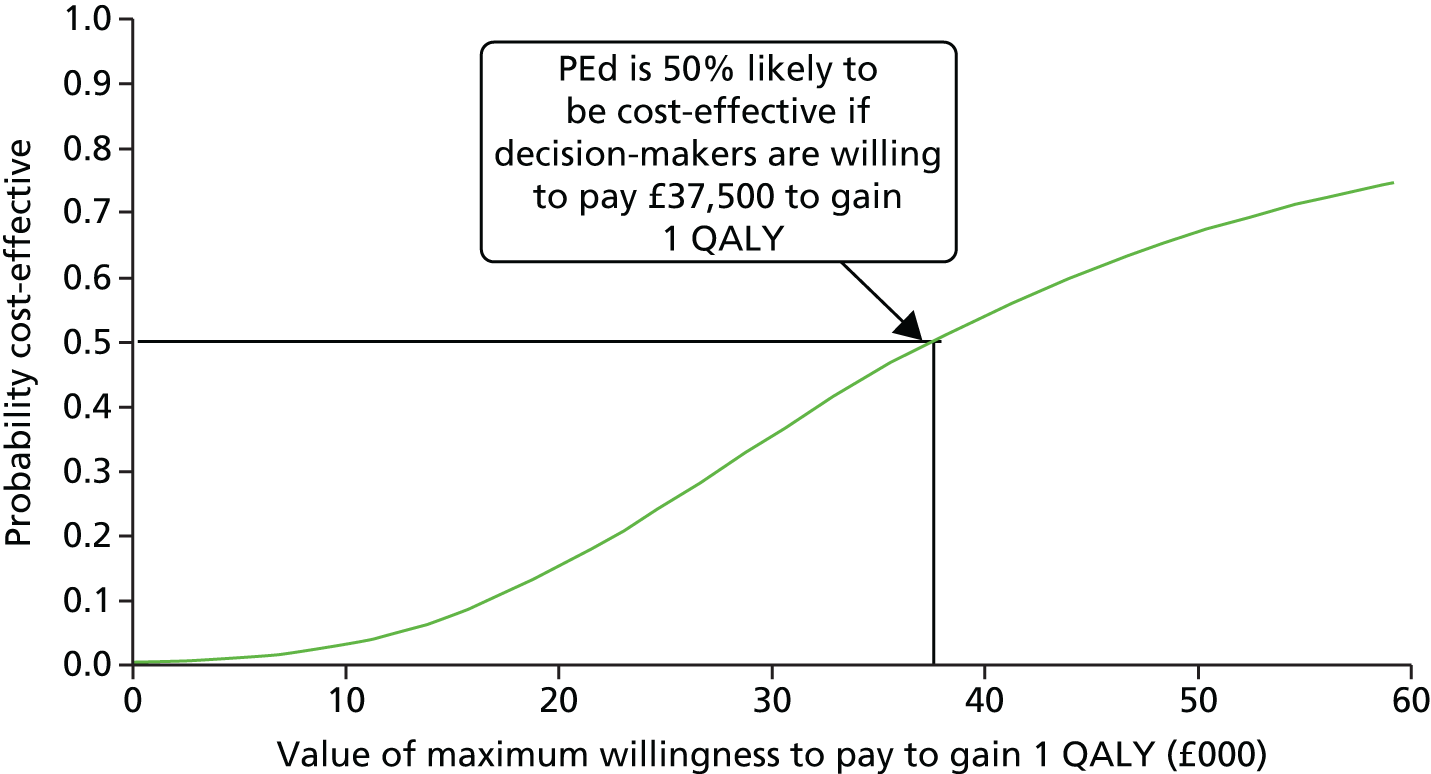
Sensitivity analyses
The results of all of the sensitivity analyses are presented in Table 23. However, Figures 11 and 12 report the cost-effectiveness acceptability curves for only those sensitivity analyses in which the conclusion differed from that of the primary analysis. All of the sensitivity and subgroup analyses report the bootstrapped net costs and QALYs that were generated from regression analyses to control for the impact of baseline characteristics. It is important to note that these analyses are exploratory and that the sample size available for each analysis may not be sufficient to identify important differences. In addition, the number of these exploratory analyses increases the chance of finding a difference between the exploratory analysis and the primary analysis because of the number of analyses, rather than because an important difference exists. These sensitivity analyses used an intention-to-treat approach. They were used to assess whether or not there was uncertainty as a result of the choices made about the design of the economic evaluation (e.g. choice of health benefit measure, range of costs included) and analysis methods (e.g. method of dealing with missing observations and missing follow-up).
| Sensitivity analysis | Net cost (£) (95% CI) | Net QALY (95% CI) | Net cost per QALY gained (£) | Probability PEd cost-effective if willing to pay £30,000 to gain 1 QALY |
|---|---|---|---|---|
| Primary analysis | ||||
| 1098 (252 to 1943) | 0.023 (0.001 to 0.056) | 47,739 | 0.35 | |
| Type of imputation | ||||
| Complete-case analysis (n = 55) | –588 (–4734 to 3558) | 0.049 (–0.177 to 0.274) | PEd dominates, QALY gain, net saving | 0.83 |
| Missing QALY data imputed by linear interpolation (n = 287) | 1122 (272 to 1971) | 0.024 (0.004 to 0.045) | 46,750 | 0.23 |
| Alternative measures of health benefit | ||||
| Relapse-free years (n = 287) | 1098 (252 to 1943) | 0.131 (0.08 to 0.182) | 8382 | Probability PEd cost-effective if willing to pay £30,000 to gain 1 relapse-free year: 0.99 |
| Relapse avoided (n = 287) | 1098 (252 to 1943) | 0.102 (0.68 to 136) | 10,765 | Probability PEd cost-effective if willing to pay £30,000 to avoid one relapse: 0.99 |
| QALY estimated from SF-6D utility values (n = 287) | 1098 (252 to 1943) | 0.013 (0.000 to 0.027) | 84,461 | 0.07 |
| Alternative cost measures | ||||
| Costs medications, exclude costs ‘other’ medicines (n = 289) | 1008 (162 to 1854) | 0.028 (0.001 to 0.056) | 25,200 | 0.41 |
| Costs exclude costs of PEd/PS (n = 287) | 1124 (275 to 1974) | 0.028 (0.001 to 0.056) | 40,142 | 0.34 |
FIGURE 11.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, sensitivity analyses: complete cases, relapse-free years, relapse avoided.

FIGURE 12.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, sensitivity analyses: length of follow-up.

Figures 11 and 12 summarise the cost-effectiveness acceptability curves for only the sensitivity analyses that indicate a different conclusion from the primary analysis. PEd is more likely to be cost-effective than PS in the following scenarios:
-
for the complete-case analysis (that does not include multiple imputation of missing data)
-
if participants are followed up for 64 weeks rather than for 96 weeks
-
when the clinically based outcomes of relapse avoided and relapse-free years are used as the measure of health benefit instead of QALYs.
Table 24 reports the estimated net costs, QALYs and ICER of PEd for each of the within-trial subgroup analyses conducted, along with the probability that PEd is cost-effective compared with PS. Figures 13 and 14 summarise the cost-effectiveness acceptability curves for those subgroup analyses that indicate a different conclusion (compared with the primary analysis) about the likely cost-effectiveness of PEd.
| Subgroup analysis | Net cost (£) (95% CI) | Net QALY (95% CI) | Net cost per QALY gained (£) | Probability that PEd is cost-effective if willing to pay £30,000 to gain 1 QALY |
|---|---|---|---|---|
| Primary analysis | ||||
| 1098 (252 to 1943) | 0.023 (0.001 to 0.056) | 47,739 | 0.35 | |
| Number of PEd or PS sessions attended | ||||
| < 10 (n = 112) | 149 (–555 to 852) | 0.019 (–0.025 to 0.064) | 7842 | 0.72 |
| ≥ 10 (n = 175) | 1266 (24 to 2507) | 0.023 (–0.012 to 0.057) | 55,043 | 0.25 |
| ≥ 16 (n = 118) | –844 (–1997 to 308) | 0.080 (0.036 to 0.124) | PEd dominates | 0.99 |
| 1–15 (n = 138) | 1598 (896 to 2301) | 0.001 (–0.040 to 0.043) | 1,598,000 | 0.02 |
| 0–15 (n = 169) | 955 (332 to 1577) | –0.003 (–0.040 to 0.034) | PS dominates | 0.07 |
| 0 (n = 31) | –649 (–2114 to 814) | 0.056 (–0.049 to 0.161) | PEd dominates | 0.92 |
| Number of previous episodes | ||||
| ≥ 8 (n = 249) | 1238 (270 to 2206) | 0.030 (0.001 to 0.060) | 41,267 | 0.34 |
| 1–7 (n = 38) | 1267 (–451 to 2985) | –0.056 (–0.132 to 0.019) | PS dominates | 0.04 |
| Number of previous episodes, number of sessions attended | ||||
| ≥ 8 episodes, < 16 sessions (n = 147) | 1026 (385 to 1667) | 0.012 (–0.028 to 0.052) | 85,500 | 0.21 |
| ≥ 8 episodes, 16+ sessions (n = 103) | –1109 (2504 to 287) | 0.043 (–0.003 to 0.089) | PEd dominates | 0.99 |
| 1–7 episodes, 16+ sessions (n = 15) | –713 (–1898 to 472) | 0.266 (–0.201 to 0.733) | PEd dominates | 0.99 |
| 1–7 episodes, <16 sessions (n = 23) | 6271 (3716 to 8826) | –0.133 (–0.221 to –0.044) | PS dominates | 0.00 |
| Participant completed SCID at 96-week follow-up | ||||
| SCID score (n = 212) | 983 (–25 to 1990) | 0.038 (0.009 to 0.067) | 25,868 | 0.61 |
| No SCID score (n = 75) | 470 (–273 to 1213) | –0.017 (–0.003 to 0.049) | PS dominates | 0.18 |
| Type 1 or 2 bipolar diagnosis | ||||
| Type 1 (n = 229) | 1183 (177 to 2189) | 0.006 (–0.025 to 0.038) | 197,167 | 0.009 |
| Type 2 (n = 58) | 375 (–107 to 857) | 0.129 (0.077 to 0.181) | 2907 | 1.00 |
FIGURE 13.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, subgroup analyses: number of sessions attended.

FIGURE 14.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, subgroup analyses: completed 96-week SCID assessment, BD 2 diagnosis.

The subgroup analyses exploring the impact of number of sessions attended indicate that PEd was more likely to be cost-effective than PS for the following:
-
participants who attended ≤ 9 sessions
-
participants who attended ≥ 16 sessions
-
participants who attended no sessions; however, numbers for this analysis are small, and the group of people who attended no sessions and had follow-up assessments may be atypical.
The number of previous bipolar episodes at baseline did not change the conclusions of the primary analysis. Defining subgroups according to both number of sessions and number of previous episodes indicates that PEd was likely to be cost-effective in people who had ≥ 16 sessions regardless of the number of previous episodes (1–7 previous bipolar episodes or ≥ 8 previous bipolar episodes).
PEd was also likely to be cost-effective compared with PS in:
-
the sample of participants who completed the 96-week SCID assessment
-
those participants with a BD 2 diagnosis.
The final set of subgroup analyses explored whether or not the results of the analyses using the primary clinical outcome (relapse-free years) varied by different groups of the trial participants. Table 25 shows the results of these analyses. These indicated that PEd was not likely to be a cost-effective way to delay relapse if participants did not attend any sessions (PS or PEd) or for the group of people who had eight or more previous bipolar episodes and attended < 16 sessions.
| Subgroup analysis | Net cost (£) (95% CI) | Net relapse-free year (95% CI) | Net cost per relapse-free year gained (£) | Probability that PEd is cost-effective if willing to pay £30,000 to gain 1 relapse-free year |
|---|---|---|---|---|
| Primary analysis | ||||
| 1098 (252 to 1943) | 0.131 (0.080 to 0.182) | 8382 | 0.90 | |
| Number of PEd or PS sessions attended | ||||
| < 10 (n = 112) | 149 (–555 to 852) | 0.086 (–0.0003 to 0.168) | 1733 | 0.95 |
| ≥ 10 (n = 175) | 1266 (24 to 2507) | 0.137 (0.072 to 0.203) | 9240 | 0.99 |
| ≥ 16 (n = 118) | –844 (–1997 to 308) | 0.200 (0.115 to 0.280) | PEd dominates | 1.00 |
| 1–15 (n = 138) | 1598 (896 to 2301) | 0.063 (–0.011 to 0.136) | 25,365 | 0.61 |
| 0–15 (n = 169) | 955 (332 to 1577) | 0.076 (–0.010 to 0.141) | 12,566 | 0.87 |
| 0 (n = 31) | –649 (–2114 to 814) | –0.082 (–0.196 to 0.031) | 7915 saving per relapse-free year lost | 0.19 |
| Number of previous episodes | ||||
| ≥ 8 (n = 249) | 1238 (270 to 2206) | 0.053 (0.001 to 0.106) | 23,358 | 0.61 |
| 1–7 (n = 38) | 1267 (–451 to 2985) | 0.841 (0.689 to 0.993) | 1507 | 1.00 |
| Number of previous episodes, number of sessions attended | ||||
| ≥ 8 episodes, < 16 sessions (n = 147) | 1026 (385 to 1667) | –0.014 (–0.082 to 0.055) | PS dominates | 0.09 |
| ≥ 8 episodes, ≥ 16 sessions (n = 103) | –1109 (2504 to 287) | 0.099 (0.011 to 0.186) | PEd dominates | 1.00 |
| 1–7 episodes, ≥ 16 sessions (n = 15) | –713 (–1898 to 472) | 1.290 (0.876 to 1.700) | PEd dominates | 0.99 |
| 1–7 episodes, < 16 sessions (n = 23) | 6271 (3716 to 8826) | 0.314 (–0.101 to –0.527) | 19,971 | 0.83 |
| Participant completed SCID at 96-week follow-up | ||||
| SCID score (n = 212) | 983 (–25 to 1990) | 0.072 (0.012 to 0.131) | 13,653 | 0.89 |
| No SCID score (n = 75) | 470 (–273 to 1213) | 0.379 (0.282 to 0.476) | 51 | 1.00 |
| Type 1 or 2 bipolar diagnosis | ||||
| Type 1 (n = 229) | 1183 (177 to 2189) | 0.123 (0.067 to 0.179) | 9617 | 0.99 |
| Type 2 (n = 58) | 375 (–107 to 857) | 0.187 (0.062 to 0.311) | 2005 | 1.00 |
Results: decision tree model
Table 26 summarises the decision tree events and structure and describes the probability data inputs to the economic model, and Table 27 presents the cost, utility and time data used. These parameters are derived from the within-trial analysis reported above.
| Model branch | Event sequence | Probability (95% CI) |
|---|---|---|
| 1 | Attends more than one session | 0.78 (0.71 to 0.74) |
| 1.1 | Attends 2–9 sessions | 0.19 (0.13 to 0.27) |
| 1.1.1 | First relapse, baseline–week 32 | 0.61 (0.39 to 0.79) |
| 1.1.2 | No first relapse, baseline–week 32 | 0.39 (0.21 to 0.61) |
| 1.1.2.1 | First relapse, weeks 33–64 | 0.11 (0.01 to 0.55) |
| 1.1.2.2 | No first relapse, weeks 33–64 | 0.89 (0.45 to 0.99) |
| 1.1.2.2.1 | First relapse, weeks 65–96 | 0.13 (0.01 to 0.60) |
| 1.1.2.2.2 | No first relapse, weeks 65–96 | 0.87 (0.40 to 0.99) |
| 1.2 | Attends more than 9 sessions | 0.81 (0.73 to 0.87) |
| 1.2.1 | Attends 10–15 sessions | 0.31 (0.22 to 0.41) |
| 1.2.1.1 | First relapse, baseline–week 32 | 0.43 (0.27 to 0.72) |
| 1.2.1.2 | No first relapse, baseline–week 32 | 0.57 (0.38 to 0.73) |
| 1.2.1.2.1 | First relapse, weeks 33–64 | 0.18 (0.05 to 0.45) |
| 1.2.1.2.2 | No first relapse, weeks 33–64 | 0.82 (0.55 to 0.95) |
| 1.2.1.2.2.1 | First relapse, weeks 65–96 | 0.21 (0.06 to 0.52) |
| 1.2.1.2.2.2 | No first relapse, weeks 65–96 | 0.79 (0.48 to 0.94) |
| 1.2.2 | Attends > 15 sessions | 0.69 (0.59 to 0.78) |
| 1.2.2.1 | First relapse, baseline–week 32 | 0.33 (0.23 to 0.35) |
| 1.2.2.2 | No first relapse, baseline–week 32 | 0.67 (0.55 to 0.77) |
| 1.2.2.2.1 | First relapse, weeks 33–64 | 0.13 (0.06 to 0.27) |
| 1.2.2.2.2 | No first relapse, weeks 33–64 | 0.87 (0.73 to 0.94) |
| 1.2.2.2.2.1 | First relapse, weeks 65–96 | 0.23 (0.12 to 0.39) |
| 1.2.2.2.2.2 | No first relapse, weeks 65–96 | 0.77 (0.61 to 0.88) |
| 2 | Attends 0 sessions, continue as control | 0.22 (0.16 to 0.20) |
| Parameter | Treatment group | ||
|---|---|---|---|
| PEd, mean (95% CI) | PS, mean (95% CI) | All, mean (95% CI) | |
| Utility | |||
| No relapse | 0.703 (0.635 to 0.771) | 0.669 (0.595 to 0.743) | 0.689 (0.638 to 0.739) |
| Relapse | 0.592 (0.530 to 0.654) | 0.588 (0.524 to 0.653) | 0.590 (0.543 to 0.637) |
| Cost (£) | |||
| Intervention | 450 (446 to 454) | 452 (448 to 456) | 451 (448 to 454) |
| No relapse | 1554 (1109 to 1999) | 1331 (850 to 1811) | 1458 (1117 to 1799) |
| Relapse | 3154 (2103 to 4205) | 2736 (2035 to 3436) | 2933 (2318 to 3549) |
| Weeks to relapse (within cycle) | |||
| Weeks 0–32 | 14.47 (12.11 to 16.82) | 13.04 (11.02 to 15.05) | 13.74 (12.20 to 15.28) |
| Weeks 33–64 | 18.03 (12.46 to 23.60) | 17.05 (13.69 to 20.41) | 17.39 (14.52 to 20.27) |
| Weeks 65–96 | 18.85 (14.14 to 23.56) | 18.86 (13.68 to 24.03) | 18.85 (15.44 to 22.26) |
| Weeks 0–96 | 29.63 (24.21 to 35.06) | 28.54 (23.76 to 33.32) | 29.06 (25.49 to 32.63) |
The net costs and QALYs of PEd compared with TAU are shown in Tables 28 and 29, while Figures 15–17 give the cost-effectiveness acceptability curves for the analyses.
| Analysis | Net cost (£) (95 percentile) | Net QALY (95 percentile) | Net cost per QALY (£) | Probability PEd cost-effective | |||
|---|---|---|---|---|---|---|---|
| WTPV = £15,000 per QALY | WTPV = £20,000 per QALY | WTPV = £25,000 per QALY | WTPV = £30,000 per QALY | ||||
| Probability of relapse is higher in TAU than group PEd, probability of relapse group PEd = 0.58 (95% CI 0.50 to 0.66) | |||||||
| 15% higher | 97 (–6613 to 4890) | 0.010 (–0.266 to 0.269) | 9700 | 0.47 | 0.48 | 0.50 | 0.51 |
| 20% higher | 18 (–7404 to 5065) | 0.014 (–0.277 to 0.290) | 1286 | 0.48 | 0.50 | 0.51 | 0.52 |
| 25% higher | –60 (–8142 to 5136) | 0.017 (–0.292 to 0.312) | PEd dominates | 0.50 | 0.51 | 0.52 | 0.53 |
| Analysis | Net cost (£) (95 percentile) | Net QALY (95 percentile) | Net cost per QALY (£) | Probability PEd cost-effective | |||
|---|---|---|---|---|---|---|---|
| WTPV = £15,000 per QALY | WTPV = £20,000 per QALY | WTPV = £25,000 per QALY | WTPV = £30,000 per QALY | ||||
| Time to relapse is lower in TAU than group PEd, mean time to relapse group PEd = 29.63 weeks (95% CI 24.21 to 35.06 weeks) | |||||||
| 42.5% lower | 51 (–4667 to 2765) | 0.012 (–0.154 to 0.190) | 4250 | 0.45 | 0.48 | 0.49 | 0.50 |
| 50% lower | 1 (–4802 to 2523) | 0.014 (–0.145 to 0.190) | 71 | 0.47 | 0.49 | 0.50 | 0.52 |
| 55% lower | –32 (–4885 to 2431) | 0.015 (–0.141 to 0.191) | PEd dominates | 0.47 | 0.49 | 0.51 | 0.52 |
| 60% lower | –66 (–5043 to 2336) | 0.017 (–0.136 to 0.192) | PEd dominates | 0.48 | 0.50 | 0.52 | 0.53 |
| 67.5% lower | –116 (–5266 to 2263) | 0.018 (–0.135 to 0.195) | PEd dominates | 0.50 | 0.52 | 0.53 | 0.55 |
FIGURE 15.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, comparison with TAU, probability of relapse is higher in TAU.
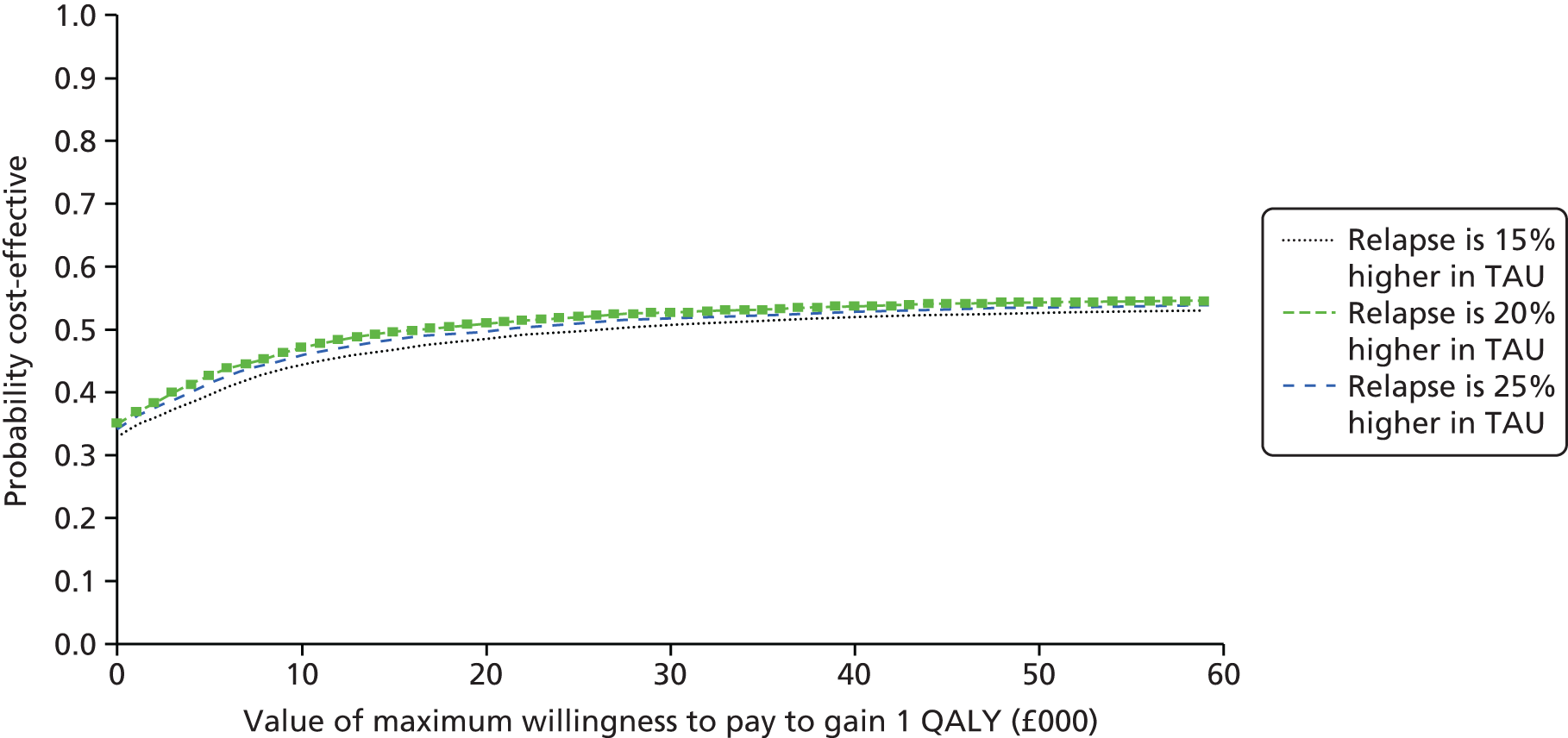
FIGURE 16.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, comparison with TAU, time to relapse is lower in TAU.

FIGURE 17.
Cost-effectiveness acceptability curves, willingness to pay to gain 1 QALY, comparison with TAU, probability of relapse is higher, time to relapse is lower in TAU.

The analyses demonstrate that, if the only effect of PEd is to reduce the probability that a person has a relapse (see Table 26 and Figure 15), then PEd may be cost-effective compared with TAU if the relapse rate in TAU is 15% higher than in PEd. This also requires that decision-makers are willing to pay £30,000 to gain 1 QALY. This translates to a probability of relapse in TAU of ≥ 0.66 for PEd to be cost-effective (i.e. the overall probability of relapse for PEd in the trial is 0.58 and a 15% increase in this probability gives a relapse rate of 0.66 for TAU).
If decision-makers are willing to pay only £15,000 to gain 1 QALY, then the probability of relapse needs to be 25% higher in the TAU group than in PEd. At 60 weeks, the probability of relapse for the PEd participants in our study was 0.50. The Bond and Anderson review32 indicates that, for TAU, the probability of relapse over an average of 60 weeks is 0.70. This is 40% higher than the PEd estimate in our study. The result indicates that the minimum difference required in the probability of relapse for PEd to be cost-effective may be achievable in routine practice.
If the only effect of PEd is to increase the time to relapse (but not the probability of relapse), then PEd may be cost-effective compared with TAU if the time to relapse in TAU is 43% lower than in PEd (see Table 27 and Figure 16). This also requires that decision-makers are willing to pay £30,000 to gain 1 QALY. The average (unadjusted) time to relapse for PEd is around 30 weeks (95% CI 24 to 35 weeks); accordingly, the average time to relapse of TAU is around 20 weeks. If decision-makers are willing to pay only £15,000 to gain 1 QALY, then the time to relapse needs to be 68% lower in the TAU group than in the PEd group. However, the data from our trial suggest that PEd has an effect on both the probability of relapse and the time to relapse.
If PEd both lowers the probability of relapse and delays the time to relapse, as seems more likely from our trial data, then PEd may be cost-effective compared with TAU. If decision-makers are willing to pay £30,000 to gain 1 QALY, the probability of relapse needs to be 10% higher in TAU, and the time to relapse 10% lower than PEd, for PEd to be cost-effective. If decision-makers are willing to pay only £15,000 to gain 1 QALY, the probability of relapse needs to be 20% higher in TAU and the time to relapse 20% lower than PEd.
Discussion
Summary of findings
Overall, the within-trial analyses of mean costs and QALYs suggested uncertainty about whether or not there were any differences between the PEd and PS groups. However, the primary analysis of net costs, QALYs and cost per QALY gained suggested a net additional cost for the PEd intervention of £1098 (95% CI £252 to £1943) and a small QALY gain of 0.023 (95% CI 0.001 to 0.056). This gives an incremental cost of £47,739 to gain 1 QALY. The additional cost of PEd indicates higher service use by the PEd participants. There was some indication that costs were higher in the PEd group in the period prior to baseline, which could mean that there were differences in the underlying need and access to health-care services. It is not clear what impact this may have on the analyses. However, as previous costs tend to predict future costs, costs for the 6 months before the baseline assessment were included as a covariate to control for baseline differences and to take any possible imbalances between the PEd and PS groups into account.
A further conclusion from the primary, within-trial analysis was that the PEd intervention, compared with PS, was likely to be cost-effective only if decision-makers are willing to pay at least £37,500 to gain 1 QALY. If decision-makers are not prepared to pay this amount, then PEd is unlikely to be more cost-effective than the equally intensive PS used for the control arm in this trial. At a willingness-to-pay threshold of £30,000, PS has a higher probability of being cost-effective (65%) than PEd (35%). As discussed in Chapter 2a, it is unclear whether or not people are able to access similar PS in routine clinical practice.
The results of the economic model analyses suggest that PEd could be cost-effective compared with TAU if PEd affects only the probability of relapse and reduces the probability of relapse by 15–25%. The probability of relapse for PEd used in the model was 0.58. This appears feasible when compared with the overall rates of relapse found in a recent systematic review of PEd. 32 If PEd affects both probability of relapse and the time to relapse, it would need to decrease the probability of relapse by 10–20% and increase the time to relapse by 10–20% to be cost-effective (depending on how much the decision-maker is willing to pay to gain 1 QALY). This translates into a probability of relapse in TAU of 0.64–0.70 and a time to relapse of 24–27 weeks.
However, the results from the economic model analyses indicated that the 95th percentiles for the net costs and net QALYs were wide and crossed zero, indicating a high level of uncertainty.
Strengths and limitations
The within-trial economic analysis was subject to the same strengths and limitations discussed for the clinical evaluation of effectiveness in terms of trial design, trial sample and length of follow-up.
Descriptive and regression analyses were used to explore the potential impact of baseline participant and service characteristics on the costs and QALYs in order to identify covariates for the cost-effectiveness analysis. The key covariates identified were similar to those identified in other economic evaluations of interventions for people with complex physical or mental health problems. 116,117,128–130
Additional issues that are relevant for the economic analysis include the range of costs considered, the measure of health benefit used for the primary analysis, and a low level of participants with complete cost and utility data.
The costs included in the economic evaluation were limited to the direct costs of providing health and social care, in accordance with the perspective taken. This will underestimate the total costs of BD for the trial participants. If there are differences in the effectiveness of the PEd or PS interventions that lead to differences in employment between the two groups, then this will also affect the net costs associated with PEd. If employment is higher in the PS group, then the net cost of the PEd group will be underestimated, and may reduce the likelihood that PEd is cost-effective yet further.
The measure of health benefit, the QALY, was used for the primary analysis and most of the sensitivity and subgroup analyses. The EQ-5D, a generic measure, was used to collect data about health status, estimate utility values and combine these with data about survival to generate QALYs. This is in line with the NICE recommendations for economic evaluations. 119 It also enables comparison between different disorders. This is relevant for policy-makers and commissioners, who have to consider the distribution of limited budgets between different health-care services. However, a generic health status measure may not be sufficiently sensitive to identify important clinical changes in participants’ mental health or bipolar status and so could underestimate the benefits of PEd.
The descriptive analyses indicated that the EQ-5D health status measure and associated utility index correlated well with the clinical measures used in the trial. In addition, QALY estimates were associated with the primary relapse outcome measures. This gives an indication that the EQ-5D and QALYs are sensitive to changes in the health of trial participants.
There was a large number of missing data, in large part owing to missing mid-follow-up observations for people who completed follow-up. This adds uncertainty to the results of the primary and sensitivity analyses of the costs and outcomes. Multiple imputation was used to impute missing values. There was evidence that the missing data owing to either missing observations or incomplete follow-up were not missing at random, which is a key threat to the validity of the multiple imputation procedure. In addition, only 55 out of 304 participants had complete cost and QALY data for all four assessments, although 54% had complete data at two or more assessments and 34% had complete data at three or more assessments.
The regression models used to impute missing data were based on initial descriptive analyses and regression analyses to identify key baseline and follow-up variables that were associated with either cost or outcomes. These were included in the chained equations to iteratively impute missing data. This goes some way to controlling for the influence of observed variables on the missing data (e.g. the influence of deteriorating mental health/relapse on the use of services, health status and willingness/ability to engage with data collection at follow-up assessments). However, the influence of unobserved variables could not be taken into account, and it is unclear the extent to which this was important.
The results of the sensitivity analyses indicated some uncertainty in the data due to the method of dealing with missing data. The analysis that used complete-case data suggested a lower net cost per QALY gained and a higher probability that PEd was cost-effective compared with PS. However, the complete case could overestimate the cost-effectiveness of PEd if the group of participants with complete data had better health, were more likely to engage with the intervention or were more likely to respond to the intervention than those with missing data. This would suggest that the analysis using multiple imputation may be conservative and underestimate the relative cost-effectiveness of PEd. The results when QALYs were imputed by linear interpolation were similar to those found in the primary analysis.
The results were also sensitive to the length of follow-up. PEd was more likely to be cost-effective at the 64-week assessment than at the 32- or 96-week assessment. This may indicate that the effect of the intervention increased between weeks 32 and 64. Alternatively, it may also indicate that missing data due to incomplete 96-week follow-up had an influence on the overall net costs and QALYs that was not accounted for by the multiple imputation process to deal with missing data.
A range of sensitivity and subgroup analyses were conducted to explore whether or not aspects of the trial and economic evaluation design may affect the likelihood that PEd is cost-effective. These analyses also explored whether or not there were specific groups of participants who may benefit more from PEd. This approach can help to identify issues for policy and further research. However, it is important to note that these analyses are exploratory, and the sample size available for each analysis may not be sufficient to identify important differences. Additionally, the number of these exploratory analyses increases the chance of finding a difference between the exploratory analysis and the primary analysis because of the number of analyses, rather than because an important difference exists.
There was limited evidence from the published literature with which to explore whether or not PEd would be likely to be cost-effective when implemented in routine practice in different settings and patient groups. This limits the extent to which any additional modelling analyses could add to the results and conclusions of the within-trial sensitivity analyses, many of which confirmed the conclusion of the primary analysis that PEd may not be cost-effective using the willingness-to-pay thresholds indicated by NICE decisions and recent commentaries. 131–136 However, a key issue noted in Chapter 2a and by NICE guidelines is the lack of evidence about the cost-effectiveness of psychological therapies compared with TAU. Accordingly, an economic model was used to explore what PEd would need to achieve in terms of effectiveness to be cost-effective compared with TAU.
There was limited evidence from the published literature to inform the structure of a full economic model that compares PEd with TAU or estimate the costs and QALYs associated with events in the TAU arm of the model. An assessment of the likely cost-effectiveness of PEd compared with TAU is important for commissioners and service providers because of the intensive nature of the control intervention (PS) in our study, which may not reflect what people receive in reality.
A simple model explored the relative reductions in the probability of relapse and increases in time to relapse that need to be achieved by PEd for it to be cost-effective compared with TAU. The advantage of the modelling approach used is that it controls for heterogeneity and uncertainty arising from using data from several sources, collected in different ways, for different samples of study participants. The simple model was focused on the relative impact of the PEd intervention on the probability of relapse and the time to relapse, compared with TAU (defined as no PEd). However, these advantages also mean that the scope of the model is constrained by the trial design, the length of follow-up and the people with BD recruited into the main RCT. Additionally, using cost and utility data from the trial also means that these are subject to the same limitations and uncertainty as missing data.
Other issues
Current evidence and commentary indicates that NICE WTPVs are < £15,000 per QALY. 113,115–120 The actual willingness to pay depends on the level of uncertainty in the evidence and the corresponding confidence that an intervention with a cost per QALY within the WTPV will occur in practice.
The net cost per QALY gained by PEd, compared with PS, is relatively high, at £47,739, which is above the commonly reported NICE threshold of £20,000–30,000 per QALY gained, based on past decisions. Whether or not NICE supports a technology also depends on the level of uncertainty associated with the cost and QALY results supporting an intervention. A range of thresholds that decision-makers are willing to pay to gain 1 QALY are reported and debated in the literature. 131–136 However, if, as seems likely, commissioners and service providers wish to focus on relapse-free years, then PEd is much more likely to be cost-effective than PS, with less uncertainty. Whether or not the NICE thresholds for cost per relapse-free year gained would be as high as those for cost per QALY gained is unclear and would depend, in part, on whether or not the relapse-free year includes all the key aspects of health status included in the QALY.
The economic model analyses indicate that PEd may be cost-effective compared with TAU if it achieves reductions in the probability of relapse and increases in the time to relapse. This is an important consideration for commissioners and service providers in areas in which TAU does not include the type of intensive group PS used as the control in our trial. Whether or not these reductions are plausible will vary from setting to setting, depending on what TAU comprises.
The quantitative and qualitative evaluations of PEd reported in Chapter 2a suggest that there may be additional reasons for implementing PEd rather than PS, which may counteract the high cost per QALY gained.
Implications for research
The exploratory sensitivity and subgroup analyses indicated a number of areas for further research.
The results were sensitive to the length of follow-up. PEd was more likely to be cost-effective at the 64-week assessment than at the 32- or 96-week assessment. Further work to identify the trajectory of costs and QALYs during the intervention phase and over the longer term is needed. This will help understanding of whether the change found in our study reflects what would happen in routine practice or whether it is an artefact produced by missing observations and incomplete follow-up.
A subgroup analysis of the economic data indicated that PEd might be cost-effective compared with PS for people with type 2 BD, those who attended ≥ 16 sessions and those who completed the 96-week SCID assessment. The subgroup analyses also indicate that the number of sessions attended affected the relative cost-effectiveness of PEd. There appeared to be differences in the utility values for those people who completed no sessions or only one session of PEd or PS compared with those who completed two or more sessions. This may indicate that those people who did not engage with the PEd or PS interventions had better overall health, which may have influenced their decisions about whether or not to participate in the group interventions. However, those people who did not complete the 96-week SCID assessment had lower utility values at baseline and follow-up than those who did complete the 96-week SCID assessment. This may indicate that these participants had lower overall health from the start of their involvement in the trial, which may have influenced whether or not they completed follow-up.
These factors suggest the need for careful identification of people who are likely to engage with and benefit from the intervention. Further work is needed to understand the influence of a patient’s overall health on decisions to participate in group PEd or PS. Combined with the process evaluation conducted as part of our study, the additional research would help to target the intervention at participants able and willing to engage in the group intervention and/or improve access to group interventions.
The baseline EQ-5D data also suggest that the participants in this trial had worse overall health than the general population (compared with population norms for the UK). The data also suggest that the trial participants had similar levels of health to those enrolled in the CUtLASS trials of antipsychotic medications for people with severe schizophrenia who required a change in medication. 116,117 However, little is known about the health of people with BD. Further work to measure and value overall health would help in the design and effective implementation of current and future interventions.
The economic model identified that PEd could be cost-effective compared with TAU. However, there was little economic evidence to inform either the structure of the model or the estimate of the parameters. The results of the economic model analyses also indicated a high level of uncertainty. This, combined with the conclusions of recent reviews, indicates that further primary data collection and evaluation is required to identify whether PEd (or PS) is effective and cost-effective in comparison with TAU.
Chapter 3 Psychological treatment of anxiety in bipolar disorder
Abstract
Despite effective psychological treatments for anxiety, research into how best to treat anxiety in the context of BD is underdeveloped. This chapter reports on three linked phases of work: (1) an exploration of SU perspectives of the type of help they require for anxiety in BD; (2) a gathering of SU and professional views on preferences for delivery and content of adapted CBT for anxiety in bipolar disorder (AIBD); and (3) a RCT feasibility and acceptability study of a new integrated psychological therapy for AIBD. The results from phase 1 indicated how important anxiety experiences were to people with BD, as well as their desire for integrated psychological therapy to address anxiety. Phase 2 indicated the importance of designing an intervention that is flexible and collaborative, combining behaviour and cognitive self-management elements. Phase 3 showed that a novel integrated intervention was feasible, acceptable and valued by SUs, although the clinical outcomes did not significantly differ from those in TAU. Anxiety is an important problem for those with BD and additional research is required to identify the optimal integrated therapy approaches to address this.
Chapter overview
This chapter first outlines previous research exploring the impact of anxiety on people with BD and its treatment. The introduction highlights the lack of specifically designed psychological therapy options for anxiety in BD, despite its substantial impact on course and outcome. Three linked phases within this research stream are then described, which (1) explored SU perspectives of the type of help they require in relation to anxiety in BD; (2) gathered SU and professional views on preferences for delivery and content of adapted CBT for AIBD; and (3) conducted a RCT feasibility and acceptability study of a new integrated psychological therapy for AIBD. Having described the methods and results associated with each element of this stream, we consider the implications and limitations of this work in relation to both future research and clinical practice.
Introduction
Prevalence of anxiety in bipolar disorder
Anxiety disorders are relatively common in the general population, with 12-month prevalence estimates of 2–18% and lifetime risk of 20–29%. 137–142 However, prevalence rates for comorbid anxiety disorders are substantially higher in those with BD, with 12-month prevalence rates of 32–53%11,12 and lifetime risk of 60–90%. 5,143 In addition, there is evidence that anxiety disorders constitute the most common comordibity in BD, being more frequent than alcohol and substance misuse or personality disorder. 144 The most commonly used diagnostic scheme in bipolar research, DSM-IV,76 identifies seven types of anxiety disorder, namely agoraphobia, generalised anxiety disorder (GAD), obsessive–compulsive disorder (OCD), panic disorder, panic disorder with agoraphobia, post-traumatic stress disorder (PTSD), social phobia and specific phobia. According to Merikangas et al. ,5 the most common types of anxiety disorder comorbid with BD are social phobia, specific phobia and GAD.
Impact of anxiety on clinical course and outcome
Comorbid anxiety is an important issue in BD because of the distress it causes patients and, specifically, the negative impact it can have on the course and outcome of the condition. Feske et al. 17 found that higher scores on a composite measure of anxiety were associated with longer time to remission and worse medication side effects in individuals with an acute BD 1 episode. Otto et al. 12 followed 1000 participants with BD over 12 months. The presence of a comorbid anxiety disorder at recruitment was associated with longer time to recovery, higher relapse risk and poorer psychosocial function across the follow-up period. This pattern of poorer psychosocial functioning in individuals with comorbid anxiety disorders was replicated in a study of 1600 participants with BD followed up over 36 months. 143 Anxiety is also associated with higher rates of suicidal ideation and attempted suicide in those with BD. 145,146
Available interventions for anxiety in bipolar disorder
Given the potential importance of anxiety in relation to clinical outcomes in BD, there has been increasing interest in how to improve anxiety symptoms. As NICE indicates, there is clear evidence for the benefits of time-limited interventions for anxiety disorders as primary diagnoses. 145–149 To date, there have been no specific psychological interventions targeted at anxiety in the context of BD. Provencher et al. 150 have explored both drug and psychological therapy outcomes studies including individuals with BD and comorbid anxiety. The majority of studies reported were not specifically designed to address anxiety as such. The exceptions were a number of single cases and small case series studies of psychological treatment of anxiety in BD, the findings of which suggest a reduction of anxiety symptoms. The main conclusion of the paper was the urgent need for a RCT to evaluate the benefits of psychotherapy for anxiety in the context of BD. Since this publication, a further paper has presented three case series in which Barlow’s unified therapy protocol for anxiety disorders was applied to three individuals with anxiety disorders comorbid with BD. 151 This indicated that, again, psychological therapy focused on anxiety was feasible, with promising outcomes in particular for two of the three participants. Again, the authors highlighted the importance of a RCT to properly evaluate the benefits of psychotherapy for anxiety in BD.
Limitations in current research
The main focus of research to date has concerned the prevalence and impact of anxiety disorders on BD. However, many individuals who do not meet full diagnostic criteria for anxiety comorbidity still experience high levels of anxiety symptoms. 18 Furthermore, both current anxiety disorder and higher levels of current anxiety symptoms (irrespective of anxiety diagnosis) are associated with worse illness course. 152 It is, therefore, a limitation of current literature that it has tended to focus more on diagnostic anxiety categories rather than experience of anxiety symptoms. The research described below therefore focuses on the latter to avoid excluding participants with BD with significant anxiety experiences that do not neatly fit with DSM-IV criteria. 76 Although there are now a number of preliminary non-RCT studies that have delivered psychological therapy for anxiety in BD, none of these adopted an integrated approach in which both bipolar and anxiety experiences were addressed. Additionally, the therapies so far proposed seem to have been developed in the absence of a detailed understanding of the experience of anxiety from the perspective of people living with BD or of their views of the type of treatment that they would prefer. In line with these issues, the research reported here places the experiences and preferences of SUs with anxiety and BD as central to the development of a novel psychological intervention and its evaluation in a RCT.
Aims
The research reported here has the following aims.
-
To understand more about the impact anxiety has on the lives of people with BD.
-
To understand what psychological help is required and by whom in relation to anxiety in people with BD.
-
To develop and evaluate the feasibility and acceptability of CBT-informed time-limited therapy for people with anxiety and BD.
Overview of methods
Phase 1
Semistructured interviews were carried out with individuals with BD 1 and BD 2 who also had experience of anxiety symptoms. The focus of the interviews was on understanding more about how individuals experience anxiety in BD, the type and delivery format of psychological treatment that people had found helpful, and what they would like in the future. A specific focus of a parallel piece of work was to analyse these interviews to explore in detail how people define the nature of anxiety in BD (this work forms an element of a linked PhD recently completed by Kay Hampshire – employed as PARADES RA on this stream153). The conduct of the interviews was shared between service user researchers (SURs) and a RA. All data were transcribed verbatim and analysed by a research team that included a RA, a SUR, an academic clinical psychologist and an academic health psychologist. An analysis was conducted in line with thematic analysis principles as described by Braun and Clark. 91
Phase 2
A series of three treatment planning focus groups were held to inform the development and delivery of the therapy to be trialled in phase 3. Based on the advice of our service user reference group (SURG), the focus groups comprised clinicians, grant holders, SUs with experience of BD and anxiety, and researchers working together. The focus groups were conducted in line with principles outlined by Rabiee. 154 Each of the three focus groups (6–10 attendees in each) was structured to elicit information on prior experiences of care in relation to anxiety, plans for the new intervention and views on format, style and timing of the intervention planned. Information from phases 1 and 2 was integrated with a review of effective treatments for anxiety and bipolar into a therapy manual for the delivery of an integrated CBT intervention for AIBD.
Phase 3
A single-blind RCT was conducted to determine the feasibility and acceptability of the AIBD intervention compared with TAU. The AIBD intervention was delivered over a period of 4 months, with participants followed up for 20 months post randomisation. The primary outcome of the study was the feasibility and acceptability of AIBD (recruitment to target, retention to follow-up, absence of untoward incidents and participants’ experience of therapy). We have also conducted preliminary analysis of anxiety and mood outcomes, cost-effectiveness and potential mechanisms for change (stigma, positive self-appraisal and stability of social rhythms). Qualitative interviews were conducted with a maximum variance sample of participants randomised to AIBD to explore acceptability.
Phase 1: a qualitative investigation of service user perspectives on treatment of anxiety in bipolar disorder
Methods
Both phase 1 and phase 2 of this WS were approved by the NHS North West Research Ethics Committee reference (10/H1015/5) on 22 January 2010.
Sample
Participants were recruited on the basis of a diagnosis of BD confirmed using the SCID77 and their willingness to talk about their experiences of anxiety. A total of 21 SUs took part, who were recruited through NHS services and self-referral in response to local press adverts in the north west. All participants were provided with a written participant information sheet and gave informed written consent to take part in one-on-one qualitative interviews.
Assessment measures
Participants completed the SCID77 to confirm a diagnosis of BD. Current mood symptoms were determined using the MAS155 and HAM-D. 82 Anxiety symptoms were measured with the HADS-A. 85 All of the observer-rated measures were completed after the semistructured qualitative interview to avoid inadvertent influence on the participants’ responses in the latter. Purposive sampling156 was used to obtain a sample including participants with varied experience of anxiety as well as both male and female and older and younger individuals.
Qualitative interview on treatment of anxiety in bipolar disorder
A flexible topic guide was developed for the semistructured interviews. This was informed by consultation with the SURG for this WS, comprising individuals with experience of both BD and anxiety. A draft topic guide developed by the research team was then piloted with two SUs (members of the PARADES SURG) to ensure that the final version had an appropriate balance of structure and flexibility, and that questions were seen as both clear and relevant to the topic at hand. The topics covered in the interview were experiences of treatment and past treatment for anxiety, strategies for self-management of anxiety, desired features of an anxiety intervention specifically designed for BD, and potential facilitators/inhibitors for accessing such an intervention.
Analysis
Each interview was recorded digitally and transcribed verbatim. Thematic analysis was applied to the interview data in line with the principles described by Braun and Clarke. 91 This approach was adopted to help identify how people respond to their anxiety experiences and what approaches are perceived as most helpful in the management of these. The research team brought their own experiences to the analysis of these data, which are also important to acknowledge. These experiences included people having had difficulties with anxiety in the past, and an aim for this WS was to understand whether or not people felt that they need more tailored help for their anxiety than that already offered. Interviews were conducted by either a RA (KH) or a SUR (PB). The research team also included an academic clinical psychologist (SJ) and an academic health psychologist (SP). Analysis occurred in parallel with data generation and, using a constant comparative approach, initial codes emerging from interviews were used to inform subsequent interviews and ensure comprehensive exploration of emerging ideas. Agreement on emergent themes and their relationship with primary interview data was ensured through regular research team meetings.
Results
Sociodemographic and clinical data
The sociodemographic and clinical characteristics of the participants are shown in Table 30. Participants were, on average, 45.75 years old (SD 10.97 years; range 25–62 years) and mostly of white British ethnicity and > 60% were female. Despite the majority of participants having had experience of tertiary education, only 24% were in paid employment. Two-thirds of participants were either divorced or single, and three-quarters were parents.
| Characteristic | n (N = 21) |
|---|---|
| Sociodemographic | |
| Ethnicity: white British | 21 |
| Sex | |
| Male | 8 |
| Female | 13 |
| Highest level of education | |
| Secondary | 3 |
| Further | 9 |
| Higher | 9 |
| Employment status | |
| Employed (paid) | 5 |
| Employed (voluntary) | 2 |
| Retired | 4 |
| Student | 1 |
| Unemployed | 9 |
| Marital status | |
| Married/cohabiting | 7 |
| Divorced/separated | 7 |
| Never married | 7 |
| Children | |
| Children aged ≤ 16 years | 4 |
| Children aged > 16 years | 12 |
| Living arrangements | |
| Spouse/partner | 5 |
| Spouse/partner and children | 2 |
| Relatives and children | 1 |
| Relatives | 4 |
| Alone | 9 |
| Clinical | |
| Bipolar diagnosis | |
| BD 1 | 20 |
| BD 2 | 1 |
| HADS-A score | |
| 0–7 (normal) | 4 |
| 8–10 (mild) | 3 |
| 11–15 (moderate) | 12 |
| 16–21 (severe) | 2 |
| Number of current anxiety disorders | |
| 0 | 6 |
| 1 | 5 |
| 2 | 5 |
| 3 | 2 |
| 4 | 2 |
| Missing | 1 |
| Current anxiety disorder | |
| Social phobia | 7 |
| Agoraphobia | 2 |
| GAD | 9 |
| PTSD | 3 |
| Panic with/without agoraphobia | 3 |
| OCD | 3 |
| Specific phobia | 2 |
| Missing | 1 |
All but one participant had a diagnosis of bipolar 1 disorder and time since diagnosis averaged > 10 years (mean age at bipolar diagnosis 34.14 years, SD 9.83 years). HAM-D score (mean 9.81, SD 7 .43) and MAS score (mean 1.57, SD 2.27) were consistent with participants being out of acute mood episode at interview. HADS-A scores (mean 11.19, SD 4.59) indicated that > 80% of participants had a current experience of at least mild anxiety. Consistent with this, most participants met the SCID criteria for at least one anxiety disorder (mean age at first mood disorder diagnosis 25.7 years, SD 10.65 years), the most common being GAD and social phobia. As nine participants had more than one anxiety disorder, the current anxiety disorders list in Table 30 totals more than 21.
Qualitative interviews
Participants were happy to discuss both their experiences of anxiety and their perspectives on relevant treatment. The quotations below all refer to responses directly related to experiences of anxiety, except where highlighted. The primary themes identified are (1) benefits and limitations of existing approaches, (2) therapeutic experiences and aspirations and (3) cognitive and behavioural approaches to managing anxiety. Each is described in turn and supported with illustrative quotations. Participant numbers and HADS-A score at the time of the interview are provided.
Benefits and limitations of existing approaches
‘You can’t just treat it with drugs’
Those people who had been prescribed medication specifically for their anxiety reported that it was ineffective and that they were also concerned about potential or recurring side effects from taking such treatment:
. . . erm they started to put me on medication, to try and erm, stop this erm sort of panic attack and anxiety attack happening. Erm, they tried me on a, a mixture of medication . . . and the first one actually made me . . . a damn sight worse. Erm . . . and made me feel really poorly as well.
A008 HADS-A = 14
. . . they [diazepam] had bad side effects I think you felt like you had been hit by a train or something the next day after taking them.
A031 HADS-A = 14
Although medication was seen to have a role in treating BD in general, it was not seen as the most appropriate treatment response to anxiety:
It’s more active, I mean I think that, medication is needed but medication alone doesn’t do anything for anxiety, and it is just not active at all.
A054 HADS-A = 9
‘I just want to know why’
Participants described receiving inadequate information about BD in general and specifically about anxiety. This lack of information was a source of anxiety in its own right, and people described a need to know more about their anxiety experiences and how to manage them:
I’m always trying, I always think knowledge is, knowledge is power. And, and the more I know, the more better able I am to handle a situation which is not within my control.
A018 HADS-A = 14
Yes, I think you know basically the more I become self-aware the more I am able to get a grip on it but when the panic takes me, then I am gone.
A023 HADS-A = 13
A minority of participants had had access to structured psychological therapy for either anxiety or BD. However, there were no consistent reports of anxiety and mood experiences being addressed in an integrated manner. Particularly stark was one participant’s account of her therapist ignoring her specific request to address her social anxiety as part of therapy:
I mean I was very fortunate in seeing clinical psychologists for a 6-year period when I was between 19 and 25. And I know probably we were too concerned about trying to survive the bipolar than looking at the social phobia so perhaps we’ll forget about that. But recently when I’ve had CAT [cognitive analytic therapy] with a clinical psychologist and I’ve mentioned social phobia, it’s just, just totally dismissed.
A009 HADS-A = 9
Despite therapy approaches typically not taking an integrated approach to anxiety and BD, some participants did describe an improvement in anxiety as a consequence of therapy for BD in general:
And that, that, that’s what cognitive–behavioural therapy helped me . . . to change my thinking to. Not necessarily fun and dull all the time but much more . . . once you’d got into a more positive frame of mind, life was far more relaxing.
A018 HADS-A = 14
Therapeutic experiences and aspirations
‘They . . . don’t realise how serious the anxiety is’
Although a number of participants said that they would have liked help with their anxiety, most found that, when they had explored this, there were significant barriers to access. These included long waiting lists, difficulty in navigating through the system to obtain the right referral and getting professionals to understand that their anxiety was a not a trivial issue:
. . . they go to the GP and the GP understands nothing about anxiety. So . . . say, tells them there’s something else wrong with them . . . some physical problem. Or gives them the wrong medication er . . . doesn’t realise how serious the anxiety is so doesn’t recommend them to a psychiatrist or a psychologist.
A018 HADS-A = 14
Yes, getting through the GP to get the referrals there is not an awful lot of places now and waiting lists are ridiculously long.
A040 HADS-A = 12
An issue raised by the minority who had accessed structured psychological help for anxiety was that the timing of the intervention was often problematic. A particular problem was people struggling to benefit from therapy because they were acutely ill at the time they received it and were not in the right place to engage with the therapeutic approach or to remember and practise the strategies suggested:
I have got so much stuff on anxiety management, CBT stuff that I did in therapy because I was in hospital . . . and there is tons of therapy but when I am that bad I can’t, I can’t do it, I have got the mindfulness for anxiety book, and I have never even used it because when I am really anxious I can’t focus on it at all.
A017 HADS-A = 15
But as I said before going back and doing maybe another course to pick up some more . . . information now that I’m stable and not anxious erm because when you’re anxious you don’t . . . erm retain the information.
A012 HADS-A = 13
‘It’s such a difficult area to address because everyone has their own individual life experience’
Most participants did not feel that they received effective help for their anxiety experiences and that for such help to be effective would require mood and anxiety experiences to be addressed in a more integrated way:
. . . anxiety and my bipolar I consider the same thing, you know. Because my bipolar makes me sensitive and vulnerable to stress, tension and anxiety and overwhelms, I get anxious very, and overwhelmed very quickly because I get anxious and overwhelmed very quickly my bipolar kicks off. So I don’t know how to separate them, I don’t think I can.
A023 HADS-A = 13
In addition to highlighting the importance of an integrated approach, participants also noted that the way in which the therapist interacted with them was crucial. Examples of how the therapeutic relationship had gone wrong for participants included when therapists had been controlling, didactic and/or apparently uninterested in the client’s experiences. Understandably, participants felt that if a collaborative, trusting therapeutic relationship was lacking they would struggle to engage and to provide the information that might be crucial to the success of the intervention:
. . . people like the A- [Therapist] guy that I used to see . . . he had his own agenda, definitely as to how much time he had, he couldn’t even remember from one meeting to the next sometimes things I had told him which would irritate me terribly.
A030 HADS-A = 14
I felt worried more worried and more worried and I felt more unable to disclose information, because I didn’t know if they were on my side you know. So I think it’s very important to establish a bit of trust . . .
A054 HADS-A = 9
Key tools and resources for therapy
Participants highlighted the importance of having appropriate information in relation to both their anxiety and their bipolar mood experiences. In addition, they described how having such information in an accessible format would help them to reflect on what they had learnt in therapy and apply it over time so that they could self-manage future anxiety issues:
I really do think that if there were simple little booklets that people could be given, errm. Because when you’re ill you can’t read a . . . you can’t tackle a book. How could a doctor tell you to go and buy this [referring to a large self-help book]? It’s ridiculous.
A013 HADS-A = 2
Yes, I could do with some, some if there was a little book or some technique, all the techniques I use I have thought of myself . . .
A030 HADS-A = 14
Self-management strategies for anxiety
‘Do something or try and get your mind working on something else’
Participants described a number of self-management strategies, most of which were based on their own experiences rather than a result of formal therapy. These strategies were, typically, behavioural approaches that they adopted to cope with an acute anxiety experience until it had diminished. Addressing the physiological aspect of anxiety was common, mainly through the use of relaxation or breathing techniques or through physical exercise. Distraction was also discussed, with participants describing how they engaged in other activities to take their mind off the anxiety experience. Generally, these strategies had developed through a process of trial and error. Whether or not they were beneficial seemed to depend on when they were implemented, with such approaches being perceived as more effective if applied in the early stages of anxiety rather than in the middle of an acute episode:
So you either have two, two ways to get rid of it [anxiety], do something or put your mind, try and get your mind working on something else.
A030 HADS-A = 14
. . . I did find that [relaxation tape] was extremely useful. Erm . . . as long as I caught it before I was in full panic mode [laughs]. It, it never used to work if I was in full panic mode.
A008 HADS-A = 14
‘It’s all about putting things into perspective’
Participants understood that the way in which they responded to the patterns of thinking associated with the development and onset of their anxiety experiences was important in dealing with these experiences effectively. However, although there was a general awareness that being able to change their responses would be helpful, very few participants felt that they had been able to develop such skills without formal therapeutic help:
When you put things into perspective it seems like, when you are less preoccupied with all the, the implications of what someone might have said or the embarrassment of having someone said something, it doesn’t, life seems so much easier I think.
A031 HADS-A = 14
But then when you are dealing with emotions like I was dealing with, it’s hard enough just to cope with that, never mind trying to analyse yourself, because there is not many people can do that, it’s the hardest analysation there is.
A022 HADS-A = 7
Phase 2: therapy manual development – treatment planning focus groups
Methods
Sample
Eleven SUs with BD and personal experience of anxiety participated in a series of three focus groups, with between four and six attending on each occasion (three participants attended on more than one occasion). Focus groups were also attended by at least two PARADES RAs (Kay Hampshire and Phil Byrne), a clinical academic (Steven Jones), a PhD researcher (Nick Todd) and a clinician (Brian Langshaw). The total numbers in each group therefore ranged from seven to nine participants.
Service user participants were recruited from mental health trusts across north-west England (Manchester Mental Health and Social Care Trust, Lancashire Care, Cumbria Partnership, Greater Manchester West, Pennine Care and Merseycare) and through self-referral from SU groups and in response to advertisements in the local press. All participants were provided with a written participant information sheet and gave their informed written consent to take part.
Focus group structure and format
At the beginning of each group, participants were introduced to the facilitators and informed of the purpose of the groups, which was to obtain their views on refining the structure, content and format of a planned, integrated, individual intervention for anxiety in BD. The group was told that the refinement of the treatment manual for the therapy would run in parallel with the focus groups, so the first group would take place as the manual was being drafted and the final group would discuss a summary of the near-final version of the manual, based on the previous groups. In addition to information from the focus groups, manual development was informed by a literature review, expert guidance and NICE guidelines for both anxiety and BD, as described in more detail below.
The topics covered in the focus groups included SU perspectives on:
-
what content should be included in an individual intervention for anxiety in BD
-
how an individual intervention of this type should be delivered
-
what type and format of supporting resources should be offered in the intervention
-
what potential barriers are to accessing this type of intervention, and how they might be overcome
-
the ways in which an intervention of the type described might be helpful
-
the potential strengths and weaknesses of the final therapy manual reviewed in session 3 based on input from sessions 1 and 2.
Results
These results provide a summary of the primary themes emerging from the focus groups. The participant number (P) and focus group number (FG) are included with quotations throughout. SU participants readily engaged with the focus group format and talked in detail about their views of the proposed approach and the contrast between this and previous experiences of therapy. There was a general view that offering an approach that was focused on integrating help with anxiety with people’s experience of bipolar mood experience was both novel and appropriate. Participants reported their frustrations at not being able to access this type of care in the past or, if they had managed to access it, the limitations of what had been offered.
Content
Service users confirmed that they did not want therapy that focused only on anxiety. Rather, there was support for an approach that also helped people to make sense of their mood history:
If a therapist is aware of the bipolar running alongside the anxiety erm they’ll probably get less frustrated, and there might be tools that they can help . . .
P6 FG1
In reviewing personal histories, it was noted that this approach should be balanced and include not only challenges experienced, but also examples of things that had gone well and ways in which people coped. Once this context was provided, there was agreement on the importance of addressing anxiety and mood together in the same therapy. This was in contrast to participants’ prior experience, which was of either not being offered therapy or receiving therapy that addressed only one aspect of their difficulties:
But what I’ve found with er the CBT that I’ve had in the past, partly because it was undiagnosed, the bipolar, at the time, was er how the CBT for anxiety wasn’t taking into consideration my er mood at the time, and how likely I was to succeed with that CBT for anxiety because my bipolar was running alongside where it was at that particular time.
P1 FG1
Although participants felt that more information about anxiety would be helpful, this was again thought to be the case only if it was individualised and provided in the context of their personal experience of BD. An important element of therapy highlighted was the need for a clear treatment plan to form a frame for the intervention from an early stage:
I want to be able to say, ‘Well, I want to be this’ by the end of the session.
P2 FG1
Yeah. Because erm [pause] I don’t know whether it could fall under individualised information you know when you go and see someone. There’d be nothing wrong in you saying ‘this is how I want this to go’. And someone else might want it to go a different way and they’d be able to negotiate it at that time rather that it being built.
P1 FG2
Specific techniques that were thought to be helpful included meditation, alternative therapies, muscular relaxation and problem-solving:
If you – you know or certain people find er meditation or hypnotism works for them. Relaxation therapies.
P2 FG1
You know the guided meditation which can be done and it’s nothing to do with religion or anything, you know. ‘Cause I do meditation and I find it chills me right down.
P4 FG2
Participants noted that tackling rather than avoiding problems was likely to be crucial when dealing with anxiety:
But you know what about this, this facing things head on. What I’m saying is if you let things like that fester and you don’t control them and deal with them, your mind will take you to all sorts of places.
P4 FG2
In helping people to engage with this type of approach, however, two linked issues were noted: (1) helping clients to recognise positive feelings experienced when challenges are overcome and (2) providing clients with support when they attempt to confront challenges (as opposed to taking a hectoring or blaming approach, which some participants reported experiencing in the past):
You get a sense of satisfaction so that’s telling, so that’s the strategy I adopt. And it’s not like a high. It’s not a sensation like that. It’s an actual sense of satisfaction and relief that you’ve actually gone and confronted something and you’ve achieved a positive result and you can look back and think that ‘well I’m glad I’m glad I didn’t delay it or hide away from it as well’.
P2 FG2
Some of the things you were talking about, your neighbours you know. I think, from my perspective, I would want some support whether it were CPN or you know learning in some kind of form of CBT, just to say well, you know, you talk to people, you can sort things out, you can rationalise things.
P4 FG2
More cognitive strategies were also noted; participants wanted to have help with approaches to reduce the impact and frequency of negative thoughts and also to acknowledge and affirm positive ones:
So there’s been a few times over 20 years when I’ve had CBT and I’ve felt it’s worked. Some of the things like errm comparing your negative thoughts to positive ones and some of the stuff when I’m shopping relating to panic attacks.
P2 FG2
Yeah that’s what I’m having at the moment and I was wondering if we can learn something in CBT that will help us to develop a shield.
P1 FG2
Following on from the importance of a clear treatment plan developed early in therapy was the monitoring of therapy progress across sessions. Again, this contrasted with people’s prior therapy experiences.
It was perceived that the focus of the therapy should be on providing clients with self-management tools. Participants welcomed the idea of an approach in which the therapist’s role was to help clients gain increased control over their experiences. In addition, it was indicated that therapy should include planning for future high-risk situations and providing help with applying coping strategies to these:
I think it’s also to do with, a lot to do with, definitely on an individual basis, taking control of your own, you know, I’m me . . . and I am sick to death of being told ‘Cut down, You go to bed at this time. You wake up at this time’. I’m not having that in my life. I’m 44 years old. I have to take control. I think . . .
P3 FG1
Yes I suppose it’s changing my mindset from being passively involved in therapy over the years to being in control of it to some extent.
P1 FG3
I think it would be a good thing. Because the more information that you’ve got, the more empowered you are to make decisions about your own health and you know deciding for yourself whether you want this or you want that, you know.
P4 FG2
Focus
A number of elements were not about specific content, but more about the tone and style of the therapy that participants highlighted. First, participants identified the importance of feeling that the client knew something about the therapist as a ‘real person’, so that they did not feel that they were working with a ‘remote’ clinical expert. This was felt to be crucial to the development of trust:
I think it’s more what we were saying before about the quality of the person who’s delivering it, you know. How empathetic they are.
P2 FG1
. . . that most important bit in that is that partnership, is that collaboration that is actually at the heart of the therapy and that it’s about two people working together and the formulations or the maps are very much around these are problems but these are your strengths and that you are actually going to draw them up yourself, with the therapist.
P5 FG3
In addition, it was noted that the approach to each client should be individual and based on understanding and working with their own experiences. This point was made to emphasise that therapy suggestions should not be driven purely by the fact that the client has a diagnosis of BD, as the experiences of people with this diagnosis are so diverse.
The importance of inspiring hope in clients was identified, in particular because otherwise participants felt that clients could develop negative feelings about their progress; this in turn was felt to be a risk to therapy, as it would be likely to reduce engagement. Linked to this was an emphasis on inspiring confidence in clients:
And we were all talking about this problem of motivation that wanes and ebbs and how we really get into something and we’ve got absolute confidence in it and see that it works and then it goes. And erm how err we know that we need to do things but we put ‘em off for something else.
P1 FG1
An important element here was that targets were realistic. An example of this was ensuring that clients realised that they did not have to achieve everything within the 10-session therapy window. To maintain this focus, participants noted that positive goals for therapy should be introduced flexibly and then revised should the client be struggling to reach them. In line with this, it was suggested that therapy should not merely focus on deficits that could be addressed, but should also highlight what people can do well:
Yeah. But it has to be my set of goals. Not you coming in and saying ‘Right I want you to be mood free . . . in 6 weeks’ time’.
P2 FG1
Yes, so, in that respect, yeah. Like my goals would be different to everyone else’s.
P1 FG2
When the mood of the client was clearly having an impact on their ability to engage with a session, it was important to allow the session to be either shortened or rescheduled without this reflecting negatively on the client. It was also noted that sometimes clients might be able to engage despite mood problems if it were possible to have sessions over the telephone rather than face to face. However, this emphasis on flexibility was not aimed at making therapy unduly easy. Rather, there was an emphasis on helping to motivate clients towards addressing challenging situations as far as this was possible:
I mean if you go in for like a session of CBT like over a 6-week period you know once for an hour every Monday afternoon or what have you. You go every Monday afternoon and the counsellor sits you down and says ‘How’s your mood today, S– ?’ and you say ‘Absolutely shite’. There’s no point in continuing. Or ‘I’m absolutely wonderful? [in a high-pitched voice].’ There’s no point in continuing.
P2 FG1
Yeah, if he or someone could identify the time you’re most receptive to take that on board, I think that would be a good thing. Just identify when your mood’s level and you can actually take things in.
P4 FG1
Format
The idea of flexibility was also relevant to the timing and location of appointments to make them, as far as possible, comfortable for the client so that they could concentrate on addressing their anxiety issues. Suggestions here included negotiating the location for sessions with each client, as views diverged on whether clinical or domestic settings would feel more appropriate:
The different ways I was treated by the different people made a difference in how I felt about going to the meetings with them. Because, you know, at one point it was like, no matter what mood I was in, I had to be there, make the effort, go in to [inaudible] and it was like she couldn’t give a shit about me she couldn’t give a monkey’s. And then I had another one and it’s like ‘All right, S–. If you don’t feel like going out, we won’t go out’.
PG2 FG1
So I don’t know, I mean people who are particularly anxious, if you could arrange the actual sessions to be somewhere where they felt more comfortable.
P4 FG1
The importance of a planned, targeted approach to therapy has been noted. However, the nature of the targets is also crucial, and it is noted here that targets should be truly collaborative. Some participants had previous experiences of being expected to meet targets that the therapist had deemed appropriate without proper reference to that client’s personal needs and goals. In such cases participants recalled either avoiding future sessions or feeling that they had to say what the therapist wanted to hear rather than engaging in genuine change:
You know, 8 weeks or whatever, and they’ve got targets and whatever, they want to see, they need to show like when you fill in a form at the beginning when you go after 8 weeks and want to see a difference. That can put pressure on people, I think.
P1 FG1
. . . if everything was set in stone before I agreed to take part in it erm I think that would put pressure on me, and what I might end up doing then is saying things to please the therapist, because it might also set up the therapist. It’s almost giving him or her targets.
P1 FG2
The therapist’s way of communicating was identified as important. Approaching the client in a ‘normal’ way rather than appearing as a distant clinical expert and, similarly, using appropriate humour in sessions were seen as signs of an engaging therapy encounter. It was also suggested that, by taking this approach, clients would be more confident to give their therapists accurate and honest sessional feedback in line with CBT principles:
I think sense of humour that the therapist has a sense of humour and that you put into practice being around people with a sense of humour and doing and watching comedies and things like that. If you’re in severe panic, I think you know you need something like that to break it.
P6 FG2
Returning to the theme of structure and clarity, participants indicated that it was important to be clear about what clients could expect from therapy in terms of both practical arrangements and the likely scale and pace of clinical gains:
There needs to be some sort of contract or some sort of an expectation, and I think that’s really important and it coming back to what you’ve just said erm I think for anybody taking any kind of therapy or doing any sort of voluntary work, or putting themselves out in any way. They’ve got to have a payback at some point erm, so I think doing like an initial, not contract but you know a statement of you know you can expect this. We’d want you to be able to quantify it at the end of your 10 weeks or whatever and say well this is what you expected to get out of it. Did you or didn’t you? And too it would make you take it more seriously because, when anything’s free you tend to have this opinion that it’s not very valuable and therapy’s going to be free isn’t it?
P4 FG2
A general atmosphere of openness and safety was also regarded as crucial to engagement:
Right, openness. You know ‘walk in, there’s a brew there, take your coat off’. Not ‘your seat is there’. Do you know what I mean?
P3 FG1
There was just one thing I was gonna say which was er expanding on the openness and knowing. Just the therapeutic relationship. Sometimes the mix isn’t right. So would there be one session where, one or two sessions where you get to know one another and just see if they’re the right person for you.
P1 FG2
The importance of practising new skills was noted, as was explaining this as part of therapy. Participants were aware that, with new strategies, levels of skill and success were likely to increase over time. However, as clients would find implementing new learning stressful, they might easily be put off if their new strategies were not instantly and completely successful. Understanding that change can take time was therefore felt important to maintain engagement and encourage positive therapeutic outcomes:
And it’s took too long for me and I think it’s because I was too quick all the times I had these skills to practise in saying they don’t work and being really negative about it. But I wonder how a therapist might work to counter that.
P1 FG2
Although it was accepted that an individual approach to therapy was appropriate, participants felt that there should be opportunities for family, friends and clinicians to support the work when needed. An example was opening up specific sessions to include a clinician or a friend when there was a specific rationale for this and it was supported by the client. Similarly, it was agreed that at the end of therapy it could be helpful to share key learning points with the clinical team, but that this should be done only with the consent and support of the client:
It’s having support from family and friends and like getting into a self-support group, which I’ve not had.
P7 FG2
Support materials
Consistent with comments about the planned and structured nature of therapy, participants indicated that it would be helpful to provide each client with a flow chart of their progress through therapy. It was also suggested that clients be provided with worksheets to summarise each session, which could be a resource after therapy and a guide to progress during it:
I just think in my – you know a simple flow chart or something of different anxieties and bipolar and how they might interplay as different might be useful.
P1 FG1
But and the worksheets as well, you know, when I was in the mood, even though a couple of weeks later it might not felt like it was working again, you can . . . keep going back to it.
P1 FG3
When you’re in a different mood or a frame of mind, positive or negative, if you’ve got something you can take or leave, put it down and then come back to it.
P1 FG1
Additionally, there was agreement that it could be helpful to provide clients with materials that they could use to remind themselves of work done in sessions and to maintain self-management after therapy ended. There was, however, some debate about the best method for this. Some participants felt that a website would be ideal to host all of the therapy information so that if any written material was lost it could be accessed after therapy. Others were adamant that they would not use a website and preferred the idea of a booklet to take away that contained the same information. In both the booklet and the website proposals, the importance of the quality of presentation was highlighted, in particular that the information should be presented in a colourful, engaging way so that it felt special in recognition of the client’s efforts to change:
But in a manual you can go back in to tap in and tap out of, I think it’s a good idea. There’s times when I can read like case studies on the internet or read peoples’ forum experience and it’s (?) like that. Oh I, I experienced that. And then there’s times where you’re not me. I don’t relate to that. And that can be erm interesting as well. So if you can keep going back to it ‘cause we don’t always get the computer up.
P1 FG1
Participants thought that, for either format, it would be helpful to provide SU accounts of living with anxiety in BD. Here it was emphasised that such accounts should be realistic and based on a coping model (rather than accounts in which people had made unrealistically miraculous progress with their anxiety issues). It was thought that this type of model would resonate more with most clients’ experiences and add to the hopefulness indicated above as a key element of engagement. Links to other resources were also thought useful for the booklet or website. A caveat here was that this could be overwhelming if too much information was provided. It was therefore argued that this would be more effective if only the most relevant and useful links were presented:
I think it would be very useful . . . I don’t know how many – words but just somebody saying ‘such-and-such has bipolar with anxiety’. Brief history of them. They’ll obviously be anonymous and so on.
P4 FG1
. . . just a summary of what they do and what they find helpful to manage their condition.
P4 FG1
And maybe if you could make it a bit more personal. Highlight who the people are, sort of. What they do, where they live. I think it makes it seem more approachable in some ways then, then you’re saying this is, I don’t know, John. It seems like it’s almost contrived if you know what I mean and it’s just been produced. If you could – like humanise it in a way. I don’t know if that rings true with anyone.
P4 FG1
Barriers and facilitators
Participants were clear that if this was a long intervention that could be offered only by, for instance, highly trained clinical psychologists, it would be of limited use, as people would just be stuck on long waiting lists. It was therefore important that it was a time-limited therapy that could be delivered by a range of clinicians with the appropriate training:
I think it would be really important for anyone delivering the programme, which sounds really good, you know, if you can get social workers and nurses and everyone to learn a package or whatever. Because I know in [town] – I don’t know where it is at the moment – but er I know 18 months ago it was a 13-month waiting list for CBT.
P1 FG1
Phase 3: a single-blind randomised controlled trial was conducted to determine the feasibility and acceptability of the anxiety in bipolar disorder intervention compared with treatment as usual
Parts of this section have been reproduced with permission from Jones et al. 157
A key question in developing approaches to anxiety comorbidity is whether to target specific anxiety diagnostic categories or anxiety symptoms. The latter course was chosen for AIBD for the following reasons.
-
Anxiety disorders themselves tend to be highly comorbid, especially in BD;150 therefore, there is a risk that single interventions focused on specific anxiety disorders would be inappropriate for this client group.
-
Evidence from the earlier phases of work in this stream, along with Hampshire,153 highlights that a substantial proportion of individuals with BD experience significant distress associated with anxiety without fitting neatly within a single anxiety diagnostic category, consistent with other previous research. 150
-
Although the detailed manifestations of anxiety disorders differ, they typically share key elements, including subjective feelings of anxiety, worry and tension, and interference with functioning. This overlap of core anxiety processes has been recognised in the development of a unified protocol by Barlow et al. 158
-
Our findings from interviews and focus groups with SUs and the advice of our SURG were that this approach would open the trial to the widest possible range of individuals with BD who might potentially benefit from it; consistent with their proposal that there is an urgent need for studies of this type, the present study is a RCT feasibility and acceptability study of a new psychological treatment for the reduction of anxiety in BD.
Methods
This study was reviewed and approved by the UK NHS Ethics Committee process (REC reference 10/H1015/83). It was preregistered with International Standard Randomised Controlled Trial Number (ISRCTN) 84288072, and a detailed research protocol has been published. 159
Trial design
The trial was a rater-blind RCT comparing up to 10 sessions of integrated CBT for anxiety in the context of BD (AIBD), together with TAU, compared with TAU alone. The trial was conducted across seven NHS trusts in north-west England, with recruitment taking place over a period of 11 months between June 2011 and May 2012.
Participants were randomised by an independent clinical trials unit (Christie Hospital, Manchester, now Manchester Academic Health Science Centre Clinical Trials Unit 9). Randomisation was carried out using randomly sized permuted blocks. Allocation was minimised on sex, number of previous mood episodes (depression and mania: ≤ 7, 8–19 or ≥ 20 sessions) and level of current anxiety [Hamilton Anxiety Rating Scale (HAM-A) score: 0–17, 18–24 or ≥ 25160], as better outcomes from psychological therapy have been associated with female sex, fewer previous mood episodes and lower levels of anxiety. 161–163
In an endeavour to maintain blinding to treatment arm among researchers, they were housed in different offices from those occupied by the therapists. In addition, they had no involvement in the randomisation process, and trial participants were reminded by researchers not to disclose which treatment arm they had been allocated to. When unblinding did occur, steps were taken to reallocate an alternative blind RA for future assessments, and this was recorded by the programme manager (see Results).
As the primary purpose of this pilot study was to evaluate the feasibility and acceptability of delivering the proposed intervention and to test trial procedures such as consent and follow-up rates, a formal power calculation for comparing interventions is not appropriate. The recruitment target was therefore set at 72 participants so that an attrition rate of 25% could be estimated with a 95% CI of ± 10%. A sample of this size will also give some indications of the potential clinical effectiveness of the intervention.
Recruitment
Participants were recruited from community and primary care mental health teams, mental health outpatient clinics, GP surgeries and voluntary services in north-west England, covering Manchester, Lancashire, Cumbria and Merseyside. The study was also advertised in local media, and posters and leaflets were distributed in both NHS and non-NHS sites to maximise participant access. All participants provided written informed consent before being recruited into the study and additional consent was obtained from those who participated in the nested qualitative study (see Qualitative interviews).
Inclusion/exclusion criteria
The inclusion criteria were:
-
having a DSM-IV diagnosis of primary BD assessed using the SCID77
-
having current significant anxiety symptoms (HADS-A score of ≥ 8)85
-
having sufficient understanding of written and spoken English to provide informed consent, engage with interviews and assessments and be able to use intervention materials
-
being ≥ 18 years old.
Exclusion criteria comprised having a manic, hypomanic, depressed or mixed episode currently or having had one in the previous 4 weeks; having current suicidal ideation with intent; and having the inability or being unwilling to provide informed consent.
A subset of 17 participants randomised to the AIBD arm were interviewed following therapy to explore their views on the acceptability of the intervention.
Primary feasibility and acceptability outcomes
The feasibility and acceptability of delivery of AIBD to participants were evaluated in terms of levels of recruitment into the trial, retention of participants in both arms of the study and adherence to, and completion of, therapy. The post-randomisation follow-up period was up to 80 weeks. The original intention was to follow up over a 48-week period to capture these outcomes; however, this period was extended to ensure that there was sufficient time to explore mood relapse episodes.
Face-to-face assessments were conducted during the initial interview (to confirm diagnosis), at baseline and at 16, 48 and 80 weeks. Interim telephone assessments were conducted at 32 and 64 weeks. Observer ratings of bipolar relapse (SCID LIFE77), depression (HAM-D82,164,165) and mania (MAS155) were obtained at each assessment point. Self-report measures of anxiety [Spielberger State–Trait Anxiety Inventory (STAI)166] and personal recovery [Bipolar Recovery Questionnaire (BRQ)98] were obtained at all except interim assessments.
Qualitative interviews
Semistructured individual interviews were undertaken with a subgroup of recipients of the AIBD intervention to provide additional information on acceptability. Participants were purposively sampled to ensure maximum variance in terms of age and sex, HADS-A score at recruitment, number of sessions attended, previous relapse and outcome. The interviews covered a range of anxiety-related topics, but for the purposes of this report we have concentrated on interviewees’ perspectives on the acceptability and impact of AIBD intervention. All interviews were audio-recorded and transcribed verbatim, before being subjected to thematic analysis following Braun and Clarke. 91
Secondary clinical outcomes
Primary clinical outcomes as prespecified in the published protocol, evaluated the impact of AIBD on observer- and self-reported anxiety (HAM-A;160,164,165 STAI166), time to relapse and level of residual symptoms of mania and depression (SCID LIFE;77 HAM-D82 and MAS155).
The following additional clinical outcomes were assessed:
-
personal recovery (BRQ98)
-
quality of life and social functioning as measured by EQ-5D,115 Quality of Life in Bipolar Disorder Questionnaire167 and the observer-rated Personal and Social Performance Scale (PSP)168
-
medication adherence as measured by Stephenson Medical Adherence Questionnaire (MEDAD). 169
It is also hypothesised that AIBD will cost no more than TAU as assessed by Client Service Receipt Inventory170 and the Economic Patient Questionnaire (Professor Linda Davies, University of Manchester 2011, personal communication) and informed by EQ-5D as a measure of health status.
However, consistent with the original programme application, the Results section will focus on measures of mood, anxiety and personal recovery.
Intervention
The AIBD intervention integrated effective approaches to reducing anxiety and addressing BD informed by NICE guidelines for both conditions. 26,147,148 Important elements in AIBD include flexible engagement in therapy, reviewing of both positive experiences and coping as well as difficulties and challenges, and applying a flexible formulation-driven approach to development of individual therapy plans. Qualitative interviews and focus groups were also conducted with individuals with lived experience of BD and anxiety. The information drawn from these informed the final version of AIBD including emphasising the importance of a flexible approach that was responsive to the client’s current priorities and giving therapists the ability to focus on both anxiety and bipolar issues as required. These interviews also highlighted the importance of therapists working in a trusting and truly collaborative manner with clients.
Anxiety-specific elements in AIBD included improving awareness of anxiety symptoms, applying mental imagery and relaxation techniques, and experiencing graded exposure to anxiety-provoking situations. To integrate this work with BD, AIBD also highlights instances for each client in which anxiety and mood experiences interact. When mood issues also represent a significant factor in the client’s presentation, these are addressed by mood-monitoring techniques, regularisation of routine and improving problem-solving strategies. In line with other suggestions from the SU consultation described above, participants were provided with SU manuals. These contained information on BD and anxiety, and contact information for support services. The manual also had space for the client to record their clinical sessions and collate any session-linked information, including details of their formulation, therapy plan and staying well plans. Each client was also able to access online all information used in therapy.
Measures to assess therapeutic alliance and adherence to treatment protocol
The Work Alliance Inventory (WAI) (short-form therapist and client versions171) was used to assess engagement in therapy. Treatment fidelity was assessed by both the Cognitive Therapy Scale-Revised (CTS-R172) version and the AIBD Fidelity Scale specifically designed for the current study (see Appendix 4).
Data analysis
The intention-to-treat principle, subject to the availability of data, was employed in all analyses of clinical and functional outcomes.
The survival methods used to analyse the PEd trial (see Chapters 2a and 2b) were applied to the anxiety AIBD trial. For the time to relapse analyses, the covariates were treatment arm, sex, number of previous bipolar episodes (8–20 and > 20 both vs. 1–7 sessions) and baseline HAM-A total score.
On inspection of the scale summary statistics for the outcomes, there was little evidence of a linear trend in improvement over time (with the highest mean scores at week 80 on the AIBD arm for HAM-A and STAI-State primary outcomes). We therefore modified the approach specified in the statistical analysis plan by fitting repeated measures models using time as a discrete variable rather than the statistical analysis plan-specified linear mixed effects models approach with time as a continuous variable. The plausibility of the normality assumption of these models was checked using a normal probability plot of model residuals. A model with a discrete time by treatment interaction was fitted to estimate the treatment effect and the 95% CI at each follow-up time point. If there was no evidence of an interaction at the 5% level, then the interaction term was omitted and the overall treatment effect was estimated from this simpler model.
The same covariates as described above were used for these models, additionally adjusting for the baseline value of the outcome. A baseline response by discrete time interaction term was also included.
Results
Primary outcomes
Feasibility
Figure 18 presents the recruitment and retention rates for participants in AIBD and TAU arms in accordance with Consolidated Standards of Reporting Trials (CONSORT) criteria. 173 These figures and the additional information on participation rates for the AIBD intervention were used to inform assessments of feasibility. Of 122 people screened for eligibility, 14 declined, 9 were uncontactable and 27 did not meet the study’s inclusion criteria. Thus, only 11% of those offered the opportunity to participate in the study declined. Of the participants recruited, 32 (44%) were recruited by clinician referral and 40 (56%) were recruited via self-referral, with no evidence for differential eligibility or refusal rates in the AIBD versus TAU arms. The two arms did differ in the proportions coming from each referral source, with two-thirds of AIBD participants coming from self-referral and two-thirds of TAU participants coming from NHS referrals (Table 31).
| Characteristic | Treatment arm, n (%) | |
|---|---|---|
| AIBD (N = 37) | TAU (N = 35) | |
| Sex | ||
| Male | 13 (35.1) | 10 (28.6) |
| Female | 24 (64.9) | 25 (71.4) |
| Number of previous episodes | ||
| ≤ 7 | 4 (10.8) | 3 (8.6) |
| 8–19 | 7 (18.9) | 6 (17.1) |
| ≥ 20 | 26 (70.3) | 28 (74.3) |
| HAM-A score | ||
| ≤ 17 | 35 (94.6) | 29 (82.9) |
| 18–24 | 1 (2.7) | 4 (11.4) |
| > 24 | 1 (2.7) | 2 (5.7) |
| HADS-A score | ||
| 8–10 (mild) | 11 (29.7) | 9 (25.7) |
| 11–15 (moderate) | 16 (43.2) | 15 (42.9) |
| 16–21 (severe) | 10 (27.0) | 8 (22.9) |
| Missing | 0 (0.0) | 3 (8.6) |
| Current anxiety disorders | ||
| Social phobia | 12 (32.4) | 9 (25.7) |
| Agoraphobia | 6 (16.2) | 4 (11.4) |
| GAD | 19 (51.4) | 18 (51.4) |
| PTSD | 10 (27.0) | 7 (20.0) |
| Panic ± agoraphobia | 9 (24.3) | 6 (17.1) |
| OCD | 5 (13.5) | 6 (17.1) |
| Specific phobia | 9 (24.3) | 6 (17.1) |
| Number of current anxiety disorders | ||
| 0 | 5 (13.5) | 6 (17.1) |
| 1 | 10 (27.0) | 15 (42.9) |
| 2 | 12 (32.4) | 6 (17.1) |
| 3 | 6 (16.2) | 4 (11.4) |
| 4 | 3 (8.1) | 3 (8.6) |
| 5 | 0 (0.0) | 1 (2.9) |
| 6 | 1 (2.7) | 0 (0.0) |
| Bipolar status | ||
| BD 1 | 31 (83.8) | 31 (88.6) |
| BD 2 | 6 (16.2) | 4 (11.4) |
| Medication at baseline | ||
| Antidepressants | 20 (54.1) | 11 (31.4) |
| Mood stabilisers | 23 (62.2) | 26 (74.3) |
| Antipsychotics | 18 (48.6) | 17 (48.6) |
| NHS service use 6 months pre baseline | ||
| Inpatient stay days | 4.27 (16.63) | 0.91 (4.74) |
| CMHT appointments | 7.19 (10.95) | 6.17 (7.49) |
| Outpatient appointments | 2.97 (4.81) | 1.63 (3.48) |
| GP appointments | 4.73 (4.17) | 3.69 (3.15) |
| Source of referral | ||
| NHS | 12 (37.5) | 20 (62.5) |
| Self | 25 (62.5) | 15 (37.5) |
| Ethnicity | ||
| White British | 33 (89.2) | 32 (91.4) |
| Other white | 0 (0.0) | 1 (2.9) |
| Asian other | 0 (0.0) | 1 (2.9) |
| British Asian | 2 (5.4) | 1 (2.9) |
| Not stated | 2 (5.4) | 0 (0.0) |
| Marital status | ||
| Married or cohabiting | 17 (45.9) | 7 (20.0) |
| Divorced/annulled/separated | 9 (24.3) | 13 (37.1) |
| Never married | 11 (29.7) | 15 (42.9) |
| Number of children | ||
| 0 | 15 (40.5) | 15 (42.9) |
| 1 | 7 (18.9) | 4 (11.4) |
| 2 | 8 (21.6) | 5 (15.3) |
| 3 | 7 (18.9) | 7 (20.0) |
| 4 | 0 (0.0) | 3 (8.6) |
| ≥ 5 | 0 (0.0) | 1 (2.9) |
| Education | ||
| Years 7–11 (no GCSE) | 3 (8.1) | 0 (0.0) |
| GCSEs or equivalent | 6 (16.2) | 8 (22.9) |
| Further or higher education not completed | 7 (18.9) | 4 (11.5) |
| Further or higher education completed | 16 (43.2) | 16 (45.7) |
| Postgraduate education not completed | 0 (0.0) | 1 (2.9) |
| Postgraduate education completed | 5 (13.5) | 6 (17.1) |
| Working | ||
| No | 21 (56.8) | 23 (65.7) |
| Yes | 16 (43.2) | 12 (34.3) |
| Type of work | ||
| Employed full-time | 5 (13.5) | 4 (11.4) |
| Employed part-time | 7 (18.9) | 4 (11.4) |
| Voluntary | 3 (8.1) | 3 (8.6) |
| Self-employed | 1 (2.7) | 1 (2.9) |
| Unemployed | 6 (16.2) | 8 (22.9) |
| Sickness/disability benefit | 11 (29.7) | 12 (34.3) |
| Retired | 3 (8.1) | 2 (5.7) |
| Student | 1 (2.7) | 1 (2.9) |
Seventy-two participants were randomised, fully meeting the prespecified recruitment target and representing 59% of those screened for eligibility. These participants were recruited over a period of 11 months, indicating an average recruitment rate of 6.5 per month. Retention was 88% (n = 64) to end of therapy follow-up (16 weeks), 75% (n = 54) to 32-week follow-up, 74% (n = 53) to 48- and 64-month follow-ups and 72% (n = 52) to the final 80-week follow-up. Two of the participants lost to follow-up died during the final follow-up period for reasons that were deemed by TSC/Data Monitoring Committee to be unrelated to the trial. Thus, retention rates across the trial were consistent with the feasibility target of 75% ± 10% up to 20 months as prespecified. 159
As for any large ongoing study, the main aim was to collect primary outcome data. As this was a pilot study, we were able to collect data on completion rates for the secondary self-report measures (Table 32). These will assist with developing subsequent studies by indicating the willingness of participants to complete such measures.
| Treatment arm | Time point, n (SD) | |||
|---|---|---|---|---|
| Baselinea (0 weeks) | 16 weeks | 48 weeks | 80 weeks | |
| AIBD | 36 (97.3) | 28 (90.3) | 20 (74.1) | 20 (76.9) |
| TAU | 34 (97.1) | 27 (84.4) | 22 (84.6) | 22 (84.6) |
As is evident from Figure 18, there is no evidence of resentful demoralisation in this trial, with retention rates at least as high in TAU as in the active intervention across assessment points.
Unblindings were reported in seven AIBD and four TAU participants (15.3% of the RCT total). Reasons for unblindings were participants in AIBD mentioning therapist by name to a RA, RAs being asked to amend therapy appointments, care co-ordinators’ accidental disclosure during risk assessment and therapist accidental disclosure. Unblindings in TAU arose from participant disclosure at follow-up interview, and knowledge that the TAU group was invited to undertake a secondary interview for another Spectrum Centre for Mental Health Research study (Everyday Momentary Observations of Thoughts and Emotions). For all 11 participants we were successfully able to reallocate an alternative blinded RA to carry out future follow-up interviews.
Participants
The mean age of the participants in both groups was > 40 years (AIBD: 45.5 years, SD 10.7 years; TAU: 42.9 years, SD 16.6 years) and most were female (see Table 2; n = 49, 68%). Although all participants had a HADS score of > 8, consistent with anxiety cases,85,174 the scores at baseline on the HAM-A were very variable, ranging from 0 to 33 (normal range to severe), and on average scoring in the upper levels of the normal range (AIBD: 12.84, SD 3.26; TAU: 12.97, SD 3.75). An exploration of anxiety disorders in the recruited participants indicated that 85% met the diagnostic criteria for at least one anxiety disorder, GAD and social phobia being the most common in both arms. More participants in the AIBD arm met the criteria for two or more anxiety disorders (59% vs. 40%). Consistent with this, numerical rates of social phobia, agoraphobia, PTSD, panic ± agoraphobia and specific phobia were all higher in the AIBD arm, although these differences were not statistically significant. The AIBD arm also reported higher rates of service use across all areas than did the TAU arm.
Participants predominantly had a chronic course of BD, with > 70% of participants having had more than 20 episodes of mania or depression at baseline. The majority of people had a diagnosis of BD 1 (n = 62, 86%). The majority were either divorced or never married (n = 48, 67%) and most were parents (n = 42, 58%). Although the majority of participants had at least commenced further education (n = 55, 76%), only just over one-third were in employment (n = 28, 39%). Of those employed, the highest proportion was in part-time work (n = 11 of 28, 39%). Of those not in work, the majority were in receipt of sickness/disability benefits (n = 23 of 44, 52%). Comparing the treatment arm with the control arm, there were no substantial differences in age, sex, number of previous episodes, bipolar status, ethnicity, number of children or education. Twice as many participants in the intervention arm were married or cohabiting, and approximately 10% more intervention group participants were in work. Two-thirds of referrals in the intervention arm were self-referrals into the study, compared with only one-third of self-referrals in the control arm. The remainder were referred by the NHS from a mix of clinicians, clinical studies approach to clients at clinics and invitation letter from GPs. Self-referrals were in response to adverts in the local press and radio, through Pendulum (the magazine of Bipolar UK) and participants’ local support groups, and from newsletters from Spectrum Centre for Mental Health Research at Lancaster University.
Treatment delivered and treatment fidelity
Participants offered AIBD were offered up to 10 sessions. The mean number of sessions attended was 7.7 (SD 2.8), with only two participants not attending any sessions. The remainder attended between 1 and 10 sessions, with 84% (n = 31) attending more than three sessions and 59% (n = 22) attending at least nine sessions.
Adherence to the therapy protocol and CTS-R was assessed independently for 26 randomly selected therapy recordings across early, mid and late sessions (sessions 1 and 2, sessions 4 and 5, and sessions 7–10, respectively). Adherence to the treatment protocol was 92% across the appropriate therapeutic stages (initial, formulation, intermediate, later and throughout). The mean score for CTS-R was 47.1 (SD 5.03), and all recordings met threshold criteria for competence. 175 Mean CTS-R showed little change across sessions: early, 47.5 (SD 5.74); mid, 47.4 (SD 5.24); and late, 46.1 (SD 3.76).
Client ratings of therapy
Therapists asked each client who received AIBD to rate the therapy on two 0–10 scales. The first was to rate how useful they thought their experience of the therapy had been from 0 (not at all) to 10 (extremely); the second was to rate whether or not they would be likely to recommend the therapy to anyone else with similar problems from 0 (definitely not) to 10 (definitely yes). These data were available for 21 participants. They were missing for nine participants and not collected from the remainder due to dropout, failure to attend or, in one case, death of the participant (for reasons unconnected to the trial).
Ratings for their own experience of therapy averaged a score of 9.14 (range 8–10). The average rating for the likelihood of recommending the therapy to others was 9.26 (range 8–10). Thus, for those who completed the ratings, no participants provided anything less than a high (≥ 8) rating for the helpfulness of the therapy and the extent to which they were likely to recommend it to someone else.
Therapeutic alliance
Therapeutic alliance was assessed using the client short-form 12-item version of the WAI, which was completed by participants after sessions 3 and 10 and scored out of a total of 28 (four items each using a seven-point Likert scale, with two items reverse coded). At session 3, agreement on the goals of treatment were similar between all three therapists whose clients returned fully completed WAI questionnaires (score range 23.3–26.7, n = 23/37); agreement on how to achieve these goals (task agreement) ranged from 22.7 to 24.6; and the development of a bond between participant and therapist ranged from 22.8 to 23.3. These scores summed provided overall scores out of 84, ranging from 68.8 to 74.3.
By session 10, 16 participants had fully completed the measure. One of our therapists had left by this point, and their participants had been assigned to one of the two remaining therapists, which might have had an impact on the alliance scores. Agreement on the goals of treatment ranged from 23.7 to 24.4; agreement on how to achieve these goals (task agreement) ranged from 23.7 to 24.0; and, as expected over a period of time, the bond score between participant and therapist had increased to range from 23.3 to 25.2. These scores summed provided overall scores of 70.8–73.6 out of 84.
Qualitative interview findings regarding acceptability
A number of key themes emerged from a preliminary analysis of in-depth qualitative interviews with 17 participants who had received AIBD. Participants indicated that they valued the intervention and contrasted this with other previous forms of support they had received. This had led many to commit wholeheartedly to the therapy:
And it is the only therapy I have had in all my . . . I have had anxiety since I was what, about 15 and that is the only thing that worked for me was that CBT therapy.
AN003
. . . the [therapy was] a real gift, and so I took it with you know both hands, six toes and my teeth because it was a gift.
AN011
Participants also recognised value in treating anxiety and BD together and contrasted this with previous experiences of having had these problems addressed separately, which they perceived as leading to a more superficial approach to both difficulties:
Normally people do them separately and trying to put them together when you are ill is just . . . not easy at all . . . if you have got them separate it’s like skirting round each issue, but putting them together and showing a person that deal with them all, and will go slowly over everything so you know what to expect, it is so much better, definitely.
AN007
The AIBD intervention, at 10 sessions over 4 months, was relatively brief compared with other forms of structured psychological therapy for significant mental health problems. For some participants, this was helpful in providing a clear structure with clear personally identified targets to work towards:
. . . it was 10 sessions, and that were it, but the 10 sessions meant that the goals that were laid out at session one, those were the goals that were worked at, and those were the goals that were achieved by the end and that is a far better way of working.
AN001
However, others felt that they would have preferred the therapy to continue for longer while they felt that they were benefiting from it, although it was not evident that specific elements were omitted from the therapy:
I remember thinking I didn’t want them to come to an end. But I couldn’t have told you, or couldn’t have probably pinpointed aspects that I needed more to cover I think I was just . . . gaining so much that I kind of felt maybe there was more to gain as well you know if I had carried on.
AN008
Another finding identified related to participant perceptions of outcomes following therapy. Participants identified that the coping strategies they had learnt in AIBD helped them escape the social isolation and functional limitations that anxiety had historically imposed on them. It was also noted that people still experienced challenges in the course of their BD, but that they felt more confident in dealing with these, including by using the self-management strategies learnt during therapy:
. . . it has been crippling for me over the years. I have had a number of breakdowns, and each time it’s always been the anxiety that has kept me prisoner in my own home, it has stopped me from socialising, and progressing so this time, I have healed better and with coping strategies that have allowed me to do things, a lot quicker than before.
AN008
. . . prior to becoming part of this study I thought my bipolar disorder owned me . . . and was in control of me and I had no control over it . . . and through this study, now I own it and I control it . . . I had wrote my life off . . . I had consigned myself to a life on benefits and you know I have got this mental illness and I am on major drugs and antipsychotic you know it doesn’t make me feel good . . . and I had just resigned myself to it, even though you know in the past I have had relatively good success in whatever endeavours I have undertaken . . . through this study and through the work that I did with [research assistant] and [therapist] it give me, renewed belief and . . . made me take control of my life again.
AN001
Secondary outcomes
Outcomes are described below with respect to 48-week follow-up, as submitted with the original application, and also 80 weeks (extended to capture mood relapse more accurately).
Self- and observer-reported outcome measures
Key clinical outcomes for the AIBD trial were self- and observer-rated anxiety symptoms, subsyndromal mood symptoms, recovery (Tables 33 and 34) and time to next mood episode (Figures 19–21).
| Measure | Week | Treatment arm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIBD | TAU | ||||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | ||
| STAI-State | 0 | 47.1 | 11.2 | 47 | 25 | 70 | 36 | 48.0 | 15.1 | 48 | 20 | 79 | 35 |
| 16 | 44.3 | 14.3 | 48 | 22 | 67 | 30 | 47.7 | 11.0 | 49 | 23 | 69 | 31 | |
| 48 | 40.8 | 12.2 | 38 | 21 | 60 | 23 | 44.5 | 14.2 | 43 | 21 | 70 | 24 | |
| 80 | 48.7 | 15.0 | 51 | 23 | 69 | 24 | 41.7 | 15.1 | 41 | 21 | 68 | 24 | |
| STAI-Trait | 0 | 53.7 | 8.2 | 54 | 32 | 66 | 37 | 53.4 | 11.3 | 53 | 30 | 71 | 35 |
| 16 | 51.5 | 11.4 | 54 | 30 | 72 | 30 | 52.6 | 9.9 | 51 | 35 | 77 | 31 | |
| 48 | 47.9 | 12.6 | 48 | 29 | 72 | 23 | 50.3 | 10.9 | 51 | 32 | 80 | 24 | |
| 80 | 49.6 | 11.2 | 50 | 34 | 70 | 24 | 46.3 | 13.1 | 43 | 23 | 79 | 23 | |
| HAM-D | 0 | 8.7 | 5.1 | 8 | 0 | 21 | 37 | 8.0 | 5.5 | 7 | 0 | 23 | 35 |
| 16 | 10.1 | 7.8 | 8 | 0 | 27 | 31 | 10.5 | 7.7 | 8 | 1 | 34 | 31 | |
| 32 | 9.2 | 7.5 | 7 | 1 | 27 | 25 | 8.9 | 7.6 | 8 | 0 | 29 | 27 | |
| 48 | 8.7 | 7.9 | 5 | 0 | 25 | 24 | 7.9 | 6.3 | 8 | 0 | 22 | 25 | |
| 64 | 9.8 | 7.0 | 9 | 0 | 25 | 26 | 7.4 | 8.6 | 6 | 0 | 30 | 23 | |
| 80 | 9.1 | 7.0 | 9 | 0 | 26 | 27 | 8.7 | 8.0 | 7 | 0 | 31 | 26 | |
| HAM-A | 0 | 9.1 | 6.1 | 8 | 0 | 33 | 37 | 9.8 | 7.8 | 7 | 0 | 31 | 35 |
| 16 | 10.3 | 9.2 | 9 | 0 | 30 | 31 | 11.0 | 7.7 | 9 | 1 | 31 | 31 | |
| 32 | 9.7 | 8.1 | 9 | 0 | 26 | 25 | 10.8 | 9.9 | 8 | 0 | 37 | 27 | |
| 48 | 10.2 | 9.1 | 7 | 0 | 34 | 24 | 10.8 | 8.8 | 8 | 0 | 37 | 25 | |
| 64 | 11.6 | 8.1 | 11 | 0 | 28 | 26 | 11.1 | 11.4 | 7 | 0 | 38 | 23 | |
| 80 | 12.3 | 9.1 | 10 | 0 | 31 | 27 | 11.3 | 10.6 | 9 | 0 | 45 | 26 | |
| MAS | 0 | 2.2 | 2.4 | 2 | 0 | 8 | 37 | 2.5 | 2.9 | 2 | 0 | 11 | 35 |
| 16 | 2.1 | 2.5 | 1 | 0 | 10 | 31 | 2.2 | 2.1 | 1 | 0 | 8 | 31 | |
| 32 | 1.5 | 2.9 | 1 | 0 | 14 | 25 | 2.6 | 4.1 | 1 | 0 | 18 | 27 | |
| 48 | 3.4 | 5.0 | 1 | 0 | 15 | 24 | 2.0 | 3.0 | 1 | 0 | 12 | 25 | |
| 64 | 2.9 | 3.3 | 3 | 0 | 16 | 26 | 3.7 | 4.9 | 2 | 0 | 20 | 23 | |
| 80 | 2.4 | 3.7 | 1 | 0 | 16 | 27 | 4.8 | 5.8 | 3 | 0 | 20 | 26 | |
| BRQ | 0 | 2107 | 374 | 2097 | 1109 | 2901 | 36 | 2161 | 405 | 2090 | 1525 | 2909 | 34 |
| 16 | 2248 | 411 | 2158 | 1458 | 3066 | 31 | 2146 | 401 | 2221 | 1272 | 2828 | 27 | |
| 48 | 2326 | 374 | 2210 | 1723 | 3073 | 21 | 2351 | 480 | 2379 | 1296 | 3252 | 23 | |
| 80 | 2318 | 379 | 2225 | 1733 | 2967 | 20 | 2398 | 497 | 2428 | 1450 | 3290 | 23 | |
| Outcome measure/model | Week | Treatment effecta | 95% CI | p-value |
|---|---|---|---|---|
| STAI-State | ||||
| Model 1b | 16 | –2.39 | –7.45 to 2.67 | 0.355 |
| 48 | –2.50 | –8.11 to 3.11 | 0.382 | |
| 80 | 8.49 | 1.58 to 15.41 | 0.016 | |
| 0.001 | ||||
| STAI-Trait | ||||
| Model 1 | 16 | –0.98 | –5.16 to 3.20 | 0.645 |
| 48 | –1.37 | –6.64 to 3.90 | 0.611 | |
| 80 | 3.70 | –1.75 to 9.15 | 0.184 | |
| 0.072 | ||||
| Model 2c | –0.40 | –4.38 to 3.57 | 0.842 | |
| HAM-A | ||||
| Model 1 | 16 | –0.09 | –3.33 to 3.16 | 0.959 |
| 62 | –0.30 | –4.00 to 3.39 | 0.872 | |
| 48 | 0.71 | –3.38 to 4.80 | 0.734 | |
| 64 | 1.43 | –3.06 to 5.92 | 0.533 | |
| 80 | 1.88 | –2.59 to 6.35 | 0.409 | |
| 0.798 | ||||
| Model 2 | 0.25 | –2.44 to 2.93 | 0.858 | |
| HAM-D | ||||
| Model 1 | 16 | –0.22 | –3.74 to 3.30 | 0.901 |
| 32 | 1.28 | –2.45 to 5.00 | 0.501 | |
| 48 | 1.68 | –2.17 to 5.53 | 0.393 | |
| 64 | 2.53 | –1.55 to 6.61 | 0.225 | |
| 80 | 0.91 | –2.68 to 4.49 | 0.620 | |
| 0.861 | ||||
| Model 2 | 0.98 | –1.46 to 3.42 | 0.431 | |
| MAS | ||||
| Model 1 | 16 | 0.05 | –1.04 to 1.14 | 0.928 |
| 32 | –0.63 | –2.25 to 1.00 | 0.450 | |
| 48 | 1.84 | –0.26 to 3.94 | 0.086 | |
| 64 | –0.89 | –3.20 to 1.42 | 0.449 | |
| 80 | –2.14 | –4.64 to 0.36 | 0.094 | |
| 0.032 | ||||
| BRQ | ||||
| Model 1 | 16 | 138.40 | –33.06 to 309.80 | 0.114 |
| 48 | –22.90 | –199.90 to 154.10 | 0.800 | |
| 80 | –86.80 | –269.10 to 95.80 | 0.352 | |
| 0.063 | ||||
| Model 2 | 47.40 | –93.10 to 187.90 | 0.508 | |
FIGURE 19.
Kaplan–Meier estimates of time to first depression- or mania-type bipolar episode. Reproduced with permission from Jones et al. 157
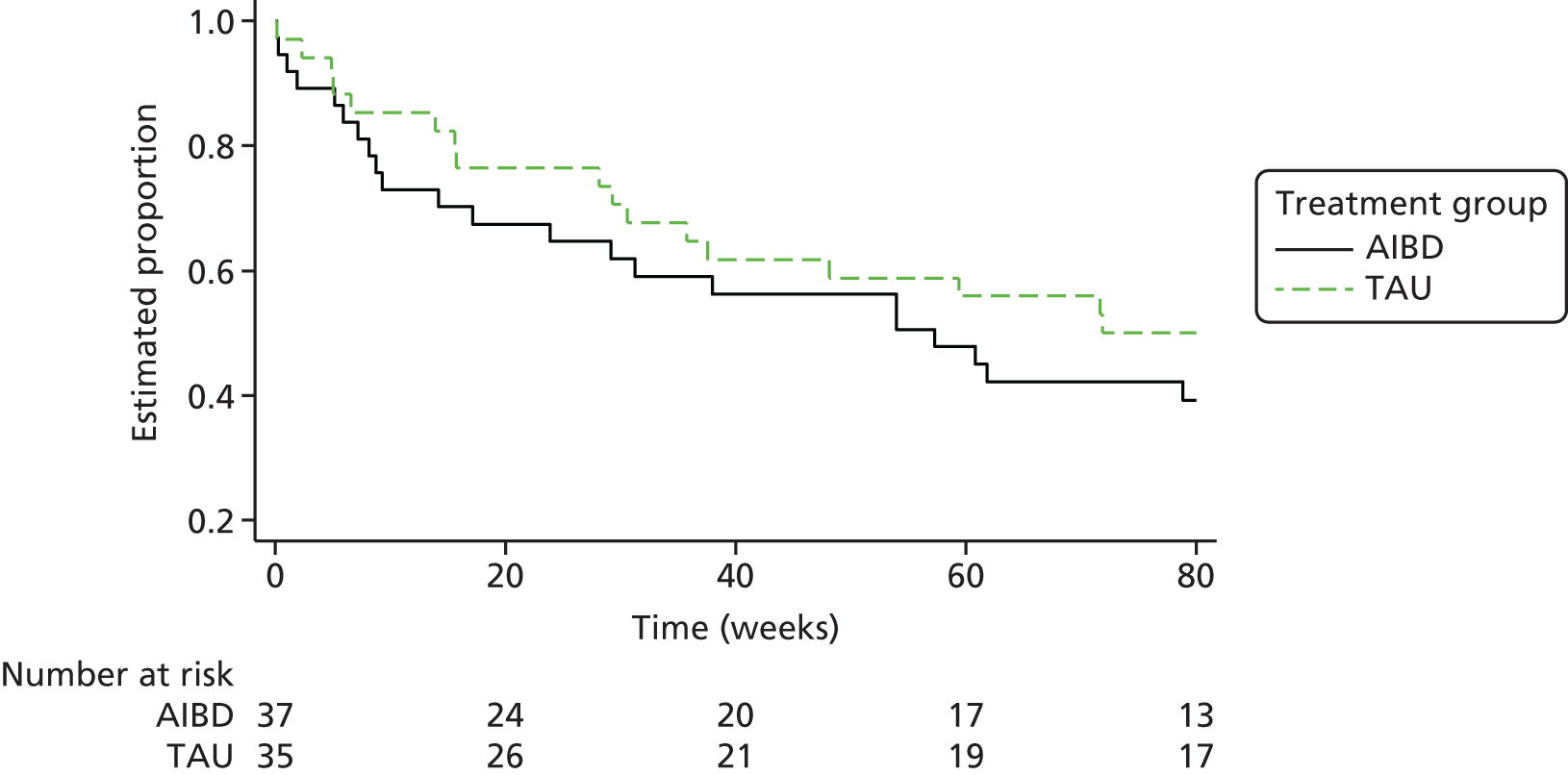
FIGURE 20.
Kaplan–Meier estimates of time to first depression-type bipolar episode. Reproduced with permission from Jones et al. 157

FIGURE 21.
Kaplan–Meier estimates of time to first mania-type bipolar episode.
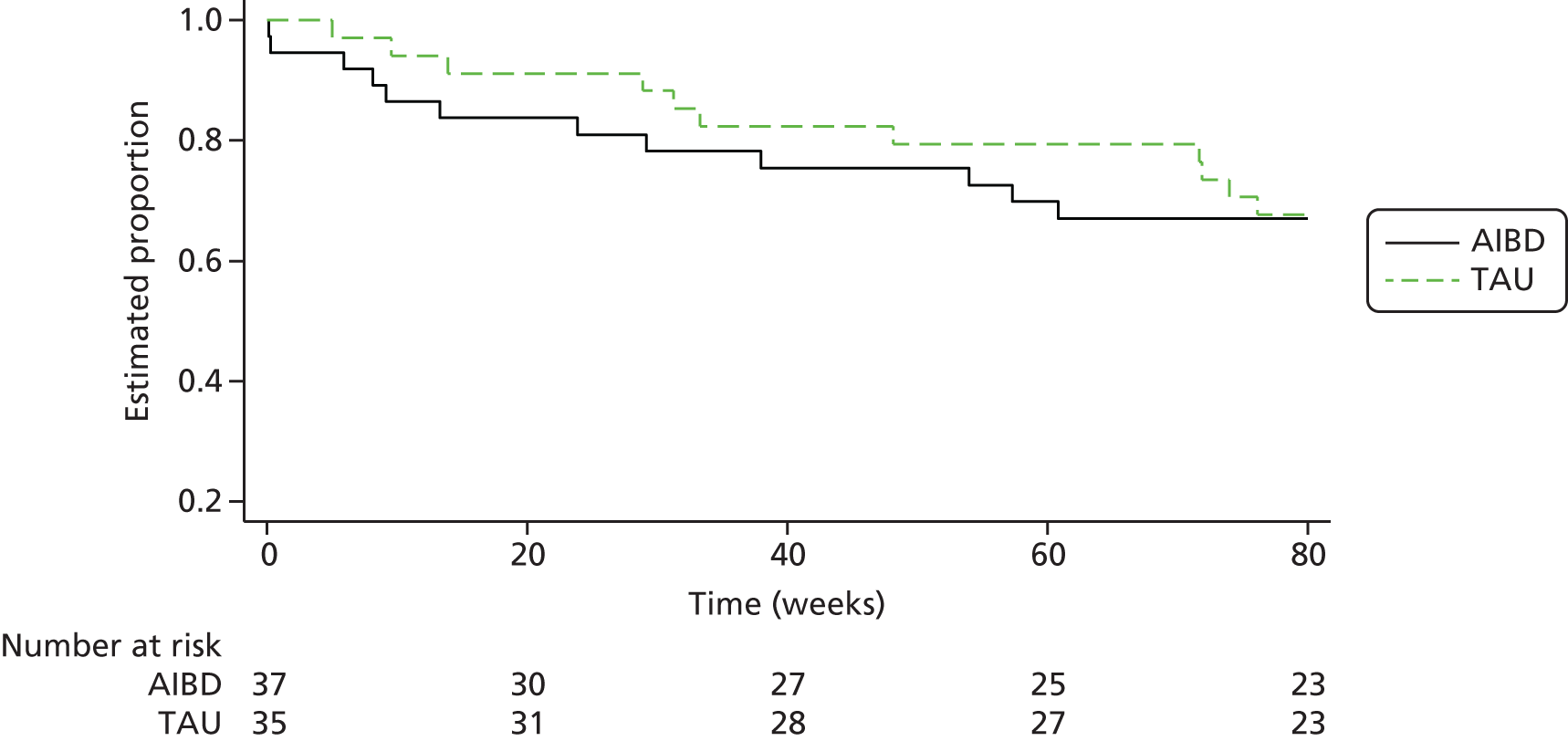
State–Trait Anxiety Inventory-State scores at baseline for both arms were elevated compared with age-matched norms,166 consistent with the intention to target an anxiety subgroup (norm means 35.88–36.03; SD 10.52–11.07). The mean score fell within the highest 20% of scores (AIBD 87th percentile; TAU 88th percentile) found among age-matched general population controls166 and were similar to the normative score for a diagnosed anxiety group (without BD) in the STAI manual (mean 49.02; SD 11.62). STAI-Trait scores were higher than those for STAI-State and substantially elevated compared with age-matched norms (mean 35.03–35.06; SD 8.88–9.31), with comparable SDs. Both arms fell within the highest 15% of scores (both 93rd percentile) of age-matched general population controls and were substantially higher than normative scores for a diagnosed anxiety group (mean 48.08; SD 10.65). These elevated scores are consistent with a successful HADS screening procedure to include individuals with clinical levels of anxiety symptoms. For both STAI-State and STAI-Trait, the trajectory of the reduction in STAI score from baseline to the 48-week follow-up was clearly steeper in the AIBD group than in the TAU group. This was the planned end point in the original application. However, this apparent gain did not persist to the 80-week follow-up.
Observer-rated anxiety assessment was conducted using the HAM-A. At baseline, both group means on the HAM-A were very low, consistent with a very mild level of anxiety (mild anxiety > 8; Matza et al. 176). Levels of observer-rated anxiety remained low throughout the follow-up period in both arms, never exceeding the mild anxiety threshold.
Observer-rated depression scores were determined using the HAM-D. 82 According to cut-offs determined by Zimmerman et al. ,177 participants were at the low end of mild depression at baseline in both arms (mild depression 8–16) and remained so throughout the trial.
Observer-rated mania scores were determined using the MAS. Bech178 defined absence of mania as a score of ≤ 6. Scores for both arms of the trial were extremely low throughout the study, consistent with participants being recruited during euthymia. The only exception was in the TAU group at weeks 64 and 80, when MAS increased above 4, although this remains within the ‘no mania’ range.
Self-reported recovery scores were measured using the BRQ. At baseline, recovery scores were substantially below those reported in the development paper for this measure98 (mean 2357.7, SD 414.0). The BRQ scores increased during therapy in the AIBD arm, in contrast to the TAU arm, but by 48 and 80 weeks the mean scores in both arms were comparable to those in the development sample previously noted.
In terms of relapse overall, 37% of the TAU group and 43% of the AIBD group had relapsed by the 48-week follow-up, and at the end of the final follow-up (80 weeks) the corresponding figures were 49% and 59% respectively. When relapses were compared by type of episode, it was found that the proportion who had experienced a depressive relapse was similar in the two groups, both at 48 weeks (31% of the TAU group and 32% of the AIBD group) and at 80 weeks (40% of the TAU group and 43% of the AIBD group). Likewise, at 48 weeks, 17% of the TAU group and 24% of the AIBD group had experienced a manic relapse, and by 80 weeks 31% of the TAU group and 32% of the AIBD group had experienced a manic relapse.
As expected for a feasibility study, the estimates of difference between the groups were very imprecise. As the CIs crossed 1 for the Cox model HRs, these were all non-significant. For the repeated measures analysis of the STAI-State outcome, the treatment by discrete time interaction was significant, with the estimated mean scores lower at weeks 16 and 48, but 8.49 units higher at week 80 in the AIBD arm than in the TAU arm.
Discussion
Phase 1
Semistructured interviews were conducted with 21 individuals with experience of BD and anxiety. The primary themes that emerged from the data highlighted:
-
‘Benefits and challenges of existing approaches’, in particular the limitations of trying to address anxiety with medication alone and the lack of information and integrated therapy approaches to anxiety and bipolar in the experience of participants.
-
‘Therapeutic experiences and aspirations’ indicated the importance of clinicians recognising how serious anxiety problems are for some people and the need for the appropriate treatment at the right time. In terms of psychological therapy, participants wanted an approach that was sensitive to their individual experiences and a therapist who was genuinely collaborative rather than didactic. Supplementing therapy sessions with information in an accessible booklet format was also highlighted.
-
‘Self-management strategies for anxiety’. Participants mainly described approaches they had discovered over time rather than through formal therapy. The primary approaches identified were behavioural approaches (such as relaxation or distraction). Although participants understood that cognitive factors were potentially important, most had not developed strategies to address unhelpful thoughts in the absence of structured psychological therapy.
These results highlight that individuals living with anxiety alongside their BD regard this as a serious problem, find current therapeutic options inadequate and would like access to structured psychological care to address this to supplement their own self-management strategies. Consistent with Frank et al.,18 even participants who did not have a current formal anxiety diagnosis were experiencing a significant impact from their anxiety symptoms. Current NICE guidelines1 indicate that individuals with BD should be offered anxiety intervention when required, based on NICE anxiety guidelines. 147,148 However, as the participants indicated in their interviews, treating anxiety without regard to the influence of BD is unsatisfactory in a number ways; these relate to the timing of therapy, the understanding of how anxiety and bipolar symptoms interact, and the primary importance of flexible collaborative approaches to engaging clients.
In terms of psychological therapy, participants wanted an approach in which there was due regard paid to the impact of anxiety on their lives, a focus on formulating individuals unique experiences and a therapy style that was collaborative and non-didactic. In contrast with findings that highlight ambivalence towards treatment in individuals with BD,179 participants expressed a clear interest in help of this type supplemented by additional materials in booklet form (or similar accessible format). Liebert180 has recently argued that the apparent ambivalence in individuals diagnosed with BD is commonly a response to the person feeling as though their actual experiences are undervalued and to inadequate and/or inappropriate treatment. Thus, in the current study, participants were clear about the core elements of an intervention that they would feel motivated to engage with. The individualised approach suggested by participants is consistent with other research into BD which, for instance, highlighted the potential benefits of a novel intervention to enhance personal recovery in BD which engaged directly with key personal goals rather than clinician-presumed therapy targets. 98,181
The majority of participants had not received psychological therapy targeting their anxiety symptoms. Despite or perhaps because of this, a range of self-management strategies was described. Behavioural strategies such as relaxation were reported to be quite effective when instigated in the early stages of an anxiety episode. Consistent with these reports, NICE guidance for GAD149 continues to recommend the use of guided relaxation as an effective intervention. However, in contrast, very few participants were able to use more cognitive strategies despite understanding that patterns of thinking were linked to their anxiety episodes. As cognitive approaches are also recognised as important in NICE guidance for anxiety disorder generally,1,147,148 this gap underscores the importance of offering people access to psychological help that can help them to identify and implement effective cognitive strategies to help manage their anxiety.
Phase 2
A series of three focus groups were conducted to elicit SU views on a planned integrated individual intervention for anxiety in the context of BD. Across the groups participants discussed the following.
-
Content: support with respect to both anxiety and BD, including individualised information, problem-solving and relaxation techniques, capitalising on current successful coping and tackling rather than avoiding problematic situations. The importance of cognitive as well as behavioural strategies was noted, and participants felt that it would be helpful to have an intervention with clear structure and monitoring of progress through this.
-
Focus: participants indicated that knowing something about the therapist as a person was important for engagement, as was instilling hope that progress could be made. Conversely, it was indicated that it would be helpful if the therapy was about developing skills that could be used beyond the intervention period, rather than there being a rush to resolve all anxiety issues within therapy. Such skills should build on client assets as well as aiming to remedy any deficits that the person was finding problematic.
-
Format: participants wanted the location and timings of therapy as well as therapy goals to be individually negotiated with each client. It was also noted that clients were likely to engage honestly with a therapist who makes an effort to communicate in ‘normal’ rather than ‘expert’ language and uses humour where this is appropriate. Likewise, participants wanted a therapeutic environment that felt safe so that new skills could be practised in a supportive environment in which immediate success was neither required nor expected. There was general agreement that an individual approach was preferable for this intervention, but that this should not exclude family or the care co-ordinator from specific sessions that the client wished them to attend.
-
Support materials: participants indicated that it would be helpful to have material that provided an overview of therapy along with worksheets to summarise each session. Although there was some disagreement about whether or not materials should be held on a website or in booklet format, there was clear consensus that materials should be well presented and include coping focused accounts from other individuals with experience of BD and anxiety.
-
Barriers and facilitators: a key issue here was accessibility. Based on prior experience of long waiting lists, participants highlighted the importance of a time-limited approach that could be delivered by a range of MHPs to increase the likelihood that those who needed the intervention could access it in a timely manner.
The information from phases 1 and 2 was broadly consistent in highlighting that individuals with BD and anxiety wanted access to a flexible, individualised, coping-focused psychological intervention that provided them with both cognitive and behavioural strategies to address their anxiety issues in the context of their BD. These views overlap with the limited research evidence to date, which indicates that the most promising psychological interventions for anxiety in BD are likely to be cognitive behavioural. 150,182 In addition, consistent with the views expressed by participants on the limitations of current treatments, there is a dearth of empirical evidence concerning the effectiveness of benzodiazepenes, mood stabilisers or antipsychotics in addressing anxiety in the context of BD. 182 These findings, therefore, converge on supporting the rationale for the evaluation of the new therapy in phase 3.
Phase 3
Primary feasibility and acceptability outcomes
The overall trial design appears to be feasible, as the study recruited to target and achieved retention of > 70% up to the 20-month follow-up. Of the people screened for eligibility, 14 (11.5%) declined the opportunity to participate in the trial, and a further nine were not contactable and so their willingness could not be ascertained. A further 22% did not meet the study inclusion criteria, so 59% of those screened were randomised into one of the arms of the feasibility trial. This inclusion rate is higher than for a similar individual cognitive therapy trial published recently181 and compares well with large-scale studies of CBT for relapse prevention, for which inclusion rates range from 17% to 48%. 79,100
In the current study, the retention rate at 20 months was 72%, which was within the target of 75% ± 10% and compares well with other CBT trials in BD. Jones et al. 73 obtained 67% follow-up to 20 months, while Scott et al. 79 retained 75% of their sample during the therapy phase and then to 18 months from baseline. Furthermore, retention appeared to be approximately the same in both arms, with retention, if anything, favouring TAU. Therefore, it does not seem that resentful demoralisation (differential dropout from trials in which the participants are not allocated to the active or preferred arm183) was a problem in this study. No adverse events were reported as a result of involvement in the trial in general or the therapy in particular.
The demographics of the sample are similar to those of previous studies of psychological therapy for participants with an established course of BD. In common with Scott et al. ,79 Lam et al. 100 and Meyer and Hautzinger,184 current participants were, on average, aged > 40 years. The current sample had experienced numerous prior mood episodes and over two-thirds had had at least 20 prior episodes, a higher proportion than reported by either Lam et al. 100 or Meyer and Hautzinger,184 even though these studies targeted relapse prevention. This suggests that the course experienced by our participants was at the more severe end of the bipolar spectrum.
The current study appears to be feasible with respect to the delivery of the AIBD intervention. On average, therapy clients attended 77% of sessions offered, indicating a significant commitment to receiving AIBD. A number of previous papers on individual therapy in BD did not specify attendance rates for the therapy arm. 79,185 Meyer and Hautzinger184 reported that 14.5% of their therapy participants dropped out of therapy owing to non-attendance, the majority after attending < 50% of possible sessions. More specific data from Jones et al. 181 indicated that individuals with recent-onset BD attended, on average, 78% of therapy sessions. These figures suggest that we achieved AIBD attendance rates at least comparable to those in the published literature. Consistent with this pattern, client ratings of the usefulness of therapy and likelihood of recommending the therapy were very high (average more than 9/10 in both cases); however, it should be noted that these ratings were not available for all participants and, therefore, should not be seen as definitive. In-depth qualitative interviews also indicated the value placed by participants on access to AIBD, particularly as it integrated approaches to BD and anxiety and was linked to improvements in both symptoms and functioning in a number of clients. It is, however, of note that some participants in the AIBD group would have preferred a longer intervention (although others were happy with it as delivered), in some cases to aid generalisation of therapy gains and in others for non-specific reasons related to enjoyment from working with the therapist.
Secondary outcomes
Although the trial was not designed to formally test the impact of AIBD on clinical outcomes, measures were taken of anxiety, mood and functional outcomes to inform a more definitive trial. Consistent with the potentially low power of this feasibility study, estimates of effects were imprecise, and none of the key comparisons was statistically significant to 48 weeks’ follow-up; the sole exception at 80 weeks was STAI. Self-reported anxiety data from STAI indicated levels of anxiety at baseline consistent with the inclusion criteria for the trial. Both trait and state forms of the measure indicate potential improvements in anxiety to 48 weeks post baseline. In contrast, observer-rated anxiety (HAM-A) was apparently low at baseline and throughout the trial. This discrepancy between observer and self-reported anxiety reports is potentially important for further trial development. Based on the screening data, the reports of clients in receipt of therapy and the self-report measurement, it seems highly likely that HAM-A underestimated the degree of anxiety experienced, which suggests that it might not be an appropriate outcome measure in a larger trial of this type.
Observer measures of mood indicated low levels of depression (HAM-D) and mania (MAS) consistent with our recruitment of participants during periods of euthymia. Given these low levels of mood problems at baseline, it is not surprising that there was no evidence for differential impact of mood during the trial. The only change of potential significance was slight elevation of mood (although still outside the clinical range) in the TAU group after 64–80 weeks. This anomaly seems unlikely to be linked to a negative impact of AIBD on mood as scores were extremely consistent in this arm.
Personal recovery, as measured by BRQ, was relatively low at baseline for participants in both arms of the trial and during the therapy period improved more rapidly among participants in the AIBD arm than among those in the TAU arm. However, by the time of later follow-up assessments, the TAU group had caught up with the AIBD group.
The overall recurrence rate of 54% for any episode of mania or depression across both arms was low compared with the recurrence rates at 2 years’ follow-up reported by Colom et al. 61 (of 92% in the PS group and 67% in the PEd group by 96 weeks). In comparison with individual psychological interventions, the relapse rate at 80 weeks was lower than that reported by Meyer and Hautzinger184 (64.5% at 80 weeks), comparable to that reported by Scott et al. 79 (52% at 72 weeks) and lower than that reported by Lam et al. ,185 who followed participants up for 48 weeks and found an overall relapse rate of 53% (compared with 43% in the current trial at the same time point). Although the recurrence rates were higher in the AIBD arm, the HR did not approach statistical significance. A moderation analysis indicated that neither number of previous episodes or baseline anxiety influenced clinical outcomes in response to AIBD was not significant, but the pattern of results suggested that response to this intervention might be clearer in those with more previous episodes.
Strengths and limitations of the research
The qualitative research in phase 1 of this WS highlighted the importance of developing an integrated psychological therapy approach for people with BD and comorbid anxiety. Although the sample size was reasonable for a thematic analysis, the study reflects the particular views of a subgroup of individuals from north-west England. Thus, although the findings were relevant to both treatment development in this WS and informing consideration of other approaches more widely, it would be sensible to explore these issues in more ethnically diverse groups and individuals earlier in their course of BD. Phase 2 had similar strengths and weaknesses in providing rich and detailed therapy development information but from a limited number of patients and clinicians in a particular region of the UK. The work conducted employed established focus group principles,154 and therefore such limitations are generally characteristic of this type of investigation. It seems that the information provided in phases 1 and 2 led to the development of an intervention (AIBD) that was feasible and acceptable based on data collected in phase 3. As a feasibility and acceptability study, the sample size was relatively small and not powered to detect statistically significant differences in clinical effects of the therapy. As such, estimates of clinical outcomes were imprecise with large standard errors. The sample was, however, adequate to provide evidence for the key feasibility and acceptability outcomes and to provide formative information about potential adaptations for a larger trial. A further definitive trial of AIBD would need to be conducted using a trial of sufficient power to reliably detect clinically significant differences in clinical outcomes.
Clinical and research implications
Information from phases 1 and 2 indicates that there is demand for improved psychological therapy for people with BD and anxiety and that this needs to be delivered with due recognition of the bipolar experiences people have had. This is relevant not only to further development of AIBD, but also to the consideration of treatment planning in NHS services.
Overall, the results from phase 3 indicate that the trial was successful in demonstrating feasibility and acceptability in that our selection and recruitment procedures worked, people took up the opportunity for therapy when this was offered to them, and retention to the trial was good. Secondary clinical data present a mixed picture, with most promising outcomes in self-reported anxiety ratings. This suggests that a future larger trial should explore anxiety outcomes in more detail. The impact on relapse and mood was expected to be less direct given the focus of the therapy on anxiety issues. It is therefore likely that a future, more definitive, trial would focus on a briefer follow-up to capture anxiety changes, with the need for lengthy follow-up required to monitor time to relapse. As most RCTs of CBT for primary anxiety disorder report a maximum follow-up period of 48 months, it is envisaged that this would be the appropriate duration for a future AIBD trial. 186 Although the intervention was generally well received by participants, some interviewed expressed a desire for more sessions to help generalisation of therapy. For a future trial, it would therefore be sensible to consider the potential to extend AIBD to 12 to15 sessions, in line with NICE guidance for high-intensity interventions for primary anxiety disorder. 149 If successful, such a definitive trial would provide the first evidence-based integrated therapy for anxiety in BD. This would be a timely addition to the therapeutic options for BD given the recognition of the importance of anxiety in recently revised NICE guidelines for BD57 and the prospective national roll-out of the Improving Access to Psychological Therapies Severe Mental Illness programme.
Chapter 4 Psychological treatment of comorbid alcohol use in bipolar disorder
Abstract
Alcohol use is a common comorbid issue in BD, and the limited number of published therapy trials to date indicate more promising outcomes for alcohol use than for use of other substances. To date there have been no trials of integrated motivational interviewing (MI) and cognitive therapy for problematic alcohol use in BD. Research was conducted across three linked phases: (1) a Q-Sort study of reasons for and experiences of cannabis and alcohol use in patients with BD; (2) the development and refinement of a therapy manual informed by pilot work, the Q-Sort study and SU focus group review; and (3) a RCT feasibility study of a novel integrated MI-CBT intervention for alcohol use in BD.
The results of phase 1 indicated important differences between cannabis and alcohol users in relation to both reasons for use and after-effects, with more negative experiences in alcohol users. Phase 2 indicated the importance of the integrated approach being collaborative, normalising and flexible. Phase 3 showed that the integrated MI and CBT intervention was feasible, acceptable and valued by recipients. The differences between the trial arms in clinical outcomes were not significant. Further research is needed to optimise the delivery of integrated psychology treatment for alcohol problems in BD.
Chapter overview
This chapter first provides an overview of research to date on comorbid substance use in BD and clarification of the rationale for focusing this stream on alcohol use in particular. The introduction illustrates that comorbid alcohol use is the most common substance use comorbidity and that the limited number of published therapy trials to date indicate more promising outcomes for alcohol use than for use of other substances. The three linked phases of this research stream are then described. These phases are (1) a Q-Sort study of reasons for, and experiences of, cannabis and alcohol use in patients with BD; (2) development and refinement of a therapy manual informed by pilot work, the Q-Sort study and SU focus group review; and (3) a RCT feasibility and acceptability study of a novel integrated MI-CBT intervention for alcohol use in BD. The primary methods and results from each element of this stream will be described, followed by consideration of the clinical and research implications of key findings.
Introduction
Prevalence of substance misuse, including alcohol, in bipolar disorder
Substance use is common in BD, with lifetime prevalence estimates ranging from 50% to 60% for BD 1. 5,187 Substance use rates are significantly higher than in the general population16 and those with other axis 1 psychiatric disorders. 188,189 For example, Schaffer et al. 190 reported past-year problematic substance use disorder (SUD) of 29% for BD and of 14.3% for major depressive disorders in a population-based sample. This high comorbidity in BD is clinically relevant because individuals with BD who use substances tend to haveworse social and neurocognitive functioning,191–193 a more severe clinical course194,195 and poorer treatment outcomes. 196,197
Alcohol is the most commonly abused substance among individuals with comorbid BD and SUD,187 with prevalence rates of 30–35% reported for alcohol use disorder in BD. 198 Comorbid alcohol misuse is associated with more severe mood disturbance199 and high levels of subsyndromal symptom,200 which are themselves associated with increased relapse risk and greater risks of suicide and violence. 201 Comorbid cannabis misuse is also common, occurring with a lifetime prevalence of 20–25% in BD. 13 Cannabis misuse is associated with earlier onset of BD, longer duration of mood episodes, greater illness severity, worse treatment adherence, greater disability and higher risk of suicide. 202–205
Even moderate alcohol consumption appears to be associated with higher risk of relapse and higher current mood symptoms. 199 Higher rates of impulsivity,206–209 suicide,191,200,210 criminality211 and violence201 are also observed for those with BD and comorbid alcohol misuse.
Available interventions for substance use in bipolar disorder
Despite the high prevalence of substance misuse in BD, and in particular comorbid cannabis and alcohol problems,5,13 there have been few developments in integrated therapy. To date there have been three published RCTs addressing this issue. Schmitz et al. 212 compared 12 weeks of individual CBT with a medication-monitoring control intervention and observed no differences between groups on substance use outcomes during treatment, but some improvement in mood. No data were collected at follow-up to explore the persistence of the mood effect. Weiss et al. 38,213 conducted two RCT evaluations of their integrated therapy compared with group counselling. Integrated group therapy delivered over 12–20 weeks led to improvements in substance abuse outcomes following treatment at 3 months’ post-treatment follow-up, particularly with respect to alcohol use. However, the findings with respect to mood were more mixed, with one trial indicating a worsening of depressive symptoms following treatment and the other indicating a reduction in risk of mood episodes and improved overall clinical outcome.
There have been no studies to date that have integrated MI with CBT approaches. This is an important omission, as ambivalence to treatment is common in BD. 180,214 There is clear evidence for the benefits of MI in addressing alcohol and drug issues in a range of client groups,215,216 as well as its particular impact of addressing treatment ambivalence. 217 By not addressing this issue, previous trials have focused on clients who are already motivated to change their substance use, therefore potentially excluding those with higher levels of clinical need. The work described in this stream therefore reports on the development of a therapy approach that integrated MI approaches to engage clients and achieve commitment to change with CBT approaches to provide tools for change and relapse prevention (MI-CBT) intended to be both inclusive and integrated, not excluding potential clients due to ambivalence.
Aims
The research reported here has the following aims:
-
to understand more about the experience and impacts of cannabis and alcohol use in BD
-
to develop an integrated therapy approach in partnership with SUs to address alcohol misuse (including in individuals who are currently ambivalent about their use of alcohol)
-
to evaluate the feasibility and acceptability of MI-CBT for alcohol use in BD with respect to recruitment, retention and feedback from participants.
Overview of methods
Phase 1
Q-Sort methodology was used to explore experiences and consequences of substance use in BD. This drew on a review of the literature, a qualitative evaluation conducted previously51 and analysis of client therapy tapes from our pilot work in preparation for this stream218 to develop the Q-concourse. Fifty individuals reporting either alcohol or cannabis use problems sorted the concourse statements in accordance with Q-methodology, first for experiences and second for consequences.
Phase 2
A series of focus group meetings were held with either SUs or health-care professionals. Focus groups were conducted in line with the principles described by Rabiee. 154 Both the SU and the professional focus groups were structured around eliciting information on prior experiences of therapy for alcohol issues, obtaining feedback on plans for the new intervention and views on format, style and timing of the intervention planned. The draft manual discussed in phase 2 was informed from a review of current literature, the phase 1 results and the therapy manual employed for our pilot work before this programme.
Phase 3
A single-blind RCT was conducted to determine the feasibility and acceptability of the integrated intervention compared with TAU. MI-CBT was delivered over 20 sessions over a period of 6 months, with participants followed up at 12 months post randomisation. Primary outcomes of the study were the feasibility and acceptability of MI-CBT (recruitment to target, retention to follow-up, absence of untoward incidents). We have also conducted preliminary analysis of alcohol and mood outcomes (frequency and severity of alcohol use and time to mood relapse).
Phase 1: substance use experiences in bipolar disorder – a Q-methodological investigation
Material and methods
Development of the Q-sets
The Q-samples for this study (the sets of statements describing reasons for, and after-effects of, use) were derived from (1) a structured literature review of studies reporting reasons for, and effects of, substance use in BD; (2) transcripts from a previous qualitative study of reasons for substance use in BD;51 and (3) review of audiotapes of therapy sessions from our pilot intervention case series. 218
The final Q-concourse (the set of all statements identified) of 296 experiences were condensed into 41 reasons for substance use and 40 after-effects of substance use by CB, SJ and NB. The final wording of each statement was then agreed in consultation with a SU group of people with experience of both BD and substance use issues.
Sample
This study was reviewed and approved by the UK NHS Ethics Committee process (REC reference 10/H1002/12).
The participants were recruited from a range of NHS (Dual Diagnosis, Community Mental Health, Assertive Outreach, Older Adult and Early Intervention) teams and voluntary sector organisations (Addiction Dependency Solutions, the Bipolar Organisation and the Mood Swings Network) in north-west England. All participants met DSM-IV criteria for bipolar affective disorders 1 or 2 as assessed by the SCID,77 and were consuming either alcohol or cannabis as follows: alcohol use exceeding 28 units for males and 21 units for females on at least half of the weeks of the previous 3 months, or use of cannabis at least two times per week in at least half the weeks in the 3 months prior to assessment. 24 Participants using both alcohol and cannabis completed the study referring to use of their most problematic substance (MPS).
Procedure
Each participant sorted first the 41 reasons statements and subsequently the 40 after-effects statements. A response grid (Figure 22) was provided on which participants could sort the statements in line with the following instructions.
FIGURE 22.
Q-Sort response matrix.

Reasons statements
Order the statements onto the response grid according to your reasons for using substance [X] by placing the statements that you feel apply to you the most on the right hand side of the grid, and the statements you feel apply to you the least on the left hand side of the grid.
Effects statements
Order the statements onto the response grid according to experiences you may have had in the 24 hours following using substance [X] by placing the statements that you feel apply to you the most on the right hand side of the grid, and the statements you feel apply to you the least on the left hand side of the grid.
For each ranking, the same procedure was applied. After constructing three sorts of statements (applies to me, does not apply to me and neutral), each participant identified the strongest ‘applies to me’ statement and placed it in the far right space on the response grid, then identified the two statements which ranked next highest in terms of relevance or importance to them, and placed these in the next column. This process was repeated until all statements from the ‘applies to me’ pile were placed on the grid. The participants were then asked to consider their ‘does not apply to me’ pile and repeat the process, starting with the statements they felt applied to them the least, placing statements on the opposite side of the response grid. They were then asked to consider any statements in their ‘neutral’ pile and place them accordingly in the centre of the grid. Following sorting, participants were given the opportunity to review their ranking before the final sort was recorded for the study.
Measures
Mood episodes and symptoms
Number of previous manic and depressed episodes were identified using the SCID interview. 77 Current mood was assessed using the HAM-D82 and the MAS,155 used in combination with the SCID interview.
Substance use
The Opiate Treatment Index219 was used to elicit the number of days of use of the MPS in the previous month, the period of use at this level (years) and the number of days alcohol was consumed in excess of UK government recommended levels in the past month. Alcohol and substance abuse/ dependence were assessed during the SCID interview. 77
Data analysis
An analysis of the Q-Sorts was performed using a dedicated software package (PQ Method, version 2.3, Schmolck, Munich, Germany) and employing principal components analysis with Varimax rotation. The resulting correlational matrix reveals relationships between individuals rather than between items and allows for the identification of factor exemplars to define each factor. PQ Method merges these factor exemplars using the weighted average of all the sorts that load significantly on each factor to produce a factor array. Chi-squared, t-tests and one-way analyses of variance were used to examine differences between participants loading on factors in terms of demographic (age, sex, marital status, living arrangements, education and employment status), clinical (number of previous manic/depressed episodes and current mood ratings) and substance use (MPS, SCID diagnosis, number of days use in past month and mean number of years use of MPS) characteristics.
Results
Participant demographics
Fifty individuals meeting the DSM-IV criteria for bipolar affective disorder 1 (n = 48) or 2 (n = 2) participated in the study, of whom 58% (n = 29) reported alcohol as the MPS and 42% (n = 21) reported cannabis as the MPS. For the alcohol MPS participants, 79% met the SCID criteria for current alcohol abuse or dependence. For the cannabis MPS participants, 57% met the criteria for current cannabis dependence and 14% met the criteria for cannabis abuse. Participants reported having used their MPS for an average of 21 days in the past month (SD 8.65 days in the past month). The time participants had used the substance before the study ranged from 3 months to 30 years.
The sample comprised 28 men (56%) and 22 women (44%), and the mean age of 40.2 years (SD 12.12 years). Most participants were of white British origin (94%), were unmarried (82%) and lived alone (54%); the remainder (46%) lived with others (partners, friends, children or others). The majority had completed further education (60%), but most were currently unemployed (80%).
Reasons for use
Principal components analysis of the Q-Sorts describing reasons for use resulted in a two-factor solution with 48 participants loaded on one or other of these factors (26 factor 1; 22 factor 2), and 41% of the variance explained (Table 35). Two participants’ sorts did not load on either of the factors.
| Q-Sort statement | Factor | |
|---|---|---|
| 1: mood management | 2: social reasons | |
| Makes me feel normal | 0 | 1 |
| Makes me feel less alone/lonely | –1 | 2 |
| Helps me to celebrate success | 0 | 0 |
| Relieves side effects of medication | –3 | –4 |
| It helps me to think | 1 | –2 |
| Satisfies my cravings/dependency | 1 | 0 |
| Slows down my racing thoughts | 3 | –1 |
| Helps me to sleep | 4 | 1 |
| Enables me to join in with what family and friends are doing | –2 | 2 |
| Helps me get along with others when pressured to | –2 | –1 |
| Helps me to feel less irritable | 2 | 1 |
| Helps me manage low mood | 2 | 3 |
| Alleviates boredom | 1 | 3 |
| Helps me to achieve an altered state of mind | 2 | 1 |
| Helps me to relax | 4 | 4 |
| Reduces my anxiety | 1 | 3 |
| Helps me manage my visions | –4 | –4 |
| Helps me to manage my anger | 0 | –3 |
| Helps me to get/stay high/elated | –2 | 0 |
| Makes me less restless | 3 | 0 |
| Increases my creativity | 0 | –1 |
| Increases my motivation | –3 | –3 |
| Helps me focus/get things done | –1 | –3 |
| Helps me cope with difficult/memories | 1 | 0 |
| Increases my energy | –4 | –1 |
| Helps me deal with problems | 1 | 0 |
| Makes me more open to new ideas | 0 | –1 |
| Boosts my confidence/self-esteem | –1 | 2 |
| Helps me to fit in | –1 | 1 |
| Helps me manage my voices | –5 | –5 |
| Relieves physical pain | 0 | –2 |
| Makes me feel good | 2 | 5 |
| Makes me feel less suspicious | –2 | –2 |
| Makes me less inhibited | –1 | 1 |
| Helps me enjoy sexual experiences more | –2 | –1 |
| Helps me to switch off | 3 | 2 |
| Makes me more sociable | –1 | 4 |
| Stops me from feeling too high/elated | 2 | –2 |
| Helps me to manage my appetite | –3 | –2 |
| Fits into my routine/lifestyle | 0 | 0 |
| Makes me feel calm | 5 | 2 |
Factor 1: mood management
Individuals who loaded on this factor reported using alcohol or cannabis to help to manage mood and to cope with distressing feelings, as one participant explained: ‘[drinking] used to be about socialising, but now it’s more about making me calm – it helps me to cope with everything that comes along with being high’. The most frequently endorsed reason for substance use by people in this group was ‘to feel calm’ (+5). Other highly endorsed items included ‘to aid sleep’ (+4), ‘to relax’ (+4) and ‘to feel less restless’ (+3). One participant explained ‘it’s the only thing that works to calm my anxiety’. Another described cannabis as ‘mood management. It’s a guarantee that I won’t get locked up [psychiatric hospital]’. This group also endorsed using substances for reasons explicitly related to the symptoms of mania or hypomania such as to stop feeling too high (+2), to slow down racing thoughts (+3), and to feel less irritable (+2). A further factor exemplar explained:
. . . today I woke up feeling high and I couldn’t have spoken to you unless I’d smoked to bring me back down to normal . . . sometimes I like the high so I don’t smoke, but if I want to stop it or slow it down, I normally can with weed.
This group did not report using substances to help them manage psychotic symptoms such as hearing voices (–5), visions (–4) or feelings of suspicion (–2). Nor did they endorse using substances to reduce the side effects of medication (–3). In keeping with the use of substances to reduce symptoms of mania, this group did not endorse using substances to increase energy (–4) or as an aid to help them get or stay high (–2). Moreover, individuals loading on this factor did not endorse socially related reasons, such as substance use to go along with others (–2), to join in with family and friends (–2) or to reduce loneliness (–1). One participant explained ‘it’s the opposite of being more sociable, it’s more about being less’. Another explained, ‘it’s not social for me, I lock myself away from people with my problems’.
Factor 2: social and enhancement reasons
Individuals loading on factor 2 primarily used substances to ‘feel good’ (+5). In contrast with those who loaded on factor 1, people in this group endorsed more socially motivated reasons for use. They used alcohol or cannabis to be more sociable (+4), to alleviate boredom (+3), to join in with family or friends (+2), to feel less lonely (+2) and to fit in (+1). They also reported using substances to boost confidence (+2) and reduce inhibitions (+1). This group were broadly neutral about using substances for their sedative such as feeling calm (+2), feeling normal (+1), reducing irritability (+1) and improving sleep (+1). As with factor 1, those who loaded on this factor did not report using substances to manage psychotic symptoms such as voices (–5), visions (–4) or suspiciousness (–2). Nor did they report using substances to manage the side effects of medication (–4). Some mood-related reasons for use were endorsed by participants loading on this factor, for example substance use to help manage low mood (+3) and reduce anxiety (+3): ‘I recently detoxed and I’m finding it very hard to stay off (alcohol) as it helps me to manage [pause] control my mood’; ‘I only drink when I get depressed – I wouldn’t bother if I felt OK’.
Although this group reported substance use to be predominantly social, some substance use was an attempt to cope with distressing feelings.
Group differences between factors
Statistical tests were employed to explore whether there were any differences between participants loading on factor 1 (mood management) and factor 2 (social enhancement) in terms of demographic, clinical and substance use variables. The majority of group 1 (mood management) were cannabis users (58%), whereas only 23% of group 2 (social enhancement) were cannabis users [χ2 (1) = 5.99; p = 0.01]. There was a trend for group 1 participants to have experienced more manic episodes [χ2 (2) = 6.18; p = 0.06].
After-effects of use
Principal components analysis of the Q-Sorts relating to the after-effects of substance use resulted in a three-factor solution (Table 36). Of the 50 participants who completed this sort, 48 loaded on one of these three factors (25 factor 1; 16 factor 2; 7 factor 3), and 49% of the variance was explained.
| Statement | Factor | ||
|---|---|---|---|
| 1: positive after-effects | 2: negative after-effects | 3: getting ‘high’ | |
| I feel more sociable | 3 | –2 | 4 |
| I feel sexually aroused | 0 | –4 | –1 |
| Stops me going high/elated | 1 | 0 | –4 |
| I feel worthless | –1 | 2 | –1 |
| I black out | –3 | –3 | 1 |
| I feel paranoid | –3 | 1 | 2 |
| I feel guilty | 0 | 4 | 0 |
| I don’t feel like talking to people | –1 | 1 | –2 |
| I have racing thoughts | –3 | 3 | 2 |
| I can concentrate better | 2 | –3 | –3 |
| I feel tired | 2 | 3 | 1 |
| I don’t feel the benefit from my medication | –1 | 0 | –1 |
| I feel depressed | –2 | 3 | 0 |
| I feel more bothered by past events | –2 | 2 | –2 |
| I feel suicidal | –4 | –3 | –5 |
| I feel better | 5 | –1 | 2 |
| I feel more likeable | 2 | –2 | 3 |
| I have a longer attention span | 1 | 0 | –3 |
| I feel more confident | 3 | –2 | 5 |
| I feel less irritable | 4 | 0 | –1 |
| I feel impulsive/disinhibited | 1 | –1 | 3 |
| I feel a buzz | 2 | –1 | 4 |
| I get high/elated | 0 | 0 | 3 |
| I feel ill | –2 | 5 | 1 |
| I have flashbacks | –4 | –1 | –1 |
| I feel out of control | –2 | 1 | 1 |
| I feel fearful/scared | –1 | 2 | 0 |
| I feel confused | –1 | 1 | 1 |
| I have hallucinations | –5 | –5 | –2 |
| I feel anxious | –2 | 4 | 0 |
| I feel my thoughts slow down | 3 | 0 | 2 |
| I feel I can do things I normally can’t | 1 | –2 | 1 |
| I feel more sensitive to highs and lows | 0 | 2 | –3 |
| I have disturbed sleep | 1 | 1 | 0 |
| I feel more motivated | 1 | –2 | –2 |
| I feel less angry | 2 | –1 | –1 |
| I have a better memory | –1 | –4 | –4 |
| I feel isolated | 0 | 2 | 0 |
| I feel I can function better | 4 | –1 | –2 |
| I have memory loss | 0 | 1 | –2 |
Factor 1: positive after-effects
Individuals loading on factor 1 generally reported feeling better (+5) as an after-effect of substance use; they felt more confident (+3), more sociable (+3) and more likeable (+2) in the time period following use of alcohol or cannabis. This group also tended to report feeling less irritable (+4) and less angry (+2) in the time following use of substances: ‘I just feel content, less bothered by people’s opinions, less affected by everything’. They felt that their thoughts slowed down (+3) and reported that they could function better (+4): ‘It means I can function effectively; without it I’m out of control’. This group also endorsed being able to concentrate better (+2) and feeling more motivated (+1) after substance use. One participant described improved functioning in specific circumstances: ‘I function generally better as a musician, but not as a driver’. This was endorsed by another participant, who reported that substance use enables him to ‘concentrate better on music, but not on other things’. Participants who loaded on factor 1 also reported feeling a buzz’ (+2) following substance use, and being more impulsive (+1). They reported that they could do things they normally could not (+1), suggesting that this group of individuals experienced both mood-related improvements and changes in their psychological state following substance use. At the same time, they also reported that substance use stopped them feeling too high (+1) and allowed them a longer attention span (+1). After-effects endorsed by individuals loading on this factor appeared mainly to be positive; however, individuals did report feeling tired (+2) and having disturbed sleep (+1), although, importantly, one individual described feeling tired as a positive after-effect.
Individuals loading on this factor did not endorse many of the negative after-effects of substance use presented in the Q-Sort. They did not experience hallucinations (–5) as an after-effect of substance use, nor did they experience flashbacks (–4), feel suicidal (–4), feel paranoid (–3) or have blackouts (–3). Likewise, they did not report feeling bothered about their past (–2), anxious (–2), ill (–2), depressed (–2) or scared (–1). They did not experience racing thoughts (–3) and, in keeping with an earlier observation that they experienced memory loss, they did not positively endorse having a better memory (–1) following substance use.
Factor 2: negative after-effects
In contrast to factor 1, individuals who loaded on factor 2 strongly endorsed the negative consequences of substance use. Individuals reported feeling ill (+5), anxious (+4), guilty (+4), depressed (+3), scared (+2) and worthless (+2) as after-effects of substance use. One participant reported that the consequences of use seem to be ‘all of the opposites to the reasons why I drink’. This group reported feeling more sensitive to highs and lows (+2), and experienced racing thoughts (+3) following use. Similarly to those loading onto factor 1, they reported feeling tired (+3) as well as experiencing memory loss (+1) and having disturbed sleep (+1) after substance use: ‘alcohol generally exacerbates low mood; if I’m low, it makes me even lower’. However, in contrast with those individuals loading on factor 1, this group also reported feeling out of control (+1) after substance use as well as feeling confused (+1), isolated (+2), paranoid (+1) and more bothered by past events (+2). They did not endorse positive social after-effects of alcohol or cannabis use; they did not feel like talking to people (+1), and placed feeling more likeable (–2), more sociable (–2) and more confident (–2) on the negative side of the response grid.
In contrast to those loading on factor 1, this group did not report the enhancing effects of substance use such as being able to concentrate (–3) or function better (–1). They did not report feeling more motivated (–2) and they did not report feeling ‘better’ generally (–1) as after-effects of alcohol or cannabis use. They also did not endorse the intoxicating effects of alcohol or cannabis use such as feeling a buzz (–1) or getting high (0).
Factor 3: getting ‘high’
Like factor 1, the majority of after-effects endorsed by people loading on factor 3 were positive. The main after-effects of substance use endorsed by this group included feeling more confident (+5), more sociable (+4) and more likeable (+3). This group also felt better (+2) following substance use and also reported thoughts slowing down (+2). However, a number of after-effects do distinguish this third factor from factor 1. Participants loading on factor 3 endorsed feeling a buzz (+4) and getting high/elated (+3) following substance use. They also felt impulsive and disinhibited (+3). Moreover, unlike those loading on factor 1, this group of individuals also endorsed some negative after-effects of use, such as feeling paranoid (+2), having racing thoughts (+2), feeling ill (+1) and blacking out (+1). Like those who loaded on factor 2, those loading on factor 3 endorsed feeling out of control (+1) and feeling confused (+1). This group tended to experience social and enhancement (intoxicating) effects, while also acknowledging the negative consequences of substance use.
Group differences between factors
Statistical tests were employed to explore whether or not there were any differences in the participants loading on the three factors. Just one difference was found. Fewer participants loading on factor 1 (positive after-effects) met criteria for alcohol use disorder (29%) than those loading on factor 2 (negative after-effects; 50%) and factor 3 (getting high; 71%) [χ2 (2) = 10.588; p = 0.03].
Relationships between reasons for substance use and after-effects
Frequency counts and percentages for how participants’ sorts of reasons for substance use relate to their sorts of after-effects of substance use were examined (Table 37). Equal numbers of participants (46%) who used substances to manage their mood (factor 1) endorsed positive and negative after-effects. Of the participants who endorsed social and enhancement reasons for use (factor 2), a higher number endorsed positive after-effects (50%) than negative ones (18%).
| Reasons for use | Factor | ||
|---|---|---|---|
| 1: positive after-effects, n (%) | 2: negative after-effects, n (%) | 3: getting ‘high’, n (%) | |
| Factor 1: mood management | 12 (46) | 12 (46) | 1 (4) |
| Factor 2: social reasons | 11 (50) | 4 (18) | 5 (23) |
Phase 2: therapy manual development – treatment planning focus groups
Sample
This study was reviewed and approved by the UK NHS Ethics Committee process (REC reference 10/H1014/40). Nine SUs with BD and personal experience of alcohol use problems and two MHPs with experience of working with people with both difficulties were recruited from two mental health trusts across the north-west (Manchester Mental Health and Social Care and five Boroughs NHS Trust). Following guidance from the PARADES SURG, separate focus groups were run for the SU and MHP participants. Both sets of focus group meetings were facilitated by PARADES researchers (NB and LB) and a clinical academic (CB).
Focus group structure and format
Each focus group began with an explanation of the purpose of the group from CB, and consent was obtained from participants to audio-record the focus group. This included an overview of the main topics to be covered in both the groups. The primary purpose of the groups was to refine the development of the integrated MI-CBT intervention for BD. To this end, participants were given an overview of the therapy development work that had been done to date. Focus group members' views were then elicited from both groups separately on:
-
therapy content
-
additional resources
-
treatment delivery
-
engagement.
The recordings were transcribed verbatim and analysed under these themes.
Results
Participants in both focus groups engaged readily in the planned discussion. SUs highlighted limitations with their current care and lack of access to an integrated approach to their alcohol problems in the context of BD. There was general approval from both groups for the intention to engage people in a collaborative and non-directive manner, as well as an acknowledgement that there was little access to this type of approach for most SUs in practice. Perspectives arising from each focus group are described in turn. Quotations are used to illustrate key points, and an identifying number is given after each quotation.
Service user perspectives
The SU focus group highlighted the importance of a good collaborative relationship with the therapist in the proposed therapy, and it was noted that it might be beneficial if the therapist had had personal experience of alcohol problems themselves. In line with these issues, there was a consensus that the therapeutic approach should be non-judgemental, collaborative and normalising:
I think you need to build a good relationship first before you try and do anything. If you don’t trust the person that you’re working with, you’re not going to go for it.
SU6
. . . this issue of not being judgmental about individuals erm . . . I’d say the key thing would be to sort of like, let the person themselves define what the problem is. I mean I know that is the intention of the formulation and whatever, but make that explicit from the outset that you know, it’s going to be your goals, your targets that we’re going be working towards and stuff. Not that we’re coming in here and tackling your problems drinking. I know on the thing it doesn’t say problem alcohol abuse does it.
SU1
. . . someone who had been there themselves, who were either bipolar or who had alcohol problems in the past. Yeah . . . that would be my ideal . . .
SU1
Discussions also indicated that SUs would welcome consideration of alternatives to alcohol if they were at the stage of considering a change in their alcohol use. Similarly, hearing other people’s stories in relation alcohol use in BD and how people have approached changes in use was thought to be a useful component of the therapy:
And then of course you’ve got the next stage which is replacing the alcohol with something else. If you don’t replace it with something else then there’s going to be a big hole.
SU1
Suggestions are quite helpful. Not telling people what to do.
SU4
Linked to this, it was suggested that offering people information on what others had found helpful in changing their alcohol use could be beneficial. For each of these suggestions, it was emphasised that they were likely to be beneficial if offered as options but that it was crucial that the therapist did not appear to be telling people what to do:
. . . would there be resources within this study for when you go out to recruit people . . . hopefully a success story, be willing to come out with the person taking consent to participate?
SU1
Flexibility in the timing and location of sessions was emphasised. In particular, offering people the option to have therapy at home was welcomed and thought likely to enhance engagement and retention:
I get in a state having to leave the house.
SU5
However, it was also cautioned that this should again be an offer rather than a prescription, as home may not be the ideal place for everyone:
But it does depend who lives there, because if you’re with people who you don’t want to know about it, it could be awkward.
SU6
. . . when I go and see my psychiatrist I have to go and see him at the outpatients place, but it’s the same place as where I was an inpatient, and there’s a certain smell in the hospital. And whenever I go back to a psychiatrist appointment, I’m taken back to the time when I was almost sectioned and I was having ECT [electroconvulsive therapy] and I think sometimes you don’t want to be in that kind of environment.
SU1
When the SU did not think the home was appropriate, they thought that an alternative should be negotiated that offered both flexibility and confidentiality:
For me, I wouldn’t want the therapy in my house because all my problems are in my house. I like to get out and get away and go somewhere neutral and talk about my problems.
SU9
Participants endorsed the importance of trying to time sessions for each client before they had begun drinking that day so that this did not detract from therapeutic work. To aid engagement, it was regarded as crucial to highlight to participants that the sessions were confidential. In general, a central feature of confidentiality was that information on quantity of alcohol consumed should not be disclosed to the SUs’ clinical team unless the client wanted to share it or this could not be avoided because of risk:
They’re going to really need to know that quite clearly . . . that nothing will be divulged about their drinking.
SU6
Clinician perspectives
The MHP focus group discussion highlighted the importance of eliciting broader goals beyond substance use to link into substance issues as therapy progresses:
. . . those broader, general goals are really really important because they are the focus of your therapy when you’re talking about things in general. You’re talking about alcohol or cannabis, you’re remembering . . . you’re collaboratively reminding each other the reason why you’re spending this time is because you’re here and you want to be there, and something’s stopping you from getting there.
MHP1
To aid this process therapists need to be very clear about eliciting people’s valued goals, which would not necessarily be reducing their alcohol use:
It’s sort of a bit more empowering than just ‘let’s get rid of this symptom’ which is kind of more of a medical approach.
MHP1
A number of alternatives were considered for eliciting goals, in particular employing a card sort approach to identify and prioritise these:
I haven’t done them yet in therapy, but also I’ve been in the past few months introduced to them . . . value cards . . . That sort of thing that helps to focus people’s minds a little bit.
MHP2
In relation to making changes in alcohol-related behaviour, it was noted that an important precondition was a clear understanding of what resources each person would need to have in place to feel able to make a change in behaviour. Once this was determined, clinicians felt that experimenting with change could be a constructive element of therapy. However, it was cautioned that it was necessary that any such experimentation was set up in a non-judgemental way so that whatever the outcome this would be seen not as success or failure but as providing useful information for therapy.
In relation to the overall process of therapy, the importance of genuine therapeutic optimism was highlighted along with empathy for the client’s situation. It was also acknowledged that there was a need for experienced therapists to be able to balance these requirements:
The therapeutic alliance. I think therapeutic optimism within that therapeutic alliance, because if you think that people will change, they’ve got more of a chance of changing . . . Saying ‘I do believe in you, you’re doing really well’, you know ‘it’s incredible how resilient you are.’ And with most of the clients I see that’s genuine because you hear their lives and you find out what they do, and it’s not ‘they’ve got problems’, it’s ‘why haven’t you got more problems?’, quite often . . . It has to be genuine, you can’t fake it.
MHP1
. . . empathy with the ambivalence is the really important . . . that you genuinely say ‘yeah it seems to be a really difficult thing to do this change, making your mind up about something is really hard, I know that . . . how uncomfortable that feels’ . . . So it’s about having those conversations which do two things at the same time, which make your relationship with them a deep and meaningful one based on empathy and warmth and honesty, . . . while at the same time leaving them uncomfortable about the ambivalence to their situation. So you want somebody leaving having seen you both comfortable with you and the relationship they have with you, and uncomfortable about the decisions they’re going to have to make, so that they’re more inclined to make them . . . And no wonder the evidence base for this is only slowly growing, because this is really quite complicated.
MHP1
In terms of maximising the therapeutic benefits of the intervention, clinicians felt that it was important to look for opportunities to engage the wider care team when possible. It was acknowledged that to achieve this requires the therapist to be flexible in exploring appropriate strategies to engage busy care co-ordinators:
I think that it has to be managed with a great deal of flexibility and opportunism towards the case manager. In other words, that you go out of your way to meet their needs in this relationship with you. You can’t expect them to move . . . you shouldn’t expect them to be able to move even a little bit towards you, that’s the reality.
MHP1
Linked to this there was general agreement that written therapy summaries and post-therapy plans developed with clients were important to ensure that gains extended beyond the immediate therapy period:
. . . you know so that it becomes more of a joint ownership thing in that it’s a three-way discussion at some point about change, and also it’s giving a very clear message to the care coordinator – actually the individual created this so this is somebody who you think is stuck but the individual has created this.
MHP1
Clinicians were clear that the planned intervention would not be appropriate for people with physical dependency owing to a lack of direct detox access for the research therapists:
I’ve thought a lot about this because I knew you’d have it, and I think you’re right to [exclude] on physical safety grounds as much as possible.
MHP1
The importance of attendance at the therapy to maximise client benefit was also considered; reducing non-attendance rates could be facilitated by a frank and honest discussion with clients about their attendance:
I guess then it’s also, you know, whether you can have open and honest discussions where people feel they can have open and honest discussions about why they’re missing appointments.
MHP2
Although clinicians acknowledged that the provision of up to 20 sessions was relatively generous for a clinical therapy trial, it was a limited period of time to explore the complexities of both BD and alcohol use. The importance of maintaining therapy momentum to move through the required work in the time period was, therefore, emphasised:
. . . it’s that early gains thing that there’s some kind of stimulus-reward going early on that you get something out of it.
MHP2
Clinicians also acknowledged that not all clients would actually want to work on their alcohol use and that, in that case, it was crucial to explore alternative goals to support ongoing engagement and offer opportunities over time to link these goals back to alcohol use when appropriate:
. . . don’t want to go to this sort of goal driven . . . our job is to reduce people’s alcohol because that is not what MI’s about. Our job is to allow people to have . . . facilitate directive, person-centred counselling around their intrinsic motivation for change, dealing with all the ambivalence they’ve got.
MHP1
I guess the general message that you’re trying to give them whilst you’re explaining the process is about you know, giving people a chance to learn about the process of change for them, and making that a very personal, you know making it more meaningful for you in terms of how you make decisions in your life. I don’t know . . . there’s something about unpacking okay this is the reason of the study, from the process you know. And yes obviously you know this is what we’re looking at, but our experiences from these trials is that all sorts of different changes happen within people’s lives or potentially can do. Or people make changes in their lives that may not relate to the study purpose. I don’t know if there’s any way of putting that succinctly.
MHP2
. . . we don’t change people, we don’t reduce their alcohol, they do that. We just have the right kind of conversations with them hopefully at the right time, which enables them to make that decision.
MHP1
Clinicians noted that it was important to provide clients with additional resources to support their therapy. In particular, as with the SU focus group, it was suggested that personal stories from other SUs were offered to clients. For example, there was a discussion about the potential benefits of video/cartoon depiction of other people’s journeys through alcohol use and behaviour change in the context of BD. In addition, it was felt that clients should be offered information about other sources of mental health support and about social and practical issues including finances.
Phase 3: a single-blind randomised controlled trial was conducted to determine the feasibility and acceptability of motivational interviewing cognitive–behavioural therapy compared with treatment as usual
The protocol has been published in Jones et al. 220 © 2018 Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
Methods
This study was reviewed and approved by the UK NHS Ethics Committee process (REC reference number 10/H1014/75). The trial was pre-registered on the ISRCTN registry (ISRCTN14774583).
Design
A pragmatic rater-blind RCT was conducted of up to 20 sessions of integrated MI-CBT for alcohol use in the context of BD compared with TAU. The trial is reported in accordance with the CONSORT guidelines for non-pharmacological trials. 221
Sample size
As the primary purpose of the study was to evaluate the feasibility and acceptability of delivering the integrated MI-CBT intervention [Alcohol in Bipolar Disorder Longitudinal Evaluation (initially), later Assessing Bipolar Lifestyle Experiences (ABLE)], a formal power calculation was not appropriate. It was estimated that 24 participants per group would be sufficient to allow the reliable determination of primary feasibility and acceptability outcomes. The study was not powered to evaluate the effectiveness of clinical outcomes but may help inform estimates of the potential treatment effect sizes (secondary objective).
Participants
Participants were recruited from adult MHS in nine NHS trusts in north-west England, covering Greater Manchester, Lancashire, Merseyside and Cheshire. The study was also advertised through voluntary organisations, local media, posters and leaflets distributed to both NHS and non-NHS sites. Inclusion criteria were the following: age ≥ 18 years; SCID diagnosis of BD 1 or 2; alcohol use exceeding 21 units for men/14 units for women on at least half of the weeks of the previous 3 months, or at least one alcohol ‘binge’ (exceeding the government recommended number of units of alcohol in a day: for men this is 8 units daily and for women this is 6 units daily) per fortnight in each of the previous 3 months (six binges in 3 months); a score of ≥ 8 on the Alcohol Use Disorders Identification Test (AUDIT222); and an ability to provide informed consent and having a fixed abode.
Procedure
Following written informed consent obtained at a face-to-face visit, diagnostic and substance use eligibility were confirmed using the SCID axis 1 disorders77 and AUDIT. 222 Participants meeting the inclusion criteria completed baseline assessment measures. Randomisation to therapy plus TAU or TAU only was conducted by the independent clinical trials unit at The Christie NHS Foundation Trust, Manchester. There is evidence that clinical outcomes from psychological therapy are typically better for women,161 preliminary evidence for better outcomes in BD for individuals with fewer previous episodes,163 and evidence that baseline AUDIT score predicts alcohol relapse. 223 Therefore, minimisation was used with respect to sex, number of previous bipolar episodes (mania, hypomania or depression) and score on the AUDIT.
Intervention
The ABLE intervention consisted of integrated MI and CBT and was developed from a treatment programme that has been shown to be beneficial to people with substance abuse and psychosis. 224 A recent case series applying key elements of this approach to BD patients218 has demonstrated its transferability to this client group. Participants in the therapy condition were offered up to 20 therapy sessions over 6 months. Sessions were typically 45–60 minutes long. Therapy was delivered at participants’ preferred location, usually their home. To ensure therapist fidelity and competence, all therapists met the British Association of Behavioural & Cognitive Psychotherapies accreditation criteria and were provided with a comprehensive 3-month training period as well as weekly supervision sessions with supervisors experienced in both MI and CBT.
The intervention was informed by both the case series work of Jones et al. 218 and the preparatory work from the earlier elements of the WS (phases 1 and 2). Initial sessions focus on engagement, employing MI to develop a shared understanding of the client’s key life goals and concerns, to elicit and selectively reinforce the client’s own self-motivational statements, and to monitor the client’s readiness to reduce their drinking. The therapist then works with the client to develop a shared understanding of how goals and concerns, and particularly how bipolar-relevant symptoms and relapses (depression, hypomania, anger, irritability, impulsiveness and disrupted social rhythms) might be related to drinking alcohol. The shared formulation that is developed from this phase of therapy is used to identify individual determinants and consequences of the client’s key problems, especially with respect to alcohol use. For clients who have motivation to change, the next stage of therapy involves developing an alcohol reduction/abstinence plan guided by the individual formulation and the needs of the client. For all participants, the plan incorporates appropriate evidence-based cognitive–behavioural strategies focused on implementing changes in drinking behaviour. This phase can also include CBT to facilitate alternative approaches to dealing with mood symptoms that have been associated with alcohol use, including depressed or elevated mood or interpersonal concerns. The intervention also includes development of a relapse prevention plan that summarises what has been learnt from therapy and provides a reference for the client following completion of treatment. For clients who are not ready to change their alcohol use, the therapist works on other client-led problems to maintain engagement, while continuing to link alcohol use to their concerns through MI techniques.
Training and monitoring of trial therapists
The four therapists employed by the trial were trained in the ABLE intervention, with CB leading training on the MI elements and SJ training in CBT in context of BD. Therapists received group supervision fortnightly, which included peer supervision and supervision led by CB. Therapists also had the opportunity to attend monthly joint sessions with therapists delivering AIBD to discuss CBT-related issues.
Within the ABLE RCT, once participants were allocated to receive therapy, the details were conveyed to the therapists, who aimed to begin therapy within a 2-week window. Participants received a letter advising them that they had been allocated to therapy and naming the therapists who would be making contact with them. The PARADES programme office managed appointments on behalf of the therapists and monitored the number of sessions that were completed with each participant.
Treatment fidelity
A random sample of 25% of session tapes (nine participants) were selected and assessed by an independent rater (SB) using MI-CTS-R. 225 Scores were recorded for section A (adherence with procedure) and section B (appropriate and strategic use of core skills).
Assessment of outcomes
At baseline, the SCID77 was completed to confirm the bipolar diagnosis and to provide information on alcohol disorder diagnoses as well as information on sociodemographic variables. The follow-up period was 12 months from initial randomisation. RAs blind to treatment allocation conducted outcome assessments for all available participants at baseline and at the 3-, 6- (end of therapy), 9- and 12-month follow-up appointments to provide an indication of the effectiveness of the intervention in increasing time to bipolar relapse, reducing frequency and severity of alcohol use, and impact on other clinical outcomes. All appointments were administered in person and recorded with the participant’s consent. Table 38 outlines the time frame for the measures. RAs trained to protocol collected missing follow-up data from clinical case notes and GP record reviews for participants who had provided written consent. Follow-up assessments were undertaken with RAs blinded to the arm to which the participant had been allocated. When unblindings occurred, these were recorded in a trial database and an alternative RA was requested to carry out further assessment visits. Reasons for unblindings were also recorded to help prevent future occurrences.
| Outcome measures | Time point of assessment | ||||
|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | |
| Primary outcome measures | |||||
| TLFB | ✓ | ✓ | ✓ | ✓ | ✓ |
| SCID LIFE | ✓ | ✓ | ✓ | ✓ | ✓ |
| Secondary outcome measures | |||||
| HAM-D | ✓ | ✓ | ✓ | ✓ | ✓ |
| MAS | ✓ | ✓ | ✓ | ✓ | ✓ |
| Internal States Scale | ✓ | ✓ | ✓ | ||
| PSP | ✓ | ✓ | ✓ | ||
| MEDAD | ✓ | ✓ | ✓ | ||
| EQ-5D | ✓ | ✓ | ✓ | ||
| PHQ-9 | ✓ | ✓ | ✓ | ||
| STAI | ✓ | ✓ | ✓ | ||
| BIS | ✓ | ✓ | ✓ | ||
| Readiness to Change Questionnaire – Alcohol | ✓ | ✓ | ✓ | ||
Levels of recruitment into the trial, retention of participants in both arms of the study, and adherence to, and completion of, therapy were evaluated to assess the feasibility and acceptability of delivering the MI-CBT to individuals with a diagnosis of BD.
Primary clinical outcomes
The Timeline Followback (TLFB) interview226 was used to measure the frequency and severity of alcohol use following MI-CBT, whereas the SCID research version (SCID DSM-IV: SCID LIFE80) was used to measure time to next bipolar episode (characterised by either depression or mania-type episodes).
Secondary outcome measures
Observer-rated mood symptoms were assessed by the HAM-D82 and MAS,155 whereas self-reported symptoms was measured by the Patient Health Questionnaire-9227 and Internal States Scale. 228 Quality of life and social functioning were measured by the PSP167 and medication adherence was measured by the MEDAD. 169
Process measures
The Barratt Impulsiveness Scale (BIS229) was used to investigate whether or not MI-CBT improved clinical outcomes through reducing impulsivity.
Measures to assess therapeutic alliance and adherence to treatment protocol
The WAI (short-form, therapist and client versions171) was used to assess engagement in therapy. Treatment fidelity was assessed by both the CTS-R172 and the MI-CBT Fidelity Scale specifically designed for the current study. 225
Qualitative interviews
Semistructured interviews were undertaken with a subgroup of recipients of the ABLE intervention selected to ensure maximum variance (e.g. number of sessions attended, age, sex, number of relapses, level of alcohol use and outcome). The interviews covered a range of topics, but for the purposes of this report we have concentrated on participants’ perspectives on the acceptability and impact of the AIBD intervention. All interviews were audio-recorded and transcribed verbatim, before being subjected to thematic analysis following Braun and Clarke. 91
Statistical methods
For the time-to-relapse Cox analyses, the covariates were treatment arm, sex, number of previous bipolar episodes (< 12 vs. ≥ 12 episodes) and AUDIT score at randomisation (8–15 vs. ≥ 16).
Repeated measures models with discrete time were fitted to the TLFB data. In model 1, a discrete time-by-treatment interaction was fitted to estimate the treatment effect and the 95% CI at each follow-up time point. If there was no evidence of an interaction at the 5% level, then the interaction term was omitted and the overall treatment effect was estimated (model 2). The same covariates as described above were used for these models, except that the AUDIT score was replaced with the baseline value of the outcome. The plausibility of the normality assumption of these models was checked using a normal probability plot of model residuals.
Results
As a pilot study, the ABLE RCT was designed to assess the feasibility and acceptability of developing this intervention into a larger-scale trial. We therefore present:
-
the feasibility of this study
-
the characteristics of the participants randomised to the two arms
-
results from the MI-CBT intervention that was developed and treatment fidelity in terms of acceptability to participants, and we present some of the views elicited from the qualitative interviews with participants who received it
-
the outcomes obtained in terms of alcohol intake and time to next bipolar recurrence.
Primary feasibility outcomes
The RCT was given the working acronym ABLE, initially meaning Alcohol and Bipolar Lifestyle Evaluation, but following SU feedback this was later changed to Assessing Bipolar Lifestyle Experiences.
Figure 23 presents the recruitment and retention rates for participants in the ABLE and TAU arms, consistent with CONSORT criteria reporting. 173 These figures were considered in combination with participation rates for the ABLE intervention in relation to the assessment of feasibility. Seventy-six potentially eligible participants were referred to the study over a period of 23 months (July 2011–June 2013). Two people could not be contacted for further assessment and 30 were excluded as they did not meet eligibility criteria at pre-screen (n = 22) or declined to participate (n = 8). Thus, only 10.5% of those offered the opportunity to participate in the trial declined.
FIGURE 23.
Assessing Bipolar Lifestyle Experiences study recruitment and retention.
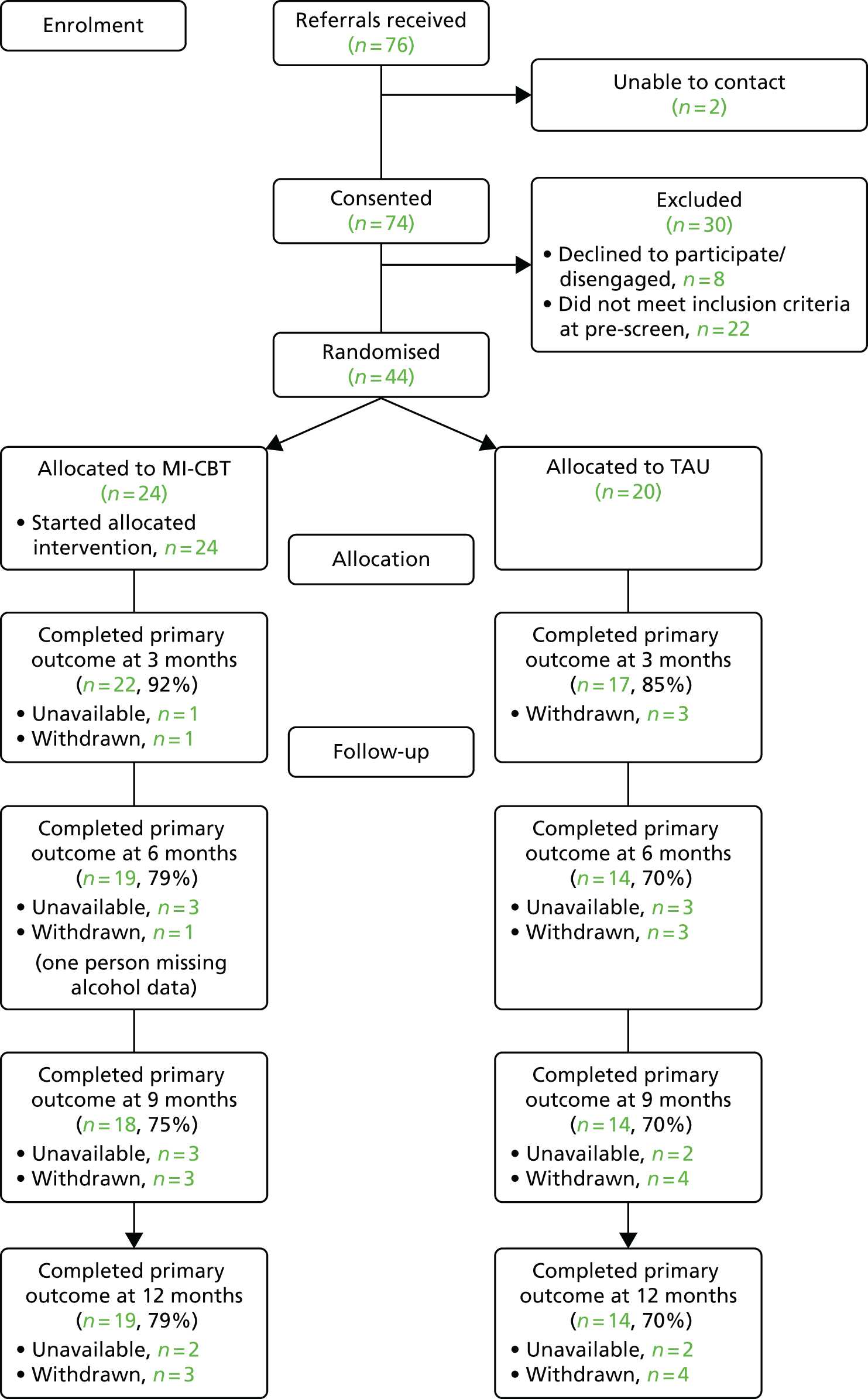
A total of 44 participants were randomised, which was 58% of those originally referred and within 92% of the target figure of 48. Data on the primary outcome measures (SCID DSM-IV: SCID LIFE and TLFB) were collected for 39 (89%) participants at 3 months, 33 (75%) participants at 6 months, 32 (73%) participants at 9 months and 33 (75%) participants at 12 months. Full data on relapse and alcohol use were available for 32 (73%) participants. Figure 23 illustrates the participant flow through the trial with respect to the MI-CBT and TAU arms of the ABLE RCT. The retention to 12 months for MI-CBT was 79% and for TAU was 70%. There was no evidence of differential retention at any of the follow-up points by arm (p > 0.25 for all χ2 comparisons) and, thus, resentful demoralisation does not seem to be a significant issue for this design.
Unblindings
Ten unblindings occurred within the trial (eight in MI-CBT, two in TAU). In all of these unblindings, we were able to ensure blind rating of outcomes measures for future follow-up assessments. Unblindings were due to a variety of reasons (six by participants mentioning their allocation to the RA and four from comments made by either care co-ordinators or programme administrative staff).
Adverse events
One adverse event was reported to the TSC chairperson for a participant allocated to MI-CBT. At the 6-month follow-up, the participant reported to the RA that they had taken an intentional overdose, although they were unable to recall the number of tablets they had taken or exactly when this had happened. This was not deemed a suicide attempt, and the participant, on revealing the information, was advised to contact their GP. The GP intervened and additional help was sought, all of which was documented in accordance with trial protocols. The participant was not hospitalised and was later able to recall more details of the event.
Participant characteristics
Table 39 displays the demographic characteristics, clinical features and baseline substance use data for the current sample. Participants were, on average, aged > 40 years and the majority were male (52%). Consistent with the inclusion criteria, all participants had an AUDIT score of ≥ 8, reflecting risky (8–12), hazardous (13–15) or harmful (≥ 16) alcohol use. The initial SCIDs indicated that, of these participants, 32 (73%) met the criteria for alcohol abuse and 18 out of the 32 (56%) met the criteria for alcohol dependence.
| Characteristic | Treatment group, n (%) | |
|---|---|---|
| MI-CBT (N = 24) | TAU (N = 20) | |
| Sex | ||
| Male | 14 (58.3) | 9 (45.0) |
| Female | 10 (41.7) | 11 (55.0) |
| Previous episodes | ||
| < 12 | 9 (37.5) | 8 (40.0) |
| ≥ 12 | 15 (62.5) | 12 (60.0) |
| AUDIT score | ||
| 8–15 | 8 (33.3) | 11 (55.0) |
| ≥ 16 | 16 (66.7) | 9 (45.0) |
| Ethnicity | ||
| White British | 24 (100.0) | 16 (80.0) |
| Other white | 0 (0.0) | 1 (5.0) |
| Black British | 0 (0.0) | 2 (10.0) |
| Asian | 0 (0.0) | 1 (5.0) |
| Marital status | ||
| Divorced/annulled/separated | 8 (33.3) | 6 (30.0) |
| Never married | 11 (45.8) | 11 (55.0) |
| Married or cohabiting | 5 (20.8) | 3 (15.0) |
| Number of children | ||
| 0 | 11 (45.8) | 10 (50.0) |
| 1 | 5 (20.8) | 7 (35.0) |
| 2 | 2 (8.3) | 3 (15.0) |
| 3 | 5 (20.8) | 0 (0.0) |
| Not stated | 1 (4.2) | 0 (0.0) |
| Living with | ||
| Spouse/partner only | 5 (20.8) | 2 (10.0) |
| Child(ren) only | 2 (8.3) | 5 (20.0) |
| Spouse/partner and child(ren) | 2 (8.3) | 1 (5.0) |
| Close relative with child(ren) | 1 (4.2) | 1 (5.0) |
| Close relative no child(ren) | 0 (0.0) | 2 (10.0) |
| Friends without child(ren) | 2 (8.4) | 0 (0.0) |
| Alone | 11 (45.8) | 10 (50.0) |
| Other | 1 (4.2) | 0 (0.0) |
| Education | ||
| Years 7–11 (no GCSEs) | 4 (17.4) | 2 (10.0) |
| GCSEs or equivalent | 4 (17.4) | 7 (35.0) |
| Further education not completed | 1 (4.3) | 2 (10.0) |
| Further education completed | 4 (17.4) | 2 (10.0) |
| Higher education not completed | 2 (8.7) | 1 (5.0) |
| Higher education completed | 5 (21.7) | 3 (15.0) |
| Postgraduate not completed | 2 (8.7) | 1 (5.0) |
| Postgraduate completed | 1 (4.3) | 2 (10.0) |
| Working | ||
| No | 17 (70.8) | 16 (80.0) |
| Yes (includes one volunteer) | 7 (29.2) | 4 (20.0) |
| Type of work | ||
| Employed full time | 2 (8.3) | 2 (10.0) |
| Employed part time | 3 (12.5) | 00.0 |
| Voluntary | 00.0 | 1 (5.0) |
| Self-employed | 1 (4.2) | 1 (5.0) |
| Unemployed | 3 (12.5) | 1 (5.0) |
| Sick/disability | 11 (45.8) | 12 (60.0) |
| Retired | 2 (8.3) | 1 (5.0) |
| Student | 1 (4.2) | 2 (10.0) |
| Not stated | 1 (4.2) | 00.0 |
| Bipolar status | ||
| BD 1 | 21 (87.5) | 19 (95.0) |
| BD 2 | 3 (12.5) | 1 (5.0) |
| Alcohol abuse | ||
| Overall | 20 (83.3) | 12 (60.0) |
| In the past month | 11 (55.0) | 7 (58.3) |
| Alcohol dependence | ||
| Overall | 15 (62.5) | 12 (60.0) |
| In the past month | 7 (46.7) | 7 (58.3) |
| Substance abuse | ||
| Overall | 4 (16.7) | 4 (20.0) |
| In the past month | 00.0 | 1 (25.0) |
| Substance dependence | ||
| In the last 12 months | 8 (33.3) | 4 (20.0) |
| In the past month | 2 (25.0) | 1 (25.0) |
Participants had established recurrent BD, with 61% reporting > 12 episodes. The large majority were white British (> 90%) and most were divorced or never married (> 80%), with just under half having no children (47.7%). Although > 60% of participants had at least begun further or higher education, three-quarters were currently unemployed (75%) and only 8% were in full-time employment. The majority of those unemployed were in receipt of sickness disability benefit. Comparing MI-CBT with TAU indicated more men and a higher proportion of more hazardous drinkers in the former at baseline; participants in the two arms were similar with respect to age, number of previous episodes, ethnicity, marital status and children. The mean age was 41.3 (SD 13.1) years in the MI-CBT group and 42.1 (SD 10.4) years in the TAU group. In the ABLE arm there were, numerically, more participants who had received higher education and were in work.
Treatment delivered and treatment fidelity
Participants offered MI-CBT were offered up to 20 sessions over a period of 6 months. All of the 24 participants allocated to the ABLE therapy arm attended at least one therapy session, up to a maximum of 20. The mean number of sessions attended was 17.6 (SD 4.5), with 21 out of 24 attending at least 15 sessions, indicating that 87.5% engaged substantially with the therapy. The proportions of participants attending sessions from 0 to 20 are as follows: 0–5 = 1 (4.2%), 6–10 = 1 (4.2%), 11–15 = 1 (4.2%) and 16–20 = 21(87.5%).
Nine sessions selected at random from the 24 participants receiving the intervention were assessed by an independent rater. The sessions selected ranged from early to late stages of therapy (sessions 4–16). The appropriate and strategic use of core skills was assessed using the Motivational Interventions for Drugs & Alcohol misuse in Schizophrenia trial MI-CBT scale, which showed that eight out of nine participants (88.9%) at least partially met the criteria. In one of the nine sessions examined, one therapist failed to meet the criterion on ‘collaboration’ (one single dimension of the 12 items examined).
Therapeutic alliance was assessed using the client short-form 12-item version of the WAI, which was completed by participants after sessions 3 and 16 and scored out of a total of 28 (four items each using a 7-point Likert scale with two items reverse coded). At session 3, agreement on the goals of treatment was similar between all three therapists whose clients returned WAI questionnaires (n = 21/24; 23.3–25.7), agreement on how to achieve these goals (task agreement) ranged from 22.3 to 24.6 (n = 21) and agreement on the development of a bond between participant and therapist ranged from 23.4 to 24.3 (n = 20; one participant had two missing items so their data could not be pro-rated). These scores summed provided overall scores out of 84 ranging from 70.0 to 72.1.
At session 16, only 18 participants completed the measure, so it may be expected that the scores increase given that some of those who found therapy less amenable would have dropped out of therapy. At this later session, agreement on the goals of treatment was available for only two of the three therapists (25.4–26.0); agreement on how to achieve these goals (task agreement) ranged from 25.4 to 25.5; and agreement on the development of a bond between participant and therapist ranged from 24.0 to 24.7. These scores summed provided overall scores out of 84 of 75.5, equal for both therapists.
The therapists completed ratings of the level of formulation achieved and perceived client outcomes. Formulation level was rated from 1 (little shared enlightenment about key concerns/impossible to facilitate discussion) to 5 (comprehensive shared and agreed formulation of all key factors including alcohol). Formulation ratings (mean 4.2) varied from 1 (for a participant who attended a single session) to 5; 20 out of 24 participants achieved a shared and agreed formulation incorporating the role of alcohol, whereas the remaining three had a partial formulation achieved but the full engagement of the client with the formulation was not definite. Therapy outcomes were rated with respect to overall benefit (0 = none and 5 = substantial). The overall ratings ranged from 0 to 5 (mean 3), with 67% achieving an outcome of ≥ 3.
Qualitative data on acceptability
Fifteen individuals who received the MI-CBT therapy took part in an in-depth qualitative interview at the end of therapy to explore their experiences of the intervention. A number of key themes were elicited from these data on preliminary analysis, which will be presented in more detail in a forthcoming qualitative paper.
Participants were positive about the therapy, identifying how it was helpful in addressing behaviours that they identified as harmful and the impact that this had on their life:
The fact that it’s worked, and it has worked for somebody who well self-confessed is really, really cut up, messed up, scarred like you know I was totally destroying myself, and the fact that it has managed to work on someone like me.
AB002
The therapy without wanting to sound melodramatic I would probably say it was fairly life-changing for me to be honest.
AB001
The intervention was developed to combine MI and CBT in recognition of the ambivalence that individuals may bring to a therapy concerning changing patterns of alcohol use. Consistent with this, participants described that they had been concerned that they would be given very directive instructions to achieve changes that would be impossible. With respect to disclosure, participants described how they did not always want to cover particular issues that they felt would have been too demanding:
I was worried . . . that it was going to be like, writing thou shalt not drink, I thought it was going to be a bit of a finger wagging erm . . . because I can’t give up alcohol, I can’t, because by giving up alcohol I would give up my whole social existence.
AB008
There is probably elements of things that we didn’t cover, not necessarily because there wasn’t time but because I didn’t want to cover them at that time. Erm . . . I think [therapist] touched on a couple of things that, quite emotional things for me, erm . . . as I was growing up and things like, that we didn’t touch on because I found it too stressful at the time.
AB010
Engagement was facilitated by a collaborative non-directive approach that aided openness and gave them a sense of control and responsibility over the decisions that they were making:
[Therapist] never came across as superior so to speak or anything like that it was like on a one-to-one equal basis. And I never felt put down by saying certain, you know things other people might find stupid, hearing things and things like that it wasn’t ever; I didn’t feel embarrassed to say anything whatever.
AB010
The biggest thing that I became aware of, out of all the therapy and speaking to you and everything, everybody who has ever come and seen me, it is down to me, it is down to me, it is not about the people who come and sit and you discuss it with them who were really kind to me and what have you it’s not at the end of the day the buck stops with me.
AB008
Participants identified benefits from the therapy in terms of use of alcohol, in particular that they no longer felt that every situation was an excuse for triggering alcohol consumption:
I feel it’s much more manageable it is not my go-to place, so it is not the first thing that I go right I need a drink . . . because always there is a reason to drink, there is a funeral, there is a wedding, you are happy, you are sad, you are stressed you are on holiday, you could link it as much as you want to, erm . . . so I feel that I have extricated myself from [that].
AB015
Benefits were also reported for bipolar symptoms and wider functioning and relationships:
I have been great. I have not had any erm . . . more episodes and I have stayed out of hospital, and I have started a little job actually.
AB006
. . . having a one-to-one and feel that it’s safe to talk, about your problems, well made me, give me the courage a bit, to go forward rather than backwards.
AB009
[My daughter] understands how I feel now rather than just mom is not well today, erm . . . she understands it, she even jokes about it now.
AB010
Clearly, no form of intervention is going to be ideal for all potential clients. One participant identified that the intervention was not what they would have defined as therapy, possibly because of its non-directive and flexible nature. Another participant felt that therapy was motivating them to change but that they over-reached during a period of elevated mood. Both of these factors will be considered in the future refinement of the approach:
I couldn’t actually say that you know I felt it was therapeutic. The conversation was therapeutic because it’s always nice to be involved in a conversation but you know apart from that you know I couldn’t say it was a therapy.
AB011
. . . as time went on and what I had set out to do I had achieved, I actually started to get overconfident, and then towards the end of the therapy I was going a bit up and trying to do too much so I got ahead of myself, and that ended up with me just overloading myself and then hitting a bit of a crisis.
AB013
Secondary outcomes
The key clinical outcomes for the ABLE study were frequency and quantity of alcohol use (see Table 40) and time to next bipolar recurrence (Figures 24–26).
FIGURE 24.
Kaplan–Meier estimates of time to recurrence of depression- or mania-type bipolar episode.

FIGURE 25.
Kaplan–Meier estimates of time to recurrence of depression-type bipolar episode.
FIGURE 26.
Kaplan–Meier estimates of time to recurrence of mania-type bipolar episode.
Alchohol data: Timeline Followback
The summary statistics are shown in Table 40 and the model results are shown in Table 41.
| Visit (months)a | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT | TAU | |||||||||||
| Mean | Median | SD | Minimum | Maximum | n | Mean | Median | SD | Minimum | Maximum | n | |
| Mean alcohol units/day | ||||||||||||
| 0 | 7.5 | 6.7 | 5.3 | 1.8 | 22.3 | 24 | 6.0 | 6.2 | 3.5 | 1.0 | 11.4 | 20 |
| 3 | 7.4 | 5.4 | 7.8 | 0.6 | 33.8 | 22 | 5.5 | 4.8 | 4.3 | 0.7 | 15.8 | 17 |
| 6 | 6.1 | 4.4 | 4.8 | 0.4 | 17.9 | 19 | 4.1 | 2.2 | 3.6 | 0.1 | 10.8 | 14 |
| 9 | 6.5 | 4.9 | 5.7 | 0.0 | 19.6 | 18 | 4.1 | 2.4 | 4.6 | 0.0 | 14.7 | 14 |
| 12 | 5.7 | 3.7 | 5.5 | 0.0 | 16.9 | 19 | 4.2 | 2.6 | 4.1 | 0.0 | 14.9 | 14 |
| Days abstinent: alcohol (%) | ||||||||||||
| 0 | 39.3 | 42.8 | 26.3 | 0.0 | 82.2 | 24 | 43.3 | 41.7 | 24.0 | 0.0 | 87.8 | 20 |
| 3 | 45.2 | 48.3 | 32.0 | 0.0 | 90.0 | 22 | 48.2 | 46.7 | 30.1 | 0.0 | 96.7 | 17 |
| 6 | 45.8 | 46.7 | 33.7 | 0.0 | 93.3 | 19 | 60.2 | 66.7 | 31.1 | 0.0 | 96.7 | 14 |
| 9 | 41.3 | 33.3 | 37.7 | 0.0 | 100.0 | 18 | 62.1 | 66.7 | 30.6 | 0.0 | 100.0 | 14 |
| 12 | 47.4 | 36.7 | 35.0 | 0.0 | 100.0 | 19 | 61.7 | 63.3 | 23.3 | 23.3 | 100.0 | 14 |
| Days abstinent: all substances (%) | ||||||||||||
| 0 | 39.2 | 42.8 | 26.1 | 0.0 | 82.2 | 24 | 43.3 | 41.7 | 24.0 | 0.0 | 87.8 | 20 |
| 3 | 44.5 | 45.0 | 31.8 | 0.0 | 90.0 | 22 | 42.5 | 43.3 | 29.5 | 0.0 | 83.3 | 17 |
| 6 | 45.4 | 46.7 | 33.2 | 0.0 | 90.0 | 19 | 59.8 | 66.7 | 30.8 | 0.0 | 96.7 | 14 |
| 9 | 41.3 | 33.3 | 37.7 | 0.0 | 100.0 | 18 | 61.7 | 66.7 | 30.0 | 0.0 | 100.0 | 14 |
| 12 | 46.0 | 36.7 | 34.1 | 0.0 | 100.0 | 19 | 56.4 | 60.0 | 22.3 | 23.3 | 93.3 | 14 |
| Binge days (%) | ||||||||||||
| 0 | 48.3 | 46.7 | 3.4 | 10.0 | 100.0 | 24 | 43.9 | 41.1 | 28.6 | 12.2 | 100.0 | 20 |
| 3 | 43.5 | 41.7 | 32.6 | 3.3 | 100.0 | 22 | 41.6 | 46.7 | 36.3 | 0.0 | 100.0 | 17 |
| 6 | 41.4 | 33.3 | 34.7 | 0.0 | 100.0 | 19 | 30.5 | 20.0 | 31.3 | 0.0 | 100.0 | 14 |
| 9 | 42.0 | 41.7 | 39.0 | 0.0 | 100.0 | 18 | 30.7 | 15.0 | 34.1 | 0.0 | 100.0 | 14 |
| 12 | 36.5 | 26.7 | 34.3 | 0.0 | 100.0 | 19 | 31.7 | 25.0 | 24.8 | 0.0 | 76.7 | 14 |
| AUDIT total | ||||||||||||
| 0 | 19.9 | 7.3 | 16.5 | 11.0 | 39.0 | 24 | 18.0 | 7.0 | 15.0 | 8.0 | 30.0 | 20 |
| 12 | 15.3 | 7.5 | 17.0 | 0.0 | 25.0 | 15 | 12.2 | 7.7 | 10.0 | 4.0 | 30.0 | 11 |
| Outcome measure/model | Month | Treatment effect | 95% CI | p-value |
|---|---|---|---|---|
| Mean alcohol units/day | ||||
| Model 1 | 3 | 0.98 | –2.04 to 4.00 | |
| 6 | 0.92 | –1.15 to 3.00 | ||
| 8 | 0.72 | –1.38 to 2.82 | ||
| 12 | 0.06 | –2.96 to 3.09 | ||
| Time × treatment interaction | 0.955 | |||
| Linear trend test | 0.641 | |||
| Model 2: no interaction | 0.68 | –0.77 to 2.14 | 0.358 | |
| Days abstinent alcohol (%) | ||||
| Model 1 | 3 | –5.39 | –21.5 to 10.7 | |
| 6 | –16.0 | –32.1 to 0.12 | ||
| 9 | –16.7 | –36.3 to 2.89 | ||
| 12 | –14.9 | –31.6 to 1.81 | ||
| Time × treatment interaction | 0.500 | |||
| Linear trend test | 0.395 | |||
| Model 2: no interaction | –12.0 | –23.5 to –0.48 | 0.041 | |
| Days abstinent all substances (%) | ||||
| Model 1 | 3 | 0.77 | –15.3 to 16.8 | |
| 6 | –15.1 | –31.1 to 0.90 | ||
| 9 | –15.7 | –34.9 to 3.41 | ||
| 12 | –10.2 | –26.5 to 6.14 | ||
| Time × treatment interaction | 0.226 | |||
| Linear trend test | 0.335 | |||
| Model 2: no interaction | –9.0 | –19.8 to 1.87 | 0.105 | |
| Binge days (%) | ||||
| Model 1 | 3 | –0.46 | –14.5 to 13.6 | |
| 6 | 7.55 | –4.80 to 19.9 | ||
| 9 | 1.86 | –15.2 to 19.0 | ||
| 12 | –1.78 | –19.5 to 16.0 | ||
| Time × treatment interaction | 0.569 | |||
| Linear trend test | 0.775 | |||
| Model 2: no interaction | 2.16 | –6.93 to 11.26 | 0.641 | |
Baseline level of mean daily alcohol consumption was in the high or very high risk range for the MI-CBT arm and on the threshold for high risk in the TAU arm;230 frequency of use and frequency of binge days were also elevated in comparison with the general UK and European Union populations. This pattern suggests that the selection criterion based on AUDIT has appropriately identified participants who have problematic patterns of drinking. Mean alcohol use declined from baseline in both arms, with no significant differences between them. Abstinence was numerically lower for alcohol and substance in the MI-CBT arm at baseline. The extent of abstinence across follow-up increased more in the TAU arm than in MI-CBT arm, although estimates were imprecise. A similar pattern was noted for all substances, but the differences between groups did not reach formal significance levels. There was no evidence of difference in change in the percentage of binge days between the MI-CBT and TAU arms.
Episode recurrence
Of the 24 participants in the MI-CBT arm, nine (37%) had a recurrence of an episode of depression or mania type. In the TAU arm, five participants (25%) had a recurrent episode. Of the recurrences, 78% and 80% were depressive episodes in the MI-CBT and TAU arms, respectively. A Kaplan–Meier plot for time to recurrence in the two groups is shown in Figure 24. The adjusted HR was 1.50 (95% CI 0.49 to 4.58; p = 0.478), indicating a non-significantly faster time to recurrence in the MI-CBT arm than in the TAU arm.
In the MI-CBT arm, a total of seven (29%) subjects had a recurrence of a depressive episode, compared with five (25%) in the TAU arm. The results of a Kaplan–Meier analysis comparing the two groups are shown in Figure 25. There was no evidence of a difference between the groups, with an estimated HR of 1.10 (95% CI 0.34 to 3.54; p = 0.878).
In each arm, only two participants had a recurrence of a mania-type episode. Because of small numbers the analyses are not very reliable (95% CIs for percentiles were not estimable).
Figure 26 shows the Kaplan–Meier estimates for the two groups. The time to recurrence of mania relapse was non-significantly faster in the MI-CBT arm than in the TAU arm (adjusted HR 1.62, 95% CI 0.21 to 12.83; p = 0.646).
Self- and observer-rated outcome measures
The completion rates for self- and observer-rated measures (Table 42) at baseline were 95.4% of those with SCID assessments: 93.4% at 6 months and 87.9% at 12 months.
| Time since baseline (months) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT | TAU | |||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | |
| HAM-D | ||||||||||||
| 0 | 5.17 | 5.64 | 3.00 | 0 | 19.00 | 24 | 3.94 | 4.37 | 3.00 | 0 | 13.88 | 20 |
| 3 | 4.87 | 5.13 | 2.50 | 0 | 15.00 | 22 | 5.41 | 5.23 | 3.00 | 0 | 16.00 | 17 |
| 6 | 4.62 | 5.88 | 2.00 | 0 | 20.88 | 20 | 6.01 | 7.47 | 3.14 | 0 | 23.00 | 14 |
| 9 | 4.36 | 5.02 | 3.39 | 0 | 20.00 | 17 | 4.43 | 6.80 | 2.00 | 0 | 20.00 | 14 |
| 12 | 4.87 | 4.65 | 3.00 | 0 | 15.00 | 19 | 5.59 | 5.16 | 5.29 | 0 | 20.30 | 14 |
| MAS | ||||||||||||
| 0 | 1.04 | 1.90 | 0.00 | 0 | 8.00 | 24 | 0.70 | 1.03 | 0.00 | 0 | 3.00 | 20 |
| 3 | 1.66 | 4.47 | 0.00 | 0 | 21.00 | 22 | 1.00 | 1.50 | 0.00 | 0 | 5.00 | 17 |
| 6 | 0.81 | 1.33 | 0.00 | 0 | 5.00 | 20 | 0.29 | 0.48 | 0.00 | 0 | 1.10 | 14 |
| 9 | 0.88 | 1.97 | 0.00 | 0 | 7.70 | 17 | 1.11 | 2.49 | 0.00 | 0 | 9.00 | 14 |
| 12 | 1.21 | 2.15 | 0.00 | 0 | 8.00 | 19 | 2.72 | 4.48 | 1.05 | 0 | 16.00 | 14 |
Social functioning was assessed using the PSP (Table 43). Participants in the MI-CBT arm were, on average, experiencing mild problems in a single area of social functioning, whereas those in the TAU arm fell into the category of good functioning in all areas, on average. PSP scores remained in the same functioning categories for both arms, with a small numerical decline in the TAU arm to 12 months’ follow-up.
| Time since baseline (months) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT | TAU | |||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | |
| 0 | 79.7 | 13.4 | 80 | 45 | 97 | 23 | 84.4 | 14.3 | 90.5 | 45 | 95 | 20 |
| 6 | 78.5 | 13.4 | 78 | 61 | 95 | 19 | 85.7 | 8.8 | 90.00 | 70 | 95 | 13 |
| 12 | 78.3 | 11.4 | 78 | 61 | 95 | 19 | 81.6 | 9.1 | 81.00 | 70 | 95 | 13 |
Readiness to change was assessed and participants were allocated to one of three stages: pre-contemplative, contemplative or action (Table 44). Scores for each item were given from –2 to +2, providing a score for each subscale. If the participant scored the same for two stages, then the lower one in the direction pre-contemplative–contemplative–action was used to categorise them.
| Time since baseline (months) | Treatment group | Pre-contemplative, n (%) | Contemplative, n (%) | Action, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| MI-CBT, n | TAU, n | MI-CBT | TAU | MI-CBT | TAU | MI-CBT | TAU | |
| 0 | 23a | 20 | 2 (8.7) | 4 (20.0) | 19 (82.6) | 11 (55.0) | 2 (8.7) | 5 (25.0) |
| 6 | 19 | 13 | 3 (15.8) | 4 (30.8) | 8 (42.1) | 1 (7.7) | 8 (42.1) | 7 (61.5) |
| 12 | 17 | 12 | 2 (11.8) | 4 (33.3) | 5 (29.4) | 4 (33.3) | 10 (58.8) | 4 (33.3) |
Self-rated mood state was ascertained using the Internal States Scale measure (Table 45). Note that data for one participant in whom one of the three well-being scores was missing were rated as meeting the criteria for depression (an activation score of < 155 already denoted either euthymia or depression, and the two well-being scores present were both zero, so, even if the score for the missing item was the maximum 100 on the 0–100 scale, this participant would still be able to meet cut-off points only for depression rather than euthymia, which had a cut-off point of ≥ 125). Pro-rating was not appropriate given that 33% of the data were missing for that subject’s subscale.
| Time since baseline (months) | Treatment group | (Hypo)mania, n (%) | Mixed state, n (%) | Euthymia, n (%) | Depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT, n | TAU, n | MI-CBT | TAU | MI-CBT | TAU | MI-CBT | TAU | MI-CBT | TAU | |
| 0 | 24 | 20 | 4 (16.7) | 7 (35.0) | 4 (16.7) | 2 (10.0) | 7 (29.2) | 5 (25.0) | 9 (37.5) | 6 (30.0) |
| 6 | 19 | 13 | 3 (15.8) | 5 (38.5) | 3 (15.8) | 5 (38.5) | 7 (36.8) | 1 (7.7) | 6 (31.6) | 2 (15.4) |
| 12 | 17 | 13 | 3 (17.6) | 3 (23.1) | 5 (29.4) | 4 (30.8) | 4 (23.5) | 3 (23.1) | 5 (29.4) | 3 (23.1) |
State and trait STAI scores were similar between the study arms and changed very little over the 12-month follow-up period (Table 46).
| Assessment time point (months) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT | TAU | |||||||||||
| Mean | SD | Median | Minimum | Maximum | n | Mean | SD | Median | Minimum | Maximum | n | |
| STAI-State | ||||||||||||
| 0 | 43.3 | 5.8 | 43.0 | 34 | 55.8 | 23 | 42.9 | 5.0 | 42 | 34 | 53.0 | 18 |
| 6 | 43.7 | 6.3 | 44.5 | 32 | 61.0 | 18 | 45.0 | 4.4 | 46 | 36 | 53.0 | 13 |
| 12 | 44.2 | 5.0 | 44.2 | 35 | 50.0 | 17 | 42.8 | 6.1 | 44 | 30 | 51.0 | 13 |
| STAI-Trait | ||||||||||||
| 0 | 46.5 | 3.7 | 47.0 | 41 | 54.0 | 23 | 44.9 | 4.5 | 45 | 34 | 55.8 | 20 |
| 6 | 45.3 | 5.4 | 45.0 | 37 | 57.0 | 17 | 46.0 | 5.0 | 46 | 39 | 54.0 | 13 |
| 12 | 44.1 | 4.8 | 44.0 | 34 | 55.0 | 17 | 44.9 | 6.7 | 44 | 31 | 59.0 | 13 |
The PHQ-9 measure was used to determine the level of depression, adopting cut-off points of 5 for mild depression, 10 for moderate depression, 15 for moderately severe depression and 20 for severe depression (Table 47). Each of the nine items was scored 0–3, and pro-rating was allowed for up to two items by substituting the average of the non-missing items.
| Assessment time point (months) | Treatment group | None/minimal, n (%) | Mild depression, n (%) | Moderate depression, n (%) | Moderately severe depression, n (%) | Severe depression, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT, n | TAU, n | MI-CBT | TAU | MI-CBT | TAU | MI-CBT | TAU | MI-CBT | TAU | MI-CBT | TAU | |
| 0 | 23 | 20 | 5 (21.7) | 6 (30.0) | 10 (43.5) | 6 (30.0) | 16 (37.2) | 3 (8.7) | 2 (8.7) | 3 (15.0) | 3 (13.0) | 2 (12.0) |
| 6 | 18 | 13 | 8 (44.4) | 4 (30.8) | 5 (27.8) | 2 (15.4) | 4 (22.2) | 4 (30.8) | 1 (5.6) | 1 (7.7) | 0.0 | 2 (15.4) |
| 12 | 17 | 12 | 7 (41.2) | 4 (33.3) | 1 (5.9) | 2 (16.7) | 8 (47.1) | 4 (33.3) | 1 (5.9) | 2 (16.7) | 0.0 | 0.0 |
The distribution between the trial arms and across the assessment time points for the attentional, motor, non-planning impulsivity and overall BIS totals was not significantly different (Table 48).
| Assessment time point (months) | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI-CBT | TAU | |||||||||||
| Mean | Median | SD | Minimum | Maximum | n | Mean | Median | SD | Minimum | Maximum | n | |
| BIS: attentional | ||||||||||||
| 0 | 17.34 | 17.00 | 5.14 | 9.0 | 28 | 22 | 18.44 | 17.00 | 5.43 | 8.0 | 28.0 | 19 |
| 6 | 16.50 | 16.00 | 4.40 | 10.0 | 25 | 16 | 16.62 | 16.00 | 3.95 | 10.0 | 23.0 | 13 |
| 12 | 19.11 | 18.00 | 7.34 | 8.0 | 39 | 17 | 16.87 | 17.00 | 3.68 | 12.0 | 23.0 | 11 |
| BIS: motor | ||||||||||||
| 0 | 24.96 | 25.15 | 3.52 | 19.0 | 32 | 22 | 24.60 | 23.00 | 5.89 | 16.0 | 38.0 | 19 |
| 6 | 25.25 | 24.00 | 5.05 | 14.0 | 35 | 16 | 24.72 | 24.00 | 4.90 | 16.0 | 36.0 | 13 |
| 12 | 24.58 | 26.00 | 4.33 | 15.9 | 35 | 17 | 23.59 | 22.00 | 4.75 | 17.6 | 31.9 | 11 |
| BIS: non-planning | ||||||||||||
| 0 | 28.45 | 28.00 | 6.51 | 17.6 | 42 | 22 | 28.60 | 30.00 | 5.52 | 19.0 | 39.0 | 19 |
| 6 | 27.89 | 28.50 | 6.20 | 18.0 | 39 | 16 | 27.94 | 28.60 | 4.42 | 21.0 | 34.0 | 13 |
| 12 | 27.34 | 27.50 | 5.88 | 15.0 | 40 | 17 | 28.21 | 29.00 | 5.24 | 18.3 | 36.0 | 11 |
| BIS: total | ||||||||||||
| 0 | 70.76 | 71.17 | 13.21 | 48.6 | 100 | 22 | 71.64 | 69.00 | 14.73 | 48.0 | 105.0 | 19 |
| 6 | 69.64 | 69.50 | 14.11 | 43.0 | 99 | 16 | 69.28 | 70.00 | 10.09 | 49.0 | 90.0 | 13 |
| 12 | 71.02 | 73.00 | 14.58 | 43.0 | 104 | 17 | 68.67 | 68.00 | 12.49 | 47.9 | 89.0 | 11 |
Discussion
Phase 1
A Q-Sort investigation of the reasons for, and consequences of, use of substances (cannabis or alcohol) was conducted in 50 participants with BD 1 or 2 and comorbid substance use.
The reasons for use identified focused on two factors. The first was mood management. For these participants, substance use primarily centred on its perceived impact on stress and anxiety symptoms, and to a lesser extent its ability to manage symptoms of elevated mood. In contrast, substances were not used to moderate more psychotic symptoms or to enhance hypomania. The majority of this group reported cannabis as their MPS.
The second factor, social and enhancement reasons, involved participants using substances to feel more sociable and less bored, rather than to mitigate symptoms of mood and anxiety. The majority of this group reported alcohol as their MPS.
After-effects of use focused on three factors. The first related to positive after-effects. For these participants, the primary after-effects related to a general sense of feeling better, including enhanced confidence, sociability and likeability. A reduction in anger symptoms and improvements in functioning were also reported. This group were predominantly cannabis users.
The second factor was negative after-effects. In contrast with those in the first factor, these participants reported feeling ill, anxious, guilty, depressed, scared and worthless as a result of substance use and they did not report positive social or functioning effects.
Finally, participants in the third group, ‘getting higher’, perceived positive impacts on sociability, confidence and likeability. This group overlapped with those in factor 1; however, they also endorsed the ‘buzz’ and elation after-effects of substance use as well as decreased inhibition.
The majority of participants in factors 2 and 3 used alcohol as their MPS.
The mood-related reasons for use reported in this study are consistent with previous self-report studies in BD and, indeed, in the wider population. 50,231–233 However, it is of note that these reasons were reported for a subset of mainly cannabis-using participants. This is consistent with previous anecdotal accounts of beneficial effects of cannabis use in relation to symptoms of mania and depression234 and a recent experience sampling study in which cannabis use was associated with moment-to-moment changes in positive affect. 235
The remainder focused on using substances for social and enhancement reasons, which have also been reported in general population, psychosis and bipolar samples. 51,236,237
There was a mixed pattern reported regarding the after-effects of substance use. Half of the sample reported predominantly positive effects, and a further 14% reported mixed positive and ‘getting high’ after-effects. Consistent with this, Weiss et al. 238 found that two-thirds of their groups of bipolar participants reported perceived symptom improvement associated with substance use, whereas Healy et al. 51 noted that the established use of substances for self-medication is perceived to be a valid self-management option for some individuals with BD. The majority of participants in the positive after-effects factor were primarily using cannabis, again consistent with the anecdotal and experience sampling method literature referred to above. Negative after-effects seem more linked to alcohol use, consistent with Goldstein’s et al. 199 finding that even lower levels of alcohol can be associated with worsening bipolar symptoms, although Weiss et al. 238 did not find that the quantity of substance use was associated with perceived outcomes. This mixed pattern of reasons and consequences highlights the importance of considering individualised therapeutic options for substance use in BD. Additionally, the greater focus of negative outcomes in relation to alcohol use than to cannabis use suggests that the former might present a more appropriate therapeutic target; this is also consistent with the results of Barrowclough et al. 24 that alcohol users with psychosis benefited significantly from a substance use intervention, compared with those using other substances, including cannabis.
Phase 2
Separate SU and clinician focus groups were held to aid the refinement of the proposed MI-CBT intervention for alcohol and BD. Participants’ views were solicited on therapy content, additional resources, treatment delivery and engagement.
Service user perspectives
Service users highlighted the importance of therapy being non-judgemental, collaborative and normalising. Information regarding alternatives to alcohol use was welcomed if it was offered as an option rather than instruction, and particularly if it was given in the context of other SUs’ experiences. SUs indicated that the setting and timing of sessions should be flexible and individually negotiated. Crucial elements of engagement were the provision of very clear information about the confidentiality of sessions and the disclosure of information to care teams only after negotiation with the SU (except when dictated by risk issues).
Clinician perspectives
Clinicians indicated that, to facilitate engagement, it was important to offer an approach that encompassed goals beyond substance use and that such other goals could then be linked to substance use to affect change in the latter. However, when changes in alcohol use were being proposed, it was noted that these should be explored in terms of the client’s resources and that both positive and negative changes should be evaluated constructively to maintain engagement. Alongside this, it was noted that the clinician should have sufficient experience to handle the competing demands of this potentially complex intervention, including balancing therapeutic optimism with genuine empathy for the client’s circumstances.
Clinicians felt that the proposed intervention was relatively brief for addressing both alcohol and bipolar issues, but that this could be achieved with appropriate focus on therapeutic momentum. To enhance generalisation of gains, it was felt that, when possible, links should be made with the wider care team, including sharing written therapy summaries and plans when the client agreed to this. Consistent with the SU perspective, it was noted that it would be helpful to have good-quality supporting materials, including SU accounts and information about alternative sources of support.
Focus group information from both SUs and clinicians clarified the importance of an individualised, integrated, flexible intervention delivered in a collaborative manner and embracing both alcohol use and wider goals, with the use of appropriate supporting materials.
Phase 3
The trial design appeared feasible, despite recruitment being 92% of the target, as only 10.5% of those offered the opportunity to participate refused. Of the 76 individuals screened, 58% both met the inclusion criteria and agreed to participate. This inclusion rate is almost identical to that for the AIBD trial and is elevated compared with previous CBT for relapse prevention trials. 79,159,185 It also compares well with the results of Weiss et al. ,38,213 who reported an inclusion rate of 47–59% for their integrated group therapy interventions for alcohol and BD.
The retention rate to 12 months’ follow-up was 75%, which was consistent with the retention target for the trial and did not differ significantly between the arms, suggesting that resentful demoralisation is not a significant issue for those in the TAU arm of this trial. 183 This retention rate is similar to that reported for in-person data collections by Weiss et al. 38,213 (73.8–74.2%), but lower than their total retention to follow-up when second tier data collection (95%: e-mail/questionnaire) was included from their trials. It is likely that this difference is due to our broader inclusion of individuals who did not report high motivation to change or adherence to mood-stabilising medication, which they required, and a longer follow-up period (12 months from baseline, compared with 6–8 months). Consistent with this, these retention rates compared favourably with those of the AIBD trial (phase 3; see Chapter 3 of this report) and with those of other CBT trials for BD delivering individual therapy (67–75% retention). 79,181
Consistent with the majority of RCT evaluations of psychological therapy for BD, most participants were aged > 40 years, had a chronic course of BD, and were unemployed despite most having education beyond General Certificate of Secondary Education (GCSE) level (see Chapter 3). 79,184,185 We are not aware of any previous psychological therapy trials that have focused specifically on alcohol use in BD, so there is no direct comparator with respect to severity of alcohol use. However, AUDIT scores for the current study indicated that the majority of participants fell into the category of harmful/hazardous use; balance between the arms was an issue here, with 20% more falling into this category in the MI-CBT arm than in the TAU arm at baseline.
The current trial also indicated that the delivery of the MI-CBT intervention is feasible. No participants refused the intervention and the average attendance at the sessions offered was 88%. This proportion is higher than for group therapy attendance in the Weiss et al. 38,213 trials (54–74%), despite these authors’ exclusion of participants without confirmed motivation to change, substance use and bipolar symptoms, and consistent with other trials of individual psychological therapy for BD (see Chapter 3183). Therapist ratings of formulation and benefit from therapy indicated that, although individuals were not required to have achieved motivation to change alcohol use in order to be included in the study, a shared formulation was achieved with 83% of clients, and moderate to substantial client benefits were seen in 67%. Consistent with these findings, in-depth qualitative interviews with recipients of the MI-CBT intervention indicated that the intervention was positively received, with engagement enhanced by the collaborative flexible approach fundamental to MI and reports of meaningful improvements in alcohol use, mood, self-management and functioning. Not all experiences were positive, however; one participant felt that the sessions were ‘nice’ but did not view them as formal therapy, and another felt that they over-reached themselves in therapy. This information will be important for further refinement of the intervention.
As a feasibility and acceptability study, this was not designed to formally test the efficacy of MI-CBT in changing clinical outcomes. Therefore, although information was collected on alcohol use, mood, relapse and functioning, any estimates are imprecise. No significant differences were observed between the groups in mean alcohol use or percentage of binge days; in both arms there was a pattern of general reduction. The number of days abstinent from alcohol and substances increased in both arms. The increase in alcohol abstinence was great in the TAU arm. It is of note here that the focus of the MI-CBT intervention was not specifically on increasing abstinence, as goals were behaviour change, collaborative, and typically focused on the reduction and management of alcohol rather than the eradication of its use. In terms of social functioning, as measured by PSP, both groups were functioning relatively well. The therapy participants had, on average, mild problems in one functioning area, whereas the TAU group had good functioning. This pattern remained the same across the intervention and follow-up periods.
The relapse rates were low in both arms of the trial. Depressive relapses were similar in MI-CBT and TAU (29% vs. 25%), as were mania relapses (8% vs. 10%). Overall, recurrence rate between the arms was approximately 31%, which is extremely low compared with previous individual therapy trials for BD (52–53% over 48–72 weeks79,185) and low in comparison with the 20-month follow-up data from the anxiety trial (see Chapter 3; 43% at 12 months183).
Strengths and limitations of the research
The Q-Sort study reported in phase 1 provided information on reasons for use and after-effects of both alcohol and cannabis. Although Q-Sort is a well-established technique, its validity depends on the validity and comprehensiveness of the Q-sets. Every effort was made to ensure its validity through the use of relevant and diverse data sources, but it is possible that there were some aspects of both domains that were not captured by the statements offered for rating. It is also possible that some participants found it difficult to distinguish between reasons for use and after-effects, although this distinction has been used successfully in previous research. 239
Phase 2 provided focus group data from both SUs and clinicians. Although this was important for refining the intervention, it is inherent in this approach that the rich data obtained come from a limited number of participants, in this case centred around north-west England. As such, it is not safe to assume that exactly the same issues would be raised in a different setting, although the overlap between findings here and from the focus groups for AIBD (see Chapter 3) does suggest a consistency of key elements around flexible collaborative engagement and provision of support materials. In phase 3, a feasibility and acceptability RCT was conducted; as such, it was underpowered to estimate the clinical and functional outcomes of the MI-CBT therapy with imprecise estimates of effect. The scale of the study was, however, sufficient to meet its intended purpose of evaluating the feasibility and acceptability of both the overall trial design and the intervention.
Clinical and research implications
Phase 1 highlighted the range of perspectives on substance use in participants with BD. In particular, it suggested that individuals using cannabis perceived more benefits than those using alcohol. This might suggest that the latter group could be better candidates for interventions designed to change behaviour. Phase 2 highlighted the importance of engaging individuals with alcohol problems in a constructive, flexible, collaborative and non-judgemental way, which allows for work on topics including, but not restricted to, alcohol use. The results of phase 3 indicate that an integrated intervention for individuals with alcohol and BD comorbidity is feasible and acceptable. Preliminary clinical outcome data did not indicate clear benefits from the intervention, despite very strong endorsement from the in-depth qualitative work. Further investigation is required to identify how this intervention can be adapted to enhance clinical outcomes so that efficacy matches the positive feasibility and acceptability data.
Chapter 5 Suicidal behaviour in bipolar disorder
Abstract
People with BD are at high risk of engaging in suicidal behaviours (i.e. suicide and self-harm). However, controversy remains over who is most at risk, which risk factors are specifically related to suicidal behaviour in BD rather than psychiatric illness in general, and how MHS can respond to reduce suicidal behaviours in this group. To address this, four discrete but related studies were conducted: (1) descriptive analyses of data from a large case series of people with BD who died by suicide; (2) case–control analyses of data on recent and current inpatients with BD who died by suicide; (3) case–control analyses and survival analyses of data on people with BD who self-harm; and (4) a thematic analysis of interviews with people who had direct experience of suicide and self-harm in BD. The results confirmed that the risk of suicide and self-harm is high in those with BD. Some characteristics and risk factors are shared with other psychiatric groups, but some may be unique to those with BD, such as longer duration of illness, high number of periods of inpatient care and problems establishing diagnosis. The interviews showed that rapid access to care was crucial in responding to suicidal behaviours in this group and that patients and carers needed to be made more aware of available services. Closer involvement of carers was seen as desirable, when appropriate, to help services respond faster to rapid changes in illness or behaviour and help prevent suicidal behaviours in people with BD.
Chapter overview
This chapter describes research designed to explore suicidal behaviour in people with BD. The introduction outlines what is already known about suicidal behaviour in those with BD from previous work, as well as priorities for future research. This leads to a description of the aims of the current work, which incorporates a series of discrete but related studies. For clarity, the methods and results for each study are described in turn. The discussion and clinical implications/conclusions sections then consider the results across studies and how the findings add to current knowledge about suicidal behaviour in those with BD. This section also reviews how the current findings might be useful from a clinical perspective.
Introduction
Prevalence of suicidal behaviours
People with BD are at increased risk of engaging in suicidal behaviours (attempted suicide, self-harm, or completed suicide). Suicide is one of the most important causes of premature mortality in this group,240,241 up to 60% of whom will self-harm at least once during their lifetime. 6,242 Figures for the prevalence of suicidal behaviour in those with BD vary depending on the methodology employed, but estimates generally agree that 5–20% will eventually die by suicide. 6,243,244 Estimates from more recent studies are generally lower, which may be a consequence of changes in diagnostic criteria over time, the inclusion of BD 2 and less focus on inpatient populations. 245,246 However, even the lowest estimates still exceed the figures for suicide in the general population (1.4% of all deaths worldwide in 2012247).
In terms of non-fatal self-harm, studies have found that up to 50% of people with BD report having made at least one suicide attempt. 248,249 A number of studies also suggest that rates of suicide attempt are 1.5–2.5 times higher in those with BD than in those with unipolar depression. 244,250,251
Risk factors for suicidal behaviour in bipolar disorders
Previous suicide attempt has repeatedly been identified as the most powerful predictor of future suicidal behaviour in those with BD. 54,243,252,253 Men with BD tend to be at higher risk of completed suicide, whereas women with BD tend to be at higher risk of suicide attempt. 54,254–256 Risk is also thought to be higher at younger ages, especially in the years immediately after diagnosis;6,20,257–259 thus, studies of outpatients have found that most serious suicidal behaviour happens within 10 years of diagnosis. 260
There is ongoing debate over which phase of bipolar illness confers the highest risk of suicidal behaviour, although it is rare in mania. 249,253 There is support for the view that depressive phases present the highest risk,249,256,261 but mixed states may combine low mood, irritability and the drive to act on suicidal thoughts. 262 However, in a systematic review, Hawton et al. 54 found no association between completed suicide and phase of illness.
Psychiatric comorbidities are often present in people with BD and may confer an increased risk of suicidal behaviour. 6 There is evidence that the risk of suicidal behaviour in those with BD is increased by the presence of lifetime substance use disorders,257,263–265 anxiety and panic disorders266,267 and cluster B personality disorders, which include borderline, narcissistic, histrionic and antisocial personality disorders. 248,264,268
Other potential risk factors that may increase suicidal behaviours in those with BD include having a family history of affective disorder or suicide,248,264,269 undergoing recent negative life events,124,243,248,254 being single,248 having a serious physical illness,258 having had a higher number of previous episodes and cyclothymic temperament254 and having a comorbid eating disorder. 248
Pharmacological treatment and the prevention of suicidal behaviour
Although antidepressants may be of value in the treatment of acute depressive episodes, their potential mood-destabilising effect may make them inappropriate for long-term treatment in people with BD. Over the long term, mood stabilisers are the preferred treatment. 258,270 In particular, lithium has received increased attention owing to a growing body of data that suggests that it may have an antisuicidal effect in those with BD. 53,242,253,271
Limitations of previous research
A lack of consensus remains on the prevalence of, and risk factors for, suicidal behaviour in people with BD. Methodological differences may account for some of this; for example, retrospective methods looking at antecedents of suicidal behaviour may be subject to recall or social desirability bias or be restricted by the accuracy of medical records, leading to an underestimate of suicidal behaviours. Prospective studies can be more accurate, but these are often limited by sample size and lack statistical power. Follow-up periods vary greatly, and, although longer follow-up may increase accuracy, there are many attendant difficulties, such as changes in diagnostic criteria and treatment interventions over time. Studies restricted to a single site or single patient type, such as recruiting only from among hospitalised patients, biases the results and limits the extent to which these can be generalised across the bipolar population.
Despite the limitations, there is general agreement that individuals with BD have a greatly increased risk of suicidal behaviour. Therefore, it is important that clinicians can reliably screen for characteristics that are indicative of high risk at different stages of the illness. There is a further need for clarity about how such characteristics might influence or precipitate suicidal acts, and how MHS can most effectively respond.
Aims
-
To understand the risk of suicide in people with BD in a NHS service setting.
-
To understand the demographic-, clinical- and management-related characteristics and risk factors for suicidal behaviour in people with BD.
-
To understand how these factors interact and influence suicidal behaviour in people with BD.
Methods and results
For ease of interpretation, this section will describe the methods and results used for each of the four studies included in this component of the programme, outlined below.
Study 1 used data from a large, national case series of individuals with BD who died by suicide. The overall characteristics of suicide in BD were explored and the risk of suicide in BD in the psychiatric population was examined. Demographic, treatment and clinical characteristics were then compared between people with bipolar disorder who had died by suicide, and people with other psychiatric diagnoses who had died by suicide.
Study 2 used a smaller case–control data set of current and recent inpatients to systematically compare characteristics identified in study 1 and establish which of them might be risk factors for suicide in people with BD, beyond those commonly found in psychiatric populations.
Study 3 used a cohort design to explore the characteristics of people with BD who self-harm, and the risk of repetition and subsequent suicide in those with BD compared with other people who self-harm.
Study 4 used qualitative methods to further explore factors found to be important in the previous studies, how these and other factors are experienced on an individual level, and the role of MHS in prevention of suicidal behaviour in BD. Rich, experiential data covering multiple perspectives were generated from a series of in-depth interviews with relatives of people with BD who died by suicide, and with people with BD who had self-harmed.
All statistical analyses were conducted using Stata/IC versions 11.0 to 12.1 and/or SPSS [versions 15.0 to 18.0 (SPSS Inc., Chicago, IL, USA) and version 19.0 (IBM Corporation, Armonk, NY, USA)] for Microsoft Windows® (Microsoft Corporation, Redmond, WA, USA).
Study 1: the National Confidential Inquiry (into Suicide and Homicide by People with Mental Illness) database
Clements C, Morriss R, Jones S, Peters S, Roberts C, Kapur N. Suicide in bipolar disorder in a national English sample, 1996–2009: frequency, trends and characteristics, Psychological Medicine, volume 43, issue 12, pp. 2593–602, reproduced with permission. 272 © Cambridge University Press 2013.
Method
Our analyses used data from the suicide database of the National Confidential Inquiry (into Suicide and Homicide by People with Mental Illness) (NCI). 273,274 Data collection by the NCI involves three stages. In stage 1 the NCI collects basic information on a complete national sample of suicide deaths and deaths that have received an open verdict at coroner’s inquest for all individuals aged ≥ 10 years from the Office for National Statistics (www.ons.gov.uk, accessed 10 February 2012) for England and Wales. It is convention in the UK to include open verdicts in official statistics and research studies, as the majority of these are suicide deaths,275 and excluding cases given open verdicts may result in a 50% underestimate of the incidence of suicide. 276 In stage 2 the NCI identifies people who died by suicide who were in contact with MHS in the 12 months before their death, and these become an ‘inquiry case’. In stage 3 the consultant psychiatrist responsible for the care of the patient is identified and sent a copy of the NCI suicide questionnaire to complete.
The suicide questionnaire collects information on social/demographic characteristics, clinical history, details of the suicide, details of care and final contact with services, and the respondents’ views on prevention. The NCI suicide database includes data on inquiry cases from 1996 onwards. The NCI suicide questionnaire has a response rate of > 97%277 and has been shown to be a reliable data collection tool. 278
Ethics approval
The NCI has ethics approval from South Manchester Medical Research Ethics Committee, the North West Research Ethics Committee, the National Information Governance Board for Health and Social Care and the Patient Information Advisory Group and approval under Section 60 (now Section 251) of the Mental Health and Social Care Act. 279 Individual research and development (R&D) approvals are also held for each trust where data were collected.
National Confidential Inquiry into Suicide and Homicide by People with Mental Illness psychiatric samples
Unless stated otherwise, the BD suicide sample used in the analyses included all cases from England on the NCI suicide database with a primary diagnosis of BD. This is also the case for other psychiatric samples used in the analyses (i.e. major depressive disorder, schizophrenia). Primary and secondary diagnoses are collected routinely, and primary diagnosis is determined by the responding clinician. Diagnostic categories are concordant with International Classification of Diseases, Tenth Edition (ICD-10) classifications. In reporting primary and secondary diagnoses, respondents may use a combination of case note review, consultation with other members of the mental health team and clinical judgement based on their own knowledge of the patient.
Trends
Poisson regression was used to carry out trend tests to identify changes over time in frequency and rate of suicide in BD. Tests were run for the whole BD sample, and for age and sex subgroups. Interactions between BD suicide deaths over time and general population suicide deaths over time were also examined, using data from the Office for National Statistics. Only suicide deaths occurring between 1997 and 2007 were included, as data completeness for these years is > 98%. Rates of suicide per year per 100,000 of the population were also calculated and examined for trends over time using Poisson regression.
Characteristics of suicide in bipolar disorder and comparisons with other groups
Descriptive statistics were used to illustrate the demographic, treatment and clinical characteristics of the bipolar suicides. Characteristics were compared between BD suicides and a general group of all other psychiatric suicides, and then with suicides in unipolar depressive disorder and schizophrenia. Any cases with unknown items were removed from the analysis of that item; therefore, the denominator in each analysis is the number of valid cases for each item. Pearson’s χ2 tests of association were used, and for two by two tables the continuity correction is given. Logistic regression was used for detailed between-diagnosis comparisons. ORs and associated 95% CIs are also presented. Because multiple testing was used, a p-value of 0.01 was selected to assess statistical significance. Only variables that reached statistical significance were retained in the model and reported. Age and sex were regarded as possible explanatory variables rather than confounders and so were not adjusted for in the primary analyses; however, in the secondary analyses carried out, all ORs were adjusted for age and sex. Rates of inpatient suicide were calculated per 1000 of the inpatient population for suicide deaths in BD, depression and schizophrenia. Inpatient population data by diagnosis, used as the denominator in these calculations, were obtained from Hospital Episode Statistics (www.hscic.gov.uk, accessed 26 April 2013), and included the overall number of new spells of inpatient care by year and by diagnostic group (ICD-10 codes: F30–31 and F34.1 for BD, F32–39 for depressive disorders, and F20–29 for schizophrenia and other delusion disorders).
Results
Characteristics of suicide in bipolar disorder
There were 1489 BD suicides in our sample. This represented just under 10% of all suicide deaths on the NCI database (9.6% of 15,465 valid cases). The average number of BD suicides per year was 116. There were no significant trends over time in the frequency of BD suicides [incidence rate ratio (IR) 0.99, 95% CI 0.97 to 1.00; p = 0.15], whereas general population suicides over the same time period decreased significantly (IR 0.98, 95% CI 0.98 to 0.98; p < 0.01). There was a significant downwards trend in the frequency of suicide among those aged < 24 years in the BD group (IR 0.84, 95% CI 0.77 to 0.93; p < 0.001) and a similar but non-significant trend among those aged 25–44 years in the BD group (IR 0.97, 95% CI 0.94 to 0.99; p = 0.015), but no trend was apparent in the older groups.
Table 49 shows the demographic and diagnostic characteristics of BD suicides, compared with those of other psychiatric suicides. BD suicides were older, less likely to be male and less likely to have a formal secondary diagnosis. The most common secondary diagnoses in BD cases were personality disorder and alcohol dependence. Those with BD were more likely than those with other psychiatric illnesses to have had their primary diagnosis for > 5 years. There were statistically significant differences between BD suicides and other psychiatric suicides in marital status, employment status and living arrangements, with bipolar suicides less likely to be widowed, unemployed and homeless, but the differences between the two groups in terms of proportion across categories were minimal.
| Variable (% valid responses) (N = 15,465) | Diagnosis, n (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| BD (N = 1489) | All other diagnoses (N = 13,976) | |||
| Age groups (years) (98) | ||||
| ≤ 24 | 57 (3.8) | 1106 (8.1) | 0.45 (0.35 to 0.59) | < 0.01 |
| 25–44 | 641 (43.0) | 6297 (46.0) | 0.89 (0.80 to 0.99) | |
| 45–64 | 635 (42.6) | 4574 (33.4) | 1.48 (1.33 to 1.65) | |
| ≥ 65 | 156 (10.5) | 1701 (12.4) | 0.82 (0.69 to 0.98) | |
| Male (98) | 833 (55.9) | 9113 (66.6) | 0.64 (0.57 to 0.71) | < 0.01 |
| Ethnic origin (93) | ||||
| White | 1373 (93.2) | 12,446 (93.0) | 1.03 (0.84 to 1.28) | 0.17 |
| Black African/Caribbean | 23 (1.6) | 289 (2.2) | 0.72 (0.47 to 1.10) | |
| Indian/Pakistani/Bangladeshi | 43 (2.9) | 302 (2.3) | 1.30 (0.94 to 1.80) | |
| Chinese | 2 (0.1) | 26 (0.2) | 0.70 (0.17 to 2.95) | |
| Marital status (96) | ||||
| Married or cohabiting | 469 (31.8) | 3976 (29.6) | 1.11 (0.99 to 1.24) | < 0.01 |
| Single or divorced | 935 (63.3) | 8537 (63.6) | 0.99 (0.89 to 1.11) | |
| Widowed | 72 (4.9) | 909 (6.8) | 0.71 (0.55 to 0.90) | |
| Employment status (94) | ||||
| Employed | 268 (18.3) | 2479 (18.8) | 0.97 (0.84 to 1.11) | < 0.01 |
| Unemployed | 547 (37.4) | 5481 (41.6) | 0.84 (0.75 to 0.94) | |
| Other | 649 (44.3) | 5228 (39.6) | 1.21 (1.09 to 1.35) | |
| Living arrangements (94) | ||||
| Living alone | 667 (45.4) | 5596 (42.2) | 1.09 (0.98 to 1.21) | < 0.01 |
| Living with family | 660 (44.9) | 6119 (46.2) | 0.94 (0.84 to 1.05) | |
| Homeless | 14 (1.0) | 271 (2.0) | 0.43 (0.26 to 0.71) | |
| Other | 128 (8.7) | 1269 (9.6) | 0.94 (0.70 to 1.14) | |
| Previous self-harm (96) | 938 (63.9) | 9039 (67.3) | 0.86 (0.77 to 0.97) | 0.01 |
| History of violence (95) | 314 (21.4) | 2754 (20.7) | 1.04 (0.91 to 1.19) | 0.59 |
| History of alcohol use (95) | 534 (36.3) | 5861 (43.8) | 0.73 (0.66 to 0.82) | < 0.01 |
| History of drug use (95) | 370 (25.2) | 4076 (30.6) | 0.77 (0.68 to 0.87) | < 0.01 |
| Method of suicide (96) | ||||
| Self-poisoning | 394 (26.6) | 3885 (28.6) | 0.90 (0.80 to 1.01) | < 0.01 |
| Hanging | 527 (35.6) | 4973 (36.6) | 0.95 (0.85 to 1.06) | |
| Jumping | 263 (17.8) | 1974 (14.5) | 1.26 (1.10 to 1.46) | |
| Other | 295 (19.9) | 2766 (20.3) | 0.98 (0.85 to 1.12) | |
| Duration of primary diagnosis (97) | ||||
| < 12 months | 147 (9.9) | 2887 (21.5) | 0.40 (0.34 to 0.48) | < 0.01 |
| 1–5 years | 276 (18.6) | 3793 (28.2) | 0.58 (0.51 to 0.67) | |
| > 5 years | 1057 (71.4) | 6766 (50.3) | 2.47 (2.19 to 2.78) | |
| Secondary diagnoses (96) | ||||
| BD | 00.0 | 89 (0.7) | – | < 0.01 |
| Depressive illness | 70 (4.8) | 1675 (12.4) | 0.35 (0.28 to 0.45) | |
| Anxiety/phobia/panic disorder/OCD | 44 (3.0) | 1125 (8.3) | 0.30 (0.25 to 0.46) | |
| Alcohol dependence | 116 (7.9) | 974 (7.2) | 1.11 (0.91 to 1.35) | |
| Drug dependence | 51 (3.5) | 642 (4.8) | 0.72 (0.54 to 0.97) | |
| Personality disorder | 143 (9.8) | 1174 (8.7) | 1.14 (0.95 to 1.36) | |
| Adjustment disorder | 28 (1.9) | 550 (4.1) | 0.46 (0.31 to 0.67) | |
| Alcohol misuse but not dependence | 59 (4.0) | 526 (3.9) | 1.04 (0.79 to 1.36) | |
| Drug misuse but not dependence | 31 (2.1) | 307 (2.3) | 0.93 (0.64 to 1.35) | |
| Other | 48 (3.3) | 507 (3.8) | 0.87 (0.64 to 1.17) | |
| No secondary diagnosis | 874 (59.7) | 5927 (43.9) | 1.89 (1.70 to 2.11) | |
Bipolar disorder suicides were more likely to be current or recent inpatients, to have more than five previous admissions to inpatient care, to be prescribed typical or atypical antipsychotic drugs and to be prescribed lithium or other mood stabilisers. They were less likely than other psychiatric suicides to be in contact with drug and alcohol services. These results are summarised in Table 50.
| Care and treatment characteristics (% valid responses) (N = 15,465) | Diagnosis, n (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| BD (N = 1489) | All other diagnoses (N = 13,976) | |||
| Inpatient at time of the suicide (98) | 286 (19.2) | 1835 (13.4) | 1.54 (1.34 to 1.76) | < 0.01 |
| Suicide within 3 months of discharge from inpatient care (84) | 339 (28.2) | 2772 (23.4) | 1.28 (1.12 to 1.47) | < 0.01 |
| One to five previous admissions to inpatient care (94) | 828 (57.2) | 7574 (57.6) | 0.98 (0.88 to 1.10) | 0.78 |
| More than five admissions to inpatient care (94) | 469 (32.4) | 1754 (13.3) | 3.11 (2.76 to 3.51) | < 0.01 |
| ECT (76) | 12 (1.1) | 73 (0.7) | 1.53 (0.83 to 2.83) | 0.24 |
| Seeing drug and/or alcohol services (94)a | 39 (5.9) | 701 (11.9) | 0.47 (0.33 to 0.65) | < 0.01 |
| Receiving psychological treatment (74) | 169 (15.0) | 1660 (16.0) | 0.92 (0.78 to 1.10) | 0.38 |
| Prescribed oral typical antipsychotic drugs (73) | 205 (18.3) | 12,885 (12.6) | 1.55 (1.32 to 1.83) | < 0.01 |
| Prescribed oral atypical antipsychotic drugs (73) | 435 (38.9) | 2683 (26.3) | 1.78 (1.57 to 2.03) | < 0.01 |
| Prescribed lithium or mood stabiliser (73) | 654 (58.2) | 707 (6.9) | 18.77 (16.30 to 21.62) | < 0.01 |
| Prescribed any antidepressant (71) | 704 (60.0) | 6606 (66.1) | 0.77 (0.68 to 0.87) | < 0.01 |
| Non-compliant with medication in the month before death (87) | 254 (18.2) | 2105 (17.2) | 1.07 (0.93 to 1.24) | 0.38 |
Table 51 shows the clinical characteristics at last contact before suicide. BD suicides were more likely to have been seen by MHS in the 7 days before suicide and to have evidence of depressive symptoms at last contact. The majority of cases (> 85%) in both groups were considered to be at low or no immediate risk of suicide. Adjustments for age and sex did not make any significant changes to the results.
| Clinical characteristics at last contact (% valid responses) (N = 15,465) | Diagnosis, n (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| BD (N = 1489) | All other diagnoses (N = 13,976) | |||
| Time between last contact and suicide (97) | ||||
| < 24 hours | 349 (23.7) | 2589 (19.2) | 1.30 (1.15 to 1.48) | < 0.01 |
| 1–7 days | 555 (37.6) | 3988 (29.6) | 1.43 (1.28 to 1.60) | |
| 1–4 weeks | 312 (21.2) | 3153 (23.4) | 0.88 (0.77 to 1.00) | |
| 5–13 weeks | 151 (10.2) | 1746 (13.0) | 0.77 (0.65 to 0.91) | |
| > 13 weeks | 108 (7.3) | 1984 (14.7) | 0.46 (0.37 to 0.56) | |
| Evidence of emotional distress (94) | 467 (32.3) | 4565 (34.8) | 0.90 (0.80 to 1.01) | 0.07 |
| Evidence of depressive episode (94) | 567 (39.2) | 3859 (29.5) | 1.54 (1.38 to 1.72) | < 0.01 |
| Evidence of hostility (94) | 62 (4.3) | 786 (6.0) | 0.70 (0.54 to 0.92) | 0.01 |
| Evidence of hopelessness (93) | 229 (16.0) | 1950 (15.1) | 1.07 (0.92 to 1.24) | 0.39 |
| Immediate suicide risk (92) | ||||
| No risk | 417 (29.2) | 3842 (30.0) | 0.96 (0.85 to 1.09) | 0.64 |
| Low | 813 (57.0) | 7162 (56.0) | 1.04 (0.93 to 1.16) | |
| Moderate | 173 (12.1) | 1520 (11.9) | 1.02 (0.87 to 1.21) | |
| High | 24 (1.7) | 270 (2.1) | 0.79 (0.52 to 1.21) | |
Suicide in bipolar disorder compared with suicide in unipolar depressive disorder and schizophrenia
Rates of suicide for inpatients were highest among those with unipolar depressive disorder, with an average of 2.2 inpatient suicide deaths per 1000 inpatient admissions for depression. Rates of inpatient suicide were similar between schizophrenia and BD: 1.8 and 1.7, respectively.
In the multiple regression model comparing BD and unipolar depression (Table 52), there was little difference in demographic variables, although bipolar cases were slightly less likely to be male. Variables independently associated with BD included having a history of violence, using a suicide method that involved jumping, having had the primary diagnosis for > 5 years, having a secondary diagnosis of drug dependence, being aged ≤ 24 years at onset of illness, having a high number of admissions to inpatient care, being prescribed an atypical antipsychotic medication and being prescribed lithium (although this result is likely to be an artefact of the analysis, as lithium is unlikely to be prescribed to people with unipolar depression). People with BD were less likely than those with unipolar depression to have a secondary diagnosis of anxiety or to be prescribed antidepressants.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Male | 0.67 (0.50 to 0.90) | 0.01 |
| History of self-harm | 0.47 (0.35 to 0.65) | < 0.01 |
| History of violence | 2.59 (1.75 to 3.82) | < 0.01 |
| Method of suicide: jumping | 2.02 (1.19 to 3.43) | 0.01 |
| Duration of diagnosis < 12 months | 0.48 (0.30 to 0.76) | < 0.01 |
| Duration of diagnosis > 5 years | 1.68 (1.19 to 2.37) | < 0.01 |
| Secondary diagnosis of anxiety | 0.17 (0.08 to 0.33) | < 0.01 |
| Secondary diagnosis of drug dependence | 5.05 (1.82 to 13.97) | < 0.01 |
| Other secondary diagnosisa | 0.43 (0.23 to 0.81) | 0.01 |
| Onset age ≤ 24 years | 3.22 (1.20 to 8.63) | 0.02 |
| One to five previous admissions to inpatient care | 1.62 (1.14 to 2.31) | 0.01 |
| Six or more admissions to inpatient care | 3.33 (1.92 to 5.75) | < 0.01 |
| Atypical antipsychotic | 1.96 (1.47 to 2.61) | < 0.01 |
| Lithium | 8.85 (6.45 to 12.13) | < 0.01 |
| Tricyclic antidepressant | 0.45 (0.28 to 0.74) | < 0.01 |
| Other antidepressant | 0.45 (0.28 to 0.71) | < 0.01 |
The multiple regression model comparing BD suicides with schizophrenia (Table 53) suggests that those with BD who died by suicide were more likely than those with schizophrenia to be older, employed and white, to have died by hanging, to have been prescribed lithium or another mood stabiliser, to have been prescribed antidepressants, to have experienced negative life events before the suicide, to have had evidence of depression at last contact with services and to have been assessed as at no immediate risk of suicide. People with BD who died by suicide were less likely than those with schizophrenia to be male, single or divorced, to have had a secondary diagnosis of depression and to have been prescribed antipsychotic medication.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Male | 0.37 (0.25 to 0.54) | < 0.01 |
| Aged ≥ 65 years | 3.41 (1.12 to 10.41) | 0.03 |
| Employed | 2.32 (1.38 to 3.90) | < 0.01 |
| White | 3.01 (1.51 to 6.00) | < 0.01 |
| Single or divorced | 0.30 (0.11 to 0.80) | 0.02 |
| Method of suicide: hanging | 1.74 (1.19 to 2.54) | < 0.01 |
| Secondary diagnosis of depression | 0.10 (0.05 to 0.20) | < 0.01 |
| Typical antipsychotic | 0. 22 (0.11 to 0.44) | < 0.01 |
| Atypical antipsychotic | 0. 56 (0.37 to 0.83) | < 0.01 |
| Lithium | 18.11 (11.54 to 28.42) | < 0.01 |
| SSRI antidepressant | 1.20 (1.03 to 1.39) | 0.02 |
| Other antidepressant | 2.35 (1.30 to 4.25) | 0.02 |
| Negative life events | 1.59 (1.11 to 2.28) | 0.01 |
| Evidence of depression | 4.69 (2.97 to 7.43) | < 0.01 |
| No immediate risk | 2.10 (1.16 to 3.80) | 0.01 |
Study 2: National Confidential Inquiry into Suicide and Homicide by People with Mental Illness case–control database (of current and recent inpatients)
Method
Data were collected as part of the NCI’s work, using the same methods, and under the same ethics and research governance approvals as those detailed for study 1. The database contained detailed information on a sample of patients who were admitted to inpatient care, or were within 3 months post discharge from inpatient care, between 1999 and 2006. Cases were people who died by suicide during this time and were identified via the NCI suicide database. Controls were people who were alive on a specific ‘index date’ (i.e. the date of suicide of their respective case) and were identified via data provided by Hospital Episode Statistics (www.hscic.gov.uk/hes, accessed 8 October 2014). There were no other matching criteria used. All cases and controls were aged between 16 and 65 years (older ages were excluded as older people tend to receive care from different services, e.g. Rubenowitz et al. 280), had a range of psychiatric diagnoses and demographic characteristics, and were not restricted by any other matching criteria.
The NCI suicide questionnaire was used to collect detailed information on cases, and an adapted version of the questionnaire was used to collect data on living controls. This adapted version made reference to an ‘index date’ instead of the date of suicide. Questionnaires were completed by clinicians involved in the patients’ care. These data were derived from four studies conducted by the NCI, and further details on each study and the data collection methods used can be found in Bickley et al. 281 and Hunt et al. 282–284
Bipolar case–control sample
Sixty-three cases and 89 controls had a primary diagnosis of BD. To create the case–control pairs necessary for this study, we used the bipolar suicide cases to create two case–control data sets. First, bipolar suicide cases and living bipolar controls were matched as closely as possible on date of suicide/index date, the maximum difference being 121 days in either direction. The mean difference was 25 days and > 60% were matched to within 20 days. Second, we used the remaining non-bipolar suicide cases as a control group, matching to the bipolar suicide cases on date of suicide to within 7 days in either direction, with a mean difference of 1 day. Over 60% matched to within 1 day of the bipolar case’s suicide date. In order to explore the full range of variables available, we did not impose any other matching criteria on either data set.
Analysis
Conditional logistic regression was used to compare cases and controls on key characteristics. Any cases with unknown items were removed from the analysis of that item; therefore, the denominator in each analysis is the number of valid cases for each item. ORs, 95% CIs and associated p-values were calculated. Tests were also run to adjust for age and sex. Characteristics that were significant at the p ≤ 0.05 level in the univariate analysis were entered into a multiple regression model. Multiple regression models were attempted, but, owing to the small number of cases included and high levels of multicollinearity (tested using the Stata collin command), the models were unreliable and are therefore not presented.
Results
Twenty-eight (44%) bipolar cases were male. Age ranged from 23 to 65 years, with a mean of 45 years. The most common method of suicide was jumping from a height or jumping/lying before a moving object (n = 20, 32%), followed by hanging (n = 19, 30%) and self-poisoning (n = 8, 13%). Twenty-four (38%) cases had a secondary diagnosis; personality disorder was the most common (n = 10, 42%), followed by alcohol dependence (n = 7, 29%). Forty-three (68%) bipolar cases were inpatients at the time of the suicide.
Bipolar disorder suicide cases versus bipolar disorder living controls
Table 54 shows the key results of the univariate conditional logistic regression analyses.
| Variable | Cases (N = 63), n (valid %) | Controls (N = 63), n (valid %) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Male | 28 (44.4) | 30 (47.6) | 0.87 (0.41 to 1.82) | 0.71 |
| Aged 16–25 years | 4 (6.4) | 5 (7.9) | 0.80 (0.21 to 2.98) | 0.74 |
| Aged 26–45 years | 26 (41.3) | 27 (42.9) | 0.95 (0.50 to 1.81) | 0.87 |
| Aged 46–65 years | 33 (52.4) | 31 (49.2) | 1.13 (0.57 to 2.21) | 0.73 |
| Lives with family | 17 (32.1) | 31 (49.2) | 0.45 (0.20 to 0.99) | 0.05 |
| Adverse life events | 36 (80.0) | 16 (25.4) | 29.00 (3.95 to 212.89) | < 0.01 |
| History of self-harm | 41 (77.4) | 18 (29.5) | 23.00 (3.11 to 170.31) | < 0.01 |
| History of violence | 15 (28.3) | 18 (29.0) | 1.00 (0.45 to 2.23) | 1.00 |
| History of alcohol use | 19 (35.9) | 14 (22.6) | 3.25 (1.06 to 9.97) | 0.04 |
| History of drug use | 12 (22.6) | 13 (21.0) | 1.80 (0.60 to 5.37) | 0.29 |
| History < 12 months | 4 (6.4) | 3 (4.8) | 1.50 (0.25 to 8.98) | 0.66 |
| History 1–5 years | 8 (12.7) | 10 (15.9) | 0.71 (0.23 to 2.25) | 0.57 |
| History ≥ 6 years | 51 (81.0) | 50 (79.4) | 1.14 (0.41 to 3.15) | 0.80 |
| One to five admissions to inpatient care | 28 (52.8) | 35 (55.6) | 1.08 (0.51 to 2.29) | 0.85 |
| Six or more admissions to inpatient care | 25 (47.2) | 27 (42.9) | 1.00 (0.46 to 2.16) | 1.00 |
| Secondary depression | 1 (4.2) | 0 (0.0) | – | – |
| Secondary alcohol dependence | 7 (29.2) | 1 (7.1) | – | – |
| Secondary drug dependence | 2 (8.3) | 2 (14.3) | – | – |
| Secondary personality disorder | 10 (41.7) | 2 (14.3) | 2.00 (0.18 to 22.06) | 0.57 |
| Lithium | 36 (57.1) | 43 (68.3) | 0.61 (0.29 to 1.29) | 0.20 |
| Tricyclic antidepressant | 10 (16.1) | 0 (0.0) | – | – |
| SSRI/SNRI | 28 (46.7) | 15 (23.8) | 2.63 (1.16 to 5.93) | 0.02 |
| Evidence of depressive illness | 24 (39.3) | 8 (13.1) | 3.29 (1.41 to 7.66) | 0.01 |
| Evidence of alcohol use | 2 (3.3) | 3 (4.9) | 0.67 (0.11 to 3.99) | 0.66 |
| Evidence of drugs use | 1 (1.7) | 3 (4.9) | 0.33 (0.03 to 3.20) | 0.34 |
| Evidence of self-harm | 10 (16.4) | 2 (3.3) | 5.00 (1.10 to 22.82) | 0.04 |
| Evidence of hopelessness | 7 (11.5) | 6 (9.8) | 1.17 (0.39 to 3.47) | 0.78 |
| Evidence of suicidal ideas | 7 (11.5) | 4 (6.8) | 2.00 (0.50 to 8.00) | 0.33 |
| Contact services < 24 hours before index date | 37 (58.7) | 31 (50.0) | 1.63 (0.67 to 3.92) | 0.28 |
| Contact services 1–7 days before index date | 22 (34.9) | 25 (40.3) | 0.73 (0.29 to 1.81) | 0.49 |
| No long-term suicide risk | 2 (3.3) | 15 (25.0) | 0.08 (0.01 to 0.59) | 0.01 |
| Low long-term suicide risk | 23 (38.3) | 35 (58.3) | 0.42 (0.20 to 0.87) | 0.02 |
| Moderate long-term suicide risk | 22 (36.7) | 10 (16.7) | 2.86 (1.21 to 6.76) | 0.02 |
| No immediate suicide risk | 11 (18.6) | 30 (49.2) | 0.29 (0.12 to 0.71) | 0.01 |
| Low immediate suicide risk | 40 (67.8) | 28 (45.9) | 1.91 (0.92 to 3.96) | 0.08 |
| Moderate immediate suicide risk | 5 (8.5) | 3 (4.9) | 1.67 (0.40 to 6.97) | 0.48 |
Odds ratios were wide for many variables, reflecting the relatively small sample size. Compared with living controls, cases who died by suicide were nearly 30 times more likely to have experienced recent adverse life events (such as recent unemployment, bereavement or relationship problems) (OR 29.99, 95% CI 3.95 to 212.89). Cases were over 20 times more likely to have a history of self-harm (OR 23.00, 95% CI 3.11 to 170.31), and three times more likely to have a history of alcohol use (OR 3.25, 95% CI 1.06 to 9.97). Those who died by suicide were also more likely to be prescribed a selective serotonin reuptake inhibitor or a serotonin–norepinephrine reuptake inhibitor (SNRI) antidepressant (OR 2.63, 95% CI 1.16 to 5.93), and to have evidence of depressive illness (OR 3.29, 95% CI 1.41 to 7.66) and evidence of self-harm (OR 5.00, 95% CI 1.10 to 22.82) at last contact with services. Cases were also more likely than living controls to be considered at moderate long-term, but not immediate, risk of suicide at last contact (OR 2.86, 95% CI 1.21 to 6.76). Living with family was less common in cases than in controls (OR 0.45, 95% CI 0.20 to 0.99), and controls were consistently rated as at low risk of suicide in evaluations of both immediate (OR 0.29, 95% CI 0.12 to 0.71) and long-term risk (OR 0.42, 95% CI 0.20 to 0.87). Adjustment for age and sex did not change the significance of any variables.
Bipolar suicide deaths versus non-bipolar suicide deaths
Cases with BD were more likely than those with other psychiatric diagnoses to have had their primary diagnosis for at least 6 years (OR 3.00, 95% CI 1.19 to 7.56) and were much more likely to have been prescribed lithium (OR 33.00, 95% CI 4.51 to 41.28). However, they were less likely to be male (OR 0.41, 95% CI 0.20 to 0.82), to have a history of drug use (OR 0.41, 95% CI 0.17 to 0.99) and to be assessed as at no long-term risk of suicide (OR 0.13, 95% CI 0.02 to 1.00). Adjusting for age and sex made history of drug use and assessment of no long-term risk non-significant, while the same adjustments made evidence of alcohol use at last contact significant (OR 0.17, 95% CI 0.03 to 0.97; p = 0.05). The results of this analysis are shown in Table 55.
| Variable | Cases (N = 63), n (valid %) | Controls (N = 63), n (valid %) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Male | 28 (44.4) | 44 (69.8) | 0.41 (0.20 to 0.82) | 0.01 |
| Aged ≤ 25 years | 4 (6.4) | 6 (9.5) | 0.67 (0.19 to 2.36) | 0.53 |
| Aged 26–45 years | 26 (41.3) | 36 (57.1) | 0.58 (0.30 to 1.13) | 0.11 |
| Aged 46–65 years | 33 (52.4) | 21 (33.3) | 1.86 (0.97 to 3.56) | 0.06 |
| Lives with family | 17 (32.1) | 17 (34.0) | 0.89 (0.34 to 2.30) | 0.81 |
| Adverse life events | 36 (80.0) | 21 (63.6) | 2.00 (0.50 to 8.00) | 0.33 |
| History of self-harm | 41 (77.4) | 36 (75.0) | 1.38 (0.55 to 3.42) | 0.49 |
| History of violence | 15 (28.3) | 13 (26.0) | 1.00 (0.42 to 2.40) | 1.00 |
| History of alcohol use | 19 (35.9) | 23 (47.9) | 0.57 (0.24 to 1.36) | 0.21 |
| History of drug use | 12 (22.6) | 21 (43.8) | 0.41 (0.17 to 0.99) | 0.05 |
| History < 12 months | 4 (6.4) | 10 (16.1) | 0.33 (0.09 to 1.23) | 0.10 |
| History 1–5 years | 8 (12.7) | 14 (22.6) | 0.50 (0.19 to 1.33) | 0.17 |
| History ≥ 6 years | 51 (81.0) | 38 (61.3) | 3.00 (1.19 to 7.56) | 0.02 |
| One to five admissions to inpatient care | 28 (52.8) | 35 (70.0) | 0.47 (0.20 to 1.09) | 0.08 |
| Six or more admissions to inpatient care | 25 (47.2) | 15 (30.0) | 2.13 (0.92 to 4.92) | 0.08 |
| Secondary depression | 1 (4.2) | 8 (22.9) | 0.33 (0.03 to 3.20) | 0.34 |
| Secondary alcohol dependence | 7 (29.2) | 2 (5.7) | 4.00 (0.45 to 5.79) | 0.22 |
| Secondary drug dependence | 2 (8.3) | 4 (11.4) | 0.50 (0.05 to 5.51) | 0.57 |
| Secondary personality disorder | 10 (41.7) | 8 (22.9) | 3.00 (0.61 to 4.86) | 0.18 |
| Lithium | 36 (57.1) | 3 (4.8) | 33.00 (4.51 to 41.28) | < 0.01 |
| Tricyclic | 10 (16.1) | 5 (8.1) | 2.00 (0.68 to 5.85) | 0.21 |
| SSRI/SNRI | 28 (46.7) | 32 (52.5) | 0.79 (0.36 to 1.73) | 0.55 |
| Evidence of depressive illness | 24 (39.3) | 16 (27.1) | 2.14 (0.87 to 5.26) | 0.10 |
| Evidence of hostility | 2 (3.4) | 9 (14.5) | 0.22 (0.05 to 1.03) | 0.05 |
| Evidence of alcohol use | 2 (3.3) | 9 (14.3) | 0.22 (0.05 to 1.03) | 0.05 |
| Evidence of drugs use | 1 (1.7) | 5 (8.2) | 0.20 (0.02 to 1.71) | 0.14 |
| Evidence of self-harm | 10 (16.4) | 9 (14.5) | 1.17 (0.39 to 3.47) | 0.78 |
| Evidence of hopelessness | 7 (11.5) | 8 (13.1) | 0.83 (0.25 to 2.73) | 0.76 |
| Evidence of suicidal ideas | 7 (11.5) | 6 (9.8) | 1.25 (0.34 to 4.65) | 0.74 |
| Contact < 24 hours before index date | 37 (58.7) | 27 (43.6) | 2.25 (0.98 to 5.17) | 0.06 |
| Contact 1–7 days before index date | 22 (34.9) | 23 (37.1) | 0.88 (0.43 to 1.79) | 0.72 |
| No long-term suicide risk | 2 (3.3) | 8 (13.3) | 0.13 (0.02 to 1.00) | 0.05 |
| No immediate suicide risk | 11 (18.6) | 16 (25.8) | 0.67 (0.27 to 1.63) | 0.37 |
Study 3: Manchester Self-Harm Project database
Reprinted from Journal of Affective Disorders, Vol 173, Clements C, Jones S, Moriss R, Peters S, Cooper J, While D, Kapur N, Self-harm in bipolar disorder: findings from a prospective clinical database, 113–19, Copyright (2014), with permission from Elsevier. © 2014 Elsevier B.V. Published by Elsevier Inc. All rights reserved. 285
Method
The study used data from the Manchester Self-Harm Project (MaSH), which is a collaboration between the University of Manchester, local MHS providers and the general hospitals that serve the population of the City of Manchester (further information is available at www.manchester.ac.uk/mash, accessed 21 February 2014). Established monitoring methods identify episodes of self-harm presenting to study hospitals. The MaSH and, subsequently, the current study define self-harm as intentional self-poisoning or self-injury, irrespective of motivation. 286 Data are collected on demographics and method of self-harm for all episodes, with more detailed clinical information obtained for those assessed by mental health specialists and/or by emergency department doctors (e.g. previous self-harm, drug or alcohol misuse, psychiatric treatment, mental state, risk assessment and clinical follow-up). Case ascertainment is essentially complete, and data cover all self-harm presentations between September 1997 and December 2010. Data completeness for individual variables is high. 287
The nested case–control sample
The MaSH database is suited to a nested case–control design, as it represents a well-defined source population, of known size, permitting cases and controls to be selected from a matched and relevant risk set, making optimal use of the data available for what is a relative rarely recorded event (e.g. self-harm by people with a diagnosis of BD).
Bipolar disorder cases were any individual with a primary diagnosis of BD recorded in the diagnosis field for any episode of self-harm. Individuals with conflicting diagnosis in other self-harm presentations were excluded from the bipolar group (i.e. three individuals were excluded due to a conflicting diagnosis of schizophrenia recorded in another episode of self-harm). Cases and controls were matched 1 : 5 on date of self-harm. If fewer than five controls were available for a particular date, then controls were selected from the following day, and so on, until the five control condition was satisfied, in order to optimise statistical power. 288 Power calculations indicated that five controls would give 99% power to detect a 30% difference at a 0.05 level of significance.
Analysis
Descriptive statistics summarised the demographic and clinical characteristics of cases and controls, denominator was the number of valid responses for that item, and ‘unknown’ responses were removed from each analysis. All variables were examined in a series of univariate conditional logistic regressions, controlling for age and sex (an examination of the distribution of the age variable indicated it was not normally distributed; therefore, age groups were used instead of a continuous age variable), and significant variables (at a p-value of ≤ 0.05) were entered simultaneously into a multiple conditional logistic regression model. Summary statistics, Kaplan–Meier survival curves and a Cox regression model were used to examine repetition of self-harm in the case–control sample.
Results
The study comprised 103 bipolar cases and 515 non-bipolar controls. Cases with a recorded diagnosis of BD made a total of 269 presentations over the study period, representing around 0.7% of all self-harm presentations. Nearly 60% (n = 60) of bipolar cases had at least one repeat presentation for self-harm recorded. The average period of follow-up after the initial self-harm episode was 7.7 years.
The key results of the univariate regression analyses are shown in Table 56. BD cases were more likely to be female, aged between 45 and 64 years, married or living with a partner, unemployed or registered as long-term sick and to have had sleep disturbance before the self-harm episode. They were nearly three times more likely to have had previous episodes of self-harm, and around seven times more likely to have current or previous psychiatric treatment. Self-harm was more than twice as likely to be considered a direct response to psychiatric symptoms in the bipolar group as in the control group, but less likely to be linked to either relationship problems or alcohol use.
| Variable | Cases (N = 103), n (valid %) | Controlsa (N = 515), n (valid %) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Female | 72 (69.9) | 283 (55.0) | 1.77 (1.12 to 2.81) | 0.02 |
| Aged ≤ 24 years | 21 (20.4) | 192 (37.4) | 0.42 (0.25 to 0.71) | < 0.01 |
| Aged 25–44 years | 49 (47.6) | 252 (49.0) | 0.97 (0.63 to 1.49) | 0.88 |
| Aged 45–64 years | 29 (28.2) | 59 (11.5) | 2.98 (1.76 to 5.05) | < 0.01 |
| Aged ≥ 65 years | 4 (3.9) | 11 (2.1) | 1.78 (0.51 to 6.23) | 0.36 |
| Single | 42 (44.2) | 271 (57.8) | 0.86 (0.51 to 1.44) | 0.56 |
| Married/partner | 38 (40.0) | 116 (24.7) | 1.75 (1.06 to 2.88) | 0.03 |
| Employed | 16 (16.7) | 115 (28.2) | 0.56 (0.30 to 1.01) | 0.05 |
| Unemployed | 45 (46.9) | 141 (34.6) | 1.89 (1.15 to 3.10) | 0.01 |
| Self-poisoning, drugs | 84 (81.6) | 414 (80.5) | 0.89 (0.51 to 1.56) | 0.68 |
| Self-injury | 14 (13.6) | 75 (14.6) | 1.06 (0.57 to 1.98) | 0.85 |
| Previous self-harm | 71 (74.0) | 187 (49.0) | 2.98 (1.73 to 5.15) | < 0.01 |
| Self-harm in previous 12 months | 33 (39.3) | 112 (37.0) | 1.04 (0.57 to 1.92) | 0.89 |
| Current psychiatric treatment | 75 (79.8) | 135 (34.9) | 7.11 (3.85 to 13.12) | < 0.01 |
| Previous psychiatric treatment | 78 (87.6) | 172 (44.4) | 6.89 (3.48 to 13.64) | < 0.01 |
| Current alcohol use | 14 (16.3) | 111 (29.5) | 0.46 (0.24 to 0.88) | 0.02 |
| Current substance misuse | 7 (8.1) | 49 (13.2) | 0.79 (0.32 to 1.98) | 0.62 |
| Feeling depressed | 58 (64.4) | 251 (65.5) | 1.03 (0.74 to 1.44) | 0.87 |
| Sleep disturbance | 61 (67.8) | 203 (53.1) | 1.74 (1.02 to 2.95) | 0.04 |
| Hopelessness | 32 (37.2) | 155 (40.9) | 0.64 (0.38 to 1.08) | 0.09 |
| Suicidal thoughts | 33 (37.1) | 144 (37.5) | 0.86 (0.51 to 1.44) | 0.57 |
| Suicide plans | 7 (8.0) | 70 (18.5) | 0.36 (0.15 to 0.90) | 0.03 |
| Precipitants of self-harm | ||||
| Relationship problems partner/friend | 23 (26.1) | 161 (42.8) | 0.48 (0.27 to 0.85) | 0.01 |
| Relationship problems parents/siblings | 15 (17.1) | 71 (18.9) | 0.91 (0.46 to 1.78) | 0.77 |
| Bullying/intimidation | 2 (2.3) | 20 (5.3) | 0.46 (0.10 to 2.11) | 0.32 |
| Bereavement | 8 (9.1) | 34 (9.0) | 0.81 (0.32 to 2.03) | 0.65 |
| Housing | 12 (13.6) | 36 (9.6) | 1.86 (0.85 to 4.06) | 0.12 |
| Work | 8 (9.1) | 42 (11.2) | 1.03 (0.45 to 2.35) | 0.95 |
| Legal | 2 (2.3) | 13 (3.5) | 0.81 (0.17 to 3.87) | 0.79 |
| Health problems | 5 (5.7) | 34 (9.0) | 0.71 (0.25 to 2.02) | 0.52 |
| Money | 10 (11.4) | 44 (11.7) | 1.40 (0.63 to 3.10) | 0.41 |
| Direct response to psychiatric symptoms | 31 (35.2) | 53 (14.1) | 2.32 (1.32 to 4.07) | < 0.01 |
| Abuse | 5 (5.7) | 22 (5.9) | 1.11 (0.34 to 3.66) | 0.87 |
| Owing to alcohol abuse | 1 (3.3) | 20 (19.6) | 0.18 (0.02 to 1.49) | 0.11 |
| Owing to substance abuse | 1 (3.3) | 6 (5.9) | 1.47 (0.10 to 22.09) | 0.78 |
The multiple regression model in Table 57 shows that bipolar cases were three times more likely than non-bipolar controls to be unemployed, to have previous psychiatric treatment and to have sleep disturbance. Cases were less likely to have relationship problems, current alcohol use or suicide plans.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Female | 1.83 (0.83 to 4.02) | 0.13 |
| Aged 25–44 years | 1.03 (0.38 to 2.77) | 0.96 |
| Aged 45–64 years | 0.93 (0.28 to 3.10) | 0.91 |
| Aged ≥ 65 years | 1.19 (0.11 to 2.81) | 0.89 |
| Married/partner | 2.06 (0.88 to 4.83) | 0.10 |
| Unemployed | 3.08 (1.21 to 7.85) | 0.02 |
| Long-term sick | 2.18 (0.68 to 6.96) | 0.19 |
| Relationship problems partner/friend | 0.31 (0.12 to 0.80) | 0.02 |
| Direct response to psychiatric symptoms | 0.64 (0.24 to 1.72) | 0.38 |
| Previous self-harm | 2.25 (0.93 to 5.48) | 0.07 |
| Current psychiatric treatment | 2.18 (0.77 to 6.16) | 0.14 |
| Previous psychiatric treatment | 2.96 (1.05 to 8.33) | 0.04 |
| Current alcohol use | 0.31 (0.10 to 0.96) | 0.04 |
| Sleep disturbance | 2.95 (1.29 to 6.74) | 0.01 |
| Suicide plans | 0.25 (0.07 to 0.86) | 0.03 |
The overall repetition rate (i.e. the proportion of the sample with at least one repeat episode at any time during follow-up) was 58% (n = 60) for cases and 25% (n = 129) for controls, and there was a significant association between diagnosis of BD and repeated self-harm (χ2 = 44.58; p < 0.001). Figure 27 shows time to first repeat in a Kaplan–Meier plot. A higher proportion of cases than controls had a re-presentation for self-harm during the study period (log-rank χ2 = 50.32; p < 0.001). However, there was little difference in the average time to first repeat (658 days for cases and 548 days for controls), and the difference was not significant [t(187) = –0.90; p = 0.37].
FIGURE 27.
Kaplan–Meier survival curves of time to next presentation for self-harm. Reprinted from Journal of Affective Disorders, Vol 173, Clements C, Jones S, Moriss R, Peters S, Cooper J, While D, Kapur N, Self-harm in bipolar disorder: findings from a prospective clinical database, 113–19, Copyright (2014), with permission from Elsevier. © 2014 Elsevier B.V. Published by Elsevier Inc. All rights reserved.
Diagnosis of BD was significant in the Cox regression model (HR 3.08, 95% CI 2.20 to 4.18; p < 0.01) adjusted for age and sex. A post hoc sensitivity analysis, restricting the control sample to those with a formal psychiatric diagnosis (n = 95), attenuated the HR for BD, but it remained significant (HR 2.40, 95% CI 1.56 to 3.70; p < 0.01). In the multiple regression model, the inclusion of additional explanatory variables reduced the HR for BD, but it still maintained significance at the 0.05 level in the adjusted analysis (HR 1.68, 95% CI 1.10 to 2.56; p < 0.02). Previous self-harm (HR 15.28, 95% CI 6.17 to 37.84; p < 0.01) and current psychiatric treatment were also significant predictors (HR 1.80, 95% CI 1.20 to 2.70; p < 0.01).
Mortality outcome data were available for 79% (n = 491) of the case–control sample (data linkage was carried out from 2001 onwards). Four (3.9%) of the bipolar cases and seven (1.4%) controls died by suicide during the follow-up period.
Study 4: qualitative interview study – experiences of suicide and self-harm
Recruitment
The original recruitment target was 20 participants: 10 relatives of people with BD who died by suicide and 10 people with a diagnosis of BD who had self-harmed. Recruitment into both groups took longer than originally planned owing to difficulties identifying and approaching people who met the inclusion criteria. The original method of recruitment for the self-harm group (via the MaSH database) resulted in three completed interviews, and the original recruitment method for the relatives group (via the NCI database and GP referrals) resulted in four completed interviews. This method was time-consuming and required a large number of research governance and R&D/patient identification centre approvals to cover recruitment areas for a small return in numbers of participants. To achieve the recruitment targets, the methods of recruitment were expanded. Applications for additional approvals from the National Research Ethics Service added to delays in completing the qualitative study.
Additional recruitment methods included the following.
-
Recruitment via clinical studies officers (CSOs) at Manchester Mental Health and Social Care Trust (for self-harm participants only). CSOs sought patient referrals via care co-ordinators, clinicians, and patient groups within the trust.
-
Bipolar UK advertising (for both self-harm and relatives participants). Two adverts were placed in Pendulum, the magazine produced by Bipolar UK. The first, run in 2012, focused on the relatives group of participants and was successful in attracting a number of participants; therefore, a second advert was run in 2013 asking for participants for the self-harm group.
-
Bipolar UK support groups and events. Two support groups based in the north-west were visited in person and study information was handed out. Flyers were distributed nationally via Pendulum magazine and information was also given out at the 2012 Bipolar UK annual conference.
-
PARADES referrals. Information was given out to people attending events related to other PARADES WSs, and staff passed on information to suitable potential participants where appropriate.
Recruitment was completed in August 2013, with a total of 22 participants, 11 in each group, thereby slightly exceeding the original targets. As recruitment expanded to cover all of England, it was necessary to use telephone interviews for some participants instead of the face-to-face interviews originally intended. The resulting interview data are, therefore, a mixture of both methods. The transcription of the first interviews was initially carried out by the RA on the study, and later interviews were transcribed by a professional service (1st Class Secretarial Services, Gorebridge, UK; www.1stclass.uk.com, accessed 20 January).
Thematic analysis
A thematic analysis was used to examine the transcripts. The method has been described in detail by Braun and Clarke. 91 Thematic analysis is unattached to any specific theoretical framework and, as such, offers the flexibility to explore and interpret detailed qualitative data with the goal of providing complete and useful accounts. The freedom and flexibility of thematic analysis was appropriate for these data, as the study was essentially exploratory in nature, allowing interviewees to guide the narratives towards topics that they felt were most important in relation to suicidal behaviour in people with BD.
An early review of the first six transcripts showed that experience of MHS and key issues around barriers and access to care were central in carer and SU accounts. Therefore, the analysis focused on these aspects of the narratives in the hope that it would give insight into how MHS respond to people with BD who are at high risk of suicidal behaviour, and if there might be missed opportunities for intervention and prevention. This focus is also consistent with studies 1–3 (clinician-generated data), and the most useful way to explore the data within the allotted time. These interviews are a rich and valuable source of data about a hard-to-reach population, and there is potential for further analysis at a later date.
Results
The three previous studies have shown that people with BD who die by suicide are more likely than other psychiatric suicides to have high levels of contact, and recent contact, with MHS before engaging in suicidal behaviours (study 1); that people with BD who die by suicide are also more likely than living BD controls to have previously self-harmed, and to have had their primary diagnosis for longer than other psychiatric suicides (study 2); and that people with BD who self-harm are more likely to repeat self-harm and to repeat sooner than other people who self-harm (study 3). If these results are reliable, the question that arises is why people with BD who are at high risk are not being identified as such by MHS and being managed appropriately to prevent further suicidal behaviour.
This study uses the lived experience of SUs and carers/relatives (the term ‘carer’ will be used throughout the discussion) to explore issues that are important in their experiences of MHS. Carers formed a more coherent group in terms of shared experiences, as they were all bereaved by the suicide of someone with BD. Therefore, the analysis is focused primarily on these interviews. SUs were a more diverse group, and their experiences of suicidal behaviour were variable across the gamut of suicidal behaviours (e.g. from superficial self-injury to medically serious suicide attempt). SU interviews were used as a source of supporting information to test initial themes, and any novel items were incorporated into the existing thematic structure.
The two main themes identified were:
-
experience of MHS at times of crisis
-
routes to and roadblocks in communication.
Each theme will be outlined and, for brevity, illustrated only by key quotations. Figures 28 and 29 showing possible hierarchies and relationships of subthemes are included to aid interpretation. Themes and subthemes are broadly related, and this analysis presents just one possible interpretation of the data. For each participant quotation, anonymous identifiers are used (S for service user and R for relative), along with transcript line numbers.
FIGURE 28.
Map of the theme ‘experience of MHS during crisis’.

FIGURE 29.
Map of the theme ‘routes to and roadblocks in communication’.
Experience of Mental Health Services at times of crisis
Subthemes within experience of MHS are grouped under two headings: (1) accessing MHS and (2) quality of care.
Accessing Mental Health Services
See Figure 28.
Access to effective mental health care was considered to be of paramount importance during times of crisis and when there is imminent risk of suicidal behaviour. Not only were the majority of SUs (and carers) aware of when they needed help, they actively took steps to secure it. However, help-seeking was often blocked at an early stage, whether or not they had been in contact with MHS before:
I spent 3 days in and out of A&E [accident and emergency] begging them to help me.
S2 30–31
[I]t used to make him angry, cross, you know, and he couldn’t get people to sort of listen, and to help him.
R8 255–256
Lack of knowledge of services available, and/or knowledge of how to access them, seemed to compound the situation:
We didn’t realise that we had a pass, if you like, back into the mental health system so we went back to the GP.
R6 75–76
Services and organisations outside the NHS were seen as beneficial, but they were not signposted and so SUs and carers had to find them independently:
Then we came across someone called Crisis Point, I ended up there for 2 weeks, now they were good, they made a massive, massive, massive difference to me.
S1 510–511
I said can I short circuit it by paying for it and she sort of said ‘I’m not allowed to recommend anyone’. So it was this, I’m trying to do the best for my wife and you won’t help me.
R2 713–715
Similarly, SUs and carers felt that there were many barriers to accessing MHS. GPs were often the first point of contact, and the first obstacle, in accessing care, and acted as gatekeepers:
I have to say the GPs were very much, ‘oh pull yourself together’, you know, sort of attitude.
R6 33–36
I went to the GP, my friend, my best mate came with me, but I wish she’d sat in on the consultation with me, because you wouldn’t believe what this doctor said to me, he was horrible.
S2 147–149
However, this was not universally the case:
I cannot fault my GP, because they [ . . . ] could not have dealt with it any better. He was wonderful and got us into the psychiatric hospital as soon, the same day, which was just unbelievable.
R3 501–503
Other people experienced problems when they were put in contact with MHS:
Him and an assistant [ . . . ] I was as honest as I am being with you and he just turned to her and he went, ‘do you believe a word she’s saying’, and this woman just looked at him and went, ‘no’, and I felt about that big, and I’m sort of thinking I need help. Help.
R3 501–503
In some cases there was a sense of being seen as ‘not ill enough’ to need MHS. This may be particularly relevant to people with BD, who tend to be higher functioning than some other psychiatric groups:
I have so much insight when I’m ill, people don’t realise how ill I am, and that’s why I struggle to get help, every time. Because I know where I am and what’s going on, and I know I can express what I think’s going on in my head they don’t realise that I’m ill, as ill as I am. I get to a critical desperate point where I have to scream for help because people, professionals don’t realise how ill I am. It’s my downfall really.
S2 130–134
Diagnosis, or the lack of a correct diagnosis, can also be a barrier to accessing appropriate care and, therefore, the correct treatment. One participant described a cycle of suicide attempts, followed by psychiatric assessments that failed to identify a problem and then being discharged home without help, describing it as, ‘just like a merry-go-round’ (S1 311). There is a danger that people could be prescribed inappropriate medication when BD is not recognised or is overlooked:
He didn’t bother looking at my history, he should have known that I had bipolar, he just handed me antidepressants without a mood stabiliser, so if I’d just had antidepressants on their own I would have gone manic.
S2 149–152
Quality of care
Quality of care seemed to be judged not necessarily on outcomes, but on how sympathetic and understanding clinicians were perceived to be:
I got him to our doctor who was very sympathetic, who came, we went to see him, and then he came round here.
R3 107–108
This was related to two factors: continuity of care over time and trust. Continuity of care was seen as crucial in the care of SUs with BD, particularly when there were clear signs of suicidal thoughts or behaviour:
I think is one of the most significant problems in the system really is that at that point the psychiatrist [ . . . ] said well I can’t follow her [ . . . ], and there was no mechanism for transferring anything, alerting anybody.
R5 141–144
With continuity of care comes the ability to build supportive relationships with MHS staff, which feeds into developing trust. Trust was seen as vital, and clinicians who were open and inclusive towards carers were considered more reliable and trustworthy:
Most of them they excluded me [ . . . ] there was one guy, he was one of the few people that both [the patient] and I trusted because he was quite happy to have us both in at the same time.
R2 656–659
When there was neither continuity nor trust between SUs (and carers) and services, the care was seen as superficial:
Some were ok, some were terrible, so they’d come every day and drop my medication off, some wouldn’t even come in and talk to me.
S2 219–220
I mean I can’t remember how many different people, social workers have dealt with him. It would be sort of a few months and then they were having a baby, fair enough the woman would be having a baby, but you know it doesn’t help the person you are working with, and then it was somebody else who was a trainee.
R8 398–401
A better understanding of the individual patient (unique knowledge of that patient) and their needs was valued:
I said [the patient] liked you, and he always used to say oh [GP] is great. I said, because you understood him as much as anybody understand [him], you understood him.
R1 504–506
He had a CPN [community practice nurse] [ . . . ] who was extremely good from the point of view that he would speak to me and ask where [the patient] was up to with his behaviour.
R4 183–184
The converse was also true, whereby the patient and their typical behaviour were not known and so there was concern that red flag behaviour might be overlooked:
But there was quite a lot too much, and from the medics, never mind anyone else, of people just listening to what [the patient] had to say and believing it, and taking it at face value and she was not in a mental state to be telling anyone a straight tale.
R5 525–529
There was also concern about SUs not being treated as individuals with individual needs, and being reduced to nothing but their illness:
I think they look at them as if, well they just look at the illness and not the whole person maybe that needs to come in.
R7 786–787
Routes to and roadblocks in communication
See Figure 29.
Essentially, this theme is concerned with how information flows between the SU, MHS and carers. It also reveals how information regarding the mental health care of people with BD can become unreliable, and where problems may be encountered that contribute to risk.
How much and how explicitly the SU communicated with both MHS and carers was frequently mentioned as a source of concern. The non-disclosure of important information was not only an obstacle in accessing care, but also a cause of conflict between MHS and carers, as it precipitated an imbalance of available information when contributing to care decisions.
Failure to disclose information was not always intentional. In one interview it was clear that the SU had a disorganised lifestyle, which led to missed appointments that the carer mistakenly blamed on MHS:
I used to get quite cross because, you know, he would say he hadn’t seen this person, but when I inquired into it, the more likely thing was that they had gone to his house and he wasn’t there.
R8 357–360
When this failure to disclose was intentional, there were a number of motives proposed, including protecting family members from distress:
She had attempted it maybe about four times, but she didn’t want us to know, she didn’t want to worry us.
R7 194–195
Another motive was protecting SUs’ privacy (also linked to issues of confidentiality), and there was evidence of reluctance to open up to MHS or carers:
I think [the patient] was quite a private person as far as, as wanting to involve other people, he’d rather keep, keep it to himself. I think that maybe that’s why social, social services didn’t make more of an effort, because he seemed to be holding it together.
R3 685–687
In a few cases there was an element of denial:
She was putting up this front that she was absolutely fine, and that was the really big message that was coming across, that she thought she was fine, the world was against her.
R5 422–423
The impact on care was that SUs often presented as being less ill than they perhaps were, which is particularly important if suicidal behaviour or intent is being hidden:
[H]e certainly, sort of, was able to give the, the consultant the impression that, you know, I’m alright.
R4 599–600
Many carers raised concerns with MHS that were not followed up owing to the SUs’ denial of suicidal thoughts or plans. The knock-on effect is that risk is not accurately assessed and the opportunity for intervention is missed:
I came down and found him in the chair he took an overdose and he’d promised [the psychiatrist] that he wasn’t suicidal. He said that he was alright now things were getting done.
R1 78–79
Carers saw themselves as having an important role in the care of patients with BD, as they often possessed additional information on behaviour changes or life events that could help to inform the person’s treatment and care:
[W]e were aware that he was drinking. I brought all this, about a month before [the patient] died, I brought all this to the attention of his CPN [ . . . ] and he was quite shocked by that, and the living conditions, how [the patient] was living.
R4 225–230
Carers wanted to be seen as partners in the care of their loved ones:
[A]s a family we were their biggest resource and they never sort of came to us to help. Either to help us, or to use what we were saying to help him.
R4 276–277
There was support for the idea that mental health care should be collaborative between the SU, carers and MHS. The key point was that direct involvement, and being kept informed of care decisions, was valued:
I was involved, and I think that helped.
R3 703
However, SUs did not always want their friends or family involved, for a number of reasons, and this could create conflict on multiple fronts, such as between MHS and the SU:
As I say each time over the years, what to me had happened, and also [partner] has said this, I mean he’s very angry about it all, is that a lot of them have just seen that he’s been at the side of me, they’ve signed me out and just gone ‘you deal with it,’ and it’s been landed on [partner’s] shoulders, every single time and he’s not medically trained, and why should it be put on someone like that?
S1 760–765
Between the SU and their carer(s):
I was allowed to live with my best mate whilst I recovered rather than be put in hospital. I begged my best mates to put me in hospital because I know how difficult I am when I’m ill.
S2 214–216
And between MHS and carers:
[I]t was just extremely unhelpful, and inflamed things enormously, and what I was trying to do was to give the doctor information that [the patient] wouldn’t have given her about the way she was behaving and the way she was thinking.
R5 131–134
Mental Health Services staff are bound by strict rules regarding confidentiality of patient information, and this may exacerbate conflict between MHS and carers:
They then sort of diagnosed her as, she is bipolar and we’re going to treat her as bipolar, and I didn’t know anything about this because patient confidentiality was one of the big problems, you do not get any information out of them. They will tell you what they want to tell you and they won’t give you the full picture.
R2 220–224
You just got told on many, many occasions that, you know, its patient confidentiality. But, when you’re dealing with somebody who’s suffering from mental health, and if I turn round and said to them, I said ‘if my son had cancer, you would have involved me, to care for him quite well at home’.
R4 311–314
This can cause carers to feel frustrated, as they are unable to help, even when the information they have to offer is important:
[W]hen he died, you know, the psychiatrist had actually said that he didn’t think it was planned suicide, because he didn’t think [the patient] was in that frame of mind to make that decision at that time, even though, even though he had been telling me that he wanted, you know, he didn’t want to live. He wanted to die for probably months.
R6 457–461
What is very clear from the interviews is that information needs to flow between all parties for optimal care. When the lack of information was revealed, carers were often left feeling misled and powerless and, most importantly, that they might have been able to do more if they had known more about their relative’s illness and care:
He spoke to [the patient] and then he asked me to go in on my own and asked me what did I know of [the patient’s] condition, and I knew absolutely nothing. He was quite surprised really that I didn’t.
R4 115–117
It was in her records and of course once I’d seen her records I was sort of able to say, why didn’t you tell me all this stuff and I could have said look these are panic attacks and made a far better picture of what was going on, but as it was I was relying on them to put everything together and they just didn’t.
R2 322–325
Discussion
Study 1
Summary of the characteristics of suicide in bipolar disorder
-
Ten per cent of psychiatric patients who died by suicide had a primary diagnosis of BD, a rate lower than for people with schizophrenia (18%) and people with unipolar depression (36%).
-
A higher proportion of female suicides.
-
Most common in those aged between 25 and 64 years.
-
More likely to have had their diagnosis for ≥ 5 years.
-
Common comorbidities were personality disorder and alcohol dependence.
-
More likely to have been recent/current inpatients and to have had five or more previous admissions.
-
Prescribed mood stabilisers.
-
Recent contact with MHS.
-
Assessed as at no or low immediate risk of suicide.
The results of this study are broadly consistent with previous research on factors found to be associated with completed suicide in people with BD. 248,256,257 Our data confirm that suicide in people BD is associated with a high number of previous admissions to inpatient care,248 although the reasons for admission were not available. The rate of suicide by inpatients with BD (who might be considered the most severely ill of those in contact with psychiatric services) was similar to that by inpatients with schizophrenia, both of which were lower than that for inpatients with unipolar depressive disorder.
The number of bipolar cases with substance misuse256,258,264 and/or anxiety disorder146,248 was smaller than might have been expected given the prevalence of comorbid substance and anxiety disorders typically found in BD populations. 6,146 It is possible that this was a recording bias; however, differences in diagnostic practices, in terms of clinical recognition of anxiety and/or substance use in BD in the USA and England, cannot be ruled out. However, our study was restricted to a clinical subgroup of people with BD who died by suicide, and previous work has suggested that substance/alcohol use is not strongly related to suicide in these individuals. 54
Fewer than 40% of BD cases had evidence of depressive symptoms at their last contact with services before the suicide. The lower than expected proportion of cases with depressive symptoms could be a result of the particular characteristics of our clinical sample, a reflection of the time interval between being seen and death, or an indication of a crucial under-recognition of depression among this group.
Mood stabilisers were the most commonly prescribed pharmacological treatment for those with BD; however, given the possible antisuicidal properties of lithium,242 an important finding was that > 40% of cases were not prescribed mood stabilisers. Data for this variable were not complete (73% complete) and should be interpreted cautiously. It is likely that people not prescribed mood stabilisers would be over-represented in the missing data group and, therefore, the proportion of individuals not prescribed appropriate medication might be even higher.
Compared with unipolar depressive disorder, people with BD who died by suicide were more likely to have a history of violence, to die by jumping, to have their primary diagnosis for > 5 years, to have up to five previous admissions to inpatient care, to be prescribed atypical antipsychotics, to have a comorbid diagnosis of drug dependence, to have a young age at onset and to have five or more previous admissions to inpatient care.
Compared with suicide cases with a primary diagnosis of schizophrenia, those with a primary diagnosis of BD were more likely to be employed, to die by hanging, to be prescribed antidepressants, to have negative life events before the suicide, to be assessed as at no immediate risk of suicide at last contact with services, to be aged ≥ 65 years, to be white and to have evidence of depression at last contact with MHS.
Although the pattern of significant characteristics differed depending on the comparison diagnosis, factors that seem to be specifically associated with BD suicides, taken together, could broadly be classified into three areas: (1) markers of illness severity (e.g. recent or current hospital admissions, comorbidity), (2) factors related to duration or the chronic nature of the illness (e.g. repeat admission to inpatient care, > 5 years post diagnosis) and (3) markers of underlying impulsivity (e.g. history of violence, more violent methods of suicide). 289
The majority of cases were seen within 7 days before the suicide and were considered to be at ‘low risk’ when assessed. This is consistent with the results of psychological autopsy studies (whereby detailed data are collected on the deceased from various sources, e.g. family members, treating clinicians and medical records), which show that suicidal intent is rarely communicated when the individual is seen by a health-care professional shortly before the suicidal act, although it may be communicated earlier to friends or family members. 290
Study 2
Summary of inpatient suicide by people with bipolar disorder
Bipolar disorder suicide cases versus living bipolar controls
-
The presence of depression and treatment with antidepressant medication.
-
History of previous self-harm and recent episode of self-harm.
-
Alcohol use and/or comorbid personality disorder.
-
Adverse life events.
Bipolar disorder suicide cases versus other psychiatric suicide controls
-
More likely to have had their diagnosis for ≥ 5 years.
-
More likely to be female.
-
Less likely to have a history of drug use.
These analyses go beyond the previous descriptive work on the characteristics of people with BD who die by suicide in describing the possible risk factors for suicide within this particularly high-risk recent and current inpatient group. To our knowledge, inpatient suicide in BD has been examined only once before,291 even though this group may be best placed to receive preventative interventions. It is important that clinicians can accurately assess and monitor risk of suicide, perhaps especially during the transition from inpatient to outpatient care. With little work on suicide in inpatients with BD, it is difficult to say whether or not the risk factors for this group differ from general risk factors for suicide in BD. Although our study is small scale, it provides important clinical information about how these patients might differ and how those most at risk might be identified.
The main results of this study are also generally consistent with those of previous studies on factors related to suicide in people with BD. 257,272 In particular, our study emphasises the high rate of previous self-harm found among those with BD who die by suicide. 252,253 However, not all previous research is supported; for example, hopelessness was not found to be significant in either comparison, and being male, which has previously been cited as a risk, was found to be protective in the second set of analyses. 54,256
Study 3
Summary of self-harm in people with bipolar disorder
-
More likely to repeat self-harm.
-
More likely to be female.
-
More likely aged between 45 and 64 years.
-
More likely unemployed/registered as long-term sick.
-
More likely history of previous self-harm.
-
More likely current/previous contact with MHS.
-
More likely sleep disturbance.
The results support work that has shown that women with BD have a higher rate of suicidal behaviour than men,256 and contrasts with the findings of studies that have shown a greater sex equality in self-harm. 292 The results showed that women were over-represented among bipolar cases, even in comparison with ‘others’ who self-harm – a group already known to be disproportionately female. 286 One possible explanation is that, although BD itself is unrelated to sex, women are more likely to seek help and, therefore, more likely to be in contact with services and given an appropriate diagnosis. Another explanation is that, although self-harm methods used by people with BD tend to be more potentially lethal in general,292 there is evidence that methods may be particularly dangerous in men with BD, with a higher case fatality. 293
Although not significant in the multiple regression analysis (owing to the presence of ‘previous psychiatric treatment’ in the model, which may be acting as a confounder), previous self-harm was significant in the univariate analysis, and the difference in proportions between cases and controls was large, at 74% versus 49%. This is in line with a number of previous studies on both lethal and non-lethal self-harm in BD. 54,253
It might also be of note that ‘direct response to psychiatric symptoms’ was the only significant predictor within the precipitants of self-harm variables, and it was more than twice as common in the bipolar cases. If this is considered, along with the tendency for the bipolar cases to be in current or previous contact with psychiatric services and to have previously self-harmed, it could be indicative of a group who are more seriously unwell and who might have had a longer duration of illness. There is some evidence that those with longer duration of untreated illness/incorrect diagnosis, greater illness severity and greater illness burden have a higher frequency of suicidal behaviour and poorer outcomes. 242,244 By contrast, those without BD were more likely to report relationship problems as a precipitant.
Although depression and hopelessness were present, to some extent, across all cases and controls, as might be expected,54 these were not significant predictors of repetition. Of the clinical characteristics described in this data set, only sleep disturbance seemed to be a robust independent predictor in BD253 and, indeed, was almost three times more likely to be present in bipolar cases than in non-bipolar controls.
Previous work looking at the association of drug and/or alcohol use with suicidal behaviour in BD has produced contradictory results. 292 We found that alcohol use was, in fact, recorded less frequently in cases than in controls. This is somewhat surprising given that alcohol and substance use is recognised as common in people with BD. 6 However, there is evidence that alcohol/substance use may not be strongly related to suicidal behaviour in BD,54 and it may be that illness-related factors play a more important role in this group. For example, suicidal plans were much less common in bipolar cases, which supports the hypothesis that impulsivity, a characteristic trait in bipolar patients, may be of particular importance. 294 Impulsivity, driven by positive or negative affect, in the presence of other factors recognised as associated with self-harm and suicide more generally, may be especially important in precipitating episodes of self-harm and may be a key area for future research.
The control sample contained data on 95 (18%) individuals who had a psychiatric diagnosis, including depressive illnesses (n = 43, 8%), anxiety and stress disorders (n = 20, 4%), alcohol misuse/dependence (n = 18, 3%) and personality disorder (n = 3, 0.6%). It was not our intention to compare self-harm between diagnostic groups in this study. Instead, we aimed to investigate factors that distinguish self-harm in BD from self-harm in the general population, which will include a proportion of people with psychiatric diagnoses. We believe that our study has potentially important implications for clinicians encountering a person with BD who has self-harmed. As the majority of controls (> 80%) had no recorded psychiatric diagnosis, it could be that some of the findings were not specific to those with BD but reflected wider risks for individuals with mental illness. However, a post hoc sensitivity analysis showed that the HR for repeat self-harm in those with BD compared with controls with a psychiatric diagnosis remained elevated, with a HR of 2.40 (compared with a HR of 3.08 for the whole sample).
Study 4
Summary of thematic analysis
-
Service users’ and carers’ primary concern is fast access to good-quality mental health care.
-
Help-seeking is common, but barriers, such as gate-keepers, are also common.
-
Correct diagnosis is essential to enable correct treatment, and SUs/carers should be given information about the diagnosis to help understand risks.
-
Diagnosis and treatment is facilitated by building trust and maintaining continuity of care.
-
Care should be collaborative, when possible, to minimise conflict and utilise the carer’s greater knowledge of the SU’s symptoms and circumstances.
Overall, SU and carer experiences of MHS were a mix of positive and negative; however, most participants described at least one occasion when they had had difficulty accessing care during times when the risk of suicidal behaviour was increased. This was generally the focus of the interviewees’ concerns, rather than any illness-related factors or behaviours of the SU. The importance of gaining a correct diagnosis was emphasised, as this seemed to facilitate access to care, and lack of diagnosis could contribute to a ‘merry-go-round’ of suicidal behaviour and missed opportunities to intervene. At least part of the cause of this pattern of behaviour identified by SUs was that being able to vocalise their symptoms was taken as proof of the lack of severity of those symptoms, leading to a situation in which the SU felt that they had to ‘scream’ for help.
Once the SU is in contact with MHS, the key issue, particularly for carers, is that care is as collaborative as possible – assuming that the SU gives permission for information about their care to be shared. There are multiple points in the flow of information at which details about care, treatment and SUs’ symptoms can become blocked, and vital information that could help prevent suicidal behaviour could be missed. Carers were keen to be included and viewed as collaborators in care, with many participants indicating that they were unable to pass on information to the care team when they became worried about changes in the SU’s symptoms. Keeping the same care team and building trust over time went a long way towards reducing these experiences. In general, mental health care was viewed more positively, regardless of outcome, when clinicians were seen as sympathetic and interested in involving carers.
Most of the factors identified as important to SUs and relatives in the narratives are experiences that may be more generally related to problems with mental health care, such as the importance of working collaboratively and maintaining continuity of care over time, and could apply equally to other high-risk psychiatric groups as to people with BD. However, this does not diminish the importance of these issues or their impact on the SU or carer, and it does not rule out the possibility that these problems are over-represented among people with BD. Some themes identified are likely to be more specifically related to a diagnosis of BD; for example, difficulties in establishing a diagnosis may be particularly relevant to this group, as BD is often initially misdiagnosed as unipolar depression, or symptoms are confused with cluster B personality disorders. Likewise, some of the barriers to accessing MHS raised by participants might be encountered more often by people with BD. A SU’s ability to express distress and describe their symptoms was taken to mean they were not ill enough to require access to MHS. There is some evidence that people with BD are higher functioning than those in other high-risk psychiatric groups (such as those with schizophrenia295,296), and do not differ from general population samples in terms of educational attainment, which might contribute to their presentation being misinterpreted by services. 297
Methodological limitations
Not all of those with BD who die by suicide will be in contact with services in the 12 months before death,298 and not all people who self-harm present to hospital following the act; therefore, the BD cases included in these studies may be a systematically different group from people who engage in suicidal acts but do not have contact with services. However, the quantitative studies compare BD cases only with others who also had contact with services. The differences identified are therefore reliable within those populations, and important, as these are the people who MHS are best positioned to identify and help. The qualitative work, however, was not restricted to people who were in contact with services at the time of suicide or self-harm and, therefore, the results may be more broadly generalisable.
A study from the Netherlands by ten Have et al. 299 found the most common reason for lack of contact with MHS was lower severity of BD symptoms; therefore, our sample may be biased towards the more seriously ill. It would have not been feasible to collect detailed data on all individuals with BD who had not been in contact with MHS or medical services. The lack of generalisability is particularly relevant in regard to study 2, which specifically examined the most severely ill patients. However, it is encouraging that the findings in study 2 are consistent with the results of study 1, which included outpatients, and there is support in the study for the idea that those who are more severely ill are at higher risk for suicidal behaviour.
The data used in studies 1 and 2 were collected from clinicians after the suicide had occurred. Completion of the NCI suicide questionnaire relies on the accuracy of case notes and the clinician’s knowledge of the patient. The clinicians were therefore not blinded to patient outcome when completing the questionnaire, and it is possible that this could have influenced the results, particularly those relating to care or treatment before death. In study 3 the self-harm data were collected at presentation, or very soon thereafter, and so recall bias was less of a problem, although accuracy was still dependent on clinicians.
Allocation to the BD case-group was dependent on the recording of primary diagnosis by clinicians, and it is possible that, when there were multiple comorbidities, primacy was attributed incorrectly. In study 1, there were 89 cases with a secondary diagnosis of BD, all of whom had a primary diagnosis of schizophrenia. We adopted a conservative approach and included these in the ‘other primary diagnosis’ comparison group, which might have reduced the between-group differences. A post hoc sensitivity analysis showed that the inclusion of the 89 individuals in our BD suicide group had little effect on the results overall. Comorbidity might also be a concern in the self-harm study (study 3); however, only 11 (11%) cases had a comorbid psychiatric diagnosis recorded; depressive illnesses were most common (n = 5, 5%), followed by anxiety and stress disorders (n = 3, 3%), alcohol misuse (n = 2, 2%) and learning difficulties (n = 1, 1%). Nineteen controls (20% of the controls with a psychiatric diagnosis, 4% of all controls) were also recorded as having comorbid psychiatric diagnoses.
Diagnosis in the quantitative studies was assigned by clinicians according to ICD-10 categories, rather than standard interview measures, and misdiagnosis is possible. In study 3, the diagnosis was given by the clinician at the time of the assessment and reflects the patient’s mental state at the time in addition to historical information taken from the patient and medical records. When a specific diagnosis is unclear, the field will remain empty, and any diagnosis given is, therefore, more likely to be based on clear evidence, which may favour those with more pronounced symptoms and more severe illness.
In study 3, the number of cases with a diagnosis of BD was small in comparison with the size of the overall data set (< 1% of total presentations). This suggests that psychiatric diagnoses in general may be under-reported on the MaSH database. This is in line with previous reviews, which suggest that only a small proportion of self-harm patients receive a psychiatric diagnosis when they present to hospitals. 300 This may be due to time pressures within the emergency department, the need for rapid assessment and turnover of self-harm patients, or a reluctance to assign a diagnosis on the basis of one assessment.
Study 4 took a qualitative approach, and the authors acknowledge that narratives were subjective and retrospective and might have been influenced by recall bias. Participants were self-selected volunteers, and this may have created selection bias within the sample, in that people who had very positive or very negative experiences with MHS might have been more motivated to participate. However, participants’ experiences of MHS were mixed overall, with participants reporting both good and bad aspects of care. Time constraints made it necessary to focus the analysis on the themes most pertinent to suicidal behaviour in BD, and other themes may be explored within this data set at a later time. Despite these limitations, these data represent a body of valuable information on a difficult-to-reach group (particularly in regard to the carers who had been bereaved by the suicide of someone with BD), and the lived experience of suicidal behaviour in BD, and may be important in illustrating where services might make improvements tailored towards this patient group.
Misdiagnosis could be a problem within BD cases, as it is known to be difficult to distinguish BD from diagnoses such as borderline personality disorder. 261,301 However, this is true of all studies of BD that rely on past records and clinical judgement, and verifying diagnoses is beyond the scope of this work. The effect of bias from such missed or misallocated BD would be to minimise between-group differences, and it is reassuring that the differences seen in the findings across the quantitative studies are consistent with those of previous work.
It is possible that the clinicians completing the questionnaire in studies 1 and 2 were not the professionals with whom the last contact was made and, consequently, variables relating to clinical state might have been under-reported (e.g. evidence of depressive symptoms). However, when filling in the NCI questionnaire clinicians generally use patient records in addition to their personal knowledge and recollection of the patient, and it would be unusual for significant changes in mood state not to be recorded as part of the clinical record.
There are some potential issues with missing data on the NCI and MaSH databases and, therefore, the results presented should be interpreted with caution. The majority of variables were > 90% complete on the NCI suicide database and > 75% complete on the MaSH self-harm database. Patient treatment variables had greater levels of missing data than demographics. In most cases this would have been because the clinician left some fields blank, but some might be historical artefacts due to the evolving nature of the suicide questionnaires/self-harm collection forms, and the addition of new questions over time that cannot be back-coded for older cases.
Variables entered into the multiple regression in study 1 were not screened for correlations or specified a priori. Given the exploratory nature of this work and the expectation that correlations would fall below the commonly recommended 0.8 level, including all relevant variables in the analysis was intended to give as broad a picture as possible of the key differences between suicides by different diagnostic groups.
There are a number of psychological characteristics that are of current research interest in relation to self-harm and suicide in BD, such as temperament302,303 and impulsivity,294,304 that may be important in suicidal behaviour in BD, but they were unable to be explored as there was not sufficient detail within the quantitative data and so they were not directly addressed in the qualitative work.
Clinical and research implications
Suicide prevention is a priority for MHS and it is, therefore, important to be able to identify those at risk of suicidal behaviour. 277 Knowledge of what characterises increased risk for specific diagnoses, rather than for psychiatric illness as a whole, might allow for improved identification of the most at-risk patients. The results of this study show that, although risk of suicide in BD is high compared with general population studies, it may be that some of the societal determinants of suicide have less of an impact in this clinical population, in whom illness-related factors may be more relevant. The possibility that individual clinical groups are not benefiting from an overall decline in suicide raises issues of equity, which warrant specific exploration in future studies. However, we also found that suicide is more common in unipolar depressive disorder and schizophrenia, which is surprising, and our results are likely to have underestimated the magnitude of this risk. For inpatients, among whom illness severity is commonly high, suicide rates for BD are similar to those in schizophrenia (1.7 vs. 1.8 per 1000 inpatient admissions by diagnosis).
In contrast to general population suicides in England, the number of bipolar suicides remained relatively stable over time. This could also be a consequence of the improved diagnosis of BD, and perhaps of BD 2 in particular, which might have caused an increase in BD suicides, as people who would previously have been diagnosed with unipolar depressive disorder are moved into the BD category.
Cohort studies of those with mental illness often describe the risk of suicide as highest at the time of diagnosis, with the risk subsequently diminishing over time. 6,299 We found consistently that in these studies suicides took place at least 5 years after diagnosis. In addition, those with BD were more likely than those in the comparison groups to self-harm or die by suicide in middle age. These findings suggest that risk of suicide in BD may be relatively persistent. Study 3 confirmed that risk of suicidal behaviour is not restricted to the short term following self-harm: the average time to repetition approached 2 years in this study. Sixteen per cent (n = 13) of bipolar cases who were in previous contact with psychiatric services were recorded as not in current contact with services. As risk appears to be enduring, continued contact with services may be appropriate.
Although there were similar levels of comorbid alcohol dependence in the BD group and in those with other primary diagnoses, the BD group were less likely to be in contact with alcohol services, highlighting a possible deficiency in the provision of dual diagnosis services despite a current policy focus on improving services for people with psychiatric disorder and drug or alcohol misuse. 305,306 Despite NICE guideline recommendations,1,58 over one-third of people with BD who died by suicide in this study were not prescribed lithium or another mood stabiliser, although there were some missing data for this variable. Given the increased risk of suicide that seems to accompany a diagnosis of BD, and the strong evidence for the protective effect of lithium,53,253,256 this might be considered a key treatment in long-term management.
A clinically important result from study 1 was that the majority of patients with BD were seen by MHS during the week before the suicide, and nearly one-quarter within the 24 hours immediately preceding it, providing a potential opportunity for services to intervene. However, 9 out of 10 people with BD who died by suicide were assessed as being at no or low risk at last contact. These data, along with those from the case–control study of recognised high-risk patients, suggest that clinical assessments may not be accurately identifying the BD patients who are most at risk. An alternative explanation is that patients with BD might be subject to rapid changes in mental state, consistent with the hypothesis that suicidal behaviour in BD is associated with impulsivity. 253,289 Impulsivity might also explain why BD cases in study 3 were less likely to have ‘suicidal plans’, even though the proportion of those with suicidal thoughts was similar in both groups of self-harmers. The impulsivity of suicidal behaviour in BD may make it more difficult to predict when patients are most at risk. However, good-quality monitoring and management of symptoms and improved approaches to detection of risk – including assessing levels of impulsivity, which is variable within the BD population – could help.
It is important that health professionals are able to accurately identify those patients who might be most at risk of suicide. Factors such as alcohol dependence/misuse, personality disorder, depressive episode, multiple psychiatric admissions and current or recent admission could characterise such a high-risk group. In our sample of BD suicide deaths, 75% had one or more of these factors, 34% had two or more, 8% had three or more and < 1% had four. In terms of risk management, a reduction in the emphasis placed on traditional indicators of risk, such as social circumstances and recent diagnosis, and an increase in the emphasis on diagnosis-specific factors may increase the utility of assessments. Ideally, clinically useful indicators of suicide risk that can be operationalised need to be identified in future studies. However, the identification of a high-risk group with BD on the basis of factors associated with suicide in this sample would be premature, as two-thirds of completed suicide had fewer than two of these risk factors. More work is required to determine causes of suicide in BD and to explore how risk factors interact to precipitate suicidal behaviour.
The results of the case–control analysis confirmed the importance of so-called ‘conventional’ risk factors for suicide, such as self-harm, markers of depressive illness, social isolation, life events and alcohol misuse. Our secondary analysis identified factors that may have been more specific to the BD group, such as long duration of illness, and poor recognition of risk despite recent contact with services.
Previous self-harm is a strong predictor of future suicidal behaviour, and in the self-harm study (study 3) we found that repetition of self-harm was particularly common in individuals with BD: nearly 6 out of 10 repeated during the follow-up period. People with BD who self-harmed typically had a number of high-risk characteristics (e.g. previous self-harm, history of psychiatric care). However, these characteristics, recognised as risk factors in general and psychiatric self-harm populations, did not comprehensively explain the increased risk of repetition associated with a diagnosis of BD.
Sleep disturbance was a common and independent predictor of self-harm in the bipolar group. This may be a marker of changes in illness, agitation or mood-state, as found in mixed depressive episodes and known to be associated with self-harm in BD. 262 Depressive symptoms were also evident in nearly 65% of the bipolar cases. We were not able to assess psychiatric comorbidity, severity or duration of illness in this study.
Given the frequency of previous self-harm and current/previous psychiatric care in the BD cases across studies, it is likely that those with BD who engage in suicidal behaviours could be distinguished from other disorders on markers of severity and chronicity (e.g. long duration of illness, multiple inpatient admissions, previous self-harm). The key precipitant that distinguished the BD group in the self-harm study (study 3) was ‘a direct response to psychiatric symptoms’, which indicates that illness-related factors may play a larger role in acts of suicidal behaviour in BD than any specific negative life events.
Many factors associated with suicidal behaviour in BD are known risk factors for suicide and self-harm in general and within the psychiatric population. However, those with BD seem to carry a particularly heavy burden of such risk factors – with very high levels of previous self-harm, multiple inpatient admissions, a long duration of illness, comorbid personality disorder and/or alcohol use – that goes beyond that of other groups. Identification of risk factors alone may not contribute much to clinical practice; however, in combination with results from across the other studies within the suicidality WS, this work provides new and valuable information about who might be most at risk and how services might better respond to suicidal behaviour in BD.
Clinicians need to be aware of the higher risk of self-harm in BD and the link between previous self-harm and suicide. Of course, these risks might not be restricted to those with BD, and those with other psychiatric disorders may also be at higher risk.
General preventative measures, such as good-quality assessment and interventions for people who self-harm, the treatment of depressive symptoms and prompt access to services after significant life events, are likely to help prevent suicidal behaviour in people with BD. However, more tailored approaches, perhaps focused on recognising the long-term risk that BD may confer, are also likely to be of benefit. The use of lithium, which has been shown to have specific benefits for prevention of suicide,256,307 and other mood stabilisers may be beneficial. Psychological approaches, such as CBTs, which have been shown to have a positive impact on suicidal behaviour in general, might also have an important role in prevention. 308
Chapter 6 Is the Mental Capacity Act changing clinical practice for patients with bipolar disorder?
A related paper has been published as Planning for incapacity by people with bipolar disorder under the Mental Capacity Act 2005. J Soc Welf Fam law 2015;38:263–86. 309
Other publications include Morriss et al. ,310 Mental Capacity Act 2005: Post-Legislative Scrutiny. Report of Session 2013–14311 and Advance Planning for People with Bipolar Disorder: A Guide to Making Decisions About Your Personal Welfare, Property and Financial Affairs (see Appendix 5). 312
Abstract
It is now more than a decade since the MCA formalised the law concerning advance decision-making and advance planning by people who go on to lose capacity. Such planning has been encouraged in the MHA Code of Practice since 2008 and by NICE guidelines on BD since 2006. This study used quantitative (544 people with BD and 650 psychiatrists) and qualitative methods to study the use of such planning among people with BD. Consistent with other studies, this study found that, notwithstanding the official encouragement, such planning was underused in this patient group. The paper considers reasons why this might be the case. For people using such advance planning, it looks at how Lasting Powers of Attorney (LPoAs), advance decisions to refuse treatment (ADRTs) and statements of wishes and feelings were used, what they contained and how they were viewed by psychiatrists.
Background
The MCA was passed in 2005 after a long legislative gestation and took effect in October 2007. 28,313 The MCA creates a statutory framework allowing decisions to be taken on behalf of persons lacking capacity. The statute adopts a functional test of capacity that is decision and time specific. It contains a statutory test of best interests that is a considered mixture of objective and subjective factors, and establishes a dedicated court for the administration of issues arising under the Act. Particularly relevant for present purposes, it expands and codifies the previous law related to advance planning in anticipation of incapacity.
The MCA has a wide-ranging remit from decisions concerning personal issues such as financial affairs, use of property and the care of dependants through to decisions about the provision of treatment. Essentially, when an individual lacks capacity to make a specific decision or set of decisions (for which there is a statutory test based on the presence of a disorder of mind or brain causing an inability to understand relevant information, to retain the information at least for a short time, to use or weigh the information in making a decision, and to communicate the decision), the statute requires that (subject to exceptions noted below) decisions are to be made in the ‘best interests’ of the individual (called ‘P’ in the statute, a convention which is continued in the discussion that follows). ‘Best interests’ is itself to be determined according to a statutorily defined protocol. The person assessing best interests must involve P in the decision as much as possible, and consider if and when P is likely to regain capacity. P’s present (i.e. incompetent) wishes and feelings are to be taken into account, as are P’s past wishes, feelings, values and beliefs, and particularly any wishes expressed in written form. P’s carers are to be consulted as to P’s best interests (and particularly these wishes, feelings, beliefs and values), as is any person P identifies to be consulted on these matters. This is not an exhaustive list of factors; however, the decision-maker is also to take into account ‘all the relevant circumstances’, so objective best interests are also relevant.
The MCA provides several mechanisms by which P, when competent, can influence the decision-making process.
-
ADRT: this allows P, when competent, prospectively to refuse medical treatment that occurs in a subsequent period of incapacity. This is an unusual mechanism, in that it is not based on the best interests of P. Subject to the terms of the MHA 1983,27 discussed below, a valid and applicable ADRT is enforceable, whether or not it is in P’s best interests. It is in law as if P had momentarily regained capacity at the relevant time and refused the treatment in question. The mechanism covers only refusals of treatment; treatment cannot be demanded using an ADRT.
-
Statements of wishes and feelings: P’s wishes, feelings, beliefs and values, however expressed, will be relevant to the determination of best interests, but the statute states that particular regard is to be had to ‘any relevant written statement made by him [P] when he had capacity’ [MCA, s 4(6) (a)]. Although these statements are meant to be influential, they are not determinative: the broader and multifactorial best interests test continues to apply. Statements of wishes may concern any decision (including medical treatment, social care and matters relating to property and affairs) and may be express views either for or against possible options.
-
LPoAs: these allow P to determine who will make decisions in the event that he or she loses capacity. Although the person selected (the ‘donee’) may be given guidance in the LPoA as to P’s preferences, the LPoA does not displace the statutory best interests test. It merely determines who will apply that test. This may, of course, have an impact on how the test will be interpreted and the result reached – the donee may, for example, weight different factors in the best interests test differently from other decision-makers or may have a different view of P’s values, preferences, wishes and feelings – but the decision is still to be based on the statutory best interests test. The LPoA forms are slightly different for care and treatment decisions and for property and affairs decisions, although nothing precludes P from creating both (with either different or the same donees). LPoAs for property and affairs are the successors of Enduring Powers of Attorney (EPoAs) created under legislation from 1985. Although no new EPoAs may be created, those validly created prior to the implementation of the MCA in 2007 are still effective. Many are still in circulation, and they remain part of the advance planning landscape.
Bipolar disorder is, of course, a mental disorder within the meaning of section 1 of the MHA 1983, and so the interrelations between the MCA and the MHA are, therefore, of particular relevance in the present study. These arise in a variety of ways.
First, an individual lacking capacity to make hospital admission decisions may be admitted if the admission is in P’s best interests as defined in the Act. If the conditions of the admission are sufficiently intrusive as to constitute a deprivation of liberty within the meaning of Article 5 of the European Convention for the Protection of Human Rights and Fundamental Freedoms,314 the general rule is that the admission may nonetheless occur if the provisions of Schedules A1 and 1A of the MCA are met – the Deprivation of Liberty Safeguards (or ‘DoLS’). These DoLS admissions cannot occur, however, when the proposed admission is in order to provide treatment that has been refused under an ADRT. This applies to all hospital admissions, including those for treatment for mental disorder. Psychiatric admissions, however, have an additional twist in the ‘eligibility’ criterion of the DoLS. These are ambiguous as to whether or not they permit admission in violation of a statement of wishes against the admission. Certainly, if the present (ex hypothesi incapable) wishes of P are against an admission for treatment within the scope of the MHA, the DoLS cannot be used. The degree to which past wishes expressed when competent are to be taken into account in determining the present wishes is not clear. The relevant part of the eligibility criteria is phrased in the present tense, but includes an additional clause that ‘regard is to be had to circumstances from the past only so far as it is still appropriate to have regard to them’ [MCA, sch 1A, para 5(7)]. This suggests that previous wishes are to be taken into account, but if and only if it is ‘appropriate’ to do so – a term which is not further defined or explained. In any event, whether or not the DoLS can be used, the civil detention provisions of the MHA remain available. These can be used to over-rule any of the MCA advance planning mechanisms as regards admissions when the MHA detention criteria are met.
Although the MCA applies regarding treatment of informal patients under the MHA, the compulsory treatment provisions of the MHA allow most treatments for mental disorder of detained patients, so long as the conditions of compulsion (most notably, after 3 months, the provision of a second opinion by a second opinion-appointed doctor – an independent psychiatrist assigned by the Care Quality Commission) are met. The exception is the provision of electroconvulsive therapy (ECT), where an ADRT or the refusal of a donee of an LPoA cannot be over-ruled except in cases of emergency, defined by the MHA as when the treatment is ‘immediately necessary’ to save the patient’s life or ‘to prevent a serious deterioration of his condition’ [MHA, s 62(1)]. The enhanced enforceability of ADRTs refusing ECT is relevant to people with BD, as ECT is an approved treatment for people experiencing severely depressive symptoms. 1
The advance planning mechanisms of the MCA also make some restrictions to the operation of community treatment orders under the MHA. The MHA states that treatment of a person lacking capacity under these orders may not be given in the community when such treatment conflicts with an ADRT or the refusal to consent of a donee of an LPoA [MHA, s 58A(5)(c)(i)]. The practical impact of this is, however, minimal, as in those circumstances, P can still be recalled to hospital and the treatment given there, by force if necessary, as long as this has been approved by a second opinion-appointed doctor or as long as the treatment occurs within 3 months of the detention giving rise to the community treatment order, or in the first 28 days of the order.
The MHA, therefore, provides considerable authority to over-rule advance plans made under the terms of the MCA. This was somewhat tempered by chapter 17 of the MHA Code of Practice introduced in 2008 (paragraph 17.8),315 which contained specific advice and detail to clinicians as to how to approach wishes and decisions expressed in advance when the decision involves treatment within the scope of MHA compulsion. Consistent with the MHA, the Code stopped well short of stating that such plans must be followed, but it did encourage clinicians to engage with patients in the making of advance plans and to follow such plans whenever possible. These provisions are largely repeated in the current Code of Practice. 27 Regarding ADRTs under the MCA, the current Code states:
Even where clinicians may lawfully treat a patient compulsorily under the Act, they should, where practicable, try to comply with the patient’s wishes as expressed in an advance decision. They should, for example, consider whether it is possible to use a different form of treatment not refused by the advance decision. If it is not, they should explain why to the patient.
Department of Health 2015, paragraph 9.927
Regarding statements of wishes and feelings, the Code states:
Encouraging patients to set out their wishes in advance will often be a helpful therapeutic tool, promoting collaboration and trust between patients and professionals. It is also a way in which effective use can be made of patients’ expertise in the management of crises in their own conditions.
Department of Health 2015, paragraph 19.15;27 see also Department of Health 2008, paragraph 17.14315
Although the Code is traditionally merely guidance, a provision introduced in the 2007 amendments to the MHA requires clinicians to ‘have regard’ to the Code [MHA s 118(2D)]. 27 Although the Code remains not formally legally binding, it must nonetheless be taken to have some legal authority or persuasive power. Clinicians who fail to follow the Code will need to be ready to explain why.
Advance planning such as that provided by the MCA ought to be of particular relevance to people with BD. Given that it is a recurrent condition, those affected by BD will often experience periods of good health and restored capacity, between occasions of mania or depression where the disorder manifests itself. An individual can expect approximately 10 such recurrences over the course of his or her life. 7 Unlike an individual with dementia, for example, who will have to speculate as to what he or she will want when an advanced plan is acted on, the person with BD will, after the first occurrence, have direct experience to rely on in developing such a plan. Consistent with this, NICE has, since 2006, advised clinicians to collaborate with patients in making such advance plans. 1,26
The objective of the present WS is to determine how advance planning is being used by people with BD and, insofar as possible, how the health, social care and legal systems are responding to such advance planning by people with BD.
Objectives
The main research questions for this WS were to:
-
determine if there is evidence that patients with BD are making use of the MCA
-
find out if psychiatrists were trained in the use of the MCA and if the amount and quality of such training were associated with use of the MCA in people with BD
-
determine whether or not the Act promotes joint care planning in the event of an emergency when capacity may be lost, for instance by enhancing and clarifying procedures established through early warning sign interventions or arrangements for looking after property
-
examine barriers and drivers at SU and staff levels to both the use of the Act in general, and specifically in relation to the assessment of capacity in BD and in relation to joint care planning in an emergency when capacity is lost for a period of time but then regained.
The second of these objectives was added after the grant was obtained. It was our view that in the event of the use of the MCA provisions being limited (as turned out to be the case), examining the training received by the relevant personnel might be relevant to understanding that limited use.
Method
These were to be investigated based on the following data:
-
a national postal survey of general adult and old age psychiatrists in England and Wales
-
a national postal survey of SUs with BD recruited through a SU organisation (Bipolar UK, previously MDF – the BiPolar Organisation)
-
a study of Court of Protection records
-
a survey and qualitative study of SUs taking part in component 1 of this programme that promotes the use of early warning sign interventions and the MCA together with qualitative interviews with key workers.
Aims 1 and 2 were successfully met, albeit with some modifications; for practical reasons, the surveys were conducted online and in person with a hard copy rather than through the post. The original objective of recruiting 500 participants for each survey was exceeded, with 650 psychiatrists and 544 SUs taking part. Interviews with those involved in component 1, and particularly SUs involved in the management of that component, had occurred as part of the process of designing the survey questionnaires, and it became clear at that time that richer qualitative data would be obtained through recruitment from the survey returns than from component 1. Interviewees from component 1 (like, as it turned out, the population of people with BD as a whole) had remarkably little experience with the MCA, and selecting people with the relevant experience of advance planning made recourse to the main survey necessary. The bulk of the qualitative data envisaged by item 4 was obtained from among those completing the quantitative survey and with experience of the MCA advance planning mechanisms (14 SUs and eight psychiatrists).
The study of the Court of Protection records (item 3) proved impractical. The application for this project was submitted in October 2007 as the Court of Protection was coming into being, and the available information at that time suggested that advance planning issues would come within its remit. However, it subsequently became clear that very little of the work of the Court was related to advance planning by people with BD, the subject of this study or, indeed, other cyclical disorders. In 2010, the Court issued only 218 personal welfare orders, and the indications were that the vast majority of these related to people with either dementia or a learning disability. 316 Consistent with this, there is no indication from either the qualitative or the quantitative aspects of the present research that the research participants had had any involvement with the Court of Protection, beyond registering LPoAs: a purely administrative process. Again, consistent with this, Bartlett et al. 317 have, for unrelated research, analysed the 154 decisions of the Court of Protection from January 2009 to June 2014 reported in Westlaw or British and Irish Legal Information Institute, the two primary public sources for Court of Protection decisions. Only one concerned a person with BD, and only two involved psychiatric conditions that might be expected to improve over time. The absence of this sort of case from the workload of the Court of Protection may itself raise interesting questions for consideration, but that is a research question for a different project. Because of the direction the Court took in its actual development, this aspect of the research was abandoned.
A strength of our chosen approach is that we collected a more integrated set of data than might otherwise have been expected from the original application, with initial qualitative pilot interviews giving rise to the quantitative survey, which in turn provided the basis for the further qualitative interviews. Although the methods of the individual substudies will be discussed separately, the findings will be discussed together, as the qualitative data are informed by the previous quantitative findings.
Work preparatory to surveys
Preparatory interviews for the design of the quantitative surveys were conducted in a convenience sample of SUs and service providers in the autumn of 2009. Recruitment for this aspect of the project was not carried out through the NHS and, therefore, the ethics policies then in effect did not require National Research Ethics Service approval. Instead, ethics approval was given by the Research Ethics Committee of the School of Law at the University of Nottingham (the home department of one of the two primary investigators, Bartlett).
The recruitment of the service providers for these preparatory interviews was largely through personal connections of the research team. Although a set of initial interview questions was prepared, these were open-ended to allow for developments according to answers provided by interviewees. As an example, one interviewee raised the argument that advance planning could be seen as a conflict of interest for clinicians, as the refusal of medical treatment is not something that clinicians preferred from their patients. This was then incorporated into the interview questions and the view was raised with subsequent interviewees.
Interviews were recorded and transcribed in each case. Differing opinions on particular issues were not considered to be problematic or in need of reconciliation, as these preparatory interviews were essentially fact-finding exercises designed to scope the range of opinions and awareness of advance planning in general.
The service providers interviewed were four lawyers, two psychiatrists, a GP, three social workers and a MCA lead for a major mental health trust. SUs were drawn in part from the SU advisory panel for the larger PARADES programme, and in part via an advertisement posted in Pendulum, the magazine of Bipolar UK. At this stage of the process, five SUs were interviewed. The initial findings of these interviews were discussed with a SU advisory group based at the Spectrum Centre for Mental Health Research at the University of Lancaster, as well as core staff of the broader PARADES programme.
The interviews provided a variety of useful insights, incorporated into the development of the overall surveys. They also made it clear that participants in WS 1 would not have a distinct experience of advance planning from the broader population of people with BD, and, to get an adequate number of interviewees, efforts would be better focused on surveys 1 and 2 to get a sense of how advance planning was in fact working among the community of people with BD.
To express our thanks to participants for completing the surveys, a guide relating to advance planning under the MCA specifically for people with BD was written by Dr Mudigonda and the team312 (see Appendix 5), which runs to approximately 6500 words. A revised version has been given a significantly improved design by the East Midlands Academic Health Sciences Network (EMAHSN) and is being distributed both online and at a variety of SU events at which the results of the overall PARADES programme are discussed. It is proving remarkably popular. For example, it was promoted in part in a tweet following the Better Care for People with Bipolar Disorder event in Nottingham, directing readers to the Better Care Together website. The booklet was downloaded 1893 times within 24 hours of the tweet. Four months after its launch, it had been downloaded 19,452 times and 4201 hard copies had been distributed. Bipolar UK, the national charity supporting people with BD, their friends and families, has ordered 3000 copies to be distributed in its new member packs. The content also forms the basis of a taught course in one recovery college (Nottingham) and is going to be adopted by the Social Care Institute for Excellence.
Research ethics approval for the surveys of psychiatrists and SUs (both qualitative and quantitative) was applied for in November 2010. This was initially refused at a Local Research Ethics Committee (LREC) meeting on 2 December on the basis, inter alia, that the quantitative studies were unnecessary and would add nothing to the qualitative findings, and that the only acceptable way to achieve a quantitative sample of SUs was with reference to clinical lists of people with BD. Notwithstanding these views of the LREC, it was decided that the project should not be fundamentally changed. Following some redrafting, the application was resubmitted to a different LREC, considered on 28 March 2011 and approved subject to minor amendments. These were eventually resolved and the study received final ethics approval in late May 2011. Applications for R&D approvals had commenced after provisional acceptance following the 28 March meeting, and continued into the spring of 2012 with eventually 23 sites participating. Recruitment of psychiatrists and SUs through the Mental Health Research Network (MHRN) occurred as the relevant research and development approvals were received.
Concurrently, an electronic version of the survey was posted on the PARADES website via the online survey design tool SurveyMonkey (www.surveymonkey.com, accessed 12 March 2015). Ethics approval for non-NHS recruitment had been given by the Research Ethics Committee of the School of Law at the University of Nottingham on 17 March 2011 and, once the study had been approved through the LREC process (to ensure that the same surveys could be used in both the online and paper recruitment), the surveys were posted on 6 April 2011. Recruitment for the electronic version was through professional organisations (for psychiatrists) and SU organisations (for people with BD).
As the research budget did not permit use of translation services, all recruitment for both qualitative and quantitative elements of this study was restricted to English speakers. Although the MCA covers both England and Wales, the administration of the MCA in Wales is devolved to the Welsh Assembly, and recruitment for this WS was therefore restricted to England.
Participants
Service users inclusion/exclusion criteria
For the survey:
-
age ≥ 18 years (no upper age limit)
-
confirmation that the potential participant had received a diagnosis of BD from a primary care or secondary mental health-care clinician
-
provision of written or electronically endorsed consent to the survey.
For the qualitative interview:
-
provision of personal contact details to research team
-
fulfilment of DSM-IV criteria for BD 1 or BD 276 following a semistructured diagnostic interview77
-
provision of further written informed consent to the first qualitative interview (and separate consent to the follow-up interview to receive feedback on the booklet; participants were not excluded if they did not give consent to follow-up).
Psychiatrists inclusion/exclusion criteria
-
Practising within England and Wales (i.e. the jurisdiction of the MCA).
-
Specialising in either general adult or old-age psychiatry.
-
A consultant psychiatrist or in training grades (CT1–CT3, ST4–ST6).
-
For qualitative interview only, provision of further written informed consent.
Procedure
Survey
We aimed to recruit a sample of 500 SU participants and 500 psychiatrists in a 12-month period. No data were available for a formal power calculation. Our aim was to collect a national sample using relatively few resources, so we designed a computerised survey on the University of Nottingham website using freely available software (SurveyMonkey; www.surveymonkey.com/, accessed 12 March 2015). Our pilot work suggested that many SU participants did not have access to the internet as they lived on a low income, or were not confident in using it. Therefore, we provided a written version of the survey that participants could post to us using a pre-paid stamped addressed envelope (see Appendix 6). The study was advertised with the help of the MHRN in England, a national network of community scientific officers employed by the National Institute for Health Research (NIHR) to enable participation in research adopted onto the national research portfolio. The study was also advertised with the help of Bipolar UK, the largest SU charity for BD, through its publication Pendulum, which is sent electronically to its members. The research team attended a national conference run by this organisation to further publicise the study. The Royal College of Psychiatrists agreed to tweet the link to the survey, and the study team attended a national conference organised by the Royal College of Psychiatrists to further publicise the study.
A significant number of surveys from both SU and psychiatrist groups were completed on paper, primarily by those recruited through the MHRN. In terms of trends emerging from the survey completion methods, there was a clear preference for the electronic version among the psychiatrist group, whereas the opposite was the case for the SUs. Of 650 psychiatrists, 196 completed a paper copy of the survey (30%), compared with 306 out of 544 SUs (56%). Manual entry of the data by the research team into SurveyMonkey was, therefore, essential, as it allowed for data from both electronic and hard copy surveys to be analysed together. All paper copies of the submitted surveys were then stored in a locked designated cabinet.
Qualitative interviews
In addition to enriching the data, the quantitative component of the study was essential for identifying participants for the qualitative interviews, which were conducted concurrently. Potential participants for the qualitative interviews were first identified from a question on the survey that asked whether or not they would be willing to be interviewed further for the project. Those who were interested were asked to leave their contact details in case they were selected, whereas those who did not want to participate further simply left the relevant fields blank.
With respect to the selection criteria for the qualitative interviews, care was taken to ensure that participants were from as diverse a range of backgrounds as possible and that there was a wide range of experience of using the MCA. In both groups, no definitive number of interviews was determined beforehand. Rather, interviews were to continue until data saturation had been reached.
The following criteria were also applied during the interview selection process.
-
Overall experience of advance planning under the MCA: given that overall knowledge and experience of the subject were limited, participants who either had made an advance plan themselves (SUs) or had patients with advance plans (psychiatrists) were likely to provide greater insight into the practicalities of advance planning. However, in both SU and psychiatrist groups, the numbers of willing interviewees without any experience of advance planning far outweighed the numbers of willing interviewees who did have this experience. Four out of 14 SU interviewees had made advance plans, and one out of seven psychiatrist interviewees had had patients who had done so. Despite this, interview data from those with no previous experience or knowledge of advance planning were also necessary to identify reasons behind this, for example trust infrastructure problems, lack of personal interest or need and lack of adequate information.
-
Location: to prevent biased data, interviewees were selected from varying locations, as identified by a survey question asking for the interviewee’s relevant NHS trust. In exceptional cases, two interviewees from the same trust were interviewed, but this was a result of particularly rich survey data that warranted further analysis via interview.
-
Ethnicity/cultural background: the majority of survey respondents categorised themselves as ‘white British’ and the ethnicity of interviewees largely reflected this. However, interviewees from black and minority ethnic backgrounds and those born overseas were also interviewed to allow analysis of whether or not ethnicity and culture had any bearing on approaches and attitudes towards advance planning.
-
Psychiatrists: consultants, senior and junior trainees in general adult and old-age psychiatry were selected to ensure maximum variance of practical clinical experience.
The overall objective of the interviews was to examine, in greater detail, the issues participants faced when making their advance plan, including making decisions about the content and any advice they had received. However, the research team also felt it important to further examine the reasons why participants had not made an advance plan and whether this was because of personal preference or practical difficulties. In addition, two interviews with SUs already working with the research team from the pilot phase of the project were included in the analysis, as these brought out further issues of importance.
Before an interview with a SU was conducted, the diagnosis of BD, in accordance with DSM-IV criteria,76 was confirmed using a SCID. 77 All qualitative interviews were conducted by a single interviewer (Mohan Mudigonda), digitally recorded and transcribed verbatim. Questioning was structured by the interviewer to cover the main topics according to a topic guide, developed during the pilot phase, that was co-produced with a panel of SUs as well as the research team, but it was also responsive to issues emerging from participants’ accounts. The interviewer used a combination of open questions to elicit free responses and focused questions for probing and prompting. Emerging themes were explored throughout the data collection process and specifically attended to and developed in further interviews. As part of the interview process, each participant received a copy of a specific guide to the MCA for people with BD at the end of the initial interview. A further audio-taped telephone interview was requested from each participant focusing on the form and content of the booklet. These interviews were structured in a similar way to the survey interviews but they were much shorter and focused entirely on the booklet. Again, there was scope for additional questions or deviation from the original questions, depending on the answers the interviewee provided. These interviews were also digitally recorded and transcribed.
Measures
The Mood Disorder Questionnaire
The Mood Disorder Questionnaire (MDQ)318 is a screening instrument for DSM-IV BD 1, BD 2 and BD not otherwise specified, with good sensitivity and specificity in outpatient clinics, that has been validated in a UK outpatient context. 319 Participants self-rate the presence or absence of 13 symptoms typical of mania or hypomania. If participants scored positively for seven or more of these symptoms, self-reported that several symptoms had occurred together, and that these had caused a moderate or severe problem, they were eligible for the study. SUs who did not meet these minimum criteria were excluded from further participation in the study unless they had a self-reported diagnosis of BD.
Survey for service users
The survey for SUs was split into seven sections that addressed the following topics (see Appendix 6).
-
Section A: preliminary information (age, ethnicity, relationship status, education, employment and membership of SU support groups). Sex was not included because of concerns from some ethics committees that too much specific information might enable participants to be identified without their consent.
-
Section B: advance decisions to refuse treatment or reasons for not making advance decisions to refuse treatment, during detention under the MHA. SUs were first asked whether or not they had ever made an advance decision to refuse treatment. A short description of an advance decision was provided in the survey so that it would be clearer to respondents what it involved.
-
Section C: lasting powers of attorney for personal welfare – why people made them or did not make them.
-
Section D: advance statements of wishes and feelings – why people made them or did not make them, whether or not they were in writing, who advised about them and what they contained.
-
Section E: financial planning – whether or not an EPoA/LPoA had been made and why, who had been told about it, and if/what other techniques had been used to take into account occurrences of bipolar-related symptoms.
Each section consisted of a mix of open and closed questions, and participants were not required to answer every question in the survey. For the online version, participants were automatically directed to the relevant question, whereas those who completed a hard copy version of the survey were directed to the relevant question via skip points. As an example, those who had previously made an advance plan of some description were directed to answer the remainder of questions in that section, whereas those who had not previously made an advance plan were asked to go straight to the following section and continue the survey.
Survey for psychiatrists
The survey for psychiatrists differed from the SU survey in both length and questions asked, although the topics addressed remained the same (see Appendix 4). The survey was divided into nine sections that addressed the following topics.
-
Section A: preliminary information [position, years since qualification, place of work (e.g. inpatient, crisis team) and geographic location].
-
Section B: MCA training – how many sessions attended, whether or not it was mandatory, how recently it had taken place, whether or not training considered advance planning, the nature of training and the quality of training.
-
Section C: ADRTs and BD – whether or not they had experience of patients making ADRTs, whether or not they had advised on making ADRTs, the content of ADRTs and the factors influencing a decision to advise regarding ADRTs.
-
Section D: ADRTs and other conditions – content of ADRTs.
-
Section E: ADRTs and the MHA 2007 – whether or not they had encountered ADRTs in context of patients admitted in psychiatric unit or sectioned under the MHA.
-
Section F: ADRTs in clinical practice – how often these should be discussed and how these were recorded.
-
Section G: advance statements of wishes and feelings – whether or not they had experience of patients using these, what was contained, and whether or not frequency was changing among people with BD.
-
Section H: ADRTs and implementation of the MCA – whether or not they had experience of patients using these and what was contained.
-
Section I: LPoAs – whether or not they had experience of patients making LPoAs and who advised on these.
Questions followed the same mix of formats used in the SU survey and, as before, participants from this group were not required to answer every question. Although the subject areas remained broadly the same, a shift in focus was necessary to produce relevant data and to take into account the different roles that both participant groups fulfilled within the project. Topics such as MCA training and the implementation of advance planning measures within clinical practice were not of relevance to the SU group and were therefore omitted from that version of the survey.
Data analysis
Descriptive statistics were used to summarise the survey data. Basic quantitative data analysis was first conducted via SurveyMonkey to provide percentages and respondent numbers. Univariate analysis, such as chi-squared analysis, was conducted using SPSS version 22. In essence, the responses of SUs can be split into those who had made some form of advance plan (advance decision to refuse treatment, advance statement, LPoA) and those who had not. In the survey of psychiatrists, the amount and the quality of training were explored in relation to experience and use of the MCA. When univariate analysis indicates that several demographic or service provision factors may be associated with the use of the MCA, binary logistic regression was applied following checks for collinearity applied by exploring the Spearman correlations between the independent variables that might enter the logistic regression. None of the independent variables was excluded because of collinearity.
The qualitative analysis was led by Mohan Mudigonda. A thematic analysis was used, allowing the themes to emerge out of the data without any prior assumptions. 320 Themes, categories and memos were coded into an NVivo document (QSR International, Warrington, UK), which was refined and elaborated in the light of incoming data and analysis, employing an inductive stance. In addition, each interview was separately analysed by at least one other researcher to check for reliability of coding. Findings and themes were discussed regularly by an interdisciplinary team comprising researchers with different professional backgrounds (law, psychiatry, psychology) and presented to a SU panel of people with BD, thereby increasing the trustworthiness of the analysis. 321 The analysis and the data generation took place in parallel, whereby further interviews were sought to test emerging patterns that were modified using constant comparison, ‘cycling’ between sets of data, the developing analysis and further sampling and interviews. The final data presentation is given to illustrate the themes that emerged from the analysis. In this report we have used the qualitative data to amplify findings from the surveys to reduce the length of the submission of data that are confirmatory and duplicative of survey findings.
Results
Demographic information in surveys of service users and psychiatrists
The survey was completed by 550 SU participants, but one participant was excluded because they had not been diagnosed with BD and five participants were excluded because they were not usually resident in England or Wales, leaving 544 participants included in the analysis. On the MDQ, 543 (98.9%) symptomatically met the criteria for possible BD, five (0.9%) did not and one answer was missing, but these participants were not excluded provided they reported that they had received a diagnosis of BD from a secondary care or primary care clinician because the MDQ is not a diagnostic instrument. The mania symptoms reported in the MDQ had led to moderate or serious problems in 518 (94.3%) participants, to minor problems in 24 (4.4%) participants and to no problems in 6 (1.1%) participants, and there was one (0.2%) missing answer. Again, the severity of these problems was not an exclusion criterion given that people with BD can have more substantial problems with depression than mania and they may feel that their hypomania symptoms even improved their daily function if they had BD 2. Only 53 (9.8%) participants were not currently visiting a MHP, and approximately 80% were seeing a psychiatrist. Therefore, the vast majority of participants not only were diagnosed with BD but had experienced moderate or serious problems as a result of mania symptoms and were currently receiving specialist mental health care. The age, ethnicity, marital status, education, employment status and current care arrangements of the 544 SU participants in the quantitative survey are in Table 58. SUs were asked to state the NHS trust that provided their care; if they did not know this, they were asked to provide the first three digits of their postcode so that their geographical location could be identified.
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| 18–34 | 103 (19.1) |
| 35–44 | 123 (22.8) |
| 45–54 | 166 (30.7) |
| 55–64 | 106 (19.6) |
| ≥ 65 | 38 (7.0) |
| Missing | 4 (0.7) |
| Ethnicity | |
| White British/Irish | 486 (90.0) |
| White other | 12 (2.2) |
| South Asian | 12 (2.2) |
| Black | 12 (2.2) |
| Mixed | 14 (2.6) |
| Chinese | 1 (0.2) |
| Missing | 3 (0.6) |
| Married or cohabiting | 251 (45.7) |
| Children | 318 (58.9) |
| Highest level of education | |
| No qualifications | 63 (11.7) |
| GCSE/NVQ | 152 (28.2) |
| A level/diploma | 153 (28.3) |
| Degree or higher | 162 (30.2) |
| Missing | 9 (1.7) |
| Employment status | |
| Employed/self-employed/student | 215 (39.8) |
| Sick/disability/retired | 215 (39.8) |
| Unemployed | 90 (16.7) |
| Missing | 20 (3.8) |
| Current care | |
| Psychiatrist | 434 (80.4) |
| Care co-ordinator | 139 (25.7) |
| CPN | 166 (30.9) |
| Social worker | 54 (10.0) |
| Counsellor | 38 (7.0) |
| Psychologist/psychotherapist | 44 (8.1) |
| None of the above | 53 (9.8) |
| Support groups | |
| NHS support group | 115 (21.3) |
| Non-NHS support group | 97 (18.0) |
Direct face-to-face contact with the CSOs working for the MHRN was the most frequent way in which SUs were recruited into the study (n = 209, 38.1%), followed by notification by their psychiatrist (n = 129, 23.5%). One hundred and nineteen people (21.7%) found out about the survey through means not otherwise categorised in Table 58. Most frequently, these involved electronic sources such as Twitter (Twitter, Inc., San Francisco, CA, USA) or websites (n = 26, 4.7%), or through Pendulum magazine (the magazine of Bipolar UK) or similar means (n = 30, 5.5%). The remainder were a variety of sources, such as being told by friends or letters through the post (presumably mainly from the MHRN, although this was not stated in the returns); in the case of Derby MHRN, the CSO publicised the research on BBC local radio, which led to Dr Mudigonda being interviewed and the project being further publicised on radio.
A total of 650 psychiatrists were recruited for the quantitative survey. Table 59 shows the grade, work setting, country of medical training and duration of time since medical qualification of this sample. Within the sample, there were 374 (57.5%) consultants in general adult or old-age psychiatry, and the remainder were trainees. A slight majority qualified in medicine outside the UK, and almost all of the sample were working in settings where contact with people who have BD and loss of mental capacity may be expected. Of the 650 participants, 573 indicated how they had become aware of the survey: the considerable majority (n = 436, 76.1%) were recruited either through the MHRN directly or through information cascaded through their trusts or colleagues. An additional 39 (6.8%) became aware of the survey through professional organisations, and 17 (3.0%) became aware through e-mails sent by the PARADES team. The responses of 81 participants (14.0%) were unclear as to how they became aware of the study; most frequently it was noted that they had found out about the survey ‘by e-mail’, without indicating the source of the e-mail. Respondents were drawn from across England, albeit with some over-representation from the Midlands and marked under-representation from the north-west. Of 607 respondents who identified the geographic location of their work, 133 (21.9%) were from the West Midlands, 116 (19.1%) were from the East Midlands, 80 (13.2%) were from the south-west, 116 (19.1%) were from the south-east, 74 (12.2%) were from the east, 46 (7.6%) were from London and 10 (1.6%) were from the north-west.
| Work characteristic | n (%) |
|---|---|
| Grade | |
| Consultant general adult psychiatry | 283 (43.5) |
| Consultant old-age psychiatry | 91 (14.0) |
| ST4–6 trainees | 111 (17.1) |
| CT1–3 trainees | 130 (20.0) |
| Missing | 35 (5.4) |
| Main work setting | |
| Community mental health team | 349 (53.7) |
| Inpatient | 216 (33.3) |
| Crisis team/EIP/ACT | 77 (11.9) |
| Missing | 8 (1.2) |
| Years since medical qualification | |
| 0–10 | 210 (32.3) |
| 11–20 | 241 (37.1) |
| 21–30 | 146 (22.5) |
| ≥ 30 | 51 (7.8) |
| Missing | 2 (0.3) |
| Country of medical qualification | |
| UK | 306 (47.1) |
| European Union | 51 (7.8) |
| Outside European Union | 288 (44.3) |
| Missing | 5 (0.8) |
Training of psychiatrists in the use of the Mental Capacity Act 2005
Table 60 shows the number of training sessions, methods used for training, source of the training, quality of training and reasons for attending the training: 595 (91.5%) had attended at least one training session on the MCA, 465 (71.5%) had attended two or more sessions and 326 (50.1%) had been to a training session in the previous year. Of the 595 psychiatrists trained in the MCA, 487 (81.8%) had been trained by their local NHS trust. The quality of the training was perceived to be high, with 446 (75.0%) rating it as good, very good or excellent (see Table 60). Insofar as there are deficiencies in the implementation of the MCA in general, therefore, they cannot simply be placed at the door of perceived inadequate training, at least among psychiatrists.
| Characteristic | n (%) |
|---|---|
| Number of training sessions | |
| 0 | 55 (8.5) |
| 1 | 128 (19.7) |
| 2 | 183 (28.2) |
| 3 | 113 (17.4) |
| > 3 | 169 (26.0) |
| Trained but missing data | 2 (0.3) |
| Method of training | |
| Case examples | 491 (75.5) |
| Role play | 82 (12.6) |
| Watch video | 44 (6.8) |
| None of these | 86 (13.2) |
| Source of training | |
| Local NHS trust | 489 (75.2) |
| Royal College of Psychiatrists | 133 (20.5) |
| Legal or solicitor | 48 (7.4) |
| Pharmaceutical company | 35 (5.4) |
| Other | 89 (13.7) |
| Perceived quality of training | |
| Excellent | 24 (4.0) |
| Very good | 153 (25.7) |
| Good | 269 (45.2) |
| Average | 134 (22.5) |
| Below average | 12 (2.0) |
| Missing | 3 (0.5) |
| Primary reason for attending | |
| Mandatory NHS trust training | 172 (28.9) |
| Approved clinician training | 194 (32.6) |
| Educational event | 128 (71.5) |
| Personal interest | 79 (13.3) |
| Other | 22 (3.7) |
Of perhaps greater concern than the current quality of MCA training, > 60% of the sample attended the training because it was mandatory (either directly or as part of section 12 approval training under the 1983 MHA27), and an additional 21.5% attended as part of educational events, which are also often effectively mandatory as part of continuing professional education requirements. Only 13.3% stated that they attended out of personal interest. Furthermore, and of particular relevance to this study, 209 (35.1% receiving training) psychiatrists stated that either minimal or no attention was paid to matters of advance planning in the training sessions. That said, when asked to identify which profession was best informed of the advance planning provisions of the MCA, the psychiatrists identified themselves by a considerable margin [psychiatrists, n = 363 (55.8%); social workers, n = 222 (34.2%); GPs, n = 28 (4.3%); other physicians, n = 20 (3.0%); and nurses, n = 18 (2.7%)]. Again, there may be issues about the adequacy of training, but it would appear that difficulties of implementation should not be attributed to deficiencies in training in a simplistic fashion: it would seem that the picture is much more complex.
The receipt of training in the MCA (overall, 595 or 91.5% psychiatrists) was associated with greater experience since medical qualification [≥ 11 years since qualification, n = 420 (95.9%), vs. up to 10 years since qualification, n = 172 (82.4%); OR 5.00, 95% CI 2.77 to 9.01; p < 0.001]. Furthermore, the number of MCA training sessions received was also associated with greater experience since medical qualification {receipt of two or more MCA training sessions: ≥ 11 years since qualification [n = 346 (82.3%) vs. up to 10 years since qualification, n = 117 (67.6%); OR 2.28, 95% CI 1.51 to 3.44; p < 0.001], qualification outside the UK [n = 258 (82.2%) vs. in the UK, n = 207 (73.7%); OR 1.63, 95% CI 1.09 to 2.44; p = 0.018]}, and the perceived quality of training received good or better [n = 363 (81.4%) vs. average or worse, n = 101 (69.2%); OR 1.95, 95% CI 1.26 to 3.01; p = 0.013]. The perception of the quality of training was not related to years of experience or qualification outside the UK. Table 61 examines the binary multiple logistic regression associations between the quality of training and the methods of training, the site of training, the number of training sessions and the topics covered in the training. Compared with average or poor training, good or excellent training was associated positively with the use of case summaries, video feedback (44 or 9.9% good or better quality of training vs. average or worse training; Fisher’s exact two-tailed test p < 0.001), role play, coverage of ADRTs and assessment of capacity, and negatively with training in their own NHS trust. In relation to the specific use of ADRTs and the need to be able to assess fluctuating capacity in conditions such as BD with highly variable severity and nature of mood and, therefore, capacity, it is notable that even good- or better-quality training covered these issues in only just over 45% and 37% of cases, respectively. Therefore, the majority of better-quality MCA training for psychiatrists is not fit for the purpose of applying the MCA to people with BD.
| Training characteristic | Quality of training, n (%) | Statistics | ||
|---|---|---|---|---|
| Good or better (N = 444) | Average or worse (N = 144) | OR (95% CI) | p-value | |
| Used role play | 76 (17.1) | 6 (4.1) | 3.32 (1.37 to 8.07) | 0.008 |
| Training in ADRT | 203 (45.6) | 26 (17.8) | 2.58 (1.54 to 4.31) | < 0.001 |
| Capacity assessment | 410 (92.3) | 107 (74.3) | 2.80 (1.56 to 5.02) | 0.001 |
| Training in their NHS trust | 355 (80.0) | 132 (91.7) | 0.39 (0.20 to 0.77) | 0.007 |
Table 62 displays the binary multiple logistic regression associations between the quality of training and the discussion of ADRT and barriers to training. Compared with average or poor training, good or excellent training was associated with fewer psychiatrists who never discussed ADRTs with patients, and fewer psychiatrists who believed that they had insufficient time to discuss ADRTs with patients. Table 62 also shows that, compared with receiving only one training session on the MCA, receiving two or more training sessions was associated with more psychiatrists discussing ADRTs at Care Programme Approach meetings and fewer psychiatrists who believed that they had insufficient training to discuss ADRTs with patients. There were no other associations between the number of MCA training sessions and reported practice or beliefs about implementing ADRTs. Of some concern is that, even after good or excellent training, 96 (21.5%) psychiatrists would never discuss ADRTs, and 206 (46.3%) would not discuss ADRTs even if the SU or carer raised it. Furthermore, 177 (39.7%) and 178 (38.3%) psychiatrists still believed that they had insufficient training and time to discuss ADRTs in clinical practice, despite good or excellent training and two or more training sessions, respectively, suggesting that more than training is required to increase the discussion and use of ADRTs with SUs who have BD.
| Training characteristic | Quality of training, n (%) | Statistics | ||
|---|---|---|---|---|
| Good or better (N = 444) | Average or worse (N = 144) | OR2 (95% CI) | p-value | |
| Never discuss ADRT | 96 (21.5) | 48 (32.9) | 0.53 (0.35 to 0.79) | 0.010 |
| Insufficient time to do ADRT | 177 (39.7) | 79 (54.1) | 0.57 (0.37 to 0.88) | 0.002 |
| Amount of training, n (%) | ||||
| ≥ 2 sessions (N = 465) | 1 session (N = 127) | |||
| Discuss ADRT routinely at CPA meetings | 77 (16.6) | 11 (8.7) | 2.37 (1.17 to 4.83) | 0.017 |
| Insufficient training to do ADRT | 178 (38.3) | 80 (63.8) | 0.41 (0.27 to 0.63) | < 0.001 |
Use of the Mental Capacity Act 2005 by service users with bipolar disorder: survey results
In the abstract, SUs viewed advance planning with considerable enthusiasm. Of the 496 SUs who answered the question about how important they considered it to be able to make plans about their personal welfare, 408 (82.3%) responded that it was very important and a further 61 (12.3%) responded that it was somewhat important. It is, therefore, striking how infrequently the advance planning mechanisms of the MCA were actually used. Table 63 shows that just over one-third (n = 199, 36.6%) of the SUs had heard of the MCA before participating in the study. Of the 544 SUs in the quantitative survey, 242 (44.5%) had used any of the mechanisms discussed in the survey – ADRTs, LPoAs or statements of wishes and feelings – suggesting that the publicity concerning the study had a small impact on the uptake of the planning mechanisms of the MCA even before completing the survey. Table 63 summarises this use of the MCA.
| Knowledge and use of MCA | (N = 544), n (%) |
|---|---|
| Knowledge of MCA | 199 (36.6) |
| Plans about personal welfare are very important | 408 (75.0) |
| Made ADRT | 54 (9.9) |
| Before 2007 | 28 (5.1) |
| After 2007 | 32 (5.9) |
| In writing | 28 (5.1) |
| Advanced statement of wishes and feelings | 62 (11.4) |
| LPoA, personal care | 31 (5.7) |
| LPoA, property and affairs | 21 (3.9) |
At first glance, there seemed a reasonable uptake of the MCA, with 242 (44.5%) making some use of it. However, 151 (28% of the total survey, 62.4% of those using advance planning mechanisms) had had only relatively informal discussions relating to their property and affairs. The more formal mechanisms in the MCA were used only infrequently: 54 (9.9% of the total survey) had made an ADRT [only 29 (54%) of which were in writing], 28 (5.1%) had an EPoA under the 1985 legislation, 21 (3.9%) had an LPoA for property and affairs and 62 (11.4%) had a statement of wishes and feelings. Thirty-one (5.7%) identified themselves as having made an LPoA for personal care, although this statistic is open to some doubt: in 10 of these cases, the participant gave the motivation as ‘financial matters’, suggesting that it may well not have been an LPoA for personal care that they had signed. If this is correct, the actual number for LPoAs for personal care may in fact be smaller than the statistic suggests, although the number of LPoAs for property and affairs may, consequently, be slightly increased. In that event, the number of LPoAs for personal welfare would reduce to 21 (3.5%) and the total number of EPoA/property LPoAs would rise to 53 (9.7%).
These low rates of usage are consistent with the psychiatrist survey data. Only 94 (14.5%) surveyed psychiatrists had encountered a patient with BD who had made an ADRT, 136 (20.9%) had encountered a patient who had made an oral or written statement of wishes and feelings and 91 (14.0%) had encountered a patient who had made an LPoA relating to health or personal welfare. As many of the psychiatrists are likely to have treated a number of patients with BD, these small numbers are perhaps even more striking than the returns from SUs. The disparity may suggest that some SUs have indeed made advance plans of which their psychiatrists are unaware. That also seems likely from the data: of the 54 SUs who made an ADRT, only 12 (22.2%) had given a copy to their psychiatrist. Nonetheless, the use of advance plans would appear to be remarkably limited.
Furthermore, the impression of psychiatrists would appear to be that the MCA has made little difference in the use of advance planning. Of the 259 psychiatrists expressing an opinion, 208 (80.3%) considered that the number of people with BD making ADRTs had remained the same since the implementation of the MCA in 2007 and 41 (15.8%) considered that it had increased by < 10%. Of the 252 psychiatrists expressing a view regarding statements of wishes and feelings by people with BD, 187 (74.2%) thought that the frequency remained the same since the MCA came into force and 46 (18.3%) thought that it had increased by < 10%.
The key reason for the underusage of advance planning would appear to be a lack of knowledge among people with BD. Only 199 of the SU respondents (36.6 %) had heard of the MCA prior to taking the survey. Of those that had not made an ADRT, 326 (65.6%) indicated that the primary reason was that they had not known about them prior to taking the survey. Of the 480 people who had not made statements of wishes and feelings and who provided a reason for not doing so, 385 (80.2%) indicated that the primary reason was that they did not know of them.
Table 64 shows the univariate and binary multiple logistic regression analyses of demographic and service use associated with the knowledge of SUs with BD about the MCA. Logistic regression shows that having degree-level education, attending NHS or non-NHS SU groups and/or attending a counsellor (as opposed to a psychotherapist or psychologist) were all associated with increased knowledge of the MCA before the survey.
| Demographic or service use factor | Knowledge of MCA, n (%) | Statistics | |||
|---|---|---|---|---|---|
| Yes (N = 196) | No (N = 344) | χ2 | df | p-value | |
| Children | 105 (53.5) | 215 (62.5) | 4.12 | 1 | 0.042 |
| Married | 84 (42.9) | 117 (34.0) | 4.18 | 1 | 0.041 |
| Degree education | 82 (41.8) | 82 (23.8) | 19.3 | 1 | < 0.001 |
| Unemployed | 22 (11.2) | 68 (19.8) | 6.56 | 1 | 0.010 |
| Service use | |||||
| NHS SU group | 52 (26.5) | 63 (18.3) | 5.03 | 1 | 0.025 |
| Non-NHS SU group | 53 (27.0) | 46 (13.3) | 15.6 | 1 | < 0.001 |
| Counsellor | 21 (10.7) | 18 (5.2) | 5.60 | 1 | 0.018 |
| Occupational therapist | 18 (9.2) | 16 (4.7) | 4.43 | 1 | 0.037 |
| Binary logistic regression ( N = 540) | |||||
| Demographic or SU factor | OR | 95% CI | p-value | ||
| Non-NHS SU group | 2.37 | 1.49 to 3.76 | < 0.001 | ||
| Degree education | 2.15 | 1.45 to 3.20 | < 0.001 | ||
| NHS SU group | 1.68 | 1.08 to 2.63 | 0.022 | ||
| Counsellor | 2.23 | 1.11 to 4.47 | 0.023 | ||
| Occupational therapist | 2.12 | 1.01 to 4.44 | 0.047 | ||
Among those SUs who did know about advance planning, it does not appear that they found out about it primarily from their psychiatrists, even when the planning was directly relevant to medical care (Table 65). Of people with ADRTs, only 10 (19%) found out about them from their psychiatrist. Of the 23 people who found out from ‘other’ sources, 12 (23.1% of the total) did not find out about ADRTs from others at all, but identified their source of knowledge as ‘myself’ or similar. It is important to note that although belonging to a NHS or non-NHS SU group and having a counsellor were associated with knowledge of the MCA, these factors probably define a group of people with BD who believed in the ADRT so strongly that they drew up an ADRT personally or with other health professionals, notably the psychiatrist. The survey suggests that NHS or non-NHS SU groups did not provide the materials to enable the SU with BD to make an ADRT.
| Source of information | n (%) |
|---|---|
| Other | 23 (44) |
| Own research (e.g. internet, library) | 17 (33) |
| Psychiatrist | 10 (19) |
| Care co-ordinator | 6 (12) |
| Non-NHS SU group | 3 (6) |
| Friend | 2 (4) |
| NHS SU group | 1 (2) |
| Lawyer | 1 (2) |
| Family member | 1 (2) |
Regarding planning beyond medical care, discussions with psychiatrists appear to be infrequent. For example, of the 161 people who had engaged in informal discussions relating to their property and affairs, only 16 (10%) had engaged in those discussions with their psychiatrist. This is, in a sense, unsurprising; financial management is not a part of a psychiatrist’s training, and it is unlikely that psychiatrists will be directly involved in the execution or implementation of such plans. Nonetheless, financial issues (most notably overspending) can cause particular distress to, and practical problems for, people with BD, and can significantly affect their mood, and the practical options open to them, during recovery. The management of financial issues is, therefore, appropriately considered a part of the care package. To that extent, at least, they are relevant to the psychiatrist, and it is appropriately the role of the psychiatrist to alert the patient to advance planning possibilities.
Psychiatric involvement was limited not merely in finding initial information about advance planning, but also in the development of plans. Only 22 of the 31 SUs who reported that they had made an advance statement of wishes relating to personal care sought advice from a psychiatrist during the development of the plan. Only 11 of the 54 SUs making ADRTs reported doing so. The more encouraging news, albeit based on very small numbers, is that, when such advice was sought, it tended to be viewed as either good or very good [19 of the 22 (86%) people consulting a psychiatrist for a statement of wishes; 7 of the 11 (64%) consulting a psychiatrist for ADRTs].
This does not mean that people making advance plans did not seek advice. The survey asked about consulting care co-ordinators, psychiatrists, lawyers, SU groups within and outside the NHS, family members, friends, text-based materials and other sources. Only two of the 29 SUs making written ADRTs consulted none of these. Of the six people consulting only one, four considered the advice received to be good or very good. Of the remaining 19 people who consulted at least two of these, all but one received at least one set of advice that was viewed as good or very good. Consistent again with the view that the problems are not just about training, it does seem that good advice is to be had, but only if the SU knows where and how to look for it.
The SU data make clear that it is not only a lack of knowledge that results in a failure to engage in advance planning. Although overwhelmingly enthusiastic about the availability of advance planning, not all SUs wanted to make advance plans.
Use of the Mental Capacity Act 2005 by service users with bipolar disorder: qualitative results
The qualitative evidence suggested that SUs’ reluctance to make advance plans was sometimes the result of simple procrastination, and that the complexity of some of the forms (most notably the LPoA) was a barrier, but the data suggest other reasons. Sometimes, a lack of advance planning would appear to flow from a desire not to foreclose or discourage treatment options, particularly when the SU had a good relationship with his or her psychiatrist, as in the following comment from the qualitative data:
Well, psychiatrists are very good at listening to you and trying out things that will both work and be acceptable to you. I’ve never had any problem with feeling that medication has been imposed on me. I’ve been occasionally, not with my current psychiatrist, but occasionally been under pressure to take a certain type of medication that I didn’t want, like lithium – but I’ve never felt like I didn’t have any choice in the matter.
SU10
Consistent with this, 61 of the 171 SUs (37%) in the quantitative study who were aware of ADRTs but did not make one did so because ‘I am confident that my doctor will make the right decisions for me’. Similarly, of the 95 people who had heard of statements of wishes and feelings but had not made one and expressed a reason why, 48 (51%) stated that this was because they did not feel strongly enough about a particular issue to make one, and 47 (50%) stated that they were unsure how to make the statement. Lack of knowledge is clearly significant, but it is not the only factor in failing to engage in advance planning.
However, even when SUs are aware of the possibilities, the interview data suggest that there is an absence of support for completing the plan. SU2, for example, described her experience as follows:
Then you mentioned that you were given a form about refusing treatment and this was a year after you were admitted as a ‘voluntary’ patient?
The advance statement, yes.
I mean . . . that’s . . .
A bit lax.
Well . . . yes.
Well, it doesn’t surprise me because the whole system seems to be sluggish . . . and unsympathetic.
So it [the plan] is all to do with refusals of treatment. And were you given any assistance or help with . . .
No. I was just given this form by my support worker and then she left. So I filled it in and handed it back to her; I took a copy.
So it was just, kind of ‘write this’? Did you get any . . .
Information?
Yes, advice or information about what is needed to make the document valid?
No, I was just presented with the form. If I could do, then would I please fill it in? Then she left.
It does not appear that meaningful advice or support was provided regarding either what the SU wanted to include in the document or the legal effect of the document.
Interviewees in the qualitative element of the project further noted the emotional cost of advance planning, as it required the revisiting of previous unpleasant and distressing times. Advance planning has many benefits, but its emotional costs must also be acknowledged: a matter that should be considered by anyone offering advice in this area. Furthermore, some SUs with BD, including some who had made advance plans, did not understand that an advance plan is a legally binding document:
Yes there was [a pro forma provided through the trust]. I used that, but just added in my own personal needs and what I wanted. Obviously it’s not a legal document and the doctors can over-ride that as far as I am aware, but at least there’s a lot of information in there that could be quite useful to a new doctor.
SU12
Service users are encouraged by many mental health professionals to write recovery plans. For some SUs who have made provisions recognised by the MCA, the distinction between a recovery plan, which has no formal legal basis and is not binding on health professionals, and provisions under the MCA, which are legally binding, is unclear. The failure of understanding is explicit in the following comment from SU13:
The only thing I’ve done is when I was working on my recovery booklet. There was a section there about your requests if you had to be sectioned again. That was supposed to be scanned and added to your care plan, but that didn’t happen I don’t think. Then I had a new care co-ordinator since . . . probably the end of last year . . . And we’ve gone through a new care plan, but again that isn’t anything to do with the MCA . . . it was a very informal . . . sort of my wishes were written down . . . I don’t think anybody would even find them on the system to be perfectly honest with you [laughs]!
That’s really interesting. So just so I have it correct – this is a recovery ‘booklet’?
Yes, it was one that was produced by [XXXX] trust. They’re quite big on recovery and they have recovery packs that the care co-ordinator works through with clients. Part of that is, you know, write it down with us . . . when you’re high or low and then . . . it’s the whole thing really . . . you just write about your illness.
This shows no understanding that the content of the relevant statements may well be relevant under the MCA. In that sense, it may well not be a case of ‘you just write about your illness’.
Tables 66–68 show the univariate and binary multiple logistic regression analyses of demographic factors and services used that were associated with SUs with BD making an ADRT, an advance statement of wishes and feelings, and any LPoA. Logistic regression shows that attending NHS or non-NHS SU groups and having a care co-ordinator were associated with making an ADRT. Only attending a NHS support group and having a care co-ordinator were associated with making an advance statement of wishes and feelings. Being > 55 years of age was the only factor associated with having a LPoA. In an analysis of any use of the MCA, where the belief in the importance of having plans about welfare was added to demographic and service use factors in logistic regression, any use of the MCA was also associated with attending NHS SU groups and having a care co-ordinator, but the belief about the importance of having plans about welfare also had an effect (Table 69). As this belief was reduced in the group of SUs who had provision for their welfare under the MCA compared with those who did not, the concern about making plans about welfare may have been reduced by making provisions under the MCA. Once this item was removed, then attending a NHS SU group or non-NHS service group or having a care co-ordinator were associated with making provisions under the MCA.
| Group | Made ADRT, n (%) | Chi-squared statistics | |||
|---|---|---|---|---|---|
| Yes (N = 54) | No (N = 488) | χ2 | df | p-value | |
| NHS SU group | 23 (42.6) | 93 (19.1) | 16.0 | 1 | < 0.001 |
| Non-NHS SU group | 16 (29.6) | 82 (16.8) | 5.40 | 1 | 0.020 |
| Psychiatrist | 49 (90.7) | 388 (79.5) | 3.93 | 1 | 0.048 |
| Care co-ordinator | 25 (46.3) | 114 (23.4) | 13.4 | 1 | < 0.001 |
| Non-NHS SU group | 14 (25.9) | 64 (13.1) | 15.2 | 1 | < 0.001 |
| Binary logistic regression (N = 542) | |||||
| Group | Made advance statement, n (%) | Chi-squared statistics | |||
|---|---|---|---|---|---|
| Yes (N = 61) | No (N = 475) | χ2 | df | p-value | |
| Care co-ordinator | 24 (39.3) | 113 (23.8) | 6.87 | 1 | 0.009 |
| NHS support group | 27 (44.3) | 88 (18.5) | 21.2 | 1 | < 0.001 |
| Binary logistic regression (n = 536) | |||||
| Demographic or SU factor | OR | 95% CI | p-value | ||
| NHS SU group | 3.36 | 1.92 to 5.89 | < 0.001 | ||
| Care co-ordinator | 1.95 | 1.10 to 3.44 | 0.022 | ||
| Demographic or SU factor | Made LPoA, n (%) | Chi-squared statistics | |||
|---|---|---|---|---|---|
| Yes (N = 31) | No (N = 503) | χ2 | df | p-value | |
| Aged > 55 years | 14 (45.2) | 130 (25.8) | 5.53 | 1 | 0.007 |
| Care co-ordinator | 13 (41.9) | 126 (25.0) | 4.32 | 1 | 0.038 |
| Occupational therapist | 5 (16.1) | 29 (5.8) | 5.26 | 1 | 0.022 |
| Binary logistic regression (N = 535) | |||||
| Demographic or SU factor | OR | 95% CI | p-value | ||
| Aged > 55 years | 2.43 | 1.15 to 5.12 | 0.020 | ||
| Demographic or SU factor | Knowledge of MCA, n (%) | Statistics | ||||
|---|---|---|---|---|---|---|
| Yes (N = 116) | No (N = 415) | χ2 | df | p-value | ||
| Aged > 55 years | 41 (35.3) | 103 (24.8) | 5.08 | 1 | 0.024 | |
| Care co-ordinator | 47 (40.5) | 90 (21.7) | 16.80 | 1 | < 0.001 | |
| Social worker | 17 (14.7) | 36 (8.7) | 3.61 | 1 | 0.057 | |
| NHS support worker | 45 (38.8) | 69 (16.6) | 26.40 | 1 | < 0.001 | |
| Non-NHS SU group | 28 (24.1) | 68 (16.4) | 3.68 | 1 | 0.055 | |
| Very important to have plans about welfare | 76 (65.5) | 321 (77.3) | 6.73 | 1 | 0.009 | |
| Binary logistic regression (n = 531) | ||||||
| Independent factor | β | Standard error | Wald | exp(β) | df | p-value |
| NHS support group | 1.130 | 0.242 | 21.75 | 0.323 | 1 | < 0.001 |
| Care co-ordinator | 0.821 | 0.237 | 11.97 | 0.440 | 1 | 0.001 |
| Non-NHS SU group | –0.600 | 0.241 | 6.91 | 1.821 | 1 | 0.013 |
The survey of SUs asked about the content of statements of wishes and feelings and of written ADRTs. These are reported in Tables 70 and 71. For ADRTs, it is notable that no one refused all medication. Instead, it was classes of medication, specific medication or ECT that were most frequently refused. Statements of wishes are a more flexible mechanism than ADRTs, extending potentially to all personal matters. Nonetheless, views regarding medical treatment figured prominently: 30 (48%) of the statements expressed wishes in favour of, and 32 (52%) against, a particular medical treatment. Even more frequent was the identification of an individual to be notified in case of illness (n = 38, 61%).
| Treatment | n (%) |
|---|---|
| All medication | 0 (0) |
| A particular medication (e.g. haloperidol) | 15 (52) |
| A particular class of medication (e.g. antidepressants) | 12 (41) |
| ECT | 9 (31) |
| Depot injections of medications | 4 (14) |
| Not stated | 2 (7) |
| Subject | n (%) |
|---|---|
| Who is to be notified in the event of illness | 38 (61) |
| Against a particular medical treatment | 32 (52) |
| In favour of a particular medical treatment | 30 (48) |
| General domestic affairs | 23 (37) |
| Wishes regarding accommodation | 19 (30) |
| Who is not to be notified in the event of illness | 17 (27) |
| Pet care | 15 (25) |
| Child care | 6 (10) |
| Dependent care | 4 (7) |
The places where written ADRTs and written statements of wishes were kept are listed in Table 72. A problem with the data arose in this question. Twelve of the 29 people indicated that ‘I just keep a copy for myself’, but 11 of these went on to identify others with whom the document was kept. This presumably means that these respondents read the response as ‘I kept a copy for myself’, although this is not a good measure for the numbers of respondents keeping a copy of their ADRT, as the other respondents presumably did not read the question in that way. It does not follow from this, however, that NHS staff were routinely informed of the ADRTs. Of the 29 responses, eight did not provide a copy of the ADRT to their psychiatrist or care co-ordinator, or had it placed in their notes (although one of these respondents provided a copy to his or her GP). Among people making statements of wishes, 17 of the 53 (32.1%) respondents did not provide a copy to their psychiatrist or care co-ordinator, or place a copy in their NHS notes (although, of these, four provided copies to their GP and one provided a copy to a CPN). This failure to notify is a problematic strategy, as it is not clear that the ADRT will come to the attention of the relevant medical personnel at the time when it is needed. That said, given the comments regarding placement of advance planning documents in patient records (see below), relying on family carers and trusted friends to bring the relevant documents to light at the relevant time may be a sensible strategy.
| Place | Written ADRT (N = 29), n (%) | Written statements of wishes and feelings (N = 53), n (%) |
|---|---|---|
| Psychiatrist | 12 (41) | 17 (32) |
| Family member | 12 (41) | 15 (28) |
| NHS records | 12 (41) | 13 (25) |
| Self only | 12 (41) | 0 (0)a |
| GP | 10 (34) | 15 (28) |
| Care co-ordinator | 5 (17) | 17 (32) |
| Friend | 3 (10) | 6 (11) |
| Carer | 2 (7) | 7 (13) |
| Lawyer | 2 (7) | 3 (6) |
| Non-NHS SU group | 1 (3) | 3 (6) |
| NHS SU group | 0 (0) | 0 (0) |
| Other | 5 (17) | 13 (25) |
The SU survey indicates little about how much account is taken of advance planning by clinicians when the circumstances envisaged in the plan arise: only two people in the survey had made an ADRT and had later been sectioned. The psychiatrists’ data are more illuminating. As Table 73 shows, 108 of the psychiatrists were aware of people with ADRTs who had later been sectioned. In more than one-third of these cases, the ADRT was relevant to the sectioning to some degree, although in only three cases (2.8%) was it the primary reason for the sectioning. Similarly, we were not given any specific examples in our qualitative data from either SUs or psychiatrists that indicated how SUs had used the formal provisions of the MCA, such as an advanced statement of wishes and feelings, to identify and act on early warnings of bipolar relapse, although some SUs did utilise these to prevent relapse.
| Level of use of MHA | n (%) |
|---|---|
| Not at all | 68 (10.5) |
| To some extent | 26 (4.0) |
| To a great extent | 11 (1.7) |
| Primary reason for use of MHA | 3 (0.5) |
| No response | 542 (83.3) |
Discussion
An overarching picture emerges from the data. Notwithstanding the general enthusiasm for advance planning among people with BD and a very high level of concern that they have plans about their welfare, which the MCA is designed to promote, the advance planning mechanisms of the MCA are rarely used by people with BD. Despite the advice of both NICE26,57 and the Code of Practice of the 1983 MHA, clinicians are not actively promoting the use of such mechanisms. Around half of psychiatrists would discuss these options if asked directly by a patient, but it is difficult to see that this is a viable strategy as so few people with BD find out about the options available through other means. If they do not know, they may well not ask and, sadly, even if they do, a substantial number of psychiatrists will still not discuss the MCA with them. When advance planning is done, a significant minority of persons making the plans do not discuss them with their psychiatrists, even when the plan is directly relevant to psychiatric care, and a significant minority do not lodge a copy of the plan with their clinicians or in their NHS notes.
Low rates of usage of advance plans are consistent with findings in a variety of studies relating to other clinical groups. 311,322–324 The reasons for these low rates of usage in a variety of clinical groups overlap with the data in the present study, reflecting both a hesitancy of clinicians to engage with advance planning, and in some cases, a hesitancy of SUs to take up the options even when they are aware of them.
For SUs, the most significant reason for the relatively low rate of advance planning would appear to be their lack of knowledge about the options available: that knowledge deficit is clear from the data in the present study. Knowledge of the MCA tended to be greatest among those with a degree-level education and among those who attended NHS and non-NHS SU support groups. However, those who were aware of the MCA often obtained their knowledge not from these support groups, but rather from their own efforts to find out information, and from a range of health professionals, most commonly psychiatrists.
The reluctance of psychiatrists to engage with advance planning is evidenced in the quantitative survey, and the interview data suggest a similar reluctance on the part of other clinical staff. The data do not, however, provide a simplistic reason for this reluctance. As noted above, few psychiatrists say that they are opposed to advance planning in principle. A substantial proportion of psychiatrists surveyed said that lack of time or lack of training were significant reasons for their failure to discuss advance planning to some extent. There are few psychiatrists who, with training, would never discuss the MCA. Training may be important in that better quality of training in the MCA was associated with discussion of ADRT, and both the number and quality of training sessions were associated with routine discussion of ADRTs at Care Programme Approach meetings and a reduction in the perception that time and training were barriers to implementing ADRTs. This does not necessarily imply that the training improved these aspects of care, because the data are observational and based on self-report. Nevertheless, a perception of sufficient good-quality training was that it involves case summaries, more than one session, and considers the assessment of capacity and ADRTs. Perhaps of particular relevance to a condition such as BD, in which mental state and capacity can change quickly, there was perhaps insufficient training on the assessment of and use of the MCA in relation to fluctuating capacity. Nevertheless, the data also suggest that training psychiatrists in the use of the MCA is not sufficient to enable SUs with BD to become aware of and use the MCA, as, even after good-quality training, nearly half of psychiatrists would not discuss ADRTs, even if the SU or their carer raised the topic with them.
Notwithstanding the low level of doctrinal disagreement to advance planning noted in the Quantitative survey section, there was significant indication in the interview data that advance planning, and particularly ADRTs, were perceived as inconsistent with the ethos of care. Interviewees of all types – SUs, psychiatrists and other professionals – acknowledged that this involved a shift towards greater patient involvement (or, in some interviews, empowerment), and this was perceived as coming into conflict with a paternalist ethos that remained in medical practice. Contrary images were also present in the interviews, and the full range of interviewees also identified cases where considerable efforts had been made by professionals to comply with advance plans. However, an ongoing perception of paternalist medical authority is also clear from the interviews, to the point where some health professionals interviewed referred to ADRTs as creating a ‘conflict of interest’ with the overall medical role of provision of appropriate treatment. Consistent with this, even the psychiatrists interviewed who were enthusiastic about ADRTs and other advance planning – and there were a number of those – did not suggest that the terms of the MHA should be altered so that ADRTs could not be over-ruled. Indeed, of some concern was the reporting by 40 (6.2%) psychiatrists of cases where the MHA was used to at least some extent to over-ride an ADRT. Although it is beyond the scope of this piece of research, the data suggest that some consideration is required among SUs and mental health and legal professionals in relation to how the ethos of recovery can be reconciled with a more paternalistic approach to care that one might hope would be used sparingly and as a last resort.
It is at best doubtful how far the concerns relating to the potential limitations on treatment of advance planning are warranted. Certainly, the psychiatrists interviewed could identify situations that could be problematic. One psychiatrist, for example, noted a patient that had requested treatment with a specific brand of medication that had not proved effective in the past, and in the end, notwithstanding attempts to comply, it became necessary to depart from the plan. The quantitative data from SUs in the present study, however, suggest that none making an ADRT refused all treatment. Although compliance with an ADRT may limit treatment options, it does not remove them entirely. Under the current law, the statement of wishes is not binding even under the MCA, and the ADRT can be over-ruled if the conditions for compulsion under the MHA are met; therefore, the reluctance of psychiatrists to engage with advance planning seems unjustified.
Engagement with advance planning requires an understanding not merely of the terms of the MCA (where the psychiatrists seemed broadly happy with their training), but also of individual trust policies. This theme arose only at the post-survey interview stage of the current project, but interviews with clinicians in particular suggest that the mechanics of implementing advance planning in individual trusts are not well understood. No one who was asked in the post-survey interviews could provide a coherent account of their trust policies in this area, nor could they explain how or where advance plans were kept in the patient record (whether that was a paper record or an electronic record). It is possible that what is holding things back is not knowledge of the MCA or doctrinal beliefs about the desirability of advance planning per se, but this set of much more mundane, practical matters.
This also raises questions about the strategy that SUs should adopt when they do make advance plans. Clearly, it is prudent to discuss the advance plan with the psychiatrist and to make sure that a copy is added to the notes, but it would also appear to be prudent to ensure that a friend, carer or family member also has a copy of the plan, as it is not obvious that the plan will be highlighted in those notes and, therefore, identified and relied on at the relevant time.
These themes also come together in considering SU understanding of the relevant advance planning mechanisms. Both the quantitative and the qualitative data suggest considerable confusion among SUs as to the nature of the various MCA advance planning mechanisms and the ways in which they relate to other initiatives in their care (most notably recovery programmes). The doubtfulness of the validity statistics in the quantitative survey regarding LPoAs for treatment and personal care has already been noted: thus, roughly one-third of people who identified themselves as making LPoAs thought that they concerned property and finances, which they do not. Reasons given for failing to make specific sorts of plan further make this confusion explicit. Some people, for example, indicated that they did not need a statement of wishes, as they had signed an LPoA, or on the basis that a family member would make the relevant decisions well. There is no understanding that family members have no formal authority to make decisions, nor that the donee of an LPoA is still bound by the best interests criteria. There appears to be no realisation that the form in question may well have been a legal document that imports duties and obligations under the MCA.
Confusion with recovery plans was particularly evident. Although the objectives of recovery programmes are related to those of advance planning under the MCA, they are not quite the same. The former are intended to promote ongoing self-reflection, leading to an articulation of goals regarding treatment and how those goals are to be achieved. Recovery programmes can be understood as a search for common ground between patient and clinical and other carers. The MCA mechanisms are instead legal mechanisms to determining future care. Sometimes such determinations will be consistent with medical views, but sometimes they will not. While the ethos of recovery is creation of mutual trust and partnership between professional carers and patients, the ethos of advance planning is SU choice. These are related, but not quite identical, concepts. Furthermore, the material given to patients as part of recovery programmes does not always refer to the MCA, and does not always provide information consistent with the MCA about the legal meaning of statements made as part of the recovery plan.
Strengths and limitations of the data
Although this study is, as far as we are aware, the largest empirical study of advance decision-making yet conducted regarding the MCA, it is not, of course, definitive. The survey seems to have reached people with a definitive diagnosis of BD, the vast majority being in contact with MHS and reporting moderate or severe difficulties as a result of hypomania or mania symptoms. The survey of psychiatrists seems to have collected a national sample of psychiatrists of different grades, with the majority being consultants in posts where they would regularly see SUs with BD. Although the quantitative elements of the study are composed of reasonable sample sizes, they are, to a considerable degree, independent samples: the SUs sampled were not necessarily the patients of the psychiatrists sampled. Even with the large numbers in the initial samples, the numbers engaged in advance planning directly was small. Although that is itself a highly important finding – the MCA advance planning mechanisms are not being used to any great extent by people with BD – it does mean that the quantitative inferences relating to the individual planning mechanisms is based on relatively small sample sizes, and it is much less obvious that these conclusions can simply be generalised uncritically. In the extreme, only two of the SUs who had made ADRTs had been subject to MHA admission afterwards. That is, of course, an insufficient number to produce relevant quantitative findings. The limitations of the statistical data are addressed in part through the use of the qualitative data: the quantitative conclusions received support through the interviews.
The result remains problematic, however. The study remains essentially a study of SUs and psychiatrists. Apart from the initial interviews on which the survey was developed, no social care professionals, other health professionals, legal professionals or non-professional carers were surveyed or interviewed. One might reasonably expect such groups to have a rather different perspective on the MCA. As a clear example to make the point, as noted above, only 2.7% of psychiatrists thought nurses were the best informed health profession on advance planning and the MCA. One might reasonably expect that, if nurses were surveyed, they might well have a different view. Although that is a particularly clear example, the issue of perspective is pervasive: this is a study of psychiatrists and SUs.
The value of the quantitative evidence is also contingent on the representativeness of the sample. The age distribution and the rates of employment are broadly consistent with that found in Morgan et al. ’s 2005325 Australian epidemiological study of BD, although the proportion of people married or civilly partnered appears somewhat higher in the present sample. Unfortunately, we do not know anything about the proportion of SUs of each sex in the survey, and if this split is representative of people with BD in the general population. If the population of people with BD matches the general population, the sample contains a smaller representation from black and minority ethnic communities than it ought (7.8% in our study, compared with 14.0% nationally). If SUs with BD match the general population – a matter not entirely free from doubt – the sample in this study was somewhat overeducated, and black and minority ethnic people were under-represented. It is not obvious how the racial disparity would affect the results. The educational disparity might perhaps suggest that the situation is in fact more extreme on key findings than the statistics would suggest. It is hard to believe that more highly educated people are less informed about their rights than the general population, and that they are less willing to press for those rights and engage with service providers in ensuring that those rights are respected. If the problems are a lack of knowledge and limited engagement with professionals, it seems unlikely that a sample with fewer educated people would result in findings of greater use of the MCA or greater engagement with professionals about advance planning.
Conclusion
Implementation of the MCA has attracted some criticism in recent months, with an ad hoc committee of the House of Lords311 noting overall poor understanding and engagement with the MCA, and poor use of advance planning in particular. The present study supports that overall criticism. At least for people with BD, it would seem that the advance planning provisions of the MCA have been of little impact. As noted early in this chapter, that is a shame, as advance planning seems particularly suited to people with cyclical conditions such as BD.
In its response to the House of Lords report, the Government has announced the formation of an interministerial working group to promote and monitor implementation of the MCA. It is to be hoped that this group will be successful at increasing the usage of advance planning; although, as the House of Lords report also suggests, the evidence from the present study is that the problem is not one of simple knowledge deficit among SUs (important though that is), but also a matter of cultural change among the relevant professional groups.
Chapter 7 Service user involvement in the PARADES programme
This chapter was written by Ms Rita Long, SUR.
Introduction to service user involvement
When asked to write this chapter I initially thought:
Oh no, I can’t write the way ‘they’ [academics] want me to,
and:
what a great opportunity to collate the PARADES (SU) collaboration journey.
With hindsight, these thoughts could be predicted considering the tension created by, on the one hand, the void between academic worlds and that of lived experience and, on the other, the drive to integrate both of these areas. The aim of this chapter is to capture the SU perspective of this journey, the tailored philosophy underpinning ‘involvement’ for this project, and the lessons learnt throughout the PARADES programme. Involving SUs in mental health research was an integral part of the entire PARADES programme from the outset. This innovative concept of holistically integrating SUs in research was in its infancy when the PARADES grant application was submitted to the NIHR in 2007. Therefore, WS leads had limited access to previous tried-and-tested models that used this approach. Instead, the research team developed a collaborative model, from including having a SUR as a grant applicant and then as a grant holder for the PARADES programme. Although the initial grant holder was then unable to commit to the project owing to other demands on their time, the programme team identified another SUR to take on this role. In practice, there have been four SURs involved at grant holder level throughout the life of the programme. Liz Pitt, Martina Kilbride, Debbie Mayes and Rita Long are all SURs who made significant contributions to the management and implementation of the PARADES programme. This senior input helped to integrate SU involvement throughout the programme, including a bimonthly SURG, paid SURs and group facilitators, and SUs contributing to staff training.
To provide some context to the history, rationale and motivation surrounding SU involvement in the PARADES programme, I interviewed the programme’s chief investigator, Professor Steven Jones. Below are the outcomes from that interview.
Steven’s previous experience of working with SUs came while teaching on the clinical psychology doctorate programme based at the University of Manchester. This programme had SU involvement embedded throughout the course, and this provided valuable experience of the potential for SUR involvement on the PARADES programme. Steven also worked collaboratively with SUs on another NIHR Programme Grants for Applied Research programme-funded project in Manchester, led by Tony Morrison.
During this interview, Steven expressed a sense that there may have been a risk for him as principal investigator and potentially for WS leads being worried about ‘getting it wrong’ or ‘not doing it the right way’. He consulted a colleague who had experience working with SUs who advised ‘to have a go and don’t be put off by the potential of getting it wrong’. Interestingly, Steven is now the co-director of the Spectrum Centre for Mental Health Research at Lancaster University, which has an established SU involvement structure rooted throughout its entire work.
Steven’s response when I asked him why he thought it was a good idea to involve SUs was that ‘It doesn’t make sense not to involve the people with the lived experience of what we are researching’. From my perspective, this begs the question ‘then why does this not make sense to other researchers?’ He added that his view of a ‘continuum mode’ of mental health, as opposed to the belief that people with mental health issues are qualitatively different in some way, might be part of the driver to involve people in what ultimately will affect them. He also felt that having SUs involved ensures that research is relevant for those who are its intended beneficiaries, and that its priorities are those of the people living with the condition.
Steven was one of five WS leads on the PARADES programme. I was interested to explore if at the outset other WS leads shared Steven’s attitude and commitment to actively involving SUs in their work for PARADES. Steven explained that the commitment to involve SUs was a shared one from the start of the ‘WS leads’ collaboration. There were, however, discussions as to how this may actually happen and its implementation did vary depending on the needs and nature of each WS. My sense is that across WSs the principles of collaborative engagement of SUs were embraced. All the WS leads had some experience and had previously demonstrated a commitment to working in close collaboration with SURs, providing the basic foundations to this ‘foggy’ process.
From the outset, WS leads, the PARADES programme manager and SURs agreed on some basic ‘involvement’ principles that were implemented throughout the whole programme. The team were all of the opinion that patient and public involvement (PPI) activities were a ‘two-way’ process where PPI members would not only contribute to the study, but they would personally gain from taking part. Initially, the SURG existed during the writing of the programme grant, which remained in place at the start of the PARADES programme, providing direction to WS1. As work began on developing the two intervention studies (WS2 and WS3), a decision was made to develop WS-specific focus groups to inform these. Latterly, more members were recruited into a larger overarching advisory panel who were a more central feature of the Spectrum Centre for Mental Health Research and provided advice for many of the studies being developed there, including for PARADES programme training and recruitment of staff, review of study materials and dissemination.
Some fundamental principles included:
-
At the outset, PPI activities were designed to be diverse so that people could contribute in a range of ways. Some SUs did not feel able to offer a long-term commitment but were keen to contribute in time-limited ways, such as by consulting on a particular project. Others were able to be involved throughout the programme to provide consistency of input.
-
When recruiting for PPI activities, the SUR involved explicitly explained the commitment expected for each activity, as well as clarifying that people could opt out or take a break at any time. Feedback from SUs regarding this flexible approach was that they felt able to participate because they were not under pressure to commit for the long term or to ‘make excuses’ if they needed or wanted to withdraw for any reason.
-
The Principal Investigator and SUR of the relevant WSs ensured that the SURG, focus group and advisory panel meetings had clear and transparent aims and objectives.
-
When recruiting for SU members, the SUR clearly explained the aims and objectives of each meeting and these were communicated to potential members before they registered to take part. This enabled SUs to make informed choices whether or not a particular activity was of interest and/or relevant to their personal experiences and interests. It also enabled WS leads and SURs to recruit panel members that had some knowledge relevant to the topic to be discussed.
-
The transparency of these aims and objectives empowered SUs to make ‘informed’ decisions that maximised personal learning and development opportunities.
-
Feedback was given to all PPI contributors as to how the information they provided would be utilised in the study. Not only did this principle validate the views and opinions of panel members, it also gave a number of SUs the confidence to ‘speak out’ in other arenas.
-
All RAs received sensitivity training from a SUR. RAs were also given the opportunity to practise their interview techniques with members of the PARADES advisory panel. These interviews were tailored to reflect the need of the study on which each RA worked. Feedback from these interviews included what it was like to take part from the participants’ perspective. The RA and SUR then reflected on this feedback as a means to developing the skills of the RA within the remit of the stream in which they were working.
Service user involvement in the development of the application
The explicit focus of the PARADES programme was to address issues of importance to people living with BD. Therefore, the WSs were developed in partnership with people with lived experience as part of the application process. The applicants were paid a small amount of PPI money from MHRN to support a series of consultation groups as the application was developed. This input highlighted the importance of the programme focus around relapse, comorbidity, suicidality and mental capacity for SUs.
Common themes for patient and public involvement across workstreams
Workstreams 1–3
A SUR provided sensitivity training relevant to therapists’ roles and level of experience. An example of this is how to ask and respond to questions on sensitive issues such as suicidal thoughts or behaviours during manic episodes. This training included ‘mock’ therapy sessions with individual therapy teams. Feedback was given to teams as a group and individually, relating to the nature and relevance of the feedback.
As with all the streams, a SUR was given training and supported to enable her to contribute to the analysis and interpretation of data. With the support of the PARADES SURG, she developed an imaginative and comprehensive SU results dissemination strategy; as with all PARADES streams, she also contributed to the writing-up of the results.
Specific patient and public involvement activity within workstreams
Workstream 1: psychoeducation
Carers, people with lived experience of BD and clinicians are aware that there is not a system or structure in place that enables people with BD to access relevant and reliable information that will:
-
help them understand what BD is in relation to their diagnosis
-
help them understand their personal experiences of the disorder and how it may affect them
-
enable them to develop or acquire tools that enable them to stay well.
Psychoeducation and PS are two structures that offer forums where information and support are made available.
A SUR and the PARADES SURG collaborated with academic leads to translate this manual into a format that was culturally sensitive to an English audience in general and to a SU audience in particular.
The introduction of the SUF to complement the clinician facilitator (CF) roles in the PEd and PS groups was a significant addition to the model used by Colom et al. 33,61,62 There was preliminary evidence that this type of role was feasible and welcomed by other SUs from an uncontrolled pilot study conducted in north-east England. 70 However, the detailed results of this preliminary evaluation were not available when the PARADES groups were being developed. Therefore, the details of how to integrate this innovative role into the therapeutic team posed some challenges for academics, SUFs and clinicians. SURs worked very closely with the WS leads to:
-
develop SUF job descriptions
-
shortlist and interview candidates for SUF posts
-
deliver a 3-month induction and training programme for newly appointed SUFs
-
disseminate accurate, accessible information about the role where it was to be advertised. This included ensuring that potential applicants could access the information required and contact the SUR to clarify concerns or aspects related to the role.
As the SUF role was a new initiative, the SUR organised and delivered peer supervision in addition to the weekly team supervision given to the therapy team. This proved to be a really beneficial support to SUFs. Topics raised in the supervision sessions were often of a sensitive and personal nature (the nature and impact of these topics varied considerably from SUF to SUF), but included exploring personal boundaries, triggers (for the facilitators own mental health issues), and roles and responsibilities (within the team of facilitators). Balancing the relationship between being a colleague and a SU in a therapeutic setting was a recurrent theme that came up throughout these sessions. The main dilemma seemed to be the potential disclosure of awkward and/or antisocial behaviour while in episode. The SUR who facilitated the peer supervision sessions used a variety of approaches to deal with this issue. Examples of approaches used were:
-
SUF identifying examples of situations (reflective or scenarios) and then together brainstorming and developing strategies that could be utilised in practice
-
brainstorming potentially challenging scenarios and working together to develop a variety of suggestions of how to manage them.
At both peer supervision and facilitator team supervisions (SUF and CF combined), there was discussion of several emotive and challenging topics that had emerged in the groups, such as approaches when people disclose buying medication over the internet and when people are discharged from their NHS care for non-attendance at appointments. On the surface, these issues appear ‘straightforward’, but in reality, an integrated SUF and ‘clinician’ team model brought diverse opinions and perspectives of how these situations could be managed in PS and PEd settings. Weekly supervision with the WS leads provided a forum where these issues could be contained and explored in a supportive yet task-focused manner. I feel it is a testament to the tenacious approach of all parties that these challenges and issues were managed in a way that enabled the SUF role to be tested and sustained throughout the entire PEd stream programme.
Service user involvement in addressing practical issues, such as what venues to use and accessible transport options, provided valuable insight into the accessibility of the therapy to participants. Two examples of this were:
-
having connections with the ‘ring and ride’ service that enabled a participant to attend therapy despite anxiety issues when using public transport
-
not having meetings before 11 a.m., as some people find early mornings difficult, which may be a result of taking certain medications.
The PEd and PS were delivered in successive waves over 2.5 years in East Midlands and 2 years in the north-west, with participants being followed up for 96 weeks. To keep participants informed as to where the research was up to, we set up ‘get-together sessions’ after the 96-week final assessments were complete. We brought members of both the PEd and PS arms together with the CF and SUFs from their session. At these sessions we shared information about the number of participants taking part and where we were up to in the recruitment and follow-up cycle. We also took this opportunity to collect participant feedback on the sessions and collated ideas on how to disseminate research results and implement findings into services. This information was used to develop a dissemination strategy.
Trust commissioners, the PARADES Principal Investigator and a SUR are working to implement evidence-based PEd into frontline services. The SUR role in this includes:
-
recruitment training and supervision of SUFs
-
sensitivity training for therapists
-
translating materials to meet the needs of specific audiences
-
recruitment of participants to PEd programmes.
Workstream 2: anxiety and bipolar disorder
Anxiety is a component of BD that SU and clinicians often see differently. Clinically, it is often seen as a separate disorder, and as a result, sometimes people are treated as if they have ‘dual diagnoses’, as opposed to BD with anxiety as a component. In reality, this translates to people being signposted to one service for BD and another for anxiety, or receiving help for only one of these issues. This practice is not something SUs have generally found helpful, as it does not help integrate these experiences or give a consistent message on how to best self-manage in relation to them.
The anxiety WS demonstrates again how engaging SUs in the initial stages of the research process provides invaluable information that can influence the relevance of the research for a particular target group. In the first phase of this WS, in-depth interviews with individuals provided invaluable insight from SUs into how important researching anxiety was to SUs and what type of support they wanted. These interviews were also conducted in part by a RA who was themselves a declared SU. The strength of these views not only provided excellent research data, but was also the momentum that drove the study forward.
In phase 2 of the study, SU focus groups provided data on SU views regarding preferences for the content and delivery of the AIBD therapy. This included where the therapy took place, how frequent and how long sessions were, the desired tone and foci of sessions and the type of support materials that people wanted.
Both RAs were involved in the analysis of the qualitative interview and focus group data, along with the rest of the research team. As one of the RAs was also a declared SU, he was able to bring this perspective to the analysis.
In phase 3 the SU contributed to liaison with referrers to aid recruitment and was involved with the interpretation of in-depth qualitative interviews with recipients of the AIBD therapy.
Workstream 3: alcohol and bipolar disorder
The associations between the use of alcohol and/or cannabis and symptoms of BD are often not made, or, at best, misunderstood by the person affected and their care team. Frequently, clinicians report believing that the person affected has a mental health problem that is separate to the use of alcohol or cannabis use. This perception is often a barrier to SUs seeking appropriate and effective services that integrates BD with that of alcohol and cannabis use.
The initial (Q-Sort) study highlighted reasons for use and consequences of alcohol and cannabis use in BD, highlighting the greater likelihood of perceived negative effects with alcohol use. This could not have been achieved without SUs being willing to share their own personal experiences. A SU-focus group then highlighted some basic principles to underpin the planned integrated therapy, including the importance of a collaborative relationship between the therapist and the client.
In phase 3 the SUR worked with the RAs as part of the recruitment team, promoting the trial to both SUs and clinicians. The SUR was also part of the qualitative team and brought a SU perspective to the analysis of the in-depth interviews with participants who had received the MI-CBT intervention. The alcohol WS has therefore not only developed an integrated MI-CBT approach but has also drawn attention to an issue that affects many people with a diagnosis of BD.
Workstream 4: suicidality
This WS involved the use of National Confidential Enquiry databases and in-depth qualitative interviews with SUs with experience of self-harm and carers who had lost a relative with BD to suicide. The WS RA sought advice from the PARADES SURG to help frame the interviews and consider how best to deal with this topic sensitively. Advertising material was also reviewed and approved by members of the PARADES SURG. These advertisements were then placed in the newsletter of the national charity for BD, Bipolar UK (Pendulum). The topic guide for the qualitative section of this study was also reviewed and approved by the PARADES SURG. The interview was piloted with a SU. Feedback was requested on the experience of taking part in the interview, its content and the competence of the RA. Feedback on this process was positive. SU and carer involvement was therefore particularly important for this WS. In response to the SURG input, an additional stage was added post-interview in the offer for a follow-up telephone call on the day following the interview for a debrief, with further support provided if required. Talking about issues around suicide is something that clinicians may avoid and they may feel anxious about making ‘clumsy’ comments that may cause distress to those affected. There is a misconception that people affected in some way by suicide do not want to talk about it. In fact, the SU and carers who contributed to and/or took part in this study indicated that this is not the reality. Given the opportunity, SU and carers often want to talk about their experiences in relation to suicidality, but fear upsetting or worrying others. Therefore, the obstacle is often that they lack access to safe non-judgemental space to engage in such conversations.
Workstream 5: Mental Capacity Act 2005
The fluctuating nature of BD complicates planning for care, including dealing with work issues and finances if a person were to become unwell. Consultations with SUs and carers confirmed that there was a lot of ambiguity, not just with people affected by BD but also clinicians and professionals in general. Initially, five SUs were recruited from the PARADES advisory panel to consider the design of quantitative surveys of professionals and SUs concerning knowledge and use of advance planning indicated by the MCA. The panel then reviewed suggestions from these discussions, and their thoughts and ideas were then incorporated into the development of the overall surveys (see Appendices 4 and 6). The interviews raised a variety of questions about the role of capacity in psychiatric practice, particularly as it relates to BD, in which capacity may only be lost temporarily during periods of relapses rather than permanently (Mudigonda et al. , 2015, unpublished).
In addition to the surveys, in-depth qualitative interviews were conducted with SUs drawing, in part, from the SU Advisory Panel for the larger PARADES programme, and in part through an advertisement posted in Pendulum, the magazine of Bipolar UK. The initial findings of these interviews were discussed with a SU advisory group based at the Spectrum Centre for Mental Health Research at the University of Lancaster. The work in this stream highlighted the need for a ‘user friendly’ and legally accurate guide to MCA. To express our thanks to participants to complete the surveys, a guide relating to advance planning under the MCA specifically for people with BD was written. A revised version has been given a significantly improved design by the EMAHSN and is being distributed both online and at a variety of SU events at which the results of the overall PARADES programme are discussed (see Appendix 5). The guide is provided to all new members of Bipolar UK, has been downloaded from the EMAHSN website over 50,000 times and been rated as useful by 95% of readers according to the EMAHSN impact report.
Outcomes for service users
The PARADES programme has employed 14 SUs across the programme, including 10 SUs who were trained to deliver therapy as part of group PEd and PS, two SU grant holders and two SURs. In addition, a number of SURG members have provided input and been reimbursed for their time spent attending meetings and out-of-pocket expenses. Three of the SUs involved in the PARADES programme progressed to postgraduate training, and several others to consistent employment after an interrupted work history. Three SUs involved with the programme have been co-authors of academic papers to date. Overall, this indicates the positive impact of the programme on the individuals involved in delivering it, in addition to those participating in the programme of research. A SUR also obtained a Winston Churchill Memorial Trust Travelling Fellowship while employed on the PARADES programme and used this opportunity to share her experiences of SU involvement and to learn from other colleagues in Canada and USA. Table 74 illustrates the extent of participant involvement in the programme overall and scale of the programme’s engagement of SUs and professionals.
| n | Activity type |
|---|---|
| People with BD | |
| 304 | PEd and PS groups |
| 72 | Anxiety RCT and 10 focus groups |
| 44 | ABLE RCT and two interviews |
| 11 | Self-harm interviews |
| 544 | Advanced directives survey |
| Relatives of people with BD who died by suicide | |
| 11 | Interviews |
| Psychiatrists | |
| 650 | Advance directive survey and eight interviews |
Dissemination
An end-of-project conference was planned right from the beginning to disseminate findings and to provide all participants with information in regards to the ‘what happens next’. Two events were planned, one in each of the two regions. The first, for the East Midlands region, took place on 3 December 2014 at Notts County Football Club (68 SUs and 49 HPCs registered; 79 attended), and the second, for the north-west region, took place on 12 January 2015 at the Manchester United football ground (43 SUs and 48 HPCs/researchers; 91 attended). The audience at both events was a mix of SUs, their carers, clinicians and researchers. Available study results and relevant implementation of the research into practice was the main focus of the day. All WS leads, the programme manager, some therapists, a SUR and trust commissioners attended. Participants that could not or chose not to attend were offered copies of the conference packs and outline results.
All delegates at the events were asked to complete a feedback form; 68 forms returned were analysed. Feedback was generally very positive, with attendees appreciating being invited to the event to hear about the various WSs (Table 75). The strengths of the events described were the opportunity for networking, meeting new people and catching up with friends, a strong SU presence, a mixture of presenters and information provided by Bipolar UK.
| Event | City (%) | |
|---|---|---|
| Nottingham | Manchester | |
| Presentations | 94 | 97 |
| Workshops | 72 | 79 |
| Overall experience | 97 | 96 |
In both locations, presentations from each of the WS leads took place in the morning. Some sample comments are presented below:
Very accessible speakers. Evidence, passion, patients and practice in one place, was quite a surprise.
Nottingham
Really interesting, learnt a lot, great to see all the enthusiasm from researchers.
Manchester
The afternoon session was set aside for workshops, with attendees being able to choose to attend two of the four workshops being run:
The workshops were good. They encouraged debate and discussion from both professionals and service users. It was good to be in an environment where there are no obvious demarcations between professionals and service users.
Nottingham
Some negative feedback was, however, received after the first event:
Workshops should be more interactive – too much talking at us.
Nottingham
Conferences need a shake up! Sitting listening for hours is not good for people, let alone people on medication? Seating needs re-arranging to make it more inclusive rather than traditional arrangement.
Nottingham
This helped us to refine our plan for the Manchester event and to make the workshops in the afternoon more interactive. SUs who had taken part in the intervention therapies developed in WS2 and WS3 were invited to share their experiences:
I loved the research and I spoke to someone with bipolar who also was a professional. She was also coping with working, life and the mental stuff. We had a laugh about our symptoms and how we cope. I realised that I had never spoken openly to someone with bipolar before, ever. I had clients with bipolar but I was in professional mode. I found the experience very moving.
Manchester, received via e-mail
Even though the afternoon workshops provided the opportunity for attendees to hear about SU participation, some felt this should have also been implemented during the morning presentations too:
Aware of the depth of service user involvement in PARADES but not reflected by the presentations.
Manchester
For clinicians and commissioners, the impact of hearing directly about the impact of participation in PARADES was clear:
As a healthcare professional the information I learnt regarding psychoeducation. I will try to take that to the service I work within and try to develop our service for better service user care.
Nottingham
I would like to see more psychological treatments being offered – groups appear to have an evidence base – could be a good way of meeting larger group in my work setting [Community Mental Health Team].
Manchester
The legacy of the PARADES programme has been that 16 NHS staff across the participating trusts have been trained to deliver group PEd and PS, three to deliver the anxiety and bipolar CBT intervention and four to deliver the alcohol and bipolar MI-CBT intervention.
Other methods of dissemination tools utilised were:
-
tweets (throughout the project as well as on the 2 days of the dissemination conferences)
-
articles in Bipolar UK’s Pendulum and group newsletters
-
attending events and giving out information (e.g. giving out the MCA booklet); examples of events attended by a SUR include (a) Stockport Carers Conference 2015; (b) RETHINK Conference 2015; and (c) Bipolar UK Conference, London 2015.
Dissemination is obviously ongoing (e.g. SUR attendance at INVOLVE conference and information disseminated through North West People in Research website). As we know, dissemination is a ‘broad’ term and happens organically when SUs are provided with accurate, relevant and accessible information.
Chapter 8 Discussion
The successful completion of the PARADES programme has significantly advanced knowledge with respect to group and individual psychological interventions, risk factors for self-harm and suicidality, and the application of mental capacity legislation in practice for BD.
Our adaption of PEd and PS groups for delivery in a UK NHS setting showed that it was possible to deliver both of these successfully in a partnership between health professionals and SUFs. We had originally intended to compare group PEd with TAU to provide a measure of the effectiveness of this intervention in relation to routine standard care. However, on advice from the Programme Grants for Applied Research selection panel, the comparison of group PEd versus PS was undertaken. This has the advantage of indicating relative benefits of one intervention over the other, but the disadvantage that it does not indicate the benefits of either with respect to routine care. We successfully recruited 304 participants to the RCT, with the interventions delivered in equal proportion by the same therapists in 22 groups in inner-city, urban and rural locations across two regions in England. Participants with BD 1 or BD 2 had a history of relapse in the preceding 2 years, but were not in a mania-type episode or a depression episode at the time of recruitment to the study. In keeping with the design of pragmatic effectiveness RCTs, there were few other exclusion criteria. Around 85% of participants referred to the study were contactable, met criteria for the study and were recruited into the study, and 78% of therapy participants attended more than one session, indicating that overall the trial design and the treatments offered were acceptable to participants. The high number of participants and groups, high acceptance rate, broad inclusion/exclusion criteria and representativeness of participants compared with other participants in previous RCTs of psychological interventions for BD indicate that the RCT has high generalisability to routine NHS clinical practice. Qualitative interviews with participants who dropped out from both PEd and PS showed that dropout from PS only was related to the lack of structure of groups and dominance by some participants.
Overall, there was no statistical difference in outcome on the primary outcome of time to next bipolar episode between the treatment arms despite PEd delaying relapse by a median of 19.1 weeks compared with PS. However, in the 13% of the sample who had experienced between one and seven previous bipolar episodes, there was a large and statistically significant moderator effect on the primary outcome in favour of PEd versus PS. In terms of secondary outcomes, there were three statistically significant differences in outcome, with improvements in time to next mania episode, interpersonal function and quality of life. There was a trend towards significant improvement in self-rated anxiety in the immediate post-treatment effect, but this was not maintained by 2 years. There was also a large effect on time to the next depression episode in those with one to seven bipolar episodes. The overall net cost of PEd was £1098 higher than that of PS, with a QALY gain of 0.023. In relation to TAU, PEd is likely be cost-effective, with a reduction of > 15% in probability of relapse.
This pattern in favour of PEd was despite both groups overall doing better than those receiving standard TAU based on comparison data from previous group and individual psychological therapy trials. 32,72,79 Attendance rates were significantly higher for PEd than for PS, and there was evidence from the qualitative interviews that the lack of structure in PS was frustrating for some participants and that these groups were also rather more vulnerable to being dominated by a small number of participants.
We employed mixed-methods approaches to investigate comorbidity in BD. With respect to comorbid anxiety, both in-depth qualitative interviews and focus group data highlighted that this was regarded as an important problem by SUs and that many wanted psychological help with this that integrated techniques for anxiety with techniques relevant to general management of BD. A novel integrated CBT intervention for anxiety in the context of BD (AIBD) was developed, informed by the qualitative and focus group work as well as the current evidence base for effective interventions for anxiety and BD. 1,149 This was evaluated in a feasibility and acceptability trial. The trial design appears feasible, with recruitment and retention to target and no evidence for differential dropout. In terms of the intervention, services users attended over 75% of sessions offered, which compares well with published therapy trials in BD. 73,184 Clients’ ratings of therapy were positive and consistent with responses obtained from in-depth qualitative interviews post therapy. There were no significant differences between the intervention and TAU arms on measures of anxiety, mood, relapse or personal recovery. There was a pattern of improved self-reported anxiety to the 48-week follow-up and improved personal recovery to end of therapy in those receiving the intervention, but these were not sustained to the final, 80-week follow-up. Observer-rated mood symptoms were low throughout the study and recurrence rates for mood episodes were low in both arms. A moderation analysis to explore whether or not number of previous episodes or baseline anxiety influenced clinical outcomes in response to AIBD was not significant, but the pattern of results suggested that response to this intervention might be clearer in those with more previous episodes.
We also investigated comorbid substance use in BD using Q-Sort and focus group methods to understand reasons for, and consequences of, use, as well as SU and clinician perspectives on psychological therapy. This work indicated that, whereas cannabis was associated with mood management reasons for use and positive after-effects, alcohol was linked to social and enhancement reasons for use and more strongly with negative after-effects. In terms of therapy, both pilot data218 and the focus group work highlight the importance of a therapy that addresses both alcohol and bipolar issues in a flexible, non-judgemental manner focusing on the individual goals of the SU. A novel intervention combining MI with CBT to address alcohol use in the context of BD was developed, informed by Q-Sort and focus group work as well as our pre-PARADES pilot work and current evidence. 1,326 A feasibility and acceptability RCT was conducted comparing this intervention with TAU. The trial design appeared feasible, with recruitment to 92% of original target and retention to target. As with the AIBD trial above, there was no significant difference in retention between arms. The intervention itself seemed to be feasible and acceptable, with participants attending 88% of sessions offered and therapists rating success in achieving shared problem formulation around alcohol in over 80% of participants and at least moderate improvement in clinical outcome in two-thirds of those offered therapy. In-depth qualitative interviews post therapy were also predominantly positive about the style of therapy, and mood and alcohol outcomes. There were no significant differences with respect to quantitative measures of alcohol use, mood, relapse or social functioning. The pattern was of reduced alcohol use and increased abstinence in both arms, although this finding was not significant. Mood recurrence rates were exceptionally low for both arms of the study. Moderation analyses with respect to number of previous episodes and severity of alcohol problem at baseline were not significant with respect to mean alcohol use or percentage binge days, although there was numerical benefit of MI-CBT for those with milder alcohol problems in reducing binge days.
Our investigations of suicide and self-harm in bipolar combined both survey data and in-depth qualitative interviews. An analysis of the NCI database indicated that 10% of psychiatric patients who died by suicide had BD. People with BD who died by suicide were more likely to be female, to have had a chronic and recurrent illness course, and to have been in recent contact with services compared with people with other diagnoses who died by suicide. Interrogation of the MaSH database indicated that, compared with other individuals who self-harm, self-harmers with BD are more likely to experience recurrent episodes, more likely to be female, middle aged, unemployed and in contact with services and more likely to suffer from sleep disturbance. In-depth qualitative interviews with carers of individuals who had completed suicide and individuals with bipolar with a self-harm history indicated that both wanted prompt access to good-quality care and access to information about diagnosis and associated risks. Consistent with findings above for therapy trials, carers also highlighted the importance of a collaborative approach to care and noted the importance of trust and continuity of care in improving outcomes with respect to self-harm and suicide.
The final stream of the programme focused on the MCA and its application in BD from the perspectives of both clinicians and SUs with BD. National survey data indicated that, although SUs were positive about advance planning, they rarely used the MCA in relation to this, largely as they were unaware of it, and clinicians did typically not promote it. Clinicians indicated that they were more likely to promote the MCA actively if they had received good-quality training, but, even then, nearly half would still not discuss it.
Thus, although training and information sharing are important in this area, there also appear to be challenges in relation to how adoption of MCA principles fit with models of routine care. Interviews with clinicians and SUs indicated that the greater autonomy offered by the MCA approach conflicts with a paternalistic approach to care in general. In addition, interviews with clinicians highlighted major shortcomings in trust policies with respect to MCA that rendered attempts to adopt its provision either difficult or impossible in many cases. Interviews also highlighted the importance of distinguishing between collaborative recovery plans and the advance planning under MCA, as some individuals appeared to view these as essentially synonymous.
Specific outputs from the programme
The first WS has developed and definitively evaluated a model of service delivery of PEd, employing the skills of a multidisciplinary group of clinicians and SUFs. This intervention was enthusiastically received by participants, and, in response to both positive trial outcomes and SU demand, the group PEd intervention is already being rolled out in the East Midlands and the north-west through Manchester Mental Health and Social Care Trust, the host trust for the PARADES programme.
From WS2 and WS3 we have provided important additional information on the importance, impact and treatment of comorbid anxiety and alcohol use in BD. We have also developed therapy manuals for the delivery of CBT-informed therapy for anxiety comorbid with BD and for integrated MI-CBT therapy for BD with alcohol use. These manuals summarise flexible, integrated collaborative approaches consistent with SU requirements, as well as clinical tools based on current evidence. Further adaptions to these manuals will be informed by additional analysis of current data to clarify appropriate targeting of interventions.
Workstream 4 has highlighted important factors associated with individual and service characteristics linked to risk for suicide and severe self-harm and their inter-relationships. This information will be summarised as part of brief ‘learning points’ for clinical practice for Manchester Mental Health and Social Care Trust as the lead trust, which can also be shared across the NHS nationally and has implications for both training and service development.
Workstream 5 has demonstrated the importance of the MCA for SUs and the key challenges in terms of clinical training and clinical ethos that need to be met to improve its adoption, particularly with respect to advance directives. The findings from this work led our team to develop a booklet on advance planning, which has been widely adopted by trusts and individual SUs and carers. This work has also fed into a House of Lords review of mental capacity.
We have disseminated our research findings through the publications listed in Acknowledgements. There is a series of trial outcome papers in preparation in relation to each of the intervention streams that will be supplemented by qualitative papers on people’s experiences of each form of therapy. Further papers are planned that draw on the programme-wide data (particularly WSs 1–3) to explore psychological, clinical and sociodemographic influences on cross-sectional and longitudinal outcomes in BD.
Future research
The results of the PEd and PS trial indicate potential benefits of group PEd in terms of acceptability, clinical outcomes and cost, which suggest that this approach should be considered for implementation in the NHS. Further research would be helpful to understand what factors facilitate or impede successful implementation in clinical practice and the impact of such implementation on routine clinical outcomes. Additionally, there is scope to further understand for whom this intervention is most helpful. Our data indicate that the strongest effects occur in individuals with fewer episodes, and an appropriately powered trial focused on early BD would be important to definitively test this. Additionally, our data indicate that not all participants engaged fully with a group approach (37% of PEd and 53% of PS attended fewer than half of the available sessions). It is, therefore, appropriate to investigate the extent to which individual PEd interventions might offer an alternative option for individuals who find it difficult to engage with a group setting.
The feasibility and acceptability outcomes for the AIBD and ABLE trials indicate that it is possible to deliver an integrated psychological approach for comorbidity. Further research is required to explore the extent to which such approaches are effective in addressing symptoms and functioning in BD. In particular, it will be relevant to explore the extent to which these approaches need to be tailored for individuals with more severe courses of illness and whether or not such interventions are more effective with individuals of more recent bipolar onset. Additionally, the research in both streams has highlighted the importance and impact of comorbid conditions in BD. In contrast with much previous actuarial research, this work presents data from the perspective of the participant. Further research could helpfully apply this SU-focused methodology to understanding more about the impacts of other comorbid psychological and physical factors in the course and outcome of BD.
The suicide and self-harm research highlighted a number of risk and protective factors associated with suicidal behaviour in BD, as well as describing a number of areas where mental health care for people with suicidal behaviour and BD has been poor. Along with contributing to the existing knowledge base, the results of this work could help researchers to develop tailored information and training that emphasise the high risk of suicidal behaviour in BD and how to access help, as well as more generally feeding into improvements in risk detection and prevention of suicidal behaviours in BD. The MCA stream indicates the importance of improving knowledge and application of advance directives in BD. Further research in this area could use the results of our work to inform improved training plans for clinicians and SUs and evaluate the impact of such work on application of the MCA in practice.
Clinical implications
This programme of research highlights the crucial importance of psychological and social factors in the course and outcome of BD. Furthermore, there is evidence that when offered the opportunity, many clients are keen to engage with psychological therapy to help understand their experiences and to develop additional adaptive coping strategies. These approaches are consistent with the national impetus towards recovery-focused interventions that enhance self-management and functioning. However, as the MCA research has indicated, there is a challenge for services in fully adopting approaches that recognise client autonomy and choice, as it tends to clash with a more patrician medical model in which the clinical expert is seen as knowing best. Across the therapy trials of the PARADES programme, participant engagement was facilitated by a flexible, collaborative approach to therapy aided by ensuring that SUs were involved in the development of each stage. Such approaches to engagement are relevant to the effective delivery of a wide range of care within clinical services, which could themselves benefit from genuine collaboration with SUs in the refinement of service content and delivery.
Overall, the RCT suggests that, given a choice between PEd and PS, there are some benefits for PEd in acceptance and effectiveness, particularly in relation to people with up to seven previous bipolar episodes. Clinical services should consider adoption of the PEd intervention based on clinical outcome and health economic data was well as evidence of client demand for this type of approach. Work on anxiety and alcohol interventions is at an earlier stage, but it is already clear that clients do not want to have these comorbid problems dealt with as separate from their BD and that they want psychological, as well as pharmacological, treatment options. Services should also be aware of the limitations of current assessments of risk in relation to suicide and self-harm. These might be improved by reference to risk indicators in the current programme. Findings from our work with relatives and individuals who have self-harmed also highlight the importance of engaging and listening to relatives as a crucial source of risk information. Finally, training staff and SUs in the application of the MCA would be both consistent with the intentions of the Act and likely to improve engagement and clinical outcomes, as clients then, as far as possible, receive interventions they have found to be helpful and avoid interventions that they do not wish to have.
Acknowledgements
We would like to thank the following: Manchester Mental Health and Social Care NHS Trust who hosted this programme (especially Matthew Collier for his support in the financial administration of this programme and the research and innovation team); the MHRN and Clinical Research Networks along with the NHS trusts and GP practices who helped promote the research among their SUs and all those who referred into our various studies; members of the PARADES PEd TSC; Shirley Reynolds, John Belcher, Thomas Meyer, Tim Rawcliffe, Andrew Gumley and David Miklowitz who advised and monitored throughout the programme on WSs 1–3; the numerous staff who have worked on the programme over its entirety (listed below); and the third-sector organisations who gave us the opportunity to attend their meetings to promote our research to their members.
We also express our sincere thanks to all those participants who shared their views which helped shape the development of this research, to those who responded to surveys, took part in the RCTs and provided extensive feedback through numerous questionnaires or agreed to be interviewed about their experiences to help further refinements.
Finally, we would like to thank Academic Health Sciences Network East Midlands and Collaboration for Leadership in Applied Health Research and Care East Midlands for their assistance in the production and promotion of our ‘Advance Planning for People with Bipolar Disorder’ booklet and running the Nottingham dissemination event at Notts County, both of which helped with the subsequent event held at Manchester United. We are delighted that following attendance at this event by part of the City Wide commissioning team, that North, South and Central Manchester Clinical Commissioning Groups have recently begun the process of rolling out group PEd. Newark and Sherwood and Nottingham City Clinical Commissioning Groups have both commissioned the group PEd programme following lobbying from patients and local mental health groups.
Staff who worked on the PARADES programme are as follows.
-
Ali Beck
-
Arun Chopra
-
Ayaz Quereshi
-
Ayesha Ahmed
-
Brian Langshaw
-
Caroline Clements
-
Chris Roberts
-
Christine Barrowclough
-
Claire Hilton
-
Dawn Knowles
-
Debbie Mayes
-
Dionysios Ntais
-
Elizabeth Camacho
-
Elly McGrath
-
Emily Goodman-Smith
-
Emma Weymouth
-
Fiona Lobban
-
Fiona Holland
-
Georgia Lykomitrou
-
Glyn Judge
-
Heather Robinson
-
Ian Wilson
-
Jane Fisher
-
Jelena Jovanoska
-
Karthik Thangavelu
-
Kat Taylor
-
Katherine Berry
-
Kathryn Reeveley
-
Kay Hampshire
-
Kiran Pindiprulu
-
Kirsten Nokling
-
Kirsty Stevenson
-
Linda Davies
-
Lisa Brown
-
Lisa Riste
-
Lizzie Tyler
-
Lorraine Getten
-
Lucy Bateman
-
Lynda Fretwell
-
Lynsey Gregg
-
Mahesh Nachami
-
Marcus Barker
-
Matthew Impey
-
Mike Fitzsimmons
-
Mohan Mudigonda
-
Nancy Black
-
Natasha Lyon
-
Nav Kapur
-
Nick Todd
-
Paul Hammersley
-
Peter Bartlett
-
Phil Byrne
-
Puru Pathy
-
Rachel Lambourne
-
Rebecca Anderson
-
Rebecca Owen
-
Rebecca Sutton
-
Richard Morriss
-
Richard Pattison
-
Rita Long
-
Ros Hartwell
-
Sara Zarbakhsh
-
Sarah Peters
-
Sian Newman
-
Steven Jones
-
Steve Kendall
-
Sue Lomas.
Contributions of authors
Professor Steven Jones (Professor of Clinical Psychology and Co-Director of Spectrum Centre for Mental Health Research at Lancaster University) was the chief investigator for the PARADES programme, contributing methodological and practical input to all programme WSs as well as being responsible for the conduct of the programme overall. He led the anxiety WS and co-led the alcohol and bipolar WS. He contributed to supervision of the group PEd and PS therapy team. He contributed to the design, conduct, data analysis and interpretation of many of the studies, and took a lead on writing this report.
Dr Lisa Riste (Research Fellow, Programme Manager, The University of Manchester & Manchester Mental Health & Social Care NHS Trust) was responsible for the day-to-day running of the programme, staff recruitment, training and supervision, oversight and reporting of all RCTs, and provided data quality and checking across the programme. She co-authored this report with the respective WS leads and checked it for accuracy and completeness.
Professor Christine Barrowclough (Emeritus Professor in Clinical Psychology, The University of Manchester) led the alcohol and bipolar WS, supervised the ABLE therapists and devised the WS. She contributed to the draft papers arising from the WS, which are due to be submitted shortly.
Professor Peter Bartlett (Professor Mental Health Law, University of Nottingham) co-led the advance directives WS providing the mental health law input, and led the write- up and preparation of these results for publication.
Ms Caroline Clements (Research Associate in the Centre for Suicide Prevention at The University of Manchester) conducted all three phases of the suicidality WS, including qualitative interviews, and co-wrote and prepared these results.
Professor Linda Davies (Professor of Health Economics, The University of Manchester) was lead for the health economic analyses provided for the programme and prepared the results for publication.
Ms Fiona Holland (Research Associate in Biostatistics at The University of Manchester) ran the statistical analyses for WSs 1–3 and prepared the results for these sections.
Professor Nav Kapur (Professor of Psychiatry and Population Health, Head of Research at the Centre for Suicide Prevention at The University of Manchester and Honorary Consultant in Psychiatry at the Manchester Mental Health and Social Care Trust) led the suicidality WS and co-wrote this chapter.
Professor Fiona Lobban (Professor of Clinical Psychology and Co-Director of Spectrum Centre for Mental Health Research at Lancaster University) was the north-west lead for the group PEd WS and wrote and prepared these results for publication, as well as providing lead supervision to the group PEd and PS therapy team in the north-west.
Ms Rita Long (SUR, Lancaster University) wrote the SU involvement chapter, co-chaired the SURG of the Spectrum Centre for Mental Health Research and advised on the development of the PARADES programme. She also contributed to thematic analysis of AIBD and ABLE outcome interviews and to the delivery of group PEd and PS in the north-west.
Professor Richard Morriss (Consultant in General Adult and Community Psychiatry with Nottinghamshire Healthcare NHS Trust and Professor of Psychiatry and Community Mental Health at the University of Nottingham) was the lead for the group PEd WS in East Midlands, and provided supervision to the group PEd and PS therapy team there. He was co-lead for the advance directives WS, and co-wrote and prepared these results for publication.
Dr Sarah Peters (Senior Lecturer, Health Psychology, The University of Manchester) led the qualitative analyses across all elements of the PARADES programme and contributed to preparation of results for the monograph and linked publications.
Professor Chris Roberts (Professor of Biostatistics at The University of Manchester) advised on the statistical design of WSs 1–3, advised and procured randomisation processes for these and oversaw the data analysis and writing of the report.
Dr Elizabeth Camacho (Research Fellow in Health Economics, Manchester Centre for Health Economics, The University of Manchester) drafted the early versions of Chapter 2b and provided substantive comments on the final version.
Dr Lynsey Gregg (Lecturer in Health and Clinical Psychology, The University of Manchester) led work on the development of the Q-Sort methodology and analysis used in WS3 and prepared the results of this for papers and this report.
Mr Dionysios Ntais (Health Economics and Outcomes Research Analyst at IMS Health, previously Research Associate in Health Economics, Manchester Centre for Health Economics, The University of Manchester) drafted the early versions of Chapter 2b and provided substantive comments on the final version.
Publications
Theses
Work completed as part of the PARADES programme has also been written up and successfully submitted in respect of the following theses:
Black N. Exploring Experiences of Substance Use in Bipolar Disorder: A Q Methodology Design. MPhil thesis. Manchester: University of Manchester; 2011.
Hampshire K. The Nature and Experience of Anxiety in Bipolar Disorder. PhD thesis. Lancaster: Lancaster University; 2014.
Robinson H. Self Regulation to Understand Escalation of Mood Swings in People with Bipolar Disorder. PhD thesis. Lancaster: Lancaster University; 2014.
Sutton R. Barriers and Facilitators to Engaging People in Clinical Trials: A Qualitative Secondary Analysis of Three Psychological Trials from the PARADES Programme for Bipolar Disorder. MRes thesis. Manchester: University of Manchester; 2015.
Workstream summaries, materials and presentations available via our programme website (www.PARADES-bipolar.co.uk) and conference presentations.
Reports
When relevant, we have also shared our findings with the relevant government organisations, including responding to a call for evidence where we were able to supply evidence directly from WS5:
House of Lords Select Committee on the Mental Capacity Act 2005. Mental Capacity Act 2005: Post-Legislative scrutiny, HL (2013 to 14) 139 Oral and Written Evidence vol II, pp. 1689–93. 2014. URL: www.parliament.uk/documents/Mental-Capacity-Act-2005/mental-capacity-act-2005-vol2.pdf (accessed October 2016).
Summary reports have been, or are in the process of, being written for the public, commissioners and clinicians in collaboration with our implementation and dissemination groups:
Long R. How Can ‘Service Users’ be Best Involved in Work? (Part 2). Presented to Manchester Mental Health & Social Care NHS Trust.
Peer-reviewed publications arising from the research programme
Morriss RK, Lobban FJS, Riste L, Peters S, Roberts C, Davies L, et al. Pragmatic randomised controlled trial of group psychoeducation versus group support in the maintenance of bipolar disorder. BMC Psychiatry 2011;11:214.
Clements C, Morriss R, Jones S, Peters S, Roberts C, Kapur N. Suicide in bipolar disorder in a national English sample, 1996–2009: frequency, trends and characteristics. Psychol Med 2013;43:2593–602.
Jones S, McGrath E, Hampshire K, Owens R, Riste L, Roberts C, et al. A randomised controlled trial of time limited CBT informed psychological therapy for anxiety in bipolar disorder. BMC Psychiatry 2013;13:54
Mudigonda M, Bartlett P, Morriss R, Chopra A, Jones S. Advance Planning for People With Bipolar Disorder. EMAHSN and EMCLAHRC. 2014. URL: http://emahsn.org.uk/mental-health/bipolar-disorder/ (accessed 12 October 2017).
Clements C, Jones S, Morriss R, Peters S, Cooper J, While D, et al. Self-harm in bipolar disorder: findings from a prospective clinical database. J Affect Disord 2015;173:113–19.
Tyler E JS, Black N, Carter L, Barrowclough C. The relationship between bipolar disorder and cannabis use in daily life: an experience sampling study. PLOS ONE 2015;10:e0118916.
Bartlett P, Mudigonda M, Chopra A, Morriss R, Jones S. Planning for incapacity by people with bipolar disorder under the Mental Capacity Act 2005. J Soc Welf Fam Law 2016;38:3, 263–86.
Morriss R, Mudigonda M, Bartlett P, Chopra A, Jones S. National survey of training of psychiatrists on advance directives to refuse treatment in bipolar disorder. BJPsych Bull 2017;41:320–4.
Jones SH, Knowles D, Tyler E, Holland F, Peters S, Lobban F, et al. The feasibility and acceptability of a novel anxiety in bipolar disorder intervention compared to treatment as usual: a randomized controlled trial [published online ahead of print July 19 2018]. Depress Anxiety 2018.
Jones S, Robinson H, Riste L, Roberts C, Peters S, Bateman L, et al. Integrated psychological therapy for people with bipolar disorder and co-morbid alcohol use: a feasibility and acceptability randomised controlled trial. Contemp Clin Trials Communn 2018;10:193–8.
There are a series of papers in preparation from WSs 1–3, including trial clinical outcome reports, health economic outcomes (WS1) and qualitative investigation of comorbidity and therapy experiences.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Bipolar Disorder: The Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care. Clinical Guidance 185. London: NICE; 2014.
- Perlick DA, Hohenstein JM, Clarkin JF, Kaczynski R, Rosenheck RA. Use of mental health and primary care services by caregivers of patients with bipolar disorder: a preliminary study. Bipolar Disord 2005;7:126-35. https://doi.org/10.1111/j.1399-5618.2004.00172.x.
- Woods SW. The economic burden of bipolar disease. J Clin Psychiatry 2000;61:38-41.
- McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price: The Cost of Mental Health Care in England to 2026. London: The King’s Fund; 2008.
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 2007;64:543-52. https://doi.org/10.1001/archpsyc.64.5.543.
- Goodwin FK, Jamison KR. Manic-Depressive Illness. New York, NY: Oxford University Press; 1990.
- Mackin P, Young AH, Wright P, Stern J, Phelan M. Core Psychiatry. Edinburgh: Elsevier Saunders; 2005.
- Judd L, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003;60:261-9. https://doi.org/10.1001/archpsyc.60.3.261.
- Judd L, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002;59:530-7. https://doi.org/10.1001/archpsyc.59.6.530.
- Kupfer DJ, Frank E, Grochocinski VJ, Cluss PA, Houck PR, Stapf DA. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. J Clin Psychiatry 2002;63:120-5. https://doi.org/10.4088/JCP.v63n0206.
- McIntyre RS, Soczynska JK, Bottas A, Bordbar K, Konarski JZ, Kennedy SH. Anxiety disorders and bipolar disorder: a review. Bipolar Disord 2006;8:665-76. https://doi.org/10.1111/j.1399-5618.2006.00355.x.
- Otto MW SN, Wisniewski SR, Miklowitz DJ, Kogan JN, Reilly-Harrington NA, Frank E, et al. Prospective 12-month course of bipolar disorder in out-patients with and without comorbid anxiety disorders. Br J Psychiatry 2006;189:20-5. https://doi.org/10.1192/bjp.bp.104.007773.
- Cerullo MA, Strakowski SM. The prevalence and significance of substance use disorders in bipolar type I and II disorder. Subst Abuse Treat Prev Policy 2007;2. https://doi.org/10.1186/1747-597X-2-29.
- Frye MA, Altshuler LL, McElroy SL, Suppes T, Keck PE, Denicoff K, et al. Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry 2003;160:883-9. https://doi.org/10.1176/appi.ajp.160.5.883.
- Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Ruan WJ, et al. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 2005;66:1205-15. https://doi.org/10.4088/JCP.v66n1001.
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264:2511-18. https://doi.org/10.1001/jama.1990.03450190043026.
- Feske U, Frank E, Mallinger AG, Houck PR, Fagiolini A, Shear MK, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry 2000;157:956-62. https://doi.org/10.1176/appi.ajp.157.6.956.
- Frank E, Cyranowski JM, Rucci P, Shear MK, Fagiolini A, Thase ME, et al. Clinical significance of lifetime panic spectrum symptoms in the treatment of patients with bipolar I disorder. Arch Gen Psychiatry 2002;59:905-11. https://doi.org/10.1001/archpsyc.59.10.905.
- Henry C, Van den Bulke D, Bellivier F, Etain B, Rouillon F, Leboyer M. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry 2003;64:331-5. https://doi.org/10.4088/JCP.v64n0316.
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004;55:875-81. https://doi.org/10.1016/j.biopsych.2004.01.022.
- Salloum IM, Thase ME. Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord 2000;2:269-80. https://doi.org/10.1034/j.1399-5618.2000.20308.x.
- Strakowski SM, Keck PE, McElroy SL, West SA, Sax KW, Hawkins JM, et al. Twelve-month outcome after a first hospitalization for affective psychosis. Arch Gen Psychiatry 1998;55:49-55. https://doi.org/10.1001/archpsyc.55.1.49.
- Drug Misuse: Psychosocial Interventions. London: NICE; 2007.
- Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ 2010;341. https://doi.org/10.1136/bmj.c6325.
- National Suicide Prevention Strategy for England. London: Department of Health; 2002.
- Avoidable Deaths. Manchester: National Confidential Inquiry into Suicide and Homicide by People with Mental Illness, University of Manchester; 2006.
- Mental Health Act 1983: Code of Practice. London: The Stationery Office; 2015.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Morriss R, Scott J, Paykel E, Bentall R, Hayhurst H, Johnson T. Social adjustment based on reported behaviour in bipolar affective disorder. Bipolar Disord 2007;9:53-62. https://doi.org/10.1111/j.1399-5618.2007.00343.x.
- Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, et al. Collaborative care for bipolar disorder: part II. Impact on clinical outcome, function, and costs. Psychiatr Serv 2006;57:937-45. https://doi.org/10.1176/ps.2006.57.7.937.
- Simon GE, Ludman EJ, Bauer MS, Unützer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry 2006;63:500-8. https://doi.org/10.1001/archpsyc.63.5.500.
- Bond K, Anderson IM. Psychoeducation for relapse prevention in bipolar disorder: a systematic review of efficacy in randomized controlled trials. Bipolar Disord 2015;17:349-62. https://doi.org/10.1111/bdi.12287.
- Colom F, Vieta E, Sanchez-Moreno J, Palomino-Otiniano R, Reinares M, Goikolea JM, et al. Group psychoeducation for stabilised bipolar disorders: 5-year outcome of a randomised clinical trial. Br J Psychiatry 2009;194:260-65. https://doi.org/10.1192/bjp.bp.107.040485.
- Perry A, Tarrier N, Morriss R, McCarthy E, Limb K. Randomised controlled trial of teaching bipolar disorder patients to identify early symptoms of relapse and obtain early treatment. BMJ 1999;318:149-53. https://doi.org/10.1136/bmj.318.7177.149.
- Donaldson L. The Expert Patients Programme. London: Department of Health; 2006.
- Clinical Guidelines for the Management of Anxiety. London: NICE; 2004.
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O’Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. Am J Psychiatry 2001;158:1706-13. https://doi.org/10.1176/appi.ajp.158.10.1706.
- Weiss RD, Griffin ML, Kolodziej ME, Greenfield SF, Najavits LM, Daley DC, et al. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. Am J Psychiatry 2007;164:100-7. https://doi.org/10.1176/ajp.2007.164.1.100.
- Drake RE, Xie H, McHugo GJ, Shumway M. Three-year outcomes of long-term patients with co-occurring bipolar and substance use disorders. Biol Psychiatry 2004;56:749-56. https://doi.org/10.1016/j.biopsych.2004.08.020.
- Gunnell D, Middleton N. National suicide rates as an indicator of the effect of suicide on premature mortality. Lancet 2003;362:961-2. https://doi.org/10.1016/S0140-6736(03)14367-X.
- Saving Lives: Our Healthier Nation. London: Department of Health; 1998.
- Lifton I, Kettl PA. Suicidal ideation and the choice of advance directives by elderly persons with affective disorders. Psychiatr Serv 2000;51:1447-9. https://doi.org/10.1176/appi.ps.51.11.1447.
- Toprac MG, Dennehy EB, Carmody TJ, Crismon ML, Miller AL, Trivedi MH, et al. Implementation of the Texas Medication Algorithm Project patient and family education program. J Clin Psychiatry 2006;67:1362-72. https://doi.org/10.4088/JCP.v67n0906.
- Strategic Analysis of UK Mental Health Research Funding. London: Mental Health Research Funders Group; 2005.
- Flood C, Byford S, Henderson C, Leese M, Thornicroft G, Sutherby K, et al. Joint crisis plans for people with psychosis: economic evaluation of a randomised controlled trial. BMJ 2006;333. https://doi.org/10.1136/bmj.38929.653704.55.
- Henderson C, Flood C, Leese M, Thornicoft G, Sutherby K, Szmukler G. Effect of joint crisis plans on use of compulsory treatment in psychiatry: single blind randomised controlled trial. BMJ 2004;329. https://doi.org/10.1136/bmj.38155.585046.63.
- Henry C, Sorbara F, Lacoste J, Gindre C, Leboyer M. Antidepressant-induced mania in bipolar patients: identification of risk factors. J Clin Psychiatry 2001;62:249-55. https://doi.org/10.4088/JCP.v62n0406.
- Strakowski SM, DelBello MP. The co-occurrence of bipolar and substance use disorders. Clin Psychol Rev 2000;20:191-206. https://doi.org/10.1016/S0272-7358(99)00025-2.
- Weiss RD. Treating patients with bipolar disorder and substance dependence: lessons learned. J Subst Abuse Treat 2004;27:307-12. https://doi.org/10.1016/j.jsat.2004.10.001.
- Weiss RD, Kolodziej M, Griffin ML, Najavits LM, Jacobson LM, Greenfield SF. Substance use and perceived symptom improvement among patients with bipolar disorder and substance dependence. J Affect Disord 2004;79:279-83. https://doi.org/10.1016/S0165-0327(02)00454-8.
- Healey C, Peters S, Kinderman P, McCracken C, Morriss R. Reasons for substance use in dual diagnosis bipolar disorder and substance use disorders: a qualitative study. J Affect Disord 2009;113:118-26. https://doi.org/10.1016/j.jad.2008.05.010.
- Conrad PJ, Stewart SH. A critical look at dual-focused cognitive-behavioural treatments for comorbid substance use and psychiatric disorders: strengths, limitations and future directions. J Cogn Psychother 2005;19:265-89.
- Angst J, Angst F, Gerber-Werder R, Gamma A. Suicide in 406 mood-disorder patients with and without long-term medication: a 40 to 44 years’ follow-up. Arch Suicide Res 2005;9:279-300. https://doi.org/10.1080/13811110590929488.
- Hawton K, Sutton L, Haw C, Sinclair J, Harriss L. Suicide and attempted suicide in bipolar disorder: a systematic review of risk factors. J Clin Psychiatry 2005;66:693-704. https://doi.org/10.4088/JCP.v66n0604.
- Highet NJ, McNair BG, Thompson M, Davenport TA, Hickie IB. Experience with treatment services for people with bipolar disorder. Med J Aust 2004;181:47-51.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96. https://doi.org/10.1016/S0140-6736(12)61729-2.
- Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care. Updated Edition. CG185. Leicester and London: British Psychological Society and Gaskell; 2014.
- Bipolar Disorder: The Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. CG 38. London: British Psychological Society and Gaskell; 2006.
- VA/DoD Clinical Practice Guideline for Management of Bipolar Disorder in Adults. Version 2.0. Washington, DC: Veterans Administration; 2010.
- Colom F, Vieta E. Psychoeducation Manual for Bipolar Disorder. Cambridge: Cambridge University Press; 2006.
- Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, et al. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 2003;60:402-7. https://doi.org/10.1001/archpsyc.60.4.402.
- Colom F, Vieta E, Reinares M, Martínez-Arán A, Torrent C, Goikolea JM, et al. Psychoeducation efficacy in bipolar disorders: beyond compliance enhancement. J Clin Psychiatry 2003;64:1101-5. https://doi.org/10.4088/JCP.v64n0917.
- Oud M, Mayo-Wilson E, Braidwood R, Schilte P, Jones SH, Morriss R, et al. Psychological interventions for adults with bipolar disorder: a systematic review and meta-analysis. Br J Psy 2016;208:213-22.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. https://doi.org/10.1191/1740774505cn076oa.
- Chiesa A, Malinowski P. Mindfulness-based approaches: are they all the same?. J Clin Psychol 2011;67:404-24. https://doi.org/10.1002/jclp.20776.
- Morselli P, Elgie R, Cesena B. GAMIAN-Europe/BEAM survey II: cross-national analysis of unemployment, family history, treatment satisfaction and impact of the bipolar disorder on life style. Bipolar Disord 2004;6:487-97. https://doi.org/10.1111/j.1399-5618.2004.00160.x.
- Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, et al. The effectiveness and cost effectiveness of a national lay-led self-care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254-61. https://doi.org/10.1136/jech.2006.053538.
- Colom F, Lam D. Psychoeducation: improving outcomes in bipolar disorder. Eur Psychiatry 2005;20:359-64. https://doi.org/10.1016/j.eurpsy.2005.06.002.
- Colom F, Vieta E. A perspective on the use of psychoeducation, cognitive-behavioral therapy and interpersonal therapy for bipolar patients. Bipolar Disord 2004;6:480-6. https://doi.org/10.1111/j.1399-5618.2004.00136.x.
- Coulthard K, Patel D, Brizzolara C, Morriss R, Watson S. A feasibility study of expert patient and community mental health team led bipolar psychoeducation groups: implementing an evidence based practice. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-301.
- Morriss RK, Lobban F JS, Riste L, Peters S, Roberts C, Davies L, et al. Pragmatic randomised controlled trial of group psychoeducation versus group support in the maintenance of bipolar disorder. BMC Psychiatry 2011;11. https://doi.org/10.1186/1471-244X-11-114.
- Lobban F, Taylor L, Chandler C, Tyler E, Kinderman P, Kolamunnage-Dona R, et al. Enhanced relapse prevention for bipolar disorder by community mental health teams: cluster feasibility randomised trial. Br J Psychiatry 2010;196:59-63. https://doi.org/10.1192/bjp.bp.109.065524.
- Jones SH, Smith G, Mulligan LD, Lobban F, Law H, Dunn G, et al. Recovery-focused cognitive–behavioural therapy for recent-onset bipolar disorder: randomised controlled pilot trial. Br J Psychiatry 2015;206:58-66. https://doi.org/10.1192/bjp.bp.113.141259.
- Kessing LV, Hansen HV, Christensen EM, Dam H, Gluud C, Wetterslev J, et al. Do young adults with bipolar disorder benefit from early intervention?. J Affect Disord 2014;152–154:403-8. https://doi.org/10.1016/j.jad.2013.10.001.
- Beshai S, Dobson KS, Bockting CL, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clin Psychol Rev 2011;31:1349-60. https://doi.org/10.1016/j.cpr.2011.09.003.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- First MB, Spitzer RL, Gibbon M, Endicott J. Structured Clinical Interview for DSM-IV Axis 1 Disorders, (SCID-I). Washington, DC: American Psychiatric Press; 1997.
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) 1997.
- Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, et al. Cognitive–behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. Br J Psychiatry 2006;188:313-20. https://doi.org/10.1192/bjp.188.4.313.
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The longitudinal interval follow-up evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 1987;44:540-8. https://doi.org/10.1001/archpsyc.1987.01800180050009.
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992;149:1148-56. https://doi.org/10.1176/ajp.149.9.1148.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. https://doi.org/10.1136/jnnp.23.1.56.
- Williams JB, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol 2008;23:120-9. https://doi.org/10.1097/YIC.0b013e3282f948f5.
- Licht RW, Jensen J. Validation of the Bech–Rafaelsen Mania Scale using latent structure analysis. Acta Psychiatr Scand 1997;96:367-72. https://doi.org/10.1111/j.1600-0447.1997.tb09931.x.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Goodwin GM. Consensus Group of the British Association for Psychopharmacology . Evidence-based guidelines for treating bipolar disorder: revised second edition-recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2009;23:346-88. https://doi.org/10.1177/0269881109102919.
- Service User Experience in Adult Mental Health: Improving the Experience of Care for People Using Adult NHS Mental Health Services. CG 136. Leicester and London: British Psychological Society and Gaskell; 2011.
- Hoyle RH, Georgesen JC, Webster JM. Analyzing data from individuals in groups: the past, the present and the future. Group Dyn 2001;5:41-7. https://doi.org/10.1037/1089-2699.5.1.41.
- Barrowclough C, Haddock G, Lobban F, Jones S, Siddle R, Roberts C, et al. Group cognitive–behavioural therapy for schizophrenia. Randomised controlled trial. Br J Psychiatry 2006;189:527-32. https://doi.org/10.1192/bjp.bp.106.021386.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Boyatzis RE. Transforming Qualitative Information. Cleveland, OH: Sage; 1998.
- Peters S. Qualitative research methods in mental health. Evid Based Ment Health 2010;13:35-40. https://doi.org/10.1136/ebmh.13.2.35.
- Henwood KL, Pidgeon NF. Qualitative research and psychological theorizing. Br J Psychol 1992;83:97-111. https://doi.org/10.1111/j.2044-8295.1992.tb02426.x.
- Morse JM. The significance of saturation. Qual Health Res 1995;5:147-49. https://doi.org/10.1177/104973239500500201.
- Indrayan A, Doi SAR, Williams GH. Methods of Clinical Epidemiology. Berlin: Springer-Verlag; 2013.
- Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol 2008;59:565-90. https://doi.org/10.1146/annurev.psych.59.113006.095941.
- Jones S, Mulligan LD, Higginson S, Dunn G, Morrison AP. The bipolar recovery questionnaire: psychometric properties of a quantitative measure of recovery experiences in bipolar disorder. J Affect Disord 2013;147:34-43. https://doi.org/10.1016/j.jad.2012.10.003.
- How Mental Illness Loses Out in the NHS: A Report for the Centre for Economic Performance’s Mental Health Policy Group. London: LSE; 2012.
- Lam DH, Hayward P, Watkins ER, Wright K, Sham P. Relapse prevention in patients with bipolar disorder: cognitive therapy outcome after 2 years. Am J Psychiatry 2005;162:324-9. https://doi.org/10.1176/appi.ajp.162.2.324.
- Office for National Statistics . 2011 Census: Ethnic Groups, Local Authorities in England and Wales 2015. www.ons.gov.uk/ons/datasets-and-tables/index.html?pageSize=50&sortBy=none&sortDirection=none&newquery=Ethnicity&content-type=Reference+table&content-type=Dataset (accessed 24 February 2015).
- Smits AJ, Hofmann SG. A meta-analytic review of the effects of psychotherapy control conditions for anxiety disorders. Psychol Med 2009;39:229-39. https://doi.org/10.1017/S0033291708003498.
- Miklowitz DJ, Goodwin GM, Bauer MS, Geddes JR. Common and specific elements of psychosocial treatments for bipolar disorder: a survey of clinicians participating in randomized trials. J Psychiatr Pract 2008;14:77-85. https://doi.org/10.1097/01.pra.0000314314.94791.c9.
- Torrent C, Bonnin Cdel M, Martínez-Arán A, Valle J, Amann BL, González-Pinto A, et al. Efficacy of functional remediation in bipolar disorder: a multicenter randomized controlled study. Am J Psychiatry 2013;170:852-9. https://doi.org/10.1176/appi.ajp.2012.12070971.
- Post RM, Rubinow DR, Ballenger JC. Conditioning and sensitisation in the longitudinal course of affective illness. Br J Psychiatry 1986;149:191-20. https://doi.org/10.1192/bjp.149.2.191.
- Javadpour A, Hedayati A, Dehbozorgi G-R, Azizi A. The impact of a simple individual psycho-education program on quality of life, rate of relapse and medication adherence in bipolar disorder patients. Asian J Psychiatr 2013;6:208-13. https://doi.org/10.1016/j.ajp.2012.12.005.
- de Barros Pellegrinelli K, de O Costa LF, Silval KI, Dias VV, Roso MC, Bandeira M, et al. Efficacy of psychoeducation on symptomatic and functional recovery in bipolar disorder. Acta Psychiatr Scand 2013;127:153-8. https://doi.org/10.1111/acps.12007.
- Kessing LV, Hansen HV, Hvenegaard A, Christensen EM, Dam H, Gluud C, et al. Treatment in a specialised out-patient mood disorder clinic v. standard out-patient treatment in the early course of bipolar disorder: randomised clinical trial. Br J Psychiatry 2013;202:212-19. https://doi.org/10.1192/bjp.bp.112.113548.
- Lobban F, Dodd AL, Dagnan D, Diggle PJ, Griffiths M, Hollingsworth B, et al. Feasibility and acceptability of web-based enhanced relapse prevention for bipolar disorder (ERPonline): trial protocol. Contemp Clin Trials 2015;41:100-9. https://doi.org/10.1016/j.cct.2015.01.004.
- Smith DJ, Griffiths E, Poole R, di Florio A, Barnes E, Kelly MJ, et al. Beating bipolar: exploratory trial of a novel internet-based psychoeducational treatment for bipolar disorder. Bipolar Disord 2011;13:571-77. https://doi.org/10.1111/j.1399-5618.2011.00949.x.
- Scott J, Colom F, Popova E, Benabarre A, Cruz N, Valenti M, et al. Long-term mental health resource utilization and cost of care following group psychoeducation or unstructured group support for bipolar disorders: a cost-benefit analysis. J Clin Psychiatry 2009;70:378-86. https://doi.org/10.4088/JCP.08m04333.
- Department of Health . National Schedules of Reference Costs 2012–2013 2013. www.gov.uk/government/uploads/system/uploads/attachment_data/file/261154/nhs_reference_costs_2012-13_acc.pdf (accessed October 2016).
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- The EuroQol group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Davies LM, Barnes TR, Jones PB, Lewis S, Gaughran F, Hayhurst K, et al. A randomized controlled trial of the cost-utility of second-generation antipsychotics in people with psychosis and eligible for clozapine. Value Health 2008;11:549-62. https://doi.org/10.1111/j.1524-4733.2007.00280.x.
- Davies LM, Lewis S, Jones PB, Barnes TRE, Gaughran F, Hayhurst K, et al. Cost-effectiveness of first generation antipsychotics to treat psychosis: results from a randomised controlled trial. Br J Psychiatry 2007;191:14-22. https://doi.org/10.1192/bjp.bp.106.028654.
- Hayhurst H, Palmer S, Abbott R, Johnson T, Scott J. Measuring health-related quality of life in bipolar disorder: relationship of the EuroQol (EQ-5D) to condition-specific measures. Qual Life Res 2006;15:1271-80. https://doi.org/10.1007/s11136-006-0059-z.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Faria R, Gomes M, Epstein D, White IR. Guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. https://doi.org/10.1136/bmj.329.7459.224.
- Briggs AH, O’Brien BJ. The death of cost minimisation analysis?. Health Econ 2001;10:179-84. https://doi.org/10.1002/hec.584.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- Sendi PP, Briggs AH. Affordability and cost-effectiveness: decision-making on the cost-effectiveness plane. Health Econ 2001;10:675-80. https://doi.org/10.1002/hec.639.
- Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D. Netherlands: Springer; 2014.
- Davies LM, Lewis S, Jones PB, Barnes TRE, Gaughran F, Hayhurst K, et al. Cost effectiveness of first- v. second-generation antipsychotic drugs: results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. Br J Psychiatry 2007;191:14-22. https://doi.org/10.1192/bjp.bp.106.028654.
- Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Clinical effectiveness, cost-effectiveness and service users’ perceptions of early, intensively resourced communication therapy following a stroke: a randomised controlled trial (the ACT NoW Study). Health Technol Assess 2012;16. https://doi.org/10.3310/hta16260.
- Davies L, Jones A, Vamvakas G, Dubourg R, Donmall M. The Drug Treatment Outcomes Research Study (DTORS): Cost-Effectiveness Analysis. London: The Home Office; 2009.
- Guthrie E, Afzal C, Blakeley C, Blakemore A, Byford R, Camacho E, et al. Choosing health options in chronic care emergencies. Programme Grants Appl Res 2017;5.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making 2000;20:332-42. https://doi.org/10.1177/0272989X0002000310.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- NICE . Carrying NICE Over the Threshold 2015 2015. www.nice.org.uk/news/blog/carrying-nice-over-the-threshold (accessed October 2016).
- Office of Health Economics . OHE Occasional Paper Critiques the Claxton Et Al. £13,000 Per QALY Estimate 2015 n.d. www.ohe.org/news/ohe-occasional-paper-critiques-claxton-et-al-%C2%A313000-qaly-estimate (accessed October 2016).
- Raftery JP. NICE’s cost-effectiveness range: should it be lowered?. PharmacoEconomics 2014;32:613-5. https://doi.org/10.1007/s40273-014-0158-6.
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:617-27. https://doi.org/10.1001/archpsyc.62.6.617.
- Kessler RC, Üstün T. The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Meth Psychiatr Res 2004;13:93-121. https://doi.org/10.1002/mpr.168.
- McEvoy PM, Grove R, Slade T. Epidemiology of anxiety disorders in the Australian general population: findings of the 2007 Australian National Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry 2011;45:957-67. https://doi.org/10.3109/00048674.2011.624083.
- WHO World Mental Health Survey Consortium . Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA 2004;291:2581-90. https://doi.org/10.1001/jama.291.21.2581.
- Wells JE, Oakley Browne MA, Scott KM, McGee MA, Baxter J, Kokaua J. Prevalence, interference with life and severity of 12-month DSM-IV disorders in Te Rau Hinengaro: the New Zealand Mental Health Survey. Aust N Z J Psychiatry 2006;40:851-4. https://doi.org/10.1080/j.1440-1614.2006.01903.x.
- Wells JE, Oakley Browne MA, Scott KM, McGee MA, Baxter J, Kokaua J. Te Rau Hinengaro: the New Zealand Mental Health Survey: overview of methods and findings. Aust N Z J Psychiatry 2006;40:835-44. https://doi.org/10.1111/j.1440-1614.2006.01902.x.
- Sala R, Goldstein BI, Morcillo C, Liu SM, Castellanos M, Blanco C. Course of comorbid anxiety disorders among adults with bipolar disorder in the U.S. population. J Psychiatr Res 2012;46:865-72. https://doi.org/10.1016/j.jpsychires.2012.03.024.
- Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med 2005;67:1-8. https://doi.org/10.1097/01.psy.0000151489.36347.18.
- Goes FS, McCusker MG, Bienvenu OJ, MacKinnon DF, Mondimore FM, Schweizer B, et al. Co-morbid anxiety disorders in bipolar disorder and major depression: familial aggregation and clinical characteristics of co-morbid panic disorder, social phobia, specific phobia and obsessive-compulsive disorder. Psychol Med 2012;42:1449-59. https://doi.org/10.1017/S0033291711002637.
- Simon NM, Pollack MH, Ostacher MJ, Zalta AK, Chow CW, Fischmann D, et al. Understanding the link between anxiety symptoms and suicidal ideation and behaviors in outpatients with bipolar disorder. J Affect Disord 2007;97:91-9. https://doi.org/10.1016/j.jad.2006.05.027.
- Post-Traumatic Stress Disorder: The Management of PTSD in Adults and Children in Primary and Secondary Care. London: NICE; 2005.
- Obsessive–Compulsive Disorder: Core Interventions in the Treatment of Obsessive–Compulsive Disorder and Body Dysmorphic Disorder. London: NICE; 2005.
- Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults: Management in Primary, Secondary and Community Care CG113. London: NICE; 2011.
- Provencher MD, Hawke LD, Thienot E. Psychotherapies for comorbid anxiety in bipolar spectrum disorders. J Affect Disord 2011;133:371-80. https://doi.org/10.1016/j.jad.2010.10.040.
- Ellard K, Deckersbach T, Sylvia LG, Nierenberg AA, Barlow D. Transdiagnostic treatment of bipolar disorder and comorbid anxiety with the unified protocol: a clinical replication series. Behav Modif 2012;36:482-508. https://doi.org/10.1177/0145445512451272.
- Goldberg D, Fawcett J. The importance of anxiety in both major depression and bipolar disorder. Depress Anxiety 2012;29:471-8. https://doi.org/10.1002/da.21939.
- Hampshire K. The Nature and Experience of Anxiety in Bipolar Disorder 2014.
- Rabiee F. Focus-group interview and data analysis. Proc Nutr Soc 2004;63:655-60. https://doi.org/10.1079/PNS2004399.
- Bech P, Rafaelsen OJ, Kramp P, Bolwig TG. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology 1978;17:430-1. https://doi.org/10.1016/0028-3908(78)90022-9.
- Patton MQ. Qualitative Evaluation and Research Methods. Thousand Oaks, CA: Sage; 1990.
- Jones SH, Knowles D, Tyler E, Holland F, Peters S, Lobban F, et al. The feasibility and acceptability of a novel anxiety in bipolar disorder intervention compared to treatment as usual: a randomized controlled trial. Depress Anxiety 2018.
- Barlow DH, Ellard KK, Fairholme CP, Farchione TJ, Boisseau CL, Allen LB, et al. Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders: Workbook. New York, NY: Oxford University Press; 2010.
- Jones S, McGrath E, Hampshire K, Owen R, Riste L, Roberts C, et al. A randomised controlled trial of time limited CBT informed psychological therapy for anxiety in bipolar disorder. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-54.
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32:50-5. https://doi.org/10.1111/j.2044-8341.1959.tb00467.x.
- Burt VK, Rasgon N. Special considerations in treating bipolar disorder in women. Bipolar Disord 2004;6:2-13. https://doi.org/10.1046/j.1399-5618.2003.00089.x.
- Gaudiano BA, Miller IW. Anxiety disorder comobidity in bipolar I disorder: relationship to depression severity and treatment outcome. Depress Anxiety 2005;21:71-7. https://doi.org/10.1002/da.20053.
- Scott J, Colom F, Vieta E. A meta-analysis of relapse rates with adjunctive psychological therapies compared to usual psychiatric treatment for bipolar disorders. Int J Neuropsychopharmacol 2007;10:123-9. https://doi.org/10.1017/S1461145706006900.
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety 2001;13:166-78. https://doi.org/10.1002/da.1033.
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988;45:742-7. https://doi.org/10.1001/archpsyc.1988.01800320058007.
- Spielberger CD. State–Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press; 1983.
- Michalak EE, Murray G. Collaborative RESearch Team to Study Psychosocial Issues in Bipolar Disorder (CREST.BD) . Development of the QoL.BD: a disorder-specific scale to assess quality of life in bipolar disorder. Bipolar Disord 2010;12:727-40. https://doi.org/10.1111/j.1399-5618.2010.00865.x.
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101:323-9.
- Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. The rational clinical examination. Is this patient taking the treatment as prescribed?. JAMA 1993;269:2779-81. https://doi.org/10.1001/jama.1993.03500210079036.
- Beecham J, Knapp M, Thornicoft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 2001.
- Tracey TJ, Kokotovic AM. Factor structure of the working alliance inventory. Psychol Assess 1989;1:207-10. https://doi.org/10.1037/1040-3590.1.3.207.
- Blackburn IM, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The revised cognitive therapy scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. https://doi.org/10.1017/S1352465801004040.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-18.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. https://doi.org/10.1016/S0022-3999(01)00296-3.
- Keen AJ, Freeston MH. Assessing competence in cognitive-behavioural therapy. Br J Psychiatry 2008;193:60-4. https://doi.org/10.1192/bjp.bp.107.038588.
- Matza L, Morlock R, Sexton C, Malley K, Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods in Psychiatr Res 2010;19:223-32. https://doi.org/10.1002/mpr.323.
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 2013;150:384-8. https://doi.org/10.1016/j.jad.2013.04.028.
- Bech P, Bech P. Clinical Psychometrics. Chichester: John Wiley & Sons; 2012.
- Hirschfeld R, Bowden CL, Gitlin MJ, Keck PE, Perlis RH, Suppes T, et al. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:64-110.
- Liebert RJ. A re-view of ambivalence in bipolar disorder research. Ethical Hum Psychol Psychiatry 2013;15:180-94. https://doi.org/10.1891/1559-4343.15.3.180.
- Jones SH, Smith G, Mulligan L, Lobban F, Law H, Dunn G, et al. Recovery focused CBT for individuals with recent onset bipolar disorder: a randomised controlled pilot trial. Br J Psychiatry 2015;206:58-66.
- Vázquez GH, Baldessarini RJ, Tondo L. Co-occurrence of anxiety and bipolar disorders: clinical and therapeutic overview. Depress Anxiety 2014;31:196-20. https://doi.org/10.1002/da.22248.
- Brewin CR, Bradley C. Patient preferences and randomised clinical trials. BMJ 1989;299:313-15. https://doi.org/10.1136/bmj.299.6694.313.
- Meyer TD, Hautzinger M. Cognitive behaviour therapy and supportive therapy for bipolar disorders: relapse rates for treatment period and 2-year follow-up. Psychol Med 2012;42:1429-39. https://doi.org/10.1017/S0033291711002522.
- Lam D, Watkins ER, Hayward P, Bright J, Wright K, Kerr N, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome of the first year. Arch Gen Psychiatry 2003;60:145-52. https://doi.org/10.1001/archpsyc.60.2.145.
- Covin R, Ouimet AJ, Seeds PM, Dozois DJ. A meta-analysis of CBT for pathological worry among clients with GAD. J Anxiety Disord 2008;22:108-16. https://doi.org/10.1016/j.janxdis.2007.01.002.
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 2011;68:241-51. https://doi.org/10.1001/archgenpsychiatry.2011.12.
- Goodwin FK, Jamison KR. Manic Depressive Illness: Bipolar and Recurrent Depression. New York, NY: Oxford University Press; 2007.
- Ostacher MJ, Perlis RH, Nierenberg AA, Calabrese J, Stange JP, Salloum I, et al. Impact on substance use disorders on recovery from episodes of depression in bipolar disorder patients: prospective data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 2010;167:289-97. https://doi.org/10.1176/appi.ajp.2009.09020299.
- Schaffer A, Cairney J, Veldhuizen S, Kurdyak P, Cheung A, Levitt A. A population-based analysis of distinguishers of bipolar disorder from major depressive disorder. J Affect Disord 2010;125:103-10. https://doi.org/10.1016/j.jad.2010.02.118.
- Cardoso BM, Kauer Sant’Anna M, Dias VV, Andreazza AC, Ceresér KM, Kapczinski F. The impact of co-morbid alcohol use disorder in bipolar patients. Alcohol 2008;42:451-7. https://doi.org/10.1016/j.alcohol.2008.05.003.
- Marshall DF, Walker SJ, Ryan KA, Kamali M, Saunders EF, Weldon AL, et al. Greater executive and visual memory dysfunction in comorbid bipolar disorder and substance use disorder. Psychiatry Res 2012;200:252-7. https://doi.org/10.1016/j.psychres.2012.06.013.
- Mazza M, Mandelli L, Di Nicola M, Harnic D, Catalano V, Tedeschi D, et al. Clinical features, response to treatment and functional outcome of bipolar disorder patients with and without co-occurring substance use disorder: 1-year follow-up. J Affect Disord 2009;115:27-35. https://doi.org/10.1016/j.jad.2008.08.019.
- Gaudiano BA, Uebelacker LA, Miller IW. Impact of remitted substance use disorders on the future course of bipolar I disorder: findings from a clinical trial. Psychiatry Res 2008;160:63-71. https://doi.org/10.1016/j.psychres.2007.05.014.
- Rakofsky JJ, Dunlop BW. Do alcohol use disorders destabilize the course of bipolar disorder?. J Affect Disord 2013;145:1-10. https://doi.org/10.1016/j.jad.2012.06.012.
- Frye MA, Salloum IM. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord 2006;8:677-85. https://doi.org/10.1111/j.1399-5618.2006.00370.x.
- van Zaane J, van den Brink W, Draisma S, Smit JH, Nolen WA. The effect of moderate and excessive alcohol use on the course and outcome of patients with bipolar disorders: a prospective cohort study. J Clin Psychiatry 2010;71:885-93. https://doi.org/10.4088/JCP.09m05079gry.
- Di Florio A, Craddock N, van den Bree M. Alcohol misuse in bipolar disorder. A systematic review and meta-analysis of comorbidity rates. Eur Psychiatry 2014;29:117-24. https://doi.org/10.1016/j.eurpsy.2013.07.004.
- Goldstein BI, Velyvis VP, Parikh SV. The association between moderate alcohol use and illness severity in bipolar disorder: a preliminary report. J Clin Psychiatry 2006;67:102-6. https://doi.org/10.4088/JCP.v67n0114.
- Bellivier F, Yon L, Luquiens A, Azorin JM, Bertsch J, Gerard S, et al. Suicidal attempts in bipolar disorder: results from an observational study (EMBLEM). Bipolar Disord 2011;13:377-86. https://doi.org/10.1111/j.1399-5618.2011.00926.x.
- Elbogen EB, Johnson SC. The intricate link between violence and mental disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2009;66:152-61. https://doi.org/10.1001/archgenpsychiatry.2008.537.
- Agrawal A, Nurnberger JI, Lynskey MT. Bipolar Genome Study . Cannabis involvement in individuals with bipolar disorder. Psychiatry Res 2011;185:459-61. https://doi.org/10.1016/j.psychres.2010.07.007.
- Lagerberg TV, Sundet K, Aminoff SR, Berg AO, Ringen PA, Andreassen OA, et al. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 2011;261:397-405. https://doi.org/10.1007/s00406-011-0188-4.
- Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE, et al. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry 2007;64:57-64. https://doi.org/10.1001/archpsyc.64.1.57.
- van Rossum I, Boomsma M, Tenback D, Reed C, van Os J. EMBLEM Advisory Board . Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J Nerv Ment Dis 2009;197:35-40. https://doi.org/10.1097/NMD.0b013e31819292a6.
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, et al. Bipolar spectrum–substance use co-occurrence: behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. J Pers Soc Psychol 2009;97:549-65. https://doi.org/10.1037/a0016061.
- Etain B, Mathieu F, Liquet S, Raust A, Cochet B, Richard JR, et al. Clinical features associated with trait-impulsiveness in euthymic bipolar disorder patients. J Affect Disord 2013;144:240-7. https://doi.org/10.1016/j.jad.2012.07.005.
- Henna E, Hatch JP, Nicoletti M, Swann AC, Zunta-Soares G, Soares JC. Is impulsivity a common trait in bipolar and unipolar disorders?. Bipolar Disord 2013;15:223-7. https://doi.org/10.1111/bdi.12034.
- Swann AC, Moeller FG, Steinberg JL, Schneider L, Barratt ES, Dougherty DM. Manic symptoms and impulsivity during bipolar depressive episodes. Bipolar Disord 2007;9:206-12. https://doi.org/10.1111/j.1399-5618.2007.00357.x.
- Oquendo MA, Currier D, Liu SM, Hasin DS, Grant BF, Blanco C. Increased risk for suicidal behavior in comorbid bipolar disorder and alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Clin Psychiatry 2010;71:902-9. https://doi.org/10.4088/JCP.09m05198gry.
- Quanbeck CD, Stone DC, Scott CL, McDermott BE, Altshuler LL, Frye MA. Clinical and legal correlates of inmates with bipolar disorder at time of criminal arrest. J Clin Psychiatry 2004;65:198-203. https://doi.org/10.4088/JCP.v65n0209.
- Schmitz JM, Averill P, Sayre S, McCleary P, Moeller FG, Swann A. Cognitive–behavioral treatment of bipolar disorder and substance abuse: a preliminary randomized study. Addict Disord Their Treat 2002;1:17-24. https://doi.org/10.1097/00132576-200205000-00004.
- Weiss RD, Griffin ML, Jaffee WB, Bender RE, Graff FS, Gallop RJ, et al. A ‘community-friendly’ version of integrated group therapy for patients with bipolar disorder and substance dependence: a randomized controlled trial. Drug Alcohol Depend 2009;104:212-19. https://doi.org/10.1016/j.drugalcdep.2009.04.018.
- Weiss RD, Najavits LM, Greenfield SF. A relapse prevention group for patients with bipolar and substance use disorders. J Subst Abuse Treat 1999;16:47-54. https://doi.org/10.1016/S0740-5472(98)00011-7.
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003;71:843-61. https://doi.org/10.1037/0022-006X.71.5.843.
- Project Match . Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. J Stud Alcohol 1997;58:7-29. https://doi.org/10.15288/jsa.1997.58.7.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York, NY: Guilford Press; 2002.
- Jones S, Deville M, Mayes D, Lobban F. Self-management in bipolar disorder: the story so far. J Ment Health 2011;20:583-92. https://doi.org/10.3109/09638237.2011.600786.
- Darke S, Ward J, Hall W, Heather N, Wodak A. The Opiate Treatment Index (OTI) Researcher’s Manual. Sydney: National Drug and Alcohol Research Centre; 1991.
- Jones S, Robinson H, Riste L, Roberts C, Peters S, Bateman L, et al. Integrated psychological therapy for people with bipolar disorder and co-morbid alcohol use: a feasibility and acceptability randomised controlled trial. Contemp Clin Trials Communn 2018;10:193-8.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Farren CK, Snee L, Daly P, McElroy S. Prognostic factors of 2-year outcomes of patients with comorbid bipolar disorder or depression with alcohol dependence: Importance of early abstinence. Alcohol Alcohol 2013;48:93-8. https://doi.org/10.1093/alcalc/ags112.
- Barrowclough C, Haddock G, Beardmore R, Conrod P, Craig T, Davies LM, et al. Evaluating integrated MI and CBT for people with psychosis and substance misuse: recruitment, retention and sample characteristics of the MIDAS trial. Addict Behav 2009;34:859-66. https://doi.org/10.1016/j.addbeh.2009.03.007.
- Haddock G, Beardmore R, Earnshaw P, Fitzsimmons M, Nothard S, Butler R, et al. Assessing fidelity to integrated motivational interviewing and CBT therapy for psychosis and substance use: the MI-CBT fidelity scale (MI-CTS). J Ment Health 2012;21:38-4. https://doi.org/10.3109/09638237.2011.621470.
- Sobell LC, Sobell MB, Litten RZ, Allen J. Measuring Alcohol Consumptiom: Psychosocial and Biological Methods. New Jersey, NJ: Humana; 1992.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Bauer MS, Crits-Christoph P, Ball WA, Dewees E, McAllister T, Alahi P, et al. Independent assessment of manic and depressive symptoms by self-rating. Scale characteristics and implications for the study of mania. Arch Gen Psychiatry 1991;48:807-12. https://doi.org/10.1001/archpsyc.1991.01810330031005.
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51:768-74. https://doi.org/10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1.
- Rehm J, Shield K, Gmel MXRG, Frick U. Alcohol Consumption, Alcohol Dependence and Attributable Burden of Disease in Europe: Potential Gains From Effective Interventions for Alcohol Dependence. Canada: Centre for Addiction and Mental Health; 2012.
- Bizzarri JV, Rucci P, Sbrana A, Gonnelli C, Massei GJ, Ravani L, et al. Reasons for substance use and vulnerability factors in patients with substance use disorder and anxiety or mood disorders. Addict Behav 2007;32:384-91. https://doi.org/10.1016/j.addbeh.2006.04.005.
- Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord 2009;115:367-75. https://doi.org/10.1016/j.jad.2008.10.003.
- Sonne SC, Brady KT, Morton WA. Substance abuse and bipolar affective disorder. J Nerv Ment Dis 1994;182:349-52. https://doi.org/10.1097/00005053-199406000-00007.
- Grinspoon L, Bakalar JB. The use of cannabis as a mood stabilizer in bipolar disorder: anecdotal evidence and the need for clinical research. J Psychoactive Drugs 1998;30:171-7. https://doi.org/10.1080/02791072.1998.10399687.
- Tyler E, Jones S, Black N, Carter LA, Barrowclough C. The relationship between bipolar disorder and cannabis use in daily life: an experience sampling study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0118916.
- Gregg L, Haddock G, Barrowclough C. Self-reported reasons for substance use in schizophrenia: a Q methodological investigation. Ment Health Subst Use 2009;2:24-39. https://doi.org/10.1080/17523280802593293.
- Stewart SH, Zeitlin SB, Samoluk SB. Examination of a three-dimensional drinking motives questionnaire in a young adult population. Behav Res Ther 1996;34:61-7. https://doi.org/10.1016/0005-7967(95)00036-W.
- Weiss RD, Kolodziej M, Griffin ML, Najavits LM, Jacobson LM, Greenfield SF. Substance use and perceived symptom improvement among patients with bipolar disorder and substance dependence. J Affect Disord 2004;79:279-83. https://doi.org/10.1016/S0165-0327(02)00454-8.
- Barkus E, Lewis S. Schizotypy and psychosis-like experiences from recreational cannabis in a non-clinical sample. Psychol Med 2008;38:1267-76. https://doi.org/10.1017/S0033291707002619.
- Baldessarini RJ, Jamison KR. Effects of medical interventions on suicidal behavior. Summary and conclusions. J Clin Psychiatry 1999;60:117-22.
- Dutta R, Boydell J, Kennedy N, Van Os J, Fearon P, Murray RM. Suicide and other causes of mortality in bipolar disorder: a longitudinal study. Psychol Med 2007;37:839-47. https://doi.org/10.1017/S0033291707000347.
- Baldessarini RJ, Pompili M, Tondo L. Suicide in bipolar disorder: risks and management. CNS Spectr 2006;11:465-71. https://doi.org/10.1017/S1092852900014681.
- Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry 1997;170:205-28. https://doi.org/10.1192/bjp.170.3.205.
- Tondo L, Lepri B, Baldessarini RJ. Suicidal risks among 2826 Sardinian major affective disorder patients. Acta Psychiatr Scand 2007;116:419-28. https://doi.org/10.1111/j.1600-0447.2007.01066.x.
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord 2002;68:167-81. https://doi.org/10.1016/S0165-0327(01)00377-9.
- Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry 2011;68:1058-64. https://doi.org/10.1001/archgenpsychiatry.2011.113.
- Preventing Suicide: A Global Imperative. Geneva: World Health Organization; 2014.
- Leverich GS, Altshuler LL, Frye MA, Suppes T, Keck PE, McElroy SL, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. J Clin Psychiatry 2003;64:506-15. https://doi.org/10.4088/JCP.v64n0503.
- Valtonen H, Suominen K, Mantere O, Leppämäki S, Arvilommi P, Isometsä ET. Suicidal ideation and attempts in bipolar I and II disorders. J Clin Psychiatry 2005;66:1456-62. https://doi.org/10.4088/JCP.v66n1116.
- Chen YW, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biol Psychiatry 1996;39:896-9. https://doi.org/10.1016/0006-3223(95)00295-2.
- Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry 1999;56:617-26. https://doi.org/10.1001/archpsyc.56.7.617.
- Marangell LB, Bauer MS, Dennehy EB, Wisniewski SR, Allen MH, Miklowitz DJ, et al. Prospective predictors of suicide and suicide attempts in 1556 patients with bipolar disorders followed for up to 2 years. Bipolar Disord 2006;8:566-75. https://doi.org/10.1111/j.1399-5618.2006.00369.x.
- Rihmer Z. Suicide risk in mood disorders. Curr Opin Psychiatry 2007;20:17-22. https://doi.org/10.1097/YCO.0b013e3280106868.
- Azorin J-M, Kaladjian A, Adida M, Hantouche E, Hameg A, Lancrenon S, et al. Risk factors associated with lifetime suicide attempts in bipolar I patients: findings from a French National Cohort. Compr Psychiatry 2009;50:115-20. https://doi.org/10.1016/j.comppsych.2008.07.004.
- Brown GK, Beck AT, Steer RA, Grisham JR. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol 2000;68:371-7. https://doi.org/10.1037/0022-006X.68.3.371.
- Tondo L, Isacsson G, Baldessarini R. Suicidal behaviour in bipolar disorder: risk and prevention. CNS Drugs 2003;17:491-51. https://doi.org/10.2165/00023210-200317070-00003.
- Dalton EJ, Cate-Carter TD, Mundo E, Parikh SV, Kennedy JL. Suicide risk in bipolar patients: the role of co-morbid substance use disorders. Bipolar Disord 2003;5:58-61. https://doi.org/10.1034/j.1399-5618.2003.00017.x.
- López P, Mosquera F, de León J, Gutiérrez M, Ezcurra J, Ramírez F, et al. Suicide attempts in bipolar patients. J Clin Psychiatry 2001;62:963-6. https://doi.org/10.4088/JCP.v62n1208.
- Tsai SY, Lee JC, Chen CC. Characteristics and psychosocial problems of patients with bipolar disorder at high risk for suicide attempt. J Affect Disord 1999;52:145-52. https://doi.org/10.1016/S0165-0327(98)00066-4.
- Dilsaver SC, Chen YW, Swann AC, Shoaib AM, Tsai-Dilsaver Y, Krajewski KJ. Suicidality, panic disorder and psychosis in bipolar depression, depressive-mania and pure-mania. Psychiatry Res 1997;73:47-56. https://doi.org/10.1016/S0165-1781(97)00109-1.
- Benazzi F. Mixed depression, suicidality, and antidepressants. J Clin Psychiatry 2006;67:1650-1. https://doi.org/10.4088/JCP.v67n1024b.
- Valtonen HM, Suominen K, Haukka J, Mantere O, Leppämäki S, Arvilommi P, et al. Differences in incidence of suicide attempts during phases of bipolar I and II disorders. Bipolar Disord 2008;10:588-96. https://doi.org/10.1111/j.1399-5618.2007.00553.x.
- Comtois KA, Russo JE, Roy-Byrne P, Ries RK. Clinicians’ assessments of bipolar disorder and substance abuse as predictors of suicidal behavior in acutely hospitalized psychiatric inpatients. Biol Psychiatry 2004;56:757-63. https://doi.org/10.1016/j.biopsych.2004.10.003.
- Oquendo MA, Currier D, Mann JJ. Prospective studies of suicidal behavior in major depressive and bipolar disorders: what is the evidence for predictive risk factors?. Acta Psychiatr Scand 2006;114:151-8. https://doi.org/10.1111/j.1600-0447.2006.00829.x.
- Sublette ME, Carballo JJ, Moreno C, Galfalvy HC, Brent DA, Birmaher B, et al. Substance use disorders and suicide attempts in bipolar subtypes. J Psychiatr Res 2009;43:230-8. https://doi.org/10.1016/j.jpsychires.2008.05.001.
- Kilbane EJ, Gokbayrak NS, Galynker I, Cohen L, Tross S. A review of panic and suicide in bipolar disorder: does comorbidity increase risk?. J Affect Disord 2009;115:1-10. https://doi.org/10.1016/j.jad.2008.09.014.
- Simon NM, Zalta AK, Otto MW, Ostacher MJ, Fischmann D, Chow CW, et al. The association of comorbid anxiety disorders with suicide attempts and suicidal ideation in outpatients with bipolar disorder. J Psychiatr Res 2007;41:255-64. https://doi.org/10.1016/j.jpsychires.2006.08.004.
- Galfalvy H, Oquendo MA, Carballo JJ, Sher L, Grunebaum MF, Burke A, et al. Clinical predictors of suicidal acts after major depression in bipolar disorder: a prospective study. Bipolar Disord 2006;8:586-95. https://doi.org/10.1111/j.1399-5618.2006.00340.x.
- Høyer EH, Olesen AV, Mortensen PB. Suicide risk in patients hospitalised because of an affective disorder: a follow-up study, 1973–1993. J Affect Disord 2004;78:209-17. https://doi.org/10.1016/S0165-0327(02)00311-7.
- Akiskal HS, Benazzi F. Family history validation of the bipolar nature of depressive mixed states. J Affect Disord 2003;73:113-22. https://doi.org/10.1016/S0165-0327(02)00330-0.
- Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry 2005;162:1805-19. https://doi.org/10.1176/appi.ajp.162.10.1805.
- Clements C, Morriss R, Jones S, Peters S, Roberts C, Kapur N. Suicide in bipolar disorder in a national English sample, 1996–2009: frequency, trends and characteristics. Psychol Med 2013;43:2593-602. https://doi.org/10.1017/S0033291713000329.
- Hunt IM, Kapur N, Robinson J, Shaw J, Flynn S, Bailey H, et al. Suicide within 12 months of mental health service contact in different age and diagnostic groups: National Clinical Survey. Br J Psychiatry 2006;188:135-42. https://doi.org/10.1192/bjp.188.2.135.
- Appleby L, Shaw J, Kapur N, Windfuhr K, Ashton A, . Avoidable Deaths: Five-Year Report of the National Confidential Inquiry into Suicide and Homicide by People with Mental Illness. London: Department of Health; 2006.
- O’Donnell I, Farmer R. The limitations of official suicide statistics. Br J Psychiatry 1995;166:458-61. https://doi.org/10.1192/bjp.166.4.458.
- Linsley KR, Schapira K, Kelly TP. Open verdict v. suicide – importance to research. Br J Psychiatry 2001;178:465-8. https://doi.org/10.1192/bjp.178.5.465.
- Appleby L, Shaw J, Kapur N, Windfuhr K, Williams A, Swinson N, et al. National Confidential Inquiry into Suicide and Homicide by People with Mental Illness: Annual Report England and Wales. Manchester: University of Manchester; 2012.
- Windfuhr K, While D, Hunt I, Turnbull P, Lowe R, Burns J, et al. Suicide in juveniles and adolescents in the United Kingdom. J Child Psychol Psychiatry 2008;49:1155-65. https://doi.org/10.1111/j.1469-7610.2008.01938.x.
- Health and Social Care Act 2001: Chapter 15. London: The Stationery Office; 2001.
- Rubenowitz E, Waern M, Wilhelmson K, Allebeck P. Life events and psychosocial factors in elderly suicides – a case-control study. Psychol Med 2001;31:1193-202. https://doi.org/10.1017/S0033291701004457.
- Bickley H, Hunt IM, Windfuhr K, Shaw J, Appleby L, Kapur N. Suicide within two weeks of discharge from psychiatric inpatient care: a case-control study. Psychiatr Serv 2013;64:653-9. https://doi.org/10.1176/appi.ps.201200026.
- Hunt I, Kapur NN, Webb RT, Robinson J, Burns J, Turnbull P, et al. Suicide in current psychiatric in-patients: a case-control study The National Confidential Inquiry into Suicide and Homicide. Psychol Med 2007;37:831-7. https://doi.org/10.1017/S0033291707000104.
- Hunt IM, Kapur N, Webb R, Robinson J, Burns J, Shaw J, et al. Suicide in recently discharged psychiatric patients: a case-control study. Psychol Med 2009;39:443-9. https://doi.org/10.1017/S0033291708003644.
- Hunt IM, Bickley H, Windfuhr K, Shaw J, Appleby L, Kapur N. Suicide in recently admitted psychiatric in-patients: a case–control study. J Affect Disord 2013;144:123-8. https://doi.org/10.1016/j.jad.2012.06.019.
- Clements C, Jones S, Morriss R, Peters S, Cooper J, While D, et al. Self-harm in bipolar disorder: findings from a prospective clinical database. J Affect Disord 2015;173:113-19. https://doi.org/10.1016/j.jad.2014.10.012.
- Hawton K, Harriss L, Hall S, Simkin S, Bale E, Bond A. Deliberate self-harm in Oxford, 1990–2000: a time of change in patient characteristics. Psychol Med 2003;33:987-95. https://doi.org/10.1017/S0033291703007943.
- Kapur N, Cooper J, O’Connor RC, Hawton K. Non-suicidal self-injury v. attempted suicide: new diagnosis or false dichotomy?. Br J Psychiatry 2013;202:326-8. https://doi.org/10.1192/bjp.bp.112.116111.
- Goldstein L, Zhang H. Efficiency of the maximum partial likelihood estimator for nested case control sampling. Bernoulli 2009;15:569-97. https://doi.org/10.3150/08-BEJ162.
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am J Psychiatry 2005;162:1680-7. https://doi.org/10.1176/appi.ajp.162.9.1680.
- Isometsä E, Heikkinen M, Henriksson M, Aro H, Lönnqvist J. Recent life events and completed suicide in bipolar affective disorder. A comparison with major depressive suicides. J Affect Disord 1995;33:99-106. https://doi.org/10.1016/0165-0327(94)00079-O.
- Finseth PI, Morken G, Andreassen Oa, Malt UF, Vaaler AE. Risk factors related to lifetime suicide attempts in acutely admitted bipolar disorder inpatients. Bipolar Disord 2012;14:727-34. https://doi.org/10.1111/bdi.12004.
- Cassidy F. Risk factors of attempted suicide in bipolar disorder. Suicide Life Threat Behav 2011;41:6-11. https://doi.org/10.1111/j.1943-278X.2010.00007.x.
- D’Ambrosio V, Salvi V, Bogetto F, Maina G. Serum lipids, metabolic syndrome and lifetime suicide attempts in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2012;37:136-40. https://doi.org/10.1016/j.pnpbp.2011.12.009.
- Watkins HB, Meyer TD. Is there an empirical link between impulsivity and suicidality in bipolar disorders? A review of the current literature and the potential psychological implications of the relationship. Bipolar Disord 2013;15:542-58. https://doi.org/10.1111/bdi.12090.
- Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, et al. Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am J Psychiatry 1997;154:1544-50.
- Dickerson F, Boronow JJ, Stallings C, Origoni AE, Cole SK, Yolken RH. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the repeatable battery for the assessment of neuropsychological status. Psychiatry Res 2004;129:45-53. https://doi.org/10.1016/j.psychres.2004.07.002.
- Schoeyen HK, Birkenaes AB, Vaaler AE, Auestad BH, Malt UF, Andreassen OA, et al. Bipolar disorder patients have similar levels of education but lower socio-economic status than the general population. J Affect Disord 2011;129:68-74. https://doi.org/10.1016/j.jad.2010.08.012.
- Merikangas KR, Pato M. Recent developments in the epidemiology of bipolar disorder in adults and children: magnitude, correlates, and future directions. Clin Psychol - SCI PR 2009;16:121-33. https://doi.org/10.1111/j.1468-2850.2009.01152.x.
- ten Have M, Vollebergh W, Bijl R, Nolen WA. Bipolar disorder in the general population in the Netherlands (prevalence, consequences and care utilisation): results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). J Affect Disord 2002;68:203-13. https://doi.org/10.1016/S0165-0327(00)00310-4.
- NHS Centre for Reviews and Dissemination, University of York . Deliberate self-harm. Eff Health Care Bull 1998;4:1-4.
- Bayes A, Parker G, Fletcher K. Clinical differentiation of bipolar II disorder from borderline personality disorder. Curr Opin Psychiatry 2014;27:14-20. https://doi.org/10.1097/YCO.0000000000000021.
- Pompili M, Rihmer Z, Akiskal H, Amore M, Gonda X, Innamorati M, et al. Temperaments mediate suicide risk and psychopathology among patients with bipolar disorders. Compr Psychiatry 2012;53:280-5. https://doi.org/10.1016/j.comppsych.2011.04.004.
- Sarısoy G, Kaçar OF, Pazvantoğlu O, Oztürk A, Korkmaz IZ, Kocamanoğlu B, et al. Temperament and character traits in patients with bipolar disorder and associations with attempted suicide. Compr Psychiatry 2012;53:1096-102. https://doi.org/10.1016/j.comppsych.2012.05.002.
- Mahon K, Burdick KE, Wu J, Ardekani BA, Szeszko PR. Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disord 2012;14:80-9. https://doi.org/10.1111/j.1399-5618.2012.00984.x.
- Dual Diagnosis: Developing Capable Practitioners to Improve Services and Increase Positive Service User Experience. London: Department of Health; 2008.
- Mental Health Policy Implementation Guide: Dual Diagnosis Good Practice Guide. London: Department of Health; 2001.
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 2013;346. https://doi.org/10.1136/bmj.f3646.
- Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: a systematic review and meta-analysis. Behav Modif 2008;32:77-108. https://doi.org/10.1177/0145445507304728.
- Bartlett P, Mudigonda M, Chopra A, Morriss R, Jones S. Planning for incapacity by people with bipolar disorder under the Mental Capacity Act 2005. J Soc Welf Fam Law 2015;38:263-86.
- Morriss R, Mudigonda M, Bartlett P, Chopra A, Jones S. National survey of training of psychiatrists on advance directives to refuse treatment in bipolar disorder. BJPsych Bull 2017;41:320-4.
- Mental Capacity Act 2005: Post-Legislative Scrutiny. Report of Session 2013–14. London: The Stationery Office; 2014.
- Mudigonda M, Bartlett P, Morriss R, Chopra A, Jones S. Advance Planning for People with Bipolar Disorder: A Guide to Making Decisions About Your Personal Welfare, Property and Financial Affairs. East Midlands: Collaboration for Leadership in Applied Mental Health Research and Care (East Midlands); 2014.
- Mental Capacity Act. London: Ministry of Justice; 2007.
- European Convention for the Protection of Human Rights and Fundamental Freedoms 213 U.N.T.S. 222 entered into force Sept. 3 1953. Strasbourg: Council of Europe; 1950.
- Mental Health Act Code of Practice. Para 17.8. London: The Stationery Office; 2008.
- Court of Protection Report 2010. London: Judiciary of England and Wales; 2010.
- Bartlett P, Morriss R, Mudigonda M, Chopra A, Jones S. Advance decisions under the Mental Capacity Act 2005 in cases of bipolar disorder. J Soc Welf Fam Law 2016;38:263-86.
- Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000;157:1873-5. https://doi.org/10.1176/appi.ajp.157.11.1873.
- Twiss J, Jones S, Anderson I. Validation of the Mood Disorder Questionnaire for screening for bipolar disorder in a UK sample. J Affect Disord 2008;110:180-4. https://doi.org/10.1016/j.jad.2007.12.235.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publishing Company; 1967.
- Stiles WB, Llewelyn S, Kennedy P. Handbook of Clinical Health Psychology. Chichester: John Wiley & Sons, Ltd; 2003.
- Bond CJ, Lowton K. Geriatricians’ views of advance decisions and their use in clinical care in England: qualitative study. Age Ageing 2011;40:450-6. https://doi.org/10.1093/ageing/afr025.
- Samsi K, Manthorpe J. ‘I live for today’: a qualitative study investigating older people’s attitudes to advance planning. Health Soc Care Community 2011;19:52-9. https://doi.org/10.1111/j.1365-2524.2010.00948.x.
- Williams V, Boyle J, Jepson M, Swift P, Williamson T, Heslop P. Making Best Interests Decisions: People and Processes. London: Mental Health Foundation; 2012.
- Morgan VA, Mitchell PB, Jablensky AV. The epidemiology of bipolar disorder: sociodemographic, disability and service utilization data from the Australian National Study of Low Prevalence (Psychotic) Disorders. Bipolar Disord 2005;7:326-37. https://doi.org/10.1111/j.1399-5618.2005.00229.x.
- Alcohol Use Disorders Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence. London: NICE; 2010.
Appendix 1 Additional participant details
| Last SCID assessment | Survival status, n (%) | |||||
|---|---|---|---|---|---|---|
| Not known | Dead | Alive | ||||
| PEd | PS | PEd | PS | PEd | PS | |
| Baseline | 0 | 17 (11) | 1 (1) | 0 | 14 (9) | 0 |
| 32 weeks | 0 | 7 (5) | 0 | 0 | 5 (3) | 0 |
| 64 weeks | 0 | 13 (9) | 0 | 3 (2) | 15 (10) | 0 |
| 96 weeks | – | – | – | – | 104 (68) | 96 (64) |
| Missing last assessment data | 14 (9) | 15 (10) | 0 | 0 | 0 | 0 |
| Total | 14 (9) | 52 (34) | 1 (1) | 3 (2) | 13 (90) | 96 (64) |
| Characteristic | Treatment group | |
|---|---|---|
| PEd (N = 153), n (%) | PS (N = 151), n (%) | |
| Living situation | N = 153 | N = 151 |
| Lives alone | 50 (33) | 68 (45) |
| Does not live alone | 99 (65) | 79 (52) |
| Other | 4 (3) | 4 (3) |
| Number of children | N = 153 | N = 151 |
| 0 | 63 (41) | 58 (38) |
| 1 | 27 (18) | 36 (24) |
| 2 | 40 (26) | 31 (21) |
| 3 | 13 (8) | 21 (14) |
| 4 | 8 (5) | 4 (3) |
| ≥ 5 | 2 (1) | 1 (1) |
| Education | N = 153 | N = 151 |
| Educated up to further education | 92 (60) | 92 (61) |
| Educated beyond further education | 61 (40) | 59 (39) |
| Employment status | N = 153 | N = 151 |
| Currently working | 45 (29) | 37 (24) |
| Not currently working | 108 (71) | 114 (76) |
Appendix 2 Utility and quality-adjusted life-years data
| EQ-5D health states | Treatment group, n/N (%) | |
|---|---|---|
| PEd (n = 153) | PS (n = 151) | |
| Baseline | ||
| No problem with mobility | 93/134 (69) | 95/131 (73) |
| No problem with self-care | 107/134 (80) | 100/132 (76) |
| No problem with usual activities | 57/133 (43) | 59/131 (45) |
| No problem with pain/discomfort | 76/133 (57) | 71/130 (55) |
| No problem with anxiety/depression | 54/133 (41) | 52/130 (40) |
| 32-week assessment | ||
| No problem with mobility | 49/75 (65) | 59/86 (69) |
| No problem with self-care | 61/75 (81) | 59/86 (69) |
| No problem with usual activities | 32/74 (43) | 27/86 (31) |
| No problem with pain/discomfort | 43/75 (57) | 40/86 (47) |
| No problem with anxiety/depression | 33/74 (45) | 25/85 (29) |
| 64-week assessment | ||
| No problem with mobility | 43/67 (64) | 42/60 (70) |
| No problem with self-care | 52/67 (78) | 43/60 (72) |
| No problem with usual activities | 29/67 (43) | 23/60 (38) |
| No problem with pain/discomfort | 36/67 (54) | 30/60 (50) |
| No problem with anxiety/depression | 24/66 (36) | 19/60 (32) |
| 96-week assessment | ||
| No problem with mobility | 32/49 (65) | 32/51 (63) |
| No problem with self-care | 34/50 (68) | 39/51 (76) |
| No problem with usual activities | 19/50 (38) | 18/51 (35) |
| No problem with pain/discomfort | 29/50 (58) | 21/51 (41) |
| No problem with anxiety/depression | 15/50 (30) | 22/50 (44) |
| EQ-5D utility values | Treatment group, mean (SD) [95% CI] | |
|---|---|---|
| PEd (N = 153) | PS (N = 151) | |
| Baseline | 0.69 (0.29) [0.64 to 0.74]; n = 133 | 0.68 (0.29) [0.63 to 0.73]; n = 125 |
| 32-week assessment | 0.70 (0.28) [0.64 to 0.77]; n = 75 | 0.63 (0.30) [0.57 to 0.70]; n = 86 |
| 64-week assessment | 0.66 (0.30) [0.59 to 0.74]; n = 66 | 0.62 (0.34) [0.53 to 0.71]; n = 60 |
| 96-week assessment | 0.63 (0.33) [0.53 to 0.72]; n = 49 | 0.70 (0.24) [0.64 to 0.77]; n = 50 |
Appendix 3 Service use and costs
| Type of service | Treatment group, used service n/N (%) | |
|---|---|---|
| PEd (n = 153) | PS (n = 151) | |
| 6 months before baseline | ||
| Hospital inpatient overnight admission | 31/149 (21) | 20/144 (14) |
| Hospital outpatient care | 137/150 (91) | 123/144 (85) |
| General practice (GP, practice and district nurses) | 137/152 (90) | 128/145 (88) |
| Other primary physical health care | 45/152 (30) | 33/144 (23) |
| Community-based mental health and social care | 48/148 (32) | 60/143 (42) |
| Used any prescription medications | 140/148 (95) | 137/144 (95) |
| Baseline–week 32 | ||
| Hospital inpatient overnight admission | 23/121 (19) | 16/112 (14) |
| Hospital outpatient care | 100/121 (83) | 85/111 (76) |
| General practice (GP, practice and district nurses) | 105/121 (87) | 97/112 (87) |
| Other primary physical health care | 23/121 (19) | 16/112 (14) |
| Community-based mental health and social care | 37/121 (31) | 35/111 (32) |
| Used any prescription medications | 111/113 (98) | 103/105 (98) |
| Weeks 32–64 | ||
| Hospital inpatient overnight admission | 13/108 (12) | 12/100 (12) |
| Hospital outpatient care | 83/108 (77) | 80/100 (80) |
| General practice (GP, practice and district nurses) | 97/108 (90) | 90/100 (90) |
| Other primary physical health care | 9/107 (8) | 14/100 (14) |
| Community-based mental health and social care | 29/108 (27) | 32/100 (32) |
| Used any prescription medications | 95/97 (98) | 85/88 (97) |
| Weeks 64–96 | ||
| Hospital inpatient overnight admission | 14/107 (13) | 12/95 (13) |
| Hospital outpatient care | 76/107 (71) | 66/95 (70) |
| General practice (GP, practice and district nurses) | 96/107 (90) | 86/95 (87) |
| Other primary physical health care | 11/107 (10) | 9/95 (10) |
| Community-based mental health and social care | 43/107 (40) | 22/95 (23) |
| Used any prescription medications | 91/92 (99) | 83/84 (99) |
| Services used | Treatment group, mean cost (£a) (95% CI) | |
|---|---|---|
| PEd (N = 153) | PS (N = 151) | |
| 6 months before baseline | ||
| Hospital inpatient admission | 1861 (842 to 2880); n = 149 | 2233 (935 to 3531); n = 144 |
| Hospital outpatient care | 609 (521 to 697); n = 150 | 613 (486 to 739); n = 144 |
| General practiceb | 180 (138 to 222); n = 152 | 168 (129 to 208); n = 145 |
| Other primary physical health care | 58 (37 to 79); n = 152 | 48 (27 to 68); n = 144 |
| Community mental health/social care | 379 (228 to 530); n = 148 | 416 (254 to 577); n = 143 |
| Prescription medications | 461 (321 to 600); n = 148 | 280 (198 to 363); n = 144 |
| Baseline–week 32 | ||
| Hospital inpatient admission | 981 (136 to 1526); n = 121 | 671 (226 to 1116); n = 112 |
| Hospital outpatient care | 481 (391 to 571); n = 121 | 502 (399 to 606); n = 112 |
| General practiceb | 187 (115 to 259); n = 121 | 199 (118 to 280); n = 112 |
| Other primary physical health care | 39 (18 to 60); n = 121 | 25 (11 to 38); n = 112 |
| Community mental health/social care | 364 (220 to 509); n = 121 | 249 (143 to 355); n = 111 |
| Prescription medications | 538 (375 to 701); n = 113 | 360 (268 to 452); n = 105 |
| Weeks 32–64 | ||
| Hospital inpatient admission | 911 (0 to 2033); n = 108 | 1120 (132 to 2017); n = 100 |
| Hospital outpatient care | 370 (293 to 447); n = 108 | 431 (338 to 523); n = 100 |
| General practiceb | 159 (118 to 200); n = 108 | 134 (97 to 170); n = 100 |
| Other primary physical health care | 14 (1 to 26); n = 107 | 33 (11 to 56); n = 100 |
| Community mental health/social care | 363 (176 to 550); n = 108 | 295 (162 to 428); n = 100 |
| Prescription medications | 504 (331 to 677); n = 97 | 361 (260 to 461); n = 88 |
| Weeks 64–96 | ||
| Hospital inpatient admission | 1360 (23 to 2697); n = 107 | 1386 (439 to 2332); n = 95 |
| Hospital outpatient care | 352 (268 to 436); n = 107 | 387 (291 to 483); n = 95 |
| General practiceb | 95 (78 to 111); n = 107 | 125 (92 to 159); n = 95 |
| Other primary physical health care | 15 (5 to 25); n = 107 | 33 (4 to 62); n = 95 |
| Community mental health/social care | 470 (276 to 664); n = 107 | 343 (91 to 594); n = 95 |
| Prescription medications | 652 (426 to 878); n = 92 | 431 (327 to 535); n = 84 |
| Assessment points | Treatment group, mean (£a) (SD) [95% CI] | |
|---|---|---|
| PEd (N = 153) | PS (N = 151) | |
| 6 months before baseline | 3589 (6672) [2482 to 4696]; n = 142 | 3878 (8573) [2419 to 5337]; n = 135 |
| Baseline–week 32 | 3015 (3863) [2291 to 3738]; n = 112 | 2241 (2263) [1792 to 2690]; n = 100 |
| Weeks 32–64 | 2254 (6226) [992 to 3515]; n = 96 | 2524 (5623) [1311 to 3737]; n = 85 |
| Weeks 64–96 | 2667 (6788) [1253 to 4080]; n = 91 | 2692 (4961) [1602 to 3782]; n = 82 |
| Total baseline–week 96 | 7576 (14,357) [4361 to 10,792]; n = 79 | 7487 (10,600) [4881 to 10,093]; n = 66 |
Appendix 4 Mental Capacity Act 2005 study: psychiatrists’ survey
Appendix 5 Advance planning for people with bipolar disorder
Appendix 6 Mental Capacity Act 2005 study: service user survey
Appendix 7 Integrated anxiety intervention-specific fidelity measure
Throughout therapy
-
Did the therapist use destigmatising language?
-
Did the therapist highlight and reflect on strengths when appropriate?
-
Were feelings of hopelessness addressed if raised?
-
Was the aim of the session consistent with the clients defined integrated therapy goals?
-
Was the therapist flexible with regard to the duration, location and timing of appointments?
-
Was the content of the session defined either by the client or in collaboration with the client?
Initial phases
-
Did the therapist discuss the integrated anxiety approach with the client?
-
Did the therapist develop a shared understanding with the client regarding the relative importance of anxiety and mood issue in relation to the goals of therapy?
-
Did the therapist allow time for exploring the impact diagnosis had for the client, if appropriate?
-
Was the client provided with appropriate psychoeducation material around anxiety and mood as appropriate?
Formulation
-
Did the therapist develop, in collaboration with the client, a balanced formulation including strengths as well as difficulties?
-
When formulating events with the client did the therapist make an effort to reduce self-criticism by validating the client’s psychological or behavioural reaction (e.g. it is understandable that somebody may feel/do X after Y, most people would think X in that situation).
-
Did the formulation reflect the primary need of the client whether this was anxiety, mood or a combination of the two?
-
Did the therapist convey a continuum model of mood and anxiety experiences as appropriate?
-
Did the therapist allow the client to make sense of their experiences using the model of the client’s choice (e.g. illness model, stress model, etc.)?
Intermediate sessions
-
Did the therapist employ relevant anxiety management techniques with the client where appropriate?
-
Were mood management techniques linked to the client’s goals?
-
Did the therapist ensure all self-management strategies covered were added to the client’s resource pack?
Later sessions
-
Did the therapist consolidate with the client their personal ways of self-managing?
-
Did the therapist highlight the importance of behaviour and activity which is meaningful to the individual?
-
Did the therapist reflect on self-management as a life-long process which the client will continue to employ after therapy has been completed?
-
Did the therapist deal with stigmatising attitudes and promote awareness of the internalisation of stigma?
-
Was a staying well plan completed?
-
If appropriate were the key outcomes of therapy disseminated to the clients support network (e.g. family, GP, care co-ordinator).
List of abbreviations
- ABLE
- Assessing Bipolar Lifestyle Experiences
- ADRT
- advanced decision to refuse treatment
- AIBD
- anxiety in bipolar disorder
- AUDIT
- Alcohol Use Disorders Identification Test
- BD
- bipolar disorder
- BIS
- Barratt Impulsiveness Scale
- BRQ
- Bipolar Recovery Questionnaire
- CBT
- cognitive–behavioural therapy
- CF
- clinician facilitator
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPN
- community psychiatric nurse
- CSO
- clinical studies officer
- CTS-R
- Cognitive Therapy Scale-Revised
- CUtLASS
- Cost Utility of the Latest Antipsychotic drugs in Schizophrenia Study
- df
- degrees of freedom
- DoLS
- Deprivation of Liberty Safeguards
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- ECT
- electroconvulsive therapy
- EMAHSN
- East Midlands Academic Health Sciences Network
- EPoA
- Enduring Power of Attorney
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GAD
- generalised anxiety disorder
- GCSE
- General Certificate of Secondary Education
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale – anxiety subscale
- HAM-A
- Hamilton Anxiety Rating Scale
- HAM-D
- Hamilton Depression Rating Scale
- HR
- hazard ratio
- ICC
- intracluster correlation coefficient
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IR
- incidence rate ratio
- IRR
- inter-rater reliability
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LIFE
- Longitudinal Interval Follow-up Evaluation
- LME
- linear random-effects model
- LPoA
- lasting power of attorney
- LREC
- Local Research Ethics Committee
- LRT
- likelihood ratio test
- MAS
- Bech–Rafaelsen Mania Scale
- MaSH
- Manchester Self-Harm Project
- MCA
- Mental Capacity Act 2005
- MDQ
- Mood Disorder Questionnaire
- MEDAD
- Stephenson Medical Adherence Questionnaire
- MHA
- Mental Health Act
- MHP
- mental health professional
- MHRN
- Mental Health Research Network
- MHS
- Mental Health Services
- MI
- motivational interviewing
- MPS
- most problematic substance
- NB
- net benefit
- NCI
- National Confidential Inquiry (into Suicide and Homicide by People with Mental Illness)
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OCD
- obsessive–compulsive disorder
- OR
- odds ratio
- PARADES
- Psychoeducation, Anxiety, Relapse, Advance Directive Evaluation and Suicidality
- PEd
- psychoeducation
- PHQ-9
- Patient Health Questionnaire-9
- PPI
- patient and public involvement
- PS
- peer support
- PSP
- Personal and Social Performance Scale
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RA
- research assistant
- RCT
- randomised controlled trial
- RR
- relative risk
- SAS
- Social Adjustment Scale
- SCID
- Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SNRI
- serotonin–norepinephrine reuptake inhibitor
- SOFAS
- Social and Occupational Functioning Assessment Scale
- STAI
- State–Trait Anxiety Inventory
- SU
- service user
- SUD
- substance use disorder
- SUF
- service user facilitator
- SUR
- service user researcher
- SURG
- service user reference group
- TAU
- treatment as usual
- TLFB
- Timeline Followback
- TSC
- Trial Steering Committee
- WAI
- Work Alliance Inventory
- WS
- workstream
- WTPV
- willingness-to-pay value
