Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0608-10073. The contractual start date was in April 2010. The final report began editorial review in March 2018 and was accepted for publication in December 2019. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Fitzmaurice et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
This programme of work comprises three interconnecting work packages:
-
a RCT of extended anticoagulation treatment versus standard treatment for the prevention of recurrent venous thromboembolism (VTE) and post-thrombotic syndrome (PTS) [the ExACT (Extended Anticoagulation) trial]
-
a qualitative study to explore existing knowledge and barriers, across the spectrum of health care from patients to health-care professionals (HCPs) [the ExPeKT (Exploration of Patient knowledge and expectation) study]
-
a health economic modelling study to evaluate the most cost-effective methods of treating and preventing recurrence of VTE.
Protocols for the ExACT trial [work package (WP) 1]1 and the ExPeKT study (WP2)2 have been published. These WPs are summarised in Table 1 and detailed in subsequent pages of the report.
| Work package | Year | |||||||
|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| WP1: the ExACT RCT | First patient recruited July 2011 | Last patient recruited February 2015 | Last patient follow-up February 2017 | |||||
| WP2: the ExPeKT studies | HCP interviews | |||||||
| Patient interviews | ||||||||
| WP3: health economic analysis | Data collection | |||||||
| Modelling June 2017 to February 2018 | ||||||||
Background
Venous thromboembolism, defined as deep-vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cardiovascular disorder, after myocardial infarction and stroke. DVT is common (i.e. an incidence of approximately 1 per 1000 people per annum) and is associated with mortality and serious morbidity, particularly PE and PTS.
The largest attributable risk for acquiring VTE is hospital admission for either medical or surgical conditions. Most hospitalised patients have one or more risk factors for VTE,3 with mortality from hospital-acquired thrombosis (HAT) greater than the combined total number of deaths from breast cancer, acquired immune deficiency syndrome (AIDS) and road traffic accidents each year in the UK. There is evidence to show that around 60% of people undergoing hip or knee replacement will suffer a DVT if they receive no preventative intervention, and that the mortality rate is around 30% if this is left untreated. 4
Venous thromboembolism is therefore a substantial health-care problem that is associated with significant mortality, morbidity and economic cost. In 2005, there was an estimated cost to the NHS of £640M for the management of VTE. 5
Some people suffer an unprovoked DVT, where no identifiable reason for the DVT can be found. These unprovoked DVTs are treated with oral anticoagulation therapy (OAT), which generally continues for between 6 weeks and 6 months. However, the optimal duration of treatment is still uncertain. 6 When OAT is discontinued, there is an annual recurrence rate of approximately 10% in the first year and around 5% thereafter, irrespective of the duration of treatment.
Prevention of venous thromboembolism
Hospital-acquired thrombosis
The largest risk for acquiring VTEs is hospital admission. 4 Current UK guidelines for preventing HAT recommend using the Department of Health and Social Care’s risk assessment tool to assess appropriate thromboprophylaxis for individuals. 7 Thromboprophylaxis has been shown to reduce the risk of VTE by 75% in surgical patients and 50% in medical patients.
In 2010, Commissioning for Quality and Innovation (CQUIN) agreements were introduced that required all UK acute trusts to assess risk for VTEs for at least 90% of patients, or risk financial penalties. 8 However, an All-Party Parliamentary Thrombosis Group (APPTG) survey found that implementation of risk assessment was poor,9 and only 58% of trusts carry out a regular clinical audit of thromboprophylaxis. Furthermore, HAT can occur up to 90 days post discharge, but there is little or no understanding of the role that primary care can play in the process; there is also no evidence of the use of care plans for community VTE prophylaxis.
Unprovoked deep-vein thrombosis
The risk of recurrence among patients with unprovoked thrombosis is higher than for those with identifiable risk factors, such as surgery or long-haul travel. The cumulative rate of recurrence for those with unprovoked VTE is about 25% at 5 years and 30% at 10 years.
The risk varies with time, with the highest risk evident during the first 6–12 months; the risk never reaches zero. Furthermore, these recurrent events are fatal in about 5–9% of people. Most recurrences can be prevented by appropriate antithrombotic therapy, but there is a concern in terms of the duration of treatment owing to the increased risk of bleeding from prolonged OAT.
Several studies have investigated the optimal duration of OAT, with the British Committee for Standards in Haematology recommending between 6 weeks and 6 months, depending on the aetiology. 6 However, the optimal duration of OAT for patients with a first unprovoked DVT remains uncertain, with randomised controlled trials (RCTs) suggesting that the duration has little effect on the rate of recurrence. 10 Research to address the most appropriate approach to preventing recurrences is still required.
Identification of patients at the highest risk of recurrence
Given the balance of risks and benefits of anticoagulation therapy (AT) described above, it may be beneficial to identify those at the highest risk of recurrence. It may be possible to stratify individual risk based on a variety of clinical factors, including sex or comorbidities, or to measure laboratory markers such as coagulation factors. Alternatively, risk prediction models can be used, such as the Vienna Prediction Model, although this is most accurate in people already at low risk of recurrence. 11 There is evidence that normal D-dimer levels, measured around 4 weeks after cessation of OAT, are associated with lower risk of recurrence. 12
D-dimer is a fibrin degradation product present in the blood after a blood clot is degraded by fibrinolysis; levels of D-dimer can be tested using laboratory and point-of-care testing devices. The primary clinical use of levels of D-dimer has been in conjunction with probability scores to determine whether or not further investigation is required for the diagnosis of VTE.
More recently, interest has been shown in studies that have investigated whether or not the levels of D-dimer can be utilised as a guide for determining who is at risk of recurrent VTE following treatment of the initial acute episode. Some small studies have suggested that the levels of D-dimer could act as a useful predictor of recurrent VTE in patients whose OAT is discontinued. 13 There are limited data on the utility of levels of D-dimer as a predictor in patients still on therapy, but Rodgers et al. 14 have shown that the levels of D-dimer can be used to predict recurrence in low-risk female patients. Two systematic reviews of D-dimer testing after cessation of treatment for VTE (seven studies with total of 1888 patients) concluded that ‘additional research is needed to establish the optimal interval between stopping anticoagulation and performing D-dimer testing, (and) to identify the optimum cut-off point that predicts recurrence’. 14
No studies to date have investigated the utility of D-dimer testing while a patient is still undergoing therapy, and there are no studies that have investigated the utility of D-dimer testing in conjunction with clinical algorithms to prevent recurrence of DVT.
Post-thrombotic syndrome
Post-thrombotic syndrome is a frequent and costly complication of DVT that can lead to chronic venous insufficiency and ulceration, and reduced quality of life for patients. Prevalence of PTS in one study was found to be 37% after 2.2 years, with 4% of people having severe PTS. 15 PTS has a cumulative incidence after 2 years of around 25%, and it has been postulated that prolonged treatment with OAT could prevent the development of PTS. 16 However, the only randomised data available suggested no association between long-term low-dose warfarin treatment and the development of PTS,15 and there are no data available for patients treated with long-term therapeutic warfarin.
Barriers to implementation of thromboprophylaxis
For the reasons discussed above, the prevention of VTE and appropriate management of the risk of recurrence is important. However, despite the introduction of guidelines8 and CQUIN agreements, it seems that the guidelines and recommendations are not universally implemented.
It is known that there is little knowledge about VTE in the public arena,4 which is perhaps unsurprising, as it is apparent that HCPs also underestimate the extent and impact of the problem. The APPTG report highlighted the role that primary care could play in improving the management of VTE. 17 General practitioners (GPs) are in a good position to deliver education to patients, and to improve the management of patients post hospital discharge. However, there is little evidence to date of the use of care plans for community VTE prophylaxis.
When considering patient barriers, diabetes studies have shown the desire to avoid injectable drugs, so low-molecular-weight heparin therapy may, therefore, introduce concordance issues. 18 Patients were educated about the risk of heparin-induced thrombocytopenia, a life-threatening, immune-mediated prothrombotic adverse drug effect. Patients appreciated the information about the potential adverse reactions to the drug; the information given did not lead to a treatment refusal, with all patients choosing treatment. 19
The lack of public awareness could be addressed through public education programmes, as improving understanding will empower patients with the knowledge to request a risk assessment on admission to hospital. 20 However, we do not know patients’ attitudes to education and information in this area. Furthermore, we do not know how best to deliver this information; no educational measures have been developed.
The HCP barriers to initiating VTE prophylaxis may be manifold. There is some evidence that non-adherence to guidelines is an issue for clinicians but barriers to implementation are unclear. Knowledge to practice translational issues are extremely important for the successful integration of thromboprophylaxis into the community and clarity around processes is necessary.
The guidelines stipulate a supporting role for GPs, based on notification that a patient has been discharged from hospital and the treatment prescribed. However, communication between care settings is known to be problematic, leading to the role performed by primary care being unclear. If primary care is to contribute more effectively to the prevention of HAT, then a better understanding of its role and of the factors that influence the role is required.
Summary
The VTE prevention and treatment programme was designed to provide an evidence base to improve the prevention and treatment of VTE in the NHS, through addressing some of the existing gaps in the evidence, as discussed above. The programme consists of a series of studies including a RCT; a survey of stakeholders; qualitative interviews with patients, doctors, nurses and other stakeholders; cost-effectiveness analyses; patient preference survey; and a number of economic modelling studies. Each work package is detailed below.
Patient and public involvement
Mrs Eve Knight, Director of Anticoagulation Europe, a patient-based charitable organisation, was involved in this programme from its conception and continued to provide input throughout the lifetime of the programme as a member of the Programme Board. She approved all patient-facing material and also contributed to the develoment of the interview schedule for WP2. The charity was not involved in the identification of patients for the programme. The programme of work was reviewed and edited by the two public representatives on the Primary Care Research Network, Central England (PCRN-CE) Management Group.
Work package 1: randomised controlled trial of extended anticoagulation treatment versus standard treatment for the prevention of recurrent venous thromboembolism and post-thrombotic syndrome in patients being treated for a first episode of unprovoked venous thromboembolism (the ExACT trial)
Overview
The background section described the existing evidence around the most appropriate treatment to prevent recurrent clots in people who have had a first unprovoked VTE, and the identification of people likely to be at the highest risk of recurrence. Uncertainty remains about the optimum duration of anticoagulation treatment following a first unprovoked VTE, and there is no clear guidance to help clinicians identify those at a high risk of recurrence. There is also a lack of data available around the use of anticoagulation treatment to prevent the development of PTS. This work package aimed to address these gaps in the knowledge.
This section reports on the ExACT trial. The cost analysis and patient preference study are presented in subsequent sections.
The ExACT trial was approved by Trent Research Ethics Committee on 21 October 2010.
Methods
The ExACT trial was a non-blinded, prospective, multicentre RCT. The full methods of the ExACT trial have been published. 1,21 The methods are further described in Appendix 1.
Aim
The aim of the ExACT trial was to investigate the effect of extending treatment with oral anticoagulation for those patients with first unprovoked proximal DVT or PE prior to discontinuing treatment, in terms of reduction in incidence of VTE and PTS.
Objectives
-
To determine the protective effect of prolonged AT on recurrent events of DVT or PE.
-
To determine the protective effect of prolonged AT on the severity of PTS.
-
To establish the performance characteristics of D-dimer testing while still on treatment as a prediction tool for recurrence of VTE in patients with first unprovoked proximal DVT or PE.
-
To establish factors that contribute to the recurrence of VTE in patients with a first unprovoked proximal DVT or PE.
-
To determine the cost-effectiveness of extended treatment with anticoagulation (described in WP3).
-
To determine patient preferences and utilities with regard to extended anticoagulation treatment (described in WP3).
Sample size calculation
The study was designed to compare 2-year VTE recurrence rates between participants in the extended versus discontinued AT arms, and also to compare these rates for a group of participants with a baseline raised levels of D-dimer. A sample size of 352 (176 per arm) would be sufficient to detect a clinically important difference between the arms with minimum 86% power, two-sided alpha = 0.05, assuming recurrence rates between 1.4% and 4.3% for the extended AT arm and 14.2% in the discontinued AT arm. Recruitment was lower than expected and, at the request of the Trial Steering Committee, the power calculation was re-estimated, as a result of which it was determined that a sample of 270 participants (allowing for 10% loss to follow-up) would provide at least 80% power to detect the planned effect sizes.
Summary of changes to the protocol (version 2.11)
-
We listed an additional objective (to determine the protective effect of prolonged AT on the severity of PTS) that was included as a planned secondary outcome in the protocol but was not detailed in the objectives.
-
We clarified that VTE and bleeding events are time-to-event outcomes.
-
We excluded the second analysis outlined in section 4.1 of protocol version 2.11 (number of recurrent thrombotic events between those with a raised level of D-dimer who were randomly allocated to the no-treatment arm and those with normal levels of D-dimer who were randomly allocated to the no-treatment arm), as the first analysis listed in this section is the only subgroup analysis required to investigate the cut-off point analysis.
Results
Participant recruitment
The first patient was randomised in July 2011 and the last patient was randomised in February 2015. The records of 8422 patients presenting to the recruiting sites with a suspected DVT/PE were screened to identify potentially eligible patients who met the inclusion criteria. Following application of the criteria, 5034 patients were identified as ineligible for invitation to participate. Invitations to participate in the trial in the form of postcards and participant information sheets were provided to 3388 potentially eligible patients. Responses were received from 1361 out of 3388 (40%) potentially eligible patients, with 993 out of 1361 (73%) notifying the research team that they were interested in participating in the study and providing their permission for the research team to contact their GP to confirm their eligibility to participate. Overall, 368 out of 3388 (11%) patients responded stating that they did not wish to participate. Responses to the invitation were not returned by 2027 out of 3388 (60%) patients (Figure 1).
FIGURE 1.
Patient flow diagram. a, Data for suspected DVT or PE are taken from site screening logs and are therefore only estimates of the total number with DVT or PE.

The GPs of the 993 potentially eligible patients were contacted to confirm patient eligibility to take part. GPs confirmed eligibility for 393 out of 993 (40%) patients, and 499 out of 993 (50%) were ineligible. GPs did not complete the eligibility checks for 101 out of 993 (10%) patients. The main reasons for exclusion were that the patient had recently stopped oral anticoagulation (20%), they had an indication for long-term OAT (15%) and they had a provoked DVT or PE (14%). The reasons for ineligibility were not reported for 112 out of 499 (22%) patients.
A total of 281 patients provided written informed consent to participate and were randomised: 141 were randomly allocated to the intervention arm of the study (group E) and 140 to the control arm (group D) of the study. All 281 trial participants attended visit 1, 273 (97%) attended visit 2, 263 (94%) attended visit 3 and 260 attended (93%) visit 4 (Figure 2). Post oral anticoagulation cessation visits were completed by 182 out of 281 (65%) participants.
FIGURE 2.
Patient flow diagram following randomisation. a, Includes four participants who withdrew consent to use their data; b, includes one participant who withdrew consent to use their data; and c, includes two patients receiving rivaroxaban (Xarelto; Bayer AG, Leverkusen, Germany) therapy.

Baseline characteristics
No differences were found in baseline characteristics between the intervention and control groups (Table 2). The mean age of participants was around 63 years, with a roughly even split between DVT and PE, and approximately two-thirds of participants were male.
| Characteristic | Trial group | Total (N = 273) | |
|---|---|---|---|
| Control (group D) (N = 134) | Intervention (group E) (N = 139) | ||
| Age at time of randomisation (years) | |||
| Mean (SD) | 63.3 (12.7) | 62.2 (13.0) | 62.7 (12.8) |
| Median (IQR) | 64.5 (55.6–74.0) | 64.4 (53.3–72.4) | 64.4 (54.4–72.7) |
| Sex, n (%) | |||
| Female | 44 (32.8) | 45 (32.4) | 89 (32.6) |
| Male | 90 (67.2) | 94 (67.6) | 184 (67.4) |
| Diagnosis (DVT/PE),a n (%) | |||
| Unprovoked DVT | 69 (51.5) | 70 (50.4) | 139 (50.9) |
| Unprovoked PE | 65 (48.5) | 69 (49.6) | 134 (49.1) |
| Ethnicity, n (%) | |||
| White | 131 (97.8) | 131 (94.2) | 262 (96.0) |
| Mixed | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Asian or Asian British | 0 (0.0) | 3 (2.2) | 3 (1.1) |
| Black or black British | 2 (1.5) | 5 (3.6) | 7 (2.6) |
| Other ethnic groups | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Smoking status, n (%) | |||
| Non-smoker | 63 (47.0) | 60 (43.2) | 123 (45.1) |
| Ex-smoker | 48 (35.8) | 60 (43.2) | 108 (39.6) |
| Current smoker | 19 (14.2) | 18 (13.0) | 37 (13.6) |
| Smokes occasionally | 4 (3.0) | 1 (0.7) | 5 (1.8) |
| Alcohol consumption, n (%) | |||
| No | 44 (32.8) | 51 (36.7) | 95 (34.8) |
| Yes | 90 (67.2) | 88 (63.3) | 178 (65.2) |
| BMI classification (kg/m2), n (%) | |||
| Underweight (< 18.5) | 2 (1.5) | 0 (0.0) | 2 (0.7) |
| Normal range (18.5–24.99) | 47 (35.1) | 47 (33.8) | 94 (34.4) |
| Overweight (25–29.99) | 51 (38.1) | 53 (38.1) | 104 (38.1) |
| Obese (≥ 30) | 33 (24.6) | 37 (26.6) | 70 (25.6) |
| Missing | 1 (0.8) | 2 (1.4) | 3 (1.1) |
| Family history of VTE, n (%) | |||
| No | 102 (76.1) | 102 (73.4) | 204 (74.7) |
| Yes | 32 (23.9) | 37 (26.6) | 69 (25.3) |
| Medical history | |||
| Stroke, n (%) | |||
| No | 130 (97.0) | 136 (97.8) | 266 (97.4) |
| Yes | 4 (3.0) | 3 (2.2) | 7 (2.6) |
| Transient ischaemic attack, n (%) | |||
| No | 129 (96.3) | 138 (99.3) | 267 (97.8) |
| Yes | 5 (3.7) | 1 (0.7) | 6 (2.2) |
| Angina, n (%) | |||
| No | 129 (96.3) | 136 (97.8) | 265 (97.1) |
| Yes | 5 (3.7) | 3 (2.2) | 8 (2.9) |
| Myocardial infarction, n (%) | |||
| No | 133 (99.3) | 134 (96.4) | 267 (97.8) |
| Yes | 1 (0.8) | 5 (3.6) | 6 (2.2) |
| Ischaemic heart disease, n (%) | |||
| No | 130 (97.0) | 136 (97.8) | 266 (97.4) |
| Yes | 4 (3.0) | 3 (2.2) | 7 (2.6) |
| Peripheral vascular disease, n (%) | |||
| No | 134 (100.0) | 134 (96.4) | 268 (98.2) |
| Yes | 0 (0.0) | 5 (3.6) | 5 (1.8) |
| PTS score (categorical), n (%) | |||
| No PTS | 70 (52.2) | 66 (47.5) | 136 (49.8) |
| Mild PTS | 42 (31.3) | 51 (36.7) | 93 (34.1) |
| Moderate PTS | 15 (11.2) | 18 (13.0) | 33 (12.1) |
| Severe PTS | 5 (3.7) | 2 (1.4) | 7 (2.6) |
| Missing | 2 (1.5) | 2 (1.4) | 4 (1.5) |
| PTS score | |||
| Mean (SD) | 5.2 (4.2) | 5.1 (3.8) | 5.2 (4.0) |
| Median (IQR) | 4.0 (2.0–7.5) | 5.0 (2.0–8.0) | 4.0 (2.0–8.0) |
| Missing | 2 | 2 | 4 |
| EQ-5D-3L | |||
| Mean (SD) | 0.8 (0.2) | 0.8 (0.3) | 0.8 (0.3) |
| Median (IQR) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) |
| Missing | 0 | 4 | 4 |
| VEINES-QOL score | |||
| Mean (SD) | 48.2 (10.7) | 49.6 (9.9) | 48.9 (10.3) |
| Median (IQR) | 51.1 (41.1–57.6) | 53.0 (44.6–56.7) | 52.1 (43.3–57.5) |
| Missing | 0 | 2 | 2 |
| Health-care utilisation because of PTS | |||
| Patient receiving primary care treatment, n (%) | |||
| No | 124 (92.5) | 128 (92.1) | 252 (92.3) |
| Yes | 9 (6.7) | 11 (7.9) | 20 (7.3) |
| Missing | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Type of nurse patients were seen by, n (%) | |||
| Practice | 2 (1.5) | 1 (0.7) | 3 (1.1) |
| District | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| HCA | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| None | 59 (44.0) | 70 (50.4) | 129 (47.3) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 73 (54.5) | 68 (48.9) | 141 (51.7) |
| Patient receiving treatment for a leg ulcer, n (%) | |||
| No | 66 (49.3) | 71 (51.1) | 137 (50.2) |
| Yes | 1 (0.8) | 2 (1.4) | 3 (1.1) |
| Missing | 67 (50.0) | 66 (47.5) | 133 (48.7) |
| Patient receiving secondary care treatment, n (%) | |||
| No | 131 (97.8) | 135 (97.1) | 266 (97.4) |
| Yes | 1 (0.8) | 4 (2.9) | 5 (1.8) |
| Missing | 2 (1.5) | 0 (0.0) | 2 (0.7) |
Primary outcome
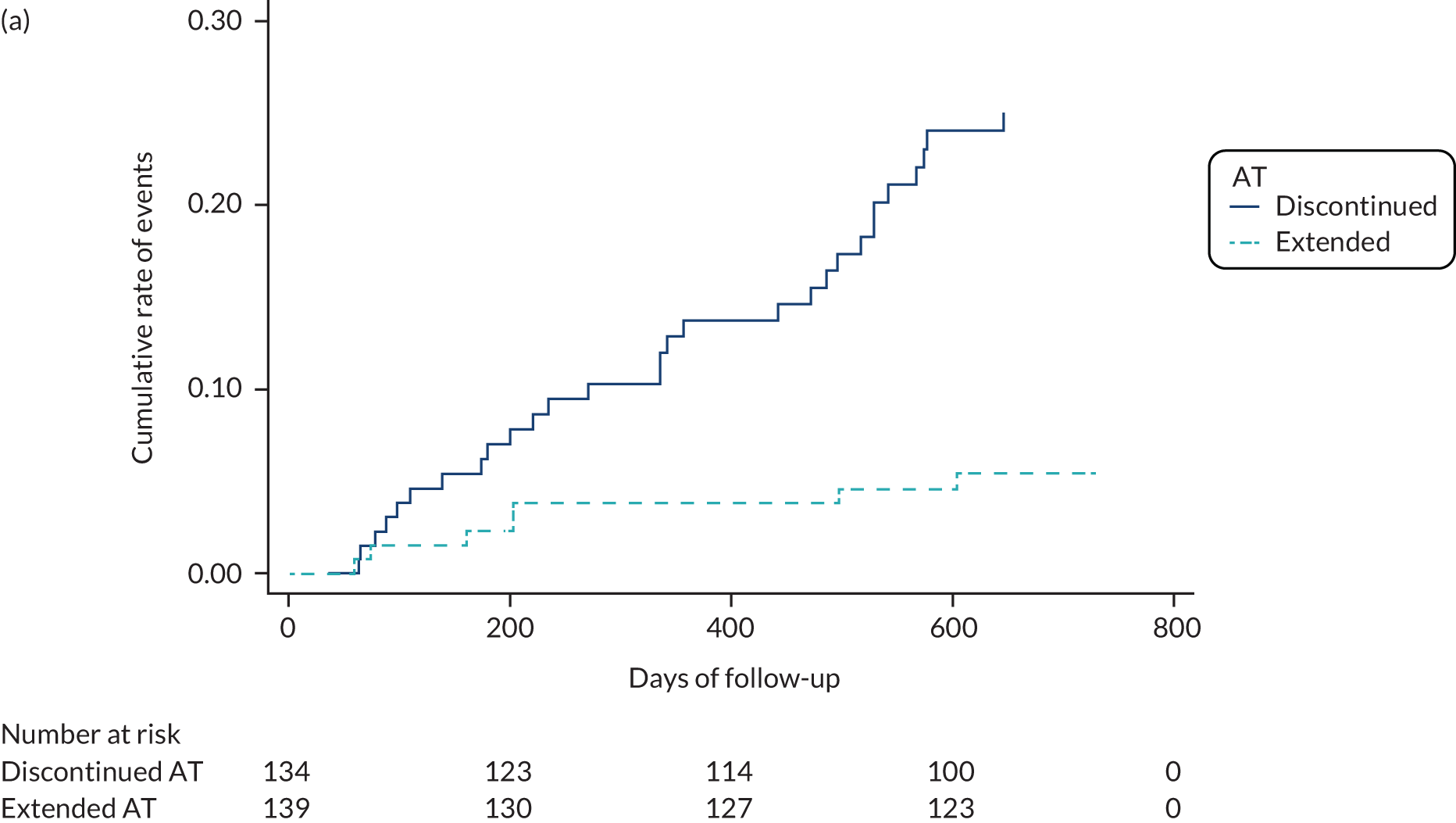
In terms of the primary outcome, there were 32 recurrent VTEs in 31 patients (13.54 events per 100 person-years) within the control group (group D) compared with seven events in seven patients (2.75 events per 100 person-years) in the intervention group (group E). This gave an adjusted hazard ratio of 0.2 [95% confidence interval (CI) 0.09 to 0.46; p < 0.001], meaning that patients receiving extended AT were 80% less likely to suffer a recurrent event than those patients who discontinued AT (Table 3 and Figure 3). Age did not affect the rate of recurrence in either group, but males had numerically more recurrences off treatment than females. The intervention effect does not differ significantly between the two age groups (p = 0.267), but the intervention group (group E) had significantly reduced VTE recurrences than the control group (group D) in males (adjusted hazard ratio 0.11, 95% CI 0.03 to 0.38) but not in females (adjusted hazard ratio 0.48, 95% CI 0.14 to 1.59), even though the associated interaction effect is not significant at the 5% level (p = 0.099) (see Table 4).
| Outcome | Trial group | Adjusted hazard ratio (95% CI)a | p-value | |
|---|---|---|---|---|
| Control (group D) (N = 134) | Intervention (group E) (N = 139) | |||
| Primary outcome | ||||
| Recurrent VTE | ||||
| Number of participants with one or more events, n (%) | 31 (23.1) | 7 (5.0) | 0.20 (0.09 to 0.46) | < 0.001 |
| Number of eventsb | 32 | 7 | ||
| Number of events per 100 person-yearsc | 13.54 | 2.75 | ||
| Secondary outcomes | ||||
| Major bleeding events | ||||
| Number of participants with one or more events, n (%) | 3 (2.2) | 9 (6.5) | 2.99 (0.81 to 11.05) | 0.100 |
| Number of events | 3 | 9 | ||
| Number of events per 100 person-yearsc | 1.18 | 3.54 | ||
| Clinically relevant non-major bleeding events | ||||
| Number of participants with one or more events and non-missing event dates,d n (%) | 19 (14.2) | 28 (20.1) | 1.51 (0.84 to 2.71) | 0.165 |
| Number of participants with one or more events,d n (%) | 21 (15.7) | 32 (23.0) | ||
| Number of eventsd | 25 | 43 | ||
| Number of events per 100 person-yearsc | 8.13 | 12.50 | ||
FIGURE 3.
Cumulative risk of the primary outcome of time to first recurrent VTE (a) and of the secondary outcomes of time to first major bleeding (b), and time to first clinically relevant non-major bleeding event (c) between discontinued AT and extended AT.

Secondary outcomes
There were three major bleeding events (1.18 events per 100 person-years) in the control group (group D) and nine major bleeding events (3.54 events per 100 person-years) in the intervention group (group E), giving an adjusted hazard ratio of 2.99 (95% CI 0.81 to 11.05; p = 0.10). There were 19 clinically relevant non-major bleeding events (8.13 events per 100 person-years) in the control group (group D) and 28 clinically relevant non-major bleeding events (12.50 events per 100 person-years) in the intervention group (group E), giving an adjusted hazard ratio of 1.51 (95% CI 0.84 to 2.71; p = 0.165). These differences were not statistically significant (see Table 3 and Figure 3). In both groups, more people aged > 65 years experienced bleeding (Table 4).
| Characteristic | Trial group | Hazard ratio (95% CI)a | p-value for interaction | |||
|---|---|---|---|---|---|---|
| Control (group D) (n = 134) | Intervention (group E) (n = 139) | |||||
| Number of events (%) | Number of events per 100 person-years | Number of events (%) | Number of events per 100 person-years | |||
| Recurrent VTE | ||||||
| Sex | 0.099 | |||||
| Male | 23 (25.6) | 15.26 | 3 (3.2) | 1.75 | 0.11 (0.03 to 0.38) | |
| Female | 8 (18.2) | 10.23 | 4 (8.9) | 4.83 | 0.48 (0.14 to 1.59) | |
| Age (years) | 0.267 | |||||
| ≤ 65 | 17 (23.3) | 13.77 | 2 (2.8) | 1.52 | 0.11 (0.03 to 0.48) | |
| > 65 | 14 (23.0) | 13.28 | 5 (7.5) | 4.07 | 0.31 (0.11 to 0.85) | |
| Major bleeding events | ||||||
| Sex | 0.961 | |||||
| Male | 2 (2.2) | 1.18 | 6 (6.4) | 3.57 | 2.92 (0.59 to 14.48) | |
| Female | 1 (2.3) | 1.18 | 3 (6.7) | 3.49 | 3.13 (0.33 to 30.12) | |
| Age (years) | 0.190 | |||||
| ≤ 65 | 2 (2.7) | 1.45 | 2 (2.8) | 1.50 | 1.01 (0.14 to 7.17) | |
| > 65 | 1 (1.6) | 0.86 | 7 (10.5) | 5.79 | 6.89 (0.85 to 56.03) | |
The D-dimer levels pre-randomisation showed no difference in terms of risk of recurrence. Although a higher percentage of those patients with VTE recurrence had a baseline D-dimer level > 0.5 µg/l, this was not statistically significant (Table 5). Further work is being done as part of an ongoing Doctor of Philosophy (PhD) project to investigate whether or not there is an alternative cut-off point for D-dimer levels while still on therapy, which might be predictive of further events.
| D-dimer level at baseline | Recurrence, n (%) | Total (n = 273), n (%) | |
|---|---|---|---|
| No recurrence (n = 235) | Recurrent VTE (n = 38) | ||
| < 0.5 µg/l (%) | 216 (91.91) | 33 (86.84) | 249 (91.21) |
| ≥ 0.5 µg/l (%) | 9 (3.83) | 3 (7.89) | 12 (4.40) |
| Missing | 10 (4.26) | 2 (5.26) | 12 (4.40) |
Similarly, there was no significant difference in time in therapeutic range for patients on extended treatment between those with or without recurrence, but the number of participants was small (Table 6).
| Therapeutic range | Recurrence | Total (n = 121) | |
|---|---|---|---|
| No recurrence (n = 116) | Recurrent VTE (n = 5)a | ||
| Mean (SD) | 76.27 (15.27) | 83.93 (13.86) | 76.59 (15.24) |
| Median (IQR) | 77.03 (67.81–86.53) | 76.95 (75.22–97.63) | 77.02 (68.21–86.66) |
In terms of the quality of life and PTS outcomes, there were no differences between the groups (Table 7).
| Outcome | AT | p-value for time–treatment interaction | |||
|---|---|---|---|---|---|
| Discontinued (n = 134) | Extended (n = 139) | ||||
| n | Adjusted meana (95% CI) | n | Adjusted meana (95% CI) | ||
| VEINES-QOL | 0.766 | ||||
| 6 months | 118 | 50.13 (48.98 to 51.29) | 126 | 49.87 (48.74 to 51.00) | |
| 12 months | 116 | 50.13 (48.97 to 51.29) | 124 | 50.34 (49.20 to 51.48) | |
| 18 months | 112 | 50.74 (49.57 to 51.91) | 117 | 50.20 (49.04 to 51.35) | |
| 24 months | 108 | 50.33 (49.14 to 51.51) | 120 | 50.30 (49.16 to 51.45) | |
| EQ-5D-3L | 0.908 | ||||
| 6 months | 118 | 0.80 (0.76 to 0.83) | 126 | 0.81 (0.78 to 0.84) | |
| 12 months | 117 | 0.81 (0.77 to 0.84) | 124 | 0.81 (0.78 to 0.85) | |
| 18 months | 113 | 0.82 (0.79 to 0.86) | 117 | 0.82 (0.79 to 0.86) | |
| 24 months | 108 | 0.82 (0.79 to 0.85) | 120 | 0.81 (0.78 to 0.85) | |
| Severity of PTSb | 0.907 | ||||
| 6 months | 117 | 4.77 (4.24 to 5.30) | 126 | 4.73 (4.22 to 5.25) | |
| 12 months | 116 | 4.68 (4.14 to 5.21) | 123 | 4.88 (4.36 to 5.40) | |
| 18 months | 111 | 4.73 (4.19 to 5.28) | 115 | 4.96 (4.43 to 5.49) | |
| 24 months | 110 | 5.00 (4.45 to 5.54) | 120 | 5.09 (4.57 to 5.62) | |
| Outcome | AT | p-value for time–treatment interaction | |||
| Discontinued (n = 134) | Extended (n = 139) | ||||
| n | % | n | % | ||
| Category of PTSb | |||||
| 6 months | |||||
| None (0–4) | 66 | 49.25 | 71 | 51.08 | |
| Mild (5–9) | 42 | 31.34 | 36 | 25.90 | |
| Moderate (10–14) | 7 | 5.22 | 15 | 10.79 | |
| Severe (≥ 15) | 2 | 1.49 | 4 | 2.88 | |
| 12 months | |||||
| None (0–4) | 67 | 50.00 | 71 | 51.08 | |
| Mild (5–9) | 38 | 28.36 | 38 | 27.34 | |
| Moderate (10–14) | 10 | 7.46 | 10 | 7.19 | |
| Severe (≥ 15) | 1 | 0.75 | 4 | 2.88 | |
| 18 months | |||||
| None (0–4) | 63 | 47.01 | 63 | 45.32 | |
| Mild (5–9) | 39 | 29.10 | 37 | 26.62 | |
| Moderate (10–14) | 8 | 5.97 | 11 | 7.91 | |
| Severe (≥ 15) | 1 | 0.75 | 4 | 2.88 | |
| 24 months | |||||
| None (0–4) | 66 | 49.25 | 65 | 46.76 | |
| Mild (5–9) | 29 | 21.64 | 37 | 26.62 | |
| Moderate (10–14) | 11 | 8.21 | 12 | 8.63 | |
| Severe (≥ 15) | 4 | 2.99 | 6 | 4.32 | |
Discussion
Work package 1 adds to the accumulating evidence base that patients with a first unprovoked VTE, either DVT or PE, can benefit from prolonged anticoagulation in terms of reducing recurrence of VTE with no statistical increased risk of bleeding. Data that have been published subsequent to the start of WP1 have also demonstrated reduction in recurrence of VTE with no increase in bleeding using lower doses of alternative agents in RCTs. 21 The previously published study used apixaban at two doses or placebo in a population who had already been treated for 6 or 12 months and in whom there remained clinical equipoise in terms of continuing or stopping the AT, with follow-up for 12 months. The event rates for recurrence were somewhat higher in the current study, at 8.8% for the placebo group in the apixaban study compared with 23.1% for the control group (group D) in the current study. In terms of the treated populations, the event rates in the apixaban study were 1.7% for both the 2.5-mg and the 5-mg group compared with 5% in the intervention group (group E) from WP1. Patients were, of course, followed up for 2 years in WP1.
There were no differences found in the current study with regard to any of the other secondary outcomes, quality of life (including both disease-specific and generic measures) or the incidence or severity of PTS.
The results of D-dimer levels at baseline, prior to cessation of AT, were not discriminatory in terms of predicting VTE recurrence.
Limitations
Work package 1 did not achieve its recruitment target, which made it difficult to draw strong conclusions on the generalisability of these data. Two similar studies are currently being undertaken in Canada [DODS (D-dimer Optimal Duration Study)22] and the Netherlands [the Venous thrombolISm: Tailoring Anticoagulant therapy duration (VISTA) study], and we have an agreement with those triallists to combine data.
Another weakness of this study was that there was no blinding for some of the end-point adjudication, particularly the evaluation of PTS. However, in terms of thrombosis and bleeding, an independent end-point adjudication committee was established.
The cost-effectiveness of the approach followed in WP1 will be explored in the subsequent sections.
Summary
Work package 1 has demonstrated that extended treatment with anticoagulation for up to 2 years following initial treatment of a first unprovoked VTE provides protection in terms of recurrent VTE with a non-significant increase in the risk of major and non-major clinically relevant bleeding. No protection was seen in terms of PTS and there was no difference on either of the quality-of-life measures. Finally, no evidence was seen in this study to support the use of measuring levels of D-dimers while the patient is still taking anticoagulant treatment in terms of predicting who will have a recurrent VTE event.
Work package 2: Exploring Prevention and Knowledge of venous Thromboembolism (ExPeKT) – surveys
In previous sections, the findings from WP1 were described and summarised. This section reports on WP2, the qualitative element of the programme. The aim of this WP was to explore existing knowledge and barriers to implementing thromboprophylaxis pre and post hospital admission.
We would like to acknowledge both the British Medical Journal23,24 and the British Journal of General Practice25 for their permission in reproducing text and data from previous publications. Some parts of this report have been reproduced with permission from McFarland et al. 23 [This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/] and Apenteng et al. 24 [This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/]. Some parts have also been reproduced with permission from Litchfield et al. 25
Overview
Venous thromboembolism is a recognised risk following hospital admission. Most hospitalised patients have one or more risk factors for VTE. Around 60% of people undergoing hip or knee replacement will suffer a DVT without preventative intervention. Appropriate thromboprophylaxis reduces the risk of VTE by up to 70% for medical and surgical conditions. The introduction of the VTE Quality Standard [National Institute for Health and Care Excellence (NICE)26] and the CQUIN27 goal has led to improved VTE risk management in the hospitals.
Objectives
The objective of this series of studies was to explore the views of primary HCPs, patients and other relevant organisations on the potential role of primary care in hospital-acquired VTE risk management. Other objectives were to assess the level of existing knowledge of VTE risk among a range of primary HCPs and patients, to assess current practice and the perceived role of primary care in thromboprophylaxis among primary HCPs and patients; and to explore the interface between primary and secondary care in terms of thromboprophylaxis.
Methods and analysis
The studies were carried out using a two-stage, mixed-methods approach using surveys with primary HCPs and patients followed by interviews with primary HCPs, patients, acute trusts and other relevant organisations. Survey and qualitative interview data were used to examine the current practice of thromboprophylaxis, and the knowledge and experience of VTE prevention.
Survey data were analysed using SPSS (Statistical Product and Service Solutions) version 20 (IBM Corporation, Armonk, NY, USA). Open-ended responses were analysed using qualitative thematic methods. The recorded and transcribed semistructured interview data were analysed using constant comparative methods.
Ethics and dissemination
Ethics approval was provided by the National Research Ethics Committee (reference: 11/H0605/5), and site-specific research and development approval was granted by the relevant research and development departments in each NHS trust.
Setting
Two acute trusts in the UK, and Oxfordshire and South Birmingham PCTs.
Participants
-
Patients were recruited from medical, surgical and orthopaedic wards from acute trusts in Oxfordshire and Birmingham.
-
All of the GPs and practice nurses in Oxfordshire and South Birmingham PCTs took part.
-
HCPs and personnel based in other organisations involved in supporting patients with thromboembolic disease contributed to the other elements of this WP.
Work package 2: surveys and interviews with patients and health-care professionals regarding prevention of venous thromboembolism
Background
In 2012, the APPTG (established in 2006 to promote awareness of VTE among parliamentarians) recommended that primary care should take an increasing role in the VTE prevention pathway including pre-assessments of VTE risk for elective hospital admissions. It also recommended that there should be an increasing role for informing patients about the risks of VTE and preventative treatment, and that a robust system be developed to ensure that primary care clinicians are informed when patients are discharged on extended VTE prophylaxis so that patients can be managed and supported effectively. 3
Subsequent to these initiatives, NICE published guidance in 2018, but this was after this WP was undertaken. 7
Venous thromboembolism is a major public health problem, with VTE-related deaths per annum estimated to be almost 550,000, more than double the 209,926 combined deaths due to AIDS, breast cancer, prostate cancer and road traffic accidents. 28 Many of these events are hospital acquired and could be prevented, given the availability of effective VTE prophylaxis. 5
One in every 100 patients undergoing total knee replacement and 1 in every 200 patients undergoing total hip replacement will experience a VTE event before hospital discharge. 4 Without thromboprophylaxis, approximately 60% of patients undergoing major orthopaedic surgery, which includes total knee or hip replacement, will experience a confirmed DVT. 29 Similarly, acute medical illness patients have a moderate (10–20%) risk of developing a DVT. 30 The association between VTE and cancer is well established,31–33 and one in seven inpatients with cancer dies of PE. 34 With appropriate thromboprophylaxis, the risk of VTE can be reduced by up to 70% for medical and surgical conditions,35 and clinical trials have shown that the use of anticoagulants reduces the risk of VTE in hospitalised acutely ill medical patients. 5,6
However, VTE is highly relevant to the primary care context as a large proportion of postoperative VTEs happen in the community. 35 One study showed that 36.8% of patients developed VTE within 3 months of hospital discharge. 36 A further study of 3039 patients admitted to post-acute care after a medical condition or surgery showed that 2.4% of patients developed VTE within an average of 13 days. 37 The average length of hospital stay for medical, surgical and critical care patients is 5.3 days. 16 After discharge from hospital, these patients may discontinue thromboprophylaxis and will remain at risk of VTE for some time. Research suggests that the risk remains for up to 90 days after hospital discharge10 Extended thromboprophylaxis for at-risk medical and surgical patients that is easily administered in a community setting is essential for a reduction in VTE rates and could be cost-effective. 8,9
Many patients do not receive appropriate VTE prophylaxis both as inpatients and post discharge,35,38–40 despite government guidelines (which recommend that all patients admitted to hospital be assessed for risk of developing DVT and be given preventative treatment) and the availability of effective thromboprophylaxis. 41 Recent studies have shown that around 37% of all at-risk patients did not receive thromboprophylaxis in hospital,40 25% of medical or surgery patients at risk of VTE did not receive thromboprophylaxis in post-acute care settings, and only 54% of orthopaedic surgery patients had a prescription for thromboprophylaxis dispensed 30 days after discharge. 40,42 The need for daily injections of low-molecular-weight heparin may create problems with compliance in the outpatient setting. 37
Objectives
The aim of the research was to explore the potential role for primary care in hospital-acquired VTE risk management.
The specific objectives were to:
-
assess the level of existing knowledge of VTE risk among a wide range of primary HCPs and patients
-
assess current practice and the perceived role of primary care in thromboprophylaxis among primary HCPs and patients
-
explore the interface between primary and secondary care in terms of thromboprophylaxis
-
identify local organisations’ perceived and actual clinical barriers to the implementation of thromboprophylaxis for high-risk patients
-
explore potential care pathways for high-risk patients prior to hospital admission in terms of assessment for thromboprophylaxis.
Methods
A mixed-methods approach was employed, which involved a postal survey of patients, GPs and practice nurses followed by interviews with a subset of survey respondents and other stakeholders. This approach was selected to obtain the views of a broad range of GPs, hospital clinicians, practice nurses and other stakeholders but also to further explore specific issues identified in the survey.
Surveys
Patient survey
A postal survey of hospitalised patients who were assessed to be at a high risk of VTE was conducted. Patients were recruited from three hospital trusts in Oxfordshire and the West Midlands.
A range of wards was recruited to ensure that orthopaedic, surgical and medical patients were represented in the sample. The specific wards included orthopaedic surgery, general surgery, cancer, upper gastrointestinal, lower gastrointestinal, cardiothoracic, renal and urology, and trauma.
Patients were eligible for the study if they were admitted to the participating wards during the recruitment period and were assessed to be at a high risk of VTE (identified by screening VTE risk assessments in hospital records). Patients requiring prophylaxis during their admission only and those requiring extended prophylaxis post discharge were eligible to participate. Research nurses approached eligible patients on the wards and those agreeing to participate were asked to provide informed consent. Recruited patients were subsequently sent a study pack immediately after discharge or 1 month following discharge, if they required extended prophylaxis. The study pack contained a questionnaire, cover letter and freepost return envelope. A reminder pack was sent to non-responders after 1 month.
The patient questionnaire was designed to explore patients’ experiences of VTE risk management prior to, during and post hospital admission. The main areas covered were knowledge and understanding of VTE risk and prevention, VTE information received and the format, and VTE prophylaxis received. The questionnaire employed multiple choice and open-response question formats and could be completed in 15 minutes.
Primary care survey
A postal survey of all GPs (n = 817) and practice nurses (n = 583) within Oxfordshire and South Birmingham was conducted simultaneously. GPs were identified from the NHS choices register43 and practice nurses were identified from practice websites or by contacting practices directly. A copy of the questionnaire with enclosed consent form, participant information sheet, cover letter and freepost return envelope was included in the study packs. Respondents were asked to indicate on the survey whether or not they would be willing to participate in an individual interview.
The GP and practice nurse questionnaires were developed using the NICE guidelines8 and the Department of Health and Social Care’s risk assessment tool. 9 The main areas covered by the GP and practice nurse survey were knowledge of hospital-acquired VTE risk and appropriate management; training and current practice of VTE risk management; views of the potential role of primary care in VTE risk management; and the barriers that exist to its implementation. The survey employed multiple choice, Likert scale and open-response question formats and could be completed in 5–10 minutes. Data were double entered into SPSS software and analysed descriptively.
Patient characteristics
A total of 878 patients were recruited and sent the study pack and 564 of these returned completed questionnaires (64.2%). The mean age of respondents was 64.3 years [standard deviation (SD) 11.8 years], 38.8% (219/564) were male and the large majority were from a white background (97.5%, 550/564). A total of 50.5% (285/564) had either a university/college degree or a professional/commercial qualification, over half (53.4%, 301/564) were retired and 33.1% (187/564) were in either full- or part-time employment.
Most admissions were planned (91.7%, 517/564) as opposed to emergencies (7.3%, 41/564). Over two-thirds of admissions were for orthopaedic surgery (69.3%, 391/564) and all other admissions were general medical or surgical cases. The mean length of stay was 8 days (SD 14 days) and the mean time since discharge from hospital to the interview was 39 days (SD 21 days).
Primary care characteristics
Of the 817 GPs and 583 practice nurses who were sent study packs, 92 GPs (11.3%, 92/817) and 19 practice nurses (3.3%, 19/583) returned the surveys. The level of seniority and experience varied within the sample. The sizes of practices ranged from 472 to 52,000 patients (mean = 10,162 patients, SD = 6425 patients). Over one-third of the respondents indicated that they had an anticoagulation clinic at their practice (36.7%, 40/111).
Interviews
General practitioner interviews
One-third of respondents (37/111) indicated on the survey that they would be willing to participate in an interview. All 37 professionals were contacted by telephone. Of these, three had retired and a further 20 were unable to find a convenient time to take part or requested an online or telephone interview, which they failed to complete. Fourteen participants were subsequently interviewed for the study. This comprised 12 GPs and two advanced nurse practitioners who were commissioned by primary care trusts to provide a 24-hour rapid-response service for suspected DVTs. Interviewees were e-mailed an additional information pack comprising a covering letter and a further participant information sheet. Additional informed consent was obtained prior to the completion of the interview or provided verbal consent for telephone interviews. Interviews lasted between 10 and 50 minutes; all interviews were digitally recorded and transcribed verbatim.
The interview schedule was developed to further explore the areas covered in the survey and explored the following: GPs’ existing knowledge of the problem of hospital-acquired VTE and whether or not they have a role in reducing this problem; GPs’ current practice in VTE prevention; and GPs’ awareness of patients’ ability to recognise the symptoms of a DVT. We examined potential care pathways for high-risk patients prior to hospital admission and the interface between primary and secondary care with regard to the responsibility for a discharged patient on extended prophylaxis. The interviews sought to identify any barriers to GPs having a role in VTE prevention and management.
Transcripts were identified by code number only. Participants were not identified in any written material resulting from the interviews. The recorded and transcribed semistructured interview data were analysed using constant comparative methods. 44 Data were managed using NVivo 9 software (QSR International, Warrington, UK). Lorraine McFarland independently reviewed all the transcripts and developed codes in an iterative process to identify emerging patterns in the data and an initial coding framework. Using constant comparison, similarity and differences were identified within and across the transcripts. By comparing each part of the data, analytical categories were established and key concepts selected. Final themes were reviewed and agreed between four of the authors to enhance reliability.
Participants
Fourteen participants from two primary care trusts in the UK were interviewed for the study: 12 GPs and two advanced nurse practitioners who were commissioned by primary care trusts to provide a 24-hour rapid-response service for suspected DVTs.
Interviews with other health-care professionals and relevant organisations
Methods and analysis
A qualitative research design was used with data collected via face-to-face or telephone interviews. Interviews took place with personnel from acute trusts and other relevant organisations and examined the current practice of thromboprophylaxis, and the knowledge and experience of VTE prevention. The interviews explored the interface between primary and secondary care in terms of VTE prevention and the perceived role of primary care, and examined interdisciplinary communication, perceived barriers to VTE management, training provision and future requirements for VTE management. Key informants were identified, followed by snowball sampling. The recorded and transcribed semistructured interview data were analysed using constant comparative methods.
Interviews were conducted with a purposive sample of staff in four acute trusts and a sample of people from relevant organisations such as the Lifeblood Charity (www.thrombosis-charity.org.uk) and Anticoagulation Europe (www.anticoagulationeurope.org).
Participants
Seventeen participants were interviewed for the study. This group comprised a primary care trust commissioner, charity personnel, clinicians and VTE nurses and trainers.
Participants were provided with an information pack comprising a covering letter and a participant information sheet. Participants were asked to complete a consent form at the time of contact or provided verbal consent for telephone interviews. All interviews were digitally recorded with the permission of each participant. Content of the recordings were transcribed verbatim.
Patient interviews
Face-to-face semistructured interviews with a purposive sample of 31 high-risk patients who responded to the survey were undertaken. Interviews explored the topics elicited from the initial survey and were carried out in patients’ homes. The interview schedule topics included patient awareness of VTE; satisfaction with VTE information and understanding of the information received; adherence to treatment; the need for primary care intervention; and issues to increase awareness of VTE.
Methods
Participants were provided with an information pack comprising a covering letter and a participant information sheet and were asked to complete a consent form at the time of contact. All interviews were digitally recorded with the permission of each participant. Voice recordings were transcribed verbatim. Transcripts were identified by code number only. Participants were not identified in any written material resulting from the interviews. The recorded and transcribed semistructured interview data were analysed using constant comparative methods. Data were managed using NVivo 9 software. All transcripts were independently reviewed and codes developed in an iterative process to identify emerging patterns in the data and an initial coding framework. Using constant comparison,21 similarity and differences were identified within and across the transcripts. By comparing each part of the data, analytical categories were established and key concepts selected. Final themes were agreed between four of the authors to enhance reliability. Representative quotations that illustrate both typical responses and a range of views have been selected to illustrate the study findings.
Ethics
Ethics approval was provided by the National Research Ethics Committee (reference 11/H0605/5). The trial grant number was NIHR RP-PG-0608-10073.
Participants
A total of 31 surgical, medical, cancer and trauma patients from five separate hospitals in two NHS trusts were interviewed.
Results
Representative quotations that illustrate both typical responses and a range of views have been selected to reflect these themes.
Summary of findings
Although the findings from the individual studies have been published23–25 for the purposes of this report an overview of the findings will be presented.
The following common themes were identified:
-
communication
-
knowledge
-
role of primary care
-
education and training
-
barriers to patient adherence.
Communication
Data from all parts of this work package highlighted the issue of communication, be it between HCPs and patients or between the HCPs themselves.
From the patient survey, 138 out of 878 (16%) patients received information regarding VTE prevention from primary care, whereas 702 out of 878 (79%) reported receiving information on hospital admission, and 471 out of 713 (66%) of those discharged on extended prophylaxis reported receiving information about this.
A total of 843 out of 878 (96%) patients reported receiving treatment for VTE prevention and 713 out of 878 (81%) reported continued treatment after they were discharged. The majority of patients received pharmacological prophylaxis alone, antiembolism stockings alone, or both pharmacological prophylaxis and stockings during their hospitalisation.
In terms of communication between HCPs, it was clear that there was dissatisfaction from both primary and secondary care.
From the primary care perspective, there was concern about both the quality and timeliness of information received on hospital discharge with delays of up to 6 weeks mentioned:
No not really, we don’t really have that much contact really. A lot of the time the correspondence that we get from hospital is quite delayed. So when a consultant’s decided that there’s going to be a planned surgery, it can sometimes be delayed by up to 4/5/6 weeks before we get told about it. And also, after the procedure, and I guess this probably makes it more difficult, after they’ve had the procedure done, again it can take up to 5 or 6 weeks before we even find out that they’ve had a surgical procedure done . . . So I guess that would be one hindrance to us actually, getting people started on things when we don’t find out sometimes 5 or 6 weeks later. And that’s probably the most critical period, when they’re going to get a DVT or a PE.
GP1
I think it’s one of these areas, it’s like a lot of areas in medicine, where there is no connection between secondary care and primary care. The secondary care somehow thinks that we’ll pick it up. Well we may not because we never get the discharge summary for 2 weeks. We don’t necessarily receive the discharge summary for a couple of weeks after the patient’s been discharged . . . Well now, because the number of appointments has risen and the number of patients on their books has risen an extra bit, it rises by the month, some patients say, well we’ve got this letter and the receptionist just takes it off the patient and so we don’t actually get to see them. It’s all to do with the amount of appointments we have is just limited.
GP6
The content and quality of patient discharge notes received by the GP vary widely and are a concern for many participants. They suggest that discharge notes are often brief and lack basic information:
Completely pot luck it just depends on the quality of the discharge summary. Some discharge summaries are very good, they tell you what the dose of Clexane that they want you to give and for how many weeks and what they’re treating it for. And then on other hand, you just don’t really get any feedback at all. And it’s only sometimes when patients ring you up and say, I’m on a Clexane injection and I’ve been told to get some more from my GP. So it varies largely.
GP1
One participant acknowledged that there is a gap in the care pathway where no one has responsibility for a discharged patient on extended prophylaxis. One GP questioned if the responsibility to make sure that a patient has been discharged with prophylaxis lies with the consultant having done the operation or with primary care:
A lot of the time . . . we get a phone call saying, oh can you come and do a home visit, leg swelling or pain in the chest. And a lot of the time, when you diagnose it, you then look back and think, actually somebody should have been responsible for looking after this patient. I think once they’ve had their operation done, I think it’s a grey area, in terms of where the responsibility lies. Does it lie with the consultants who’ve done the operation to make sure that they’ve sent patients home with prophylaxis, or whether it’s our job then to just make sure that they are on prophylaxis when they’ve come out?
GP1
It was suggested that a solution to this grey area in patient care would be an official handover where acute trusts request that primary care takes over:
Some consultants will probably say responsibility lies with them, they’ve done the operation, they should decide on prophylaxis and how long for. Whereas some consultants would say it should fall upon the GP because it is an extended course and it’s the GP’s responsibility to follow it up. But yes, I think up until we probably get good communication or some kind of pro forma where there’s an official handover occurring saying, right, I’ve done this operation, this is what I’ve started them on, please would you carry on either prescribing this or looking after the patient. So I think that probably needs to be improved.
GP1
Secondary care should be responsible until they have effectively communicated the patient’s needs to primary care and their GP has agreed to take over responsibility.
GP9
However, participants confirmed that they do not make contact with a discharged patient on the assumption that the hospital had made the necessary arrangements. Shortfalls in the procedure are put down to hospitals not doing their job properly:
We wouldn’t make active contact, no, if somebody was discharged, I would assume that the hospital had sorted it all out really. We don’t really have time to phone people up just in case the hospital hasn’t done their job properly.
GP5
Generally, if I think they’re a fit young person or they haven’t got any physical or mental issues, then I would tend to leave the patient alone, unless they contact me with any issues, but otherwise, no.
GP1
The GPs in rural practices are more likely to contact a patient discharged from hospital on low-molecular-weight heparin:
In our practice, when we see they’re on low-molecular-weight-heparin, sometimes I’ll give them a ring and say, are you alright giving yourself a jab or do you want to come up to the surgery to have it done? But that’s because we’re a rural practice. But if you’re in a big city or a big town practice, no you don’t have the time to even breathe let alone ring them.
GP6
Delays in receiving discharge notification could make prescribing difficult for primary care and one participant felt that commissioning should not be split between providers:
Sometimes you don’t hear until the patient has been discharged for a couple of weeks and if you can provide a week’s anticoagulation I really can’t see why you can’t provide a month’s anticoagulation because it would be a much neater package commissioning it this way rather than splitting it between various providers.
GP7
Similar issues were raised by secondary care clinicians with regard to communication both to and from primary care. It was pointed out that VTE occurs and is usually diagnosed in the community and, thus, the GPs have a role to play:
I think we’ve missed a trick here – there’s this belief that thrombosis is an issue that is seen and dealt with within the hospital. Most people who will have a hospital-acquired thrombosis will have it in the community because you don’t tend to get a DVT or a PE until you are discharged. So before you even look at prevention of VTE – the majority of thrombosis will be diagnosed in the community. Prevention even more so . . . So primary care has a large role to do here. Now maybe it’s unfair on them that it’s all being dumped into the community but actually that is the price you pay for having less beds and it’s the price you pay for having to free up beds to admit the acutely ill patients.
Consultant and VTE lead
Hospital staff expressed concerns regarding whether or not patients complete the course of extended prophylaxis when they have been discharged from the hospital as ‘a bit of a grey area’:
We send patients home on Clexane, they’ve been shown how to do it, but they [midwives] feel they are not actually administering it at home. Some will be visited by community midwives more regularly and they can check up on that but you might have a lady that isn’t seen and no one seems to check up that they are actually taking their thromboprophylaxis or not. It could be the same in other patients.
VTE prevention lead
From our root cause analysis we’ve done on patients most of them have gone home with the necessary prophylaxis and certainly now we’ve developed a standard pro forma root cause analysis form. One of the questions on it is now, ‘did you complete the course?’ or ‘did the patient complete the course?’ and we’ve started asking them that if we can, if we are able to contact them. So yes that is a bit of a grey area. You can’t know for certain if they are self-administering. I suppose that is something we could ask when they come back to clinic but then it’s after the horse has bolted then, isn’t it?
Clinical nurse tutor
There also remains a problem of used sharps disposal for the patient:
Used sharps disposal is also a challenge and often the patients are asked to bring them back to hospital.
Consultant nurse for anticoagulation
It was suggested that ‘patients need to know what to expect’. When this participant was asked who could do this, they responded with: ‘The GP’ (consultant nurse for anticoagulation).
There were few positive comments regarding communication between secondary and primary care, with the one notable example being in maternity services:
It seems to work very well for maternity-related thromboprophylaxis with very few problems between secondary and primary care.
Community pharmacist
The need for effective communication between every level of the NHS for the safe management of extended thromboprophylaxis has been recognised at the commissioning level:
Extended thromboprophylaxis can be needed for quite a long time. The whole system needs to manage that safely and that’s about communication . . . Whilst the individual provider or the individual surgery thinks that everything is fine – it’s only when you look at the overall picture you realise, actually patients came out of this clinic and information wasn’t sent to the GP and, therefore, the GP doesn’t know what’s going on, and so we try and monitor that system from an overall point of view . . . the incentives are much more focused on the business of a hospital to deliver a business bottom line in terms of funding than working across a whole system to actually ensure that all the safety and all the protocols are properly joined up with other providers outside their business. There is something there about actually the market system around secondary and primary care it’s not joined up, and that’s a problem.
Commissioner
In areas of high risk such as anticoagulation and that type of management that the commissioner needs to be really understanding, looking at what’s going on and looking at where the risks are and really trawling, quite actively, trawling to try and see where they can identify risks . . . given that things like anticoagulation and the top 10 risks and the top 10 examples of avoidable admission we should be focusing, as commissioners, much more upon those clinical areas we’re being encouraged to do than to focus on other things that commissioners get involved in, which might be less outcome based.
Commissioner
Knowledge
Health-care professionals
As part of the primary care survey, respondents were asked to estimate the annual number of deaths from hospital-acquired VTE. According to the House of Commons 2005 Health Committee Report, the figure is approximately 25,000. 45 Answers ranged from 100 to 90,000 within our sample (mean = 29,619). Respondents were also asked to correctly identify the risk factors for hospital-acquired VTE and the patient groups requiring extended thromboprophylaxis according to the Department of Health and Social Care’s risk assessment tool. The majority correctly identified all risk factors.
From the interviews, one GP suggested that there is very little awareness of VTE in primary care. The results demonstrated that health-care professionals’ experience of DVT ranges from many cases (the advanced nurse practitioner, specifically responsible for attending suspected DVTs, saw one case per day) to the GPs who have seen only a few, or one or two per year (the more common experience). Participants who see many cases of DVT suggested they are related to hospitalisation and orthopaedic surgery. GPs seeing one or two cases every month tended to be those working with patients with drug addiction and compromised veins. Seeing few patients with DVT in primary care was attributed to the protocols being followed in hospitals:
There is very little awareness; there is little awareness amongst primary care staff.
GP7
I see many cases of DVT in the community and the majority are related to previous hospitalisation.
GP10
I see probably one a day . . . connected to orthopaedic surgery mainly.
Advanced nurse practitioner 11
So I mean I don’t see many cases personally of patients coming out of hospital suffering a VTE, and I guess that’s because protocols are being followed in hospital . . . we see very few cases.
GP3
Patients
Patient understanding and awareness of hospital-acquired VTE appeared vague and incomplete. Many patients had an elusive idea about their VTE preventative treatment but they generally deferred to the health professional ‘knowing what they were doing’:
All I had was morphine and my usual tablets. That’s all I had.
Did you say you had stockings, surgical stockings?
Yep, yeah, those nice white ones.
Did you understand why you needed to use those?
Well, it’s obviously to stop the blood clots forming. I don’t understand why, it’s just there and not anywhere else on the body. But there we go, they must know. Yeah.
When I went to see the surgeon, in the follow-up interview, I asked him why I’d been given these anticoagulants, and he said that that was something that everybody was given who had an operation.
P1 Orthopaedic patient
Recollection of information was unspecific. P11 suggested the required information would have been given among all the other information received but the amusing anecdote evokes the best recall:
When you saw the surgeon, he mentioned all of the risks, he went from, you’re going to have this, you’re going to have this but this is what could happen, you could get an infection, you could get this, and he listed so much stuff and by the time he’d finished I went ‘Oh my God I’m gonna die.’. And in there somewhere would’ve been blood clots. But I don’t remember specifically talking to the nurse about, at the hospital.
P11 Orthopaedic patient NOC53
Many patients said that they knew about blood clots but did not link them to their own situation. Recall of the information provided in hospital was vague, even suggesting that they were told about blood clots when flying:
I’m quite ignorant about blood clots. I mean you always think when you’re flying, people say you can get blood clots.
P19 Orthopaedic patient ORH31
Can you tell me about the stockings? Did they explain to you why you were wearing them? Not really, it was to do with the aircraft.
P22 Medical patient ORH60
A patient was aware of the connection between immobility and blood clots:
Nowadays you’re not kept in bed for any longer than absolutely necessary to avoid blood clots. ’Cause years ago they used to keep people in bed and then they had thrombosis and things as a result which wasn’t very good but they know more now.
P24 Orthopaedic patient NOC120
A medical patient felt that the hospital was very informative regarding VTE information but was too poorly on the first day to consider the information:
I was aware that they risk assess and also they actually, even though I was having aspirin, I had the injections . . . because I’d been in hospital probably 6 months earlier as well, I’d gone through all that anyway. So I actually, actually I felt too poorly to be bothered the first day, but they were very informative.
P27 Medical patient UHB47
One orthopaedic patient felt that there was too much emphasis placed on blood clot prevention. This patient experienced haemorrhaging after hospital discharge:
If anything I would say probably, over, people are really, really quite frightened of it, the staff are and things like that, there’s no doubt about it. They concentrate very heavily on it . . . What the mistake was, it was the medics in the hospital that were warfarinising me too heavily too quickly, which comes full circle to my analysis of, they’re too paranoid about the blood clots.
P4 Orthopaedic patient NOC99
From the patient survey, it was found that, prior to admission, 15.7% received information from GPs or practice nurses in primary care. Secondary care health professionals provided advice to 79.3% of patients on admission to hospital and to 66.4% of those patients discharged from hospital on extended prophylaxis. Post discharge, 12.8% received information regarding blood clot prevention from GPs or practice nurses in primary care.
Role of primary care
The large majority of the GPs and practice nurses reported that they never or only occasionally conducted VTE risk assessments (94.6%, 105/111), yet 34.2% (38/111) believed that this role should fall within the remit of primary care. Even more respondents (50.5%, 56/111) believed that they should be providing advice to patients prior to elective hospital admissions, but, in practice, 79.3% (88/111) never or only occasionally do this. Involvement in VTE risk management post hospitalisation was higher in those surveyed, with 39.6% (44/111) reporting that they never or only occasionally managed extended thromboprophylaxis and 64.2% reporting that they never or only occasionally provided advice about VTE risk to their patients following discharge from hospital. Three-quarters of respondents (74.8%, 83/111) believed that primary care should manage patients requiring extended prophylaxis and 58.6% (65/111) believed that primary care should provide advice to patients requiring extended prophylaxis following hospital admission.
Despite a substantial proportion of respondents indicating that primary care should take on a greater role, the majority of GPs and practice nurses perceived barriers to conducting VTE risk assessments (84.7%, 94/111), managing extended prophylaxis (73.0%, 81/111) and providing VTE management advice (69.4%, 77/11) in primary care. The main barriers identified were lack of time, resources and expertise; lack of continuity of care and poor communication between primary and secondary care; lack of awareness of planned hospitalisations; lack of knowledge of exact regimes and risk/benefit ratio of prophylaxis; lack of understanding of risk associated with different procedures and the potential complications; lack of consistency over VTE risk management protocols at different hospitals, the cost of prophylaxis and confusion over whether primary or secondary care is responsible; the mobility/wellness of patients to attend surgery for prophylaxis; and the pressure from many other areas in primary care.
One participant who was aware of the NICE guidelines7 for VTE felt that they were hospital focused:
NICE guidance exists but I think current guidance is predominantly hospital focussed.
GP15
Some participants felt that they, as GPs, should have a role in reducing the risk of VTE, but were unaware of any specific guidelines and were vague regarding what that role would entail or how it could be implemented. They suggested that their role should only encompass preventative medicine and that primary care should not be considered as the safety net for VTE prevention. Participants quoted a series of reasons why their involvement would be difficult, in particular the logistical problem of having no contact with patients before hospitalisation and the burden of adding to their already exhaustive workload:
All GPs should have a role in reducing risk of VTE. However cannot recollect any specific guidelines for general practice.
GP8
A good question, we should do [have a role in reducing hospital-acquired VTE]; I don’t think we know what our role is at the moment.
GP4
I don’t think it’s a safe system to rely on primary care alone because there are times when we don’t have anything to do with the patients before they go in. So I think that it’s something that we could be doing, sort of preventative medicine, but I don’t think we could necessarily be the safety net.
GP4
Additional funding and resources to enable primary care to take on a role in VTE prevention was a major factor for several participants. It was felt that primary care was well placed to improve outcomes, particularly as they had access to patient medical details and histories, but their involvement would require clear planning to encompass training, resources and regular audit:
Limited role for primary care due to capacity in general practice to take on additional work which is not funded.
GP8
A small role – but not one that I am prepared to take on without extra time and remuneration.
GP10
Primary care is well placed to improve outcomes in this area but clear planning, training provision and resource, with regular audit, would be needed to ensure this was done effectively and safely.
GP9
A lot of the time we know the patients and their background history. I can’t see why we can’t do a risk assessment. I guess the cynics amongst us would probably say, well where are we going to get the time to sit down with every patient who’s going in for a planned elective admission, to go through the risk assessment? I guess that’s just a matter of time and resources really.
GP1
However, one participant would not want to take on risk assessment in general practice because it would complicate the process and impose additional appointments on their workload. In addition, there could then be a problem in linking the patient record between general practice and the hospital:
In terms of assessing people who go up to hospital, I would not want to take that on in general practice. I think that that would be complicated; it would be an unnecessary appointment for the patient. So it would be duplicating patient’s, you know, it would make things more complicated for the patient. What should happen is, that should happen either when somebody is listed for surgery, that they should go and see a nurse at that appointment, or they should, if they’re having a pre op clinic, they should be discussed at the pre op clinic. For them to come and see us about it, that means a third appointment, which is inappropriate. So it’s not good for the patient, it’s not good for us and it doesn’t make sense from the hospital’s point of view because they haven’t got a record of whether we’ve done it or not. So on no count does that make sense.
GP5
Some participants felt that primary care could step in if there was a breakdown in the care pathway but essentially thought that acute trusts should be the main provider:
In terms of context – side effects experience or if there is a breakdown and the district nurse does not turn up then primary care has a role to play there but in routing provision of that, that should be commissioned as one package delivered by the same team and in terms of delivery I don’t think primary care should take over that.
GP7
Not our role, should be in their routine provision of information from hospital.
GP14
Similarly, participants felt that there was a role for primary care in maintaining patient compliance:
There is some role for them [GPs], from my point of view, this is just on a personal level as a nurse, if we have supplied the stocking for a prophylaxis, we should be following that through, it’s a prescription. If I prescribe an antibiotic, because when I go out to these patients, some of them don’t have a DVT, they’ve got cellulitis, I prescribe an antibiotic for them. If they’re not then taking their course of antibiotic, then I have to do something about that. But just supplying a prescription for a tens [TED stockings (thromboembolic deterrent)] stocking, and then the patient doesn’t do anything about it, then, you know, there’s a failure in the system.
Advanced nurse practitioner 11
I think there’s a role in trying to maintain compliance, yes totally. But whether we can do much more than that, I don’t know.
GP4
Raising awareness of hospital-acquired VTE risk was a more acceptable role for primary care to take on, but, as the task is already set up in acute trusts, participants were reluctant to see why they should take it on as well:
I think we should make them more aware of the risks definitely, so that, I guess that’s another way to tackle it, whether we somehow collate data on patients who are going in to hospital and send them this information beforehand. And get the patients to prompt their consultants and question them whether they should be coming home with any prophylaxis. That could be another strategy, yes.
GP1
Patient awareness, that would be fine but only if there is a system for doing this systematically, otherwise it will be haphazard.
GP13
One participant felt happy to provide prescriptions to patients and send out district nurses to help elderly patients with injections:
Yes, we’re more than happy to give that out to our patients. And patients who are confident enough to self-administer it will be quite happy to ask us for some more. Those patients who are elderly and are unable to administer it, we also get our district nurses to go out and give them their Clexane injections. So yes, we’re quite happy prescribing it.
GP1
A specific concern was when GPs accepted hospital procedures without question:
I think a lot of the time the ones that probably do get missed are the ones who’ve been released by hospital and we’ve probably not questioned why they haven’t been sent home, especially if they’re at high risk of DVTs, on Clexane or stockings. So I think we, as GPs, should question discharges probably a bit more and especially after big operations. And I think probably, the tendency at the moment is that we do leave it in the hands of the consultants. And if they are on prophylaxis afterwards we accept that and if they’re not, again we just accept that. So I think we should probably question it a bit more.
GP1
Time pressures and financial resources were cited as the main barriers to primary care having a role in VTE management:
The key barrier, I think for me, would be the time and the resources because I’ve done risk assessments before and I’ve not had any difficulty in doing that. And I think especially if you use something that’s been validated, like the Wells’ scoring system, it’s mostly a tick-box exercise and it gives you a score at the end. So I don’t see why we shouldn’t be able to but I think, like I said it’s just the time and the resources.
GP1
One participant suggested that any funding provided to primary care for their involvement in VTE management should be set against a system of target outcomes that shows that DVT rates have gone down. Furthermore, it is felt that acknowledging the cost of VTE in terms of health burden would offset the financial resources required:
I think it’s just highlighting the costs of post-operative DVTs, PEs, not just the financial cost but also the health burden as well. And getting the health professionals to acknowledge the costs and kind of balancing it up with the time and the resources that they’ll need to put in to prevent those DVTs and PEs from occurring.
GP1
Education and training
The issues of education and training, across the range of health professionals and patients, was repeatedly noted.
There was little evidence of any training in VTE across the primary care participants:
No, no official training. I think that would be the same across the whole number of staff. We’ve not really had any official training.
GP1
It’s non-existent actually.
GP7
Not specifically, no.
GP3
Participants had varied suggestions as to how training could be implemented. This included outside speakers attending the practice at lunch time, training using a CD (compact disc) or YouTube presentation (www.youtube.com; YouTube, LLC, San Bruno, CA, USA), a purpose-written online module, training in the form of continuing professional development, and training form information published in GP magazines:
Training I think would be good generally across all staff members, nurses and doctors. And how could it be implemented? I mean yes, I think either outside speakers could come in and give the practice a talk during the lunch time session or probably inviting GPs to go to a seminar that’s being held. That would probably be a way of approaching it.
GP1
The easiest thing is for a, somebody to come and deliver in-house training to the whole team.
GP2
Theory and practical aspects provided as a day course or over the internet.
GP8
Junior doctor training in terms of risk assessment was repeatedly highlighted as an area of concern. Ongoing training may be necessary to continue to promote awareness and develop understanding to prevent the risk assessment becoming merely a tick-box exercise. It is necessary for clinical staff to understand why the exercise is so important. Engaging with the problem may subsequently ensure that there is follow-on action from the risk assessment:
‘There is very little awareness; there is little awareness amongst primary care staff . . . Also amongst secondary care staff because many see this as a chore, as a tick-box exercise.
Clinician
It can just end up being another piece of paper another tick-box exercise. I think that’s where the importance of the training comes in because you need people to understand why it’s so important.
Nurse tutor, VTE committee member
There was concern regarding current risk assessment guidelines7 in that CQUIN payments are based on the number of patients risk assessed with disregard as to whether or not there has been any action based on that assessment:
One of the weaknesses of the current strategy is that the outcome that is being measured for the moment is – the number of risk assessment forms completed. The focus needs to be on whether they have been completed correctly and clinicians have acted on that assessment. People seem to think that it is all about identifying whether a patient is at risk of thrombosis but a risk assessment tool is also there to identify whether a patient is at risk of complications of thromboprophylaxis and, therefore, it is essential that the information is used to guide practice.
Consultant and VTE lead
Individual trusts have developed their own literature for the education of both junior doctors and patients. In some cases, staff information has been adapted for patient use. Previously expressed concerns regarding low reading ability and poor levels of understanding among patients suggest that a nationally developed leaflet, targeted at an appropriate reading level, may be a simple but important requirement. Equally, standardised training material for clinical staff would help to regulate the VTE assessment and care:
We’ve adapted it [leaflet] to be appropriate to our patients.
Nurse
Bigger trusts have specific thrombosis teams or VTE nurses . . . we don’t have that but we have just put together a document that is going through the approval process so hopefully that will help. I’m not saying patients just don’t get the appropriate treatment but I think maybe the actual process gets a bit blurred sometimes.
Nurse tutor, VTE committee member
We have followed the NICE guidance and written our own local trust guidance and that’s available on the intranet and available in a little booklet form that we give to the junior doctors when they start working here.
VTE trainer
There were examples of low levels of knowledge of VTE risk and prevention among staff in some acute trusts, including hospitals where the majority of patients would be assessed to be at high risk:
The major deficiencies are actually among health professionals and that we need to address those first before we start educating patients anymore.
Consultant
When I’m doing training . . . it’s only an awareness not high fluting – signs, symptoms, prevention, risk assessment, all the key things. It is improving now because we’ve done a lot of work but even the knowledge amongst people who work in hospitals, in an orthopaedic hospital where it’s always been higher risk, is low. If it’s low for that group then the patients themselves are unlikely to have a huge amount of knowledge and then things like on your medical training, nurse training there should be a whole module on VTE and the risks associated with it.
Nurse tutor, VTE committee member
A considerable variation in VTE teaching for a range of medical staff has been identified, highlighting a call for improvements:
I’ve been looking at education and its huge variability in the amount of teaching that medical students get in haematology where most of the VTE teaching is concerned so it varies from virtually nothing to 8 weeks haematology teaching between the different medical schools and if one looks at the nursing syllabus – the midwives have nothing, there’s no module at all on VTE and the nursing modules vary so there is a huge need for improvement in education.
Charity executive
Critical care staff who see one or two VTEs per month felt that they did not know enough about thromboprophylaxis:
I see one or two cases of VTE a month. I don’t think I have enough knowledge or information about VTE and thromboprophylaxis.
Critical care charge nurse
Similarly, training to cover the management of VTEs may be inadequate:
Possibly the thing we don’t cover so well at the moment is the management of suspected or actual VTEs . . .
Nurse tutor, VTE committee member
Even when there is a clear training programme in place, a nurse tutor suggested that attention can slip and compliance rates drop off:
You almost have to police it. You do things and you think, ‘right they’ve got that now, they know that every patient needs to be risk assessed’ but then something else will come along that takes their attention for a while and before you know it, it’s starting to drop off again.
Nurse tutor, VTE committee member
There were examples of excellence in staff commitment, responsibility and training. In several acute trusts training was mandatory. The staff were well trained and knowledgeable, keeping up with new policies and guidelines to maintain an up-to-date knowledge base. The most successful implementation exists where members of the team are allocated to the task and feel dedicated to the role:
VTE training is mandatory in our organisation and this is a very useful driver.
Consultant nurse for anticoagulation
In this trust we ask, not just nurses, but everyone that has front line direct patient contact to complete the e-learning VTE learning module, which is mandatory and we also provide, for the nurses specifically, some VTE awareness sessions.
Clinical nurse tutor
We provide slots on all induction programmes for new doctors and nurses, regular lunchtime teaching for pharmacists and an established link nurse/midwife network with study days and monthly lunchtime meetings incorporating teaching. Teaching of new FY1’s who are involved with VTE trust wide audit.
Consultant nurse for anticoagulation
In addition to specific medical training, there were examples where the importance of effective communication with the patient was emphasised:
We have e-learning or face-to-face training. When we do face to face we put more emphasis on the importance of telling the patient the importance of giving them the written information, when to tell them. Getting them to think about, when some ones refusing their TED [thromboembolism-deterrent] stockings. Getting them to think ‘have you actually explained to this person what they are for’. If you haven’t, ‘why are they going to wear them?’. Why would you wear them if they put them on you and you didn’t know what they were for?
VTE prevention lead
It was recognised that documentation outlining the appropriate treatment was required in some hospitals because they do not have specialist teams to manage VTE. This was most evident in specialist orthopaedic hospitals, where staff skills are appropriate to their specialist nature with little access to specialist skills in other medical conditions. Explicitly, orthopaedic surgeons are knowledgeable with regard to risk factors related to surgery and anaesthetics but do not see cases of VTE because they are referred to a general hospital and they may be unfamiliar with the risk factors associated with cancers and other comorbidities. This includes the management of suspected or actual VTEs. This situation also calls for the provision of standardised, nationally available information:
For some patients, there are other risk factors. It would be hard to have a form that covers every eventuality. Even though we are orthopaedic speciality only, within orthopaedics there are actually spinal, oncology patients, your hips and knees, etc. So even within that small group there are lots of different risk factors and if you think about big trusts you might have medical neuro’ [neurology] as well. It would be difficult to have something that covered absolutely every risk factor.
Clinical nurse tutor
The thing we don’t cover so well at the moment is the management of suspected or actual VTEs. Because, we are a specialist orthopaedic trust so we don’t have the input of, I mean a lot of bigger trusts have specific thrombosis teams or VTE nurses or . . . Whereas in this trust we don’t have that but we have just put together a document that is going through the approval process so hopefully that will help. I’m not saying patients just don’t get the appropriate treatment but I think maybe the actual process gets a bit blurred sometimes.
Clinical nurse tutor
Specific training requirements in acute care
Critical dose clarity
Participants presented specific examples where medical knowledge appeared to be lacking with regard to VTE prevention and medication. One example was the apparent confusion around giving reduced dosage appropriate to age and renal function:
The concerns that I sometimes have is that, it’s the definite guidelines for when you give a reduced dose, between 40 and 20. And I think a lot of the more junior clinical staff, junior doctors, don’t quite understand when to go for 40 versus 20, when you’re looking at age and renal function and things like that. And it’s sort of a bit, it’s a bit arbitrary. I would think it would be junior doctors needing the training in their medical, somewhere.
Acute trust pharmacist
A reduced dose may be warranted but some members of staff do not understand the significance. Improved documentation could provide a useful checking system when a dose has been changed:
Sometimes the consultants might reduce a patient’s Enoxaparin dose to 20 [mg], we’re not always sure why. So maybe some documentation somewhere in the notes to understand why the VTE medication has been reduced because normally it’s reduced if their renal function’s poor, but sometimes it’s reduced and their renal function’s fine, or it can be reduced if a wound is oozing. But sometimes neither of those are there and we’re left to like, there’s no information as to why the patient’s dose has been reduced.
Medicines management pharmacist
When asked whether or not there were any concerns regarding the required provision of thromboprophylaxis, a critical care charge nurse enquired, ‘if patient is on warfarin do we still give it?’. When asked if training was required, a participant enquired, ‘do we need TEDs and Enoxaparin?’. There is an apparent need for further training involving exceptions to the rules, combining treatments, reducing doses according to comorbidities and understanding the implications of a patient being on warfarin.
Immobility is a causal mechanism for VTE and there is some confusion regarding a patient’s apparent mobility that requires clarification across the NHS. The following statement could indicate that some patients are not receiving appropriate thromboprophylaxis:
The risk assessment that we used is very much based on the Department of Health [and Social Care’s] risk assessment tool. The main problem that we find causes confusion is with regard to the medical patients and the definition of mobility. We’ve done quite a lot of training on that recently and amended the risk assessment tool to add in the definition of mobility as defined by the NICE guidance. ‘Cause we’ve found a lot of people were thinking if the patients not bed bound then they’ve got normal mobility. They’re missing out on thromboprophylaxis. We’ve done a lot of work with that definition of mobility to try and increase awareness. I still feel that it’s, maybe, a little bit confusing.
VTE prevention lead
A charity executive insisted that GPs have an important role to play in the education of patients, and that risk assessment should be an ongoing practice so that it is up to date when a patient accesses the NHS:
Primary care should play an important part in the education of their patients and also in ensuring that a person’s risk factors for VTE are regularly looked at and updated if necessary and that the information goes with the patient every time they access the services of the NHS.
Charity executive 2
Timely education from a GP could help patients with medicines compliance. It was suggested that patients may develop a VTE after failing to take their medication because they have not understood the importance of it:
If we find out that the patient wasn’t taking it [medication] because they haven’t received enough education, and that’s why they’ve developed a VTE, then obviously if they’d had some information right at the beginning, maybe from primary care, then that might have helped them to ensure that they comply and they took their regime, the medicine.
Medicines management pharmacist
The GPs’ perceptions of their patients’ knowledge and awareness of DVT were varied. The media appear to have created awareness with the public, but it is suggested that some people think that a DVT will never happen to them. Participants feel that the majority of patients are unable to recognise the symptoms and a general worry about DVT within the community prompts people to attend general practice with a knock to the legs or varicose veins with the concern that they have a DVT. There was a consensus among participants that hospital patients receive inadequate education about DVT but it was also recognised that many patients do not understand the information that they are given.
Barriers to patient adherence
Participants gave several reasons for thinking that they did not need to wear their surgical stockings. Being mobile very quickly constituted a substitution theory. Furthermore, patients did not wear stockings because they were irritating, they were uncomfortable, they were too tight, the patient’s legs were too swollen, the patient did not think that they were necessary, or the patient was having anticoagulant injections:
I was walking again within a week, so I was sort of up and about more, so it wasn’t as if I was just lying still for, yeah, so I didn’t wear them when I came out no, no. Well they did give me four or five pairs. Do you think perhaps they’d intended you to wear them? Yeah probably. So why did you not? I just think ‘cause I was out, up and about and, feeling y’know, that I’m moving so everything is moving sort of thing so, yeah.
P7 Orthopaedic patient ORH46
No I didn’t. I was carrying on for a little while but they made my legs worse, I felt, they were too tight and that. My daughter in law is a physiotherapist, because I was so active, she thought I would be alright without so, ‘cause they were so uncomfortable they fit in too tight around the top leg here. I was getting up two or three, about four times a night, I was always, on the move, and all the time I didn’t — wasn’t lying in bed for days on end without getting out of bed.
P3 Orthopaedic patient NOC82
To be honest, I found them incredibly irritating. So I wore it for the first week and then I just scrapped it, and I thought, well I’ve got the injections anyway. So what I did, my view was, I won’t wear the stockings but what I’ll do is I’ll move a lot more. Even if I was in bed I was doing leg exercises and keeping movement going there, I kind of thought I’d rather do more of that, than wear the stockings, now whether or not that’s advisable who knows but, I can only apply my own logic.
P5 Orthopaedic patient ORH35
No no I didn’t wear them. ’Cause I was up and about.
P15 Medical/cancer patient
I came home with the ones from the hospital and I was told to keep then on for, I think it’s something like a couple of weeks. But I didn’t, not with these. No.
P16 Orthopaedic patient ORH38
I was told I wouldn’t need the stockings anymore just use the fragmin injections.
P9 Orthopaedic patient NOC138
Several participants suggested that, although they had been given stockings to take home, they did not wear them because they had not been specifically told to. These participants also suggested that they did not wear the stockings because they were unpleasant. Without clear instructions, participants are unsure whether they have to wear the stockings and for how long they should wear them. Several participants thought that it was not necessary to wear stockings once they were home:
I don’t recall being told, you’ve got to wear these for X amount of time. They’re not easy to get on at all, I had to put that on before and I don’t think I’d put it on right, it was all ruckled and they said it’s got to be smooth. And I had that on for a few days but I can’t remember, I don’t think it was on when I came out and I was only in for 4 days. But nobody said, ‘we’re giving you these because we want you to put them on’.
P26 Orthopaedic patient ROH68
They were, my legs were going in and out like this, and I think I took them off myself later on that evening. Nobody actually said to me, take them off or, do you want them taken off? I think I knew what they were for – they may’ve said, but I think I can’t really remember.
P17 Orthopaedic patient ORH61
I was given the tight socks to wear. I’ve got two at home, I was wearing them when I came home. Yeah. I haven’t worn any since I got home at all.
P18 Medical patient ORH17
For 6 weeks supposedly after. Not completely the 6 weeks no, 4 weeks of that I should imagine. I stopped wearing them because of irritation and, you know, just general tightness of the leg sort of thing with a stocking on, not being used to a full stocking.
P33 Orthopaedic patient ROH65
In hospital. I didn’t when I came out. I think I probably should have done for a bit longer. They were so uncomfortable, they are so uncomfortable, they really are. And yes I got them at home but I did stop using them as soon as I ever thought I could . . . I knew they were important but I didn’t think they were totally necessary when I came out.
P34 Orthopaedic patient ROH104
Both medical and orthopaedic patients felt that there was too much information to take in at a time when they were concerned about their hospitalisation. Knowing what to prioritise would help:
I think the difficulty is when you’re going in for an operation like this, there’s so much information that you need to take in anyway, and you’re worried about y’know, going into hospital, who’s going to look after your animals/children/husband or whatever, so, I think it would have to be carefully sort of planned in to, to have an impact really.
P1 Orthopaedic patient ORH4
You don’t know what is important in that information, so if you’re given a load, you can’t prioritise, and nobody else prioritises it for you.
P15 Medical patient ORH8
Several orthopaedic patients suggest that conflicting advice was given with regard to wearing stockings, which usually led to the stockings not being worn once the patient had left hospital:
I suppose that’s probably the most unclear part of the whole procedure, the injections were fine, I was quite happy with, y’know, doing and administering it and that whole process. Obviously the stockings were worn in the hospital sort of continuously and then I think the information you get, the information sheets is: wear of up to 4 weeks post op[eration], but there was definitely conflicting advice from the nursing staff. Some of the nurses were, ‘well it’s not that serious if you don’t wear them’ others were ‘absolutely must wear them’. So, y’know there was definitely conflicting advice.
P5 Orthopaedic patient ORH35
I did ask should I wear them, when I came home and nobody seemed to know whether I should or shouldn’t but I presumed it was OK, y’know you’ve had them on for a fortnight.
P19 Orthopaedic patient ORH31
The nurse said wear them for a fortnight which is what I did and then reading the leaflet afterwards it said keep them, wearing the stockings for after 6 weeks but, I only wore them for a fortnight.
P24 Orthopaedic patient NOC120
Many participants found the stockings uncomfortable to wear and difficult to put on. The nature of hip and knee surgery meant that patients did not have the flexibility to reach their feet:
Very uncomfortable and, very hot but I knew I had to keep using them. I wore them for the full time. Oh I hated them, hated them. I found that the design was awful because they had this little hole in the bottom, which, at night got trapped around your toes, so you’d wake up and your toes were freezing because they’d been stopping the circulation, and then when you were wearing shoes to go out, they trap around your toes as well, they’d work up, no matter what we did, and of course my husband had a terrible job getting them on, I couldn’t.
P6 Orthopaedic patient NOC100
Never managed to do it, now I’ve got short arms unfortunately and of course if you’ve got hip problems it’s a bit difficult to do that, so I had to have help with those.
P8 Orthopaedic patient NOC110
Help with putting the stockings on came from family members:
My wife done it. Even like, with socks, I can’t bend. So anything below the knee normally, my wife does it.
P2 Orthopaedic patient NOC58
I have a wife for that. It was difficult, and that was an area which I think somehow that can be improved, how anybody on their own does it, I don’t know. You cannot do it on your own, and however much you’ve got this, the gadget.
P4 Orthopaedic patient NOC99
I’m very fortunate because my husband’s very good, he does the cooking and cleaning.
P9 Orthopaedic patient NOC138
Oh, it was awkward getting them on. Oh, my son came in. I can’t even get my tights on now.
P22 Medical patient ORH60
All participants given a course of injections to self-administer after hospitalisation claimed to have completed the course. Most participants claimed to have received one training session, were uncertain about giving themselves an injection and would have liked reassurance that they were injecting themselves correctly.
A medical patient did not know why she had been given the injections and complained that the alternative was a daily trip to the doctors. It was not until the research interview that she was made aware that the injections were to prevent blood clots and not for pain relief:
You’ve done it for about a week, and you go, ‘I can’t do this anymore’, don’t be silly of course you can, you’ve done it for a week, of course you can do it and then you go, just do it but there does seem to be this, oh I really don’t want to put this thing in me again. But, realising effectively that, there isn’t a choice, just get on and do it.
P9 Orthopaedic patient NOC138
Not at all happy. But they said that alternative was going to the doctors every day. Well you need a lift for that, you know, when you don’t feel good. So I just had to do it for a month. Yes, bruises everywhere. They just gave me the thing. Injections in the stomach are to prevent blood clots. Oh, are they? See that’s what it was for. Thought it was for relief.
P22 Medical patient ORH60
The nurse did the injections when I was in hospital and then explained that I’d have to do it for a month afterwards. And, my wife wouldn’t do so I had to do it myself.
P24 Orthopaedic patient NOC120
Some participants experienced no problems with the course of injections and complied with the treatment advice:
I injected my belly every day and when I came out I had to do it for 2 weeks I think after. No trouble at all. They give you a quick lesson.
P33 Orthopaedic patient ROH65
I was quite aware of all this blood clotting, so I didn’t need to ask. I was just aware that it was there and you just do what you’re told. It’s doing what you’re told really isn’t it?
P34 Orthopaedic patient ROH 104
I did the whole course. Yeah, I was gonna complete the course, never miss.
P3 Orthopaedic patient NOC82
It was clear from the patient interviews that there were issues relating to retention of information, whereby patients cannot remember, they forget or the information does not sink in. Patients did not remember if anyone talked to them about VTE prevention:
I take the wife with me, she tends to ask the questions and remembers more than I do, because I have troubles, I can remember things but I’m not that great in remembering things.
P2 Orthopaedic patient NOC58
I honestly don’t remember but I’m getting quite forgetful in my old age.
P18 Medical patient ORH17
I don’t remember any particular discuss[ion] but at the same time, I mean what I’ve been told, it didn’t sink in. I’m sorry to be so vague about it.
P18 Medical patient ORH17
I’m pretty certain we were given injections for, to prevent blood clotting, but I mean I was, there was so much, happening to me, that I, can’t a hundred per cent remember.
P20 Medical patient ORH51
Just an operation can cause DVTs, so when you come out you’ll be, I suppose, cared for enough. I can’t remember exactly what was said.
P4 Orthopaedic patient NOC99
They did say blood clots, you know, stops your blood clotting, but they didn’t tell you what symptoms you react from it. Well to be honest, I mean whether they’ve told me I don’t know because I was under a lot of anaesthetic in the hospital for a few days. Whether it was mentioned earlier on and, you know, obviously my minds all over the place, they could have mentioned something.
P28 Medical emergency patient UHB23
One patient suggested that most people are aware because airlines have taken up the issue and if they have done so it must be important:
I think probably more people know about it now because the airlines have taken it up and I think that’s really where you learn most, that if they think it’s worth telling you to wear their stockings or whatever, then, yes it is important.
P8 Orthopaedic patient NOC110
When told the symptoms of a DVT during the interview, a participant remembered reading the information but did not take it in because he did not recognise the importance or significance. This suggests that information needs emphasis and prioritisation for the patient:
That’s certainly was in that information I read, which just shows you that, but, there was no real emphasis put on that. Y’know, it’s most probably quite important to you.
P21 Orthopaedic patient NOC125
There were concerns raised regarding the volume of information given, with one patient overwhelmed by the volume of information provided by the hospital. It was suggested that information was given for the sake of ticking a box with little concern given to the patient’s capacity to understand or take in the information:
You can say you’ve given the information, you can’t say that people have understood it or you’ve actually explained it. I’ve had so much information not just about blood clots but about, cancer and chemotherapy and radiotherapy and I’m a nurse and honestly, I cannot make head and tail of it. And there’s things that, you just think, can’t they just give me a priority like, number one do this do this, do that, you’re just given so much information, I’ve got piles of it upstairs and now and again I might say, ‘Oh I think I’ll, let’s have a look at that now and see if it makes sense now’. I just think it’s, complete waste of time to be honest. I think it’s people saying, they feel they can tick the boxes that, we’ve told you but, actually, what goes in is very different. And what’s sits in your brain’s very different so, I don’t think it’s very impressive myself.
P15 Medical/cancer patient ORH8
Participants suggested that there may be an optimal time frame to receive VTE information to avoid times when it is difficult to take in details when recovering from an operation. The first day was considered to be the worst time to receive information:
I would say that I think the most useful thing would’ve been, maybe a sort of, about 48 hours after having had the operation, to have had a nurse come in who specialised in that sort of thing and explain it to me at that stage.
P1 Orthopaedic patient ORH4
I don’t think I would’ve done [read leaflet]. Probably not the first day, ‘cause you’re still a bit drowsy, probably on the second, third day or something like that.
P2 Orthopaedic patient NOC58
Furthermore, participants suggested that, rather than just being handed the leaflets, it would be helpful if there was someone to go through and point out the key points:
Although you get the booklet of information, to be honest you’re not always that, mentally, alert when you’re still on heavy meds, you, y’know you might read a bit but you don’t really process it, so, maybe just sort of having someone there saying, this is the information what’s in this pack is, there’s this, this and this. Let’s go through these key important things again. It makes a massive difference having someone physically go through it with you rather than just giving you a batch of paper to read. You read it and on the medication at the time you probably don’t process it all. Then you forget where you’ve put it when you need it. It’s much better I think if you have one on one, and something that may be even better would be 2 or 3 days once you’re home. Or you could even have someone do it over the phone.
P5 Orthopaedic patient ORH35
Participants suggested that before the operation would be an appropriate time to do this:
Before the operation yeah.
P7 Orthopaedic patient ORH46
Sometimes when they give it you, you’re not actually at your best to take it in. Some of the information they give you obviously at your pre-op[eration]. I don’t recall the blood clot bit being given to me at the pre-op[eration]. Now that might’ve been a good place to mention it because you’re a bit more aware at that point of things, because they’ve already gone through all the other risks and things.
P9 Orthopaedic patient NOC138
However, the worry about an operation could blot out the ability to take in information:
Too much of everything else is going on isn’t it? And you’re worried about your operation as it is anyway. All they basically say is you’ve gotta have your injections for blood clots. They come round and give you that but there’s not a lot else said after that about it really.
P7 Orthopaedic patient ORH46
All you’re thinking about is the operation so you’re not really yeah, not really switched on to it are you.
P7 Orthopaedic patient ORH46
When you’re not dozy from the operation. To be given some of the information at that point’s probably not the best time to get to read it.
P9 Orthopaedic patient NOC138
Just prior to discharge was also suggested as the best time to receive the information. The information is then relevant to the requirement for patients to self-inject when at home:
They didn’t tell me the detail of how to inject myself until the day I was going home. So by that time I was feeling reasonably sort of fit and looking forward to going home, and I was in a mood for sort of taking those sorts of things in. The first days after the operation, when I was on morphine and things like that, then I possibly wouldn’t have wanted too much detail.
P25 Orthopaedic patient ROH55
Summary
Through a series of surveys and interviews, five common themes were identified with regard to the prevention of HAT. These themes were communication, knowledge, role of primary care, education and training, and barriers to patient adherence.
Although the implementation of risk assessment in hospitals has been successful over the past 10 years, it is not so clear that this has had a major impact on the reduction of HAT. Further research should be undertaken to address the issues identified within this work package to ensure the maximum impact on the reduction of HAT in the future.
Work package 3: health economics and patient preferences
Work package 3 comprised four projects:
-
a within-trial health economic analysis
-
a health economic modelling study to evaluate the cost-effectiveness of extended anticoagulation, extrapolating the results of the ExACT RCT
-
a patient preference study of extended oral anticoagulation for first unprovoked VTE
-
a health economic modelling study of extended anticoagulation for treatment and prevention of recurrent VTE, using a decision rule for extended treatment with direct oral anticoagulants (DOACs).
The health economic modelling in projects 2 and 4 differ in their approach and comparators. Project 2 concerns the overall strategy of extending anticoagulation and directly extrapolates the results of the ExACT RCT. Project 4 builds on previous work concerning the use of a decision rule (using a prognostic model) to determine the cut-off risk for extending anticoagulation comparing several levels of risk. This analysis explored the use of DOACs in this context.
The full details of these studies are provided in Appendix 2. A summary is provided in the following section.
Within-trial health economic analysis (work package 3)
This subsection provides an overview of the within-trial economic analysis conducted alongside the ExACT trial to evaluate the cost-effectiveness of prolonged oral anticoagulation treatment beyond 3–6 months for patients with a first unprovoked proximal DVT or PE.
Methods
The economic evaluation took the form of a cost–utility analysis using quality-adjusted life-years (QALYs) based on the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), instrument. Relevant patient costs used in the economic evaluation alongside the clinical trial were based on individual-level data collected during the 2-year follow-up period of the trial. Costs were estimated from an NHS perspective and were based on 2012/13 values, consistent with the time period of data collection. In line with the main trial analysis, 273 patients were included in the economic evaluation, with 139 randomised to the extended anticoagulation group (137 receiving warfarin and two receiving rivaroxaban) and 134 to the group discontinuing anticoagulation.
The cost analysis adopted a UK health-care perspective. Resource use data were collected alongside the trial. The total costs for each participant consisted of treatment costs (anticoagulation and monitoring), PTS costs, and adjudicated adverse event costs (thrombotic and haemorrhagic). Resource use concentrated on primary care, medicines, hospital consultant appointments, accident and emergency (A&E) attendances and hospital admissions (see Appendix 2).
For adverse events, only events that were relevant to this study were costed, based on the resource use requirements of each event. Therefore, only adjudicated thrombotic events, haemorrhagic events and death (if resource use was utilised) were included in the costing; unrelated adverse events were not included.
Quality of life
The outcomes for the health economic analyses of the ExACT trial were based on QALYs estimated using the EQ-5D-3L instrument. All QALY scores reported in the base-case analysis reflect imputation and adjustment. As the follow-up period was 2 years, all QALYs for year 2 were discounted at a rate of 3.5%.
Cost-effectiveness analysis
An incremental cost-effectiveness analysis was conducted in accordance with the intention-to-treat principle, to determine the difference in costs and outcomes between extended and discontinued anticoagulation. The unit of outcome was the incremental cost per QALY gained (incremental costs divided by incremental QALYs).
Results
Costs
The costing analysis based on the trial data showed that the mean cost per participant during the 24-month trial period was £326.30 for the extended anticoagulation arm and £350.38 for the discontinued anticoagulation arm (Table 8). This demonstrates an overall mean cost saving of £24.08. As expected, mean costs in the intervention arm were greater for anticoagulation and haemorrhagic events, but much lower for thrombotic events. Costs for treatment of PTS were very similar, in line with clinical results demonstrating no differences in PTS severity.
| Resource use category | AT, mean (SD) | Difference (bootstrapped 95% CI) | |
|---|---|---|---|
| Extended (n = 139) | Discontinued (n = 134) | ||
| Anticoagulation | 202.42 (189.61) | 57.96 (95.59) | 144.46 (115.22 to 183.16) |
| PTS treatment | 71.47 (306.75) | 76.57 (200.28) | –5.10 (–67.10 to 58.88) |
| Thrombotic events | 70.80 (299.19) | 272.11 (584.31) | –201.31 (–321.21 to –98.38) |
| Haemorrhagic events | 53.08 (183.69) | 20.31 (74.00) | 32.77 (3.33 to 70.50) |
| Total NHS costs | 326.30 (439.49) | 350.38 (616.39) | –24.08 (–165.47 to 102.20) |
Outcomes
The baseline EQ-5D-3L score was higher in the discontinued anticoagulation arm (0.814) than in the extended anticoagulation arm (0.807) and slightly higher for the majority of time points. Complete EQ-5D-3L data at all time points were available for 218 participants (80%), with a slightly higher rate in the extended anticoagulation arm (81%) than in the discontinued anticoagulation arm (78%).
The differences in the mean imputed QALY score (adjusted for baseline differences) over 24 months’ follow-up were very similar in the two trial arms, although slightly favouring extended anticoagulation, with 0.009 additional QALYs. The complete-case analysis without any imputation and adjustment slightly favoured the discontinued anticoagulation arm. In all cases the 95% CIs around the mean differences did not suggest significance.
Base-case analysis
Table 9 provides the base-case results of the economic evaluation alongside the ExACT trial and reports the within-trial cost-effectiveness analysis undertaken using the adjusted and imputed data. Although differences in both cost and QALYs were small, the results favour the extended anticoagulation arm, with slightly lower costs and more QALYs.
| Difference in mean (intervention – control) | Bootstrapped 95% CI | Interpretation | |
|---|---|---|---|
| NHS costs (£) | –24.08 | –165.47 to 102.20 | Intervention less costly and more effective |
| QALYsa | 0.009 | –0.055 to 0.071 |
However, there is uncertainty surrounding these results at a willingness-to-pay threshold of £20,000 per additional QALY; there is a 62% probability of extended anticoagulation being the cost-effective option, with a 61% probability at £30,000 per QALY.
Discussion
These results suggest that, over the period of the trial, extended anticoagulation was marginally cheaper because of the additional costs of anticoagulation being offset by a reduction in costly thrombotic events, and quality of life was very similar, suggesting that this may be a cost-effective option. However, there was a great deal of uncertainty around the results, particularly the QALYs. This may be due to the EQ-5D-3L only capturing quality of life at specific time points rather than measuring short-term disutility of thrombotic and haemorrhagic events at the time of occurrence. Therefore, the clinical benefits demonstrated in the main results of the trial are not translated into large quality-of-life gains. As a within-trial analysis can take into account only the costs and outcomes that have occurred within the trial follow-up period, Markov modelling to extrapolate results over a lifetime horizon was undertaken and is presented in the next section.
Health economic modelling study to evaluate the cost-effectiveness of extended anticoagulation (work package 3)
Methods
Model population
The patient population comprised adult individuals having already completed at least 3 months of AT in response to their first unprovoked VTE. Individual patients were generated from the ExACT patient data set with characteristics created by randomly sampling the patient-level data by means of a uniform distribution. Patient characteristics comprised age, sex and type of index VTE event (DVT or PE).
Model pathways and clinical events
The economic model extrapolated the results of the ExACT trial and compared the strategy of discontinuing anticoagulation with lifetime extended anticoagulation. The model contained the same potential pathways in both strategies (Figure 4), and their characteristics in part determined the probability of clinical events, costs and utilities. The model had a 1-month time cycle and lifetime time horizon.
FIGURE 4.
Model patient pathways.

In 1 month, an individual had a probability of experiencing a clinical event: death from other causes, recurrent VTE (non-fatal DVT, fatal or non-fatal PE), fatal or non-fatal major bleeds (intracranial bleed, gastrointestinal bleed, and other major bleeds). Recurrent VTE carried a risk of PTS. A recurrent VTE could be a PE or DVT and the recurrent VTE type was assumed to be influenced by an individual’s initial VTE site. Once a patient suffered a recurrent VTE, they were put on AT for life with cessation of therapy only if a later major bleeding event occurred. VTE events were assumed to incur a one-off quality-of-life reduction, with a proportion of surviving patients assumed to suffer from severe PTS for life.
All major bleeding events had short-term costs and quality-of-life decrements. In addition, an intracranial bleed was assumed to be associated with ongoing costs and a permanent quality-of-life decrement along with a sustained increased lifetime risk of other-cause mortality. For the ‘other major bleeds’ category, it was assumed that this heterogeneous category of bleeds should have the same costs and quality-of-life decrement as a gastrointestinal bleed.
Any major bleeding event led to discontinuation of AT. A recurrent VTE in a later cycle was assumed to restart therapy. It was assumed that there was no effect of AT on VTE recurrence risk more than 1 month post cessation of therapy.
Model type
A Markov patient-level simulation was developed in TreeAge 2014 (TreeAge software, Williamstown, MA, USA) and built on previous work on extended anticoagulation. 46 The patient-level simulation allowed the use of individual patient characteristics (age, sex, index VTE type) from the ExACT trial. The model was run with a large number of simulated patients (50,000) to account for interpatient variability.
Clinical parameters
The risk of a patient’s first recurrent VTE in the first 2 years was calculated by converting the trial primary outcomes for both arms from the rates per 100 person-years to an annual probability. 14 The annual risk of a further VTE event after a VTE recurrence on and off therapy was obtained from the PREVENT trial. 11 Only major bleeds were included in the model, and the risk of major bleeding on and off therapy was obtained from the ExACT trial data, converting rates per 100 person-years to annual probabilities. All annual probabilities were subsequently converted to monthly probabilities.
Resource use and costs
Costs of therapy and clinical events were included in the model. Costs of VTE and bleeding events were obtained from NHS Reference Costs 2012/13. 47 In line with the within-trial analysis, the cost hospital international normalised ratio (INR) monitoring was assumed and the cost per visit was obtained from previous research and updated (see Appendix 2). All costs were updated to 2012/13 prices using the Hospital and Community Health Services (HCHS) Index. 18
Quality of life
Quality-of-life (utility) values were assumed to be age related as they entered the model using EQ-5D-3L UK normative values. 12 Utility values for clinical events were multiplied by the age-specific utility to derive quality-of-life reductions for patients experiencing a clinical event. The model assumed no disutility with warfarin in the base case.
Assessment of cost-effectiveness
The incremental analysis calculated the cost per QALY gained for extended anticoagulation versus discontinued anticoagulation. Cost-effectiveness was assessed in relation to the NICE lower threshold of £20,000 per QALY, where a value of £20,000 per QALY is judged to be cost-effective. 48 All costs and outcomes were discounted at the recommended 3.5%. 13
Deterministic sensitivity analysis
To test the robustness of base-case results, deterministic sensitivity analyses were run to determine the impact of changing key parameters on results.
Probabilistic sensitivity analysis
Where available, data were input into the model as distributions to assess parameter uncertainty in the form of a probabilistic sensitivity analysis (PSA). The model was rerun with 10,000 simulations for each trial of 1000 simulated patients and the results expressed as cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs).
Results
Base-case results
Under base-case assumptions, lifetime extended anticoagulation was more costly but more effective than extended anticoagulation, with an incremental cost-effectiveness ratio (ICER) of £9530 per QALY gained.
Deterministic sensitivity analysis results
Shortening the time horizon to 5 years increased the ICER to £15,975 per QALY, which was still below the £20,000 per QALY threshold. Any further increase in time horizon beyond 5 years led to a reduction in the ICER. Increasing the risk of death from PE improved the cost-effectiveness of extended anticoagulation to £5836 per QALY. Attaching a disutility of 0.997 for being on anticoagulation increased the ICER, but it was still within the acceptable threshold. Changing the type and, therefore, cost of anticoagulation monitoring had the greatest impact on the ICER, with the scenario of all practice-led monitoring giving an ICER of £20,970 per QALY gained, slightly above the threshold, and an equal split of hospital and practice monitoring was £15,219 per QALY.
Probabilistic sensitivity analysis results
At a willingness-to-pay threshold of £20,000 per additional QALY, there is a 56% probability of extended anticoagulation being the cost-effective option, with a 60% probability at £30,000 per QALY.
Discussion
The economic evaluation assessed the cost-effectiveness of lifetime extended anticoagulation in patients with a first unprovoked VTE. The base-case results indicate that this intervention would be cost-effective, and this result was robust to sensitivity analysis except for a more extreme scenario of all general practice-based INR monitoring. However, quality of life on treatment, mortality risk after PE and the time horizon all had an impact on the cost-effectiveness results. There is considerable uncertainty around the base-case result, and this is most likely to be due to using trial-based estimates of recurrent VTE and bleeding. This uncertainty would reduce with the incorporation of data from a synthesis of these data with similar studies.
A key strength of the analysis is the use of an individual patient simulation, which uses characteristics of the participants of the ExACT trial. This was preferable to the more common cohort model with a homogeneous set of characteristics as the model results were more representative of a realistic patient population. The modelling method was also more efficient by using tracker variables to hold information on characteristics than requiring the construction of a large number of Markov health states.
As the purpose of the model was extrapolation beyond the ExACT trial period, data on VTE risk, type of VTE and bleeding risk were utilised from the trial. This allowed the uncertainty within the trial data to be represented. Ideally, decision models should use all of the available evidence on effectiveness of an intervention. Therefore, it is suggested that a future application of the model should use individual patient data synthesised from a number of studies to provide more generalisable results.
A patient preference study of extended oral anticoagulation for first unprovoked venous thromboembolism (work package 3)
The aim of this research was to determine patient preferences for extended anticoagulation treatment, taking into account both the benefits (reduction in thrombotic events) and risks (bleeding events) associated with anticoagulation.
Methods for data collection and analysis
A questionnaire was developed to elicit patient preferences for the extension of anticoagulation (specified as warfarin; see Report Supplementary Material 1). 49
Questionnaires were completed by patients during their final follow-up study appointment, with assistance from the study nurses. Completed questionnaires were then entered onto a database.
An initial descriptive analysis was undertaken to determine the distribution of responses for both questions separately. Subsequently, both questions were considered together to determine whether a participant showed (1) a preference for warfarin (i.e. more willing to risk a higher risk of bleeding than DVT), (2) no warfarin (i.e. more willing to take a higher risk of having DVT than bleeding) or (3) no preference (i.e. chose equivalent risks).
These data were analysed taking into account the following patient characteristics:
-
age
-
sex
-
trial arm
-
thrombotic event during the trial
-
bleeding event during the trial.
This was to determine whether or not a pattern was emerging in relation to demographics, and whether allocation of treatment or an event during the trial had influenced their choice.
Results
A total of 218 patients completed questionnaires, which was a response rate of 80%.
Risk of serious bleeding with extended warfarin to avoid deep-vein thrombosis/pulmonary embolism
A total of 72 patients (37%) reported that they were willing to take a 100% risk of a bleed to stay on warfarin for a further 5 years to avoid a further DVT/PE. A total of 155 patients (80%) were willing to take greater than the stated 5% risk of a bleed, and only 15% (n = 28) were not willing to take any risk of serious bleed.
Risk of deep-vein thrombosis without extended warfarin to avoid serious bleeding
A total of 24 patients (12%) were willing to take a 100% risk of a DVT/PE to avoid a serious bleed. A total of 44 patients (23%) were willing to take greater than the stated 30% risk of a DVT/PE, and 84 patients (44%) were not willing to risk a DVT/PE to avoid a serious bleed.
Comparison of risk of bleeding and risk of deep-vein thrombosis
A total of 63 patients (33%) showed a very strong preference for extended warfarin by being willing to take a 100% risk of a bleed, but were not willing to take any risk of a DVT/PE. A total of 21 patients (11%) chose the opposite (i.e. had a very strong preference for not extending warfarin). Comparing the risk of DVT/PE with the risk of bleeding, the majority (135 patients, 70%) had a preference for avoiding DVT/PE (and extending warfarin); 40 patients (21%) preferred to avoid a serious bleed and the remaining 18 patients (9%) gave very similar values for both DVT/PE and bleeding.
The majority of participants were willing to take a higher risk of a bleed (compared with DVT/PE) to receive warfarin for 5 years. Twenty out of the 24 patients (83%) who suffered a DVT/PE and responded to the questionnaire preferred extended warfarin. Those who had suffered a major bleed reported only a slightly lower preference for extended warfarin (6/9, 67%).
Discussion
The results of this exploratory work on patient preferences for extended warfarin show that there was a higher preference for long-term anticoagulation than short-term treatment (i.e. people were generally willing to take a higher bleeding risk). Unsurprisingly, this was particularly true for those who had a recurrent VTE during the trial follow-up period.
Health economic modelling of extended anticoagulation for treatment and prevention of recurrent venous thromboembolism using a decision rule (work package 3)
Methods
This study aimed to build on previous modelling that considered extended anticoagulation with warfarin to evaluate the cost-effectiveness of a decision rule for extending anticoagulation with three alternative anticoagulants, referred to as DOACs (i.e. apixaban, dabigatran and rivaroxaban), in patients after a first unprovoked VTE. Results for warfarin are also presented. Details of the model are provided in Appendix 2.
Model pathways and clinical events
The economic model compared a strategy of no therapy (usual care) with a number of decision rule strategies, where therapy was restarted (effectively continued) if the predicted annual risk of VTE recurrence was equal to or greater than the given threshold risk. In line with the extended warfarin analysis, arbitrary but clinically relevant thresholds were explored in the analyses (1%, 3%, 5%, 7.5% 10%, 12.5%, 15%, 17.5%, 20%, 22.5% and 25%) and a treat-all strategy was also included as a comparator. The specified VTE risks were then used as different decision rule comparators.
Any major bleeding event led to discontinuation of AT and a subsequent recurrent VTE in a later cycle was assumed to restart therapy.
Clinical parameters
The model was run for each DOAC separately (i.e. apixaban, dabigatran, rivaroxaban) as well as warfarin. Owing to weak calibration statistics of the prognostic model after 3 years, beyond this point an annual constant risk for the first recurrent VTE event off therapy was used. 41
Resource use and costs
Costs of therapy and clinical events were included in the model, and details are provided in Appendix 2.
Quality of life
Quality-of-life (utility) values were assumed to be age related because they enter the model using EQ-5D-3L UK normative values. Utility values for clinical events and disutility on warfarin were multiplied by the age-specific utility to derive quality-of-life reductions for patients on warfarin or experiencing a clinical event. It was assumed that all three DOACs did not result in any disutility. Warfarin was assigned a disutility of 0.997.
Assessment of cost-effectiveness
A sequential incremental analysis was designed to calculate the cost per QALY gained for applying a decision rule versus the next most effective option, applying the rules of dominance and extended dominance. Cost-effectiveness was assessed in relation to NICE lower threshold of £20,000 per QALY, where a value of £20,000 per QALY is judged to be cost-effective.
Deterministic sensitivity analysis
To test the robustness of base-case results, deterministic sensitivity analyses were run to determine the impact of changing key parameters on results:
-
The probability of death from a PE was increased to 30% because of uncertainty among clinical experts.
-
Subgroup analysis was undertaken for index PE patients, as the subgroup of PE patients were at a higher risk of recurrence and mortality.
Results
Base-case results
Under base-case assumptions, restarting warfarin therapy for patients with a predicted annual VTE recurrence risk of 25% gave the lowest cost per QALY of £2814. This recurrence risk was also the most cost-effective for apixaban, dabigatran and rivaroxaban, with ICERs ranging between £5520 and £6312 per QALY.
When considering a threshold of £20,000 per QALY gained, resuming AT with warfarin for patients with a predicted annual VTE recurrence risk of 17.5% yielded the highest number of QALYs while also being considered cost-effective with an ICER of £15,572 per QALY gained. Both dabigatran and rivaroxaban were cost-effective at a higher VTE recurrence risk of 20%, with a risk of 17.5% resulting in ICERs above the £20,000 threshold but below £30,000. The lowest risk threshold at which apixaban was cost-effective was even higher, at 22.5%.
Deterministic sensitivity analysis results
Increasing the risk of death from PE led to lower risk decision rule strategies also being cost-effective. This was ≥ 12.5% in the case of warfarin, and ≥ 17.5% for apixaban, dabigatran and rivaroxaban.
All decision rule strategies of ≥ 10% were cost-effective with extended warfarin when the patients’ index event was a PE, reflecting the high-risk nature of such index events. All strategies of ≥ 12.5% were cost-effective for dabigatran and rivaroxaban, and all strategies ≥ 15% were cost-effective for apixaban.
Discussion
The economic evaluation assessed the cost-effectiveness of utilising a decision rule for extended AT in patients with a first unprovoked VTE, and explored three DOACs (i.e. apixaban, dabigatran, rivaroxaban) in addition to warfarin. Base-case results suggest that treating patients with a predicted 1-year VTE risk of ≥ 17.5% with warfarin could be cost-effective compared with the next most effective option. The risk at which therapy was cost-effective was higher for all three DOAC drugs, at 20% for dabigatran and rivaroxaban, and 22.5% for apixaban, which was the most expensive. Increasing the risk of death from PE and running the model for only those whose index event was a PE reduced the level of risk of recurrence at which a therapy became cost-effective.
To build on this model, there is a need for robust long-term data on the risk of recurrent VTE in unprovoked index VTE patients, and bleeding risk data.
Summary of the venous thromboembolism programme
The overarching aim of the current programme was to investigate methods to improve both the treatment and prevention of VTE. It is fair to say that there has been significant progress in both of these areas during the lifetime of the programme and, to a large extent, the original aims and objectives have been met.
With regard to treatment, there have been advances in the availability of new drugs for the treatment of VTE, with four new drugs being licensed since the beginning of the programme (i.e. dabigatran, rivaroxaban, apixaban, edoxaban), which have shown evidence of effectiveness in both the acute stage and the preventative stage.
The current programme utilised a RCT, a within-trial health economic analysis, health economic modelling and a patient preference study to explore the potential for extended treatment of up to 2 years after the initial treatment period with anticoagulation (primarily warfarin) to prevent recurrent VTE and assess the impact of this strategy on prevention of PTS. With 281 patients recruited (fewer than the planned 352), the trial demonstrated significant reduction in recurrence of VTE.
In terms of the primary outcome, there were 32 recurrent VTEs in 31 patients (13.54 events per 100 person-years) within the control group (group D) compared with seven events in seven patients (2.75 events per 100 person-years) in the intervention group (group E), giving an adjusted hazard ratio of 0.2 (95% CI 0.09 to 0.46; p < 0.001). This meant that patients receiving extended AT were 80% less likely to suffer a recurrent event than those patients who discontinued AT.
The within-trial health economic analysis and extrapolation beyond the trial data suggested that the extended strategy was cost-effective, while the patient preference study demonstrated a strong preference for the extended strategy, based on a fear of recurrence compared with a fear of major bleeding. However, further health economic modelling (which considered extended anticoagulation with warfarin and DOACs using a decision rule) was less conclusive, with cost-effectiveness only demonstrated at an annual recurrence risk between 17.5% and 22.5%. These levels are very high, with current risk models of continuing treatment using annual recurrence rates of ≤ 5%. The model developed will, however, be useful in terms of utilising data from future research in determining the annual rate of recurrence at which it is cost-effective to continue treatment. The reduction in recurrence of VTE was offset by a small, non-significant, increase in bleeding.
It is clear, therefore, that further research is needed to help to identify those patients in whom it is safe to discontinue therapy after an initial treatment phase of 3–6 months. The current trial did not identify any specific demographic markers to help with this, although there was a numerically higher rate of recurrence in males. One objective of the current trial was to establish whether or not the measurement levels of D-dimer prior to cessation of therapy could be helpful in this regard. Based on the current analysis, it would seem that measurement of D-dimer levels at the current cut-off points is not predictive of recurrence, but there is further work being pursued as part of an ongoing PhD that will analyse these data further.
A further aim of the current trial was to establish whether or not extended therapy with anticoagulation could be effective in preventing PTS. The initial analysis suggested that there was no difference in either the incidence or the severity of PTS between extended therapy or standard therapy. This will be the subject of further analysis.
In terms of prevention of HAT, there has been a rapid increase in risk assessment for hospital inpatients since the beginning of this programme, but our work with patients, HCPs and other stakeholders identified that there remain significant barriers to successful implementation of preventative strategies. These include issues around communication, knowledge, education and training, and barriers to patient adherence. These issues are most apparent at a primary care level, with the role of primary care in enabling improved thromboprophylaxis poorly specified. Future work will build on these findings to further improve the progress that has already been made.
In summary, the programme has been largely successful in meeting its aims and objectives and will make a significant contribution to the current body of knowledge around the prevention and treatment of VTE both in hospitals and in the community.
Limitations
The major limitation for this programme was the failure to achieve the recruitment target for WP1. This was discussed on an ongoing basis with the Trial Steering Committee, and the main strategy to overcome this was to increase the number of recruitment sites. Despite this, only 281 patients were recruited despite an initial sample size calculation of 352. The failure to recruit to target limits the strength of the findings within WP1, and this is particularly true in terms of bleeding events.
The failure to reach the required sample size also had an impact on the strength of findings available through the health economic modelling as part of WP3, with a large amount of uncertainty around all the results. Similarly, the costs attributed within the health economic models are subject to a large amount of uncertainty.
A further limitation was that data were collected only within the ExACT RCT for PTS, with no data collected for chronic thromboembolic pulmonary hypertension. Although this is a severe chronic long-term complication of VTE, it is relatively rare and it was felt that it was impractical to collect data on this in the current programme.
Implications
The main implication of the work included in this programme is that it is clinically effective to prolong treatment for patients with a first unprovoked VTE, and it may be cost-effective, but this will need further exploration because of the large amount of uncertainty around the health economic findings. This is borne out by patient preferences, but it is important to try to improve our current understanding of who is at an increased risk of recurrence and, by contrast, who is at a low risk and, thus, for whom it is safe to stop anticoagulation after a short period of treatment.
The second implication of the research is that although there has been good progress with implementation of risk assessment for HAT, more needs to be done, particularly in terms of education for both patients and primary care HCPs. As always, communication is highlighted as a particular weakness.
In terms of lessons learned, the main barrier to successful completion of the research programme was the slower than anticipated recruitment for WP1. It is difficult to known how this could have been improved and the situation was monitored closely by the Trial Steering Committee. In retrospect, more sites should have been recruited initially.
Recommendations for future research
-
Further research is required to elucidate the factors (i.e. clinical, demographic and biomarker factors) that can predict those patients in whom it is safe to stop AT following a first unprovoked VTE.
-
Further research is required to identify the best methods for the prevention of PTS.
-
Further work is required in elucidating the best role for primary care in terms of VTE prevention.
-
More robust data are required on the risks of recurrent PE following a first unprovoked PE to build on the health economic modelling provided within this programme.
-
More robust data on bleeding events in routine practice are required to build on the health economic modelling provided within this programme.
-
Data are required on the incidence and prevalence of chronic thromboembolic pulmonary hypertension following a first unprovoked episode of VTE.
Acknowledgements
We would like to acknowledge the contributions of all the hospitals and general practices, patients, HCPs and other stakeholders in enabling the completion of this ambitious programme.
It is with sadness that we acknowledge the contribution of Patrick Kesteven, who sadly passed away in 2017.
There are several people who contributed significantly to the development of this programme, who have subsequently retired, in particular Roger Holder, who worked tirelessly to provide the initial statistical support, Ellen Murray, who was essential both in developing the ideas and writing the initial grant proposal, and Peter Rose, who provided constant expert support.
We would also like to acknowledge Ander Cohen and Beverley Hunt, who provided additional clinical and epidemiological expertise throughout the lifetime of the programme.
Governance
The programme was overseen by a Programme Management Board comprising the following:
Professor David Fitzmaurice, University of Birmingham.
Dr Ellen Murray, University of Birmingham.
Professor Richard Hobbs, University of Oxford.
Professor Carl Heneghan, University of Oxford.
Dr Alison Ward, University of Oxford.
Professor Sheila Greenfield, University of Birmingham.
Dr Sue Jowett, University of Birmingham.
Dr Roger Holder, University of Birmingham.
Miss Jo Leggat, Secretary, University of Birmingham.
Dr Rick Roberts, South Birmingham PCT.
Dr Will Lester, University Hospital, Birmingham.
Mrs Eve Knight, Anticoagulation Europe.
Dr Chris Gardiner, University of Oxford.
Professor Jonathan Mant, University of Cambridge.
Dr Peter Rose, University Hospital, Warwick.
Independent observer
Professor Paul Moss, University of Birmingham.
Trial Steering Committee (established for work package 1: the ExACT trial)
Dr David Keeling (chairperson), University of Oxford.
Dr Patrick Kesteven, Freeman Hospital Newcastle.
Mrs Eve Knight (PPI), Anticoagulation Europe.
Dr Carl Heneghan, University of Oxford.
Professor David Fitzmaurice (chief investigator), University of Birmingham.
Dr Ellen Murray, University of Birmingham.
Mr Roger Holder (Lead Statistician until August 2013), University of Birmingham.
Mrs Andrea Roalfe (Lead Statistician after August 2013), University of Birmingham.
Data Safety and Monitoring Board (established for work package 1: the ExACT trial)
Dr Trevor Baglin (chairperson), Addenbrookes Hospital, Cambridge.
Professor Rafael Perera (Independent Statistician), University of Oxford.
Professor Jonathan Mant, University of Cambridge.
Frequency of meetings were determined by the chairperson of the Data Safety and Monitoring Board.
Contributions of authors
Professor David Fitzmaurice (https://orcid.org/0000-0002-9104-6252) (Professor of Primary Care with a special interest in VTE) was chief investigator with responsibility for the delivery on the overall programme and was lead for WP1. He led on the initial draft of the manuscript and is corresponding author.
Dr Kate Fletcher (https://orcid.org/0000-0001-7895-7572) (Trials Co-ordinator) was the programme manager with principal responsibility for delivery of WP1.
Dr Sheila Greenfield (Professor of Primary Care Sociology with a special interest in qualitative methodology) was the co-lead for WP2.
Dr Sue Jowett (https://orcid.org/0000-0001-8936-3745) (Health Economist) was the lead for WP3.
Dr Alison Ward (https://orcid.org/0000-0002-6343-9382) (Medical Sociologist) was the co-lead for WP2.
Dr Carl Heneghan (https://orcid.org/0000-0002-1009-1992) (Academic General Practitioner) provided methodological expertise for WP1 and sat on the TSC for WP1.
Mrs Eve Knight (chairperson of Anticoagulation Europe) provided PPI input.
Dr Chris Gardiner (https://orcid.org/0000-0002-2318-0062) (Biomedical Scientist) provided methodological expertise for WP1 regarding D-dimer measurement.
Dr Andrea Roalfe (https://orcid.org/0000-0003-1622-2639) (Statistician) provided statistical support in developing the programme.
Dr Yongzhong Sun (Statistician) provided statistical support for the analysis of WP1.
Dr Pollyanna Hardy (https://orcid.org/0000-0003-2937-8368) (Statistician) provided statistical support for the analysis of WP1.
Dr Deborah McCahon (https://orcid.org/0000-0003-2768-293X) (Research Fellow) was the project manager for WP1.
Mrs Gail Heritage (https://orcid.org/0000-0003-3247-8765) (Project Officer) undertook data collection and site management for WP1.
Mrs Helen Shackleford (Project Officer) undertook data collection and site management for WP1.
Professor FD Richard Hobbs (https://orcid.org/0000-0001-7976-7172) (Professor of Primary Care with a special interest in cardiology) provided methodological expertise for WP1 and WP2.
All named authors have contributed to the drafting of this report.
Publications
McFarland L, Ward A, Greenfield S, Murray E, Heneghan C, Harrison S, Fitzmaurice D. ExPeKT – exploring prevention and knowledge of venous thromboembolism: a two-stage, mixed-method study protocol. BMJ Open 2013;3:e002766.
Tullett J, Murray E, Nichols L, Holder R, Lester W, Rose P, et al. Trial protocol: a randomised controlled trial of extended anticoagulation treatment versus routine anticoagulation treatment for the prevention of recurrent VTE and post thrombotic syndrome in patients being treated for a first episode of unprovoked VTE (The ExACT Study). BMC Cardiovasc Disord 2013;13:16.
McFarland L, Murray E, Harrison S, Heneghan C, Ward A, Fitzmaurice D, Greenfield S. Current practice of venous thromboembolism prevention in acute trusts: a qualitative study. BMJ Open 2014;4:e005074.
Apenteng PN, Fitzmaurice DA, Litchfield I, Harrison S, Heneghan C, Ward A, Greenfield S. Patients’ perceptions and experiences of the prevention of hospital acquired thrombosis: a qualitative study. BMJ Open 2016;6:e013839.
Litchfield I, Fitzmaurice D, Apenteng P, Harrison S, Heneghan C, Ward A, Greenfield S. Prevention of hospital-acquired thrombosis from a primary care perspective: a qualitative study. Br J Gen Pract 2016;66:e593–602.
Bradbury C, Fletcher K, Sun Y, Heneghan C, Gardiner C, Roalfe A, et al. A randomised controlled trial of extended anticoagulation treatment versus standard treatment for the prevention of recurrent venous thromboembolism (VTE) and PTS in patients being treated for a first episode of unprovoked VTE (the ExACT study). Br J Haematol 2020;188:962–75.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Please note exclusive use will be retained until the publication of major outputs. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Tullett J, Murray E, Nichols L, Holder R, Lester W, Rose P, et al. Trial protocol: a randomised controlled trial of extended anticoagulation treatment versus routine anticoagulation treatment for the prevention of recurrent VTE and post thrombotic syndrome in patients being treated for a first episode of unprovoked VTE (The ExACT Study). BMC Cardiovasc Disord 2013;13. https://doi.org/10.1186/1471-2261-13-16.
- McFarland L, Ward A, Greenfield S, Murray E, Heneghan C, Harrison S, et al. ExPeKT – exploring prevention and knowledge of venous thromboembolism: a two-stage, mixed-method study protocol. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002766.
- All-Party Parliamentary Thrombosis Group . Addressing the Challenges, Maintaining the Momentum: An Analysis of the Progress to Date and a Vision for 2015 and Beyond, October 2015 2012.
- Januel JM, Chen G, Ruffieux C, Quan H, Douketis JD, Crowther MA, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA 2012;307:294-303. https://doi.org/10.1001/jama.2011.2029.
- Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008;371:387-94. https://doi.org/10.1016/S0140-6736(08)60202-0.
- Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. PREVENT Medical Thromboprophylaxis Study Group . Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004;110:874-9. https://doi.org/10.1161/01.CIR.0000138928.83266.24.
- NICE . Venous Thromboembolism in Over 16s: Reducing the Risk of Hospital-Acquired Deep Vein Thrombosis or Pulmonary Embolism n.d. www.nice.org.uk/guidance/NG89 (accessed 16 October 2019).
- Huo MH, Muntz J. Extended thromboprophylaxis with low-molecular-weight heparins after hospital discharge in high-risk surgical and medical patients: a review. Clin Ther 2009;31:1129-41. https://doi.org/10.1016/j.clinthera.2009.06.002.
- Sarasin FP, Bounameaux H. Out of hospital antithrombotic prophylaxis after total hip replacement: low-molecular-weight heparin, warfarin, aspirin or nothing? A cost-effectiveness analysis. Thromb Haemost 2002;87:586-92. https://doi.org/10.1055/s-0037-1613053.
- Sweetland S, Green J, Liu B, Berrington de González A, Canonico M, Reeves G, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009;339. https://doi.org/10.1136/bmj.b4583.
- Shrivastava S, Ridker PM, Glynn RJ, Goldhaber SZ, Moll S, Bounameaux H, et al. D-dimer, factor VIII coagulant activity, low-intensity warfarin and the risk of recurrent venous thromboembolism. J Thromb Haemost 2006;4:1208-14. https://doi.org/10.1111/j.1538-7836.2006.01935.x.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Rodger MA, Scarvelis D, Kahn SR, Wells PS, Anderson DA, Chagnon I, et al. Long-term risk of venous thrombosis after stopping anticoagulants for a first unprovoked event: a multi-national cohort. Thromb Res 2016;356:152-8. https://doi.org/10.1016/j.thromres.2016.03.028.
- Altman DG, Doré CJ. Randomisation and baseline comparisons in clinical trials. Lancet 1990;335:149-53. https://doi.org/10.1016/0140-6736(90)90014-V.
- Amin A, Spyropoulos AC, Dobesh P, Shorr A, Hussein M, Mozaffari E, et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE start). J Thromb Thrombolysis 2010;29:326-39. https://doi.org/10.1007/s11239-009-0361-z.
- The All-Party Parliamentary Thrombosis Group n.d. http://apptg.org.uk/ (accessed February 2020).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Jowett S, Bryan S, Murray E, McCahon D, Raftery J, Hobbs FD, et al. Patient self-management of anticoagulation therapy: a trial-based cost-effectiveness analysis. Br J Haematol 2006;134:632-9. https://doi.org/10.1111/j.1365-2141.2006.06243.x.
- n.d. www.e-dendrite.com/files/13/file/Pages%20from%20VERITY%202007.pdf (accessed 18 October 2016).
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708. https://doi.org/10.1056/NEJMoa1207541.
- Kearon C, Parpia S, Spencer FA, Schulman S, Stevens SM, Shah V, et al. Long-term risk of recurrence in patients with a first unprovoked venous thromboembolism managed according to D-dimer results: a cohort study. J Thrombosis Hemostasis 2019;17:1144-52. https://doi.org/10.1111/jth.14458.
- McFarland L, Murray E, Harrison S, Heneghan C, Ward A, Fitzmaurice D, et al. Current practice of venous thromboembolism prevention in acute trusts: a qualitative study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005074.
- Apenteng PN, Fitzmaurice D, Litchfield I, Harrison S, Heneghan C, Ward A, et al. Patients’ perceptions and experiences of the prevention of hospital-acquired thrombosis: a qualitative study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-013839.
- Litchfield I, Fitzmaurice D, Apenteng P, Harrison S, Heneghan C, Ward A, et al. Prevention of hospital-acquired thrombosis from a primary care perspective: a qualitative study. Br J Gen Pract 2016;66:e593-602. https://doi.org/10.3399/bjgp16X685693.
- NICE . Rivaroxaban for the Treatment of Deep Vein Thrombosis and Prevention of Recurrent Deep Vein Thrombosis and Pulmonary Embolism (NICE Technology Appraisal TA261) 2012.
- NHS . Commissioning for Quality and Innovation n.d. www.england.nhs.uk/nhs-standard-contract/cquin/ (accessed 23 April 2020).
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Berggvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. Thromb Haemost 2007;98:756-64. https://doi.org/10.1160/TH07-03-0212.
- Ageno W, Spyropoulos AC, Turpie AG. Role of new anticoagulants for the prevention of venous thromboembolism after major orthopaedic surgery and in hospitalised acutely ill medical patients. Thromb Haemost 2012;107:1027-34. https://doi.org/10.1160/TH11-11-0787.
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381-453. https://doi.org/10.1378/chest.08-0656.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e44-e88. https://doi.org/10.1378/chest.11-2292.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. https://doi.org/10.1001/archinte.160.6.809.
- Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol 2009;20:1619-30. https://doi.org/10.1093/annonc/mdp068.
- Shen VS, Pollak EW. Fatal pulmonary embolism in cancer patients: is heparin prophylaxis justified?. Southern Med J 1980;73:841-3. https://doi.org/10.1097/00007611-198007000-00005.
- Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2005;76:1534-8. https://doi.org/10.1136/jnnp.2004.055145.
- Spencer FA, Emery C, Lessard D, Goldberg RJ. Worcester Venous Thromboembolism Study. Upper extremity deep vein thrombosis: a community-based perspective. Am J Med 2007;120:678-84. https://doi.org/10.1016/j.amjmed.2006.06.046.
- Laporte S, Mismetti P, Décousus H, Uresandi F, Otero R, Lobo JL, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation 2008;117:1711-16. https://doi.org/10.1161/CIRCULATIONAHA.107.726232.
- Warwick D, Friedman RJ, Agnelli G, Gil-Garay E, Johnson K, FitzGerald G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br 2007;89:799-807. https://doi.org/10.1302/0301-620X.89B6.18844.
- Caprini JA, Hyers TM. Compliance with antithrombotic guidelines. Manag Care 2006;15:49-50.
- Sterne JA, Bodalia PN, Bryden PA, Davies PA, López-López JA, Okoli GN, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21090.
- NICE . Venous Thromboembolism: Reducing the Risk for Patients in Hospital n.d. www.nice.org.uk/Guidance/CG92 (accessed March 2020).
- Scannapieco G, Ageno W, Airoldi A, Bonizzoni E, Campanini M, Gussoni G, et al. Incidence and predictors of venous thromboembolism in post-acute care patients. A prospective cohort study. Thromb Haemost 2010;104:734-40. https://doi.org/10.1160/TH10-03-0169.
- NHS Choices . Improving Health, Improving Lives 2011. www.nhs.uk/about-the-nhs-website/professionals/developments/documents/annual-report/annual_report_2011_digital.pdf (accessed March 2020).
- Glaser BG. The constant comparative method of qualitative analysis. Social Problems 1965;12:436-45. https://doi.org/10.2307/798843.
- House of Commons Health Committee . The Prevention of Venous Thromboembolism in Hospitalised Patients. Second Report of Session 2004–05 n.d. https://publications.parliament.uk/pa/cm200405/cmselect/cmhealth/99/99.pdf (accessed March 2020).
- Monahan M, Ensor J, Moore D, Fitzmaurice D, Jowett S. Economic evaluation of strategies for restarting anticoagulation therapy with warfarin based on Venous Thromboembolism (VTE) risk after an index unprovoked VTE event. J Thrombosis Haemostasis 2017;15:1591-600. https://doi.org/10.1111/jth.13739.
- Department of Health and Social Care . NHS Reference Costs 2012 13 n.d. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed March 2020).
- Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. BMJ 2007;335:358-9. https://doi.org/10.1136/bmj.39308.560069.BE.
- NICE . Atrial Fibrillation: Medicines to Help Reduce Your Risk of a Stroke – What Are the Options? n.d. www.nice.org.uk/guidance/cg180/resources/cg180-atrial-fibrillation-update-patient-decision-aid-243734797 (accessed 14 April 2018).
- Kahn SR, Lamping DL, Ducruet T, Arsenault L, Miron MJ, Roussin A, et al. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol 2006;59:1049-56. https://doi.org/10.1016/j.jclinepi.2005.10.016.
- Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost 2009;7:884-8. https://doi.org/10.1111/j.1538-7836.2009.03339.x.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Pancani C, Coccheri S. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002;87:7-12. https://doi.org/10.1055/s-0037-1612936.
- Baglin T, Palmer CR, Luddington R, Baglin C. Unprovoked recurrent venous thrombosis: prediction by D-dimer and clinical risk factors. J Thromb Haemost n.d.;6:577-82.
- Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med 2004;23:2509-25. https://doi.org/10.1002/sim.1815.
- Joint Formulary Committee . British National Formulary 2013.
- n.d. www.solent.nhs.uk/_store/documents/woundformularyfeb13finalupdateapril2013.pdf (accessed 18 October 2016).
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;11:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Office for National Statistics . National Life Tables, England &Amp; Wales, 1980–82 to 2010–12 2013.
- Chitsike RS, Rodger MA, Kovacs MJ, Betancourt MT, Wells PS, Anderson DR, et al. Risk of post-thrombotic syndrome after subtherapeutic warfarin anticoagulation for a first unprovoked deep vein thrombosis: results from the REVERSE study. J Thromb Haemost 2012;10:2039-44. https://doi.org/10.1111/j.1538-7836.2012.04872.x.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Oxford Vascular Study . A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343-51. https://doi.org/10.1161/STROKEAHA.112.667204.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Locadia M, Bossuyt PM, Stalmeier PF, Sprangers MA, van Dongen CJ, Middeldorp S, et al. Treatment of venous thromboembolism with vitamin K antagonists: patients’ health state valuations and treatment preferences. Thromb Haemost 2004;92:1336-41. https://doi.org/10.1160/TH04-02-0075.
- Ensor J, Riley RD, Jowett S, Monahan M, Snell KIe, Bayliss S, et al. Prediction of risk of recurrence of venous thromboembolism following treatment for a first unprovoked venous thromboembolism: systematic review, prognostic model and clinical decision rule, and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20120.
- Douketis J, Tosetto A, Marcucci M, Baglin T, Cushman M, Eichinger S, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523-31. https://doi.org/10.7326/0003-4819-153-8-201010190-00009.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. https://doi.org/10.1056/NEJMoa0906598.
- Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-7. https://doi.org/10.1056/NEJM199903253401201.
- Castellucci LA, Le Gal G, Rodger MA, Carrier M. Major bleeding during secondary prevention of venous thromboembolism in patients who have completed anticoagulation: a systematic review and meta-analysis. J Thromb Haemost 2014;12:344-8. https://doi.org/10.1111/jth.12501.
- Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363-72. https://doi.org/10.1161/CIRCULATIONAHA.110.004747.
- EQ-5D . Population Norms n.d. https://euroqol.org/eq-5d-instruments/population-norms/ (accessed 23 April 2020).
- Joint Formulary Committee . British National Formulary 2017.
- Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 1996;156:1829-36. https://doi.org/10.1001/archinte.1996.00440150083009.
Appendix 1 Methods for work package 1 (the ExACT trial)
Trial design
The ExACT trial was a non-blinded, multicentre, two-arm, parallel-group RCT.
Participant eligibility
Inclusion criteria
People aged ≥ 18 years with a diagnosis of first unprovoked* proximal** DVT or PE on treatment with anticoagulants.
Patients who had completed a minimum of 3 months of anticoagulant therapy (target INR 2–3 for those taking warfarin).
*Unprovoked was defined as, within the preceding 3 months, no history of major surgery, lower limb trauma (i.e. fracture, limping for 3 days), use of combined oral contraceptive pill, hormone replacement therapy, pregnancy, significant immobility (i.e. confined to bed for 3 days), or active cancer.
**Proximal was defined as a DVT in the trifurcation area, popliteal, superficial femoral, deep femoral, common femoral and iliac veins.
Exclusion criteria
-
Patients aged < 18 years.
-
Patients with another indication for long-term AT (e.g. atrial fibrillation).
-
Patients with a diagnosis of first unprovoked proximal DVT or PE who were no longer receiving AT.
-
Patients with a high risk of bleeding as evidenced by any of the following:
-
Patients with a previous episode of major bleeding where the cause was not effectively treated.
-
Known thrombocytopenia with a platelet count of < 120 × 109/l.
-
Known chronic renal failure with a creatinine level of > 150 µmol/l (1.7 mg/dl) or an estimated glomerular filtration rate of < 30 ml/minute.
-
Known chronic liver disease with a total bilirubin level of > 25 µmol/l (1.5 mg/dl).
-
Active peptic ulcer.
-
-
Patients requiring antiplatelet therapy (e.g. aspirin and/or clopidogrel).
-
Patients with a vena cava filter in place.
-
Patients who have had a D-dimer test performed within 2 months of potential enrolment other than for evaluation for suspected recurrent VTE that was not confirmed.
-
Patients whose GP expected their life expectancy to be < 5 years.
-
Patients unable to attend follow-up visits because of geographic inaccessibility.
-
Patients participating in a competing investigation.
-
Patients with known antiphospholipid syndrome.
-
Patients with known inherited protein C and/or protein S or antithrombin deficiency.
-
Patients with a diagnosis of active cancer.
-
Patients unwilling to give consent.
Study setting
Patients were identified from participating anticoagulation clinics in NHS secondary care sites, and primary care sites running large anticoagulation clinics identified through the National Centre for Anticoagulation Training. Randomisation, study intervention and data collection were primarily undertaken within primary care.
List of recruitment sites
-
University Hospital Birmingham (UHB).
-
Heartlands, Solihull and Good Hope Hospitals (Heart of England NHS Foundation Trust).
-
City and Sandwell Hospitals in Birmingham (Sandwell and West Birmingham Hospitals NHS Trust).
-
Walsgrave Coventry and Rugby St Cross Hospitals (University Hospital Coventry and Warwickshire NHS Foundation Trust).
-
Warwick and Stratford-upon-Avon Hospitals (South Warwickshire NHS Foundation Trust).
-
Leicester General Hospital, Leicester Royal Infirmary and Glenfield Hospital (University Hospitals of Leicester NHS Trust).
-
University Hospital of North Staffordshire.
-
Sir Robert Peel and Samuel Johnson Community Hospitals (Burton Hospitals NHS Foundation Trust).
-
Worcestershire Royal Hospital, Kidderminster Hospital and Alexandra Hospital, Redditch (Worcestershire Acute Hospitals NHS Trust).
-
Russells Hall Hospital, Corbett Hospital and Guest Hospital (The Dudley Group NHS Foundation Trust).
-
George Eliot Hospital (George Eliot Hospital NHS Trust).
There were also 10 primary care sites running anticoagulation clinics identified through the National Centre for Anticoagulation Training, and SouthDoc who provided primary care-based anticoagulation services:
-
Ann Jones Family Health Centre
-
Bellevue Medical Centre
-
Cape Hill Medical Centre
-
Grange Hill Surgery
-
Greenridge Surgery
-
Hall Green Health
-
Harborne Medical Practice
-
Ridgeacre House Surgery
-
Riverbrook Surgery
-
Shipston Medical Centre.
Recruitment
During their anticoagulation clinic appointment, people identified as being potentially eligible for the study were given a patient information sheet and a postcard to return to the research department giving permission for the team to request confirmation of eligibility criteria from their GP. GPs were then contacted by the team, given information about the study and asked to complete a form providing the eligibility information required. GPs were also asked if they would be willing to provide ongoing information around adverse events if their patient enrolled into the study, and a room in the practice in which the follow-up visits could take place. Where the GP was unable to provide the information, the patient’s hospital consultant was approached to complete the form; where the practice was unable to provide a room for follow-up appointments, arrangements were made at the recruiting hospital.
People who did not return their initial postcard were given a second postcard approximately 1 month after the first through the anticoagulation clinic; anyone still not responding was not contacted further. Patients confirmed by their GP or consultant as being eligible were telephoned by the research team (at least 1 month before planned cessation of AT) to arrange their baseline clinic appointment. The baseline appointment had to be carried out prior to cessation of OAT.
Intervention
The intervention comprised extended anticoagulation for a period of 24 months following diagnosis of a first unprovoked VTE. Participants were allocated to either continue AT for a further 2 years (group E) or to discontinue AT (group D) as planned. Compression stockings could be used by participants as advised by the clinician treating the VTE.
For participants allocated to group E and who were taking warfarin, their warfarin continued to be prescribed through their GP or anticoagulation clinic, as per usual practice. The anticoagulation clinic lead was asked to extend their clinic visits for a further 2 years. All participants randomised to the group E receiving warfarin continued to have their INR monitored through their existing service provider. Participants allocated to the extended anticoagulation group (group E) and who were receiving rivaroxaban continued to have their AT managed by their existing health-care provider.
Participants randomised to the control group (group D) received standard care, which involved discontinuation of AT as planned. The GP and any specific hospital consultant involved with the participant’s care (e.g. haematologists) were informed that the patient was entering a study (Figure 5).
FIGURE 5.
Eligibility.
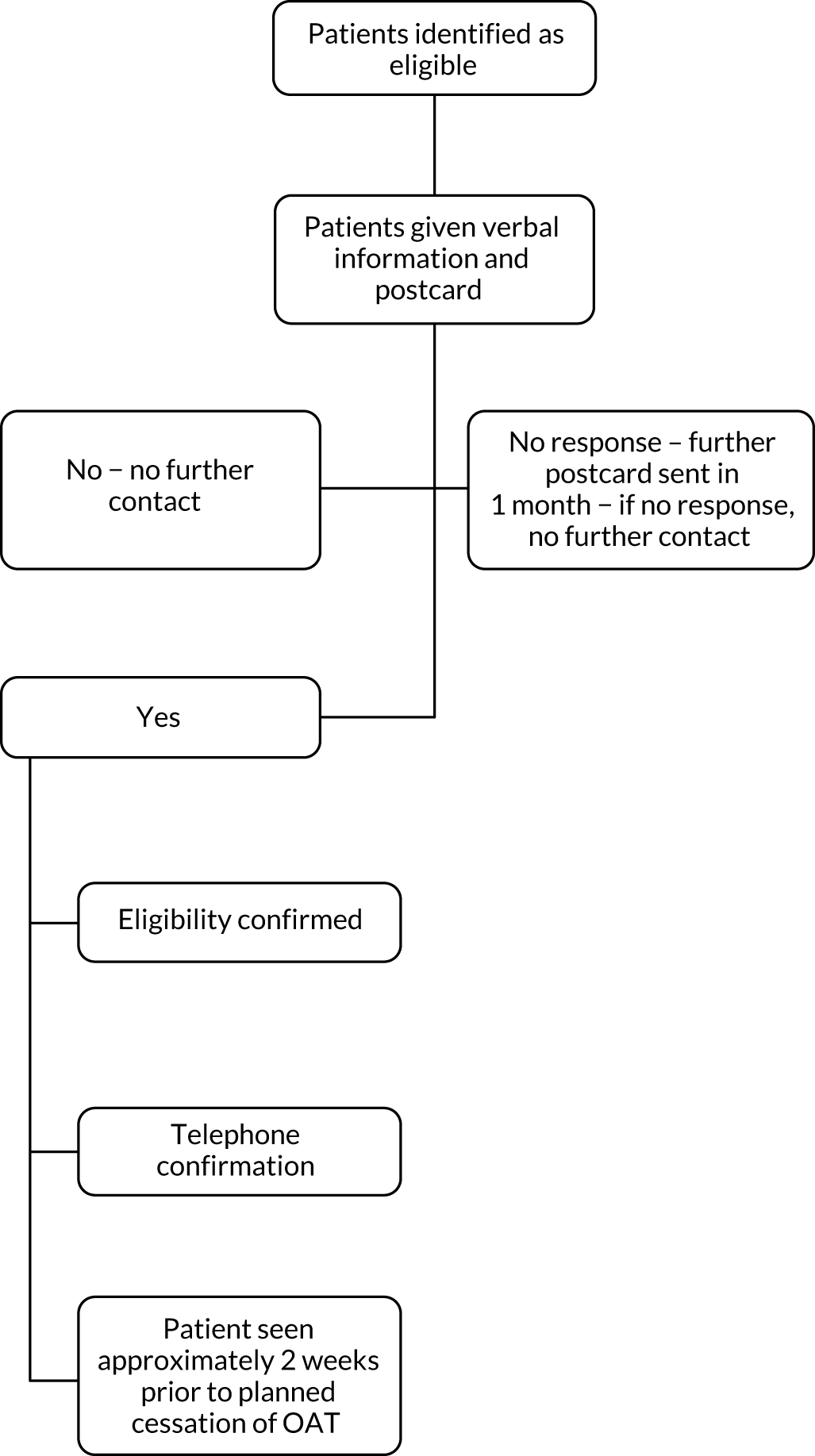
Randomisation
Randomisation was performed within the web-based computerised clinical case report form produced at the Department of Primary Care Clinical Sciences, University of Birmingham. When a patient was identified as being eligible for the study and had given written consent to take part, the researcher entered details into the computerised case report form and was issued immediately with a dedicated randomisation code allocating the patient to either extended AT (intervention, group E) or stopping AT as planned (control, group D).
Patients were randomised to the allocated intervention with a 1 : 1 allocation ratio. The randomisation sequence was created by a statistician (RH) who had no clinical involvement in the trial. The randomisation software used random permuted blocks with a block of size of 4 to ensure that the staff recruiting patients to the trial could not reliably predict the next allocation. Randomisation was stratified by diagnosis (DVT or PE) to ensure balance across intervention groups.
Outcomes measures
Primary outcome
-
Time to first recurrent VTE between randomisation and 24 months.
Secondary outcomes
-
Severity of PTS.
-
Bleeding events (major and clinically relevant non-major).
-
INR control in group E.
-
Identification of a cut-off point for levels of D-dimer in the diagnosis of DVT/PE.
-
Assessing whether or not levels of D-dimer are a predictor of PTS.
-
Patient quality of life [assessed via the Venous Insufficiency Epidemiological and Economic study – Quality of Life (VEINES-QOL)50 and EQ-5D-3L questionnaires].
-
Serious adverse events.
-
Cost-effectiveness.
Data collection
Baseline clinic appointment
At the baseline clinic appointment, the researcher confirmed eligibility with the patient, obtained written informed consent and randomised the patient. Additional information was collected and blood tests were performed (Box 1).
Ethnicity.
Sex.
Date of birth.
Smoking status.
Alcohol consumption.
Body mass index.
Family history of VTE.
Medical history.
Stroke.
Transient ischaemic attack.
Angina.
Myocardial infarction.
Ischaemic heart disease.
Peripheral vascular disease.
Concomitant medications.
DVT or PE.
QuestionnairesVillalta PTS scale.
EQ-5D-3L.
VEINES-QOL/symptoms.
Cost questionnaire.
SamplesHeparinised venous blood sample. a
Citrated venous blood sample. b
For D-dimer test on point-of-care device (Cobas h 232; Roche Diagnostics, Lewes UK). Participant and researcher were blind to these results.
For storage at central laboratories for later testing.
Participants were clinically examined for signs and symptoms of PTS using the Villalta scale, a validated clinical scoring system. 51 The Villalta scale was applied to both legs to establish presence and severity of PTS. The scale comprises six signs of PTS (i.e. swelling, hyperpigmentation, skin induration, redness, pain on calf compression and venous ectasia), the presence and severity of five self-reported symptoms (i.e. pain, cramping, heaviness, paraesthesia and pruritus), and each sign and symptom was graded on a scale of 0 to 3. A score of 5–9 signifies mild disease, 10–14 signifies moderate disease and ≥ 15 (or the presence of a venous ulcer) signifies severe disease.
Follow-up data collection
All participants were asked to attend 6-monthly study follow-up clinic appointments for 2 years (five visits in total). One additional appointment was carried out 1 month post anticoagulation cessation to collect an off-treatment D-dimer test. Data collected during these follow-up appointments can be seen in Box 2.
New medical diagnoses.
New medications.
Current anticoagulation status.
Adverse events.
QuestionnairesVillalta PTS scale.
EQ-5D-3L.
VEINES-QOL/symptoms.
SamplesHeparinised venous blood sample. a
Citrated venous blood sample. b
For D-dimer test on point-of-care device (Cobas h 232). Patient and researcher were blind to these results.
For storage at central laboratories for later testing.
Participants’ NHS numbers were flagged at the NHS Digital and the research team were informed about deaths and cause of death. A copy of the death certificate was obtained where appropriate.
Ascertainment of outcomes
During each study follow-up appointment and at the end of the study, participants’ GP records were reviewed for evidence of any thrombotic or haemorrhagic events (including fatalities), whether or not the participant was taking an anticoagulant, and any hospital admissions. Where a hospital admission was identified, researchers collected a copy of the discharge summary, admission and discharge dates and, where possible, discharge destination. Where a recurrent clot was identified, the nature of the diagnosis was noted (i.e. defined radiologically).
Outcome validation
To obtain objective confirmation of the primary outcomes, all potential thrombotic and haemorrhagic events were scrutinised by an Independent Adjudication Committee who were blind as to the intervention allocation. The Adjudication Committee consisted of three clinicians (i.e. a neurologist, a haematologist and a professor of primary care). On a quarterly basis, each member of the committee was given redacted and anonymised information about possible events and asked to categorise the event as thrombotic (DVT or PE), haemorrhagic (major), haemorrhagic (non-major) or no event. Where two out of the three members agreed on a definition, that definition was taken as correct. However, where all three members disagreed, the event was revisited and the adjudicators asked to discuss, re-assess and provide a unanimous decision.
Participant withdrawal and loss to follow-up
At the start of each follow-up clinic appointment, participants were asked if they were happy to continue their involvement in the study; those who said yes were followed up as described earlier. For participants who no longer wished to continue taking part, there were a number of options: complete withdrawal (participant no longer wished to take the study medication, did not want to attend study clinics, and no longer gave permission for researchers to access their medical records), and follow-up through notes only (participants who did not wish to attend further study follow-up appointments, but who were happy for us to continue to access their medical records to gather outcome and adverse event data). Participants who were no longer taking their allocated treatment were able to continue all follow-up visits as per protocol.
Sample size
At the design stage of the study, one of the intended aims was to have sufficient statistical power to detect an intervention effect on the primary outcome for the group of participants who were showing a positive level of D-dimer at baseline. The sample size was therefore determined to address this aim but, on completion of the study, the number of participants with a raised D-dimer level at baseline was considered too small to perform any meaningful comparisons.
A sample size of 79 in each arm would have been sufficient to detect a difference in recurrence rates between those showing a positive level of D-dimer on treatment who were allocated to extended AT and those showing a positive level of D-dimer who were allocated to discontinue AT (i.e. 4.3% vs. 21.4%)52 with 90% power at the 5% significance level. Assuming a 50% positive D-dimer rate at baseline and a 10% loss to follow-up at 2 years, the total sample size required was 79 × 2 × 2/0.9 = 352 participants (and 316 completing the study). With such a sample size an overall comparison of recurrence rates between the extended AT group and the discontinued AT group could be made with power of between 86% and 99%, assuming recurrence rates of between 1.4% and 4.3% for the extended AT group and 14.2% for the discontinued AT group.
One objective was to determine an optimal cut-off point on a D-dimer result taken when the participant was receiving treatment. In a study of 272 patients, Baglin et al. 53 report that 170 out of 272 patients had a D-dimer level in excess of 500 µg/l at around 1 month following cessation of therapy and their receiver operating characteristic curve analysis suggests an optimal cut-off point of 500 µg/l. 31 The circumstances of their study differed from ours but using their data gives some indication of the precision with of the estimate of an optimal cut-off point with slightly more patients than Baglin et al. 53 With some assumptions, a rough calculation of the precision with which a cut-off point in the region of 500 ng/ml could be estimated with our sample size of 316 would be within about 7%.
Analysis populations
All primary analyses (primary and secondary outcomes including safety outcomes) were performed on an intention-to-treat basis. Participants were analysed in the intervention group to which they were randomised, and all participants were included whether or not they received the allocated treatment, excluding the following prespecified post-randomisation exclusions:
-
participants who were subsequently found to have antiphospholipid syndrome, protein C and/or protein S or antithrombin deficiency
-
participants for whom consent to use their data were withdrawn.
Baseline characteristics
The following demographic variables were described for the total population and for the two randomised groups separately: age, sex, ethnicity, smoking status, alcohol consumption, body mass index classification, family history of VTE, previous medical history (stroke, transient ischaemic attack, angina, myocardial infarction, ischaemic heart disease, peripheral vascular disease), PTS (score and category), EQ-5D-3L, VEINES-QOL score, diagnosis (DVT/PE) and health-care utilisation because of PTS (receiving primary or secondary care treatment, type of nurse and treatment for leg ulcer).
Categorical data were summarised by frequencies and percentages. Continuous data were summarised by the number of responses, mean and SD if deemed to be normally distributed, and the median and interquartile range (IQR) if data appear skewed. Tests of statistical significance have not been undertaken. 15
Analysis methods: primary outcome
The number of participants with at least one recurrent VTE is presented by trial arm. Cox regression analysis was used to compare the time to first recurrent VTE between randomisation arms and hazard rates presented, censoring for deaths, losses to follow-up and withdrawals of consent to use data. The analysis was adjusted for diagnosis (DVT/PE) at baseline. The proportional hazards assumption was tested by visual assessment of cumulative log-hazard plots between the two treatment arms, and also by testing the statistical significance of an interaction parameter of the log of time with the treatment covariate. The comparison between treatment arms is summarised as a hazard ratio with associated 95% CIs, and p-values are also reported. The total number of events and the number of events per 100 person-years are presented to aid interpretation of the data.
Analysis methods: secondary outcomes
Severity of post-thrombotic syndrome
Repeated measures mixed modelling was used to compare the PTS score between treatment and control groups over the 2-year follow-up. The analysis allowed for the repeated nature of the data measured at 6, 12, 18 and 24 months, and an interaction term for the treatment and time point was fitted. At each time point, the worst score from both of the participant’s legs was counted as the score for the participant at that particular time point. The model was adjusted for the baseline PTS score, the time of assessments and diagnosis (DVT/PE) at baseline as fixed effects. Model assumptions were checked for evidence of non-normality in the residuals. The adjusted mean PTS scores at each time point are presented by trial arm. The presence and severity of PTS is also reported, using frequencies and percentages, according to the following cut-off points (0–4, no PTS; 5–9, mild PTS; 10–14, moderate PTS; ≥ 15, severe PTS).
Bleeding events (major and clinically relevant non-major)
Cox regression analysis was used to compare the time to the first major and clinically relevant non-major bleeding events (as separate outcomes) between randomisation arms. The analysis was performed as per the primary outcome. The total number of events and the number of events per 100 person-years are presented to aid interpretation of the data.
International normalised ratio control in the extended anticoagulation therapy group
The relationship between the percentage of time in range, measured using INR data, and the recurrence of VTE as a binary outcome will be investigated using logistic regression models, adjusting for diagnosis (DVT/PE). In a similar manner, the effect of percentage time in range on major bleeding events as a binary outcome will be investigated. This analysis will be carried out at a later date and published separately. However, the descriptive statistics for the therapeutic range between the groups of VTE recurrence or not will be presented.
Identification of cut-off point for levels of D-dimer in diagnosis of deep-vein thrombosis/pulmonary embolism
The optimum cut-off point for levels of D-dimer at randomisation was investigated using a Cox proportional hazards model and fractional polynomials,54 with time to first VTE event as the dependent variable and treatment allocation and diagnosis (DVT/PE) at baseline included as covariates. The cut-off point was then used to create a binary variable and the Cox analysis repeated with an interaction term for the binary variable and randomised group to confirm validity. In particular, we were interested in the following subgroup analysis:
-
time to first recurrent thrombotic event between those showing a raised level of D-dimer at point of randomisation who were randomly allocated to the treatment arm and those showing raised levels of D-dimer at point of randomisation who were randomly allocated to the no-treatment arm.
Hazard ratios within each D-dimer subgroup were to be presented with the corresponding 95% CIs. The p-value for the interaction effect was to be reported. As there was no meaningful pattern observed with the baseline levels of D-dimer data, these analyses were not performed.
Assessing whether levels of D-dimer are a predictor of post-thrombotic syndrome
Mixed linear or ordered logistic regression model analysis were used to model the relationship between level of D-dimer and PTS score. Age, sex, treatment group, diagnosis (DVT/PE) and the PTS score at baseline will be included as covariates, PTS and level of D-dimer being time-dependent variables. PTS score will be considered in two ways: (1) as a continuous score and (2) as a binary or ordinal categorical using published classifications. At each time of assessment, the worst score from both of the participant’s legs was counted as the overall PTS score for the participant.
Patient quality of life (as measured using the VEINES-QOL and EQ-5D-3L questionnaires)
The analysis method followed that described for the PTS score.
Planned subgroup analyses
Subgroup analyses were limited to events (either VTE recurrences or major bleeding events) and the following subgroups:
-
sex
-
age (two groups: aged ≤ 65 years and aged > 65 years).
The effects of these subgroups were examined by Cox regression analysis, using independent variables as the interaction between intervention arm and each of the two covariates (age and sex), separately, together with their main effects and diagnosis (DVT/PE). Subgroup-related estimates and 95% CIs are presented with interaction results alongside.
Statistical software
Stata version 12 was used for all analyses.
Levels of confidence and p-values
For all the primary and secondary outcome measures, estimates of treatment effects are presented with 95%, two-sided CIs. p-values are reported from two-sided tests. No adjustment for multiplicity was made.
Interim analyses and stopping rules
A data safety monitoring board met every 6 months to review numbers of event recurrences and major bleeds in each arm. Stopping rules were in place for benefit and for harm. The following rule was agreed by the data safety monitoring board (on 21 October 2013).
For benefit
The difference in recurrence between the extended AT group and discontinued AT group would be much greater than the expected 17.1% (4.3% and 21.4% respectively for individuals with raised levels of D-dimer at baseline).
An interim analysis using a modified Haybittle–Peto3 formulation of 4 SDs for benefit, the minimum number of events required for an evaluation that would identify a signal would be of 16 recurrences with all of these occurring in the discontinued AT group (16 : 0). An alternative option would be to evaluate once at least 19 recurrences have been observed; then, only if there was a 18 : 1 or 19 : 0 difference in the event frequency, this would trigger a review to potentially stop the trial early for benefit.
Options:
-
Review data for benefit after 16 recurrences have occurred in total and assume a signal if 16 : 0 in the discontinued AT versus extended AT group (respectively).
-
Review data for benefit after 19 recurrences have occurred in total and assume a signal if 18 : 1 or 19 : 0 in the discontinued AT versus extended AT group (respectively).
For safety
The number of major bleeds in the extended AT group is much greater than the estimated 1–3% per year.
-
Review data for safety if 11 major bleeds have been observed in the trial and assume a signal if 11 : 0 in the extended AT versus discontinued AT group (respectively).
VERITY (venous thromboembolism registry) database
In the original application we intended to analyse data in the VERITY (venous thromboembolism registry) database to derive a clinical algorithm for prediction of recurrent thrombosis. VERITY is a UK-based prospective observational registry of patients with VTE that aims to develop and improve the management of VTE through increasing knowledge and best practice. The registry includes data on patient demographics and the diagnostic strategies and treatment approaches taken in the management of patients. 20
When the data held within the registry were examined in detail, it was found that there were a number of key data items that were not included, particularly levels of D-dimer. It was therefore decided that it was not possible to use VERITY for this purpose, and this aspect of the project was not undertaken.
Appendix 2 Work package 3
Within-trial health economic analysis (work package 3)
Overview of section
This section provides a detailed description of the within-trial economic analysis conducted alongside the ExACT trial to evaluate the cost-effectiveness of prolonged oral anticoagulation treatment beyond 3–6 months for patients with a first unprovoked proximal DVT or PE.
Methods
The economic evaluation took the form of a cost–utility analysis using QALYs based on the EQ-5D-3L instrument. Relevant patient costs used in the economic evaluation alongside the clinical trial were based on individual-level data collected during the 2-year follow-up period of the trial. Costs were estimated from an NHS perspective, and were based on 2012/13 values, consistent with the time period of data collection. In line with the main trial analysis, 273 patients were included in the economic evaluation, with 139 randomised to the extended anticoagulation group and 134 to the group discontinuing anticoagulation.
Cost and resource use
The cost analysis adopted a UK health-care perspective. Resource use data were collected alongside the trial. The total costs for each participant consisted of treatment costs (anticoagulation and monitoring), PTS costs, and adjudicated adverse event costs (thrombotic and haemorrhagic). Resource use concentrated on primary care, medicines, hospital consultant appointments, A&E attendances and hospital admissions.
As part of the treatment cost, the cost of anticoagulation has two components:
-
The cost of the medication was assumed to be the unit cost per tablet (per day) multiplied by the duration of treatment. An assumption on the average dose of warfarin as being 5 mg per day was made for costing purposes. Patients who were on a different anticoagulant (rivaroxaban) were costed in the same way using unit costs of that medication.
-
Visit for an INR test (if on warfarin). The total number of INR test visits per patient, based on individual resource use data, was multiplied by the unit cost per visit, assuming a hospital clinic visit.
For participants on anticoagulation, where data on anticoagulation visits were not available because of loss to follow-up, it was assumed that treatment continued for the full 24 months. The mean overall frequency of monitoring was assumed for the time frame where INR visit data were missing. Tables 10–12 present the unit costs related to treatment. PTS costs were based on visits to (or by) HCPs, and specified dressings and medications.
| Item | Cost (£) | Description | Source |
|---|---|---|---|
| Warfarin | 0.95 | Per pack of 28 5-mg tablets | BNF, 201355 |
| Rivaroxaban | 210 | Per pack of 100 20-mg tablets | BNF, 201355 |
| INR monitoring visit | 8.59 | Per hospital INR clinic visit (updated to 2012/13 costs) | Jowett et al., 201619 |
| Resource use category | AT, mean (SD) | |
|---|---|---|
| Extended [n = 139 (warfarin 137, rivaroxaban 2)] | Discontinued (n = 134) | |
| Anticoagulation visits | 18.47 (12.26) | 6.28 (10.45) |
| PTS treatment | ||
| GP | 0.50 (0.06) | 0.57 (0.98) |
| GP home visit | 0.06 (0.46) | 0.01 (0.09) |
| Practice nurse | 0.06 (0.42) | 0.96 (8.13) |
| Nurse home visit | 0.25 (2.97) | 0.01 (0.09) |
| A&E | 0.04 (0.19) | 0.08 (0.30) |
| Outpatient | 0.06 (0.27) | 0.13 (0.41) |
| Product name | Cost (£) | Source |
|---|---|---|
| ActiFormCool® (Lohman & Rauscher UK Ltd, Burton on Trent, UK) – 10- × 10-cm dressing | 2.66 | BNF, 201355 |
| VISCOPASTE™ (Smith & Nephew plc, London, UK) bandages | 3.69 | BNF, 201355 |
| Doublebase™ (Dermal Laboratories Ltd, Hitchin, UK) gel – 500 ml | 5.83 | BNF, 201355 |
| Paraffin cream – 500 g | 3.66 | BNF, 201355 |
| Betnovate (GlaxoSmithKline UK, Middlesex, UK) – 30 g | 1.43 | BNF, 201355 |
| IODOFLEX™ (Smith & Nephew plc, London, UK) and IDOSORB™ (Smith & Nephew plc, London, UK) | 9.18 | BNF, 201355 |
| Fusidic acid – 30 g | 3.10 | BNF, 201355 |
| ALLEVYN™ Adhesive (Smith & Nephew plc, London, UK) – 7.5- × 7.5-cm dressing | 3.53 | BNF, 201355 |
| Biatain® Adhesive (Coloplast Ltd, Peterborough, UK) – 10- × 10-cm dressing | 1.77 | BNF, 201355 |
| Sorbsan (Pharma-Plast Ltd, Deeside, UK) – 10- × 10-cm dressing | 3.10 | BNF, 201355 |
| PRIMAPORE (Smith & Nephew plc, London, UK) – 8 × 15 cm | 0.34 | BNF, 201355 |
| Mepore® ultra (Mölnlycke Health Care Ltd, Milton Keynes, UK) – 7 × 8 cm | 0.14 | BNF, 201355 |
| Sodium chloride injection (20 ampoules) | 8.15 | BNF, 201355 |
| K-Four (Urgo Ltd, Loughborough, UK) multilevel compression kit (bandage) | 6.90 | BNF, 201355 |
| Class 2 compression stockings | 10.54 | Basingstoke Wound Formulary, 201356 |
| Ciprofloxacin 250 mg | 0.75 | BNF, 201355 |
For adverse events, only events that were relevant to this study were costed, based on the resource use requirements of each event. Therefore, only adjudicated thrombotic events, haemorrhagic events and death (if resource use was utilised) were included in the costing, and unrelated adverse events were not included. For this reason, no data on chronic pulmonary hypertension were included in the analysis as no data for this outcome were collected within the ExACT trial.
For each event, data were collected on any relevant health-care contacts in primary and secondary care, and, although data on the ExACT trial length of hospitalisation were not reported, information was given if the stay in hospital was prolonged. Therefore, in the absence of data on actual length of stay for hospitalisation, the unit costs of thrombotic events were based on whether or not the event required hospitalisation and, if so, whether the hospitalisation was prolonged. If the event had not led to hospitalisation, the ‘event only’ cost was calculated as the difference between the long-stay cost and the product of weighted average length of stay in long stay and unit cost of an excess bed-day. If the adverse event occurred as a complication to an elective procedure, then only the cost of an estimated additional amount of time in hospital was included. Costs were based on NHS Reference Costs 2012/1347 (Table 13) and Unit Costs of Health and Social Care 201357 for visits to HCPs, as shown in Table 14. As the follow-up period was 2 years, all costs for year 2 were discounted at a rate of 3.5%.
| Event | Cost (£) | Source |
|---|---|---|
| PE (no hospitalisation) | 751 |
Difference of: weighted average of length of stay in days in ‘Non-Elective Inpatients (Long Stay)’ multiplied by the weighted average of excess bed-days ‘DZ09’ in ‘Non-Elective Inpatients (Long Stay – Excess bed days)’ AND weighted average of ‘DZ09’ in ‘Non-Elective Inpatients (Long Stay)’ |
| PE (hospitalisation) | 1197 | Weighted average of ‘DZ09’ in ‘Non-Elective Inpatients (Short Stay)’ |
| PE (prolonged hospitalisation) | 2143 | Weighted average of ‘DZ09’ in ‘Non-Elective Inpatients (Long Stay)’ |
| DVT (no hospitalisation) | 739 |
Difference of: weighted average of length of stay in days in ‘Non-Elective Inpatients (Long Stay)’ multiplied by the weighted average of excess bed-days ‘QZ20’ in ‘Non-Elective Inpatients (Long Stay – Excess bed days)’ AND weighted average of ‘QZ20’ in ‘Non-Elective Inpatients (Long Stay)’ |
| DVT (hospitalisation) | 1225 | Weighted average of ‘QZ20’ in ‘Non-Elective Inpatients (Short Stay)’ |
| DVT (prolonged hospitalisation) | 2034 | Weighted average of ‘QZ20’ in ‘Non-Elective Inpatients (Long Stay)’ |
| Percutaneous coronary intervention, zero to two stents: excess stay due to adverse event | 607 | Difference in length of stay between ‘EA31B’ and ‘EA31D’ multiplied by weighted average excess bed-day cost £353 |
| Major open prostate or bladder neck procedures (male): excess stay due to adverse event | 591 | Difference in length of stay between ‘LB21A’ and ‘LB21B’ multiplied by weighted average excess bed-day cost £492 |
| Minor nose procedures, 19 years and over with CC: hospitalisation for epistaxis | 1494 | CZ12B |
| Enoxaparin 40 mg over 12 weeks | 255 | BNF, 201355 |
| Item | Cost (£) | Description | Source |
|---|---|---|---|
| GP practice consultation | 45 | Per patient contact lasting 11.7 minutes (based on GP-led consultations) | PSSRU 201357 |
| Practice nurse consultation | 13.43 | Per patient contact lasting 15.5 minutes | PSSRU 201357 |
| GP home visits | 114 | Per home visit | PSSRU 201357 |
| Nurse home visit | 39 | Per home visit | PSSRU 201357 |
| A&E attendance | 115 | A&E services | NHS Reference Costs 2012/13 Index sheet47 |
| Outpatient visit | 127 | Consultant-led outpatient attendances – for patients who were ‘admitted to hospital’ but not ‘rehospitalisation’ | NHS Reference Costs 2012/13 Index sheet47 |
Quality of life
The outcomes for the health economic analyses of the ExACT trial were based on QALYs estimated using the EQ-5D-3L instrument. A health utility is a continuum where values of zero and one are assigned to health conditions judged equivalent to death and optimal health, respectively. The EQ-5D-3L instrument comprises five questions dealing with aspects of physical and mental health (i.e. mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), for which the response is one of three possible degrees of impairment. The questionnaire was administered to study participants at baseline, 6, 12, 18 and 24 months. Data from the EQ-5D-3L were converted into utility values using UK preference-based utility weights. 58 Mean values for completed questionnaires were presented for each follow-up time point. Multiple imputation was used in cases of missing EQ-5D-3L data at one or more of the time points. 59 For participants who had died, a utility score of 0 was applied from the date of death and these participants were included in the complete-case analysis. The total QALY score for each study participant over their 24 months’ follow-up in the trial was estimated by calculating the area under each patient’s health utility curve using linear interpolation. To avoid bias, adjustment for differences in baseline EQ-5D-3L scores was undertaken using a regression-based adjustment. 60 All QALY scores reported in the base-case analysis reflect imputation and adjustment. As the follow-up period was 2 years, all QALYs for year 2 were discounted at a rate of 3.5%.
Cost-effectiveness analysis
An incremental cost-effectiveness analysis was conducted according to the intention-to-treat principle, to determine the difference in costs and outcomes between extended and discontinued anticoagulation. The unit of outcome was the incremental cost per QALY gained (incremental costs divided by incremental QALYs). Although the cost data were skewed, the arithmetic mean was calculated along with its non-parametric 95% CI. Mean differences in costs and outcomes were also presented with their non-parametric 95% CIs. To account for uncertainty because of sampling variation in cost-effectiveness, non-parametric bootstrapping was applied to the patient-level data to derive 5000 paired estimates of mean differences in costs and QALY scores. These were presented graphically on a cost-effectiveness plane and were used to construct a CEAC.
Results
Costs
The costing analysis based on the trial data showed that the mean cost per participant during the 24-month trial period was £326.30 for the extended anticoagulation arm and £350.38 for the discontinued anticoagulation arm (Table 15). This demonstrates an overall mean cost saving of £24.08. As expected, mean costs in the intervention arm were greater for anticoagulation and haemorrhagic events, but much less for thrombotic events. Costs for treatment of PTS were very similar, in line with clinical results demonstrating no differences in PTS severity.
| Resource use category | AT, mean (SD) | Difference (bootstrapped 95% CI) | |
|---|---|---|---|
| Extended (n = 139) | Discontinued (n = 134) | ||
| Anticoagulation | 202.42 (189.61) | 57.96 (95.59) | 144.46 (115.22 to 183.16) |
| PTS treatment | 71.47 (306.75) | 76.57 (200.28) | –5.10 (–67.10 to 58.88) |
| Thrombotic events | 70.80 (299.19) | 272.11 (584.31) | –201.31 (–321–21 to –98.38) |
| Haemorrhagic events | 53.08 (183.69) | 20.31 (74.00) | 32.77 (3.33 to 70.50) |
| Total NHS costs | 326.30 (439.49) | 350.38 (616.39) | –24.08 (–165.47 to 102.20) |
Outcomes
The baseline EQ-5D-3L score was higher in the discontinued anticoagulation arm (0.814) than in the extended anticoagulation arm (0.807) and slightly higher for the majority of time points (Table 16). Complete EQ-5D-3L data at all time points were available for 218 participants (80%), with a slightly higher rate in the extended anticoagulation arm (81%) compared with the discontinued anticoagulation arm (78%).
| Complete cases by time point | AT | Bootstrapped mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Extended (N = 139) | Discontinued (N = 134) | ||||
| n | Mean | n | Mean | ||
| EQ-5D-3L | |||||
| Baseline | 135 | 0.808 | 134 | 0.814 | –0.006 (–0.076 to 0.064) |
| 6 months | 127 | 0.794 | 119 | 0.789 | –0.006 (–0.070 to 0.051) |
| 12 months | 126 | 0.800 | 119 | 0.797 | 0.002 (–0.072 to 0.072) |
| 18 months | 119 | 0.793 | 116 | 0.822 | –0.028 (–0.093 to 0.045) |
| 24 months | 122 | 0.786 | 111 | 0.823 | –0.037 (–0.108 to 0.027) |
| Total QALYs | 113 | 1.562 | 105 | 1.642 | –0.080 (–0.207 to 0.035) |
| Imputed | |||||
| Total QALYs | 139 | 1.565 | 134 | 1.563 | 0.002 (–0.110 to 0.125) |
| Adjusted Total QALYs | 139 | 1.567 | 134 | 1.558 | 0.009 (–0.055 to 0.071) |
The differences in the mean imputed QALY score (adjusted for baseline differences) over 24 months’ follow-up were very similar in the two trial arms, although slightly favouring extended anticoagulation, with 0.009 additional QALYs (see Table 16). The complete-case analysis without any imputation and adjustment slightly favoured the discontinued anticoagulation arm. In all cases the 95% CIs around the mean differences did not suggest significance.
Base-case analysis
Table 17 provides the base-case results of the economic evaluation alongside the ExACT trial and reports the within-trial cost-effectiveness analysis undertaken using the adjusted and imputed data. Although differences in both cost and QALYs were small, the results favour the extended anticoagulation arm, with slightly lower costs and more QALYs.
| Difference in mean (intervention – control) | Bootstrapped 95% CI | Interpretation | |
|---|---|---|---|
| NHS costs (£) | –24.08 | –165.47 to 102.20 | Intervention less costly and more effective |
| QALYsa | 0.009 | –0.055 to 0.071 |
The cost-effectiveness plane in Figure 6 highlights the uncertainty around the result, with cost–QALY difference points in all four quadrants. The CEAC in Figure 7 reports the probability of cost-effectiveness for extended anticoagulation over a range of willingness-to-pay thresholds up to £50,000 per QALY. The CEAC shows that at a willingness-to-pay threshold of £20,000 per additional QALY, there is a 62% probability of extended anticoagulation being the cost-effective option, with a 61% probability at £30,000 per QALY.
FIGURE 6.
Cost-effectiveness plane of extended AT vs. discontinued AT.
FIGURE 7.
The CEAC of extended AT vs. discontinued AT.
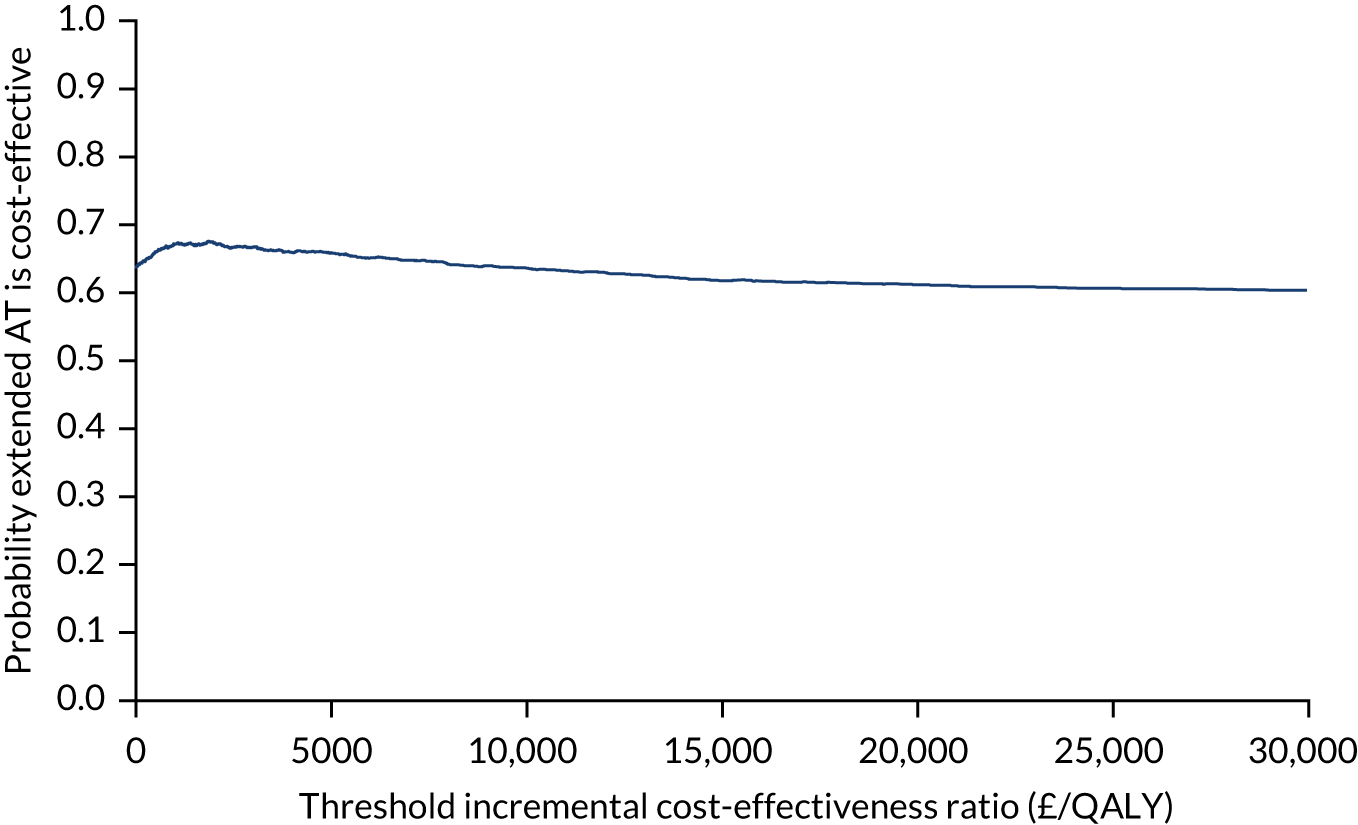
Discussion
These results suggest that, over the period of the trial, extended anticoagulation was marginally cheaper because of the additional costs of anticoagulation being offset by a reduction in costly thrombotic events. In addition, quality of life was very similar, which suggested that this may be a cost-effective option. However, there was a great deal of uncertainty around the results, particularly the QALYs. This may be because the EQ-5D-3L captures quality of life only at specific time points rather than measuring short-term disutility of thrombotic and haemorrhagic events at the time of occurrence. Therefore, the clinical benefits demonstrated in the main results of the trial are not translated into large quality-of-life gains. As a within-trial analysis can only take into account costs and outcomes that have occurred within the trial follow-up period, Markov modelling to extrapolate results over a lifetime horizon was undertaken and is presented in the next section.
Health economic modelling study to evaluate the cost-effectiveness of extended anticoagulation (work package 3)
Methods
Model population
The patient population comprised adult individuals having already completed at least 3 months of AT in response to their first unprovoked VTE. Individual patients were generated from the ExACT patient data set with characteristics created by randomly sampling the patient-level data by means of a uniform distribution. Patient characteristics comprised age, sex and type of index VTE event (DVT or PE).
Model pathways and clinical events
The economic model extrapolated the results of the ExACT trial and compared the strategy of discontinuing anticoagulation with lifetime extended anticoagulation. The model contained the same potential pathways in both strategies (Figure 8), and their characteristics in part determining the probability of clinical events, costs and utilities. The model had a 1-month time cycle and lifetime time horizon.
FIGURE 8.
Model patient pathways.

In 1 month, an individual had the probability of experiencing a clinical event: death from other causes, recurrent VTE (non-fatal DVT, fatal or non-fatal PE), fatal or non-fatal major bleeds (intracranial bleed, gastrointestinal bleed, and other major bleeds). Recurrent VTE carried a risk of PTS. Other-cause mortality was dependent on the current age and sex of the patient and was taken from UK life tables. 61 Recurrent VTE risk depended on time spent in the model, previous history of a recurrent VTE event taking place in the model, and treatment status. A recurrent VTE could be a PE or DVT and the recurrent VTE type was assumed to be influenced by an individual’s initial VTE site. Once a patient suffered a recurrent VTE, they were put on AT for life with cessation of therapy only if a later major bleeding event occurred. VTE events were assumed to incur a one-off quality-of-life reduction, with a proportion of surviving patients assumed to suffer from severe PTS for life.
The risk factors for a major bleed in the model were presence of anticoagulation treatment and age if on treatment, with major bleeds categories as gastrointestinal bleeds, intracranial bleeds and ‘other major bleeds’. All major bleeding events had short-term costs and quality-of-life decrements. In addition, an intracranial bleed was assumed to be associated with ongoing costs and a permanent quality-of-life decrement along with a sustained increased lifetime risk of other-cause mortality. For the ‘other major bleeds’ category, it was assumed that this heterogeneous category of bleeds should have the same costs and quality-of-life decrement as a gastrointestinal bleed.
Any major bleeding event led to discontinuation of AT. A recurrent VTE in a later cycle was assumed to restart therapy. It was assumed that there was no effect of AT on VTE recurrence risk more than 1 month post cessation of therapy.
Model type
A Markov patient-level simulation was developed in TreeAge 2014 and built on previous work on extended anticoagulation. 46 The model was adapted was to estimate the cost-effectiveness of extending AT versus discontinuing AT (usual care) in patients with a first unprovoked VTE event. A Markov model was appropriate here as it can represent a clinical situation where patients move between health states over a long period of time. The patient-level simulation allowed the use of individual patient characteristics (age, sex, index VTE type) from the ExACT trial, and these characteristics and clinical events that affect subsequent risks were remembered in the model with tracker variables. The model was run with a large number of simulated patients (50,000) to account for interpatient variability.
A time cycle of 1 month was selected to represent an assumption that this reflects a period in which a single clinical event might occur. Costs, utilities and probabilities were transformed into monthly equivalents as per the time cycle length. A half-cycle correction was applied to costs and effects. The base-case cost–utility analysis was undertaken from a UK NHS/Personal Social Services (PSS) perspective and considered a lifetime horizon.
Clinical parameters
Parameter estimates and their sources are listed in Table 18. The risk of a patient’s first recurrent VTE in the first 2 years was calculated by converting the trial primary outcomes for both arms from the rates per 100 person-years to an annual probability. This probability of VTE recurrence on therapy was assumed to be the same beyond 2 years, whereas the risk of VTE recurrence off therapy beyond 2 years was assumed to fall to 5%. 14 The annual risk of a further VTE event after a VTE recurrence on and off therapy was obtained from the PREVENT trial. 11 The probability of the type of any recurrent VTE being a PE was dependent on the index event, using primary data from the ExACT trial. Only major bleeds were included in the model, and the risk of major bleeding on and off therapy was obtained from ExACT trial data, converting rates per 100 person-years to annual probabilities. All annual probabilities were subsequently converted to monthly probabilities.
| Parameter (distribution type) | Estimate (distribution parameters) | Source |
|---|---|---|
| Clinical parameters | – | |
| Annual risk of recurrent VTE off therapy (first 2 years) (beta) | 12.66% (α = 16.96, β = 134) | ExACT trial |
| Long-term annual risk of VTE recurrence beyond 2 years off therapy (beta) | 5.0% (α = 5, β = 95) | Rodger et al., 201614 |
| Annual risk of recurrent VTE on therapy (first 2 years) (beta) | 2.71% (α = 3.77, β = 139) | ExACT trial |
| Long-term annual risk of VTE recurrence beyond 2 years on therapy (beta) | 2.71% (α = 3.77, β = 139) | ExACT trial |
| Annual risk of further VTE after previous recurrent VTE (beta) | Shrivastava et al., 200611 | |
| Off therapy | 12.0% (α = 11, β = 81) | |
| On therapy | 5.0% (α = 5, β = 95) | |
| Probability a recurrent VTE is a PE by index event (beta) | ||
| Index event DVT | 0.11 (α = 2, β = 17) | ExACT trial |
| Index event PE | 0.70 (α = 14, β = 6) | ExACT trial |
| Probability of death from PE (first month) (beta)21 | 0.2 (α = 2, β = 8) | Clinical consensus |
| Proportion of recurrent VTEs resulting in severe PTS (beta) | 1.1% (α = 4, β = 345) | Chitsike et al., 201262 |
| Annual risk of major bleed by age group (beta) | ||
| Not on therapy | 1.17% (α = 1.57, β = 134) | ExACT trial |
| On therapy | ExACT trial | |
| < 65 years | 1.50% (α = 2.09, β = 139) | |
| 65–74 years | 5.63% (α = 7.83, β = 139) | |
| ≥ 75 years | 5.63% (α = 7.83, β = 139) | |
| Split of major bleeds by bleed type (dirichlet) | Laporte et al., 200837 | |
| Gastrointestinal bleed | 36.5% | |
| Intracranial bleed | 17.9% | |
| Other major bleed | 45.6% (α1; α2; α3) = (499; 245; 622) | |
| Risk of death from major bleed (first month) (beta) | Laporte et al., 200837 | |
| Gastrointestinal bleed | 18.4% (α = 92, β = 407) | |
| Intracranial bleed | 32.2% (α = 79, β = 166) | |
| Other major bleed | 10.5% (α = 65, β = 557) | |
| Standardised mortality ratio for after an intracranial bleed (log-normal)a | 2.2 (95% CI 2.0 to 2.4) | Fogelholm et al., 200535 |
Resource use and costs
Costs of therapy and clinical events were included in the model (Figure 9 and Table 19). Costs of VTE and bleeding events were obtained from NHS Reference Costs 2012/1347. In line with the within-trial analysis, the cost hospital INR monitoring was assumed and the cost per visit was obtained from previous research and updated. 45 All costs were updated to 2012/13 prices using the HCHS Index. 18
FIGURE 9.
Model patient pathways.
| Cost (distribution type) | Cost estimate | Source |
|---|---|---|
| PE (fixed) | £1519 | NHS Reference Costs 2012/13 47 |
| DVT (fixed) | £732 | NHS Reference Costs 2012/13 47 |
| Proximal DVT (fixed) | £732 | NHS Reference Costs 2012/13 47 |
| 12 months’ warfarin monitoring (fixed). Assumes 27 visits per year at £8.59 per hospital visit (the ExACT trial) | £231.93 | Jowett et al., 201619 |
| Warfarin (5 mg per day, 12 months) (fixed) | £12.38 | BNF, 201355 |
| Gastrointestinal bleed (fixed) | £1092 | NHS Reference Costs 2012/13 47 |
| Other major bleed (fixed) | £1092 | Assumed same as GI bleed |
| Intracranial bleed: acute cost (gamma) | £8350 (α = 31.0, β = 269.4)a | Luengo-Fernandez et al., 201263 |
| Intracranial bleed: annual cost (fixed) | £1300 | Luengo-Fernandez et al., 201263 |
Quality of life
Quality-of-life (utility) values were assumed to be age related as they entered the model using EQ-5D-3L UK normative values. 64 As patients aged in the model, their utility score changed to reflect their updated quality of life for their age. Utility values for clinical events (Table 20) were multiplied by the age-specific utility to derive quality-of-life reductions for patients experiencing a clinical event. The model assumed no disutility with warfarin in the base case.
| Health state/clinical event | Median utility value (IQR) | Beta distribution | Duration of disutility | Source |
|---|---|---|---|---|
| DVT | 0.84 (0.64–0.98) | α = 2.0, β = 0.6 | 1 month | Locadia et al., 200465 |
| PE | 0.63 (0.36–0.86) | α = 1.2, β = 0.8 | 1 month | Locadia et al., 200465 |
| Non-fatal intracranial bleed | 0.33 (0.14–0.53) | α = 1.2, β = 2.1 | Permanent | Locadia et al., 200465 |
| GI bleed | 0.65 (0.49–0.86) | α = 1.2, β = 0.8 | 2 weeks | Locadia et al., 200465 |
| Other bleeds | 0.65 (0.49–0.86) | α = 1.2, β = 0.8 | 2 weeks | Assumed same as GI bleeds |
| Severe PTS | 0.82 (0.66–0.97) | α = 3.0, β = 0.9 | Permanent | Locadia et al., 200465 |
| Warfarin | 1 | Treatment length | Assumption |
Assessment of cost-effectiveness
The incremental analysis calculated the cost per QALY gained for extended anticoagulation versus discontinued anticoagulation. Cost-effectiveness was assessed in relation to the NICE lower threshold of £20,000 per QALY, where a value of £20,000 per QALY is judged to be cost-effective. 48 All costs and outcomes were discounted at the recommended 3.5%. 13
Deterministic sensitivity analysis
To test the robustness of base-case results, deterministic sensitivity analyses were run to determine the impact of changing key parameters on results.
-
The model time horizon was restricted to 5, 10 and 15 years.
-
The utility of warfarin therapy was reduced to 0.997 from 1.
-
The probability of death from a PE was increased to 30% because of uncertainty among clinical experts on the true value.
-
The cost of anticoagulation was increased to assume all patients were monitored in primary care and the site of monitoring was equally split between hospital- and general practice-led monitoring.
Probabilistic sensitivity analysis
When available, data were input into the model as distributions to assess parameter uncertainty in the form of a PSA. The model was rerun with 10,000 simulations for each trial of 1000 simulated patients and the results expressed as cost-effectiveness planes and CEACs.
Results
Base-case results
Under base-case assumptions, lifetime extended anticoagulation was more costly but more effective than extended anticoagulation, with an ICER of £9530 per QALY gained (Table 21).
| AT | Mean cost (£) | Mean QALYs | Cost difference (£) | QALY difference | ICER (cost per QALY) (£) |
|---|---|---|---|---|---|
| Discontinued | 3136 | 9.963 | |||
| Extended | 4575 | 10.114 | 1439 | 0.151 | 9530 |
Deterministic sensitivity analysis results
Deterministic sensitivity scenario results are shown in Table 22. Shortening the time horizon to 5 years increased the ICER to £15,975 per QALY, but this was still below the £20,000 per QALY threshold. Any further increase in time horizon beyond 5 years led to a reduction in the ICER. Increasing the risk of death from PE improved the cost-effectiveness of extended anticoagulation to £5836 per QALY. Attaching a disutility of 0.997 for being on anticoagulation increased the ICER, but it was still within the acceptable threshold. Changing the type and, therefore, cost of anticoagulation monitoring had the greatest impact on the ICER, with the scenario of all practice-led monitoring giving an ICER of £20,970 per QALY gained, which was slightly above the threshold, and an equal split of hospital and practice monitoring was £15,219 per QALY.
| Sensitivity analysis scenario | Mean cost (£) | Mean QALYs | Cost difference (£) | QALY difference | ICER (cost per QALY) (£) |
|---|---|---|---|---|---|
| Time horizon: 5 years | |||||
| Discontinued anticoagulation | 865 | 3.388 | |||
| Extended anticoagulation | 1504 | 3.428 | 639 | 0.040 | 15,975 |
| Time horizon: 10 years | |||||
| Discontinued anticoagulation | 1522 | 5.792 | |||
| Extended anticoagulation | 2563 | 5.862 | 1041 | 0.070 | 14,871 |
| Time horizon: 15 years | |||||
| Discontinued anticoagulation | 2060 | 7.450 | |||
| Extended anticoagulation | 3305 | 7.547 | 1245 | 0.097 | 12,835 |
| 30% risk of death from PE | |||||
| Discontinued anticoagulation | 2986 | 9.685 | |||
| Extended anticoagulation | 4480 | 9.941 | 1494 | 0.256 | 5836 |
| Utility loss with warfarin (0.997) | |||||
| Discontinued anticoagulation | 3136 | 9.950 | |||
| Extended anticoagulation | 4575 | 10.087 | 1439 | 0.137 | 10,504 |
| GP INR monitoring | |||||
| Discontinued anticoagulation | 4688 | 9.963 | |||
| Extended anticoagulation | 7845 | 10.114 | 3157 | 0.151 | 20,907 |
| Equal split GP: hospital monitoring | |||||
| Discontinued anticoagulation | 3912 | 9.963 | |||
| Extended anticoagulation | 6210 | 10.114 | 2298 | 0.151 | 15,219 |
Probabilistic sensitivity analysis results
The cost-effectiveness plane in Figure 10 highlights the uncertainty around the result, with cost–QALY difference pairs all both north-west and north-east quadrants. The CEAC in Figure 11 reports the probability of cost-effectiveness for extended anticoagulation over a range of willingness-to-pay thresholds up to £100,000 per QALY. The CEAC shows that at a willingness-to-pay threshold of £20,000 per additional QALY, there is a 56% probability of extended anticoagulation being the cost-effective option, with a 60% probability at £30,000 per QALY.
FIGURE 10.
Cost-effectiveness plane of extended AT vs. discontinued AT.
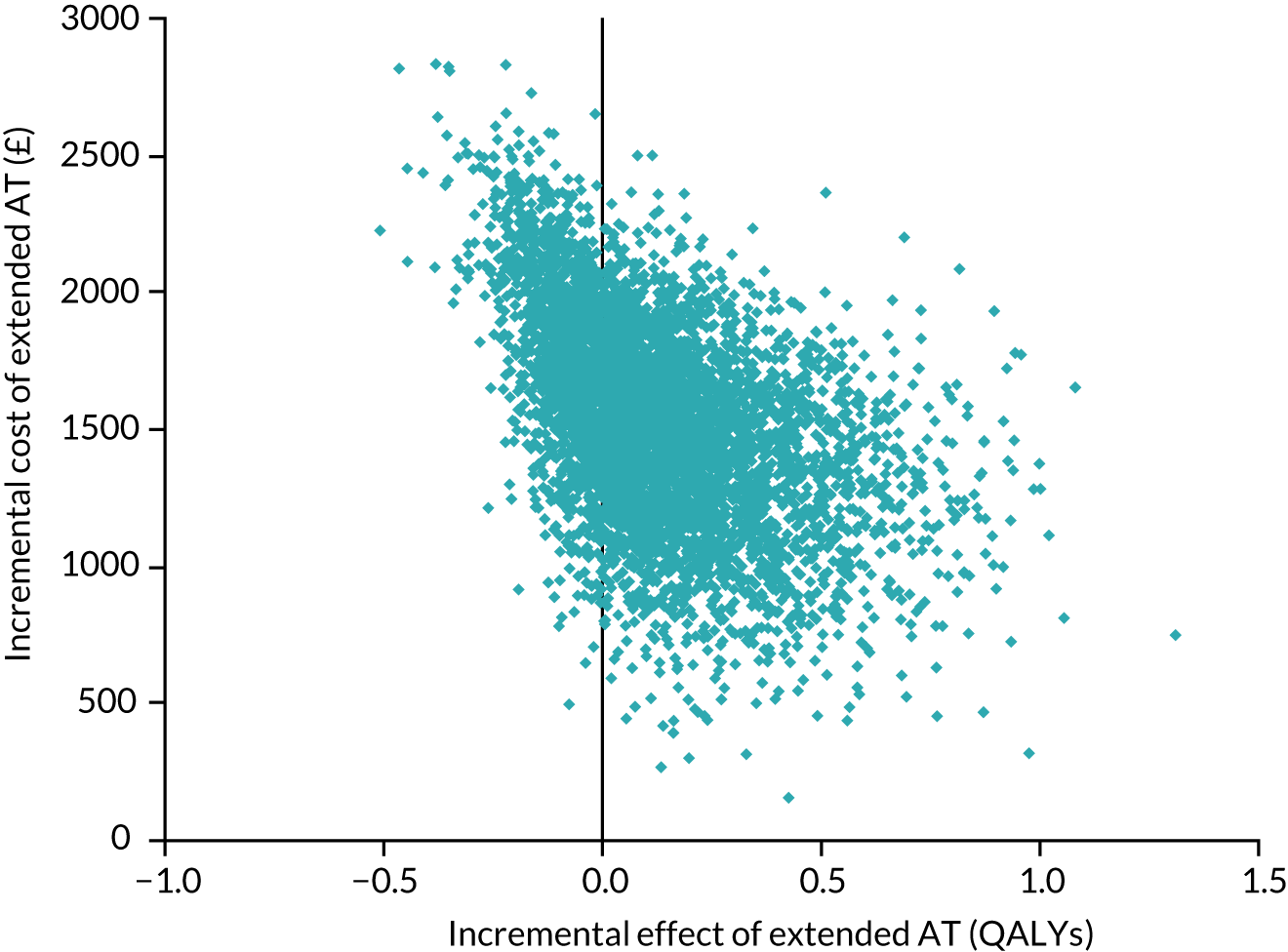
FIGURE 11.
The CEAC of extended AT vs. discontinued AT.
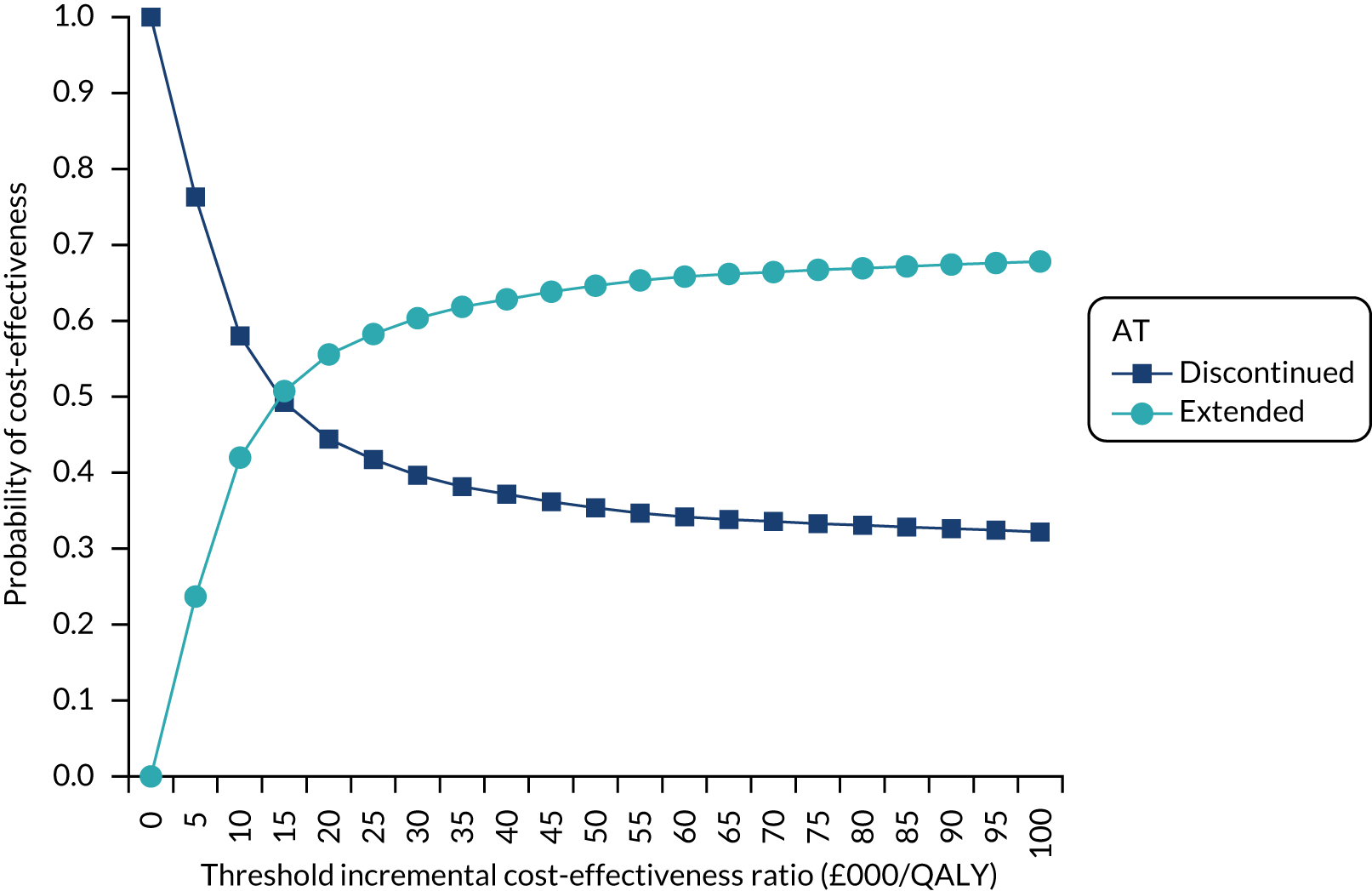
Discussion
The economic evaluation assessed the cost-effectiveness of lifetime extended anticoagulation in patients with a first unprovoked VTE. The base-case results indicate that this intervention would be cost-effective, and this result was robust to sensitivity analysis except for a more extreme scenario of all practice-based INR monitoring. However, quality of life on treatment, mortality risk after PE and the time horizon all had an impact on the cost-effectiveness results. The PSA results suggest considerable uncertainty around the base-case result, and this is most likely to be due to using trial-based estimates of recurrent VTE and bleeding. This uncertainty would reduce with the incorporation of data from a synthesis of these data with similar studies.
A key strength of the analysis is the use of an individual patient simulation, which uses characteristics of the participants of the ExACT trial. This was preferable to the more common cohort model with a homogeneous set of characteristics as the model results were more representative of a realistic patient population. The modelling method was also more efficient by using tracker variables to hold information on characteristics than requiring the construction of a large number of Markov health states.
As the purpose of the model was extrapolation beyond the ExACT trial period, data on VTE risk, type of VTE and bleeding risk were utilised from the trial. This allowed the uncertainty within the trial data to be represented. Ideally, decision models should use all the available evidence on effectiveness of an intervention; therefore, it is suggested that a future application of the model should use individual patient data synthesised from a number of studies to provide more generalisable results.
A patient preference study of extended oral anticoagulation for first unprovoked venous thromboembolism (work package 3)
Patient preferences
Research aims
The aim of this research was to determine patient preferences for extended anticoagulation treatment, taking into account both the benefits (reduction in thrombotic events) and risks (bleeding events) associated with anticoagulation.
Methods for data collection and analysis
A questionnaire was developed to elicit patient preferences for the extension of anticoagulation (specified as warfarin) (see Appendix 1). This used a technique regularly used to represent risks and benefits on anticoagulation as part of a decision tool, where 100 faces were represented. 49
The questionnaire was in two parts, looking at the risk of clots and risk of bleeding separately. Participants were first shown a representation of current evidence where the risk of DVT was 30% over 5 years without warfarin, and 0% with warfarin. They were then asked for the risk of serious bleeding they were willing to take over the 5 years by staying on warfarin (and, therefore, avoiding DVT), by crossing out faces on the picture. The second half of the questionnaire showed a representation of current evidence on serious bleeding, with 0% risk with no warfarin and 5% risk with warfarin. They were then asked what risk of DVT they were willing to take to avoid being on warfarin (and, therefore, avoiding serious bleeding), again by crossing out faces on the picture.
Questionnaires were completed by patients during their final follow-up study appointment, with assistance from the study nurses, with completed questionnaires entered onto a database.
An initial descriptive analysis was undertaken to determine the distribution of responses for both questions separately. Subsequently, both questions were considered together to determine whether a participant showed (1) a preference for warfarin (i.e. willing to risk a higher risk of bleeding than DVT), (2) a preference for no warfarin (i.e. willing to take a higher risk of DVT than bleeding) or (3) no preference (i.e. chose equivalent risks). Answers where it was clear that the participant had not understood the questionnaire, by offering no answer or responding 0% or 100% to both options, were reported separately. A descriptive analysis of counts and percentages was then presented. Finally, these data were analysed taking into account the following patient characteristics:
-
age
-
sex
-
trial arm
-
thrombotic event during the trial
-
bleeding event during the trial.
This was to determine whether or not any pattern was emerging in relation to demographics, and whether or not allocation of treatment or an event during the trial had influenced their choice.
Results
A total of 218 patients completed questionnaires, giving a response rate of 80%. Response rates were higher for those in the extended warfarin arm, women and those aged ≥ 65 years (Table 23).
| Characteristic | Response rate, % (n/N) |
|---|---|
| All patients | 79.9 (218/273) |
| Extended warfarin | 84.2 (117/139) |
| Discontinued warfarin | 75.4 (101/134) |
| Sex | |
| Male | 77.2 (142/184) |
| Female | 85.4 (76/89) |
| Age (years) | |
| < 65 | 75.2 (100/133) |
| ≥ 65 | 79.7 (118/148) |
Four participants did not feel that they could answer the question and a further 21 gave responses that indicated that they did not understand the task. Of those who appeared to not understand the task, nine chose a 0% risk of both types of event (i.e. not willing to risk either type of event) and a further 12 stated they were willing to take a 100% risk of both types of event, therefore giving an inconsistent response. The analysis presented here was undertaken on the remaining 193 responses.
Risk of serious bleeding with extended warfarin to avoid deep-vein thrombosis/pulmonary embolism
Here responses ranged from 0% to 100%, with 72 participants (37%) reporting they were willing to take a 100% risk of a bleed to stay on warfarin for a further 5 years and to avoid a further DVT/PE. A total of 80% (n = 155) were willing to take greater than the stated 5% risk of a bleed, and only 15% (n = 28) were not willing to take any risk of serious bleed.
Risk of deep-vein thrombosis without extended warfarin to avoid serious bleeding
Again, responses ranged from 0% to 100% risk, with 24 participants (12%) willing to take a 100% risk of a DVT/PE to avoid a serious bleed. A total of 23% (n = 44) were willing to take greater than the stated 30% risk of a DVT/PE, and 44% (n = 84) were not willing to risk a DVT/PE to avoid a serious bleed.
Comparison of risk of bleeding and risk of deep-vein thrombosis
The responses to both questions were then considered together. A total of 63 participants (33%) showed a very strong preference for extended warfarin by being willing take a 100% risk of a bleed, but not willing to take any risk of a DVT/PE. Only 21 (11%) chose the opposite (i.e. a very strong preference for not extending warfarin). Comparing the risk of DVT/PE with bleeding, the majority (70%, n = 135) showed a preference for avoiding DVT/PE (and extending warfarin), 21% (n = 40) preferred to avoid a serious bleed, and the remaining 18 (9%) gave very similar values for both DVT/PE and bleed.
The majority of participants were willing to take a higher risk of a bleed (compared with DVT/PE) to receive warfarin for 5 years (Table 24). This did not differ greatly in terms of sex, age group or trial arm, although men and those aged ≥ 65 years had a slightly greater preference for extended warfarin. Incidence of a DVT/PE in the trial period had an impact, with 20 out of the 24 (83%) who suffered a DVT/PE and responded to the questionnaire preferring extended warfarin. Those who had suffered a major bleed only reported a slightly lower preference for extended warfarin (68%, 6/9).
| Characteristic | Preference for lower risk of DVT/PE (favours extended warfarin) |
|---|---|
| All | 135 (69.9%) |
| Sex | |
| Male | 90 (72.0%) |
| Female | 45 (66.0%) |
| Trial arm | |
| Extended warfarin | 72 (69.2%) |
| No warfarin | 63 (70.8%) |
| Age (years) | |
| < 65 | 61 (67.0%) |
| ≥ 65 | 74 (72.5%) |
| DVT/PE | |
| Yes | 20 (83.3%) |
| No | 115 (68.0%) |
| Major bleed | |
| Yes | 6 (66.7%) |
| No | 129 (70.1%) |
Discussion
The results of this exploratory work on patient preferences for extended warfarin show that there was a higher preference for long-term anticoagulation (i.e. people were generally willing to take a higher bleeding risk). Unsurprisingly, this was particularly true for those who had a recurrent VTE during the trial follow-up period. There were challenges in measuring patient preferences, with a number of participants not understanding the tasks and giving inconsistent answers, despite the use of a standard visual aid.
Health economic modelling of extended anticoagulation for treatment and prevention of recurrent venous thromboembolism (work package 3)
Overview
The original research proposal aimed to undertake health economic modelling to evaluate the most cost-effective methods of preventing and treating VTE. Since the original proposal was written, considerably more evidence has been published on the prevention and treatment of VTE and the landscape of VTE prevention has changed considerably. 26
In 2010, NICE guidance on VTE hospital-based thromboprophylaxis was published (updated in 2015), and this included a comprehensive model-based health economic analysis. 8,26 VTE risk assessment was subsequently introduced as part of the CQUIN scheme in 2010/11,27 and nationally mandated Quality Requirements, with associated financial consequences, were later introduced in the 2014/15 NHS Standard Contract. Therefore, the proposed modelling of the health economics impact of improving the take-up of guidelines was deemed to be redundant because of the high take-up of risk assessment. Furthermore, in terms of treatment of VTE, additional treatments (DOACs) became available after assessment for clinical effectiveness and cost-effectiveness by the NICE Technology Appraisal process. Recent published research has determined the best oral anticoagulants for primary prevention, treatment and secondary prevention of VTE, again using economic modelling. 40 Therefore, this section aims to build on our previous published work, which assessed the cost-effectiveness of using a decision rule for continued anticoagulation, concentrating on warfarin. Although the original report briefly considered dabigatran and rivaroxaban in a sensitivity analysis, the subsequent paper only considered warfarin in the fully incremental analysis comparing different risk thresholds with each other. 66 This work now allows us to determine the most cost-effective VTE risk thresholds for continuing anticoagulation with dabigatran, rivaroxaban and apixaban, assuming non-inferiority to warfarin.
Methods
A previously developed prognostic model estimated an individual patient’s risk of a further unprovoked VTE without treatment. 46 Using this prognostic model, a decision rule was developed to determine the strategy for treatment based on a threshold of VTE recurrence risk (e.g. 5% VTE recurrence risk at 1 year post therapy). This study aims to build on previous modelling that considered extended anticoagulation with warfarin to evaluate the cost-effectiveness of a decision rule for extending anticoagulation with three alternative DOACs (i.e. apixaban, dabigatran and rivaroxaban) in patients after a first unprovoked VTE. Results for warfarin will also be presented.
Model type
The Markov patient-level simulation was developed in TreeAge 2014 (TreeAge software, Williamstown, MA, USA) to estimate the cost-effectiveness of using a decision rule for restarting AT with a DOAC versus no AT (usual care) in patients with a first unprovoked VTE event. The patient-level simulation allowed the use of patient-level data to create individual patients, each with a set of varying characteristics, and to be assigned a risk of VTE recurrence. Patient characteristics and clinical events that affect subsequent risks were remembered in the model with tracker variables. The model was run with a large number of simulated patients (50,000) to account for variability between patients.
A time cycle of 1 month was selected to represent a period in which a single clinical event might occur. Costs, utilities and probabilities were transformed into monthly equivalents as per the time cycle length. A half-cycle correction was applied to costs and effects. The base-case cost–utility analysis was undertaken from a UK NHS/PSS perspective and considered a lifetime horizon.
Model population
The modelled patient population was adult individuals having already completed at least 3 months of AT after their first unprovoked VTE. Patients entered the model having already had their D-dimer level measured 30 days after stopping at least 3 months of AT. Individual patients were generated from patient data (recurrent VTE collaborative database), which was used to develop the prognostic model. 67 Each patient had characteristics created by randomly sampling the patient-level data by means of a uniform distribution. Patient characteristics comprised age (mean 61.7 years), sex (61.8% male), type of index VTE event (distal DVT 9.2%, proximal DVT 58.5%) and PE (32.3%), and post-anticoagulation D-dimer level (mean 667.3 µg/l). The individual’s risk of a recurrent VTE within 12 months was then determined by inputting their newly created characteristics into the prognostic model risk equation.
Model pathways and clinical events
The economic model compared a strategy of no therapy (usual care) with a number of decision rule strategies, where therapy was restarted if the predicted annual risk of VTE recurrence was equal to or greater than the given threshold risk. In line with the extended warfarin analysis, arbitrary but clinically relevant thresholds were explored in the analyses: 1%, 3%, 5%, 7.5% 10%, 12.5%, 15%, 17.5%, 20%, 22.5%, 25% and a treat-all strategy was also included as a comparator. The specified VTE risks were then used as different decision rule comparators. Once the decision rule was applied, all the patients encountered the same potential pathways in all strategies (see Figure 9), with their characteristics determining the probabilities of clinical events, costs and utilities.
In each 1-month time cycle, an individual had the probability of experiencing one clinical event: death from other causes, recurrent VTE (non-fatal distal or proximal DVT, fatal or non-fatal PE), or fatal or non-fatal major bleeds (intracranial bleed, gastrointestinal bleed and other major bleeds). A recurrent VTE carried a risk of PTS. Other-cause mortality was dependent on the current age and sex of the patient and was taken from UK life tables. 61 Recurrent VTE risk depended on a patient’s characteristics, time spent in the model, previous history of a recurrent VTE event taking place in the model, and treatment status. A recurrent VTE could be a PE, distal DVT or proximal DVT. The recurrent VTE type was assumed to be affected by the site of an individual’s initial VTE. Once a patient suffered a recurrent VTE, they were put on AT for life, with therapy cessation only occurring with a later major bleeding event. VTE events were assumed to incur a one-off quality-of-life reduction, with a proportion of surviving patients assumed to suffer from severe PTS for life.
Treatment status and age (if on treatment) were considered to be risk factors for a major bleed, and bleeds were split into gastrointestinal bleeds, intracranial bleeds and other major bleeds. All major bleeding events had short-term costs and quality-of-life decrements. In addition, an intracranial bleed was assumed to be associated with ongoing costs and a permanent quality-of-life decrement along with a sustained increased lifetime risk of other-cause mortality. For model simplification purposes, it was agreed that the other major bleeds category should have the same costs and quality-of-life decrement as a gastrointestinal bleed.
Any major bleeding event led to discontinuation of AT, and a subsequent recurrent VTE in a later cycle was assumed to restart therapy. It was assumed that there was no effect of AT on VTE recurrence risk after 30 days post cessation of therapy.
Clinical parameters
The model was run for each DOAC separately (i.e. apixaban, dabigatran, rivaroxaban) as well as warfarin. The risk of a patient’s first recurrent VTE off therapy was calculated using the prognostic model for up to 3 years post D-dimer measurement (30 days after initial therapy cessation). Owing to weak calibration statistics of the prognostic model after 3 years, beyond this point an annual constant risk for the first recurrent VTE event off therapy was used. 46 Annual risk of a further VTE event after a VTE recurrence was an average of values for patients with normal and elevated D-dimer levels, on and off therapy respectively in the PREVENT trial. 14 The original model uses estimates on warfarin, and in this model it is assumed that these same risks of VTE and bleed on treatment are applied to each DOAC.
Resource use and costs
Costs of therapy and clinical events were included in the model (Table 25). The cost of a D-dimer test was incurred by the decision rule strategies as the D-dimer information was needed to enact the decision rules (Table 26). The 2012/13 costs in the original model were inflated to 2016/2017 prices using the HCHS Index, so that the most recent drug costs could be included. 68
| Parameter (distribution type) | Estimate (distribution parameters) | Source |
|---|---|---|
| Clinical parameters | – | |
| Annual risk of recurrent VTE off therapy (fixed) | Prognostic model equation | Ensor et al., 201666 |
| Short-term 6-month risk of recurrent VTE on AT (beta) | 2.1% (α = 27, β = 1239) | Schulman et al., 200969 |
| Long-term annual risk of VTE recurrence beyond 6 months on therapy (beta) | 1.3% (α = 1, β = 78) | Kearon et al., 199970 |
| Long-term annual risk of VTE recurrence beyond 2 years off therapy (beta) | 5.0% (α = 5, β = 95) | Rodger et al., 201614 |
| Annual risk of further VTE after previous recurrent VTE (beta) | Shrivastava et al., 200611 | |
| Off therapy | 12.0% (α = 11, β = 81) | |
| On therapy | 5.0% (α = 5, β = 95) | |
| Probability a recurrent VTE is a PE by index event (beta) | Douketis et al., 201067 | |
| Index event DVT | 0.15 (α = 15, β = 88) | |
| Index event PE | 0.52 (α = 30, β = 28) | |
| Probability of death from PE in the first month (beta)21 | 0.2 (α = 2, β = 8) | Clinical consensus |
| Proportion of recurrent VTEs resulting in severe PTS (beta) | 1.1% (α = 4, β = 345) | Chitsike et al., 201262 |
| Annual risk of major bleed by age group (beta) | ||
| Not on therapy | 0.45% (α = 25, β = 5593) | Castellucci et al., 201471 |
| On therapy | Eikelboom et al., 201172 | |
| < 65 years | 2.43% (α = 23, β = 929) | |
| 65–74 years | 3.25% (α = 86, β = 2554) | |
| ≥ 75 years | 4.37% (α = 106, β = 2324) | |
| Split of major bleeds by bleed type (Dirichlet) | Laporte et al., 200837 | |
| Gastrointestinal | 36.5% | |
| Intracranial | 17.9% | |
| Other major | 45.6% (α1 = 499; α2 = 245; α3 = 622) | |
| Risk of death from major bleed (first month) (beta) | Laporte et al., 200837 | |
| Gastrointestinal | 18.4% (α = 92, β = 407) | |
| Intracranial | 32.2% (α = 79, β = 166) | |
| Other major | 10.5% (α = 65, β = 557) | |
| Standardised mortality ratio for after an intracranial bleed (log-normal)a | 2.2 (95% CI 2.0 to 2.4) | Fogelholm et al., 200535 |
| Cost (distribution type) | Cost estimate (£)a | Source |
|---|---|---|
| PE (fixed) | 1598 | NHS Reference Costs 2012/13 47 |
| DVT (fixed) | 770 | NHS Reference Costs 2012/13 47 |
| Proximal DVT (fixed) | 770 | NHS Reference Costs 2012/13 47 |
| 12 months’ warfarin monitoring (fixed) | 355 | NICE, 201226 |
| Warfarin (5 mg per day, 12 months) (fixed) | 9.13 | BNF, 201774 |
| Apixaban (2.5 mg twice daily, 12 months) (fixed) | 693.50 | BNF, 201774 |
| Dabigatran (150 mg twice daily 12 months) (fixed) | 620.50 | BNF, 201774 |
| Rivaroxaban (20 mg daily, 12 months) (fixed) | 657 | BNF, 201774 |
| Gastrointestinal bleed (fixed) | 1149 | NHS Reference Costs 2012/13 47 |
| Other major bleed (fixed) | 1149 | Assumed same as GI bleed |
| Intracranial bleed: acute cost (gamma) | 8786 (α = 31.0, β = 269.4)b | Luengo-Fernandez et al., 201263 |
| Intracranial bleed: annual cost (fixed) | 1368 | Luengo-Fernandez et al., 201263 |
Quality of life
Quality-of-life (utility) values were assumed to be age related as they enter the model using EQ-5D-3L UK normative values. 73 As patients aged in the model, their utility score changed to reflect the updated quality of life for their age. Utility values for clinical events (Table 27) and disutility on warfarin were multiplied by the age-specific utility to derive quality-of-life reductions for patients on warfarin or experiencing a clinical event. It was assumed that all three DOACs did not result in any disutility. Warfarin was assigned a disutility of 0.997.
| Health state/clinical event | Median utility value (IQR)a | Beta distribution | Duration of disutility | Source |
|---|---|---|---|---|
| DVT | 0.84 (0.64–0.98) | α = 2.0, β = 0.6 | 1 month | Locadia et al., 200465 |
| PE | 0.63 (0.36–0.86) | α = 1.2, β = 0.8 | 1 month | Locadia et al., 200465 |
| Non-fatal intracranial bleed | 0.33 (0.14–0.53) | α = 1.2, β = 2.1 | Permanent | Locadia et al., 200465 |
| GI bleed | 0.65 (0.49–0.86) | α = 1.2, β = 0.8 | 2 weeks | Locadia et al., 200465 |
| Other bleeds | 0.65 (0.49–0.86) | α = 1.2, β = 0.8 | 2 weeks | Assumed same as GI bleed |
| Severe PTS | 0.82 (0.66–0.97) | α = 3.0, β = 0.9 | Permanent | Locadia et al., 200465 |
| Warfarin | 0.997 (0.953–1.0) | α = 16.4, β = 0.3 | Treatment length | Gage et al., 199675 |
Assessment of cost-effectiveness
A sequential incremental analysis was designed to calculate the cost per QALY gained for applying a decision rule versus the next most effective option, applying the rules of dominance and extended dominance. Cost-effectiveness was assessed in relation to the NICE lower threshold of £20,000 per QALY, where a value of £20,000 per QALY is judged to be cost-effective. 26 Strategies were compared by increasing effectiveness, and ICERs were calculated from the difference in costs and effects between a decision rule strategy and the next best alternative. A strategy is said to be dominated if it is more expensive and less effective than a comparator. All costs and outcomes were discounted at the recommended 3.5%. 26
Deterministic sensitivity analysis
To test the robustness of base-case results, deterministic sensitivity analyses were run to determine the impact of changing key parameters on results. Results from the original model suggested that the model was particularly sensitive to parameters relating to PE; therefore, additional analysis concentrated on these parameters.
-
The probability of death from a PE was increased to 30% because of uncertainty among clinical experts.
-
Subgroup analysis was undertaken for index PE patients, as the subgroup of PE patients were at a higher risk of recurrence and mortality.
Results
Base-case results
Tables 28–31 present the base-case results for each anticoagulant, where a sequential incremental analysis was undertaken. Under base-case assumptions, restarting warfarin therapy for patients with a predicted annual VTE recurrence risk of 25% gave the lowest cost per QALY of £2814. This recurrence risk was also the most cost-effective for apixaban, dabigatran and rivaroxaban, with ICERs ranging between £5520 and £6312 per QALY.
| Strategy | Mean cost (£) | Mean QALYs | ICER (cost per QALY) (£) |
|---|---|---|---|
| Treat all | 5964 | 10.4448 | Dominated |
| Decision rule: 1% | 5888 | 10.4516 | Dominated |
| Decision rule: 3% | 5560 | 10.4805 | Dominated |
| Decision rule: 5% | 5079 | 10.5089 | Dominated |
| Treat no one | 3347 | 10.5448 | – |
| Decision rule: 7.5% | 4478 | 10.5449 | Dominated |
| Decision rule: 25% | 3392 | 10.5607 | 2814 |
| Decision rule: 22.5% | 3413 | 10.5628 | 10,330 |
| Decision rule: 10% | 4036 | 10.5640 | Dominated |
| Decision rule: 20% | 3453 | 10.5662 | 11,547 |
| Decision rule: 17.5% | 3510 | 10.5699 | 15,572 |
| Decision rule: 12.5% | 3770 | 10.5705 | Dominated |
| Decision rule: 15% | 3603 | 10.5709 | 90,176 |
| Strategy | Mean cost (£) | Mean QALYs | ICER (cost per QALY) (£) |
|---|---|---|---|
| Treat all | 9740 | 10.4719 | Dominated |
| Decision rule: 1% | 9606 | 10.4783 | Dominated |
| Decision rule: 3% | 9031 | 10.5054 | Dominated |
| Decision rule: 5% | 8187 | 10.5312 | Dominated |
| Treat no one | 5005 | 10.5566 | – |
| Decision rule: 7.5% | 7113 | 10.5639 | Dominated |
| Decision rule: 25% | 5108 | 10.5729 | 6312 |
| Decision rule: 22.5% | 5153 | 10.5752 | 19,773 |
| Decision rule: 20% | 5230 | 10.5789 | 20,822 |
| Decision rule: 10% | 6316 | 10.5804 | Dominated |
| Decision rule: 17.5% | 5342 | 10.5830 | 27,610 |
| Decision rule: 15% | 5518 | 10.5846 | 106,950 |
| Decision rule: 12.5% | 5828 | 10.5853 | 445,518 |
| Strategy | Mean cost (£) | Mean QALYs | ICER (cost per QALY) (£) |
|---|---|---|---|
| Treat all | 8903 | 10.4719 | Dominated |
| Decision rule: 1% | 8782 | 10.4783 | Dominated |
| Decision rule: 3% | 8262 | 10.5054 | Dominated |
| Decision rule: 5% | 7498 | 10.5312 | Dominated |
| Treat no one | 4637 | 10.5566 | – |
| Decision rule: 7.5% | 6529 | 10.5639 | Dominated |
| Decision rule: 25% | 4728 | 10.5729 | 5520 |
| Decision rule: 22.5% | 4767 | 10.5752 | 17,504 |
| Decision rule: 20% | 4836 | 10.5789 | 18,573 |
| Decision rule: 10% | 5811 | 10.5804 | Dominated |
| Decision rule: 17.5% | 4936 | 10.5830 | 24,593 |
| Decision rule: 15% | 5093 | 10.5846 | 95,736 |
| Decision rule: 12.5% | 5372 | 10.5853 | 400,210 |
| Strategy | Mean cost (£) | Mean QALYs | ICER (cost per QALY) (£) |
|---|---|---|---|
| Treat all | 9322 | 10.4719 | Dominated |
| Decision rule: 1% | 9194 | 10.4783 | Dominated |
| Decision rule: 3% | 8646 | 10.5054 | Dominated |
| Decision rule: 5% | 7843 | 10.5312 | Dominated |
| Treat no one | 4821 | 10.5566 | – |
| Decision rule: 7.5% | 6821 | 10.5639 | Dominated |
| Decision rule: 25% | 4918 | 10.5729 | 5916 |
| Decision rule: 22.5% | 4960 | 10.5752 | 18,638 |
| Decision rule: 20% | 5033 | 10.5789 | 19,697 |
| Decision rule: 10% | 6063 | 10.5804 | Dominated |
| Decision rule: 17.5% | 5139 | 10.5830 | 26,102 |
| Decision rule: 15% | 5306 | 10.5846 | 101,343 |
| Decision rule: 12.5% | 5600 | 10.5853 | 422,864 |
When considering a threshold of £20,000 per QALY gained, resuming AT with warfarin for patients with a predicted annual VTE recurrence risk of 17.5% yielded the highest number of QALYs while also being considered cost-effective with an ICER of £15,572 per QALY gained. Both dabigatran and rivaroxaban were cost-effective at a higher VTE recurrence risk of 20%, with a risk of 17.5% resulting in ICERs above the £20,000 threshold but below £30,000. The lowest risk threshold where apixaban was cost-effective was even higher, at 22.5%.
Deterministic sensitivity analysis results
Deterministic sensitivity scenario results are shown in Tables 32 and 33. These results illustrate that increasing the risk of death from PE led to lower risk decision rule strategies also being cost-effective. This was ≥ 12.5% in the case of warfarin, and ≥ 17.5% for apixaban, dabigatran and rivaroxaban.
| Strategya | Mean cost (£) | Mean QALYs | ICER (cost per QALY) (£) |
|---|---|---|---|
| Warfarin | |||
| Treat no one | 3236 | 10.3742 | |
| Decision rule: 12.5% | 3686 | 10.4448 | 14,683 |
| Apixaban | |||
| Treat no one | 4827 | 10.3855 | |
| Decision rule: 17.5% | 5193 | 10.4390 | 12,881 |
| Dabigatran | |||
| Treat no one | 4474 | 10.3855 | |
| Decision rule: 17.5% | 4799 | 10.4390 | 11,502 |
| Rivaroxaban | |||
| Treat no one | 4650 | 10.3855 | |
| Decision rule: 17.5% | 4996 | 10.4390 | 12,191 |
| Strategya | Mean cost (£) | Mean QALYs | ICER (cost per QALY) (£) |
|---|---|---|---|
| Warfarin | |||
| Treat no one | 3369 | 10.1690 | |
| Decision rule: 10% | 4144 | 10.2888 | 19,298 |
| Apixaban | |||
| Treat no one | 4934 | 10.8010 | |
| Decision rule: 15% | 5539 | 10.2709 | 12,840 |
| Dabigatran | |||
| Treat no one | 4558 | 10.8010 | |
| Decision rule: 12.5% | 5440 | 10.2884 | 18,065 |
| Rivaroxaban | |||
| Treat no one | 4761 | 10.8010 | |
| Decision rule: 10% | 5666 | 10.2884 | 19,111 |
All decision rule strategies of ≥ 10% were cost-effective with extended warfarin when the patient’s index event was a PE reflecting the high-risk nature of such index events. All strategies of ≥ 12.5% were cost-effective for dabigatran and rivaroxaban, with all strategies ≥ 15% cost-effective for apixaban.
Discussion
The economic evaluation assessed the cost-effectiveness of utilising a decision rule for extended AT in patients with a first unprovoked VTE, and explored three DOACs (i.e. apixaban, dabigatran, rivaroxaban) in addition to warfarin. Base-case results suggest that treating patients with a predicted 1-year VTE risk of ≥ 17.5% with warfarin could be cost-effective compared with the next most effective option. The risk at which therapy was cost-effective was higher for all three DOAC drugs, at 20% for dabigatran and rivaroxaban and 22.5% for apixaban, the most expensive. Although the new drugs do not require regular monitoring, all three are still more expensive than warfarin plus INR monitoring. Increasing the risk of death from PE and running the model for only those whose index event was a PE reduced the level of risk of recurrence at which a therapy became cost-effective.
This analysis builds on the first economic evaluation to consider using a decision rule to weigh up the advantages and disadvantages of resuming anticoagulation treatment in unprovoked VTE patients. There were a number of simplifying assumptions required in the original modelling; for example, the prognostic model used to calculate individual risk predictions was applied at 30 days post cessation of AT, which is not clinically ideal as some patients will have recurrence in these 30 days. In addition, in the absence of data, constant VTE recurrence risks were used beyond 3 years, after a subsequent VTE and on treatment. In practice, recurrent VTE risk is likely to vary by patient characteristics. Furthermore, the use of the prognostic model for the economic analysis implicitly assumes that the risk prediction tool is perfectly accurate but, in reality, there will be a degree of error between predictions and reality. In addition, the strategy for treatment resumption and cessation after a major bleeding event may differ between patients. The other key assumption made in this model was the non-inferiority of three DOACs compared with warfarin; therefore, the risk of recurrent VTE and bleeds, obtained from studies concerning warfarin, was also for all treatments, so the cost of treatment was primarily driving the results.
Sensitivity analyses in the original published model demonstrated the large uncertainty underlying many of the parameters and their effect on results. Therefore, there is a need for robust long-term data on the risk of recurrent VTE in unprovoked index VTE patients, and bleeding risk data.
List of abbreviations
- A&E
- accident and emergency
- AIDS
- acquired immune deficiency syndrome
- APPTG
- All-Party Parliamentary Thrombosis Group
- AT
- anticoagulation therapy
- CEAC
- cost-effectiveness acceptability curve
- CQUIN
- Commissioning for Quality and Innovation
- CI
- confidence interval
- DOAC
- direct oral anticoagulant
- DODS
- D-dimer optimal duration study
- DVT
- deep-vein thrombosis
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ExACT
- Extended Anticoagulation
- ExPeKT
- Exploration of Patient Knowledge and expectation
- GP
- general practitioner
- HAT
- hospital-acquired thrombosis
- HCHS
- Hospital and Community Health Services
- HCP
- health-care professional
- ICER
- incremental cost-effectiveness ratio
- INR
- international normalised ratio
- IQR
- interquartile range
- NICE
- National Institute for Health and Care Excellence
- OAT
- oral anticoagulation therapy
- PE
- pulmonary embolism
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PTS
- post-thrombotic syndrome
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SPSS
- Statistical Product and Service Solutions
- VEINES-QOL
- Venous Insufficiency Epidemiological and Economic study – Quality of Life
- VTE
- venous thromboembolism
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar08050).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
