Notes
Article history
The research reported in this issue of the journal was funded by the PGfAR programme as project number RP-PG-0612-20010. The contractual start date was in March 2015. The draft report began editorial review in August 2019 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Downs et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
SYNOPSIS
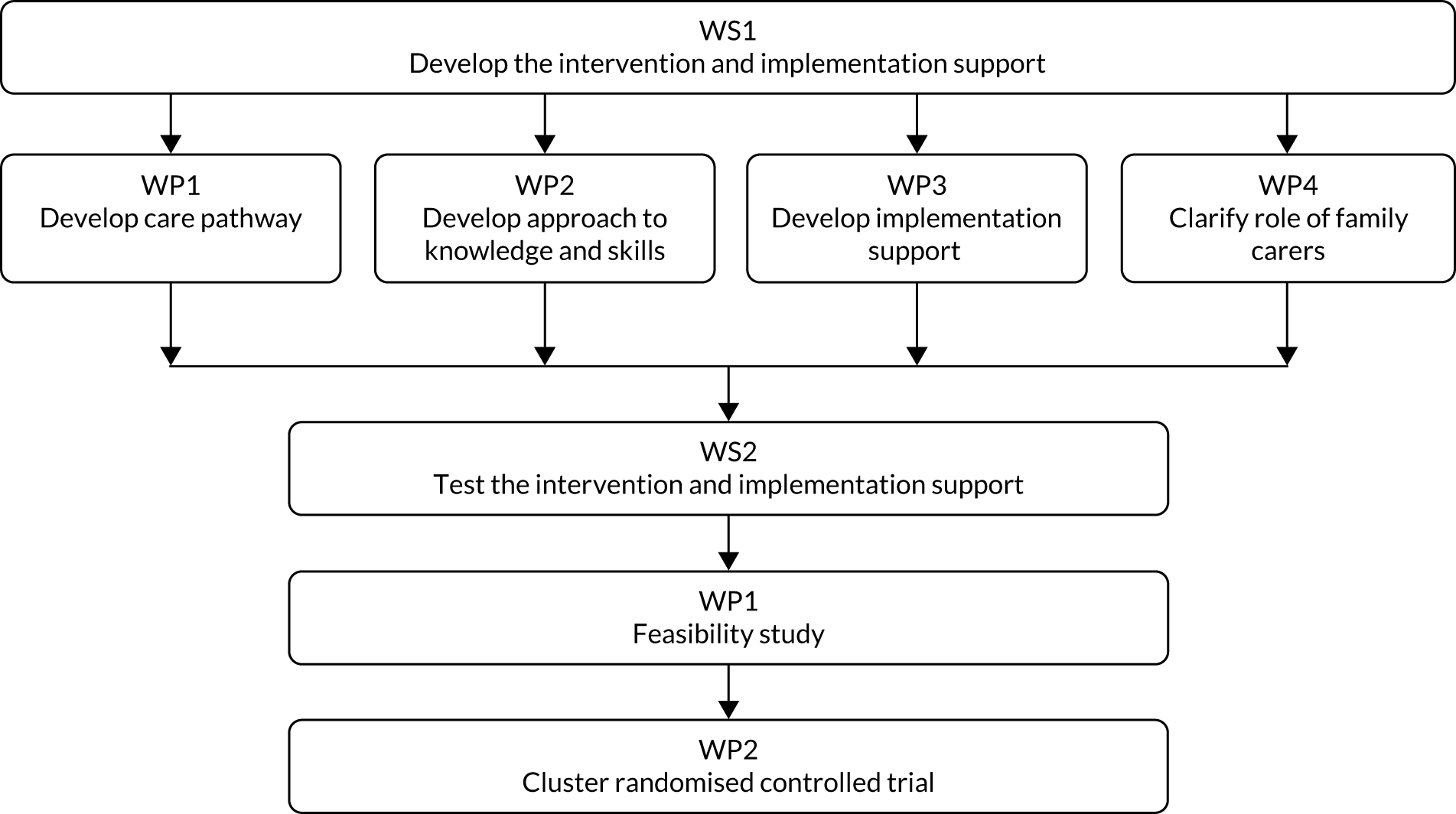
Research pathway diagram
Figure 1 depicts the research pathway.
FIGURE 1.
Research pathway diagram. WP, work package; WS, workstream.
Alterations to programme aims or design
The main alterations to the programme include:
-
aim
-
use of interviews and informal conversations rather than focus groups to gather feedback during the feasibility study
-
location of Yorkshire pilot homes
-
duration of post-intervention pilot study period
-
number of, and approach to, clinical record reviews
-
use of laptops for data collection and entry
-
use of additional human resources.
Aim
In the original proposal, the aim was to reduce rates of hospital admissions from nursing homes for ambulatory care-sensitive conditions (ACSCs), conditions whose early detection and active management in nursing homes could have prevented the exacerbation that led to the unplanned admission. We aimed to optimise and pilot test a pragmatic, acceptable, evidence-based intervention for ensuring active management of ACSCs in nursing homes, thus reducing avoidable hospital admissions.
The overall aim was revised to the following: to develop and pilot test an intervention to improve rates of early detection and treatment for four conditions. This allowed us to have an explicit focus on the active management needed for early detection and treatment in order to reduce hospitalisations. This change in emphasis meant that the project was more closely aligned with policy on improving health care for residents as a route to decreasing hospitalisations. Therefore, we called it the Better Health in Residents in Care Homes with Nursing (BHiRCH-NH) study.
Use of interviews and informal conversations rather than focus groups to gather feedback during the feasibility study
We had intended to conduct two focus groups with staff in the two feasibility homes to further refine the intervention and implementation support. These proved difficult to arrange as the staff in both homes were under significant time pressure and unable to take time out to join a group. Indeed, even seeking one-to-one time with them proved difficult, because of pressure of work. Instead, we conducted one semistructured interview (during which there were frequent interruptions), which we transcribed, and otherwise made notes during impromptu conversations with staff. We took a pragmatic approach to analysis, noting key points to share with the team.
Location of Yorkshire pilot homes
In Yorkshire, we had to recruit nursing homes outside Bradford for the pilot study. Following a presentation we made to the Bradford and District Clinical Commissioning Group (CCG) in autumn 2017, tools similar to the project intervention tools were sent to all nursing and care homes in the district, meaning that these homes were essentially pre-exposed to the intervention. We therefore had to move the intervention to areas that had not had this exposure.
Duration of post-intervention pilot study period
In the original proposal, we expected to have a 12-month post-intervention study period. Owing to delays of approximately 10 months in obtaining ethics approval, the post-intervention study period was reduced to 6 months. This meant that we had a shorter period in which to embed the intervention and were unable to look at the seasonal effects on the primary and secondary outcome measures.
Number of, and approach to, clinical record reviews
We reviewed 11, as opposed to 30, nursing home residents’ records because of the shortened post-intervention period and the lower than anticipated numbers of hospital admissions. We had planned to review a random sample of residents’ records, using a structured audit template comparing the care pathways developed for the intervention with a pathway analysis of the events leading to hospitalisation or treatment for an ACSC, and noting if changes were made to the care pathway documentation.
This approach depended on the intervention being adopted as planned. However, in the event, only 16 Stop and Watch Early Warning Tool (S&W) forms were completed in the study period, with 11 of them coming from a single home. Furthermore, we were able to locate only three of the eight completed care pathway forms. During interviews, some nursing home staff stated ‘we do this anyway’, and some were able to point to local practices that formalised and supported those claims.
We therefore changed the approach to focus on any reference to the observations or actions in the care pathway. We hoped that this would allow us to confirm (or refute) the claim that the principles of the intervention were already in use as normal practice. We also hoped that it would give us insights into which aspects of the intervention were seen as most useful. We thought that this would be the most helpful approach in deciding whether or not to pursue a definitive trial.
Use of laptops for data collection and entry
We had intended that we would enter data while in the field using laptops. We instead relied on completing paper-based case report forms, which were then manually entered into the database prepared by University College London’s Clinical Trials Unit, Priment.
Use of additional human resources
Research facilitator
To facilitate access to residents’ records, we identified a research facilitator in each home who could assist. Fieldworkers reported positive experiences in working with such staff.
Clinical Research Network colleagues
We had intended to recruit nursing homes from the Bradford area. However, having presented an overview of the project to the Bradford and District CCG, the project details were distributed to care homes, meaning that they were essentially pre-exposed to the intervention. This meant that we had to recruit nursing homes from outside the Bradford area. Colleagues from the Yorkshire and Humber Clinical Research Network (CRN) helped with recruiting nursing homes and with data collection from these homes.
Background to the research programme
Introduction
Too many residents living in nursing homes go to hospital for conditions that, if detected and treated earlier, could have been managed in the nursing homes. In the UK, significant attention has been paid to the lack of consistent, equitable and adequate NHS support to nursing homes to ensure enhanced health care in nursing homes. 1 NHS England has had a programme of vanguard sites modelling innovative practice to enhance health care in nursing homes. Few robust studies of hospital admission reduction programmes in the UK were available when we started this study. Our work addressed the NHS policy focus on reducing avoidable hospital admissions and the lack of proactive and timely health care for frail older people living in nursing homes.
Number of frail older people with comorbidities in care homes
In England, approximately 450,000 adults aged > 65 years live in care homes; of these, slightly more than 220,000 live in nursing homes. Older people living in nursing homes have significant levels of comorbidity, frailty and complex physical and mental health needs. 2–9 Residential care home residents also have significant health-care needs. 6 Victor et al. 10 found that most of their residential care home study participants had three to four diagnosed conditions.
Health-care needs poorly met in care homes
A consistent and concerning picture of care home residents’ varied and inequitable access to primary and specialist NHS services has been documented for > 15 years,3,5,11–15 with efforts continuing to address this concern. 8 Iliffe et al. 14 demonstrated persistent variable and inadequate NHS service provision to these settings. It is equally well established that unmet health-care needs can result in unplanned, unnecessary and avoidable hospital admission. 8,11
Ambulatory care-sensitive conditions and hospitalisation
Ambulatory care-sensitive conditions are conditions that can lead to unplanned hospital admissions that may have been avoidable or manageable by timely access to medical care in the community. 16 The conditions include angina, asthma, cellulitis, chronic obstructive pulmonary disease, congestive heart failure (CHF), dehydration, diabetes mellitus, gastroenteritis, epilepsy, hypertension, hypoglycaemia, urinary tract infections (UTIs), pneumonia, and severe ear, nose and throat infections. 15
In the UK, ACSCs account for one-sixth of hospital admissions in all age groups. 17 The ageing population has led to a 40% increase in admissions between 2001 and 2011,18 and all-cause hospital admissions from nursing homes rose by 63% between 2011 and 2015. 19 Four ACSCs contribute to a large proportion of hospitalisations from nursing homes (i.e. respiratory infections,16,20–22 acute exacerbation of chronic heart failure,23,24 UTIs16,25 and dehydration16) and may underlie other problems such as falls and delirium.
Avoidable hospital admissions are not unique to the UK. For example, Jeon et al. 26 examined insurance claims for long-term care residents in Japan and found that > 70% of potentially avoidable hospital admissions were related to respiratory infections, UTIs or CHF. 26
Negative effects of hospitalisation
As well as causing distress to residents, their families and staff, hospitalisation is expensive for the health and social care system. Hospital admission increases the risk of decline in functional ability, delirium, adverse events and prolonged stays. 27,28 Areas with many nursing homes tend to have higher rates of unplanned hospital admission among those aged > 75 years. 29 The King’s Fund17 and the British Geriatrics Society5 have raised concerns about the quality of health care to nursing homes. In economic terms, avoidable hospital admissions are costly to the NHS. The Care Quality Commission (CQC)30 described suboptimal experiences for residents moving between care homes and hospitals. The NHS has made reducing avoidable hospital admission from nursing homes a policy imperative. 8
Reducing avoidable hospital admissions
Interventions have been developed to address avoidable hospital admissions. These fall into two categories: multicomponent interventions (implementation of a range of tools) and single-component interventions (predominantly advance care planning or single-disease care pathways, e.g. pneumonia). Multicomponent interventions show significant reductions in avoidable admissions. 31–35 Key characteristics of these include enhancing the knowledge and skills of nursing home staff,36 clinical guidance and decision-support tools (care pathways), engaging with families,37 and specialist input from geriatricians or nurse practitioners. 31,38 In addition, research highlights the importance of collaborative development of interventions with nursing home staff,39 residents and families,37 consider implementation support,24,40 including using local champions.
In the USA, financial incentives and penalties have prompted co-ordinated care across care settings to reduce hospital admissions. 41 One quality improvement project to reduce avoidable hospitalisations in the USA is the Interventions to Reduce Acute Care Transfers (INTERACT) programme. 42 This focuses on improving well-being and quality of care through (1) (training in) prompt recognition, identification (diagnosis) and management (treatment) of acute conditions; (2) communication, documentation and decision support tools; and (3) advance care planning. Although a pilot study of INTERACT in 25 homes demonstrated reduced rates of hospitalisations,24 a more robust design [cluster randomised controlled trial (RCT)] failed to find such effects. 40 Rantz et al. 38,43 studied the effects on hospitalisation rates of embedding an advanced practice registered nurse in nursing homes. Such an approach was not pursued in this programme as its sustainability in times of austerity may have proven difficult.
In England, the NHS Five Year Forward View44 made a commitment to improve health-care services to residents to reduce avoidable hospital admissions from care homes. The new care models included a commitment that general practitioners (GPs) be assigned to care homes and be required to make at least weekly visits. In 2016, NHS England established vanguards, demonstration sites for the new care models, the Enhanced Health in Care Homes7 initiative. The aim was to redress the inadequacy of current health care in care homes and to ensure that residents received adequate health care, in order to reduce the resulting avoidable hospitalisations. The care home vanguards have had a positive effect, with reports of lower growth in emergency admissions than the rest of England.
Baylis and Perks-Baker7 conducted interviews with care providers about progress on closer working between health and social care. They found positive results, although most benefits were measured with respect to reduced NHS costs, rather than improved quality of care and quality of life for residents. They noted that successful projects had worked in partnership with care providers and were not just about quality improvement in the home, but were engaged in changing practice at the interface between care homes and the NHS. In this project, we were keen to measure quality of life, as well as rates of hospital admission. This project focused on quality improvement in the nursing home, including improving nurse communication with primary care. We focused on nursing homes, as nursing expertise on site allowed for local ownership and relevant clinical expertise to drive change.
Enhancing health care to care homes
Concerns were raised by Baylis and Perks-Baker,7 the British Geriatrics Society,3,5,45 and a joint working party from the Royal College of Physicians, Royal College of Nursing and the British Geriatrics Society45 about health-care provision to the care home sector.
Six of the vanguard projects focused on enhanced health in care homes, proactively monitoring residents’ health, improving multidisciplinary support to care homes and improving workforce skills. In 2014, a project in Rushcliffe, Nottinghamshire, which implemented an enhanced health-care model (aligning care homes with general practices, regular visits from a named GP, improved support from community nurses, independent advocacy and support from the third sector, and a programme of work to engage and support care home managers), demonstrated a reduction in emergency hospital admissions and accident and emergency (A&E) attendances. 46 A programme implemented between 2013 and 2017, in east London, which provided residents of nursing homes with increased GP access and continuity of primary care, with support to nursing home staff, also reduced the rate of emergency hospital admissions and A&E attendances. 47 It is noteworthy that there have been several system-based changes to the provision of health care in nursing homes during the BHiRCH-NH programme.
It is only relatively recently that empirical research has been conducted to ensure proactive health care in UK care homes (e.g. Devi et al. 48 and Goodman et al. 49). Furthermore, the quality of intervention studies is highly variable. Insufficient attention has been paid to key methodological issues, particularly issues of implementation, adherence to the intervention or the clustering effect within nursing homes. There is a lack of robustly conducted RCTs.
Research on enhancing health for residents in UK care homes, published since we started this project, has focused on identifying service delivery models that optimise the integration between the care home, the NHS and social care services. For example, the Optimal study49,50 explored how nursing homes worked with the NHS and how different delivery models affected residents’ and staff access to health care. They concluded that NHS professionals and care home staff need dedicated time and resource to work together to discuss, plan and review health care for individuals and all residents. Staff from both sectors need to be trained to provide collaborative care.
An ongoing quality improvement collaborative in the UK, Proactive HEAlthcare of Older People in Care Homes (PEACH),48 focuses on implementing and delivering comprehensive geriatric assessment to care homes. This collaborative project includes health-care and social care professionals, care home staff and specialists in clinical aspects, quality improvement methodology and research. Chadborn et al. ’s51 realist review identified three elements required to ensure effective health care for older people in UK care homes: comprehensive geriatric assessment, care planning and working towards a patient’s goals. Health care in care homes will be improved by ongoing contact between health care and care home staff and opportunities for care home staff to liaise with a wider system of health care, including access to dementia-specific expertise. 52
When we developed and conducted this study (2010–13), there was a policy imperative to reduce avoidable hospital admissions. This project focused on developing an intervention that would enhance the quality of health care in care homes to reduce resident hospital admissions.
Implementing change in care homes
A range of implementation strategies that optimise engagement with the intervention have been identified. These include establishing local implementation teams in the form of quality collaboratives and identifying local intervention champions and ensuring resident and family involvement. These approaches are consistent with the literature on practice development in nursing homes in the UK and international frameworks for knowledge translation [e.g. Promoting Action on Research Implementation in Health Services (PARiHS)]. 53 In this programme, we used PARiHS as the overarching framework to ensure local ownership of the change process and to introduce the intervention. 53–56
The PARiHS framework proposes that implementation is a function of a dynamic relationship between:
-
Evidence57 – in this study, the evidence implemented was the synthesis of findings from the systematic literature review, additional research, and clinical and family experience.
-
Context56 – in this study, issues such as organisational commitment and leadership, role alignment, and decision-making were dimensions of the context to be addressed.
-
Facilitation54 – in this study, we used the implementation method of continuous quality improvement; within that approach, we elected to use the intervention champion role and quality collaboratives as the facilitation processes.
Studies into why evidence is not used in practice consistently raise the challenge of ‘the black box of context’. 58,59 Despite robust studies demonstrating efficacy, translation into practice is not straightforward. The most successful implementation occurs when evidence is robust, the context is receptive to change and when the change process is facilitated. 53,60 However, finding a method that pays attention to context and that is facilitated is a challenge in nursing homes, because of limited resources. 61 Studies of nursing home organisational culture change conducted in the USA concluded that interventions need to be aligned with the administrative, operational and management structure of nursing homes. Furthermore, nurses needed to work with and mentor care assistants, helping them to apply new knowledge and supporting them in decision-making. 62
We used intervention champions and quality collaboratives to drive forward practice change from within the home. 63,64 These reflected the evidence identified in the programme about the role of local champions and local implementation teams. Quality collaboratives had been applied internationally and in many sectors, including health care. A quality collaborative involves diverse stakeholders working together to close the gap between actual and potential practice, in this case the proactive detection and management of ACSCs in frail nursing home residents. Quality collaboratives provide a framework for quality improvement that is not dependent on external facilitators. Instead, the method draws on the existing expertise of stakeholders who are familiar with the context and culture of the setting, and thus have greater insight into the factors that can enable or hinder improvements. In a recent study, Harvey et al. 62 identified the importance of recruiting and retaining staff who have facilitation skills and support from managerial leaders as key criteria for achieving practice change in nursing homes. 61
In this programme, we promoted local ownership of the change process, including how to introduce and embed the intervention. We collaborated with stakeholders to design and evaluate the intervention and the implementation guidance.
We identified the likely elements of a multicomponent intervention:
-
observation, communication and decision-making tools and documentation for care of ACSCs in UK nursing homes
-
knowledge and skills development of nurses and care assistants
-
involvement of family members
-
nurse confidence, empowerment and leadership
-
robust implementation guidance.
Workstream 1: develop the complex intervention
Aim
The aim of workstream (WS) 1 was to develop and manualise a pragmatic and acceptable multicomponent intervention to ensure proactive health care in nursing homes for four ACSCs associated with avoidable hospital admissions for care home residents: respiratory infection, exacerbation of CHF, UTI and dehydration.
There were four work packages (WPs):
-
WP1 – develop care pathways for ACSCs (respiratory infection, exacerbation of CHF, UTI and dehydration) in UK nursing homes
-
WP2 – develop optimal approaches to enhancing the knowledge and skills of nursing home staff
-
WP3 – develop optimal approaches to implementation support and an implementation support package
-
WP4 – clarify the role of family in early detection.
Work package 1: develop care pathways for ambulatory care-sensitive conditions in UK nursing homes
Led by health-care specialists in primary care (LR) and geriatrics (JY), we sought to develop a care pathway to ensure early detection and treatment of four index conditions: respiratory infection, exacerbation of CHF, UTI and dehydration.
Methods of data collection and analysis
We worked with stakeholders, including nursing home staff and our Carer Reference Panel (CRP), to develop and adapt the INTERACT tools for use in the UK.
The following were conducted:
-
Co-applicant specialists in geriatric medicine (JY) and primary care (LR) reviewed care pathways identified in a search for care pathways (see Appendices 1 and 2). This led to version 1 of the care pathway.
-
We obtained expert panel input via e-mail on this version of the care pathway (see Appendix 3). Experts included three international advisory group members, two members of the British Geriatrics Society and three members of the Royal College of General Practitioners. This input was reviewed by John Young and Louise Robinson, and led to version 2.
-
We held a consensus workshop with 17 stakeholders with diverse areas of expertise, including eight nurses (four community/district nurses, three nursing home nurses and one nurse consultant), two nursing home managers, three care assistants, one geriatrician and three family members (see Appendix 4). This led to the final draft of the care pathway.
Limitations
The main limitation was the limited amount of previous research to guide our approach. Only 22 papers were identified in the literature review. Overall, the quality of intervention studies was rated as being highly variable. There was a lack of robustly conducted RCTs (only two trials were rated as being of ‘strong’ quality). Insufficient attention was paid to key methodological issues, particularly the clustering effect within nursing homes. Furthermore, there were few studies conducted in the UK, thereby having questionable relevance to the UK context.
Key finding
We identified care pathways for ACSCs in nursing homes for our four conditions (respiratory infection, exacerbation of CHF, UTI and dehydration) and adapted them in consensus co-design workshops with staff and family carers. We developed a care pathway to facilitate assessment and diagnosis of the four index conditions from existing care pathways used to facilitate diagnosis and treatment of health conditions in care home residents. The final version of the care pathway is presented in Appendix 5.
Inter-relationship with other parts of the programme
The care pathway was one of three intervention tools that were included in the feasibility study.
Work package 2: develop approaches to enhancing staff knowledge and skills
We sought to identify:
-
the knowledge and skills required to ensure early detection and treatment of the four index conditions
-
effective approaches to enhancing the knowledge and skills of nursing home staff
-
knowledge and skills enhancement programmes for active management of ACSCs in nursing homes.
Methods for data collection and analysis
We used three methods of data collection: semistructured interviews, rapid research review and consensus-building.
Semistructured interviews
We conducted semistructured interviews with 18 key informants to identify their perspectives on the knowledge and skills required and best practice in enhancing care staff knowledge and skills, nationally and internationally. Key informants included 10 nursing home staff, three members of the National Dementia Strategy Workforce Advisory Group and five members of the international advisory group. Interview schedules were developed with feedback from our CRP (see Appendix 6).
Interviews were carried out via telephone or face to face and were audio-recorded and transcribed. Notes were also taken.
We used thematic analysis, which involved becoming familiar with the verbatim transcripts and assigning preliminary codes to each transcript. 65 We then identified patterns or themes across the transcripts.
Rapid research review
We conducted a rapid research review of the knowledge and skills required and approaches to knowledge and skills enhancement. The following databases were searched from 1990 to 2015: the Cochrane Library, EMBASE™ (Elsevier, Amsterdam, the Netherlands), PubMed, Social Care Online (Social Care Institute for Excellence, London, UK), Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE, PsycInfo® (American Psychological Association, Washington, DC, USA), Applied Social Science Index (ASSIA) and Web of Science™ (Clarivate Analytics, Philadelphia, PA, USA) (see Appendix 7).
The review focused on the following three areas for both nurses and care assistants:
-
knowledge and skills required for staff managing complex health conditions
-
training to develop these knowledge and skills
-
evaluations of training in care homes and other settings.
Abstracts were considered for relevance and duplicates were discarded. From the rapid review, we identified knowledge and skills for staff managing complex conditions, including:
-
subject specific –
-
palliative care
-
psychological/psychosocial assessment
-
dementia care/awareness
-
pain management.
-
-
generic –
-
communication skills
-
dealing with challenging behaviour
-
patient-centred approach
-
awareness of the principles of ethical health care
-
involving patients and carers
-
use of evidence-based knowledge
-
leadership
-
teaching skills
-
use of restraints/sedation and associated risks
-
professional self-care
-
nutritional assessment.
-
Combining findings from our rapid research review with findings from the interviews, we developed a set of knowledge and skills for nursing home staff for early detection of health conditions (see Appendix 8).
Consensus workshop
We provided a user-friendly overview of these findings to participants 2 weeks before the consensus workshop. The 10 participants comprised three community/district nurses, three family carers, two care assistants, one care home manager and one GP. We sought consensus on participants’ views in relation to the knowledge and skills required for, and evidence-based approaches to, knowledge and skills enhancement. In addition to nursing home staff, we considered whether or not family carers could become involved in timely detection, and what skills this would require.
We audio-recorded and made notes during the workshop. Using the verbatim transcripts, we conducted a thematic analysis. 66 This involved familiarisation during which initial codes were applied. We then categorised these codes into themes.
We identified ways to enhance knowledge and skills of nursing home staff, including:
-
shadowing for hands-on experience
-
college courses
-
training to reinforce learning on an ongoing basis.
Any limitations
There was a limited literature to inform us about the knowledge and skills required for nursing home care assistants and nurses to achieve early detection, assessment and reporting of acute changes in residents’ health.
Key findings
We derived a set of key knowledge and skills for nurses (see Appendix 8) and for care home staff and family members that are required for early detection of the four ACSCs. These included:
-
knowledge of how ACSCs may manifest
-
ability to detect these changes during daily care
-
knowledge of residents’ medical conditions, care plans and their baseline behaviour and symptoms
-
continuing assessment skills
-
leadership skills
-
communication skills.
Inter-relation with other parts of the programme
We examined the use of the knowledge and skills matrix in our feasibility study. We expected Practice Development Champions (PDCs) would use it to conduct a gap analysis and to direct care staff and family members to learning resources that could help to address these gaps.
Work package 3: develop implementation support
We sought to:
-
Agree criteria for recruiting PDCs who would act as internal facilitators of the intervention in each implementation site. We sought to agree the purpose, role and person specification for PDCs.
-
Agree the purpose and composition of the practice development support groups (PDSGs) and guidelines for establishing and supporting them (in each of the sites, to support PDCs with implementation).
-
Develop implementation support resources for care home owners, managers, PDCs and PDSGs including the purpose and key contents of the project handbooks for staff and stakeholders.
Three weeks prior to the third consensus workshop, we sent the 12 participants a draft of our approach to identifying the PDCs and setting up the PDSG, and a draft of the implementation guidance workbooks. 64 The participants comprised one community/district nurse, three family carers, two nursing home managers, one nursing home nurse, two care assistants, one acute care nurse and three GPs. The workshop was facilitated by our co-applicant experts in practice development in nursing homes (KF and BM).
Methods for data collection
We made detailed notes of the consensus workshop discussions and participants generated written notes.
Method for data analysis
We analysed the notes, along with written materials generated by the participants, looking for key themes.
Key findings
The criteria for PDCs were thought to be appropriate. Participants identified situations in which there could be gaps in coverage of staff implementing the intervention. In particular, staff from evening and weekend shifts would need to be recruited to the PDSGs to ensure coverage across the week. Further issues with the language and structure of the staff handbooks were noted, in particular with respect to readability for care assistants and family members.
Participants reported that the purpose of the PDCs and the training methods needed to be clearer. The staff handbooks, which had been designed to provide information about the intervention, and about introducing and embedding change, were felt to be in need of improvement, including the following: be more visually attractive, use simpler language and provide less information.
Based on these findings, we developed the final version of the purpose, role and person specification of PDCs (see Appendix 9). We agreed the key topics to cover in the preparation workshop, using both presentations and interactive exercises. Box 1 presents the topics for the PDC preparation workshop.
-
Considering evidence, context and facilitation in developing practice: the PARiHS framework.
-
What are we putting into practice?
-
Strategies for engaging people.
-
Collective learning, evaluation and critique.
-
Teamwork and leadership in developing practice.
-
Giving and receiving feedback.
-
Working with families and family carers.
-
Process review.
-
Planning and developing individual action plans for moving forward.
Limitations
We intended to provide a 2-day preparation workshop for PDCs. Owing to a diary mix-up with one of the PDCs, we reduced the workshop to 1 day.
Inter-relationship with other parts of the programme
We finalised our approach to recruiting and supporting PDCs and identified the personal characteristics and professional skills required of them. The support to be provided included a preparation workshop, monthly telephone support (with AB, the programme manager) and a website with additional resources for nursing home staff. We tested this implementation support in our feasibility study in WS2, WP1.
Work package 4: clarify the role of family members
Aim
The aim was to explore family members’ perspectives on their involvement in the timely detection of their family members’ changes in health in UK nursing homes. Specifically, we sought to address:
-
How are family carers involved in timely detection of changes in their relatives’ health in nursing homes?
-
How can family carers be supported to engage in timely detection of their family members’ changes in health?
Methods for data collection
We conducted 14 semistructured interviews with family carers of residents living in 13 different nursing homes (see Appendix 10). This allowed us to obtain in-depth information on their perceived and preferred role. All interviewees were adult children and lived near the nursing home. We recruited family members who were regularly involved with their relative. We used telephone interviews to gather data from family members who were unable to attend a face-to-face interview.
In addition, we finalised drafts of the following:
-
a handbook on the intervention and its implementation for the PDCs
-
a handbook about the intervention and its implementation for care home owners and managers
-
a workbook on implementation for the PDSGs.
Method for data analysis
Interview data were transcribed verbatim and analysed using thematic analysis. 67
Any limitations
We were unable to identify family members who lived further away from the care home, despite advertising nationally. We had hoped to include these, as they provide an additional perspective on family involvement.
Key findings
Families were involved in the timely detection of changes in health in three key ways:
-
noticing signs of changes in health
-
informing care staff about what they noticed
-
educating care staff about their family members’ changes in health and how they manifest.
Families suggested that they could be supported to detect early changes in health by developing effective working practices with care staff. 66
Inter-relation with other parts of the programme
Family involvement was examined in the feasibility study in WS2, WP1. The intention was for care staff to establish how best to work with family members who would like to be involved in the intervention, recording preferences in each resident’s care record, as well as explaining the purpose and application of the S&W.
Draft 1 of the complex intervention
In summary, the complex intervention we developed comprised five components, plus its implementation, facilitated by identifying and supporting PDCs.
Five components of the complex intervention
1. Stop and Watch Early Warning Tool
The intervention will use an adapted version of Atlantic Florida University’s S&W (version 4.0). 68 This tool is used widely in the USA. It highlights simple signs and behaviours to identify common, but non-specific, changes in a resident’s condition that seem out of the ordinary for the resident. The tool is intended to be used as an alert to determine if further assessment of a resident by a registered nurse (with the care pathway) is necessary. It helps nurses and care assistants to check for potential warning signs of deterioration in health. It can be used whenever anyone in the care home has a concern about a resident.
2. Care pathway
The care pathway is to be used by nurses whenever a change has been noted with the S&W. It is a clinical guidance and decision-support system that includes primary and secondary assessment of respiratory infection, acute exacerbation of chronic heart failure, UTI and dehydration. Primary assessment comprises screening questions and secondary assessment requires more detailed investigation.
3. Modified situation, background, assessment, recommendation technique
The situation, background, assessment, recommendation (SBAR) technique is to be used by nurses to help structure communication with primary care services. We adapted this for use in care homes.
4. Knowledge and skills
We have identified training resources that addresses potential gaps in the knowledge and skills necessary for implementation of the intervention tools. These resources are available using a web link to care home staff and family carers. We have developed a knowledge and skills matrix identifying the key knowledge and skills required of nurses, care assistants and family carers.
5. Involvement of (and support for) family carers
Care staff are expected to ascertain the level of involvement family carers of residents wish to have in the early detection of changes in the health of their relatives.
Implementation support
Implementation support included asking nursing home managers to engage in a change management programme, which included:
-
identifying two PDCs
-
establishing a PDSG to support the work of the PDCs in introducing and embedding the intervention tools.
The study team provided training for the PDCs’ role. We provided two implementation support handbooks:
-
for care home staff (for managers and PDCs)
-
for staff and PDSG members.
The handbooks were to be used in the nursing home to help staff to understand the intervention and support its implementation. The handbooks offer background information on some of the approaches to change used in this project, promote an understanding of how the intervention can be implemented in the differing contexts of each home, facilitate and improve the learning of others in the nursing home, help teams learn and act alongside the people for whom they care, maximise opportunities for all team members to enhance their leadership capabilities, and offer information for residents and their family carers to help them become active participants in developing practice in their care homes.
Workstream 2, work package 1: test the intervention – feasibility and acceptability study
Aim
The primary aim of the feasibility and acceptability study (conducted from October 2016 to January 2017) was to refine the intervention, the implementation support and the study procedures to inform the pilot cluster RCT. The secondary aim was to identify data to be collected in the pilot study.
Research questions
-
Can the intervention be delivered as intended?
-
What further refinements are required to the implementation guidance?
-
Is the approach to collecting data feasible?
-
What are the resource requirements to collect and analyse the data?
Ethics approval
The feasibility study received ethics approval from London Queen Square Research Ethics Committee (reference number 16/LO/1361). It was registered in the International Standard Randomised Controlled Trial Number (ISRCTN) registry (as ISRCTN86811077) (registration date: 12 September 2017).
Results
Can the intervention be delivered as intended?
At the end of the 3 months, we carried out a semistructured interview with one intervention champion to learn about their experience of taking part in the feasibility study, the barriers to and facilitators of using the intervention, the training workshop and the implementation support. In addition, we held informal conversations with six further members of staff from the two nursing homes to explore what had worked well, what had worked less well, and what facilitated and hindered adoption of the intervention. Notes were taken during the conversations. The interview was analysed alongside the notes from impromptu conversations with care staff.
Stop and Watch Early Warning Tool
The S&W was used 25 times across both nursing homes, completed by both care assistants and nurses. The S&W was sometimes used retrospectively rather than at the time the change was observed. One PDC indicated that they valued the S&W as an additional resource in their nursing homes. Another noted that a key benefit of the S&W was that it enabled junior staff members to notice changes in residents’ health conditions. In both nursing homes, its key strength was that it was clear, quick and easy to use. That said, a care assistant reported that there was not enough time to use it as intended. Information about the S&W was not always well communicated. Many staff learned about it through word of mouth, and staff who worked at the weekend stated that the PDC had not sufficiently explained the BHiRCH-NH study and purpose.
Care pathway
The care pathway was used five times across both nursing homes over the 3-month study period. PDCs reported that they had to continually remind their nursing colleagues to use the tool. Nurses sometimes used the care pathway for concerns about deterioration in health for conditions other than the four target conditions. PDCs from both nursing homes reported that staff did not see that it provided any additional benefit to what they were already doing. In some cases, the care pathway was used informally as a prompt to indicate what steps should be taken next, such as when to contact primary care. Some nurses reported that the layout of the care pathway was difficult to follow. Furthermore, PDCs felt that they had not successfully communicated why it was being introduced into their nursing home.
The situation, background, assessment, recommendation technique
Practice Development Champions in both nursing homes reported that they already used a different version of the SBAR technique and were happy to replace this with the modified, nursing home-specific version provided in this study. They reported that it was used as intended by nurses in both nursing homes, suggesting that it is acceptable to nursing home staff. PDCs in both nursing homes indicated that staff members were aware of the key points of the SBAR technique when contacting primary care. The PDCs believed that the SBAR technique gave nurses increased confidence to speak to GPs, for example in ensuring that antibiotics were obtained from a GP. The SBAR technique was viewed as a way of empowering nurses, as it gave them more confidence in speaking with primary care. One of the PDCs mentioned the importance of building rapport and having a good relationship with GPs for the benefit of the residents.
Family involvement
Practice Development Champions in one nursing home did not see the need to collaborate with family to improve detection of changes in residents’ health. PDCs in the other nursing home reported that they made some efforts to include family carers in monitoring the health of residents, but did so inconsistently and without systematically documenting their efforts. Family carers were not informed about how they could be involved in the intervention during the feasibility study. This was because of the lack of a systematic method for communicating with family members regarding the intervention.
Enhancing knowledge and skills
The knowledge and skills matrix was used minimally in one nursing home and not at all in the other.
What further refinements are required to the implementation guidance?
Practice Development Champions
We identified two PDCs, whose role was to train, support and work alongside nurses and care staff to ensure uptake of the intervention. PDCs felt that their role in empowering and motivating staff was key to introducing and embedding this new way of working. PDCs were to establish and co-ordinate the work of the PDSG and maintain momentum for change and development. In the context of the PARiHS framework, the PDSG acts as a collective internal facilitator supporting the PDCs. It was intended that the detail of how PDCs worked with the PDSG would be negotiated at each nursing home site to ensure a mutually supportive relationship, with clear lines of accountability.
Training workshop
Two PDCs from each home attended the 1-day training course to prepare them for their role. The training day was facilitated by our practice development experts (BM and KF). Our geriatrician co-applicant (JY) provided an overview of how to detect changes in health and one of our patient and public involvement (PPI) colleagues (SN or BWC) presented on the role of family in health care for relatives in nursing homes. The presentations were video-recorded to be used as an online resource for the PDCs.
Practice Development Champions in both nursing homes reported that the training workshop was highly informative, in particular the input regarding early detection of changes in residents’ health. In one of the homes, the PDC used the video-recorded session from the training day when cascading information about the intervention to nurses and care assistants. PDCs felt that previous training (e.g. in end-of-life care) had covered similar knowledge and skills, and that no further training was required.
Practice development support group
Three of the four PDCs were managers of the homes and they relied on a top-down approach to implementation. They did not establish a PDSG. Furthermore, PDCs believed that family involvement in PDSGs was unnecessary, as they believed that staff already monitor residents’ health effectively.
Time to embed the intervention
One of the key ways staff members felt that the intervention could be better implemented was through the nursing home having more time and resources. They noted that low levels of staffing in the nursing home had hampered implementation of the intervention. The duration of the feasibility study was also thought to be a hindrance. Three months was considered too short a time frame to implement complex changes to working practices. One of the PDCs stated that an intervention of 1 year would enable changes to be made in a nursing home. They felt that the short time frame was demotivating.
Handbooks
The handbooks were viewed as being overly long and complex. Nevertheless, the PDCs reported that the implementation handbooks contained useful reference material.
Is the approach to collecting data feasible?
Numbers recruited
The managers in both homes assisted with identification of residents, their family carers, staff and PDCs. All residents who were not receiving palliative or end-of-life care were eligible for participation in the study. We recruited 12 residents, 22 staff (six nurses, 13 care assistants and three domestic staff) and eight family carers.
Data collected
We were able to gather individual-level data (for residents, staff and family carers) (Table 1), process-level data (Table 2) and care home-level data for each month over the 3 months. Although we were able to collect data from residents’ care records, this was more time-consuming than anticipated.
| Data collected | Total number of individuals (n) |
|---|---|
| Residents (N = 12) | |
| Demographics | 12 |
| Resident quality of life (DEMQOL-U) | 12 |
| Resident quality of life using the EQ-5D-5L | 12 |
| Medical consumables, such as prescription medication and prosthetics | 12 |
| Use of health and social care services (CSRI) (A&E, GP, etc.) | 12 |
| Serious adverse events | 1 |
| Functional ability of residents assessed by the Barthel Index69 | 6 |
| Staff (N = 22) | |
| Demographics | 22 |
| Degree of perceived organisational support for providing person-centred care (P-CAT; Edvardsson et al.70) | 6 |
| Nurse ratings of communication with primary care (Tjia et al.71) | 6 |
| Context Assessment Index (McCormack et al.72) | 6 |
| Family carers (N = 8) | |
| Demographics | 8 |
| Carer quality of life using the EQ-5D-5L | 8 |
| Carer-perceived quality of life of the resident (EQ-5D-5L Proxy) | 8 |
| Carer-perceived quality of life of the resident (DEMQOL-U Proxy) | 8 |
| Identify preferred role of family carer | 8 |
| Process-level data | Total (n) |
|---|---|
| S&W | |
| Number of completed S&W forms | 25 |
| Most common changes observed | |
| Ate less | 11 |
| Skin change | 8 |
| Talks less | 2 |
| Seems different | 7 |
| Who completed the form | |
| Nurse | 6 |
| Care assistant | 19 |
| Number of S&W forms for which no changes were noted after using the form | 2 |
| Care pathway | |
| Number of S&W forms that resulted in a care pathway being actioned and completed by the nurse | 5 |
| Number of care pathways that were completed by the nurse who was initially informed of a change | 2 |
| Number of primary assessments conducted using the care pathway | 3 |
| Number of secondary assessments conducted using the care pathway | 3 |
| Number of primary and secondary assessments using the care pathway that resulted in an ambiguous outcome | 0 |
| Number of care pathways administered 6 hours after an ambiguous outcome | 0 |
| Outcome of care pathway assessment | |
| Further general monitoring using S&W | 0 |
| Monitoring for specific symptoms | 0 |
| Treatment initiated in care home | 2 |
| Condition communicated with primary care | 1 |
What are the resource requirements to collect and analyse the data?
Although we could collect individual-, process- and care home-level data, it was more time-consuming than expected.
Final draft of complex intervention
In summary, following the feasibility study, the final draft of the complex intervention comprised three intervention tools: the S&W, the care pathway and the SBAR technique.
Stop and Watch Early Warning Tool
We agreed to emphasise the purpose and use of the S&W during the training workshop for PDCs, including our expectation that family carers be offered the opportunity to have a role in alerting staff to any changes they noticed in their relatives’ health.
Care pathway
We clarified the links between sections and changed the form so that data could be entered directly. We expected that PDCs would provide further education to their nursing colleagues about its purpose and aims.
The situation, background, assessment, recommendation technique
We expected that nursing home nurses would use the SBAR technique, when communicating with primary care, as an integral part of their engagement with the intervention.
Involving families
We integrated family involvement into use of the S&W by emphasising the role of family members in early detection of changes in health. Family members were expected to notify staff if they observed any changes in their relative, which, in turn, would prompt the staff member to use the S&W.
We expected nursing home staff to inform family carers about the research at the start of the project and invite them to participate. Nursing home staff were expected to describe to family carers, who had expressed an interest in participating, the potential roles they could play in the project.
Knowledge and skills development
As the PDCs had not engaged in this aspect of the intervention, we decided to use the knowledge and skills matrix as a way of describing nurses’ knowledge and skills at the outset of the study. This would provide us with useful contextual information.
Final draft of the implementation strategy
Components
Practice Development Champions
We revised the content of the training workshop (see Appendix 11). The information provided to PDCs was simplified, was reduced in length and was more focused on directly useable skills and tools.
Practice development support group
We refined the implementation support and training for PDCs to ensure inclusion of all aspects of implementation support. We noted that, in the feasibility study, PDSGs were not established in either nursing home. Greater emphasis was to be placed on supporting PDCs to set up PDSGs.
Handbooks
The length and complexity of the implementation handbooks were significantly reduced. Two separate handbooks for PDCs and PDSGs were integrated into a single volume (see Appendix 12). We agreed that the monthly coaching telephone calls would be provided by co-applicants with expertise in practice development (BM and KF) rather than by the programme manager (AB), as he would be involved in data collection.
Data collection
Recruitment
In identifying potential care homes for the pilot trial, we established that the study had the full support of the nursing home manager and that the home was capable of implementing a complex intervention (e.g. noting the level of enthusiasm of the nursing home manager, nursing home involvement in other trials). We refined the recruitment process for residents to facilitate access to personal or professional consultees when necessary. We incorporated a study launch day, detailed set-up procedures (see Appendix 13) and identification of a research facilitator in the nursing home to ensure efficient recruitment and optimal data collection.
We expected a CRP member to input regarding family involvement and the S&W at each launch event, but scheduling proved difficult such that only one person did. We ensured publicity about the study in the nursing home environment. For example, we provided posters (see Appendix 14), leaflets and a newsletter (see Appendix 15).
Resource requirements
We recruited a research facilitator from the nursing home staff to support the research team with recruiting residents and family carers and with accessing residents’ records (see Appendix 16). We also had support from the local CRN with recruitment and consent processes. We reduced the number of questionnaires for use in the pilot trial. For example, we removed the Context Assessment Index as it was too burdensome. Furthermore, we found that some of the questionnaires used in the feasibility study did not provide a great deal of additional information; for example, there was overlap between the Dementia Quality of Life (DEMQOL) scale and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), as measures of quality of life. We retained the EQ-5D-5L and the EQ-5D-5L proxy (reported by a carer).
Workstream 2, work package 2: pilot cluster randomised controlled trial
Parts of this section appear in Sampson et al. 73. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Aim
The aim of the pilot cluster RCT was to test the intervention and its implementation guidance (developed in WS1 and refined in the feasibility study) to:
-
establish whether or not a future definitive trial is warranted and, if it is, to inform the design, sample size and other key parameters needed
-
further refine the intervention and its implementation guidance.
Methods
For detailed methods, see Appendix 17. Methods are briefly summarised in the following sections.
Trial design
We conducted a pilot cluster RCT of the BHiRCH-NH study intervention in nursing homes and an embedded process evaluation.
Study population
Nursing homes
We recruited 14 nursing homes (eight in West Yorkshire and six in Greater London). Nursing homes were identified via local CRNs and the Enabling Research in Care Homes (ENRICH) network.
Inclusion criteria
Nursing homes that fulfilled the following criteria were recruited: expressed an interest in the project; had adequate staffing for the intervention; and had the capacity to implement the different components and take part in, and support, research activities.
Exclusion criteria
Nursing homes that had been placed in special measures by the CQC, the English body responsible for assuring quality of care, were excluded.
As this was a pilot study to inform the sample size calculation for a definitive trial, no formal sample size calculation was conducted. Nursing homes were purposively selected to include a range of providers (large and small chains, independent providers), locations (urban, suburban and rural) and sizes.
Staff and residents
All English-speaking staff and residents aged > 65 years and their family carers were invited to participate in the collection of individual-level data until we recruited approximately 20 residents and staff from each nursing home (for recruitment figures and reasons for exclusion, see Appendices 18–21).
Randomisation and masking
Nursing homes were randomised prior to the intervention starting (considered to be the day after the training workshop); four in West Yorkshire and three in Greater London (seven in total) were randomised to the intervention arm and four in Yorkshire and three in Greater London (seven in total) were randomised to the treatment-as-usual (TAU) arm, stratified by location, by an independent statistician from the Priment Clinical Trials Unit (CTU). The statistician and health economists analysed the data while remaining blind to randomised allocation. Data were not collected by people who were blind to allocation because of resource issues and the visibility of the intervention in nursing homes.
Ethics and consent
Ethics approval was obtained from the Queen Square London Research Ethics Committee (reference number 17/LO/1542). We gained written permission from the manager, regional manager or owner for the intervention to be implemented at nursing home level. We obtained individual consent for the collection of individual-level outcome data from residents. If they lacked capacity, we consulted a personal or professional consultee, as per the Mental Capacity Act 2005,74 for their agreement for the person to participate. A member of the nursing home staff identified potentially eligible residents. Adhering to the Mental Capacity Act 2005,74 the research team conducted a capacity assessment with respect to participation. Consent was sought from family carers associated with residents recruited to the study and from nursing home staff to answer questionnaires and/or take part in qualitative interviews.
Care record reviews
To explore fidelity to the intervention, two nurse researchers (SG, HP) reviewed a convenience sample of five records for residents who had been admitted to hospital, or received treatment in the nursing home, for an ACSC. The nurse researchers used free text to record reference to the observations and actions in the care pathway.
To ascertain whether or not hospital admissions were avoidable, one of the co-applicants (JY) and two nurse researchers (SG, HP) extracted data from the care home records of 11 residents who had been hospitalised, and completed the first section of the structured implicit record review (SIRR) tool. 75
Trial procedures
The trial ran for 10 months between November 2017 and August 2018. Each nursing home appointed a research facilitator to support the research team with recruitment activities and to ensure that nursing home-level data collected without consent from resident files were pseudoanonymised prior to being given to the research team. The intervention was delivered as described in Workstream 2, work package 1: test the intervention – feasibility and acceptability study. Nursing homes in the control arm received TAU according to existing local policy and practice. Data were collected for 3 months prior to the intervention phase starting and for 6 months after. All medications and treatments were permitted.
Trial measures
We collected data across three domains (Table 3):
-
Individual-level data on nursing home residents, their family carers and staff – these were collected from staff, family carers or residents who had given informed consent or from residents from whom we obtained agreement from a consultee.
-
System-level data – collected from each nursing home as a whole using existing record systems, pseudoanonymised and provided by the nursing home manager or research facilitator.
-
Process-level data – we examined how the intervention was implemented in practice, including fidelity. To understand the effectiveness of the trial procedures, we collected data on recruitment rates and consent and the numbers of family carers who wished to be involved in the residents’ care, and assessed the completeness of outcome measures, data collection and the return rate of questionnaires.
| Data collected and tool used | Collected by, at time point | |||
|---|---|---|---|---|
| Pre intervention | Monthly | At 6 months only | Post intervention | |
| Resident | ||||
| Sociodemographics | ||||
| Age, gender, ethnicity, marital status, highest level of education | Researcher | – | – | – |
| Service use in the previous month | ||||
| CSRI. Estimates health-care service use and helps to calculate the total health-care costs | Researcher | Researcher | Researcher | Researcher |
| Functional status | ||||
| The Barthel Index69 | Researcher | – | Researcher | Researcher |
| Resident quality of life – self-rated | ||||
| EQ-5D-5L self-rated health index and visual analogue scale of current health state | Participant | – | Participant | Participant |
| Resident quality of life – proxy rated | ||||
| EQ-5D-5L Proxy family carer or staff member view of the resident’s quality of life | Family carer/care home staff | – | Family carer/care home staff | Family carer/care home staff |
| Family carer | ||||
| Sociodemographics | ||||
| Age, gender, ethnicity, marital status, years of schooling, highest level of education | Family carer | – | Family carer | Family carer |
| Quality of life | ||||
| EQ-5D-5L | Family carer | – | Family carer | Family carer |
| Preferred role | ||||
| How much and how they like to be involved in the residents care | Family carer | – | – | – |
| Staff | ||||
| Staff sociodemographics | ||||
| Age, gender, ethnicity, number of years of education | Researcher | – | – | – |
| Staff work characteristics | ||||
| Highest qualification, role in care home, duration of service, shift pattern, first language | Researcher | – | – | – |
| Organisational support for person-centred care | ||||
| P-CAT | Care home staff | – | Care home staff | Care home staff |
| Communication with primary care | ||||
| Nurse–physician communication in the long-term care setting | Care home staff | – | Care home staff | Care home staff |
| Perceived knowledge and skills for early detection of changes in health | ||||
| Developed from feasibility study. Assesses key knowledge and skills needed to implement the intervention. Rated on a 5-point Likert scale | Care home staff | – | Care home staff | Care home staff |
| System-level data | ||||
| Number of hospital admissions | ||||
| Respiratory infection, exacerbation of CHF, UTI and dehydration | Care home staff | Care home staff | Care home staff | Care home staff |
| ‘Avoidability’ of admissions | ||||
| SIRR76 | Care home staff | Care home staff | Care home staff | Care home staff |
| Use of primary assessment tool | ||||
| Respiratory infection, exacerbation of CHF, UTI and dehydration | Care home staff | Care home staff | Care home staff | Care home staff |
| Use of secondary assessment | ||||
| Respiratory infection, exacerbation of CHF, UTI and dehydration | Care home staff | Care home staff | Care home staff | Care home staff |
| Out-of-hours GP contacts | ||||
| GP visits or telephone contact | Care home staff | Care home staff | Care home staff | Care home staff |
| Ambulances and hospital use | ||||
| Number and length of hospital stays (days), A&E attendances and re-admissions | Care home staff | Care home staff | Care home staff | Care home staff |
| Deaths in the previous calendar month | Care home staff | Care home staff | Care home staff | Care home staff |
| Staff turnover | Care home staff | Care home staff | Care home staff | Care home staff |
| Care home occupancy level | ||||
| Number of available beds to potential new residents | Care home staff | Care home staff | Care home staff | Care home staff |
We conducted semistructured interviews with five nursing home managers, six PDCs, three nurses, seven care assistants and five family carers from across the five homes that were able to deliver the intervention. We explored participants’ views on their knowledge of, engagement with, and views on effectiveness of, the intervention. Family carers were purposively sampled to ensure a range of genders, ages and types of family carer. The interviews lasted ≈ 40 minutes, and were conducted either face to face or by telephone.
Serious adverse events
Participants were monitored each month for potential adverse events. Serious adverse events (SAEs) were defined, using the standard operating procedures of the Priment CTU, as any untoward occurrence that resulted in death, was considered life-threatening at the time of the event, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity or was any other important medical condition. Each untoward event was then considered and designated as ‘related’, that is it resulted from administration of any of the research procedures, or ‘unexpected’, that is the type of event is not listed in the protocol as an expected occurrence.
Statistical analysis
We followed Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting randomised trials. Given that this was a pilot trial, analyses were mainly descriptive, focusing on recruitment, participant characteristics, other baseline and outcome variables, loss to follow-up and tabulation of SAEs. We summarised completeness of data collection on all outcome measures. Analyses were carried out in Stata® version 14 (StataCorp LP, College Station, TX, USA). We aimed to consider the rates of hospital admission for ACSCs to inform the sample size calculation for a full trial.
We calculated costs associated with the intervention. Resource use associated with NHS and social care provision was collected and costs were calculated from the NHS and Personal Social Services (PSS) perspective. We assessed the feasibility of calculating quality-adjusted life-years (QALYs) for residents and family carers. The health economic analysis was conducted using utility values (to calculate QALYs) from resident self-reported EQ-5D-5L questionnaires.
We provided an initial estimate of the incremental mean cost per QALY gained in the intervention arm, compared with the TAU arm, and reported the probability that the intervention is cost-effective for a range of willingness-to-pay (WTP) values for a QALY gained. We then estimated the price that a health-care decision-maker would be willing to pay to have perfect information regarding all factors that influence which treatment choice is preferred as the results of a cost-effectiveness analysis in the form of expected value of perfect information (EVPI) and expected value of partial perfect information (EVPPI). 76,77
Qualitative analysis
Verbatim transcripts of the interviews were made. Key themes were coded using framework analysis. 66 This involved the following stages:
-
Familiarisation – reading through each transcript and making notes.
-
Identifying a thematic framework – identifying key themes, issues or discussion points. We used the topic guide as a starting point.
-
Indexing – annotating transcripts to identify patterns.
-
Charting – rearranging the data and framework to create order.
-
Mapping and interpretation – understanding how themes relate to one another.
The process was assisted by the use of NVivo qualitative data analysis software, version 10 (QSR International, Warrington, UK). For the family carer transcripts, stages 1 and 2 were conducted by the CRPs in collaboration with the research team. A workshop on framework data analysis was held with the CRP and members were also offered the opportunity to send input by post (see Box 2).
Context: management and care home practice.
Knowledge: knowledge about the study and the intervention tools.
Engagement: use of the intervention tools. Note that this aspect of the framework applied only to nursing home staff.
Impact: impact of the intervention tools on staff, nursing home practice and family carers.
Stages 3–5 were conducted by members of the research team. A sample of the interviews was independently coded by an additional member of the team to check levels of agreement. For interview transcripts from nursing home staff, the research team conducted stages 1–5. The thematic framework developed at stage 2 for the family carers was adapted for these staff transcripts. We explored the extent of engagement with the intervention in terms of knowledge about, and use of, its three elements: the S&W, the care pathway and the SBAR technique. We explored the effectiveness of the key elements of the implementation strategy: PDCs, the training workshop, monthly coaching telephone calls, the handbook and the web-based resources. We presented the qualitative data under these headings as they directly address the questions posed in the pilot study about the knowledge of, and engagement with, the intervention and its implementation strategy. We have added commentary to the discussion about how these findings link to the PARiHS framework, with an emphasis on context and facilitation.
Findings
We describe the trial settings and participants: residents, staff and family carers at baseline and after randomisation. We then highlight the key findings according to each of the objectives for this WP.
Trial settings
Fourteen (which was the target number) nursing homes were recruited and randomised as planned: seven to the intervention (three in London and four in West Yorkshire) and seven to the control (three in London and four in West Yorkshire). One Yorkshire intervention nursing home dropped out during pre intervention as it was closed by its owners. A further intervention group nursing home in London dropped out following PDC training as it was unable to implement any aspects of the intervention.
Nursing homes were predominantly privately managed; one was a local authority nursing home. They had a median of 50 residents [interquartile range (IQR) 34–68 residents]. The majority (73%) were ‘Dementia Registered’ and were rated overall as ‘good’ by the CQC. Table 4 allows comparison with the national average overall CQC rating for care homes: 3% are rated ‘inadequate’, 31% are rated as ‘requires improvement’, 65% are rated as ‘good’ and 1% as ‘outstanding’. Therefore, overall CQC ratings for nursing homes in this trial were above average, when compared with UK care homes.
| Nursing Home ID | Rating | |||||
|---|---|---|---|---|---|---|
| Safe | Effective | Caring | Responsive | Well led | Overall | |
| 1 | Good | Good | Good | Good | Good | Good |
| 2 | Requires improvement | Good | Good | Good | Good | Good |
| 3 | Requires improvement | Good | Requires improvement | Good | Requires improvement | Requires improvement |
| 4 | Good | Good | Good | Good | Good | Good |
| 5 | Good | Good | Good | Good | Good | Good |
| 6 | Requires improvement | Good | Good | Good | Good | Good |
| 7 | Good | Requires improvement | Good | Good | Good | Good |
| 8 | Good | Good | Good | Good | Good | Good |
| 9 | Good | Outstanding | Outstanding | Outstanding | Outstanding | Outstanding |
| 10 | Requires improvement | Good | Good | Good | Good | Good |
| 11 | Requires improvement | Good | Good | Good | Requires improvement | Requires improvement |
| 12 | Good | Good | Good | Good | Good | Good |
| 13 | Good | Good | Good | Good | Good | Good |
| 14 | Requires improvement | Good | Good | Good | Good | Good |
| Trial average |
|
|
|
|
|
|
Table 5 shows nursing home characteristics at baseline. There were 13 nursing homes, with a median of 60 resident places (IQR 36–71 resident places). A median of 20 residents had dementia (IQR 15–33 residents) in each nursing home. There were relatively few hospital admissions, ambulances called and A&E attendances at the nursing home level in the month before baseline. There was a median of three hospital admissions per participating home (IQR 2–5), three ambulances called (IQR 2–6) and three resident A&E attendances (IQR 2–5).
| Characteristic | Median | IQR |
|---|---|---|
| Residents and beds | ||
| Beds available to new residents (N = 12a) | 1 | 1–3 |
| Resident places (N = 12a) | 60 | 36–71 |
| Number of residents present in home (N = 13) | 50 | 34–68 |
| Number of residents with dementia present in home (N = 13) | 20 | 15–33 |
| Number of residents currently in hospital (N = 13) | 0 | 0–1 |
| Medical attendances | ||
| Number hospital admissions (N = 12a) | 3 | 2–5 |
| Number of ambulances called (N = 12a) | 3 | 2–6 |
| Unscheduled (out-of-hours) GP visits or telephone contacts (N = 12a) | 1 | 1–3 |
| A&E attendances (N = 12a) | 3 | 2–5 |
| Staffing | ||
| Number of qualified nursing staff who were rostered on during the day (N = 13) | 3 | 2–5 |
| Number of care staff who were rostered on during the day (N = 13) | 11 | 9–13 |
| Number of qualified nursing staff who were rostered on during the night (N = 13) | 2 | 1–3 |
| Number of care staff who were rostered on during the night (N = 13) | 3 | 3–7 |
| Number of staff in 24-hour period who were agency/bank staff (N = 13) | 1 | 0–3 |
| Number of permanent registered nursing staff (including those on sick/carer/compassionate leave) (N = 13) | 10 | 8–13 |
| Number of permanent other care staff (including those on sick/carer/compassionate leave) (N = 13) | 26 | 24–57 |
| Number of registered nursing staff from those above on sick/carer/compassionate leave (N = 13) | 0 | 0–1 |
| Number of other care staff from those above on sick/carer/compassionate leave (N = 13) | 0 | 0–2 |
| Nursing home | n/N | % |
| Privately managed | 12/13 | 92 |
| Local authority managed | 1/13 | 8 |
| Nursing | 6/13 | 46 |
| Nursing and personal care | 7/13 | 54 |
| Dementia registered | 8/11 | 73 |
| Dementia specialist | 2/12 | 17 |
As can be seen in Table 6, all nursing homes had a dedicated GP and access to chiropody. Support from other external agencies varied. For example, only one-quarter of the nursing homes received regular input from a geriatrician and 38% had input from a district nurse.
| Regular care home attendance from | n/N | % |
|---|---|---|
| Physiotherapist | 7/12a | 58 |
| Geriatrician | 3/12a | 25 |
| District nurse | 5/13 | 38 |
| Tissue viability nurses | 7/12a | 58 |
| Dietitian | 9/13 | 69 |
| Speech and language therapist | 8/13 | 62 |
| Optician | 11/13 | 85 |
| Ophthalmologist | 4/13 | 31 |
| Chiropodist | 13/13 | 100 |
| Occupational therapist | 7/13 | 54 |
| Dentist | 8/12a | 67 |
| Audiologist | 2/12a | 17 |
| Dedicated GP for all residents | 13/13 | 100 |
As can be seen in Table 7, few nursing homes reported having used the interventions in this trial before. Prior to the trial, one nursing home used the S&W, two nursing homes used care pathways for ACSCs and four used the SBAR technique to aid communication with primary care.
| Prior use of intervention tools | n/N | % |
|---|---|---|
| S&W | 1/13 | 8 |
| Care pathway | 2/13 | 15 |
| SBAR technique | 4/13 | 31 |
Table 8 presents the nursing home characteristics at baseline, by randomised group. There was a higher median number of resident places in the TAU group than in the intervention group, and a higher median number of residents with dementia in the TAU group, possibly because the TAU group had dementia-specialist homes, whereas there were no such homes in the intervention group.
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Residents and beds | ||||
| Beds available to new residents | 2 | 0–2 | 1 | 1–3 |
| Resident places | 67 | 47–84 | 49 | 28–64 |
| Number of residents present in home | 50 | 47–83 | 46 | 25–63 |
| Number of residents with dementia present in home | 33 | 15–44 | 20 | 13–23 |
| Number of residents currently in hospital | 1 | 0–2 | 0 | 0–1 |
| Medical attendances | ||||
| Number hospital admissions | 4 | 2–7 | 3 | 2–4 |
| Number of ambulances called | 4 | 2–9 | 3 | 2–6 |
| Unscheduled (out-of-hours) GP visits or telephone contacts | 2 | 1–3 | 1 | 1–3 |
| A&E attendances | 3 | 1–4 | 3 | 2–6 |
| Staffing | ||||
| Qualified nursing staff rostered on during the day | 3 | 2–5 | 3 | 2–5 |
| Care staff rostered on during the day | 11 | 9–14 | 12 | 6–13 |
| Qualified nursing staff rostered on during the night | 2 | 1–3 | 2 | 1–3 |
| Care staff rostered on during the night | 3 | 3–7 | 4 | 2–7 |
| Number of staff in 24-hour period who were agency/bank staff | 1 | 0–3 | 1 | 0–3 |
| Number of permanent registered nursing staff (including those on sick/carer/compassionate leave) | 11 | 10–13 | 8 | 7–15 |
| Number of permanent other care staff (including those on sick/carer/compassionate leave) | 26 | 24–70 | 33 | 23–57 |
| Number of registered nursing staff from those above on sick/carer/compassionate leave | 0 | 0–1 | 0 | 0–1 |
| Number of other care staff from those above on sick/carer/compassionate leave | 1 | 0–6 | 0 | 0–2 |
| Nursing home | n/N | % | n/N | % |
| Privately managed | 6/7 | 86 | 6/6 | 100 |
| Local authority managed | 1/7 | 14 | 0/6 | 0 |
| Nursing | 4/7 | 57 | 2/6 | 33 |
| Nursing and personal care | 3/7 | 43 | 4/6 | 67 |
| Dementia registered | 5/6 | 83 | 3/5 | 60 |
| Dementia specialist | 2/6 | 33 | 0/6 | 0 |
As can be seen in Table 9, there is little difference at baseline between the homes in terms of receipt of medical and allied health services.
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| Physiotherapist | 4/7 | 57 | 3/5 | 60 |
| Geriatrician | 2/7 | 29 | 1/5 | 20 |
| District nurse | 3/7 | 43 | 2/6 | 33 |
| Tissue viability nurses | 3/7 | 43 | 4/5 | 80 |
| Dietitian | 4/7 | 57 | 5/6 | 83 |
| Speech and language therapist | 4/7 | 57 | 4/6 | 67 |
| Optician | 5/7 | 71 | 6/6 | 100 |
| Ophthalmologist | 1/7 | 14 | 3/6 | 50 |
| Chiropodist | 7/7 | 100 | 6/6 | 100 |
| Occupational therapist | 3/7 | 43 | 4/6 | 67 |
| Dentist | 5/7 | 71 | 3/5 | 60 |
| Audiologist | 1/7 | 14 | 1/5 | 20 |
| Dedicated GP for all residents | 7/7 | 100 | 6/6 | 100 |
There were minor differences between intervention and TAU nursing homes at baseline in terms of reported use of intervention tools prior to the trial (Table 10). None of the intervention homes reported using the S&W or the care pathway; three nursing homes in the TAU group reported using the SBAR technique.
Residents
As can be seen in Table 11, we recruited 237 residents: two-thirds were female, they were predominantly white (90%) and the median age was 86 years (IQR 80–91 years). The median Barthel Index score was low, at 27 points (IQR 9–64 points), indicating a high level of dependence. Only 3% of the residents had an admission for an ACSC in the month before baseline, 11 (5%) residents had had at least one ambulance called and 12 (5%) had had an unscheduled GP visit or telephone contact.
| Characteristic | n/N or median | % or (IQR) |
|---|---|---|
| Demographics | ||
| Male | 73/234 | 31 |
| Age (years) (N = 231) | 86 | (80–91) |
| Ethnicity | ||
| White | 203/225 | 90 |
| Black | 14/225 | 6 |
| Asian | 5/225 | 2 |
| Other | 3/225 | 1 |
| Marital status | ||
| Married | 49/223 | 22 |
| Single | 59/223 | 26 |
| Divorced or widowed | 115/223 | 52 |
| Education | ||
| Completed years of education (N = 181) | 11 | (9–12) |
| No qualifications or GCSE or equivalent | 107/184 | 58 |
| A level/NVQ/HNC/HND or equivalent | 18/184 | 10 |
| Degree or higher degree | 23/184 | 13 |
| Other qualification | 36/184 | 20 |
| Function | ||
| Barthel Index score (N = 232) | 27 | (9–64) |
| Admissions and events | ||
| At least one admission in the previous month | 6/235 | 3 |
| Respiratory infection admission | 1/235 | 0.4 |
| UTI admission | 2/235 | 1 |
| Dehydration admission | 0/235 | 0 |
| CHF admission | 1/235 | 0.4 |
| At least one ambulance called | 11/234 | 5 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 12/235 | 5 |
| At least one A&E attendance | 10/235 | 4 |
| Died | 1/234 | 0.4 |
There were similar resident characteristics between the intervention and TAU groups at baseline (Table 12).
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| n/N or median | % or (IQR) | n/N or median | % or (IQR) | |
| Demographics | ||||
| Male | 46/137 | 34 | 27/97 | 28 |
| Age (years) | 86 | (80–91) | 84 | (78–91) |
| Ethnicity | ||||
| White | 117/131 | 89 | 86/94 | 91 |
| Black | 9/131 | 7 | 5/94 | 5 |
| Asian | 4/131 | 3 | 1/94 | 1 |
| Other | 1/131 | 1 | 2/94 | 2 |
| Marital status | ||||
| Married or cohabiting | 28/127 | 22 | 21/96 | 22 |
| Single | 40/127 | 32 | 19/96 | 20 |
| Divorced or widowed | 59/127 | 46 | 56/96 | 58 |
| Education | ||||
| Completed years of education | 11 | (10–12) | 11 | (9–11) |
| No qualifications or GCSE or equivalent | 63/113 | 56 | 44/71 | 62 |
| A Level/NVQ/HNC/HND or equivalent | 11/113 | 10 | 7/71 | 10 |
| Degree or higher degree | 14/113 | 12 | 9/71 | 13 |
| Other qualification | 25/113 | 22 | 11/71 | 15 |
| Function | ||||
| Barthel Index score | 27 | (9–66) | 30 | (8–63) |
| Admissions and events | ||||
| At least one admission in the previous month | 2/139 | 1 | 4/96 | 4 |
| Respiratory infection admission | 0/139 | 0 | 1/96 | 1 |
| UTI admission | 1/139 | 1 | 1/96 | 1 |
| Dehydration admission | 0/139 | 0 | 0/96 | 0 |
| CHF admission | 0/139 | 0 | 1/96 | 1 |
| At least one ambulance called | 4/138 | 3 | 7/96 | 7 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 11/139 | 8 | 1/96 | 1 |
| At least one A&E attendance | 6/139 | 4 | 4/96 | 4 |
| Died | 1/138 | 1 | 0/96 | 0 |
Family carers
As can be seen in Table 13, we recruited 91 family carers, two-thirds of whom were female. Their median age was 63 years (IQR 57–71 years). The majority wanted a role in noticing changes in their relative’s health and informing staff of this. Other roles that family carers wished to take on included informing staff of their relative’s background, personal history and preferences, managing finances and taking the person out for visits. Table 13 presents details of family carer characteristics at baseline, and Table 14 presents family carer characteristics at baseline by randomised group.
| Characteristic | n/N or median | % or (IQR) |
|---|---|---|
| Demographics | ||
| Male | 31/91 | 34 |
| Age (years) (N = 88) | 63 | (57–71) |
| Ethnicity | ||
| White | 72/87 | 83 |
| Black | 12/87 | 14 |
| Asian | 3/87 | 3 |
| Marital status | ||
| Married or cohabiting | 65/87 | 75 |
| Single | 10/87 | 11 |
| Divorced or widowed | 12/87 | 14 |
| Education | ||
| Completed years of education (N = 87) | 11 | (11–12) |
| No qualifications or GCSE or equivalent | 35/86 | 41 |
| A Level/NVQ/HNC/HND or equivalent | 13/86 | 15 |
| Degree or higher degree | 26/86 | 30 |
| Other qualification | 12/86 | 14 |
| Preferred role | ||
| Noticing early signs of changes in health | 79/87 | 91 |
| Informing care staff about the early signs of changes in health | 77/87 | 89 |
| Educating care staff about how the early signs of changes in health present | 51/87 | 59 |
| Educating care staff about the history of the health of their family member | 57/87 | 66 |
| Prefer not be involved | 5/87 | 6 |
| Other | 18/87 | 21 |
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| n/N or median | % or (IQR) | n/N or median | % or (IQR) | |
| Demographics | ||||
| Male | 17/56 | 30 | 14/35 | 40 |
| Age (years) | 62 | (57–71) | 64 | (58–74) |
| Ethnicity | ||||
| White | 43/52 | 83 | 29/35 | 83 |
| Black | 7/52 | 13 | 5/35 | 14 |
| Asian | 2/52 | 4 | 1/35 | 3 |
| Marital status | ||||
| Married or cohabiting | 36/53 | 68 | 29/34 | 85 |
| Single | 8/53 | 15 | 2/34 | 6 |
| Divorced or widowed | 9/53 | 17 | 3/34 | 9 |
| Education | ||||
| Completed years of education | 12 | (11–13) | 11 | (11–12) |
| No qualifications or GCSE or equivalent | 21/53 | 40 | 14/33 | 42 |
| A Level/NVQ/HNC/HND or equivalent | 9/53 | 17 | 4/33 | 12 |
| Degree or higher degree | 14/53 | 26 | 12/33 | 36 |
| Other qualification | 9/53 | 17 | 3/33 | 9 |
| Preferred role | ||||
| Noticing early signs of changes in health | 49/52 | 94 | 30/35 | 86 |
| Informing care staff about the early signs of changes in health | 48/52 | 92 | 29/35 | 83 |
| Educating care staff about how the early signs of changes in health present | 28/52 | 54 | 23/35 | 66 |
| Educating care staff about the history of the health of their family member | 33/52 | 63 | 24/35 | 69 |
| Prefer not be involved | 2/52 | 4 | 3/35 | 9 |
| Other | 9/52 | 17 | 9/35 | 26 |
Staff
We recruited 132 staff, with a median age of 42 years (IQR 30–53 years); 12% were male, 50% of nurses spoke English as a first language and 59% of staff described themselves as white. Most staff had worked at the nursing home for ≥ 1 years (71%) and 30% were qualified nurses (Table 15).
| Characteristic | n/N or median | % or (IQR) |
|---|---|---|
| Demographics | ||
| Male | 16/130 | 12 |
| Age (years) (N = 129) | 42 | (30–53) |
| Ethnicity | ||
| White | 76/129 | 59 |
| Black | 29/129 | 22 |
| Asian | 16/129 | 12 |
| Other | 8/129 | 6 |
| Marital status | ||
| Married or cohabiting | 71/129 | 55 |
| Single | 47/129 | 36 |
| Divorced or widowed | 11/129 | 9 |
| Education | ||
| Completed years of education (N = 126) | 11 | (11–12) |
| No qualifications or GCSE or equivalent | 16/128 | 13 |
| A Level/NVQ/HNC/HND or equivalent | 46/128 | 36 |
| Degree or higher degree | 49/128 | 38 |
| Other qualification | 17/128 | 13 |
| Type of staff | ||
| Registered nurse | 16/125 | 13 |
| Manager/matron/charge nurse | 12/125 | 10 |
| Staff nurse | 8/125 | 6 |
| Agency nurse | 2/125 | 2 |
| Not a nurse | 87/125 | 70 |
| Nurses’ working patterns | ||
| Works days | 28/40 | 70 |
| Works evenings | 2/40 | 5 |
| Works nights | 9/40 | 23 |
| Works mornings/early 12-hour shifts | 12/40 | 30 |
| Works afternoons/late 12-hour shifts | 3/40 | 8 |
| Works weekends | 3/40 | 8 |
| Number of hours per week typically working in this care home | ||
| < 8 | 1/40 | 3 |
| 8–12 | 0/40 | 0 |
| 13–40 | 13/40 | 33 |
| > 40 | 26/40 | 65 |
| Length of time working (nurses only) | ||
| How long have you worked at this nursing home? | ||
| < 2 months | 1/40 | 3 |
| 2–6 months | 4/40 | 10 |
| 7–11 months | 7/40 | 18 |
| 1–5 years | 17/40 | 43 |
| 6–10 years | 6/40 | 15 |
| > 10 years | 5/40 | 13 |
| How long have you worked in nursing homes? | ||
| 7–11 months | 2/39 | 5 |
| 1–5 years | 14/39 | 36 |
| 6–10 years | 7/39 | 18 |
| > 10 years | 16/39 | 41 |
| How long have you been a registered nurse? | ||
| 1–5 years | 8/40 | 20 |
| 6–10 years | 10/40 | 25 |
| > 10 years | 22/10 | 55 |
| Language | ||
| English is first language | 20/40 | 50 |
Scores on the Person-centred Care Assessment Tool (P-CAT) (possible range 13–65 points) were generally positive, with a median score of 49 points (IQR 46–53 points) (see Appendix 22). The majority of nurses were positive about the quality of communications they had with the residents’ GP (see Appendix 23). Nurses were positive about their knowledge and skills (see Appendix 24).
As can be seen in Table 16, the intervention group had a greater percentage of white staff. Two-thirds of participating TAU staff and three-quarters of intervention staff were not nurses.
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| n/N or median | % or (IQR) | n/N or median | % or (IQR) | |
| Demographics | ||||
| Male | 9/70 | 13 | 7/60 | 12 |
| Age (years) | 42 | (32–53) | 39 | (27–50) |
| Ethnicity | ||||
| White | 32/69 | 46 | 44/60 | 73 |
| Black | 21/69 | 30 | 8/60 | 13 |
| Asian | 9/69 | 13 | 7/60 | 12 |
| Other | 7/69 | 10 | 1/60 | 2 |
| Marital status | ||||
| Married or cohabiting | 40/69 | 58 | 31/60 | 52 |
| Single | 22/69 | 32 | 25/60 | 42 |
| Divorced or widowed | 7/69 | 10 | 4/60 | 7 |
| Education | ||||
| Completed years of education | 12 | (11–12) | 11 | (11–12) |
| No qualifications or GCSE or equivalent | 9/69 | 13 | 7/59 | 12 |
| A Level/NVQ/HNC/HND or equivalent | 18/69 | 26 | 28/59 | 47 |
| Degree or higher degree | 28/69 | 41 | 21/59 | 36 |
| Other qualification | 14/69 | 20 | 3/59 | 5 |
| Type of staff | ||||
| Registered nurse | 9/67 | 13 | 7/58 | 12 |
| Manager/matron/charge nurse | 8/67 | 12 | 4/58 | 7 |
| Staff nurse | 5/67 | 7 | 3/58 | 5 |
| Agency nurse | 1/67 | 1 | 1/58 | 2 |
| Not a nurse | 44/67 | 66 | 43/58 | 74 |
| Nurses only | ||||
| Works days | 16/24 | 67 | 12/16 | 75 |
| Works evenings | 1/24 | 4 | 1/16 | 6 |
| Works nights | 4/24 | 17 | 5/16 | 31 |
| Works mornings/early 12-hour shifts | 5/24 | 21 | 7/16 | 44 |
| Works afternoons/late 12-hour shifts | 0/24 | 0 | 3/16 | 19 |
| Works weekends | 1/24 | 4 | 2/16 | 13 |
| Number of hours per week typically working in this care home | ||||
| < 8 | 0/24 | 0 | 1/16 | 6 |
| 8–12 | 0/24 | 0 | 0/16 | 0 |
| 13–40 | 8/24 | 33 | 5/16 | 31 |
| > 40 | 16/24 | 67 | 10/16 | 63 |
| How long have you worked at this care home? | ||||
| < 2 months | 1/24 | 4 | 0/16 | 0 |
| 2–6 months | 3/24 | 13 | 1/16 | 6 |
| 7–11 months | 4/24 | 17 | 3/16 | 19 |
| 1–5 years | 7/24 | 29 | 10/16 | 63 |
| 6–10 years | 6/24 | 25 | 0/16 | 0 |
| > 10 years | 3/24 | 13 | 2/16 | 13 |
| How long have you worked in care homes? | ||||
| 7–11 months | 1/24 | 4 | 1/15 | 7 |
| 1–5 years | 6/24 | 25 | 8/15 | 53 |
| 6–10 years | 7/24 | 29 | 0/15 | 0 |
| > 10 years | 10/24 | 42 | 6/15 | 40 |
| How long have you been a registered nurse? | ||||
| 1–5 years | 3/24 | 13 | 5/16 | 31 |
| 6–10 years | 7/24 | 29 | 3/16 | 19 |
| > 10 years | 14/24 | 58 | 8/16 | 50 |
| Language | ||||
| English is first language | 12/24 | 50 | 8/16 | 50 |
Objective 1: establish whether or not resident consent procedures allow the collection of sufficient individual-level data
We recruited 245 residents to the study (see Appendices 18–21). We screened a total of 680 residents, 557 of whom met eligibility criteria. Thus, there was a 35% recruitment rate. The recruitment target was 20 residents per care home. Taking into account the fact that two homes dropped out, leaving 12 in the study, the target of 240 residents was reached. The majority of eligible residents (n = 364, 65%) did not have capacity to give consent to participate in the study. We recruited 135 of the final 245 participants (55%) by using a personal or professional consultee.
During the 6-month study period, in the TAU group, 19 residents died, four moved to different nursing homes and four withdrew. In the intervention group, 13 residents were lost when two nursing homes withdrew, 14 residents died, two residents moved to a different home and two did not wish to continue in the study. Thus, 73% of recruited residents completed the study, with 10% lost to follow-up as a result of mortality. We conclude that we had good retention throughout the study and that the consent procedures did allow the collection of sufficient individual-level data throughout the study period.
Objective 2: assess the effectiveness of the implementation strategy
Practice Development Champions
The implementation strategy involved establishing two PDCs working with a PDSG in each participating care home to introduce and embed the three intervention tools: the S&W, the care pathway and the SBAR technique. PDCs were supported in their role with a training workshop, a handbook, web-based resources and monthly telephone coaching from the co-applicant practice development experts (KF and BM).
One of the intervention homes withdrew from the study after their two PDCs had participated in the training workshop. They were unable to comply with the requirement that the PDCs lead the implementation of the intervention. This was a result of a combination of a lack of knowledge, skills and expertise on the part of the PDCs, and a lack of management support.
Across the five remaining intervention homes, none introduced the intervention as expected, although most PDCs engaged in some kind of information sharing and training about the project. One PDC described her role as:
To come back and train staff on what we’d learned and implement the tool and monitor its effectiveness within our setting.
Nursing home 6, PDC3
Some reported that they had used a cascade model of training the nurses to train the care staff in using the S&W and had noticed that care assistants were now reporting changes in residents’ health quicker. In another home, the PDC took time out to brief nurses one to one and then used time at the start of each shift to remind small groups of staff to be aware of possible changes in the residents’ health.
The PDCs’ effectiveness at implementing the intervention (introducing and integrating it into everyday practice) was variable. In one home, PDCs had integrated elements of the complex intervention into their regular morning meetings, where they reviewed each resident identifying any concerns and integrated it into the electronic care records.
Practice development support groups
None of the PDCs set up PDSGs. Some tried but found timetabling very difficult. One PDC chose not to set one up as they had only five nurses in total. Therefore, they thought that there was no need to set up a separate group as they already had a mechanism for effective communication with staff. One PDC got support for the intervention from her/his nurses, as they were the ones using the tools and reporting back at staff meetings. One PDC felt that, had the groups been set up, they would have been able to more effectively engage family carers in the BHiRCH-NH study.
Training workshop
One PDC reported that the training workshop at the outset of the study was very helpful. They noted, in particular, the input from the consultant geriatrician (JY) regarding signs of early deterioration and input from the family member. They also valued the discussion about how to share knowledge with colleagues:
It actually was very nice to have a relative there giving her personal experience, which was that little bit of an extra insight. That is one of the main things.
Nursing home 9, PDC1
Several PDCs suggested that the study team communicating more directly with staff about the project and providing staff training would have been helpful.
The handbook
Engagement with the handbook was variable. One PDC said they had not had time to look at it, whereas another considered it to be ‘brilliant’ and others pointed to it being helpful to have information about the research underlying the project for when their colleagues asked them questions. The handbook provided them with a reference point. They felt that having the handbook made it easier to communicate the goals of the BHiRCH-NH study to their colleagues, as they could present the underlying research evidence:
I had to deliver it [details of the intervention] to the carers and other nurses. I knew exactly what the goals were and then, when actually delivering it, I knew a little bit more knowledge instead of just saying ‘this is this sheet, this is’. I could answer some of their questions and then I had it [the handbook] by my side, so if they asked me something I didn’t know, I could quickly flick through it [the handbook] and say ‘this is the reason why’.
Nursing home 9, PDC1
The monthly coaching telephone calls
Several of the PDCs pointed to the helpfulness of the monthly telephone calls with the practice development expert. They found these to be encouraging, providing useful reminders, and found it reassuring to know there was someone at the end of the telephone if they had queries:
[The telephone call] was helpful because I told them [the practice development coaches] what I’ve done. They said to me ‘well done’. When I do something good, and someone says to you ‘well done’, then our work is not for nothing.
Nursing home 12, PDC2
It keeps it fresh in your mind as well, because you come to the training day having not spoken to anyone again I probably would have just forgotten about it. It would go to the bottom of the pile. The fact that I knew she was ringing me every month kept my mind fresh and I knew that I would still keep using it because I was going to have this conference call with her.
Nursing home 6, PDC3
One of the practice development coaches thought that the offer of monthly calls was not welcome in the home that dropped out. Despite numerous attempts at making contact and establishing a schedule of telephone calls, no telephone calls were established. He/she concluded that continuing the process potentially breached the ethics procedures of voluntary participation; thus, further attempts were withdrawn.
Support from the manager
The PDCs pointed to the critical role of the manager in lending them authority, putting their weight behind the project and being someone with who they could discuss progress. One PDC felt that the manager should have had helped more to disseminate the BHiRCH-NH intervention across the care home because she had more authority. Those who were given more time by their managers felt more able to implement the BHiRCH-NH intervention. The PDC also felt that staff lacked motivation and would only undertake training and complete forms that were mandatory and part organisational policy:
I found it very difficult to chase everyone and to explain, and that’s why I asked my manager a few times . . . ‘when you have a meeting or can you please just pass it over?’ because it is different when it comes from the managers than when it comes from me . . . it’s the boss . . . not all the staff considered [it] really important because . . . everyone saw [it as] something optional.
Nursing home 4, PDC4
Relationships with primary care and out-of-hours services
Relationships with primary care and out-of-hours services also had an impact on implementation. Most PDCs reported excellent relationships with their primary care providers. Although one PDC felt that they were able to communicate changes well, at the weekend they had greater difficulty getting the needed services:
The only problem now in our area is that, over the weekend, the Rapid Response, there is a wait time. They don’t have a nurse prescriber. Sometimes they will say ‘I am a nurse like you, it’s your responsibility in that nursing home’. They don’t have enough doctors over the weekend. So they say ‘I’m so sorry, we don’t have a doctor. If you consider it an emergency, just send them to the hospital’. So we don’t have any antibiotics or anything over the weekend. We have to wait until Monday.
Nursing home 12, PDC2
Objective 3: assess fidelity to the intervention
It was not possible to assess fidelity to the intervention in the way we had intended. During the study period, only 16 S&W forms and eight care pathways were completed in the intervention nursing homes. This included both residents who had given consent to participate and anonymised S&W forms and care pathways collected from residents who had not given consent (Table 17).
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| Median | IQR | Median | IQR | |
| Medical attendance | ||||
| Number hospital admissions | 12 | 12–16 | 12 | 7–18 |
| Number of ambulances called | 12 | 11–17 | 19 | 7–22 |
| Number of Unscheduled (out-of-hours) GP visits or telephone contacts | 8 | 7–13 | 9 | 4–25 |
| Number of A&E attendances | 12 | 11–13 | 8 | 7–13 |
| Rate of hospital admissions per 100 person-months | 5.3 | 2.3–8.3 | 5.9 | 1.7–7.1 |
| Rate of ambulances called per 100 person-months | 5.7 | 2.3–8.0 | 6.0 | 2.0–9.3 |
| Rate of unscheduled (out-of-hours) GP visit or contacts per 100 person-months | 2.5 | 1.8–4.0 | 5.1 | 1.1–6.0 |
| Rate of A&E attendances per 100 person-months | 4.3 | 2.5–8.3 | 3.9 | 2.0–5.6 |
| Use of intervention tools | ||||
| Number of S&Ws completed for those who did and those who did not consent | N/A | N/A | 1 | 0–3 |
| Number of care pathways completed for those who did and those who did not consent | N/A | N/A | 0 | 0–2 |
| Hours spent by PDCs on the intervention | N/A | N/A | 2.1 | 0–3.8 |
| Hours spent by members of the PDSG on the intervention | 0 | 0–1 | ||
We found limited evidence that the S&W or care pathways were used, either using our forms or using the principles of early detection, assessment and reporting. In a few cases, routine clinical observations (e.g. temperature, blood pressure) were carried out, but these were not reported systematically or presented as part of a coherent assessment plan. One home had a policy of recording routine observations once per month.
Objective 4: assess the level of nursing home staff (and family carer) engagement with the intervention
There were mixed perspectives on the level of engagement of nursing home staff with the intervention. A clearer picture emerged about the involvement, or lack of involvement, of family carers.
Nursing home staff engagement
No engagement, no impact
In one care home, the PDC felt that there was very little engagement in the project:
I don’t think [the BHiRCH-NH study] made too much difference because . . . 90% of the staff didn’t have a clue about this project.
Nursing home 4, PDC4
Stop and Watch Early Warning Tool
When exploring participants’ knowledge and use of the intervention tools, the S&W was almost always mentioned. Most staff saw value in the S&W, especially for care assistants and domestic staff who had not previously been trained to look for early signs of residents’ deteriorating health. They felt that, since the S&W had been introduced, care assistants were more aware of the key role they played in the medical care of residents:
For the carers [care assistants], the most important bit was the Stop and Watch.
Nursing home 9, PDC1
The PDCs described that use of the S&W meant that everyone who came into contact with a resident was involved in helping with early detection of changes in residents’ health:
The Stop and Watch we did and everybody was aware of it and everybody used it. I do think it worked really well and everybody liked it and it’s good for your relatives and your carers and your domestic staff and people that aren’t trained to notice those changes.
Nursing home 6, PDC3
One of our cooks, he was going to join this team because he goes around with the tea trolley. He’s another set of eyes, if he sees something that’s different . . . He is aware because he attended the [daily review of each resident]. Whereas, quite often, staff like cook or housekeeping might’ve thought they didn’t have any part to play. Whereas now, they are all at the [daily review of each resident] and they all know about.
Nursing home 2, PDC5
I think the Stop and Watch is really good and we’ve still got it up in the home. I think it’s tailed off slightly in the care plan, but only because it was put in originally for the residents that consented, so obviously, as new residents have come in . . . but it’s still up in the home and it’s still up in the nurses’ office. So it’s still there to prompt staff and the staff are aware of it so it’s not something we’re just going to take down and put in the bin. It’s a good tool to follow; it’s a good prompt.
Nursing home 6, PDC3
Care home staff frequently reported that they were ‘already doing it’, namely already engaging in early detection of deteriorating health. Moreover, according to PDCs, care assistants were already aware of the need for early detection, and were already able to notice and communicate changes in residents’ health. Although some staff said that they were already looking out for changes in residents’ health, they noted that the BHiRCH-NH study had formalised and provided a structure and emphasis on this aspect of their care.
It was frequently reported that, although they found S&W useful, they did not use it to record their observations. Some saw the S&W form as unnecessary additional paperwork. Staff felt that they already had a place in the care record to make a note of any changes they noticed. Furthermore, nurses reported that care assistants came directly to them with any concerns they might have about a resident. Alternatively, care assistants could pass on this information at shift handover or at a regular morning meeting during which each resident’s well-being was reviewed.
We expected that family carers could play a role in early detection by alerting the care assistant or nurse to complete a S&W form. Although family carers did mention noticing changes in their relatives’ health, they did not attribute this to the BHiRCH-NH study. Encouragement of family carer involvement from staff was mixed, with some family carers having never been approached by staff and others given specific information about the S&W.
Some PDCs felt that family carers were more aware of the need to notice and communicate changes in their relatives’ health. However, they did not necessarily use the S&W form:
Yes, they might not come and necessarily say it’s the Stop and Watch tool, but I think it highlighted noticing a change in people to them. Like ‘oh, she doesn’t seem quite right’, which is on the Stop and Watch, isn’t it, but they’re not necessarily saying ‘the Stop and Watch tool has said this’, but I think, by explaining it to them, it made them more aware to notice changes.
Nursing home 6, PDC3
Care pathway
Nurses reported that the content of the care pathway was fundamental to their training, and, as such, was not necessary. That said, some of the nurses we spoke to found the pathway helpful in reassuring them that they were doing the right thing, and felt that it provided them with an audit trail. They said that the care pathway served as a useful prompt, aide memoir, of what they had learned when training to be a nurse.
Communication with primary care: use of the situation, background, assessment, recommendation technique
The SBAR technique was intended to be used by nurses when communicating with primary care. Staff in several of the nursing homes reported that they were already using it, prior to the BHiRCH-NH study. Others reported using it to communicate with a range of professionals, and one nurse reported that she could delegate some of her communications with primary care to senior care assistants, as it provided the necessary structure and detail.
One manager reported the added value of the SBAR technique to their work:
The SBAR was the main catch for me – at least from feedback of staff themselves, especially the nursing staff. It gives them confidence when they were communicating with others – doctors or the GP – even among themselves – they were able to give more detail rather than make assumptions – they were more prepared. They had a checklist already there for themselves to know exactly what they needed to talk to another professional. I think that SBAR is really good. The feedback is really good. Everybody is using that. Although we were doing things, it was just, like, the icing on the cake.
Nursing home 9, Manager 1
The SBAR technique was already being used in some of the nursing homes prior to the BHiRCH-NH intervention (see Table 10). The PDCs felt that, as nurses, they were already noticing early signs of changes in health and able to communicate with primary care about these changes. One PDC reported that they were already communicating information about residents to the GP using the SBAR technique, which had enabled them to communicate information in a structured way. The implication of formalising this communication was that the GPs were now more likely to regard the nurses’ comments as valid concerns:
So I could literally just go bang, bang, bang, bang, and they just said ‘yes we are sending someone now’. Now, if I had possibly missed things out, stumbled, faltered or forgotten something, you know, they may not have taken me so seriously. It was just very concise. And then, when they actually came to pick the patient up, they had all this information. They already had a proper and clearer idea than me just saying ‘oh, I think the BM [bowel movement] was this’ or I kind of scrambled to read my handwriting on a scrap of paper.
Nursing home 9, PDC1
Stop and Watch Early Warning Tool; care pathway; and the situation, background, assessment, recommendation technique
One nurse reported that the S&W, the care pathway and the SBAR technique provided reassurance whenever they felt unsure about the correct path to follow:
Because we’re nurses, I think we just did it automatically. We would notice somebody wasn’t right and we would suspect a urine infection, or if they had been passing urine frequently and were confused, we would do what the pathway tells you to do anyway. But it just gave you it in a more structured way and something to follow . . . it prompts you, doesn’t it? It prompts people. So if the nurse saw somebody wasn’t quite right, but didn’t suspect a UTI, didn’t really know what was going on, they would follow the Stop and Watch, do the observations that the pathway tells you to and probably come to a more conclusive conclusion, before you ring the GP, rather than just ringing them and saying the resident doesn’t feel right.
Nursing home 6, Nurse 2
Family carer involvement
Family involvement in the BHiRCH-NH study was minimal, and was primarily focused on taking part in the research, including completing questionnaires and engaging in interviews, and not so much with the intervention itself. It was not clear to them how they could be involved beyond their role as a research participant. They knew that the aim of the project was to notice changes in health and reduce hospitalisations, yet they had little knowledge of the BHiRCH-NH intervention tools, including the S&W.
The professional health-care background of family carers may have had an influence on how much they were involved in the BHiRCH-NH study. In one home, for example, the BHiRCH-NH study had been introduced to family carers who had a background in health or social care. Three family carers had a health-care background and mentioned how, at least in part, this had enhanced their interest in BHiRCH-NH study.
One family carer had learnt about the BHiRCH-NH study from the nursing home manager. She was not aware of PDCs and had assumed that the manager was leading the project. The manager had shown her the S&W, and she knew about the care pathway and the SBAR technique:
It’s about taking that time, isn’t it, just to observe or listen . . . I thought it was good. It’s very clear and concise. Good pointers. I dare say, you might say some of it is common sense. I think we can all be guilty of not stopping and watching when you’ve got things to get on with and other residents to get up or whatever.
Nursing home 6, Family Carer 2
Interestingly, this perspective on the S&W, that it is about taking time to look rather than thinking about each sign of health change on the tool, was strongly advocated by a care assistant in the same home, suggesting that his message might have spread across the home to family carers.
Family carers mentioned that they had already been involved in noticing and communicating signs of health change in their relative, before the BHiRCH-NH study. A family carer was noticing signs of changes in health in her relative and communicating it to staff prior to the BHiRCH-NH study. Moreover, she described an occasion when she alerted staff to a change, but felt she was confirming what the staff already knew.
Other family carers had little understanding of the BHiRCH-NH study beyond meeting with research fieldworkers or the interviewer:
[The aim of the study is] to observe and to notice the care of the residents, any difference in, perhaps, their temperature, appetite, just general observations of how they are, whether they’re drinking enough, their urine output and food intake. And any sores, anything that might become infected that could be dealt with before the need to go to a hospital, before it got out of hand . . . I don’t know anything about [the BHiRCH-NH tools], because I presume the nurses were told about this, to implement it and tell the staff and train them.
Nursing home 9, Family Carer 1
One family carer knew that the BHiRCH-NH study was meant to involve family in early detection, yet she had not been involved in the BHiRCH-NH study beyond answering the research questions and taking part in an interview. She had no knowledge of the BHiRCH-NH tools. No staff member had approached her about the BHiRCH-NH study. All her knowledge about the BHiRCH-NH study had come from the fieldworker:
As I understand it, it was designed for staff and carers, family. Everybody was meant to be part of the project to try and detect early detection and change and to help the person that we were looking after.
Nursing home 4, Family Carer 3
There was a feeling that the BHiRCH-NH study could have done more to connect with family, and that an improved visual prompt, such as a checklist displayed in the care home, could have helped to create this connection:
[Family] need to be aware that they can say their relative is on BHiRCH-NH and ask questions to the care home about who will be involved in it.
Nursing home 2, Family Carer 5
Family carers were unsure of the effectiveness of the BHiRCH-NH intervention, which could be related to their lack of knowledge about the study. One family carer describes how she had a feeling that the system of early detection was working, but she would not be able to give any evidence that the BHiRCH-NH study was responsible for this system:
[BHiRCH-NH] wasn’t explicitly referred to and I wasn’t invited to part of a group or anything, but it certainly felt like that system was happening . . . but they didn’t describe them as tools as part of, or described how they were doing them, but it was very evident that they were doing all those things.
Nursing home 4, Family Carer 3
It was thought that the staffing in the care home may have affected the implementation of the BHiRCH-NH intervention. One family carer described how changes in his mother’s health had not been acted on quickly enough by an agency nurse. He did not see any evidence of the BHiRCH-NH system in place on this occasion:
The problems were that the [agency] nurse didn’t really escalate it quick enough, even though my Mum’s temperature was somewhere around the 38–39 [°C] . . . I understand that the home uses the BHiRCH-NH system, but I don’t see evidence of it on this particular occasion, and that may be because it was an agency nurse and she may have been unfamiliar with the care pathway.
Nursing home 2, Family Carer 5
He also suspected that other staff in the nursing home, such as the handyman, would not feel empowered enough to speak out about changes in health.
One family carer described instances when signs of ill health had been quickly detected by the nursing home. However, there was a sense that nothing in the nursing homes had changed since the BHiRCH-NH study had started:
Her urine was very offensive, and she wasn’t her usual perky self. But they picked up on that and got her antibiotics . . . In our experience, since mum was in there, she went in last July, and it’s been our experience really that they have always been very proactive in all those areas. It’s months, definitely, rather than just recently.
Nursing home 6, Family Carer 2
Objective 5: investigate whether or not the intervention would be sustainable outside the context of a trial
Given the findings of objectives 3 and 4, we conclude that the intervention in its current form would not be sustainable outside the context of this trial. That said, at least two of the five intervention nursing homes said they would continue with using the tools after the study had finished.
Objective 6: assess potential primary and secondary outcome measures for a definitive trial
We studied the following outcome measures:
-
primary measure – admission to hospital for one of the four ACSCs
-
secondary measure – use of out-of-hours services.
We assessed their appropriateness by examining the rates of ACSC admissions, the accuracy of clinical records and reports of SAEs.
Rates of hospitalisation for ambulatory care-sensitive conditions
The aim of the study was to develop and pilot test an intervention to improve early detection, assessment and treatment of four common health-care conditions (respiratory infection, exacerbation of CHF, UTI and dehydration) to reduce potentially avoidable hospital admissions for these conditions. At baseline, the rates of hospitalisation for any of these conditions were low: 0.4% of the cohort had an admission for respiratory infections, 1% for UTI, none for dehydration and 0.4% had an admission for chronic heart failure in the month before the trial started. There were six admissions in total from the 235 residents in the study. Considering the whole 6-month study period, 25 study participants (15%) had an unplanned hospital admission for one of the ACSCs studied. Rates of events (per 100 person-months) are given in Table 17. The incidence of the primary outcome of interest suggests that this may not be the best potential primary outcome measure in a future study (see Table 17).
Considering secondary outcome measures for a definitive trial, we found that a slightly higher proportion of participants (n = 38, 16%) had an A&E attendance during the follow-up period, 42 (18%) had at least one ambulance called, 29 (12%) had an unscheduled (out-of-hours) GP visit and 21 (11%) had died. The incidence of these events was still relatively low and not sufficiently frequent to be used as a primary outcome in a definitive study. Comparing TAU with intervention nursing homes, the difference for those who had an admission who were in the study at baseline was 3.0% [95% confidence interval (CI) –6.4% to 12.4%]. This comparison is limited because both of the nursing homes that dropped out were in the intervention arm; thus, their residents were lost to follow-up, resulting in fewer admissions from that arm (see Appendix 25).
Test of the assumption that a hospitalisation for an ambulatory care-sensitive condition is a proxy for avoidable hospital admission
One nursing home had no eligible residents, because those admitted to hospital had died and/or their care records were no longer available. We intended to review only residents admitted for ACSCs, but we expanded our sample to include any hospital admission, partly because it was not always possible to identify if an admission was for an ACSC and because of low numbers overall. For the same reason, we also included residents who died in hospital, as long as their records were still available.
Three of the 10 admissions that could be assessed were deemed to be potentially avoidable. This is consistent with national findings79 that suggest that about one-third of admissions for older people are potentially avoidable. This provides some support for the validity of our approach.
None of the care records provided a complete picture of a resident’s health in the period leading up to admission. Nonetheless, the panel agreed on 10 of the 11 records. Only one was classified as ‘unable to decide’ because of lack of information. These included definitely yes (n = 1), probably yes (n = 2), probably not (n = 5), definitely not (n = 2) and insufficient information in records (n = 1). Only 3 of the 10 hospital admissions rated were judged to be avoidable. From this review of care records, we concluded that an ACSC admission is not a proxy for avoidable admission.
Serious adverse events
There were no differences in SAEs between the TAU and intervention groups (Table 18). There were 104 SAEs in 74 residents during the study, and 34 residents died (19 in the TAU group and 15 in the intervention group). Of the 103 SAEs, hospitalisation (any cause) was the most common (n = 50, 48% of SAEs).
| Type of adverse event | Adverse event (n) | |
|---|---|---|
| TAU (N = 59) | Intervention (N = 44) | |
| A&E visits | ||
| Change in consciousness | 1 | 0 |
| Collapse | 1 | 0 |
| Cough | 1 | 0 |
| Fall | 5 | 4 |
| Gastritis | 1 | 0 |
| UTI | 2 | 1 |
| Hernia | 0 | 1 |
| Died, any cause | 0 | 1 |
| Total | 11 | 7 |
| Admissions- returned to nursing home | ||
| Respiratory infection | 8 | 6 |
| UTI | 2 | 0 |
| CHF | 0 | 2 |
| Anaemia | 1 | 0 |
| Bradycardia | 1 | 0 |
| Breathless | 0 | 1 |
| ‘Assessment’ | 1 | 0 |
| Choking | 0 | 1 |
| Depression | 0 | 1 |
| Deep-vein thrombosis | 0 | 1 |
| Epistaxis | 1 | 1 |
| Fall | 0 | 1 |
| Fever | 0 | 1 |
| Hypokaleamia | 0 | 1 |
| Neck mass | 1 | 0 |
| Pulmonary embolism | 1 | 0 |
| Per rectum bleed | 1 | 0 |
| Urinary retention | 1 | 0 |
| Seizure | 1 | 0 |
| Sepsis | 1 | 3 |
| Shortness of breath | 1 | 0 |
| Stroke | 0 | 1 |
| Visual changes | 0 | 1 |
| Warfarin management | 0 | 1 |
| Total | 21 | 22 |
| Admitted to hospital and died | ||
| Respiratory infection | 0 | 2 |
| Frailty | 1 | 0 |
| Myocardial infarction | 1 | 0 |
| Unresponsive | 0 | 1 |
| Cause unknown | 0 | 1 |
| Hypotension | 0 | 1 |
| Total | 2 | 5 |
| Events in nursing home | ||
| Respiratory infection | 0 | 1 |
| Transient ischaemic attack | 0 | 1 |
| Blood in urine | 1 | 0 |
| Deterioration in diabetes | 1 | 0 |
| Pyelonephritis | 1 | 0 |
| Sepsis | 2 | 0 |
| UTI | 1 | 0 |
| Total | 6 | 2 |
| Died in nursing home | ||
| Respiratory infection | 4 | 4 |
| Frailty | 5 | 2 |
| Dementia | 3 | 0 |
| Cardiac arrest | 1 | 0 |
| Influenza | 1 | 0 |
| Prostate cancer | 1 | 0 |
| Parkinson’s disease | 1 | 0 |
| Squamous cell carcinoma | 1 | 0 |
| Myocardial infarction | 0 | 1 |
| Cardiac failure | 0 | 1 |
| Unknown cause | 0 | 1 |
| Total | 17 | 9 |
Objective 7: collect cost and outcome data
The aim of the economic evaluation was to provide a preliminary estimate of the cost-effectiveness of delivering the intervention, compared with TAU. We compared a range of costs, for example primary, community and emergency health-care services, between the TAU and intervention groups. We assumed that differences in costs between the two groups were due to the intervention. Data regarding health-care service use were collected at baseline (covering the preceding month) and monthly for the following 6 months. Data on quality of life were collected using the EQ-5D-5L questionnaire at baseline and 6 months (resident self-reported, carer’s perception and carer’s own quality of life). The economic analysis was from the NHS and PSS perspective and was conducted using utility values (to calculate QALYs) from resident self-reported EQ-5D-5L questionnaires (highest return rate of questionnaires). The incremental cost per QALY gained was calculated and the probability of cost-effectiveness of the intervention for a range of values of WTP for a QALY gained was reported (see Appendix 26).
Objective 8: establish the key cost components through economic analysis
The cost of the intervention included the cost of training the PDCs and the cost of delivering the intervention. It did not include the costs of monthly telephone coaching. The total cost of training and delivery of the intervention at each nursing home was recorded (see Appendix 26, Tables 21 and 25). One nursing home withdrew after randomisation; therefore, we considered the cost of the intervention there to be £0 (as ‘per randomised’ approach). Assuming that the intervention would be offered to all 89 residents randomised to the intervention group, the mean cost per resident would be £74 (95% CI £64 to £84).
During the 6 months’ follow-up, there were no significant differences in any component of health-care resource use between the intervention and TAU groups. The most common type of contacts were primary care, community health or emergency services for both groups, with a mean of 9.6 [standard deviation (SD) 7.6] visits per resident in the intervention group and 8.3 (SD 6.1) visits per resident in the TAU group. However, the difference in the cost of outpatient appointments is significantly greater (£27, 95% CI £1.20 to £53.50) in the intervention group; this could be a result of more costly service use by the residents in the intervention group, but the CIs are wide, indicating that this may be a chance finding due to multiple comparisons (see Appendix 26, Table 22).
The mean total cost of health-care resource use per resident (after accounting for missing data) over the 6 months was £1458 (95% CI £1351 to £1566) in the intervention group and £1233 (95% CI £1171 to £1295) in the TAU group (see Appendix 26, Table 25).
Objective 9: estimate the probability that the intervention is cost-effective
The non-parametric bootstrapping produced a mean total cost per resident in the intervention group of £1479 (95% CI £757 to £2200), compared with £1271 (95% CI £975 to £1566) in the TAU group. The mean difference in cost between the intervention group and the TAU group was £208 (95% CI –£561 to £977), which was not statistically significant (see Appendix 26, Table 29).
Non-parametric bootstrapping after multiple imputation produced 0.315 (95% CI 0.304 to 0.326) QALYs in the intervention group and 0.298 (95% CI 0.290 to 0.307) QALYs in the TAU group, generating a mean difference in QALYs of 0.016 (95% CI 0.003 to 0.300), which was statistically significant. However, this finding should be interpreted with caution as this was a secondary outcome, we made multiple comparisons and this was not significant in the complete-case analysis for which missing values were not imputed (see Appendix 26, Table 29).
The incremental cost per QALY gained of the intervention, compared with TAU, was £12,633. Residents receiving the intervention accrued a non-significantly higher cost, and a very small increase in QALYs, which was statistically significant; the intervention has a 65% probability of being cost-effective at a WTP threshold of £20,000 and a 77% probability of being cost-effective at a WTP of £30,000 (see Appendix 26, Figure 6).
Objective 10: measure the completeness of data collection, completion of documentation and return rate of questionnaires
Care staff-related data
We were able to collect data on most of the recruited care staff at baseline (n = 132). For example, 129 (98%) care staff gave demographic details and 127 (96%) completed the P-CAT. All nurses (n = 40) completed the nurse–GP communication tool and 34 out of 40 (85%) completed the knowledge and skills tool. Subsequent attrition in response to these scales was secondary to nursing homes dropping out of the study, rather than staff being unwilling or unable to complete the forms.
Resident-related data
Demographic and functional ability data were available on most residents at baseline (n = 235), including gender (99%), ethnicity (95%), marital status (98%) and functional ability (Barthel Index) (98%). Data on years of education were more difficult to collect, as nursing home staff often did not know this about the residents. We cannot be sure that no admissions or visits to acute hospital were missed, as nursing homes did not have centralised systems for collecting these data.
At subsequent time points, one or two nursing homes from both intervention and TAU groups did not report some data. The number of data reported decreased over time, notwithstanding nursing home dropout.
Patient and public involvement
Aim
The aim of the PPI work in the BHiRCH-NH study was to:
-
collaboratively involve family carers of people living in nursing homes, or family carers of people with dementia, at all levels of and stages of the BHiRCH-NH study
-
use family carers’ and people with dementia’s experiences to inform the content and form of written and oral information given about the study and assist in interpretation of data.
Methods
We worked with two PPI co-applicants (SN and BWC), both people with experience of a relative living in a care home, who provided strategic and operational management of the PPI work in the study. Barbara Woodward-Carlton had been involved in the study RP-DG-0610-10034 (Developing an evidence-based intervention to improve health care for, and prevent avoidable hospital admission of, older care home residents with frailty or dementia).
The main way in which we facilitated PPI in the BHiRCH-NH study was through establishing two CRPs: one based in London (chaired by SN) and one group based in Yorkshire (chaired by BWC). We sent out initial invitations to members of the Alzheimer’s Society Research Networks in London and the South East; and Yorkshire, Humber and North Lincolnshire. Following initial meetings in London and Bradford, two groups, comprising seven and nine members, respectively, were convened. All family carers had personal experience, past and/or current, of supporting a family member living in a care home. We were also joined by two people living with dementia who attended the Yorkshire group for varying lengths of time; for one person with dementia, their deteriorating condition prevented their ongoing involvement. Each group met every 6 months, initially in the same month, and then ensuring that the project had input from PPI representatives every 3 months. The key areas of input concerned:
-
preparation of appropriate study documentation
-
provision of a family perspective in the PDC training
-
study recruitment
-
data analysis
-
report writing.
Resident involvement
We also sought to integrate the perspective of care home residents to inform how we introduced and implemented the intervention to the care home setting, with specific reference to a review of the study information materials for residents in the pilot trial.
We conducted an internal evaluation to review the process and outcomes of the PPI work by the CRPs. 80 The evaluation of the PPI involvement was conducted at two time points: baseline data were collected in January 2017 and data towards the end of study were collected in December 2018.
Further PPI representation was present on the National Institute for Health Research (NIHR) Programme Steering Committee (n = 1) and the International Advisory Group (n = 2).
Results
The PPI work contributed in several ways throughout the study development, management and completion.
Pre-funding preparation
Shirley Nurock and Barbara Woodward-Carlton initiated the focus on family involvement in health care in nursing homes. They were involved in writing the grant application, ensuring a prominent focus on family involvement. At this stage, we drew on our study experience of working with a CRP and expanded it to use two panels, one at each study site.
Supporting the study
There are five areas in which PPI was influential in the study’s delivery.
Preparation of appropriate study documentation
At meetings, we reviewed the following documents with panel members: project leaflet, participant information sheets, consent forms and the project handbook for the intervention homes. We received a number of suggestions as to how to make the wording more comprehensible and to avoid professional jargon. After the research team received feedback, we responded by identifying where we would make changes. In the event that we did not act on the suggestions, we explained our thinking to the CRP members. An example of information circulated back to the CRP members is shown in Appendix 27.
We held a meeting with seven care home residents and one activity co-ordinator in a nursing home in Lancashire, away from the sites for the feasibility and pilot trial, in May 2016. The residents specifically looked at the project leaflet. The discussion identified specific changes required to the document with respect to font size and the use of images that reflect UK settings and staff. In terms of its usefulness for residents, the residents suggested that the leaflet was more appropriate for family members because of the high level of communication challenges for nursing home residents.
Study recruitment
We offered CRP members the opportunity to attend launch events in the participating nursing homes at the start of the study. The aim was to:
-
offer a perspective on how family can play a role in detection in changes in health
-
offer a friendly face to residents and family members
-
ensure that the research was presented in an accessible way.
One launch event was attended by a CRP representative. Unfortunately, owing to staffing changes and the logistics of arranging dates with the nursing homes, it was not possible to involve CRP members further, despite their willingness to be involved and their subsequent disappointment.
Attendance at the Practice Development Champions’ training workshop
We ensured that one of the PPI leads attended each PDC training workshop (two workshops for the intervention homes and one workshop for the TAU homes). In this workshop, the PPI leads talked about the importance of family involvement in delivering the intervention. Prior to the PPI leads’ attendances at the training workshops, we discussed the role of families in the intervention at the CRP meeting. This material informed what the PPI representatives talked about, providing additional information to their personal accounts of experiences of care in nursing homes.
In addition, the CRP suggested that we change the terms used to describe the nursing home staff responsible for implementing the intervention in the nursing home. They found that the term ‘intervention champions’ was not clear. It was therefore agreed that we would refer to these nurses as PDCs. Consequently, it was also agreed that the ‘quality collaborative’ who support their work would be referred to as a PDSG.
Data analysis
We offered CRP members the opportunity to engage in data analysis of family carer interviews conducted during the pilot study. A training session on framework analysis was given to each CRP and then an analysis workshop was held, attended by six members. A further four members undertook analysis of the interviews from a distance. As a result of the workshop, an analytical template was developed, which was used initially by the research team to structure the analysis of the family carer interviews and also as a basis for the analysis of manager, PDC and care staff interviews.
Report writing, publications and dissemination
We have sought to write this report with Barbara Woodward-Carlton, Shirley Nurock and Katherine Froggatt working together. We offered the CRP members the opportunity to review a draft of the final report. This was undertaken by 11 people.
We have also supported dissemination activity by the PPI leads. PPI colleagues have written two articles for a lay audience about their involvement in the study in the Alzheimer’s Society Research newsletter (see Appendices 28 and 29).
The PPI leads have been co-authors on some study publications,66,81 took part in a presentation82 and took part in the BHiRCH-NH market stall at the Alzheimer’s Society Public Involvement workshop in 2017.
Evaluation of the patient and public involvement
Evaluation of the PPI work in the study was undertaken at two points, January 2017 and December 2018, using a survey questionnaire, based on the Public Involvement Impact Assessment Framework (PiiAF) approach. 80 In January 2017, we sent invitations to the 11 active CRP members. Eight members responded (seven females, one male; seven were a family member of a person living/lived in a care home and one was a person living with dementia). Their prior involvement in PPI ranged from 2 to > 20 years (not specified). This was an experienced PPI group, whose members had all been involved in other research studies or as monitors for the Alzheimer’s Society, with many advocating for and supporting care for people with dementia in their communities.
In December 2018, 11 evaluation forms were circulated to CRP members. The evaluation addressed the nature of involvement, what had gone well from a PPI perspective and what could have been improved, and asked for panel members to identify ways in which the PPI element influenced the outcomes of the study. Nine responses were returned.
Discussion
Patient and public involvement was integrated from the start and had informed the study. The approach of using a CRP, which had worked well in the Programme Development Grant (RP-DG-0610-10034), was continued in this study, and expanded to establish a panel at each study site. The plans for PPI in the project in both grant applications were seen as strengths at funding application stage and by the Queen Square Research Ethics Committee.
The areas where PPI made a difference to study outcomes from the study team perspective was with respect to study documentation, input into the training workshop for the PDCs and qualitative data analysis. We reviewed the study documentation with CRP members and with residents; through this process, we developed documentation that was clear and easy to read (see Appendix 27). The ‘wording of documentation and project products’ (London CRP member) was identified as an area in which the panel members had made a difference:
. . . I really enjoyed being a part of this project and felt that our contributions as a group were valuable to the team. I was thinking, in particular, in regard to the poster letting residents and carers know about the BHiRCH-NH project. They took on board what our feedback to the draft was; they appreciated our comments and were happy to change or amend accordingly.
London CRP member, 2018
In engaging with the CRP regarding data analysis of family carer interviews, we worked with it to develop a coding template that was used to structure the findings.
The process of PPI and how we engaged with PPI members were strengths of this study, identified by the PPI members themselves:
This has been a very worthwhile project. The PPI involvement has been integral (not always the case!) to the BHiRCH-NH team’s plans, and as a member of the London Carer Reference Panel I felt fully informed, appropriately consulted, and therefore valued, throughout. Our comments on all aspects of the project – whether work planning, the content and wording of documents, the analysis of data – were meticulously noted and taken into account. My direct experience as a carer of three close relatives in nursing homes, two of whom had probably avoidable hospitalisations due to advanced infections, meant that I felt able to contribute positively to this research.
London CRP member, 2018
The study team were able to engage with criticism and deficits identified by the PPI members:
All our voices are heard . . . Even the very critical comments taken note of and I feel the panel’s views are valued by the PPI lead.
London CRP member, 2018
There were some limitations in what we achieved with respect to recruitment and engaging with residents. One area where we did not manage to embed the PPI perspective was in study recruitment. The logistics of setting dates for family information events in the participating nursing homes and the availability of CRP members meant that this did not happen, except in one instance. This was a disappointment to the members.
Recruitment through the Alzheimer’s Society Research Network, although leading to the involvement of a committed and enthusiastic group of members, raised other challenges. Initially, members of that panel believed that the project was focused on dementia (as well as our four target conditions), so there was a need to ensure that we maintained a focus on all residents in nursing homes. There was also considerable discussion around inclusion of additional chronic conditions, which were out of the scope of the study. The decision to keep the focus on our four conditions led to one CRP member leaving the study. We resolved this issue by taking care to ensure that we provided clarity about the scope of decision-making required of panel members and the reasons underlying the conditions of interest in this study.
Our engagement with residents in nursing homes was limited. It was not possible to undertake longitudinal engagement with residents. Only one group was convened to look at one element of the information for potential resident participants. This experience showed us the challenges of working with residents with complex needs and the communication challenges that we were unable to meet.
We also had to tailor engagement with PPI members to reflect their other commitments, while providing timely input into the study progress. We recognised that some of the PPI colleagues had a heavy workload in terms of involvement with other research and non-research projects. To work around this, we sought to ensure that meetings were scheduled at appropriate times, and that we used other means of keeping colleagues informed of the work. We offered different ways to respond to consultations, offering face-to-face meetings and postal responses, to ensure that everyone could make contributions in a way that worked for them.
We initially held meetings with the London and Yorkshire CRPs within 1 week of each other. However, this resulted in unnecessary duplication of work, as we were receiving similar feedback from each group. We therefore agreed to stagger the timing of the CRPs. Meetings were then held every 3 months, alternating between the Yorkshire and London panels. Both panels were kept informed of the work and outcomes from each other’s meetings.
This reflection from a PPI co-applicant captures the overall feedback from panel members:
Certainly from my viewpoint and never having been on, let alone chaired, a Carer Reference Panel, it was a revelation as to what could be achieved by a group with common experiences of care homes. And great to see that the London panel developed into such a harmonious group, never afraid to express views, positive or negative, and clearly enjoying being on the panel. Indeed they are very sorry that BHiRCH-NH is drawing to its conclusion and would have cheerfully continued in their role!
London CRP member 2018
Reflections
The PPI element has been an important part of the research. The human and financial resource invested in the PPI work ensured that we were able to plan sustained engagement with our PPI advisers throughout the study. Investment in two CRPs meant that we were ensured input every 3 months. The experienced PPI members were able to confidently engage with the work asked of them. As a research team, we were continually challenged to ensure that our materials and briefing were clear and succinct, to ensure that we brought members up to date with study progress, through appropriate updates provided using both face-to-face and written formats.
In seeking PPI representation for this study, the Alzheimer’s Society Research Network Volunteers offered an established route to identify people with relevant experience for the study, but it was a challenge at times to ensure that the work was considered from a care home, rather than a dementia-specific, perspective. This was discussed at the final meeting and the benefits and challenges of this avenue for recruitment in this study were explored together.
We were requested to embed engagement with residents in the PPI work as well. This proved more challenging and it was not possible to sustain this beyond a single meeting. Further work needs to establish a viable forum for residents, one that can accommodate the significant frailty and changing nature of the membership of such a group.
Discussion
Key findings
In this study, we developed and tested a complex intervention for early detection, assessment and reporting of acute changes in residents’ health in UK nursing homes. We developed an evidence-based complex intervention and theory-informed implementation support, in collaboration with diverse stakeholders. The evidence-based complex intervention included:
-
an early warning tool to be completed by care assistants, with prompting by family carers, as appropriate
-
care pathways to guide the nurse with conducting and recording a detailed assessment
-
a tool to help nurses structure their report to primary care on acute changes in a resident’s health.
The theory-informed implementation support included:
-
identification of PDCs to introduce and embed the intervention (internal facilitation)
-
support for PDCs to introduce and embed the intervention, including a training workshop, knowledge and skills matrix, project handbook, web-based resources and monthly telephone coaching calls (external facilitation).
We developed the intervention and support for its implementation by engaging with the literature and collaborating with a wide variety of stakeholders using multiple methods, including semistructured interviews and consensus workshops. Stakeholders brought a range of personal and professional expertise. They included nursing home managers, nursing home nurses and care assistants, community nurses, GPs, acute care nurses, a geriatrician and a quality improvement lead.
We found a broad base of support for our focus on early detection, assessment and reporting. Stakeholders endorsed the reliance on both external and internal facilitation for introducing and embedding the intervention. The feasibility study in two nursing homes led to us simplifying the intervention, focusing our implementation support on the essential aspects, refining our approach to recruitment and reducing the burden of data capture.
We recruited sufficient numbers of nursing homes (n = 14), staff members (n = 148), family members (n = 95) and residents (n = 245). We retained a majority of those recruited. Monitoring of adverse events and qualitative data did not suggest that the intervention caused harm. There was little evidence of engagement with the intervention. Only 16 S&W forms were completed and the care pathway was used eight times over the 6-month post-intervention period. The S&W was mentioned by staff as being useful, even when it was not used to record observations. Both nurses and care assistants said they were already doing something similar to the intervention: care assistants said they already noticed early changes and reported them to nurses; nurses told us that they already conducted detailed assessment and often used the SBAR technique when communicating with primary care, as they had done prior to the BHiRCH-NH study. We saw limited engagement with the tools and the activities the tools required, and there was little family involvement.
The implementation strategy was only partially effective. Five of the six intervention homes identified two PDCs who attended the 1-day training workshop. Following the workshop, one of these homes withdrew from the study. PDCs shared information about the project with their teams and found the training and monthly calls helpful. Several felt the need for additional support, whether from management or the study team. At least one home had integrated the focus on early detection of changes in residents’ health into their daily resident review meeting and said they would continue to do so. Additional homes reported that they would continue to display posters about the S&W in residents’ rooms and in the nurses’ station.
The CRP worked with us throughout the study, advising on study information and data collection and engaging in data analysis and interpretation. Despite excellent recruitment and retention, the limited engagement with the intervention tools and support for their implementation in the pilot trial leads us to conclude that a definitive trial of this intervention is not warranted.
Interpretation of findings
We discuss interpretation of findings, first, with respect to the development of the intervention, and, second, with respect to the testing of the intervention.
Development of the intervention
It is surprising that, as we developed a complex intervention in collaboration with key stakeholders, the intervention had little traction. NIHR have stressed the importance of effective partnership-working as essential for practice development in nursing homes. 83 We explicitly sought end-user input into the design of the intervention and its implementation support.
It is possible that the collaborative approach we took did not fulfil many of the key tenets of co-production. 84 The intervention may have been more acceptable and effective had we developed it collaboratively with each nursing home. In this way, we could have addressed the specific issue any particular nursing home was facing, if any, with respect to avoidable hospital admissions. Such an approach would be closer to the ambitions of quality improvement, rather than research per se, albeit cognisant of its challenges. 85 An example of this is ‘My Home Life’ [http://myhomelife.org.uk (accessed 20 February 2020)], which focuses on working in partnership with care home providers to identify and collaborate on improving the quality of care in care homes. Furthermore, such an approach would have entailed closer collaboration with all levels of nursing home staff, including care assistants, domestic and estates staff. 86–88 Certainly, closer working with care assistants would have been of benefit, whether as advisors or participants. In retrospect, we had limited engagement with them, even though they play a key role in early detection.
In essence, we came to the consensus workshops with a proposed solution based on findings from our study, which we sought to refine, which suggests a power imbalance both in terms of the definition of the problem and its proposed solution. We succeeded in including all perspectives and skills, but perhaps would have benefited from having more detailed engagement from a reference/advisory group solely consisting of nursing home nurses and care assistants. We respected and valued each person’s knowledge. Indeed, we purposely sought stakeholders with expertise to complement our own. We did not assess stakeholders’ perspectives on what they got from engaging in consensus workshops, but attendance figures suggest that they derived benefit. Over the period of three consensus workshops, we had 17, 10 and 12 participants, respectively. The collaboration with the CRP has led to establishing close relationships within the panel and between the panel and the research team.
Although we applied a theory-informed approach to implementation, it is possible that, with additional external facilitation and better-resourced internal facilitation, we could have engaged in closer co-design of the implementation support. Furthermore, it may have been beneficial to offer different levels of support for different PDCs working in different contexts. PDCs did differ with respect to levels of support needed and acquired, whether from internal colleagues or external facilitation.
Despite working closely with a diverse range of stakeholders, we did not achieve our ambition to develop an intervention, the implementation of which would be led by the nursing home and adapted to each home’s context. Arguably, we imposed a solution from outside the nursing home, rather than identifying the problem and its potential solution from within.
Testing of the intervention
In testing the intervention, we examined engagement with the tools and views on the implementation support.
Limited engagement with tools
There are several possible reasons for the limited engagement with the BHiRCH-NH study tools. First, it is possible that, as reported, staff engaged with the principles of the tools, but did not use them to record their observations. It is noteworthy that using them to record observations was integral to our expectation of their use of the tools.
Second, it is possible that our nursing homes had no need for tools to guide them in early detection, detailed assessment or reporting because they had existing working practices that ensured early detection and assessment. There is some support for this, as evidenced by the low rates of hospitalisation and health service use in the sample. Furthermore, the intervention tools may duplicate existing records already used to note observations of changes in residents’ health. Staff may view existing approaches as meeting the aims of the project. This is consistent with several staff reporting that they were ‘already doing it’, whether in terms of care assistants noticing early signs or nurses conducting detailed assessments and using structured tools to report changes in residents’ health.
Third, it may be because the recruited nursing homes were atypical. The evidence we have on their CQC ratings suggest that these are an above-average sample of homes. Fourth, it is possible that the residents in the sample were atypical, in not being very frail and thus being less likely to develop any of the four index conditions. Certainly we had more residents who were able to consent for themselves than might be expected in a nursing home population. 89 Fifth, it is possible that the limited use of the intervention tools was because of the lack of potency of our support for implementation.
Limited engagement with implementation strategy
There are a variety of interdependent explanations for nursing homes’ limited engagement with the implementation strategy of establishing and supporting PDCs to lead practice change. We expected that PDCs would receive internal support from management and their PDSG and external facilitation from our co-applicants’ (KF and BM) telephone coaching.
First, there is some suggestion that the nursing home managers were not sufficiently visible in their endorsement of this new way of working. The need for effective leadership and support from management in achieving culture change is well established. 90–93 Although managers had signed up to participate in the study, there is some evidence that they may have seen it more as a research project requiring their co-operation with making data available, rather than a practice development project that required their sustained and visible leadership.
Second, nursing home managers may not have provided pragmatic support in terms of release from normal duties. Effective internal facilitation of change requires considerable resource,40,61,94,95 with at least one of these studies40 focusing explicitly on the implementation challenges of hospital use reduction programmes in nursing homes. A 2019 paper96 reporting on the implementation of a complex intervention in nursing homes provides a comprehensive overview of the barriers and facilitators at the level of the individual, the intervention and the care home. Barriers to implementation at the level of the care home included insufficient support from management with respect to staff workload allocation.
Third, we may have provided insufficient external support to PDCs to lead practice change. In our study, we had initially intended to provide PDCs with a 2-day training programme, but, following feedback from the homes that participated in the feasibility study, we agreed to a 1-day training workshop.
Although monthly coaching telephone calls were offered, their take-up was variable, usually low, suggesting that embedding this intervention was low priority or that it was conducted in the midst of competing priorities. It is likely that more face-to-face time with practice development coaches was needed, but this would both have to fit within the PDCs’ existing workloads and be affordable, should it prove to be effective and later commissioned by the CCG.
Fourth, it is possible that the PDCs lacked the knowledge and skills of facilitation to bring about quality improvement in these complex environments. Nurses may be less skilled in how adults learn, how change happens and how to use evidence to effect change than expected. We have to ask is it reasonable to expect staff in the care home to bring about complex change. Nurse-led initiatives are reported in the literature employing a variety of strategies. 97,98
One of the reasons we were keen to have internally led facilitation for introducing and embedding the intervention tools was to ensure the sustainability of the intervention outside the trial. We had viewed the internal facilitator role as twofold: facilitating introduction and embedding of the quality improvement project, and increasing the knowledge, skills and expertise of staff to continue that improvement over time, to achieve sustainability. Without sufficient investment in either internal or external facilitation, we have to ask if it is possible to bring about the kinds of changes we sought. 61
Active management of health-care needs and reducing rates of hospitalisation from nursing homes needs to be seen in the broader context of the drive to improve the quality of life and quality of care in care homes and nursing homes. This has been a consistent area of focus for at least 10 years. 99–107 In England, the CQC108,109 has raised consistent concerns about the quality of care in nursing homes. Quality of care in care homes is a priority for the National Quality Board and the National Commissioning Board. Increasing research in care homes was an explicit aim in the Prime Minister’s Challenge on Dementia 2020. 100 It is also a key aim of NIHR. 83
It is noteworthy that we are not alone in finding insufficient engagement with an intervention designed to improve the quality of care in care homes. Care homes are challenging environments, not just for conducting research,83 but also for the staff working in them. 110–112 A recent trial funded by the NIHR Health Technology Assessment programme [Enhancing Person-centred care In Care homes (EPIC)]113 also relied on staff in the home leading the intervention, with facilitation from an external expert. The team found suboptimal implementation of the intervention and, perhaps not surprising, no benefit on the primary outcome of agitation. 113 In exploring the barriers to and facilitators of adopting the intervention, the team found that these were not just a function of the nature of the intervention, but also functioned at the level of the individual and the care home. 96 This reminds us of the many layers of influence on achieving effective ways to introduce and embed complex new ways of working.
A recent NIHR-funded [Well-being and Health for people with Dementia (WHELD)] study114–118 provides further evidence for the need for sufficiently resourced and targeted external facilitation to achieve positive benefits. In the WHELD study, a therapist provided coaching, supervision and regular review over a 9-month period. This achieved positive effects, albeit modest, on resident outcomes of quality of life, agitation and neuropsychiatric symptoms.
Although in this study we provided some external facilitation, it may have been insufficient to effectively support the internal facilitators, namely the PDCs. For example, the training workshop might have been better as 2 days with follow-on in-person days to support a community of practice approach to implementation. We offered 1-day training in response to feedback from nursing home staff. We were aware of the challenge of requiring attendance at a 2-day programme with follow-up days for a staff group already under-resourced.
In the USA, the Green House119 is recognised as one of the most comprehensive and standardised models of quality care and quality of life in nursing homes, and is arguably the most rigorously researched. Bowers et al. 119 developed a conceptual model to explain differences in the care processes associated with homes that have high numbers of hospital admissions and homes that have low numbers of hospital admissions. They describe the tension between achieving the aims of person-centred care (e.g. a homely, democratic environment) and the goal of achieving quality clinical outcomes (e.g. prompt care for early changes in a resident’s health). There is a risk that our intervention, which involved an extra burden of observation and documentation, may challenge staff who are already pressed for time and detract from the delivery of person-centred care. Longo et al. 120 have also studied the process of illness identification and have identified factors that promote or delay such identification. Uppermost among these are communication and teamwork.
Recent quality improvement programmes have sought to introduce advance care planning into care homes, especially for frail, older people living with dementia. 121 Kezirian et al. 121 describe a practice improvement initiative with 15 care homes in Canada over a period of 6 months. They succeeded in ensuring that the homes engaged in routine data collection of key outcomes in order to inform practice change. They pointed to the need to have a critical mass of clinicians engaged in plan–do–study–act cycles to effect change in practice. 121–124 Other examples of effective partnerships include the Centre for Nursing Innovation’s teaching care home initiative [www.fons.org/programmes/teaching-care-homes (accessed 20 February 2020)] and an increasing range of collaborations between NHS organisations and care homes to develop practice, create more person-centred cultures and maintain residents’ health.
Study successes
We have contributed to research in UK nursing homes, identified as lacking by NIHR. 83 Our successes centre on working with a diverse range of stakeholders to build and sustain relationships in the field. This led to excellent recruitment and retention. We demonstrated constructive engagement with diverse stakeholders throughout the development of the intervention. Most notable has been the valuable contribution from CRPs in both London and Yorkshire over the study period. Their largely positive reports of engagement are probably due to the relationships that have built up over the past few years, not just between the research team and the panel, but also within the panel itself.
The effective recruitment of nursing homes, residents, staff and family carers was due to several interdependent factors. We emphasised relationship-building in training our fieldwork staff. We had assistance from the CRNs local to both recruitment sites. CRN colleagues’ pre-existing relationships with field sites facilitated their consent to participate. In addition, given its policy priority, the topic was of great interest to homes. Our recent dissemination event was oversubscribed, suggesting that this remains an area of care practice of concern to the sector.
The excellent retention of homes and consented participants was, in part, due to the fieldworkers’ visibility and positive presence. On a monthly basis, they gathered data in each home. At our recent dissemination event, one of the managers noted how it enhanced the home to have fieldworkers present. She thought it brought fresh energy into the home and served as a stimulant for both residents and staff. An additional factor that helped with data collection was our innovative approach to asking homes to identify someone on their staff (a research facilitator) who would facilitate access to data, including care home records, for consented residents. Managers, staff and family carers endorsed the importance of the study’s focus. This has no doubt helped in recruitment and retention.
Through these innovative and effective approaches to recruitment, retention and data collection, this study made a significant contribution to the field of care home research. 83 This study has contributed to the move to redress the unacceptably low levels of research into the care of the frailest and most vulnerable members of society. Despite the significant number of older people living in nursing homes, there is relatively little research conducted in these settings. Our study was part of the initiative to ensure parity of hospital and care home research. We have contributed to the NIHR mission of identifying effective ways to improve the care and experience of nursing home residents. Furthermore, we have been successful in addressing NIHR’s aim of building effective partnerships between all professions involved in care for residents: health and social care; and researchers, care providers and family carers.
These findings can add to the guidance from NIHR to support better research in care homes [see ENRICH: www.enrich.nihr.ac.uk (accessed 20 February 2020)]. In particular, we can guide future researchers in addressing the challenges to conducting research in care homes. 125 We have demonstrated effective partnership working across a range of stakeholders, identified by NIHR as essential for practice development in care homes. 83 This programme has successfully married theory and research with the expertise and real-world concerns and experiences of care providers and family carers. As a result, it directly addresses the aim of NIHR to design, conduct and disseminate applied research in collaboration with the full range of people affected. 126,127
Study challenges
The key challenges centre on the lack of engagement with the intervention tools and the variable engagement with the support provided for their implementation. It is possible that, since we began this study in spring 2015, the focus of policy and practice development on reducing hospitalisations and enhanced health care in care homes has led to significant levels of activity. NHS England’s demonstration projects (vanguards) and other quality improvement initiatives mean that usual care will be improving, making trials challenging to conduct. Although this level of activity is to be celebrated, it does make conducting research in such a fast-moving landscape challenging. For example, the appetite to try anything that might help with reducing hospitalisations meant that our presentation to the CCG in one fieldwork site resulted in the study tools being sent to all care home managers in that site. This meant that we needed to identify additional sites for recruitment.
It is possible that the intervention nursing homes were not ready to engage in a change research project. Goodman et al. 128 describe 10 essential readiness characteristics:
-
fit with care home priorities
-
senior management support
-
staff have time to do change
-
ongoing processes for change present
-
level of recent changes
-
identified champions
-
pre-existing relationships in place
-
perception of change as positive
-
fit with current training and work patterns
-
tailored to current processes.
From our interviews, we can say that there was widespread support for the importance of early detection and assessment in the homes. Although we identified champions, senior management support was not always evident, nor were the champions always allocated time to lead and embed the change. Arguably, we were not sufficiently aware of how the intervention and its implementation support could be tailored to current processes, training and work patterns. For this we perhaps relied too heavily on the PDCs without providing sufficient support.
A further and related challenge was the ineffective communication within some of the homes regarding the project. Although PDCs reported informing their staff about the project using a variety of approaches, it is noteworthy that, during qualitative interviews, many staff spoke about the S&W as though it was the only element of the BHiRCH-NH study intervention.
The intervention was developed in 2015, solely based on evidence available at that time around upskilling and developing the role of nursing home staff in delivering enhanced health care and, as such, was monodisciplinary. Key components for resident health care, identified in the 2017 Optimal49 study were GP involvement supported by integrated external services. Further evidence from a realist review of Comprehensive Geriatric Assessment in care homes published in 2019,51 after our study had been completed, suggests that the engagement of a multidisciplinary team that uses care planning as a mechanism to seek external specialist support is required to make Comprehensive Geriatric Assessment work.
Study limitations
The study limitations centre on being unable to confirm the veracity of the data, the suitability of a cluster RCT for a quality improvement initiative of this kind and an intervention that focused solely on nursing home staff. With respect to veracity of the data, we have no way of checking the data gathered. For example, nurses rated their knowledge and skills regarding early detection of health conditions and their communication with primary care more highly than would be expected. This may reflect a growth in knowledge and skills in the field or perhaps a case of nurses not knowing what they do not know.
We gathered data on nursing home characteristics, including residents and beds, hospital attendances, staffing and support from external health-care services. We cannot, however, be sure that information has not been missed, as it is difficult to cross-check this against independent records. Most nursing homes did not keep logs of resident admissions or ambulance and GP call-outs.
It is possible that the cluster RCT design was too constraining for practice development of this nature. 129,130 Had we been engaged in quality improvement, we could have worked in closer partnership with each home to better understand the problem(s) they had with respect to hospitalisations; to co-design potential solutions; and, perhaps most importantly, to co-design implementation strategies to ensure embedding of these solutions. 85 During the feasibility study, nursing home staff were unable to make themselves available to engage in semistructured interviews. This may have limited our understanding of the feasibility and acceptability of the intervention at that stage. We have some evidence that participating homes saw themselves as involved in research rather than involved in practice development; as a result, they may not have seen a need for local ownership of, nor commitment to, introducing and embedding the intervention. It is well documented that interventions designed and implemented for research purposes do not always make their way into mainstream practice. 131–133 An alternative research design to the cluster RCT may be more appropriate. For example, Estabrooks et al. ,131,132,134,135 Rycroft-Malone et al. 133 and Cranley et al. 136 have used research evidence feedback to care home staff to increase care quality. In this study, we drew on the PARiHS framework to support staff in introducing and embedding the intervention.
The intervention required changes in staff behaviour and practice; it did not consider the wider health and social care system surrounding the nursing home, nor how the nursing home staff and residents interact and relate with this. The relationship of the care home manager with their local GP is particularly important,137 as this facilitates anticipatory care and nursing home staff and GPs to establish common goals for the care of the residents. 138 Although we collected data on whether or not care homes were served by multiple or single general practices, we did not collect more fine-grained information on the quality of this relationship that may have influenced residents’ health care. Had we continued with our intended use of the Context Assessment Index in the pilot trial, we would have a better understanding of how the context affected nursing home practices. Furthermore, these data would have helped us identify distinguishing features of homes that demonstrated some indication of sustainability.
We drew on the PARiHS framework to develop our approach to implementation. It is possible that engaging with the behaviour change literature, such as the capability, opportunity and motivation behaviour (COM-B) model,139 may have added to our understanding of the change process.
Implications for future research
Future research should address the following questions.
What are effective approaches to ensure that NHS and care home staff work collaboratively?
Although our study was conducted within nursing home, more attention needs to be paid to effective collaboration between the NHS and care homes. In this study, we wanted to improve nursing home staff detection, assessment and reporting of changes in residents’ health.
What is the extent of the problem of avoidable admissions from care and nursing homes?
We need to establish hospitalisation rates for all conditions from nursing homes and care homes in the UK. This will enable future studies to target high-admitting homes and the conditions that lead to these high rates of admission. In this study, we focused on ACSCs, yet found relatively low hospitalisation rates for these conditions. This may be because of the lack of routine data collection in nursing homes and under-reporting of acute hospital admissions. New automated methods have recently been developed and may give a more reliable and scalable technique to assess nursing home resident acute hospital resource use. 140 Since we designed this intervention study, the research field has moved forward and routinely collected health and social care data have been used to give insight into the effects of interventions that aim to improve care. For example, the Health Foundation briefing on the Rushcliffe vanguard project46 linked care home data with Hospital Episode Statistics to conduct a matched comparison study between residents who received the enhanced health-care model and similar individuals residing in similar care homes in comparable areas of England. Such data are less subject to the possible recruitment bias found in RCTs and give a ‘real-world’ picture of change. They do, however, have disadvantages. For example, the data are based on aggregate service-level data and do not give information on contextual variables or on what the ‘active ingredient’ of the new intervention may be.
In addition, we need to establish the extent to which care homes problematise detection and recurrent hospital admissions, as the NHS does.
What are the current processes of care for residents who experience deterioration in their health?
We need a better understanding of the processes of care for residents who experience ACSCs that result in hospitalisation and for those who experience ACSCs that do not result in hospitalisation. Bowers et al. 119 used a qualitative grounded theory to develop a conceptual model to explain common and divergent (from the Green House model) care practices. Such an approach could be used to propose a conceptual model to explain differences in care processes between homes with low admission rates and homes with high admission rates in the UK.
Future research would benefit from a breadth of data, including care record reviews, participant or non-participant observation and interviews with key personnel. Further work might also usefully consider the value of nationally standardised record formats for early detection and assessment in nursing homes, which would reduce variation around what is recorded in everyday practice.
What are the important contextual variables to consider in achieving early detection?
Bowers et al. 119 remind us of a host of contextual variables to consider when seeking to establish and embed care processes associated with low rates of hospital admission. These include having opportunities to communicate, and having a culture of taking advantage of such opportunities. Small-scale housing models of nursing home care provide these more easily than large-scale provision. Opportunities to communicate include communication with the full range of stakeholders, including residents, family carers, all levels of nursing home staff and visiting health professionals, including primary care.
The legitimate role and involvement of care assistants is a particularly important contextual variable. Bowers et al. 119 argue that the notion of ‘empowering’ care assistants needs to be tempered with them being accountable to a care team. Future research should, at a minimum, gather data on the primary care context and the role of care assistants in health care in each home. Describing the primary care context would include describing the NHS service delivery model, whether weekly clinics or individual GPs visiting individual residents. Accessing their perspective on the adequacy and completeness of nurse reports of resident ill health would be an important perspective not sufficiently included in the current study.
What resources are needed for internal and external facilitation of change?
We need to identify the resources required to ensure effective support for both external and internal facilitation of change. 61,113 With respect to externally supported facilitation, although previous studies have attempted to determine the optimal dose and mechanism (e.g. Seers et al. 95), further studies need to adequately resource internal–external facilitator relationships. With respect to internal facilitation, research could identify the human resources (personal qualities and professional knowledge and skills regarding facilitation of change), as well as the time required, for leadership in introducing and embedding change in care practice.
Might this intervention be useful to residential care homes?
We found a lower than expected rate of admission to acute hospitals in our nursing home sample. This may be because these are not documented or may reflect very recent evidence that the overall rate of admissions from nursing homes is lower than that found in residential care homes. 141 Our intervention may be more suitable in residential care homes, where there is no on-site nursing care and more potential to reduce emergency care use, something suggested during interviews with nursing home staff. 142
Victor et al. 10 query whether or not residential care home staff have the expertise that residents assume they have: expertise in both noticing changes in health and determining whether or not they warrant medical attention. Residents relied on care home staff (less commonly family) as intermediaries in arranging access to GPs. Almost half expected that care home staff would monitor their health; knew them sufficiently well to notice health changes; and would initiate help from the GP, without prompting by the resident. They conclude that care home staff may need support in fulfilling this ascribed role of day-to-day monitoring of residents’ health. They argue that more attention needs to be paid to this aspect of care home staff training and that we need evidence about the best ways to equip care home staff in this area. We sought to support nursing home staff in detecting and reporting early signs of health deterioration in residents. Some of our interviewees and several of the participants at our dissemination event mentioned this.
During the interviews conducted as part of the process evaluation, the S&W was frequently mentioned. It is possible that using this tool in combination with family involvement and knowledge and skills enhancement may prove useful, particularly in residential care homes. 142 This focus could be enhanced by adopting approaches used in the growing body of research on implementing advance care planning in care homes (e.g. see Kezirian et al. 121). Our intervention, however, was not succesfully implemented in any of the participating nursing homes; therefore, we cannot assume that implementation would be successfull in residential care homes.
What is the cost to care homes and nursing homes of reducing hospital admissions?
Future research incorporating economic models of hospital reduction programmes should measure the cost of care to the care home.
How can we effectively engage in co-production of interventions and implementation strategies with diverse stakeholders?
To optimise impact, we need to facilitate engagement in co-production of applied research with the full range of stakeholders, for example care and nursing homes, GPs and paramedics. 84 This includes assessing care home readiness and capacity to engage, including releasing all levels of staff to engage in intervention design. 143
Acknowledgements
The authors thank the care home managers, nurses, care assistants, residents and family carers for participating in this project.
We are also grateful for the input and support received from the project’s CRPs, International Advisory Group and Programme Steering Committee.
Contributions of authors
Murna Downs (https://orcid.org/0000-0003-3062-5223) (Chair in Dementia Studies) was the chief investigator and led WS1 to develop the intervention.
Alan Blighe (https://orcid.org/0000-0002-1016-5218) (Programme Manager 2015–18) carried out recruitment, retention, data collection and analysis.
Robin Carpenter (https://orcid.org/0000-0002-8845-7819) (Senior Data Manager) set up and monitored the project databases.
Alexandra Feast (https://orcid.org/0000-0001-9072-3023) (Research Fellow) conducted WS1 and WS2 recruitment, retention, data collection and analysis, and prepared the ethics application.
Katherine Froggatt (https://orcid.org/0000-0003-0339-3877) (Chair in End of Life Care) co-led the PPI, co-led the implementation support, co-facilitated training workshop for PDCs, offered monthly telephone coaching calls to PDCs, and provided expert input regarding qualitative analysis.
Sally Gordon (https://orcid.org/0000-0003-1866-2857) (Research Nurse) contributed to the clinical record review.
Rachael Hunter (https://orcid.org/0000-0002-7447-8934) (Associate Professor, Health Economics) led the economic analysis.
Liz Jones (https://orcid.org/0000-0003-3832-1101) (Programme Manager 2018–19) contributed to the clinical record review.
Natalia Lago (https://orcid.org/0000-0002-4820-7567) (Senior Trial Manager) ensured robust trial management and monitoring systems were in place.
Brendan McCormack (https://orcid.org/0000-0001-8525-8905) (Head of the Divisions of Nursing, Occupational Therapy and Arts Therapies, Associate Director Centre for Person-centred Practice Research, Vice President Omega Xi Chapter Sigma Global) co-led WS1, WP3 regarding implementation support, co-facilitated the training workshop for PDCs and offered monthly telephone coaching calls to PDCs.
Louise Marston (https://orcid.org/0000-0002-9973-1131) (Principal Research Associate) developed the analysis plan for the pilot study and carried out the statistical analysis of the feasibility and pilot studies.
Shirley Nurock (Alzheimer’s Society Research Network Volunteer) co-led the PPI, chaired the London CRP and engaged in dissemination.
Monica Panca (https://orcid.org/0000-0002-4031-6478) (Health Economist) carried out the health economics analysis of the feasibility study and pilot study.
Helen Permain (https://orcid.org/0000-0001-9279-6728) (Research Nurse) contributed to the clinical record review.
Catherine Powell (https://orcid.org/0000-0001-7590-0247) (Research Fellow) conducted WS1 and WS2 recruitment, retention, data collection and analysis, conducted interviews and data analysis regarding process evaluation and interviews with family carers and was the lead author on a paper66 about family involvement.
Greta Rait (https://orcid.org/0000-0002-7216-7294) (Professor of Primary Care and Health Services Research; Joint Director Priment CTU) was the triallist for the WS2 feasibility and pilot studies.
Louise Robinson (https://orcid.org/0000-0003-0209-2503) (Professor of Primary Care and Ageing) co-led WS1, WP1 regarding developing care pathway.
Barbara Woodward-Carlton (Alzheimer’s Society Research Network Volunteer) co-led the PPI, chaired the Yorkshire CRP and engaged in dissemination.
John Wood (https://orcid.org/0000-0003-2334-3172) (Statistician) developed an analysis plan for the project data.
John Young (https://orcid.org/0000-0003-4085-9306) (Professor of Elderly Care Medicine) co-led WS1, WP1 regarding developing the care pathway, and led the clinical record review.
Elizabeth Sampson (https://orcid.org/0000-0001-8929-7362) (Professor of Dementia and Palliative Care and Old Age Psychiatrist) led the WS2 pilot study and was the lead author on the trial protocol and trial results papers. 73,81
All authors provided a critical review and final approval of the report.
Publications
Powell C, Blighe A, Froggatt K, McCormack B, Woodward-Carlton B, Young J, et al. Family involvement in timely detection of changes in health of nursing homes residents: a qualitative exploratory study. J Clin Nurs 2018;27:317–27.
Sampson EL, Feast A, Blighe A, Froggatt K, Hunter R, Marston L, et al. Evidence-based intervention to reduce avoidable hospital admissions in care home residents (the Better Health in Residents in Care Homes (BHiRCH) study): protocol for a pilot cluster randomised trial. BMJ Open 2019;9:e026510.
Sampson EL, Feast A, Blighe A, Froggatt K, Hunter R, Marston L, et al. Pilot cluster randomised trial of an evidence-based intervention to reduce avoidable hospital admissions in nursing home residents (Better Health in Residents of Care Homes with Nursing—BHiRCH-NH Study). BMJ Open 2020;10:e040732.
Data-sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- The King’s Fund . Enhanced Health Care in Care Homes 2017. www.kingsfund.org.uk/projects/enhanced-health-care-homes (accessed 20 February 2020).
- Care Quality Commission . State of Care Report 2018. https://webarchive.nationalarchives.gov.uk/20190112070317/https://www.cqc.org.uk/publications/major-report/state-care (accessed on 19 October 2020).
- British Geriatrics Society . Failing the Frail: A Chaotic Approach to Commissioning Healthcare Services for Care Homes 2012.
- McDougall FA, Matthews FE, Kvaal K, Dewey ME, Brayne C. Prevalence and symptomatology of depression in older people living in institutions in England and Wales. Age Ageing 2007;36:562-8. https://doi.org/10.1093/ageing/afm111.
- British Geriatrics Society . Quest for Quality. British Geriatrics Society Joint Working Party Inquiry into the Quality of Healthcare Support for Older People in Care Homes: A Call for Leadership, Partnership and Quality Improvement 2011.
- Dudman J, Meyer J. Understanding residential home issues to meet health-care needs. Br J Community Nurs 2012;17:434-8. https://doi.org/10.12968/bjcn.2012.17.9.434.
- Baylis A, Perks-Baker S. Enhanced Health in Care Homes: Learning from Experiences so Far. London: The King’s Fund; 2017.
- NHS England . NHS Outcomes Framework 2016 to 2017 2016.
- NHS England . NHS Outcomes Framework 2017–2018 2017.
- Victor C, Davies S, Dickinson A, Morbey H, Masey H, Gage H, et al. ‘It just happens’. Care home residents’ experiences and expectations of accessing GP care. Arch Gerontol Geriatr 2018;79:97-103. https://doi.org/10.1016/j.archger.2018.08.002.
- Bowman CE, Elford J, Dovey J, Campbell S, Barrowclough H. Acute hospital admissions from nursing homes: some may be avoidable. Postgrad Med J 2001;77:40-2. https://doi.org/10.1136/pmj.77.903.40.
- Davies SL, Goodman C, Bunn F, Victor C, Dickinson A, Iliffe S, et al. A systematic review of integrated working between care homes and health care services. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-320.
- Glendinning C, Jacobs S, Alborz A, Hann M. A survey of access to medical services in nursing and residential homes in England. Br J Gen Pract 2002;52:545-8.
- Iliffe S, Davies SL, Gordon AL, Schneider J, Dening T, Bowman C, et al. Provision of NHS generalist and specialist services to care homes in England: review of surveys. Prim Health Care Res Dev 2016;17:122-37. https://doi.org/10.1017/S1463423615000250.
- Grabowski DC, O’Malley AJ, Barhydt NR. The costs and potential savings associated with nursing home hospitalizations. Health Aff 2007;26:1753-61. https://doi.org/10.1377/hlthaff.26.6.1753.
- Carter MW, Porell FW. Vulnerable populations at risk of potentially avoidable hospitalizations: the case of nursing home residents with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2005;20:349-58. https://doi.org/10.1177/153331750502000605.
- Tian Y, Dixon A, Gao H. Emergency Hospital Admissions for Ambulatory Care-sensitive Conditions: Identifying the Potential for Reductions. London: The King’s Fund; 2012.
- Bardsley M, Blunt I, Davies S, Dixon J. Is secondary preventive care improving? Observational study of 10-year trends in emergency admissions for conditions amenable to ambulatory care. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002007.
- Hospital Episode Statistics Analysis Team . Counts of Finished Admission Episodes (FAEs) With an Emergency Admission by Source of Admission from 2009–10 to 2015–16 2016.
- Walsh EG, Wiener JM, Haber S, Bragg A, Freiman M, Ouslander JG. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home- and community-based services waiver programs. J Am Geriatr Soc 2012;60:821-9. https://doi.org/10.1111/j.1532-5415.2012.03920.x.
- Purdy S, Griffin T, Salisbury C, Sharp D. Ambulatory care sensitive conditions: terminology and disease coding need to be more specific to aid policy makers and clinicians. Public Health 2009;123:169-73. https://doi.org/10.1016/j.puhe.2008.11.001.
- Krüger K, Jansen K, Grimsmo A, Eide GE, Geitung JT. Hospital admissions from nursing homes: rates and reasons. Nurs Res Pract 2011;2011. https://doi.org/10.1155/2011/247623.
- Ouslander JG, Diaz S, Hain D, Tappen R. Frequency and diagnoses associated with 7- and 30-day readmission of skilled nursing facility patients to a nonteaching community hospital. J Am Med Dir Assoc 2011;12:195-203. https://doi.org/10.1016/j.jamda.2010.02.015.
- Ouslander JG, Lamb G, Tappen R, Herndon L, Diaz S, Roos BA, et al. Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. J Am Geriatr Soc 2011;59:745-53. https://doi.org/10.1111/j.1532-5415.2011.03333.x.
- Alessi CA, Harker JO. A prospective study of acute illness in the nursing home. Aging 1998;10:479-89. https://doi.org/10.1007/BF03340162.
- Jeon B, Tamiya N, Yoshie S, Iijima K, Ishizaki T. Potentially avoidable hospitalizations, non-potentially avoidable hospitalizations and in-hospital deaths among residents of long-term care facilities. Geriatr Gerontol Int 2018;18:1272-9. https://doi.org/10.1111/ggi.13458.
- Mukadam N, Sampson EL. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr 2011;23:344-55. https://doi.org/10.1017/S1041610210001717.
- Watkin L, Blanchard MR, Tookman A, Sampson EL. Prospective cohort study of adverse events in older people admitted to the acute general hospital: risk factors and the impact of dementia. Int J Geriatr Psychiatry 2012;27:76-82. https://doi.org/10.1002/gps.2693.
- Smith P, Sherlaw-Johnson C, Ariti C, Barsley M. Quality Watch. Focus on: Hospital Admissions from Care Homes. London: Health Foundation and Nuffield Trust; 2015.
- Care Quality Commission . Cracks in the Pathway: People’s Experiences of Dementia Care As They Move Between Care Homes and Hospitals 2014.
- Ouslander JG, Perloe M, Givens JH, Kluge L, Rutland T, Lamb G. Reducing potentially avoidable hospitalizations of nursing home residents: results of a pilot quality improvement project. J Am Med Dir Assoc 2009;10:644-52. https://doi.org/10.1016/j.jamda.2009.07.001.
- Ouslander JG, Berenson RA. Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med 2011;365:1165-7. https://doi.org/10.1056/NEJMp1105449.
- Lisk R, Yeong K, Nasim A, Baxter M, Mandal B, Nari R, et al. Geriatrician input into nursing homes reduces emergency hospital admissions. Arch Gerontol Geriatr 2012;55:331-7. https://doi.org/10.1016/j.archger.2011.10.014.
- Codde J, Arendts G, Frankel J, Ivey M, Reibel T, Bowen S, et al. Transfers from residential aged care facilities to the emergency department are reduced through improved primary care services: an intervention study. Australas J Ageing 2010;29:150-4. https://doi.org/10.1111/j.1741-6612.2010.00418.x.
- Kane RL, Keckhafer G, Flood S, Bershadsky B, Siadaty MS. The effect of Evercare on hospital use. J Am Geriatr Soc 2003;51:1427-34. https://doi.org/10.1046/j.1532-5415.2003.51461.x.
- Mor V, Intrator O, Fries BE, Phillips C, Teno J, Hiris J, et al. Changes in hospitalization associated with introducing the Resident Assessment Instrument. J Am Geriatr Soc 1997;45:1002-10. https://doi.org/10.1111/j.1532-5415.1997.tb02973.x.
- Young Y, Inamdar S, Dichter BS, Kilburn H, Hannan EL. Clinical and nonclinical factors associated with potentially preventable hospitalizations among nursing home residents in New York State. J Am Med Dir Assoc 2011;12:364-71. https://doi.org/10.1016/j.jamda.2010.03.006.
- Rantz MJ, Popejoy L, Vogelsmeier A, Galambos C, Alexander G, Flesner M, et al. Reducing avoidable hospitalizations and improving quality in nursing homes with APRNs and interdisciplinary support: lessons learned. J Nurs Care Qual 2018;33:5-9. https://doi.org/10.1097/NCQ.0000000000000302.
- Olsan T, Sweet R, Tourje N, Biuso J. Reducing avoidable rehospitalisations by improving residents’ transitions to the nursing home 2011;12. https://doi.org/10.1016/j.jamda.2010.12.071.
- Kane RL, Huckfeldt P, Tappen R, Engstrom G, Rojido C, Newman D, et al. Effects of an intervention to reduce hospitalizations from nursing homes: a randomized implementation trial of the INTERACT program. JAMA Intern Med 2017;177:1257-64. https://doi.org/10.1001/jamainternmed.2017.2657.
- Ouslander JG, Lamb G, Perloe M, Givens JH, Kluge L, Rutland T, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: [see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp. 760–761]. J Am Geriatr Soc 2010;58:627-35. https://doi.org/10.1111/j.1532-5415.2010.02768.x.
- Bonner A, Tappen R, Herndon L, Ouslander J. The INTERACT Institute: Observations on Dissemination of the INTERACT Quality Improvement Program Using Certified INTERACT Trainers. Gerontologist 2015;55:1050-7. https://doi.org/10.1093/geront/gnu103.
- Rantz MJ, Popejoy L, Vogelsmeier A, Galambos C, Alexander G, Flesner M, et al. Impact of advanced practice registered nurses on quality measures: the Missouri Quality Initiative Experience. J Am Med Dir Assoc 2018;19:541-50. https://doi.org/10.1016/j.jamda.2017.10.014.
- NHS . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 17 October 2020).
- Royal College of Physicians . The Health and Care of Older People in Care Homes: Report of a Joint Working Party of the Royal College of Physicians, the Royal College of Nursing and the British Geriatrics Society 2000.
- Lloyd T, Wolters A, Steventon A. Briefing: The Impact of Providing Enhanced Support for Care Home Residents in Rushcliffe n.d. www.health.org.uk/sites/default/files/IAURushcliffe.pdf (accessed 20 February 2020).
- Sherlaw-Johnson C, Crump H, Curry N, Paddison C, Meaker R. Transforming Health Care in Nursing Homes: An Evaluation of a Dedicated Primary Care Service in Outer East London n.d. www.nuffieldtrust.org.uk/research/transforming-health-care-in-nursing-homes-an-evaluation-of-a-dedicated-primary-care-service-in-outer-east-london.
- Devi R, Meyer J, Banerjee J, Goodman C, Gladman JRF, Dening T, et al. Quality improvement collaborative aiming for Proactive HEAlthcare of Older People in Care Homes (PEACH): a realist evaluation protocol. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-023287.
- Goodman C, Davies SL, Gordon AL, Dening T, Gage H, Meyer J, et al. Optimal NHS service delivery to care homes: a realist evaluation of the features and mechanisms that support effective working for the continuing care of older people in residential settings. Health Serv Deliv Res 2017;5. https://doi.org/10.3310/hsdr05290.
- Gage H, Dickinson A, Victor C, Williams P, Cheynel J, Davies SL, et al. Integrated working between residential care homes and primary care: a survey of care homes in England. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-71.
- Chadborn NH, Goodman C, Zubair M, Sousa L, Gladman JRF, Dening T, et al. Role of comprehensive geriatric assessment in healthcare of older people in UK care homes: realist review. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026921.
- Gordon AL, Goodman C, Davies SL, Dening T, Gage H, Meyer J, et al. Optimal healthcare delivery to care homes in the UK: a realist evaluation of what supports effective working to improve healthcare outcomes. Age Ageing 2018;47:595-603. https://doi.org/10.1093/ageing/afx195.
- Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care 1998;7:149-58. https://doi.org/10.1136/qshc.7.3.149.
- Harvey G, Loftus-Hills A, Rycroft-Malone J, Titchen A, Kitson A, McCormack B, et al. Getting evidence into practice: the role and function of facilitation. J Adv Nurs 2002;37:577-88. https://doi.org/10.1046/j.1365-2648.2002.02126.x.
- Kitson AL, Rycroft-Malone J, Harvey G, McCormack B, Seers K, Titchen A. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci 2008;3. https://doi.org/10.1186/1748-5908-3-1.
- McCormack B, Kitson A, Harvey G, Rycroft-Malone J, Titchen A, Seers K. Getting evidence into practice: the meaning of ‘context’. J Adv Nurs 2002;38:94-104. https://doi.org/10.1046/j.1365-2648.2002.02150.x.
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-33.
- Graham I, Logan J, Harrison M, Straus S, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map?. J Contin Educ Health Prof 2006;26:13-24. https://doi.org/10.1002/chp.47.
- Rycroft-Malone J. Theory and knowledge translation. Nurs Res 2007;56:S78-S85. https://doi.org/10.1097/01.NNR.0000280631.48407.9b.
- Rycroft-Malone J, Kitson A, Harvey G, McCormack B, Seers K, Titchen A, et al. Ingredients for change: revisiting a conceptual framework. Qual Saf Health Care 2002;11:174-80. https://doi.org/10.1136/qhc.11.2.174.
- Harvey G, McCormack B, Kitson A, Lynch E, Titchen A. Designing and implementing two facilitation interventions within the ‘Facilitating Implementation of Research Evidence (FIRE)’ study: a qualitative analysis from an external facilitators’ perspective. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0812-z.
- Stone RI, Reinhard SC, Bowers B, Zimmerman D, Phillips CD, Hawes C, et al. Evaluation of the Wellspring Model for Improving Nursing Home Quality. New York, NY: The Commonwealth Fund; 2002.
- Øvretveit J, Bate P, Cleary P, Cretin S, Gustafson D, McInnes K, et al. Quality collaboratives: lessons from research. Qual Saf Health Care 2002;11:345-51. https://doi.org/10.1136/qhc.11.4.345.
- Øvretveit J, Leviton L, Parry G. Increasing the generalisability of improvement research with an improvement replication programme. BMJ Qual Saf 2011;20:i87-91. https://doi.org/10.1136/bmjqs.2010.046342.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2013.
- Powell C, Blighe A, Froggatt K, McCormack B, Woodward-Carlton B, Young J, et al. Family involvement in timely detection of changes in health of nursing homes residents: a qualitative exploratory study. J Clin Nurs 2018;27:317-27. https://doi.org/10.1111/jocn.13906.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Florida Atlantic University . Stop and Watch Early Warning Tool n.d. https://pathway-interact.com/wp-content/uploads/2018/09/INTERACT-Stop-and-Watch-v4_0-June2018_June-2018.pdf (accessed 19 October 2020).
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5. https://doi.org/10.1037/t02366-000.
- Edvardsson D, Fetherstonhaugh D, Nay R, Gibson S. Development and initial testing of the Person-centered Care Assessment Tool (P-CAT). Int Psychogeriatr 2010;22:101-8. https://doi.org/10.1017/S1041610209990688.
- Tija J, Mazor KM, Field T, Meterko, Spenard A, Gurwitz JH. Nurse–physician communication in the long-term care setting: perceived barriers and impact on patient safety. J Patient Saf 2009;5:145-52. https://doi.org/10.1097/PTS.0b013e3181b53f9b.
- McCormack B, McCarthy G, Wright J, Slater P, Coffey A. Development and testing of the Context Assessment Index (CAI). Worldviews Evid Based Nurs 2009;6:27-35. https://doi.org/10.1111/j.1741-6787.2008.00130.x.
- Sampson EL, Feast A, Blighe A, Froggatt K, Hunter R, Marston L, et al. Pilot cluster randomised trial of an evidence-based intervention to reduce avoidable hospital admissions in nursing home residents (Better Health in Residents of Care Homes with Nursing–BHiRCH-NH Study). BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-040732.
- Great Britain . Mental Capacity Act 2005 2005.
- Saliba D, Kington R, Buchanan J, Bell R, Wang M, Lee M, et al. Appropriateness of the decision to transfer nursing facility residents to the hospital. J Am Geriatr Soc 2000;48:154-63. https://doi.org/10.1111/j.1532-5415.2000.tb03906.x.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27. https://doi.org/10.1177/0272989X04263162.
- Coyle D, Oakley J. Estimating the expected value of partial perfect information: a review of methods. Eur J Health Econ 2008;9:251-9. https://doi.org/10.1007/s10198-007-0069-y.
- Care Quality Commission . The State of Health Care and Adult Social Care in England 2017 18 n.d. www.cqc.org.uk/sites/default/files/20171011_stateofcare1718_report.pdf (accessed 23 September 2020).
- National Audit Office . Reducing Emergency Admissions. Report by the Comptroller and Auditor General (HC 833) 2018.
- Popay J, Collins M. The Public Involvement Impact Assessment Framework Guidance. Lancaster, Liverpool, Exeter: Universities of Lancaster, Liverpool and Exeter; 2014.
- Sampson EL, Feast A, Blighe A, Froggatt K, Hunter R, Marston L, et al. Evidence-based intervention to reduce avoidable hospital admissions in care home residents (the Better Health in Residents in Care Homes (BHiRCH) study): protocol for a pilot cluster randomised trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026510.
- Downs M, Woodward-Carlton B, Feast A, Froggatt K. Better Health in Residents in Care Homes n.d.
- National Institute for Health Research Dissemination Centre . Advancing Care – Research With Care Homes. Themed Review 2017.
- NIHR INVOLVE . Guidance on Co-Producing a Research Project n.d. www.invo.org.uk/wp-content/uploads/2019/04/Copro_Guidance_Feb19.pdf (accessed 20 February 2020).
- Sutton E, Dixon-Woods M, Tarrant C. Ethnographic process evaluation of a quality improvement project to improve transitions of care for older people. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010988.
- Abrahamson K, Davila H, Hountz D. Involving nursing assistants in nursing home QI. Am J Nurs 2018;118. https://doi.org/10.1097/01.NAJ.0000530228.48458.09.
- Cranley LA, Norton PG, Cummings GG, Barnard D, Batra-Garga N, Estabrooks CA. Identifying resident care areas for a quality improvement intervention in long-term care: a collaborative approach. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-59.
- Kontos PC, Miller KL, Mitchell GJ. Neglecting the importance of the decision making and care regimes of personal support workers: a critique of standardization of care planning through the RAI/MDS. Gerontologist 2010;50:352-62. https://doi.org/10.1093/geront/gnp165.
- Bowman C, Whistler J, Ellerby M. A national census of care home residents. Age Ageing 2004;33:561-6. https://doi.org/10.1093/ageing/afh177.
- Scott-Cawiezell J, Schenkman M, Moore L, Vojir C, Connoly R, Pratt M, et al. Exploring nursing home staff’s perceptions of communication and leadership to facilitate quality improvement. J Nurs Care Qual 2004;19:242-52. https://doi.org/10.1097/00001786-200407000-00011.
- Dumas LG, Blanks C, Palmer-Erbs V, Portnoy FL. Leadership in nursing homes-2009: challenges for change in difficult times. Nurs Clin North Am 2009;44:169-78. https://doi.org/10.1016/j.cnur.2009.03.005.
- Lynch BM, McCormack B, McCance T. Development of a model of situational leadership in residential care for older people. J Nurs Manag 2011;19:1058-69. https://doi.org/10.1111/j.1365-2834.2011.01275.x.
- Martin JS, McCormack B, Fitzsimons D, Spirig R. Evaluation of a clinical leadership programme for nurse leaders. J Nurs Manag 2012;20:72-80. https://doi.org/10.1111/j.1365-2834.2011.01271.x.
- Rycroft-Malone J, Seers K, Eldh AC, Cox K, Crichton N, Harvey G, et al. A realist process evaluation within the Facilitating Implementation of Research Evidence (FIRE) cluster randomised controlled international trial: an exemplar. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0811-0.
- Seers K, Rycroft-Malone J, Cox K, Crichton N, Edwards RT, Eldh AC, et al. Facilitating Implementation of Research Evidence (FIRE): an international cluster randomised controlled trial to evaluate two models of facilitation informed by the Promoting Action on Research Implementation in Health Services (PARIHS) framework. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0831-9.
- Griffiths AW, Kelley R, Garrod L, Perfect D, Robinson O, Shoesmith E, et al. Barriers and facilitators to implementing dementia care mapping in care homes: results from the DCM EPIC trial process evaluation. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1045-y.
- Marsden E, Craswell A, Taylor A, Coates K, Crilly J, Broadbent M, et al. Nurse-led multidisciplinary initiatives to improve outcomes and reduce hospital admissions for older adults: The Care coordination through Emergency Department, Residential Aged Care and Primary Health Collaboration project. Australas J Ageing 2018;37:135-9. https://doi.org/10.1111/ajag.12526.
- Jordan S, Banner T, Gabe-Walters M, Mikhail JM, Round J, Snelgrove S, et al. Nurse-led medicines’ monitoring in care homes study protocol: a process evaluation of the impact and sustainability of the adverse drug reaction (ADRe) profile for mental health medicines. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-023377.
- Department of Health and Social Care . Living Well With Dementia: A National Dementia Strategy 2009.
- Department of Health and Social Care . The Prime Minister’s Challenge on Dementia 2020 2015.
- Department of Health, Social Services and Public Safety . Nursing Homes Minimum Standards 2008.
- Department of Health, Social Services and Public Safety (DHSSPS) . Residential Care Homes: Minimum Standards 2011.
- National Audit Office . Improving Dementia Services in England – An Interim Report 2010.
- National Audit Office . Improving Services and Support for People With Dementia 2007.
- Care Quality Commission . Regulating the Quality and Safety of Health and Adult Social Care 2012.
- House of Commons Committee of Public Accounts . Improving Services and Support for People With Dementia 2008.
- House of Commons Committee of Public Accounts . Improving Dementia Services in England: An Interim Report 2010.
- Care Quality Commission . Essential Standards of Quality and Safety 2010.
- Care Quality Commission . Health Care in Care Homes – A Special Review of the Provision of Health Care to Those in Care Homes 2012.
- Harrad R, Sulla F. Factors associated with and impact of burnout in nursing and residential home care workers for the elderly. Acta Biomed 2018;89:60-9. https://doi.org/10.23750/abm.v89i7-S.7830.
- Costello H, Walsh S, Cooper C, Livingston G. A systematic review and meta-analysis of the prevalence and associations of stress and burnout among staff in long-term care facilities for people with dementia. Int Psychogeriatr 2019;31:1203-16. https://doi.org/10.1017/S1041610218001606.
- Yeatts DE, Seckin G, Shen Y, Thompson M, Auden D, Cready CM. Burnout among direct-care workers in nursing homes: Influences of organisational, workplace, interpersonal and personal characteristics. J Clin Nurs 2018;27:3652-65. https://doi.org/10.1111/jocn.14267.
- Surr C, Holloway I, Walwyn REA, Griffiths AW, Meads D, Kelley R, et al. Dementia Care Mapping to reduce agitation in care home residents with dementia: the EPIC cluster RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24160.
- Fossey J, Garrod L, Tolbol Froiland C, Ballard C, Lawrence V, Testad I. What influences the sustainability of an effective psychosocial intervention for people with dementia living in care homes? A 9–12 month follow-up of the perceptions of staff in care homes involved in the WHELD randomised controlled trail. Int J Geriatr Psychiatry 2019;34:674-82. https://doi.org/10.1002/gps.5066.
- Romeo R, Zala D, Knapp M, Orrell M, Fossey J, Ballard C. Improving the quality of life of care home residents with dementia: cost-effectiveness of an optimized intervention for residents with clinically significant agitation in dementia. Alzheimers Dement 2019;15:282-91. https://doi.org/10.1016/j.jalz.2018.08.010.
- Ballard C, Corbett A, Orrell M, Williams G, Moniz-Cook E, Romeo R, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002500.
- Ballard C, Orrell M, Sun Y, Moniz-Cook E, Stafford J, Whitaker R, et al. Impact of antipsychotic review and non-pharmacological intervention on health-related quality of life in people with dementia living in care homes: WHELD-a factorial cluster randomised controlled trial. Int J Geriatr Psychiatry 2017;32:1094-103. https://doi.org/10.1002/gps.4572.
- Ballard C, Orrell M, Moniz-Cook E, Woods R, Whitaker R, Corbett A, et al. Improving mental health and reducing antipsychotic use in people with dementia in care homes: the WHELD research programme including two RCTs. Programme Grants Appl Res 2020;8. https://doi.org/10.3310/pgfar08060.
- Bowers B, Roberts T, Nolet K, Ryther B. THRIVE Research Collaborative . Inside the Green House ‘Black Box’: opportunities for high-quality clinical decision making. Health Serv Res 2016;51:378-97. https://doi.org/10.1111/1475-6773.12427.
- Longo DR, Young J, Mehr D, Lindbloom E, Salerno LD. Barriers to timely care of acute infections in nursing homes: a preliminary qualitative study. J Am Med Dir Assoc 2004;5:4-10. https://doi.org/10.1097/01.JAM.0000027250.76379.B2.
- Kezirian AC, McGregor MJ, Stead U, Sakaluk T, Spring B, Turgeon S, et al. Advance care planning in the nursing home setting: a practice improvement evaluation. J Soc Work End Life Palliat Care 2018;14:328-45. https://doi.org/10.1080/15524256.2018.1547673.
- Aasmul I, Husebo BS, Sampson EL, Flo E. Advance care planning in nursing homes – improving the communication among patient, family, and staff: results from a cluster randomized controlled trial (COSMOS). Front Psychol 2018;9. https://doi.org/10.3389/fpsyg.2018.02284.
- Kinley J, Denton L, Levy J. Improving the approach to future care planning in care homes. Int J Palliat Nurs 2018;24:576-83. https://doi.org/10.12968/ijpn.2018.24.12.576.
- Sævareid TJL, Lillemoen L, Thoresen L, Førde R, Gjerberg E, Pedersen R. Implementing advance care planning in nursing homes – study protocol of a cluster-randomized clinical trial. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0869-1.
- Lam HR, Chow S, Taylor K, Chow R, Lam H, Bonin K, et al. Challenges of conducting research in long-term care facilities: a systematic review. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0934-9.
- Froggatt K, Goodman C, Morbey H, Davies SL, Masey H, Dickinson A, et al. Public involvement in research within care homes: benefits and challenges in the APPROACH study. Health Expect 2016;19:1336-45. https://doi.org/10.1111/hex.12431.
- Goodman C, Davies S. ENRICH: a new innovation to facilitate dementia research in care homes. Br J Community Nurs 2012;17. https://doi.org/10.12968/bjcn.2012.17.6.277.
- Goodman C, Sharpe R, Russell C, Meyer J, Gordon A, Dening T, et al. Care Home Readiness: A Rapid Review and Consensus Workshops on How Organisational Context Affects Care Home Engagement With Health Care Innovation 2017. https://uhra.herts.ac.uk/bitstream/handle/2299/18200/Vanguard_Care_home_readiness_report_FINAL.pdf?sequence=2 (accessed 17 October 2020).
- Severs M. Complex service evaluation. Age Ageing 2000;29:5-7. https://doi.org/10.1093/oxfordjournals.ageing.a008099.
- Dixon Woods M, Martin G. Does quality improvement improve quality?. Future Healthc J 2018;3:191-4. https://doi.org/10.7861/futurehosp.3-3-191.
- Estabrooks PA, Brownson RC, Pronk NP. Dissemination and implementation science for public health professionals: an overview and call to action. Prev Chronic Dis 2018;15. https://doi.org/10.5888/pcd15.180525.
- Estabrooks CA, Squires JE, Cummings GG, Teare GF, Norton PG. Study protocol for the translating research in elder care (TREC): building context – an organizational monitoring program in long-term care project (project one). Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-52.
- Rycroft-Malone J, Dopson S, Degner L, Hutchinson AM, Morgan D, Stewart N, et al. Study protocol for the translating research in elder care (TREC): building context through case studies in long-term care project (project two). Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-53.
- Estabrooks CA, Teare GF, Norton PG. Should we feed back research results in the midst of a study?. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-87.
- Estabrooks CA, Knopp-Sihota JA, Cummings GG, Norton PG. Making research results relevant and useable: presenting complex organizational context data to nonresearch stakeholders in the nursing home setting. Worldviews Evid Based Nurs 2016;13:270-6. https://doi.org/10.1111/wvn.12158.
- Cranley LA, Birdsell JM, Norton PG, Morgan DG, Estabrooks CA. Insights into the impact and use of research results in a residential long-term care facility: a case study. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-90.
- Goodman C, Davies SL, Gordon AL, Meyer J, Dening T, Gladman JR, et al. Relationships, expertise, incentives, and governance: supporting care home residents’ access to health care. An interview study from England. J Am Med Dir Assoc 2015;16:427-32. https://doi.org/10.1016/j.jamda.2015.01.072.
- Robbins I, Gordon A, Dyas J, Logan P, Gladman J. Explaining the barriers to and tensions in delivering effective healthcare in UK care homes: a qualitative study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003178.
- Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Housley G, Lewis S, Usman A, Gordon AL, Shaw DE. Accurate identification of hospital admissions from care homes; development and validation of an automated algorithm. Age Ageing 2018;47:387-91. https://doi.org/10.1093/ageing/afx182.
- Lloyd T, Conti S, Santos F, Steventon A. Effect on secondary care of providing enhanced support to residential and nursing home residents: a subgroup analysis of a retrospective matched cohort study. BMJ Qual Saf 2019;28:534-46. https://doi.org/10.1136/bmjqs-2018-009130.
- Little S, Rodgers G, Fitzpatrick JM. Managing deterioration in older adults in care homes: a quality improvement project to introduce an early warning tool. Br J Community Nurs 2019;24:58-66. https://doi.org/10.12968/bjcn.2019.24.2.58.
- Bunn F, Goodman C, Corazzini K, Sharpe R, Handley M, Lynch J, et al. Setting priorities to inform assessment of care homes’ readiness to participate in healthcare innovation: a systematic mapping review and consensus process. Int J Environ Res Public Health 2020;17. https://doi.org/10.3390/ijerph17030987.
- Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Checklist n.d. www.equator-network.org/wp-content/uploads/2013/04/Revised-CHEERS-Checklist-Oct13.pdf (accessed 15 April 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Beecham JK, Knapp MRJ, Thornicroft G, Brewin C, Wing JK. Measuring Mental Health Needs. London: Gaskell; 1992.
- Department of Health and Social Care Payment by Results Finance and Costing Team . National Schedule of Reference Costs 2015–16 2017.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 20 February 2020).
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost–utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- EuroQol Research Foundation . EQ-5D Instruments n.d. https://euroqol.org/eq-5d-instruments/ (accessed 15 April 2020).
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Sinharay S, Stern HS, Russell D. The use of multiple imputation for the analysis of missing data. Psychol Methods 2001;6:317-29. https://doi.org/10.1037/1082-989X.6.4.317.
- Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess 1999;3. https://doi.org/10.3310/hta3020.
- Strong M, Oakley JE, Brennan A. Estimating multi-parameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a non-parametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
Appendix 1 Search terms for identifying care pathways
MEDLINE® [via Ovid® (Wolters Kluwer, Alphen aan den Rijn, the Netherlands)]
Date range searched: 1946 to June Week 1 2015.
Without care homes search strategy
-
practice guideline/ (20,201)
-
clinical protocols/ (21,088)
-
algorithms/ (188,266)
-
critical pathways/ (4923)
-
exp guideline/ (26,446)
-
Health Planning Guidelines/ (3891)
-
guideline adherence/ (22,798)
-
(compliance adj (protocol? or policy or guideline?)).tw. (65)
-
(guideline? adj2 (introduc* or issu* or impact or effect? or disseminat* or distribut* or implement*)).tw. (5784)
-
nursing protocol?.tw. (110)
-
professional standard*.tw. (975)
-
(practice guidelin* or practice protocol* or practice parameter or clinical practice guidelin*).tw. (14,792)
-
(consensus development conference or consensus development conference nih).pt. (9785)
-
((clinical or critical or care) adj3 path).tw. (765)
-
((care adj map*) or plan*).tw. (677,527)
-
or/1-15 [guidelines] (944,931)
-
hospitalization/ and (avoid* or prevent* or unnecessary).tw. (7908)
-
Health Services Misuse/(3871)
-
17 or 18 [avoidable hospitalisation] (11,692)
-
ambulatory care/ (36,848)
-
chronic disease/ (224,731)
-
exp pulmonary disease, chronic obstructive/ (38,800)
-
exp heart failure/ (91,251)
-
exp respiratory tract infections/ (299,937)
-
exp urinary tract infections/ (39,673)
-
exp dehydration/ (10,763)
-
(COPD or chronic obstructive pulmonary disease or respiratory infect* or respiratory tract infect* or congest* heart failure or dehydrat* or urinary tract infect* or UTI).mp. (191,968)
-
or/20-27 [ambulatory care sensitive terms] (771,218)
-
19 or 28 [avoidable hospitalisation or ambulatory care sensitive terms] (780,792)
-
exp aged/ (244,7169)
-
geriatrics/ (26,985)
-
geriatric nursing/ (12,538)
-
(gerontol* or ageing or aging or aged or elder* or geriatric* or seniors or senior citizen* or old age or late* life).mp. (4,385,665)
-
(older adj (person* or people or adult* or patient*)).tw. (77,780)
-
veterans/ (10,477)
-
veteran*.mp. (27,581)
-
or/30-36 [older adults] (4,405,212)
-
16 and 29 and 37 [guidelines and avoid hosp or ambulatory care sensitive terms and elderly] (9704)
-
limit 38 to (english language and yr=”2000 -Current”) (6709)
* etc. etc.
With care homes search strategy
-
practice guideline/ (20,201)
-
clinical protocols/ (21,088)
-
algorithms/ (188,266)
-
critical pathways/ (4923)
-
exp guideline/ (26,446)
-
Health Planning Guidelines/ (3891)
-
guideline adherence/ (22,798)
-
(compliance adj (protocol? or policy or guideline?)).tw. (65)
-
(guideline? adj2 (introduc* or issu* or impact or effect? or disseminat* or distribut* or implement*)).tw. (5784)
-
nursing protocol?.tw. (110)
-
professional standard*.tw. (975)
-
(practice guidelin* or practice protocol* or practice parameter or clinical practice guidelin*).tw. (14,792)
-
(consensus development conference or consensus development conference nih).pt. (9785)
-
((clinical or critical or care) adj3 path).tw. (765)
-
((care adj map*) or plan*).tw. (677,527)
-
or/1-15 [guidelines] (944,931)
-
hospitalization/and (avoid* or prevent* or unnecessary).tw. (7908)
-
Health Services Misuse/ (3871)
-
17 or 18 [avoidable hospitalisation] (11,692)
-
ambulatory care/ (36,848)
-
chronic disease/ (224,731)
-
exp pulmonary disease, chronic obstructive/ (38,800)
-
exp heart failure/ (91,251)
-
exp respiratory tract infections/ (299,937)
-
exp urinary tract infections/ (39,673)
-
exp dehydration/ (10,763)
-
(COPD or chronic obstructive pulmonary disease or respiratory infect* or respiratory tract infect* or congest* heart failure or dehydrat* or urinary tract infect* or UTI).mp. (191,968)
-
or/20-27 [ambulatory care sensitive terms] (771,218)
-
19 or 28 [avoidable hospitalisation or ambulatory care sensitive terms] (780,792)
-
Homes for the Aged/ (11,446)
-
exp Nursing Homes/ (32,977)
-
nursing home?.tw. (21,734)
-
or/30-32 [nursing home terms] (43,502)
-
Nursing Care/ (26,994)
-
Rehabilitation Nursing/ (1175)
-
Community Health Nursing/ (18,583)
-
Hospitals, Convalescent/ (270)
-
Rehabilitation Centers/ (6980)
-
Institutionalization/ (4824)
-
Group Homes/ (857)
-
Assisted Living Facilities/ (974)
-
Residential Facilities/ (4769)
-
Long-Term Care/ (22,346)
-
care home?.tw. (1849)
-
((group or residential) adj home?).tw. (1360)
-
((residential or long-term or longterm or long-stay) adj5 (care or facility or facilities or ward? or institution)).tw. (22,874)
-
((sheltered or retirement or residential) adj5 (hous* or home? or accommodation)).tw. (2623)
-
(life care cent*or continuing care cent*or extended care facility or extended care facilities).tw. (209)
-
((skilled or intermediate) adj2 (nursing facility or nursing facilities)).tw. (1522)
-
assisted living.tw. (1326)
-
or/34-50 [institutional care/nursing care terms] (103,301)
-
exp aged/ (2,447,169)
-
geriatrics/ (26,985)
-
geriatric nursing/ (12,538)
-
(gerontol* or ageing or aging or aged or elder* or geriatric* or seniors or senior citizen* or old age or late* life).tw. (641,501)
-
(older adj (person* or people or adult* or patient* or inpatient*)).tw. (77,861)
-
veterans/ (10,477)
-
or/52-57 [aged terms] (2,809,474)
-
51 and 58 [institutional care or nursing care and aged terms] (31,907)
-
33 or 59 [nursing homes or all other institutional care or nursing care and aged terms] (66,135)
-
16 and 29 and 60 [guidelines and ambulatory care or avoidable admissions or nursing homes or other aged facilities/care] (477)
-
limit 61 to yr=“2000 -Current” (366)
Appendix 2 Care pathways identified in search
| Number | Health condition | Care pathway name | Care setting | Source |
|---|---|---|---|---|
| 1 | Heart failure | Heart failure logic model | Outpatient | Dunn PL. Steps to Reducing Heart Failure Hospital Readmissions Through Improvement in Outpatient Care. Unpublished PhD thesis. Minneapolis, MN: Walden University; 2015 |
| 2 | Heart failure | Timeline | Outpatient | Dunn PL. Steps to Reducing Heart Failure Hospital Readmissions Through Improvement in Outpatient Care. Unpublished PhD thesis. Minneapolis, MN: Walden University; 2015 |
| 3 | Heart failure | Flow sheet | Outpatient | Dunn PL. Steps to Reducing Heart Failure Hospital Readmissions Through Improvement in Outpatient Care. Unpublished PhD thesis. Minneapolis, MN: Walden University; 2015 |
| 4 | Heart failure | Pathways | Outpatient | Dunn PL. Steps to Reducing Heart Failure Hospital Readmissions Through Improvement in Outpatient Care. Unpublished PhD thesis. Minneapolis, MN: Walden University; 2015 |
| 5 | UTI | Figure 1 Evaluation and management of UTI | Nursing home | Unwin B, Porvaznik M, Spoelhof GD. Nursing home care: part II. Clinical aspects. Am Fam Physician 2010;81:1229–37 |
| 6 | Respiratory | Figure 2 Evaluation and management of pneumonia | Nursing home | Unwin B, Porvaznik M, Spoelhof GD. Nursing home care: part II. Clinical aspects. Am Fam Physician 2010;81:1229–37 |
| 7 | Generic | Table 5 Evaluation of fever and infection in long term care facilities | Nursing home | Unwin B, Porvaznik M, Spoelhof GD. Nursing home care: part II. Clinical aspects. Am Fam Physician 2010;81:1229–37 |
| 8 | Respiratory | Figure 2 Clinical pathway for treating residents of nursing homes with lower respiratory tract infection and pneumonia | Nursing home | Loeb M, Carusone SC, Goeree R, Walter AD, Brazil K, Kruegar P, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA 2006;295:2503–10 |
| 9 | Respiratory | CARE PATH symptoms of lower respiratory infection | Nursing home | Tools: INTERACT – CARE PATH Symptoms of Lower Respiratory Infection. URL: https://interact2.net/docs/INTERACT%20Version%204.0%20Tools/INTERACT%20V%204%20Care-Path-Lower-Respiratory-Infection_Nov%2017%202014.pdf (accessed 17 June 2015) |
| 10 | UTI | CARE PATH symptoms of UTIs | Nursing home | Tools: INTERACT – CARE PATH Symptoms of Urinary Tract Infection (UTI). URL: https://interact2.net/docs/INTERACT%20Version%204.0%20Tools/INTERACT%20V%204%20Care_Path_UTI%20Nov%2017%202014.pdf (accessed 17 June 2015) |
| 11 | Delirium | CARE PATH symptoms of acute mental status change | Nursing home | Tools: INTERACT – CARE PATH Symptoms of Acute Mental Status Change. URL: https://interact2.net/docs/INTERACT%20Version%204.0%20Tools/INTERACT%20V%204%20Care_Path_MENTAL_STATUS_CHANGE_Nov%2017%202014.pdf (accessed 17 June 2015) |
| 12 | Generic | S&W | Nursing home | Tools: INTERACT. Stop and Watch Early Warning Tool. URL: https://interact2.net/docs/INTERACT%20Version%204.0%20Tools/INTERACT%20V%204%20Stop-and-Watch%20Nov%2017%202014.pdf (accessed on 17 June 2015) |
| 13 | Heart failure | CARE PATH symptoms of congestive heart failure (CHF) | Nursing home | Tools: INTERACT – CARE PATH Symptoms of Congestive Heart Failure (CHF). URL: https://interact2.net/docs/INTERACT%20Version%204.0%20Tools/Care_Path_SYMPTOMS_CHF_v8.pdf (accessed 17 June 2015) |
| 14 | Heart failure | CHF clinical pathway | Nursing home | Infusion Resource. Congestive Heart Failure Clinical Pathway Care Resource. Riverside, RI: Infusion Resource, LLC; 2015 (personal communication) |
| 15 | Heart failure | Heart failure care | Home care | BrightStar Care. Heart Failure Care. URL: www.brightstarcare.com/range-of-care/brightstar-clinical-pathways/heart-failure/ (accessed 22 June 2015) |
| 16 | Respiratory | COPD | Home care | BrightStar Care. Chronic Obstructive Pulmonary Disease (COPD) Care Program. URL: www.brightstarcare.com/range-of-care/brightstar-clinical-pathways/chronic-obstructive-pulmonary-disease-copd/ (accessed 22 June 2015) |
| 17 | Delirium | Delirium/dementia care | Home care | BrightStar Care. Delirium/Dementia Care. URL: www.brightstarcare.com/range-of-care/brightstar-clinical-pathways/delirium-dementia-care-program/ (accessed 22 June 2015) |
| 18 | Respiratory | Pneumonia care | Home care | BrightStar Care. Pneumonia Care. URL: www.brightstarcare.com/range-of-care/brightstar-clinical-pathways/pneumonia/ (accessed 22 June 2015) |
| 19 | Generic | National Early Warning Score (NEWS) | Generic | Royal College of Physicians. National Early Warning Score (NEWS): Standardising the Assessment of Acute Illness Severity in the NHS. Report of a Working Party. 2012. URL: www.rcplondon.ac.uk/sites/default/files/documents/national-early-warning-score-standardising-assessment-acute-illness-severity-nhs.pdf (accessed 17 June 2015) |
| 20 | UTI | Lower UTI in men care pathway | Hospital | Bera A, Fowler C, Boomla K. Lower Urinary Tract Infection in Men Care Pathway. London: Clinical Effectiveness Group, Centre for Primary Care and Public Health, Blizard Institute Barts and The London School of Medicine and Dentistry; 2012. URL: www.blizard.qmul.ac.uk/ceg-resource-library/clinical-guidance/care-pathways/20-lower-urinary-tract-symptoms-in-men-january-2012/file.html (accessed 17 June 2015) |
| 21 | Respiratory | Respiratory tract infections – antibiotic prescribing. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care | National Institute for Health and Care Excellence. Respiratory Tract Infections – Antibiotic Prescribing. Prescribing of Antibiotics for Self-limiting Respiratory Tract Infection in Adults and Children in Primary Care. 2008. URL: www.nice.org.uk/guidance/cg69/resources/guidance-respiratory-tract-infections-antibiotic-prescribing-pdf (accessed 17 June 2015) | |
| 22 | General | Rehabilitation after critical illness | Hospital and 2–3 months after discharge | National Institute for Health and Care Excellence. Rehabilitation after Critical Illness. NICE Clinical Guideline 83. 2009. URL: www.nice.org.uk/guidance/cg83/resources/guidance-rehabilitation-after-critical-illness-pdf (accessed 17 June 2015) |
| 23 | Heart failure | Chronic heart failure management of chronic heart failure in adults in primary and secondary care | Primary and secondary care | National Institute for Health and Care Excellence. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary. 2010. URL: www.nice.org.uk/guidance/cg101/resources/guidance-chronic-obstructive-pulmonary-disease-pdf (accessed 17 June 2015) |
| 24 | Respiratory | Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary | Primary and secondary care | National Institute for Health and Care Excellence. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary. 2010. URL: www.nice.org.uk/guidance/cg101/resources/guidance-chronic-obstructive-pulmonary-disease-pdf (accessed 17 June 2015) |
| 25 | Delirium | Delirium diagnosis, prevention and management | Hospital or long-term care | National Institute for Health and Care Excellence. Delirium Diagnosis, Prevention and Management: NICE Clinical Guideline 103. 2010. URL: www.nice.org.uk/guidance/cg103/resources/guidance-delirium-pdf (accessed 17 June 2015) |
| 26 | Respiratory | COPD pathway | All | British Lung Foundation. COPD Pathway. 2013. URL: https://copdpathway.blf.org.uk/ (accessed 17 June 2015) |
| 27 | UTI | Power urinary tract in females | Map of Medicine. Lower Urinary Tract Infection (UTI). Map of Medicine. 2014. URL: https://app.mapofmedicine.com/mom/58/page.pdf?department-id=4%26specialty-id=1014%26pathway-id=11618%26page-id=11620%26pathway-prov-cert=/attachments/18177_provcert.pdf%26history=clear%26notes=yes (accessed 17 June 2015) | |
| 28 | UTI | Male UTI symptoms | Map of Medicine. Male Lower Urinary Tract Symptoms. International View. London: Map of Medicine; 2013 (Issue 4). URL: file:///C:/Users/cpowell2/Downloads/maleUTIpage%20(1).pdf (accessed 17 June 2015) | |
| 29 | Dehydration | Altered nutritional status in the long-term care setting | Care homes | National Guideline Clearinghouse. Altered Nutritional Status in the Long-term Care Setting. URL: www.guideline.gov/content.aspx?id=32490%26search=dehydration (accessed 17 June 2015) |
| 30 | UTI | Treatment of UTI in non-pregnant women | National Guideline Clearinghouse. Treatment of Urinary Tract Infection in Non Pregnant Women. URL: www.guideline.gov/content.aspx?id=12628 (accessed 17 June 2015) | |
| 31 | UTI | UTI | National Guideline Clearinghouse. Urinary Tract Infection. URL: www.guideline.gov/content.aspx?id=34419%26search=urinary+tract+infection (accessed 17 June 2015) | |
| 32 | Heart failure | Management of chronic heart failure | Scottish Intercollegiate Network (SIGN). Management of Chronic Heart Failure: A National Clinical Guideline. 2007. URL: www.sign.ac.uk/pdf/sign95.pdf (accessed 17 June 2015) | |
| 33 | UTI | Management of suspected bacterial urinary tract infection in adults | Scottish Intercollegiate Network (SIGN). SIGN Guideline 88: Management of Chronic Heart Failure. 2013. URL: www.sign.ac.uk/guidelines/fulltext/88/recommendations.html (accessed 1 June 2015) | |
| 34 | Heart failure | CHF triage algorithms | US Department of Veterans Quality Enhancement Research Initiative (QUERI). CHF Triage Algorithms.URL: www.queri.research.va.gov/chf/products/hf_toolkit/CHF-TriageAlgorithms_MinneapolisVA.pdf (accessed 28 June 2015) | |
| 35 | Heart failure | CHF clinical pathway inpatient | US Department of Veterans Quality Enhancement Research Initiative (QUERI). HF Toolkit for Providers: CHF Clinical Pathway Inpatient. 2010. URL: www.queri.research.va.gov/chf/products/hf_toolkit/ClinicalPathways-Inpatient-Chart-DurhamVA.pdf (accessed 29 June 2015) | |
| 36 | Heart failure | Grand Junction Veteran Affairs Medical Centre-Clinical Pathway | US Department of Veterans Affairs Quality Enhancement Research Initiative (QUERI). HF Toolkit for Providers: Grand Junction VAMC-Clinical Pathway. 2010. URL: www.queri.research.va.gov/chf/products/hf_toolkit/CHF-Pathway-GrandJunction-VA.pdf (accessed 29 June 2015) | |
| 37 | Dehydration | Dehydration best practice in the care home | Care home | Campbell N. Dehydration: best practice in the care home. Nurs Resid Care 2012;14:21–5 |
Appendix 3 E-mail to expert panel re first draft of care pathway
Appendix 4 Information sheet for consensus workshop participants
Appendix 5 The care pathway
Appendix 6 Semistructured interview schedule with key informants regarding knowledge and skills
Key informants: academics and policy colleagues
Introduction
Thank you very much for agreeing to act as a key informant for ‘Better Health in Residents in Care Homes’ (BHiRCH). This interview will take approximately 30 minutes to complete.
-
Are you happy with the interview being recorded? If not, I can take notes.
-
You do not have to answer all the questions if you do not wish to. If you would like to end or take a break from the interview at any time, please let me know.
Context
As you know, our primary aim is to improve the early detection and timely referral of readily treatable health conditions (respiratory infection, exacerbation of CHF, UTI and dehydration) affecting residents in nursing homes.
The project is over a 3-year period and we are currently in year 1. In year 1 we are developing and testing the best approach to reduce avoidable admissions from care homes. In years 2 and 3 we will test the intervention, first of all in two nursing homes, and then in 14 nursing homes. We will be interested to see whether the complex intervention affects rates of avoidable hospital admissions and outcomes of importance to residents, staff and families.
At the moment we are finalising a care pathway that can be used in nursing homes that will involve senior care assistants, nurses in care homes, care home managers, family members and primary care professionals.
We would like your assistance with the next part of our project, which involves developing an optimal approach to enhancing knowledge and skills of nursing home staff, as well as implementation support.
Therefore, I will ask you questions about:
-
key knowledge and skills for health care in care homes
-
literature you are aware of in this area
-
best practice you are aware of nationally and internationally.
Do you have any further questions before we start?
For all
Are you aware of any literature that addresses:
-
perspectives on core competencies required for ensuring proactive health care
-
effective approaches to enhancing the knowledge and skills of nursing home staff
-
existing knowledge and skills enhancement programmes for active management of ACSCs in nursing homes
-
optimal approach to implementation support?
Can you give examples of best practice you are aware of nationally and internationally?
-
What do you think are the core competencies that are required for nursing home staff to (ensure proactive health care)?
Prompts: what skills do you think are required for staff to recognise ACSCs? (Heart failure, UTI, respiratory infection, dehydration.)
-
What are the current provisions of specific training required for medical professionals (physicians, nurses, geriatricians) who are seeing nursing home residents?
Prompts: prevention, health gain, health maintenance and palliative care.
-
How do you think specific knowledge about older people’s medical conditions could be improved to recognise ACSCs?
-
What are the current provisions of specific training in care for older people required for nursing home staff?
-
How can competencies be enhanced within the environment of a nursing home?
Prompts: (e.g. around existing time pressures).
Use of telecare? Could this be developed to assist communication between staff and primary care? Are there training requirements here?
How can workload be balanced across GPs, nurses and care assistants?
-
What else is needed in addition to medical care to manage the four ACSCs we are concerned with (CHF, respiratory infection, UTI, dehydration)?
Prompts:
Access to physiotherapy, occupational therapy, speech and language therapy, pharmacy
Psychologist, dentist, social work
Do these systems exist already?
International advisory group members
-
What do you think are they key knowledge and skills needed to provide good health care in care homes?
-
What do you think are the best ways to get these knowledge and skills?
-
What do you think is the best way to ensure early detection of changes in a resident’s condition?
Care home staff
-
What do you think you need to know in order to help a resident who is about to become ill?
-
How do you get this information? [e.g. telemedicine; types of training, e.g. online; digital versatile disc (DVD); induction; supervision; peers/colleagues; handover; magazines; web].
-
How might you get this information?
-
-
What signs do you notice in a resident about to become ill?
-
What skills do you think you need in order to help a resident who is about to become ill?
-
How do you get these skills?
-
How might you get these skills?
-
Nurses
-
What do you think nurses need to know in order to help a resident who is about to become ill?
-
How do they get this information?
-
How do you think they can best get this information?
-
-
What skills do you think nurses need in order to help a resident who is about to become ill?
-
How do they get these skills?
-
How do you think they can best get these skills?
-
Care assistants
-
What do you think care assistants need to know in order to help a resident who is about to become ill?
-
How do they get this information?
-
How do you think they can best get this information?
-
-
What skills do you think care assistants need in order to help a resident who is about to become ill?
-
How do they get these skills?
-
How do you think they can best get these skills?
-
Domestic staff
-
What do you think domestic staff need to know in order to help a resident who is about to become ill?
-
How do they get this information?
-
How do you think they can best get this information?
-
-
What skills do you think domestic staff need in order to help a resident who is about to become ill?
-
How do they get these skills?
-
How do you think they can best get these skills?
-
Appendix 7 Search terms for rapid review
Search terms included:
‘Dementia’, ‘Alzheimer’s disease’, ‘nursing home’, ‘nursing home staff’, ‘care home’, ‘care home staff’, ‘nurses’, ‘nurs*’, ‘training’, ‘effective training’, ‘modelling’, ‘demonstration’, ‘effective approaches’, ‘programme’, ‘program’, ‘education’, ‘continuing education’, ‘evaluation’, ‘competencies’, ‘core competencies’, ‘knowledge’, ‘skills’, ‘knowledge and skills’, ‘staff’, ‘health conditions’ and ‘ambulatory care sensitive’.
Appendix 8 Knowledge and skills nurses need for early detection of acute deterioration of residents’ health
Has up-to-date knowledge of key symptoms of, and best approach to management of, respiratory infections, exacerbation of CHF, UTI and dehydration?
-
Has knowledge of how CHF may manifest in older people.
-
Has knowledge of how respiratory infections may manifest in older people.
-
Has knowledge of how dehydration may manifest in older people.
-
Has knowledge of how UTIs may manifest in older people.
Has the ability to detect these signs and symptoms during daily care?
-
Is able to identify changes in physiology or behaviour, compared with baseline or normal function for that resident.
-
Is able to identify changes in physiology or behaviour for people who cannot communicate verbally.
-
Is able to carry out ongoing, informal assessments of changes in physiology or behaviour during day-to-day care.
Knows residents’ medical conditions, care plans addressing these, and their normal functioning.
-
Knows each resident’s existing medical conditions.
-
Knows each resident’s care plans with respect to these medical conditions.
-
Knows what is normal for each resident in terms of physiology, abilities and behaviour (i.e. their baseline).
-
Able to carry out immediate interventions (e.g. adjusting fluid levels).
-
Able to recognise verbal and non-verbal signs of acute deterioration.
-
Able to carry out bedside observations (vital signs, etc.).
-
Able to utilise basic clinical skills (use of medication, etc.).
-
Knowledge of when to seek additional support (e.g. asking a nurse, or calling a GP or ambulance).
-
Able to set and monitor staff’s work to ensure quality of health care is provided.
-
Able to encourage or support staff in their care for acute deterioration in resident’s health.
-
Able to negotiate and plan a course of action with other staff.
-
Able to record a plan of action, for example draft and update care plans.
-
Able to ensure acute changes in a resident’s health status are communicated to staff in written or oral form.
-
Able to ensure effectiveness of treatment for acute changes in a resident’s health status are communicated to staff and family in written or oral form.
-
Able to convey information about changes in health accurately and sensitively to family.
-
Able to adapt assessment and communication for people with dementia or communication difficulties.
-
Able to communicate the nature of change in a resident’s health status.
Appendix 9 Purpose, role and person specification for Practice Development Champions
The following criteria are essential and are equally important:
-
be a registered nurse
-
have been working in the nursing home for at least 6 months
-
has some knowledge of good practice in supporting health care and has an interest in the topic (can demonstrate some essential knowledge of the management of the four conditions: dehydration, deterioration of CHF, lower respiratory tract infection and UTI)
-
knows co-workers (has been in the organisation long enough to know the staff and how they work)
-
knows the environment (has some insight into the culture of the setting)
-
knows the organisation (knows their way around the organisation, e.g. who’s who, policies in place, decision-making structures)
-
possesses effective communication skills (could include attributes of being open-minded, being creative, has experience of managing meetings/groups, able to talk in front of groups)
-
is self-aware and resilient (has insight into their support needs, but is also not afraid of challenge/conflict; willing to engage in own professional development)
-
is reliable and dependable [has time they can dedicate to this work (in writing from their manager); carries through with responsibilities, meets deadlines or negotiates otherwise; is not intending to be on extended leave during intervention period]
-
is respected by co-workers (has a good relationship with co-workers, which means they will be listened to with respect to new ideas).
Appendix 10 Interview schedule for family carers regarding their role in health care in nursing homes
Introduction
Thank you very much for agreeing to take part in this interview.
Our study
As you know we are engaged in a study to develop and test an intervention to improve health care in care homes with nursing.
What will happen today?
Today we are interested in understanding the role you play or would like to play in the care of your relative’s health.
We will ask you questions about:
-
the role you play and the role you would like to play
-
what knowledge and skills you have and would like to have to play a role in the care of your relative’s health
-
what ways of communicating with care home staff and primary care that you find helpful and not helpful.
We would like to record your answers so that we have an accurate account.
Reminder about our promise to you
As you may remember from the information sheet about the study:
-
You do not have to answer any questions you do not wish to.
-
You can withdraw from the study at any time.
-
We will not use your name in any of our findings.
-
We will provide you with an honorarium.
Ground rules
There are no wrong answers. Please respect each other’s opinions. Would you like to establish any ground rules yourselves?
Any questions?
Introducing ourselves
Let’s introduce ourselves by telling us your name and about your involvement in the care of your relative (relative’s health) living in a care home with nursing.
Role in the care of your relative’s health
-
What role do you play in the care of your relative’s health?
-
What role would you like to play in the care of your relative’s health?
Knowledge and skills for your role in the care of your relative’s health
-
What skills and knowledge do you need in order to be involved in the care of your relative’s health?
-
Do you know what health conditions your relative has?
-
How do you know when your relative is becoming ill? What do you notice?
-
Do you have information about your relative that might be helpful to care home staff?
Communication with care home staff about your relative’s health
-
Please describe a time when you have had useful communication about your relative’s health with nursing home staff?
-
What was useful about it?
-
What role did staff play?
-
What role did you play?
-
-
Please describe a time when communication was not so good – what might have made it better?
Communication with primary care staff and general practitioners
-
Please describe a time when you have had useful communication about your relative’s health with primary care staff – GPs?
-
What was useful about it?
-
What role did staff play?
-
What role did you play?
-
-
Please describe a time when communication was not so good – what might have made it better?
One last comment
Do you have any further comments or questions? (each person asked)
Many thanks
Thank you very much for your time.
Next steps
We will transcribe what was said today and see where there are some clear themes about families’ involvement in care for their relative’s health in nursing homes.
Please provide information about payment of honorarium, time scales and web page link and Twitter account so people can follow the study’s progress.
Appendix 11 Training workshop for Practice Development Champions
Summary
Welcome to the PDCs Development and Support Programme for the BHiRCH project. The aim of the Development and Support Programme is to help you as a PDC to enable participative engagement in the implementation of the BHiRCH intervention in your care home. The programme is focused on helping you to become better equipped to work with others in overcoming obstacles to put the BHiRCH intervention into practice.
We hope you find the workshop stimulating, challenging, creative, exciting and, most of all, a worthwhile experience that equips you with knowledge, skills and expertise that can be used effectively in this BHiRCH project.
Learning outcomes
Learning outcomes
By the end of the workshop you will be able to:
-
Understand and implement the BHiRCH intervention.
-
Identify the key staff needed to facilitate the change process, who will go on to form the PDSG.
-
Have the skills necessary to negotiate with managers/leaders and the membership of the PDSG.
-
Collaborate in identifying changes that need to be made within the care home to embed the BHiRCH intervention.
-
Engage with all staff and family members.
These outcomes are worked towards through a focus on specific aims pertaining to the three key elements of facilitation, evidence and context.
Facilitation
-
Demonstrate the skills of a facilitator who adopts a person-centred approach.
-
Enable members of the PDSG to participate in all aspects of the implementation activity.
Evidence
-
Enable the PDSG to understand the BHiRCH Intervention.
-
Help other staff to develop their own knowledge and skill in better supporting care home residents in maintaining their health care.
Context
-
Enable members of the PDSG to understand the importance of developing:
-
a conducive context, for example collaborative decision-making processes, enabling power and authority processes, sharing resources, effective communication and feedback processes
-
workplace cultures of effectiveness
-
effective leadership and team-working
-
effective care review processes at individual, team and systems levels.
-
| Programme for the day | |
|---|---|
| 08.45 | Registration and coffee |
| 09.00 | Welcome, introductions, overview |
| Negotiate terms of engagement of whole group (Brendan and Katherine) | |
| 09.30 | Overview of the BHiRCH project (Alan Blighe) |
| 09.50 | Considering evidence, context and facilitation in developing practice – The BHiRCH Project Handbook (Katherine and Brendan) |
| 10.20 | What are we putting into practice:
|
| 11.20 | Coffee |
| 11.30 | Putting the BHiRCH project into Practice 1: (Brendan and Katherine)
|
| 12.30 | Lunch |
| 13.15 | Putting the BHiRCH project into Practice 2: (Brendan and Katherine)
|
| 14.45 | Coffee |
| 15.00 | Action-planning, getting started and moving forward (Katherine and Brendan) |
| Telephone support and using the available resources | |
| 16.00 | Sharing our learning, evaluation and feedback |
| 16.30 | Closure |
Appendix 12 Project handbook
Reproduced with permission from Bradford Teaching Hospitals Foundation Trust.
Reproduced with permission from Bradford Teaching Hospitals Foundation Trust.
Appendix 13 Study set-up plan
Appendix 14 Sample project poster
Appendix 15 Sample project newsletter
Appendix 16 Research facilitator role description
-
Has been working in the nursing home for at least 6 months.
-
Has up-to-date knowledge of residents in the care home.
-
Knows co-workers (has been in the organisation long enough to know the staff and how they work).
-
Knows the environment (has some insight into the culture of the setting, understands how to access records).
-
Knows the organisation (knows their way around the organisation, e.g. who’s who, policies in place, decision-making structures).
-
Possesses effective communication skills (could include attributes of being open-minded, being creative, has experience of working with family members and external health and social care staff).
-
Is reliable and dependable [has time they can dedicate to this work (in writing from their manager); carries through with responsibilities, meets deadlines or negotiates otherwise; is not intending to be on extended leave during intervention period].
-
Is respected by co-workers (has a good relationship with co-workers, which means they will be listened to with respect to new ideas).
Appendix 17 Trial protocol
Sampson EL, Feast A, Blighe A, Froggatt K, Hunter R, Marston L, et al. Evidence-based intervention to reduce avoidable hospital admissions in care home residents (the Better Health in Residents in Care Homes (BHiRCH) study): protocol for a pilot cluster randomised trial. BMJ Open 2019;9:e026510. https://doi.org/10.1136/bmjopen-2018-026510
Appendix 18 Pilot study CONSORT flow diagram: residents’ recruitment
Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
FIGURE 2.
Pilot study CONSORT flow diagram: residents’ recruitment.

Appendix 19 Pilot study CONSORT flow diagram: residents’ retention
Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
FIGURE 3.
Pilot study CONSORT flow diagram: residents’ retention.
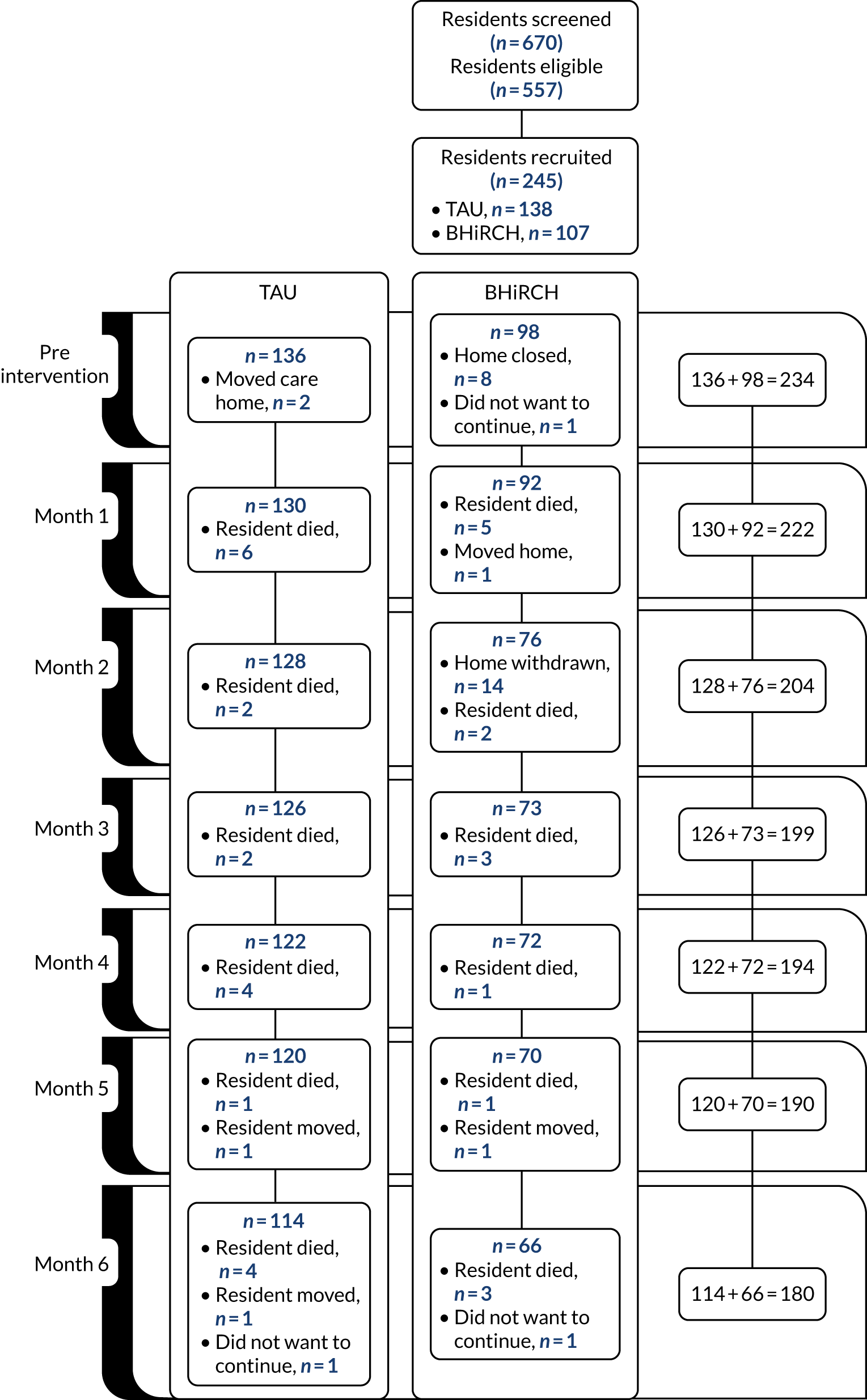
Appendix 20 Pilot study CONSORT flow diagram: nursing home staff
Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
FIGURE 4.
Pilot study CONSORT flow diagram: nursing home staff.
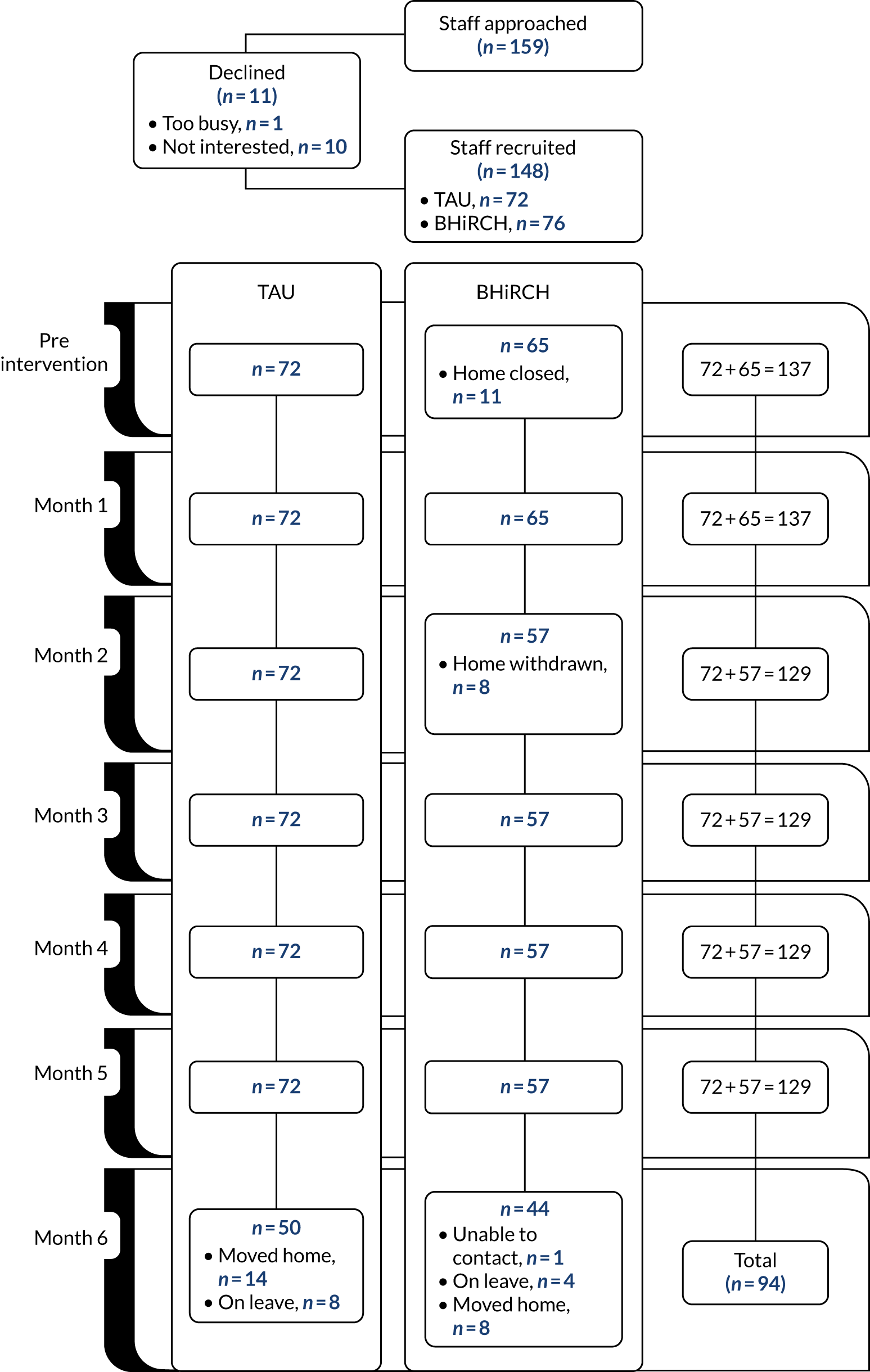
Appendix 21 Pilot study CONSORT flow diagram: family carer
Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
FIGURE 5.
Pilot study CONSORT flow diagram: family carer. CP, care partner.
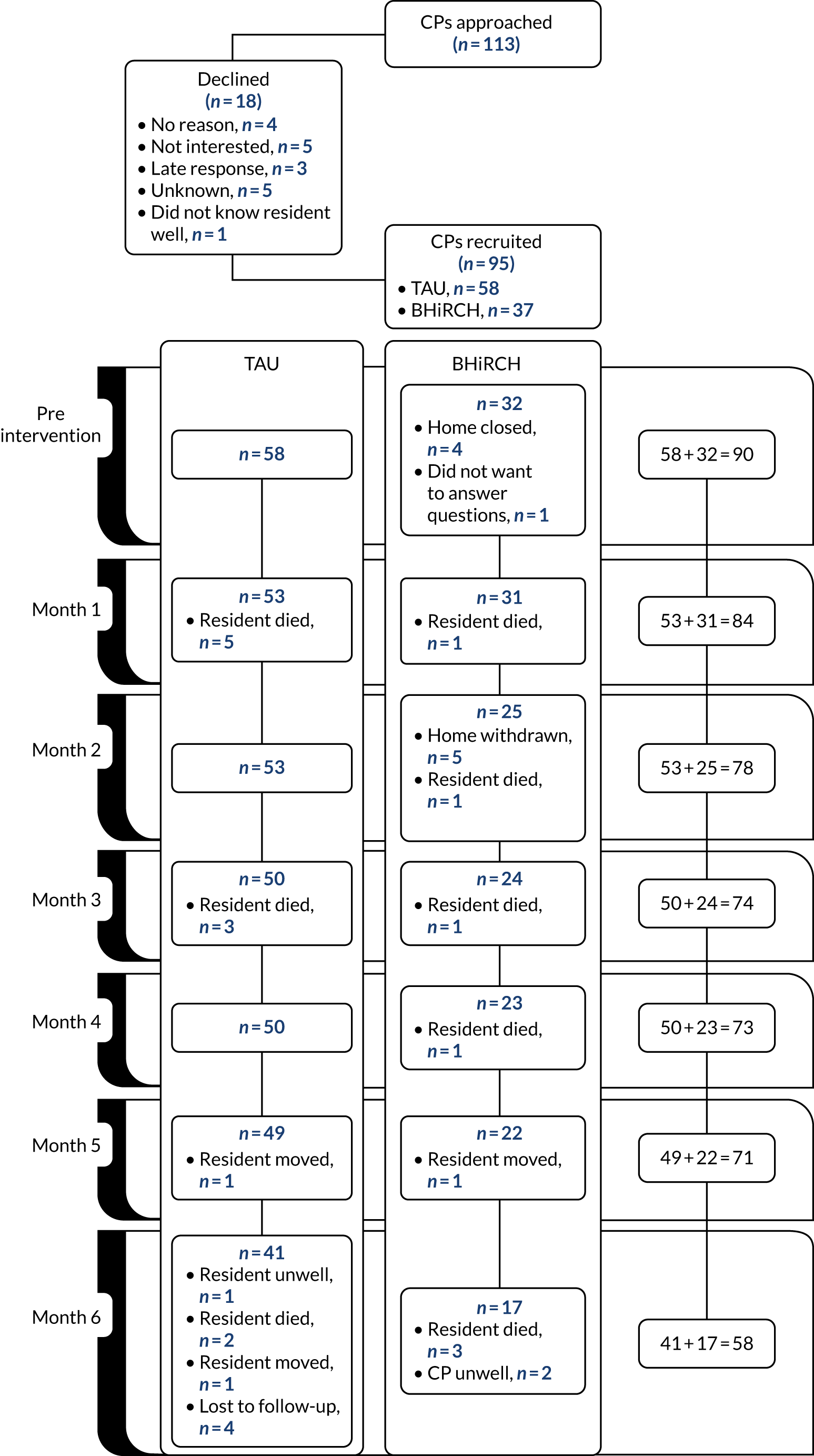
Appendix 22 Nurse ratings on the Person-centred Care Assessment Tool at baseline
Staff ratings
| Question | n/N or median | % or (IQR) |
|---|---|---|
| We often discuss how to give person-centred care | ||
| Disagree | 2/127 | 2 |
| Neither agree nor disagree | 2/127 | 2 |
| Agree | 60/127 | 47 |
| Completely agree | 63/127 | 50 |
| We have formal team meetings to discuss residents’ care | ||
| Completely disagree | 1/127 | 1 |
| Disagree | 3/127 | 2 |
| Neither agree nor disagree | 10/127 | 8 |
| Agree | 55/127 | 43 |
| Completely agree | 58/127 | 46 |
| The life history of the residents is formally used in the care plans we use | ||
| Disagree | 2/126 | 2 |
| Neither agree nor disagree | 7/126 | 6 |
| Agree | 53/126 | 42 |
| Completely agree | 64/126 | 51 |
| The quality of the interaction between staff and residents is more important than getting the tasks done | ||
| Completely disagree | 2/127 | 2 |
| Disagree | 3/127 | 2 |
| Neither agree nor disagree | 20/127 | 16 |
| Agree | 53/127 | 42 |
| Completely agree | 49/127 | 39 |
| We are free to alter work routines based on residents’ preferences | ||
| Completely disagree | 0/125 | 0 |
| Disagree | 4/125 | 3 |
| Neither agree nor disagree | 16/125 | 13 |
| Agree | 56/125 | 45 |
| Completely agree | 49/125 | 39 |
| Residents are offered the opportunity to be involved in individualised everyday activities | ||
| Completely disagree | 0/126 | 1 |
| Disagree | 1/126 | 7 |
| Neither agree nor disagree | 59/126 | 47 |
| Agree | 57/126 | 45 |
| Completely agree | 0/126 | 0 |
| I simply do not have the time to provide person-centred care | ||
| Completely disagree | 78/125 | 62 |
| Disagree | 29/125 | 23 |
| Neither agree nor disagree | 12/125 | 10 |
| Agree | 5/125 | 4 |
| Completely agree | 1/125 | 1 |
| The environment feels chaotic | ||
| Completely disagree | 42/127 | 33 |
| Disagree | 36/127 | 28 |
| Neither agree nor disagree | 31/127 | 24 |
| Agree | 14/127 | 11 |
| Completely agree | 4/127 | 3 |
| We have to get the work done before we can worry about a homelike environment | ||
| Completely disagree | 40/127 | 32 |
| Disagree | 38/127 | 30 |
| Neither agree nor disagree | 20/127 | 16 |
| Agree | 24/127 | 19 |
| Completely agree | 5/127 | 4 |
| This organisation prevents me from providing person-centred care | ||
| Completely disagree | 71/128 | 55 |
| Disagree | 35/128 | 27 |
| Neither agree nor disagree | 13/128 | 10 |
| Agree | 8/128 | 6 |
| Completely agree | 1/128 | 1 |
| Assessment of residents’ needs is undertaken on a daily basis | ||
| Completely disagree | 1/127 | 1 |
| Disagree | 5/127 | 4 |
| Neither agree nor disagree | 9/127 | 7 |
| Agree | 60/127 | 47 |
| Completely agree | 52/127 | 41 |
| It is hard for residents in this facility to find their way around | ||
| Completely disagree | 40/127 | 32 |
| Disagree | 48/127 | 38 |
| Neither agree nor disagree | 23/127 | 18 |
| Agree | 13/127 | 10 |
| Completely agree | 3/127 | 2 |
| Residents are able to access outside space as they wish | ||
| Completely disagree | 4/128 | 3 |
| Disagree | 13/128 | 10 |
| Neither agree nor disagree | 25/128 | 20 |
| Agree | 54/128 | 42 |
| Completely agree | 32/128 | 25 |
| PCAT score (N = 120) | 49 | (46–53) |
Appendix 23 Nurse–general practitioner communication ratings at baseline, by randomised group
Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
| Nurse–GP communication | TAU | Intervention | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| Have difficulty understanding what GP means because of medical jargon | 2/24 | 8 | 0/16 | 0 |
| A GP’s language or accent makes it hard for you to understand what they are saying | 1/24 | 4 | 1/16 | 6 |
| A GP has difficulty understanding what you are saying because of your language or accent | 2/24 | 8 | 0/16 | 0 |
| GPs interrupt you before you have finished reporting on a resident | 2/24 | 8 | 2/16 | 13 |
| GPs consider nurses’ views when making decisions about residents | 18/23 | 78 | 11/16 | 69 |
| GPs are rude to you when you call them about a resident | 2/23 | 9 | 0/16 | 0 |
| You feel respected after an interaction with a GP? | 19/24 | 79 | 10/16 | 63 |
| You feel frustrated after an interaction with a GP? | 3/24 | 13 | 1/16 | 6 |
| Difficulty reaching the GP | 5/24 | 21 | 4/16 | 25 |
| Uncertainty about what to tell the GP | 1/23 | 4 | 0/15 | 0 |
| Feeling that the GP does not want to deal with the problem | 1/24 | 4 | 2/16 | 13 |
| Finding time to make the call | 8/24 | 33 | 3/16 | 19 |
| Finding a quiet place to make the call | 7/24 | 29 | 3/16 | 19 |
| Anticipating that the GP will be rude or unpleasant | 0/24 | 0 | 1/16 | 6 |
| Feeling hurried by the GP | 4/24 | 17 | 3/16 | 19 |
| Feeling that I am bothering the GP | 2/24 | 8 | 2/15 | 13 |
| Worrying that the GP may order something inappropriate or unnecessary | 2/24 | 8 | 2/16 | 13 |
| Feeling that I do not have enough time to say everything that I need to say | 1/24 | 4 | 2/15 | 13 |
| Nurses get good training in this care home | 16/24 | 67 | 12/15 | 80 |
| Nurses in this care home are willing to try new protocols | 20/24 | 83 | 13/15 | 87 |
| Nurses in this care home sometimes have to ignore protocols to get everything done | 4/24 | 17 | 2/15 | 13 |
| When this care home makes changes to improve resident care, they follow up to see if the changes worked | 18/24 | 75 | 13/15 | 87 |
| It is hard to make changes to improve resident care in this care home | 1/24 | 4 | 3/15 | 20 |
| This care home often wants nurses to follow protocols that do not really help residents | 1/24 | 4 | 2/15 | 13 |
| New protocols often make it harder for nurses to do their job | 2/24 | 8 | 2/15 | 13 |
| The communication between nurses and GPs in this care home is open | 20/24 | 83 | 14/16 | 88 |
| Feel comfortable communicating with GPs? | 23/24 | 96 | 13/16 | 81 |
| Feel comfortable communicating with nurse practitioners? | 23/24 | 96 | 11/16 | 69 |
Appendix 24 Nurse self-rated knowledge and skills at baseline, by randomised group
Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
| Question | TAU | Intervention | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| I know how acute episodes of chronic heart failure may manifest in older people | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 2/13 | 15 |
| Agree | 12/21 | 57 | 6/13 | 46 |
| Completely agree | 9/21 | 43 | 5/13 | 38 |
| I know how respiratory infections may manifest in older people | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 7/21 | 33 | 6/13 | 46 |
| Completely agree | 14/21 | 67 | 7/13 | 54 |
| I know how dehydration may manifest in older people | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 7/21 | 33 | 7/13 | 54 |
| Completely agree | 14/21 | 67 | 6/13 | 46 |
| I know how UTIs may manifest in older people | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 9/21 | 43 | 6/13 | 46 |
| Completely agree | 12/21 | 57 | 7/13 | 54 |
| I am able to identify changes in physiology or behaviour, compared with baseline or normal function, for that resident | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 8/21 | 38 | 6/13 | 46 |
| Completely agree | 13/21 | 62 | 7/13 | 54 |
| I am able to identify changes in physiology or behaviour for people who cannot communicate verbally | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 1/13 | 8 |
| Agree | 11/21 | 52 | 6/13 | 46 |
| Completely agree | 10/21 | 48 | 6/13 | 46 |
| I know each resident’s existing medical conditions | ||||
| Disagree | 0/21 | 0 | 1/13 | 8 |
| Neither agree nor disagree | 1/21 | 5 | 1/13 | 8 |
| Agree | 15/21 | 71 | 7/13 | 54 |
| Completely agree | 5/21 | 24 | 4/13 | 31 |
| I know each resident’s care plans with respect to these medical conditions | ||||
| Disagree | 0/21 | 0 | 1/13 | 8 |
| Neither agree nor disagree | 1/21 | 5 | 1/13 | 8 |
| Agree | 10/21 | 48 | 7/13 | 54 |
| Completely agree | 10/21 | 48 | 4/13 | 31 |
| I know what is normal (i.e. their baseline) for each resident in terms of physiology, abilities and behaviour | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 1/21 | 5 | 0/13 | 0 |
| Agree | 11/21 | 52 | 8/13 | 62 |
| Completely agree | 9/21 | 43 | 5/13 | 38 |
| I am able to carry out immediate interventions (e.g. adjusting fluid levels) | ||||
| Disagree | 1/21 | 5 | 0/12 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/12 | 0 |
| Agree | 10/21 | 48 | 11/12 | 92 |
| Completely agree | 10/21 | 48 | 1/12 | 8 |
| I am able to recognise verbal and non-verbal signs of acute deterioration | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 10/21 | 48 | 9/13 | 69 |
| Completely agree | 11/21 | 52 | 4/13 | 31 |
| I am able to carry out bedside observations (vital signs, etc.) | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 6/21 | 29 | 5/13 | 38 |
| Completely agree | 15/21 | 71 | 8/13 | 62 |
| I am able to utilise basic clinical skills (use of medication, etc.) | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 5/21 | 24 | 6/13 | 46 |
| Completely agree | 16/21 | 76 | 7/13 | 54 |
| I know when to seek additional support (e.g. asking a nurse, calling a GP or ambulance) | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 4/21 | 19 | 6/13 | 46 |
| Completely agree | 17/21 | 81 | 7/13 | 54 |
| I am able to set and monitor staff work to ensure quality of health care is provided | ||||
| Disagree | 1/21 | 5 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 5/21 | 24 | 9/13 | 69 |
| Completely agree | 15/21 | 71 | 4/13 | 31 |
| I am able to encourage or support other staff in their care for acute deterioration in resident’s health | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 2/21 | 10 | 0/13 | 0 |
| Agree | 5/21 | 24 | 9/13 | 69 |
| Completely agree | 14/21 | 67 | 4/13 | 31 |
| I am able to negotiate and plan a course of action with other staff | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 3/21 | 14 | 1/13 | 8 |
| Agree | 6/21 | 29 | 7/13 | 54 |
| Completely agree | 12/21 | 57 | 5/13 | 38 |
| I am able to record a plan of action (e.g. draft and update care plans) | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 10/21 | 48 | 6/13 | 46 |
| Completely agree | 11/21 | 52 | 7/13 | 54 |
| I am able to ensure that acute changes in a resident’s health status are communicated to staff in written or oral form | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 7/21 | 33 | 7/13 | 54 |
| Completely agree | 14/21 | 67 | 6/13 | 46 |
| I am able to ensure that effectiveness of treatment for acute changes in a resident’s health status are communicated to staff and care partners in written or oral form | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 1/21 | 5 | 1/13 | 8 |
| Agree | 6/21 | 29 | 6/13 | 46 |
| Completely agree | 14/21 | 67 | 6/13 | 46 |
| I am able to convey information about changes in health accurately and sensitively to care partners | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 1/13 | 8 |
| Agree | 10/21 | 48 | 6/13 | 46 |
| Completely agree | 11/21 | 52 | 6/13 | 46 |
| I am able to adapt assessment and communication for people with dementia or communication difficulties | ||||
| Disagree | 1/21 | 5 | 0/13 | 0 |
| Neither agree nor disagree | 1/21 | 5 | 0/13 | 0 |
| Agree | 11/21 | 52 | 6/13 | 46 |
| Completely agree | 8/21 | 38 | 7/13 | 54 |
| I am able to communicate the nature of change in a resident’s health status | ||||
| Disagree | 0/21 | 0 | 0/13 | 0 |
| Neither agree nor disagree | 0/21 | 0 | 0/13 | 0 |
| Agree | 12/21 | 57 | 5/13 | 38 |
| Completely agree | 9/21 | 43 | 8/13 | 62 |
Appendix 25 Resident characteristics at subsequent months
Tables 19 and 20 show that the numbers of admissions, ambulances called and unscheduled GP visits or telephone contacts were highest in the first couple of months (as were the percentages). This coincided with when there were more care homes and residents in the study and also when weather was cooler. The Barthel Index score decreased to a median of 20 points (IQR 15–35 points).
| Characteristic | n/N or median | % or (IQR) |
|---|---|---|
| Month 1 | ||
| At least one admission in the previous month | 13/224 | 6 |
| Respiratory infection admission | 5/224 | 2 |
| UTI admission | 4/223 | 2 |
| Dehydration admission | 0/223 | 0 |
| CHF admission | 0/223 | 0 |
| At least one ambulance called | 17/224 | 8 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 11/225 | 5 |
| At least one A&E attendance | 16/224 | 7 |
| Month 2 | ||
| At least one admission in the previous month | 12/218 | 6 |
| Respiratory infection admission | 4/218 | 2 |
| UTI admission | 2/218 | 1 |
| Dehydration admission | 1/218 | 0.5 |
| CHF admission | 1/218 | 0.5 |
| At least one ambulance called | 8/218 | 4 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 6/218 | 3 |
| At least one A&E attendance | 8/218 | 4 |
| Month 3 | ||
| At least one admission in the previous month | 7/202 | 3 |
| Respiratory infection admission | 2/202 | 1 |
| UTI admission | 0/202 | 0 |
| Dehydration admission | 0/202 | 0 |
| CHF admission | 0/202 | 0 |
| At least one ambulance called | 7/202 | 3 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 6/202 | 3 |
| At least one A&E attendance | 6/202 | 3 |
| Month 4 | ||
| At least one admission in the previous month | 8/197 | 4 |
| Respiratory infection admission | 2/197 | 1 |
| UTI admission | 1/197 | 0.5 |
| Dehydration admission | 0/197 | 0 |
| CHF admission | 0/197 | 0 |
| At least one ambulance called | 8/197 | 4 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 4/197 | 2 |
| At least one A&E attendance | 7/197 | 4 |
| Month 5 | ||
| At least one admission in the previous month | 5/193 | 3 |
| Respiratory infection admission | 1/193 | 0.5 |
| UTI admission | 0/193 | 0 |
| Dehydration admission | 0/193 | 0 |
| CHF admission | 0/193 | 0 |
| At least one ambulance called | 4/193 | 2 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 4/193 | 2 |
| At least one A&E attendance | 4/193 | 2 |
| Month 6 | ||
| At least one admission in the previous month | 2/184 | 1 |
| Respiratory infection admission | 0/184 | 0 |
| UTI admission | 0/184 | 0 |
| Dehydration admission | 0/184 | 0 |
| CHF admission | 0/184 | 0 |
| At least one ambulance called | 6/184 | 3 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 3/184 | 2 |
| At least one A&E attendance | 5/184 | 3 |
| Barthel Index score (n = 179) | 20 | (15–35) |
| Over the 6 months for those who were in the study for the whole 6-month period | ||
| At least one admission in the previous month | 21/182 | 12 |
| Respiratory infection admission | 7/182 | 4 |
| UTI admission | 5/182 | 3 |
| Dehydration admission | 1/182 | 0.6 |
| CHF admission | 0/182 | 0 |
| At least one ambulance called | 28/182 | 15 |
| At least one unscheduled (out-of-hours) GP visits or telephone contact | 19/183 | 10 |
| At least one A&E attendance | 25/182 | 14 |
| Over the 6 months for those who were in the study at baseline | ||
| At least one admission | 35/235 | 15 |
| Respiratory infection admission | 13/235 | 6 |
| UTI admission | 7/235 | 3 |
| Dehydration admission | 1/235 | 0.4 |
| CHF admission | 1/235 | 0.4 |
| At least one ambulance called | 42/235 | 18 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 29/235 | 12 |
| At least one A&E attendance | 38/235 | 16 |
| Dieda | 34/214 | 16 |
| Characteristic | TAU | Intervention | ||
|---|---|---|---|---|
| n/N or median | % or (IQR) | n/N or median | % or (IQR) | |
| Month 1 | ||||
| At least one admission in the previous month | 6/132 | 5 | 7/92 | 8 |
| Respiratory infection admission | 3/132 | 2 | 2/92 | 2 |
| UTI admission | 2/131 | 2 | 2/92 | 2 |
| Dehydration admission | 0/131 | 0 | 0/92 | 0 |
| CHF admission | 0/131 | 0 | 0/92 | 0 |
| At least one ambulance called | 7/132 | 5 | 10/92 | 11 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 6/133 | 5 | 5/92 | 5 |
| At least one A&E attendance | 8/132 | 6 | 8/92 | 9 |
| Month 2 | ||||
| At least one admission in the previous month | 8/129 | 6 | 4/89 | 4 |
| Respiratory infection admission | 3/129 | 2 | 1/89 | 1 |
| UTI admission | 2/129 | 2 | 0/89 | 0 |
| Dehydration admission | 0/129 | 0 | 1/89 | 1 |
| CHF admission | 0/129 | 0 | 1/89 | 1 |
| At least one ambulance called | 6/129 | 5 | 2/89 | 2 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 2/129 | 2 | 4/89 | 4 |
| At least one A&E attendance | 5/129 | 4 | 3/89 | 3 |
| Month 3 | ||||
| At least one admission in the previous month | 3/127 | 2 | 4/75 | 5 |
| Respiratory infection admission | 0/127 | 0 | 2/75 | 3 |
| UTI admission | 0/127 | 0 | 0/75 | 0 |
| Dehydration admission | 0/127 | 0 | 0/75 | 0 |
| CHF admission | 0/127 | 0 | 0/75 | 0 |
| At least one ambulance called | 2/127 | 2 | 5/75 | 7 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 0/127 | 0 | 6/75 | 8 |
| At least one A&E attendance | 0/127 | 0 | 6/75 | 8 |
| Month 4 | ||||
| At least one admission in the previous month | 5/126 | 4 | 3/71 | 4 |
| Respiratory infection admission | 1/126 | 1 | 1/71 | 1 |
| UTI admission | 1/126 | 1 | 0/71 | 0 |
| Dehydration admission | 0/126 | 0 | 0/71 | 0 |
| CHF admission | 0/126 | 0 | 0/71 | 0 |
| At least one ambulance called | 5/126 | 4 | 3/71 | 4 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 3/126 | 2 | 1/71 | 1 |
| At least one A&E attendance | 4/126 | 7 | 3/71 | 4 |
| Month 5 | ||||
| At least one admission in the previous month | 2/122 | 2 | 3/71 | 4 |
| Respiratory infection admission | 1/122 | 1 | 0/71 | 0 |
| UTI admission | 0/122 | 0 | 0/71 | 0 |
| Dehydration admission | 0/122 | 0 | 0/71 | 0 |
| CHF admission | 0/122 | 0 | 0/71 | 0 |
| At least one ambulance called | 2/122 | 2 | 2/71 | 3 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 3/122 | 2 | 1/71 | 1 |
| At least one A&E attendance | 1/122 | 1 | 3/71 | 4 |
| Month 6 | ||||
| At least one admission in the previous month | 1/116 | 1 | 1/68 | 1 |
| Respiratory infection admission | 0/116 | 0 | 0/68 | 0 |
| UTI admission | 0/116 | 0 | 0/68 | 0 |
| Dehydration admission | 0/116 | 0 | 0/68 | 0 |
| CHF admission | 0/116 | 0 | 0/68 | 0 |
| At least one ambulance called | 3/116 | 3 | 3/68 | 4 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 1/116 | 1 | 2/68 | 3 |
| At least one A&E attendance | 2/116 | 2 | 3/68 | 4 |
| Barthel Index score | 20 | (15–37) | 20 | (15–34) |
| Over the 6 months for those who were in the study for the whole 6-month period | ||||
| At least one admission | 14/114 | 12 | 7/68 | 10 |
| Respiratory infection admission | 5/114 | 4 | 2/68 | 3 |
| UTI admission | 4/114 | 4 | 1/68 | 1 |
| Dehydration admission | 0/114 | 0 | 1/68 | 1 |
| CHF admission | 0/114 | 0 | 0/68 | 0 |
| At least one ambulance called | 15/114 | 13 | 13/68 | 19 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 10/115 | 9 | 9/68 | 13 |
| At least one A&E attendance | 13/114 | 11 | 12/68 | 18 |
| Over the 6 months for those who were in the study at baseline | ||||
| At least one admission | 19/139 | 14 | 16/96 | 17 |
| Respiratory infection admission | 8/139 | 6 | 5/96 | 5 |
| UTI admission | 5/139 | 4 | 2/96 | 2 |
| Dehydration admission | 0/139 | 0 | 1/96 | 1 |
| CHF admission | 0/139 | 0 | 1/96 | 1 |
| At least one ambulance called | 20/138 | 14 | 22/96 | 23 |
| At least one unscheduled (out-of-hours) GP visit or telephone contact | 14/139 | 10 | 15/96 | 16 |
| At least one A&E attendance | 17/139 | 12 | 21/96 | 22 |
| Died | 19/132 | 14 | 15/82 | 18 |
The difference in percentages for those who had an admission who were in the study at baseline is (group 1 – group 0) 3.0 (95% CI –6.4 to 12.4). Clearly, this is not a fair comparison as a couple of care homes from the same arm dropped out (and, by definition, the residents had to drop out too), so there were fewer admissions in that arm than there might have been.
Appendix 26 Health economics analysis
Cost-effectiveness of an intervention for reducing hospital admissions in people living in nursing homes: a cluster randomised controlled trial (BHiRCH-NH)
Aim
The aim of the economic evaluation was to evaluate the cost-effectiveness of delivering the BHiRCH-NH intervention, compared with TAU, over 6 months. This is reported as the incremental cost per QALY gained and the probability of cost-effectiveness for a range of values of WTP for a QALY gained.
Outcomes
-
The EQ-5D-5L scores at baseline and 6 months [residents’ self-completed questionnaires and carer-perception (about the resident) questionnaires].
-
Resource use at baseline (covering the previous month) and monthly for the following 6 months.
The economic analysis was conducted from the NHS and PSS perspective. We calculated the incremental cost per QALY gained over 6 months and the probability of cost-effectiveness for a range of values of WTP for a QALY gained. Analyses followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Good Reporting Practices. 144
Analyses
Cost of delivery of the BHiRCH-NH intervention
The cost of the BHiRCH-NH intervention included the costs of training PDCs, materials and delivery of training, nursing home staff time for delivery of the intervention, preparation and travel time. Research staff providing training gave details of the number of sessions they delivered and the duration of the sessions, any preparation and travel time. The cost of materials was provided by the study team. Other resources used included catering, venue hire and administration. Hourly costs for care home staff were taken from the Personal Social Services Research Unit (PSSRU). 145
Health-care resource use and costs
Health-care resource use was collected, including information about primary, community or emergency services, hospital admissions, ambulance and A&E services, using a specifically designed questionnaire based on Client Service Receipt Inventory. 146 The questionnaire was completed by research assistants at baseline for the previous 1 month and monthly for the following 6 months.
Service use was costed using published costs (NHS reference costs147 and PSSRU145). The cost of prescribed medication was taken from the British National Formulary. 148
Linear regression including covariates for randomisation to BHiRCH-NH or TAU, baseline service use costs and clustering for care home with 1000 bootstrap replications (bias corrected) was used to calculate the mean difference in costs between BHiRCH-NH and TAU and 95% CIs.
Utilities and quality-adjusted life-years
Quality-adjusted life-years were calculated as the area under the curve, adjusting for baseline differences,149 gained over 6 months in the BHiRCH-NH intervention, and were compared with the TAU group. QALYs were calculated using the EQ-5D-5L and the formula developed by EuroQol. 150–152 We ran some analyses using carers’ perception values for EQ-5D-5L for comparison. Carers’ quality of life was also assessed using the EQ-5D-5L questionnaire. Both carer-completed questionnaires had a poor return.
Linear regression including covariates for randomisation to BHiRCH-NH or TAU, baseline utility and clustering for care home with 1000 bootstrap replications (bias corrected) was used to calculate the mean difference in QALYs between BHiRCH-NH and TAU and 95% CIs.
Missing data
Data were missing for resource use and utility scores. Multivariate imputation was used to impute missing cost and QALY values by chained equation. We generated 20 imputed data sets. For each of the 20 imputed data sets, we ran 1000 bootstrap replications using non-parametric bootstrapping, resampling observations with replacements. 153
All analyses were conducted using Stata version 14.
Incremental cost-effectiveness ratio
The mean incremental cost per QALY gained of BHiRCH-NH, compared with TAU, was calculated by dividing the group randomisation coefficient calculated in the cost bootstrap analysis by the randomisation coefficient in the QALY bootstrap analysis. There was no discounting of costs or QALYs, given that they were reported over 6 months only. All costs are reported in 2016/17 Great British pounds.
Cost-effectiveness plane and cost-effectiveness acceptability curve
The joint distribution of the incremental expected costs and incremental expected QALYs of the two interventions was plotted on a cost-effectiveness plane. To report the probability that the BHiRCH-NH intervention is cost-effective, compared with TAU, for a range of values of WTP for a QALY gained, the bootstrap results have been used to generate a cost-effectiveness acceptability curve,154 and the probability that the BHiRCH-NH intervention is cost-effective, compared with TAU, at a £20,000 and £30,000 WTP for a QALY gained is reported.
Expected value of perfect information and expected value of partial perfect information for parameters
We modelled the lifetime costs and outcomes of the BHiRCH-NH intervention, compared with TAU. This involved assessing the quality of the published information available, the development of an initial model and the identification of which cost and outcome components would benefit most from further research, that is EVPI and EVPPI analysis for a single group and groups of parameters using the Sheffield Accelerated Value of Information model. 155
We converted EVPI for an individual patient to a population-level estimate based on the number of potential patients expected to benefit from a chosen intervention over a certain time horizon. 76 If the EVPI is low, it is improbable that gathering more information will be worth it, or the cost of obtaining the data has to remain modest.
If further research seems potentially worthwhile, based on the population EVPI, it would be useful to identify the particular aspects worth studying to resolve the uncertainty surrounding them. 77 A parameter with a higher EVPPI is more uncertain, and further research can be designed and focused to get a more precise estimate of its value.
Results
Cost of the BHiRCH-NH intervention, total costs and quality-adjusted life-years
The total cost of training and delivery of the intervention at each nursing home was recorded and is presented in Table 21. One nursing home withdrew after randomisation; therefore, we used an ‘as randomised’ approach for the analysis and considered the cost of intervention in this nursing home to be £0. Assuming that BHiRCH-NH intervention would be offered to all 89 residents randomised to the intervention group, the mean cost per resident would be £74 (95% CI £64 to £84).
| Cost | Nursing homes | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 (withdrawn) | |
| Number of residents randomised to the intervention | 20 | 15 | 22 | 7 | 20 | |
| Traininga (£) | 912 | 696 | 744 | 884 | 628 | 0 |
| Materials used in trainingb (£) | 4 | 4 | 4 | 4 | 4 | 0 |
| Delivery of interventionc (£) | 300 | 1090 | 1597 | 344 | 38 | 0 |
| Total (£) | 1216 | 1790 | 2345 | 1232 | 670 | 0 |
Health-care resource use and costs
During the 6 months’ follow-up, there were no significant differences in any component of health-care resource use between the BHiRCH-NH and TAU groups (Table 22). The most common types of contact were primary care, community health or emergency services for both groups, with a mean of 9.6 (SD 7.6) visits per resident in the BHiRCH-NH group and 8.3 (SD 6.1) visits per resident in the TAU group. However, the difference in cost of outpatient appointments is significantly greater (£27, 95% CI £1.20 to £53.50) in the BHiRCH-NH group; this could be because of more costly service use by the residents in the intervention group.
| Type of resource use (unit) | Randomised group, mean (SD); n | Difference (intervention vs. TAU) | Mean cost (£) | Incremental difference (intervention vs. TAU), mean cost (95% CI) (£) | |||
|---|---|---|---|---|---|---|---|
| TAU | Intervention | Mean | p-value | TAU | Intervention | ||
| Primary care, community health or emergency services (visits) | 8.3 (6.1); 114 | 9.6 (7.6); 71 | 1.3 | 0.20 | 491.30 | 694.90 | 203.6 (–67.4 to 474.6) |
| Outpatient appointments (visits) | 0.4 (1.0); 114 | 0.7 (1.5); 68 | 0.3 | 0.11 | 22.60 | 49.90 | 27.4 (1.2 to 53.5) |
| Inpatient services (admissions) | 0.2 (0.5); 114 | 0.2 (0.6); 68 | 0.001 | 0.99 | 356.90 | 426.60 | 69.6 (–333.8 to 472.9) |
| Length of stay in hospital (days) | 0.8 (3.9); 114 | 0.9 (3.9); 68 | 0.2 | 0.76 | |||
| Ambulance service (calls) | 0.2 (0.6); 114 | 0.2 (0.5); 68 | 0.04 | 0.61 | 1.30 | 1.60 | 0.3 (–0.9 to 1.4) |
| A&E (attendances) | 0.1 (0.5); 114 | 0.3 (0.6); 68 | 0.1 | 0.21 | 23.10 | 38.70 | 15.6 (–8.9 to 40.1) |
| Prescriptions (packages) | 54.1 (23.8); 114 | 60.5 (26.6); 68 | 6.33 | 0.10 | 325.70 | 381.60 | 55.9 (–43.8 to 155.6) |
| Resource use | Source | Unit cost (£) |
|---|---|---|
| Outpatient appointments | ||
| Anticoagulant clinic | NHS reference costs OP | 16 |
| Biopsy | NHS reference costs OP | 135 |
| Breast surgeon | NHS reference costs OP | 99 |
| Cardiology | NHS reference costs OP | 52 |
| Colposcopy | NHS reference costs OP | 244 |
| CT | NHS reference costs OP | 98 |
| Dermatologist | NHS reference costs OP | 77 |
| Diabetic clinic | NHS reference costs OP | 129 |
| Elderly care practitioner | NHS reference costs OP | 92 |
| ENT | NHS reference costs OP | 78 |
| Gastroscopy | NHS reference costs OP | 104 |
| General surgery | NHS reference costs OP | 95 |
| Neurology | NHS reference costs OP | 146 |
| Oncologist | NHS reference costs OP | 100 |
| Ophthalmology | NHS reference costs OP | 63 |
| Optometrist | NHS reference costs OP | 62 |
| Orthopaedic clinic | NHS reference costs OP | 98 |
| Orthotics | NHS reference costs OP | 121 |
| Psychiatrist | NHS reference costs OP | 40 |
| Psychologist | NHS reference costs OP | 130 |
| Radiologist | NHS reference costs OP | 145 |
| Respiratory nurse | NHS reference costs OP | 117 |
| Ultrasonography | NHS reference costs OP | 94 |
| Urology | NHS reference costs OP | 94 |
| Vascular clinic | NHS reference costs OP | 139 |
| A&E attendances, ambulance services (calls) | ||
| A&E visit | NHS reference costs AMB | 155 |
| A&E call | NHS reference costs AMB | 7 |
| Non-emergency paramedic ambulance | NHS reference costs AMB | 181 |
| Primary care, community health or emergency services | ||
| Audiologist | NHS reference costs CHS | 53 |
| Best-interest assessor | PSSRU | 619 |
| Cancer nurse | NHS reference costs CHS | 69 |
| Care home assessment team | PSSRU | 193 |
| Chiropodist/podiatrist | NHS reference costs CHS | 40 |
| Community dietitian | NHS reference costs CHS | 81 |
| Community mental health nurse | PSSRU | 39 |
| Community nursing team | PSSRU | 193 |
| Community psychiatric nurse | NHS reference costs CHS | 198 |
| Community rehabilitation team | NHS reference costs CHS | 94 |
| Continence care nurse | NHS reference costs CHS | 80 |
| Crisis intervention | PSSRU | 40 |
| Dementia team/memory clinic | PSSRU | 435 |
| Dentist | PSSRU | 101 |
| Enteral nurse | NHS reference costs CHS | 82 |
| Deprivation of liberty safeguards | PSSRU | 1438 |
| GP: surgery | PSSRU | 38 |
| GP: telephone | PSSRU | 12 |
| Occupational therapist | NHS reference costs CHS | 79 |
| Palliative care team | NHS reference costs CHS | 92 |
| Parkinson’s nurse | NHS reference costs CHS | 70 |
| Physiotherapist | NHS reference costs CHS | 49 |
| Pharmacist | PSSRU | 28 |
| Phlebotomist | NHS reference costs DAPS | 3 |
| Practice nurse | PSSRU | 36 |
| Rapid response team | PSSRU | 80 |
| Social care worker | PSSRU | 48 |
| Speech and language therapist | NHS reference costs CHS | 88 |
| Stoma nurse | NHS reference costs CHS | 41 |
| Tissue viability nurse | NHS reference costs CHS | 51 |
| Wheelchair service | NHS reference costs CHS | 92 |
| Inpatient services (reasons for admissions) | ||
| Bradycardia | NHS reference costs NEL | 1812 |
| Collapse/syncopal episode | NHS reference costs NEL | 1209 |
| Deep-vein thrombosis | NHS reference costs NEL | 1453 |
| Diabetic ketoacidosis | NHS reference costs NEL | 1351 |
| Fall | NHS reference costs NEL | 1847 |
| Haematuria | NHS reference costs NEL | 1395 |
| Haemorrhage | NHS reference costs NEL | 2279 |
| High INR levels | NHS reference costs NEL | 2332 |
| Hypertension | NHS reference costs NEL | 1369 |
| Hypokalaemia/acute kidney injury | NHS reference costs NEL | 3872 |
| Low potassium levels, dehydration | NHS reference costs NEL | 4919 |
| Metabolic acidosis secondary to sepsis | NHS reference costs NEL | 2570 |
| Respiratory infection | NHS reference costs NEL | 2618 |
| Seizure | NHS reference costs NEL | 2202 |
| Sepsis due to cellulitis | NHS reference costs NEL | 3510 |
| Stroke | NHS reference costs NEL | 3109 |
| Unable to see, no response to light | NHS reference costs NEL | 3058 |
| UTI | NHS reference costs NEL | 1570 |
| Worsening peripheral oedema | NHS reference costs NEL | 1905 |
| Medication | Route | Cost/package (£) |
|---|---|---|
| Colecalciferol (Aciferol®, Rhodes Pharma Ltd) | Tablets | 3.21 |
| Nifedipine (Adalat®, Bayer plc) | Tablets | 7.89 |
| Calcium carbonate/colecalciferol (Adcal D3®, Kyowa Kirin Ltd) | Tablets | 2.95 |
| Alendronic acid (Accord Healthcare Ltd) | Tablets | 2.95 |
| Allopurinol (A A H Pharmaceuticals Ltd) | Tablets | 3.65 |
| Alverine citrate (A A H Pharmaceuticals Ltd) | Tablets | 4.90 |
| Rivastigmine (Alzest®, Dr Reddy’s Laboratories UK Ltd) | Patch | 19.68 |
| Amiodarone hydrochloride (Accord Healthcare Ltd) | Tablets | 0.74 |
| Amisulpride (Milpharm Ltd) | Tablets | 1.56 |
| Amitriptyline hydrochloride (Accord Healthcare Ltd) | Tablets | 0.77 |
| Amlodipine (Accord Healthcare Ltd) | Tablets | 0.85 |
| Amoxicillin (Kent Pharmaceuticals Ltd) | Tablets | 14.80 |
| Anusol cream® (Church & Dwight UK Ltd) | Cream | 19.97 |
| Apixaban (Bristol-Myers Squibb Pharmaceuticals Ltd) | Tablets | 1.53 |
| Apomorphine hydrochloride (Britannia Pharmaceuticals Ltd) | Inhaler | 1.91 |
| Aripiprazole (Milpharm Ltd) | Tablets | 0.61 |
| Aspirin (Medreich Plc) | Tablets | 1.09 |
| Atenolol (Accord Healthcare Ltd) | Tablets | 0.59 |
| Atorvastatin (Almus Pharmaceuticals Ltd) | Tablets | 0.73 |
| Azathioprine (Ennogen Pharma Ltd) | Tablets | 0.76 |
| Azithromycin (Accord Healthcare Ltd) | Tablets | 0.89 |
| Baclofen [Alliance Healthcare (Distribution) Ltd] | Tablets | 1.26 |
| Beclometasone (Mylan) | Inhaler | 2.49 |
| Bisacodyl (Dr Reddy’s Laboratories UK Ltd) | Tablets | 5.05 |
| Bisoprolol fumarate (Teva UK Ltd) | Tablets | 11.30 |
| Hyoscine butylbromide (Buscopan®, Sanofi) | Tablets | 0.30 |
| Buprenorphine (Butec®, Qdem Pharmaceuticals Ltd) | Patch | 0.71 |
| Buprenorphine (Butrans®, Napp Pharmaceuticals Ltd) | Patch | 0.75 |
| Calcium carbonate (Takeda UK Ltd) | Tablets | 15.40 |
| Calcium carbonate/colecalciferol (BR Pharmaceuticals Ltd) | Tablets | 1.62 |
| Carbamazepine (Medreich Plc) | Tablets | 1.55 |
| Carbimazole (Flamingo Pharma UK Ltd) | Tablets | 2.70 |
| Cefalexin (Milpharm Ltd) | Tablets | 1.23 |
| Cefradine [Alliance Healthcare (Distribution) Ltd] | Tablets | 1.39 |
| Celiprolol hydrochloride (Mylan) | Tablets | 1.76 |
| Cetirizine hydrochloride (Dexcel-Pharma Ltd) | Tablets | 9.75 |
| Chloramphenicol eye (Almus Pharmaceuticals Ltd) | Drops | 1.96 |
| Chlorphenamine maleate (Bristol Laboratories Ltd) | Tablets | 0.78 |
| Ciprofoloxacin (Accord Healthcare Ltd) | Tablets | 0.79 |
| Ciprozole (Accord Healthcare Ltd) | Tablets | 0.87 |
| Citalopram Rivopharm (UK) Ltd) | Tablets | 4.32 |
| Clobetasol propionate (Accord Healthcare Ltd) | Cream | 1.64 |
| Clonazepam (A A H Pharmaceuticals Ltd) | Tablets | 4.37 |
| Clotrimazole (The Boots Company PLC) | Tablets | 17.60 |
| Clozapine (Mylan) | Tablets | 2.92 |
| Co-amoxiclav® (Medreich Plc) | Tablets | 7.92 |
| Co-beneldopa® (Roche Products Ltd) | Tablets | 14.20 |
| Co-careldopa® (Zentiva) | Tablets | 17.60 |
| Co-codamol® (A A H Pharmaceuticals Ltd) | Tablets | 25.86 |
| Codeine phosphate® [Alliance Healthcare (Distribution) Ltd] | Tablets | 31.55 |
| Co-dydramol®[Alliance Healthcare (Distribution) Ltd] | Tablets | 17.60 |
| Colchicine (Ria Generics Ltd) | Tablets | 3.88 |
| Colecalciferol (Alissa Healthcare Research Ltd) | Tablets | 3.65 |
| Colistimethate (Teva UK Limited) | Tablets | 6.75 |
| Co-tenidone® (A A H Pharmaceuticals Ltd) | Tablets | 2.14 |
| Co-trimoxazole® (A A H Pharmaceuticals Ltd) | Tablets | 3.65 |
| Cyanocobalamin (RPH Pharmaceuticals AB) | Injection | 9.33 |
| Dexamethasone (Consilient Health Ltd) | Tablets | 4.69 |
| Diazepam (Teva UK Ltd) | Tablets | 6.75 |
| Digoxin (Teva UK Ltd) | Tablets | 62.37 |
| Dipyridamole (Accord Healthcare Ltd) | Tablets | 7.49 |
| Docusate sodium (UCB Pharma Ltd) | Tablets | 0.81 |
| Domperidone [Alliance Healthcare (Distribution) Ltd] | Tablets | 0.82 |
| Donepezil (A A H Pharmaceuticals Ltd) | Tablets | 3.54 |
| Dosulepin (A A H Pharmaceuticals Ltd) | Tablets | 7.69 |
| Doxazosin (A A H Pharmaceuticals Ltd) | Tablets | 0.84 |
| Doxycycline (Ennogen Pharma Ltd) | Tablets | 1.43 |
| Enalapril (Merck Sharp & Dohme Ltd) | Tablets | 19.20 |
| Erythromycin (A A H Pharmaceuticals Ltd) | Tablets | 5.05 |
| Felodipine (Chiesi Ltd) | Tablets | 3.70 |
| Fentanyl (Janssen-Cilag Ltd) | Patch | 16.17 |
| Ferrous fumarate [Alliance Healthcare (Distribution) Ltd] | Tablets | 3.70 |
| Ferrous gluconate (Kent Pharmaceuticals Ltd) | Tablets | 2.69 |
| Ferrous sulphate (Accord-UK Ltd) | Tablets | 27.69 |
| Fesoterodine (Pfizer Ltd) | Tablets | 1.33 |
| Fexofenadine [Alliance Healthcare (Distribution) Ltd] | Tablets | 1.33 |
| Finasteride (Teva UK Ltd) | Tablets | 3.85 |
| Flucloxacillin (A A H Pharmaceuticals Ltd) | Tablets | 2.95 |
| Fluticasone (Sandoz Limited) | Inhaler | 6.56 |
| Fluticasone/Formoterol (Napp Pharmaceuticals Ltd) | Inhaler | 6.91 |
| Fluticasone/Salmeterol (Aspire Pharma Ltd) | Inhaler | 11.19 |
| Folic acid (Essential-Healthcare Ltd) | Tablets | 7.30 |
| Furosemide [Alliance Healthcare (Distribution) Ltd] | Tablets | 7.01 |
| Gabapentin (A A H Pharmaceuticals Ltd) | Tablets | 9.58 |
| Galantamine (Teva UK Ltd) | Tablets | 0.81 |
| Galantamine (Gatalin®, Aspire Pharma Ltd) | Tablets | 0.93 |
| Gaviscon® (GlaxoSmithKline plc, Brentford, UK) | Liquid | 1.46 |
| Glycerol (A A H Pharmaceuticals Ltd) | Suppositories | 3.65 |
| Haloperidol (Crescent Pharma Ltd) | Liquid | 15.50 |
| Hyoscine (Bayer plc) | Tablets | 10.91 |
| Hyoscine butylbromide (Sanofi) | Tablets | 12.93 |
| Hyoscine hydrobromide (Bayer plc) | Tablets | 8.75 |
| Ibuprofen (A A H Pharmaceuticals Ltd) | Tablets | 14.50 |
| Isosorbide mononitrate (Dexcel-Pharma Ltd) | Tablets | 11.36 |
| Lansoprazole (Teva UK Ltd) | Tablets | 2.25 |
| Latanoprost eye (Kent Pharmaceuticals Ltd) | Drops | 10.06 |
| Lercanidipine hydrochloride (A A H Pharmaceuticals Ltd) | Tablets | 7.79 |
| Letrozole [Alliance Healthcare (Distribution) Ltd] | Tablets | 7.99 |
| Leuprorelin (Takeda UK Ltd) | Tablets | 0.71 |
| Levetiracetam [Alliance Healthcare (Distribution) Ltd] | Tablets | 1.11 |
| Levomepromazine (Sanofi) | Tablets | 1.10 |
| Levothyroxine (Accord Healthcare Ltd)) | Tablets | 0.74 |
| Lisinopril (A A H Pharmaceuticals Ltd) | Tablets | 2.10 |
| Lithium (Essential Pharma M) | Tablets | 2.94 |
| Loperamide (McNeil Products Ltd) | Tablets | 11.66 |
| Loratadine (Accord Healthcare Ltd) | Tablets | 1.01 |
| Lorazepam (A A H Pharmaceuticals Ltd) | Tablets | 0.84 |
| Losartan (Sandoz Ltd) | Tablets | 0.90 |
| Lymecycline [Alliance Healthcare (Distribution) Ltd] | Tablets | 30.27 |
| Mebeverine (A A H Pharmaceuticals Ltd) | Tablets | 5.03 |
| Meloxicam (Niche Generics Ltd) | Tablets | 0.75 |
| Memantine (A A H Pharmaceuticals Ltd) | Tablets | 4.14 |
| Meptazinol (Almirall Ltd) | Tablets | 1.45 |
| Metformin [Alliance Healthcare (Distribution) Ltd] | Tablets | 16.72 |
| Methotrexate (Nordic Pharma Ltd) | Tablets | 26.31 |
| Metoclopramide (Peckforton Pharmaceuticals Ltd) | Tablets | 24.36 |
| Metronidazole [Alliance Healthcare (Distribution) Ltd] | Tablets | 4.21 |
| Fentanyl (Mezolar®, Sandoz Ltd) | Patch | 6.31 |
| Midazolam (Novell Pharmaceutical Laboratories) | Tablets | 1.00 |
| Mirabegron (Astellas Pharma Ltd) | Tablets | 3.50 |
| Mirtazapine (Milpharm Ltd) | Tablets | 3.73 |
| Morphine sulphate (A A H Pharmaceuticals Ltd) | Injection | 25.78 |
| Naproxen (A A H Pharmaceuticals Ltd) | Tablets | 2.17 |
| Fluticasone propionate (Nasofan®, GlaxoSmithKline UK Ltd) | Inhaler | 29.96 |
| Nebivolol (A A H Pharmaceuticals Ltd) | Tablets | 41.75 |
| Nicorandil (Zentiva) | Tablets | 16.51 |
| Nifedipine (Advanz Pharma) | Tablets | 30.00 |
| Nitrofurantoin (A A H Pharmaceuticals Ltd) | Tablets | 55.00 |
| Ofloxacin (Mylan) | Tablets | 0.80 |
| Olanzapine (A A H Pharmaceuticals Ltd) | Tablets | 2.37 |
| Omeprazole (A A H Pharmaceuticals Ltd) | Tablets | 7.48 |
| Oxybutynin (A A H Pharmaceuticals Ltd) | Tablets | 0.39 |
| Oxycodone (Morningside Healthcare Ltd) | Tablets | 0.40 |
| Oxytetracycline (Crescent Pharma Ltd) | Tablets | 0.69 |
| Pantoprazole (Takeda UK Ltd) | Tablets | 21.00 |
| Paracetamol (Medreich Plc) | Tablets | 1.91 |
| Paracetamol (Phoenix Labs Ltd) | Suppositories | 2.82 |
| Paroxetine (GlaxoSmithKline UK Ltd) | Tablets | 29.32 |
| Penicillin (Sandoz Limited) | Tablets | 25.94 |
| Pentoxifylline (Sanofi) | Tablets | 32.45 |
| Perindopril erbumine (Accord Healthcare Ltd) | Tablets | 3.23 |
| Phenytoin [Alliance Healthcare (Distribution) Ltd] | Tablets | 4.46 |
| Piracetam (UCB Pharma Ltd) | Tablets | 0.55 |
| Piroxicam (Pfizer Ltd) | Tablets | 3.36 |
| Pivmecillinam (A A H Pharmaceuticals Ltd) | Tablets | 11.52 |
| Pramipexole (Mylan) | Tablets | 9.95 |
| Pravastatin (Teva UK Ltd) | Tablets | 7.16 |
| Prednisolone (Teva UK Ltd) | Tablets | 1.04 |
| Prednisone (Teva UK Ltd) | Tablets | 0.84 |
| Promethazine (Sanofi) | Tablets | 3.93 |
| Propranolol (Teva UK Ltd) | Tablets | 7.12 |
| Quetiapine (Accord Healthcare Ltd) | Tablets | 3.00 |
| Ramipril (Brown & Burk UK Ltd) | Tablets | 2.92 |
| Ranitidine (Zentiva) | Tablets | 5.23 |
| Ranolazine (A. Menarini Farmaceutica Internazionale SRL) | Tablets | 1.99 |
| Risedronate (Milpharm Ltd) | Tablets | 1.70 |
| Risperidone (Sandoz Ltd) | Tablets | 15.76 |
| Rivaroxaban (Bayer Plc) | Tablets | 15.10 |
| Rivastigmine (Sandoz Ltd) | Tablets | 17.50 |
| Rosuvastatin (Mylan) | Tablets | 15.10 |
| Rotigotine (UCB Pharma Ltd) | Tablets | 35.28 |
| Roxatidine (Aventis Pharma Ltd) | Tablets | 5.56 |
| Safinamide (Profile Pharma Ltd) | Tablets | 6.54 |
| Salbutamol (GlaxoSmithKline UK Ltd) | Tablets | 2.19 |
| Saline nebuliser (A A H Pharmaceuticals Ltd) | Inhaler | 3.40 |
| Salmeterol (GlaxoSmithKline UK Ltd) | Inhaler | 2.72 |
| Seretide (GlaxoSmithKline UK Ltd) | Inhaler | 0.58 |
| Simvastatin (Merck Sharp & Dohme Ltd) | Tablets | 41.5 |
| Sinemet® (Merck Sharp & Dohme Ltd) | Tablets | 1.40 |
| Sodium chloride (Baxter Healthcare Ltd) | Solution | 1.26 |
| Sodium valproate (Sanofi) | Solution | 1.98 |
| Spironolactone (A A H Pharmaceuticals Ltd) | Tablets | 5.50 |
| Symbiocort (AstraZeneca UK Ltd) | Inhaler | 1.66 |
| Tamoxifen (Genesis Pharmaceuticals Ltd) | Tablets | 2.91 |
| Tamsulosin (Sanofi) | Tablets | 12.75 |
| Carbamazepine (Tegretol®, Novartis Pharmaceuticals UK Ltd) | Tablets | 68.21 |
| Temazepam (Mylan) | Tablets | 88.98 |
| Tetracycline (A A H Pharmaceuticals Ltd) | Tablets | 7.00 |
| Thiamine (Essential-Healthcare Ltd) | Tablets | 33.26 |
| Thrimetropin (Crescent Pharma Ltd) | Tablets | 0.79 |
| Diltiazem hydrochloride (Tildiem LA®, Sanofi) | Tablets | 0.83 |
| Tinzaparin [Alliance Healthcare (Distribution) Ltd] | Tablets | 5.79 |
| Tiotropium (Boehringer Ingelheim Ltd) | Tablets | 0.33 |
| Tizanidine (Niche Generics Ltd) | Tablets | 0.96 |
| Tolterodine (Sandoz Ltd) | Tablets | 2.15 |
| Torasemide (Mylan) | Tablets | 0.28 |
| Tramadol (Brown & Burk UK Ltd) | Tablets | 2.59 |
| Trazodone (A A H Pharmaceuticals Ltd) | Tablets | 3.54 |
| Venlafaxine (Torrent Pharma UK Ltd) | Tablets | 15.39 |
| Verapamil (Accord Healthcare Ltd) | Tablets | 25.50 |
| Vitamin B (A A H Pharmaceuticals Ltd) | Tablets | 43.13 |
| Vitamin D3 (Ennogen Healthcare Ltd) | Tablets | 46.88 |
| Warfarin [Alliance Healthcare (Distribution) Ltd] | Tablets | 1.00 |
| Co-codamol [Zapain®, Alliance Healthcare (Distribution) Ltd] | Tablets | 37.64 |
| Zolpidem (Zentiva) | Tablets | 7.53 |
| Zopiclone (Kent Pharmaceuticals Ltd) | Tablets | 20.13 |
| Zoton (Zentiva) | Tablets | 34.60 |
In the complete-case analysis, the mean total cost of health-care resource use per resident over 6 months was £1560 (95% CI £912 to £2209) in the BHiRCH-NH group and £1250 (95% CI £900 to £1599) in the TAU group. Accounting for missing data using multiple imputation, the values were £1458 (95% CI £1351 to £1566) in the BHiRCH-NH group and £1233 (95% CI £1171 to £1295) in the TAU group (Table 25).
| Type of cost | Randomised group, cost (£) | Difference (Intervention vs. TAU) (£) | |
|---|---|---|---|
| TAU | Intervention | ||
| Mean (SD) cost | Mean (SD) cost | ||
| Training | 0 (0) | 39 (28) | 39 |
| Materials used in training | 0 (0) | 0.2 (0.10) | 0.2 |
| Delivery of the intervention | 0 (0) | 34 (32) | 34 |
| BHiRCH-NH intervention costs per participant | 0 (0) | 74 (50) | 74 |
| Total cost (95% CI) | Total cost (95% CI) | Difference (95% CI) | |
| Total cost per resident of health-care resource use over 6 months | |||
| Complete cases | 1250 (900 to 1599); n = 105 | 1560 (912 to 2209); n = 66 | 310 (–361 to 981); n = 171 |
| With imputation | 1233 (1171 to 1295) | 1458 (1351 to 1566) | 225 (108 to 342) |
| Total cost per resident of BHiRCH-NH intervention cost plus health-care resource use over 6 months | |||
| Complete cases | 1250 (900 to 1599); n = 105 | 1640 (989 to 2291); n = 66 | 390 (–283 to 1063); n = 171 |
| With imputation | 1233 (1171 to 1295) | 1532 (1424 to 1640) | 299 (182 to 417) |
Adding the cost of the intervention to the cost of health-care resource use over the 6 months, the mean total cost in the BHiRCH-NH group became £1640 (95% CI £989 to £2291) in the complete case and £1532 (95% CI £1424 to £1640) after accounting for missing data (see Table 25).
Utilities and quality-adjusted life-years
Using responses from resident self-reported EQ-5D-5L questionnaires
The differences in mean utility values per resident at baseline (–0.123, 95% CI –0.230 to –0.016) and in QALYs (–0.078, 95% CI –0.148 to –0.008) were statistically significant when resident self-reported questionnaires were administered.
Accounting for missing data, mean utility values per resident in the BHiRCH-NH group increased from 0.504 (95% CI 0.491 to 0.518) to 0.649 (95% CI 0.637 to 0.661) at 6 months. The mean utility values per resident in the TAU group increased from 0.617 (95% CI 0.607 to 0.628) to 0.652 (95% CI 0.641 to 0.662) at 6 months. The differences in mean QALYs at 6 months favoured TAU when resident self-reported questionnaires were administered (–0.029, 95% CI –0.037 to –0.022) (Table 26).
| Utility values | TAU | Intervention | Incremental difference | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Complete cases | ||||||
| Baseline | 0.610 (n = 69) | 0.540 to 0.680 | 0.487 (n = 58) | 0.403 to 0.570 | –0.123 | –0.230 to –0.016 |
| 6 months | 0.685 (n = 59) | 0.610 to 0.759 | 0.614 (n = 32) | 0.501 to 0.727 | –0.071 | –1.995 to 0.058 |
| QALYs | 0.337 (n = 46) | 0.297 to 0.377 | 0.259 (n = 26) | 0.196 to 0.321 | –0.078 | –0.148 to –0.008 |
| With imputations | ||||||
| Baseline | 0.617 | 0.607 to 0.628 | 0.504 | 0.491 to 0.518 | –0.113 | –0.130 to –0.097 |
| 6 months | 0.652 | 0.641 to 0.662 | 0.649 | 0.637 to 0.661 | –0.003 | –0.202 to 0.012 |
| QALYs | 0.317 | 0.312 to 0.322 | 0.289 | 0.283 to 0.294 | –0.029 | –0.037 to –0.022 |
Using responses from carers’ perception of EQ-5D-5L questionnaires
The differences in mean utility values per resident at 6 months (0.195, 95% CI 0.013 to 0.377) were statistically significant when carers’ perception questionnaires were administered.
Accounting for missing data, mean utility values per resident in the BHiRCH-NH group increased from 0.274 (95% CI 0.264 to 0.285) to 0.441 (95% CI 0.428 to 0.453) at 6 months. The mean utility values per resident in the TAU group decreased from 0.344 (95% CI 0.337 to 0.352) to 0.257 (95% CI 0.247 to 0.267) at 6 months.
The differences in mean QALYs at 6 months favoured the BHiRCH-NH intervention when carers’ perception questionnaires were administered (0.028, 95% CI 0.023 to 0.034) (Table 27).
| Utility values | TAU | Intervention | Incremental difference | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Complete cases | ||||||
| Baseline | 0.357 (n = 49) | 0.294 to 0.419 | 0.277 (n = 29) | 0.178 to 0.376 | –0.079 | –0.189 to 0.030 |
| 6 months | 0.291 (n = 28) | 0.177 to 0.405 | 0.485 (n = 17) | 0.335 to 0.636 | 0.195 | 0.013 to 0.377 |
| QALYs | 0.160 (n = 25) | 0.113 to 0.208 | 0.176 (n = 13) | 0.101 to 0.252 | 0.016 | –0.066 to 0.098 |
| With imputations | ||||||
| Baseline | 0.344 | 0.337 to 0.352 | 0.274 | 0.264 to 0.285 | –0.070 | –0.083 to –0.058 |
| 6 months | 0.257 | 0.247 to 0.267 | 0.441 | 0.428 to 0.453 | 0.183 | 0.168 to 0.199 |
| QALYs | 0.150 | 0.147 to 0.154 | 0.179 | 0.174 to 0.183 | 0.028 | 0.023 to 0.034 |
Using responses from carers’ own quality-of-life EQ-5D-5L questionnaires
The differences in mean utility values and QALYs were not statistically significant when carers’ quality-of-life questionnaires were administered. Accounting for missing data, mean utility values per carer in the BHiRCH-NH group increased from 0.925 (95% CI 0.919 to 0.927) to 0.944 (95% CI 0.940 to 0.949) at 6 months. However, the mean utility values per carer in the TAU group decreased from 0.908 (95% CI 0.902 to 0.914) to 0.812 (95% CI 0.800 to 0.823) at 6 months (Table 28).
| Utility values | TAU | Intervention | Incremental difference | |||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Complete cases | ||||||
| Baseline | 0.903 (n = 53) | 0.859 to 0.947 | 0.914 (n = 35) | 0.870 to 0.947 | 0.011 | –0.054 to 0.075 |
| 6 months | 0.788 (n = 30) | 0.669 to 0.907 | 0.919 (n = 18) | 0.846 to 0.992 | 0.131 | –0.030 to 0.292 |
| QALYs | 0.419 (n = 28) | 0.376 to 0.462 | 0.455 (n = 18) | 0.427 to 0.483 | 0.036 | –0.021 to 0.093 |
| With imputations | ||||||
| Baseline | 0.908 | 0.902 to 0.914 | 0.925 | 0.919 to 0.927 | 0.016 | 0.008 to 0.024 |
| 6 months | 0.812 | 0.800 to 0.823 | 0.944 | 0.940 to 0.949 | 0.133 | 0.119 to 0.147 |
| QALYs | 0.430 | 0.426 to 0.433 | 0.467 | 0.465 to 0.469 | 0.037 | 0.033 to 0.042 |
Differences in mean QALYs at 6 months favoured the BHiRCH-NH intervention when carers’ own quality-of-life questionnaires were administered (0.037, 95% CI 0.033 to 0.042) (see Table 28).
Analysis using utility values based on resident self-reported questionnaires
The non-parametric bootstrapping produced a mean total cost per resident in the BHiRCH-NH group of £1479 (95% CI £757 to £2200), compared with £1271 (95% CI £975 to £1566) for the TAU group. The mean difference in cost between BHiRCH-NH and the TAU group was £208 (95% CI –£561 to £977), which was not statistically significant (Table 29).
| Incremental cost (£) | QALYs gained | |||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Resident self-completed | ||||
| Base casea | 208 | –561 to 977 | 0.016 | 0.003 to 0.300 |
| Complete caseb | 352 | –745 to 1448 | 0.018 | –0.012 to 0.048 |
| Carers’ perception | ||||
| Base casea | 214 | –554 to 982 | 0.052 | 0.044 to 0.060 |
| Complete caseb | 352 | –745 to 1448 | 0.039 | –0.028 to 0.057 |
Non-parametric bootstrapping after multiple imputation produced 0.315 (95% CI 0.304 to 0.326) QALYs in the BHiRCH-NH group and 0.298 (95% CI 0.290 to 0.307) QALYs in the TAU group, generating a mean difference in QALYs of 0.016 (95% CI 0.003 to 0.300), which was statistically significant (see Table 29).
Analysis using utility values based on carers’ perception questionnaires
The non-parametric bootstrapping produced a mean total cost per resident in the BHiRCH-NH group of £1468 (95% CI £742 to £2193), compared with £1254 (95% CI £973 to £1534) for the TAU group. The mean difference in cost between the BHiRCH-NH and the TAU groups was £214 (95% –£554 to £982), which was not statistically significant (see Table 29).
Non-parametric bootstrapping after multiple imputation produced 0.193 (95% CI 0.187 to 0.198) QALYs in the BHiRCH-NH group and 0.140 (95% CI 0.135 to 0.146) QALYs in the TAU group, generating a mean difference in QALYs of 0.052 (95% CI 0.044 to 0.060), which was statistically significant (see Table 29).
Incremental cost-effectiveness ratio, cost-effectiveness plane and cost-effectiveness acceptability curve
Using utility values based on resident self-reported questionnaires
The incremental cost per QALY gained of the BHiRCH-NH intervention, compared with TAU, was £12,633.
The majority of incremental cost-effectiveness pairs (and the point estimate of the incremental cost-effectiveness ratio) fall in the north-east quadrant of the incremental cost-effectiveness plane, indicating that the BHiRCH-NH intervention is more costly and more effective than TAU. However, a proportion of the points lie in the south-east quadrant, indicating that the BHiRCH-NH intervention is less costly and more effective than TAU (Figure 6). This confirms that there is some uncertainty concerning whether or not and at what value the BHiRCH-NH intervention is cost-effective.
FIGURE 6.
Incremental cost-effectiveness plane (1000 bootstrapped replicates).
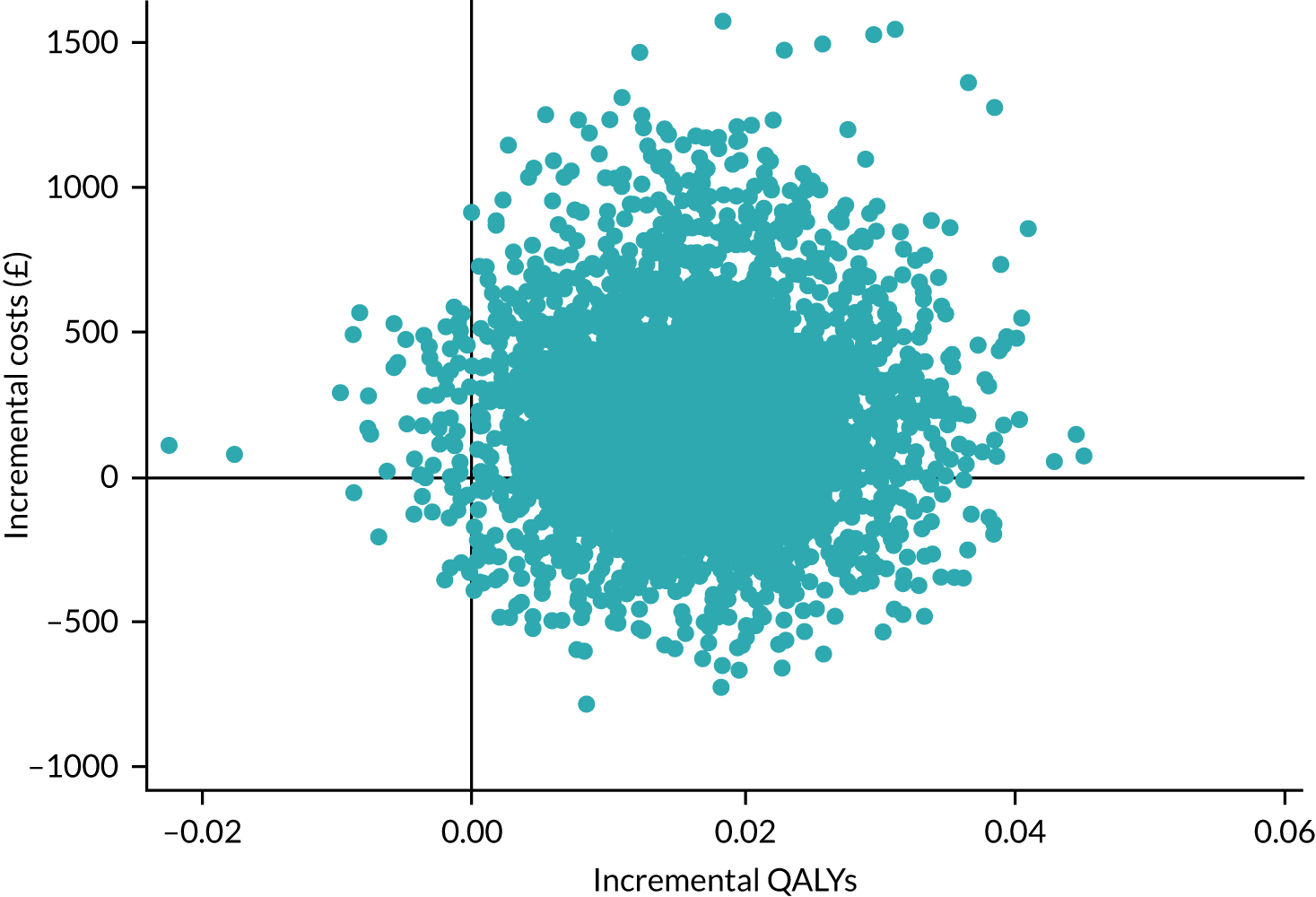
Residents receiving the BHiRCH-NH intervention accrued a non-significantly higher cost, and a very small increase in QALYs, which was statistically significant; the BHiRCH-NH intervention has a 65% probability of being cost-effective at a WTP value of £20,000 and a 77% probability of being cost-effective at a WTP value of £30,000 (Figure 7).
FIGURE 7.
Cost-effectiveness acceptability curve showing the probability that the BHiRCH-NH intervention is cost-effective, compared with TAU, at different values of WTP for a QALY, n = 237. Reproduced with permission from Sampson et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
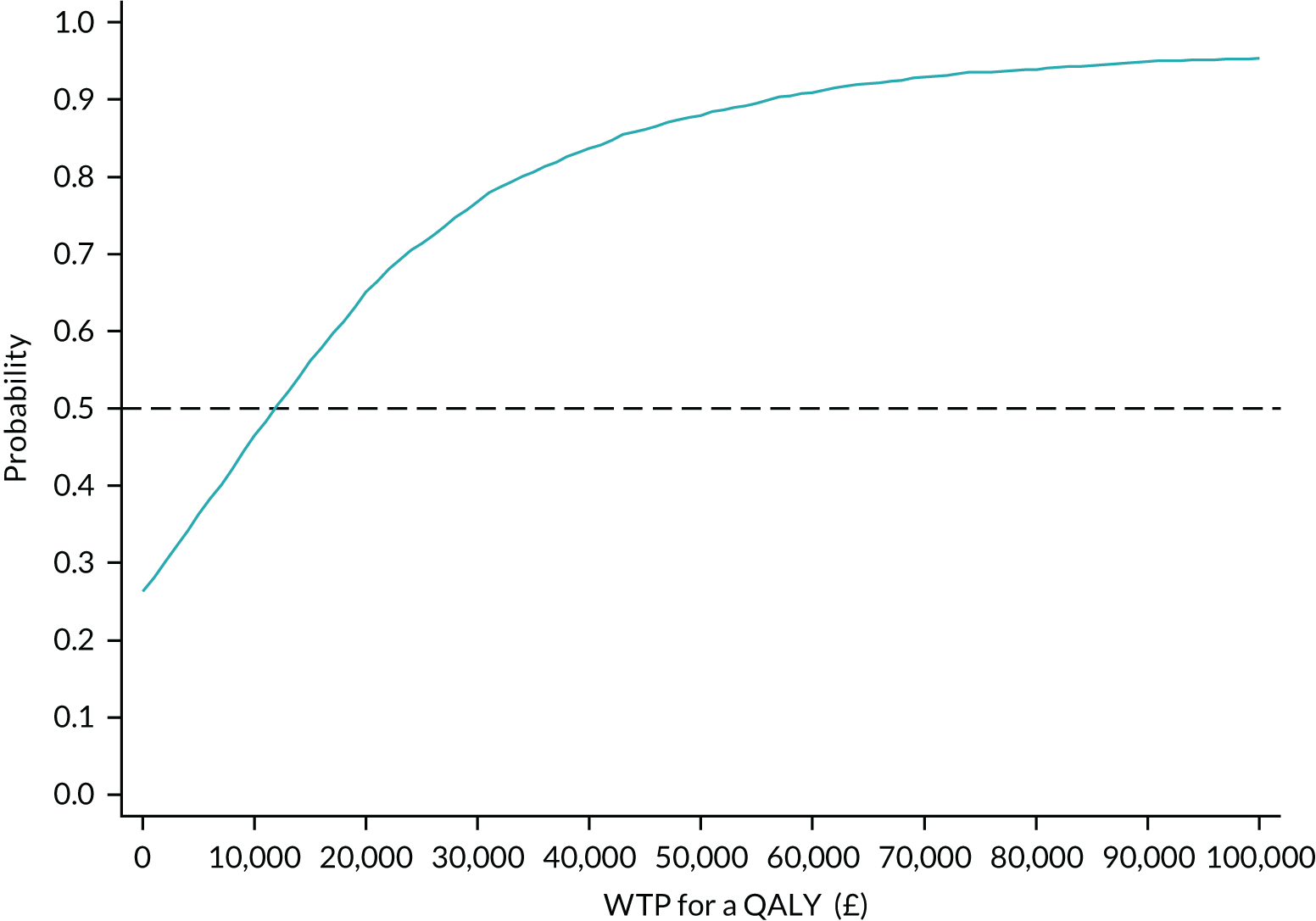
Expected value of perfect information
The overall EVPI per person affected by the decision is estimated to be £94.88. This is equivalent to 0.005 QALYs per person on the health effects scale (Figure 8).
FIGURE 8.
Overall EVPI (on costs scale).
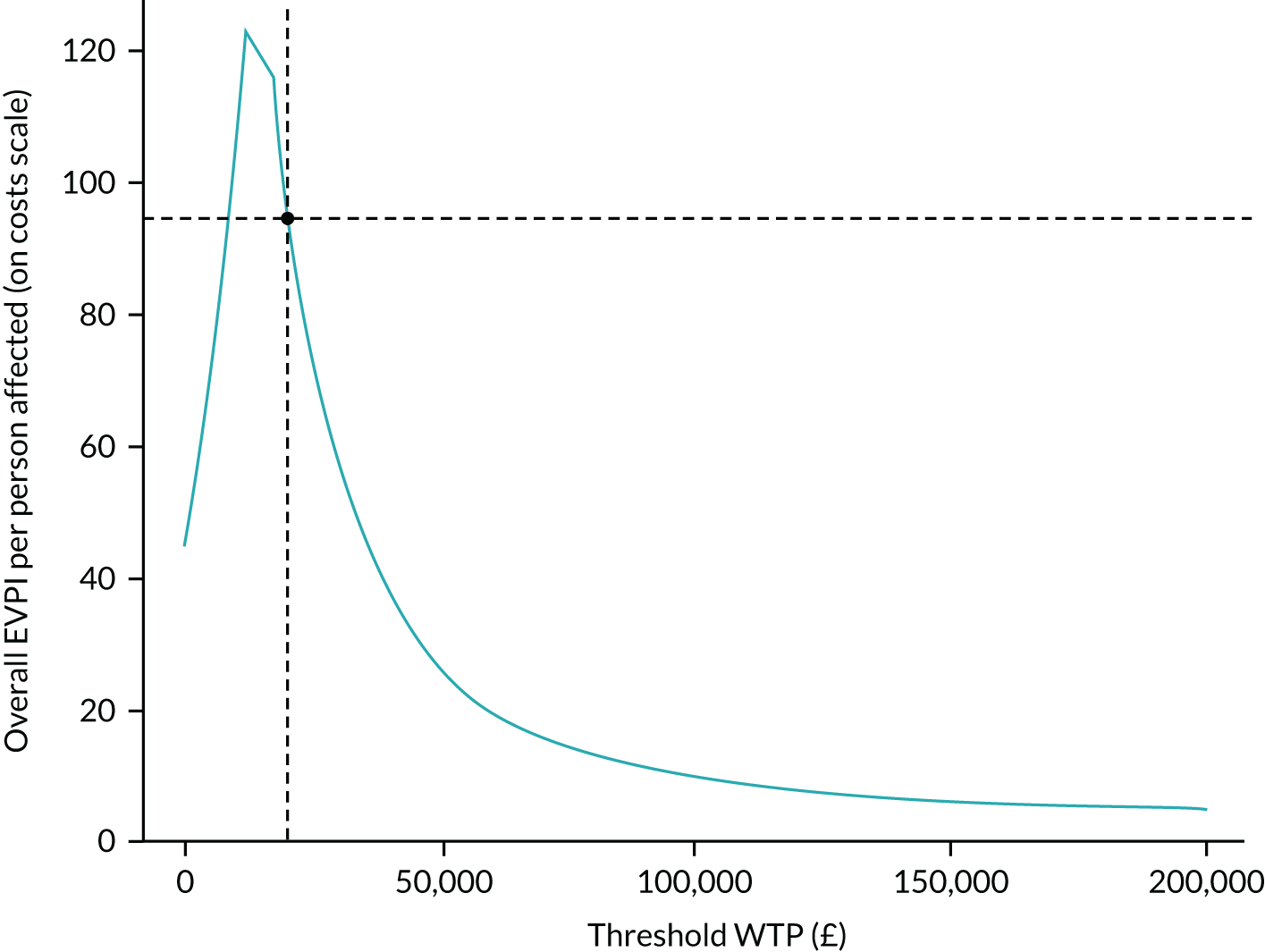
If the number of people affected by the decision per year is 1000, then the overall EVPI per year is £94,878 for England.
When thinking about the overall expected value of removing decision uncertainty, one needs to consider how long the current comparison will remain relevant. If the decision relevance horizon is 10 years, then the overall expected value of removing decision uncertainty for England would be £948,783. Research or data collection exercises costing more than this amount would not be considered an efficient use of resources.
Expected value of partial perfect information for parameters
Expected value of partial perfect information enables identification of those parameters that contribute significantly to decision uncertainty.
In this study, cost parameters are causing most of the decision uncertainty and the potential value of reducing the uncertainty by collecting more data is estimated at £53.99 per person (Figure 9).
FIGURE 9.
The EVPPI per resident by model parameters at £20,000 per QALY gained.
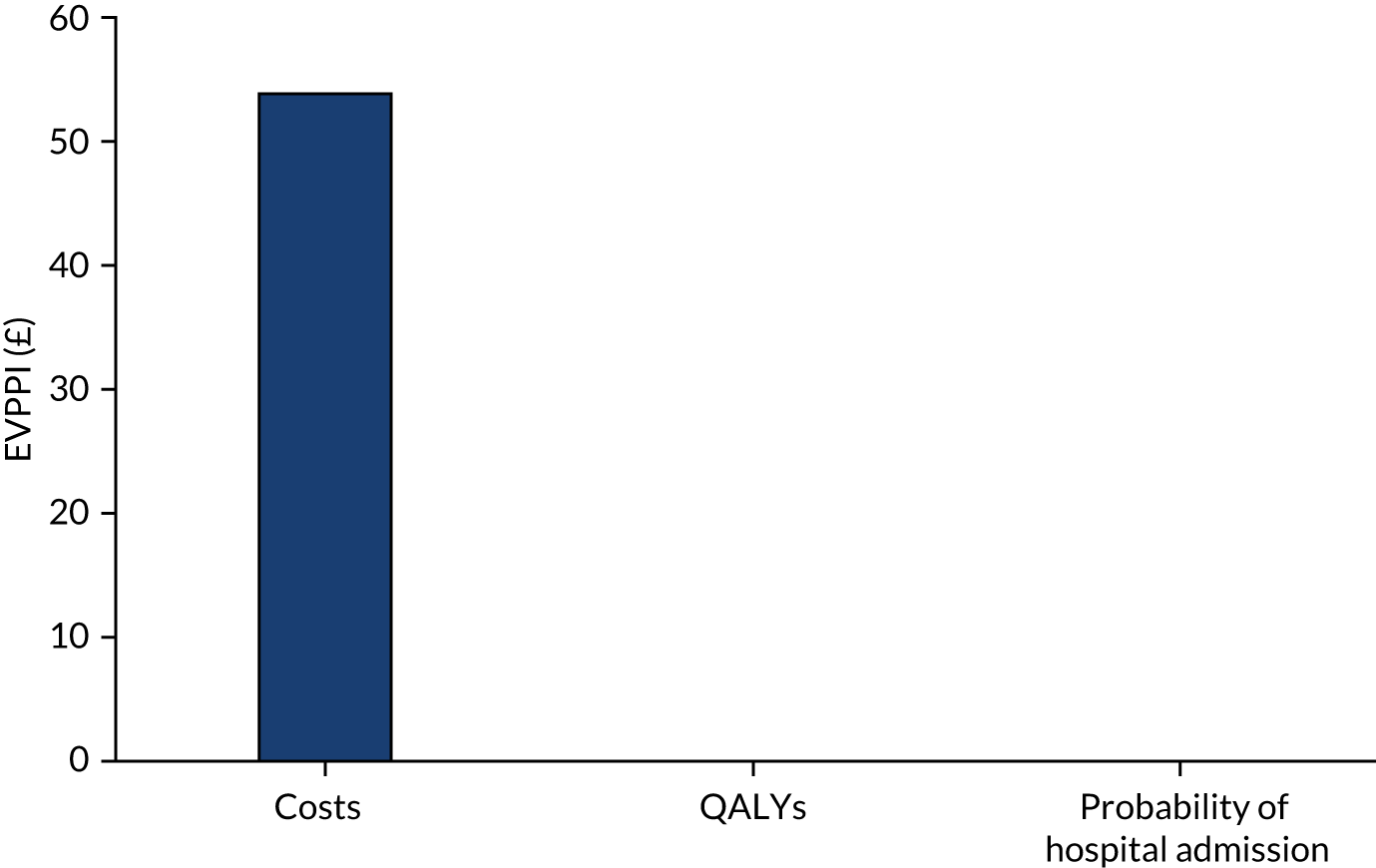
Economic implications
The economic results showed that the intervention is more costly than TAU and has a very small increase in QALYs.
Whether or not the EQ-5D-5L questionnaire and QALYs are the correct denominator for our intervention can be questioned.
A tendency for carer perception values to be lower than self-reported values is likely to result from the fact that the cognitively impaired people are less able to recognise deteriorations in their own health. The carer ratings are going in the opposite direction (decrease) in the TAU group in our sample; the reason might be that the number of people with dementia is higher in this group. There was a higher median number of residents with dementia in the TAU group, possibly because the TAU group contained dementia-specialist homes, whereas the intervention group did not.
These findings are set against a backdrop of uncertain cost-effectiveness for the majority of complex interventions delivered in nursing homes.
Appendix 27 An example of our process of listening to Carer Reference Panel feedback
A project poster, pilot study patient information sheet and consent form were circulated to the CRP members; our response to their feedback is summarised in this appendix.
Feedback from the Carer Reference Panel on pilot study patient information sheet and consent form, December 2016/January 2017
| CRP member | Issue | Team response |
|---|---|---|
| SB (Yorkshire) | I think that the information sheets/consent forms all read very well. I seem to think that in previous docs we have said something to reassure people that should hospital admission be necessary it will still take place? | This sentence ‘Please be assured that where a hospitable admission is needed, this will take place’ has been added to resident, care partner and consultee information sheet |
| Will all care partners be given their info sheet as a matter of course or will residents be asked if they want their family members to be involved (apologies if this has been discussed previously)? And depending on the answer to that question, do we need to mention in the Residents’ info sheet that they can have their care partner involved if they wish, or if all care partners get the sheet that we have informed the care partner . . . hope this makes sense! | All care partners will be invited to take part in the project if their relative/friend agrees to take part. It may not be appropriate to add that we will be contacting care partners as some residents may not have a care partner | |
| I think the poster and launch event document are fine | Thank you for your feedback | |
| WM (Yorkshire) | BHiRCH-NH trial poster:Love this one :) | Thank you for the positive feedback |
| Launch event:This one will look fine, especially when the photo of a smiley face is added | Thank you for the positive feedback | |
| Resident patient information sheet:With this one – there’s an awful lot of words and little colour – it doesn’t look very friendly – could it be broken up with an image or two? And I always think a smiley photo of the researcher helps to engage, even if it’s just at the end, or maybe the beginning to say hello | A picture of the local researcher will be added | |
| GR (Yorkshire) | BHIRCH-NH trial poster:Very good | Thank you for the positive feedback |
| BHIRCH-NH launch event:No problem | Thank you for the positive feedback | |
| Resident consent form:Final paragraph page 1. I do not like the word ‘testing’. Much prefer any of ‘pilot studying’ or investigating or evaluating. Residents might infer guinea pig connotation otherwise | This was discussed during the London CRP and the consensus was that ‘trying out’ would be better than ‘testing’. This has been replaced | |
| Final paragraph again. Would ‘more drowsiness’ and ‘ increasing loss of appetite’ be better since most residents will have instances of drowsiness and loss of appetite anyway | This was also discussed at the London CRP, where it was agreed that we are interested in a change in these presentations, rather than it being in one direction, such as increasing. This was made clearer on the information sheet | |
| Page 3. The paragraph re insurance is, of course, necessary but I wonder if the insurance is specific to this project or an umbrella one for the University activities as a whole. The way it reads implies there are risks in taking part and prospective participants might be then unnecessarily waryIf it is an umbrella policy then wording could be different and less implicit in risks for this particular project. In either case I wonder if the last sentence is really necessary of ‘Participants may be able to claim compensation etc.’. The first sentence relates itself to claims anyway | Unfortunately we were told to include this paragraph by our sponsor; therefore we are unable to change this | |
| Care Partners consent form:No problem | ||
| Information sheet and declaration form for PC:Page 5 Permission sought to inform relative’s/friend’s GP of participation. I assume that if permission is not given then the resident involved cannot take part. If that is true then does it need stating? | If the relative/friend does not wish for the resident’s GP to be informed, the resident can still take part in the study | |
| VC (Yorkshire) | I think the phrase ‘end up’ in terms of a patient in residential care going into hospital would be better if it was less colloquial, for example, it could read, ‘Sometimes, this results in people in care-homes being admitted to hospital.’ I could be nit-picking! | This has been changed on the launch poster |
| Overall, I think the documents are easy to read, the order of content is just right. The layout is clear; there isn’t any jargon that I feel isn’t understandable – although having read lots of this type of document I may have got used to some jargon! | Thank you for the positive feedback | |
| I think the ethics application forms for each group are well-written and I look forward to hearing about the launch | Thank you for the positive feedback | |
| SN (London) | The heading on each information sheet perhaps include . . . ‘in Nursing Care Homes’ and after that just refer to care homes | Unfortunately unable to change the heading as this would no longer fit with the acronym of BHiRCH-NH. However, the first subheading now includes ‘Nursing care home’ |
| The care home where . . . live is trying out a new way . . . | ‘Testing’ has been replaced with ‘trying out’ | |
| ‘. . . observing you more closely for changes in your physical health such as x x x x > If specific changes occur care home staff will follow new guidelines to investigate the problem . . .’ | May leave the text as it is, as the intervention is not a ‘new guideline’ | |
| Drop the pseudo bit and just use anonymously . . . | The sponsor will wish for us to use this terminology. However, a definition has been added, and the research staff will explain the term during the consent process | |
|
This has been changed | |
| Poster is too wordy. Possibly the photo of the resident and a child could be a bit smaller? Agree that if possible could have one version for staff room in care home and another on the main notice-board for the residents to see | We have created two new versions of the poster, for staff and for general use in public areas. We have also changed the paragraph order, and made some small changes to text to clarify our aims and what we will implement | |
| Switch round the first and second paragraphs | ||
| 3rd para . . . ‘anyone who is involved in the day-to-day living of the care home may be able to contribute by alerting nurses and staff if they notice unusual signs or a change in a resident’s behaviour . . .’ | ||
| CH | Launch poster:Change ‘common ill-health conditions’ to common ill-health problems’ | This has been changed |
| Change ‘emergency presentation at hospital’ to emergency attendance at hospital’ | This has been changed | |
| Resident information sheet:Change ‘Health illnesses’ to ‘health issues’ | This has been changed | |
| Change ‘testing’ to ‘ trying out’ | ‘Testing’ has been replaced with ‘trying out’ | |
| Change ‘Staff will regularly check for early warning signs of illness such as changes in drowsiness, change in appetite, changes in how you communicate or change in your heart rate’ to ‘Staff will regularly check for early warning signs of illness such as changes in dehydration, urine infections, chest infections, drowsiness, loss of appetite, changes in how you communicate or a change in your heart rate’ | The team would like to keep early warnings signs as generic as possible in order for staff to complete the S&W | |
| GS | Launch poster:Change ‘ill-health conditions’ to ‘ill-health problems’ | This has been changed |
| Move ‘People living in care homes can sometimes be admitted to hospital for conditions which, if noticed and treated earlier, could have been managed in the care home’ to the beginning of the paragraph | This has been changed | |
| Add ‘Research shows’ to the beginning of ‘People living in care homes can sometimes be admitted to hospital for conditions which, if noticed and treated earlier, could have been managed in the care home’ | This has been changed | |
| Early detection and active management of these conditions has the potential to prevent deterioration in physical health leading to an emergency at attendance hospital | ‘Physical’ has been inserted into the sentence | |
| Early detection and active management of these conditions has the potential to prevent deterioration in physical health leading to an emergency attendance referral to hospital | In the general discussion, the term emergency attendance appeared to be a more popular choice | |
| Research shows that people living in care homes can sometimes be admitted to hospital for conditions which, if noticed and treated earlier, could have been managed more successfully in the care home | This has been changed | |
| Care partner information sheet:Care partner is considered jargon | This term was believed to cover both friends and relatives and cannot be changed at this point | |
| This new project involves care home staff being trained to observeing residents more closely for changes in their physical health | These revisions have been implemented. The sentence, ‘observing residents more closely than normal’ was not added as this may not be the case in each care home | |
| At a time convenient to you, a researcher will ask you some questions about how your quality of life are feeling so that the research team can assess whether the project positively impacts is beneficial to you as well as your relative/friend | These revisions have been made as the word ‘feeling’ was believed to be ambiguous, and ‘positively impacts’ was believed to be jargon | |
| You will be helping to improve the care that all care home residents receive in the future | This revision has been made | |
| Resident information sheet:Before you say ‘yes’ or ‘no’ to taking part in the project, we would like you to know why the research is being done and what would happen if you were to take part you would probably want to know why the research is being done and what it will involve. (Original text was perceived as patronising) | All suggestions have been actioned other than:
|
|
| Replaced health ‘illnesses’ with health ‘issues’ throughout | ||
| Early identification of changes in your health is essential to ensure active health management in care homes, so that you do not need to go to hospital unless absolutely necessary this may also lead to hospital admissions being avoided | ||
| The care home where you live might be trying out a new way to ensure better health in residents in care homes and reduce hospital admissions The care home where you live is testing a new way to ensure better health in residents in care homes and reduce hospital admissions | ||
| A sentence needs to be added regarding the use of a consultee ‘If you are unable to decide for yourself if you would like to take part in the project, we will ask someone who knows you to decide whether it would be in your best interests to take part in the project’ | ||
| We hope that you will not wish to, but if you do decide to so, you can withdraw from the project at any time You can withdraw from the project at any time. This will not affect your care in any way. If you wish to withdraw from the project, we would use the information collected up to the time of your withdrawal unless you tell us that you want all information to be destroyed | ||
| Personal consultee information sheet:Term consultee is considered jargon | All suggestions have been actioned other than:
|
|
| Invitation to take part in a nursing care home research project | ||
| Replaced health ‘illnesses’ with health ‘issues’ throughout | ||
| This project is about the early identification of changes in residents’ health which can ensure active health care in nursing homes and may also avoid the need to admit your relative/friend to hospital unnecessarily. Please be assured that where a hospitable admission is needed, this will take place | ||
| Discussion at London CRP, 11 January 2017 | The term pseudo anonymous is confusing | A definition has been added |
| More detail should be added regarding how data are pseudo-anonymised and how this impacts on anonymity and confidentiality | A clearer, more comprehensive paragraph has been added in all patient information sheets | |
| ‘Before you decide if you would like to take part, you probably want to know why the research is being done and what it will involve’ was perceived as condescending | This sentence has been changed to:Before you say ‘yes’ or ‘no’ to taking part in the project, we would like you to know why the research is being done and what would happen if you were to take part | |
| The term ‘health issues’ was preferred to ‘health illnesses’ | This was amended in the resident, care partner and personal consultee information sheets | |
| With respect to the resident information sheet, the panel wanted the resident to be informed that a personal or professional consultee may be asked to provide proxy consent if they do not have capacity, and to include that the research team may also ask the care partner questions about their quality of life |
This has been addressed by inserting: All points regarding the launch poster were implemented |
|
| The headings ‘what if there is a problem’, ‘Who is organising the research’ and ‘who has reviewed and approved the research’ were thought to be better placed at the end of the document rather than in the middle | These sections were moved towards the end of the information sheet | |
Launch event poster:
|
All of these points have been addressed |
Appendix 28 Paper by patient and public involvement co-applicants
Woodward-Carlton B, Nurock S. Better Health in Residents in Care Homes. Network News, produced for the Alzheimer’s Society Research Network. 2017; issue 14. Reproduced with permission from the Alzheimer’s Society.
Shirley and Barbara are involved in the Better Health in Residents in Care Homes (BHiRCH-NH) project. The project is funded by the National Institute for Health Research (NIHR). In the following article they elaborate on the project and their role.
Alzheimer’s Society Research Network Volunteers in Yorkshire and Humberside have over the years formed a close working relationship with the research staff of the University of Bradford’s School of Dementia Studies, which has enabled us to participate in a number of research projects with them. One of the most recent collaborations has been involvement in the 3-year 3-month NIHR-funded BHiRCH-NH (Better Health in Residents in Care Homes) project led by Professor Murna Downs, along with clinicians and researchers from UCL, Queen Margaret University, Edinburgh, Newcastle University and Lancaster University.
It is a major concern to the NHS and government that so many people who live in care homes, not just those with dementia, are sent to hospital to be treated for conditions that could have been treated in the care homes, if they had been picked up earlier. Evidence has shown that noticing changes in health of residents earlier is essential to ensure good health for residents.
The aim of the project is to develop and test new ways of identifying and so treating earlier any small changes in the health of residents in order to maintain better health in residents and also reduce rates of avoidable admissions to hospitals from care homes. Research Network Volunteers from Yorkshire and London with experience of a relative living in a care home were invited to join one of the two Carer Reference Panels (CRPs) where their knowledge and observations could enhance the study. Professor Katherine Froggatt of Lancaster University facilitates all CRP meetings, with Shirley Nurock chairing the London CRP and Barbara Woodward-Carlton the Yorkshire panel.
Members of both CRPs, now up and running, have been able to make contributions to the development of the education and care programme, which is based on previous evidence and consultation with care staff, medical staff and family members. To date we have been in discussions on clarifying the role of family members and how, if at all, they wish to be involved in their relatives’ care, with special consideration to communication between families and care home staff, reviewing patient information sheets, choosing a logo and other aspects of the project. Our meetings are interesting and stimulating: a genuine forum for exchanging ideas.
This is a complex project with a number of work streams. It is still in the early stages but ethics approval has been granted to try out the staff training programme and use of the newly devised programme of care in care homes in Yorkshire. Further testing will occur in 2017 in care homes in Yorkshire and London.
Reproduced with permission from the Alzheimer’s Society. © Queen’s Printer and Controller of HMSO 2021. This work was produced by Forster et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Appendix 29 Paper by patient and public involvement co-applicants
Nurock S, Woodward-Carlton B. Update on the Better Health in Residents in Care Homes project. Network News, produced for the Alzheimer’s Society Research Network. 2018; issue 23. Reproduced with permission from the Alzheimer’s Society.
Two years ago, Barbara Woodward-Carlton and Shirley Nurock talked to Network News about the BHiRCH-NH study (Better Health in Residents in Care Homes). Data collection is now well under way in Yorkshire and London, and in this article the pair reflect on their involvement so far.
The BHiRCH-NH project is about identifying and managing small changes in residents’ health to keep them well and prevent avoidable admission to hospital. It’s a complex project which sparked extensive debate around the ethics of research in care homes, and which has also faced recruitment challenges.
The work is led by Professor Murna Downs (University of Bradford) with colleagues from UCL, Queen Margaret University, Edinburgh, Lancaster and Newcastle Universities. Barbara (Yorkshire) and Shirley (London) each chair a Carer Reference Panel (CRP) of Research Network Volunteers (RNVs) with experience of a relative living in a care home. Each CRP meets twice a year with the research team to discuss progress, review documents and consider the role of family members in their relatives’ care.
Shirley writes:
Since we last wrote it has been an interesting experience getting more fully involved with the project. I attended a launch event at a care home in London, where Alex, a researcher from UCL, and I gave a presentation about BHiRCH-NH to recruit residents, their relatives and staff to the project.
I spoke about my experiences when my late husband was in a care home and staff failed to heed my concerns about his health. He was admitted to A&E three times, usually at night, for reasons that could have been picked up earlier. I saw many relatives nodding in recognition of the situation.
I’ve also been involved in implementing the intervention. Barbara and I attended training days for care home staff who had agreed to act as Practice Development Champions (PDCs) to put the intervention into practice.
Some CRP members (including me) want to be involved with data analysis. We had a training session to introduce the topic and I am grateful to the seven enthusiastic RNVs who regularly attend the London CRP meetings; you have great ideas and are never reticent about expressing your views, negative or otherwise! Thank you all.
Barbara writes:
I too, have a fantastic group of RNVs as advocates for the BHiRCH-NH project. Their willingness to travel to Bradford from across Yorkshire, North Lincolnshire, Hull and Humberside indicates outstanding dedication to this project.
The Yorkshire PDC training day that I attended promised to be ‘stimulating, creative, exciting and most of all a worthwhile experience that equips you (the champions) with knowledge, skills and expertise that can be used effectively . . .’. It certainly achieved that aim.
The PDCs were quiet at first and a little quizzical. What had they, or their managers, let them in for? But by the end of the day, all of them were happily contributing and discussing how they could implement the guides, confident that their managers would support them.
The excellent presentations from the team were key to unlocking this interest. Brendan McCormack spoke of the importance of stories as evidence and so I shared my experiences and others gave examples of theirs. One particularly poignant story from two nurses gave us all pause for thought.
We left with our copies of the project handbook knowing so much more than when we arrived. The nurses were enthused and set off home buoyed up by access to research that would improve the lives of those they cared for, and likewise their own lives too.
Reproduced with permission from the Alzheimer’s Society. © Queen’s Printer and Controller of HMSO 2021. This work was produced by Forster et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Glossary
- Care home
- Used to refer to residential care homes and care homes with nursing. Residential care homes do not have a nurse on site 24 hours a day. Care homes with nursing have a nurse on site 24 hours a day. This study was concerned with practice in care homes with nursing. These settings are referred to as nursing homes.
- Family carers
- Refers to family, partners or friends closest to the person. Initially, the term ‘care partner’ was used; it was changed following a request from the Carer Reference Panel, which felt that the term care partner could also apply to care staff.
List of abbreviations
- A&E
- accident and emergency
- ACSC
- ambulatory care-sensitive condition
- BHiRCH-NH
- Better Health in Residents in Care Homes with Nursing
- CCG
- Clinical Commissioning Group
- CHF
- congestive heart failure
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CQC
- Care Quality Commission
- CRN
- Clinical Research Network
- CRP
- Carer Reference Panel
- CTU
- Clinical Trials Unit
- ENRICH
- ENabling Research In Care Homes
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- GP
- general practitioner
- INTERACT
- Interventions to Reduce Acute Care Transfers
- IQR
- interquartile range
- NIHR
- National Institute for Health Research
- PARiHS
- Promoting Action on Research Implementation in Health Services
- P-CAT
- Person-centred Care Assessment Tool
- PDC
- Practice Development Champion
- PDSG
- practice development support group
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SBAR
- situation, background, assessment, recommendation
- SD
- standard deviation
- SIRR
- structured implicit record review
- S&W
- Stop and Watch Early Warning Tool
- TAU
- treatment as usual
- UTI
- urinary tract infection
- WHELD
- Well-being and Health for people with Dementia
- WP
- work package
- WS
- workstream
- WTP
- willingness to pay
