Notes
Article history
The research reported in this issue of the journal was funded by the PGfAR programme as project number RP-PG-0611-20010 from route sheet. The contractual start date was in October 2013. The draft report began editorial review in May 2019 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Forster et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
SYNOPSIS
Background, aims and objectives of the Improving Longer Term Stroke Care (LoTS2Care) programme
Research into, and the care of, patients after stroke has been transformed in recent years. Rapid clinical change has been underpinned by a dynamic research culture, and the recommended stroke care pathway in the first weeks after stroke is becoming established. Despite this, however, longer-term outcomes remain poor for many. 1–3 Post-hospital services, such as early supported discharge (ESD), are not universally available and are usually time limited, and stroke survivors and their families can feel abandoned without the knowledge or information to access services or support. 4
Almost two-thirds of stroke survivors leave hospital with a disability,1 the prevalence of depression is 31%,5 inactivity is common6 and health-related quality of life (QoL) deteriorates post stroke. 7 Data from the South London Stroke Register indicate that 20–30% of stroke survivors have a poor outcome over a range of physical, social and psychological domains up to 10 years after the event,8 underlining the requirement for a longer-term care strategy.
Many stroke survivors require assistance from informal carers, often family members, for activities of daily living (ADL), including bathing, dressing and toileting. 9 This burden of care has an important effect on carers’ physical and psychosocial well-being,10,11 with up to 48% of carers reporting health problems and two-thirds reporting a decline in social life, and with high self-reported levels of strain. 12
Any strategy for longer-term care needs to be feasible and centred on identified needs, and the outcomes of importance to stroke survivors and their carers. These needs are multifaceted, and influenced by a range of social and environmental factors. Our previous survey (n = 1251 participants) investigated the prevalence of unmet needs in community-dwelling stroke survivors 1–5 years after stroke, reporting that nearly half of respondents had one or more unmet long-term needs. 13 These related to information provision (54%), mobility problems (25%), falls (21%), incontinence (21%), pain (15%) and fatigue (43%). Over half reported a reduction in leisure activities.
Few detailed data are available on the specific needs (and barriers to and facilitators of addressing them) and service requirements for stroke survivors and their carers in the longer term after stroke. Our programme was configured to address this evidence gap. Our Consumer Research Advisory Group (CRAG) was instrumental in prioritising and shaping this research question and has been central to the delivery of this research. We were mindful of the ever-changing NHS and social care environment and wished to work with stakeholders to generate data that would inform the feasible provision of care to enhance the lives of people after stroke.
We sought to develop and test a longer-term integrated stroke care strategy focused on improving the QoL of stroke survivors and their carers by addressing unmet needs and maintenance and enhancement of participation (i.e. involvement in life situations).
Participation is defined in the International Classification of Functioning and Disability (ICF)14 as ‘an individual’s involvement in life situations in relation to health conditions, body functions and structures, activities and contextual factors’ (environmental and personal factors).
It was planned to address the following research questions:
-
What do stroke survivors and their carers identify as the key barriers and enablers that influence unmet needs and participation after stroke?
-
How can services and personnel (health, social care and voluntary sector) be informed and configured to deliver a replicable system of stroke service?
-
Using the framework of intervention mapping, can evidence- and theory-based practical care strategies and associated materials be developed to address unmet need, and maintain and enhance participation for all those affected by stroke?
-
Can key study design considerations for a future large-scale randomised controlled trial (RCT) of the proposed longer-term care strategy be addressed by undertaking a feasibility cluster RCT?
The objectives were to:
-
develop the content of the care strategy through qualitative exploration with stroke survivors and their carers and review of the evidence relating to content and delivery
-
inform feasible means of delivery through a national survey and more detailed examination of four services
-
use the framework of intervention mapping to develop a care strategy, supporting materials and training programmes (for stroke survivors and staff)
-
refine content and test implementation of the care strategy through case studies in three stroke services
-
undertake a feasibility cluster RCT to refine procedures for a future large-scale trial.
The programme was delivered through five overlapping workstreams (WSs) (Figure 1).
FIGURE 1.
Inter-relationship between the different WSs of the LoTS2Care programme. LoTS2Care, Improving Longer Term Stroke Care.

Workstream 1a: an exploration of the predictors of longer-term unmet needs and participation post stroke
Semistructured interviews with stroke survivors and their carers
Introduction
To develop patient-centred services for stroke survivors in the longer-term, a comprehensive understanding of the needs, experiences and priorities of those living with stroke was required. 15
Although the existing literature provided an understanding of the level of unmet needs,13 how stroke is experienced, some of the challenges faced by stroke survivors16,17 and the way these challenges are managed by stroke survivors are not fully understood. Evidence suggested that stroke survivors do play an active role in their recovery;18 however, little is known about the processes that influence whether or not stroke management strategies are carried out successfully, particularly in the longer term.
Therefore, the first study in the programme aimed to address these gaps by exploring the specific longer-term needs of stroke survivors (e.g. type of information) from their own perspectives. The barriers to and facilitators of behaviours which impact on these longer-term needs and participation14 were also explored. The findings informed the intervention development process for the longer-term care strategy.
Aims and objectives
The objectives of this study were to:
-
gain further insight into specific longer-term needs (e.g. type of information required) of stroke survivors and their carers
-
explore the barriers to and enablers of the behaviours that affect longer-term needs and participation
-
explore how stroke survivors and carers develop strategies for managing problems/resolving the issues that they face post stroke.
Methods
Full details of methods are provided in Report Supplementary Material 1 (the research protocol).
Study design
This was a qualitative study involving semistructured interviews and a thematic approach to data analysis.
Participants
Participants were community-dwelling stroke survivors and their carers at two different time points: 9–12 months post stroke and between 2 and 4 years post stroke. This allowed for the identification of ongoing needs and provided an opportunity to reflect on what information and support had been useful and how this could be improved.
Potential participants were identified from an established research database held at Bradford Teaching Hospitals NHS Foundation Trust. In brief, stroke survivors were eligible for the study if they had a confirmed primary diagnosis of new stroke, were > 9 months post stroke, resided in the community and were able to provide informed consent (or consultee assent). Carers were eligible if they were identified by the stroke survivor as the main informal carer who provides support a minimum of once per week.
A maximum variation purposive sampling strategy was used to select a heterogeneous population with a range of disability levels (assessed by the Barthel Index19), high and low levels of unmet need,20 differing socioeconomic status (assessed by postcode), living circumstances (alone/with carer) and age range at the two time points.
Semistructured interviews
Unmet needs and the barriers and facilitators experienced in trying to overcome these were explored across all of the interviews (topic guides are provided in Report Supplementary Material 2).
Efforts were made by the researchers to tailor the interviews for stroke survivors with communication difficulties (by using pictures/adapting topic guides/use of keywords). The interviews were audio-recorded and transcribed verbatim. A thematic approach21 to data analysis was taken, with the transcripts analysed in two categories (those who were 9–12 months post stroke and those who were > 24 months post stroke), to establish any differences in the experiences at different time periods post stroke.
For the purpose of this analysis, needs were defined as those that stroke survivors perceived as challenges to overcome or address. Standard approaches to demonstrating trustworthiness and quality in qualitative research were used. 22 Throughout data collection and analysis, data, codes and emerging categories and theories were presented to and discussed with the research team and Programme Management Group (PMG) at regular intervals.
Results
A full report is provided in Appendix 1.
Twenty-eight stroke survivors and 11 carers (eight wives and three husbands) were recruited to the study from November 2013 to April 2014. Thirteen of the stroke survivors were between 9 and 12 months post stroke, and 15 were between 32 and 47 months post stroke. They represented a range of ages, disability levels and reported unmet needs (see Appendix 1, Table 8). All of the stroke survivors and their carers decided to be interviewed together.
Identified needs, barriers and facilitators
The needs identified and the barriers and facilitators that the stroke survivors and their carers experienced are summarised in Figure 2. This figure highlights where barriers and facilitators relate to more than one need. For example, ‘building a support network’ is a facilitator across nine of the 13 needs. Other common barriers and facilitators include lack/loss of support, stigma, acceptance as a process and creative problem-solving.
FIGURE 2.
Stroke survivor needs, barriers and facilitators.
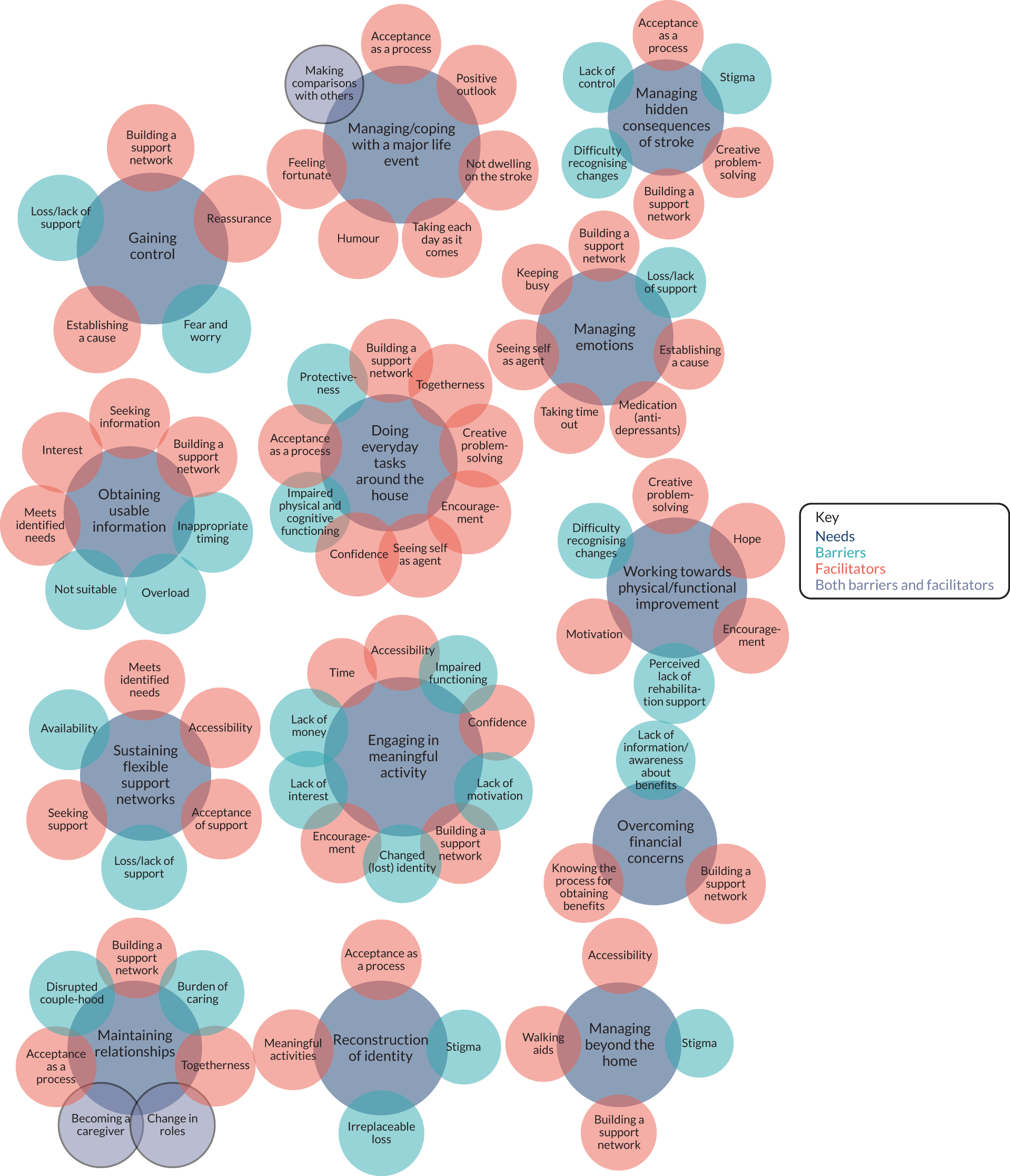
Thirteen key needs were identified:
-
managing and coping with a major life event
-
gaining control
-
managing emotions
-
reconstruction of identity
-
doing everyday tasks around the house
-
working towards physical and functional improvement
-
managing hidden consequences of stroke (e.g. difficulties with concentration and processing of information, memory impairments and mood swings)
-
obtaining usable information
-
sustaining flexible support networks
-
engaging in meaningful activity
-
overcoming financial concerns
-
maintaining relationships
-
managing beyond the home.
Some differences were recognised in the two time frames. Feelings of hope in terms of the need to work towards physical functioning were more common amongst those who were 9–12 months post stroke. These stroke survivors also talked more often about establishing a cause for their stroke as part of understanding and making sense of their situation (linked to the need to gain control and facilitate this process). More of the participants who were at least 24 months post stroke talked about the process of reaching acceptance. This was important for addressing many of their needs, including doing everyday tasks around the house, managing and coping with the major life event, managing the hidden consequences of stroke, reconstruction of their identity and maintaining relationships. Aside from these slight differences at each time point post stroke, the identification and management of needs remained similar across the two time points.
Four of the unmet needs related to emotional needs: gaining control; managing and coping with a major life event; managing daily stressors and strains; and reconstruction of identity.
Key findings
This qualitative study examined the needs of 28 stroke survivors at two time points [9–12 months (n = 13) and > 24 months (n = 15)]. Thirteen needs were identified from the perspectives of the stroke survivors and their carers. Participants’ accounts of their lives comprised complex and interacting factors that shaped how they managed their needs post stroke. The existing literature has previously indicated the areas where stroke survivors and their carers commonly experience unmet needs. 13,23 Although insightful, such research has not provided a comprehensive understanding of how needs can be addressed, or acknowledged the factors that may facilitate or hinder this process. It also neglects the notion that needs may change over time. A unique approach to understanding the stroke experience was taken through exploring specific needs across all areas of the stroke survivors’ lives and investigating the factors that influence whether or not these needs are addressed. This study contributes to the wider body of literature by gaining an in-depth understanding of the broad scope of needs experienced by stroke survivors in the longer term, from their own perspectives, and explored the barriers and facilitators that stroke survivors and their carers faced as they worked to manage and overcome these needs.
The participating stroke survivors still had needs that were unaddressed, even up to 3 years post stroke. Across both time points, emotional needs were emphasised, supporting findings from a previous qualitative review. 24 In the current study, stroke survivors felt that there was a lack of emotional support, which often led to feelings of neglect, particularly when they initially returned home. Some stroke survivors and their carers also experienced a sense of abandonment following withdrawal of support from health professionals (e.g. physiotherapists). Stroke survivors expressed a range of emotional difficulties, which included, for some, frustration and anger as they tried to manage the impacts of the stroke, indicating the need for services and interventions to encompass longer-term emotional support.
This work highlighted the importance of understanding needs in different contexts. Perceived and actual stigma was a barrier to going out in public areas, for example with regards to walking aids, for which a distinction was made between perceived stigma (because they felt that people would make negative judgements) and their own lived experiences of being stigmatised. Interestingly, some stroke survivors who actively managed their impairments in their own homes were reluctant to spend much time out of their home because of some of the difficulties they faced in interactions with others. Such findings suggest that efforts must be made to increase public awareness around stroke in order to increase social participation amongst stroke survivors. Alternatively, techniques for stroke survivors and their carers could be encouraged to reduce feelings of perceived stigma.
Although stroke survivors and their carers faced barriers to addressing their needs, the findings indicated that stroke survivors and carers do play an active role in managing their circumstances/situation, using both practical and mental coping strategies, supporting findings from previous research. 18,25,26 Although previous literature has provided examples of such strategies (e.g. mobilising support networks26), this study identified how these are used to address specific needs. This study confirmed the importance of support networks and identified this (‘sustaining flexible support networks’) as an unmet need after stroke. This was identified as one of the needs of stroke survivors and also one of the key mechanisms for addressing other identified needs, for example engaging in meaningful activities, overcoming financial concerns, doing everyday tasks around the house and managing beyond the home.
A more nuanced understanding of the role of the stroke survivor in seeking and maintaining support was gained, particularly in circumstances in which this could be vulnerable to change. Interestingly, many of the stroke survivors were reluctant to join support groups, often because they did not feel that they were a ‘group person’ or because they did not feel that their stroke was ‘bad’ enough.
The need to ‘obtain usable information’ supported findings from prevalence studies in which information is commonly reported as an unmet need. 13,23 From the stroke survivors’ accounts, it was clear that they were given some information following their discharge from hospital; however, there was a general sense of negativity attached to this, as concerns were raised about both the timing and the amount of information provided. This may explain this being regarded as an unmet need, despite information being available. The findings indicated that stroke survivors and their carers continue to need information in the longer term following the stroke as they draw on this information to resolve a specific problem, as and when it arises.
Many of the needs experienced by stroke survivors who were 9–12 months post stroke were similar to those of stroke survivors at 3 or 4 years post stroke, suggesting that some needs are persistent. However, there were some subtle differences apparent across the two time points, an example being around reaching acceptance. This emerged as a key facilitator for managing life after stroke, supporting research that highlighted acceptance as a critical factor in being able to cope. 18 Those who were at least 24 months post stroke talked about this more than those who were 9–12 months post stroke, suggesting that those who have more recently had their stroke may have had less time to reach the point of acceptance.
Some stroke survivors spoke about acceptance in broad terms, of accepting the stroke and moving forward, whereas others talked about this more specifically in terms of accepting that tasks take longer around the house and accepting their new identity. Some stroke survivors struggled to accept the changes to their lives and themselves following the stroke. One stroke survivor made an interesting distinction between realisation and acceptance. She realised that things were different, yet she failed to accept this. This suggested that acceptance has both an emotional and a cognitive/intellectual meaning. The process of achieving it is one aspect of adjustment to long-term illness, a process that must be worked towards over time, which was reflected in other accounts from the stroke survivors. Amongst those who had managed to accept their stroke, there was a sense that they felt that they had little choice but to do this to move forward.
Evidently, acceptance is complex and a number of factors shape whether or not this is possible. Supporting stroke survivors in reaching acceptance is important, as it affects a number of needs, for example maintaining relationships, managing and coping with a major life event and reconstruction of identity.
Relationship with other parts of the programme
Consistent with the aims of this workstream (WS), longer-term needs have been identified. These findings suggested that stroke survivor needs should be routinely monitored during the recommended routine reviews. The needs were captured in behavioural terms and the barriers to and facilitators of addressing needs have been identified, providing a useful insight into how stroke survivors develop strategies for managing difficulties that they face post stroke. However, it is important to note that stroke survivors did not always talk in terms of specific behaviours as part of their narratives. This had implications for how the next steps of intervention mapping were managed.
To obtain a comprehensive picture of unmet needs after stroke, the programme team undertook two additional pieces of work, as outlined in the following section.
Exploration of the literature in which stroke survivors identify unmet needs
With the assistance of an experienced information scientist, a detailed search of the literature was undertaken in August 2014. No language or date limitations were applied. See Report Supplementary Material 3 for the search strategy. The strategy was appropriately modified and applied to MEDLINE, Cumulative Index to Nursing and Allied Health Literature, PsycINFO, Allied and Complementary Medicine Database, and Web of Science; 897 papers were identified. Following review, 34 papers were taken forward to full-text scrutiny and seven were identified as describing unmet needs of stroke survivors and/or their carers2,8,13,15,23,27,28 (see Report Supplementary Material 3). The research team recorded the unmet needs reported in these papers. The unmet needs identified are summarised elsewhere (see Figure 5 and Report Supplementary Material 3).
In addition, to inform our work on unmet needs, all of the enquiries received by the UK Stroke Association helpline between 1 April 2013 and 31 March 2014 were collated. 29
Workstream 1b: review of literature to inform content and delivery of the care strategy
The aim of this WS was to complete literature reviews to inform content and delivery of the care strategy. The objectives comprised:
-
identifying community-based interventions that enhance mood, QoL or participation of stroke survivors, their carers or both at least 6 months after incident stroke, and any adverse events
-
scoping the literature on mechanisms of delivery in stroke and other long-term conditions, including identification of:
-
models of care
-
success factors for supported self-management
-
mediators and assessment tools
-
methods for engaging with participants with communication and cognitive problems
-
-
identifying other appropriate candidate primary outcome measures
-
updating the Cochrane review of information provision after stroke. 30
Because of the diverse nature of community-based post-stroke interventions, an overview of Cochrane reviews was conducted to identify effective interventions that may be relevant to longer-term stroke survivors or their carers, and to provide an initial framework for such interventions, followed by a systematic review of community-based interventions for survivors or their carers at least 6 months after stroke.
See Report Supplementary Material 4 for protocols of both reviews.
Systematic overview of Cochrane reviews to identify effective interventions that may be relevant to long-term stroke survivors or their carers
Methods
All reviews produced under the remit of the Cochrane Stroke Group (May 2014) were screened to identify systematic reviews of community interventions for stroke survivors or carers.
Reviews were included if any of the participants in the included studies were at least 6 months post stroke at the start of intervention delivery. ‘Community interventions’ were defined pragmatically as follows: not delivered to inpatients (i.e. outpatient and wider community), and not pharmaceutical, surgical, radiological, radiotherapy or ‘medical devices’ [including acupuncture, transcutaneous electrical nerve stimulation, repetitive transcranial magnetic stimulation, robotics].
In keeping with our long-term focus, main outcomes were participation, QoL, health status, mood and, additionally for carers, burden. We also incorporated the primary outcomes of the included reviews as secondary outcomes in our overview, using the ICF as a framework. We grouped interventions by the post-stroke problems that they were intended to address. One reviewer extracted data, which another checked. Quality was assessed by National Institute for Health and Care Excellence (NICE) criteria. 31 Effectiveness was summarised per intervention based on our main and secondary outcomes. We assessed the evidence as good or limited based on the precision and consistency of effects, or very limited in the case of evidence from single studies.
Key findings
Through our search strategy, 329 reviews were retrieved, and 28 (see Appendix 2) were included in the review, encompassing 352 studies. Seventeen reviews met all quality criteria; 11 met five of the six criteria.
The main findings of this review are diagrammatically presented in Table 1. There was very little evidence of effect on mood, participation, health status, QoL or carer burden. This was primarily because few studies measured these outcomes.
| Intervention | Survivors | Carers | ||||||
|---|---|---|---|---|---|---|---|---|
| Perceived health status | Mood | Participation | QoL | Perceived health status | Mood | Participation | QoL | |
| CIMT | ||||||||
| Circuit class therapy | ||||||||
| Hands-on therapy | ||||||||
| Home-based therapy | ||||||||
| Information provision | ○ | ○ | – | ○ | ○ | ○ | – | |
| Inspiratory muscle training | ○ | |||||||
| Interventions for visual-field defects | – | |||||||
| Mental practice | ||||||||
| Mirror therapy | ||||||||
| Music therapy | ||||||||
| Non-pharmaceutical interventions for attention deficits | – | – | ||||||
| Non-pharmaceutical interventions for fatigue | ||||||||
| Non-pharmaceutical interventions for problems faced by caregivers | ○ | ○ | ||||||
| OT for ADL | – | – | – | |||||
| OT in care homes | ||||||||
| Overground gait training | ||||||||
| Physical fitness training | ○ | ○ | ||||||
| Physical rehabilitation | ||||||||
| Psychotherapy | – | |||||||
| Rehabilitation at home < 1 year after stroke | – | – | – | |||||
| Rehabilitation at home > 1 year after stroke | – | – | – | – | ||||
| Repetitive task training | – | |||||||
| Speech and language therapy | – | |||||||
| Stroke liaison workers | – | – | – | |||||
| Telerehabilitation | ○ | |||||||
| Treadmill training | ||||||||
| Virtual reality | ||||||||
| Water-based exercises | ||||||||
For stroke survivors:
-
Ten reviews30,32–40 reported perceived health status – information provision,30 inspiratory muscle training,34 fitness training33 and telerehabilitation40 reported limited evidence of improvements.
-
Nine reviews30,32,33,35–37,39,41,42 reported mood, with limited evidence of improvements found for information provision30 and fitness training. 33
-
Two reviews30,43 reported measures of QoL: information provision had very limited evidence of improvement. 30
-
One review30 reported no evidence of effect of information provision on participation.
For carers, very limited evidence from one study reported in two reviews30,44 suggested that teaching procedural knowledge could improve perceived health status and reduce strain and depressive symptoms. However, our own work45 has superseded those reviews; we found that the intervention was not effective when evaluated in a large pragmatic trial.
Looking at our secondary outcomes, there was evidence that interventions targeting ICF mobility (e.g. walking, handling objects), self-care (ADL) and domestic and interpersonal life (extended ADL) could improve these outcomes.
The schematic in Figure 3 provides a graphical illustration of the distribution of research activity, which is not necessarily in the areas of most importance to stroke survivors and their carers. The lines link reviews to the studies they include, indicating when studies are included in more than one review (e.g. a study included in the liaison workers’ review and the support for caregivers review).
FIGURE 3.
Schematic demonstrating linkage between Cochrane reviews.
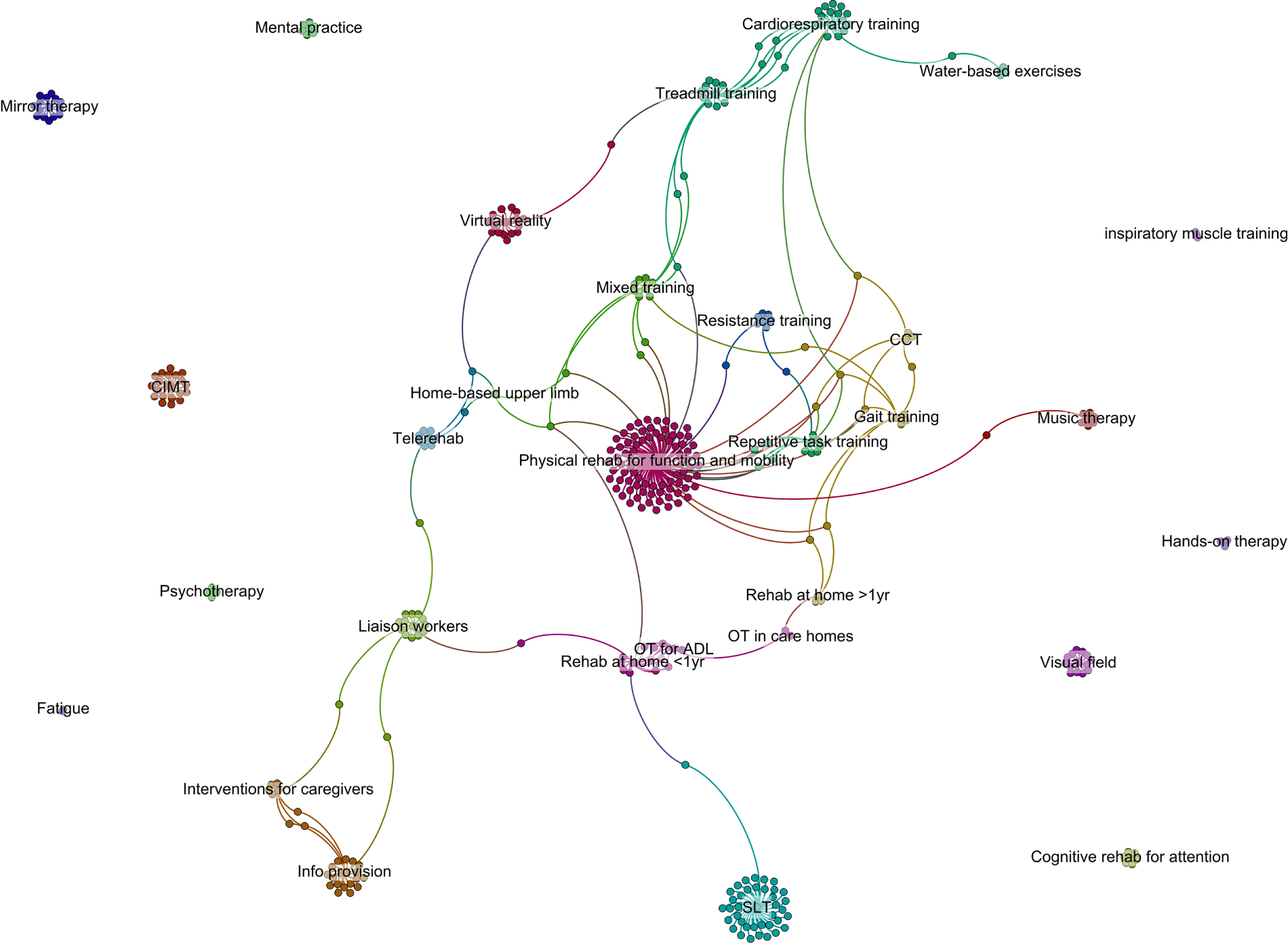
Thus, despite considerable efforts, we were unable to identify any pre-existing community-based interventions with good evidence for improving the QoL, participation, mood or health status of stroke survivors or their carers.
This overview has now been registered as a Cochrane review. 46
Review of studies
Methods
In this review, all stroke studies identified by the Cochrane Stroke Group [via AskDORIS (Database Of Research Into Stroke)], except those examining medicine, surgery, radiology and radiography, were screened. Studies were excluded when they were not a RCT or cluster RCT; patients were, on average, < 6 months post stroke at the start of the intervention; the intervention was not community based; and < 20 participants were included. The review of studies considered the risk of bias, the type of intervention and adherence to the intervention (when this was reported). Effectiveness of the studies was assessed based on the outcomes of interest to our research programme: stroke survivor QoL, health status, mood, participation, ADL, extended ADL, communication, other measures of behaviour and, additionally for carers, burden. We thematically grouped the components of each intervention to enable access to relevant details during intervention development. We also catalogued all measures used in the studies to identify possible alternative primary outcomes and indicators of behaviour change.
Key findings
Through our search strategy, 8054 studies were identified, reduced to 3501 when medicine, surgery, radiology and radiography interventions were excluded. Of 3501 studies screened on title/abstract, 630 were identified as being potentially eligible; 2871 were excluded. Following full-text retrieval, 105 studies were included in the review. Sixty-four studies were primarily of impairment-focused physical training, which we grouped into 15 intervention types. Other kinds of intervention included speech and language therapy (n = 11), combinations of educational, social and recreational activities (n = 8), tailored occupational rehabilitation (n = 4), social work or social network therapy (n = 3), provision of aids and devices (n = 2), ADL practice (n = 2), vision training (n = 2), combined exercise and education (n = 2), care co-ordination and interdisciplinary management (n = 2), a multifaceted long-term rehabilitation programme (n = 1) and carer-focused interventions (n = 4).
Figure 4 builds on Figure 3 and shows the studies that were included in both the overview and review of studies, indicating the overlap between these two reviews and the comparative lack of longer-term studies. Approximately half (52/105) of the trials included in the review of studies also featured in the overview. Among those not included in Cochrane reviews were specific physical rehabilitation interventions, such as pelvic floor training and bilateral arm therapy, as well as combinations of interventions, such as aerobic and strength training, or physical activity and education. The evidence for other intervention types (e.g. mobility training; combinations of educational, social and recreational activities) is split between several Cochrane reviews and excludes multiple longer-term studies.
FIGURE 4.
Schematic demonstrating linkage between the overview and review of studies.
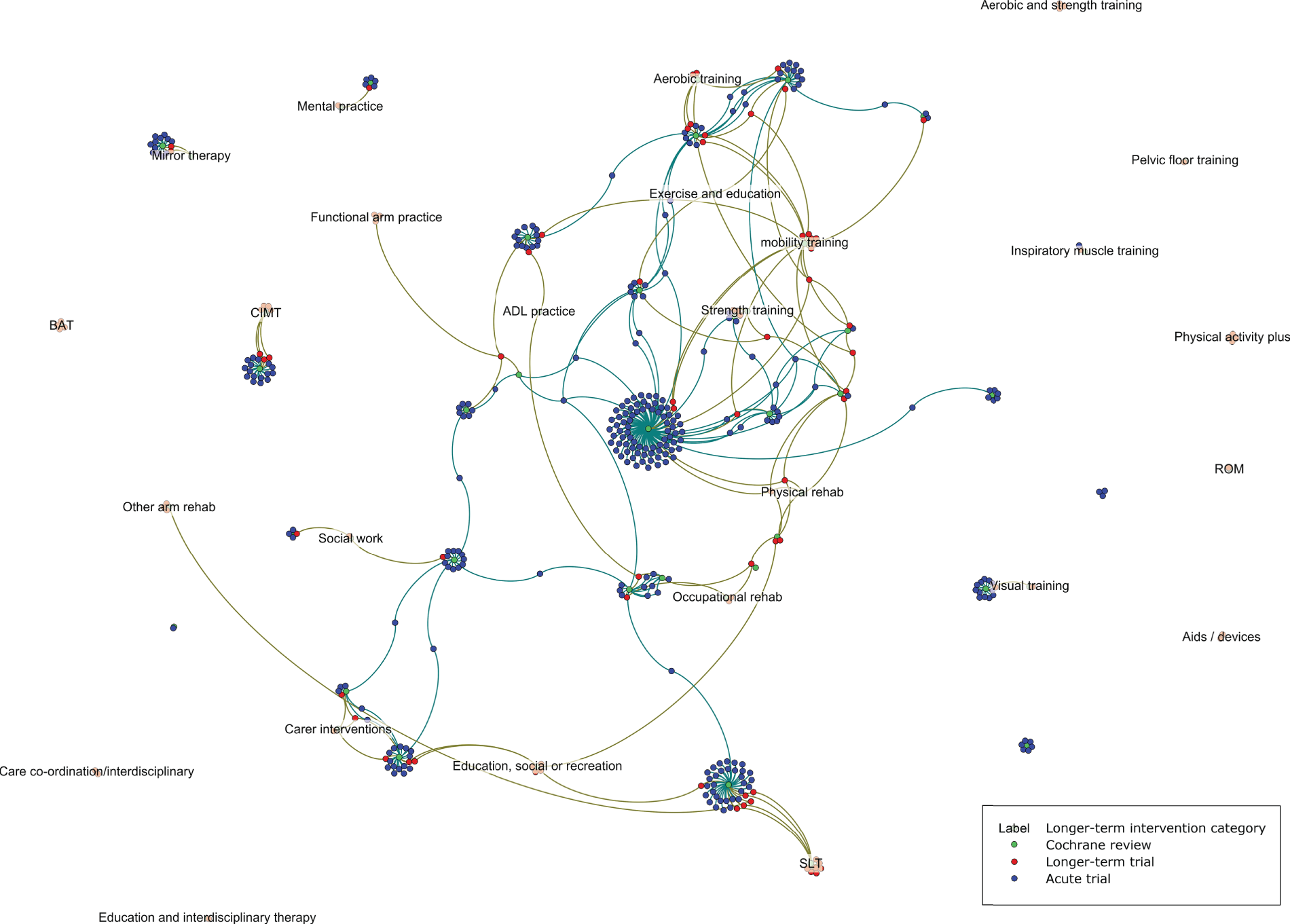
Sixty-two studies reported at least one outcome of interest, 24 of which reported a significant difference between groups in one of these outcomes in favour of the intervention:
-
Two carer interventions47,48 and one care co-ordination and interdisciplinary management intervention49 reported an effect on carer mood and burden.
-
Of the eight interventions in the category of education, social engagement and recreational activity, four50–53 reported effects on outcomes including mood, participation and communication.
-
Of 33 physical training trials reporting an outcome of interest, 1354–66 reported effects on outcomes including health status, mood, participation, ADL and extended ADL.
-
One67 of two interventions combining exercise and education reported improved health status.
-
One study68 of long-term rehabilitation and counselling with social, educational and recreational courses reported effects on mood and extended ADL.
-
Two69,70 of four tailored occupational rehabilitation interventions were reported to improve extended ADL and participation.
-
No studies reported an effect on QoL, although only six relatively small studies68,71–75 measured this.
No association between risk of bias and ‘successful’ interventions was identified.
Although measures of ability were routinely reported, measures of specific, everyday behaviours were rarely reported. Only two studies70,76 reported an effect: reduced incontinence following pelvic floor muscle training and76 increased number of outdoor journeys for people receiving an outdoor mobility intervention. 70
The majority of trials related to physical exercise, and there was a noticeable lack of trials evaluating other interventions for longer-term stroke survivors and their carers. Although many studies reported significant effects, trials were small and there were no consistent patterns to indicate effective types of intervention. Some trials of promising interventions have recently been replicated and demonstrated no effect. 45,77,78 However, trials incorporating aspects of education, social engagement and recreational activity and those incorporating functional training appeared more promising than others.
These comprehensive literature reviews demonstrated that relatively little research has been undertaken to try to enhance longer-term outcome after stroke, focusing on the outcomes of importance to stroke survivors and their families. There was no strong evidence on the most effective components of an intervention.
Outcome measures
Outcome measures were extracted from the reported studies and reviewed by the PMG and research team. A shortlist of measures was reviewed by the CRAG. These discussions and consultation with the Programme Steering Committee (PSC) informed the outcomes selected for our evaluation work.
Delivery mechanisms
A scoping review of reviews addressing delivery mechanisms of health care in chronic illness was undertaken. Seven eligible review studies, including two additional papers from the references, were identified. 79–85 Details are provided in Appendix 3. Relevant policy documents were also identified. 86–92
Looking across the identified reviews, the majority of the primary evidence being synthesised was focused on diabetes, and the most convincing evidence was of supported self-management.
Following the completion of the overview and review of studies, the Cochrane review Self management programmes for quality of life in people with stroke was published. 93 This reported possible small effects on QoL and self-efficacy, with further research likely to have an important impact on these results.
Materials development for people with communication problems
This was recognised as an ongoing challenge, not only for our programme, but across the spectrum of stroke research. To address this issue, our colleague Faye Wray undertook a linked Doctor of Philosophy (PhD) focused on assessing the appropriateness of self-management for stroke survivors with impaired communication. 94 Insights into amendment of materials for people with communication impairment were provided. This included detailed guidelines previously developed by the Stroke Association, which can be summarised as making sets of messages that are short, with clear sentences, using easy words and having a good layout. 95 We also sought guidance from an aphasia group in Grimsby on their specific needs and means of addressing those needs. In addition, we formed a collaboration with colleagues at the Greater Manchester Collaborations for Leadership in Applied Health Research and Care (CLAHRC) who have extensive experience in this area and provided expert guidance.
Update on Cochrane review of information provision
The Cochrane review was updated while the grant was under consideration; the results were published in 2012. 30
The review30 indicated that there is evidence that information improves patient and carer knowledge of stroke and aspects of patient satisfaction, and that it reduces patient depression scores. However, the reduction in depression scores was small and may not be clinically significant.
At the time of publication of this report, an update has been submitted to the editors and is being revised accordingly.
Workstream 2: national survey of post-discharge stroke services, and focus groups and interviews to identify service needs, barriers and enablers
An exploration of care models for longer-term stroke care in England
The objectives of this WS were to gather information on:
-
mechanisms (if any) in place for identifying and/or maintaining contact with stroke survivors, up to and after the recommended 6-month review
-
current arrangements for the 6-month and annual reviews
-
the configuration of local stroke services in the light of the development of Clinical Commissioning Groups (CCGs), including identification of stroke lead and governance arrangements
-
services available, for example Stroke Association provision, fitness classes.
This information was gathered to inform the development of the longer-term care strategy by ensuring that any proposed strategy was feasible in current service models by determining methods of case ascertainment, the optimal timing of delivery and who might be best placed to undertake the review process and deliver the care strategy.
Methods
Development of the survey
Using the information collated in the LoTSCare programme of work, a detailed six-page survey tool was formulated that collected data on the community stroke team (CST): where they received referrals from, what services they offered and for how long, and what other services were available in their area. This survey was sent to two community teams for feedback, and then e-mailed to the 29 Community Stroke Services that participated in the LoTSCare trial. The survey was sent out in August 2013 and a reminder was sent at the beginning of October 2013; however, only eight (28%) of the surveys were returned. The research team and PMG concluded that this pilot survey was too long and too difficult to complete in Microsoft Word (Microsoft Corporation, Redmond, WA, USA) and that the method of contact was not effective.
At the same time that the survey was being prepared, the Sentinel Stroke National Audit Programme (SSNAP) started to plan collection of national data on the 6-month stroke reviews and the Stroke Association offered the research team data on its service provision across England as commissioned from April 2014. Consequently, it was agreed that the SSNAP data would be utilised to inform our understanding of objectives 1 and 2, and a simple one-page survey would be sent out to all CCGs to achieve objectives 3 and 4. The Stroke Association data would be used to complement the survey findings.
The survey asked how long stroke services were commissioned for, whether or not any other providers commissioned services in the longer term and whether or not an annual review was commissioned (see Appendix 4).
Survey participants
Clinical Commissioning Groups
A directory of contacts for all CCGs (n = 203) listed on the NHS England website in November 2013 (www.england.nhs.uk/ccg-details/; accessed 18 August 2020) was compiled.
Community stroke teams
A directory of CSTs, as listed in the Stroke Association website UK Stroke Contacts Map (www.stroke.org.uk/professionals/uk-stroke-contacts-map; accessed November 2013) in November 2013, was compiled.
The e-mail addresses of all National Institute for Health Research (NIHR) Stroke Research Network (SRN) managers were also gathered from the NIHR Clinical Research Network (CRN) website in November 2013.
Survey administration
The survey (see Appendix 4, Figure 10) and a cover letter, signed by Anne Forster (lead investigator) and co-investigator Matthew Fay, were sent via e-mail on 18 December 2013. The e-mail to the CCGs was addressed to the lead for longer-term conditions/stroke. Completion of the survey could be via an electronic Word document or a SurveyMonkey® (Palo Alto, CA, USA) link. A written reminder was sent to all CCGs, and an e-mail reminder was sent to all community teams 1 month later on 20 January 2014.
The same e-mail and copy of the survey was also sent to the NIHR SRN managers who were asked to forward the survey to local CSTs. The survey was also promoted at the UK Stroke Forum Exhibition Stand (December 2013), and completed then by a number of community teams.
Sentinel Stroke National Audit Programme data
The first round of data collection on 6-month reviews, collected between October 2013 and March 2014, was reported in August 2014. 96 SSNAP data are collected on every stroke survivor admitted to hospitals across England and Wales, and are not summarised at the level of service provision (CCGs). Data on the 6-month reviews were collected and reported for each patient. These data were reviewed to explore objectives 1 and 2.
Stroke Association data
These data were provided to the research team by the Stroke Association director for the North of England Life After Stroke Services (co-investigator Elaine Roberts) and detailed all Stroke Association services commissioned across CCGs in England from April 2014 to March 2015, including the number of CCGs that commissioned the Stroke Association or other services to complete 6-month reviews. These data were reviewed to explore objectives 2 and 4.
Data synthesis
Survey data were entered into a database and the length of service provision was categorised as (1) up to 6 months post stroke, (2) up to 12 months post stroke or (3) > 12 months post stroke. This service consisted of stroke or neuro-specific community rehabilitation teams. Commissioning of general community rehabilitation teams was not accepted as provision of support to stroke survivors.
Provision of other services was taken from the free text in the survey and grouped according to the type of service: (1) Stroke Association or (2) other support, including exercise classes, stroke clubs, etc. Provision of Stroke Association services was verified by the Stroke Association data; when there were discrepancies, the Stroke Association data were taken as being correct.
Information on the provision of 6-month reviews was taken from the SSNAP data report, and also from information on the commissioning of 6-month reviews in the Stroke Association data.
Provision of annual reviews was collated from the survey. The survey did not distinguish between 12-month general practitioner (GP) health checks and annual health and social care reviews.
Results
Service provision
Of 203 identified CCG areas, 116 (57%) responded to the survey (see Appendix 4, Figure 11, and Report Supplementary Material 5). Of these, 30% (35/116) provided a stroke or neuro-specific community rehabilitation team service up to 6 months post stroke, 40% (46/116) provided a service up to 12 months post stroke and 29% (34/116) reported a service beyond 12 months post stroke.
Stroke Association Life after Stroke services were commissioned in 74% of CCGs, providing support to stroke survivors up to 12 months post stroke. In the survey, other support, such as exercise groups and stroke clubs, was reported by 57% (66/116) of CCGs.
Six-month and annual reviews
The return rate for the SSNAP 6-month review data was low (only 22.8% of patient records had an answer in this section). Overall, only 15% (3360/22,273) of survivors received a 6-month review.
In the Stroke Association data, 12% of CCGs commissioned the Stroke Association to complete 6-month reviews, and a further 16% of CCGs commissioned NHS community services to complete the 6-month reviews.
In the survey, 39% (45/116) of CCGs commissioned an annual review for stroke survivors (including 12-month health checks completed by GPs and annual health and social care reviews).
Key findings
The national survey identified three types of service provision – up to 6 months post stroke, up to 12 months post stroke and beyond 12 months post stroke – with the most common model being provision up to 12 months. The Stroke Association Life After Stroke services were commissioned by 74% of services and other support, such as groups and clubs, by 57% of CCGs.
The provision of longer-term support for stroke survivors was highly variable across the country, with a substantial number of survivors potentially receiving no support after hospital discharge. Our survey results suggested that, 12 months post stroke, approximately 70% of services provide no formal support to stroke survivors. This is in stark contrast to research indicating a high number of stroke survivors with unmet need beyond the first year post stroke. 13
Despite current policy recommendations of a 6-month and annual health and social care reviews,97,98 the SSNAP data revealed that only 15% of survivors had received a 6-month review. The Stroke Association data indicated that 6-month reviews were commissioned in fewer than one-third of CCGs in 2014, and our survey demonstrated a similarly low number of annual reviews being commissioned.
Strengths and weaknesses
Our survey was completed by 57% of CCGs across England. CCGs completing the survey may have differed from those that did not; for example, responses may have included a larger proportion of exemplar CCGs keen to demonstrate commissioning of services beyond 12 months, because CCGs that commissioned no service and/or one up to 6 months may have been less motivated to respond.
It is important to note that reporting the proportion of CCGs that commissioned a particular service did not necessarily equate to the number of stroke survivors actually receiving this service. Many community rehabilitation teams and 6-month review procedures had eligibility criteria, meaning that the service was not available to all.
The completion of the 6-month review data from the SSNAP was very low in 2014. This was the first round of 6-month review data collection for the SSNAP, and it demonstrates how challenging the collection of community survey data can be.
Relationship with other parts of the programme
This survey demonstrated that the provision of longer-term support for stroke survivors was highly variable across the country, with a substantial number of survivors potentially receiving no support after hospital discharge, and with fewer than one-third of services completing 6-month reviews with stroke survivors. Findings from this research indicate that strategies for longer-term stroke care need to be developed and implemented across the country. It was concluded that attempting to establish a longer-term service beyond 12 months post stroke, when many services were struggling to commission anything beyond 6 months, was unlikely to be feasible in the current climate. This programme of work subsequently focused on the 6-month review, to provide support to stroke survivors based on the needs identified at this time point.
An exploration of care models for longer-term stroke care in England and the barriers to and enablers of longer-term service provision
Aim
This study aimed to gain further insights and understanding from service deliverers about barriers to and enablers of the development and implementation of our care strategy by conducting focus groups with a purposive sample of different models of service identified in the national survey.
Methods
Full details of the methods are provided in the protocol (see Report Supplementary Material 6).
Study design
Having identified different models of post-discharge support, we originally intended to gain insights, through focus groups, from CSTs that exemplified the three models. However, given the diversity of stroke service provision and the professional make-up of CSTs, additional focus groups were required to gain a fuller, more informed understanding of the barriers to and enablers of delivering longer-term support.
On review of the survey data, eight CSTs were purposively selected to be invited to participate in focus groups on the basis of their location and the pattern of post-discharge support they provided. CSTs were selected from different English regions, including both urban and rural districts. Initial contact and invitations were extended to a lead member of the CSTs identified from the survey data (i.e. stroke service co-ordinator, team leader, head of service, service manager or therapy consultant), who subsequently discussed the invitation with team members and invited fellow team members to participate. There were no set inclusion criteria for participation other than to include secondary care clinicians as well as community-based professionals who undertook clinical, organisational or managerial roles in supporting stroke survivors.
Research governance
Approval was obtained from the relevant NHS trusts’ research and development departments.
Focus groups
Focus group methodology was used to explore the views of CST members as to their understanding of stroke survivors’ longer-term (i.e. ≥ 6 months post stroke) unmet needs, barriers that inhibit CSTs from addressing them and enablers that support them to do so. Written informed consent was taken from all participants, who were assured that all data would be treated confidentially, and that all reporting would be anonymised.
The focus groups were facilitated by a researcher, with a fellow researcher taking notes. An identical topic guide was used for each focus group (see Report Supplementary Material 7). The staff mix supported an exploration of different perspectives and produced insights into the service group dynamic. All focus group discussions were audio-recorded and transcribed, and data were thematically analysed. 21
Data analysis
The researcher (AP) began by familiarising himself with the transcripts, and the contextual nature of the materials contained in them. Subsequently, the researcher engaged in an interrogative process of sifting carefully through the data and identifying initial codes, later grouped into themes, relating to barriers to longer-term support for stroke survivors, and enablers of longer-term support for stroke survivors. Ongoing analysis then refined the specifics of each theme. Further analysis of these themes uncovered distinct categories in the overarching contextual framework of each theme. Hence, the complexity of service providers’ perceptions of their needs, and the factors that presented barriers to their being met, could be captured and explored. Finally, a selection of compelling extracts was used to exemplify identified themes.
Similarly, practical examples of how identified service provider needs were being met in the context of current organisational practice were classified as ‘actual enablers’. In addition, practices that potentially enabled service providers’ needs being met were classified as ‘potential enablers’.
Results
All eight sites that were approached agreed to participate and hosted focus group discussions. Five of the CSTs provided post-stroke support for up to 12 months (in accordance with the common model), two provided support for up to 6 months (model 2) and one for up to 3 years (model 3) (Table 2). Sixty-five participants occupying a multidisciplinary range of predominantly clinical roles took part in focus groups between April and June 2014, with eight participants per group on average (Table 3).
| Site | Model type | Geographical region |
|---|---|---|
| B | Up to 12 months | North-west (urban) |
| N | Up to 6 months (no Stroke Association) | North-east (rural) |
| S | Up to 12 months | North Lincoln (rural) |
| C | Up to 12 months | South London (urban) |
| W | > 12 months | Midlands (urban) |
| A | Up to 12 months | Yorkshire (rural) |
| P | Up to 12 months | Peninsula (urban/rural) |
| NA | Up to 12 months | Peninsula (rural) |
| Service | Physiotherapist | Occupational therapist | Speech and language therapist | Therapy assistant | Nurse | Senior manager | Stroke co-ordinator | Rehabilitation/therapy consultant | Stroke Association IAS | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| B (n = 8) | 2 | 1 | 1 | 1 | 1 | 1 | Social worker | |||
| N (n = 8) | 3 | 1 | 1 | 1 | 1 | Psychologist | ||||
| S (n = 9) | 1 | 1 | 1 | 1 | 1 | 2 | GP, dietitian | |||
| C (n = 8) | 2 | 1 | 2 | 2 | 1 | |||||
| W (n = 7) | 1 | 1 | 1 | 1 | 2 | 1 | ||||
| A (n = 7) | 2 | 2 | 1 | 1 | 1 | |||||
| P (n = 9) | 1 | 3 | 1 | 1 | 2 | CCG commissioner | ||||
| NA (n = 9) | 4 | 2 | 1 | 1 | 1 |
Social familiarity within CSTs meant that opinions were generally expressed in a frank and open manner. Although each discussion was intended to continue for approximately 1 hour, they often over-ran considerably.
Subsequent to the group discussions, it was intended to conduct semistructured interviews with purposively sampled individuals identified through the focus groups as having a key role in community-based service delivery. The aim of such interviews would have been to cover topics in greater depth and to explore nuances of opinion, in a manner that the constraints of the focus group format would not allow. However, initial analysis of the focus groups indicated that the key issues had been explored in considerable detail, that differences of opinion had been identified and that there would be little substantive benefit in undertaking supplementary interviews. Hence these proposed interviews were not undertaken.
Key findings
This qualitative study used a focus group approach to explore the perspectives of eight English CSTs regarding unmet needs of stroke survivors and their carers and the barriers to and enablers of delivering longer-term support to stroke survivors. The research uncovered a complex and interwoven range of barriers and enablers.
Perceived needs of stroke survivors
The key longer-term needs of stroke survivors were identified by focus group members, and are presented as a summary here and in Appendix 5:
-
a meaningful role and sense of identity
-
psychological support
-
ongoing information and advice
-
non-stroke-specific wider community engagement opportunities
-
stroke-specific group engagement
-
appropriate health and social care at home
-
longer-term supported self-management
-
support with personal relationships
-
support with physical health needs
-
employment and financial support
-
effective transportation to facilitate access to services and activities
-
social acceptance by society at large.
Barriers and enablers
The barriers generally fell into the following broad categories:
-
deficits of skills and resources
-
availability of training
-
prevailing cultural systems and organisational processes in the NHS
-
failure of multiagency partnership-working.
Enablers were often antithetical to the barriers and could be grouped into the following categories:
-
creative in-house approaches to training and educational enhancement
-
flexible operational, managerial and cultural approaches
-
effective multiagency partnerships
-
strong training and educational links with other agencies.
Barriers relating to resources and skills deficits were beyond the control of CSTs. These included the absence of sufficient funded psychologist posts in their teams, inadequate public transport systems that meant survivors found it difficult to access community facilities and unwillingness on the part of employers to support survivors in undertaking retraining in order for them to return to work. Similarly, a lack of nationally funded opportunities to support survivors and employers in maintaining employment, bureaucratic procedures concerning state benefits and the way in which mainstream community facilities, such as gyms, are not mindful of the needs of survivors were additional barriers that CSTs were unable to influence.
Cultural and organisational processes in the NHS created additional barriers. A target-driven, largely inflexible approach that placed adherence to the requirements of the SSNAP database over a clinically driven, person-centred approach created considerable barriers. The organisational shift from a traditional community rehabilitative approach to a short-term ESD-style intervention approach reduced the potential for longer-term support. Additional barriers rooted in NHS organisational systems included bureaucratic telephony processes that inhibited survivors from contacting CSTs. The orientation of NHS commissioning, with a perceived relatively low emphasis on funding rehabilitative support in the community, and the absence of evidence-based guidance on the components of a good longer-term support structure, represented further barriers.
The absence of effective multiagency partnerships lay at the heart of key barriers inhibiting longer-term support. Poor strategic relationships between the NHS and social services manifested itself in the lack of a shared vision for longer-term support, with social services seen to be pursuing a narrower, more limited goal centred on social care packages in the home. High staff turnover, insufficient time spent in survivors’ homes and, on occasion, delivery of incorrect advice to survivors characterised the perceived social care agency approaches. Largely piecemeal joint budgetary arrangements, and a reluctance to engage with voluntary-sector bodies, provided further evidence of ineffective multiagency partnership-working.
Shortfalls in training involved both in-house training and training issues pertaining to other agencies. A lack of in-house training in CSTs meant that the capacity to support survivors affected by psychological difficulties was diminished. Staffing pressures and organisational patterns of work made it very difficult to deliver training to private care agency staff. GPs were felt to have inadequate training in spasticity management, and were poor at signposting survivors to other services. These training issues collectively reduced longer-term support for survivors.
Creative in-house promotion of education and training was delivered in various formats, and helped to enable longer-term support. In-house psychology training programmes to promote basic understanding of techniques to support survivors affected by low mood, and multidisciplinary team (MDT) clinics to promote a culture of interdisciplinary learning across professional boundaries were examples of enablers of longer-term support. Similarly, a culture that supports work-based learning and self-study, and the development of educational programmes for survivors that involve the participation of different agencies (e.g. therapists, benefit advisers, pharmacists), can also advance longer-term support in creating greater awareness among survivors on how to cope more successfully. Self-management programmes, such as Bridges,99 and the delivery of the Expert Patient Programme100 in a group environment were also cited as helping to achieve this goal. The delivery of peer support by survivors, who had undergone rigorous training, to offer guidance to survivors was also highlighted, in addition to the potential value of a keyworker approach in acting as a mentor/guide to more recent survivors.
Flexible operational and managerial approaches to delivering health care to survivors also seemed to enable longer-term support. Hence, an open-door referral policy that facilitated ease of access to therapists, collective working patterns within CSTs that ensured that the geographical region was adequately covered and the use of MDT clinics were indicative of flexible operational systems. Interdisciplinary methods that enabled more timely fitting of necessary equipment aids in survivors’ homes and a more clinically driven approach for delivering short bursts of therapy according to survivors’ needs further illustrated a flexible managerial approach that advanced longer-term support.
Although reported relatively infrequently, there were examples of multiagency partnerships that facilitated longer-term support. A post-stroke physiotherapy group, managed by leisure services and the local NHS, was an example of this. Furthermore, some services reported positive links with local employment services, and positive engagement with national programmes supporting disabled people into work. Community-based stroke groups, often run by the Stroke Association or other voluntary groups, sometimes involved different agencies coming together to support aphasic and younger survivors. A day centre that involved the participation of different voluntary groups, as well as a gym, and that hosted a cafe that promoted social interaction among survivors, was also cited.
Some support built around training and educational activities could also be effective. The positive impact of training of re-enablement workers by therapists to promote independent living at home could be considerable. Training survivors to utilise information technology (IT) methods to promote independence, particularly through using Skype™ (Microsoft Corporation, Redmond, WA, USA) and e-mail to enhance communication, was considered a positive approach to enhancing longer-term support.
Strengths and weaknesses of the study
A key strength of the study was the involvement of therapeutic and non-therapeutic staff with direct experience of interacting with stroke survivors, who were able to deliver informed perspectives regarding barriers and enablers, based on a wealth of collective experience. The content of the discussions drew on a broad range of cross-cutting issues that enabled participants to reflect on operational, managerial and cultural factors that influenced outcomes for longer-term stroke survivors. This produced rich and varied material that, in reflecting on different issues confronting survivors, appeared more insightful than might have been revealed by other qualitative methods, given the cross-cutting themes that emerged from the thematic analysis.
A weakness of the study concerns the non-participation of some key members of the stroke teams, in particular consultant stroke physicians and local commissioners of services. Their participation may have highlighted additional issues that this study has not considered.
Relationship with other parts of the programme
The study highlighted the value of 6-month and annual reviews for maintaining contact with stroke survivors so that changing circumstances can be monitored and managed accordingly. The potential benefits of interdisciplinary team learning and practice across professional boundaries in clinical and non-clinical settings, innovative in-house training programmes for CSTs (combined with an open-door self-referral policy), survivor-to-survivor peer support schemes to help overcome practical and emotional challenges and the delivery of self-management programmes focused on a goal-setting approach to problem-solving informed our subsequent thinking.
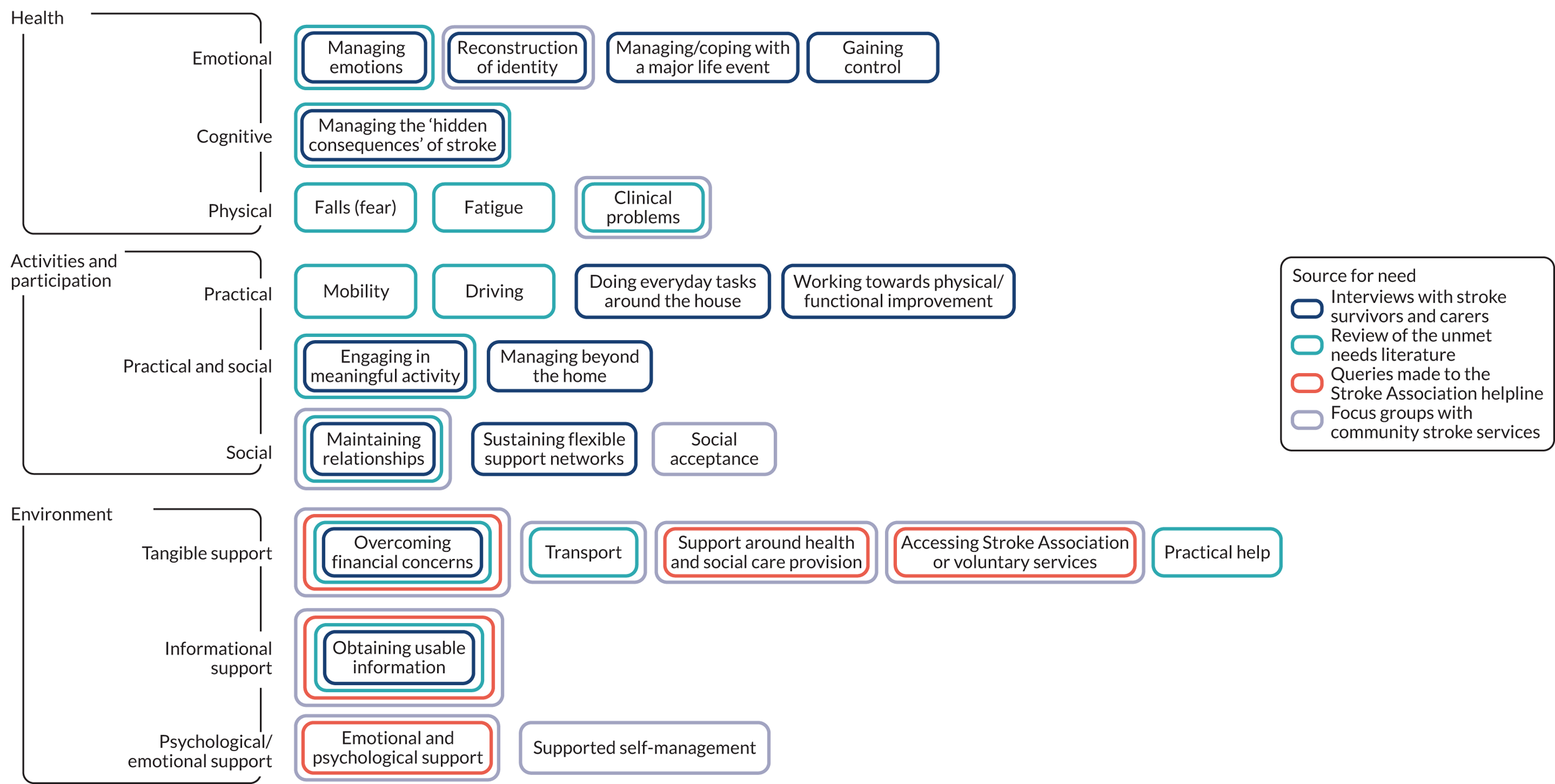
Synthesis of unmet needs after stroke
At the completion of this WS, we reviewed all data gathered in WS1 and WS2 to synthesise the unmet needs of people after stroke (Figure 5).
FIGURE 5.
Unmet needs after stroke.
Workstream 3: development of a theory-based supported self-management intervention based on targeted behaviours
Aim
The aim was to develop an intervention with appropriate supporting materials and plans for implementation that was grounded in existing research and practice, and focused on improving QoL by addressing unmet needs and maintenance and enhancement of participation of stroke survivors, while not increasing the burden on their carers (as outlined in the grant application).
Methods
Although, for ease of comprehension, the research is described as a linear process, in reality, the work was completed iteratively using a participatory approach involving the PMG, the CRAG and the following groups, established for this WS:
-
The project working group comprised Anne Forster, Rosemary McEachan, Josie Dickerson, Thomas F Crocker, Jessica Hall and Arvin Prashar, with additional input sought from Allan House, Jenny Hewison, Robbie Foy, David Clarke, Rebecca J Hawkins, Mary Godfrey, Katie Grenfell, Christopher McKevitt and members of the PMG. The working group and associated academics gave us insight from a range of disciplines, including stroke rehabilitation, psychology, psychiatry, social anthropology, health services research and implementation science, as well as previous experience of the intervention mapping approach.
-
A reference group (RG) comprised the project working group; representatives from commissioning (GR), local authority social care (JW and AW), a community integration service run by the Stroke Association and Momentum Skills (AM), Carers’ Resource (AJ), the Stroke Association (LH) and community neurophysiotherapy (SM); and a stroke survivor (SF). This RG gave us access to tremendous experience and a range of insights on how longer-term stroke services should be shaped with particular respect to what was wanted and needed and what would be practicable and fit with existing services. The RG met monthly for 5 months and was instrumental in framing the intervention.
Clarifying general features and needs to be met by the intervention
Methods
With input from the RG and PMG, the principles for the intervention were developed based on those outlined in the grant proposal.
The total list of 23 needs, identified in WS1a from the qualitative work, literature reviews and helpline data and from the focus groups in WS2 (see Figure 5), was prioritised by members of our RG and our established CRAG. Members sorted cards of the needs into piles of ‘most important’ and ‘less important’ and provided explanations for their choices. The selection of needs were iteratively reviewed and refined in discussion with these groups.
Results
Principles for the intervention were developed, and included the overall aims of improving QoL and participation for stroke survivors; that the intervention be relevant and accessible to all stroke survivors and their carers; that it be responsive to context, be feasible and sustainable; and that it can be developed in a context of existing health and social care resources.
The RG and CRAG emphasised that the importance of specific needs would vary widely between individuals. However, they provided guidance as to the needs that our intervention should concentrate on, emphasising psychosocial needs and arguing that these were less likely to be addressed by current services, and, moreover, were most likely to influence QoL and participation.
The prioritised needs to address were gaining control, managing emotions, maintaining relationships, managing or coping with a major life event, reconstruction of identity, managing hidden consequences of stroke, communication, mobility, clinical problems, support around health and social care provision, obtaining usable information, sustaining flexible support networks and engaging in meaningful activity (see Appendix 6).
Of the 10 remaining needs from the list of 23, six were needs with existing service responses, and therefore remained as needs to be addressed by referral and not directly by the intervention (e.g. falls, driving, financial). Four needs had barriers and enablers similar to those already included, and were therefore likely to be encompassed in the intervention (e.g. ‘managing beyond the home’ has barriers and enablers that are similar to those of ‘engaging in meaningful activity’).
The RG discussed the following challenges:
-
Complex psychological and relationship issues – it was agreed that these were beyond the scope of the intervention and should be managed through referral to existing psychological services and organisations (e.g. Relate).
-
Carer-specific needs – although acknowledging the importance of recognising carers’ needs (and implementation of the Carer Act 2014101) it was agreed that the intervention would focus on survivors’ needs. Those delivering the intervention should be equipped to recognise problems and to help carers find alternative appropriate services. Detailed exploration of carer needs was not undertaken to inform an intervention. Carers’ Resource were an active participant in our research programme and ensured that we were aware of the needs and perspectives of carers and other family members at all times. Our research team had undertaken considerable previous work addressing carer needs. 45 A colleague in our unit undertook a linked PhD specifically continuing that work, focused on developing an intervention to reduce carers’ burden. 102
Developing specific objectives for the intervention
Methods
In accordance with the intervention mapping framework,103,104 behavioural outcomes for the 13 prioritised needs of survivors and their families were identified. For each of these behavioural outcomes, performance objectives were developed (what participants and agents in the environment need to do to achieve the behavioural outcome). Matrices of change objectives were developed, identifying specific determinants from the theoretical domains framework (TDF)105 that were likely to influence achievement of these performance (e.g. self-efficacy, skills) objectives in a range of domains. Fourteen domains of the refined TDF were used, along with two further domains particularly relevant to a stroke population: physical function and communication.
Results
A catalogue of 263 performance objectives and hundreds of change objectives designed to address the identified needs was developed (an example is provided in Appendix 7). As a result, the intervention mapping approach became too overwhelming in the face of the complexity, the interindividual variation and the lack of several specific behaviours that longer-term stroke survivors’ outcomes of QoL and participation could be focused on. The hundreds of change objectives that were generated highlighted the limited applicability of intervention mapping for an intervention designed to meet such a wide range of needs, each with a large variety of possible barriers and enablers. The working group agreed that there were too many performance and change objectives to take through the proposed steps of intervention mapping. In addition, the relevance of these objectives to overcoming the needs of survivors or carers would be very dependent on the particular needs and circumstances of the survivors and carers. There appeared to be some similarity between many of the performance objectives organised under different behavioural outcomes.
Instead of identifying methods and applications for each change objective in accordance with intervention mapping, the intervention plan was developed using:
-
the similarities identified in the performance objectives above
-
problem-structuring and shared knowledge creation, shaped by discussion with the PMG and RG
-
information gained from all WSs and from the expertise of stroke survivors and carers
-
consultation with health, social and academic professionals
-
the research evidence review (see Appendix 8).
These sources converged to produce three intervention components: problem solving, building and sustaining a flexible support network, and obtaining usable information. When compared with the interim outputs from the intervention mapping work that had attempted to merge performance objectives across multiple needs, the approaches were compatible in cases for which it was feasible to check.
Recognised approaches to each component of the intervention were selected, consulting the relevant literature, the RG and the PMG. Practical applications to enact the components of the intervention were then considered and identified.
Problem-solving
To clarify, problem-solving was intended to address prioritisation of needs, and developing and enacting solutions that were specific and feasible for each survivor’s lived experience and circumstances. This would be allied with opportunities to access existing therapies when this was desired, deemed necessary and available. Existing approaches were adapted, because social problem-solving is a well-developed method with underpinning models, established components and approaches to application (e.g. problem-solving therapy106).
Building and sustaining a flexible support network
Building and sustaining a flexible support network enabled current problem-solving, but was also intended to provide a protective effect against the negative consequences of future challenges. Relevant methods and applications to incorporate into the intervention were identified.
Obtaining usable information
Access to usable information would also enable problem-solving, as well as satisfy the need to understand the personal and mutual ramifications of stroke and future prospects for recovery. In specifying this component, the RG and CRAG recognised what the evidence base suggested: that provision of large swathes of material would tend to overwhelm, rather than support, survivors, and that assistance in identifying and interpreting relevant information would better fit the principles of self-management.
Delivery format
Because the intervention was being designed to cater for all stroke survivors beyond 6 months after stroke, and given that the preceding work had demonstrated that some survivors are averse to attending groups, it was concluded that individual person-centred delivery must be the starting point. In addition, owing to the incorporation of facilitated problem-solving and the importance of tailoring the delivery to a survivor’s needs, it seemed likely that the intervention would include face-to-face delivery. Individual delivery was also deemed helpful in catering for ‘hard-to-reach’ groups, such as people affected by aphasia or cognitive impairment. The benefit that many survivors and service providers report from peer support was recognised. However, given that peer support was already available across the UK (for example, through the Stroke Association), raising awareness and encouraging engagement with it was incorporated in the intervention rather than incorporated as a component in itself.
Timing
Although the original grant suggested that this intervention would probably incorporate the annual review, the exploration of current services suggested that relatively few survivors were receiving a 6-month review; therefore, it was concluded that the 6-month review was an appropriate anchor point.
Producing materials
Methods
The literature was searched for existing self-management interventions in stroke and other chronic conditions (details of the search strategy can be found in Report Supplementary Material 8). Existing materials were reviewed to determine whether or not they fitted with the selected approaches. When this was the case, these were adapted to the target group. When this was not the case, new materials were developed in accordance with the selected approach. The RG, PMG and CRAG contributed to this process.
Results
The comprehensive search of the literature relating to self-management in stroke produced 2492 titles. From these, 171 papers were scrutinised in detail. The form and content of numerous intervention materials were considered, in particular the Chronic Disease Self-Management Programme; the West Lothian Stroke Workbook; a problem-solving approach previously developed by Professor Allan House for stroke/diabetes; the Greater Manchester CLAHRC Patient-Led Assessment for Network Support (PLANS) website; Professor Lewin’s The Heart Manual; and The Pain Management Plan, a stroke recovery video guide developed in New Zealand.
For the problem-solving section, materials previously developed by Professor Allan House for stroke/diabetes patients were selected and adapted with reference to the latest iteration of problem-solving therapy. Further materials to support building a sustainable support network were developed and added. Stroke Association literature was selected and adapted to provide essential information for stroke survivors to enable them to understand their stroke and its repercussions. A short list of key national contacts was also developed to provide opportunities for survivors and carers to access relevant information. Furthermore, work was carried out with the Greater Manchester CLAHRC in adapting its PLANS website as an information resource identifying local activities and services. These materials were brought together in a booklet format, which was branded the ‘Moving Forward Guide’.
The Longer-term Unmet Needs after Stroke (LUNS) tool,20,107 originally designed to elicit needs for which there was a clear service response, was extended to incorporate the full range of needs identified in earlier work (LUNS+). The original intention to send this tool out to survivors in advance was considered, but, in discussion with the CRAG, PMG and PSC, it was decided that to do so would be overwhelming or perceived as negative by some survivors. From the extended LUNS tool, a priming tool was developed to explain the purpose of the meeting between survivor and site facilitator, including a sample of life domains that might be of interest and to provide space to record important changes since the stroke and challenges overcome.
Planning for implementation
Based on the proposed intervention, Professor Lewin’s The Heart Manual108 and The Pain Management Plan,109 the process of developing behavioural outcomes and performance and change objectives for the individuals who would implement the intervention was worked through. Implementation was also analysed against an alternative framework specific to this purpose110 and the RG and CRAG were consulted. This was developed into a plan for training, processes and resources that was further developed in WS4.
Description of the intervention
In brief, at this point, the intervention consisted of a review approximately 6 months after stroke. Survivors were to be offered an initial face-to-face meeting to identify any potential unmet needs. The priming tool, which detailed the date and time of the meeting, would be sent out in advance. This tool highlighted some of the issues that may be covered, and prompted the survivor to note down those that may be relevant to them. At the meeting, these issues and needs were to be discussed, and a supported self-management approach introduced with the aim of addressing needs through individualised problem-solving. The process involved action-planning and goal-setting, facilitated by use of the Moving Forward Guide. The Guide provided general information about stroke and its effects, and up-to-date contact information for a range of organisations. Sections about a survivor’s life and the challenges they have overcome, social network mapping tools, current challenges facing the survivor, and tools to aid goal-setting and action-planning were to be added to the guide in a flexible manner, should they become relevant. The intervention also promoted the use of an online information resource (PLANS111), which acted as a directory of local activities and services (see Appendix 9).
Workstream 4: refinement and pilot implementation of the care strategy
Aims and objectives
The aim of this WS was to evaluate and refine the care strategy developed in WS3, prior to proceeding to a larger feasibility trial. The focus was to test mechanisms for, and feasibility of, embedding the service in routine care. This included identifying and training staff to deliver the core components of the care strategy to a small sample of stroke survivors. We also took the opportunity to assess the use of the outcome measures.
Methods
Identification of sites
Interest for participation was elicited by circulation of an expression-of-interest form to all stroke services in Yorkshire. Sites were selected to ensure a range of populations and longer-term service provision. Criteria for eligibility to participate as a pilot site were demonstration of multiagency working, not participating in similar research studies, willingness to engage proactively with the research process and readiness to explore different ways of working.
Refining and piloting the intervention
A number of approaches were applied to refine and test the intervention.
Action groups
An action research, service improvement approach was employed to engage staff in a cyclical process to preliminarily test and refine the care strategy. Action groups were established at each site and the action research process involved input or dialogue, action, transformation, review and refinement. Each site was asked to identify facilitators who would be trained to lead local implementation (henceforth called the ‘site facilitator’). Each action group meeting was facilitated by a researcher from the central research team (henceforth called the ‘action research facilitator’) (further details are provided in Appendix 10).
Semistructured interviews
Semistructured interviews were undertaken with stroke survivors and their carers (when applicable) to obtain insights of their experiences of the intervention and their views regarding the care strategy. Interviews were conducted in survivors’ homes using a study-specific topic guide. Interviews were audio-recorded and transcribed verbatim. Field notes documenting contextual factors were collected by the researchers to aid analysis.
Semistructured exit interviews were also undertaken with the pilot site facilitators to explore their experiences and thoughts with regard to delivering different elements of the care strategy.
Expert panel
An independent panel of specialist clinicians, who would be available to participate in supervision and case discussion if required, was established. The idea of using an expert panel arose owing to concerns that the intervention might fail if stroke survivors’ problems were too difficult for the site facilitators to address and/or required specialist services (e.g. psychology and pain clinics). Site facilitators were instructed to submit anonymised case studies of any patients or problems they were having difficulty with to the independent panel, which would review the case and determine if it required very specialist input or if, with guidance, the facilitator could have addressed the problem. This process was introduced to enable the feasibility of the intervention to be assessed (i.e. would the majority of stroke survivors actually require specialist input?).
Focus groups and expert seminars
Focus groups comprising staff from the pilot sites and expert seminars with national experts were held at the end of the study to draw together the experiences and lessons learnt to refine the intervention and delivery mechanisms.
Stroke survivor and carer recruitment
The service changes implemented as part of the care strategy were service improvements and, as such, stroke survivors and their carers received the service as part of their routine care and were not approached for consent. However, stroke survivors and their carers were approached for consent to complete outcome assessments, as well as semistructured interviews. They were first approached by clinical staff on entry to the stroke care strategy (e.g. during the 6-month review or a visit from a Stroke Association worker) to discuss participation in outcome assessments and/or interviews. Those who were interested in participating were asked to complete a consent-to-contact form, which was passed on to the research team.
On receipt of a participant’s consent-to-contact form, the research team posted the stroke survivors and carers a study information sheet and baseline questionnaire pack. Potential participants for selected semistructured interviews were approached approximately 2–4 months later.
Results
Expressions of interest were received from six sites; three were selected to represent a range of services, as detailed in the following list. The three pilot sites were able to demonstrate commitment to multiagency working, and had the following characteristics:
-
one service with and two services without a longer-term stroke service or 6-month review (in the one service that delivered a 6-month review, this comprised an initial telephone contact, followed by a home visit during which a structured assessment took place, involving signposting to therapeutic and social support services)
-
one service with and two services without open access to services
-
one rural and two urban geographical areas.
Action groups
Action groups were successfully established in the three sites, and each site identified facilitators to lead local implementation. Two sites each identified one member of staff (an occupational therapist and a physiotherapist), whereas the other site identified a health and well-being worker and an administrator. Membership of the action groups varied between sites, but, along with the site facilitator, generally comprised local service managers, Stroke Association representatives and stroke survivors and their carers. Initial meetings took place in January and February 2015 at each site to introduce the research team, provide an overview of the developed care strategy and explain the action research process.
During early action group meetings, similar topics were discussed in all three sites. These included the configuration of the existing stroke service, methods of identifying and including all stroke survivors in the 6-month review process, the intervention materials and how they could be used effectively, and how to implement the intervention in their site.
The action groups met fortnightly for 3 months in two sites and for 2 months in one site, and met monthly thereafter. A total of 40 action group meetings took place across the three sites. All sites maintained active engagement with the research team, identifying barriers to and facilitators of intervention delivery, which contributed to the further refinement of the intervention and materials. Fortnightly meetings of the action research facilitators ensured that there was transfer of knowledge between the sites and maintained momentum with regard to the development of a common and flexible care strategy.
Training
Facilitators from all sites attended the provided training, which comprised an initial 2-day workshop held in April 2015. The training was developed and delivered in collaboration with the central research team and Enabling Self-Care,112 the latter led by an independent specialist physiotherapist and consultant clinical health psychologist, both with years of experience in providing training and support in self-management knowledge and skills to health and social care professionals. Training focused on self-management and the role of the facilitator to support self-management in long-term conditions, specifically in relation to stroke. The training was delivered through presentations, free-think sessions and role-play. Enabling Self-Care provided supporting materials for the training workshops, which were subsequently developed into a comprehensive training manual by the central research team.
A follow-up training half-day took place in July 2015, providing site facilitators with a forum to discuss their experiences with each other, after having had a chance to practise delivering the intervention in their sites. It was also an opportunity to revise what was taught during the initial 2-day workshop. In preparation for the training session, the facilitators were asked to prepare a couple of case studies to stimulate thoughts about challenging situations and cases that went well.
Intervention delivery
Facilitators from all sites went on to deliver the intervention and recorded all patient contact on the purposely developed data collection forms.
The intervention was delivered to 62 stroke survivors across the three sites. The average total contact time was 114 minutes and average total work time per survivor was 162 minutes. Fifty-eight per cent of stroke survivors received the priming tool, 46% received the guide book and 22% used PLANS. Information was provided in 58% of cases, and social networks and unmet needs were discussed in 76% of cases. The intervention prompted referral to external services in one-quarter of cases (e.g. to a Blue Badge scheme, a hospital specialist and organisers of a stroke exercise class). An example of a completed record is provided in Appendix 11.
Semistructured interviews
Interviews were undertaken with 15 stroke survivors [site 1, n = 4; site 2, n = 5; and site 3, n = 6; four female and 11 male; six were interviewed with their carers (five female, one male)] and four site facilitators. Although purposive sampling had been planned, insufficient numbers were recruited to enable this, meaning that all consenting stroke survivors were interviewed.
Most interviewees remembered being contacted about having a first meeting and were happy to do so in their home. The facilitator tended to be described as good at listening or easy to talk to, someone who tried to understand them and didn’t bombard them with information, pleasant, knowledgeable and encouraging. Some participants described the process of listing the challenges faced and overcome as encouraging. Owing to problems with their hearing aid, one person had difficulty communicating with the facilitator, and these communication difficulties weren’t identified by the facilitator.
Many participants either did not remember or did not have specific views on the intervention materials. However, some described the priming tool as helpful, triggering thoughts about what to discuss and helping them ‘think outside the box’ (Nigel). Worksheets were described as clear and easy to use, with well-sized text. Some participants described the worksheets as prompting them to think through what bothered them, to problem-solve and to recognise progress made and the supportive network they had around them. However, some found the size of the booklet a bit overwhelming and, although some found writing things down helpful, others did not want to. Some participants described the contacts section as useful, and another found the Understanding My Stroke section helpful in conjunction with the facilitator’s explanation of their stroke.
Social mapping was completed by some, but not all, interviewees. Two participants described it as uncomfortable/difficult to complete: one said this was because it was the big issue for them and the other said it was because they didn’t have many people in their support network, but that it did help them feel grateful for the support they did have.
Goals set were often related to getting out and about (e.g. on the bus to the community centre, walking to shops, going to the pub), driving, domestic activities and leisure/hobby activities. Most participants were comfortable with the goal-setting process, although two were not. One participant (Stuart) described the difficulty of goal-setting when apathetic: ‘the motivation has gone, the humour has gone, the desire to do things that you really enjoy doing . . . I just find it meaningless’. They found writing goals down demeaning and it reminded them of being at work. Another participant (Susan) (who had been left with the materials to self-complete) found ‘setting goals’ daunting and would have preferred ‘aims’, which would convey less pressure to achieve them.
Participants described action-planning, which ranged the full spectrum of being developed entirely independently of the facilitator, collaboratively developed and suggested by the facilitator. Participants also reported progress monitoring in some cases, including adjusting and setting new goals/plans. Participants were signposted to the local Different Strokes group, to their GP about their depression and to the availability of free continence products. Referrals were made to a blue badge scheme, a hospital specialist and organisers of a stroke exercise class.
The closure phase of the intervention sparked a range of emotions. Although some participants felt that the intervention had ran its natural course, others felt abandoned again (following previous post-stroke health-care withdrawals), and still others expressed mixed emotions, disappointed that they didn’t receive more visits, but understanding the withdrawal in the context of constrained resources. Most participants were aware that they were able to opt back in to the service and many described this as reassuring. However, some were not aware of this and no participants had done so, despite the desire expressed for continuing contact by some participants.
Participants described the benefits they perceived from being able to talk about their circumstances with someone knowledgeable and understanding. They also described the importance of the relationship with the facilitator, some in terms of trust, openness and even friendship. Others mentioned the importance of the facilitator aligning with their current situation and the goals they had. One carer (John) said the facilitator had helped by encouraging him to do less and did his wife ‘more good than any of the others, because she took no nonsense from her’.
Many participants described how they were reassured, encouraged and motivated by the facilitator, and were imbued with confidence both through conversation and through attempting goals. Other participants said they were already self-motivated or did not feel motivated by the intervention.
Participants often linked improved confidence or a shift in attitude to the numerous things that they were now doing, or doing differently, as a result of the intervention. Eight interviewees/dyads out of 15 reported doing something as a direct result of the intervention. These included dressing themselves, walking more, using a mobility scooter or the bus, getting out with the help of family/neighbours, organising a sponsored run, participating in groups, reading, other hobbies and domestic tasks, and setting their own goals. In turn, for some participants, this was linked with improved mood.
Expert panel
None of the site facilitators submitted case studies to the expert panel.
Focus groups and expert seminars
Focus groups comprising staff from the pilot sites and two expert seminars were held. The seminars included academics from across the UK (Fiona Jones, St George’s London; Rebecca Fisher, University of Nottingham; David Clarke, University of Leeds; and Maggie Lawrence, Glasgow Caledonian University), all chosen for their expertise in self-management and community services. Other attendees included a local commissioner and senior community staff. Outputs from these meetings and the interviews with stroke survivors and their families and the site facilitators contributed to the key findings described in Key findings.
Stroke survivor and carer outcomes
Twenty-nine consent-to-contact forms were received, and questionnaire packs were posted out to those patients and their carers. Eleven (38%) baseline packs were returned. Approximately 3 months later, follow-up packs were sent to eight of those who had returned baseline packs (two participants had withdrawn, and one participant had been recruited at a late stage in the study and the follow-up was due beyond the time period of the study). Six (75%) follow-up packs were returned. Recruitment and return rates were lower than anticipated; however, the majority of outcomes were fully completed and no particular challenges were identified, although this may have been a reflection of the select sample rather than the characteristics of the outcome measures.
Key findings
Action research process
The action research process enabled iterative development of the intervention materials with input from those delivering the intervention and stroke survivors/carers (i.e. intended recipients). It also provided insight into local processes and the apparatus of local service delivery, opportunity to try out different parts of the intervention, regular feedback from facilitators delivering the intervention and socially produced knowledge (i.e. social setting produces a different kind of knowledge). There was particular advantage in bringing together both lay and clinical perspectives to promote greater shared understanding.
Challenges of the action research process
Challenges to the action research process included disagreement within action groups, challenges of moving forward without consensus, relative power of the action groups to engineer change given existing service frameworks and patterns of working, and differences in autonomy/freedom from established ways of working.
Existing services
In one of the sites, tensions emerged between the planned ‘new’ service and a recently commissioned service, which was partially resolved by extensive discussions.
In another site, during initial discussions, there was a lack of clarity on who (how many) and at what time point stroke survivors were being approached for a 6-month review. It emerged that multiple assessments were being undertaken, with some staff discussing unmet needs and administering the Patient Health Questionnaire-9 items at approximately 6 months after stroke and a third-sector organisation undertaking an assessment of unmet needs and medical review at 6 months after stroke and a similar review at 6 months after hospital discharge. There appeared to be little shared knowledge about these multiple reviews and information was not passed between the services that carried them out.
The need to have detailed conversations with as many stakeholders as feasibly possible prior to set-up of a new service was highlighted.
Intervention implementation
Through this WS, we were able to establish that it was feasible to introduce the new intervention into existing services. Some particular points of note were highlighted regarding implementation. These were as follows.
Number of facilitators
Although the action group provided a supportive structure, in two of the three sites, one person was tasked with delivering the intervention. It was recognised that this was not ideal as information provided from the sites indicated that the associated workload would preclude all stroke survivors being seen. Therefore, for the feasibility trial, it was emphasised that at least two people should be trained in the new approach.
Identification of stroke survivors
All three services established mechanisms to identify stroke survivors at the appropriate time point. However, at one of the sites, the facilitator undertook a detailed audit to check the throughput and noted that a number of stroke survivors had been missed off of the referral lists for no apparent reason. ‘Talking through’ the care pathway as it currently exists might highlight gaps and duplication.
Timing of delivery
Timing of delivery of the intervention was confirmed to co-ordinate with or act in place of the recommended 6-month review.
Building a relationship with the stroke survivor
Members of the action groups and stroke survivors, in their interviews, both emphasised the importance of building a rapport in order to successfully deliver the intervention.
Dealing with specific stroke survivor problems
The facilitators requested that training should include a focus on dealing with people with anxiety and depression, as that had proved challenging on occasions.
Expert panel
The research team had concerns that problems might arise that were (1) outside the expertise of the facilitators or (2) required specialist input. The availability of the expert panel was highlighted through the action groups, but no cases emerged. This provided reassurance that addressing the identified unmet needs of stroke survivors was within the remit of these facilitators.
Patient-led Assessment for Network Support
Colleagues in the Greater Manchester CLAHRC had developed the web-based resource tool, PLANS. This was an interactive tool in which someone could enter their home address, and local leisure/social needs relevant to their interests would be identified. 113 Difficulties emerged during this feasibility work, as local information had to be manually uploaded to the site and routinely checked/updated. This was hugely time-consuming. Use was also compromised as internet access was not always available in the stroke survivors’ homes. Of the three sites, one site created the resource, one began doing so but expressed concerns about vetting the local information, and the other did not. This, together with challenges of maintaining the web platform, led to this component of the intervention being dropped.
Recruitment of ‘research’ participants
Identification of stroke survivors for interview and completion of outcome measures through the clinical staff was not successful and limited purposeful sampling of participants. Consequently, the number of stroke survivors interviewed was lower than anticipated. Resources available through the CRN for the feasibility trial enabled a different model to be successfully implemented in WS5.
Outputs
Challenges of 6-month review
There was considerable discussion in the expert seminars and during the feasibility work on the interface between the proposed new intervention and the existing 6-month review, and whether it should replace or be alongside existing procedures. It was concluded that we would aim for the new intervention to replace the current review process, and thus become a component of our care strategy.
Stroke population
On reviewing the findings of this and previous WSs, and following discussion at the expert seminars, it was agreed that the intervention would be offered to all stroke survivors. Therefore, there would not be any triaging of the stroke population. It was recognised that this may mean that information about the potential benefits for a subset of the population might be masked, but there was insufficient information to appropriately determine which subset of the population might benefit. It was agreed that outputs from the feasibility trial might inform this.
Developed intervention
The primary activity and outcome of WS4 was continuous refinement of the intervention, intervention materials and associated logic model. Members of the action groups and the site facilitators had substantial input into refining the intervention materials, which included producing a priming tool (based around the invitation to the appointment); producing worksheets; and clarifying language, for example referring to problems or challenges and trying to generate more positive reflection rather than an immediate negative focus on unmet needs. Following feedback from stroke survivors via the action groups, the manual was replaced by individual worksheets.
The intervention was initially called ‘Moving Forward’, but, to avoid confusion with an intervention delivered by the Stroke Association of the same name, was renamed New Start.
The developed intervention, New Start, consists of the following components:
-
needs assessment delivered through a face-to-face review at approximately 6 months post stroke
-
supported self-management care strategy
-
materials to support needs assessment, self-management, goal-setting and action-planning, as well as the provision of usable information (the ‘priming tool’ and ‘New Start Guide’)
-
structured training programme for staff [face-to-face modules, supported by training worksheets and video content, as well as online learning resource through Google (Google Inc., Mountain View, CA, USA) hub/website and e-mailed links to training videos developed by the team and uploaded to YouTube (YouTube, LLC, San Bruno, CA, USA)].
A logic model was also developed for the intervention and implementation (Figure 6). In addition, an outcomes chain114 was developed to represent, diagrammatically, a theory of change, that is what must be achieved in implementing the intervention to bring about the overall outcome: improved QoL and increased participation for stroke survivors (see Appendix 12). A matrix to accompany the outcomes chain was also developed to describe details about success criteria for each outcome and factors that may aid or impede its achievement (available from the authors). The Template for Intervention Description and Replication framework115 was also completed to report the components of the intervention.
FIGURE 6.
New Start intervention logic model. Reproduced with permission from Hardicre et al. 142 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
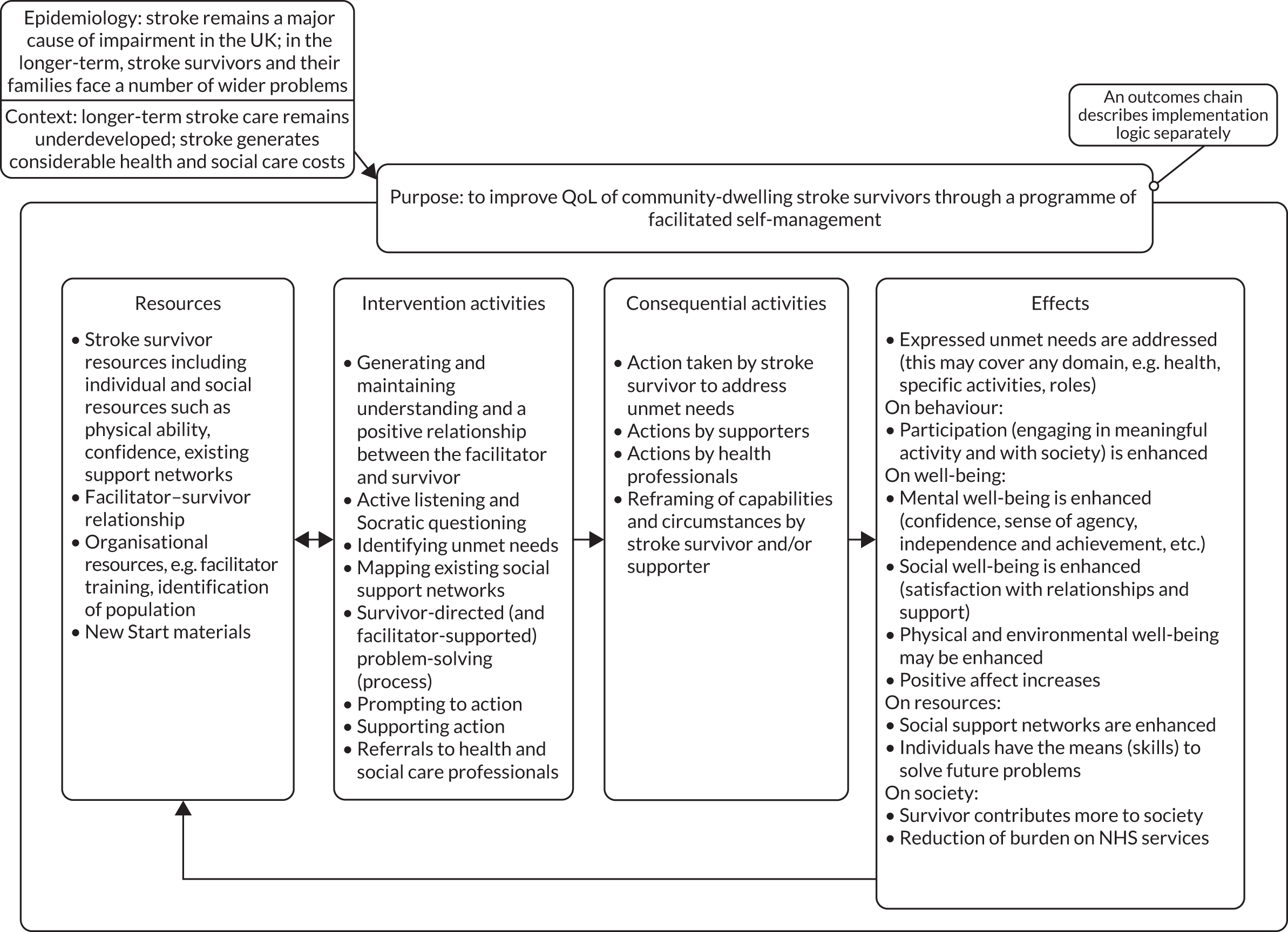
Competency assessment
Members of the action groups and, later, colleagues in the expert seminar emphasised the importance of competency assessment. A detailed competency assessment framework was therefore developed. This included observing delivery of the intervention, conducting an oral or written test of understanding of the intervention and assessing structured reflective reports completed by facilitators to describe situations in which they have delivered the intervention (see Appendix 13).
Key criteria for sites for the feasibility trial (workstream 5)
It was emphasised to sites interested in participating that they would have to establish a robust mechanism to identify all stroke survivors in an identified service, not just those stroke survivors who had been in receipt of post-discharge services such as ESD or community rehabilitation.
In recognition of the workload, it was emphasised that at least two people, in a supportive team, should be trained in the new approach.
It was identified as important to obtain information on current throughput of stroke survivors, staffing levels and details of associated and referral services available to staff in the service. This informed the development of a site survey, which was implemented (in WS5) when sites first expressed an interest, just prior to randomisation, at 3-month intervals during the trial and at the conclusion of the study.
Finalised outcome measures
The choice of appropriate outcome measures to use in the feasibility trial was reviewed in the PMG and PSC. The list of outcome measures to be used in the feasibility trial was finalised, and included measures that may be the primary outcome in the full trial, as well as other measures to capture possible mediator and moderator effects, which included the Patient Activation Measure116,117 assessed at 3 and 6 months (as described in Appendix 14).
Workstream 5: feasibility cluster randomised controlled trial of New Start
Aims and objectives
Parts of this section have been reproduced Forster et al. 118 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The aim was to conduct a pragmatic, multicentre, cluster randomised controlled feasibility trial of the Improving Longer Term Stroke Care (LoTS2Care) intervention (New Start), compared with usual care. The key objectives related to evaluating the feasibility and acceptability of implementing a future definitive cluster RCT:
-
recruitment methods (stroke service and stroke survivor)
-
intervention implementation and delivery
-
definition of usual care
-
assessment of outcome measures (completeness, follow-up rates, potential for effectiveness) and estimation of the intracluster correlation coefficients (ICCs)
-
preliminary assessment of cost and cost-effectiveness
-
safety.
Objective 2 was additionally evaluated via the embedded process evaluation (see Workstream 5: process evaluation). Objective 5 was assessed via health economic data (see Workstream 5: economic evaluation).
Summary of study design
The LoTS2Care feasibility trial was a cluster RCT conducted in English and Welsh stroke services. Ten stroke services were randomised (1 : 1) to implement New Start or continue with usual care only. New Start was delivered by trained facilitators and offered to all stroke survivors in services allocated to the intervention. Stroke survivors were invited to participate in the trial by post. A total of 269 stroke survivors were registered to the trial, which satisfied the required minimum of 200 stipulated in the protocol. Outcome measures and health and social care service use were collected via post at 3, 6 and 9 months after recruitment. An embedded process evaluation was undertaken on an ongoing basis in each intervention stroke service throughout the study.
Recruitment
Stroke services
Stroke services were eligible if they:
-
agreed to undertake a robust mechanism to identify all stroke survivors at 4–6 months post stroke
-
had the facilities and capacity to deliver the New Start intervention (i.e. staff available to undertake training and provide face-to-face contact with community-based stroke survivors who were at least 6 months post stroke).
Stroke services were excluded if they:
-
had previously participated in research contributing to the New Start intervention development
-
were currently implementing or intending to implement a service comparable to the New Start intervention (e.g. a self-management focused approach) within the study duration.
A number of approaches was used to identify stroke services for participation; Figure 7 shows the Consolidated Standards of Reporting Trials (CONSORT) diagram. CCGs covering three geographical areas were contacted (n = 133), as well as NIHR CRNs covering four areas. In addition, 29 sites that had participated in a previous (unrelated) stroke trial (LoTSCare23) were also approached.
FIGURE 7.
The CONSORT diagram showing the flow of stroke services and stroke survivors throughout trial. ETC, excess treatment costs.
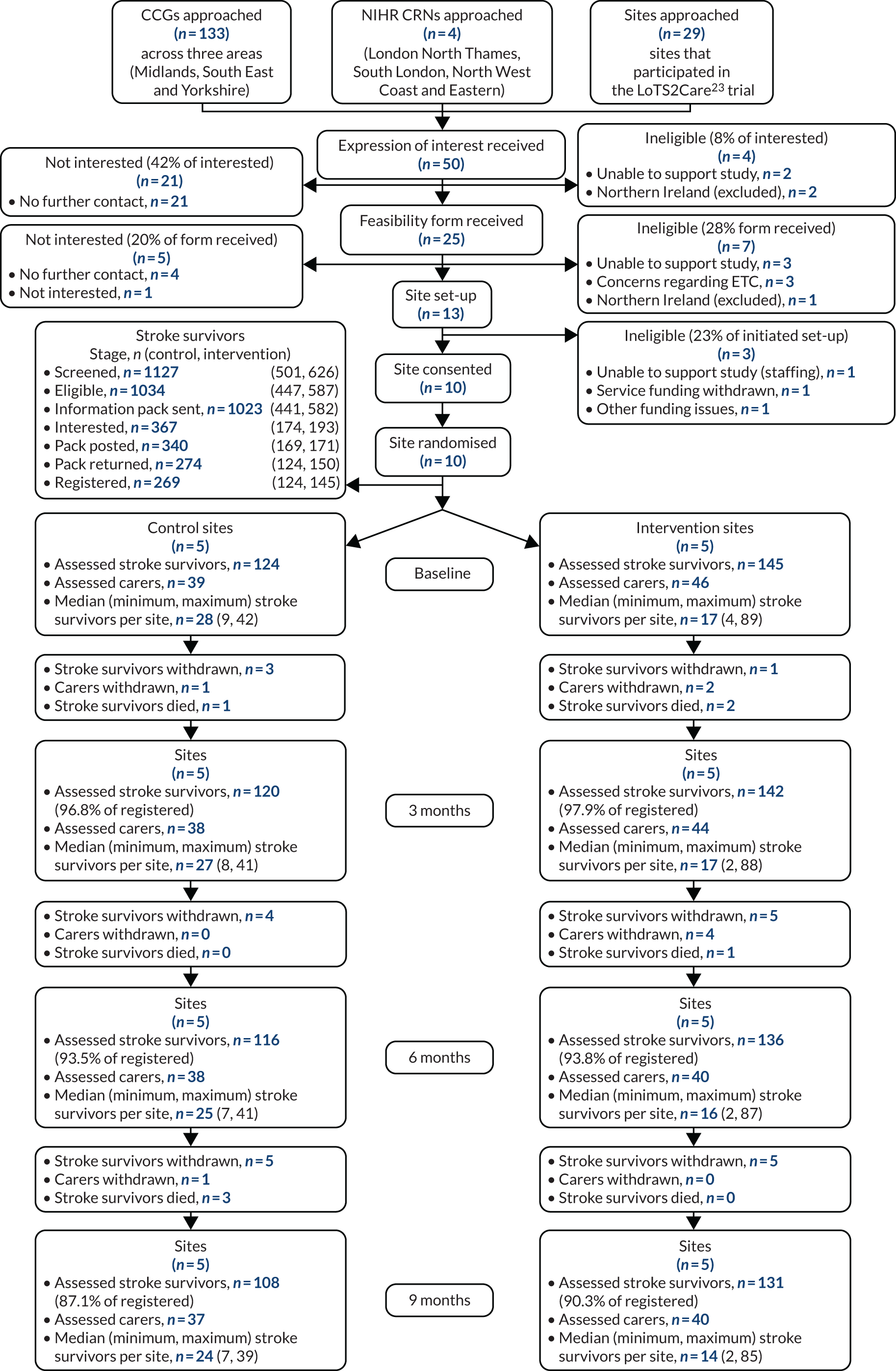
Stroke survivors
The New Start intervention was offered to all stroke survivors in the stroke services allocated to the intervention. Trial screening and recruitment of stroke survivors were undertaken by research staff, blind to treatment allocation, and independent of site staff delivering any interventions to participants (in either arm). Eligible stroke survivors were asked to consent to outcome assessment completion and to permit access to their electronic health-care records.
Stroke survivors were eligible for inclusion in the study if they:
-
were between 4 and 6 months since confirmed primary diagnosis of new stroke
-
resided in the community (i.e. not in a nursing or residential care home)
-
lived among the defined population covered by the stroke service
-
provided informed consent or consultee declaration
-
returned a completed baseline questionnaire.
No exclusion criteria were applied. Reasons for not being offered or provided the New Start intervention (within intervention services) were documented, to inform eligibility criteria for a future definitive trial.
To minimise treatment bias, New Start facilitators and usual care staff were not informed as to which of their patients were participating in the trial.
Screening and approaching stroke survivors for trial inclusion commenced at all stroke services approximately 12 weeks after services had been randomised, to allow for New Start intervention training. A consecutive sample of stroke survivors was identified by clinical or research staff (providing that governance procedures allowed the research staff to access the clinical records) and screened for eligibility; those eligible were initially approached via a trial invitation letter. Research staff had the option to chase any non-responders via telephone. Interested stroke survivors were provided with a baseline questionnaire pack and consent form, by their preferred method (face to face or by post). Participants were asked to consent to data collection only.
Carers
Carer involvement in the trial was optional. All carers identified by the stroke survivor as the main informal caregiver (providing the stroke survivor with support a minimum of once per week) and who provided consent (implied via return of completed baseline questionnaire) were eligible for study inclusion.
Carer information packs, including the baseline questionnaire, were provided to the stroke survivor with their baseline questionnaire pack, with a request to pass on to a carer if appropriate.
Randomisation
Stroke services were randomised on a 1 : 1 basis by the Clinical Trials Research Unit (CTRU) statistician using a computer-generated minimisation programme incorporating a random element. Minimisation factors were as follows:
-
the number of stroke survivors seen by community teams per annum (above and below the median across all recruited services)
-
whether or not recruitment and intervention were delivered at separate trusts (yes/no).
Recruiting teams were not informed of the randomisation result, in order to minimise selection bias during recruitment.
Intervention
New Start
The intervention is described in Workstream 4: refinement and pilot implementation of the care strategy,Developed intervention.
Stroke services randomised to the intervention identified New Start facilitator(s) who were trained in the intervention. Training comprised attending a structured training course involving face-to-face training supported by additional written materials. Facilitators learnt relevant theory about a self-management approach and communication skills, as well as specific details about the intervention and how to deliver it to stroke survivors.
New Start facilitators were assessed for competency in intervention delivery, through review of patient activity records, reflective reports, interviews and observation (when practically possible), approximately 16 weeks after completing the initial training course.
Usual care
Stroke services randomised to usual care (control) continued to deliver care as determined by local policy and practices.
Methods for data collection
Data were collected at the level of the service (including staff) and from individual consenting participants at baseline and at 3, 6 and 9 months post recruitment.
Stroke service-level data
Participating services were requested to complete a site survey documenting current service provision (including the number of patients offered their service in the preceding 6 months) when expressing interest in the study, and then at pre randomisation (baseline), pre recruitment and every 3 months during recruitment and follow-up. This survey captured usual care and assessment, and changes in stroke service provision during the trial (aside from the New Start intervention). Publicly available SSNAP data were reviewed to assess usual throughput in each service against trial recruitment rates.
New Start facilitator data
New Start facilitators provided information on their usual role and level of stroke experience. They completed the Self-Efficacy and Performance in Self-management Support (SEPSS)119 instrument prior to training, after the initial training course and further training day, and at the end of the trial. They completed the normalisation process theory (NPT)120,121 toolkit during the early, middle and end stages of implementation. As part of ongoing training and development, facilitators were asked to participate in reflective practice by submitting self-reflection reports monthly.
Stroke survivor data
Screening data
Screening data were collected by research staff for all stroke survivors identified as potentially eligible, and included basic demographic details (age, sex, ethnicity), dates of hospital admission and discharge, National Institutes of Health Stroke Scale (NIHSS) score at admission, modified Rankin Scale (mRS) score at discharge and availability of a carer. If applicable, reasons for ineligibility and reasons for declining participation were also recorded.
Baseline data
For those stroke survivors registered to take part in the trial, the following baseline data were collected by research staff: ethnicity, date of birth, sex, NHS identifier (ID), living arrangements (i.e. alone/with another person), carer identified by stroke survivor, address and telephone number, GP details, date of stroke, stroke severity (e.g. NIHSS score at time of admission), mRS score at discharge, level of impairment at recruitment (e.g. speech impairment, difficulties with communication) and preferred method(s) of contact. Stroke survivor baseline assessments were administered by research staff in person or by post (according to stroke survivor preference).
Follow-up data
The central trial team followed up stroke survivors via postal questionnaires at 3, 6 and 9 months post recruitment, supported by postal, telephone and text reminders if questionnaires were not returned in 2 weeks. Proxy completion of questionnaires was permitted. If outcome measures were not returned by post, telephone interviews were conducted to maximise data collection.
The questionnaires included:
-
the World Health Organization Disability Assessment Schedule (WHODAS) 2.0,122–124 36-item version
-
the Warwick–Edinburgh Mental Well-being Scale (WEMWBS)125–128
-
the EuroQoL-5 Dimensions, five-level version (EQ-5D-5L)129–132
-
the 13-item Short Form Patient Activation Measure (PAM) survey116,117
-
the LUNS tool20
-
relevant questions adapted from the Millennium Survey of Poverty and Social Exclusion138 and GP Patient Survey. 139
The questionnaires included a single overall life satisfaction question and a request for information about who completed the pack (stroke survivor/proxy) and how much help was provided.
Health, social care and voluntary- or third-sector service use was collected, together with costs, from participants using a resource use questionnaire.
The possibility of using NHS Digital data to provide information on stroke survivors’ hospital admissions and outpatient attendance during the trial was also explored.
Carer data
Carer baseline assessments were administered by post, unless the carer attended the face-to-face visit requested by the stroke survivor/consultee. Carers were followed up by the central trial team via postal questionnaires at 3, 6 and 9 months after stroke survivor recruitment, with reminders and telephone interviews, as for stroke survivor questionnaires.
The questionnaires included the Caregiver Burden Scale (CBS),140 the EQ-5D-5L and ICECAP-A.
Study within a trial: participant questionnaire format
Stroke survivor and carer follow-up questionnaires consisted of a large number of outcome measures alongside a resource use questionnaire. A study within a trial was conducted to determine the most acceptable questionnaire format to maximise follow-up rates for a future definitive trial. Stroke survivors (and carers when available) were randomised (1 : 1) by the CTRU, prior to the 6-month follow-up time point, to receive one of two alternative questionnaire formats: (1) a single comprehensive booklet containing all measures or (2) two shorter booklets (one containing the outcome measures, the other containing the resource use questionnaire). Stroke survivors were sent the allocated questionnaire formats at the 6- and 9-month follow-up time points (when applicable, the carer received the format allocated to the stroke survivor).
Intervention data
Compliance with the New Start intervention was monitored throughout the trial via observations and regular collection of activity records, used by facilitators to record intervention delivery. These records enabled audit of the number of stroke survivors in receipt of the New Start intervention, and assessment of adherence to, and fidelity of, the intervention delivery. Training sessions were observed and fully documented. All contacts between the research team and facilitators regarding the implementation of the intervention were recorded (considered as implementation enhancement activities). These data were interpreted alongside the parallel process evaluation, providing a comprehensive evaluation of training and implementation processes (see Workstream 5: process evaluation).
Usual care data
The site survey captured details of usual care at each participating site. Stroke services (intervention and control) were asked to record their procedures for offering 6-month reviews, including means of identification and methods of contact (telephone/mail). Stroke service clinical staff in all participating sites were asked to keep a usual care activity record for each stroke survivor they offered/provided a service to 6 months after their stroke, where they could record whether or not the stroke survivor could be contacted and whether or not they agreed to having a review, as well as details of the input received (staff were asked to provide details of any visits or contacts they had with stroke survivors), when applicable.
Recruitment
Colleagues undertaking the process evaluation interviewed staff undertaking recruitment in each site to gain feedback on procedures.
Safety reporting
Data on related and unexpected serious adverse events were collected. Death of a stroke survivor/carer or institutionalisation were also recorded as expected events. We explored the feasibility of using routine data for collecting this information.
Statistical methods
See Report Supplementary Material 9 for the statistical analysis plan.
Analyses and data summaries were conducted on the intention-to-treat population and focused on descriptive statistics and confidence interval (CI) estimation, rather than formal hypothesis testing.
Criteria for progression to a definitive trial
The criteria for progression to a definitive trial were predefined and based on recruitment, follow-up and intervention implementation and delivery. A traffic-light system of green (go), amber (review) and red (stop) was applied (Table 4).
| Criteria | Green | Amber | Red |
|---|---|---|---|
| Average recruitment of participants per site over 6 months | ≥ 20 (range 12–30) | < 20 but ≥ 10 | < 10 |
| Rate of return of follow-up questionnaires (at 9 months) | ≥ 75% | < 75% but ≥ 60% | < 60% |
| Intervention training | At least two members of staff from each stroke service attended training days and were assessed as being competent | ||
| Intervention delivery (% of recruited stroke survivors who were offered at least one session of the intervention) | ≥ 75% | < 75% but ≥ 50% | < 50% |
| Intervention implementation (% of stroke services that were deemed competent and went on to deliver the intervention to participants) | ≥ 80% (i.e. four services) | 60% (i.e. three services) | < 60% (i.e. 0–2 services) |
Key findings
Recruitment and follow-up
Stroke services
Fifty stroke services responded to the initial contact with an expression of interest, of which 25 (50% of interested) returned feasibility forms, 13 (26% of interested) entered site set-up, 10 (20% of interested) consented to participating in the trial, and all 10 sites were randomised. During screening and recruitment, 14 services (28% of interested) were found to be ineligible, and 26 services (52% of interested) lost interest or ceased contact.
Five services were randomised to each arm, with minimisation characteristics balanced across arms (see Appendix 15, Table 10). Other site characteristics show the range of service provision across sites (Table 5).
| Site | Average monthly referralsa (n) | Recruited, (n) | 4- to 8-month service | 6-month review | Provide service for all patients | How is review delivered |
|---|---|---|---|---|---|---|
| New Start sites | ||||||
| 1 | 4 | 13 | Yes | Yes | No | Telephone |
| 2a | 12 | 4 | Yes | Yes | Yes | At a patient’s home |
| 3 | 119 | 89 | Yes | Yes | Yes | At a patient’s home/clinic |
| 4 | 27 | 22 | Yes | Yes | Yes | At a patient’s home/clinic |
| 5 | 25 | 14b | Yes | Yes | Yes | At a patient’s home |
| Usual care sites | ||||||
| 6 | 32 | 32b | Yes | Yes | Yes | At a patient’s home/clinic |
| 7 | 20 | 10b | Yes | Yes | Yes | Telephone |
| 8 | 92 | 24 | Yes | Yes | Yes | At a patient’s home |
| 9 | 82 | 24 | Yes | Yes | Yes | At a patient’s home/clinic |
| 10 | 18 | 8 | Yes | No | No | N/A |
Participants and carers
Of 1127 stroke survivors who received care across the 10 services and were screened for participation, 1034 (91.7%) were eligible, 367 were interested (32.6% of screened, 35.5% of eligible) and 269 were registered to participate in the study (23.9% of screened, 26.0% of eligible; see Figure 7). There was variation across services in the proportion of eligible participants who were registered (7.7–34.5%) (see Appendix 15, Table 11): it was slightly lower in the New Start services [145/587 (24.7%)] than in the usual care services [124/447 (27.7%)] (see Appendix 15, Table 12). The number of participants recruited per site ranged from 4 to 89 (usual care, 10–42; New Start, 4–89), with the mean number of participants per site being higher in the intervention arm (usual care, 24.8; New Start, 29).
Eighty-five carers of registered participants were also recruited to the study: 39 in the usual care arm and 46 in the New Start arm.
Screening characteristics
Registered stroke survivors had characteristics similar to those of the whole screened population (see Appendix 15, Table 13).
Baseline characteristics
Baseline characteristics for registered patients were broadly similar across the two arms (see Appendix 15, Table 14). However, participants in the New Start arm had marginally better WHODAS (simple and complex), WEMWBS and PAM scores than participants in the usual care arm. Participants in the New Start arm were more likely to respond ‘A lot’ to all of the stroke-specific questions from the GP survey, which ask how much support a person would currently receive in various situations. Conversely, a higher proportion of New Start participants reported five or more unmet needs [60/145 (41.4%) vs. 41/124 (33.1%)].
Carers recruited to the trial completed the CBS questionnaire. Carers in the usual care arm reported slightly higher levels of caregiver strain at baseline, with a mean score of 48.7 points, compared with a mean score of 45.6 points in the New Start arm (see Appendix 15, Table 15). Alongside the overall score, five subscale scores were also calculated. Four of these subscale scores were, on average, similar across arms at baseline; however, the general strain subscale had higher scores in the usual care arm than in the New Start arm (20.1 vs. 17.9).
Participant retention
Overall retention of stroke survivors during the study period was high, with 239 out of 269 (88.8%) participants being available for follow-up at 9 months (see Appendix 15, Table 16). The proportion of available stroke survivors varied considerably across services (50.0–95.5%; see Appendix 15, Table 17). Losses to follow-up were due to deaths or withdrawals from the study; seven participants died during the study period (see Appendix 15, Table 18).
Comparison of recruitment rates with Sentinel Stroke National Audit Programme data
A table comparing SSNAP-reported figures with recruitment and clinical screening figures can be found in Appendix 16.
Intervention delivery and usual care
Facilitator training and competency assessment
Intervention training comprised an initial 2-day training session and two follow-up sessions. Facilitators then had the opportunity to practise intervention delivery before the trial commenced and were asked to complete two structured reflective reports focusing on New Start delivery during this practice phase. Training was adhered to fairly well, with only a small number of facilitators not completing or attending all aspects of training (see Appendix 15, Table 19). Facilitators were appraised regarding their competency in delivering the New Start intervention. All were deemed competent (see Appendix 15, Table 20).
The New Start intervention was delivered via face-to-face meetings with the stroke survivor. Overall, 86 out of 145 (59.3%) intervention trial participants had at least one intervention meeting. These participants attended an average of 1.14 meetings, each lasting approximately 1 hour (see Appendix 15, Table 21). Twelve of the 15 trained facilitators went on to deliver the intervention to registered participants, with a high degree of variability in the number of patients seen (range 1–24), number of visits (range 1–25), average number of visits per participant (range 1–2) and average duration of visit (range 20–82 minutes) (see Appendix 15, Table 23).
Baseline characteristics were compared between trial participants who did and trial participants who did not receive the New Start intervention. Notable differences included levels of higher education (49.2% among those who did not receive New Start vs. 39.5% among those who did receive New Start) and in the number of unmet needs, which was lower in those not receiving the intervention (see Appendix 15, Table 25). Those receiving the intervention had, on average, a longer stay in hospital as a result of their stroke (13 vs. 8 days). There were also differences apparent in the levels of stroke severity between these groups (measured via mRS and NIHSS) and in the level of language ability after stroke, but large numbers of missing data for these measures make interpretation difficult.
New Start sessions: non-trial participants
In keeping with the cluster trial design, all stroke survivors at intervention sites were offered the intervention, not all of whom consented to outcome data collection. Of the 442 non-study participants contacted, 294 (66.5%) had at least one intervention meeting (see Appendix 15, Table 22), which is slightly higher than the rate for study participants (59.3%). This population of stroke survivors attended, on average, a similar number of meetings as study participants (1.16), but these were, on average, shorter, at around 52 minutes. There was, again, a high degree of variability in the number of patients seen (range 3–43), the number of visits (range 4–47), the average number of visits per participant (range 1–2.33) and the average duration of a visit (range 31–84 minutes) for each of the 13 facilitators who delivered the intervention to non-study participants (see Appendix 15, Table 24).
Usual care
Only four of the five usual care sites offered a 6-month review, compared with all five intervention sites (see Appendix 15, Table 26). Across usual care sites, 86% of stroke survivors who were offered stroke care between 6 and 12 months post stroke were seen or spoken to; the majority (87.1%) of these had one contact, with an average duration of contact of 54 minutes (see Appendix 15, Table 27). Variation in usual care was observed across study sites (see Appendix 15, Table 28).
Uptake of reviews: New Start and usual care
The uptake of 6-month reviews across all services was 58.7%; however, it varied widely, from 9.7% to 100% (see Appendix 17).
A telephone invitation with an opt-in review was the most common approach to offer; however, a letter of invitation with a pre-booked appointment (opt out) resulted in the highest levels of uptake, on average.
Home was the most common location of review delivery, and resulted in higher levels of uptake, on average.
Unblinding
There were 14 reported occasions of New Start facilitator unblinding to research participation, occurring across three sites and 13 patients (see Appendix 15, Table 29). Most unblindings occurred within the first month post registration, an average of 20 days after registration (see Appendix 15, Table 30 and Figure 12).
Assessment of outcome measures
Response rates to self-reported questionnaires
Return rates of patient-completed questionnaires were assessed by arm at all time points. Return rates were consistently higher in the New Start arm than in the usual care arm for all questionnaires at all time points (see Appendix 15, Table 31). This difference was particularly visible in the return rates of the WHODAS, WEMWBS and LUNS tool questionnaires at 9 months (usual care, 81.5–84.3%; New Start, 88.5–91.6%).
Completion rates of outcomes
The WHODAS and WEMWBS questionnaires were administered at baseline and at 6 and 9 months. There was a higher proportion of partially completed questionnaires at 9 months for the WHODAS, a 36-item questionnaire, than for the 14-item WEMWBS (66.9% vs. 10.4% of available participants) (see Appendix 15, Table 32). However, the flexibility of the WHODAS simple score (it can be reduced to 32-items for those not working) meant that, at 9 months, the proportion of partially completed questionnaires that could not be scored was higher for the WEMWBS than for the WHODAS with simple scoring (44.0% vs. 20.0%). This was not the case for the more rigid complex scoring of the WHODAS, for which 50.0% of partially completed questionnaires were unscored because of missing items. At each time point for both questionnaires, return rates in the New Start arm were slightly higher than those in the usual care arm.
The PAM was administered at baseline and at 3 and 6 months. Of the partially completed PAM questionnaires at 6 months, 47.6% (20/42) were unscored because of missing items.
Study within a trial assessing method of administration
At 6 months, 126 participants were randomised to receive a single booklet and 128 participants were randomised to receive two shorter booklets – 254 participants in total; one of the participants randomised to receive a single booklet withdrew shortly after randomisation, hence 253 participants in total were sent booklets. At 9 months, after accounting for deaths and withdrawals between 6 and 9 months, 116 participants (92.1% of those randomised) were sent a single booklet and 123 participants (96.1% of those randomised) were sent two shorter booklets.
At 9 months, participants were more likely to return follow-up questionnaires when these were administered as a single booklet than as two shorter booklets. At 9 months, 215 participants returned the booklets by post (90.0% of those sent). A total of 108 participants returned the single booklet (93.1% of those sent); however, among those sent two booklets, only 105 returned both (85.4% of those sent) and two returned a single booklet containing the outcome measures only (1.6% of those sent).
For those returning questionnaires at 9 months, item completion rates were also affected by questionnaire format. Health-care resource use alone and the full battery of measures (i.e. all health outcomes and resource use) were more likely to be completed in the single-booklet arm than in the two-booklets arm. Completion rates of health-care resource use alone in the single-booklet arm and the two-booklets arm were 98.1% and 89.7%, respectively (91.4% and 78.0% of those sent, respectively); full outcomes completion rates were 97.2% and 87.9%, respectively (90.5% and 76.4% of those sent, respectively) (see Appendix 15, Table 33).
Similar trends were also observed at 6 months.
Carers were also more likely to return the questionnaires when sent a single booklet. At 9 months, 30 carers returned the single booklet (88.2% of those sent), whereas 30 returned both (81.1% of those sent). For those returning questionnaires, there were no differences in item completion rates between groups at 9 months and a very small difference between groups at 6 months; however, when assessed as a proportion of those sent questionnaires, all types of outcomes at both time points were more likely to be complete for those sent a single booklet. The number of carers available and assessed in this analysis is low; thus, this finding should be interpreted more cautiously than that for stroke survivors (see Appendix 15, Table 34).
Overall, these results suggest that outcome follow-up rates are maximised when sending all required questionnaires in a single, longer booklet rather than when splitting them across two, shorter booklets.
Statistical outcomes
Questionnaire outcomes were measured at both patient (see Appendix 15, Table 35) and cluster level, with significance testing performed on the cluster-level point estimates of the WHODAS and WEMWBS questionnaires, at the 5%, 33% and 49% significance levels. The cluster-level estimates in the intervention arm used data from only four of the five intervention sites, as one site recruited only four participants and numbers with outcome data were insufficient for such analysis. At the cluster level, the WHODAS score with simple scoring was found to be significantly lower in the intervention arm than in the usual care arm at 6 months (mean difference 3.14, 67% CI 0.76 to 5.51) at the 33% significance level (see Appendix 15, Table 36). This finding was not observed at 9 months, and consistent significant differences were not observed at any other time point or for any other outcome.
The change in PAM levels between time points was assessed graphically via bubble plots (Figure 8). This shows, by arm, how many participants moved between PAM levels at baseline and at 3 and 6 months, and also summarises missing data. We can see that, between baseline and 6 months, 26.5% (30/113) of stroke survivors in the New Start arm with complete data improved their PAM level, compared with only 20% (17/85) of usual care participants. This pattern was also seen between 3 and 6 months [New Start, 28.8% (32/111); usual care, 26.8% (22/82)] and between baseline and 3 months [New Start, 23.4% (29/124); usual care, 20.0% (20/100)]. Participants in the usual care arm were also more likely to have missing PAM data than those in the New Start arm at all time points. The mean PAM scores in both groups (see Appendix 15, Table 35) did not indicate change in line with the reported 4-point minimal clinically important difference. 141
FIGURE 8.
Change in PAM level: (a) between baseline and 3 months; (b) between 3 and 6 months; and (c) between baseline and 6 months. Note that, in these plots, the numbers on both axes represent the PAM categorical level at each specified time point, with 1 being the lowest, least active level (individuals tend to be passive and feel overwhelmed by managing their own health. They may not understand their role in the care process) and 4 being the highest, most active level (individuals have adopted many of the behaviours needed to support their health, but may not be able to maintain them in the face of life stressors). Zero represents a missing value at the time point labelled on the respective axis. The size of, and number in, each bubble represent the number of patients in each group. The bubbles above the diagonal lines show patient groups whose PAM levels have improved over time.
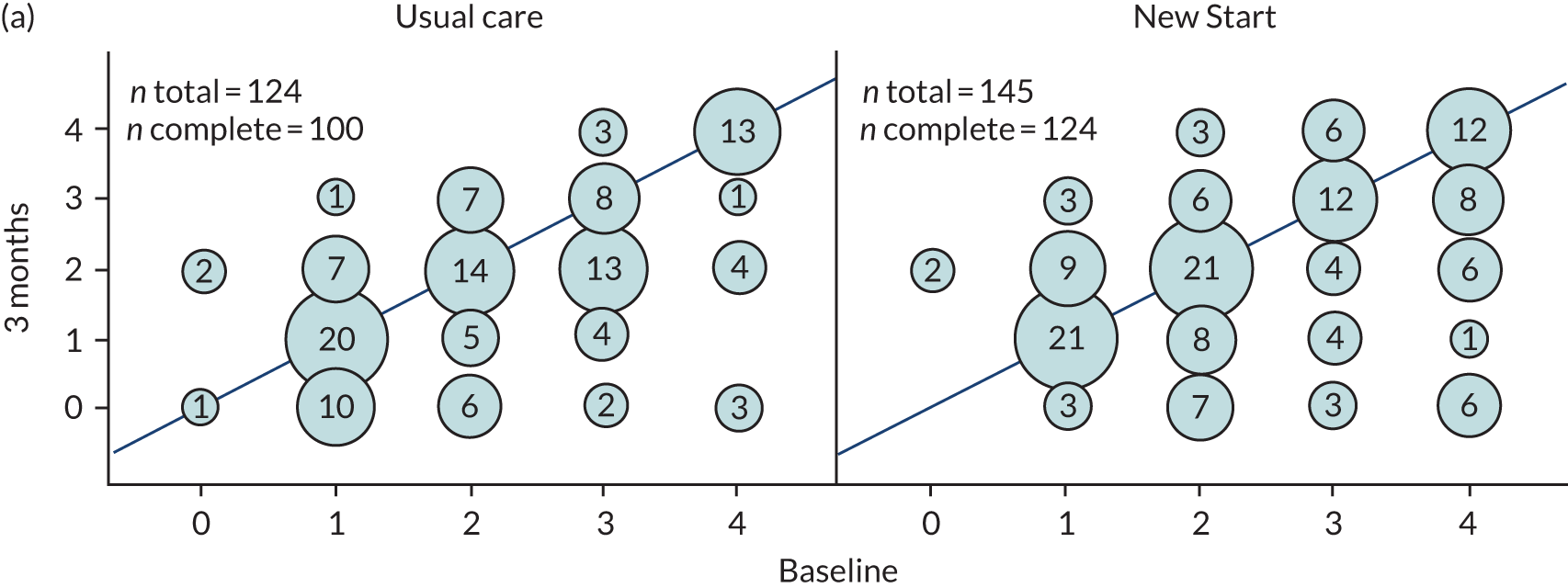
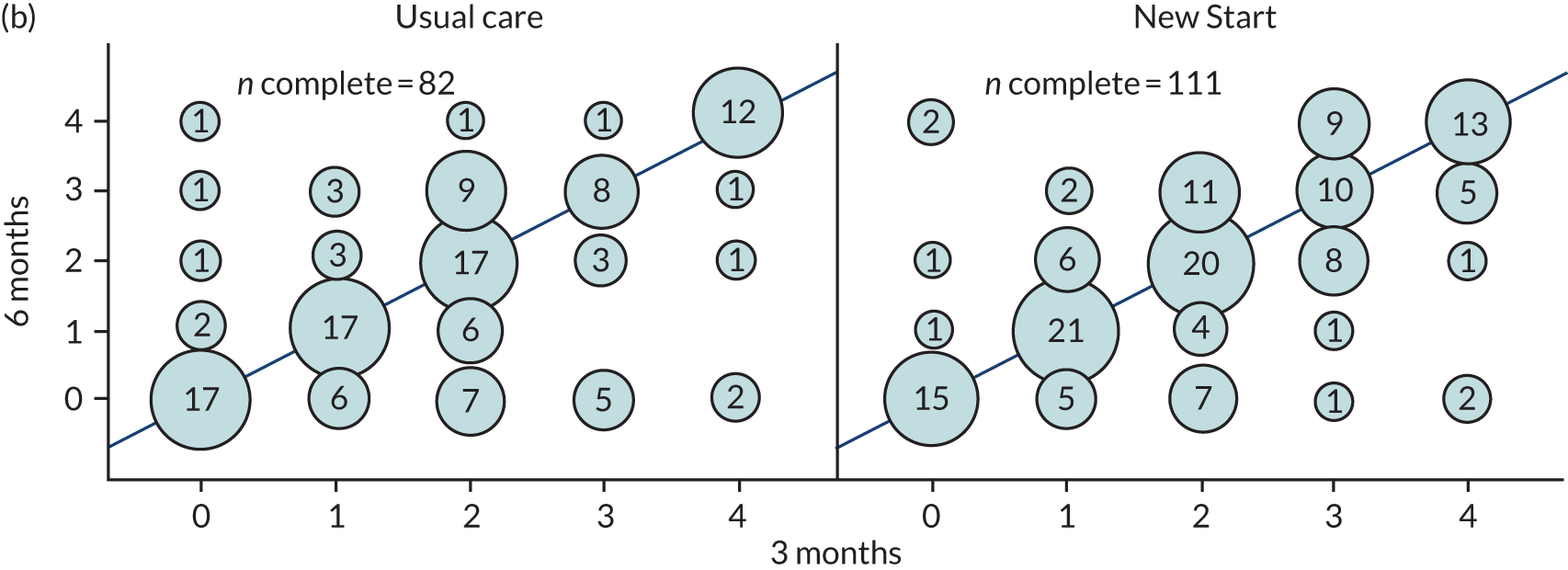
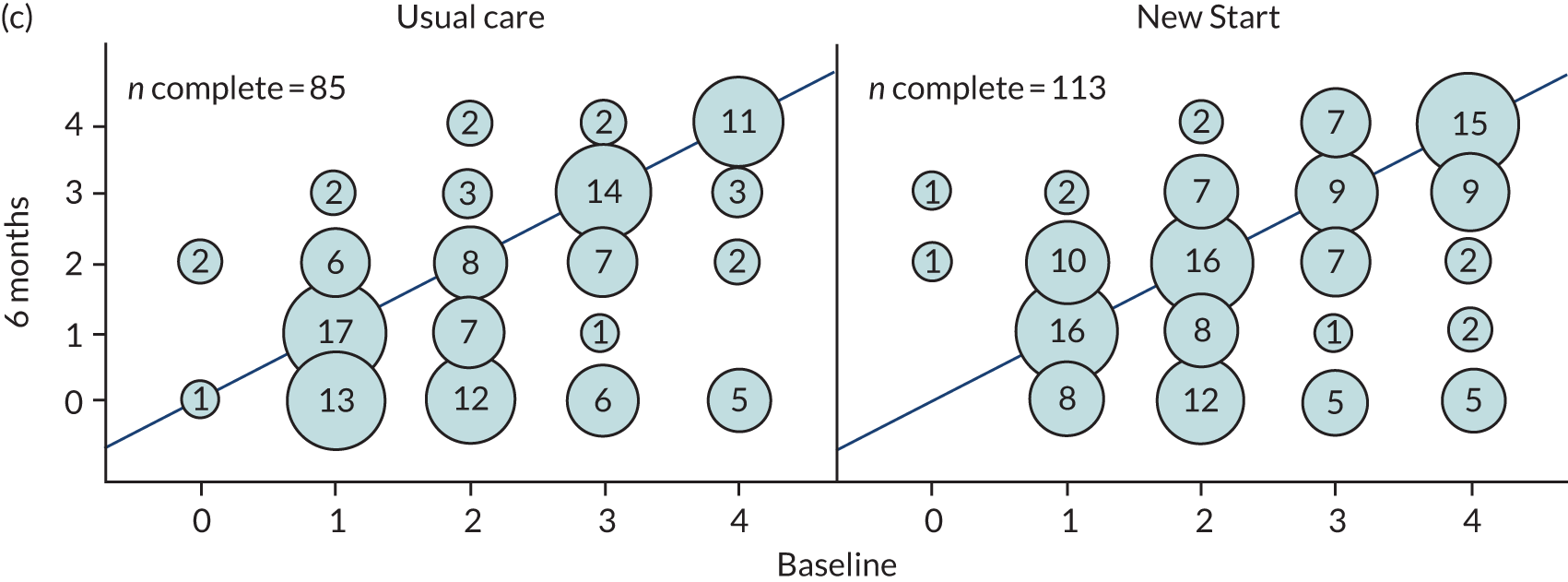
Carer outcomes are summarised in Appendix 15, Table 37. The CBS was used to measure carer burden, with a higher score indicating a higher level of burden. At baseline, carers in the usual care arm had higher average CBS scores (usual care, 48.7 points; New Start, 45.6 points), as was the case at 3 months (usual care, 48.7 points; New Start, 47.7 points), but this reversed at 6 months (usual care, 43.7 points; New Start, 46.8 points) and 9 months (usual care, 44.9 points; New Start, 48.2 points) (see Appendix 15, Table 37). An explanation for this change is the relatively small sample size (85 carers) and large numbers of missing data, especially in the usual care arm. It is also thought that more severely affected stroke survivors, and thus more burdened carers, are less likely to be followed up as the trial progresses.
Sample size estimation
To inform the sample size estimation for a definitive trial, information is required on the average cluster size and coefficient of variation, as well as an estimate of the ICC for the proposed outcomes.
The mean cluster size, as defined by the number of registered participants per site, was 29 [standard deviation (SD) 34.18] in the intervention arm (145 registered participants; five sites) and 24.8 (SD 12.64) in the usual care arm (124 registered participants; five sites). These correspond to a coefficient of variation in cluster size of 1.18 in the intervention arm and 0.51 in the usual care arm. The high level of variance observed in the intervention arm is due to the inclusion of one small site (site 2a, four participants) and one large site (site 3, 89 participants). If the small site is removed, as was done in the cluster-level significance tests, the mean cluster size in the intervention arm changes to 35.3 (SD 36.02) (141 registered participants; four sites), and the coefficient of variation of cluster sizes changes to 1.02.
Intracluster correlation coefficients were calculated for the WHODAS (simple and complex scoring) and the WEMWBS questionnaires. They were calculated by treating recruiting sites as clusters, and evaluating the ICC for each questionnaire score at the given time points. Site 2a was omitted from the calculations as it contained only four participants. Because of the small number of clusters and participants, it was difficult to produce reliable ICC estimates for the outcome measures. The range of ICC values produced was between 0.01 and 0.02. Results are provided in Appendix 15, Table 38; cases for which the ICC estimate is zero do not have an accompanying 95% CI. Estimates for the coefficient of within-cluster variation were also produced with 95% CIs, to also aid in the sample size estimation for a definitive trial.
Recruitment procedures
A summary of the findings on views about barriers to and facilitators of recruitment of participants by staff undertaking the recruitment is provided in Appendix 18. In general, all reported that the procedures, primarily recruitment by post, were implemented smoothly.
Safety
At baseline, a higher proportion of participants in the usual care arm than in the New Start arm had hospital inpatient stays during the previous 3 months (17.2% vs. 10.6%), with the stays being longer and more frequent (see Appendix 15, Table 39) for participants in the usual care arm. The proportions of hospitalisations in both arms decreased at follow-up, and were fairly balanced across arms at 3, 6 and 9 months.
The proportion of admissions to accident and emergency (A&E) at baseline was higher in the usual care arm (13.8% vs. 10.6%), but this changed at follow up, with a higher proportion of A&E admissions in the New Start arm at 3, 6 and 9 months (10.5% vs. 14.4%, 7.2% vs. 9.7%, 5.8% vs. 14.7% at 3, 6 and 9 months, respectively).
When stroke survivors were unable to self-report, hospitalisations and institutionalisations were reported via proxies (see Appendix 15, Table 40). Interpretation is limited because of small numbers.
Seven deaths were recorded in the population during the study period: four in the usual care arm (3.2%) and three in the New Start arm (2.1%) (see Appendix 15, Table 18).
Progression to a definitive trial
Progression was assessed separately based on three criteria: recruitment, follow-up, and intervention delivery and implementation. Guidelines for progression to a definitive trial are based on a traffic-light system of green (proceed to trial design), amber (review trial design and/or implementation, then proceed), red (stop and do not proceed) (see Table 4).
Recruitment
More than half of sites had recruitment periods of > 6 months, so, in addition to assessing the total number of stroke survivors recruited per site, a prorated figure is also evaluated against the progression criteria (see Appendix 15, Table 41).
The overall average number of recruited stroke survivors per site, prorated to a 6-month period, was 24.1, fulfilling the green requirements for this criterion (i.e. ≥ 20). Five of the 10 sites achieved green status, recruiting at least 20 patients when scaled to a 6-month period. Another four sites achieved amber status (≥ 10 patients); however, three of these fell within the ‘acceptable range’, recruiting at least 12 patients over a scaled 6-month period. One of the 10 recruiting sites was assessed as red in this criterion, as it failed to recruit an average of 10 patients over a 6-month period.
Follow-up
Follow-up is assessed via the number of questionnaire booklets received at 9 months post registration. Of the 269 patients in the trial, 216 booklets were returned at the 9-month time point, giving a follow-up rate of 80.3% and achieving green status for this criterion (see Appendix 15, Table 42). This includes all modes of administration, and both survivor- and proxy-completed booklets.
The follow-up rates are further split by treatment allocation, showing that follow-up rates were slightly higher in the New Start arm than in the usual care arm, with 84.1% of New Start participants returning the booklet, compared with 75.8% of usual care participants. Both groups achieved green status on this criterion, however, with follow-up rates of > 75%.
Intervention implementation and delivery
Intervention delivery is evaluated as the number of stroke survivors being offered at least one session of the intervention. When looking at data returned on the relevant case report forms, the proportion of stroke survivors offered the intervention varies significantly by site. Only three sites achieved the 75% proportion required for green status, with the overall proportion standing at 70%, placing it in the amber region (see Appendix 15, Table 43). However, there were issues with sites completing and returning these forms, and so these data are not believed to be complete or accurate.
Additional data were collected from the sites to see if more patients were offered the intervention than reported. When combining these data with those reported on forms, we see a much improved picture of intervention delivery, with all sites offering at least 75% of patients a session of the intervention, and with an overall proportion of 95.2%. This highlights a potential data collection burden on facilitators, which should be noted when planning a definitive trial.
In total, all five intervention sites had at least two facilitators deemed competent, delivered the New Start intervention and provided it to patients, securing green status for this criterion (see Appendix 15, Table 44). However, at some sites, there were concerns regarding the number of patients being offered, accepting and receiving the intervention.
Discussion
The feasibility cluster RCT of the New Start intervention has been successfully completed.
Summary of results
We successfully recruited 10 services, which recruited 269 participants. In the five services randomised to the intervention, facilitators implemented the intervention. No safety concerns were identified and return rates of at least 78% were achieved for the outcome assessments, with retention of 88.8% of participants for the study period.
Sites
We sought to offer the study to a wide range of stroke services; therefore, invitations to consider the study were sent to CCGs across three areas (the Midlands, the South East, and Yorkshire), and four NIHR CRNs (London North Thames, South London, North West Coast, and Eastern) were contacted explicitly. This process was time-consuming, and it was impossible to know whether or not the correspondence had reached the most appropriate person (or anyone). Although some CCGs expressed interest, the translation from interest to recruitment was low, primarily because the initial expression of interest was at a higher level, removed from the clinical setting. A number of sites in the CRNs expressed interest, but did not appreciate the requirement for clinical engagement in intervention delivery. The most successful approach was to sites who had engaged in our previous work. 23
We recruited 10 sites reflecting a wide geographical and cultural spread from across England and Wales. Detailed information was obtained from the sites during the site recruitment process through completion of site surveys and interviews. Of the 10 sites, nine provided 6-month reviews prior to the study. None of the sites reported that it provided an intervention with components similar to our own. The level of service provision up to the 6-month review varied considerably between the sites. This reflected the variety of service models identified in WS2 and provides confidence that the sites were a reasonable representation of current practice.
Information obtained informed the stratification procedures for site randomisation. Despite this, however, one site (site 3) had considerably higher recruitment levels than the other sites.
Design
The design was robust and rigorous. A number of steps were taken to reduce bias. A key feature of the design was a clear separation between staff undertaking the recruitment in each site and the clinical staff delivering the intervention. Recruiting teams were not informed of the randomisation result, to minimise selection bias during recruitment, and the research team tried to ensure that this blinding was maintained (e.g. in site visits, conduct of the process evaluation, guidance to clinical staff). To minimise treatment bias, New Start facilitators and usual care staff (clinical team) were not informed as to which of their patients were participating in the trial.
Methods for the identification of potential participants by these two groups (clinical teams for the review, and recruitment teams to identify participants for the trial) generated considerable debate. This included clarification of information governance procedures (that no name and address of a patient would be forwarded to staff undertaking recruitment without consent), gaining sufficient information to ensure that contact was not attempted with those people who had not survived their stroke and ensuring that people were within the geographical service area. Despite some technical difficulties and the time-consuming nature of the process, identification of participants was established and worked well at the majority of sites. Recruitment was perceived to have been supported by the close relationship between the recruiters and the research team and chief investigator, which included site visits and teleconferences.
Initial concern that there would be a mismatch between the stroke survivors offered the intervention and those invited into the trial was ill-founded.
Recruitment
Methods
Colleagues undertaking recruitment initially voiced concern at the feasibility of recruiting people predominately by post. However, the process progressed more smoothly than envisaged. No potential participants complained about being approached in this way. The research/service configurations were slightly different in each site. In one site, recruitment was undertaken by staff who were able to give potential participants ‘advance warning’ during their hospital stay that they would be contacting them in the future. However, this was not the case in the largest recruiting site (site 3).
Screening and baseline data collection was kept deliberately minimal and mirrored the information provided for the SSNAP (NIHSS and mRS scores). However, for some sites, staff from the research directorate did not have access (for appropriate information governance reasons) to the SSNAP record; therefore, these data are missing. For similar reasons, the recruiting staff were not always aware of any communication and cognition difficulties the stroke survivor may have, or the presence or absence of a carer. Postal recruitment influenced the entry criteria for the study. Our preference was to exclude stroke survivors in receipt of palliative care; however, we concluded that there was no appropriate way to frame that ‘qualifying’ question in a postal invitation.
Recruiting participants to longer-term stroke studies has previously been challenging, as there is no central register of stroke survivors and, once provision of services is ended, which might be soon after stroke for some, there are limited opportunities to identify and engage with this population. We believe that this study is one of the first to successfully recruit a cohort of post-stroke survivors by post. Colleagues in the recruitment teams found the process smooth and resource-efficient.
Rates
Participants were recruited over 8 months, which was shorter than envisaged in the original grant application, with considerable variation between the sites. One site recruited a large number of participants; however, it was also a large service with a higher throughput of stroke survivors. In one rural site, the throughput was much smaller; consequently, the rate of recruitment was lower. Recruitment remained low at one urban site (n = 4), despite site visits and involvement of the CRN. In this geographical area, one community service received referrals from three acute hospitals. The community service (site 2a) received referrals as usual during the study, providing the intervention to 85 individuals, and the process evaluation reported that the New Start intervention was delivered with a relatively high level of fidelity. Although one of the recruitment staff had a period of absence towards the end of the recruitment period, we were largely unable to identify why the clinical services were not able to identify stroke survivors and invite them to participate in the study.
The recruiters themselves suggested a number of different reasons for variability in uptake, including socioeconomic factors and the context provided by existing stroke service provision. Capacity of the recruiting staff negatively affected recruitment in only one site, where a large number of research projects (not in stroke) were being undertaken, thereby reducing the resources available for this study.
Comparison with Sentinel Stroke National Audit Programme data
We found it problematic to compare the site data with information provided on the SSNAP, as patients may be double-counted (e.g. on discharge from an acute trust, then on discharge from a community trust). Tracking patient flow was not as straightforward as we had thought. Going forward, following the planned upgrade of the SSNAP, this might be easier.
Characteristics of participants
The majority of the patients had had no further education since leaving school (54%), which is similar to previous study cohorts. 45 Retention of participants in the study and completion of outcome measures met the progression criteria.
Take-up of the intervention
The detailed data collection undertaken in this study provides a unique data set to inform the longer-term care of people after stroke. These data (see Appendix 17) provide insight into the different methods the clinical teams used to contact stroke survivors (telephone/letter) and different approaches (opt in/opt out). Not surprisingly, procedures in which stroke survivors had to actively opt out of a visit demonstrated the greatest take-up. The level of take-up in some sites was surprising (< 10% in one site). However, when considering the care of people after stroke, and set in the context of many feeling abandoned, any contact, such as a telephone call offering them a visit from a health professional, could be considered an intervention of sorts. It is interesting to note that participants choosing to not receive the New Start intervention review had higher levels of higher education (New Start not received 49.2% vs. New Start received 39.5%) and lower reported levels of unmet needs than those receiving intervention.
As a cluster RCT, the intervention (or usual care) was provided to all stroke survivors in receipt of the service. This enabled us to gain comprehensive (anonymised) data on intervention delivery to explore whether or not trial participants received treatment that was different from that received by stroke survivors not included in the trial. The detailed process evaluation (see Workstream 5: process evaluation) has highlighted a number of challenges with the delivery of the intervention and is discussed in detail in the next chapter.
Workstream 5: process evaluation
Aims and objectives
The aim of the process evaluation was to gain an understanding of how New Start was implemented and received by stroke survivors in a range of settings, to inform the optimisation of its future design and evaluation. The objectives were to:
-
assess implementation fidelity
-
explore and clarify causal assumptions regarding implementation
-
investigate the contextual factors associated with variations in intermediate outcomes between sites
-
explore the views, perceptions and acceptability of the intervention to facilitators, stroke survivors and carers
-
identify barriers to and facilitators of trial recruitment
-
test and refine methods of data collection and interrogation in preparation for a process evaluation alongside a future effectiveness trial.
Design/methods
Full details of the methods are available in the published protocol. 142
We adopted a mixed-methods approach to data collection. Non-participant observation of facilitator training, intervention delivery and local organisational processes (e.g. facilitator work patterns and their interactions with colleagues in the wider stroke services) was undertaken. Semistructured interviews took place with stroke survivors, New Start facilitators and relevant site staff (managers, administrators and trial recruiters). Documentation of intervention activity, facilitator activity and usual care was used to capture specific information about New Start activities. Reports by facilitators were also used. First, the SEPSS119 instrument was administered prior to training, twice following training and again at the end of the trial. Second, NPT (http://normalizationprocess.org/; accessed 8 August 2020) toolkits were completed by facilitators during the early, middle and end stages of implementation. Facilitators were also asked to complete monthly self-reflection reports supported by a reflective framework. Additional data accessed by process evaluators included structured site surveys conducted throughout the feasibility trial containing details of current service provision. Numerical data from activity records, the SEPSS and a visual inspection of NPT toolkit reports were entered into a database. Recorded interviews were transcribed verbatim, anonymised and managed alongside anonymised observational field notes and additional data listed above using the qualitative data analysis tool NVivo (version 10.0/11.00) (QSR International, Warrington, UK).
Analysis
Familiarisation with the qualitative data was followed by data reduction, during which the researchers engaged in transforming the data to identify patterns and themes between sets of data in order to make sense of them and generate descriptions and explanations relevant to the research objectives. 143 To manage the data, a coding framework was created, based on emerging themes and informed by the research questions. Standard approaches to demonstrating trustworthiness and quality in qualitative research were used including clearly documenting the research process, transparently developing interview topic guides in the light of ongoing analysis, documenting contextual features in which research was carried out, exploring deviant cases and discussing emerging findings among the process evaluation team. Following initial analysis, the consolidated framework for implementation research110 was used as a sensitising theoretical framework to place the findings in a wider context.
Results
Ten sites throughout England and Wales were recruited to the trial, of which five were randomised to deliver the intervention. Fifteen clinicians received training in New Start and were assessed as being competent to deliver it. Non-participant observation of facilitator training was undertaken during the initial 2-day event and then at both follow-up days. Observation of organisational processes took place during 10 visits to intervention sites. During the delivery phase of the trial, 377 stroke survivors received New Start across active sites (as a cluster trial, all stroke survivors seen received the intervention, regardless of their participation in the trial). Fourteen facilitators were observed delivering New Start on 31 occasions (see Appendix 19, Figure 14). At one site, two facilitators were observed a second time following a 4-month interval to assess whether or not their delivery changed after a visit by a trial implementation team member. Interviews were undertaken with 15 facilitators, 26 stroke survivors (see Appendix 19, Figure 15), three managers involved in implementing New Start, three other local staff supporting New Start delivery at three intervention sites, and 22 recruiters across all 10 sites (see Appendix 19, Tables 50 and 51, and Appendix 18, Table 45, for the demographic characteristics of participants).
Key findings
This section contains key process evaluation findings. See Appendix 19 for more detailed findings. See Appendix 18 for findings related to the trial recruiter interviews.
Implementation fidelity
-
Recruitment of sites and delivery of the intervention. Site recruitment took place mostly as intended; however, implementation of the intervention was most successful at sites where senior service managers were involved in agreeing to participate in the trial. The intervention was delivered to sites as intended.
-
Adoption of intervention by sites. Sites did not always adopt the intervention as intended; ideally, staff should have been ring-fenced to support implementation of the reviews, but this was problematic in busy clinical settings. Organisational support or support from colleagues for facilitators to deliver New Start was not always made available, and adaptations were made to intervention implementation. One site recruited facilitators specifically to deliver New Start (site 1), which was associated with a high degree of fidelity of intervention delivery.
-
Delivery of intervention training and ongoing support. Facilitators were trained as intended, although a more prescriptive approach to New Start delivery may have helped facilitators implement the intervention more easily. Facilitators were offered ongoing support from the LoTS2Care team during the delivery phase. Difficulties were experienced by facilitators at some sites in accessing the online support available (due to local trust IT policies); however, facilitators did not take up opportunities for other accessible support from the LoTS2Care team, such as an NHS e-mail messaging group or teleconference.
-
Adoption of the intervention by facilitators. Facilitators were encouraged to develop skills and confidence by practising New Start delivery during a 3-month period following the initial training event before they underwent competency assessment and the trial went ‘live’ (in total, 116 stroke survivors received a visit during this practice period; see Appendix 19, Table 46). Although facilitators demonstrated detailed knowledge of the principles underlying New Start, some struggled to deliver aspects of the intervention as intended. Facilitators could find it hard to adopt a collaborative approach to problem-solving and goal-setting. This was associated with difficulties in facilitating active engagement in these processes on the part of the stroke survivor. Facilitators sometimes found the flexible design confusing, completing records of their activity was considered onerous and they sometimes did not have confidence in all of the elements of the intervention or the potential benefit of the intervention for most of the population. Nevertheless, activity records and observations suggest that, at times, facilitators did deliver New Start as intended. This was noted at two sites where facilitators reported previous experience of goal-setting/self-management, support from their organisation and high levels of self-efficacy and buy-in.
-
Although most facilitators expressed positive attitudes to completing reflections, half reported lacking sufficient time to do this.
-
Identification of stroke survivors and invitation to receive New Start. Stroke survivors were generally identified as intended. However, data presented in Appendix 17 and in Appendix 19, Table 47, demonstrate the variation in take-up of the invitation to the review.
Dose
Appendix 20 presents a summary of New Start activity data, as recorded by facilitators. In most cases, New Start was not delivered entirely as intended:
-
Although 69.7% (377/541, where 541 is based on the number of stroke survivors with completed New Start activity records) of stroke survivors approached during the delivery phase received New Start, 75% (n = 284/377) of these did not receive the minimum dose, defined a priori as an initial visit plus follow-up.
-
New Start was often introduced in such a manner that the underlying purposes and expectations were unclear.
-
Facilitators only briefly explored social networks, rather than rigorously mapping them to identify supporters for future action-planning; this potentially narrowed problem-solving options. There is little evidence that facilitators were able to help stroke survivors develop social networks or use them to address ongoing needs.
-
Although observational data suggest that unmet needs were identified on many occasions, activity records indicate that collaborative goal-setting took place during 5% (n = 20) of cases.
Nevertheless, it should be noted that several facilitators did successfully deliver aspects of the intervention, such as goal-setting/action-planning, and one facilitator delivered the intervention with a high level of fidelity.
Stroke survivor response
Stroke survivors could be unclear about aspects of New Start, that is they assumed that they were receiving a standard follow-up appointment, rather than an intervention containing elements of self-management. Consequently, the ongoing needs they identified were generally related to their physical health. Although they could struggle to engage in problem-solving and goal-setting, and a minority changed their activities as a result, most stroke survivors reported benefiting from the intervention because they felt supported and understood. Occasionally, they appeared to fully understand the purposes of the New Start intervention and engaged in all aspects of the intervention as intended.
Exploration and clarification of causal assumptions regarding implementation
The findings mostly support the theory of change articulated by the outcomes chain (see Appendix 12), although some of the relationships between intermediate outcomes were not as expected; for example, the lack of established referral pathways at one of the sites did not prevent facilitators delivering New Start with a high level of fidelity.
Facilitator, stroke survivor and carer views of New Start
-
More than half the facilitators and several stroke survivors reported that 6 months was too late to introduce New Start, with several advocating the introduction of self-management earlier. Facilitators reported that many stroke survivors had either recovered by this time or adapted their lifestyle in their own fashion in response to their ongoing disabilities.
-
One-third of facilitators across all sites reported that too many New Start materials were provided, the content of these worksheets was repetitive and it was difficult to choose which worksheet to use. Facilitators across all sites reported that recording details of the delivered intervention and completing documentation relating to the trial were onerous.
-
Two facilitators favoured delivering New Start in clinic, on the basis that distractions are less likely to occur and stroke survivors investing time and effort in attending were likely to value the intervention more. In contrast, two-thirds of facilitators favoured delivery at a recipient’s home because it provided context and because stroke survivors were spared travelling to clinic and were more likely to feel relaxed. When interviewed, stroke survivors with a high degree of recovery and an absence of mobility issues were generally happy to attend clinic, whereas those with physical impairment and/or a lack of transport valued the opportunity to be seen at home.
-
Most stroke survivors and almost all facilitators reported that New Start provided the opportunity to talk about their experience of stroke and reflect. Many stroke survivors reported that they valued the opportunity to talk about their experiences and felt supported and understood. Several reported feeling reassured that their experiences had been normal and that they had taken an appropriate approach to recovery.
-
Although almost all facilitators reported that self-management had the potential to increase the QoL of stroke survivors, several reported that they felt that this had occurred rarely and that this approach was suitable for a minority of stroke survivors. When interviewed, stroke survivors rarely mentioned self-management explicitly, although they occasionally reported increased confidence and improved motivation to engage in planned actions and other tasks.
Testing/refining methods of data collection
Observations of New Start delivery and interviews with stroke survivors, facilitators and other staff were successfully undertaken. Some facilitators appeared to modify their delivery when being observed. In future, more observations of delivery may be useful in lessening the degree to which this takes place and in providing a more accurate view of facilitator practice. During stroke survivor interviews, difficulties with recall were noted; consequently, facilitator photographs were used to prompt memories, which were found to be useful. Collection of facilitator self-reported data (SEPSS questionnaires and NPT toolkit reports) was generally successful; however, there were a few missing data because questionnaires were either incomplete or not returned (see Appendix 19, Tables 48 and 49). Despite the occasional difficulties mentioned, methods of data collection and analysis were effective in meeting the study objectives.
Recommendations
Recommendations for intervention development
-
Simplify the intervention: reduce materials and clearly define the intervention’s intended purpose to avoid confusion. Reconsider presentation of the priming tool, which was intended to prompt stroke survivors and facilitators to consider the broad range of unmet needs.
-
Consider offering/introducing elements of the intervention such as self-management earlier in the stroke care pathway.
Recommendations for future implementation
-
Consider the degree to which sites support implementation at an organisational level.
-
Clarify the key components of the intervention, ensuring that training and implementation are focused on delivering those components.
-
Consider professional background, and experience of self-management strategies, during selection of facilitators, recognising that training might need to be tailored appropriately.
-
Think about ways to bolster survivor involvement, interest or confidence.
-
Consider provision of strategies to support patients’ self-management earlier in the stroke pathway.
-
Introduce intervention clearly to stroke survivors so they understand what is expected of them. It is a difficult balance, encouraging the patient and their families to take ownership of their care without increasing anxiety.
Recommendations for a future process evaluation
-
Undertake more observations of intervention delivery.
-
Interview stroke survivors closer to receipt of intervention.
-
Reduce burden of self-report for facilitators (reflections can be useful for both facilitators and process evaluators, but avoid enforcing frequency).
Conclusions
This process evaluation found that, although New Start was delivered across a range of sites, it was often not implemented entirely as intended. A range of factors explaining why success in implementing New Start was limited have been identified and data collection methods have been tested and refined. This increased understanding will assist in the further development and evaluation of New Start and other similar interventions.
Workstream 5: economic evaluation
Background
The exploratory economic evaluation was conducted in two parts: a within-trial economic evaluation was conducted to evaluate the costs and benefits associated with the New Start intervention that occurred during the trial, and an economic model was developed to analyse future costs and benefits beyond the trial time horizon. The analysis was guided by the recommendations of the NICE methods guide. 144
Within-trial analysis
Methods
For full description of the within-trial analysis methods, see Appendix 21.
The exploratory within-trial economic evaluation evaluated the effect of the New Start intervention on the QoL and health-care costs of stroke survivors in the UK. Costs, estimated from the societal perspective (direct and indirect), outcomes and quality-adjusted life-years (QALYs) of stroke survivors at centres randomised to the New Start intervention versus usual care were compared over the 9-month time horizon of the trial. As the time frame was < 1 year, discounting of the costs and benefits was not required.
Measurement of outcomes, resource use and costs
Quality-adjusted life-years were calculated based on patient and carer health state utility values obtained from the EQ-5D-5L questionnaire at baseline and at 3, 6 and 9 months. 145
Information on all health-care resource use during the trial was collected using patient- and carer-completed questionnaires at 3, 6 and 9 months, and converted to costs using appropriate UK unit costs146,147 (see Appendix 21, Table 52). The cost of the intervention was estimated as the cost of the 6-month review meeting along with any associated follow-ups. Total costs for each patient were calculated as the sum of costs assigned from hospital, community health and social services and the intervention cost, along with out-of-pocket costs incurred by patients and their informal carers.
Adjusting for baseline imbalance
A multiple regression analysis was used to estimate differential mean QALYs and predict adjusted QALYs controlling for utility at baseline.
Missing data
When there were missing QoL or cost follow-up data, multiple imputation methods were used to generate estimates of missing values based on the distribution of observed data, as per recommended best practices for economic evaluation alongside clinical trials. 148
Cost-effectiveness analysis
The primary analysis consisted of a cost–utility analysis over the 9-month trial period and included adjustment for baseline variables and imputation of missing data. The incremental cost per QALY gained from the New Start intervention compared with usual care was calculated, producing an incremental cost-effectiveness ratio (ICER). 149
Sensitivity analyses were conducted to explore the impact of assumptions made in the primary analysis and alternative perspectives for analyses.
The level of sampling uncertainty around the ICER was explored using a non-parametric bootstrap to generate 10,000 estimates of incremental costs and benefits. This was used to illustrate the probability that the New Start intervention is cost-effective at a range of threshold values.
Results
For a full description of the results, see Appendix 21.
Resource use and costs
Resource use throughout the trial, broken down by item and associated costs, is presented in Appendix 21, Tables 53 and 54. Use of health-care services was higher in the New Start arm, but use of private health-care services (paid for out of pocket) was higher in the usual care arm. The multiple regression analysis indicated that the difference in observed costs between groups was not statistically significant (p > 0.05, 95% CI –899.646 to 3211.452).
Quality of life
Patient and carer EQ-5D-5L and ICECAP-A scores are presented in Appendix 21, Tables 55 and 56, respectively. There was little difference in EQ-5D-5L scores over the trial period in either arm, and the multiple regression analysis indicated that there was no significant difference in total QALYs gained between groups (p > 0.05, 95% CI –0.043 to 0.014).
Missing data
Complete and missing resource use and EQ-5D-5L data are presented in Appendix 21, Tables 57 and 58, respectively. EQ-5D-5L scores were complete at all follow-up points for 195 (72%) patients and 35 (41%) carers. Resource use questionnaires were complete at all follow-up points for 180 (67%) patients.
Cost-effectiveness results
Cost-effectiveness results are presented in Table 6. This indicates that New Start would not be the favourable option as the treatment for stroke survivors, as the QALY gain is lower in the New Start arm. However, if a whole health-care system approach is taken, the New Start intervention has the potential to be cost-effective in a resource allocation sense, given that New Start is less costly but not much less effective (small difference in QALYs), that is the money saved has the potential to generate more ‘health’ elsewhere in the system. 149 However, these results should be viewed with caution, as this is an exploratory analysis of feasibility data only.
| Treatment allocation | Cost (£), mean (SD) | Incremental cost (£) | QALYs, mean (SD) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Usual care | 4846.89 (3335.88) | 0.504 (0.011) | |||
| New Start | 4056.86 (2038.72) | –790.02 | 0.502 (0.015) | –0.002 | 395,010 |
Bootstrapped estimates of the incremental costs and effects indicated that the New Start intervention is unlikely to be a cost-effective use of resources (see Appendix 21, Figure 17). At a cost-effectiveness threshold of £20,000 per QALY gained, the New Start intervention showed a 48% probability of being cost-effective (see Appendix 21, Figure 18).
The results of the sensitivity analyses are presented in Appendix 21, Table 59. These results showed a great deal of variation in the cost-effectiveness estimates for each scenario explored, demonstrating substantial uncertainty around the results.
Health economic model
Methods
A cohort Markov decision model was developed to analyse future costs and benefits of New Start compared with usual care beyond the trial time horizon and to identify the areas of greatest uncertainty to inform future research.
The outcome measure for the model was the QALY. The analysis was conducted from a societal perspective to analyse the costs and benefits of New Start, compared with usual care, over a lifetime horizon. Costs and outcomes were discounted to present value using a discount rate of 3.5% and ICERs were estimated.
Model
The model (Figure 9) is based on QoL rather than clinical events. This approach was deemed appropriate for modelling the impact of the New Start intervention, as the outcomes related directly to changes in QoL. It does, however, create an unusual co-dependency between QoL and health states (because QoL defines both health state and QALYs gained). The model was populated using data from the trial to inform transition probabilities, health-state costs and utilities as treatment costs associated with New Start and usual care. For model parameters that could not be collected within the trial, including long-term mortality following stroke, recommended best practices for identifying and synthesising evidence from the literature were followed. Treatment costs for each arm were taken from the LoTS2Care trial data and represent the average cost in each arm for health-care consultations and visits associated with New Start or the usual follow-up care 6 months post stroke. Model parameters are presented in Appendix 21, Table 60.
FIGURE 9.
Lifetime decision-analytic model to compare New Start with usual care following stroke.

Validation, sensitivity analysis and value-of-information analysis
Model validation was conducted with reference to the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) best-practice guide for model transparency and validation. 150 As in the within-trial analysis, the incremental cost per QALY gained was estimated and compared with a cost-effectiveness threshold of £20,000 per QALY. Deterministic sensitivity analysis was used to explore possible alternative scenarios to those used in the base-case analysis. A value-of-information analysis was conducted to explore the costs associated with the uncertainty in the results, and the expected value of perfect information (EVPI), representing an upper bound on the value of conducting further research, was estimated.
A full description of the methods, including the parameter values, and the results is presented in Appendix 21.
Results
The cost-effectiveness results from the lifetime analysis are presented in Table 7 for the base case and for each scenario explored in the sensitivity analyses. Appendix 21, Figures 19 and 20, show the cost-effectiveness plane and cost-effectiveness acceptability curve (CEAC) produced from the lifetime analysis.
| Treatment allocation | Lifetime cost (£) | Incremental cost (£) | Lifetime QALYs | Incremental QALY | ICER (£/QALY) |
|---|---|---|---|---|---|
| Base case | |||||
| Usual care | 131,038 | 17.354 | Usual care dominates | ||
| New Start | 131,082 | 44 | 17.354 | 0.000 | |
| Alternative estimation of treatment costs | |||||
| Usual care | 131,042 | 17.354 | Usual care dominates | ||
| New Start | 131,149 | 107 | 17.354 | 0.000 | |
| No assessment in usual care arm (treatment cost = 0) | |||||
| Usual care | 131,022 | 17.354 | Usual care dominates | ||
| New Start | 131,082 | 60 | 17.354 | 0.000 | |
| Minimally important difference = 0.05 (0.1 in base case) | |||||
| Usual care | 135,792 | 17.236 | |||
| New Start | 135,636 | –155 | 17.247 | 0.011 | New Start dominates |
The population EVPI, at the cost-effectiveness threshold value of £20,000 per QALY gained, is > £110M. The population EVPI for other values of the cost-effectiveness threshold is presented in Appendix 21, Figure 21. The greatest uncertainty was seen around the number of patients whose improvement in QoL is not maintained (the transition from ‘recovered’ to ‘post-stroke relapse’ in the model) (see Appendix 21, Figure 22).
Mediators and moderators
Potential mediators and moderators for consideration in future analyses were identified using the results from the work undertaken in the development of the intervention (WSs 1 and 2) (see Appendix 21, Tables 61 and 62). The results of the exploratory moderated regression analysis for the selected exemplars are summarised in Appendix 21, Table 63 (see also Appendix 21, Figures 23–26).
Discussion
Principal findings
The primary within-trial cost-effectiveness analysis and long-term evaluation of lifetime costs and benefits in the economic model were both exploratory. The within-trial analyses indicated that, although the New Start intervention may be a cost-effective use of resources, the results were not robust to alternative assumptions explored in sensitivity analyses. There was considerable variation in the cost-effectiveness estimates, with each variation in assumptions indicating substantial uncertainty around the results.
Fewer QALYs were gained in the New Start arm than in the usual care arm, but the mean difference was small in real terms and the difference was not statistically significant. Furthermore, total costs were lower in the New Start arm than in the usual care arm, although this difference was not statistically significant. The difference in costs was particularly driven by lower costs incurred by stroke survivors and their carers for private health care paid for out of pocket. It is also of note that the health-care resource use covered by the NHS was greater in the New Start arm. This could demonstrate an unmet need for health care in usual care practices, which is being addressed with the New Start intervention. Therefore, by increasing NHS provision for stroke survivors, New Start may reduce private expenditure for health care, which could have equity benefits.
The results obtained from the longer-term analysis of costs and benefits using the decision-analytic model indicated that New Start was unlikely to be cost-effective compared with usual care. As in the within-trial analysis, there was uncertainty in the results, which was driven by the small differences between the treatment options in terms of both costs and QALYs. The EVPI indicated that further research conducted at an expected cost of < £110M would be required to reduce the uncertainty in the results. The EVPI indicated that, in the model, the greatest uncertainty was around the number of patients whose improvement in QoL was not maintained, namely in the transition from ‘recovered’ to ‘post-stroke relapse’.
Strengths and weaknesses of the economic analysis
A strength of this analysis lies in the randomised controlled design of the trial, which enabled the collection of good-quality data that were used to explore the feasibility of a full trial and the analysis to be conducted. This has shown that a within-trial analysis and an analysis of longer-term outcomes would be feasible, but has also highlighted areas where changes in the data collected or the way that they are collected could allow for more robust evidence collection and analysis in a full trial.
One limitation of the analysis is the lack of available data on stroke recurrence and long-term survival data following stroke. This meant that assumptions had to be made to enable long-term modelling of costs and outcomes. Although the modelling was still possible, it could perhaps be more robust if it was possible to obtain good-quality data to inform these aspects.
Meaning of the feasibility trial
This analysis has shown that a within-trial cost-effectiveness analysis would be feasible as part of a definitive trial. However, it is noted that, for both QoL and resource use data, compliance decreased over the duration of the feasibility trial. Compliance from carers in QoL questionnaires was particularly low. Consequently, if a full trial was conducted, ways to maintain compliance should be explored, for example altering the frequency to address questionnaire fatigue and exploring ways to ensure that carers are engaged.
In addition, it has been shown to be feasible to conduct a long-term analysis of costs and outcomes using a decision-model framework. However, it may be possible to make improvements to the structure of the model if it were possible to obtain or collect certain data such as stroke recurrence and the impact of stroke recurrence on QoL, for example in a definitive trial.
Unanswered questions and further research
This analysis has provided preliminary estimates of cost-effectiveness; however, the primary purpose was to assess the feasibility of conducting such analyses as part of a definitive trial. This feasibility trial was not powered to provide definitive answers; consequently, a full trial would be required to reduce the uncertainty around the cost-effectiveness estimates.
The results from the within-trial analysis indicated that there may be a decrease in out-of-pocket costs for private health care and an increase in use of NHS services with the New Start intervention. This could have equity implications, which could be explored further in a definitive trial.
The results from the EVPI indicated considerable uncertainty around the number of patients whose improvement in QoL was not maintained (the transition from ‘recovered’ to ‘post-stroke relapse’). This is likely to be a valuable area for future research, aiming to reduce the uncertainty around the cost-effectiveness of the New Start intervention, and could be addressed with additional long-term follow-up of patients.
Discussion
We have completed a large and complex programme of research focused on improving longer-term outcomes for people after stroke. Through literature reviews and qualitative and quantitative exploration, a complex intervention with aligned materials and training was developed.
This intervention has been feasibility tested in 10 sites geographically spread across England and Wales, with an embedded process evaluation and parallel health economic analysis.
The work began by exploring the perspective of stroke survivors and their carers about their experiences in the longer term after stroke. A comprehensive understanding of unmet needs, the change over time, how these needs can be addressed and the factors that may facilitate or hinder this process was successfully developed. Although stroke survivors and their carers faced barriers to addressing their needs and rarely spoke of specific behaviours, the findings indicate that stroke survivors and their carers play an active role in managing using both practical and mental coping strategies. The work highlighted the importance of support networks as a need and as one of the key facilitators for addressing other identified needs. As in previous studies, the need for information was highlighted. There was a general sense of negativity attached to the information provided, as concerns were raised about the timing and the amount. The need for longer-term interventions was supported, as unmet needs were identified even 3–4 years post stroke. Through additional literature review and investigation of the Stroke Association Helpline, 23 unmet needs were identified and prioritised by our Reference and Consumer Groups to underpin the development work.
The national survey and detailed focus group work with colleagues in stroke services evidenced the wide variety, disparity and challenges faced in the provision of community-based post-stroke services.
The concurrent comprehensive review of the literature did not identify any interventions that successfully addressed unmet needs or enhanced participation for people after stroke. The graphic presentation of the reported research demonstrated that there has been considerable focus on physical rehabilitation and less focus on psychosocial aspects of stroke, which were identified as being important by CRAG.
The development work captured the views and experiences of stroke survivors and their carers, and health and social care professionals. The literature reviews and national survey highlighted the evidence and service gaps. All informed intervention development. The provisionally developed intervention and training were further refined during implementation in WS4, and we were successful in undertaking a feasibility trial in WS5, which was enhanced by a considerably more detailed process evaluation than originally planned. Some specific challenges, strengths and weaknesses of the programme are drawn out below.
Intervention development
The intervention was developed using a bottom-up approach, allowing the needs of the stroke survivors and the existing evidence base to define the care strategy, rather than starting with a predetermined idea of the intervention. Members of the PMG and our existing CRAG were actively involved in the intervention development process. We also convened a RG, which included a wide spectrum of stakeholders, including a stroke survivor, health and social care workers, a commissioner, and colleagues from third-sector organisations (Carers’ Resource and Stroke Association).
The original ambition was to develop the intervention using components of the early WSs and the framework of intervention mapping. Intervention mapping103,104 consists of procedural steps: needs assessment; creating behavioural programme outcomes, developing behavioural and outcomes, performance objectives and change objectives (desired outcomes in terms of health, behaviour, sub-behaviour and behavioural influence, respectively); developing theory-based intervention methods and practical applications; devising the intervention with associated plans for implementation; and, as a final step, evaluation.
We undertook work to inform this process. Comprehensive and detailed identification of unmet needs was undertaken. The identified unmet needs were then prioritised by stakeholder groups to inform the intervention development. In keeping with the intervention mapping process, behavioural outcomes were identified for the prioritised needs, performance objectives (what participants and agents in the environment need to do to achieve the behavioural outcome) were identified, and then matrices of change objectives, identifying specific factors likely to influence achievement of these performance objectives in a range of domains, were developed.
However, the process was complex (and complicated) as we were not able to identify a small number of specific behaviours that long-term stroke survivors’ outcomes of QoL and participation could be focused on. Although the needs identified in the qualitative interviews in WS1 were written broadly in behavioural terms, for example managing emotions, it was difficult to elicit more specific behaviours that would have supported the intervention mapping process. The output from this careful, time-consuming work was hundreds of performance objectives and thousands of change objectives, too many to process through all the stages of intervention mapping. It was also acknowledged that the relevance of these objectives to overcoming the needs of survivors or carers would be very dependent on both the particular needs and circumstances of the survivors and carers. This would make the task of selecting and configuring any resultant components to each survivor/carer very difficult.
Intervention mapping was therefore problematic to implement in the context of this research programme, in which a heterogeneous population reported a number of unmet needs, with a range of barriers and enablers, and no widely applicable behaviours that were specific to these needs. In addition, although structured and transparent, the process was difficult to conceptualise to the RG. However, the performance objectives for many of the behavioural objectives were specific examples of a problem-solving process, and this informed our amended approach.
With the PMG and RG, who were central to the intervention development process, we therefore amended our approach to intervention development. Utilising the outputs from the earlier WSs, the crucial input of the RG and continuing the focus on the prioritised unmet needs, we developed the intervention plan using problem-structuring and shared knowledge creation. This provisional intervention was compatible with the interim outputs from our intervention mapping work, when it was feasible to check.
Stakeholder engagement
Patient and public involvement was (and is) central to our work and was crucial to this programme. The long-established CRAG helped to develop the original research questions and the grant application. It has remained engaged throughout (meeting quarterly) and provided considerable input into all WSs, such as reviewing interview guides and summaries of literature reviews, prioritising unmet needs, informing choice of outcome measures and aiding interpretation of the results. Mick Speed (stroke survivor) is a valued colleague who provides advice and guidance, and facilitates access to the local Stroke Group. All new members of staff attend a CRAG meeting as routine to become familiar with the challenges of post-stroke life. Gill Carter is a member of the PMG and Tony Oliver is a member of the PSC. Both provided much appreciated insights and advice reflecting on their own experiences of life after stroke.
In addition, the purposely convened RG shaped the focus of the intervention. The action groups in the three sites comprised a range of personnel, including stroke survivors and carers. Their work was central to refinement and practical implementation of the intervention. The facilitators in these sites were from a range of backgrounds and provided considerable insights into the challenges of delivering the intervention. They shared their learning in the training of the facilitators in the feasibility trial. Stroke survivors who participated in WS4 also provided feedback. The combined outputs included refinement of the intervention materials moving from a manual-based intervention to worksheets, which enabled the people providing the intervention to pick and mix components that were required for that particular patient.
Timing of the intervention
The survey (WS2) informed the decision to frame our intervention around the 6-month time point after stroke. Only a minority of areas provided any service beyond 12 months after stroke. It therefore seemed unfeasible to establish an entirely new service, and it was recognised that many post-stroke survivors may not have had any contact with services since soon after discharge from hospital. The intent was to develop a longer-term stroke strategy. However, both facilitators and some stroke survivors suggested that 6 months was too late and that self-management would be of more benefit to stroke survivors if delivered earlier.
Framing of the intervention
Data from the process evaluation suggested that the manner in which the intervention was introduced may influence subsequent engagement. The term ‘6-month review’ was established in the stroke lexicon and difficult to move away from. The initial approach to the stroke survivors, particularly if by letter, with the priming tool, may have unintentionally given the impression that the interaction offered was consistent with standard health-care approaches. During the initial meeting, the absence of a clear definition of intended facilitator and stroke survivor roles and the manner in which facilitators could frame questions about unmet need may have also fuelled the assumption that the intervention was similar to familiar clinical interactions. The intent of the priming tool was to aid the stroke survivor in identifying their needs, but this seems to have led to a more checklist approach from which some facilitators and stroke survivors found it difficult to move away, to consider a more problem-solving approach. Self-management proved to be a difficult concept to convey, although our early work demonstrated that stroke survivors play an active role in managing, using both practical and mental coping strategies.
The interaction between a ‘6-month review’ and our new approach was subject to considerable discussion at all stages of intervention development and implementation of the feasibility trial. However, it seems that it remained problematic. The data requirements for the SSNAP meant that some centres felt obliged to ask more clinically focused questions. Some facilitators were able to successfully interweave the clinical assessment with the self-management approach, but others found that more challenging.
Assessment of competency
A detailed scheme of competency support and assessment was implemented in the trial. This included completing the SEPSS prior to training, after the initial training course and further training day, and at the end of the trial. The facilitators completed the NPT toolkit during the early, middle and end stages of implementation; as part of ongoing training and development, facilitators were asked to participate in reflective practice by submitting self-reflection reports monthly. Following initial training, a period of 3 months was allowed to encourage practice of intervention delivery; challenges faced were discussed at a further training day. Following this, assessment of competency was undertaken by structured interview. Despite this, it seems that some colleagues struggled to deliver the intervention. A longer period of in-service practice might have helped, but this is difficult within the time constraints and risks losing engagement from control sites. One site was provided with additional training, but few used the online resources available.
Engagement with the intervention
Stroke survivors did not engage with the intervention at a number of time points. The reasons are multifaceted.
Population
To be inclusive and because of the dearth of any contrary evidence, we sought to offer the intervention to all stroke survivors. Perhaps inevitably, this meant that the population was heterogeneous; some people felt that they no longer required input from stroke services. It is of note that the people who did not receive the New Start intervention (mainly because they actively declined it or did not respond to the initial invitation) had on average slightly higher levels of higher education, shorter length of hospital stay and lower levels of unmet need than those who did receive the intervention.
Service context
One might speculate that stroke survivors who had had a high level of support prior to their 6-month review might feel that all their needs had already been addressed and no further input was required. Conversely, people who had formed an ongoing relationship with their service provider might welcome further contact.
Context
The context of service change is crucial;81 although we sought to consider the wider health and social care systems, this is challenging to address successfully. One specific challenge was the perceived pressure in some services to collect clinical data for the SSNAP. This led to the intervention developing a more clinical focus, and some facilitators struggled to combine this with the New Start approach.
Mechanisms of the process
Whether stroke survivors were invited to the review by opt-in or opt-out mechanisms impacted on the take-up.
Unmet needs
It may be that some stroke survivors did not have many unmet needs. In the LUNS data captured at baseline, one-quarter of respondents reported one or no unmet needs (n = 76 in total; n = 37 in intervention group).
It emerged during earlier WSs that people with less salient disabilities (e.g. cognitive/emotional) did not always recognise that their current issues were caused by the stroke (i.e. being tired/irritable/unable to concentrate), but considered them part of getting old. The priming tool presented to the stroke survivor prior to their review was intended to act as a prompt to consider this broader range of needs. Nevertheless, it seems that some participants did not identify any unmet needs or tended to favour reporting needs reflecting physical mobility.
Implementation
Despite training and their clear understanding of the principles underpinning New Start, some facilitators struggled to adapt to a more collaborative approach to problem-solving and goal-setting. This was associated with difficulties in facilitating active engagement in these processes on the part of the stroke survivor. Learning from the process evaluation will inform consideration of the staff groups most suited to deliver a self-management-style intervention and a more tailored training programme for the facilitators. Delivery of the intervention was suboptimal in some sites and will need addressing prior to a definitive trial.
Trial procedures
A strength of this work is the rigorously robust implementation of the feasibility trial, with clear separation of clinical delivery and research procedures. We have demonstrated that it is achievable to recruit participants by post (recruiting more than the initially suggested sample size). There was considerable variation in the numbers screened and recruited in the clusters, some of which could be explained (rural population with fewer throughputs), others of which could not (urban area with potential for larger throughput), despite exploring factors (screening: number of competing trials; recruitment: socioeconomic aspects of the population). Interviews and surveys were undertaken with all services as part of the eligibility assessment; this is key to understanding the patient population and provision of usual care. Although all services fulfilled the eligibility criteria of not delivering a self-management intervention, the Bridges intervention was commissioned in one site towards the end of the study. This led to the exclusion of a small number of potential participants.
The intervention was available to all stroke survivors in the services so randomised, which has provided us with a large and informative data set relating to clinical delivery and need.
Outcome assessments
Completion of outcome assessment was largely successful, although there were increasing withdrawals at the 9-month time point. This was because the outcomes were too close together, at 3, 6 and 9 months, leading to participants reporting that they had nothing new to report at 9 months and, therefore, had not returned the assessment booklet. In a larger trial, outcome assessment would be more spaced out. We have reported new evidence that outcome assessments provided in one booklet (rather than two separate booklets) generate greater return rates.
Conclusion
Implications for practice
A considerable number of new data, including identification of unmet needs, have been generated to inform the longer-term challenges faced by people after stroke, and the variability of service provision has been highlighted. It is appropriate that all stroke survivors be offered the opportunity for a review/contact with services at 6 months after their stroke; however, not all stroke survivors feel that they need such a review. The procedures (initial approach by telephone/letter/opt-in/opt-out) and framing of the review (checklist/problem-solving) influences take-up. Facilitators could find it hard to adopt a collaborative approach to problem-solving and goal-setting, and to integrate this approach with the clinical data collection required for the SSNAP. These factors, and, for the latter, the associated skill set required, should be considered carefully as services are established.
The findings suggest that services may benefit from reviewing their stroke service pathways, to try and optimise continuity of care for the stroke survivors and their families, ensuring that transfers of care between services is as co-ordinated as recommended.
Research recommendations
This research highlighted the dearth of evidence-based interventions for the longer term after stroke, particularly of interventions that are focused on the priorities of stroke survivors.
Further work is required to promote co-ordinated delivery of the stroke care pathway.
Despite extensive development work, the process evaluation highlights challenges in implementation of the intervention. Key components of the intervention need to be refined.
To enhance delivery, it is necessary to identify which staff groups are best suited to deliver what. This should include exploring the interface with primary care.
Refinement of the target population for this style of intervention is needed, as well as ensuring that the unmet needs of the wider stroke population are met. This could be, for example, by administering a survey of unmet needs to stroke survivors.
Trial procedures were successfully implemented. The intervention requires further refinement and for the target population to be more clearly specified prior to evaluation in a large-scale trial.
Acknowledgements
We thank all of the stroke survivors and their carers who participated in all stages of this research: your contribution is much appreciated; thank you.
We acknowledge the ongoing help, support and friendship from the CRAG.
We would like to thank all of the stroke services and associated research offices that participated in this study. Your help and support was very much appreciated.
-
Airedale General Hospital.
-
Croydon Health Services NHS Trust.
-
Torbay and South Devon Health and Care Trust.
-
Northumbria Healthcare NHS Foundation Trust.
-
Pennine Acute Hospitals NHS Trust.
-
Scunthorpe General Hospital.
-
Walsall Healthcare NHS Trust.
-
Plymouth Community Healthcare.
-
South West Yorkshire Partnership NHS Foundation Trust.
-
Sheffield Teaching Hospitals NHS Foundation Trust.
-
Bronglais General Hospital, Hywel Dda University Health Board.
-
Barnet Intermediate Care Services, Central London Community Healthcare NHS Trust.
-
Doncaster Royal Infirmary, Rotherham Doncaster and South Humber NHS Foundation Trust.
-
Peterlee Community Stroke Rehabilitation Team, County Durham and Darlington NHS Foundation Trust.
-
University Hospital of North Tees, North Tees and Hartlepool NHS Foundation Trust.
-
Sunderland Royal Hospital, City Hospitals Sunderland NHS Foundation Trust.
-
Gloucester Care Services, Gloucestershire Care Services NHS Trust.
-
Gloucestershire Royal Hospital, Gloucestershire Hospitals NHS Foundation Trust.
-
Norwich Community Hospital, Norfolk Community Health and Care NHS Trust.
-
South Petherton Community Hospital, Somerset Partnership NHS Foundation Trust.
-
Gorseinon Hospital, Abertawe Bro Morgannwg University Health Board.
-
West Park Hospital, The Royal Wolverhampton NHS Trust.
We thank colleagues in the stroke services of Bradford Teaching Hospitals NHS Foundation Trust who have supported our work and specifically assisted us in implementing WS1 of the programme.
We thank representatives from commissioning (Gillian Richardson), local authority social care (Jackie Wilks and Anita Wolstencroft), a community integration service run by the Stroke Association and Momentum Skills (Alistair Miller), Carers’ Resource (Anna Jackson), a stroke survivor (Sarah Free), the Stroke Association (Louise Haigh) and community neurophysiotherapy (Sophie Makower) who participated in the RG for WS3.
We are extremely grateful to the members of the PSC who have supported this work throughout implementation of this programme. Their input has been invaluable. The PSC were Professor Richard McManus, Mr Tony Oliver, Professor Chris Weir, Professor Gillian Parker and, in the first 2 years, Professor Bob Lewin.
We would like to thank colleagues in the Academic Unit for Ageing and Stroke Research and the CTRU who have provided support and advice over the last few years.
We thank Eve Jenner and Patrick Hill of Enabling Self Care for their assistance in developing and delivering training to the New Start facilitators.
We would like to pay tribute to our colleague Professor Bipin Bhakta who assisted us in the development of this programme and implementation of the first WSs prior to his untimely death.
Patient, public and professional engagement
Presentations
Forster A, Richardson G. Improving Longer-term Stroke Care. Wakefield Primary Care, Wakefield, March 2015.
Crocker T. The LoTS2Care Programme So Far. Stroke Study Day, Hallamshire Hospital, Sheffield, June 2015.
Exhibition stands
Gaining Expressions of Interest, 8th UK Stroke Forum Conference, Harrogate, 3–5 December 2013.
Making Research Real, Bradford, May 2015.
UK Stroke Assembly, East Midlands Conference Centre, Nottingham, 9–10 June 2015.
Stroke Study Day, Hallamshire Hospital, Sheffield, 30 June 2015.
Celebrating 30 Years of Stroke Research Within the Academic Unit, 12th UK Stroke Forum Conference, Liverpool, 28–30 November 2017.
Consumer Research Advisory Group
CRAG meetings held quarterly.
Consumer conference (York)
The programme work was presented to a group of 30 post-stroke survivors and their carers. The issue of access to routine data was discussed and awareness was raised of the difficulties of undertaking research.
Contributions of authors
Anne Forster (https://orcid.org/0000-0001-7466-4414) (Professor of Stroke Rehabilitation) was the lead grant holder and chief investigator for the programme and oversaw the implementation, completion and write-up of this programme.
Seline Ozer (https://orcid.org/0000-0002-5791-5469) (Senior Research Fellow) was the trial manager from September 2016. She oversaw the operational delivery of the feasibility trial at the Academic Unit for Ageing and Stroke Research.
Thomas F Crocker (https://orcid.org/0000-0001-7450-3143) (Senior Research Fellow) assisted with writing of the grant application, led the literature review, and substantially contributed to WSs 1–4.
Allan House (https://orcid.org/0000-0001-8721-8026) (Professor of Liaison Psychiatry) and Jenny Hewison (https://orcid.org/0000-0003-3026-3250) (Professor of the Psychology of Healthcare) were co-applicants on the research programme grant, were members of the PMG and contributed to the conceptualisation and delivery of all components of the implementation of this programme.
Elaine Roberts (Director of Stroke Support for England for the Stroke Association) was a member of the PMG and provided insight and advice over all of the programme, particularly providing insights from the Stroke Association and the user view.
Josie Dickerson (https://orcid.org/0000-0003-0121-3406) (Research Programme Manager) was the programme manager from October 2013 to April 2015 and oversaw the operational delivery of WSs 1–3.
Gill Carter (stroke survivor) contributed to development and delivery of all components of this programme.
Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Professor of Health Economics) was a co-applicant on the research programme grant, was a member of the PMG, led the design of the health economic components of the feasibility trial and was responsible for the health economic analysis, including reporting.
Matthew Fay (https://orcid.org/0000-0002-0274-536X) (GP) was a co-applicant on the grant, was a member of the PMG and contributed to the delivery of the programme.
Gillian Richardson (https://orcid.org/0000-0002-5601-3447) (Health Services Manager) was a co-applicant on the grant and a member of the PMG. She contributed considerable knowledge on service delivery in stroke to this programme.
Alan Wright (https://orcid.org/0000-0002-5787-293X) (Research Fellow) led and undertook the process evaluation reported in WS5.
Christopher McKevitt (https://orcid.org/0000-0002-5290-4613) (Professor of Social Sciences and Health) was a co-applicant on the research programme grant and a member of the PMG. He provided input to the delivery of the whole programme, specifically the qualitative aspects.
Rosemary McEachan (https://orcid.org/0000-0003-1302-6675) (Director of Born in Bradford) was a co-applicant on the research programme grant and a member of the PMG. She provided input to the delivery of the whole programme, specifically around concepts of behaviour change.
Robbie Foy (https://orcid.org/0000-0003-0605-7713) (Professor of Primary Care) was a co-applicant on the research programme grant and a member of the PMG. He provided input to the delivery of the whole programme, specifically around concepts of implementation science.
Lorna Barnard (https://orcid.org/0000-0001-5436-6882) was the senior trial co-ordinator at the CTRU until July 2017, and contributed to the set-up and delivery of the feasibility trial.
Lauren Moreau (https://orcid.org/0000-0002-0280-6345) was the trial co-ordinator at the CTRU from January 2016 until December 2018, and contributed to the set-up, delivery and completion of the feasibility trial.
Arvin Prashar (https://orcid.org/0000-0003-3349-4353) (Research Fellow) led and reported the focus groups in WS2, assisted with intervention development (WS3) and was instrumental in the delivery of WS4.
David Clarke (https://orcid.org/0000-0001-6279-1192) (Associate Professor in Stroke) provided advice and guidance to the research team over the lifetime of this programme, particularly relating to qualitative components.
Natasha Hardicre (https://orcid.org/0000-0002-3639-5556) (Research Fellow) assisted in the delivery of WS4, contributed to the development and delivery of the intervention training, and conceptualised and developed the process evaluation.
Ivana Holloway (https://orcid.org/0000-0002-9542-883X) (Statistician) contributed to grant development, was a member of the PMG and wrote the statistical analysis plan for the feasibility trial.
Richard Brindle (https://orcid.org/0000-0002-1848-6219) (Statistician) was responsible for the statistical analysis and the reporting of the feasibility trial.
Jessica Hall (https://orcid.org/0000-0003-3622-9598) (Research Fellow) led and undertook the qualitative work reported in WS1.
Louisa-Jane Burton (https://orcid.org/0000-0003-3617-1410) (Research Fellow) assisted with data collection for the process evaluation and contributed to its analysis and reporting.
Ross Atkinson (https://orcid.org/0000-0001-8976-2754) (Programme Manager) managed the programme from May 2015 to August 2016, overseeing implementation of WS4.
Rebecca J Hawkins (https://orcid.org/0000-0003-1811-1369) (Lecturer in Qualitative Health Research) was a co-applicant and member of the PMG; contributed to the design, set-up and acquisition and analysis of data in WS1; and offered support in the design of the qualitative elements of the process evaluation.
Lesley Brown (https://orcid.org/0000-0001-5499-9145) (Senior Research Fellow) contributed to the literature reviews in WS2.
Nicola Cornwall (https://orcid.org/0000-0003-2207-859X) (Research Fellow) contributed to organisation and delivery of WSs 3 and 4.
Bryony Dawkins (https://orcid.org/0000-0002-7038-1975) (Research Fellow) contributed to the health economic analysis of the feasibility trial, including the write-up.
David Meads (https://orcid.org/0000-0003-1369-2483) (Associate Professor of Health Economics) contributed to the health economic modelling of the feasibility trial.
Laetitia Schmitt (https://orcid.org/0000-0003-1052-488X) (Research Fellow) contributed to the design of the health economic component of the feasibility trial.
Marie Fletcher (https://orcid.org/0000-0001-7545-1314) (Data Manager) contributed to the design, delivery and collection of all data for the feasibility trial.
Michael Speed (stroke survivor) was a co-applicant on the grant and contributed to the development of the programme.
Katie Grenfell (https://orcid.org/0000-0002-8078-7047) (Research Fellow) contributed to the systematic overview and review of studies in WS1b, assisting with screening, data extraction and analysis.
Suzanne Hartley (https://orcid.org/0000-0003-2346-9461) (Head of Trial Management at the CTRU) was project delivery lead for the feasibility trial (for the CTRU).
John Young (https://orcid.org/0000-0003-4085-9306) (Professor of Elderly Care) was a co-applicant on the research programme grant and shared clinical and methodological expertise throughout the programme.
Amanda Farrin (https://orcid.org/0000-0002-2876-0584) (Professor of Clinical Trials and Evaluation of Complex Interventions) was a co-applicant on the research programme grant, was the programme methodologist and was responsible for the main statistical analysis and reporting of the feasibility trial.
Publications
Forster A, Brown L, Smith J, House A, Knapp P, Wright JJ, Young J. Information provision for stroke patients and their caregivers (review). Cochrane Database Syst Rev 2012;11:CD001919.
Dickerson J, Forster A. Questions people ask about stroke: what’s changed in 20 years? SAGE Open Med 2015;3:2050312115591623.
Forster A, Hartley S, Barnard L, Ozer S, Hardicre N, Crocker T, et al. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomised controlled feasibility trial. 2018;19:317.
Hardicre NK, Crocker TF, Wright A, Burton LJ, Ozer S, Atkinson R, et al. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for the process evaluation of a cluster randomised controlled feasibility trial. 2018;19:368.
Crocker TF, Ozer S, Brown L, Hall JF, Forster A. Non-pharmacological interventions for longer-term stroke survivors or their carers: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2019;4:CD013317.
Conferences
Forster A. Long-term Management of Patients with Stroke. Platform presentation. 9th World Stroke Congress, Istanbul, 22–25 October 2014.
Forster A, Dickerson J, Hall J, Crocker T, House A, Hewison J, et al. Development and Evaluation of Strategies to Provide Longer-term Health and Social Care for Stroke Survivors and their Carers. Poster presentation. 9th World Stroke Congress, Istanbul, 22–25 October 2014.
Dickerson J, Forster, A on behalf of the LoTS-2-Care Collaboration. A National Survey of Community Stroke Service Provision Beyond Six Months Post Stroke. Poster presentation. 9th UK Stroke Forum Conference, Harrogate, 2–4 December 2014.
Forster A, Dickerson J, House A, McKevitt C, Hulme C, Richardson G, et al., on behalf of the LoTS-2-Care Collaboration. Development and Evaluation of Strategies to Provide Longer-term Health and Social Care for Stroke Survivors and their Carers (LoTS-2-Care). Poster presentation. 9th UK Stroke Forum Conference, Harrogate, 2–4 December 2014.
Hall J, Hawkins R, Gillian J, Dickerson J, Crocker T, McEachan R, Forster A, on behalf of the LoTS-2-Care Collaboration. Improving Longer Term Outcomes Post Stroke: Exploring the Barriers and Enablers that Influence Unmet Need, Life Quality and Participation after Stroke. Poster presentation. 9th UK Stroke Forum Conference, Harrogate, 2–4 December 2014.
Crocker T, Brown L, Hall J, Grenfell K, Dickerson J, Forster A, on behalf of the LoTS-2-Care Collaboration. A Systematic Overview of Cochrane Reviews of Interventions Feasible for Stroke Survivors and Carers in the Community Beyond 6 Months. Poster presentation. Inaugural European Stroke Organisation Conference, Glasgow, 17–19 April 2015.
Dickerson J, Hall J, Prashar A, Crocker T, Hawkins R, McEachan R, Forster A, on behalf of the LoTS-2-Care Collaboration. A Detailed Exploration of the Longer-term Unmet Needs of Stroke Survivors. Poster presentation. Inaugural European Stroke Organisation Conference, Glasgow, 17–19 April 2015.
Dickerson J, Forster A. Questions People Ask about Stroke: Revisiting the UK Stroke Association Helpline Data. Poster presentation. Inaugural European Stroke Organisation Conference, Glasgow, 17–19 April 2015.
Prashar A, on behalf of the LoTS-2-Care Collaboration. What Factors Inhibit and Enable English Community Stroke Teams from Meeting the Needs of Longer-term Stroke Survivors? Findings from a Focus Group Study. Poster presentation. Inaugural European Stroke Organisation Conference, Glasgow, 17–19 April 2015.
Forster A. Development of Strategies to Provide Longer-term Health and Social Care for Stroke Survivors and their Carers. Platform presentation. Combined 26th Annual Scientific Meeting of the Stroke Society of Australasia and 11th Australasian Nursing and Allied Health Stroke Conference SMART STROKES, Melbourne, VIC, 2–4 September 2015.
Crocker T, Brown L, Hall J, Grenfell K, Dickerson J, Forster A, on behalf of the LoTS-2-Care Collaboration. A Systematic Overview of Cochrane Reviews of Interventions Feasible for Stroke Survivors and Carers in the Community Beyond 6 Months. Poster presentation. 10th UK Stroke Forum Conference, Liverpool, 1–3 December 2015.
Crocker T, Forster A, on behalf of the LoTS-2-Care Collaboration. Moving Forward: An Intervention for Longer-term Health and Social Care for Stroke Survivors. Poster presentation. 10th UK Stroke Forum Conference, Liverpool, 1–3 December 2015.
Dickerson J, Hall J, Prashar A, Crocker T, Hawkins R, McEachan R, Forster A, on behalf of the LoTS-2-Care Collaboration. A Detailed Exploration of the Longer-term Unmet Needs of Stroke Survivors. Poster presentation. 10th UK Stroke Forum Conference, Liverpool, 1–3 December 2015.
Prashar A, Dickerson J, Forster A, on behalf of the LoTS-2-Care Collaboration. What Factors Inhibit and Enable English Community Stroke Teams from Meeting the Needs of Longer-term Stroke Survivors? Poster presentation. 10th UK Stroke Forum Conference, Liverpool, 1–3 December 2015.
Dickerson J, Hall J, Prashar A, Crocker T, Hawkins R, McEachan R, Forster A, on behalf of the LoTS-2-Care Collaboration. A Detailed Exploration of the Longer-term Unmet Needs of Stroke Survivors. Poster presentation. Bradford Institute for Health Research – Research Conference ‘Research that Changes a City’, Bradford, 7 October 2016.
Hall J, et al. Improving Longer Term Outcomes Post Stroke: Exploring the Barriers and Enablers that Influence Unmet Need, Life Quality and Participation after Stroke. Poster presentation. Bradford Institute for Health Research – Research Conference ‘Research that Changes a City’, Bradford, 7 October 2016.
Hardicre N, on behalf of the LoTS-2-Care Collaboration. Who is the Person in ‘Person-centred’? Challenging Therapists’ Views of Person-centred Goal-setting through a Self-management Approach. Poster presentation. 11th UK Stroke Forum Conference, Liverpool, 28–30 November 2016.
Exhibition stand celebrating 30 years of stroke research within the Academic Unit. 12th UK Stroke Forum Conference, Liverpool, 28–30 November 2017.
Barnard L, Moreau L, Forster A, Atkinson R, Crocker T, Fletcher M, et al. , on behalf of the LoTS2Care collaboration. Recruiting Stroke Survivors to a Clinical Trial Long-term Post-stroke: Challenges and Lessons Learned. Poster presentation. 12th UK Stroke Forum Conference, Liverpool, 28–30 November 2017.
Crocker T, Forster A, Brown L, on behalf of the review team. Update of Cochrane Review of Information Provision for Stroke Patients and their Caregivers. Poster presentation. 13th UK Stroke Forum Conference, Telford, 4–6 December 2018.
Ozer S, Forster A, Hartley S, Barnard L, Crocker T, Fletcher M, et al. , on behalf of the LoTS2Care Programme Management Group. Uptake of Six Month Post-stroke Review: Findings from the LoTS2Care Feasibility Trial. Poster presentation. 13th UK Stroke Forum Conference, Telford, 4–6 December 2018.
Wright A, Burton L, Hardicre N, Crocker T, Ozer S, House A, et al. , on behalf of the LoTS-2-Care Programme Management Group. A Longer-term Care Strategy to Support Stroke Survivors and their Carers (LoTS-2-Care) – A Process Evaluation. Poster presentation. 13th UK Stroke Forum Conference, Telford, 4–6 December 2018.
Moreau L, Holloway I, Ozer S, Forster A, Hartley S, Brindle R, Farrin A. Maximising Follow-up Rates of Patient Reported Outcome Measures: A Study Within a Trial (SWAT) – Results from the LoTS2Care Feasibility Trial. Poster presentation. 5th International Clinical Trials Methodology Conference, Brighton, 6–9 October 2019.
Hardicre N, Crocker T, Ozer S, Wright A, Burton L, on behalf of the LoTS2Care Programme Management Group. Essential Functions in Complex Interventions: Lessons from the Implementation of New Start. Platform presentation (awarded the Society for Research in Rehabilitation prize for best rehabilitation abstract). 14th UK Stroke Forum Conference, Telford, 3–5 December 2019.
Forster A, Ozer S, Moreau L, Hartley S, Barnard L, Fletcher M, et al. , on behalf of the LoTS-2-Care Programme Management Group. A Cluster Randomised Controlled Feasibility Trial of an Intervention to Support Stroke Survivors and their Carers in the Longer-term. Poster presentation. 14th UK Stroke Forum Conference, Telford, 3–5 December 2019.
Crocker T, Wright A, Burton L, Hardicre N, Ozer S, House A, et al. , on behalf of the LoTS-2-Care Programme Management Group. Process Evaluation of New Start, a 6-month Review Incorporating Problem-solving and Self-management to Improve Stroke Survivors’ Quality of Life. Poster presentation. 14th UK Stroke Forum Conference, Telford, 3–5 December 2019.
Moreau L, Holloway I, Hartley S, Brindle R, Farrin A, Ozer S, et al. , on behalf of the LoTS-2-Care Programme Management Group. Reducing the Burden of Patient Reported Outcome Measures? A Study Within a Trial (SWAT) – Results from the LoTS2Care Feasibility Study. Poster presentation. 14th UK Stroke Forum Conference, Telford, 3–5 December 2019.
Data-sharing statement
Workstreams in this programme grant involved qualitative methodologies; therefore, the data generated are not suitable for sharing beyond those contained in this report. Further information can be obtained from the corresponding author.
As WS5 was a feasibility trial to inform a definitive trial, sharing of the trial data set is not anticipated; however, any data requests should be sent to the corresponding author and would be subject to review by a subgroup of the trial team, which will include the data guarantor, Professor Farrin. All data-sharing activities would require a data-sharing agreement.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Stroke Association . State of the Nation: Stroke Statistics 2018. www.stroke.org.uk/sites/default/files/state_of_the_nation_2018.pdf (accessed 8 August 2020).
- Murray J, Young J, Forster A. Review of longer-term problems after a disabling stroke. Rev 2007;17:277-92. https://doi.org/10.1017/S0959259808002608.
- Salter K, Hellings C, Foley N, Teasell R. The experience of living with stroke: a qualitative meta-synthesis. J Rehabil Med 2008;40:595-602. https://doi.org/10.2340/16501977-0238.
- Pindus DM, Mullis R, Lim L, Wellwood I, Rundell AV, Abd Aziz NA, et al. Stroke survivors’ and informal caregivers’ experiences of primary care and community healthcare services – a systematic review and meta-ethnography. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0192533.
- Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014;9:1017-25. https://doi.org/10.1111/ijs.12357.
- Tieges Z, Mead G, Allerhand M, Duncan F, van Wijck F, Fitzsimons C, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil 2015;96:15-23. https://doi.org/10.1016/j.apmr.2014.08.015.
- Kwok T, Pan JH, Lo R, Song X. The influence of participation on health-related quality of life in stroke patients. Disabil Rehabil 2011;33:1990-6. https://doi.org/10.3109/09638288.2011.553709.
- Wolfe CD, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, et al. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLOS Med 2011;8. https://doi.org/10.1371/journal.pmed.1001033.
- Guidetti S, Andersson K, Andersson M, Tham K, Von Koch L. Client-centred self-care intervention after stroke: a feasibility study. Scand J Occup Ther 2010;17:276-85. https://doi.org/10.3109/11038120903281169.
- Rigby H, Gubitz G, Phillips S. A systematic review of caregiver burden following stroke. Int J Stroke 2009;4:285-92. https://doi.org/10.1111/j.1747-4949.2009.00289.x.
- Cameron JI, Naglie G, Silver FL, Gignac MA. Stroke family caregivers’ support needs change across the care continuum: a qualitative study using the timing it right framework. Disabil Rehabil 2013;35:315-24. https://doi.org/10.3109/09638288.2012.691937.
- Forster A, Fillit H, Rockwood K, Young J. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. Philadelphia, PA: Elsevier, Inc.; 2016.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398-403. https://doi.org/10.1161/STROKEAHA.110.598839.
- World Health Organization . International Classification of Functioning and Disability: ICIDH-2, Beta-2 Draft – Short Version 1999.
- Sumathipala K, Radcliffe E, Sadler E, Wolfe CD, McKevitt C. Identifying the long-term needs of stroke survivors using the International Classification of Functioning, Disability and Health. Chronic Illn 2012;8:31-44. https://doi.org/10.1177/1742395311423848.
- Ellis-Hill C, Robison J, Wiles R, McPherson K, Hyndman D, Ashburn A. Going home to get on with life: patients and carers experiences of being discharged from hospital following a stroke. Disabil Rehabil 2009;31:61-72. https://doi.org/10.1080/09638280701775289.
- Robison J, Wiles R, Ellis-Hill C, McPherson K, Hyndman D, Ashburn A. Resuming previously valued activities post-stroke: who or what helps?. Disabil Rehabil 2009;31:1555-66. https://doi.org/10.1080/09638280802639327.
- Ch’ng AM, French D, McLean N. Coping with the challenges of recovery from stroke: long term perspectives of stroke support group members. J Health Psychol 2008;13:1136-46. https://doi.org/10.1177/1359105308095967.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3. https://doi.org/10.3109/09638288809164103.
- LoTSCare LUNS Study Team . Validation of the longer-term unmet needs after stroke (LUNS) monitoring tool: a multicentre study. Clin Rehabil 2013;27:1020-8. https://doi.org/10.1177/0269215513487082.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2008;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Lincoln YS, Guba EG. But is it rigorous? Trustworthiness and authenticity in naturalistic evaluation. New Directions for Program Evaluation 1986;1986:73-84. https://doi.org/10.1002/ev.1427.
- Forster A, Mellish K, Farrin A, Bhakta B, House A, Hewison J, et al. Development and evaluation of interventions and tools to improve patient and carer centred outcomes in longer-term stroke care (LoTS care) and exploration of adjustment post stroke. Programme Grants Appl Res 2014;2. https://doi.org/10.3310/pgfar02060.
- Murray J, Young J, Forster A, Ashworth R. Developing a primary care-based stroke model: the prevalence of longer-term problems experienced by patients and carers. Br J Gen Pract 2003;53:803-7.
- Ahuja SS, Clark S, Morahan EM, Ono M, Mulligan H, Hale L. The journey to recovery: experiences and perceptions of individuals following stroke. N Z J Physiother 2013;41:36-43.
- Pound P, Gompertz P, Ebrahim S. Social and practical strategies described by people living at home with stroke. Health Soc Care Community 1999;7:120-8. https://doi.org/10.1046/j.1365-2524.1999.00168.x.
- Hare R, Rogers H, Lester H, McManus R, Mant J. What do stroke patients and their carers want from community services?. Fam Pract 2006;23:131-6. https://doi.org/10.1093/fampra/cmi098.
- Kersten P, Low JT, Ashburn A, George SL, McLellan DL. The unmet needs of young people who have had a stroke: results of a national UK survey. Disabil Rehabil 2002;24:860-6. https://doi.org/10.1080/09638280210142167.
- Dickerson J, Forster A. Questions people ask about stroke: what’s changed in 20 years?. SAGE Open Med 2015;3. https://doi.org/10.1177/2050312115591623.
- Forster A, Brown L, Smith J, House A, Knapp P, Wright JJ, et al. Information provision for stroke patients and their caregivers. Cochrane Database Syst Rev 2012;11. https://doi.org/10.1002/14651858.CD001919.pub3.
- National Institute of Health and Care Excellence . The Guidelines Manual: Appendix B: Methodology Checklist: Systematic Reviews and Meta-Analyses 2012.
- Loetscher T, Lincoln NB. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev 2013;5. https://doi.org/10.1002/14651858.CD002842.pub2.
- Saunders DH, Sanderson M, Brazzelli M, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD003316.pub5.
- Xiao Y, Luo M, Wang J, Luo H. Inspiratory muscle training for the recovery of function after stroke. Cochrane Database Syst Rev 2012;5. https://doi.org/10.1002/14651858.CD009360.pub2.
- Legg LA, Drummond AE, Langhorne P. Occupational therapy for patients with problems in activities of daily living after stroke. Cochrane Database Syst Rev 2006;4. https://doi.org/10.1002/14651858.CD003585.pub2.
- Outpatient Service Trialists . Therapy based rehabilitation services for stroke patients at home. Cochrane Database Syst Rev 2003;1.
- Aziz NA, Leonardi-Bee J, Phillips M, Gladman JR, Legg L, Walker MF. Therapy-based rehabilitation services for patients living at home more than one year after stroke. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD005952.pub2.
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD006073.pub2.
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta-analysis. Cochrane Database Syst Rev 2010;5. https://doi.org/10.1002/14651858.CD005066.pub2.
- Laver KE, Schoene D, Crotty M, George S, Lannin NA, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD010255.pub2.
- Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Syst Rev 2008;4. https://doi.org/10.1002/14651858.CD003437.pub3.
- Brady MC, Kelly H, Godwin J, Enderby P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 2012;5. https://doi.org/10.1002/14651858.CD000425.pub3.
- Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, et al. Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev 2011;10. https://doi.org/10.1002/14651858.CD008388.pub2.
- Legg LA, Quinn TJ, Mahmood F, Weir CJ, Tierney J, Stott DJ, et al. Non-pharmacological interventions for caregivers of stroke survivors. Cochrane Database Syst Rev 2011;10. https://doi.org/10.1002/14651858.CD008179.pub2.
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost-effectiveness analysis. Lancet 2013;382:2069-76. https://doi.org/10.1016/S0140-6736(13)61603-7.
- Crocker TF, Ozer S, Brown L, Hall JF, Forster A. Non-pharmacological interventions for longer-term stroke survivors or their carers: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2019;4. https://doi.org/10.1002/14651858.CD013317.
- Draper B, Bowring G, Thompson C, Van Heyst J, Conroy P, Thompson J. Stress in caregivers of aphasic stroke patients: a randomized controlled trial. Clin Rehabil 2007;21:122-30. https://doi.org/10.1177/0269215506071251.
- Hartke RJ, King RB. Telephone group intervention for older stroke caregivers. Topics Stroke Rehabil 2003;9:65-81. https://doi.org/10.1310/RX0A-6E2Y-BU8J-W0VL.
- Lincoln NB, Walker MF, Dixon A, Knights P. Evaluation of a multiprofessional community stroke team: a randomized controlled trial. Clin Rehabil 2004;18:40-7. https://doi.org/10.1191/0269215504cr700oa.
- Thomas SA, Walker MF, Macniven JA, Haworth H, Lincoln NB. Communication and Low Mood (CALM): a randomized controlled trial of behavioural therapy for stroke patients with aphasia. Clin Rehabil 2013;27:398-40. https://doi.org/10.1177/0269215512462227.
- Elman RJ, Bernstein-Ellis E. The efficacy of group communication treatment in adults with chronic aphasia. J Speech Lang Hear Res 1999;42:411-9. https://doi.org/10.1044/jslhr.4202.411.
- Johnson J, Pearson V. The effects of a structured education course on stroke survivors living in the community. Rehabil Nursing 2000;25:59-65. https://doi.org/10.1002/j.2048-7940.2000.tb01864.x.
- Mulders AHM, de Witte LP, Diederiks JPM. Evaluation of a rehabilitation after-care programme for stroke patients. J Rehabil Sci 1989;2:97-103.
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, et al. Exercise to enhance mobility and prevent falls after stroke: the Community Stroke Club Randomized Trial. Neurorehabil Neur Repair 2012;26:1046-57. https://doi.org/10.1177/1545968312441711.
- Flansbjer UB, Miller M, Downham D, Lexell J. Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. J Rehab Med 2008;40:42-8. https://doi.org/10.2340/16501977-0129.
- Jeong S, Kim MT. Effects of a theory-driven music and movement program for stroke survivors in a community setting. Appl Nurs Res 2007;20:125-31. https://doi.org/10.1016/j.apnr.2007.04.005.
- Lin KC, Wu CY, Wei TH, Lee CY, Liu JS. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: a randomized controlled study. Clin Rehabil 2007;21:1075-86. https://doi.org/10.1177/0269215507079843.
- Lin KC, Wu CY, Liu JS. A randomized controlled trial of constraint-induced movement therapy after stroke. Acta Neurochir Suppl 2008;101:61-4. https://doi.org/10.1007/978-3-211-78205-7_10.
- Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized controlled trial. Neurorehabil Neur Repair 2012;26:85-9. https://doi.org/10.1177/1545968311418675.
- Olney SJ, Nymark J, Brouwer B, Culham E, Day A, Heard J, et al. A randomized controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke 2006;37:476-81. https://doi.org/10.1161/01.STR.0000199061.85897.b7.
- Ouellette MM, LeBrasseur NK, Bean JF, Phillips E, Stein J, Frontera WR, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke 2004;35:1404-9. https://doi.org/10.1161/01.STR.0000127785.73065.34.
- Smania N, Aglioti SM, Girardi F, Tinazzi M, Fiaschi A, Cosentino A, et al. Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurol 2006;67:2050-2. https://doi.org/10.1212/01.wnl.0000247279.63483.1f.
- Tseng CN, Chen CCH, Wu SC, Lin LC. Effects of a range-of-motion exercise programme. J Adv Nurs 2007;57:181-91. https://doi.org/10.1111/j.1365-2648.2006.04078.x.
- Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil 1996;75:114-20. https://doi.org/10.1097/00002060-199603000-00006.
- Wu C, Chen C, Tsai W, Lin K, Chou S. A randomized controlled trial of modified constraint-induced movement therapy for elderly stroke survivors: changes in motor impairment, daily functioning and quality of life. Archiv Phys Med Rehabil 2007;88:273-8. https://doi.org/10.1016/j.apmr.2006.11.021.
- Yelnik AP, Le Breton F, Colle FM, Bonan IV, Hugeron C, Egal V, et al. Rehabilitation of balance after stroke with multisensorial training: a single-blind randomized controlled study. Neurorehabil Neur Repair 2008;22:468-76. https://doi.org/10.1177/1545968308315996.
- Zedlitz AM, Rietveld TC, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke 2012;43:1046-51. https://doi.org/10.1161/STROKEAHA.111.632117.
- Pitkanen K. Stroke rehabilitation in the elderly: a controlled study of the effectiveness and costs of a multidimensional intervention [Dissertation]. Kuopio: University of Kuopio; 2000.
- Desrosiers J, Noreau L, Rochette A, Carbonneau H, Fontaine L, Viscogliosi C, et al. Effect of a home leisure education program after stroke: a randomized controlled trial. Arch Phys Med Rehabil 2007;88:1095-100. https://doi.org/10.1016/j.apmr.2007.06.017.
- Logan PA, Gladman JR, Avery A, Walker MF, Dyas J, Groom L. Randomised controlled trial of an occupational therapy intervention to increase outdoor mobility after stroke. BMJ 2004;329:1372-5. https://doi.org/10.1136/bmj.38264.679560.8F.
- Aben L, Heijenbrok-Kal MH, van Loon EM, Groet E, Ponds RW, Busschbach JJ, et al. Training memory self-efficacy in the chronic stage after stroke: a randomized controlled trial. Neurorehabil Neur Repair 2013;27:110-7. https://doi.org/10.1177/1545968312455222.
- Chinchai P, Bunyamark T, Sirisatayawong P. Effects of caregiver education in stroke rehabilitation on the quality of life of stroke survivors. WFOT Bulletin 2010;61:56-63. https://doi.org/10.1179/otb.2010.61.1.015.
- Roth T, Sokolov AN, Messias A, Roth P, Weller M, Trauzettel-Klosinski S. Comparing explorative saccade and flicker training in hemianopia: a randomized controlled study. Neurology 2009;72:324-31. https://doi.org/10.1212/01.wnl.0000341276.65721.f2.
- Sims J, Galea M, Taylor N, Dodd K, Jespersen S, Joubert L, et al. Regenerate: assessing the feasibility of a strength-training program to enhance the physical and mental health of chronic post stroke patients with depression. Int J Geriatr Psychiatry 2009;24:76-83. https://doi.org/10.1002/gps.2082.
- Towle D, Lincoln NB, Mayfield LM. Evaluation of social work on depression after stroke. Clin Rehabil 1989;3:89-96. https://doi.org/10.1177/026921558900300201.
- Tibaek S, Gard G, Jensen R. Pelvic floor muscle training is effective in women with urinary incontinence after stroke: a randomised, controlled and blinded study. Neurourol Urodyn 2005;24:348-57. https://doi.org/10.1002/nau.20134.
- Logan PA, Armstrong S, Avery TJ, Barer D, Barton GR, Darby J, et al. Rehabilitation aimed at improving outdoor mobility for people after stroke: a multicentre randomised controlled study (the Getting out of the House Study). Health Technol Assess 2014;18. https://doi.org/10.3310/hta18290.
- Sackley CM, Walker MF, Burton CR, Watkins CL, Mant J, Roalfe AK, et al. An Occupational Therapy intervention for residents with stroke-related disabilities in UK Care Homes (OTCH): cluster randomised controlled trial with economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20150.
- Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag 2003;6:63-71. https://doi.org/10.1089/109350703321908441.
- Boult C, Green AF, Boult LB, Pacala JT, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine’s ‘retooling for an aging America’ report. J Am Geriatr Soc 2009;57:2328-37. https://doi.org/10.1111/j.1532-5415.2009.02571.x.
- Coleman K, Mattke S, Perrault PJ, Wagner EH. Untangling practice redesign from disease management: how do we best care for the chronically ill?. Annu Rev Public Health 2009;30:385-408. https://doi.org/10.1146/annurev.publhealth.031308.100249.
- Dennis SM, Zwar N, Griffiths R, Roland M, Hasan I, Powell Davies G, et al. Chronic disease management in primary care: from evidence to policy. Med J Aust 2008;188:S53-6. https://doi.org/10.5694/j.1326-5377.2008.tb01745.x.
- Goodwin N, Curry N, Naylor C, Ross S, Duldig W. Managing People with Long-term Conditions. London: The King’s Fund; 2010.
- Singh D. Surrey and Sussex Primary Care Trust Alliance . Transforming chronic care: a systematic review of the evidence. Evid Based Cardiovasc Med 2005;9:91-4. https://doi.org/10.1016/j.ebcm.2005.03.011.
- Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7370.925.
- Coulter A, Roberts S, Dixon A. Delivering Better Services for People with Long-term Conditions: Building the House of Care. London: The King’s Fund; 2013.
- Department of Health and Social Care . The National Service Framework for Long-Term Conditions 2005.
- Department of Health and Social Care . Supporting People With Long-Term Conditions: An NHS and Social Care Model to Support Local Innovation and Integration 2005.
- Department of Health and Social Care . Supporting People With Long Term Conditions to Self Care: A Guide to Developing Local Strategies and Good Practice 2005.
- Mathers N, Roberts S, Hodkinson I, Karet B. Care Planning: Improving the Lives of People with Long-term Conditions. London: Royal College of General Practitioners; 2011.
- NHS England . Transforming Participation in Health and Care: The NHS Belongs to Us All 2013.
- Skills for Care . Common Core Principles to Support Self-Care 2015. www.skillsforcare.org.uk/Document-library/Skills/Self-care/Commoncoreprinciples.pdf (accessed 19 March 2019).
- Fryer CE, Luker JA, McDonnell MN, Hillier SL. Self management programmes for quality of life in people with stroke. Cochrane Database Syst Rev 2016;8. https://doi.org/10.1002/14651858.CD010442.pub2.
- Wray FD. Designing a Self-Management Intervention for Stroke Survivors With Communication Difficulties 2017.
- Stroke Association . Accessible Information Guidelines 2012.
- Royal College of Physicians . SSNAP Clinical Audit: October 2013–March 2014 n.d. www.strokeaudit.org/results/Clinical-audit/National-Results.aspx (accessed 8 September 2020).
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 2008.
- Department of Health and Social Care (DHSC). National Service Framework for Older People 2001.
- McKenna S, Jones F, Glenfield P, Lennon S. Bridges self-management program for people with stroke in the community: a feasibility randomized controlled trial. Int J Stroke 2015;10:697-704. https://doi.org/10.1111/ijs.12195.
- Donaldson L. Expert patients usher in a new era of opportunity for the NHS. BMJ 2003;326:1279-80. https://doi.org/10.1136/bmj.326.7402.1279.
- Great Britain . Carer Act 2014 2014.
- Hall JF. The Development of an Intervention for Carers of Stroke Survivors Using an Intervention Mapping Approach 2017.
- Bartholomew Eldridge LK, Markham CM, Ruiter RA, Fernandez ME, Kok G, Parcel GS. Planning Health Promotion Programs: An Intervention Mapping Approach. Hoboken, NJ: Wiley; 2016.
- Bartholomew LK, Parcel GS, Kok G, Gottlieb NH, Fernandez ME. Planning Health Promotion Programs: An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass; 2011.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Nezu AM, Maguth Nezu C, D’Zurilla TJ. Problem-Solving Therapy: A Treatment Manual. New York, NY: Springer Publishing Company; 2012.
- LoTSCare LUNS. Study Team . Longer-Term Unmet Needs After Stroke (LUNS) 2008. https://journals.sagepub.com/doi/suppl/10.1177/0269215513487082/suppl_file/suppl-survey.pdf (accessed 19 August 2020).
- NHS Lothian . The Heart Manual n.d. www.theheartmanual.com/ (accessed 21 May 2019).
- Npowered . Chronic Pain: The Pain Management Plan n.d. www.pain-management-plan.co.uk/ (accessed 21 May 2019).
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Blickem C, Kennedy A, Jariwala P, Morris R, Bowen R, Vassilev I, et al. Aligning everyday life priorities with people’s self-management support networks: an exploration of the work and implementation of a needs-led telephone support system. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-262.
- Enabling Self Care n.d. www.enablingselfcare.com/ (accessed 21 May 2019).
- Blickem C, Kennedy A, Vassilev I, Morris R, Brooks H, Jariwala P, et al. Linking people with long-term health conditions to healthy community activities: development of Patient-Led Assessment for Network Support (PLANS). Health Expect 2013;16:e48-59. https://doi.org/10.1111/hex.12088.
- Funnell SC, Rogers PJ. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models. San Francisco, CA: Jossey-Bass; 2011.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39:1005-26. https://doi.org/10.1111/j.1475-6773.2004.00269.x.
- Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res 2005;40:1918-30. https://doi.org/10.1111/j.1475-6773.2005.00438.x.
- Forster A, Hartley S, Barnard L, Ozer S, Hardicre N, Crocker T, et al. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomised controlled feasibility trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2669-5.
- Duprez V, Van Hooft SM, Dwarswaard J, van Staa A, Van Hecke A, Strating MM. The development and psychometric validation of the self-efficacy and performance in self-management support (SEPSS) instrument. J Adv Nurs 2016;72:1381-95. https://doi.org/10.1111/jan.12918.
- May CR, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Rehm J, Üstün TB, Saxena S, Nelson CB, Chatterji S, Ivis F, et al. On the development and psychometric testing of the WHO screening instrument to assess disablement in the general population. Int J Methods Psychiatr Res 1999;8:110-22. https://doi.org/10.1002/mpr.61.
- Ustün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 2010;88:815-23. https://doi.org/10.2471/BLT.09.067231.
- World Health Organization . WHO Disability Assessment Schedule 2.0 (WHODAS 2.0), 36 Item Version 2010. www.who.int/icidh/whodas/ (accessed 25 February 2020).
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick–Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-63.
- Stewart-Brown S, Janmohamed K. Warwick–Edinburgh Mental Well-Being Scale (WEMWBS) User Guide. Version 1 2008. www.healthscotland.com/scotlands-health/population/Measuring-positive-mental-health.aspx (accessed 25 February 2020).
- Putz R, O’Hara K, Taggart F, Stewart-Brown S. Using WEMWBS to Measure the Impact of Your Work on Mental Wellbeing: A Practice-Based User Guide 2012. www.corc.uk.net/media/1244/wemwbs_practitioneruserguide.pdf (accessed 8 September 2020).
- Taggart F, Stewart-Brown S, Parkinson J. Warwick–Edinburgh Mental Well-Being Scale (WEMWBS) User Guide. Version 2 2015. www.healthscotland.com/scotlands-health/population/Measuring-positive-mental-health.aspx (accessed 25 February 2020).
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Krabbe P, Weijnen T, Brooks R, Rabin R, De Cahrro F. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht: Springer Netherlands; 2003.
- EuroQol Research Foundation . EQ-5D-5L User Guide 2019. https://euroqol.org/publications/user-guides (accessed 8 September 2020).
- Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2012;21:167-76. https://doi.org/10.1007/s11136-011-9927-2.
- Al-Janabi H, Keeley T, Mitchell P, Coast J. Can capabilities be self-reported? A think aloud study. Soc Sci Med 2013;87:116-22. https://doi.org/10.1016/j.socscimed.2013.03.035.
- Al-Janabi H, Peters TJ, Brazier J, Bryan S, Flynn TN, Clemens S, et al. An investigation of the construct validity of the ICECAP-A capability measure. Qual Life Res 2013;22:1831-40. https://doi.org/10.1007/s11136-012-0293-5.
- Flynn TN, Huynh E, Peters TJ, Al-Janabi H, Clemens S, Moody A, et al. Scoring the ICECAP-A capability instrument. Estimation of a UK general population tariff. Health Econ 2015;24:258-69. https://doi.org/10.1002/hec.3014.
- Keeley T, Al-Janabi H, Lorgelly P, Coast J. A qualitative assessment of the content validity of the ICECAP-A and EQ-5D-5L and their appropriateness for use in health research. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0085287.
- Millennium Survey of Poverty and Social Exclusion. Universities of Bristol, York: Loughborough, Heriot-Watt and the Office for National Statistics; 1999.
- Ipsos MORI. GP Patient Survey n.d. https://gp-patient.co.uk/SurveysAndReports (accessed 25 February 2020).
- Elmståhl S, Malmberg B, Annerstedt L. Caregiver’s burden of patients 3 years after stroke assessed by a novel caregiver burden scale. Arch Phys Med Rehabil 1996;77:177-82. https://doi.org/10.1016/S0003-9993(96)90164-1.
- Anderson JK, Wallace LM. Evaluation of uptake and effect on patient-reported outcomes of a clinician and patient co-led chronic musculoskeletal pain self-management programme provided by the UK National Health Service. Br J Pain 2018;12:104-12. https://doi.org/10.1177/2049463717734015.
- Hardicre NK, Crocker TF, Wright A, Burton LJ, Ozer S, Atkinson R, et al. An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for the process evaluation of a cluster randomised controlled feasibility trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2683-7.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: SAGE Publications, Inc.; 1994.
- National Institute of Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Department of Health and Social Care . NHS Reference Costs 2016 to 2017 2017. https://improvement.nhs.uk/resources/reference-costs/ (accessed 1 September 2017).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making 2012;32:733-43. https://doi.org/10.1177/0272989X12454579.
- Becker G, Kaufman SR. Managing an uncertain illness trajectory in old age: patients’ and physicians’ views of stroke. Med Anthropol Q 1995;9:165-87. https://doi.org/10.1525/maq.1995.9.2.02a00040.
- Burton CR. Living with stroke: a phenomenological study. J Adv Nurs 2000;32:301-9. https://doi.org/10.1046/j.1365-2648.2000.01477.x.
- Shannon RL, Forster A, Hawkins RJ. A qualitative exploration of self-reported unmet need one year after stroke. Disabil Rehabil 2016;38:2000-7. https://doi.org/10.3109/09638288.2015.1107784.
- Elmståhl S, Sommer M, Hagberg B. A 3-year follow-up of stroke patients: relationships between activities of daily living and personality characteristics. Arch Gerontol Geriatr 1996;22:233-44. https://doi.org/10.1016/0167-4943(96)00696-6.
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167-82. https://doi.org/10.1111/1467-9566.ep11339939.
- Faircloth CA, Boylstein C, Rittman M, Young ME, Gubrium J. Sudden illness and biographical flow in narratives of stroke recovery. Sociol Health Illn 2004;26:242-61. https://doi.org/10.1111/j.1467-9566.2004.00388.x.
- Walsh M, Galvin R, Macey C, Mccormack C, Horgan F. National Survey of Stroke Survivors: Documenting the Experiences and Levels of Self-Reported Long-Term Need in Stroke Survivors in the First 5 Years. Systematic Review: Factors Associated With Community Re-Integration in the First 12 Months Post Stroke: A Qualitative Synthesis 2013.
- English C, Hillier SL. Circuit class therapy for improving mobility after stroke. Cochrane Database Syst Rev 2010;7. https://doi.org/10.1002/14651858.CD007513.pub2.
- Sirtori V, Corbetta D, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in stroke patients. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD004433.pub2.
- McGeough E, Pollock A, Smith LN, Dennis M, Sharpe M, Lewis S, et al. Interventions for post stroke fatigue. Cochrane Database Syst Rev 2009;3. https://doi.org/10.1002/14651858.CD007030.pub2.
- Winter J, Hunter S, Sim J, Crome P. Hands-on therapy interventions for upper limb motor dysfunction following stroke. Cochrane Database Syst Rev 2011;6. https://doi.org/10.1002/14651858.CD006609.pub2.
- Coupar F, Pollock A, Legg LA, Sackley C, van Vliet P. Home-based therapy programmes for upper limb functional recovery following stroke. Cochrane Database Syst Rev 2012;5. https://doi.org/10.1002/14651858.CD006755.pub2.
- Barclay-Goddard RE, Stevenson TJ, Poluha W, Thalman L. Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst Rev 2011;5. https://doi.org/10.1002/14651858.CD005950.pub4.
- Thieme H, Mehrholz J, Behrens J, Pohl M, Dohle C. Mirror therapy for improving motor function after stroke [full review]. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD008449.pub2.
- Bradt J, Magee WL, Dileo C, Wheeler BL, McGilloway E. Music therapy for acquired brain injury. Cochrane Database Syst Rev 2010;7. https://doi.org/10.1002/14651858.CD006787.pub2.
- Fletcher-Smith JC, Walker MF, Cobley CS, Steultjens EM, Sackley CM. Occupational therapy for care home residents with stroke. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD010116.pub2.
- States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev 2009;3. https://doi.org/10.1002/14651858.CD006075.pub2.
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev 2014;4. https://doi.org/10.1002/14651858.CD001920.pub3.
- Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD002840.pub3.
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2011;9. https://doi.org/10.1002/14651858.CD008349.pub2.
- Mehrholz J, Kugler J, Pohl M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database Syst Rev 2011;1. https://doi.org/10.1002/14651858.CD008186.pub2.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-15.
- Kennedy A, Rogers A, Chew-Graham C, Blakeman T, Bowen R, Gardner C, et al. Implementation of a self-management support approach (WISE) across a health system: a process evaluation explaining what did and did not work for organisations, clinicians and patients. Implement 2014;9. https://doi.org/10.1186/s13012-014-0129-5.
- Mudge S, Kayes N, McPherson K. Who is in control? Clinicians’ view on their role in self-management approaches: a qualitative metasynthesis. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007413.
- Norris M, Kilbride C. From dictatorship to a reluctant democracy: stroke therapists talking about self-management. Disabil Rehabil 2014;36:32-8. https://doi.org/10.3109/09638288.2013.776645.
- Levack WM, Dean SG, Siegert RJ, McPherson KM. Navigating patient-centered goal setting in inpatient stroke rehabilitation: how clinicians control the process to meet perceived professional responsibilities. Patient Educ Couns 2011;85:206-13. https://doi.org/10.1016/j.pec.2011.01.011.
- Jones F, McKevitt C, Riazi A, Liston M. How is rehabilitation with and without an integrated self-management approach perceived by UK community-dwelling stroke survivors? A qualitative process evaluation to explore implementation and contextual variations. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014109.
- Mäkelä P, Gawned S, Jones F. Starting early: integration of self-management support into an acute stroke service. BMJ Qual Improv Rep 2014;3. https://doi.org/10.1136/bmjquality.u202037.w1759.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Devlin N, Shah K, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Gov.uk . Ethnicity Facts and Figures. Average Hourly Pay 2018. www.ethnicity-facts-figures.service.gov.uk/work-pay-and-benefits/pay-and-income/average-hourly-pay/latest (accessed 25 February 2020).
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost–utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Hayes RJ, Moulton LH. Cluster Randomised Trials. New York, NY: Chapman and Hall/CRC; 2009.
- National Institute of Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 25 February 2020).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377-401. https://doi.org/10.1146/annurev.publhealth.23.100901.140534.
- Chen P, Lin KC, Liing RJ, Wu CY, Chen CL, Chang KC. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual Life Res 2016;25:1585-96. https://doi.org/10.1007/s11136-015-1196-z.
- Office for National Statistics (ONS) . National Life Tables, UK: 2015 to 2017 2018. www.ons.gov.uk/releases/nationallifetablesuk2015to2017 (accessed 20 October 2020).
- Guilhaume C, Saragoussi D, Cochran J, François C, Toumi M. Modeling stroke management: a qualitative review of cost-effectiveness analyses. Eur J Health Econ 2010;11:419-26. https://doi.org/10.1007/s10198-010-0228-4.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- Sentinel Stroke National Audit Programme (SSNAP) . CCG and LHB Results Portfolio (annual) April 2017–March 2018 n.d. www.strokeaudit.org/results/Clinical-audit/Clinical-CCG-LHB-LCG.aspx (accessed 8 September 2020).
- Aiken LS, West SG, Reno RR. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: SAGE Publications, Inc.; 1991.
- Frazier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. J Couns Psychol 2004;51:115-34. https://doi.org/10.1037/0022-0167.51.1.115.
- Aguinis H, Beaty JC, Boik RJ, Pierce CA. Effect size and power in assessing moderating effects of categorical variables using multiple regression: a 30-year review. J Appl Psychol 2005;90:94-107. https://doi.org/10.1037/0021-9010.90.1.94.
Appendix 1 Workstream 1a: full report
Introduction
An understanding of the needs, experiences and priorities of those living with stroke is required for the development of patient-centred services for stroke survivors in the longer-term. 15 The lived experiences of stroke have been the focus of many qualitative studies that have allowed for an insight into the challenges stroke survivors face, including feelings of uncertainty, loss of identity, being unable to drive, the impact on social relationships and a loss of previously valued activities. 16,17,151,152 Ch’ng et al. 18 also addressed challenges over time; acceptance emerged as a critical factor in being able to cope. Although these studies provide an understanding of how stroke survivors experience their illness, they do not address their specific needs nor the factors that influence how they address their needs.
A number of survey-based studies have been conducted to assess the prevalence of problems and unmet needs among stroke survivors in the community up to 5 years post stroke. McKevitt et al. 13 found that nearly half of respondents had one or more unmet longer-term needs relating to information provision (54%), mobility problems (25%), falls (21%), incontinence (21%), pain (15%) and fatigue (43%). Over half also reported a reduction in leisure activities. A study using the LUNS questionnaire found that the average number of unmet needs amongst 850 stroke survivors was four. The three mostly commonly reported were around more information about stroke, fear of falling and difficulties with forgetfulness and concentration. 20
Survey-based studies highlight the varied needs experienced by stroke survivors, but they do not consider the meaning attached to needs and why they are important for carers and stroke survivors. There can be misinterpretations using survey measures because a study using the LUNS tool indicated that a large proportion of stroke survivors experienced no unmet needs, yet they were not ‘problem-free’. 20 Further qualitative work by Shannon et al. 153 aimed to gain greater insight into stroke survivors who have residual impairment and the reasons why they report low or no unmet needs. Findings indicated that stroke survivors negotiate their identification of unmet needs through their perceptions of their experiences. The findings also highlighted reasons why people may not identify as having needs, despite experiencing issues. These included their level of acceptance and expectations, and experiences of services. The complexities attached to understanding and addressing needs were emphasised, including some recognition that there may be a shift in how needs are experienced over time and the resultant impact on the required resources and strategies for managing. This suggests that more needs to be understood from a qualitative perspective in addition to studies of prevalence about needs and how individuals go about managing needs.
Although there is a lack of explicit focus on needs and how these are managed, evidence suggests that stroke survivors are active in their response to illness. Common strategies for managing include mobilising informal social networks, information-seeking, finding creative ways of carrying out tasks, doing things more slowly, beginning to relearn, engaging in exercise and other activities, and ‘covering up’ some of their physical disabilities. 18,25,154 There is also some indication that stroke survivors experience a biographical disruption155 before transitioning towards a biographical flow. 156 Together, this evidence challenges the notion that stroke survivors are passive recipients of care. Once stroke survivors return home, they often start to actively manage the challenges they face as part of their recovery following the stroke (as do carers in many cases).
Although a general understanding of beneficial strategies have been highlighted, there is limited research exploring the barriers to and facilitators of addressing needs, particularly in the longer term. Sumathipala et al. 15 provided a greater understanding of perceived longer-term needs among those between 1 and 11 years post stroke and the barriers to and facilitators of functioning, rather than a range of different needs. In their qualitative synthesis, Walsh et al. 157 categorised the barriers to and facilitators of re-integrating into the community into four main themes: the primary effects of the stroke (e.g. physical and communication difficulties), personal factors (e.g. emotional challenges, perseverance), social factors (e.g. sense of belonging vs. perceived stigmatisation) and those related to professionals (e.g. being supported vs. being abandoned). These, however, were limited to stroke survivors within the first year post stroke
The current evidence indicates that understanding and addressing needs is a complex picture that warrants further investigation. This qualitative study aims to explore stroke survivors’ longer-term needs from their own perspectives, with attention to the barriers and facilitators that are influential in whether or not their needs are addressed, and the stroke survivors’ levels of participation. 14 The findings will inform the intervention mapping process, for developing the longer-term care strategy.
Methods
Aims and objectives
The objectives of the study were to:
-
gain further insight into specific longer-term needs (e.g. type of information)
-
explore the barriers to and enablers of the behaviours that affect longer-term needs and participation
-
explore how stroke survivors and caregivers develop strategies for managing problems/resolving the issues that they face post stroke.
Study design
This was a qualitative study involving semistructured interviews to explore participants’ longer-term experiences at two different time points post stroke. Two researchers conducted all the interviews between November 2013 and April 2014, and the data were analysed using a thematic approach. 21
Ethics approval
This study has NHS permission and was approved by the North Wales Ethics Committee (reference number 13/WA/0301).
Participants
Semistructured interviews were conducted with two groups of community-dwelling stroke survivors and their carers. One group of stroke survivors (group 1) was interviewed when they were between 9 and 12 months post stroke. A separate group (group 2) was interviewed when the survivors were at least 24 months post stroke. Across all of the interviews, stroke survivors’ needs, and the barriers to and facilitators of addressing their needs, were explored. Gaining an understanding of experiences at two different time points allowed ongoing needs to be identified. It also provided an opportunity to reflect on what had been useful and where improvements could be made.
Although the study primarily focused on longer-term needs after stroke from the perspective of the stroke survivor, it is acknowledged that carers are an important part of improving longer-term outcomes. Therefore, carers of stroke survivors who expressed an interest in participating were also invited to take part.
Eligibility criteria
Stroke survivors were eligible for the study if they:
-
had a confirmed diagnosis of new stroke
-
were either between 9 and 12 months post stroke or between 24 and 36 months post stroke
-
were residing in the community
-
were able to provide informed consent (or be consented via a consultee).
Stroke survivors were excluded if they were residing in a nursing or residential care home, or if their main requirement was palliative care.
Carers were eligible if they:
-
were identified by the stroke survivor as the main informal carer who provides help and support (practical and/or emotional) at least once a week
-
could provide written informed consent and consultee declaration for the stroke survivor (when appropriate).
Carers were excluded if the stroke survivor did not consent to taking part.
Recruitment
Across both groups of interviewees, potential participants were identified from an established research register of stroke survivors, held by the Academic Unit for Ageing and Stroke Research at Bradford Teaching Hospitals NHS Foundation Trust. At the time of recruitment, the database held information on > 300 stroke survivors between 0 and 50 months post stroke who consented to inclusion in this database while they were in hospital and agreed to be contacted regarding participation in future research at the Academic Unit for Ageing and Stroke Research. Using this database, carers can be contacted via the stroke survivor.
Potential participants were identified from the established database. Following checks to assess their survival status and living circumstances (e.g. in a care home or living in the community), study information sheets, consent to further contact forms, stamped addressed envelopes and a questionnaire pack were posted out to eligible participants. The questionnaire included the LUNS monitoring tool20 and the Barthel Index19 to assess their level of independence. It also provided the opportunity to collect data on age and living circumstances (i.e. alone/with carer).
Interested stroke survivors were asked to return the consent to contact form in the stamped addressed envelope provided. Potential participants were informed that not all those returning forms would be involved in the study, and their standard of care would not be affected by taking part. The details of those who returned their forms were entered into a database. Maximum variation purposive sampling was used to guide the selection of participants based on age, level of deprivation (indices of deprivation), living circumstances (alone/with carer), level of independence (Barthel Index) and number of unmet needs (assessed by LUNS monitoring tool).
Following identification of potential participants, a researcher telephoned carers who consented to being contacted and explained the study. If participants were willing to take part, an interview was arranged. At this point, they were also asked if they would like the carer to take part in the interview. If the stroke survivor wanted the carer to take part, information was sent via post for them to consider prior to the interview. Carers confirmed whether or not they would like to participate on the day of the interview. When carers opted to participate in the study, they were given the option to be interviewed with or separately from the stroke survivor.
Interview topic guide
The topic guide was devised from themes in the existing literature and discussions with stroke survivors and their carers. It was also informed by the aims of this study, and therefore sought to gain an understanding of needs in the longer term post stroke and the barriers and facilitators that stroke survivors face in addressing their needs. Topics included life before the stroke, the consequences of the stroke (e.g. emotional, physical and social), life after their stroke and the factors that hindered or enabled how their impairments/difficulties were managed.
The same topic guide was used across the two groups to ensure that all stroke survivors were asked similar questions, enabling comparisons during the analysis process. It also allowed for some flexibility based on the carers’ responses, and further questions could be asked to gain richer, detailed accounts of their experiences.
The topic guide was structured to put the participants at ease, starting with questions such as ‘could you tell me a little bit about yourself?’ and ‘how would you describe yourself before the stroke happened?’. Following these, questions were asked about the impact of stroke from the point of returning home, to life at the point of interview, then thoughts about the future. Stroke survivors were asked directly about their needs, for example ‘what were your needs at this point?’ and ‘how have your needs changed?’. They were also asked about their needs less directly in questions such as ‘what do you need support with?’. To attend to the barriers and facilitators, questions such as ‘what has helped the process of adapting to changes?’ and ‘what has hindered this process?’ were asked.
Data collection and consent procedures
Interviews were conducted face to face in the stroke survivors’ own homes. Prior to commencing interviews, the purpose of the study was explained again and participants were asked if they had read the information sheets and had the opportunity to ask questions.
Written consent for stroke survivors and carers was obtained face to face during the interview visit. If stroke survivors were unable to read or sign the consent form due to impairments, but had the capacity to consent, the consent procedure was witnessed by a carer or significant other. If stroke survivors lacked capacity to consent, consultee declaration was provided by the carer. It was made clear to participants that they had the right to withdraw at any point and that their personal information would remain confidential.
Once the appropriate consent procedures were completed, interviews commenced following the structure outlined in the previous section. In all cases, regardless of whether or not carers were present, the questions remained focused on the stroke survivors. However, carers were free to contribute as they wished. To ensure that stroke survivors were given the opportunity to express their views, interviews were adapted for those with communication difficulties by writing down keywords and adapting questions in accordance with their needs.
At the end of each interview, participants were thanked for their time and asked if they had any further questions. The researcher also ensured that they were provided with contact details in case they had any further questions or wanted to withdraw from the study.
Data analysis
All interviews were audio-recorded and transcribed verbatim. A thematic analysis was undertaken drawing on phases proposed by Braun and Clarke:21 (1) familiarisation, (2) generating initial codes, (3) searching for themes, (4) reviewing themes, (5) defining and naming themes and (6) producing the report. However, adaptations to the traditional analysis process were made between phases 4 and 5 to allow for an understanding of carers’ needs and the barriers to and facilitators of addressing needs, rather than a thematic description of experiences.
The transcripts were analysed in two categories (those who were 9–12 months post stroke and those who were > 24 months post stroke). They were separated to establish any differences in the experiences at different times post stroke.
Familiarisation
All participants were provided with pseudonyms and any identifiable data were removed from the transcripts. All transcripts were read and re-read as part of the familiarisation process.
Generating initial codes
A data-driven approach was adopted to avoid losing a contextual understanding of experience. Line-by-line coding was conducted on each transcript, using an active voice to ensure that codes remained close to the text.
Searching for themes
Emerging ideas were documented alongside the line-by-line codes to establish similarities and differences in each transcript. For each transcript, data segments from the initial coding were organised into meaningful groups, supported with memos. Thematic maps were created (one for each transcript) to establish inter-relationships between the codes and themes for each participant.
Reviewing themes
The themes identified in each transcript were considered in terms of whether or not they captured the essence of what was in them. Progression to across-case analysis involved looking across the data set from each interview participant to establish the similarities and differences in the experiences of stroke survivors in the longer-term post stroke. An overall thematic map was developed for each time point, documenting where themes inter-related. Documenting the research process in this way enhanced dependability and confirmability. 22
Defining and naming themes
As the thematic analysis progressed and the themes were refined, the focus was placed on ‘needs’ identified in the stroke survivors’ and caregivers’ narratives and the barriers to and facilitators of addressing needs. For the purpose of this analysis, needs were defined as those that the individual see as a challenge to overcome or address. These carry importance because of the meaning associated with them. In some cases, these needs were dyadic and co-constructed by the stroke survivors and the carers.
A secondary analysis was conducted that involved re-examining the themes for each participant and developing a series of diagrams to establish needs, and the barriers to and facilitators of addressing needs, at each of the two time points.
Standard approaches to demonstrating trustworthiness and quality in qualitative research were used, including the clear documentation of the research process (methods, analysis and any problems encountered and solutions found). The software program NVivo 10 was used to store, organise and code the data. Memos concerning coding and emerging themes and theories were also recorded throughout the analysis process. Throughout data collection and analysis, data, codes and emerging categories and theories were presented to and discussed with the Study Management Group and PMG at regular intervals. The emerging findings were also presented to stroke professionals, as well as to stroke survivors and their caregivers in the CRAG. Comments received were considered alongside the ongoing analysis.
Results
A total of 166 questionnaires were sent out to eligible stroke survivors from the established databases. Questionnaires were returned by 95 (57.2%) stroke survivors. Twenty-eight stroke survivors were purposively sampled to the study from November 2013 to April 2014. Their 11 caregivers (eight wives and three husbands) also participated. Thirteen of the stroke survivors were between 9 and 12 months post stroke and 15 were between 32 and 47 months post stroke. Interviews lasted between 43 and 105 minutes. All stroke survivors spoke English. Eleven caregivers took part in the interviews; in all cases, interviews were conducted together. The caregivers’ contributions to the interviews were considered in the analysis process. However, their input was largely confirmatory about the caregivers’ perceptions of the stroke survivors’ experiences, rather than a rich insight into their own experiences. For this reason, the needs remain focused on the stroke survivor or any shared needs rather than caregiver-specific needs. Table 8 outlines the characteristics of the stroke survivors included in the study sample.
| Name (pseudonym) | Age (years) | Time post stroke (months) | Barthel/LUNS | Living circumstances | Summary of life pre stroke (as reported in interviews) | Stroke impairments | Summary of life after stroke (as reported in interviews) |
|---|---|---|---|---|---|---|---|
| Marilyn | 70 | 43 | Moderate 12/low needs 0 | Lives with husband | She and her husband were ramblers, went line dancing |
|
Watches television occasionally, goes to watch line dancing, attends weekly exercise group for multiple sclerosis |
| Mavis | 60 | 43 | Moderate 17/low needs 1 | Lives with husband |
|
|
Watches television, goes out to the theatre and cinema, goes out for meals |
| Gordon | 63 | 38 | Moderate 17/multiple needs 4 | Lives with wife and adult son | Working delivering prescriptions, used to be happy |
|
Volunteers as a hospital appointments driver, listens to music, would rather be at home than go out socially |
| Ellie | 90 | 37 | Moderate 18/multiple needs 3 | Lives alone | Has been a widow for > 20 years; her husband died of cancer | Numbness on left side | Goes shopping, sees one or two friends, plays Scrabble, still able to drive, goes to church |
| Roger | 74 | 42 | Independent 20/multiple needs 3 | Lives with wife |
|
|
No longer able to drive, does exercises everyday, swims once a week, goes for a walk, gardens and goes for days out with wife |
| Robert | 84 | 34 | Moderate 13/low needs 0 | Lives alone |
|
|
Goes shopping, does not drive quite as far, sees his brother regularly, spends time gardening |
| Michael | 63 | 39 | Severe 9/multiple needs 2 | Lives with wife |
|
|
Goes out for a meal occasionally, watches television, spends most of his time in the house, can no longer drive |
| Lizzy | 72 | 32 | Independent 20/multiple needs 6 | Lives alone |
|
|
Belongs to the British Sugarcraft Guild, gardens, listens to music and does not drive as far |
| Jimmy | 69 | 47 | Independent 20/low needs 1 | Lives with wife and daughter | Retired from working as an accounts manager, did not have an active hobby, did a lot of reading, spent time with daughter who has Down Syndrome |
|
Attends stroke groups with his wife, goes to an active life class, can no longer drive. Spends time at home with his wife |
| Iris | 76 | 40 | Moderate 18/multiple needs 6 | Lives alone |
|
|
Takes care of her home, leads dances now she is unable to participate, uses computer regularly, occasionally goes out with friends |
| Daphne | 78 | 32 | Independent 19/low needs 1 | Lives with husband | Played bowls, did the gardening and housework |
|
Returned to playing bowls, does the gardening, does the housework, knitting |
| Cedric | 75 | 36 | Moderate 16/multiple needs 9 | Lives with wife | Going away in the caravan with his wife |
|
Goes away on short mini breaks with his wife in the UK, attends an IT class |
| Carla | 54 | 36 | Severe 10/multiple needs 12 | Lives alone |
|
|
No longer works, spends a lot of time in her house, does not drive any more, spends time with boyfriend |
| Bob | 73 | 43 | Independent 20/low needs 1 | Lives with wife | Retired from teaching, spent time looking after young granddaughter, in the process of selling their house, role as the cook | Some speech impairment, lost confidence and motivation, lacks strength in legs | Able to drive again, does the gardening, goes on holiday with his wife |
| Arnie | 75 | 34 | Severe 2/multiple needs 6 | Lives with wife | Prior to first stroke of six, he was president of the rugby club, worked full time lecturing, had busy social life | Impaired speech, impaired mobility, impaired vision, short-term memory loss, weakened right side, walking difficulties, uses stick and wheelchair | Can no longer drive, spends time with young grandson, goes for days out, watches television, reads the paper |
| Timmy | 66 | 9 | Moderate 15/multiple needs 4 | Lives alone | Working as an engineer |
|
Goes walking every day, sees his family regularly. Spends a lot of time at home |
| Paddy | 53 | 9 | Independent 20/multiple needs 7 | Lives with wife, son and daughter |
|
|
Spends most of his time at home, watches television, goes for the occasional walk to the local shop |
| Maude | 79 | 10 | Independent 19/multiple needs 5 | Lives with husband | Attended U3A group, did the cleaning and shopping |
|
Able to continue as she did before |
| Malcolm | 71 | 12 | Independent 20/low needs 1 | Lives alone | Going to car rallies, drinking at the social club, socialising | No physical impairment | Goes walking near his house, still goes to social club but does not drink alcohol, still goes to car rallies |
| Lisa | 54 | 9 | Moderate 16/multiple needs 11 | Lives with husband | She and her husband did not work prior to the stroke; spent a lot of time at home looking after the grandchildren |
|
Majority of time spent at home, still sees family, goes out occasionally |
| Julia | 84 | 10 | Moderate 18/multiple needs 2 | Lives with husband |
|
|
|
| Jane | 82 | 10 | Independent 20/low needs 1 | Lives alone | Did activities such as camping, knitting, bowls | No physical impairment | Continues with all her hobbies as before, spends time looking after the house |
| Evelyn | 82 | 9 | Moderate 18/multiple needs 5 | Lives alone | Widowed, spent time drinking with husband, used to go to bowls, did the house work | Weakened left side initially, balance affected, experienced depression, fatigue | Goes shopping with her son, goes out to lunch with friends, does the crosswords, reads the paper, does some housework |
| Denise | 47 | 11 | Independent 20/multiple needs 1 | Lives with husband |
|
|
|
| David | 76 | 10 | Independent 20/low needs 0 | Lives with wife | Went on holidays with wife, had a hotel, helped son out with his business |
|
Still goes on holidays with wife, goes walking, spends time with family |
| Charlie | 62 | 10 | Independent 19/multiple needs 19 | Lives with wife | Worked in a recycling plant, loved watching rugby, spent a lot of time socialising |
|
|
| Cathy | 54 | 11 | Independent 20/multiple needs 8 | Lives with teenage daughter | Worked full time as a teaching assistant, was a governor at the school |
|
|
| Alfred | 78 | 9 | Independent 19/multiple needs 2 | Lives with wife | Played table tennis five times a week, spent time reading |
|
Goes to the gym, walks at least every other day, no longer able to drive, goes on holidays, reads novels |
Identified needs
Stroke survivors experienced 13 needs related to different aspects of their experience. For the purpose of this study, needs were defined as something meaningful for stroke survivors and their caregivers in terms of the impact on ‘doing of everyday life’ and significance for who they are as a person (sense of self).
The 13 needs identified were (1) managing and coping with a major life event, (2) gaining control, (3) managing emotions, (4) doing everyday tasks around the house, (5) working towards physical and functional improvement, (6) managing hidden consequences of stroke, (7) obtaining usable information, (8) sustaining flexible support networks, (9) engaging in meaningful activity, (10) overcoming financial concerns, (11) maintaining relationships, (12) reconstruction of identity and (13) managing beyond the home.
Some differences were recognised between those who were 9–12 months post stroke and those who were > 24 months post stroke. Feelings of hope, in terms of the need to work towards physical functioning, were more common amongst those who were 9–12 months post stroke. At this time point, stroke survivors more often talked about establishing a cause for their stroke as part of understanding and making sense of their situation. This was attached to the need to gain control.
Alternatively, more of those who were at least 24 months post stroke talked about the process of reaching acceptance. This was important for addressing many of the needs, including doing everyday tasks around their house, managing and coping with the major life event, managing the hidden consequences of stroke, reconstruction of identity and maintaining relationships. Aside from the slight differences at each time point post stroke, much of their talk around needs and managing these needs remained similar across the two time points.
This section outlines each of the 13 needs and the barriers to and facilitators of addressing needs that are documented in Figure 2.
Managing and coping with a major life event
The stroke had affected the survivors and their caregivers to some extent. Some were more affected initially, but quickly reached a point where they were continuing as they did previously, for example Jane and David. Others took longer to cope and manage with the effects of the stroke across different aspects of their lives, for example Carla. For the majority of the stroke survivors, the stroke was viewed as unexpected and shocking and caused some level of disruption to their own, and their family’s, lives, even temporarily. Managing and coping thereafter is a longer-term issue; therefore, stroke survivors and their caregivers needed to be able to find ways of managing and coping in terms of moving forward from this major life event.
Stroke survivors and their caregivers adopted many beneficial strategies (‘facilitators’) for coping with the ‘major life event’. Making comparisons with others was an interesting strategy as this can have both negative and positive consequences for stroke survivors and their caregivers. For some, making these comparisons enabled some realisation that they are lucky to be alive or that their difficulties are not as bad as they could be. This left them feeling fortunate, despite experiencing a major life event:
I was so delighted to be OK when I’d heard of people who are, you know, disabled or speech problems and all sorts of things that you hear after and I was so grateful that I was very happy, actually.
Julia
In contrast, some stroke survivors felt that they were not doing as well as they could be when they compared themselves with others. Marilyn felt this way when she compared herself to someone who had experienced a milder stroke:
Yeah, and I said to [husband] ‘oh, she’s walking already’ and he said ‘yeah, but her stroke weren’t as serious as yours’.
Marilyn
Having a sense of humour and a positive attitude were important facilitators for coping with life after stroke. Being able to achieve a positive outlook was associated with re-evaluating their lives. After her stroke, Evelyn became depressed, but through re-evaluating her situation and realising she had something to live for, she coped better. There was also a sense that a stroke survivor’s outlook was influenced by their personality prior to the stroke. For example, Denise described herself as determined and expressed that she is not one for ‘moping around’. She acknowledged that another stroke could happen, yet she is willing to take a risk and actively continue with her life rather than passively sitting at home:
So what do you think’s helped you do so well since the stroke?
I don’t know really, probably because I’m just determined to get on with things, I’m not one for sitting around moping so . . . And I’m sure I could have gone other way if, you know, I could have been getting up and going out and doing things, then I could have had another, I’m not doubting that but I’d still rather that chance and get on with life than sit around waiting for it.
Not dwelling on the stroke was another strategy adopted by some of those who were 9–12 months post stroke, for example Timmy, Denise, Jane and Evelyn. Jane did not feel the need to look back in terms of the stroke because she knew how lucky she had been. By taking this approach, these stroke survivors saw it as a way of moving forward and not focusing too much on the event itself. Although this is a positive approach to managing for some of the stroke survivors, others struggled to project too far into the future, especially when their circumstances were more unpredictable. This unpredictability occurred among those who were more impaired by the stroke and faced uncertainty in the extent of their recovery. Taking each day as it comes was a way of managing this uncertainty to some extent. Paddy has experienced three strokes since 2007; since having these, he no longer plans or thinks about the future.
Acceptance was another strategy that stroke survivors and their caregivers employed to cope with the stroke and the changes to their lives. Those who were at least 24 months post stroke talked about this more during the interviews than those who were 9–12 months post stroke. Acceptance was discussed in the context of different aspects of their recovery and in terms of coping overall with the major life event. Cedric and his wife recognised the importance of acceptance and took the view that this is something you have to ‘learn’ to do as part of a process over time:
Oh you’ve to accept it, you’ve to have a positive outlook, and you have to learn to accept it.
Cedric’s wife
Being unable to reach acceptance can be a barrier to managing and coping. Carla was 3 years post stroke at the time of the interview, but she openly stated ‘I still haven’t accepted it’. She made a distinction between realisation and acceptance:
And when do you think you started to sort of realise things would be different?
When did I realise or when did I accept?
Yeah, well whichever sort of suits?
I realised just how little I, not how little, no, how different it was all, what, how different my life is going to be when I didn’t succeed at going back to work. Because work’s a great healer, int it? You go to work you can forget it, can’t you? But no.
Failing to successfully return to work allowed her to realise that her life was going to be different, but she still struggled to accept it.
Gaining control
During the time that stroke survivors spend in hospital, control is in the hands of medical professionals, and stroke survivors become passive agents of information and support. Caregivers have not always fully taken on their roles and much of the responsibility for the stroke survivor is out of their hands. The transition from hospital to home can lead to a loss of control for both the stroke survivor and their caregivers (or other family members) through having to manage the uncertainties that living with life after stroke brings. The key areas discussed among the stroke survivors and their caregivers around control included the need to feel able to manage and the need to address uncertainties (e.g. fear of the stroke happening again and understanding why the stroke happened).
With regards to managing, there was often a mismatch between stroke survivors and caregivers in the transitional period from hospital to home. For example, stroke survivor Bob wanted control by coming home from hospital, but his wife felt that this transition would lead to a loss of control. This is when she would fully embark on her caregiving role and she was unprepared at this stage:
Again, I suppose it’s the control situation, isn’t it, of whichever side you’re of, you wanted to be in control in coming home and I wanted somebody else to be in control because I didn’t feel in control.
Bob’s wife
Both stroke survivors and caregivers needed to feel a sense of control; however, there were differences in what allowed them to feel in control. It was common for both caregivers and stroke survivors to feel abandoned and dismissed once they left hospital because of the lack of support. Marilyn’s husband would have liked someone to visit following her stroke to provide reassurance about managing initially:
You’re guessing a bit on what you’re doing, whereas you feel you need some reassurance to, for somebody to either say ‘no, don’t do that’ or ‘no, that’s fine, you keep doing that’, isn’t it?
Marilyn’s husband
Seeking and building support networks helped some of the caregivers and stroke survivors to manage once the formal support had diminished. Engaging with this support helped them to cope and feel more in control. Charlie’s wife sought informal support from their daughter when she struggled with her situation:
I get very stressed, I get very upset, I get very emotional, my daughter gets it all [laughs].
Charlie’s wife
The lack of control experienced by stroke survivors and their caregivers was also driven by the possibility of another stroke. Those who were 9–12 months post stroke still expressed fears about another stroke:
Yeah, she sounds nice. So do you worry about having another stroke?
Yeah, I do. What causes strokes?
However, this expression of fear was less common among those who were > 24 months post stroke. The stroke survivors were often unable to gain an accurate prognosis about the likelihood of re-occurrence, which added to their uncertainties. To attempt to gain control over their unpredictable situations (particularly amongst those who were 9–12 months post stroke), stroke survivors established their own cause to the stroke. Some looked to external events to explain why it happened and others considered the possibility of lifestyle choices. When possible, some of the stroke survivors actively changed their health behaviours. For example, Evelyn consciously cut down her drinking following the stroke in an attempt to reduce the chances of this happening again.
Managing emotions
The degree of emotional impact for the stroke survivors and their caregivers varied greatly. Some were really struggling, whereas others felt that the stroke had left them with no emotional impacts at all. Some of the emotions felt by the stroke survivors included shock, fear, anger, worry, anxiety, stress, depression and guilt. Some of the caregivers also experienced many of these emotions, particularly stress and strain as a result of taking on their new role.
A distinction can be made between emotional responses to the actual event and emotional responses to living with and managing the impairments and impacts of the stroke. There was a need to be able to find ways of managing, as, for many, these emotional responses were ongoing and still apparent up to 3 years post stroke. Examples of the emotions experienced by stroke survivors and their caregivers are discussed in the following distinct categories, in addition to the barriers to and facilitators of addressing these.
Initial emotional responses to the stroke
As documented in the previous theme about gaining control, fear and worries about re-occurrence of stroke were common and led to stroke survivors establishing their own cause of the stroke.
Depression was also felt by some stroke survivors. Cedric experienced this in the initial period post stroke. He described being close to suicide until he got antidepressants, which his wife said gave him a ‘lift’. In many cases, it is difficult to distinguish between depression caused by the stroke and depression as a response to the stroke. At the time of the interview, he was around 3 years post stroke, but he has continued to take antidepressants; these, alongside his positive attitude, allowed him to overcome the depression.
Emotional responses to living with and managing the impairments and impacts of the stroke
Living with and managing the consequences of the stroke evoked stress among some of the stroke survivors. Carla, for example, has become more tired since her stroke and often has bad days, meaning she is vulnerable to becoming more stressed. Seeing themselves as an agent helped the management of stress. Carla learned to acknowledge that, on a bad day, she needs to employ strategies to manage her stress levels. For example, she avoided picking up the telephone if she felt that she would become stressed. It is important for stroke survivors to be able to be aware of their emotional state to employ such strategies:
I mean, if I’m having a bad day or I’m tired and I know I, if the phone rings I just don’t answer it. So I do keep myself out of situations that I could get stressed in, so yeah I suppose in a way I have started to do that, yeah.
Carla
Mavis provides an example of the ‘everydayness of managing’ in the context of her relationship with her husband. She started to take back some of the jobs from her husband, which has facilitated a reduction in her feeling sorry for herself. This was beneficial for him as he suffered with depression and became exhausted by the burden of taking on extra duties alongside work.
Caregivers also experienced negative emotions including depression and feelings of stress, as a result of taking on their new roles. To cope with the strain, caregivers often devised strategies. For example, Arnie’s wife ‘takes time out’ by purposefully getting up early before preparing everything for him:
Yeah. I’m up at the crack of dawn, I mean this morning I was up at 20 past four, I like to get, that’s my time of day with meself and I have me coffee and me fag and I prepare everything.
Arnie’s wife
Many caregivers and stroke survivors lacked emotional support, which made managing emotions more difficult. When possible, some carers built their own informal support networks to cope with some of the strain attached to caring. They also recognised useful avenues of formal support (e.g. counselling); however, this was not freely available.
Doing everyday tasks around the house
Many stroke survivors (e.g. Paddy and Gordon) spent more time in their homes than they did before their stroke, as a result of being physically impaired or becoming resigned to this way of living. It was common for stroke survivors to be motivated by the need to function to a point where they could carry out purposeful tasks, for example making food or getting dressed or washed. For some ‘house-proud’ individuals, being able to carry out tasks was important for maintaining a well-kept home environment. For others, this was important as it formed part of their daily routines, which often centred on home-based activities.
Building a support network was important in cases where individuals were unable to carry out household tasks independently (e.g. cooking, vacuuming, washing). Timmy, for example, had support from his daughter when he first had his stroke as a way of coping with the demands of running his home. This was appropriate for him initially and eventually he was able to manage on his own, but just did things at a steadier pace:
So can you remember what you were doing on a typical day when you first got home from hospital?
Well me daughter stopped with me for a few days to make sure I could do things, and I was doing things gradually. Well, like I said, I had them, well ready meals just you put in the microwave, so I just had them to do, you see, but coming from the oven on this side onto the breakfast was enough.
Building a sense of togetherness was also important for some of the stroke survivors and caregivers. For example, stroke survivor Lisa and her partner worked together in managing the home environment since she had become physically disabled by the stroke:
. . . So I do the cleaning up and stuff like that, anything that needs polishing . . . or washing floors down, I do all that, then Lisa will do like surfaces that can be done while you’re stood, Lisa will do those, so yeah, yeah Lisa does what she can and then yeah I just do the other stuff.
Lisa’s husband
Encouragement from a partner was also useful for some of the stroke survivors as they managed tasks around the home. Jimmy’s wife let him try tasks before stepping in to support him. Confidence was also influential in whether or not some stroke survivors could carry out everyday tasks around the house. Cathy did cooking in hospital before she was allowed home. She perceived this as an exam that gave her the confidence to feel prepared for her return home. Repeatedly carrying out tasks without assistance increased her confidence:
Was there certain things you did to prepare or were you just ready . . .?
I said, ‘once I’ve passed those exams in hospital, I’ll be alright’, you know and then they put me in a room, quite a big room that was carpeted for like home-from-home, so you’re on your own and you have to do everything yourself, so when I’d done that for about 4 weeks they thought, ‘oh she must be OK’.
Some stroke survivors talked about acceptance in the context of knowing that jobs around the house take longer and they commonly managed daily tasks by pacing or creatively problem-solving. It was also common among the stroke survivors to start to see themselves as agents in their adjustment and recovery. This involved taking some responsibility for their actions and recovery process. For example, Paddy purposefully uses his weak arm in tasks of daily living (e.g. shaving) to be able to manage day to day and facilitate improvements.
Caregivers being overprotective was sometimes a barrier to stroke survivors carrying out tasks around the home. Caregivers were inclined to be most protective in ‘risky’ areas around the home, for example the kitchen and the bathroom. These were places they associated with greater risks of falls or injuries.
Working towards physical/functional improvement
Many stroke survivors in the sample were physically affected by the stroke to some extent. Some (e.g. Cathy) were unable to walk without a walking aid. Others experienced numbness in their limbs or loss of function/co-ordination (e.g. Robert). Some had no physical impairment at all (e.g. Jane). Stroke survivors who had been affected physically by the stroke demonstrated a need to continue improving once the formal rehabilitation ended. These improvements were often recognised in the context of their abilities to carry out purposeful tasks or meaningful activities and were important for gaining some normality.
A number of facilitators of improvement were identified among the stroke survivors. Creative problem-solving helped enable physical functioning, despite physical difficulties. Marilyn and her husband had an exercise bike that she was unable to use using conventional methods, but her husband found a beneficial way for her to adjust her technique, so she could do her exercises:
Well, because what we do is, we turn it the other way round, ’cause Marilyn can’t sit on it, so I turn it with the handlebars facing her and then she can pedal it sat in her chair the other way round.
Marilyn’s husband
Some stroke survivors also talked about the encouragement they gained from significant others/partners/friends/peers. Some caregivers suggested exercises for the stroke survivors, and prompted movement either when formal therapy ended or when this was never provided:
My husband’s very good, like I said, he thought of the ball idea and he’d say to me, ‘lift your leg, lift your leg’, every so often, ‘lift your leg, keep doing that’, you know, and ‘move your foot, bend your foot like that and that, push it up and down’ so many times a day . . .
Mavis
Among those at 9–12 months post stroke, hope was important, particularly when improvements were uncertain:
Oh, I hope so, yeah. I hope my legs will get working better, that’s the only thing to go now.
Cathy
It was not as common for those who were at least 24 months post stroke to remain hopeful because they had managed for longer and come to terms with the extent of improvement in their recovery. These individuals may have reached a new sense of normal at this stage with regards to their physical functioning.
Motivation was an important facilitator for physical improvements. Those who were highly motivated recognised improvements and emphasised the importance of continued improvement be able to carry out meaningful tasks. On the other hand, difficulties recognising improvements were a barrier to continuing to work towards improvements. If stroke survivors failed to recognise improvements, they became disheartened and lacked motivation. For example, Marilyn could not see that she had improved, despite her husband telling her. She needed reassurance from a professional to be able to see that she was doing ‘alright’:
I’m alright once I went to physio[therapy] and they say ‘yes you are’, you know, ‘you’re doing alright’, if they say I was doing alright, I mean, I felt totally different then about it all.
Marilyn
Unfortunately, the input Marilyn received from the physiotherapy team ended, which made this situation more difficult, as her husband could do little to persuade her that she was improving. This is closely linked to another barrier: the perceived lack of physical rehabilitation support. Some stroke survivors thought that they could have benefited from more input from physiotherapists to facilitate a better physical recovery.
Managing hidden consequences of stroke
Many stroke survivors experienced consequences of the stroke that could be considered more ‘hidden’, for example difficulties with concentration and processing of information, memory impairments and mood swings. These were things that were often hidden from caregivers, the public and health professionals. They were also sometimes hidden from the stroke survivors if they were unable to recognise changes in themselves, for example a change in temperament. Stroke survivors needed to find ways to manage these hidden consequences.
Creative problem-solving facilitated the management of hidden consequences. For example, Denise found it useful to write things down to cope with the demands of her job at the opticians. By using this strategy, she did not feel that her memory loss interfered with her working duties:
So it doesn’t interfere with anything at work, like, it’s not a problem or anything if you don’t remember something?
No, because I have a little tray and I write things down and put notes in me little tray.
Acceptance was important for coming to terms with the longer-term consequences of stroke, such as difficulties processing information, and the resultant impacts that these can have on their lives. Driving is an example of an activity that many stroke survivors struggled with as a result of both physical and cognitive impairments. Carla experienced ‘hidden’ consequences, for example problems with spatial awareness and processing of information, that contributed to her decision to stop driving. Her doctor helped her to realise that she must be truthful and face her circumstances following the stroke. She accepted that she could not deal with the physical or mental side of driving, although she still misses this aspect of her pre-stroke life.
Roger also experienced difficulties with his ability to process information. Building a support network was a way in which he and other stroke survivors have managed this problem. Rogers’s wife wrote instructions for him for when he is using the printer. This worked to some extent, but he often put the instruction card away, meaning his wife had to prompt him to get this out:
I’ve written a little notice by the printer, telling him which order to do everything, but he puts the card away. [Laughs] I said if I write it out I’ll know which way to do it but it’s alright, when we need I say, ‘shall we get the card out?’, you know.
Roger’s wife
Although there are positive ways in which some of the stroke survivors have managed, they also faced barriers to managing at times, one of which was difficulty recognising changes as a result of the stroke. Roger has also had impaired concentration since the stroke, yet he does not recognise this. He and his wife experience conflict around his driving abilities, as he thinks that, if he was physically fine, he would be able to drive. His wife has noticed his lack of concentration, yet this is hidden from him. This makes things difficult because he is unrealistic about what he can still do:
Mm, so for you it’s all about getting your feet working?
Feet, yes. I mean if a car was the opposite way round I could drive it, I’m sure I could.
It’s the concentration though Roger, you don’t see things quite the same way.
Well, you and I disagree on this one.
Experiencing a lack of control over difficulties such as mood swings and irritability can also serve as a barrier to managing these issues. For example, Carla experienced these problems for some time following her stroke. She often became very irritable but felt that she could not stop herself. Over time, she is more aware of these difficulties and can control these changes in mood more easily than she did initially. She can keep her emotions in check, but it doesn’t happen as much as it used to. This is something she talked about as a past behaviour and on reflection described this as ‘horrid.’
Stigma was also problematic in managing the hidden consequences of stroke. Lizzy experienced ‘public stigma’ as she found that people struggle to see that she is ill and associated her stammer from the stroke with being drunk:
Well it’s this problem that people can’t see that you’re ill, you know, they don’t realise that, it’s a bit like being deaf, they can’t see you’re deaf, they can’t see your speech is trouble, so they don’t know whether you’re, whether you’ve got a stammer or whether you’re drunk!
Lizzy
When she is out in public, she prefers that people know what her problems are, so that they cannot make these assumptions.
Obtaining usable information
There was a general sense of negativity attached to the timing and content of the information that was given to stroke survivors and their caregivers in hospital. Despite this, stroke survivors and caregivers expressed a need to obtain usable information. The extent of usability was dependent on different circumstances, preferences and needs. Some of the stroke survivors expressed a preference for more specific information to meet needs such as managing incontinence and making lifestyle and dietary changes. Some also wanted to be more informed about what to expect following the stroke.
An example of a common barrier to finding the information usable initially was ‘information overload’. Some of the stroke survivors felt that they had been bombarded with information to the point where they failed to look at it:
Oh yeah, you get all sorts of information, leaflets you know, but you don’t tend to take it all in because you get that much of it, you know.
Malcolm
Others found the information unsuitable because it was specific, too general or too obvious.
Timing of information was also problematic for some stroke survivors and their caregivers. Lisa and her husband felt that the timing of information from the speech and language therapist was poor because they had already devised their own communication methods by this point. Her husband would have liked some advice while she was in hospital on how to communicate with her, and found that the cards given to them later by the speech and language therapist would have been more appropriate at that stage:
. . . she brought the charts with her, the letter charts so that and then she brought charts that had pictures and stuff so you could, so Lisa could just be shown at it, so she could just point at things, like I say I’m hungry or I want a cup of tea, so I looked at them and I thought, well they’d have been handier in the hospital.
Lisa’s husband
Once they got the communication aids, they could have benefited from some more training, as Lisa struggled with getting used to the tablet and they were offered little choice.
Actively seeking information via asking questions or using the internet was a facilitator of ensuring that information was more usable. This was common when stroke survivors or their caregivers faced a problem and wanted to learn more to address the problem. Maude’s husband used the internet to gain information and reassurance about his wife’s diagnosis and prognosis of stroke while she was in hospital:
Well just look at the different types of stroke and the, and the difference between, you know, what the symptoms are of a transient ischemic attack and a full stroke and things of that nature, and the different types of stroke, whether they’re haemorrhagic or not and that was what I looked at, and it did seem that if you had to have a stroke, the best sort of stroke to have would be the one that Maude had so.
Maude’s husband
Building a support network is closely linked to actively seeking information. This enabled some of the stroke survivors and their caregivers to obtain the types of information they needed from a certain source of support. Jimmy and his wife attended a group for carers where they took part in a course over a number of weeks. They had the opportunity to engage in a one-to-one session at the end of the course to gain information that they may not have wanted to discuss in front of the wider group:
By the end of the course you could go up to them and say, you know, a one-to-one with anyone and say, ‘is there . . .?’, you know, and if you’ve got something that you’re thinking about you might wanted to have keep it private you know, between the person, not let anybody else on the course know about it, but you can speak to her, you know?
Jimmy
The level of interest and extent to which it meets their needs also plays a part in whether or not some of the stroke survivors make use of information. Alfred subscribed to a monthly magazine regarding his health and found it interesting to see how different people deal with their strokes. Cathy used information she was given in a folder by one of the community nurses. This comprised numerous sections, examples being ‘life after stroke’ and ‘social care and support.’ This information met her needs in terms of providing her with some understanding about the stroke and useful contacts for managing specific issues, for example benefits.
Sustaining flexible support networks
Stroke survivors used both formal and informal support networks as part of managing their ‘recovery’. Most of the stroke survivors talked positively about the formal support they received from health-care professionals while they were in hospital. Following discharge from hospital, there was a shift in the types of support they received. Examples of post-discharge support included family, friends, community nurses, other external agencies (e.g. Age UK, Stroke Association) and support groups. Once the stroke survivors left hospital, they had more choice in the support they wished to mobilise, although they were still restricted to some extent by access and availability. Support was also influenced by changes in circumstances of support providers (e.g. friends and family). Stroke survivors and their caregivers needed to find ways of sustaining support in the event of unexpected changes.
Stroke survivors and their caregivers experienced a range of barriers to being able to sustain support. Formal support such as physiotherapy was not available to all stroke survivors; this left some (e.g. Mavis) feeling angry because they wished they could have had this support. Many stroke survivors also suffered from a lack of support as, sadly, many family members and friends had died. Lizzy lost her husband, who died of cancer very close to the time of her stroke; this made managing with the stroke more difficult because he wasn’t around to help her. She described this as a ‘double whammy’ because she had to cope with being widowed and manage the impacts of the stroke:
Yeah. It was the double whammy of having, being widowed and the stroke so close together that it’s taken a bit longer. They did suggest that I’d had the stroke was maybe because of I hadn’t grieved properly for my husband.
Lizzy
Acceptance (or not) of support was one of the facilitators of being able to sustain support. Stroke survivors and their caregivers talked very little about support from social care and talked mostly about health care, third-sector organisations and informal support. Individuals were often accepting of support from friends, caregivers and relatives, yet more formal, group-based support was not as readily accepted. The extent to which support was accepted related to how appropriate it was for addressing their needs. David and Jane were not interested in attending groups because they did not think their strokes were bad enough.
As with the information, actively seeking support is a facilitator of gaining useful flexible networks. Lizzy had some input from a speech and language therapist, but did not feel that she was getting on very well, so she proactively got in touch with her to overcome this problem. She was given more exercises to try following this:
I called her back about 4 months after because I didn’t feel that I was getting on very well and she gave me some more exercises.
Lizzy
Accessibility can be both a barrier to and a facilitator of being able to engage with some types of support. Some stroke survivors wanted to be able to attend groups, but they were limited by their location and transport options. Others could be visited at home for health-related support, in which case accessibility was not problematic.
Engaging in meaningful activity
Stroke survivors needed to engage in meaningful leisure and home-based activities including golf, board games and housework. The meaning attached to these activities varied for different individuals. Some stroke survivors valued the social aspects of activities, others engaged in activities for enjoyment, to maintain a purpose or to keep busy.
Some individuals could engage in the same activities as before their stroke, some took part in some aspects of previous activities and others changed their activities as a result of the impairments caused by the stroke. For example, before her stroke Cathy was a teaching assistant. She was unable to continue in this role because of her physical and communication difficulties, but she had more time to engage in housework. She found new enjoyment in her role around the home, which keeps her busy following the stroke:
Oh, I’m just as busy in the house; I never thought I’d like housework! So I do that, I’ve been in the cloakroom with a vacuum this morning and I’ve got a bread machine so I make bread for us.
Cathy
Engagement in previously enjoyable activities was influenced by their physical abilities and level of confidence. Daphne played bowls before her stroke and lacked confidence initially. However, her captain encouraged her to keep playing, which gave her the confidence to continue, despite some physical impairments post stroke.
Accessibility was also influential in whether or not stroke survivors could continue to engage in meaningful activities. Some stroke survivors had informal support networks that could provide transport to enjoyable activities, which facilitated ongoing engagement post stroke. However, not all stroke survivors could rely on such support. Carla wanted to be able to attend a group, but, because of her rural location, lack of family close by, limited bus routes and inability to drive, her access was limited.
For some, the level of engagement in new or previous activities was influenced by motivation. Some stroke survivors lacked motivation following their stroke, which meant that they were reluctant to engage in activities. A change in or loss of identity as a result of physical changes to the self also served as a barrier to engagement in previously enjoyable activities. Paddy became physically impaired following his strokes, leading to changes in his self-concept. He described himself as active before the stroke and this loss of self has been a bit of a ‘comedown’ for him. To meet the needs of his new defined self as ‘less active’, he changed his activities, meaning he spends prolonged periods watching rather than playing football.
Some stroke survivors benefited from being encouraged to take on new activities. Cedric’s wife bought him a computer because she believed that it would be useful as part of his cognitive recovery. He later joined a computer class, which he finds enjoyable. Linked to this was the importance of building a support network for engaging in meaningful activities. This included support in a social sense or for physically attending an event. Lizzy is a representative of a sugarcraft club. She finds people who she can ‘double up’ with to get to her regular meetings, as she has lost her confidence with driving longer distances:
Yes. I’m a rep[resentative] for the sugar people as well, sugarcraft people and if we’re having meetings down in Brighton or something like that, I usually try and find someone I can double up with.
Lizzy
It was more difficult for stroke survivors to continue with activities if they experienced a lack of interest. Alfred no longer plays table tennis since he had his stroke; finding a potential replacement for this activity has been difficult, as table tennis provided him with enjoyment. It is the physical aspect of this activity that he has struggled to replace and he lacks interest in alternative activities such as bowls. Lack of money can also have an impact on the level of activity that stroke survivors can engage in. Carla lost her job as a nurse as a result of the stroke. She was caught up in a vicious circle as she is limited in what she can do without money, yet she was struggling to figure out what job she would be able to do instead.
Overcoming financial concerns
Some of the stroke survivors and their caregivers talked about financial difficulties during the interviews. Some stroke survivors and their caregivers had to give up work following the stroke and others were forced to increase their working hours to be able to cope financially (e.g. Paddy’s wife).
Obtaining benefits allowed some of the stroke survivors and their caregivers to manage, but the process for obtaining these was often problematic. Therefore, stroke survivors and caregivers had a need to find ways of overcoming this financial concern.
A common barrier to being able to obtain benefits was lack of information or awareness:
He said ‘I’m not being funny but you’ll get £200 a month for what you are’. ‘Oh’, I said ‘I wouldn’t know where to start or where to go’.
Timmy
The facilitator of overcoming this issue was ‘knowing the process’. Cathy emphasised the importance of knowing about the process of getting benefits earlier on. She knew about this only because she was given an information pack, but she found it stressful managing financial issues alongside adjusting to the impact of the stroke:
I think definitely people need to know about and they should be told that, I think, quite early on because I think it was November or no, October, I got in touch with the Independent Living Allowance and they send the form and it had to be in and again they can take as long as they want and you have a deadline, you have to have it with them.
Cathy
Building a support network was a facilitator of overcoming some of the issues around benefits. Some of the stroke survivors made links with charities such as Age UK that provide financial support (e.g. Lisa and her husband).
Maintaining relationships
There was a need among stroke survivors to be able to maintain relationships with significant others, including partners, other family members, colleagues and friends. The interview accounts reflect the diverse effects that a stroke can have on relationships. Some of the stroke survivors and caregivers in the sample experienced difficulties, but did not face a complete breakdown in their relationships as a result of the stroke. For example, Charlie’s wife recognised that he has become more ‘chompy’ since the stroke, and described her new life as a caregiver as stressful. Alternatively, others have become closer since the stroke. For example, Lisa’s husband believes marriage is about caring for each other so he takes the view that there is a change in that care. He does not think of himself as her carer and understands that she would do the same if the situation was reversed. This supports the importance of maintaining relationships for both the stroke survivors and the caregivers:
You don’t think of yourself as a carer?
No, it’s just one of those things, when you’re a married couple you care for each other anyway, all it is is just a little bit of change in that care, that’s all, for me that’s all it is, it’s that the only . . .
The thing that’s different, yeah, it’s a nice way of looking at it.
If it were the other way around, I know damn well she’d be exactly the same, you know what I mean, if things ever changed, if she had to do things that I’d normally do, she’d do it, so just that, just how it should be, isn’t it?
The concept of togetherness facilitated a maintenance of relationships. For Lisa’s husband, the sense of togetherness is reflected in his understanding that he is there for his wife and he knows this would be reciprocated if the situation was different. Cedric and his wife similarly talk about ‘looking after each other’, yet they talk about this in terms of how they work together as a team on a daily basis. There is a sense of appreciation and understanding, which has allowed them to maintain a positive relationship following Cedric’s stroke.
Acceptance also played a significant part in the maintenance of relationships. Some stroke survivors talked about this as a process that they worked towards over time. Paddy provided an account of how he reached acceptance. This involved consciously making a decision to accept the stroke because he did not want to become bitter and thinks he would have driven people away had he not accepted it:
. . . I think you’ve got to accept it, otherwise you’ll be snapping at everyone and falling out with everyone. You drive everyone away. You’d end up with no friends because people would get fed up of you, wouldn’t they?
Paddy
‘Building a support network’ of informal support was another approach to maintaining relationships with family members and friends beyond the relationships between the stroke survivors and their partners. Malcolm’s wife passed away 4 years prior to the stroke. He continues to maintain relationships with his friends, who have been an important part of his life since he has lived alone.
‘Disrupted couplehood’ is one of the barriers to maintaining a positive relationship. Conflict is one of the factors that can lead to disruption in relationships. Roger and his wife had disagreements regarding driving. He has struggled to come to terms with the loss of driving and, according to his wife, has become a back-seat driver. She believes this has caused a few problems and throughout the interview it was evident that they have had a number of arguments since Roger’s stroke:
No, but there’s still . . . But the one thing with the driving is, Roger so misses the driving and my driving according to Roger is hopeless so . . . [Laughs].
Yeah, mine is hopeless according to my husband as well.
So it’s like having a back-seat, a side-seat and a front-seat driver! [Laughs] And it does cause a few problems.
‘Couplehood’ can also be disrupted by the loss of sexual relationships. Charlie and his wife recognised that their sexual relationship had suffered following the stroke. Paddy also mentioned that the stroke has affected the sex life of him and his partner, although he stated that they have both accepted this. His partner was not present in the interview; therefore, her perspective on this remains unknown.
The ‘burden of caring’ can also serve as a barrier to maintaining the relationship that some of the stroke survivors had with their partners before the stroke. In terms of managing each day, the stroke had an impact on some of the partners. When Lisa is feeling down, it also knocks her husband down. Charlie’s wife also feels the stress in coping with life after stroke. She thinks he can be very demanding now and it can be difficult making sure he is OK:
Yeah, how is it stressful? What are the most stressful things about it?
Well it’s making ends meet and still running the house and making sure he’s OK and Charlie can be demanding at times, can’t you, love? When you’re in, when he’s having a bad day he can be . . .
Despite their caregivers feeling this burden at times, Charlie and Lisa have managed to maintain relationships, albeit that the dynamic has changed since the stroke. It is clear that the impact of the stroke can lead to a change in roles. This change was experienced more positively by some than others. This can be problematic for a relationship when the partners are no longer happy with their new role. For example, Michael’s wife took on a new gardening role, which would not be her preferred choice prior to the stroke. In such circumstances, it was important for couples to work together to try and embrace their new roles.
Becoming a caregiver is one of the most significant role changes. Their role typically involves spending most of their time looking after their loved one, who is dependent on them for support every day. Maintaining relationships can become difficult when there is a negative perception of the resultant change in dynamics. However, this new dynamic in the relationship can be positive when aspects of their marital relationships can be maintained. Arnie and his wife still chat together and have a cuddle as they would have done before the stroke.
Reconstruction of identity
A loss of functioning in part of the body or loss of confidence or memory following the stroke can lead to a change in a stroke survivor’s sense of self. Paddy experienced a loss of identity after the first of his three strokes left him physically impaired down one side. His sense of self as an active person has been lost since he has been unable to engage in physical activity. During the interview, he described his new self as ‘restricted.’ A reconstruction of identity is often required to address the loss of or change in identity as part of adjustment to life after stroke. Some require little reconstruction if they can maintain aspects of their previous self, for example independence. For others this can be more difficult and it means adjusting to take on new self-concepts that they did not previously hold.
Stroke survivors expressed the barriers to and facilitators of reaching the construction of a new identity. Acceptance was an important facilitator of achieving this. Iris knew that she was going to be disabled by her impaired functioning in her arm and leg, but she did not feel that there was anything that she could do, so saw no point in getting worried.
Self-concepts can be related to previous activities. It was therefore important for stroke survivors to find meaningful activities that suited their new identities, or allowed their previous identities to remain. Iris refers to herself as disabled following the stroke; when she was asked about herself as a person, she talked about herself in terms of the physical disability she has acquired. As she has adjusted to this new identity, she has looked for ways of meeting her needs, such as a holiday for people with a disability:
I have but I’ve never, I’ve said, I’ve told you about holiday flats or houses or that, well that’s not what I want, it’s something where I can go as an individual, that I could be taken there and sort of looked after there, carefully looked after and then brought back as well, but who understands what it is to be disabled.
Iris
Irreplaceable loss was a barrier to managing the new identity following the stroke. This refers to loss of what allowed them to define themselves as in a certain way prior to the stroke (e.g. as an independent person) and is related to different aspects of their lives (e.g. driving and working). Carla valued her independence in terms of being able to do what she wanted. Following the stroke, she has experienced a sense of irreplaceable loss in terms of this aspect of her identity. She acknowledged that she will not be what she was before (physically) and finds this really hard.
Stigma was also challenging for stroke survivors as they worked through a process of reconstructing their identities. During the interviews, stroke survivors talked about stigma in relation to being old, being like a baby, looking drunk and being less intelligent. Cathy perceived herself as intelligent before she had her stroke. The stroke caused her to become physically impaired and she needs to use a wheelchair when she goes out beyond her home. Visibly, she entered the social world with a new identity, but her intelligence was unaffected by the stroke. She noticed people acting differently towards when she used the wheelchair:
Well, just that they talk down to you. They don’t think you’re as intelligent and it’s not affected my intelligence.
Cathy
Fortunately, this did not stop Cathy from going out, but it could be problematic for other stroke survivors.
Managing beyond the home
Managing with life after stroke goes beyond the home environment. The home can be a safe space for regaining some of the control that is lost at the time of the stroke. In contrast, stroke survivors and their caregivers can feel less in control beyond their own home, as the outside world leads to numerous challenges. These include those that are within the built environment (e.g. inappropriate access for disabled people and lack of handrails) and those that occur in interactions with others. It is therefore important for stroke survivors to be able to manage beyond the home environment.
Cedric and his wife provide an example of a situation that reflects the difficulties that stroke survivors can experience beyond the home, both physically and in interactions with others. The built environment meant that the wheelchair became stuck at the airport, leading him to fall. People did not know what to do, so he was left struggling in this situation. Cedric tried to account for why people did not help him and suggests that this is a result of a fear of being sued. He also talked about lack of public awareness in terms of handling a stroke survivor, which complicated matters further for him:
And he’s, he’s difficult to get up now; for instance, yesterday, when we were coming off the plane, we always have a wheelchair for Cedric and there was a young girl, like, you, god love her, I felt right sorry for her, pushing Cedric, and there was a kind of a little hump and didn’t the wheels get stuck and he fell straight out of the wheelchair, right out on his legs, ‘cause he can’t save himself. So you know, I’m saying, ‘Don’t panic now, don’t panic,’ and I’m worse than anybody does, so once I can get him around he can get on his knees, then I can help him up, you know, and that’s a . . . you know, there was people at the back of us and they just stood looking at us.
People don’t want to get involved because they’re that afraid, I think, of being sued.
Yeah, that’s true.
We’re not that kind of people, I took them into Morrison’s [Morrison’s Supermarkets plc, Bradford, UK] and he had a hot cup of tea.
Yeah, but they don’t know, a lot of people how to lift a person that’s had a stroke, you know, they get hold of your hand and try and pull it, well you don’t, you know what I mean [laughs]?
Examples of barriers to and facilitators of managing beyond the home are discussed as follows.
Accessibility can be both a barrier to and facilitator of managing beyond the home. Iris has no problem getting to places, as she makes use of taxis. However, she struggles when she arrives at her destinations. Therefore, she is reliant on support from others to manage. This links to the importance of ‘building support networks’, a facilitator for managing beyond the home. Stroke survivors often needed such support to feel safe:
Yeah, that’s what me daughter says you see, I sometimes take her out shopping and that on a weekend and she says, ‘You’re looking for your trolley when you get out, “Where’s the trolley?”. You don’t need your trolley, you’ve got me here’, which is right because, should something go wrong, she’s there.
Timmy
Walking aids were also a facilitator of physically managing for some of the stroke survivors (e.g. Timmy, Cathy). This was another way of protecting their safety and facilitating movement. Timmy has his trolley as a walking aid when he walks longer distances alone. This stops him from panicking in the absence of support.
Some barriers were also apparent for managing beyond the home where stroke survivors faced interactions with others. Some stroke survivors were reluctant to use wheelchairs and sticks because of the stigma attached to these. As a result, they did not often go outdoors. Paddy has limited mobility following his stroke. He uses a stick around the home and for short distances beyond the home. However, he is reluctant to use his wheelchair for longer distances because he thinks he looks like a baby if his wife pushes him around:
If she’s pushing me about, it’s like being a baby in a pushchair.
Paddy
Paddy assumes that people will think in this way; he has never had an experience where someone has told him he looks like a baby. He admitted he could be wrong and he might just be ‘being silly’, but it still stops him from going to some places, which affects the activities he can engage in.
Discussion
This qualitative study examined the needs of 28 stroke survivors (13 stroke survivors at 9–12 months post stroke and 15 stroke survivors at > 24 months post stroke). Thirteen needs have been identified from the perspectives of the stroke survivors and their caregivers. Participants’ accounts of their lives comprised complex and interacting factors that shaped how they managed their needs post stroke. The existing literature has previously indicated the areas where stroke survivors and their caregivers commonly experience unmet needs. 13,20 Although insightful, such research did not provide a comprehensive understanding of how needs can be addressed or, acknowledge the factors that may facilitate or hinder this process. It also neglects the notion that needs may change over time.
This study contributes to the wider body of literature by gaining an in-depth understanding of the broad scope of needs experienced by stroke survivors in the longer term, from their own perspectives. Furthermore, this study has explored the barriers and facilitators stroke survivors and their caregivers face as they work to manage and overcome these needs.
The study highlighted that the participating stroke survivors still have needs that are unaddressed, even up to 3 years post stroke. Across both time points, emotional needs were emphasised, supporting findings from a previous qualitative review. 24 In this study,24 stroke survivors felt that there was a lack of emotional support, which often led to feelings of neglect, particularly when they initially returned home. Abandonment was also experienced by some of the stroke survivors and their caregivers following withdrawal of support from health professionals (e.g. physiotherapists). Stroke survivors expressed a range of emotional difficulties that included, for some, frustration and anger as they tried to manage the impacts of the stroke. Services and interventions in the longer term need to encompass emotional support, as this is an ongoing need for some of the stroke survivors up to 3 years after their stroke.
This research highlighted the importance of understanding needs in different contexts. The findings suggest that managing beyond the home environment can pose different challenges for stroke survivors and their caregivers. Stigma emerged as one of the key barriers to going out in public areas. Survivors talked about this with regards to walking aids where a distinction was made between perceived stigma and their own experiences of being stigmatised. Interestingly, some of the stroke survivors who actively managed their impairments in their own homes were reluctant to spend much time out of their home owing to some of the difficulties they faced in interactions with others. Such findings suggest that efforts must be made to increase public awareness around stroke to increase social participation among stroke survivors. Alternatively, management techniques for stroke survivors and their caregivers could be encouraged to reduce feelings of perceived stigma. These could include strategies for articulating their difficulties to enable more positive interactions in society.
Although stroke survivors and their caregivers faced barriers to addressing their needs, the findings indicate that they do play an active role in managing using both practical and mental coping strategies, supporting findings from previous research. 18,25,26 Although previous literature has provided examples of such strategies (e.g. mobilising support networks),26 this study has identified how these strategies are used to address specific needs. This study supports the importance of support networks, as ‘sustaining flexible support networks’ was identified as one of the needs and as one of the key facilitators of addressing other identified needs (e.g. engaging in meaningful activities, overcoming financial concerns, doing everyday tasks around the house and managing beyond the home).
A more nuanced understanding of the role of the stroke survivor in seeking and maintaining support was gained, particularly in circumstances in which this could be vulnerable to change. Interestingly, many of the stroke survivors were reluctant to join support groups, often because they did not feel that they were a group person or because they did not feel that their stroke was ‘bad’ enough. Such findings have implications for the types of support that is made available to stroke survivors and their caregivers in the longer term.
The need to ‘obtain usable information’ supports findings from prevalence studies in which information is commonly reported as an unmet need. 13,20 From the accounts of the stroke survivors, it is clear that they are given information of some sort following their discharge from hospital; however, there is a general sense of negativity attached to this as concerns were raised about the timing and the amount of information. These issues may account for this being regarded as an unmet need, despite the information being available. The findings indicate that stroke survivors and their caregivers continue to need information in the longer term following their stroke. They draw on this information to resolve a specific problem, as and when it arises. Such findings have implications for how information should be made available to stroke survivors and their caregivers in the longer term.
Interestingly, many of the needs experienced by stroke survivors who were 9–12 months post stroke were similar to the needs of those who were > 24 months post stroke, suggesting that some of their needs are ongoing. This supports the need for longer-term interventions and expands on existing understandings in the literature, where the focus is often on the first year. Despite this, there were some subtle differences apparent across the two time points, an example being around reaching acceptance. This emerged as a key facilitator of managing life after stroke, supporting research which highlighted acceptance as a critical factor in being able to cope. 18 Those who were at least 24 months post stroke talked about this more than those who were 9–12 months post stroke. This suggests that those who have more recently had their stroke may have had less time to reach the point of acceptance.
Some of the stroke survivors spoke about acceptance in broad terms, of accepting the stroke and moving forward, whereas others talked about this more specifically in terms of accepting that tasks take longer around the house and accepting their new identity. Some of the stroke survivors struggled to accept the changes to their lives and themselves following the stroke. One of the stroke survivors made an interesting distinction between realisation and acceptance. She realises that things are different, yet she fails to accept this. This suggests that it is a process that must be worked towards over time, which is reflected in other accounts from the stroke survivors. Among those who have managed to accept, there was a sense that they had little choice but to do this to move forward.
Evidently, acceptance is complex and a number of factors shape whether or not this is possible. Supporting stroke survivors in reaching this is important, as it affects a number of needs, for example maintaining relationships, managing and coping with a major life event and reconstruction of identity.
Strengths and weaknesses
This study drew on a thematic approach to qualitative research whereby semistructured interviews were carried out to gain an in-depth nuanced understanding of stroke survivors’ needs and the management of these needs post stroke. These were addressed from the perspectives of the stroke survivor, rather than being predetermined by a questionnaire. A further strength was that caregivers were also invited to attend and provide their perspectives of the stroke survivors’ needs. Twenty-eight stroke survivors and 11 caregivers participated in the study. Including caregivers in the study meant that the stroke survivor–caregiver dyad was addressed; therefore, a sense of their different perspectives and also of their relationships was gained.
A unique approach to understanding the stroke experience was taken through exploring specific needs across all areas of the stroke survivors’ lives and investigating the factors that influence whether or not these needs are addressed. This detailed understanding was gained for the purpose of the intervention mapping process,104 whereby behaviours and determinants of these behaviours are outlined and practical methods are selected to address them as part of a longer-term care strategy.
The sample was also purposively selected to ensure variation in key characteristics that are known to shape longer-term adjustment and participation post stroke. These included age, socioeconomic status and whether the stroke survivor lived at home or with others. Participants were also selected at two different time points post stroke, 9–12 months and at least 24 months up to 4 years, to ensure that an understanding of needs at different stages post stroke was captured. They were also chosen from different geographical areas (variation in services across the UK) and selected based on needs and level of independence using the Barthel and LUNS questionnaires. Recruitment of the participants via an established research register meant that the study included a wider range of stroke survivors who were residing in the community, not just those who attend stroke groups.
Although the purposive sampling strategy enabled the recruitment of a diverse group of participants, the sample lacked those in more difficult and complex circumstances. The measures used to assess needs and level of independence lacked an indication of those with communication or emotional difficulties, which made it difficult to purposely select these individuals.
In addition, very few of the stroke survivors had dependent children. It is possible that such individuals have different needs that may not be reflected in these findings.
Implications for health care and social care
These findings suggest that stroke survivor needs should be routinely monitored during the 6- and 12-month reviews. These could be incorporated into a discussion around the factors that enable or hinder them in addressing their needs. Stroke survivors would then be able to be referred for specialist support, when appropriate, to meet their perceived needs or provided with opportunities to learn skills in self-management to address their needs independently.
This support would work with both stroke survivors and their caregivers to find creative ways of problem-solving and managing impairments. They would be supported to gain some control over their situation in order to cope with losses to their lives and their identities. Working through the process of acceptance would allow them to rebuild a meaningful life through engaging in meaningful activities, maintaining relationships and sustaining flexible support networks. This would also help to overcome ongoing disruption for stroke survivors and their caregivers. Emotional and psychological needs occur in the longer term and are not often addressed by current services. Stroke survivors often struggle to overcome these needs themselves, which suggests that support must respond to individuals with these needs.
Appendix 2 Reviews included in workstream 1b overview
| Reference | Review | Studies (participants) | Time post stroke | Controls |
|---|---|---|---|---|
| English and Hillier 2010158 | Circuit class therapy, 2010 | 6 (292) |
|
Various |
| Sirtori et al. 2009159 | CIMT, 2009 | 19 (619) |
|
Various or no intervention |
| Loetscher and Lincoln 201332 | Cognitive rehabilitation for attention, 2013 | 6 (223) |
|
Usual care |
| Hackett et al. 200841 | Depression, 2008 (psychotherapy interventions) | 4 (448) | Means ranged from ‘within a few days’ to 25 months | Usual care and/or attention control |
| McGeough et al. 2009160 | Fatigue, 2009 (self-management programme) | 1 (125) | Unclear | No intervention |
| Saunders et al. 201333 | Fitness training, 2013 | 45 (2188) | Means ranged from 8.8 days to 7.7 years | Various or no intervention |
| Winter et al. 2011161 | Hands-on therapy, 2011 | 3 (86) | Mean 3.5 years | Various or no intervention |
| Coupar et al. 2012162 | Home-based upper limb therapy, 2012 | 4 (166) | Mean 157 days | Usual care |
| Forster et al. 201230 | Information provision, 2012 | 21 (2289 survivors, 1290 carers) |
|
Unclear |
| Xiao et al. 201234 | Inspiratory muscle training, 2011 | 2 (66) |
|
Unclear |
| Legg et al. 201144 | Interventions for caregivers, 2011 | 8 (1007) | Unclear, but 2 > 6 months | Unclear |
| Barclay-Goddard et al. 2011163 | Mental practice, 2011 | 6 (119) |
|
Usual care or usual physiotherapy/OT |
| Thieme et al. 2012164 | Mirror therapy, 2012 | 14 (567) | Means ranged from 5 days to 5 years | Various or no intervention |
| Bradt et al. 2010165 | Music therapy, 2010 | 7 (184) | Unclear, 2 acute, 2 > 6 months | Various or no intervention |
| Legg et al. 200635 | OT for ADL, 2006 | 9 (1258) | Unclear, 1 trial > 1 year | Usual care or no care |
| Fletcher-Smith et al. 2013166 | OT in care homes, 2013 | 1 (118) | Unclear | Usual care |
| States et al. 2009167 | Overground gait training, 2009 | 9 (499) | All > 6 months | No intervention or a control |
| Pollock et al. 2014168 | Physical rehabilitation for function and mobility, 2014 | 99 (10,401) |
|
Various or no intervention |
| Outpatient Service Trialists 200336 | Rehabilitation at home for < 1 year, 2003 | 14 (1617) | All with mean < 1 year: 9 recruited at discharge, 4 within 6 months | Usual care |
| Aziz et al. 200837 | Rehabilitation at home for > 1 year, 2008 | 5 (487) | All with mean > 1 year | Usual care |
| French et al. 200738 | Repetitive task training, 2007 | 14 (680) |
|
Various or no intervention |
| Brady et al. 201242 | Speech and language therapy, 2012 | 39 (2518) |
|
Various or no intervention |
| Ellis et al. 201039 | Stroke liaison workers, 2010 | 16 (4759) | Unclear but predominantly < 3 months | Usual care or alternative service |
| Laver et al. 201340 | Telerehabilitation, 2013 | 10 (933) |
|
Various including usual care |
| Mehrholz et al. 2014169 | Treadmill training, 2014 (treadmill only) | 16 (823) | Unclear but where reported 4 < 6 months and 3 > 6 months | Various or no intervention |
| Laver et al. 2011170 | Virtual reality, 2011 | 19 (565) |
|
Usually an intervention using a conventional approach |
| Pollock et al. 201143 | Visual field interventions, 2011 | 13 (344) | Unclear, 1 acute, 3 > 6 months | Placebo or no intervention |
| Mehrholz et al. 2011171 | Water-based exercises, 2011 | 4 (94) |
|
Various or no intervention |
Appendix 3 Findings from literature review of delivery mechanisms (workstream 1b)
A scoping review of reviews addressing delivery mechanisms of health care in chronic illness was undertaken.
An information specialist searched 10 databases using keywords and controlled vocabulary developed for the following strategy: chronic illnesses and methods of delivery of health care and community care setting and evaluation and review.
Following de-duplication, 2080 records were screened, 700 of which were identified as being of interest. These were categorised by the primary study types they included, the interventions/service aspects and the conditions/participants on which they focused. Most of the identified reviews focused on specific conditions. To make this review focused and more manageable, the PMG decided to limit the scope to review papers that considered a range of delivery mechanisms across a wide range of long-term conditions. This resulted in seven review studies, including two additional papers identified from the references. Relevant policy documents were also identified. 86–92
Boult et al. 80 identified 15 models of comprehensive health care for older persons with chronic conditions with some evidence of effectiveness: multidisciplinary care, add-ons to primary care (case management, disease management, preventive home visits, comprehensive geriatric assessment, pharmaceutical care, chronic disease self-management, proactive rehabilitation, caregiver education and support), transitional care, hospital-at-home, care in nursing homes, prevention and management of delirium, and comprehensive hospital care.
Singh84 conducted a wide-ranging overview examining interventions that change the organisation of care (e.g. integrated care), interventions that target systems (e.g. care pathways) and those targeting patients. Singh84 identified evidence to support providing information, self-management education, involving people in decision-making, identifying those at most risk, self-monitoring and referral, electronic monitoring and telemonitoring, nurse-led strategies, primary care-led strategies, integration of community and hospital care, and broad models of care [e.g. the Chronic Care Model (CCM)].
The review by Bodenheimer79 summarises the effectiveness of components of the CCM. It highlighted self-management education and some provider education interventions for their potential to improve outcomes without being particularly expensive.
Similarly, Dennis et al. 82 used the CCM as a framework for their review and found that self-management support (education or motivational counselling), MDTs and their combination have some evidence of effectiveness, but that there is little evidence relating to effects of health-care organisation or involving community resources.
Weingarten et al. 85 pooled the effects of disease management programmes aimed at providers (feedback, education, reminders) and patients (education, reminders and financial incentives) on guideline adherence and disease control. These may all be effective, but estimates vary by disease.
Coleman et al. 81 conducted a narrative review of disease-management programmes and claimed that ‘carve-out’ interventions that target only patient behaviour change may be less effective than those that also work to develop the skills of providers or to redesign care delivery (e.g. creating linkage to ancillary/community-based services).
Goodwin et al. 83 examined the role of general practice in the management of long-term conditions. Among other recommendations, they claimed that integration and co-ordination are essential to ensure that patients do not fall through gaps and so that professionals know what services are available in their area. They also emphasised the importance of personalisation and flexibility in delivery, to enable patients to tailor the service to them.
Appendix 4 Workstream 2: national survey
FIGURE 10.
Workstream 2 national survey pro forma.

FIGURE 11.
Map of returned surveys.
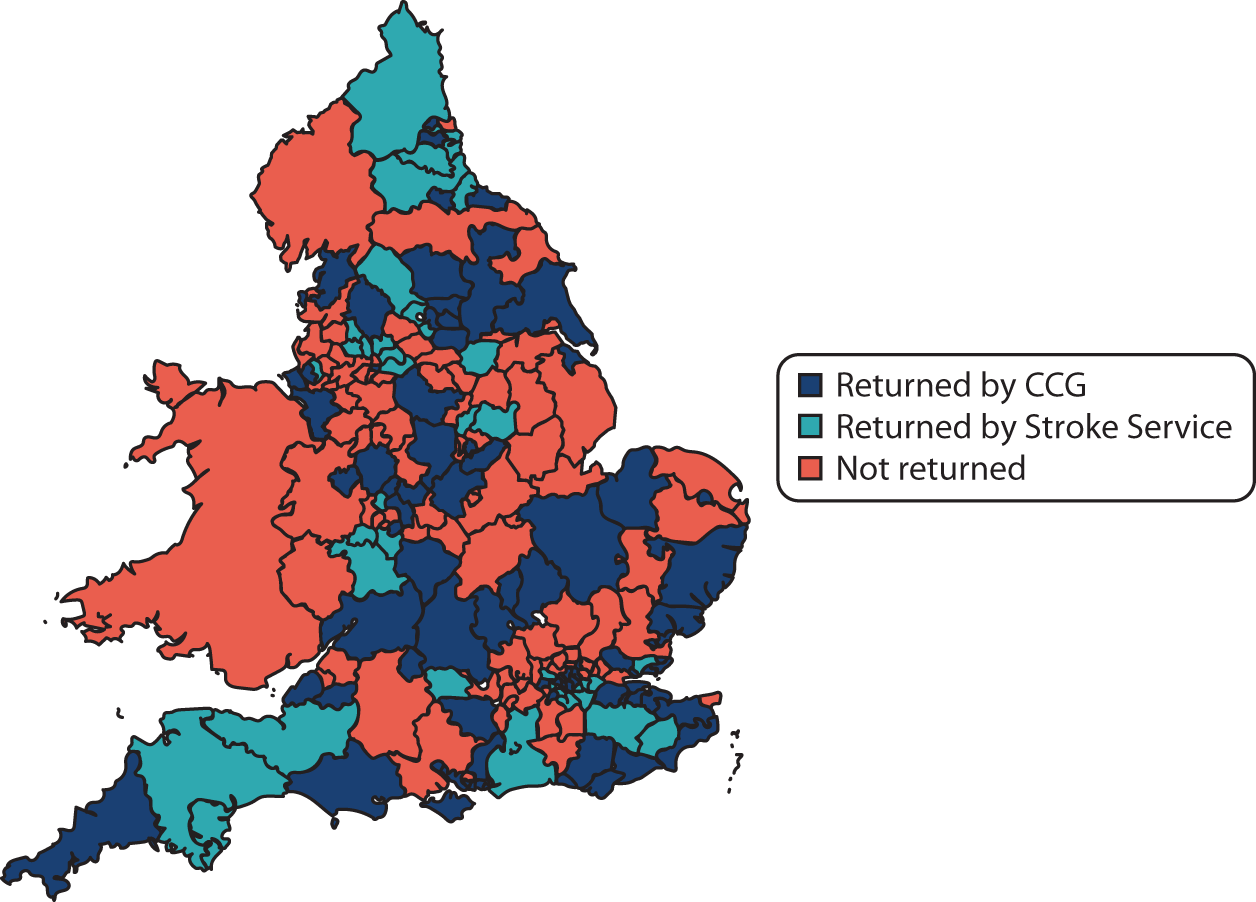
Appendix 5 Perceived needs of stroke survivors identified by workstream 2 focus groups
The key longer-term needs of stroke survivors were identified by focus group members. These are discussed in the following sections.
A meaningful role and sense of identity
The loss of role and identity through the onset of disability, together with the potential loss of employment, could lead to a lack of motivation, boredom, low mood and frustration. Creating new roles, so that a survivor is able to regain some control of their life, was considered important.
Psychological support
This reflected the challenges of adjusting to changed circumstances, such as moving from hospital to home, and coping with low mood/depression. The withdrawal of community stroke services meant that survivors were often felt to be socially isolated. Limited psychological support and the impact of cognitive problems posed additional emotional challenges for survivors.
Ongoing information and advice
Obtaining appropriate information and advice at the point of discharge from hospital was identified as a need, as was gaining advice to address fears of recurring stroke. Understanding risk factors for stroke was an important feature.
Non-stroke-specific wider community engagement opportunities
Social isolation suggested the need for wider community engagement opportunities, such as appropriate gym and social facilities, as well as opportunities for engaging in voluntary work.
Stroke-specific group engagement
Support groups were seen as valuable in reducing social isolation for some survivors, yet there were perceived challenges in enabling transportation to such groups, and providing care for survivors at support groups. Groups for aphasic survivors, as well as younger survivors, were particularly helpful.
Appropriate health and social care at home
Co-ordinated support at home from health and social care teams in ways that were not too burdensome or onerous for the survivor and their family was felt to be important.
Longer-term supported self-management
Supported self-management may include maintaining links with professional therapeutic support as necessary, so that stroke survivors can better maintain their exercise routines and goal-centred focus. Ultimately, this was seen as enabling survivors to become as independent as possible, and do as many activities as they used to do pre stroke, as well as develop new activities.
Support with personal relationships
The emotional strain placed on relationships as a consequence of a stroke with the shift in the dynamics of the home, with the move from partner to carer, allied to the behavioural changes on the part of the survivor, creates a powerful need for relationship support.
Support with physical health needs
Changing physical health over time since discharge can create a need for maintaining health through addressing issues such as seating, positioning and manual handling. Linked to this is the need for personal acceptance of disability arising from stroke, and adapting to fatigue.
Employment and financial support
A key perceived need was to address issues arising from a loss of paid employment, such as accessing benefits advice and seeking to re-enter the labour market or take up voluntary employment opportunities.
Effective transportation to facilitate access to services and activities
Poor public transportation, particularly for those living in more isolated, rural communities, limited access to community services and group activities. The cost of private transport could also be prohibitive.
Social acceptance by society at large
In general terms, the need for wider societal change that enables understanding and acceptance of the disabilities affecting survivors was also identified.
Appendix 6 Prioritising the needs of stroke survivors and their families (workstream 3)
Members of the LoTS2Care RG (n = 6) and members of the CRAG (n = 4) were asked to categorise the 23 identified longer-term needs of stroke survivors and their families into ‘most important’ and ‘less important’. The results of this exercise are presented in the following table.
| Expressed need | Individual | Total votesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L | N | T | G | GR | AJ | SM | LH | AM | AF | ||
| Engaging in meaningful activity | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Emotional/psychological support | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||
| Gaining control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||
| Managing emotions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||
| Maintaining relationships | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||
| Managing hidden consequences of stroke | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||
| Managing/coping with a major life event | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||
| Reconstruction of identity | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||
| Obtaining usable information | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |||
| Mobility | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |||
| Support around health and social care provision | 1 | 1 | 1 | 1 | 1 | 5 | |||||
| Clinical problems | 0 | 1 | 1 | 1 | 1 | 1 | 5 | ||||
| Sustaining flexible support networks | 1 | 1 | 1 | 1 | 4 | ||||||
| Working towards physical/functional improvement | 0 | 1 | 1 | 1 | 1 | 4 | |||||
| Overcoming financial concerns | 0 | 1 | 1 | 1 | 1 | 4 | |||||
| Transport | 1 | 1 | 1 | 1 | 4 | ||||||
| Doing everyday tasks around the house | 0 | 1 | 1 | 1 | 1 | 4 | |||||
| Accessing Stroke Association/voluntary services | 1 | 1 | 1 | 1 | 4 | ||||||
| Driving | 0 | 1 | 1 | 1 | 3 | ||||||
| Managing beyond the home | 1 | 1 | 1 | 3 | |||||||
| Fatigue | 0 | 1 | 1 | 2 | |||||||
| Falls (fear) | 0 | 1 | 1 | 2 | |||||||
| Practical help | 0 | 1 | 1 | 2 | |||||||
| Total | 12 | 10 | 11 | 16 | 11 | 12 | 12 | 12 | 7 | 12 | |
Appendix 7 Example of behavioural outcomes, performance objectives and change objectives for prioritised need ‘engaging in meaningful activities’
Engaging in meaningful activities
Finding ways of engaging in some form of meaningful activity to occupy the day (socialising, exercising, driving, working, etc.).
Overall outcome
For every stroke survivor who wants to enhance their engagement in meaningful activities, the survivor will engage in one or more new (or previous) meaningful activities, or adapt one or more ongoing activities to make it become meaningful within 6 months of starting the intervention.
Linked outcomes
-
Reconstruction of identity.
-
Building and sustaining a support network.
| Behavioural outcomes and performance objectives | Domains | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Knowledge | Skills | Social/professional role and identity | Beliefs about capabilities | Optimism | Beliefs about consequences | Reinforcement | Intentions | Goals | Memorya | Environmentb | Social influences | Emotion | Behavioural regulation | Physical impairment | |
| 2. Survivor identifies meaningful activities that suit their current circumstances (including functioning, identity, accessibility, available time and money) | |||||||||||||||
| 2.1 Survivor identifies any pre-stroke activities they would like to resume | C2.1 believes they may still be able to do pre-stroke activities | M2.1 wants to be able to resume pre-stroke activities | |||||||||||||
| 2.2 Survivor recognises new opportunities as a result of the stroke | S2.2 can identify changes in relationships, personal interests, interests of current social network and time available | A2.2 open to considering new opportunities | C2.2 believes they can engage in new opportunities/activities | OE2.2 believes considering these changes will assist in engaging in meaningful activities | M2.2 wants to find new opportunities to engage in meaningful activity | ||||||||||
| 2.3 Survivor identifies activities they would consider meaningful |
|
|
M2.3 wants to identify new activities | ||||||||||||
| 2.4 Survivor identifies barriers (moral, social, emotional, practical) that currently limit engagement in these activities | K2.4 can describe the kinds of barriers to activities and how they relate to specific examples | S2.4 can analyse and describe why they cannot currently engage in meaningful activities | C2.4 believes they can identify the barriers to engaging in meaningful activities | OE2.4 believes considering changes will assist in engaging in meaningful activities | |||||||||||
| 2.5 Survivor identifies which barriers can be overcome by strategies, including using support; problem-solving; adapting the activity to reduce/remove the barriers, while retaining its meaningfulness | K2.5 is aware of strategies and the parameters of the problems they solve | S2.5 can match problems to strategies | C2.5 believes they can identify relevant strategies for their problems | OE2.5 believes many barriers can be overcome with the right strategies | M2.5 wants to overcome barriers | ||||||||||
| 2.6 Survivor develops meaningful activities using available time | |||||||||||||||
| 2.7 Survivor identifies which meaningful activities they could try to engage in | K2.7 knows the activities they would like to do | S2.7 can consider the suitableness of possible activities |
|
OE2.7 believes identifying suitable activities will increase the likelihood of engaging in meaningful activities | |||||||||||
| 3. Survivor engages in meaningful activity | |||||||||||||||
| 3.1 Survivor addresses barriers in order to be able to engage in meaningful activity | K3.1 knows how to address barriers (see PO2.5) | S3.1 can solve problems, harness resources and social networks and generate change | C3.1 believes they can solve problems, harness resources and social networks and generate change | A3.1 feels positive about overcoming barriers | M2.5/3.1 wants to overcome barriers |
|
|||||||||
| 3.2 Survivor sets achievable goals for achieving/working towards the activity | K3.2 knows how to set SMART objectives | S3.2 recognises their current capabilities, identifies potential for improvement and can phrase goal | A3.2 feels positive about goal-setting | C3.2 believes they can set effective goals | OE3.2 believes goal-setting will enhance likelihood of engaging in meaningful activity | M3.2 wants to set goals | |||||||||
| 3.3 Survivor engages in meaningful activity or achieves goals towards the activity | S3.3 demonstrates the ability to participate in the activity | C3.3 believes they can engage in the activity | A3.3 feels positive about engaging in meaningful activity | OE3.3 believes they will be more fulfilled if they engage in the activity | M3.3 wants to engage in the activity | CA3.3 has the cognitive ability to engage in the activity | H3.3 develops new meaningful activity into a habit | PA3.3 has the physical ability to engage in the activity | |||||||
| 4. Survivor adapts the strategy over time as appropriate | |||||||||||||||
| 4.1 Survivor monitors achievement of goals | K4.1 knows how to measure goal achievement | S4.1 can compare behaviour with goals | C4.1 believes they can monitor achievement of goals | M4.1 believes monitoring goals will help achievement of goals | |||||||||||
| 4.2 Survivor adapts goals in the face of problems | S4.2 can consider and plan alternative strategies | C4.2 believes they can adapt goals in the face of problems | |||||||||||||
| 5. Survivor accepts loss of some meaningful activities | |||||||||||||||
| 5.1 Survivor works towards coming to terms with a loss of some activities | K5.1 Survivor recognises the meaningful activities that they are currently unable to do after the stroke | S5.1 acceptance | |||||||||||||
Behavioural outcomes
-
2. Survivor identifies meaningful activities that suit their current circumstances (including functioning, identity, accessibility, available time and money).
-
3. Survivor engages in meaningful activity.
-
4. Survivor adapts the strategy over time as appropriate.
-
5. Survivor accepts loss of some meaningful activities.
(When behavioural outcomes were first drafted, a behavioural outcome 1 was written, which we later agreed to remove but without altering the numbering in the working documents.)
Environmental conditions
-
Support helps survivor to reflect on meaningful activities.
-
Support helps survivor to identify feasible meaningful activities.
-
Support helps survivor to set goals.
-
Support helps stroke survivor to resume meaningful activities.
-
Support helps survivor to accept loss of some activities.
-
Significant other avoids over protectiveness (physical and emotional).
-
Support encourages significant other to avoid over protectiveness (physical and emotional).
-
Commissioner funds rehabilitation therapy that focuses on achieving the ability to do a meaningful activity.
-
Rehabilitation therapy focuses on achieving the ability to do a meaningful activity.
-
Transport is available to get to the activity.
-
Activity organiser uses an accessible location.
-
Local government makes public spaces accessible.
-
Activity organiser, health services, social services, survivor: provides adaptations to the environment and/or necessary aids to do the activity.
-
Activity organiser adapts the activity to accommodate impaired functioning.
Appendix 8 Problem-structuring, priority-setting for services and knowledge mobilisation
| Unmet needs | Modifying influences | Desirable services |
|---|---|---|
| Interviews | Grey literature | Literature reviews + WS2 |
| Survey | Service users | Service users |
| Literature | Expert knowledge | Expert knowledge |
| Stroke Association queries | ||
| 23 initially identified | Resources | Flexible and modifiable |
| Prioritised needs | Expectations/values | Affordable |
| Theory | Sustainable | |
| Policy | Compatible |
Appendix 9 Provisional summary of the framework for the intervention
Problem-solving self-management with survivors and carers
This would involve:
-
Ensuring that the survivor and carers have an understanding of
-
their cause of stroke, risk factors, recurrence risk (gaining control)
-
the full range of their stroke impairments – physical, emotional, communication, cognitive (managing emotions and hidden consequences)
-
the consequences of the stroke on identity, relationships, sexual functioning, roles (identity), meaningful activities.
-
-
Identifying what needs they have that they would like to address.
-
Identifying barriers to and facilitators of these needs.
-
Generating strategies to resolve problems.
-
Using strategies.
-
Evaluating the effectiveness of the strategies
What is covered in this would be determined by what the survivor/carer saw as their most important needs to address, but this will help to address all of the prioritised needs of gaining control, managing/coping with a major life event, managing emotions, managing hidden consequences, maintaining relationships, reconstruction of identity, mobility, clinical problems, communication, access to voluntary/paid work [as well as the other needs of falls (fear), fatigue, driving, transport, financial concerns].
Help with obtaining usable information
Someone or some service needs to provide and facilitate access to information at the right time, on the right subjects, in a format that is helpful to the survivor and carers, and without overloading them. An important aspect of this information provision would be a tool that identifies all potential activities that are available in the local area (and also access to national resources such as online forums, etc.) [i.e. the Patient-Led Assessment for Network Support (PLANS) tool]. 113
Access to usable information would help to address all of the above needs. The use of the PLANS tool113 or similar would, in particular, address the needs of access to health and social care and voluntary services, engaging in meaningful activities and building a sustainable support network.
Help survivors and their carers build sustainable flexible support networks
This is a key facilitator of addressing unmet needs. For those survivors and carers who do not have an existing sustainable flexible support network, the intervention will help them to build one.
Even if a survivor has no unmet needs, they would be provided with access to usable information, and, when necessary, help with building a support network, both of which could help to resolve future emerging problems.
Appendix 10 Workstream 4 action groups methods
Each site was asked to identify facilitators who would be trained to lead local implementation (henceforth called the ‘site facilitator’). Following an initial meeting, fortnightly action group meetings were planned for 4 months and monthly thereafter. Each meeting was facilitated by a researcher from the central research team (action research facilitator), who documented consent and attendance, minutes of the meeting, actions agreed, responsibilities, and target and completion dates. An action research pro forma for each meeting was populated, and the meetings were audio-recorded and transcribed verbatim with the consent of participants.
Once they had been trained, site facilitators began to implement the intervention in their sites in the homes of stroke survivors and to deliver feedback on any problems encountered and their experiences to the local action group meetings. The research team developed a data collection tool for site facilitators to complete following each patient contact. It recorded the number and duration of each episode of contact with the patient, what materials they were provided with, what was discussed in relation to the intervention materials, whether or not goals and action plans were completed (and focus of these) and if any referrals were made.
Additional data on the implementation process was obtained through independent observation of action groups and shadowing the site facilitators’ visits to stroke survivors. Throughout the WS, the action research facilitators documented barriers faced in each of the sites, how these were overcome, success stories that they witnessed and other helpful hints and tips that may be useful for future implementation of the intervention. These were collated as a master list.
Fortnightly meetings were held between the action research facilitators for each site to help maintain a common sense of the intervention, compare progress and problem-solve difficulties at their sites.
Appendix 11 Example of a completed workstream 4 activity record
Appendix 12 New Start intervention outcomes chain
Reproduced with permission from Hardicre et al. 142 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.

Appendix 13 Overview of assessment of progress: New Start care strategy delivery
The training package consisted of a 2-day workshop plus 2 additional follow-up days. These were delivered over a 3- or 4-month period, allowing facilitators time to implement the care strategy in their service and to practise delivering the review to stroke survivors. Prior to commencing recruitment to the trial, the progress of the facilitators needs be checked to ensure that they understand the care strategy and are delivering it as intended.
The nature of the care strategy is such that it is flexible and emergent. This means that assessment of progress needs to explore whether or not the individuals working as facilitators understand the key features, the key purposes and the key activities of the care strategy; we also need to know whether or not facilitators are able to apply these in practice. The resources available to the LoTS2Care research team, alongside the geographical spread of the sites, mean that observation of delivery of the New Start review has not been possible for all facilitators at all sites. Moreover, it is recognised that observation of one review provides a ‘snapshot’ of a facilitator’s understanding and ability to apply this. For these reasons, the approach to assessing the progress of facilitators uses multiple components. These are outlined in the following sections.
Observation (when possible)
If possible, observing delivery of the care strategy is beneficial because it allows the researcher to see whether or not facilitators are able to apply their knowledge and skills of the New Start care strategy in practice. Natasha Hardicre (research fellow leading on training and implementation of the New Start care strategy) observed delivery of the review in the facilitators’ services [these observations took place during the time between the initial training course (September 2016) and commencement of recruitment to the trial (January 2017)]. The benefit of this method is that the review was done in its ‘natural’ setting. This allowed other factors to be observed, such as the ways in which facilitators and stroke survivors interacted with the environment and the interactions that occurred before and after the review, for example doorstep interactions or navigating clinical settings. Natasha observed four reviews in three services, delivered by three facilitators. Three of these reviews took place in a clinic within a hospital setting and one took place at the home of the stroke survivor participating in the review. All stroke survivors gave their verbal consent that they were happy for someone to observe their review.
An observation guide was created to provide observers with a structured framework when observing a review. This guide draws on the logic model. It contains sections to report what has been observed and a section where feedback can be noted down.
Oral or written test
Observation, although helpful, can provide only limited insight into a facilitator’s understanding of the care strategy, and their ability to apply key principles in that particular situation. Therefore, testing understanding through an oral interview or written test will be used to supplement any observation that is done, or as the primary means of assessing knowledge and understanding if observation is not possible. Specific questions will be asked to explore the facilitators’ understanding of the key features of the New Start care strategy, the differences between New Start and their previous/other ways of providing reviews to stroke survivors, the key purposes of the care strategy and how these are achieved, and the activities associated with their role as a facilitator. There are a number of ways of administering these questions.
They can be given as a written test for facilitators to complete and return. The advantage of this method is that facilitators have an opportunity to reflect on what they have learnt and spend time articulating their answers in ways that reflect their knowledge and understanding. There is, however, some risk that facilitators will rely too heavily on their notes or other resources, resulting in a presentation of their knowledge that does not reflect their actual understanding of the care strategy.
Moreover, facilitators need to be familiar enough with the care strategy and the materials to be able to deliver it effectively and flexibly with stroke survivors and their carers. Alternatively, then, facilitators could complete this under ‘test conditions’ to examine the degree to which they can accurately recall what they know and understand about New Start. However, sitting a test under exam conditions can be a cause of anxiety and stress for some people, and this may inhibit their ability to recall information. It may not, therefore, accurately reflect their ability to actually deliver the care strategy to stroke survivors.
Another alternative is to interview facilitators and ask the questions verbally. This would enable the facilitators to describe their understanding of the care strategy in a conversation. This enables the researcher to prompt or probe the facilitators in order to explore their understanding and go beyond surface-level description. The conversation could be recorded and transcribed for records or further analysis if required in the future. Oral interviews provide a way of checking that a facilitator is familiar enough with the care strategy that they are able to recall its key features, while allowing facilitators the time and opportunity to describe them through the course of a conversation. The researcher administering the interview can also seek clarification about points they are unsure about and this can provide a more robust and comprehensive assessment of progress and competency. It was agreed by the Trial Management Group that interviews would be carried out via telephone between 11 and 20 January 2017. Louisa Burton (research fellow) carried out these interviews. The Trial Management Group agreed that, if assessments were needed in a future trial, they would be happy for the questions to be administered and completed via interview in person or by telephone, or as a written test.
Reflective reports
Reflective practice is something that the facilitators are being asked to engage in on a regular basis. At the training course, weekly reflection was suggested as good practice, although reflective reports are requested only monthly so as to reduce burden on facilitators. Facilitators are able to choose what they reflect on for these monthly reports, although guidance was given at the training workshop (e.g. implementation issues, occasions when reviews went well, occasions when reviews were challenging). Facilitators are also able to choose how to present their reflections (e.g. visually, structured report, diary/journal entry).
As part of the assessment of progress of the New Start facilitators, they are being asked to complete two additional structured reflective reports. Each of these focuses on an occasion when the facilitators have delivered the New Start review. The report asks facilitators to describe the situation, reflect on their experience, compare it with their expectations and apply their theoretical knowledge, and suggest ways that they could learn from the experience. Facilitators were sent these reports on Thursday 5 January 2017; the deadline for completion and return was Wednesday 18 January 2017.
Assessing competency
Assessing progress is part of the training package, but, for the purposes of the trial, it is also important to establish whether or not facilitators are competent at delivering the intervention as intended. To use these means of assessment, structured guides have been created to allow a researcher to conduct the assessment and decide whether or not a facilitator is ‘competent’. Possible outcomes of the assessment are:
-
Facilitator is considered ‘competent’ – they have sufficient knowledge and understanding of the key features, aims and activities of the New Start care strategy, and they can demonstrate an ability to apply their knowledge in practice.
-
Facilitator is not considered ‘competent’ yet – if a facilitator does not yet have sufficient knowledge and understanding of the New Start review, or has not been able to demonstrate an ability to apply their knowledge in practice (e.g. insufficient practice at delivering it), a training plan will be put in place. Reassessment will then take place at a future time point.
Reassessing competency
Reassessment will use the same components as the original assessment: assessment questions and review of a reflective reports and activity record. It is expected that the facilitator will deliver at least one review between the original assessment and reassessment; this will give them an opportunity to apply any additional knowledge or understanding that they have gained. It will also provide them with an experience to reflect on. Only one additional reflective report will be required for reassessment.
Appendix 14 The LoTS2Care outcome measures
| Measure | Domain(s)/explanation | Possible primary outcome | Mediator/ moderator | Likelihood of responding to this intervention in this population? | Other for/against | Time point |
|---|---|---|---|---|---|---|
| WHODAS 2.0 |
|
Yes | No |
|
|
|
| EQ-5D | ‘Health-related quality of life’‘A cardinal index of health’‘Health state in five dimensions’ | No | Although the underlying constructs are likely to change somewhat, they are not our main focus and are likely to be relatively insensitive |
|
|
|
| ICECAP-A | Capability of well-being, that is the ability to have stability, to have attachment, to have autonomy, to achieve, to enjoy | No | Although each item seems relevant, it is perception of capability that is asked about. Although perception of capability may well change, the intervention is not directed at context, but what people do and how they feel |
|
||
| WEMWBS |
|
Unclear |
|
For: being used currently in many public health initiatives, so should become well recognised |
|
|
| Stroke PAM |
|
Yes |
|
|
|
|
| Two questions from GP patient survey |
|
Yes | For: should be responsive and intermediate in the link to QoL/participation outcomes |
|
|
|
| Social questions | How much support someone might be able to elicit in times of need (from family, friends, etc.) | Yes |
|
|||
| Health economics resource | Covers employment, stroke-related activities and resource use | No | Unlikely employment will respond substantially (but would be good if it did) | Mainly designed to assess inputs rather than outcomes |
|
|
| LUNS tool | Longer-term needs after stroke (that could be addressed by existing services) |
|
|
Appendix 15 Workstream 5: trial key findings, figures and tables
| Stratification factor | Randomised service, n (%) | ||
|---|---|---|---|
| Usual care (n = 5) | New Start (n = 5) | Total (n = 10) | |
| Recruitment and intervention at separate trusts | |||
| Yes | 1 (20.0) | 1 (20.0) | 2 (20.0) |
| No | 4 (80.0) | 4 (80.0) | 8 (80.0) |
| Size of service (number of referrals in previous 12 months) | |||
| ≤ 300 | 3 (60.0) | 3 (60.0) | 6 (60.0) |
| > 300 | 2 (40.0) | 2 (40.0) | 4 (40.0) |
| Site | Screened (N) | Stroke survivors, n (%) | Registered (of those eligible) (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Eligible (of those screened) | Information pack sent (of those eligible) | Interested in taking part (of those to whom a pack was sent) | Baseline questionnaire posted (of those interested) | Baseline questionnaire returned (of posted) | Registered (of those who returned the pack) | |||
| New Start sites | ||||||||
| 1 | 39 | 38 (97.4) | 37 (97.4) | 16 (43.2) | 16 (100.0) | 13 (81.3) | 13 (100.0) | 34.2 |
| 2b | 13 | 13 (100.0) | 13 (100.0) | 1 (7.7) | 1 (100.0) | 1 (100.0) | 1 (100.0) | 7.7 |
| 2c | 16 | 14 (87.5) | 14 (100.0) | 3 (21.4) | 1 (33.3) | 3 (100.0) | 3 (100.0) | 21.4 |
| 3 | 350 | 347 (99.1) | 345 (99.4) | 121 (35.1) | 110 (90.9) | 93 (78.2) | 89 (95.7) | 25.6 |
| 4 | 106 | 94 (88.7) | 92 (97.9) | 28 (30.4) | 27 (96.4) | 23 (82.1) | 22 (95.7) | 23.4 |
| 5 | 102 | 81 (79.4) | 81 (100.0) | 24 (29.6) | 16 (66.7) | 17 (70.8) | 17 (100.0) | 16.7 |
| Usual care sites | ||||||||
| 6 | 183 | 168 (91.8) | 168 (100.0) | 68 (40.5) | 67 (98.5) | 43 (64.2) | 42 (97.7) | 21.0 |
| 7 | 30 | 29 (96.7) | 29 (100.0) | 10 (34.5) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 34.5 |
| 8 | 112 | 111 (99.1) | 110 (99.1) | 36 (32.7) | 35 (97.2) | 29 (82.9) | 29 (100.0) | 26.1 |
| 9 | 116 | 89 (76.7) | 84 (94.4) | 42 (50.0) | 39 (92.9) | 28 (66.7) | 28 (100.0) | 31.5 |
| 10 | 60 | 50 (83.3) | 50 (100.0) | 18 (36.0) | 18 (100.0) | 15 (83.3) | 15 (100.0) | 30.0 |
| Total | 1127 | 1034 (91.7) | 1023 (98.9) | 367 (35.9) | 340 (92.6) | 274 (75.5) | 269 (98.2) | 26.0 |
| Treatment arm | Screened (N) | Stroke survivor, n (%) | Registered (of those eligible) (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Eligible (of those screened) | Information pack sent (of those eligible) | Interested in taking part (of those to whom a pack was sent) | Baseline questionnaire posted (of those interested) | Baseline questionnaire returned (of posted) | Registered (of those who returned the pack) | |||
| Usual care | 501 | 447 (89.2) | 441 (98.7) | 174 (39.5) | 169 (97.1) | 124 (72.1) | 124 (99.2) | –27.70 |
| New Start | 626 | 587 (93.8) | 582 (99.1) | 193 (33.2) | 171 (88.6) | 150 (78.5) | 145 (97.3) | –24.70 |
| Total | 1127 | 1034 (91.7) | 1023 (98.9) | 367 (35.9) | 340 (92.6) | 274 (75.5) | 269 (98.2) | –26.00 |
| Characteristic | Screened | Clinical screening | Registered | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Total | Usual care | New Start | Total | Usual care | New Start | Total | |
| Total, n (%) | 501 (44.5) | 626 (55.5) | 1127 (100) | 386 (33.7) | 761 (66.3) | 1147 (100) | 124 (46.1) | 145 (53.9) | 269 (100) |
| Age (years) | |||||||||
| Mean (SD) | 73.9 (13.40) | 73.4 (12.91) | 73.6 (13.12) | 72.9 (14.17) | 73.7 (13.07) | 73.5 (13.44) | 72.2 (11.65) | 71.6 (10.88) | 71.9 (11.22) |
| Missing (n) | 8 | 1 | 9 | 23 | 3 | 26 | 1 | 0 | 1 |
| Sex, n (%) | |||||||||
| Male | 264 (52.7) | 355 (56.7) | 619 (54.9) | 220 (57.0) | 421 (55.3) | 641 (55.9) | 68 (54.8) | 81 (55.9) | 149 (55.4) |
| Missing | 6 (1.2) | 2 (0.3) | 8 (0.7) | 1 (0.3) | 0 (0.0) | 1 (0.1) | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Ethnicity, n (%) | |||||||||
| White | 275 (54.9) | 314 (50.2) | 589 (52.3) | 339 (87.8) | 696 (91.5) | 1035 (90.2) | 76 (61.3) | 115 (79.3) | 191 (71.0) |
| Black | 1 (0.2) | 7 (1.1) | 8 (0.7) | 4 (1.0) | 9 (1.2) | 13 (1.1) | 1 (0.8) | 1 (0.7) | 2 (0.7) |
| Asian | 10 (2.0) | 10 (1.6) | 20 (1.8) | 15 (3.9) | 16 (2.1) | 31 (2.7) | 2 (1.6) | 1 (0.7) | 3 (1.1) |
| Mixed | 5 (1.0) | 1 (0.2) | 6 (0.5) | 3 (0.8) | 1 (0.1) | 4 (0.3) | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Other ethnic group | 3 (0.6) | 1 (0.2) | 4 (0.4) | 6 (1.6) | 4 (0.5) | 10 (0.9) | 2 (1.6) | 0 (0.0) | 2 (0.7) |
| Not stated | 90 (18.0) | 182 (29.1) | 272 (24.1) | 4 (1.0) | 15 (2.0) | 19 (1.7) | 23 (18.5) | 26 (17.9) | 49 (18.2) |
| Missing | 117 (23.4) | 111 (17.7) | 228 (20.2) | 15 (3.9) | 20 (2.6) | 35 (3.1) | 19 (15.3) | 2 (1.4) | 21 (7.8) |
| Length of hospital stay (days) | |||||||||
| Mean (SD) | 13 (41) | 12 (28) | 13 (34) | 14 (20) | 11 (17) | 12 (19) | |||
| Missing (n) | 94 | 146 | 240 | 12 | 5 | 17 | |||
| mRS at discharge, n (%) | |||||||||
| 0 | 20 (4.0) | 58 (9.3) | 78 (6.9) | 7 (5.6) | 12 (8.3) | 19 (7.1) | |||
| 1 | 63 (12.6) | 77 (12.3) | 140 (12.4) | 24 (19.4) | 23 (15.9) | 47 (17.5) | |||
| 2 | 82 (16.4) | 35 (5.6) | 117 (10.4) | 25 (20.2) | 7 (4.8) | 32 (11.9) | |||
| 3 | 83 (16.6) | 32 (5.1) | 115 (10.2) | 19 (15.3) | 8 (5.5) | 27 (10.0) | |||
| 4 | 51 (10.2) | 25 (4.0) | 76 (6.7) | 15 (12.1) | 2 (1.4) | 17 (6.3) | |||
| 5 | 9 (1.8) | 12 (1.9) | 21 (1.9) | 2 (1.6) | 1 (0.7) | 3 (1.1) | |||
| 6 | 0 (0.0) | 1 (0.2) | 1 (0.1) | ||||||
| Missing | 193 (38.5) | 386 (61.7) | 579 (51.4) | 32 (25.8) | 92 (63.4) | 124 (46.1) | |||
| NIHSS score at admission | |||||||||
| Mean (SD) | 5.6 (5.79) | 6.1 (6.05) | 5.8 (5.90) | 4.9 (5.46) | 4.4 (4.55) | 4.7 (5.11) | |||
| Missing (n) | 181 | 375 | 556 | 36 | 88 | 124 | |||
| Availability of carer, n (%) | |||||||||
| Yes | 62 (12.4) | 43 (6.9) | 105 (9.3) | 17 (13.7) | 22 (15.2) | 39 (14.5) | |||
| Missing | 79 (15.8) | 116 (18.5) | 195 (17.3) | 19 (15.3) | 12 (8.3) | 31 (11.5) | |||
| Characteristic | Usual care | New Start | Total |
|---|---|---|---|
| Total, n (%) | 124 (46.1) | 145 (53.9) | 269 (100) |
| Age (years) | |||
| Mean (SD) | 73 (12) | 72 (11) | 73 (11) |
| Missing (n) | 0 | 0 | 0 |
| Sex, n (%) | |||
| Male | 69 (55.6) | 81 (55.9) | 150 (55.8) |
| Female | 54 (43.5) | 64 (44.1) | 118 (43.9) |
| Missing | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Ethnicity, n (%) | |||
| White | 78 (62.9) | 115 (79.3) | 193 (71.7) |
| Black | 1 (0.8) | 1 (0.7) | 2 (0.7) |
| Asian | 2 (1.6) | 1 (0.7) | 3 (1.1) |
| Mixed | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Other ethnic group | 2 (1.6) | 0 (0.0) | 2 (0.7) |
| Not stated | 26 (21.0) | 28 (19.3) | 54 (20.1) |
| Missing | 14 (11.3) | 0 (0.0) | 14 (5.2) |
| Marital status, n (%) | |||
| Single | 5 (4.1) | 9 (6.2) | 14 (5.2) |
| Married | 66 (54.1) | 87 (60.0) | 153 (57.3) |
| Living as married | 7 (5.7) | 5 (3.4) | 12 (4.5) |
| Separated | 0 (0.0) | 3 (2.1) | 3 (1.1) |
| Divorced | 8 (6.6) | 11 (7.6) | 19 (7.1) |
| Widowed | 33 (27.0) | 29 (20.0) | 62 (23.2) |
| Missing | 5 (4.0) | 1 (0.7) | 4 (1.5) |
| Living arrangement, n (%) | |||
| Living alone | 34 (27.4) | 40 (27.6) | 74 (27.5) |
| Living with another person | 74 (59.7) | 100 (69.0) | 174 (64.7) |
| Missing | 16 (12.9) | 5 (3.4) | 21 (7.8) |
| Education level, n (%) | |||
| None | 2 (1.6) | 2 (1.4) | 4 (1.5) |
| Primary school | 5 (4.1) | 7 (4.8) | 12 (4.5) |
| Secondary school | 55 (45.1) | 74 (51.0) | 129 (48.3) |
| Further/higher education | 55 (45.1) | 62 (42.8) | 117 (43.8) |
| Missing | 7 (5.6) | 0 (0.0) | 5 (1.9) |
| Time since stroke (months) | |||
| Mean (SD) | 5.3 (0.83) | 5.4 (0.71) | 5.4 (0.77) |
| Missing (n) | 1 | 1 | 2 |
| Level of language ability after stroke, n (%) | |||
| Normal | 40 (32.3) | 47 (32.4) | 87 (32.3) |
| Dysphasia | 1 (0.8) | 5 (3.4) | 6 (2.2) |
| Dysarthria | 0 (0.0) | 3 (2.1) | 3 (1.1) |
| Not known | 80 (64.5) | 90 (62.1) | 170 (63.2) |
| Missing | 3 (2.4) | 0 (0.0) | 3 (1.1) |
| Length of hospital stay (days) | |||
| Mean (SD) | 15 (24) | 11 (18) | 13 (21) |
| Missing | 9 | 4 | 13 |
| Time between onset/awareness of stroke and hospital admission (days)a | |||
| Mean (SD) | 0.5 (4.84) | 0.1 (0.84) | 0.3 (3.34) |
| Missing | 4 | 3 | 7 |
| mRS at discharge,b n (%) | |||
| 0 | 5 (4.0) | 11 (7.6) | 16 (5.9) |
| 1 | 24 (19.4) | 22 (15.2) | 46 (17.1) |
| 2 | 27 (21.8) | 7 (4.8) | 34 (12.6) |
| 3 | 20 (16.1) | 8 (5.5) | 28 (10.4) |
| 4 | 14 (11.3) | 1 (0.7) | 15 (5.6) |
| 5 | 2 (1.6) | 1 (0.7) | 3 (1.1) |
| Missing | 32 (25.8) | 95 (65.5) | 127 (47.2) |
| NIHSS score at admissionb | |||
| Mean (SD) | 5.0 (5.51) | 4.5 (4.51) | 4.8 (5.12) |
| Missing | 38 | 88 | 126 |
| Availability of carer, n (%) | |||
| Yes | 41 (33.1) | 52 (35.9) | 93 (34.6) |
| No | 53 (42.7) | 79 (54.5) | 132 (49.1) |
| Not known | 29 (23.4) | 14 (9.7) | 43 (16.0) |
| Missing | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| WHODAS simple score (higher score indicates higher level of disability) | |||
| Mean (SD) | 26.2 (20.84) | 23.7 (18.10) | 24.9 (19.44) |
| Missing | 20 | 28 | 48 |
| WHODAS complex score (higher score indicates higher level of disability) | |||
| Mean (SD) | 26.9 (24.30) | 25.6 (19.34) | 26.2 (21.74) |
| Missing | 56 | 68 | 124 |
| WEMWBS score (higher score indicates better state of well-being) | |||
| Mean (SD) | 46.6 (12.63) | 47.5 (11.61) | 47.1 (12.06) |
| Missing | 6 | 0 | 6 |
| PAM score (higher score indicates higher level of activation) | |||
| Mean (SD) | 56.7 (16.93) | 58.6 (17.72) | 57.7 (17.35) |
| Missing | 3 | 2 | 5 |
| PAM level (categorised PAM score), n (%) | |||
| (≤ 47.0) does not believe that activation is important | 38 (30.6) | 36 (24.8) | 74 (27.5) |
| (47.1–55.1) a lack of knowledge and confidence to take action | 32 (25.8) | 45 (31.0) | 77 (28.6) |
| (55.2–67.0) beginning to take action | 30 (24.2) | 29 (20.0) | 59 (21.9) |
| (≥ 67.1) taking action | 21 (16.9) | 33 (22.8) | 54 (20.1) |
| Missing | 3 (2.4) | 2 (1.4) | 5 (1.9) |
| LUNS (number of long-term unmet needs), n (%) | |||
| 0 | 19 (15.3) | 16 (11.0) | 35 (13.0) |
| 1 | 20 (16.1) | 21 (14.5) | 41 (15.2) |
| 2 | 16 (12.9) | 21 (14.5) | 37 (13.8) |
| 3 | 15 (12.1) | 15 (10.3) | 30 (11.2) |
| 4 | 11 (8.9) | 12 (8.3) | 23 (8.6) |
| 5 | 7 (5.6) | 13 (9.0) | 20 (7.4) |
| ≥ 6 | 34 (27.4) | 47 (32.4) | 81 (30.1) |
| Missing | 2 (1.6) | 0 (0.0) | 2 (0.7) |
| GP patient survey, n (%) | |||
| Help around house when ill | |||
| A lot | 65 (52.4) | 96 (66.2) | 161 (59.9) |
| Some | 34 (27.4) | 32 (22.1) | 66 (24.5) |
| Not much | 9 (7.3) | 9 (6.2) | 18 (6.7) |
| None at all | 13 (10.5) | 8 (5.5) | 21 (7.8) |
| Missing | 3 (2.4) | 0 (0.0) | 3 (1.1) |
| Help with heavy jobs | |||
| A lot | 63 (50.8) | 89 (61.4) | 152 (56.5) |
| Some | 32 (25.8) | 37 (25.5) | 69 (25.7) |
| Not much | 12 (9.7) | 10 (6.9) | 22 (8.2) |
| None at all | 12 (9.7) | 9 (6.2) | 21 (7.8) |
| Missing | 5 (4.0) | 0 (0.0) | 5 (1.9) |
| Advice on important changes | |||
| A lot | 44 (35.5) | 57 (39.3) | 101 (37.5) |
| Some | 29 (23.4) | 34 (23.4) | 63 (23.4) |
| Not much | 12 (9.7) | 15 (10.3) | 27 (10.0) |
| None at all | 25 (20.2) | 20 (13.8) | 45 (16.7) |
| Missing | 14 (11.3) | 19 (13.1) | 33 (12.3) |
| Problems with spouse | |||
| A lot | 37 (29.8) | 58 (40.0) | 95 (35.3) |
| Some | 24 (19.4) | 33 (22.8) | 57 (21.2) |
| Not much | 9 (7.3) | 8 (5.5) | 17 (6.3) |
| None at all | 25 (20.2) | 18 (12.4) | 43 (16.0) |
| Missing | 29 (23.4) | 28 (19.3) | 57 (21.2) |
| Feeling depressed | |||
| A lot | 45 (36.3) | 68 (46.9) | 113 (42.0) |
| Some | 43 (34.7) | 45 (31.0) | 88 (32.7) |
| Not much | 18 (14.5) | 12 (8.3) | 30 (11.2) |
| None at all | 13 (10.5) | 13 (9.0) | 26 (9.7) |
| Missing | 5 (4.0) | 7 (4.8) | 12 (4.5) |
| Help caring for someone | |||
| A lot | 30 (24.2) | 40 (27.6) | 70 (26.0) |
| Some | 15 (12.1) | 16 (11.0) | 31 (11.5) |
| Not much | 6 (4.8) | 4 (2.8) | 10 (3.7) |
| None at all | 29 (23.4) | 24 (16.6) | 53 (19.7) |
| Missing | 44 (35.5) | 61 (42.1) | 105 (39.0) |
| Need someone to look after your home when away | |||
| A lot | 52 (41.9) | 71 (49.0) | 123 (45.7) |
| Some | 31 (25.0) | 34 (23.4) | 65 (24.2) |
| Not much | 12 (9.7) | 5 (3.4) | 17 (6.3) |
| None at all | 16 (12.9) | 22 (15.2) | 38 (14.1) |
| Missing | 13 (10.5) | 13 (9.0) | 26 (9.7) |
| Characteristic | Usual care | New Start | Total |
|---|---|---|---|
| Total, n (%) | 39 (45.9) | 46 (54.1) | 85 (100) |
| Carer age at registration (years) | |||
| Mean (SD) | 63.4 (12.95) | 67.6 (10.73) | 65.7 (11.92) |
| Median (range) | 64.1 (32.2–87.2) | 69.5 (46.2–84.0) | 66.9 (32.2–87.2) |
| Missing (n) | 1 | 1 | 2 |
| Carer sex, n (%) | |||
| Male | 11 (28.2) | 17 (37.0) | 28 (32.9) |
| Female | 28 (71.8) | 29 (63.0) | 57 (67.1) |
| Who caring for, n (%) | |||
| Spouse/partner | 23 (59.0) | 37 (80.4) | 60 (70.6) |
| Son/daughter (including in-law, step child) | 8 (20.5) | 1 (2.2) | 9 (10.6) |
| Parent | 7 (17.9) | 5 (10.9) | 12 (14.1) |
| Other relative | 1 (2.6) | 1 (2.2) | 2 (2.4) |
| Friend | 0 (0.0) | 2 (4.3) | 2 (2.4) |
| Total CBS score (points) | |||
| Mean (SD) | 48.7 (15.32) | 45.6 (15.28) | 47.0 (15.28) |
| Median (range) | 46.0 (23.0–79.0) | 42.5 (24.0–83.8) | 43.5 (23.0–83.8) |
| Missing (n) | 1 | 0 | 1 |
| CBS subscore (points): general strain | |||
| Mean (SD) | 20.1 (7.02) | 17.9 (6.32) | 18.9 (6.69) |
| Median (range) | 19.5 (8.0–32.0) | 16.0 (8.0–31.0) | 17.0 (8.0–32.0) |
| Missing (n) | 1 | 0 | 1 |
| CBS subscore (points): isolation | |||
| Mean (SD) | 7.3 (2.46) | 6.9 (2.50) | 7.1 (2.47) |
| Median (range) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) |
| Missing (n) | 3 | 0 | 3 |
| CBS subscore (points): disappointment | |||
| Mean (SD) | 11.3 (4.25) | 10.8 (4.31) | 11.0 (4.27) |
| Median (range) | 10.5 (5.0–19.0) | 10.0 (5.0–20.0) | 10.0 (5.0–20.0) |
| Missing (n) | 1 | 0 | 1 |
| CBS subscore (points): emotional involvement | |||
| Mean (SD) | 4.8 (1.74) | 4.9 (2.27) | 4.8 (2.03) |
| Median (range) | 4.0 (3.0–10.0) | 4.0 (3.0–11.0) | 4.0 (3.0–11.0) |
| Missing (n) | 1 | 0 | 1 |
| CBS subscore (points): environment | |||
| Mean (SD) | 5.3 (2.00) | 5.0 (2.10) | 5.1 (2.04) |
| Median (range) | 5.0 (3.0–9.0) | 5.0 (3.0–12.0) | 5.0 (3.0–12.0) |
| Missing (n) | 1 | 2 | 3 |
| Availability | Treatment allocation, n (%) | ||
|---|---|---|---|
| Usual care (N = 124) | New Start (N = 145) | Total (N = 269) | |
| Available at 3 months | |||
| Yes | 120 (96.8) | 142 (97.9) | 262 (97.4) |
| No | 4 (3.2) | 3 (2.1) | 7 (2.6) |
| Available at 6 months | |||
| Yes | 116 (93.5) | 136 (93.8) | 252 (93.7) |
| No | 8 (6.5) | 9 (6.2) | 17 (6.3) |
| Available at 9 months | |||
| Yes | 108 (87.1) | 131 (90.3) | 239 (88.8) |
| No | 16 (12.9) | 14 (9.7) | 30 (11.2) |
| Availability | New Start, n (%) | Usual care, n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 13) | 2a (N = 4) | 3 (N = 89) | 4 (N = 22) | 5 (N = 17) | Total (N = 145) | 6 (N = 42) | 7 (N = 10) | 8 (N = 29) | 9 (N = 28) | 10 (N = 15) | Total (N = 124) | |
| Available at 3 months | ||||||||||||
| Yes | 13 (100.0) | 2 (50.0) | 88 (98.9) | 22 (100.0) | 17 (100.0) | 142 (97.9) | 41 (97.6) | 9 (90.0) | 29 (100.0) | 27 (96.4) | 14 (93.3) | 120 (96.8) |
| No | 0 (0.0) | 2 (50.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 3 (2.1) | 1 (2.4) | 1 (10.0) | 0 (0.0) | 1 (3.6) | 1 (6.7) | 4 (3.2) |
| Available at 6 months | ||||||||||||
| Yes | 12 (92.3) | 2 (50.0) | 87 (97.8) | 19 (86.4) | 16 (94.1) | 136 (93.8) | 41 (97.6) | 8 (80.0) | 28 (96.6) | 25 (89.3) | 14 (93.3) | 116 (93.5) |
| No | 1 (7.7) | 2 (50.0) | 2 (2.2) | 3 (13.6) | 1 (5.9) | 9 (6.2) | 1 (2.4) | 2 (20.0) | 1 (3.4) | 3 (10.7) | 1 (6.7) | 8 (6.5) |
| Available at 9 months | ||||||||||||
| Yes | 11 (84.6) | 2 (50.0) | 85 (95.5) | 19 (86.4) | 14 (82.4) | 131 (90.3) | 39 (92.9) | 8 (80.0) | 26 (89.7) | 24 (85.7) | 11 (73.3) | 108 (87.1) |
| No | 2 (15.4) | 2 (50.0) | 4 (4.5) | 3 (13.6) | 3 (17.6) | 14 (9.7) | 3 (7.1) | 2 (20.0) | 3 (10.3) | 4 (14.3) | 4 (26.7) | 16 (12.9) |
| Treatment allocation | Site | Patient number | Registration date | Date of death | Place of death | Primary cause of death | Time between registration and death | |
|---|---|---|---|---|---|---|---|---|
| Days | Months | |||||||
| Usual care | 10 | 219 | 17 July 2017 | 9 March 2018 | Hospital | Not known (not recorded). Admitted to hospital YC GCS98 after being found unconscious and not seen for 2 days | 235 | 7.7 |
| Usual care | 10 | 89 | 13 April 2017 | 1 May 2017 | Unknown | Unknown | 18 | 0.6 |
| Usual care | 8 | 216 | 13 July 2017 | 27 March 2018 | Unknown | Suicide by hanging | 257 | 8.4 |
| Usual care | 6 | 63 | 27 March 2017 | 20 December 2017 | Unknown | Unknown | 268 | 8.8 |
| New Start | 2c | 1 | 7 February 2017 | 10 March 2017 | Unknown | Bronchopneumonia, ischaemic and vascular heart disease | 31 | 1 |
| New Start | 2c | 10 | 23 February 2017 | 24 April 2017 | Home | Malignant tumour of breast, cause of death 94b | 60 | 2 |
| New Start | 4 | 136 | 09 May 2017 | 22 September 2017 | Unknown | Unknown | 136 | 4.5 |
| Site | Facilitators (n) | Received training manual | Access to online materials | Attended initial session | Attended follow-up session | Practised intervention | Completed reflective report | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| 1 | 3 | 3 | 100.00 | 1 | 33.30 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 |
| 2a | 3 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 |
| 3 | 4 | 4 | 100.00 | 3 | 75.00 | 4 | 100.00 | 4 | 100.00 | 4 | 100.00 | 4 | 100.00 |
| 4 | 2 | 2 | 100.00 | 0 | 0.00 | 1 | 50.00 | 1 | 50.00 | 2 | 100.00 | 1 | 50.00 |
| 5 | 3 | 3 | 100.00 | 3 | 100.00 | 3 | 100.00 | 2 | 66.70 | 3 | 100.00 | 3 | 100.00 |
| Total | 15 | 15 | 100.00 | 10 | 66.70 | 14 | 93.30 | 13 | 86.70 | 15 | 100.00 | 14 | 93.30 |
| Site | Facilitators (n) | Deemed competent | |
|---|---|---|---|
| n | % | ||
| 1 | 3 | 3 | 100.0 |
| 2a | 3 | 3 | 100.0 |
| 3 | 4 | 4 | 100.0 |
| 4 | 2 | 2 | 100.0 |
| 5 | 3 | 3 | 100.0 |
| Total | 15 | 15 | 100.0 |
| Site | Number of recruited trial participants | Survivors contacted (informal)a (n) | Survivors contacted (formal) | Completed at least one New Start visit | Total number of visits | Average number of visits per survivor | Average duration of visit (minutes) | ||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||||
| 1 | 13 | 11 | 10 | 76.90 | 7 | 53.80 | 13 | 1.86 | 80.4 |
| 2a | 4 | 4 | 4 | 100.00 | 3 | 75.00 | 3 | 1 | 51.7 |
| 3 | 89 | 87 | 65 | 73.00 | 65 | 73.00 | 71 | 1.09 | 57 |
| 4 | 22 | 22 | 9 | 40.90 | 9 | 40.90 | 9 | 1 | 41.7 |
| 5 | 17 | 14 | 14 | 82.40 | 2 | 11.80 | 2 | 1 | 60 |
| Total | 145 | 138 | 102 | 70.30 | 86 | 59.30 | 98 | 1.14 | 58.6 |
| Site | Survivors contacteda (n) | Completed at least one New Start visit | Total number of visits | Average number of visits per survivor | Average duration of visit (minutes) | |
|---|---|---|---|---|---|---|
| n | % | |||||
| 1 | 34 | 13 | 38.20 | 17 | 1.31 | 73.8 |
| 2a | 86 | 82 | 95.30 | 110 | 1.34 | 48 |
| 3 | 148 | 147 | 99.30 | 162 | 1.1 | 55 |
| 4 | 41 | 38 | 92.70 | 38 | 1 | 40 |
| 5 | 133 | 14 | 10.50 | 14 | 1 | 52.9 |
| Total | 442 | 294 | 66.50 | 341 | 1.16 | 51.9 |
| Facilitator ID | Site | Number of patients | Total number of visits | Average number of visits per survivor | Average duration of visit (minutes) |
|---|---|---|---|---|---|
| 2 | 1 | 4 | 8 | 2 | 81.9 |
| 11 | 1 | 3 | 5 | 1.67 | 78 |
| 12 | 1 | – | – | – | – |
| 3 | 2a | – | – | – | – |
| 4 | 2a | 2 | 2 | 1 | 67.5 |
| 5 | 2a | 1 | 1 | 1 | 20 |
| 6 | 3 | 24 | 25 | 1.04 | 60.2 |
| 8 | 3 | 11 | 12 | 1.09 | 56.3 |
| 9 | 3 | 14 | 16 | 1.14 | 51.9 |
| 10 | 3 | 16 | 18 | 1.13 | 57.5 |
| 15 | 4 | 4 | 4 | 1 | 35 |
| 17 | 4 | 5 | 5 | 1 | 47 |
| 13 | 5 | 1 | 1 | 1 | 60 |
| 14 | 5 | 1 | 1 | 1 | 60 |
| 18 | 5 | – | – | – | – |
| Total | 86 | 98 | 1.14 | 58.6 | |
| Facilitator ID | Site | Number of patients | Total number of visits | Average number of visits per survivor | Average duration of visit (minutes) |
|---|---|---|---|---|---|
| 2 | 1 | 10 | 10 | 1 | 66.5 |
| 11 | 1 | 3 | 7 | 2.33 | 84.3 |
| 12 | 1 | – | – | – | – |
| 4 | 2a | 35 | 40 | 1.14 | 52.6 |
| 5 | 2a | 28 | 41 | 1.43 | 44.3 |
| 3 | 2a | 19 | 29 | 1.53 | 46.8 |
| 6 | 3 | 43 | 46 | 1.07 | 56 |
| 8 | 3 | 42 | 47 | 1.12 | 45.9 |
| 9 | 3 | 19 | 24 | 1.26 | 76.3 |
| 10 | 3 | 43 | 45 | 1.05 | 52.2 |
| 15 | 4 | 18 | 18 | 1 | 37.2 |
| 17 | 4 | 20 | 20 | 1 | 42.5 |
| 13 | 5 | 6 | 6 | 1 | 72.5 |
| 14 | 5 | – | – | – | – |
| 18 | 5 | 4 | 4 | 1 | 31.3 |
| Unknown | 5 | 4 | 4 | 1 | 45 |
| Total | 294 | 341 | 1.16 | 51.9 | |
| Characteristic | Baseline | ||
|---|---|---|---|
| Intervention not received | Intervention received | Total | |
| Participants, n (%) | 59 (40.7) | 86 (59.3) | 145 (100) |
| Age (years) | |||
| Mean (SD) | 74 (12) | 71 (10) | 72 (11) |
| Missing (n) | 0 | 0 | 0 |
| Sex, n (%) | |||
| Male | 32 (54.2) | 49 (57.0) | 81 (55.9) |
| Female | 27 (45.8) | 37 (43.0) | 64 (44.1) |
| Ethnicity, n (%) | |||
| White | 47 (79.7) | 68 (79.1) | 115 (79.3) |
| Black | 1 (1.7) | 0 (0.0) | 1 (0.7) |
| Asian | 0 (0.0) | 1 (1.2) | 1 (0.7) |
| Not stated | 11 (18.6) | 17 (19.8) | 28 (19.3) |
| Marital status, n (%) | |||
| Single | 3 (5.1) | 3 (3.5) | 6 (4.1) |
| Married | 32 (54.2) | 49 (57.0) | 81 (55.9) |
| Living as married | 3 (5.1) | 2 (2.3) | 5 (3.4) |
| Separated | 0 (0.0) | 3 (3.5) | 3 (2.1) |
| Divorced | 2 (3.4) | 8 (9.3) | 10 (6.9) |
| Widowed | 18 (30.5) | 20 (23.3) | 38 (26.2) |
| Missing | 1 (1.7) | 1 (1.2) | 2 (1.4) |
| Living arrangement, n (%) | |||
| Living alone | 16 (27.1) | 24 (27.9) | 40 (27.6) |
| Living with another person | 41 (69.5) | 59 (68.6) | 100 (69.0) |
| Missing | 2 (3.4) | 3 (3.5) | 5 (3.4) |
| Education level, n (%) | |||
| None | 1 (1.7) | 0 (0.0) | 1 (0.7) |
| Primary school | 4 (6.8) | 3 (3.5) | 7 (4.8) |
| Secondary school | 25 (42.4) | 48 (55.8) | 73 (50.3) |
| Further/higher education | 29 (49.2) | 34 (39.5) | 63 (43.4) |
| Missing | 0 (0.0) | 1 (1.2) | 1 (0.7) |
| Time since stroke (months) | |||
| Mean (SD) | 5.2 (0.77) | 5.4 (0.66) | 5.4 (0.71) |
| Missing (n) | 0 | 1 | 1 |
| Level of language ability after stroke, n (%) | |||
| Normal | 24 (40.7) | 23 (26.7) | 47 (32.4) |
| Dysphasia | 0 (0.0) | 5 (5.8) | 5 (3.4) |
| Dysarthria | 1 (1.7) | 2 (2.3) | 3 (2.1) |
| Not known | 34 (57.6) | 56 (65.1) | 90 (62.1) |
| Length of hospital stay (days) | |||
| Mean (SD) | 8 (11) | 13 (21) | 11 (18) |
| Missing (n) | 1 | 3 | 4 |
| Time between onset/awareness of stroke and hospital admission (days) | |||
| Mean (SD) | 0.2 (0.70) | 0.0 (0.93) | 0.1 (0.84) |
| Missing (n) | 1 | 2 | 3 |
| mRS at discharge, n (%) | |||
| 0 | 7 (11.9) | 4 (4.7) | 11 (7.6) |
| 1 | 15 (25.4) | 7 (8.1) | 22 (15.2) |
| 2 | 3 (5.1) | 4 (4.7) | 7 (4.8) |
| 3 | 4 (6.8) | 4 (4.7) | 8 (5.5) |
| 4 | 1 (1.7) | 0 (0.0) | 1 (0.7) |
| 5 | 0 (0.0) | 1 (1.2) | 1 (0.7) |
| Missing | 29 (49.2) | 66 (76.7) | 95 (65.5) |
| NIHSS score at admission | |||
| Mean (SD) | 5.2 (4.75) | 3.7 (4.14) | 4.5 (4.51) |
| Missing (n) | 27 | 61 | 88 |
| Availability of carer, n (%) | |||
| Yes | 17 (28.8) | 35 (40.7) | 52 (35.9) |
| No | 31 (52.5) | 48 (55.8) | 79 (54.5) |
| Not known | 11 (18.6) | 3 (3.5) | 14 (9.7) |
| WHODAS simple score (higher score indicates higher level of disability) | |||
| Mean (SD) | 25.6 (18.62) | 22.3 (17.76) | 23.7 (18.10) |
| Missing (n) | 12 | 16 | 28 |
| WHODAS complex score (higher score indicates higher level of disability) | |||
| Mean (SD) | 26.9 (20.31) | 24.3 (18.56) | 25.6 (19.34) |
| Missing (n) | 22 | 46 | 68 |
| WEMWBS score (higher score indicates better state of wellbeing) | |||
| Mean (SD) | 48.4 (11.73) | 46.9 (11.56) | 47.5 (11.61) |
| Missing (n) | 0 | 0 | 0 |
| PAM score (higher score indicates higher level of activation) | |||
| Mean (SD) | 58.6 (18.00) | 58.6 (17.62) | 58.6 (17.72) |
| Missing (n) | 0 | 2 | 2 |
| PAM level (categorised PAM score), n (%) | |||
| (≤ 47.0) Not believing activation is important | 14 (23.7) | 22 (25.6) | 36 (24.8) |
| (47.1–55.1) A lack of knowledge and confidence to take action | 21 (35.6) | 24 (27.9) | 45 (31.0) |
| (55.2–67.0) Beginning to take action | 9 (15.3) | 20 (23.3) | 29 (20.0) |
| (≥ 67.1) Taking action | 15 (25.4) | 18 (20.9) | 33 (22.8) |
| Missing | 0 (0.0) | 2 (2.3) | 2 (1.4) |
| LUNS (number of long-term unmet needs), n (%) | |||
| 0 | 9 (15.3) | 7 (8.1) | 16 (11.0) |
| 1 | 8 (13.6) | 13 (15.1) | 21 (14.5) |
| 2 | 9 (15.3) | 12 (14.0) | 21 (14.5) |
| 3 | 5 (8.5) | 10 (11.6) | 15 (10.3) |
| 4 | 8 (13.6) | 4 (4.7) | 12 (8.3) |
| 5 | 5 (8.5) | 8 (9.3) | 13 (9.0) |
| ≥ 6 | 15 (25.4) | 32 (37.2) | 47 (32.4) |
| GP patient survey, n (%) | |||
| Help around house when ill | |||
| A lot | 41 (69.5) | 55 (64.0) | 96 (66.2) |
| Some | 15 (25.4) | 17 (19.8) | 32 (22.1) |
| Not much | 2 (3.4) | 7 (8.1) | 9 (6.2) |
| None at all | 1 (1.7) | 7 (8.1) | 8 (5.5) |
| Help with heavy jobs | |||
| A lot | 42 (71.2) | 47 (54.7) | 89 (61.4) |
| Some | 11 (18.6) | 26 (30.2) | 37 (25.5) |
| Not much | 3 (5.1) | 7 (8.1) | 10 (6.9) |
| None at all | 3 (5.1) | 6 (7.0) | 9 (6.2) |
| Advice on important changes | |||
| A lot | 30 (50.8) | 27 (31.4) | 57 (39.3) |
| Some | 11 (18.6) | 23 (26.7) | 34 (23.4) |
| Not much | 4 (6.8) | 11 (12.8) | 15 (10.3) |
| None at all | 6 (10.2) | 14 (16.3) | 20 (13.8) |
| Missing | 8 (13.6) | 11 (12.8) | 19 (13.1) |
| Problems with spouse | |||
| A lot | 30 (50.8) | 28 (32.6) | 58 (40.0) |
| Some | 11 (18.6) | 22 (25.6) | 33 (22.8) |
| Not much | 2 (3.4) | 6 (7.0) | 8 (5.5) |
| None at all | 6 (10.2) | 12 (14.0) | 18 (12.4) |
| Missing | 10 (16.9) | 18 (20.9) | 28 (19.3) |
| Feeling depressed | |||
| A lot | 35 (59.3) | 33 (38.4) | 68 (46.9) |
| Some | 15 (25.4) | 30 (34.9) | 45 (31.0) |
| Not much | 4 (6.8) | 8 (9.3) | 12 (8.3) |
| None at all | 4 (6.8) | 9 (10.5) | 13 (9.0) |
| Missing | 1 (1.7) | 6 (7.0) | 7 (4.8) |
| Help caring for someone | |||
| A lot | 21 (35.6) | 19 (22.1) | 40 (27.6) |
| Some | 4 (6.8) | 12 (14.0) | 16 (11.0) |
| Not much | 3 (5.1) | 1 (1.2) | 4 (2.8) |
| None at all | 5 (8.5) | 19 (22.1) | 24 (16.6) |
| Missing | 26 (44.1) | 35 (40.7) | 61 (42.1) |
| Need someone to look after your home when away | |||
| A lot | 35 (59.3) | 36 (41.9) | 71 (49.0) |
| Some | 13 (22.0) | 21 (24.4) | 34 (23.4) |
| Not much | 1 (1.7) | 4 (4.7) | 5 (3.4) |
| None at all | 5 (8.5) | 17 (19.8) | 22 (15.2) |
| Missing | 5 (8.5) | 8 (9.3) | 13 (9.0) |
| Site | 4- to 8-month service | 6-month review | Provide service for all patients | How is review delivered? | Number of contacts | Telephone follow-up | Is the service open-ended? | Use of self-management | Use of action-planning | Use of goal-setting |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | No | Telephone | 1 | No | Yes | No | No | No |
| 2a | Yes | Yes | Yes | At patient’s home | 1–5 | Occasionally | No | No | No | No |
| 3 | Yes | Yes | Yes | At patient’s home/clinic | 1 | No | No | No | No | No |
| 4 | Yes | Yes | Yes | At patient’s home/clinic | 1 (usually) | No | Yes | Yes | Yes | No |
| 5 | Yes | Yes | Yes | At patient’s home | 1 | No | Yes | |||
| 6 | Yes | Yes | Yes | At patient’s home/telephone | Yes | Yes | Yes | Yes | No | |
| 7 | Yes | Yes | Yes | Telephone | 1 | No | No | Yes | ||
| 8 | Yes | Yes | Yes | At patient’s home | 1 | If needed | Yes | |||
| 9 | Yes | Yes | Yes | At patient’s home/clinic | 1 | No | Yes | No | No | Yes |
| 10 | Yes | No | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Description of usual care | Usual care | New Start | Total |
|---|---|---|---|
| Participants, n (%) | 124 (46.1) | 145 (53.9) | 269 (100) |
| Is stroke survivor appropriate to approach?, n (%) | |||
| Yes | 110 (88.7) | 140 (96.6) | 250 (92.9) |
| Was stroke survivor offered a post-stroke review?, n (%) | |||
| Yes | 108 (87.1) | 138 (95.2) | 246 (91.4) |
| If offered a post-stroke review, was stroke survivor seen or spoken to?, n (%) | |||
| Yes | 93 (86.0) | 24 (17.4) | 117 (47.6) |
| No | 15 (14.0) | 31 (22.5) | 46 (18.7) |
| Not applicablea | 0 (0.0) | 83 (60.1) | 83 (33.7) |
| If seen or spoken to | |||
| Number of stroke survivor contacts, n (%) | |||
| 1 | 81 (87.1) | 11 (45.8) | 92 (78.6) |
| 2 | 5 (5.4) | 3 (12.5) | 8 (6.8) |
| 3 | 4 (4.3) | 6 (25.0) | 10 (8.5) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 1 (4.2) | 1 (0.8) |
| 15 | 1 (1.2) | 0 (0.0) | 1 (0.8) |
| Missing | 2 (2.2) | 3 (12.5) | 5 (4.0) |
| Total | 93 (100) | 24 (100) | 117 (100) |
| Average duration of contacts (minutes) | |||
| Mean (SD) | 54 (27) | 44 (18) | 47 (22) |
| Median (range) | 45 (15–130) | 45 (3–90) | 45 (3–130) |
| Missing (n) | 64 | 8 | 72 |
| Average time between first and last contact (weeks) | |||
| Mean (SD) | 1 (6) | 12 (12) | 4 (9) |
| Median (range) | 0 (0–24) | 6 (0–45) | 0 (0–45) |
| Missing (n) | 2 | 4 | 6 |
| Number of stroke survivors referred to another service, n (%) | |||
| Yes | 23 (24.7) | 12 (50.0) | 35 (29.9) |
| Missing | 19 (20.4) | 1 (4.2) | 20 (17.1) |
| Not applicable | 18 (19.4) | 7 (29.2) | 25 (21.4) |
| Description of usual care | Site | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2a | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Total, n (%) | 13 (4.8) | 4 (1.5) | 89 (33.1) | 22 (8.2) | 17 (6.3) | 42 (15.6) | 10 (3.7) | 29 (10.8) | 28 (10.4) | 15 (5.6) | 269 (100) |
| Is stroke survivor appropriate to approach?, n (%) | |||||||||||
| Yes | 13 (100.0) | 4 (100.0) | 87 (97.8) | 22 (100.0) | 14 (82.4) | 41 (97.6) | 10 (100.0) | 28 (96.6) | 28 (100.0) | 3 (20.0) | 250 (92.9) |
| Was stroke survivor offered a post-stroke review?, n (%) | |||||||||||
| Yes | 11 (84.6) | 4 (100.0) | 87 (97.8) | 22 (100.0) | 14 (82.4) | 41 (97.6) | 10 (100.0) | 28 (96.6) | 26 (92.9) | 3 (20.0) | 246 (91.4) |
| If offered a post-stroke review, was stroke survivor seen or spoken to?, n (%) | |||||||||||
| Yes | 0 (0.0) | 3 (75.0) | 0 (0.0) | 10 (45.5) | 11 (78.6) | 36 (87.8) | 8 (80.0) | 21 (75.0) | 25 (96.2) | 3 (100.0) | 117 (47.5) |
| No | 3 (27.3) | 1 (25.0) | 21 (24.1) | 3 (13.6) | 3 (21.4) | 5 (12.2) | 2 (20.0) | 7 (25.0) | 1 (3.8) | 0 (0.0) | 46 (18.7) |
| Not applicablea | 8 (72.7) | 0 (0.0) | 66 (76.7) | 9 (40.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 83 (33.7) |
| Number of stroke survivor contacts, n (%) | |||||||||||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (90.0) | 2 (18.2) | 36 (100.0) | 8 (100.0) | 14 (66.7) | 22 (96.0) | 1 (33.3) | 92 (74.2) |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (9.5) | 3 (4.0) | 0 (0.0) | 8 (6.5) |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (54.5) | 0 (0.0) | 0 (0.0) | 3 (14.3) | 0 (0.0) | 1 (33.3) | 10 (8.1) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| 15 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (0.8) |
| Missing | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (9.5) | 0 (0.0) | 0 (0.0) | 5 (4.0) |
| Average duration of contacts (minutes) | |||||||||||
| Mean (SD) | 21 (20) | 63 (16) | 79 (23) | 15 (0) | 45 (.) | 35 (11) | 47 (6) | 47 (22) | |||
| Median (range) | 5 (3–60) | 60 (30–90) | 80 (20–130) | 15 (15–15) | 45 (45–45) | 30 (20–60) | 45 (45–60) | 45 (3–130) | |||
| Missing (n) | 0 | 3 | 0 | 5 | 0 | 0 | 0 | 28 | 0 | 0 | 36 |
| Average time between first and last contact (weeks) | |||||||||||
| Mean (SD) | 0 (0) | 19 (10) | 0 (0) | 0 (0) | 1 (11) | 1 (4) | 2 (2) | 4 (9) | |||
| Median (range) | 0 (0–0) | 24 (0–28) | 0 (0–0) | 0 (0–0) | 0 (0–24) | 0 (0–19) | 2 (0–5) | 0 (0–45) | |||
| Missing (n) | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 6 |
| Number of stroke survivors referred to another service, n (%) | |||||||||||
| Yes | 0 (0.0) | 1 (33.3) | 0 (0.0) | 2 (20.0) | 9 (81.8) | 9 (27.8) | 0 (0.0) | 11 (52.4) | 2 (8.0) | 1 (33.3) | 35 (29.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 9 (0.0) | 2 (25.0) | 1 (4.8) | 7 (28.0) | 0 (0.0) | 20 (17.1) |
| Not applicable | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (50.0) | 2 (18.2) | 10 (0.0) | 0 (0.0) | 1 (4.8) | 7 (28.0) | 0 (0.0) | 25 (21.4) |
| Site (recruited trial participants) | First unblinding, n (%) | Second unblinding: informed by stroke survivor, n (%) | |
|---|---|---|---|
| Informed by stroke survivor | Other | ||
| 1 (13) | 1 (7.7) | 0 (0.0) | 0 (0.0) |
| 2a (4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 (89) | 10 (11.2) | 1 (1.1) | 0 (0.0) |
| 4 (22) | 1 (4.5) | 0 (0.0) | 1 (4.5) |
| 5 (17) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 (42) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 (10) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 8 (29) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 9 (28) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10 (15) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total (269) | 12 (4.5) | 1 (0.4) | 1 (0.4) |
| Time from registration to unblinding | Time (days) | |
|---|---|---|
| First unblinding | Second unblinding | |
| Mean (SD) | 19.6 (20.17) | 34.0 (–) |
| Mean (95% CI) | 19.6 (8.65 to 30.58) | 34.0 (–) |
| Median (range) | 14.0 (–5.0 to 76.0) | 34.0 (–) |
FIGURE 12.
Time between patient registration and facilitator unblinding.
| Questionnaire | Baseline, n (%) | 3 months, n (%) | 6 months, n (%) | 9 months, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | Usual care | New Start | Usual care | New Start | |
| Available participants | 124 (46.1) | 145 (53.9) | 120 (45.8) | 142 (54.2) | 116 (46.0) | 136 (54.0) | 108 (45.2) | 131 (54.8) |
| WHODAS | ||||||||
| Yes | 122 (98.4) | 145 (100.0) | 101 (87.1) | 127 (93.4) | 91 (84.3) | 117 (89.3) | ||
| No | 2 (1.6) | 0 (0.0) | 15 (12.9) | 9 (6.6) | 17 (15.7) | 14 (10.7) | ||
| WEMWBS | ||||||||
| Yes | 119 (96.0) | 145 (100.0) | 95 (81.9) | 121 (89.0) | 88 (81.5) | 116 (88.5) | ||
| No | 5 (4.0) | 0 (0.0) | 21 (18.1) | 15 (11.0) | 20 (18.5) | 15 (11.5) | ||
| PAM | ||||||||
| Yes | 121 (97.6) | 143 (98.6) | 105 (87.5) | 128 (90.1) | 95 (81.9) | 117 (86.0) | ||
| No | 3 (2.4) | 2 (1.4) | 15 (12.5) | 14 (9.9) | 21 (18.1) | 19 (14.0) | ||
| LUNS | ||||||||
| Yes | 121 (97.6) | 145 (100.0) | 89 (82.4) | 120 (91.6) | ||||
| No | 3 (2.4) | 0 (0.0) | 19 (17.6) | 11 (8.4) | ||||
| Baseline | 3 months | 6 months | 9 months | |||||
|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | Usual care | New Start | Usual care | New Start | |
| Available participants, n (%) | 124 (46.1) | 145 (53.9) | 120 (45.8) | 142 (54.2) | 116 (46.0) | 136 (54.0) | 108 (45.2) | 131 (54.8) |
| WHODAS simple score | ||||||||
| Questionnaire completion, n (%) | ||||||||
| Completed | 25 (20.2) | 23 (15.9) | 25 (21.6) | 23 (16.9) | 20 (18.5) | 28 (21.4) | ||
| Partially completed | 97 (78.2) | 122 (84.1) | 76 (65.5) | 104 (76.5) | 71 (65.7) | 89 (67.9) | ||
| Not completed | 2 (1.6) | 0 (0.0) | 15 (12.9) | 9 (6.6) | 17 (15.7) | 14 (10.7) | ||
| Partially completed, n (%) | ||||||||
| Prorated | 79 (81.4) | 94 (77.0) | 61 (80.3) | 80 (76.9) | 55 (77.5) | 73 (82.0) | ||
| Score missing | 18 (18.6) | 28 (23.0) | 15 (19.7) | 24 (23.1) | 16 (22.5) | 16 (18.0) | ||
| Number of missing items | ||||||||
| Mean (SD) | 4.2 (3.50) | 4.5 (4.00) | 4.7 (5.28) | 4.5 (4.44) | 4.2 (3.89) | 4.9 (6.14) | ||
| Missing (n) | 2 | 0 | 14 | 8 | 17 | 11 | ||
| WHODAS complex score | ||||||||
| Questionnaire completion, n (%) | ||||||||
| Completed | 25 (20.2) | 23 (15.9) | 25 (21.6) | 23 (16.9) | 20 (18.5) | 28 (21.4) | ||
| Partially completed | 97 (78.2) | 122 (84.1) | 76 (65.5) | 104 (76.5) | 71 (65.7) | 89 (67.9) | ||
| Not completed | 2 (1.6) | 0 (0.0) | 15 (12.9) | 9 (6.6) | 17 (15.7) | 14 (10.7) | ||
| Partially completed, n (%) | ||||||||
| Prorated | 43 (44.3) | 54 (44.3) | 35 (46.1) | 48 (46.2) | 36 (50.7) | 44 (49.4) | ||
| Score missing | 54 (55.7) | 68 (55.7) | 41 (53.9) | 56 (53.8) | 35 (49.3) | 45 (50.6) | ||
| Number of missing items | ||||||||
| Mean (SD) | 4.2 (3.50) | 4.5 (4.00) | 4.7 (5.28) | 4.5 (4.44) | 4.2 (3.89) | 4.9 (6.14) | ||
| Missing (n) | 2 | 0 | 14 | 8 | 17 | 11 | ||
| WEMWBS score | ||||||||
| Questionnaire completion, n (%) | ||||||||
| Completed | 109 (87.9) | 135 (93.1) | 85 (73.3) | 106 (77.9) | 82 (75.9) | 104 (79.4) | ||
| Partially completed | 13 (10.5) | 10 (6.9) | 17 (14.7) | 22 (16.2) | 9 (8.3) | 16 (12.2) | ||
| Not completed | 2 (1.6) | 0 (0.0) | 14 (12.1) | 8 (5.9) | 17 (15.7) | 11 (8.4) | ||
| Partially completed, n (%) | ||||||||
| Prorated | 9 (69.2) | 10 (100.0) | 9 (52.9) | 13 (59.1) | 5 (55.6) | 9 (56.3) | ||
| Score missing | 4 (30.8) | 0 (0.0) | 8 (47.1) | 9 (40.9) | 4 (44.4) | 7 (43.8) | ||
| Number of missing items | ||||||||
| Mean (SD) | 0.5 (2.28) | 0.1 (0.32) | 1.2 (3.65) | 1.0 (3.25) | 0.6 (2.61) | 0.8 (3.01) | ||
| Missing (n) | 2 | 0 | 14 | 8 | 17 | 11 | ||
| PAM score | ||||||||
| Questionnaire completion, n (%) | ||||||||
| Completed | 111 (89.5) | 132 (91.0) | 92 (76.7) | 116 (81.7) | 85 (73.3) | 104 (76.5) | ||
| Partially completed | 11 (8.9) | 13 (9.0) | 18 (15.0) | 20 (14.1) | 17 (14.7) | 25 (18.4) | ||
| Not completed | 2 (1.6) | 0 (0.0) | 10 (8.3) | 6 (4.2) | 14 (12.1) | 7 (5.1) | ||
| Partially completed, n (%) | ||||||||
| Prorated | 10 (90.9) | 11 (84.6) | 12 (66.7) | 10 (50.0) | 9 (52.9) | 13 (52.0) | ||
| Score missing | 1 (9.1) | 2 (15.4) | 6 (33.3) | 10 (50.0) | 8 (47.1) | 12 (48.0) | ||
| Number of missing items | ||||||||
| Mean (SD) | 0.3 (1.41) | 0.5 (2.20) | 0.8 (2.87) | 1.0 (3.20) | 1.1 (3.36) | 1.5 (3.90) | ||
| Missing (n) | 2 | 0 | 10 | 6 | 14 | 7 | ||
| LUNS score | ||||||||
| Questionnaire completion, n (%) | ||||||||
| Completed | 97 (78.2) | 117 (80.7) | 63 (58.3) | 86 (65.6) | ||||
| Partially completed | 25 (20.2) | 28 (19.3) | 28 (25.9) | 34 (26.0) | ||||
| Not completed | 2 (1.6) | 0 (0.0) | 17 (15.7) | 11 (8.4) | ||||
| Number of missing items | ||||||||
| Mean (SD) | 0.8 (2.87) | 0.4 (1.30) | 1.5 (4.28) | 0.9 (2.54) | ||||
| Missing (n) | 2 | 0 | 17 | 11 | ||||
| Variable | 6 months, n (% of returned) [% of sent] | 9 months, n (% of returned) [% of sent] | ||||
|---|---|---|---|---|---|---|
| Single booklet (N = 125) | Two separate booklets (N = 128) | Total (N = 253) | Single booklet (N = 116) | Two separate booklets (N = 123) | Total (N = 239) | |
| Returned | 114 | 108 (both booklets); 4 (one booklet only) | 226 | 108 | 105 (both booklets); 2 (one booklet only) | 215 |
| Completion rate by type of measure | ||||||
| Outcomes | 113 (99.1) [90.4] | 108 (96.4) [84.4] | 221 (97.8) | 105 (97.2) [90.5] | 103 (96.3) [83.7] | 208 (96.7) |
| Health economics | 111 (97.4) [88.8] | 102 (91.1) [79.7] | 213 (94.2) | 106 (98.1) [91.4] | 96 (89.7) [78.0] | 202 (94.0) |
| Both | 110 (96.5) [88.0] | 99 (88.4) [77.3] | 209 (92.5) | 105 (97.2) [90.5] | 94 (87.9) [76.4] | 199 (92.6) |
| Variable | 6 months, n (% of returned) [% of sent] | 9 months, n (% of returned) [% of sent] | ||||
|---|---|---|---|---|---|---|
| Single booklet (N = 37) | Two separate booklets (N = 38) | Total (N = 75) | Single booklet (N = 34) | Two separate booklets (N = 37) | Total (N = 71) | |
| Returned | 32 | 29 (both booklets) | 61 | 30 | 30 (both booklets) | 60 |
| Completion rate by type of measure | ||||||
| Outcomes | 31 (96.9) [86.5] | 27 (93.1) [71.1] | 58 (95.1) | 29 (96.7) [85.3] | 29 (96.7) [78.4] | 58 (96.7) |
| Health economics | 30 (93.8) [81.1] | 26 (89.7) [68.4] | 56 (91.8) | 29 (96.7) [85.3] | 29 (96.7) [78.4] | 58 (96.7) |
| Both | 30 (93.8) [81.1] | 26 (89.7) [68.4] | 56 (91.8) | 29 (96.7) [85.3] | 29 (96.7) [78.4] | 58 (96.7) |
| Outcome measure | Baseline | 3 months | 6 months | 9 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Total | Usual care | New Start | Total | Usual care | New Start | Total | Usual care | New Start | Total | |
| Simple WHODAS score | ||||||||||||
| Mean (SD) | 26.2 (20.84) | 23.7 (18.10) | 24.9 (19.44) | 23.3 (20.35) | 20.6 (17.44) | 21.8 (18.81) | 22.0 (20.17) | 21.0 (18.15) | 21.4 (18.98) | |||
| Median (range) | 25.3 (0.0–86.1) | 20.8 (0.0–81.3) | 20.8 (0.0–86.1) | 18.1 (0.0–92.4) | 17.0 (0.0–78.5) | 17.7 (0.0–92.4) | 18.1 (0.0–84.7) | 17.7 (0.0–72.9) | 18.1 (0.0–84.7) | |||
| Missing | 20 | 28 | 48 | 38 | 41 | 79 | 49 | 43 | 92 | |||
| Complex WHODAS score | ||||||||||||
| Mean (SD) | 26.9 (24.30) | 25.6 (19.34) | 26.2 (21.74) | 23.9 (24.18) | 22.0 (20.51) | 22.9 (22.20) | 23.7 (24.82) | 22.6 (20.14) | 23.1 (22.22) | |||
| Median (range) | 21.9 (0.0–88.9) | 23.2 (0.0–66.1) | 22.5 (0.0–88.9) | 15.7 (0.0–91.3) | 16.6 (0.0–85.7) | 16.6 (0.0–91.3) | 16.8 (0.0–85.0) | 19.2 (0.0–83.8) | 17.8 (0.0–85.0) | |||
| Missing | 56 | 68 | 124 | 64 | 74 | 138 | 68 | 73 | 141 | |||
| WEMWBS score | ||||||||||||
| Mean (SD) | 46.6 (12.63) | 47.5 (11.61) | 47.1 (12.06) | 46.0 (12.18) | 46.5 (11.41) | 46.2 (11.73) | 47.6 (11.73) | 46.8 (11.71) | 47.1 (11.70) | |||
| Median (range) | 46.0 (14.0–70.0) | 47.0 (18.0–70.0) | 46.0 (14.0–70.0) | 46.0 (14.0–70.0) | 46.3 (25.0–69.0) | 46.0 (14.0–70.0) | 48.0 (18.0–70.0) | 47.0 (15.0–70.0) | 47.4 (15.0–70.0) | |||
| Missing | 6 | 0 | 6 | 30 | 25 | 55 | 37 | 31 | 68 | |||
| PAM score categorical, n (%) | ||||||||||||
| (≤ 47.0) Not believing that activation is important | 38 (30.6) | 36 (24.8) | 74 (27.5) | 29 (23.4) | 34 (23.4) | 63 (23.4) | 25 (20.2) | 27 (18.6) | 52 (19.3) | |||
| (47.1–55.1) A lack of knowledge and confidence to take action | 32 (25.8) | 45 (31.0) | 77 (28.6) | 40 (32.3) | 42 (29.0) | 82 (30.5) | 25 (20.2) | 36 (24.8) | 61 (22.7) | |||
| (55.2–67.0) Beginning to take action | 30 (24.2) | 29 (20.0) | 59 (21.9) | 17 (13.7) | 29 (20.0) | 46 (17.1) | 22 (17.7) | 28 (19.3) | 50 (18.6) | |||
| (≥ 67.1) Taking action | 21 (16.9) | 33 (22.8) | 54 (20.1) | 16 (12.9) | 21 (14.5) | 37 (13.8) | 15 (12.1) | 24 (16.6) | 39 (14.5) | |||
| Missing | 3 (2.4) | 2 (1.4) | 5 (1.9) | 22 (17.7) | 19 (13.1) | 41 (15.2) | 37 (29.8) | 30 (20.7) | 67 (24.9) | |||
| PAM score continuous | ||||||||||||
| Mean (SD) | 56.7 (16.93) | 58.6 (17.72) | 57.7 (17.35) | 54.5 (15.08) | 55.0 (15.48) | 54.8 (15.27) | 56.4 (18.64) | 57.5 (17.99) | 57.1 (18.23) | |||
| Median (range) | 51.0 (20.5–100.0) | 51.0 (20.5–100.0) | 51.0 (20.5–100.0) | 51.0 (22.6–100.0) | 51.0 (14.5–100.0) | 51.0 (14.5–100.0) | 51.0 (9.0–100.0) | 53.2 (0.0–100.0) | 51.0 (0.0–100.0) | |||
| Missing | 3 | 2 | 5 | 22 | 19 | 41 | 37 | 30 | 67 | |||
| LUNS: number of unmet needs (count), n (%) | ||||||||||||
| 0 | 19 (15.3) | 16 (11.0) | 35 (13.0) | 21 (16.9) | 33 (22.8) | 54 (20.1) | ||||||
| 1 | 20 (16.1) | 21 (14.5) | 41 (15.2) | 16 (12.9) | 20 (13.8) | 36 (13.4) | ||||||
| 2 | 16 (12.9) | 21 (14.5) | 37 (13.8) | 14 (11.3) | 19 (13.1) | 33 (12.3) | ||||||
| 3 | 15 (12.1) | 15 (10.3) | 30 (11.2) | 7 (5.6) | 16 (11.0) | 23 (8.6) | ||||||
| 4 | 11 (8.9) | 12 (8.3) | 23 (8.6) | 10 (8.1) | 10 (6.9) | 20 (7.4) | ||||||
| 5 | 7 (5.6) | 13 (9.0) | 20 (7.4) | 6 (4.8) | 5 (3.4) | 11 (4.1) | ||||||
| 6+ | 34 (27.4) | 47 (32.4) | 81 (30.1) | 17 (13.7) | 18 (12.4) | 35 (13.0) | ||||||
| Missing | 2 (1.6) | 0 (0.0) | 2 (0.7) | 33 (26.6) | 24 (16.6) | 57 (21.2) | ||||||
| LUNS: number of unmet needs (average) | ||||||||||||
| Mean (SD) | 3.8 (3.59) | 4.1 (3.19) | 4.0 (3.37) | 3.1 (3.08) | 2.7 (3.20) | 2.9 (3.15) | ||||||
| Median (range) | 3.0 (0.0–15.0) | 3.0 (0.0–14.0) | 3.0 (0.0–15.0) | 2.0 (0.0–11.0) | 2.0 (0.0–17.0) | 2.0 (0.0–17.0) | ||||||
| Missing | 2 | 0 | 2 | 33 | 24 | 57 | ||||||
| Questionnaire and time point | Cluster point estimates | Mean difference (95% CI) (67% CI) (51% CI) | t-test p-value;a significant at 5%, 33%, 49% level | |||
|---|---|---|---|---|---|---|
| New Start | Usual care | |||||
| n/Nb | Mean (SD) | n/N | Mean (SD) | |||
| WHODAS simple (score 0–100; higher score = higher level of disability) | ||||||
| Baseline | 4/5 | 26.2 (4.34) | 5/5 | 23.9 (5.81) | –2.3 | 0.53 |
| (–10.60 to 6.01) | No | |||||
| (–5.97 to 1.38) | No | |||||
| (–4.85 to 0.26) | No | |||||
| 6 months | 4/5 | 21.12 (2.93) | 5/5 | 24.2 (3.69) | 3.14 | 0.21 |
| (–2.23 to 8.50) | No | |||||
| (0.76 to 5.51) | Yes | |||||
| (1.48 to 4.79) | Yes | |||||
| 9 months | 4/5 | 24.2 (4.71) | 5/5 | 23.34 (4.38) | –0.87 | 0.52 |
| (–8.05 to 6.30) | No | |||||
| (–4.05 to 2.30) | No | |||||
| (–3.08 to 1.34) | No | |||||
| WHODAS complex (score 0–100; higher score = higher level of disability) | ||||||
| Baseline | 4/5 | 28.0 (5.34) | 5/5 | 24.7 (7.72) | –3.26 | 0.49 |
| (–14.05 to 7.53) | No | |||||
| (–8.04 to 1.51) | No | |||||
| (–6.59 to 0.06) | No | |||||
| 6 months | 4/5 | 23.9 (4.56) | 5/5 | 26.0 (6.89) | 2.07 | 0.62 |
| (–7.46 to 11.59) | No | |||||
| (–2.15 to 6.29) | No | |||||
| (–0.87 to 5.00) | No | |||||
| 9 months | 4/5 | 26.2 (6.22) | 5/5 | 26.0 (5.99) | –0.16 | 0.97 |
| (–9.82 to 9.50) | No | |||||
| (–4.44 to 4.11) | No | |||||
| (–3.14 to 2.82) | No | |||||
| WEMWBS (score 14–70; higher score = better state of mental well-being) | ||||||
| Baseline | 4/5 | 46.9 (2.06) | 5/5 | 47.2 (1.55) | 0.29 | 0.82 |
| (–2.54 to 3.12) | No | |||||
| (–0.96 to 1.54) | No | |||||
| (–0.58 to 1.16) | No | |||||
| 6 months | 4/5 | 47.2 (1.80) | 5/5 | 44.4 (5.70) | –2.87 | 0.37 |
| (–9.96 to 4.21) | No | |||||
| (–6.01 to 0.26) | No | |||||
| (–5.06 to –0.69) | Yes | |||||
| 9 months | 4/5 | 45.8 (2.71) | 5/5 | 47.2 (2.80) | 1.42 | 0.47 |
| (–2.96 to 5.80) | No | |||||
| (–0.52 to 3.36) | No | |||||
| (0.07 to 2.77) | Yes | |||||
| CBS outcomes | Baseline | 3 months | 6 months | 9 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care (n = 39) | New Start (n = 46) | Total (n = 85) | Usual care (n = 39) | New Start (n = 46) | Total (n = 85) | Usual care (n = 39) | New Start (n = 46) | Total (n = 85) | Usual care (n = 39) | New Start (n = 46) | Total (n = 85) | |
| Total CBS score (points) | ||||||||||||
| Mean (SD) | 48.7 (15.32) | 45.6 (15.28) | 47.0 (15.28) | 48.7 (14.76) | 47.7 (14.23) | 48.1 (14.30) | 43.7 (14.22) | 46.8 (14.46) | 45.5 (14.32) | 44.9 (16.05) | 48.2 (15.68) | 46.8 (15.78) |
| Median (range) | 46.0 (23.0–79.0) | 42.5 (24.0–83.8) | 43.5 (23.0–83.8) | 45.5 (25.0–82.0) | 48.0 (28.0–84.0) | 46.0 (25.0–84.0) | 42.0 (22.0–79.0) | 48.5 (23.0–79.0) | 42.0 (22.0–79.0) | 43.5 (22.0–83.0) | 49.0 (22.0–81.0) | 46.1 (22.0–83.0) |
| Missing (n) | 1 | 0 | 1 | 19 | 15 | 34 | 14 | 10 | 24 | 15 | 13 | 28 |
| Subscale scores (points) | ||||||||||||
| General strain | ||||||||||||
| Mean (SD) | 20.1 (7.02) | 17.9 (6.32) | 18.9 (6.69) | 20.2 (7.01) | 18.3 (5.82) | 19.1 (6.32) | 17.8 (6.41) | 18.9 (5.87) | 18.5 (6.07) | 18.5 (7.37) | 19.5 (6.52) | 19.1 (6.84) |
| Median (range) | 19.5 (8.0–32.0) | 16.0 (8.0–31.0) | 17.0 (8.0–32.0) | 18.5 (11.0–32.0) | 19.0 (9.0–32.0) | 18.5 (9.0–32.0) | 17.0 (8.0–32.0) | 20.5 (9.0–29.0) | 18.0 (8.0–32.0) | 18.0 (8.0–32.0) | 19.0 (8.0–31.0) | 18.5 (8.0–32.0) |
| Missing (n) | 1 | 0 | 1 | 19 | 16 | 35 | 14 | 10 | 24 | 16 | 13 | 29 |
| Isolation | ||||||||||||
| Mean (SD) | 7.3 (2.46) | 6.9 (2.50) | 7.1 (2.47) | 7.4 (2.81) | 7.1 (2.64) | 7.2 (2.68) | 6.2 (2.60) | 6.9 (2.67) | 6.6 (2.64) | 6.2 (3.14) | 7.4 (2.82) | 6.8 (3.00) |
| Median (range) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) | 6.0 (3.0–12.0) | 7.0 (3.0–12.0) | 6.0 (3.0–12.0) | 5.0 (3.0–12.0) | 7.0 (3.0–12.0) | 7.0 (3.0–12.0) |
| Missing (n) | 3 | 0 | 3 | 19 | 17 | 36 | 14 | 11 | 25 | 15 | 15 | 30 |
| Disappointment | ||||||||||||
| Mean (SD) | 11.3 (4.25) | 10.8 (4.31) | 11.0 (4.27) | 11.5 (3.90) | 11.4 (4.13) | 11.4 (3.99) | 10.5 (3.57) | 11.1 (4.00) | 10.8 (3.81) | 10.8 (4.02) | 11.3 (4.50) | 11.1 (4.27) |
| Median (range) | 10.5 (5.0–19.0) | 10.0 (5.0–20.0) | 10.0 (5.0–20.0) | 11.0 (5.0–19.0) | 10.0 (6.0–20.0) | 11.0 (5.0–20.0) | 10.0 (5.0–19.0) | 11.0 (5.0–20.0) | 10.0 (5.0–20.0) | 11.0 (5.0–20.0) | 12.0 (5.0–19.0) | 11.0 (5.0–20.0) |
| Missing (n) | 1 | 0 | 1 | 19 | 17 | 36 | 14 | 11 | 25 | 15 | 13 | 28 |
| Emotional involvement | ||||||||||||
| Mean (SD) | 4.8 (1.74) | 4.9 (2.27) | 4.8 (2.03) | 4.6 (1.79) | 5.3 (2.34) | 5.0 (2.15) | 4.3 (1.77) | 4.6 (2.04) | 4.5 (1.93) | 4.3 (1.86) | 4.8 (2.06) | 4.6 (1.99) |
| Median (range) | 4.0 (3.0–10.0) | 4.0 (3.0–11.0) | 4.0 (3.0–11.0) | 4.5 (3.0–9.0) | 5.0 (3.0–12.0) | 5.0 (3.0–12.0) | 4.0 (3.0–9.0) | 4.0 (3.0–11.0) | 4.0 (3.0–11.0) | 4.0 (3.0–10.0) | 4.0 (3.0–10.0) | 4.0 (3.0–10.0) |
| Missing (n) | 1 | 0 | 1 | 19 | 16 | 35 | 14 | 10 | 24 | 16 | 13 | 29 |
| Environment | ||||||||||||
| Mean (SD) | 5.3 (2.00) | 5.0 (2.10) | 5.1 (2.04) | 5.1 (2.04) | 5.0 (1.97) | 5.0 (1.98) | 4.8 (1.91) | 5.3 (2.04) | 5.1 (1.98) | 5.0 (2.07) | 5.0 (2.06) | 5.0 (2.05) |
| Median (range) | 5.0 (3.0–9.0) | 5.0 (3.0–12.0) | 5.0 (3.0–12.0) | 5.0 (3.0–10.0) | 5.0 (3.0–10.0) | 5.0 (3.0–10.0) | 5.0 (3.0–9.0) | 5.0 (3.0–10.0) | 5.0 (3.0–10.0) | 4.5 (3.0–9.0) | 5.0 (3.0–9.0) | 5.0 (3.0–9.0) |
| Missing (n) | 1 | 2 | 3 | 20 | 18 | 38 | 14 | 12 | 26 | 15 | 15 | 30 |
| Outcome and time point | Number of non-missing observations | Estimated coefficient of reliability, ICC (95% CI) | Estimated coefficient of within-subject variance (95% CI) |
|---|---|---|---|
| WHODAS simple score | |||
| Baseline | 217 | 0.02 (0.00 to 0.23) | 0.77 (0.65 to 0.90) |
| 6 months | 188 | 0.00 (–) | 0.86 (0.73 to 1.00) |
| 9 months | 175 | 0.00 (–) | 0.88 (0.74 to 1.04) |
| WHODAS complex score | |||
| Baseline | 142 | 0.01 (0.00 to 0.95) | 0.81 (0.67 to 0.97) |
| 6 months | 130 | 0.00 (–) | 0.94 (0.77 to 1.15) |
| 9 months | 127 | 0.00 (–) | 0.93 (0.76 to 1.14) |
| WEMWBS score | |||
| Baseline | 259 | 0.00 (–) | 0.26 (0.23 to 0.28) |
| 6 months | 212 | 0.00 (–) | 0.25 (0.23 to 0.28) |
| 9 months | 199 | 0.00 (–) | 0.25 (0.22 to 0.28) |
| Hospitalisation and institutionalisation | Baseline | 3 months | 6 months | 9 months | ||||
|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | Usual care | New Start | Usual care | New Start | |
| Total completed health economics booklets, n (%) | 116 (95.1) | 142 (97.9) | 105 (96.3) | 132 (98.5) | 97 (97.0) | 124 (97.6) | 86 (96.6) | 116 (97.5) |
| Hospital inpatient stay | ||||||||
| Yes, n (%) | 20 (17.2) | 15 (10.6) | 14 (13.3) | 13 (9.8) | 8 (8.2) | 13 (10.5) | 8 (9.3) | 10 (8.6) |
| If yes, number of days in hospital | ||||||||
| Mean (SD) | 12.7 (12.0) | 11.0 (18.3) | 6.4 (5.3) | 8.5 (10.6) | 8.7 (10.1) | 11.5 (19.9) | 2.9 (1.7) | 6.9 (5.3) |
| Median (range) | 11.0 (1.0–45.0) | 5.0 (2.0–63.0) | 5.5 (1.0–20.0) | 3.0 (1.0–30.0) | 4.0 (1.0–28.0) | 5.0 (1.0–70.0) | 2.0 (1.0–6.0) | 8.0 (1.0–15.0) |
| Missing (n) | 4 | 4 | 2 | 2 | 1 | 2 | 1 | 3 |
| If yes, total number of visits during previous 3 months | ||||||||
| Mean (SD) | 2.0 (1.5) | 1.7 (1.0) | 1.6 (1.5) | 1.4 (0.8) | 1.3 (0.5) | 1.2 (0.4) | 1.0 (0.0) | 1.8 (1.7) |
| Median (range) | 1.0 (0.0–5.0) | 1.0 (1.0–3.0) | 1.0 (1.0–5.0) | 1.0 (1.0–3.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 1.5 (0.0–4.0) |
| Missing (n) | 7 | 9 | 7 | 6 | 4 | 7 | 3 | 6 |
| Admitted to hospital A&E department | ||||||||
| Yes, n (%) | 16 (13.8) | 15 (10.6) | 11 (10.5) | 19 (14.4) | 7 (7.2) | 12 (9.7) | 5 (5.8) | 17 (14.7) |
| If yes, total number of visits during previous 3 months | ||||||||
| Mean (SD) | 1.6 (0.7) | 1.5 (1.6) | 1.4 (0.5) | 1.2 (0.4) | 1.5 (0.8) | 1.7 (2.2) | 2.0 (0.0) | 1.2 (0.4) |
| Median (range) | 1.5 (1.0–3.0) | 1.0 (1.0–6.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–3.0) | 1.0 (1.0–8.0) | 2.0 (2.0–2.0) | 1.0 (1.0–2.0) |
| Missing (n) | 4 | 5 | 6 | 6 | 1 | 2 | 3 | 6 |
| Admitted to nursing/residential home | ||||||||
| Yes, n (%) | 0 (0.0) | 3 (2.1) | 1 (1.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| If yes, number of days in nursing/residential home | ||||||||
| Mean (SD) | 29.0 (–) | 90.0 (–) | 14.0 (–) | |||||
| Median (range) | 29.0 (29.0 to 29.0) | 90.0 (90.0 to 90.0) | 14.0 (14.0 to 14.0) | |||||
| Missing (n) | 2 | 0 | 1 | |||||
| If yes, total number of visits during previous 3 months | ||||||||
| Mean (SD) | – | – | – | |||||
| Median (range) | – | – | – | |||||
| Missing (n) | 3 | 1 | 2 | |||||
| Hospitalisation and institutionalisation | Baseline | 3 months | 6 months | 9 months | ||||
|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | Usual care | New Start | Usual care | New Start | |
| Total completed health economics booklets, n (%) | 6 (4.9) | 3 (2.1) | 4 (3.7) | 2 (1.5) | 3 (3.0) | 3 (2.4) | 3 (3.4) | 3 (2.5) |
| Hospital inpatient stay | ||||||||
| Yes, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) |
| If yes, number of days in hospital | ||||||||
| Mean (SD) | 6.0 (–) | 10.0 (–) | ||||||
| Median (range) | 6.0 (6.0–6.0) | 10.0 (10.0–10.0) | ||||||
| Missing (n) | 0 | 0 | ||||||
| If yes, total number of visits during previous 3 months | ||||||||
| Mean (SD) | – | 1.0 (–) | ||||||
| Median (range) | – | 1.0 (1.0–1.0) | ||||||
| Missing (n) | 1 | 0 | ||||||
| Hospital A&E department | ||||||||
| Yes, n (%) | 2 (33.3) | 1 (33.3) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 2 (66.7) |
| If yes, total number of visits during previous 3 months | ||||||||
| Mean (SD) | 1.0 (0.0) | 1.0 (–) | 1.0 (0.0) | 1.0 (–) | 1.0 (0.0) | |||
| Median (range) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | |||
| Missing (n) | 0 | 0 | 0 | 0 | 0 | |||
| Nursing/residential home | ||||||||
| Yes, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
FIGURE 13.
Patient-level CONSORT diagram.
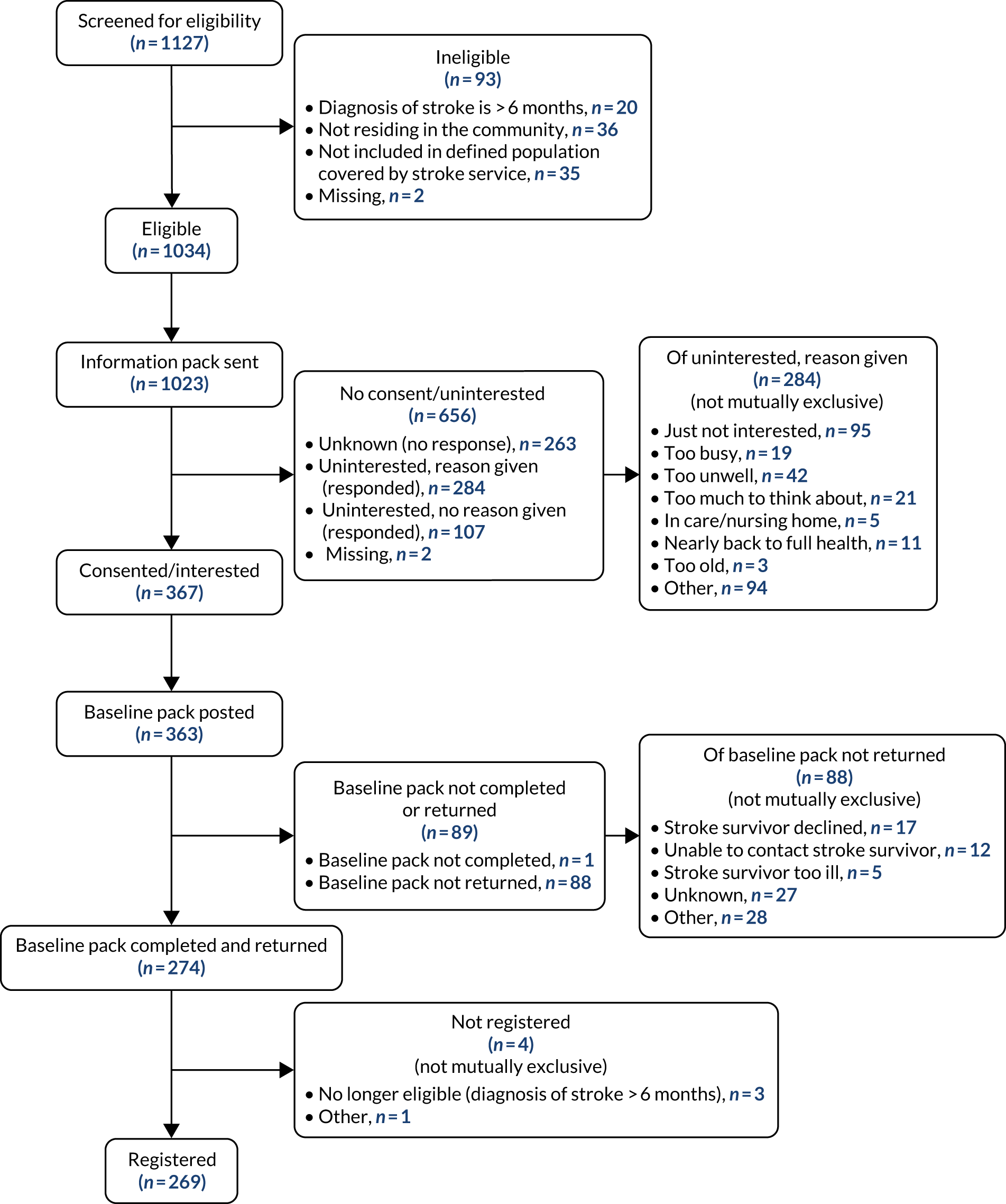
| Site | Total number recruited | Screening length (months) | Average monthly recruitment (n) | 6-month prorated recruitment |
|---|---|---|---|---|
| 3 | 89 | 5.1 | 17.5 | 105.0 |
| 6 | 42 | 8.1 | 5.2 | 31.1 |
| 4 | 22 | 4.6 | 5.0 | 28.7 |
| 8 | 29 | 7.4 | 3.9 | 23.5 |
| 9 | 28 | 8.0 | 3.5 | 21.1 |
| 1 | 13 | 5.3 | 2.5 | 14.7 |
| 10 | 15 | 6.5 | 2.3 | 13.9 |
| 5 | 17 | 8.1 | 2.1 | 12.6 |
| 7 | 10 | 5.9 | 1.7 | 10.2 |
| 2a | 4 | 7.9 | 0.5 | 3.0 |
| Total | 269 | 66.9 | 4.0 | 24.1 |
| Treatment allocation | Participants (n) | Booklets returned | Follow-up rate (%) |
|---|---|---|---|
| Usual care | 124 | 94 | 75.80 |
| New Start | 145 | 122 | 84.10 |
| Total | 269 | 216 | 80.30 |
| Site | Recruited stroke survivors (n) | Stroke survivors offered intervention | Stroke survivors received intervention | ||||
|---|---|---|---|---|---|---|---|
| Recorded via activity record (n) | Recorded via activity record (n) | Including data from site (%) | Data from site (%) | n | % | ||
| 1 | 13 | 10 | 76.90 | 11 | 84.60 | 7 | 53.80 |
| 2a | 4 | 4 | 100.00 | 4 | 100.00 | 3 | 75.00 |
| 3 | 89 | 65 | 73.00 | 87 | 97.80 | 65 | 73.00 |
| 4 | 22 | 9 | 40.90 | 22 | 100.00 | 9 | 40.90 |
| 5 | 17 | 14 | 82.40 | 14 | 82.40 | 2 | 11.80 |
| Total | 145 | 102 | 70.30 | 138 | 95.20 | 86 | 59.30 |
| Site | Participants recruited (n) | Participants receiving intervention (at least one visit) (n) | Participants receiving intervention (%) | Facilitators deemed competent (n) |
|---|---|---|---|---|
| 1 | 13 | 7 | 53.80 | 3 |
| 2a | 4 | 3 | 75.00 | 3 |
| 4 | 22 | 9 | 40.90 | 2 |
| 3 | 89 | 65 | 73.00 | 4 |
| 5 | 17 | 2 | 11.80 | 3 |
| Total | 145 | 86 | 59.30 | 15 |
Appendix 16 Sentinel Stroke National Audit Programme-reported data versus recruitment and clinical screening figures
| LoTS2Care site | SSNAP data | Trial data | ||
|---|---|---|---|---|
| Number of patients applicable for 6-month follow-up (M4.1) | 6-month follow-up completed (M4.4) | Clinical screening figures (data correct as of 2 February 2018) | Recruitment screening figures (data correct as of 10 January 2018) | |
| 1 | N/A | N/A | 22 | 18a |
| 2a | 32 | 27 | 41 | 18 |
| 3 | 271 | 137 | 297 | 167a |
| 4 | NR | NR | 67 | 60a |
| 5 | 66 | 64 | 55 | 16 |
| 6 | 24 | 21 | 62 | 71 |
| 7 | NR | NR | 74 | 3b |
| 8 | 125 | 123 | Not received from site | 66 |
| 9 | NR | NR | Not received from site | 47 |
| 10 | N/A | N/A | 10 | 23 |
Appendix 17 Uptake of 6-month post-stroke reviews
Appendix 18 Factors that aided and impeded trial recruitment
Twenty-two recruiters from all 10 sites were interviewed and their views on recruiting for the LoTS2Care trial were sought. Although no major difficulties were reported at any site, a range of barriers to and facilitators of recruitment were identified.
Barriers to and facilitators of recruitment
Availability of eligible participants
A lack of potential participants could be a barrier; recruiters at three sites reported that the numbers of stroke survivors eligible for inclusion in the trial were limited in their locality. At one site, this was because recruiters relied on patients attending a single stroke co-ordinator clinic. In contrast, recruiters elsewhere commented that having access to a large number of potential participants on the ‘list’ plus an efficient and well-established team with experience of stroke research was helpful. Socioeconomic factors could influence recruitment rates; recruiters at one site where English may not have been stroke survivors’ first language suggested that cultural/linguistic factors contributed to non-participation. Elsewhere, poverty and low levels of literacy were identified as exerting a negative influence on recruitment. At a third site, recruiters suggested that the prevailing stoicism characterising the local population could result in limited motivation to be involved in research. In contrast, access to a well-educated and affluent older population was identified during interviews as a facilitating factor by recruiters at two other sites.
Access to participant ‘lists’ and clinical information
The SSNAP database was used successfully by recruiters to generate lists at three sites. Clinical records were used at the remaining seven sites. Minor technical difficulties and the time-consuming nature of the latter approach were reported at two sites and substantial difficulties were reported at another relating to accessing lists of potential participants directly from the stroke co-coordinator’s patient list via an electronic record system, which proved complicated and inefficient. On the other hand, several recruiters reported that accessing clinical information relating to participants from clinical records/SSNAP could be difficult without specific skills/permissions.
Methods of inviting stroke survivors to participate
Recruiters at half the sites commented that high levels of recovery for many survivors at this time point was associated with a lack of motivation for survivors to participate because they were getting on with their lives and no longer wanted to think about their stroke. Recruiters at three sites reported that survivors had contacted them because they had recovered well and wondered if they were eligible for the study. Thus, there was agreement between the recruiters at most sites that inviting stroke survivors to participate in the trial at 4–6 months post stroke had a negative effect on recruitment.
Recruiters at one site commented that a flexible trial design had enabled them to decide to introduce the project to stroke survivors when they were still in hospital, which they felt facilitated recruitment. Similarly, recruiters at another site commented that they were based on the stroke unit and they found that familiarity with stroke survivors resulted in prompt responses to invitation. Inviting stroke survivors to participate in the trial by letter was considered a barrier by several recruiters on the grounds that the invitation letter was overly long and difficult to understand. Recruiters at two sites thought that the information sheet was too long and resulted in the stroke survivor being overloaded with information. However, contacting non-responders by telephone was considered difficult at half the trial sites because of the use of withheld numbers and call minder systems. At two sites, recruiters described this method of chasing up non-responders as time-consuming because of the large numbers of stroke survivors involved.
Aspects of the study design
The use of questionnaires was identified as a potential barrier to recruitment and retention of participants at several sites. Recruiters at two sites suggested that the prospect of completing questionnaires may have been off-putting for some potential participants and several recruiters suggested that receiving a lengthy and complex questionnaire was a factor in participants either not taking part or withdrawing from the study. One recruiter suggested that it would be easier to gather information from participants if they were seen in person; she felt that many of them wanted to speak to somebody and discuss their progress.
Relationship with the trial team
Recruiters at half the sites reported a positive relationship with the trial management team, which included support and the provision of information relating to trial design. Furthermore, recruiters at three sites identified site visits by the chief investigator as useful. Similarly, recruiters at almost all sites reported that they had found the clinical trials research unit to be easily contacted, helpful, efficient, approachable and responsive. In contrast, one recruiter reported that a lack of practical guidance from the trial team had made the task of recruitment more difficult than it might otherwise have been.
Key points
-
Factors impeding trial recruitment included the following: a limited pool of eligible participants (at some sites), recruiting at the 4–6 month post stroke time point and using postal invitations that overloaded stroke survivors with information and introduced the prospect of questionnaires.
-
Factors aiding trial recruitment included the following: having personnel skilled in accessing the SSNAP database available to access participant ‘lists’, a flexible trial design allowing adaptation of recruitment approaches and pre-existing stroke survivor familiarity with the trial and recruiters.
Summary of recruiter demographic data
Twenty-two recruiters took part in interviews. All recruiters were white, most were female (91%; n = 20), aged > 41 years (86%; n = 19) and most were employed at NHS band 6 or 7 (68%; n = 15). Recruiters had a range of experience in research, with most having spent either 1 or 2 years (41%; n = 9) or ≥ 5 years in research (36%; n = 8) (Table 45).
| Demographic factor | Recruiters (N = 22) (n) |
|---|---|
| Age (years) | |
| 18–30 | 0 |
| 31–40 | 3 |
| 41–50 | 6 |
| ≥ 51 | 13 |
| Gender | |
| Male | 2 |
| Female | 20 |
| Ethnicity | |
| White | 22 |
| Other | 0 |
| NHS band | |
| 8 | 0 |
| 7 | 5 |
| 6 | 10 |
| 5 | 1 |
| < 5 | 0 |
| Other | 6 |
| Time in research (years) | |
| < 1 | 1 |
| 1 or 2 | 9 |
| 3–5 | 4 |
| ≥ 5 | 8 |
Appendix 19 Process evaluation: detailed findings
The protocol for the process evaluation has been published,142 with methods summarised in Workstream 5: process evaluation. The intervention as intended is described in Figure 6 and in Appendix 9, Figure 6, and Appendix 12. The first section of this appendix describes the extent to which the intervention was implemented as intended, how it was adapted during implementation and its acceptability to stroke survivors and facilitators. The form in which findings are presented is based on Grant et al. ’s172 framework for designing and reporting process evaluations for cluster randomised trials of complex interventions, describing implementation at site level, then at facilitator level and to the target population (i.e. stroke survivors). In section two, the testing and refinement of process evaluation methods are discussed. Finally, demographic data are presented in section three.
Section one: implementation of the intervention
Implementation at site level
Recruitment of sites
Ten sites were recruited to participate in the trial and randomised 1 : 1 into five active and five control sites. Provision of agreement to participate in the trial varied between the five sites randomised to deliver the intervention, and this shaped its implementation. At sites 1 and 2a, agreement to participate was provided by senior managers/clinicians with an interest in developing self-management/psychosocial approaches to care in their services, which ensured organisational buy-in to implementation. At the remaining three sites, clinicians, rather than service managers, agreed to participate; consequently, there was less organisational involvement. Sites 1, 2a and 3 were geographically large. Site 1 was predominantly rural with a population concentrated in small market towns and coastal resorts, whereas sites 2a and 3 had a mixture of rural and urban communities. Sites 4 and 5 were smaller and predominantly urban. At sites 2a, 4 and 5, there was access to a range of services via established referral pathways.
Delivery of the intervention to sites
The intervention was largely delivered to sites as intended. During conversations with service managers and during the training sessions, the LoTS2Care team provided guidance to facilitators about the required skills and background of facilitators, what equipment and facilities would be needed, and that the intervention should be offered to all stroke survivors as an opt-out service, initially via telephone. The LoTS2Care team asked for New Start to be delivered without the addition of medical assessment components in survivors’ homes and for facilitators to avoid wearing uniforms in order to avoid positioning themselves as expert and reinforcing a medical mindset. The LoTS2Care team also provided intervention-specific standard operational procedures and assisted with a small amount of funding for administrative support at two sites.
Adoption of the intervention by sites
Provision of resources for facilitators
Stroke services at all active sites allocated resources to appoint facilitators and support them to attend training and deliver New Start. However, the majority of sites did not ring-fence staff time to work as New Start facilitators.
Management support for implementation
The degree to which management supported the intervention varied considerably between sites. At site 1, service managers were particularly active in driving the intervention implementation forward by directly supervising facilitators during the practice phase. Ample organisational support was also available at site 2a, including additional administrative assistance and clinical resources in anticipation of referrals generated as a consequence of New Start. This level of support was not available at the remaining sites. At site 4, there was insufficient organisational support to ensure that facilitator posts were filled for the intended duration and, owing to considerable organisational change, New Start delivery was disrupted when a facilitator left 2 months before the end of the delivery phase because of an expiring contract. This resulted in high caseload pressure for the remaining facilitator, resulting in six stroke survivors being seen by her colleague who was not trained to deliver New Start.
Support from colleagues
Adequate support from colleagues, including peer support, was reported everywhere except at site 4, where a communication breakdown within stroke services led to some stroke survivors receiving invitations to both New Start and standard 6-month review appointments, confusing both clinicians and stroke survivors. This lack of contact with other facilitators in site 4 may have been a factor in the lead facilitator adapting intervention delivery in an unplanned manner by deciding during the opening minutes of the appointment whether or not to attempt problem-solving and action-planning based on her assessment of their potential to benefit from the intervention.
Access to equipment and facilities
Facilitators generally had access to adequate equipment, facilities and resources. This was not the case at site 4, where New Start was delivered in a clinic and facilitators reported a lack of consistent access to suitable wheelchair-accessible clinic facilities, which rendered delivering New Start stressful. However, this was in the context of the ongoing organisational change and was not noted as a problem at site 5, which also delivered New Start exclusively in a clinic setting.
Standard operating procedures
Legal and ethics approvals to access and use clinical records to identify and contact stroke survivors were in place and understood. Facilitators at all sites reported that clinical records were accessible and that standard operating procedures already existed that were relevant to the intervention.
Referral pathways
Facilitators at all sites except site 1 reported accessing a wide range of services via clear referral pathways. The lack of referral pathways at site 1 did not prevent relatively high levels of fidelity during New Start delivery.
Unintended changes to implementation by sites
Changes to aspects of the intervention were made at four sites. First contact was made by letters at three sites (2a, 3 and 5) instead of by telephone, as was initially suggested, and occurred at sites 1 and 4. At all five sites, New Start was delivered face to face as intended; one site delivered New Start in both stroke survivor homes and clinic (site 3). As a result of service pressure and clinical needs at sites 2a and 3, hybridisation of the intervention took place, with elements of a medical review such as blood pressure monitoring as well as items required to fulfil SSNAP data collection being delivered at the same meeting as New Start. Facilitators wore uniforms at site 2a because they had already seen stroke survivors in their clinical capacity and thought it would appear unprofessional to arrive in their own clothes; there is no evidence that this had a detrimental impact on the response to New Start.
Implementation at facilitator level
Recruitment of facilitators
Fifteen facilitators were either recruited (site 1) or identified from an existing service to engage with New Start delivery. The degree of facilitator selection varied between sites and this contributed to some sites having facilitators that were more experienced in patient-led approaches, and, therefore, more prepared for delivering New Start. At site 1, facilitators were specifically recruited; a senior service manager sought guidance on the desired facilitator attributes from members of the trial team and facilitators were chosen on the basis of their experience and abilities to deliver a self-management intervention. At sites 2a, 3 and 5, facilitators were appointed based on their existing roles as clinicians embedded in stroke services and experienced in delivering standard 6-month reviews. At site 4, recruitment of facilitators was complicated by the inability of those initially identified to attend training. After some uncertainty, the site principal investigator (a senior clinician working in the traumatic brain injury service) took on the role of facilitator, alongside a second facilitator who was involved in delivering speech and language therapy to stroke survivors.
Delivery of the intervention to facilitators
New Start training
Facilitator training was completed mostly as intended. Facilitators were to receive 2 days of initial training, to be followed by 3 months of practice delivering the intervention in their services, with follow-up training to troubleshoot problems during this period and prior to an assessment of progress (competency assessment). Fourteen facilitators attended the initial 2-day training in September 2016, with the first day devoted to principles of self-management and the second to the specifics of the New Start intervention. Thirteen facilitators attended the first follow-up in November 2016 and 12 facilitators attended the second follow-up day in January 2017, which were run as troubleshooting sessions. Members of the LoTS2Care team travelled to site 4 to provide facilitator training to those who had been unable to attend the initial training. Training records and observational data suggest that facilitators received adequate training on the theories of self-management, effective communication techniques and the delivery of the intervention. A range of supporting materials were provided to the facilitators. For example, templates were provided of content for letters to be sent out to stroke survivors prior to their initial meeting.
Although substantial guidance was given to facilitators about what elements of New Start were ‘usual’, such as number of visits, they were advised that it was a flexible intervention. This flexibility extended to the timing of appointments and the use of telephone contacts, as well as face-to-face interactions for follow-up appointments. This flexibility may have contributed to the difficulties that some facilitators had later in delivering the intervention.
Continuing professional development
During the training, facilitators were asked to complete monthly reflective reports to support their development and were provided with templates and guidance to support this.
Ongoing support from the LoTS2Care team
Evidence from training observations suggests that facilitators were offered continued support for implementing New Start from the LoTS2Care team during both the practice and delivery phases. First, an online discussion board where facilitators could meet to access peer support was established on the NIHR hub community. Second, an e-mail messaging group was established that could be accessed via the NHS messaging account. Third, online video resources were made available. Fourth, facilitators were provided with feedback following the competency assessment, with details of what was being done well and what aspects could be further developed. A newsletter was sent out highlighting areas in which facilitators had scored well in the competency assessment, as well as the aspects that were commonly not reported by facilitators as part of the intervention, as a prompt/reminder to consider these aspects in their practice.
Finally, a teleconference co-ordinated by the trial manager designed for facilitators to discuss any issues and share their experience was offered halfway through the delivery phase.
Adoption and implementation of the intervention by facilitators
Practising New Start delivery
Facilitators were encouraged to develop skills and confidence by practising New Start delivery during a 3-month period following the initial training event before they underwent competency assessment and the trial went ‘live’. During this practice period and the subsequent trial delivery period, the facilitators were asked to complete activity records documenting their input to all the stroke survivors they saw. In total, records suggest that 158 stroke survivors were approached for New Start in the pre-competency assessment period and, of these, 116 (73%) received a visit. The breakdown by site is shown in Table 46.
| Site | Approached (n) | Received visit, n (% of approached) |
|---|---|---|
| 1 | 25 | 11 (44) |
| 2a | 5 | 5 (100) |
| 3 | 88 | 87 (99) |
| 4 | 3 | 3 (100) |
| 5 | 37 | 10 (27) |
| Total | 158 | 116 (73) |
Facilitators at two sites reported having limited opportunities to develop skills during this time. At site 5, this was a result of limited uptake (described in Identification of stroke survivors and invitation to receive New Start). It is unclear why only three participants were approached at site 4. It is also unclear why, at site 2a, only five stroke survivors are reported as being approached and seen. However, this may be a reflection of data entry/return rather than what actually happened.
Facilitators’ responses to available support from the LoTS2Care team
In most cases, facilitators either did not access support from online resources initially provided or could not access them because of local trust IT policies. They also did not take up the NHS e-mail messaging account and the offer of a teleconference halfway through the delivery phase, despite having access to both. Several facilitators commented that they did not feel the need to engage with colleagues at other sites. A site visit was undertaken by a member of the LoTS2Care trial team in order to provide support and clarify aspects of New Start delivery, following expressed concerns from facilitators at one site. Members of the LoTS2care team, including the chief investigator, provided ongoing support and advice to site 3, which found delivery of the intervention challenging. Facilitators at two sites reported that they would have benefitted from more training on completing data entry sheets.
Competency
All 15 facilitators were assessed as competent based on predefined criteria following interviews and review of reflective reports.
Underlying theoretical and practical knowledge
All facilitators demonstrated an understanding of intervention processes and key components of the intervention during competency assessment interviews conducted prior to delivering the intervention. Similarly, when interviewed at the end of the delivery phase, facilitators generally reported that they had sufficient knowledge of the intervention, local resources and referral pathways to deliver New Start.
Operationalising New Start
Facilitators reported that, although they had sufficient theoretical knowledge, they found delivering some aspects of New Start difficult. First, they found it hard to facilitate a stroke survivor’s active engagement in problem-solving because of the stroke survivor’s expectations of being ‘done to’ or their apparent need for medically specialised information. Second, working collaboratively could be difficult from their perspective because of their professional background. Some found aspects of the recommended communication style, such as allowing prolonged silences, uncomfortable because they were unfamiliar with these approaches. Similarly, several facilitators with nursing backgrounds reported difficulty with goal-setting, as this was outside their usual practice. In contrast, most of the facilitators with physiotherapy backgrounds reported that, although goal-setting was familiar to them, it was hard not to be directive in their approach. As anticipated, some of the facilitators who had been appointed because they previously delivered 6-month reviews reported difficulty altering their routine approaches. Some facilitators felt torn between fulfilling the requirements of the SSNAP (which requested clinical information) and the more patient-centred approach of New Start.
A further difficulty related to the volume and complexity of New Start materials. One-third of the facilitators from across sites suggested that there were too many New Start worksheets and that content was repetitive. Some facilitators commented that the flexible design of the intervention made it hard to select which worksheets to use. Facilitators at site 3 referred to the ‘People I know’ sheet as the ‘circle of love’, describing the social mapping materials as ‘patronising’ and ‘schooly’, and declaring them inappropriate for the stroke survivors seen at that site. Facilitators at site 2a reported not using social mapping sheets because they already knew the stroke survivors’ circumstances. On the other hand, several facilitators reported finding the priming tool and the ‘Understanding my Stroke’ booklet useful. The difficulties experienced by facilitators engaging with New Start materials appear to have been compounded by the flexibility of the intervention. Instead of tailoring it to the needs of the individual, as intended, they tended to adopt elements that fit with existing practice or that they felt comfortable with.
It is notable that, although facilitators at site 2a were able to provide a hybrid intervention in which elements of New Start were delivered with fidelity, this was not the case at site 3, where observations and interviews suggested that including clinical components of standard 6-month reviews (which they were nervous of omitting) limited effective delivery of the problem-solving elements of New Start. At site 3, the facilitators were unable to keep the process ‘on track’ and avoid immediately providing solutions when stroke survivors identified physical health problems and expected the facilitator to solve them. In contrast, at site 2a, facilitators were observed to collaboratively engage stroke survivors in needs elicitation and problem-solving, alongside questions about current medication and checking blood pressure. They were aware of the effect that discussing medication and checking blood pressure had on medicalising the interaction, and therefore left these aspects of the appointment until last (as did facilitators at site 1).
Most facilitators did not complete monthly reflective reports as intended: they often found the completion of reflections unhelpful and the request to undertake them on a monthly basis too prescriptive. NPT toolkit reports and SEPSS questionnaires were generally completed as intended during training, implementation and at the end of the trial, with the support of the researcher conducting facilitator interviews.
Facilitators across all sites also expressed the view that completing documentation associated with the delivery of the intervention for research purposes (usual care activity records and New Start activity records) was onerous and time-consuming.
Facilitator self-efficacy
Overall SEPSS scores suggest that there was a significant increase in reported self-efficacy from pre training to the end of implementation. Relatively high levels of self-efficacy were reported at sites 1 and 2a, where facilitators were supported and had the necessary skills. In contrast, at site 3, facilitators reported low self-efficacy and discussed having difficulties with delivering New Start because their nursing backgrounds had not prepared them for collaborative goal-setting. One commented that their prolonged involvement in standard 6-month reviews had ‘tainted’ them and made slipping back into what she described as ‘old ways’ likely.
Facilitator engagement in implementing the intervention
According to NPT toolkit scores, there was some variation in levels of ‘buy-in’ between facilitators. High levels of overall engagement were consistently reported throughout the implementation phase at three sites (1, 2a and 3), whereas levels of reported buy-in varied between facilitators at the remaining two sites, with some facilitators reporting limited levels of willingness of staff to support the intervention. Variability in levels of reported engagement at these sites may reflect the limited value that some facilitators ascribed to New Start. When discussing potential benefits of the intervention, almost all facilitators expressed the view that New Start provided stroke survivors the opportunity to talk about their experiences and reflect, and had the potential to increase QoL. However, many suggested that it was suitable for a minority of the stroke survivors who had received the intervention. Several facilitators suggested that the intervention was more suitable for young and motivated stroke survivors. One facilitator commented that she felt that the facilitator role encroached on psychologists’ territory, and this made her uneasy. When discussing the timing of intervention delivery, more than half of all facilitators across four sites reported that 6 months post stroke was too late to introduce a self-management intervention, and felt that this approach would benefit from being introduced earlier in the care pathway.
Changes to services/practice as a result of delivering New Start
When interviewed, facilitators from all sites reported that delivering New Start had resulted in positive effects on their clinical practice. The following changes were cited: communicating more effectively with stroke survivors, being more person-centred, promoting self-management and adopting a more collaborative/person-centred approach. Facilitators reported that no changes to local stroke services, such as referral pathways, had occurred as a result of New Start.
Identification of stroke survivors and invitation to receive New Start
Identification of stroke survivors
The intended population consisted of all stroke survivors in the stroke services of active sites who were 4–6 months post stroke. Stroke services at all sites were able to identify stroke survivors at the required time point. At two sites (1 and 4) this was undertaken by accessing data from the SSNAP. At the remaining three sites, stroke survivors were identified as patients were discharged from hospital. At every site, facilitators reported that all eligible stroke survivors were offered the intervention.
Method of initial contact
During implementation, uptake could be affected by the manner in which sites chose to invite stroke survivors to receive New Start. At site 2a, where stroke survivors were informed of a pre-booked home appointment (opt out of home visit approach by letter), a particularly high percentage (94.4%) of eligible survivors received an initial meeting (Table 47). This was despite the initial invitation being made by letter. Uptake was lower at sites where stroke survivors were invited by letter to contact services if they were interested in New Start to arrange meetings (opt-in approach by letter). The lowest uptake rate of all sites (10.9%) was at site 5, where stroke survivors received a letter inviting them to telephone and make an appointment at a hospital-based clinic (see Table 47). Facilitators attributed lack of uptake to low levels of ongoing need at this site due to comprehensive stroke services. During the practice phase, a member of the trial team advised facilitators at this site to allocate appointments in an attempt to increase uptake, but no changes were made and the uptake rate remained low. At sites 1 and 4, the offer of New Start was made by telephone, but as an opt-in, with a home visit offered at site 1 and a clinic appointment offered at site 4 (see Table 47). At these sites, uptake rates were low and facilitators described difficulties with contacting individuals when they were home and believed this was because many people are reluctant to answer telephone calls when the number is withheld.
| Site | Presentation | Meeting location | Medium | Uptakea (%) |
|---|---|---|---|---|
| 1 | Opt-in | Home visit | Telephone | 45.5 |
| 2a | Opt-out | Home visit | Letter | 94.4 |
| 3 | Opt-in | Choice | Letter | 99.5b |
| 4 | Opt-in | Clinic | Telephone | 93.8b |
| 5 | Opt-in | Clinic | Letter | 10.9 |
Unintended changes that may have limited reach
During the study, the Bridges intervention (www.bridgesselfmanagement.org.uk/; accessed 9 October 2020) was commissioned by the local health board and was rolled out to part of the catchment area covered by site 1. Following discussions with the research team (and the Bridges team), the facilitator excluded stroke survivors who lived in the area where the Bridges programme was being implemented during the later stages of recruitment to this study. At site 4, the lead facilitator did not attempt New Start with stroke survivors whom she felt either had no unmet needs or lacked the potential to benefit from it owing to cognitive difficulties or a perceived lack of ability to engage. A further six stroke survivors at site 4 did not receive New Start because of the loss of a facilitator 2 months before the end of the delivery phase, as previously described. Reach (uptake) may have been limited at three sites (sites 3, 4 and 5) where New Start was delivered in a clinic, rather than in stroke survivors’ homes. Findings from stroke survivor interviews suggest that those experiencing a high degree of recovery and who were untroubled by mobility issues were generally happy to attend clinic appointments, whereas those with physical impairment or lack of access to transport valued the opportunity to be seen at home. The continued delivery of standard 6-month reviews (offered on an opt-out basis and delivered at home) was likely to be a further factor limiting uptake of New Start at site 5.
Delivery of New Start
A first New Start meeting was held with 377 stroke survivors (anonymised data were collected on all who were offered the service, not just trial participants) during the delivery phase, with a facilitator who had been assessed as competent once the trial had opened. Percentages reported in this section use this denominator (377), unless otherwise stated. Data are reported from the activity records completed by facilitators.
Dose
Most stroke survivors received a smaller dose of New Start than intended. It was anticipated that at least an initial meeting and two further contacts would be needed to allow the delivery of key intervention components (i.e. identification of unmet needs, goal-setting, action-planning and review). Only 13% of cases (n = 48/377) were reported as receiving this level of input. A further ‘minimum dose’ of New Start was defined as an initial meeting and at least one follow-up contact. According to activity records, 25% (n = 93/377) of stroke survivors received this amount of the intervention.
Delivery of New Start elements
Introducing the intervention
During observations of intervention delivery, New Start was introduced as a self-management intervention by two facilitators, whereas approximately half the facilitators introduced New Start as a form of ‘review’, without any further explanation of the remaining components of the intervention and the intended roles of facilitator and stroke survivor. This terminology was consistent with the training and the pre-meeting letter, but may have contributed to stroke survivors’ difficulties in engaging collaboratively in the intervention.
Establishing a relationship with the stroke survivor
Observations, facilitator reflections and stroke survivor interviews all suggest that facilitators regularly succeeded in establishing rapport with stroke survivors and were skilful in their use of questions and active listening, although it was noted that, occasionally, facilitators did not challenge unrealistic expectations. Facilitators (particularly those from nursing backgrounds) reported not having enough knowledge to challenge unrealistic expectations, for example if a stroke survivor wanted to walk again. It was often the first time they had met the stroke survivors and/or they did not feel that they had the knowledge to say whether or not this could be a goal; usually, the resolution was a referral for physiotherapy review. In some cases, when the facilitator was a physiotherapist, physical assessments were undertaken to check this (which made the appointment appear more medical in nature).
Facilitators were seen to treat stroke survivors with dignity and respect. In most observations, facilitators invited participants to complete the worksheets themselves (intended to establish shared ownership). However, it was noted that their approach was infrequently collaborative and often resulted in facilitator-directed, rather than survivor-directed, needs identification and problem-solving. This may be a consequence of the difficulties that some facilitators had in acting as ‘co-pilot’.
Identification of ongoing needs
Although activity records suggest that ongoing needs were discussed during 73% of cases and ongoing needs were identified in approximately two-thirds of observed meetings, this rarely took place using New Start worksheets. Facilitators at three sites reported significant numbers of stroke survivors being ‘stuck in the medical model’ during their meeting, and consequently expecting guidance from an expert. During training, facilitators were told to expect this and given advice about dealing with this through active listening and Socratic questioning. Sometimes the manner in which questions were observed to be framed by facilitators encouraged stroke survivors to think about their physical health. The identification of ongoing needs relating to physical health may also have been encouraged by the clinical settings and the medicalisation of the intervention whereby facilitators were delivering a hybridised intervention that included checking medication lists for the SSNAP and taking blood pressure. Facilitators’ abilities to explore psychosocial issues varied and appeared to be linked to their professional role; occupational therapists and nurses experienced in mental health care appeared relatively well equipped to do this. Observations suggested that, in some other cases, ongoing psychosocial needs that stroke survivors mentioned were not explored by facilitators or identified as a focus for action. It was unclear whether this was because they did not recognise the need, did not see it as their responsibility or were uncertain about how to respond.
Social mapping
Activity records indicated that facilitators discussed social networks in 59% of cases. However, this was undertaken using specific worksheets (E, F or G) by only two of the 15 facilitators, with only a handful of stroke survivors (n = 1 and n = 4 for the two facilitators). As mentioned in Operationalising New Start, the New Start materials relating to social mapping were the least popular among facilitators. Although facilitators were observed to routinely discuss social relations, on several occasions opportunities to explore stroke survivors’ social networks through formal social mapping appeared to be missed. This may have been because these worksheets were relatively unpopular with facilitators (see Operationalising New Start). Observations suggest that another possible reason was the nature of the needs identified: physical health needs could be considered through medical model-style solutions. There is little evidence suggesting that facilitators were able to help stroke survivors utilise and develop social networks as a consequence of New Start or use them to support problem-solving and action-planning.
Goal-setting and action-planning
Facilitators recorded goals being set in 20% of cases. Facilitators were observed inviting stroke survivors to set goals on numerous occasions and a collaborative approach to goal-setting was attempted to some extent by facilitators across all sites during observations. However, in some sites, stroke survivors were sometimes not supported to take an active role in setting goals, and the identification of unmet physical health needs often prompted facilitators to respond with referrals, rather than with collaborative goal-setting and action-planning.
Referrals to health and social care professionals
Activity records indicate that referrals took place in 34% (n = 129) of cases. As ongoing unmet needs identified were largely related to physical health, referrals were directed at health-care professionals, although facilitators did also refer for other reasons such as social and psychological support. Occasionally, stroke survivors were advised to self-refer, for example for psychological support or for access to exercise programmes/gyms via the GP, or for community transport services.
Review activity
From the completed activity records, follow-up and review activity took place in 18% of cases. Lack of follow-up, in which actions relating to goals were reviewed and amended if necessary, may mean that planned actions fail to take place and any initial success is not built on.
Provision of information
‘Understanding my Stroke’ and ‘Finding Information’ booklets were provided in 10% and 14% of cases, respectively, according to activity records. In contrast, information about available services was observed as being regularly provided, as well as advice on managing the physical effects of stroke. Information about psychosocial effects was discussed less often. Varying rates of information provision were expected, as information was supposed to be relevant and made usable.
Provision of stroke survivor support
Observations and activity records suggest that facilitators often provided their contact details and emphasised that stroke survivors could contact them: stroke survivors reported valuing this offer of support.
Combination of essential New Start elements
When predefined criteria were strictly applied to activity record data (priming tool provided + discussed life + discussed social network + needs were discussed + goals set), only 7% of cases were recorded as having all of these elements. When the additional activities of action planned + review were added to the criteria, only 3% of cases had all of these elements that should have been delivered to people with ongoing needs. However, the accuracy of the activity records is unclear and facilitators reported finding them difficult to complete as described previously.
It should be noted that, although on most occasions New Start was not delivered entirely as intended, there was considerable variation in the way that the intervention was delivered between both sites and individual facilitators, and that it was occasionally delivered with a high fidelity.
Cost of delivering New Start
A full economic analysis is reported in Workstream 5: economic evaluation and Appendix 21.
Stroke survivors’ response to New Start
Understanding of the intended purposes of the intervention
Observations of New Start meetings and interviews with stroke survivors suggest that they could sometimes be unclear about aspects of the intended purposes of New Start, specifically that it was not wedded to the medical model probably underpinning most of the previous care they had received and that it included an element of self-management. They often assumed that they were receiving a standard follow-up appointment, rather than an exploration of needs followed by a facilitated self-management intervention based on problem-solving for those with non-clinical unmet needs. Stroke survivors’ lack of understanding may have been due to several factors, although these were not described by facilitators or stroke survivors. In sites where they received a formal appointment letter rather than the suggested initial telephone call, this may have contributed to the assumption that the interaction offered was consistent with standard health-care approaches. During the initial meeting, the use of the term ‘review’ may have also fuelled the assumption that the intervention was similar to familiar clinical interactions.
Engagement in the intervention
Stroke survivors were observed to struggle to engage with aspects of New Start. Although this was expected, the facilitator, process and materials were intended to support and develop their engagement over multiple meetings. However, this often did not happen as intended. As expected, the offer to complete worksheets was almost always turned down by stroke survivors; however, this invitation did not seem to affect their engagement in the intervention either way. Although the priming tool could be useful in providing permission for the discussion of sensitive issues such as sexual function, its use as a checklist by most of the facilitators, rather than a basis for discussion and means of helping the stroke survivor prepare for their review by reflecting on what issues they faced, may have limited survivors’ identification of unmet needs.
It appeared that when stroke survivors identified ongoing needs, they could be given opportunities to problem-solve and set goals. However, they could struggle to take an active part in this and many declined to attempt these activities. There appear to be several reasons for these difficulties. They could struggle when offered a leading role in problem-solving and goal-setting out of deference to facilitators. Several facilitators suggested that this was because they had been disempowered previously during their acute stroke care and subsequent contacts with the health services. Some stroke survivors also reported difficulty because problem-solving and goal-setting were unfamiliar to them. Facilitators also reported that stroke survivors could struggle to engage in problem-solving and goal-setting because of cognitive impairment or lack of understanding. Facilitators also reported that fatigue could result in difficulties with enacting goal-setting and planning actions. When stroke survivors were able to understand that an active role in problem-solving was expected of them, they were sometimes unable to do so effectively, particularly when problems were related to their physical health, because they were given insufficient support from facilitators to explore the nature of their problems and consider solutions other than accessing available services. When stroke survivors struggled to engage, there was a tendency for facilitators to take control of the problem-solving/goal-setting process; consequently, in some cases, the only actions planned during the meeting were referrals to be undertaken by the facilitator. Nevertheless, with facilitator guidance, stroke survivors occasionally embraced all aspects of the intervention: they identified ongoing needs; set specific, measurable, achievable, relevant and time-bound goals; solved problems; and performed actions.
Reported benefits of receiving New Start
Most stroke survivors reported some benefit from their New Start meeting. Many reported feeling supported and some valued the opportunity to talk about their experiences. Several reported feeling reassured that their experiences were normal and that they had taken an appropriate approach to recovery. Improved knowledge of available services and stroke management, including the prevention of further episodes, was reported by some. Others reported benefits associated with their physical health, such as medication review, resulting from contact with services. Occasionally, psychosocial benefits, such as increased confidence and motivation, were mentioned, although usually in relation to addressing health issues such as making appointments to visit their GP. A significant minority of stroke survivors interviewed reported an increase in their QoL following receipt of New Start. This was attributed to perceived support from the facilitator and, in one case, the provision of mobility equipment.
Actions completed by stroke survivors as a result of receiving New Start were reported by some of those interviewed. These actions were mostly directed at improving physical health and function and reflect the predominance of health-related needs identified during meetings (walking, attending appointments). Occasionally, individuals recognised positive outcomes as a result of these activities, such as reduced leg pain and weight loss. Reasons given for lack of action included adverse weather, preventing walking outdoors, and the lack of contact details for services they had agreed to engage with.
Summary of contextual factors associated with variations in intermediate outcomes between sites
Several factors influenced how New Start was implemented at sites:
-
The direct involvement of key managers in New Start implementation aided implementation at two sites by providing organisational support such as facilitator supervision, adequate facilities, and administrative support. At site 4, lack of managerial involvement impeded delivery.
-
A facilitator’s professional background and experience of delivering standard 6-month reviews could influence their ability to deliver New Start as intended. Site 1 – selected for purposes of New Start delivery; interest in and ability to deliver self-management; new to review process; occupational therapy background. Site 2 – occupational therapy, physiotherapy and nursing backgrounds with self-management experience, which enabled collaborative goal-setting and action-planning. At site 3, nurses/physiotherapist had years of experience of undertaking a standard review, but lacked confidence and experience with goal-setting/collaborative approaches. Although additional training was provided, they continued to have difficulties in delivering aspects of New Start as intended. Site 4 – speech and language therapist comfortable with goal-setting and action-planning and a nurse who reported finding it difficult to avoid a medicalised approach and adopting a co-pilot role because of her background. Site 5: occupational therapist, physiotherapist and nurse prescriber. Here, facilitators were used to working across professional boundaries, so background was less significant. This team was highly experienced in delivering standard 6-month reviews; one team member was observed to deliver a standard review rather than the intended intervention.
-
Delivery of a hybrid intervention combining elements of a standard review alongside New Start took place at two sites. At site 3, where facilitators lacked confidence in delivering the intervention, this medicalisation of the intervention added to the difficulties that facilitators were experiencing with collaborative problem-solving and goal-setting.
-
Capacity/caseload pressure varied considerably between sites. High case-load pressure and pre-booked 45-minute appointments led to stress for facilitators at site 4. In contrast, ample time and a lack of pressure at site 1 enabled an unhurried approach and flexible appointments.
Key points
-
Recruitment of sites was successful and the intervention was delivered to sites largely as intended.
-
Sites did not always respond as intended; support for implementation of the intervention from organisations and colleagues was not always available and adaptations to intervention implementation were made that would influence intervention delivery.
-
Although recruitment of facilitators took place as intended, ultimately, the circumstances in which it was undertaken influenced how New Start was implemented.
-
Facilitator training was delivered mostly as intended.
-
Stroke survivors were identified as intended.
-
Methods of approach (for the review) may have influenced uptake.
-
The delivery of New Start in clinic had a negative impact on uptake for some participants.
-
Facilitators demonstrated detailed knowledge of the principles underlying New Start, but could struggle to deliver New Start as intended. Nevertheless, when facilitators possessed a professional background that included goal-setting/self-management and were supported by their organisation, high levels of buy-in and self-efficacy ensued.
-
Facilitators found completing records of their activity onerous; this may have led to under-reporting of the components of the intervention delivered.
-
Some facilitators required support to complete the NPT toolkits and SEPSS questionnaires and could struggle to undertake reflections as intended.
-
Stroke survivors could be unclear about the intended purposes of New Start. Although they identified ongoing unmet needs, they struggled to engage in problem-solving and goal-setting. A minority changed their activities as a result of receiving the intervention; nevertheless, most stroke survivors reported benefitting from the intervention because they felt supported. Occasionally, they appeared to fully understand the purposes of New Start and engaged in all aspects of the intervention as intended. This appears to have been associated with the receipt of a clear explanation of the intervention.
Discussion
In this feasibility study, we sought to test a range of materials and approaches to deliver the intervention. However, it seems that this flexibility was confusing for stroke survivors and some facilitators. A future iteration could consider mandating more elements and/or providing a simplified version of the intervention with clear guidelines for use during the training phase.
One of the key findings was that New Start was delivered most effectively at sites where intervention delivery was supported by the wider organisation. The importance of multilevel involvement within the organisation hosting self-management interventions is recognised in the literature. 173,174 Consequently, it is recommended that these aspects of organisations that may potentially deliver future iterations of New Start are carefully scrutinised.
It was clear from the findings that delivering New Start could be challenging for facilitators. Some reported that promoting self-management was hard because they found relinquishing control and avoiding the role of expert difficult. We found that the degree to which facilitators experienced these difficulties and their response to them could be shaped by their professional background and experience in this area. When it became clear to facilitators that delivering New Start as intended was proving difficult, there was a tendency for some to revert to familiar professional roles. Others have noted that recognising a patient’s ability to self-manage represents a paradigm shift for clinicians,174 and that relinquishing control when delivering self-management can be challenging175. The finding that goal-setting took place only occasionally may reflect this, as well as other factors. Levack et al. 176 investigated goal-setting in an inpatient stroke rehabilitation setting and noted that it was a complex activity involving potentially awkward and time-consuming conversations that staff could find difficult. Facilitators reported that aspects of the intervention (e.g. copious New Start materials) could augment these difficulties. A strategy providing an intermediate phase in which facilitators gained confidence through delivering a simplified version of the final intervention may have helped. It is recommended that careful consideration is given to the experience and backgrounds of clinicians delivering self-management interventions.
We found that stroke survivors could also struggle to engage with New Start. Facilitators attributed the lack of stroke survivor engagement to an absence of unmet needs, the lack of ability to set goals and a lack of motivation to self-manage. Others have reported that people living with long-term conditions appear to lack interest in supported self-management approaches because they lack confidence in professionals’ ability to provide useful input. 173 However, the factors cited by facilitators were sometimes contradicted by observations, and the reasons why stroke survivors in our study struggled to engage may be complex and may include the facilitator’s approach. Jones et al. 177 commented that stroke survivors may be dealing with a range of emotional and cognitive difficulties that could make it hard for them to engage in self-management, and that apparent passivity when receiving interventions may reflect the result of minimal opportunities to build confidence. Norris and Kilbride175 noted that stroke survivors inevitably struggle with self-management because, during the acute phase of their condition, they are positioned as passive recipients of care. Consideration should therefore be given to bolstering stroke survivors’ confidence and empowering them to fully engage.
We noted that the purpose of New Start meetings was often framed (and perceived) as a form of review, rather than as a supported self-management intervention in which the stroke survivor took control. The lack of a clear explanation may reflect the unfocused nature of a complex intervention presented to facilitators during their training as a flexible and open process. An absence of shared understanding regarding the purposes of New Start represents a further obstacle to stroke survivors engaging fully in the intervention. Ensuring that recipients of New Start fully understand that this differs from interventions based on the medical model of care may not be straightforward. Those delivering self-management interventions for stroke survivors elsewhere advise care when introducing them and report avoiding the term ‘self-management’ because it can have negative connotations. 177 Instead, they advise including more familiar terms such as ‘control’ or ‘responsibility’ in explanations. It is recommended that facilitators receive greater guidance to ensure that New Start is more clearly introduced to stroke survivors and that its purposes are fully understood by all concerned.
Both facilitators and some stroke survivors suggested that 6 months post stroke was too late, and that self-management would be of more benefit to stroke survivors if delivered earlier. Integrating self-management into an acute stroke setting has been found to be feasible. 178 However, others have pointed out that, although the principles of self-management should be introduced in the acute setting, the community setting was more likely to lead to engagement. 175 It is therefore recommended that further consideration be given to when it is offered and how it can be more needs based and survivor driven.
Section 2: testing and refining process evaluation methods
Non-participant observation of New Start delivery
Opportunity sampling was successfully used to identify a diverse sample of participants and enabled us to address the research questions. Almost all stroke survivors who were approached by facilitators and invited to participate in the study agreed to do so readily (Figure 14). Only two stroke survivors declined to participate. Facilitators reported feeling comfortable being observed, with several commenting that, as clinicians, they were used to having students and visitors present while performing clinical activities. One facilitator commented that she might not have felt as comfortable about being observed delivering New Start if she had been in somebody’s home, rather than in clinic. We noted that more goal-setting activity occurred during observed New Start delivery than was recorded at other times, suggesting that facilitator behaviour changed as a result of researcher presence or that their activity recording was poor. Changing behaviour in the presence of researchers is unsurprising, as observations occurred during isolated occasions when modifying natural behaviour would have been straightforward for facilitators. Facilitators were also aware that researchers observing them worked in the unit that devised the intervention, and so demand characteristics would have been likely. In an attempt to minimise these effects, researchers attempted to build rapport with facilitators to make them feel comfortable, and informed them that they had no involvement in developing the intervention and were separate from the trial team. It is recommended that more observations of usual care prior to implementation and intervention delivery are undertaken to lessen these effects.
FIGURE 14.
Recruitment of stroke survivors and carers for observations. C, carer; SS, stroke survivor.
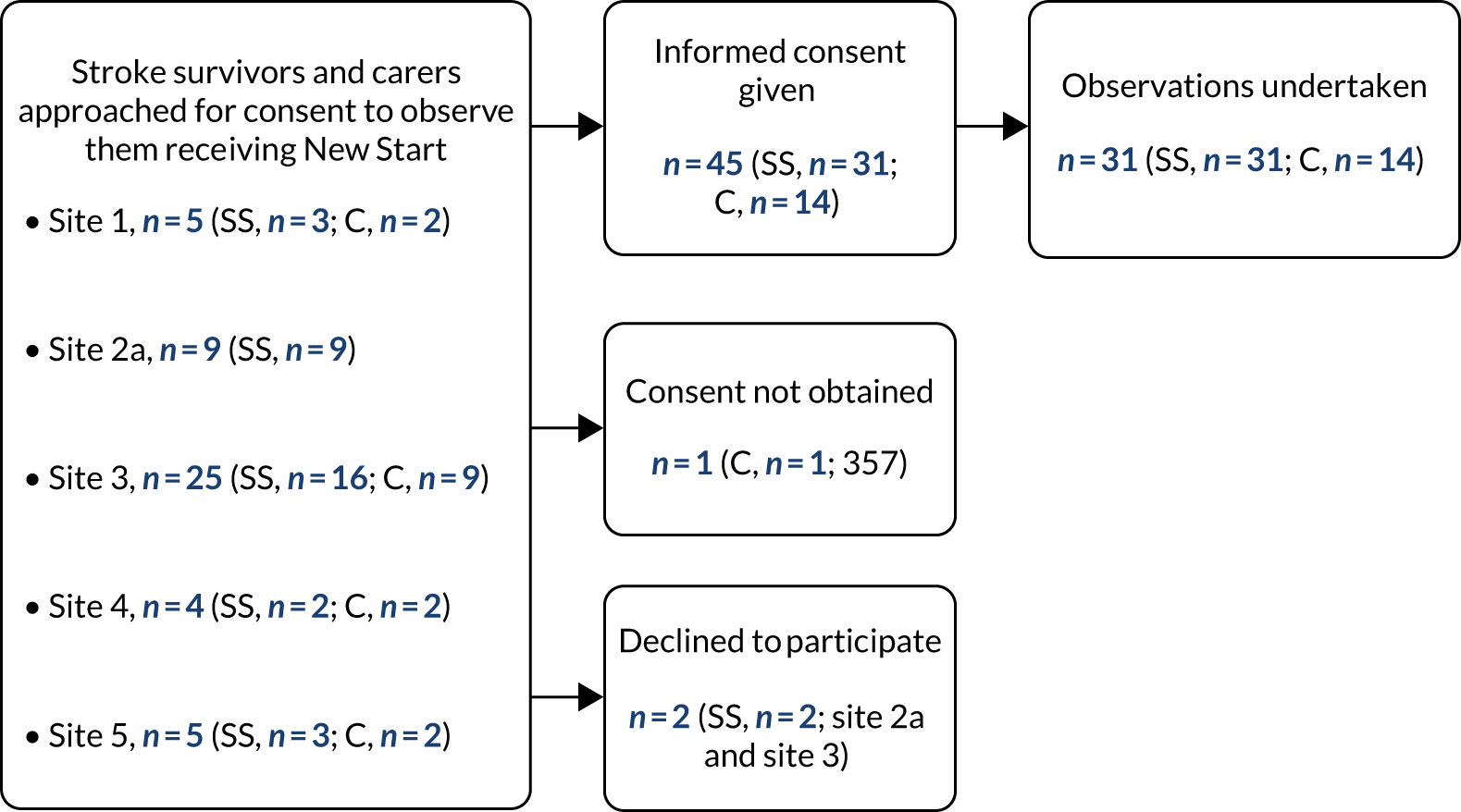
Interviews with stroke survivors
New Start activity records were used by the process evaluation team when visiting active sites to purposively select a diverse sample of stroke survivors who had received New Start. Trial participants were successfully eliminated from the list of potential informers, in most cases to avoid influencing trial outcomes. This was undertaken without facilitator involvement, to avoid unblinding them to stroke survivors’ trial status. Once a list of potential interview participants was created, process evaluators gave the list to site staff who then sent invitation letters, information sheets and response forms to these individuals. Stroke survivors were asked to return a completed response form directly to the research team. Process evaluators then contacted those agreeing to participate in an interview and directly confirmed details of the interview time and location. If the process evaluators did not receive a response within 2 weeks, site staff were asked to telephone the stroke survivors. This process of relying on facilitators to send out information packs to a list of stroke survivors identified by researchers by their New Start ID number alone was occasionally problematic because of the potential for human error related to completing complex administrative tasks in busy clinical environments and because stroke survivors had sometimes been allocated more than one ID number. Subsequent attempts by process evaluators to identify potential candidates for interview remotely in between site visits was made difficult by a reliance on the timely return of completed activity records, which allowed potential stroke survivors to be identified. Twenty-six interviews were completed (Figure 15).
FIGURE 15.
Recruitment of stroke survivors and carers for interviews. Purple shading signifies invited/approached. Orange shading signifies interview did not take place (declined/excluded/cancelled/unable to contact/deemed inappropriate). Green shading indicates agreed/interview completed.
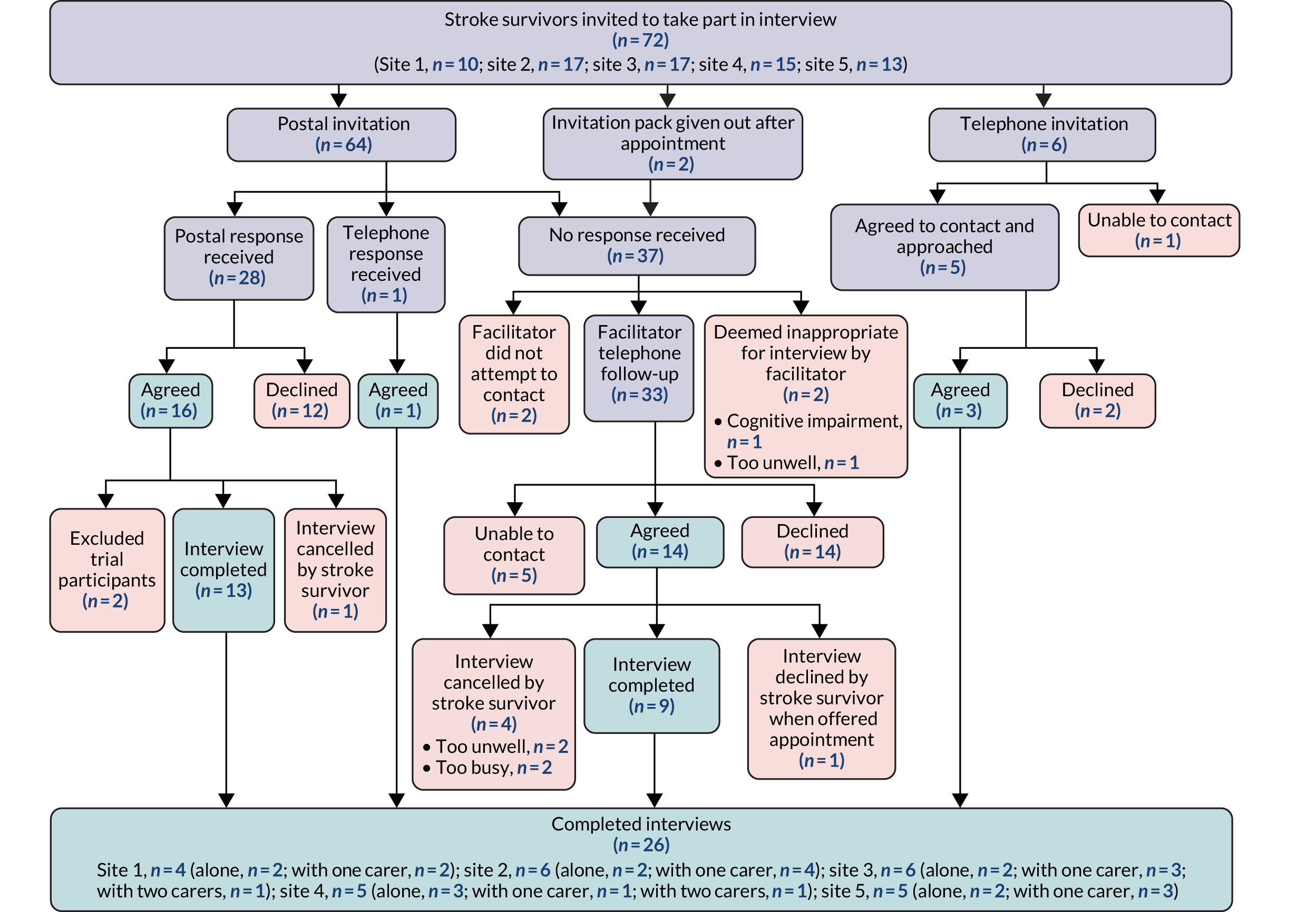
Difficulties with stroke survivor recall were sometimes noted during interviews. Stroke survivors could have difficulty remembering details of their New Start appointments. These details included meeting duration, whether or not they had received materials either prior to or during their appointment, worksheets that had been completed and the name of the facilitator who had seen them. Stroke survivors sometimes had difficulties differentiating between New Start facilitators and other health-care professionals, and could struggle to remember which health-care professional had been responsible for which element of input. Stroke survivors also had difficulty identifying whether positive changes that had taken place were due to interaction with the facilitator or with other health professionals/services. These difficulties appear to be related to memory difficulties, cognitive impairment, the amount of time elapsed since the appointment in question (which could have occurred up to 6 months previously) and having received advice and practical assistance from a range of different people. Some data suggest that carers were sometimes better at differentiating between health professionals. Using photographs of facilitators helped to jog memories in some interviews. At one site, organising interviews within 1 week of completing the intervention led to very clear and detailed recall of the appointment. This was made possible by the assistance of a facilitator who invited the stroke survivor to participate in an interview at the end of the initial New Start appointment. This approach may be a more efficient method of recruiting stroke survivors to participate than the one used in the majority of cases. It is recommended that interviews with stroke survivors are undertaken closer to the time of delivery.
Interviews with facilitators and relevant site staff
These were conducted without incident, mostly face to face or, occasionally, by telephone.
Self-report by facilitators
Facilitators were asked to complete SEPSS questionnaires at four time points during the trial: at the beginning and end of initial training, after completing the third training event and at the end of the delivery phase. This was intended partly as a basis for them to reflect on their delivery of New Start. Few comments were made regarding the completion of the SEPSS tool. When opinions were occasionally offered, they were either non-committal (e.g. completing the tool felt ‘fine’) or expressed the view that questions contained in the tool were difficult to answer. Facilitators completed NPT toolkits on three occasions during the trial: before delivering the intervention, mid-way through delivery and at the end of the delivery phase. Several facilitators were critical of the toolkit, stating that the wording was confusing or vague and that completing the questions was of limited value to them. In contrast, three facilitators expressed the view that completing the toolkit had been useful; one spoke about it ‘re-validating’ what the intervention was about and helping them to reflect on the manner in which they delivered New Start. Several facilitators stated that the purpose of completing the tool was not made clear to them. Some data were missing because facilitators either did not complete SEPSS questionnaires and NPT toolkits or failed to return them to the LoTS2Care team (Tables 48 and 49)
| Time point | SEPSS | Missing NPT toolkits | |
|---|---|---|---|
| Missing questionnaires | Missing self-efficacy/performance scale totalsa | ||
| TP0 (n) | 0 | 0 | 3 |
| TP1 (n) | 1 | 10 | 2 |
| TP2 (n) | 3 | 12 | 1 |
| TP3 (n) | 1 | 5 | N/A |
| Total, n/N (%) | 5/60 (8) | 27/120 (23) | 6/45 (13) |
| Time point | Number of facilitators with missing dataa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subscale | ||||||||||||
| Assess | Advise | Agree | Assist | Arrange | Overall | |||||||
| Self-efficacy | Performance | Self-efficacy | Performance | Self-efficacy | Performance | Self-efficacy | Performance | Self-efficacy | Performance | Self-efficacy | Performance | |
| TP0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 1 |
| TP1 | 1 | 6 | 2 | 8 | 2 | 8 | 1 | 7 | 3 | 6 | 2 | 8 |
| TP2 | 4 | 6 | 4 | 4 | 6 | 6 | 5 | 6 | 5 | 6 | 6 | 7 |
| TP3 | 3 | 2 | 3 | 3 | 5 | 4 | 3 | 2 | 4 | 3 | 3 | 2 |
Reflective reports
Facilitators were asked to complete reflections as a means of exploring their responses to delivering New Start on a monthly basis from the initial training until the end of the delivery phase (approximately 15 months); this met with mixed success. With the exception of one facilitator who completed 40, the majority of facilitators completed between one and seven. Most facilitators reported favourable attitudes to reflective reports; several facilitators with nursing backgrounds commented that this was something that they were used to doing as part of their professional validation (although two nurses who had been qualified for several decades reported finding the process of writing difficult and said that they would prefer to talk about their practice). However, approximately half of the facilitators reported that they struggled to find time to complete the reflections. In response to these difficulties, a verbal reflection over the telephone was trialled with one facilitator, which proved successful. Monthly completion was also considered too frequent by some facilitators, whereas others stated that they had lacked the support necessary for completing reflections, such as a reminder from the LoTS2Care team. One facilitator commented that requesting monthly reflections was antithetical to the reflective process, in which reflections are completed as a response to a specific incident. Two facilitators reported that writing reflective reports helped them to deliver the intervention.
Documentation of intervention activity
As mentioned previously, there was a degree of misunderstanding on the part of some facilitators relating to recording New Start activity; this lack of a consistent approach and a degree of inaccuracy was considered to potentially adversely affect the evaluation of New Start delivery. To explore this further, a comparison was made between researcher field notes in which elements of New Start delivered during observations were recorded and activity records completed by facilitators during the same appointments. Field notes and activity records from 31 appointments were compared and the degree of consistency between what was observed and was recorded was classified by the researcher as ‘good’, ‘fair’ or ‘poor’. Consistency in 20 appointments was classified as ‘good’, in nine appointments as ‘fair’ and in two appointments as ‘poor’. We found that the main discrepancy between what was observed and recorded related to facilitators’ use of New Start materials. On eight occasions, facilitators under-recorded the amount of New Start materials used, compared with what was observed, and, on five occasions, facilitators over-recorded the use of New Start materials. These findings imply that in one-third of observed appointments there was some degree of error in recorded activity. Facilitators recounted finding record-keeping onerous, and it is possible that these errors occurred because facilitators recorded their activities some time after New Start meetings. Simplifying activity records may be possible, but this might compromise data collection for trial purposes.
Key points
Observations of New Start delivery and interviews with stroke survivors, facilitators and other staff were successfully undertaken. Some facilitators may have modified their delivery when being observed. In future, more observations of delivery may be useful in lessening the degree to which this takes place and providing a more accurate view of their practice. Some stroke survivors struggled to recall details of their New Start meeting, particularly when asked about events that had taken place several weeks or even months previously; therefore, interviewing closer to time of delivery is advisable. Most facilitators failed to complete reflections on a regular basis because they forgot or lacked time. Facilitators found completing intervention documentation onerous; simplifying paperwork would therefore be likely to make implementing New Start more acceptable for those delivering it in the future.
Section 3: summary of demographic data
Stroke survivors and carers
Thirty-three stroke survivors and 15 carers were approached for consent to observations as part of the process evaluation. Two stroke survivors declined and one carer did not provide informed consent. Thirty-one stroke survivors and 14 carers were subsequently observed receiving the New Start intervention (see Figure 14). Of the participating stroke survivors, most were white (97%, n = 30) and male (71%, n = 22). Most (58%, n = 18) lived with a spouse or partner; 26% (n = 8) lived alone; and others lived with a parent, son or daughter, friend, live-in carer or a combination of these (16%, n = 5). Most were aged > 60 years (87%; Table 50). All carers were white, and most were female (79%, n = 12) and aged > 60 years (86%, n = 12).
| Age (years) | Interview participants (n) | Observation participants (n) | ||
|---|---|---|---|---|
| Stroke survivors | Carers | Stroke survivors | Carers | |
| 18–40 | 0 | 1 | 0 | 1 |
| 41–59 | 4 | 5 | 4 | 1 |
| 60–79 | 11 | 7 | 16 | 6 |
| ≥ 80 | 11 | 4 | 11 | 6 |
| Total | 26 | 17 | 31 | 14 |
Twenty-six stroke survivors and 17 carers took part in interviews. All participants were white. Of the stroke survivor participants, 58% (n = 15) were male; 47% (n = 8) of carers were also male. Thirty-one per cent of stroke survivors lived alone (n = 8); the remaining 69% (n = 18) lived with a spouse or partner, a minority of whom also lived with a live-in carer (n = 1), or son or daughter (n = 2). Eighty-five per cent of stroke survivors were aged > 60 years (n = 22), as were 65% of carers (see Table 50). Most carers were the spouse or partner of a stroke survivor participant (65%, n = 11); others were a son or daughter (24%, n = 4), grandchild (4%, n = 1) or live-in carer (4%, n = 1).
Facilitators
Fifteen facilitators were recruited to deliver New Start. All facilitators were white and female; most were aged > 41 years (87%; n = 13) and employed at NHS band 6 or 7 (87%; n = 13) (Table 51).
| Demographic factor | Facilitators (N = 15) (n) | Other staff (N = 6a) (n) |
|---|---|---|
| Age (years) | ||
| 18–30 | 1 | 0 |
| 31–40 | 1 | 0 |
| 41–50 | 7 | 3 |
| ≥ 51 | 6 | 3 |
| Gender | ||
| Male | 0 | 6 |
| Female | 15 | 0 |
| Ethnicity | ||
| White | 15 | 6 |
| Other | 0 | 0 |
| NHS band | ||
| 8 | 0 | 1 |
| 7 | 9 | 2 |
| 6 | 4 | 0 |
| 5 | 1 | 0 |
| < 5 | 1 | 3 |
| Other | 0 | 0 |
Other staff
Six other relevant members of site staff took part in interviews, including administrators and managers (two of whom were also facilitators). All other staff interviewed were white, female and aged > 41 years (see Table 51).
Appendix 20 New Start intervention delivery data
FIGURE 16.
New Start intervention delivery data. a, Based on number of stroke survivors with completed New Start activity records. We are aware that ≈245 more stroke survivors were approached based on contact log data [site 3 found it unfeasible to complete a New Start activity record for all stroke survivors who were sent an invitation letter and site 4 completed form 20s (usual care activity forms) rather than New Start activity records for around half of stroke survivors, who received a standard review rather than New Start]. b, Collaborative goal-setting = priming tool provided + discussed life + discussed social network + discussed needs + set goals + planned action. Data are reported for all stroke survivors who were first approached once facilitators were deemed to be competent (i.e. implementation phase).
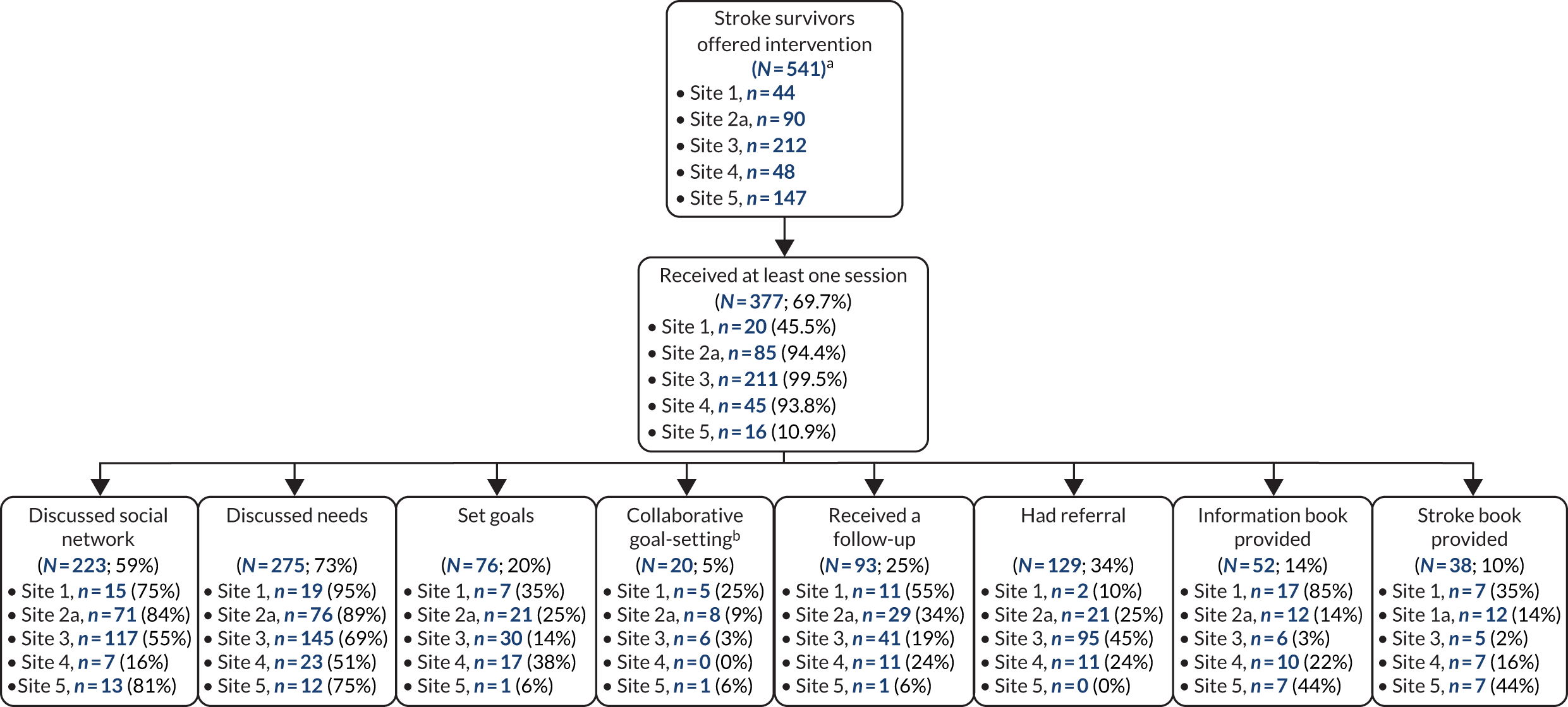
Appendix 21 The LoTS2Care health economics analysis
Background
The exploratory economic evaluation was conducted in two parts: a within-trial economic evaluation was conducted to evaluate the costs and benefits associated with the New Start intervention that occurred during the trial, and an economic model was developed to analyse future costs and benefits beyond the trial time horizon. The analysis was guided by the recommendations of the NICE methods guide. 144
Within-trial analysis
An exploratory economic evaluation was conducted alongside the LoTS2Care feasibility trial to produce preliminary estimates of the cost-effectiveness of the New Start intervention, compared with usual care, for stroke survivors.
Methods
Aims and end points
The primary aim of this analysis was to produce preliminary estimates of the cost-effectiveness of the New Start intervention to inform future research. The primary end point was the cost per QALY gained from the New Start intervention, compared with usual care, at 9 months post randomisation. 179
Perspective and time frame
The study adopted a societal perspective for the main analysis, and an additional analysis was undertaken from a health-care provider perspective. Costs (direct and indirect) and outcomes of stroke survivors at centres randomised to the New Start intervention versus usual care were compared over the 9-month time horizon of the trial. As the time frame was < 1 year, discounting of the costs and benefits was not required.
Measurement of outcomes
Health-state utility values were obtained from patient responses to the EQ-5D-5L questionnaire,145 which was administered at baseline and at 3, 6 and 9 months post randomisation. Patient responses were converted to utility values using the standard UK general population tariff values. 180 The utility values represent patients’ QoL and were multiplied by duration (t) in each health state to generate QALYs, which were used as the main outcome measure for this analysis, using an area under the curve approach:
where EQ-5DBaseline, EQ-5D3, EQ-5D6 and EQ-5D9 are the EQ-5D-5L scores at baseline, month 3, month 6 and month 9, respectively. If an individual died during the trial, we assumed that their utility value was 0 from the date of death to trial end and assumed a linear transition to this value from their last completed EQ-5D-5L.
Multivariate regression was used to analyse the difference in QALYs between treatment groups, controlling for baseline QoL, age, gender and site.
Health-state utility values were also obtained for carers from their responses to the EQ-5D-5L questionnaire, which was administered at the same time points and analysed using the same methods as for patients.
In addition, patients and carers completed the ICECAP-A questionnaire at each of the follow-ups. 133,136 These data were analysed in the same way as the EQ-5D-5L data, in a sensitivity analysis, to explore the effect of alternative health-related QoL questionnaires.
Measurement of resource use and costs
All health-care resource use was collected for the trial period of 9 months from randomisation using patient- and carer-completed questionnaires administered at 3, 6 and 9 months. This included use of primary and secondary care services along with voluntary and third-sector provider services used. Patient and carer out-of-pocket costs were also collected and time taken off work by patients and/or carers as a result of patient health was recorded.
Cost analysis
All use of health-care services during the trial period was converted to costs using appropriate UK unit costs estimated for the price year 2017. Unit costs were assigned to health-care resource use from the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2017147 and the Department of Health and Social Care’s National Schedule of Reference Costs. 146 All unit costs used in the analysis are presented in Table 52. The cost of the intervention was estimated as the cost of the 6-month review meeting, along with any associated follow-ups, each calculated based on the duration of the appointment, where it took place and the health-care professional seen. Patients’ use of health-care resources and total costs were calculated for the intention-to-treat population.
| Item | Location | Unit cost (£) | Source | Details |
|---|---|---|---|---|
| Community health and social services | ||||
| GP surgery | Clinic | 37.00 | PSSRU 2017147 | Per patient contact lasting 9.22 minutes |
| Home | 85.00 | PSSRU 2017147 | (Per patient contact lasting 9.22 minutes + average 12-minute travel time) × £4.00 per minute | |
| Telephone | 14.80 | GP-led triage, per call lasting 4 minutes | ||
| Nurse | Clinic | 10.85 | PSSRU 2017147 | Per 15.5-minute consultation (PSSRU 2015181), based on £42 per hour (PSSRU 2017147) |
| Home | 18.05 | Consultation + £7.20 (based on 12 minutes’ travel time) | ||
| Telephone | 7.90 | PSSRU 2017147 | Practice nurse, nurse-led triage, per call lasting 6.56 minutes | |
| Psychiatrist or psychologist | Clinic | 53.00 | PSSRU 2017147 | Clinical psychologist, band 7, per working hour |
| Home | 63.60 | Consultation + £10.60 (average 12-minute travel time) | ||
| Telephone | 21.20 | Assumeda | ||
| Physiotherapist | Clinic | 53.00 | NHS Reference Costs 2016–17146 | Physiotherapist, adult, one to one |
| Home | 63.60 | Consultation + £10.60 (average 12-minute travel time) | ||
| Telephone | 21.20 | Assumeda | ||
| Occupational therapist | Clinic | 78.00 | NHS Reference Costs 2016–17146 | Occupational therapist, adult, one to one |
| Home | 93.60 | Consultation + £15.60 (based on 12-minute travel time) | ||
| Telephone | 31.20 | Assumeda | ||
| Speech and language therapist | Clinic | 96.00 | NHS Reference Costs 2016–17146 | Speech and language therapist, adult, one to one |
| Home | 115.20 | Consultation + £19.20 (based on 12-minute travel time) | ||
| Telephone | 38.40 | Assumeda | ||
| Social worker | Clinic | 59.00 | PSSRU 2017147 | Per hour of client-related work |
| Home | 70.80 | Consultation + £11.80 (based on 12-minute travel time) | ||
| Telephone | 23.60 | Assumeda | ||
| Counsellor | Clinic | 53.00 | PSSRU 2017147 | Counsellor, band 7, per working hour |
| Home | 63.60 | Consultation + £10.60 (average 12-minute travel time) | ||
| Telephone | 21.20 | Assumeda | ||
| Home help or care worker | Clinic | 26.00 | PSSRU 2017147 | Per hour weekday |
| Home | 31.20 | Consultation + £5.20 (based on 12 minutes’ travel time) | ||
| Telephone | 10.40 | Assumeda | ||
| Day centre | Clinic | 62.00 | PSSRU 2017147 | Local authority own-provision day care for older people, per day |
| Telephone | 1.53 | PSSRU 2017147 | Assume 6.56 minutes (as per practice nurse) at £14 per client hour | |
| Family or support groupsb | Clinic | 54.00 | PSSRU 2017147 | Family support worker, per hour of client-related work |
| Home | 64.80 | Consultation + £10.80 (based on 12 minutes’ travel time) | ||
| Telephone | 21.60 | Assumeda | ||
| Hospital services | ||||
| Inpatient stay (24 hours) | 384.50 | NHS Reference Costs 2016–17146 | Elective inpatients excess bed-days | |
| Hospital day centre | 735.62 | NHS Reference Costs 2016–17146 | Day case | |
| Outpatient clinic | 135.53 | NHS Reference Costs 2016–17146 | Outpatient procedures | |
| A&E | 148.36 | NHS Reference Costs 2016–17146 | Emergency medicine | |
| Nursing/residential home | 82.00 | NHS Reference Costs 2016–17146 | Intermediate care home-based services | |
| Other health-care services (specified by participants) | ||||
| 111 service | 8.00 | www.bbc.co.uk/news/health-22370621 (accessed 9 October 2020) | ||
| 24-hour blood pressure monitor | 52.00 | NHS Reference Costs 2016–17146 | Electrocardiographic monitoring or stress testing | |
| Blood test | 13.85 | PSSRU 2017147 and NHS Reference Costs 2016–17146 | 15.5-minute consultation with practice nurse at £42 per hour, plus blood test at £3 – directly accessed pathology services – haematology | |
| Chiropractor | 78.00 | NHS Reference Costs 2016–17146 | Other therapist, adult, one to one | |
| Computerised tomography | 83.00 | NHS Reference Costs 2016–17146 | Computerised tomography scan of one area, without contrast, ≥ 19 years | |
| Cystoscopy | 232.96 | NHS Reference Costs 2016–17146 | Diagnostic flexible cystoscopy, ≥ 19 years | |
| Dentist | 85.00 | NHS Reference Costs 2016–17146 | General dental service attendance | |
| Diabetic clinic | 145.31 | NHS Reference Costs 2016–17146 | Outpatient attendance, diabetic medicine | |
| Dietitian | 85.00 | NHS Reference Costs 2016–17146 | Dietitian | |
| Disability assessment | 131.80 | NHS Reference Costs 2016–17146 | Assessment for rehabilitation, multidisciplinary, specialist | |
| Diabetes nurse | 65.00 | NHS Reference Costs 2016–17146 | Specialist nursing, diabetic nursing, adult, face to face | |
| Echocardiography | 70.36 | NHS Reference Costs 2016–17146 | Simple echocardiography, ≥ 19 years | |
| Endoscopy | 191.00 | NHS Reference Costs 2016–17146 | Wireless capsule endoscopy, ≥ 19 years | |
| External handrail | 45.00 | PSSRU 2017147 | Fit handrail – external: total average cost | |
| Eye clinic | 90.90 | NHS Reference Costs 2016–17146 | Outpatient attendance, ophthalmology | |
| Hearing clinic | 54.00 | NHS Reference Costs 2016–17146 | Audiology: audiometry or hearing assessment, ≥ 19 years | |
| Live-in care | 311.00 | PSSRU 2017147 | Social care support for people with physical disability, home care average cost per week | |
| Optician | 21.31 | Department of Health and Social Care, NHS Commissioning Board | NHS sight test fee | |
| Orthoptic stroke clinic | 64.03 | NHS Commissioning board | Outpatient attendance, orthoptics | |
| Permanent residential care | 158.00 | PSSRU 2017147 | Local authority own-provision residential care for older people, per permanent resident day | |
| Podiatrist | 41.00 | NHS Reference Costs 2016–17146 | Podiatrist, tier 1, general podiatry | |
| Phlebotomy | 71.00 | NHS Reference Costs 2016–17146 | Phlebotomy (£3) + specialist nurse, adult, face to face (£68) | |
| Rehabilitation – respiratory | 322.98 | NHS Reference Costs 2016–17146 | Rehabilitation for respiratory disorders | |
| Rehabilitation – stroke | 88.00 | NHS Reference Costs 2016–17146 | Stroke community rehabilitation teams | |
| Specialist nurse | 68.00 | NHS Reference Costs 2016–17146 | Community health services, other specialist nursing, adult, face to face | |
| Stoma clinic | 51.00 | NHS Reference Costs 2016–17146 | Specialist nursing, stoma care services, adult, face to face | |
| Stroke-related shoulder procedure | 171.00 | NHS Reference Costs 2016–17146 | Stroke medicine – minimal shoulder procedures | |
| Stroke specialist | 170.00 | NHS Reference Costs 2016–17146 | Stroke medicine – consultant-led face-to-face attendance | |
| Vascular department | 156.05 | NHS Reference Costs 2016–17146 | Outpatient attendance, vascular surgery | |
| Wheelchair assessment | 127.00 | NHS Reference Costs 2016–17146 | Wheelchair services adults: assessment low need | |
| Wheelchair repairs | 32.00 | PSSRU 2017147 | Wheelchair maintenance | |
| Wheelchair review | 156.00 | NHS Reference Costs 2016–17146 | Wheelchair services, adult: review, all needs | |
Total costs for each patient were calculated as the sum of costs assigned from hospital, community health and social services, and the intervention cost, along with out-of-pocket costs incurred by patients and their informal carers. Time taken off work by patients and carers was included in the societal perspective analysis using a human capital approach and a median hourly pay for UK adults of £11.31. 182 Multivariate regression was used to analyse the difference in costs between treatment groups, controlling for age, gender (as observed demographic variables that may affect outcomes) and site (as this is a cluster randomised trial).
Adjusting for baseline imbalance
As patients’ baseline utility is likely to be correlated with their utility over the follow-up period, any imbalance in baseline utilities must be accounted for when calculating differential effects between treatment groups. 183,184 Multiple regression analysis was used to estimate differential mean QALYs and to predict adjusted QALYs controlling for utility at baseline.
Missing data
Based on descriptive analysis of the missing data, the analysis was conducted under the assumption that the missing data were missing at random. 185 Consequently, when data were missing for QoL or cost follow-up, multiple imputation methods were used to generate estimates of missing values based on the distribution of observed data, as per recommended best practices for economic evaluation alongside clinical trials. 148
When choosing the level at which to impute missing data (more or less aggregated), a balance needs to be struck between maintaining the data structure and achieving a stable imputation model. 185 Consequently, for QoL data, missing EQ-5D-5L index values were imputed at each follow-up. For costs, missing data were imputed for each follow-up at the level of total community health and social care costs and total hospital costs, not at the unit of resource level. Missing baseline EQ-5D-5L values were imputed using mean imputation to ensure that imputed values were independent of treatment allocation. 186 EQ-5D-5L index values were recorded as missing if any EQ-5D-5L items were missing for a given time point. Costs were counted as missing if all resource items on the case report form were missing.
The imputation was performed in Stata® version 15 (StataCorp LP, College Station, TX, USA) using predictive mean matching to perform multiple imputation by chained equations to predict missing values based on baseline variables (age, sex, QoL and site) and observed outcomes. Predictive mean matching ensures that only plausible values of the missing variable are imputed, as the imputed value is drawn from another individual whose predicted value is close to the predicted value of the individual with the missing observation. 185
Cost-effectiveness analysis
Primary analysis
The cost-effectiveness analysis adopted an intention-to-treat perspective for analysing and summarising the health economic trial data. The primary analysis consisted of a cost–utility analysis over the 9-month trial period and included adjustment for baseline variables and imputation of missing data. The incremental cost per QALY gained from the New Start intervention, compared with usual care, was calculated, producing an ICER149 as follows:
The ICER was calculated using the two-stage method, as recommended for cluster randomised trials with fewer than 20 clusters. 187 Therefore, patient-level costs and QALYs were used to estimate average costs and QALYs for each care home, and care home-level average costs and QALYs were used in the ICER calculation above.
The NICE consider a cost per QALY within the range of £20,000–30,000 to be acceptable. 144 Therefore, the lower limit of this threshold (£20,000) was used to determine cost-effectiveness.
Sensitivity analyses
Sensitivity analyses were conducted to explore the impact of assumptions made in the primary analysis and alternative perspectives for analysis. ICERs from each of the sensitivity analyses were compared with the main trial results to identify areas of uncertainty.
The effect of adjusting for baseline imbalance on cost-effectiveness was explored in an analysis with no adjustment for baseline differences between groups. In addition, the effect of not imputing missing data was considered in an analysis including only complete cases. Analysis using ICECAP-A133,136 to measure health-related QoL (rather than the EQ-5D-5L) was explored, along with an analysis to explore the effect of combining patient and carer QALYs. As the primary analysis was conducted from the societal perspective, additional analyses explored the impact on the result if the analysis was conducted from a health and social care provider perspective. In November 2018, NICE updated their position statement with regards the use of the EQ-5D-5L in reference case analyses, and recommended using a mapping function to map EQ-5D-5L responses to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), value set. 188 Consequently, we conducted an additional sensitivity analysis using EQ-5D-3L values obtained from the van Hout et al. 189 cross-walk. As outlined in the protocol, we had intended to map WHODAS 2.0 scores to the EQ-5D-5L scores. However, given the challenges with the EQ-5D-5L values and changes in recommendations around their use, this was not undertaken, as any results from such mapping would not be robust.
Uncertainty analysis
The level of sampling uncertainty around the ICER was determined using a non-parametric bootstrap to generate 10,000 estimates of incremental costs and benefits. The bootstrapped estimates were plotted on the cost-effectiveness plane to illustrate the uncertainty surrounding the cost-effectiveness estimates. 190 A CEAC illustrating the probability that the New Start intervention is cost-effective at a range of threshold values (£0–100,000) was also constructed using the bootstrapped samples. 191
Results
Sample
Of the 269 participants recruited to the trial, 169 had complete resource use and EQ-5D-5L results for all follow-ups.
Resource use and costs
Table 53 shows the average resource use of participants in each trial arm over the 9-month duration of the trial.
| Item | Location | Baseline | 3 months | 6 months | 9 months | ||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | Usual care | New Start | Usual care | New Start | ||
| Community health and social services | |||||||||
| GP consultation | Clinic | 1.805 (2.78), n = 118 | 1.633 (1.758), n = 139 | 1.486 (1.749), n = 105 | 1.496 (1.855), n = 129 | 1.323 (1.762), n = 96 | 1.413 (1.711), n = 121 | 1.471 (1.784), n = 87 | 1.298 (1.585), n = 114 |
| Home | 0.22 (0.668), n = 118 | 0.108 (0.445), n = 139 | 0.343 (1.48), n = 105 | 0.132 (0.63), n = 129 | 0.292 (1.353), n = 96 | 0.099 (0.455), n = 121 | 0.126 (0.567), n = 87 | 0.053 (0.417), n = 114 | |
| Telephone | 0.508 (1.182), n = 118 | 0.633 (1.352), n = 139 | 0.971 (2.471), n = 105 | 0.574 (1.753), n = 129 | 0.479 (1.005), n = 96 | 0.471 (1.342), n = 121 | 0.54 (1.139), n = 87 | 0.5 (1.471), n = 114 | |
| Nurse consultation | Clinic | 0.716 (1.508), n = 116 | 0.791 (1.788), n = 129 | 0.969 (1.83), n = 98 | 0.748 (1.596), n = 119 | 0.663 (0.842), n = 92 | 0.741 (1.002), n = 112 | 0.667 (1.468), n = 87 | 0.845 (1.621), n = 110 |
| Home | 0.336 (1.164), n = 116 | 1.822 (16.159), n = 129 | 2.398 (18.381), n = 98 | 1.748 (16.53), n = 119 | 0.25 (2.197), n = 92 | 0.295 (1.393), n = 112 | 0.08 (0.651), n = 87 | 1.718 (17.158), n = 110 | |
| Telephone | 0.129 (0.666), n = 116 | 0.101 (0.392), n = 129 | 0.133 (0.62), n = 98 | 0.126 (0.53), n = 119 | 0.13 (0.474), n = 92 | 0.08 (0.448), n = 112 | 0.011 (0.107), n = 87 | 0.064 (0.339), n = 110 | |
| Psychologist consultation | Clinic | 0.134 (1.151), n = 112 | 0.055 (0.477), n = 127 | 0 (0), n = 99 | 0.034 (0.224), n = 118 | 0.011 (0.104), n = 92 | 0.164 (0.873), n = 110 | 0 (0), n = 85 | 0.066 (0.442), n = 106 |
| Home | 0.098 (0.949), n = 112 | 0.031 (0.355), n = 127 | 0 (0), n = 99 | 0.025 (0.158), n = 118 | 0.076 (0.73), n = 92 | 0 (0), n = 110 | 0 (0), n = 85 | 0 (0), n = 106 | |
| Telephone | 0.116 (1.228), n = 112 | 0 (0), n = 127 | 0 (0), n = 99 | 0.017 (0.184), n = 118 | 0 (0), n = 92 | 0.018 (0.191), n = 110 | 0 (0), n = 85 | 0 (0), n = 106 | |
| Physiotherapist consultation | Clinic | 0.754 (4.351), n = 114 | 0.515 (2.734), n = 130 | 0.257 (0.934), n = 101 | 0.639 (2.037), n = 119 | 0.174 (0.689), n = 92 | 0.851 (3.617), n = 114 | 0.253 (0.922), n = 83 | 0.541 (2.662), n = 109 |
| Home | 1.237 (3.764), n = 114 | 1.223 (5.109), n = 130 | 0.861 (3.544), n = 101 | 0.454 (2.881), n = 119 | 1 (9.383), n = 92 | 0.544 (3.592), n = 114 | 0 (0), n = 83 | 0.165 (1.221), n = 109 | |
| Telephone | 0.246 (1.659), n = 114 | 0.285 (1.744), n = 130 | 0.02 (0.14), n = 101 | 0.025 (0.157), n = 119 | 0.043 (0.417), n = 92 | 0.026 (0.209), n = 114 | 0.024 (0.22), n = 83 | 0 (0), n = 109 | |
| Occupational therapist consultation | Clinic | 0.5 (3.763), n = 114 | 0.25 (1.386), n = 128 | 0.196 (1.081), n = 102 | 0.119 (0.396), n = 118 | 0.187 (1.382), n = 91 | 0.07 (0.413), n = 115 | 0.048 (0.265), n = 84 | 0.16 (1.18), n = 106 |
| Home | 0.439 (1.47), n = 114 | 0.625 (3.219), n = 128 | 0.245 (1.41), n = 102 | 0.076 (0.572), n = 118 | 0 (0), n = 91 | 0.096 (0.546), n = 115 | 0.024 (0.218), n = 84 | 0.019 (0.137), n = 106 | |
| Telephone | 0.184 (1.301), n = 114 | 0.258 (1.713), n = 128 | 0.088 (0.565), n = 102 | 0.034 (0.29), n = 118 | 0.044 (0.419), n = 91 | 0.052 (0.346), n = 115 | 0 (0), n = 84 | 0.038 (0.273), n = 106 | |
| Speech and language therapist consultation | Clinic | 0.142 (0.789), n = 113 | 0.117 (0.542), n = 128 | 0.039 (0.195), n = 102 | 0.078 (0.42), n = 116 | 0.022 (0.149), n = 89 | 0.078 (0.58), n = 115 | 0.012 (0.11), n = 83 | 0.028 (0.215), n = 107 |
| Home | 0.354 (2.838), n = 113 | 0.516 (3.423), n = 128 | 0.098 (0.536), n = 102 | 0.207 (1.867), n = 116 | 0.067 (0.636), n = 89 | 0.035 (0.263), n = 115 | 0.072 (0.659), n = 83 | 0 (0), n = 107 | |
| Telephone | 0.018 (0.188), n = 113 | 0.102 (1.064), n = 128 | 0.01 (0.099), n = 102 | 0 (0), n = 116 | 0.045 (0.424), n = 89 | 0.026 (0.28), n = 115 | 0 (0), n = 83 | 0.009 (0.097), n = 107 | |
| Social worker appointment | Clinic | 0.045 (0.249), n = 110 | 0.031 (0.173), n = 131 | 0.02 (0.141), n = 100 | 0.009 (0.093), n = 116 | 0 (0), n = 90 | 0.043 (0.244), n = 115 | 0 (0), n = 84 | 0 (0), n = 105 |
| Home | 0.136 (1.153), n = 110 | 0.031 (0.213), n = 131 | 2.71 (26.999), n = 100 | 0.034 (0.371), n = 116 | 0 (0), n = 90 | 0.017 (0.187), n = 115 | 0 (0), n = 84 | 0.057 (0.362), n = 105 | |
| Telephone | 0.045 (0.314), n = 110 | 0.046 (0.325), n = 131 | 0.02 (0.2), n = 100 | 0.017 (0.186), n = 116 | 0 (0), n = 90 | 0.026 (0.208), n = 115 | 0 (0), n = 84 | 0.067 (0.422), n = 105 | |
| Counsellor appointment | Clinic | 0.009 (0.095), n = 111 | 0 (0), n = 130 | 0.01 (0.1), n = 100 | 0.017 (0.186), n = 116 | 0.044 (0.332), n = 90 | 0.018 (0.19), n = 111 | 0.024 (0.218), n = 84 | 0 (0), n = 103 |
| Home | 0.018 (0.19), n = 111 | 0 (0), n = 130 | 0.01 (0.1), n = 100 | 0.009 (0.093), n = 116 | 0.011 (0.105), n = 90 | 0 (0), n = 111 | 0.012 (0.109), n = 84 | 0 (0), n = 103 | |
| Telephone | 0 (0), n = 111 | 0 (0), n = 130 | 0.05 (0.5), n = 100 | 0 (0), n = 116 | 0 (0), n = 90 | 0 (0), n = 111 | 0.048 (0.436), n = 84 | 0.029 (0.296), n = 103 | |
| Home help/care worker appointment | Clinic | 0.982 (9.592), n = 113 | 0.061 (0.24), n = 131 | 0.061 (0.241), n = 98 | 3.033 (32.861), n = 120 | 0.076 (0.267), n = 92 | 0.079 (0.271), n = 114 | 0.643 (4.728), n = 84 | 3.897 (35.112), n = 107 |
| Home | 9.336 (46.943), n = 113 | 10.603 (51.923), n = 131 | 16.204 (67.525), n = 98 | 16.992 (66.479), n = 120 | 11.87 (56.488), n = 92 | 3.596 (33.975), n = 114 | 7.786 (48.84), n = 84 | 10.28 (46.531), n = 107 | |
| Telephone | 0.062 (0.571), n = 113 | 0.397 (3.766), n = 131 | 0.408 (4.041), n = 98 | 0 (0), n = 120 | 0 (0), n = 92 | 0 (0), n = 114 | 0.071 (0.655), n = 84 | 0 (0), n = 107 | |
| Day care | Clinic | 0.063 (0.576), n = 111 | 0.008 (0.088), n = 129 | 0.052 (0.418), n = 97 | 0 (0), n = 116 | 0.273 (1.727), n = 88 | 0.138 (1.166), n = 109 | 0.265 (1.704), n = 83 | 0.01 (0.099), n = 103 |
| Home | 0 (0), n = 111 | 0 (0), n = 129 | 0 (0), n = 97 | 0 (0), n = 116 | 0 (0), n = 88 | 0 (0), n = 109 | 0.012 (0.11), n = 83 | 0 (0), n = 103 | |
| Telephone | 0 (0), n = 111 | 0 (0), n = 129 | 0 (0), n = 97 | 0 (0), n = 116 | 0 (0), n = 88 | 0 (0), n = 109 | 0 (0), n = 83 | 0 (0), n = 103 | |
| Family/support groups | Clinic | 0.045 (0.209), n = 110 | 0.063 (0.349), n = 128 | 0.061 (0.241), n = 98 | 0.043 (0.205), n = 115 | 0.023 (0.15), n = 88 | 0.045 (0.314), n = 110 | 0.427 (3.315), n = 82 | 0.049 (0.216), n = 103 |
| Home | 0.364 (3.814), n = 110 | 0.305 (2.448), n = 128 | 0 (0), n = 98 | 0.052 (0.56), n = 115 | 0.273 (2.558), n = 88 | 0 (0), n = 110 | 0.146 (1.325), n = 82 | 0.019 (0.197), n = 103 | |
| Telephone | 0 (0), n = 110 | 0.336 (2.821), n = 128 | 0 (0), n = 98 | 0 (0), n = 115 | 0 (0), n = 88 | 0 (0), n = 110 | 0 (0), n = 82 | 0.058 (0.591), n = 103 | |
| Hospital services | |||||||||
| Inpatient days | 1.963 (6.402), n = 107 | 0.969 (5.982), n = 129 | 0.752 (2.677), n = 105 | 0.872 (4.029), n = 117 | 0.705 (3.55), n = 88 | 1.2 (6.856), n = 115 | 0.256 (0.927), n = 82 | 0.505 (2.152), n = 107 | |
| Day centre | 0.09 (0.404), n = 100 | 0.192 (1.41), n = 120 | 0.112 (0.403), n = 98 | 0.112 (0.705), n = 107 | 0.071 (0.259), n = 84 | 0.071 (0.29), n = 113 | 0.075 (0.382), n = 80 | 0.142 (0.999), n = 106 | |
| Outpatient visit | 2.31 (3.85), n = 116 | 1.538 (3.053), n = 132 | 1.305 (1.693), n = 105 | 1.252 (1.827), n = 123 | 1.376 (2.064), n = 93 | 1.788 (3.836), n = 118 | 1.465 (2.227), n = 86 | 1.381 (2.778), n = 113 | |
| A&E visit | 0.238 (0.581), n = 105 | 0.165 (0.614), n = 127 | 0.126 (0.388), n = 103 | 0.209 (0.468), n = 115 | 0.118 (0.448), n = 85 | 0.177 (0.804), n = 113 | 0.21 (0.586), n = 81 | 0.139 (0.502), n = 108 | |
| Residential care | 0 (0), n = 100 | 0.46 (3.132), n = 124 | 0.909 (9.045), n = 99 | 0 (0), n = 108 | 0.326 (2.122), n = 86 | 0.297 (2.23), n = 111 | 0.177 (1.575), n = 79 | 0.132 (1.36), n = 106 | |
Average health-care costs over the trial period are presented in Table 54. A range of out-of-pocket costs were recorded, including cost of private health care, travel for appointments (e.g. mileage, parking), additional household expenses as a result of patients’ health (e.g. additional cleaning/laundry products, paid help for household tasks) and home modification (e.g. adding rails internally/externally). Multiple regression analysis indicated that the difference in costs between groups was not statistically significant (p > 0.05, 95% CI –899.646 to 3211.452).
| Total costs | Usual care | New Start | ||
|---|---|---|---|---|
| Mean (SD) | Minimum, maximum | Mean (SD) | Minimum, maximum | |
| Community health and social services | 1659.81 (4779.07), n = 115 | 0, 33,773.5 | 1484.2 (4131.88), n = 138 | 0, 34,320 |
| Hospital services | 1299.37 (2206.49), n = 115 | 0, 12,554.05 | 1633.36 (3846.38), n = 136 | 0, 29,359.36 |
| Other NHS/social care services | 130.25 (255.96), n = 32 | 0, 1056 | 281.79 (1203.18), n = 44 | 0, 7992.70 |
| Intervention cost | 19.85 (40.69), n = 124 | 0, 418.75 | 67.8 (185.22), n = 145 | 0, 2152.62 |
| Total cost (health-care provider) | 2988.44 (5556.58), n = 116 | 0, 35,818.61 | 3251.09 (5816.41), n = 138 | 2.83, 34,591.06 |
| Patient out-of-pocket costs | 682.5 (3024.06), n = 124 | 0, 28,037.02 | 775.14 (2740.78), n = 145 | 0, 28,368.30 |
| Patient time off worka | 214.48 (178.24), n = 123 | 0, 508.95 | 173.16 (189.11), n = 145 | 0, 508.95 |
| Carer out-of-pocket costs | 80.27 (177.56), n = 39 | 0, 915.25 | 318.3 (566.18), n = 46 | 0, 2742.81 |
| Carer time off worka | 280.57 (962.08), n = 39 | 0, 5768.1 | 562.43 (2378.76), n = 46 | 0, 15,438.15 |
| Total cost (societal) | 4066.75 (7612.23), n = 116 | 0, 54,621.36 | 4541.06 (6763.32), n = 138 | 2.83, 37,846.06 |
Quality of life
The mean (SD) patient and carer EQ-5D-5L and ICECAP-A scores for each trial arm at each follow-up are presented in Tables 55 and 56, respectively. There is little difference in EQ-5D-5L scores over the trial period in either arm, and multiple regression analysis indicated that were was no significant difference in total QALYs gained between groups (p > 0.05, 95% CI –0.043 to 0.014). In each arm, the EQ-5D-5L scores of patients appear to peak at the 3-month follow-up, followed by subsequent decline in the following months. The change from baseline to 3 months is larger in the New Start arm, but the subsequent decline is also larger for New Start patients.
| Time point | Usual care, mean (SD) | New Start, mean (SD) | ||
|---|---|---|---|---|
| EQ-5D-5L score | Change from baseline | EQ-5D-5L score | Change from baseline | |
| Patient EQ-5D-5L score | ||||
| Baseline | 0.666 (0.274), n = 120 | 0.655 (0.26), n = 142 | ||
| 3 months | 0.706 (0.254), n = 110 | 0.029 (0.157), n = 107 | 0.697 (0.246), n = 134 | 0.033 (0.171), n = 132 |
| 6 months | 0.682 (0.28), n = 99 | 0.016 (0.178), n = 96 | 0.68 (0.266), n = 125 | –0.005 (0.201), n = 122 |
| 9 months | 0.698 (0.27), n = 92 | 0.011 (0.202), n = 90 | 0.667 (0.275), n = 123 | –0.009 (0.214), n = 121 |
| Carer EQ-5D-5L score | ||||
| Baseline | 0.808 (0.254), n = 37 | 0.803 (0.219), n = 45 | ||
| 3 months | 0.847 (0.128), n = 18 | 0.016 (0.137), n = 16 | 0.829 (0.166), n = 31 | 0.028 (0.087), n = 30 |
| 6 months | 0.875 (0.135), n = 27 | 0.024 (0.133), n = 25 | 0.841 (0.137), n = 33 | 0.002 (0.109), n = 33 |
| 9 months | 0.877 (0.133), n = 24 | –0.013 (0.077), n = 22 | 0.78 (0.228), n = 32 | –0.013 (0.148), n = 32 |
| Time point | Usual care, mean (SD) | New Start, mean (SD) | ||
|---|---|---|---|---|
| ICECAP-A score | Change from baseline | ICECAP-A score | Change from baseline | |
| Patient ICECAP-A score | ||||
| Baseline | 0.696 (0.309), n = 119 | 0.741 (0.258), n = 143 | ||
| 3 months | 0.771 (0.255), n = 107 | 0.054 (0.275), n = 103 | 0.797 (0.243), n = 128 | 0.049 (0.265), n = 127 |
| 6 months | 0.72 (0.278), n = 96 | 0.018 (0.302), n = 92 | 0.716 (0.271), n = 122 | –0.027 (0.273), n = 121 |
| 9 months | 0.72 (0.28), n = 93 | 0.008 (0.327), n = 91 | 0.746 (0.268), n = 118 | –0.002 (0.283), n = 117 |
| Carer ICECAP-A score | ||||
| Baseline | 0.714 (0.296), n = 39 | 0.759 (0.258), n = 46 | ||
| 3 months | 0.653 (0.302), n = 17 | –0.009 (0.3), n = 17 | 0.75 (0.252), n = 31 | 0.006 (0.251), n = 31 |
| 6 months | 0.735 (0.327), n = 26 | –0.005 (0.229), n = 26 | 0.715 (0.257), n = 35 | –0.034 (0.292), n = 35 |
| 9 months | 0.765 (0.332), n = 24 | 0.017 (0.324), n = 24 | 0.696 (0.337), n = 32 | –0.047 (0.176), n = 32 |
Missing data
Complete and missing resource use and EQ-5D-5L data are presented in Tables 57 and 58, respectively. A total of 180 patients completed resource use questionnaires for all follow-ups. A total of 195 patients had complete EQ-5D-5L scores for all follow-ups. At the start of the trial, completion of resource use and EQ-5D-5L was good, but this tailed off over the trial.
| Item | Location | Baseline | 3 months | 6 months | 9 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | Usual care | New Start | Usual care | New Start | ||||||||||
| n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | ||
| Community health and social services | |||||||||||||||||
| GP | Clinic | 118 (6) | 95 | 139 (6) | 96 | 105 (19) | 85 | 129 (16) | 89 | 96 (28) | 77 | 121 (24) | 83 | 87 (37) | 70 | 114 (31) | 79 |
| Home | 118 (6) | 95 | 139 (6) | 96 | 105 (19) | 85 | 129 (16) | 89 | 96 (28) | 77 | 121 (24) | 83 | 87 (37) | 70 | 114 (31) | 79 | |
| Telephone | 118 (6) | 95 | 139 (6) | 96 | 105 (19) | 85 | 129 (16) | 89 | 96 (28) | 77 | 121 (24) | 83 | 87 (37) | 70 | 114 (31) | 79 | |
| Nurse | Clinic | 116 (8) | 94 | 129 (16) | 89 | 98 (26) | 79 | 119 (26) | 82 | 92 (32) | 74 | 112 (33) | 77 | 87 (37) | 70 | 110 (35) | 76 |
| Home | 116 (8) | 94 | 129 (16) | 89 | 98 (26) | 79 | 119 (26) | 82 | 92 (32) | 74 | 112 (33) | 77 | 87 (37) | 70 | 110 (35) | 76 | |
| Telephone | 116 (8) | 94 | 129 (16) | 89 | 98 (26) | 79 | 119 (26) | 82 | 92 (32) | 74 | 112 (33) | 77 | 87 (37) | 70 | 110 (35) | 76 | |
| Psychologist | Clinic | 112 (12) | 90 | 127 (18) | 88 | 99 (25) | 80 | 118 (27) | 81 | 92 (32) | 74 | 110 (35) | 76 | 85 (39) | 69 | 106 (39) | 73 |
| Home | 112 (12) | 90 | 127 (18) | 88 | 99 (25) | 80 | 118 (27) | 81 | 92 (32) | 74 | 110 (35) | 76 | 85 (39) | 69 | 106 (39) | 73 | |
| Telephone | 112 (12) | 90 | 127 (18) | 88 | 99 (25) | 80 | 118 (27) | 81 | 92 (32) | 74 | 110 (35) | 76 | 85 (39) | 69 | 106 (39) | 73 | |
| Physiotherapist | Clinic | 114 (10) | 92 | 130 (15) | 90 | 101 (23) | 81 | 119 (26) | 82 | 92 (32) | 74 | 114 (31) | 79 | 83 (41) | 67 | 109 (36) | 75 |
| Home | 114 (10) | 92 | 130 (15) | 90 | 101 (23) | 81 | 119 (26) | 82 | 92 (32) | 74 | 114 (31) | 79 | 83 (41) | 67 | 109 (36) | 75 | |
| Telephone | 114 (10) | 92 | 130 (15) | 90 | 101 (23) | 81 | 119 (26) | 82 | 92 (32) | 74 | 114 (31) | 79 | 83 (41) | 67 | 109 (36) | 75 | |
| Occupational therapist | Clinic | 114 (10) | 92 | 128 (17) | 88 | 102 (22) | 82 | 118 (27) | 81 | 91 (33) | 73 | 115 (30) | 79 | 84 (40) | 68 | 106 (39) | 73 |
| Home | 114 (10) | 92 | 128 (17) | 88 | 102 (22) | 82 | 118 (27) | 81 | 91 (33) | 73 | 115 (30) | 79 | 84 (40) | 68 | 106 (39) | 73 | |
| Telephone | 114 (10) | 92 | 128 (17) | 88 | 102 (22) | 82 | 118 (27) | 81 | 91 (33) | 73 | 115 (30) | 79 | 84 (40) | 68 | 106 (39) | 73 | |
| Speech and language therapist | Clinic | 113 (11) | 91 | 128 (17) | 88 | 102 (22) | 82 | 116 (29) | 80 | 89 (35) | 72 | 115 (30) | 79 | 83 (41) | 67 | 107 (38) | 74 |
| Home | 113 (11) | 91 | 128 (17) | 88 | 102 (22) | 82 | 116 (29) | 80 | 89 (35) | 72 | 115 (30) | 79 | 83 (41) | 67 | 107 (38) | 74 | |
| Telephone | 113 (11) | 91 | 128 (17) | 88 | 102 (22) | 82 | 116 (29) | 80 | 89 (35) | 72 | 115 (30) | 79 | 83 (41) | 67 | 107 (38) | 74 | |
| Social worker | Clinic | 110 (14) | 89 | 131 (14) | 90 | 100 (24) | 81 | 116 (29) | 80 | 90 (34) | 73 | 115 (30) | 79 | 84 (40) | 68 | 105 (40) | 72 |
| Home | 110 (14) | 89 | 131 (14) | 90 | 100 (24) | 81 | 116 (29) | 80 | 90 (34) | 73 | 115 (30) | 79 | 84 (40) | 68 | 105 (40) | 72 | |
| Telephone | 110 (14) | 89 | 131 (14) | 90 | 100 (24) | 81 | 116 (29) | 80 | 90 (34) | 73 | 115 (30) | 79 | 84 (40) | 68 | 105 (40) | 72 | |
| Counsellor | Clinic | 111 (13) | 90 | 130 (15) | 90 | 100 (24) | 81 | 116 (29) | 80 | 90 (34) | 73 | 111 (34) | 77 | 84 (40) | 68 | 103 (42) | 71 |
| Home | 111 (13) | 90 | 130 (15) | 90 | 100 (24) | 81 | 116 (29) | 80 | 90 (34) | 73 | 111 (34) | 77 | 84 (40) | 68 | 103 (42) | 71 | |
| Telephone | 111 (13) | 90 | 130 (15) | 90 | 100 (24) | 81 | 116 (29) | 80 | 90 (34) | 73 | 111 (34) | 77 | 84 (40) | 68 | 103 (42) | 71 | |
| Home help/care worker | Clinic | 113 (11) | 91 | 131 (14) | 90 | 98 (26) | 79 | 120 (25) | 83 | 92 (32) | 74 | 114 (31) | 79 | 84 (40) | 68 | 107 (38) | 74 |
| Home | 113 (11) | 91 | 131 (14) | 90 | 98 (26) | 79 | 120 (25) | 83 | 92 (32) | 74 | 114 (31) | 79 | 84 (40) | 68 | 107 (38) | 74 | |
| Telephone | 113 (11) | 91 | 131 (14) | 90 | 98 (26) | 79 | 120 (25) | 83 | 92 (32) | 74 | 114 (31) | 79 | 84 (40) | 68 | 107 (38) | 74 | |
| Day care | Clinic | 111 (13) | 90 | 129 (16) | 89 | 97 (27) | 78 | 116 (29) | 80 | 88 (36) | 71 | 109 (36) | 75 | 83 (41) | 67 | 103 (42) | 71 |
| Home | 111 (13) | 90 | 129 (16) | 89 | 97 (27) | 78 | 116 (29) | 80 | 88 (36) | 71 | 109 (36) | 75 | 83 (41) | 67 | 103 (42) | 71 | |
| Telephone | 111 (13) | 90 | 129 (16) | 89 | 97 (27) | 78 | 116 (29) | 80 | 88 (36) | 71 | 109 (36) | 75 | 83 (41) | 67 | 103 (42) | 71 | |
| Family/support groups | Clinic | 110 (14) | 89 | 128 (17) | 88 | 98 (26) | 79 | 115 (30) | 79 | 88 (36) | 71 | 110 (35) | 76 | 82 (42) | 66 | 103 (42) | 71 |
| Home | 110 (14) | 89 | 128 (17) | 88 | 98 (26) | 79 | 115 (30) | 79 | 88 (36) | 71 | 110 (35) | 76 | 82 (42) | 66 | 103 (42) | 71 | |
| Telephone | 110 (14) | 89 | 128 (17) | 88 | 98 (26) | 79 | 115 (30) | 79 | 88 (36) | 71 | 110 (35) | 76 | 82 (42) | 66 | 103 (42) | 71 | |
| Hospital services | |||||||||||||||||
| Inpatient days | 107 (17) | 86 | 129 (16) | 89 | 105 (19) | 85 | 117 (28) | 81 | 88 (36) | 71 | 115 (30) | 79 | 82 (42) | 66 | 107 (38) | 74 | |
| Day centre | 100 (24) | 81 | 120 (25) | 83 | 98 (26) | 79 | 107 (38) | 74 | 84 (40) | 68 | 113 (32) | 78 | 80 (44) | 65 | 106 (39) | 73 | |
| Outpatient | 116 (8) | 94 | 132 (13) | 91 | 105 (19) | 85 | 123 (22) | 85 | 93 (31) | 75 | 118 (27) | 81 | 86 (38) | 69 | 113 (32) | 78 | |
| A&E | 105 (19) | 85 | 127 (18) | 88 | 103 (21) | 83 | 115 (30) | 79 | 85 (39) | 69 | 113 (32) | 78 | 81 (43) | 65 | 108 (37) | 74 | |
| Residential care | 100 (24) | 81 | 124 (21) | 86 | 99 (25) | 80 | 108 (37) | 74 | 86 (38) | 69 | 111 (34) | 77 | 79 (45) | 64 | 106 (39) | 73 | |
| Time point | Patient EQ-5D-5L | Carer EQ-5D-5L | ||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | New Start | Usual care | New Start | |||||
| n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | n valid (n missing) | % complete | |
| Baseline | 120 (4) | 97 | 142 (3) | 98 | 37 (2) | 95 | 45 (1) | 98 |
| 3 months | 110 (14) | 89 | 134 (11) | 92 | 18 (21) | 46 | 31 (15) | 67 |
| 6 months | 99 (25) | 80 | 125 (20) | 86 | 27 (12) | 69 | 33 (13) | 72 |
| 9 months | 92 (32) | 74 | 123 (22) | 85 | 24 (15) | 62 | 32 (14) | 70 |
Eighty-five carers were recruited to the trial, representing 87% of the patients who were known to have a carer. There were 132 patients without carers and a further 39 patients for whom it was not known whether or not they had a carer. Of the 85 carers recruited to the trial, 35 had complete EQ-5D-5L scores for all follow-ups. This represents 41% of the consented carers, and just 36% of the known carers.
Cost-effectiveness results
Cost-effectiveness results are presented in Table 6. The usual care group had the highest QALY gain over the trial period, whereas the mean total cost was lowest for the New Start group. The small difference in QALYs relative to the cost saving indicates that the New Start intervention may be a cost-effective use of resources (if the money saved is expected to generate more health elsewhere in the system). However, these results should be viewed with caution as this is an exploratory analysis of feasibility data only.
Uncertainty analysis
Bootstrapped estimates of the incremental costs and incremental effects are plotted on the cost-effectiveness plane in Figure 17. This shows the joint distribution of the incremental costs and effects for New Start, compared with usual care. The majority of points lie in the two west quadrants, indicating that New Start is unlikely to increase QALYs gained. The spread of points in the north and south quadrants demonstrates the uncertainty around the impact on costs of the New Start intervention.
FIGURE 17.
Cost-effectiveness plane: New Start vs. usual care. CET, cost-effectiveness threshold.
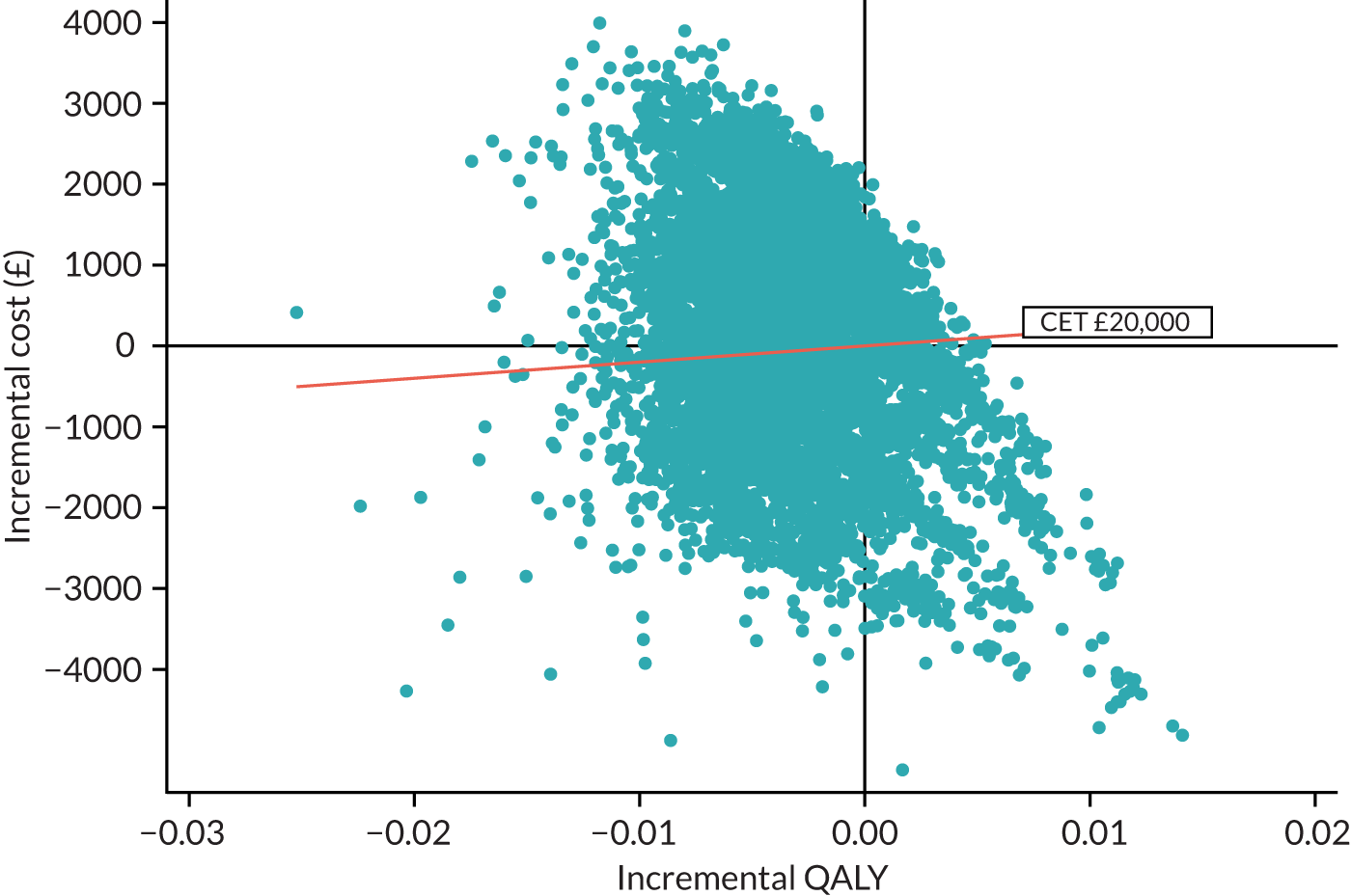
Exploratory analysis of the probability that the New Start intervention is cost-effective is presented on the CEAC shown in Figure 18. Based on data collected during the feasibility trial, at a cost-effectiveness threshold of £20,000 per QALY, the New Start intervention has a 48% probability of being cost-effective. As usual care has been shown to produce more QALYs, the probability that the New Start intervention is cost-effective decreases as the cost-effectiveness threshold increases.
FIGURE 18.
The CEAC: New Start vs. usual care.

Sensitivity analyses
The cost-effectiveness results for each scenario explored in the sensitivity analyses are presented in Table 59. The results of the primary analysis are not robust to the sensitivity analyses conducted and a great deal of variation in the cost-effectiveness estimates is observed for each scenario explored. Owing to the large numbers of missing data for carers (only 35 carers had complete data), the sample size was not sufficient to undertake robust sensitivity analyses including carers’ data.
| Treatment allocation | Cost (£), mean (SD) | Incremental cost (£) | QALY, mean (SD) | Incremental QALY | ICER (£ per QALY) |
|---|---|---|---|---|---|
| Intention to treat: unadjusted | |||||
| Usual care | 4846.89 (3335.88) | 0.496 (0.063) | |||
| New Start | 4056.86 (2038.72) | –790.02 | 0.484 (0.132) | –0.012 | 65,835 |
| Complete case | |||||
| Usual care | 4445.87 (4218.97) | 0.547 (0.006) | Usual care dominates | ||
| New Start | 6288.99 (3003.43) | 1843.12 | 0.542 (0.004) | –0.005 | |
| Alternative measures of health-related QoL: ICECAP-A | |||||
| Usual care | 4846.89 (3335.88) | 0.539 (0.003) | New Start dominates | ||
| New Start | 4056.86 (2038.72) | –790.02 | 0.555 (0.004) | 0.02 | |
| Health-care provider perspective | |||||
| Usual care | 3608.59 (2351.40) | 0.504 (0.011) | |||
| New Start | 3088.31 (1767.74) | –520.28 | 0.502 (0.015) | –0.002 | 260,140 |
| Map EQ-5D-5L to EQ-5D-3L | |||||
| Usual care | 4849.46 (3330.24) | 0 | 0.449 (0.013) | ||
| New Start | 4084.02 (2012.42) | –765.44 | 0.441 (0.017) | –0.008 | 95,680 |
Health economic model
A cohort Markov decision model was developed to analyse future costs and benefits of New Start compared with usual care beyond the trial time horizon and to identify areas of greatest uncertainty to inform future research.
The outcome measure for the model was the QALY. The analysis was conducted from a societal perspective to analyse the costs and benefits of New Start compared with usual care over a lifetime horizon. Costs and outcomes were discounted to present values using a discount rate of 3.5%.
Model framework
The model schematic in Figure 9 shows a simplified patient pathway and describes how patients can move between health states (indicated by arrows). Alternative model structures were considered that explicitly modelled stroke recurrence; however, it was felt that the intervention would not affect the risk of recurrence or survival. In addition, data to parameterise the more complex structure were not available, and so the modelling of long-term outcomes under those frameworks would not have been feasible.
The model starts with all patients aged 72.5 years, representing the mean age of patients at baseline in the LoTS2Care trial, and in the health state ‘post stroke (6 months)’ that represents the average QoL of patients 6 months after a stroke. From this health state, depending on the treatment and support they receive (e.g. from the health system, friends and family), their QoL may improve, represented by a transition to the ‘recovered’ health state. The ‘recovered’ health state represents an improvement in QoL from baseline. This is defined by a minimally important difference of 0.1 in improvement in QoL for stroke patients in rehabilitation programmes. 192 It is also possible that these improvements in QoL may not last, depending on the type, duration or intensity of the treatment/support received, and so a return to the QoL experienced at 6 months post stroke is possible. This is represented by the transition from the ‘recovered’ health state to the ‘post-stroke relapse’ health state. ‘Post-stroke relapse’ has the same costs and utility as ‘post stroke (6 months)’, but it is not possible to return to ‘recovered’ from ‘post-stroke relapse’. Death is an absorptive state. Health-care costs and health benefits are associated with each health state and patients accumulate these costs and benefits over 3-month cycles.
It is irregular for model health states to be based on QoL (rather than clinical events). Yet this approach was appropriate for modelling the impact of the New Start intervention, as the outcomes related directly to changes in QoL. This creates an unusual co-dependency between QoL and health states (because QoL defines both health state and QALYs gained).
Model parameters
The model was populated using data from the trial to inform transition probabilities, health-state costs and utilities, and treatment costs associated with New Start and usual care. Parameter values for the model were obtained from the trial data based on a reported minimally important difference of 0.1 in QoL (the smallest change that could be noticed by patients). 192 For model parameters that could not be collected during the trial, including long-term mortality following stroke, recommended best practices for identifying and synthesising evidence from the literature were followed. Treatment costs for each arm were taken from the LoTS2Care trial data and represent the average cost in each arm for health-care consultations and visits associated with New Start or the usual follow-up care 6 months post stroke. Model parameters are presented in Table 60.
| Parameter | Value | Distribution | α | β | Source | Notes/description |
|---|---|---|---|---|---|---|
| Transition probabilities: New Start | ||||||
| Post stroke (6 months) to recovered | 0.24137931 | Beta | 35 | 110 | Lots2Care trial data | Patients in New Start arm whose EQ-5D score increases by at least 0.1 |
| Recovered to post-stroke relapse | 0.213793103 | Beta | 31 | 114 | Lots2Care trial data | Patients in New Start arm whose EQ-5D score decreases by at least 0.1 |
| Transition probabilities: usual care | ||||||
| Post stroke (6 months) to recovered | 0.208a | Beta | 26 | 99 | Lots2Care trial data | Patients in the usual care arm whose EQ-5D score increases by at least 0.1 |
| Recovered to post-stroke relapse | 0.208a | Beta | 26 | 99 | Lots2Care trial data | Patients in the usual care arm whose EQ-5D score decreases by at least 0.1 |
| Mortality rate | Age dependant | ONS mortality data193 plus weighting for stroke patients194 | Mortality rate applied to whole cohort regardless of health state | |||
| Health-state utilities | ||||||
| Post stroke | 0.6956204 | Beta | 366.7682635 | 160.4851976 | Lots2Care trial data | Based on mean baseline EQ-5D score |
| Recovered | 0.7180197 | Betab | 0.338321387 | 14.76578398 | Lots2Care trial data | Based on EQ-5D score 9 months post stroke of patients whose EQ-5D score had increased by at least 0.1 |
| Post-stroke relapse | 0.6956204 | Beta | 1.385977726 | 0.60645626 | Lots2Care trial data | Equal to post stroke (6 months) health state |
| Death | 0 | Fixed | Assumed | |||
| Health-state costs | ||||||
| Post stroke | 4203.19 | Gamma | 61.1028993 | 68.78871621 | Lots2Care trial data | Average costs associated with patients, excluding those whose EQ-5D score increases by at least 0.1 |
| Recovered | 3870.28 | Gammac | 0.56868394 | 585.4042582 | Lots2Care trial data | Average costs associated with patients whose EQ-5D score increases by at least 0.1 |
| Post-stroke relapse | 4203.19 | Gamma | 61.1028993 | 68.78871621 | Lots2Care trial data | Equal to post stroke (6 months) health state |
| Death | 0 | Fixed | Assumed | |||
| Treatment costs | ||||||
| New Start | 67.799 | Gamma | 0.133993856 | 505.9858861 | Lots2Care trial data | Intervention cost New Start arm |
| Usual care | 19.849 | Gamma | 0.240374822 | 82.57520404 | Lots2Care trial data | Intervention cost usual care arm |
Model feasibility and assumptions
The feasibility of parameterising the model was explored using data from the trial and information from the wider literature. As most of the parameters for the model were informed by the trial data, it was feasible to parameterise most of the model. However, in the development of the model structure it was expected that some data would be available from the SSNAP and NHS Digital to inform long-term mortality rates following stroke and stroke recurrence rates. Owing to difficulties in the process, these data were not obtained and this information had to be found from other sources. Targeted literature searches were used to identify relevant evidence to inform long-term mortality rates following stroke. However, owing to a lack of data on which (if any) patients suffered a stroke recurrence, and, consequently, no data on the QoL and cost implications that would be required to model stroke recurrence explicitly, it was assumed that the parameter values used incorporate the effect of recurrences. (Parameter values are informed by LoTS2Care trial data, which likely incorporate some stroke recurrences, but we cannot identify them.)
It was also assumed that costs and utilities associated with health states stay the same over the lifetime. This is a simplification and unlikely to happen in reality, but the assumption was the same for both arms, so the impact on incremental values is likely to be minimal.
Model validation
Model validation was conducted with reference to the ISPOR best practice guide for model transparency and validation. 150 Verification and face validity were tested by conducting a structured ‘walk-through’ with other modellers. Internal validity was also tested by conducting extreme value analysis. A further test of technical validity was conducted by comparing results produced by the model with results obtained by running all parameters through the Sheffield Accelerated Value of Information (SAVI) tool. 195 External validation is difficult to test as the events in the model correspond to changes in QoL. In addition the LoTS2Care trial was the first to test the New Start intervention, which is not yet widely used in practice, meaning that real-world event data are not available. However, some level of validation was tested by comparing model outputs at 9 months with the results of the within-trial analysis.
Analysis
As in the within-trial analysis, the incremental cost per QALY gained was estimated and compared with a cost-effectiveness threshold of £20,000 per QALY. Deterministic sensitivity analysis was used to explore possible alterative scenarios to those used in the base-case analysis. The treatment costs associated with New Start and usual care were estimated from the trial data; there was considerable variation as the treatment received was not standardised. One scenario analysis explored a more standardised treatment, which consisted of four consultations for New Start treatment and one consultation for usual care (the number and types of consultation for each case were informed by the trial). Data from the SSNAP indicate that only ≈30% of patients eligible for a 6-month assessment are assessed. We explored this in a scenario that had zero treatment costs for usual care (a fuller analysis of this scenario would also have adjusted estimates of effectiveness in usual care; this was not possible with available data, but should be explored in a full trial if appropriate data can be obtained, e.g. from the SSNAP). A further scenario analysis was explored that re-estimated parameters based on a minimally important difference value of 0.05. Parameter uncertainty was addressed through probabilistic sensitivity analysis using Monte Carlo simulation. The outputs of the analysis are presented as a scatterplot on the cost-effectiveness plane and on the CEAC.
A value-of-information analysis was conducted to explore the costs associated with the uncertainty in the results and the EVPI, representing an upper bound on the value of conducting further research, was estimated. The EVPI was estimated based on the annual number of patients applicable to be assessed at 6 months post stroke,196 and assuming the decision is relevant for a period of 10 years, after which time it is reasonable to assume that the treatment pathway may have changed. A discount rate of 3.5% was used to discount the future value of additional research to the present value. In addition, the expected value of perfect parameter information (EVPPI) was also explored using the SAVI tool. 195 This allows us to identify the parts of the model where there is greatest uncertainty, to inform future research.
Results
The cost-effectiveness results from the lifetime analysis are presented in Table 7 for the base case and for each scenario explored in the sensitivity analyses. Figures 19 and 20 show the cost-effectiveness plane and CEAC, respectively, produced from the lifetime analysis.
FIGURE 19.
Cost-effectiveness plane: lifetime analysis.
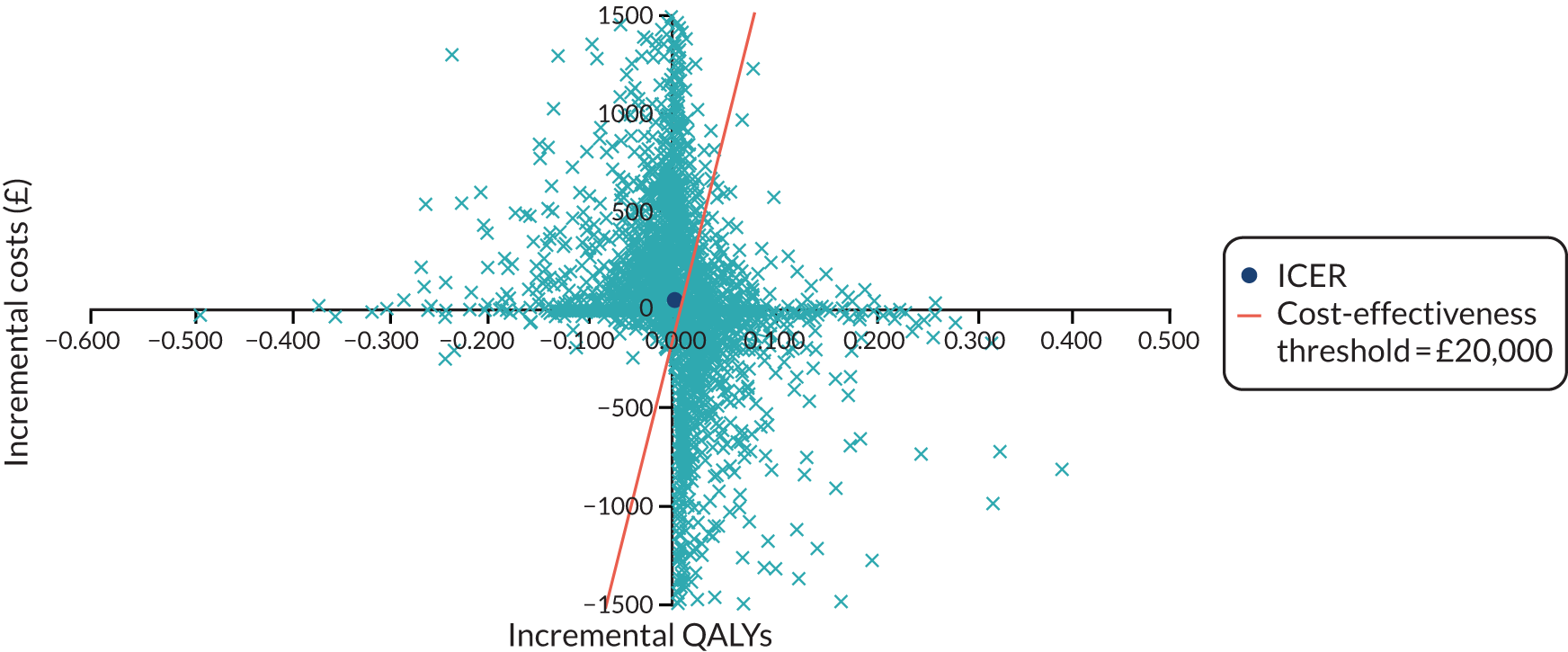
FIGURE 20.
The CEAC: lifetime analysis.
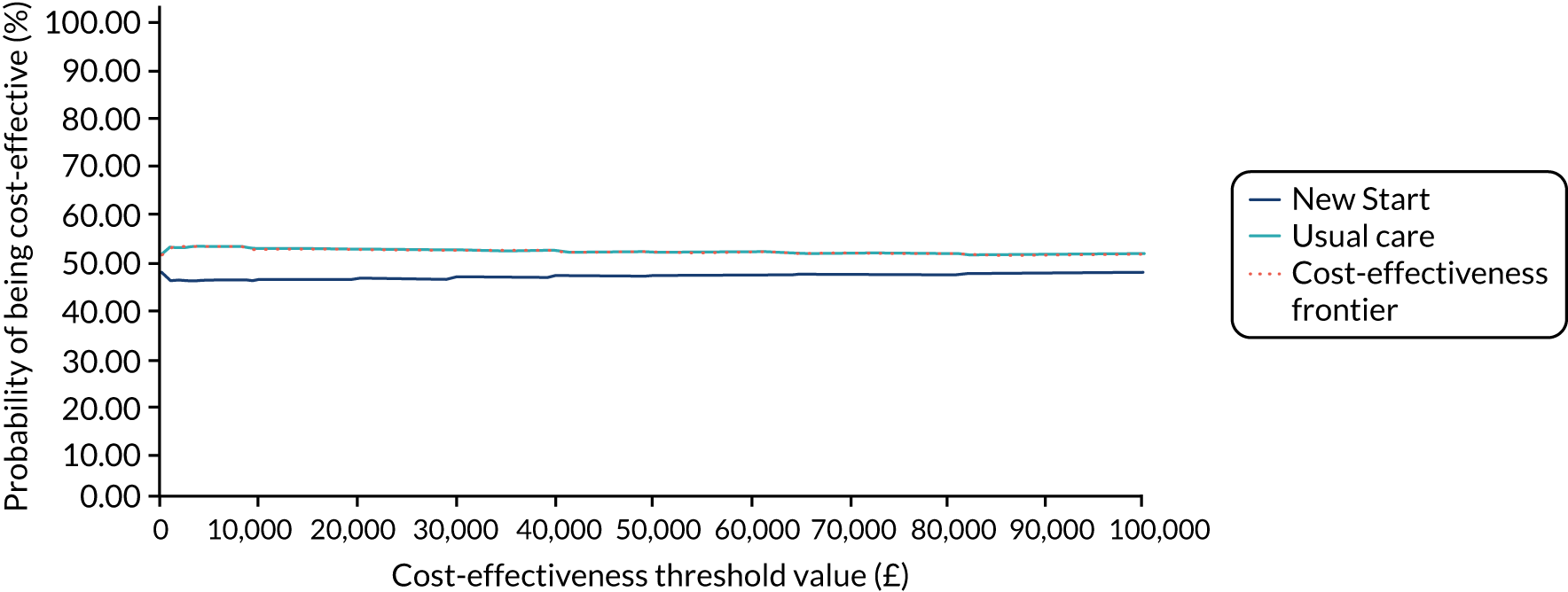
The population EVPI, at the cost-effectiveness threshold value of £20,000 per QALY gained, is > £110M. The population EVPIs for other values of the cost-effectiveness threshold are presented in Figure 21.
FIGURE 21.
The EVPIs at various cost-effectiveness thresholds.
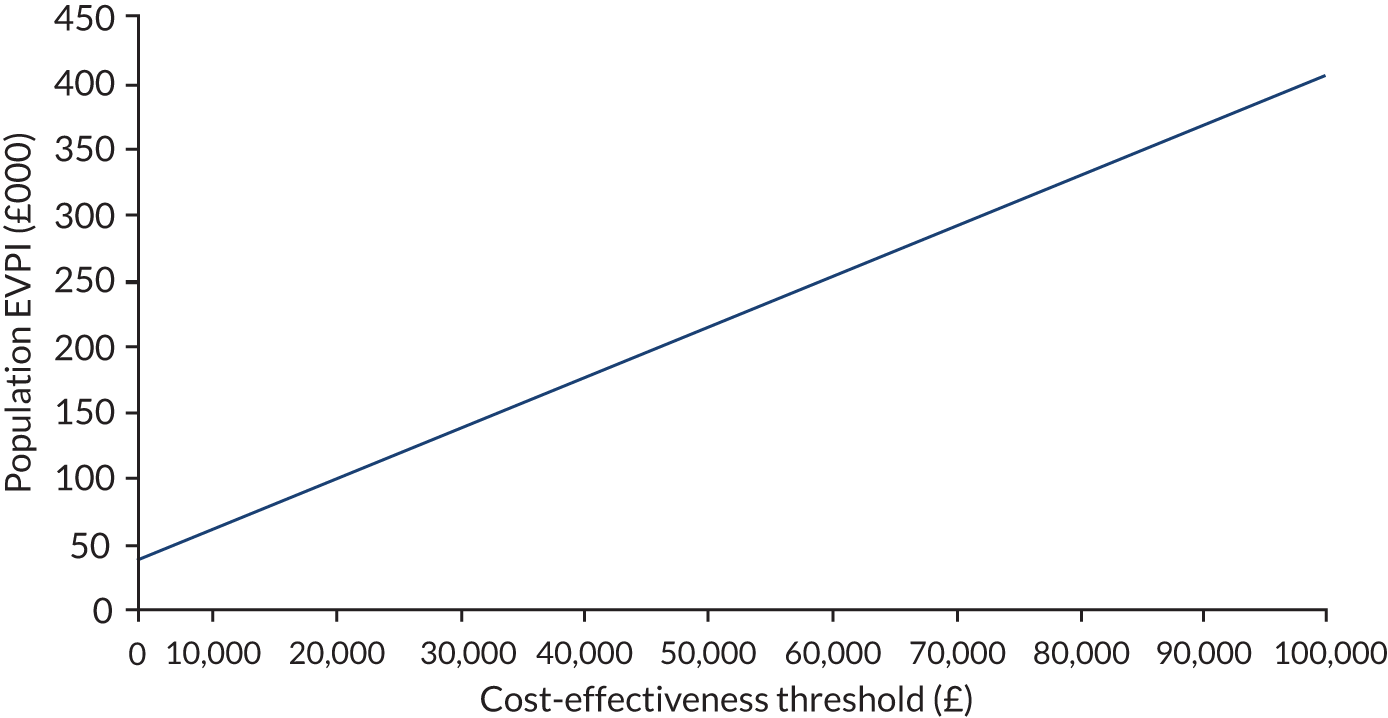
The EVPPI for individual parameters is presented in Figure 22. This shows that there is greatest uncertainty around the number of patients whose improvement in QoL is not maintained (the transition from ‘recovered’ to ‘post-stroke relapse’).
FIGURE 22.
The EVPPIs for individual parameters.
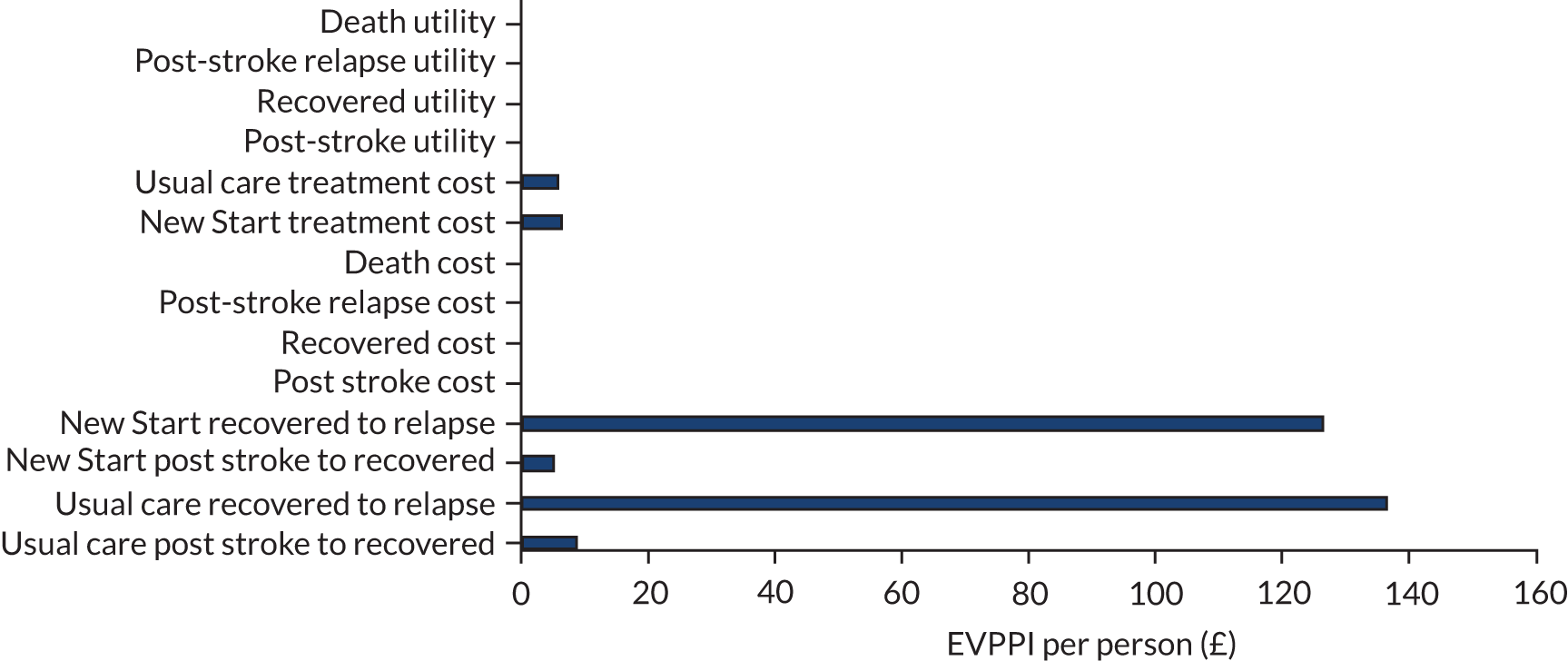
Mediators and moderators
Mediators
Potential mediators for consideration in future economic analysis (and more widely) were identified using the results from the work undertaken in the development of the intervention (WSs 1 and 2). Choice was informed by identified barriers to and enablers of behaviours affecting needs and participation of stroke survivors in the intervention; barriers to and enablers of staff delivery of service models; training needs of staff to deliver the service models; and the subsequent logic model. The proposed mediators are presented in Table 61, together with examples of the key inhibitors/enablers that informed their inclusion. The final column of Table 61 outlines the data collection requirements in a definitive trial for each mediator.
| Mediator | Mediator informed by (logic model/barriers/enablers WSs 1 and 2) | Data required in definitive trial |
|---|---|---|
| Patients do not attend the interview | Accessibility, inadequate public transport, stigma, motivation, lack of money, time, impaired function, confidence, support network, changed identify, encouragement | CRF |
| Patients do not take up activities | Meet identified needs, meaningful activities, motivation, lack of money, time, impaired function, confidence, support network, changed identify, encouragement, limited provision of activities to engage with, poor information provision | Patient questionnaire |
| Location/setting of the visit | Accessibility, confidence, lack of money, time, impaired function, building a support network | CRF |
| PAM | Motivation, rehabilitation support, encouragement, hope, problem-solving, seeking information, positive outlook, acceptance as a process, seeking support, fear and worry | Part of outcome measurement |
| Social support | Building a support network, loss or lack of support, acceptance of support, burden of caring, togetherness, change in roles, becoming a caregiver, acceptance as a process, disrupted couplehood | CRF/patient questionnaire |
| Review relating to stroke | Identify emergent problems and provide necessary support | CRF/patient questionnaire/EHR [e.g. HES, CPRD, EMIS Health (Leeds, UK), ResearchOne] |
| Professional support in previous 6 months | Sustaining flexible support networks, meets identified needs, accessibility, acceptance of support, loss/lack of support, seeking support, availability, accessibility, stigma | CRF/patient questionnaire/HER (e.g. HES, CPRD, EMIS Health, ResearchOne) |
| WEMWBS | Fear and worry, reassurance, loss/lack of support, building a support network, medication, taking time out, seeing self as agent, keeping busy, burden of caring, change in roles | Part of outcome measurement |
| WHODAS | Rehabilitation support, encouragement, hope, creative problem-solving, difficulty recognising changes, motivation, impaired functioning | Part of outcome measurement |
| Charlson Comorbidity Index | Rehabilitation support, encouragement, hope, creative problem-solving, difficulty recognising changes, motivation, impaired functioning | CRF/HES |
Moderators
Potential moderators for consideration in a future economic analysis (and more widely) were identified using the results from the work undertaken in the development of the intervention (WSs 1 and 2), and are detailed in Table 62. A selection of the identified moderators were chosen as exemplars for which exploratory analyses were conducted. The chosen exemplars are indicated in Table 62 with notes on the reasons for or against selection.
| Moderator | Data required to enable moderation analysis in definitive trial | Selected for exemplar analysis | Reason |
|---|---|---|---|
| Gender | CRF | Yes | Good completion |
| Age | CRF | Yes | Good completion |
| Site | CRF | No | Clustering by site already accounted for in cost-effectiveness analysis |
| Ethnicity | CRF | No | Not representative, small numbers |
| Employment | CRF | No | Linked with age |
| Patient has a carer (yes/no) | CRF | Yes | Well completed, patients excluded if it was not known whether or not they had a carer (n = 43) |
| Patient’s carer is a live-in carer (yes/no) | CRF | No | Not well completed |
| If the carer is family/friend | CRF | No | Not well completed, and, of those with complete data, very small numbers whose carer was not family (n = 5) |
| Baseline QoL | CRF (e.g. ED-5D score) | No | Adjust for EQ-5D in main analysis |
| Stroke score at admission/discharge | Hospital records (HES) (e.g. NIHSS score at admission, mRs at discharge) | No | Not well completed (NIHSS, n = 143; mRs, n = 142) |
| Length of stay for stroke admission | Hospital records (HES) | No | Difficult to derive from available data – discrepancies between onset of stroke and hospital admission |
| Language ability at baseline | CRF | No | Not known for a large proportion of patients |
| Level of unmet need at baseline | LUNS, baseline CRF | Yes | Good completion |
| Mobility at baseline | Baseline CRF (e.g. EQ-5D, WHODAS) | No | Adjust for baseline EQ-5D score in main analysis |
| Ability to self-care at baseline | Baseline CRF (e.g. EQ-5D) | No | Adjust for baseline EQ-5D score in main analysis |
| Pain at baseline | Baseline CRF (e.g. EQ-5D) | No | Adjust for baseline EQ-5D score in main analysis |
| Ability to undertake usual activities at baseline | Baseline CRF (e.g. EQ-5D) | No | Adjust for baseline EQ-5D score in main analysis |
| Depression/anxiety at baseline | Baseline CRF (e.g. EQ-5D) | No | Adjust for baseline EQ-5D score in main analysis |
| Cognitive problems at baseline | Baseline CRF [e.g. WHODAS (understanding and communicating)] | No | Some overlap with EQ-5D domain |
| Clinical problems | Charlson Comorbidity Index | No | Some overlap with EQ-5D |
| Emotional problems at baseline | CRF (e.g. WEMWBS) | No | Some overlap with EQ-5D domain |
| Benefits/finance | CRF | No | Data not collected |
Exploratory moderation analysis was conducted using the regression approach outlined by Aiken et al. :197
where Y is the outcome variable, I is the intercept, X is the causal variable (in our case treatment: New Start or usual care), M is the moderator variable and XM is an interaction term of X and M, and e is the error term. Here, coefficient c measures the moderation effect and coefficient a measures the simple effect of X (treatment) when M = 0.
As the economic analysis is concerned with two outcomes together, costs and outcomes (QALYs), the moderation analysis was conducted using ordinary least squares bivariate regression analysis for costs and QALYs, accounting for the correlation between the two.
Of the potential moderators selected for exemplar analyses, gender and the patient having a carer were analysed as dichotomous variables (dummy coded). Age was centred to mean = 0 before analysis to allow results to be interpreted at the mean observed value, and to reduce issues with multicollinearity. 198 Level of unmet need is measured on the LUNS scale, with values from 0 to 15. Centring the LUNS variable was considered; however, as the results are more easily interpreted without centring in this case, and as LUNS = 0 is still an interpretable value, moderated regression for level of unmet need is presented without centring the LUNS variable.
The results of the exploratory moderated regression analysis for the selected exemplars are summarised in Table 63. For each analysis, the treatment estimate shows the effect of the New Start intervention on costs and/or QALYs when the moderator = 0. The moderator estimate shows the effect on costs and/or QALYs when treatment = 0 (usual care). The interaction estimate measures the moderation and shows the change in the effect of treatment on the outcome for a 1-unit change in the moderator.
| Moderator | Total costs | Total QALYs | ||
|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | |
| Gendera | ||||
| Treatment | –594.68 | 0.667 | 0.003 | 0.928 |
| Gender | –625.16 | 0.692 | –0.029 | 0.456 |
| Interaction | 1943.06 | 0.351 | –0.034 | 0.513 |
| Intercept | 4452.16 | 0.000 | 0.543 | 0.000 |
| Carer (yes/no)b | ||||
| Treatment | 648.07 | 0.583 | –0.029 | 0.429 |
| Carer | 5262.11 | 0.002 | –0.143 | 0.000 |
| Interaction | –1453.03 | 0.506 | 0.044 | 0.415 |
| Intercept | 2651.90 | 0.003 | 0.572 | 0.000 |
| Agec | ||||
| Treatment | 296.24 | 0.771 | –0.012 | 0.621 |
| Age | 145.70 | 0.027 | –0.005 | 0.001 |
| Interaction | –53.89 | 0.554 | 0.003 | 0.249 |
| Intercept | 4178.59 | 0.000 | 0.530 | 0.000 |
| Baseline level of unmet need (LUNS) | ||||
| Treatment | –2676.84 | 0.073 | –0.032 | 0.339 |
| LUNS | 970.29 | 0.000 | –0.033 | 0.000 |
| Interaction | –700.06 | 0.022 | 0.007 | 0.272 |
| Intercept | 724.87 | 0.512 | 0.648 | 0.000 |
The analysis indicates that gender could be a possible antagonistic moderator for cost, as the predictor and moderator have the same direction of effect on the outcome, but the interaction effect is in the opposite direction. For QALYs, gender appears to be a buffering moderator, as it weakens the effect of the predictor variable on the outcome. These effects are illustrated in Figure 23. Patients having a carer appears to be an antagonistic moderator for both costs and QALYs, and indicates that having a carer is associated with lower costs and an increase in QALYs for patients in the New Start arm. These effects are illustrated in Figure 24. Age appears to be an antagonistic moderator for both costs and QALYs. At the mean age, the New Start intervention is associated with higher costs and lower QALYs (treatment estimate); as age increases, costs increase and QALYs decrease (moderator estimate), but age increasing reduces the costs associated with New Start (interaction estimate). These effects are illustrated in Figure 25 at values for age 1 SD above and below the mean observed age. Level of unmet need appears to be an enhancing moderator for cost, as the predictor and moderator effects on the outcome have a stronger than additive effect, and an antagonistic moderator for QALYs. For low unmet needs, New Start increases costs and reduces QALYs; however, for high unmet needs, New Start reduces costs and increases QALYs. These effects are illustrated in Figure 26.
FIGURE 23.
Analysis of moderators: gender. (a) Total cost; and (b) total QALYs.
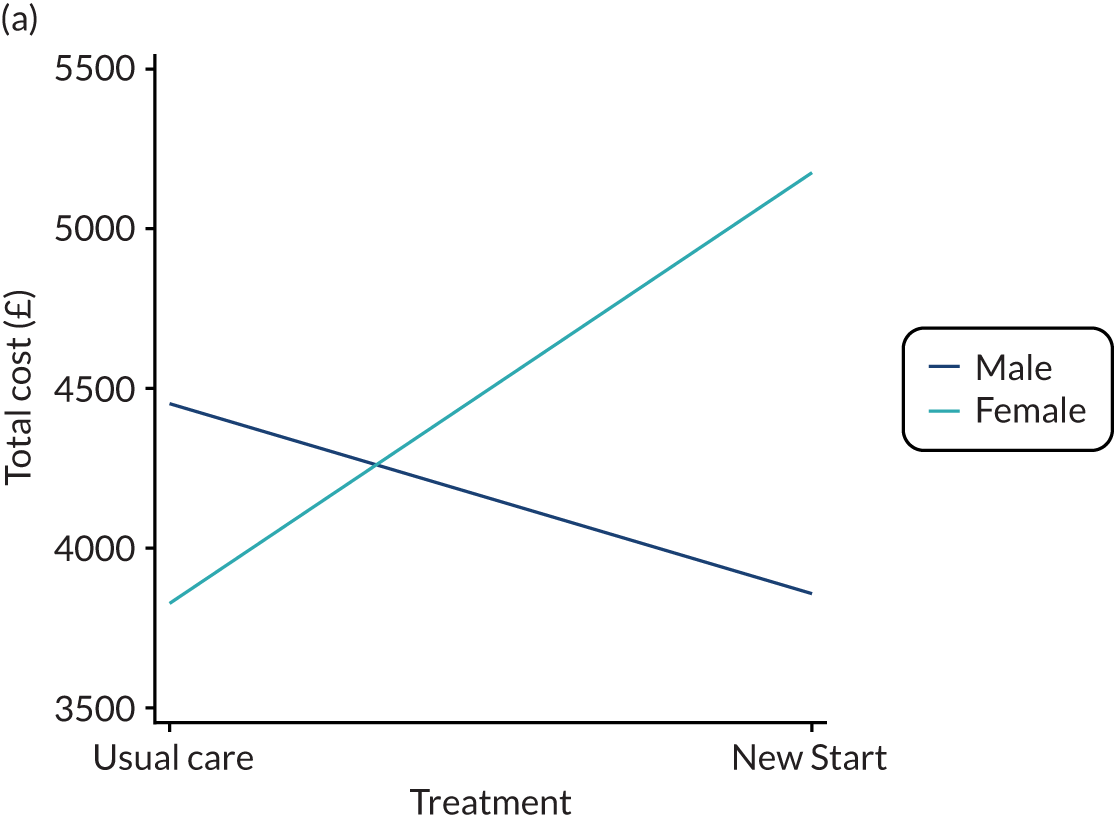
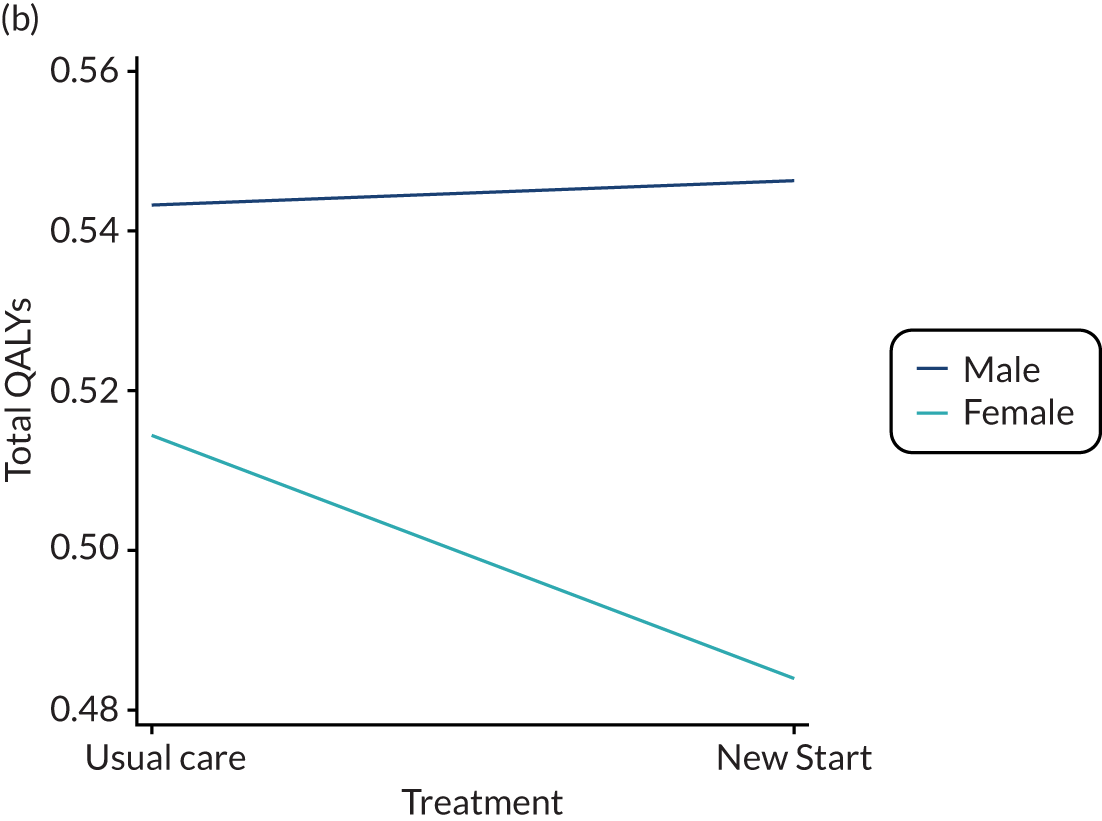
FIGURE 24.
Analysis of moderators: carer. (a) Total cost; and (b) total QALYs.
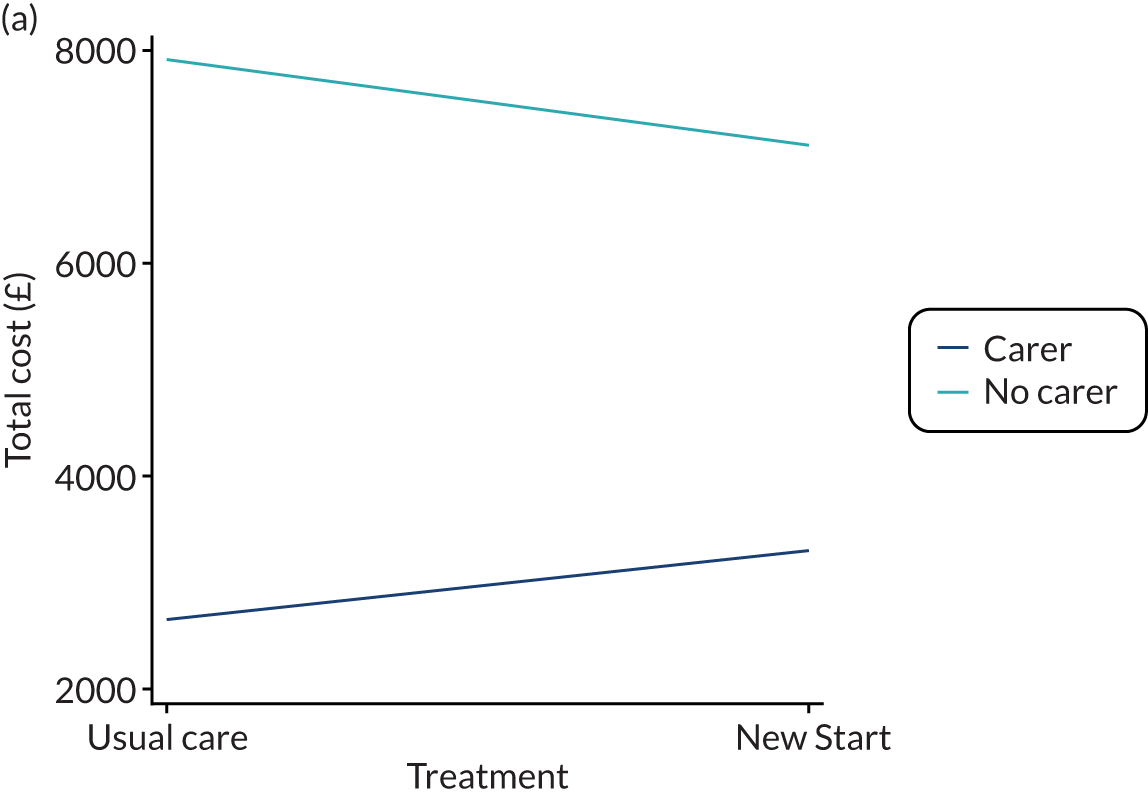
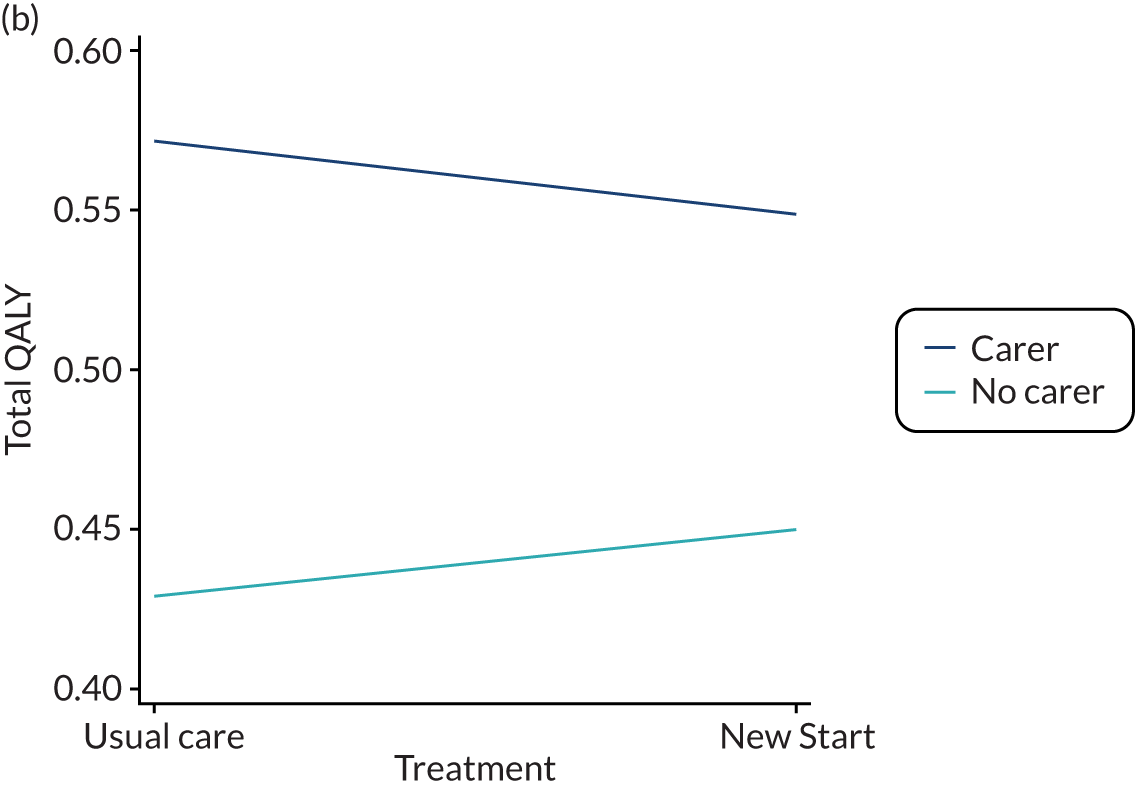
FIGURE 25.
Analysis of moderators: age. (a) Total cost; and (b) total QALYs.
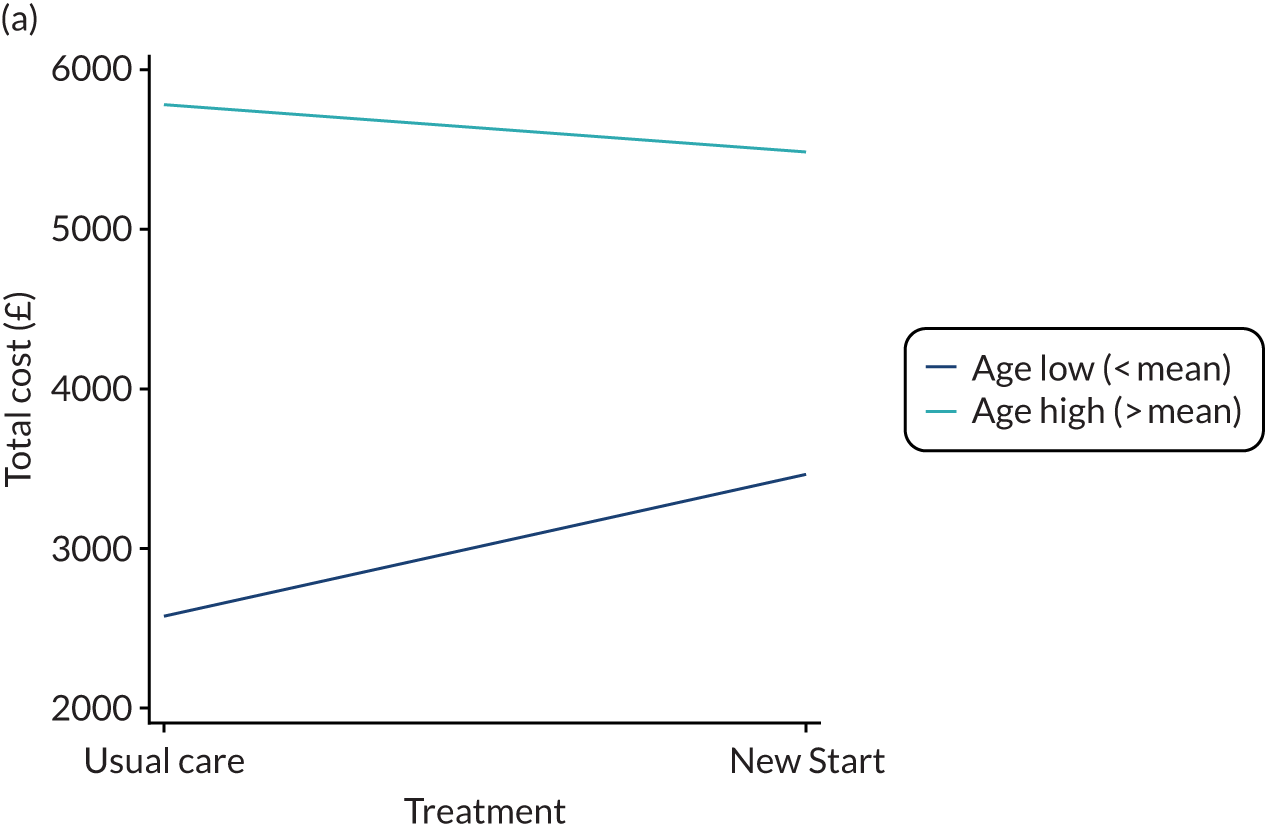
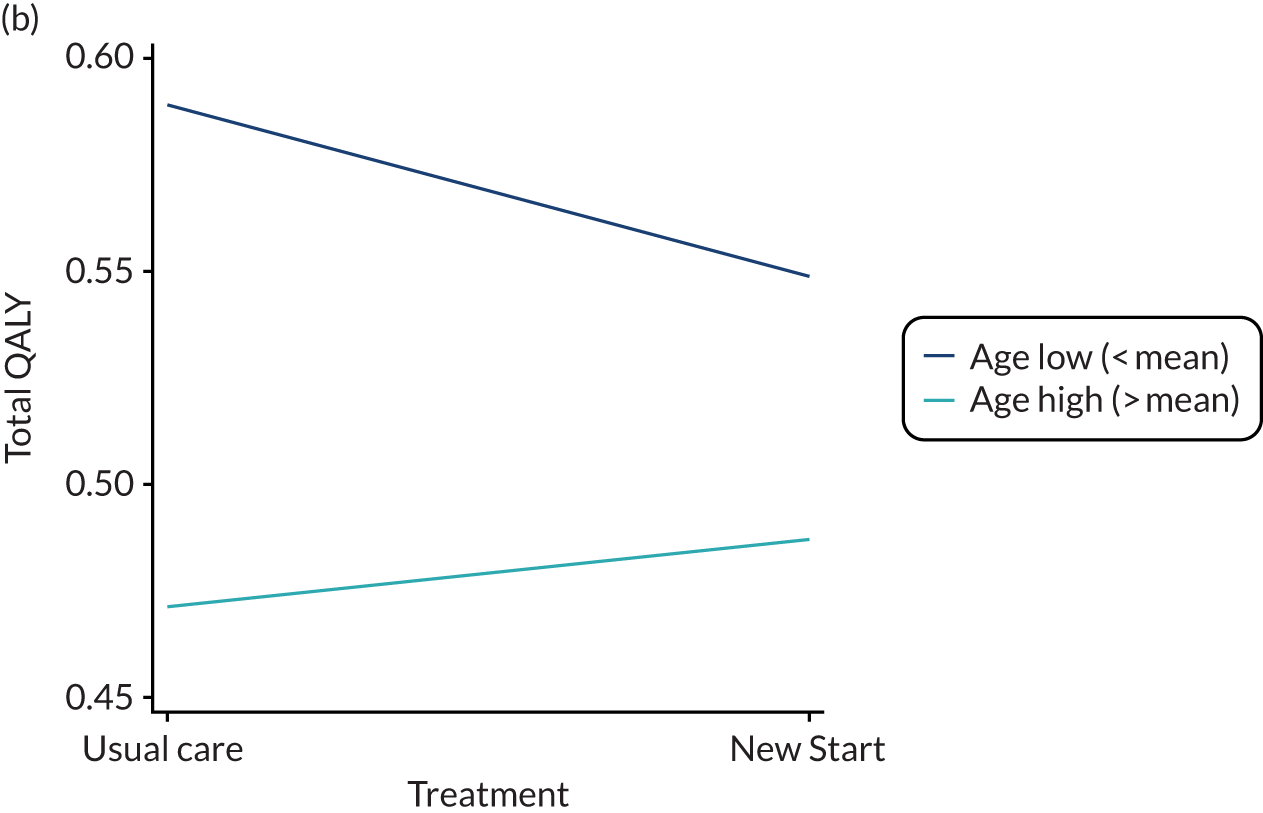
FIGURE 26.
Analysis of moderators: level of unmet need. (a) Total cost; and (b) total QALYs.
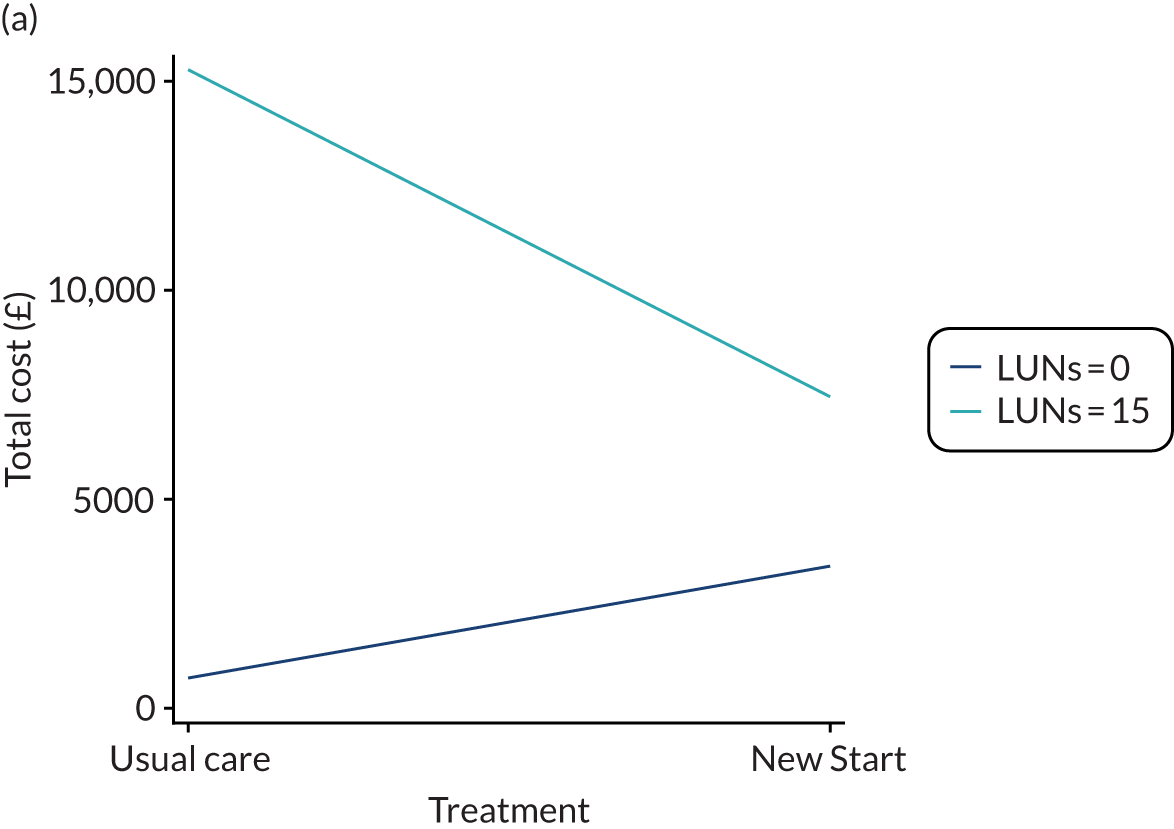
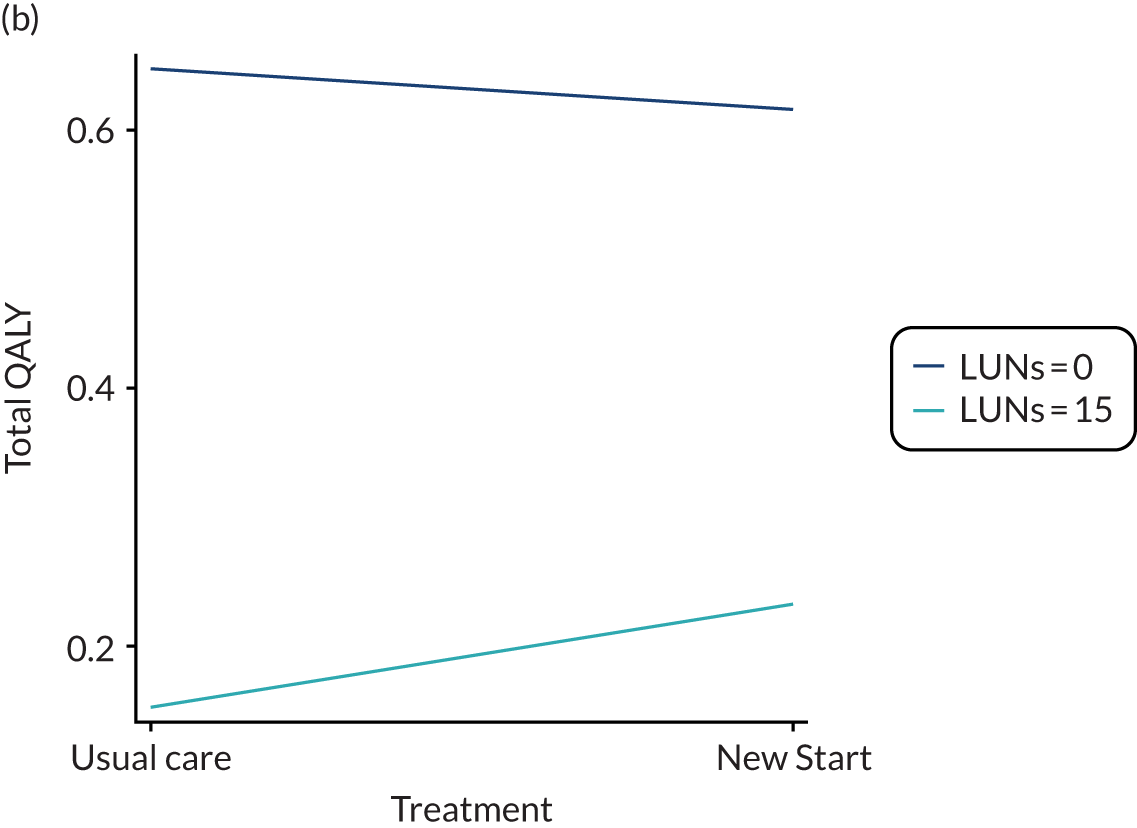
Statistical significance is presented in Table 63; however, the difficulties in interpreting significance that are common to moderation analyses198,199 are further exemplified by this being an exploratory analysis that is not powered for definitive results. Consequently, discussion of the results is based on the direction of the estimates without consideration of statistical significance. Further analysis of moderators conducted in a full trial should ensure that moderators to be considered are identified at the design stage of the trial, to ensure that appropriate steps are taken in the study design and data collection to allow more meaningful analysis. As this is an exploratory analysis, we did not control for confounding. However, we recommend that, in a larger trial with full power, any further analysis of moderators should consider incorporating such controls.
Discussion
Principal findings
The primary within-trial cost-effectiveness analysis and long-term evaluation of lifetime costs and benefits in the economic model were both exploratory. The within-trial analyses indicated that, although the New Start intervention may be a cost-effective use of resources, the results were not robust to alternative assumptions explored in sensitivity analyses. There was considerable variation in the cost-effectiveness estimates, with each variation in assumptions indicating substantial uncertainty around the results.
Fewer QALYs were gained in the New Start arm than in the usual care arm, but the mean difference was small in real terms and the difference was not statistically significant. Total costs were also lower in the New Start arm than in the usual care arm, although this difference was not statistically significant. The difference in costs was particularly driven by lower costs incurred by stroke survivors and their carers for private health care paid for out of pocket. Furthermore, it is notable that health-care resource use covered by the NHS was greater in the New Start arm. This could demonstrate an unmet need for health care within usual care practices, which is being addressed with the New Start intervention, consequently reducing private expenditure for health care, which could have equity benefits.
The results obtained from the longer-term analysis of costs and benefits using the decision-analytic model indicated that New Start was unlikely to be cost-effective, compared with usual care. As in the within-trial analysis, there was uncertainty in the results, which was driven by the small differences between the treatment options in terms of both costs and QALYs. The EVPI indicated that further research conducted at an expected cost of < £110M would be warranted to reduce the uncertainty in the results. The EVPPI indicated that, in the model, there was greatest uncertainty around the number of patients whose improvement in QoL was not maintained, namely in the transition from ‘recovered’ to ‘post-stroke relapse’.
Strengths and weaknesses of the economic analysis
A strength of this analysis lies in the randomised controlled design of the trial, which has enabled the collection of good-quality data that were used to explore the feasibility of conducting analysis in a future full trial. This has shown that a within-trial analysis and an analysis of longer-term outcomes would be feasible, but has also highlighted areas where changes in the data collected, or in the ways in which they are collected, could allow for more robust evidence collection and analysis in a full trial.
One limitation of the analysis was the lack of available data on stroke recurrence and long-term survival data following stroke. This meant that assumptions had to be made to enable long-term modelling of costs and outcomes. Although the modelling was still possible, it could perhaps be more robust if it were possible to obtain good-quality data to inform these aspects.
Meaning of the feasibility trial
This analysis has shown that a within-trial cost-effectiveness analysis would be feasible as part of a definitive trial. However, it is noted that, for both QoL and resource use data, compliance decreased over the duration of the feasibility trial. Compliance from carers in QoL questionnaires was particularly low. Consequently, if a full trial was conducted, ways to maintain compliance should be explored, for example altering the frequency to address questionnaire fatigue and exploring ways to ensure that carers are engaged.
In addition, it has been shown to be feasible to conduct a long-term analysis of costs and outcomes using a decision-model framework. However, it may be possible to make improvements to the structure of the model if it were possible to obtain or collect certain data, such as stroke recurrence and the impact of stroke recurrence on QoL, in a definitive trial.
Unanswered questions and further research
This analysis has provided preliminary estimates of cost-effectiveness; however, the primary purpose was to assess the feasibility of conducting such analyses as part of a definitive trial. This feasibility trial was not powered to provide definitive answers; consequently, a full trial would be required to reduce the uncertainty around the cost-effectiveness estimates.
The results from the within-trial analysis indicated that there may be a decrease in out-of-pocket costs for private health care and an increase in use of NHS services with the New Start intervention. This could present equity benefits of the New Start intervention, which should be explored further in a definitive trial.
The results from the EVPPI indicated considerable uncertainty around the number of patients whose improvement in QoL was not maintained (the transition from ‘recovered’ to ‘post-stroke relapse’). This is likely to be a valuable area for future research aiming to reduce the uncertainty around the cost-effectiveness of the New Start intervention, and could be addressed with additional long-term follow-up of patients.
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- CBS
- Caregiver Burden Scale
- CCG
- Clinical Commissioning Group
- CCM
- Chronic Care Model
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CLAHRC
- Collaborations for Leadership in Applied Health Research and Care
- CONSORT
- Consolidated Standards of Reporting Trials
- CRAG
- Consumer Research Advisory Group
- CRN
- Clinical Research Network
- CST
- community stroke team
- CTRU
- Clinical Trials Research Unit
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- ESD
- early supported discharge
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- GP
- general practitioner
- ICC
- intracluster correlation coefficient
- ICECAP-A
- ICEpop CAPability measure for Adults
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning and Disability
- ID
- identifier
- ISPOR
- International Society for Pharmacoeconomics and Outcomes Research
- IT
- information technology
- LoTS2Care
- Improving Longer Term Stroke Care
- LUNS
- Longer-term Unmet Needs after Stroke
- MDT
- multidisciplinary team
- mRS
- modified Rankin Scale
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- NPT
- normalisation process theory
- PAM
- Patient Activation Measure
- PhD
- Doctor of Philosophy
- PLANS
- Patient-led Assessment for Network Support
- PMG
- Programme Management Group
- PSC
- Programme Steering Committee
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RG
- reference group
- SAVI
- Sheffield Accelerated Value of Information
- SD
- standard deviation
- SEPSS
- Self-Efficacy and Performance in Self-management Support
- SRN
- Stroke Research Network
- SSNAP
- Sentinel Stroke National Audit Programme
- TDF
- theoretical domains framework
- WEMWBS
- Warwick–Edinburgh Mental Well-being Scale
- WHODAS
- World Health Organization Disability Assessment Schedule
- WS
- workstream
Notes
-
Literature search exploring unmet needs after stroke (workstream 1a)
-
Workstream 3 literature search strategy for review of existing self-management interventions in stroke and other chronic conditions
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09030).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
