Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1212-20018. The contractual start date was in January 2015. The final report began editorial review in April 2021 and was accepted for publication in February 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Underwood et al. This work was produced by Underwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Underwood et al.
Synopsis
Background
Headaches are second only to low back pain as a global cause of years lived with disability, accounting for 6.4% of the total years lived with disability. 1 Only dental caries are more common than migraine and tension-type headaches. 1 Headaches are the most common neurological disorder treated in primary care, accounting for around 3% of general practitioner (GP) consultations; however, 70% of these patients do not get a formal diagnosis. 2,3 Two-thirds (64%) of people seen in a specialist headache clinic have not had a headache diagnosis from their GP. Of those with migraine, fewer than half have been offered specific migraine treatment. 4
For many people with headache disorders their symptoms are intermittent and they can be managed with symptomatic treatment as required. However, for a substantial group of people, headaches become a chronic disabling disorder. This group contributes disproportionally to the health burden, and economic cost, of headache disorders.
Treatment guidelines for headache disorders are typically formulated in a biomedical framework, with the main focus being on drug treatments for those with a diagnosed headache disorder. For example, the only non-pharmacological intervention recommended for people with chronic tension-type headache or chronic migraine in the 2012 National Institute for Health and Care Excellence (NICE) guidelines5 published was acupuncture. There is a need for more non-pharmacological treatments to help those living with headache disorders.
In 2015, when we started this programme of work, very little was known about how best to support those who have developed a chronic problem consequential to a primary headache disorder. We anticipated that any supportive self-management programme would need to include helping more people to get a headache diagnosis, avoiding medication overuse headaches, providing medication appropriate for the headache type and supportive self-management for a chronic painful disorder.
Headache researchers usually expect those individuals studied to have an established headache diagnosis prior to study entry. 6,7 This work, however, started from the premise that most people with frequent headaches do not have an established diagnosis, and was developed from the perspective of headaches as a chronic disorder. There is not, however, a recognised clinical entity of chronic headaches. In epidemiological studies researchers use a definition of chronic headaches based on the definition of chronic migraine or chronic tension-type headache, specifically headaches on ≥15 days per month for the previous 3 months. 8–11 This matches neither the conventional definition for chronicity of pain used by International Association for the Study of Pain (IASP), of pain persisting for greater than 3 months,12 nor the definitions of chronicity used for other headache disorders, for example chronic cluster headache. 9 In addition, the chronicity of headaches defined in this manner is labile. 9,12 In a community study of migraine,13 three-quarters [386/526 (73%)] of people with chronic migraine had one or more 3-month period in the following year when their headache frequency was consistent with episodic (<15 headache days per month) rather than chronic migraine. 13 Nevertheless, this epidemiological definition is a useful shorthand to describe our population of interest: people living with headaches affecting them on most days. Our overarching aim was to develop and test a supportive education and self-management group intervention implementable in primary care for people with chronic headaches. This is an area with little previous methodological work and an absence of substantial previous UK experience of recruiting people from primary care for studies of headache interventions. A broad programme of research was needed to set the scene for our randomised controlled trial (RCT).
Key areas of uncertainty were as follows:
-
Was it possible to recruit people from primary care who met our definition of chronic headache?
-
How easy would it be to identify people who might have other headache types not suitable for our intervention package?
-
What is the patient experience of living with chronic headaches (including chronic migraine)?
-
What should be the content of the self-management support intervention?
-
What format should be used for the delivery of the self-management support intervention?
-
How acceptable would our intervention package be to people living with chronic headaches?
-
How should we measure outcomes?
These were all addressed in the phase 1 feasibility study. In phase 2 we ran a full RCT, with a cost-effectiveness analysis and an embedded process evaluation.
Phase 1: trial feasibility
The trial feasibility phase was made up of four core areas of work, each of which mapped onto our work packages (WPs):
-
WP1 – developing a strategy to identify people with chronic headaches from primary care.
-
WP2 – developing and evaluating a telephone headache classification interview.
-
WP3 – developing and evaluating an education and self-management support intervention for people living with chronic headaches.
-
WP4 – selecting the most appropriate patient-reported outcome measure (PROM).
The trial feasibility phase aimed to answer the questions of what can be done, what should be done, and how best it can be done for a future RCT. Here we describe these packages of work and how these informed the trial feasibility (see Figure 1).
FIGURE 1.
Components of the trial feasibility phase. PPI, patient and public involvement. Reproduced with permission from White et al. 14 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The figure includes minor additions and formatting changes to the original.
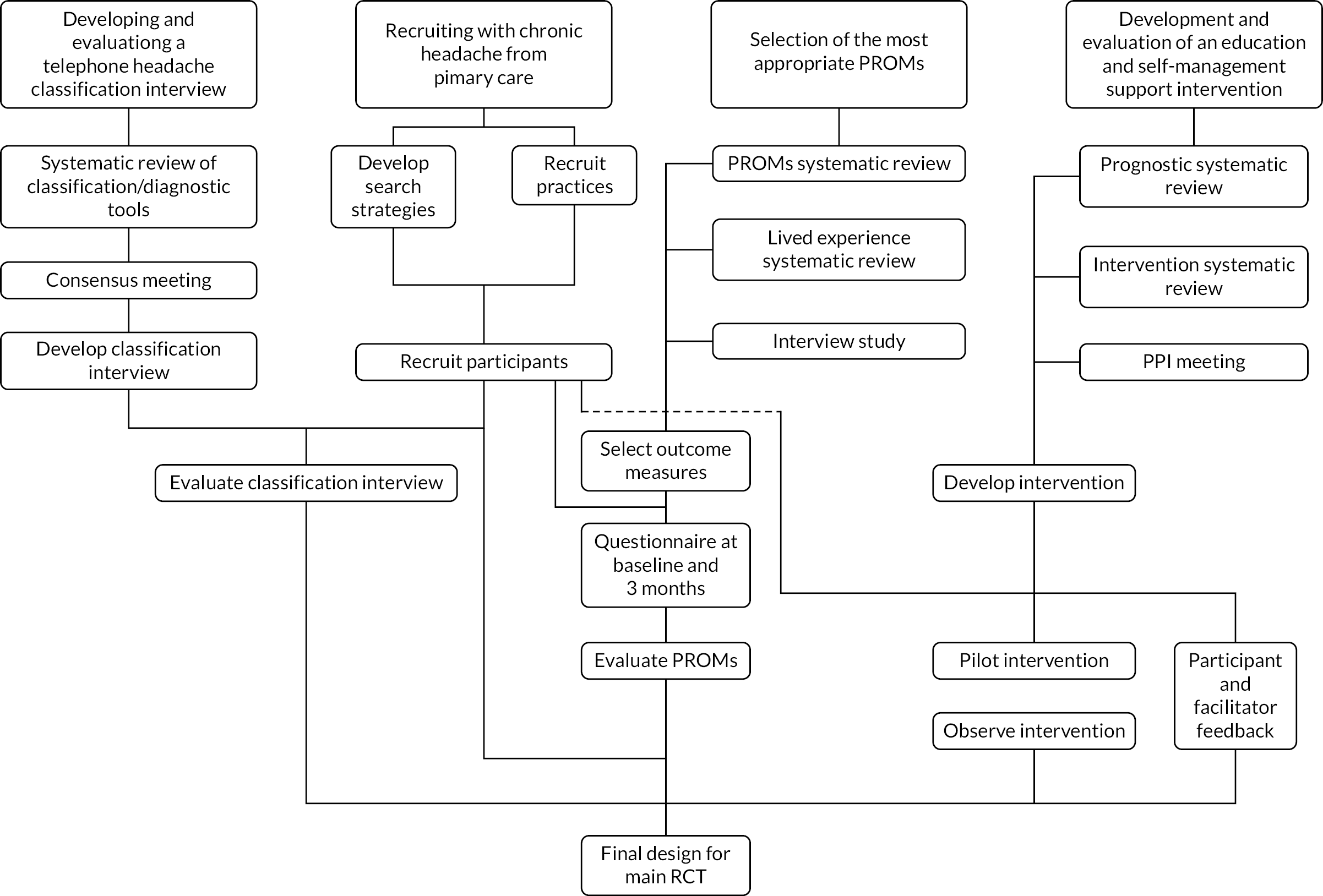
Recruiting feasibility sample (work package 1)
Objective: to develop and test strategies for recruiting people living with chronic headaches from primary care.
To determine recruitment feasibility, we needed to:
-
Develop a strategy to identify people with chronic headaches from primary care. Our scoping work identified that the standard clinical terminology system (Read codes) for coding chronic headache disorders in general practice was rarely used. We developed a search strategy incorporating age, consultation for headaches and prescription of headache-specific medication to identify our target population.
-
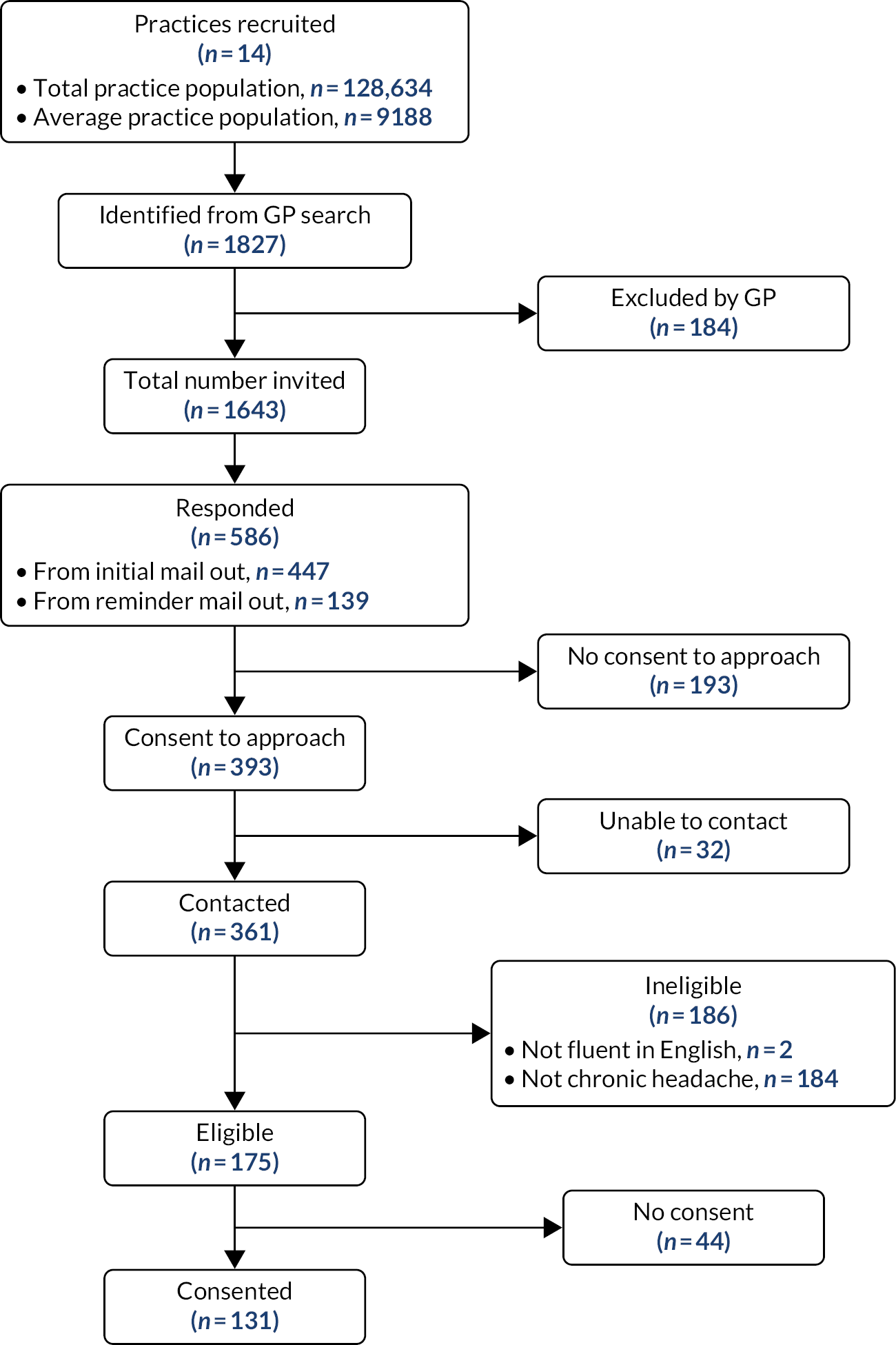
For any future trial, we needed to be sure we could recruit practices. To test this, during the feasibility phase we aimed to recruit practices in the West Midlands. With the help of our local Clinical Research Network (CRN) we recruited 14 practices, giving us a total practice population of 128,634. A detailed account of the recruitment process is given in our published paper (see Appendix 1). 14
From the practice population of 128,634 we obtained informed consent from 131 participants (see Figure 2). 14 Participants’ mean age was 49 years [interquartile range (IQR) 38.5–58 years, standard deviation (SD) 13.3 years], 108 (82%) were female and 125 (95%) were white. These participants consented to:
FIGURE 2.
Practice and participant recruitment for the feasibility study. Reproduced with permission from White et al. 14 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The figure includes minor additions and formatting changes to the original.
-
completing an electronic (smartphone/web-based) headache diary (a paper version was also available) for 3 months
-
filling out a baseline, 2-week and 3-month questionnaire
-
taking part in two telephone interviews
-
the research team sharing the headache assessments with the participants’ GPs.
They also agreed to potentially being invited to two further studies:
-
an interview study to explore the experience of living with frequent headaches, how we might refine our proposed support programme, and what outcomes are important to people living with frequent headaches
-
a pilot study in which we invite people to take part in our chronic headache education and self-management support programme.
The sample size for the feasibility study was driven by the need for sufficient data to measure the level of agreement of the classification interviews in WP2. The original sample size was 170; this was revised to 153 paired interviews owing to a change in planned analyses approved by the Programme Steering Committee (PSC) and the funder in March 2016. Later, the PSC agreed that recruitment could stop at 131 participants after reviewing the agreement analysis for the first 100 paired classification interviews. This cohort of 131 participants provided us with a sampling frame for the feasibility work; full details are in our published paper (see Appendix 1). 14
Developing and evaluating a structured telephone interview for diagnosing common chronic headache disorders (work package 2)
Objective: to develop and evaluate a brief diagnostic interview to support diagnosis for people with chronic headaches, focusing on the diagnosis of the three common chronic headache disorders – migraine, chronic tension-type headache and medication overuse headache.
Within this WP it was important not only to be able to identify the population of interest for the main trial, but also to feel confident that those who have other headache types not suitable for our trial were appropriately identified, and referred for relevant support. We first reviewed the literature on diagnostic tools.
Systematic review of diagnostic tools for chronic headache
We searched for studies aiming to validate tools for diagnosis and/or classification of headaches. We searched the published literature between January 1988 and June 2016 using MEDLINE® (National Library of Medicine, Bethesda, MD, USA), Applied Social Sciences Index and Abstracts (ASSIA), Embase® (Elsevier, Amsterdam, the Netherlands), Web of Knowledge™ (Clarivate™, Philadelphia, PA, USA) and PsycInfo® (American Psychological Association, Washington, DC, USA). Methodological quality was assessed using items from the Quality of Diagnostic Accuracy Studies (QUADAS-2) tool. We identified 4348 titles and removed 2459 duplicates; after screening the remaining titles we obtained full-text results for 195 papers. Thirty-eight papers met our inclusion criteria validating 30 tools designed to diagnose, classify or screen for headache disorders. Of these, 21 tools were for classification of one headache type and nine were for multiple headache types. Full details are given in our published paper (see Appendix 2). 15
We did not find any tools that we could use for our proposed trial and felt that it was important to develop our classification tool based on the evidence as well as a collaboration with clinicians and patients. We therefore organised a consensus conference. Full details are in our published paper (see Appendix 3). 16
Consensus conference
The aim of the day was to reflect on the evidence from the review and draw on the expertise of the delegates to help inform the content of the Chronic Headache Education and Self-management Study (CHESS) nurse telephone classification interview.
The purpose of the meeting was to develop a classification interview that would:
-
confirm study eligibility – participants aged ≥18 years with chronic headache, defined as a headache on ≥15 days per month for at least 3 months
-
classify the participant’s headache type as part of the active intervention to inform treatment and advice (this was done during the face-to-face part of the intervention).
Twenty-six delegates attended (10 neurologists with specialist interest in headaches, three general neurologists, five headache specialist nurses, one GP with a special interest in headaches and seven people living with chronic headaches) attended our ‘Classification Consensus Conference’ at the University of Warwick in August 2015.
They were split into four multidisciplinary groups. Using facilitated discussions each group was asked to address the following four questions:
-
What do we need to know from a person to exclude secondary headaches?
-
What do we need to know from a person to exclude primary headaches other than chronic migraine and tension-type headache?
-
What do we need to know from a person to distinguish between chronic tension-type headache and chronic migraine?
-
What do we need to know from a person to identify medication overuse headache?
The facilitators aimed to get consensus on discussed items; when there was uncertainty, items were taken to a plenary session in which they were further discussed followed by voting to gain consensus. Further work by the research team developed and refined the final classification logic model underpinning the classification interview (see Figure 3).
FIGURE 3.
Classification logic model. a, Tension-type headache, has mild nausea. NSAID, non-steroidal anti-inflammatory drug. Reproduced with permission from Potter et al. 16 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The figure includes minor additions and formatting changes to the original.
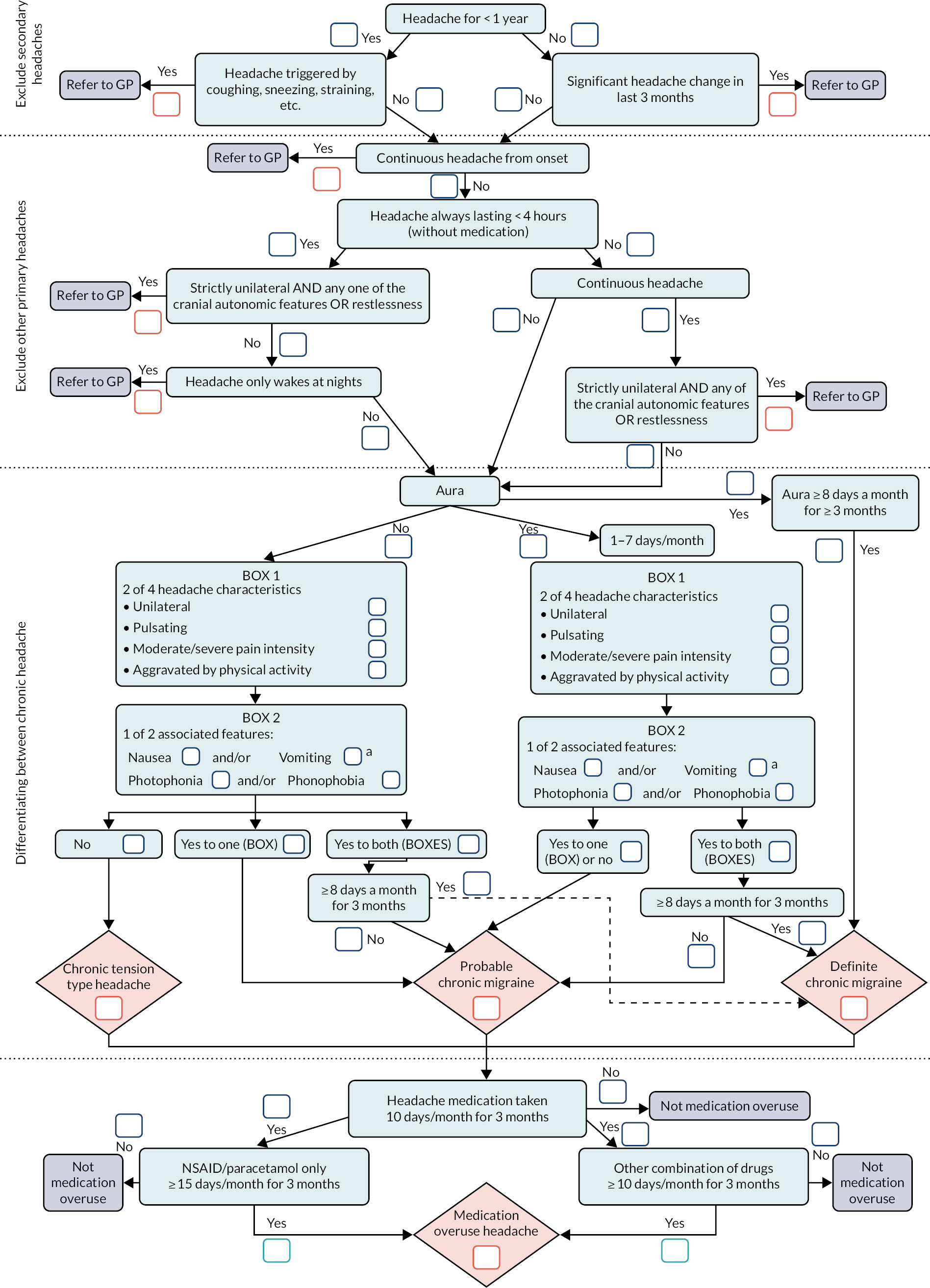
The classification logic model is presented as a linear process. However, it was developed to be completed non-sequentially as information was obtained during the interview and it is not a diagnostic algorithm.
Having developed the classification tool, the next phase was to validate the tool by training nurses to use the tool.
Training and validation
On 22 February 2016 six research nurses who would complete the telephone classification interviews attended a training day. Nurses were provided with a detailed manual and further one-to-one support to ensure that they felt confident in conducting the interviews (see Report Supplementary Material 1).
To validate the telephone classification tool, we did paired interviews whereby participants received an interview with the nurse and soon after a second interview with a headache specialist doctor from the National Migraine Centre (NMC). 17 These doctors used their standard approach to telephone assessment and did not use the CHESS logic model. 16
Level of agreement was measured using proportion of concordance, the kappa statistic and prevalence-adjusted bias-adjusted kappa. 18 The sample size calculation was based on the kappa statistic of the level of agreement between the nurse interview and the specialist doctor. Nurses carried out 111 classification interviews and the doctors carried out 108 interviews. We obtained paired data on 107 participants.
There was generally good agreement between nurse and doctor interviews (proportion of concordance > 0.75). 16 We reviewed cases in which both parties disagreed on the classification and those in which both classified the headaches as ‘other’ (non-chronic migraine or chronic tension-type headache). 16 Typically, the disagreements were around whether the headaches were episodic or were chronic migraine. Four people had an excluded headache type: two people had cluster headaches, one had a hemicrania continua and one had a primary stabbing (ice pick) headache. This confirmed that, although ineligible primary headache types are uncommon, they were sufficiently common to justify identifying them prior to randomisation.
A striking, and unexpected, observation from this work was that only a very small proportion of those we assessed had chronic tension-type headaches: only 6 out of 107 (6%). This had consequences for our approach to the primary analysis for the RCT described in phase 2.
Developing and evaluating the intervention (work package 3)
Objective: to develop and pilot an education and self-management support intervention for the management of common chronic headache disorders (the CHESS intervention) that is both theoretically informed and based on best evidence.
Details of our intervention development are in our published paper (see Appendix 4). 19
We first reviewed the existing literature to understand the experience of chronic headaches from the patient perspective, what content and approaches might be effective for this population and what modifiable prognostic factors exist to be targeted in future interventions.
Systematic reviews
Details of each review are in our published papers (see Appendices 5–7). 20–22
Lived experience of chronic headache (see Appendix 5)20
We systematically reviewed and appraised the qualitative literature on the lived experiences of those living with chronic headaches. We included qualitative studies of adults (aged ≥18 years) with chronic headaches. We searched MEDLINE, Embase, ASSIA, PsycInfo, Scopus® (Elsevier, Amsterdam, the Netherlands) and Web of Science™ (Clarivate) between January 1988 and July 2016. We included studies that used qualitative methodology, or mixed methodology if the qualitative findings were reported separately. We excluded studies that did not have a patient’s perspective, theses, dissertations and conference papers. We appraised the included studies for risk of bias. We used a thematic analysis across the studies followed by a meta-ethnographic approach.
Four studies met our inclusion criteria. Analysis identified three overarching themes:
-
‘headache as a driver of behaviour’ – forcing patients to stop activities or take increasing medication to function
-
‘the spectre of headache’ – the worries, fear and guilt that patients carry
-
‘strained relationships’ – the effect their headaches and behaviour have on those around them.
Although chronic tension-type headaches were represented in the data, they may have been overshadowed by chronic migraine features.
Prognostic factors in chronic headache (see Appendix 6)21
We included prospective cohort studies and RCTs of chronic headaches, published in English. We included adults with chronic migraine, or chronic tension-type headache, with or without medication overuse headache disorders. We excluded studies with participants < 18 years old, dissertations and conference proceedings. We searched Cochrane, MEDLINE/PubMed® (National Library of Medicine), Embase, PsycInfo, Web of Science and ASSIA, from January 1980 to June 2016. Two reviewers independently extracted data and assessed the methodological quality. RCTs were included only if a subgroup analysis was reported or enough data to perform subgroup analysis were presented. We assessed the adequacy of any moderator analyses.
Twenty-seven studies met our inclusion criteria: 17 prospective cohort studies and 10 RCTs with subgroup analyses. There was moderate evidence for depression and anxiety, poor sleep, stress, medication overuse and poor self-efficacy predicting a poor outcome. There was inconclusive evidence for treatment expectations, age and age at onset, body mass index, employment and headache features predicting a poor outcome.
Broadly speaking, the factors identified were consistent with prognostic factors seen in people with chronic painful musculoskeletal disorders, supporting the notion that adapting approaches used to help people live better with other chronic disorders can be applied to people living with chronic headaches. 23,24
Style and content of intervention programmes (see Appendix 7)22
Our aim in this review was to identify the components and method of delivery used in non-pharmacological educational and self-management interventions for headache disorders. We included RCTs comparing a relevant educational and/or self-management intervention for headache disorders with usual care. We excluded studies with participants aged < 18 years old, invasive treatments such as acupuncture, interventions purely focusing on physical exercise, dissertations and conference proceedings. We searched the Cochrane Library, MEDLINE, Embase, Web of Science and PsycInfo from January 1980 to June 2016.
We included 16 studies in the review. We found positive overall effects of self-management interventions over usual care for pain intensity, headache-related disability and quality of life. A moderate effect was seen on mood. A greater effect on mood was observed in interventions that included a cognitive–behavioural therapy (CBT) component than those without, and for group interventions when compared with one-to one delivery.
Interview study
We conducted the interview study to build on our understanding from the systematic reviews to aid the development of the intervention. More details are included in our published paper (see Appendix 4). 19
We had planned to conduct interviews on the sampling frame developed in WP1. At the time we needed to do these interviews to inform intervention development, this sampling frame was not established. Therefore, our sample was obtained through Migraine Action, one of our charity partners (Migraine Action merged with The Migraine Trust in 2018) and approved by the PSC and funder in October 2015. We sent 100 invitations leading to 21 responses. Of these, seven met our inclusion criterion of headaches on ≥15 days per month for at least 3 months. A topic guide was informed by the literature review. The guide allowed the exploration of perceptions of helpful and unhelpful treatment strategies. All interviews were audio-recorded for transcription.
The results suggested that participants had tried a range of therapies and interventions, some of which were helpful while others were not. Access to education and peer support was deemed positive, as was learning new skills such as relaxation, mindfulness and stress management.
Developing the intervention package
The reviews and the interview study were summarised and presented at a multidisciplinary intervention development day held in November 2015 at the Royal College of General Practitioners in London. The aim of the day was to start to scope out what the CHESS intervention should look like. Eighteen people attended, bringing together clinical, academic and lay expertise. The facilitated discussions were factored around four core areas (see Box 1).
-
Tailored headache education
-
How can the classification interview be used for the intervention for supporting optimisation of drug treatment?
-
What written information is needed (for GP and patient)?
-
What should be the structure of this consultation?
-
What should it be the content of this consultation?
-
How long should it last?
-
Where should it be conducted and by whom?
-
-
Generic chronic headache self-management
-
What format should the self-management intervention take?
-
What should the content be?
-
How should it be delivered (format, length)?
-
Who should it be delivered by?
-
Where should it be delivered?
-
What material do we need to develop for the intervention arm?
-
Do we need any material for the GP?
-
-
Control group
-
What would be deemed an acceptable control arm?
-
What material do we need to develop for the control arm?
-
Do we need any material for the GP?
-
-
Ongoing support
-
How should any ongoing post intervention support be provided?
-
How should this be standardised?
-
How should this be recorded?
-
There was overall agreement that the intervention should be a group education and self-management intervention with an integrated one-to-one consultation. The group intervention would be for 8–10 participants and be modular, but participants should attend all the sessions. Suggestions were to run the programme during school hours in community settings when possible. It was agreed that the intervention should be delivered by a nurse and a layperson living with chronic headaches and delivered in a non-didactic manner. The content should include educational material, self-management material, medication advice, plus a digital versatile disc (DVD) suitable to share with friends and family. Providing a DVD for family and friends was a suggestion from patient partners that the academic team had not previously considered. Ongoing support was agreed as up to 8 weeks of telephone support by the nurse, individually negotiated with participants.
The group felt strongly that there should be a comparator control group arm and not just usual care. As the literature22 suggested beneficial use of relaxation, this was deemed a good control intervention. It was agreed that a relaxation compact disc (CD), adapted from a previous study,25 would be developed for CHESS. Control participants were also to be provided with customised information on their headache type after a classification interview.
In the feasibility phase the intervention was designed to be delivered, by a nurse and a layperson, over 2.5 days with a one-to-one nurse consultation and individualised follow-up.
Testing and refining the intervention package
Having developed the intervention package and a facilitator manual to accompany this (see Report Supplementary Materials 2 and 3), we delivered three groups using the 2.5-day intervention format described. Groups were run between July and September 2016. We approached 79 participants from our sampling frame (see Appendix 1). 14 Thirteen participants attended: six attended the first group, three the second group and four the third group. Difficulty with attendance was as a result of commitment of time over 2 consecutive days.
As part of the formative evaluation the process evaluation team observed group two and concluded that the intervention had been delivered as per protocol. They interviewed 12 out of the 13 participants who attended these feasibility groups. Some participants had also completed a feedback form at the end of each day.
Overall, everyone had appreciated connecting with others with the same condition, and this was a driver to return. The course content and pace were well received. Group discussions were appreciated, as was the lay facilitation. The nurse one-to-one sessions were highly valued by most, with the majority wanting telephone follow-ups. The majority said that they would recommend the course to others. Only two had some reservations: one felt that it would be useful for people newly diagnosed and one wanted more individual tailoring of advice.
We interviewed three nurses and two lay facilitators to ask about their experiences of running the groups. This feedback resulted in the removal of the half-day follow-up because participants found it difficult to get the time off to attend. The final and the third feasibility group piloted the 2-day revised format intervention, which was subsequently adopted for the main RCT (see Figure 4). For the main study we also provided additional facilitator training on medication use.
FIGURE 4.
Intervention structure. (a) Feasibility phase and (b) main study.
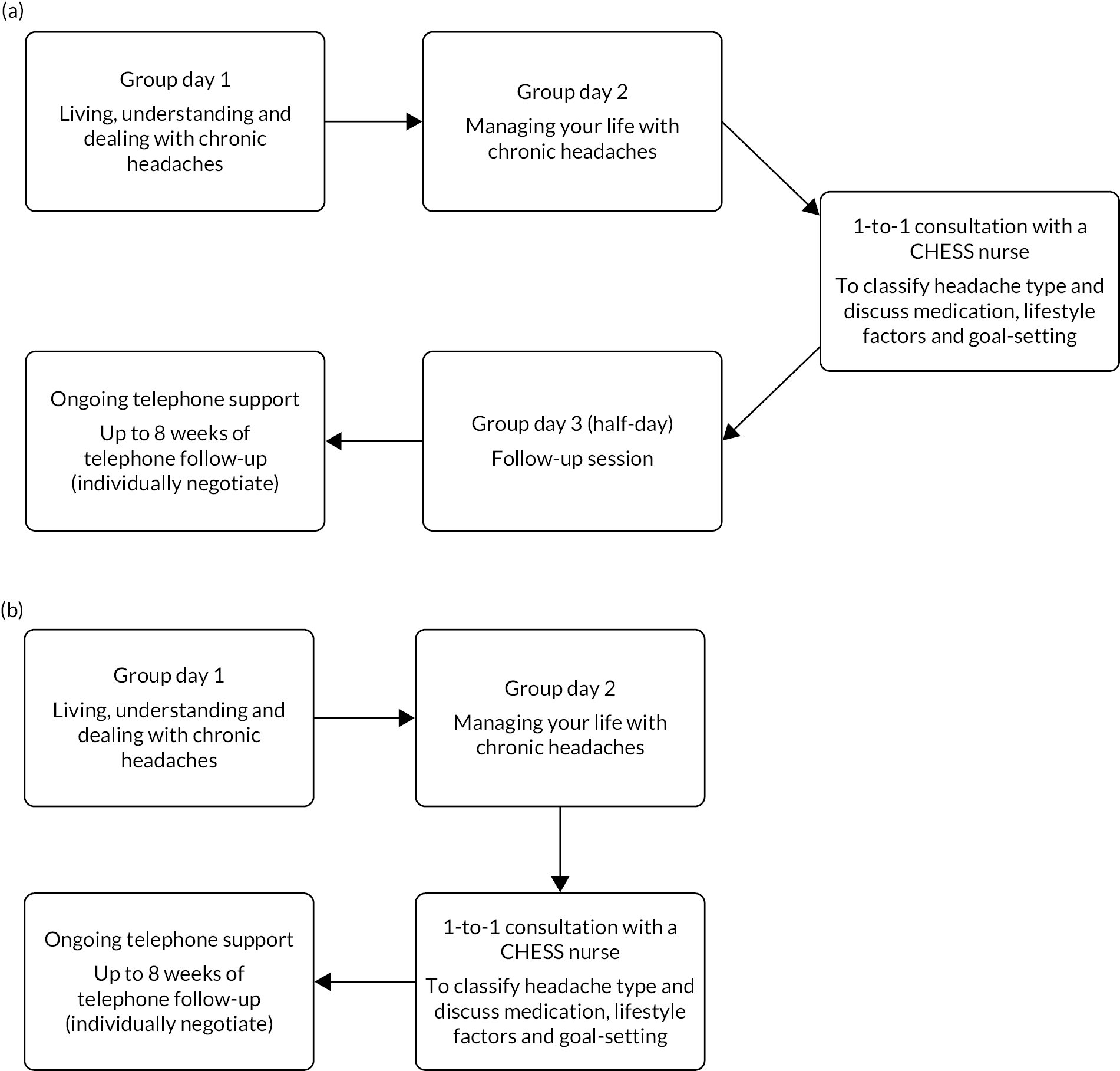
During the feasibility phase it became clear that recruiting laypeople with chronic headaches as facilitators would be challenging because of the unpredictable nature of the condition. This was discussed at a trial management meeting in June 2016, at which there was agreement to recruit allied health professionals to co-facilitate with the nurse.
The theoretical underpinnings and behaviour change rational and techniques for the CHESS intervention, and course content, are described elsewhere (see Appendix 4). 19 The CHESS intervention materials are available from http://wrap.warwick.ac.uk/171671.
Choice of clinical effectiveness and cost-effectiveness outcome measures (work package 4)
Objective: to select most appropriate outcome measures for the RCT of the CHESS intervention package.
This work is described in our published papers and appendices (see Appendices 8–10). 26,27
Systematic review of patient-reported outcome measures (see Appendix 8)26
We wanted to assess the quality and acceptability of outcome measures for chronic and episodic headache. We searched for multi-item PROMs evaluated following completion by adults aged ≥ 18 with episodic or chronic headache. We searched published literature between January 1980 and December 2016 using MEDLINE and Embase. We assessed study methodological quality using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist, and PROM measurement quality and acceptability by reference to accepted international standards. 28–32
We included 46 papers providing evidence for 23 PROMs. Six measures looked at the impact of headaches overall and five were specific to the impact of migraines. Six assessed responses to, and/or satisfaction with, migraine-specific drug treatments. A further six generic measures had been assessed in headache populations. Assessment of reliability was generally limited with acceptable evidence for the six-item Headache Impact Test-6 (HIT-6). 33 Assessment of responsiveness was rare and patient involvement was limited and poorly reported. Overall, the HIT-6, the Migraine-Specific Quality of Life Questionnaire (MSQ) version 2.134 and the Patient Perception of Migraine Questionnaire – Revised (PPMQ-R)35 had acceptable evidence of reliability and validity, although that was still limited. For a generic ‘headache’ population only the HIT-6 had acceptable evidence of validity and reliability.
Based on the review, and cognitive interviews, detailed below, the HIT-6 was selected as the primary outcome measure for the RCT. Many of the assessed measures had a migraine focus, making them challenging to apply to a generic chronic headache population. However, among the team, patient and public involvement (PPI) research partners and the lay advisory group, there was a sense that a lot of the questions in the 14-item MSQ v2.1 had relevance to our population.
Outcomes for the trial (see Appendix 9)27
With the permission of GSK plc (formerly GlaxoSmithKline plc; Brentford, UK), the developers of the MSQ v2.1, we modified this measure, changing the focus of each item from ‘migraine’ to ‘headache’. The adapted measurement was renamed as the Chronic Headache Quality of Life Questionnaire (CHQLQ).
We did a mixed-methods comparative evaluation of the CHQLQ and HIT-6. Feasibility study participants completed the postal questionnaires at three time points: baseline and at 2 and 12 weeks. This provided the raw data necessary to inform the psychometric evaluation. A range of analyses informed the determination of data quality, reliability, validity, responsiveness to important change in health and score interpretation. The questionnaire included headache-specific, generic and several domain-specific measures. In addition, we carried out semistructured cognitive interviews with 14 participants within 24 hours of them completing the 2-week questionnaire to explore the relevance, acceptability, clarity and comprehensiveness of the headache-specific (and generic) measures. 36–39 We wanted to explore what participants felt was missing and how individuals determined any improvement in their headache. Interviews were audio-recorded and transcribed.
Both the CHQLQ and HIT-6 were well completed, had good psychometric properties and were relevant to the experience of headaches. The CHQLQ captured the wide-ranging impact of chronic headache, in particular the emotional impact, to a greater extent than HIT-6.
Our original intention was to make a final decision on the primary outcome for the trial informed by our quantitative study. It was not possible to complete this work before starting the main trial. For this reason, we set HIT-6 as the primary outcome for the trial and the CHQLQ as a secondary outcome.
Electronic data capture (see Appendix 10)
We wanted to explore electronic means of capturing data on the frequency, severity and duration of headaches. The application (app) was developed by Clinvivo Ltd, a University of Warwick spin–out. We developed three questions to capture headache frequency, duration and severity. The developers drafted a version that was initially tested by the research team and members of the lay advisory group.
We agreed a secure data management process to enable data captured from the app to be tracked against each participant’s trial number. A system of flagging participants who had not responded for more than 3 weeks was also implemented. Eight feasibility participants were asked to complete the app over an 11-week period. The overall feedback was positive and completion rates reasonable: the team agreed to include the smartphone app as part of the main trial.
Professor Martin Underwood, the chief investigator, is a director and shareholder of Clinvivo Ltd. The use of this company was suggested in the original application for funding. Professor Underwood subsequently recused himself from all contracting decisions, which followed University of Warwick standard financial procedures. He had no involvement in this aspect of the work from either a University of Warwick or a Clinvivo Ltd perspective during the lifetime of the study. He is not an author on the draft paper describing this work. He has edited this report with respect to the use of the smartphone app.
Mapping study of health outcomes in people living with chronic headaches (see Appendix 11)40
A piece of work mapping between health-specific outcomes and health utility measures was included in the original proposal embedded in our existing data collection. During the lifetime of the programme we concluded that it would be better to collect data for this outside the main trial. Recruiting an external cohort of chronic headache patients (separate from the main trial participants) meant that externally generated mapping coefficients could be obtained to inform the economic evaluation of the CHESS intervention. We set up a separate substudy to collect data for headache clinics. This additional work was approved by the funder in September 2019 but was considerably delayed because of the COVID-19 pandemic. In the mapping substudy, mapping or crosswalk algorithms were developed to estimate EuroQol-5 Dimensions, three-level version (EQ-5D-3L), and Short Form questionnaire-6 Dimensions (SF-6D) health utilities from responses to the HIT-6 and the CHQLQ. Data from cross-sectional cohort of 349 people living with chronic headaches in England were used to develop the mapping functions while baseline data from CHESS participants served as a validation sample. Appendix 11 presents further details of the methods, analyses, and results. Overall, censored least absolute deviations models generated the best performance in terms of accuracy of predictions. EQ-5D-3L and SF-6D utilities were best predicted from the HIT-6 without the need for additional patient-level information, whereas predictions for the CHQLQ required age and gender in addition to the summary score.
Other related work
We describe here other activities done as part of the CHESS programme that are outside the main narrative.
Core Outcome Set in Migraine (see Appendix 12)39
In WP2 we identified inconsistencies in outcome reporting alongside often poorly defined outcomes. We recognised the need and opportunity to develop a core outcome set for migraine trials. We decided that this would have wider relevance than focusing on the needs of just our trial. We made the decision to focus on a core outcome set for migraine trials, rather than for headache trials, informed by the overwhelming proportion of those we recruited in WP1 having migraines.
In a two-step process, we defined the core domain set (what to measure), followed by the core measurement set (how to measure specified domains). We identified >50 domains from our systematic reviews and our qualitative work. These data were presented in two questionnaires, one for episodic migraine, the other for chronic migraine. We did a modified, three-round electronic-Delphi study with patients and professionals.
Professor Underwood, the chief investigator for this study, is a director and shareholder of Clinvivo Ltd, who provided the Delphi platform. He recused himself from any discussions related to the choice of Delphi platform for this study.
The results of this Delphi study (see Report Supplementary Material 4). were discussed at a consensus day, at which the aim was to ratify the core domains, agree on the core measurement set and recommend the core outcome set. Through group facilitation and discussion, a two-domain core outcome set was agreed for chronic and episodic migraine:
-
migraine-specific pain – to be assessed with an 11-point numerical pain rating scale, and frequency as the number of headache/migraine days over a specified period
-
migraine-specific quality of life to be assessed with the Migraine Functional Impact Questionnaire. 41
Although the Migraine Functional Impact Questionnaire is a new PROM, it has strong evidence of face and content validity and essential measurement properties (when compared with existing measures of headache-specific quality of life). Participants in the consensus meeting felt that it better represented the important elements of headache-specific quality of life that were identified during the Delphi process. The Migraine Functional Impact Questionnaire had not been published when we started the CHESS RCT and so we were not able to use this as an outcome.
Relationship between chronic headaches and chronic low back pain (see Appendix 13)42
Our approach to seeking to help people with chronic headaches draws on approaches used to treat people with chronic musculoskeletal pain. As an additional piece of work, we did a systematic review of studies looking at the association between chronic headache and chronic back pain, full details published elsewhere. 42 We identified 14 studies reporting on our primary outcome: the association between chronic headache disorders and persistent low back pain (LBP). Different papers found odds ratios ranging between 1.55 [95% confidence interval (CI) 1.13 to 2.11] and 8.00 (95% CI 5.3 to 12.1). The strength of these findings was constrained by the variable approaches used by the original authors to define both chronic headaches and back pain. This supports our decision to use a biopsychosocial approach, grounded in previous work on chronic musculoskeletal pain, to inform our headache intervention.
Multicentre trial (work package 5)
Objective: to run a multicentre RCT, including an economic evaluation, of the CHESS intervention package.
Details of the trial have been published elsewhere (see Appendices 14 and 15). 43,44 Further information is available in our original application, final protocol, data management plan, statistics analysis plan and health economics analysis plan (see Report Supplementary Material 5–8). Here we address the research question:
-
Is the CHESS intervention package clinically effective and cost-effective when compared with a usual-care control?
Clinical methods
Practices identified people who had consulted with headaches or who had been prescribed a migraine-specific drug (triptans/pizotifen) in the previous 2 years. 14 The list of people was screened by a GP in the practice to identify people it would not be appropriate to approach, for example people with a severe uncontrolled mental health problem or a terminal illness. Table 1 provides a list of the full inclusion/exclusion criteria. The practice then sent out packs inviting people to express an interest in the trial. Those who were interested returned an expression of interest form to the research team. The research team telephoned the potential participant to confirm their eligibility and obtain verbal consent to start the smartphone app, or paper headache symptom diary. A study pack with the participant information leaflet, consent form and baseline questionnaire was sent to the potential participant. Once the consent form and baseline questionnaire were received, a classification interview call was arranged (see Figure 5).
| Inclusion criteria | Exclusion criteria |
|---|---|
| Able and willing to comply with the study procedures and provide written informed consentAged ≥18 years (no upper limit)Living with chronic headache: defined as headache on ≥ 15 or more days per month for at least the preceding 3 monthsThe nurse telephone classification interview confirms headache type to be chronic migraine, or chronic tension-type headache, with or without medication overuse headacheFluent in written and spoken English | Unable to attend the group sessionsNo access to a telephone (for classification interview)Has an underlying serious psychological disorder with ongoing symptoms that preclude or significantly interfere with participation in the group interventionPrevious entry or randomisation in the present trialCurrently participating in another clinical trial of headache treatments or unregistered medicinal product, or < 90 days have passed since completing participation in such a trial |
FIGURE 5.
Study flow chart. NMC, National Migraine Centre. Adapted with permission from Patel et al. 43 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

Nurses used the classification tool (see Figure 3) to confirm study eligibility and flagged any people with suspected non-eligible headaches for a second telephone interview with a doctor from the NMC. Participants classified as having an eligible headache type were then eligible for randomisation. If there was not a group an individual could attend, they were not randomised.
Our population of interest was people meeting an epidemiological definition of chronic headaches, that is, people with headaches for ≥ 15 days per month for at least 3 months. 8,10,11 At our classification day, it was decided to focus the trial just on people with migraine or tension-type headaches. People suspected of having other chronic headache types were directed to their GPs. For reporting we present three primary headache phenotypes:
-
definite chronic migraine – people meeting International Classification of Headache Disorder, third edition (ICHD-3) criteria for chronic migraine, that is at least 8 days per month with a migraine attack with or without aura9
-
chronic tension type headache and episodic migraine – people meeting ICHD-3 chronic tension-type headache and ICHD-3 criteria for episodic migraine
-
chronic tension-type headache.
Each group included those with and without medication overuse headache. Because not all migraine attacks meet the strict criteria for a migraine attack either because of early treatment or because they are mild,9 we report chronic migraine and chronic tension-type headache with episodic migraine together for our primary analysis. People meeting our definition of probable chronic migraine are typically managed in the same manner as people with definite chronic migraine and received the same advice within the trial.
In the feasibility study, 97 out of 103 (94%) of those assessed as having an eligible headache type had ‘chronic migraine’. 16 With the agreement of the Trial Steering Committee, the Data Monitoring Committee and the funder, we specified that, if at least 85% of our participants had ‘migraine’, that our primary analysis would just be on the population with ‘migraine’, with sample size inflated, if necessary, to ensure adequate statistical power for this analysis.
Between April 2016 and March 2018, we trained 30 facilitators. Quality assurance for the intervention was monitored in several ways, including observations of sessions, audio-recordings of sessions, participant feedback and facilitators’ personal reflections (see Report Supplementary Material 9).
The control intervention quality was assessed by keeping record of when packs had been sent to participants, any contact with control participants, attrition rates, when letters had been sent to the GP and date of classification interview.
Randomisation and masking
The unit of randomisation for this parallel-group study was the individual participant. The randomisation allocation ratio was 1 : 1.07 in favour of the intervention group to account for clustering in one arm. Randomisation was done using minimisation, stratifying by geographical locality (Midlands and Greater London) and headache type (chronic migraine, chronic migraine with episodic migraine or chronic tension-type headache only, with or without medication overuse headache). To maintain allocation concealment all baseline data were collected prior to randomisation. Randomisation was done using Warwick Clinical Trials Unit’s randomisation programme by a person independent of the research team. It was not possible to mask the study team, facilitators or participants from the treatment allocation.
Groups of four or five geographically proximate practices were clustered with the aim of starting recruitment around the same time in several practices. Participants were randomised in batches, with a target size of around 20 to ensure sufficient participants to populate a group and reduce any delay between randomisation and starting the intervention group. Participants unable to attend the group that they were originally allocated to were offered attendance at another group if available.
Participants were informed of their allocation by the study team via a telephone call. Written confirmation of the randomisation and headache classification was also sent to the participant and to their GP.
Post-randomisation withdrawals and exclusions
All participants were followed up when possible, and data were collected in accordance with the trial protocol until the end of the trial. No further data were collected for participants who explicitly withdrew their consent, and only the data collected up the point of withdrawal were used in the final analysis.
Outcome measures
Primary outcome measure
Our primary outcome was the HIT-6 score at 1 year. 33 It consists of six questions with five responses (never to always: score 6, 8, 10, 11 and 13 points). The score ranges from 36 to 78 points, with higher scores indicating greater headache severity (see Report Supplementary Material 10).
Secondary outcome measures
We used the CHQLQ as a secondary headache disability outcome. Our other secondary outcome measures were headache days in the preceding 28 days; typical headache duration and severity in previous 28 days; EuroQoL-5 Dimensions, five-level version (EQ-5D-5L);45 SF-12 version 2,46 reported as physical and mental component scores; Hospital Anxiety and Depression Scale (HADS);47 Pain Self-Efficacy Questionnaire (PSEQ);48 and Social activity: Social Integration Subscale (SIS) of the Health Education Impact Questionnaire (heiQ). 49
Baseline data collected included basic demographic data and data on the troublesomeness of other bodily pains. 50
We collected data on total headache days, average duration of headache and headache severity from participants weekly for 6 months and then monthly, starting from the initial eligibility call. These outcomes were collected according to patient preference using a smartphone app or paper diary records (not both).
We sent postal questionnaires at 4, 8 and 12 months. HIT-6 scores, headache days and EQ-5D-5L scores were, if needed, collected by telephone. To maximise follow-up rates, we used several strategies, including sending high street vouchers with each initial questionnaire and study pens with reminder questionnaires and a shorter questionnaire being sent as a second reminder (see Report Supplementary Material 11).
Sample size
We estimated the sample size using Moerbeek and Wong’s51 method, which accounts for clustering in one arm. Based on similar trials52 we used an intracluster correlation coefficient of 0.01.
The sample size (n = 689: relaxation arm, n = 333; self-management arm, n = 356) was estimated to assess the clinical effectiveness in the migraine population, providing 90% power to detect a target (worthwhile) between-group difference of 2 points (SD 6.87, from the feasibility study) in the HIT-6 outcome at 12 months using a two-sided test and a 5% significance level with 20% loss to follow-up. 14 Some support for this being a plausible effect size came from a pilot study53 of a similar intervention for migraine. Some overrun on sample size was expected to allow all groups to be adequately populated.
Primary and secondary analyses
The primary analysis approach was intention to treat. Data were summarised and reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines for RCTs. 54 Our statistical analysis plan is available in Report Supplementary Material 7.
Participant characteristics and outcomes were summarised as mean and SD for continuous data or frequency and percentage for categorical data, summarised by treatment arm. The median and IQR were presented if data deviated substantially from a normal distribution.
The primary end point was 12 months. For the primary and secondary analyses, treatment effects were estimated using linear mixed-effects models with partial clustering to account for the trial design with clustering in the self-management arm [command ‘mixed’ from Stata® (StataCorp LP, College Station, TX, USA)]. Analyses were adjusted for age, gender, the baseline value of the dependent variable and baseline stratification factors (type of headache and geographical locality). The adjusted treatment effect estimates and associated 95% CI s were presented for all analyses. All statistical tests were two-sided at the 5% significance level. Analyses were conducted using the statistical software package Stata 15 and R (The R Foundation for Statistical Computing, Vienna, Austria) version 4.0.3.
Complier-average causal effect analyses
We carried out complier-average causal effect (CACE) analyses for both levels of adherence for the primary outcome only. Minimal adherence was defined as attending day 1 of the intervention plus the one-to-one session. Full adherence was defined as the participant attending both days, plus individualised contact with the nurse.
Subgroup analyses
We carried out prespecified subgroup analyses using formal statistical tests for interaction to examine whether baseline anxiety (HADS anxiety score, ≤ 10 and > 10 points), depression (HADS depression score, ≤10 and >10 points) and severity (HIT-6 score, ≤ 64 and > 64 points) moderated treatment effect. 55
Symptom diary (total headache days, average duration of headache and headache severity)
These data were analysed using longitudinal analyses adjusting for the same variables as those used in the primary analyses (fixed effects) and participant as random effects.
Additional analyses
We assessed treatment effects in terms of the primary outcome for the whole population, including those with tension-type headache only, for chronic migraine and chronic tension-type headache with episodic migraine separately, and for those with or without medication overuse headache. These results will contribute to future meta-analyses and inform future guidelines.
Sensitivity analyses
We performed two sensitivity analyses: one excluding those interviewed for the process evaluation and another excluding those who reported <15 headache days on their baseline questionnaire.
Adverse events and serious adverse events
The frequency and percentage of adverse events (AEs) and serious adverse events (SAEs) in the trial are reported.
Clinical results
The results are in Appendix 15. 44 A detailed statistical report is available as Report Supplementary Material 12. A separate statistical report is also available for all 736 randomised participants, including those without migraine, as Report Supplementary Material 13.
Screening and recruitment
Between April 2017 and March 2019 we recruited from 164 general practices with a combined patient population of 1,529,684. Of the 32,998 potential participants identified from screening we approached 31,020 (94%). We received 2179 expressions of interest (including 41 self-referrals). We contacted 1871 (86%); of these, 1159 (62%) were eligible. Of these, 92 (8%) did not proceed because there were no suitable groups for them to attend and 785 of the remaining 1067 (74%) returned consent forms. We did classification calls with 751 (96%) of these. Nine people (1%) withdrew at this time. Six (<1%) were excluded because of a non-eligible headache (two cluster headache, two new daily persistent headache, one cervicogenic headache and one hemicrania continua; Figure 6). The final number of recruited participants was therefore 736 (including 27 self-referrals).
FIGURE 6.
Consolidated Standards of Reporting Trials flow chart. a, Participants withdrew from intervention only and continued to be on follow-up; b, Complete withdrawal. One withdrawn on the day of randomisation, and one 2 days after randomisation; c, cumulative number of complete withdrawals. Adapted with permission from Underwood et al. 44 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
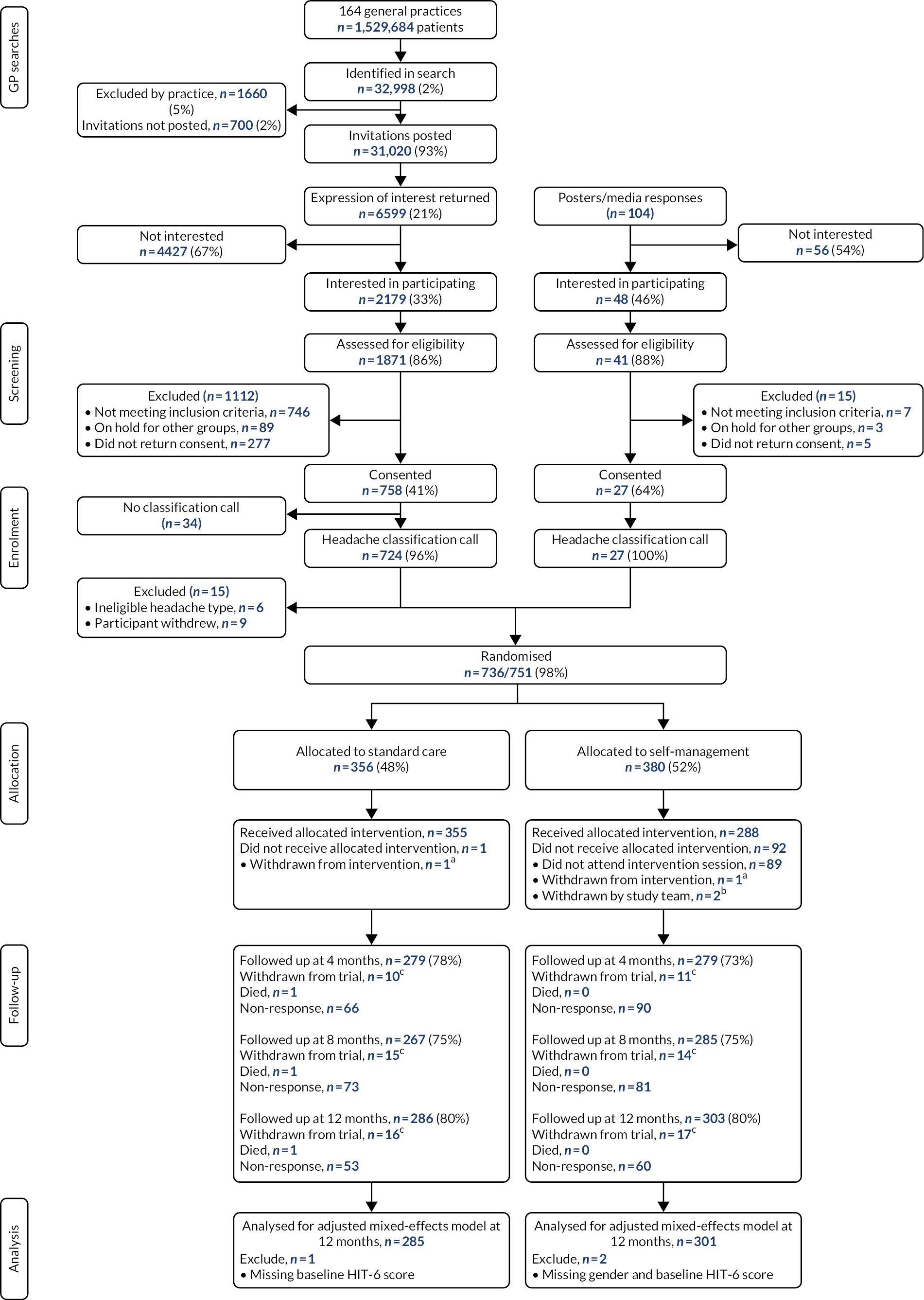
Of these, 727 (99%) had chronic migraine or chronic tension type headache and episodic migraine. Henceforth, unless otherwise specified, all the results refer to this group. Two participants were withdrawn from the trial by the study team soon after randomisation; one because they were living with someone already randomised to the trial, the other because the person made known that they had recently started in a trial of a calcitonin gene-related peptide (CGRP) monoclonal antibody for their headaches.
Participant baseline characteristics
Baseline characteristics were well matched between treatment arms. Most participants were white (586/727; 81%) and the majority were female (604/727; 83%) and the mean age was 48 years (SD 15 years). Just over half (396/727; 55%) had definite chronic migraine, 46% (331/727) had chronic migraine or chronic tension type headache with episodic migraine and 407 out of 727 (56%) had medication overuse headache. The median number of headache/migraine days over the last 4 weeks reported at baseline was 16 (IQR 11–20) days. Thirty-eight per cent (274/721) reported <15 headache days in the preceding 4 weeks.
We reached a diverse population that was representative of national averages in terms of ethnic mix and levels of deprivation, with a good mix of rural and urban areas.
Medication use was similar across both groups. At baseline, 662 out of 727 (91%) had used acute treatments, and 235 out of 727 (32%) had used prophylactic medications.
The overall mean HIT-6 score (primary outcome) at baseline was 64.5 points (scale range 36–78 points; SD 5.5 points), suggesting that most participants had severe symptoms. People with chronic migraine had greater headache severity, lower quality of life, less self-efficacy and less social interaction than those with chronic tension type headache and episodic migraine. Many participants had chronic pain other than their headaches: 375 out of 727 (52%) had at least moderately troublesome neck and 277 out of 727 (38%) had at least moderately troublesome back pain (see Report Supplementary Material 12).
Participant follow-up
Follow-up rates for the primary outcome were 76% (551/727) at 4 months, 75% (546/727) at 8 months and 80% (582/727) at 12 months. Three participants had missing baseline data. The primary analysis included data from 579 out of 727 (80%) participants. Thirty-two (4%) participants withdrew completely from the study including follow-up.
Adverse events
There were two SAEs, both deaths unrelated to the trial. There were seven AEs: five in the self-management arm that occurred during the intervention sessions and were related to developing a migraine or becoming upset during a session. Two were in the usual-care arm: one related to the content of the relaxation CD, and one participant became upset during a process evaluation interview.
Intervention data
We had 42 intervention groups, run by 20 facilitators, at 35 venues in the Midlands and London. Median group size at randomisation was nine (IQR 7–12) and median attendance on day 1 was 6.5 (IQR 5–9). The first session was attended by 286 out of 376 (76%) of those randomised, 259 out of 376 (69%) achieved partial adherence and 216 out of 376 (57%) full adherence (see Report Supplementary Material 12).
Effect of COVID-19
One-year follow-up was due to be completed soon after the UK national lockdown on 23 March 2020. Inability to access the office during this time meant that we were unable to manage reminder questionnaires. For this reason, more 12-month core outcomes were collected by telephone. The COVID-19 pandemic also made it impossible for the study team to visit general practices to collect data for the health economics analyses. We developed new processes to allow practice teams to extract these data remotely on our behalf.
Primary outcome: six-item Headache Impact Test
We found no evidence of a positive effect at 12 months, the primary end point (mean difference –0.3 points, 95% CI –1.23 to 0.67 points), or at 8 months (mean difference 0.07 points, 95% CI –0.95 to 1.09 points) (see Table 2). At 4 months participants in the self-management support group had statistically significantly lower HIT-6 scores (better headache-related quality of life: mean difference of –1.0 point; 95% CI –1.91 to –0.006 points) than participants in the standard-care group. The intracluster correlation coefficients were very small (< 0.0001) in all our analyses. The findings from our CACE analyses, sensitivity analyses and additional analyses were not materially different. There was no evidence of treatment effect modification in our prespecified subgroup analyses.
| Model | Time point (months) | ||
|---|---|---|---|
| 4 | 8 | 12 | |
| ITT | |||
| Mean difference (95% CI) | –1.0 (–1.91 to -0.006) | 0.07 (–0.95 to 1.09) | –0.3 (–1.23 to 0.67) |
| p-value | 0.049 | 0.888 | 0.560 |
| CACE (minimum adherence) | |||
| Mean difference (95% CI) | –1.3 (–2.57 to -0.02) | 0.04 (–1.22 to 1.31) | –0.4 (–1.67 to 0.87) |
| p-value | 0.046 | 0.945 | 0.540 |
| CACE (full adherence) | |||
| Mean difference (95% CI) | –1.6 (–3.10 to -0.01) | 0.05 (–1.46 to 1.56) | –0.5 (–2.00 to 1.05) |
| p-value | 0.048 | 0.945 | 0.540 |
Secondary outcomes
There was no evidence of a difference in headache days between the groups at the 8-month and 12-month follow-ups. However, at 4 months participants in the self-management support group reported 1.5 (95% CI 0.48 to 2.56) more headache days in the previous 28 days than participants in the standard-care group. The mean difference of EQ-5D visual analogue scale score at 12 months was 3.9 points (95% CI 0.90 to 6.88 points), favouring the self-management support group, but there were no statistically significant differences at 4 or 8 months. At 4 and 12 months, but not at 8 months, those in the self-management group had stronger self-efficacy beliefs as measured by the PSEQ. The mean differences were 2.3 points (95% CI, 0.51 to 4.00 points) and 2.1 points (95% CI 0.17 to 3.96 points) at 4 and 12 months, respectively. We did not find any differences in the role restrictions, limitations or emotional impact domains of the CHQLQ, EQ-5D-5L, the SF-12 mental and physical component scores, the HADS, or the SIS of the heiQ at any time point (see Figure 7).
FIGURE 7.
Treatment differences and 95% CI s for secondary outcomes adjusted for age, gender, baseline value of the dependent variable, headache type and geographical locality at the 4-, 8- and 12-month follow-ups. MCS, mental component score; PCS, physical component score; SF-12/36, Short Form questionnaire-12/36 items. Adapted with permission from Underwood et al. 44 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
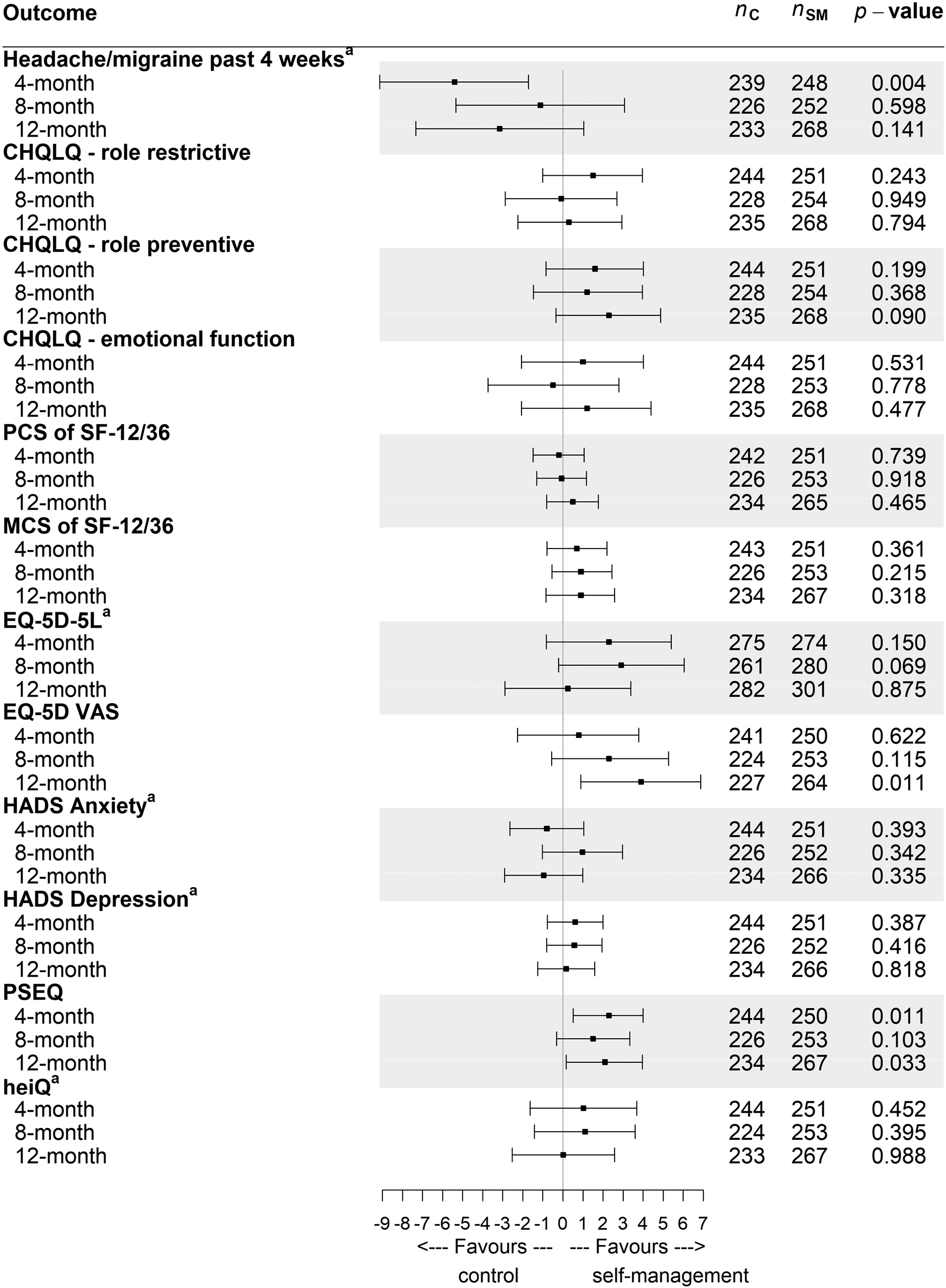
At 8 months the mean headache duration in the self-management group was 9.2 hours (SD 7.3 hours), whereas the mean duration in the standard-care group was 8.0 hours (SD 6.9 hours) (difference, 2.0 hours; 95% CI 0.55 to 3.42 hours). There were no statistically significant differences at 4 or at 12 months (see Report Supplementary Material 12).
Estimates and 95% CI rescaled to range from 0 to 100 for graphical representation purposes only (see Figure 7). To obtain the estimated difference and its 95% CI in its original scale, the value from graph is multiplied by (maximum value/100). For example, the estimated difference for the HADS anxiety score at the 4-month follow-up was (−0.801 × 21/100) −0.16821. Full details of results are available from Appendix 15 and Report Supplementary Material 12).
Medication use
At baseline, 91% of participants were using acute treatments (painkillers/triptans) and 32% were using preventative medications. There was no change over time or any between-group differences in proportions using acute prescribed and over-the-counter acute medications or prophylactic medications. Neither were there any statistically significant between-group differences in the defined daily doses used by those using acute and prophylactic drug treatments.
There were two statistically significant differences in drug use in those reporting use of that group of drugs; more defined daily doses of beta-blockers (p = 0.005) at 4 months and fewer defined daily doses of opioids (p = 0.02) were used at 8 months in the self-management support group (see Appendix 15 and Report Supplementary Material 12).
There were no differences in proportions using acute medications for ≥10 or ≥15 days in the last 28 days at any time point. Overall, at 12 months, 43%, and 21% participants, respectively, used painkillers/triptan for headaches on ≥10 or ≥15 days out of the last 28 days. This compares with 63% and 38%, respectively, at baseline (see Report Supplementary Material 12).
Second-line prophylactic drugs [Botox® (AbbVie Inc., North Chicago, IL, USA) and CGRP monoclonal antibodies] were used by five participants. Four received Botox injection (self-management support group, n = 2; standard-care group, n =2) and two people from the self-management support group were prescribed erenumab. One participant received both Botox and erenumab.
Headache symptom diary
No statistically significant differences were observed between the two groups from the longitudinal analyses for any of these outcomes. The estimated between-group difference for the number of headache days over 1 year was 0.2 (95% CI –0.11 to 0.46) days, for the duration of headache the estimated difference was 0.4 (95% CI –0.47 to 1.28) hours and for headache severity the estimated difference was 0.2 (95% CI –0.08 to 0.46) points on a 0–11 scale.
Health economics methods
Health economic analyses
Our health economic analyses are reported in more detail in Appendix 16. For a full report of our economic analyses, see Report Supplementary Material 14.
We did a prospective within-trial economic evaluation to estimate the cost-effectiveness of the CHESS intervention. For costs we used 2019 Great British pounds and for outcomes we used quality-adjusted life-years (QALYs). Our base-case analysis was conducted from the perspective of the NHS and Personal Social Services (PSS). 56
We estimated resource use using the intervention costs, calculated in a micro-costing exercise, and NHS health and social care costs estimated from participant questionnaires and general practice records. We derived the unit costs of community health and social services from the Unit Costs of Health and Social Care 2019,57 published by the PSS Research Unit. Drug costs were estimated from the Prescription Cost Analysis58 and the British National Formulary. 59
For our analyses we converted the EQ-5D-5L into health utilities based on the UK tariff for the EQ-5D-3L using the van Hout et al. 60 and Hernandaez-Alarva and Pudney61 crosswalk algorithms. 60,62,63 We used the van Hout et al. 60 crosswalk method for our base-case analysis. For our sensitivity analyses, we used the Hernandaz-Alava and Pudney61 method to estimate QALYs from the ED-5D-5L and Brazier and Roberts’64 algorithm to generate these from the Short Form questionnaire-12 items (SF-12).
To account for missing data we used multiple imputation by chain equations implemented through the R package MICEmic65 assuming that data were missing at random. We imputed missing costs and health utility values at the level of resource category and health-related quality of life assessment, stratified by intervention arm. 66 We pooled parameter estimates across 50 imputed data sets using Rubin’s rules. 67
Base-case cost-effectiveness
Our base-case cost-effectiveness analysis estimated the cost–utility of the CHESS intervention compared with usual care from the perspective of the NHS and PSS. We calculated economic costs and QALYs for each patient over a 12-month post-randomisation time horizon. We calculated total costs by summing costs associated with the delivery of the intervention and utilisation of broader hospital- and community-based health and social care services.
We fitted bivariate generalised linear mixed-effects regressions assuming a gamma distributed error structure and logarithmic link function to imputed data in R using methods for cost-effectiveness analyses of cluster-randomised and multinational trial data. The models account for the within- and between-cluster correlation between costs and effects measured from the same individuals.
We calculated the incremental cost-effectiveness ratio (ICER) for the CHESS intervention compared with standard care by dividing the between-group difference in adjusted mean total costs by the between-group difference in adjusted mean QALYs. We calculated the incremental net (monetary) benefit of the intervention compared with usual care for cost-effectiveness thresholds ranging from £15,000 to £200,000 per QALY gained.
We estimated the uncertainty of our cost-effectiveness estimates using the Monte Carlo method using 2000 bootstrapped replications. 68 We did the following sensitivity analyses:
-
QALYs generated from EQ-5D-5L utilities using the Hernandez-Alava and Pudney61,62 crosswalk function
-
utilities generated from via the SF-12/SF-6D tariff for the UK64
-
costs calculated from a societal perspective
-
unadjusted analysis of the multiple imputation data
-
adjusted complete-case analysis.
We did the following subgroup analyses:
-
medication overuse (yes vs. no)
-
geographical locality (London vs. Midlands)
-
gender (female vs. male)
-
age (<40 vs. ≥40 years).
Health economics results
The acquisition cost of the CHESS intervention was £266.95 per participant. In our base-case analysis the ICER was £8617. There was an 83% probability that the CHESS analysis was cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained. This finding was robust to either of the EQ-5D-5L algorithms. However, using data from the SF-12 the ICER was £32,083. From a societal perspective the ICER was just £765 (see Table 3).
| Analysis | Incremental estimates (95% CI) | ICER (£) | |
|---|---|---|---|
| Costs (£) | QALYs | ||
| Base casea | 268 (176 to 377) | 0.031 (–0.005 to 0.063) | 8617 |
| EQ-5D-5L,a Hernandez-Alava and Pudney61 | 269 (170 to 388) | 0.028 (–0.001 to 0.055) | 9535 |
| SF-12 (SF-6D) utilitya | 269 (162 to 399) | 0.008 (–0.02 to 0.035) | 32,083 |
| Societal costsa | 25 (–702 to 1231) | 0.033 (–0.001 to 0.063) | 765 |
| Unadjusted analysis | 229 (82 to 432) | 0.033 (–0.112 to 0.127) | 6895 |
| Adjusted complete-case analysisa | 321 (202 to 465) | 0.017 (–0.01 to 0.042) | 18,968 |
In our subgroup analyses we found lower ICERs for those aged ≥40 years, females, those with medication overuse headache, and those living in the Midlands (see Appendix 16, table 3).
Process evaluation
This process evaluation protocol and results are available in Appendices 17 and 18,69,70 and as an archived full report available in Report Supplementary Material 15, prepared ahead of the main results being available. For the process evaluation we have included all 736 randomised participants.
The aims of the process evaluation of the main trial were to:
-
assist in the interpretation of the results of the main effectiveness trial
-
develop a set of transferable principles regarding the intervention to inform its implementation on a wider scale, if the intervention proves to be effective.
Methods
We used a mixed-methods approach. Quantitatively, we described reach/context, recruitment, dose delivered, dose received and fidelity. Qualitatively, we explored the experiences of participants, intervention facilitators and GPs about their involvement in the trial (see Table 4).
| Key process evaluation components | Source of data | Type of data |
|---|---|---|
| Reach and context | NHS GP practice data and trial data | Practice numbers and location. Census and national statistics |
| Recruitment | Trial recruitment data | Routine trial dataSample of expression of interest forms from those who declined to participate |
| Dose delivered | Trial intervention delivery records | Groups delivered/not delivered and whyLocation of groups |
| Dose received | Trial intervention attendance sheetsTrial data | Attendance dataReasons given for not attending |
| Fidelity | Intervention group audio-recordingsParticipants one-to-one consultation forms GP feedback forms | Audio-recording data10% form completion check for adherence |
| Impact of intervention | Participant interviews | Interview transcripts |
| Experience of participating in the trial | Staff interview/focus groups Participant interviews Participant feedback forms GP feedback forms | Intervention staff focus group notes and recordings Patient interview recordings/transcripts Participant feedback GP feedback |
Results
Reach/context
The intervention team delivered 42 (2-day) group sessions. Of the 380 (migraine plus tension-type headache) participants allocated to the 2-day group sessions, 288 (76%) attended at least part of the 2-day course, and 92 (24%) were not exposed to the CHESS intervention at all. Of the 288 who did attend the group sessions, 227 (79%) attended both days, whereas 61 (21%) only attended day 1. Of the 288 who took part in at least one session, 261 (91%) had a one–to-one interaction with the nurse. Overall, 261 out of 380 (69%) participants achieved the predefined minimum dose (attended at least some of the course and the one-to-one discussion with the nurse). Only 217 (57%) fully adhered to the intervention. Intervention fidelity was good, with adherence being slightly better than competence [adherence, 83% (IQR 67–100%); competence, 70% (IQR 50–90%)].
Assessment of a 10% (n = 27) random sample of the case report forms completed during the one-to-one sessions found that the sessions were fully completed as required by the protocol.
Written feedback on the 2-day group session and the one-to-one session with the nurse was provided by 117 participants. There were high levels of satisfaction with the course overall and with the facilitators, although there were lower satisfaction scores for the venues, the relaxation and taster sessions, and the mindfulness session. Similar views were expressed during interviews.
During focus groups, intervention facilitators reported that they found some sessions more challenging to deliver than others, notably sessions on acceptance, impact of thoughts mood and emotions on headaches, mindfulness and relaxation for headaches, medication management and managing setbacks.
Twenty-eight participants took part in the interview study exploring their experience of the trial (self-management support group, n = 17; standard-care group, n = 9; randomised to intervention but did not attend any sessions, n = 2) soon after receiving the intervention and 12 months after randomisation. In the first interview, all participants were generally positive about the intervention they experienced. Group participants liked the group format and valued meeting with others to share information. Those with advanced headache knowledge found the group confirmatory, but they were positive about the opportunity to discuss experiences with others. Some interviewees felt that people earlier in their headache trajectory might have more to gain from the intervention. There were people, all with high levels of knowledge about headache, who would have liked a session on where to find information about cutting-edge treatments. The most popular sessions were on lifestyle, stress and anxiety, and sleep, as interviewees felt that they had gained understanding of how these may affect headaches. Many participants did not like the sessions on mindfulness and relaxation for headaches and on managing setbacks. Participants also valued being able to review their headaches and management at the one-to-one sessions with the nurse.
Interview data at 12 months indicated that, although some participants had made changes to how they managed their headaches, such as changing medications and recognising triggers, most had changed very little. Among those who had made little change, there were some for whom life had become more problematic with family issues, work and other health issues.
Conclusion of process evaluation
The process evaluation suggests that CHESS reached a diverse population across different geographical settings. Attendance reached our predefined dose, but many participants were not exposed to the intervention. The interventions were delivered with fidelity and, although generally well received, with some sessions liked more than others, both intervention facilitators and participants had reservations about several components of the course. There is evidence that participants valued the group and one-to-one aspects of the intervention giving them the opportunity to explore and review their headaches and its management. The process evaluation provides no clear explanation as to why the CHESS intervention appears ineffective.
Patient and public involvement
There has been substantial PPI contributing to the design, conduct, and interpretation of the CHESS programme. This is reported in detail in our published paper (see Appendix 19). 71
Throughout the programme we worked closely with three UK migraine charities and a lay advisory group to help direct the research and ensure that the patient voice had been embedded in our work. We used guidance from the National Institute for Health and Care Research (NIHR) standards for public involvement to help support us in establishing and implementing PPI. 72 We started from the premise that PPI representatives were full members of the team and we sought, as far as possible, to reduce power differential. 73 We sought to work in an environment of mutual respect and with each team member valuing others’ contributions.
Our lay patient partners were paid for their time and travel; we reimbursed our charity partners for their travel. We summarise the PPI contribution throughout the trial.
Development of research idea
The original research question, ‘Will an education and self-management programme help people living with chronic headache?’, is based on a research recommendation in the 2012 NICE guidelines on the management of headaches. 5 Members of the lay guideline development group helped identify the research question.
Grant application
Our co-applicants included representatives of the three leading UK migraine charities. The involvement at this stage was from our charity partners. It did not include direct patient involvement.
Establishing a lay advisory group
We established a lay advisory group, from our larger lay reference group, at the start of the programme. We sought to establish a group of 8–10 people living with chronic headaches. Members needed access to e-mail but not specific skills. Our charity partners approached a diverse group of people with chronic headaches. We also approached people through a University of Warwick PPI initiative. We contacted responders to identify their headache type and basic demographic details. Ten people joined the CHESS lay advisory group. This group then developed their own rules of engagement (see Appendix 19, box 2). 71
Trial oversight
We invited representatives of each of the three charities to our monthly trial management groups. The charities found it difficult to commit their staff to meeting times. Our independent PSC had two lay members who contributed to eight meetings over the duration of the programme. One lay representative contributed to our Data Monitoring Committee.
Feasibility study research ethics application
When we sought research ethics approval for the feasibility study our lay advisory group was not established. Our charity partners helped us to find five people who critically reviewed our patient-facing documents.
Headache classification day
Our lay reference group contributed to the classification day, ensuring that the key questions generated were relevant to people with chronic headache and health professionals. Seven lay people attended on the day.
Electronic data collection
The CHESS lay advisory group helped to refine the questions used in the electronic diary and provided feedback on the acceptability, the user experience and the app. A suggestion from our lay advisory group included the addition of a calendar indicating the period of recall.
We provided participants with a summary of their diary data once data collection was complete. The group commented on, and made recommendations to improve, the individual data summary sent to main trial participants at the end of their data collection period.
Intervention design and development
Seven members of Migraine Action took part in an individual, face-to-face interview about their headaches and the treatments they had tried, to inform the intervention development.
Our intervention development day was chaired by one of our professional charity partners and attended by three lay representatives, suggested by our charity partners.
Patient-reported outcome measures
During the feasibility study three members of the lay advisory group helped analyse data from interviews exploring participants’ views on the relevance, clarity, comprehensiveness and acceptability of four PROMs.
Core outcome set for migraine
Three PPI members actively collaborated in all stages of the core outcome set for migraine (COSMIG) project, allowing co-development of a three-stage international Delphi survey, subsequent data interpretation, co-facilitation of the consensus meeting and co-authorship of the subsequent publication. 39
Public and media engagement
Following a university press release we were invited onto The Victoria Derbyshire Show (BBC News, London, UK). We were represented by one of our lay members and the chief investigator. Our lay member was able to speak about their experience of chronic headaches and the impact this had on their life. This generated public interest in CHESS.
Post-results patient and public (lay) discussion group
Once the trial outcome was known we sought an additional patient and public perspective on the findings. We ran a 2-hour virtual discussion group with 10 people drawn from the CHESS reference group. The process evaluation team presented an overview of the process evaluation results followed by the trial outcomes. The group were disappointed with the outcome but not completely surprised. Although the CHESS intervention provided useful knowledge and tools, the group thought that developing new skills and behaviours takes time. Participants thought a longer intervention may be needed to reinforce learning and to support behaviour change.
Patient and public involvement learning points
Our thinking on patient involvement has developed substantially since we submitted the first application for funding in June 2012. At that time, we had planned a formal evaluation of PPI in the programme. We were asked to remove this by the funding board at the outline stage. This does mean we have missed the opportunity to do a more in-depth prospective evaluation. Nevertheless, we have identified some key learning points:
-
More training in the research process for both our lay and professional PPI partners.
-
Lay input, in addition to charity input, into the development of the grant may have provided a different perspective.
-
A designated team member to be the PPI contact could have improved engagement.
-
Regular correspondence to update the lay advisory group throughout the duration of the programme.
Discussion
The CHESS programme has been a substantial amount of work over several years. We have advanced our understanding of the challenges of living with chronic headaches and made some progress in developing the methodology for running RCTs of complex interventions for people living with chronic headaches. Specifically, we have:
-
reviewed and added to the qualitative literature on the experience of living with chronic headaches
-
demonstrated that we can recruit people from the community for trials of headache treatments
-
developed an approach to classifying headache disorders using a nurse telephone interview
-
reviewed the existing literature on quality-of-life assessment for people with chronic headache
-
developed a core outcome set for people living with migraine
-
developed a mapping algorithm to improve health economics analyses in headache studies
-
reviewed the literature on prognostic factors for people living with chronic headaches
-
validated a patient-centred outcome measure for studies of people living with chronic headaches, as opposed to people living with migraine.
Nevertheless, our intervention had no meaningful impact on headache-related quality of life or headache days. This is intensely disappointing for the research team and, more importantly, we do not have anything new to offer people living with what can be a profoundly debilitating, and poorly understood, disorder.
In phase 1 we did all our preparatory work for the planned RCT. This can be broadly divided into new fieldwork and systematic reviews. The fieldwork demonstrated that is possible to recruit people with headache disorders from general practice, providing participants to evaluate our classification interviews, our package of outcome measures and pilot our intervention ahead of the main RCT.
We found in our systematic reviews ample evidence that the disability caused by chronic headaches fits within the same broad theoretical framework used to approach other chronic painful disorders. Although the available literature was sparse, our review of qualitative studies had resonance with that found in other chronic pain disorders such as LBP. It is perhaps surprising that we identified only four qualitative studies of the experience of living with chronic headaches. This contrasts with 187 such studies, largely of chronic musculoskeletal pain, found in a review of reviews of the experience of living with chronic pain. 74
There is a need for further work to explore the experience of living with, and receiving care for, chronic headaches. In our review of prognostic factors, we identified potentially modifiable prognostic factors that are common to the wider pain literature: anxiety, depression, poor sleep, stress and poor self-efficacy. 21,23 Further evidence supporting the relationship between chronic headaches and chronic musculoskeletal pain comes from our review demonstrating the association between primary headache disorders and persistent LBP. 42 Looking more widely at the headache literature, including episodic and chronic migraine and tension-type headache, we found evidence that self-management interventions could have positive effects on headache-related disability, mood and pain intensity, but not on headache frequency. 22 A subsequent Cochrane systematic review75 of psychological therapies for episodic or chronic migraine, however, concluded that high-quality research to determine if psychological therapies were effective was absent. The evidence for the use of self-management support programmes for chronic musculoskeletal pain is mixed. 25,76 Nevertheless, we had sufficient evidence to consider that a self-management support intervention based on psychological principles might be effective for our population of interest. We were also anticipating that the provision of a diagnostic classification, advice on specific drug treatments for migraine and addressing medication overuse would enhance the overall effect.
Our review of patient-reported outcomes identified a limited evidence base for patient-reported outcomes for use in headache studies. 26 Only the HIT-6 had sufficient evidence to support its use in a mixed headache population. We therefore adapted the MSQ v2.1 as a new measure, the CHQLQ, that is about headaches in general. We demonstrated that it had good measurement properties. This may be a better measure than the HIT-6 for trials for studies of people with chronic headaches.
Our work on COSMIG identified that headaches days and headache-related quality of life should be given equal standing. 39 For these reasons, it was important to look again at how to measure patient-centred outcomes for our trial. When this work was done, an additional migraine-specific measure became available: the Migraine Functional Impact Questionnaire. This has superior measurement properties to the MSQ v2.1 and was also seen to have greater face validity by our patient partners. 77 For these reasons we are recommending this as part of the core outcome set for migraine studies.
As is usual in NIHR trials we included the EuroQoL-5 Dimensions (EQ-5D) to allow the calculation of health utilities for our health economics analysis. 44 We had concerns about the use of this in our population because of the unpredictable and rapidly changing pattern of headache disorders. The EQ-5D measures health state ‘today’ and does not take into account the, sometimes large, differences in health states within, or between, days. In our systematic review of PROMs we found only limited evidence to support the use of EQ-5D in headache disorders. 26 We did find acceptable evidence of construct validity for the Short Form questionnaire-36 items78 and some limited evidence for the SF-12. 46 The longer measurement period of the short-form suite of measures may make them a more appropriate outcome measure. In the light of our concerns about the EQ-5D we included the SF-12 in our final package of outcome measures to also allow us to calculate health utility from the SF-6D. In our mapping study we were to show that health utility values for economic evaluations could be predicted from the HIT-6 or the CHQLQ.
Overall, our work on outcome measures for headache studies has informed the approaches we should use to measure outcomes in headache trials. Importantly we have shown that patient-centred outcomes that assess quality of life need to be given equal weighting with headaches days in future trials of interventions for headaches/migraine. We have identified, and for chronic headaches validated, appropriate outcomes that can be used in future studies. By involving patients in all stages of this work we have been able to ensure its relevance to our population of interest.
The core of phase 2 was our RCT. Because we exceeded our planned sample size, and the effects of clustering in the intervention arm were negligible, we had ample statistical power to identify any clinically important between-group differences. There was no evidence of any positive effect on clinically relevant outcomes at 12 months, when we measured our primary outcome, or at 8 months, with the limits of the 95% CI excluding our target (worthwhile) effect size. Others, in a study of episodic migraine, have suggested that the minimal clinically important between-group difference for the HIT-6 should be 1.5 points. 79 Even against these stricter criteria, not directly applicable to our population, we have excluded a worthwhile benefit at 12 months. Neither did any of our sensitivity, or pre-planned subgroup, analyses at 12 months show a possible benefit. However, there was a small beneficial effect on HIT-6 at 4 months (shortly after the individualised one-to-one nurse consultation and follow-up of self-management programme), around half of our target (worthwhile) difference of 2 points. However, the 95% CI limits effectively excluded any possibility of achieving the target difference: –1.0 points (95% CI, –1.91 to –0.006 points). At the same time point there was also an increase in headache days (mean 1.5 days, 95% CI 0.48 to 2.56 days) in the past 28 days. Findings from the overall analysis of 736 randomised participants were not materially different.
It is possible that the HIT-6 was not the best primary outcome measure to use for this trial. We would now prefer the CHQLQ to better capture the overall impact of chronic headaches. However, no difference was seen on any of its three domains at any time point.
Among our secondary outcomes for pain self-efficacy, there was a treatment benefit at 4 and 12 months but not at 8 months. It is possible this indicates that our intervention affected one of our main intermediate outcomes without feeding through as patient benefit, although these may also be chance observations because of the large number of comparisons made.
It was disappointing that only three-quarters of those randomised to the self-management support intervention attended any sessions. All participants had confirmed availability for sessions prior to randomisation. Poor attendance is common in trials of group interventions for chronic pain. In two similar previous studies we observed non-attendance rates of 11%80 and 17%. 25 However, the minimal adherence rate observed in CHESS (69%) compares favourably with that observed on our two previous studies (70%25 and 63%). 80 The effect sizes observed in our CACE analysis did not differ materially from the primary analysis, suggesting that poor attendance was not the explanation for our disappointing findings.
Surprisingly, although there was only a modest delay (median 8 days) between being assessed for study entry, when the presence of chronic headache was established, and completion of the baseline questionnaire, just 62% of those randomised reported headaches on at least 15 of the previous 28 days. This might reflect some response shift in questionnaire completions, or short-term variability in headache days. 13,81 In practical terms, it is the population we recruited that would be offered this intervention if it had been successful. So this is not of great concern.
Another important aspect of the preparatory work we did for our trial was to develop an approach to classification that allowed us to phenotype our trial entrants. Although many headache classification tools are available, we did not find any fit for our purpose. 15 We needed a tool that would both screen out people with headache disorders other than migraine and tension-type headache, and positively phenotype those with migraine. We therefore needed to develop our own tool. These classification interviews were not a substitute for a full diagnostic consultation informed by a prospectively completed headache diary. Nevertheless, at the point in the care pathway where there was a need to identify those who might need further consideration of headache disorders other than migraine or tension-type headache, and to identify those likely to have migraine, they represented the unstructured approach typically used in primary care. Our approach has the potential for implementation in primary care. Beyond this current grant we have secured additional Programme Grants for Applied Research (PGfAR) Programme Development Grant (PDG) funding from NIHR (PGfAR PDG NIHR202614) to adapt this for online use by people with headaches to allow self-classification of migraine.
Developing our intervention programme was another success for the feasibility stage. Finding that we could not use lay co-facilitators was disappointing, but finding this out in the feasibility phase allowed adaption for the main study.
After performing analyses of multiple outcomes at multiple time points and including multiple sensitivity and subgroup analyses, we could not find any evidence of any clinically relevant positive effects from the CHESS intervention. We have conclusively demonstrated that our intervention is ineffective. This is a surprising finding, as the CHESS intervention had a good theoretical underpinning, targeted modifiable psychological variables and was well regarded by the participants (see Appendix 18). Furthermore, the educational and classification components of the intervention should have led to increased use of prophylactic medications and a reduction in medication overuse.
The control intervention was more than just usual care: we gave the results of the classification interview to participants and their GPs. If people in the control group used medication more appropriately as a consequence of this information, then this might have reduced the apparent effect size. That there was no difference in the use of prophylactic medications in either group over time makes this unlikely.
Our base-case cost-effectiveness analysis shows a high probability that the CHESS intervention is cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained. This finding is robust in a sensitivity analysis using the Hernandez-Alava EQ-5D-5L conversion algorithm,61 but not when utilities were calculated from SF-12 data. This may reflect tentative evidence in the external literature that suggests that the EQ-5D generates larger utility gains associated with improvements in headache-related outcomes. 82,83 Before starting this work we had concerns that the EQ-5D might not be the best measure of health utility to use in a headache population because its 1-day measurement window would not adequately capture substantial day-to-day, or within-day, fluctuations in health state affecting people with migraine. Data derived from the SF-12 might be more stable. Whatever the explanation for this difference, it does raise the possibility that our base-case analysis may be overestimating the cost-effectiveness of the CHESS intervention. Conversely, the much reduced ICER found when we used a societal perspective suggests that the CHESS intervention might represent better value for money than our base-case analysis if a different perspective is used.
It is not clear why the CHESS intervention appears to generate additional QALYs when it has no meaningful effect on our headache-specific outcomes. It is possible that the EQ-5D-5L, but not the SF-12, is measuring non-specific effects from attending the CHESS intervention not measured using headache-specific outcomes. Or it might be that there is an early benefit, evidenced by the 4-month HIT-6 findings, that is having proportionally larger impact in the area under the curve analysis. Although these health economic findings are important, it is not clear how they can be used to inform treatment choices in the absence of clear clinical benefit.
Not all trials of complex interventions have a process evaluation run independent from the main trial. This work has given us good insights into the experiences of people living with chronic headaches and of their experiences within the trial. Although this process evaluation was thorough, it did not provide us with any insights as to why the intervention was ineffective.
The dose delivered by the intervention was good, in that sessions were delivered as planned. Intervention fidelity was good, and consistent with that observed for other, similar, interventions. 14
The premise of our intervention was that those living with migraine had the potential to improve their experience of headache through what they chose to do or not do. Our results add to the evidence that this is not the case. This may come as a relief to those living with migraine, as it reduces the burden on them to control a problem that our process evaluation indicates can feel uncontrollable. It allows migraine to be recognised as an intermittently and unpredictable disabling condition that requires a flexible response from society.
It is unclear why a multifaceted, theory-driven (best-practice), evidence-based intervention, specifically designed for those with chronic headache conditions, that was well delivered and well received failed to make a difference.
Patient and public involvement has helped shape the overall design and development of this programme of work. It has provided important input into the style and content of our intervention, and how we should measure and assess patient outcomes in chronic headache disorders. We have been able to reflect on our experiences of PPI in the research programme. Our thinking has, of course, developed over the lifetime of the CHESS research programme. For the future we think that we can make our processes more efficient and ensure that PPI is rewarding and supportive for all those involved.
Reflections on what was and what was not successful in the programme
Overall, the programme was delivered well, and we achieved our overarching aim of developing and testing a self-management support programme for people living with chronic headaches. We successfully undertook a series of systematic reviews to set the scene for the rest of the work. Our feasibility study was complicated as we were seeking to achieve multiple objectives within the same framework. This did make it difficult to explain to participating general practices and study participants. Recruitment rates for the main trial from general practice were smaller than originally thought. In our original application we thought we would need to work with 30–40 general practices. In the end we needed to work with 164 practices.
Limitations relating to the method or execution of the research
This was an ambitious programme of work that in phase 1 needed integration of interlinked work packages. The original timelines were, in some areas, too ambitious, needing some changes as to how we collected data, notably recruiting for some interviews through our charity partners rather than from feasibility study participants. In addition, work on validating the CHQLQ was not completed prior to starting the main trial, meaning that we set the HIT-6 as our primary outcome, although our view now is that this is not the most suitable outcome for studies of this nature. This has not, however, affected our final conclusion as the results from the two measures are consistent. The COVID-19 pandemic had a limited impact on the trial. Follow-up finished early in the first wave of the pandemic, meaning that we had some reduction in the data available for our secondary outcomes and we needed to make changes to how we collected data from GP surgeries.
Conclusions from the whole programme
Our data effectively exclude the possibility that this short intervention is effective for the treatment of people with chronic migraines, or chronic tension-type headache and episodic migraine. Nevertheless, the health burden of chronic headache disorders, principally chronic migraine, is debilitating. Further advances in this field must be driven by new theoretically and/or biologically informed intervention models.
Recommendations for future research
-
New work to better understand the health impact of chronic headache disorders and to identify modifiable risk factors for a poor outcome.
-
Development and testing of new non-pharmacological interventions for a tightly phenotyped group with chronic migraine.
-
Work is needed to improve classification of headache disorders in primary care to allow better targeting of the available drug treatments of proven effectiveness, and reduce medication overuse.
Implications for practice and any lessons learnt
There is no need, at this time, to implement formal supportive self-management support programmes, of the type tested here, for people living with chronic migraine.
Acknowledgements
Trial team
-
Felix Achana (Associate Professor).
-
Dawn Carnes [Director: National Council for Osteopathic Research (UK)].
-
Sandra Eldridge (Professor).
-
David Ellard (Associate Professor).
-
Barbara Gougoulis (Administrator).
-
Frances Griffiths (Professor of Medicine in Society).
-
Elizabeth Habershon (Research Nurse).
-
Kirstie Haywood (Reader).
-
Siew Wan Hee (Senior Research Fellow).
-
Helen Higgins (Senior Project Manager).
-
Sobhash Jhuree (Research Nurse).
-
Manjit Matharu (Professor).
-
Hema Mistry (Associate Professor).
-
Dipesh Mistry (Trial Statistician).
-
Sian Newton (Trial Manager).
-
Vivien Nichols (Research Fellow).
-
Chloe Norman (Trial Co-ordinator).
-
Emma Padfield (Senior Project Manager).
-
Shilpa Patel (Senior Research Fellow).
-
Stavros Petrou (Professor).
-
Tamar Pincus (Professor).
-
Rachel Potter (Senior Research Fellow).
-
Harbinder Sandhu (Professor).
-
Kimberley Stewart (Trial Manager).
-
Stephanie Taylor (Professor).
-
Martin Underwood (Professor).
Other co-applicants
-
Giles Elrington.
-
Brendan Davies.
-
Joanna Hamilton-Colclough.
-
Mary Bright.
-
Wendy Thomas.
Trial Steering Committee
-
David Fitzmaurice.
-
Simon Kyle.
-
Louise Longworth.
-
Andrea Manca.
-
Stephen Morley.
-
Allison Ogden-Newton.
-
Fran Reynolds.
-
Leone Ridsdale (chairperson).
-
Alexandra Sinclair.
-
Linda Williams.
-
Nicola Williams.
Data Monitoring and Ethics Committee
-
Brian Burns.
-
Professor Lee Shepston (chairperson).
-
Tracey Young.
Grant-holding body
Ceri Jones, Giovanni Bucci, and the finance team within University Hospitals Coventry and Warwickshire.
Support staff
University of Warwick
Sharisse Alleyne, Rebecca Ayling, Dongquan Bi, Katie Booth, David Boss, Reece Carlington, Loraine Chowdhury, James Crawford, Claire Daffern, Penny Goode, Danielle Hale, Catherine Hill, Amy Ismay, Xiaoli Jia, Jenny Lee, Chockalingam Muthiah, Kerry Raynes, Sonia Sandhu, Marta Spocinska, Craig Turner, Jill Wood and Ziyu Zhong; and Adrian Willis and the finance team within Warwick Medical School.
Queen Mary University of London
Ryan Grainger, Charlotte Edwards Roscamp and Muriel Mcaughtrie.
Classification day
Angela Abdalla, Maha Ahmed, Debi Alvares, Jane Anderson, Bal Athwal, Mohammed Belhag, Barbara Box, Phoebe Brobby, Sam Chong, Katie Dodd, Paul Dorman, Julie Edwards, Roland Etti, Jithin George, Rachel Hanson, Susan Hirst, Graham Howkins, Sarah Irving, Zaza Katsarava, Francesca Kelly, Susie Lagrata, Nassif Mansour, Lynne Muldoon, Gemma Napul, Niran Nirmalananthan, Sally Reckert, Deb Smith, Imad Soryal, Tim Steiner, Anthony Thomas, Alok Tyagi, Jitka Vanderpol, Ben Wakerley, Tom Walker, Stuart Weatherby, Lara York and Tim Young.
Core Outcome Set in Migraine consensus day
Maha Ahmed, Bal Athwal, Barbara Box, Keith Griffiths, Asha Hareendran, Amanda Harrison, Kirstie Haywood, Rigor Jensen, Jessica Jones, Susie Lagrata, Richard Lipton, Anne-Marie Logan, Manjit Matharu, Vivien Nichols, Niranjanan Nirmalananthan, Shilpa Patel, Gemma Pearce, Stavros Petrou, Rachel Potter, Helen Power, Regina Rendas-Baum, Susan Stubbs, Martin Underwood, Amit Vora and Lara York.
Clinvivo
Johnathan Foss and Rob Froud.
Additional authors of publications
Fiona Caldwell, Tom Mars and Arani Vivekanantham.
Classification calls
Tracey Adcock, Elaine Butcher, Jon Davies, Pauline Derbyshire, Linda Field, Eleanor Hoverd, Sarah Joshi, Claire Talbot, Sobhash Jhuree and Elizabeth Habershon.
Lay advisory group members
Barbara Box, Sarah Alix, Graham Howkins, Lynne Muldoon, Gemma Pearce, Sally Reckert, Deb Smith, Lisa Stubbs, Christine Williams and Lara York.
Lay contributors to feedback on feasibility Research Ethics Committee application
Rachel Clegg, Claire Horton, Fran Kelly and Sheila Kelly.
Lay contributors to intervention design day
Elinor Bailey, Andrew Cook and Meghan Fay.
Facilitators
Jeanette Allison, Sue Carrington, Olivia Neely, Emma Randle and Gemma Pearce. Denis Anthony, Melissa Baldey, Claire Balmer, Sarah Bridgewater, Sue Carrington, Sinead Clarke O’Neill, Penny Crofts, Rebecca Davies, Linda Field, Elizabeth Habershon, Joanna Haywood, Sobhash Jhuree, Debbie Kelly, Sue Kenney, Anne-Marie Logan, Janet Lowe, Vinod Mahtani, Anna Molares, Katherine Priddis, Katrin Probyn, Jayshireen Singh, Karlene Stimpson, Priya Varma, Claire Winch and Sarah Wytrykowski.
General practices
London
Akerman Medical Practice, Albion Street Group Practice, Argyle Health Practice, Artesian Health Centre, Aylesbury Medical Centre, Balham Park Surgery, Belsize Priory Medical Practice, Binfield Road Surgery, Blackfen Medical Centre, Blithehale Medical Centre, Brayford Square Surgery, Bridge Lane Group Practice, Brockley Road Surgery, Brondesbury Medical Centre, Chrisp Street Health Centre, City Wellbeing Practice, Clapham Park Group Practice, Commercial Way Surgery, Crosslands Surgery, Crown Dale Medical Centre, Decima Street Surgery, Dr Driver & Partners, Dun Cow Surgery, Essex Lodge Surgery, Forest Hill Group Practice, Fortune Green Practice, Gill Medical Practice, Greengate Medical Centre, Grove Park Terrace Surgery, Hampstead Group Practice, Harford Health Centre, Herne Hill Road Medical Practice, Heston Living Care, Hounslow Family Practice, James Wigg Group Practice, Jubilee Street Practice, Keats Group Practice, Knoll Medical Practice, Lambeth Walk Group Practice, Manor Place Surgery, Minet Green Health Practice, Mocketts Wood Surgery, Morden Hill Surgery, Mount Medical Centre, New Eltham Medical Centre, Northgate Medical Practice, Nexus Health Group, Old Dairy Health Centre, Open Door Surgery, Park Group Practice, Parliament Hill Surgery, Prince of Wales Medical Centre, Princess Street Group Practice, Queens Crescent Practice, Sir John Kirk Close Surgery, Southfields Group Practice, St Paul’s Way Medical Centre, Stratford Health Centre, Stanford Medical Centre, Streatham Common Practice, Surrey Docks Health Centre, Talbot Medical Centre, The 301 East Street Surgery, The Beeches Surgery, The Bromley Common Practice, The Exchange Surgery, The Spitalfields Practice, The Woodlands Practice, Twickenham Park Medical Centre, Upton Lane Medical Centre, West Hampstead Medical Practice, Whitechapel Health Centre and Woodgrange Medical Practice.
Midlands
Abingdon Surgery, Alcester Health Centre, All Saints Medical Centre, All Saints Surgery, Alrewas Surgery, Apollo Surgery, Aspley Medical Practice, Avonside Health Centre, Balance Street Practice, Barton Family Practice, Bennfield Surgery, Berinsfield Health Centre, Boathouse Surgery, Broad Street Surgery, Broadshires Health Centre, Budbrooke Medical Centre, Bulkington Surgery, Burbury Medical Centre, Bushloe Surgery, Castle Medical Centre, Chase Meadow Health Centre, Church Street Practice, Churchfields Surgery, Clifton Hampden Surgery, Cobbs Garden Surgery, College Road Surgery, Copsewood Medical Centre, Corbett Medical Centre, Coventry Road Medical Practice, Cradley Surgery, Cripps Health Centre, Dordon & Polesworth Group Practice, Downsfield Medical Centre, Dr Rasib and Partners, Dr Reily and Partners, Dr Singh & Partners, Dr Sood and Partners, Dr Walji & Partners, Dudley Park Medical Centre, East Leicester Medical Practice, Eaton Wood Medical Centre, Eden Court Medical Practice, Elmswood Surgery, Erdington Medical Centre, Eynsham Medical Group, Farrier House Surgery, Forrest Medical Centre, Gate Medical Centre, Great Witley Surgery, Greet Medical Practice, Holbrooks Health Team, Hall Green Health, Hazelwood Group Practice, Henley Green Medical Centre, High Street Surgery, Hockley Farm Medical Practice, Jockey Road Medical Centre, Kingsfield Medical Centre, Kingsmount Medical Centre, Lapworth Surgery, Leen View Surgery, Limbrick Wood Surgery, Long Furlong Medical Centre, Manor Court Surgery, Lisle Court Medical Centre, Marcham Road Family Health Centre, Maypole Health Surgery, Mere Green Surgery, Mill View Surgery, Mortimer Medical Practice, Moseley Avenue Surgery, New Road Surgery, Northgate Surgery, Norton Canes Health Centre, Omnia Practice Yardley, Green Medical Centre, Park Leys Medical Practice, Peel Croft Surgery, Phoenix Family Care, Priory Gate Practice, Queslett Medical Centre, Red House Surgery, River Brook Medical, Rivergreen Medical Centre, Riversley Road Surgery, Rother House Medical Centre, Salters Medical Practice, Sherbourne Medical Centre, Shipston Medical Centre, Spring Gardens Health Centre, St Johns House Medical Centre, St Wulfstan Surgery, Sunrise Medical Practice, The Atherstone Surgery, The Chiltern Surgery, The Fairfields Practice, The Forum Health Centre, The Grange Medical Centre, The Malthouse Surgery, The Marches Surgery, The New Dispensary, The Old Priory Medical Centre, The Surgery at Aylestone, The Westgate Practice, Thornloe Lodge Surgery, Trinity Court Surgery, Wallingford Medical Practice, Walsgrave Health Centre, Westfield Surgery, Westside Medical Centre, Wetmore Road Surgery, Whaddon Medical Centre, White Horse Medical Practice, Willenhall Primary Care Centre 1, Windrush Medical Practice, Woodlands Medical Centre and Yardley Wood Health Centre.
Clinical Commissioning Groups
-
Coventry and Rugby.
-
South Warwickshire.
-
North Warwickshire.
-
Birmingham Cross City.
-
East Staffordshire.
-
South East Staffordshire and Seisdon Peninsula.
-
Birmingham South Central.
-
Nottingham City.
-
Nottingham North & East.
-
Nottingham West.
-
Leicester City.
-
East Leicester & Rutland.
-
Camden.
-
Hounslow.
CHESS digital versatile disc
Slate & Mortar Ltd (Birmingham, UK).
CHESS mindfulness compact disc and handout
Mindlab Goodworks Ltd (Teddington, UK) and Gill Davies.
Relaxation compact disc production
Warwick Audio Visual Department.
National Migraine Centre
Paul Booton, David Bloomfield, Jessica Briscoe, Charlotte Burr, Nazeli Manukyan, Katy Munro, Judith Pearson, Swati Raina, Lauren Shirazi and Heather Sims.
Migraine Trust
Angus Baldwin, Susan Haydon, Wendy Thomas and Arlene Wilkie.
Migraine Action
Simon Evans.
Clinical Research Networks
-
Leicester.
-
North London.
-
Nottingham.
-
South London.
-
Thames Valley South Midlands.
-
West Midlands.
Contributions of authors
Martin Underwood (https://orcid.org/0000-0002-0309-1708) was the chief investigator for the programme. Martin Underwood and Manjit Matharu (https://orcid.org/0000-0002-4960-2294) devised the original concept and Manjit Matharu was clinical lead. Dawn Carnes (https://orcid.org/0000-0002-3152-3133), Sandra Eldridge (https://orcid.org/0000-0001-5638-2317), David R Ellard (https://orcid.org/0000-0002-2992-048X), Frances Griffiths (https://orcid.org/0000-0002-4173-1438), Kirstie Haywood (https://orcid.org/0000-0002-5405-187X), Siew Wan Hee (https://orcid.org/0000-0002-0415-263X), Harbinder Sandhu (https://orcid.org/0000-0003-1522-8078), Stavros Petrou (https://orcid.org/0000-0003-3121-6050), Tamar Pincus (https://orcid.org/0000-0002-3172-5624), Stephanie JC Taylor (https://orcid.org/0000-0001-7454-6354) and Manjit Matharu were the original co-applicants for the programme grant.
Sian Newton (https://orcid.org/0000-0001-8035-0411), Choe Norman (https://orcid.org/0000-0002-3275-3141) and Kimberley Stewart (https://orcid.org/0000-0002-9814-8331) were trial managers and Helen Higgins (https://orcid.org/0000-0002-7095-4542) and Emma Padfield (https://orcid.org/0000-0003-4832-9609) were senior project managers. Sandra Eldridge, Siew Wan Hee and Dipesh Mistry (https://orcid.org/0000-0002-0875-9260) led the statistical analysis and interpretation. Felix Achana (https://orcid.org/0000-0002-8727-9125), Hema Mistry (https://orcid.org/0000-0002-5023-1160) and Stavros Petrou led the health economic analysis and interpretation. Dawn Carnes, Shilpa Patel (https://orcid.org/0000-0003-0726-4888), Harbinder Sandhu, Tamar Pincus and Stephanie JC Taylor and led the development of the self-management intervention, and Rachel Potter (https://orcid.org/0000-0001-6655-8996) and Manjit Matharu led the headache classification and one-to-one consultations. Kirstie Haywood led the outcome measurement work. David R Ellard, Frances Griffiths, Vivien Nichols (https://orcid.org/0000-0002-3372-1395) and Stephanie JC Taylor led the process evaluation.
All authors made substantial contributions to the design of the work or the acquisition, analysis or interpretation of the data, and provided a critical review and final approval of the report.
Publications
2016
Fifth European Headache and Migraine Trust International Congress, Glasgow
Haywood K, Mars TS, Potter R, Patel S, Underwood M. Assessing the Impact of Chronic and Episodic Headache and Treatment Outcomes: A Systematic Review of Patient-Reported Outcome Measures (PROMS). 5th European Headache and Migraine Trust International Congress, Glasgow, UK, 15–18 September 2016.
Patel S, Carnes D, Matharu M, Pincus T, Sandhu H, Underwood M, Probyn K. Development of an Educational and Self-management Intervention for Chronic Headache – Chronic Headache Education and Self-Management Study (CHESS). 5th European Headache and Migraine Trust International Congress, Glasgow, UK, 15–18 September 2016.
Probyn K, Caldwell F, Bowers H, Matharu M, Patel S, Sandhu H, Underwood M, Pincus T. Style and Content of Educational and Self-management Interventions for Chronic Headache – A Systematic Review. 5th European Headache and Migraine Trust International Congress, Glasgow, UK, 15–18 September 2016.
Probyn K, Caldwell F, Bowers H, Matharu M, Sandhu H, Underwood M, Pincus T. Predictors of Chronic Headache – A Systematic Review. 5th European Headache and Migraine Trust International Congress, Glasgow, UK, 15–18 September 2016.
Potter R, Dodd K, Matharu M, Probyn K, Pincus T, Underwood M. Development of a Chronic Headache Classification Interview – Chronic Headache Education and Self-management Study (CHESS). 5th European Headache and Migraine Trust International Congress, Glasgow, UK, 15–18 September 2016.
2017
The British Pain Society’s 50th Anniversary Annual Scientific Meeting, Birmingham
Nichols V, Ellard D, Griffiths F, Atiya K, Underwood M, Taylor S. The Lived Experience of Chronic Headache and Its Treatment: A Qualitative Review and Synthesis of Qualitative Studies. The British Pain Society’s 50th Anniversary Annual Scientific Meeting, Birmingham, UK, 3–5 May 2017.
Patel S, Sandhu H, Carnes D, Matharu M, Pincus T, Potter R, Probyn K, Taylor S, Underwood M. Development of an Educational and Self-management Intervention for Chronic Headache – The Chronic Headache Education and Self-management Study (CHESS). The British Pain Society’s 50th Anniversary Annual Scientific Meeting, Birmingham, UK, 3–5 May 2017.
Probyn K, Mistry D, Bowers H, Caldwell F, Patel S, Sandhu H, Underwood M, Matharu M, Pincus T. Non-Pharmacological Self-management For People Living With Migraine or Tension-Type Headache: A Systematic Review Including Analysis of Intervention Components. The British Pain Society’s 50th Anniversary Annual Scientific Meeting, Birmingham, UK, 3–5 May 2017.
Probyn K, Bowers H, Mistry D, Caldwell F, Patel D, Sandhu H, Underwood M, Matharu M. Prognostic Factors for Chronic Headache: A Systematic Review. The British Pain Society 50th Anniversary Annual Scientific Meeting, Birmingham, UK, 3–5 May 2017.
South East Regional Society for Academic Primary Care Conference, Cambridge
Vivien Nichols, Stephanie Taylor, David Ellard, Frances Griffiths. “Maybe the next one will work?” How People with Chronic Headache Manage. A Qualitative Study. South East Regional Society for Academic Primary Care Conference, Cambridge, UK, 26–27 January 2017.
Society for Academic Primary Care Conference, Warwick
Patel S, Sandhu H, Carnes D, Taylor S, Matharu M, Pincus T, Probyn K, Potter R, Underwood M. Development of an Educational and Self-management Intervention for Chronic Headache – Chronic Headache Education and Self-Management Study (CHESS). Society for Academic Primary Care Conference, Warwick, UK, 12–14 July 2017.
Potter R, Matharu M, Dodd K, Wan Hee S, Underwood M. Development and Validation of a Chronic Headache Classification Interview – Chronic Headache Education and Self-management Study (CHESS). Society for Academic Primary Care Conference, Warwick, UK, 12–14 July 2017.
Nichols V, Ellard D, Griffiths F, Underwood M, Taylor S. What Is It Like for Patients Living with Chronic Headache? A Systematic Review and Synthesis of Qualitative Studies. Society for Academic Primary Care Conference, Warwick, UK, 12–14 July 2017.
Probyn K, Bowers H, Mistry S, Caldwell F, Underwood M, Matharu M, Pincus T. Can We Identify Factors Predicting Prognosis and Influencing Response to Preventative Interventions for Chronic Headache? Society for Academic Primary Care Conference, Warwick, UK, 12–14 July 2017.
Probyn K, Bowers H, Mistry D, Caldwell F, Underwood M, Patel S, Sandhu H, Matharu M, Pincus T. Non-pharmacological Self-management with CBT, Patient Education, Mindfulness and Relaxation for Migraine and Tension-type Headache: A Systematic Review. Society for Academic Primary Care Conference, Warwick, UK, 12–14 July 2017.
10th Congress the of European Pain Federation, Copenhagen
Probyn K, Bowers H, Mistry D, Caldwell F, Underwood M, Patel S, Sandhu H, Matharu M, Pincus T. Non-pharmacological Self-management for People Living with Migraine or Tension-type Headache: A Systematic Review Including Analysis of Intervention Components. European Pain Federation 10th Congress of European Pain Federation, Copenhagen, Denmark, 6–9 September 2017.
Potter R, Matharu M, Wan Hee S, Underwood M. Development and Validation of a Chronic Headache Classification Interview – Chronic Headache Education and Self-management Study (CHESS). European Pain Federation 10th Congress of European Pain Federation, Copenhagen, Denmark, 6–9 September 2017.
Nichols V, Ellard D, Griffiths F, Underwood M, Taylor S. The Lived Experience of Chronic Headache: A Systematic Review and Synthesis of Qualitative Studies. European Pain Federation 10th Congress of European Pain Federation, Copenhagen, Denmark, 6–9 September 2017.
Probyn K, Bowers H, Mistry D, Caldwell F, Underwood M, Matharu M, Pincus T. Can We Identify Factors Predicting Prognosis and Influencing Response to Preventative Interventions for Chronic Headache? European Pain Federation 10th Congress of European Pain Federation, Copenhagen, Denmark, 6–9 September 2017.
2018
Society for Academic Primary Care 2018 Conference, London
Potter R, White K, Matharu M, Taylor S, Sandhu H, Underwood M. Chronic Headache Education and Self-management Study (CHESS) – A Feasibility Study. Society for Academic Primary Care Conference, London, UK, 10–12 July 2018.
Potter R, White K, Matharu M, Taylor S, Sandhu H, Ellard D, Underwood M. Chronic Headache Education and Self-management Study (CHESS). Society for Academic Primary Care Conference, London, UK, 10–12 July 2018.
17th International Association for the Study of Pain World Congress on Pain, Boston, MA
Potter R, Matharu M, Dodd K, Wan Hee S, Hoverd E, Underwood M. Development and Validation of a Telephone Classification Interview for Chronic Headache. 17th International Association for the Study of Pain World Congress on Pain, Boston, MA, USA, 12–16 September 2018.
Patel S, Sandhu H, Carnes D, Taylor S, Matharu M, Pincus T, Probyn K, Potter R, Underwood M. Development of an Educational and Self-management Intervention for Chronic Headache. 17th International Association for the Study of Pain World Congress on Pain, Boston, MA, USA, 12–16 September 2018.
Potter R, White K, Matharu M, Taylor S, Sandhu H, Ellard D, Underwood M. Design and Chronic Headache Education and Self-management Study (CHESS): Trial Design. 17th International Association for the Study of Pain World Congress on Pain, Boston, MA, USA, 12–16 September 2018.
2019
UK Trial Managers’ Network Annual Conference, Birmingham
White K, Potter R, Underwood M on behalf of the CHESS team. Chronic Headache Education and Self-management Study (CHESS) – Challenges and Facilitators to Study Recruitment and Delivery. UK Trial Managers’ Network Annual Conference, Birmingham, UK, 2019.
Norman C, Patel S on behalf of the CHESS Team. Chronic Headache Education and Self-management Study (CHESS) – Smartphone Application. UK Trial Managers’ Network Annual Conference, Birmingham, UK, 2019.
2022
Ellard D, Nichols V, Griffiths F, Underwood M, Taylor S on behalf of the CHESS team. A process evaluation of the chronic headache education and self-management study (CHESS) Association for the Study of Pain 19th IASP World Congress on Pain 2022 Toronto, Canada, 2022.
Underwood M, on behalf of the CHESS team. The CHESS trial. A supportive self-management programme for people livings with chronic headaches: a randomised trial and economic evaluation. Association for the Study of Pain 19th IASP World Congress on Pain 2022 Toronto, Canada, 2022.
Data-sharing statement
All requests for data should be sent to the Warwick Clinical Trials Unit data access team (wctudataaccess@warwick.ac.uk). Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
Appendix 1 Chronic Headache Education and Self-management Study (CHESS): a mixed-methods feasibility study to inform the design of a randomised controlled trial
This appendix is reproduced with permission from White et al14 (https://doi.org/10.1186/s12874-019-0672-5). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
Background
Self-management support programmes are effective in a range of chronic conditions; however, there is limited evidence for their use in the treatment of chronic headaches. The aim of this study was to test the feasibility of four key aspects of a planned, future evaluative trial of a new education and self-management intervention for people with chronic headache: (1) recruiting people with chronic headache from primary care, (2) a telephone interview for the classification of chronic headaches, (3) the education and self-management intervention itself, and (4) the most appropriate patient-reported outcomes (PROMS).
Methods
Participants were identified and recruited from general practices in the West Midlands region of the UK. We developed a nurse-led chronic headache classification interview and assessed agreement with an interview with headache specialists. We developed and tested a group-based education and self-management intervention to assess training and delivery receipt using observation, facilitator, and participant feedback. We explored the acceptability and relevance of PROMs using postal questionnaires, interviews and a smartphone app.
Results
Fourteen practices took part in the study and participant recruitment equated to 1.0/1000 registered patients. Challenges to recruitment were identified. We did 107 paired headache classification interviews. The level of agreement between nurse and doctor interviews was very good. We piloted the intervention in four groups with 18 participants. Qualitative feedback from participants and facilitators helped refine the intervention including shortening the overall intervention and increasing the facilitator training time. Participants completed 131 baseline questionnaires, measurement data quality, reliability and validity for headache-specific and generic measures were acceptable.
Conclusion
This study indicated that recruiting people with chronic headache from primary care is feasible but challenging, our headache classification interview is fit for purpose, our study intervention is viable, and our choice of outcome measures is acceptable to participants in a future randomised controlled trial (RCT).
Appendix 2 Diagnostic and classification tools for chronic headache disorders: a systematic review
This appendix is reproduced with permission from Potter et al15 (https://doi.org/10.1177/0333102418806864). This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (http://us.sagepub.com/en-us/nam/open-access-at-sage). The text below includes minor additions and formatting changes to the original text.
Background or aim
Despite guidelines and the International Classification of Headache Disorders (ICHD-III beta) criteria, the diagnosis of common chronic headache disorders can be challenging for non-expert clinicians. The aim of the review was to identify headache classification tools that could be used by a non-expert clinician to classify common chronic disorders in primary care.
Methods
We conducted a systematic literature review of studies validating diagnostic and classification headache tools published between January 1988 and June 2016 from key databases: MEDLINE, ASSIA, Embase, Web of Knowledge and PsycINFO. Quality assessment was assessed using items of the Quality of Diagnostic Accuracy Studies (QUADAS-2).
Results
The search identified 38 papers reporting the validation of 30 tools designed to diagnose, classify or screen for headache disorders: nine for multiple headache types, and 21 for one headache type only. We did not identify a tool validated in primary care that can be used by a non-expert clinician to classify common chronic headache disorders and screen for primary headaches other than migraine and tension-type headache in primary care.
Conclusions
Despite the availability of many headache classification tools we propose the need for a tool that could support primary care clinicians in diagnosing and managing chronic headache disorders within primary care, and allow more targeted referral to headache specialists.
Appendix 3 Development and validation of a telephone classification interview for common chronic headache disorders
This appendix is reproduced with permission from Potter et al16 (https://doi.org/10.1186/s10194-018-0954-z). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Background
For a trial of supportive self-management for people with chronic headache we needed to develop and validate a telephone classification interview that can be used by a non-headache specialist to classify common chronic headache types in primary care. We aimed to specifically exclude secondary headaches other than medication overuse, exclude primary headache disorders other than migraine and tension-type headache (TTH), distinguish between chronic migraine and chronic TTH, and identify medication overuse headache.
Methods
We held a headache classification consensus conference to draw on evidence and expertise to inform the content of a logic model underpinning the classification interview. Nurses trained to use the logic model did telephone classification interviews with participants recruited from primary care. Doctors specialising in headache did a second validation interview.
Results
Twenty-six delegates attended the headache classification conference including headache specialist doctors, nurses and lay representatives (with chronic headache). We trained six nurses to do the classification interviews and completed 107 paired interviews; median days between interviews was 32 days (interquartile range 21–48 days). We measured level of agreement between the nurse and doctor interviews using proportion of concordance, simple kappa and prevalence-adjusted bias-adjusted kappa (PABAK). Proportion of concordance of agreement between nurse and doctor interviews was 0.76, simple kappa coefficient κ = 0.31 (95% CI 0.09 to 0.52), and PABAK 0.51 (95% CI 0.35 to 0.68), a moderate agreement. In a sensitivity test following review of headache characteristics recorded, concordance was 0.91, κ = 0.53 (95% CI 0.28 to 0.79), and PABAK 0.81 (95% CI 0.70 to 0.92), a very good agreement.
Conclusion
We developed and validated a new evidence-based telephone classification interview that can be used by a non-headache specialist to classify common chronic headache types in primary care.
Appendix 4 Development of an education and self-management intervention for chronic headache – CHESS trial (Chronic Headache Education and Self-management Study)
This appendix is reproduced with permission from Patel et al19 (https://doi.org/10.1186/s10194-019-0980-5). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Background
Self-management interventions are well recognised and widely used in chronic conditions. Their application to chronic headaches has been limited and generally of low quality. We describe here our process for developing an evidence-based, and theory driven, education and self-management intervention for those living with chronic headache.
Methods
Our intervention was designed using several core information sources: the results of three systematic reviews; qualitative material from those living with chronic headaches; our knowledge from existing self-management interventions; and finally collaborative input from a multidisciplinary team of clinicians, academics, patients and charity partners. We manualised the intervention and associated training as a package for use in a feasibility study. We made adaptations for its use in a randomised controlled trial.
Results
We piloted the intervention in four groups with a total of 18 participants. Qualitative feedback from 12 participants and five facilitators allowed the intervention to be refined for the main randomised controlled trial. Some of the key changes included shortening of the overall intervention, changes to the originally planned facilitators and spreading the facilitator training over 3 days rather than 2.
We are now testing the final revised intervention in a randomised controlled trial of its clinical effectiveness and cost effectiveness. The group component of the intervention is delivered over 2 days with the first day focused on living, understanding and dealing with chronic headaches and the second day exploring how to adapt and take control of one’s life with chronic headaches.
Conclusion
Our pilot work indicates that our intervention is feasible to deliver, and with the relevant changes would be acceptable for use with this population. Our randomised control trial is ongoing. We anticipate publishing final results in 2021. The CHESS intervention materials are available from http://wrap.warwick.ac.uk/171671/1/Chronic-Headache-Education-and-Self-management-Support-study-CHESS-additional-information.zip.
Appendix 5 The lived experience of chronic headache: a systematic review and synthesis of the qualitative literature
This appendix is reproduced with permission from Nichols et al20 (https://doi.org/10.1136/bmjopen-2017-019929). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Objective
To systematically review the qualitative literature of the lived experience of people with a chronic headache disorder.
Background
Chronic headaches affect 3–4% of the population. The most common chronic headache disorders are chronic migraine, chronic tension-type headache and medication overuse headache. We present a systematic review and meta-ethnographic synthesis of the lived experience of people with chronic headache.
Methods
We searched seven electronic databases, hand-searched nine journals and used a modified Critical Appraisal Skills Programme checklist to appraise study quality. Following thematic analysis we synthesised the data using a meta-ethnographic approach.
Results
We identified 3586 unique citations; full texts were examined for 86 studies and four were included in the review. Included studies differed in their foci: exploring, patient-centred outcomes, chronic headache as a socially invisible disease, psychological processes mediating impaired quality of life and the process of medication overuse. Initial thematic analysis and subsequent synthesis gave three overarching themes: ʻheadache as a driver of behaviour’ (directly and indirectly), ‘the spectre of headache’ and ‘strained relationships’.
Conclusion
This meta-synthesis of published qualitative evidence demonstrates that chronic headaches have a profound effect on people’s lives, showing similarities with other pain conditions. There were insufficient data to explore the similarities and differences between different chronic headache disorders.
Appendix 6 Prognostic factors for chronic headache: a systematic review
This appendix is reproduced with permission from Probyn et al21 (https://doi.org/10.1212/WNL.0000000000004112). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND), which permits downloading and sharing the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Objective
To identify predictors of prognosis and trial outcomes in prospective studies of people with chronic headache.
Methods
This was a systematic review of published literature in peer-reviewed journals. We included (1) randomized controlled trials (RCTs) of interventions for chronic headache that reported subgroup analyses and (2) prospective cohort studies, published in English, since 1980. Participants included adults with chronic headache (including chronic headache, chronic migraine, and chronic tension-type headache with or without medication overuse headache). We searched key databases using free text and MeSH terms. Two reviewers independently extracted data and assessed the methodologic quality of studies and overall quality of evidence identified using appropriate published checklists.
Results
We identified 16,556 titles, removed 663 duplicates, and reviewed 199 articles, of which 27 were included in the review—17 prospective cohorts and 10 RCTs with subgroup analyses reported. There was moderate-quality evidence indicating that depression, anxiety, poor sleep and stress, medication overuse, and poor self-efficacy for managing headaches are potential prognostic factors for poor prognosis and unfavorable outcomes from preventive treatment in chronic headache. There was inconclusive evidence about treatment expectations, age, age at onset, body mass index, employment, and several headache features.
Conclusion
This review identified several potential predictors of poor prognosis and worse outcome postinterventions in people with chronic headache. The majority of these are modifiable. The findings also highlight the need for more longitudinal high-quality research of prognostic factors in chronic headache.
Appendix 7 Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components
This appendix is reproduced with permission from Probyn et al22 (https://doi.org/10.1136/bmjopen-2017-016670). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Objectives
To assess the effect of non-pharmacological self-management interventions against usual care, and to explore different components and delivery methods within those interventions.
Participants
People living with migraine and/or tension-type headache.
Interventions
Non-pharmacological educational or psychological self-management interventions, excluding biofeedback and physical therapy. We assessed the overall effectiveness against usual care on headache frequency, pain intensity, mood, headache-related disability, quality of life and medication consumption in meta-analysis. We also provide preliminary evidence on the effectiveness of intervention components and delivery methods.
Results
We found a small overall effect for the superiority of self-management interventions over usual care, with a standardised mean difference (SMD) of –0.36 (–0.45 to –0.26) for pain intensity, –0.32 (–0.42 to –0.22) for headache-related disability, 0.32 (0.20 to 0.45) for quality of life and a moderate effect on mood [SMD 0.53 (–0.66 to –0.40)]. We did not find an effect on headache frequency [SMD –0.07 (–0.22 to 0.08)].
Assessment of components and characteristics suggests a larger effect on pain intensity in interventions that included explicit educational components [–0.51 (–0.68 to –0.34) vs. –0.28 (–0.40 to –0.16)], mindfulness components [–0.50 (–0.82 to –0.18) vs. 0.34 (–0.44 to –0.24)] and in interventions delivered in groups versus one-to-one delivery [0.56 (–0.72 to –0.40) vs. –0.39 (–0.52 to –0.27)] and larger effects on mood in interventions including a cognitive–behavioural therapy (CBT) component with an SMD of –0.72 (–0.93 to –0.51) compared with those without CBT –0.41 (–0.58 to –0.24).
Conclusion
Overall we found that self-management interventions for migraine and tension-type headache are more effective than usual care in reducing pain intensity, mood and headache-related disability, but have no effect on headache frequency. Preliminary findings also suggest that including CBT, mindfulness and educational components in interventions, and delivery in groups, may increase effectiveness.
Appendix 8 Assessing the impact of headaches and the outcomes of treatment: a systematic review of patient-reported outcome measures (PROMs)
This appendix is reproduced with permission from Haywood et al26 (https://doi.org/10.1177/0333102417731348). This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (http://us.sagepub.com/en-us/nam/open-access-at-sage). The text below includes minor additions and formatting changes to the original text.
Aims
To critically appraise, compare and synthesise the quality and acceptability of multi-item patient-reported outcome measures for adults with chronic or episodic headache.
Methods
Systematic literature searches of major databases (1980–2016) to identify published evidence of PROM measurement and practical properties. Data on study quality (COSMIN), measurement and practical properties per measure were extracted and assessed against accepted standards to inform an evidence synthesis.
Results
From 10,903 reviewed abstracts, 103 articles were assessed in full: 46 provided evidence for 23 PROMs, eleven specific to the health-related impact of migraine (n = 5) or headache (n = 6), six assessed migraine-specific treatment response/satisfaction and six were generic measures. Evidence for measurement validity and score interpretation was strongest for two measures of impact, Migraine-Specific Quality of Life Questionnaire (MSQ v2.1) and Headache Impact Test 6-item (HIT-6), and one of treatment response, the Patient Perception of Migraine Questionnaire (PPMQ-R). Evidence of reliability was limited, but acceptable for the HIT-6. Responsiveness was rarely evaluated. Evidence for the remaining measures was limited. Patient involvement was limited and poorly reported.
Conclusion
While evidence is limited, three measures have acceptable evidence of reliability and validity: HIT-6, MSQ v2.1 and PPMQ-R. Only the HIT-6 has acceptable evidence supporting its completion by all ‘headache’ populations.
Appendix 9 Measuring health-related quality of life in chronic headache: a comparative evaluation of the Chronic Headache Quality of Life Questionnaire and Headache Impact Test
This appendix is reproduced with permission from Haywood et al27 (https://doi.org/10.1177/03331024211006045). This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage). The text below includes minor additions and formatting changes to the original text.
Objective
To compare the quality and acceptability of a new headache-specific patient-reported measure, the Chronic Headache Quality of Life Questionnaire (CHQLQ) with the six-item Headache Impact Test (HIT-6), in people meeting an epidemiological definition of chronic headaches.
Methods
Participants in the feasibility stage of the Chronic Headache Education and Self-management Study (CHESS) (n = 130) completed measures three times during a 12-week prospective cohort study. Data quality, measurement acceptability, reliability, validity, responsiveness to change and score interpretation were determined. Semistructured cognitive interviews explored measurement relevance, acceptability, clarity and comprehensiveness.
Results
Both measures were well completed with few missing items. The CHQLQ's inclusion of emotional well-being items increased its relevance to participant's experience of chronic headache. End effects were present at item level only for both measures. Structural assessment supported the three and one-factor solutions of the CHQLQ and HIT-6, respectively. Both the CHQLQ (range 0.87 to 0.94) and HIT-6 (0.90) were internally consistent, with acceptable temporal stability over 2 weeks (CHQLQ range 0.74 to 0.80; HIT-6 0.86). Both measures responded to change in headache-specific health at 12 weeks [CHQLQ smallest detectable change (improvement) range 3 to 5; HIT-6 2.1].
Conclusions
While both measures are structurally valid, internally consistent, temporally stable and responsive to change, the CHQLQ has greater relevance to the patient experience of chronic headache.
Appendix 10 Headache smartphone app – development and application in the Chronic Headache and Self-management Study (CHESS)
Appendix 11 Mapping between headache specific and generic preference-based health-related quality of life measures
This chapter has been reproduced with permission from Khan et al. 40 © The Author(s) 2022. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Background
The Headache Impact Test (HIT-6) and the Chronic Headache Questionnaire (CH-QLQ) measure headache-related quality of life but are not preference-based and therefore cannot be used to generate health utilities for cost-effectiveness analyses. There are currently no established algorithms for mapping between the HIT-6 or CH-QLQ and preference-based health-related quality-of-life measures for the chronic headache population.
Methods
We developed algorithms for generating EQ-5D-5L and SF-6D utilities from the HIT-6 and the CHQLQ using both direct and response mapping approaches. A multi-stage model selection process was used to assess the predictive accuracy of the models. The estimated mapping algorithms were derived to generate UK tariffs and was validated using the Chronic Headache Education and Self-management Study (CHESS) trial dataset.
Results
Several models were developed that reasonably accurately predict health utilities in this context. The best performing model for predicting EQ-5D-5L utility scores from the HIT-6 scores was a Censored Least Absolute Deviations (CLAD) (1) model that only included the HIT-6 score as the covariate (mean squared error (MSE) 0.0550). The selected model for CH-QLQ to EQ-5D-5L was the CLAD (3) model that included CH-QLQ summary scores, age, and gender, squared terms and interaction terms as covariates (MSE 0.0583). The best performing model for predicting SF-6D utility scores from the HIT-6 scores was the CLAD (2) model that included the HIT-6 score and age and gender as covariates (MSE 0.0102). The selected model for CH-QLQ to SF-6D was the OLS (2) model that included CH-QLQ summary scores, age, and gender as covariates (MSE 0.0086).
Conclusion
The developed algorithms enable the estimation of EQ-5D-5L and SF-6D utilities from two headache-specific questionnaires where preference-based health-related quality of life data are missing. However, further work is needed to help define the best approach to measuring health utilities in headache studies.
Appendix 12 A core outcome set for preventative intervention trials in chronic and episodic migraine (COSMIG): an international, consensus-derived and multi-stakeholder initiative
This appendix is reproduced with permission from Haywood et al39 (https://doi.org/10.1136/bmjopen-2020-043242). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Objective
Typically, migraine prevention trials focus on reducing migraine days. This narrow focus may not capture all that is important to people with migraine. Inconsistency in outcome selection across trials limits the potential for data pooling and evidence synthesis. In response, we describe the development of core outcome set for migraine (COSMIG).
Design
A two-stage approach sought to achieve international, multistakeholder consensus on both the core domain set and core measurement set. Following construction of a comprehensive list of outcomes, expert panellists (patients, health-care professionals and researchers) completed a three-round electronic-Delphi study to support a reduction and prioritisation of core domains and outcomes. Participants in a consensus meeting finalised the core domains and methods of assessment. All stages were overseen by an international core team, including patient research partners.
Results
There was a good representation of patients [episodic migraine (n = 34) and chronic migraine (n = 42)] and health-care professionals (n = 33) with high response and retention rates. The initial list of domains and outcomes was reduced from >50 to 7 core domains for consideration in the consensus meeting, during which a two-domain core outcome set was agreed.
Conclusion
International and multistakeholder consensus emerged to describe a two-domain core outcome set for reporting research on preventive interventions for chronic and episodic migraine: migraine-specific pain and migraine-specific quality of life. Intensity of migraine pain assessed with an 11-point Numerical Rating Scale and the frequency as the number of headache/migraine days over a specified time period. Migraine-specific quality of life assessed using the Migraine Functional Impact Questionnaire.
Appendix 13 The association between headache and low back pain: a systematic review
This appendix is reproduced with permission from Vivekanantham et al42 (https://doi.org/10.1186/s10194-019-1031-y). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Background
To systematically review studies quantifying the association between primary chronic headaches and persistent low back pain (LBP).
Main text
We searched five electronic databases. We included case-control, cross-sectional and cohort studies that included a headache and back pain free group, reporting on any association between persistent LBP and primary headache disorders. Methodological quality was assessed using the Newcastle–Ottawa Scale. Our primary outcome was the association between primary headache disorders and persistent LBP. Our secondary outcomes were any associations between severity of LBP and severity of headache, and the relationship between specific headache sub-types classified as per International Classification of Headache Disorders (ICHD) criteria and persistent LBP.
We included 14 studies. The sizes of the studies ranged from 88 participants to a large international study with 404,206 participants. Odds ratios for the association were between 1.55 [95% confidence interval (CI) 1.13 to 2.11] and 8.00 (95% CI 5.3 to 12.1). Study heterogeneity meant statistical pooling was not possible. Only two studies presented data investigating persistent LBP and chronic headache disorders in accordance with ICDH criteria.
Conclusions
We identified a positive association between persistent LBP and primary headache disorders. The quality of the review findings is limited by diversity of populations, study designs and uncertainty about headache and LBP definitions.
Appendix 14 Usual care and a self-management support programme versus usual care and a relaxation programme for people living with chronic headache disorders: a randomised controlled trial protocol (CHESS)
This appendix is reproduced with permission from Patel et al43 (https://doi.org/10.1136/bmjopen-2019-033520). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Introduction
Chronic headaches are poorly diagnosed and managed and can be exacerbated by medication overuse. There is insufficient evidence on the non-pharmacological approaches to helping people living with chronic headaches.
Methods and analysis
Chronic Headache Education and Self-management Study is a pragmatic randomised controlled trial to test the effectiveness and cost-effectiveness of a self-management education support programme on top of usual care for patients with chronic headaches against a control of usual care and relaxation. The intervention is a 2-day group course based on education, personal reflection and a cognitive–behavioural approach, plus a nurse-led one-to-one consultation and follow-up over 8 weeks. We aim to recruit 689 participants (356 to the intervention arm and 333 to the control arm) from primary care and self-referral in London and the Midlands. The trial is powered to show a difference of 2.0 points on the Headache Impact Test, a patient-reported outcome measure at 12 months post randomisation. Secondary outcomes include health-related quality of life, self-efficacy, social activation and engagement, anxiety and depression, and health-care utilisation. Outcomes are being measured at 4, 8 and 12 months. Cost-effectiveness will be expressed in terms of incremental cost per quality-adjusted life-year gained.
Ethics and dissemination
This trial will provide data on effectiveness and cost-effectiveness of a self-management support programme for chronic headaches. The results will inform commissioning of services and clinical practice. North West – Greater Manchester East Research Ethics Committee have approved the trial. The current protocol version is 3.6 date 7 March 2019.
Appendix 15 Supportive Self-Management Program for People With Chronic Headaches and Migraine: A Randomized Controlled Trial and Economic Evaluation
This appendix is reproduced with permission from Underwood et al44. (https://doi.org/10.1212/WNL.0000000000201518). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Background and objectives
Chronic headache disorders are a major cause of pain and disability. Education and supportive self-management approaches could reduce the burden of headache disability. We tested the effectiveness of a group educational and supportive self-management program for people living with chronic headaches.
Methods
This was a pragmatic randomised controlled trial. Participants were aged 18 years or older with chronic migraine or chronic tension-type headache, with or without medication overuse headache. We primarily recruited from general practices. Participants were assigned to either a 2-day group education and self-management program, a one-to-one nurse interview, and telephone support or to usual care plus relaxation material. The primary outcome was headache related-quality of life using the Headache Impact Test (HIT)-6 at 12 months. The primary analysis used intention-to-treat principles for participants with migraine and both baseline and 12-month HIT-6 data.
Results
Between April 2017 and March 2019, we randomised 736 participants. Because only 9 participants just had tension-type headache, our main analyses were on the 727 participants with migraine. Of them, 376 were allocated to the self-management intervention and 351 to usual care. Data from 586 (81%) participants were analyzed for primary outcome. There was no between-group difference in HIT-6 (adjusted mean difference = −0.3, 95% CI −1.23 to 0.67) or headache days (0.9, 95% CI −0.29 to 2.05) at 12 months. The Chronic Headache Education and Self-management Study intervention generated incremental adjusted costs of £268 (95% CI, £176–377) [USD383 (95% CI USD252 to USD539)] and incremental adjusted quality-adjusted life years (QALYs) of 0.031 (95% CI −0.005 to 0.063). The incremental cost-effectiveness ratio was £8,617 (USD12,322) per QALY gained.
Discussion
These findings conclusively show a lack of benefit for quality of life or monthly headache days from a brief group education and supportive self-management program for people living with chronic migraine or chronic tension-type headache with episodic migraine.
Appendix 16 CHESS health economic evaluation summary
Appendix 17 The CHESS trial: protocol for the process evaluation of a randomised trial of an education and self-management intervention for people with chronic headache
This appendix is reproduced with permission from Nichols et al69 (https://doi.org/10.1186/s13063-019-3372-x). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Background
Process evaluation is increasingly common alongside complex randomised controlled trials (RCTs). This evaluation helps in understanding the mechanisms of impact and how the study processes were executed, and it includes any contextual factors which may have implications for the trial results and any future implementation. This process evaluation is for the Chronic Headache Education and Self-management Study (CHESS) RCT, which is evaluating an education and self-management group behavioural intervention for people with chronic headache. Chronic headache is defined as headaches which are present for ≥15 days per month. The most common types are chronic migraine and chronic tension-type and medication overuse headaches.
Methods
We will use a mixed-methods approach. Quantitative data will be taken from routine trial data which will help us to assess the reach of the study (i.e. did we reach those whom we expected and from where?). Intervention attendance (dose received) and attrition and qualitative data will augment our understanding about reasons why people may not wish to take part in or failed to attend sessions. Interviews with intervention facilitators and trial participants will gain different perspectives on taking part in the trial.
Fidelity will be assessed through listening to audio-recordings for adherence to course content and competence of the facilitation of a sample of sessions.
Discussion
Our process evaluation will allow us to gain insight into how the trial was delivered, the obstacles and enablers encountered and the possible reasons why the interventions may or may not be effective.
Appendix 18 Chronic Headache Education and Self-Management Study (CHESS): a process evaluation
This appendix is reproduced with permission from Ellard et al70 (https://doi.org/10.1186/s12883-022-02792-1). Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Background
The Chronic Headache Education and Self-Management Study (CHESS) multicentre randomised trial evaluated the impact a group education and self-management support intervention with a best usual care plus relaxation control for people living with chronic headache disorders (tension type headaches or chronic migraine, with or without medication overuse headache). Here we report the process evaluation exploring potential explanations for the lack of positive effects from the CHESS intervention.
Methods
The CHESS trial included 736 (380 intervention: 356 control) people across the Midlands and London UK. We used a mixed methods approach. Our extensive process evaluation looked at context, reach, recruitment, dose delivered, dose received, fidelity and experiences of participating in the trial, and included participants and trial staff. We also looked for evidence in our qualitative data to investigate whether the original causal assumptions underpinning the intervention were realised.
Results
The CHESS trial reached out to a large diverse population and recruited a representative sample. Few people with chronic tension type headaches without migraine were identified and recruited. The expected ‘dose‘ of the intervention was delivered to participants and intervention fidelity was high. Attendance (‘dose received‘) fell below expectation, although 261/380 (69%) received at least at least the pre-identified minimum dose. Intervention participants generally enjoyed being in the groups but there was little evidence to support the causal assumptions underpinning the intervention were realised.
Conclusions
From a process evaluation perspective despite our extensive data collection and analysis, we do not have a clear understanding of why the trial outcome was negative as the intervention was delivered as planned. However, the lack of evidence that the intervention causal assumptions brought about the planned behaviour change may provide some insight. Our data suggests only modest changes in managing headache behaviours and some disparity in how participants engaged with components of the intervention within the timeframe of the study. Moving forwards, we need a better understanding of how those who live with chronic headache can be helped to manage this disabling condition more effectively over time.
Appendix 19 Patient and public involvement in a UK National Institute for Health and Care Research Programme Grant for Applied Research: experiences from the Chronic Headache Education and Self-management Study (CHESS)
This appendix is reproduced with permission from Nichols et al71 (https://doi.org/10.1017/S1463423621000670). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Patient and public involvement (PPI) plays a crucial role in ensuring research is carried out in conjunction with the people that it will impact on. In this article, we present our experiences and reflections from working collaboratively with patients and public through the lifetime of a National Institute for Health and Care Research (NIHR) programme grant, the Chronic Headache Education and Self-management Study (CHESS), which took place between 2015 and 2020.
Patient and public involvement over the course of CHESS
We worked closely with three leading UK migraine charities and a lay advisory group throughout the programme. We followed NIHR standards and used the Guidance for Reporting Involvement of Patients and the Public checklist. We consulted our PPI contacts using a variety of methods depending on the phase of the study and the nature of the request. This included emails, discussions and face-to-face contact.
PPI members contributed throughout the study in the programme development, in the grant application, ethics documentation and trial oversight, during the feasibility study in supporting the development of a classification interview for chronic headache by participating in a headache classification conference, assessing the relevance and acceptability of patient-reported outcome measures by helping to analyse cognitive interview data, and testing the smartphone application making suggestions on how best to present the summary of data collected for participants. Due to PPI contribution, the content and duration of the study intervention were adapted and a Delphi study with consensus meeting developed a core outcome set for migraine studies.
Conclusions
The involvement of the public and patients in CHESS has allowed us to shape its overall design, intervention development and establish a core outcome set for future migraine studies. We have reflected on many learning points for the future application of PPI.
List of abbreviations
- AE
- adverse event
- app
- application
- ASSIA
- Applied Social Sciences Index and Abstracts
- CACE
- complier-average causal effect
- CBT
- cognitive–behavioural therapy
- CD
- compact disc
- CGRP
- calcitonin gene-related peptide
- CHESS
- Chronic Headache Education and Self-management Study
- CHQLQ
- Chronic Headache Quality of Life Questionnaire
- CI
- confidence interval
- COSMIG
- core outcome set for migraine
- CRN
- clinical research network
- DVD
- digital versatile disc
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- heiQ
- Health Education Impact Questionnaire
- HIT-6
- Headache Impact Test-6
- ICER
- incremental cost-effectiveness ratio
- ICHD-3
- International Classification of Headache Disorder, third edition
- IQR
- interquartile range
- LBP
- low back pain
- MSQ
- Migraine-Specific Quality of Life Questionnaire
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMC
- National Migraine Centre
- PDG
- Programme Development Grant
- PGfAR
- Programme Grants for Applied Research
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSC
- Programme Steering Committee
- PSEQ
- Pain Self-Efficacy Questionnaire
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality of Diagnostic Accuracy Studies
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- SIS
- Social Integration Subscale
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/PLJL1440).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
