Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as award number RP-PG-0514-20002. The contractual start date was in March 2016. The draft manuscript began editorial review in December 2022 and was accepted for publication in May 2024. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 MacArthur et al. This work was produced by MacArthur et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 MacArthur et al.
Synopsis
Summary of alterations to the programme’s original aims/design
Cluster randomised controlled trial changed to pilot and feasibility cluster randomised controlled trial
The major change within this programme was a change from a full cluster randomised controlled trial (RCT) to a pilot and feasibility RCT – details of this change and the reasons for it are described below.
The original Antenatal Preventative Pelvic floor Exercises and Localisation (APPEAL) programme was of 5 years’ duration and included four work packages (WPs) with the ultimate aim of developing and testing an intervention to train midwives to advise and support women during antenatal care in undertaking pelvic floor muscle exercises (PFME) to reduce post-partum urinary incontinence (UI). There is high-quality Cochrane evidence1 that antenatal interventions to increase PFME substantially reduce the proportion of women who experience post-partum UI. However, many midwives do not offer advice and support to women, and many pregnant women do not undertake these exercises. Thus, it was considered important to develop an intervention to integrate this advice and support into routine midwifery antenatal care and investigate its effectiveness.
The programme was planned to develop an intervention in the earlier WPs and to undertake a definitive cluster RCT within WP4. The aim was to assess whether women in community midwife teams (clusters) allocated to receive intervention care reported less UI at 10–12 weeks post partum than women in clusters allocated to receive standard care. Approximately 40 community midwife teams were to be recruited and randomised in 8–10 NHS Trusts across England.
Piloting the trial data collection instruments was the first phase of WP4 (4.1) but it was in the first stage of the programme (stage 1 being assessed to progress to stage 2) and a low rate of return of women’s questionnaires was found, at 31%. Shortly after commencing the programme, the team examined recent surveys in similar populations and realised that the proposed estimate in the grant application of 60% of women returning a postal questionnaire in the pilot study and 80% in the full trial (after developing additional online methods) was substantially over-estimated: recent return rates were around 30–35%. 2,3 The team considered what might maximise return rate. A Cochrane Review (‘Methods to increase response to postal and electronic questionnaires’) showed that giving incentives, and ones not dependent on returning the questionnaire, both increased response rates. 4 As a consequence, it was decided to send women a £10 voucher with the first questionnaire rather than on questionnaire receipt. The Cochrane Review also found a higher response from shorter questionnaires. The questionnaire developed for the pilot study was only four pages, but given that women who have recently given birth are busy, it was decided to pilot whether an even shorter questionnaire just including main outcomes might elicit a higher return rate. Thus, the WP4.1 pilot study was designed as a RCT to test a longer versus a shorter questionnaire. Women who had antenatal care from 15 community midwife teams in two NHS Trusts were randomly allocated to either long or short questionnaires. Those who returned the short questionnaire were then sent the remainder of the questions in another questionnaire. No difference in return rate was found between those randomised to the long or short questionnaire.
Possibly it was this pilot trial, of a long versus a short questionnaire, that led to a misunderstanding by National Institute for Health and Care Research (NIHR) stage 1 assessors that the pilot study had been a pilot trial assessing the proposed intervention, and that there was no difference across trial arms in the proportion of women doing PFME often enough (a few times a week or more) to possibly reduce post-partum UI. There had been no PFME intervention, but the misunderstanding was that there had been but with a lack of difference in PFME adherence. This, together with the low return rate, meant that the assessors considered that there was too much of a risk for a full trial to go ahead in WP4.2. Following the pilot trial of a long versus a short questionnaire, it was decided, in consultation with NIHR stage 1 assessors, that a full trial was a risk because, in addition to the low return rate, it may be burdensome to staff. The midwifery staffing levels and workload difficulties occurring subsequent to the programme being funded may have meant that additional midwifery tasks may be more problematic.
The APPEAL team therefore proposed a pilot and feasibility trial to collect additional process outcomes to determine whether a full trial could be feasible. A variation to contract (VTC) was submitted and NIHR agreed to this proposed change. This reduced second stage also included dropping the health economic analysis.
Work package 2 was discontinued following funding review
COVID-19 pandemic
The intervention developed in the early WPs of the programme was a training package for community midwife teams to enable them to advise and support pregnant women in undertaking PFME. The first face-to-face training session was delivered to six midwives in the week before lockdown (March 2020).
The APPEAL trial was then paused, along with all non-COVID trials, until January 2021. Following restart, it was likely that routine maternity care would continue to be affected by the pandemic, so during the trial pause it was necessary to take steps to ensure that the training intervention was ‘COVID-proof’. This meant amending the intervention so it could be delivered to midwives online and organising for women to be provided with resources in ways that reflected some routine maternity care contacts not being face to face. This was approved in a VTC, and some additional funding was given, which together with use of underspend, allowed the programme to be completed.
Implications for full trial
Members of the APPEAL team had been liaising with the Maternity and Women’s Health Policy Team at NHS England (NHSE) since 2018. As part of the NHSE Ten-Year Long Term Plan there was an intention to set up new perinatal pelvic health services across England, and NHSE were aware of our work and its relevance to the planned roll-out of this service. A significant component of the new pelvic health service is to ensure that midwives are trained in how to advise and support women to achieve better pelvic health, a central part of which includes women undertaking PFME in pregnancy and post partum. Had it not been for the pandemic, this service would not have been set up until the results from the APPEAL programme had been available. The APPEAL team are pleased that this new service is going ahead, members having previously undertaken research providing findings to show its need. It does mean, however, that a full RCT is no longer possible since many elements of the intervention will become part of standard maternity care.
The APPEAL team have continued to work with the NHSE Maternity and Women’s Health Policy Team in the early phases of the new NHS services being introduced. In July 2021 14 ‘early adopter’ local maternity and neonatal systems (LMNSs) were funded to develop a service, and 9 more ‘fast follower’ LMNSs were funded in July 2022. The aim is that by March 2024 all LMNSs will be required to set up a new perinatal pelvic health service. The APPEAL team are already working with numerous of these LMNSs, and more details are given later in the report.
Work package 1 particular context awareness: identifying barriers and enablers of change
Work package 1.1: critical interpretive synthesis
See also protocol Salmon et al. 5 (https://doi.org/10.1186/s13643-017-0420-z) and full paper Salmon et al. 6 (https://doi.org/10.1002/nau.24256).
Aims
The aim of WP1.1 was to undertake a systematic review using critical interpretive synthesis of what is known about the complex individual, professional, and organisational issues that enable or hinder the implementation of PFME training during women’s childbearing years.
Methods
Sources for inclusion were identified through structured database searches supplemented by purposive searches. Titles and abstracts were screened for eligibility and critically appraised using the Mixed Methods Appraisal Tool7 by two independent reviewers. Following data extraction using a structured template, the findings of included studies were coded using a framework based on the initial research questions. Patterns and themes were identified. New (synthetic) constructs were generated and linked to broader theory to explain the overall findings.
Key findings
Fifty quantitative and qualitative sources were included. The concept of agency (people’s ability to effect change through their interaction with other people, processes and systems) provided an overarching explanation of how PFME can be implemented during childbearing years. Women and healthcare professionals (HCPs) (individually or in groups), maternity services and organisations, funders, and policy-makers all have agency, although their capacity to implement PFME is enhanced or diminished by the professional, organisational, and policy environment.
Numerous factors constrained women’s and HCPs’ capacity to implement PFME. It is unrealistic to expect women and HCPs to implement PFME without reforming policy and service delivery to genuinely support women and HCPs in this endeavour. The implementation of evidence-based PFME requires policy-makers, organisations, HCPs, and women to value the prevention of incontinence by using low-risk, low-cost and proven strategies as part of women’s reproductive health care across the lifespan.
Limitations
For the reviewed quantitative studies, limitations included small sample size and lack of reporting of any sample size calculation; too few details regarding reasons for declining participation; lack of details regarding calibre of survey tools/outcome measures; and variable response rates. For the reviewed qualitative studies, many did not consider impact or relevance of contextual factors or did not demonstrate reflexivity.
Work package 1.2: focused ethnographic observation of clinical practice
See also Terry et al. 8 (https://doi.org/10.1016/j.midw.2020.102647).
Aims
The main aim of WP1.2 was to explore individual, HCP and organisational barriers and enablers to implementation of PFME and assessment in current UK practice. Further aims related to understanding how individual, professional and organisational factors interact to enhance or reduce effective PFME implementation in the UK context with a view to informing subsequent WPs in the APPEAL programme.
Methods
Designed with input from patient and public involvement and engagement (PPIE) activities, a variety of methods were planned: interviews from three differing sites with antenatal women and, for a subset of women, postnatal follow-up interviews; interviews with midwives and other HCPs; and observations in antenatal clinics. Additional data sources included field notes, photographs, websites, leaflets, clinic documents and antenatal service policies, all obtained from visits to the study sites.
Analysis used reflexivity and reflexive accounting (key aspects of ethnographic research9,10), with initial coding to develop a coding framework and then identify emerging themes (following principles of thematic analysis11) and adopted a process similar to the ‘constant comparative method’12 to guide theme development. Analysis was inductive (data driven) but also deductive in terms of addressing the specific objectives of the APPEAL research programme.
Extensive discussions about emerging findings took place among the research team, including with Jean-Hay Smith and Helena Frawley (our international specialist academic physiotherapy collaborators from New Zealand and Australia, respectively). These were ongoing remote and in-person meetings during June 2018.
Key findings
Data were collected at three sites (large city, small city, coastal town). A total of 23 midwives and 15 pregnant women were interviewed, with 12 also interviewed postnatally. Interviews were carried out with physiotherapists (n = 4), a link worker/translator (n = 1) and obstetricians (n = 2). Seventeen antenatal clinic observations took place.
Key findings based on all data, including clinic observation notes and interviews, were that women and midwives know that PFME training is important, but that midwives may not communicate fully to women the relatively large ‘gains’ available from engaging with PFME. There was widespread lack of confidence among women and midwives to initiate a discourse about PFME and UI. This was exacerbated by both misunderstandings and assumptions and by a lack of clear guidelines and policy, further impacting on communication between women and midwives.
Ethics
Ethical approval was obtained for the ethnography {HRA approval 15 December 2016 [Integrated Research Application System (IRAS) project ID 215180]}.
Limitations
A criticism of ethnographic research is that the presence of a researcher will impact the behaviour of those observed, creating an observer effect bias. 13 The midwives were fully informed of the purpose of this study, so it is possible that this bias occurred. It is argued, however, that even if this happens, ethnographies can still expose honest accounts about the topic under investigation. 14 Ethnographies do provide a detailed account and thus findings cannot be generalised to other women or HCPs; furthermore, we were not able to carry out interviews with women who did not speak English.
Inter-relationship of work package 1 with other parts of the programme
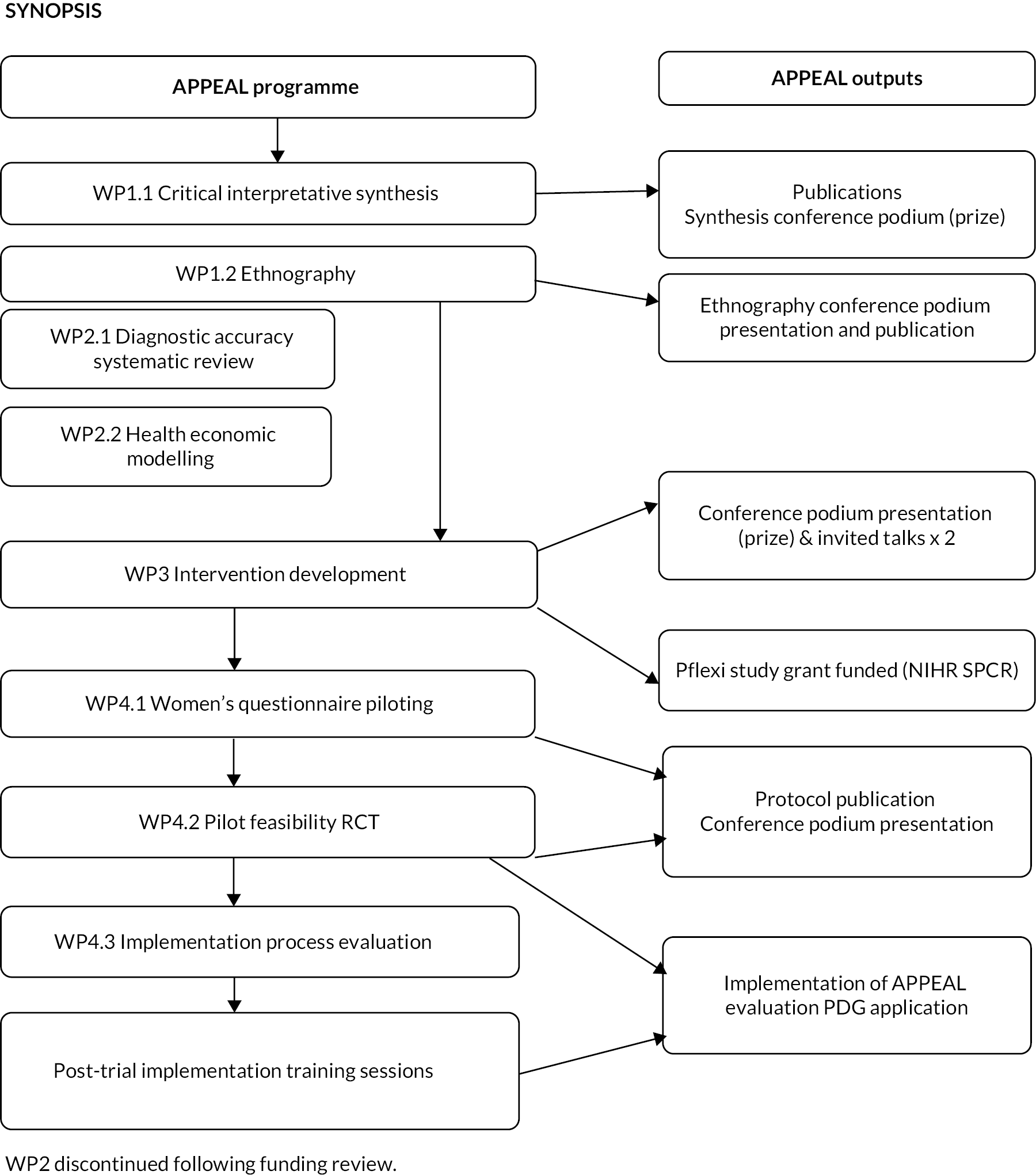
Both studies from WP1, one evidence synthesis and one empirical, were combined to create a platform of research to inform WP3 activities for the development of the APPEAL intervention, which would then be tested in the feasibility and pilot trial in WP4. Figure 1 shows diagrammatically how the whole programme fits together.
FIGURE 1.
Research pathway diagram. PDG, Programme Development Grant.
Work package 2: performance measurement: determining relevant measures of performance
Work package 2.1: diagnostic accuracy systematic review and data synthesis to identify the accuracy of tests for assessment of pelvic floor muscle localisation and contraction in women
Aims
The main aim of this study was to determine whether there were any tests for assessment of pelvic floor muscle (PFM) localisation that might be used to assist in the trial intervention.
Methods
A diagnostic test accuracy systematic review was undertaken to compare the accuracy of tests for assessment of PFM localisation and contraction in women. The population was not restricted to pregnant women to maximise data available; however, pregnancy was considered as a potential source of heterogeneity.
The secondary objectives of the review were to investigate other sources of heterogeneity; that is, factors which may affect test accuracy. Potential sources of heterogeneity included population characteristics (e.g. presence or absence of UI, obesity, menopausal status, parity, current pregnancy); index test characteristics (e.g. index test technology, expertise of test operator); and reference standard characteristics: whether digital vaginal palpation was conducted by a specialist [e.g. physiotherapists, obstetricians, gynaecologists, general practitioners (GPs) with accreditation/specialist interest in women’s health, specialist midwife or health visitor] or a non-specialist HCP. A further objective was to combine findings from WP1 to identify which of the most accurate tests are likely to be most acceptable to pregnant women. A comprehensive search strategy was developed in consultation with an international PFME expert. The following databases were searched: MEDLINE (Ovid), MEDLINE In Process (Ovid), EMBASE (Ovid), PsycINFO (Ovid), Cochrane Library (Wiley) CDSR, DARE, HTA, NHS EED and CENTRAL; CINAHL (EBSCO); SCI Web of Science SSCI. Ongoing trials registers (CT.gov and the World Health Organization’s International Clinical Trials Registry Platform register) were searched and abstracts and conference proceedings sought using Conference Proceedings Citation Index (CPCI) – Science and CPCI – Social Science and Humanities (Web of Science). Electronic searches were supplemented by citation searching and searching references of included studies and relevant systematic reviews. Databases were searched from inception to October 2016. No language limits were applied. In anticipation of a paucity of studies with a primary objective of estimating test accuracy, all study design types were considered for inclusion, except for diagnostic case–control studies as these are known to overestimate test accuracy. Test accuracy study design filters were avoided as these have been shown to miss studies. Titles and abstracts and full-text articles were screened for eligibility by two independent reviewers, with disagreements resolved by a third. A data extraction proforma and a quality assessment tool based on the QUAlity of Diagnostic Accuracy Studies tool15 tailored to the topic were prepared a priori.
Key findings
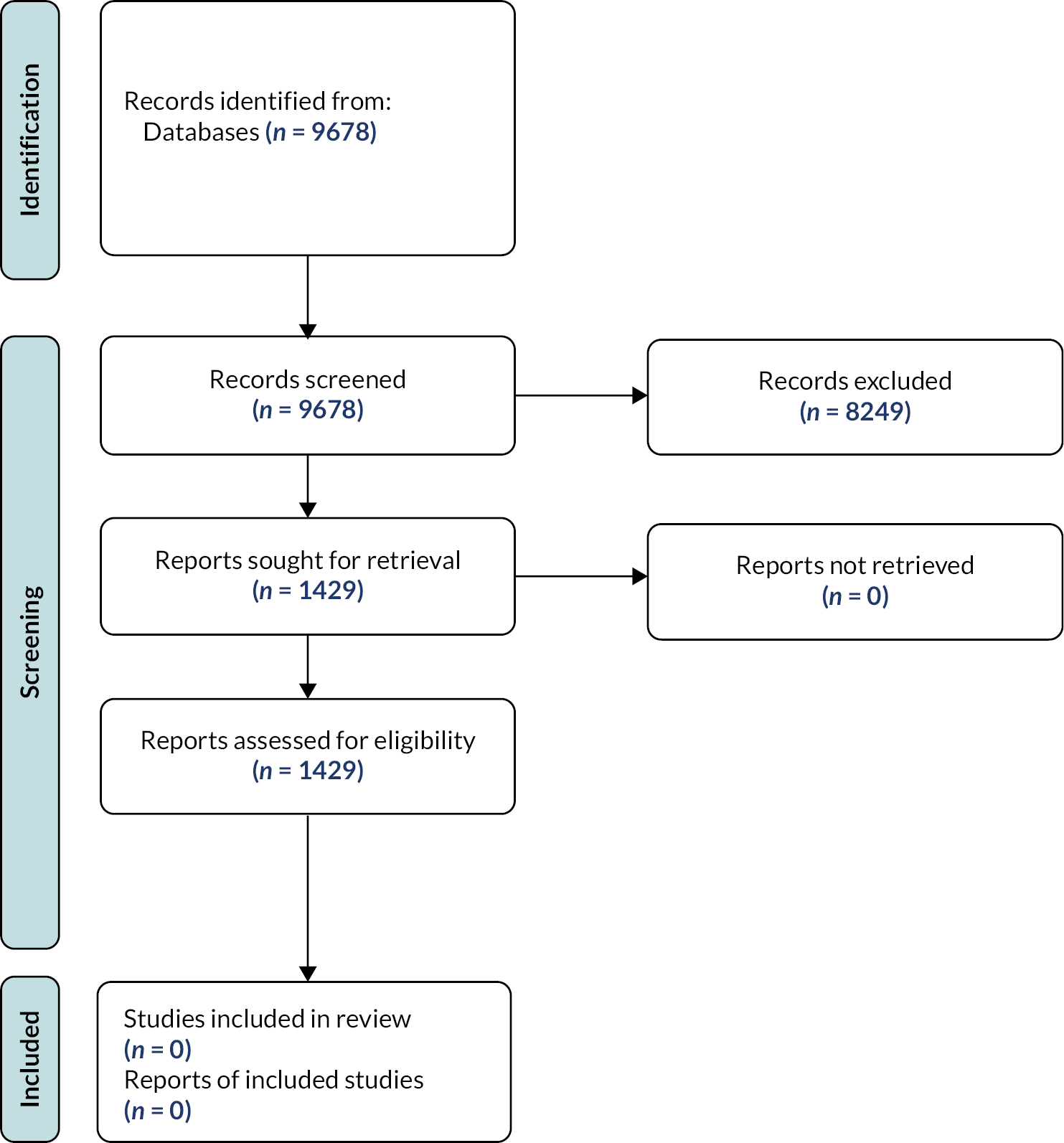
A total of 9678 unique title and abstracts were screened, and 1429 full-text articles subsequently retrieved. No studies met the review inclusion criteria. Most commonly, this was because of the absence of an index test (one or more of: methods for observation, palpation, pressure measurement, measurement of electrical activity by muscles, imaging with ultrasound) in parallel with the reference standard of digital vaginal palpation. In studies where an index test was conducted in parallel with the reference test, the paper did not provide information from which to derive a 2 × 2 diagnostic table and therefore an estimate of accuracy. This was because, except for one test accuracy study (that did not use an acceptable reference standard), all other studies identified used either an index test or the reference standard to measure the outcome of an intervention to improve PFM function. Furthermore, in the majority of these studies women were educated how to contract the pelvic floor musculature correctly so that any improvement in strength and endurance following an intervention could be measured. These studies therefore only had women who were able to contract their pelvic floor; thus, the ability of index tests to identify women unable to localise and contract their PFM could not be estimated (Figure 2).
FIGURE 2.
PRISMA flow chart of included studies.
Limitations
The lack of any available studies to assist in assessment of PFM localisation that might be used in the trial intervention was a major limitation.
Inter-relationship with other parts of the programme
The intention of this WP was to provide a diagnostic test to use in the intervention for midwives to check whether women were able to locate their PFM.
Work package 2.2: constructing a health economic decision-analytic model
Aims
The aim of this study was to collate relevant evidence to inform and develop a preliminary decision-analytic model (DAM) which would compare a range of alternative tests and treatment pathways for reducing UI among pregnant women. The overall objective of the preliminary model-based evaluation was to identify gaps in the evidence that required specific focus in the full definitive trial.
Methods
Systematic review
A pragmatic search of identified literature from WP2.1 showed insufficient evidence for the proposed model, and a scoping search did not find any economics studies on PFME among pregnant women. Hence a systematic review was conducted with the broader purpose of identifying evidence for economic evaluations of interventions to prevent and treat UI in general, not just in pregnancy, to inform a preliminary model.
The systematic review was conducted according to the guidelines of the UK’s Centre for Reviews and Dissemination. 16 A search strategy was developed using the population, intervention, comparison and outcomes framework. 17 Five electronic databases – MEDLINE, EMBASE, Web of Science, NHS Economic Evaluation database and CINAHL – were searched.
Formal economic evaluation and cost analysis studies were included if participants were females suffering from UI; the intervention was PFME to prevent or treat UI; the comparison was any alternative test and/or treatment strategy to prevent UI in women; and the main outcome was cost per quality-adjusted life-year (QALY). A two-stage categorisation process was used to screen studies for inclusion using published methods. 18,19 A quality appraisal was not undertaken because the review’s objectives required a description of all economic evidence.
Information on resource use and associated costs were extracted from reviewed studies. In addition, effectiveness data and health-related quality-of-life (QoL) data were sought from the published Cochrane Review1 and pragmatic literature searches. The collated evidence was used to estimate a baseline DAM which explored potential issues and/or gaps relating to either the intervention or QoL.
Preliminary model
We constructed a model in TreeAge (TreeAge Software, Inc., Williamstown, MA, USA) to compare the APPEAL intervention with usual care, based on a series of potential pathways to represent the wide range of viable pathways that women could follow. The pathways included scenarios in the intervention arm where pregnant women were referred to physiotherapists when they had UI or had problems locating their pelvic floor. Group and individual physiotherapy sessions were included in the modelled scenarios.
Effectiveness data were obtained from a Cochrane Review1 identified from a pragmatic literature search and an alternative study20 identified through hand searching. Similarly, utility values were obtained from a pragmatic search. 21,22 Cost and resource-use data used in the model were based on published sources. 23 Assumptions were made about all model parameters to form a complete set of values for the analysis, and these parameters were varied in sensitivity analyses to see the effect on the results.
Key findings
Systematic review
The search identified 10 studies22,24–32 out of a possible 1163. Overall, all the studies evaluated the cost/cost-effectiveness of PFME as treatment rather than as prevention for UI. The delivery of PFME differed between the studies with variations in the number, duration and frequency of physiotherapy sessions.
The studies were mostly formal economic evaluations (n = 8), with only two cost analyses. The majority (n = 6) of the economic evaluations were cost–utility analyses (with outcomes presented in terms of cost per QALY). There were variations, however, in the instruments used to measure QoL. Three of the studies (two cost–utility analyses and one cost-effectiveness analysis) were model-based. All studies included direct medical costs, and six studies included some form of direct non-medical costs (e.g. the cost of incontinence pads). Only three studies included indirect non-medical costs such as time forgone by not working and time for extra laundry loads. All but one study recommended PFME as a cost-effective intervention for treating UI.
Economic analysis
Initial results from the pre-trial economic analysis suggested some potentially helpful information for the trial design and proposed data collection. An example of such information was that the time spent by midwives providing the intervention was not likely to be a key driver in the results; this allowed the trial team to be non-prescriptive about midwives recording the time spent with women, which was initially a cause for concern. However, the preliminary results were extremely sensitive to both the effectiveness of the intervention and the QoL data inputted into the model.
Limitations
The systematic review and additional reviews provided some evidence for the pre-trial model. There were insufficient data, however, to carry out a complete economic evaluation without some fundamental assumptions. Many of the data used were proxy values from other studies of related (but not the same) populations.
Inter-relationships with other parts of the programme
The purpose of the pre-trial model was to identify gaps in the literature and areas worthy of particular focus, such as data collection, in the definitive trial. When the second stage of the programme was changed to be a feasibility and pilot RCT rather than a full definitive trial, however, the health economic analysis was discontinued.
Work package 3: plans for change: developing the constituents and means of delivery of the Antenatal Preventative Pelvic floor Exercises and Localisation intervention
Some text in this section has been reproduced with permission from Dean et al. 33 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Aims
Work undertaken in WP3 aimed to develop a training package and resources for midwives to give them the confidence to teach and support women to do PFME within routine antenatal care. The aim also included developing resources for midwives to give to women, providing further support for women to do PFME during pregnancy. The developed intervention was planned to operate at two levels: to change midwives’ behaviour (to teach and support women to do PFME) and to change women’s behaviour (to do PFME during pregnancy). The training package and resources would then be evaluated in the pilot and feasibility cluster RCT described in WP4.2 and WP4.3.
Methods
Development followed the Medical Research Council guidance for complex interventions34 and was guided by the Consolidated Framework for Implementation Research. 35 Designed over four iterative phases, the intervention development included a stakeholder event and multiple PPIE activities.
Ethics approval was obtained for WP3 phases 1 and 3 (University of Exeter Medical School Research Ethics Committee 18/06/169; HRA approval: IRAS project ID 238874).
Phase 1: focus groups
Two participant groups were planned: pregnant women or those who had given birth within the previous 12 months, and midwives providing antenatal care. They were invited to take part in separate focus groups in a range of sites in England. Data were analysed using thematic analysis. 11
Phase 2: development of a training programme including intervention mapping
The intervention was developed using data from WP3 phase 1 and WP1. A comprehensive mapping exercise was undertaken using the behaviour change wheel (BCW), theoretical domains framework36 and behaviour change technique (BCT) taxonomy (v1). 37 PPIE activity included APPEAL advisory group meetings and a wider group of advisers undertaking a citizens’ jury style assessment of mobile phone apps to determine which might be recommended as part of the women’s resources to support PFME. 38 A national stakeholder event was arranged to consider midwife training needs and antenatal service provision.
Phase 3: practice training event
An in-person practice training event was carried out with a cohort of midwives located in a different region to the future trial. A 5-point (scoring 0–4) Likert scale eight-item questionnaire was designed to assess midwives’ confidence before and after training regarding pelvic floor knowledge and teaching PFME. Focus group style discussions took place after the training session to obtain feedback on intervention format, content, and methods of delivery. An anonymous evaluation questionnaire with options to rate the training and provide free-text comments was also prepared. Researchers facilitated these activities and made field notes to record discussions and recommendations.
Phase 4: intervention refinement
Findings from WP3 phase 3 and further PPIE were used to refine the format and content of the intervention package. Additional refinements were made in response to COVID-19.
Key findings
In WP3 phase 1, 12 women (age 20–44 years; education range: secondary education to postgraduate degree) and 14 midwives (age 25–59 years; midwifery experience: 3–32 years) took part in six focus groups.
The practice training in WP3 phase 3 was attended by a different cohort of 18 midwives (age 25–60 years; midwifery experience: 2–20 years). Of the 32 midwife participants in WP3 phases 1 and 3 of the study, 13 (41%) reported no previous PFME training (formal or informal), 2 (6%) had attended specific pelvic floor rehabilitation courses, 9 (28%) reported varying amounts of training and 8 (25%) did not provide data on this.
The PPIE advisory group included six mothers with young children, with nine meetings held. The citizens’ jury included 10 women who had given birth within the previous 5 years, with two meetings held.
The stakeholders were 20 delegates representing 18 organisations across the UK with an interest in maternity services (see Acknowledgements).
Phase 1
Four themes emerged from the six focus groups. These were: ‘knowing’, ‘doing’, ‘remembering’ and ‘supporting’ antenatal PFME. Illustrative quotes for each theme are available in Appendix 1.
Knowing about pelvic floor muscle exercises and urinary incontinence
Analysis showed a lack of information and promotion of antenatal PFME meaning that women were unaware of the importance of PFME, and a lack of training and guidance for midwives. Midwives needed more knowledge on anatomy and muscle training physiology, and women needed to know why PFME were important and the consequences on UI of not doing them. Both groups agreed that midwives needed the confidence to raise the topic and make sure all women understood about PFME and their relationship to UI. For midwives, knowing the importance of PFME included wider implications, such as economic implications.
Doing pelvic floor muscle exercises
Midwives and women wanted guidance on how and when to teach and perform PFME; analysis made it clear that this should be done early in antenatal care and followed up at every subsequent appointment. Midwives and women were ambivalent about doing vaginal examination for checking the correct muscle contraction was happening. This was not regarded as something universally needed but could be offered if required.
Remembering pelvic floor muscle exercises
Prompts were seen as critical to support women to do PFME and to support midwives to implement PFME teaching. Both wanted consistent and regular reminders to help women put PFME into a daily routine. Women emphasised the need for meaningful dialogue to support them, not just simply asking whether they are doing their exercises. Women suggested signposting to smartphone apps, and midwives suggested a variety of cues, such as key chains, lanyards, and visual prompt cards.
Supporting implementation and delivery of pelvic floor muscle exercises
Women wanted multimedia options for delivery of PFME information, including online and written resources. Although debated by women and midwives, there was overall agreement that a paper leaflet, given at the booking appointment, would be acceptable provided it was accompanied by detailed verbal instruction at a subsequent appointment, hence acting as a reminder.
Midwives wanted PFME training to be delivered face to face, with demonstrations from experts on how to teach effective PFME. E-learning was not regarded as acceptable for initial training but could be an option for refresher training, as it was likely to improve accessibility. Finding time to fit in PFME training was clearly going to be challenging, and many midwives believed it should be mandatory. Implementation of training would require local organisational support to enable midwives to attend.
Further suggestions for maximising implementation included using a ‘train the trainer’ model and having a midwife PFME champion within each midwife team. Midwives also wanted to know more about local referral options and strengthening of referral pathways to physiotherapy services for women they identified as needing further support. Both midwives and women felt the need to raise awareness and strengthen support for antenatal PFME at a wider societal level, and suggested a national campaign.
Midwives were also asked to consider methods for assessing fidelity to the proposed intervention in the future trial; they suggested a PFME tick box on antenatal appointment checklists, audit of antenatal notes, and observations of midwifery practice. They did not consider audio or video recording of appointments acceptable.
Phase 2
In this phase, data from WP3 phase 1 and the earlier APPEAL research6,8 were mapped onto the BCW. This helps identify intervention functions (the broad categories of things that can be done to change behaviour) and intervention content and BCTs and these were all discussed by the research team.
One example relates to the theoretical domain of knowledge (awareness of the existence of something), which is a psychological capability component of the capability–opportunity–motivation–behaviour (COM-B) model. 36 For this to be implemented, women need to develop understanding of PFME and their relationship with pelvic health and UI, and understand the principles of how PFME might reduce UI. Similarly, midwives need to develop knowledge and understanding of the rationale for antenatal PFME for promoting PFM health during pregnancy and childbirth.
The proposed intervention functions (in this example the methods by which these knowledge gains can be achieved) through education, with the policy category (vehicle) being service provision and guidelines, and marketing-style communications. Thus, the ideas for intervention content (application) were designed to follow these principles at the two levels. At the level of women, the intervention needed to ensure that midwives could provide information and facts relating to what the pelvic floor is, its role relating to UI, what happens in pregnancy, what PFME are, why they are important, how to do them, and what the benefits are, including information on their effectiveness for preventing UI as well as other benefits of PFME. At the level of midwives, the intervention needed to cover a review of anatomy, physiology and function of PFMs, presentation, and discussion of evidence for prevalence of UI during/after pregnancy, including effectiveness of antenatal PFME for reducing UI. Furthermore, the intervention needed to impart new knowledge and understanding to midwives about the impact of UI on physical and mental health, giving examples of women’s stories (e.g. social isolation, shame, embarrassment) as well as including information on other benefits of PFME (e.g. sex, reducing time of second-stage labour).
These knowledge elements of the intervention were then coded to the BCT taxonomy, namely: shaping knowledge (instruction on how to perform a behaviour, BCT 4.1) and informing about natural consequences (information about health consequences, BCT 5.1; salience of consequences, BCT 5.2; and information about social and environmental consequences, BCT 5.3).
Finally, comments or issues arising from PPIE advisers and stakeholders were added to the map. In this example they commented that ‘knowledge is power’ for both midwives and women, but also had wider importance within society, as it would help raise the profile, and thus result in a clearer understanding of why PFME is important.
The full mapping exercise is included in Report Supplementary Material 1. Once mapping was completed, the first iteration of the intervention was prepared. Intervention materials included a midwife training programme and resources for midwives to support PFME implementation, and a resource package for women to be given by midwives during the antenatal booking appointment.
The training programme for midwives included five steps for putting PFME into antenatal clinical practice: (1) raise the topic of PFME; (2) screen for UI; (3) teach PFME; (4) prompt/remind women about how to perform PFME; and (5) refresh women’s understanding about PFME and refer to specialist services if required. A manual containing training session slides, summary leaflets and additional resources about PFME and UI was prepared for midwives.
The PPIE advisers helped co-develop the resources for women. In addition, the citizens’ jury work considered smartphone apps from a list provided by the app review undertaken by APPEAL’s collaborators in New Zealand. 38 Jury members tried out various apps and the three most highly rated were added to an app decision card with QR codes supplied to ensure ease of access. Other resources were a leaflet with information about PFME and how to perform a correct PFM contraction, and stickers to use as prompts/reminders for PFME. These, with the app decision card, were placed in a small bag to be given by midwives to women at their antenatal booking appointment.
Phase 3
Following the practice training event, evaluation scores were positive for content and delivery, and participating midwives (n = 18) found it useful. Free-text responses indicated that midwives acknowledged the importance of taking the lead regarding PFME, but lack of time, confidence, and skills to raise the issue presented challenges for implementing PFME in practice. However, these midwives reported an increase in total confidence relating to PFME from 2.70 (range 1.18–3.50) before training to 3.68 (range 3.37–4.00) after training, suggesting that with additional refinements the training had potential to address some of these challenges. The midwives raised additional considerations for implementation of PFME, time constraints of antenatal appointments, not wanting to overload women with too much information early in maternity care, and whether insufficient continuity of care would impact on their ability to develop rapport with women to teach and support PFME throughout pregnancy. The midwives also highlighted the importance of establishing PFME champions within midwifery teams and the need for buy-in from senior midwives/clinical managers to support implementation.
Phase 4
Intervention materials and content were modified and refined by the research team and PPIE advisory group in response to feedback from the practice training event. Examples were more detail for the anatomy refresher and for the physiology to cover muscle exercise training principles; and to package resources for women in a cloth bag big enough to hold a clean nappy (not a box, which would take too much space for midwives). A second version of the midwives’ manual was prepared as the trainer’s manual, with extensive speaker notes, to facilitate the plan for using a ‘train the trainer’ model in future implementation. The training session was shortened from half a day to 2 hours. Additional training and resources were developed to support a PFME champion midwife role. Further modifications were made at the onset of the COVID-19 pandemic to enable training delivery via the Zoom online platform (Zoom Video Communications, San Jose, CA, USA), and to support midwives to deliver intervention elements via telephone appointments. The final output of WP3 phase 4 was the development of the logic model for the pilot feasibility trial in WP4 (see Appendix 2, Figure 4).
Limitations
The challenges identified by midwives regarding wider system pressures,6 past difficulty accessing training, and appointment time constraints did limit intervention range and complexity. Despite these constraints, midwives and women believed that implementing PFME in antenatal care would be beneficial for large numbers of women.
At this stage, evaluation of the APPEAL intervention has used observational, before–after or qualitative data; findings from the pilot feasibility RCT in WP4.2 and 4.3 add to this evaluation.
Inter-relationship with other parts of the programme
We benefitted from continuing to work closely with PPIE advisers and drew upon known BCTs throughout the four phases to develop a comprehensive understanding of the needs of midwives, women, and stakeholders within current organisational contexts. Findings from WP1, and behaviour change theory helped us bring WP3 activities together into a training package for midwives and resources for supporting women to address these needs, to be used in WP4.
Work package 3 aimed to ensure that whatever the APPEAL intervention asked of women and midwives for implementing PFME was evidence-based, with sound theoretical underpinnings and consensus acceptance from experts and lay members of the public. Our extensive PPIE, plus national stakeholder involvement, added credibility to intervention development, which has been piloted, refined, and adapted for remote delivery.
Work package 4: piloting the intervention
Some text in this section has been reproduced with permission from Webb et al. 39 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Work package 4.1: what is the response rate for the trial questionnaire and the intraclass correlation coefficient?
See also Appendix 3 for full report of WP4.1 and Tables 5–8.
Aims
The main aim of WP4.1 was to test the data collection instruments in the questionnaire to be sent to women at 10–12 weeks post partum and, in so doing, to test what the return rate might be in a full trial. We also wanted to estimate the intraclass correlation coefficient (ICC) for a full trial. This study was completed in the first stage of the programme before there was a change to a feasibility and pilot trial for the second stage.
Recent studies in similar populations2,3 showed that response rates had been dropping, so it was decided to also assess whether a long or shorter questionnaire might give a higher response.
Methods
This study was an individually RCT undertaken in the two NHS Trusts which would be part of the subsequent pilot cluster trial. Eligible women (all except those under 16 years or with no live baby on hospital discharge) cared for in 15 community midwife teams who gave birth in two Trusts during the study period were included.
A sample size of 800 women with an anticipated response rate of between 30% and 60% would allow a return of between 240 and 480 questionnaires. With a sample size of 800, with 400 allocated to each trial arm, at 80% power and 5% significance, the study would be able to detect a 10% absolute difference in the percentage response rate between the shorter and longer questionnaires.
The first 54 consecutive women to give birth during July 2018 from each of the 15 midwifery teams were informed by their midwife at their first postnatal home visit that they would receive a postal questionnaire at 10–12 weeks post partum about incontinence and PFME.
The questionnaires comprised several validated measures to assess: UI [International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form (ICIQ-UI SF40)]; faecal incontinence (FI) [Revised Faecal Incontinence Scale (RFIS41)] (FI was included in APPEAL as PFME are generally considered to be a help but RCT evidence to show that antenatal PFME can reduce post-partum FI is not of high quality, hence not a major focus of APPEAL); confidence in PFME [Pelvic Floor Muscle Exercises Self-Efficacy Scale (PFMESES42) and Exercise Adherence Rating Scale (EARS43)]; and general health [Short Form questionnaire-12 items (SF-1244)]; as well as questions about advice that women may have received on PFME in pregnancy and their PFME practice. The long questionnaire comprised two double-sided pages with all the above measures. The short questionnaire comprised one double-sided page and did not include PFMESES, EARS or SF-12. For women who returned this questionnaire a second questionnaire, which included PFMESES, EARS and SF-12, was then sent.
Individual randomisation to the long or the short questionnaire was undertaken using a computer programme at the Birmingham Clinical Trials Unit (BCTU), stratified by community midwifery team to account for potential variation between different sociodemographic areas.
Local Trust research midwives sent the questionnaires with a cover letter, a £10 voucher, and a stamped return envelope, then sent a reminder questionnaire to women who had not responded by 2 weeks.
Permission was obtained from the Proportionate Review Subcommittee of the London – Brighton & Sussex Research Ethics Committee (18/LO/0934).
Key findings
A total of 777 women were randomised to receive a long (n = 387) or short (n = 390) questionnaire. Overall response rate was 31.3% (243/777). Response rate in the long questionnaire arm was 30.8% (119/387) and 31.8% (124/390) in the short questionnaire arm [absolute difference in return rate −1.05%, 95% confidence interval (CI) −7.6% to 5.5%]. While not statistically significant, this finding rules out large differences between response rates. The ICC of response rate was 0.007 (95% CI 0.0005 to 0.094). The baseline characteristics of the women who returned a long questionnaire were similar to those who returned the shorter version.
Of total responders, 49% (119/243) reported some leaking of urine based on responses to the ICIQ-UI SF. 40 A total of 64.2% (156/243) of women reported receiving advice to perform PFME during pregnancy from their midwife. There were 42.4% (103/243) of women who reported doing PFME often enough (a few times a day, once a day, a few times a week) to possibly reduce post-partum UI. All these responses were similar between the long and short trial arms.
The questionnaire in general was well completed by the women, with low rates of missing values, but the response was low, despite having incorporated Cochrane Review4 recommendations to increase responses to postal questionnaires.
The estimated ICC for the return rate was 0.007 (95% CI 0.0005 to 0.094) The upper limit of the 95% CI was taken as a conservative estimate of the ‘true’ return rate ICC and was used to inform the sample size calculation for the pilot trial in WP4.2.
Limitations
The main limitation in this study was the low return rate of questionnaires from the women.
Inter-relationship with other parts of the programme
This WP was planned to test the data collection instruments and procedures ready to undertake a full trial. The low rate of questionnaire return, however, increased the risk of proceeding to a full trial (see Summary of alterations to the programme’s original aims/design). The data obtained from WP4.1 were used to inform the feasibility and pilot trial described in the next section.
Work package 4.2: feasibility and pilot cluster randomised controlled trial of an antenatal preventative pelvic floor muscle exercise intervention led by midwives to reduce postnatal urinary incontinence (Antenatal Preventative Pelvic floor Exercises and Localisation Programme)
Some text in this section has been reproduced with permission from Bick et al. 45 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Aims
The aim of WP4.2 was to assess the feasibility of undertaking a future trial of a midwife-led antenatal intervention to support women to perform PFME in pregnancy to reduce post-partum UI. Other aims were to assess intervention acceptability to midwives and the women they supported. Objectives were:
-
Provide training for community midwife teams randomised to the intervention arm to encourage incorporation of a PFME care package into their usual antenatal care (outcomes related to these objectives reported in WP4.3).
-
Assess whether training, intervention implementation and trial processes were acceptable to midwives (outcomes related to these objectives reported in WP4.3).
-
Assess whether midwife characteristics (e.g. years qualified) were similar across trial arms.
-
Assess questionnaire return rates from women at 10–12 weeks post partum overall and in both trial arms to assess feasibility and allow sample size estimation for a full-scale RCT.
-
Assess characteristics of women overall and within trial arms who returned questionnaires compared with all those who gave birth in the same midwife teams over the same study period but did not respond, using anonymised routine data.
-
Assess whether baseline characteristics collected following birth (self-reported UI at pregnancy commencement, maternal and obstetric characteristics collated from maternity records) were similar across trial arms.
-
Assess midwife support for PFME in both trial groups using women’s questionnaire data and qualitative interviews with midwives and women.
-
Assess women’s practice of PFME during and after pregnancy (outcomes related to qualitative interviews reported in WP4.3) using women’s postnatal questionnaire and interview data.
-
Assess prevalence of UI and FI at 10–12 weeks post partum using women’s questionnaire data to inform the sample size calculation for a full RCT (FI was included in APPEAL as PFME is generally considered to be a help but RCT evidence to show that antenatal PFME can reduce post-partum FI is not of high quality, hence not a major focus of APPEAL).
-
Undertake any necessary revisions to the APPEAL training package and following this, recommend roll-out by midwives to all pregnant women as part of the NHS Long Term Plan (outcomes related to this objective reported in the section on practice implications).
Methods
The study design was a feasibility and pilot cluster RCT, with community midwife teams forming the clusters. This design was chosen as randomisation of individual women would likely lead to contamination because midwives would have to provide two forms of care; and randomisation of individual midwives was not possible because midwives provide care on a team basis.
Midwife teams (clusters) were randomised in a 1 : 1 ratio to standard care only or standard care plus intervention. A minimisation algorithm ensured approximate balance over the variables:
-
midwife team size, defined by number of monthly births
-
NHS Trust.
Blinding of midwives providing care was not possible. Women receiving antenatal care were not explicitly blinded but the PFME support they experienced was the usual care provided by their midwives. Due to the nature of recorded data, those responsible for conducting trial analysis could not be blind to allocation.
Ethics approval
Protocol version 4.2, dated 21 November 2021, approved by West Midlands – Edgbaston Research Ethics Committee (19/WM/0368, approval date 10 January 2022). Study sponsor is Birmingham Women’s and Children’s Hospital NHS Foundation Trust.
Intervention
Two research midwives led and facilitated online training sessions developed from earlier WPs which lasted approximately 2 hours. Midwives in intervention teams were asked to introduce the topic of pelvic floor health at antenatal ‘booking’ appointments or as early as possible after this to all women. All women were given an APPEAL resource pack, including an APPEAL leaflet with PFME information, a link to APPEAL developed videos, recommended apps to support PFME, and APPEAL logo stickers to use as reminders. Women were to be asked by the midwife at all subsequent antenatal appointments about PFME progress and any problems with PFME or incontinence symptoms. In teams where a high proportion of women were non-English-speaking, the maternity support workers (MSWs) who provided translation were also trained. Midwives and MSWs had 2–3 months to practise implementing the PFME intervention into their routine care.
Midwife ‘champions’ in each intervention team received additional training on how to support and manage women with more severe UI symptoms, referred to them by team colleagues, and recommend appropriate specialist referral; and provide reminders and advice for their team to implement the intervention.
Feasibility and pilot outcomes
Feasibility of undertaking a definitive future trial was assessed by:
-
questionnaire return rates from women who gave birth over a preselected 1-month period, at 10–12 weeks post partum overall and across trial arms
-
prevalence of UI at 10–12 weeks using the ICIQ-UI SF,40 and FI at 10–12 weeks using the RFIS41
-
women’s practice of PFME, their adherence (predefined as PFME a few times a week or more) and PFME confidence using the PFMESES,42 and EARS43 (as data on QoL were not relevant for a feasibility trial, the SF-1244 scale was not included in the questionnaire used in WP4.2). During data entry, it was noted that responses to four items from the 17-item PFMESES were missing. An error occurred when the questionnaire was downloaded from FORMAP (online tool for designing case report forms for clinical trials), which omitted these four items. However, when viewing the FORMAP version online, all four items were visible. The missing items were raised with the trial statisticians and the Trial Management Group decided to send out a corrected questionnaire containing the full set of questions to women who had not returned the first questionnaire. As this was a feasibility trial, when reporting summary data for the PFMESES questionnaire, no distinction was made between missing responses from women and missing responses due to women being sent incomplete questionnaires. Sites were asked to stop sending reminder copies of the questionnaires until a corrected version was available.
Process outcomes included whether the intervention could be implemented within routine antenatal care; whether midwives provided support for PFME in both trial arms; and women’s and midwives’ experiences in both trial arms of advice and support to perform PFME during pregnancy.
For the original aim of a full trial to go ahead, the following progression criteria had to be met:
-
Questionnaire return rate from women across trial arms did not result in substantial bias, indicated by either a high overall return rate and/or that women who returned questionnaires in both trial arms had similar baseline characteristics.
-
Women’s self-reported adherence to performing PFME was higher among women in intervention clusters than in controls.
-
Midwife support for PFME was reported as greater among women in intervention clusters than in controls.
Sample size
Sample size was based on the number and size of clusters needed to estimate the return rate of questionnaires (across trial arms) to an acceptable level of precision. In WP4.1 the estimated ICC for return rate was 0.007 (95% CI 0.0005 to 0.094). Assuming a conservative ICC of 0.1, the width of the 95% CI for different rates (e.g. return rate of questionnaires) was estimated based on a t-distribution. 46 To set a conservative upper bound on the required sample size, we determined the widest 95% CI for a given sample size which will occur for a rate of 50%.
To reflect changes from WP4.1 in the number of midwife teams and women cared for by the teams, the overall sample size target was around 1400 (17 clusters of average size 82) to estimate the 95% CI for return rate to a maximum width of 17.2%.
Data collection and analysis
Analyses were based on the intention-to-treat principle. Data analysis was descriptive, mainly focused on CI estimation, with no hypothesis testing. Analysis methods included:
-
Continuous end points summarised using means and standard deviations (SDs), by arm.
-
Categorical (dichotomous) end points (e.g. return rates of questionnaires, adherence to PFME). The number of participants and percentages experiencing the event were summarised by arm.
For all total scores and dichotomous feasibility outcomes, summary measures and 95% CIs per trial arm were estimated using a cluster-level analysis based on t-distributions with K − 1 degrees of freedom (K denotes the number of clusters in each group) and appropriate transformation where necessary (and weighting if there was variation in cluster sizes). Quantitative data were summarised using descriptive statistics.
Women’s questionnaires
Trust research midwives were asked to exclude women who had a stillbirth or neonatal death, those whose infants were taken into care, and women who had severe mental health problems, providing these data were available in maternity records. All women were to be told by their midwives at their first postnatal home contact that they would receive a questionnaire at 10–12 weeks postnatally enquiring about any incontinence they may have experienced and their PFME performance during and after pregnancy. If more than 2 weeks had elapsed since the initial questionnaire was sent but not returned, another copy was posted to the woman. A £10 voucher was offered to women who returned their questionnaire (questionnaire shown in Report Supplementary Material 2).
Trust research midwives were also asked to identify women who did not speak English as a first language from their maternity record. If the woman’s first language was Urdu, Polish, Romanian or Arabic (main non-English languages in the areas), a shortened version of the questionnaire and a cover letter translated into the relevant language were sent. As only the ICIQ-UI40 SF is translated in these languages, questionnaires sent to women who required a translated version did not include the RFIS,41 the PFMESES,42 or the EARS. 43
Responses from completed questionnaires were entered by a research midwife at the NHS Trust sites, who provided a list of the hospital/NHS numbers of women who consented for their maternity records to be accessed, to obtain information on:
-
woman’s age, ethnicity, parity, onset of labour (spontaneous/induced), mode of birth (spontaneous vaginal, instrumental, caesarean section), anaesthetic/analgesia used, perineal trauma, episiotomy, duration of active second stage
-
baby’s gestation at birth, birthweight and head circumference.
Key findings
As this was a feasibility trial, no statistical tests were used for comparisons between groups for any outcomes with only group-specific point estimates and 95% CIs used.
Women’s questionnaire return rate
Appendix 4, Tables 9 and 10 present information on sending/receiving women’s questionnaire by group and the total number of women who gave birth at both study sites during the 1-month birth period. Appendix 4, Table 11 presents women excluded from receiving a copy of the questionnaire by study site. After exclusions, a total of 998 women who received antenatal care from 1 of the 17 study clusters randomised to the trial and who met inclusion criteria were sent a questionnaire; 175 (17.5%) women returned a questionnaire. Of 531 questionnaires sent to women in intervention clusters, 88 (16.6%) were returned and of 467 in the control clusters, 87 (18.6%) were returned (Figure 3). Ninety women were sent a translated questionnaire, and eight women (five in intervention clusters and three in control) returned this.
FIGURE 3.
Work package 4.2 CONSORT flow diagram. a, Figures based on the total number of women who received antenatal care and gave birth across both study sites during the 1-month sample birth period.
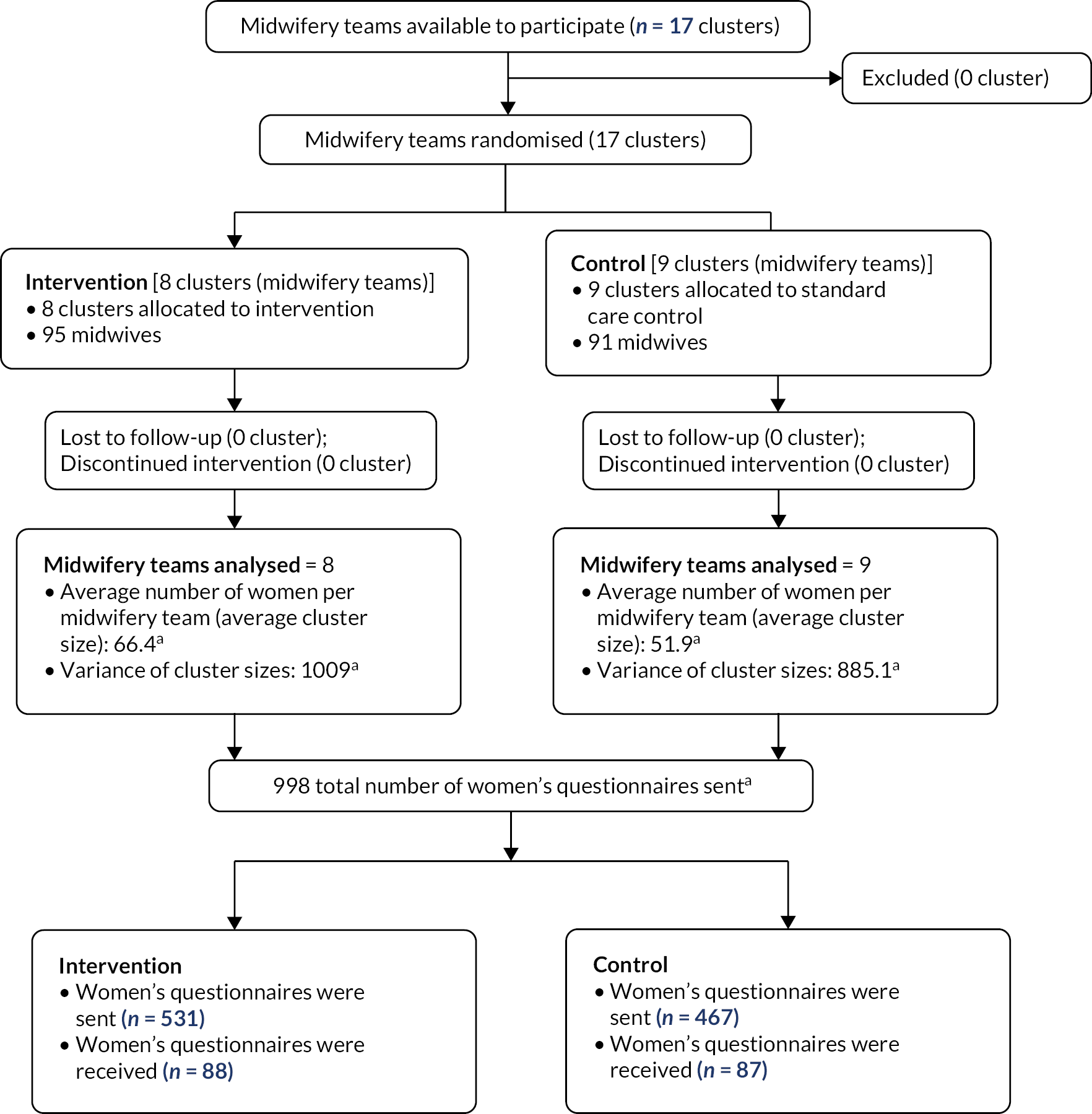
Table 1 presents the estimation of the return rate of women’s questionnaires pooled across both arms. As the return rate was low, an overall estimation provides more representative information than separate estimates by trial arm.
| Intervention: 8 clusters (n = 531) | Control: 9 clusters (n = 467) | Overall: 17 clusters (N = 998) | |
|---|---|---|---|
| % return rate estimationa | % return rate estimationb | % return rate estimation (95% CI)c | |
| Percentage returning women’s questionnaire | 15.8 | 16.4 | 16.0 (11.6 to 21.4) |
A summary of the minimisation variables by treatment arm and those not returning a questionnaire is shown in Appendix 4, Table 12.
Characteristics of women who did not return a questionnaire (see Appendix 4, Table 13)
Characteristics of women who gave birth in the study sample month but did not return a questionnaire were obtained and, using anonymised routine obstetric data, were compared with those who did return a questionnaire. Around one-third of women who did not return a questionnaire were of White British ethnicity (371/34.9%), which is a lower proportion than in the group who did return a questionnaire, and 205 (19.3%) women were of Pakistani origin, which is a higher proportion than among those who did return a questionnaire. Ethnicity data were unavailable for 205 women (19.3%) who did not return a questionnaire. Of the women who did not return a questionnaire, a higher proportion were having a second or higher birth order baby than those who did return a questionnaire. Obstetric and infant characteristics were similar among women who did and did not return a questionnaire.
Women’s baseline characteristics (see Appendix 4, Table 13)
The overall mean age of women who returned a questionnaire was 31.8 years (SD 5.2 years). Of these women, 77 (50%) were recorded as White British, 10 (6.5%) Pakistani, 7 (4.6%) Black African or Black Caribbean, and the remainder as ‘other’ ethnic minority groups. Data on ethnicity were not available for 21 women (12%). Overall, 62 women had given birth for the first time (41%), 61 (40.4%) for the second time, 28 (18.6%) had two or more previous births. Data on parity were missing for 24 (13.7%) women.
Women’s obstetric outcomes were similar across trial arms except for induction of labour: 23 (29.5%) women had induction in control clusters and 12 (15.8%) in intervention clusters. Data on mode of birth were missing for 22 women. Infant outcomes including gestation, birthweight and head circumference were similar across trial arms.
When asked in the questionnaire about their symptoms of UI at pregnancy commencement, 24 women in intervention and 24 women in control clusters reported some degree of UI, although in most cases this was infrequent (Table 2).
| How often did you leak urine at the start of your pregnancy? | Intervention (n = 88) | Control (n = 87) |
|---|---|---|
| Never | 60 (68.1%) | 61 (70.0%) |
| About once a week or less often | 13 (14.7%) | 12 (13.8%) |
| Two or three times a week | 4 (4.6%) | 6 (6.9%) |
| About once a day | 3 (3.4%) | 4 (4.6%) |
| Several times a day | 4 (4.6%) | 1 (1.2%) |
| All of the time | 0 (0%) | 1 (1.2%) |
| Can’t remember | 4 (4.6%) | 2 (2.3%) |
Midwife support for performing pelvic floor muscle exercises in pregnancy
Women were asked in the questionnaire about midwife support for PFME in pregnancy. Overall, 73 (83%) women in the intervention and 54 (62.1%) in the control arms reported that their midwives had advised them to perform PFME. The critical information, however, and the main prespecified assessment of midwife support, was ‘did your midwife explain how to perform PFME when pregnant?’ and 57 (64.8%) of women in the intervention and 33 (37.9%) in the control arms reported that they had (Table 3). Thirteen (14.8%) women in the intervention arms reported that a midwife never talked to them about PFME; this figure was 30 (34.5%) women in the control arms. Other aspects of midwife support are shown in Table 3.
| Outcomes from questionnaire | Response | Intervention (n = 88) | Control (n = 87) |
|---|---|---|---|
| Key pilot outcomes | |||
| Did your midwife explain how to perform pelvic floor muscle exercises when you were pregnant? | Yes | 57 (64.8%) | 33 (37.9%) |
| 95% CI | 56.9 to 72.4a | 24.6 to 51.2b | |
| Women’s predefined adherence in performing PFME in pregnancyc | Yes | 43 (50.0%) | 33 (38.4%) |
| Missing | 2 | 1 | |
| 95% CI | 24.1% to 77.1%d | 12.4% to 67.1%e | |
| Prevalence of UIf | Yes | 39 (44.3%) | 47 (54.0%) |
| 95% CI | 32.0% to 56.1%g | 42.2% to 65.8%h | |
| Further outcomes | |||
| Did your midwife advise you to perform pelvic floor muscle exercises when you were pregnant? | Yes | 73 (83.0%) | 54 (62.1%) |
| Did your midwife give you a pack of information on pelvic floor muscle exercises when you were pregnant? | Yes | 49 (57.7%) | 17 (19.8%) |
| Missing | 3 | 1 | |
| When did your midwife give you the pack of information on pelvic floor muscle exercises? | Never given | 35 (42.7%) | 65 (77.4%) |
| At first (booking) appointment | 15 (18.3%) | 10 (11.9%) | |
| At second antenatal appointment | 15 (18.3%) | 3 (3.6%) | |
| At later antenatal appointment | 17 (20.7%) | 6 (7.1%) | |
| Missing | 6 | 3 | |
| How often did your midwife talk to you about pelvic floor muscle exercises when you were pregnant? | Never | 13 (14.8%) | 30 (34.5%) |
| Only at booking appointment | 20 (22.7%) | 15 (17.2%) | |
| Occasionally | 33 (37.5%) | 22 (25.3%) | |
| Every antenatal appointment | 18 (20.5%) | 10 (11.5%) | |
| Can’t remember | 4 (4.5%) | 10 (11.5%) | |
| Did your midwife ever ask you if you had any difficulties with performing pelvic floor muscle exercises? | Yes | 27 (31.0%) | 8 (9.3%) |
| Missing | 1 | 1 | |
| Before you were pregnant, have you ever been taught or learned how to perform pelvic floor muscle exercises? | Yes | 36 (41.4%) | 39 (45.4%) |
| Missing | 1 | 1 | |
| How often did you perform pelvic floor muscle exercises when you were pregnant? | Never – not advised to | 9 (10.2%) | 18 (20.7%) |
| Never – other reasons | 9 (10.2%) | 4 (4.6%) | |
| Few times a month | 19 (21.7%) | 27 (31.0%) | |
| Once a week | 6 (6.8%) | 4 (4.6%) | |
| Few times a week | 23 (26.1%) | 17 (19.5%) | |
| Once a day | 11 (12.5%) | 5 (5.8%) | |
| Few times a day | 9 (10.2%) | 11 (12.6%) | |
| Can’t remember | 2 (2.3%) | 1 (1.2%) | |
| Do you currently perform pelvic floor muscle exercises? | Yes | 46 (66.7%) | 45 (64.3%) |
| Missing | 19 | 17 | |
| How often did you do pelvic floor muscle exercises over the last month? | Never – not advised to | 9 (10.2%) | 12 (13.8%) |
| Never – other reasons | 10 (11.4%) | 13 (14.9%) | |
| Few times a month | 25 (28.4%) | 23 (26.4%) | |
| Once a week | 5 (5.7%) | 4 (4.6%) | |
| Few times a week | 24 (27.3%) | 17 (19.5%) | |
| Once a day | 6 (6.8%) | 9 (10.4%) | |
| Few times a day | 9 (10.2%) | 9 (10.4%) | |
Women’s reporting of pelvic floor muscle exercise practice in pregnancy
The main prespecified assessment of undertaking PFME was the women’s responses to the question ‘How often did you perform pelvic floor muscle exercises when you were pregnant?’, with PFME adherence in a manner likely to improve symptoms predefined as a few times a week or more (see Table 3). There were 43 women (50%) in intervention clusters who reported this level of adherence and 33 women (38.4%) in control clusters.
Table 3 also shows whether they currently were performing PFME and how often they had done so in the last month: 39 (44.3%) women in intervention clusters reported performing PFME a few times a week or more during the month prior to completing the questionnaire and 35 (40.2%) in control clusters.
Women’s confidence in performing PFME, assessed using PFMESES42, is shown in Appendix 4, Table 14. Womens excercise adherence was further assessed using EARS43 with adherence score a little higher among women in the intervention arm. As described earlier four items from the PFMESES were omitted rendering the full score invalid.
Prevalence of urinary incontinence and faecal incontinence
Based on women’s completion of the ICIQ-UI SF40 in the 10–12 weeks questionnaire, UI prevalence was categorised into a binary ‘yes’/’no’ outcome, and 39 (44.3%) intervention women and 47 (54%) control women reported they did leak urine (see Table 3). The full ICIQ-UI SF data are shown in Appendix 4, Table 14.
Based on the RFIS,41 completed in the 10–12-week questionnaire, 15 women in intervention (18.1%) and 11 in control clusters (13.3%) reported FI. The full RFIS data are shown in Appendix 4, Table 15.
Midwives’ characteristics
Data relating to midwives’ experience in the intervention and control clusters are shown in Table 4. The number of years working as a midwife and as a community midwife were similar in intervention and control groups, and most midwives in both groups were band 6 level.
| Intervention (n = 73) | Control (n = 31) | ||
|---|---|---|---|
| Current midwifery band | 5 | 3 (4.4%) | 0 (0%) |
| 6 | 62 (91.2%) | 26 (92.9%) | |
| 7 | 3 (4.4%) | 2 (7.1%) | |
| Missing | 5 | 3 | |
| Number of years working as a midwife | n | 68 | 28 |
| Mean (SD) | 11.3 (9.2) | 13.0 (9.5) | |
| Missing | 5 | 3 | |
| Number of years working as a community midwife | n | 68 | 28 |
| Mean (SD) | 6.2 (6.4) | 8.8 (7.7) | |
| Missing | 5 | 3 |
Limitations
Despite several initiatives, including a short questionnaire, translated versions, £10 voucher on questionnaire return and their midwife telling them that a questionnaire would arrive, the proportion of women who returned a questionnaire was low. This proportion, however, was similarly low in both trial arms, and the characteristics of women in both trial arms were similar. The proportion of women who reported UI was higher than is generally found in studies, so it may be that more women who returned a questionnaire did so because they experienced UI.
Although the intention was, and trial protocol stated, that women may be ‘offered other options of completing the questionnaire, including online or mobile-friendly versions’, when online versions were explored with BCTU, their decision was that this was not possible without women’s prior consent because University of Birmingham would then hold the woman’s internet provider address (potentially identifiable data) and hence would not meet General Data Protection Regulations (GDPRs) requirements.
Not all the midwives completed questionnaires, and fewer did so in the control clusters. This may have been because of the lengthy (six-page) participant information leaflet and consent form that were necessary before coming to the questionnaire because of BCTU’s requirements, which intervention midwives were familiar with. In addition, in both groups some midwives had technical difficulties accessing the questionnaire.
Another limitation was that the BCTU omitted to print four of the questions in the questionnaire from the PFMESES,42 which assessed women’s confidence in their practice of performing PFME, so this scale was invalid.
The pilot and feasibility trial had been set up to assess whether a future definitive trial would be possible. However, as described in Summary of alterations to the programme’s original aims/design, a future definitive trial would not be possible due to changes introduced as part of the NHSE Long Term Plan to improve women’s pelvic health. This meant that we were not able to go on to undertake a study to obtain the best evidence of effectiveness.
Inter-relationship with other parts of the programme
Work package 4.2 piloted the intervention developed in earlier WPs (WP1, 3 and 4.1) and ran in parallel with the trial process evaluation (WP4.3).
Work package 4.3: process evaluation
Aims
This WP explored the feasibility and acceptability of APPEAL training and implementation. Aims included identification of facilitators and concerns or challenges to implementation during the trial, and recommendations for refinement to the training in any subsequent practice implementation (see Implications for practice and Appendix 12). Four objectives listed in WP4.2 relevant to this WP are repeated here with additional details to reflect the theory of change presented in the logic model (see Appendix 2, Figure 4) plus a fifth objective. Objectives were to:
-
Provide training for community midwife teams randomised to the intervention arm to encourage incorporation of a PFME care package into their usual antenatal care.
-
Assess whether training, intervention implementation and trial processes were acceptable to midwives by identifying and investigating factors impacting on:
-
fidelity of training delivery to midwives by trainers
-
uptake of training by midwives
-
effectiveness of the training intervention for improving implementation of PFME by midwives in antenatal care
-
fidelity of PFME intervention delivery to women by midwives.
-
-
Assess women’s practice of PFME during and after pregnancy using women’s postnatal questionnaire and interview data to evaluate potential effectiveness of the PFME intervention for improving women’s practice of, adherence to, and confidence with PFME.
-
Assess midwife support for PFME in both trial groups using qualitative interviews with midwives and women, and women’s questionnaire data, to inform feasibility, including possible acceptability of collecting outcome data from women.
-
Investigate possible intervention contamination between trial arms.
Methods
Multiple methods were used with qualitative and quantitative data sources and analytical methods (see Appendix 5). Research questions, methods used to answer questions, and data source and type are described and were mapped to relevant components of the process evaluation framework for designing and reporting process evaluations in cluster trials. 47 This mapping was tabulated (see Appendix 5, Tables 16 and 17) to show how the multiple methods of data collection fitted together to inform the overall analyses reported here. Reporting followed this sequence: first, the processes involving trial clusters (1a delivery to, and 1b response of, midwifery teams); second, the processes involving the target population of midwives (2a delivery to, and 2b response of, midwives); and third, for the target population of women (3a delivery to, and 3b responses of, women) to meet the first four objectives.
Key findings
Delivery to trial clusters (midwifery teams) (objective 1)
Training was delivered to midwives as intended (mean score 86.4%, SD 9.2%) for training session fidelity observed in the three sessions evaluated during the main training period (January–March 2021), although the first training session observation revealed lower fidelity to the training protocol (75.8%) compared to sessions observed later (92.4%). 48 Only minor refinements were needed: addition of a myth-buster slide, and adjusting the ‘red flag’ screening slide. Throughout the intervention period, 3750 resource packs were provided to intervention midwife teams.
Response of trial clusters (midwifery teams) (objectives 1 and 2)
Uptake of training was successful, with all 95 midwives working in intervention teams during the implementation period receiving training. Those on short-term sick leave were trained when they returned to work. Seven intervention team midwives were on long-term sick leave or maternity leave so did not work during the implementation period. In addition, 11 MSWs were trained because in some teams they provided translation for women who did not speak English. Each team recruited an APPEAL midwife champion.
Delivery to midwives and response of midwives (objectives 2 and 4)
Delivery and content of initial training sessions were evaluated48 among the midwives (n = 65/71, 91.5% of those in the initial training phase). Most midwives reported acceptability for most training aspects, although some expressed preference for in-person training (see Appendix 6, Tables 19 and 20). Positive factors affecting acceptability of training delivery were: flexibility of booking in; offering training at different times of day/days of week; not having to travel to training session. Reasons for six midwives not completing the evaluation were internet connectivity or system login problems; none refused to participate.
Midwives showed clear increases in confidence about PFME following training, with median increase of at least 1 point (on a 0–4 scale) for each of the eight questions. A Wilcoxon signed-rank test produced z-scores for each question which were converted to p-values (see Appendix 6, Table 21). All p-values (< 0.001) indicated a significant change in confidence following training.
Champions were provided with a role description (see Report Supplementary Material 3) and found meetings useful for peer support. Champion activity was monitored and summarised (see Appendix 7, Tables 22–25). Interviews with intervention midwives revealed benefits of having a champion for support and advice, and that self-nominated champions were more enthusiastic about the role, which was not regarded as a big commitment (see Appendix 9, Table 29).
At the end of the intervention period, an implementation evaluation questionnaire was completed by intervention midwives and MSWs (n = 59, 62% of those originally trained and remaining in team) (see Appendix 8, Table 26). Respondents self-reported which steps of APPEAL they delivered to women (never, few, some, most, or all, of the women): raising the topic (89%), giving the resource bag (68%), teaching a PFME contraction (68%), and practising a contraction in antenatal clinic (45%) with most/all of the women. In free-text space (see Appendix 8, Table 27), respondents also reported the APPEAL resource bag (n = 31), prompt cards (n = 17) and team champions (n = 16) as being the most important resources to support implementation. The top three most reported barriers to implementation were: ‘lack of time’ (n = 39), ‘forgetting’ (n = 29), and ‘language barriers’ (n = 26), with ‘other priorities’ and not being on maternity record system ‘to act as a prompt’ also mentioned. Midwives (n = 18) reported making 44 referrals to physiotherapy services.
Interviews were conducted with a sample of intervention midwives (n = 13) during trial implementation (see Appendix 9, Table 28). Responses to initial structured questions about acceptability, engagement, knowledge, attitude and suggestions to improve training are shown in Appendix 9, Table 29. They indicated positive responses (see Appendix 9, Table 30): ‘I’m enthusiastic about it’ and ‘it’s a very good fit’ (with personal and professional values) and importance to women; it was perceived to be effective (‘I think it should help get that message across’) and to increase personal self-efficacy [‘I do feel confident’ (about the intervention)]; and it made sense [‘I’ve never thought about the impact of pregnancy on the pelvic floor’ (before the training)]. However, there was some ambivalence – the burden of delivery (‘it just feels a bit impossible’) mainly due to workload volume, limited appointment time, system pressures, remembering everything and opportunity costs (‘so many other priorities’) – and a sense of hopefulness rather than certainty, with some concerns that midwives would not be able to put it into practice or that women would not do it (see Appendix 9, Table 31).
Post-trial interviews were conducted with a sample of intervention (n = 6) and control midwives (n = 12) (see Appendix 10, Tables 32 and 33). Findings from intervention midwives reiterated their positive experiences of the training but there was an increasing inconsistency with implementation as time passed since training. Control midwives reported a range of previous PFME experience, from no PFME training to extensive knowledge, but they confirmed lack of consistency for implementing into standard care. Control midwives reiterated earlier WP findings regarding the challenges of teaching PFME and how to best support implementation (see Appendix 10, Table 33).
Delivery to women and response of women (objectives 3 and 4)
Considering responses of women in the intervention group who returned the trial outcome questionnaire (see Table 3), it is clear that delivery to women did often occur: most indicated that they were advised to do PFME by their midwives (83%); many were taught how to do PFME (64.8%) and given a resource pack (57.7%); and some reported that their midwives talked to them about PFME occasionally (37.5%) or at every appointment (20.5%). All these figures were higher than responses made by women from the control group, suggesting an impact of APPEAL implementation, with trained midwives delivering more PFME teaching and support than was occurring in standard care during the same period.
Findings from post-trial interviews with women (intervention n = 13, control n = 16) (see Appendix 11, Tables 34 and 35) revealed that some aspects of PFME advice were delivered in both groups, but it was apparent that remembering was problematic. Intervention group women recalled specific APPEAL resources: the bag, leaflet or App QR codes (see Appendix 11, Table 36). A much stronger and more consistent finding from all interviews (including midwives’ interviews) was consensus acknowledgement of the importance of understanding why and how to do PFME and that an APPEAL-type intervention is what is wanted.
Reflecting further on the WP4.2 results, 50% of intervention group women self-reported practice of and adherence to PFME during pregnancy (see Table 3), indicating a degree of fidelity to the intended APPEAL intervention (see Appendix 5, Table 18). Dilution of fidelity was not unexpected: both quantitative and qualitative data sources indicated some midwives did not deliver the APPEAL intervention fully and even when it was delivered, some women did not change their behaviour to do PFME. However, women’s level of PFME adherence was higher than the standard care group women reported (see Table 3). Differences in PFME adherence levels between intervention and control groups were similar to the differences these groups reported regarding receipt of information and support from their midwife to do PFME (see Table 3). As these results are all aligned, it suggests that an explanatory factor for the observed between-group differences is the APPEAL intervention training and implementation. Although women’s responses reported imperfect fidelity for intervention delivery, there was an indication that PFME adherence by the intervention group had been sufficient for more women to report benefits in UI symptoms than those receiving standard care. Such promising findings indicated APPEAL was facilitating required behaviour changes, but more work is needed to optimise and synchronise these change processes at all levels: services, midwives and women.
Feasibility and acceptability of collecting outcome data from women (objective 4)
Only a small proportion of women (17.5%) returned the questionnaire; therefore, as already described, a future trial would not be feasible. However, most respondents completed the questionnaire fully. Furthermore, a very high proportion (132/175, 77%) indicated permission to be approached for follow-up interview. We cannot determine whether low questionnaire return rates were due to the burden imposed by the data collection method (paper-based, requiring return by prepaid post) or to that imposed by the questions asked. However, the lack of missing data in returned questionnaires suggests that to increase return rates in any future evaluations the first step would be to improve data collection methods (e.g. using mobile phones) rather than changing the number and type of questions.
Contamination between trial arms (objective 5)
Minimal evidence of between-group contamination was found in the post-trial interviews with midwives and women. There was an occurrence of one midwife who changed from intervention to a control team, but this occurred very late in the intervention period. There was no indication from interviewed women assigned to standard care that any received resources badged as APPEAL, but some did report receiving advice about PFME (as per standard care).
Limitations
The APPEAL training was adapted for remote delivery due to NHS requirements in response to COVID-19 that researchers could not meet staff on site, and while this was acceptable to most midwives, some would have preferred in-person training. We cannot assess whether in-person training would have resulted in higher levels of implementation, but evaluations of the training itself were positive.
Several processes were compromised by factors outside the control of the researchers, for example: internet problems (for midwives completing online consent forms and questionnaires); excessive length of information sheets (prescribed by BCTU) affecting time available for midwives to complete the implementation questionnaire and inability to offer the women’s questionnaire electronically (due to BCTU consideration of data protection requirements); failure to include all items of the self-efficacy scale meant this theory of change could not be evaluated; and delays resulting from hospital trust governance policies in obtaining women’s contact details for interview increased the time lag for women to recall their antenatal care.
Inter-relationship with other parts of the programme
Work package 4.3 was mainly informed by WP3 but also WP1 and WP4.1 and ran in parallel with WP4.2. Findings from WP4.3 showed that during the pilot feasibility trial (WP4.2) the APPEAL intervention was delivered to midwives as planned and that they responded with positive evaluations of their training. This increased midwives’ confidence regarding teaching of PFME to women in their care and there was evidence from WP4.3 findings that some midwives changed their practice behaviour, which also translated into some women changing their behaviour (WP4.2 results). As reported in earlier WPs, the main challenge with delivering the intervention was lack of time in appointments, suggesting that service-level constraints still need to be addressed in any post-programme implementation.
Account of involvement of patients and the public
The APPEAL team aimed to embed patient and public involvement throughout the programme, from funding application preparation (2012–4) to dissemination (2022). Multiple methods were used depending on work stage: an existing adviser joined the co-applicant team to help prepare the application; an adviser joined the programme steering group (2016–22); a local group (n = 7) was convened to support WPs 1 and 3 (2016–8); and there were further groups for WP 4 (2022).
We used a variety of approaches to facilitate engagement during the early WPs. We used in-person ‘focus group’ style community meetings (n = 9) to incorporate women’s perspectives into the research process, with mothers able to bring their children to facilitate engagement. We held a citizens’ jury style event to select electronic resources, and there was wider public consultation in the form of a national stakeholder event with 20 delegates representing 18 UK organisations with an interest in maternity services (including APPEAL public advisers, clinicians, charities, and health professional bodies) to inform and help endorse implementation. Subsequently, when COVID-19 impacted, we held small-group follow-up meetings virtually to review results of WP4 and help plan dissemination.
Impact was highly positive. Our co-applicant adviser designed the waterdrop logo, after consulting with pregnant women who were clear the logo should not depict a baby or pregnant mother as they did not want to ‘blame the baby’ for incontinence. During WP1 ethnography, public involvement was an integral part of the process, ensuring the research was relevant and important to them. The seven women were from an established mother and toddler group at a community centre and remained as advisers throughout this WP. They represented a diverse socioeconomic group, but we were not able to recruit a diverse ethnic group. This group’s input had direct impact on the ethnography: they critiqued interview schedules, discussed challenges of implementing PFME into antenatal care, and contributed to emerging themes. During WP3, advisers co-developed resources for women: a citizens’ jury style event considered what smartphone apps should be included; advisers helped design the women’s leaflet and recommended that all resources be contained in a cloth bag big enough to hold a clean nappy. Following the practice training event (for midwives), the advisory group contributed to improving the video clips used for the training. Wider impacts have arisen from the huge enthusiasm we experienced from our advisers: additional public advisory groups were convened to discuss implementing APPEAL-style education for teenagers and young women (i.e. before pregnancy) and more recently to support related funding to implement APPEAL-style training into primary care.
Public involvement was critical to ensuring we met our aim to embed patient and public perspectives from the outset. It informed the content of what resources women received during antenatal care and what they said they needed from their midwives.
Reflections on our public involvement work included a strong sense that our research was doing what mattered: that what we were asking of pregnant women (and midwives) was not just evidence-based but reflected what mattered to them. By including them in all the development work and choice of resources, we had an intervention and supporting materials that had credibility and would be acceptable and feasible for pregnant women and midwives to make use of, and we would not be asking too much of these busy people. We also benefitted from working with the highly experienced Peninsula Applied Research Collaboration (ARC) and ARC West Midlands public involvement teams who supported recruitment and running of our local activities.
The degree of contributions and commitment from the public were also important for motivating the research team when hitting barriers to progressing the research (funding review, COVID-19 pauses and lockdown complications, governance, and data collection/data access issues). These problems impacted our ability to sustain, during WP4, the high level of public involvement achieved in earlier WPs. Sustainability was also an issue from the representativeness perspective, in that over the 10 years from inception to completion of APPEAL our advisers were not always going to be recent antenatal care recipients; and after serving a period of time, the co-applicant adviser did resign. Thus, there is a trade-off between achieving continuity in representation versus ongoing recruitment of newly pregnant women, which we partly overcame by having criteria to include women who had recently experienced antenatal care (toddlers under 5 years) and keeping the invitation open to anyone who wished to remain.
Several of our earlier advisers remained and, with new recruits, have helped plan dissemination. In this they have made many suggestions (see Appendix 12, Tables 37 and Table 39) for how we can get the main messages of APPEAL into the public space, rather than for people to necessarily attend dissemination events which they felt could be too much of a time burden. Examples of their suggestions include: videos and posters for women’s health events, GP surgeries, back of toilet doors and TV adverts; to approach charities, incontinence product suppliers (e.g. TENA®) and folic acid vitamin suppliers to include key PFME messages, including QR codes for further information; and for midwives to wear badges saying ‘Have you done your PFME?’. Finally, the group reviewed the Lay Summary and Abstract of this report, and one adviser also reviewed the Scientific Summary. We thank members of the APPEAL public advisory group for their insights and commitment.
Reflections on what was and was not successful in the programme
What was successful?
Our major success within the programme was that we have developed an intervention of training midwives to advise and support women in undertaking PFME which most midwives were able to implement, in full or at least in part, within routine antenatal care. Almost 100 midwives were trained in our programme, and our evaluation showed that their knowledge and confidence after the training were substantially increased relative to before training.
We feel that this success was based on having a large multidisciplinary international research team that comprised academics with experience in childbirth-related incontinence, psychologists, qualitative researchers, trialists, clinical experts (obstetric physiotherapists, midwives, obstetricians and GPs) and implementation scientists. Our international collaborators included a world-leading academic obstetric physiotherapist in PFME (Jean-Hay Smith from New Zealand), who has led several Cochrane Reviews on PFME. We were also able to co-opt another world-leading clinical academic expert on pelvic floor function and dysfunction (Helena Frawley, University of Melbourne) part way through the programme.
Due to the commencement of the NHSE Ten-Year Long Term Plan, there have been new ‘early adopter’ and ‘fast follower’ perinatal pelvic health services funded in 23 LMNSs across England, and we have been able to use our findings in implementation work sooner than would typically be the case. To date, around 170 specialist health professionals from these LMNSs have attended workshops we have provided for them to consider how they might adopt the APPEAL training intervention for midwives in their areas.
We managed to include substantial PPIE and stakeholder engagement at various stages, as described above.
We have won conference prizes with our abstracts, specifically at the International Continence Society which leads multidisciplinary continence research and education worldwide. We have also been successful in obtaining NIHR School of Primary Care funding for spin-off research to develop a similar intervention for use in general practices.
What was not successful?
In attempting to do PFME, we know that some women are unable to locate their PFMs. Our attempt to find a diagnostic test to assist midwives in assessing whether women were able to locate their PFMs was not successful. A comprehensive systematic review was undertaken but failed to find a single diagnostic test for use in this way.
The other lack of success was our ability to obtain a high return rate from questionnaires sent to women. This, together with the NHS Long Term Plan roll-out, means that we would be unable to undertake a full cluster RCT as originally planned.
To obtain the highest possible return rate, we had hoped to be able to use optional online questionnaire completion methods as women in earlier WPs had recommended. However, BCTU protocols meant that they would not allow us to provide this online version. The reason was that women’s consent to take part was by returning a completed questionnaire and completion by using online questionnaire methods would have allowed each woman’s IP address to be detected, hence it was not considered to be compliant with GDPR. The APPEAL team were extremely disappointed by this. Since COVID-19 and lockdown, people’s use of online methods has become even more prevalent, and it was considered that many women busy with young babies would have preferred it and had it been available a higher proportion of questionnaires would likely have been returned.
Reflections on issues relating to equality, diversity and inclusion
Participant representation
There were two main sets of participants in our research: the midwives who provided antenatal care and the PFME intervention to the women, and the women included in the trial.
We included all midwives who were employed to deliver antenatal care by the Trusts where the trial was undertaken. These participants were all women and ranged in terms of their ages and ethnic backgrounds. All those in the intervention team clusters received the APPEAL training and, among those who completed training evaluation and included demographic details, there was a wide range of ethnic and age groups.
It was realised early on in training the midwives that in caring for women who did not speak English, MSWs in their team would assist in translating or interpreting, and would hence be involved in care. In the teams where this occurred, we also trained a small number of MSWs so that they could assist in intervention delivery for non-English-speaking women.
In sending out the questionnaires, to maximise the likelihood that non-English speakers could participate, we translated the main parts of the questionnaire into the four main languages prevalent in the local areas.
Research team
Our co-applicants were mostly women, with only three males. None were from ethnic minority groups. Among the researchers appointed to the work, all were women and two were from an ethnic minority group. There was a wide range of expertise and experience, including both clinical and non-clinical backgrounds, in the team. Junior research staff were encouraged and supported to develop their expertise through mentoring from senior team members and supported to attend training opportunities. One research midwife was appointed to a senior position in the Royal College of Midwives. Another research midwife has just been successful in obtaining an advanced NIHR fellowship. Another research midwife has been appointed to another NIHR programme research post. Another junior staff member has obtained promotion to another organisation. The team supported five interns to undertake some of the research, one of whom was successful in having an abstract and presentation of her APPEAL programme work accepted at a prestigious international conference.
The PPIE representatives included during the research were all women because we wanted lived experience of pregnancy. Steps were taken to ensure that we included representatives from underserved populations, in terms of women living in deprived areas and ethnic minority groups. We were successful in the former but, despite our efforts, only had limited ethnic minority representation.
Limitations relating to the method or execution of the research
Limitations in the research methods varied for the different WPs in the programme. In the critical interpretive synthesis review in WP1.1, the quantitative studies found were limited by small sample size, few details regarding reasons for declining participation, lack of details regarding calibre of survey tools/outcome measures, and variable response rates. For qualitative studies, many did not consider the impact or relevance of contextual factors or demonstrate reflexivity.
Ethnographic research in WP1.2 suffered from the general limitation of this methodology: that the presence of a researcher can impact the behaviour of those being observed. This could result in the midwife care observed in relation to PFME being more extensive than would usually occur in their practice. In terms of our interviews, a limitation throughout was that we were not able to interview women who did not speak English.
In WP2 the lack of any studies to assist in assessment of PFM localisation that might be used to assist in the trial intervention was an obvious limitation such that any inclusion of assessment of correctness of pelvic muscle contraction was not possible.
The main limitations of WP3 were the challenges identified by midwives regarding wider system pressures, and time constraints within clinic appointments limiting the range and complexity of the intervention that could be produced. Despite these constraints, midwives and women believed that implementing PFME in routine antenatal care would be beneficial for many women.
Once we reached WP4, the main limitation was the low proportion of women who returned a trial questionnaire. As described earlier, in the data collection pilot (WP4.1) the return rate from women was 31% at the ‘data lock’ point, rising to 35% with latecomers, in keeping with other recent post-partum studies. 2,3 The original application had proposed a much higher return rate, so the second stage of the programme was amended to a feasibility and pilot cluster trial with substantial additional process outcomes (WP4.3), still with the aim of consideration of a full cluster trial to follow. The return rate in the feasibility trial was even lower, at only 17%, though with balance between trial arms, meaning that a trial with the same design would not have been worthwhile. We had not been allowed by the BCTU to try to increase return rate by using online data collection methods due to GDPR concerns (see What was not successful?). However, because of the new perinatal pelvic health service being established by NHSE, the cluster trial originally planned would not anyway have been possible in view of the change in the standard care comparator; a definitive randomised controlled cluster trial would have provided best evidence of effectiveness of the APPEAL intervention. Evidence on how this new perinatal pelvic health service should be developed, however, is minimal, such that even with the limitation of a low questionnaire return rate from women, the overall findings and outputs of the APPEAL programme will make an important contribution to assist in the new service development.
Conclusions from the whole programme
This programme has demonstrated that training and resourcing standard antenatal care midwives appropriately to support women to undertake PFME in pregnancy is feasible, acceptable and could improve PFME adherence and reduce post-partum UI. To do this required the development of a complex intervention to influence behaviour change at two levels: the midwives and the women. Although there were limitations in the programme as described above, this probably represents the best available evidence on whether it is feasible to embed a PFME intervention in standard antenatal care in England.
Recommendations for future research
It is now no longer possible to undertake a future definitive RCT in England, because of the changes occurring as part of NHSE’s new perinatal pelvic health services. Numerous ‘early adopter’ and ‘fast follower’ local maternity systems are in the process of setting up this new service, and we have already provided some with our intervention package. More research evaluation is required to follow up on this and we are proposing working with NHSE to assist in undertaking this. It may be that a future definitive RCT could take place in other similar maternity care settings internationally, such as Australia or New Zealand, or in other parts of the UK.
Research is also needed to evaluate the adaptation and roll-out of PFME training to other HCPs so that others as well as midwives can provide appropriate pelvic health care for women across their life course (in accordance with the NHS Long Term Plan). Finally, women and midwives have consistently told us that they think the prevention of UI should be raised with much younger women; this concurs with recent National Institute for Health and Care Excellence (NICE) guidance about pelvic floor dysfunction which recommends research into promoting this topic in schools. 50
In view of our findings in WP2 that there was no diagnostic test to help midwives identify whether women can locate their PFMs and the knowledge that some women are not able to locate these muscles, this is an area of practice in which further research is needed. In addition, it may have been that explanation of how to contract the pelvic floor given by the midwives would have been more difficult for women whose first language was not English. This is another area for further research; although we did train MSWs who were able to speak other languages to reduce the impact of this, other methods need to be investigated.
Implications for practice
The programme of work undertaken for APPEAL shows much promise for changing the practice of midwives who are clearly keen to do this and, after receiving the APPEAL training, much more confident to take on this role. As is often the case in this type of research, the APPEAL team cannot claim that optimal behaviour change occurred for all midwives. There were various barriers for midwives in attempting to change their practice at service and policy level. With the changes now happening as part of the NHSE new perinatal pelvic health services, many of the opportunities for optimising implementation into practice, raised by midwives and women during the APPEAL programme, could be actioned. For midwives, priority for PFME care, prompts on maternity record systems, regular training updates, and improved understanding of what happens in physiotherapy consultations to aid interprofessional communication are some examples. Women wanted resources to be more accessible, for example, digital format for leaflets and app card, videos in clinic waiting rooms, and reminder texts between appointments (see Appendix 12, Table 39).
Our promising findings indicate the APPEAL intervention is operating on the right lines. With imminent changes to NHS service provision, there is now opportunity to optimise behaviour change by both midwives and the women they care for. Supporting all pregnant women to do their PFME, by implementation of APPEAL, has potential for reducing UI for large numbers of women.
Additional information
Contributions of authors
Christine MacArthur (https://orcid.org/0000-0003-0434-2158) (Professor of Maternal and Child Epidemiology) was chief investigator and, with the help of other co-applicants, conceived the programme. She supervised the Birmingham-based research team and took overall responsibility for the programme. She led the writing of this final report.
Debra Bick (https://orcid.org/0000-0002-8557-7276) (Professor of Evidence Based Midwifery) was midwife lead involved in conceiving the programme, chief investigator for the pilot and feasibility trial in WP4.2 and contributed to the writing of this final report.
Victoria Salmon (https://orcid.org/0000-0002-1536-4750) (Research Fellow) was involved in the research in WP1, WP3 and WP4.3, led the design of the training intervention and resources for midwives and women and assisted in the preparation of this final report.
Ellie Jones (https://orcid.org/0000-0003-2552-8322) (Research Midwife/Fellow) was involved in conducting the pilot and feasibility cluster trial in WP4.2 trial, training intervention midwives, supporting Trust research midwives and assisted in the preparation of this final report.
Jean Hay-Smith (https://orcid.org/0000-0002-9009-2812) (Professor in Rehabilitation and Professor in Women’s Health, University of Otago, New Zealand) was involved in conceiving the programme, contributed across the programme, attended the National Stakeholder event, contributed the mobile phone app review in the APPEAL resources for women and commented on the draft report.
Jon Bishop (https://orcid.org/0000-0003-1789-5886) (Trial Statistician) undertook the analyses in WP4.1, supervised the analyses of the pilot and feasibility cluster trial in WP4.2 and contributed to the writing of this final report.
Eleni Gkini (https://orcid.org/0000-0001-8083-2947) (Trainee Trial Statistician) undertook the pilot and feasibility trial analysis in WP4.2 supervised by the other statisticians, was involved in preparing the report of WP 4.2 and commented on the draft report.
Karla Hemming (https://orcid.org/0000-0002-2226-6550) (Professor of Biostatistics) was co-applicant and senior statistician with specific expertise in cluster trial analysis; was responsible for this methodology and commented on the draft report.
Sara Webb (https://orcid.org/0000-0002-4924-3017) (Head of Research at Royal College of Midwives) was research midwife in the earlier parts of the programme, was lead for WP4.1 and commented on the draft report.
Mark Pearson (https://orcid.org/0000-0001-7628-7421) (Reader in Implementation Science) was lead for WP1.1, gave expert implementation science input across the programme and commented on the draft report.
Tim Coleman (https://orcid.org/0000-0002-7303-4805) (Professor of Primary Care) gave expert support of behaviour change and trial methodology and general support across the programme and commented on the draft report.
Rohini Terry (https://orcid.org/0000-0003-4188-8504) (Research Fellow) was involved in the research in WP1.2, supported the intervention development and practice training event (WP3), was involved with interviews in WP4.3 and assisted in the preparation of this final report.
Libby Edwards (https://orcid.org/0000-0001-9380-7732) (Research Midwife) was involved in training the intervention midwives in WP4.2, assisted with interviews in WP4.3 and supporting Trust research midwife work and commented on the draft report.
Helena Frawley (https://orcid.org/0000-0002-7126-6979) (Associate Professor, Women’s Health Physiotherapy, University of Melbourne, Australia) contributed expert advice on intervention development and delivery, attended the National Stakeholder event, contributed to the APPEAL video resources for women and commented on the draft report.
Eivor Oborn (https://orcid.org/0000-0003-0566-4327) (Professor of Implementation Science) gave expert implementation science support across the work of the programme and commented on the draft report.
Sarah Dean (https://orcid.org/0000-0002-3682-5149) (Professor of Psychology Applied to Rehabilitation and Health) was involved in conceiving the programme, led the Exeter-based research in WP1, WP3 and WP4.3, supported the research in WP2 and WP4.2 and co-ordinated the international team involvement. In addition, she is senior author of this final report.
Acknowledgements
We would like to thank all the midwives who took part in the intervention, all the women who returned the questionnaires and all other participants and Trusts who supported the programme in various ways.
The study team acknowledges the study delivery support given by Birmingham Clinical Trials Unit, in particular William McKinnon and Mary Nulty.
We also acknowledge the work undertaken by early-phase researchers Lucy Hope, Rachael Jarvie and Chidubem Okeke Ogwulu and by students on supervised research placements with the University of Exeter team: Lauren Chandler, Alex Sherrell, Saskia Chapman, Clodagh Smith and Rohan May.
We acknowledge the support by the NIHR Applied Research Collaborations in the West Midlands, Southwest Peninsula, and South London.
Patient and Public Advisory Group members
Thanks to the APPEAL public advisory group for their ongoing valuable contribution and commitment to this project. Thanks to the PenARC Public Involvement Team, researchers Kate Boddy and Emma Cockcroft, for their support recruiting PPI members, setting up and running the PPI advisory group meetings.
Programme Steering Committee members
Professor Nadine Foster (Chair) Professor Suzanne Hagen, Professor Helen Cheyne, Ms Sarah Skinner.
National Stakeholder Group members
We would like to thank the women, healthcare professionals and the following organisations for their contribution to the national stakeholder consultation event: Birmingham Women’s and Children’s NHS Foundation Trust (Grete Drewett, Sue Smithson); Camden Clinical Commissioning Group; Chartered Society of Physiotherapy; Gusset Grippers (Elaine Miller); Mumsnet; Mumsnet guest blogger (Sarah Haselwood); National Childbirth Trust; NHS Education for Scotland (Helene Marshall); NHS England, Head of Maternity, Children and Young People (Jacqueline Dunkley-Bent); NHS England Maternity team (Clare Capito); Pelvic, Obstetric and Gynaecological Physiotherapy; Royal College of Midwives President (Kathryn Gutteridge); Royal College of Midwives professional adviser for education (Gail Johnson); Royal College of Obstetricians and Gynaecologists (RCOG); RCOG Women’s Voices; Sandwell and West Birmingham NHS Trust; University Hospitals Birmingham; Virgincare/University College London (Health visitor).
We would also like to acknowledge Professor Jane Daniels, Professor Tracy Roberts, Professor Doug Tincello, Dr Clare Davenport and Ms Joanne Kidd-Chadwick who were involved as co-applicants in the first stage of the programme but did not continue into the second stage of the work.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
Ethics statements have been included in the WP to which they are relevant.
Information governance statement
The University of Birmingham and The University of Exeter are joint data controllers and are committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679.
Under the Data Protection Legislation The University of Birmingham is the Data Processor and the Data Controller for information relating to midwife participants’ data and we process personal data in accordance with their instructions. The University of Birmingham is the Data Controller and Data Processor for information relating to outcomes of pregnant participants’ data. The University of Exeter is the Data Processor and the Data Controller for information relating to midwife participants’ data including audio files/transcripts arising from the telephone interviews. Birmingham Women’s and Children’s NHS Foundation Trust is also a Data Processor for information relating to outcomes of pregnant participants’ data.
You can find out more about how The University of Birmingham handle personal data, including how to exercise your individual rights, by contacting The University of Birmingham’s Data Protection Officer by e-mail: dataprotection@contacts.bham.ac.uk and about how The University of Exeter handle personal data, including how to exercise your individual rights, by contacting The University of Exeter’s Data Protection Officer by e-mail: dataprotection@exeter.ac.uk
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/TJDH7946.
Primary conflicts of interest: Christine MacArthur was a member of the NIHR Policy Research Units Commissioning Panel 2017–8 and 2022–3 and declares NIHR funding grants (NIHR202869, NIHR129182 and NIHR200165). Sarah Dean is on the NIHR Programme Grant for Applied Research funding Panel Committee and The Stroke Association Funding Panel and declares NIHR funding grants (NIHR151938, NIHR204099, NIHR202020, NIHR201038, NIHR201070 and NIHR 200428). Debra Bick was a HS&DR Researcher-Led Panel Member (1 April 2020–30 April 2023) and is Chair of Trustees of MASIC charity and declares NIHR funding grants (NIHR202172, NIHR206660, NIHR202165, NIHR134298, NIHR150979, NIHR131250, NIHR131352, NIHR128721, NIHR16/77/02 and NIHR131161). Victoria Salmon has received funding from the Academic Health Science Network South-West to develop video resources related to APPEAL Grant. Ellie Jones has received a NIHR Advanced Fellowship (NIHR1906817). Rohini Terry declares NIHR funding grants (NIHR204030, NIHR151938, NIHR856766 and NIHR200428). Mark Pearson is a member of the NIHR HS&DR Funding Committee and declares NIHR funding grants (NIHR 131606, NIHR132931, NIHR135128, NIHR206122, NIHR203682, NIHR158758, NIHR203123, NIHR204349, NIHR204312 and NIHR206252). Karla Hemming declares NIHR funding grants (NIHR204156, NIHR202826 and NIHR203062). Tim Coleman is on the NIHR HTA Clinical Evaluation and Trials Committee (2015–9), Programmes for Applied Health Research Committee (2022 to date) and NIHR Research for Patient Benefit Committee (2022 to date) and declares NIHR funding grants (NIHR129210, NIHR206513, RP-PG-0109-10020 and RP-PG-0615-20003).
Publications
Salmon V, Hay-Smith J, Jarvie R, Dean S, Oborn E, Bayliss S, et al. ; APPEAL study. Opportunities, challenges and concerns for the implementation and uptake of pelvic floor muscle assessment and exercises during the childbearing years: protocol for a critical interpretive synthesis. Syst Rev 2017;6:18. https://doi.org/10.1186/s13643-017-0420-z
Salmon VE, Hay-Smith J, Jarvie R, Dean S, Oborn E, Bayliss SE, et al. Opportunities, challenges and concerns for implementing pelvic floor muscle assessment and training during childbearing years: a critical interpretive synthesis. ICS conference, Florence Sept 2017. Neurourol Urodyn 2017;36:S280–1. https://doi.org/10.1186/s13643-017-0420-z. Won best scientific abstract prize for health services research.
Hay-Smith J PL, Farmery D, Dean S, Grainger R. Apps-olutely fabulous? – the quality of PFMT smartphone app content and design rated using the Mobile App Rating Scale, behaviour change taxonomy, and guidance for exercise prescription. ICS 2019 Gothenburg Scientific Programme. Neurourol Urodyn 2019;38:S1–532. https://doi.org/10.1002/nau.24118
Terry R, Jarvie R, Hay-Smith J, Salmon V, Pearson M, Boddy K, Salmon V, Pearson M, MacArthur C, Dean S. Are you doing your pelvic floors? An ethnographic exploration of discussions between health professionals and pregnant women about pelvic floor muscle exercise during pregnancy. International Continence Society (ICS) Conference 2019, Gothenburg. Neurourol Urodyn 2019;38:S1–532 https://doi.org/10.1002/nau.24118.
Terry R, Jarvie R, Hay-Smith J, et al. ‘Are you doing your pelvic floor?’ An ethnographic exploration of the interaction between women and midwives about pelvic floor muscle exercises (PFME) during pregnancy. Midwifery 2020;83:102647. https://doi.org/10.1016/j.midw.2020.102647
Salmon V, Hay-Smith EJ, Jarvie R, Dean S, Terry R, Frawley H, et al. Implementing pelvic floor muscle training in women’s childbearing years: a critical interpretive synthesis of individual, professional, and service issues. Neurol Urodyn 2020;39(2):863–70. https://doi.org/10.1002/nau.24256
Bick D, Bishop J, Coleman T, Dean S, Edwards E, Frawley H, et al. Antenatal preventative pelvic floor muscle exercise intervention led by midwives to reduce postnatal urinary incontinence (APPEAL): protocol for a feasibility and pilot cluster randomised controlled trial. Pilot Feas Stud 2022;8:231. https://doi.org/10.1186/s40814-022-01185-y
Dean S, Salmon V, Terry R, Hay-Smith J, Frawley H, Chapman S, et al. ; on behalf of the Research Programme Team. Teaching effective pelvic floor muscle exercises in antenatal care: design and development of a training package for community midwives in the UK. ICS Conference 2022, Vienna. Won Best in Category prize. Continence 2022;2:100204. https://doi.org/10.1016/j.cont.2022.100204
Smith C, Salmon V, Jones, E, Edwards L, Hay-Smith J, Frawley H, et al. ; on behalf of the APPEAL Programme Team. Training for midwives to support women to do their exercises during pregnancy. A mixed method evaluation of the midwife training during a feasibility and pilot randomised controlled trial. ICS Conference 2022, Vienna. Continence 2022;2:100206. https://doi.org/10.1016/j.cont.2022.100206
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Boyle R, Hay-Smith EJ, Cody JD, Mørkved S. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochr Datab Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD007471.pub2.
- Harrison S, Alderdice F, Henderson J, Quigley MA. You and Your Baby: Pilot Survey. Oxford: National Perinatal Epidemiology Unit; 2019.
- Harrison S, Alderdice F, Henderson J, Quigley MA. You and Your Baby: A National Survey of Health and Care. Oxford: National Perinatal Epidemiology Unit; 2020.
- Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Woolf B, Perkins C. Cochr Datab Syst Rev. 2009.
- Salmon VE, Hay-Smith EJC, Jarvie R, Dean S, Oborn E, Bayliss SE, et al. Syst Rev. 2017.
- Salmon VE, Hay‐Smith EJC, Jarvie R, Dean S, Terry R, Frawley H, et al. Implementing pelvic floor muscle training in women’s childbearing years: a critical interpretive synthesis of individual, professional, and service issue. Neurourol Urodyn 2020;39:863-70. https://doi.org/10.1002/nau.24256.
- Pace R, Pluye P, Bartlett G, Macaulay AC, Salsberg J, Jagosh J, et al. Testing the reliability and efficiency of the pilot mixed methods appraisal tool (MMAT) for systematic mixed studies review. Int J Nurs Stud 2012;49:47-53.
- Terry R, Jarvie R, Hay-Smith J, Salmon V, Pearson M, Boddy K, et al. ‘Are you doing your pelvic floor?’ An ethnographic exploration of the interaction between women and midwives about pelvic floor muscle exercises (PFME) during pregnancy. Midwifery 2020;83. https://doi.org/10.1016/j.midw.2020.102647.
- Mays N, Pope C. Qualitative research in health care: assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Savage J. Ethnographic evidence: the value of applied ethnography in healthcare. J Res Nurs 2006;11:383-93. https://doi.org/10.1177/1744987106068297.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. London: SAGE Publications Ltd; 2014.
- LeCompte MD, Preissle Goetz J. Problems of reliability and validity in ethnographic research. Rev Educ Res 1982;52:31-60.
- Monahan T, Fisher JA. Benefits of ‘observer effects’: lessons from the field. Qual Res 2010;10:357-76. https://doi.org/10.1177/1468794110362874.
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-9.
- Tacconelli E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect Dis 2010;10. https://doi.org/10.1016/S1473-3099(10)70065-7.
- Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:A12-3.
- Roberts T, Henderson J, Mugford M, Bricker L, Neilson J, Garcia J. Antenatal ultrasound screening for fetal abnormalities: a systematic review of studies of cost and cost effectiveness. BJOG: Int J Obstetr Gynaecol 2002;109:44-56.
- Jackson LJ, Auguste P, Low N, Roberts TE. Valuing the health states associated with Chlamydia trachomatis infections and their sequelae: a systematic review of economic evaluations and primary studies. Value Health 2014;17:116-30.
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet 2011;378:328-37.
- Mittmann N, Trakas K, Risebrough N, Liu AB. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics 1999;15:369-76.
- Richardson ML, Sokol ER. A cost-effectiveness analysis of conservative versus surgical management for the initial treatment of stress urinary incontinence. Am J Obstetr Gynecol 2014;211:565.e1-6.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Kent: University of Kent; 2017.
- Brunenberg DE, Joore MA, Veraart CP, Berghmans BCM, van der Vaart CH, Severens JL. Economic evaluation of duloxetine for the treatment of women with stress urinary incontinence: a Markov model comparing pharmacotherapy with pelvic floor muscle training. Clin Therap 2006;28:604-18.
- Costantini E, Lazzeri M, Bini V, Zucchi A, Scarponi E, Porena M. Managing female urinary incontinence: a regional prospective analysis of cost–utility ratios (CURs) and effectiveness. Arch Ital Urol Androl 2014;86:112-7.
- Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess 2010;14:1-188.
- Jha S, Walters SJ, Bortolami O, Dixon S, Alshreef A. Impact of pelvic floor muscle training on sexual function of women with urinary incontinence and a comparison of electrical stimulation versus standard treatment (IPSU trial): a randomised controlled trial. Physiotherapy 2018;104:91-7.
- Lamb S, Pepper J, Lall R, Jørstad-Stein EC, Clark MD, Hill L, et al. Group treatments for sensitive health care problems: a randomised controlled trial of group versus individual physiotherapy sessions for female urinary incontinence. BMC Womens Health 2009;9:1-9.
- Neumann PB, Grimmer KA, Grant RE, Gill VA. The costs and benefits of physiotherapy as first‐line treatment for female stress urinary incontinence. Austral New Zeal J Publ Health 2005;29:416-21.
- Sjöström M, Lindholm L, Samuelsson E. Mobile app for treatment of stress urinary incontinence: a cost-effectiveness analysis. J Med Intern Res 2017;19.
- Sjöström M, Umefjord G, Lindholm L, Samuelsson E. Cost‐effectiveness of an internet‐based treatment program for stress urinary incontinence. Neurourol Urodyn 2015;34:244-50.
- Williams KS, Assassa RP, Cooper NJ, Turner DA, Shaw C, Abrams KR, et al. Leicestershire MRC Incontinence Study Team . Clinical and cost-effectiveness of a new nurse-led continence service: a randomised controlled trial. Br J Gener Pract 2005;55:696-703.
- Dean S, Salmon V, Terry R, Hay-Smith J, Frawley H, Chapman S, et al. 14 Teaching effective pelvic floor exercises in antenatal care: design and development of a training package for community midwives in the United Kingdom. Continence 2022;2. https://doi.org/10.1016/j.cont.2022.100204.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50-1. https://doi.org/10.1186/1748-5908-4-50.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Hay-Smith J, Peebles L, Dean S, Grainger R. ICS 2019 Gothenburg scientific programme. Neurourol Urodyn 2019;38:S1-532. https://doi.org/10.1002/nau.24118.
- Webb S, Jones E, Bishop J, MacArthur C. A comparison of a short versus long postal questionnaire on childbirth-related incontinence: a randomised controlled trial to evaluate response rates. Res Square 2020. https://doi.org/10.21203/rs.3.rs-89366/v1.
- Avery K, Donovan TJ, Shaw C, Gotoh M, Abrams P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30.
- Sansoni J, Hawthorne G, Fleming G, Marosszeky N. The Revised Faecal Incontinence Scale: a clinical validation of a new, short measure for assessment and outcomes evaluation. Dis Colon Rectum 2013;56:652-9. https://doi.org/10.1097/DCR.0b013e318279c2ac.
- Chen SY. The development and testing of the pelvic floor muscle exercise self-efficacy scale. J Nurs Res 2004;12:257-66. 10.1097/01.jnr.0000387510.52243.c8.
- Newman-Beinart NA, Norton S, Dowling D, Gavriloff D, Vari C, Weinman JA, et al. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: the Exercise Adherence Rating Scale (EARS). Physiotherapy 2017;103:180-85. https://doi.org/10.1016/j.physio.2016.11.001.
- Ware J, Kosinski M, Keller S. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1196;34:220-33.
- Bick D, Bishop J, Coleman T, Dean S, Edwards E, Frawley H, et al. Antenatal preventative pelvic floor muscle exercise intervention led by midwives to reduce postnatal urinary incontinence (APPEAL): protocol for a feasibility and pilot cluster randomised controlled trial. Pilot Feas Stud 2022;8. https://doi.org/10.1186/s40814-022-01185-y.
- Eldridge SM, Costelloe CE, Kahan BC, Lancaster GA, Kerry SM. How big should the pilot study for my cluster randomised trial be?. Stat Methods Med Res 2016;25:1039-56. https://doi.org/10.1177/0962280215588242.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-15.
- Smith C, Salmon V, Jones E, Edwards E, Hay-Smith J, Frawley H, et al. 16 Training for midwives to support women to do their exercises during pregnancy: a mixed method evaluation of the midwife training during a feasibility and pilot randomised controlled trial. Continence 2022;2. https://doi.org/10.1016/j.cont.2022.100206.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- National Institute For Health and Care Excellence . Pelvic Floor Dysfunction Prevention and Non-Surgical Management 2021.
- Bengtsson M. How to plan and perform a qualitative study using content analysis. NursingPlus Open 2016;2:8-14. https://doi.org/10.1016/j.npls.2016.01.001.
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Method 2006;5:80-92. https://doi.org/10.1177/160940690600500107.
Appendix 1 Work package 3 phase 1 focus groups: quotes from four themes arising from the focus groups with women and midwives
| Major theme | Subtheme | Example data |
|---|---|---|
| 1. ‘Knowing’ about PFME and UI | 1.a What: Information/guidance required by midwives and women about pelvic floor health | … a reminder about the anatomy of the muscle structure, I know it sounds really back to student days but it’s quite complex and that … which would be a good prompt as to why it’s so important because of its complexity. Midwife, FGMid1a … understanding what the pelvic floor is and does … like, how pregnancy affects it, how age affects it as well is, kind of, quite important. Woman, FGW2 |
| 1.b Why: Importance of PFME and its relationship to pelvic floor health | … you really want to know why are you doing them [PFME], you don’t just want to be told that you should be doing this because you want to know why you should keep doing them afterwards, why you should do them during pregnancy, […] and what the consequences can be if you’re not doing it. Women, FGW1b At the end of the day, whatever we can do that saves the NHS some money down the line, it’s worth investing in us being given the time to have the training, because potentially we’re saving tens of thousands with a woman having surgery later on in life because of something that could potentially have been prevented. Midwife, FGMid1a |
|
| 2. ‘Doing’ PFME | 2.a How to teach/assess/perform PFME |
Technique
(Midwife) It’s knowing that you’ve got the correct information, because there is no sort of formal anything at the moment [about teaching PFME]. There’s no updates, there’s nothing. (Midwife) And the best way to teach them [PFME]. […] So if you know it’s actually the right way, then you’re probably more confident. Midwives, FGMid1a Explaining which bits you should feel tightening and which bits you shouldn’t because I’ve, I don’t know, I’ve heard all kinds of things about how your tummy should be tensed or your tummy should be relaxed or this should be this and this. Woman, FGW1b Assessment I think that [pelvic floor assessment skills] would be useful, it would be useful for any midwife to have that […] knowledge and understanding wouldn’t it, so that she could use it with women, yeah. Midwives, FGMid2 I wouldn’t ever [do a vaginal assessment], that to me is a completely unnecessary internal examination. Midwife, FGMid1b I think they [midwives] need to have it [PFME assessment] in their repertoire to be able to do really […] If you have someone who says, ‘Actually, I don’t get it’, you know, ‘I don’t mind you examining me’. Woman, FGW1a |
| 2.b When to teach PFME | I think you have to mention it [PFME and UI] at booking because it gives it importance … if you don’t it’s ‘Oh it’s something that’s not bothered until now’. Midwife, FGMid1a Almost the initial talk about it [PFME/UI] has to be quite gentle so it might be at your booking appointment, yeah, you mention it and say perhaps you’d like to go and look it up, here’s the website or whatever, at our next appointment perhaps we’ll go through it, you know, or if you’ve got any questions and sort of feed it through, maybe like a drip feed at the beginning sort of and put that idea in there that it might be something that’s really important, obviously say about the consequences but even if you’re embarrassed by something it might get you thinking. Woman, FGW1b |
|
| 3. ‘Remembering’ PFME | 3.a Prompting engagement and participation in PFME by women, and implementation of PFME teaching by midwives | … a little reminder at each appointment to say, are you remembering to do them because I find that every time. I kind of need a reminder and then I start doing them for a while and then it kind of fades out and I lose track. Woman, FGW1b I also struggle without a prompt when somebody says, do something twice [e.g. being asked to teach PFME], like I’ll remember the first time without a doubt but if you’ve not told me when to do it the next time and some kind of prompt on that appointment then I will have every intention of doing it, but I will likely forget. Midwife, FGMid2 |
| 4. ‘Supporting’ PFME implementation and delivery | 4.a Methods and resources to deliver information and training to midwives and information to women | Delivery of information to women: Multimedia visual resources I suppose like a properly done video of some sort, you wouldn’t have to take up any more time in your appointments then, you could watch the videos […] like you’d get on the NHS website or something. Woman, FGW1b The other thing, I know in some waiting rooms they’ve got screens up, would it be worthwhile […], if you had just a short video with your pelvic floor exercises, […]. And probably a conversation starter as well with them, did you see the video, and if there’s a link that we can provide for them, for that video. Midwife, FGMid2 Leaflets I, personally, prefer, like, a leaflet or something, and if they did it in at an appointment, they would go through it with you. Woman, FGW1a … if you give someone a leaflet then they’ll be like, ‘OK, great, thanks for the leaflet’, – put them in their bag, and they’ll find it maybe at the bottom of their bag, […] I mean, like, oh well, you never look at that, and just kind of, throw it away […] Woman, FGW2 Practising PFME in the appointment (Woman) In the first [antenatal class] session we went through pelvic floor exercises, and she got everyone to stand up. So, we stood up behind chairs, and then she talked, talked us through it. So, it was like, you weren’t just sitting there, getting information, you actually had to, had to at least physically move yourself. (Woman) That’s a really good idea to actually, sort of, make somebody stand up, and actually physically, do it in front of you, which some people, you know, wouldn’t want to do it, but I do think that’s a good idea. Women, FGW1a Smartphone apps … the biggest game-changer for me, and I still wasn’t that good at doing them, was someone telling me to download the Squeezy app. Woman, FGW2 (Midwife) … we know women access apps, you know, very readily when they are pregnant. […] (Midwife) So a really good one [to recommend] would be useful. Midwives, FGMid2 Delivery of training to midwives: Credible source I think we perhaps have quite a bit of myths that we say, and to see a practitioner who’s expert in pelvic floor videoed doing a consultation with a woman, I think would be really useful. Midwives, FGMid1a Training duration (Midwife) You’d get an hour, tops [on a mandatory study day]. […] (Midwife) Half an hour is more realistic. (Midwife) They’re usually 45 minutes each slot, aren’t they on study day. Midwives, FGMid1a … it’s [PFME] not entirely new, it’s something that, I think, if a really good programme is put together, then, actually, half an hour, 45 minutes could be enough. Midwife, FGMid1b Face to face vs. online training (Midwife) I don’t think it’s appropriate to do it with something like e-learning. Because it’s too … (Midwife) No. It’s the practical-ness, a skill, yeah. Midwives, FGMid1a Online might help, but I do think it’s often people engage better with something when they’ve had perhaps a half a day study, or a whole day study initially which is quite in-depth and gives them a good knowledge and understanding, and then, yes you could catch up or top it up with some online training, … Midwife, FGMid2 Role play to facilitate learning (Midwife) So it should almost be a prompt scenario, you know, […]. (Midwife) Like a role play and how you would explain, you know, how to do. […] (Midwife) And the impact on the woman, as well. Midwives, FGMid2 I hate role-plays! Midwife, FGMid1a |
| 4.b Support requirements for implementation of training and delivery in antenatal care |
Access to training So you’d need support from a director of midwifery, […]. So either midwives decide to pay for the study day themselves, or the Trust might pay for the study day, but that’s highly unlikely given the current budgets. If you were to go to some external agency that has a vested interest like the Health Education [name of location], that that sort of organi … that sort of body might be able to fund it, but money is going to be a challenge. Midwife, FGMid2 Mandatory training (Midwife) […] so if something like this [PFME training] was made as part of that mandatory study day you know that after a year you technically should have delivered that training to all midwives in the Trust. […] (Midwife) The other thing about having it as part of our mandatory days is that everybody has to do it. Midwives, FGMid1a I don’t think it’s going to be made mandatory, I think realistically … mandatory training is absolutely chock-a-block full in terms of the training package and it’s been pared down to, used to be four days, it’s gone down to three days … so I think incorporating this into mandatory training is going to be a big ask, at this hospital, I can’t speak for other hospitals. Midwife, FGMid2 I think, I know it’s really, you know, difficult with mandatory training but if something’s so important that’s why, that’s where it should be, because otherwise you don’t do it. Midwife, FGMid2 Train the trainer and PFME champions I think so maybe you need a kind of like, a core of people who know perhaps and then who can then … And then disseminate that knowledge out. Midwife, FGMid1a (Midwife) I think to not have it [training] being passed down. (Midwife) Like Chinese whispers [laughs]. (Midwife) Yeah, and you train your trainers and then your trainers are training someone else and then they’re then training women to try and keep that step-down as short as possible so that it doesn’t get diluted as it goes down the line to the actual women who need that information. Midwives, FGMid1a (Midwife) Yeah, we have different champions for different things, like there’s somebody that does a [group] training for each team, […] (Midwife) Usually there’s somebody who’s a bit interested in it, like having a lead makes a big difference in all honesty. Midwives, FGMid1a Multidisciplinary approach I’d need all my GPs who I would probably refer them to antenatally to understand the pathway and to be able to know that this isn’t normal. Midwife, FGMid1a (Midwife) I also think you need multidisciplinary buy-in, because there’s no point midwives alone doing this, anything that’s going to be rolled out effectively needs to have input from … (Midwife) GPs as well. (Midwife) … GPs, obstetricians, gynaecologists, and anybody who comes into contact with the women during the antenatal period really. Midwives, FGMid2 National public health campaign It [promoting PFME] needs to be a massive public health campaign really, doesn’t it? On buses and things like that, so it’s just normalised, and integrated into normal life, rather than just when you get pregnant and you realise, ‘Oh, it’s quite important!’ Woman, FGW2 Ideas for fidelity checking No, you’re not having me on a video, but you can listen to me, you can watch me and you can tape me Midwife, FGMid1a That’s the only way, is to actually have it as a checklist item on an antenatal clinic appointment and then you can audit the notes to see whether that’s been ticked or not, I can’t think how else you could do it, … Midwife, FGMid2 |
Appendix 2 Logic model illustrating theory of change for APPEAL training intervention
FIGURE 4.
Logic model illustrating theory of change for APPEAL training intervention. AN, antenatal; MW, midwife; PFD, pelvic floor dysfunction; PFMC, pelvic floor muscle contraction.
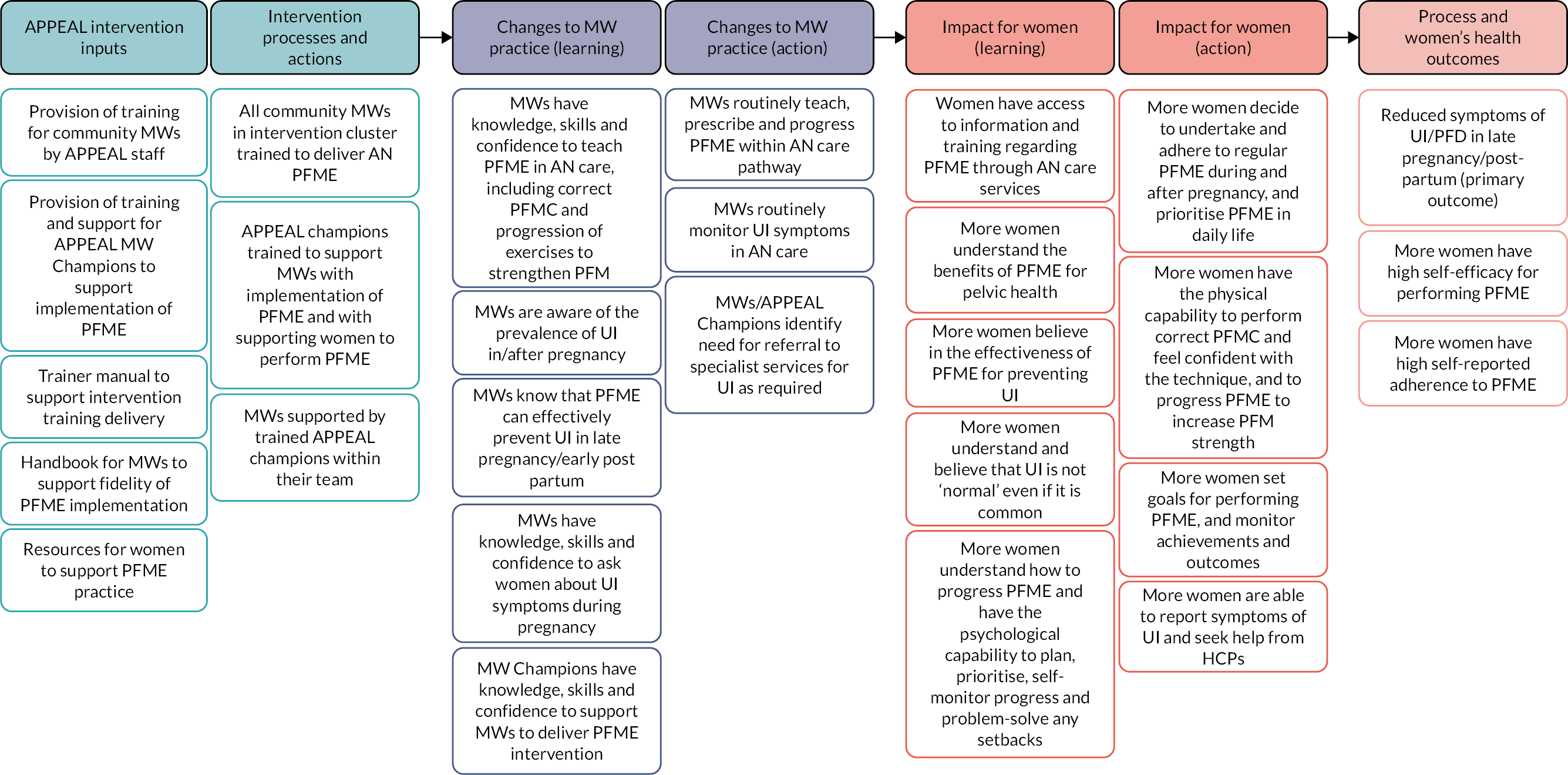
Appendix 3 Full report of work package 4.1
What is the response rate for trial questionnaire and intraclass correlation coefficient?
Aims
The primary aim of WP4.1 was to consider what the response rate to the study questionnaire would be and in so doing to estimate the ICC for a full trial. Because it was realised from other studies in similar populations that response rates have been dropping, it was decided to assess whether a long or shorter questionnaire would give a higher response in a subsequent full cluster RCT. A second objective was to assess the prevalence of urinary stress incontinence to be used in the subsequent cluster trial. This study was in the first stage of the programme before there was a change to a feasibility and pilot trial for the second stage of the programme.
Methods
This study was a parallel group trial which took place in two NHS Trusts with maternity services which would be part of the subsequent trial. Women who delivered while under the care of all 15 community midwifery teams in the Trusts during the study period who met the eligibility criteria were included. Not eligible were those under 16 years, or who did not have a live baby on hospital discharge.
The first 54 consecutive women to give birth during July 2018 in each midwife team (see later for sample size) were informed by their midwife at their first postnatal home visit that they would receive a postal questionnaire 10–12 weeks after birth. There were 54 women in each team to ensure representativeness, as teams varied in size and in the demographics of the population they served.
The questionnaires comprised several validated measures to assess UI (ICIQ-UI SF40), FI (RFIS41),self-efficacy of PFME (PFMESES42), and adherence (EARS43) and general health (SF-1244) and questions about advice that women may have received on PFME in pregnancy and their PFME practice.
The long questionnaire comprised two double-sided pages and included all the above measures. The short questionnaire comprised one double-sided page and did not include PFMESES, EARS or SF-12 (part A). For women who returned this questionnaire, a second questionnaire (part B) which included PFMESES, EARS and SF-12 was sent.
Randomisation was by a computer program at the BCTU. Individual participants were randomised to the long or the short questionnaire. The randomisation procedure was stratified by community midwife team to account for potential variation between the midwife teams in different sociodemographic areas.
Based on allocation, women were sent the long or short questionnaire by local Trust research midwives 10–12 weeks after birth, with a cover letter, £10 voucher, and stamped return envelope. The Trust research midwives sent a reminder questionnaire to women if they had not responded within 2 weeks. Women allocated to the short questionnaire arm were only sent the second part of the questionnaire if they returned their initial questionnaire.
An original sample size of 800 women with anticipated response rate between 30% and 60% would allow return of between 240 and 480 questionnaires. With a sample size of 800, with 400 allocated to each trial arm, at 80% power and 5% significance, the study would be able to detect a 10% absolute change in the percentage response rate.
Urinary incontinence was assessed using the ICIQ-UI SF40 questionnaire, which asks four questions about aspects of UI; based on which, women were dichotomised into two groups with ‘absence’ or ‘presence’ of UI. ‘Absence’ was a response of ‘never’, ‘none’ or score 0 to all four urinary questions, and ‘presence’ was a response of anything other than ‘never’, ‘none’ or score 0 to any of the four urinary questions. Analysis was performed using SAS® (SAS Institute Inc., Cary, NC, USA; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries; ® indicates USA registration). Permission was obtained from the Proportionate Review Subcommittee of the London – Brighton & Sussex Research Ethics Committee (18/LO/0934).
Results
There were 777 women randomised and sent either a long or short questionnaire, and the overall response rate was 31.3% (243/777). The number of questionnaires sent was less than the planned 810 because two small midwife teams had not reached 54 eligible births by the end of the sample month. There was no difference in the response rate either overall or to the UI questions according to questionnaire length. In the long compared with the short questionnaire arm, 30.8% (119/387) women responded overall relative to 31.8% (124/390) (odds ratio 0.95, 95% CI 0.70 to 1.29, absolute difference in return rate: −1.05%, 95% CI −7.6% to 5.5%), and 30.8% (119/387) answered the UI questions relative to 31.5% (123/390) (odds ratio 0.96, 95% CI 0.71 to 1.31, absolute difference in return rate: −0.79%, 95% CI −7.3% to 5.7%). Of the 124 women in the short questionnaire arm who responded, only 31 (25%) returned the second part (part B) of the questionnaire. Figure 5 shows the CONSORT flow diagram for WP4.1.
Table 5 shows baseline characteristics of all women who returned a questionnaire and compares those who returned a long questionnaire to those who returned the short part A questionnaire. Baseline characteristics were similar between groups.
Of total responders, 49% (119/243) reported some leaking of urine in the 4-week period prior to questionnaire completion and this was similar between trial arms (Table 6). There were 20.6% who reported some degree of FI (Table 7).
A total of 64.2% (156/243) women reported that they had been advised to perform PFME during their pregnancy by a midwife, and this was similar between trial arms. There were 42.4% (103/243) of women who reported doing PFME often enough (a few times a day, once a day, a few times a week) to possibly reduce post-partum UI (Table 8).
Conclusion
The overall questionnaire response was low at 31.3%, despite incorporating Cochrane Review recommendations to increase responses to postal and electronic questionnaires, namely: first-class postage; first-class stamped envelopes for return; £10 voucher sent with initial mailing as incentive; and reminder questionnaire.
This pilot study showed, therefore, that questionnaire response rate was relatively low, and a different length questionnaire made no difference. The estimate of the ICC for the return rate was 0.007 (95% CI 0.0005 to 0.094).
FIGURE 5.
Work package 4.1 CONSORT flow diagram.
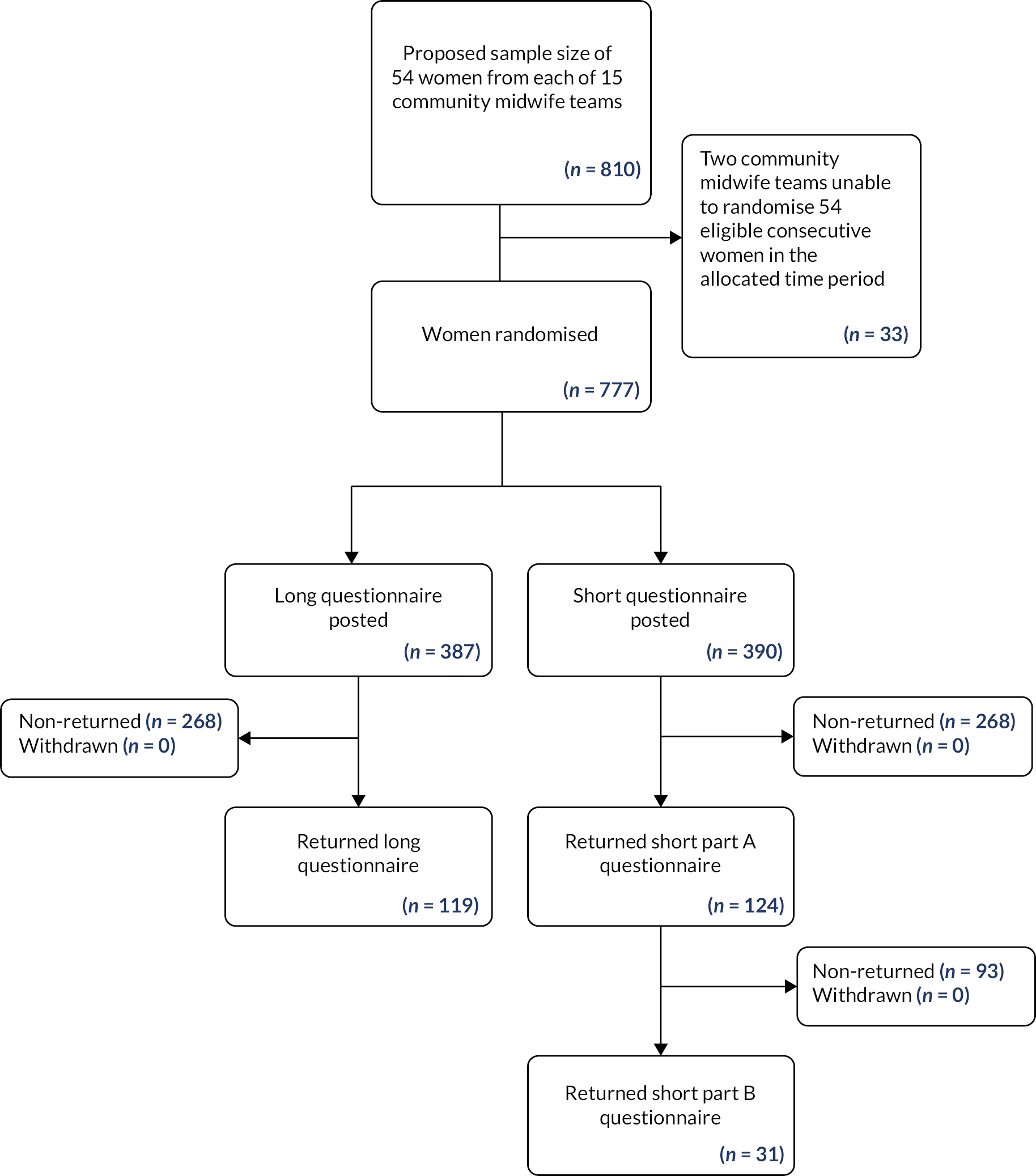
| Birth baseline characteristic | Long (N = 119) | Short part A (N = 124) | Total (N = 243) |
|---|---|---|---|
| Labour onset | |||
| Spontaneous onset | 55 (46.2%) | 61 (49.2%) | 116 (47.7%) |
| Induced | 26 (21.8%) | 22 (17.7%) | 48 (19.8%) |
| Not applicable – elective caesarean section | 14 (11.8%) | 12 (9.7%) | 26 (10.7%) |
| Missing | 24 (20.2%) | 29 (23.4%) | 53 (21.8%) |
| Mode of birth | |||
| Spontaneous vaginal | 63 (52.9%) | 65 (52.4%) | 128 (52.7%) |
| Instrumental vaginal | 13 (10.9%) | 14 (11.3%) | 27 (11.1%) |
| Caesarean section | 21 (17.6%) | 20 (16.1%) | 41 (16.9%) |
| Missing | 22 (18.5%) | 25 (20.2%) | 47 (19.3%) |
| Analgesia | |||
| Yes | 26 (21.8%) | 28 (22.6%) | 54 (22.2%) |
| No | 52 (43.7%) | 48 (38.7%) | 100 (41.2%) |
| Missing | 41 (34.5%) | 48 (38.7%) | 89 (36.6%) |
| Perineum | |||
| Intact perineum | 31 (26.1%) | 28 (22.6%) | 59 (24.3%) |
| Labial tear | 0 (0%) | 1 (0.8%) | 1 (0.4%) |
| First-degree tear | 7 (5.9%) | 10 (8.1%) | 17 (7%) |
| Second-degree tear | 34 (28.6%) | 38 (30.6%) | 72 (29.6%) |
| Third- and fourth-degree tear (OASI) | 3 (2.5%) | 1 (0.8%) | 4 (1.6%) |
| Not applicable | 19 (16%) | 18 (14.5%) | 37 (15.2%) |
| Missing | 25 (21%) | 28 (22.6%) | 53 (21.8%) |
| Episiotomy | |||
| Yes | 16 (13.4%) | 16 (12.9%) | 32 (13.2%) |
| No | 76 (63.9%) | 80 (64.5%) | 156 (64.2%) |
| Missing | 27 (22.7%) | 28 (22.6%) | 55 (22.6%) |
| Birthweight ( n ) | (n = 97) | (n = 100) | (n = 197) |
| Mean (SD), g | 3232 (457) | 3348 (482) | 3291 (472) |
| Missing | 22 (18%) | 24 (19%) | 46 (19%) |
| Head circumference ( n ) | (n = 90) | (n = 95) | (n = 185) |
| Mean (SD), cm | 34 (1.44) | 34 (1.40) | 34.1 (1.42) |
| Missing | 29 (24%) | 29 (23%) | 58 (24%) |
| Length of active second stage ( n ) | (n = 53) | (n = 50) | (n = 103) |
| Median (IQR), minutes | 15 (7–53) | 19 (5–42) | 19 (6–49) |
| Missing | 66 (55%) | 74 (60%) | 140 (58%) |
| Outcome | Long (N = 119), n (%) | Short part A (N = 124), n (%) | Total (N = 243), n (%) |
|---|---|---|---|
| Urinary incontinence | |||
| Present | 59 (49.5) | 59 (48.4) | 119 (49.0) |
| Absent | 60 (50.4) | 64 (51.6) | 124 (51.0) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
| Bladder function questions | |||
| How often do you leak urine? | |||
| Never | 65 (54.6) | 69 (55.6) | 134 (55.1) |
| About once a week or less often | 30 (25.2) | 33 (26.6) | 63 (25.9) |
| Two or three times a day | 12 (10.1) | 8 (6.5) | 20 (8.2) |
| About once a day | 5 (4.2) | 4 (3.2) | 9 (3.7) |
| Several times a day | 6 (5) | 7 (5.6) | 13 (5.3) |
| All of the time | 0 (0) | 1 (0.8) | 1 (0.4) |
| Missing | 1 (0.8) | 2 (1.6) | 3 (1.2) |
| How much urine do you usually leak? | |||
| None | 64 (53.8) | 63 (50.8) | 127 (52.3) |
| A small amount | 47 (39.5) | 46 (37.1) | 93 (38.3) |
| A moderate amount | 6 (5) | 8 (6.5) | 14 (5.8) |
| A large amount | 0 (0) | 0 (0) | 0 (0) |
| Missing | 2 (1.7) | 7 (5.6) | 9 (3.7) |
| Overall, how much does leaking urine interfere with your everyday life? | |||
| 0 (not at all) | 72 (60.5) | 73 (58.9) | 145 (59.7) |
| 1 | 14 (11.8) | 20 (16.1) | 34 (14) |
| 2 | 9 (7.6) | 8 (6.5) | 17 (7) |
| 3 | 7 (5.9) | 8 (6.5) | 15 (6.2) |
| 4 | 5 (4.2) | 2 (1.6) | 7 (2.9) |
| 5 | 1 (0.8) | 3 (2.4) | 4 (1.6) |
| 6 | 2 (1.7) | 3 (2.4) | 5 (2.1) |
| 7 | 3 (2.5) | 2 (1.6) | 5 (2.1) |
| 8 | 2 (1.7) | 0 (0) | 2 (0.8) |
| 9 | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| 10 (a great deal) | 0 (0) | 2 (1.6) | 2 (0.8) |
| Missing | 3 (2.5) | 2 (1.6) | 5 (2.1) |
| When does urine leak? | |||
| Never | 60 (50.4) | 68 (54.8) | 128 (52.7) |
| Before you can get to the toilet | 22 (18.5) | 19 (15.3) | 41 (16.9) |
| When you sneeze | 40 (33.6) | 31 (25) | 71 (29.2) |
| When you are asleep | 3 (2.5) | 1 (0.8) | 4 (1.6) |
| When you are physically active/exercising | 21 (17.6) | 16 (12.9) | 37 (15.2) |
| When you have finished urinating and are getting dressed | 7 (5.9) | 7 (5.6) | 14 (5.8) |
| For no obvious reason | 6 (5) | 4 (3.2) | 10 (4.1) |
| All of the time | 0 (0) | 1 (0.8) | 1 (0.4) |
| Outcome | Long (N = 119), n (%) | Short part A (N = 124), n (%) | Total (N = 243), n (%) |
|---|---|---|---|
| Faecal incontinence | |||
| Present | 31 (26) | 19 (15.3) | 50 (20.6) |
| Absent | 88 (74) | 104 (83.9) | 192 (79) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
| Bowel function questions | |||
| Do you leak, have accidents or lose control with solid stool? | |||
| Never | 101 (84.9) | 117 (94.4) | 218 (89.7) |
| Rarely | 9 (7.6) | 5 (4) | 14 (5.8) |
| Sometimes | 6 (5) | 0 (0) | 6 (2.5) |
| Often or usually | 2 (1.7) | 1 (0.8) | 3 (1.2) |
| Always | 1 (0.8) | 0 (0) | 1 (0.4) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
| Do you leak, have accidents or lose control with liquid stool? | |||
| Never | 100 (84) | 110 (88.7) | 210 (86.4) |
| Rarely | 11 (9.2) | 10 (8.1) | 21 (8.6) |
| Sometimes | 5 (4.2) | 1 (0.8) | 6 (2.5) |
| Often or usually | 2 (1.7) | 2 (1.6) | 4 (1.6) |
| Always | 1 (0.8) | 0 (0) | 1 (0.4) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
| Do you leak stool if you don’t get to the toilet in time? | |||
| Never | 99 (83.2) | 114 (91.9) | 213 (87.7) |
| Rarely | 10 (8.4) | 6 (4.8) | 16 (6.6) |
| Sometimes | 9 (7.6) | 1 (0.8) | 10 (4.1) |
| Often or usually | 0 (0) | 0 (0) | 0 (0) |
| Always | 1 (0.8) | 2 (1.6) | 3 (1.2) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
| Does stool leak so that you have to change your underwear? | |||
| Never | 100 (84) | 114 (91.9) | 214 (88.1) |
| Rarely | 11 (9.2) | 5 (4) | 16 (6.6) |
| Sometimes | 6 (5) | 2 (1.6) | 8 (3.3) |
| Often or usually | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Always | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
| Does bowel or stool leakage cause you to alter your lifestyle? | |||
| Never | 107 (89.9) | 116 (93.5) | 223 (91.8) |
| Rarely | 6 (5) | 2 (1.6) | 8 (3.3) |
| Sometimes | 3 (2.5) | 2 (1.6) | 5 (2.1) |
| Often or usually | 2 (1.7) | 1 (0.8) | 3 (1.2) |
| Always | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Missing | 0 (0) | 2 (1.6) | 2 (0.8) |
| Outcome | Long (N = 119), n (%) | Short part A (N = 124), n (%) | Total (N = 243), n (%) |
|---|---|---|---|
| Did your midwife advise you to perform PFME when you were pregnant? | |||
| Yes | 77 (64.6) | 79 (63.7) | 156 (64.2) |
| No | 21 (17.7) | 22 (17.7) | 43 (17.7) |
| Missing | 21 (17.7) | 23 (18.6) | 44 (18.1) |
| How often did you perform PFME when you were pregnant? | |||
| Never – was never advised to | 17 (14.3) | 22 (17.7) | 39 (16.0) |
| Never – other reasons | 9 (7.6) | 12 (9.7) | 21 (8.6) |
| A few times a month | 38 (31.9) | 22 (17.7) | 60 (24.7) |
| Once a week | 4 (3.4) | 4 (3.2) | 8 (3.3) |
| Few times a week | 24 (20.2) | 29 (23.4) | 53 (21.8) |
| Once a day | 10 (8.4) | 12 (9.7) | 22 (9.1) |
| A few times a day | 13 (10.9) | 15 (12.1) | 28 (11.5) |
| Can’t remember | 3 (2.5) | 8 (6.5) | 11 (4.5) |
| Missing | 1 (0.8) | 0 (0) | 1 (0.4) |
| Do you currently perform PFME? | |||
| Yes | 73 (61.3) | 73 (58.8) | 146 (60.1) |
| No | 36 (30.3) | 38 (30.7) | 74 (30.4) |
| Missing | 10 (8.4) | 13 (10.5) | 23 (9.5) |
| How often did you do PFME over the last month? | |||
| Never – was never advised to | 10 (8.4) | 13 (10.5) | 23 (9.5) |
| Never – other reasons | 15 (12.6) | 18 (14.5) | 33 (13.6) |
| A few times a month | 41 (34.5) | 33 (26.6) | 74 (30.5) |
| Once a week | 25 (21.0) | 22 (17.7) | 47 (19.3) |
| Few times a week | 5 (4.2) | 8 (6.4) | 13 (5.4) |
| Once a day | 11 (9.2) | 14 (11.3) | 25 (10.3) |
| A few times a day | 12 (10.1) | 15 (12.1) | 27 (11.1) |
| Missing | 0 (0) | 1 (0.8) | 1 (0.4) |
Appendix 4 Additional work package 4.2 tables
| Number of women in the database | 1304 |
| Number of women in the database who received maternity care from one of the midwifery teams randomised to APPEAL | 1005 |
| Number of out of area women in the database | 269 |
| Number of new team’sa women in the database | 4 |
| Number of women in the database for whom no midwifery team recorded | 26 |
| Number of women to whom the questionnaire was sent | 1294 b |
| Number of women to whom the questionnaire was sent, who received maternity care from one of the midwifery teams randomised to APPEAL | 998 |
| Number of out of area women to whom the questionnaire was sent | 266 |
| Number of new team’sa women to whom the questionnaire was sent | 4 |
| Number of women to whom the questionnaire was sent but no midwifery team recorded | 26 |
| Number of women who returned the questionnaire | 231 |
| Number of women who returned the questionnaire, who received maternity care from one of the midwifery teams randomised to APPEAL | 175 |
| Number of out of area women who returned the questionnaire | 56 |
| Number of new team’sa women who returned the questionnaire | 0 |
| Number of women to whom the short, translated version of the questionnaire was sent | 111 |
| Number of women to whom the short, translated version of the questionnaire was sent, who received maternity care from one of the midwifery teams randomised to APPEAL | 90 |
| Number of out of area women to whom the short, translated version of the questionnaire was sent | 17 |
| Number of new team’sa women to whom the short, translated version of the questionnaire was sent | 3 |
| Number of women to whom the short, translated version of the questionnaire was sent but no midwifery team recorded | 1 |
| Number of women who returned the short, translated version of the questionnaire | 10 |
| Number of women who returned the short, translated version of the questionnaire and who received maternity care from one of the midwifery teams randomised to APPEAL | 8 |
| Number of out of area women who returned the short, translated version of the questionnaire | 2 |
| Number of new team’sa women who returned the short, translated version of the questionnaire | 0 |
| Number of women to whom the standard questionnaire was sent | 1179 |
| Number of women to whom the standard questionnaire was sent, who received maternity care from one of the midwifery teams randomised to APPEAL | 905 |
| Number of out of area women to whom the standard questionnaire was sent | 248 |
| Number of new team’sa women to whom the standard questionnaire was sent | 1 |
| Number of women to whom the standard questionnaire was sent but no midwifery team recorded | 25 |
| Number of women who returned the standard questionnaire | 221 |
| Number of women who returned the standard questionnaire and who received maternity care from one of the midwifery teams randomised to APPEAL | 167 |
| Number of out of area women who returned the standard questionnaire | 54 |
| Number of new team’sa women who returned the standard questionnaire | 0 |
| Intervention (N = 531) | Control (N = 467) | Overall (N = 998) | |
|---|---|---|---|
| Number of women to whom the questionnaire was sent | 531 | 467 | 998 |
| Number of women to whom the short, translated version of the questionnaire was sent | 52 | 38 | 90 |
| Number of women to whom the standard questionnaire was sent | 479 | 426 | 905 |
| Number of women to whom the questionnaire was sent one time | 98 | 76 | 174 |
| Number of women to whom the questionnaire was sent one time, who returned the questionnaire | 62 | 48 | 110 |
| Number of women to whom the questionnaire was sent two times | 433 | 391 | 824 |
| Number of women to whom the questionnaire was sent two times, who returned the questionnaire | 26 | 39 | 65 |
| Total number of questionnaires returned | 88 | 87 | 175 |
| Number of women who returned the short questionnaire | 5 | 3 | 8 |
| Number of women who returned the standard questionnaire | 83 | 84 | 167 |
| Number of women recorded on the database and the questionnaire was not sent to thema: | 6 | 1 | 7 |
| Site 1 | Site 2 | |
|---|---|---|
| Stillbirth | 0 | 0 |
| Neonatal death | 0 | 5 |
| Infant death | 0 | 1 |
| Fetal abnormality | 1 | 0 |
| Women whose infants were taken into care due to safeguarding concerns | 1 | 0 |
| Women who have severe mental health problems | 8 | 0 |
| Restricted address | 1 | 0 |
| Twin pregnancy – one twin intrauterine death | 0 | 1 |
| Minimisation variables | Women who returned questionnaire | Women who did not return questionnaire | |||
|---|---|---|---|---|---|
| Intervention (N = 88), n (%) | Control (N = 87), n (%) | Overall (N = 175), n (%) | Overall (N = 823), n (%) | ||
| Midwifery team size | Small | 35 (39.8) | 25 (28.7) | 60 (34.3) | 276 (33.5) |
| Large | 53 (60.2) | 62 (71.3) | 115 (65.7) | 547 (66.5) | |
| Trust | Site 1 | 46 (52.3) | 23 (26.4) | 69 (39.4) | 300 (36.5) |
| Site 2 | 42 (47.7) | 64 (73.6) | 106 (60.6) | 523 (63.5) | |
| Women who returned questionnaire | Women who did not return questionnaire | ||||
|---|---|---|---|---|---|
| Intervention (N = 88) | Control (N = 87) | Overall (N = 175) | Overall (N = 1063) | ||
| Women’s demographics and other baseline characteristics | |||||
| Maternal | |||||
| Age, years | n | 78 | 77 | 155 | 1063 |
| Mean (SD) | 31.6 (4.9) | 31.9 (5.5) | 31.8 (5.2) | 29.6 (N/Aa) | |
| Minimum, maximum | 22, 45 | 19, 48 | 19, 48 | 15, 46 | |
| Median (IQR) | 32 (28–35) | 32 (29–35) | 32 (28–35) | Ν/Αa | |
| Missing | 10 | 10 | 20 | 0 | |
| Ethnicity | n | 88 | 87 | 175 | 1063 |
| British | 40 (52.0%) | 37 (48.1%) | 77 (50.0%) | 371 (34.9%) | |
| Irish | 1 (1.3%) | 0 (0%) | 1 (0.7%) | 5 (0.5%) | |
| White other | 5 (6.5%) | 1 (1.3%) | 6 (3.9%) | 46 (4.3%) | |
| White and Black Caribbean | 0 (0%) | 0 (0%) | 0 (0%) | 11 (1.0%) | |
| White and Black African | 0 (0%) | 0 (0%) | 0 (0%) | 4 (0.4%) | |
| White and Asian | 0 (0%) | 0 (0%) | 0 (0%) | 5 (0.5%) | |
| Mixed other | 0 (0%) | 0 (0%) | 0 (0%) | 8 (0.8%) | |
| Indian | 0 (0%) | 3 (3.9%) | 3 (2.0%) | 33 (3.1%) | |
| Pakistani | 3 (3.9%) | 7 (9.1%) | 10 (6.5%) | 205 (19.3%) | |
| Bangladeshi | 2 (2.6%) | 0 (0%) | 2 (1.3%) | 16 (1.5%) | |
| Asian other | 6 (7.8%) | 7 (9.1%) | 13 (8.4%) | 29 (2.7%) | |
| Black Caribbean | 0 (0%) | 1 (1.3%) | 1 (0.7%) | 10 (0.9%) | |
| Black African | 1 (1.3%) | 1 (1.3%) | 2 (1.3%) | 37 (3.5%) | |
| Black other | 3 (3.9%) | 1 (1.3%) | 4 (2.6%) | 16 (1.5%) | |
| Chinese | 0 (0%) | 0 (0%) | 0 (0%) | 6 (0.6%) | |
| Any other ethnic group | 6 (7.8%) | 1 (1.3%) | 7 (4.6%) | 56 (5.3%) | |
| Not stated | 6 (7.8%) | 4 (5.2%) | 10 (6.5%) | 193 (18.2%) | |
| Declined to answer | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Not known | 4 (5.2%) | 14 (18.2%) | 18 (11.7%) | 12 (1.1%) | |
| Missing | 11 | 10 | 21 | 0 | |
| Parity | n | 88 | 87 | 175 | 1063 |
| 0 | 34 (45.3%) | 28 (36.8%) | 62 (41.0%) | 367 (34.5%) | |
| 1 | 28 (37.3%) | 33 (43.4%) | 61 (40.4%) | 317 (29.8%) | |
| 2 | 9 (12.0%) | 7 (9.2%) | 16 (10.6%) | 181 (17.0%) | |
| 3 | 4 (5.4%) | 5 (6.6%) | 9 (6.0%) | 91 (8.6%) | |
| 4 | 0 (0%) | 1 (1.4%) | 1 (0.7%) | 35 (3.3%) | |
| 5 or more | 0 (0%) | 2 (2.6%) | 2 (1.3%) | 72 (6.8%) | |
| Missing | 13 | 11 | 24 | 0 | |
| Obstetric | |||||
| Onset of labour | n | 88 | 87 | 175 | 1063 |
| Spontaneous | 42 (55.3%) | 39 (50.0%) | 81 (52.6%) | 429 (41.7%) | |
| Induced | 12 (15.8%) | 23 (29.5%) | 35 (22.7%) | 307 (29.8%) | |
| N/A – elective caesarean section (including failed induction) | 22 (28.9%) | 16 (20.5%) | 38 (24.7%) | 293 (28.5%) | |
| Missing | 12 | 9 | 21 | 34 | |
| Mode of birth | n | 88 | 87 | 175 | 1063 |
| Ventouse | 10 (13.3%) | 11 (14.1%) | 21 (13.7%) | 61 (5.7%) | |
| Forceps | 4 (5.3%) | 4 (5.1%) | 8 (5.2%) | 49 (4.6%) | |
| Caesarean section | 26 (34.7%) | 24 (30.8%) | 50 (32.7%) | 405 (38.1%) | |
| Spontaneous vaginal birth | 35 (46.7%) | 39 (50.0%) | 74 (48.4%) | 547 (51.5%) | |
| Missing | 13 | 9 | 22 | 1 | |
| Anaestheticb | n | 88 | 87 | 175 | 1063 |
| Spinal | 23 (79.3%) | 23 (76.7%) | 46 (78.0%) | 345 (69.4%) | |
| Epidural | 5 (17.2%) | 7 (23.3%) | 12 (20.3%) | 122 (24.5%) | |
| General | 1 (3.5%) | 0 (0%) | 1 (1.7%) | 30 (6.0%) | |
| Missing | 59 | 57 | 116 | 566 | |
| Analgesiac | n | 88 | 87 | 175 | 1063 |
| Yes | 39 (72.2%) | 50 (80.7%) | 89 (76.7%) | 524 (49.3%) | |
| No | 15 (27.8%) | 12 (19.3%) | 27 (23.3%) | 539 (50.7%) | |
| Missing | 34 | 25 | 59 | 0 | |
| Perineal trauma | n | 88 | 87 | 175 | 1063 |
| First degree | 6 (8.3%) | 7 (9.1%) | 13 (8.7%) | 86 (15.2%) | |
| Second degree | 21 (29.2%) | 27 (35.1%) | 48 (32.2%) | 223 (39.5%) | |
| 3a | 1 (1.4%) | 1 (1.3%) | 2 (1.3%) | 5 (0.9%) | |
| 3b | 1 (1.4%) | 0 (0%) | 1 (0.7%) | 2 (0.4%) | |
| 3c | 1 (1.4%) | 0 (0%) | 1 (0.7%) | 0 (0%) | |
| Fourth degree | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.2%) | |
| Labial lacerations | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| None | 42 (58.3%) | 42 (54.5%) | 84 (56.4%) | 248 (43.9%) | |
| Missing | 16 | 10 | 26 | 498 | |
| Episiotomy | n | 88 | 87 | 175 | 1063 |
| Yes | 17 (23.3%) | 16 (21.1%) | 33 (22.2%) | 143 (24.0%) | |
| No | 56 (76.7%) | 60 (78.9%) | 116 (77.8%) | 453 (76.0%) | |
| Missing | 15 | 11 | 26 | 467 | |
| Duration of second stage (minutes) | n | 26 | 37 | 63 | 809 |
| Minimum, maximum | 0, 1260 | 0, 1860 | 0, 1860 | 0, 300 | |
| Median (IQR) | 35 (2–143) | 36 (10–77) | 36 (8–94) | Ν/Αa | |
| Missing | 62 | 50 | 112 | 254 | |
| Infants | |||||
| Gestation at birth (weeks) | n | 78 | 78 | 156 | 1063 |
| Mean (SD) | 39.6 (1.6) | 39.3 (2.2) | 39.4 (1.9) | 36.0 (N/Aa) | |
| Minimum, maximum | 34.6, 42.1 | 24.1, 41.9 | 24.1, 42.1 | 23.0, 42.0 | |
| Missing | 10 | 9 | 19 | 0 | |
| Birthweight (g) | n | 78 | 78 | 156 | 1063 |
| Mean (SD) | 3356 (435) | 3325 (499) | 3340 (467) | 3171 (N/Aa) | |
| Minimum, maximum | 2170, 4550 | 700, 4150 | 700, 4550 | 550, 4850 | |
| Missing | 10 | 9 | 19 | 0 | |
| Head circumference (cm) | n | 73 | 74 | 147 | 1015 |
| Mean (SD) | 34.5 (1.3) | 34.2 (1.2) | 34.4 (1.2) | 33.3 (N/Aa) | |
| Minimum, maximum | 32.0, 38.0 | 30.5, 36.5 | 30.5, 38.0 | 21.0, 52.0 | |
| Missing | 15 | 13 | 28 | 48 | |
| Prevalence of UI using the ICIQ-UI SF | Intervention (n = 88) | Control (n = 87) | |
|---|---|---|---|
| Total ICIQ-UI SF scorea | N | 87 | 86 |
| Mean (SD) | 3.3 (4.5) | 4.2 (4.3) | |
| Missing | 1 | 1 | |
| 95% CI | 2.1 to 4.5b | 2.9 to 5.6c | |
| How often do you leak urine? | Never | 49 (55.7%) | 40 (46.0%) |
| About once a week or less | 22 (25.0%) | 21 (24.1%) | |
| Two or three times a week | 8 (9.0%) | 15 (17.2%) | |
| About once a day | 5 (5.7%) | 4 (4.6%) | |
| Several times a day | 2 (2.3%) | 7 (8.1%) | |
| All of the time | 2 (2.3%) | 0 (0%) | |
| Missing | 0 | 0 | |
| How much urine do you usually leak (whether you wear protection or not)? | None | 49 (53.3%) | 36 (41.8%) |
| A small amount | 35 (40.2%) | 44 (51.2%) | |
| A moderate amount | 3 (23.5%) | 6 (7.0%) | |
| A large amount | 0 (0%) | 0 (0%) | |
| Missing | 1 | 1 | |
| Overall, how much does leaking urine interfere with your everyday life? Please tick a number between 0 (not at all) and 10 (a great deal) | 0 | 53 (60.2%) | 39 (45.3%) |
| 1 | 6 (6.8%) | 10 (11.6%) | |
| 2 | 5 (5.7%) | 9 (10.5%) | |
| 3 | 8 (9.1%) | 10 (11.6%) | |
| 4 | 3 (3.4%) | 4 (4.7%) | |
| 5 | 6 (6.8%) | 6 (7.0%) | |
| 6 | 0 (0%) | 4 (4.7%) | |
| 7 | 2 (2.3%) | 2 (2.3%) | |
| 8 | 3 (3.4%) | 0 (0%) | |
| 9 | 0 (0%) | 0 (0%) | |
| 10 | 2 (2.3%) | 2 (2.3%) | |
| Missing | 0 | 1 | |
| When does urine leak?d | Never – urine does not leak | 47 (53.4%) | 36 (41.4%) |
| Leaks before you can get to the toilet | 16 (18.2%) | 25 (28.7%) | |
| Leaks when you sneeze | 25 (28.4%) | 34 (39.1%) | |
| Leaks when you are asleep | 1 (1.1%) | 4 (4.6%) | |
| Leaks when you are physically active/exercising | 20 (22.7%) | 21 (24.1%) | |
| Leaks when you have finished urinating and are getting dressed | 7 (8.0%) | 11 (12.6%) | |
| Leaks for no obvious reason | 5 (5.7%) | 3 (3.5%) | |
| Leaks all of the time | 1 (1.1%) | 2 (2.3%) | |
| Missing | 0 | 1 | |
| Prevalence of UIe | Yes | 39 (44.3%) | 47 (54.0%) |
| No | 49 (55.7%) | 40 (46.0%) | |
| Missing | 0 | 0 | |
| 95% CI | 32.0% to 56.1%f | 42.2% to 65.8%g | |
| Women’s EARS score | N | 82 | 82 |
| Mean (SD) | 10.8 (5.6) | 9.4 (5.4) | |
| Missing | 6 | 5 | |
| 95% CI | 9.1 to 12.4f | 5.3 to 15.0g | |
| Total score of self-efficacy (PFMESES): women’s confidence in their practice of performing PFMEh | N | 19 | 22 |
| Mean (SD) | 65.7 (9.1) | 65.6 (8.1) | |
| Missing | 69 | 65 | |
| 95% CI | 58.0 to 73.2e | 61.0 to 70.3f | |
| Subscore of self-efficacy (PFMESES): women’s belief in PFME execution and its benefitsi | N | 79 | 76 |
| Mean (SD) | 41.6 (7.5) | 41.6 (6.8) | |
| Missing | 9 | 11 | |
| 95% CI | 38.9 to 44.2e | 37.9 to 45.4f | |
| Subscore sum of self-efficacy (PFMESES) in performing PFME as scheduled and despite barriersj | N | 19 | 24 |
| Mean (SD) | 22.4 (4.5) | 23.2 (4.0) | |
| Missing | 69 | 63 | |
| 95% CI | 17.9 to 27.0e | 21.3 to 25.1f |
| Intervention (n = 88) | Control (n = 87) | ||
|---|---|---|---|
| Prevalence of FI at 10–12 weeks using the RFIS41 | |||
| Total RFI scorea | N | 82 | 82 |
| Mean (SD) | 1.2 (3.1) | 0.7 (2.2) | |
| Missing | 6 | 5 | |
| Total score < 4 | 72 (87.8%) | 78 (95.1%) | |
| 4 ≤ Total score ≤ 6 | 4 (4.9%) | 2 (2.5%) | |
| 7 ≤ Total score ≤ 12 | 4 (4.9%) | 1 (1.2%) | |
| Total score ≥ 13 | 2 (2.4%) | 1 (1.2%) | |
| 95% CI | 0.2 to 2.0b | 0.2 to 0.9c | |
| Prevalence of FId | Yes | 15 (18.1%) | 11 (13.3%) |
| No | 68 (81.9) | 72 (86.7%) | |
| Missing | 5 | 4 | |
| 95% CI | 6.6% to 28.9%e | 4.8% to 21.2%f | |
| Prevalence of flatus incontinence | |||
| Prevalence of flatus incontinenceg | Yes | 34 (41.0%) | 30 (35.7%) |
| No | 49 (59.0%) | 54 (64.3%) | |
| Missing | 5 | 3 | |
| 95% CI | 17.9% to 64.6%e | 25.5% to 45.8%f | |
Appendix 5 Data collection and analysis methods (work package 4.3)
Work package 4.3 comprised a process evaluation undertaken in parallel with the WP4.2 pilot feasibility trial (see Bick et al. for protocol45). This process evaluation focused on exploring the feasibility and acceptability of the intervention and implementation structures, in line with Medical Research Council guidance. 34 It aimed to identify facilitators and concerns or challenges regarding implementation that might be addressed prior to a future evaluation of intervention effectiveness.
Research questions and methods used to answer the questions
Tables 16 and 17 summarise the data collection sources and analysis methods. The left-hand column of the tables lists the research questions which have been broken down and mapped onto relevant components of the Grant et al. (2013) framework for designing and reporting process evaluations:47 processes involving clusters: delivery to clusters, response of cluster (see Table 16); processes involving target population: delivery to individuals and response of individuals (see Table 17). Data collection sources are listed across the tables (top row) according to four stages of the study: during training, post training, implementation phase and end of study. The second row indicates whether the data source was predominantly quantitative (Qt) or qualitative (Ql). The tables therefore present an overview of how the multiple methods of data collection fitted together to inform overall understanding as it showed which research question they aimed to address and which phase of the study they referred to, and the type of data source.
Data collection and analysis methods
The following refers to data sources presented in column headings in Tables 16 and 17.
Training attendance
Data sample: attendance records were recorded by research midwives for all training sessions they delivered.
To analyse training attendance, we descriptively summarised the number of midwives who attended training from each community midwife team in the intervention cluster [e.g. 89 of 92 (97%) of midwives from n = xx community teams].
Training observation
One of two members of the research team joined and silently observed four of the main training sessions. The researchers checked whether key statements were articulated, which were listed in bold on the midwives’ training presentation slide notes. The key statements were ticked off once delivered, permitting quantitative monitoring of training fidelity. The researcher also qualitatively described the spirit of delivery as intended by the protocol.
The quantitative data constituted the number of key statements delivered – this was summarised descriptively via the proportion (%) of key statements delivered, as well as any key statements that were consistently omitted by the facilitators.
The qualitative data gathered from this source were evaluated via content analysis51 to assess: (1) what went well, (2) whether any aspects of the mode of delivery needed improvement and (3) what barriers could pose a challenge to training implementation. The data were coded deductively, as well as checked inductively, to establish the main themes that arose from that data set.
| RQ no | RQ | During training | Post training | Implementation phase | End of study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training attendance | Training observation | Pre-/post-training questionnaires | Training evaluation questionnaire | I/V MW interviews | Champion interviews | Champion monitoring | Distribution of resource packs | Implementation questionnaires | I/V MW interview | Champion interview | Control MW interview | Women’s questionnaires and interviews | ||
| Qt | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Ql | Qt | ||
| Delivery to clusters | ||||||||||||||
| 2a | What training is delivered to community MWs? | Qt | Qt and Ql | Ql | Ql | Ql | Ql | Ql | ||||||
| 2b | Who receives training? | Qt | Ql | Ql | Qt and Ql | Ql | Ql | Ql | ||||||
| 2b | What are the opportunities/challenges for MWs receiving training as planned? | Qt | Ql | Ql | ||||||||||
| Response of clusters | ||||||||||||||
| 2c | Do MWs feel more confident to implement PFME in AN care following training? | Qt | Ql | Ql | Ql | Ql | ||||||||
| 1a | Is training delivery and content and implementation of PFME acceptable to midwives/champions? | Qt and Ql | Ql | Ql | Qt and Ql | Ql | Ql | |||||||
| 2c, 1a | What factors affect the acceptability of training delivery and content for MWs/champions? | Ql | Ql | Ql | Qt and Ql | Ql | Ql | |||||||
| RQ no | RQ | During training | Post training | Implementation phase | End of study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training attendance | Training observation | Pre-/post-training questionnaires | Training evaluation questionnaire | I/V MW interviews | Champion interviews | Champion monitoring | Distribution of resource packs | Implementation questionnaire | I/V MW interview | Champion interview | Control MW interview | Women’s questionnaires | Women’s interviews | ||
| Qt | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Ql | Qt | Ql | ||
| Delivery to individuals | |||||||||||||||
| 2c, 1a | Do training resources for MWs/champions aid implementation of the PFME intervention for women? | Qt and Ql | Ql | Ql | Qt and Ql | Ql | Ql | Ql | Ql | ||||||
| 1a, 2d, 2e | What are the challenges, concerns and opportunities for MWs implementing PFME following training? | Ql | Ql | Qt and Ql | Ql | Ql | |||||||||
| 1a, 2e | Did MWs seek support from champions for implementing PFME? | Qt and Ql | Qt and Ql | Ql | Ql | ||||||||||
| 1a, 2e | Was champions’ support for implementation of PFME intervention helpful/unhelpful? | Qt and Ql | Qt and Ql | Ql | Ql | ||||||||||
| 2d, 2e, 3 | Were resources for women delivered as expected? | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Ql | Qt | Ql | ||||||
| 2d, 2e, 3 | Were more women told about PFME in intervention vs. control group? | Qt | |||||||||||||
| 2d, 2e, 3 | What factors might explain/contribute to differences in number of women told about PFME in intervention vs. control group? | Qt | Qt | Qt | Qt and Ql | Ql | Ql | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Ql | Qt | Ql |
| 2e | How many women did MWs want to/were able to refer for specialist advice/management of UI? | Qt | Qt | ||||||||||||
| 2d, 2e | Were women who perform PFME now, and those who practised regularly (≥ a few times a week) during pregnancy and in the past month, told about PFME by their MW? | Qt | Ql | ||||||||||||
| 2d, 2e | How often were women who perform PFME now, and those who practised regularly (≥a few times a week) during pregnancy and in the past month, told about PFME? | Qt | Ql | ||||||||||||
| 2d, 2e | Were women who perform PFME now, and those who practised regularly (≥a few times a week) during pregnancy and in the past month, told how to do PFME? | Qt | Ql | ||||||||||||
| 2d, 2e, 3 | Were women who perform PFME now, and those who practised regularly (≥ a few times a week) during pregnancy and in the past month, given a resource bag to support PFME? | Qt | Ql | ||||||||||||
| Response of individuals | |||||||||||||||
| 4 | How many women return outcomes questionnaire? | Qt | |||||||||||||
| 4 | Do women who return questionnaires complete the PFME practice, adherence and self-efficacy questions? | Qt | |||||||||||||
| 2d, 2e | Did more women practise PFME during pregnancy in intervention vs. control group? | Qt | |||||||||||||
| 2d, 2e | Did more women adhere to PFME during pregnancy in intervention vs. control group? | Qt | |||||||||||||
| 2d, 2e | Did women report more self-efficacy for PFME in intervention group? | Qt | |||||||||||||
| 2d, 2e | What factors might explain differences in women’s practice, adherence, and self-efficacy for PFME (if any) between intervention and control group? | Qt | Qt and Ql | Qt | Qt and Ql | Ql | Ql | Qt | Qt | Qt and Ql | Ql | Ql | Ql | Qt | Ql |
Both analyses were synthesised narratively, highlighting any points for training refinement.
Pre-/post-training confidence questionnaires
The questionnaires were completed by consenting intervention midwives before and after the training sessions. There were eight questions that were a part of the survey, scored using a 5-point Likert scale (0 = not at all confident to 4 = completely confident), giving a range from 0 to 34. Therefore, a higher overall score implies a greater confidence in discussing and teaching PFME in antenatal care. The data collected from this source allowed for individual question quantitative summaries.
A summary score was produced for each question for both the pre- and post-training questionnaire. The question summary scores were summarised with descriptive statistics (e.g. median, IQR, mean, SD). Box charts and bar charts were used to visualise these findings. The pre- and post-training confidence scores were used to calculate any change in confidence before and after training, per question. The appropriate parametric or non-parametric repeated measures inferential statistic was used, with p < 0.05 indicating a significant change in confidence.
Training evaluation questionnaire
An anonymous questionnaire was completed post training by intervention midwives. The questionnaire involved six 10-point Likert scale questions (0 = negative response, through to 10 = very positive response, to the training) as well as free-text responses about the acceptability of the training content and delivery. The data from this questionnaire allowed quantitative analyses for each question, but the six Likert scale questions were not designed to be summed; the free-text data were analysed qualitatively.
Each Likert scale question was summarised descriptively (e.g. median, IQR, mean, SD). The free-text responses were analysed using content analysis51 for each question individually and then administered on the data set as a whole. It was initially an inductive analysis, using a subset of questionnaires to develop categories and a coding framework for use with the remaining questionnaires. Text was analysed for the appearance and frequency of specific content, and the process followed the four steps outlined below.
-
Inductive analysis to identify codes. Coding focused on capturing manifest content (i.e. content which was visible and obvious rather than implicit). This is the most appropriate level of analysis where brief, possibly single-word, responses were recorded. Codes were grouped into categories for each question.
-
The first round of analysis grouped codes into categories for each question to create a coding framework, using a subset of questionnaires.
-
The coding framework was applied to the remaining questionnaires and the frequency of occurrence of coding categories was recorded.
-
A second round of analysis was conducted to look at the data set as a whole (rather than by question). Data were analysed deductively based on the BCW, and inductively to identify any additional themes arising within the data.
Quantitative and qualitative analyses were synthesised narratively, highlighting any points for training refinement.
Interviews were conducted with intervention midwives and champions (post training and end of study), and control midwives (end of study) and women (end of study, post questionnaire completion).
Thirteen interviews, each for 30–60 minutes, were conducted with intervention midwives and champions post training. They aimed to gather information on the midwives’ experience of the training and of implementing the training into their practice, including any limitations they experienced and suggestions to improve the training. The interviews also focused on midwives’ views on the acceptability of the training session and its content. Interview topic guides were informed by the logic model (see Appendix 2, Figure 4), BCW theoretical framework and Sekhon’s acceptability framework. 49
Data were analysed using a hybrid thematic approach, combining deductive and inductive analyses. 52 Deductive (or theoretical) thematic analysis was driven by the logic model for the intervention (see Appendix 2, Figure 4), the BCW theoretical framework that was used to guide intervention development,36 and Sekhon’s acceptability framework,49 which helped inform the topic guides. Inductive thematic analysis enabled identification and analysis of any novel themes that did not fit into the predefined theoretical framework. 11 Telephone interviews were transcribed by an approved transcription service. Online interviews conducted via Zoom were auto transcribed and checked for accuracy.
The phases of thematic analysis were as follows:
-
checking and reading of transcripts and data familiarisation
-
initial coding using deductive and inductive approaches
-
10% of transcripts will be coded by a second analyst
-
identification of themes
-
reviewing of themes
-
theme definition and naming
-
finalising and reporting of analysis.
Analysis was discussed by analysts and research team members to support the interpretation of the data.
Champion monitoring
Data source: champions recorded their activities related to this role using a template form provided in the champions’ manual. Entries included, for example, the number and nature of requests for support from the midwives in their team (number of requests for advice or onward referral) plus any open-text data reflective journal detailing the support they offered to midwives, any observations regarding any possible contamination between intervention and control teams, as well as their general experiences of being a champion.
Quantitative data were summarised descriptively. Free-text and qualitative data were summarised using content analysis (as detailed previously).
Distribution of resource bags for women
Data source: the trial research team recorded the number of resource bags given to teams. These data were summarised descriptively.
Fidelity of intervention implementation questionnaire
An anonymous questionnaire was completed during the implementation period by 59 intervention midwives. The questionnaire involved seven 5-point scale questions about implementing APPEAL training to the women in their care (4 = ‘yes, all women’ through to 0 = ‘no women’); one 4-point scale question about appointments (3 = ‘at all appointments’ through to 0 = ‘never’); one ‘yes/no’ question about referrals made (including frequency of referral if yes); two questions, one on barriers and one on facilitators to implementation, each with nine categorical response options (tick as many that apply) and a twelfth question inviting any other comments (free-text responses). The data from this questionnaire allowed quantitative analyses for each question, but the questions were not designed to be summed; the free-text data were analysed qualitatively using content analysis as detailed previously.
Quantitative and qualitative analyses were synthesised narratively, highlighting any points for training refinement.
Women’s questionnaires
Sections 3–5 of the APPEAL outcomes questionnaire for women were further analysed within the process evaluation in conjunction with, and after, the preliminary statistical analysis had taken place. Summaries of intervention and control arm responses to sections 3–5 (PFME performance, information received from midwife, PFME self-efficacy and adherence) were undertaken as part of the main trial analyses and then incorporated into this process evaluation.
Responses to section 3 supported identification of midwife fidelity to the PFME intervention for women and possible contamination between clusters (see Table 18); section 4 would indicate women’s self-efficacy for PFME; and section 5 would indicate women’s adherence to PFME. Response rates to these sections indicated the feasibility and possible acceptability of collecting PFME outcome data from women.
Responses to questions in section 3 were reported narratively using descriptive summaries (e.g. range with mean and SD or mode and median) where appropriate.
Section 4 contained the 17-item Chen PFMESES. 42 Items are scored using a 5-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, and 5 = strongly agree), with a possible summed score range from 17 to 85 (higher score indicating greater self-efficacy for performing PFME). The scale contains two factors: (1) belief in PFME execution and benefits (items 1–4, 10, 12–17); and (2) belief in performing PFME as scheduled and despite barriers (items 5–9, 11). Missing data were examined and reported, and any imputation undertaken in accordance with the main trial analysis. A total self-efficacy score and subscores for each factor were summarised for the intervention and control arms. (NB as described earlier, the omission by BCTU of four questions rendered this full scale invalid.)
Section 5 contained the six-item EARS. 43 Items are scored using a 5-point Likert scale (from 0 = completely agree to 4 = completely disagree) with a possible summed score range from 0 to 24. Items 1 and 5 are reverse scored. A higher overall adherence score indicated better adherence to exercise. Missing data were examined and reported, and any imputation undertaken prior to statistical analysis in accordance with the main trial analysis.
Associations between variables that may explain differences in PFME practice, adherence and self-efficacy were explored descriptively. For example, for women who performed regular PFME: (1) what proportion were told about PFME by their midwife? (including how often they were told to perform PFME), and (2) what proportion were given a resource bag? Did women who reported being told about PFME by their midwife and/or who were given a resource bag, also report greater adherence and/or self-efficacy for PFME? These data were summarised descriptively.
Reporting
Results for training attendance, training observation, and distribution of resource bags for women were reported as text. Quantitative questionnaire results (pre- and post-training confidence; training evaluation, fidelity of intervention implementation, and women’s outcomes) were presented in tables. Qualitative results (questionnaire free text or interviews) were presented in tables for content analyses and in text boxes for thematic analyses. Results arising from the champion monitoring notes were presented in accordance with the data source arising (e.g. in tables for numerical summaries and for free-text content analyses).
| Section 3 | Intervention | Control |
|---|---|---|
| How often did you perform pelvic floor muscle exercises when you were pregnant? (Tick one option) | Never advised = off protocol Never (other), a few times a month, once a week = off protocol ≥ a few times a week = on protocol |
≥ a few times a week = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| Do you currently perform pelvic floor muscle exercises? (Tick one option) | Never advised = off protocol Never (other), a few times a month, once a week = off protocol ≥ a few times a week = on protocol |
≥ a few times a week = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| How often did you do pelvic floor muscle exercises over the last month? (Tick one option) | Never advised = off protocol Never (other), a few times a month, once a week = off protocol ≥ a few times a week = on protocol |
≥ a few times a week = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| Did your midwife advise you to perform pelvic floor muscle exercises when you were pregnant? (Tick one option) | No = off protocol | Yes = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| Did your midwife explain how to perform pelvic floor muscle exercises when you were pregnant? (Tick one option) | No = off protocol | Yes = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| Did your midwife give you a pack of information on pelvic floor muscle exercises when you were pregnant? (Tick one option) | No = off protocol | Yes = indicative of possible contamination? (but it is in NICE guidelines so they may have given some other leaflet/information) |
| When did your midwife give you the pack of information on pelvic floor muscle exercises? (Tick one option) | Never, at another appointment = off protocol At my first or second appointment = on protocol |
At my first or second appointment = indicative of possible contamination? (but it is in NICE guidelines so they may have given some other leaflet/information) |
| How often did your midwife talk to you about pelvic floor muscle exercises when you were pregnant? (Tick one option) | Never, once only, occasionally = off protocol At every appointment = on protocol |
Yes = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| Did your midwife ever ask you if you had any difficulties with performing pelvic floor muscle exercises? (Tick one option) | No = off protocol | Yes = indicative of possible contamination? (but it is in NICE guidelines so they may have received some other leaflet/information) |
| Before you were pregnant have you ever been taught or learned how to perform pelvic floor muscle exercises? (Tick one option) | Compare intervention vs. control to assess baseline self-reported PFME performance |
Appendix 6 Summary of training evaluation
| Midwives reported PFME training and experience (n = 68) | Mean (SD) | Median (IQR) |
|---|---|---|
| Years as a midwife | 11.3 (9.2) | 8 (5–14) |
| Years as a community midwife | 6.2 (6.4) | 5 (1–8.25) |
| Midwives’ previous PFME training (n = 47)a | Frequency, n | Percentage, % |
| None | 34 | 72.3 |
| Non-specific | 3 | 6.4 |
| Specific – minimal | 7 | 14.9 |
| Specific – substantial | 3 | 6.4 |
| Topic ratings from midwives’ post-training evaluation questionnaire | Numerical response (range 0–10) Mean (SD) |
|---|---|
| Overall, did you find the training useful? (n = 64) | 9.4 (1.2) |
| How did you find the training content? (n = 64) | 9.3 (1.0) |
| How helpful did you find the method of training delivery? (n = 64) | 8.7 (2.0) |
| Will the midwives’ resource pack be useful? (n = 63) | 9.1 (1.4) |
| Overall, would you recommend the training? (n = 65) | 9.4 (1.0) |
| Midwives’ confidence ratings questiona | Pre training | Post training | z scoreb, (p-value) |
|---|---|---|---|
| Median confidence score (IQR) | |||
| 1. Raising the topic | 3 (2–3) | 4 (3–4) | −5.3 (p < 0.001) |
| 2. Raising the topic of UI | 3 (2–3) | 4 (3–4) | −5.3 (p < 0.001) |
| 3. Understanding pelvic floor anatomy | 2 (1–2) | 3 (3–4) | −6.3 (p < 0.001) |
| 4. Assessing a PFM contraction | 2 (1–2) | 3 (3–4) | −6.4 (p < 0.001) |
| 5. Teaching pregnant women to do PFME | 2 (2–3) | 4 (3–4) | −5.9 (p < 0.001) |
| 6. Giving further advice about how to do PFME | 2 (2–2) | 3 (3–4) | −6.2 (p < 0.001) |
| 7. Referring women who cannot do a PFM contraction for further help | 2 (1–2) | 3 (3–4) | −6.1 (p < 0.001) |
| 8. Advising women on how to manage UI | 2 (1–2) | 3 (2–4) | −6.2 (p < 0.001) |
Appendix 7 Champion monitoring data (including interview data)
| April | May | June | July | August | September | October | November | December | |
|---|---|---|---|---|---|---|---|---|---|
| Mean time spent on champion role (minutes) (n = 8 teams) | 48.4 | 63.8 | 64.4 | 61.3 | 52.5 | 63.0 | 75.0 | 40.0 | 32.9 |
| Range of time (minutes) spent on champion role (n = 8 teams) | 27–60 | 30–120 | 15–120 | 0–240 | 0–180 | 15–120 | 10–180 | 0–60 | 0–60 |
| Total APPEAL queries recorded by champions (n = 8 teams) | 7 | 1 | 5 | 1 | 1 | 0 | 0 | 0 | 0 |
| Total referrals to pelvic health physiotherapist reported to champions (n = 8 teams) | 2 | 3 | 4 | 0 | 2 | 5 | 1 | 2 | 1 |
| Activity/nature of query | Frequency |
|---|---|
| Supporting training dates | 1 |
| Answering general team queries/discussing/reminding team re APPEAL in team meetings | 9 |
| Sending reminders to team, for example via WhatsApp | 1 |
| Attending champion meeting | 1 |
| Ordering resources for women, including resource bags and leaflets in different languages | 2 |
| Addressing queries: | |
| What appointment do I raise topic of PFME? | 1 |
| Do I start PFME if someone transfers to me at 34/40 weeks’ gestation | 1 |
| Do I discuss APPEAL at every appointment? | 1 |
| Referral criteria for specialist pelvic health physio, including how and when to refer | 3 |
| Reminder of what to say in APPEAL | 1 |
| How to document PFME/APPEAL | 3 |
| Troubleshooting/peer support, for example fitting APPEAL into busy appointments | 2 |
| Support for MSWs/assistants trained in APPEAL | 1 |
| Summary of findings | Illustrative quotes (participant identification number not included to avoid possibility of identification due to small numbers of champions interviewed) |
|---|---|
| Not a big commitment: Role does not take too much time Approx 1 hour per month |
|
| Less enthusiasm if did not volunteer for the role |
|
| Champion training was useful: Helped understand documentation and referral processes Champions highlighted gaps in APPEAL training re documentation and referral – modified accordingly |
|
| Champion meetings are useful for peer support and shared learning |
|
| Champions see their role to support team with reminders for APPEAL and support for referrals |
|
| Champions may experience challenges motivating teams |
|
| Challenges | Suggested solutions |
|---|---|
| Language barriers |
|
| Time – being able to fit into booking/16-week appointment |
|
| Reluctant team members/hostility of team Feels like nagging the team |
|
| Gaining trust as new member in the team |
|
| Worry about contamination |
|
| Lack of opportunities to ask multiparous women |
|
| Remembering to do the intervention/behaviour change for midwives |
|
| Getting the women on board – women paying lip service to the midwives when asked about PFME |
|
| Lack of face-to-face time with team to keep APPEAL fresh in team members’ minds |
|
| Needing quick answers to PFME issues in clinic |
|
| Getting feedback from team midwives re implementation of APPEAL |
|
Appendix 8 Implementation questionnaire results
| Response options | Questions from the implementation questionnaire | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1. PFME raised first antenatal appointment? (n = 52) | Q2. Women given resource bag? (n = 56) | Q3. Contents of the resource bag discussed? (n = 55) | Q4. How often do you ask women about UI? (n = 55) | Q5. Teach women to do a PFM contraction? (n = 57) | Q6. Women practise PFM contraction at appointment? (n = 56) | Q7. Do you set a PFME programme? (n = 54) | Q8. Do you review progress throughout pregnancy? (n = 53) | |
| No women | 0 (0%) | 2 (4%) | 0 (0%) | 2 (4%) | 0 (0%) | 5 (9%) | 5 (9%) | 3 (6%) |
| Yes, a few women | 2 (4%) | 4 (7%) | 3 (5%) | N/Aa | 4 (7%) | 8 (14%) | 5 (9%) | 9 (17%) |
| Yes, some women | 4 (8%) | 12 (21%) | 3 (5%) | 18 (33%) | 14 (25%) | 18 (32%) | 12 (22%) | 16 (30%) |
| Yes, most | 21 (40%) | 28 (50%) | 22 (38%) | 30 (55%) | 24 (42%) | 21 (38%) | 25 (46%) | 21 (40%) |
| Yes, all | 25 (48%) | 10 (18%) | 27 (51%) | 5 (9%) | 15 (26%) | 4 (7%) | 7 (13%) | 4 (8%) |
| Topics from implementation questionnaire | Numerical response summary | Comments reported in association with numerical responses | Additional free-text comments |
|---|---|---|---|
| Women practise pelvic floor muscle contractions at appointment (Q6: Are you managing to get women to practise a PFM contraction during an antenatal appointment with you?) (n = 56) |
Of those midwives that completed the question, 45% stated that all or most women were practising pelvic floor muscle contractions at their appointments compared to the other response options. | ‘Not time to do in clinic’ ‘Clinic is constantly over-run’ ‘We don’t have enough time to discuss this regularly, or to spend assessing PFME’ ‘Language barrier is sometimes an issue’ ‘Quite time-consuming with already limited time and language barriers’ |
‘Most women are receptive to PFME information and advice’ ‘Most if not all women I have discussed PFME with have been receptive and enthusiastic about PFME and I have found it a very easy topic to discuss’ ‘Concerns regarding restrictions’ ‘A video would be helpful’ ‘Need a prompt on Badgernet so I remember’ |
| Referrals to a physiotherapist (Q9: Have you needed to refer women to the physiotherapy department for managing incontinence? If yes, how many?) (n = 50) |
Of those midwives that completed the question, 36% referred women to physiotherapy. The total number referred was 44. Mean = 2 SD = 1.5 |
N/A | N/A |
| Barriers to implementation (Q10: Are there any barriers to you implementing the APPEAL intervention and if so, what?) |
Top three:
|
‘Generally, I feel like I don’t have time to complete this and all the other checks we have to do’ ‘It is very difficult to teach PF during a 20/30 [minute] appointment still’ ‘It would eventually be very helpful if the APPEAL intervention was available via a tick box on Badgernet. This would save precious time and more likely not to be forgotten. Sometimes on odd occasion would discuss PFM with women but forget to document on Badger’ ‘Short staffing within the team therefore picking up more visits made it difficult to remember’. ‘Language barriers were also a major impact on asking about UI and PFME’ |
‘I do not think the resource pack was effective’ ‘I think more leaflets and different languages as not all people have access to a smartphone to download app. Visual leaflets would be more beneficial. Statistics would also help encourage women’ ‘Need more resource packs’ |
| Helping implementation (Q11: Is there anything that helps you implement the APPEAL intervention?) |
Top three:
|
There were no comments associated with the numerical responses for this question. | ‘I think this will really benefit women and will have a big impact on improving UI’ ‘This intervention has helped increase my knowledge and understanding e.g. women with hyperemesis. I would not have originally referred to physio’ |
Appendix 9 Interviews with intervention midwives during implementation
| Intervention midwives: implementation phase (N = 13)a | |
|---|---|
| Role description (n) | Team lead (2) Community midwife (7) APPEAL champion (4) |
| Years as midwife, mean (range) | 10.62 (2–31) |
| Midwives with > 5 years’ experience as midwife, n | 8 |
| Midwives with ≤ 5 years’ experience as midwife, n | 5 |
| Years as community midwife, mean (range) | 4.69 (0–12) |
| Midwives with > 5 years’ experience in community, n | 5 |
| Midwives with ≤ 5 years’ experience in community, n | 8 |
| Major themes | Subthemes | Supporting quotes |
|---|---|---|
| Theme 1: past training and experiences | Range of past experience and training history | ‘Um, 3 years ago, so yeah, 2018’ (124) ‘20 years this year’ (103) ‘No, … no actual training’ (120) ‘Um, I remember making models … with like Playdoh of the pelvic floor … we all had like a session where we were all doing pelvic floor exercises’. (140) |
| Theme 2: acceptability of online delivery of training session | Benefits of online delivery, positive comparisons with in- person delivery Limitations of online delivery, negative comparisons with in- person delivery |
‘uh, and I think it probably works better for some people, as opposed to getting to venues. Um, it’s a little bit easier’. (124) ‘I found it really good especially how they were able to share their screen. I felt like was able to concentrate a little bit more…’ (112) ‘So, I actually think it worked better virtually. Yeah’. (103) ‘I think anything is better face to face’. (120) ‘I think while on the training was difficult because there was a group of us and, you know, connections, and it dropping out … people saying the same thing about 10 times … it would have been much better to just do it face to face’. (137) ‘I think you do lose some of the non-verbal things that you would have in a face-to-face environment’. (113) ‘I think a lot of people tend to be more quiet online … we don’t like the online speaking and seeing ourselves. I think that makes a difference’. (120) |
| Theme 3: midwives’ engagement | Engagement with content Engagement with delivery style |
‘I thought we probably should have had something like that during our actual midwifery training, rather than kind of years and years on. It would have been really helpful’. (122) ‘I thought it was very much a two-way thing, rather than just being lectured at or talked to…’ (136) |
| Theme 4: how the training influenced PFME knowledge | Knowledge New attitude/skills |
‘ … especially because I’ve got no children myself … you always hear like I can’t cough or I can’t sneeze … but actually looking at the statistics it was mind-blowing how many women are actually affected by it’. (112) ‘I swear when I was a student we was only ever taught like postnatally’. (162) ‘I don’t know, even if they told me they were leaking urine, I wouldn’t have even considered referring them to a physiotherapist. I wouldn’t have known what to … I’d have been like, “do your pelvic floors. It’ll get better, if not speak to your GP”’. (112) |
| Theme 5: midwives’ attitude towards training | Positive attitude towards training Negative attitude towards training |
‘I thought it was really helpful. I think [PFME] is really important. I think … pelvic floor exercises can make a massive difference to women in their whole life, not just in pregnancy’. (122) ‘I think it should be something that we should include in mandatory training as well’. (120) ‘Boring’ (162) (References were also made to the statistics being confusing, and the video scenario not being representative of practice conditions.) |
| Theme 6: suggestions to improve training | Training on anatomy and physiology is important for PFME to support labour | ‘I think all of that is essential really as part of the training, you need to know anatomy and physiology to understand what you’re teaching, and then the practical advice as well … I think it was all essential’. (140) ‘I think that’s what swaying a [lot] of ladies as well, because a lot are interested in labour and how they can improve labour…’ (167) |
| Construct of acceptabilitya | Summary of findings | Illustrative quotes |
|---|---|---|
| Affective attitude ‘I’m enthusiastic about it’ |
|
|
| Burden ‘it just feels a bit impossible’ |
|
|
| Ethicality ‘it’s a very good fit’ |
|
|
| Intervention coherence ‘I’ve never thought about the impact of pregnancy on the pelvic floor’ |
|
|
| Opportunity costs ‘so many other priorities’ |
|
|
| Perceived effectiveness ‘I think it should help to get that message across’ |
|
|
| Self-efficacy ‘I do feel confident’ |
|
|
| Theme | Summary of findings | Illustrative quotes |
|---|---|---|
| Challenges |
|
|
| Concerns |
|
|
| Opportunities |
|
|
Appendix 10 Interviews with intervention and control midwives at the end of the APPEAL study
| Intervention midwives: end of study (N = 6)a | Control midwives: end of study (N = 12) | |
|---|---|---|
| Role description (n) | Team lead (1) Community midwife (3) APPEAL champion (2) |
Team lead (1) Community midwife (11) |
| Years as midwife, mean (range) | 12.33 (5–31) | 12.42 (2–32) |
| Midwives with > 5 years’ experience as midwife, n | 4 | 8 |
| Midwives with ≤ 5 years’ experience as midwife, n | 2 | 4 |
| Years as community midwife, mean (range) | 5.5 (1–16) | 7.42 (0–22) |
| Midwives with > 5 years’ experience in community, n | 3 | 5 |
| Midwives with ≤ 5 years’ experience in community, n | 3 | 7 |
| Theme | Intervention midwives | Control midwives | ||
|---|---|---|---|---|
| Summary of findings | Illustrative quotes | Summary of findings | Illustrative quotes | |
| Background/experience of PFME/UI |
|
|
|
|
| Consistency of teaching PFME/asking about UI |
|
|
|
|
| Acceptability of APPEAL/teaching PFME in AN care |
|
|
|
|
| Feasibility of research/evidence of contamination |
|
|
|
|
| Making it happen – ideas for supporting implementation adoption and spread |
|
|
|
|
Appendix 11 Findings from postnatal interviews with women in the APPEAL feasibility and pilot trial
| APPEAL group | NHS Trust 1 | NHS Trust 2 | Age (years), mean (range) | Parity, mean (range) | Total interviewed |
|---|---|---|---|---|---|
| Control | 8 | 8 | 31.3 (23–27) | 1.7 (1–3) | 16 |
| Intervention | 8 | 5 | 33.3 (23–40) | 1.7 (1–3) | 13 |
| Total | 16 | 13 | 32.2 (23–40) | 1.7 (1–3) | 29 |
| Theme | Intervention group quotes: receiving APPEAL-based PFME antenatal care | Control group quotes: receiving standard PFME antenatal care |
|---|---|---|
| Degrees of fidelity (delivery and receipt of PFME interventions) | ‘She gave me a bit of information about kind of doing the exercises, didn’t go into too much around kind of the anatomy and the purpose, because I think it was assumed I already knew that, and then gave me a little bag and some leaflets in that about kind of different things you could get to help train with the pelvic floor and told me the kind of repetitions that I needed to do, so when I was cleaning my teeth in the morning, those kind of things. That was about it, that I got’. (355) ‘I got a leaflet about pelvic floor exercises and they talked me through it, and that was, I think, the first midwife face-to-face visit, and then the second appointment, I think subsequent appointments they just asked, have you got any problems, are you managing to do your exercises, that kind of thing. So they sort of just checked in with whether I was managing the exercises and if I had any problems …’ (026) ‘When I was about 28 weeks I got given like a pack of, I don’t really remember exactly what was in there, but there was also like a QR code for an app to download. Yeah it was like near the end of the pregnancy … I liked the resources that I was given, but by the midwife I wasn’t told much about it. Like she didn’t tell me what it was for, she just gave it to me and mentioned how she got in trouble for not giving it to someone else before … didn’t really know what the pack was for until I got home and, yeah and then I read it but, but I feel like if she would’ve told me and talked to me about it as well then I would have been more able to, be more excited about it, if that makes sense, like read it and understand it more’. (117) ‘Oh gosh, I think she mentioned it once, you know, she mentioned just to do the pelvic floor exercises and that’s it really to be honest’. (030) ‘I remember that she did speak to me about the pelvic floor exercises, [in a previous pregnancy] whereas this time it was just sheets given out’. (Interviewer: ‘We have, the study is a sort of quite a big study it’s called APPEAL and it has got the, it’s got a little logo on it with a water droplet, and that’s on the information sheet and I think there’s some stickers and some an app card and things, can you remember any of that?’) ‘I don’t recall but I wouldn’t be 100% certain, I just, I don’t remember being given any of that’. (121) |
‘ … after I had the baby and then when I had my midwife and health visitor come and see me at home, then I was, I was told then. They just said, they said, you know, make sure that you start doing your pelvic floor exercises and that you’re regularly doing them and even if it’s, you know, just for a couple of minutes a day’ (19) ‘No, no, all I was told is after having my daughter that, the midwife goes, she goes, “oh, when you just sit down, it’s like you just squeeze your bottom together as you’re sitting down”. And that’s basically like supposed to put your pelvic muscles back together’. (011) ‘ … my midwife asked me if I was doing pelvic floor exercises. I can’t remember the exact nature of the conversation but I think because I said yes and expressed sort of knowledge about it we didn’t go any further, but she was a friend, very friendly woman and I think if I had said what on earth’s that then she would have given me more information, but I think because I just said, yes I know what to do, it’s fine, we didn’t go any further but I think the opportunity was there’. (098) ‘I would say it was very rushed. It was kind of “oh make sure you do your pelvic floor exercises”, “yeah”, and there wasn’t much more information than that in terms of what the midwife said or what was expected of me in pregnancy’. (128) ‘I mean probably briefly in my midwife appointment, I think, I definitely remember the midwife encouraging me to do them at least in one of my appointments, maybe more of them, and I did kind of ask for clarification around it … did I get a piece of paper, I don’t know, yeah, definitely verbally, I might have got an information sheet although I don’t think I did’ (319) ‘I wasn’t given any information about pelvic floor exercises because I felt that I knew it already. So I believe there would have been, if I’d have asked I would have been given bountiful information but I didn’t ask, so. And it was mentioned, you know, my midwife did say “oh, you know, make sure you’re doing your pelvic floors” and there might have been a point where she said, “do you know how to do those?” and I would have said yes and I think that’s why no more further information was given’. (248) |
| Themes | Intervention group quotes: receiving APPEAL-based PFME antenatal care | Control group quotes: receiving standard PFME antenatal care |
|---|---|---|
| Making PFME a priority would help (The impact of midwives' lack of prioritisation acting as a barrier) |
‘I think if the midwife would have mentioned it probably every appointment, like have you been doing your, your pelvic floor, I think, if she would have mentioned it, then I probably would have done it more regularly’. (30) | ‘I started off enthusiastically and then I just kind of gave up because I just, you know, it hadn’t been stressed as being important and I had many other things on my mind’. (128) |
| PFME knowledge and awareness matters (Lack of knowledge hinders or negatively impacts on doing PFME) |
‘ … and then as soon as I knew more about, about it, like, I would do it more often as well, because before I knew information about it, I would just do it like once a week or once every couple of months, to just, whenever I would remember or read about it or see about it, or whatever. But yeah, and now I do it more often now that I know more information about it’. (117) | ‘I’d say I didn’t find them easy because I wasn’t overly sure about what I’m supposed to be doing. So it kind of left me feeling a bit silly I guess because I was just a bit like “well I don’t really know what I’m doing right now”, so it became a thing that in the end I just didn’t bother to do because of that reason’. (128) But knowledge is not always enough: ‘I think hearing the midwife say to me that as I get older, my pelvic floor muscles won’t be as strong, therefore the risk of me leaking as I’m older gets higher, so you should do them. That was enough for me to go, “OK, yeah, I should really do them”. But for me, that’s the reason why I want to do them because when I get older, I don’t want to leak … I don’t ever want to be incontinent so I know I should do it but I don’t’. (017) |
| Knowing how to do PFME (mastery) (Lack of mastery reduces motivation to do the exercises) |
‘I think, knowing about how important it is to relax those muscles and understanding how to relax them, would have been useful, but I don’t know how easy that is to do through a leaflet, because it wasn’t really until someone examined me and saying, now those muscles are relaxing, that I understood how to do it. I think, you know, sometimes you’re holding on and you don’t realise’. (026) | ‘ … if there were like diagrams, maybe, or, you know, just if there was, like, some kind of like leaflet with diagrams on there or information telling you exactly how to perform them and, you know, how to get the best, you know, the most effectiveness out of them as well, even like if they, you know, gave us some links to videos that we could watch, demonstrating it, you know, even, yeah, leaflets, even…’ (019) |
| Knowing how and why matters (The need to understand ‘why’ reinforces the doing) |
‘Probably how to do the pelvic floors and why they were important … like it’s a pretty standard thing to do. But you see it’s only easy if you know how to do it. I think people, women would probably benefit to learn how to do it in the first place, which then would enable them to just sort of bring it into their daily routine I guess’. (032) ‘I think if you speak to any pregnant woman they know that you need to do pelvic floor, but I don’t think they understand the reasons why you have to do it during pregnancy and why it’s important. I think it becomes more apparent once you’ve given birth what kind of the main function and things is of it…’ (355) ‘I don’t think you want to scare women but I think it might be valuable to sort of warn the, sort of warn what the risks are if you don’t do enough exercise … I’m not sure, and, you know, and then the midwives who delivered my baby were, you know, were very, they were sure to tell me how important it would be to do my pelvic floor after that. But I can’t, I perhaps hadn’t realised prior to having my second baby that, you know, that was something that might happen…’ (544) |
‘Honestly, I would have loved to receive more information like how to do it. Because it’s good, at least when they explain it to you it’s good, rather than having to go on YouTube and look at it yourself. So I prefer like the, just like physical exercise they can show it to you or if they can tell you there’s like antenatal classes you can go do and they can show you what exercises you can do, that would really help’. (50) |
| Importance of resources to support women to do PFME (Examples: leaflets, apps or other physical resources) |
‘Yes, definitely, I think my midwife said from the get-go that I should be doing my pelvic floor exercises, sent me a video to watch and, yeah, sent me links that were really useful, so I think that was quite clear’. (314) ‘ … then gave me a little bag and some leaflets in that about kind of different things you could get to help train with the pelvic floor and told me the kind of repetitions that I needed to do, so when I was cleaning my teeth in the morning, those kind of things. That was about it, that I got’. (355) ‘I got given like a pack of, I don’t really remember exactly what was in there, but there was also like a QR code for an app to download. Yeah it was like near the end of the pregnancy … I liked the resources that I was given…’ (117) |
‘I think that the leaflet that I received the first time was very, very helpful so I think everyone should have that, like, no matter whether it’s their first or ninth pregnancy, I think that should always be provided’. (347) ‘I should have just had stickers in places where I sit down, reminding me to do them. And I had every intention of doing that, I just never got round to it’. (017) ‘I think maybe if, if we were given like some kind of, like a checklist or something that, you know, this many times a day, try to complete them, that would have been good’. (019) ‘ … if there was like some kind of like leaflet with diagrams on there or information telling you exactly how to perform them and, you know, how to get the best, you know, the most effectiveness out of them as well’. (019) |
| Importance of additional support from services and clinicians (Examples: classes, physical checks) |
‘ … like antenatal appointments and, you know, where you all get together and you talk about pain relief, things like that, it would be, it would’ve been quite useful to have, you know, maybe a session on pelvic floors and, because then I think we’d see it as more important, because, you know, just being given a leaflet in passing just doesn’t show how severe it is and it was only when people tell you “oh, you need to do it because it will affect you later in life”, so I think maybe like a bit of a little group session talking about it, you know, opportunities to, if it was, you know, … sessions to talk about things and actually just time with somebody to ask questions because I feel sometimes the midwives, particularly when it’s antenatal appointments, they’re so rushed and by the time you see them they’re already late with their appointments so you feel like you don’t want to keep up any more of their time, so I think dedicated sessions would be really good and would show how important they are to do’. (006) ‘ … so the information was fine. I felt like it would have been, yeah, it might have been nice to sit with your midwives, perhaps, and just gone through a few exercises together’. (413) ‘I would say to be perhaps given some material and then a conversation had, so it’s in your mind so that then you, you don’t then just put it, the leaflet, you know, down and never look at it again, that it’s, the conversation would, the discussion would, with your midwife would then prompt you to actually read the leaflet and, you know, keep it at the forefront of your mind because I think you do tend to get quite a lot of material around pregnancy and birth, so I think it’s easy to sort of just sort of stash it all away in a folder and not really pay that much attention to it, but if you have a conversation with your midwife I think that, about it, that would encourage you to pay more attention to it’. (544) |
‘ … even if there were like online Zoom classes, because I remember I joined them for breastfeeding support and like baby massage classes, I joined them on Zoom. So, even if there were classes like that, that would have been really useful’. (019) ‘But in some ways it’s kind of physical demonstration, of kind of sitting with women and talking them through it and making them aware of the feeling of it being correct etc., because I think something like a leaflet for that is very difficult and hands-off and doesn’t necessarily do the job’. (128) ‘But then I think without someone examining so that you know that you’re doing them correctly it’s difficult to reassure yourself that you’re doing them right. But then you know, you can’t really examine women during pregnancy, you know, because of the risk of infection and things so it’s difficult’. (52) ‘I went to this private physio which obviously is not available to everybody … a quick procedure for her to check internally the strength of your pelvic floor muscles and also any gaps in your abdominal muscles, … and it’s really quick to check and I feel like it would be really helpful it that was offered by the GP at the six-week check-up, you know to start women on that path to recovery again, really early on, would be really helpful’. (098) |
| Women’s request for ongoing support to maintain PFME (Support and reminders to continue and go beyond pregnancy) |
‘I think keep reminding and come back to us when we’ve got the breastfeeding sorted and we’re not as, you know, completely absorbed in our babies’. (026) | ‘I feel that you need until you get into a good habit, so anything that can be continually exposing women to the idea of it and the benefits I suppose, and I guess also the risks, not to scare people, but the risks of not doing it, or of having weak pelvic floor muscles, you know, what that means after pregnancy, incontinence and whatever, I think, yeah, just constant exposure to the whole thing, probably in different ways because I think if you constantly see the same something, that it’s the same, you just become sort of immune to it don’t you’. (098) |
Appendix 12 APPEAL refinements and recommendations for future development
| Changes to APPEAL following training | Factors that facilitated implementation in clinical practice during APPEAL |
|---|---|
|
|
| Midwife recommendations for ongoing improvement |
|---|
|
| Recommendations | Suggestions for improvements to supporting PFME by control arm women | Suggestions for improvements to supporting PFME by APPEAL intervention arm women |
|---|---|---|
| Presentation of resources related to PFME | Written formata Video for presentation formata |
Eye-catching resources Resources should be provided online (e.g. videos) Fluidity in information provision |
| Types of physical resources required by women | Useful apps (i.e. ones that work), appropriate reminders to develop habitsa Checklist to complete daily |
Classes Online information |
| Input from health professionals to support PFME | Taught by midwifea Constant reminders during pregnancy to highlight importance, making sure they were ‘keeping on top of them’a Midwife ‘sitting down with you’ to make sure you can feel it working; physical demonstration to accompany any leaflets etc.a |
Midwife showing resourcesa Option for physical examinationa Follow-up post partum Signposting to range of resources suitable for the individual womana Early mention antenatally (then repeated)a Health professional asking questions to the woman about how they are getting ona Referral options, switching between health professionalsa Detailed conversation by midwives in antenatal perioda |
| Guidance and support regarding performing (how to do) PFME | Preparation for labour, description of range of benefitsa When to do them (i.e. not on the toilet)a Description of why and how they are helpfula |
Learning how to relax musclesa Relaxation Information on benefits, importance, not just UI, prolapse, sexual function, post-partum healinga |
List of abbreviations
- APPEAL
- Antenatal Preventative Pelvic floor Exercises and Localisation programme
- BCT
- behaviour change technique
- BCTU
- Birmingham Clinical Trials Unit
- BCW
- behaviour change wheel
- DAM
- decision-analytic model
- EARS
- Exercise Adherence Rating Scale
- FI
- faecal incontinence
- GDPR
- General Data Protection Regulation
- GP
- general practitioner
- HCP
- healthcare professional
- ICC
- intraclass correlation coefficient
- ICIQ-UI SF
- International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form
- IRAS
- Integrated Research Application System
- LMNS
- local maternity and neonatal system
- MSW
- maternity support worker
- NHSE
- National Health Service England
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PFM
- pelvic floor muscle
- PFME
- pelvic floor muscle exercises
- PFMESES
- Pelvic Floor Muscle Exercises Self-Efficacy Scale
- PPIE
- patient and public involvement and engagement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RFIS
- Revised Faecal Incontinence Scale
- SF-12
- Short Form questionnaire-12 items
- UI
- urinary incontinence
- URL
- uniform resource locator
- VTC
- variation to contract
- WP
- work package
Notes
-
World package 3 mapping of behaviour change wheel, theoretical domains framework and behaviour change techniques to inform intervention development
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/TJDH7946).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
