Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0707-10059. The contractual start date was in October 2008. The final report began editorial review in September 2013 and was accepted for publication in July 2014. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Elaine McColl is a member of the National Institute for Health Research (NIHR) Programme Grants for Applied Research subpanel and also a member of the NIHR Journals Editorial Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Thomas et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Urinary incontinence (UI) following stroke is common, with prevalence estimates suggesting around half of stroke survivors are affected in the acute phase. Findings are similar across countries (e.g. UK 48%,1 Denmark 47%,2 Germany 53%3). As many as 43.5% and 38% stroke survivors remain incontinent at 3 months and 1 year respectively. 4 In longer-term stroke survivors (on average 9 years post stroke), prevalence has been reported as 17%. 5
Problems with continence have been shown to be amenable to early intervention, particularly in the 3 months following stroke. 6 Stroke outcome may be better in those stroke survivors who remain continent or regain continence. 7 Although there are problems with attributing better stroke outcome to improvements in continence, it is possible early intervention aimed at promoting recovery from incontinence may improve morale and self-esteem and therefore speed overall stroke recovery. 7,8 It is also possible that the recovery of continence reduces barriers to participation in rehabilitation activity.
Despite the availability of clinical guidelines for the management of UI in women9 and after stroke,10 national audit data11 suggest incontinence is often poorly managed. In the latest Sentinel audit,11 63% of patients had a plan for continence management, an increase of only 5% since 2004. Improvements in continence have not kept pace with those in other aspects of stroke care, for example establishing a safe swallow, where the proportion assessed has increased from 63% to 83% over the same period. Although continence is already recognised as a component of organised stroke care, it is known that nurses find managing continence in the context of stroke challenging,12 with over-reliance on urinary catheterisation as a management strategy especially in the acute phase of illness. 13 There are medical therapies which can be appropriately used to assist continence but these need to be based on appropriate first-line assessment and behavioural management in line with national guidelines. 10
The more severe the stroke, the greater the likelihood of UI;14,15 other factors linked to UI include older age or cognitive impairment. 16 Problems experienced include urinary retention or complete incontinence. The most likely pattern of incontinence is urinary frequency, urgency (a sudden compelling desire to pass urine which is difficult to defer) and urge incontinence (involuntary leakage immediately following, or concurrent with, an urgent sensation of needing to void). 6 Urge incontinence is the most common type after stroke,17 but the cerebral lesion may also lead to practical difficulties with bladder control caused by, for example, motor impairment, depression and aphasia18 (termed functional incontinence).
The symptoms of UI are reported to be more severe and have more of an effect on the lives of stroke survivors, when compared with other groups of people. 19 Incontinence is not just a physical problem, but impacts on what people can do, for example participate in rehabilitation activities, and how they feel. Depression is twice as common in stroke survivors who are incontinent20 and there may be a link between depression associated with urinary symptoms and suicide. 21 Continuing incontinence is associated with poor outcome in both stroke survivor and carer. 2 Furthermore, the negative social consequences of dealing with incontinence for both survivor and carer cannot be ignored, as both may become isolated and marginalised. 22 If post-stroke incontinence is targeted early, not only is there the potential to reduce the poor outcome of stroke associated with incontinence, but also the negative social consequences associated with it post-hospital discharge.
Evidence to guide the management of UI after stroke is poor; our systematic review23 found no rigorously conducted studies evaluating interventions in secondary care. No published trials of behavioural interventions for UI after stroke were found other than a single trial of pelvic floor muscle training (PFMT). 24
Available conservative interventions for UI include bladder training (BT),25 timed voiding (TV),26 prompted voiding (PV),27 habit retraining (HT)28 and PFMT. 29 BT is generally used for urge incontinence and aims to increase the time interval between voids so continence is regained. It involves patient education, scheduled voiding and positive reinforcement, but can also include self-monitoring and urge suppression techniques. PV and TV have mainly been used with people who have cognitive deficits. They are based on a system of scheduled voids, with PV including reminders and reinforcement for self-initiation of toileting. To date, trials of PV have mainly taken place in US nursing homes; however, there was no a priori reason why this approach should not be introduced into the care of stroke patients in secondary care in the UK.
The effectiveness of conservative interventions has been systematically reviewed in adults. The review of TV26 included only two trials of poor methodological quality and concluded there was no empirical evidence for or against the intervention. Similarly, the review of HT28 found insufficient evidence of an effect on continence outcomes to recommend this approach. In the review of BT,25 trials tended to favour BT and there was no evidence of adverse effects. The review of PV27 found evidence of increased self-initiated voiding and decreased incontinent episodes in the short term.
Pelvic floor muscle training may also be effective in assisting the individual to manage urge, stress or mixed incontinence29 and has been shown to be effective as a combined intervention with BT. 30,31
As urge strategies have been shown to be effective in stress incontinence32 and stress strategies in urge incontinence,33 a number of trials have tested combined behavioural interventions (CBIs) for both stress and urge incontinence, on the premise that combining techniques may be more effective than single techniques. Existing reviews have considered mixed types of interventions (e.g. physical + behavioural) for UI. 34–36 There are also two reviews that have included pooled results for CBIs,37,38 but these reviews are specific to women and include studies relating to the prevention of incontinence, i.e. including continent people. There is no current review of CBIs for UI.
Despite a growing evidence base, existing evidence for continence management has not been widely implemented in clinical practice, even by stroke specialist teams working on recognised stroke units. 12 This lack of implementation in stroke clinical practice is in keeping with a recent and growing recognition that the implementation of research in practice is influenced not only by individual clinicians, but also by the organisational context in which they operate. 39–44 Organisational context has been defined as ‘the environment or setting in which the proposed change is to be implemented’. 45 At its simplest level, context may refer to the physical environment where health care takes place. However, Rycroft-Malone et al. 45 concluded from their concept analysis that contexts conducive to research implementation included a range of less tangible process elements: ‘clearly defined boundaries; clarity about decision-making processes; clarity about patterns of power and authority; resources; information and feedback systems; active management of competing “force fields”. . . and systems in place that enable dynamic processes of change and continuous development’. 45
Theories underpinning organisational influence include those of learning organisations (with characteristics encompassing hierarchical structure, information systems, human resource practices, organisational culture and leadership46) and knowledge management (how organisational mechanisms affect knowledge uptake and use47–49). Successful implementation of an intervention to improve the management of post-stroke UI is likely to be mediated not only by individual members of staff and availability of evidence-based guidance, but also by the complexity of the intervention as well as the interplay of patient, social and organisational factors. 49,50 Careful attention needs to be paid to the specific barriers to change in any given setting, identified through ‘diagnostic analysis’ at levels that may include the individual, groups or teams, organisations and the wider health-care system. 51 Strategies then need to be ‘tailored’ to overcome barriers identified. 52
The intervention in our programme focused on conservative strategies shown to have some effect with participants in studies included in Cochrane systematic reviews,25,27,29,53,54 but which had not had their effectiveness demonstrated with stroke patients. These strategies included a combined package of BT and (where possible) PFMT and PV.
We also evaluated whether or not supported implementation, through targeted organisational development aimed at ‘normalising’ the intervention,55–58 showed more preliminary evidence of effectiveness than introduction of the intervention alone, as well as evaluating both in comparison to usual care.
Programme aims
The programme aimed to develop, implement and evaluate the preliminary clinical effectiveness and cost-effectiveness of a systematic voiding programme (SVP), with or without supported implementation, for the management of UI after stroke in secondary care. The programme was structured in line with the Medical Research Council (MRC) framework for the evaluation of complex interventions59,60 and comprised two phases:
Phase I (MRC development phase):
-
evidence synthesis of quantitative and qualitative literature on combined approaches to manage UI post stroke
-
case study of the introduction of the SVP in one stroke service.
Phase II (MRC feasibility and piloting phase):
-
cluster randomised controlled exploratory trial, incorporating a process and health economic evaluation.
Structure of the monograph
Chapter 2 summarises the aims, methods and findings of the evidence synthesis. Development of the interventions (SVP and supported implementation) is described in Chapter 3. The case study of the introduction of the SVP in one stroke service is reported in Chapter 4. Phase II comprised the exploratory cluster randomised controlled trial (RCT) and is reported in Chapters 5 and 7 (methods and findings, respectively); evaluation of process in Chapters 6 and 8 (methods and findings, respectively) and health economic evaluation in Chapter 9. Finally, we report the methods and evaluation of patient, public and carer (PPC) involvement (see Chapter 10). Chapter 11 discusses implications of the programme for the Phase III trial.
Chapter 2 Combined behavioural interventions for urinary incontinence: systematic review of effectiveness, acceptability, feasibility and predictors of treatment outcome
Introduction
Overview
Our systematic review of interventions to promote urinary continence after stroke showed a lack of evidence to inform practice. 23 Current guidelines recommend behavioural strategies targeted to the type of incontinence as a first-line therapy in UI for both men and women, and also suggest that combining behavioural interventions may be useful. 9 This chapter presents the evidence for combined interventions from three linked reviews: a descriptive review of intervention content, an effectiveness review including meta-analysis of randomised and quasi-RCTs specific to voiding function and a narrative review of barriers and enablers to successful behavioural interventions.
Description of the intervention
Behavioural interventions aim to improve bladder control by altering the behaviour of the recipient. This may include changing attitudes, knowledge or skills in order to encourage or enable the implementation of alternative strategies to manage voiding activity (e.g. using distraction, muscle clamping). Behavioural components specifically targeting voiding activity can include PFMT, bladder inhibition training, PV, urge suppression techniques (urge strategies), urethral occlusion techniques (stress strategies), urethral emptying techniques, or lifestyle management such as altering dietary or fluid intake. Additional behavioural components may be directed at enhancing adherence to therapy by increasing sensory or cognitive awareness [e.g. biofeedback (BIO), or by motivational techniques such as coaching]. A meta-study of systematic reviews of behavioural interventions has called for clarity in the theory underpinning the use of behavioural interventions for UI. 53,54
Systematic reviews of the effectiveness of single behavioural interventions already exist for BT;25 TV;26 PV;27 HT28 and PFMT. 29 As urge strategies have been shown to be effective in stress urinary incontinence (SUI)32 and stress strategies in urge urinary incontinence (UUI),33 a number of trials have tested CBIs for both SUI and UUI, on the premise that combining techniques may be more effective than single techniques. Existing reviews have considered mixed types of interventions (e.g. physical + behavioural) for UI. 34–36 There are also two reviews that have included pooled results for CBIs,37,38 but these reviews are specific to women and include studies relating to the prevention of incontinence, i.e. including continent people. There is therefore no current review of CBIs for UI.
Our review found no published trials of behavioural interventions for UI after stroke other than a single trial of PFMT,24 so a systematic review limited to stroke is not an option. In addition, the conditions and contexts for successful implementation of behavioural interventions for UI have not been reviewed. This review therefore aimed to evaluate the effectiveness of CBIs in the general population together with the evidence for factors influencing adherence and outcome, to inform the design of an intervention specific to post-stroke UI.
An effective intervention could be more easily replicated if it were explicitly described. The focus of this review is a complex intervention, combining multiple behavioural intervention components targeting UI with additional cognitive and/or behavioural components to improve uptake or adherence. Maximising the potential for the success of the intervention will depend on clear specification of, and fidelity to, distinctive techniques. A secondary purpose of this review is therefore to construct a standard intervention, by clear description and categorisation of intervention content from existing research.
To maximise the potential for success, staff implementing the intervention will need to tailor it to the characteristics of their client group and setting. The third purpose of the review is to identify moderators of successful outcome of behavioural interventions for UI.
Objectives
To identify best practice in the delivery of an optimal behavioural intervention for UI, the objectives of the review were to:
-
Determine whether or not combined/complex behavioural interventions improve urinary continence in adults, compared with usual care or another/single intervention.
-
A secondary objective was to determine the effect of CBIs on:
-
– subjective or objective improvement in severity or symptoms
-
– quality of life (QoL)
-
– treatment satisfaction
-
– adverse effects; and
-
– socioeconomic outcomes (e.g. cost).
-
-
-
Describe and define the potential components/mechanisms of action of the intervention.
-
Identify the barriers to and enablers of the successful implementation of a behavioural intervention for UI in adults.
Scope of the review
First, a descriptive review delineates intervention content using a standardised model. The effectiveness review includes a meta-analysis of randomised and quasi-RCTs of CBIs that are specific to urinary voiding function. The narrative review considers three separate types of information relating to barriers and enablers of successful behavioural interventions for UI:
-
studies reporting client or staff views of barriers and enablers
-
data relating to rates of uptake and adherence; and
-
studies identifying independent predictors of adherence or outcome.
Structure of the review
The following section outlines the review methods. The results of the review are presented in four sections:
-
Description of the included studies and the content of the behavioural interventions
-
Findings: studies of effectiveness
-
Findings: narrative review of acceptability and feasibility, comprising three subsections:
-
Client views
-
Staff views
-
Studies of feasibility.
-
-
Findings: predictors of adherence and treatment outcome.
The report’s conclusions will draw together the findings from the different sources of information and evaluate the implications for the design of an intervention for post-stroke UI.
Review methods
Search strategy for the identification of studies
A composite search was used to underpin all of the review components, drawing on the search developed by the Cochrane Incontinence Review Group for terms related to UI. Specific terms related to behavioural interventions or terms for relevant research aims/designs (e.g. behaviour therapy, predictor, behavioural research, etc.) were collated from the Cochrane Effective Professional and Organisational Care Review Group search strategy, and from previous Cochrane reviews on behaviour change. The searches above were combined, and then limited for exclusions related to age (child), condition (pregnancy, prostatectomy) and language (non-English). The search was designed for MEDLINE (see Appendix 1) and then adapted for other databases.
The following sources were searched:
-
Databases of published material, including Cochrane Central Register of Controlled Trials (latest issue), MEDLINE (1966 to October 2008), EMBASE (1980 to October 2008), Cumulative Index to Nursing and Allied Health Literature (1982 to October 2008), PsycINFO (1966 to October 2008), Allied and Complementary Medicine Database (1985 to October 2008).
-
Databases of unpublished trials and theses, including metaRegister of Controlled Trials, CRISP, CentreWatch, National Institutes for Health Research (including back searches on National Research Register/Research Findings Register), Index to Theses and Dissertation Abstracts International.
-
Conference proceedings of the International Continence Society (ICS) (2006–8).
-
Forward and lateral citation searching, via ISI Web of Knowledge for all included studies, and on references for included studies from existing systematic reviews of behavioural interventions for UI (traced via Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, and International Health Technology Assessment).
Searches on smaller databases used the free-text terms ‘incontinence’ or ‘urinary incontinence’ depending on suitability.
After removal of duplicate records and records obviously not relevant to the review by one reviewer (BF), two reviewers (BF, LT) independently screened the remaining records on title and abstract (see Appendix 2 for screening criteria). Full-text papers were obtained for screened records identified by either reviewer. Two reviewers (BF, LT) also independently filtered all full-text papers for inclusion, using the filtration pro-forma (see Appendix 3).
Data extraction templates for different types of study were designed with suitable outcome formats and criteria for critical appraisal (see Appendix 4), together with coding frames and guidance (see Appendix 5). After training and inter-rater reliability checks for coding and quality assessment, critical appraisal and data extraction were undertaken independently by two reviewers (BF, LT).
Inter-rater reliability for such complex data extraction was difficult to maintain at a consistently high level. In particular, despite a detailed coding frame, difficulties were experienced with reliabilities in the classification of the behavioural strategies used in interventions and the predictor variables tested in multivariate analyses, mainly because of inadequate detail in the original studies. Therefore all differences in data extraction and classification between the two reviewers were discussed and agreed throughout the process of data extraction, with one of two additional reviewers (ML/CS) checking outcome data extraction and predictor classification.
We contacted triallists to obtain data collected but not reported, or where data were reported in a form that could not be used. Only further details of study design were obtained, with no additional outcome data gained via this route.
Narrative review search additions
The search undertaken for the full review was also used to identify studies for the narrative review. The identification of studies relating to predictors was not found to be reliable during filtering because it did not include studies testing predictors of adherence for single behavioural interventions, or trials including subgroup analysis. Therefore, all trials included in existing systematic reviews of single behavioural interventions identified by the original search were checked. The review of predictor variables by Goode61 was also used to trace further studies, with forward and lateral citation searching for all included studies.
Criteria for considering studies for inclusion
Criteria for included study types (i.e. trials, observational/qualitative studies) were different for each component of the review, but other aspects could also be slightly different. For example, the effectiveness review was limited to a tight definition of CBIs to ensure homogeneity of included studies, whereas the narrative review of barriers and enablers included any study collating people’s views of any behavioural intervention for UI. The definitions used for the effectiveness review will be given first, followed by any differences in inclusion criteria for other review components.
Review of effectiveness
Participants
Adults aged ≥ 18 years, diagnosed either by symptom classification or urodynamic study as having any type of UI, excluding people with short-term incontinence for physiological reasons (e.g. within 1 year of urological surgery or childbirth). UI was defined in its widest sense to include people with signs, symptoms or urodynamic evaluation of overactive bladder or urine leakage, as defined by the study authors. People with or without cognitive impairment were included, on condition that the person had an active role in the behavioural intervention (e.g. behaviour modification).
Interventions
Interventions with more than one behavioural technique directly targeted at improving the management of different types of incontinence were included (e.g. PFMT + BT, PFMT + urge strategies, BT + stress strategies).
Pelvic floor muscle training was included as a behavioural intervention, because it could be argued that it targets behaviour change to develop muscle training as an established habit. Although the mechanism of action of PFMT on UI is possibly physical, this is unlikely to be effective without sustained practice over a period of time. Encouraging and sustaining behavioural practice is therefore a focus of the intervention in many PFMT trials, as much as ensuring correct physical technique.
Prompted voiding was included because the behavioural component is primarily targeted to influencing the behaviour of the person with UI.
Trials using BIO could be included if BIO was used as an intermittent assessment or aid to teaching the correct use of pelvic muscles.
Trials using BIO as a continuous component of the intervention were excluded, as this could be categorised as a physiological treatment rather than a behavioural intervention. Trials using physical mechanisms to augment or enhance muscle training, such as the use of vaginal cones or electrical stimulation, were excluded for the same reason.
Habit retraining or TV were excluded as behavioural techniques because the behavioural component targets the behaviour of staff or carers as much as the person with UI.
Interventions where an additional behavioural mechanism was targeted towards improving adherence to a single behavioural intervention for UI (e.g. reminders for PFMT) were excluded, as their primary outcome for comparison was adherence, with secondary impact on incontinence. Interventions composed of mixed behavioural interventions (e.g. BT + exercise, PFMT + lifestyle adaptations) were also excluded, because they include components not designed to target different types of incontinence.
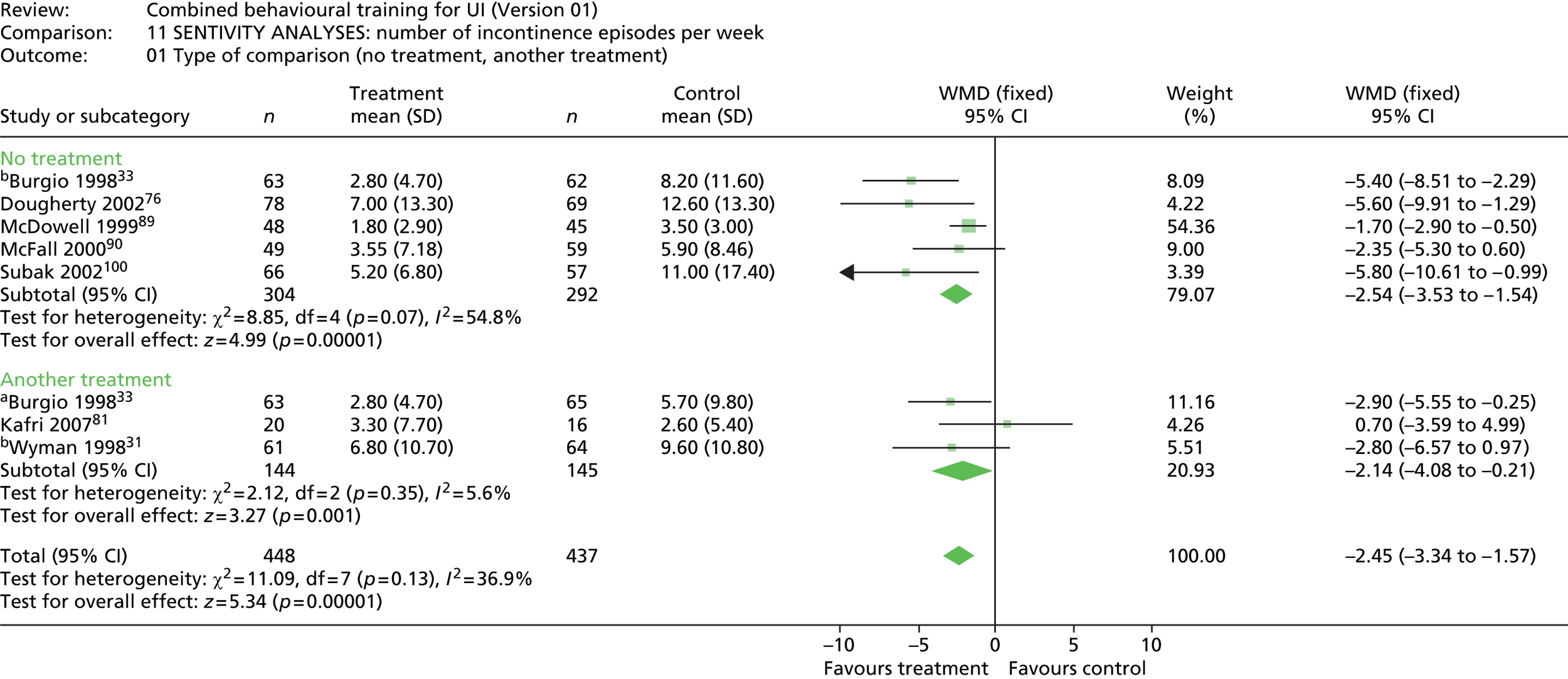
The specific comparisons to be made in the effectiveness review included:
-
multicomponent behavioural intervention compared with no treatment, attention control or usual care
-
multicomponent behavioural intervention compared with another intervention.
If enough comparisons were available, the second group would be split into:
-
CBI compared with single behavioural intervention
-
CBI compared with another treatment (e.g. drug therapy).
Randomised or quasi-RCTs where one arm includes a CBI, compared with no treatment control, or another treatment/single behavioural intervention.
The primary outcome for the meta-analysis of the impact of CBIs on UI was the number of people who reported continuing UI. This was defined by subjective measures (e.g. the number of incontinent episodes as measured in a urinary diary, mean per week) or objective measures (e.g. pad test of quantified leakage).
Secondary outcomes included:
-
patient/carer perceptions of improvement
-
objective measures of severity (e.g. grams of urine lost per 24 hours on pad test)
-
patient/carer perceptions of severity of incontinence
-
urinary symptoms
-
QoL or symptom distress
-
satisfaction with treatment
-
adverse effects; and
-
costs for the client or service.
Short-term (up to 12 months post treatment) follow-up measures were collated for primary and secondary outcomes. If data from multiple follow-up time points were available from a single study, the time point nearest to 6 months post treatment was used because this was judged to be a reasonable length of time to assess whether or not behavioural change has been embedded.
Review of acceptability and feasibility
Acceptability
Study designs included were qualitative or quantitative, where data were collected from service users or from staff about their perceptions or experiences of behavioural interventions, including information on factors influencing:
-
choice or uptake of behavioural interventions for UI
-
adherence to/maintenance of a behavioural programme
-
withdrawal/dropout from a behavioural programme.
Studies exploring client experience of self-management strategies for UI in general were excluded if behavioural interventions (i.e. BT, PFMT, PV) were not referred to specifically.
Feasibility and implementation data
We had planned to review information about implementation of behavioural interventions from studies reporting the process of development or implementation of a behavioural intervention for UI. These studies were screened and filtered, but owing to the high number of studies found (n = 33), detailed information on implementation was not extracted and processed. However, data on rates of uptake, treatment adherence and withdrawal were extracted from any study implementing a behavioural intervention for UI.
Review of predictors of treatment adherence or outcome
Studies of predictors of adherence or treatment outcome of CBIs, or studies of predictors of adherence to single behavioural interventions were included. Predictors of adherence for single interventions were included because they were thought to be generalisable to behavioural adherence to combined interventions. Predictors of treatment outcome of single interventions were not included because they were not judged to be reliably predictive of treatment outcome for combined interventions, due to the potential for differences in physiological mechanisms of action.
Study designs included were:
-
prospective longitudinal cohort studies or clinical trials
-
RCTs that include subgroup analysis of factor(s) influencing adherence/outcome
-
retrospective cohort or cross-sectional studies.
To be included, studies had to include a description of the method of data analysis, and provide data on the relationship between the predictor and outcome based on individual study participants (other than the baseline value of that variable). For full data extraction of results for predictor variables, studies had to identify independent predictors using multivariate analysis. Studies using univariate analysis were only partially data extracted, for the identification and listing of potential predictor variables.
The dependent variables included were any of the following:
-
intention to adhere/short- or long-term adherence
-
treatment failure/non-response
-
cure
-
improvement
-
psychological status/QoL.
Any time points for outcome measurement were considered.
Methods of the review
Review of effectiveness
Data relevant to the pre-stated outcome measures, characteristics of the study, interventions and participants were extracted. The elements of the voiding intervention were categorised based on a previous meta-study. 53,54 The categorisation of the client behaviour change intervention was based on a taxonomy of behaviour change techniques. 62
Assessment of methodological quality was undertaken using the Cochrane Collaboration Risk of Bias Tables to include assessment of adequate sequence generation; allocation concealment; blinding of outcome assessors; incomplete data addressed; freedom from selective reporting; and freedom from other bias (see Appendix 4).
Where appropriate, data were quantitatively combined using meta-analysis to determine the typical effect of the intervention. Intention-to-treat analysis was used, where participants are analysed in the group to which they are randomised. Trial data were processed as described in the Cochrane Collaboration Handbook63 using the Cochrane Collaboration statistical package RevMan 4.2.8 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
For individual clinical indicators, a fixed-effects model was used to calculate pooled estimates of treatment effects with 95% confidence intervals (CIs) using:
-
relative risk (RR) for binary data
-
weighted mean difference (WMD) for continuous data using similar measurement
-
standardised mean difference (SMD) for continuous data from different measurement sources
-
standardised effect (SE) when generic inverse variance (GIV) was used to pool binary and continuous outcomes.
For trials with missing data, primary analysis was based on observed data, without imputation. Assessment of heterogeneity of intervention effects was made using the I2 statistic. If substantial heterogeneity of treatment effects was evident (I2 ≥ 50%), a random-effects model was used.
A priori subgroup analyses were planned as follows.
Client group factors:
-
type of incontinence – SUI only, UUI only, mixed urinary incontinence (MUI)
-
sex – female only, male only, mixed
-
age – mean age < 65 years, ≥ 65 years
-
cognitive status – people with cognitive incapacity excluded/not excluded.
Intervention factors:
-
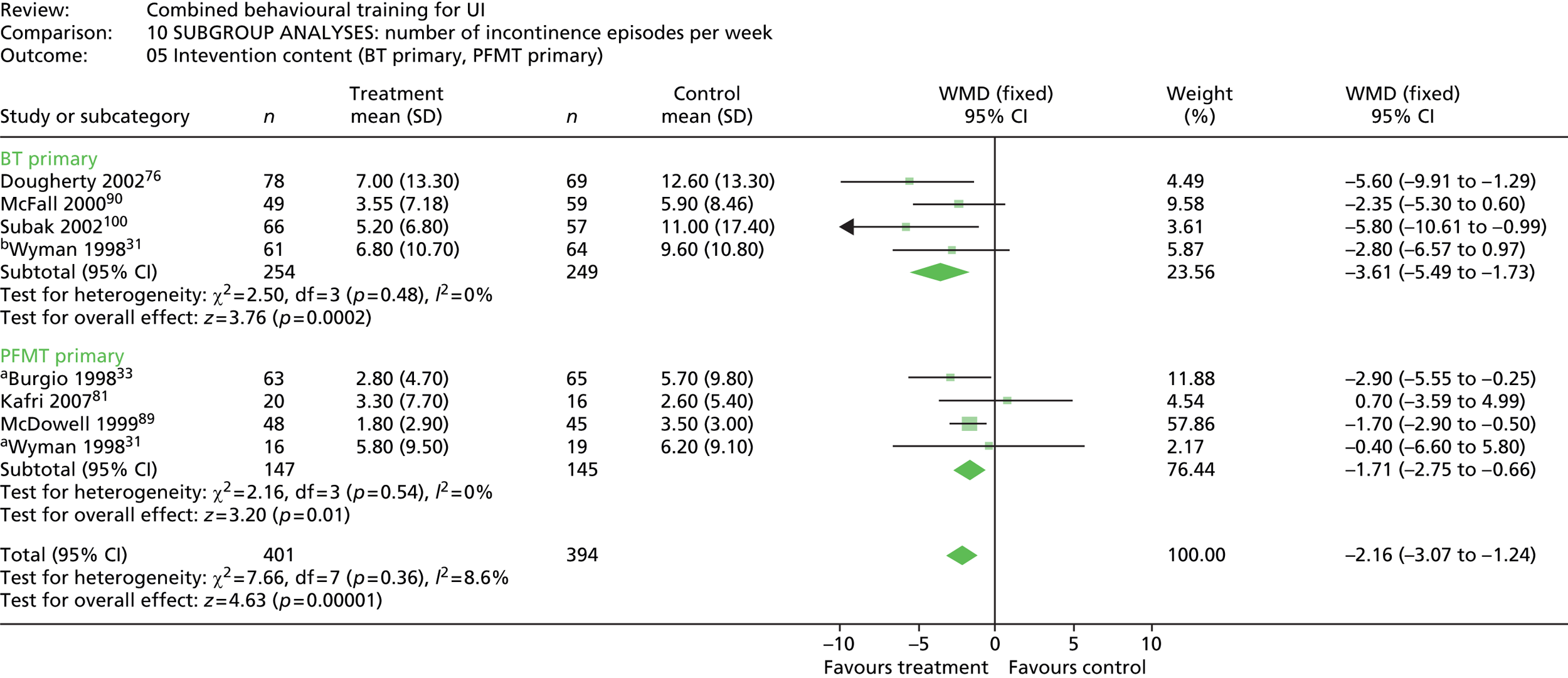
intervention content – BT primary, PFMT primary, PV primary
-
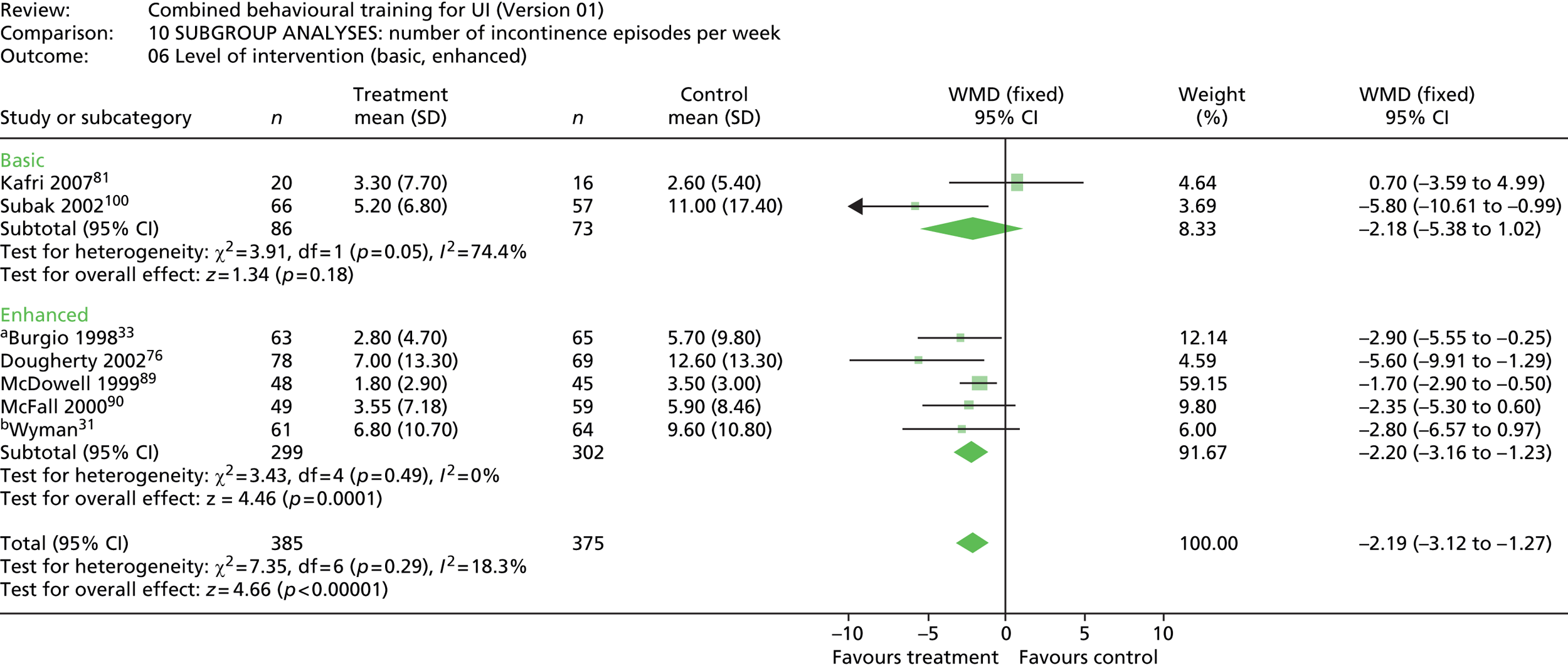
intervention level – basic (i.e. the delivery of multiple strategies aimed at increasing the effectiveness of urinary function activities); enhanced (i.e. the addition of strategies aimed at tailoring an intervention to the specific needs of the individual or enhancing adherence or commitment to practice/activities, e.g. goal-setting, reminder systems, coaching)
-
intervention duration – i.e. length of time in weeks in contact with intervention delivery (≥ 8 weeks, > 8 weeks)
-
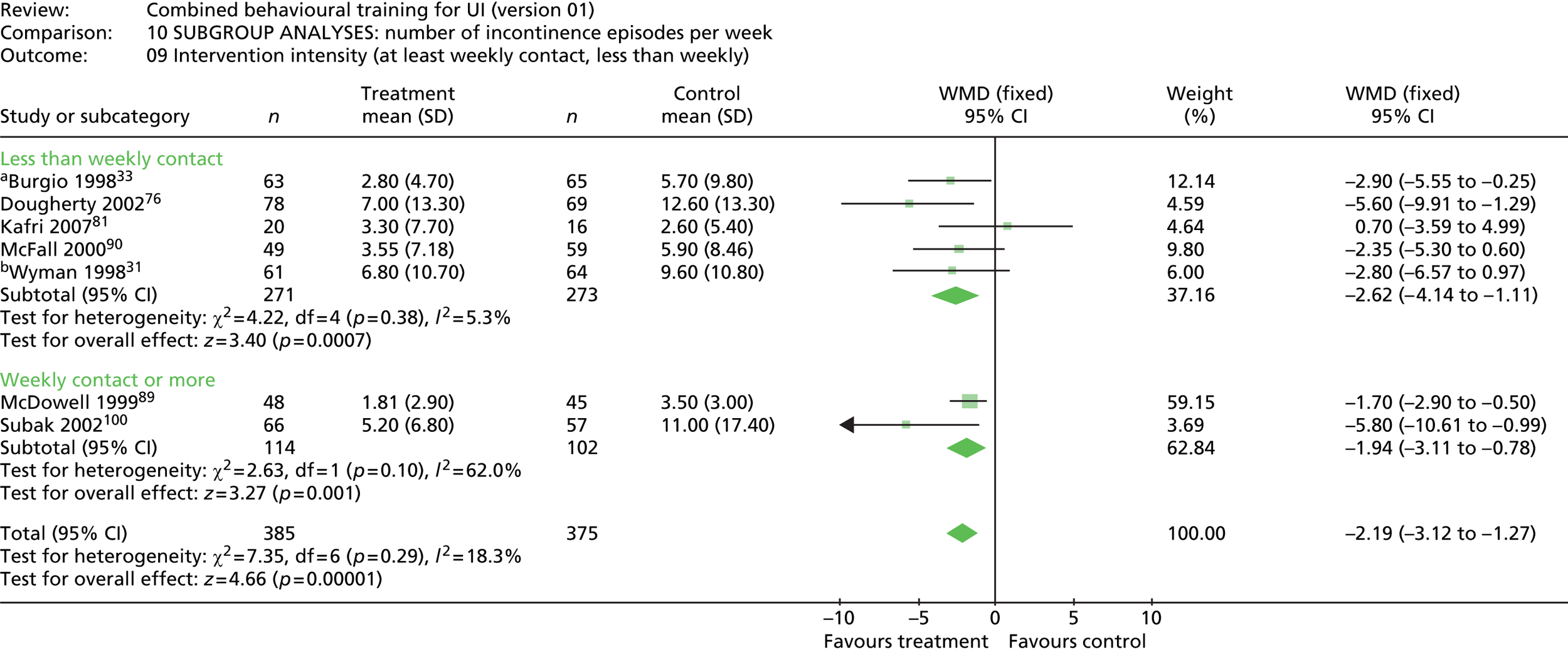
intervention intensity – i.e. number of contacts with the person providing the intervention for content delivery or monitoring/feedback (at least weekly, less than weekly).
A priori sensitivity analyses were planned for type of comparison group (no treatment vs. another treatment), study quality to include allocation concealment (adequate, unclear/not adequate) and loss to follow-up (≤ 20%, > 20%).
Subgroup and sensitivity analyses were performed using a chi-squared test for heterogeneity (via the decomposition of the Q-statistic).
Review of acceptability and feasibility
Acceptability
Data were extracted as follows:
-
client group recruitment and inclusion/exclusion criteria (age, ethnicity, sex, UI type, cognitive status, functional ability)
-
research design classification (qualitative study, survey, process evaluation, action research)
-
intervention classification (combined, PFMT, BT, PV, generic)
-
data collection and analysis methods (framework/model)
-
findings [researcher theme(s), categories and codes].
Findings were identified from secondary data, i.e. the study authors’ aggregate themes, categories or codes relating to potential barriers and enablers to behavioural interventions, and not at the level of the original data (e.g. quotes from respondents). Findings were categorised based on Davidson et al. 64 and National Institute for Health and Care Excellence (NICE) guidance on interventions to support behaviour change65 as follows:
-
intervention – combined, PFMT, BT, PV, generic behavioural
-
influencing factor source – client, intervention or context
-
influencing factor direction – enabler or barrier
-
outcome – choice/uptake, participation/adherence, longer-term sustainability, withdrawal/dropout.
Descriptive data extraction was undertaken by one reviewer and checked by a second reviewer. Data extraction and categorisation of findings, and quality appraisal were undertaken by two reviewers independently. Quality assessment was based on quality criteria for qualitative studies or observational designs,66 including criteria related to participant selection and representativeness, data collection and analysis, methods of representation and testing the robustness of findings (see Appendix 4).
Feasibility
Rates of non-participation (i.e. people who were eligible to participate and who did not opt to do so), treatment adherence and withdrawal or dropout (short and long term) were extracted, together with the reasons if given. Data were tabulated, averaged and reported in the context of intervention type, client group and setting.
Review of predictors of treatment adherence or outcome
Data were extracted for (independent) predictor variables relating to characteristics of the client group as follows:
-
sociodemographic variables, i.e. age, ethnicity, sex, education/income
-
physiological variables, i.e. gynaecologic/obstetric status and history, weight/body mass index (BMI), urodynamic variables, prior treatment, type, duration of UI, severity of UI/symptoms
-
health/functional variables, i.e. general health status/comorbidities, self-care ability, functional ability, cognitive status, mental health
-
psychological variables, i.e. health/treatment perceptions; perceived QoL, self-efficacy/esteem, attributions of control, prior adherence, knowledge/skill, motivation/attitude, goal orientation
-
social variables, i.e. social influences and demands.
A coding frame for the definition and classification of predictor variables was used (see Appendix 4). Using a standardised protocol, data extraction for studies using multivariate analysis included:
-
research design classification (prospective cohort/clinical trial, RCT, retrospective cohort/cross-sectional study)
-
client group classification (age range, sex, UI type, cognitive status)
-
intervention classification (combined, PFMT, BT, PV)
-
selection and measurement of independent variables (hypothesis/model for selection, definition, who/how measured, timing, validity and reliability of measurement)
-
measurement of outcome variables (who/how measured, timing, validity and reliability of measurement, definition of outcome categorisation)
-
statistical analysis method
-
variables entered into univariate analysis
-
variables entered into multivariate analysis
-
statistically significant results from univariate analysis not subsequently confirmed as an independent predictor
-
statistically significant results for independent predictor variables for:
-
intention to adhere/adherence behaviour
-
treatment failure
-
cure
-
improvement
-
psychological status
-
QoL.
-
Descriptive data extraction was undertaken by one reviewer and checked by a second reviewer. Data extraction of predictor and outcome variables and quality appraisal was undertaken by two reviewers independently. Quality assessment was based on quality criteria for observational studies67 and for regression studies,68 and included criteria related to participant selection and representativeness, predictor and outcome variable selection, definition and measurement, adequacy of sample size, follow-up and analysis (see Appendix 4).
Stakeholder involvement in the review process
Review Management Group
The Review Management Group was composed of the named authors on the review. They met quarterly during the review process and their input included:
-
discussion of studies referred by reviewers where inclusion was unclear, with subsequent refinement of the criteria for inclusion and exclusion
-
feasibility testing of the classification structures for the review and review of the data extraction proforma
-
checking back to the original study data from the results to comment on robustness of interpretation
-
reading and commenting on all review outputs.
Service User Group
The PPC involvement group were involved at three stages for consultation on the review:
-
to advise on the parameters and scope of the review, and the included interventions, comparisons and outcomes
-
to consider the draft results of the review and comment on their perceptions and priorities for the components of the intervention and mediating factors
-
once the review was completed, to assist in the translation of the findings into practical products for implementation.
Trial Management and Steering Groups
The review findings were presented to the Identifying Continence OptioNs after Stroke (ICONS) Trial Management and Steering Groups, who then made suggestions for:
-
the content of the behavioural intervention for UI to be used with people after stroke
-
optimal conditions of implementation on which to base the tailoring of the intervention to client groups and settings
-
hypotheses about potential mediators and moderators for consideration in the design of the pilot trial.
Their suggestions were then used to adapt the intervention and data collection protocols for use in the case study.
Description of included studies
Results of the search
The main database search identified 8289 records. Duplicate records (n = 1807) and records which were clearly irrelevant on title (n = 4224) were removed, leaving 2258 records for screening. Another 31 records were added from additional searches of trial registers, databases of unpublished studies and conference proceedings, plus 68 records from secondary references. Of the 2357 records screened for inclusion, 538 full-text papers were retrieved. Four records could not be traced.
The 538 papers were filtered independently by two reviewers who discussed any disagreement and were coded as relevant to one or more of the review components. Exclusions were as follows:
-
not English language (n = 1)
-
not research (n = 80)
-
not behavioural (n = 46)
-
not UI (n = 25)
-
excluded client group (e.g. pregnancy, post prostatectomy) (n = 1)
-
not CBI (n = 65)
-
single UI intervention plus adherence intervention (n = 9)
-
compares methods of delivery of behavioural intervention (n = 54)
-
confounded intervention (n = 25)
-
not appropriate research design (n = 75); and
-
review (n = 47); these were combed for secondary references.
Excluded records totalled 428.
Of the remaining 110 papers, 33 related to the implementation of behavioural interventions, either from reports of intervention development, process evaluations or feasibility studies. Owing to the volume of material, these studies were not reviewed in detail, other than to extract data from the feasibility studies on rates of uptake, adherence and withdrawal.
Table 1 details the remaining papers, identifying published, unpublished and ongoing studies exclusive to each component of the review. The number of studies that the published papers refer to are given in brackets.
| Status of paper | Meta-analysis | Narrative review | Predictors | Total | |
|---|---|---|---|---|---|
| Effectiveness | Acceptability | Feasibility | |||
| Published | 20 (10) | 12 (11) | 2 (2) | 11 (10) | 45 (33) |
| Unpublished | 0 | 3 (1) | 0 | 4 (3) | 7 (4) |
| Ongoing | 3 (3) | 0 | 0 | 0 | 3 (3) |
| Excluded | 14 (8) | 6 (6) | 2 (2) | 0 | 22 (16) |
| Total papers | 37 (21) | 21 (18) | 4 (4) | 15 (13) | 77 (56) |
In total, 77 papers detailing 56 studies were included at filtration. The table also identifies the number of studies excluded after filtering. The rationale for exclusion is given in a table of excluded studies at Appendix 7. No exclusions are shown for the review of predictors, as filtering was reapplied specifically for predictor studies after the main filtering was completed.
Thirty-three published studies contributed data to different review components (Table 2). Details of the individual studies are given in a table of included studies at Appendix 6.
| Study | Effectiveness | Acceptability | Feasibility | Predictors |
|---|---|---|---|---|
| Alewijnse et al. 2001,69 200370 | ✓ | |||
| Aslan et al. 200871 | ✓ | ✓ | ||
| Baigis-Smith et al. 198972 | ✓ | |||
| Bear et al. 199773 | ✓ | ✓ | ||
| Burgio et al. 199833 | ✓ | ✓ | ||
| Burgio et al. 200374 | ✓ | |||
| Dingwall and McLafferty 200675 | ✓ | |||
| Dougherty et al. 200276 | ✓ | ✓ | ||
| Mather and Bakas 200277 | ✓ | |||
| Gerard 199778 | ✓ | |||
| Hay-Smith et al. 200779 | ✓ | |||
| Johnson et al. 200180 | ✓ | |||
| Kafri et al. 2007,81 200882 | ✓ | ✓ | ||
| Kincade et al. 199983 | ✓ | |||
| Kincade et al. 200184 | ✓ | |||
| Lee et al. 200585 | ✓ | |||
| Lekan-Rutledge et al. 199886 | ✓ | |||
| Macaulay et al. 198787 | ✓ | ✓ | ||
| McDowell et al. 199288 | ✓ | |||
| McDowell et al. 199989 | ✓ | ✓ | ✓ | |
| McFall et al. 200090,91 | ✓ | |||
| MacInnes 200892 | ✓ | |||
| Milne and Moore 200693 | ✓ | |||
| O’Dell et al. 200894 | ✓ | |||
| Oldenberg and Millard 198695 | ✓ | |||
| Perrin et al. 200596 | ✓ | |||
| Remsburg et al. 199997 | ✓ | |||
| Resnick et al. 200698 | ✓ | |||
| Rose et al. 199099 | ✓ | |||
| Subak et al. 2002100 | ✓ | ✓ | ✓ | |
| Svengalis et al. 1995101 | ✓ | |||
| Tadic et al. 2007102 | ✓ | |||
| Wyman et al. 199831 | ✓ | ✓ | ✓ | |
| Total per review component | 10 | 11 | 11 | 13 |
Description of studies of effectiveness
Included studies
Of the 21 studies identified for the effectiveness review, eight were excluded (reasons are given in the table of excluded studies in Appendix 7). Three studies are ongoing. 82,103,104 There were no unpublished studies. The remaining 10 studies are detailed in Table 3.
| Study | Study design | Comparison(s) | Client group/setting |
|---|---|---|---|
| Aslan et al. 200871 (Turkey) | Quasi-RCT (n = 64) | Attention control | F, aged ≥ 65 years, rest home |
| Bear et al. 199773 (USA) | Quasi-RCT (n = 24) | No treatment control | F, aged ≥ 55 years, home |
| Burgio et al. 199833 (USA) | RCT (n = 197) |
|
F, aged ≥ 55 years, UUI, community |
| Dougherty et al. 200276 (USA) | RCT (n = 178) | No treatment control | F, aged ≥ 55 years, rural area, home |
| Kafri et al. 2007,81 200882 (Israel) | Quasi-RCT (n = 44) | Medication | F, UUI, community |
| Macaulay et al. 198787 (UK) | RCT (n = 50) |
|
F, UUI |
| McDowell et al. 199989 (USA) | RCT (n = 105) | Attention control | M/F, aged ≥ 60 years, home bound |
| McFall et al. 200090,91 (USA) | RCT (n = 145) | Waitlist control | F, aged ≥ 65 years, community |
| Subak et al. 2002100 (USA) | RCT (n = 152) | Waitlist control | F, aged ≥ 55 years, community |
| Wyman et al. 199831 (USA) | RCT (n = 204) |
|
F, community |
Study design
The 10 studies included 1163 participants in 13 intervention–comparison pairs. Table 3 details the trial arms compared against CBIs. Of the seven control comparisons, three were attention controls,33,71,89 two were waitlist controls90,91,100 and two were no treatment controls. 73,76 The remaining six treatment comparisons included three medications [propantheline bromide (Pro-Banthine®, Roxane Laboratories Inc.) or oxybutinin (Ditropan, several manufacturers) (× 2)],33,81,82,87 two single behavioural interventions (BT or PFMT)31 and one psychotherapy comparison. 87
Seven of the trials were undertaken in the USA31,33,73,76,89–91,100 one in the UK,87 one in Turkey71 and one in Israel. 81,82 Three were quasi-RCTs. 71,73,81 All of the quasi-RCTs and the oldest trial87 had fewer than 100 participants. The remaining trials all had more than 100 participants. One of the quasi-RCTs73 was an external pilot for a larger RCT. 89 One study did not provide outcome data suitable for pooling. 87
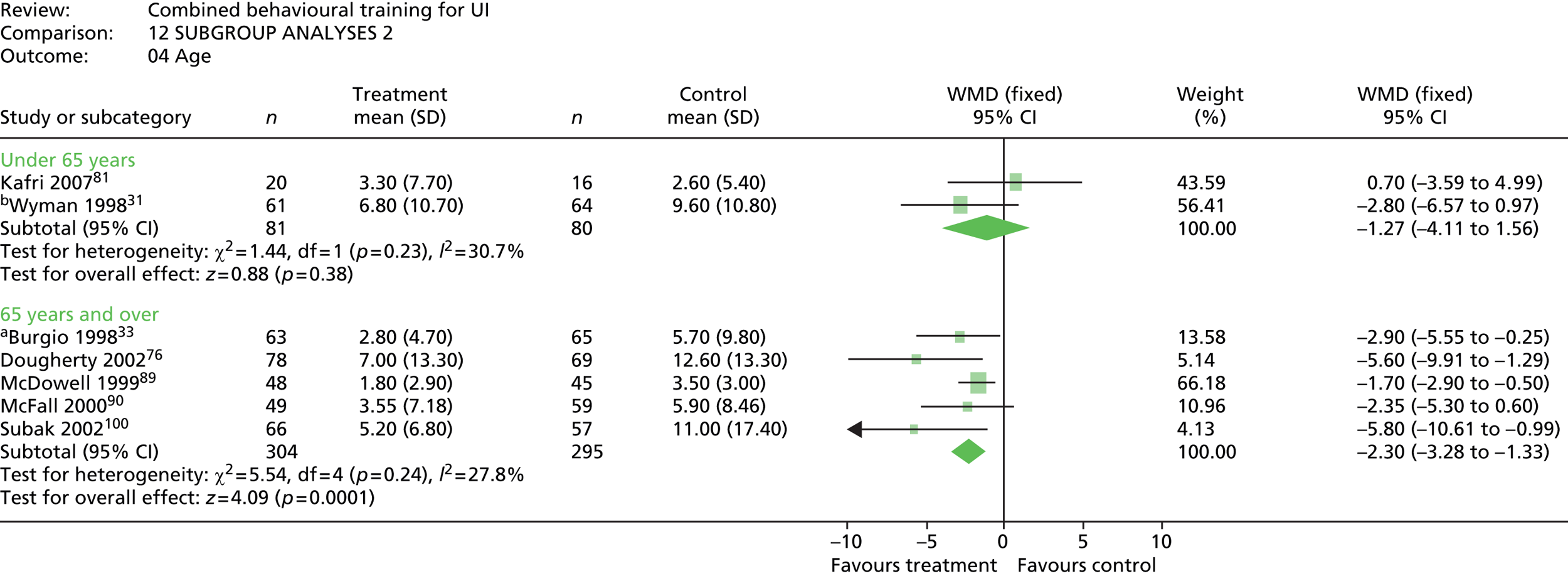
All of the trials except one89 were limited to female participants, and the sample for McDowell et al. 89 was also 90% female. Only three trials included people aged > 55 years, and two of these had samples with a mean age of ≥ 55 years. 31,81
Three trials were undertaken with participants with UUI. 33,81,87 The remaining trials were undertaken with people with all types of incontinence. One trial provides outcome data for intervention subgroups based on urodynamic diagnosis. 31
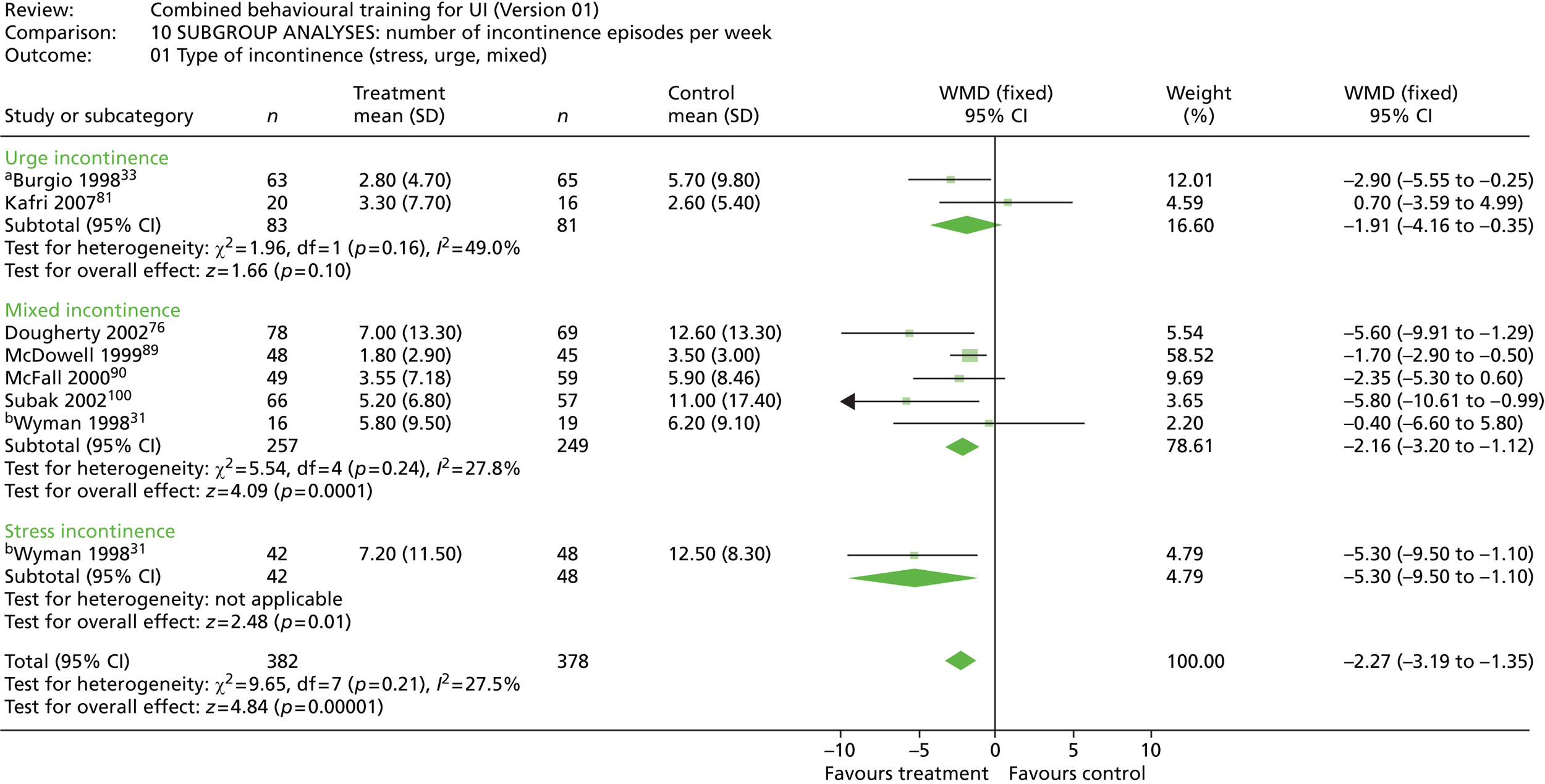
The definition of incontinence differs slightly: four trials specifying that UI episodes had to occur at least twice a week,33,73,76,89 one trial specifying at least once a week31 and one trial specifying more than two episodes a month. 71 Of the remaining four trials, two confirmed UUI by urodynamic testing,81,87 McFall et al. 90 used self-report of UI for 3 months or more as an inclusion criteria and Subak et al. 100 did not define UI but referred to standard diagnostic criteria sourced from US guidelines.
Two related studies73,89 were undertaken with people who were housebound and a further study was undertaken using home visits to women from rural areas of the USA. 76 One study was undertaken with people in rest homes. 71 Five studies involved community samples with interventions delivered in clinic visits. 31,33,81,90,100 The setting for one study was unclear. 87
Three trials did not exclude people with cognitive impairment. Two of these required that a person with cognitive impairment had a caregiver present who was willing to undertake PV. 73,76 One other trial did not exclude people with cognitive impairment,89 but outcome data are only reported for people without cognitive impairment.
Description of urinary incontinence interventions
Table 4 summarises details of the interventions used in the 10 trials, including the components of the intervention, method of delivery, and the duration and intensity of contact with professionals. Some of these details were provided by contact with study authors.
| Study | UI intervention components | Method of delivery | Duration/intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PV | BT | PFMT | Urge strategies | Stress strategies | Other | I or G | H or C | N or O | Number of weeks | Number of contacts | Intensity of contact (weekly = 1) | |
| Aslan et al. 200871 | ✓ | ✓ | ✓ | I | H | N | 8 | 8 | 1 | |||
| Bear et al. 199773 | ✓ | ✓ | ✓ | ✓ | I | H | N | 6–24 | 2–40 | – | ||
| Burgio et al. 199833 | ✓ | ✓ | ✓ | I | C | N | 8 | 4 | 0.5 | |||
| Dougherty et al. 200276 | ✓ | ✓ | ✓ | I | H | N | 6–24 | NS | – | |||
| Kafri et al. 200781 | ✓ | ✓ | ✓ | ?G | C | O | 12 | 5 | 0.4 | |||
| Macaulay et al. 198787 | ✓ | ✓ | NS | C | N | 12 | 7 | 0.6 | ||||
| McDowell et al. 199989 | ✓ | ✓ | ✓ | ✓ | I | H | N | 8 | 8 | 1 | ||
| McFall et al. 200090 | ✓ | ✓ | ✓ | ✓ | G | C | N/O | 9 | 5 | 0.6 | ||
| Subak et al. 2002100 | ✓ | ✓ | G | C | N | 6 | 6 | 1 | ||||
| Wyman et al. 199831 | ✓ | ✓ | ✓ | ✓ | I | C | N | 12 | 9 | 0.8 | ||
All of the trials included PFMT, albeit to various degrees. All of the trials except one33 included BT, with one trial73 including BT or PV, depending on the cognitive status of the individual. Six trials included the teaching of either urge strategies (e.g. distraction) or stress strategies (e.g. muscle clamping), with three trials teaching both31,33,89 and three trials teaching one or the other. 71,81,90 However, description and labelling of the techniques used tended to be inconsistent. Three trials included other strategies, such as advice about alterations to diet and/or fluid intake. 73,76,90
Interventions in two trials were delivered to groups. 90,100 The delivery format was unclear in two trials81,87 and the remainder were delivered to individuals. Eight out of 10 interventions were delivered by nurses, with another intervention predominantly delivered by nurses but including other professions. 90 One intervention was delivered by physical therapists. 81
Most of the interventions ran over 6–12 weeks, with interventions in two related trials running over a minimum of 6 weeks and a maximum of 24 weeks. 73,76
Three trials had weekly contacts with a health-care professional for the duration of the intervention,71,89,100 with four trials having at least bi-weekly contact. 31,33,87,90 The number of contacts was stated as being in the range of 2 to 40 contacts in an intervention lasting a minimum of 6 weeks and a maximum of 24 weeks in one pilot trial. 73 This is unstated, but likely to be similar in the related trial. 76 Most of the trials stated a requirement for practice of the techniques between contacts.
Intensity of intervention was defined as the ratio of the number of contacts with a person delivering the intervention to the length of the intervention period. An intensity of 1 is weekly contact. Intensity could not be derived for the two trials that did not specify the exact number of contacts. 73,76 Three trials had at least weekly contact71,89,100 and four trials had at least bi-weekly contact. 31,33,87,90 Only one trial had less than bi-weekly contact. 81
Features of urinary incontinence intervention components
Table 5 details the features of the BT and PFMT interventions in the included trials. A dash in the table means that the feature was not stated in the paper. The level of description of the interventions was variable and lack of description cannot be interpreted as absence of the feature in practice.
| Study | BT | PFMT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient education | Scheduled voiding | Positive reinforcement | Self-monitoring | Urge suppression | Confirm correct technique | Individual instruction | Adherence check | Monitoring progress | Longer training | |
| Aslan et al. 200871 | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | – | – | ✓ |
| Bear et al. 199773 | – | ✓ | – | ✓ | – | ✓ | ✓ | – | ✓ | ✓ |
| Burgio et al. 199833 | ✓ | ✓ | – | ✓ | – | |||||
| Dougherty et al. 200276 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ |
| Kafri et al. 200781 | – | ✓ | – | ✓ | – | ✓ | ✓ | – | – | ✓ |
| Macaulay et al. 198787 | – | ✓ | – | – | – | – | – | – | – | ✓ |
| McDowell et al. 199989 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – |
| McFall et al. 200090 | ✓ | – | – | ✓ | ✓ | – | – | – | – | – |
| Subak et al. 2002100 | ✓ | ✓ | – | ✓ | – | – | – | – | – | – |
| Wyman et al. 199831 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Bladder training
Core features of BT were identified from the Cochrane systematic review of BT,25 to include patient education, scheduled voiding, positive reinforcement, self-monitoring and urge suppression.
-
Patient education about basic urinary physiology and function was stated as included in six out of nine trials. 31,71,76,89,90,100 Eight out of nine trials described a system of scheduled voiding, where voiding intervals were specified. 31,71,73,76,81,87,89,100
-
Six trials31,71,73,76,89,100 described the use of a system of gradually increasing void intervals tailored to the baseline and progress of the individual, as described by Wyman and Fantl. 105 Two trials described gradual increases in voiding interval81,87 without describing tailoring to the individual. The remaining trial just described the intervention as bladder retraining without further detail. 90
-
Four out of nine trials specifically described positive reinforcement for progress. 31,71,76,89 One other trial73 is likely to have included positive reinforcement because they were using the same protocol, but it is not specifically mentioned in the trial report.
-
All of the trials except one87 described using bladder diaries for self-monitoring of voiding patterns. Two trials73,76 used 3-day diaries and the remainder used daily diaries.
-
Four out of nine trials specifically detail instruction in urge suppression techniques such as distraction. 31,76,89,90
Pelvic floor muscle training
Core features of PFMT were identified from the review by Bo,106 including details of the exercises (e.g. type of exercise, frequency, intensity and duration). In terms of PFMT, this relates to whether contractions are maximal or submaximal, the duration of exercise and relaxation periods, the speed and duration of muscle contraction, and the amount of exercise in the form of repetitions and duration. Additional data were extracted about whether or not exercise was generalised to different body positions and activities/situations, whether or not practice was progressive in terms of intensity or amount, and the method of teaching. Table 6 gives details of PFMT teaching regimes included in the trials.
| Study | Exercise detail | Positions | Activities | Increased pressures | PFMT teaching method | Number of teaching sessions |
|---|---|---|---|---|---|---|
| Aslan et al. 200871 | NS | ✓ | – | – | Digital | 1 |
| Bear et al. 199773 | NS | – | – | – | BIO | NS |
| Burgio et al. 199833 | 15, three times a day, aim for a 10-second contraction | ✓ | ✓ | ✓ | BIO | 2–4 |
| Dougherty et al. 200276 | 45 per day, three times per week | – | – | – | BIO | 1 |
| Kafri et al. 200781 | 12, two times a day, aim for a 10-second submaximal contraction | ✓ | – | ✓ | Digital | 1 |
| Macaulay et al. 198787 | NS | – | – | – | NS | NS |
| McDowell et al. 199989 | 10–15, three times a day, aim for a 10-second contraction | ✓ | ✓ | – | BIO | ≤ 4 |
| McFall et al. 200090 | NS | – | – | – | NS | NS |
| Subak et al. 2002100 | 100 per day, 2–3 second tighten/relax five times, as quickly as possible | – | – | – | Verbal | – |
| Wyman et al. 199831 | 50 per day, 10 fast, 40 sustained | – | – | – | BIO | ≤ 4 |
Four trials did not give details of the exercises. 71,73,87,90 Three of the remaining trials aimed for 30–50 repetitions daily. 31,33,89 Two trials had lower intensities of practice76,81 and one trial had a higher intensity of practice. 100 Most of the trials specified aiming for 10-second maximum sustained contractions, except Subak et al. 100 which used sets of rapid 2–3-second contractions. Wyman et al. 31 used a mix of fast and sustained contractions.
-
Three trials did not describe confirming correct initial pelvic floor muscle contraction technique, either by digital palpation or BIO. 87,90,100 One other trial did use digital palpation, but 30% of older women living in a nursing home refused. 71
-
Three trials did not describe individual instruction for PFMT. 87,90,100 Of these, two used group teaching. 90,100
-
Two out of nine trials report the level of adherence of the individual to the prescribed exercise regime. 31,89
-
Five trials describe repeating sessions (or the opportunity to repeat session dependent on progress) of BIO during the intervention. 31,33,73,76,89
-
Of the 10 trials using PFMT, five had a longer intervention period (i.e. ≥ 12 weeks) where sustained impact on muscle performance is more likely to be achieved. 31,73,76,81,87
Features of behavioural intervention components
Table 7 details and categorises the behavioural component of the interventions, as per the taxonomy of behavioural interventions described by Abrahams and Michie. 62 The categorisation was based only on the published accounts given of the interventions. The level of description of intervention components was variable. Given the restrictions on length of publication, absence of description of a feature may not constitute its absence in practice.
| Study | Behavioural intervention components | ||||||
|---|---|---|---|---|---|---|---|
| Information provision | Self-monitoring | Adherence reminders | Tailoring/goal-setting | External monitoring | External motivation | Counseling | |
| Aslan et al. 200871 | ✓ | ✓ | – | – | ✓ | ✓ | – |
| Bear et al. 199773 | ✓ | ✓ | – | ✓ | ✓ | – | – |
| Burgio et al. 199833 | ✓ | ✓ | – | – | ✓ | ✓ | – |
| Dougherty et al. 200276 | ✓ | ✓ | – | ✓ | ✓ | – | – |
| Kafri et al. 200781 | ✓ | ✓ | – | – | – | – | – |
| Macaulay et al. 198787 | – | – | – | – | – | – | – |
| McDowell et al. 199989 | ✓ | ✓ | – | ✓ | ✓ | – | – |
| McFall et al. 200090 | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ |
| Subak et al. 2002100 | ✓ | ✓ | – | – | ✓ | – | – |
| Wyman et al. 199831 | ✓ | ✓ | – | – | ✓ | ✓ | ✓ |
Information provision
Information provision could include providing general information on health, health behaviour, or the consequences of behaviour; or providing explicit instruction on how to perform a behaviour. All except one of the trials87 described some level of information provision, although the content varied markedly.
Nine of the 10 trials described skills instruction on behavioural techniques. Two trials describe only skills instruction. 33,81 Additional educational content described by other trials included:
-
Structure and function of the urinary tract, normal voiding mechanisms and the causes and symptoms of incontinence. 71,100
-
Two trials31,76 that describe patient education as per the protocol for BT by Fantl et al. 32 This includes discussion of normal bladder control; explanation of the pathophysiology underlying different types of incontinence; and stressing the importance of continence as a learned behaviour and brain control over lower urinary tract function. Three other trials used this protocol71,73,89 but do not refer to the content of their information provision. Two of these trials identify giving lifestyle advice on dietary or fluid intake behaviour, or environmental adaptations. 71,73,89 McFall et al. 90 also taught definitions and types of UI, identified resources which provided educational material on UI and included the aim ‘to learn that the condition is treatable’. No trial reported including information about the consequences of behavioural techniques (e.g. pros and cons), although McFall et al. 90 did include discussion of coping strategies that help control incontinence or its negative consequences.
Self-monitoring and adherence reminders
Self-monitoring involves keeping a record of specified behaviours. Nine trials included a behavioural technique for self-monitoring of urinary function by the inclusion of a bladder diary – only the early trial by Macaulay et al. 87 did not include a regular bladder diary during treatment. Two trials also included self-monitoring of treatment adherence behaviour. 31,89
Adherence reminders include the use of passive or interactive devices or systems to self-prompt practice (e.g. sheets to fill in, computerised counters, display items such as fridge magnets). No trial specifically mentioned a reminder system, or a method of recording that served a dual purpose of data collection and behavioural prompting, although it is likely that the simple presence of the bladder diary did have a reminder function. One trial did use audio cassettes for PFMT practice, which could have a reminder function. 31
Tailoring/goal-setting
A number of techniques are relevant to tailoring and goal-setting, including intention formation, barrier identification, relapse prevention, setting graded tasks, detailed goal-setting, review of behavioural goals and agreeing a behavioural contract.
By their nature, both BT and PFMT involve setting goals and graded tasks based on operant conditioning principles, but these are also based on physiological reasons related to bladder capacity or muscle fibre action. The setting of goals and the incremental nature of the targets are not necessarily behavioural techniques to assist learning and do not tend to meet the definitions given in Abraham and Michie62 as described below.
Intention formation
Intention formation involves encouraging the person to set a general goal or resolution to decide to change. It does not involve planning exactly what will be done, when and how, which would be classified as goal-setting. Two trials included reference to people being asked about their own goals for continence. 73,76
Barrier identification
Barrier identification involves thinking about potential barriers and planning ways to overcome them. One trial included reference to strategies to remove environmental barriers (e.g. installing night lights, assistive mobility devices such as grab bars, etc.). 89
Relapse prevention
Relapse prevention involves identifying situations that increase the likelihood or failing to perform a new health behaviour and planning to manage the situation. One trial referred to reviewing the voiding diary for ‘problem-solving’. 90
Setting graded tasks
Setting graded tasks involves planning a sequence of actions or task components that increase in difficulty over time until the target behaviour is reached. No trials explicitly met this criterion for what appeared to be behavioural reasons, but separating physiological from behavioural rationales was almost impossible to do. Details of intervention components (other than straightforward BT or PFMT schedules where void intervals/exercise intensity increases over time) that could be seen as graded in difficulty for behavioural reasons are described here.
Seven trials sequenced the introduction of components of the intervention,31,33,73,76,81,89,90 but only three of these appear to base sequencing on increasing task difficulty. One trial referred to teaching BT after urge strategies had been taught, so that participants could use the skills learned to suppress urge sensations during BT. 89 Two trials included practising PFMT against increasing bladder pressure, once PFMT had been learned. 33,81 Four trials referred to generalising skills, by practising exercises in different positions, and during different activities. 33,71,81,89 This could be interpreted as practising learned skills in situations of increasing challenge.
Goal-setting and behavioural contracts
This requires detailed planning of what the person will do, where, when and how. Both BT and PFMT include detailed instruction, so all trials could be said to include an element of this. However, using behavioural principles for the learning and application of the techniques by detailed planning of goals for the individual subject is not a strong feature of any of the trials. Two trials included formal review of individual goals at each stage of the programme,73,76 but because this goal-setting did not include detailed planning, it was categorised as intention formation, and reported earlier.
Monitoring, motivation and reinforcement
Adherence interventions included in this section include feedback on performance, provision of general encouragement or contingent reward, teaching to use prompts and cues, prompting practice and use of follow-up prompts.
Six trials described regular external review and feedback on performance by a health professional, via the bladder diary. Three trials included weekly review,71,89,100 and two trials reported bi-weekly review. 33,89 In one other trial,90 the review was weekly, but it is not clear if the bladder diary review was done on an individual basis, as the teaching was done in fairly large groups. Two trials stated a review of progress was done at the end of each phase of the intervention73,76 and one trial did not refer to feedback. 87
Four trials describe providing general encouragement to adhere to the programme. 31,33,71,90 The other six trials do not explicitly describe providing encouragement or reinforcement, but, of these, four were following the BT protocol by Fantl et al. ,32 which includes the requirement for positive reinforcement. 73,76,89,100 No trials included the provision of contingent rewards.
Two trials referred to embedding behavioural practices into daily routines,89,90 but none of the trials included prompts to practice other than regular contact with professionals. Two trials used written recording of adherence to the programme. 31,89
These include prompting self-talk, identification as a role model, planning social support, using social comparison, motivational interviewing and techniques for stress and time management. Wyman et al. 31 used affirmations and self-statements. McFall et al. 90 referred to the opportunities for modelling of behaviour and the social support provided by delivering the intervention in a group setting.
Allocation of interventions
Not all participants received the same interventions in all trials. In two trials73,76 participants received intervention components dependent on need. Participants were allocated to a self-monitoring phase if they had problematic fluid or caffeine intake, excessive daytime void intervals, nocturia or constipation. Participants were then allocated to BT, or to PV if functionally or mentally dependent on a caregiver. Finally, participants progressed to PFMT if insufficient progress had been made in earlier stages. How many participants progressed through each phase of the intervention is not reported for Bear et al. ,73 but Dougherty et al. 76 report that out of 94 people in the intervention group, the number progressing through each phase was as follows: self-monitoring (n = 41), BT (n = 89), PFMT (n = 45).
In the trial by McDowell et al. ,89 urge strategies were taught to participants who reported involuntary urine loss following a strong urge to void (85/105), stress strategies were taught to those who reported leaking urine with sudden increases in abdominal pressure (44/105), and only participants who reported frequent voiding got BT. However, in a related paper Engberg et al. 107 reported that many participants had high urinary frequency.
Outcomes
The 10 trials used a range of outcome measures, measurement statistics and time intervals for follow-up. Outcomes measured are detailed in Table 8 and summarised below.
| Study | Cure | Improvement: diary | Improvement: subjective | Severity: pad test | Symptoms: frequency | Symptoms: urgency | Symptoms: nocturia | QoL | Satisfaction | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Aslan et al. 200871 | – | ✓ | – | ✓ | ✓ | ✓ | ✓ | – | – | – |
| Bear et al. 199773 | – | ✓ | – | ✓ | – | – | – | – | – | – |
| Burgio et al. 199833 | ✓ | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | – |
| Dougherty et al. 200276 | – | ✓ | – | ✓ | ✓ | – | – | ✓ | – | – |
| Kafri et al. 200781 | – | ✓ | – | – | ✓ | – | ✓ | ✓ | – | ✓ |
| Macaulay et al. 198787 | – | – | – | – | – | – | – | – | – | – |
| McDowell et al. 199989 | – | ✓ | – | – | – | – | – | – | – | – |
| McFall et al. 200090 | ✓ | ✓ | – | – | ✓ | – | ✓ | ✓ | – | – |
| Subak et al. 2002100 | – | ✓ | ✓ | – | ✓ | – | ✓ | – | ✓ | – |
| Wyman et al. 199831 | ✓ | ✓ | ✓ | ✓ | – | – | – | ✓ | ✓ | – |
Primary outcome
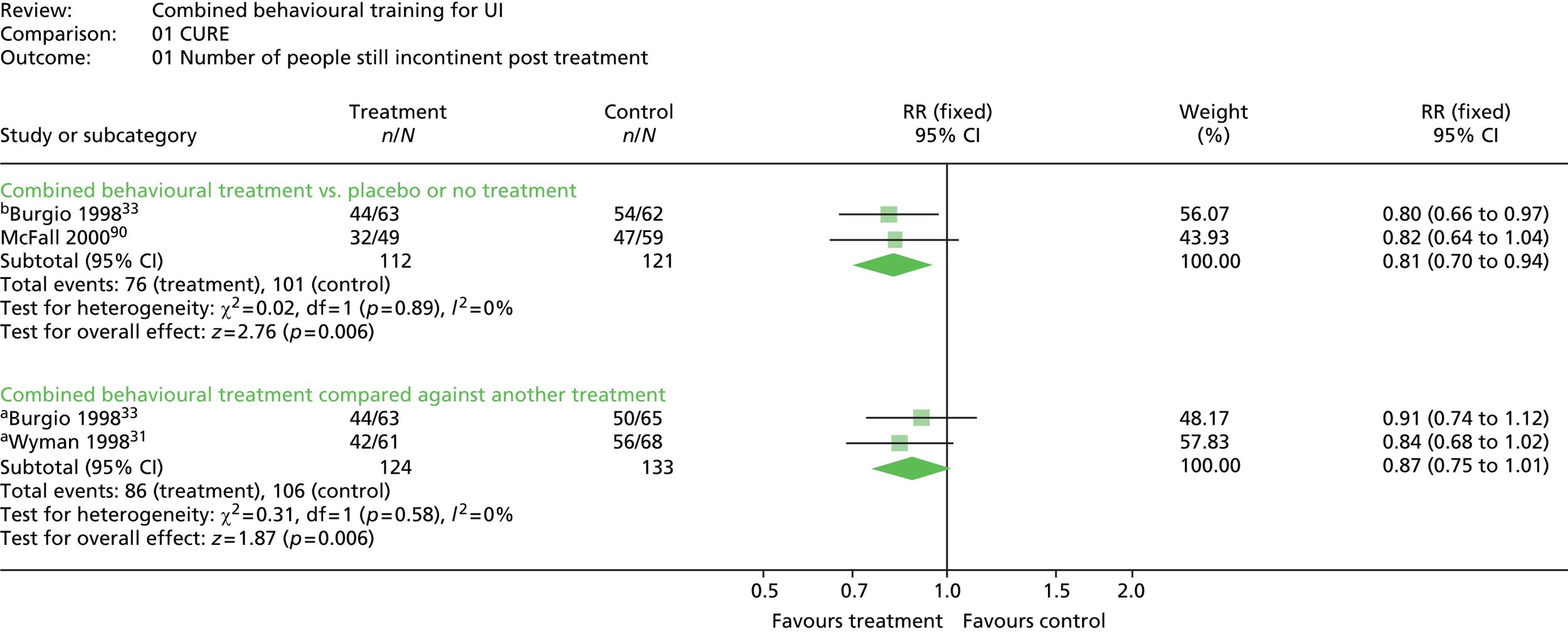
The primary outcome was the number of people continent after treatment (i.e. cured). Three trials included a measure of cure,31,33,90 all defined as the number of people reporting 100% improvement in the number of incontinent episodes as measured in a urinary diary (mean per week). One additional trial87 reported the percentage of patients with UI in graphical form, but did not provide numerical results other than p-values for difference between groups.
No trials reported cure using objective measures (e.g. number of people reporting 0% leakage using a pad test of quantified leakage).
Secondary outcomes
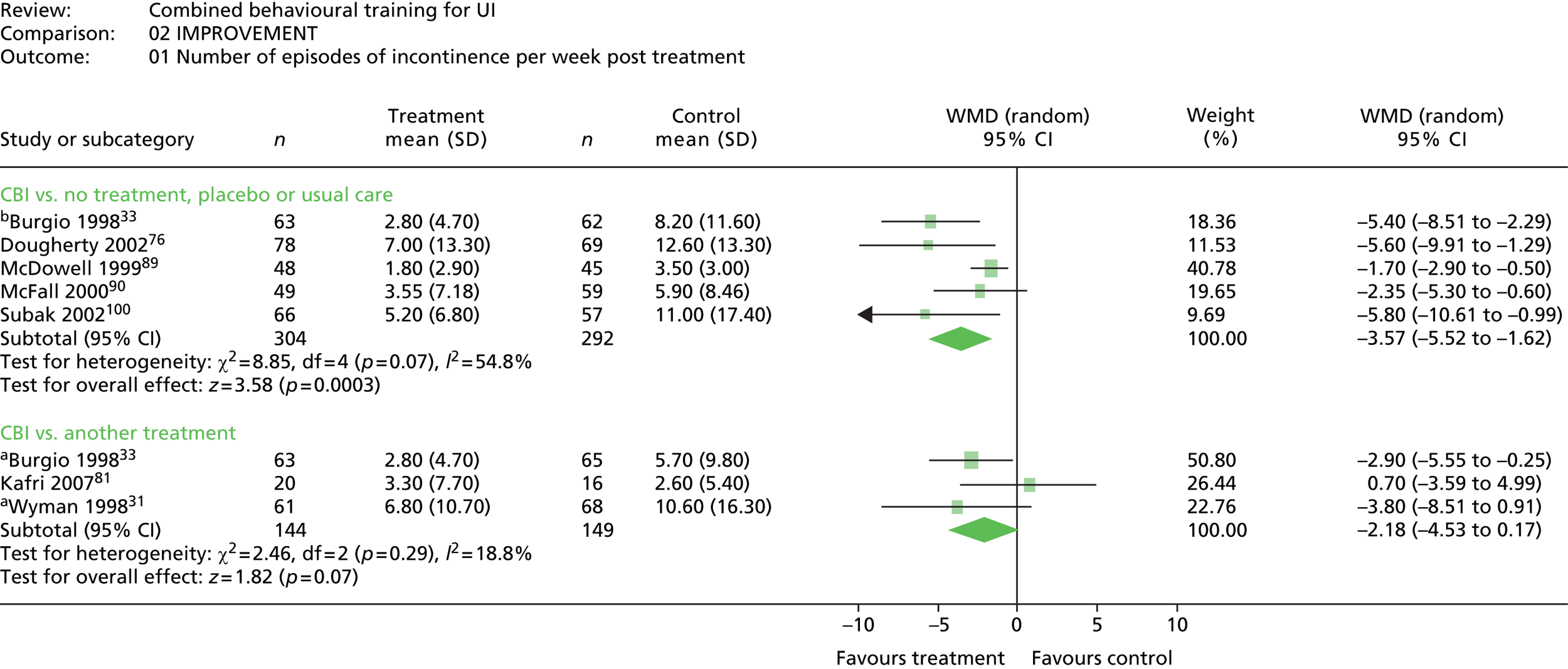
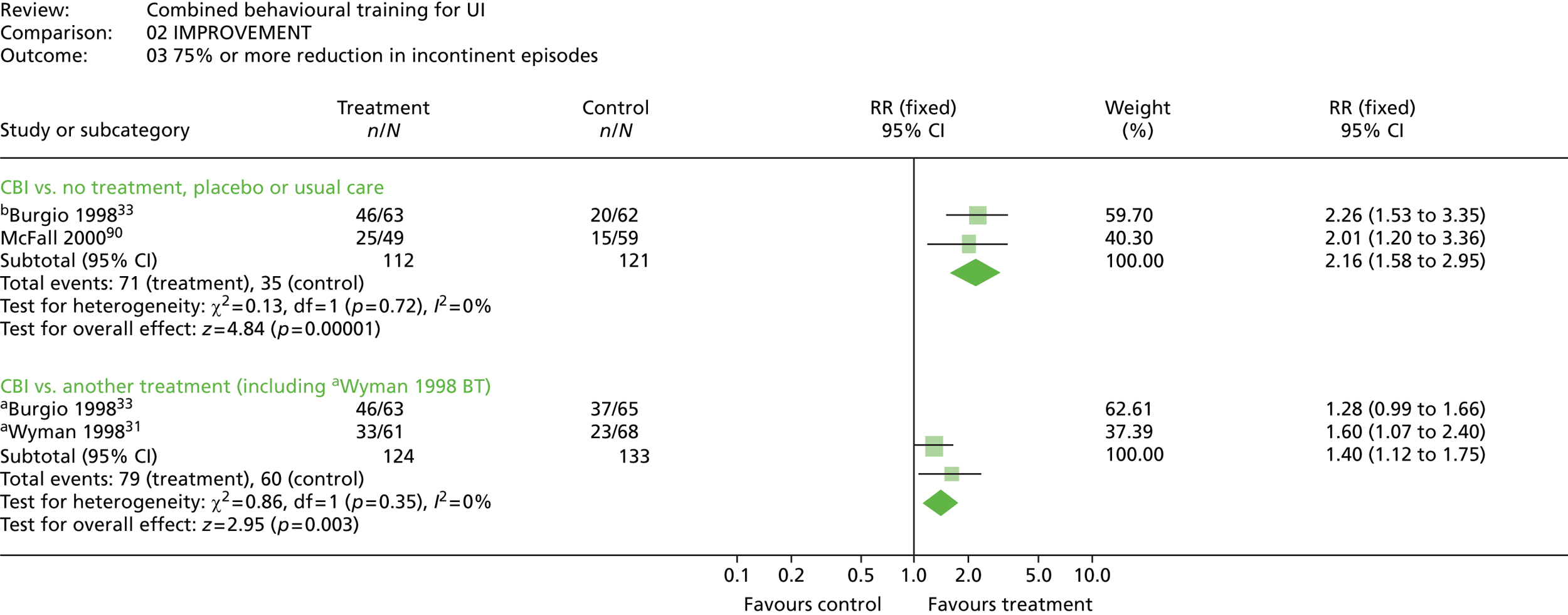
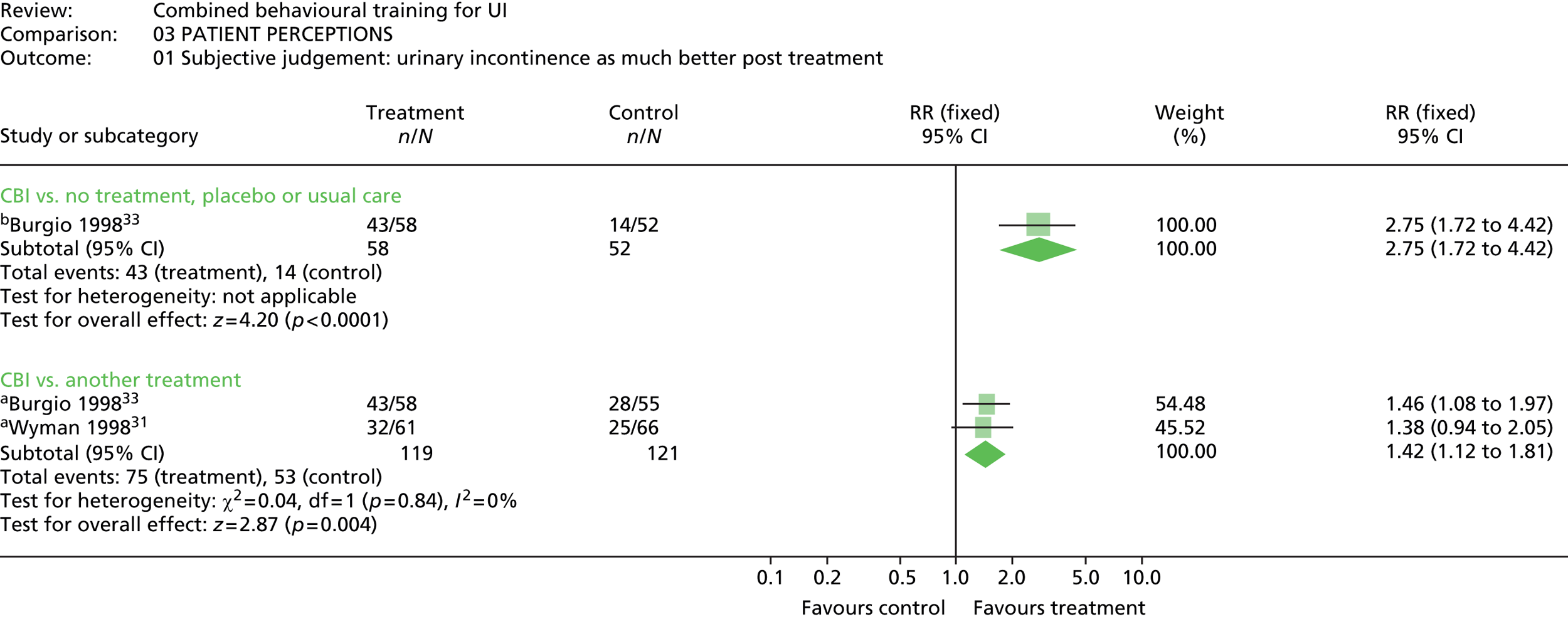
The most common method used to express the degree of improvement in UI was reporting the number of incontinent episodes per day or week. This measure of improvement was included in all trials except Macaulay et al. 87 Three trials also included the participants’ perception of improvement; two using a scale of much better, better, no change, or worse;31,33 and one trial reporting whether the intervention had helped a great deal, moderately, slightly, or not at all. 100
Of the four trials that used a pad test, two did not report data. 31,73 One trial reported grams of urine lost in 24 hours. 76 The same trial also reported a subjective assessment of the severity of urine loss, rated from 1 to 7 from ‘the best bladder control you can imagine’ (1), to ‘the worst bladder control you can imagine’ (7). Another trial71 reported binary data on the number of people with improved (vs. no change or worse) results on a pad test.
Six trials reported urinary symptoms. Five trials reported frequency: four trials reporting frequency of voiding per day or week;76,81,90,100 and one trial reporting number of people reporting urinary frequency as better, unchanged or worse. 71 The same trial reported number of people reporting urinary urgency as better, unchanged or worse; and the number of people reporting nocturia as better, unchanged or worse. Four other trials measured frequency of nocturnal micturition. 33,81,90,100
Five trials included a measure of QoL, but data could not be extracted from one trial because the data were not provided separately for intervention and control groups. 90 Of the remaining four trials, two used the Incontinence Impact Questionnaire (IIQ), which measures symptom distress,31,76 one trial used the Incontinence Quality of Life (Questionnaire) (I-QOL),81 and one trial used the Urogenital Distress Inventory (UDI). 31
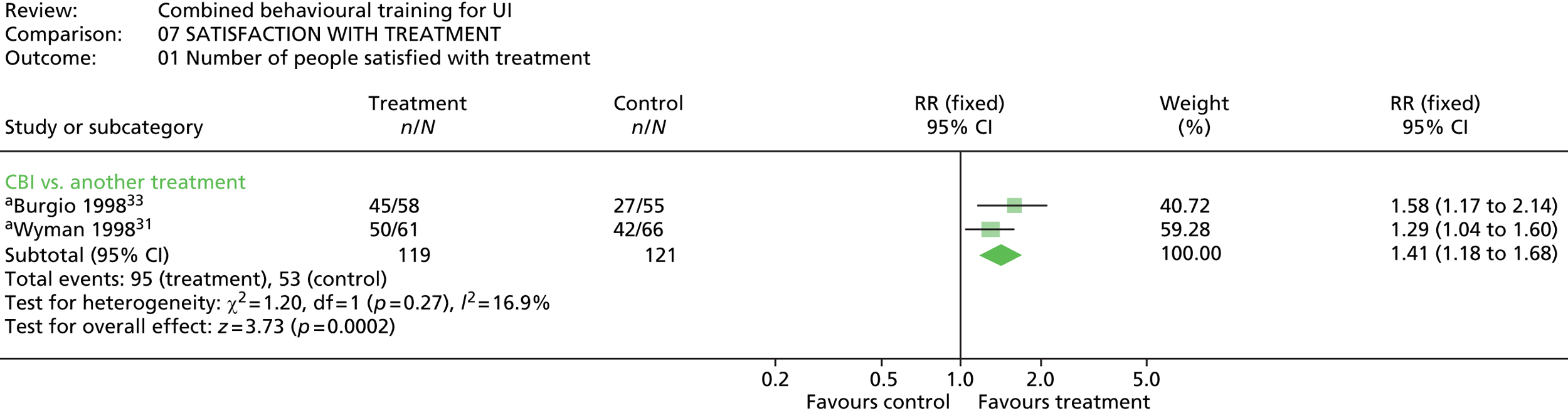
Two trials reported on satisfaction with treatment,31,33 using a four-point scale from not at all satisfied, to very satisfied. One other trial100 asked for participant’s reports on how the behavioural therapy had helped them in dealing with their urine leakage problem (rated not at all, slightly, moderately, or a great deal).
One trial reported total number of adverse events (e.g. discomfort, fatigue, side effects of drugs). 81
Outcome measurement timing
Post-treatment measurement timing was variable (Table 9), with post-treatment measurement at 6 weeks in one trial,100 8–10 weeks in four trials,33,71,89,90 3 months in three trials31,81,87 and 6 months in two trials. 73,76
| Study | < 3 months | 3 months | 6 months | 9 months | 12 months | 15 months | 18 months | 21 months | 24 months |
|---|---|---|---|---|---|---|---|---|---|
| Aslan et al. 200871 | PT (8 weeks) | – | ✓ | – | – | – | – | – | – |
| Bear et al. 199773 | – | – | PT | – | – | – | – | – | – |
| Burgio et al. 199833 | PT (10 weeks) | – | – | – | – | – | – | – | – |
| Dougherty et al. 200276 | – | – | PT | – | ✓ | – | ✓ | – | ✓ |
| Kafri et al. 200781 | – | PT | ✓ | – | – | – | – | ✓ | – |
| Macaulay et al. 198787 | – | PT | ✓ | – | – | – | – | – | – |
| McDowell et al. 199989 | PT (8 weeks) | ✓ | ✓ | ✓ | ✓ | – | – | – | – |
| McFall et al. 200090 | PT (9 weeks) | – | ✓ | – | – | ✓ | – | – | – |
| Subak et al. 2002100 | PT (6 weeks) | – | ✓ | – | – | – | – | – | – |
| Wyman et al. 199831 | – | PT | ✓ | – | – | – | – | – | – |
Follow-up timing also varied, with the most common being 6 months, which was included in seven trials. 31,71,81,87,89,90,100 Long-term follow-up of ≥ 12 months was included in four trials. 76,81,89,90
Quality of included effectiveness studies
The quality of the included studies was assessed against the Cochrane criteria of adequate sequence generation and allocation concealment; completeness of data reporting and blinding for each main class of outcome measure; selective outcome reporting; and any other sources of bias.
Of the 10 studies, three trials had adequate description of the random sequence generation procedure;33,89,100 five trials stated that sequence allocation was random but did not describe the procedure;31,73,76,87,90 and two trials used non-random sequence generation processes, i.e. alternate allocation. 71,81
Allocation was judged to be adequately concealed in one trial,100 unclear in seven trials31,33,73,76,87,89,90 and not adequately concealed in two trials. 71,81
In the three trials that used objective measures of urine loss, blinding of analysts to the results was judged to be unclear in two trials71,73 and adequate in one trial. 76 Blinding of analysts to the results of the bladder diary was judged adequate in two trials,33,100 unclear in five trials71,73,76,87,89 and not adequate in three trials. 31,81,90 In the seven trials that used subjective outcome measurements, blinding of outcome assessors to the results was deemed unclear in two trials76,87 and not adequate in five trials. 31,33,81,90,100
Objective outcome data were judged to be complete in two trials71,73 and not complete in one trial. 76 Data from bladder diaries were judged to be complete in four trials,33,73,89,90 unclear in two trials31,71 and not complete in three trials. 76,81,100 Data from subjective measurements were judged to be unclear in three trials31,33,87 and not complete in four trials. 76,81,90,100
Outcome reporting was judged to be free of the suggestion of selective outcome reporting in five trials,31,33,76,81,87 unclear in one trial89 and selective in four trials. 71,73,90,100
Generalisability of the included effectiveness studies
All except one of the studies were limited to females, with the remaining study being 10% male. 89 The majority of studies were also limited to older women.
All types of incontinence were included, but there are more data relating to participants with UUI (46%) than with MUI (40%) or SUI (14%). Most of the studies were undertaken with participants who had established and moderate to severe incontinence. Only one study related to people with mild or very mild symptoms. 90 This was the only study to recruit mainly from community populations rather than from clinical settings, although a few other trials did include community advertising as an additional route of recruitment.
In general, people with cognitive impairments were excluded, although two trials did not exclude people with cognitive impairment if a carer was available and willing to be involved. 73,76 One specifically targeted older people who were homebound89 and one was undertaken in a nursing home,71 suggesting that behavioural UI interventions could at least be feasible with frailer client groups.
Most of the studies have been undertaken in a North American or Middle Eastern setting, with only one early study in a European setting. However, given that these are mostly clinic or home delivered interventions, there is no reason to believe that they are not transferable.
Description of studies of acceptability and feasibility
Description of studies of client experience
Table 10 lists the studies identified by the search. Eight studies were identified: two studies were unpublished and therefore no data extraction was undertaken. 108,109
| Study | Client | Method | Focus | Uptake | Adherence | Dropout |
|---|---|---|---|---|---|---|
| Johnson et al. 200180 USA (n = 79) |
Frail older adults, UI | Postal survey | UI treatment | ✓ | – | – |
| Milne and Moore 200693 Canada (n = 38) |
Individuals, UI | Qualitative, interviews and focus groups | Self-care strategies | ✓ | ✓ | – |
| O’Dell et al. 200894 USA (n = 25) |
Older women, UI | Qualitative, interviews | Pelvic floor care | ✓ | ✓ | – |
| Hay-Smith et al. 200779 New Zealand (n = 20) |
Women, SUI | Qualitative, interviews | PFMT | – | ✓ | – |
| MacInnes 200892 UK (n = 12) |
Women, SUI | Qualitative, telephone interviews | PFMT | – | – | ✓ |
| Kincade et al. 199983 USA (n = 10) |
Women, UI | Qualitative, interviews | Combined intervention | – | – | ✓ |
Of the remaining six studies, three were completed in the USA,80,83,94 one in Canada,93 one in New Zealand79 and one in the UK. 92
Study type
There are five qualitative studies,79,83,92–94 and one survey. 80 All studies collected data from clients, but Johnson et al. 80 collected data from proxy respondents and also collected data from family members and nursing staff. Four studies used face-to-face interviews79,83,93,94 and one of these studies also used focus groups. 93 One study used telephone interviews92 and one study used postal questionnaires. 80
Participants
The samples for two studies were designed to include both men and women,80,93 the rest included women only. Two studies targeted older adults: community dwelling80 and in residential care. 94 Two studies were specific to women participating in a programme of PFMT for SUI;79,92,110 the remaining studies included people with mixed types of UI.
Kincade et al. 83 included both men and women in the overall study, but only interviewed women who had not completed their programme. One study did not exclude participants who were continent at the time of interview (9/38 participants reported no or rare wetness), but who had experience of self-care strategies;93 and one study included proxy respondents who did not themselves have UI. 80
Interventions
Two studies were not specific to a particular type of behavioural intervention, but included material relevant to uptake of or adherence to behavioural self-care strategies. 93,94 One study elicited preferences for treatment, including behavioural options. 80 Two studies concerned client experiences with PFMT79,92 and one study concerned client experience of a combined intervention using PFMT and BT. 83
Outcomes
Client perceptions or experiences could relate to factors influencing choice or programme uptake; participation, maintenance and adherence during the programme; sustainability in the longer term; or failure/withdrawal from the programme. One study (n = 79) explored the treatment preferences of frail older nursing home residents80 and two studies (n = 22) explored reasons for dropout/withdrawal from treatment. 83,92 The remaining three studies (n = 83) were more wide ranging, covering factors influencing uptake and/or adherence.
Quality of included studies
In the main, sampling methods were clear although one study did not include an explanation of the final sample or reasons for non-response92 and one survey did not provide characteristics of the respondents because proxy respondents were used. 80 In general, analysis of data was poorly described, with four studies providing very little description of the analysis process including how findings were selected and managed. 80,83,92,94 However, findings in these studies are predominantly descriptive. One study referred to a process for testing the validity of interpretation with respondents,94 with two other studies using an external researcher to check coding. 79,93 Two studies explicitly considered the potential for bias in the methods used. 79,94
In summary, two studies met most of the appraisal criteria, where any weaknesses were unlikely to impact on the credibility of findings. 79,93 Two studies had weaknesses mainly in the description of analysis such that weaknesses had the potential to impact on the credibility of the findings. 83,94 The qualitative component in two studies was poorly described. 80,92 However, it should be noted that both of these studies were mainly descriptive in nature.
Generalisability of included studies
The included studies are mostly generalisable only to women. Two studies included men: a study of treatment preferences in residential care80 where no details are given of the sample; and a study of factors impacting on self-care93 that included five men out of 38 respondents.
All of the studies required participants to be cognitively able to participate. In the main, respondents were a staff or self-selected volunteer sample – with the possibility that they would not be representative of the wider population.
Two studies are generalisable to relatively younger women with SUI. 79,92 Two studies are generalisable to older women83,93 with mixed types of incontinence. The study by O’Dell et al. 94 included residents with pelvic floor dysfunction including disorders of urination, defaecation or vaginal prolapse. Twenty-three people out of 25 had UI, but 13 out of 25 also had other problems. Findings are defined by the different conditions and in the main it is clear when findings are referring to UI. Johnson et al. 80 included proxy respondents without UI.
Description of studies of staff experience
Six studies elicited the opinions of staff about aspects of delivering behavioural interventions for UI (Table 11).
| Study | Staff group | Data collection method | Focus |
|---|---|---|---|
| Lekan-Rutledge et al. 199886 | NA, LTC (n = 141) | Questionnaire | PV |
| Remsburg et al. 199997 | Nursing, LTC (n = 88) | Questionnaire | PV |
| Johnson et al. 200180 | Nursing, LTC (n = 66) | Group interviews | Continence care |
| Mather and Bakas 200277 | NA, LTC (n = 31) | Focus groups | Continence care |
| Dingwall and McLafferty 200675 | Nursing, acute (n = 63) | Focus groups, interviews | Continence care |
| Resnick et al. 200698 | Nursing, LTC (n = 38) | Focus groups | Continence care |
One of the studies80 is also included in the client experience section. Five studies were completed in long-term care (LTC) facilities in the USA77,80,86,97,98 and one in acute care in the UK. 75 Two of the studies were within the last 3 years,75,98 four studies were conducted between 12 and 16 years ago. 77,80,86,97
Two studies used questionnaires to collect data;86,97 four studies used group interviews or focus groups, with two of these studies also using a small number of individual interviews. 75,80 All of the studies collected data from nurses, four studies collected data from mixed grades of nursing staff, with two studies specific to nursing assistants (NAs). 77,86 Two studies86,97 were undertaken to elicit the views of staff about a PV intervention that they had participated in; the remaining four studies were about views on aspects of general continence care that could include behavioural intervention. 75,77,80,98
Quality of included studies
Four studies collected qualitative data. 75,77,80,98 Sample selection was adequately described in all studies, but two studies provided insufficient detail of the final sample. 80,98 All studies adequately detailed data collection methods, but analysis processes were only adequately detailed by one study. 98 Results were clearly presented in all studies, but methods of testing credibility of the findings were not described in two studies. 75,98
Two studies collected quantitative data. 86,97 Both studies adequately described sample selection, but details of the final sample were insufficient in Remsburg et al. ’s study. 97 Methods of data collection and analysis were insufficiently described in both studies.
Generalisability of included studies
In the main, findings are most relevant to nursing staff working in LTC settings in the USA. 77,80,86,97,98 There is only one study relevant to acute care in the UK. 75 However, most studies are reporting barriers to the provision of adequate continence care (including forms of behavioural intervention) to older people.
Description of studies measuring predictors of treatment adherence or outcome
Table 12 lists the 16 studies identified by the search. There were 13 studies where the primary focus was analysis of predictors and three RCTs from the effectiveness review that also included regression testing for moderators of outcome. Three studies were unpublished and did not progress to data extraction. Two linked studies,69,70 considered as one because they relate to the same sample, measured intention to adhere and long-term adherence at different time points.
| Study | Type of intervention | Type of analysis | Dependent variable | Unpublisheda |
|---|---|---|---|---|
| Alewijnse et al. 2001,69 200370 | Combined | M | A | – |
| Baigis-Smith et al. 198972 | Combined | U | O | – |
| Burgio et al. 200374 | Combined | M | O | – |
| Chen 2001 | Single (PFMT) | M | A | ✓ |
| Gerard 199778 | Combined | U | O | – |
| Kartha 1989 | Combined | M | A | ✓ |
| Kincade et al. 200184 | Combined | U | A | – |
| McDowell et al. 199288 | Combined | U | O | – |
| McDowell et al. 199989 | Combined | M | O | – |
| Oldenburg and Millard 198695 | Combined | M | O | – |
| Rose et al. 199099 | Combined | U | O | – |
| Shishani 2003 | Single (PFMT) | M | A | ✓ |
| Subak et al. 2002100 | Combined | M | O | – |
| Svengalis et al. 1995101 | Single (PFMT) | U | A | – |
| Tadic et al. 2007102 | Combined | M | O | – |
| Wyman et al. 199831 | Combined | M | O | – |
Of the 13 studies that progressed to data extraction, seven studies used multivariate analysis,31,69,70,74,89,95,100,102 one measured predictors of adherence69,70 and six measured predictors of outcome. 31,74,89,95,100,102 Six studies used univariate analysis only:72,78,84,88,99,101 two measuring predictors of adherence,84,101 and four measuring predictors of outcome. 72,78,88,99
Only descriptive data on the variables tested were extracted from univariate analyses, as univariate analysis does not provide robust information on independent predictors. However, it is useful to know which predictors have been selected and tested as potentially predictive, to understand what the confirmed independent predictors have been selected from and compared against. Therefore, variables included in all univariate analyses are described separately in the results.
Table 13 summarises the main details of the studies using multivariate analysis to predict different dependent variables, including adherence, improvement in UI, cure and QoL. The predictors of each independent variable will be considered in turn.
| Study | Study design | Dependent variable | Client group/setting |
|---|---|---|---|
| Alewijnse et al. 2001,69 200370 (Netherlands) | CS, RCT (n = 129) | Adherence | F |
| Burgio et al. 200374 (USA) | RCT (n = 197) | Cure (n = 49) Improvement (n = 128) |
F, aged ≥ 55 years, UUI |
| McDowell et al. 199989 (USA) | RCT (n = 105) | Improvement (n = 105) Responder vs. non-responder (NS) |
M/F, aged ≥ 60 years, home-bound |
| Oldenburg and Millard 198695 (Austraila) | CT (n = 53) | Improvement | F, UUI |
| Subak et al. 2002100 (USA) | RCT (n = 152) | Improvement | F, aged ≥ 55 years |
| Tadic et al. 2007102 (USA) | RCT (n = 42) | QoL | F, aged ≥ 60 years, UUI |
| Wyman et al. 199831 (USA) | RCT (n = 204) | Cure (n = 62) Improvement QoL |
F |
Predictors of treatment adherence
Two linked studies undertaken in the Netherlands measured predictors of different aspects of adherence in the same sample (n = 129) at different time points. 69,70
Study type
Alewijnse et al. 69 is a cross-sectional study undertaken on a sample of women from primary care who self-reported problems with continence. Alewijnse et al. 70 reports data from women who subsequently agreed to participate in a RCT.
Participants
Participants were women recruited from 23 practice registers in the Netherlands between 1995 and 1998, selected by a recorded risk factor for UI, and who then self-reported UI. Women unable to fill out questionnaires, or those suffering from neurological conditions, were excluded.
Interventions
The behavioural intervention consisted of PFMT on an individual basis with a physiotherapist, together with a self-help guide modelled on health education theory.
Outcomes
The outcome measured in the first study69 was ‘intention to adhere’, measured by two questions: ‘Do you intend to adhere to the exercise advice?’ and ‘Do you intend to exercise every day?’, using a seven-point scale and summed to form one score.
The outcome measured in the second study70 was long-term adherence behaviour, measured by self-report in a 7-day diary of number of days per week women had followed the physiotherapist’s behavioural advice, categorised as optimal, moderate or poor adherence, and validated by three items in a self-report questionnaire.
Time points of outcome measurement
Measurement of intention to adhere in the first study was prior to the trial, and measurement of long-term adherence in the second study was 1 year post treatment.
Predictor variables
Variables included in multivariate analysis for prediction of intention to adhere or long-term adherence behaviour were:
-
physiological: severity of UI, type of UI
-
general health: subjective general health
-
psychological: health perceptions; history of sexual abuse after 18 years of age; health knowledge; sex education at school; self-efficacy (abilities and difficulties); attitudes (pros and cons); pre-trial intention to adhere; self-report of adherence behaviour during treatment
-
social: social norms (normative beliefs of important persons about PFMT); social modelling (how many other women known with PFMT experience); social support (how many other women discussed UI and therapy); social demands (hours per week paid labour).
Quality of included studies
The first study69 was cross-sectional with data on predictors and outcome from self-report, collected at the same time point, including UI type and severity. Ordering of questions is unclear. The selection of predictor and outcome variables is model based, with clear definition of variables but the authors acknowledge some problematic measurement issues. Sample size was sufficient for the reduced number of variables included in multivariate analyses, but not for the number of variables included in the initial univariate model. Analysis was judged inadequate because of choice of statistical tests for data type, and a potentially inappropriate approach to adjustment for confounders.
The second study70 had the same problems of measurement of predictor variables, but outcome variable definition and measurement was stronger. Sample size was again sufficient for multivariate analysis but not for the number of variables included in the univariate model. There was 80% follow-up from the original trial cohort, but more than 20% loss to analysis from outliers and missing data, with only 75 out of 103 included in the multivariate analysis.
Generalisability of included studies
Both studies were women (only) with self-reported UI and who agreed to participate in a behavioural intervention trial.
Predictors of treatment outcome
Six studies were included: one from more than 20 years ago,95 four over 10 years old,31,74,89,100 and one from the past 10 years. 102 Five studies were from the USA31,74,89,100,102 and one from Australia. 95 There were 814 participants in the six studies for whom data were available. Two studies89,100 do not report separate results according to type of incontinence (n = 257); the remaining studies reported results separately for participants with SUI (n = 205) and urinary urgency/UUI or detrusor instability (n = 352).
Study type
One study was a clinical trial of a behavioural treatment programme95 and the remaining five were analyses within RCTs of behavioural training. 31,74,89,100,102
Participants
Only one study included both men and women. 89 These participants were older (mean age 76 years) housebound people. The other five trials were all limited to female participants, with two studies reporting samples with a mean age > 70 years,100,102 two studies having samples with a mean age 60–70 years,31,74 and Oldenburg and Millard95 having a much younger sample with a mean age of 43 years.
Type of UI was urodynamically confirmed in four studies. 31,74,95,102 The remaining two studies diagnosed UI type by history. 89,100 Two studies present separate results for women with SUI and UUI,31,74 two studies present results for women with UUI only95,102 and two studies present results for MUI types. 89,100
The definition of UI differed slightly between studies. Three studies specified a minimum of two episodes of UI per week74,89,102 and two studies specified one episode of UI per week31,100 (as minimum for inclusion). One study did not define minimum standards, instead referring to participants suffering from ‘excessive frequency and urgency of micturition’. 95 All studies required participants to be mentally/cognitively intact or able to participate.
Interventions
All trials used PFMT with some degree of BIO. An exception was Subak et al. ’s trial,100 where the low intensity intervention provided verbal and written instruction on exercises. All trials except Burgio et al. 74 and Tadic et al. 102 included BT, and three trials74,89,102 also include stress and/or urge strategy training.
Outcomes
Two studies reported cure of UI (defined as 100% reduction in UI episodes and measured using self-report bladder diaries). 31,74
All six studies measured degree of improvement using self-report bladder diaries: two studies defined improvement as ≥ 75% reduction in incontinent episodes;31,74 two studies89,100 measured percentage reduction in UI episodes; and McDowell et al. 89 also classified people as responders (> 0% improvement) and non-responders (0% improvement). Oldenburg and Millard95 reported patient rating of degree of improvement in UI (defining success as cure or significant improvement) and patient rating of severity of urological symptoms [defining scores 1 standard deviation (SD) above the group mean as failure and scores less than this as success]. Severity was measured using a Bladder Symptom Score (no further details).
All studies included a post-treatment measure of outcome. One study95 only included therapist perception of outcome post treatment, but used all patient-derived measurement at 18 months post treatment. Wyman et al. 31 also included measurement at 3 months post-treatment.
Variables entered into multivariate analyses as predictors of treatment outcomes for CBIs are detailed in Table 14.
| Variable category | Indicator | Oldenburg and Millard 198695 | Wyman et al. 199831 | McDowell et al. 199989 | Burgio et al. 200374 | Subak et al. 2002100 | Tadic et al. 2007102 |
|---|---|---|---|---|---|---|---|
| Sociodemographic | Sex | ✓ | |||||
| Education | – | – | ✓ | ✓ | – | – | |
| Physiological | Severity of UI | ✓ | ✓ | ✓ | ✓ | – | |
| Symptom severity | ✓ | – | – | – | – | – | |
| Type of UI | ✓ | – | ✓ | ||||
| Duration of UI | ✓ | – | – | – | – | – | |
| Bladder capacity | – | – | – | ✓ | – | – | |
| Previous treatment | ✓ | – | – | ✓ | – | – | |
| Health/functional | Medical history: arthritis | – | – | – | ✓ | – | – |
| Uses assistive device | – | – | ✓ | – | – | – | |
| Self-care: functional status | – | – | ✓ | – | – | – | |
| Caregiver requirement | – | – | ✓ | – | – | – | |
| Psychological | Adherence | ✓ | – | ✓ | – | – | – |
| Psychological problems | ✓ | – | ✓ | – | – | ✓ | |
| Perceptions of seriousness | ✓ | – | – | – | – | – | |
| Perceptions of control | ✓ | – | – | – | – | – | |
| Social | Lives alone/with others | – | – | ✓ | – | – | – |
All studies except two89,102 included the major predictor variable of severity of UI. McDowell et al. 89 was the only study not limited to one type of UI or analysed in subgroups according to type of UI. Previous treatment was included in two studies,74,95 and duration of UI95 and bladder capacity74 in one study.
One study included an aspect of medical history (arthritis)74 and one study included measures of functional ability or independence. 89
Two studies that included measures of adherence and measures of psychological problems as predictor variables89,95 investigated the impact of history of depression and current depressive status on QoL and UI improvement.
Only the study by McDowell et al. 89 with older housebound people included a social predictor of lives alone/with others.
Participant selection and characteristics were clear in all studies except Oldenburg and Millard. 95 The rationale for selection of predictors was unstated in all studies, although all except McDowell et al. 89 and Tadic et al. 102 included the major variables of type/severity of UI. Definition and measurement of predictor variables was clear in four studies. 31,89,100,102 In the other two studies, descriptive details and evidence of validity and reliability were lacking for some measurements, or some variable parameters were not clearly specified. 74,95 Outcome measurement was clear in four studies31,74,100,102 and unclear in two studies. 89,95 No study described blinding of predictor and outcome measurement, although one study indicated that analysts were blinded. 100
Predictor variables were present in a significant proportion of the population in all studies, except Tadic et al. 102 Sample size was inadequate for the number of variables entered into multivariate analysis in two studies. 89,95 The number lost to follow-up and/or reasons for dropouts are not reported in three trials. 74,95,100 All studies used appropriate statistical tests except Oldenberg and Millard. 95 Three trials accounted for important confounders,31,74,100 but only two trials provided data on the precision of estimates in the analysis. 74,89
In summary, only two trials had a low number of design flaws that were unlikely to impact on internal validity. 31,100 One trial from the 1980s95 was considerably flawed, such that results were unlikely to be valid. The remaining studies had significant weaknesses in either variable definition, sample size or confounding, such that internal validity was also likely to be compromised to some extent.
Findings: studies of effectiveness
Results are presented for primary outcomes and then secondary outcomes. For each outcome, post-treatment results will be presented, then results for follow-up to 12 months. Results of the subgroup and sensitivity analyses will be presented at the end of the section.
For each outcome, results are split into subtotals for:
-
comparisons against no treatment, usual care, placebo or attention control
-
comparisons against another treatment.
Subtotals are not pooled to give an overall treatment effect, as these were thought likely to differ between no treatment and another treatment comparisons.
To avoid repetition, ‘no treatment’, placebo or usual care comparison groups are described using the generic term ‘no treatment control’. The term also includes waitlist or attention control groups. The comparisons in each trial are described in Description of included studies.
Two trials31,33 have three arms and therefore include two different intervention–comparison pairs. To avoid including the same trial twice in a pooled effect, they have been dealt with as follows.
The trial by Burgio et al. 33 includes two comparison groups: an attention control comparison; and another treatment comparison (drug). These two comparison groups will be pooled separately in the comparison subgroups above, so will not be counted twice.
In the trial by Wyman et al. ,31 there are two comparison groups against another treatment (i.e. CBI vs. BT or PFMT). To avoid overinflation of the pooled effect, the comparison least favourable to the combined intervention on the primary outcome has been selected for inclusion, i.e. the comparison against BT (referred to in the forest plots as aWyman 199831). This choice will be carried through all analyses. However, if the comparison with PFMT (referred to in the forest plots as bWyman 199831) would be less favourable to the combined intervention for any particular outcome, this will be given preference to preserve the most conservative estimate of treatment effect. Although the difference in treatment effect for the two comparison groups was usually very small, a sensitivity analysis including the other comparator was always undertaken, and is presented where influential.
Results are presented for unfavourable events in the main. A reduction in unfavourable events is a positive treatment impact. This is graphically displayed to the left of a forest plot (see Figure 1). However, three outcomes are presented as a gain in favourable events (i.e. degree of improvement, subjective perceptions of improvement and satisfaction with treatment). For these outcomes, a positive treatment impact is displayed to the right of the forest plot (see Figures 4, 5 and 15). The exception is QoL which is displayed as a reduction (i.e. to the left of the forest plot, because most QoL scales are scaled so that lower scores are better). QoL scores which do not follow this rule, for example I-QOL have been multiplied by –1 so that they can be pooled. Favourable results for QoL are therefore displayed to the left of a forest plot (e.g. less impact of incontinence on QoL, less symptom distress).
Results are presented as:
-
RR for binary (dichotomous) outcomes (e.g. continent/not continent)
-
WMD for continuous data (e.g. grams of urine lost, number of incontinent episodes)
-
SMD for continuous data where outcomes have been measured using different scales (e.g. QoL)
-
SE when GIV has been used to pool binary and continuous outcomes.
All results are presented with 95% CIs.
Interpretations of value have been made as follows.
Effect sizes are statistically significant if p ≤ 0.05, and are described as marginally statistically and non-significant from p = 0.05 to 0.07. This small range was chosen for clarity, but it should be noted that many of the p-values for treatment effects are below p = 0.10 and p-values in this range are therefore reported for information.
Standardised mean differences and SEs are categorised as small (≤ 0.34), moderate (0.35–0.65) and large (> 0.65), based on Cohen’s108 rules of thumb guidance from the social sciences of a SMD of 0.20 as a small effect, 0.50 as a moderate effect and 0.80 as a large effect.
Primary outcome
Cure
Number of people remaining incontinent (post treatment)
Three trials31,33,90 gave results for the proportion of participants remaining incontinent at post treatment, by self-report in 1- or 2-week bladder diaries (i.e. not achieving 100% reduction in incontinent episodes). The pooled results are presented in Figure 1.
For two trials33,90 with no treatment comparisons (n = 275, data available for 85%), the pooled effect was statistically significant, favouring the CBI (RR 0.81, 95% CI 0.70 to 0.94).
For two trials31,33 with alternative treatment comparisons (n = 267, data available for 96%), the results were marginally statistically non-significant (RR 0.87, 95% CI 0.75 to 1.01; p = 0.06).
Number of people remaining incontinent (follow-up to 12 months)
Wyman et al. 31 (n = 204, data available for 91%) reported the proportion of participants not achieving continence at 3 months post treatment (6 months post baseline). Results were not statistically significant (RR 0.87, 95% CI 0.72 to 1.05).
Secondary outcomes
Improvement
Number of incontinent episodes per week (post treatment)
Eight trials31,33,73,76,82,89,90,100 including nine intervention comparison pairs reported the number of episodes of incontinence per week at post treatment (between 6 and 12 weeks), measured by self-report in 1- or 2-week bladder diaries (except two trials73,76 where 3-day diaries were used). Bear et al. 73 did not provide data suitable for pooling. The results of the remaining eight studies are summarised in Figure 2.
Five trials33,76,89,90,100 included a no treatment comparison (n = 750, data available for 79%). Pooled results show a statistically significant mean reduction in episodes of incontinence per week in CBI trials (WMD –3.57, 95% CI –5.52 to –1.62).
Three trials (n = 309, data available for 95%) included comparison against another intervention. 31,33,81 Pooled results were marginally statistically non-significant (WMD –2.18, 95% CI –4.53 to 0.17; p = 0.07).
Number of incontinent episodes per week (follow-up to 12 months)
Three trials31,76,81 reported follow up data (Figure 3). One trial with a no treatment comparison76 reported statistically significant results favouring the CBI at 6 months post treatment (12 months post baseline) (WMD –5.60, 95% CI –9.92 to –1.28).
FIGURE 3.
Number of incontinent episodes: follow-up to 12 months. aWyman 199831 = combined intervention vs. BT. df, degrees of freedom.
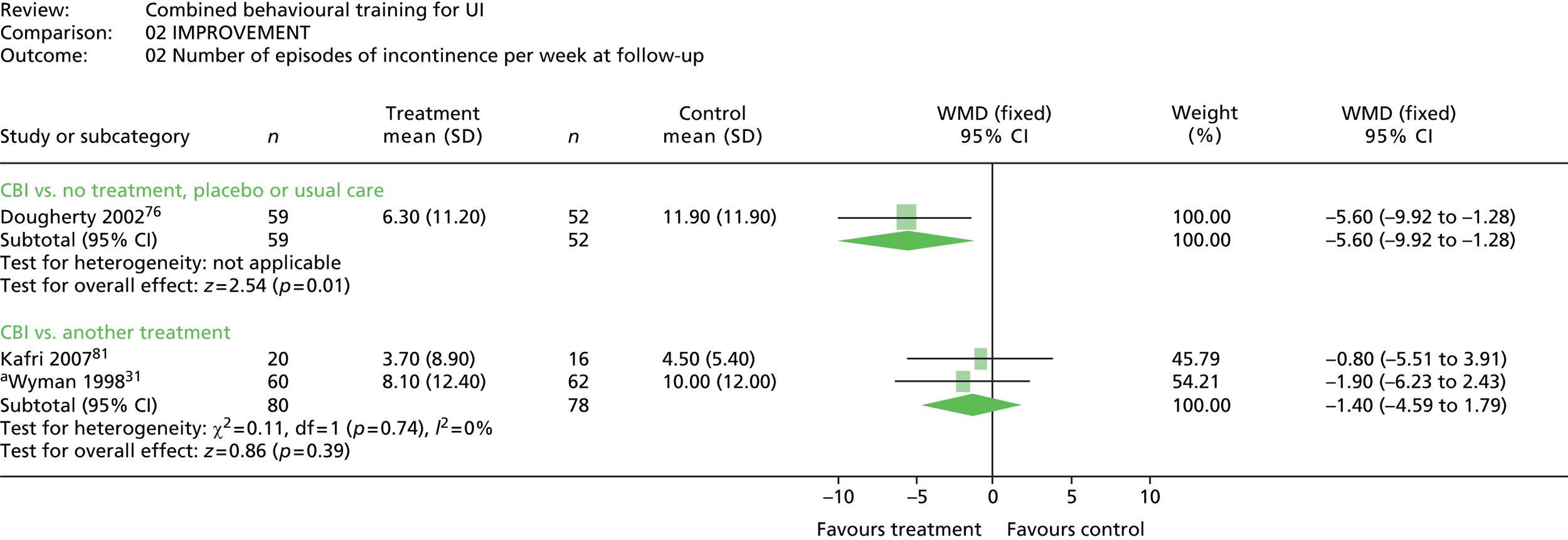
Two trials31,81 with comparisons against other treatments (n = 179, data available for 88%) report results for 3 months post treatment (6 months post baseline). The pooled effect was not statistically significant (WMD –1.40, 95% CI –4.59 to 1.79).
Proportion of people achieving 75% or more reduction in incontinent episodes (post treatment)
Three trials31,33,90 with four relevant intervention comparison pairs gave results for the proportion of participants achieving 75% or more reduction in incontinent episodes at post treatment, by self-report in 1- or 2-week bladder diaries (see Figure 4).
For two trials33,90 including no treatment comparisons (n = 275, data available for 85%), the pooled effect was statistically significant favouring the CBI (RR 2.16, 95% CI 1.58 to 2.95).
Two trials31,33 included another treatment comparison. The pooled effect (n = 265, data available for 97%) was statistically significant favouring the CBI for the comparison including the Wyman et al. 31 BT comparison group (RR 1.40, 95% CI 1.12 to 1.75; as illustrated in Figure 4); but statistically non-significant with the inclusion of the Wyman et al. 31 PFMT comparison group (RR 1.60, 95% CI 0.94 to 2.73; p = 0.08).
Proportion of people achieving 75% or more reduction in incontinent episodes (follow-up to 12 months)
One of the trials31 also reported results for 75% or more reduction in incontinent episodes at 6 months post baseline (3 months post treatment). The effect size (n = 204, data available for 92%) was statistically significant favouring the CBI for the comparison with BT (RR 1.79, 95% CI 1.16 to 2.78); but not statistically significant for the comparison with PFMT (RR 1.32, 95% CI 0.92 to 1.91).
Subject perceptions of improvement
Post treatment
Two trials31,33 with three relevant comparison groups gave results for the proportion of participants who classified their incontinence as ‘much better’ at post treatment, by self-report on four- or five-point scales. Results are summarised in Figure 5.
One trial33 reported comparison against a placebo control group (n = 130, data available for 85%), with a statistically significant effect size favouring the CBI (RR 2.75, 95% CI 1.72 to 4.42).
Two trials31,33 included comparisons against another treatment (n = 265, data available for 91%). The pooled effect was also statistically significant favouring the CBI (RR 1.42, 95% CI 1.12 to 1.81).
Follow-up to 12 months
One trial31 comparing a CBI with either BT or PFMT (n = 204, results available for 90%) gave results for patient perception of UI as ‘much better’ at 6 months post baseline (3 months post treatment). The effect size was just statistically significant for the BT comparison group favouring the CBI (RR 1.53, 95% CI 1.00 to 2.32; p = 0.05); but not statistically significant for the PFMT comparison group (RR 1.43, 95% CI 0.96 to 2.12; p = 0.08).
Severity of incontinence
Grams of urine lost in 24 hours (post treatment)
Four studies used a pad test to evaluate severity of urine loss31,71,73,76,109 (Wyman et al. 31 reported in Elser et al. 109). However, Elser et al. 109 did not report data separately for each treatment group, and Bear et al. 73 reported mean grams of urine loss per day, but no SDs. Results for the remaining trials using no treatment comparison groups71,76 are illustrated in Figure 6.
FIGURE 6.
Severity of incontinence (grams of urine loss in 24 hours): post treatment. df, degrees of freedom.
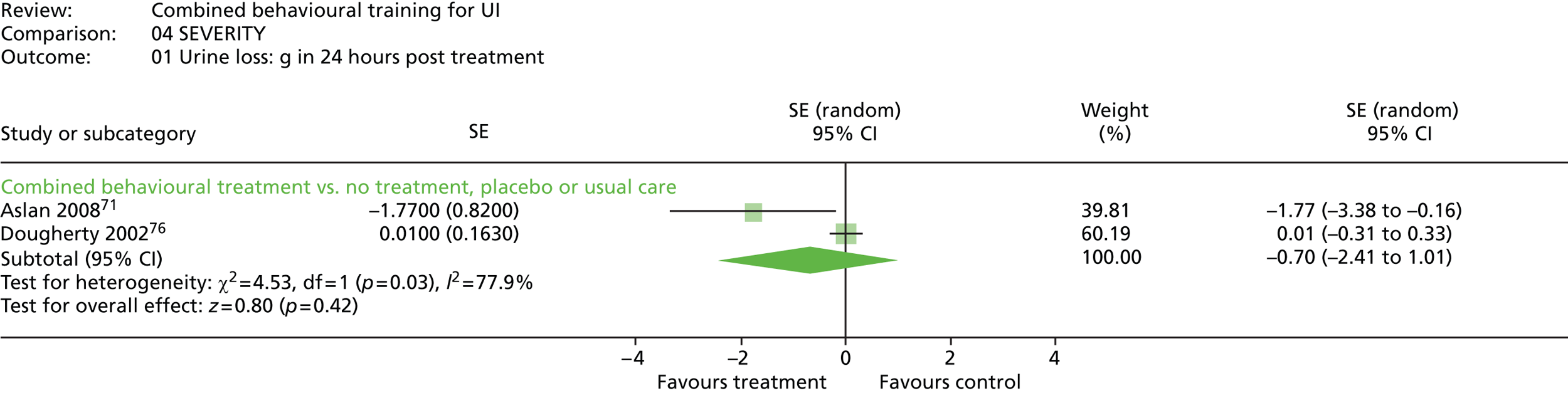
Results were pooled using GIV and a random-effects model given the substantial heterogeneity of treatment effect (I2 = 77.9%) (see Figure 6). The result was not statistically significant (SE –0.70, 95% CI –2.41 to 1.01).
Dougherty et al. 76 also provided a subjective measure of severity of urine loss, rated 1–7, with 7 defined as ‘the best bladder control you can imagine’ and 1 defined as ‘the worst bladder control you can imagine’. Treatment effects were significantly different between experimental and control conditions favouring the CBI (SMD –1.21, 95% CI –0.86 to –1.56).
Grams of urine lost in 24 hours (follow-up to 12 months)
The same two trials included follow-up data: Dougherty et al. 76 at 12 months post baseline (6 months post treatment) and Aslan et al. 71 at 6 months post baseline (4 months post treatment).
The pooled effect (Figure 7) using GIV and a fixed-effects model was statistically significant favouring the CBI (SE –0.43, 95% CI –0.80 to –0.06). However, by using a random-effects model to facilitate comparison at post treatment and follow-up, the pooled effect was not statistically significant (SE –0.60, 95% CI –1.47 to 0.26).
FIGURE 7.
Severity of incontinence (grams of urine loss in 24 hours): follow-up to 12 months. df, degrees of freedom.
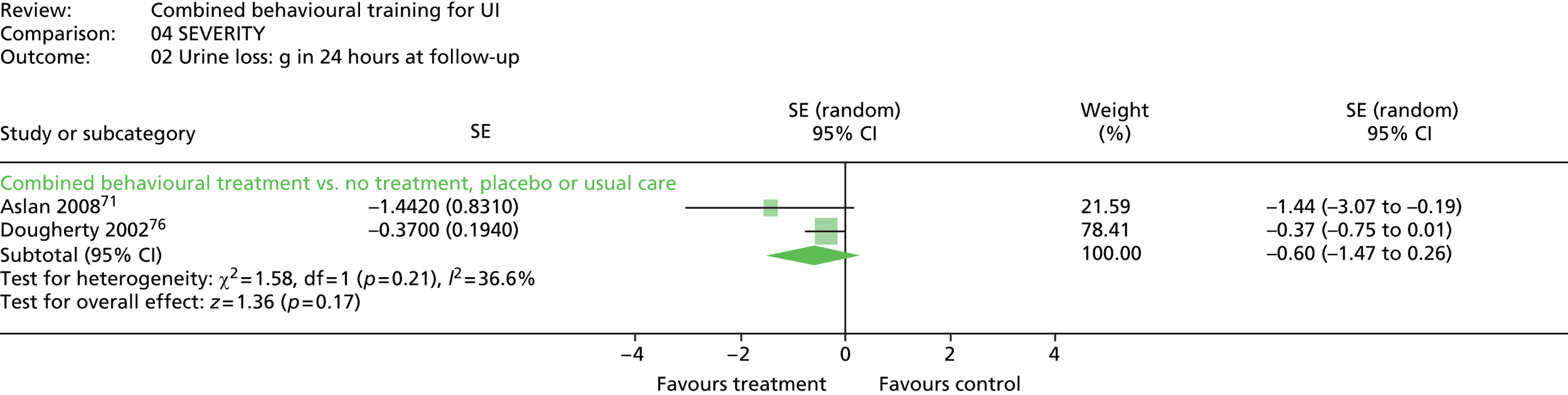
Symptoms
Urinary frequency (post treatment)
Five studies provided data on the number of voids during the day (Figure 8). Four studies (n = 579, data available for 75%) compared a CBI with no treatment. 71,76,90,100 GIV was used to combine dichotomous outcomes from Aslan et al. 71 with the continuous outcome data from the other three trials. Using a random-effects model given the substantial heterogeneity of treatment effects (I2 = 74.9%), the pooled result was statistically significant favouring the CBI (SE –0.55, 95% CI –0.97 to –0.13).
FIGURE 8.
Frequency (number of voids during the day): post treatment. df, degrees of freedom.

The result for one quasi-randomised trial81 comparing a CBI against GIV was used to combine dichotomous outcomes from Aslan et al. 71 with the continuous outcome another treatment (n = 44, data available for 82%) was not statistically significant (SE –0.04, 95% CI –0.70 to 0.62; WMD –0.10, 95% CI –1.83 to 1.63).
Urinary frequency (follow-up to 12 months)
Three studies reported follow-up results for frequency of micturition during the day. Two studies (n = 282, data available for 57%) compared combined behavioural training against a no-treatment control (Figure 9).
FIGURE 9.
Frequency (number of voids during the day): at follow-up to 12 months. df, degrees of freedom.
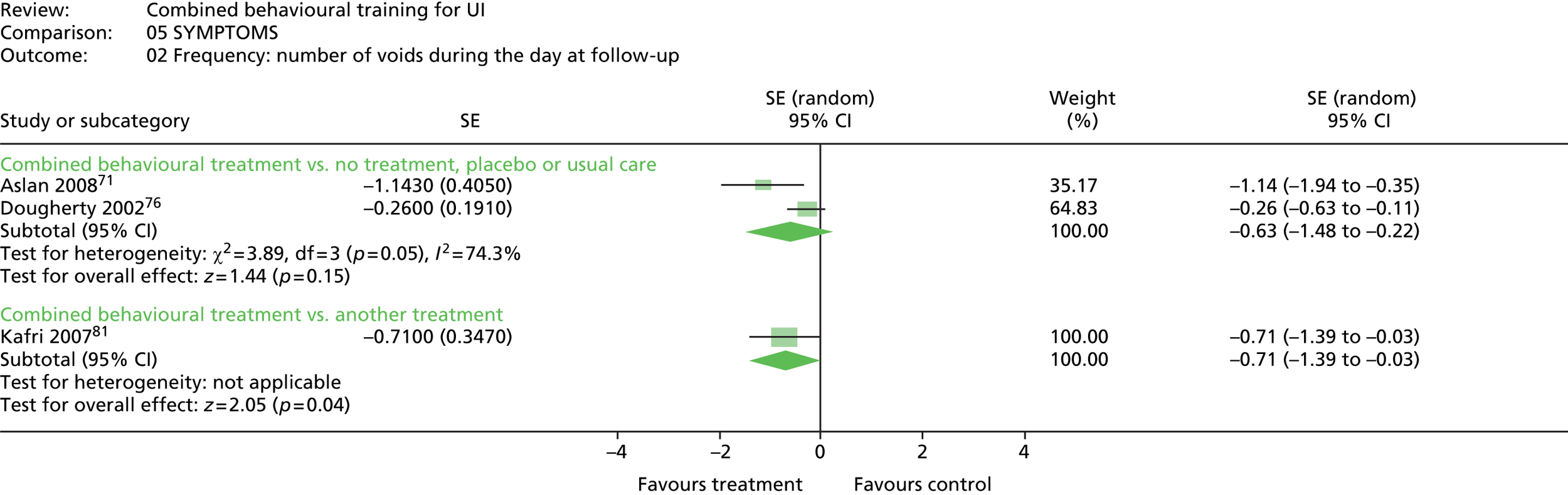
Aslan et al. 71 reported data for 6 months post baseline (4 months post treatment). Dougherty et al. 76 reported data for 12 months post-baseline (6 months post treatment). Using a random-effects model given the substantial heterogeneity of treatment effects (I2 = 74.3%), the pooled effect was not statistically significant (SE –0.63, 95% CI –1.48 to 0.22).
In a quasi-experimental study comparing a CBI against medication, Kafri et al. 81 reported a statistically significant treatment effect favouring the CBI for 6 months post-baseline (3 months post treatment) (SE –0.71, 95% CI –1.39 to –0.03; WMD –1.70, 95% CI –3.26 to –0.14).
Nocturia (post treatment)
Six trials reported results for the number of voids during the night. The results for five trials33,71,76,90,100 using a no treatment comparison are illustrated in Figure 10.
Generic inverse variance was used to combine dichotomous and continuous outcomes, and substantial heterogeneity of treatment effect (I2 = 89.5%) necessitated the use of a random-effects model. Pooled results (n = 709, data available for 72%) were not statistically significant (SE –0.33, 95% CI –0.95 to 0.29).
Two trials33,81 compared a CBI against another treatment (drug therapy). Results (n = 176, data available for 73%) were statistically significant favouring the CBI (SE –0.46, 95% CI –0.81 to –0.11; WMD –0.36, 95% CI –0.67 to –0.04).
Nocturia (follow-up to 12 months)
Three trials reported data for nocturia at follow-up. 71,76,81
Two trials reported no treatment comparisons: Aslan et al. 71 reported results for 6 months post baseline (4 months post treatment); Dougherty et al. 76 reported results for 12 months post baseline (6 months post treatment). Results are illustrated in Figure 11.
FIGURE 11.
Nocturia (number of voids during the night): follow-up to 12 months for no treatment comparisons. df, degrees of freedom.
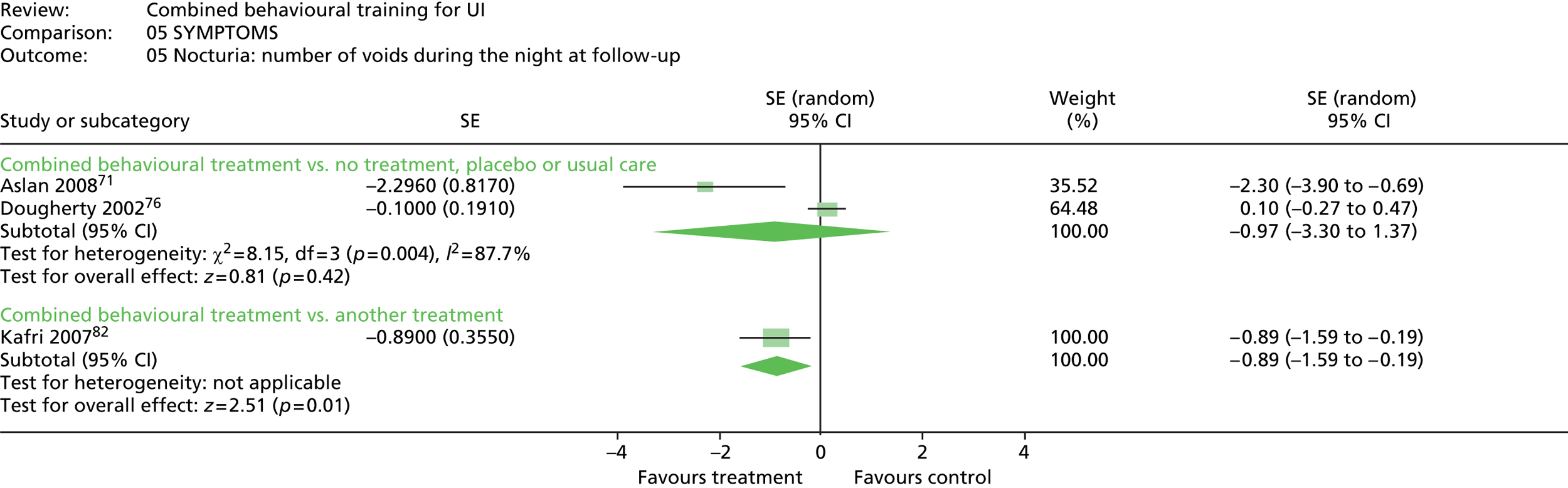
Pooled results (n = 282, data available for 59%) were not statistically significant (SE –0.97, 95% CI –3.30 to 1.37).
One quasi-randomised trial81 compared combined behavioural training with another treatment. Results (n = 44, data available for 82%) at 6 months post baseline (4 months post treatment) were statistically significant favouring the CBI (SE –0.89, 95% CI –1.59 to –0.19; WMD –1.00, 95% CI –1.75 to –0.25).
Urgency (post treatment)
Only one quasi-randomised trial71 reported results for urinary urgency (n = 64, data available for 78%). The number of people reporting that symptoms of urgency were unchanged or worse was statistically significant post-treatment favouring the CBI, compared with a no treatment control group (RR 0.57, 95% CI 0.37 to 0.89).
Urgency (follow-up to 12 months)
Aslan et al. 71 also reported results for urinary urgency at 6 months post baseline (4 months post treatment). The number of people (n = 64, data available for 78%) reporting that symptoms of urgency were unchanged or worse was statistically non-significant (RR 0.67, 95% CI 0.41 to 1.07; p = 0.09).
Quality of life
Post treatment
Two different aspects of QoL were measured in the trials: scales measuring the impact of incontinence; and scales measuring symptom distress. Owing to the difference in the underlying concepts, these measures were not pooled and results are presented separately.
Impact of incontinence (post treatment)
Five trials included a measure of disease-specific QoL but two of these trials did not report data in a form suitable for pooling. Aslan et al. 71 used the King’s Health Questionnaire, but post-treatment data were not reported. McFall et al. 90 used the Short Form questionnaire-36 items (SF-36), but do not report data separately for treatment and control group, other than mean scores on one subscale.
Three trials reported data suitable for pooling. Dougherty et al. 76 and Wyman et al. 31 used the IIQ (lower score = improvement). Kafri et al. 81 used the I-QOL (higher score = improvement). To harmonise the direction of scores, results for Kafri et al. 81 were entered as negative. Substantial between trial heterogeneity (I2 = 75.8%) necessitated the use of a random-effects model for pooling. Results are presented in Figure 12.
FIGURE 12.
Quality of life (impact of incontinence): post treatment. aWyman 199831 = combined intervention vs. BT. df, degrees of freedom.
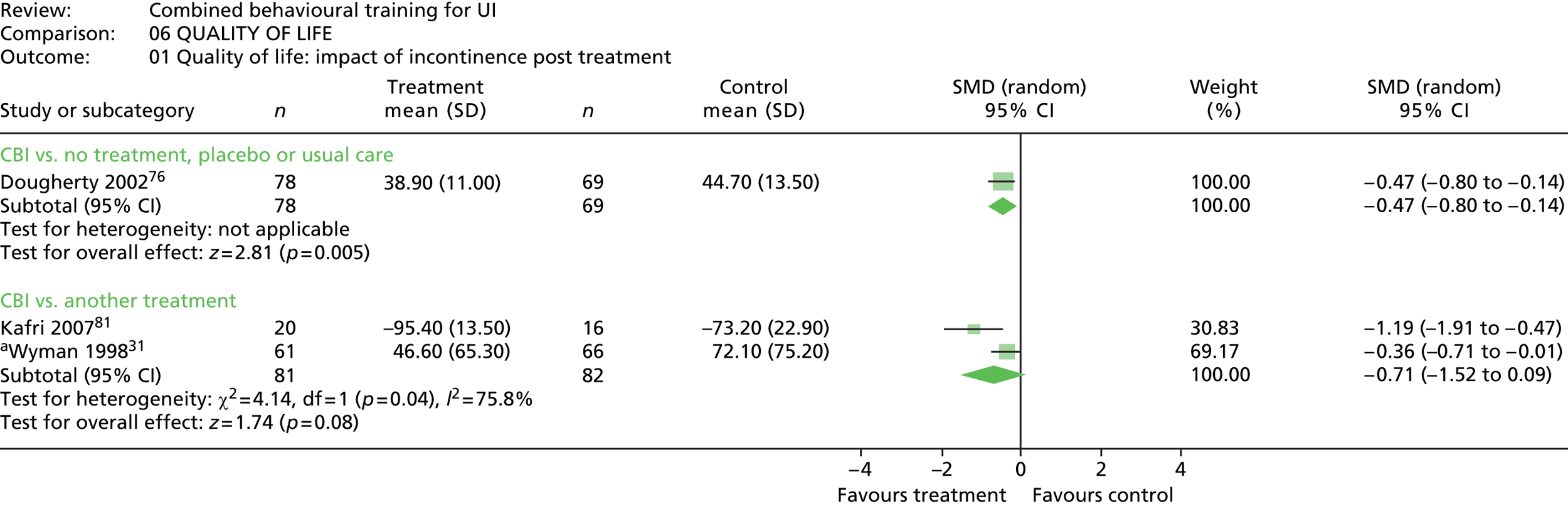
Impact of incontinence (follow-up to 12 months)
The same three trials measured impact of incontinence at follow-up: Dougherty et al. 76 at 12 months post baseline (6 months post treatment); and the other two trials31,81 at 6 months post baseline (3 months post treatment) (Figure 13).
FIGURE 13.
Quality of life: impact of incontinence: follow up to 12 months. aWyman 199831 = combined intervention vs. BT. df, degrees of freedom.
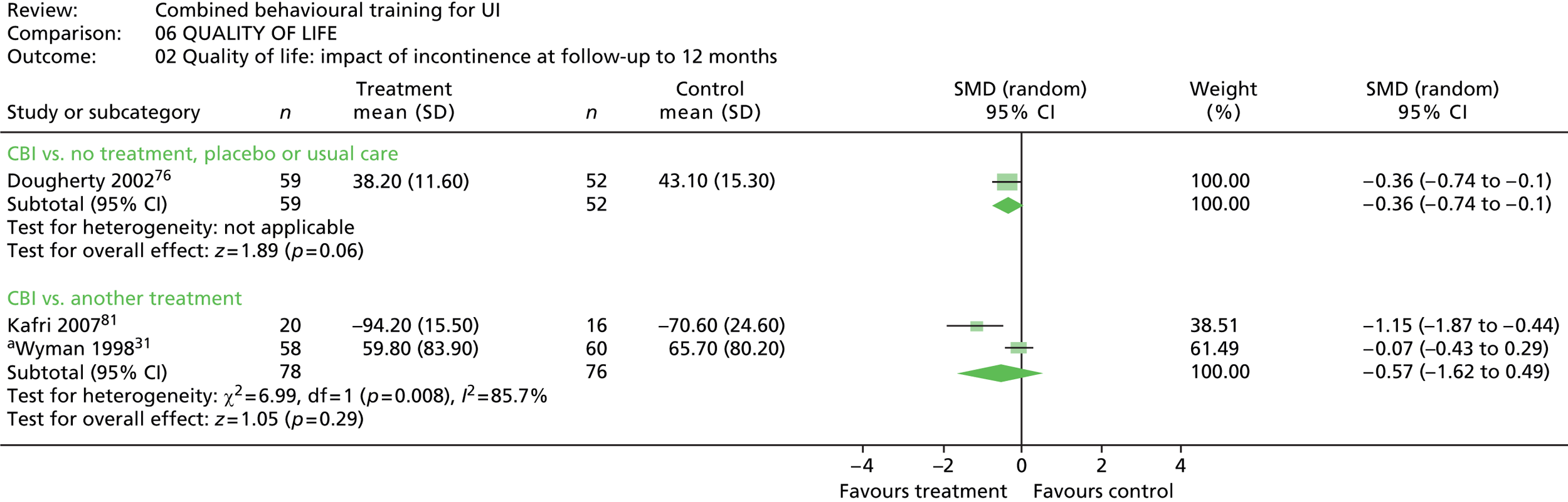
Dougherty et al. 76 compared a CBI with no treatment control. The effect (n = 218, data available for 51%) was marginally statistically non-significant (SMD –0.36, 95% CI –0.74 to 0.01; p = 0.06).
A random-effects model was used due to the heterogeneity of treatment effect (I2 = 85.7%) to pool the two trials using comparison against another treatment. 31,81 The pooled effect size (n = 179, data available for 86%) was not statistically significant (SMD –0.57, 95% CI –1.62 to 0.49).
Symptom distress (post treatment)
Two studies used measures of symptom distress or impact. Burgio et al. 33 used the Symptom-Checklist-90-Revised; Wyman et al. 31 used the UDI. Results are summarised in Figure 14.
One trial33 compared combined behavioural training with a placebo control group (n = 130, data available for 61%). Results showed no statistically significant difference (SMD –0.05, 95% CI –0.44 to 0.34).
A random-effects model was used to pool the results of the two trials using comparisons against another treatment because of substantial heterogeneity in treatment effects (I2 = 92.5%). Results (n = 265, data available for 89%) showed no statistically significant between-groups difference in treatment effects (SMD –0.46, 95% CI –1.41 to 0.50).
Symptom distress (follow-up to 12 months)
One trial31 assessed symptom distress at 6 months post baseline (3 months post treatment). The treatment effect (n = 135, data available for 89%) was marginally statistically non-significant for the BT comparison group (SMD –0.36, 95% CI –0.72 to 0.01; p = 0.06); but not statistically significant for the comparison with PFMT (SMD –0.24, 95% CI –0.59 to 0.12).
Satisfaction with treatment
For the outcome of satisfaction, only comparisons with another treatment were included, as satisfaction for a non-treatment condition is not a meaningful outcome.
Two studies using comparisons against another treatment reported rates of satisfaction. Burgio et al. 33 used a three-point scale (completely, somewhat, or not at all satisfied) with a comparison group who received the drug oxybutinin. Wyman et al. 31 used a four-point scale (very, slightly, neither, dissatisfied, or very dissatisfied) in comparison groups receiving single behavioural interventions, i.e. BT or PFMT. Results are presented for people who scored ‘completely satisfied’ in Burgio et al. 33 and for people who scored ‘very satisfied’ in Wyman et al. 31 (Figure 15).
Satisfaction with a CBI was statistically significantly higher than for other treatments (RR 1.41, 95% CI 1.18 to 1.68).
Adverse effects
Two studies33,81 comparing a CBI against drug therapy were the only studies to measure adverse events (n = 241, data available for 66%). Burgio et al. 33 used an adverse events checklist for the side effects of oxybutinin, and also asked women whether or not they were comfortable enough with treatment to continue indefinitely.
Although 96% of the group receiving the behavioural intervention were happy to continue, 55% of the drug therapy group and 43% of the placebo group were happy to continue.
One quasi-experimental study81 comparing a CBI against drug treatment with oxybutinin measured the total number of adverse events per patient for the study period (n = 44, data available for 82%). The effect size was just statistically significant favouring the CBI (WMD –1.20, 95% CI –2.40 to 0.00; p = 0.05).
Summary: review of effectiveness
Pooled effect sizes for all outcomes are provided in Table 15. Pooled results for comparison with another treatment will be summarised first. If results are not significant for any outcome, results from the no treatment comparison will be considered.
| Outcome | Pooled effect size (95% CI) | |||
|---|---|---|---|---|
| Number of comparisons | No treatment comparison | Number of comparisons | Another treatment comparison | |
| Not cured: number of people remaining incontinent | 2 | RR 0.81 (0.70 to 0.94)b | 2 | RR 0.87 (0.75 to 1.01)a |
| Improvement: number of incontinence episodes | 5 | WMD –3.57 (–5.52 to –1.62)b | 3 | WMD –2.18 (–4.53 to 0.17)a |
| Improvement: ≥ 75% reduction in UI episodes | 2 | RR 2.16 (1.58 to 2.95)b | 2 | RR 1.60 (0.94 to 2.73) |
| Subject perceptions of improvement (much better) | 1 | RR 2.75 (1.72 to 4.42)b | 2 | RR 1.42 (1.12 to 1.81)b |
| Severity of incontinence (grams urine lost per 24 hours) | 2 | SE –0.70 (–2.41 to 1.01) | 0 | – |
| Symptoms: frequency | 4 | SE –0.55 (–0.97 to –0.13)b | 1 | SE –0.04 (–0.70 to 0.62) |
| Symptoms: nocturia | 5 | SE –0.33 (–0.95 to 0.29) | 2 | SE –0.46 (–0.81 to –0.11)b |
| Symptoms: urgency | 1 | RR 0.57 (0.37 to 0.89)b | 0 | – |
| QoL: impact of incontinence | 1 | SMD –0.47 (–0.80 to –0.14)b | 2 | SMD –0.71 (–1.52 to 0.09) |
| QoL: symptom distress | 1 | SMD –0.05 (–0.44 to 0.34) | 2 | SMD –0.46 (–1.41 to 0.50) |
| Satisfaction with treatment | 0 | – | 2 | RR 1.41 (1.18 to 1.68)b |
| Adverse events | – | – | 1 | WMD –1.20 (–2.40 to 0.00)b |
Post treatment
Table 15 shows a summary of pooled effect sizes per outcome.
Comparison with another treatment
Pooled results for the number of people remaining incontinent were marginally statistically non-significant (RR 0.87, 95% CI 0.75 to 1.01; p = 0.06).
Results were statistically significant favouring the CBI for:
-
subject perceptions of improvement
-
nocturia
-
satisfaction with treatment
-
number of adverse events.
Results were marginally statistically (non)-significant for:
-
number of incontinence episodes.
Results were not statistically significant for:
-
75% or more reduction in incontinent episodes
-
urinary frequency
-
QoL – impact of incontinence, symptom distress.
Comparison against no treatment
The pooled effect for the chance of a person remaining incontinent was statistically significant favouring the CBI (RR 0.81, 95% CI 0.70 to 0.94).
The secondary outcomes that were not statistically significant (marginal or otherwise) in comparison with another treatment that were statistically significant favouring the CBI when compared with placebo, no treatment or usual care, are:
-
number of incontinence episodes
-
75% or more reduction in incontinent episodes
-
urinary frequency
-
QoL – impact of incontinence.
One other outcome that was not tested against another treatment was statistically significant favouring the CBI when compared against placebo, no treatment or usual care:
-
urinary urgency.
Two outcomes were not statistically significant in any comparison:
-
severity of incontinence (grams of urine lost per 24 hours)
-
QoL – symptom distress.
Follow-up to 12 months
Table 16 shows a summary of pooled effect sizes per outcome.
| Outcome | Pooled effect size (95% CI) | |||
|---|---|---|---|---|
| Number of comparisons | No treatment comparison | Number of comparisons | Another treatment comparison | |
| Not cured: number of people remaining incontinent | 0 | – | 1 | RR 0.87 (0.72 to 1.05) |
| Improvement: number of incontinence episodes | 1 | WMD –5.60 (–9.92 to –1.28)b | 2 | WMD –1.40 (–4.59 to 1.79) |
| Improvement: ≥ 75% reduction in UI episodes | 0 | – | 1 | RR 1.32 (0.92 to 1.91) |
| Subject perceptions of improvement (much better) | 0 | – | 1 | RR 1.43 (0.96 to 2.12) |
| Severity of incontinence (grams urine lost per 24 hours) | 2 | SE –0.60 (–1.47 to 0.26) | 0 | – |
| Symptoms: frequency | 2 | SE –0.63 (–1.48 to 0.22) | 1 | SE –0.71 (–1.39 to –0.03)b |
| Symptoms: nocturia | 2 | SE –0.97 (–3.30 to 1.37) | 1 | SE –0.89 (–1.59 to –0.19)b |
| Symptoms: urgency | 1 | RR 0.67 (0.41 to 1.07) | 0 | – |
| QoL: impact of incontinence | 1 | SMD –0.36 (–0.74 to 0.01)a | 2 | SMD –0.57 (–1.62 to 0.49) |
| QoL: symptom distress | 0 | – | 1 | SMD –0.24 (–0.59 to 0.12) |
Comparison against another treatment
The pooled effect size for number of people remaining incontinent at follow-up was not statistically significant (RR 0.87, 95% CI 0.72 to 1.05).
Two outcomes showed a statistically significant difference favouring the CBI at follow-up:
-
urinary frequency
-
nocturia.
Outcomes that showed no statistically significant difference at follow-up included:
-
number of incontinence episodes
-
75% or more reduction in incontinent episodes
-
subject perceptions of improvement
-
QoL – impact of incontinence, symptom distress.
Comparison against no treatment
No trial provided data on the number of people remaining incontinent at follow-up, in comparison against no treatment.
Of the outcomes that were marginal or not statistically significant in comparison against another treatment, outcomes that were statistically significant favouring the CBI in comparison against no treatment included:
-
number of incontinence episodes.
One outcome showed a marginal (non)-statistically significant difference:
-
QoL – impact of incontinence.
Outcomes that showed no statistically significant difference in either comparison condition included:
-
severity of incontinence (grams of urine lost in 24 hours)
-
urinary urgency.
Quality of results
The results presented above need to be considered in the light of the quality of evidence to support them. Tables 15 and 16 illustrate that not many trials contributed data to each outcome. Trials were also judged to be of good, moderate, or poor quality as follows:
-
good quality (++): studies where the results are unlikely to be affected by any weaknesses in study design or conduct
-
moderate quality (+): studies where weakness in study design or conduct has the potential to impact on the validity or reliability of the results
-
poor quality (–): studies were the results are likely to be affected by weaknesses of study design or conduct.
For each outcome, results that are supported by trials of moderate or good quality will be summarised, together with any other quality issues (number of respondents, per cent of respondents data are available for, heterogeneity of pooled treatment effect) that could influence interpretation of the quality of the evidence. For each outcome, results for comparison with another treatment will be presented first, followed by results for no treatment comparisons.
Number of people remaining incontinent
The result of borderline statistical non-significance when compared against another treatment was supported by two trials of moderate quality. 31,33 In the no treatment comparison, the treatment effect was statistically significant favouring the CBI, supported by one trial of moderate quality33 and one of poor quality. 90
Number of incontinence episodes
The borderline non-significant result when compared against another treatment was supported by two trials of moderate quality. 31,33 In the no treatment comparison, this outcome was statistically significant favouring the CBI, supported by four trials of moderate quality. 33,76,89,100
Seventy-five per cent or more reduction in incontinent episodes
The comparison against another treatment was not statistically significant and was supported by two trials of moderate quality. 31,33 The no treatment comparison was statistically significant favouring the CBI and supported by one trial of moderate quality. 33
Subject perceptions of improvement
The statistically significant result for this outcome favouring the CBI in comparison with another treatment was supported by two trials of moderate quality,31,33 and by one trial of moderate quality in the no treatment comparison. 33
Severity of incontinence
This outcome was not tested in a comparison against another treatment. In a no treatment comparison, the result was not statistically significant, supported by one trial of moderate quality76 and one of poor quality. 71 There was significant heterogeneity of treatment effect in these two trials.
Symptoms
Findings for urinary symptoms were variable.
Effect size for urinary frequency was not statistically significant in comparison against another treatment, but this result was from a small study of poor quality. 81 Frequency was statistically significant favouring the CBI in comparison against no treatment, supported by two trials of moderate quality76,100 and two of poor quality. 71,90 However, there was substantial heterogeneity of treatment effects.
There was a statistically significant difference in between-groups effect size for nocturia favouring the CBI for another treatment comparison, supported by one study of moderate quality33 and one study of poor quality. 81 Results for no treatment comparisons were not statistically significant, supported by five trials, three of moderate quality33,76,100 and two of poor quality. 71,90 However, there was substantial heterogeneity of treatment effects in these five studies.
Urgency was only tested against a no treatment comparison. The statistically significant result favouring the CBI is supported by one small quasi-experimental trial of poor quality. 71
Quality of life
The non-significant effect for impact of incontinence on QoL in comparison with another treatment was supported by one study of moderate quality31 and one of poor quality. 81 The statistically significant effect for impact of incontinence in the no treatment comparison group favouring the CBI was supported by one study of moderate quality. 76
The non-significant effect for symptom distress in comparison against another treatment was supported by two studies of moderate quality,31,33 and was also not statistically significant in the no treatment comparison, supported by one study of moderate quality,33 although data were only available for 51% of participants.
Satisfaction with treatment
The statistically significant effect for treatment satisfaction in comparison against another treatment favouring the CBI was supported by two studies of moderate quality,31,33 although data were only available for 63% of participants.
Adverse events
The result of marginal statistical significance for the risk of adverse events in comparison with another treatment was only supported by one small trial of poor quality81 and was not measured in any trial with no treatment comparison.
Generalisability of results
Comparisons with another treatment
All of the outcome results relate only to females. Most outcomes are from groups that include people with different types of incontinence, except results for frequency, nocturia and adverse events, which relate solely to people with urge incontinence. None of the results are specific to a particular age group.
Comparison with no treatment
Most of the outcomes relate only to females. Number of incontinent episodes was the only outcome to include males in the sample, from one out of five trials that contributed results. Results for most outcomes related to people with different types of incontinence (except those for subject perceptions of improvement) and symptom distress (which relate only to women with urge incontinence). All of the results relate to women aged ≥ 55 years, but the results for urinary urgency relate only to women aged ≥ 65 years.
Subgroup analyses
Planned subgroup analyses included investigating the effects of client group factors of type of incontinence, age, sex and cognitive status, and intervention factors of content, level, duration and intensity. Subgroup analyses for sex and cognitive status could not be conducted due to a lack of relevant trials in a subgroup.
All subgroup analyses were conducted on the outcome of improvement measured as ‘number of incontinent episodes per week’, as this was the only outcome with sufficient trials to make subgroup analysis viable. Data on number of incontinent episodes was not presented in a form suitable for pooling for two out of the nine trials that collected outcome data from bladder diaries. 71,73
If there was more than one comparison group in a trial, the group least favourable to the combined intervention on the outcome ‘number of incontinent episodes per week (post treatment)’ was chosen for inclusion in the subgroup analyses. Therefore, the drug comparison group was included from the trial by Burgio et al. 33 and the PFMT comparison group was chosen from the trial by Wyman et al. 31 The exception to this is the subgroup analysis for type of incontinence, where a probable error was detected in the results for the PFMT group in Wyman et al. 31 with a SD of 0.00 for the stress incontinence subgroup. Results for the BT group were therefore used for the subgroup analysis for type of incontinence.
Type of incontinence
Two trials included only people with UUI as the predominant pattern. 33,81 Four trials presented results for combinations of UI type (i.e. MUI, SUI or UUI) without subgroup data. 76,89,90,100 One trial presented subgroup data for people with SUI or MUI. 31 Results are presented in Figure 16.
Age
Two trials had younger samples (i.e. with a mean age of less than 65 years)31,81 and five trials had relatively older samples (i.e. aged ≥ 65 years). 33,76,89,90,100 Results are presented in Figure 17.
No statistically significant difference in treatment effects was found between the three types of UI (p = 0.34).
No statistically significant difference in treatment effects was found for younger versus older age groups (p = 0.50).
Type of intervention
Four intervention–comparison pairs31,76,90,100 were judged to have an initial or primary emphasis on BT and four intervention–comparison pairs were judged to have an initial or primary emphasis on PFMT. 31,33,81,89
Results are presented in Figure 18. No statistically significant difference in treatment effects was found for type of intervention, but there was a trend towards BT (p = 0.08).
Level of intervention
Trials were classified dependent on whether they were judged to be basic (i.e. delivery of behavioural strategies aimed at increasing the effectiveness of urinary function activities) or enhanced (i.e. additional behavioural strategies aimed at tailoring an intervention to the specific needs of the individual or enhancing adherence or commitment to practice/activities, e.g. goal-setting, reminder systems, coaching).
Two trials were judged to be focused on basic delivery of strategies aimed at voiding function. 81,100 The remaining five trials were judged to have at least some enhancement to basic delivery of a voiding function intervention,31,33,76,89,90 although the relative emphasis on additional behavioural strategies varied. Results are illustrated in Figure 19.
No statistically significant difference in treatment effects was found for level of intervention (p = 0.92).
Duration of intervention
Trials were categorised into those with 8 weeks or less intervention delivery, or more than 8 weeks. Three trials had an intervention delivery period of 8 weeks or less. 33,89,100 Four trials had intervention delivery periods of more than 8 weeks. 31,76,81,90 Results are presented in Figure 20.
No statistically significant difference in treatment effects was found for duration of intervention (p = 0.71).
Intensity of intervention
Intensity of intervention was defined as the ratio of the number of contacts with a person delivering the intervention to the length of the intervention period, with subgroups defined as contact at least weekly or less than weekly. Five trials had less than weekly contact,31,33,76,81,90 and two had at least weekly contact. 89,100 Results are illustrated in Figure 21.
No statistically significant difference in treatment effects was found for duration of intervention (p = 0.48).
Sensitivity analyses
Planned sensitivity analyses included type of comparison group (no treatment vs. another treatment), study quality, allocation concealment (adequate, unclear/not adequate) and loss to follow-up (≤ 20%, > 20%). Planned sensitivity analyses for study quality could not be undertaken, as only one trial was judged to have adequate allocation concealment100 and only one contributing trial had more than 20% loss to follow-up. 90
Type of comparison group
Five trials had no treatment intervention–comparison pairs. 33,76,89,90,100 Three trials had intervention versus another treatment comparison groups. 31,33,81 Results are illustrated in Figure 22.
No statistically significant difference in treatment effects was found for type of comparison group (p = 0.48).
Findings: narrative review of acceptability and feasibility
Client views
Research aims
Table 17 details the stated aims of the six included studies: two studies mainly referred to factors influencing choice/uptake of UI treatments in older people in residential care;80,94 two studies provided information about factors influencing participation and adherence to behavioural therapies;79,93 and two studies focused on reasons for dropout from a UI treatment programme. 83,84,92
| Study | Choice/uptake | Participation/adherence | Failure/withdrawal |
|---|---|---|---|
| Johnson et al. 200180 | To describe and compare preferences for different UI treatments in LTC from groups likely to act as proxy decision-makers | ||
| O’Dell et al. 200894 | Self-perceived needs and preferences for pelvic floor dysfunction care | ||
| Milne and Moore 200693 | Factors influencing self-care choices and factors that impede or facilitate maintenance of behavioural therapies | ||
| Hay-Smith et al. 200779 | To seek women’s experiences of PFMT, their understandings of the exercises and the way they exercised | ||
| MacInnes 200892 | To understand why some women with SUI do not complete therapy | ||
| Kincade et al. 199983 | To explore why patients withdrew from a behavioural programme |
Findings
Choice/uptake
Table 18 details the results of the two studies considering factors impacting on choice or uptake of behavioural treatments for UI. 80,94 Both of these studies considered the treatment preferences of older adults in LTC facilities in the USA. Results suggest that clients may have a higher tolerance for symptoms and a lower tolerance for disturbance, with a preference for interventions promoting independence and comfort, and resistance to any invasive intervention. Behavioural interventions such as PV can be viewed as embarrassing and resulting in dependence on others, with residents in care facilities disliking the subsequent reliance on nursing staff.
| Barrier | Enabler |
|---|---|
| Client factor | |
|
|
|
|
| Intervention factor | |
|
|
|
|
| Context factor | |
|
|
|
|
|
|
Both of these studies did not limit data collection to respondents with UI. In one study, other problems with elimination were not differentiated94 and in the other study, respondents without UI were used as proxies. 80
Participation/adherence
Table 19 details the results of two studies considering factors impacting on participation in and adherence to behavioural interventions. Hay-Smith et al. 79 was specific to PFMT, whereas Milne and Moore93 referred to client factors impacting on adherence to both BT and PFMT.
| Barrier | Enabler | |
|---|---|---|
| Client factor | ||
| BT |
|
|
|
||
|
||
| PFMT |
|
|
|
||
|
||
| Intervention factor | ||
|
||
|
|
|
| Context factor | ||
|
|
|
|
||
For BT, a barrier to adherence was increased fear of accidents, whereas for both BT and PFMT, respondents identified difficulty with developing a routine and fitting the intervention into daily life, but a feeling of mastery and control if successful. Enablers included realistic goals and adaptation of daily routines.
There were negative perceptions of PFMT, including the difficulty of learning the exercises and knowing whether or not they were done correctly. Respondents valued feedback and follow-up. Contextual features that impacted on adherence included the requirement for privacy. Both of the studies were conducted with women, with one study specific to women with SUI. 79
Withdrawal/dropout
Two studies considered women’s reasons for withdrawal from behavioural UI programmes. PFMT was a major component of both programmes. The findings are detailed in Table 20.
| Barrier | Enabler |
|---|---|
| Client factor | |
| Intervention factor | |
|
|
|
|
|
|
|
|
| Context factor | |
Women cited other health problems, competing pressures, the inconvenience of attending clinics and negative perceptions of PFMT as barriers. Due to the difficulty of knowing whether or not practice was successful, feedback was viewed as helpful by some respondents in both studies. Both of these studies were completed on non-attenders of established continence clinics, so the results may not be generalisable beyond these specific examples.
Summary: client experiences
Table 21 brings together the evidence for barriers and enablers to behavioural UI therapies overall, grouped as client, intervention or contextual factors.
| Barrier | Enabler |
|---|---|
| Client | |
|
|
|
|
|
|
|
|
|
|
| Intervention | |
|
|
| Context | |
Client factors Uptake and maintenance of behavioural therapies could be affected by clients’ values and lifestyle preferences, prior experiences with behavioural therapies and their perceptions of the potential consequences – both positive and negative. Adherence was helped by having realistic goals and expectations and experiencing the positive consequences of success.
Intervention factors Barriers included difficulty knowing whether or not PFMT exercises were being done correctly and fitting interventions into daily life. Professional follow-up and feedback helped adherence, as did tailoring interventions to the individual’s needs and routines.
Contextual factors The convenience or cost of treatment options could affect adherence, as could the availability of a suitable environment for practice. People in residential care valued independence and preferred to avoid increased reliance on nursing staff. They could therefore show a preference for containment strategies for UI, rather than behavioural therapies.
Quality of findings
As well as the number of studies supporting a particular finding, the quality of the original study needs to be considered. Table 22 takes the main category of findings from Table 21 and attaches levels of evidence. The number of studies are identified, with a quality classification as follows:
-
studies where the credibility of the findings are unlikely to be affected by any weaknesses in study design or conduct (++)
-
studies where weaknesses in study design or conduct have the potential to impact on the credibility of the findings (+)
-
studies were the credibility of the findings is likely to be affected by weaknesses of study design or conduct (–).
| Barrier | Enabler |
|---|---|
| Client | |
|
|
|
|
|
|
|
|
|
|
| Intervention | |
|
|
|
|
|
|
| Context | |
|
|
|
|
Researcher conclusions and implications for practice
As well as extracting the original data, we extracted any suggestions by the researchers on their conclusions about suggested ways of improving practice. Although not primary data originating from clients, these conclusions are useful to researchers who are designing future interventions. The data was sourced from the conclusions and implications for practice sections of research reports. The suggestions were simply classified as either relating to the structure of health care (e.g. resources, staff training), or the process of health care (i.e. what should be done). Suggestions were identified as relevant to the stage of informed choice and assessment of suitability for an intervention, or encouraging adherence and preventing dropout.
Informed choice and assessment
Table 23 presents researcher suggestions impacting at the stage of initial client choice and uptake of treatment options.
| Structure | Process |
|---|---|
| Informed choice/assessment | |
|
|
Suggestions about improvements to health-care structures related to the timing, siting and labelling of interventions, and ensuring a basic level of staff knowledge to enable informed choice to happen.
Suggestions about process improvements included eliciting and honouring client preferences and values (particularly with older people), eliciting clients’ goals and their expectations of treatment, and including an assessment of self-efficacy and barriers to uptake of UI treatments in the initial stages of treatment.
Encouraging adherence and preventing withdrawal from treatment
Table 24 presents researcher suggestions relevant to maintaining client participation in treatment.
| Structure | Process |
|---|---|
| Encouraging adherence and preventing dropout | |
|
|
Suggestions about improvements to health-care structures that could impact on client participation and withdrawal included the provision of adequate written information to enable adherence and consideration for delivering treatment in a group setting.
Suggestions to improve the processes of health care included client-focused teaching and realistic goals; personalised practice schedules adapted to the clients daily routine; regular professional follow-up with feedback of objective data and goal evaluation; consideration of the use of strategies such as reminders or motivational interviewing; with early follow-up and alternative options for people who fail to attend.
Staff views
Research aims
Table 25 details the stated aims of the six included studies. One study mainly referred to factors influencing choice/uptake of UI treatments for older people in residential care. 80 Three studies detailed the factors influencing the provision of continence care: one in acute care settings75 and two in LTC settings. 77,98 Two studies (from the last decade) focused on staff perceptions of delivering a PV intervention in LTC settings. 86,97
| Study | Therapy uptake | Continence care | PV programmes |
|---|---|---|---|
| Johnson et al. 200180 | Criteria for choice of therapy | ||
| Dingwall and McLafferty 200675 | Nurses’ views of promotion of continence in acute care | ||
| Mather and Bakas 200277 | NAs’ perceptions of ability to provide continence care in LTC | ||
| Resnick et al. 200698 | Barriers and enablers to UI management in LTC | ||
| Lekan-Rutledge et al. 199886 | NAs’ perceptions of problems of implementing PV | ||
| Remsburg et al. 199997 | Staff perceptions of a PV programme |
Although the studies had slightly different aims, they all included factors that potentially impacted on the methods selected and used for the promotion of continence by staff, including factors relating to clients, interventions and context. Factors are presented as relating to generic continence promotion, except for those factors specific to an intervention, which are labelled as such in the tables.
Findings
Client factors
The included studies identified characteristics of the client that would affect the continence promotion strategies that staff used, or their chances of success, as detailed in Table 26.
| Barrier | Enabler |
|---|---|
|
|
|
|
|
|
|
From a staff perspective, the success of continence promotion strategies was affected by clients’ views on UI and their past experiences; their functional, cognitive and communication abilities; their motivation; and whether or not their continence improved. Ensuring functional ability to participate and the appropriate assessment of clients’ suitability for participation were seen as enablers to appropriate continence promotion.
Intervention-related factors
Table 27 identifies the factors that staff identified as potentially influencing the use of specific interventions.
| Barrier | Enabler |
|---|---|
|
|
|
|
Contextual factors
Contextual factors were most frequently identified by staff as impacting on their ability to promote continence, as detailed in Table 28.
| Barrier | Enabler |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
In summary, factors which could act as a barrier to continence promotion by staff included:
-
views on UI in older people
-
different views on aims of UI therapy than clients or family
-
referral and admission routes
-
nursing assessment procedures
-
staff motivation and education
-
lack of staff and conflicting work priorities
-
the requirements of manual handling
-
perceptions of treatment effectiveness
-
scheduling conflicts.
Factors that could act as enablers to continence promotion included:
-
education
-
teamwork
-
adequate staffing
-
methods of work allocation
-
sufficient and appropriate equipment and supplies
-
experience of success.
The two studies specific to the NA role in the promotion of continence or the implementation of PV also identified methods to improve management of the NA contribution to care, including:
-
regular assignments77
-
inclusion in the plan of care, and in reports on mobility and functional status77
-
increased accountability for adhering to a toileting plan98
-
more autonomy and freedom to prioritise, work as a team77
-
recognition and reward of contribution to continence promotion and management77,98
-
identification of role models. 98
Quality of findings
The quality of the studies related to staff views was poor overall, with only one study of good quality (1 ++)98 and two studies of moderate quality (1 +). 75,77 However, all of the findings were supported by at least one study of moderate quality, except those that are given in italics relating to the differences between staff, clients and family preferences, and some specific enablers for PV implementation such as assessment, allocation and staff monitoring.
Generalisability of findings
The generalisability of most of the findings related to barriers is confirmed in both acute and LTC settings, in the USA and the UK. However, the generalisability of findings related to enablers is mostly confined to NAs working in LTC settings in the USA.
Researcher conclusions and implications for practice
Researcher suggestions to improve the general delivery of continence interventions extracted from the discussion or conclusions included:
-
changing the philosophy from one of accepting incontinence, and the use of self-efficacy-based motivational interventions to help staff and residents believe continence can be improved98
-
identifying residents most likely to benefit from routine toileting77,97
-
staff education97 including ways in which behavioural interventions may help improve urinary control98
-
staff skills in promoting mobility and continence, the experience of caregiving and strategies for mutual support86
-
presenting realistic expectations of outcome to staff
-
supervising/monitoring nursing staff performance86,97 and providing appropriate incentives to ensure adherence to behavioural-based continence care programmes by staff, patient and family98
-
sufficient staff77,86 and a team approach to continence care77,98
-
new technologies to facilitate documenting continence care and new technologies such as bladder scanners98
-
examination of routines that promote or hinder productivity. 86
The two studies specific to the NA role also suggested:
-
consistency of NA assignments to allow the development of relationships77
-
a substantial role for NAs in developing continence care plans for residents for whom they are responsible77,97
-
including NAs in the daily nursing report77
-
nursing recognition and commendation of the contributions of NAs to successful continence care. 77
Studies of feasibility
Eleven studies provided details of uptake, adherence or withdrawal for a CBI (as detailed in Table 29). All of the studies were quasi-randomised or RCTs except Perrin et al. ,96 which was a feasibility study. Only one of the samples included men. 89 One feasibility study was designed to test a CBI with women aged ≥ 75 years, recruited from urology clinics. 96 An additional six studies recruited people aged ≥ 55 years. 33,71,73,76,89,100 Of the studies recruiting older people, one71 was undertaken in a nursing home; one89 with homebound people, and two related studies were undertaken with women from rural areas. 73,76
| Study | Client group | % declined to participate | % non-adherence during treatment | % non-adherence at follow up | Withdrawal from programme |
|---|---|---|---|---|---|
| Aslan et al. 200871 | F, aged ≥ 65 years, nursing home | 16 | 24 | ||
| Bear et al. 1997 (quasi)73 | F, aged ≥ 55 years, rural | 50 | |||
| Burgio et al. 199833 | F, aged ≥ 55 years, UUI | 17 | 6 | ||
| Dougherty et al. 200276 | F, aged ≥ 65 years, rural | 18 | |||
| Kafri et al. 2008 (quasi)82 | F, UUI | 17 | 13 | ||
| Lee et al. 200585 | F | 28 | |||
| Macaulay et al. 198787 | F, UUI | 4 | 6 | ||
| McDowell et al. 199989 | M/F, aged ≥ 60 years, housebound | 18 | 24 | 15 | |
| Perrin et al. 2005 (CT)96 | F, aged ≥ 75 years | 38 | 18 | 30 | |
| Subak et al. 2002100 | F, aged ≥ 55 years | 19 | |||
| Wyman et al. 199831 | F | 20 | 60a | 6 |
Six of the studies provided information about rates of refusal to participate,33,71,82,87,89,96 of which four reported rates of 16–18%. 33,71,82,89 One study reporting a refusal of 4% was recruiting participants from within an existing study. 87 The feasibility study96 reporting a refusal rate of 38% was recruiting older people than those in other studies.
Information on rates of non-adherence to recommended behavioural treatment components was given in four studies,31,85,89,96 with rates ranging from 18% to 28%. Only one study31 reported longer-term adherence, with 60% non-adherence to BT and 47% non-adherence to PFMT at 3 months after treatment completion.
Ten of the 11 studies reported loss to follow-up, but not all studies differentiated between withdrawals/dropouts and other reasons for loss to follow-up (e.g. illness). Dropout is from the treatment group receiving the behavioural intervention at first follow-up if data were available, or from treatment and control if both groups received the same intervention. Mean loss to follow-up was 18.7% (SD 13.6) but rates varied widely. Four studies with samples of younger women31,33,82,87 reported loss to follow-up of less than 15% (mean 7.75% SD 3.5). All of the studies with loss to follow-up rates of 15% and over had samples of older people (mean 26%, SD 12.9).
In summary, based on information in previous trials of combined behavioural therapy predominantly undertaken with older women with established levels of UI, it is expected that rates would be approximately:
-
20% for refusal to participate
-
20–30% for non-adherence to exercise/therapy recommendations
-
30% for loss to follow-up from therapy.
Findings: predictors of adherence and treatment outcome
Studies that include multivariate analysis
Eight papers31,69,70,74,89,95,100,102 report separate multivariate analyses. Two papers69,70 report data taken from the same sample at different time points and are therefore treated as one study. Results are presented first for predictors of adherence and then for measures of treatment outcome including improvement in UI, UI cure, QoL and psychological outcomes.
Predictors of adherence
Two papers relating to one study reported multivariate analyses of predictors of adherence. Alewijnse et al. 69 tested predictors of intention to adhere to a behavioural programme, prior to the intervention starting. At 1-year post treatment, Alewijnse et al. 70 measured predictors of long-term adherence (i.e. up to 1 year post treatment) to a behavioural exercise regime in the same cohort of women, in a RCT.
Three factors were found to be independent predictors of intention to adhere: severity of UI (more urine loss per wet episode); self-efficacy difficulties (perceived ability to perform exercises as required); and self-efficacy abilities (perceived ability to perform required exercises in various situations).
At 1 year post treatment, severity of UI was again a predictor of long-term adherence behaviour. Two other variables were independent predictors of long-term adherence: not having sex education at school; and adherence behaviour during treatment.
Predictors of improvement in urinary incontinence
Four studies tested predictors of improvement in UI. 74,89,95,100 Improvement was defined and measured in various ways and at different time points. Studies also presented results for different subgroups, and one study included two regression models. 95
Predictors of improvement in urinary incontinence at post treatment
One trial100 did not identify any variables as significant. Table 30 summarises the results at post treatment for the remaining three trials identifying predictors of improvement in UI. 74,89,95 Results are summarised as negatively or positively associated with improvement.
| Negative predictor | Positive predictor |
|---|---|
| Socioeconomic | |
| Male89 | |
| Education89 | |
| Physiological | |
| Severity of UI74 | |
| Previous treatment74 | |
| Health/functional | |
| Use of an assistive device89 | |
| Improved functional status89 | |
| Partial caregiver requirement89 | |
| Psychological | |
| Fewer psychological problems89 Adherence89,95 |
|
Six variables were identified as independent predictors that were negatively associated with improvements in UI. The trial by McDowell et al. 89 of a behavioural intervention in older housebound men and women found that male sex and more years of education were negatively associated with improvement (defined as percentage change in UI episodes). Male sex and use of an assistive device were also an independent predictor of poorer outcome. Improved functional status was also negatively associated with improvement.
Burgio et al. 74 identified two variables as negatively associated with more than 75% improvement in UI episodes in women with SUI: severity of UI (defined as greater frequency of UI episodes per week) and previous treatment for UI (consisting of any treatment or evaluation, surgery or medication). Burgio et al. 74 also found severity of UI (defined as use of garment protection) to be negatively associated with more than 75% improvement in UI in women with UUI.
Three variables were independent predictors that were positively associated with improvements in UI. In McDowell et al. ,89 having fewer psychological problems (defined as lower scores on the Geriatric Depression Scale), was positively associated with improvement in UI. Partial caregiver requirement (vs. none or full) and adherence were also positively associated with reduction in UI episodes. Adherence (measured by clinic attendance, recording and self-report) was also positively associated with therapist evaluation of cure or significant improvement in UI in Oldenburg and Millard. 95
Predictors of improvement in urinary incontinence at follow-up
Two studies explored the relationship between predictors and treatment outcome at follow-up time points. Wyman et al. 31 tested severity and type of UI as predictors of treatment outcome of a CBI at 3 months post treatment and found no association. In a study measuring outcome at 18 months post treatment,95 treatment adherence was positively associated with patient perception of degree of improvement in UI. Previous surgical treatment, chronic urological symptoms and perceptions of seriousness as measured by the Health Worry Index were all negatively associated with scores for urge symptoms and UUI on the Bladder Symptom Score.
Predictors of urinary incontinence cure
Two studies measured cure (defined as 100% reduction in UI episodes). Wyman et al. 31 found no significant association between rates of cure and severity and type of UI in their RCT of CBIs, at post treatment, or at 3 months post treatment. In women with UUI, Burgio et al. 74 found a positive association between rates of cure and previous surgery; severity of UI as measured by baseline diary (but not as measured by self-report); use of garment protection; and a lower number of years of education.
Other outcomes
Wyman et al. 31 found a positive association between type of UI and QoL measures. At post treatment, women with SUI reported less life impact (IIQ) and women with urge incontinence reported less symptom distress (UDI). No significant associations were found at 3 months post treatment.
Tadic et al. 102 identified history of depression to be a predictor of QoL (as measured by the Urge Impact Scale) in older women with UUI.
Testing of predictor variables
The results above identify independent predictors of adherence or treatment outcome, but these have to be viewed against the number of times the variable relationship has been included and tested in univariate and multivariate analyses, and the proportion of studies where the variable was confirmed to be an independent predictor.
Univariate analyses
Thirteen studies report a univariate analysis. 69,70,72,74,78,84,88,89,95,99–102 The number of times variables have been included in a univariate analysis is identified in Table 31. Variables included in the three studies measuring predictors of adherence are presented separately to the ten studies measuring predictors of treatment outcome.
| Variable category | Variable | Adherence (three studies) | Outcome (10 studies) |
|---|---|---|---|
| Sociodemographic | Sex | – | 3 |
| Age | 3 | 6 | |
| Ethnicity | 1 | 2 | |
| Education/income | 3 | 4 | |
| Physical | Physiological variables | 1 | 1 |
| Weight/BMI | 1 | 1 | |
| Urodynamic variables | 1 | 3 | |
| Previous treatment | 1 | 5 | |
| Duration of UI | 3 | 6 | |
| Type of UI | 2 | 5 | |
| Severity of UI | 3 | 7 | |
| Health/functional | General health/comorbidities | 2 | 3 |
| Functional status | – | 2 | |
| Cognitive status | – | 3 | |
| Mental | Health perceptions | 1 | 1 |
| Psychological symptoms | – | 3 | |
| Condition/treatment perceptions | 1 | 2 | |
| Self-efficacy/esteem | 1 | 1 | |
| Attributions of control | 1 | 2 | |
| Adherence | 3 | 2 | |
| Knowledge/skill | 1 | – | |
| Attitude/motivation | 1 | – | |
| Social | Social influences | 3 | 1 |
Sociodemographic variables
Of the 13 studies, only five were not sex specific. 72,84,88,89,99 Three of these five tested sex as a variable. 72,89,99 Age was tested in 10 out of 13 studies,69,70,72,74,78,84,88,89,99,101 but ethnicity has only been included in three out of 13 studies. 74,84,89
Physical variables
The influence of the major variables of type and severity of UI have been included in most studies. Severity of UI has been included in 10 out of 13 studies. 69,70,72,74,84,88,89,95,100,101 Five studies targeted people with a specific type of UI,78,95,99,101,102 and of the remaining eight studies, seven included type of UI as a predictor variable. 69,70,74,84,88,89,100 Bagis-Smith et al. 72 did not include UI as a predictor variable.
Six studies considered the influence of prior treatment. 74,88,89,95,99,101 A small proportion of studies have included other urodynamic or physiological variables such as bladder capacity, or weight.
Health/functional variables
Only three studies included measures of general health or function as potential predictors of treatment outcome. Of the six studies that did not state exclusion of people with significant levels of cognitive impairment, two studies89,99 included mental capacity [e.g. as measured by Mini Mental State Examination (MMSE) scores] as a predictor variable. One other study74 also included mental status scores in a sample that excluded people with a lower score.
Mental variables
One study of predictors of adherence69,70 measured a wide array of psychological variables at different time points. Two other studies included a measure of adherence as a predictor of treatment completion. 84,101 Few studies have included psychological variables as a predictor of outcome.
Multivariate analyses
Independent predictors of intention to adhere and treatment adherence were measured in one study. 69,70 Severity of UI and self-efficacy were found to be predictors of intention to adhere, but only severity of UI was also a predictor of adherence at 1 year post treatment, together with lack of sex education at school and treatment adherence behaviour.
Table 32 summarises the number of times predictors have been tested against treatment outcome in a multivariate analysis. Results are summarised for each category of predictor variable in terms of how many studies have included the variable in multivariate analysis, and the results across studies. Associations between variables are described as positive or negative.
| Predictor variable | Wyman et al. 199831 | Burgio et al. 200374 | McDowell et al. 199989 | Subak et al. 2002100 | Oldenburg and Millard 198695 | Tadic et al. 2007102 | Dependent variable | |
|---|---|---|---|---|---|---|---|---|
| Socioeconomic | ||||||||
| Sex | N | Male | < IMP | |||||
| Education | P | Less education | > C | |||||
| N | More education | < IMP | ||||||
| Physical | ||||||||
| Urodynamic variables | ✗ | |||||||
| Previous treatment | N | Prior medication | < IMP (SUI) | |||||
| N | Prior surgery | < IMP | ||||||
| P | > C (UUI) | |||||||
| Type of UI | P | ✗ | ✗ | Type of UI | < impact on QoL | |||
| Severity of UI | ✗ | N | ✗ | ✗ | Greater severity | < IMP | ||
| P | Lower severity | > C | ||||||
| Duration of UI | N | Chronic symptoms | < IMP | |||||
| Health and function | ||||||||
| General health status | ✗ | |||||||
| Functional status | N | Assistive device | < IMP, < C | |||||
| N | Greater function | < IMP | ||||||
| Mental | ||||||||
| Psychological problems | P | P | Less symptoms | > C | ||||
| P | More depression | > impact on QoL | ||||||
| Perceptions of problem | N | More worry | < IMP | |||||
| Perceptions of control | ✗ | |||||||
| Adherence | P | P | More adherence | > IMP, > C | ||||
| Social | ||||||||
| Social influences | P | Social situation | > IMP, > C | |||||
Socioeconomic variables
Socioeconomic variables were included in two studies of treatment outcome. 74,89 One study found male sex to be predictive of less improvement in UI in older housebound adults. 89 Level of education was included as a variable in two studies. More education was found to be predictive of less improvement in older housebound adults;89 and lower educational level was found to be predictive of likelihood of cure in older women with urge UI. 74 Age, ethnicity and socioeconomic status have not been tested as independent predictors.
Physiological variables
Physiological variables have been tested in four studies. The urodynamic variable bladder capacity was tested in one study but not found to be significant. 74 Previous treatment has been tested in two studies, with varying results. Previous treatment with medication was found to be predictive of less improvement in women with SUI. 74 Previous treatment with surgery was found to be predictive of less improvement in younger women with UUI,95 but predictive of greater likelihood of cure in older women with UUI. 74 Weight/BMI has not been tested.
Type of UI was included in two studies and was not found to be a significant predictor of improvement or cure,100 but was related to symptom distress and symptom impact on QoL in one study. 31 Severity of UI was included in four studies. 31,74,95,100 In three out of four studies correlating severity with degree of improvement in UI,31,95,100 severity was not found to be an independent predictor, but was found to be an independent predictor of worse outcome by Burgio et al. 74 in women with stress or UUI. In three studies correlating severity with likelihood of cure, two studies including women with stress and urge incontinence did not find severity to be an independent predictor of likelihood of cure,31,95 while one study confirmed lower severity of UI at baseline as a positive predictor of cure in women with UUI. 74 Chronic urological symptoms were predictive of less improvement in younger women with UUI in one study. 95
Health and functional status variables
Aspects of general health status were measured in one study and not found to be a predictor of UI improvement. 74 Measures of functional status were included in one study. 89 Use of an assistive device for mobility was predictive of less improvement, but greater functional status was reported to be correlated with less likelihood of improvement or cure. Cognitive status has not been tested.
Psychological variables
Psychological problems have been tested in three studies. Less affective symptoms in older housebound people correlated with more likelihood of cure89 and a history of depression was associated with greater improvement in QoL. 102 A greater degree of worry was correlated with less improvement in younger women with UUI. 95 Perceptions of control were measured in one study but not found to be predictive of improvement. 95 Self-reported degree of adherence was measured in two studies, and found to be predictive of improvement and cure in older housebound people89 and predictive of cure in younger women with urge UI. 95
Social variables
Social variables were included in one study. The partial presence of a caregiver was found to be predictive of improvement with older housebound people. 89 Not living alone was also predictive of the likelihood of cure in the same study.
Quality of evidence
The previous section identified that many of the variables have only been included in one or two studies, with the most tested variable (severity of UI) included in three studies. Evidence for each predictor variable is therefore relatively weak, but the quality of the study also has to be taken into account in interpreting the strength of evidence. The description of the quality of included studies identified two studies as of reasonably good quality (++),31,100 three studies of moderate quality (+),74,89,102 and one study of poor quality (–). 95 The final table (Table 33) summarises the strength of evidence for each predictor of adherence or treatment outcome of behavioural interventions for UI.
| Association between predictor and outcome | Not a significant predictor | |
|---|---|---|
| Negative | Positive | |
| Socioeconomic | ||
| (IMP) Male (1 +) | ||
| (IMP) More education (1 +) | (A, C) Less education (2 +) | |
| Physiological | ||
| (IMP) Bladder capacity (1 +) | ||
| (IMP) Prior medication (1 +) | ||
| (IMP) Prior surgery (1 –) | (C) Prior surgery (1 +) | |
| (IMP) Duration of UI (1 –) | (C) Duration of UI (1 –) | |
| (QoL) Type of UI (1 +) | (IMP) Type of UI (2 ++) | |
| (IMP, C) Severity of UI (1 +) | (A) Severity of UI (1 +) | (C) Severity of UI (1 ++, 1 –) (IMP) Severity of UI (1 ++) |
| Health + function | ||
| (A, IMP) Health status (2 +) | ||
| (C) Assistive device (1 +) | ||
| (IMP) Greater function (1 +) | ||
| Psychological | ||
| (IMP) Greater worry (1 –) | (C) Psychological problems (1 +) | (QoL) Perceptions of control (1 –) |
| (QoL) Depression (1 +) | ||
| (IMP) Adherence (2 +, 1 –) | ||
| Social | ||
| (IMP, C) Social situation (1 +) | ||
Socioeconomic variables
Male sex is a significant predictor of less improvement in UI in one study of moderate quality. 89 Level of education was measured in two studies of moderate quality. 74,89
Physical variables
Prior treatment with medication is a significant predictor of less improvement in one study of moderate quality. 74 The same study also found prior surgery to be predictive of more chance of cure, but one study of poor quality found prior surgery to be predictive of less improvement. 95
Duration of UI was measured in one study of low quality and found to be predictive of degree of improvement, but not cure. 95 Type of UI has not been confirmed as a significant predictor of outcome in two studies of good quality,74,100 but was predictive of less symptom distress and impact of UI on QoL in one study of good quality. 31
Severity of UI was also not found to be a significant predictor of improvement or cure in the same two good quality studies31,100 together with one study of poor quality. 95 Severity of UI was found to be a significant predictor of greater adherence in one study of moderate quality, but less improvement or cure in another study of moderate quality. 74
General health and function variables
General health status was not found to be a significant predictor of adherence in one study of moderate quality,69 or of improvement in one study of moderate quality. 74 One study of moderate quality89 provided mixed results around functional status, with use of an assistive device for mobility associated with less chance of cure, but greater overall functional status associated with less improvement.
Psychological variables
Greater worry is predictive of less improvement in one study of poor quality. 95 Fewer psychological problems are associated with greater likelihood of cure in one study of moderate quality,74 but a history of depression was associated with greater improvement in QoL in one study of moderate quality. 102 Adherence has been found to be a significant predictor of improvement in two studies of moderate quality69,89 and one study of poor quality. 95 Perceptions of control are not a significant predictor of outcome in one study of poor quality. 95
Social variables
One study of moderate quality89 found social situation (defined as living arrangements of partial presence of a caregiver) to be predictive of improvement and cure.
Generalisability of evidence
There was variation in the client groups included in the studies, so variables may only be confirmed predictors in specific client groups. Results for predictors of adherence are generalisable to women with self-reported UI. 69,70 Severity of UI was a predictor of worse outcome in women with SUI or UUI. 74,95 Previous treatment was a predictor of worse outcome in women with UUI. 74,95 Sex, functional and social status variables were predictors of outcome in older housebound people. 89 Fewer psychological problems were also predictors of improvements in UI in older housebound people and improvements in perceived QoL in women. 102 Type of UI was also found to be related to QoL in women. 31
Modelling predictor variable relationships
Although individual studies can provide information about individual predictor variables, none of the studies tested predictive models, so there is little information available about how predictor variables might interact.
Figure 23 summarises the independent predictor variable relationships from at least one study of moderate quality (dotted line). Black lines indicate negative impact, i.e. worse outcomes, while blue lines indicated positive impact. Dark green dotted lines indicate where evidence for the direction of correlation is mixed.
FIGURE 23.
Draft model of predictor variable relationships.
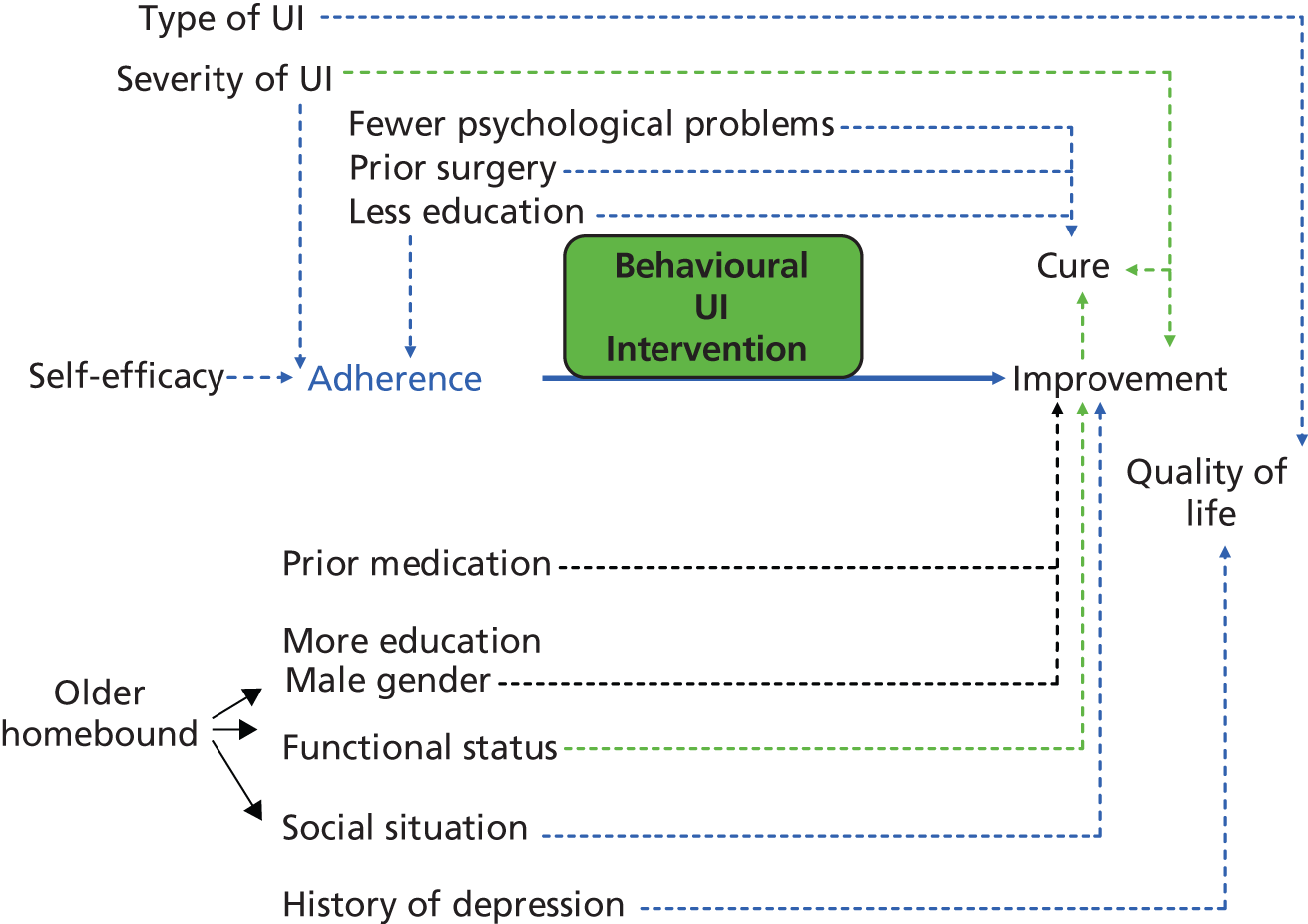
The only correlation to be confirmed in more than one study (illustrated by a solid line) is the link between adherence and improved outcome.
Discussion
Review of effectiveness: summary of results
Ten studies (n = 1163)31,33,71,73,76,81,87,89,91,100 with 13 intervention–comparison pairs were included in the review. Two studies did not provide data suitable for pooling. 73,87
Primary outcome
Results for comparisons with another treatment in the number of people remaining incontinent at post treatment were marginally not statistically significant (RR 0.87, 95% CI 0.75 to 1.01) in two trials of moderate quality. 31,33 Follow-up results had a similar estimate of effect size but were not statistically significant for comparison with another treatment (RR 0.87, 95% CI 0.72 to 1.05). Results for no treatment comparisons were statistically significant favouring the CBI (RR 0.81, 95% CI 0.70 to 0.94) from two trials33,90 (one of moderate quality33), with no measurements available at follow-up.
Secondary outcomes
Results for number of incontinent episodes at post treatment were marginally statistically non-significant (WMD –2.18, 95% CI –4.53 to 0.17) in comparisons with another treatment in three trials,31,33,81 two of which were of moderate quality. 31,33 Follow-up results were also not statistically significant in two trials,31,81 one of these was of moderate quality31 (WMD –1.40, 95% CI –4.59 to 1.79). Results were statistically significant favouring the CBI (WMD –3.57, 95% CI –5.52 to –1.62) in five trials with no treatment comparisons, four of which were of moderate quality. Follow-up results were also statistically significant favouring the CBI (WMD –5.60, 95% CI –9.92 to –1.28) in one trial of moderate quality.
Results for comparison with another treatment were statistically significant favouring the CBI for improvement in UI at post treatment in terms of subject perceptions of improvement (RR 1.42, 95% CI 1.12 to 1.81) in two trials of moderate quality. 31,33 At follow-up to 12 months the effect was of similar magnitude but was not statistically significant (RR 1.43, 95% CI 0.96 to 2.12). In comparison with another treatment, the effect size for 75% or more reduction in incontinent episodes was not statistically significant at post treatment (RR 1.60, 95% CI 0.94 to 2.73) or follow-up (RR 1.32, 95% CI 0.92 to 1.91). The no treatment comparison was statistically significant post treatment favouring the CBI (RR 2.16, 95% CI 1.58 to 2.95), but was not measured at follow-up.
Results for severity of UI for comparison with another treatment were not measured at post treatment or follow-up. No treatment comparison in two trials71,76 (one of moderate quality76) was not statistically significant at post treatment (SE –0.70, 95% CI –2.41 to 1.01) or follow-up (SE –0.60, 95% CI –1.47 to 0.26).
From a single trial,81 the effect for symptoms in terms of urinary frequency was not statistically significantly different from that of another treatment in one trial (SE –0.04, 95% CI –0.70 to 0.62), but was statistically significantly different from the no treatment comparison favouring the CBI (SE –0.55, 95% CI –0.97 to –0.13) in four trials,71,76,91,100 two of which were of moderate quality. 76,100 Results for nocturia were statistically significant favouring the CBI (SE –0.46, 95% CI –0.81 to –0.11) in comparison against another treatment in two trials,33,81 one of which was of moderate quality. 33 Results for nocturia were also statistically significant at follow-up favouring the CBI (SE –0.71, 95% CI –1.39 to –0.03) in comparison with another treatment, in one study of poor quality. 81 Urinary urgency was not measured at post treatment in any trial using comparison against another treatment, but results were statistically significant for a no treatment comparison favouring the CBI (RR 0.57, 95% CI 0.37 to 0.89) in one trial of poor quality. Effect differences for urgency at follow-up were not statistically significant (RR 0.67, 95% CI 0.41 to 1.07).
Results for QoL were not statistically significant for either impact of incontinence or symptom distress in comparison against another treatment at post treatment or at follow-up. For a no treatment comparison, reduction in impact of incontinence on QoL was statistically significant at post treatment favouring the CBI (SMD –0.47, 95% CI –0.80 to –0.14) in one trial of moderate quality,76 and was marginally statistically non-significant at follow-up (SMD –0.36, 95% CI –0.74 to 0.01) in the same trial.
The chance of satisfaction with treatment was statistically significantly different favouring the CBI (RR 1.41, 95% CI 1.18 to 1.68) when compared against other treatments in two trials of moderate quality. 31,33
Results for number of adverse events were also just statistically significant favouring the CBI (WMD –1.20, 95% CI –2.40 to 0.00), with more adverse events in the drug comparison group in one trial of poor quality. 81
In summary, there is evidence that, in comparison against no treatment, CBIs show gains in the number of people cured, objective and subjective measures of the degree of improvement in UI, reduction in some symptoms and impact of incontinence on QoL, but not severity of incontinence or symptom distress.
In comparison with other treatments, CBIs seem to be more advantageous for subjective perceptions of improvement and satisfaction with treatment. CBIs are possibly more advantageous than other treatments in terms of reducing the number of incontinent episodes and nocturia, but there is insufficient evidence for gains in rates of cure, frequency of micturition, or improvements in QoL.
There is evidence that in comparison with usual care, treatment effects on the number of incontinent episodes, the severity of incontinence and the impact of incontinence on QoL, can persist into the longer term. In comparison with other treatments, the magnitude of effect at follow-up is similar to the post-treatment effect, but only achieves statistical significance favouring the CBI for urinary symptoms of nocturia and urgency.
Review of effectiveness: quality of the evidence
In general, results were supported by at least one trial of moderate quality. 73 However, out of the 10 trials, one did not provide data suitable for pooling and two quasi-experimental trials reported some large treatment effects that were not consistent with other trial results. 71,81 Although eight31,33,73,76,87,89,90,100 out of 10 trials reported random allocation, only three provided an adequate description of the procedure;33,89,100 and allocation concealment was judged adequate in only one trial. 100 However, judgements about the quality of trials are based on the trial reports and not on contact with trials authors, so may refer more to the quality of trial reporting. This effectiveness review is the basis for a Cochrane review, and trial authors will be contacted as part of that process.
Although most of the results are supported by at least one trial of moderate quality, there are some inconsistencies in the results, which need exploring. These are in part due to the small number of trials contributing to each outcome, meaning that the direction and magnitude of effect does not necessarily show consistency across no treatment and another treatment comparisons, or across post treatment and follow-up results for the same outcomes. This may be in part due to the fact that different trials contribute to the comparisons, with variation in trial quality, client groups and timing of outcome measurement. The statistical significance of an effect is often variable across different outcome measurement time points, whereas the effect size is relatively similar.
Rates of cure, number of people achieving 75% of more reduction in incontinent episodes and number of incontinent episodes were all sourced from the same data in bladder diaries. In comparing CBIs against another treatment, the difference in the number of incontinent episodes and the number of people cured were marginally statistically non-significant, whereas the number of people gaining 75% or more reduction was not statistically significant. However, the general pattern is for statistically significant difference favouring the CBI in the number of incontinent episodes when compared against no treatment, but marginal or statistically non-significant differences of smaller size when compared against other treatments.
Subject perceptions appear to be more positive than relatively more objective data such as bladder diaries. One explanation might suggest increased contact with a health professional affecting subjective perceptions. However, this explanation would not be consistent with the evidence. The review of PFMT identifies average time of intervention as 8–12 weeks,29 whereas the average time of CBI delivery is also 8–12 weeks. 79 There could be increased intensity of contact within the programme duration, but the PFMT review identified weekly clinic contacts as the average, whereas CBI interventions had slightly more than weekly contact, on average. Alternatively, the differences seen could be an artefact of comparing severely right-skewed distributions for which the SDs are very large relative with the means (effectively making the mean for number of incontinent episodes insensitive to change).
Results for urinary symptoms are at times inconsistent and also subject to considerable heterogeneity, which is in some part attributable to the results from two quasi-experimental trials. 71,81 For urinary frequency, effects are heterogenous for no treatment comparisons at post treatment, mainly due to the large treatment effects from one quasi-experimental trial. 71 The inconsistency in statistically significant results favouring the CBI for nocturia in comparisons with another treatment, but not in no treatment comparisons, could be because the significant difference is sourced only from a small quasi-experimental trial. 81 However, there is also a high degree of heterogeneity in the treatment effects of the no treatment trials, attributable to large treatment effects from Aslan et al. 71 as before, but also results favouring the control group from two trials. 90,100 In both trials, this was attributable to a rise in nocturnal micturitions in the treatment group. Neither author offers a possible explanation. Last, only one quasi-experimental trial contributes to the statistically significant treatment effects favouring the CBI seen for urinary urgency. 71 Owing to the inconsistency and heterogeneity of treatment effects without plausible clinical explanation, results for urinary symptoms therefore appear unreliable.
Results for QoL are more consistent, in that there is no statistically significant difference seen in either impact of incontinence or symptom distress on QoL for two trials using comparisons against another treatment,31,81 but results for impact of incontinence are statistically significant favouring the CBI in one trial using comparison with no treatment,76 and marginally statistically non-significant with smaller effect size at follow-up for the same trial. However, there was significant heterogeneity of treatment effect observed for both impact of incontinence and symptom distress, in some part attributable to large treatment effects observed in one quasi-experimental trial. 81 Owing to the small number of trials contributing data and the heterogeneity of treatment effects, results for QoL also appear unreliable.
Results for satisfaction with treatment are based on two trials of moderate quality,31,33 but results for adverse effects are based on only one quasi-experimental trial,81 so should be interpreted with caution.
Review of effectiveness: overall completeness and applicability of evidence
The results would suggest that, in terms of effectiveness immediately after treatment, the choice between CBIs and other treatments (mainly drugs or single interventions) is based on greater subject perceptions of improvement, greater satisfaction with treatment, and the potential for more people to achieve greater levels of improvement (75% or more) in UI. Results for symptom reduction or improvements in QoL are inconsistent. We found no trials that included a comparative assessment of the cost of delivering CBIs compared with other interventions, but the contact time and patterns do not appear significantly different to PFMT trials.
The results for effectiveness over the longer term are less clear, with no clear benefit over other treatments, other than in symptom reduction, where the evidence is potentially unreliable. The effect size estimates at follow-up are generally consistent with post-treatment effect sizes, with only a small estimated reduction over time. However, the small number of trials and the smaller sample size at follow-up makes interpretation very difficult.
Few trials used objective measures of UI, such as a pad test for grams of urine lost per day. Most trials included the number of incontinent episodes, so could report degree of improvement and number of people cured, but only three trials reported on the primary outcome of cure. Requests for further outcome data were made to some authors, with no success.
Overall, the results are more relevant to people with UUI or MUI, as only 14% of participants were classified as having genuine SUI only. In the main, results are applicable to people with established, moderate to severe UI, with only one trial including people with mild UI. 90 The results are also mainly applicable to women, with only a few men included in one trial. 89 Participants had a mean age > 55 years in 9 out of 10 trials, > 60 years in eight trials and > 70 years in three trials. Most participants were cognitively able.
The review documents the degree of diversity in the content, main focus, duration and intensity, enhancement, and allocation of interventions, and in what they are compared against. However, there is sufficiently similar content in terms of main components and methods of delivery that CBIs can be easily differentiated from single interventions and their results feasibly combined. However, there are not sufficient trials using the same outcome measure in any one comparison of main features to clarify what might be the dominant mechanisms of effect. None of the subgroups of intervention characteristics showed any significant difference and, in truth, it was difficult to allocate interventions to such broad brush categories as basic or enhanced delivery. The benefit of such an in-depth description of intervention content may lie more in delineating potential mechanisms of action to be tested in future trials.
In terms of acceptability and safety of the intervention, there was no evidence of adverse effect, although few trials explicitly monitored them. 33,81 Although trials have mostly been conducted in US settings,31,33,73,76,89,90,100 there is no reason to think that they might not be acceptable to either staff or clients in the UK, or feasible in a UK health-care context. However, only one trial used a truly community derived sample,76 and most participants were self-selecting after being in contact with health-care services. Three trials included people who were older and home-bound or in nursing homes,71,73,89 so interventions seem feasible with this client group, although loss to follow-up was higher, and there were aspects of the interventions that were not fully accepted, such as the internal examination for PFMT.
Review of effectiveness: potential limitations of the review process
For the purpose of gaining an overall picture, the review structure has leaned towards combining the results of trials, but the degree of heterogeneity in treatment effects for some outcomes suggests that the pooled results may be unreliable. Subgroup analyses were only possible on one outcome, and were in the main not statistically significant, so did not provide much information about potential sources for the observed heterogeneity. However, the explanation is likely to be in the combination of differences in client group, intervention type, comparison group, setting and study quality in the included trials. The detailed description of the included interventions may help to detect potential mechanisms of action for exploration in future trials.
The alternative to pooling is to consider each trial in isolation, and to undertake narrative review for each outcome. In effect, the review has also done this, in terms of a detailed description and comparison of the interventions, and narrative consideration of likely sources of heterogeneity for each outcome. Another alternative would be to structure the review comparisons differently; in particular, to use types of incontinence as subgroups rather than type of comparison group. However, even though the sensitivity analysis for type of comparison group was not statistically significant and there is therefore no evidence for not pooling results, the review group prefers to maintain the distinction. Only three trials offer the potential to analyse results by type of incontinence,31,33,81 and the effect of this has been checked in a subgroup analysis.
The choice of primary outcome measure was a matter for considerable discussion, as the review group felt that cure was not necessarily the most appropriate outcome to be expected of a behavioural intervention, although it might be the most desirable. Behavioural interventions were thought more likely to incur improvement in continence and urinary symptoms. There was also discussion about whether or not subject perceptions of improvement should be preferenced over relatively less subjective measures such as bladder diaries. If subject perceptions had been chosen as the primary outcome, CBI would be viewed as successful, whereas less benefit is observed in more objective measures such as number of incontinent episodes, or number of people regaining continence. The measure of cure was eventually chosen as the primary outcome because it is the end target of treatment for urinary continence, and as such is a gold standard against which all treatments can be compared. There remains the potentially valid criticism that we may have chosen the wrong outcome measure as primary, and that an alternative comparison structure should have been used.
The choice of post-treatment timing and up to 12 months follow-up are not perhaps the most desirable choice, as long-term continence would be the most valuable end point. However, few trials measure longer-term outcomes, and those that do measure at variable time points. Post-treatment scores were thought likely to be the most comparable. In this review, a number of trials measured medium-term (i.e. up to 12 months) outcomes,76,89,90 but only three trials provided outcome data past 12 months follow-up. 76,81,90
Narrative review of acceptability and feasibility: summary of findings
Client views
Six studies of the experiences of clients were found: five qualitative studies (n = 105)79,83,92–94 and one postal survey (n = 79). 80
Factors identified by at least one study of moderate quality that could act as a barrier to continence promotion for clients included:
-
perceptions of UI as a problem and level of tolerance for symptoms
-
preference for interventions that promote independence and avoiding reliance on staff in residential care
-
increased fear of being wet with BT, negative attitudes to and experiences of PFMT and difficulty of doing exercises properly
-
competing interests/demands, and difficulty developing routines and fitting them into daily life
-
convenience and cost of treatment
-
delivery mode (e.g. group vs. individual).
Factors identified by at least one study of moderate quality that could act as an enabler to continence promotion for clients included:
-
having realistic goals and expectations, and gaining a sense of mastery and control if successful
-
adapting daily routines to include PFMT with feedback on correct performance of exercises and professional follow-up
-
availability of an accessible and clean bathroom in residential care.
Additional suggestions by researchers to facilitate treatment choice and adherence included:
-
considering the timing, siting and labelling of interventions, and the training of staff
-
eliciting and honouring client preferences and their goals and expectations for treatment
-
including an assessment of self-efficacy and barriers to uptake of treatment in the initial stages
-
the provision of adequate written information
-
consideration of the use of strategies such as reminders or motivational intervention, with early follow-up and alternative options for people who fail to attend
-
consideration of group teaching as a strategy.
Staff views
Six studies of the views of nursing staff were also included about continence care including behavioural strategies in general (n = 273);75,77,80,98 or about involvement in PV interventions (n = 154). 86,97
Factors identified by at least one study of moderate quality that could act as a barrier to continence promotion for clients included:
-
views of staff about the aims of treatment
-
likelihood of continence assessment affected by routes of admission or referral
-
lack of suitable assessments and involvement of the multidisciplinary team (MDT)
-
staff motivation and education
-
conflicting work priorities, lack of staff, requirements of manual handling, scheduling conflicts
-
perceptions of treatment effectiveness.
Factors identified by at least one study of moderate quality that could act as enabler to continence promotion for clients included:
-
education
-
teamwork, adequate staffing, methods of work allocation
-
sufficient and appropriate equipment and supplies
-
experience of success.
Specific suggestions relating to enablers for the involvement of NAs in the promotion of continence included:
-
regular allocation of clients
-
inclusion in planning and reporting of care
-
increased accountability for adherence, more autonomy and freedom to prioritise
-
recognition and reward for contribution
-
identification of role models.
Additional suggestions by researchers to facilitate continence promotion by staff included:
-
changing philosophy of care away from accepting continence
-
the use of self-efficacy-based motivational interventions for both staff and clients
-
targeting clients most likely to benefit
-
providing realistic expectations of outcome to staff
-
supervision and monitoring or staff performance
-
incentives to adherence to behavioural programmes
-
technology to support continence care (e.g. bladder scanners).
Narrative review of acceptability and feasibility: quality of the evidence
The quality of studies was mixed, with the potential for bias originating in the main from poor description of methods of data analysis, or methods of testing the robustness of the findings. However, most of the qualitative studies were descriptive rather than in-depth studies, so the identification and listing of potential barriers and facilitators to the delivery or uptake of behavioural interventions is unlikely to be problematic, in terms of interpretation or synthesis. Only a small number of data was extracted from the descriptive component of the two surveys,80,97 such as the frequency of agreement with barriers to the use of behavioural methods for continence promotion, or additional barriers identified in free-text responses. However, most of the findings were supported by at least one study of moderate quality.
Narrative review of acceptability and feasibility: overall completeness and applicability of the evidence
Although there were 12 studies included in the narrative analysis focusing on broadly the same topic, there was relatively little overlap between their specific focus, with three studies of treatment choice;80,93,94 three of treatment adherence;79,93,94 two of treatment withdrawal,83,92 two of PV implementation;86,97 two of continence promotion in LTC;80,94 and one in acute care. 75 Only one study79 collected detailed information about clients’ experiences of a particular behavioural therapy (i.e. PFMT), so there is in fact very little in-depth exploration of client responses to behavioural therapies.
The available evidence for clients relates to cognitively able women in the main, but apart from that similarity, the samples differed. Two of the studies were restricted to women with SUI,79,92 and the samples in these two studies were younger. Two of the studies related to older women in residential care,80 and one study was a community sample. 93 The mix of samples and the different focus of the studies means that the barriers and enablers identified should be viewed as context specific, rather than generalisable to any client group or setting.
There is no information relating to client experiences of behavioural therapies other than PFMT, and none of the studies were longitudinal, so not much is known about how views might change and develop over time. There is very little information about particular subgroups, for example those with severe or mild incontinence, or about men’s experience of behavioural therapies.
The available evidence for staff all relates to nursing staff, predominantly those working in long-term residential care, although one study was undertaken in acute care. 75 The samples of four of the studies included a mix of grades from NA through to charge nurse and director of nursing. The identified barriers and enablers related in the main to the direct delivery of care and although two studies did take a rather wider view that included some system features, there is not a strong whole systems approach overall. The focus on behavioural therapies in the general studies of continence care was rather small, with the impression being that nurses did not know much about them. There were no studies in a community setting, and none relevant to the delivery of a specific behavioural therapy other than PV.
Narrative review of acceptability and feasibility: limitations of the review process
The synthesis of qualitative data is not a straightforward mechanical process, and some issues were encountered that need to be taken into consideration when reading the synthesis.
The extraction of the findings
In the main, we identified as ‘findings’ the study authors’ themes, categories and codes (i.e. secondary data). We did not collate respondents’ quotes, specific examples, or detailed data. The level at which we were most likely to identify and extract ‘factors’ was at the level of the category in the original study – although this differed depending on whether the original study was purely descriptive, or more interpretive. There were occasional differences in the level of data extraction by reviewers, where one reviewer might extract a barrier or facilitator that was described as an example from a single client. Identified factors could therefore be based on one or many respondents’ views, and we have not differentiated or made any interpretation of relative importance or size of impact. We have only grouped similar or related factors and identified where multiple studies have described the same factor.
The classification of the findings
We used different classifications of the data to facilitate its presentation and synthesis, but these were imposed by us rather than being inherent in the original data.
-
Factors were classified as either relating to client, therapy or context. Contextual issues were easy to differentiate, but it was sometimes difficult to identify whether characteristics were inherent to interventions, or whether they were solely attributable to client perceptions (e.g. the statement that PFMT exercises were difficult). Reports of client experiences or feelings tended to be categorised as client attributes, whereas more concrete features tended to be linked to the intervention.
-
Factors were also classified as barriers and enablers. However, many more barriers were identified with fewer enablers, and sometimes factors could be interpreted as either. Our decision was to classify according to the expression in the text as far as was possible and duplicate factors in both if necessary. If it was unclear whether a factor acted as a barrier, enabler or both, our decision was to go with whatever was our agreed interpretation by both reviewers, rather than using a consistent rule.
-
Factors were classified as relating to different stages in the process of treatment: uptake, participation and withdrawal. This was sometimes explicit in the study design or reporting (e.g. a study of factors influencing withdrawal from behavioural treatment), but other studies included factors relating to multiple stages – sometimes explicitly, and sometimes not. Again, we used our judgement to assign factors to stages. However, we did not try to duplicate factors in multiple stages, but rather to assign a factor to the most appropriate stage.
-
Factors were classified according to which behavioural therapy they were reported to affect (i.e. BT, PFMT, PV). However, some studies were more related to generic management of incontinence with behavioural therapy as one of the considered options. It was not possible in these studies to narrow down to a specific therapy, but where it was possible factors are reported as related to a specific therapy.
These issues of classification and interpretation of the original data need to be taken into account when reading the synthesis, as does the inevitable loss of detail when summarising studies.
Review of predictors of adherence or outcome: summary of results
Independent predictors confirmed in at least one study of moderate quality include, for:
-
intention to adhere – self-efficacy
-
longer-term adherence behaviour – greater severity of UI, lack of sex education at school and treatment adherence behaviour
-
improvement in UI – social situation, educational level, gender in older housebound people, and adherence behaviour and prior treatment with medication in women
-
cure – fewer psychological problems, prior surgery for SUI, less education
-
QoL – history of depression, type of UI.
Results for functional status and severity of UI are conflicting.
The only predictor confirmed in more than one study was adherence as a predictor of improvement.
Review of predictors of adherence or outcome: quality of the evidence
Only two studies were of good quality,31,100 and these two studies only measured the two major variables of type and severity of UI, and did not confirm either variable as an independent predictor of improvement, although type of UI was correlated to QoL in one study. 31 The remaining studies were of moderate quality overall, although all had some weakness in the definition or measurement of predictor or outcome variables, and none had adequate blinding of assessors.
As most of the predictors have only been confirmed in one study, the results need to be treated with caution. In particular, results for prior treatment or severity of UI are mixed, and are probably related to the type of UI. The only result that can be viewed with any level of confidence is the relationship between adherence and treatment outcome.
Review of predictors of adherence or outcome: completeness and applicability of the evidence
Although many variables have been included in univariate analyses, the majority of variables tested in multivariate analysis have been physiological, with few studies including social or psychological variables. Only three studies have included mental or cognitive status,89,95,102 and only one study has considered functional status. 89
The studies also cover different populations, with one study specific to older housebound people, and the remainder specific to either older versus younger women, or women with different types of UI. Women made up the majority of the study samples, with only a few males included in one study. 89
Conclusions
Results of the review were presented to and discussed by the ICONS Management and Steering Groups, and also to the Patient, Carer and Public Involvement Groups. From those sources, the implications for the ICONS trial were drawn up.
Implications for practice
-
Include the teaching of stress strategies as a component of the PFMT protocol.
-
Consider how to check the performance of PFMT exercises.
-
Use behavioural headings in the protocol for the intervention, so that nurses have clear content to direct them on behavioural components, i.e.
-
patient education: provision of instruction, modelling behaviour
-
review: intention formation, barrier identification, tailoring to individual’s goals, provision of feedback on review
-
maintenance: reminders, encouragement, motivation, dealing with anxieties/fears.
-
-
Reflect the same structure in the patient booklet by adding a section about ‘What other people have said’, and a section about ‘keeping up the practice’, together with strategies for this, for example have small goals in mind, try to do the exercises at certain times of the day so you remember them, do not get disheartened if there is not immediate improvement, etc.
Implications for research
-
Include questions on confidence in instruction/doing PFMT exercise, and whether or not feedback was available in interviews with both staff and patients.
-
Review the measurement of adherence in the trial to ensure that adherence to different components of the intervention is monitored.
-
Include measurement of staff adherence, i.e. per cent completion of scheduled activity, fidelity of adherence to protocol.
-
Consider alternatives to bladder diaries for people with communication problems.
-
Include questions related to subjective experience in interviews with patients:
-
fears, negative attitudes to interventions
-
expectations
-
tailoring intervention to individual and circumstance
-
mastery/control
-
assessment of correct performance, receipt of feedback.
-
-
Include questions related to subjective experiences in interviews with staff:
-
named barriers/enablers
-
staff training in the assessment of incontinence
-
availability of continence assessments
-
workload, team allocation, etc.
-
-
Include information from the review in staff education.
-
Definition and measurement of outcome varies considerably in the trials included in the review, and the ICONS trial should take into consideration the Cochrane recommendations for outcome measurement.
-
Predictor variables with sufficient consistent evidence to be taken into consideration in trial design include type of incontinence and adherence. Evidence related to the severity of incontinence is inconsistent, but should also be measured.
-
Predictor variables hypothesised to be influential but with insufficient testing include:
-
cognitive status
-
functional status
-
psychological status
-
perceptual and sensory difficulties
-
difficulties with learning
-
self-efficacy
-
attitude
-
perceptions of control
-
educational background.
-
-
Data collection on these variables should be considered.
-
There is insufficient evidence in the review on the use of BT or PFMT in men. One excluded trial of mixed behavioural strategies does include men, but does not provide data on incontinence. 110 In the absence of any data from the review, we may need to refer back to the literature on the use of conservative methods for UI after prostatectomy for intervention type, timing and intensity to be used with men.
Chapter 3 Developing the interventions
Overview
In this chapter, we report the development and rationale behind interventions used in the research programme and how they were informed by the evidence synthesis. The effectiveness component of the evidence synthesis suggested CBIs impact on rates of cure, objective and subjective measures of degree of improvement, reduction in some symptoms and impact of incontinence on QoL. In comparison with other treatments, CBIs are more advantageous for subjective perceptions of improvement and satisfaction with treatment, and are possibly more advantageous than other treatments in terms of reducing the number of incontinent episodes and nocturia. We therefore selected a combined approach comprising BT and PFMT as a key component of the intervention.
Systematic voiding programme
Assessment of continence status
The assessment phase comprised two components, a 3-day bladder diary and a comprehensive continence assessment. Ward staff completed a 3-day bladder diary (see Appendix 8) for all patients admitted to the participating unit with a confirmed stroke. The aim of the diary was twofold: to identify patients who were incontinent and to use the pattern of incontinence to inform the initial voiding interval on the BT or PV programme.
All patients who were not continent by the end of the 3-day diary underwent a full assessment of their incontinence based on a set of evidence-based assessment criteria and conducted by nursing staff (see Appendix 9). Assessment criteria were taken from a systematic review of methods of diagnosis and assessment of UI,111 these included an initial assessment (including history taking and physical examination); urine dipstick examination and (if indicated) a mid-stream urine specimen tested by microscopic examination, culture and sensitivities; an estimation of post-void residual urine volume, when indicated by the history/3-day diary (using the bladder scanner provided by the project); and an identification of the type of incontinence [UUI, SUI, MUI (both UUI and SUI), ‘functional’ UI or unclear]. Following this assessment, nursing and medical staff determined the route most appropriate to each patient using the algorithm provided (see Appendix 10).
Conservative interventions
The intervention comprised algorithm-driven individualised SVPs tailored to the physical and cognitive capabilities of each patient. The algorithm specified two routes: a combined package including BT and PFMT for those patients who are cognitively able, and PV for those with cognitive impairment. Protocols for ward staff are shown in Appendices 11–13. BT included three main components: (1) focused education for patients and carers [including information on the anatomy and physiology of the lower urinary tract, the rationale behind the programme and strategies to suppress the urge to void (e.g. distraction and relaxation)31,112]; (2) individualised voiding regimens designed to restore normal voiding patterns by progressively lengthening the time interval between voids, based on assessment of participants’ normal voiding patterns and self-monitoring; and (3) patient-held voiding diary, a cognitive intervention designed to promote self-awareness of voiding habits. 100,113
Pelvic floor muscle training was designed to strengthen types I and II muscle fibres in the pelvic floor. It was intended that patients would perform five fast (3 seconds) and 10–20 sustained (10 seconds) contractions with 10-second relaxation periods between contractions twice a day in line with the protocol by Wyman et al. 31
For those patients with cognitive impairments, the programme consisted of elements traditionally classified as PV. Unlike BT and PFMT, PV is not designed to affect bladder function but to avoid or minimise episodes of incontinence. 114 Participants were approached according to individualised schedules (e.g. every 2 hours during waking hours), asked if they are dry or wet, and prompted to use the toilet. 115 Verbal praise was offered for correct reporting of dryness/wetness and successful toileting. Participants with cognitive impairments were given the opportunity to participate in the education and patient-held diary components of the intervention.
Participants not able to walk to the toilet were assisted by nursing staff. Weekly review of progress, with adjustment or change of route as appropriate, was recommended. Participants were provided with written information about their SVP to enable them to continue with it after discharge from hospital.
Table 34 shows how the intervention was informed by the findings of the evidence synthesis on the barriers and enablers to successful implementation of conservative interventions for UI (see Chapter 2), in line with the MRC framework for developing and evaluating complex interventions. 59,60
| Recommendation for practice | Action taken |
|---|---|
| (1) Include teaching of stress strategies in PFMT protocol | Exercises to strengthen pelvic floor muscles included in protocol |
| (2) Check performance of PFMT exercises | Guidance provided for men and women in protocol |
| (3) Use behavioural headings in intervention protocols | Headings such as ‘patient education’, ‘review’ and ‘maintenance’ included in protocols |
| (4) Add section to the patient education booklet entitled ‘what other people have said’ and ‘keeping up the practice’, together with strategies for doing this | Sections added to the patient education booklet |
The SVP was incorporated into routine practice by all staff in day-to-day contact with patients. All nursing staff [including health-care assistants (HCAs), night staff and student nurses] were provided with an education programme of both theory and practice (developed by the research team and the research programme’s two dedicated PPC groups) enabling them to implement the programme. Training was largely web-based to facilitate easy access and flexibility, but face-to-face sessions were offered to cover the practical aspects of intervention delivery and recording. The online training programme has been endorsed by the UK Stroke Forum Education and Training (UKFST, URL: http://ukfst.org/, reference number QM0056) and can be accessed at URL: http://breeze01.uclan.ac.uk/p9llwxl5z18/, with the username ‘iconsuser@uclan.ac.uk’ and the password ‘stroke’.
Additional 2.8 whole-time equivalent HCAs (i.e. one extra HCA per daytime shift, including weekends) were employed on the unit for the duration of the intervention period. Participating units in both the case study and trial phase were also provided with a bladder scanner and training in its use from the supplier (Verathon®, Medical UK Ltd).
Systematic voiding programme plus supported implementation
This trial arm received the intervention as outlined above, plus supported implementation using facilitation.
Designing the implementation strategy
The implementation literature highlights the non-linear flow of knowledge, including evidence from research, into and within health-care organisations. 41 This emphasises the need to embed the investigation of implementation issues within evaluation programmes,59,60 including consideration of how use of the SVP could be maximised within this randomised trial and subsequently.
As a science, knowledge translation lacks conceptual and theoretical clarity,116 where implementation, improvement, innovation, change management and organisational learning can be blurred. Although all these fields include some elements of change processes, variations exist in the nature and sources of knowledge driving change. Although components of the SVP have been shown to be effective in some (mainly primary care) settings,25,27 its adoption within acute stroke services is new. Specific implementation challenges may be anticipated through variation in both beliefs about the transferability or clinical ‘fit’ between the SVP and the acute stroke care context,117 the credibility of underpinning evidence,45 and the quality of processes used to support implementation. 118
Considerable effort has been spent in exploring the utility of psychological theories of behaviour change in explaining individual clinician’s use of evidence119,120 and across organisational settings. 121 However, there is growing recognition that the implementation of evidence in practice is also influenced by the organisational context in which clinicians operate. 40–43,122 Organisational context has been defined as ‘the environment or setting in which the proposed change is to be implemented’. 123 At its simplest level, context may refer to the physical environment where health care takes place. However, Rycroft-Malone et al. concluded from their concept analysis that contexts conducive to research implementation included a range of less tangible process elements: ‘clearly defined boundaries; clarity about decision-making processes; clarity about patterns of power and authority; resources; information and feedback systems; . . . and systems in place that enable dynamic processes of change and continuous development’. 45,124
Theories underpinning organisational influences on implementation include that of learning organisations (with characteristics encompassing hierarchical structure, information systems, human resource practices, organisational culture and leadership46) and knowledge management (how organisational mechanisms affect knowledge uptake and use). 47,48,125 Although barriers and enablers may be identified through evaluation of these aspects of the implementation context, other factors associated with the target practice and its organisation will also need assessment and management.
In this study, the implementation of systematic voiding regimes may be influenced by staff knowledge and skills, resource availability, competing clinical demands and the systems that may influence practice in this area. Although both contextual perspectives may be a legitimate focus of attention within implementation initiatives, tensions exist between designing multicomponent implementation strategies that have maximum impact126 and ensuring explanatory power through theoretical integrity in strategy selection.
Successful implementation of an intervention to improve the management of post-stroke UI is likely to be mediated by individual members of staff and availability of evidence-based guidance, the complexity of the intervention as well as the interplay of patient, social and organisational factors. 49,50 The literature suggests that careful attention needs to be paid to the specific barriers to change in any given setting, identified through ‘diagnostic analysis’ at levels that may include the individual, groups or teams, organisations and the wider health-care system. 51 Tailoring in implementation can refer both to the adaptation of evidence to local clinical settings,116 and the selection of strategies to change professional practice that take account of recognised obstacles to, or enablers of, implementation. 52 A common theoretical approach used in implementation research draws on Lewin’s127 classic work on force field analysis, where competing fields require active management for successful change. Although consideration of context provides some indication as to where influencing factors may be identified, how they influence implementation will only become evident (and potentially managed) as the new practice is ‘normalised’, or embedded within other clinical work.
Approaching implementation from a ‘normalisation’ theoretical lens55–58 is expected to provide real-time information on barriers and enablers of change as clinicians engage in the work of implementing the SVP. We anticipated this information would be a useful resource for supporting implementation at a local level. In addition, this focus on implementation work would address the limitations of considering contextual influences associated with either the acute stroke service configuration or the implementation context described earlier.
We therefore evaluated whether or not supported implementation, through targeted organisational development aimed at ‘normalising’ the intervention, was more effective than introduction of the intervention alone.
Services randomised to the supported implementation arm of the trial received the SVP together with supported implementation comprising diagnostic analysis of context at the level of the organisation; identification of barriers (defined as ‘factors that impede the implementation of change in professional practice’52); and facilitators to the intervention, as well as targeted organisational development activities.
Facilitation
To support the process of implementing the SVP, we used a form of facilitation, a model that has been used successfully in secondary care settings128,129 and is currently the focus of an international trial of ‘technical’ and ‘enabling’ facilitation in a nursing home context. 130 The process of facilitation involves supporting and enabling people to change their practice. 128,129 Although approaches to facilitation vary, they are based on the principle that ownership is with the group. 131,132 The facilitator guides the group towards accomplishing a goal, helping members identify the obstacles that may impede progress and enabling them to identify strategies to overcome them. 132
Facilitation has not received much attention in systematic reviews of implementation strategies to change professional practice,133 although it is likely there is overlap between this strategy and the use of local opinion leaders and educational outreach (or academic detailing), respectively, identified as interventions of variable and consistent effectiveness. 133 It has come to prominence principally as a component of the Promoting Action on Research Implementation in Health Services130 framework. 43,130,134
Although there was a focus on goal attainment (defined as the normalisation of the SVP), our approach to facilitation primarily focused on ‘enabling’ rather than ‘doing for’ others,128,129 with an emphasis on developing and empowering both individuals and teams.
We used both internal and external facilitation. External facilitation aimed to help internal facilitators at the research sites to understand how to bring about change in health professional practice in order to embed the SVP within usual practice. External facilitators (EFs) also supported local facilitators in the form of encouragement, mentoring and providing feedback. These activities are in line with Stetler et al. ’s132 findings from their retrospective evaluation of facilitation from the viewpoint of implementation change agents. Enablers of external facilitation were providing motivation and leadership; team understanding and support of the role; maintaining contact with internal change agents and appointing facilitators with the requisite skills; and experience and/or personal attributes.
We employed the expertise and experience of at least one specialist practitioner (staff members expert in the field of stroke and incontinence) per stroke service allocated to the ‘supported implementation’ arm to serve as internal facilitators. Internal facilitators were required to possess characteristics outlined in Box 1. Their aim was to help teams work together, provide the necessary information and training, maintain motivation and give feedback and practical help when needed.
-
Has some knowledge of good practice in continence care and has an interest in the topic (has a positive attitude towards evidence and how evidence can help develop this aspect of care and can demonstrate some essential knowledge of continence promotion and key aspects of best practice in continence management, e.g. assessment, use of continence aids).
-
Knows coworkers (has been in the organisation long enough to know the staff and how they work).
-
Knows the environment (has some insight into the culture of the setting).
-
Knows the organisation (knows their way around the organisation, e.g. who’s who, policies in place, decision-making structures).
-
Occupies a clinical leadership position (one where they have authority or are able to negotiate authority to make decisions about practice; how practice is organised; resources impacting on practice).
-
Possesses effective communication skills (could include attributes of being open minded, being creative, has experience of managing meetings/groups, able to talk in front of groups).
-
Is self-aware and resilient (has insight into their support needs, but is also not afraid of challenge/conflict; willing to engage in own professional development).
Underpinning the facilitation process was the facilitation manual (see Appendix 14). The manual was loosely based on an action planning framework which allows barriers and enablers to be made explicit, and addressed through facilitation activities. Barriers and enablers were identified a priori, drawing on findings from the case study and the soft systems analysis of the continence system; there was also scope for these to emerge through ongoing internal facilitation activities. The manual also included a ‘toolkit’ of facilitation interventions and strategies based on the recent taxonomy developed by Dogherty et al. 135 Internal facilitators were asked to record which strategies they used and to reflect on these in terms of their usefulness in enabling and supporting change.
External facilitators worked with each site to develop an action plan (see Appendix 15) to structure facilitation work and encourage the development of objectives, action plans and an analysis of barriers and facilitators to implementing these. EFs provided support throughout the intervention period through a mixture of face-to-face meetings, teleconferences and e-mail correspondence.
Chapter 4 Case study
Overview
Our research programme aimed to develop, implement and explore the potential clinical effectiveness and cost-effectiveness of a SVP (including BT and PFMT for patients who are cognitively able and PV for patients with cognitive impairments), with or without supported implementation, for the management of UI after stroke in secondary care. The programme design was based on the UK MRC framework for the evaluation of complex interventions. 59,60 This chapter presents the development phase, a case study of the introduction of the SVP in one stroke service in north-west England.
Objectives
Objectives were to inform the Phase II trial by:
-
identifying systems affecting the likelihood of the SVP becoming embedded in mainstream stroke clinical care
-
exploring health professionals’ views about the acceptability of the SVP
-
measuring presence/absence of UI and frequency of UI episodes at baseline and 6 weeks post stroke
-
investigating factors affecting discharge UI
-
assessing adherence to intervention paperwork (3-day diaries and daily clinical logs).
Methods
Design
A mixed-methods single case study approach136 was chosen for its suitability in investigating highly contextualised and complex phenomena. 137 Methods included analysis of context using interviews with clinical leaders analysed using soft systems methodology; a process evaluation using focus or group interviews with staff delivering the intervention and analysed using normalisation process theory (NPT); and outcome evaluation using data collected from patients receiving the SVP and analysed using descriptive statistics.
Setting
An 18-bedded acute stroke unit in a large trust serving a population of 370,000 and with teaching and foundation trust status.
Subjects and sampling
Patient inclusion criteria:
-
aged ≥ 18 years with a diagnosis of stroke based on the World Health Organization (WHO) criteria138 (no upper age limit)
-
UI as defined by the ICS139 as ‘involuntary loss of urine’
-
conscious (defined as a Glasgow Coma Score140 of ≥ 12)
-
medically stable as judged by the clinical team
-
incontinence classified as SUI, UUI, MUI or ‘functional’ UI. 141
Health professional and clinical leader inclusion criteria:
-
health professionals either delivering the intervention or linking with the intervention in any capacity.
Consent
Local research ethical approval was granted by Bolton Research Ethics Committee on 24 April 2009 (09/H1009/15). Research and development and site-specific approval was given on 26 November 2009; approval was also obtained from the University of Central Lancashire Faculty of Health and Social Care Ethics Committee (FHEC) on 2 July 2009 (CA 138).
Participant consent process
All patients admitted to the unit during the intervention period were screened for eligibility; patients were recruited as early as possible following admission. Informed consent to collect baseline and outcome data was sought from all patients meeting the inclusion criteria. Nursing staff asked these patients whether or not their name could be given to the research team. If the patient agreed, a member of the research team visited the patient to explain the project, answer any questions the patient (and their families/carers) had and provided a participant information sheet (see Appendix 16). Patients were given at least 24 hours to consider participation and were visited by a member of the research team after this period; patients choosing to participate signed the consent form at this stage.
For patients unable to consent for themselves, a person able to advise on the presumed wishes of the patient was approached to act in the role of consultee. This is in line with the recommendations of the Mental Capacity Act 2005 (www.legislation.gov.uk/ukpga/2005/9/contents). 142
Health professional and clinical leader consent process
Health professionals and clinical leaders were identified by the research team in collaboration with senior nurses, medical staff and other health professionals working in the stroke service. Health professionals and clinical leaders were approached by a member of the research team. An initial appointment was made with each potential participant, where the study was explained and a participant information sheet provided (see Appendix 17). Health professionals and clinical leaders indicated they may like to take part by returning a card to the research team. A further appointment was made with those indicating they might like to take part, where signed consent was obtained.
The intervention
The intervention (see Chapter 3) comprised a SVP including assessment (including a 3-day diary and comprehensive continence assessment), algorithm-driven individualised conservative interventions tailored to the physical and cognitive capabilities of each patient and weekly review.
All eligible patients admitted to the participating stroke service were treated using the SVP, regardless of whether or not they consented to collection of outcome data: successful implementation of the intervention was deemed more likely if all eligible patients were included. Risks to patient safety were minimal, although there was a potential risk of demoralisation if the programme was unsuccessful.
The ward manager, two ward sisters, 12 staff nurses and 13 HCAs completed the online training programme. Additional training was provided by a clinical nurse specialist in the workplace and comprised one half-day session (repeated several times to ensure the majority of ward staff had the opportunity to participate). The content of face-to-face training is shown in Table 35; training materials are available on request.
| Topic | Content |
|---|---|
| Introduction to the ICONS study | Study aim and methods of the case study phase |
| Definitions of ‘continence’ and ‘incontinence’ | |
| Anatomy and physiology of the lower urinary tract | Normal urinary functioning
|
| Types of UI | UUI SUI MUI and SUI Overflow incontinence |
| UI following stroke | Detrusor hyper-reflexia (overactive bladder) Detrusor hyporeflexia (underactive bladder) Outflow obstruction |
| Assessment of UI | |
| Introduction to conservative interventions | BT PV PFMT |
Methods of evaluation
Four methods of evaluation were used.
Identifying organisational context for embedding the systematic voiding programme
A soft systems approach143 was used to identify systems affecting the likelihood of the SVP becoming embedded in mainstream stroke care. The stroke pathway can be conceptualised in terms of the patient’s journey through constituent services which vary in purpose, structure, location and workforce. The patient’s experience of UI is similarly longitudinal, and has the capacity to span the entire pathway. This poses a challenge to achieving continuity in all aspects of incontinence care and associated patient management, which is similarly multifaceted drawing on different interventions delivered by different professional groups in different contexts. These contexts include, among others, different settings (acute stroke unit, rehabilitation unit, community, care home) and different practice paradigms (emergency/acute, rehabilitation, continuing, palliative).
The ICONS study clinical intervention can be conceptualised as one component of a whole incontinence care system and our aim was to ensure that the intervention could be embedded within this. The first step was to understand the incontinence care system. This information was used to inform subsequent stages of the research which focus on implementation of the new intervention, and the degree to which it is embedded in systems and clinical practice. To ensure this phase of the research programme was manageable within planned resources, the analysis focused in detail on the stroke unit and primary discharge destinations, and considered other components of the pathway in general.
In systems theory, services are considered as complex human activity systems143 which can be understood in terms of the relationships between their structure, process and outcomes. The aim of systems analysis was to describe relationships and use them to generate a definition of how the service or, as in this context, groups of services worked. This is known as the root definition of the service and describes the relationship between six factors:
-
customers (patients and their family carers)
-
actors (providers of UI care)
-
transformations (key changes occurring as a result of the service)
-
world view (the value system and policy context in which the service operates or the justification for the service)
-
owners or stakeholders of the system (agencies involved in commissioning the service)
-
environment (local conditions that enable or limit UI care).
Although not a hypothesis to be tested in this research programme, understanding the whole system within which the intervention was delivered may have enhanced the degree to which implementation was successful. In addition, when led by theory, this ‘diagnostic analysis’ information may help in the design of transferable implementation strategies by highlighting barriers and facilitators to implementation, workforce education and training issues, and process factors such as requirements for clinical documentation.
A purposive sample of staff engaged in managing and delivering the incontinence systems were approached for inclusion in this study component. Subjects were selected to ensure breadth of coverage in terms of the range of health professionals who provide UI interventions in both primary and secondary care.
The aim of the systems analysis was to evaluate CATWOE (customers, actors, transformations, worldview, ownership and environment) elements and use them to generate a definition of how continence provision worked as a clinical system, highlighting the barriers and facilitators that were anticipated to impede or enhance implementation of the SVP. Qualitative data relating to the evaluation of organisational context were analysed using a directed content analytic approach. 144 This involved drawing deductively on categories derived from pre-existing theory to guide the analysis of interview data within the soft systems framework. 145 The purpose of analysis was to develop new and more specific insights into implementation context by extending the theoretical position within the particular clinical focus of the case study.
Practically, interviews were coded using the major concepts of the framework. Each data set was analysed by two people independently. At regular stages of the analysis, agreement was explored, disagreements discussed and coding frame guidance revised accordingly as more nuanced understandings of the concepts in the study context emerged. During the final stages of analysis, a site summary was created by merging findings across interviews, linking related text chunks together for key concepts and condensing to remove overlap.
Stroke unit staff views of embedding the systematic voiding programme
Six audio-taped focus or group interviews were conducted with health professionals involved in delivering the programme at 1 to 2 monthly intervals throughout the case study. Interviews explored views of the behavioural approaches and their acceptability and feasibility, and the perceived impact of different components of care (e.g. focused education, individualised voiding regimens).
Normalisation process theory55–58 provided the theoretical framework for implementing and evaluating the SVP. The model is designed to facilitate understanding of the practical issues involved in embedding complex interventions into routine practice (e.g. ease of use and integration), and has been used in a range of settings. 55,146 It provides a theoretical framework for both implementation and its evaluation and our intervention provided a good fit with May et al. ’s definition of complex interventions as comprising ‘multiple behavioural, technological, and organisational components’. 57 In addition, the model’s view of change as resulting from collective, rather than individual, action57 is in line with our aim of bringing about change through group activity. The NPT also has a similar emphasis on organisational context. It comprises 16 dimensions in four categories, illustrated in simplified form147 in Table 36.
| Sense-making | Cogntive participation | Collective action | Reflexive monitoring |
|---|---|---|---|
| Could people see how the new practice differs? | Who were the key people driving the new practice forward? | Could people do what the new practice required? | Could people determine the effects of the new practice? |
| Did people agree with the new practice? | Did people agree they should be involved? | Did people feel confident in each other’s work and expertise? | Do people agree about the worth of the new practice? |
| Did people understand what they were supposed to do? | Did people organise themselves to undertake the work required? | Did people have the right skills and training? | Do the people involved think the new practice is worth doing? |
| Do people think the new practice has value for them? | Did people work together to build the procedures required? | Was the new practice adequately supported and resourced? | Did people make changes to the new practice? |
Initially, focus or group interviews were coded using the NPT framework by two people independently; at the completion of coding each interview, agreement was explored, disagreements discussed and coding frame guidance revised accordingly (see Appendix 18). Multiple comments relating to the same point were condensed for each NPT dimension to remove overlap and redundancy. Comments were labelled by respondent to facilitate identifying any variation in findings by staff grade (i.e. HCA for health-care assistant, or Q for qualified staff).
Second, a site summary was created by merging the findings across the six interviews, linking related text chunks together for each NPT dimension and condensing down to remove overlap and redundancy, taking care to avoid loss of meaning or viewpoint. Findings were categorised and reported as either barriers or facilitators to implementation of the SVP. Implications for the trial phase were built from these by the research team and discussed at Steering Group meetings. These were used to refine the SVP intervention, facilitation manual and implementation plan.
Patient outcome and factors affecting discharge continence status
Baseline data
Baseline information about consented participants included:
-
age
-
gender
-
ethnicity
-
date of admission
-
date of stroke onset
-
date baseline questionnaire completed
-
location when recruited into the study
-
consciousness level (defined as either ‘alert’ or ‘drowsy’ on the ‘Clinical Status on Admission’ item of the European Stroke Database)
-
type of stroke
-
stroke subtype [Oxford Community Stroke Project (OCSP) classification]148
-
comorbidities
-
Barthel Index at baseline and day 7 post stroke149
-
Pre-stroke modified Rankin Scale (mRS) handicap score150
-
Incontinence Severity Index (ISI)151
-
Leicester Urinary Symptom Questionnaire (LUSQ) – pre and post stroke152
-
functional incontinence
-
cognitive ability (Abbreviated Mental Test Score)153
-
fluid intake
-
bowel function
-
relevant clinical investigations (e.g. mid-stream urine, bladder scan)
-
medications.
Outcome data
The ICONS research nurse recorded the following information for patients on discharge from the unit or discharge from the SVP, whichever was sooner:
-
date questionnaire completed
-
date of discharge (if applicable)
-
discharge status (alive or dead)
-
discharge destination
-
Barthel Index149
-
LUSQ152
-
ISI. 151
Questionnaires were scanned using ABBYY form reader optical character recognition software (version 6.4, ABBYY Software Ltd, London), transferred into the Statistical Product and Service Solutions (SPSS; IBM SPSS Statistics, Armonk, NY, USA) and analysed using descriptive statistics. For the purpose of analysis, UI was defined as a response other than ‘never’ on the ISI question ‘How often do you experience urinary leakage?’. UUI was defined as the response ‘yes’ to the LUSQ question ‘When you get the urge to pass urine, does any leak before you get to the toilet?’. SUI as the response ‘yes’ to the LUSQ question ‘Do you ever leak when you do any of the following?’. MUI as both SUI and UUI; and functional UI as mobility or balance restrictions stopping patients reaching the toilet on time.
Urinary incontinence at discharge or 6 weeks (whichever was sooner) for patients who started the programme was explored using descriptive statistics; factors affecting discharge/6-week incontinence outcomes were investigated using regression modelling. Using presence/absence of incontinence and number of incontinent episodes in the last 5 days, we performed various analyses to help identify characteristics predictive of outcome. Given the limited sample available, we used findings from the evidence synthesis and discussions with experts, including members of the Steering Group, to identify a set of characteristics previously found to be predictive of outcome among those incontinent following a stroke. These were age, gender, functional ability, prior treatment for UI and prior surgery for UI. The main analysis for identification of characteristics predictive of outcome was based on number of UI episodes in the 5 days prior to discharge. This was based on the following assumptions: for those discharged prior to 6 weeks post stroke, continence levels will be maintained from discharge (as most discharged substantially earlier than this will be fully continent); cases dying between entry into the study and 6 weeks should be excluded. Sensitivity analysis was applied to determine whether or not these assumptions affected the conclusions.
Modelling used approaches appropriate for count data. Given the expected clustering at zero (among those who had regained full continence prior to discharge) and consequent over dispersion, it was anticipated that a negative binomial model might provide a better fit than a Poisson (or normal) model; this was explored during the data analysis which was based around a flexible forward stepwise selection procedure, but always including the number of incontinence episodes in the first 5 days following recruitment. Analysis was repeated for presence/absence of incontinence using logistic regression modelling based initially on terms included in the model for number of incontinent episodes in the last 5 days prior to discharge. Subsequently, terms were selected using stepwise backward elimination (using a 10% significance level for exclusion and a 5% level for reclusion) and any differences in effect sizes investigated and interpreted in the context of the case study.
Adherence to the intervention
Implementation fidelity has been defined as ‘the degree to which an intervention was implemented as was intended’. 154 Measuring the extent to which key components of the SVP, the 3-day diary, BT and PV, were delivered as per protocol was particularly relevant given the exploratory nature of this phase of the research programme. Adherence to intervention paperwork was used to measure fidelity; data was extracted from daily clinical logs specific to each route on the programme and from the 3-day diary.
Daily clinical logs
A clinical log was a single sheet of paper on which nursing staff recorded the patient’s activity on the programme for 1 day. One clinical log should therefore have been completed for each patient for each day that they received the ICONS programme. Given that there are two different types of regime, PV and BT, there were two different types of clinical logs, corresponding to each regime. It was the role of clinical staff to determine which regime a patient should receive (see Chapter 3).
In order to assess adherence to the daily BT or PV regime, all clinical logs were collected for consented patients. The method used to input and analyse data from the clinical logs was based on the identification of key quality indicators of adherence. Indicators were assessed in stages, and are shown in Appendix 19.
Summary measures of the key quality indicators for each clinical log were entered using a proforma to facilitate data input (see Appendix 20). A filtering system was developed, whereby data input for an individual clinical log could be terminated at one of two stages (stage 1 or 2; see Appendix 19). This meant that for each clinical log details of how it performed at each stage were entered, with later stages omitted if earlier stages did not meet the quality indicator.
Data input was initially undertaken by one researcher. Any issues encountered or assumptions made were recorded. Following initial data input, this researcher met with a senior researcher to address any issues that had arisen and verify whether or not assumptions made were reasonable. Any duplicate clinical logs (arising from photocopying errors) were identified and removed from the data set. Finally, the senior researcher checked 10% of the clinical logs for inputting errors. As this revealed an error rate of less than 5%, it was agreed that the team could be confident about the accuracy of the data input and no further data verification was deemed necessary.
The final analysis of the data was undertaken jointly by two researchers. A simple descriptive analysis was undertaken, exploring how well clinical logs performed against the different quality indicators.
Three-day diary
Research nurses were asked to submit a copy of 3-day diaries for all participants for whom one was recorded (i.e. participants who were incontinent at baseline or whose catheter was removed before discharge; participants catheterised throughout their stay were not eligible to complete the diary). Each diary was assessed using a filtering system, with data input for an individual terminated at stage 1 to stage 4 if it failed to achieve the stage’s key quality indicator. These were:
-
Is there a paper copy of the 3-day diary present?
-
Is the diary complete?
-
Is there an entry on each of the 3 days of the diary?
-
Are there three or more entries on EACH day with a time recorded in the ‘time went to the toilet’ column?
-
Are there three or more entries on EACH day where a ‘time went to the toilet’ entry has a value in the ‘leaked’ column?
The assessment of ‘yes’ or ‘no’ for each applicable stage was entered into the SPSS.
Findings
Centre
The original centre chosen as the case study site was not able to supply the excess treatment costs required; this only became apparent after all the initial engagement and training work had been done. It was therefore necessary to find another site and obtain research and development approval, resulting in a delay of around 9 months to the start of this phase. The centre chosen was an 18-bedded acute stroke unit in a large trust serving a population of 370,000 and with teaching and foundation trust status.
Organisational context for embedding the systematic voiding programme
Four group interviews were conducted between February and July 2010. Eighteen staff took part in interviews; Table 37 shows the level of staff present in each interview.
| Interview | Interviewer | Staff present | Number of respondents | Interview length (minutes) |
|---|---|---|---|---|
| 1 | CB/LT | Staff nurse | 2 | 57 |
| HCA | 2 | |||
| Total | 4 | |||
| 2 | CB/HD | Speech and language therapist | 1 | Audio-recording failure – notes written post interview |
| Physiotherapy student | 2 | |||
| Occupational therapist | 3 | |||
| Dietitian | 1 | |||
| Total | 7 | |||
| 3 | CB/LT | HCA | 2 | 35 |
| Stroke specialist nurse | 1 | |||
| Ward sister | 1 | |||
| General manager for medicine | 1 | |||
| Total | 5 | |||
| 4 | CB/HD | District nursing area team co-ordinator | 1 | 51 |
| Continence advisor | 1 | |||
| Total | 2 |
Customers
Incontinence was viewed as a significant problem in terms of prevalence, and these problems were compounded by the complexity of stroke-related disease consequences and the high prevalence of comorbidities such as dementia. Other challenges related to a lack of patient awareness, where nurses perceived that
a lot of people don’t know [they’re incontinent].
(interview number) 1; (transcript line) 118
In the following example, the complexities associated with delivering safe moving and handling processes challenged the delivery of effective incontinence care:
with the hoisting of the patients as well even though if they can tell you that yes I need the toilet . . . by the time you have got the machine, you’ve got the sling in, you’ve hoisted them up onto the bed, got the garments down and hoisted them back up again over the bed pan it could be too late because they can’t hold. So then you can get embarrassment and things like that.
1;41
The focus on containment of incontinence related to views about the inpatient and community contexts of continence care, and variations in clinical priorities. Although there was a recognition that different patients may attach different levels of importance to continence issues, continence was considered more of a priority for community services:
what is a major thing to one patient is small to another and vice versa and I think it’s whatever’s most really concerning that patient at that time . . . maybe immediately post stroke that isn’t the least of their worries really and maybe that is something that as they get home continence is more of a problem.
3;49
Actors
Responsibility for assessment of continence issues appeared to lay primarily with a ‘link nurse’: a registered nurse with lead responsibility for practice and an appointed source of practice-related knowledge. At the time of the interview this individual worked night shifts and there were no reported links with community-based specialist continence service. As such, the effectiveness of this approach to knowledge transfer was questionable:
there isn’t a clear strategy for gaining expertise up to date information about best approaches to incontinence are mostly through the link nurse.
2;3
Multiple sources of information for assessment and care planning were available in addition to any assessments completed by the link nurse, although the utility of these was questionable. They included information from family members (although this was reported to be unreliable); other clinical documentation such as fluid balance charts (which reported patients as wet or dry at various times); and from other services accessed during a patient’s journey through the hospital:
it’s in one of the questions inside the kardex on the front sheet – how is your continence, how were you before. That’s mostly down to MAU [Medical Assessment Unit] anyway.
1;134
Different contributions to the assessment of UI appeared to be provided by different allied professional groups relating to their domain of professional practice (rather than some framework underpinning needs assessment). Although this was reported to have some beneficial impacts for care planning and delivery, it was not always clear how these contributions were integrated:
A lot of people, different therapies do some assessment work in relation to incontinence that’s fed into the notes and discussed within the clinical team on a regular basis but that information is also then conveyed into and discussed within multidisciplinary team meetings.
2;3
In any case, discussions of continence care within a MDT context were not goal oriented, and usually occurred within the context of ‘preventing discharge’ or where it ‘limits people’s ability to engage in the rehabilitation process’ (2;3).
Staff were perceived to have a ‘generalist’ role in relation to incontinence, drawing on:
their experience, knowledge and expertise that they have developed through their professional career.
2;3
However, nurses reported their learning in this area to be limited by the service model, with:
limited opportunity to see how patients progress beyond the ward because of the nature of the stroke pathway.
2;3
Transformations
Within the acute stroke context, the overarching aims of (in)continence care related primarily to containment:
make sure they are all clean, dry, comfortable whatever they need whether if there is a continent patient that needs help taking to the toilet you still have to make the time to take them so they don’t have an accident, become stressed and embarrassed.
1;18
Continence issues were rarely discussed in terms of rehabilitation or recovery goals. For example, nursing handover sheets primarily focused on what needed to be done in relation to incontinence:
. . . at handovers they’ll say [someone has] been grossly incontinent. They wouldn’t say right we’ve got to do this we’ve got to that. It sort of highlights who you’ve got to think about as being potentially wet or dry.
1;164
In this respect, the espoused role for nursing in continence care would appear to relate primarily to work associated in dealing with incontinence.
Transformations were addressed mainly in routinised, patterned practice around ‘2-hourly back rounds’. These were thought helpful as they:
-
mimicked routines at home (e.g. going to the toilet after lunch)
-
integrated with patterns of mobility-oriented interventions (e.g. hoisting patterns)
-
integrated with other work routines (e.g. personal hygiene and meal times):
We do back rounds or what we call back rounds which are 2-hourly and especially for incontinent ones or if they need like for the skin and integrity you know they have 2–4 hour back rounds. Try to get it where like you would at home, when you get up the first thing you do is you want the bathroom, it’s same before lunch, after lunch you know in between mid-afternoon if they are going back on bed rest.
1;28
Worldview
The predominant view shared in group interviews was that community services should be the focus of continence care, with acute care delivering incontinence care around containment, balanced with other clinical priorities:
the degree to which it features is influenced by individual problems that a patient may have, but it may fall down the list when there are other issues, particularly ones like patient’s safety, cognition or wandering.
2;3
The views that appeared to justify this delineation in service responsibility related to the ‘home’ as the setting in which people would have to adjust to the consequences of incontinence:
that’s why perhaps the hospital haven’t placed such a big importance on that assessment in that things do change when they get home.
3.42
The focus on containment may relate to other views about incontinence care as being ‘time consuming’:
. . . we can come in in the morning and somebody is wet, bed bath and tidy them up get them dry and go away to somebody else and you have to keep going back to people which it’s not their fault;
1;7
it’s the time and if you’re tied up accidents will happen you can’t be everywhere at once so you know.
1;23
The demand of this ‘incontinence work’ was felt to have negative consequences for other patients:
so many patients incontinent, so many that aren’t . . . the patients that aren’t incontinent don’t get the time that we could spend with them in patient care. They don’t get that one to one.
1;11
Consequently, staff appeared to make trade-offs between the level of continence care that can be provided and other pressures:
We are still doing medicines which can take an hour and a half . . . obviously you know you’ve got to focus on your medicines, you can’t be doing your toileting.
1;53
Ownership
Continence care was variously described as a key nursing function, but with shared responsibility for assessment and monitoring:
the trigger questions need to be asked by whoever that patient and carer come in contact with.
3;70
This was viewed as important when problems associated with UI may be hidden. However, this key function did not translate easily into professional practice in the following ways:
-
A lack of nursing input into MDT meetings made it difficult to highlight continence issues:
So what in your experience about incontinence does go on in MDT?
. . . not a lot really. Not a clue never sat in one. I’ve only done one so. We are not allowed in them.
-
Confusion regarding decision-making in some aspects of continence care [e.g. trial without catheter (TWOC)]:
Would a TWOC . . . be sort of a nursing decision?
Not normally just a nursing decision. Occasionally a doctor’s. It’s usually higher level.
-
Decisions regarding the selection of incontinence aids:
How would you decide which one that you would go for? That’s for management really. There is only one type at the moment.
1;216
Environment
Specialist continence services and expertise were located within the community, reflecting where the majority of the sample felt that continence care beyond containment occurred. Two key characteristics of the environment underpinning continence care within the acute stroke period were highlighted: (dis)continuity and teamworking.
Numerous potential areas for discontinuity were identified at both clinical practice (e.g. assessment during inpatient stay) and organisational (e.g. between inpatient, hospital and community services) levels. Strategic links between specialist continence services in the community and the inpatient stroke service that existed were opportunistic and historical, dependent on existing relationships between individuals:
[Name] sometimes does, is based at . . . and yes I think the proximity to the hospital allows for that in an advisory capacity. [It’s] much easier and its historical.
3;36
Teamworking around continence care was felt to be difficult due the scope of the problem, the complexity of the relevant services, and a focus on traditional ways of ‘enabling’ teamwork:
when you’re looking at really effective continence care post stroke there is such a lot of different specialities, professionals involved and there are a lot of people, we are talking about quite a large number of patients. So to have a specialist multidisciplinary meeting where you looked at every patient would be completely, we just couldn’t do it I don’t think.
3;86
The root definition generated from the soft systems analysis is shown in Table 38.
| CATWOE heading | Definition | Root definition |
|---|---|---|
| Customer | System beneficiaries | Incontinence is a prevalent problem, compounded by stroke-related disease consequences and comorbidities Patients have different priorities around incontinence |
| Actors | People that carry out activities within the system | Lead nursing responsibility for assessment Multiple professional inputs around assessment (driven by domains of professional practice) Integration of information and inputs unclear Knowledge transfer underpinning practice perceived as ineffective |
| Transformations | Changes brought about by the system | Containment within the acute stroke period Interventions to manage containment Focused on routinised, patterned practice |
| Worldview | What views justify the system? | Clinical issue viewed as ‘incontinence work’, the priority of which is relative to other aspects of clinical work Community services were viewed as the place for continence care |
| Ownership | Who drives the system? | Diffuse: generalist responsibility of all staff Claims around the nursing role not always represented in decision-making processes Confused responsibility for some clinical decisions (e.g. TWOC) |
| Environment | Constraints on the system | Expertise and specialist practice existed in community settings Significant potential for discontinuity Teamworking challenging |
Stroke unit staff views of embedding the systematic voiding programme
Interviews were conducted at 1 or 2 monthly intervals throughout the intervention period. Twenty-one staff took part in interviews. Of these, two were ward sister/ward manager level (band 6 and band 7, respectively); seven were staff nurses (band 5) and 12 were HCAs (band 2 or 3). Table 39 shows the level of staff present in each interview.
| Interview | Interviewer | Staff present | Number of respondents | Interview length (minutes) |
|---|---|---|---|---|
| 1 (month 2) | LT | Ward sister (band 6) | 1 | 54 |
| Staff nurse | 2 | |||
| HCA | 2 | |||
| Total | 5 | |||
| 2 (month 4) | LT | Staff nurse | 1 | 35 |
| HCA | 2 | |||
| Total | 3 | |||
| 3 (month 6) | LT | Staff nurse | 1 | 57 |
| HCA | 2 | |||
| Total | 3 | |||
| 4 (month 8) | HD | Staff nurse | 1 | 27 |
| HCA | 2 | |||
| Total | 3 | |||
| 5 (month 9) | HD | Staff nurse | 1 | 41 |
| HCA | 2 | |||
| Total | 3 | |||
| 6 (month 10) | HD | Ward manager (band 7) | 1 | 41 |
| Staff nurse | 1 | |||
| HCA | 2 | |||
| Total | 4 |
Coherence: the sense-making work that people do when faced with a new practice
Differentiation
Differentiation is about whether or not staff perceive a difference between what they were doing before and the new practice. Components of the intervention such as positive praise, bladder diaries and PV were not seen as new:
the prompted voiding thing, we did that anyway . . . it was just never recorded.
Q1
However, staff recognised how the components of the intervention were used more frequently,
I suppose it wasn’t as often . . .
Q3
and that the programme also made them more aware of time:
you realise how quickly time goes.
HCA3
Practice was seen as different in terms of time spent focusing on the issue of continence,
at least you’re . . . sitting down and discussing things with them
Q2
and the outcome was also seen as different:
the patients – many of them become continent . . . it is different from the normal experience.
Q2
Communal specification
Communal specification is about whether or not people have a shared understanding of the new practice. There was no evidence that staff did not have a shared understanding of the intervention on the basis of the training received; there was also no evidence of disagreement about the overall aim of the intervention. In fact, the intervention could act as a focus for patients to work with staff towards a common goal:
Plus it gives the patient the incentive as well doesn’t it, cos you’re saying right, 2-hourly . . . to keep you dry.
Q4
There was some comment relating to initial changes in the intervention:
it was supposed to be physio doing bladder training.
Q3
Also, the patients’ families could misunderstand the purpose or intended outcome of the intervention, thinking it might prolong treatment:
I’ve had two patients come to me and say, oh, they’re not doing that well why? It’ll prolong the treatment. I want them to come home.
HCA6
There was some indication that the intervention was interpreted quite widely, as in the case of a gentleman who couldn’t hold his bottle but was continent otherwise:
I would think why was he on it because he is not incontinent? He may have a few accidents but he knows he is having an accident . . . but like [staff name] said, it’s all about how best for him to use his bottle.
HCA2
Individual specification
Individual specification is about whether or not individuals understand what the new practice requires of them. There were numerous examples of initial misunderstanding over who was responsible for delivering the intervention by all grades of staff. There were also a number of examples of staff not being involved from the beginning so missing explanations of the intervention, or missing the training. However, there were also perceptions that staff were choosing not to be aware of what the new practice required:
. . . some people haven’t got a clue what they are supposed to be doing . . . cos they’re not bothered.
HCA5
The algorithm was perceived as clear and helpful and the view was that staff should not have difficulty understanding what to do:
to be honest, the laminated sheets that are up behind the nurse station tell you everything you need to do.
Q3
However, even though the instructions were seen to be clear, not everybody appears to have been informed, although this was perceived by other staff as an issue of motivation rather than knowledge:
you have the laminated sheets as big as that you can’t miss it, and even last week a staff nurse said to me ‘I didn’t know anything about it’. . . so unless it’s actually saying you are responsible for that particular piece of work they said I didn’t know.
Q3
Although there was a perception that there had been adequate initial preparation, staff commented on the need to keep up with changes in how the protocol was administered over time:
That (weekly review) was the result of the last meeting, because things were being missed, and I didn’t know I had to do that.
Q3
It is possible that people might not have been informed of changes in how the protocol was being done on the ward because they were off duty. So, despite the fact that there was a simple and easily understood algorithm, there may also be a need for a procedure or responsibility for keeping new or temporary staff involved and for keeping all staff updated.
The paperwork and documentation appeared to be an area for lack of understanding, such as the change to a 7-day diary and the scoring system for amount of UI. However, the paperwork might also have been acting as a prompt sheet for tasks that qualified staff were not fully aware of responsibility for:
you know you’re going through the blue sheet . . . has this been done, has that been done . . . is this meaning that should have been done?
Q4
The impact of the training on people’s understanding of what they were supposed to do appeared to be low; many commented that the training should be more practically orientated, rather than theoretical. However, specific examples of areas of confusion in understanding included confusion about the types of incontinence and how these relate to the different interventions. There appeared to be some difficulty in choosing a time schedule for voiding,
you look at it and think, maybe 3 (hours), maybe 2,
Q6
and a lack of clarity about the method of increasing the time between voids, with most staff talking in terms of increasing time in 1-hour slots whatever the patient’s voiding pattern:
Well if they’re incontinent every 5 minutes then you do it every 2 hours, but if they’re not, then you could leave it every 3 hours.
Q4
Internalisation
Internalisation concerns whether or not people see the potential value of a new practice. The ICONS intervention appears to be valued under specific conditions:
I would say that it’s worth doing cos it does benefit . . . I’d give it a go. If you’ve got the staff it works.
HCA4
However, some aspects of the new practice were not uniformly valued: and may not have been used:
I didn’t self-praise them at the end, I think it belittled them.
HCA5
Continence care was not recognised as a priority over other aspects of care on a routine day-to-day basis:
You can’t just leave one dependent person, just to put somebody on the toilet.
HCA1
Continence was also viewed as less important than other needs:
you know their condition is not going to deteriorate, I know it sounds horrible, but nobody’s going to die if. . ., but if somebody can’t cannulate them or give them a drug, yes they will.
Q3
The acute care setting also impacted on the priority given to continence:
the busier, the more acute the ward gets, the more the toileting programmes get put to one side, which is understandable.
Q3
Staff could see the benefit of the intervention for some patients and the importance of continence to patients was recognised:
. . . it’s devastating even for an old person . . . it’s not the fact that they can’t move so much or the speech has gone a little deteriorated . . . its someone’s continence that brings them to tears.
Q3
Success with the intervention could increase the priority of continence:
I think as a result of the ICONS, everybody on the ward realised how important it was because we can see improvements, so you can see it works.
Q2
However, staff did not view the intervention as of value for everyone, particularly those who did not make any progress, but they could not necessarily predict who would benefit. Some patients also did not want to be involved:
Some of the patients just don’t want to do it anyway, do they?
HCA4
while another patient was reported as asking to continue the intervention after discharge from the ward.
In general, staff could easily see the value of the intervention for patients, but not so easily for themselves. The success of the intervention could increase feelings of guilt for staff if the intervention was not able to be delivered properly:
You feel bad because [patient’s name] knows and she is trying to ask you . . . and it’s a shame when you can’t get to her . . .
HCA4
Cognitive participation: the relational work that people do to build and sustain a new practice
Initiation
Initiation is about whether or not key individuals drive the new practice forward. The ICONS HCAs reported taking responsibility for keeping the paperwork available and informing other staff:
I would make sure I went round each ICONS patient, make sure they had a sheet, and then inform whoever is in that team if they go to the loo, . . . if I don’t get back, will you do it? Just go through it with them. We have a lot of bank staff as well.
HCA2
Qualified staff were also involved in inducting new staff, and ensuring that everyone was aware of ICONS on the morning hand over:
you just have to point it out in the mornings . . . and identify anybody that is on the ICONS study and just try and push that forward.
Q1
A link nurse for continence was also involved in increasing awareness of the ICONS programme. The ward manager had been reported as stating that they wanted to continue the programme once ICONS had finished. There was also some evidence that qualified staff were to some extent balancing negative comment with positive comment, particularly around positive outcomes for patients and the work saved for staff in terms of not having to change beds.
Enrolment
Enrolment is about whether or not people agree that the new practice should be part of their work. There was consistent reference to initial difficulty in knowing who was responsible for ICONS work and numerous examples of confusion, although these appear to have been resolved over time.
The major issue of enrolment of patients centred around who should be eligible for the programme, with numerous comments by staff about whether or not patients with cognitive difficulties should be involved:
I think it’s the individual patient as well, their cognitive issues, and that’s made a big difference hasn’t it on how the patient responds. Its the cognitively impaired patients that we don’t seem to have a great amount of success.
HCA1
Staff thought there were patients who would never be successful on the programme:
no matter how long you do this with certain people I don’t think it’s going to work, I don’t think you can get the continence back so I think that needs to be looked at really.
Q1
Staff were also not completely convinced that patients should be put on the programme in the very early stages:
. . . in the early days, when they are just not with it at all, then there’s no way they can learn, it’s too early.
HCA4
I agree, they’re putting them on you’re thinking why? They haven’t got a clue. But maybe then later on 2 or 3 weeks, when they come round a bit more . . .
HCA4
We’re not giving them time to come to terms with having a stroke.
HCA4
Staff debated whether the programme would work better on a rehabilitation rather than acute ward, and expressed uncertainty about whether nursing homes would carry on with the programme.
In terms of enrolment to different routes of the programme, not many people seemed to be on BT. Staff said this was because they lost them from the ward before they reached the stage of being able to undertake this route. One member of staff thought BT would not be useful,
because of the type of patient we had on ICONS, they didn’t understand 100% what we were saying.
HCA2
Staff thought that patients probably agreed to enrolment in the programme, but were not interested in the paperwork:
they don’t have the concentration span to sit there and read. The families – they read it, but I’m not sure about the patients.
HCA1
Some patients or their relatives were not willing to be involved, but one family tried to enrol their relative on the programme even though she wasn’t incontinent. One family refused incontinence assessment as they found it intrusive. A further patient was taken off the programme because her daughter did not want her mother labelled as incontinent, and felt that 2-hourly toileting was ‘mythering’ her. Staff referred to the difficulty of talking to families about the continence status of their relative:
I was asking a man about his mother, whether they were [incontinent] before, it was a bit embarrassing . . .
Q5
Legitimation
Legitimation refers to whether or not people buy into the new practice, and whether or not they are willing and able to organise themselves. There was evidence that, on the whole, staff were organising themselves to undertake the work required although there was repeated reference to the reluctance of some staff to get involved, and some ongoing areas of difficulty such as the hard work of organising two members of staff to toilet patients together in the mornings:
it can be hard work, especially if everyone is doing washes and things.
HCA1
There were also areas where responsibility was still being negotiated or worked out between qualified staff and HCAs; these were altering voiding intervals and completing continence assessments.
Staff commented on the inability or unwillingness of some patients to undertake the work required by the new practice. They referred to patients not putting the effort in, not co-operating, manipulating the programme or not wanting to walk to the toilet, and suggested that patients might not want the programme to interfere with visiting time. Staff thought that patients were not able to fill in their own bladder diaries and their families were not happy filling in diaries either.
There were specific aspects of the intervention that both staff and patients reported difficulty with. Staff referred to the patients’ difficulty with dealing with urinary urgency:
getting them to hold on for 2 hours that’s challenging sometimes, and then they might think they’re taking a backward step if they are incontinence at some time, its like ‘Oh, I can’t do it’.
Q4
This was also challenging for staff to deal with in terms of knowing how to distract the patient. It was also suggested that patients were sometimes incapable of participating because of communication difficulties:
some of the patients can’t give you an answer if you go up to them and said do you want to go to the toilet, some stroke patients’ can’t answer you properly can they?
HCA2
Activation
Activation refers to whether or not people work together to develop the new practice. There was evidence that after initial difficulties, people were working together to develop and embed the new practice:
. . . it was very disruptive at first. Everybody else presumed everybody else was doing it. They’d go to the toilet and you wrote it down and then there would be no chart there, and then you’d go find it and you are backtracking on through fluid balance. It was confusing. I think we got the swing of it in the end.
HCA2
Staff talked about getting into a routine (especially with the paperwork) and working out responsibilities between themselves. They also described a system of allocating daily responsibility and informing staff of people allocated to the programme. Night staff were involved in preparing paperwork; this was driven by the link nurse for continence, who worked permanent night shifts. Staff also talked about adapting the discharge information to include ICONS-related material. They also talked about how they were dealing with problems such as what to do if you miss a toileting time:
well it helps us to put down ‘short staffed’, or ‘had an emergency’; [than to] not put anything because then we’re none the wiser as to what’s going on.
Q3
Some of the collective procedures needed to sustain the new practice were still under development. Qualified staff referred to the difficulty in filling out the assessment because of lack of information either due to patients’ communication difficulties or lack of family input, and stop–start points where the assessment was stopped while waiting for information and then took longer than the suggested 3 days. There was also some discussion around the difficulty of negotiating extended toileting times in collaboration with patients who were reluctant to agree to longer intervals:
it’s harder to get them to go any longer than 2 hours.
HCA1
Once you get going you seem to be going every hour and a half, we’re not making it longer, we are making it shorter.
Q1
There was some level of discord between qualified staff and HCAs about when people should be taken off the programme if it wasn’t working. HCAs perceived that they were doing the work, and that qualified staff were making the decision to continue even though the programme was perceived as fruitless:
it’s us that are doing it and you’re thinking, its make no difference, even though we’re doing it, but they’re keeping them on it, why? . . . It’s the ones in blue making the decision, but it’s the ones in green doing the work.
HCA5
The main difficulty with activation was related to certain aspects of the paperwork. Staff questioned whether or not the screening register needed to be filled in and whether or not totals on daily logs needed to be calculated. There was confusion about the scoring system for amount of UI and some staff talked about wanting something on the paperwork to indicate whether or not someone had used the toilet. There was also some confusion over filling in the time on the diary and also on the daily log.
Collective action: the operational work that people do to enact a new practice
Interactional workability
Interactional workability concerns whether staff or patients are able to do the tasks required of the new practice. There was a perception that the ICONS programme was
being done as much as we can do.
Q1
but that ‘fitting it in around everything else’ was the main challenge. There was also a recognition that despite their efforts it didn’t always work out, and that, like anything new, there was work involved in developing a routine, and
getting it into your workload.
Q6
The dominant theme in interactional workability was that ICONS was ‘extra work’ (Q3), including extra paperwork. Challenges to staff being able to execute the programme successfully included the nature of the acute care setting with staff allocated to other priorities such as thrombolysis; the number and level of dependency of the patients on the ward at any one time coupled with the availability of the staff resource; staff perception of priorities of need:
we could be feeding seven or eight patients . . . nutrition is more important in my mind.
HCA1
Managing the time-constrained nature of the intervention was challenging, including consistency of effort within time limits:
. . . it can be hard work and difficult to do it all the time . . . ;
Q1
or at busy times of day. The extra paperwork was questioned, particularly in relation to admission:
we have so much to do when people come in admitted . . . and we‘ve got all that paperwork . . . ;
Q1
and also at other busy times such as the morning. There was a reference to overlap in paperwork between the fluid balance charts and some ICONS forms, but it was noted that this could also work to advantage if one was missed.
The physical nature of the intervention was a consideration. Staff referred to the ‘hard work’ of trying to integrate the intervention with other physical tasks like washing, and the need to schedule toileting with other required positional changes, but stated that it got better with practice. They referred to the requirement for two people to move someone and the sheer physical difficulty of moving people with hoists, especially if speed was required:
When women are in chairs really it’s a problem . . . you’ve missed it by the time you get them on the bed.
HCA1
The short time frame for toileting was sometimes difficult to meet and sometimes staff were not always able to get there in time. This had consequences for patients, but staff also felt guilty. One qualified nurse referred to being honest in recording what was not done, and why.
Specific difficulties staff saw for patients included not being able to hold on for 2 hours. Staff referred to the difficulty of extending the time, with distraction seen as ineffective:
regardless of how you distract them, they’re sat clock watching. There’s nothing else for them to do . . .
Q6
Staff referred to the need to work toileting times around visiting times, as patients did not want to go to the toilet while visitors were present, and the difficulty of knowing whether a patient had actually used the toilet. Patients were thought to be unable to fill out bladder diaries.
In terms of the intervention as a whole, there was some difficulty in assessing within 3 days if the staff had to wait to talk to relatives, and some difficulty of maintaining continuity between individual patients and staff:
. . . there’s lots of stop–start points . . . like speaking to relatives, leaving the literature out, and when you look at nurses’ rotas . . . being dotted here there and everywhere, it’s very difficult to be part of the process the whole way through.
Q6
Staff also did not think there was much opportunity for people to progress to BT within the short time frame in the acute setting.
Relational integration
Relational integration refers to whether or not staff or patients are confident in each other’s work and expertise in relation to the new practice. There was initial confusion in roles:
everybody presumed everybody else was doing it,
HCA2
and a continuing lack of understanding in some staff:
. . . even now 6 months into it not all the staff on the ward are aware of what they should be doing;
Q3
although this was also interpreted as making excuses for not doing the work.
There were a number of actions staff were taking so that people knew about and were doing what they were supposed to do, including meetings to discuss and resolve issues; checks that the work was being done when it was supposed to be done; clarifying roles and responsibilities between teams; writing on the front of the Kardex that ICONS was everyone’s responsibility; and systems for communicating between staff, for example at discharge. There was also some acknowledgement that things were not always followed through, and that with a new practice people forget and need reminding.
There was underlying grumbling reference by HCAs that qualified staff did not perceive toileting to be their job and that they were avoiding it:
sometimes you get it . . . ’no, I’m the trained staff, I’ve done my bit, you’re ICONS nurse.’ And yet they could have toileted that person while you’ve been on break.
HCA3
An opposing view was that for:
some HCAs on the ward . . . they don’t know what pilot study means and because it’s a research study that’s trained staff.
Q3
Staff on the acute ward were confident that staff in the community had the skills and knowledge to undertake the programme (and a report from one patient confirmed this was happening), but they also referred to the possibility that nursing homes might not continue the programme, or that the continuation of ICONS in the community might not work:
because there is no obvious crossover between the hospital side of the study and the community side.
Q6
Skill set workability
Skill set workability refers to whether or not the work of the new practice is appropriately allocated to people with the rights skills or training. There was again reference to initial confusion over the allocation of work to ICONS nurses and also the allocation of work to specific grades of staff:
. . . we assumed at the beginning it would be the ICONS nurses that would set up the sheet and everything, the assessment, but then it turned out to be trained, but most of the trained didn‘t know this did they?
HCA2
Lack of clarity was cleared up, and:
somebody would take responsibility on each shift as being an identified ICONS nurse . . .
HCA2
This HCA referred to taking responsibility for communicating with other staff who may not know the system, like bank staff. However, it was not clear if this responsibility was seen as endemic practice, or just the initiative of this individual. Other systems to ensure allocation of work included laminated sheets to ensure that staff were aware of the ICONS process and the ward manager putting a notice on the front of the Kardex.
Some aspects of the allocation of work were perhaps not as well embedded; these were articulated as requests within interviews that things should happen in a certain way in the future, including:
if you’re dealing with the person fill the paperwork in . . . ;
HCA3
when you roll this out as full blown research, right from day one . . . make sure you emphasise that it’s every nurse on the ward that takes part in it.
Q3
Like we had the trained staff that do the initial assessments yeah? And then you direct us what pathways, so from then its everybody . . . so it doesn’t matter what rank, forget ranks . . .
HCA3
The division of labour between qualified staff and HCAs was a relatively consistent theme, including who took responsibility for changes to an individual patient’s programme and responsibility for assessment.
Night staff had been drawn into involvement by doing nightly reviews which were reported as being completed most of the time. Other work to integrate ICONS into existing systems for review included specifying that charts were reviewed every night and programmes were reviewed every weekend at night.
The involvement of senior and junior staff in the administration of ICONS as a research programme was hampered by other responsibilities:
I’m coming here, then all of a sudden I’ve got the stroke bleep, I’ve got called to another meeting, and the same applies to other staff . . . [name HCA] can’t today, we’ve got a thrombolysis.
Q6
The response to the training provided was mixed; in general qualified staff thought it was adequate, whereas unqualified staff found it too difficult. The consensus was that it should include more practical detail on the ICONS programme.
Contextual integration
Contextual integration refers to whether or not the new practice is adequately supported by the host organisation. The main message was about staffing:
it’s fine if you’ve got the staff to do it,
Q1
and that the ward did not always receive the extra staff funded by the study. There was a recognition that consistency of staffing was also needed, although demand depended on how many patients were on the programme at once and the requirement for multiple staff to be available for positional changing.
Identifying Continence OptioNs after Stroke-funded staffing was not always protected, with lack of control over staff movement between wards, especially by inexperienced staff nurses at weekends. There was a perception that the protection of staffing had got worse over time, and also a recognition of the need for fairness in staffing and whether or not keeping extra staff would always be fought for:
it depends on how somebody else is struggling.
Q6
The programme was seen to be supported by the involvement of the ward manager. There was some perception that the ICONS programme was perhaps more suitable in a rehabilitation than an acute setting, because staff were pulled away for other priorities in an acute setting, whereas there was more routine in a rehabilitation environment.
Training time was not seen to be supported by the organisation and there was some suggestion that it was not perceived to be acceptable for HCAs to spend time on it:
there’s no way they are going to let you sit behind a desk for 3 hours.
HCA5
Reflexive monitoring: the appraisal work that people do to assess and understand how a new practice affects them and others
Systematisation
Systematisation refers to the ability of people to determine the effectiveness of an intervention. Staff could recognise success,
. . . we’ve had some good successes,
HCA3
and the contribution made by the programme towards overall recovery:
. . . you just see what you’re doing is working . . . they come in they’re unconscious . . . then 3 or 4 weeks later they walk off the ward . . . we’ve done something right.
Q3
Staff could also broadly differentiate levels of progress:
. . . they have all done well out of it, more or less,
HCA2
and quicker improvement in people on BT (Q3). Staff could also identify factors that influenced programme success such as staffing levels.
Although staff had an overall sense that the programme worked for some patients, some of the time, they struggled to remember individuals and could not always see the connection between the decisions they made and patient progress:
the assessments . . . did they help you decide what regime the patients went on?
Erm, erm, well they must have up to a point, yeah, I don’t know. I can’t think of any individual . . .
They also did not think patients necessarily appreciated the significance of the activity being undertaken for their benefit:
. . . you don’t seem to get a reaction from patients do you? Like they don’t really understand what is going on.
HCA2
The evaluation paperwork was an aspect of the programme that drew comment. There was a lack of understanding about the purpose of some data collection, including the screening register, and that some information required on the forms was confusing, including:
the blue sheet, the things that it’s asking you, is this meaning that it should have been done? . . . a PV examination, a PR . . . ?
Q4
and the scoring system for amount of UI. Staff did not see the value of all of the data collection forms:
I think they were a bit repetitive . . . the daily ones.
HCA5
Staff pointed out that forms do not capture all required information:
. . . you asked whether they want to go to the toilet, doesn’t necessarily mean she went . . . you don’t know if she has gone to the toilet between 9 a.m. and 6 p.m. . . .
HCA2
Although this information was on the fluid balance chart and did not record useful summary information:
And it would help the next shift coming on . . . whether they can use a bed pan . . . or toilet.
HCA2
One respondent commented that not all possible evaluation information was collected:
it’s a shame we didn’t do a pressure sore survey concurrently . . .
Q6
The bladder diaries were identified as useful, even though they were completed by staff; however, staff did not get to see summary information from diaries:
I think you would have to ask the ICONS nurse because they take them away, don’t they.
HCA2
Communal appraisal
Communal appraisal concerns whether or not people can use formal monitoring to collectively evaluate if a practice is worthwhile. In the case study, because staff were not necessarily involved in the analysis, this was interpreted as whether people could identify or receive feedback from formal measurement of outcomes.
When asked if they would like more feedback information, staff said that they would, and their comments seemed to indicate that they could not judge whether or not their efforts to implement the programme were successful:
We don’t know how we’re doing . . . just let us know if we are doing it right. You could say for example, the 3-hourly toileting regime prompting, that is being done, that’s lovely, and the paperwork’s being done.
Q3
Staff also did not seem to know generally about incontinence prevalence and their comparative performance against a baseline. In response to examples of success, one respondent asked the interviewer:
OK, that sounds really positive. I was just wondering, is this different to your normal experience . . .?
Q2
One of the reasons why process feedback might be needed is that staff do not necessarily receive outcome feedback, especially over the longer term: patients move on before completing the programme, or before moving onto the BT regime:
We’re possibly losing a big percentage of the group before some progressed to that point.
Q6
One consequence of patients moving on is that staff do not see the benefits of their efforts:
patients are moved on . . . so we don’t reap the benefits do we? . . . So we don’t see the total outcome. We do all the hard work.
Q1
Individual appraisal
Individual appraisal is about whether or not individuals think a practice is worth doing. Staff could appreciate the benefits of the programme for patients, including improved self-esteem, QoL and independence; and less complications, anxiety, agitation and embarrassment. Staff thought the programme gave patients a goal, and that patients could see improvement, which was a boost to morale. They did not think it necessarily impacted on destination at discharge, and that a negative consequence was that patients could be ‘stressed out’ by the programme (Q4).
Staff did not think that the programme worked for everyone, but they could not necessarily predict who it would work for from the beginning. Progress was summed up as ‘hit and miss’ (HCA5), with the programme having worked overall ‘up to a point’ (Q5). Their main comment on programme effectiveness was that it related mainly to cognitive awareness:
it just depended on the individual really. If they were with it, it was no problem . . . There’s no answer for everybody.
Q5
Qualified staff were concerned about trying to improve who the programme was targeted at and the HCAs commented about when the programme was not working:
Carrying it on with some of them . . . it’s making no difference whatsoever and they’re still doing it and you’re thinking, what a waste . . .
HCA5
In contrast, staff did not immediately see benefits for themselves:
I can’t see any benefit for staff, sorry but I can’t.
Q1
Staff did comment about possible benefits for them in terms of ‘saving effort later’ (HCA6), such as less washing, changing beds and less treatment of pressure sores. The main benefit was in seeing improvement in patients and the satisfaction of seeing the documentation completed well. There was also a benefit of having a goal, and learning. Possible negative aspects for staff included stress due to the pressure of additional work balanced against the benefit of seeing patients progress.
Reconfiguration
Reconfiguration is about how people modify their work in response to their evaluation of the new practice. There was some suggestion that individual members of staff were slightly modifying aspects of the programme they found difficult, such as not making patients resist feelings of urinary urgency for long, and also modifying their own approach to managing continence:
. . . whereas before you didn’t really clock-watch, now . . . you realise how quickly time goes.
HCA3
There was also some indication that aspects of the programme were being modified perhaps because they were misunderstood, such as starting people on a 3 hourly schedule, and if that did not work, moving them to 2 hourly (Q4).
There were also reports of formal meetings where aspects of process (i.e. how the work was to be done) were modified, including involving night staff and starting daily reviews of forms and weekly reviews of progress. Staff requested specific modification to some aspects of the paperwork, such as the screening register and daily logs.
Summary of facilitators and barriers to introducing the systematic voiding programme
Facilitators and barriers to introducing the programme are shown in Table 40.
| NPT constructs | Facilitator | Barrier |
|---|---|---|
| Coherence | ||
| Differentiation | Can see the newness of the programme as a whole, and the emphasis on continence | Do not view the programme components as new |
| Communal specification | General agreement on the aim of the programme in terms of impact on continence | Possibility of wide interpretation of intervention purpose/outcome in staff and in patients/relatives |
| Individual specification | Good algorithm clearly explains programme. Most staff understand what to do | Some difficulty in keeping up with changes and in specific aspects of programme delivery – mainly paperwork – but also determining voiding interval |
| Internalisation | Value of the programme for some patients is clearly recognised, but benefit is only achievable with additional staffing | Value of prioritising continence may not be agreed for all patients. Benefit is balanced against other priorities in acute care, and the effort needed |
| Cognitive participation | ||
| Initiation | Qualified staff, nominated HCAs and ward manager influential in embedding and supporting programme | Some qualified staff appear less involved |
| Enrolment | Some patients and their relatives agreed to enrolment in the programme, if not every detail. Staff agreed to enrolment on condition of extra staffing | Eligibility of patients with cognitive or communication difficulty, those showing no progress, or those very early in recovery was disputed |
| Legitimation | Staff were organising themselves to carry out the programme, including issues of responsibility for or input into decision making about programme changes for individual patients | Some patients were perceived as unwilling or unable to participate, and both staff and patients had difficulty dealing with urinary urgency |
| Activation | After initial difficulties, staff were working together to develop new routines and procedures. Some collective procedures are still under development | There is some discord between HCAs and qualified staff about involvement in toileting, and discontinuing the programme for individuals |
| Collective action | ||
| Interactional workability | The programme is being done as well as they are able | The programme is extra physical work, which is difficult to manage within time constraints in an acute setting, where other tasks take precedence |
| Relational integration | People are clear about their responsibilities, and in the main things are being done when they should be | There was some suspicion that the programme would not be continued in other settings |
| Skill set workability | There are systems in place to allocate work | There are some tensions between HCAs and qualified staff |
| Contextual integration | The programme was seen to be supported by management at ward level | Extra staffing and training time requirements were not seen to be consistently supported |
| Reflexive monitoring | ||
| Systematisation | Staff could recognise success, broad levels and speed of progress, and influencing factors | Staff did not understand or see the value of all of the data collection |
| Communal appraisal | On the whole, staff can see the value of the intervention in individual patient’s progress to continence | Staff do not receive sufficient feedback on process or longer-term outcome |
| Individual appraisal | Staff can appreciate the benefits for certain patients | Staff do not easily appreciate the benefits for themselves |
| Reconfiguration | The formal organisation of programme modification is ongoing in meetings, etc. | Individual staff report trying to informally modify aspects of the programme they find difficult to manage, but there is no formal system for this feedback loop |
Patient outcomes and predictors of discharge continence status
Patient characteristics
Forty-three patients were recruited between January and September 2010. The total number of patients screened was 263. Of those screened, 163 (62%) were continent, 31 (11.8%) were medically unstable and three (1.1%) had a Glasgow Coma Score less than 12. The remaining 23 patients were not recruited for various reasons including refusal to consent (n = 1), transferred (n = 4), or discharged (n = 1). Table 41 shows characteristics of all patients recruited and all patients who were put on the programme.
| Characteristica | All patients (n = 43) | Patients on programme (n = 28) |
|---|---|---|
| Age (years): mean (SD) | 75.1 (13.5) | 76.8 (13.1) |
| Sex: male | 16 (37.2%) | 10 (35.7%) |
| Ethnicity | ||
| Caucasian | 41 (95.3%) | 26 (93%) |
| Indian | 1 (2.3%) | 1 (3.5%) |
| Black African | 1 (2.3%) | 1 (3.5%) |
| Stroke subtype | ||
| TACSb | 15 (34.9%) | 9 (32.1%) |
| PACS | 18 (41.9%) | 12 (42.9%) |
| LACS | 9 (20.9%) | 6 (21.4%) |
| POCS | 1 (2.3%) | 1 (3.6%) |
| Barthel Index at baseline: median (IQR) (n = 41, 26) |
3 (1–6.5) | 4 (1–7) |
| Barthel Index at day 7: median (IQR) (n = 41, 27) |
4 (1–7) | 5 (1–7) |
| Pre-stroke mRS (n = 41, 27) | ||
| No symptoms | 1 (2.4%) | 1 (3.7%) |
| Minor symptoms | 1 (2.4%) | 0 (0%) |
| Minor handicap | 15 (36.6%) | 12 (44.4%) |
| Moderate handicap | 11 (26.8%) | 5 (18.5%) |
| Moderate severe handicap | 8 (19.5%) | 7 (25.9%) |
| Severe handicap | 5 (12.2%) | 2 (7.4%) |
| LUSQ | 1 (2.4%) | 1 (3.7%) |
| Pre-stroke SUI (n = 35, 24) | ||
| No | 19 (54.3%) | 14 (58.3%) |
| Yes | 8 (22.9%) | 5 (20.8%) |
| Not known | 9 (25.7%) | 5 (20.8%) |
| Pre-stroke UUI (n = 40, 26) | ||
| No | 19 (47.5%) | 14 (53.8%) |
| Yes | 11 (27.5%) | 7 (26.9%) |
| Not known | 10 (25%) | 5 (19.3%) |
| Pre-stroke MUI (n = 41, 27) | ||
| No | 19 (46.3%) | 14 (51.9%) |
| Yes | 8 (19.5%) | 5 (18.5%) |
| Not Known | 14 (34.1%) | 8 (29.6%) |
Patient trajectory
Twenty-eight patients commenced the SVP and 15 patients did not (Figure 24); some were ineligible due to becoming continent on the 3-day diary, discharge before completing the 3-day diary and, in one case, death on the day of recruitment. A further three patients might have been eligible for the programme after catheter or penile sheath removal; it is unclear why these patients did not commence the programme.
FIGURE 24.
Route taken by recruited patients.

The majority of patients (19, 68%) commenced a PV routine, four (14%) commenced BT, three (11%) had both regimes (in two cases moving from PV to BT) and for two (7%) the route was unknown. Most patients remained on the programme between 0 and 13 days (8, 30.8%) and 14 and 27 days (13, 50%) [median 16.5 days, interquartile range (IQR) 5.75–26.25 days, range 0–64 days].
Implementing PFMT required the support of the physiotherapy team in terms of assessing whether or not participants were able to exercise their pelvic floor muscles. The physiotherapy team were reluctant to participate in this and it was therefore not possible to implement this part of the SVP.
Characteristics of recruited patients are shown in Table 41. Mean age of patients recruited was 75 years; the range was 42–98 years. Two-thirds were female and the majority of patients were Caucasian (41/43, 95.3%). Most patients (33/43, 76.8%) had middle cerebral artery strokes [total anterior circulation syndrome (TACS) or partial anterior circulation syndrome (PACS)] and minor to severe handicap pre stroke, with only two patients (4.8%) having no or minor symptoms. Eleven were incontinent prior to stroke, with eight (22.9%) having SUI, 11 (27.5%) UUI and eight (19.5%) MUI (UUI and SUI).
Table 42 shows baseline continence status of all patients on the programme. Most patients had functional incontinence (23/25, 92%), with 5 out of 27 (19%) having both UUI and SUI. Nearly half of patients for whom data were available and who were put on the programme (12/25, 48%) had very severe incontinence as measured by the ISI.
| Characteristica | Patients on programme (n = 28) |
|---|---|
| Median (IQR) UI episodes in the 5 days following recruitment (n = 26) | 8.5 (4–13) |
| Baseline continence status (n = 27) | |
| Continent | 2 (7%) |
| Incontinent | 25 (93%) |
| Baseline SUI (n = 26) | |
| No | 5 (19%) |
| Yes | 6 (23%) |
| Not known | 15 (58%) |
| Baseline UUI (n = 27) | |
| No | 5 (19%) |
| Yes | 13 (48%) |
| Not known | 9 (33%) |
| Baseline MUI (n = 27) | |
| No | 5 (19%) |
| Yes | 5 (19%) |
| Not known | 17 (62%) |
| Baseline functional incontinence (n = 25) | |
| No | 1 (4%) |
| Yes | 23 (92%) |
| Not known | 1 (4%) |
| Baseline ISI category (n = 25) | |
| None (0) | 2 (8%) |
| Slight (1–2) | 3 (12%) |
| Moderate (3–6) | 5 (20%) |
| Severe (8–9) | 3 (12%) |
| Very severe (12) | 12 (48%) |
| Time on programme in days (n = 26) | |
| 0–13 | 8 (31%) |
| 14–27 | 13 (50%) |
| 28–41 | 3 (12%) |
| ≥ 42 | 2 (8%) |
Patient outcome
Table 43 shows patient outcomes for patients who undertook the SVP.
| Outcomea | Patients on programme (n = 28) |
|---|---|
| Median (IQR) number of UI episodes in the 5 days prior to discharge (n = 19) | 5 (2–8) |
| Change in number of UI episodes (5 days prior to discharge minus 5 days following recruitment) (n = 18) | 3 (–0.3 to 8.3) |
| Change in number of UI episodes (5 days prior to discharge minus 5 days following recruitment) for patients with 1–7 UI episodes in first 5 days (n = 5) | 0 (–1 to –2) |
| Change in number of UI episodes (5 days prior to discharge minus 5 days following recruitment) for patients with 8–17 UI episodes in first 5 days (n = 12) | 7 (0.75–10.5) |
| Stroke unit discharge status | |
| Alive | 27 (96%) |
| Dead | 1 (4%) |
| Stroke unit discharge destination (n = 23) | |
| Private address (not alone) | 4 (17%) |
| Residential home | 3 (13%) |
| Nursing home | 3 (13%) |
| Rehabilitation unit | 5 (22%) |
| Other hospital | 2 (9%) |
| Other ward (same hospital) | 6 (26%) |
| Median (IQR) Barthel Index (n = 27) | 8 (5–11) |
| Continence status (n = 28) | |
| Continent | 6 (21%) |
| Incontinent | 20 (71%) |
| Catheterised | 1 (1%) |
| Missingb | 1 (%) |
| ISI category (n = 17) | |
| None (0) | 4 (15%) |
| Slight (1–2) | 5 (19%) |
| Moderate (3–6) | 5 (19%) |
| Severe (8–9) | 3 (11%) |
| Very severe (12) | 9 (35%) |
| SUI (n = 12) | |
| No | 6 (50%) |
| Yes | 6 (50%) |
| UUI (n = 14) | |
| No | 7 (50%) |
| Yes | 7 (50%) |
| MUI (n = 12) | |
| No | 9 (75%) |
| Yes | 3 (25%) |
Of the 28 patients on the programme, six (21%) reported being continent at 6 weeks post stroke. Similar numbers of patients reporting the reason for their incontinence reported UUI, SUI, MUI and other incontinence problems. More than half of those with continence problems (12/22, 55%) reported severe or very severe problems on the ISI. The median reduction in the number of incontinence episodes (over a 5-day period) was three episodes overall, rising to seven in patients with the highest number of incontinent episodes at baseline. Only four (17%) patients were recorded as being discharged to a private address, none of whom returned to living alone.
Factors affecting discharge continence status of patients on the programme
Forward selection led to gender (p < 0.0001), baseline Barthel Index category (p = 0.001) and age (p = 0.078) being included in the best-fitting Poisson model; OCSP category was non-significant (p = 0.14).
These terms were then considered in the logistic regression model for presence/absence of incontinence. Age (p = 0.96) and baseline Barthel index category (p = 0.27) were eliminated from the model at the first two steps, leaving only sex as a predictor of incontinence. Moreover, when considered as a potential additional predictor, OCSP category was again non-significant (p = 0.720) and age did not re-enter the model on removal of baseline Barthel Index category (p = 0.99) (Table 44).
| Characteristica | Continent | Incontinent | Multiple logistic regression | |
|---|---|---|---|---|
| OR (95% CI) | p-value | |||
| Sex | 0.091 | |||
| Maleb | 4 (40.0%) | 6 (60.0%) | – | |
| Female | 2 (11.1%) | 16 (88.9%) | 5.33 (0.77 to 37.09) | |
| Baseline Barthel Index (n = 26) | 0.27 | |||
| 0–6b | 3 (15.8%) | 16 (84.2%) | – | |
| 7–13 | 3 (42.9%) | 4 (57.1%) | 3.13 (0.41 to 24.02) | |
| Mean (SD) agec | 77.0 (13.6) | 76.7 (13.3) | 0.99 (0.46 to 2.16) | 0.99 |
| OCSP classification (n = 27) | 0.72 | |||
| TACSb | 2 (22.2%) | 7 (77.8%) | – | |
| PACS | 3 (25.0%) | 9 (75.0%) | 3.19 (0.14 to 74.85) | |
| LACS | 1 (16.7%) | 5 (83.3%) | 1.22 (0.08 to 19.37) | |
Adherence to the intervention
Three-day diary
Table 45 shows quality of completion of the 3-day diary. Completed diaries were present for 30 patients. Two patients who commenced a regime did not have a diary, while four patients had a diary but did not start a regime: in three cases they were continent by the end of the diary period and for one patient the reason is not clear. The majority of diaries completed (27, 90%) had an entry on each of the 3 days. Few patients (7, 23.3%) had three or more entries on each day with a time recorded in the ‘time went to the toilet’ column. Of these, six (20%) patients had three or more entries on each day where a ‘time went to the toilet’ entry had a value in the ‘leaked’ column.
| Stage | Key quality indicator | Number (%) meeting quality indicator | Number (%) not meeting quality indicator |
|---|---|---|---|
| 1. Diary present | Is there a paper copy of the 3-day diary present? | 30 | 0 |
| 2. Diary completed | Is the diary completed? | 30 (100) | 0 |
| 3. Entry on each of 3 days | Is there an entry on each of the 3 days of the diary? | 27 (90) | 3 (10) |
| 4. ‘Time went to the toilet’ completed | Are there three or more entries on EACH day with a time recorded in the ‘time went to the toilet’ column? | 7 (23.3) | 23 (76.7) |
| 5. Values in ‘leaked’ column completed | Are there three or more entries on EACH day where a ‘time went to the toilet’ entry has a value in the ‘leaked’ column? | 6 (20) | 24 (80) |
Daily clinical logs
Clinical logs were received and analysed for 331 days from 22 patients; 261 (78.9%) were for PV and 70 (21.1%) were for BT. Table 46 shows quality of completion of the daily logs. Although the majority (80.1%) had a regime interval documented, only 39.3% had a regime interval and correct schedule of proposed times. For these clinical logs, it was documented that patients were taken to the toilet within 30 minutes of the scheduled time on 59.0% of occasions; on average, it was documented that patients on PV were asked if they were dry or wet on 74.7% of occasions, and encouragement was documented as given on 75.2% of occasions.
| Stage | Quality indicator | Result |
|---|---|---|
| 1 | % (of clinical logs processed) with regime interval present and correctly documented | 80.1 |
| 2 | % (of clinical logs processed) with both regime interval and schedule of proposed times present and correctly documented | 39.3 |
| For clinical logs that achieved both stage 1 and stage 2 (n = 130) | ||
| 3(b) | On average, how often was a ‘time toileted’ documented that was within 30 minutes of the proposed time? (%) | 59.0 |
| 4(a) | On average, how often was it documented that the patient had been asked if they were wet?a (%) | 74.7 |
| 4(b) | On average, how often was encouragement documented as given? (%) | 75.2 |
Implications for the trial phase
The proportion of all patients admitted with incontinence (100/263, 38%) was at the lower end of expected prevalence4,16 and of these, fewer than expected were recruited (43/100, 43%). Recruited patients tended to be at the more severe end of the spectrum in terms of stroke and incontinence severity; a possible reason could be that patients with mild incontinence and less severe strokes were among those transferred or discharged before they could be recruited. This has implications for the trial in terms of extending estimates of the recruitment period. Trial participants were also likely to be at the severe end in terms of both stroke and incontinence severity posing practical challenges in implementing the SVP with patients needing considerable assistance with toileting.
Findings from the soft systems analysis suggest the intervention was introduced into an environment not conducive to therapeutic continence management. The overarching aim of continence care was keeping patients ‘clean, dry and comfortable’, with continence rarely discussed in terms of rehabilitation or recovery goals. A similar focus on containment rather than rehabilitative activities has been reported elsewhere. 12,75 Continence care was usually couched in terms of being ‘time consuming’ and staff appeared to make trade-offs between the level of continence care that could be provided, and other pressures.
Soft systems analysis revealed a context where incontinence was viewed as a significant problem, but this was not reflected in the organisation and delivery of continence care. There was a mismatch between stated importance and clinical practice, with continence peripheral to, rather than embedded in, rehabilitation. The site was therefore starting from a low base characterised by a lack of clinical and organisational structures facilitating continence management. It was clear implementation effort required in the trial phase were considerable if sites were comparable to the case study site.
Normalisation process theory findings showed that although there was misunderstanding and confusion during initial implementation of the SVP in terms of roles and tasks, over time there was embedding of processes facilitated by new routines and procedures. The value of the programme was recognised for some patients and visible examples of success motivated staff to continue, although benefits for cognitively impaired patients were less obvious. Staff broadly agreed about the purpose of the intervention and found the programme understandable, although aspects of the paperwork and determining the voiding interval, posed some difficulty. Adherence data also reflects problems staff encountered with paperwork: 3-day diaries were completed, and the majority had entries on all 3 days, but detailed completion of voiding times and wet episodes was rare. Similarly, although the majority of daily clinical logs had a voiding interval documented and there was evidence of variation in toileting interval, only a third had proposed times present and correctly documented. A more in-depth training in SVP paperwork and processes, with adequate time and support to practice, was recommended for the trial phase.
In terms of building and sustaining the new practice, qualified staff (in particular the link nurse), HCAs and the ward manager were key, with some qualified staff less involved. Methods of engaging qualified staff will need consideration prior to the trial phase; this is required if active continence management is to be viewed as a specific therapeutic intervention155 and given increased status comparable with other rehabilitation activities.
The acute setting meant continence care had to be balanced against other priorities and for some staff was of less importance at this stage of the patients’ trajectory. Furthermore, outcomes of continence work were not visible, as patients were often transferred before an improvement could be seen. Early intervention is recommended in the latest policy10 and we therefore included acute units in the trial phase, but also the rehabilitation units linked to these to enhance exposure to the SVP across both phases of the patients’ stay in hospital.
Only 28/43 (65%) of recruited patients began the SVP. Catheterisation (or penile sheath use) was cited as the reason for 10 of these; however, it is not clear (a) why patients catheterised throughout did not have catheters removed and (b) why patients catheterised at baseline were not subsequently put on the SVP. Attention to catheter removal needed to be made explicit in the trial protocol in line with policy;10 systems for documenting reasons why patients do not begin the SVP also needed revising.
The case study did not set out to assess the effectiveness of the programme, and the acute unit context meant few patients received the SVP for longer than 4 weeks; at least 6 weeks is recommended. 25,31 One-quarter of patients became continent and there was a median reduction of three incontinent episodes over a 5-day period, with a median reduction of seven episodes in patients with more incontinent episodes at baseline; this was viewed as clinically significant by our ICONS PPC involvement groups.
Adherence to the intervention paperwork left room for improvement with only six (20%) of the 3-day diaries fully completed and only 39.3% of daily logs with both regime interval and schedule of proposed times documented. Problems with paperwork were also highlighted in ward staff NPT interviews and indicated revisions and clarifications were needed prior to the trial phase.
Although implementing the programme was viewed by the research team as encompassing all the MDT, and efforts were made to spread awareness and encourage participation in ICONS training, in practice it fell almost exclusively to nursing staff. In particular, physiotherapy staff did not feel able to assess if patients were able to exercise their pelvic floor muscles, a necessary requisite before beginning PFMT, therefore it was not possible to introduce the combined intervention. Lack of therapist involvement in identifying, assessing and managing UI after stroke is also highlighted by Dumoulin et al. ;156 this is concerning given the key role recommended by both evidence157–159 and policy,141 and highlighted the need for enhanced strategies to promote ‘buy in’ in the trial phase.
Chapter 5 Exploratory cluster randomised controlled trial: methods
Overview
This chapter describes methods used in Phase II of the research programme (MRC feasibility and piloting phase), a cluster randomised controlled exploratory trial.
Aim
The trial aimed to assess the feasibility of a full-scale cluster randomised trial through testing the interventions for preliminary evidence of clinical effect and providing information to enable estimates of the number of sites and patients who would need to be recruited for a full-scale cluster randomised trial to evaluate effectiveness.
Objectives
The trial objectives were to:
-
assess feasibility in terms of rates of participant recruitment and retention (cluster level)
-
assess fidelity to the intervention (cluster level)
-
conduct a qualitative assessment of feasibility from the perspective of multiple stakeholders (cluster level)
-
conduct a preliminary evaluation of supported implementation and intervention alone, relative to usual care (cluster level)
-
investigate patient-related factors affecting patient outcome (cluster and individual patient level)
-
investigate stroke service-level factors potentially affecting stroke service outcomes to estimate the amount of unexplained variability in outcomes between trusts and between patients (cluster and individual patient level)
-
confirm the choice of primary and secondary outcome measures for a full-scale cluster randomised trial to evaluate effectiveness (cluster level)
-
develop and test data collection tools for an economic evaluation within a full-scale cluster randomised trial (cluster level).
Design
A three-arm, parallel, open, exploratory, pragmatic, cluster RCT of a SVP (including BT and PFMT for patients who are cognitively able and PV for patients with cognitive impairments), with or without supported implementation, for the management of UI after stroke in secondary care. The intention was for the whole stroke unit team to implement the SVP so, to minimise contamination, allocation was based on clusters, and the unit of randomisation and analysis was the stroke service.
Study setting
Twelve NHS stroke services in England and Wales. For the purpose of the trial, a stroke service comprised both acute and rehabilitation stroke units. Stroke units were defined according to the definition provided by the Royal College of Physicians, London, for the National Sentinel Stroke Audit. 160
Inclusion and exclusion criteria
Stroke services
Inclusion criteria
-
Stroke services with specialist acute and rehabilitation stroke services (either separate or combined units).
-
Access to appropriate excess treatment costs.
Exclusion criteria
-
Stroke service without specialist acute and rehabilitation stroke units (either separate or combined).
Patients
Inclusion criteria
-
Aged ≥ 18 years with a diagnosis of stroke based on the WHO criteria138 (no upper age limit).
-
UI as defined by the ICS139 as ‘involuntary loss of urine’.
-
Conscious (defined as either ‘alert’ or ‘drowsy’ on the ‘Clinical Status on Admission’ item of the European Stroke Database).
-
Medically stable as judged by the clinical team.
-
AND
-
-
Incontinence classified as SUI, UUI, MUI or ‘functional’ UI.
-
OR
-
-
Catheterised in the acute phase of the stroke.
Participants who had incontinence before the index stroke were included. Given the expected age range of the population, there was likely to be a high prevalence of pre-stroke incontinence among potential participants. Furthermore, there is evidence to suggest patients with longstanding incontinence may benefit from a programme of behavioural interventions. 25,27
Participants who were catheterised were recruited; if the catheter was removed, they were assessed as per protocol and began the SVP if they were still incontinent. Participants who were continent after catheter removal and those discharged with a catheter still in situ were not put onto the SVP.
Exclusion criteria
-
Pre-existing long-term catheter.
-
Routine self-catheterisation prior to stroke.
-
Patients who refused consent.
-
Patients unable to consent for whom a consultee did not agree that the patient would wish to be included.
Health professionals and clinical leaders
Inclusion criteria
-
Health professionals either delivering the intervention or linking with the intervention in any capacity.
Centre recruitment process
The trial was adopted onto the Stroke Research Network (SRN) portfolio and was identified as open to new sites using SRN procedures. The original intention was to include sites in north-west England only and the research team made contact with all stroke services in this region and invited expressions of interest. Six stroke services in Lancashire and Cumbria were able to find the excess treatment costs; service support costs were provided by the Comprehensive Local Research Network (CLRN). Eight stroke services in Cheshire and Merseyside expressed an interest in participating, but were unable to supply excess treatment cost funding. It was not feasible to include a further 10 services in Greater Manchester as they used a ‘hub and spoke’ model with multiple rehabilitation units linked to one acute unit. Consequently, an approach was made to the Welsh National Institute for Social Care and Health Research (NISCHR); excess treatment costs are held centrally by the Welsh Office rather than with each health board and initial discussions suggested there was likely to be a favourable response to supporting the trial. Potential sites in Wales were invited to attend a meeting to discuss suitability for inclusion; six sites met the inclusion criteria and agreed to participate.
Following randomisation, one Welsh site declined participation due to changes taking place within their stroke service, leaving little scope for supporting a new study. A replacement site with similar throughput and Sentinel Stroke Audit score was recruited (the rehabilitation unit accepting patients from the acute unit used in the case study phase). Newcastle Clinical Trials Unit advised substituting the Welsh site with the recruited site in the randomisation stratum and rerandomising this stratum. Subsequently, a further Welsh site was found to have a much lower number of admissions per annum than anticipated (around 120 compared with an original estimate of 300). A chance meeting with the assistant lead nurse of a potential site in West Anglia led to the inclusion of a replacement site. Newcastle Clinical Trials Unit advised substituting the old with the new site rather than rerandomising at this stage, as training had begun in several sites randomised to receive the intervention by this time.
Participant recruitment process
All patients admitted to participating stroke units were screened within 72 hours of admission using a screening form in line with the inclusion criteria (see Appendix 21).
Patients not catheterised
In intervention units, potential participants who met the inclusion criteria began a 3-day bladder diary (see Appendix 8). The consent process then began for all patients where the diary showed evidence of involuntary leakage of urine and who were medically stable. In usual care, potential participants identified as incontinent by ward nursing staff were screened for eligibility. In all trial arms, potential participants who were not yet medically stable were reassessed by the research nurses and the ward team at regular intervals until they were deemed to meet this criterion.
Patients catheterised
These patients began the consent process as soon as they were medically stable.
Nursing staff asked each eligible patient whether or not his/her name could be given to the research team. If the patient agreed, a member of the research team visited the patient, explained the project, answered any questions and provided a participant information leaflet (see Appendix 22). Patients were given at least 24 hours to consider participation and were visited by a member of the research team after this period; patients choosing to participate signed the consent form at this stage (see Appendix 23).
For patients unable to consent for themselves, a person able to advise on the presumed wishes of the patient was approached to act in the role of consultee. This is in line with the recommendations of the Mental Capacity Act (2005)142 and in line with the expressed wish of the study PPC involvement groups that everyone who was eligible should have the opportunity to participate.
Recruitment rates in each cluster were monitored on a regular basis to identify both quantitative (i.e. numbers of participants recruited) and qualitative (i.e. patient characteristics) imbalance across clusters. 161 If identified, imbalance was thought to be indicative of likely selection bias (e.g. cluster with low proportion of stroke patients identified as eligible but high proportion of those eligible with severe incontinence), we reviewed the recruitment process and addressed any issues identified.
Interventions
Systematic voiding programme
Participants received the SVP (see Chapter 3) comprising assessment (including a 3-day diary and comprehensive continence assessment); algorithm-driven individualised conservative interventions tailored to the physical and cognitive capabilities of each patient; and weekly review. In the light of case study findings, the following changes were made:
-
greater focus on preparing ward staff adequately before the intervention began
-
increased focus on obtaining therapist ‘buy in’, through meetings with the programme co-ordinator and encouragement to attend training sessions
-
more emphasis on practical aspects of implementing the SVP in face-to-face training, including detailed explanation of paperwork
-
simplification of daily clinical logs.
Systematic voiding programme plus supported implementation
This trial arm received the intervention as outlined above, plus supported implementation using facilitation (see Chapter 3).
Usual care (control group)
Participants in this group received usual care provided by the stroke service. This could comprise checking for urinary tract infection; checking for overflow incontinence (using the bladder scanner provided); containment using a variety of devices (e.g. absorbent products) with regular changes and some form of toileting schedule.
Outcomes
The primary effectiveness outcome was participant incontinence (presence/absence). Presence or absence, rather than severity of incontinence, was chosen on the advice of the PPC groups based on their view that achievement of full continence was key for people with stroke. Measures were taken at 6, 12 and 52 weeks post stroke. The primary analysis was initially of the 6-week data as conservative interventions for continence typically last 6 weeks,25,31 and an effect was most likely to be seen at this time point. However, it quickly became apparent that 6 weeks after commencement of the intervention was more in line with 12 weeks post stroke owing to participants not being recruited as soon after stroke as anticipated. The Trial Steering Group therefore approved a change to primary analysis of 12-week data.
Secondary effectiveness outcomes were QoL, frequency and severity of incontinence, urinary symptoms, activities of daily living (ADLs) and death; at discharge, 6, 12 and 52 weeks post stroke.
Ascertainment of outcomes
Presence/absence of incontinence was measured by the International Consultation on Incontinence Modular Questionnaire – Urinary Incontinence (ICIQ-UI) Short Form. 162 Absence of incontinence was defined as the response ‘never’ to question 3, ‘How often do you leak urine?’; presence of incontinence was defined as any other response to question 3 (ranging from ‘about once a week or less often’ to ‘all the time’). The ICIQ-UI Short Form has received a grading of Anew – highly recommended from the International Consultation on Incontinence Symptoms and Quality of Life Committee indicating published reports of acceptable reliability, validity and responsiveness in at least one study. 163 To our knowledge, the ICIQ-UI Short Form has not been used in the post-stroke population. We have conducted preliminary validation of the tool with six stroke survivors from our PPC involvement group using the approach recommended by the ICIQ developers (Dr Nikki Cotterill, University of Bristol, 2009, personal communication); all thought the tool was appropriate for use post stroke and few problems were identified.
Quality of life was measured using the I-QOL164,165 and the European Quality of Life-5 Dimensions (EQ-5D). 166 As the I-QOL has only been validated for people with incontinence, data were not analysed for patients who were continent or catheterised. Frequency and severity of incontinence was ascertained using the ISI. 151 Urinary symptoms were measured using the LUSQ152 and ADL using the Barthel Index. 149
In addition, the following baseline information about the patient were recorded following consent: date of birth (age to be calculated*); sex; ethnicity; date of admission; date of stroke onset; date baseline questionnaire completed; location when recruited into the study; consciousness level (defined as either ‘alert’ or ‘drowsy’ on the ‘Clinical Status on Admission’ item of the European Stroke Database); side of body affected by stroke; type of stroke; stroke subtype (OCSP classification148); day 7 Barthel Index;149 pre-stroke mRS*;150 pre-stroke living circumstances*; LUSQ;152 type of UI (UUI, SUI, MUI, ‘functional’ UI or unclear); cognitive ability (six-item Cognitive Impairment Test167); fluid intake; bowel function; relevant clinical investigations (e.g. mid-stream urine, bladder scan); medications; living circumstances; verbal subsection of the Glasgow Coma Scale*;140 ability to lift both arms off the bed*; ability to walk independently*; ICIQ-UI Short Form;162 ISI;151 EQ-5D. 166
The six factors highlighted with a ‘*’ above form the Edinburgh stroke case-mix adjuster168 and were collected to enhance patient-level prognostic adjustment of the statistical modelling of outcomes.
Data collection
Baseline data were collected on entry to the trial by the research nurse. Outcome data were collected at 6, 12 and 52 weeks via postal questionnaires (or researcher administered questionnaires if participants were still in hospital at 6 or 12 weeks; see Appendix 24). All participants with aphasia were offered a face-to-face interview with the research nurse to collect outcome data; this was conducted using appropriate communication aids (e.g. pictorial cards with a ‘thumbs up’ picture indicating ‘yes’ and a ‘thumbs down’ card indicating ‘no’). Specialist help was available from speech and language therapists and Speakeasy, a specialist aphasia charity based in Ramsbottom, Bury (www.buryspeakeasy.org.uk/), if this was needed.
Postal and telephone reminders were used if questionnaires were not returned within 2 weeks. Where completion of postal questionnaires was not possible, participants (or carers if a proxy was needed) were invited to complete assessments over the telephone. If neither postal or telephone completion was possible, a face-to-face assessment in the participant’s home was offered.
Sample size
The sample size was chosen pragmatically, rather than on the basis of a formal sample size calculation. Our aim was to balance practicalities and the need for reasonable precision in the estimation of effects to inform the sample size calculation for a full-scale trial to test effectiveness.
The use of four stroke services per arm (12 in total) was deemed to suffice for an indication of likely effectiveness and helped us address any feasibility issues relating to the delivery of the interventions. It also provided some degree of confirmation regarding the size of the intra-class correlation coefficient and enabled us to perform a review of the number of sites (and the number of patients needed per site) for each arm of the trial for a full-scale trial. It also provided some information on stroke service-level factors which may help explain the variability in outcome between stroke services in each arm, thus potentially helping to reduce the intraclass correlation coefficient and hence achieve efficiency in terms of the number of stroke services required for a full-scale trial.
We estimated that 12 stroke services would admit around 4500 patients per year, of whom we expected around 20% would meet the trial inclusion criteria and consent to participate. To achieve better balance in the number of participants recruited per trust/health board, we planned to recruit for 12 months in services recorded as admitting 300 or less patients per year and for 9 months in trusts recorded as admitting more than 300 patients per year; our initial target for recruitment was 780 patients across the 12 services.
Centre randomisation
Sequence generation
In order to ensure comparability of trial arms with respect to type of unit, quality of care and throughput, stroke services were placed into four strata. These were based, in order of priority, on (i) whether they had separate or combined acute and rehabilitation units at the time (in one site separate units had combined by the start of recruitment); (ii) their average performance on the ‘nine key indicators of stroke care’ in the National Sentinel Stroke Audit Phase II11 (clinical audit); and (iii) the number of stroke patients admitted per year. Services were randomly allocated to intervention (n = 4), supported implementation (n = 4) and usual care (n = 4) arms by the Newcastle Clinical Trials Unit. After allocating hospitals to the strata, the randomisation schedule was generated using block randomisation (block length of three) to allocate one site to each arm within every stratum. The software package Stata (version nine; StataCorp LP, College Station, TX, USA) was used.
Allocation concealment
Allocation was based on clusters. Within each stratum, stroke services were not informed of their intervention allocation until ALL stroke services within that stratum were recruited to take part in the trial. However, when two sites required substitution the rest of the stratum were already aware of their allocation.
Blinding
Once stroke services within a stratum were recruited, services were then made aware of their allocation, as were staff identifying and recruiting trial participants from within that service. Outcome assessment for participants still inpatients at 6 and 12 weeks post stroke was done by research nurses; therefore blinded outcome assessment was not possible. Although we originally intended research nurses to collect outcome data in sites other than their own (and in which they were unaware of allocation), the geographical spread of sites meant this was not feasible.
The trial statistician was not blinded during the analysis, although the statistical analysis plan was finalised prior to any outcome data being available to the trial statistician. Some post-hoc subgroup analyses and sensitivity analyses were, however, determined subsequently.
Data analysis
Recruitment
Each month, proportions of stroke patients meeting the inclusion criteria for the trial and proportions of these patients actually recruited were compared descriptively between clusters and intervention groups.
Baseline data
Baseline characteristics were summarised using means (with SDs), or median (IQR) if the data were continuous or counts, respectively, or frequency (percentage) if the data were dichotomous or categorical. All outcome data were summarised in a similar manner.
Outcome data
The primary analysis was performed using intention to treat. All stroke services randomised were retained in the trial. Outcome data were collected from all consented patients whenever possible, whatever their level of subsequent engagement with the allocated intervention programme. For the 6-week outcome time point, outcome data received no later than 10 weeks post stroke were included in the primary analysis; for 12- and 52-week outcomes, all data received were included.
To account for the cluster randomisation, we used mixed-effects modelling for continuous, ordinal and dichotomous outcomes to compare the two groups on primary and secondary outcome data. Baseline measures of the outcomes variables (where appropriate), stroke subtype and the other prognostic patient-level information (from the Edinburgh case-mix adjuster168) were included as individual-level covariates in the models for outcome data.
Missing outcome data were imputed according to the particular outcome. For the primary analysis, dichotomous and ordinal outcomes for those who withdrew, died or were otherwise lost to follow-up were imputed using a worst-case scenario (e.g. for the primary outcome variable, all those for whom incontinence status was not recorded at the respective time points post stroke were assumed to be incontinent). For continuous outcomes (I-QOL), the primary analysis used a non-parametric multiple imputation approach. 169 Missing baseline data were not imputed.
The effect of stroke service-level factors was also explored in the modelling with the intention of investigating potential stratification factors for a future effectiveness trial, and to potentially reduce the size of the intrastroke service correlation coefficient by reducing the unexplained component of the variability between stroke services.
Subgroup analyses
Pre-planned exploratory subgroup analyses were for the following variables:
-
pre-stroke incontinence (with/without)
-
sex (male/female)
-
type of incontinence (UUI; SUI; MUI; ‘functional’ UI; unclear)
-
sex combined with type of incontinence
-
OCSP subtypes [unconscious; TACS; PACS; posterior circulation syndrome (POCS); lacunar stroke (LACS); unclassifiable]
-
baseline bowel function (7-day Barthel item: incontinent or occasional accident; fully continent)
-
baseline ISI (dry; slight; moderate; severe)
-
pre-stroke mRS (0–2 little or no handicap; 3–5 moderate or severe handicap)
-
type of stroke (ischaemia; haemorrhage)
-
side of stroke (left; right; both; neither)
-
type of intervention (catheterised throughout; intervention with BT at some stage; intervention but never BT).
Additional post-hoc exploratory subgroup analyses were performed for age (as a continuous variable), baseline cognitive impairment status (not cognitively impaired; cognitively impaired or unknown cognitive status), SUI (presence; absence) and UUI (presence; absence).
Subgroup analyses were performed by adding to the model an interaction between the (three-group) intervention factor and each of the factors listed above individually. Interactions significant at α = 0.1 were deemed indicative of a potential subgroup effect. It was also planned to explore jointly any interactions individually significant at α = 0.1.
Sensitivity analysis
Various sensitivity analyses were performed on the primary outcome measure. These analyses included:
-
the use of alternative analytical approaches, given the small number of clusters
-
complete case analysis
-
alternative imputation methods using a non-parametric multiple imputation169 approach for dichotomous and ordinal outcomes data
-
per-protocol analysis, using varying definitions of ‘receipt of sufficient of the intervention’
-
varying the eligibility criteria for timely response at each outcome time point
-
excluding patients with pre-stroke incontinence
-
excluding patients catheterised throughout their hospitalisation.
Ethical aspects
The trial was approved by Bradford Research Ethics Committee (Reference number 10/H1302/60), which has a lead responsibility for studies with mental capacity issues, on 10 August 2010; site research and development departments; and by the University of Central Lancashire FHEC on 11 August 2010 (CA168).
Informed consent to participate in the trial was sought from participants themselves or from a consultee, as outlined above. All patients were informed that participation was voluntary and that they were able to withdraw at any time. UI is a sensitive issue and the approach taken with participants needed to reflect this. Our study had two dedicated PPC involvement groups; both provided advice on recruitment strategies as well as assisting in the development of patient documentation.
Trial management and monitoring
The Trial Management Group (comprising all programme applicants) met every 3 months to discuss the day-to-day management and running of the programme. The Trial Steering Committee met every 6 months with a remit including review of objectives and progress against plans and objectives and monitoring the quality of the research to help ensure it contributes to knowledge at a national and international level.
The Independent Data Monitoring Committee (IDMC) were responsible for safeguarding the interests of trial participants, assessing the effect of the interventions during the trial, and for monitoring the overall conduct of the clinical trial. The IDMC were advisory to the trial sponsor and the Trial Steering Group and met biannually.
Chapter 6 Process evaluation: methods
Overview
This chapter presents methods of the process evaluation. We introduce the logic model underpinning the evaluation and describe the evaluation structure in line with Grant et al. ’s170 framework for process evaluations of cluster RCTs.
Process evaluation of implementation
An integrated multiple component evaluation was conducted in order to describe implementation and assist in explaining why the intervention and its components were, or were not, successful. Process evaluations examine what the intervention comprises and how it is delivered to target participants;154 they are designed to evaluate fidelity and provide explanatory evidence around trial outcomes. Fidelity is defined as ‘the degree to which programs . . . are implemented as intended by the program developers’. 171 At its simplest level, fidelity is measured in terms of adherence to the intervention (i.e. content, coverage, frequency and duration154). However, potential moderators of adherence, such as intervention complexity, strategies used to facilitate implementation, quality of delivery and participant responsiveness,172 also need consideration. Moderators may also work together to contribute to outcome.
Reflecting best practice in complex intervention research, we developed a logic model to underpin the process evaluation using Hasson’s154 adaptation of Carroll et al. ’s172 framework. The model represents practitioners’ implementation activities (Figure 25), expected impacts (Table 47) and contextual mediators of change (Box 2). To increase explanatory power of the model, we synthesised principles from theoretical frameworks underpinning the study (e.g. principles underlying the implementation strategy, facilitation and NPT) into mechanisms of action to explain conditions necessary for activities to impact on outcomes. Mechanisms were:
-
thinking: conceptual work associated with the SVP (e.g. increasing awareness)
-
planning: organising systems or processes to align and drive new practice
-
doing: enacting the SVP
-
evaluating: reflecting on performance and progress.
FIGURE 25.
Logic model a: practitioners’ implementation activities.
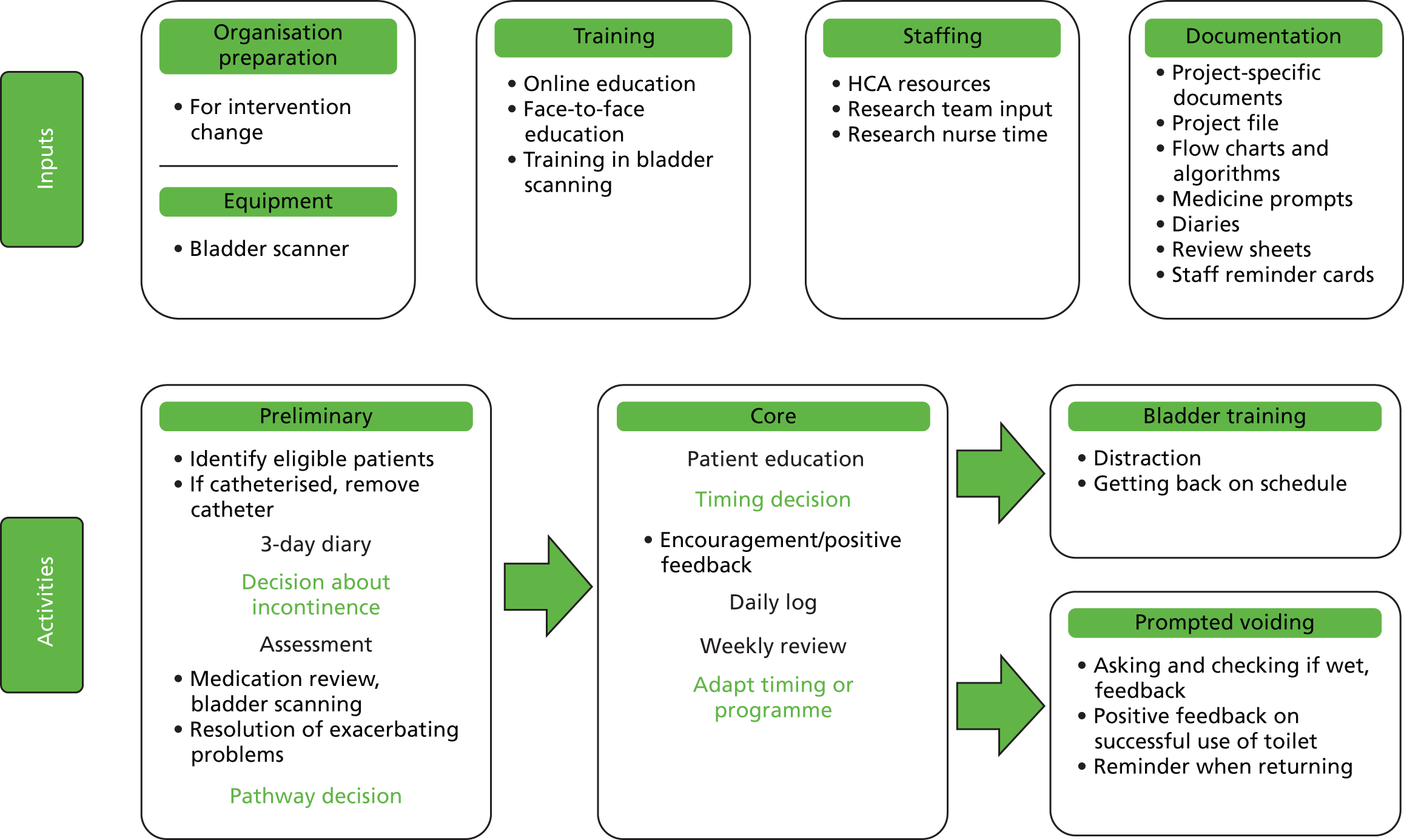
| Stage | Impact | ||
|---|---|---|---|
| Patient | Staff | Organisation | |
| Thinking | Aware of continence status Sensory awareness |
Awareness of continence Knowledge of continence Knowledge of individual need Decision-making |
Collective understanding and knowledge of:
|
| Planning | Know a plan is in place Know their regime |
Know who is incontinent Know who is on SVP Know what to do for patient Can establish a voiding pattern |
Better management of workload/patient need |
| Doing | Increased continence talk Skill in managing urgency Lengthening time intervals between voids |
Increase in:
|
More consistency of good practice More efficiency |
| Evaluating | Aware of progress Positive emotions Satisfaction |
Knowledge of performance Knowledge of impact More aware of need |
Knowledge of performance and impact |
| Outcomes | Increased bladder capacity Reduced harm, e.g. urinary tract infection Less incontinent episodes Less incontinent amount More likely to go home |
Increased therapeutic role | Less waste Less use of inappropriate products, e.g. catheters, pads Research citizenship/kudos |
-
Stroke severity.
-
Patient gender.
-
Ward layout.
-
Presence of ward routines.
-
Team working.
-
Therapist engagement.
-
Degree of change required to do the SVP.
-
Leadership.
-
Use of resources.
Although our original intention was to use Hasson’s model154 to underpin the process evaluation, Grant et al. 170 have recently published a framework specific to cluster RCTs. Most domains are found in both; however, Grant et al. 170 also include maintenance (sustainability of processes over time), unintended consequences and theory used to develop the intervention. Table 48 shows domains and methods used to address each domain in our trial.
| Domain | Research question | Research methods used: intervention arm | Additional research methods used: supported implementation arm |
|---|---|---|---|
| Recruitment of clusters | How were clusters sampled and recruited? | Documentation of recruitment process Documentation of cluster characteristics |
|
| Delivery to clusters | Which intervention was delivered for each cluster? | Analysis of intervention documentation | Analysis of meeting notes and internal facilitator records Interviews with internal and EFs |
| Response of clusters | How was the intervention adopted by clusters? | Interviews with ward staff | Interviews with internal and EFs |
| Recruitment and reach in individuals | Who received the intervention in each setting and are they representative? | Baseline data on patient characteristics Interviews with ward staff |
|
| Delivery to individuals | Which intervention is delivered in each cluster? | Interviews with ward staff | Interviews with internal and EFs |
| How well did staff adhere to the intervention? | Analysis of intervention documentation Monitoring adherence to protocol |
Analysis of meeting notes and internal facilitator records Interviews with internal and EFs |
|
| Response of individuals | How did the target population respond? | Interviews with patients and families Interviews with ward staff |
|
| Maintenance | How were processes sustained over time? | Interviews with ward staff | Interviews with internal and EFs |
| Theory | What theory was used to develop the intervention? | Soft systems NPT |
Implementation theory: facilitation |
| Context | What was the wider context in which the trial was conducted? | Soft systems methodology |
Cluster characteristics
Key characteristics of each site were recorded. These comprised type of hospital; catchment population of trust or health board; number of stroke admissions per annum; type of unit; total number of beds; number of dedicated stroke beds; 2008 Sentinel Audit score (average of nine key indicators of stroke care); and the average number of qualified, unqualified and total nursing staff on duty on morning and afternoon shifts (including ICONS funded HCAs).
Delivery of the intervention to individuals
Staff adherence was assessed through an examination of:
-
completion of intervention documentation (3-day diaries and daily clinical logs for participants on BT and PV)
-
adherence to the protocol in terms of allocation of participants to the appropriate regime and the management of catheterisation.
Completion of intervention documentation
Three-day diary
Research nurses were asked to submit a copy of the 3-day diary for all participants for whom one was recorded, i.e. participants who were incontinent at baseline or whose catheter was removed before discharge. Participants catheterised throughout their stay were not eligible to complete the diary. Each diary was assessed using a filtering system, with data input for an individual diary terminated at any stage if it failed to achieve that stage’s key quality indicator. The assessment of ‘yes’ or ‘no’ for each applicable stage was entered into the SPSS. For a summary of the stages and key quality indicators, see Chapter 4.
Daily clinical logs for bladder training and prompted voiding
We collected daily clinical logs for all participants in the intervention groups during three (for sites with a 9-month intervention period) or four (for sites with a 12-month intervention period) randomly selected weeks during each 3-month period in which the stroke service was recruiting participants into the trial. The methods used to process, input and analyse the clinical logs collected from the main trial sites were identical to the methods used for the clinical logs collected in the case study phase (see Chapter 4), with two additions.
Addition 1
In the main trial, for some clinical logs the quality of the photocopy was insufficient and as a result some parts of these clinical logs were impossible to read. A code was created, entitled ‘unable to process’. This code was applied to a clinical log if any data pertaining to the first three quality indicators (regime interval, proposed times and ‘times toileted’) was unreadable due to poor photocopy quality. Once the code was applied, the clinical log was not processed further.
Addition 2
For ‘times toileted’ that were either more than 30 minutes earlier or later than the proposed time or that were missing, one researcher scrutinised any comments documented in order to identify whether or not a ‘clinically justifiable’ explanation was provided. The main criteria for comments to be deemed ‘clinically justifiable’ were agreed with a key group of researchers from the ICONS trial. Examples of ‘clinically justifiable’ and ‘clinically non-justifiable’ comments are shown in Table 49. Comments recorded as ‘clinically justifiable’ were reviewed by a senior researcher in order to check validity. An additional analysis was then performed for stage 3b (quality indicator: ‘times toileted – within schedule’), in which an exemption was made for occasions on which clinically justifiable explanations were given for early, late or missing ‘times toileted’.
| Type of comments deemed to be ‘clinically justifiable’ in relation to an early, late or missing ‘time toileted’ | Type of comments not deemed to be ‘clinically justifiable’ |
|---|---|
| Patient sleeping Patient catheterised Patient not on the ward. (Only if location is either out of hospital, or in hospital NOT with an AHP, or is non-specified, AND if time off ward is either non-specified OR is of sufficient length to preclude toileting at scheduled time) Patient refused/declined/did not want toilet. (The underlying assumption is that the patient was asked) |
Staffing issues, e.g. staff too busy Patient at physiotherapy or occupational therapy Patient lost buzzer Toileting time fell during meal time Patient did not need to go. (The underlying assumption is that, although the patient did not need to go to the toilet, they should still have been asked to go – giving them the opportunity to decline if they so wished) |
Adherence to the protocol: allocation of participants to the appropriate regime and the management of catheterisation
The protocol required all participants who were incontinent during the 3-day diary period to be assessed for their suitability for BT or PV, with the appropriate regime commencing the day after completion of the 3 day diary. The recommended route for participants with cognitive impairment or no control over their bladder was PV; for all other participants it was BT. Ward staff were encouraged to move participants onto BT once they regained some bladder control or their cognitive function improved. For participants catheterised in the acute stage, ward staff were asked to conduct a TWOC as early as possible unless there was a valid clinical reason not to do so.
Our assessment of site adherence to the protocol included the following:
-
Length of time from the last day of the 3-day diary to commencement of regime.
-
Proportion of eligible patients allocated to regime.
-
Proportion of eligible patients allocated to the correct regime, based on the following criteria:
-
– PV: patients with cognitive impairment, defined as a score of 8 or more on the 6-Item Cognitive Impairment Test; OR patients who had no control over their bladder, defined as answering ‘all the time’ to the ICIQ question ‘how often do you leak urine?’
-
– BT: patients with no cognitive impairment, defined as a score of 0–7 on the 6-Item Cognitive Impairment Test, and some control over their bladder, defined as answering ‘several times a day’, ‘about once a day’, ‘two or three times a week’ or ‘about once a week or less often’ to the ICIQ question ‘how often do you leak urine’?
-
-
Length of time spent on the regime.
-
Number of participants catheterised in the acute stage.
-
Number of participants catheterised at discharge.
-
Time from entry to removal of catheter.
Response of individuals
Semistructured interviews were conducted with patients at discharge and sought patients’ experiences of their treatment for incontinence and their views of the effectiveness of treatment (see Appendix 25 for interview schedule).
We used maximum variance sampling173 to generate a range of participants in terms of gender, age, ethnicity, type of incontinence and stroke severity. Participants were also chosen to reflect those with a range of outcomes at discharge (defined in terms of the frequency of incontinent episodes).
Interviews were conducted by a researcher or research nurse. They were audiotaped and fully transcribed; all transcripts were checked against the recording to ensure accuracy. If the participant declined to be recorded, written notes were made by the researcher.
Initial coding was undertaken using thematic analysis. Codes were then clustered and findings integrated within the evolving logic model.
Response of clusters, recruitment and reach in individuals, delivery to and response of individuals and maintenance of processes over time
As in the case study phase, NPT55–58 provided the theoretical framework for implementing and evaluating the SVP. The aim was to facilitate understanding of the practical issues involved in embedding the intervention into routine practice.
Qualitative, semistructured interviews were performed with health professionals involved in the intervention to explore experiences of implementing the intervention, and drivers and barriers to successful implementation (n = 15–20 per intervention group, but with the numbers determined by data saturation). The interview schedule is shown in Appendix 26.
Each site was rated by the trial manager according to key parameters with a theoretical link to participant outcome, with the aim of checking the validity of the NPT analysis. These parameters included the extent of engagement with the programme, the level of research nurse involvement and the extent to which the ward manager provided leadership and direction in programme implementation. The full list of parameters and scoring system is shown in Appendix 27.
Interview summaries were initially constructed (interviews could include up to three respondents). First, the transcript was coded into the NPT framework (see Chapter 4). The respondents’ comments were then condensed for each NPT dimension to remove overlap and redundancy (such as when multiple respondents repeated broadly the same point, or the same respondent said something more than once); summarised to retain the essence of the points being made; and given an identifying label for the site (letter) and interview (number), for example AA2.
Site summaries were then created across all respondent interviews (an example is shown in Appendix 28). This involved merging all interview summaries for one site, linking related text chunks together for each NPT dimension and condensing down to remove overlap and redundancy, taking care to avoid loss of meaning or viewpoint. A short sentence summary was made for each unique salient point keeping as closely as possible to the expression of the respondents, with the number of respondents making a similar point in brackets [e.g. It is running smoothly (3) but it adds to the pressure/frustration if we can’t do it (3). It is (1)/isn’t (1) being done properly. Sometimes we just can’t do it because of staffing or workload (2)]. This allowed demonstration of convergent or divergent views within a site, or outlier viewpoints.
Across site summaries were made for each NPT dimension, by merging the site summaries so that the range of views expressed across all sites, and the main agreements and differences between sites, could be identified. The number of sites contributing to a particular finding is indicated in brackets at the end of the sentence, for example ‘Sites reported some difficulties with assessment (4), weekly reviews (2), and daily logs (1)’, or is reported in the text, for example ’Four sites had a toileting regime in place’.
Data analysis quality checks
All coding was undertaken by two coders independently (BF, JMc). Inter-rater reliability of coding was checked using side-by-side comparison, discussion of difference, agreement on final code(s) and accuracy of summarising, and adaptation of the coding framework if necessary. Identification and extraction of key points for coding across both coders was consistently reliable. Initially, there were regular differences in allocation to some codes between the two coders; a main code was discussed and agreed as differences arose. After main codes were agreed, internal consistency of coding was high.
The across site summary was populated by one researcher (BF) with direct quotes from respondents, to illustrate meanings. A second researcher (JMc) then checked back to the original transcripts, to ensure that the sense of meaning in the quotes used had been maintained, and to verify the number of sites supporting a statement. This was done to check reliability of interpretation.
The second researcher also checked the spread of quotes used to ensure that there was a balance of perspective from across the sites and from different respondent grades (e.g. qualified and unqualified staff). Within-site divergence between respondents of different grade could be lost to some extent in the site summary174 (e.g. if a particular grade of staff were more expressive, numerous or dominant in group interviews). To ensure that any differences of opinion between staff of different grades was maintained, a subgroup analysis was undertaken to check whether or not divergent views could be attributed to respondent grade for any NPT dimension. This is pointed out in the findings if present.
External consistency of interpretation of the data was reviewed by a management team member with experience of using the NPT framework in other research studies (FC).
Data synthesis
Barriers and facilitators to implementation
The main aim of using the NPT framework was to identify factors in the implementation of the intervention which might have influenced its success. Findings were categorised as barriers/difficulties or facilitators/suggestions for improving the implementation of the SVP within each NPT dimension. Implications for a future trial were built from these by the research team, and discussed at Steering Group meetings.
Potential mechanisms of action
To investigate links between features of the intervention and outcome, we extracted respondents’ views about how the intervention might work to impact on outcome. These potential mechanisms of action were mapped against the logic model of the intervention, described earlier in the chapter.
Context in which the trial was conducted
A soft systems analysis was used to define the clinical system within which ICONS SVP interventions were to be implemented (or not), and to uncover complex, messy systems, within which multiple perspectives may be found. Consequently, an exploratory research design and inclusive approach to sampling was required. Soft systems methodology143 was used to describe relationships between structure, process and outcome within the stroke service and to generate a definition of how the service worked (see Chapter 4). Objectives were to:
-
provide a description of the incontinence system
-
explore the ‘fit’ between the SVP and existing services in primary and secondary care environments
-
highlight the barriers and facilitators that might impede or enhance programme implementation within a continuous system of continence care.
A purposive sample of participants engaged in managing and delivering the clinical system were approached for inclusion in this study component. The sample was selected to ensure breadth of coverage in terms of the system and the range of professional roles that provide UI interventions.
Qualitative interviews were held with clinical leaders in the intervention and supported implementation trial arms before the intervention phase began. Health professionals in usual care were also interviewed to facilitate comparison in terms of continence management across trial arms. In order to minimise any change in practice, interviews in this trial arm were conducted at the end of the data collection period. The interview schedule is shown in Appendix 29.
Evaluation of supported implementation
This concerned the supported implementation trial arm only. Evaluation comprised two strands.
First strand: key informant semistructured interviews175 with internal facilitators, their deputies and external facilitators
Interviews explored experiences of implementing facilitation using the four domains of NPT as a framework: coherence (negotiating the intervention); cognitive participation (developing the intervention processes); collective action (implementing the new processes); and reflexive monitoring (evaluating the intervention processes).
All interviews were audio taped, transcribed and subjected to thematic analysis using NPT as a framework. The qualitative data analysis programme NVivo (QSR International, Warrington, UK) was used to code and categorise text; validity of interpretation was assessed through discussion by the project team.
Second strand: internal facilitator records
Descriptive analysis of weekly logs focused on which strategies were implemented; evidence from meeting notes was again analysed using NPT assisted by NVivo as above.
Chapter 7 Exploratory cluster randomised controlled trial: findings
Overview
In this chapter we present quantitative findings from the RCT. Findings from the process and health economic evaluations are presented in Chapters 8 and 9.
Recruitment
Twelve sites commenced recruitment between January 2011 and January 2012 (Figure 26). No site dropped out, each recruiting participants either for at least their planned duration of 9 or 12 months or until recruitment ceased at all sites on 31 July 2012 (see Figures 30 and 31). Site recruitment periods ranged from 9 to 14 months (Figure 27; site identifiers have been removed to protect anonymity).
FIGURE 26.
Flow of clusters and participants through the trial.
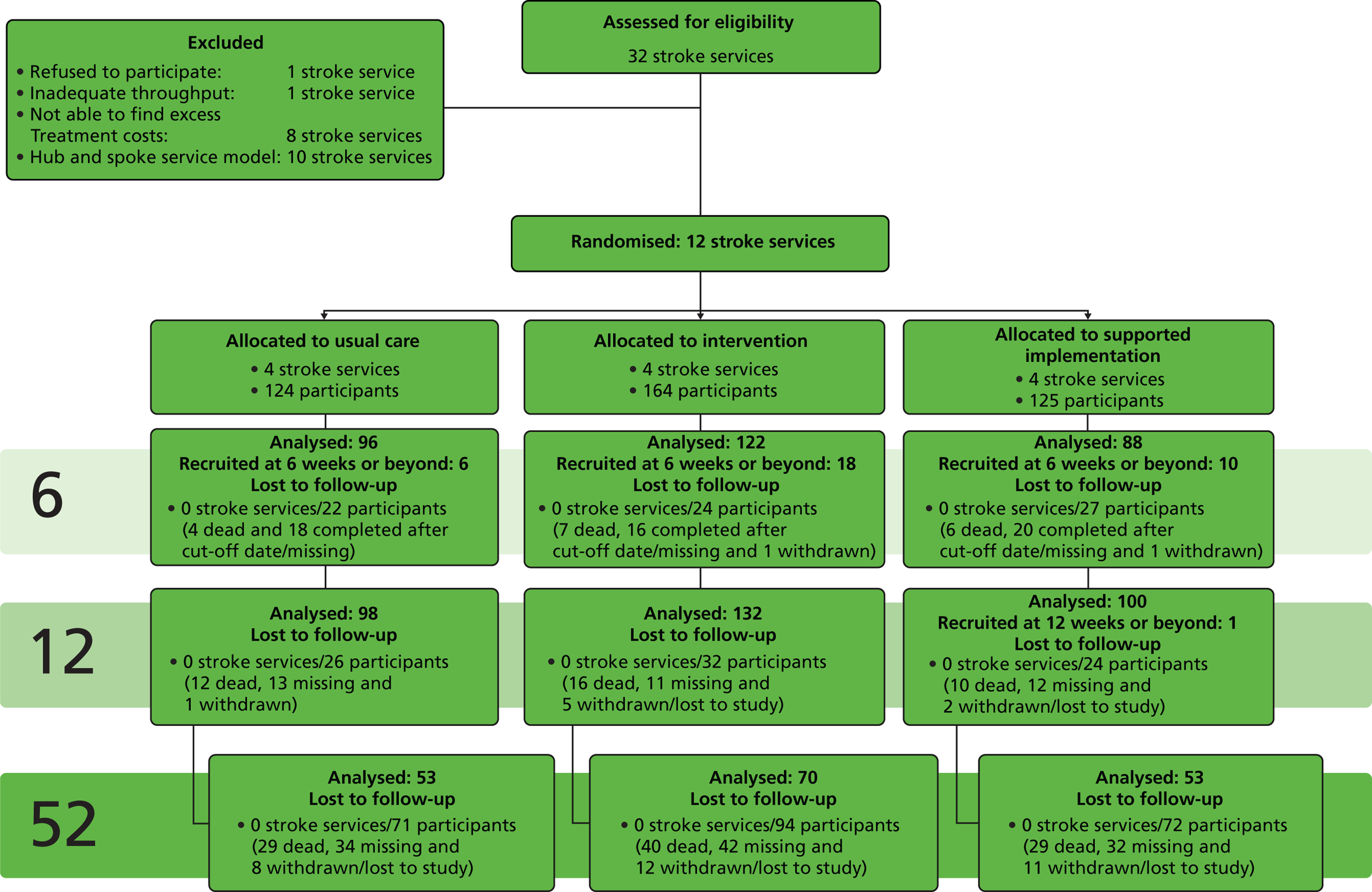
FIGURE 27.
Participant recruitment chart.
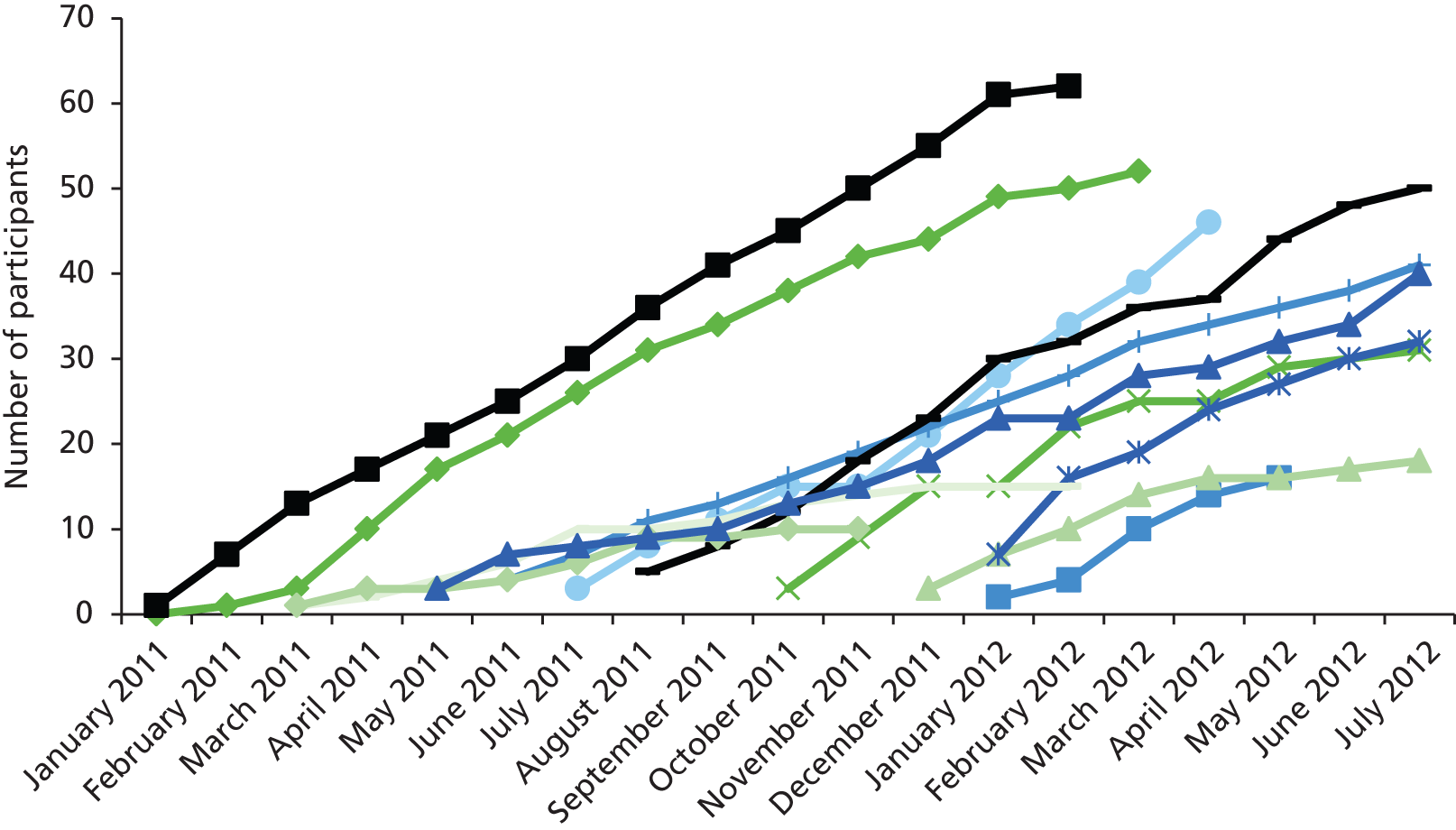
Four hundred and thirteen patients were recruited into the trial; 124 usual care, 164 intervention and 125 supported implementation. A total of 6060 patients were screened for eligibility; of these 2675 (44%) had not had a confirmed stroke. The number of non-stroke patients screened was highest in the intervention (1515/3078, 49%) and supported implementation (981/1999, 49%) arms and lowest in usual care (179/983, 18%). There was large variation across sites, with non-stroke patients screened ranging from 2 (1%) to 448 (79%).
The proportion of patients eligible for recruitment was similar across trial arms (usual care 155/804, 19%; intervention 259/1563, 17%; and supported implementation 176/1018, 17%). Of these, 80% (124/155) were recruited in usual care, 63% (164/259) in intervention and 71% (125/176) in supported implementation; the proportion of eligible patients recruited ranged from 10 (50%) to 46 (98%) across sites.
Data completeness
Baseline data were collected for all patients. The overall response rate at 6 weeks was 85% (306/362), excluding 34 patients recruited at more than 6 weeks post stroke and 17 who had died (usual care 96/114, 84%; intervention 122/139, 88%; supported implementation 88/109, 81%). At 12 weeks, the overall response rate was 88% (330/374), excluding one patient recruited at 12 weeks and 38 who had died (usual care 98/112, 88%; intervention 132/148, 89%; supported implementation 100/114, 88%). At 52 weeks, the overall response rate was 56%, excluding 98 who had died (usual care 53/95, 56%; intervention 70/124, 57%; supported implementation 53/96, 55%).
Baseline characteristics
Participant characteristics at baseline are shown in Table 50. Median age was 79 (IQR 70.5–85.0) years and was similar between arms. Overall, nearly half were male (189, 46%); with slightly more males in intervention (86, 52%) compared with the usual care (51, 41%) and supported implementation (52, 42%) arms. Median 7-day Barthel Index was 4 (IQR 2–7) and similar across arms. The number of patients with no symptoms on the pre-stroke mRS was 139 (34%) overall; the proportion was slightly higher in usual care (52, 42%) compared with the intervention (54, 33%) and supported implementation (33, 27%) arms. The median probability of survival free of dependency at 6 months (measured by the Edinburgh Case mix-adjuster168) overall was 0.02 (IQR 0.01–0.08) and was similar across arms.
| Measure | All sites (n = 413) | Usual care (n = 124) | Intervention (n = 164) | Supported implementation (n = 125) | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 79 | (70.5–85.0) | 80 | (72–86) | 77 | (68–83) | 81 | (74–85) |
| Male, n % | 189 | 46% | 51 | 41% | 86 | 52% | 52 | 42% |
| Ethnicity, n % | ||||||||
| White British | 397 | 97% | 123 | 99% | 155 | 96% | 119 | 95% |
| Other | 14 | 3% | 1 | 1% | 7 | 4% | 6 | 5% |
| Type of stroke, n % | ||||||||
| Ischaemic | 350 | 85% | 101 | 81% | 143 | 88% | 106 | 85% |
| Haemorrhagic infarct | 49 | 12% | 17 | 14% | 14 | 9% | 18 | 15% |
| Primary intracerebral haemorrhage | 12 | 3% | 6 | 5% | 6 | 4% | 0 | 0% |
| OCSP classification, n % | ||||||||
| TACS | 185 | 46% | 37 | 30% | 80 | 51% | 68 | 54% |
| PACS | 118 | 29% | 54 | 44% | 31 | 20% | 33 | 26% |
| LACS | 88 | 22% | 28 | 23% | 44 | 28% | 16 | 13% |
| POCS | 14 | 3% | 3 | 2% | 3 | 2% | 8 | 6% |
| Side of body affected by stroke | ||||||||
| Left side | 207 | 50% | 58 | 47% | 86 | 53% | 63 | 50% |
| Right side | 176 | 43% | 55 | 44% | 69 | 43% | 52 | 42% |
| Both sides | 6 | 1% | 2 | 2% | 2 | 1% | 2 | 2% |
| Neither side | 21 | 5% | 9 | 7% | 4 | 2% | 8 | 6% |
| 7-day Barthel Index, median (IQR) | 4 | (2–7) | 5 | (2–9) | 3 | (2.0–6.3) | 5 | (3.0–7.5) |
| Pre-stroke mRS, n % | ||||||||
| No symptoms | 139 | 34% | 52 | 42% | 54 | 33% | 33 | 27% |
| No significant disability | 166 | 41% | 39 | 31% | 72 | 44% | 55 | 45% |
| Slight disability | 30 | 7% | 4 | 3% | 19 | 12% | 7 | 6% |
| Moderate disability | 54 | 13% | 21 | 17% | 10 | 6% | 23 | 19% |
| Moderately severe disability | 18 | 4% | 7 | 6% | 6 | 4% | 5 | 4% |
| Severe disability | 2 | 0% | 1 | 1% | 1 | 1% | 0 | 0% |
| Pre-stroke living type, n % | ||||||||
| House | 324 | 79% | 94 | 76% | 134 | 84% | 96 | 77% |
| Flat | 47 | 12% | 21 | 17% | 11 | 7% | 15 | 12% |
| Sheltered housing | 20 | 5% | 4 | 3% | 8 | 5% | 8 | 6% |
| Residential home | 15 | 4% | 5 | 4% | 4 | 3% | 6 | 5% |
| Nursing home | 1 | 0% | 0 | 0% | 1 | 1% | 0 | 0% |
| Other | 1 | 0% | 0 | 0% | 1 | 1% | 0 | 0% |
| Pre-stroke living circumstances, n % | ||||||||
| Alone | 162 | 40% | 51 | 41% | 64 | 40% | 47 | 38% |
| With partner | 194 | 47% | 57 | 46% | 77 | 48% | 60 | 48% |
| With other family | 37 | 9% | 11 | 9% | 14 | 9% | 12 | 10% |
| With other | 16 | 4% | 5 | 4% | 5 | 3% | 6 | 5% |
| Speech, n % | ||||||||
| None | 47 | 11% | 10 | 8% | 22 | 14% | 15 | 12% |
| Incomprehensible | 63 | 15% | 19 | 15% | 22 | 14% | 22 | 18% |
| Inappropriate | 72 | 18% | 25 | 20% | 32 | 20% | 15 | 12% |
| Cognitive ability, n % | ||||||||
| Confused | 83 | 20% | 26 | 21% | 22 | 14% | 35 | 28% |
| Orientated | 146 | 36% | 44 | 35% | 64 | 40% | 38 | 30% |
| Edinburgh case-mix probability of survival free of dependency at 6 months, median (IQR) | 0.02 | (0.01–0.08) | 0.02 | (0.01–0.05) | 0.02 | (0.01–0.11) | 0.02 | (0.01–0.08) |
Stroke subtype classified according to the OCSP classification148 is shown in Figure 28. The majority of patients had middle cerebral artery strokes (TACS or PACS 303, 74%), although the proportion of patients with PACS was higher in usual care (54, 44%) compared with the intervention (31, 19%) and the supported implementation (33, 26%) arms.
FIGURE 28.
Baseline stroke subtype by trial arm.
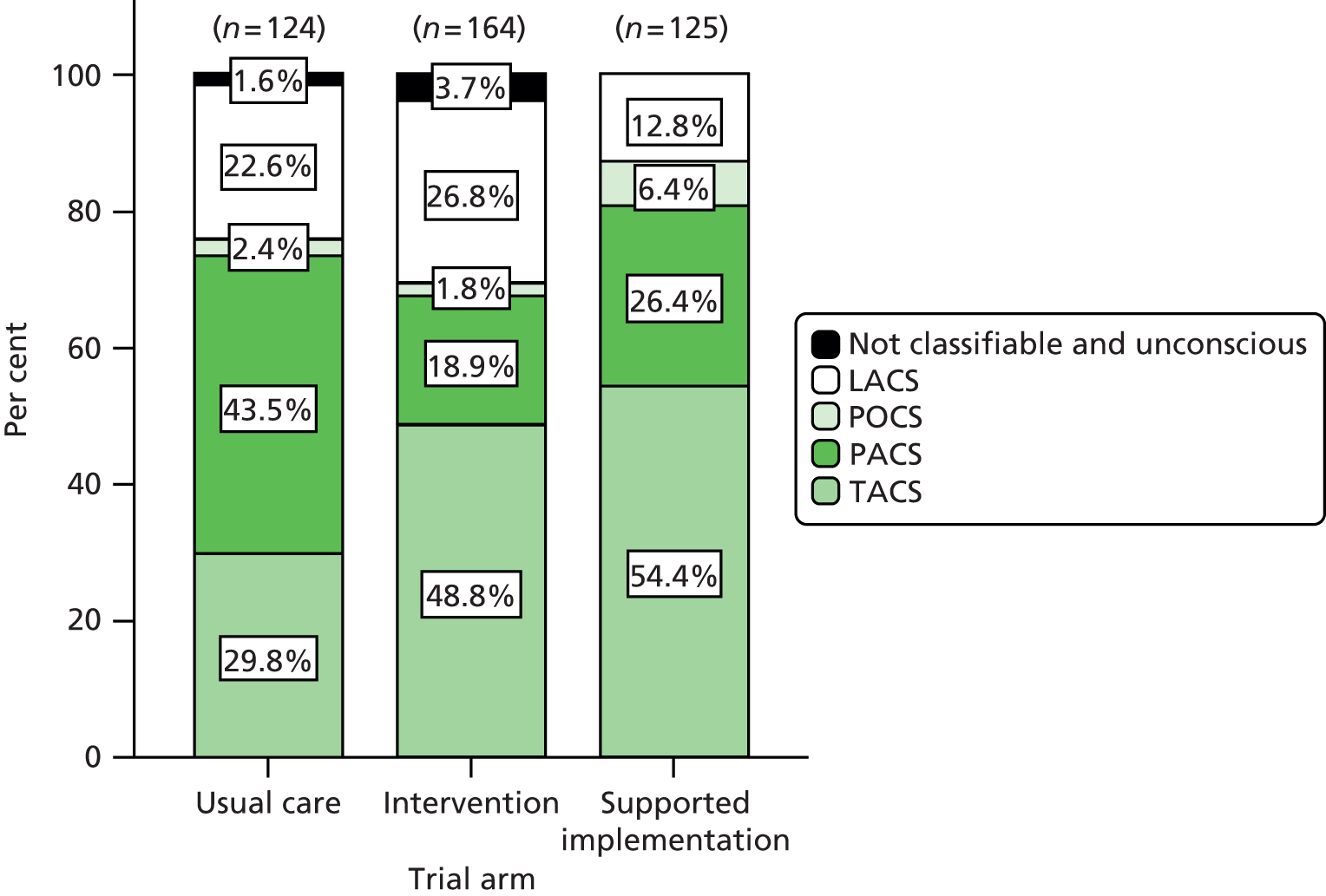
The majority of patients lived in their own home pre stroke (371/408, 91%), with nearly half of patients living alone (162/409, 40%). At baseline, only two-fifths of patients were able to converse and were orientated (146/411, 36%), only 33/413 (8%) were able to lift their arms and only 31/413 (8%) were able to walk independently.
Tables 51 and 52 show continence and EQ-5D status at baseline.
| Outcome | Trial arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | Supported implementation | All sites | |||||
| Catheterised (% of returned) | 21 | 17% | 33 | 20% | 18 | 14% | 72 | 17% |
| Potential respondents for incontinence measures (% of due) | 103 | 83% | 131 | 80% | 107 | 86% | 341 | 83% |
| Primary outcomes | ||||||||
| Presence/absence of incontinence (ICIQ-UI Short-Form Q3) | ||||||||
| Continenta | 10 | 10% | 20 | 15% | 12 | 11% | 42 | 12% |
| Incontinentb | 93 | 90% | 111 | 85% | 95 | 89% | 299 | 88% |
| ICIQ-UI Short Form | ||||||||
| Never | 10 | 11% | 20 | 18% | 12 | 13% | 42 | 14% |
| About once a week or less often | 6 | 7% | 3 | 3% | 1 | 1% | 10 | 3% |
| Two or three times per week | 11 | 12% | 12 | 11% | 9 | 10% | 32 | 11% |
| About once a day | 9 | 10% | 20 | 18% | 17 | 19% | 46 | 16% |
| Several times a day | 63 | 70% | 70 | 64% | 56 | 62% | 189 | 64% |
| All the time | 2 | 2% | 0 | 0% | 2 | 2% | 4 | 1% |
| Secondary outcomes | ||||||||
| ISI score | ||||||||
| Median (IQR) | 8 | 6–8 | 8 | 6–8 | 8 | 4–8 | 8 | 4–8 |
| None (0) | 10 | 10% | 20 | 16% | 12 | 12% | 42 | 13% |
| Slight (1–2) | 2 | 2% | 1 | 1% | 0 | 0% | 3 | 1% |
| Moderate (3–4) | 12 | 12% | 9 | 7% | 29 | 30% | 50 | 15% |
| Severe (6–8) | 77 | 76% | 96 | 76% | 57 | 58% | 230 | 71% |
| LUSQ | ||||||||
| Frequency of toilet visits during daytime | ||||||||
| At least every 30 minutes | 0 | 0% | 2 | 2% | 0 | 0% | 2 | 1% |
| Every hour | 9 | 10% | 6 | 5% | 5 | 6% | 20 | 7% |
| Every 90 minutes | 23 | 26% | 10 | 9% | 3 | 3% | 36 | 12% |
| Every 2 hours | 42 | 47% | 59 | 54% | 35 | 39% | 136 | 46% |
| Less often than every 2 hours | 20 | 22% | 33 | 30% | 47 | 52% | 100 | 34% |
| Type of incontinence | ||||||||
| UUI present | 86 | 85% | 97 | 78% | 82 | 85% | 265 | 82% |
| SUI present | 65 | 80% | 30 | 50% | 43 | 74% | 138 | 69% |
| Functional incontinence present | 91 | 90% | 110 | 85% | 88 | 89% | 289 | 88% |
| Classification of continence status | ||||||||
| Continent | 10 | 8% | 20 | 12% | 11 | 9% | 41 | 10% |
| UUI | 7 | 6% | 9 | 6% | 3 | 3% | 19 | 5% |
| SUI | 1 | 1% | 0 | 0% | 0 | 0% | 1 | 0% |
| MUI | 64 | 51% | 30 | 18% | 43 | 34% | 137 | 33% |
| Functional incontinence only | 0 | 0% | 1 | 1% | 1 | 1% | 2 | 1% |
| Unclear incontinence type | 21 | 17% | 71 | 43% | 49 | 39% | 141 | 34% |
| Catheterised | 21 | 17% | 33 | 20% | 18 | 14% | 72 | 17% |
| EQ-5D item | Usual care | Intervention | Supported implementation | All sites | ||||
|---|---|---|---|---|---|---|---|---|
| Mobility, n % | ||||||||
| No problems | 8 | 8% | 6 | 5% | 5 | 6% | 19 | 6% |
| Some problems | 53 | 52% | 35 | 29% | 48 | 56% | 136 | 44% |
| Confined to bed | 40 | 40% | 79 | 66% | 33 | 38% | 152 | 50% |
| Self-care, n % | ||||||||
| No problems | 5 | 5% | 9 | 8% | 11 | 13% | 25 | 8% |
| Some problems | 50 | 50% | 45 | 38% | 36 | 42% | 131 | 43% |
| Unable to wash or dress self | 46 | 46% | 65 | 55% | 39 | 45% | 150 | 49% |
| Usual activities, n % | ||||||||
| No problems | 2 | 2% | 4 | 3% | 3 | 4% | 9 | 3% |
| Some problems | 46 | 46% | 35 | 30% | 36 | 42% | 117 | 38% |
| Unable to perform | 53 | 52% | 79 | 67% | 46 | 54% | 178 | 59% |
| Pain or discomfort, n % | ||||||||
| None | 68 | 67% | 55 | 47% | 54 | 46% | 177 | 58% |
| Moderate | 33 | 33% | 54 | 46% | 28 | 24% | 115 | 38% |
| Extreme | 0 | 0% | 8 | 7% | 3 | 3% | 11 | 4% |
| Anxiety or depression, n % | ||||||||
| None | 61 | 61% | 63 | 53% | 40 | 47% | 164 | 54% |
| Moderate | 36 | 36% | 45 | 38% | 44 | 52% | 125 | 41% |
| Extreme | 3 | 3% | 10 | 8% | 1 | 1% | 14 | 5% |
Forty-seven per cent (195/413) of patients were catheterised at recruitment and for these patients, questions about continence status were collected on catheter removal. Median time to catheter removal was 19 days, although the IQR was wide (IQR 8.5–34.0 days). Seventeen per cent (72/413) remained catheterised throughout. Over four-fifths of patients (300/341, 88%) were incontinent at baseline, with 41/341 (12%) continent, usually after catheter removal but in a few cases due to delay in completing the baseline assessment. The majority of patients were incontinent several times a day (189/299, 63%). Median score on the ISI was 8 in all trial arms (IQR 4–8 overall), with 230/325 (71%) falling within the ‘severe’ category. Over four-fifths of patients had UUI (265, 82%) and functional incontinence (289, 88%).
The majority of patients were dependent in terms of mobility, self-care and usual activities at baseline as measured by the EQ-5D, with only 19 out of 307 (6%), 25 out of 306 (8%) and 9 out of 304 (3%) having no problems with mobility, self-care and usual activities respectively. Two-fifths of patients (126/303, 42%) had pain or discomfort and nearly half (139/303, 46%) were anxious or depressed.
Adverse events
Events most likely to be related to the ICONS intervention were falls, urinary tract infections and bladder catheterisations. A total of 91 adverse events were recorded, 30 in usual care, 33 in intervention and 28 in the supported implementation arm.
Sixty-six patients had a recorded fall, urinary tract infection, bladder catheterisation or a combination of these. Twenty of these patients were in usual care (20/124, 16%); 25 out of 164 (15%) in intervention and 21 out of 125 (17%) in the supported implementation arm. Table 53 shows the number and proportion of adverse events by trial arm. As some patients had more than one fall or urinary tract infection, Tables 54 and 55 show the number of patients with one or more of these adverse events in each trial arm.
| Trial arm (n participants) | Adverse event | |||
|---|---|---|---|---|
| Fall | Urinary tract infection | Bladder catheterisation | Total | |
| Usual care (124) | 16 (53.3%) | 13 (43.3%) | 1 (3.3%) | 30 (100%) |
| Intervention (164) | 11 (33.3%) | 18 (54.5%) | 4 (12.1%) | 33 (100%) |
| Supported implementation (125) | 4 (14.3%) | 23 (82.1%) | 1 (3.6%) | 28 (100%) |
| Trial arm (n participants) | Number of falls | Total | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | ||
| Usual care (124) | 4 | 2 | 1 | 1 | 8 |
| Intervention (164) | 5 | 3 | 0 | 0 | 8 |
| Supported implementation (125) | 2 | 1 | 0 | 0 | 3 |
| Trial arm (n participants) | Number of UTIs | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Usual care (124) | 13 | 0 | 0 | 13 |
| Intervention (164) | 14 | 2 | 0 | 16 |
| Supported implementation (125) | 16 | 2 | 1 | 19 |
Overall, the number of adverse events was similar across trial arms. There were more falls reported in usual care, and more urinary tract infections in both intervention arms compared with usual care. The intervention arm had more catheterisations, although numbers reported were small.
Length of stay, discharge destination and discharge continence status
Length of stay
Overall median (IQR) length of stay in the stroke unit was 47 (30–68) days; 43.0 (28.3–59.0) days in usual care; 52.5 (35.0–70.8) days in intervention; and 47 (27–78) days in supported implementation.
Discharge destination
Table 56 shows discharge destination from the participating hospital. Overall, nearly half of patients were discharged home (185, 45.4%); the proportion was lower in supported implementation (47, 38.2%) due to the more frequent practice of discharging patients to community hospitals.
| Patient destination on discharge from participating hospital | Trial arm | Overall (n = 407) | ||
|---|---|---|---|---|
| Usual care (n = 120) | Intervention (n = 164) | Supported implementation (n = 123) | ||
| Home alone | 20 (16.7%) | 14 (8.5%) | 17 (13.8%) | 51 (12.5%) |
| Home not alone | 36 (30%) | 68 (41.5%) | 30 (24.4%) | 134 (32.9%) |
| Nursing or residential home | 30 (25.0%) | 63 (38.4%) | 25 (20.3%) | 118 (29.0%) |
| Other hospital | 26 (21.7%) | 7 (4.3%) | 39 (31.7%) | 72 (17.7%) |
| Dead | 8 (6.7%) | 12 (7.3%) | 12 (9.8%) | 32 (7.9%) |
Before discharge from hospital, 21 patients (5.1%) were discharged from the stroke unit to another ward in the same hospital (usual care 10, 8.1%; intervention 8, 4.9%; supported implementation 3, 2.4%). Twenty-eight patients (6.8%) died on the stroke unit (usual care 6, 4.8%; intervention 11, 6.7%; supported implementation 11, 8.9%), a further four patients died after discharge to another ward in the same hospital.
For patients admitted from their own home, one quarter overall (105, 27.2%) were discharged to a residential or nursing home (Table 57). More intervention patients were discharged to a residential or nursing home (usual care 27, 23.5%; intervention 56, 36.1%; supported implementation 22, 18.8%), whereas patients in usual care and supported implementation were more likely to be discharged to community hospitals for further rehabilitation.
| Patient destination from participating hospital for patients who were admitted from home | Trial arm | Overall (n = 387) | ||
|---|---|---|---|---|
| Usual care (n = 115) | Intervention (n = 155) | Supported implementation (n = 117) | ||
| Home alone | 20 (17.4%) | 13 (8.4%) | 17 (14.5%) | 50 (12.9%) |
| Home not alone | 36 (31.3%) | 67 (43.2%) | 30 (25.6%) | 133 (34.4%) |
| Nursing or residential home | 27 (23.5%) | 56 (36.1%) | 22 (18.8%) | 105 (27.1%) |
| Other hospital | 26 (22.6%) | 7 (4.5%) | 37 (31.6%) | 70 (18.1%) |
| Dead | 6 (5.2%) | 12 (7.7%) | 11 (9.4%) | 29 (7.5%) |
Continence status at discharge
At discharge, 38 (31%) of usual care, 72 (44%) of intervention and 51 (41%) of supported implementation participants were continent. Relative to usual care, the intervention arm had an odds ratio (OR) of 1.47 (95% CI 0.81 to 2.67) of being discharged continent, with supported implementation having an OR of 1.54 (95% CI 0.83 to 2.85); the overall difference between trial arms was non-significant (p = 0.32). Overall, combined intervention arm participants had 1.50 (95% CI 0.88 to 2.57, p = 0.13) times the odds of continence at discharge than usual care participants.
Patient outcomes at 6 weeks post stroke
Table 58 shows patient outcomes at 6 weeks post stroke. An OR > 1 favours the intervention (intervention or supported implementation arms). Only 66 (29%) patients reported being continent, with another 76 (25%) reporting being catheterised. There was no real suggestion of a beneficial effect of either intervention relative to usual care on outcome, with adjusted OR estimates for the dichotomised form of ICIQ question 3 of 0.94 (95% CI 0.46 to 1.94) for intervention and 0.62 (95% CI 0.28 to 1.37) for supported implementation arms, and, for the original ordinal form of the question, 0.83 (95% CI 0.49 to 1.38) and 0.89 (95% CI 0.52 to 1.51) respectively. These were reflected in the ORs for secondary outcomes, with most suggesting no positive effect of either intervention relative to usual care. Overall, almost 50% of the non-catheterised respondents reported severe incontinence on the ISI, with similar percentages with severe incontinence in each trial arm (47% usual care; 45% intervention; 40% supported implementation). However, almost 50% of patients had received less than 2 weeks of their allocated intervention by 6 weeks post stroke and over 25% had spent less than 7 days on the programme by this time point.
| Measure | Trial arm | All sites | ICCb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | Intervention vs. usual care, OR (95% CI) | Supported implementation | Supported implementation vs. usual care, OR (95% CI)a | |||||||
| Questionnaires returned | 96 | 122 | 88 | 306 | |||||||
| Catheterised (% of returned) | 21 | 22% | 37 | 30% | 18 | 20% | 76 | 25% | |||
| Potential respondents for incontinence measures (% age of returned) | 75 | 78% | 85 | 70% | 70 | 80% | 230 | 75% | |||
| Primary outcomes | |||||||||||
| Presence/absence of incontinence (ICIQ-UI Short-Form Q3) | 0.94 (0.46 to 1.94) | 0.62 (0.28 to 1.37) | 0 | ||||||||
| Continentc | 21 | 28% | 29 | 34% | 16 | 23% | 66 | 29% | |||
| Incontinentd | 54 | 72% | 56 | 66% | 54 | 77% | 164 | 71% | |||
| ICIQ-UI Short Form (usual care 75; intervention 83; supported implementation 70; all 228)e | 0.83 (0.49 to 1.38) | 0.89 (0.52 to 1.51) | 0 | ||||||||
| Never | 21 | 30% | 29 | 41% | 16 | 24% | 66 | 31% | |||
| About once a week or less often | 8 | 11% | 7 | 10% | 11 | 16% | 26 | 12% | |||
| Two or three times per week | 12 | 17% | 10 | 14% | 8 | 12% | 30 | 14% | |||
| About once a day | 9 | 13% | 11 | 15% | 12 | 18% | 32 | 15% | |||
| Several times a day | 25 | 35% | 25 | 35% | 22 | 32% | 72 | 34% | |||
| All the time | 0 | 0% | 1 | 1% | 1 | 1% | 2 | 1% | |||
| Secondary outcomes | |||||||||||
| ISI score (usual care 75; intervention 84; supported implementation 68; all 227)e | 0.87 (0.48 to 1.57) | 0.84 (0.46 to 1.56) | 0 | ||||||||
| Median (IQR) | 4 | (0–8) | 4 | (0–8) | 4 | (1–8) | 4 | (0–8) | |||
| None (0) | 21 | 28% | 29 | 35% | 16 | 24% | 66 | 29% | |||
| Slight (1–2) | 6 | 8% | 6 | 7% | 7 | 10% | 19 | 8% | |||
| Moderate (3–4) | 13 | 17% | 11 | 13% | 18 | 26% | 42 | 19% | |||
| Severe (6–8) | 35 | 47% | 38 | 45% | 27 | 40% | 100 | 44% | |||
| Leicester Urinary Symptoms | |||||||||||
| Frequency of toilet visits during daytime (usual care 71; intervention 71; supported implementation 68; all 210)e | 0.43 (0.22 to 0.84) | 0.60 (0.30 to 1.22) | 0.017 | ||||||||
| At least every 30 minutes | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |||
| Every hour | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |||
| Every 90 minutes | 2 | 3% | 2 | 3% | 1 | 1% | 5 | 2% | |||
| Every 2 hours | 2 | 3% | 2 | 3% | 10 | 15% | 14 | 7% | |||
| Less often than every 2 hours | 67 | 94% | 67 | 94% | 57 | 84% | 191 | 91% | |||
| Types of incontinence | |||||||||||
| UUI present (usual care 75; intervention 84; supported implementation 68; all 227)e | 52 | 70% | 50 | 59% | 1.35 (0.67 to 2.71) | 48 | 71% | 0.89 (0.41 to 1.91) | 150 | 66% | 0 |
| SUI present (usual care 71; intervention 78; supported implementation 57; all 206)e | 36 | 51% | 32 | 41% | 1.01 (0.42 to 2.43) | 33 | 58% | 0.82 (0.34 to 1.61) | 101 | 49% | 0 |
| EQ-5D | |||||||||||
| Mobility (usual care 96; intervention 114; supported implementation 88; all 298)e | 1.27 (0.69 to 2.34) | 1.37 (0.74 to 2.53) | |||||||||
| No problems | 13 | 14% | 10 | 9% | 6 | 7% | 29 | 10% | |||
| Some problems | 51 | 53% | 44 | 39% | 48 | 55% | 143 | 48% | |||
| Confined to bed | 32 | 33% | 60 | 53% | 34 | 39% | 126 | 42% | |||
| Self-care (usual care 96; intervention 114; supported implementation 88; all 298)e | 0.61 (0.34 to 1.09) | 0.75 (0.40 to 1.39) | 0 | ||||||||
| No problems | 13 | 14% | 13 | 11% | 13 | 15% | 39 | 13% | |||
| Some problems | 49 | 51% | 47 | 41% | 36 | 41% | 132 | 44% | |||
| Unable to wash or dress | 34 | 35% | 54 | 47% | 39 | 44% | 127 | 43% | |||
| Usual activity (usual care 96; intervention 114; supported implementation 88; all 298)e | 0.42 (0.22 to 0.81) | 0.79 (0.41 to 1.51) | 0 | ||||||||
| No problems | 6 | 6% | 5 | 4% | 5 | 6% | 16 | 5% | |||
| Some problems | 44 | 46% | 30 | 26% | 34 | 39% | 108 | 36% | |||
| Unable to perform | 46 | 48% | 79 | 69% | 49 | 56% | 174 | 58% | |||
| Pain or discomfort (usual care 95; intervention 111; supported implementation 84; all 290)e | 0.66 (0.38 to 1.16) | 0.63 (0.34 to 1.14) | 0 | ||||||||
| None | 62 | 65% | 58 | 52% | 52 | 62% | 172 | 59% | |||
| Moderate | 32 | 34% | 48 | 43% | 29 | 35% | 109 | 38% | |||
| Extreme | 1 | 1% | 5 | 5% | 3 | 4% | 9 | 3% | |||
| Anxiety or depression (usual care 95; intervention 107; supported implementation 83; all 285)e | 0.56 (0.33 to 0.95) | 0.86 (0.49 to 1.51) | 0 | ||||||||
| None | 51 | 54% | 47 | 44% | 47 | 57% | 145 | 51% | |||
| Moderate | 42 | 44% | 54 | 50% | 33 | 40% | 129 | 45% | |||
| Extreme | 2 | 2% | 6 | 6% | 3 | 4% | 11 | 4% | |||
| I-QOL, mean (SD)f (usual care 53; intervention 43; supported implementation 38; all 134)e | 75 | (56.7–90.9) | 78.4 | (37.5–89.8) | –10.1 (–29.5 to 9.3) | 74.4 | (48.3–92.1) | –10.1 (–28.6 to 8.3) | 76 | (51.7–91.2) | 0.184 |
| Barthel Index, median (IQR) (usual care 96; intervention 117; supported implementation 88; all 301)e | 8.5 | (3–14) | 7 | (3–11) | 0.68 (0.43 to 1.07) | 7.5 | (4–12) | 0.70 (0.43 to 1.13) | 8 | (3–12) | 0 |
| Dead (usual care 118; intervention 145; supported implementation 114; all 377)e | 4 | 3% | 7 | 5% | 1.01 (0.43 to 2.39) | 6 | 5% | 0.72 (0.31 to 1.71) | 17 | 5% | 0.0087 |
Results were relatively insensitive to assumptions regarding missing data; for example, complete case analysis showed continence OR estimates of 1.02 (95% CI 0.50 to 2.12) for intervention and 0.91 (95% CI 0.42 to 1.96) for supported implementation relative to usual care, and hot deck multiple imputation gave continence OR estimates of 1.09 (95% CI 0.52 to 2.31) for intervention and 0.90 (95% CI 0.43 to 1.87) for supported implementation. Per-protocol analysis suggested that those who received the intervention according to protocol had better outcomes than usual care, particularly as requirements for protocol adherence were strengthened; for those who had received at least 14 days of intervention, the estimated continence OR relative to usual care was 2.60 (95% CI 0.99 to 6.82), although that for supported implementation increased only to 0.98 (95% CI 0.29 to 3.28). Moreover, an analysis of the effect of duration of treatment on outcomes showed that, in the intervention arm, there was a potential effect of duration of intervention, with a continence OR estimate of 1.26 (95% CI 0.94 to 1.69) for each additional week on treatment; however, the comparative effect of supported implementation was only 1.06 (95% CI 0.72 to 1.55).
Findings were insensitive to the exclusion of patients who were either catheterised throughout their time in the stroke unit, never incontinent following removal of a catheter, or both; however, excluding patients with pre-stroke incontinence led to a reduction in the continence OR estimates relative to usual care for both intervention (OR 0.76, 95% CI 0.33 to 1.76) and supported implementation (OR 0.37, 95% CI 0.14 to 0.96) arms.
Six (50%) of the centres were in the bottom quartile on the average performance on the ‘nine key indicators of stroke care’ in the National Sentinel Stroke Audit Phase II11 (clinical audit), with only one centre in the top quartile. Findings for supported implementation were relatively sensitive to adjustment, seemingly due to the respective performance of the supported implementation sites (three from the bottom quartile and one from the top quartile of the National Sentinel Stroke Audit performance score). There was no strong evidence that either the annual admission rate or type of unit (combined/separate acute and rehabilitation units) impacted on continence rates at 6 weeks post stroke, although the OR of 1.84 (95% CI 0.69 to 4.86) suggested a potentially greater chance of continence at 6 weeks among the larger units, whereas for type of stroke unit, the estimated OR of 0.70 (95% CI 0.36 to 1.36) suggested a lower chance of continence in the combined units.
There was little or no evidence of clustering effects, with almost all intracluster correlation coefficient (ICC) estimates being very close to 0. Owing to the small number of clusters, CIs for the ICC were wide and highly sensitive to the estimation approach.
Subgroup analyses
Table 59 shows subgroup analyses for 6-week ICIQ absence of incontinence and frequency of incontinence outcomes. There is some evidence to suggest patients with pre-stroke incontinence were more likely to be continent at 6 weeks in supported implementation (p = 0.069); however, this finding was not replicated in the analysis of the ordinal form of the outcome. There was a stroke subtype interaction effect with intervention arm on the dichotomised (but not the ordinal) form of the outcome; in usual care (p = 0.017), outcome appeared generally better for TACS than LACS, with a reversal of this patter in the intervention and supported implementation arms. Baseline ISI category interacted significantly with the intervention arm in the analysis of the ordinal form of outcome; this suggests that baseline severity may have had less impact on incontinence frequency at 6 weeks post stroke in the intervention and supported implementation arms than in usual care. In terms of component of the SVP received, BT may have led to better outcome than PV or usual care, particularly in the intervention arm (p = 0.070 dichotomised; p = 0.094 ordinal categorisation). None of the other subgroup effects of the intervention were significant (p > 0.1), although some investigations were not possible due to sparse categories (see Table 59).
| Subgrouping variable | Absence of incontinence (p-value) | Frequency of incontinence (p-value) | Reason for non-convergence |
|---|---|---|---|
| Pre-stroke incontinence: yes; no | 0.069a | 0.34 | |
| Sex: male; female | 0.53 | 0.90 | |
| Pre-stroke mRS: independent (0–2); dependent (3–5) | b | b | Of the 66 patients continent at 6 weeks post stroke, only one (supported implementation) patient was not independent pre stroke |
| Type of stroke: ischaemia; primary intracerebral haemorrhage | b | b | No participant with primary intracerebral haemorrhage in supported implementation |
| Side of body affected by stroke: left; right (non-specific side excluded) | 0.87 | 0.88 | |
| OCSP:c TACS; PACS; LACS | 0.017a | 0.31 | |
| Baseline bowel function: incontinent or occasional accident; continent | b | b | All supported implementation patients who were continent at 6 weeks post stroke were fully continent for bowel function at baseline |
| Type of incontinence: UUI; SUI; MUI; functional only; unclear | b | b | Only one participant (usual care) had SUI alone and two patients had functional incontinence alone (none in usual care) |
| Type of incontinence by sex interaction | b | b | As above |
| Baseline ISI: none; mild or moderate; severe | 0.19 | 0.050a | |
| Leicester Urinary Symptoms UUI: yes; no | 0.19 | 0.21 | |
| Leicester Urinary Symptoms SUI: yes; no | 0.74 | 0.30 | |
| Treatment plan type: catheterised throughout; BT at some stage; not BT at any stage; no intervention | 0.070a | 0.094a | |
| Aged | 0.14 | 0.38 | |
| Baseline cognitive impairment: yes; no | 0.29 | 0.42 | |
| Site annual stroke patient numbers: high: ≥ 300; low: < 300 | 0.54 | 0.62 |
Patient outcomes at 12 weeks post stroke
Table 60 shows patient outcomes at 12 weeks post-stroke. There was no evidence of better outcomes on the ICIQ or ISI in either intervention arm, with all OR estimates close to 1; OR estimates for the dichotomised form of ICIQ question three were 1.02 (95% CI 0.54 to 1.93) for intervention and 1.06 (95% CI 0.54 to 2.09) for supported implementation arms compared with usual care, and, for the original ordinal form of the question, 0.97 (95% CI 0.58 to 1.61) and 1.22 (95% CI 0.72 to 2.08) respectively. However, both intervention arms had a higher estimated odds of continence than usual care with respect to urgency (intervention OR 1.58, 95% CI 0.83 to 2.99; supported implementation OR 1.73, 95% CI 0.88 to 3.43); there was a greater estimated odds of continence with respect to stress in supported implementation (OR 1.82, 95% CI 0.82 to 4.01) but not intervention (OR 1.04, 95% CI 0.45 to 1.82) compared with usual care. Although none of these increases was statistically significant, such increases are suggestive of a potential reduction in the odds of specific types of incontinence; evidence is more consistent across the arms for UUI. None of the other incontinence outcomes showed a strong suggestion of a substantial improvement in outcomes in the intervention and/or supported implementation arms relative to usual care. However, there was a consistent pattern of worse estimated effects of the intervention on the EQ-5D outcomes, but only the CIs for the effect of intervention (relative to usual care) on mobility and on self-care suggested ORs < 1 (poorer QoL).
| Outcome | Trial arm | All sites | ICCb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | Intervention vs. usual care, OR (95% CI) | Supported implementation | Supported implementation vs. usual care, OR (95% CI)a | |||||||
| Questionnaires returned | 98 | 132 | 100 | 330 | |||||||
| Catheterised (% of returned) | 18 | 18% | 27 | 20% | 13 | 13% | 58 | 17% | |||
| Potential respondents for incontinence measures (% age of returned) | 80 | 82% | 105 | 80% | 86 | 86% | 271 | 82% | |||
| Primary outcomes | |||||||||||
| Presence/absence of incontinence (ICIQ-UI Short-Form Q3) | |||||||||||
| Continentc | 24 | 30% | 43 | 41% | 1.02 (0.54 to 1.93) | 27 | 31% | 1.06 (0.54 to 2.09) | 94 | 35% | 0 |
| Incontinentd | 56 | 70% | 62 | 59% | 59 | 68% | 177 | 65% | |||
| ICIQ-UI Short Form (usual care 80; intervention 104; supported implementation 86; all 270)e | 0.97 (0.58 to 1.61) | 1.22 (0.72 to 2.08) | 0 | ||||||||
| Never | 24 | 30% | 43 | 41% | 27 | 31% | 94 | 35% | |||
| About once a week or less often | 12 | 15% | 9 | 9% | 10 | 12% | 31 | 12% | |||
| Two or three times per week | 12 | 15% | 13 | 12% | 11 | 13% | 36 | 13% | |||
| About once a day | 12 | 15% | 6 | 6% | 10 | 12% | 28 | 10% | |||
| Several times a day | 18 | 23% | 25 | 24% | 27 | 31% | 70 | 26% | |||
| All the time | 2 | 3% | 8 | 8% | 1 | 1% | 11 | 4% | |||
| Secondary outcomes | |||||||||||
| ISI score (usual care 80; intervention 102; supported implementation 86; all 268)e | 0.86 (0.50 to 1.50) | 0.92 (0.52 to 1.64) | 0 | ||||||||
| Median (IQR) | 3 | (0–6) | 2.5 | (0–8) | 4 | (0–8) | 3 | (0–8) | |||
| None (0) | 24 | 30% | 43 | 42% | 28 | 33% | 95 | 35% | |||
| Slight (1–2) | 10 | 13% | 8 | 8% | 6 | 7% | 24 | 9% | |||
| Moderate (3–4) | 19 | 24% | 12 | 12% | 17 | 20% | 48 | 18% | |||
| Severe (6–8) | 27 | 34% | 39 | 38% | 35 | 41% | 101 | 38% | |||
| Leicester Urinary Symptoms | |||||||||||
| Frequency of toilet visits during daytime (usual care 73; intervention 88; supported implementation 71; all 232)e | 0.85 (0.47 to 1.54) | 1.09 (0.60 to 1.96) | 0.0075 | ||||||||
| At least every 30 minutes | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |||
| Every hour | 0 | 0% | 0 | 0% | 1 | 1% | 1 | 0% | |||
| Every 90 minutes | 5 | 7% | 2 | 2% | 4 | 6% | 11 | 5% | |||
| Every 2 hours | 10 | 14% | 11 | 13% | 6 | 8% | 27 | 12% | |||
| Less often than every 2 hours | 58 | 79% | 75 | 85% | 60 | 85% | 193 | 83% | |||
| Type of incontinence | |||||||||||
| UUI present (usual care 79; intervention 103; supported implementation 85; all 267)e | 53 | 67.1% | 52 | 50.5% | 1.58 (0.83 to 2.99) | 49 | 57.6% | 1.73 (0.88 to 3.43) | 154 | 57.7% | 0 |
| SUI present (usual care 72; intervention 83; supported implementation 72; all 227)e | 38 | 52.8% | 29 | 34.9 | 1.04 (0.45 to 1.82) | 31 | 43.1 | 1.82 (0.82 to 4.01) | 98 | 43.2% | 0 |
| EQ-5D | |||||||||||
| Mobility (usual care 96; intervention 129; supported implementation 92; all 317)e | 0.92 (0.52 to 1.62) | 0.79 (0.44 to 1.41) | 0 | ||||||||
| No problems | 19 | 20% | 16 | 12% | 10 | 11% | 45 | 14% | |||
| Some problems | 53 | 55% | 62 | 48% | 57 | 62% | 172 | 54% | |||
| Confined to bed | 24 | 25% | 51 | 40% | 25 | 27% | 100 | 32% | |||
| Self-care (usual care 97; intervention 126; supported implementation 92; all 315)e | 0.45 (0.26 to 0.79) | 0.65 (0.36 to 1.16) | 0 | ||||||||
| No problems | 25 | 26% | 21 | 17% | 18 | 20% | 64 | 20% | |||
| Some problems | 42 | 43% | 47 | 37% | 40 | 43% | 129 | 41% | |||
| Unable to wash or dress | 30 | 31% | 58 | 46% | 34 | 37% | 122 | 39% | |||
| Usual activities (usual care 97; intervention 126; supported implementation 91; all 314)e | 0.49 (0.27 to 0.90) | 0.63 (0.34 to 1.17) | 0 | ||||||||
| No problems | 10 | 10% | 9 | 7% | 8 | 9% | 27 | 9% | |||
| Some problems | 43 | 44% | 39 | 31% | 32 | 35% | 114 | 36% | |||
| Unable to perform | 44 | 45% | 78 | 62% | 51 | 56% | 173 | 55% | |||
| Pain or discomfort (usual care 95; intervention 123; supported implementation 93; all 311)e | 0.73 (0.43 to 1.23) | 0.88 (0.50 to 1.54) | 0 | ||||||||
| None | 55 | 58% | 51 | 41% | 50 | 54% | 156 | 50% | |||
| Moderate | 37 | 39% | 58 | 47% | 34 | 37% | 129 | 41% | |||
| Extreme | 3 | 3% | 14 | 11% | 9 | 10% | 26 | 8% | |||
| Anxiety or depression (usual care 95; intervention 122; supported implementation 92; all 309)e | 0.67 (0.39 to 1.13) | 0.95 (0.54 to 1.67) | 0 | ||||||||
| None | 53 | 56% | 47 | 39% | 47 | 51% | 147 | 48% | |||
| Moderate | 37 | 39% | 66 | 54% | 37 | 40% | 140 | 45% | |||
| Extreme | 5 | 5% | 9 | 7% | 8 | 9% | 22 | 7% | |||
| I-QOL,f mean (SD) (usual care 51; intervention 47; supported implementation 35; all 133)e | 72.6 | (58.3–83.0) | 76.1 | (42.5–94.3) | –5.5 (–24.1 to 13.1) | 67.1 | (51.1–85.2) | –1.9 (–21.2 to 17.4) | 72.6 | (52.9–87.5) | 0.216 |
| Barthel Index, median (IQR) (usual care 94; intervention 128; supported implementation 95; all 317)e | 11 | (4–16) | 8 | (4–13) | 0.71 (0.46 to 1.11) | 11 | (6–15) | 0.97 (0.61 to 1.54) | 10 | (4–15) | 0 |
| Dead (usual care 123; intervention 159; supported implementation 122; all 404)e | 12 | 10% | 16 | 10% | 1.04 (0.56 to 1.92) | 10 | 8% | 1.15 (0.60 to 2.19) | 38 | 9% | 0 |
Results were relatively insensitive to assumptions regarding missing data; for example, complete case analysis showed continence ORs of 1.02 (95% CI 0.52 to 1.98) for intervention and 1.02 (95% CI 0.50 to 2.07) for supported implementation relative to usual care, and hot deck multiple imputation gave continence OR estimates of 0.91 (95% CI 0.48 to 1.75) for intervention and 0.95 (95% CI 0.47 to 1.92) for supported implementation. Per-protocol analysis suggested that those who had received the intervention according to protocol had better outcomes than usual care, although this did not appear to hold for supported implementation; for those who had received at least 14 days of intervention, the estimated continence OR relative to usual care was 1.54 (95% CI 0.69 to 3.47), and that for supported implementation was 1.07 (95% CI 0.40 to 2.90). However, an analysis of the effect of duration of treatment on outcomes showed little or no evidence of an effect, with OR of 1.04 (95% CI 0.90 to 1.21) and 0.96 (95% CI 0.83 to 1.12) in the intervention and supported implementation arms respectively.
Excluding patients who were catheterised throughout their time in the stroke unit or never incontinent following removal of a catheter, or both, had relatively little impact in supported implementation, although the continence OR estimate increased slightly when each exclusion criteria was applied separately, and further to 1.30 (95% CI 0.65 to 2.61) in intervention when both exclusion criteria were applied. On excluding patients with pre-stroke incontinence, a reduction in the continence OR estimates for both intervention (OR 0.84, 95% CI 0.34 to 2.05) and, particularly, supported implementation (OR 0.71, 95% CI 0.28 to 1.79) relative to usual care was observed.
As for the 6-week outcome, findings for supported implementation were relatively sensitive to adjustment for the centre factors used in the stratification. There was no strong evidence that either the annual admission rate or type of unit (combined/separate acute and rehabilitation units) impacted on continence rates at 12 weeks post stroke, although the OR of 0.62 (95% CI 0.15 to 2.64) suggested a potentially lesser chance of continence at 12 weeks among the larger units, whereas for type of stroke unit, the estimated OR of 1.40 (0.51 to 3.79) suggested a potentially greater chance of continence in the combined units.
There was, again, little or no evidence of clustering effects, with almost all ICC estimates being very close to 0. Owing to the small number of clusters, CIs for the ICC were wide and highly sensitive to the estimation approach.
Subgroup analyses
Table 61 shows subgroup analyses for 12-week ICIQ absence of incontinence and frequency of incontinence outcomes. Stroke subtypes other than TACS may be more likely to be continent in intervention and supported implementation (p = 0.054) arms. Patients with pre-stroke UI and older participants may be relatively more likely to be continent in supported implementation than the other trial arms (p = 0.048 and p = 0.024 respectively). In terms of other characteristics, participants with stroke affecting the right side of the body may be more likely to have greater incontinence frequency at 12 weeks (p = 0.080) and patients with baseline faecal incontinence may be relatively more likely to have UI at 12 weeks in supported implementation than the other trial arms.
| Subgrouping variable | Absence of incontinence (p-value) | Frequency of incontinence (p-value) | Reason for non-convergence |
|---|---|---|---|
| Pre-stroke incontinence: yes; no | 0.048a | 0.12 | |
| Sex: male; female | 0.76 | 0.25 | |
| Pre-stroke mRS: independent (0–2); dependent (3–5) | b | b | Only six patients dependent pre stroke were continent at 12 weeks, none of whom were in usual care |
| Type of stroke: ischaemia; primary intracerebral haemorrhage | b | b | There were no participants with primary intracerebral haemorrhage in supported implementation |
| Side of body affected by stroke: left; right (non-specific side excluded) | 0.19 | 0.080a | |
| Stroke subtype:c TACS; PACS; LACS | 0.054a | 0.27 | |
| Baseline bowel function: incontinent or occasional accident; continent | 0.003a | 0.46 | |
| Type of incontinence: UUI; SUI; MUI; functional only; unclear | b | b | Only one participant (usual care) had SUI alone and two patients had functional incontinence alone (none in usual care) |
| Type of incontinence by sex interaction | b | b | As above |
| Baseline ISI: none; mild or moderate; severe | 0.42 | 0.24 | |
| Leicester Urinary Symptoms UUI: yes; no | 0.17 | 0.34 | |
| Leicester Urinary Symptoms SUI: yes; no | 0.49 | 0.55 | |
| Treatment plan type: catheterised throughout; BT at some stage; not BT at any stage; no intervention | 0.15 | 0.34 | |
| Aged | 0.024a | 0.24 | |
| Baseline cognitive impairment: yes; no | 0.78 | 0.65 | |
| Site annual stroke patient numbers: high ≥ 300; low < 300 | 0.54 | 0.96 |
Patient outcomes at 52 weeks post stroke
Table 62 shows participant outcomes at 52 weeks post stroke. Mortality and attrition rates were both high at 52 weeks, with outcomes were available for only 176 out of 413 (43%) of participants. The primary analysis suggests that the intervention and supported implementation participants may have worse outcomes than those who received usual care, with estimated continence ORs of 0.56 (95% CI 0.27 to 1.16) for intervention and 0.60 (95% CI 0.27 to 1.30) for supported implementation. However, despite high but similar rates of missing data across the three trial arms (usual care 71/124, 57%, intervention 94/164, 57%, supported implementation 72/125, 58%, including those who died or withdrew) findings were not particularly sensitive to the assumptions made in the handling of missing data: continence ORs for complete case analysis were 0.63 (95% CI 0.27 to 1.45) for intervention and 0.60 (95% CI 0.25 to 1.47) for supported implementation, and hot deck multiple imputation gave ORs of 0.53 (95% CI 0.18 to 1.62) for intervention and 0.63 (95% CI 0.19 to 2.03) for supported implementation, the wider CIs reflecting the high percentages of missing data. For the secondary outcomes, estimated ORs tended to be below 1 for the intervention arm (relative to usual care) with the supported implementation arm having OR estimates closer to and sometimes above 1, although 95% CIs were almost always consistent with ‘no differential effect’. Findings consistent with improved outcomes for UUI and SUI in intervention arms observed at earlier outcome time points were not replicated at 52 weeks post stroke.
| Outcome | Trial arm | All sites | ICCb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | Intervention vs. usual care, OR (95% CI) | Supported implementation | Supported implementation vs. usual care, OR (95% CI)a | |||||||
| Questionnaires returned | 53 | 70 | 53 | 176 | |||||||
| Catheterised (% of returned) | 3 | 6% | 7 | 10% | 2 | 4% | 12 | 7% | |||
| Potential respondents for incontinence measures (% age of returned) | 50 | 94% | 62 | 89% | 50 | 94% | 162 | 92% | |||
| Primary outcomes | |||||||||||
| Presence/absence of incontinence (ICIQ-UI Short Form Q3) | 0.56 (0.27 to 1.16) | 0.60 (0.27 to 1.30) | 0 | ||||||||
| Continentc | 21 | 42% | 23 | 37% | 15 | 30% | 59 | 36% | |||
| Incontinentd | 29 | 58% | 39 | 63% | 35 | 70% | 103 | 64% | |||
| ICIQ-UI Short Form (usual care 50; intervention 62; supported implementation 49; all 161)e | 0.65 (0.36 to 1.15) | 0.87 (0.47 to 1.59) | 0 | ||||||||
| Never | 21 | 42% | 23 | 37% | 15 | 31% | 59 | 37% | |||
| About once a week or less often | 9 | 18% | 6 | 10% | 15 | 31% | 30 | 19% | |||
| Two or three times per week | 4 | 8% | 9 | 15% | 4 | 8% | 17 | 11% | |||
| About once a day | 4 | 8% | 6 | 10% | 1 | 2% | 11 | 7% | |||
| Several times a day | 8 | 16% | 11 | 18% | 9 | 18% | 28 | 17% | |||
| All the time | 4 | 8% | 7 | 11% | 5 | 10% | 16 | 10% | |||
| Secondary outcomes | |||||||||||
| ISI score (usual care 50; intervention 62; supported implementation 49; all 161)e | 0.67 (0.36 to 1.25) | 0.84 (0.44 to 1.60) | 0 | ||||||||
| Median (IQR) | 2 | (0–6) | 3 | (0–6.5) | 2 | (0–8) | 2 | (0–6) | |||
| None (0) | 21 | 42% | 23 | 37% | 15 | 31% | 59 | 37% | |||
| Slight (1–2) | 8 | 16% | 5 | 8% | 14 | 29% | 27 | 17% | |||
| Moderate (3–4) | 7 | 14% | 12 | 19% | 5 | 10% | 24 | 15% | |||
| Severe (6–8) | 14 | 28% | 22 | 35% | 15 | 31% | 51 | 32% | |||
| Leicester Urinary Symptoms | |||||||||||
| Frequency of toilet visits during daytime (usual care 39; intervention 52; supported implementation 40; all 131)e | 0.81 (0.48 to 1.38) | 1.06 (0.60 to 1.84) | 0 | ||||||||
| At least every 30 minutes | 0 | 0 | 0 | 0% | 0 | 0% | 0 | 0% | |||
| Every hour | 0 | 0 | 0 | 0% | 0 | 0% | 0 | 0% | |||
| Every 90 minutes | 2 | 5% | 3 | 6% | 3 | 8% | 8 | 6% | |||
| Every 2 hours | 5 | 13% | 7 | 13% | 3 | 8% | 15 | 11% | |||
| Less often than every 2 hours | 32 | 82% | 42 | 81% | 34 | 85% | 108 | 82% | |||
| UUI present (usual care 49; intervention 61; supported implementation 47; all 157)e | 23 | 47% | 33 | 54% | 0.60 (0.30 to 1.23) | 27 | 57% | 0.64 (0.30 to 1.40) | 83 | 53% | 0 |
| SUI present (usual care 49; intervention 58; supported implementation 46; all 153)e | 7 | 14% | 20 | 34% | 0.19 (0.05 to 1.08) | 5 | 11% | 1.08 (0.28 to 4.19) | 32 | 21% | 0.108 |
| I-QOL (usual care 26; intervention 33; supported implementation 28; all 87)e | 5.5 (–30.0 to 40.9) | –12.2 (–49.2 to 24.9) | 0.278 | ||||||||
| Median (IQR) | 69.8 | (56.3–88.4) | 81.6 | (46.5–92.0) | 80.7 | (48.3–88.3) | 77.3 | (52.3–88.8) | |||
| Barthel Index (usual care 50; intervention 63; supported implementation 49; all 162)e | 0.69 (0.42 to 1.14) | 0.83 (0.49 to 1.39) | 0 | ||||||||
| Median (IQR) | 14 | (7–18) | 8 | (4–16) | 11 | (6.5–16) | 11 | (5–16.25) | |||
| EQ-5D | |||||||||||
| Mobility (usual care 51; intervention 66; supported implementation 51; all 168)e | 0.67 (0.36 to 1.25) | 1.22 (0.67 to 2.25) | 0 | ||||||||
| No problems | 8 | 16% | 9 | 14% | 8 | 16% | 25 | 15% | |||
| Some problems | 34 | 67% | 37 | 56% | 37 | 73% | 108 | 64% | |||
| Confined to bed | 9 | 18% | 20 | 30% | 6 | 12% | 35 | 21% | |||
| Self-care (usual care 53; intervention 68; supported implementation 51; all 172)e | 0.40 (0.20 to 0.77) | 0.88 (0.46 to 1.68) | 0 | ||||||||
| No problems | 18 | 34% | 14 | 21% | 15 | 29% | 47 | 27% | |||
| Some problems | 23 | 43% | 20 | 29% | 23 | 45% | 66 | 38% | |||
| Unable to wash or dress | 12 | 23% | 34 | 50% | 13 | 25% | 59 | 34% | |||
| Usual activities (usual care 51; intervention 68; supported implementation 52; all 171)e | 0.54 (0.27 to 1.08) | 0.92 (0.46 to 1.82) | 0 | ||||||||
| No problems | 10 | 20% | 7 | 10% | 5 | 10% | 22 | 13% | |||
| Some problems | 23 | 45% | 22 | 32% | 21 | 40% | 66 | 39% | |||
| Unable to perform | 18 | 35% | 39 | 57% | 26 | 50% | 83 | 49% | |||
| Pain or discomfort (usual care 51; intervention 69; supported implementation 52; all 172)e | 0.87 (0.50 to 1.53) | 1.10 (0.61 to 1.98) | 0 | ||||||||
| None | 22 | 43% | 25 | 36% | 22 | 42% | 69 | 40% | |||
| Moderate | 25 | 49% | 40 | 58% | 24 | 46% | 89 | 52% | |||
| Extreme | 4 | 8% | 4 | 6% | 6 | 12% | 14 | 8% | |||
| Anxious or depressed (usual care 50; intervention 69; supported implementation 50; all 169)e | 0.84 (0.47 to 1.47) | 1.28 (0.71 to 2.33) | 0 | ||||||||
| None | 31 | 62% | 32 | 46% | 22 | 44% | 85 | 50% | |||
| Moderate | 16 | 32% | 30 | 43% | 26 | 52% | 72 | 43% | |||
| Extreme | 3 | 6% | 7 | 10% | 2 | 4% | 12 | 7% | |||
| Dead (usual care 116; intervention 152; supported implementation 114; all 382)e | 29 | 25% | 40 | 26% | 1.20 (0.72 to 2.00) | 29 | 25% | 0.99 (0.58 to 1.69) | 98 | 26% | 0 |
Results from per-protocol analyses were similar to those from the primary approach, although OR estimates in the intervention arm tended to be a little closer to 1 and those in the supported implementation arm tended to be a little further from 1. However, an analysis of the effect of duration of treatment on outcomes showed little or no evidence of an effect; the ORs of 0.59 (95% CI 0.25 to 1.43) and 0.63 (95% CI 0.27 to 1.48) in the intervention and supported implementation arms, respectively, were very similar to those obtained in the primary analysis.
Excluding patients who were catheterised throughout their time in the stroke unit or never incontinent following removal of a catheter, or both, had relatively little impact on the intervention effect estimates in either trial arm. Findings showed a similar pattern to those of the per-protocol analysis. On excluding those with pre-stroke incontinence, a reduction in the continence OR estimates for both intervention (OR 0.44, 95% CI 0.19 to 1.00) and supported implementation (OR 0.54, 95% 0.23 to 1.28) relative to usual care was again observed.
As for the 6- and 12-week outcomes, findings, particularly for supported implementation arms, were relatively sensitive to adjustment for the centre factors used in the stratification. With only 12 centres, there was little evidence provided as to the effects of the stratification factors on outcome; the estimated effects of admission to a large unit (OR 1.58, 95% CI 0.08 to 30.94) and of admission to a combined unit (OR 2.08, 95% CI 0.40 to 10.69) were both positive.
As for the 6- and 12-week outcomes, there was little or no evidence of clustering effects, with ICC estimates mostly being very close to 0 and estimated with poor precision, with sensitivity to the estimation approach used.
Subgroup analyses
Table 63 shows subgroup analyses for 52-week ICIQ absence of incontinence and frequency of incontinence outcomes. Participants with stroke affecting the right side of the body may be more likely to have greater frequency of incontinence at 52 weeks (p = 0.0012) or, similarly, to be less likely to be continent (p = 0.034). The effect of baseline ISI category on continence at 52 weeks post stroke appeared less strong in the intervention arm than the other two trial arms (p = 0.086), a finding replicated in the analysis of incontinence frequency (p = 0.022). The effect of baseline UUI on the risk of incontinence at 52 weeks may be less strong in the intervention arm than the other two arms (p = 0.066), although the corresponding effect on incontinence frequency was not quite significant (p = 0.13). None of the other subgroup effects were statistically significant (p > 0.1), although some investigations were not possible due to sparse categories (see Table 63).
| Subgrouping variable | Absence of incontinence (p-value) | Frequency of incontinence (p-value) | Reason for non-convergence |
|---|---|---|---|
| Pre-stroke incontinence: yes; no | 0.59 | 0.96 | |
| Sex: male; female | 0.83 | 0.72 | |
| Pre-stroke mRS: independent (0–2); dependent (3–5) | a | 0.27 | There were only two participants who were dependent pre stroke and who had a good outcome |
| Type of stroke: ischaemia; primary intracerebral haemorrhage | a | a | There were no participants with primary intracerebral haemorrhage in supported implementation |
| Side of body affected by stroke: left; right (non-specific side excluded) | 0.034b | 0.0012b | |
| Stroke subtype:c TACS; PACS; LACS | 0.55 | 0.59 | |
| Baseline bowel function: incontinent or occasional accident; continent | 0.38 | 0.83 | |
| Type of incontinence: UUI; SUI; MUI; functional only; unclear | a | a | Only one participant (usual care) had SUI alone and two patients had functional incontinence alone (none in usual care) |
| Type of incontinence by sex interaction | a | a | As above |
| Baseline ISI: none; mild or moderate; severe | 0.086b | 0.022b | |
| Leicester Urinary Symptoms UUI: yes; no | 0.066b | 0.13 | |
| Leicester Urinary Symptoms SUI: yes; no | 0.64 | a | Sparse categories across multiple variables cause problems with convergence |
| Treatment plan type: catheterised throughout; BT at some stage; not BT at any stage; no intervention | 0.29 | 0.31 | |
| Aged | 0.88 | 0.50 | |
| Baseline cognitive impairment: yes; no | 0.92 | 0.67 | |
| Site annual stroke patient numbers: high ≥ 300; low < 300 | 0.20 | 0.92 |
Additional analysis: maintenance of continence status after discharge
In order to explore whether those patients continent at discharge maintained this status after leaving the hospital, we examined whether or not patients discharged before their 6- or 12-week outcome assessment remained continent at these time points. At 6 weeks, data were available for 138 out of 140 patients; of these, 53 (38.4%) were continent, 64 (46.4%) were incontinent and 21 (15.2%) were catheterised. 6-week continence status was available for 46 out of 53 patients continent at discharge; eight of these completed their 6-week questionnaire on the day of or before discharge and were therefore excluded from this analysis (Table 64).
| Continence status at 6 weeks | Trial arm | Overall (n = 38) | ||
|---|---|---|---|---|
| Usual care (n = 13) | Intervention (n = 16) | Supported implementation (n = 9) | ||
| Continent | 6 (46.2%) | 8 (50%) | 1 (11.1%) | 15 (39.5%) |
| Incontinent | 7 (53.8%) | 8 (50%) | 7 (77.8%) | 22 (57.9%) |
| Catheterised | 0 | 0 | 1 (11.1%) | 1 (2.6%) |
Overall, over half the patients continent at discharge were incontinent at 6 weeks (22/38, 57.9%), with a higher proportion incontinent in supported implementation (7/9, 77.8%).
For patients continent at discharge but incontinent at 6 weeks, median (IQR) length of time from discharge from the stroke unit to the completion of the 6-week questionnaire overall was 23.5 (14.0–34.5) days. The median (IQR) length of time by trial arm was 18 (6–33) days for usual care, 29.5 (21.0–37.5) days for intervention and 17 (14–34) days for supported implementation. For patients who were continent at discharge and at 6 weeks, the median length of time from discharge from the stroke unit to the completion of the 6-week questionnaire overall was 23 (7–34) days. The median length of time by trial arm was 28.00 (18.25–36.75) days for the usual care arm, 22.5 (4.0–33.5) days for the intervention arm and 15 days (only one patient) for the supported implementation arm. Length of time since discharge does not therefore seem to affect whether patients retain continence at 6 weeks.
At 12 weeks, data were available for 169 out of 171 patients; of these, 67 (39.6%) were continent, 57 (33.7%) were incontinent and 45 (26.6%) were catheterised. 12-week continence status was available for 54 out of 67 patients discharged before 12 weeks and continent at discharge and is shown in Table 65. No patients continent at discharge were catheterised at 12 weeks.
| Continence status at 12 weeks | Trial arm | Overall (n = 54) | ||
|---|---|---|---|---|
| Usual care (n = 14) | Intervention (n = 24) | Supported implementation (n = 16) | ||
| Continent | 6 (42.9%) | 17 (70.8%) | 9 (56.2%) | 32 (59.3%) |
| Incontinent | 8 (57.1%) | 7 (29.2%) | 7 (43.8%) | 22 (40.7%) |
Overall, two-fifths of patients continent at discharge were incontinent at 12 weeks (22/54, 40.7%); a smaller proportion of patients were incontinent in the intervention arm (7, 29.2%) compared with usual care (8, 57.1%) and supported implementation (7, 43.8%).
For patients who were continent at discharge and at 12 weeks, median (IQR) length of time from discharge from the stroke unit to the completion of the 12-week questionnaire overall was 31.00 (23.75–41.00) days. The median length of time was 30.00 (17.75–54.50) days for usual care, 39.0 (26.0–45.5) days for intervention and 28 (20–34) days for the supported implementation arm.
For patients continent at discharge but incontinent at 12 weeks, median (IQR) length of time from discharge from the stroke unit to the completion of the 12-week questionnaire overall was 27.50 (18.75–54.00) days. The median length of time by trial arm was 23.5 (17.5–28.0) days for usual care, 51 (24–66) days for intervention and 27 (18–29) days for supported implementation. Intervention patients who became incontinent had been discharged for a much longer period of time; this might explain the greater proportion incontinent in this trial arm at 12 weeks.
Chapter 8 Exploratory cluster randomised controlled trial: findings from the process evaluation
Overview
In this chapter, we report findings from the process evaluation structured according to Grant’s framework. 170
Cluster characteristics
Characteristics of clusters not recruited appeared broadly similar to recruited clusters in terms of stroke admissions per annum and average of nine indicators in the 2008 Sentinel Audit. There were two hospitals in both usual care and intervention admitting ≥ 300 stroke patients per annum, but only one in supported implementation. Usual care was the only arm without a university hospital; these sites also tended to have higher Sentinel Audit scores.
Table 66 shows characteristics of participating stroke services; site codes have been removed to protect anonymity.
| Type of hospital | Catchment population of trust/health board | Number of stroke admissions per annum | Type of unit | Unit layout | Total number of beds | Number of stroke beds | 2008 Sentinel Audit score (average of nine indicators) | Average number of staff during recruitment period (including ICONS funded HCAs) | Participation in other initiatives during recruitment phase | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morning shift | Afternoon shift | |||||||||||||
| Total staff | Qualified staff | HCAs | Total staff | Qualified staff | HCAs | |||||||||
| Usual care | ||||||||||||||
| District general | R: 320,000 C: 350,000 |
212 | Acute and rehabilitation | Long ward with central desk, four-bedded bays and single rooms | 16 | 16 | 58 | |||||||
| District general | (NS) 340,000 | 163 | Rehabilitation | Four-bedded bays, Nightingale style | 14 | 14 | 73 | 5 | 2 | 3 | 5 | 2 | 3 | Safety Express – November 2012 onwards; Intentional Rounding – September 2012 onwards |
| District general | (NS) 676,000 | 302 | Acute and rehabilitation | L-shaped ward, four- and one-bedded rooms | 30 | 30 | 60 | 8 | 4 | 4 | 6 | 3 | 3 | |
| District general | (NS) 676,000 | 304 | Acute and rehabilitation | Hub and spoke, two by eight-bedded bays and five single rooms | 21 | 21 (6 acute, 15 rehabilitation) | 62 | 8 | 4 | 4 | 6 | 3 | 3 | Intentional Rounding – October 2011 onwards |
| Intervention | ||||||||||||||
| Teaching; foundation trust | R: 440,000 | 459 | Acute and rehabilitation | Long ward, central desk, four-bedded bays and single rooms | 31 | 31 (15 acute, 16 rehabilitation) | 61 | 6–9 | 2–4 | 3–7 | 5–7 | 2–3 | 2–4 | None |
| District general | R: 370,000 | 194 | Rehabilitation | Hub and spoke, six-bedded bays and single rooms | 23 | Also admitted medical patients | 33 | 7 | 3 | 4 | 5 | 3 | 2 | Intentional Rounding – September 2011 onwards |
| University | (NS) 340,000 | 127 | Rehabilitation | Long ward, six four-bedded bays and single rooms | 29 | 29 | 76 | |||||||
| District general | R: ≥ 500,000 | 577 | Acute and rehabilitation (split site) | Acute: hub and spoke, four-bedded bays and single rooms Rehab: L-shaped, four-, two- and one-bedded rooms |
22/24 | 22 in acute unit; 24 in rehabilitation unit | 61 | 8a | 3 | 5 | 5 | 2 | 3 | Safety Express and Intentional Rounding – dates not known |
| Supported implementation | ||||||||||||||
| District general | R: 320,000 C: 350,000 |
258 | Acute and rehabilitation | Hub and spoke, eight-, four-, two- and one-bedded rooms | 24 | 24 | 58 | 7 | 3 | 4 | 5 | 2 | 3 | None |
| University; foundation trust | C: 450,000 | 552 | Rehabilitation | L-shaped ward, all four-bedded bays (new build) | 26 | 18 | 80 | 8 | 4 | 4 | 7 | 3 | 4 | |
| District general | R: 289,400 | 201 | Acute and rehabilitation (split site) | Acute: hub and spoke Rehab: L-shaped ward with two central desks |
28/27 | 12 in acute unit; 27 in rehabilitation unit | 56 | 8 | 3 | 5 | 6 | 3 | 3 | None |
| University | R: 445,000 | 159 | Acute and rehabilitation | Nightingale ward with additional four- and two-bedded rooms | 23 | 23 (6 acute, 17 rehabilitation) | 55 | 8 | 4 | 4 | 6 | 3 | 3 | None |
Delivery of the intervention to individuals
Staff adherence to the intervention
Staff adherence was assessed through an examination of:
-
intervention documentation (3-day diaries and daily clinical logs for participants on BT and PV)
-
adherence to the protocol in terms of allocation of participants to the appropriate regime and the management of catheterisation.
Table 67 shows adherence to the intervention by trial arm. Information on catheterisation practice was also available for usual care sites and is included in this table.
| Adherence to the SVP | Usual care (n = 124) | Intervention (n = 164) | Supported implementation (n = 125) |
|---|---|---|---|
| Catheterisation | |||
| Patients catheterised in acute stage | 56 (45.2) | 80 (48.8) | 59 (47.2) |
| Time to removal of catheter (days) | Mean 25.22; median 20; SD 17.12; range 4–66; IQR 10.25–32.00 | Mean 26.83; median 20; SD 26.12; range 1–130; IQR 8.75–35.25 | Mean 21.92; median 13; SD 21.11; range 3–78; IQR 5–35 |
| Patients catheterised in acute stage and at discharge | 16 (12.9) | 35 (21.3) | 19 (15.2) |
| 3-day diary | |||
| Present | N/A | 70 (68.6) | 66 (80.5) |
| Completed | N/A | 70 (68.6) | 61 (74.4) |
| Entry on each of 3 days | N/A | 52 (51.0) | 54 (65.9) |
| ‘Time went to toilet’ completed | N/A | 13 (12.7) | 17 (20.7) |
| ‘Leaked’ column completed | N/A | 10 (9.8) | 16 (19.5) |
| Allocation to regime | |||
| Patients eligible for regime | N/A | 114 (69.5) | 93 (74.4) |
| Patients put on regime | N/A | 102 (89.5) | 82 (88.2) |
| Allocation to regime | N/A | PV: 86 (86.0) BT: 14 (14.0) |
PV: 72 (90.0) BT: 8 (10.0) |
| Allocation to the correct regime | N/A | Yes: 42 (42.0) No: 58 (58.0) |
Yes: 44 (55.0) No: 36 (45.0) |
| Patients changing regime | 10 patients (9 PV to BT, 1 BT to PV) | 1 patient (PV to BT) | |
| Time to start regime from end date of 3-day diary (days) | N/A | Mean 2.57; median 2; SD 4.31; range –8 to 23; IQR 1–4 | Mean 2.74; median 1; SD 5.23; range –4 to 30; IQR 1.00–2.25 |
| Number of days on regime | N/A | Mean 27.99; median 24; SD 19.08; range 1–93; IQR 14–37 | Mean 37.14; median 21.5; SD 41.05; range 1–186; IQR 9.50–26.25 |
| Weekly review | |||
| At least one weekly review completed (for those on programme for at least 7 days) | N/A | 74 (83.1) | 47 (78.3) |
| Staff adherence to intervention paperwork | |||
| Clinical logs adherence | |||
| Total no. of clinical logs received | 405 | 331 | |
| Total no. of clinical logs processed (NB. This excludes clinical logs that were coded as ‘unable to process’ due to insufficient quality of photocopy) | 396 | 320 | |
| No. of patients | 40 | 31 | |
| Percentage of clinical logs processed according to type of regime | PV: 90.4; BT: 9.6 | PV: 100.0; BT: 0.0 | |
| Stage 1: % (of clinical logs processed) with regime interval present and correctly documented | 83.3 | 89.4 | |
| Stage 2: % (of clinical logs processed) with both regime interval and schedule of proposed times present and correctly documented | 38.9 | 31.9 | |
| No. of processed clinical logs that achieved both stages 1 and 2 | 154 | 102 | |
| For the processed clinical logs that achieved both stages 1 and 2 | |||
| Stage 3: On average, how often was a ‘time toileted’ documented that was within 30 minutes of the proposed time? (NB. Occasions on which a clinically justifiable explanation was given for an early/late/missing time toileted were exempted from this analysis) | 54.8% (SD 29.3) | 56.0% (SD 34.1) | |
| Stage 4a: On average, how often was it documented that the patient had been asked if they were wet?a | 57.9% (31.2) | 65.9% (32.0) | |
| Stage 4b: On average, how often was encouragement documented as given? | 58.4% (34.6) | 57.5% (33.2) | |
Completion of intervention documentation
Three-day diary
Diaries were received for two-thirds of patients who commenced the SVP in the intervention group (70/102, 68.6%) and four-fifths of patients in the supported implementation group (66/82, 80.5%). All diaries in the intervention group were completed; five diaries in the supported implementation group were returned blank. Half of the diaries in the intervention group (52/102, 51%) and two-thirds in the supported implementation group (54/82, 65.9%) had an entry on each of the 3 days. Few diaries in the intervention group had three entries for ‘time went to the toilet’ on each day (13/102, 12.7%) or three entries in the corresponding ‘leaked’ columns completed (10/102, 9.8%). Figures were slightly higher in the supported implementation group, 17 out of 82 (20.7%) and 16 out of 82 (19.5%) respectively.
Daily clinical logs for bladder training and prompted voiding
Similar numbers of clinical logs were processed for both the intervention and the supported implementation groups (396 vs. 320 respectively). On 38.9% of processed clinical logs in the intervention group, both a correct regime interval and a correct schedule of proposed times (two prerequisites to be able to undertake the daily programme) were recorded. A lower proportion of processed clinical logs in supported implementation (31.9%) were found to have achieved this.
Processed clinical logs for which both key quality indicators (stages 1 and 2) had been successfully achieved were then examined further. For these clinical logs it was documented that patients were taken to the toilet within 30 minutes of the scheduled time on 54.8% of occasions in intervention and on 56.0% of occasions in supported implementation.
Two key aspects of ‘best practice’, asking the patient if they are dry or wet and giving the patient encouragement, were done relatively well. For the processed clinical logs that had achieved stages 1 and 2, it was, on average, documented that patients were asked if they were dry or wet on 57.9% of occasions in intervention and on 65.9% of occasions in supported implementation. On average encouragement was documented as given on 58.4% of occasions in intervention and on 57.5% of occasions in supported implementation.
Adherence to the protocol: management of catheterisation and allocation of participants to the appropriate regime
Participants catheterised
Nearly half of patients in intervention arms were catheterised in the acute stage (139/289, 48.1%); fifty-four patients (54/289, 18.7%) remained catheterised at discharge, a higher proportion of these were in intervention (35, 21.3%) compared with supported implementation (19, 15.2%). There is also evidence that catheters were not removed promptly, with a median (IQR) time from entry to removal for combined intervention arms of 16.0 (7.5–35.0) days; removal appeared to be quicker in supported implementation (median 13 days, IQR 5–35 days) compared with intervention (median 20 days, IQR 8.75–35.25 days).
Commencing bladder training or prompted voiding
Almost three-quarters of patients in both groups were eligible to begin a regime (114, 69.5% intervention; 93, 74.4% supported implementation). Patients catheterised throughout and those continent after catheter removal were not eligible. In both intervention arms, the majority of patients eligible to receive BT or PV actually received it (intervention 102/114, 89.5%; supported implementation 82/93, 88.2%). Furthermore, there is evidence that conservative interventions started promptly after completion of the 3-day diary in line with the protocol (intervention median 2 days, IQR 1–4 days; supported implementation median 1 day, IQR 1.00–2.25 days). Using the criteria outlined previously (see Chapter 6), only around half of patients received the correct regime (86/180, 47.8%); fewer intervention patients (42/100, 42%) received the correct regime compared with supported implementation (44/80, 55%).
Median number of days on the programme was 24 days in intervention and 21.50 days in supported implementation. The IQR was wide in both groups: 14–37 days and 9.50–26.25 days respectively.
Response of individuals
Patient interviews
Patient characteristics
Twelve interviews were undertaken with participants from across six ICONS study sites, eight from the intervention arm and four from the supported implementation trial arm. Patient characteristics are shown in Table 68.
| Site | Gender | Age | Ethnicity | Pre-stroke incontinence | Type of incontinence | ISI score baseline | ICIQ-UI score baseline | Continence status at discharge | Interview with: | Communication or cognitive impairment |
|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | ||||||||||
| None | ||||||||||
| SVP | ||||||||||
| AA | F | 73 | White | Yes | UUI | 8 | 14 | Continent | Patient | None |
| AA | F | 80 | White | No | Functional | 8 | 14 | Continent | Patient | Possible cognitive impairment |
| AA | M | 74 | White | Unknown | N/A – continent after catheter removal | 0 | 1 | Continent | Patient | None |
| AA | M | 79 | White | No | UUI; functional | 8 | 16 | Continent | Patient and wife | Aphasia |
| BB | F | 88 | White | Yes | MUI | 8 | 19 | Incontinent | Patient and daughter | Aphasia |
| BB | M | 68 | White | No | Functional | 8 | 9 | Missing | Patient and wife | None |
| BB | F | 73 | White | Yes | Functional | 6 | 6 | Incontinent | Patient | None |
| EE | M | 60 | White | Yes | UUI; functional | 8 | 16 | Continent | Patient | None |
| SVP plus supported implementation | ||||||||||
| HH | M | 85 | White | Not known | Not known | Missing | Missing | Continent | Patient | None |
| KK | M | 84 | White | No | MUI; functional | 8 | 16 | Continent | Patient | None |
| LL | M | 69 | White | Yes | UUI | 3 | 9 | Incontinent | Patient and daughter | None |
| LL | F | 80 | White | Yes | MUI | 4 | 7 | Continent | Patient | None |
Seven participants were male, five were female. The median age was 76.5 years (range 60–88 years) and all participants were of white British ethnicity. Half the participants had pre-stroke UI. Post-stroke UI types included functional UI (n = 3), MUI (n = 3), UUI (n = 2), UUI and functional UI (n = 2). One participant was continent after catheter removal and for one continence status was unknown. Continence status at discharge from the stroke unit was continent (n = 8), incontinent (n = 3) and unknown (n = 1).
Participants could, if they wished, be interviewed with a carer. Eight patients were interviewed alone; two with their wife, and two with their daughter.
Most participants (n = 9) had no communication problem or cognitive impairment. Two had aphasia; one was noted to have apparent cognitive problems during the interview. Two interviews were not digitally recorded: one owing to a noisy environment and one at the request of the participant, who had aphasia.
Findings
Preliminary phase: making a decision
There were three key themes discussed by patients and carers relating to making a decision about UI in the ‘preliminary’ phase of the model:
Participants talked about their UI in terms of lack of control, or lack of awareness.
Urinary incontinence was felt to have physically unpleasant effects, notably wet bedding or wet clothing. Skin discomfort was also mentioned:
Lying in it is just absolutely dreadful, I got so sore.
AA061
it affects me tremendously, my skin feels as if it’s burning, which meant when I went for a shower I had to be very careful, in fact on one or two occasions I thought I wonder if I’ve got scalded with the shower
AA061
There were many negative emotions relating to the experience of UI, such as the worry, upset or embarrassment of having UI:
the more you do it the more frustrated you get . . .and the more depressed you get.
BB004
it can get you down . . . it feels like it’s . . . took part of your life, you know what I mean
EE036
Its impact on a partner was also a concern for some:
I’d be very, very worried . . . ruining a good bed, and . . . wetting a nice partner
AA061
Some participants held the belief that incontinence was an inevitable consequence of stroke:
I just felt it was just part for the course really . . . I suppose so many people do have incontinence problems when they’ve had a stroke
BB031
However, there was also an opposing view, held by some participants who had experienced pre-stroke UI or urinary frequency, that their symptoms had improved since the stroke:
I think it’s just a natural effect of the stroke I think, I have heard it said that people who have had a stroke often urinate less frequently afterwards
HH042
The assessment phase
Many patients did not remember much about the initial assessment, perhaps because it had taken place early after the stroke when they were still acutely unwell. As with the clinical assessment of UI, the assessment process was seen as enabling the patient to acknowledge the problem and the need for intervention:
your programme gets the person to admit they’ve got incontinence, and once they admit they’ve got it and that they need help, that’s the big thing
LL002
The benefit of staff engaging in discussion of UI as part of the therapeutic relationship was also identified:
I think it’s probably helped a lot, with somebody else taking an interest
AA061
Patients and carers also engaged actively in the process of assessment in the preliminary phase
in the first week we wrote it in the booklet and we kept a note of things
LL002
There was also some evidence of misunderstanding in the assessment phase regarding the diagnosis of UI, with some people erroneously believing that functional UI was not a form of UI and that it did not warrant going on the ICONS programme:
Once they realised that it weren’t a ‘bladder out of control’, then they chose not to put me on the ICONS.
BB004
Core phase: timing decision
There was, perhaps inevitably, an element of trial and error in selecting the appropriate TV interval:
the 2 and a half hour was, I couldn’t make it, but the 2 hour I could do it
AA049
It was also recognised that participation in ICONS meant that nursing staff had to devote considerable time and effort to the TV programme:
So the fact that the nurses consciously set a time aside and go and ask and do it regularly
BB002
However, it was challenging to co-ordinate TV with other activities in the patient’s day, such as therapy sessions or off-ward visits for investigations or interventions:
I was surprised the nurses would think about it often enough, cos you’re out and about all over the place
LL002
Core phase: adapting timing or programme
Participants identified that the SVP was not a quick fix. They realised that although setbacks might arise, it was important to keep motivated during the programme:
if you have an accident, fair enough it can’t be helped, but you don’t give up on yourself
AA049
Particularly for those who needed practical assistance with toileting, timing was crucial. If they requested help too early, they would find that they were unable to urinate after all; if too late, they might have an episode of UI. As well as the ability to recognise the urge to urinate, they needed to develop detailed knowledge of how quickly the nursing staff were likely to respond, which might vary at different times of day depending on the ward routine.
I’d normally press the buzzer just in time – not too early because nothing might happen – so I time it as best I can
AA061
As with the initial assessment, some participants took an active role in self-monitoring throughout the programme. However, the ‘official’ ICONS paper work was found to be somewhat cumbersome. Ownership of their progress monitoring was demonstrated by those who adapted the process for their own needs:
I found the notepad was easier ‘cos it’s a smaller piece of paper and you can just flick through the days
LL002
Bladder training and prompted voiding
Distraction techniques were found to be of limited use. Exercises such as ‘counting backwards from 100 in sevens’, suggested in the programme, were not well received. It was felt that such measures were challenging even as a stand-alone exercise, and were very hard to achieve in the context of BT:
it was so hard to do, it was one of the memory tests they gave him as well counting in sevens . . . I couldn’t, couldn’t do it
LL002
The PV programme was found to be useful in re-establishing a regular pattern of micturition:
It meant you got very good attention, frequent reminders, that you got back into the habit of going
AA062
Participants also found that the PV programme enabled them to gradually take control of their own toileting:
they asked you if you wanted the bedpan, and now you’re more ringing for it yourself
BB002
Prompted voiding was found to be especially useful for people with post-stroke aphasia, who might have otherwise had difficulty in formulating and expressing a request for toileting. The regular regime made it comparatively easy for them to respond to a staff query about their toileting needs:
it might have taken longer to know what she wanted to say; it is sometimes difficult to make us understand.
BB002
Context in which the trial was conducted
Fifty semistructured interviews were conducted with staff across the 12 study sites. Of the 12 sites, four sites were allocated to each of the three pilot trial arms: intervention; supported implementation; and usual care. Forty-three interviews were conducted individually and seven interviews were undertaken as a small group interview comprising two or three staff members. In total, the 50 interviews involved 59 respondents across four main staff groups as illustrated in Table 69.
| Staff group | Number of respondents |
|---|---|
| Nursing | 36 (61%) |
| Nursing managerial (matrons and above) | 4 (6.8%) |
| AHPs | 14 (23.7%) |
| Medical | 5 (8.5%) |
| Total | 59 (100%) |
Nursing respondents included nursing staff ranging in grade from HCAs through to ward manager level. This group also comprised SRN nurses, specialist nurses and stroke nurse co-ordinators. Three nurses performed a dual role incorporating the role of ICONS research nurse. Nursing managerial respondents included nursing staff at grade of matron and above including deputy chief nurse and directorate manager. Medical staff respondents comprised solely of consultant level staff. Allied Health Professional (AHP) respondents included lead physiotherapists, lead occupational therapists, physiotherapists, occupational therapists and one speech and language therapist.
Data collection
Potential participants were invited to participate by letter. Arranged by study administrators, interviews were conducted within clinical sites participating in the study at a time to minimise disruption. Participants were able to participate in individual or group interviews according to personal preference and logistics. The interview spine was developed around the six dimensions of a root definition of a soft system, and refined through a pilot study. Interviews were digitally recorded with the participants’ consent and fully transcribed. All transcripts were checked against the original recording to ensure accuracy.
All interviews were conducted between March 2011 and November 2012 by senior researchers from University of Central Lancashire (LT, JG and DF). Three joint interviews were conducted by LT and CB. Interview lengths varied by staff group, study location and individual interview versus group interview. Table 70 illustrates the total and mean (SD) interview length of the 43 individual interviews by staff group.
| Staff group (n) | Individual interviews, total time (minutes) | Mean interview time (minutes) | SD |
|---|---|---|---|
| Nursing (27) | 732.0 | 27.1 | 7.3 |
| Nursing managerial (3) | 97.0 | 32.3 | 1.5 |
| AHPs (9) | 305.0 | 33.9 | 7.5 |
| Medical (4) | 96.0 | 24.0 | 24.8 |
| Total (43) |
Seven group interviews were conducted across four study sites (FF; HH; MM; JJ). The mean group interview length was 40.7 minutes (SD 8.2 minutes). Respondents were from varied staff groups as illustrated in Table 71.
| Study site (number of group interviews) | Total site interviews (minutes) | Number of respondents | Staff groups |
|---|---|---|---|
| FF (2) | 82 | Six (three in each group) | Nursing and medical AHPs |
| HH (2) | 75 | Four (two in each group) | Nursing managerial and ICONS research nurse AHPs |
| MM (1) | 57 | Two | Nursing |
| JJ (2) | 71 | Four (two in each group) | Nursing Dual role nurses |
| Total time of group interviews (minutes) | 285 | ||
Interview lengths across the 12 study sites ranged from 15 minutes to a maximum of 57 minutes with a mean interview length of 30.3 minutes (SD 9.3 minutes). Table 72 illustrates number of interviews, mean interview length, SD and total minutes of interviews by site and by trial arm.
| Trial arm | Study site | Number of interviews | Mean interview length (minutes) | SD | Total minutes/site |
|---|---|---|---|---|---|
| Intervention | AA | 6 | 26.2 | 6.6 | 157 |
| EE | 4 | 29.8 | 3.3 | 119 | |
| CC | 1 | 23.0 | – | 23 | |
| BB | 2 | 37.5 | 6.4 | 75 | |
| Total minutes (arm): | 374 | ||||
| Intervention and facilitation | KK | 8 | 34.6 | 10.3 | 277 |
| LL | 3 | 28.7 | 14.2 | 86 | |
| FF | 6 | 32.5 | 9.8 | 195 | |
| HH | 3 | 35.3 | 3.8 | 106 | |
| Total minutes (arm): | 664 | ||||
| Usual care | MM | 2 | 47.0 | 14.1 | 94 |
| GG | 5 | 28.6 | 3.4 | 143 | |
| JJ | 3 | 34.7 | 2.9 | 104 | |
| DD | 7 | 19.4 | 2.1 | 136 | |
| Total minutes (arm): | 477 | ||||
| Total sample: number of interviews, mean interview length, SD and total number of minutes of interviews | 50 | 30.3 | 9.3 | 1515 | |
Data management and analysis
Interview transcripts were managed using Atlas-Ti software (version 6, Scientific Software Development GmbH, Berlin, Germany). Completed by two analysts, who jointly developed and refined a coding framework, the initial phase of the analysis required immersion in each transcript through multiple readings, noting initial reflections in the form of memos. The coding framework (Table 73) was developed from the six soft systems dimensions outlined earlier, and was refined through joint coding of interview transcripts across three study sites.
| Major code | Description |
|---|---|
| CUSTOMER | Data relating to descriptors of service recipients (both patients/family carers; and at individual and group level). These data will include stroke-related problems; needs; aspirations; experiences; demographics, as they relate to matters of urinary continence |
| ACTOR | Data relating to professional and support staff who have responsibility/performed activities or health interventions that are directly or indirectly relevant to urinary continence |
| TRANSFORMATION | Data which describe changes that, by reporting or logical inference, are made as a result of the interventions and responsibilities completed by ‘actors’ Assessment and diagnosis; patient education; routinised care |
| WORLDVIEW | Data about any aspect of the continence care system that have potential explanatory value; clinical priorities Statements about patient and family carer factors (e.g. empowerment) when these reflect critiques of the system (e.g. is currently disempowering) |
| OWNERSHIP | Data relating to leadership of the system Finance issues; financial flows relevant to the urinary continence system Systems and processes that control resources and equipment Feedback from service users and staff that drives service redesign/practice development |
| ENVIRONMENT | Data relating to clinical and other environments in which the continence care system operates. The environment may be multidimensional, as follows: Equipped/unequipped to deliver urinary continence care Geography (e.g. physical layout, social and emotional meaning) Team culture/working Continuity across settings Absence or presence of clinical information Absence or presence of knowledge and skills/learning culture |
The coding framework was then applied to the transcripts, focusing on elements of text that covered a specific issue or perspective. Special attention was paid to the following:
-
data that report first-hand experience of the clinical system, rather than general statements of opinion, and
-
data obtained from participants working within those components of the acute stroke pathway directly involved in the ICONS study.
The analysis then reflected the principles of framework approach,176 including the production of charts for each dimension across each study site to allow within and cross-site comparison. These charts were then used to identify patterns of meaning within and across interviews in the form of themes. Themes have been used to construct a narrative synthesis which describes the post-stroke UI system, and the range of perspectives within each domain across the sites as a whole. This thematic analysis was used to identify elements of the clinical system that could logically be used to characterise ICONS sites. Following theoretical triangulation with other sources of data, these elements were considered within four overarching mechanisms that explained SVP fidelity and impacts within the trial.
Findings
Findings are presented as themes which characterise participants’ responses within each of the six dimensions of the post-stroke UI clinical system. Exemplary quotations are included to highlight the meaning and scope of each theme. For auditing purposes, quotations are accompanied by a code indicating the participating site, participant and location of the quotation in the interview transcript.
Customer themes
The ‘prevalence’ of incontinence
Although there was variation in the reported proportions of patients with UI, there was consistent reporting of the significant size of the clinical population who were thought to be incontinent of urine after stroke:
the majority of our patients do have continence issues . . . We do use a lot of continence products really when they are here.
DD07; 4
Generally, the presence of UI was linked in the minds of participants to stroke severity and the complexity of associated patients’ needs:
Other patients who have had strokes and are quite good, they’re up and about walking and things, they are generally continent it’s just the patients who’ve had large strokes
DD05; 32
our patients . . . who maybe are doubly incontinent they are likely to be severely globally impaired in a cognitive state and/or the language state so you are talking about a complex patient who you know needs a high level of nursing care
EE02; 204
Importantly, staff recognised that UI may have been pre-existing, linked to a complex picture of underlying poor health:
some of the patients are known . . . to the continence service already and they come in with the pad that’s been supplied by them so they’ve already had their assessment undertaken.
AA03; 36
The hidden nature of incontinence
Despite consistent reports of the high prevalence of post-stroke UI, many staff participants mentioned instances where incontinence was actively hidden by some patients:
patients if they are mobile tend to hide it.
GG02; 33
This may reflect the patterns of coping that some individuals had developed to address a pre-stroke continence problem:
some of the gentlemen with urge incontinence and things like that prostate problems, and again some of the ladies that’ll just go and buy pads from Boots and keep quiet about it.
FF09; 38
Particularly in relation to older groups of patients, there was agreement that the topic of continence could be taboo for some:
I don’t know whether many patients would be happy for it to be discussed
EE01; 76
think some of our patients especially the older generation, they don’t actually like their family to know that they’ve got a continence problem.
AA05; 44
There was some evidence that staff felt they were adept at uncovering incontinence:
a lot of patients are quite good at covering up problems that they have . . . but the staff are usually pretty good picking things up.
JJ02; 67
However, there were instances where it was evident that problems with continence had been missed. For example, a medical consultant reported that:
sometimes it’s the patient who raises it as a problem, like a patient did today and nobody else had mentioned it.
FF08; 8
Discussions about prevalence highlighted a complex interplay between UI, comorbidity, and the consequences of stroke which made it difficult for staff to understand the nature of UI from the patient perspective. Many participants referred to ‘cognitive impairments’ as being a significant problem for patients who ‘perhaps don’t grasp the implications of [UI] very well’ (AA02, 242). Participants discussed active strategies used by patients, such as denial and problems with mental capacity, that compounded the assessment of urinary continence. It also appeared hard for participants to be able to distinguish between denial and mental capacity, as articulated in the following excerpt:
they generally go and ask the patients [about incontinence] which is fine if the patient has got capacity and things, but if they’re in denial and that, even with capacity if they’re in denial, they tend not to see it as it is.
LL01; 29
For some participants there was a view that increasing patient age was associated with a lower personal importance attached to continence:
other people, depends on the ages, they’re not always interested, the more elderly ones aren’t sometimes, just want to be left alone.
JJ04; 528
Some participants reported that patients could be ‘quite happy to be quite passive can’t they? You know would happily stay in bed’. However, this participant, a physiotherapist, also reflected on the same individual who would be able to ‘toilet herself independently if we had a raised toilet seat and a frame was around it’ (HH04, 93), highlighting that unmet needs could complicate engagement in other aspects of care around incontinence. There was also recognition of the disruptive nature of stroke on pre-existing coping mechanisms ‘by virtue of the fact they’ve now had a catastrophic event’ (EE02, 18).
Family carers as ‘customers’ and ‘actors’
Although most of the interview discussions focused on patients, there was a recognition of the importance of engaging family carers as recipients of aspects of care within the clinical system. Family carers were seen as an important source of information on a patient’s pre-stroke life, including any continence issues or incontinence coping mechanisms:
we have a family meeting and continence is nearly always discussed.
GG05; 41
However, the main substance of this talk focused on repositioned family carers as both recipients of care and ‘actors’ supporting the delivery of the clinical system in practice:
they get involved sometimes towards discharge planning if they’re going to go home with catheters. They come in and get involved in catheter management, how to empty the bags, when to change the bags, how to apply the . . . night bags.
GG01; 91
Actors themes
Integrated working around (in)continence
Reflecting the complexity of post-stroke UI, the data demonstrate a wide range of professional groups involved in the system:
stroke’s so wide ranging you . . . can find people . . . cognitively impaired as a result of the stroke, you can have people with speech and language difficulties . . . and you can certainly have . . . people with comorbidity, so they might have dementia or confusion, they might have other underlying pathologies that mean that their wishes aren’t easily accessible.
LL03; 22
Nurses were generally seen as the ‘glue’ of the clinical system, largely owing to the continuous professional involvement in inpatient settings:
well its normally the . . . nursing staff, but the physiotherapy and the occupational therapy staff get involved as well. Predominantly it’s the nursing staff ‘cos they’re here twenty-four hours.
MM01; 174
Much of the data relating to the nature of working relationships between nursing and support workers focused on the delegation of work around the management of UI:
there is somebody who needs toileting every so many hours then it usually tends to be the delegated job for a clinical support worker.
DD06; 36
There were instances where other professional groups were reported to contribute to this work:
it would be the health care assistants and the nurses . . . possibly physios . . . or OTs [occupational therapists] if they had them in they would take them to the toilet as well,
AA05; 89
but these instances were exceptional. Although the predominant model of practice was team-working, the challenges of co-ordinating work through the clinical system were evident:
communication . . . somebody thinks the other person’s done it and the other person hasn’t done it and you’re just . . . you know it’s been assumed. I don’t think it’s been done intentionally.
DD01; 173
However, the data also demonstrated evidence of co-ordinated and integrated working in other aspects of work within the clinical system. Physiotherapists described how addressing toileting needs and goals were integrated into functional rehabilitation:
we’re involved . . . in washing and dressing, so toileting is obviously part of that
HH04; 6
and for sharing continence-related information across the care team:
we’re all responsible for completing the assessment each time we toilet a patient or do a change. The whole team’s responsible for that, from the health cares to the therapists
GG01; 101
part of our rehab is involved in looking at toilet transfers and once we‘ve, I suppose bed to chair transfers we start off with and then we would look at doing toilet transfers and then looking at educating with the nursing staff on how best to transfer onto the toilet or commode.
HH04; 5
Although aspects of team-working and information sharing across professional groups were evident in the data, there were instances in which integrated working was discussed in more nuanced terms, often in relation to professional interests. There were some views expressed that incontinence issues were really only addressed by some professional groups when it impinged on other aspects of their contribution:
therapists only really if it affects their ability to deliver their care
AA06; 41
if we’re aware as a medic that there’s a problem then we will do medical assessment as well.
LL02; 29
There was evidence that different professional groups were reflecting on a greater need for engagement in line with changes to the service model:
we’re discharging a lot earlier . . . we need to be thinking about how we can how we outreach into them if our patients have been moved very fast. We probably need to be getting more involved.
GG05; 275
However, these concerns were contrasted with a lack of evidence and policy driving further contributions to the clinical system:
we looked at the RCP [Royal College of Physicians’] guidelines to see what we as OTs [occupational therapists] should be doing in the hospital with our stroke patients and it’s not you know [UI is] not a big thing for OTs . . . In fact I’m not even sure it’s mentioned.
FF03; 29
Generally, discussions about integrated working around post-stroke UI mirrored broader structures and processes for team-working in the study sites. Key features of these discussions focused on the ‘multidisciplinary team meeting’, and how this was organised. For example, when multidisciplinary meetings had little nursing involvement, UI may have received less attention:
It isn’t really addressed because it’s more of a therapy handover than a nursing handover . . . because we haven’t really got a nurse on the team as such.
EE04; 12
This reflected a view that:
within the multidisciplinary team . . . there are certain areas which are deemed as the responsibility of certain disciplines, and there the twain meets, so . . . nursing is continence and medication, and so we’re a bit silo-oriented within the MDT.
LL01; 44
On the other hand, there was evidence that UI was ‘a continuing thing that crops up in goal planning meetings’ (LL03, 16). In these situations there appeared to be a more integrated approach to multidisciplinary meetings:
its probably something that comes up in our multidisciplinary meeting . . . we’ve got nursing and therapists there, so when we feel that the patient’s reached a point at which we should expect them to at least be able to manage continence from a . . . medical condition point of view.
MM02; 53
Transformation themes
The importance of assessment
A considerable amount of ‘talk’ within interviews focused on the importance of the assessment of incontinence, and generally participants reported satisfaction with the ability to develop an understanding of each patient’s problems and needs:
they’re first assessed . . . is quite good, because you can look at that and you can get a clear picture whether or not they do have any incontinence problems . . . so that’s managed quite well.
EE01; 205
Early assessment appeared to be important, although this often reflected institutional norms:
there’s an actual incontinence assessment sheet, so all patients that hit the unit or . . . hit any of the wards within [name of hospital] that would have a continence assessment within 24 hours of being on the ward.
AA05; 6
Within the stroke service, there was evidence that assessments were used to develop a management plan:
we put them on a 3-day chart to monitor continence, how many times we’re changing . . . incontinence products or how many times they’re needing to be toileted, keep that for 3 days and then we set up a management plan.
GG01; 26
Although this quotation also demonstrates a willingness to adapt approaches to patient management, this was far from the norm. A number of examples were identified in the data that demonstrated a lack of connection between assessment, problem identification and the development of a continence plan for an individual patient:
I don’t think we . . . actually diagnose what causes the continence, whether it’s stress, urge, functional or whatever, we do tend to be a little bit . . . I suppose anecdotal in some respects as we get to know the patients and then you find out what . . . you know what usually starts it off and why they are incontinent, that tends to be the way it’s . . . kind of managed.
LL01; 17
just gives us an overall . . . picture really of when they’re incontinent, but then we don’t do anything off the back of it you know as effectively, we don’t do voiding or anything like that
LL01; 11
sometimes they’re padded up and there isn’t really a full assessment done as to what the problem is
FF08; 14
As most patients’ pathways included access to emergency or early assessment facilities outside of the stroke service, there was potential for interventions that complicated the assessment of urinary continence:
when we receive them sometimes they’ve been catheterised . . . in A&E [accident and emergency] or AMU [acute medical unit].
JJ04; 62
Variations in practice were observed around the use of assessment tools, with little reported knowledge of sensitivity or specificity:
there are several continence assessment tools . . . and basically you can choose which one you use, and . . . in the past we have used the continence assessment tool that they used in the day hospital continence clinic, so we . . . have tried to use that.
LL01; 90
In this sense, the selection and use of tools was associated more with custom and practice.
There was a recognition that, despite systematic approaches to assessment, some patients had the potential to ‘fall through the net’, including those with communication difficulties:
if they can’t communicate it the chances are that they may actually be too severely impaired to use a reference point to communicate [incontinence].
EE02; 171
Consequently, there was a recognition that flexible approaches to ongoing assessment were required. This enabled staff to be able to identify changing needs across the patient pathway:
we promote continence and we’re actively monitoring the patient regularly, and when there’s a change in the patient we . . . change the intervention to match the patient I suppose.
DD01; 164
The importance of routine
Approaches to work within the clinical system could be characterised as organisational routines associated principally with both ‘checking’ whether patients were wet or dry, or helping patients to use toilet facilities:
we do the checks, we have four-hourly checks and maybe 2-hourly checks.
DD01; 107
We’re all pretty good at doing that, we toilet them every 2 hours if we have to and, that’s all I can say about it really.
DD03; 96
There was some evidence that these routines were structured to coincide with other activities associated with patient care, or activities such as mealtimes, that could have broader meaning for patients:
we go round and do rounds like at certain times, unless like they ring the bell and ask for the toilet. We normally do rounds like in the morning when we’re sitting them up, and then we check them like when we’re washing them, and check them before dinner, then we check them after dinner.
DD03; 17
Balancing routine and individuals’ needs
The data demonstrated beliefs that more proactive approaches:
stroke patients can benefit from being asked to go to the toilet rather than just leaving them when they’re . . . and then they do become incontinent, so more regular toileting maybe
DD01; 59
and tailored approaches:
we all know that timed toileting is the best way to proceed
HH02; 5
were potentially more effective. Examples of approaches to tailoring included varying practice across day and night shifts; increasing the gap between toileting activities; and beginning to build patterns that would reflect routines in other care settings or at home:
the intentional rounding we have for patients which we go to them every 2 hours during the day to check their continence, . . . and then at night the . . . night staff will check them as well and if need be they will toilet them at the same times so . . . but then, once we have a pattern usually things improve
JJ01; 20
you get him up in a morning . . . take him over to the loo before breakfast, so . . . then you kind of try and stretch it to just before lunch or something like that you know, so that so they know when they’re hopefully going to go, and . . . its good then, because if he goes home with care and the other times perhaps and the people will be coming in at, and they could manage to do it with him as well
JJ04; 156
we . . . start a chart on the end of the bed and we ask all the staff to toilet them whether we decide it’s half hourly, hourly or 2 hourly. And then over time we build that up in time length in between so that hopefully they’re starting to go more like a normal person
GG01; 68
There was a view that timed approaches to toileting could be particularly effective for ‘people with cognitive problems’ (FF10, 57). However, where participants had received some information about the SVP interventions being evaluated within the ICONS research programme, there was some uncertainty regarding the balance between routinised and tailored approaches, with the potential for individual regimes to regress towards an organisational pattern:
There might be a lot of patients on 2-hourly toileting, but it’s not because we’ve decided everybody should be on 2-hourly toileting, it’s because when we’ve done their diaries that’s what they require.
AA05; 177
Worldview themes
The clinical paradigm underpinning the management of post-stroke urinary continence was mixed, with participants essentially ascribing different levels of priority to incontinence relative to other aspects of care within the acute stroke pathway; attaching different levels of status to practice in continence care; holding different views on the natural progression of UI; and, consequently, holding different views about the legitimacy of UI as a focus for rehabilitation effort.
Balancing clinical priorities
There was some recognition of the importance of urinary continence for patients:
continence is top of the list of priorities of things, they find it hugely embarrassing to be incontinent.
LL03; 97
However, there were varying views about the priority of continence care within the context of the full range of patient problems and needs within the acute stroke period where ‘you’re doing things to save lives you know, the patients are medically unstable’ (EE01, 139). Consequently, other aspects of care were considered to be more important at different stages of a patient’s journey, particularly when staffing resources were considered to be low:
there’s a lot of input needed from the nursing staff in the majority of patients, so I think if it came down to whether they have the . . . their IV [intravenous] drug infusion that was due, or you know taking them a . . . bedpan, I think that would come first. . . it’s just in the nature of . . . nurses. If you . . . could get more staff then it would be very much at the forefront of their minds, but I think they have to prioritise
EE01; 142
it isn’t always an ideal world as you can imagine on a 36 bedded ward, it is really busy and we try our best. But sometimes with the staffing that we have, it’s difficult to get to everybody at the right times
DD07; 14
Importantly, some participants reflected that the priority attached to continence issues was also shaped by non-clinical issues such as the:
emphasis to complete . . . this new paperwork. Sometimes that takes precedence over things that perhaps would be more useful . . . there’s pressure to . . . make sure that’s completed.
JJ01; 44
There was also some discussion about the influence of staffing resources on the priorities attached to different aspects of continence care, specifically where delivering the work of managing incontinence was prioritised to prevent harm to patients:
if . . . you’re under staffed and . . . you’re prioritising . . . continence care based on the people that are going to suffer the most as a result of being incontinent . . . the likes of your very bed bound patients who need to be turned to avoid pressure sores and pressure damage if they’re in a pad and you need to keep going back to check them.
LL03; 40
Other factors which dampened the priority attached to continence issues in the acute stroke period related to the validity of the clinical picture, and concerns about:
whether, what the patients presenting will be as a true picture of their stroke-related continence.
EE02; 12
There was some evidence that staff considered that a lower priority attached to continence in the acute stroke period reflected the perspectives of patients and family members:
I think most patients and their families wouldn’t automatically consider continence in the first few hours of stroke.
EE03; 32
This contrasted with the view that:
if the patients see [incontinence] as a problem it’s more prioritised probably than if perhaps the patients hadn’t mentioned it.
JJ01; 62
The generalist nature of stroke incontinence practice
Reflecting the varying degrees of priority attached to UI issues, there was mixed opinion about the status of practice within the clinical system. There was a view that UI care within stroke services would be no different to any other clinical area:
I think from a general point of view they would be treated like any other patient
EE04; 82
and that the status of this care was low:
[continence care is] just the basic things
FF09; 20
Reinforcing this view, this participant, a qualified nurse, went on to say later that:
the clinical support workers are the ones that normally do the basic, I don’t mean to be disrespectful but the basic nursing care.
FF09; 104
As such, it was felt that, within nursing, continence care has:
probably never been perhaps perceived as . . . an attractive aspect of care
MM01; 256
Views about the generalist nature of continence care were associated with views about the importance of experiential forms of knowledge, rather than evidence from research:
if it’s somebody fairly senior that’s had . . . lots of . . . experience with looking after incontinent patients then, and can use past . . . remedies if you like for it.
LL01; 20
you can often use your intuition, . . . you’ve got the idea that if a patient’s agitated, if they’re fidgeting, if . . . they’re trying to get up and walk around . . . one of the first things on the list of questions that you’ll ask that patient is, do you want to use the toilet
LL03; 49
In some sites there was a clear strategic shift away from specialisation around continence issues where nurses:
lose ownership of it as an issue . . . first thing they do is oh we’ve got a continence problem and we’ll ring the continence nurse rather than what are we [participant’s emphasis] going to do about it.
HH01; 27
The inevitability and intractability of incontinence after stroke
The lack of specialisation in this clinical system appeared to be mirrored by views that post-stroke UI was either an inevitable problem, an intractable problem, or both:
a lot of our patients will be getting incontinence care rather than continence care, because their condition is such that that’s all that’s appropriate at that time.
MM01; 328
Some participants provided more nuanced perspectives on this issue:
I don’t think they see it as inevitable, but I think they see it as almost intractable . . . it’s a different mind-set really to think this is a . . . problem and we can ameliorate the effects of this problem, as opposed to thinking we can cure this problem.
AA06; 86
This doctor went on to say that post-stroke UI is ‘not really rehabilitable as such’ (AA06, 92), which reflects other views about the general futility of practice in this area:
I think there’s just an acceptance on the ward that people are incontinent you know and it’s a statement rather than a kind of well this is an issue that we might need to deal with.
FF03; 11
Feelings of the futility of intervention appeared to be justified with views that any improvement in, or resolution of problems with continence were unrelated to professional input:
and if you lucky all of a sudden you’ll become continent, or if you’re unlucky you’ll spend the rest of your life incontinent, and your option is get a catheter put in, and that sounds very harsh, but that is a true picture.
AA05; 123
they improve as [JJ04] was just saying as patients improve . . . it resolves itself
JJ02; 40
The legitimacy of incontinence as a focus for rehabilitation
As a whole, the data provide evidence of mixed views about the status of UI, either as a legitimate focus for rehabilitation endeavour, as a mediator of the success of functional rehabilitation, or timely transfer of care. There were clear examples of the importance of rehabilitation to resolve or reduce the impacts of UI in the data:
every person’s allocated a key contact and that might be a therapist, and I can just think of one patient now where a therapist agreed a goal with the patient that was around promoting continence
MM01; 168
The importance of a practice framework to underpin this rehabilitation approach was also evident, where staff did:
try and set goals and things like that. But . . . there isn’t much of a plan to try to toilet them more frequently or ask them more frequently if they need the toilet. There isn’t an actual form like a formal plan so to speak that we use.
DD07; 28
This lack of a practice framework within which to organise continence promotion was evident in other interviews:
I don’t think that continence was promoted . . . they are incontinent and that’s it. We . . . change them when they’re wet, there was no way of promoting . . . no set procedure in place for incontinent patients. I don’t think it was dealt with . . . in a systematic way at all
EE01; 43
In interviews where the dominant perspective was on the mediation of rehabilitation, the discussion focused on the management of incontinence to prevent harm:
looking at . . . their skin to make sure that not going to be any pressure damage for incontinence
DD01; 35
and the impact of UI on the success of rehabilitation where:
[UI] would . . . cut into the therapy session
HH04; 5
Where this was the case, there was evidence that this could be addressed constructively:
during the multidisciplinary team meetings [which] will flag that up as an issue, which we have to try and sort out about it some way . . . in order to try and allow the therapy to progress etc. So it might be simple things like pads.
AA06; 38
Other discussions focused on the mediating effect of UI on the ability to arrange a timely transfer of care from the stroke service:
although we do try to manage continence, it’s not effectively managed at admission. It generally tends to be on discharge. It’s the reason why we tend to not discharge people home
LL03; 5
I think some people think it as a, they do really think of it as a discharge necessity rather than an in-patient requirement.
FF10; 84
There were some instances in the data which showed that, although staff approached UI from a rehabilitation perspective, there were significant environmental deficiencies, such as the distance to useable toilet facilities, that made this problematic:
I do have an issue . . . making people walk to the toilet when they are actually desperate for the toilet and instead of wheeling them and walking them back. That’s an issue with me you know. There’s no dignity there is there if you’re going to wet yourself on the way to the toilet.
DD02; 130
Ownership themes
Distributed leadership
Interviews demonstrated multiple, distributed sources of leadership within the clinical system around issues relevant to continence. Nursing was ascribed a persistent source of clinical leadership, although this was predominantly discussed in relation to the management of incontinence:
continence is seen as a nursing issue historically isn’t it.
EE04; 10
Within the nursing profession, participants debated the practical challenges of delegating work to support staff:
auxiliaries tend to take the lead. Sometimes the staff nurses may have other jobs to do, but we try and keep a check . . . But isn’t very regularly that it ever happens to be honest.
DD07; 34
However, there was also evidence of strategic leadership across both registered and support staff around the development of practices around urinary continence:
You need to have somebody who’s passionate about the continence like I say, I mean I’ve always tried to . . .
AA03; 149
I think there’s several HCAs who are I think who are very proactive and who have been here quite a long time and they have a system at work
HH04; 74
we’ve got lots of nurses who do subscribe to the Nursing Times, who do subscribe to all sorts of applications, and they’re quite happy to bring that level of information to their colleagues and to share that learning
LL03; 146
The focus on leadership around incontinence work was further highlighted in the processes of onward referral for specialist advice and support where:
. . . it’s likely to be the consultant that would refer. It might be the nursing staff that flag up an issue but realistically I think that’s more likely to be the consultant or the medical staff.
EE03; 64
This quotation is characteristic of a more general lack of clarity about, and potentially mismatch between, clinical leadership and authority for decision-making around UI.
Clinical impact of stock control
Exploring stock flows around the clinical system highlighted some reported disparities between patients’ needs, demand and responsibility for planning care, largely to control costs:
the trust as a whole . . . want us to save fifty thousand pounds in continence products.
MM03; 12
This meant that some staff participants felt that appliances and products to manage UI had limited availability, or were not of sufficient quality:
we have very limited pads and things what we can use, I think we have two different sorts, we have these horrible net knickers . . . We used to get really nice continence products but were not allowed anymore so yeah it does limit it yeah definitely
DD05; 42
there’s only one sheath that we can have in stock . . . I think, we’re quite restricted from that point of view
JJ01; 125
a lot of the time we don’t have the products that we need on the ward either . . . and then we use conveens on the gentlemen and half the time it’s never topped up . . . and the sizes aren’t there that we need
GG03; 232
There were some instances where family members were complementing ward resources to address concerns about quality:
we’ve had some relatives buy pads in you know because they’ve felt that the ones that we had weren’t appropriate. . . which you know costs a lot of money to that patient’s relative.
DD07; 22
Organisational strategy
Although the cost of products to support the management of incontinence was an important driver of practice around post-stroke UI, there was clear evidence of organisational drivers which influenced the importance attached to, and content of, this practice system. These related primarily to ‘intentional rounding’ programmes, where nursing and support staff were tasked with checking patients on a regular basis:
we fill in . . . if they’ve been wet, dry . . . had their bowels open. So we go round every 2 hours and check the patients . . . unless like they go to the toilet themselves, or if they ring the bell and let us know, you know but if they’re incontinent we check them every two hours really
DD03; 23
Consistent reference was made to performance management strategies that included, or were relevant to, UI. These included participation in national audits of stroke care:
we . . . do the RCP [Royal College of Physicians’] Sentinel Audit every year which obviously covers continence, well . . . basically is the patient continent or not and do we succeed in managing the continence.
LL01; 117
Other examples of audits were driven by local stakeholders to meet commissioners quality requirements:
we recently did the nursing quality assessment tool . . . and we failed drastically on continence . . . both urinary and . . . faecal incontinence because the assessments that we were doing weren’t adequate enough for the PCT [primary care trust].
FF09; 17
However, there was also evidence of home-grown performance management programmes:
Continence is one of the clinical indicators that we audit monthly, and it’s also one of the Essence of Care areas that’s audited but I think that’s just once a year now, unless there’s problems and then it’s audited more frequently, but certainly the . . . clinical indicators are monthly. It’s an audit of five patients at random, picked at random . . . do they have appropriate management tools in place, have they been assessed, do they have the care plans . . . have they got review dates, that kind of thing
GG01; 164
These were the approaches to performance management that were most closely linked by participants to practice development where:
they do offer us advice across the trust . . . from the point of view of promoting continence.
HH01; 42
Environmental themes
Unsurprisingly, the environmental attributes of the clinical system were multiple, providing both opportunities and challenges to staff and patients alike. These influences related to the layout of clinical settings within which the system operated; the history of change; differing approaches to risk management across the system; the availability of knowledge, skills and expertise; information quality; and the continuity of provision and patient experience throughout the system relative to their recovery pathway.
Clinical geography
The evaluation of clinical facilities by participants focused principally on issues of access and privacy:
we never have enough . . . toilets . . . we’ve got . . . fourteen men up in this . . . area with one toilet at one end of that . . . area and two toilets in the corridor beyond that for the men to use, so if you’re not very mobile . . . you’ve got quite a long run
LL01; 147
we haven’t got a lot of space . . . to chat with patients privately
JJ04; 348
The data demonstrated that staff were creative in their use of clinical settings to meet an individual patient’s needs:
I think they do put people if they are just starting to walk a little bit independent, they’ll position them in the bed by the nearest toilet on the ward . . . the environment isn’t perfect.
FF04; 163
Rather than simply an issue of the number of toilet facilities available in clinical areas, patterns of access associated with individuals’ needs appeared to be important in shaping demand and use:
we do use the disabled toilet at both ends for male and female quite a lot because it’s got quite a lot of hand rails for patients . . . sometimes you get a queue at them toilets . . . so if they had a couple then that would be helpful
DD05; 124
The toilets are too low, there’s not enough room. We’ve got 4 toilets down near the ladies’ and they tend to use just one particular toilet because it has the bars around it
DD02; 120
Generally, the degree of fit between the physical layout of clinical areas and the work associated with continence care appeared to depend on whether clinical areas were purpose-built, or whether stroke services had developed within existing care facilities:
ward wasn’t built as a rehab facility, so we’ve had to do some minor adjustments as best we can to some of the toilets to make sure that they’re disability friendly, but they’re not, still not ideal . . . they wouldn’t . . . allow somebody in a wheelchair to toilet themselves independently
MM01; 362
There was evidence that in some sites, staff had been able to influence the design of clinical settings around their experiences of care provision, specifically with reference to access issues:
when they did the plans they had a toilet and then a bathroom and a toilet but we’ve asked them to do it as . . . an en suite type of thing, so that they’ve got a wash basin there as well, so the patient can go in there and do their ablutions in . . . privacy and you know without having to . . . have people barging in behind curtains
JJ01; 161
History of change
Another attribute of the clinical environment on which participants drew to evaluate the potential to alter aspects of the clinical system, including use of the SVP, was the history of change, and how change had been experienced:
with all of the ward moves and everything else it has been a very, very, very hard last 2 years . . . 18 months to 2 years.
HH02; 115
Some of the features of successful change included generating broad commitment to change:
it was a big push to get everybody on board it worked well.
HH02; 137
Strong leadership that set change within the context of a clear rationale, including performance relative to peer organisations and services, was highlighted as being particularly important:
I do think that continence care is possibly one of the weak areas and certainly that is what the Sentinel stroke audit’s telling us. So we really need to unpick that
EE03; 104
the ward manager tends to discuss with us what’s been happening and what’s come from the audits and how well we’re doing in comparison to other trusts
DD06; 80
We’re trying to see what the other hospitals do around the [region] and see how they manage an actual plan if there’s a formal plan that they use cos we just don’t have one at all . . . the Cardiac and Stroke Network have been helping us in that way
DD07; 68
Embedding change within national initiatives was generally discussed in positive terms:
being part of 90–10 Improvement Programme was a big lever as well in terms of improvements.
FF10; 152
However, not all change initiatives reported in interviews had been positive, particularly where some of the more positive features of the organisational context for change cited earlier were not evident:
trying to get change is very difficult. I’ve just recently done some constraint induced movement therapy which is the best evidence that a stroke rehab there is but we need to plan it and do it properly before we can implement it and you know. I e-mailed all the senior managers and haven’t had a reply for 3 weeks.
FF03; 57
Balancing risk between hospital and home
The data demonstrated clear differences in how risk was managed within the clinical system across different settings within the stroke pathway. At home there was more opportunity for specificity to an individual patient’s needs:
the home environment can be set up for that particular patient. So if they need a rail or a bar or a toilet raiser, that may be appropriate for them, but they won’t always be available in hospital.
LL02; 143
Consequently, there was a recognition that long-term approaches to promoting continence were hard to achieve in the hospital setting:
often we get right up to the stage of discharge and patients are still pressing the bell to go. It’s kind of learned behaviour I guess, institutionalisation. But it doesn’t help us when we’re saying, ‘well if you’ . . . you know, ‘why aren’t you going on your own because that’s what you’re going to have to do at home’
FF03; 121
Every patient has a manual handling risk assessment and as a matter of course, and again, this may be custom and practice, patients are asked to ring the bell before they go to the toilet because they are having to walk across a ward with lots of obstacles in the way.
FF03; 121
Importantly, there was evidence of tailoring rehabilitation input to address anticipated continence needs associated with transfer of care:
we would support the nursing staff in handling . . . so that Mr X can go to his weak side every time he goes to the toilet because that’s what he’s gonna need to do when he gets home.
HH04; 11
Contrastingly, approaches to managing incontinence developed within the hospital setting sometimes had to be drastically altered to accommodate transfer of care:
sometimes we manage them here with pads, but going home they can’t be managed back home with them because they haven’t got the carers or family to go in and do the changes that we would do. So we then have to make a decision to catheterise
GG01; 53
so we might be able to get patients continent with prompted voiding in hospital, but then who’s going to prompt them at home if they haven’t got 24-hour care?
MM01; 72
Availability of knowledge, skills and expertise
A considerable amount of discussion within interviews focused on the availability of knowledge, clinical skills and expertise in the right place, and at the right time to meet patients’ needs. Many participants focused on their ability to access education and training provided principally by specialist services:
continence service here developed workshop days that you’d go to and attend . . . and we can also gain you know the . . . policies and things like that they use,
MM03; 177
or by commercial suppliers of incontinence products:
[education and training] tends to be product related
GG03; 148
Although there were opportunities for nursing and support staff:
we try and send all, both . . . and I have the support workers and our qualified can go on, but that’s it really with training
LL01; 62
there was little evidence of access to education and training for other professional groups. In the absence of formalised approaches, there was strong evidence of reliance on experiential sources of knowledge:
well I’ve been here for that . . . it’s the nurses who can do it . . . I haven’t been on any incontinence course.
DD04; 95
In addition to attendance at education and training events, there was some limited evidence of organisational approaches to link specialist knowledge and skills to clinical practice. These included ward-based resources:
there’s a communication folder that’s in the staff room and anything that’s new, any articles, any research or any information that needs to be passed onto staff we tend to put it into the big folder so it’s available for all the staff to read so that’s quite a good idea that we have on the ward.
DD06; 76
Other approaches relied on the availability of specialist staff as a source of support and advice:
there is a lady who works in the community that works with continence and I remember a year or two ago we used to ask her for advice and things and she came in [to deliver training].
DD06; 50
there’s a community continence nurse that we can access but only generally post discharge . . . I did ask them to come and see a patient on the ward recently and on discussion she said she would but it was for faecal incontinence actually not urinary . . . but I think afterwards when the staff contacted her she doesn’t come into the hospital
LL02; 38
we’ve always got that link with the continence service in the community you know to ring and they’ve always got the knowledge of where to, you know who to contact
AA03; 193
There was some evidence of strategies that attempted to bridge expertise in specialist services or agencies with the stroke service, but these were limited in scope:
we’ve got link nurses for every aspect and whatever you go and learn on your training day it’s then up to you to filter down, but it’s equally up to us if we want to know something ask . . . your link nurses.
GG02; 81
In this case, the ‘link nurse role’ appeared to extend little further than providing a higher level of education and training for one or two members of staff within a clinical team.
Availability and use of clinical information
The data appear to indicate that, in terms of post-stroke UI, the clinical system could be characterised as ‘data rich, information poor’:
at the moment if I am being totally honest, I get very little information coming out about continence.
AA02; 24
Services peripheral to the stroke service provided limited, detailed information on continence:
when they come from the medical admissions unit . . . we get like a handover sheet if they’re continent, incontinent.
DD03; 11
Within the stroke clinical settings, pathway-specific documentation tended to include more detailed information, including pre-stroke continence status:
they have got an integrated stroke pathway and from day one and from the beginning . . . continence is as it were identified pre . . . stroke and post-stroke.
FF09; 23
Generally there was little, if any, evidence of access to patient-specific information on ongoing continence issues or outcome after discharge from hospital, preventing evaluation of care provision:
we don’t really know what happens to them when they get home is what I’m trying to say.
MM01; 79
There was some evidence that clinical documentation played a role in sharing relevant clinical information; however, this tended to be reported as burdensome in terms of volume:
we’ve got that much paperwork take for instance we’ve got a patient that’s . . . has continence issues, we’ve got the assessment to do, we’ve got intentional rounding which . . . is the problem and then there’s another . . . fluid balance chart if we’re looking at the output and then they’re on a diet chart . . . so there’s about four pieces of paper before you start and all of them you know are probably saying the same thing.
JJ01; 125
Perhaps unsurprisingly there was evidence of reliance on oral, and consequently fragile, modes of communication:
when a problem’s identified really, and then you know we pass it back to we go through the communications through handover and you know lets all staff become aware of that.
DD01; 65
Oral communication was also prone to shaping by the priorities of individual staff members, meaning that continence issues could be lost:
we don’t really get a handover from the acute or rehab about particularly about a patient’s continence issues it’s more around therapy issues.
EE04; 8
Continuity across the clinical system (experience, provision and service)
There was a clear recognition of the potential mismatch between the configuration of care services within the stroke pathway, and the delivery of continuity within the clinical system. Unsurprisingly, particular challenges to continuity were identified at the point of transfer of care to rehabilitation or community-based services:
On discharge for some patients if they need like continence assessments in the community, district nurses tend to do them and look at if they need like continence equipment when they go home
DD07; 46
if we do . . . want district nursing service to follow patients up at home there’s quite a gap between the time that they leave and the time that the district nurse gets round to doing the continence assessment and supply the pads, it can be up to 6 weeks
LL01; 23
This may reflect a lack of knowledge about service availability:
I know the NHS has . . . an incontinence nurse, but I’ve never seen them, unless they get when they’re discharged they see them,
DD03; 74
or perhaps a more fundamental lack of integration of services around UI.
Synthesis
Within soft systems methodology, the development of a ‘root definition’ of the system under consideration represents a stage in the change process underpinning soft systems work. Specifically, the use of the six soft systems domains identified earlier provides a structured opportunity to identify and evaluate the assumptions underpinning a system. Box 3 shows the root definition of the clinical system supporting service provision around incontinence, whereas Table 74 shows emerging themes compared against what could be viewed as the ‘gold standard’ system.
The clinical paradigm reflected in the organisation and delivery of the system is mixed, dependent on the degree to which individual staff members see UI as the legitimate focus of rehabilitation, or as a barrier to meaningful engagement in rehabilitation activities. As a complex clinical problem, UI is a prevalent concern for acute stroke patients, which may be compounded by other health conditions, and may be actively hidden by patients. The degree of priority attached to UI is varied across patients and staff, and may differ between individual cases according to beliefs about clinical significance relative to other clinical work, the nature of recovery of continence and organisational pressures. Clinical staff work with patients, and occasionally family members, to accurately evaluate the causes and patterns of incontinence, in order to deliver multiple impacts around patient safety, dignity and urinary function. A key characteristic of service provision around the management of UI is routinised, patterned practice, which has the capacity to support regimes that are more tailored to the needs of individuals. This practice may reflect other patterned or routinised approaches to both clinical work and organisational strategies to enhance service quality. The management of incontinence is primarily a nursing responsibility, addressed with the health-care support workforce. Members of the MDT may provide active support within the system depending on individuals’ understandings of UI, and reflecting broader processes of multidisciplinary working within the acute stroke service. A complex, multifactoral organisational context surrounds this complex system, including the configuration of clinical and organisational geography; continuity of provision and experience; the availability of information, knowledge and skills; a culture of evaluation and service improvement; financial and strategic influences.
| Root definition domains | Themes | Descriptors (against ICONS gold standard) |
|---|---|---|
| Customers | The prevalence of incontinencea | |
| The hidden nature of continencea | ||
| Family members as customers and actorsa | ||
| Actors | Integrated working around (in)continence | Integrated working around continence; different professional perspectives; ‘everyone’s business’ |
| Transformations | The importance of assessmenta | |
| The importance of routine | Evidence of regularised approaches to managing continence; patterning care | |
| Balancing routine and individuals’ needsa | ||
| Worldview | Balancing clinical priorities | Incontinence being important (in whatever context) relative to other interventions |
| The generalist nature of stroke incontinence practicea | ||
| The inevitability and intractability of incontinencea | ||
| The legitimacy of continence as a focus for rehabilitation | Continence as an outcome (rather than mediator) of rehabilitation endeavour; goal setting; progress review | |
| Ownership | Distributed leadership | Multiple people leading continence issues; co-ordinated approaches |
| Clinical impact of stock controla | ||
| Organisational strategy | Understanding of the organisational importance of continence care; audit and feedback; quality review | |
| Environment | Clinical geography | Synergy between the clinical environment and continence work |
| History of changea | ||
| Balancing risk in hospital and home settingsa | ||
| Availability of knowledge, skills and expertise | Co-ordinated and structured approaches to education and training | |
| Availability of clinical informationa | ||
| Continuity within the service model | Integrated community and hospital services; continuity of goals and interventions |
The analysis and findings from the soft systems analysis focused initially on the development of a rich understanding of the organisation and delivery of intervention around post-stroke UI. This understanding is useful in pointing to factors which may inhibit or enhance implementation of any intervention relevant to continence in subsequent clinical trials, and informing the development of bespoke implementation strategies.
Response of clusters, recruitment and reach of individuals, delivery to and response of individuals and maintenance of processes over time
Normalisation process theory
The intervention is referred to as the SVP. Findings for each of the 16 NPT dimensions are presented, with illustrative quotes. Single numbers in brackets, for example (4) identify the number of sites supporting a finding. Letters and numbers in brackets after an italicised quote are the reference identifier for a specific interview, for example (AA2) refers to the second interview from site AA. Table 75 provides the number of quotes used from each site, to illustrate that the spread was equitable, and that each site contributed some material to all sections, except for one site (AA), where no quote was used related to coherence. However, the number of quotes from other sites tended to be low in this NPT category.
| Site | Number of interviews | NPT category | |||
|---|---|---|---|---|---|
| Coherence | Cognitive participation | Collective action | Reflexive monitoring | ||
| AA | 4 | 0 | 6 | 7 | 6 |
| BB | 3 | 6 | 5 | 20 | 11 |
| CC | 2 | 2 | 5 | 15 | 8 |
| EE | 5 | 2 | 5 | 15 | 6 |
| FF | 3 | 2 | 5 | 16 | 16 |
| HH | 5 | 5 | 10 | 19 | 10 |
| KK | 4 | 2 | 10 | 8 | 8 |
| LL | 6 | 1 | 11 | 15 | 8 |
| Total | 32 | 20 | 57 | 115 | 73 |
The discussion presents a short summary of the main findings and evaluates the strength of the evidence for the main mechanisms of action, suggesting which might be the dominant explanations in the logic model as a basis for testing in a future trial. Implications of the barriers and facilitators for intervention and implementation design in a future trial are discussed later in the report (see Chapter 11).
Demographic data
Demographic data for the interview respondents are summarised in Table 76. Some respondents were interviewed in small groups of two or three. Thirty-eight members of staff were interviewed in total, during 32 interviews.
| Grade of staff | Intervention site | Supported implementation site | ||||||
|---|---|---|---|---|---|---|---|---|
| AA | BB | CC | EE | FF | HH | KK | LL | |
| Band 7: ward manager | 1 | 1 | 1 | 2 | 1 | 1 | 1 | |
| Band 6: sister/charge nurse | 1 | 1 | 2 | |||||
| Band 5/6: staff nurse | 1 | 1 | 1 | 1 | 2 | 2 | ||
| Band 5: research nurse | 1 | |||||||
| Band 2/3: HCA | 2 | 1 | 3 | 2 | 2 | 3 | 4 | |
| Number of interviewees per site | 4 | 3 | 2 | 7 | 5 | 5 | 6 | 6 |
| Number of interviews per site | 4 | 3 | 2 | 5 | 3 | 5 | 4 | 6 |
Findings
Coherence: the sense-making work that people do when faced with a new practice
Sites differed in how much continence care they were providing prior to introduction of the SVP: some were doing very little (4):
Prior to ICONS there wasn’t any managed systems of continence assessment. Patients came to us with a catheter in, when that was taken out they either regained continence individually, or remained incontinent.
BB2
Other sites had regular toileting schedules (4). One respondent said that patients who were more likely to succeed were chosen for toileting.
The most common changes identified were that the SVP was more structured and formal (5), timed (4) and documented (8), for example:
I think ICONS put it into a more formal setting, you had your protocol to follow, initial assessment, weekly reviews, you’d set programs for patients to work to. It was a more formalised way of doing it.
CC2
Basic toileting tasks were not seen as different, but the routine and the documentation were (5), particularly the assessment of continence that staff were required to undertake as part of the SVP (3). There was a strong suggestion in the findings that staff (HCAs in particular) did not necessarily differentiate between the SVP and regular toileting (3):
We had quite a lot of dissent towards it [the SVP]. Whether people didn’t fully understand what we were trying to do or just thought, ‘Well, we already do this, do we need to go down this avenue?’.
CC2
However, staff did report that attitudes towards continence management were changing:
Before ICONS we would wait for people to buzz us, or ask before mealtimes . . . You are more conscious of it now;
FF2
with more therapeutic intention:
What we’re doing now is to get them where we can help them be continent, and not be wet, and feeling better about themselves.
BB1
Most respondents felt that staff agreed with the aim of the programme of prompting patients’ continence (6), and that it was part of the nursing role and rehabilitation:
I know it’s been brought in because of research but really we should be looking at continence and how it affects patients’ lives.
BB1
The programme components were seen as logical:
It’s a thorough assessment to begin with, and then you plan the interventions you’re going to take, and then there is an evaluation as well, so it does seem a good circle of events – continuous assessment and planning. It’s a thorough way of doing it.
FF2
Four sites agreed with the SVP and did not mind doing it. Respondents said that being able to see benefit (1) and having enough staff (2) influenced staff views. They perceived that some staff would not like it because it was hard work, and more paperwork (2), ‘I’d say it’s about 70/30 for and against’ (L5). Respondents from two sites said the SVP was not popular with staff because of the extra demands incurred, but respondents from both also recognised some benefit, so lack of agreement was not severe in any site, and there was reasonable agreement with the principle aims of the SVP overall. However, respondents from most sites (6) were quite negative about the paperwork, particularly the assessment:
The paperwork is ridiculous, we don’t need to know all that,
HH1
with one respondent suggesting possible negative effects for patients:
As part of the assessment, we wouldn’t want to compare their life before to what it is now, because it will be different and I think that can be quite damaging emotionally.
HH3
Respondents disagreed about the suitability of the SVP for some patient groups specified in the inclusion criteria (3), especially those who were unwell, people with dementia, or long-term continence problems:
I couldn’t understand why some patients with catheters were signed up for ICONS. Even those with long term prostate problems we still included, and that isn’t something you are going to solve. That was where our sticking point wasn’t it, it was the long-term prostate problems and ladies with long-term catheters.
FF4
Respondents commented that it took a while to settle in and know what they were doing (5), but that the SVP was logical and made sense:
It’s not rocket science. It’s actually quite a simple process: steps and documentation.
HH1
However, although seen as straightforward, there was a suggestion that aspects of the SVP were sometimes misinterpreted, including:
The three day diary to start with was fairly self-explanatory, wasn’t too taxing, then we take it from the next level whether they don’t need it or they do, and then it’s just prompted voiding – fairly straightforward to be honest.
BB3
Respondents routinely referred to the programme as ‘regular toileting’.
I was struggling at first because we were told to put everybody on the programme then the research nurse said not people who are continent or catheterised.
EE2
Confusion also arose in the same site around including younger people,
we knew they were continent and didn’t need it,
EE1
and people with urinary frequency.
Initially there was a lot of confusion, there was a different process for different patients. Staff didn’t realise that you had to do this 3-day diary first and then the assessments, to choose the correct procedure;
BB2
In bladder training some staff will not let the person go to the toilet if it isn’t 2-hourly whereas we understand it’s more that you encourage them not to go;
HH4
I was just doing it every week. I didn’t look back to the week before, perhaps I should have done though.
KK6
The understanding of certain staff groups was questioned:
I don’t think the auxiliaries understood for about the first half of the programme that there was a process. It was just ‘Here’s ICONS’, and they’re put on prompted voiding.
BB2
Bank staff were also identified as lacking understanding, because of their lack of training and experience with the SVP:
The problem was occasionally you’d get bank staff haven’t worked here before and then you have to go through it all again.
KK6
Staff thought that most patients could understand the SVP to an extent (3), although some did not understand:
The patient lacked understanding of what the whole purpose of the programme was about – he just wanted to get continent again, he wasn’t interested in anything else. So he lacked understanding of the process with it.
HH4
One respondent said that the structured plans could help patients to understand, as well as staff. Two respondents commented that most relatives understood the programme (3), but staff felt that explaining the programme to relatives could be difficult, especially explaining the research component (1); and the requirement for extending the voiding interval, which is a part of BT (1).
A commonly cited benefit of the SVP was an increase in the priority of continence care (5), and highlighting to nurses that incontinence as a problem is amenable to change (3). There was recognition of the importance of continence for patients (4), particularly in relation to community living, QoL and discharge destination (3). Other potential benefits mentioned included increasing comfort, improved self-esteem and dignity, and avoiding embarrassment and the adverse effects of incontinence. One respondent thought the SVP was particularly suitable for people after stroke because of its structured approach, two other respondents referred to the benefits of training for regaining normal routines.
Another commonly cited benefit was in some rebalancing of control between patient and staff (3):
As nurses, you tend to do everything, so this is a way of giving the patient back ownership and getting them to start clicking in.
BB1
Staff recognised that continence control signals wider recovery from stroke (2) and gives the patient hope (4):
It’s not ‘Oh, I’ve had a stroke and now I’m incontinent’, it’s ‘something can be done about this’.
KK6
This also linked to nurses feeling that they could help patients (4), and that the programme gave nurses an increased therapeutic role:
I think patients on the programme felt quite secure, they knew they were incontinent and they knew that we were addressing the situation, and that there was a plan to try and help them.
CC2
Staff also identified potential benefits for themselves. They saw the SVP as providing them with structure and guidance (6), making staff think more about continence (3), and reducing workload in the long run (3). Staff could see potential benefit, but the added work was unpopular (3). Overall value was summarised by one respondent as:
It’s definitely better for the patient, but it does take more work and that was the biggest thing.
KK6
Cognitive participation: the relational work that people do to build and sustain a new practice
External people seen as responsible for initiating the programme included the research team (2), matron (2) and stroke network nurse (2), with the trial manager involved in training and ongoing support (3).
Senior ward staff were consistently referred to as being responsible for pushing the new practice forward (8). Their functions in embedding the new practice included:
-
promoting the programme:BB2
The key people were the trained nurses encouraging it and explaining it to the auxiliaries and trying to get everybody behind the programme, they were very good at promoting it;
-
providing direction and reminders:AA4
Management and senior staff were influential by reminding and explaining;
-
education and supervision:EE4
The ward sister has an overall view and a supervisory and educational role making sure that the team knows what they’re doing;
-
organisation and delegation:HH4
The senior sister is the main one organising the programme, saying the programme is everyone’s responsibility, making sure we are all up-to-date with the programme;
-
monitoring and feedback:LL1
If we don’t know what we’re doing we’re soon told, we’re soon picked up on it by the ward manager. She’s pretty good – keeps an eye on all that.
The style of management and direction was commented on by HCAs, including involvement:
The ward manager facilitated time to learn the programme and did not just delegate work to HCAs but involved them in learning why;
AA3
and the availability of support:
The support we got was fantastic, it meant a lot to us. If you were stuck you could either go to the ward manager or research nurse.
KK2
Ward managers commented on the key role of proactive senior staff nurses in three sites:
. . . we’ve also got some of the more senior staff nurses who are really confident in delivering the same sort of thing: they were the ones who initiated in governance meetings what we needed to do to make the programme more visible.
FF1
Two sites commented on the lack of a key member of ward staff driving the programme:
We don’t seem to have an ICONS champion on the ward. We’ve got the research nurse but there isn’t a nominated band 5 or 6.
HH5
The overall ward approach to embedding new practices was also obliquely referred to, with three sites crediting the whole ward team as being responsible for and involved in implementation.
In six out of eight sites the research nurse was identified as a valuable resource, although their role differed considerably across sites. In some sites, the research nurse managed the programme almost independently of ward staff:
The research nurse would come in and do all the paperwork for the trained staff on the ward;
LL2
in other sites the research nurse supported the ward staff to deliver the programme:
We’ve got the research nurse for any queries, or to push the staff forward a little bit, that helped.
LL5
Roles undertaken by the research nurses included:
-
teaching, explaining the programme, answering questions
-
highlighting, reminding, acting as a trigger for what needed doing
-
driving, co-ordinating, directing, helping everything run smoothly
-
talking with the patient, working out the regime
-
keeping on top of who should be on the programme
-
working with HCAs, demonstrating the paperwork
-
checking charts were updated, making sure assessments and weekly reviews were done
-
taking control, doing all the paperwork if not done
-
acting as a link between units
-
updating ward staff of changes.
A research nurse presence was not consistent over the whole of the programme in a number of sites, and their role and impact was influenced by whether they were successful in integrating with the ward staff:
Initially the research nurse got in the way of it working well, but now the clinical leader on the ward is our link nurse with regards to research. If the research nurse needs anything implementing she asks the ward managers to make sure it gets done, and we will delegate it;
FF1
and the timing of their presence:
The research nurse leaving three-quarters of the way through had a bit of an impact but it didn’t make a massive difference on patients. It just made everything else a little bit more different, other people coming on the ward. She was so good that we relied on her quite a bit really.
KK1
It was difficult to discern how leadership by the ward staff integrated with the research nurse role, being an interaction of personalities, role definitions, level of ownership of the programme, and continuity of presence. Research nurse characteristics commented on included being: ‘. . . somebody visible, on the ward fairly regularly, a named link’ (CC1); ‘. . . approachable’ (EE4); ‘. . . known and popular’ (AA1); ‘. . . clued up’ (EE1); and patient:
. . .she would reiterate and reiterate just to help the flow through our heads.
HH5
Ward staff on one site commented favourably on the impact a research nurse who became involved at a practical level had on their perceptions of the programme:
She did shifts with the ICONS nurses, her doing that warmed us up to it a little bit more.
HH4
The perspective provided by external research nurses was valued, for co-ordination:
We were lucky to have a strong research nurse. It might not have worked if we hadn’t had the research nurse pulling everyone together;
AA3
for monitoring performance,
The research nurses have been a big help because we were floundering, we had somebody to bounce off and see that we were doing it right;
BB1
or to counteract established perceptions,
People you wouldn’t think would be a candidate, somebody from the outside would come in and say to us give it a go and see how they do. And yes they did well.
BB1
Five sites commented on the role of the extra staff provided to the sites (known as ICONS HCAs or band 3s) in promoting the programme:
The key people driving it were the research nurse, sister, and three ICONS nurses.
KK2
Management external to the ward was not seen as providing input to the programme (2), for example:
There’s been no input from management.
FF2
Two sites out of the four that received external facilitation as part of the trial commented on the help provided by the facilitator, and one site commented on the perceived lack of external facilitation:
We haven’t had much external support, somebody was supposed to be visiting four times but they only visited once.
HH1
This is described in more detail later in this chapter, see Supported implementation.
There was recognition that staff needed to be involved in the programme and motivated (4):
We needed to involve all of the staff, get ourselves more on board.
EE1
Four sites thought their staff were on board with the programme, or at least not negative. Staff attributed willingness to be involved to enjoyment (1), a decrease in workload in the long run (1), or wanting to be involved in the research (2).
Three sites reported that there was quite a lot of dissent in the initial stages, and that it took time to get the programme going, get people on board and keep them motivated:
I struggled to get people on board and to keep it up. It was fine if certain people were on shift who really knew about it but if somebody wasn’t here it would lapse a bit.
BB1
Respondents from two of these sites went on to say that once staff had been involved, they realised that the SVP did not require much extra work. Facilitators to enrolment included whether or not staff saw that the programme could be done, and their experience of success. In general, staff appeared prosaic about involvement over time,
I don’t think people mind doing it. It’s just something you get on and do,
FF2
although the paperwork remained a significant barrier for some (2).
There was a general recognition across sites (6) that there is always some resistance to taking up new practices:
There is a core of people but then it’s not just the ICONS – it’s anything; it’s whatever we’ve introduced they’ve been very anti. It’s a case of saying ‘this is what is in now and this is what we’re doing and you have to do it, you are accountable’. But it’s the same ones you are going to hit a brick wall with.
BB1
A proportion of staff remained resistant and could significantly influence uptake, but even they could come round eventually,
It was quite interesting to see how influential one person and their negative thoughts could be, but bizarrely this person is now the first person to start suggesting regular toileting.
CC2
Nurses thought that AHPs such as physiotherapists and occupational therapists were not involved, possibly because they had not approached them:
Therapists don’t get involved because we haven’t asked.
FF2
This may have been because the SVP was seen as a nursing role:
It was very difficult to get any of the therapists on board – they do seem to think that continence is a nursing issue. The head OT [occupational therapist] and physio should have been involved in the beginning to make sure they participated as well. They knew about it but didn’t think they were part of it. They have got OT assistants or physio assistants who do hands-on personal care but they weren’t used to help towards the continence programme.
KK3
In one site, therapists accommodated the SVP in the daily routine:
The therapist was very good about it. They would look in the diary and change the times that they would take the patient down to the Department,
KK6
but would not necessarily get involved:
We could have worked the timetable round the therapy sessions. They should have thought over there ‘It’s 11 o’clock now the patient is on ICONS’, but they’d bring them back for the toilet even though they’ve got the facilities there.
KK3
Doctors were also not involved:
The doctor didn’t know who was on ICONS.
KK6
Respondents from four sites commented that most patients were quite happy to be involved, although staff also commented that some were not. Staff thought this was possibly because it was research or because it might extend the hospital stay:
I think maybe they’re a bit worried that going on the programme will prolong their stay. They want to get out of hospital as quickly as possible and go back home;
LL6
or because it drew attention to incontinence:
I think it might be drawing attention to their problem as well. Sometimes in the early stages they’ve got so much else going on its making them focus on another problem.
CC1
One respondent commented that patients were happy to be involved until it came down to the practicalities:
Sometimes patients agree to it and then when you put it into practice they don’t want to know, when you are saying every 2 hours come to the loo, that’s different.
FF2
The main feature of ward organisation altered by ICONS was the introduction of new staff. Each ward was able to hire 2.8 band 3 HCAs to cover daytime shifts. Recruitment of additional staff differed between sites: five sites recruited new HCAs to target and maintain a reasonable presence over the length of the SVP; two sites failed to hire new staff or had severe staffing problems throughout; one site mainly used bank staff or ward staff overtime because of hiring difficulties at the trust. The way the new staff were used also differed between sites: three sites relied totally on ICONS HCAs or bank staff to deliver the programme; two sites integrated ICONS HCAs into the ward staffing such that there was no role differentiation; and three sites moved from relying on ICONS HCAs at first to shared responsibility for the programme. There was some evidence of role conflict for ICONS HCAs who were not fully integrated into the ward staff:
If somebody phoned in sick they would be one short to go and wash in the morning, then I was in-between which way to go.
KK2
Five sites referred to role differentiation between trained staff and HCAs in terms of delivering the SVP,
The staff nurses set it up for the HCAs, do the checks, and progress the patient to the next level, so it’s there for HCAs to fill in on the right page,
AA3
although not all paperwork was done by trained staff:
HCAs do the 3-day diary, staff nurses decide which programme the patient goes on.
EE1
Responsibilities did need attention to sort out:
It takes a while to get into who is doing the paperwork and three day assessment,
EE4
and role demarcation was not without a hint of resentment about the familiar split between cognitive and physical work in nursing:
The nurse looking after the patient is doing the two main parts, and everybody else is just doing the hard work.
FF2
Three sites identified role demarcation between the research nurse and ward staff for undertaking programme activities, with the research nurse generally involved in recruitment, but also undertaking the continence assessment and weekly reviews in two sites, and SVP design and co-ordination in another:
The research nurse puts out the three day diary, or tells us who to put on it, then she formulates the toileting plan.
FF2
Although senior ward staff were seen as having overall responsibility for monitoring and co-ordinating programme delivery in all eight sites on a day-to-day basis, responsibility for specific aspects of the paperwork differed: staff nurses or junior sisters were responsible for paperwork associated with their allocated patients in six sites, one site relied almost totally on the research nurse to manage the programme and paperwork, and on one acute ward the ward sister undertook the assessment and trained staff did everything else.
Role allocation was influenced by the type of ward,
I think it’s more difficult in this acute setting, maybe in rehab it would have been a lot more involvement from the staff nurses, but here it was more the nursing assistants that would get involved,
KK1
and also by the amount of time the research nurse was present on the ward,
The Band 6s tend to know what’s going on with the patients, more so than the research nurses. The Band 6s go around on the doctor’s round so know the catheter is coming out.
HH5
In the site where the research nurse was running the programme, role allocation was attributed to ward pressures:
It is running smoothly and the paperwork is getting completed, but the research nurse is doing the assessment and the weekly reviews rather than nursing staff doing them. If that didn’t happen they probably wouldn’t get done. It’s because it’s so busy it just doesn’t get round to being done by the staff nurse looking after the patient. If there is one staff nurse looking after 18 people they are not going to fill in the questionnaire. As far as they’re concerned that’s not important at the moment due to direct care.
LL5
Overall, the SVP was not perceived to have made a significant difference to patterns of workload allocation on the wards,
ICONS hasn’t affected how work is allocated, or the ward routine,
LL5
although it did make a difference to workload,
It has affected the ward in trying to make sure that it’s done properly, making sure we have taken the people who need to go to the toilet regularly and that it is noted down.
LL4
All of the sites had undertaken activities to incorporate the SVP into the ward routines and procedures, including having ICONS symbols on the ward whiteboard and on individual boards behind the patient’s bed to discretely remind staff who was on the programme:
We have handover sheets where ICONS is highlighted, and triangles that we report by somebody’s name above their bed to highlight, because we do have a lot of bank agency staff. It’s easier to say if they’ve got a triangle they are on a toileting programme, you need to look at the notes at the bottom of the bed.
CC1
Including ICONS on the handover charts and sheet was particularly important in reminding staff on a daily basis which stage of the SVP patients had reached, and whether or not new patients were ready to start yet:
The 3-day diary will tell us the person isn’t ready for intervention, and it’s reported on the handover sheet to do a reassessment.
HH2
The handover sheet was also used as an impromptu data collection form:
We also put tick boxes on the handover sheet to say that things had been done in case we didn’t have time to fill in the paperwork.
HH5
Staff recognised that it was important to start the programme early. Wards had developed routines for the night staff or ICONS HCAs to put out paperwork at the start of the morning shift (2). The SVP itself was not seen as complex, but staff recognised that it needed embedding into the ward routines or it was in danger of being forgotten. Prompting mechanisms included use of care clocks to help remind staff about the timing of toileting (1) and leaving reminder notes in diaries for weekly reviews (1). Paperwork for the SVP had to compete with other tasks and paperwork for attention:
It was really hard to keep vigilant about ICONS because it was getting lost within all the other paperwork . . . It needs to be visual.
BB1
One site rationalised the overlap in paperwork between fluid balance charts and the SVP records:
We did have the fluid balance chart and now we have the ICONS chart, so people said if they don’t need their fluid recording just use the ICONS, so we got better organised really.
CC2
During the period when the SVP was operating, intentional rounding was introduced into the NHS with the aim of ensuring that all patients were seen by staff on a regular basis to meet basic needs, including fluid intake, skin care and toileting. On the one hand, this worked in favour of the SVP because staff were required to pay attention to the toileting needs of all patients on a regular basis:
The Proactive Patient Rounds initiative [intentional rounding] made it easier with ICONS because people were looking at charts every two hours anyway.
HH4
However, it could also work against implementing the SVP as it was designed: the SVP tended to be merged with the regular toileting required by intentional rounding, rather than an individualised timing regime:
When we’re going back doing the rounding which is done on a 2-hourly basis we’ll ask as well, ‘Do you want the toilet?’ so we try tying the two together.
BB3
There was some suggestion that fidelity to other aspects of the SVP may have been problematic in some sites. For example, it is not clear if BT was used as a first choice for people who were cognitively able. At least one site might have used PV as a generic initial stage for everyone:
Yeah, I think we started off with the prompting, then bladder training for the people who are cognitively okay.
LL5
Respondents from another site said:
Most people go on PV.
HH1
We don’t get a lot of people on bladder training.
HH4
However, it was also evident that staff on other sites did individualise programme timings:
The programme was good. It was more structured, something to work to, obviously people are individual and sometimes something didn’t work but then you had structure to tailor it, you adjusted a little.
KK6
Collective action: the operational work that people do to enact a new practice
Every site referred to initial difficulties in the first few weeks, but that the programme became embedded and routine over time,
It was running smooth towards the end. Because we’ve done it for a good period of time it’s become second nature, it’s now part of our daily routine;
BB3
although reminders were still needed (2):
You’ve got to keep on top of it all the time and keep pushing it, keep the momentum, because once it goes off the boil, something else takes over,
EE1
and it might not be done properly all the time (3). Time constraints and staffing levels influenced whether or not the programme was done (3):
If it wasn’t done it’s because of time pressures and being short staffed.
LL4
Too many people on the programme at once could also overwhelm capacity (2), but too few meant staff could lose focus:
We will have a run of continent people . . . and get out of the habit of doing the programme.
HH3
The SVP was seen to place extra demand on nurses, both physically,
It’s hard work to toilet people every 2 hours . . . You seem to be toileting them forever,
EE2
and cognitively:
I always say the nurses on the ward haven’t got time to think. They can think about things they’ve got to, but they can’t think why they should be doing this.
KK1
The programme needed to be simple, quick, and easy to do:
There’s so much to do in a day’s work, they like it being simple, it’s easier managed and maintained.
HH1
There were four main decision points in the SVP at which any difficulties in fidelity to the programme as designed would be evident: decisions about eligibility; pathway (BT or PV); timing; and adaptations.
The SVP required the completion of a screening register for everyone admitted to the unit, to ensure that all eligible patients were identified and started on the algorithm. Patients with UI or those who were catheterised in the acute phase were eligible for recruitment, once they were conscious and medically stable. All eligible patients were started on a 3-day bladder diary after catheter removal.
Ward staff had to ‘maintain vigilance’ about eligibility with a changing patient population as new patients were admitted:
It’s just being vigilant on top of patients coming over to us and are they accounted for on ICONS, are they somebody you could do it with?
BB1
One strategy was to view the screening register and 3-day diary together as a blanket screening process:
There weren’t very many patients being put on the programme. By screening every patient that came in, by putting everybody on the three day diary we captured a lot more patients. Most patients now go on the programme, it’s embedded, part and parcel of stroke practice.
EE1
Maintaining vigilance also required management at different time points for each patient:
One glitch is that people are not put on the 3-day diary when the catheter comes out.
HH5
The SVP was seen as sufficiently flexible to be able to adapt when a patient’s health status was changeable,
The programme works with poorly patients because it can be stopped for periods of illness then gradually started again,
AA3
but it could also be difficult for staff to keep the SVP in mind over time:
The patient goes backwards and forwards – catheterised, not catheterised, starts the programme, goes into retention, is re-catheterised, comes back, starts the programme again. This can happen a few times because our patients do go up and down. They’re the ones that can be easily left.
HH2
The SVP paperwork did not provide a way of managing this ‘surveillance’ activity for each patient. It generally fell to senior ward staff to keep track of which stage each patient was at, which they found hard to manage:
On return from annual leave there was a massive list of people that could be started on 3-day diaries.
HH3
One site enrolled HCAs into monitoring the programme start for specific patients:
Because then if the HCAs have a name they’ll keep saying to me, ‘How is Mr X, is he ready?’, and that’s how we’ve been working it. It’s just getting them to think like that.
BB1
Health-care assistants were responsible for diary completion in most sites, and although the diary process was not a problem to them, it could be forgotten,
A lot of them tend to forget the 3-day diary and go straight into the forms,
BB1
or not completed consistently over 3 days:
A 3-day diary it’ll start one day and it doesn’t get filled in so we have to start the next day and it doesn’t get filled in and then you’re here so you do it 2 days and then the third day it doesn’t get filled so you have to start it again.
HH4
Patient transfer between acute and rehabilitation wards could also cause problems with continuity of diary completion:
If part of the diary is being done on the acute unit we didn’t know whether to start again. We started again because we didn’t know whether it was reliable, because it was only part done, or done too early.
EE4
Staff in acute wards reported difficulties with diary completion:
By the time we had done the 3-day diary they were going to rehab so we didn’t get involved with it as much as we’d like to have.
KK1
Respondents from one acute site thought involvement in the SVP was a good thing, but questioned diary completion for 3 days when people were found to be continent,
Sometimes you start the 3-day diary off and after 2 days we noticed the patient is continent so it was ‘Do we need to carry this on?’;
KK6
or when people were found to be incontinent:
The three day diary is a bit too long to be assessing people when they could be at risk of excoriation. I would rather start two-hourly prompting earlier. The 3-day diary is brilliant if they are just having little accidents, but when they are actually incontinent it’s too long to keep them on that.
KK1
There were patient groups that some staff thought were not suitable for the SVP at all, including:
-
continuous incontinence:
Some patients are a bit impossible to manage on toileting because they are wet all the time;
LL3
-
poorly patients and patients with dementia:
A lot of patients have dementia and were confused and didn’t understand what we were trying to do and in that respect it wasn’t appropriate at all, they couldn’t learn anything from it;
AA4
-
the frail elderly:
We seem to be having general rehab patients who were not up to rehabilitation, not well enough even to come out of the bed, more dependent, need to be fed, a lot of older care patients;
KK3
-
lack of awareness:
We had one lady . . . no knowing of when she needed to go and she was wet so many times and her skin started to break, it was very distressing for her to have the catheter put back in but she felt it had to be done.
BB1
The protocol required that patients who met the criteria for eligibility began care according to the algorithm, which included a continence assessment, and a decision about whether to start the patient on PV (cognitive impairment or no bladder control), or BT (little or no cognitive impairment and some bladder control). The continence assessment was a 30-page document used to assess the type, duration and severity of continence, and any influencing factors. Part of the assessment process included medication review, and staff were provided with cards to summarise the effects of the most common drugs on continence. Wards were also provided with a bladder scanner and training in its use to aid assessment of urinary retention, a common reason for catheter use. Although not explicit in the protocol, staff were encouraged in training to remove catheters as soon as possible.
Staff liked the bladder scanner:
Having the bladder scanning has made care more technical, and improved explaining things to patients. It’s reduced the panic about the patient being in retention because you can see how much they have in their bladder.
EE4
Nothing was said in the interviews about managing the consequences of assessment, medication review or bladder scanning in terms of activities to resolve problems. It is not known if this was because these tasks were uneventfully completed, but it may be that they were not completed because there was no requirement for them to be recorded in the SVP.
Completing the assessment was generally disliked (5). Staff attributed difficulty to lack of time,
Realistically it would take an hour to an hour and a half to fill it out properly which is an enormous amount when you’ve got all the other workload that you’ve got to do;
HH2
lack of privacy,
Finding the time and privacy to do the continence assessments has been a bit of a struggle;
CC1
and because staff often could not obtain the information easily:
The thing at the front seems to be the hardest because most of the patients aren’t able to talk to you or can’t remember, so you’re waiting for families to come in.
BB1
Staff questioned whether or not all of the information in the assessment was needed:
Whether the assessment is giving them a more detailed picture or whether it’s overwhelming them with information I’m not sure. People say this is too much about one specific area of this person’s care when I’ve got this other huge backlog of history.
HH2
The sequence of events from bladder diary, through assessment, to programme choice had to be remembered and managed over a number of days:
Sometimes we’d start the two-hourly prompting and I’d think ‘Oh I can’t, I haven’t done the assessment.’ Or we’d put it on the board – ICONS assessment needs doing, and perhaps it wouldn’t get done for a few days . . . so you have to go back then . . . to the assessment and then back to the toileting again, but we got into it after a few weeks – we weren’t too bad.
EE6
Sites had different ways of managing this continuity, including monitoring by a nominated person,
One of the ANPs [Adult Nurse Practitioners] is going to be keeping on top of it. She’s in charge of the new catheter forms we have to fill in now to keep a check on how long the catheters are in and when the bags are changed so ICONS can run alongside of that;
BB1
monitoring by the ward sister or research nurse,
It is running smoothly and the paperwork is getting completed, but the research nurse is doing the assessment and the weekly reviews rather than nursing staff doing them. If that didn’t happen they probably wouldn’t get done;
LL5
and using handover and whiteboards to record status, although these were not perfect:
Sometimes the handover sheet got missed for a few days but they were more or less up-to-date with what was happening.
CC2
Programme choice was not explicitly referred to, but PV seemed to be the more common option:
I think we ended up doing more prompted voiding than bladder training. Possibly because the patients who were incontinent tended to be people with more cognitive problems, they needed the prompting rather than being able to take charge of their own destiny.
CC2
This was not necessarily just for people with cognitive problems, but for everyone:
We start off with prompting and then bladder training for the people who are cognitively okay.
LL5
It is unclear whether or not this was due to misunderstanding or was a purposive deviation from the protocol.
The BT protocol asked staff to encourage patients to follow their voiding timetable as closely as possible. If able, patients were encouraged to complete a 7-day voiding diary as part of the programme. If the patient was unable to complete it, staff recorded their progress. Although staff were generally positive about patient involvement, one member of staff commented that patients could get over-involved:
We’ve got one patient who is too obsessed with his bladder training – he’s constantly on the buzzer after you’ve just seen to him.
LL6
Three sites identified that distraction (as it was being implemented) was challenging for the staff and the patient,
We are saying try and hold it as long as you can, they are saying ‘Please why won’t you let me go?’ and getting quite agitated about it.
HH4
One respondent said,
Very agitated patients who want to go to the toilet every 5 minutes, I feel a bit awkward saying you’ve been now and you got two hours to go, it feels a bit hard. I do tell them and then they get anxious more and more and get quite irate so you’ve got to give them a bottle. You keep them calm – they’ve already had one stroke you don’t want them to have another.
LL1
Respondents from two sites commented that it also looks bad to relatives when staff appear to be stopping people from going to the toilet. The comments suggest that the principles of extending the voiding interval by small increments in BT may not have been fully understood by all staff.
In the initial stage of PV, the person was asked if they were wet, then the nurse would check and give feedback on whether the patient’s awareness was correct. Patients could lack awareness,
Some can’t tell you if they’re wet or not, they don’t realise, you ask them but they don’t realise,
FF4
but repeatedly asking people if they were wet was uncomfortable for staff,
I don’t like asking the patient if they’re wet because they are embarrassed, but I know it’s got to be done;
LL1
and possibly for the patient:
Asking the same question about whether they’re wet or not is perhaps a bit tedious after a while for the patient.
LL4
Regular prompting to use the toilet could be difficult with some patients:
There were a couple of patients that we started on the programme and we stopped it because they have such huge problems, they were confused. I think they just got to the point where every time you asked them to go to the toilet they were getting very angry, frustrated, so we just backed off because it was distressing them . . . I think maybe it was the frequency that they couldn’t deal with, the last thing they remembered was you asking them to go to the toilet, and here you were again.
CC2
Staff talked about avoiding confusion, ‘moving the bed can really mix them up’ (E4), and using methods of asking which encouraged participation:
I think it depends how you ask them. It says on the thing to ask ‘are you wet? Do you want to go to the toilet?’ They quite often say no, but if you say ‘Come along, we’ll take you to the toilet’ they will come, so it depends how you word it.
FF4
Staff thought that regular toileting could also be difficult for people who needed hoisting:
You have to hoist them onto the bed, remove their clothes, hoist them onto the toilet, hoist them back on the bed, and then back into the chair and the whole process can take up to 45 minutes and patients aren’t always compliant because of that.
CC1
Using bedpans was not as effective,
Because of the cognition again, very often if you sit patients on a toilet they’ll go, but a bedpan doesn’t have the same effect – it’s harder for them.
FF4
Other conditions affecting patients who staff found challenging for regular prompting included:
-
depression:BB1
She was quite capable of being continent. It was just that she had got herself in such a low mood and didn’t want to bother;
-
fatigue:FF1
there are some patients who you can try to walk to the toilet every two hours and they are absolutely shattered as a result of it. Fatigue can be really problematic for some people;
-
dependency:BB2
A lot of the elderly patients who perhaps needed a bit of care prior to the stroke perhaps weren’t as motivated to regain their continence;
-
communication problems:HH4
for patients who have a lack of communication it’s difficult to get them to understand the programme if they’re not able to tell you when they need to go;
-
urge incontinence:AA4
We sometimes can’t get there quickly enough for people with urge incontinence.
For BT or PV, staff had to choose a timing interval (the time span between voids), based on the bladder diary. This was translated into scheduled voiding times throughout the day and recorded daily on a treatment log. Staff then had to complete the log for each scheduled voiding time throughout the day, including the actual time they attended the patient, whether or not care had been delivered as prescribed, the outcome (i.e. whether the patient was wet or dry), and if they had successfully used the toilet or bedpan.
Timing was the most commented on aspect of the SVP. As all of the documentation surrounding the SVP was based at an individual patient level, the first point about timing was remembering who (out of up to 32 patients on a ward) was on a TV programme:
People were having difficulty remembering who was on ICONS despite the little red triangle on the name board.
CC2
Timing could be difficult to schedule, remember and adhere to,
It’s hard to keep track of who needs doing at what time,
HH4
especially in relation to therapy, visiting times, or mealtimes:
Mealtimes are protected so usually we work around them, and take patients to the toilet before but that doesn’t always work. Patients sometimes don’t want to delay going, and you can’t not take them because it looks bad, so that doesn’t work.
LL1
Keeping to time for patients with physical limitations was a challenge:
Toileting people 2-hourly if they are very dependent is difficult, because of the time it takes to finish, the two hours are nearly up again.
CC1
The programme timings set up expectations which could have negative consequences for patients if not met, but also for staff:
If you haven’t got enough staff some days you’re called everywhere, you can’t get there in time which is quite frustrating.
LL1
Staff were aware that patients lacked confidence in their ability to keep to timings,
That’s one thing you must remember to do if you‘ve promised that you’re going to come back, you must go back,
BB1
and that patients could be reluctant to buzz:
Some people won’t press the buzzer for assistance, they don’t like to use their buzzers.
LL6
Staff identified strategies to keep to timings, such as enrolling patients:
We make sure they’ve got the buzzer and say ‘Right, we’re due to come back at such a time, if you press 10 minutes before then we’re not leaving you on the last-minute,’ especially if they have to walk to the toilet.
BB1
Staff said they sometimes could not complete the daily treatment logs immediately after they had attended to the patient, and might forget to go back:
In some cases you would find that the documentation had been forgotten to be completed, but when you asked had people been to the toilet they had at the times they should have gone.
CC2
One problem with the completion of the daily treatment logs was that staff did not know how to record refusal,
If the patient has refused you’ve got to write no and that looks like that we haven’t bothered to toilet them,
CC1
or accidents:
The patients who can walk to the toilet are the ones we’ve found hard to manage . . . We don’t know what to write down for patients who make an attempt to go to the toilet, and then have an accident, although they did instigate going.
EE4
Ambulant patients also created recording difficulties because staff did not necessarily know if they had actually used the toilet. There was overlap between the daily treatment logs and fluid balance charts, and one suggestion that recording on the daily treatment logs sometimes might not match actual practice:
There are occasional days where the charts aren’t filled in as accurately and you wonder whether it has been done routinely.
HH3
The tendency to merge the SVP with intentional rounding had benefits for workload,
Because the ICONS and the skin and safety round came at the same time they support each other, it’s not an extra job. This was fortunate otherwise it would have been a nightmare,
FF1
but perhaps created problems for individualised timings if everyone was checked at the same time:
when the ward rounds came in, that made it easier, because it’s become the norm to check everybody every 2 hours.
HH2
The protocol suggested reviewing patient progress at weekly intervals using the daily treatment logs and the 7-day bladder diary completed by the patient. The voiding schedule for the following week was to be documented in the back of the patient information booklet, the nursing care plan, and the weekly review form. The weekly review was identified as an ideal time to discuss progress with patients, and provide support and encouragement to continue.
Patients’ completion of a 7-day bladder diary seemed useful for those patients who could manage it:
Those people who went on bladder training quite enjoyed being in charge of their piece of paper and their pen. It was something that they felt they had some control over in this environment where everything is so completely different.
CC2
There was no reference to discussing weekly progress reviews with the patient.
Despite placing reminders in the diary, weekly reviews could be forgotten:
We’re not doing very well with the weekly assessments. That should be done automatically, part of the risk assessment, so . . . it’s as important as your falls assessment every week because that gives you a scale of where you are up to. We are having to go back and fill them in which is just giving ourselves extra work.
BB1
Two sites suggested that weekly reviews should all happen at the weekend so that they were the same as reviews of other aspects of care.
At least one respondent might have misunderstood the process of weekly review,
I was just doing it every week. I didn’t look back to the week before, perhaps I should have done though,
KK6
but comments on the consequences of missing reviews suggests that its purpose was clear to others:
It didn’t matter if people didn’t change very much but there is the chance that you might have missed a couple of weeks where somebody might have moved a lot faster if you’d got the assessment done on time.
CC2
The protocol was not explicit about whether or not the SVP could be stopped, and staff from one site commented that they did not know how to stop it:
Sometimes we’ve carried on for days because we didn’t know we could discontinue it.
EE4
Change was recognised as bringing challenge,
Change in routines is difficult, people have got to be supportive of it mentally,
AA1
with specific challenges for nursing culture,
Nurses have a reactive rather than proactive nature – they expect incontinence and use pads. Changing that culture is quite a struggle;
HH3
and in respect of individualising care:
we’re asking people not just walk into work and blanket think about everyone, and that means – you’ve really got to think.
HH2
At the start of the trial when they were beginning to implement the SVP, most sites had a period of learning, where they were unsure:
We were a bit muddled about it initially because people were saying different things . . . It took a few weeks for us to understand it.
AA4
In some sites, the initial rollout was rushed,
The initial rollout could have been done better. It wasn’t a flow of information, somebody knew about it on one shift but didn’t pass it over to another shift.
BB1
By the time of the interviews, staff were expressing confidence in each other,
We can rely on each other in regards to how it gets done each day,
AA2
I think the team as a whole are doing pretty good,
FF2
although some staff remained less enthusiastic than others:
It was explained well enough but it was whether the staff took it. Here’s something else for us we’ve got to do again, and they just had a negative [attitude] from there on, so it was overcoming that but as time’s gone on they have got better.
FF2
Individual staff required ongoing monitoring for some aspects:
Documentation can be left to some nurses but not others, whether that’s down to confidence or personalities, I’m not sure.
EE4
Respondents from three sites expressed confidence that the programme was being run properly, and respondents from two sites thought that the programme would not run properly all the time without being driven:
It’s hard to judge if people are genuinely on-board with it, I like to think that people are fully engaged but when I’m not here I couldn’t vouch for that.
FF1
Respondents from one site thought that this was just the process of developing routine:
You need to keep saying to people every day this is what we’re doing . . . And then eventually it sinks in.
HH2
Reasons suggested for lack of compliance included maintaining attention in the face of competing priorities,
It’s almost like persistent nagging making sure that the ICONS stays there and the focus stays at that level;
FF1
the necessity to complete paperwork,
It’s more the paperwork that you’re constantly nagging certain people for;
FF1
and shortage of time:
you’ve pretty much got to encourage people constantly to get involved but it’s not because they don’t want to do it, it’s just they haven’t got time.
KK1
There was improvement in compliance over time:
Gradually we got to where the named nurses were making sure that things were up-to-date as far as possible, people took more ownership as time went on, they didn’t need as much chasing up in the end so I think people did start to embrace it more.
CC2
Lack of staff involvement with the programme in one site was attributed to maintenance of separate roles for the ICONS HCAs:
The major issue on the ward is communication between staff. If we were all sat down and told it’s everybody’s responsibility we would just do it like normal, like everything else we do on the ward.
HH4
Bringing inexperienced new staff into the ward as ICONS HCAs was seen as ineffective in influencing existing ward routines:
It was very difficult for the ICONS nurses who were young girls to walk into a new workforce and know what to do . . . because you are trying to change people’s mentality and maybe challenge their work ethic,
HH2
as was the use of an external research nurse:
For the new research nurse it is very hard to come on to a ward two days a week and have any sort of presence.
HH2
The practical aspects of the SVP became an accepted part of routine ward practice and staff gained more confidence in their own knowledge of continence,
I certainly learnt a lot and I thought I was quite knowledgeable about continence,
HH3
and their ability to manage it, ‘Nurses have been empowered by it’ (EE1). Extra staff impacted on staff confidence that they could do the programme consistently:
I think the staff getting on board has helped, and having the extra staff. As long as we’ve got the staff to do it, I think everyone’s quite happy to do it.
LL4
One practical aspect that remained problematic in two sites was getting staff to maintain vigilance for eligible patients for the SVP:
It’s just making that part of the admission process, trying to look at ways of making it more in the front of their minds, triggering the staff to think about it.
BB1
Respondents from one site thought that the SVP had adversely impacted on ward relationships,
There were negative interactions because of it. The auxiliaries were in high demand, quite rightfully overstressed regarding it, and it did cause some bad morale and some bad attitudes on the ward, but I think they were resolved further down the line and things began to work better,
BB2
but respondents from two sites thought the number of positive interactions between staff had increased:
It has brought the team closer together for the simple fact that they are talking, as well as continence, about how they plan their day. It has made people co-operate more with each other.
CC1
Respondents from three sites also thought that the programme had improved liaison with therapists,
The occupational therapists are thinking is there anything they can help a person to use the toilet. One of the ladies we were struggling with, the physiotherapist did extra work with her so she could get onto a standard hoist,
FF1
with respondents from one site also identifying increased nursing input to MDT meetings:
Continence is something that a nurse now expects to be asked about, or expects to share in the multidisciplinary team ward rounds.
FF1
Although it was thought that staff were aware of the need to promote continence (1), some skill deficits were acknowledged such as moving and handling skills for new ICONS HCAs (2), communication about continence (2), and assessing continence (2).
The SVP was seen as relatively difficult to learn at first (3):
ICONS was more challenging to get to grips with than other research projects.
AA2
Training given by the research staff or senior ward staff was seen as meeting needs by some staff,
We had a presentation, what it was all about, why we were doing it . . . That was it. I don’t think she could have explained anymore, or highlighted anything differently
LL5
but there were often mixed views in the same site. Some staff would have liked more training,
We didn’t get enough direction at first, we only had one meeting which wasn’t enough information,
LL3
or training for specific staff grades:
The education was focussed on the qualified nurses. There wasn’t enough direction for HCAs at the beginning. There should be more support and more advice for unqualified people.
AA3
The timing of the training relative to starting the SVP varied between sites, with some having a delay of a few weeks, and others having a rapid start. There were problems with delaying the start,
There was a gap between the initial training and the start of the trial, it would be better to have the training just as we were about to start,
FF2
but staff from three sites also suggested that a more gradual lead in to the programme would have been beneficial,
We should have done a couple of trial cases, learnt and reflected on that. You could do with a few months being on the program and not actually entering the results sort of a trial,
EE4
or that cascade training might have been better:
Because the trial manager did the training it doesn’t become your own. It might be better to cascade training by senior staff. I struggled to get my head round it at first, so as senior nurses we should have understood it more.
EE4
Although suggesting potential improvements to the training, staff did think that they had the skills and knowledge to deliver the programme (4). They also thought that they had learned new things, such as the potential for intervention,
Nurses are more aware that continence doesn’t have to be a big problem if you can get it in the early stages;
EE1
assessment,
The bladder scanning was a skill we never had before, it’s a skill we’ve got now, continue to use;
KK3
and improved ability to talk to patients about continence:
Because we have more knowledge we were having more informed conversations with patients.
FF1
There were positive comments about the educational resources, including the information packs that staff received (3) and the medication cards (1). Only one person reported that they had completed the online training, and they thought it was good. Six other respondents said they had not done the online training: two said they did not know about it; one blamed lack of time but with a hint that online training was perhaps not the most preferred delivery route:
We’ve had so much online training coming through from the trust itself. We’ve got to do it for the BM [measures blood sugar] machines, for nutrition. They’re pushing through so many online but it’s finding time. I don’t get time, I have never read my e-mails.
KK3
Some wards identified staff to take on specific roles within the programme:
I specifically picked staff nurse to be that educator because if one person kept up, making sure all the paperwork was there, and then it’s a case of her cascading it down to everybody.
BB1
Comments on inappropriate staff allocation included the use of bank staff, ‘It’s got to be permanent staff doing it’ (FF4); disagreement with the ward sister being involved in trial recruitment,
A ward sister looking after a team of 12 patients hasn’t got time to get consent for a clinical trial;
FF2
and using HCAs to complete reviews:
Staff nurses got us to do the weekly reviews at the end of each week: the new research nurse doesn’t let us do that because we’re not supposed to.
HH4
Although at least two sites did not manage to recruit or maintain adequate extra staffing, all eight sites said that having extra staff helped:
With three extra staff . . . We thought we’d died and gone to heaven.
EE6
Extra staffing was perceived as a negotiating device to involve staff in delivering the SVP, ‘The extra nurses were like a bargaining tool’ (H1), and meant that staff could deliver the programme consistently:
If you were caught up with something else perhaps you couldn’t get back there to make sure there was consistency. The extra staff made sure you could follow it through.
KK3
However, having extra staff did not seem to affect perceptions that workload had increased. Seven out of eight sites commented on the extra work of the programme on what were already busy wards, six identified inadequate staffing as a barrier to delivering the programme, five identified problems with sickness or short staffing during the programme delivery period:
We struggled when we were short staffed to try and make sure everybody was going to the toilet at the right time, and you didn’t always have time to do the paperwork and weekly reviews as you should do.
CC2
The extra staffing provided by the research programme was generally well protected from trust demand, but not always:
And what happened with the ICONS staff they were counted in the numbers. If someone was short, one of ours would have to go off to cover, so we were no better off. I know that’s not what you want to hear because the university funded it, but that’s the reality, that’s what happens.
FF4
Owing to the presence of extra staff supplied by the research, support from the trust for adequate staffing was something that had to be actively defended,
That’s when I have to speak to people about my finances and they wonder why, they look at the ward level staff, they are needed because of this programme;
BB1
or creatively used:
One of the things that helped us with ICONS is enhanced support, bank carers who are allocated a bay of patients, and they tend to do a lot of the intentional rounding, and attempt to do a lot of the ICONS as well.
BB2
However, new or bank staff could be difficult to integrate:
If you’ve got your own staff who know about ICONS and what they are supposed to do, it’s fine – but when there’s three or four of you and the rest are bank staff, it’s difficult.
FF4
Adequate staffing appeared to be important in whether or not staff felt positive about the programme:
The programme has worked generally as long as we’ve got enough staff to make sure that all the paperwork is done, and chasing it up – I think it’s good.
LL4
Problems meant that staff felt bad about not being able to deliver the programme properly:
If you haven’t got enough staff some days you can’t get there in time which is quite frustrating.
LL1
Delivery of the SVP during a shift was also perceived to require relentless effort,
We never stopped from start to finish there isn’t a break because we’re constantly doing all the time;
BB1
and needed to compete with other demands on the attention and effort of ward staff:
The paperwork for the CQC [Care Quality Commission] visits was phenomenal. It was really hard to keep vigilant about ICONS because it was getting lost within all the other paperwork.
BB1
Three sites identified difficulties with the environment or equipment, such as lack of toileting facilities,
Toilet facilities are a problem because of the mix of patients we have only one male toilet;
CC1
lack of space for hoisting equipment,
They added extra beds up here. The space for manoeuvring the machinery around [is limited], and the patients are very needy;
LL2
or lack of equipment:
Lack of equipment can be a barrier, we sometimes have to wait for it, or they are broken.
EE6
Reflexive monitoring: the appraisal work that people do to assess and understand how a new practice affects them and others
Success was variously defined by respondents as:
-
getting continent, no accidents, dry every time:HH4
I would say success is no leaking, no accidents;
-
partial continence:FF4
If someone had the odd accident I wouldn’t say it was a failure. If you can get it right 80% of the time it’s better than nothing at all;
-
less wet:CC2
those who it didn’t work with were more continent doing ICONS than they were without it. They were drier more of the time when they were being toileted regularly than they were without it so that was obviously better than being wet all the time;
-
not getting urgency, aware of need:BB1
Success is knowing that you don’t get that urgency, you can walk them to the toilet and they’ve got time and that’s because they’re getting that feeling of going to the toilet but the urgency is not there;
-
asking to go to the toilet, not wearing pads:LL6
Success is when a person has gone from wearing a pad, to asking to go to the toilet, and actually being dry every time.
Staff from five sites said they could see change in the patient’s progress,
Staff can see the patient’s reaction to success, and can see improvement over time,
AA3
and in patient outcome,
I could see it had a positive effect on quality of life and discharge destination and for that reason I liked it.
BB2
One acute site talked about the disappointment in not being able to see outcome:
It was a bit disappointing at first because we didn’t know the outcome most of the time, we didn’t have any feedback for how well we had done . . . in acute all you’re seeing are the very ill, you can’t think what’s to come, what the person who can do nothing is going to be like three months down the line.
KK6
The long-term outcome for any patient was also not known because of lack of follow up into the community:
We don’t follow anybody up. It might be beneficial for the community stroke teams to follow them up.
EE1
Staff could see patient progress reflected in the paperwork:
Once they started noticing a lot of the patients we did get them triggered back into timing and it was only as you were discharging and having it in paperwork, the fact is we got them into a routine and it makes a big difference.
BB1
Sometimes, staff were surprised that particular patients progressed,
We have seen people who were incontinent who finished up being continent or better than they were. There have been a few that we have been quite surprised at,
HH3
but also that incontinence was not necessarily as prevalent as was expected in acute stroke:
You have this perception that incontinence goes hand-in-hand with stroke, but since we’ve been doing the trial we’ve noticed that incontinence as a whole isn’t as bad as we first thought.
FF1
Visible success was important for staff motivation:
We did have some success stories over an 18 month period. As auxiliaries started to realise and started seeing more of the benefit because they weren’t constantly going back to these patients it did become more popular over time.
BB2
Focusing on success was used proactively to encourage and motivate HCAs:
Some of the people who were in the further training were my stronger more proactive staff nurses. They worked with the clinical support workers and tried to link continence to mortality and discharge destination, making them think about outcome rather than the task in front of them at the time.
FF1
Feedback from the family was also influential:
It’s when the family start saying oh she’s continent now, that made the difference, that started people thinking.
BB1
Senior staff and research nurses found the programme hard to monitor, ‘It was hard to keep a handle on the programme’ (A1). Four sites talked about informal evaluation of outcome:
We haven’t formally assessed the value of the programme other than through observation: just watching and seeing what’s happening on the ward such as less use of resources, less wet beds, less wet clothes, less nursing time, less buzzers going off.
F1
The programme appeared to influence the amount of monitoring of continence,
I suppose we are monitoring their continence more closely, that gives us a better picture,
LL5
which confirmed fewer incontinent patients than expected:
I was personally surprised how few there was. A lot of patients from the acute unit do the 3-day diary and we find they’re not actually incontinent.
CC1
More formal evaluation was not frequently referred to, but included the auditing of skin and safety rounds; buzzer audits; emotional touch point groups, where patients are asked about their concerns; and Care Quality Commission (CQC) visits. Stakeholders external to the ward required measurement of progress: from the research team,
Having the research nurses coming in from the University is really good because they’ll keep us on our toes;
BB1
and from the trust:
Skin and safety rounds are being audited on a weekly basis, and fed back to the trust board. We’ve been told to hold people to account if they are not filling in their paperwork. Auditing the skin and safety rounds every week tells the ward manager whether or not we’re achieving what we are supposed to be achieving, and that is fed down to the staff and the governance meetings . . . and nobody likes to be seen to fail do they?
FF1
The ICONS research was seen to feed into external monitoring, ‘ICONS provides evidence for the Sentinel Audit’ (EE1). One site referred to increased nursing discussion of continence in team meetings:
It’s discussed at MDT [Multidisciplinary Team Meetings], it’s become our nursing issue. It is a big issue at discharge planning.
EE4
One respondent questioned whether ICONS had resulted in increased attention to evaluating continence care in their trust:
Whether it’s to do with this or other Trust issues they are looking at the type of pads we use, which are now changing; there is a feeling that continence is increasing in importance within the Trust.
CC1
One bar to continuing evaluation was the lack of paperwork for continence other than that supplied by the research:
I hope our hospital might create our own paperwork: we can’t use any paperwork that hasn’t been approved. Without ICONS we haven’t got anything to monitor patients’ progress: there needs to be a care plan issued specifically for this.
HH3
Awareness was shown of the need to underpin practice with research evidence:
There is such a lack of research out there, the trials that have been done previously were small, and we didn’t really have any good clinical evidence to support what we were doing.
FF1
Respondents recognised that linking the SVP to outcome was challenging,
It’s difficult to say whether people who have been successful on ICONS might have been successful anyway,
HH3
including wider outcomes:
A recent audit done by the Stroke Association has said we are in the upper quarter for patient satisfaction and experience, but linking that to the ICONS trial is difficult.
FF1
The research outcome was needed to support continued use of the SVP:
If we can demonstrate it [the SVP] promotes continence we would adapt and use it but confirmation is needed because of the commitment needed to enforce implementation. If it is successful, there would be a lot of pressure to implement it from specialist nurse, consultants, senior management.
AA1
People generally thought that – despite the extra work – the programme was better than previous continence practice,
It is definitely better for the patient, it does take more work and that was the biggest thing,
KK6
conditional on having the staff to do it,
As long as we’ve got the staff to do it, yeah, I think it’s good.
LL4
All eight sites reported that the intervention worked for a proportion of people,
It has promoted continence in lots of people so ultimately it is good . . . I think you can see that it works,
LL4
with some attempting to put a figure on the proportion:
I’d say they made improvements about 75% of the time.
KK2
There was a degree of surprise about the perceived effectiveness of the intervention from both qualified staff,
What we do now is better – no question. I’ve been surprised, I think it has worked,
HH1
and non-qualified staff:
I got a bit upset at first, it was like here we go again, but this time I’ve actually seen a few benefits.
LL1
There was general agreement (8) that some patient groups tended to do better:
It worked for patients with less cognitive impairment, more mobility, better communication and understanding, younger people.
EE6
However, two respondents pointed out that it could also work for people with cognitive difficulties:
Quite a few people did end up being continent before they were discharged which was quite good for the patients who were more cognitively impaired and didn’t necessarily know exactly what we were doing.
CC2
One respondent speculated about the reasons:
Sometimes the ones with cognitive impairment were the ones that would respond better to the routine. In some ways it helped the ones that were more cognitively impaired . . . who are quiet and withdrawn and don’t demand attention – it gives them attention. The ones that are more alert, verbal, demanding of attention – they get more of the attention on a ward. The ones with communication difficulties would lose out. Whereas if they are on ICONS they are getting that input as well.
FF4
Regaining continence was important in determining discharge destination:
One frail elderly man came in really poorly, doubly incontinent. We did ICONS with him and persevered and by the end of his stay he was continent, going to the toilet regularly himself, able to recognise when he needed to go, which had a massive impact on his discharge destination because he was deemed safe to go to his home environment whereas if he’d been mobile and incontinent that can be more problematic because it’s a higher risk of patients falling and they might need a higher package of care or a nursing home.
BB2
Even without full continence, the development of a routine for cognitively impaired people was valuable for their discharge – whether going home or to a nursing home:
The fact is we got them into a routine and it makes a big difference, especially when you’re handing over . . . It may be you would always have to be the one to go to them and say would you like to try the toilet, maybe they would never trigger themselves but if you had that routine you would keep the patient dry and it would give them self-respect.
BB1
Some patients also managed to wait for longer before going to the toilet:
And some you got lengthened time between toileting, you could get 3- to 4-hourly with some.
FF4
Patients could respond fairly quickly to the intervention,
It’s worked really really well for other patients, straightaway you’ve seen an improvement,
HH4
but some might take longer to respond:
It has worked with some but not for others, although it could still be working.
HH1
Two respondents said that patients did not always become continent at night:
One of the ladies has gone from being totally incontinent, to more or less continent, but at night time they revert back because they seemed to relax.
LL1
Mobility was a major issue affecting the likelihood of success:
It’s usually the ones that progress who are sitting quite well in the chair, so then you could transfer them onto the toilet. Those types of patients came out far better than those who were medically unwell and not coming out of the bed.
KK3
There was disagreement about the impact of gender, with two respondents seeing more improvement in men, and another more improvement in women. One respondent thought that the impact of not having to use pads was greater in men:
The men had been more impressed because for them to be continent has made a big difference because they don’t want to be having pads at any time;
BB1
whereas women still liked to use pads:
We managed to get a few ladies home and be continent, but even though they were continent they still like to use Tena pads, they’ve always had them and they’ll always have them.
BB1
The same respondent speculated about why men do better:
The men seem to have got better than the ladies, they seem to be quicker in getting themselves into a routine. I’m wondering whether it’s just because it’s easier to manage a bottle.
BB1
There was a fairly general view, (4) expressed that the programme would not work with some patients:
It wasn’t going to work with some people no matter how much we toileted them.
EE6
Respondents attributed non-response to pre-existing incontinence or lack of awareness (3), or cognitive problems (3), but thought that response was to some extent unpredictable:
Some it didn’t have any impact on at all. You couldn’t get any pattern or rhyme or reason to what was happening. It wasn’t a particular type of patient, it was variable; it depends on the mental capacity, the cognition – but it could vary even with that.
FF4
One respondent said:
But it’s a fact that sometimes you . . . do have to implement it to see does it work?
AA2
However, although patient response to the SVP was known to be unpredictable, this attitude of needing to try the SVP with everyone did not appear to be a commonly held view.
Five respondents identified that patients felt better, physically and emotionally:
Improvement is not only in incontinence but in personality, they become mentally a lot better, happier as well because they aren’t getting emotional about being wet.
HH4
Three respondents thought that there were benefits for self-esteem, independence and dignity of the patient, and five respondents felt that more involvement, ownership and control of the patients’ recovery improved their confidence:
Patients are getting self-esteem and confidence in themselves because they are getting back to their normal ways like they would at home.
LL6
One respondent thought this helped patients feel that their needs were being met:
We are pre-empting what might be coming by addressing needs on a regular basis, patients feel their needs are being met. We do emotional touch point groups and things like helplessness used to come up quite a lot, but that hasn’t come up for quite a while.
FF1
Benefits for nurses and nursing care included:
-
Increased nursing awareness, knowledge or confidence. Six respondents identified improved nursing awareness, interest, knowledge or confidence:HH3
They [the assessments] make you think a lot more about the type of continence problems people are having. Educationally it’s very good there’s been a lot of teaching, it’s made people interested;
-
Making nursing care easier, reducing workload. Five respondents identified that nursing workload had reduced:HH5
Workload – I still think it’s made our lives easier. A full bed change takes two staff half an hour maybe three-quarters of an hour. Standing the person to the toilet takes five minutes – the time difference is a plus for us,
or that the work had shifted from less physical work, to more paperwork:
So it has made an impact in nursing time. It’s more paperwork but the actual physical work has eased off a little bit;
BB1
-
Reduction in pad use. Five respondents identified reductions in pad use:
Our pad usage seems to be going down because we are taking people to the bathroom;
FF1
-
Improved communication with patients and relatives. Three respondents identified improved communication with patients:
I think we’re much more open about continence with patients, people actually discussing why we’re doing things, and the importance of it, and asking for the patient’s opinion more. If patients are having problems still with their incontinence we are giving them much more choices as well as the toileting, which pads to use and things like that,
CC1
and with relatives:
Involving the relatives in assessment takes a lot of time;
KK6
-
Improved communication between staff. Two respondents identified more interaction between staff about continence:
With the time management, people are talking about that with each other and planning, and I hear staff saying, ‘Well we’ve got so and so will need to go on at such a time,’ so they are interacting and planning between themselves, rather than responding all the time which is what I think they tended to do before;
CC1
-
Changing nursing attitudes towards incontinence. Three respondents thought nurses now saw incontinence as more amenable to intervention:
It did alter attitudes towards strokes and continence that something could be done;
KK6
-
Increased therapeutic role for nursing. Two respondents identified improvements in nurses’ ability to proactively assist patient recovery:
It is showing that we’ve looked at it and we are identifying that there is a problem, and we can help you with it, or at least we can try;
FF2
-
Changes to care planning. Three respondents identified changes to care planning and continuity:
It certainly helped us improve the consistency of care. It’s given us knowledge and a structured plan of action. Nurses like to have a clear management plan to follow: you’re more likely to get consistency and continuity;
FF1
-
Increase in use of bladder scanner. Three respondents talked about increases in bladder scanning:
I thought the bladder scanner was excellent too. We do scan bladders much more frequently than we used to;
CC2
-
Reduction in catheter use. Two respondents thought that there was less use of catheters,
There’s less catheterisation;
EE4
-
Calmer ward, fewer buzzers going off. Two respondents said that there were fewer requests from patients:
It didn’t take long to work out that buzzers were going off less, so you were pre-empting care, being proactive rather than reactive;
FF1
-
Increase in investigations. One respondent identified a change in continence assessment:
Increased consciousness of continence results in more investigations e.g. dipping urine, sending samples off. They probably do this more now than before.
EE4
The extra staff were seen to impact on aspects of care wider than just continence:
We have got the extra staff, which is brilliant. That has certainly had an impact on the care that we can deliver . . . Not just continence but all aspects of care.
FF1
Views on the impact of the SVP on cost were mixed. One respondent thought it would save money:
Costs have probably balanced out: having to send the patient home with a pack of pads at 60 or 70 pounds a pack and the costs in changing patients versus the paid costs of the labour. If it ran long term you would probably find significant savings but you would have to get more wards on board;
HH5
another thought the costs would probably even out:
But at the end of the day the time and nursing time we are saving by toileting these patients and not having wet clothes and beds to change if you added it up in a total week, it would actually reduce nursing time and hopefully break even.
FF1
Staff at seven out of eight sites thought that the SVP should be continued, and that it was worth doing. Staff at five out of eight sites identified that they were still doing the physical components of the SVP, at least in terms of regular toileting. Only one site suggested that the SVP was not continuing, with some expressed regret:
It probably wouldn’t be a popular decision to carry it on but personally I think it’s a shame it has stopped. Since the trial is finished it’s not in place anymore. We manage it with nappy pads like we did before. Some patients have been encouraged to use urinals and bedpans as much as they can. There is no formal assessment in place anymore.
BB2
However, despite this overwhelmingly positive evaluation of the impact of the SVP and its continuation in some form in over half of the sites, even without extra staffing, this wasn’t unconditional, per protocol, or wholesale. Respondents said that staffing levels would affect whether or not the programme was continued (4), toileting was to be merged with skin and safety rounds (2), and the paperwork would not be continued in its present form (4). In two sites, the programme was continued, but only with those patients thought likely to succeed. One site thought that doing the programme intermittently when staffing allowed was probably not worth it:
Maybe not continuing it if we haven’t got staffing levels, because we’re doing it one day and then not doing it another day and then it’s pointless.
LL5
The structure provided by the programme was identified as motivating (1), as was experience of success (3):
It’s all down to education, confidence, and knowing the result of it really, knowing that it’s going to work.
AA2
There were some deviations from the protocol that might have been due to misunderstanding or misinterpretation, such as putting everyone on the diary (1), then on PV (2); regular as opposed to individualised toileting (2); promoting the use of the toilet instead of a bedpan (1); and stopping the programme for people who were incontinent (1). Other deviations appeared to be in response to perceived problems with the protocol fit with individual patient need or context, including adaptation of the process for unco-operative patients (1); or fitting in to the reality of ward routines such as mealtimes and visiting times (2). Other deviations appeared to be creative reinterpretations of the SVP protocol, such as starting prompting before the diary finished in acute areas (1).
People made suggestions for how the programme should be modified, including a focus on those people for whom it was likely to work:
If we were going to change anything it would be concentrated on the ones that we know are going to do well, and less time on the ones that we know aren’t,
KK3
although there was a recognition that this would miss some people for whom it might work:
. . . there has got to be the odd one or two who we thought wouldn’t do well who actually do.
KK3
One respondent wondered about the necessity of repeatedly asking people if they were wet:
I wonder whether it’s a bit too much sometimes for the patient constantly saying they are wet, each time you have to ask them – but then they’ve got to be aware haven’t they.
LL4
Two respondents suggested extending the programme to the night-time.
The main changes suggested were to the paperwork, including simplifying the assessment (2), moving the weekly review to the weekend (1) and designing a care plan to take over recording when ICONS finished (1). However, three respondents suggested not changing anything until the programme had run for longer.
People also suggested improvements to the implementation of the programme, including having a co-ordinator in the early stages (1); doing an initial rollout to senior staff first (1); having a longer interval between training and starting the programme (1); and changing the training to a full day course (2). Other suggestions to aid implementation included providing symbols for boards (1); visual aids for people with aphasia (1); and badges to identify ICONS HCAs (1).
Four respondents suggested that the programme was being, or should be used for other patient groups, such as those with other medical or neurological conditions, or suggested continuing the programme in community settings.
Summary of findings related to the implementation of the systematic voiding programme
Coherence: the sense-making work that people do when faced with a new practice
Sites differed in how much continence care they were providing before implementation of the SVP, some having very little (4), others using regular toileting (4). The SVP was seen as structured (5), timed (4) and documented (8). Nursing tasks were similar, but routine and documentation were different (5), particularly the assessment (3). The SVP was not consistently differentiated from regular toileting.
Staff agreed with the aim of the programme (6) and its role in nursing care and rehabilitation, but were negative about some aspects such as the paperwork (6) and extra work (2). Having enough staff and seeing positive results facilitated agreement with the SVP (4). Some staff disagreed with the suitability of including all patient groups (3), especially people who were unwell, or who had cognitive or long-term continence problems.
Although it took some time to learn and settle in, the SVP was seen as easy to understand (5). It was felt that most staff understood the programme (6), although there were indications that some misinterpretation of components of the SVP occurred, particularly around the differentiation between regular toileting and programmes tailored to individuals. Staff thought that most patients and relatives understood (3), but that explaining the programme to patients and relatives could be difficult (3).
Staff could see the value of the new practice in terms of increasing the priority of continence care (5), and highlighting that continence is amenable to change (3). There was recognition of the importance of continence for patients (4), and that continence control could also signal wider recovery to the patient (2). Nurses felt they were helping patients (4). The SVP provided structure and guidance (6) and reduced workload in the long run (3). Although staff recognised the benefits, the extra work was unpopular (3).
Cognitive participation: the shared work that people need to do to build and sustain a new practice
Key individuals introducing the new practice included the research team, matron, stroke network nurse and trial management. Senior ward staff were seen as responsible for pushing the new practice forward (8). Their role included promoting the programme; providing direction and reminders; education and supervision; organisation and delegation; and monitoring and feedback.
Proactive senior staff nurses were also seen as influential in driving the programme forward (3).
Although not consistent over the whole of the programme in a number of sites, the research nurse was recognised as a valuable resource (6). Their external perspective was valued for co-ordination, monitoring performance and countering established views. The extra HCAs (5) and the external facilitation (2) provided by the ICONS research study also influenced perceptions of the programme.
Staff in half of the sites were said to be involved and motivated. Positive influences on staff engagement included enjoyment, work reduction and seeing success. Three sites reported a lot of dissent in the initial stages, with people taking some time to become involved and motivated. Some of this dissent related to how the SVP was implemented. Therapists were not directly involved (3), but could act to facilitate the SVP in scheduling therapy sessions around toileting times (1). Patients were generally happy to be involved (4); reluctance to get involved was attributed to fear of extending hospital stay, or drawing attention to incontinence.
The main feature of ward organisation altered by introduction of the SVP was the recruitment of new staff, and sites had variable success with this. Sites also differed in how they utilised the new staff, from complete integration into the ward team, to allocating them full responsibility for implementing the SVP. The research nurse also had varying responsibilities for co-ordination and paperwork between sites. Overall, the SVP was not perceived to have made a significant difference to patterns of workload allocation, with normal role differentiation between staff nurses and HCAs (5). However, respondents referred to potential enhancements to the HCA role, in response to increased training, involvement and perceived impact.
All of the sites had constructed reminders to facilitate delivery of the SVP (5) such as whiteboard symbols; entries in the diary; recording status in the handover sheets; or involving night-staff in preparing paperwork. Respondents also talked about the need to harmonise SVP paperwork with other recording systems (4). The co-ordinating and informing functions of the paperwork were seen as valuable (3), as were the information resources provided by the ICONS research programme (2).
Collective action: the operational work that people need to do to enact a new practice
After initial teething problems, staff felt the SVP ran smoothly most of the time (7), although reminders were still required (2). Staff reported problems with delivering some aspects of the intervention: maintaining surveillance to put new people on the SVP and scheduling people through the different stages was hard to keep tabs on (4); the assessment was difficult to complete (5); 3-day diaries were thought too long in some instances (2); and the weekly reviews could be forgotten (3). Timed toileting could be challenging to schedule, remember and adhere to within the ward routine (4), and staff did not know how to record some occurrences (3). Frequent regular toileting was difficult to manage with some patient groups (3), especially those with physical or cognitive difficulties. Using distraction and extending the time interval before use of the toilet (3), or repeatedly asking patients if they were wet (2) was thought difficult or uncomfortable for staff and patients. Staff made suggestions about how to better manage the SVP on a daily basis, including how to involve patients, and schedule care to take account of ward practices.
Change was recognised as bringing challenge (3), and most sites had a period of learning during which they felt unsure, but confidence had increased in themselves (2), and in the programme being done properly (3), although perhaps not all the time (2). Senior staff felt it was hard to maintain motivation and attention in relation to continence because of competing priorities and the need to provide constant reminders (4), especially around recruitment to the SVP (2). However, staff felt more confident and skilled in their ability to manage continence (2). They talked to each other more about continence (3), although relationships could also suffer around new responsibilities (1). There was increased communication about continence with the wider team (2), although continuity between separate acute and rehabilitation areas could be a problem (1).
The SVP was felt to be difficult to learn at first (3) and suggestions were made to adapt training and implementation methods. Although some specific skill deficits were acknowledged in communication and assessment, overall, staff felt they had learned new things (3) and had the skills and knowledge to deliver the programme (4). The training (4) and educational resources (3) provided were useful but there was poor uptake of the online training (6). Ongoing updating of new staff needed monitoring (3), and there were problems with using bank staff unfamiliar with the SVP (2).
Although respondents in all eight sites valued having the extra staff, the majority also commented on the extra work required by the SVP on already busy wards (7). Delivery of the SVP had been affected by inadequate staffing or sickness at some points in time (7), which adversely impacted on staff morale if they then could not deliver the SVP properly. Trust support was needed to protect extra staffing resources. Three sites reported difficulties with environmental resources for toileting, but staff liked the bladder scanner provided by the research (2).
Reflexive monitoring: the appraisal work that people do to assess and understand how a new practice affects them and others
Success was defined by respondents as patients becoming continent, but they also saw partial continence or being less wet as worthwhile outcomes. Their definition of success also included patients not having urgency, being aware of their need and asking to go to the toilet. Staff said they could see changes in patients (5), which were highlighted by the paperwork (6). Visible success was important for staff motivation (5), staff also noted comments from families (2).
The programme was monitored by senior staff and research nurses (5), but staff found monitoring difficult (3). They referred to observation (4), and monitoring continence more closely. More formal evaluation from the trust and external bodies such as skin and safety audits or CQC visits were ongoing and ICONS was seen to contribute to Sentinel Audit results (1), but linking the SVP to wider changes in outcome was thought problematic (2). One site referred to increased nursing discussion of continence at MDT meetings and in discharge planning.
Despite the extra work – the SVP was deemed to be better than previous practice, conditional on having the staff to do it (2). Respondents from sites were unanimous in saying that the intervention worked for a proportion of people (8). There was general agreement that people without problems of understanding, communication or mobility were likely to do better (3). However, people with cognitive problems could benefit from attention and being less incontinent (2) or the development of routine (1) and continence status could have a big impact on discharge destination (2).
There was variation in speed of recovery, and people could regain continence in the day time but still need pads at night (2). People had different views on the impact of gender (3). There was a fairly consistent view that the programme would not work with some patients (4), with failure attributed to pre-existing continence problems, lack of awareness, or cognitive problems, although it was recognised that the response of individual patients to the SVP was unpredictable, and could be surprising (2).
Staff identified physical and emotional benefits of regaining continence for patients (5), as well as benefits for self-esteem, ownership and control of recovery (5). Benefits for nursing and nursing care included changed attitudes towards continence (3); increased awareness, knowledge and confidence (6); reduced workload (5); improved communication between staff (4) and with patients (2); increased therapeutic role for nursing (2); better assessment and care planning (4); and a calmer ward (2). Staff thought costs might be lower (1); respondents from five sites said pad use had reduced (5).
Although respondents from the majority of sites thought the programme worth continuing (7), and respondents from five out of eight did maintain the core aspects of the programme after the research was completed, respondents said that staffing would affect continuation (4); or that the programme would not be maintained without pressure from senior staff (2). Respondents from four sites indicated that toileting would be merged into skin and safety rounds; respondents from two sites said the programme would be done with those patients thought likely to succeed. Although the structure of the programme was valuable, respondents said the paperwork was unlikely to continue in its current form (4).
People made suggestions for ways in which the SVP content could be modified and for improving the implementation of the programme, around co-ordination (3), timing of implementation (1), training (2) and additional resources (3). Respondents from three sites said they planned to use the programme with other patient groups.
Supported implementation
External and internal facilitators
The EFs were Dr Chris Burton (CB), Senior Research Fellow in Evidence-Based Practice at Bangor University, Gwynedd, UK, and Dr Jane Williams (JW), Consultant Nurse in Stroke Care and Chief of Service, Clinical Service Centre of Medicine for Older People, Rehabilitation and Stroke, Portsmouth Hospitals NHS Trust, Portsmouth, UK. CB and JW have many years’ experience of running a successful leadership programme for stroke service managers for the Department of Health; CB has a special interest and track record in implementation science. Responsibility for sites was allocated solely on practical grounds, with facilitators linked to sites closest to their place of work: CB worked with sites FF and HH, and JW with sites KK and LL.
Each site was asked to choose their internal facilitator based on the criteria outlined previously (see Chapter 3); sites were also encouraged to nominate one or more deputy facilitators to help share the work and ensure cover for annual leave and sickness. Table 77 shows the number and grade of facilitators in supported implementation sites.
| Site (codes removed to protect anonymity) | Internal facilitators | ||
|---|---|---|---|
| Number | Grade | Comments | |
| Site 1 | 1 | Band 7 – ward managera | |
| 1 | Band 6 – ward sistera | ||
| Site 2 | 1 | Band 7 – ward managera | ICONS research nurse (band 7) also acted in a facilitation role between August 2011 and 31 January 2012 |
| 1 | Band 6 – ward sister | ||
| Site 3 acute unit | 1 | Band 7 – ward manager | |
| 1 | Band 6 – stroke co-ordinator | ||
| Site 3 rehabilitation unit | 1 | Band 6 – staff nurse | |
| 1 | Band 5 – staff nurse | ||
| Site 4 | 1 | Band 7 – ward managera | |
| 2 | Band 6 – ward sister; staff nurse | Ward sister absent from unit throughout intervention period | |
Documents supplied to the research team
Table 78 shows documents received. Meeting notes indicate that sites HH, KK and LL met with their EFs on a regular basis throughout the intervention period. FF, KK and LL completed a diary of weekly activities, although these were only completed for 5 and 6 weeks at LL and FF respectively. Weekly diaries showed internal facilitators were engaging in the full range of activities, from planning to evaluating change; a more detailed analysis of these was not undertaken owing to the large variability in terms of number of diaries submitted.
| Meeting notes, action plans and weekly facilitation logs | ||||||
|---|---|---|---|---|---|---|
| Site | Meetings with EF | Action plans: implementation objectives | Weekly facilitation logs (weeks beginning) | Other | ||
| Site 1 | 22 May 2012 (notes of telephone conversation) | |||||
| 6 weeks (no dates available, but known to be consecutive) | ||||||
| Site 2 | 29 June 2011 19 April 2012 17 May 2012 12 June 2012 18 July 2012 |
|
None completed | |||
| Site 3 | 10 January 2012 28 February 2012 23 May 2012 (telephone update) 12 June 2012 10 July 2012 |
Acute unit:
|
2 January 2012 | 30 April 2012 | ||
| 16 January 2012 | 14 May 2012 | |||||
| 6 February 2012 | 28 May 2012 | |||||
| 20 February 2012 | 4 June 2012 | |||||
| 5 March 2012 | 11 June 2012 | |||||
| 26 March 2012 | 18 June 2012 | |||||
| 16 April 2012 | 25 June 2012 | |||||
| 23 April 2012 | 2 July 2012 | |||||
Rehabilitation unit:
|
23 January 2012 | 9 April 2012 | ||||
| 30 January 2012 | 16 April 2012 | |||||
| 6 February 2012 | 24 April 2012 | |||||
| 13 February 2012 | 1 May 2012 | |||||
| 20 February 2012 | 7 May 2012 | |||||
| 27 March 2012 | 14 May 2012 | |||||
| 5 March 2012 | 21 May 2012 | |||||
| 12 March 2012 | 28 May 2012 | |||||
| 19 March 2012 | 4 June 2012 | |||||
| 26 March 2012 | 11 June 2012 | |||||
| 3 April 2012 | 25 June 2012 | |||||
| Site 4 | 10 January 2012 28 February 2012 12 June 2012 10 July 2013 |
21 May 2012 28 May 2012 11 June 2012 25 June 2012 9 July 2012 |
||||
| Weekly activities undertaken by internal facilitators | ||||||
| Activity | Site FF (6 weeks) | Site HHa | Site KK acute unit (16 weeks) | Site KK rehabilitation unit (22 weeks) | Site LL (5 weeks) | |
| Planning for change | ||||||
| Increasing awareness | 6 | 9 | 4 | 5 | ||
| Developing plan | 4 | 9 | 1 | 3 | ||
| Leading and managing change | ||||||
| Knowledge management | 6 | 9 | 11 | 2 | ||
| Project management | 6 | 9 | 8 | 4 | ||
| Recognising context | 6 | 8 | 19 | 4 | ||
| Team building | 6 | 8 | 21 | 5 | ||
| Project support | 6 | 8 | 11 | 2 | ||
| Monitoring progress and ongoing implementation | ||||||
| Problem-solving | 5 | 8 | 8 | 1 | ||
| Providing support | 5 | 8 | 15 | 5 | ||
| Effective communication | 5 | 8 | 18 | 5 | ||
| Evaluating change | ||||||
| Assessment | 3 | 13 | 10 | 3 | ||
Meetings between internal and external facilitators
Both EFs met internal facilitators either before or at the beginning of the intervention period. The purpose of the initial meeting was to orientate them to their new role using the facilitation handbook and to help them develop a set of ‘higher level’ implementation objectives. The number of meetings held is shown in Table 78 and ranged from three (FF) to five (HH and KK). Meeting notes were analysed using NPT.
Findings: analysis of meeting notes
Coherence: the sense-making work people do when faced with a new practice
Discussion with facilitators in the first meeting at HH suggested that the SVP looked like a return to task allocation and 2 hourly ‘toilet rounds’; although this was not viewed as problematic, staff stressed the need to emphasise the individual nature of the ICONS programme.
Developing a shared understanding of the practical implications of delivering the programme, as well as ensuring staff knew what was expected of them, was discussed at HH and subsequently incorporated into an action plan with the objective ‘Ensuring the appropriate staff know “who is doing what” within the systematic voiding programme’.
There was evidence from LL suggesting all staff were aware of what was involved in delivering the programme, with HCAs singled out particularly as actively involved and beginning to take on the role of prompting others to initiate parts of the programme.
The value of the programme in raising the profile of continence management was discussed in meetings at HH and LL; the ICONS study was viewed as providing a focus for continence and also as a trigger for people to think more about continence (LL) and adopt therapeutic, rehabilitative strategies rather than containment (HH). The relevance of continence management in facilitating early supported discharge was raised in KK acute unit.
Cognitive participation: the relational work people do to build and sustain a new practice
Nurses were viewed as key to introducing the ICONS programme, described by facilitators at KK (rehabilitation unit) as ‘very much nurse led to date’. Nurses singled out as key leaders were the research nurse (HH), band 5 staff (HH), HCAs (LL) and the lead nurse (KK, rehabilitation unit). The research nurse in HH was particularly highly regarded for her practice development skills, which led facilitators to view her as a ‘massive driver’ of the programme and as providing reinforcement for the facilitation work undertaken by the designated internal facilitator and her deputy. Her presence on the unit every day was highly valued and she was viewed as particularly good at encouraging staff to complete paperwork.
The lead nurse was regarded as supportive in KK (rehabilitation unit). Band 5 staff were identified pre implementation as having a leading role in the initiative in HH, whereas at LL HCAs were singled out as ‘good champions’ of the project.
At LL, there was evidence that relatives were taking a more proactive role in terms of checking their relative was receiving the continence care prescribed. Meeting notes report this was welcomed by staff:
It doesn’t hurt that relatives sometimes ‘have a go’ at the nursing staff for not taking their relative to the toilet at the right time.
Meeting notes, LL
Meeting notes suggested staff were using a variety of strategies to organise the work of implementing the SVP. A common strategy to facilitate this was discussion in meetings, including weekly meetings (HH), multidisciplinary meetings (HH), unit planning meetings (LL) and ward rounds (KK rehabilitation unit). Allocating specific responsibility was also discussed at KK acute unit and LL, where one HCA at each end of the ward had responsibility for ensuring paperwork was completed. The system was more diffuse at KK rehabilitation unit where no one designated person was responsible for ensuring patients on the programme received the care they needed. Informing bank staff about ICONS also fell within the remit of HCAs in KK (rehabilitation unit).
Ward staff discussed techniques to ensure patients on the programme were visible; these included identifying patients on the patient board (HH, KK acute unit, LL) and ensuring they were included in handovers (LL). There was some evidence that completion of paperwork had improved over time (KK rehabilitation unit), with night staff also involved in weekly reviews (LL).
Collective action: the operational work people do to enact a new practice
Too many patients suitable for and on the programme was cited as a barrier in KK rehabilitation unit, whereas at HH, KK acute unit and LL there were occasions on which there were not enough patients suitable; at KK this was due to norovirus closing the ward to new admissions, whereas at LL it was due in part to the addition of four medical beds to the unit. Paperwork was viewed as something staff were not getting round to doing (HH) and as putting pressure on regular staff (LL), but LL staff also commented that the continence assessment had become embedded in routine practice. Staff on KK acute unit liked and were using the documentation to good effect by June 2012.
Patients’ PV schedules were disrupted by therapy sessions (LL). As sessions were not timetabled, it was not possible to plan scheduled voiding times to fit around these.
In two sites (KK and LL) there were sustained attempts to involve the MDT in the ICONS programme. For example, transferring and mobilising were identified in KK (rehabilitation unit) as areas where a multiprofessional approach could enhance care; facilitators were also keen for physiotherapists to review use of pelvic floor exercises for suitable patients and for occupational therapists to incorporate continence into their rehabilitation planning. Both occupational therapy and physiotherapy teams were encouraged to become involved in patients’ toileting regimes to facilitate adherence throughout the day, and processes were put into place to expedite this, for example informing therapists every Monday who was on the programme. Championing the multidisciplinary approach was of particular importance to the facilitator at KK (rehabilitation unit), as she describes:
I said now look this is supposed to be a multidisciplinary approach, not a nursing one, so [they] said ‘oh yeah we’re all willing you know’, I said ‘the thing is you all need to know how to do the paperwork, it’s no good you just being’, she said ‘you know one of the therapists said, oh we always take them to the toilet’, I said ‘it’s not as simple as taking them to the toilet, there’s a plan’.
Internal facilitator, KK
The research nurse, who covered both KK and LL, was also instrumental in ‘planting the seed’ of MDT involvement by attending MDT meetings as well as inviting therapists to ICONS facilitation meetings. Both acute and rehabilitation units at KK added MDT involvement to their action plans in the second month of the intervention period. LL identified many benefits of working more closely with therapists, including increasing nursing staff knowledge of and ability to solve problems around positioning and handling; more proactive multidisciplinary meetings; early identification of continence goals; and more effective discharge planning. To facilitate this, the team agreed to pilot multidisciplinary ward based working.
In LL, HCAs were reported to be taking the initiative in implementing the programme and prompting other staff, for example when patients were ready to go on the 3-day diary.
Towards the end of the intervention period, the facilitator at LL stated that staff morale was low, contributing to lethargy towards change.
The research nurse covering KK and LL undertook initial training with the majority of the staff in the three units, including some night staff. ‘Refresher’ training was provided in February 2012. Staff were also keen for members of the MDT to access training, as knowledge was viewed as a means of increasing motivation to engage with ICONS (KK rehabilitation unit). However, although meeting notes indicated the research nurse was investigating the feasibility of doing this, there is no evidence it actually took place. Meeting notes from KK acute unit in February 2012 suggested staff were completing the online training; however, in June staff were prompted further as many had not completed it. MDT members also requested access to the online training (KK acute unit), but it is not known if any completed it.
Training in using the bladder scanner was well received and staff commented on the positive influence this had on continence management (KK rehabilitation unit).
The EF at HH provided additional training focusing on leadership within the work of internal facilitators. Staff were invited to complete the Multifactor Leadership Questionnaire; however, as none had done so in advance, the session comprised a presentation on transformational leadership.
Inadequate staff was identified as an issue in all sites. In FF the problem was particularly acute, with the unit working with 66% establishment in May 2012.
The availability of ICONS-funded HCAs was discussed at meetings in HH, KK acute and rehabilitation units and LL. In KK and LL, the full complement of ICONS HCAs were working on the units and, despite these being bank staff at KK, there were regular staff assigned. However, the perception at both KK (both units) and LL was that staffing was ‘a struggle’ due to staff sickness (KK rehabilitation unit) and insufficient bank staff to provide cover (LL). Staff at KK rehabilitation unit commented on the employment of therapy nurses on their unit; these covered the whole week and their role was to work alongside nursing and therapy staff in the provision of therapy. Their input was viewed as an asset in assisting patients to progress. In HH, one ICONS-funded HCA left in March 2012 and there was discussion about employing bank nurse cover for this post.
Staffing issues were cited as hindering the ability of staff to implement the programme. Use of many bank staff, all needing to know about ICONS, posed a communication challenge (KK rehabilitation unit). High levels of temporary staff also gave rise to poor continuity (LL). A further issue specific to supported implementation arose in LL, with two out of three facilitators not present on the unit, in one case for the duration of the intervention period. This was addressed by asking a band 5 staff nurse to stand in, although this was not entirely satisfactory as this person had many other commitments.
Reaction to change was mentioned in discussions at HH. In the first meeting at HH, internal facilitators articulated how the unit was very used to and able to cope with change. By April 2012, however, the impetus for the programme had ‘fallen off’ due to the original research nurse leaving and being replaced by a new person who spent only 2 days a week on the unit and did not have a similar background in practice development.
Environmental issues were raised by staff in KK rehabilitation unit. The therapy area was viewed as not conducive to toileting patients, therefore they were returned to the ward for this purpose which mitigated against nursing staff efforts to involve therapy staff in continence activities. The environment at KK acute unit was also regarded as highly unsuitable, with poor access to toilet facilities and lack of space within toilets leading to dignity, privacy and safe transfer issues.
Equipment issues regarding bladder scanners were discussed at both KK units. The bladder scanner was greatly valued at the rehabilitation unit. As the project was only able to provide one scanner per trial site, KK acute unit did not have one but the research nurse liaised with the equipment library to ensure the unit could access one when required.
Reflexive monitoring: the appraisal work that people do to assess and understand how a new practice affects them and others
At KK rehabilitation unit, success of the intervention was discussed; there was a strong belief that practice had changed for the better, and continence history taking and assessment were viewed as being much more in-depth and accurate towards the end of the intervention period. This assessment also led to a greater number of referrals to urology.
Staff at KK (both units) and LL believed the programme was working in terms of helping patients regain continence, with this outcome directly attributed to the programme. There was a feeling at KK rehabilitation unit that the programme may not work quite so well with cognitively impaired patients:
we’ve had a few though, . . . like a gentleman with cognitive problems, and . . . we’ve tried, and we’ve tried you know like you say to him well you’re nice and dry now try the toilet, no I’m dry, and we’re not getting through to him, so that’s been a difficult one.
KK rehabilitation unit
At LL, the programme was also credited with changing the ‘silo’ culture between nurses and therapists, albeit slowly.
On KK rehabilitation unit, there was general agreement around the benefits of the programme, with staff having ‘nothing negative to say’; this is also evidenced by their intention to continue with assessment and PV when the study ended. At LL also, successes with patients had encouraged staff to continue with the approach.
There was evidence of a dip in enrolment at HH 3 months before the intervention period ended; a ‘re-launch’ was planned to emphasise achievements and successes so far and encourage everyone to keep going until the end of the intervention period.
Findings: interviews with internal and external facilitators
Interviews were conducted with both EFs and with internal facilitators indicated with ‘a’ in Table 77.
Coherence: the sense-making work people do when faced with a new practice
In FF, the internal facilitators viewed facilitation work as part of their everyday job; there was no change in role, just a change of focus.
In one site, understanding of the scope and nature of external facilitation was not shared between the external and internal facilitators. The EF expected to visit the site at the beginning, middle and end of the intervention period, with ways of working and reporting mechanisms being established at the first visit. However, the site expected monthly meetings and, when these did not happen in the early stages, there was a feeling that the site was not receiving what they were entitled to:
if you’re going to say you’re going to do something then you do it, and I think when something is said that people are going to do and they don’t, then it becomes quite difficult
IF-HH
The initial plan for visits and reporting needed revision based on the demands of the site; given the emphasis put on face-to-face meetings, these were increased to monthly even though progress with action plans around multidisciplinary working and the visibility of the SVP was good. Meetings therefore focused on aspects other than implementing the SVP, for example leadership development and peer review of the internal facilitator’s leadership style. These activities were viewed by the EF as:
relevant in terms of enhancing the background of implementation by not necessarily related to . . . what we were doing
EF1
An additional issue was the introduction of an additional implementation mechanism, peer networking, where two internal facilitators visited another trial site to see how the SVP was operating. This was suggested by another member of the project team without consultation with the EF, and was viewed as potentially ‘muddying the waters’:
encouraging the sites to network . . . so they visited other sites which you know networking, peer networking is a . . . keen mechanism for supporting service improvement which isn’t facilitation
EF1
The integration of ‘normalisation’ was also viewed by EF1 as blurring the theoretical basis of facilitation through the addition of another theoretical framework.
Issues around understanding of the EF role also arose in the other site led by EF1. This site identified issues for discussion related to running the trial rather than implementing the SVP, for example recruiting patients. The facilitator role became one of broker, blurring the boundaries between supporting the research and supporting implementation:
so there I was sort of more facilitating the conversations between research support, research support managers, the clinical staff, the . . . internal facilitators, not really around issues to do with the trial, but in terms of sorting out you know personality issues and problems and you know resource issues around the trial rather than around the . . . intervention per se
EF1
In HH, there was uncertainty about what the internal facilitator role would involve.
This was not mentioned.
Cognitive participation: the relational work people do to build and sustain a new practice
HH singled out the research nurse, who also worked in a facilitator role:
although I was a facilitator I had another band 7 with me, she was absolutely exceptional, she was absolutely fantastic and she just really got on with the job
IF-HH
Both EFs commented that strategies used to encourage engagement of internal facilitators (other than meetings) did not seem to be working, for example there was little correspondence by telephone or e-mail in between meetings. EF2 concluded that internal facilitators did not take advantage of support available:
I did site visits with [LT] fairly early on, and . . . then . . . further visits as . . . the . . . research was about to get underway, so I set up e-mail . . . distribution lists and e-mailed people, said you know I’m here, I gave people my . . . e-mail, my . . . direct telephone number to contact me, I e-mailed to ask how people were getting on, but . . . there it was I have to say that the communication was all one way from me to them, and . . . often I . . . heard nothing back, very little came back . . . at all.
EF2
EF2 believed that although there was a flurry of activity before meetings, internal facilitators lost focus in between. As well as little contact between EF2 and internal facilitators, there was also little discussion or support between facilitators working in the acute and rehabilitation units within the same site. EF2 expressed disappointment that the internal facilitators had not really grasped the research and were not pushing it forward; they were viewed as being in ‘victim mode’ and perhaps hampered by not being advanced in terms of clinical leadership. Internal facilitators focused on engagement with ward staff implementing the SVP rather than with EFs.
In terms of expectations about how facilitation would be managed, EF1 believed it would include problem-solving, supporting the staff and developing organisational context, for example leadership. Initially, the focus for EF1 was on building relationships, establishing ways of working and introducing internal facilitators to the model of facilitation embodied in the facilitation manual. This included thinking through how to implement the SVP in their stroke service, identifying and prioritising challenges and selecting which ones to develop into action plans:
a . . . handbook for them as internal facilitators, and that included . . . an action planning framework for them to use to highlight issues or challenges that they as internal facilitators came up against when they were trying to get the algorithm into practice, and then helping them to think through those action plans what you know the barriers and enablers to achieving the action plan, what the objectives would be you . . . know breaking it down into small manageable chunks of activity that they could do to get round the . . . problem that they were facing, or to . . . change the problem . . . in terms of getting the algorithm into practice.
EF1
For EF2, there was also a focus on directing staff towards project-specific and external resources, for example ICONS online training and the Stroke Improvement Programme. Keeping up momentum was important to both EFs through flexibility and willingness to help (EF1), seeking (EF1) and giving feedback (EF2), reassurance and positive reinforcement (EF2).
Internal facilitators focused on challenges in engaging both the organisation (LL), nursing staff and the MDT (FF and LL). Informal approaches were used to roll out the programme, for example discussions over ‘coffee in the staff room’ in FF, and a ‘try it and see’ approach in HH:
it was very much fed to the staff that . . . you know . . . just let’s roll it, let’s just roll, roll it out see where we go, pick up the problems as we . . . go along.
IF-HH
Momentum was increased by ICONS being ‘high on the agenda’ (FF), focusing on positive aspects at ward meetings and handovers, maintaining a positive attitude (LL) and providing feedback and positive reinforcement (FF).
Action plans were mentioned in FF and LL; FF cited the process through which the EF guided their development and implementation, whereas in LL the action plan described what was going to be implemented, how practice was going to change and how this would be communicated with staff.
Communication between external and internal facilitators took the form of visits (EF1, EF2, FF and LL) and e-mail contact (EF1, EF2, FF). Although EF1, EF2 and FF viewed e-mail as a means of keeping up momentum, the facilitator at HH stated there was no e-mail contact, and also that visits did not happen. LL also pointed out that there was little telephone contact with the EF.
In terms of internal facilitator ‘work’ at the sites, at LL there were weekly discussions between facilitators and the research nurse, with issues fed back to staff in ward meetings.
Collective action: the operational work people do to enact a new practice
Interactional workability (whether or not staff and patients are able to do the tasks required of a new practice).
EF2 commented that in some units the SVP was quickly absorbed into routine work, whereas in others this was less successful. Delays in starting ICONS and the consequent time lag between initial site visits and the intervention starting were problematic, with units ‘going off the boil’ by this time (EF2).
No internal facilitators mentioned whether or not they were able to undertake facilitation tasks, but focused instead on factors affecting whether or not units were able to do the SVP, for example staffing issues forcing a temporary halt to recruitment (FF) and problems engaging staff and the organisation (LL).
Relational integration (whether or not staff are confident in each others’ work and expertise in relation to the new practice).
External facilitators had differing perceptions of their role. For EF1, it was viewed as encouraging staff to use theory to think through problems and conflict resolution, whereas for EF2 it was more practical: supporting the delivery of the intervention, providing clinical support and pointing staff in the direction of project-specific and external resources. Both viewed the role as bringing about service development through developing leaders. Although EFs worked largely independently because their sites started at different times, there was liaison to check consistency (EF2).
Both EFs commented on the internal facilitator role. EF1 was unclear how internal facilitators had been selected:
. . . I’m not sure for example whether the internal facilitators that I worked with are ones that had been selected for the role or put themselves forward, whether they’d just agreed to do it or whether they were told to do it you know . . . we actually didn’t, we weren’t involved in identifying how the internal facilitators would be recruited and worked out, our job was very much, I thought our job was as supporting them really and that perhaps is a limitation.
EF1
Furthermore, EF1 did not view their role as informing internal facilitators what their role was; this meant working with their own perceptions of the role. In one site this was problematic, as there was unwillingness to engage other members of the team, whereas in another site EF1 was successful in developing a team of facilitators, including an experienced HCA.
In terms of relationships between internal and EFs, for EF1 the key to managing this was flexibility and consistency in communication; EF2 did not comment on this specifically but suggested that the research nurse role was more important than her role in terms of keeping ICONS current with internal facilitators. In FF there was uncertainty about the role of the EF and how this fitted with the internal facilitator role; however, in LL the EF was viewed as a colleague with whom to discuss issues and problems.
Internal facilitators viewed their role as facilitating the smooth running of the trial (FF) and introducing the SVP (LL), taking the lead in supporting staff, ensuring they had the necessary knowledge and skills (FF and LL), ‘seeing the whole picture’ and making sure things were done; knowing the team helped in this regard (FF). In two sites the facilitator role was shared with the research nurse (HH) and staff nurses and HCAs (LL); this was perceived to be both useful and necessary as they were ‘hands on’ and better able to keep track compared with the ward manager. Consequently, there was little role change for the internal facilitator in HH:
although I was a facilitator I had another band 7 with me, she was absolutely exceptional, she was absolutely fantastic and she just really got on with the job, and in actual fact I think . . . I could say she probably took all of that role off me
IF-HH
Relationships with the members of the project team were cited as important by EF1:
there was certainly good relationships . . . developed with [name] and [name] you know which you know sort of enabled some discussion about these sorts of issues to be addressed in a . . . more positive and fruitful way.
EF1
Credibility was an important attribute of EFs (EF1), and knowledge and skills brought to the role included knowledge of leadership theory and the theory underpinning facilitation; expertise in supporting service development; and communication skills (EF1). For EF2 skills centred around practical experience, for example of tackling staffing issues and introducing early supported discharge. EF2 also mentioned interest in the staff and their units and an approachable manner as requisites.
Internal facilitators described the following knowledge and skills as necessary: knowledge of and rationale behind the project (HH and LL); leadership and management skills (FF); ability to ‘sell’ to the team (HH); practice development skills (HH); and the ability to assess what is important (LL). For FF, ICONS developed existing skills rather than teaching new ones. Skills of EFs were recognised, these included facilitation skills; the ability to ask the right questions and draw out solutions from internal facilitators; and the immediate understanding of problems (FF and LL).
In terms of skills development, internal facilitators in HH and LL pointed to a lack of training for the facilitator role; however, the internal facilitator at HH did not believe any additional knowledge was necessary.
EF2 questioned the nature of the internal facilitators themselves and if they were indeed the right people for the job.
Difficulties in engaging the organisation was highlighted by both the internal and EF at LL; although it was involved at the stage of organising funding, there were problems navigating the system to recruit HCAs leading to complex negotiations. In terms of internal facilitators, EF1 commented that they did not have designated time for their role.
Reflexive monitoring: the appraisal work that people do to assess and understand how a new practice affects them and others
Success of facilitation was assessed by both EFs through examining the extent of achievement of action plans:
solely through . . . the . . . action plans, whether action plans are being achieved or not, that was the framework that we were going to use . . . so . . . looking at you know whether the actions were resolving, if they weren’t resolving how could we . . . either look at barriers and enablers
EF1
Internal facilitators talked largely about success of the SVP, rather than facilitation; HH mentioned that staff feeling positive about what they were doing was a mechanism for judging success, but whether this was success of facilitation or implementation of the SVP was unclear.
Responses from internal facilitators focused on appraisal of the SVP rather than on facilitation, with the exception of HH, who did not view EF visits as helpful.
Issues raised by EFs included lack of involvement in the stage of recruiting internal facilitators,
we actually didn’t, we weren’t involved in identifying how the internal facilitators would be recruited and worked out, our job was very much, I thought our job was as supporting them really and that perhaps is a limitation.
EF1
increased demands from study sites, becoming diverted into helping resolve personality and resource issues (with suggested dilution of the facilitation intervention) (EF1) and lack of engagement of internal facilitators both in dialogue (e.g. e-mail correspondence) and in active use of action plans as a tool to implement facilitation. EF2 concluded that internal facilitators did not take full advantage of the support on offer:
I don’t think the teams used me as . . . well as they could have done you know I was willing to . . . give a lot more support . . . and . . . I think they yeah they . . . could have used me more effectively.
EF2
The internal facilitator at HH did not view supported implementation, or assistance from the EF, as successful:
I’d have been probably just as happy not to have had external facilitation.
HH-IF
She did, however, find the visit to another ICONS site ‘incredibly helpful’ in preparing her for the role of facilitator, and also valued the input of the research nurse highly in terms of sharing the internal facilitator role.
There was mention of the facilitation manual in LL:
I would say I was slightly shocked how big that was.
IF-LL
A greater focus on negotiating roles and relationships between internal and EFs was suggested by EF1, including involvement in recruiting internal facilitators; more attention to role definition and relationships between internal and EFs; and shared expectations of roles:
making sure that there is a clear framework that . . . provides a . . . common ground, absolutely crucial . . . being clear about expectations with all partners, that all partners are . . . you know have shared the same expectations of the role would be important, I think trying to get internal and EFs working together from, right up front would be really important.
EF1
Closer working with other EFs was also advocated in terms of sharing learning about which strategies are or are not working. Finally, EF1 suggested greater clarity was needed about what was actually being facilitated: ‘getting the trial up and running’ or ‘getting the intervention into practice’.
EF2 suggested practical solutions for strengthening links with internal facilitators:
what I could have done . . . in future rather than just e-mails is perhaps log book, and some we did do some by telephone, but whether you know you just have more of a weekly touching base.
EF2
EF2 also commented that facilitation visits should begin when the site is ready to start rather than (as happened in practice) 6 months beforehand:
we got all very excited and . . . dashed off and visited the units sort of in I think it was about July or something, and then actually we didn’t get started till . . . November, and then a couple of units . . . into the sort of January I think it was, so I think there was . . . an element of the . . . units had gone off the boil a bit really.
EF2
Suggestions from internal facilitators included simplification rather than using ‘big jargon’ (HH) and utilising a supernumerary, ward-based facilitator:
if we’d have had a ward based facilitator that was not in . . . the numbers and that was solely you know implementing this . . . research and what have you it would have . . . been implemented a lot more effectively, a lot more effectively, cos it was an extra role for us.
IF-LL
Barriers and facilitators to implementing facilitation are shown in Table 79.
| Barriers, difficulties | Facilitators, suggestions |
|---|---|
| Differentiation: can people see how the new practice differs? | |
| No change in role perceived as necessary | |
| Communal specification: do people agree with the new practice? | |
| View of external facilitation not shared between internal and EFs | |
| Introduction of peer networking as additional mechanism | |
| Individual specification: do people understand what the new practice requires of them? | |
| Uncertainty about what internal facilitator role would involve | |
| Internalisation: do people see the potential value of the new practice? | |
| No comments | |
| Initiation: who are the key individuals driving the new practice forward? | |
| Research nurse (experienced in change management) also worked in facilitator role | |
| Enrolment: do people agree that the new practice should be part of their work? | |
| Little correspondence by telephone or e-mail between internal and EFs | |
| Little discussion between internal facilitators within same site | |
| Legitimation: do people organise themselves to undertake the work required by the new practice? | |
| EFs kept up momentum through flexibility and willingness to help | |
| Internal facilitators adopted strategies to engage the organisation, nursing staff and MDT | |
| Facilitation handbook and action plans provided a focus for facilitation activity | |
| Activation: do people work together to build the procedures needed to sustain the new practice? | |
| Not enough EF visits | EF site visits |
| Little e-mail and telephone contact with EF in between visits | E-mail and telephone contact with EF in between visits |
| Interactional workability: can people do what the new practice requires? | |
| Time lag between initial visits and intervention starting | |
| Relational integration: are people confident in each other’s work and expertise? | |
| Internal facilitators uncertain about the role of the EF | EF role in bringing about service development through developing leaders |
| Development of team of internal facilitators | |
| Skill set workability: do people have the right skills and training? | |
| Internal facilitators may not have been right people | Skills of EFs recognised |
| Lack of training in for internal facilitators | |
| Is the new practice adequately supported and resourced? | |
| Internal facilitators not given designated time for the role | |
| Systematisation: can people determine the effects of the new practice? | |
| Success assessed through achievement of action plans | |
| Communal appraisal: do people agree about the worth of the new practice? | |
| EF visits not viewed as helpful | |
| Individual appraisal: do people think it is worth doing? | |
| Lack of involvement of EFs in internal facilitator recruitment | Visit by internal facilitators to another ICONS site good preparation for role |
| Unrealistic demands from study sites | |
| Lack of engagement of internal facilitators | |
| Reconfiguration: do people make changes to the new practice? | |
| Greater attention to negotiating roles of both internal and EFs | |
| Closer working between EFs | |
| Begin facilitation visits when site ready to begin implementation | |
Exploration of individual site rankings
In order to explain which processes potentially contributed to patient outcome, we examined pooled process data related to level of embedding and adherence. This was done at the level of the individual site (intervention groups only) rather than by trial arm, as heterogeneity across sites meant it would be misleading to provide an overall score by trial arm. Aggregated data for quantitative data on adherence to intervention protocols and qualitative data (organisational context assessed using soft systems analysis and embedding of the intervention in practice, together summarised in an ‘embedding’ score) are shown in Table 80; in all rankings 1 = best and 8 = worst. Both sites where the intervention was well embedded were in supported implementation (HH and KK), and there was only one site in this trial arm where the intervention was poorly embedded (LL). In contrast, no intervention sites had an embedding score of 1, with two showing conflicted or neutral and two poor embedding. Three sites with best adherence to the protocol were in the supported implementation trial arm.
| Embedding and adherence measures | Intervention | Supported implementation | ||||||
|---|---|---|---|---|---|---|---|---|
| Site AA | Site BB | Site CC | Site EE | Site FF | Site HH | Site KK | Site LL | |
| Embedding score (soft systems and NPT analysis) | 2 | 3 | 3 | 2 | 2 | 1 | 1 | 3 |
| 3-day diary: number with entry on all 3 days | 6 | 5 | 4 | 7 | 8 | 3 | 2 | 1 |
| Daily clinical logs | 6 | 4 | 8 | 3 | 5 | 1 | 2 | 7 |
| Number of patients catheterised in acute stage | 5 | 4 | 2 | 8 | 7 | 6 | 1 | 3 |
| Time to catheter removal | 4 | 6 | 1 | 7 | 3 | 2 | 8 | 5 |
| Number of patients catheterised at baseline and at discharge | 6 | 5 | 1 | 8 | 7 | 4 | 1 | 3 |
| Number of eligible patients put on regime | 3 | 6 | 8 | 4 | 5 | 7 | 1 | 2 |
| Length of time from last day of 3-day diary to regime commencing | 2 | 5 | 8 | 3 | 6 | 7 | 1 | 4 |
| Allocation to correct regime | 6 | 5 | 4 | 3 | 7 | 1 | 2 | 8 |
| Combined ranking | 5 | 6 | 4 | 7 | 8 | 2 | 1 | 3 |
Chapter 9 Economic analysis
Study design
Study question
Background
The potential effectiveness of the ICONS SVP has been explored and described (see Chapter 7). In order to obtain a more complete picture of whether or not this voiding programme is worth implementing in practice, it is necessary to understand the potential effect on costs as well as outcomes. There is currently a lack of economic evaluations in the area of continence management after stroke. In this chapter the cost of delivering the intervention (a SVP; hereafter, the programme) will be explored and described, as well as the impact of the programme on subsequent health and social service costs. Obtaining data on costs requires the development of methods to record resource use in a variety of settings and from a range of sources. Therefore, this chapter is also concerned with exploring the feasibility of the methods for collection of cost data. The results from this chapter are aimed at informing the approach to data collection in a definitive trial.
Study aims and objectives
Develop and test data collection methods for an economic evaluation within a full-scale cluster randomised trial.
-
develop data collection methods based on discussion with staff and patients
-
explore completeness of the data
-
identify if there are areas of resource use relevant to the programme not covered by the data collection
-
describe the costs associated with the ICONS SVP
-
explore the data for evidence of potential cost-effectiveness
-
identify lessons learned to inform a definitive clinical trial.
Perspective
The analysis follows the current technology appraisal guidelines used by the NICE and so will take the perspective of the UK NHS and Personal Social Services (PSS). 177
Selection of alternatives
Current treatment
As outlined in the introduction (see Chapter 1), UI following acute stroke is common, affecting between 40% and 60% of people in hospital after a stroke. 16 Despite the availability of clinical guidelines for the management of UI after stroke,10 national audit data11 suggest incontinence is often poorly managed. Current usual care does not necessarily include a continence management plan (63% patients had one in the latest Sentinel audit11) so although there are recommendations in guidelines, such recommendations are not systematically implemented in practice. Often, the aim of usual care for incontinence involves containment rather than developing a proactive plan to promote continence. The programme is described in detail elsewhere (see Chapter 3) and focuses on those conservative strategies shown to have some effect with participants in studies included in Cochrane systematic reviews,25,27,29,53,54 but which had not had their effectiveness demonstrated with stroke patients. These strategies included BT and PV. We also evaluated whether or not supported implementation, through targeted organisational development aimed at ‘normalising’ the programme,55–58 showed more preliminary evidence of effectiveness than introduction of the programme alone. The programme aimed to develop, implement and evaluate the preliminary clinical effectiveness and cost-effectiveness of a SVP, with or without supported implementation, for the management of UI after stroke in secondary care as compared with usual care.
Form of evaluation
In order to explore the potential range of benefits of the SVP, we are considering cost–utility analyses and cost-effectiveness approaches. The cost–utility analysis will assess gains in quality-adjusted life-years estimated from the EQ-5D. 166 Although such a cost–utility analysis will allow an exploration of the impact of the programme on QoL, the generic nature of the assessment means that it may not be an outcome that meaningfully reflects the impact of programme to stroke patients. Consequently, we will perform a cost-effectiveness analysis by assessing urinary frequency and exploring symptom-free days: this will be estimated using the first question from the ISI151 – for the purpose of this study, this question also had an option of ‘never,’ and the first question from the ICIQ-SF. 162
Data collection
Efficacy data
The efficacy data used in the analyses are those from the exploratory trial reported elsewhere (see Chapter 7). Measures of efficacy will be those recorded at 52 weeks.
Benefit measurement and valuation
Two measures of health outcome have been used: quality-adjusted life-years gained and symptom-free days.
Quality of Life
The EQ-5D,166 a generic QoL measure, which measures health on five items (mobility; self-care; usual activities; pain and discomfort; anxiety and depression) was recorded on the outcome questionnaire. It is being used to facilitate comparison with other diseases and interventions and will allow us to estimate the potential benefit of the programme in terms of quality-adjusted life-years gained using the UK tariff value,178 and subsequently estimate a cost per quality-adjusted life-year gained. Calculation of the tariff and subsequent adjustment of the outcome quality-adjusted life-year for baseline value179 is described below. However, it has been suggested that the EQ-5D has a large ceiling effect and poor responsiveness in a non-stroke sample of women with UI. 180 We therefore also used a condition-specific measure, the incontinence-specific QoL instrument I-QOL,164,165 which has been shown to be the best continence-specific measure for use in clinical trials in terms of reliability, validity and responsiveness to change. 180 However, the I-QOL has been validated only for those who are incontinent. We aim to explore the association between the I-QOL and the utility values calculated from the EQ-5D using Spearman’s rank-order coefficient, overall and by trial arm.
Quality-adjusted life-year calculation and adjustment
For each of the five items in the EQ-5D there are three levels: no problems, some problems and extreme problems. Each EQ-5D health state obtained from the EQ-5D descriptive system was converted in to a single summary index – the tariff. The tariff was calculated using a formula that attaches weights to each of the three levels in each of the five dimensions. Deducting the appropriate weights from the value of 1 which indicates full health (i.e. state 11111) will produce a corresponding index for this health state.
The weights used in the UK are given in Table 81. For participants who are of full health (i.e. state 11111), no weight is deducted (including the morbidity constant of 0.081), hence giving a tariff of 1. For all other health states, a morbidity constant of 0.081 is also deducted in addition to appropriate weights. Finally, for participants who have answered level 3 to at least one of the five dimensions (i.e. state 11133) a further weight of 0.269 is also deducted in addition to the appropriate weights. The quality-adjusted life-years calculated for each participant were based on the tariff value computed from their response to the EQ-5D at 52 weeks.
| Attribute | Level | Decrement |
|---|---|---|
| Constant | –0.081 | |
| Mobility | No problems (1) | 0 |
| Some problems (2) | –0.069 | |
| Confined to bed (3) | –0.314 | |
| Self-care | No problems (1) | 0 |
| Some problems (2) | –0.104 | |
| Unable to (3) | –0.214 | |
| Usual activities | No problems (1) | 0 |
| Some problems (2) | –0.036 | |
| Unable to (3) | –0.094 | |
| Pain/discomfort | None (1) | 0 |
| Moderate (2) | –0.123 | |
| Extreme (3) | –0.386 | |
| Anxiety/depression | None (1) | 0 |
| Moderate (2) | –0.071 | |
| Extreme (3) | –0.236 | |
| N3 (level 3 at least once) | –0.269 |
A mixed model was used to control for baseline utility. The following equation was applied:
where i is the patient identifier (i = 1, 2, . . . , N), ti is the trial arm (1 = usual care, 2 = intervention, 3 = supported implementation), Oi is the OCSP classification, Ei is the Edinburgh case-mix and Qib is the baseline utility value.
Symptom-free days
The symptom-free days were based on the response to the ISI questionnaire and the ICIQ-UI. With regards to frequency of incontinence, the ISI questionnaire has four levels, and the numbers of symptom-free days assigned to each of these levels were 28 ‘less than once a month;’ 24 ‘one or several times a month’; 20 ‘one or several times a week’; and 0 ‘every day and/or night’. For the purpose of this study, because the outcome questionnaire was being sent to people who may not have incontinence, we included a response option of ‘never’ which was assigned a value of ‘0’. The ICIQ-UI has six levels, which were assigned the values 28 ‘never’; 24 ‘about once a week or less often’; 18 ‘two or three times a week’; 4 ‘about once a day’; 0 ‘several times a day’; 0 ‘all the time’. Owing to minor inconsistencies in responses to the two questionnaires we developed a contingency table where we estimated the number of symptom-free days based on the scores on the two questionnaires (Table 82). Twenty-eight was used as a maximum value because it reflects 4 weeks.
| ISI response | ICIQ-UI response | |||||
|---|---|---|---|---|---|---|
| Never | About once a week or less often | Two or three times a week | About once a day | Several times a day | All the time | |
| Nevera | 28.0 | 26.0 | 23.0 | 16.0 | 14.0 | 14.0 |
| Less than once a month | 27.5 | 25.5 | 22.5 | 15.5 | 13.5 | 13.5 |
| One or several times a month | 26.0 | 24.0 | 21.0 | 14.0 | 12.0 | 12.0 |
| One or several times a week | 24.0 | 22.0 | 19.0 | 12.0 | 10.0 | 10.0 |
| Every day and/or night | 14.0 | 12.0 | 9.0 | 2.0 | 0.0 | 0.0 |
Assessment of feasibility
For each of the different data collection methods we recorded response rates. The postal questionnaire had a section at the back where respondents could add comments about the questionnaire. The admission data recorded were collected from both the postal questionnaire and the individual centres. The data from the two sources were compared using Cohen’s kappa statistic. 181
Costing
Seven areas of resource use were identified from hospital: (i) research staff providing training around the programme, staff receiving training [(ii) ward staff and (iii) internal facilitators]; (iv) EFs; (v) staff performing the programme; and after discharge, (vi) community health and social service input; (vii) admissions to hospital, subsequent to the admission for the stroke. For each of these areas, data collection forms were constructed to record data.
In-hospital resources
Staff training: ward staff
Training was provided by the ICONS research team, which allowed an estimate of time spent in delivering and receiving training. Hospital staff were asked to provide details on the amount of time spent on the online training.
Staff training: internal facilitators
For those staff acting as internal facilitators, we asked them to estimate time spent being trained by the EFs and the time they subsequently spent on internal facilitation.
External facilitators
The EFs were asked to record the number of site visits and travel costs.
Staff performing the programme
It was originally intended that staff would be asked to record the amount of time they spent performing the programme on a case-by-case basis. However, following initial discussions with hospital sites, it was felt that there was a danger of overburdening ward staff with paperwork, the consequences of which might have diluted provision of the programme: because the programme itself requires completion of paperwork (see Chapter 6, Delivery of the intervention to individuals). As a result it was decided to make estimates of the likely resource required for the programme by asking each of the 12 centres about the staff input required for toileting a patient. At each centre a member of the senior ward staff was asked to complete a paper questionnaire, which asked them to consider for a single occasion of toileting a patient, the method of toileting (toilet, commode, bed-pan), number of staff involved in the toilet process, whether or not this number of staff would be involved throughout the process and the levels (bands) of staff involved. The staff member was asked to consider the input required for four different types of patients, based on differing levels of dependency. The four levels were based around the Barthel149 transfer item: independent; transfers with the help/supervision of one; needs help of two people; bed-bound. We asked staff to make the estimates based on their experience/knowledge of the toileting process and not specifically in relation to the ICONS programme. Questionnaires were received from eight of the sites and an average figure for time spent and staff involved in toileting each type of patient on a single occasion was estimated.
The nature of the programme means that patients are toileted on multiple occasions throughout the day. The number of occasions is dependent on the regime interval recorded on the daily clinical logs (see Chapter 6). As it was not possible to identify the actual number of occasions every patient was toileted on a case-by-case basis, we used the sample of daily clinical logs (described in Chapter 8) to make estimates of the likely daily number of toileting occasions for each patient receiving the programme. Where we had daily clinical logs for a patient, the average number of occasions they were toileted each day was based on the average of all their available daily clinical logs. For all other patients, we based the number of occasions that patients were toileted each day on the average calculated from all of the daily clinical logs available at that site. This daily average was then multiplied by the number of days each person was known to have received the programme (or ‘time spent on the regime’, see Chapter 6) to estimate the total number of toileting episodes per patient.
Post-hospital resources
Community health and social service input
A postal questionnaire designed for self-completion was sent to patients and carers. The postal questionnaire was based on a design used previously by the applicants when querying input after discharge in a cohort of stroke patients. 182 The questionnaire was developed further for ease of use by patients and carers through discussion with the ICONS PPC groups. So as not to overburden patients, at 12 weeks the resource use questionnaire was sent out 2 weeks after the outcome questionnaire. Therefore, this resource use questionnaire was sent out at 14-weeks post stroke: to maintain consistency between the resource use and outcome questionnaires, this resource use questionnaire will still be referred to as 12 weeks. At 14 weeks, questionnaires were only sent to patients discharged by week 12, because the questionnaire was designed to record resource use in the community, i.e. patients had to have been in the community for at least 2 weeks to merit sending out a questionnaire. If patients did not return their questionnaire within 2 weeks a further questionnaire was sent out. If there was still no response, the patient would be contacted by telephone. The postal questionnaire recorded use of the following resources: general practitioner (GP) contacts, practice nurse contacts, number of admissions to hospital (subsequent to the index admission), physiotherapy, occupational therapy, chiropody, district nurse, home care (home help), continence advisor, stroke family support worker and day centre visits. Patients living in non-residential care also provided information about aids and adaptations to their residence: walking stick, wheelchair, hoist (or similar), height adjustable bed, mattress cover, chair raiser, toilet seat raiser, bedpan/urinal, commode, toilet rails, adapted bath/shower, alarm call. In addition, patients had the option to list any other aids not covered in the list above.
Admissions
In addition to obtaining the number of admissions from patients, centres were asked to send details of any patient admissions to hospital, which were subsequent to the index admission, up to 52 weeks. Due to a pre-planned process (and consequently allocation of resources) this data retrieval was only possible for those patients who should have completed the 52 weeks resource use questionnaire by 19 November 2012. The dates and reasons for the admissions were recorded. If the patient only attended the A&E department, the date and reason were recorded. If an admission was elective it was excluded from the analysis. The hospital data were considered to be ‘correct’, but not all hospitals provided data. Comparisons were made between patient-reported readmissions and hospital data and agreement was explored using the kappa statistic. In order to keep the estimates of costs for readmissions simple, readmissions were split into two types: short stay (an admission for 1 night) and long stay (an admission of ≥ 2 nights).
Currency and price data
Unit costs were obtained from national data,183 Lancashire Teaching Hospitals’ Aids and Adaptations Catalogue184 or Boots online. 185 All costs are reported in pounds sterling at 2011/12 prices, unit costs can be seen in Table 83.
| Item of resource use | Cost (£) | Source |
|---|---|---|
| Post discharge | ||
| Health care | ||
| GP | 52.00 | Curtis (2012)183 |
| Practice nurse | 14.00 | Curtis (2012)183 |
| Physiotherapy | 33.00 | Curtis (2012)183 |
| Occupational therapy | 33.00 | Curtis (2012)183 |
| District nurse | 70.00 | Curtis (2012)183 |
| Continence advisora | 49.00 | Curtis (2012)183 |
| Chiropody | 30.00 | Curtis (2012)183 |
| Social care | ||
| Home care | 23.00 | Curtis (2012)183 |
| Stroke family support worker | 29.00 | Curtis (2012)183 |
| Day centre visits | 40.00 | Curtis (2012)183 |
| Aids and adaptations | ||
| Walking stick | 6.70 | LTHTR184 |
| Wheelchair | 172.00 | Curtis (2012)183 |
| Hoist (or similar) | 319.00 | Curtis (2012)183 |
| Height adjustable bedb | 27.53 | LTHTR184 |
| Mattress cover | 48.90 | Boots185 |
| Chair raiser | 21.62 | LTHTR184 |
| Toilet seat raiser | 15.00 | LTHTR184 |
| Bedpan/urinal | 6.50 | LTHTR184 |
| Commode | 25.00 | LTHTR184 |
| Toilet rails | 6.00 | Curtis (2012)183 |
| Adapted bath/shower | 407.00 | Curtis (2012)183 |
| Alarm call | 54.00 | Curtis (2012)183 |
| A&E attendance (not admitted) | 130.00 | Curtis (2012)183 |
| Admissions to hospital | ||
| Short stay | 586.00 | Curtis (2012)183 |
| Long stay | 2461.00 | Curtis (2012)183 |
Performing the programme
In order to value the cost of the time performing the programme we made estimates of the cost for a minute of staff time, which could then be multiplied up as necessary. The costs for different bands of staff were based on the Pay-Circular (AforC) 2/2011. 186 Adjustments were made for on-costs and overheads and a cost per minute was calculated (Table 84).
| Level | Point | Basic (£) | On-costs (£)a | Overheads (staff) (£)b | Overheads (non-staff) (£)c | Cost per annum (£) | Hours per annumd | Cost per minute (£) |
|---|---|---|---|---|---|---|---|---|
| Band 2 | 5 | 15,444 | 3707 | 3089 | 6178 | 28,417 | 1593 | 0.30 |
| Band 3 | 9 | 17,368 | 4168 | 3474 | 6947 | 31,957 | 1593 | 0.33 |
| Band 4 | 14 | 20,183 | 4844 | 4037 | 8073 | 37,137 | 1593 | 0.39 |
| Band 5 | 20 | 24,554 | 5893 | 4911 | 9822 | 45,179 | 1573 | 0.48 |
| Band 6 | 25 | 29,464 | 7071 | 5893 | 11,786 | 54,214 | 1573 | 0.57 |
| Band 7 | 30 | 35,184 | 8444 | 7037 | 14,074 | 64,739 | 1573 | 0.69 |
The cost per minute was then combined with the estimates of time taken to toilet a patient (see Appendix 30) to calculate a cost to toilet the different patient types (Table 85).
| Item of resource use (in hospital) | Cost (£) | Reference |
|---|---|---|
| An occasion of toileting patient types | ||
| Independent | 2.19 | See Appendix 30 |
| Transfers with the help/supervision of one | 4.49 | See Appendix 30 |
| Needs help of two people | 9.65 | See Appendix 30 |
| Bed-bound | 12.84 | See Appendix 30 |
Cost of training
The cost of the training was based on staff time in delivering the training and the cost of staff time undertaking the training. The unit costs related to delivering and receiving training can be found in Table 86. As the initial training was only provided in 30-minute sessions, an hourly rate was used as a basis from which to calculate the costs of delivering and receiving the training. For staff who were being trained to be internal facilitators an hourly rate was used. The structure of the process for delivery of facilitator training meant that the EFs were costed at a daily rate. One of the EFs was paid on a consultancy basis, whereas for the other EF, a daily rate was calculated from the original application.
| Item of resource use | Cost (£) | Reference |
|---|---|---|
| Delivery of training | ||
| Staff trainer 1 | 34 | From application |
| Staff trainer 2 | 28 | From application |
| EF 1 | 379a | From application |
| EF 2 | 500a | Consultancy rate |
| Research nurse | 25 | From application |
| Travel (delivery of training only) | 45b | Average per site |
| Receipt of training | ||
| Ward managers/sisters | 58 | Curtis (2012)183 |
| Ward sister | 50 | Curtis (2012)183 |
| Staff nurse | 41 | Curtis (2012)183 |
| Research nurse | 25 | From application |
| HCA | 21 | Curtis (2012)183 |
| Physiotherapists | 34 | Curtis (2012)183 |
| Occupational therapists | 34 | Curtis (2012)183 |
| Assistant practitioners | 22 | Curtis (2012)183 |
The uptake of training at the eight sites (intervention and supported implementation) is described elsewhere in the report as part of the process evaluation (see Chapter 8). As the cost of training staff is dependent on the number of staff in a particular centre it was felt that estimating an average cost across all centres would better reflect the likely cost of the (training part of the) programme. The numbers of staff ranged from 13 to 30, the type of staff included nurses and therapists. Details of costs attributed to different staff types attending the training can be found in Appendix 31. Staff spent up to 2 hours (usually four 30-minute sessions) undertaking face-to-face training with ICONS senior research staff. In addition, staff also had the opportunity to undertake online training: no data in relation to the uptake of the online training were available. We therefore made the assumption that the staff attending the face-to-face training would also spend 1 hour performing the online training. The average cost of face-to-face training per centre with regards to ward staff receiving training was estimated to be £1550 (see Appendix 31). This is then added to the estimated cost of online training (£775 and the sum is multiplied by four to produce a cost for the trial arm, approximately £9300 [see Table 86 (includes rounding error)]. The cost of all aspects of training staff in the different trial arms can be found in Table 87. Facilitation, internal and external, added £9642 to cost of the supported implementation arm (including travel). In calculating the mean cost per patient we used the annual number of patients admitted with an acute stroke and who would have been eligible for the programme as the denominator because it was felt that this would better represent the cost of training in practice rather than base it on the numbers in the trial arms, which is more of a reflection of the research process.
| Resource | Usual care (£) | Intervention (£) | Supported implementation (£) |
|---|---|---|---|
| Training development | 0 | 1770 | 1770 |
| Delivering training to hospital staff | |||
| Staff costs | 0 | 933 | 933 |
| Travel | 0 | 180 | 180 |
| Hospital staff receiving training (including online) | 0 | 9302 | 9302 |
| Internal facilitators (being trained and providing training) | 0 | 0 | 3922 |
| EFs | |||
| Staff costs | 0 | 0 | 4653 |
| Travel | 0 | 0 | 1067 |
| Staff training total | 0 | 12,185 | 21,828 |
| Mean cost per patient of staff training | 0 | 13 | 25 |
Cost of the systematic voiding programme
The estimated input required for the SVP and the associated cost can be seen in Table 88. The table shows, by trial arm and for each patient type, the number of patients in that category and the estimated average number of occasions that patients of that type were toileted as part of the programme. SVP had a higher cost in the supported implementation arm, which was due to the high number of occasions of toileting bed-bound patients.
| Resource | Intervention | Supported implementation | ||||
|---|---|---|---|---|---|---|
| n a | Occasionsb | Cost (£) | n | Occasions | Cost (£) | |
| Patient type | ||||||
| Independent | 6 | 233 | 510 | 7 | 145 | 317 |
| Transfers with the help/supervision of one | 22 | 124 | 557 | 10 | 121 | 541 |
| Needs help of two people | 60 | 184 | 1775 | 52 | 192 | 1854 |
| Bed-bound | 13 | 159 | 2045 | 10 | 300 | 3857 |
| Overall mean | 171 | 1469 | 193 | 1805 | ||
Analysis and interpretation of results
Adjustments for timing of costs and benefits
The cost of stroke tends to be higher in the acute stage, when patients are cared for in hospital: these costs are borne by the NHS. Once care is transferred to the community, there are costs for the NHS, generally through GP contacts and subsequent hospital admissions and costs for PSS, through social support and residential care. On the whole, the cost of stroke tends to lessen as time since the event lengthens. Any intervention in the acute stage such as the ICONS SVP will incur costs early after the stroke event. Any benefits of this programme are more likely to be seen after this initial stage. For a definitive trial it would be important to consider resource use and measures of effectiveness beyond the acute stage. Consequently, one of the aims of this exploratory trial is to consider longer-term data capture. In addition, this trial aims to explore evidence of potential cost-effectiveness. Consequently, for the purpose of this exploratory trial the time horizon for the cost analysis will be from admission to the stroke unit to 52 weeks post stroke. All health and social care resources are included in the analysis because it is unclear which ones incontinence might affect. As a result of this 1-year time horizon, no discounting will be performed.
Allowance for uncertainty
Resource use and cost data are described separately for each trial arm and described using means and SDs, or numbers and percentages where appropriate. The outcome effectiveness data can be found in full elsewhere in the report (see Chapter 7) and are summarised in the Results section of this chapter.
Resource use data are described in three ways.
Complete cases
Data are described using all data available at each of 12 and 52 weeks.
Base case
For the base-case analysis we report data for all patients. We used the data from the 52-week resource use questionnaires when available and for admissions to hospital we used the data from the hospitals when it had been supplied. If data were missing from either source, we performed imputations. The method of imputation was as follows: the average usage for each of the post-hospital resources was estimated from the available data; this average figure was estimated separately for stroke types, based on the OCSP classification (see Chapter 5). This method was chosen because it was assumed that resource use was likely to be affected by severity of stroke. The average value calculated for each resource within a stroke type was imputed for the missing values within that resource.
Sensitivity analyses
From the resource use data in the base case we used the lower and upper limits of the 95% CIs to explore the potential range of resources used. For the training, we did not have CIs and so we varied the cost of the training by ± 25%. From the outcome data we used the lower and upper limits of the 95% CIs to explore the potential range of effects.
Results
Assessment of feasibility (completeness of data)
For the requests to provide estimates of how long it would take to toilet a patient, 8 out of 12 (66.7%) sites provided a response (usual care, n = 2; intervention, n = 3; supported implementation, n = 3). Admission data were provided by 9 out of 12 (75%) sites (usual care, n = 2; intervention, n = 4; supported implementation, n = 3). One usual care site and one intervention site provided no data to either request.
Postal questionnaires: resource use
At 12 weeks, post-hospital resource use data were collected for 193 out of 263 (73.4%) patients eligible to be sent a postal questionnaire. Table 89 shows the number of responses in each of the trial arms. One-hundred and fifty patients were not contacted regarding resource use because of withdrawal (n = 9), death (n = 45) or still being in hospital due to the index stroke (n = 96). Considering eligible patients only, the proportion of respondents was similar across all three trial arms (between 72% and 75%).
| Record status at 12 weeks | Usual care (n = 124) | Intervention (n = 164) | Supported implementation (n = 125) | Overall (n = 413) |
|---|---|---|---|---|
| Missing: lost to follow-up at this time point | 22 (17.7%) | 24 (14.6%) | 19 (15.2%) | 65 (15.7%) |
| Withdrawn | 1 (0.8%) | 4 (2.4%) | 4 (3.2%) | 9 (2.2%) |
| Dead | 13 (10.5%) | 21 (12.8%) | 11 (8.8%) | 45 (10.9%) |
| Received and entered | 57 (46%) | 83 (50.6%) | 53 (42.4%) | 193 (46.7%) |
| Missing lost to study | 0 | 5 (3.1%) | 0 | 5 (1.2%) |
| In other hospital (from ICONS) | 31 (25%) | 27 (16.5%) | 38 (30.4%) | 96 (23.3%) |
The completion rates of the individual items within the 12-week postal questionnaires are shown in Tables 90 and 91. The health and social care items (see Table 90) had an overall completion rate of 95%; for the individual items the completion rates were above 90%, with some items being completed by all participants. For most of the items, the completion rate in the usual care arm tended to be the highest, with the intervention arm generally completing a higher proportion of items than the supported implementation arm.
| Resource | Usual care (n = 57) | Intervention (n = 83) | Supported implementation (n = 53) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Health-care contacts | ||||||
| GP | 55 | 96.5 | 80 | 96.4 | 49 | 92.5 |
| Practice nurse | 56 | 98.2 | 81 | 97.6 | 48 | 90.6 |
| Physiotherapy | 54 | 94.7 | 78 | 94.0 | 49 | 92.5 |
| Occupational therapy | 56 | 98.2 | 76 | 91.6 | 51 | 96.2 |
| District nurse | 53 | 93.0 | 80 | 96.4 | 50 | 94.3 |
| Continence advisor | 55 | 96.5 | 80 | 96.4 | 49 | 92.5 |
| Chiropody | 56 | 98.2 | 81 | 97.6 | 49 | 92.5 |
| Social care contacts | ||||||
| Home carea | 36 | 97.3 | 46 | 97.9 | 32 | 94.1 |
| Stroke family support worker | 56 | 98.2 | 78 | 94.0 | 49 | 92.5 |
| Day centre visitsa | 36 | 97.3 | 47 | 100 | 33 | 97.1 |
| Hospital admissions | 57 | 100.0 | 75 | 90.4 | 50 | 94.3 |
| Overall mean | 97.1 | 95.7 | 93.6 | |||
| Resource | Usual care (n = 37) | Intervention (n = 47) | Supported implementation (n = 34) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Aids and adaptations | ||||||
| Walking stick | 36 | 97.3 | 43 | 91.5 | 31 | 91.2 |
| Wheelchair | 31 | 83.8 | 44 | 93.6 | 30 | 88.2 |
| Hoist (or similar) | 31 | 83.8 | 41 | 87.2 | 25 | 73.5 |
| Height adjustable bed | 31 | 83.8 | 41 | 87.2 | 25 | 73.5 |
| Mattress cover | 31 | 83.8 | 41 | 87.2 | 25 | 73.5 |
| Chair raiser | 33 | 89.2 | 40 | 85.1 | 26 | 76.5 |
| Toilet seat raiser | 33 | 89.2 | 42 | 89.4 | 30 | 88.2 |
| Bedpan/urinal | 29 | 78.4 | 42 | 89.4 | 24 | 70.6 |
| Commode | 33 | 89.2 | 44 | 93.6 | 27 | 79.4 |
| Toilet rails | 33 | 89.2 | 41 | 87.2 | 29 | 85.3 |
| Adapted bath/shower | 34 | 91.9 | 42 | 89.4 | 30 | 88.2 |
| Alarm call | 35 | 94.6 | 41 | 87.2 | 27 | 79.4 |
| Overall mean | 87.9 | 89.0 | 806.0 | |||
For the aids and adaptations (see Table 91), the overall response rate to the items in the 12-week postal questionnaire was 85.8%. There was some variability in the completion rates, which ranged from 70.6% up to 97.3%. Completion rates for the usual care and intervention arms were similar, with the supported implementation arm generally having a lower proportion of responses.
At 52 weeks, post-hospital resource use data were collected for 167 out of 299 (55.9%) patients eligible to be sent a postal questionnaire. Table 92 shows the number of responses in each of the trial arms. One-hundred and fourteen patients were not contacted regarding resource use because of withdrawal (n = 16) and death (n = 98). Considering those patients eligible to be sent a postal questionnaire, a similar proportion responded in each trial arm [50/91 (54.9%) usual care; 66/118 (55.9%) intervention; and 51/90 (56.7%) supported implementation].
| Record status at 52 weeks | Usual care (n = 124) | Intervention (n = 164) | Supported implementation (n = 125) | Overall (n = 413) |
|---|---|---|---|---|
| Missing: lost to follow-up at this time point | 37 (29.8%) | 46 (28.0%) | 34 (27.2%) | 117 (28.3%) |
| Withdrawn | 4 (3.2%) | 6 (3.7%) | 6 (4.8%) | 16 (3.9%) |
| Dead | 29 (23.4%) | 40 (24.4%) | 29 (23.2%) | 98 (23.7%) |
| Received and entered | 50 (40.3%) | 66 (40.2%) | 51 (40.8%) | 167 (40.4%) |
| Missing lost to study | 4 (3.2%) | 6 (3.7%) | 5 (4.0%) | 15 (3.6%) |
The completion rates of the individual items within the 52-week postal questionnaires are shown in Tables 93 and 94. For the health and social care items (see Table 93), the overall completion rate was 95.3%, with only a modest variation between items, ranging from 87.8% to 100.0%. The intervention arm showed the most variability in completion of items. The supported implementation arm had, fractionally the highest response rate.
| Resource | Usual care (n = 50) | Intervention (n = 66) | Supported implementation (n = 51) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Health-care contacts | ||||||
| GP | 47 | 94.0 | 66 | 100.0 | 51 | 100.0 |
| Practice nurse | 47 | 94.0 | 64 | 97.0 | 50 | 98.0 |
| Physiotherapy | 49 | 98.0 | 64 | 97.0 | 50 | 98.0 |
| Occupational therapy | 46 | 92.0 | 63 | 95.5 | 49 | 96.1 |
| District nurse | 48 | 96.0 | 64 | 97.0 | 50 | 98.0 |
| Continence advisor | 46 | 92.0 | 62 | 93.9 | 48 | 94.1 |
| Chiropody | 49 | 98.0 | 64 | 97.0 | 50 | 98.0 |
| Social care contacts | ||||||
| Home carea | 34 | 97.1 | 40 | 97.6 | 34 | 94.4 |
| Stroke family support worker | 46 | 92.0 | 62 | 93.9 | 47 | 92.2 |
| Day centre visitsa | 33 | 94.3 | 36 | 87.8 | 33 | 91.7 |
| Hospital admissions | 46 | 92.0 | 63 | 95.5 | 47 | 92.2 |
| Overall mean | 94.5 | 95.6 | 95.7 | |||
| Resource | Usual care (n = 35) | Intervention (n = 41) | Supported implementation (n = 36) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Aids and adaptations | ||||||
| Walking stick | 33 | 94.3 | 37 | 90.2 | 32 | 88.9 |
| Wheelchair | 31 | 88.6 | 40 | 97.6 | 32 | 88.9 |
| Hoist (or similar) | 27 | 77.1 | 38 | 92.7 | 26 | 72.2 |
| Height adjustable bed | 27 | 77.1 | 38 | 92.7 | 30 | 83.3 |
| Mattress cover | 27 | 77.1 | 38 | 92.7 | 30 | 83.3 |
| Chair raiser | 31 | 88.6 | 38 | 92.7 | 30 | 83.3 |
| Toilet seat raiser | 30 | 85.7 | 35 | 85.4 | 31 | 86.1 |
| Bedpan/urinal | 26 | 74.3 | 34 | 82.9 | 28 | 77.8 |
| Commode | 30 | 85.7 | 40 | 97.6 | 31 | 86.1 |
| Toilet rails | 32 | 91.4 | 34 | 82.9 | 30 | 83.3 |
| Adapted bath/shower | 30 | 85.7 | 34 | 82.9 | 31 | 86.1 |
| Alarm call | 29 | 82.9 | 35 | 85.4 | 29 | 80.6 |
| Overall mean | 84.0 | 89.6 | 83.3 | |||
For the aids and adaptations (see Table 94), the overall response rate to items in the 52-week postal questionnaire was 85.6%. There was a wide range of completion rates for the individual items, ranging from 72.2% up to 97.6%. On average, the completion rate for the intervention arm was highest at 89.6%, with the supported implementation arm having an average completion rate of 83.3%.
Of the 12- and 52 week questionnaires, 9 out of 193 (4.7%) and 12 out of 167 (7.2%), respectively, had comments written in the free-text box. Most of the comments were either general statements around care input or related to descriptions of difficulties faced by the patient and their families following the stroke. There were five comments that were relevant to completion of the form and can be seen below:
I am an Age UK Community Officer and I took 30 minutes to complete this form (asking client and son the questions) so 10 minutes to complete this is an unreasonable estimate either by client or with help from family/Age UK.
No, I have no problems completing these forms they are self-explanatory.
Stapling double-sided forms together makes it very difficult to complete all the forms fully.
This form took at least an hour to complete. Fortunately doctors/therapist appointments were marked on a calendar so dates are accurate – other points have to be recalled which all take time.
Well set out. Covers all aspects of having had a stroke. Thank you.
A further five comments indicated the potential problem of using a postal questionnaire to gather information in this client group:
A lot of the questions appeared to presume that I had returned home to a domestic setting – they do not really cater for people who do not fit that box. However, I have tried to give as much information as possible to assist your project. Thank you.
Quite a number of the questions are not relevant for someone who has no communication and as his wife I also can’t answer them on his behalf.
Sorry some answers are vague but they are not relevant to this lady.
. . . cannot communicate adequately to complete this form. Filled in by RGN [registered general nurse] to best ability.
. . . was unable to complete this survey – hence, help was provided.
Postal questionnaires: outcome data
The response rates for the outcome variables relevant to economic analyses are presented in Table 95. At 12 weeks the response rates for the EQ-5D items were all above 90%. The participants in the usual care arm tended to have higher completion rates. We were able to estimate symptom-free days for approximately 80% of participants, with similar numbers responding in each of the trial arms. The responses to the I-QOL at 12 weeks was variable across trial arms, with only a 59.3% response rate in the supported implementation arm compared with 91.1% in usual care. At 52 weeks the completion rate for the EQ-5D items was again high, with a > 94% response rate for all items in all of the trial arms. We were able to estimate symptom-free days in nearly 100% of patients. The completion rate of the I-QOL was, overall, lower than at 12 weeks with less variability across trial arms, ranging from 54.5% (supported implementation) to 65.4% (usual care).
| Outcome | Usual care | Intervention | Supported implementation | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Questionnaires returned (12 weeks) | n = 98 | n = 132 | n = 100 | |||
| EQ-5D (12 weeks) | ||||||
| Mobility | 96 | 98.0 | 129 | 97.7 | 92 | 92.0 |
| Self-care | 97 | 99.0 | 126 | 95.5 | 92 | 92.0 |
| Usual activity | 97 | 99.0 | 126 | 95.5 | 91 | 91.0 |
| Pain | 95 | 96.9 | 123 | 93.2 | 93 | 93.0 |
| Anxiety | 95 | 96.9 | 122 | 92.4 | 92 | 92.0 |
| Symptom-free days at 12 weeks (non-catheterised patients only) | 80 | 81.6 | 104 | 78.8 | 86 | 86.0 |
| I-QOL relevant (incontinent) | n = 56 | n = 62 | n = 59 | |||
| I-QOL at 12 weeks | 51 | 91.1 | 47 | 75.8 | 35 | 59.3 |
| Questionnaires returned (52 weeks) | n = 50 | n = 66 | n = 51 | |||
| EQ-5D (52 weeks) | ||||||
| Mobility | 49 | 98.0 | 63 | 95.5 | 49 | 96.1 |
| Self-care | 50 | 100 | 65 | 98.5 | 50 | 98.0 |
| Usual activity | 48 | 96.0 | 65 | 98.5 | 50 | 98.0 |
| Pain | 48 | 96.0 | 66 | 100 | 50 | 98.0 |
| Anxiety | 47 | 94.0 | 66 | 100 | 48 | 94.1 |
| Symptom-free days at 52 weeks (non-catheterised patients only) | n = 47 | n = 58 | n = 48 | |||
| 47 | 100 | 58 | 100 | 47 | 97.9 | |
| I-QOL relevant (incontinent) | n = 26 | n = 35 | n = 33 | |||
| I-QOL at 52 weeks | 17 | 65.4 | 20 | 57.1 | 18 | 54.5 |
Postal questionnaires: hospital data
Admission data were sought for 200 patients from across the 12 centres. Only nine centres provided admission data and in these centres, 37 patients were reported to have been readmitted, with an additional five A&E department attendances. The patient reported admissions for all sites indicated 19 admissions to hospital (including two A&E department attendances), with 15 admissions (or A&E department attendance) being reported by patients for those sites that had returned hospital data. It was possible to compare hospital and patient report for 51 patients. The comparison of the two sources of data, showed reasonable agreement between the numbers reported (Table 96). Analysis of these data revealed very good agreement (kappa = 0.66; 95% CI 0.39 to 0.94).
| Admission within 52 weeks (from patient report) | Admission within 52 weeks (from hospital records) | Total | |
|---|---|---|---|
| No | Yes | ||
| No | 33 | 4 | 37 |
| Yes | 3 | 11a | 14 |
| Total | 36 | 15 | 51 |
Resource use: complete cases
Resource use in each of the trial arms at 12 weeks can be seen in Tables 97 and 98.
| Resource | Usual care | Intervention | Supported implementation | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Health-care contacts | ||||||||
| GP | 34 | 2.62 (2.17) | 45 | 2.22 (1.57) | 34 | 2.15 (1.42) | 113 | 2.32 (1.73) |
| Practice nurse | 14 | 4.07 (5.00) | 22 | 1.77 (1.54) | 12 | 5.33 (3.80) | 48 | 3.33 (3.70) |
| Physiotherapy | 26 | 9.27 (7.91) | 32 | 7.54 (6.83) | 23 | 10.94 (16.12) | 81 | 9.06 (10.54) |
| Occupational therapy | 6 | 11.17 (11.45) | 12 | 5.58 (5.17) | 6 | 8.00 (11.69) | 29 | 7.66 (9.34) |
| District nurse | 7 | 8.20 (6.46) | 13 | 23.58 (32.02) | 10 | 5.43 (6.07) | 30 | 13.94 (22.78) |
| Continence advisor | 4 | 4.55 (2.72) | 4 | 0.5 (0.58) | 5 | 6.01 (8.74) | 13 | 3.87 (5.77) |
| Chiropody | 12 | 3.85 (9.30) | 27 | 0.95 (0.84) | 8 | 1.06 (0.93) | 47 | 1.71 (4.78) |
| Social care contacts | ||||||||
| Home care | 22 | 42.43 (28.17) | 19 | 74.11 (51.90) | 18 | 63.73 (75.91) | 59 | 59.13 (54.15) |
| Stroke family support worker | 2 | 1.5 (0.71) | 11 | 3.77 (5.04) | 7 | 4.53 (5.22) | 20 | 3.81 (4.77) |
| Day centre visits | 4 | 6.82 (3.34) | 2 | 11.36 (8.18) | 0 | 0 | 6 | 8.33 (5.06) |
| A&E department attendance (not admitted) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hospital admissions | ||||||||
| Short stay | 0 | 0 | 2 | 1 (0) | 1 | 1 (0) | 3 | 1 (0) |
| Long stay | 6 | 1 (0) | 17 | 1.12 (0.33) | 6 | 1.17 (0.41) | 29 | 1.10 (0.31) |
| Resource | Usual care (n = 35)a | Intervention (n = 42) | Supported implementation (n = 33) | Overall (n = 110) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Aids and adaptations | ||||||||
| Walking stick | 18 | 51.4 | 23 | 54.8 | 18 | 54.5 | 59 | 53.6 |
| Wheelchair | 11 | 31.4 | 24 | 57.1 | 14 | 42.4 | 49 | 44.5 |
| Hoist (or similar) | 2 | 5.7 | 7 | 16.7 | 1 | 3.0 | 10 | 9.1 |
| Height adjustable bed | 4 | 11.4 | 6 | 14.3 | 2 | 6.1 | 12 | 10.9 |
| Mattress cover | 7 | 20.0 | 15 | 35.7 | 6 | 18.2 | 28 | 25.5 |
| Chair raiser | 9 | 25.7 | 10 | 23.8 | 6 | 18.2 | 25 | 22.7 |
| Toilet seat raiser | 12 | 34.3 | 11 | 26.2 | 14 | 42.4 | 37 | 33.6 |
| Bedpan/urinal | 4 | 11.4 | 14 | 33.3 | 0 | 0 | 18 | 16.4 |
| Commode | 18 | 51.4 | 27 | 64.3 | 13 | 39.4 | 58 | 52.7 |
| Toilet rails | 14 | 40.0 | 9 | 21.4 | 15 | 45.5 | 38 | 34.5 |
| Adapted bath/shower | 10 | 28.6 | 12 | 28.6 | 12 | 36.4 | 34 | 30.9 |
| Alarm call | 5 | 14.3 | 14 | 33.3 | 7 | 21.2 | 26 | 23.6 |
Although more of the intervention arm patients saw a therapist, the average number of contacts was smaller, this was similar for the practice nurse contacts. Only a couple of people in the usual care arm saw a stroke family support worker. No one in the supported implementation arm visited a day centre. Slightly more of the intervention arm saw a district nurse, and the average number of contacts was much higher.
No one in the supported implementation arm had a bedpan/urinal and a smaller proportion received a commode; however, a higher proportion received toilet rails. A higher proportion of patients in the intervention arm had mattress covers.
Resource use in each of the trial arms at 52 weeks can be seen in Tables 99 and 100. The mean values in Table 99 are based on patients who accessed those resources. The data indicate that patients who saw their GP or practice nurse tended to see them on an average of five occasions. The number of contacts with therapy services is very high in all trial arms, particularly the intervention arm. This may be a consequence of how these data were recorded in the questionnaires, which consequently informed how they were calculated. Contacts with the district nurse and support with home care were markedly higher in the intervention arm, and again the method of recording in the questionnaire is likely to have affected the estimates of the magnitude of the input. The distribution of aids and adaptations is mostly similar across trial arms except for wheelchairs, mattress covers, bedpans/urinals and commodes, which were more frequently supplied to patients in the intervention arm.
| Resource | Usual care | Intervention | Supported implementation | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Health-care contacts | ||||||||
| GP | 30 | 5.2 (4.37) | 49 | 5.04 (3.93) | 30 | 3.6 (2.90) | 109 | 4.69 (3.83) |
| Practice nurse | 17 | 5.41 (5.24) | 30 | 6.67 (9.82) | 22 | 7.41 (10.05) | 69 | 6.59 (8.91) |
| Physiotherapy | 23 | 24.67 (23.94) | 35 | 45.97 (57.54) | 28 | 36.37 (41.92) | 86 | 37.15 (45.88) |
| Occupational therapy | 6 | 15.17 (18.00) | 17 | 52.05 (64.65) | 10 | 29.69 (37.94) | 33 | 38.57 (52.62) |
| District nurse | 11 | 19.57 (18.25) | 16 | 63.49 (92.33) | 15 | 12.29 (13.89) | 42 | 33.7 (61.91) |
| Continence advisor | 1 | 4.27 (0.00) | 7 | 9.11 (14.77) | 2 | 2.74 (1.57) | 10 | 7.35 (12.41) |
| Chiropody | 26 | 6.79 (5.00) | 32 | 6.05 (3.58) | 23 | 6.28 (4.93) | 81 | 6.35 (4.42) |
| Social care contacts | ||||||||
| Home care | 16 | 391.46 (373.52) | 19 | 678.83 (480.07) | 15 | 281.01 (335.74) | 50 | 467.53 (435.23) |
| Stroke family support | 4 | 11.73 (19.38) | 9 | 10.25 (14.41) | 7 | 1.23 (0.93) | 20 | 7.39 (12.99) |
| Day centre visits | 5 | 60.17 (47.88) | 3 | 15.29 (20.94) | 1 | 13.57 (0) | 9 | 40.03 (42.74) |
| A&E department attendance (not admitted) | 0 | 2 | 1.50 (0.71) | 3 | 1 (0.00) | 5 | 1.20 (0.45) | |
| Hospital admissions | ||||||||
| Short stay | 0 | 8 | 1.13 (0.35) | 1 | 1 | 9 | 1.11 (0.33) | |
| Long stay | 4 | 1.25 (0.50) | 27 | 1.44 (0.75) | 5 | 1 (0.00) | 36 | 1.36 (0.68) |
| Resource | Usual care (n = 35)a | Intervention (n = 41) | Supported implementation (n = 36) | Overall (n = 112) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Aids and adaptations | ||||||||
| Walking stick | 22 | 62.9 | 22 | 53.7 | 18 | 50.0 | 62 | 55.4 |
| Wheelchair | 14 | 40.0 | 27 | 65.9 | 19 | 52.8 | 60 | 53.6 |
| Hoist (or similar) | 5 | 14.3 | 12 | 29.3 | 6 | 16.7 | 23 | 20.5 |
| Height adjustable bed | 6 | 17.1 | 11 | 26.8 | 9 | 25.0 | 26 | 23.2 |
| Mattress cover | 7 | 20.0 | 15 | 36.6 | 7 | 19.4 | 29 | 25.9 |
| Chair raiser | 13 | 37.1 | 11 | 26.8 | 10 | 27.8 | 34 | 30.4 |
| Toilet seat raiser | 13 | 37.1 | 10 | 24.4 | 13 | 36.1 | 36 | 32.1 |
| Bedpan/urinal | 5 | 14.3 | 16 | 39.0 | 5 | 13.9 | 26 | 23.2 |
| Commode | 12 | 34.3 | 25 | 61.0 | 16 | 44.4 | 53 | 47.3 |
| Toilet rails | 12 | 34.3 | 13 | 31.7 | 14 | 38.9 | 39 | 34.8 |
| Adapted bath/shower | 9 | 25.7 | 13 | 31.7 | 10 | 27.8 | 32 | 28.6 |
| Alarm call | 8 | 22.9 | 8 | 19.5 | 10 | 27.8 | 26 | 23.2 |
Resource use: base case
The imputed data, showed slightly different patterns to the data in the returned questionnaires. The use of post-hospital resources was similar in the three trial arms, with the exception of district nurse input, which was higher in the intervention arm and day centre visits, which were higher in the usual care arm (Table 101).
| Resource | Usual care (n = 124) | Intervention (n = 164) | Supported implementation (n = 125) |
|---|---|---|---|
| Health-care contacts | |||
| GP | 3.55 (2.69) | 3.76 (2.57) | 3.18 (1.89) |
| Practice nurse | 2.59 (2.58) | 3.15 (4.66) | 3.15 (4.78) |
| Physiotherapy | 24.57 (13.94) | 30 (30.21) | 29.09 (23.57) |
| Occupational therapy | 15.11 (10.00) | 16.89 (26.33) | 15.76 (14.63) |
| District nurse | 8.66 (8.18) | 14.92 (33.4) | 9.1 (5.34) |
| Continence advisor | 0.39 (0.58) | 0.92 (3.36) | 0.64 (0.59) |
| Chiropody | 3.81 (2.91) | 3.98 (2.10) | 4.13 (2.53) |
| Social care contacts | |||
| Home carea | 262.57 (155.34) | 295.52 (237.64) | 239.5 (128.76) |
| Stroke family support worker | 1.33 (3.63) | 1.47 (3.89) | 1.15 (0.61) |
| Day centre visitsa | 7.16 (16.87) | 4.1 (3.95) | 3.8 (7.15) |
| A&E department attendance (not admitted) | 0.03 (0.08) | 0.03 (0.18) | 0.06(0.17) |
| Hospital admissions | |||
| Short stay | 0.06 (0.04) | 0.09 (0.25) | 0.06 (0.09) |
| Long stay | 0.34 (0.26) | 0.44 (0.56) | 0.29 (0.23) |
There were some differences between the trial arms in terms of receipt of aids and adaptations (Table 102). Calculation of the mean number of aids and adaptations was based on the patients not in residential care. Provision of bedpans/urinals and commodes were slightly higher in the intervention arm compared with the other trial arms.
| Resource | Usual care (n = 108) | Intervention (n = 136) | Supported implementation (n = 110) |
|---|---|---|---|
| Aids and adaptations | |||
| Walking stick | 0.39 (0.39) | 0.35 (0.38) | 0.35 (0.38) |
| Wheelchair | 0.30 (0.36) | 0.43 (0.40) | 0.38 (0.39) |
| Hoist (or similar) | 0.10 (0.23) | 0.13 (0.28) | 0.09 (0.23) |
| Height adjustable bed | 0.13 (0.26) | 0.16 (0.29) | 0.15 (0.27) |
| Mattress cover | 0.17 (0.29) | 0.25 (0.35) | 0.19 (0.30) |
| Chair raiser | 0.23 (0.33) | 0.20 (0.31) | 0.20 (0.31) |
| Toilet seat raiser | 0.25 (0.34) | 0.20 (0.30) | 0.24 (0.35) |
| Bedpan/urinal | 0.14 (0.26) | 0.23 (0.35) | 0.12 (0.22) |
| Commode | 0.28 (0.35) | 0.38 (0.39) | 0.30 (0.36) |
| Toilet rails | 0.28 (0.35) | 0.23 (0.32) | 0.27 (0.35) |
| Adapted bath/shower | 0.20 (0.30) | 0.21 (0.31) | 0.27 (0.36) |
| Alarm call | 0.17 (0.29) | 0.15 (0.27) | 0.19 (0.31) |
Combining resource use and costs
The combined resource use and cost data at 52 weeks can be seen in Table 103. The hospital costs were higher in the supported implementation arm mainly due to the cost of the programme. The cost of the staff training was less than 2% of the in-hospital costs. Health and social care costs were higher in the intervention arm, whereas the supported implementation had the lowest social care costs. The cost of admissions was higher in the intervention compared with the other trial arms. The cost of the aids and adaptations were marginally lower in the usual care arm and similar in the other trial arms. The mean cost per patient in the usual care arm was lower than in either of the intervention arms, with the cost in the intervention arm higher than that in the supported implementation arm.
| Resource | Usual care (£) | Intervention (£) | Supported implementation (£) |
|---|---|---|---|
| In hospital | |||
| Staff training | 0 | 13 | 25 |
| Cost of programme | 0 | 1469 | 1805 |
| Mean in-hospital costs | 0 | 1482 | 1830 |
| Post discharge | |||
| Health care | 2270 | 2996 | 2481 |
| Social care | 6230 | 6590 | 5630 |
| A&E department attendance | 0 | 0 | 0 |
| Hospital admissions | 884 | 1145 | 753 |
| Aids and adaptations | 178 | 210 | 219 |
| Mean post-discharge costs | 9563 | 10,941 | 9083 |
| Total mean costs | 9563 | 12,423 | 10,913 |
The distribution of costs for by each trial arm can be seen in Figures 29–31. In the usual care arm, the costs went up to around £35,000 but this was only for one patient (see Figure 29). The majority of patients contributed less than £10,000 to the total cost. In the intervention arm, the range of costs was much wider, ranging from around £2500 up to £45,000 (see Figure 30). Although some patients had higher costs, the majority of patients had costs of under £12,500. The supported implementation arm had a similar range of cost to the intervention arm, spanning from a minimum of around £4000 to a maximum of £34,000 (see Figure 31). Over half the patients in this trial arm had costs less than £12,000.
FIGURE 29.
Distribution of costs in the usual-care arm.
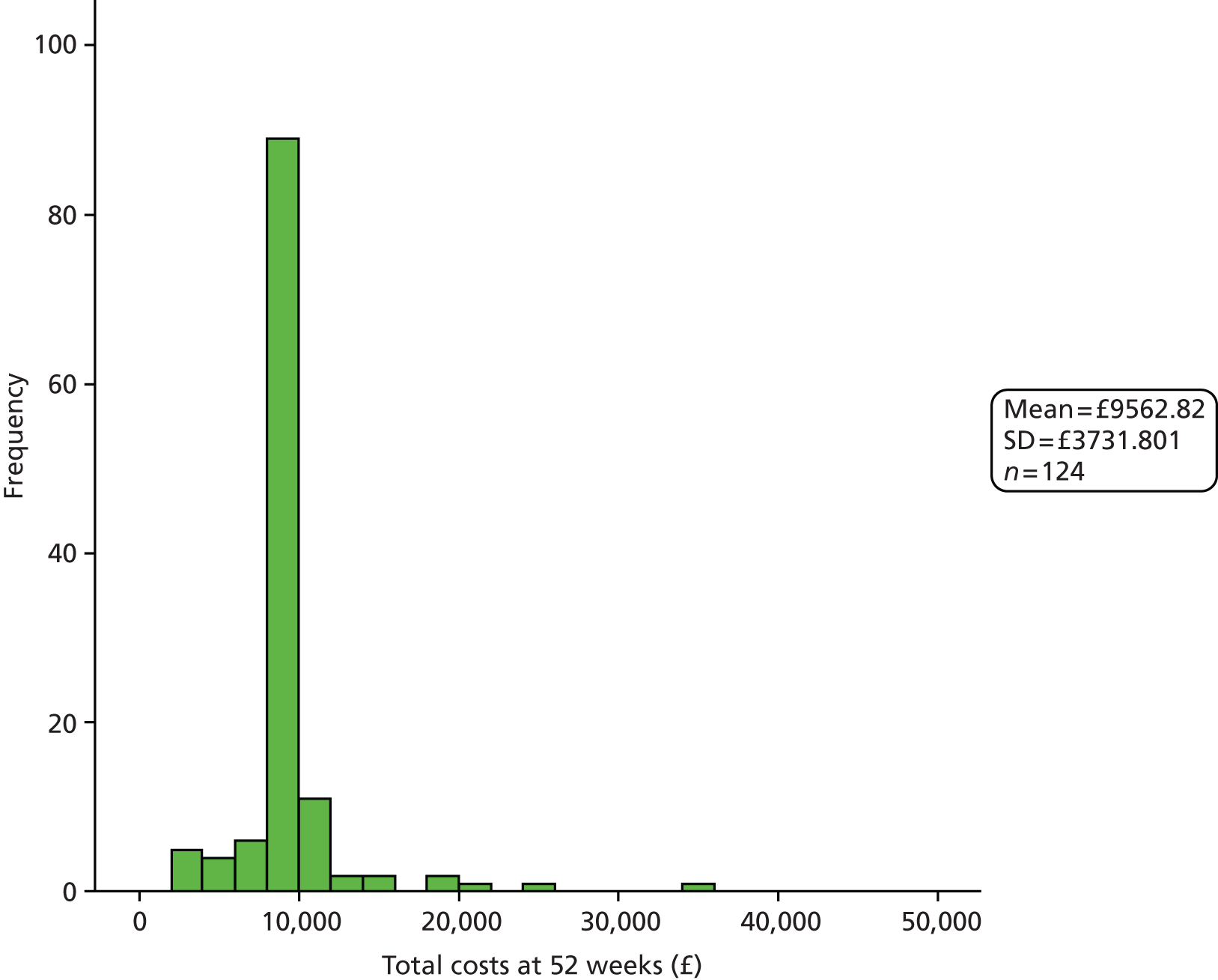
FIGURE 30.
Distribution of costs in the intervention arm.
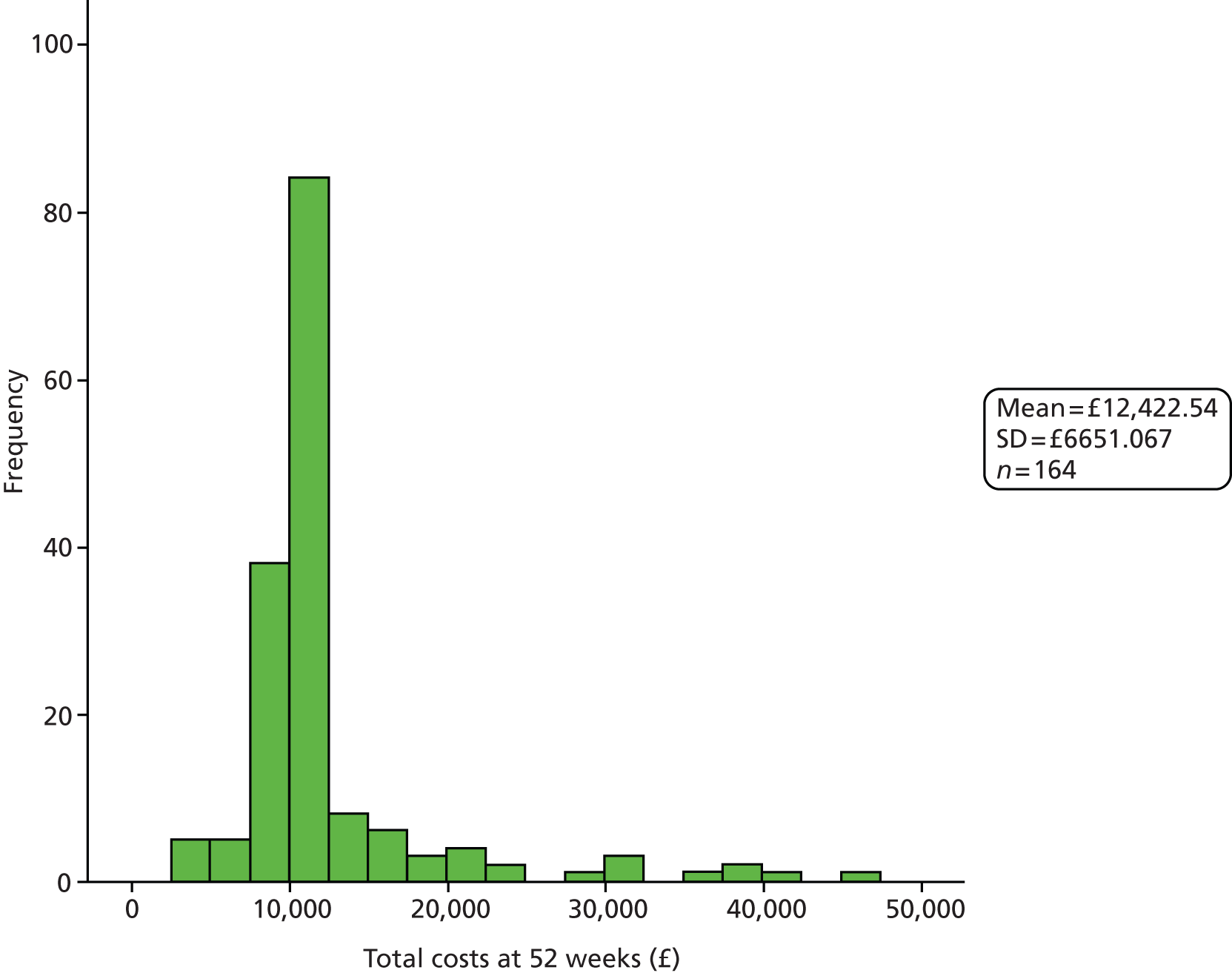
FIGURE 31.
Distribution of costs in the supported-implementation arm.
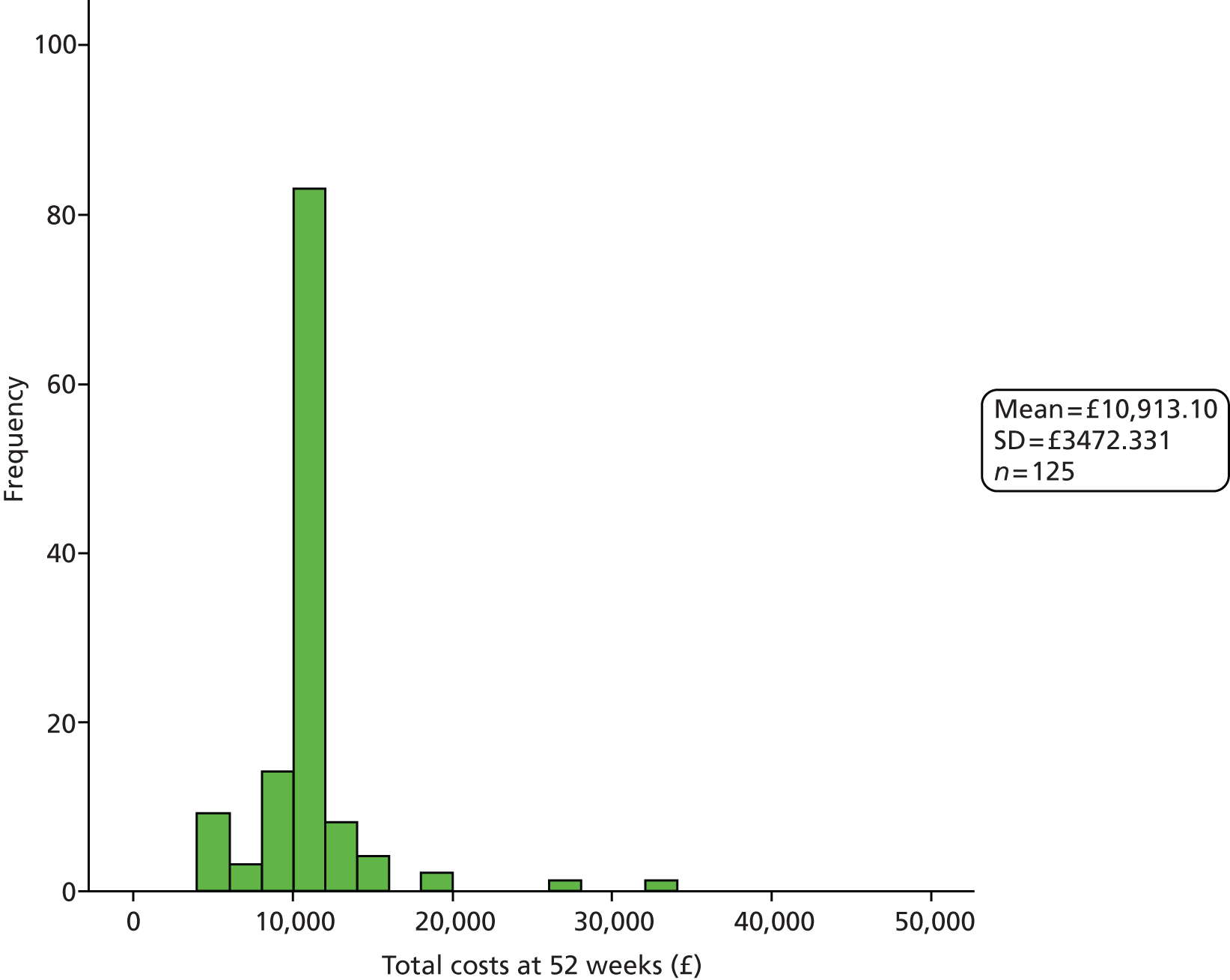
Patient outcomes
The efficacy outcomes relevant to the economic analysis are summarised in Table 104. There were clear differences between the groups at baseline, with the intervention arm having much lower utility values. The unadjusted utility values were higher at 52 weeks in all groups. However, once data were imputed and utilities adjusted for baseline variables there was a loss of quality-adjusted life-years in all groups, with the lowest value seen in the usual-care arm. The loss of quality-adjusted life-years was similar whether imputed or complete cases were used, although in contrast to the imputed data, the usual-care arm had the highest value. The number of symptom-free days was similar across groups, with the usual-care arm having the highest values. There was a marked difference between the symptom-free days when imputed data as opposed to complete cases were used: values for the latter were twice those of the former. The I-QOL was similar across groups.
| Variable | Usual care | Intervention | Supported implementation |
|---|---|---|---|
| Utility values at baseline | 0.31 (0.34), n = 100 | 0.13 (0.35), n = 116 | 0.3 (0.36), n = 83 |
| Utility values at 52 weeks | 0.46 (0.36) n = 47 | 0.26 (0.39), n = 65 | 0.39 (0.38), n = 49 |
| Utility at 52 weeks (imputed and adjusted for baseline) | –0.14 (0.20), n = 99 | –0.24 (0.20), n = 113 | –0.11 (0.19), n = 83 |
| Quality-adjusted life-years gained (imputed) (52 weeks – baseline) | –0.45 (0.20), n = 99 | –0.36 (0.22), n = 113 | –0.41 (0.24), n = 83 |
| Utility at 52 weeks (complete case, adjusted for baseline) | 0.06 (0.29), n = 41 | –0.29 (0.24), n = 47 | –0.11 (0.22), n = 39 |
| Quality-adjusted life-years gained (complete case) (52 weeks – baseline) | –0.33 (0.36), n = 41 | –0.42 (0.21), n = 47 | –0.47 (0.25), n = 39 |
| Symptom-free days at 52 weeks (using worst case imputed values) | 7.53 (11.69), n = 124 | 6.26 (10.86), n = 164 | 7.04 (11.48), n = 125 |
| Symptom-free days at 52 weeks (using complete case values) | 18.68 (11.42), n = 50 | 16.55 (11.91), n = 62 | 17.96 (11.85), n = 49 |
| I-QOL at 52 weeks | 68.12 (25.00), n = 26 | 68.71 (27.10), n = 33 | 67.37 (28.05), n = 28 |
Comparing the Incontinence Quality of Life questionnaire with the quality-adjusted life-year
The association between the I-QOL and the utility values from the EQ-5D was explored overall and within trial arms. At 52 weeks the overall comparison revealed a significant correlation [ρ = 0.36 (n = 84, p = 0.001)]. The association between the two measures differed between trial arms, with the usual-care arm showing a non-significant correlation [ρ = 0.30; (n = 24, p = 0.16)], the intervention arm showed a significant correlation [ρ = 0.47 (n = 32, p = 0.007)], with a marginally non-significant effect for the supported-implementation arm [ρ = 0.34 (n = 28, p = 0.07)].
Estimates of cost–utility and cost-effectiveness
In Table 105 a summary of the costs and outcomes is presented. The table shows the base-case values and associated lower and upper estimates, based on the 95% CIs, which are used in the sensitivity analysis.
| Variable | Usual care | Intervention | Supported implementation |
|---|---|---|---|
| Cost per patient (£) | |||
| Mean | 9563 | 12,423 | 10,913 |
| Lower estimate | 8647 | 10,558 | 9486 |
| Upper estimate | 10,479 | 14,287 | 12,340 |
| Quality-adjusted life-years gained | |||
| Mean | –0.45 | –0.36 | –0.41 |
| Lower estimate | –0.49 | –0.41 | –0.46 |
| Upper estimate | –0.41 | –0.32 | –0.36 |
| Symptom-free days | |||
| Mean | 7.53 | 6.26 | 7.04 |
| Lower estimate | 5.47 | 4.60 | 5.03 |
| Upper estimate | 9.59 | 7.92 | 9.05 |
Table 106 summarises the results of the cost–utility and cost-effectiveness analysis for the base case. The analysis revealed that when compared with usual care, both intervention trial arms had an incremental cost-effectiveness ratio (ICER) that exceeded £30,000. This was also true when the supported-implementation arm was compared with the intervention arm. The cost per symptom-free day showed that usual care was dominant over both intervention trial arms. The supported-implementation arm was dominant over the intervention arm.
| ICERs | Usual care | Intervention | Supported implementation |
|---|---|---|---|
| Cost per quality-adjusted life-year gained | |||
| Compared with usual care | – | £31,775 | £33,755 |
| Compared with intervention | – | – | £30,191 |
| Cost per symptom-free day | |||
| Compared with usual care | – | Usual care dominant | Usual care dominant |
| Compared with intervention | – | – | Supported implementation dominant |
Resource use and costs: sensitivity analysis
The effect of changing costs while keeping the effect sizes at base-case values can be seen in Table 107. The lower cost estimates resulted in ICERs of around £21,000 for both intervention and supported-implementation arms when compared with usual care. The supported-implementation arm had an ICER of £21,446 compared with the intervention arm. For the symptom-free days, usual care was dominant over both intervention trial arms. The supported-implementation arm was dominant over the intervention arm.
| Variable | Lower cost estimates | Upper cost estimates | ||
|---|---|---|---|---|
| Intervention | Supported implementation | Intervention | Supported implementation | |
| Cost per quality-adjusted life-year gained | ||||
| Compared with usual care | £21,240 | £20,983 | £42,309 | £46,526 |
| Compared with intervention | – | £21,446 | – | £38,935 |
| Cost per symptom-free day | ||||
| Compared with usual care | Usual care dominant | Usual care dominant | Usual care dominant | Usual care dominant |
| Compared with intervention | – | Supported implementation dominant | – | Supported implementation dominant |
The effect of changing estimates of effectiveness, while keeping the costs at base-case values can be seen in Table 108. For the lower estimates of effectiveness, both intervention trial arms have ICERs above £30,000 when compared with usual care, whereas the supported-implementation arm has an ICER of £24,718 compared with the intervention arm. These data contrast with the results from the upper estimates of effectiveness. When compared with usual care, the ICER for the intervention arm has changed little, but the ICER for the supported-implementation arm is less than £30,000. Compared with the intervention arm, the ICER for the supported-implementation arm is £38,776. Within the symptom-free days, usual care is dominant over the intervention trial arms, while the supported-implementation arm is dominant over the intervention arm.
| ICERs | Lower estimates of effect | Upper estimates of effect | ||
|---|---|---|---|---|
| Intervention | Supported implementation | Intervention | Supported implementation | |
| Cost per quality-adjusted life-year gained | ||||
| Compared with usual care | £32,190 | £48,620 | £31,370 | £25,851 |
| Compared with intervention | – | £24,718 | – | £38,776 |
| Cost per symptom-free day | ||||
| Compared with usual care | Usual care dominant | Usual care dominant | Usual care dominant | Usual care dominant |
| Compared with intervention | – | Supported implementation dominant | – | Supported implementation dominant |
Discussion
We aimed to explore the feasibility of assessing the cost-effectiveness of a SVP for people with incontinence after stroke. The assessment of feasibility was through examining the data collection methods and an analysis of the potential costs and cost-effectiveness of the programme.
We identified problems with the data recording procedures at all stages of the process. Having originally planned to ask staff to record the time they spent providing the programme and time spent on activities related to toileting, it became clear from our discussions with clinical teams that this approach would not be feasible. Therefore, in order to calculate the cost of the programme we relied on estimates of the time taken to toilet patients, rather than asking staff to keep records, and extrapolated the number of times patients were toileted from other data, i.e. sampling from the clinical logs used in the assessment of fidelity (see Chapter 6). This pragmatic approach was adopted because it was felt that the onerous nature of staff making and keeping notes would have affected whether they completed the documentation required for the programme – the clinical logs. Given that the clinical logs were not always completed fully, this belief has some degree of credibility. An alternative approach would have been for a third party to record the toileting process when staff were conducting the programme. Although this would have provided more accurate data it would have been much more resource intensive, likely requiring full-time commitment over a number of weeks in order to obtain a representative sample of data. In a definitive trial it is imperative that the right balance is found between the input required for collection of resource data and the representativeness of those data.
Having an individual specifically recording data prospectively, should result in more complete data being obtained. The method adopted in this study meant that four (one-third) of the sites did not return questionnaires about the time take to toilet patients. A similar problem was found with the admission data, where again two-thirds of the sites did not return data. Dedicated research staff are required in order to ensure that the hospital database can be interrogated up to 52 weeks after the last patient is recruited.
The admission data revealed problems with other methods of recording data. Patients (and carers) were asked to record the number of hospital admissions on their postal questionnaire. When this was compared with the data from the hospitals there was a reasonable level of agreement, but agreement was not total. A potential explanation for this disagreement is that patients’ admissions abstracted from hospital records were only considered in relation to the hospital where they were admitted with the index stroke. If they had been admitted to a different hospital this would not have been identified. However, patients both under and overestimated the number of admissions and so this does not explain all the disagreements. In the postal questionnaire we only asked patients to record the number of admissions and not the dates of the readmissions or the reasons. Using the hospital records meant that we were able to record more detail about the admission, which helps to develop a more comprehensive picture of the costs. It is possible that patients would not have sufficient recall at the 52-week assessment to detail exactly when they were admitted to hospital, the reasons for the admission and if it was an admission or just a visit to the A&E department.
The completion rate of the postal questionnaires was good, with over a 70% response rate at 12 weeks. The response rate at 52 weeks was lower at approximately 56%. The lower response rate for the long-term follow-up may be a result of sending the outcome and resource use questionnaires at the same time. Alternatively, the response rate may be a reflection of a reduced familiarity with the trial and its processes by 52 weeks, whereas at 12 weeks a commitment to the study would have been tangible. Given that the resource use questionnaire is requiring a recall of inputs, patients may have felt better able to answer such questions at 12 weeks when the inputs would have been more recent, fewer, and possibly still ongoing. In contrast, at 52 weeks patients may have less recall about the trial, have had further morbidities and been less sure about inputs, particularly those that had been completed, and so were more reticent to complete a questionnaire. At both 12 and 52 weeks, similar proportions in each trial arm completed their questionnaires, suggesting estimates of resource use are unlikely to be biased by differential reporting between the trial arms.
When questionnaires were returned, there were some differences in the completion rates of the individual items. At 12 weeks the health and social care items had an overall completion rate of 95%. There were slight differences between the trial arms but completion rates did not vary by more than 10%. The proportion of responses for the aids and adaptations items was generally lower, averaging around 85%, and with few items having more than a 90% completion rate. This may be a reflection of the format of responses for these items, which were presented in a list (i.e. participants may have focused on only those items that were relevant to them). At 52 weeks the overall completion rates were similar to those at 12 weeks.
When calculating the number of units used for each resource, the data informing the calculations were taken at face value from the questionnaires. For certain resources, this led to improbable estimates of usage, for example physiotherapy and occupational therapy. The estimates of use for other resources appeared more plausible, for example GP and practice nurse contacts. This difference is likely to relate to how the patients are asked to supply information. For contacts that were recorded as single events patients had to record the number of visits, whereas for ongoing resources, such as therapy and social support, patients were asked to give start and end dates as well as contacts per week. In future, these recurring inputs may be best obtained through either interviewing patients, where questioning may allow a more accurate recording of data, or by contacting the service providers such as GPs, or interrogating the hospital information systems.
Of the questionnaires that were returned, not many had comments about the format and structure. There was one positive comment and two negative comments. One of the negative comments related to use of a double-sided form: it is understood that single-sided forms are better,187 but this does translate into thicker and heavier questionnaires. The completion rates for the individual items did not appear to have been influenced by having double-sided forms. The other comment related to the suggested time taken to complete the questionnaire, suggesting it was a gross underestimation, but this was the only complaint along these lines and was made by a third party helping the patient rather than by an individual completing the form themself. Some minor rewording around the estimates of completion time may be appropriate.
For the follow-up at 12 weeks we had split the outcome and resource use questionnaires up so as not to overburden the patients. This worked reasonably well given the response rate. In addition, we also decided not ask the same question on both questionnaires. Therefore, because we had included place of residence on the outcome assessment questionnaire it was not included on the resource use questionnaire. This led to problems at 12 weeks because it was clear that some patients had been discharged from hospital when they completed their resource use questionnaire, but they were recorded as being in hospital from their outcome questionnaire, which had been sent earlier.
The costs in the three trial arms showed some differences between the usual-care arm and the two trial arms providing the programme where the costs were higher. This was partly due to the cost of the programme. The cost of post-hospital care was actually lowest in the supported-implementation arm and highest in the intervention arm. The biggest single contributor to the programme cost was due to staff training (when considering the individual elements – data not shown), although when averaged out across staff, the overall contribution of the training cost to the mean cost per patient was small. Social care made the biggest contribution to the post-hospital costs. The health and admission costs were higher in the intervention group. The SVP contributed nearly all the costs of the programme, with the cost of the training contributing very little. The SVP cost is the area for which we have the least robust cost data, so we need to be cautious in interpreting these findings. For the post-hospital costs, the patient report information has some measure of reliability as evidenced by the similarity between patient-reported and hospital data for the admissions to hospital. The admission data demonstrated differing costs in the three trial arms, with the costs in the intervention arm nearly 50% more than those in the supported-implementation arm. The analysis of the admission data was kept simple by splitting the admissions into short and long stay. Potentially these data could have been explored in further detail to identify the exact reasons for the admissions and assign more detailed costs based on the length of stay. However, if an analysis explored the data to this level, it would be worth considering clinical review of the admission information and including only those admissions linked to incontinence.
The total cost of the training was estimated to be around £3000 at each of the 12 sites, which translated into a cost of just over £12,000 for each trial arm. When the cost of facilitation was added on to this, the figure was nearly doubled. For the purpose of the cost analyses, an average cost per patient needed to be calculated. This average cost was based on patient throughput rather than numbers recruited into the trial arms so that the cost was more a reflection of clinical practice than the research process. If we had used the number of patients in the trial arms as the denominator it would have increased the average cost of training by a factor of six, but this would have had little impact on the cost analyses because the average cost for the staff training is a small proportion of the total average cost. Although the training did not contribute greatly to the overall costs it would still need to be included in any future study exploring cost-effectiveness. Having now developed this resource, the cost of training would be reduced from the delivery side; although the materials may need updating and the online resource would require some maintenance. The receipt of training would still make a substantial contribution to the training costs, with ward staff taking time out of their day for face-to-face or online training. Further costs may be incurred when new staff start on the ward, primarily for the online training. If considered to be of value, the training could be embedded in practice as part of staff induction, which may have less of an impact on subsequent staff–patient contact time.
One of the aims of this study was to explore the potential cost–utility and cost-effectiveness of the SVP. The base-case analysis used data from 167 out of 299 (55.9%) patients who had returned resource use questionnaires. The number of questionnaires returned was similar across groups. For the patients who did not return questionnaires and for those that died or withdrew, data were imputed. This meant that for the analysis, data were being imputed for circa 60% of the patients who entered the trial. Consequently, any interpretation of the cost-effectiveness analysis has to be treated with caution.
Using unadjusted EQ-5D data for available cases showed an increase from the baseline values. However, once the EQ-5D was adjusted for baseline data, the utility values were worse than baseline and this was consistent across the trial arms, with usual care having the highest value. This could reflect that either the EQ-5D is showing that the intervention package does not work or that the EQ-5D is not sensitive to any impact of the intervention. The outcome data at 52 weeks did demonstrate an improvement since baseline – contrasting with the EQ-5D, although the usual-care arm did tend to have (non-significantly) better outcomes – similar to the EQ-5D. Our uncertainty over the sensitivity of this measure to reflect the impact of the intervention was explored further by comparing it with the I-QOL, a continence-specific QoL assessment.
The exploration of the association between the I-QOL and the utility values, calculated from the EQ-5D, revealed mixed results. Overall, at 52 weeks there was a significant association, although the coefficient was less than 0.4. There were differences when this association was explored within trial arms, with the intervention arm revealing a significant correlation, and the supported-implementation arm a marginally non-significant correlation. The usual-care arm showed a non-significant correlation. The differences in these results make interpretation of the findings difficult. Looking overall, the data suggest that the EQ-5D is potentially able to reflect the impact of incontinence on QoL. However, there are two caveats to this suggestion. First, the associations explored QoL at 52 weeks and not changes from baseline because no data were collected on the I-QOL at baseline. Second, the analysis has only been conducted in those patients who were incontinent because the I-QOL is intended for use in those people with incontinence, and so by definition these respondents have a poor outcome. Where data allow, it would be worth exploring the association between the measures in all patients to determine the extent to which the EQ-5D reflects the impact of incontinence on QoL. Likewise, future work could record the I-QOL in the early stages to allow an analysis of the change scores as well as a comparison of the measures at each time point, particularly if there is a marked difference in utility values at baseline as was seen in this study. The lack of association between the measures in the usual-care arm is somewhat at odds with the other trial arms and merits further exploration, although the coefficients for the usual-care and supported-implementation arms are only marginally different.
Initial inspection of the costs and quality-adjusted life-years revealed that compared with the usual-care arm the intervention and supported-implementation arms had ICERs of just over £30,000, a value greater than the £30,000 threshold used by NICE. 177 However, when using the lower estimates of the costs in the sensitivity analysis the ICERs were around £20,000. In the other sensitivity analyses, the ICERs varied, depending on the assumptions made, but the usual-care arm did not dominate either of the intervention arms, nor were any of the ICERs greater than £50,000.
Initial inspection of the costs and symptom-free days revealed a different pattern of data to the quality-adjusted life-year analysis. The usual-care arm dominated both the intervention groups. The supported-implementation arm dominated the intervention arm. There are no figures to compare these data with, but they may inform discussions with patient groups to explore a willingness to pay.
We aimed to be inclusive in collecting resource use data after hospital and consequently sought data about a range of health and social care inputs. This inclusive approach means that a number of resources unlikely to be related to incontinence or a regaining of continence will have been included. This has the potential to bias the cost estimates if the non-related costs are much higher than the related costs, thereby masking the impact of the programme on resource use. For this reason we have not included costs related to residential care, because a cost of circa £1000 per week has the potential to bias the results, particularly for longer-term costs. An alternative to this blanket coverage, and alluded to earlier, is to investigate the reasons for resource use, particularly those related to health (e.g. GP visits and hospital admissions), and only include those related to incontinence. This approach has the potential benefit of identifying relevant costs but it requires a judgement call by a researcher and would also be much more labour intensive.
Patients in the intervention arm had more admissions to hospital. This may be due to the fact that there were more severe patients in this trial arm (see Chapter 7). Some patients had multiple admissions and this may result in higher costs in one trial arm. This could lead to bias, particularly if the reasons for admission are unlikely to be due to the outcome or programme. This may support the suggestion that there should be a review of the reasons why resources are used, and consequently exclude those resources considered to be not relevant to the outcome.
When data items were missing we performed imputations using mean values and basing these means around the OCSP classification. Although this approach is one method of performing imputations, it is has its limitations and reduces the variability within parameters. A future trial would need to consider a range of methods to impute outcome and cost data such as multiple imputation approaches. 169 Where possible, the methods of imputing data could mimic the methods used in the imputation of outcome data from the trial, including last observation carried forward; although it would be difficult to agree how to represent a value based on a worst-case scenario. Parameters to consider when making imputations might include patient factors such as age, sex and dependency.
In exploring the feasibility of assessing the cost-effectiveness of a SVP to promote continence after stroke we have identified a number of factors, described above, that need consideration in a full trial. One of the areas where we will be able to inform a future trial is the cost of resource items. The data in this report could be used in a future study, with the inclusion of a factor for inflation, or the sources of the data could be reaccessed for updated information.
Limitations
One of the purposes of an exploratory trial such as this study is to identify limitations to the research processes. A number of limitations have been identified.
With regards to the programme, the estimates of resources were not based on empirical data rather they used expert opinion, which is considered to be suboptimal. 188 The estimates were made independently and averaged so there was no undue influence that might occur with an expert panel. Related to this issue is the additional cost of equipment or consumables that might be used to support someone regaining their continence, for example pads. Although we can gather estimates on how much this contributes to the costs, this would be better identified by observation and recording in real life. A clear understanding of the programme cost is critical in order to obtain an accurate assessment of its cost-effectiveness. At the same time it is important to know the cost of not providing the programme: to do so, we would need to consider the costs attributable to incontinence in the usual care arm (i.e. how much staff time and consumables are required to support someone that has an episode of incontinence). This was not undertaken in this study, but would be important in future research. These assessments are likely to be resource intensive for the research, particularly because the recording of the cost of incontinence would also need to be performed in the trial arms providing the programme.
The calculation of the 1-year costs included data provided by the postal questionnaires. Asking patients to recall resource use over such a long period may lead to inaccurate data capture. This issue may be overcome by either more frequent follow-up, although this may increase dropout rates or by obtaining data from service providers, which may be more resource intensive.
We have calculated utility values and consequently quality-adjusted life-years using published weights. A future trial should consider an alternative approach such as the area under the curve approach, using responses at baseline, 12 and 52 weeks to map out the curves. 189
Summary
The training associated with the programme has been developed and costed, and the process of costing the training would not need to be repeated in a future trial. The training would still contribute to the costs of the programme in a future trial but, because it has been developed, the training would command less of a cost. Although the training contributes little to the overall cost, there would still be some cost associated with it through the maintenance of the online resource.
The cost and source of the individual resource use items has been identified.
In-hospital episodes of incontinence and the resources required to respond to such episodes should be recorded in all trial arms.
The resources required to perform the programme should be identified through direct observation, although some extrapolation may still be required.
Postal questionnaires may be useful to record resource use for patients, and consideration should be given whether or not they are single sided and the estimates of time taken to complete them.
Although postal questionnaires are relatively inexpensive to use, consideration should be given to obtaining post-hospital resource use data by asking patients to maintain diaries or going directly to the providers of services.
In those patients with incontinence, the EQ-5D appears to reflect the impact of incontinence on QoL as assessed by the I-QOL, but this requires further exploration.
Identifying resource use items more directly related to the programme and the effects of incontinence may allow a more realistic conclusion to be drawn around cost-effectiveness.
Any cost-effectiveness analysis needs to have a time horizon of at least 1 year after stroke.
Chapter 10 Patient, public and carer involvement
Overview
This chapter describes the process of PPC involvement within ICONS. We describe how PPC involvement was integrated into the study from the stage of developing the research proposal to the dissemination phase.
Background
A ‘service user’ is defined by the Department of Health190 as any person who has, is, or may access NHS or independent sector health services in the UK. Service users have been central to the NHS research strategy since the publication of Best Research for Best Health;191 patient and public involvement is fundamental to its vision of ‘conducting leading-edge research focused on the needs of patients and the public’. This was reflected in the creation of two funding streams, Research for Patient Benefit and Programme Grants for Applied Research, and the central role of INVOLVE (formerly Consumers in NHS Research) in promoting and empowering the public to become involved in research. Best Research for Best Health191 goes on to specify that patients and the public must be involved at all stages of the research process, from setting priorities, through selecting research methodology and patient recruitment, to dissemination of findings.
According to the Department of Health,191 the outcome of patient and public involvement is research that is more relevant to people’s needs and concerns, more reliable and more likely to be implemented in practice. This view of service user involvement leading to ‘better’ research is echoed by Beresford,192 who further adds that involvement can ensure research methods are more sensitive to the needs of research participants and therefore facilitate greater engagement. However, Smith et al. ’s193 review of service user involvement in nursing, midwifery and health visiting research found few studies with an explicit rationale or set of objectives for service user involvement.
The classification adopted by INVOLVE identifies three categories: consultation (views are sought out but with no guarantee of a change in outcomes); collaboration (service users work together with providers of services); or user control (users can shape the process at all levels). However, debate has recently focused on whether or not it is ever possible for anyone to have ultimate power over a project, with funding considerations and accountability influencing the process.
INVOLVE194 uses the term ‘diversity’ to reflect the growing range of people and groups, backgrounds and characteristics found in the UK population. Diverse groups are increasingly involved in research, but there is a growing realisation that there are sectors of society whose members are frequently excluded. Within stroke research, people with aphasia (a relatively unknown and complex communication difficulty which can affect the ability to use speech and understand the speech of other people) have traditionally been less likely to be invited to become actively involved in research, despite the fact that they make up 34% of stroke survivors. 195 In fostering true participation for people with aphasia there must be a ‘paradigm shift’ so that inclusion is ‘more than the addition of new practices to an existing toolkit’ and where ‘inclusionary practices are seen as a fundamental rethinking of values and practices’. 196 Recent aphasia research projects,197,198 many from within aphasia-specific organisations, demonstrate that when the required changes are made to values and practices then there can be successful involvement. Reflection on this involvement indicates both benefits and challenges for both the project and the individuals involved. 197 However, the over-riding theme for these studies is the importance of adhering to the principles of reducing barriers and providing facilitators to enable people with aphasia to participate.
Patient, public and carer involvement in Identifying Continence OptioNs after Stroke
Involvement prior to submission of the research proposal
Although the idea and plan for the research programme originated from the research team, prior to submission of the proposal, the team began forming links with existing local groups of stroke survivors [e.g. the Royal Preston Hospital Stroke Club, Preston; Speakeasy, a specialist aphasia charity based in Ramsbottom, Bury (www.buryspeakeasy.org.uk/)]; and other groups including service users in their membership, for example the North West Users Research Advisory Group. We also consulted the University of Central Lancashire COMENSUS (COMmunity ENgagement and Service User Support) project. Operating at the level of consultation,199 the researchers hoped to obtain feedback on the proposal, and also to develop links with individuals who might be called on to join programme-specific advisory groups if the programme was successful in obtaining funding.
Feedback from these groups helped shape the section within the proposal on ‘patient involvement within the proposed research’. For example, one member of the North West Users Research Advisory Group commented that although a wide range of expertise was represented among the co-applicants, there was no nominated individual to manage PPC involvement; this was viewed as a significant deficiency in the current climate. Accordingly, the research team approached a named individual (JV) to lead the PPC involvement in the programme and to chair the PPC group. Commenting on plans to introduce the conservative interventions for continence management, service users also emphasised the importance of debating issues such as delivery, uptake and patient and health professional adherence with a group of patients and carers before finalising plans. The finished version of the PPC involvement section of the research proposal is shown in Box 4.
Representatives from local stroke support groups, the North West Users Research Advisory Group (facilitated by SM, Steering Group member) and Bury Speakeasy have been involved in the development of the grant application by meeting with the research team and commenting on drafts of the proposal.
The programme will have a dedicated Patient, Public and Carer Involvement (PPC) Group, led by Jacqui Vella (recent chairperson, Preston PPI) and comprising 6–8 members; the Group will contribute to all components of the programme. Two members will sit on the Steering and Management Groups. Roles throughout the programme will include participating in the interpretation of data and research dissemination.
The intervention, and how delivery, uptake and adherence could be improved, will be debated by the Group before finalising the intervention. The Group will be provided with structured summaries of the available research based on the Canadian Institute for Health Services Research model. The Group will also be invited to participate in the systematic review using techniques from the Social Care Institute for Excellence.
Given the sensitivity of the topic, the Group will advise on appropriate ways to involve patients in the research: advice will be sought on recruitment strategies, and ways in which researchers can build rapport and trust with patients before beginning any data collection.
The Group will assist health professionals in delivering the education programme in each phase. Group members will evaluate findings from the programme and assess the effectiveness of the intervention and its components.
PPC Group members will be paid £100 for attending Steering and Management Group meetings, £35.60 for education activities and £10 per hour for all other activities. They will also receive travel expenses.
The University of Central Lancashire COMENSUS (COMmunity ENgagement and Service User Support) project will provide key links with service users, carers and user groups.
Forming the patient, public and carer involvement groups
The original plan in the proposal was for one dedicated PPC group. Following notification that funding for the research programme had been secured, the team visited groups consulted in the proposal preparation phase, explained the programme and asked for volunteers to form the PPC group. Four members were recruited from the Royal Preston Hospital Stroke Club, one from the North West Users Research Advisory Group and one service user who was already active within COMENSUS (the PPC group chairperson). Other founding group members included two lay members, one representative from the Stroke Association, the director of Speakeasy and a nurse specialising in continence from a local primary care trust; a total of 12 members. The group comprised stroke survivors, carers, lay members and health professionals with an interest in UI after stroke (hereafter known as the Preston Group).
The research team originally planned to include stroke survivors with aphasia as part of the Preston Group. However, further discussion with the director of Speakeasy led to the decision to form another group (hereafter known as the Speakeasy Group). Meeting separately enabled the involvement of people with aphasia to be supported in an appropriate manner through an experienced facilitator, Gill Pearl (GPe). To recruit volunteers for this group, LT visited the routine Speakeasy Group meetings and discussed recruiting participants for the ICONS programme through an ongoing process including a presentation to the whole group and one-to-one discussions with members who expressed an interest. The Speakeasy Group comprised eight stroke survivors with aphasia, one carer, one volunteer with Speakeasy and the director. LT led each meeting; members were supported to contribute by GPe.
The Preston Group began meeting in January 2009. Over the course of the programme, one member died, one health professional was no longer able to dedicate time to the group, one had a further stroke and was unable to participate further and four members left for other reasons. Four additional members joined the group, bringing membership to nine. The Speakeasy Group also began meeting in January 2009 and had only one change of membership, with the volunteer with Speakeasy leaving the group approximately half way through the 5-year period.
Aims of the groups
Patient, public and carer involvement groups were established to:
-
contribute to all aspects of the research programme
-
produce a publication using the ICONS project as an example of good practice in PPC involvement.
Group structures and processes
The ‘getting to know you’ process began before the first formal meetings of both groups. This approach is recommended by INVOLVE194 as a means of developing trust with people you would like to work with. It is particularly recommended when recruiting people not often involved in research who may view a university researcher as an ‘elite, authority figure or remote from their everyday lives’. 194 This process was continued in the first meeting of the Preston Group, with carefully planned activities to facilitate members getting to know each other and the research team. These included an informal lunch prior to the meeting, and an initial ‘icebreaker’ activity involving members chatting about themselves with a partner for 2 minutes, changing over, and introducing their partner to the group. The agenda was formed in discussion between the group chairperson (JV) and LT. LT and JV also agreed to have a section at the end of each meeting for evaluation: members were asked to report, in turn, three aspects of the meeting that had gone well and three aspects that could have gone better. This information was then used by LT to build on positive comments and address negative ones in subsequent meetings. The information was also reported in the minutes in an anonymised form. After the initial meeting, group members commented:
very friendly meeting; provided a valuable and different perspective; enlightening and enjoyable experience; very positive; great enthusiasm among the group; learnt a lot, very interesting.
The first meeting also involved an introduction to the research programme, presented by LT. Much debate ensued, for example about the methodology to be adopted, but members were also keen to share their own experiences of living with stroke and, in some cases, incontinence, as well as their experiences of the hospital system.
Although the Preston Group met at the ‘home’ of the research team at the university, the Speakeasy Group met in the Speakeasy offices on the same day as the Speakeasy Group’s usual Speakeasy meeting. This ensured members did not need to travel some distance to the university and also that members did not have to make an additional trip for ICONS meetings. Issues discussed above in relation to group formation did not apply to the Speakeasy Group, as all members knew one another and the majority had worked together on another large research study, Accessing Communication Therapy in the North West (ACTNoW). 197 However, there were many other challenges for the researcher in terms of facilitating involvement. For example, the process of arranging meetings needed to accommodate the needs of people with aphasia: simply writing to members with meeting dates in an aphasia-friendly format was not sufficient; GPe advised telephoning all members 1 day in advance of the meeting to remind them of the meeting details.
The agenda for all meetings was set by the ICONS Programme Co-ordinator (LT). In contrast to the Preston Group, which was chaired by a group member (JV), the Speakeasy Group was chaired by GPe, an experienced speech and language therapist and the director of Speakeasy. All materials and presentations for this group were developed in an aphasia-friendly format, for example using pictures and white space in between text. Meetings were also designed to ensure all members were able to contribute through a process of skilled facilitation provided by GPe. This included strict adherence to a set of ‘meeting rules’, for example: no one person was allowed to ‘hog the limelight’ and only one person was allowed to speak at any one time.
Patient, public and carer role in programme management
Two members of the Preston Group attended all Programme Steering and Management Group meetings; they acted at the interface between programme management and the PPC group in order to ensure co-ordination and transparency. Both the Steering Group (who meet bi-annually) and the Management Group (who meet quarterly) had regular agenda slots where PPC representatives reported on the work of the group. PPC members were encouraged to contribute to all discussions in both groups and their input was actively encouraged and valued by the project team.
In the data analysis and interpretation phase of the programme, two members from the Speakeasy Group were also invited to join joint Steering and Management Group meetings to enable them to contribute their views on how data should be interpreted. Although not totally aphasia friendly, the project team tried to adhere to Speakeasy Group rules, paying particular attention to the rule of only one person speaking at any one time. All presentations were prepared in both aphasia-friendly and ‘normal’ format and shown simultaneously during the meeting.
Examples of the work of the groups
Review of documents and development of aphasia-friendly versions
A key task for both groups was critical review of documents prior to submission to the Research Ethics Committee. These included patient, carer and consultee information leaflets and case study interview questionnaires for patients, carers and health professionals. The Speakeasy Group focused on compiling aphasia-friendly versions of consent forms and information leaflets, whereas the Preston Group critically reviewed documents for patients and carers without aphasia.
Both groups reviewed interview schedules and substantially revised the clarity of the questions compiled by the research team. Box 5 shows how questions asking patients for their experiences of the study intervention, have been shaped by PPC input. An example of an aphasia-friendly consent form is shown in Appendix 32.
-
How easy (or difficult) did you find it to follow the regime? What was the biggest challenge for you?
-
Did you feel that the regime you were being asked to follow had been individually designed for you?
-
Did you resent the regime at all?
-
What helped you to stick to the regime?
-
Was the regime explained to you clearly enough? What suggestions would you have to help make the regime clearer to people in your situation?
-
Was the written information you were given clear enough (in terms of language, readability, clarity)?
-
What was the most surprising aspect of the regime?
-
What do you think of the programme?
-
What was the biggest challenge?
-
What was the easiest part?
-
Did you feel that the programme was designed specially for you?
-
Did you have enough support?
-
How well did you understand what you had to do?
-
What could have been made clearer?
-
Was anything about the programme a surprise to you?
-
What was this?
-
Preliminary validation of the main outcome measure for use with patients with stroke
Six members of the Preston Group participated in some preliminary validation of the main outcome measure to be used in the trial phase, the ICIQ-UI Short Form. 162 To our knowledge, the ICIQ-UI Short Form has not been used in the post-stroke population. We conducted preliminary validation of the tool with six stroke survivors from the Preston Group using the approach recommended by the ICIQ developers (Dr Nikki Cotterill, personal communication). This involved asking participants to complete the ICIQ Short Form and to answer some questions relating to ease of completion, clarity of items and instructions and adequacy of coverage of issues related to urinary leakage.
Development of online training programme for ward staff
Two members of the Preston Group completed an early version of the online training programme designed for ward staff to complete before beginning to deliver the intervention. Their suggestions for improvement were incorporated in the final version.
The Speakeasy Group helped develop a section for the online training programme. The group identified the need for this section based on their experiences (both positive and negative) of communicating with stroke unit staff following their stroke. The group defined two aims for this training: helping ward staff identify patients with aphasia and outlining strategies for communicating effectively with them. Content was designed to meet these and included sections on common communication problems after stroke; difficulties faced by people with aphasia after stroke; what nurses can do to help people with aphasia communicate; how to help people understand and express themselves; and overcoming associated difficulties (e.g. visual problems, auditory problems, attention span and fatigue). Finally, presentation and layout was finalised and the material incorporated into the online programme.
Speakeasy Group members expressed the view that the best way to improve nurses’ communication with patients with aphasia would be for them to go out to study sites and share techniques, together with the opportunity to practise. However, this did not prove feasible as it was not included in expenditure requests in the original bid and there were practical issues given the wide geographical spread of study sites.
Involvement in facilitating implementation of the systematic voiding programme
Following feedback from the research team about problems embedding the SVP in some intervention sites, the groups proposed a novel implementation strategy in the form of a series of ‘motivational’ visits to stroke units. The visits aimed to encourage staff to think about continence from another angle and motivate staff to improve their continence management practices. Both groups developed a short presentation to share their perspectives on incontinence and to trigger discussion of what it is like to experience incontinence from a patient point of view. One member of each group visited four sites with LT or GPe. The presentation was followed by a question and answer session with nursing staff and, in some sites, members of the MDT. Twenty-nine evaluation forms were returned. Examples of comments were:
-
very informative
-
[presenters were] honest and open
-
I thought it was very good that those giving the presentation had an inside and personal knowledge
-
Made me think!
-
Really interesting to have an insight into ICONS. Able to understand how patients feel and how they can cope after stroke.
Dissemination and publicity
Both groups were involved in discussions regarding appropriate channels for dissemination, including identifying relevant conferences. The groups combined to present at a range of national and international conferences, including a section on user involvement in ICONS within symposiums at the Royal College of Nursing International Research Conferences in 2010 and 2014 and a showcase entitled ‘User involvement in the ICONS: Identifying Continence OptioNs after Stroke study: a model of consultation and collaboration’ at the INVOLVE annual conference in Nottingham (2010).
The groups have also commented on all conference abstracts and programme publications prior to submission, a substantial task given there have been over 50 international, national and local presentations from the ICONS programme. Their contribution has been recognised through authorship on all published conference abstracts and research publications.
A further example of dissemination was an interview on BBC Radio Cumbria on 14 September 2011, where a PPC group representative and LT discussed challenges involved in changing continence practice.
Discussion
Group processes
The process of putting together a project-specific group through the Speakeasy organisation was viewed as highly successful by group members. It enabled us to ‘reach out’ to people with aphasia who had previous experience of working with researchers as well as those for whom this was the first time. In working with this group, the researcher adopted the ‘Speakeasy culture’, for example using ‘tried and tested’ group rules to facilitate contribution and inclusion of all members, and procedures for setting up meetings (e.g. telephoning all participants the day before). According to the group, these systems worked well and, in their view, should be adopted more widely.
The two groups’ different ways of working did, however, pose some challenges when the groups asked to meet together so they could get to know one another. This worked well when the meeting had a social focus, for example one member of the Speakeasy Group completed a sponsored cycle ride in India in aid of charity and presented his findings to the combined groups. Issues arose, however, when a joint meeting was scheduled outside Speakeasy and where the groups were required to complete a specific task, putting together a draft publication for the INVOLVE newsletter. The Preston Group were not familiar with the Speakeasy rules, or indeed communicating with people with aphasia, and the researcher had not asked GPe to facilitate the combined group. Consequently, the Preston Group unwittingly broke the rules, for example by finishing off the sentences of the Speakeasy Group members, and the meeting was generally viewed by the Speakeasy Group as not successful. Following expert guidance by GPe, the Preston Group learned to conform to meeting rules and subsequent joint meetings were productive and enjoyable.
Reasons for lack of broader involvement at the proposal development stage
INVOLVE199 recommend that involvement with patients, carers and others should begin at the stage of identifying topics for research, prioritising these and advising on what research should be commissioned. This may be practically difficult unless researchers have immediate access to a number of individuals or groups with relevant experience. In ICONS, PPC input began at the ‘designing research’ stage; user involvement in the proposal development phase fitted the INVOLVE definition of ‘consultation’,199 generally regarded as a low level of involvement with the researcher maintaining control over the agenda. 193 The grant application arose out of several smaller studies (including a systematic review for the Cochrane Collaboration), none of which involved service users. The researcher who designed the study and wrote the proposal had no experience of service user involvement and was ‘feeling her way’ in terms of both finding appropriate existing groups to approach and also working with service users. The possibility exists that had service users been involved at the proposal development stage, the research might have taken a different direction: our plan was to focus on continence management in secondary care, but one member commented that incontinence largely became a problem when adapting to life at home after discharge, as in hospital there was ready availability of toilet facilities.
Issues of empowerment
Since obtaining funding we based our approach on a model of full collaboration (according to the INVOLVE classification199). Views of user involvement as a hierarchy with increasing levels of empowerment up to the point where service users lead the research have been criticised for not reflecting the fact that involvement could be occurring at multiple levels simultaneously, or at different levels depending on the phase of the research study. 193 For example, in the ICONS study two PPC group members were part of the programme management structure and attended Programme Management and Steering Group meetings, as well as PPC group meetings. This increased to four PPC group members in the data analysis and interpretation phase of the programme. As with other members of the programme team, it is likely that the level of decision-making they contributed to differed depending on the group in which they were working.
There were no power struggles between the team and the PPC groups with members keen to allow the research team to set the agenda. However, this did not mean that members were entirely malleable: differences existed on the relevance and importance of the sociological philosophising about empowerment and hierarchical issues in user involvement in research. For the users participating in this study, their only concern was that they were allowed to contribute to the best of their ability as mutually respected partners with the research team.
Members: problem of recruiting members for the Preston Group
The process of recruiting and retaining members for the Preston Group was challenging. Despite visiting and sending out publicity to all the local groups involving stroke survivors and the help of COMENSUS and wider groups such as the North West Users Research Advisory Group, only six members were recruited over an initial 3-month period; this suggests there may be a serious lack of availability of service users and carers willing to take part in research. As the original aim was for the group to comprise eight members, we took the decision to supplement the group with members of professional bodies: the Stroke Association, NHS community stroke services and PromoCon, a national organisation for the promotion of continence (and part of Disabled Living). Two and a half years into the programme, only two of the original service users remained in the group. Given the demographic of our members, people leaving the group through illness, death (in one case) and inability to spare the time because of other caring commitments, was not unforeseen. Although new recruits joined, lack of a stable membership presented a continuing challenge in terms of group dynamics and people ‘getting up to speed’ with a complex research programme.
The death of one member of the group, although not totally unexpected given the demographics of group members, was nonetheless disturbing. It reminded members of their mortality and the particular vulnerability of those who have suffered a stroke. The impact was especially poignant because the deceased was the youngest member of the Preston Group. It says much for the maturity and resilience of the other group members that they came to terms with the sad loss without the need for external support but constitutes an important lesson for other researchers aiming to work with similar groups of patients with long-term (or terminal) conditions to be alert to this risk and the distress it may cause.
Tension between group forming processes and ‘doing the work’
Group members welcomed the opportunity to share their experiences of stroke and their recovery trajectory. On reflection, discussions such as these are likely to be fundamental to the process of forming a new group, but in the researcher’s mind there was a tension between focusing on the tasks allocated for the meeting (in the first meeting working on the participant information sheet) and going through the group forming phase. Part of the learning process for the researcher has involved a recognition of the need to allow much more time to complete each task and focussing on only one or two tasks in each meeting. Understanding the characteristics of stroke survivors is also important; all are different, but there may be commonalities such as fatigue, meaning they tire easily, and limited powers of concentration.
Financial issues
INVOLVE200 outlines the benefits of paying members of the public for their involvement in research, for example as supporting equity of power between the research team and the public, supporting inclusion and reducing barriers to involvement, such as ability to cover the cost of transport to meetings. Our policy at the proposal stage was to pay all members an hourly fee for meeting attendance, a fixed sum for attending Management and Steering Group meetings and all expenses. However, several factors emerged during the course of the project that meant the funds allocated for patient and public involvement were not adequate to cover expenditure. These included forming two groups rather than the expected single group; PPC group members attending conferences to present papers about ICONS (with costs including conference fees, overnight accommodation, travel and a fee of £60 per day) and members undertaking site visits to talk to stroke unit staff about the importance of continence from a patient perspective. Fortunately, the National Institute for Health Research (NIHR) allowed virement between different budget headings and this enabled us to make payments to all PPC group members as well as funding attendance at conferences and site visits.
A further issue was reconciling the needs of PPC members with the university system. For example, members in the Speakeasy Group were not able to complete the standard university claim form; in negotiation with the university finance department, a simple table with details of what each person was due was compiled by the researcher; all members had to do was sign against their name. The university system of paying in advance for travel, etc., and claiming this back proved problematic for some members, but payments were allowed on the university credit card to cover these.
Conclusion
Patient, public and carer Involvement has been a particular strength of the ICONS research programme and has been recognised by INVOLVE as incorporating many elements of good practice. This was evidenced in 2011 by an invitation to contribute to an INVOLVE project developing guidelines for encouraging greater diversity and better inclusion for public involvement in research. This has now been published with an acknowledgement of the contribution of the ICONS team. 36
Many activities, for example motivational visits to study sites, were triggered by the groups themselves. Other suggestions from the groups (e.g. members of the Speakeasy Group doing ‘hands on’ training in communicating with people with aphasia) proved not to be feasible due to financial and practical constraints. Future studies would benefit from a greater focus on relevant PPC activities at the planning stage, with all activities included in expenditure estimates within the proposal.
Chapter 11 Discussion
Overview
In this chapter, we discuss findings according to the each of the objectives of the exploratory trial, and present recommendations for potential modifications to the intervention, addressing issues of feasibility identified, and design of a full-scale evaluation of its clinical effectiveness and cost-effectiveness.
Feasibility: centre and participant recruitment and retention
Recruitment
Centres
We originally planned to begin recruiting into the Phase II trial in October 2010, including stroke services solely in north-west England; however, stroke services in Cheshire and Merseyside were unable to supply excess treatment costs. Having gained agreement from NIHR, we began the process of recruiting sites in Wales, as funds are held centrally by the NISCHR and we were given early in-principle agreement that funds would be forthcoming. Further delays in starting the Phase II trial were due to:
-
negotiating different research and development approval systems in England and Wales and the variable time scales (with the process in Wales taking over 7 months for all sites and, in some cases, over a year)
-
obtaining excess treatment costs to fund the additional staff required to implement the intervention [2.8 whole-time equivalent (WTE) HCAs in each stroke service] in a climate of cost savings in the NHS
-
recruiting HCAs and research nurses in a climate of vacancy control procedures (in some sites, this took around 1 year).
Despite all of these challenges, recruitment only started 3 months later than planned, in January 2011.
Two centres dropped out following randomisation; one withdrew due to changes taking place within their stroke service leaving little scope for supporting a new study, the other was found to have a much lower number of admissions per annum than anticipated (around 120 compared with an original estimate of 300). As both clusters were in intervention arms, this could have posed a problem in terms of new sites needing to catch up with sites who had already started their training. However, due to the varying lengths of time taken to receive site-specific approvals and recruitment of the additional 2.8 WTE HCAs, sites started as soon as the above were in place and the majority of staff had completed their training. The issue of replacement sites being behind in terms of their training therefore did not arise.
The feasibility trial has addressed the issue of cluster dropout and lessons learned should minimise the risk of occurrence in the full trial. We will not recruit small centres, obtain more robust estimates of the annual numbers of stroke patients and not employ additional 2.8 WTE HCAs. We will also increase the number of centres randomised by around 10% to account for dropout post randomisation due to other unforeseen reasons.
Participants
The number of potential participants available for recruitment was affected by the capacity of participating stroke units and the large proportion of people who did not have a stroke being admitted; nearly half of people admitted to intervention (49%) and supported implementation (49%) units had not had a stroke. In one service the acute unit had only 12 dedicated stroke beds. Other units may have had ineffective screening in the A&E department, and/or a lack of nurse specialist and/or stroke-specific consultant physician roles.
The actual numbers of people admitted with a confirmed stroke, according to our screening of consecutive people admitted at each of the participating units, were up to 65% lower per annum than initial figures provided. This difference was particularly marked in intervention groups with proportions 33% and 34% lower than estimates in intervention and supported implementation, respectively, compared with just 4% lower in usual care. This reduced the number of patients eligible to participate in this study, and needs to be accounted for in our future trial, and in stroke trials more generally.
The proportion of people admitted with stroke who were eligible for the trial was half that expected based on prevalence reported in previous studies,4,16 with 19% in usual care and 17% in implementation and supported implementation units. Possible explanations for this are a larger than expected proportion of patients who were (a) not medically stable and receiving end-of-life care and (b) continent by the time they were classed as medically stable.
The percentage of eligible people recruited ranged from 50% to 98% across sites. A higher percentage of eligible people tended to be recruited in sites where research nurses were project specific and funded from the ICONS programme (three sites in each trial arm), compared with locations where CLRN or SRN research nurses were recruiting participants in addition to their other workload (one site in each trial arm).
Interpretation of the inclusion criterion ‘medically stable’ was variable across sites and contributed to delays in recruiting patients by up to 6 weeks (34 patients) and, in one case, up to 12 weeks post stroke. These delays could explain the lower than expected number of people with mild incontinence, which could have resolved by the time people were deemed to be medically stable. Although some of these people may have experienced spontaneous recovery, guidance suggests all those who are incontinent 2 weeks after diagnosis, should be reviewed and a treatment plan developed. 10
Issues with recruitment necessitated a reduction in the target sample size from 752 to 500 in July 2011.
Retention
There were an acceptable proportion (< 20% attrition), as specified by the Oxford Centre for Evidence-Based Medicine,201 of participants for whom outcome data were available at 6 and 12 weeks; and a particular achievement given the nature of the patient population. Strategies for minimising loss to follow-up were revised in December 2011 to include reminder telephone calls when questionnaires were 1- to 2-weeks overdue, with the option to complete over the telephone. At 6 weeks, the response rate was 85% (306/362), excluding participants recruited at 6 weeks and those who had died (usual care 96/114, 84%; intervention 122/139, 88%; supported implementation 88/109, 80.7%). At 12 weeks, the response rate was 88% (330/374), excluding one participant recruited at 12 weeks and those who had died (usual care 98/112, 88%; intervention 132/148, 89%; supported implementation 100/114, 88%). At 52 weeks, the overall response rate was 56%, excluding 98 who had died (usual care 53/95, 56%; intervention 70/124, 57%; supported implementation 53/96, 55%).
The response rate at 52 weeks is disappointing. One explanation might be that outcome and resource use questionnaires were sent together at this time point, unlike at 12 weeks where the resource use questionnaire was only sent once the outcome questionnaire had been returned. Receiving the two together may have been viewed as burdensome. Also, participants would not have been contacted about the study for almost 9 months so their interest in the study may have lessened. Possible ways of increasing the response rate long term might involve maintaining greater contact with participants over the fully study period, possibly including the addition of an interim time point (e.g. 6 months post stroke), or seeking outcome data over the telephone and sending the resource use questionnaire if the participant agrees to this.
Fidelity to the intervention
Adherence to the systematic voiding programme: quantitative findings
The protocol recommended avoiding catheterisation (except for the management of urinary retention or where fluid balance was critical) and reviewing and removing catheters as soon after stroke as possible in line with current guidance. 10 Nearly half of patients in intervention arms were catheterised in the acute stage (139/289, 48.1%); although this is much higher than the 20% reported in the 2010 National Sentinel Audit,11 this percentage is of those recruited and cannot necessarily be extrapolated to all people admitted to the stroke unit. Some aspects of catheterisation appeared closer to protocol recommendations in the supported implementation arm in terms of catheter removal (median 13 days, IQR 5–35 days compared with 20 days, IQR 8.75–35.25) and participants still catheterised at discharge (n = 19, 15.2% compared with n = 35, 21.3%). This finding must be viewed with caution, however, as information on reasons for catheterisation were not collected systematically after baseline and may have been legitimate.
In terms of adherence to clinical logs, documentation of the regime interval and the schedule of proposed voiding times was done on less than half of occasions (38.9% in intervention; 31.9% in supported implementation). Given that the regime interval and schedule are essential for undertaking the programme, these figures are disappointingly low. Only clinical logs for which the regime interval and schedule had been documented correctly were then examined further: documented voiding times showed that, on average, patients were toileted within 30 minutes of the proposed time on 54.8% of occasions in intervention and 56.0% of occasions in supported implementation. For these select clinical logs (on which a regime interval and schedule were correctly documented), documentation of two key components of ‘best practice’ was also examined: asking the patient if they were dry or wet (PV programmes only) and giving the patient encouragement. These ‘best practice’ components were done on over half of occasions (asking the participant if they were wet: 57.9% in intervention; 65.9% in supported implementation; giving encouragement: 58.4% in intervention; 57.5% in supported implementation). It is important to acknowledge that only clinical logs that had a regime interval and schedule of proposed times correctly documented were examined for documentation of the actual voiding times and of the best practice components: this select group of clinical logs is not necessarily representative of all the clinical logs collected.
Completion of daily clinical logs has been used as a measure of adherence to the SVP. Evidence from the qualitative process evaluation suggests nursing staff did not necessarily document everything they did, and completion of clinical logs may therefore underestimate the true level of adherence to the programme.
In both intervention arms, the majority of people eligible to receive BT or PV actually received it (intervention 102/114, 89.5%; supported implementation 82/93, 88.2%). Furthermore, there is evidence that conservative interventions started promptly after completion of the 3-day diary in line with the protocol (intervention: median 2 days, IQR 1–4 days; supported implementation: median 1 day, IQR 1.00–2.25 days).
Just under half of participants received the correct intervention (BT or PV, 86/180, 47.8%); this was similar across intervention arms (intervention 42/100, 42%; supported implementation 44/80, 55%). The majority of participants in both trial arms (158/180, 87.8%) were put on PV with only 22 (12.2%) subsequently going onto BT. It is not clear whether this was a conscious deviation from the protocol, perhaps because staff found PV ‘easier’ to implement, or whether staff misunderstood the guidance provided. Although the purpose of BT is to improve bladder function with the aim of regaining continence, PV has a different aim of minimising incontinent episodes through prompt intervention by nursing staff. 114 BT assumes an active role on the part of the person and may not have been possible given the level of functional ability and also the priority afforded to this part of their rehabilitation by participants, particularly in the early stages. Unit staff might have preferred PV for participants with no cognitive impairment given that our patient population may have had many other disabilities precluding active involvement in BT, for example inability to complete their own bladder diaries or read the time. In the future trial, more emphasis needs to be placed on training nursing staff how to interpret the 3-day diary and how to tailor a voiding schedule for BT. Specific criteria for transferring participants to BT when appropriate will also be required. At 6 weeks, there is some evidence that BT may have led to a better outcome than PV or usual care, particularly in the intervention arm (p = 0.070 dichotomised, p = 0.094 ordinal categorisation), therefore encouraging BT with suitable patients may maximise chances of regaining continence.
Despite extensive liaison with therapy staff, no intervention sites included PFMT as part of a combined intervention with BT. Lack of therapist involvement in identifying, assessing and managing UI after stroke was highlighted in the case study (see Chapter 4) and is out of alignment with the key role recommended by both evidence157–159 and policy. 141 Delivering the intervention as intended (i.e. as a combined intervention of BT and PFMT) may have led to more improvement in participant outcomes; this needs to be addressed in the future trial through specific training for physiotherapists, occupational therapists and nursing staff in the latter type of therapy.
Given the high rate of catheterisation found in this study, avoidance and management of catheterisation needs to be incorporated into the SVP. More detailed guidance needs adding to intervention protocols in the future trial, particularly around avoiding unnecessary catheterisation, conducting a ‘trial without catheter’ as soon as possible and reviewing catheterised patients on a weekly basis.
Qualitative assessment of feasibility from the perspective of multiple stakeholders
Soft systems analysis
The organisational and clinical work contexts of implementation activity, described within a clinical system, can be characterised as a rich matrix of forces127 that may constrain or enable implementation. Within implementation research, current approaches for the consideration of context focus primarily on a diagnostic analysis of barriers and enablers which may be addressed through tailored implementation approaches,52 or through the measurement of context as an explanatory variable at specific time points. 130
Thinking about context from a soft systems perspective may provide new insights into the challenge of implementation at the interface of organisational attributes and systems, and clinical work. Although generated solely through the perspectives of health-care professionals, our data demonstrate that the management of post-stroke UI is characterised as a soft system with multiple and potentially competing understandings, embedded with other clinical microsystems within the stroke service. The data identify multiple influences on implementation, not least the degree of synergy across systems. Our data do not demonstrate how these influences might play out in practice as implementation progresses, but wider theory provides some indication of those that may be most important to consider.
Across these interviews, there is an obvious lack of a consistent, and fully shared, clinical paradigm into which the ICONS SVP is to be implemented, with essentially two ‘competing’ paradigms potentially reflecting differences in views about clinical priorities and other contextual influences. One paradigm sees incontinence care as a legitimate focus for rehabilitation, around which careful assessment and goal-oriented approaches to intervention can be organised. The other paradigm sees post-stroke UI as a barrier to rehabilitation, limiting the ability of people with stroke to engage actively in planned rehabilitation activities. Paradigms are enacted through the decisions and practices of individual staff members, and unsurprisingly there is evidence that both clinical paradigms can exist within individual clinical sites. There was some evidence that these practices were associated with wider organisational cultures and approaches to rehabilitation. The SVP intervention is associated with a shift in practice away from haphazard assessment and an organisational routine approach to the management of post-stroke UI, towards a more individually tailored regime with embedded monitoring and feedback of continence. This shift in practice appears to be more consistent with the first clinical paradigm, and implementation may be more successful where this clinical paradigm predominates. 202
The way that health-care professional participants describe the nature of their clinical work in interviews suggests that, other than for the assessment of UI, the active management of post-stroke urinary continence within clinical sites can be characterised by organisational routines. These routines form the basic structure of clinical work around which individualised approaches to patient care, and multidisciplinary practices, can be negotiated. The organisational emphasis on routines appears to be growing alongside the introduction of ‘intentional rounds’ which address wider political concerns about the quality of health care. 203 The SVP requires staff to move from organisational routine to personalised practice, for example in tailoring PV to a pattern of continence highlighted in a 3-day diary. Implementation will therefore require considerable ‘tinkering around the edges’ of these organisational routines. Clearly, there will be significant potential to regress towards organisational routine, justified by the efficient use of resources where individual voiding patterns being implemented in clinical settings are actually quite similar.
The data indicate varying degrees of priority attached to urinary continence relative to other aspects of practice, with some staff clearly of the view that the acute stroke period is not the time to be focusing on this aspect of care. This runs counter to the narrative around the significant workload attached to reacting to episodes of incontinence. Logically, time spent dealing with incontinence may limit the ability to perform essential activities in other areas of care, particularly where staffing resources are limited. It may also be linked to wider views about the intractability of UI, or perceptions of the futility of professional interventions within this area of practice. Regardless of which explanations apply, and all probably do to some extent, successful implementation of the ICONS interventions may be dependent on raising the importance of continence issues within the acute stroke period. These data would suggest that success in limiting the workload impacts of UI will be an important mediator of implementation.
Integrated working around continence by a wide range of health-care professionals was clearly evident in those sites which espoused rehabilitation approaches to managing post-stroke urinary continence. Although effective teamworking is seen as an essential prerequisite for good stroke care,10 it is also seen as a positive attribute of organisational contexts conducive to implementation. 40 It can be assumed that integrated working is characteristic of some shared endeavour around which different staff groups can collaborate. Integrated working appeared to manifest in collaborative action around continence in a number of ways, and from which collaborative action on implementation within the ICONS programme could build. Specific examples included therapists’ embedding continence work into nursing strategies targeting broader, functional issues such as washing and dressing, and mobility; and the reorganisation of clinical schedules to accommodate different contributions to continence care.
The data provide examples of multiple potential drivers for good practice around continence within the clinical microsystem, many consistent with the principles underpinning the ICONS interventions. However, maintenance of the clinical system appears to be somewhat fragile, largely dependent on the professional interests of individuals such as link nurses. In addition, despite knowledge and skills around post-stroke UI being key components of the Stroke-Specific Education Framework,204 the provision of education and training for staff appeared to be haphazard. This situation may reflect a generalist view of what is required of staff in this clinical system, and is consistent with reports in the wider literature on the nursing role in stroke care. 155 In the data, there were examples of talk which demonstrated that there was little intellectual challenge in addressing continence needs in clinical practice. Inevitably, there appears to be an over-reliance on experiential sources of knowledge in clinical practice. Although an important source of ‘evidence’ for practice, it is essential that this is complemented by other forms of evidence, including research, evaluation and performance feedback. 205 Organisational approaches which synthesise different forms of knowledge for practice through, for example, structured education and training and performance review may be associated with implementation of the ICONS programme. 40
Although there is no potential to redesign clinical environments to support implementation of the ICONS interventions, the data demonstrate that staff, particularly nurses, are not passively constrained by the clinical environment and facilities within which they are working. Although the nursing role has always included attention to maintaining an environment conducive to rehabilitation,155 there is also evidence of creative use of facilities when these were lacking. These structural aspects of implementation should not be overlooked, and highlight the importance of engagement with staff and understanding clinical microsystems before any redesign takes place.
Our data do not map out the clinical systems in their entirety; this would require an alternative approach to sampling within clinical sites. However, the data indicate the complexity of the system, with multiple staff members, teams and organisations contributing to work within the system. Inevitably then, this multiplicity is associated with increased potential for problems and error. 206 Interestingly, there is some evidence of the strong influence of individuals from outside the immediate clinical work associated with urinary continence, specifically in relation to purchasing related stock and equipment. It is important to ensure that these perspectives are accounted for within implementation activity within the ICONS programme, and any subsequent roll-out of trial interventions.
Normalisation process theory
The aim of the NPT analysis was to identify factors in the implementation of the SVP which might have influenced the success of the programme. It was very clear from the analysis that the staff thought the programme had been successful in improving patient outcome, so the findings were further analysed to consider the mechanisms of action potentially linking the SVP to outcome. Figure 32 summarises the potential mechanisms.
FIGURE 32.
Potential mechanisms of action linking SVP process to outcome.

Potential mechanisms of action of the systematic voiding programme
The SVP processes are in the clear outlined boxes (see Figure 32). The main feature of the SVP that respondents referred to was its logical and structured approach to organising the management of continence (see Figure 32). Three major mechanisms of action are labelled across the top of the diagram as increased priority, increased ownership, and different care, with their component causal chains illustrated by the blue, dark green and light green coloured boxes. Some of the components are linked together.
Mechanism 1: increased priority
The SVP resulted in changed perceptions about incontinence, from an inevitable and intractable consequence of stroke, towards a symptom that could be responsive to intervention. Owing to the research, there was a strong drive from senior staff to focus on continence care, resulting in junior grade staff being consistently reminded, supported, monitored and encouraged to take responsibility. Staff had a heightened awareness of continence: they talked about being surprised at the lower prevalence of incontinence than expected; and the potential for improvement in people with stroke thought unlikely to benefit. Those with stroke and relatives were also more aware of continence, and staff were conscious of their expectations.
Mechanism 2: increased ownership
A major feature of the findings was staff talking about the benefits of their intervention. They saw improvement in individual people’s continence and they also saw a trajectory of improvement in the paperwork. Staff talked about their enjoyment in seeing people progress and the consequences of developing a continence routine sufficient for people to go home safely. After experiencing the SVP, staff also noted that the extra work was balanced out by a reduction in workload from changing beds, and also that wards were calmer, with fewer buzzers going off because they were pre-empting care. This also linked to people with stroke being aware that continence problems were being attended to, and having knowledge and confidence that staff would attend them when scheduled.
Staff thought the structured approach of the SVP was beneficial for people with stroke as well as for staff, and that it was suitable for people after stroke. They talked about people with stroke being actively involved in working towards control of continence, which gave them hope for wider recovery from stroke. Nurses talked about being more skilled in assessing and managing continence, and in talking to people with stroke. They felt that they were providing constructive help and were proud of their therapeutic role. This was especially the case with HCAs, who liked the training and were often described by senior staff as taking ownership and driving the programme. Senior staff talked about increased discussion of continence by nurses at MDT meetings and in discharge planning.
Mechanism 3: different care
Increased priority and ownership of continence contributed towards differences in the kind and amount of care provided. The logical and structured nature of the SVP meant that staff knew what they were doing. The SVP also provided a basis for co-ordinating care delivery so that everyone was working towards the same goals. The regularity of the intervention meant that it was likely to be remembered and delivered more consistently. Increased vigilance about continence meant that intervention was probably occurring with a wider group of people with stroke than previously; and staff were persevering for longer with people who they might have given up on previously. Extra staff meant that care was able to be delivered consistently, staff were more proactive in intervening and active in managing rather than containing incontinence, and people were also getting more attention. As a result of the assessment and bladder scanning, staff may also have been delivering different care, such as increased investigations, or referrals.
Evaluation of supported implementation
Sites were free to choose their own facilitators, assuming they met the criteria outlined (see Chapter 3). In practice, seven facilitators were in ward manager/ward sister posts and perhaps did not have sufficient time to commit to the role alongside managerial commitments. This may have contributed to a lack of engagement between internal and EFs in between meetings. However, this was true of all internal facilitators, none of whom had dedicated time for facilitation activities. Involvement of EFs in choosing appropriate people may have identified less obvious candidates.
The facilitation manual (see Appendix 14) was the main resource for internal facilitators and outlined both their role and that of EFs. Perceptions of what the internal facilitator role would comprise, and how this would interact with that of EFs, was generally unclear, perhaps suggesting the manual had not been consulted for guidance. This is concerning, as little understanding of the facilitator role has been identified as mitigating against successful facilitation. 132 A consequence was unrealistic expectations of the EF, particularly in two sites.
All sites developed action plans, although there is little evidence these were systematically used to monitor progress. There is no evidence that the many implementation strategies outlined in the manual, for example using ‘rich pictures’ to visualise the intervention, or role modelling, were used in practice. Emphasis within the manual on underpinning theory (e.g. NPT) could have inhibited engagement and there was some suggestion that ‘big jargon’ should be simplified.
Data from interviews and meeting notes suggested internal and EFs were using the key components of the process identified by Stetler et al. ,132 interactive problem-solving and supporting staff through the process. The supportive role fits with the concept analysis by Harvey et al. ,129 and also empirical work by Stetler et al. 132 and Cheater et al. ,207 and may have contributed to evidence from process evaluation suggesting embedding the intervention and some aspects of care were more likely to be done well in this trial arm. However, with the exception of action planning and progress meetings with EFs, it is difficult to determine the extent to which facilitators used processes different to those used in the intervention group in introducing the SVP. The similarity of techniques used to manage change in both intervention groups highlighted in NPT analysis may suggest little difference.
Patients’ perspectives
We were able to undertake only 12 interviews with patients and carers in the trial phase, and not all sites were represented. The small number of participants consenting to an interview may have been partly due to the recruitment process. Patients or consultees were asked to consent to a possible interview at the time of initial recruitment into the study. At this time point, soon after the acute stroke event, participants and consultees may have been reluctant to agree, even provisionally, to giving an interview in the future. The study protocol did not allow for participants to defer this decision to a later date, when some may have regained the capacity to consent. The geographical spread of sites also meant that even when participants did consent to be interviewed, it was not always possible for a member of the research team to do this before the person was discharged.
Data collection was limited to those who had experienced the SVP. It is possible those from usual care may have reported similar experiences to the sample interviewed, but there is no data to support or refute this. Interviews were undertaken in the hospital setting, and we were unable to explore participants’ experiences of the long-term impact of the programme post discharge.
It was apparent that some participants with functional UI held a view that this was not ‘true’ UI, and were therefore unsure why they had been included in the programme. Educational materials may need modification to address this misconception.
Proposed theory explaining how the systematic voiding programme worked in practice
Considering all the data from the process evaluation, we developed hypotheses relating to each main mechanism in the logic model (thinking, planning, doing and evaluating) and considered the evidence for what might have led to mechanisms ‘firing’ consistently and whether or not each hypothesis was supported by the data. Proxy outcomes (i.e. how the effects of the SVP might be seen) were used to articulate if hypothesised mechanisms operated or not. Appendices 33–35 provide evidence from soft systems and NPT analysis to support hypotheses.
Thinking
Table 109 shows the hypotheses and proposed mechanisms of action.
| Main mechanism | Submechanisms: how the SVP might work | Proxy outcomes: how effects of the SVP might be seen |
|---|---|---|
| Hypothesis: The SVP changed thinking about UI from it being a barrier to rehabilitation to a legitimate focus of planned, therapeutic activity |
||
Contextual barriers:
|
||
| Changed perceptions: UI, role | Therapeutic potential | Increased relative priority/attention Changed attitude – guilt, pride |
| Incontinence role (nursing) | Increased nursing knowledge and skill | |
| MDT contribution | More nursing input to MDT | |
Prior to the intervention, nursing was ascribed with expertise in continence management but in reality there was no evidence that expertise was being used and nursing practice revolved around containment. Nurses did not consistently view UI as an important priority in care delivery relative to other aspects of care and what we were proposing was potentially at odds with their approach to supporting people with continence problems.
There were three possible explanations for how thinking might have been changed by the SVP. First, taking part in ICONS and introducing the SVP led to changed perceptions of continence as a legitimate focus for rehabilitative practice. The increased focus on continence and training in underpinning theory and practice led to a change of paradigm: incontinence was now viewed as amenable to change, rather than intractable. There was evidence increased knowledge and skill in promoting continence led to this work being valued and pride in the rehabilitative, therapeutic nursing role. The SVP delivered increases in knowledge and skill and an opportunity for nurses to demonstrate this, providing legitimacy for claims that extended the nursing contribution around UI management. However, this was more apparent in rehabilitation than acute units where competing priorities (i.e. more technical interventions) still came first.
A second potential explanation was a change in nurses’ perception of their specialist role. As well as changed attitudes towards continence, role change and increased status was evident for HCAs, who often took the lead driving and co-ordinating continence care. This change was less obvious and widespread for qualified staff.
There is some evidence to suggest qualified staff continued to view incontinence work as low level, suggesting their work was less discernibly different to their existing role and may have failed to provide reward. Although the potential for continence to reconfigure the nursing role within the MDT was recognised, there were fewer examples of the change occurring. This could be owing to less involvement from qualified staff: their role typically focused on assessment and they were not necessarily actively involved in other continence activities. Lack of engagement in the whole cycle meant their practice was not sufficiently different to trigger an observable shift in their recognition and enactment of a specialist nursing role within continence care.
A third potential explanation was a change in the perception of nurses about their role and contribution within the MDT. Although there was some discussion at the MDT meeting and increased expectation of the requirement of nurses to report on continence, the uptake and promotion of a specialist nursing role in the management of continence within the MDT was not seen.
In summary, consistency across data sources and settings suggest there was a regular pattern of impact around changed thinking about continence, particularly its relative priority and value in rehabilitation settings. However, role change was largely specific to HCAs rather than qualified staff, and perceptions of a specialist nursing role for continence care within the MDT remained an aspiration rather than reality.
Planning
Table 110 shows the hypotheses and proposed mechanisms of action for possible changes in the planning of care.
| Main mechanism | Submechanisms: how the SVP might work | Proxy outcomes: how effects of the SVP might be seen |
|---|---|---|
| Hypothesis: The SVP made a structure for UI care explicit, enhancing consistent, knowledge-based delivery |
||
Contextual barriers:
|
||
| Logical structure: | Codifying and embedding care at an individual level | More people receiving UI care |
| Improved organisational consistency | Less variation | |
| More continence talk | ||
| Fewer system failures | ||
Prior to the intervention, and reflecting the disparity between ascribed and practised roles in continence, the default position regarding services was a lack of focus for clinical leadership of continence care and a mismatch between skills, knowledge and practice. In addition, configuration of services did not facilitate collective working and planning. The logical structure provided by the SVP enabled a route to improved planning of care at two levels: individual patient and organisational.
At the individual patient level, protocols designed to codify and embed the new practice appeared to engender improvements in consistency and organisation, and increased communication between staff and between staff and people with stroke. Findings suggest the logical structure was hugely successful for both participants and staff, with more people identified as needing and receiving continence care and less variation in practice. However, some elements of the SVP were difficult to implement, for example distraction.
Enacting the plan at an organisational level was not without difficulty, for example assessment proved unpopular and the SVP was not perceived as helpful with all client groups (e.g. people with functional incontinence). The logical structure ensured there were fewer system failures at an organisational level, such as patients not having a management plan. However, organising and ensuring consistent delivery of a tailored SVP to individual people within the ward environment and routine was problematic, with some over-rigid adherence to 2 hourly toileting following the introduction of national safety initiatives (e.g. intentional rounding) and a tendency to regress to routinised care when under pressure.
In summary, changes in the ability to plan care has explanatory power at the individual level but findings illustrated more work would be needed to help staff to embed the SVP into organisational planning.
Doing
Table 111 shows the hypothesis and proposed mechanisms of action for changes in what nurses did in terms of continence care.
| Main mechanism | Submechanisms: how the SVP might work | Proxy outcomes: how effects of the SVP might be seen |
|---|---|---|
| Hypothesis: The SVP helped staff to make the shift from an organisational approach to continence that was unsystematic, routine and selective to one that promoted regularity, inclusion and individualised management |
||
Contextual barriers:
|
||
| Changed clinical work: | Selection | Different patients receiving care |
| Diagnosis | Differentiated/correct care | |
| Routine | More care regularity | |
| Perseverance | Sustained delivery | |
| ‘Different talking’ | ||
Pre intervention, there were strong contextual barriers to individualised continence management, including insurmountable routine systems and focus at an individual, rather than an organisational, level. An explanatory hypothesis across the data was that the SVP helped staff make the shift from a routinised organisational approach to continence to integrated working around individualised management. Two main mechanisms around this shift in practice were changes to who received care, and to the care received.
The selection and diagnosis of patients changed from opportunistic to protocol driven: different patients (including young and cognitively impaired patients) received different care (assessment, bladder scanning, consistent and systematic attention and individualised regimes). However, although there was evidence patients were put on different regimes (BT or PV), individualising voiding intervals based on assessment data was difficult to achieve, with practice moving from unsystematised to routinised around 2 hourly toileting. Care was less selective with more people receiving a structured approach, but individualisation was difficult to implement, although it appeared easier to achieve with people able to participate fully in their own regime.
Changes to care included delivery on a more regular basis for different time periods (longer contact time; prolonged involvement) and a change in discourse around continence issues between staff and people with stroke (persuading, negotiating, expressing). Staff recognised that changes in people’s outcomes were the result of doing the SVP over a sustained period. Staff also recognised the reduced workload associated with catching people in time (i.e. managing incontinence rather than increasing continence), but sustained delivery of the SVP proved to be an uphill struggle.
In summary, although there was some explanatory power that the SVP promoted regularity and inclusion, individualisation was difficult to implement.
Evaluating
Table 112 shows the hypothesis and proposed mechanisms of action for evaluating continence care.
| Main mechanism | Submechanisms: how the SVP might work | Proxy outcomes: how effects of the SVP might be seen |
|---|---|---|
| Hypothesis: The SVP and its interpretation increased visibility and enabled staff and patients to evaluate process trajectory, workload performance and outcome |
||
Contextual barriers:
|
||
| Visibility: | Process | More surveillance |
| Trajectory/outcome | More evaluation | |
| Workload | Recognition | |
| Patient and family | More patient control | |
| More relative involvement | ||
Findings from interviews with stakeholders revealed that at the start of the intervention period, services within the trial demonstrated little, if any, attention to systematic evaluation of clinical practice or patient outcomes around UI. The data demonstrated that the SVP increased the visibility of continence management in three ways: increased surveillance of process; greater evaluation of patients’ trajectories and outcomes (including scrutiny of nurse performance in achieving these); and closer attention to workload.
For people with stroke and their families, documentation provided a visible means of monitoring progress. Families perusing daily clinical logs provided an incentive for nurses to ensure toileting was timely, and also documented. People with stroke liked the fact that nurses were actively managing their continence and the control the SVP afforded. In addition to triggering the provision and review of care, the paperwork provided a means through which performance of individual nurses could be monitored by other clinical staff and managers.
The second mechanism involved making patient trajectories and changes in outcome obvious through the daily and weekly paperwork. Although there was evidence of increased monitoring, systems were at an individual, rather than an organisational level. The SVP was less effective in facilitating systemic outcome monitoring over time and across organisational boundaries, i.e. after discharge to the community. Contextual barriers included progress being dependent on participant’s responsiveness. This explanation did not apply consistently in the acute setting where staff had limited opportunity to see success due to the relatively short length of stay
Third, staff appeared to be motivated to continue by a visible reduction in workload, for example where there was less incontinence to deal with.
The hypothesis that the SVP enabled staff and participants to evaluate increased visibility of process, trajectory, workload, performance and outcome was well supported.
A preliminary evaluation of intervention and supported implementation relative to usual care
Primary outcome
There was no suggestion of a beneficial effect of the SVP (with or without supported implementation) on outcome at 6 weeks post stroke. However, almost 50% of patients had received less than 2 weeks of their allocated intervention by this time point and over 25% had spent less than 7 days on the programme. Findings were similar at 12 weeks post stroke (intervention vs. usual care: OR 1.02, 95% CI 0.54 to 1.93; supported implementation vs. usual care: OR 1.06, 95% CI 0.54 to 2.09, respectively) and the intervention arm outcomes were no better at 52 weeks (intervention vs. usual care: OR 0.56, 95% CI 0.27 to 1.16; supported implementation vs. usual care: OR 0.60, 95% CI 0.27 to 1.30).
Overall, 161 (39.7%) of participants were continent at discharge; 38 (31%) in usual care, 72 (44%) in intervention and 51 (41%) in supported implementation. Relative to usual care, the intervention arm had an OR of 1.47 (95% CI 0.81 to 2.67) of being discharged continent, with supported implementation having an OR of 1.54 (95% CI 0.83 to 2.85); the overall difference between trial arms was non-significant (p = 0.32). Overall, participants in the two intervention arms had 1.50 (95% CI 0.88 to 2.57) (p = 0.13) times the odds of continence at discharge than usual care participants. This suggests intervention arms may have been more successful in helping patients regain continence before they left the stroke unit. Difference is adjusted for stroke subtype prognosis, albeit assuming that the intervention is equally effective for all.
However, exploratory evidence suggests that continence status may not have been maintained when the SVP ended on discharge from the stroke unit, with over half of participants continent at discharge (and discharged before 6 weeks) incontinent at 6 weeks (22/38, 57.9%) and two-fifths of participants continent at discharge (and discharged before 12 weeks) incontinent at 12 weeks (22/54, 40.7%). Ways in which the SVP can be extended to facilitate maintenance of continence after discharge need exploring and, if feasible, incorporating into a future trial.
Per-protocol analysis suggested that those who received the intervention according to protocol may have had better 12-week outcomes than those in the usual care arm, although this did not appear to hold for supported implementation; for those who received at least 14 days of intervention, the estimated continence OR relative to usual care was 1.54 (95% CI 0.69 to 3.47), and that for supported implementation was 1.07 (95% CI 0.40 to 2.90). However, this apparent relatively better outcome in the intervention arm was not maintained at 52 weeks, with estimated continence ORs for intervention and for supported implementation relative to usual care both below 1.
Secondary outcomes
There was no evidence of better outcomes on the ICIQ or ISI at 6 weeks post stroke. At 12 weeks post stroke, there was some evidence of better outcomes on the ICIQ in supported implementation (OR 1.22, 95% CI 0.72 to 2.08). Both intervention arms had a higher estimated odds of continence for UUI than usual care (intervention: OR 1.58, 95% CI 0.83 to 2.99; supported implementation: OR 1.73, 95% CI 0.88 to 3.43). There was a similar increase in the estimated odds of continence for SUI in supported implementation (OR 1.82, 95% CI 0.82 to 4.01), but this was not so marked in intervention (OR 1.04, 95% CI 0.45 to 1.82). Although none of these increases was statistically significant, they are suggestive of a potential reduction in the odds of specific types of incontinence. The evidence is more consistent across the arms for UUI. This finding is encouraging, but consistent implementation of BT (recommended for those with urge incontinence) was limited, potentially attenuating the effect estimate. However, disappointingly, there was no real suggestion that this apparent effect was maintained to 12 months post stroke.
In summary, there appears to be a trend towards more favourable continence outcomes in intervention arms at 12 weeks, particularly in those participants with UUI and (to a lesser extent) SUI. Per protocol analysis also demonstrated that participants in intervention who received at least 14 days of intervention were one and a half times more likely to be continent than those in usual care at 12 weeks. However, it is unclear whether this is an indication of effectiveness, selection or reporting bias. A higher proportion of participants were continent at discharge in intervention groups.
Linking process and outcome data
Three of the highest ranking sites on process indicators were in supported implementation; in two of these the intervention was well embedded. In contrast, no intervention sites had a score indicating high embedding, with two showing conflicted or neutral and two poor embedding. It is tempting to link these processes with the trend towards improved outcomes at 12 weeks in this trial arm. Process data were not uniformly positive, however, with one supported implementation site achieving the lowest ranking in terms of adherence data. This site was characterised by severe staffing shortages throughout the intervention period which affected their ability to maintain the SVP despite their best efforts.
Measurement issues
The amount of missing data on the LUSQ question used to identify SUI at baseline suggests this might not be the right tool for diagnostic purposes. Furthermore, participants’ answers in terms of whether they had UUI or SUI correlated poorly with nurses’ opinions of type of UI. Obtaining a valid assessment of type of UI is further complicated in participants with cognitive impairment, as UUI in particular is not easily established if the participant is unable to answer questions themselves.
Adverse events
As people receiving the SVP were theoretically taken to the toilet more often, an increase in the number of falls might have been evident; however, this was not the case and indeed there were slightly fewer falls in supported implementation (three, compared with eight in other arms).
Methodological issues
Risk of bias
Randomisation was carried out by an external trials unit ensuring researchers were unable to influence the randomisation process. Stroke services were aware of their allocation, as were staff identifying and recruiting trial participants who also conducted outcome assessment for participants who were still in hospital at 6 and 12 weeks post stroke. Although we originally intended research nurses to collect outcome data in sites other than their own (and in which they were unaware of allocation), the geographical spread of sites meant this was not feasible. The trial statistician was not blinded during the analysis, although the statistical analysis plan was finalised prior to any outcome data being available. Some additional exploratory analyses were performed, but these are identified clearly as post hoc.
Heterogeneity
There were some differences in baseline characteristics across trial arms, with relatively more males in intervention (86, 52%) compared with usual care (51, 41%) and supported implementation (52, 42%). The proportion of participants with no symptoms on the mRS was slightly higher in usual care (52, 42%) compared with the intervention (54, 33%) and supported implementation (33, 27%) arms. There were also fewer patients with the most severe stroke subtype (TACS) in usual care [37/124 (29.8%)] compared with intervention [80/164 (48.8%)] and supported implementation [68/125 (54.4%)].
This may have occurred due to consent bias, with research nurses in usual care selecting all people with stroke meeting the inclusion criteria, including those with milder strokes and UI, even though their stay in hospital may have been short; the observation that the length of stay was typically slightly shorter in usual care is consistent with this possibility. In intervention arms, it could be that research nurses recruited patients who they considered would be worthwhile and feasible to take part in the SVP rather than all those eligible for the trial. An alternative explanation could be that intervention arms admitted less ‘milder’ strokes.
Patient-related factors affecting patient outcome
At 6 weeks, there is some evidence to suggest people with pre-stroke incontinence were more likely to be continent in supported implementation (p = 0.069). In terms of participant characteristics, 65 out of 66 participants continent at 6 weeks were independent pre stroke as measured by the mRS. ISI category may have had less impact on 6-week ICIQ incontinence frequency in intervention and supported implementation than in usual care; a similarly lesser impact of baseline severity was evident in the intervention (but not the supported implementation) arm at 52 weeks.
At 12 weeks, stroke subtypes other than TACS may be more likely to be continent in intervention and supported implementation (p = 0.054) arms. People with UI pre stroke, and older people may be more likely to be continent in supported implementation (p = 0.048 and p = 0.02 respectively). Only six participants dependent pre stroke were continent at 12 weeks; none of these were in usual care. In terms of other participant characteristics, people with right-sided weakness may be more likely to have greater incontinence frequency (p = 0.080) at 12 weeks; a similar effect was detected at 52 weeks.
There is evidence from the process evaluation that all people with UI received the intervention regardless of pre-stroke continence status or stroke severity, in contrast to usual care, where continence provision may have been less systematic and determined by staff perceptions of who might benefit. It is possible the SVP contributed to people with pre-stroke incontinence regaining continence in supported implementation at 6 weeks, and also those with stroke subtypes other than TACS in both intervention groups at 12 weeks, but not those with severe pre-stroke disability.
Choice of primary and secondary outcome measures for a full-scale cluster randomised trial to evaluate effectiveness
Findings from the exploratory trial suggest presence/absence of incontinence as the primary outcome measure is appropriate to use in the future trial. Given the level of both incontinence and stroke severity in the population recruited, as well as the proportion of those incontinent before stroke, measurement of reduction in incontinent episodes could be measured by the ICIQ question ‘How often do you leak urine?’ This is preferable to objective measurement using the 24-hour pad test, as pad use may lead to more incontinent episodes in this patient group. Furthermore, the ICIQ has been shown to correlate well with the 24-hour pad test in women with urodynamic SUI. 208
The main outcome point selected initially in the exploratory trial, 6 weeks post stroke, requires revision as people were recruited later than expected and almost 50% had received less than 2 weeks of their allocated intervention by this time point. Setting the main outcome point at 12 weeks will provide better evidence of the effectiveness of the SVP in a future trial, particularly if removing the exclusion criterion ‘medically stable’ (as outlined above) contributes to earlier recruitment and a longer length of exposure to the intervention.
Using an incontinence-specific QoL measure, the I-QOL, proved problematic as the majority of questions apply only to those with incontinence and are not all applicable to patients who regain continence. However, it has been recommended for use due to its responsiveness to change in comparison with the EQ-5D180 and, in the absence of a more psychometrically robust alternative, it could be retained with guidance for people who regain continence to answer ‘not at all’ to questions that do not apply.
Developing and testing data collection tools for an economic evaluation within a full-scale cluster randomised trial
We have explored the feasibility of different methods to collect resource use data, summarised the data recorded and have identified issues that will need to be addressed in a future trial.
In order to record fully the costs associated with the programme it would be necessary to have research staff observe and record what the clinical staff are doing – the latter do not have the time to keep comprehensive records, particularly when they are also completing the paperwork necessary for the programme. The benefits of the programme within the hospital may include reduced episodes of incontinence but data reflecting such reductions were not recorded. A future trial will need to consider methods to obtain data reflecting the cost of incontinence from all groups.
The resource use postal questionnaires had a reasonable response rate at 12 weeks and most of the items were answered. At 52 weeks the response rate was lower, and completion of the individual items was also slightly lower, this reduction was more marked for the health and social care items than the aids and adaptations. The response rates to the resource use questionnaires overall were similar between groups at 12 and 52 weeks. Completion of the individual items was similar at 52 weeks compared with 12 weeks. The lower response rate for the questionnaires at 52 weeks needs further consideration and alternative methods of collecting resource use data may need to be considered.
The programme aimed at promoting continence after stroke is resource intensive and generated substantial costs in the short term. Although patients were followed up at 52 weeks, there were substantial amounts of missing data, partly due to around one-quarter of patients having died, but this meant that limited resource use data were available. Consequently, cost-effectiveness was explored using imputed data. The ICERs generated were of a magnitude to suggest that the programme has the potential to be cost-effective, but caution should be taken in interpreting these results given the large amount of imputed data. The need to identify strategies that allow more complete data to be collected is clear.
A definitive trial will need to consider a range of strategies to record resource use data. It is imperative that the right balance is found between the level of input required for collection of resource data and the accuracy of those data. This may include time spent observing and recording clinical practice – in relation to the research – asking patients to keep diaries, and sourcing resource information from the providers. Consideration may also need to be given whether or not the post-programme data collection focuses on those resource items related directly to the programme’s impact.
Recommendations
Recruitment
Centres
Only stroke services with 300 or more people admitted with confirmed stroke per annum should be considered for inclusion in the future trial. This should enhance embedding of the intervention through greater number of patients receiving the intervention and reduce costs in terms of staff training. Homogeneity of sample size within clusters will also minimise the number of clusters required.
Patients
The inclusion criterion ‘medically stable’ should be removed, and the recruitment process should begin as soon as people are identified as incontinent or catheterised. The assessment process can then begin as early as possible, with conservative interventions beginning when the unit team judge the person to be ready.
Intervention design
Potential adaptation to the systematic voiding programme design
All of the individual linkages described above (see Figure 32) are reflected to some extent in the findings, but link together into themes that suggest potential adaptations to the SVP and its implementation in the future trial. These adaptations are consistency, visibility and individualisation.
Consistency
The major strength of the SVP appeared to be the focus and knowledge of continence management that it gave to staff and patients, in a format that was logical and documented. Despite problems with the documentation, it did form the locus of attention for defining action and monitoring outcome. It meant that staff and patients worked together on the same plan, and that people had role clarity. Care was delivered consistently each day, and over the whole trajectory of the patient’s recovery. The SVP was also very accessible to HCAs, giving more meaning and value to a major component of their daily activity. A future intervention could focus on ensuring SVP components stress the value of planning, co-ordination and management of continence care.
Visibility
Perhaps the most surprising finding related to the importance of visibility of outcome on motivation and effort, and the powerful effect of visibility on proactive and therapeutic intent in nursing. Being able to link the effect of nursing actions in improving patients’ lives in the longer term was a powerful driver. It is notable that senior staff found the programme hard to monitor informally, relying on the research nurse to monitor performance. Owing to the holistic nature of nursing care, attention is often diffuse, and it is rare to receive feedback on outcome that can be directly attributed to nursing action. A future trial could focus on ensuring SVP components make continence process and outcome linkages more visible.
Individualisation
The findings suggested a lack of differentiation between regular toileting and the SVP. This, together with the linking of the SVP with intentional rounding, means it is unclear if any improvement in outcome is attributable to the SVP as a whole, or rather to regular and consistent toileting. There was evidence that staff were individualising care to some extent, but it was also evident that this aspect of the SVP was not carried out perfectly. A future trial could focus on comparing regularised with individualised continence care.
Barriers and facilitators to implementation
Appendix 36 summarises the barriers or difficulties, and facilitators or suggestions, for each question posed by the NPT framework. The four main implications for any future trial are discussed below and summarised in Figure 33, for each decision stage in the SVP pathway.
FIGURE 33.
Suggestions for improving the SVP pathway in future trials.
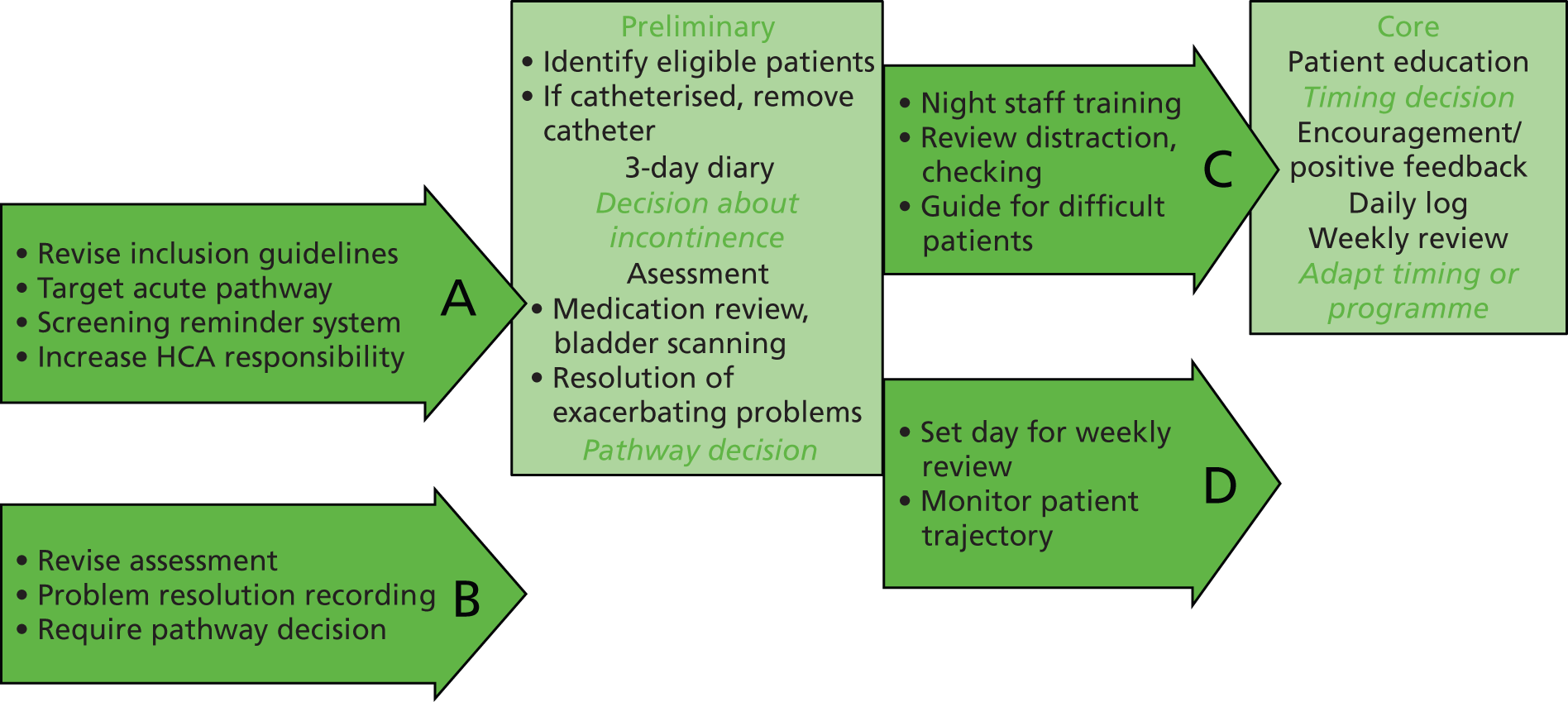
The first stage of the SVP requires the identification of eligible people, removal of catheter and completion of a 3-day diary to make a decision about whether or not a person is incontinent. Difficulties and suggested solutions for this stage of the SVP included:
-
Revise inclusion guidelines There was some confusion and disagreement around the eligibility of people with long-term continence problems, catheters or urinary symptoms without incontinence for inclusion in the SVP. One site suggested improving the information about who to put on the SVP; another site started screening everyone that consented, and said they captured more people, earlier.
Supporting the decision about incontinence
-
Target the acute pathway On sites with acute and rehabilitation wards, a 3-day diary completed in the acute ward could be perceived as unreliable in the rehabilitation ward because it might be partial, or too early. One acute area also thought that a 3-day diary was too long for a short stay area, and that they would be better starting to prompt, but they did think it was worth doing the assessment in the acute area. Review of the use of the 3-day diary in an acute setting is needed.
-
Screening reminder system Maintaining surveillance of potential recruits who were not ready for the 3-day diary because of catheterisation or being unwell was difficult in the absence of a co-ordinating research nurse, because no-one was responsible. One site allocated this responsibility to a HCA for individual people, another to a staff nurse. Either role allocation or some form of trajectory recording could be considered.
-
Increase HCA responsibility Two sites commented that HCAs had responded positively to their involvement with the SVP, two sites thought it important that HCAs saw the link between their input and outcome for the patients to maintain motivation. HCAs were important in continuously monitoring eligibility; tailoring the SVP approach to the needs of individuals and responding positively and persuasively to people who were anxious, demanding or reluctant; and completing the 3-day diary and daily logs. Some sites saw ICONS HCAs take a lead role in promoting the programme. An increased therapeutic role for the HCA in managing continence care seems feasible.
Supporting the pathway decision
The second stage of the SVP requires completion of the assessment and a decision about which pathway the participant will follow – whether PV or BT – and the timing interval based on the 3-day diary. Difficulties and suggested solutions for this stage included:
-
Revise the assessment The assessment was problematic because it was long and because staff had to consult the family to gain the required information. However, staff liked their increased skill and confidence in assessing continence. Streamlining the assessment with staff and linking this to training would be essential.
-
Require a pathway decision Although the programme was seen as providing a clear structure and management plan, there was some evidence of a lack of understanding about different processes for different people, the individualisation of toileting routines based on the bladder diary, and BT processes. Making the link between assessment and the management plan more explicit within protocols might be helpful.
Supporting the timing decision
The third stage of the SVP requires implementing the chosen pathway, scheduling of the timing intervals on a daily basis, and keeping a record of care processes. Difficulties and suggested solutions for this stage included:
-
Night staff training It was important that daily plans were initiated early in the day, and some wards had involved night staff in the preparation of paperwork to facilitate this. Staff also noted that people could regain continence during the day, but still require pads at night. At a minimum, night staff training could help the correct completion of daily individualised toileting plans. Training and involving night staff may also facilitate extending the SVP time period for those people who are continent during the day but not at night.
-
Review distraction and checking methods Staff reported difficulty with the practice of distraction and delay in BT: participants did not like it, and relatives did not understand it. Staff also reported discomfort with repeatedly asking people if they were dry or wet during PV. These aspects of the SVP need reviewing to help staff to manage them better.
-
Methods for encouraging participation Staff reported difficulties with managing people who were over anxious; irritated by being repeatedly asked if they wanted to go to the toilet; or reluctant to follow the programme. Staff had some ideas about how to phrase requests, and examples could be included in future training.
Supporting adaptation of the systematic voiding programme
The fourth stage of the SVP requires reviewing a person’s progress weekly and revising the programme and/or schedule based on the daily logs. Difficulties and suggested solutions for this stage included:
-
Reschedule the weekly review Because the weekly review could fall on different days for different people, it was sometimes missed. One staff nurse scheduled review due dates in the ward diary. Two sites suggested doing all reviews at the weekend, when other reviews traditionally took place.
-
Monitor patient trajectory Staff did not know how deal with people who had to stop and restart the programme – perhaps because of a setback. They also did not know if and how to stop the programme for people who were not making progress, or had a complete lack of awareness. Guidance for these situations could be given. For patients who had had a break in the SVP, there was then the problem of maintaining awareness of when and how to restart. Being able to track each person’s progress in the paperwork had a positive impact on staff motivation. This seems an easy thing to strengthen, perhaps by the use of graphic methods that make improvement in outcome easily visible, such as the average number of incontinent episodes per day. A trajectory summary might also serve the purpose of making breaks in the programme visible, as a reminder for restarting.
Other barriers
The findings also illustrated more generic facilitators that were noteworthy, in particular the crucial role of senior ward staff and the research nurse role in programme overseeing and co-ordination. Involvement of physiotherapy and occupational therapy staff was seen as lacking, and more effort could be directed to exploring their potential contribution. The integration of ward and research staff evolved differently to cater for local circumstances and had a significant impact on the implementation of the SVP, and potentially on differences in outcome between sites. The observation that employing relatively inexperienced HCAs as ICONS nurses had little influence on ward staff is also an important point for integration in the future trial.
Senior staff discussed the difficulty of ‘keeping a handle’ on the programme overall, and some attention could be given to supporting the work of monitoring the SVP in the paperwork, both at an individual person and ward level.
The importance of adequate staffing and the impact of workload was a perennial comment; monitoring staffing levels and patient dependency should be a component of process evaluation. Given the importance of visible improvement, perhaps making the reduction in workload more visible (e.g. fewer bed changes, fewer buzzers) by ward audit might be useful.
Keeping new and bank staff trained and updated about the research programme also needs specific attention, as does the lack of uptake of the online training resource. Training processes need revision to ensure fit with working routines. The training given to staff in explaining the SVP needs to be checked to avoid potential misunderstanding by people with stroke and relatives about the consequences of involvement in the SVP on length of stay.
Summary of recommendations for the design and implementation of the systematic voiding programme
Recommendations for the design and implementation of the SVP are shown in Table 113.
| Recommendation | Hypothesised mechanisms | |||
|---|---|---|---|---|
| Thinkinga | Planningb | Doingc | Evaluatingd | |
| Intervention design |
|
|
|
|
| Implementation |
|
|
|
|
Design
The intervention was successful in changing perceptions about incontinence from an inevitable consequence of stroke to a symptom amenable to intervention as part of rehabilitative activity. Future implementation of the SVP needs to reinforce this focus on continence as a legitimate focus of rehabilitation. A shift in thinking is also required to legitimate continence work with people with stroke who may never achieve unassisted continence; for these people the aim should be ‘managing incontinence’, i.e. ‘catching’ people before incontinent episodes.
Guidance about how the SVP should be used for certain groups of patients, for example those with long standing continence problems or functional incontinence (e.g. inability to manipulate bottles), needs adding to protocols. More specific guidance on minimising catheterisation on the stroke unit and introducing a more systematic approach to TWOC is also required.
For people on the programme, strategies for encouraging participation of ‘difficult’ people, for example those who found regular prompting irritating, need consideration. Further guidance is also required on when to take people off all or part of the programme, for example if people have become continent or if the programme is thought not to be working.
Linkages between SVP documentation need to be more explicit to enable understanding of how different components inform each other, for example pattern of incontinence in the 3-day diary should be used in decisions about the initial voiding interval. The continence assessment was widely regarded as too long to use in routine practice; this and other documentation need revising in collaboration with ward staff and linking with the training programme.
Training in pelvic floor muscle exercises (PFMEs) for physiotherapists and nursing staff, in particular assessing whether or not people with stroke are able to exercise their pelvic floor muscles and whether or not exercises are being performed correctly, needs to be considered.
Implementation
Methods of increasing uptake of training need consideration; given the low uptake of online training, other approaches may be more fruitful, for example transferring online content into ward-based hard copies or extending face-to-face training and incorporating this within mandatory training days.
Implementing the programme in an acute setting proved problematic; consideration needs to be given to which elements of the SVP should be delivered in this context, for example the 3-day diary and continence assessment, with conservative interventions starting in the rehabilitation phase and extending into the community.
Sites where the intervention became well embedded tended to be those where there was strong leadership from the research nurse in managing, co-ordinating and systematising the intervention. HCAs were also instrumental in driving implementation in many sites. Future implementation could be led and managed by a ‘link nurse’ (possibly continence specific), with designated time for the role, working with and supporting HCAs within the ward team. Given the difficulties internal facilitators had in making time for the role, facilitation activities (and dedicated time for these) could also be incorporated within the link nurse role.
Embedding mechanisms, for example organisational monitoring of outcome over time, were not part of the SVP but were instigated in sites where the intervention was well embedded by research nurses. These provided a means of linking nursing input to patient outcome which served as a powerful driver in terms of motivating staff to continue implementation. Future implementation needs to incorporate systematic means of monitoring patient progress at an organisational level and feeding results back to staff. This role could also fall within the remit of the link nurse.
Engaging the MDT in the SVP is essential and processes to facilitate this, for example formal reporting within a multidisciplinary context, need incorporating into future implementation strategies.
Estimates of the number of sites and participants required for a full-scale cluster randomised trial to evaluate effectiveness
In our original application for funding, we provided the following sample size calculation for our main trial:
In an individually-randomised trial, detection of a minimally clinically important reduction of 10% (from 70% [people expected to have UI at 6 weeks in the usual care group] to 60% [people expected to have UI at 6 weeks in the intervention groups]) would be achieved with 80% power by a sample size of 356 or 90% power by a sample size of 477 (based on a chi-square test using a 5% significance level). This difference has been found in similar studies in the elderly [32,33]. Inflating this to account for the design effect based on an a priori assumption of an intra-cluster correlation coefficient of 0.02* for the presence/absence of incontinence, suggests that recruiting an average of 125 participants to 30 clusters will achieve 80% power (an average of 200 people randomised to 36 clusters would be required for 90% power). After Phases I and II, we will review our estimate of the ICC and the prevalence of incontinence and revise our sample size estimate accordingly. Should our a priori estimate of the ICC lead to an under-powering of the study, we shall review the study design and consider including more clusters, possibly with a shorter recruitment period (i.e. less patients).
*It is suggested (Campbell et al. , 2000) that ICCs for patient outcome variables are generally between 0 and 0.05; we expect that the ICC for incontinence will be towards the lower end of this range after adjustment for cluster-level factors.
Given the estimated ICC from our feasibility trial, there is no suggestion that the ICC of 0.02 used here is anticonservative. We have suggested that a 12-week post-stroke outcome is more appropriate, given the observed distribution of intervals between stroke and recruitment and the necessary duration of intervention to potentially affect those recruited. We found that, in usual care, only approximately 20% (24/124) of participants reported being continent; given the broadening of our inclusion criteria to include those catheterised at recruitment, we also suggest a somewhat smaller than 10% improvement should now be considered minimally clinically important (e.g. 7%). This suggests that an OR of 1.48 or greater would be viewed as clinically important to detect, which is consistent with some of the ORs observed in our study. Using a two-arm trial, with equal number of clusters randomised to each in a parallel design:
-
to detect an OR of this magnitude with 80% power, assuming an ICC of 0.02, would require 34 clusters, each with 126 participants
-
if is deemed appropriate to assume a somewhat lower ICC, such as 0.0125, this sample size would achieve slightly over 90% power, or to achieve 80% power the sample size could be reduced to 65 per cluster, or the number of clusters reduced to 13 per arm.
Alternative designs should also be considered. A stepped-wedge design would potentially improve efficiency by reducing the effect of the ICC by incorporating both between- and within-cluster estimation. However, given our problems with gaining funding and approvals to start sites at comparable times in this exploratory trial, improvements in governance processes and staggered starting times would appear to be necessary for this to be practical. Given the assumptions inherent in stepped-wedge designs, the practicality and corresponding limitations to evidence would bring into question their applicability for a full-scale evaluation of a SVP in the NHS.
Recommendations for future trial design
The exploratory trial has demonstrated it is feasible to conduct a full-scale cluster RCT. The future trial will adopt this design with the following modifications.
Trial arms
-
Include two trial arms only, intervention and usual care.
-
Including a third trial arm may not be feasible given the number of sites required and is not warranted given the difficulty identifying the distinctive contribution of supported implementation in the exploratory trial.
-
Recruitment
-
Screen all potential participants within 72 hours of admission to the stroke unit.
-
Obtain consent as soon as possible, regardless of whether or not participants are medically stable and without completion of the 3-day bladder diary.
-
This will minimise imbalances at baseline caused by different recruitment procedures between intervention and usual care, and ensure earlier recruitment.
-
Data collection
-
Include reduction in incontinence episodes as a secondary outcome.
-
Retain the I-QOL, but add guidance for people who regain continence to answer ‘not at all’ to questions that do not apply.
-
Seek an alternative to the LUSQ to identify type of incontinence.
-
Consider approaches to increasing response rate at long-term follow-up, for example:
-
use an interim data collection point at 26 weeks post stroke
-
obtain outcome data in telephone interviews
-
delay collection of resource use data until outcome data is obtained.
-
-
Introduce more rigorous procedures for monitoring catheterisation (including ‘trial without catheter’).
Health economic component
-
Record in-hospital episodes of incontinence and the resources required to respond to such episodes.
-
Identify resources required to perform the programme through direct observation.
-
Consider obtaining post-hospital resource use data by asking patients to maintain diaries or going directly to providers of services.
-
Identify resource use items more directly related to the programme and the effects of incontinence to allow a more realistic conclusion to be drawn around cost-effectiveness.
Acknowledgements
The ICONS study research costs were funded by the NIHR Programme Grants for Applied Research scheme. Excess treatment costs and research support costs were funded by participating NHS trusts and health boards, Lancashire and Cumbria and East Anglia CLRNs and the Welsh NISCHR. The North West SRN provided research nurses to assist with the identification of eligible participants, recruitment and data collection, organised by Judy Ford.
The authors would like to express their heartfelt thanks to the following:
People with stroke and their families who took part in the study.
All the staff from participating stroke units, in particular those in the intervention arms, for all their support in implementing the interventions.
Members of the ICONS PPC involvement groups: Dave and Pat Brand, David Britt, May Griffiths, Philip Helvin, Brian James, Gill Pearl, Jane Whitewood, Jacqui Vella, Audrey and Richard Childs, Sean Crosby, Steve Hall, Anj Lewin, Liz Royle, Carole Scott and Jean Wright. Special thanks are due to David Britt, Jacqui Vella, Sean Crosby and Anj Lewin for also sitting on the Management and Steering Committees, and to Gill Pearl for facilitating the Speakeasy PPC group.
External members of the Steering Committee: John Norrie, Jo Rycroft-Malone, Katie Swarbrick, David Barer, Mo Wilkinson and Anil Sharma.
External members of the IDMC: Ian Ford (chairperson), Peter Langhorne and Martin Dennis.
Our project secretary, Alison Hadley.
Our research nurse in the case study phase, Hazel Dickinson.
Members of the Clinical Practice Research Unit who provided invaluable help with data collection, preparation and analysis: Mike Bullock, Joanna McAdam, Josephine Gibson, Brigit Chesworth, Laura Howell, Jacqueline Coupe, Svetlana Tishkovskaya, Jane Burnell, Edmore Chamapiwa, Sharon Howarth, Mal and Sue Auton, Kateryna McDonald, Jane Fitzgerald, Heather Cadd, Susan Miller, Zuzana Filova and Joanna Pye.
Contribution of authors
Lois H Thomas (Reader in Health Services Research) conceived the idea, wrote the proposal, was responsible for the design and conduct of the study (under the mentorship of Caroline Watkins, Professor of Stroke and Older People’s Care) and led the PPC involvement strand. She sat on Management and Trial Steering Committee and drafted the report.
Beverley French (Reader in Evidence-based Practice) led the evidence synthesis and the NPT elements of the study, contributed to the overall process evaluation and sat on the Management Group.
Christopher J Sutton (Principal Lecturer and Lead, Lancashire Clinical Trials Unit) was the study statistician; he designed and analysed the quantitative results, oversaw their reporting and interpretation and sat on the Trial Steering Committee and the IDMC.
Denise Forshaw (Senior Clinical Trials Manager) was the trial manager responsible for the day-to-day running of the exploratory trial; she sat on the Management Group.
Michael J Leathley (Senior Research Fellow) designed and led the health economic component of the trial under the mentorship of Andrew Walker (Health Economist, Glasgow University), drafted the Health Economics chapter and sat on the Management Group and Trial Steering Committee.
Christopher R Burton (Health Improvement Fellow, Bangor University) led the soft systems elements of the study, contributed to the overall process evaluation and sat on the Management Group.
Brenda Roe (Professor of Health Research, Edge Hill University) contributed to the evidence synthesis, provided specialist advice on continence and sat on the Management Group.
Francine M Cheater (Professor in Nursing Sciences, University of East Anglia) provided advice throughout the study, particularly in relation to the implementation of study interventions, and sat on the Management Group.
Jo Booth (Reader in Applied Health Research) provided advice throughout the study, particularly in relation to continence, and sat on the Management Group.
Elaine McColl (Professor of Health Services Research and Director, Newcastle Clinical Trials Unit) provided advice on trial design and conduct and sat on the Management Group.
Bernadette Carter (Professor of Children’s Nursing) provided advice on qualitative aspects of the study and sat on the Management Group.
Andrew Walker (Health Economist, Glasgow University) helped design the health economic component, provided advice throughout and mentored Michael Leathley.
Katie Brittain (Lecturer in Social Gerontology, Newcastle University) contributed to all aspects of the study and sat on the Management Group.
Gemma Whiteley (Head of Research and Innovation) managed the study as a representative of our sponsor, Lancashire Teaching Hospitals NHS Foundation Trust.
Helen Rodgers (Clinical Professor of Stroke Care, Newcastle University) provided guidance on the design and conduct of the trial and sat on the Management Group.
James Barrett (Professor of Health Care of Older People) provided clinical advice throughout the study and sat on the Management Group.
Caroline L Watkins (Professor of Stroke and Older People’s Care) was principal investigator, contributing to all aspects of the study and mentoring Lois Thomas. She sat on the Management Group and Trial Steering Committee.
All authors have contributed to analyses or interpretation of results and drafts of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd, AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001;32:1279-84. http://dx.doi.org/10.1161/01.STR.32.6.1279.
- Nakayama H, Jorgensen HS, Pedersen PM, Raaschou HO, Olsen TS. Prevalence and risk factors of incontinence after stroke. The Copenhagen Stroke Study. Stroke 1997;28:58-62. http://dx.doi.org/10.1161/01.STR.28.1.58.
- Kolominsky-Rabas PL, Hilz MJ, Neundoerfer B, Heuschmann PU. Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn 2003;22:322-7. http://dx.doi.org/10.1002/nau.10114.
- Williams MP, Srikanth V, Bird M, Thrift AG. Urinary symptoms and natural history of urinary continence after first-ever stroke--a longitudinal population-based study. Age Ageing 2012;41:371-6. http://dx.doi.org/10.1093/ageing/afs009.
- Jorgensen L, Engstad T, Jacobsen BK. Self-reported urinary incontinence in noninstitutionalized long-term stroke survivors: a population-based study. Arch Phys Med Rehabil 2005;86:416-20. http://dx.doi.org/10.1016/j.apmr.2004.05.011.
- Marinkovic SP, Badlani G. Voiding and sexual dysfunction after cerebrovascular accidents. J Urol 2001;165:359-70. http://dx.doi.org/10.1097/00005392-200102000-00003.
- Barer DH. Continence after stroke: useful predictor or goal of therapy?. Age Ageing 1989;18:183-91. http://dx.doi.org/10.1093/ageing/18.3.183.
- Patel M, Coshall C, Lawrence E, Rudd AG, Wolfe CD. Recovery from poststroke urinary incontinence: associated factors and impact on outcome. J Am Geriatr Soc 2001;49:1229-33. http://dx.doi.org/10.1046/j.1532-5415.2001.49242.x.
- Urinary Incontinence in Women: The Management of Urinary Incontinence in Women. NICE Clinical Guideline CG171. London: National Institute for Health and Care Excellence; 2013.
- National Clinical Guideline for Stroke. London: Royal College of Physicians; 2012.
- National Sentinel Stroke Audit 2010. London: Royal College of Physicians; 2011.
- Booth J, Kumlien S, Zang Y, Gustafsson B, Tolson D. Rehabilitation nurses practices in relation to urinary incontinence following stroke: a cross-cultural comparison. J Clin Nurs 2009;18:1049-58. http://dx.doi.org/10.1111/j.1365-2702.2008.02688.x.
- Cowey E, Smith LN, Booth J, Weir CJ. Urinary catheterization in acute stroke: clinical realities. A mixed methods study. Clin Rehabil 2012;26. http://dx.doi.org/10.1177/0269215511426160.
- Brittain KR, Peet SM, Castleden CM. Stroke and incontinence. Stroke 1998;29:524-8. http://dx.doi.org/10.1161/01.STR.29.2.524.
- Burney TL, Senapati M, Desai S, Choudhary ST, Badlani GH. Acute cerebrovascular accident and lower urinary tract dysfunction: a prospective correlation of the site of brain injury with urodynamic findings. J Urol 1996;156:1748-50. http://dx.doi.org/10.1016/S0022-5347(01)65498-3.
- Barrett JA. Bladder and bowel problems after stroke. Rev Clin Gerontol 2002;12:253-67. http://dx.doi.org/10.1017/S0959259802012388.
- Pettersen R, Stien R, Wyller TB. Post-stroke urinary incontinence with impaired awareness of the need to void: clinical and urodynamic features. BJU Int 2007;99:1073-7. http://dx.doi.org/10.1111/j.1464-410X.2007.06754.x.
- Brittain KR, Peet SM, Potter JF, Castleden CM. Prevalence and management of urinary incontinence in stroke survivors. Age Ageing 1999;28:509-11. http://dx.doi.org/10.1093/ageing/28.6.509.
- Brittain KR, Perry SI, Peet SM, Shaw C, Dallosso H, Assassa RP, et al. Prevalence and impact of urinary symptoms among community-dwelling stroke survivors. Stroke 2000;31:886-91. http://dx.doi.org/10.1161/01.STR.31.4.886.
- Brittain KR. Urinary symptoms and depression in stroke survivors. Age Ageing 1998;27:116-17.
- Brittain KR, Castleden CM. Suicide in patients with stroke. Depression may be caused by symptoms affecting lower urinary tract. BMJ 1998;317:1016-17. http://dx.doi.org/10.1136/bmj.317.7164.1016b.
- Brittain KR, Shaw C. The social consequences of living with and dealing with incontinence – a carers perspective. Soc Sci Med 2007;65:1274-83. http://dx.doi.org/10.1016/j.socscimed.2007.04.002.
- Thomas LH, Cross S, Barrett J, French B, Leathley M, Sutton CJ, et al. Treatment of urinary incontinence after stroke in adults. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.CD004462.pub3.
- Tibaek S, Gard G, Jensen R. Pelvic floor muscle training is effective in women with urinary incontinence after stroke: a randomised, controlled and blinded study. Neuro Urodyn 2005;24:348-57.
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 2004;1.
- Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev 2004;1.
- Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev 2000;1.
- Ostaszkiewicz J, Johnston L, Roe B. Habit retraining for the management of urinary incontinence in adults. Cochrane Database Systematic Rev 2004;2.
- Dumoulin C, Hay-Smith EJ, Mac Habee-Seguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5.
- Williams KS, Assassa RP, Cooper NJ, Turner DA, Shaw C, Abrams KR, et al. Clinical and cost-effectiveness of a new nurse-led continence service: a randomised controlled trial. Br J Gen Pract 2005;55:696-703.
- Wyman JF, Fantl JA, McClish DK, Bump RC. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Continence Program for Women Research Group. Am J Obstet Gynecol 1998;179:999-1007. http://dx.doi.org/10.1016/S0002-9378(98)70206-6.
- Fantl JA, Wyman JF, McClish DK, Harkins SW, Elswick RK, Taylor JR, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991;265:609-13. http://dx.doi.org/10.1001/jama.1991.03460050063021.
- Burgio KL, Locher JL, Goode PS, Hardin M, McDowell J, Dombrowski M, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995-2000. http://dx.doi.org/10.1001/jama.280.23.1995.
- Berghmans LC, Hendriks HJ, de Bie RA, van Waalwijk van Doorn ES, Bo K, van Kerrebroeck PE. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU Int 2000;85:254-63. http://dx.doi.org/10.1046/j.1464-410x.2000.00434.x.
- Teunissen TA, de Jonge A, van Weel C, Lagro-Janssen AL. Treating urinary incontinence in the elderly – conservative therapies that work: a systematic review. J Fam Pract 2004;53:25-30.
- Wilson D, Herbison P. Conservative management of incontinence. Curr Opin Obstet Gynecol 1995;7:386-92. http://dx.doi.org/10.1097/00001703-199510000-00012.
- Urinary Incontinence: The Management of Urinary Incontinence in Women. London: Royal College of Obstetricians and Gynaecologists; 2006.
- Shamliyan T, Wyman J, Bliss DZ, Kane RL, Wilt TJ. Prevention of urinary and fecal incontinence in adults. Evid Rep Technol Assess 2007;161:1-379.
- Estabrooks C, Morse J, Swanson J, Kuzel A. The Nature of Qualitative Evidence. Thousand Oaks, CA: Sage; 2001.
- French B, Thomas LH, Baker P, Burton CR, Pennington L, Roddam H. What can management theories offer evidence-based practice? A comparative analysis of measurement tools for organisational context. Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-28.
- Greenhalgh T, Robert G, Bate P, Kyriakidou O, Macfarlane F, Peacock R. How to Spread Good Ideas. A Systematic Review of the Literature on Diffusion, Dissemination and Sustainability of Innovations in Health Service Delivery and Organisation. London: National Coordinating Centre for NHS Service Delivery and Organisation (NCCSDO); 2004.
- McCormack B, Kitson A, Harvey G, Rycroft-Malone J, Titchen A, Seers K. Getting evidence into practice: the meaning of ‘context’. J Adv Nurs 2002;38:94-104. http://dx.doi.org/10.1046/j.1365-2648.2002.02150.x.
- Rycroft-Malone J. The PARIHS framework – a framework for guiding the implementation of evidence-based practice. J Nurs Care Qual 2004;19:297-304. http://dx.doi.org/10.1097/00001786-200410000-00002.
- Lukas CV, Holmes SK, Cohen AB, Restuccia J, Cramer IE, Shwartz M, et al. Transformational change in health systems: an organizational model. Health Care Manag Rev 2007;32:309-20. http://dx.doi.org/10.1097/01.HMR.0000296785.29718.5d.
- Rycroft-Malone J, Kitson A, Harvey G, McCormack B, Seers K, Titchen A, et al. Ingredients for change: revisiting a conceptual framework. Qual Saf Health Care 2002;11:174-80. http://dx.doi.org/10.1136/qhc.11.2.174.
- Iles V, Sutherland K. Organisational Change: A Review for Health Care Managers, Professionals and Researchers. London: National Coordinating Centre for NHS Service Delivery and Organisation (NCCSDO); 2001.
- Argote L, McEvily B, Reagans R. Managing knowledge in organizations: an integrative framework and review of emerging themes. Manag Sci 2013;49:571-82. http://dx.doi.org/10.1287/mnsc.49.4.571.14424.
- Scarbrough H, Swan J, Easterby-Smith M, Lyles MA. Handbook of Organizational Learning and Knowledge Management. London: Wiley-Blackwell; 2003.
- Gabbay J, le May A. Evidence based guidelines or collectively constructed ‘mindlines?’ Ethnographic study of knowledge management in primary care. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.329.7473.1013.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. http://dx.doi.org/10.1136/qshc.2004.011155.
- Ferlie EB, Shortell SM. Improving the quality of health care in the United Kingdom and the United States: a framework for change. Milbank Q 2001;79:281-315. http://dx.doi.org/10.1111/1468-0009.00206.
- Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2010;3. http://dx.doi.org/10.1002/14651858.CD005470.pub2.
- Roe B, Milne J, Ostaszkiewicz J, Wallace S. Systematic reviews of bladder training and voiding programmes in adults: a synopsis of findings on theory and methods using metastudy techniques. J Adv Nurs 2007;57:3-14. http://dx.doi.org/10.1111/j.1365-2648.2006.04098.x.
- Roe B, Ostaszkiewicz J, Milne J, Wallace S. Systematic reviews of bladder training and voiding programmes in adults: a synopsis of findings from data analysis and outcomes using metastudy techniques. J Adv Nurs 2007;57:15-31. http://dx.doi.org/10.1111/j.1365-2648.2006.04097.x.
- May CR, Mair FS, Dowrick CF, Finch TL. Process evaluation for complex interventions in primary care: understanding trials using the normalization process model. BMC Fam Pract 2007;8. http://dx.doi.org/10.1186/1471-2296-8-42.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: Normalization Process Theory. Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-29.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-148.
- May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res 2006;6. http://dx.doi.org/10.1186/1472-6963-6-86.
- A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: MRC; 2000.
- Developing and Evaluating Complex Interventions: New Guidance. London: MRC; 2008.
- Goode PS. Predictors of treatment response to behavioral therapy and pharmacotherapy for urinary incontinence. Gastroenterology 2004;126:141-5. http://dx.doi.org/10.1053/j.gastro.2003.10.003.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 2011. www.cochrane-handbook.org (accessed 2 January 2011).
- Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, et al. Evidence-based behavioral medicine: what is it and how do we achieve it?. Ann Behav Med 2003;26:161-71. http://dx.doi.org/10.1207/S15324796ABM2603_01.
- Behaviour Change. NICE Public Health Guidance 6. London: NICE; 2007.
- Critical Appraisal Skills Programme n.d. www.casp-uk.net/ (accessed 1 October 2008).
- Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666-76. http://dx.doi.org/10.1093/ije/dym018.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37. http://dx.doi.org/10.7326/0003-4819-144-6-200603210-00010.
- Alewijnse D, Mesters I, Metsemakers J, Adriaans J, van den BB. Predictors of intention to adhere to physiotherapy among women with urinary incontinence. Health Educ Res 2001;16:173-86. http://dx.doi.org/10.1093/her/16.2.173.
- Alewijnse D, Mesters I, Metsemakers J, van den BB. Predictors of long-term adherence to pelvic floor muscle exercise therapy among women with urinary incontinence. Health Educ Res 2003;18:511-24. http://dx.doi.org/10.1093/her/cyf043.
- Aslan E, Komurcu N, Beji NK, Yalcin O. Bladder training and Kegel exercises for women with urinary complaints living in a rest home. Gerontology 2008;54:224-31. http://dx.doi.org/10.1159/000133565.
- Baigis-Smith J, Smith DA, Rose M, Newman DK. Managing urinary incontinence in community-residing elderly persons. Gerontologist 1989;29:229-33. http://dx.doi.org/10.1093/geront/29.2.229.
- Bear M, Dwyer JW, Benveneste D, Jett K, Dougherty M. Home-based management of urinary incontinence: a pilot study with both frail and independent elders. J Wound Ostomy Continence Nurs 1997;24:163-71.
- Burgio KL, Goode PS, Locher JL, Richter HE, Roth DL, Wright KC, et al. Predictors of outcome in the behavioral treatment of urinary incontinence in women. Obstet Gynecol 2003;102:940-7. http://dx.doi.org/10.1016/S0029-7844(03)00770-1.
- Dingwall L, McLafferty E. Do nurses promote urinary continence in hospitalized older people? An exploratory study. J Clin Nurs 2006;15:1276-86. http://dx.doi.org/10.1111/j.1365-2702.2006.01381.x.
- Dougherty MC, Dwyer JW, Pendergast JF, Boyington AR, Tomlinson BU, Coward RT, et al. A randomized trial of behavioral management for continence with older rural women. Res Nurs Health 2002;25:3-13. http://dx.doi.org/10.1002/nur.10016.
- Mather KF, Bakas T. Nursing assistants’ perceptions of their ability to provide continence care. Geriatr Nurs 2002;23:76-81. http://dx.doi.org/10.1067/mgn.2002.123788.
- Gerard L. Group learning behavior modification and exercise for women with urinary incontinence. Urol Nurs 1997;17:17-22.
- Hay-Smith EJC, Ryan K, Dean S. The silent, private exercise: experiences of pelvic floor muscle training in a sample of women with stress urinary incontinence. Physiotherapy 2007;93:53-61. http://dx.doi.org/10.1016/j.physio.2006.10.005.
- Johnson TM, Ouslander JG, Uman GC, Schnelle JF. Urinary incontinence treatment preferences in Long-Term Care. J Am Geriatr Soc 2001;49:710-18. http://dx.doi.org/10.1046/j.1532-5415.2001.49146.x.
- Kafri R, Langer R, Dvir Z, Katz-Leurer M. Rehabilitation vs drug therapy for urge urinary incontinence: short-term outcome. Int Urogynecol J 2007;18:407-11. http://dx.doi.org/10.1007/s00192-006-0163-1.
- Kafri R, Shames J, Raz M, Katz-Leurer M. Rehabilitation versus drug therapy for urge urinary incontinence: long-term outcomes. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:47-52. http://dx.doi.org/10.1007/s00192-007-0397-6.
- Kincade JE, Johnson TM, Ashford-Works C, Clarke MK, Busby-Whitehead J. A pilot study to determine reasons for patient withdrawal from a pelvic muscle rehabilitation program for urinary incontinence. J Appl Gerontol 1999;18:379-96. http://dx.doi.org/10.1177/073346489901800307.
- Kincade JE, Peckous BK, Busby-Whitehead J. A pilot study to determine predictors of behavioral treatment completion for urinary incontinence. Urol Nurs 2001;21:39-44.
- Lee C, Johnson C, Chiarelli P. Women’s waterworks: evaluating an early intervention for incontinence among adult women. Aust N Z Continence Jl 2005;11:11-6.
- Lekan-Rutledge D, Palmer MH, Belyea M. In their own words: nursing assistants’ perceptions of barriers to implementation of prompted voiding in long-term care. Gerontologist 1998;38:370-8. http://dx.doi.org/10.1093/geront/38.3.370.
- Macaulay AJ, Stern RS, Holmes DM, Stanton SL. Micturition and the mind: psychological factors in the aetiology and treatment of urinary symptoms in women. BMJ 1987;294:540-3. http://dx.doi.org/10.1136/bmj.294.6571.540.
- McDowell BJ, Burgio KL, Dombrowski M, Locher JL, Rodriguez E. An interdisciplinary approach to the assessment and behavioral treatment of urinary incontinence in geriatric outpatients. J Am Geriatr Soc 1992;40:370-4.
- McDowell BJ, Engberg S, Sereika S, Donovan N, Jubeck ME, Weber E, et al. Effectiveness of behavioral therapy to treat incontinence in homebound older adults. J Am Geriatr Soc 1999;47:309-18.
- McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: episodes of incontinence and other urinary symptoms. J Aging Health 2000;12:250-67. http://dx.doi.org/10.1177/089826430001200206.
- McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: health-related quality of life. J Aging Health 2000;12:301-17. http://dx.doi.org/10.1177/089826430001200302.
- MacInnes CL. Why women leave therapy for stress incontinence. Nurs Times 2008;104:50-3.
- Milne JL, Moore KN. Factors impacting self-care for urinary incontinence. Urol Nurs 2006;26:41-5.
- O’Dell KK, Jacelon C, Morse AN. ‘I’d rather just go on as I am’ – pelvic floor care preferences of frail, elderly women in residential care. Urol Nurs 2008;28:36-47.
- Oldenburg B, Millard RJ. Predictors of long term outcome following a bladder re-training programme. J Psychosom Res 1986;30:691-8. http://dx.doi.org/10.1016/0022-3999(86)90103-0.
- Perrin L, Dauphinee SW, Corcos J, Hanley JA, Kuchel GA. Pelvic floor muscle training with biofeedback and bladder training in elderly women: a feasibility study. J Wound Ostomy Continence Nurs 2005;32:186-99. http://dx.doi.org/10.1097/00152192-200505000-00008.
- Remsburg RE, Palmer MH, Langford AM, Mendelson GF. Staff compliance with and ratings of effectiveness of a prompted voiding program in a long-term care facility. J Wound Ostomy Continence Nurs 1999;26:261-9.
- Resnick B, Keilman LJ, Calabrese B, Parmelee P, Lawhorne L, Pailet J, et al. Continence care. Nursing staff beliefs and expectations about continence care in nursing homes. J Wound Ostomy Continence Nurs 2006;33:610-18. http://dx.doi.org/10.1097/00152192-200611000-00004.
- Rose MA, Baigis-Smith J, Smith D, Newman D. Behavioral management of urinary incontinence in homebound older adults. Home Healthcare Nurse 1990;8:10-5. http://dx.doi.org/10.1097/00004045-199009000-00004.
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002;100:72-8. http://dx.doi.org/10.1016/S0029-7844(02)01993-2.
- Svengalis S, Nygaard IE, Cervone D, Kreder KJ. Perceived self-efficacy as a predictor of outcome of pelvic muscle exercises in the treatment of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1995;6:262-6. http://dx.doi.org/10.1007/BF01901521.
- Tadic SD, Zdaniuk B, Griffiths D, Rosenberg L, Schafer W, Resnick NM. Effect of biofeedback on psychological burden and symptoms in older women with urge urinary incontinence. J Am Geriatr Soc 2007;55:2010-15. http://dx.doi.org/10.1111/j.1532-5415.2007.01461.x.
- Richter HE, Burgio KL, Goode PS, Borello-France D, Bradley CS, Brubaker Lvl, et al. Non-surgical management of stress urinary incontinence: ambulatory treatments for leakage associated with stress (ATLAS) trial. Clin Trials 2007;4:92-101. http://dx.doi.org/10.1177/1740774506075237.
- Sran M, Watson C. Regaining Bladder Control in Postmenopausal Women With Osteoporosis (NCT00323245) 2008. www.clinicaltrials.gov (accessed October 2009).
- Wyman JF, Fantl JA. Bladder training in ambulatory care management of urinary incontinence. Urol Nurs 1991;11:11-7.
- Bo K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work?. Int Urogynecol J 2004;15:76-84. http://dx.doi.org/10.1007/s00192-004-1125-0.
- Engberg S, McDowell BJ, Donovan N, Brodak I, Weber E. Treatment of urinary incontinence in homebound older adults: interface between research and practice. Ostomy Wound Manag 1997;43:18-22.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988.
- Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC. The effect of bladder training, pelvic floor muscle training, or combination training on urodynamic parameters in women with urinary incontinence. Continence Program for Women Research Group. Neurourol Urodyn 1999;18:427-36. http://dx.doi.org/10.1002/(SICI)1520-6777(1999)18:5<427::AID-NAU3>3.0.CO;2-0.
- Brown CT, Yap T, Cromwell DA, Rixon L, Steed L, Mulligan K, et al. Self management for men with lower urinary tract symptoms: randomised controlled trial. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39010.551319.AE.
- Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chappie C, et al. Systematic review and evaluation of methods of assessing urinary incontinence. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10060.
- Milani R, Scalambrino S, Carrera S, Quadri G, Riva D, Casolati E. A Randomised Trial of Bladder Retraining Versus Oxybutynin in the Treatment of Idiophatic Urge Syndrome: Early Results 1986.
- Lentz G, Plevnik S, Stanton SL. Vaginal Cones Versus Bladder Drill for Sensory Urgency Treatment. Proceedings of the International Continence Society (ICS), 24th Annual Meeting 1994.
- Ostaszkiewicz J, Eustice S, Roe B, Thomas LH, French B, Islam T, et al. Toileting assistance programmes for the management of urinary incontinence in adults (protocol). Cochrane Database Systematic Rev 2013;6.
- O’Donnell PD. Behavioral modification for institutionalized individuals with urinary incontinence. Urology 1998;51:40-2. http://dx.doi.org/10.1016/S0090-4295(98)90008-5.
- Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map?. J Contin Educ Health Prof 2006;26:13-24. http://dx.doi.org/10.1002/chp.47.
- Cain M, Mittman R. Diffusion of Innovation in Healthcare. Oakland, CA: California Healthcare Foundation; 2002.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-50.
- Ramsay CR, Thomas RE, Croal BL, Grimshaw JM, Eccles MP. Using the theory of planned behaviour as a process evaluation tool in randomised trials of knowledge translation strategies: a case study from UK primary care. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-71.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Rogers EM. Diffusion of Innovations. New York, NY: Free Press; 1983.
- Estabrooks CA. Modeling the individual determinants of research utilization. West J Nurs Res 1999;21:758-72. http://dx.doi.org/10.1177/01939459922044171.
- Kitson AL, Harvey G, McCormack B. Approaches to implementing research in practice. Qual Health Care 1998;7:149-59. http://dx.doi.org/10.1136/qshc.7.3.149.
- Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care 1998;7:149-58. http://dx.doi.org/10.1136/qshc.7.3.149.
- Nicolini D, Gherardi S, Yanow D. Knowing in Organizations: A Practice-Based Approach. London: ME Sharpe; 2003.
- Wensing M, Grol R, Grol R, Wensing M, Eccles M. Improving Patient Care: The Implementation of Change in Clinical Practice. Edinburgh: Elsevier; 2005.
- Lewin K. Defining the ’field at a given time’. Psychol Rev 1943;50:292-310. http://dx.doi.org/10.1037/h0062738.
- Cheater FM, Baker R, Reddish S, Spiers N, Wailoo A, Gillies C, et al. Cluster randomized controlled trial of the effectiveness of audit and feedback and educational outreach on improving nursing practice and patient outcomes. Med Care 2006;44:542-51. http://dx.doi.org/10.1097/01.mlr.0000215919.89893.8a.
- Harvey G, Loftus-Hills A, Rycroft-Malone J, Titchen A, Kitson A, McCormack B, et al. Getting evidence into practice: the role and function of facilitation. J Adv Nurs 2002;37:577-88. http://dx.doi.org/10.1046/j.1365-2648.2002.02126.x.
- Seers K, Cox K, Crichton NJ, Edwards RT, Eldh AC, Estabrooks CA, et al. FIRE (Facilitating Implementation of Research Evidence): a study protocol. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-25.
- Robson M, Beary C. Facilitating. Aldershot: Gower Publishing Ltd; 1995.
- Stetler CB, Legro MW, Rycroft-Malone J, Bowman C, Curran G, Guihan M, et al. Role of ‘external facilitation’ in implementation of research findings: a qualitative evaluation of facilitation experiences in the Veterans Health Administration. Implement Sci 2006;1. http://dx.doi.org/10.1186/1748-5908-1-23.
- Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ 1998;317:465-8. http://dx.doi.org/10.1136/bmj.317.7156.465.
- Kitson AL, Rycroft-Malone J, Harvey G, McCormack B, Seers K, Titchen A. Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci 2008;3. http://dx.doi.org/10.1186/1748-5908-3-1.
- Dogherty EJ, Harrison MB, Graham ID. Facilitation as a role and process in achieving evidence-based practice in nursing: a focused review of concept and meaning. Worldviews Evid Based Nurs 2010;7:76-89. http://dx.doi.org/10.1111/j.1741-6787.2010.00186.x.
- Yin RK. Case Study Research: Design and Methods. Thousand Oaks, CA: Sage; 2009.
- Stake RE. The Art of Case Study Research. Thousand Oaks, CA: Sage; 1995.
- Goldstein M, Barnett HJM, Orgogozo JM, Sartorius N, Symon L, Vereshchagin NV. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407-31. http://dx.doi.org/10.1161/01.STR.20.10.1407.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116-26. http://dx.doi.org/10.1067/mob.2002.125704.
- Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34:45-5. http://dx.doi.org/10.1007/BF01405862.
- Abrams P, Cardozo L, Khoury S, Wein A. Incontinence: 4th International Consultation on Incontinence. London: Health Publications Ltd; 2009.
- Mental Capacity Act 2005. Elizabeth II. London: The Stationery Office; 2005.
- Checkland P, Scholes J. Soft Systems Methodology in Action. Chichester: John Wiley & Sons; 1999.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88. http://dx.doi.org/10.1177/1049732305276687.
- Mayring P. Qualitative content analysis. Forum Qual Soc Res 2000;1. www.qualitative-research.net/index.php/fqs/article/view/1089.
- Gask L, Rogers A, Campbell S, Sheaff R. Beyond the limits of clinical governance? The case of mental health in English primary care. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-63.
- May C, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-245.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. http://dx.doi.org/10.1016/0140-6736(91)93206-O.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud 1988;10:61-3. http://dx.doi.org/10.3109/09638288809164103.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van GJ. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. http://dx.doi.org/10.1161/01.STR.19.5.604.
- Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J Pelvic Floor Dysfunct 2006;17:520-4. http://dx.doi.org/10.1007/s00192-005-0060-z.
- Shaw C, Matthews RJ, Perry SI, Assassa RP, Williams K, McGrother C, et al. Validity and reliability of an interviewer-administered questionnaire to measure the severity of lower urinary tract symptoms of storage abnormality: the Leicester Urinary Symptom Questionnaire. BJU Int 2002;90:205-15. http://dx.doi.org/10.1046/j.1464-410X.2002.02893.x.
- Hodgkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. http://dx.doi.org/10.1093/ageing/1.4.233.
- Hasson H. Systematic evaluation of implementation fidelity of complex interventions in health and social care. Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-67.
- Burton CR. A description of the nursing role in stroke rehabilitation. J Adv Nurs 2000;32:174-81. http://dx.doi.org/10.1046/j.1365-2648.2000.01411.x.
- Dumoulin C, Korner-Bitensky N, Tannenbaum C. Urinary incontinence after stroke: identification, assessment, and intervention by rehabilitation professionals in Canada. Stroke 2007;38:2745-51. http://dx.doi.org/10.1161/STROKEAHA.107.486035.
- Dumoulin C, Korner-Bitensky N, Tannenbaum C. Urinary incontinence after stroke: does rehabilitation make a difference? A systematic review of the effectiveness of behavioral therapy. Top Stroke Rehabil 2005;12:66-7. http://dx.doi.org/10.1310/ENMX-RUV5-15WL-VNA2.
- Wikander B, Ekelund P, Milsom I. An evaluation of multidisciplinary intervention governed by functional independence measure (FIMSM) in incontinent stroke patients. Scand J Rehabil Med 1998;30:15-21. http://dx.doi.org/10.1080/003655098444273.
- Tibaek S, Gard G, Jensen R. Pelvic floor muscle training is effective in women with urinary incontinence after stroke: a randomised, controlled and blinded study. Neurourol Urodyn 2005;24:348-57. http://dx.doi.org/10.1002/nau.20134.
- National Sentinel Stroke Audit. Organisational Audit 2009. London: Royal College of Physicians; 2010.
- Giraudeau B, Ravaud P. Preventing bias in cluster randomised trials. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000065.
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. http://dx.doi.org/10.1002/nau.20041.
- Avery KN, Bosch JL, Gotoh M, Naughton M, Jackson S, Radley SC, et al. Questionnaires to assess urinary and anal incontinence: review and recommendations. J Urol 2007;177:39-4. http://dx.doi.org/10.1016/j.juro.2006.08.075.
- Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagner TH, Buesching DP. Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I-QOL). Urology 1999;53:71-6. http://dx.doi.org/10.1016/S0090-4295(98)00454-3.
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996;47:67-71. http://dx.doi.org/10.1016/S0090-4295(99)80384-7.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry 1983;140:734-9. http://dx.doi.org/10.1176/ajp.140.6.734.
- Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke 2002;33:1041-7. http://dx.doi.org/10.1161/hs0402.105909.
- Taljaard M, Donner A, Klar N. Imputation strategies for missing continuous outcomes in cluster randomized trials. Biom J 2008;50:329-45. http://dx.doi.org/10.1002/bimj.200710423.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-15.
- Dusenbury L, Brannigan R, Falco M, Hansen WB. A review of research on fidelity of implementation: implications for drug abuse prevention in school settings. Health Educ Res 2003;18:237-56. http://dx.doi.org/10.1093/her/18.2.237.
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-40.
- Patton M. Qualitative Evaluation and Research Methods. Newbury Park, CA: Sage; 1990.
- Benzer JK, Beehler S, Cramer IE, Mohr DC, Charns MP, Burgess JF. Between and within-site variation in qualitative implementation research. Implement Sci 2013;8. http://dx.doi.org/10.1186/1748-5908-8-4.
- Gilchrist VJ, Williams RL, Crabtree BF, Miller WL. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. London: Routledge; 1994.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for Euroqol: Results from a UK General Population Survey. Centre for Health Economics Discussion Paper 138. York: Centre for Health Economics; 1995.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Haywood KL, Garratt AM, Lall R, Smith JF, Lamb SE. EuroQol EQ-5D and condition-specific measures of health outcome in women with urinary incontinence: reliability, validity and responsiveness. Qual Life Res 2008;17:475-83. http://dx.doi.org/10.1007/s11136-008-9311-z.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37-46. http://dx.doi.org/10.1177/001316446002000104.
- Watkins CL, Leathley MJ, Sharma AK. Stroke Interface Audit: Pre/Post Discharge Audit of Stroke Services and Care in Liverpool and Sefton: Delivery Timeliness and Targeting – 36 month report. Preston: University of Central Lancashire; 2002.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2012.
- Aids and Adaptations Catalogue. Preston: Lancashire Texhing Hospitals NHS Trust; 2012.
- Boots n.d . Deluxe Mattress Protector – Double1115790 n.d. www.boots.com/en/Deluxe-matress-protector-Double_1236635/ (accessed 4 September 2013).
- Pay Circular (AforC) 2 2011 n.d. www.nhsemployers.org/∼/media/Employers/Publications/Pay%20circulars/Pay-Circular-AforC-2-2011.pdf (accessed 12 August 2013).
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5310.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Patient and Public Involvement in the NHS. London: Department of Health; 1999.
- Best Research for Best Health. London: Department of Health; 2006.
- Beresford P. User involvement, research and health inequalities: developing new directions. Health Soc Care Community 2007;15:306-12. http://dx.doi.org/10.1111/j.1365-2524.2007.00688.x.
- Smith E, Ross F, Donovan S, Manthorpe J, Brearley S, Sitzia J, et al. Service user involvement in nursing, midwifery and health visiting research: a review of evidence and practice. Int J Nurs Stud 2008;45:298-315. http://dx.doi.org/10.1016/j.ijnurstu.2006.09.010.
- Diversity and Inclusion: What’s it About and Why is it Important for Public Involvement in Research?. Eastleigh: INVOLVE; 2012.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988;19:1083-92. http://dx.doi.org/10.1161/01.STR.19.9.1083.
- Pound C, Duchan J, Penman T, Hewitt A, Parr S. Communication access to organisations: inclusionary practices for people with aphasia. Aphasiology 2007;2:23-38. http://dx.doi.org/10.1080/02687030600798212.
- Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Clinical effectiveness, cost-effectiveness and service users’ perceptions of early, well-resourced communication therapy following a stroke: a randomised controlled trial (The ACT Now Study). Health Technol Assess 2012;16.
- Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Effectiveness of enhanced communication therapy in the first four months after stroke for aphasia and dysarthria: a randomised controlled trial. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4407.
- Briefing Notes for Researchers: Involving the Public in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2012.
- Payment for Involvement: A Guide for Making Payments to Members of the Public Actively Involved in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2010.
- Centre for Evidence-Based Medicine n.d. www.cebm.net/ (accessed 21 August 2013).
- Grol R, Bosch M, Hulscher M. Planning and studying improvement in patient care: the use of theoretical perspectives. Milbank Q 2007;85:93-138. http://dx.doi.org/10.1111/j.1468-0009.2007.00478.x.
- Bartley A. The Hospital Pathways Project. Making it happen: Intentional Rounding. London: The King’s Fund Point of Care and The Health Foundation; 2011.
- Watkins C, Jenkinson D, Scoular P. Stroke-Specific Education Framework (SSEF) 2009. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/@sta/@perf/documents/digitalasset/dh_116343.pdf (accessed January 2013).
- Rycroft-Malone J, Seers K, Titchen A, Harvey G, Kitson A, McCormack B. What counts as evidence in evidence-based practice?. J Adv Nurs 2004;47:81-90. http://dx.doi.org/10.1111/j.1365-2648.2004.03068.x.
- Philibert I, Leach DC. Re-framing continuity of care for this century. Qual Saf Health Care 2005;14:394-6. http://dx.doi.org/10.1136/qshc.2005.016170.
- Cheater FM, Hearnshaw H, Baker R, Keane M. Can a facilitated programme promote effective multidisciplinary audit in secondary care teams? An exploratory trial. Int J Nurs Stud 2005;42:779-91. http://dx.doi.org/10.1016/j.ijnurstu.2004.11.002.
- Karantanis E, Fynes M, Moore KH, Stanton SL. Comparison of the ICIQ-SF and 24-hour pad test with other measures for evaluating the severity of urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2004;15:111-16. http://dx.doi.org/10.1007/s00192-004-1123-2.
Appendix 1 MEDLINE final search
Date of search: 10 October 2008 (3048).
Search strategy
-
exp urinary incontinence/
-
Urination/ or urodynamics/
-
Urinary catheterization/
-
Urinary bladder, neurogenic/
-
Urinary bladder, overactive/
-
Urination disorders/
-
Toilet training/
-
Incontinence pads/
-
Dysuria/ or nocturia/
-
Toilet training/
-
Incontinence pads/
-
Pelvic floor/
-
toilet$.tw.
-
(incontinen$ or continen$).tw.
-
urodynamic$.tw.
-
((bladder or detrusor or vesic$) adj5 (instability or stab$ or unstable or irritab$ or hyperreflexia or dys?ynerg$ or dyskinesia or overactive$)).tw.
-
(void$ adj5 (prompt$ or diar$)).tw.
-
(urin$ adj2 leak$).tw.
-
dribbl$.tw.
-
diaper$.tw.
-
(bladder$ adj2 (neuropath$ or neurogen$ or neurolog$)).tw.
-
bodyworn$.tw.
-
underpad$.tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
-
((pelvic or habit or bladder or toilet or sensory) adj5 (train$ or re?train$ or re?educat$ or drill)).tw.
-
(timed void$ or prompted void$).tw.
-
25 or 26
-
exp behavior therapy/
-
(behav$ adj25 (therap$ or intervention$ or train$ or re?train$ or modif$)).tw.
-
exp cognitive therapy/
-
(cognit$ adj25 (therap$ or intervention$ or train$ or re?train$)).tw.
-
Combined Modality Therapy/
-
(skill$ adj5 (train$ or re?train$)).tw.
-
*Health promotion/
-
Health Education/ or Patient Education as Topic/
-
exp *Exercise/
-
Motor skills/
-
Group processes/
-
Psychotherapy, group/
-
Social support/
-
((group or social) adj5 support).tw.
-
Self care/
-
Cues/
-
Reminder Systems/
-
Tape recording/
-
exp motivation/
-
Feedback/
-
(monitor$ or feedback or goal$).tw.
-
28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48
-
27 and 24
-
49 and 24
-
27 and 49
-
52 or 50 or 51
-
Randomized Controlled Trials/
-
random allocation/
-
Controlled Clinical Trials/
-
control groups/
-
clinical trials/ or clinical trials, phase i/ or clinical trials, phase ii/ or clinical trials, phase iii/ or clinical trials, phase iv/
-
Clinical Trials Data Monitoring Committees/
-
double-blind method/
-
single-blind method/
-
Placebos/
-
placebo effect/
-
cross-over studies/
-
Multicenter Studies/
-
Therapies, Investigational/
-
Drug Evaluation/
-
Research Design/
-
Program Evaluation/
-
evaluation studies/
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
clinical trial.pt.
-
multicenter study.pt.
-
evaluation studies.pt.
-
meta analysis.pt.
-
meta-analysis/
-
random$.tw.
-
(controlled adj5 (trial$ or stud$)).tw.
-
(clinical$ adj5 trial$).tw.
-
((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
-
(surgical adj5 group$).tw.
-
(quasi-random$ or quasi random$ or pseudo-random$ or pseudo random$).tw.
-
((multicenter of multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
-
((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
-
((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
-
(coin adj5 (flip or flipped or toss$)).tw.
-
latin square.tw.
-
versus.tw.
-
(cross-over or cross over or crossover).tw.
-
placebo$.tw.
-
sham.tw.
-
(assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
-
controls.tw.
-
(treatment$ adj6 order).tw.
-
(meta-analy$ or metaanaly$ or meta analy$ or systematic review or systematic overview).tw.
-
or/54-96
-
53 and 97
-
exp epidemiologic studies/
-
Intervention studies/ or Feasibility studies/ or Pilot projects/
-
exp Controlled Clinical Trial/
-
Nursing evaluation research/
-
Evaluation studies/ or multicenter study/
-
Program development/
-
behavioral research/ or empirical research/
-
(determinant$ or factor$ or barrier$ or enabler$ or facilitator$ or predictor$ or characteristic$).tw.
-
Guideline adherence/ or exp "outcome and process assessment (health care"/ or exp program evaluation/ or Guidelines as topic/ or Clinical Protocols/
-
Health plan implementation/
-
Organizational innovation/
-
Diffusion of innovation/
-
Patient compliance/
-
Patient satisfaction/
-
exp health behavior/
-
exp consumer satisfaction/
-
exp patient acceptance of health care/
-
Information dissemination/
-
99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 or 107 or 108 or 109 or 110 or 111 or 112 or 113 or 114 or 115 or 116
-
53 and 117
-
exp Pregnancy/
-
98 or 118
-
120 not 119
-
Child/ or Adolescent/
-
Adult/
-
122 not 123
-
121 not 124
-
limit 125 to english language
-
exp *Prostatectomy/
-
126 not 127
Appendix 2 Identifying Continence OptioNs after Stroke screening criteria
| Inclusion criteria | Guidance/details |
|---|---|
| Relevant to any form of incontinence (i.e. SUI, UUI or MUI), except incontinence associated with prostate surgery or incontinence as a consequence of childbirth | Studies with inclusion criteria within 12 months of surgery or childbirth should be excluded |
| Intervention includes one of the core behavioural interventions for UI, i.e. PFMT (BT/retraining, PV) | At the screening stage, include studies referring to HT, scheduled or TV or toileting, even though these interventions are excluded – because terminology is not used consistently |
| Intervention includes more than one cognitive, behavioural or psychosocial component | Cognitive interventions are those interventions which attempt to influence the knowledge, thinking or attitudes of the client, and can include things like education, or motivation strategies Behavioural interventions are non-physical interventions designed to influence the behaviour of the client, for example training Psychosocial interventions are those interventions designed to influence the feelings of the client towards the intervention, for example acceptability, group delivery. These last are not strictly the focus of the review but can be a component of a complex or mixed intervention |
| Intervention is not combined with another physical intervention, for example drugs, surgery, vaginal cones, electrostimulation | NB: BIO can be a component of PFMT and can be included if it is just used to teach PFMT. Some exercise to encourage toileting mobility may also be acceptable as a component of the overall intervention |
| Relating to adults | Aged ≥ 18 years |
| Intervention is compared against no treatment/usual care, or any other treatment, or a single behavioural intervention | Nurse delivery of a CBI can be included if compared against usual care, for example GP care |
| Research designs | |
| For the effectiveness review | |
|
Include RCTs or quasi-RCTs of CBIs (i.e. more than one behavioural method) or enhanced behavioural interventions (a CBI plus a method of enhancing uptake/adherence, e.g. reminder system) |
| For the narrative review | |
|
This can be client or professional views of any cognitive–behavioural, or psychosocial intervention for UI |
|
These are likely to be concerned with moderators of treatment uptake or success. Trial data can also be used |
|
Include reports of the design, development or process evaluation of any behavioural intervention (single or combined). Do not include outcome evaluations if they are just uncontrolled clinical trials |
| For citation searching | |
|
|
| Exclude | |
|
|
Appendix 3 Identifying Continence OptioNs after Stroke review: filtration sheet (version 5)
Appendix 4 Identifying Continence OptioNs after Stroke review: data abstraction form (version 2)
Appendix 5 Identifying Continence OptioNs after Stroke review: guide to data extraction
Appendix 6 Table of included studies: review of effectiveness
Aslan et al. 200871
| Aim | To determine the efficiency of BT and Kegel exercises for older women living in a rest home | |
| Study details | 2002–4. Quasi-randomised trial comparing behavioural training with a no treatment control group | |
| Country and participants | Country | Turkey |
| Number of participants | 64 participants, 33 received behavioural intervention, 31 in control group | |
| Sample | Women, aged ≥ 65 years, cognitively able and with sufficient functional ability to participate, living in one rest home in Turkey, self-reporting UI or urinary symptoms, and agreeing to participate | |
| Inclusion criteria | Incontinence (two or more episodes per month) or other urinary symptoms (frequency, urgency, nocturia). 8 out of 64 women were continent but had urinary symptoms | |
| Exclusion criteria | Chronic or neurological illness affecting daily life | |
| Mean age (years) | Treatment group = 78 (SD 4.8) Control group = 79 (SD 5.3) |
|
| Type of incontinence | SUI 40%; MUI 10%; UUI 42%; continent 8% | |
| Severity of incontinence | 52% had one or more UI episodes per day | |
| Intervention | Behavioural intervention | BT, urge suppression techniques and Kegel exercises implemented by individual visits from a nurse practitioner over 6–8 weeks |
| Comparison group(s) | ‘Control group’ details NS | |
| Outcomes | Primary outcome | Volume of UI: measured by 1-hour pad test |
| Secondary outcome(s) | Pelvic floor muscle strength, QoL, urinary symptoms | |
| Timing | 8 weeks, 6-month follow-up | |
| Notes | Study quality | Quasi-randomised: alternate allocation, concealment and blinding of outcome assessment unclear, some incomplete outcome reporting, some secondary outcome data not fully reported |
| Intervention quality | Pelvic muscle contraction checked if participants were willing. Follow-up visits checked performance and tailored intervention | |
Bear et al. 199773
| Aim | To test the effectiveness of known behavioural UI techniques in a home setting with older rural women | |
| Study details | May–December 1993 Pilot study (for Dougherty et al. 200276) with random assignment to behavioural management or a control group |
|
| Country and participants | Country | USA |
| Number of participants | n = 24 | |
| Sample | Older women, living independently in a rural area. Extensive outreach efforts made to reach eligible women. Frail elders targeted but participants had few functional limitations, and elder/carer dyads difficult to recruit | |
| Inclusion criteria | Women, aged ≥ 55 years, resident in the community, involuntary urine loss twice a week or more, of 1 gm/day or more, any type of UI. People with cognitive deficits not excluded, but required a caregiver to be willing to be involved | |
| Exclusion criteria | Residual urine volume more than 75 ml, urine infection, bladder or kidney disease | |
| Mean age (years) | 68 | |
| Type of incontinence | All | |
| Severity of incontinence | NS | |
| Intervention | Behavioural intervention | BMC intervention, consisting of three sequenced phases: (1) self-monitoring; (2) BT or PV; (3) pelvic muscle exercise. Intervention was individualised, so not all participants completed all phases. Nurse delivery at home, over 24 weeks |
| Outcomes | Comparison group(s) | Control group received initial and final visit |
| Primary outcome | NS | |
| Secondary outcome(s) | Urine loss: gms/day, volume, number of incontinent episodes per day | |
| Timing | 24 weeks | |
| Notes | Study quality | Sequence generation and allocation concealment unclear, blinding unclear. Numbers and general reasons for attrition are reported, with 50% loss to follow-up from experimental group. Data for incontinence impact profile not reported, and SDs for episodes and volume of urine loss not reported |
| Intervention quality | It is not clear how many people received each phase of the intervention, or how often they were visited | |
Burgio et al. 199833
| Aim | Comparison of the effectiveness of BIO-assisted behavioural treatment with both a standard drug treatment (oxybutynin chloride) and a control condition for the treatment of UUI | |
| Study details | 1989–95 RCT, with three arms: behavioural treatment; drug treatment; and placebo control. Participants stratified by type and severity of incontinence | |
| Country and participants | Country | USA |
| Number of participants | 197 participants: 65 behavioural intervention, 67 drug treatment, 65 placebo control | |
| Sample | Volunteer sample, recruited via local adverts and professional referrals | |
| Inclusion criteria | Women, aged ≥ 55 years, community dwelling, with UUI incontinence predominant (at least two accidents per week persisting for 3 months) and urodynamic evidence of bladder dysfunction | |
| Exclusion criteria | Continual leakage, post-void residual urine > 200 ml, uterine prolapse, impaired mental status, medical contra-indications | |
| Mean age (years) | 67.7 (SD 7.5) | |
| Type of incontinence | UUI 48.7%; MUI/SUI + UUI 51.3% | |
| Severity of incontinence | Mild 18.3%; moderate 28.9%; severe 52.8% | |
| Intervention | Behavioural intervention | BIO-assisted behavioural treatment implemented by nurse practitioners, consisting of PFMT and urge strategies Intervention included information provision, self-monitoring, external monitoring, individualised tailoring of the intervention and external motivation/reinforcement Participants had four clinic visits at 2-week intervals. Anorectal BIO was used on the first visit to teach correct pelvic muscle contraction, and repeated on the third visit if necessary (26%). Urge strategies were taught on the second visit. Home practice included three sets of 15 exercises every day, practised in various positions, gradually increasing individualised duration of contraction to 10 seconds |
| Comparison group(s) | Drug treatment: oxybutinin dosage adjusted for the individual + clinic visits + bladder diary Placebo condition: clinic visits + bladder diary |
|
| Outcomes | Primary outcome | % reduction in frequency of incontinent episodes, measured by bladder diary |
| Secondary outcome(s) | Perception of improvement and comfort, adverse events, satisfaction | |
| Timing | 2 weeks post treatment | |
| Notes | Study quality | Adequate sequence generation, allocation concealment unclear, blinding of outcome analysis and complete data for primary outcome. Attrition for secondary outcomes unclear. All prespecified outcomes reported |
| Intervention quality | Correct pelvic muscle contraction checked. Follow up visits checked performance and tailored intervention to individual | |
Dougherty et al. 200276
| Aim | To evaluate a BMC intervention to manage UI symptoms with older rural women in their homes | |
| Study details | RCT comparing BMC intervention against no treatment control | |
| Country and participants | Country | USA |
| Number of participants | 218 participants. Number at first follow-up: 78 BMC intervention; 69 control group | |
| Sample | Women living in seven rural counties in Florida, recruitment method NS | |
| Inclusion criteria | Older women (aged ≥ 55 years), private residence in a rural area, urine loss at least twice a week of 1 g per 24 hours or more, urine negative for bacteria, experiencing symptoms of SUI, UUI or MUI incontinence | |
| Exclusion criteria | Bladder cancer or kidney disease, indwelling urinary catheter, residual urine 100 cc or more, caregiver needed but unavailable | |
| Mean age (years) | 67.9 (SD 8.2) | |
| Type of incontinence | 68% MUI; 16% SUI; 16% UUI | |
| Severity of incontinence | NS | |
| Intervention | Behavioural intervention | BMC designed to structure the efforts of a nurse and a woman to implement procedures to manage UI in the woman’s home, consisting of three sequenced phases: (a) 2–4 weeks self-monitoring (for women assessed against lifestyle criteria); (b) 6–8 weeks BT; and (c) 12 weeks pelvic muscle exercise (if required) |
| Comparison group(s) | No treatment control group | |
| Outcomes | Primary outcome | Urine loss in grams over 24 hours |
| Secondary outcome(s) | Frequency of incontinent episodes, subjective report of urine loss, micturition frequency, voiding interval, subjective report of QoL, IIQ, goal achievement | |
| Timing | Post treatment 6 months; follow-up at 12, 18 and 24 months | |
| Notes | Study quality | No details of allocation sequence and allocation concealment. Blinding of primary outcome measure, but not secondary outcome measures. Attrition reasons incomplete |
| Intervention quality | No details of teaching methods or content | |
Kafri et al. 200781
| Aim | To compare the effectiveness of rehabilitation treatment with a standard drug treatment for urge UI in women | |
| Study details | Parallel clinical trial with alternate allocation, comparing behavioural or drug treatment | |
| Country and participants | Country | Israel |
| Number of participants | 44 participants, number at first follow-up: 16 in behavioural training group; 21 in drug treatment group | |
| Sample | Women, attending an outpatient urogynaecologic clinic in Tel Aviv | |
| Inclusion criteria | Women, aged ≥ 18 years, diagnoses with UUI, who demonstrated an overactive bladder in urodynamic testing | |
| Exclusion criteria | Residual urine greater than 100 ml, urinary tract infection, previous retropubic suspension surgery, urethral obstruction, cognitive or psychiatric impairment or drug treatment for depression | |
| Mean age (years) | 55 (SD 9) | |
| Type of incontinence | UUI | |
| Severity of incontinence | NS | |
| Intervention | Behavioural intervention | Educational programme teaching PFMT, urge suppression and lengthening the interval between voiding, delivered to individuals in five meetings over 12 weeks by an experienced nurse. Participants maintained a urinary diary, but it is unclear whether or not this was continuous or intermittent |
| Comparison group(s) | Oxybutinin 5 mg daily for 12 weeks, attending clinic for baseline and post-treatment outcome measurements | |
| Outcomes | Primary outcome | Frequency of void per day, and per night |
| Secondary outcome(s) | Frequency of episodes of incontinence, adverse events, QoL (I-QOL) | |
| Timing | 3 months (post treatment), 6 months, 21 months | |
| Notes | Study quality | No reports of blinding, incomplete outcome data |
| Intervention quality | Author contacted for details of intervention protocol, but no further details available | |
Macaulay et al. 198787
| Aim | Comparison of psychotherapy, bladder drill and propantheline for detrusor instability or sensory urgency | |
| Study details | Randomised trial | |
| Country and participants | Country | UK |
| Number of participants | n = 50 | |
| Sample | Women attending a urodynamic clinic who agreed to take part in a treatment trial | |
| Inclusion criteria | Detrusor instability or sensory urgency | |
| Exclusion criteria | NS | |
| Mean age | NS | |
| Type of incontinence | Detrusor instability or sensory urgency | |
| Severity of incontinence | NS | |
| Intervention | Behavioural intervention | BT + PFMT: participants were seen by the unit nurse fortnightly for 3 months, and again after 1 month (i.e. seven sessions) |
| Comparison group(s) | Comparison group: brief eclectic psychotherapy 8–12 weekly sessions for 50 minutes. Control group: propantheline | |
| Outcomes | Primary outcome | NS |
| Secondary outcome(s) | Bladder capacity, first sensation, nocturia, urgency, incontinence, somatic symptoms | |
| Timing | Post treatment = 12 weeks, 6 months | |
| Notes | Study quality | No details of randomisation method or of allocation concealment. Blinding NS. Numeric outcome data not reported |
| Intervention quality | Intervention content not described in any detail | |
McDowell et al. 199989
| Aim | To examine the short-term effectiveness of behavioural therapies in homebound older adults | |
| Study details | Randomised crossover trial comparing CBI against attention control | |
| Country and participants | Country | USA |
| Number of participants | n = 124 [data only reported for cognitively intact individuals (n = 105)] | |
| Sample | Older homebound adults living within the catchment area of two large home health-care agencies | |
| Inclusion criteria | Individuals aged ≥ 60 years, homebound, who understand and speak English, are willing to participate, and report at least two urinary accidents per week, with incontinence persisting for at least 3 months | |
| Exclusion criteria | Severe pelvic prolapse, terminal illness, post void residual urine greater than 100 ml, unable to toilet independently and with no caregiver willing and able to assist, unable to provide satisfactory self-report bladder diary data after three attempts | |
| Mean age (years) | 76.8 (SD 7.2) | |
| Type of incontinence | Any | |
| Severity of incontinence | Most participants (n = 76, 72.4%) had severe incontinence (10 or more accidents/week) | |
| Intervention | Behavioural intervention | PFMT + electromyography BIO, with urge or stress strategies dependent on the type of incontinence, and BT for people reporting frequency. The programme could also include environmental and lifestyle advice including restricting caffeine, timing fluid intake and advice/treatment for lower limb oedema and constipation. The programme was delivered over 8 weeks by a nurse practitioner |
| Comparison group(s) | The control group received visits from a nurse practitioner every 1–2 weeks to provide social interaction, but no advice about continence was given and participants were not asked to keep a continuous bladder diary | |
| Outcomes | Primary outcome | Reduction in number of incontinent episodes per day |
| Secondary outcome(s) | Adherence | |
| Timing | Post treatment (8 weeks), and 3, 6, 9 and 12 months post treatment | |
| Notes | Study quality | Sequence generation and completeness of outcome reporting are adequate, but the details of allocation concealment and blinding are not reported |
| Intervention quality | PFMT BIO used and repeated if necessary, and advice given to maintain practice after 8-week intervention ended. Weekly monitoring and feedback provided | |
McFall et al. 200090
| Aim | To assess a community-based small group behavioural intervention for UI in older women | |
| Study details | RCT with waitlist control group | |
| Country and participants | Country | USA |
| Number of participants | n = 145 | |
| Sample | Women in four US states responding to a call for participation as part of public health education | |
| Inclusion criteria | Women, aged ≥ 65 years, self-reporting UI for 3 months or more | |
| Exclusion criteria | Urological conditions such as prolapse or infection, raised blood glucose; sensory, functional or disability problems; cognitive impairment | |
| Mean age | 74 | |
| Type of incontinence | MUI | |
| Severity of incontinence | Mild | |
| Intervention | Behavioural intervention | BT, PFMT and urge strategies, delivered over five sessions by trained personnel in small group community settings |
| Comparison group(s) | Waitlist control | |
| Outcomes | Primary outcome | % reduction in UI episodes |
| Secondary outcome(s) | UI symptoms, QoL, UI impact, satisfaction | |
| Timing | Post treatment (9 weeks), and 3 and 12 months post programme | |
| Notes | Study quality | Methods of randomisation, allocation concealment and blinding are unclear. Data are reported on 108 participants. No outcome data are reported for QoL measures |
| Intervention quality | No details of the content of the BT or PFMT programme, or of teaching methods | |
Subak et al. 2002100
| Aim | To evaluate the effect of a low-intensity behavioural therapy programme (BT) on UI in older women | |
| Study details | Randomised clinical trial comparing low-intensity behavioural intervention against no treatment control | |
| Country and participants | Country | USA |
| Number of participants | 152 participants, number at first follow-up: 66 (86%) behavioural intervention, 57 (76%) control group | |
| Sample | Women aged ≥ 55 years with UI recruited at a northern California health maintenance organisation | |
| Inclusion criteria | (1) Women, aged ≥ 55 years; (2) ambulatory, living independently in the community; (3) functionally capable of independent toileting; (4) reporting at least one UI episode weekly over the past 6 months | |
| Exclusion criteria | (1) Uncontrolled diabetes mellitus; (2) urinary tract infection; (3) history suggestive of urinary obstruction or overflow, functional incontinence, or urinary tract anomalies | |
| Mean age (years) | 69 (SD 7) | |
| Type of incontinence | SUI; UUI; MUI | |
| Severity of incontinence | Approximately 50% mild, 25% moderate, 25% severe | |
| Intervention | Behavioural intervention | Educational programme, BT and PFMT, delivered by nurse educators over six weekly sessions of 30 minutes, to groups of three to five women. Participants maintained a daily urinary diary |
| Comparison group(s) | Women received no instruction, but kept urinary diaries for 6 weeks, then received the behavioural training over the next 6 weeks | |
| Outcomes | Primary outcome | Number of incontinent episodes per week |
| Secondary outcome(s) | Number of diurnal and nocturnal voids per week, subject report of how much the behavioural therapy programme had helped them | |
| Timing | Post treatment at 6 weeks and 6 months | |
| Notes | Study quality | Adequate sequence generation and allocation concealment. Blinding of statistical analysts to group allocation for primary outcome, but unclear for outcome data collection for subject report. Analysis only included women completing 6 weeks. Subject report not presented, and outcomes not reported separately at 6 months |
| Intervention quality | PFMT by verbal and written instruction only. No details of adherence check, although bladder diaries used as basis for weekly review | |
Wyman et al. 199831
| Aim | To evaluate the relative efficacy of BT, pelvic muscle exercises with BIO-assisted instruction, and combination therapy incorporating both interventions in women | |
| Study details | Randomised clinical trial with three arms | |
| Country and participants | Country | USA |
| Number of participants | 204 participants: 67 combination therapy, 68 BT, 69 PFMT | |
| Sample | Sample recruited via adverts and referrals to two centres in Southeastern USA | |
| Inclusion criteria | Community-dwelling women, aged ≥ 45 years, who were ambulatory, cognitively intact, able to toilet independently, reporting urine loss at least once per week, and with urodynamic evidence of SUI or detrusor instability | |
| Exclusion criteria | Reversible causes of UI, uncontrolled metabolic conditions, residual urine volume > 100 ml, urinary tract infection, genitourinary fistula or indwelling catheterisation, inability to correctly perform a pelvic muscle contraction | |
| Mean age (years) | 61 (SD 1) | |
| Type of incontinence | 71% SUI; 14% UUI; 15% MUI | |
| Severity of incontinence | No details given | |
| Intervention | Behavioural intervention | Combination therapy included a structured programme of education (audiovisual presentation with written and verbal instructions) delivered by trained nurses over 12 weeks. The programme covered BT, PFMT, urge suppression and stress leakage prevention techniques, and included self-monitoring of voiding behaviour with daily treatment logs, compliance assessment and positive reinforcement techniques. Clinic visits were on weeks 1–6, with bi-weekly telephone follow-up and mailing of diaries weeks 7–12 |
| Comparison group(s) |
|
|
| Outcomes | Primary outcome | Number of incontinent episodes per week |
| Secondary outcome(s) | QoL, perception of improvement, adherence, satisfaction | |
| Timing | 3 months (post treatment), and 6 months | |
| Notes | Study quality | No details of randomisation or concealment method given, blinding not undertaken for practical reasons, outcomes only reported for participants with follow up data |
| Intervention quality | Attention given to correct procedure, adherence, follow-up, self-monitoring and positive reinforcement | |
Table of included studies: review of barriers and enablers
Dingwall and McLafferty 200675
| Aim | To explore whether or not nurses working in older peoples’ or acute medical care settings promote urinary continence in older people | |
| Study details | Qualitative: focus groups and one to one semistructured interviews | |
| Country and participants | Country | UK, Scotland |
| Number of participants | n = 21 | |
| Sample | Convenience sample from two Scottish NHS regions of all registered (Grades G to C), and non-registered nurses (HCAs and nursing auxiliaries) from seven acute medical wards and one acute medical ward for older people within one teaching hospital and 15 medical wards for older people from three hospitals in another region, including acute, assessment, rehabilitation and continuing care wards | |
| Characteristics | Seven charge nurses from continuing care, seven qualified staff from continuing care and assessment, and three non-registered nurses from medical care for older people participated in focus groups, with another four charge nurses interviewed from acute areas. High number of staff from one region were continence link nurses | |
| Data collection | Method | Five focus groups arranged by area and grade of staff, and four individual interviews to capture views from acute area where focus groups did not happen |
| Questions/areas | Questions developed from the literature review | |
| Data analysis | Method | Transcripts were divided into comprehensible units. Features and patterns identified and labelled as themes by one researcher. Emergent categories and themes checked by a second researcher |
| Themes | Assessment of urinary continence Barriers to promoting continence Nurses perceptions of patients’ attitudes towards UI Interventions to promote urinary continence |
|
| Notes | Study quality | NHS regions chosen based on convenience, but all registered nurses (Grades G to C) and non-registered nurses (HCAs and nursing auxiliaries) invited to participate. Sample was approximately 3% of the population. Questions used were based on previous research. Data analysis not described in detail. Categories and themes checked by second researcher |
Lekan-Rutledge et al. 199886
| Aim | To investigate NAs perceptions of problems associated with the implementation of PV in a LTC nursing facility | |
| Study details | Survey, questionnaire | |
| Country and participants | Country | USA |
| Number of participants | n = 141 | |
| Sample | Convenience sample of 165 NAs from 33 LTC facilities in two US states, who were participating in an educational workshop. Response rate 85% | |
| Characteristics | NS | |
| Data collection | Method | Questionnaire. Primary objective of questionnaire was to assess the NAs’ perception of barriers to their ability to implement the PV programme Questionnaire was given to NAs 6 weeks after PV programme was implemented Questionnaires distributed by staff development nurses and returned anonymously |
| Questions/areas | 11 multiple choice items assessing knowledge attitudes and skills related to UI care. Questionnaire based on literature review and consultation with nurse researchers and clinicians | |
| Data analysis | Method | Descriptive statistics and correspondence analysis |
| Themes | Barriers to implementing PV Enablers to implementing PV |
|
| Notes | Study quality | Sampling clearly described, but sample characteristics unknown. No information on content, or validity/reliability of data collection tool, or analysis procedure. Impact of subgroups assessed by cluster analysis, and percentage responses given |
Mather and Bakas 200277
| Aim | To describe NAs perceptions of the specific factors that promote or hinder their ability to promote urinary continence in nursing home residents | |
| Study details | Qualitative, focus groups | |
| Country and participants | Country | USA |
| Number of participants | n = 31 | |
| Sample | NAs currently employed at two LTC facilities (one privately owned with 188 beds, one non-profit with 240 beds) in a metropolitan region of the Midwest USA were invited to participate, using flyers that listed focus group dates and times | |
| Characteristics | 100% female, 77% African American, 68% on day shift, age range from 21 to 52 years, experience mean 5 years | |
| Data collection | Method | 60-minute, semistructured focus groups with three to seven women. Interview questions provided |
| Questions/areas | Influences on decision to work in nursing home, experiences of helping residents stay dry, things helpful in helping residents stay continent, things which make it difficult to provide continence care, what advice would you give to new NAs about this part of your work, what other experience have you had that might affect your ideas about promoting continence care | |
| Data analysis | Method | Individual transcripts were analysed for trends, patterns and recurring themes using concept analysis |
| Themes | Desire to promote continence/keep residents dry Barriers to continence care Promoters of continence care |
|
| Notes | Study quality | Convenience sample. Questions clearly described. Response rates per group and total given. Description of analysis is not in-depth, and possible bias not discussed, but some checking of internal consistency |
Resnick et al. 200698
| Aim | To consider the current beliefs of NAs and directors of nursing about UI management in nursing home residents | |
| Study details | Qualitative, focus groups | |
| Country and participants | Country | USA |
| Number of participants | n = 38 | |
| Sample | Directors of nursing (n = 11) recruited from the members of the Maryland State Chapter of the National Association of Directors of Nursing Administration by postal invitation. NAs (n = 27) recruited from two urban not-for-profit nursing homes in Maryland, USA (flyers distributed) | |
| Characteristics | NS | |
| Data collection | Method | Focus groups facilitated by two people, tape recorded and transcribed |
| Questions/areas | Interview guide provided, covering causes of UI, treatment options available, challenges and support when working with residents to maintain continence, barriers and enablers to helping residents maintain continence | |
| Data analysis | Method | Content analysis using ‘in vivo’ or ‘grounded’ coding, which used informants own words to capture a particular idea. One nurse coded, one reviewed codes. Coding was then reviewed by two external researchers with experience in the topic. Description of findings to focus group participants who confirmed the themes that were developed |
| Themes | Resident influence on UI Staff/family and other contributors to UI System problems Recommended solutions to UI faculty/staffing related:
|
|
| Notes | Study quality | Some details of recruitment not clear, for example proportion and representativeness of the NA and directors of nursing samples. Data collection and analysis are in the main clearly described, but there is no description of the representativeness of responses. Second researcher reviewed and revised the codes, with external evaluator and respondent feedback |
Remsburg et al. 199997
| Aim | To assess staff perceptions of overall effectiveness of a PV programme, and to compare staff perceptions of continence outcomes with actual continence outcomes | |
| Study details | Survey, questionnaire | |
| Country and participants | Country | USA |
| Number of participants | n = 64 | |
| Sample | NAs and qualified nursing staff on four units within one 255-bed geriatric medical centre campus (one rehabilitation unit and three continuing care units), which was running a 12-week PV programme implemented by the NAs, with weekly feedback to nursing staff on compliance | |
| Characteristics | 64/88 nurses who participated in the PV intervention responded to the survey (response rate 73%), including registered nurses (n = 12), licensed practical nurses (n = 11) and NAs (n = 41) | |
| Data collection | Method | Questionnaire circulated at the end of the 12-week PV programme to all nursing staff on units that had been involved |
| Questions/areas | Questions were: Overall, the residents on your unit who participated in the programme were better/no change/worse Overall, the staff on your unit completed the toileting most of the time (80–100%), some of the time (50–79%), occasionally (< 50%), do not know Do you think the residents who are drier are happier? Yes/no Do you think that the programme should be continued? Yes/no Rating of residents response to the PV programme (better/worse, no difference, do not know) Open response option NAs completed self-monitoring forms to document the number + result of PVs, translated into a weekly compliance rate and dryness rate by research staff |
|
| Data analysis | Method | Descriptive statistics |
| Themes | Staff perceptions of the effectiveness of PV Staff reasons why the programme should not be continued |
|
| Notes | Study quality | The sampling was clear with a high response rate to survey. No report of testing data collection. Data analysis did not clearly report response rates |
Johnson et al. 200180
| Aim | To describe and compare preferences for different UI treatments in LTC from groups likely to serve as proxy decision-makers for LTC residents, i.e. residents, staff, family | |
| Study details | Descriptive comparative study, mailed survey | |
| Country and participants | Country | USA |
| Number of participants | n = 171/403 family members n = 66 nurses n = 70 volunteer residents, plus nine cognitively intact nursing home residents |
|
| Sample | Family members of residents with UI in four LTC facilities Licensed practical and registered nurses in the same four LTC facilities Residential care residents were recruited from a different facility by putting a table inviting people to volunteer in the dining room. Several cognitively intact nursing residents were also approached from the four facilities |
|
| Characteristics | Residents were average age of 87 years, 80% female and most were continent | |
| Data collection | Method | Tool tested with focus group of eight family members. Survey mailed out, with reminder after 2 weeks. Staff at the four facilities were interviewed in groups during nursing in-services. A small proportion were interviewed individually. Trained research assistants administered the survey, mostly by individual interview but some groups of two to five residents |
| Questions/areas | Tool to elicit choice using hypothetical scenarios based on a balanced description of the advantages and disadvantages of five potential UI treatments: catheters, PV, nappies, electrical stimulation, medications. Seven questions, pairing two of the five possible UI treatments. Measured on a graphic rating scale with 11 points anchored at 0 (do not know or uncertain). 5 = definitely would prefer this treatment, 3 = probably would prefer this treatment. Open question for qualitative response | |
| Data analysis | Method | Qualitative responses were categorised by inductive content analysis, i.e. post hoc, data driven |
| Themes |
|
|
| Notes | Study quality | There is adequate explanation of the targeted sample and the quantitative data collection in the survey. The final sample is not detailed, analysis of qualitative data are less clearly explained. No reference to testing the robustness of the findings/analysis |
Milne and Moore 200693
| Aim | To enhance understanding of self-care strategies individuals employ, the perceived benefits of these strategies, the factors that influence their self-care choices, and the factors that impede or facilitate maintenance of behavioural therapies | |
| Study details | 2002. Qualitative descriptive study, using individual and focus group interviews | |
| Country and participants | Country | Canada |
| Number of participants | n = 38 | |
| Sample | Advertising in health clinics, newspapers and womens’ health educational sessions (n = 14), and purposive sampling from three local clinics specialising in the treatment of UI (n = 300). Participants assigned to individual or focus group interviews based on the order of their phone call to researcher. First 15 interviewed individually; subsequent callers (n = 23) invited to attend group interviews. Inclusion criteria were (a) community-dwelling adults aged ≥ 18 years, (b) history of UI, (c) independent in ADLs, (d) able to provide informed consent in English, (e) able to articulate self-care strategies they had initiated. Sample includes people continent at the time of interview but with experience of UI | |
| Characteristics | 33 women, 5 men. Mean age 65 (range 24–86) years, 14/38 had sought help from the same physiotherapist in private practice; 2/38 recently undergone surgery and were continent; 3 had had surgery; 6/38 had tried medication | |
| Data collection | Method | 15 individual interviews and 3 focus group interviews. Interviews were conducted in participants home, and taped. Notes taken were validated with respondent. Focus groups were held at a University, and three individuals who knew each other met at home |
| Questions/areas | Topic guide: self-care strategies for UI, factors participants believed had impacted self-care choices | |
| Data analysis | Method | Content analysis, preliminary coding of broad substantive category of content (e.g. psychosocial issues, help-seeking) for each unit of analysis. Categories then re-examined for patterns Codes/categories reviewed with another researcher. Third level of analysis: links or threads existing within/between categories |
| Themes | Dietary modification, BT barriers and facilitators, PFME barriers and facilitators | |
| Notes | Study quality | Participants recruited through advertising in health clinics and local newspapers, followed by purposive sampling of physiotherapy clients. Sample selection, and methods of data collection and analysis are adequately explained |
Hay-Smith et al. 200779
| Aim | To seek the women’s experiences of PFMT, exploring the women’s understandings of the exercises and the way they exercised, thereby providing insights for interpreting the trial findings | |
| Study details | 2001. Qualitative study with women who had participated in a trial of PFMT | |
| Country and participants | Country | New Zealand |
| Number of participants | n = 20 | |
| Sample | Purposive sampling of women participating in an trial of PFMT, to select women from both intervention groups (two different types of muscle exercise), with a range of treatment outcomes, ages, referral sources and date of trial entry | |
| Characteristics | Women were from one city in New Zealand. Three-quarters had self-referred, but two-thirds of women in the trial had been referred from a health-care professional. Women were English speaking and willing to be involved. Mean age 51 years (SD 14). All had SUI | |
| Data collection | Method | Respondents were interviewed between 1 and 16 months after trial completion by first author. Respondent chose interview place, interviews lasted 30–90 minutes |
| Questions/areas | Prompt sheet used: content of the PFMT programme used during and after the trial, adaptations to the recommended training programme, incentives and disincentives to training, knowledge and beliefs about PFMT | |
| Data analysis | Method | Descriptive content analysis, blocks of text coded and grouped into categories. Categories grouped into themes. Theme development was based on similarities (consistency and strength of data across interviews), and differences (i.e. diversity and breadth) in the blocks of text and categories of meaning. Independent researcher (KR) checked the validity of the identified categories and themes |
| Themes |
|
|
| Notes | Study quality | Methods of sampling, data collection and analysis are clear, although the spread and distribution of responses is not presented. An independent researcher was used to verify analysis, no description of method is given. Researchers may have been involved in both trial management and interviewing |
O’Dell et al. 200894
| Aim | To increase understanding of the views of frail, older women in residential care about their QoL; how their pelvic floor dysfunction influenced their QoL and their self-perceived needs and preferences for pelvic floor dysfunction care | |
| Study details | Qualitative study, interviews | |
| Country and participants | Country | USA |
| Number of participants | n = 25 | |
| Sample | Study participants were recruited from assisted living and LTC facilities in one US state. Facilities were purposefully selected for diversity in setting, size, organisation (single or multiple care levels) and location (urban/rural). In assisted living facilities recruitment was by flyers through in house mail. In LTC facilities, staff asked potential participants. Inclusion criteria were current signs or symptoms of urinary or faecal dysfunction or vaginal prolapse, minimum age 65 years, conversant in English, cognitively intact. People were excluded if declined audiotaping, or were severely hearing or speech impaired | |
| Characteristics | Pelvic floor dysfunctions include problems with urination, defaecation or pelvic organ prolapse 23/25 had UI, 13/25 had other problems. All respondents were female. Mean age of people in assisted living facilities 87 (range 73–97) years; and in LTC, mean age 81 (range 65–89) years |
|
| Data collection | Method | Semistructured interviews, taped |
| Questions/areas | Questions for interview included: how does pelvic floor dysfunction affect your life? Are there things you would like to do that you cannot because of your pelvic floor dysfunction? Have you sought care for your pelvic problems before? What would you want for any treatment? What change would you want to see that would make the effort worth it? | |
| Data analysis | Method | Coding of descriptive categories, ‘searching for representation of participants views’ Peer review with geriatrician, urogynecologist and qualitative researcher. Clarity and validity of interpretation and analysis checked with three willing participants |
| Themes |
|
|
| Notes | Study quality | Purposive sampling used to increase diversity of settings and type of pelvic floor dysfunction. Description of sampling, data collection and testing for robustness were adequate, description of analysis method lacked detail. Clarity and validity of interpretation and analysis reviewed by three participants |
MacInnes 200892
| Aim | To understand why some women with SUI do not complete their therapy, and to make recommendations to improve treatment rates | |
| Study details | Qualitative, telephone interviews | |
| Country and participants | Country | UK, Scotland |
| Number of participants | n = 12 | |
| Sample | Convenience sample of women with SUI aged ≥ 16 years who had attended a nurse-led continence promotion clinic but not completed the SUI pathway (i.e. missed two consecutive follow-up appointments), over a 6-month period. SUI pathway based on conservative treatment, progressing to the prescription of duloxetine. Informed consent was prospective – at first clinic visit, and they were then included if they later dropped out. Women were excluded if they had predominant symptoms of another kind of incontinence; learning disability or cognitive impairment, or had been treated by the researcher | |
| Characteristics | 11/12 white British. Mean age 51.8 years (SD 11.6). All had SUI | |
| Data collection | Method | Telephone interviews, using topic guide |
| Questions/areas | NS | |
| Data analysis | Method | Results were compared and checked for emerging themes |
| Themes | Perceptions and satisfaction with the pathway Main reasons for failing to complete therapy |
|
| Notes | Study quality | It is unclear what proportion the 12 participants are of the total number of women who dropped out, and there are no details of non-responders. Data collection was by telephone interview. No description of data collection topic guide. The analysis method is not described. No description of validating findings |
Kincade et al. 199983
| Aim | To explore why patients who have presented for treatment for UI withdrew from a behavioural programme, by determining their perceptions of causes of UI, beliefs about UI treatments and their effectiveness, and stated reasons for failure to complete the programme | |
| Study details | Qualitative, interviews | |
| Country and participants | Country | USA |
| Number of participants | n = 25 | |
| Sample | People who did not complete a programme of behavioural treatment were contacted until 10 were found who agreed to be interviewed | |
| Characteristics | Respondents were all white, all female, mean age 68–8 (range 48–85) years. Those who agreed to be interviewed had been incontinent for longer, with slightly higher educational attainment than people who withdrew from the programme but did not agree to be interviewed | |
| Data collection | Method | Interviews conducted by trained interviewer not involved in the care at the clinic |
| Questions/areas | Semistructured interview guide used including patients definitions of UI and the ways that they are incontinent, their knowledge and beliefs about the causes, symptoms and treatments of incontinence, their perceptions of their health-care providers attitudes towards UI, their reasons for not completing treatment at the continence clinical, the behaviours and procedures they use to control or accommodate UI and the social or interpersonal aspects of UI | |
| Data analysis | Method | NS |
| Themes | Treatments to address UI Reasons for discontinuing treatment |
|
| Notes | Study quality | Sampling is clear, except for reasons for non-response. The data collected is explained, details of method of collection are less clear. No details given of analysis method, testing for robustness of analysis, or of methods of validating findings |
Table of included studies: review of feasibility
Perrin et al. 200596
| Aim | To determine the feasibility of using physical therapies to treat UI in older women | |
| Study details | 2002. Feasibility study | |
| Country and participants | Country | Canada |
| Number of participants | n = 10 | |
| Sample | Women aged ≥ 75 years, with UI, recruited from an outpatient urology clinic and waiting list for incontinence surgery | |
| Characteristics | Mean age 77.3 years (SD 2.9), all female. Four SUI; two UUI; four MUI | |
| Intervention | Details | Six treatments at a clinic over 6–9 weeks consisting of BT and PFMT with BIO, with a home exercise programme |
| Data collection | Method | Exercise journal in the form of a calendar in which participants marked an ‘X’ each time exercises were performed |
| Questions/areas | Data were collected on participation rates, treatment adherence, recording adherence, dropout/follow-up and long-term sustainability rates | |
| Data analysis | Method | Compliance was reviewed by calculating the proportion of women reporting execution of the prescribed exercises, completion of the diary and pad test, and attendance at sessions |
Table of included studies: review of moderators of adherence and treatment outcome
Alewijnse et al. 200169
| Aim | To elucidate the relative importance of determinants of adherence to PFME therapy among women | |
| Study details | 1995 and 1998. Cross-sectional survey, data obtained as a pretest of an RCT of a behavioural intervention | |
| Country and participants | Country | Netherlands |
| Number of participants | n = 129 | |
| Sample | Women recruited from 23 GP practice registers in the Netherlands | |
| Inclusion criteria | Risk factor for UI, self-reported UI | |
| Exclusion criteria | Neurological conditions, venereal disease, vaginal infection, women using medication for UI or that influences UI, women pregnant or within 3 months of delivery, operated on for UI condition, physical impairment making PFME impossible, unable to fill out questionnaires | |
| Mean age (years) | 55.3 (SD 10.8) | |
| Type of incontinence | 39% SUI; 49% MUI; 12% UUI | |
| Severity of incontinence | 21% mild (max one episode per week), 42% moderate (two to seven episodes per week), 37% severe (several times a day) | |
| Basis for choice of predictors | Theory | Attitude–social influence self-efficacy theory |
| Literature | Extensive literature search for determinants of intention to adhere to PFME therapy + interviews/focus group with women with and without PFME experience, and one focus group with physiotherapists specialising in PFME therapy | |
| Outcomes | Independent variables (predictors) | Attitude (pros, cons); social influences (norms, modelling, support, pressure); self-efficacy (expectations); incontinence frequency, duration; type of UI; subjective severity (I-QOL, IIQ); self-esteem; body esteem; history of sexual abuse; subjective general health; morbidity; use of medication; use of health-care resources; social desirability; sociodemographic variables |
| Dependent variables | Intention to adhere | |
| Notes | Study quality | Predictor selection was based on a theoretical model, and in the main, categorisation and measurement was clearly described. Outcome and predictors based on self-report. Outcome derived without methodological rigour within this study so psychometric properties are unclear. Some scales were found to have poor validity or reliability, i.e. attitude, social desirability, body esteem. Ordering of questioning unclear so difficult to assess potential for different forms of information bias. Poor methodology for selection of confounders, failing to provide a clear assessment of independent effect of self-efficacy variables. Rare predictor variables were excluded from analysis, and the sample included at least 10 cases with the lesser outcome for each predictor variable in the multivariate analysis, but not the univariate analysis |
Alewijnse et al. 200370
| Aim | To reveal predictors of long-term adherence among women with UI involved in PFME therapy | |
| Study details | 1995 and 1998. Longitudinal prospective study in which participants were exposed to either PFME therapy alone or PFME therapy supplemented with health education self help guide | |
| Country and participants | Country | Netherlands |
| Number of participants | n = 129 | |
| Sample | Women recruited from 23 GP practice registers | |
| Inclusion criteria | Risk factor for UI, self-report UI | |
| Exclusion criteria | Neurological conditions, venereal disease, vaginal infection, women using medication for UI or that influences UI, women pregnant or within 3 months of delivery, operated on for UI condition, physical impairment making PFME impossible, unable to fill out questionnaires | |
| Mean age (years) | 55.6 (SD 10.9) | |
| Type of incontinence | 37% SUI; 31% MUI; 9% UUI; 23% missing diagnosis | |
| Severity of incontinence | Mean 24.5 (SD 25) wet episodes per week | |
| Basis for choice of predictors | Theory | Attitude–social influence self-efficacy theory |
| Literature | Extensive literature search for determinants of intention to adhere to PFME therapy + interviews/focus group with women with and without PFME experience, and one focus group with physiotherapists specialising in PFME therapy | |
| Outcomes | Independent variables (predictors) | Intention to adhere; post-test adherence; attitude (pros, cons); social influences (norms, modelling, support, pressure); self-efficacy (expectations); incontinence frequency, duration; type of UI; subjective severity (I-QOL, IIQ); self-esteem; body esteem; sexual experiences; sex education; subjective general health; social desirability; sociodemographic variables; intervention (self-help guide or not) |
| Dependent variables (outcomes) | Adherence (7-day diary, questionnaire) | |
| Notes | Study quality | Predictor selection was based on a theoretical model, and in the main, categorisation and measurement was clearly described, although there is lack of clarity in definition of the outcome. Some measurement scales were found to have poor validity or reliability, i.e. attitude, social desirability, body esteem. Rare predictor variables were excluded from analysis, and the sample included at least 10 cases with the lesser outcome for each predictor variable condition included. Exclusion of large numbers of the original sample for various reasons, including data-driven reasons (outliers) limits the interpretation, particularly of any non-significant variables |
Burgio et al. 200374
| Aim | To identify predictors of outcome of a multicomponent behavioural training programme for UUI and SUI in community-dwelling women | |
| Study details | Secondary analysis of data from three randomised clinical trials of behavioural interventions for UI | |
| Country and participants | Country | USA |
| Number of participants | n = 258 | |
| Sample | Community-dwelling women recruited from the community and by professional referral | |
| Inclusion criteria | Average of at least two UI episodes per week and received behavioural treatment for persistent UI | |
| Exclusion criteria | Continual leakage, elevated post-void residual urine, uterine prolapse, heart failure, impaired mental status | |
| Mean age (years) | 64 | |
| Type of incontinence | 23% SUI; 76% UUI | |
| Severity of incontinence | Approximately 19% mild (five or less episodes per week), 29% moderate (5–10 episodes per week), 52% severe (more than 10 episodes per week) | |
| Basis for choice of predictors | Variables thought to have potential for influencing the outcome of behavioural treatment | |
| Outcomes | Independent variables (predictors) | Demographic characteristics; type, frequency and volume of incontinence; duration of UI; use of garment protection; previous evaluation and treatment; frequency of urination; impact of UI; medical history; medications; obstetric history; BMI; pelvic examination; urodynamic parameters; mental status; psychological distress; self-efficacy; mobility |
| Dependent variables (outcomes) | Cure (100% reduction in UI episodes as defined by bladder diary) Improvement (75% of more reduction in UI episodes, as defined by bladder diary) |
|
| Notes | Study quality | The selection of predictors was based on what was thought likely to act as predictors of outcome of behavioural training. No details of dropouts from trials. Unclear as to validity and reliability of predictor variables, with exception of details provided for use of bladder diaries. Outcome variable was self-report and therefore not collected blind to predictor variable collection. Analysis was not completed on variables with too few respondents, and was restricted to at least 10 cases for each predictor variable in the multivariate analysis, but not the univariate analysis |
McDowell et al. 199989
| Aim | To identify characteristics of short-term responders and non-responders in a trial of behavioural therapies for UI in homebound older adults | |
| Study details | Observational study within a prospective controlled clinical trial with cross-over design and two arms (cognitively intact and cognitively impaired participants). Within each arm, participants were randomised to a control or treatment group. Only results for cognitively intact participants are included | |
| Country and participants | Country | USA |
| Number of participants | n = 105 | |
| Sample | Individuals with UI identified by nurses in two large health organisations | |
| Inclusion criteria | Aged 60 years or older, homebound, report at least two urinary accidents per week, UI persisting at least 3 months | |
| Exclusion criteria | MMSE scores of less than 24, prolapse, terminal illness, elevated post-void residual urine, unable to toilet independently and with no caregiver available and willing to assist, fewer that two urinary accidents per week recorded in diary, unable to provide satisfactory self report bladder diary data | |
| Mean age (years) | 76.8 (SD 7.2) | |
| Type of incontinence | All | |
| Severity of incontinence | NS | |
| Basis for choice of predictors | NS | |
| Outcomes | Independent variables (predictors) | Sociodemographics; social influences; self-care ability; general health; cognitive ability; previous treatment; type, duration and severity of UI |
| Dependent variables (outcomes) | % reduction in number of UI episodes, treatment responder vs. non-responder | |
| Notes | Study quality | Data sources and measurement appear good, although predictor response categories are unclear in places. It is also not clear exactly which variables were considered for univariate models and subsequently multivariate models. The sample does not include enough cases for the number of predictor variables tested. Key prognostic variables (severity of UI, toileting skills, cognitive ability) are not included in final model. Independence of chosen predictors therefore somewhat unclear relative to these variables |
Oldenburg and Millard 198695
| Aim | Examination of the relationship between a number of clinical, demographic, social and psychological factors, and outcome immediately following treatment and at 18 months follow-up | |
| Study details | Clinical trial of a behavioural treatment programme | |
| Country and participants | Country | Australia |
| Number of participants | n = 53 | |
| Sample | Consecutive attenders of one clinic who entered a treatment programme consisting of BT with or without BIO techniques | |
| Inclusion criteria | Female, suffering from chronic excessive frequency and urgency of micturition, and UI without any definitive organic basis | |
| Exclusion criteria | None stated | |
| Mean age (years) | 43 (range 18–77) | |
| Type of incontinence | NS, but may be predominantly urge UI (see discussion) | |
| Severity of incontinence | NS | |
| Basis for choice of predictors | None stated, other than ‘factors thought likely to be predictive or either immediate or long-term treatment outcome’ | |
| Outcomes | Independent variables (predictors) | Severity/degree of UI; psychological problems; previous treatment; duration of UI; perceptions of control; perceptions of seriousness; adherence |
| Dependent variables (outcomes) |
|
|
| Notes | Study quality | Lack of clarity in definition of and justification for predictors. Lack of stated validity of both predictors and outcome. Invalid use of multiple linear regression in analysis of outcome. Lack of power so non-significant variables not necessarily unimportant |
Subak et al. 2002100
| Aim | To identify moderators of treatment outcome of a CBI in older women | |
| Study details | Secondary analysis of RCT | |
| Country and participants | Country | USA |
| Number of participants | n = 152 | |
| Sample | Women, recruited from a health maintenance organisation, referred by health professionals | |
| Inclusion criteria | Female, aged ≥ 55 years, living independently in the community, functionally capable of independent toileting, and reporting at least one episode of UI weekly in the last 6 months | |
| Exclusion criteria | Uncontrolled diabetes mellitus, urinary tract infection, history suggestive of urinary obstruction, overflow or functional incontinence, or urinary tract abnormalities | |
| Mean age (years) | 69 | |
| Type of incontinence | 24% SUI; 38% UUI; 37% MUI | |
| Severity of incontinence | Approximately 50% mild (one to seven episodes per week); 25% moderate (8–15 episodes per week); 25% severe (15 or more episodes per week) | |
| Basis for choice of predictors | None stated | |
| Outcomes | Independent variables (predictors) | Severity of UI, type of UI |
| Dependent variables (outcomes) | Number of UI episodes per week | |
| Notes | Study quality | Differential between-group dropout is a minor concern. Reported findings limited (no estimates, precision, etc., or p-values for tests of effect modification) |
Tadic et al. 2007102
| Aim | To determine whether or not prior depression or current depressive symptoms affect response to PFMT therapy | |
| Study details | Secondary analysis of RCT | |
| Country and participants | Country | USA |
| Number of participants | n = 42 | |
| Sample | Participants recruited from the community through local adverts | |
| Inclusion criteria | Functionally independent, community-dwelling women aged ≥ 60 years with at least two UUI episodes per week for at least 3 months, despite correction of potentially reversible causes. UI had to be pure or predominantly urge according to history and voiding diary | |
| Exclusion criteria | Women with significant cognitive impairment, i.e. MMSE ≤ 24, history of bladder/urological conditions, spinal cord lesions or multiple sclerosis, expected medication change or medically unstable | |
| Mean age (years) | 73.2 (SD 8.1) | |
| Type of incontinence | 100% urge-predominant | |
| Severity of incontinence | Mean 12.9 (SD 7.4) episodes of UI over 3 days | |
| Basis for choice of predictors | Hypothesis that the psychological burden of UUI is greater in older women, so they may respond better to therapy | |
| Outcomes | Independent variables (predictors) | Psychological burden (Urge Impact Scale UUI); current depression status (Mental Component Subscale) |
| Dependent variables (outcomes) | UUI frequency | |
| Notes | Study quality | The analysis does not seem to include severity of incontinence as a covariate. Measures of current and previous depression have acknowledged limitations, and assessor blinding is NS. The magnitude of the effects and the corresponding strength of evidence for the chosen confounders is not presented, nor is the rationale for their choice. No estimates of precision given |
Wyman et al. 199831
| Aim | To examine the relative efficacy of three forms of behavioural intervention: (1) BT; (2) pelvic muscle exercises with BIO-assisted instruction; and (3) combination therapy incorporating both interventions | |
| Study details | Subgroup analysis of RCT results | |
| Country and participants | Country | USA |
| Number of participants | n = 204 | |
| Sample | Participants recruited from two academic health science centres. Recruitment sources included newspaper articles and advertisements (41%), investigators practices (17%), other health-care provider referral (19%), and miscellaneous sources (23%) | |
| Inclusion criteria | Community-dwelling women aged ≥ 45 years who were ambulatory, mentally intact (MMSE score > 23), able to perform toileting independently, reported urine loss at least once per week, and had urodynamic evidence of genuine stress incontinence, detrusor instability or both | |
| Exclusion criteria | Reversible causes of UI, uncontrolled metabolic conditions, elevated residual urine volume, urinary tract infection, genitourinary fistula or indwelling catheterisation, and inability to correctly perform a pelvic muscle contraction on digital examination | |
| Mean age (years) | 61 (SD 10) | |
| Type of incontinence | 71% SUI; 15% MUI; 14% UUI | |
| Severity of incontinence | NS | |
| Basis for choice of predictors | None stated: model included terms for stratification variables: urodynamic categorisation, baseline incontinence severity (+ treatment site) + treatment group and baseline value of outcome variable | |
| Outcomes | Independent variables (predictors) | Type of incontinence, severity of incontinence |
| Dependent variables (outcomes) | Number of weekly UI episodes, pad weight, symptom impact (IIQ), symptom distress (UDI) | |
| Notes | Study quality | All outcomes self-reported by patients. Results were analysed for the two variables most likely to act as predictors of outcome. Variable conditions were specified and assessors would have been blind to treatment outcome at the time of data collection. The sample was of a sufficient size for the number of variables and variable conditions included in the analysis, and follow-up was over 80% at both time points |
Appendix 7 Table of excluded studies
| Author/date | Reason for exclusion |
|---|---|
| Studies of effectiveness | |
| Berghmans 2002 | UI was not included as an outcome measure |
| Brown 2007; Yap 2006 | UI was not included as an outcome measure |
| Fonda 1994, 1995 | Intervention predominantly combined BT + PFMT, but also included medication review and prescription if necessary |
| Kincade 2005, 2007 | Randomisation was in two stages, initially to self-monitoring vs. waitlist, and subsequently to different versions of a single behavioural intervention |
| Lagro-Janssen 1992, 1998 | Only MUI group got combined intervention, no separate outcome data available |
| Lee 2005 | No comparison group data |
| O’Brien 1991, 1995, 1996 | Only people with UUI got combined intervention, no separate outcome data available |
| Song 2006 | UI was not included as an outcome measure |
| Studies of barriers and enablers | |
| Diokno and Yuhico 1995 | Only provides data relating to frequencies of choice, no data relating to barriers and enablers of choice |
| St John 2006 | Not related to behavioural treatment |
| Simons 2005 | Collects data to test reliability of different response formats, no data relating to barriers and enablers of choice |
| Levy Storms 2007 | Collects data to test reliability of different response formats, no data relating to barriers and enablers of choice |
| McVean 2003 | Confounded by medical condition, therefore cannot attribute data to UI |
| Tannenbaum 2008 | Collects data to compare client and professional ratings, no data relating to barriers and enablers of choice |
| Studies of feasibility | |
| Pfister and Dougherty 1994 | Feasibility of a single intervention |
| Ouslander 2005 | Feasibility of a mixed intervention |
Appendix 8 3-day bladder diary
Appendix 9 Continence assessment completed by nursing staff
Appendix 10 Algorithm for ward staff
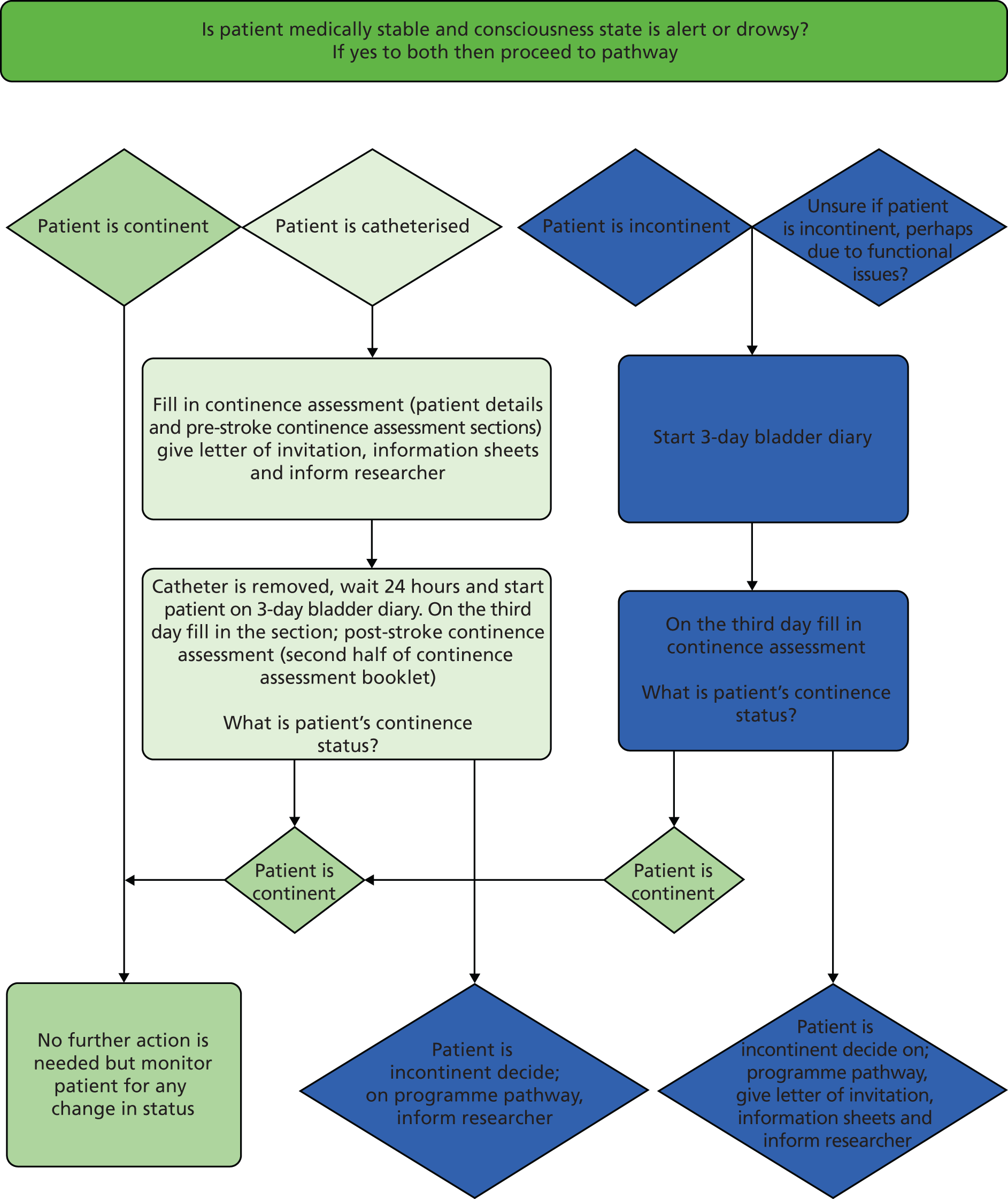
Appendix 11 Bladder training protocol
Appendix 12 Prompted voiding protocol
Appendix 13 Pelvic floor muscle training protocol
Appendix 14 Facilitation manual
Appendix 15 Action plan
Appendix 16 Case study participant information sheet
Appendix 17 Case study health professional information sheet
Appendix 18 Coding frame for analysing normalisation process theory interviews
Appendix 19 Daily clinical logs: summary of stages and quality indicators
| Stage | Key quality indicator | Key quality indicator: descriptor | Terminate data input if not achieved? | Additional information gathered |
|---|---|---|---|---|
| 1 | Regime interval (This ‘regime interval’ determines the frequency of toileting throughout the day) |
Is the regime interval present and appropriately documented? ‘Appropriate’ documentation refers to the documentation of an individual number (such as ‘2-hourly’) and NOT as a range (such as ‘2–3 hourly’) |
Yes | What is the regime interval? |
| 2 | Proposed times (‘Proposed times’ should be documented at the start of each day, based on the regime interval. The proposed times form a schedule of times for toileting, which clinical staff should then try to follow) |
Are (in-range) proposed times present and documented correctly? There should be no missing entries between the first and last documented in-range proposed time Each interval between consecutive proposed times should be identical to the regime interval (e.g. 2 hours between proposed times for a regime interval of 2-hourly) |
Yes | How many (in-range) proposed times are documented? |
| 3(a) | Times toileted Documentation |
For how many (in-range) proposed times is a corresponding ‘time toileted’ documented? The ‘times toileted’ are the actual times at which the patient was toileted and are recorded by clinical staff |
No | |
| 3(b) | Times toileted Within schedule |
For how many (in-range) proposed times is the ‘time toileted’ WITHIN 30 minutes? The ‘gold standard’ for the ICONS programme is that a ‘time toileted’ should be within 30 minutes of a proposed time |
No | For how many (in-range) proposed times is the ‘time toileted’ OUTSIDE OF 30 minutes? For how many is it MISSING? |
| 4(a) | Good practice: asking the patient if they are wet a | ‘Did you ask the patient if they were wet?’ – For how many (in-range) proposed times is ‘YES’ documented? For each toileting occasion, clinical staff are required to indicate on the clinical log if they have undertaken a number of ‘best practice’ components of the regime. These include asking the patient whether or not they are wet (if on PV regime) and giving encouragement to the patient |
No | The number of occasions on which ‘NO’ is documented and number of occasions on which answer is MISSING |
| 4(b) | Good practice: encouragement | ‘Did you give encouragement?’ – For how many (in-range) proposed times is ‘YES’ documented? As above |
No | The number of occasions on which ‘NO’ is documented and number of occasions on which answer is MISSING |
Appendix 20 Daily clinical logs: proforma used for data input
Appendix 21 Exploratory trial: participant screening form
Appendix 22 Exploratory trial: participant information sheet
Appendix 23 Exploratory trial: patient consent form
Appendix 24 Exploratory trial: outcome assessment questionnaire
Appendix 25 Patient interview schedule: intervention groups
Identifying Continence OptioNs after Stroke: Phase II exploratory trial – questions for interviews with patients, version 1
Overview of interview: consent, guidance and thanks
Consent will already have been gained for participation in the study. So prior to the interview, the researcher will check (a) that the person is still willing to participate; (b) that they understand the interview; and (c) to determine if the person is still willing to be audio-recorded. Once the researcher is assured that the person is informed and still willing to participate, the semistructured interview would commence.
These questions aim to be prompts to allow the researchers to broadly cover the same ground with each person, but the schedule will not necessarily be rigidly adhered to.
If the person has already addressed a topic, then a question covering that topic later in the interview may well be skipped (unless the researcher feels that asking it will result in an additional perspective).
If a person becomes tired or indicates they wish to terminate the interview, then the remaining questions will not be asked. If the person would like to continue with the interview at a later date, either face-to-face or over phone, then this could be arranged at a mutually convenient time.
If a person chooses not to answer a question or appears reluctant to answer a question or provide more detail, then they will not be pressed to do so.
The person will then be thanked for agreeing to take part in the interview, told that we think that their views are really important to us and that they should feel free to be frank about the things they tell us and that we will assure their anonymity and confidentiality.
The questions have been grouped to help focus the person’s thoughts about each component of the package with some introductory questions to help engage and settle the person into the interview. Before the start of the conversational aspect of the interview some key data will be collected.
Please note: the researcher should establish early on the preferred language to use in relation to terms such as voiding; for example, if the carer prefers to talk about emptying their bladder or passing water or having a wee, etc., and then use this language as appropriate. If the term ‘going to the toilet’ is used, the researcher should be sure that the participant does mean voiding rather than defecating and they should be aware that ‘going to the toilet’ is not necessarily the same as going to the toilet and voiding.
Section 1: introductory questions
These first questions are about how you feel about your urinary symptoms:
-
Can you tell me a little bit about how your urinary symptoms have affected you?
-
What impact have they had on you?
-
How have you been feeling about your urinary symptoms?
-
How confident are you that you will be able to get over these symptoms?
Section 2: questions about the continence programme
The next questions are fairly general ones that help us to find out about your general impressions of the continence programme (the programme that is helping you train your bladder):
-
How has the programme helped you?
-
Imagine that a friend asked you to explain the programme. What would you tell them?
-
What are your expectations of the programme for yourself?
-
What would you like to see happening by the time you leave hospital?
-
-
What do you think has been the best thing of taking part in the programme?
-
Has there been anything that you found difficult?
-
Please explain.
-
What did you do to overcome this?
-
How did this help?
-
What could we have done to help?
-
-
How have you found sticking to the programme?
-
Has it been hard to stick to the programme at times?
-
Can you tell me a bit more about this?
-
-
What has helped you stick to the programme?
-
How are you feeling about continuing with the programme?
-
Have you spoken about the programme with family or friends?
-
Who have you discussed it with?
-
If you haven’t discussed it, would you like to?
-
-
If someone had a similar problem to you, would you suggest that they followed the programme?
-
What advice would you give them?
-
What would you do to encourage them?
-
-
Progress can be slow. If your symptoms are not better before you leave hospital, how would you feel about that?
-
Would you carry on with the programme?
-
Section 3: questions about the information you were given
I’d like to know about the information you were given. This was about how your bladder works and how a stroke can give you bladder problems. You learned about the programme and the reasons why it could be helpful. You also learned about how to improve emptying your bladder:
-
What was the most useful thing you learned from the education leaflet?
-
How was this useful?
-
We’d like to know what you think about the education leaflet.
-
What did you like about the education leaflet?
-
What did you not like about the education leaflet?
-
-
How could we make the education leaflet better?
-
Too much information, or too little, or about right?
-
Too difficult? Too boring?
-
Couldn’t remember it all?
-
-
How helpful was the section on how the bladder works?
-
How was it helpful?
-
-
You were taught about distraction and relaxation strategies. How well did the nurses explain how they work?
-
How helpful is it to know how things are supposed to work?
-
Is there anything else you would like in the education leaflet?
-
How well did the nurses answer any questions that you had?
Section 4: questions about the voiding programme/bladder training component
These questions are about the BT part of the programme to help you get back to the pattern of voiding (passing urine/having wee/passing water/emptying bladder) before you had your stroke:
-
What do you think of the programme?
-
What was the biggest challenge?
-
What was the easiest part?
-
-
Did you feel that the programme was designed especially for you?
-
Did you have enough support?
-
How well did you understand what you had to do?
-
What could have been made clearer?
-
-
Was anything about the programme a surprise to you?
-
What was this?
-
-
Sometimes people following a programme have ‘bad or not so good days’ when it doesn’t seem to be working.
-
Have you had a day like this?
-
What did you do?
-
What helped you to get ‘back on track’?
-
Section 5: questions about the diary component
These questions ask you to think about the diary you have been filling in about your voiding habits:
-
How did you find filling in the diary? (Easy/difficult, interesting/boring/chore?)
-
When did you fill it in? (Every few hours, every day, kept forgetting about it?)
-
Did the diary help you become more aware of when you have a wee/when you pass urine/your voiding pattern?
-
Tell me more.
-
Has the diary shown you what helps and what doesn’t help?
-
Please tell me more.
-
-
-
How could we improve the diary?
Section 6: questions about the pelvic floor exercises
These questions ask you to think about the pelvic floor exercises:
-
How easy was it to learn the exercises?
-
How easy is it for you to do the exercises?
-
Tell me about how you have fitted the exercises into your day.
-
When do you do them?
-
Does someone remind you?
-
Does someone check you have done them?
-
-
Do you always do the exercises twice a day?
-
If not, what stops you?
-
-
How much have the exercises helped?
-
When did you first notice a difference?
-
What has changed?
-
Section 7: questions about the outcome criteria
These questions ask you about the ways the researchers can find out if the programme is working:
-
How has the programme helped your problems with your bladder?
-
We hope that the programme will stop patients being incontinent, or reduce the number of times that they are incontinent. Are there any other things that the researchers need to know?
-
You might not feel the need to go to the toilet so often.
-
You can ‘hold on’ for longer.
-
You might like home support (visit from a specialist).
-
You might like a telephone number for advice and support.
-
You might want advice about coping with your bladder problems when you go out.
-
You might want to know about other things that can help you manage with your bladder problems.
-
Section 8: final question(s)
If they are still having problems:
-
How confident are you that you can stick to the programme over the next 4 weeks?
-
Do you have any anxieties about it?
-
What kind of things will help you to stick to the programme?
-
What kind of things may stop you sticking to the programme?
-
These questions help to round-off the interview:
-
What is the most important thing we should know about your experience of the programme?
-
What could make the programme better for you?
-
Is there anything else you would like to tell us?
Thank you so much for taking part; it’s been very interesting talking to you.
Appendix 26 Health professional normalisation process theory interview schedule
Health professional questions using normalisation process theory framework (intervention groups)
| Dimensions | Outcome questions | NPT questions | Prompts |
|---|---|---|---|
| Coherence/sense-making: how is a complex intervention made coherent by its users? | |||
| Differentiation | What has the SVP changed in your current practice? Is it worse or better that what you were doing before? |
Do you think your practice is different now from what it was before ICONS? What is the same, and what is different? |
What has the SV programme changed? For you? For patients? For different staff groups? |
| Individual specification | Does the programme make sense to people? What do you understand the purpose of the SVP to be? Has using the SVP helped you as an individual in providing continence care? |
Do you think people understand the SVP and what they have to do? | Are there any groups who have particular difficulties? Senior staff? HCAs? Patients? |
| Communal specification | How did the team look after patients with UI after stroke before ICONS? Has using the SVP helped the team in providing continence care? Is the SVP compatible with what you do? |
Do you think people agree about the SV programme in terms of its: Purpose? How it works? |
Do all stakeholders agree on what to do and why? Qualified/non-qualified? Nursing/medical? Patient/relative? Different wards? |
| Internalisation | What were your initial impressions of the programme? Does it make sense to use the SVP to look after patients with UI? What do you think of the content of the SVP? |
Do you think people like the new programme, or not? What are the costs and benefits of doing things this way? Would everyone agree? |
Are there particular aspects that are liked more or less? Content: PV? BT? Praise? Paperwork? Support? |
| Cognitive participation: the relational work that people do to build and sustain a new practice | |||
| Initiation People drive the new practice forward |
How did you find out about your unit’s involvement in the SVP? Has there been enough direction for the programme? |
Who are the key people driving the implementation of the programme? Has this changed over time? |
How have different groups been influential? Management? External staff? Ward staff? |
| Enrolment Agreement on the new practice |
How do you see your role with respect to the SVP? Do you see this as part of your role/someone else’s role? Has involvement been sufficient? |
Do people think they should be doing this? Is everyone on board, or are some people more involved than others? |
Are any areas not involved that you think should be? For example: A&E? Nurses? Medical/others? |
| Legitimation People buy in and organise themselves |
How have the team worked together to change their practice? How have the team organised themselves to deliver the SVP? Did anything get in the way of the programme working well? |
Is the SVP running smoothly now, or are there still glitches? What sort of changes have you had to make to get the programme running smoothly on your ward? |
Has it affected groups differently? For example: Ward managers? Qualified staff? HCAs? |
| Activation People work together to develop the new practice |
What has helped staff introduce the SVP? How has the introduction of this SVP compared with other practice development initiatives? |
Has it affected how work is organised on the ward? How? |
What about how: People are allocated to different areas of the ward? Communication occurs? The routine of the day works? Night/day staff work? |
| Collective action: the operational work that people do to enact a new practice | |||
| Interactional workability (How does the work get done?) |
How did using the SVP affect your interactions with patients? How did using the SVP affect your interactions with other staff? How did you act to solve problems? Is it realistic to do the SVP on a day-to-day basis? |
Can people do what is being asked of them? Have there been any problems other than the ones you’ve mentioned (summarise)? | Can different groups do it? Staff on all shifts? All days of the week? Patients? |
| Relational integration (Staff trust each other’s work and expertise) |
How did introducing the SVP change the management of continence on the unit? Have you noticed any other changes (or spin-offs) from the introduction of the SVP on the unit? Do you think everyone would feel confident that things are being done right? |
Are you confident that the programme is being done as it should be? By everyone? |
Has it affected interactions between: Staff and patients? Qualified/HCA? Different ward areas? Different professions? Day and night staff? |
| Skill set workability (How is the work distributed?) |
How did using the SVP affect ‘who does what’ in the management of continence? What did you think about the training you got? |
Is the work allocated to the right people? Do people have the right skills and knowledge now? Is everyone trained up as much as they need to be? |
Do different groups have the ability to do the SV programme? HCAs? Patients? |
| Contextual integration (How is the work supported?) |
Are there resource implications of the SVP? Are there any other implications of using the SVP? Is there sufficient support and resources for the programme? |
What sort of things have supported the implementation of the SVP? | Time? Money? Staff? |
| Reflexive monitoring: the appraisal work that people do to assess and understand how a new practice affects them and others | |||
| Systematisation | How did you assess the value of the SVP? How does this fit with other systems in place to monitor and evaluate practice? Is the programme working? |
How do you know if the programme is working or not? Do you know this for everyone? |
What would success be for you? What would failure be? Do you think this would be the same for the patients? |
| Communal appraisal | What factors might affect the decision of the team to support the SVP? What factors might affect the decision of the team to continue to use the SVP when our project ends? On the basis of what you have seen of its results, would the ward staff think it worth continuing? |
Do you think people would agree about whether it works or not? | Ward managers? All groups of staff? Patients? |
| Individual appraisal | What factors might affect your decision to support the SVP? What factors might affect your decision to continue to use the SVP when our project ends? |
If it was up to you, would you carry on doing it? | What would affect your decision? |
| Reconfiguration | Do you think using the SVP has affected the way clinical practice is organised? How easy was it to implement? If you could change one thing to improve the programme, what change would you make? |
Do you think it is being done according to the instructions? Has the programme been modified in any way to suit the ward, other than what you have already mentioned? |
For better? For worse? How compatible was it with other aspects of stroke patients’ care? |
Appendix 27 Trial manager’s report
Trial manager’s report
Site name:
| Narrative report (describe any major features of set up and running of the trial in this site that could be pertinent to outcome): | ||||||
| Dimension | Low | Neutral/mixed | High | Comment, justification | ||
|---|---|---|---|---|---|---|
| Agreement/engagement | Ward staff resistant, reluctant to engage with programme | Staff enthusiastic, strongly motivated to engage | ||||
| Recruitment | Low or inconsistent recruitment | High steady recruitment rate | ||||
| Research nurse | Weak involvement, problematic or inconsistent presence | Strong, competent, consistent research nurse presence | ||||
| Ward leadership | Inconsistent leadership at ward level | Ward manager consistently drove and supervised programme | ||||
| Fidelity | Deviations from programme, problems with implementation | Programme delivered smoothly, few problems | ||||
| Training/support | Training/support missed, lack of attendance | Training/support well attended | ||||
| Staffing | Frequent/extended staffing problems with permanent staff | Ward staffing maintained at a reasonable level | ||||
| ICONS staffing | Frequent/extended staffing problems with ICONS staff | ICONS staffing maintained at a reasonable level throughout | ||||
| Workload | Heavy: high turnover, dependency, number | Moderate: uncommented on | ||||
| Adverse Incidents | Ward disruptions, events that interfered with programme | No unforeseen adverse incidents | ||||
Appendix 28 Example of normalisation process theory site summary
Site code:
| NPT code | Dimension | Low | Neutral/mixed | High | Comment, justification | Agree with trial manager/soft systems analysis? | ||
|---|---|---|---|---|---|---|---|---|
| Differentiation | No previous intervention: mainly containment | ✗ | Programme components already in place | We were doing very little before (ID1.1) | ||||
| Agreement Understanding |
Definition | Tight: only frank incontinence | ✗ | Loose: includes people with frequency | No reference | |||
| Understanding/agreement | Problems with interpretation/agreement | ✗ | ✗a | Good understanding and agreement | Some problems with understanding reviews | |||
| Value, importance | Value | Continence not a priority | ✗ | High value placed on continence | Incontinence wasn’t always a priority | |||
| Key people | Champion: RN | Inconsistent or distant championing | ✗ | Strong and consistent programme leadership | RN was good, but she left | |||
| Champion: ward | Inconsistent or distant championing | ✗ | Strong and consistent programme leadership | RN and ICONS HCAs appeared to be more referenced | ||||
| Enrolment | Involvement | RN plus ICONS HCA run programme | ✗ | Ward staff fully involved in programme | Most days covered by ICONS or bank staff (ID6.5) | |||
| Recruitment | Low or inconsistent recruitment | High steady recruitment rate | ||||||
| Legitimation Activation |
Work allocation | HCAs deliver toileting alone unhappy about it | ✗ | All ward staff involved in delivering programme | HCAs in the main did toileting | |||
| Workability | Fidelity | Deviations from programme, problems | ✗ | Programme delivered smoothly, few problems | ‘It worked well’ some confusion about weekly review | |||
| Skills | Training/support | Training/support missed, inadequate | ✗ | Training/support adequate, well received | ‘The training was fine’ | |||
| Resources | Staffing | Frequent/extended staffing problems | ✗ | Staffing maintained at a reasonable level | ||||
| Workload | Heavy: high turnover, dependency, number | ✗ | Moderate: uncommented on | Comment on busy ward | ||||
| Appraisal | Outcomes | Lack of visible success, patchy, few | ✗ | Visible success, staff agree it is working | ‘It’s better’ | |||
| Continuation | Not continued | ✗ | Continued | Toileting continued | ||||
Appendix 29 Soft systems analysis interview schedule
Systems analysis of post-stroke continence management
The interviews aim to:
-
explore participants’ understanding of the organisation and delivery of urinary continence care throughout the stroke pathway
-
identify how organisational issues (patient, staff, team, service and setting) shape the delivery of post-stroke continence care
-
determine barriers and facilitators which are anticipated to influence the degree to which a SVP algorithm can be embedded in acute stroke care.
Interview schedule
Introductions
Introduction of research staff.
Explain purpose of the interview.
Check provision of relevant study information sheet.
Confirm informed consent.
Clarification of role
Ask for a description of the participant’s role.
Confirm role setting and operational boundaries in terms of stroke pathway:
-
prevention/acute/rehabilitation/LTC
-
hospital/outpatients/community.
Check any previous involvement in:
-
clinical management of UI
-
relevant education and training
-
relevant service development
-
research studies.
Systems analysis
Provide visual map of generic stroke pathway.
Confirm appropriateness of map (to be developed iteratively during interviews).
Confirm that the interview will now focus on the management of a patient’s post-stroke UI across the whole pathway.
Continue to use the map as a visual prompt.
Customers (size and significance of the problem)
When in the pathway is patients’ urinary continence status assessed?
How is urinary continence assessed? Any differences in assessment in pathway components (e.g. acute and rehabilitation units; hospital and community)?
Who assesses continence?
Are any patients or problems with UI missed?
To what extent do you think current continence care meets needs? Are all needs met? Whose needs are not met by this service and why?
Are families involved in continence management? Does involvement differ in different components of the pathway (e.g. hospital and community)?
For patients who experience UI (and their families), what information is provided, when and by whom?
Who monitors the information for patients (before it is given out)? How is this done?
Actors
Which staff are responsible for aspects of the management of UI? Consider:
-
assessment
-
planning/goal-setting
-
delivering generalist interventions (providing continence care within the stroke team)
-
delivering specialist interventions (integrating specialist continence practitioners or services)
-
-
co-ordinating individual patients’ continence care.
What are the core activities of staff? Do these differ across the pathway?
What education and training do staff have in relation to continence care?
What links are there with specialist continence services across the pathway?
For each link:
-
When are patients referred and why?
-
How are patients referred and what information is shared (at referral and end of specialist intervention)?
-
How is specialist and generalist (delivered by stroke service staff) continence care integrated?
Transformations
What are the main aims of continence management at different parts of the stroke pathway (e.g. cure/containment)?
How easy is it for patients to receive different continence interventions at different times (e.g. for stroke patients in community settings, how can they reaccess continence assessments?)
Can you tell me about relevant documentation/protocols which describe plans for continence management across the pathway?
-
Referral processes?
-
Records, methods, storage, access?
Are any aspects patient held?
How are records monitored or audited? Who is involved in this?
World view
What aspects of post-stroke UI do you think should be able to managed within the stroke service?
What is good about the management of post-stroke UI?
Why is this? Who else thinks so?
What is not so good about the management of post-stroke UI care?
Why is this? Who else thinks so?
What opportunities are there for sharing information (e.g. new research) about continence management to relevant staff across the pathway?
Are data about UI (e.g. audit and evaluation information) shared across the pathway? Who takes responsibility for this?
What would be the key levers for change if you wished to alter the management of post-stroke UI in the acute stroke phase?
Who would be the key individuals that you would need to influence?
What do you think the key obstacles would be?
Are there any examples of successful research or service development projects about post-stroke UI?
Have any projects not been successful? If so, who do you think they were not?
Owners
Have commissioners made any specific recommendations or requirements about the management of post-stroke UI?
Are any aspects of post-stroke UI included in service specifications or business plans?
Who do you anticipate would be involved in considering the results or feedback from any evaluation studies or audits (e.g. Sentinel Stroke Audit) that included aspects of continence management?
Has continence ever been the key/sole issue, or is it included as one aspect of the whole package of stroke care? To what degree to commissioners get involved in these types of activity?
In your experience, have issues around UI cropped up in any patient/public consultation activity?
Environment
What do you think the different environmental challenges in delivering post-stroke urinary continence care across the stroke pathway?
-
Equipment and resources.
-
Practicalities of managing UI in hospital compared with home.
Interview closure
Is there anything else you’d like to tell me about the management of UI after stroke that you feel we should have asked you?
Many thanks for your time.
Confirm any arrangements for checking accuracy of interpretation of views.
Appendix 30 Total staff time spent toileting one patient on one occasion, with associated cost
| Patient 1a | Patient 2b | Patient 3c | Patient 4d | ||
|---|---|---|---|---|---|
| Average total staff time with patient (minutes) | 5.75 | 11.79 | 25.30 | 33.69 | |
| Proportion of time | |||||
| Band 2 | 0.46 | 2.62 | 5.37 | 11.52 | 15.33 |
| Band 3 | 0.18 | 1.02 | 2.10 | 4.50 | 5.99 |
| Band 4 | 0.05 | 0.29 | 0.59 | 1.26 | 1.68 |
| Band 5 | 0.20 | 1.14 | 2.35 | 5.04 | 6.71 |
| Band 6 | 0.08 | 0.48 | 0.98 | 2.11 | 2.81 |
| Band 7 | 0.03 | 0.20 | 0.41 | 0.88 | 1.17 |
| Associated cost (£) | |||||
| Band 2 | 0.30 | 0.78 | 1.60 | 3.42 | 4.56 |
| Band 3 | 0.33 | 0.34 | 0.70 | 1.50 | 2.00 |
| Band 4 | 0.39 | 0.11 | 0.23 | 0.49 | 0.65 |
| Band 5 | 0.48 | 0.55 | 1.12 | 2.41 | 3.21 |
| Band 6 | 0.57 | 0.28 | 0.56 | 1.21 | 1.61 |
| Band 7 | 0.69 | 0.14 | 0.28 | 0.60 | 0.81 |
| Cost per patient per occasion (£) | 2.19 | 4.49 | 9.65 | 12.84 | |
Appendix 31 Mean cost of receiving face-to-face training per site
| Staff type | Cost per staff type per hour (£) | Total staff (from four centresa) | Cost (£) |
|---|---|---|---|
| Ward managers/sisters | 58 | 4 | 464 |
| Ward sister | 50 | 7 | 700 |
| Staff nurse | 41 | 38 | 3116 |
| Research nurse | 25 | 2 | 99 |
| HCA | 21 | 29 | 1218 |
| Physiotherapists/therapy assistants | 34 | 3 | 204 |
| Occupational therapists | 34 | 2 | 136 |
| Assistant practitioners | 22 | 6 | 264 |
| Average cost/centre | 1550 |
Appendix 32 Aphasia-friendly consent form
Appendix 33 Evidence to support hypotheses from soft systems analysis
| Thinking | |
|---|---|
| Hypothesis: the SVP changed hearts and minds about UI from it being a barrier to rehabilitation to a legitimate focus of planned, therapeutic activity | |
| Balancing clinical priorities | Incontinence being important (in whatever context) relative to other interventions |
| Rehabilitation | Continence as an outcome (rather than mediator) of rehabilitation endeavour; goal-setting, progress review |
| Planning | |
| Hypothesis: the SVP made a structure for UI care explicit, enhancing consistent, knowledge-based delivery | |
| Distributed leadership | Multiple people leading continence issues; co-ordinated approaches |
| Knowledge and skills | Needs-led education and training around continence |
| Clinical geography | Synergy between the clinical environment and continence work |
| Doing | |
| Hypothesis: the SVP helped staff to make the shift from an organisational approach to continence that was haphazard, routine and selective to one that promoted regularity, inclusion and individualised management | |
| The importance of routine | Evidence of regularised approaches to managing continence; patterning care |
| Integrated working | Integrated working around continence; different professional perspectives; ‘everyone’s business’ |
| Continuity | Integrated community and hospital services; continuity of goals and interventions. |
| Evaluating | |
| Hypothesis: the SVP and its interpretation increased visibility and enabled staff and patients to evaluate process trajectory, workload performance and outcome | |
| Organisational strategy | Understanding of the organisational importance of continence care; audit and feedback; quality review |
Appendix 34 Qualitative assessment of clinical sites relative to anticipated mechanisms of change
| Themes | Site | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | BB | CC | DD | EE | FF | GG | HH | JJ | KK | LL | MM | |
| Thinking | ||||||||||||
| Hypothesis: the SVP changed hearts and minds about UI from it being a barrier to rehabilitation to a legitimate focus of planned, therapeutic activity | ||||||||||||
| BCP | ||||||||||||
| Rehab | ||||||||||||
| Planning | ||||||||||||
| Hypothesis: the SVP made a structure for UI care explicit, enhancing consistent, knowledge-based delivery | ||||||||||||
| DL | ||||||||||||
| K&S | ||||||||||||
| Clin Geog | ||||||||||||
| Doing | ||||||||||||
| Hypothesis: the SVP helped staff to make the shift from an organisational approach to continence that was haphazard, routine and selective to one that promoted regularity, inclusion and individualised management) | ||||||||||||
| Routine | ||||||||||||
| Int Work | ||||||||||||
| Continuity | ||||||||||||
| Evaluating | ||||||||||||
| Hypothesis: the SVP and its interpretation increased visibility and enabled staff and patients to evaluate process, trajectory, workload performance and outcome | ||||||||||||
| Org Strat | ||||||||||||
Appendix 35 Evidence to support hypotheses from normalisation process theory
| SVP | Main mechanism | Submechanisms | Proxy outcomes | Evidence of effect |
|---|---|---|---|---|
| Thinking | ||||
| Hypothesis: the SVP changed hearts and minds about UI from it being a barrier to rehabilitation to a legitimate focus of planned, therapeutic activity | ||||
| Changed perceptions: UI, role | ||||
| It’s made us mini specialists(H3.1) | Incontinence role (nursing) | Increased nursing knowledge and skill | Positive | |
| The staff are more focused on promoting continence as opposed to other things being potentially more important(F1.2) | Therapeutic potential | Increased priority/attention | Positive in rehabilitation settings | |
| It’s made people realise that continence is important, it’s not a nice thing for the patient to go through(E1.15) | Changed attitude: guilt, pride | Positive | ||
| It’s increased the interest and knowledge of the HCAs: it’s not just a job, they are more aware of why they’re doing it(E4.19) | Role change | Present for HCAs only | ||
| Staff have been going over to the acute unit to the MDT meeting to pull patients through, but continence is never mentioned(E4.25)Continence is something that a nurse now expects to be asked about, or expects to share in the multidisciplinary team ward rounds(F1.18) | More nursing input to MDT | Negative | ||
| Planning | ||||
| Hypothesis: the SVP made a structure for UI care explicit, enhancing consistent, knowledge-based delivery | ||||
| Logical structure | ||||
|
Codifying and embedding | People receiving UI care | Positive | |
|
Consistency | Less variation | Positive | |
|
Organisation | Fewer system failures | Some people fell outside structure or were trapped within it | |
| It got better just by communicating between each other, making sure everyone’s aware how to fill the forms out(L4.10) | Communication | More continence talk | Positive | |
| Doing | ||||
| Hypothesis: the SVP helped staff to make the shift from an organisational approach to continence that was haphazard, routine and selective to one that promoted regularity, inclusion and individualised management | ||||
| Changed clinical work | ||||
|
Selection | Different patients receiving care | Negative | |
|
Diagnosis | Differentiated/correct care | Positive | |
|
Routine | More care regularity | Positive | |
|
Perseverance | Sustained delivery | Positive | |
|
‘Different talking’ | Positive | ||
Appendix 36 Barriers and facilitators to systematic voiding programme implementation
| Barriers, difficulties | Facilitators, suggestions |
|---|---|
| Can people see how the new practice differs? | |
| Routine and documentation seen as different | Some sites had regular toileting in place |
| Do people agree with the new practice? | |
| Extra work and paperwork influences agreement Assessment was disliked SVP seen as unsuitable for some patient groups |
Being able to see benefit influences agreement Having enough staff influences agreement |
| Do people understand what the new practice requires of them? | |
| Some staff groups might not understand fully Some components of the SVP misinterpreted Explaining the SVP to relatives was difficult |
SVP seen as logical and thorough The SVP was easily understood, made sense Most patients accepted/understood the SVP Structured plans could help patients understand |
| Do people see the potential value of the new practice? | |
| Added work was unpopular | SVP seen to increase priority of continence Incontinence seen as amenable to change Rebalances control between staff and patient Continence control signals recovery to patient Increase nurse therapeutic role Gives nursing care structure and guidance Cuts workload in the long run |
| Who are the key individuals driving the new practice forward? | |
| Lack of staff member driving programme | Senior staff seen as key to driving the new practice Management style facilitated involvement Availability of support facilitated involvement Proactive senior staff nurses played a key role Research nurse identified as a valuable resource ICONS HCAs promoted the programme Some help provided by external facilitation |
| Do people agree that the new practice should be part of their work? | |
| It took time to get people on board and motivated Paperwork remained a significant barrier Some staff remained resistant Therapists were not involved Fear of extending hospital stay blocks involvement |
Enjoyment and reducing work helped staff engage Involvement showed not much extra work required Seeing that SVP can be done facilitated involvement Experience of success facilitated involvement Therapists did accommodate SVP in daily routine |
| Do people organise themselves to undertake the work required by the new practice? | |
| Role responsibilities need sorting out | |
| Do people work together to build the procedures needed to sustain the new practice? | |
| Symbols on whiteboard and bedboard SVP status included on handover sheet Night staff help to put paperwork out Use of care clocks as a reminder system Reminder notes in diary for weekly reviews Rationalise overlap in paperwork Co-ordinating function of paperwork valuable Other information resources valuable |
|
| Can people do what the new practice requires? | |
| Extra work: physical and cognitive | Needs reminders to run smoothly |
| Maintaining surveillance for screening | Use 3-day diary as blanket screening |
| Remembering/managing stages over time Keeping track of SVP progress overall Hard to remember who is on SVP |
Flexibility for change in health status Using whiteboards and handover Monitoring by nominated person |
| Difficulties with diary completion Assessment disliked – too long Weekly review can be misunderstood or forgotten |
Suggest not using diaries in acute areas Shorten time of diary completion Schedule weekly reviews at weekend |
| Timing difficult to schedule, remember, adhere to Staff did not know how to record refusal, accidents Ambulant patients hard to monitor Daily logs and fluid balance charts overlap Staff did not know how to stop the programme |
Patient completion of 7-day diary useful Enrol patients to remind staff Merge SVP timing with intentional rounding |
| Distraction/delay challenging for staff and patient Repeatedly asking about wetness disliked Some patients dislike regular prompting Regular toileting difficult for certain patients Some patient groups thought unsuitable for SVP |
Use methods to encourage participation and avoid confusion |
| Are people confident in each other’s work and expertise? | |
| Challenging to individualise care on a busy ward Some staff required ongoing monitoring Hard to maintain focus against competing priorities Adverse impact on ward relationships Continuity between acute and rehabilitation areas |
Increased confidence in managing continence Positive impact on interaction between staff Improved liaison with therapists Increased nursing input to MDT meetings |
| Do people have the right skills and training? | |
| Skill deficits in continence management SVP relatively difficult to learn at first Staff would have liked more training Problems with timing training and implementation Poor take up of online training Training for bank or new staff needs maintaining |
Suggest more training Suggest use of cascade training methods Suggest gradual lead in to implementation Improved skill in continence management Educational resources were useful Nominate staff for specific roles |
| Is the new practice adequately supported and resourced? | |
| Inadequate staffing or sickness Poor delivery impacts on staff morale Problems with use of bank staff Equipment or space could be lacking |
Extra staff facilitated consistent care Support from trust to protect staffing Staff liked the bladder scanner |
| Can people determine the effects of the new practice? | |
| Senior staff found the SVP hard to monitor There is a lack of paperwork for continence |
Visible success is important for motivation Feedback from the family was influential External stakeholders require evaluation |
| Do people agree about the worth of the new practice? | |
| Benefits for patients are recognised Benefits for staff are recognised Extra staff also impact on wider aspects of care |
|
| Do people think it is worth doing? | |
| Staffing levels would affect continuation Paperwork would affect continuation |
The programme structure is motivating |
| Do people make changes to the new practice? | |
| Need for a co-ordinator in the early stages Do an initial roll out to senior staff first Have a longer interval between training and start Change training to full day course Provide visual aids for people with aphasia |
Focus on patients likely to succeed Extend programme to night-time Simplify the assessment Design a care plan for recording Provide symbols for boards, badges for ICONS HCAs |
List of abbreviations
- A&E
- accident and emergency
- ADL
- activity of daily living
- AHP
- Allied Health Professional
- BIO
- biofeedback
- BMI
- body mass index
- BT
- bladder training
- CATWOE
- customers, actors, transformations, worldview ownership and environment
- CBI
- combined behavioural intervention
- CI
- confidence interval
- CLRN
- Comprehensive Local Research Network
- COMENSUS
- COMmunity ENgagement and Service User Support
- CQC
- Care Quality Commission
- EF
- external facilitator
- EQ-5D
- European Quality of Life-5 Dimensions
- FHEC
- Faculty of Health and Social Care Ethics Committee
- GIV
- generic inverse variance
- GP
- general practitioner
- HCA
- health-care assistant
- HT
- habit retraining
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICIQ
- International Consultation on Incontinence Modular Questionnaire
- ICIQ-UI
- International Consultation on Incontinence Modular Questionnaire – Urinary Incontinence
- ICONS
- Identifying Continence OptioNs after Stroke
- ICS
- International Continence Society
- IDMC
- Independent Data Monitoring Committee
- IIQ
- Incontinence Impact Questionnaire
- I-QOL
- Incontinence Quality of Life (Questionnaire)
- IQR
- interquartile range
- ISI
- Incontinence Severity Index
- LACS
- lacunar stroke
- LTC
- long-term care
- LUSQ
- Leicester Urinary Symptom Questionnaire
- MDT
- multidisciplinary team
- MMSE
- Mini Mental State Examination
- MRC
- Medical Research Council
- mRS
- modified Rankin Scale
- MUI
- mixed urinary incontinence
- NA
- nursing assistant
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NISCHR
- National Institute for Social Care and Health Research
- NPT
- normalisation process theory
- OCSP
- Oxford Community Stroke Project
- OR
- odds ratio
- PACS
- partial anterior circulation syndrome
- PFME
- pelvic floor muscle exercise
- PFMT
- pelvic floor muscle training
- POCS
- posterior circulation syndrome
- PPC
- patient, public and carer
- PSS
- Personal Social Services
- PV
- prompted voiding
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SE
- standardised effect
- SMD
- standardised mean difference
- SPSS
- Statistical Product and Service Solutions
- SRN
- Stroke Research Network
- SUI
- stress urinary incontinence
- SVP
- systematic voiding programme
- TACS
- total anterior circulation syndrome
- TV
- timed voiding
- TWOC
- trial without catheter
- UDI
- Urogenital Distress Inventory
- UI
- urinary incontinence
- UUI
- urge urinary incontinence
- WHO
- World Health Organization
- WMD
- weighted mean difference
- WTE
- whole-time equivalent